Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as award number 15/48/20. The contractual start date was in April 2017. The draft manuscript began editorial review in December 2022 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Henriksen et al. This work was produced by Henriksen et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Henriksen et al.
Chapter 1 Introduction
Background
Anthracyclines (doxorubicin and epirubicin) are used to treat a wide range of cancers, including breast cancer and lymphoma. Anthracycline administration is associated with dose-related cardiomyocyte injury and left ventricular dysfunction and heart failure leading to death. 1–3 Follow-up studies of breast cancer and lymphoma survivors demonstrate excessive cardiac events, including early and late development of heart failure. Prognosis from heart failure is poor. 4 The progression from heart muscle injury at the time of chemotherapy to development of clinical heart failure is not understood, and no preventive treatments are available.
Monitoring and identification of anthracycline-induced cardiotoxicity
Imaging
International guidelines recognise the challenge of variable patient susceptibility to anthracycline cardiotoxicity. 5 Extremes of age, cumulative anthracycline dose and underlying cardiac disorders such as hypertension, pre-existing cardiomyopathy and valve disease, are established risk factors. Baseline evaluation of cardiac function is recommended to provide a reference point and exclude hitherto unidentified disease. Recommendations for further monitoring are based on expert consensus and indicate additional evaluation of cardiac function during and following the completion of anthracycline chemotherapy depending on cumulative dose and plans for further cardiotoxic therapy. 5,6 However, cardiac imaging conducted too soon after the completion of anthracycline chemotherapy may miss the nadir of the fall in left ventricular ejection fraction (LVEF). Indeed, immediate post-treatment scanning (within 1 month) might have been a factor in the smaller-than-expected fall in LVEF observed in the control group of the Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) study. Here, patients with breast cancer receiving anthracycline with or without trastuzumab were randomised to treatment with candesartan or metoprolol. Overall decline in LVEF on cardiac magnetic resonance was only 2.6% [95% confidence interval (CI) 1.5% to 3.8%] in the placebo group. 7 Cardiotoxicity monitoring guidelines recognise the potential for late changes in cardiac function and advocate additional serial cardiac imaging monitoring in high-risk and paediatric populations. The uptake of late monitoring with cardiac imaging is variable, and there are cost implications associated with the follow-up of large populations of cancer survivors. UK patients receiving the anthracycline regimes studied in the Cardiac CARE (High-Sensitivity Cardiac Troponin I-Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy) trial routinely receive risk factor assessment and cardiac imaging before treatment together with follow-up cardiac imaging conducted at a variable interval of between 6 and 12 months following the completion of chemotherapy. Better methods are required to identify most patients who are at low risk of cardiotoxicity and do not require close follow-up.
Humoral biomarkers
Cardiac troponin (cTn) I and T are markers of myocardial injury, and plasma concentrations have been used to detect early anthracycline-induced cardiomyocyte toxicity. 8 High-sensitivity cTn (hs-cTn) assays can accurately quantify low plasma concentrations below the 99th centile upper reference limit. These lower concentrations contain important prognostic information that can identify individuals at increased risk of cardiovascular events and death. 9 In pilot work for the Cardiac CARE trial, we demonstrated that plasma cardiac troponin I (cTnI) concentrations exhibit an anthracycline dose-dependent increase in patients with breast cancer. 10 More than one-third of patients developed biochemical evidence of sustained myocardial injury with plasma troponin concentrations above the 99th centile upper reference limit. Early changes in this marker accurately predicted final concentrations at the end of chemotherapy, suggesting that this represents a patient-specific marker of on-treatment myocardial injury. Monitoring of cTn concentration during anthracycline therapy has been advocated in guidelines and adopted widely into clinical protocols despite having no broad mandate from randomised trials. 11,12 Furthermore, the reference 99th centile upper reference limit concentration and lowest concentration at which a 10% coefficient of variation is obtained varies considerably between vendor-specific platforms for both contemporary and current hs-cTnI and troponin T assays, preventing a meaningful comparison of recorded concentrations and thresholds for intervention between studies and protocols using different assay platforms. 13 A recent meta-analysis confirmed that increased plasma cTn concentrations during or after anthracycline treatment are associated with a sevenfold increase in the likelihood of developing left ventricular systolic dysfunction as well as a 93% negative predictive value for concentrations below the 99th centile. 14
More than three-quarters of patients in this meta-analysis had cTn concentrations quantified with contemporary (non-high sensitivity) assays. It is worth noting that both the chemotherapy dose and the consequent magnitude of myocardial injury recorded with cTn quantification were greater in many of the earlier studies included in this meta-analysis. 15,16
N-terminal pro-B-type natriuretic peptide quantification has been proposed to anticipate late development of heart failure in anthracycline-treated patients. 5 N-terminal pro-B-type natriuretic peptide may be considered a marker of myocardial stretch, and there are no data to support a role for monitoring and detection of early, on-treatment myocardial injury. The UK centres participating in Cardiac CARE do not routinely monitor cTn or N-terminal pro-B-type natriuretic peptide concentrations during treatment with anthracycline chemotherapy.
Existing research
Previous trials have investigated whether the administration of medications established for the treatment of heart failure can prevent systolic dysfunction in patients receiving chemotherapy. These studies are limited by (1) prescribing therapy to all patients resulting in substantial overtreatment and (2) using either an angiotensin-converting enzyme inhibitor (ACEi)/angiotensin II type I receptor antagonist (ARB) or a B-blocker rather than co-prescription, which has the most robust evidence base for improving function and survival among patients with left ventricular systolic dysfunction. 17
The combination of inhibiting the renin-angiotensin system and blocking β-adrenoreceptors has shown significant benefits in reducing morbidity and mortality in heart failure patients with reduced ejection fraction, including those with chemotherapy-related heart muscle disease. These therapies have also proven effective for asymptomatic left ventricular systolic dysfunction, the most common form of anthracycline cardiotoxicity. For instance, enalapril has been shown to decrease the risk of death and hospitalisation for heart failure in patients with asymptomatic left ventricular dysfunction. 18 Additionally, the combination of carvedilol and an ACE inhibitor has been found to reduce overall mortality in patients with left ventricular dysfunction following acute myocardial infarction. 19 However, randomised controlled trials (RCTs) investigating the potential of neurohormonal blockade for preventing anthracycline cardiotoxicity have yielded mixed results.
In the PRADA study, breast cancer patients receiving anthracycline treatment, with or without trastuzumab, were randomly assigned to receive the ARB candesartan, the beta-blocker metoprolol, or a placebo. 7 Whereas candesartan showed protection against myocardial dysfunction measured by LVEF on cardiac magnetic resonance imaging (MRI), metoprolol did not demonstrate the same effect. The decline in LVEF immediately after chemotherapy was 2.6 percentage points in the placebo group compared with 0.8 percentage points in those receiving candesartan (p = 0.021). However, in a subsequent extended follow-up study, a small decline in LVEF persisted, but there was no significant difference between the treatment groups. 20
A recent meta-analysis21 of 17 trials involving patients receiving anthracycline-based chemotherapy and randomised to neurohormonal blockade showed a 4% higher LVEF in the blockade group, along with a non-significant trend towards fewer clinical events. However, these trials were often single-centre, exhibited significant heterogeneity and displayed evidence of publication bias. Moreover, patient inclusion and randomisation did not take into account stratification for elevated risk of cardiotoxicity, and the trials frequently examined single therapeutic agents. Therefore, it remains unclear whether the potential treatment effect was diluted by including lower-risk patients exposed to different treatments.
The International Cardio oncology Society-One (ICOS-ONE) multicentre trial22 focused on patients treated with anthracycline and randomised them to receive enalapril either upfront or triggered by elevated cardiac troponin levels measured using either a traditional or a high-sensitivity assay. The trial lacked a placebo group, and changes in cardiac function were evaluated based on the development of cardiotoxicity defined by a decrease in LVEF of > 10% and an overall LVEF of < 50% on surveillance echocardiography up to 1 year after chemotherapy. This categorical definition of cardiotoxicity using echocardiography may not capture smaller yet clinically significant changes in LVEF following chemotherapy. The study found no significant difference between the two treatment approaches, but the most notable observation was the low rate of cardiotoxicity, with only three cases among the 273 patients in the trial and no instances of congestive heart failure or cardiovascular death. It remains uncertain whether initiating cardioprotective medications solely based on changes in high-sensitivity cTn concentrations affects the development of left ventricular dysfunction or heart failure.
Rationale for choice of intervention
Candesartan has an established role in the treatment of patients with left ventricular dysfunction, and in the PRADA study it demonstrated an early protective effect on LVEF in this patient population. 7 The avoidance of cough as a side effect is considered an advantage of angiotensin receptor blockade (candesartan) over ACE inhibition in this immunocompromised cancer population. The target dose and dose titration schedule for candesartan were identical in Cardiac CARE and the PRADA study. Carvedilol was tested previously in a similar population of anthracycline-treated breast cancer patients in the Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity (CECCY) trial. 23 There was no difference in the primary cardiotoxicity end point compared with placebo. Carvedilol treatment was associated with lower circulating cTnI concentrations and less diastolic dysfunction. In the Cardiac CARE trial, the target carvedilol dose of 25 mg twice-daily was the same but the interval between dose titration was much shorter, at 3 days compared with 3 weeks in the CECCY trial. The key difference in approach between the Cardiac CARE trial and previous cardioprotection studies is the focus in the former on maximum neurohormonal blockade on high-risk patients with coprescription of candesartan and carvedilol rather than a single agent.
Risks
Candesartan (ARB) and carvedilol (B-blocker) are widely used in the NHS with an established safety profile and cost-efficacy. No toxicity was reported in the PRADA study, which examined ARB and B-blocker combination in an identical study population to ours. ARBs and B-blockers are in widespread use for hypertension and other serious conditions including heart failure and following myocardial infarction. Patients in the study who were randomised to cardioprotection had their renal function and blood pressure monitored at dose titration clinics supervised by oncology research nurses. We followed a dose titration protocol used in the PRADA study. B-blockers may exacerbate psoriasis and asthma. Common side effects include lethargy and cold peripheries. For these reasons we believed that identifying an at-risk population of breast cancer and non-Hodgkin lymphoma (NHL) patients to target cardioprotection and closer monitoring was key.
Benefits
The long-term follow-up of breast cancer and NHL survivors demonstrates an increase in late cardiac events among this population, including symptomatic heart failure. 1 Clinical studies that record cardiac events during the period of chemotherapy therefore underestimate the magnitude of the problem. Improved survival has led to the recognition of the late impact of cardiac disease related to breast cancer therapies, and consensus statements from the European Society of Cardiology highlight the need for improved monitoring and preventive treatment. 5,12 Current clinical protocols for cardiotoxicity monitoring are suboptimal, aiming to identify cardiac muscle dysfunction after it has become established. Cardiotoxicity is identified by assessing cardiac function, using imaging to measure ejection fraction with echocardiography or radionuclide scans. By demonstrating that early cardiac injury can be detected with a high-sensitivity cardiac troponin I (hs-cTnI) assay, it will be possible to screen out a population of low-risk patients who do not exhibit elevation of this marker during chemotherapy and so do not require surveillance imaging.
Rationale for study
In this study, we used surveillance with high-sensitivity hs-cTnI blood testing in patients receiving cardiotoxic systemic therapy to identify early muscle injury, enabling targeted protective treatment with ARB and B-blocker. Our group’s prospective cohort studies including 6304 patients presenting to hospital with suspected acute coronary syndrome and 155 patients with moderate to severe aortic stenosis have confirmed that low cTnI concentrations (< 5 ng/l) are associated with low risk of future cardiac events. 9,24 In two independent validation cohorts of patients presenting with suspected acute coronary syndrome, we demonstrated that cTnI concentrations of < 5 ng/l had a negative predictive value of 99.4% for myocardial infarction or death at 30 days. 9 We further demonstrated that using the high sensitivity cTnI assay to define a gender-specific upper reference limit (99th centile: ≥ 16 ng/l for women and ≥ 34 ng/l for men) in patients presenting with chest pain identifies a population of women at increased risk of cardiac events who would be missed using older contemporary cTnI assays. 25 Our pilot study for the Cardiac CARE trial in anthracycline-treated breast cancer patients demonstrated an increase in hs-cTnI concentrations with progressive cycles of treatment, with many patients developing circulating concentrations of > 16 ng/l. 10 There is a continuum of heart muscle injury, and the PRADA study illustrated the potential for cardiac MRI to detect smaller, less severe changes in left ventricular function in these patients. 26 To capture patients with lesser degrees of cardiac dysfunction, we selected a hs-cTnI concentration threshold that randomised at least 33% of patients recruited into the study. Patients with hs-cTnI concentrations above the threshold were randomised to receive candesartan and carvedilol or to continue with routine clinical care.
Our research examined the clinical efficacy of ARB and B-blockade in preventing the development of heart muscle failure in breast cancer and NHL patients receiving anthracycline-containing chemotherapy. These treatments have an established role in the treatment of patients with left ventricular dysfunction. ARB and B-blockade have an additive treatment effect, and a strong treatment response has been demonstrated in patients with different heart failure aetiologies, including chemotherapy-related heart muscle disease. Response to ARB and B-blockade includes improved survival, improved symptoms and recovery of LVEF. LVEF is a potent prognostic indicator of heart failure,27,28 and changes resulting from therapy or disease progression are closely associated with outcomes. 29,30 All patients had LVEF monitored with serial cardiac MRI scans. Cardiac MRI is the most precise measure of cardiac function31,32 and provides additional measures of systolic volume and cardiac strain that will inform early mechanisms of chemotherapy-induced cardiac muscle injury. We therefore chose change in LVEF recorded 6 months following completion of anthracycline chemotherapy as the primary end point and surrogate marker of future heart failure events. The hypothesis was that carvedilol and candesartan will prevent the development of cardiac dysfunction in at-risk patients identified by elevated plasma hs-cTnI concentrations. Additional outcomes included treatment effect on ongoing cardiac injury (persistence of cTnI elevation), death and heart failure (definition is provided in version 11.0 of the protocol; link to publication is in Additional information) and a provisional health economic analysis of this selective intervention strategy to prevent chemotherapy-related heart failure.
The event rate was low in the PRADA study, with an average LVEF decline of only 2.6 (95% CI 1.5 to 3.8) percentage points in the placebo group. 7 Sixty per cent of patients received low-dose anthracycline (cumulative epirubicin dose of 240 mg/m2). Around 20% of patients also received trastuzumab, and the final MRI scan was conducted variably according to the end of adjuvant therapy: either immediately following 4–5 months’ treatment with anthracycline or at 15 months following treatment with anthracycline and trastuzumab. We set out to ensure a higher event rate by (1) only approaching patients scheduled for higher anthracycline doses (≥ 300 mg/m2 cumulative dose of epirubicin) and (2) restricting randomisation to high-risk patients identified by hs-cTnI elevation.
The hypothesis was that hs-cTnI monitoring would improve the detection of heart muscle injury compared with previous studies with the more insensitive contemporary assays. Increased concentrations of this plasma marker appear before the development of reduced left ventricular function and heart failure. Patients recruited into the study who exhibited a plasma hs-cTnI concentration above a threshold defined by our cTnI monitoring study were randomised to receive both carvedilol and candesartan (B-blocker and ARB) or to continue with standard care.
Our research plan was relevant to NHS cancer care pathways, recruiting patients receiving anthracycline and taking a precision medicine approach by targeting cardioprotective treatments only to at-risk patients.
Objectives
The study hypotheses were as follows:
-
The development of heart muscle failure measured by cardiac ejection fraction change in breast cancer and NHL patients receiving anthracycline will be prevented by carvedilol and candesartan.
-
All patients at risk of developing heart muscle failure will be detected by elevation of hs-cTnI on serial testing, validating the test as a simple screening tool for selecting patients for protective therapy and closer monitoring.
Primary objective
The primary objective was to determine whether known treatments for heart failure can prevent or reduce myocardial injury and the development of left ventricular systolic dysfunction.
Secondary objectives
The secondary objectives were to establish whether a novel highly sensitive plasma marker of myocardial injury can anticipate the development and monitor the progression of left ventricular systolic dysfunction.
Chapter 2 Methods
Trial design
We published an article examining the rationale for the Cardiac CARE trial design as well as the full protocol prior to the end of the study. 33
The study was a multicentre, prospective, randomised, open-label, blinded end-point (PROBE) RCT, with 1 : 1 individual randomisation to the treatment arm or standard care. Patients with troponin elevation were randomised. Non-randomised patients were allocated to receive standard care and were also followed up.
The clinical pathway for Cardiac CARE is illustrated in Figure 1. After enrolling in the study and receiving a baseline MRI scan, patients had serial blood tests for hs-cTnI concentration performed prior to each cycle of anthracycline. Patients could be randomised at each cycle from cycle 2 to cycle 6 for those patients receiving 3–6 cycles of anthracycline. The anthracycline cycle/cTnI concentration thresholds for randomisation were anthracycline cycle 2, ≥ 5 ng/l; and anthracycline cycles 3–6, ≥ 23 ng/l.
FIGURE 1.
Clinical pathway for the Cardiac CARE trial. a, Target doses: candesartan 32 mg o.d.; carvedilol 25 mg b.d.
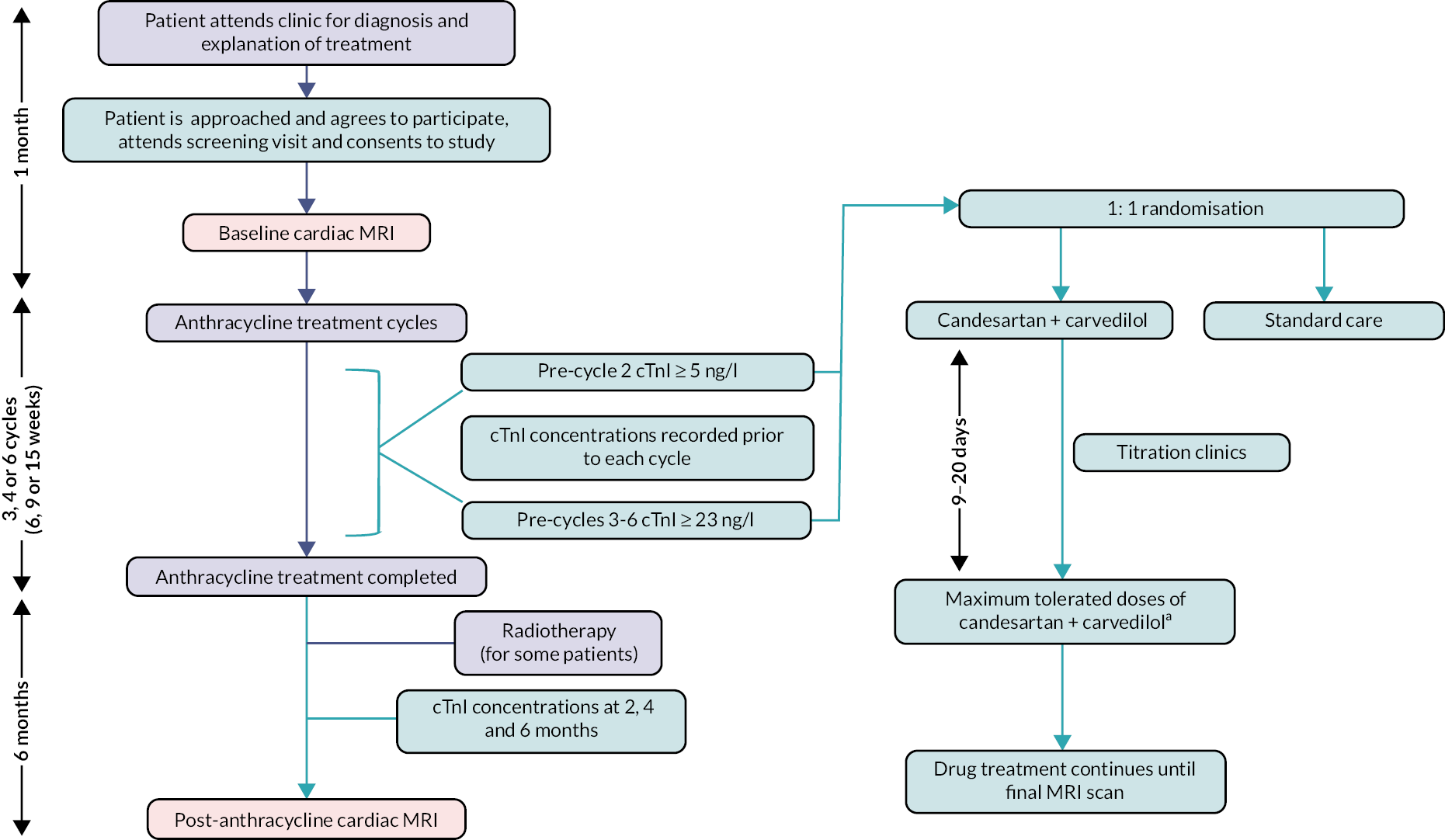
Cardiac MRI results were made available to inform clinical care. Two analysts, independent of the research teams and blinded to scan sequence (pre- or post-anthracycline scan) and treatment, conducted cardiac MRI measurements for the primary and secondary outcomes in the Core Image Analysis laboratory (Edinburgh Imaging, University of Edinburgh) in accordance with the Society for Cardiovascular Magnetic Resonance guidelines on dedicated software (CVI42 version 5.14, Circle Cardiovascular Imaging). The Cardiac CARE Cardiovascular Magnetic Resonance imaging manual has been included as Report Supplementary Material 1.
Changes to trial design
In 2018 the Trial Management Group made changes to the trial design to increase both recruitment and randomisation. Our co-applicants from the original application, the Breast Cancer Team in Leeds under Professor Chris Twelves, were projected to enrol up to one-third of patients for the study. The Leeds site did not open, and early recruitment from other sites indicated that we would need more sites and a broader population of eligible patients. Protocol version 6.0 (14 March 2018) included a major amendment to enrol patients with NHL who receive a similar anthracycline regime to breast cancer patients. A review of randomisation numbers in early 2018 indicated that ˂ 30% of trial patients were being randomised. To increase randomisations, in protocol version 7.0 (8 August 2018), the threshold concentration was reduced from 6 ng/l to 5 ng/l at cycle 2 and randomisations were allowed at cycles 3, 4, 5 and 6 with a threshold of 23 ng/l (compared with just cycle 6).
A complete list of all protocol amendments can be found in Appendix 2.
Participants
Patients aged ≥ 18 years commencing anthracycline for adjuvant or neo-adjuvant treatment of breast cancer or NHL were invited to participate in the study. Anthracycline cardiotoxicity is dose dependent, and only patients scheduled for ≥ 300 mg/m2 cumulative dose of epirubicin (or equivalent) over 3, 4 or 6 cycles were approached. The sites recorded the patients approached on the sponsor subject pre-screening log.
The research study was explained by the consultant oncologist, haematologist or research nurse at the treatment planning clinic, and the patient was invited to participate. Patients were given information on the research study to take away after the diagnosis visit. They were contacted by telephone at least 24 hours after receiving the information, and if they agreed to take part they were invited to a screening visit where they were asked to provide consent. Full written consent was obtained by physicians on the research team, the research nurse or a deputy. If appropriate, the research nurse booked a MRI scan before anthracycline chemotherapy was started.
Patient eligibility was verified by a clinical trial physician after written informed consent was obtained. Confirmation of eligibility was recorded in patients’ medical records.
Inclusion criteria
-
Female or male aged ≥ 18 years.
-
Histological diagnosis of invasive breast cancer or NHL.
-
European Cooperative Oncology Group (ECOG) performance status 0–1.
-
Planned to commence anthracycline-containing therapy.
-
For adjuvant or neo-adjuvant treatment of breast cancer: breast cancer patients scheduled for ≥ 300 mg/m2 cumulative dose of epirubicin or equivalent over 3, 4 or 6 cycles, or NHL patients planned to commence ≥ 3 cycles of CHOP or R-CHOP therapy containing ≥ 300 mg/m2 epirubicin equivalent cumulative dose of anthracycline.
-
A life expectancy of at least 12 months.
-
LVEF of ≥ 50% on baseline MRI.
-
Systolic blood pressure (SBP) of ≥ 105 and ≤ 170 mmHg.
-
An estimated glomerular filtration rate (eGFR) of > 45 ml/minute/1.73 m2.
-
Provided written consent to take part in the study.
Exclusion criteria
-
Pregnancy or breastfeeding.
-
Human epidermal growth factor receptor-2 (HER2) positive breast disease with planned trastuzumab therapy.
-
Uncontrolled arterial hypertension defined as SBP on treatment of > 170 mmHg.
-
Already taking B-blockers, ACEi or ARBs.
-
Contraindication to ARBs (eGFR of ≤ 45 ml/minute/1.73 m2, previous hypersensitivity, renal artery stenosis) or B-blockers (asthma, pathological heart block and pathological sinus bradycardia).
-
Clinically proven intolerance to lactose monohydrate.
-
A history of symptomatic heart failure.
-
Contraindication to or inability to tolerate MRI scanning.
-
Suspected poor drug compliance (suspected poor drug compliance and active alcohol or drug abuse was determined from history documented in the potential patient’s medical notes).
-
Active alcohol or drug abuse (suspected poor drug compliance and active alcohol or drug abuse was determined from history documented in the potential patient’s medical notes).
-
Previously treated with anthracyclines or trastuzumab.
-
Uncontrolled concomitant serious illness, as determined by the investigator.
-
Female or male aged < 18 years.
-
Not provided written consent to take part in the study.
-
Previously randomised into this trial.
Participant withdrawal from the trial
Patients were free to withdraw from the study at any point or a patient could be withdrawn by the investigator or responsible clinician. If withdrawal occurred, the primary reason for withdrawal was documented in the patient’s case record form. The patient had the options of (1) allowing use of the data collected up until the time of withdrawal and allowing future access to central NHS registers for future record linkage; (2) allowing use of the data collected up until the time of withdrawal but not allowing future record linkage; and (3) not allowing use of the data already collected and having these data removed from final analysis.
Withdrawal from study treatment was distinguished from withdrawal from the study. Patients who were continuing with the study but had stopped taking the investigational medicinal product (IMP) were allowed to restart at the discretion of the supervising clinician. Patients could withdraw from some study procedures or study medication but remain in the trial without a change of status. Patients who had withdrawn from the study (i.e. change of status) were not permitted to restart the study.
Anthracycline cardiotoxicity is dose-dependent. Recent studies have confirmed that cardiotoxicity is negligible at low doses, and there was no benefit to patients of continuing in Cardiac CARE if they had a change in treatment plan and did not receive anthracycline as planned. Patients who for clinical reasons stopped anthracycline-containing chemotherapy before receiving their second dose of anthracycline were withdrawn from Cardiac CARE by the investigator.
Criteria for discontinuing or modifying study medications
An investigational product could be discontinued under the following circumstances:
-
at the request of the patient or if the patient withdraws from the study
-
by the investigator or the responsible clinician if this was felt to be in the best interests of the patient
-
on completion of the study.
Following the introduction of candesartan, eGFR and serum creatinine concentration were monitored at each dose titration clinic by the oncology research nurses. A decrease in eGFR of up to 25% from baseline or an increase in serum creatinine concentration of up to 30% was accepted. Patients exhibiting changes in renal function from baseline within these limits had further dose increases at the clinical team’s discretion or remained on established doses. Patients exhibiting changes in renal function beyond these thresholds or an eGFR of < 45 ml/minute/1.73 m2 had candesartan discontinued. Patients unable to reach target dose because of symptomatic or asymptomatic hypotension (SBP of < 90 mmHg) or bradycardia [heart rate (HR) of < 50 beats per minute] continued in the study on maximal tolerated doses.
Study settings
The study recruited patients from UK regional cancer centres. All the cancer centres involved in the study had an established clinical trial infrastructure with oncology research nurses who were accustomed to co-ordinating the identification and recruitment of patients attending both the main and the satellite centres.
Interventions
This study compared standard care with standard care plus candesartan and carvedilol treatment.
Treatment arm
Standard care plus oral candesartan and carvedilol. Candesartan was started at 8 mg once-daily and increased at a minimum of 3-day intervals to 16 mg and 32 mg once-daily. Carvedilol was initiated simultaneously at 6.25 mg twice-daily and increased to 12.5 mg twice-daily and 25 mg twice-daily. The IMPs were dispensed as close as possible and ideally within 14 days of randomisation and continued until completion or withdrawal from the study. Drug prescription and dose titration visits in all sites were coordinated by oncology research nurses and supervised by oncologists.
Standard care
Standard care alone.
Outcomes
Primary outcome
Change in LVEF on cardiac MRI scan conducted 6 months after final anthracycline dose compared with baseline cardiac MRI scan conducted before anthracycline therapy compared between randomised groups.
Secondary outcomes: efficacy of candesartan and carvedilol treatment
The following biomarker, cardiac imaging and clinical end points were compared between randomised groups:
-
hs-cTnI concentrations
-
hs-cTnI concentration change from baseline to 2 months following the completion of anthracycline chemotherapy.
-
-
cardiac MRI
-
change in global longitudinal strain (GLS) and global circumferential strain (GCS) measured with feature tracking on cardiac MRI
-
change in left ventricular mass (LVM), left ventricular end-diastolic volume (LVEDV) and left atrial area (LAA).
-
Chapter 3 Clinical outcomes
-
Cardiovascular death and heart failure. Heart failure was defined by the diagnosis of clinical (symptomatic) heart failure (see protocol version 11.0 for definition, links for which are in Additional information).
-
HR and blood pressure at baseline and at 2 and 6 months following final dose of anthracycline.
The following clinically relevant thresholds for grading anthracycline cardiotoxicity were summarised by treatment but no formal statistical testing was performed.
hs-cTnI concentrations:
-
Chronic myocardial injury defined as persistent elevations of hs-cTnI above the gender-specific 99th centile at 2 months. If the 2-month sample was not available, then hs-cTnI elevation above this threshold at any point beyond this was counted.
-
Any hs-cTnI measurement of >80 ng/l during or after treatment. This concentration threshold measured with a contemporary assay has been used previously to define patients at risk of severe and early on-treatment cardiotoxicity. 8
Change in LVEF:
-
A fall in LVEF of 10% points AND a fall in ejection fraction < 50%.
-
Any fall in LVEF < 50%.
-
Any fall in LVEF < 40%.
Change in GLS and GCS myocardial strain:
-
A > 15% fall in GLS or GCS on 6-month post-anthracycline cMRI.
Clinical outcomes:
-
Death, cardiovascular death or heart failure. Heart failure was defined as the diagnosis of clinical (symptomatic) heart failure.
Specificity of hs-cTnI assay for cardiotoxicity
The trial aimed to identify patients at high risk of cardiotoxicity from early changes in hs-cTnI concentration on anthracycline treatment. The following comparisons were made within the low-risk non-randomised group. An additional exploratory comparison was made between the non-randomised and the high-risk randomised-to-standard-care groups.
Cardiac magnetic resonance imaging
Change in LVEF. This is the first secondary end point and main secondary objective. The aim was to demonstrate zero LVEF% change within the low-risk non-randomised group with equivalence limits of ± 2%:
-
change in GLS and GCS
-
change in LVM, left ventricular volume and LAA.
hs-cTnI concentrations
Compare baseline, final anthracycline cycle, 2, 4 and 6 months post anthracycline.
Health economics analysis
Health economics for all enrolled patients. Confirm the feasibility of data capture and assess the quality of data obtainable in this patient population. Provide information that can inform the design of further research including sample size calculation and/or value-of-information analysis.
Investigational medicinal product safety end points
The following were compared between the randomised groups:
-
Hypotension: SBP of < 90 mmHg.
-
Bradycardia: HR of < 50 bpm.
-
Hyperkalaemia (K+ ≥ 5.0 mmol/l).
-
Worsening renal function: decrease in eGFR of > 25% from baseline or an increase in creatinine of > 30% from baseline.
-
Acute kidney injury: an eGFR drop to < 45 ml/minute/1.73 m2.
-
Fatigue grade of ≥ 2 using Common Terminology Criteria for Adverse Events (CTCAE) classification.
-
New diagnosis of atrial fibrillation.
The protocol did not require all adverse events (AEs) to be recorded and reported in the eCRF. The safety assessments carried out for the trial and the pharmacovigilance reporting requirements are detailed in the protocol.
Changes to outcomes
No changes were made to the primary and main secondary outcomes during the study.
Sample size
A review of LVEF changes (measured by radioisotope scan) in 48 patients receiving adjuvant chemotherapy at Edinburgh Cancer Centre between 2010 and 2012 demonstrated that 15% sustained at least a 10-percentage-point change fall after anthracycline treatment and 31% exhibited the same magnitude of ejection fraction fall during trastuzumab treatment. Given the capacity of cardiac MRI to detect smaller changes in LVEF, we assumed that at least 20% of breast cancer patients receiving anthracycline chemotherapy would develop reduced LVEF. We planned to randomise at least 33% of patients using the hs-cTnI concentration threshold defined in the pilot study. We assumed that this threshold would select all patients developing meaningful reductions in LVEF. From these figures we would randomise 23 patients per group to detect a difference of 5 percentage points between the groups (standard deviation 5), at 90% power (p = 0.05). 34 A standard deviation of 5 was used in the PRADA study sample size calculation. 35 Allowing for 17% missing data (in line with PRADA) brings this to 28, and a total randomised trial size of 56. Thirty-three per cent of patients in the group initially enrolled were expected to be randomised, so the total enrolled was to be at least 168.
To assess the specificity of the plasma hs-cTnI assay for left ventricular systolic dysfunction in non-randomised patients, we wished to show that there is zero LVEF% change (with equivalence limits of ± 2%). Using a paired t-test to test for a zero change, using two-sided p-value of 0.05, 90% power and a standard deviation (SD) of differences of 5%, we needed complete paired MRI scans in 68 non-randomised patients.
Interim analyses and stopping guidelines
There was no planned interim analysis or stopping guideline.
Randomisation: sequence generation
After enrolment in the study and the baseline MRI scan, patients had serial blood tests for hs-cTnI concentration performed prior to each cycle of anthracycline. Patients could be randomised at any cycle with hs-cTnl concentration above the predetermined threshold from cycle 2 to cycle 6 for those patients receiving 3–6 cycles of anthracycline. The corresponding anthracycline cycle/hs-cTnI concentration thresholds for randomisation were:
-
anthracycline cycle 2, ≥ 5 ng/l
-
anthracycline cycles 3–6, ≥ 23 ng/l.
The allocation sequence was generated by Edinburgh Clinical Trials Unit (ECTU), using a web-based system to prevent bias. The study had a 1 : 1 randomised group design comparing standard care with standard care plus candesartan and carvedilol treatment in patients who exhibit elevated hs-cTnI concentrations during anthracycline treatment.
Randomisation: type
Patients were allocated to trial treatments using minimisation, with the following binary criteria:
-
age ≥ 65 or < 65 years
-
baseline LVEF of ≥ 60% or < 60%
-
planned cumulative epirubicin equivalent (for doxorubicin the epirubicin equivalent is double, e.g. 300 mg/m2 of doxorubicin is equivalent to 600 mg/m2 of epirubicin) dose = 300 mg/m2 or > 300 mg/m2.
Patients were allocated to the allocation recommended by the minimisation algorithm 80% of the time, and to the opposite treatment group 20% of the time, with this choice decided randomly (with no restrictions or blocking).
Randomisation: allocation concealment mechanism
Randomisation allocation was not concealed from the patient or the clinical team.
Randomisation: implementation
The allocation sequence was generated by ECTU using a web-based system that communicated the randomisation result to the local randomising clinical team.
Following randomisation, the patient and the investigator were notified of the assigned treatment allocation.
Blinding
The study had a PROBE design and there was no blinding procedure. The image analysts at Edinburgh Imaging who performed the detailed MRI analysis were blinded to treatment allocation.
Similarity of interventions
Not relevant.
Statistical methods
Edinburgh Clinical Trials Unit statisticians were responsible for analysis of the study data. The full Cardiac CARE statistical analysis plan is included as Report Supplementary Material 2.
The MRI scans from all sites were transferred to Edinburgh Imaging for detailed MRI analysis by two cardiac MRI analysts. The image analysts at Edinburgh Imaging were blinded to treatment allocation and were not involved with scanning or contact with patients. Edinburgh Imaging provided these data for the study database.
The primary analysis was change in LVEF on cardiac MRI 6 months following completion of anthracycline between randomised treatment groups, using linear regression, adjusted for age at consent, baseline LVEF and planned cumulative epirubicin equivalent dose. These were adjusted for binary fixed effects: age ≥ 65 or < 65 years; baseline LVEF ≥ 60% or < 60%; planned cumulative epirubicin equivalent dose = 300 mg/m2 or > 300 mg/m2. Baseline LVEF was the finalised Edinburgh Imaging value. This was an intention-to-treat analysis, and treatment effect was expressed by a point mean difference estimate and its 95% CI. This approach was also used for the efficacy of candesartan and carvedilol treatment secondary outcomes from cardiac MRI and hs-cTnI concentrations at 2 months.
We endeavoured to keep missing values to a minimum, and the primary analysis was a complete-case analysis. If there were sufficient missing data to cause concern, multiple imputation was to be used as a sensitivity analysis, but the primary outcome did not have sufficient missing data to cause concern (> 10%), and so multiple imputation was not necessary.
The specificity of the hs-cTnI assay for left ventricular dysfunction in non-randomised patients was assessed by calculating the mean of the within-person changes between patients’ pre- and post-anthracycline MRI scans, plus its 95% CI at the end of the study. This CI was compared with the equivalence limits of ± 2%. Specificity was evaluated on all available non-randomised patients.
Other secondary outcomes were analysed appropriately: linear regression for continuous outcomes, logistic regression for binary outcomes and Cox proportional hazards for survival analysis, adjusted as for the primary analysis. A full statistical analysis plan was finalised before database lock.
Additional analyses
The statistical analysis plan included exploratory comparisons across all cardiotoxicity measures between the low-risk non-randomised group and the high-risk randomised-to-standard-care group. This analysis was performed to quantify the magnitude of hs-cTnI concentration increase in the randomised groups compared with non-randomised patients. The comparison of cardiac MRI measures of structure and function provided an indication of how effectively the trial protocol identified a population at increased risk of cardiotoxicity.
Co-enrolment
Patients were not to take part in other clinical trials of IMPs (or devices) until 2 weeks after they finished trial medication and/or final assessments, unless agreed otherwise in advance. This was to be considered even if patients had finished the trial medication of another study but were still technically enrolled in that study for follow-up visits and so on.
Co-enrolment between Cardiac CARE and Clinical Trial of Investigational Medicinal Product (CTIMP) studies Add-Aspirin and MonarchE were agreed in writing between the sponsors and investigators for each study. Co-enrolment with other CTIMP studies was recorded in the eCRF. Participation in other research (e.g. non-CTIMP or observation studies) while taking part in this study was permissible and was recorded in the patient’s medical records but not recorded in the eCRF.
Chapter 4 Results
Patient flow
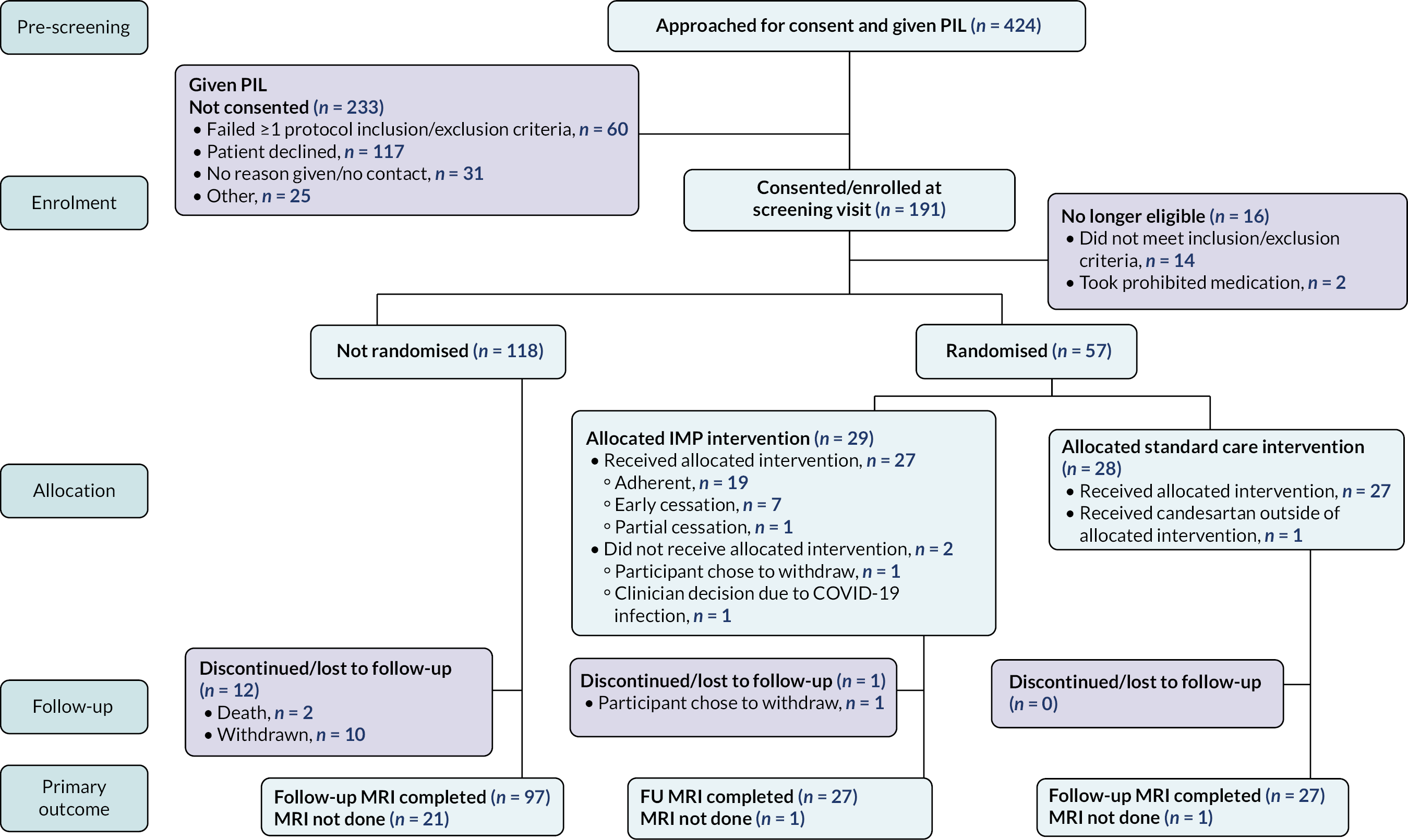
Figure 2 is the CONSORT (Consolidated Standards of Reporting Trials) flow diagram for the Cardiac CARE trial.
FIGURE 2.
CONSORT flow diagram for the Cardiac CARE trial.
Losses and exclusions
Between 4 October 2017 and 30 June 2021, 424 patients were approached across seven of the nine centres open for recruitment. One hundred and one (45.0%) of patients approached were consented. Sixteen patients from this group were subsequently excluded owing to exclusion criteria (n = 14) and the subsequent prescription of prohibited medication (n = 2). Fifty-seven (32.6%) of the remaining 175 patients were randomised. Twenty-nine were allocated to cardioprotection, with two patients in this group not completing the final follow-up MRI scan. Twenty-eight were allocated to standard care, with one patient not completing the final follow-up MRI scan. Within the remaining group of 118 non-randomised patients, 21 patients did not complete the final follow-up MRI scan. This included two deaths and 10 withdrawals from the study.
Recruitment
Cardiac CARE opened for patient recruitment across nine UK centres. Edinburgh opened on 4 October 2017, followed by Glasgow (5 December 2017), Velindre, Cardiff (9 May 2018), University Hospital Wales (20 September 2018), Oxford (25 February 2019), Mount Vernon (19 November 2019), Christie Hospital, Manchester (25 November 2019), Milton Keynes (18 December 2019) and New Victoria Hospital, Glasgow (21 March 2021). Table 1 provides details about screening, recruitment and disposition across the nine centres. Table 2 provides details of post-randomisation disposition by centre.
| Centre | Start date | End date | Days recruiting | Screened | Consented | Eligible | Randomised |
|---|---|---|---|---|---|---|---|
| Christie | 25 November 2019 | 30 June 2021 | 583 | 10 (2.4) | 3 (1.6) | 3 (1.7) | 1 (1.8) |
| Edinburgh | 4 October 2017 | 30 June 2021 | 1365 | 276 (65.1) | 119 (62.3) | 109 (61.6) | 31 (54.4) |
| Glasgow | 5 December 2017 | 30 June 2021 | 1303 | 67 (15.8) | 36 (18.8) | 34 (19.2) | 9 (15.8) |
| Milton Keynes | 18 December 2019 | 30 June 2021 | 560 | 0 (0.0) | – | – | – |
| Mount Vernon | 19 November 2019 | 17 March 2020 | 119 | 2 (0.5) | 2 (1.0) | 2 (1.1) | – |
| New Victoria Hospital | 21 March 2021 | 30 June 2021 | 101 | 0 (0.0) | – | – | – |
| Oxford | 25 February 2019 | 30 June 2021 | 856 | 37 (8.7) | 12 (6.3) | 11 (6.2) | 5 (8.8) |
| Velindre | 9 May 2018 | 30 June 2021 | 1148 | 18 (4.2) | 12 (6.3) | 11 (6.2) | 6 (10.5) |
| Wales | 20 September 2018 | 30 June 2021 | 1014 | 14 (3.3) | 7 (3.7) | 7 (4.0) | 5 (8.8) |
| All | 4 October 2017 | 30 June 2021 | 1365 | 424 (100.0) | 191 (100) | 177 (100) | 57 (100) |
| Centre | Non-randomised (n = 118) | Cardioprotection (n = 290) | Standard care (n = 28) | All randomised (n = 570) | All groups (n = 175) |
|---|---|---|---|---|---|
| Edinburgh | 77 (65.3) | 17 (58.6) | 14 (50.0) | 31 (54.4) | 108 (61.7) |
| Glasgow | 24 (20.3) | 3 (10.3) | 6 (21.4) | 9 (15.8) | 33 (18.9) |
| Velindre | 5 (4.2) | 4 (13.8) | 2 (7.1) | 6 (10.5) | 11 (6.3) |
| University Hospital Wales | 2 (1.7) | 1 (3.4) | 4 (14.3) | 5 (8.8) | 7 (4.0) |
| Oxford | 6 (5.1) | 4 (13.8) | 1 (3.6) | 5 (8.8) | 11 (6.3) |
| Mount Vernon | 2 (1.7) | 0 | 0 | 0 | 2 (1.1) |
| Christie | 2 (1.7) | 0 | 1 (3.6) | 1 (1.8) | 3 (1.7) |
Baseline data
Table 3 provides details of patient demographics and cancer treatment by randomised group.
| Characteristic | Non-randomised | Cardioprotection | Standard care |
|---|---|---|---|
| n = 118 | n = 29 | n = 28 | |
| Age (years), mean (minimum, maximum) | 52.1 (26–73) | 54.0 (23–77) | 53.5 (27–76) |
| Female sex, n (%) | 107 (90.7) | 23 (79.3) | 22 (78.6) |
| Height (cm), mean (SD) | 165.4 (7.9) | 166 (8.1) | 168 (8.4) |
| Weight (kg), mean (SD) | 76.6 (16.5) | 70.7 (15.0) | 82.5 (16.7) |
| Cancer type, n (%) | |||
| Breast | 93 (78.8) | 17 (58.6) | 15 (53.6) |
| NHL | 25 (21.2) | 12 (41.4) | 13 (46.4) |
| Risk markers for cardiovascular disease | |||
| Smoker, n (%) | |||
| Current | 12 (10.2) | 2 (6.9) | 5 (17.9) |
| Ex for < 1 year | 9 (7.6) | 2 (6.9) | 0 |
| Ex for > 1 year | 29 (24.6) | 5 (17.2) | 5 (17.9) |
| Never | 68 (57.6) | 20 (69) | 18 (64.3) |
| Diabetes mellitus, n (%) | |||
| Insulin dependent | 3 (2.5) | 0 | 0 |
| Tablet controlled | 1 (0.8) | 0 | 0 |
| Diet controlled | 0 | 0 | 0 |
| Hypertension, n (%) | 10 (8.5) | 2 (6.9) | 4 (14.3) |
| Coronary disease, n (%) | 2.5 (3) | 0 | 2 (7.1) |
| Kidney disease, n (%) | 0 | 0 | 0 |
| Concomitant cardiovascular medications,a n (%) | 9 (7.6) | 2 (6.7) | 6 (21) |
| Cancer therapy | |||
| Cumulative anthracycline dose (mg/m2) | 424 | 469 | 479 |
| Mean (Q1, Q3) | (300, 480) | (300, 600) | (330, 600) |
| 3 cycles, n (%) | 48 (40.7) | 2 (6.9) | 7 (25) |
| 4 cycles, n (%) | 35 (29.7) | 14 (48.3) | 14 (50) |
| 6 cycles, n (%) | 35 (29.7) | 13 (44.8) | 7 (25) |
| Radiotherapy, n (%) | 79 (71.2) | 16 (57.1) | 15 (53.6) |
| Radiation target location, n (%) | |||
| Left breast | 36 (45.6) | 7 (43.8) | 5 (33.3) |
| Right breast | 35 (44.3) | 6 (37.5) | 8 (53.3) |
| Both breasts | 3 (3.8) | 0 | 0 |
| Outside chest/mediastinumb | 5 (6.3) | 3 (18.8) | 2 (13.3) |
Mean (SD) patient age in non-randomised, cardioprotection and standard care groups was 52.1 (11.0) years, 54 (14.1) years and 53.5 (13.3) years, respectively. Mean mass (SD) was higher in the standard care group (82.5 kg; 16.7 kg) than in the cardioprotection (70.7 kg; 16.5 kg) and non-randomised groups (76.6 kg; 6.5 kg); 71.2% of patients had received a diagnosis of breast cancer. NHL patients were more frequently randomised than breast cancer patients, making up 43.9% of randomised and 21.2% of non-randomised groups. Cardiovascular risk markers and concomitant cardiovascular medication prescription were uncommon across all three groups. Hypertension and coronary disease were more common in the standard care group (14.3% and 7.1%, respectively) than in the non-randomised (8.5% and 3%) and cardioprotection groups (6.9% and 0%). Mean anthracycline dose was higher in the cardioprotection (469 mg/m2) and standard care group (479 mg/m2) than in the non-randomised group (424 mg/m2). Radiotherapy was more commonly prescribed in the non-randomised group (71.2%) than in the cardioprotection (57.1%) and standard care groups (53.6%).
Numbers analysed
Fifty-four (94.7%) randomised and 97 (82.2%) non-randomised patients completed MRI scanning at 6 months for analysis of the respective primary and main secondary end points.
Outcomes and estimation
Prospective data on patients’ LVEF, GLS, GCS, LVEDV, LVM, LAA, HR and systolic and diastolic systemic arterial pressure are given in Table 4.
| Patient measures (units) | Baseline, mean (SD) | 6 months post-final anthracycline dose, mean (SD) | Change from baseline to 6 months, mean (SE) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-randomised (n = 118) | Cardioprotection (n = 29) | Standard care (n = 28) | Non-randomised (n = 97) | Cardioprotection (n = 27) | Standard care (n = 27) | Cardioprotection (n = 27) | Standard care (n = 27) | Non-adjusted mean difference (95% CI) | |
| LVEF (%) | 69.3 (5.7) | 69.4 (7.4) | 69.1 (6.1) | 66.4 (6.3) | 65.7 (6.6) | 64.9 (5.9) | –4.2 (1.1) | –4.3 (1.1) | 0.1 (–3.1 to 3.4) |
| GLS (%) | –17.1 (1.9) | –16.7 (2.7) | –16.1 (2.6) | –16.7 (1.8) | –16.2 (2.3) | –14.9 (2.0) | 0.6 (0.5) | 1.2 (0.5) | –0.6 (–1.9 to 0.7) |
| GCS (%) | –19.6 (2.3) | –18.9 (3.4) | –18.0 (3.1) | –19.1 (2.2) | –18.8 (2.8) | –18.3 (2.7) | 0.0 (0.6) | 0.3 (0.6) | –0.3 (–2.0 to 1.4) |
| LVM (g/m2) | 46.2 (8.4) | 47.6 (12.1) | 50.0 (8.2) | 48.2 (8.0) | 51.4 (11.2) | 49.7 (7.4) | 3.2 (1.9) | 0.0 (1.9) | 3.2 (–2.1 to 8.5) |
| LVEDV (ml/m2) | 62.5 (11.1) | 63.4 (15.4) | 63.9 (9.9) | 63.6 (10.8) | 69.4 (13.8) | 64.1 (11.5) | 5.6 (1.8) | 0.2 (1.8) | 5.4 (0.3 to 10.5) |
| LAA (cm2/m2) | 11.6 (2.6) | 11.9 (2.5) | 11.4 (2.4) | 11.7 (2.5) | 11.9 (1.8) | 10.8 (2.0) | 0.0 (0.4) | –0.5 (0.4) | 0.5 (–0.8 to 1.8) |
Primary end point
Patients randomised to cardioprotection or standard care had mean (SD) LVEF 6 months after completion of anthracycline chemotherapy of 65.7% (6.6%) and 64.9% (5.9%), respectively. Adjusted estimated mean change in primary end-point and secondary cardiac MRI measures are shown in Table 5. After adjusting for age, pre-treatment LVEF and planned anthracycline dose, the estimated mean difference in 6-month LVEF between the cardioprotection and standard care groups was –0.4 percentage points (95% CI –3.6 to 2.8 percentage points; p = 0.82). The outcome was no different using a non-adjusted linear regression model (estimated mean difference 0.2 percentage points, 95% CI –3.1 to 3.4 percentage points; p = 0.93).
| Patient measures (units) | Adjusteda estimated change from baseline to 6 months, mean (SE) | Estimated mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Cardioprotection (n = 27) | Standard care (n = 27) | |||
| LVEF (%) | –1.3 (1.6) | –0.9 (1.9) | –0.4 (–3.6 to 2.8) | 0.82 |
| GLS (%) | 0.1 (0.7) | 0.5 (0.8) | –0.4 (–1.8 to 1.0) | 0.59 |
| GCS (%) | –2.0 (0.8) | –2.2 (1.0) | 0.2 (–1.5 to 1.8) | 0.84 |
| LVM (g/m2) | 1.9 (2.8) | –1.9 (3.3) | 3.8 (–1.9 to 9.4) | 0.20 |
| LVEDV (ml/m2) | 3.4 (2.7) | –2.6 (3.2) | 6.0 (0.6 to 11.4) | 0.03 |
| LAA (cm2/m2) | 0.4 (0.7) | 0.1 (0.8) | 0.3 (–1.0 to 1.6) | 0.65 |
We examined the per-protocol primary efficacy outcome between randomised groups in a post hoc sensitivity analyses. When only 19 cardioprotection patients who were adherent to treatment were included, there was no change in the primary outcome. The estimated mean difference in the change in 6-month LVEF between the cardioprotection and standard care groups was –0.7 percentage points (95% CI –4.3 to 2.9 percentage points; p = 0.70).
Main secondary end point
In non-randomised patients the baseline and 6-month LVEF (SD) were 69.3% (5.7%) and 66.4% (6.3%), respectively. The estimated mean difference was 2.9 percentage points (95% CI 1.4 to 4.3 percentage points; p = 0.92). The main secondary objective of demonstrating zero percentage-point change with equivalence of ± 2% was not met.
Additional secondary end points
Secondary analysis (see Table 5) identified a difference between the cardioprotection and standard care groups in adjusted LV end-diastolic volume indexed for body surface area of 6.0 ml/m2 (95% CI 0.6 to 11.4 ml/m2; p = 0.03). There was no difference between the groups for global longitudinal and circumferential strain, LVM or LAA.
hs-cTnI concentrations from baseline through chemotherapy to 6 months post chemotherapy are presented in Table 6. hs-cTnI concentrations were higher in the randomised groups. Adjusted change in hs-cTnI concentration from baseline to 2 months in cardioprotection and standard care groups was 27.3 ng/l (7.4 ng/l) and 28.8 ng/l (8.8 ng/l) [estimated mean (SE)]. The estimated mean difference was –1.6 ng/l (95% CI –17.6 to 14.4 ng/l; p = 0.85).
| Non-randomised (n = 118) | Cardioprotection (n = 29) | Standard care (n = 28) | |
|---|---|---|---|
| Baseline | 0.5 (0.5, 2.0) | 2 (1, 2) | 2 (1, 4) |
| Cycle 2 | 2 (2, 3) | 6 (5, 7.5) | 7 (5, 9) |
| Cycle 3 | 3 (2, 4) | 7 (5, 11) | 6 (5, 8) |
| Cycle 4 | 4 (3, 6) | 9 (7, 16) | 9.0 (7, 12) |
| Cycle 5 | 8 (6, 12) | 24 (11, 34) | 22 (14, 34) |
| Cycle 6 | 12 (9, 18) | 28.5 (18.5, 51.5) | 36 (24.5, 59) |
| 2 months | 14 (7, 26) | 13.5 (8.5, 36) | 28 (13, 47) |
| 4 months | 3 (2, 5) | 4.5 (3, 10.5) | 6 (4, 10) |
| 6 months | 2 (2, 4) | 3 (2, 9) | 5 (3, 10.5) |
Additional analyses
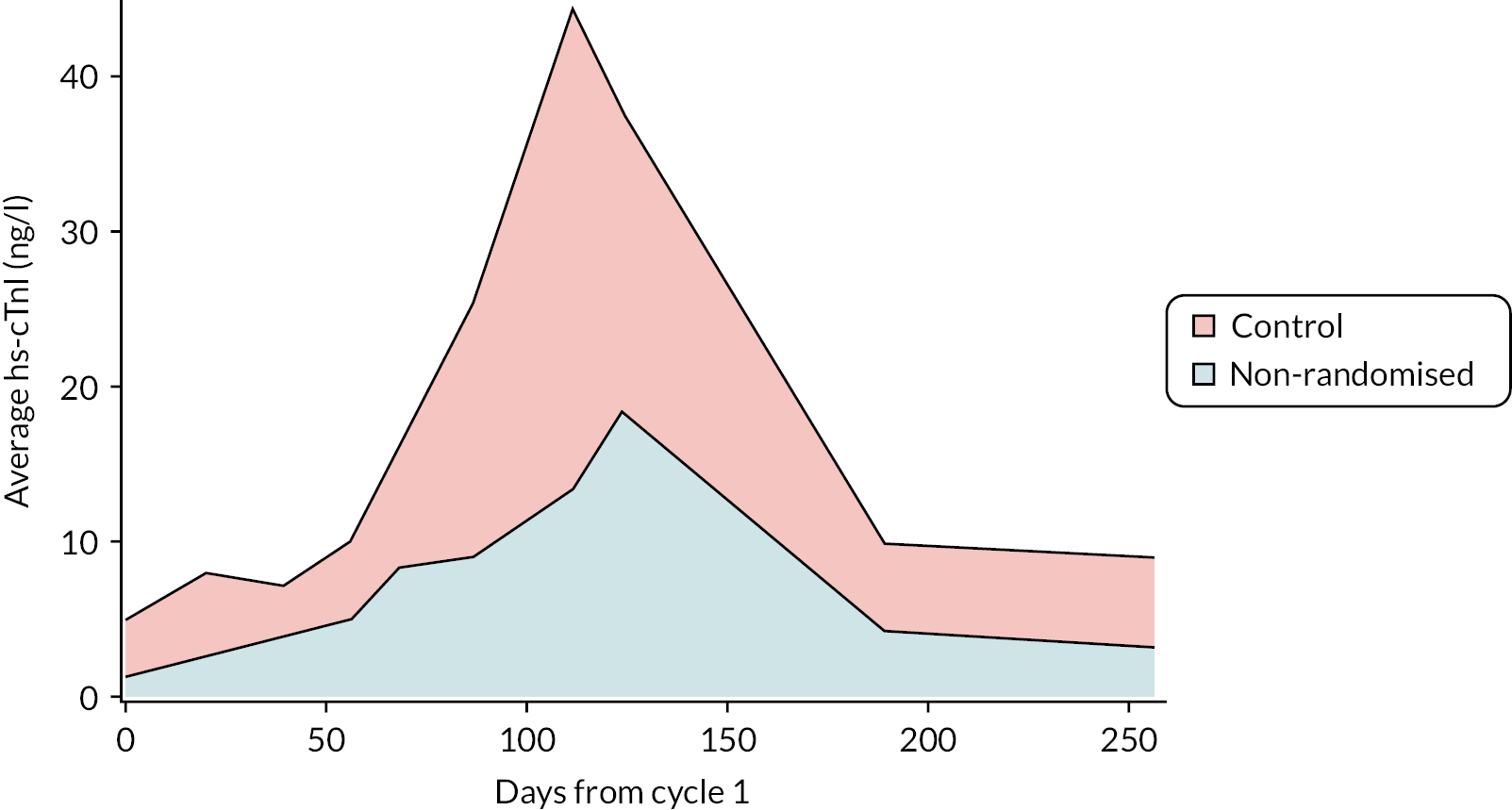
Exploratory comparisons were conducted between the high-risk standard care group and the low-risk non-randomised group. Figure 3 illustrates the increased average hs-cTnI concentrations from baseline to 6 months in the standard care group compared with the non-randomised group. Estimated mean difference in area-under-the-curve quantification of hs-cTnI concentrations from anthracycline treatment cycles 3–6 between groups are given in Table 7. Patients in the non-randomised group had mean (SD) LVEF 6 months after completion of anthracycline chemotherapy of 66.4% (6.3%). The mean (SD) change in LVEF was –2.9% (6.1%) compared with –4.3% (4.4%) in the standard care group. The difference in LVEF decline between groups was not significant. Adjusted estimated mean difference in 6-month LVEF and other cardiac MRI measures of cardiotoxicity between standard care and non-randomised groups are given in Table 8. There was no difference in these measures between the groups.
FIGURE 3.
Increased average hs-cTnI concentrations from baseline to 6 months in the standard care group compared with the non-randomised group area under the curve.
| hs-cTnI AUC log (n; standard care, n; non-randomised) | Estimated mean difference | 95% CI | p-value |
|---|---|---|---|
| Cycle 3 (7, 32) | 7.0 | 6.8 to 7.1 | 0.14 |
| Cycle 4 (7, 46) | 7.1 | 6.9 to 7.4 | 0.009 |
| Cycle 6 (14, 32) | 7.6 | 7.4 to 7.9 | 0.004 |
| Patient measures (units) | Adjusteda estimated change from baseline to 6 months, mean (SE) | Estimated mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Non-randomised (n = 97) | Standard care (n = 27) | |||
| LVEF (%) | 0.7 (1.6) | –0.6 (1.8) | –1.3 (–3.7 to 1.10) | 0.29 |
| GLS (%) | –1.0 (0.6) | –0.3 (0.6) | 0.8 (–0.1 to 1.6) | 0.10 |
| GCS (%) | –0.8 (0.6) | –1.2 (0.7) | –0.4 (–1.3 to 0.6) | 0.44 |
| LVM (g/m2) | 1.2 (1.8) | –0.5 (2.0) | –1.7 (–4.5 to 9.4) | 0.21 |
| LVEDV (ml/m)2 | 5.2 (2.7) | 4.4 (3.1) | –0.8 (–5.0 to 3.4) | 0.71 |
| LAA (cm2/m2) | 1.6 (0.7) | 0.8 (0.8) | –0.9 (–2.0 to 0.2) | 0.12 |
Binary outcomes
Clinical end points and measures of cancer therapy-related cardiac dysfunction (CTRCD) are presented in Table 9. There were no cardiovascular deaths or new atrial fibrillation recorded during the trial. One patient in the standard care treatment group developed congestive cardiac failure. This patient received heart failure treatment including candesartan and their ejection fraction recovered on the 6-month cardiac MRI scan. No patients met the criteria for asymptomatic CTRCD of a 10-percentage-point LVEF fall and fall to an absolute LVEF below 50%. Similarly, the CTRCD criterion of > 15% fall in GLS was uncommon across groups. Chronic myocardial injury 2 months after the completion of chemotherapy was not uncommon and it was similar in the non-randomised (32.1%) and cardioprotection (35.7%) groups. The proportion with chronic myocardial injury was higher (60%) in the standard care treatment group. Any recording of high hs-cTnI concentration was confined to randomised groups.
| Outcome | Non-randomised (N =118), n (%) | Cardioprotection (N = 29), n (%) | Standard care (N = 28), n (%) |
|---|---|---|---|
| Cardiovascular death | 0 | 0 | 0 |
| Any new heart failure | 0 | 0 | 1 (3.6) |
| Any new atrial fibrillation | 0 | 0 | 0 |
| Any ≥ 10 % point fall AND absolute LVEF fall below 50% | 0 | 0 | 0 |
| Any fall in LVEF below 50% | 0 | 0 | 0 |
| GLS fall of > 15% | 6 (6.5) | 4 (14.8) | 1 (3.7) |
| Chronic myocardial injury | 34 (32.1) | 10 (35.7) | 15 (60) |
| Any hs-cTnI concentration of > 80 ng/l | 0 | 3 (10.3) | 5 (17.9) |
Ancillary analyses
Clinical Trial of Investigational Medicinal Product adherence
Twenty patients (69%) were adherent to cardioprotection treatment, although one stopped candesartan within 2 months and continued with carvedilol alone. Two patients randomised to cardioprotection did not receive any medication owing to intercurrent illness and COVID-19 infection. A further seven (24%) stopped both cardioprotection drugs within 2 months owing to symptoms of light-headedness and dizziness, possibly related to low blood pressure.
Pulse and blood pressure
Pulse and blood pressure from baseline and across all visits after chemotherapy out to 6 months are reported in Table 10. Blood pressure and HR were lower in the cardioprotection treatment group at 6 months. Post hoc analysis confirmed greater reduction in HR at 6 months in the cardioprotection group (estimated mean difference – 11 bpm, 95% CI –18 to –4 bpm; p = 0.003). Although reductions were observed, there was no significant difference in SBP (–7 mmHg, 95% CI –17 to 2.0 mmHg; p = 0.12) and diastolic blood pressure (DBP, –6 mmHg, 95% CI –12 to 0.2 mmHg; p = 0.06) in the cardioprotection treatment group.
| Parameter (units) | Baseline, mean (SD) | 2 months, mean (SD) | 4 months, mean (SD) | 6 months, mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|
| Cardioprotection | Standard care | Cardioprotection | Standard care | Cardioprotection | Standard care | Cardioprotection | Standard care | |
| HR (bpm) | 77 (12) | 82 (13) | 80 (12) | 84 (14) | 72 (11) | 80 (10) | 74 (9) | 85 (13) |
| SBP (mmHg) | 131 (17) | 132 (18) | 120 (22) | 132 (17) | 121 (14) | 131 (18) | 119 (17) | 128 (15) |
| DBP (mmHg) | 80 (12) | 80 (11) | 68 (11) | 81 (9) | 75 (9) | 80 (9) | 72 (11) | 79 (9) |
Harms
Safety end points related to cardioprotection therapy
Safety end points relevant to cardioprotection treatment were recorded for all groups. There was no protocol-defined hypotension or bradycardia at baseline or at 2, 4 and 6 months after chemotherapy.
Hyperkalaemia occurred in 10.3% of non-randomised patients. Hyperkalaemia was more common in the randomised groups: 20.7% of the cardioprotection group and 17.9% of the standard care group. Worsening renal function at any point beyond baseline occurred in 2.7%, 6.9% and 7.1% of the non-randomised, cardioprotection and standard care groups. Two patients in the non-randomised and none in the randomised groups developed acute kidney injury. Fatigue was reported by 12.1%, 3.4% and 25% of the non-randomised, cardioprotection and standard care groups.
Adverse event reporting
Table 11 presents a summary of AE reporting. AEs were more commonly reported in the cardioprotection group, with 71.4% of patients having at least one AE compared with 12.7% non-randomised and 10.3% standard care patients. A total of 62.5% (20 out of 32) of AEs in the cardioprotection group were possibly related to CTIMP, with dizziness and syncope listed in 17 out 20 possibly related AEs and hypotension, palpitation and venous thromboembolism listed for the remaining three AEs that had a possible causal link with the CTIMP.
| Category | Non-randomised (n = 118) | Cardioprotection (n = 28) | Standard care (n = 29) |
|---|---|---|---|
| AEs, n | 18 | 32 | 3 |
| Patients with at least one AE, n (%) | 15 (12.7) | 20 (71.4) | 3 (10.3) |
| Serious AE, n (%) | 11/18 (61.1) | 12/32 (37.5) | 2/3 (66.7) |
| AE possibly related to CTIMP, n (%) | 1/18 (5.6) | 20/32 (62.5) | 0 |
Chapter 5 Health economics evaluations
Study question
The health economics study question, as outlined in the Cardiac CARE protocol, is the following: ‘what are the important drivers of differences in costs and quality-adjusted life-years (QALYs) between standard care and hs-cTnI-guided cardioprotection?’.
The study also aims to confirm the feasibility of data capture, assess data quality and provide insight into designing future cost-effectiveness research of hs-cTnI-guided cardioprotection.
Selection of alternatives
In the health economic analysis, costs and QALYs in the hs-cTnI-guided cardioprotection group (n = 29) are compared with those in the randomised standard care group (n = 28). The aim of the analysis is to estimate an incremental cost-effectiveness ratio (ICER) between the two groups within the study horizon, consistent with National Institute for Health and Care Excellence (NICE) guidelines, based on the assumption that costs and QALYs would not differ in patients who do not reach the hs-cTnI threshold for randomisation other than the basic cost of the assay. 36
Form of evaluation
The health economics analysis plan is included as Report Supplementary Material 3. The evaluation takes the form of a within-trial economic analysis, in which costs and QALYs are calculated for each patient from trial healthcare resource use (HRU) and EQ-5D-5L data. Estimates of 6-month and 1-year mean costs and QALYs are calculated for each arm and used to estimate the ICER. Results are presented from NHS and societal perspectives in accordance with NICE guidelines. 36
Data collection
The methods of collecting data for the purposes of cost and QALY calculation in the within-trial economic analysis relied on case report forms and patient questionnaires; full details are given in Appendix 1.
Allowance for uncertainty
In line with the objectives as a feasibility analysis, no modelling or sensitivity analysis was conducted as part of the current Cardiac CARE economic analysis. This was in part due to underpowered results for costs, life-years and QALYs limiting the utility of a modelling exercise to extrapolate these results.
Presentation of results
Quality of life (quality-adjusted life-year) analysis
Table 12 shows the results of a linear regression, where ‘beta’ represents the difference in expected 6-month and 1-year QALYs gained by the intervention group (hs-cTnI-guided cardioprotection) compared with the randomised standard care group, controlling for baseline utility. The regression model follows the methodology outlined by Manca. 37 Table 13 shows the expected adjusted 6-month and 1-year QALYs for each arm as predicted by the regression model. The detailed methodology behind these calculations is given in Appendix 1.
| Characteristic | Beta | 95% CI | p-value |
|---|---|---|---|
| Intervention | |||
| 6 months | 0.01 | –0.02 to 0.04 | 0.5 |
| 1 year | 0.004 | –0.10 to 0.11 | >0.9 |
| Standard care | Intervention | |
|---|---|---|
| 6 months | 0.405 QALYs | 0.414 QALYs |
| 1 year | 0.796 QALYs | 0.800 QALYs |
Cost analysis
Table 14 shows the mean values and 95% CIs of NHS-perspective and societal-perspective costs for both randomised groups. Detailed cost and HRU breakdowns, as well as cost data for the whole Cardiac CARE patient population, can be found in Appendix 1. The largest direct (NHS perspective) costs include the costs of inpatient stays, anthracycline therapy, radiotherapy and hospital doctor visits, with large differences between trial arms in each category.
| Perspective | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) |
|---|---|---|
| NHS | ||
| 6 months | 9142 (6906 to 11,377) | 9074 (6658 to 11,491) |
| 1 year | 11,451 (8749 to 14,154) | 13,378 (8873 to 17,882) |
| Societal | ||
| 6 months | 14,428 (10,196 to 18,660) | 11,403 (8055 to 14,752) |
| 1 year | 20,511 (13,019 to 28,004) | 17,594 (12,162 to 23,026) |
Incremental cost-effectiveness ratio
Based on the study results, from the NHS perspective, hs-cTnI-guided cardioprotection has an ICER of £481,500 per QALY gained compared with standard care among patients marked as high risk by the troponin test with a time horizon of 1 year. From the societal perspective, however, hs-cTnI-guided cardioprotection ‘dominates’ standard care (i.e. the intervention’s costs are lower while the QALY benefits are higher). However, the ICER estimates are limited by the fact that the QALY and cost estimates are not statistically significant.
Health economics evaluation discussion
The within-trial analysis confirms the feasibility of capturing data that are suitable for a future clinical trial adequately powered for a cost-effectiveness end point. These data could also contribute to scenario analysis based on modelling to aid the understanding of how efficient cardiac protection strategies could be developed in the future.
The study could benefit from further research into the uncertainty surrounding specific estimates of the main drivers of cost-effectiveness, which would include longer-term survival and quality-of-life estimates as well as focused cost estimation on inpatient stays, anthracycline therapy, radiotherapy and hospital doctor visits. Full discussion and recommendations for future economic analysis of hs-cTnI-guided cardioprotection are presented in Discussion of the within-trial analysis in Appendix 1.
Chapter 6 Discussion
We found no evidence that cardioprotection therapy with combined candesartan and carvedilol therapy prevents LVEF decline in breast cancer and NHL patients who exhibit elevated hs-cTnI concentrations during anthracycline chemotherapy. The LVEF decline observed in our multicentre study was smaller than that in previous studies using echocardiographic monitoring but similar to that in recent multicentre studies of similar patients using cardiac MRI. 7,20 Moreover, LVEF decline was similar in low-risk non-randomised and high-risk randomised patient groups despite substantial differences in plasma cardiac troponin concentrations during anthracycline treatment. The implications of a small (< 5%) mean fall in ejection fraction across this population of cancer patients are uncertain. It is possible that a larger trial, randomising more patients, could have detected a small treatment effect with cardioprotection. Again, the long-term clinical benefit of such a small putative treatment effect on LVEF is uncertain.
The central hypothesis behind the Cardiac CARE trial design was that on treatment hs-cTnI concentrations would anticipate change in LVEF at 6 months. Overall, our findings question the benefits of early cardioprotection in patients with the highest levels of cardiac injury and indicate that the correlation between on-treatment cardiac troponin concentration and changes in 6-month LVEF and other cardiac is not strong.
In this trial we observed small deteriorations in both global and longitudinal strain across all groups. There was no difference in these early markers of ventricular dysfunction between randomised groups, and the cardiotoxicity threshold of > 15% relative fall in strain was uncommon at 6 months. To account for differences in body size, LVM, left ventricular end- diastolic volume and LAA data were indexed for body surface area. There was a small but significant increase in LVEDV in the cardioprotection group. This may reflect the impact of B-blockers slowing HR, with consequent increased filling and stroke volume.
In additional secondary analysis, cardioprotection therapy did not reduce hs-cTnI concentration change from baseline to 2 months post chemotherapy. We identified this time point to examine for treatment effect when hs-cTnI concentrations are still elevated after the completion of chemotherapy and patients randomised to cardioprotection will have received therapy for at least 2 months. High concentrations (any measurement of > 80 ng/l) were confined to randomised groups, and chronic myocardial injury, defined as a persistent elevation in hs-cTnI above the 99th centile upper reference limit at 2 months after chemotherapy, was not uncommon in all three groups. We believe that this is the first time this persistent signal of myocardial injury, present in 60% of the randomised to standard care group, has been demonstrated in a large population of patients receiving anthracycline cardiotoxicity.
Thirty-one per cent of patients stopped or did not start cardioprotection therapy within 2 months of randomisation. Symptoms possibly related to cardioprotection medication such as dizziness were frequently listed in AE reporting as the reason for early cessation. By contrast, the rate of non-adherence was lower in the PRADA study, with 7% of patients assigned to the combined metoprolol and candesartan therapy arm discontinuing medication. 7 This higher level of adherence may reflect use of placebo control in PRADA. As in PRADA, we found no evidence for a signal of excess harm related to cardioprotection therapy in the safety end points. Indeed, fatigue was more common in the randomised-to-standard-care group. This result was unexpected, given that fatigue is a side effect commonly attributed to B-blockade.
Blood pressure and HR were lower in the cardioprotection group at 6 months. Fall in HR was significantly greater than in the standard care group. These observations are consistent with drug effect in the cardioprotection arm. Similarly, the per-protocol analysis examining only patients that were adherent to medication out to 6 months demonstrated no difference in the primary outcome, indicating that lack of protection from cardiotoxicity was not caused by incomplete adherence. Our results are in accordance with recent RCTs investigating neurohormonal blockade in anthracycline-treated patients. The PRADA study found no difference in LVEF decline or cardiac troponin I concentrations with metoprolol or candesartan treatment on extended follow-up out to 23 months after commencing anthracycline chemotherapy. 26 Carvedilol had a neutral effect on LVEF decline in the CECCY trial, although secondary analysis revealed reduced cardiac troponin concentrations with treatment. 23
Limitations
There are limitations to our trial. Several patients discontinued cardioprotection medication within 2 months of randomisation and this might have had some influence on treatment effect. Despite excluding patients receiving low-dose anthracycline regimes and using hs-cTnI to select patients at risk of more substantial cardiotoxicity, the degrees of LVEF decline and cardiotoxicity were mild compared with those in studies completed over 10 years ago. One patient in the standard care arm developed acute heart failure during the trial. By 6 months this patient had an LVEF > 50%, and it is striking that no patients crossed the cardiotoxicity threshold of an LVEF decline < 50%. The trial was powered to detect a 5 percentage-point difference in LVEF between the randomised groups. There was no trend towards greater or less LVEF decline with cardioprotection, but a small treatment effect was not excluded. Finally, patients randomised to cardioprotection therapy received their first doses after at least one cycle of anthracycline. It is uncertain whether prescribing cardioprotection therapy earlier (as in PRADA and CECCY) would have altered the outcome.
Generalisability
Our findings have good external validity. We enrolled and consented 45% of 424 breast cancer and NHL patients presenting for anthracycline treatment to seven centres across England, Wales and Scotland. Patients can feel overwhelmed at the time of a cancer diagnosis, and 35% declined to participate. There were key exclusion criteria and patient groups who are not represented in this trial. We excluded low-dose anthracycline regimes because of the low risk of cardiotoxicity. The trial findings may not be applicable to children and patients receiving very high anthracycline doses or with established heart disease, who are particularly vulnerable to anthracycline cardiotoxicity. HER2-positive breast cancer patients were excluded. This group constitute up to 20% of those with breast cancers and go on to receive anti-HER2 therapy. Anti-HER2 treatment has established effects on LVEF decline and cardiotoxicity, and patients receiving anthracycline followed by anti-HER2 treatment are at increased cardiotoxicity risk.
Interpretation
hs-cTnI concentrations and risk of anthracycline cardiotoxicity
The hs-cTnI concentration thresholds used in the trial protocol failed to define true high- or low- risk populations in this study. Anthracycline chemotherapy was associated with LVEF decline in breast cancer and NHL patients 6 months after completion of chemotherapy. The degree of LVEF decline was smaller than expected in the randomised groups and not significantly greater than in the hypothesised low-risk, non-randomised group. This finding suggests that the correlation between on-treatment hs-cTnI concentrations and subsequent LVEF decline is not strong. That patients in the non-randomised group also had a detectable decline in LVEF indicates future risk of further deterioration.
Prevention of LVEF decline with cardioprotection therapy
We did not find evidence that combined candesartan and carvedilol therapy prevents LVEF reduction or reduces other markers of cardiotoxicity such as GLS and circulating hs-cTnI concentrations. However, the mean deterioration in LVEF with chemotherapy was smaller than anticipated, and no participant’s LVEF deteriorated by > 10% points and the randomised sample was not at higher risk. Therefore, a larger trial would be needed to detect the prevention of this smaller LVEF decline, or higher-risk participants would have to be recruited in future trials.
Impact of patient and public involvement on Cardiac CARE trial
Patients and public representatives were engaged from the start and several protocol design features were influenced by patient recommendations. Our lead patient representative and co-applicant was Professor Abigail Marks. Professor Marks has experience of treatment for early breast cancer including chemotherapy and monitoring for cardiac toxicity. It was helpful to have affirming comments from the patient and public involvement (PPI) committee of the National Institute for Health and Care Research (NIHR) Diagnostic Evaluation Cooperative and the national patient advocacy charity Independent Cancer Patients’ Voice. Both groups recognised the concern that cancer patients have about cardiotoxicity from successful cancer treatment:
… I like the idea of having treatment that could provide protection against developing heart muscle problems. I also like the idea of being able to avoid a dose of radiation.
… an excellent study and good use of drugs which although do have side effects, these are well known and can in most cases be dealt with. I am a great fan for looking at using drugs differently – it is not only economic, but sensible.
Professor Marks participated in some Trial Steering Committee meetings but found attendance challenging owing to her own professional commitments. Difficulties and challenges during the study centred on problems opening sites and access to research infrastructure during the COVID-19 pandemic. Whenever patients were approached they were keen to participate and there was no strong need for PPI during the study.
We hosted a webinar with the Cardiac CARE patients 5 days after presenting the results at the European Society of Cardiology congress on 1 September 2022. Fifteen trial patients signed in to the webinar. The message that cardiotoxicity levels were low in the study is good news for patients and this was well received. Patients asked about ongoing cardiac follow-up in their respective centres and were interested in the possibility of further follow-up cardiac imaging studies. They appreciated the opportunity to learn about the trial results. Several noted that they had not been offered this opportunity in previous clinical trials. A key comment was that results communication about research scan results should be expedient even when the scan is not being conducted to inform patient care. Patients receiving cancer care are, naturally, concerned to receive results even if these confirm that no surprising or concerning findings have been made.
Chapter 7 Conclusions
The recently published European Society of Cardiology Cardio-Oncology guidelines provide a class I recommendation for the use of cTn monitoring in anthracycline patients at high risk of cardiotoxicity. 12 The Cardiac CARE trial findings raise doubt over whether this monitoring strategy is helpful when both patients with low-risk and patients with high-risk hs-cTnI concentration profiles developed small reductions in LVEF. Although the pathological link between cTn as a biomarker of anthracycline myocardial injury is clear, we found no evidence that elevated concentrations strongly predict cardiotoxicity, inform management or improve care when added to current treatment pathways. Further analysis of the data will establish the correlation between hs-cTnI concentrations and change in LVEF and GLS. We will also examine whether there is a threshold hs-cTnI concentration below which patients do not develop a decline in LVEF.
We found no evidence of a cardioprotection effect with combined candesartan and carvedilol. This combination was associated with side effects, and discontinuation of therapy was not uncommon. Our findings do not support the European Society of Cardiology guidelines that give a class II recommendation for use of either an angiotensin blocker or B-blockers for high-risk anthracycline-treated patients. Furthermore, the small decline in LVEF at 6 months in all groups together with the low levels of other cardiotoxicity measures cast doubt over whether any form of broadly administered cardioprotection therapy is required for these patients.
An LVEF decline of 4.3% at 6 months after chemotherapy may not have immediate clinical implications for an individual patient. Applied across a population, this magnitude of LVEF decline is likely to confer a generalised increased risk of future cardiac dysfunction and heart failure. Future research should be directed at understanding factors determining the evolution of cardiac dysfunction with monitoring and longer-term follow-up studies.
Additional information
Contributions of authors
Peter Henriksen (https://orcid.org/0000-0003-4974-0344) (Cardiology Consultant) was the chief investigator and site lead for Edinburgh and St John’s Hospital, conceived and planned the study and acted as study lead. He led writing of the report and all aspects of study design, conduct and analysis and designed and led the training of research staff.
Morag MacLean (https://orcid.org/0000-0002-4037-0247) (Senior Trial Manager) provided expertise in study management, contributed to protocol design, provided staff training and supervision, was project manager and contributed to the writing of the report.
Marek Atter (https://orcid.org/0000-0002-1628-7427) (Health Economist) co-designed the health economics analysis, carried out the analysis of the health economics data and contributed to writing the report.
Steff Lewis (https://orcid.org/0000-0003-1210-2314) (Professor of Medical Statistics) was the trial statistician, co-designed the protocol, led the design of the statistical analysis plan, oversaw the statistical analyses and contributed to the writing of the report.
Aryelly Rodriguez (https://orcid.org/0000-0002-1352-3922) (Statistician) was a trial statistician and contributed to the development of the statistical analysis plan, undertook the statistical analyses and contributed to writing of the report.
Acknowledgements
The authors would like to thank all the patients who give up their time to take part in the study. We would also like to thank all the staff at the nine Cardiac CARE sites, in particular the principal investigators, co-investigators and research nurses and co-ordinators. The authors acknowledge the support of the staff at Edinburgh Clinical Trials Unit, the Trial Steering Committee, the Data Monitoring and Ethics Committee, the Patient Advisory Group and the sponsor in delivering the trial. All grant co-applicants were involved in the conceptualisation and design of the study. All report authors have contributed to the development of this report and commented on the final drafts.
The full Cardiac CARE Investigator group are listed below.
The Cardiac CARE Investigator Group Chief Investigator: Dr Peter Henriksen.
Principal Investigators: Dr Peter Henriksen, Dr Ninian Lang, Dr Annabel Borley, Dr Clare Rowntree, Dr Graham Collins, Professor John Radford, Dr Amy Guppy, Dr Lucy Scott and Professor Attila Kardos.
Trial research teams
Edinburgh Cancer Centre and St John’s Hospital: Angus Broom, Peter Hall, Olga Oikonomidou, Fiona Scott, Sarah Beverstock, Clare Brown, Caroline Bruce, Jenny Buxton, David Cameron, Helen Creedon, Tze-en Ding, Fieke Froeling, Julie Gillies, Larry Hayward, Danka Lintott, Robbie McNeil, Desmond Maitland, Caroline Michie, Christopher Mullen, Rachel Nirsimloo, Robert Noble, Aista Oswald, Ashley Pheely, Karin Purshouse, Ahmed Rehan, Kelly Rust, Barbara Stanley, Mark Stares, Monica Szabo Xiangfei Yan, Jennifer Carruthers, Susan Crate, Stephanie Duncan, Lois Eddie, Heather Howie, Harriet Knott, Paula Little, Rachel MacAngus, Louise McFadyen, Jennifer MacPherson, Heather McVicars, Louise Maguire, Elaine Murray, Lorraine Primrose, Ruaridh Buchan, Annette Cooper, Ben Elliot, Carol Falcon, Hannah McKinlay, Claire McNicol and Mahéva Vallet.
Glasgow West of Scotland Cancer Centre and Queen Elizabeth University Hospital: Alan Cameron, Pam McKay, Iain MacPherson, Abdulla Alhasso, Sophie Barrett, Stephen Dobbin, Judith Fraser, Claire Glen, Ailsa Houroyd, Maria McGrath, Michael Manson, John Payne, Iffet Rabnawaz, Mark Rafferty, Matthew Wilson, Kate Beattie, Sophia Campbell, Annette Charlick, Alice Coy, Karen Ferguson, Gail Lynch, Linda McGarry, Pauline McIlroy, Rosie Morrison, Lynne Stirling, Marissa Varley, Martin Ball, Laura Dymock, Jacqui Gourlay, Tracey Hopkins, Evonne McLennan, Maria Nicol, Fiona Savage, Linda Taylor, Nicola Tynan and Rosie Woodward.
Velindre Cancer Centre: Jacinta Abraham, Tasia Aghadiuno, Maleeha Anwer, Peter Barrett-Lee, Claire Bartholomew, Clair Brunner, Charlie Crocker, Gwenllian Edwards, Nida Hassan, Theresa Howe, Ali Hussnain, Aida Kamarudin, Joanita Ocen, Helen Passant, Catherine Pembroke, Srijim Sashidharan, Anshu Wadhawan, Sarah Walters, Simon Waters, Richard Webster, Clare Boobier, Esther Bryant, Karen Davies, Maria Evans, Claire Lang, Katie Tanner, Rachel Williams, Michael Brown, Felix Cenal, Rob Henley, Stephanie Hughes, Colette Kemp, Jayne Richards, Emily Rumney and Zoe Lingham.
University Hospital Wales: Nagah Elmusharaf, Simona Gatto, Emily Hopkins, Zaheer Yousef, Hilary Benny, Charlotte Bloodworth, Jenni Cullen, Emma Williams, Katja Williams, Stuart Cunningham, Juliet Mort, Kathryn Murray and Catrin Truan.
Oxford Cancer and Haematology Centre: Simon Lord, Stephen Booth, Kieran Burton, Hugo De La Pena Gomez, Toby Eyre, Katrina Fordwor, Nicola Gray, Angela Hamblin, Catherine Hildyard, Sarah Jaafar, Rajesh Kharbanda, Murali Kesavan, Andrew King, Heather Leary, Paolo Polzella, Catherine Prodger, Richard Salisbury, Emma Weaver, Henna Wong, Xiao-Yin-Zhang, Gemma Austin, Cecilia Magallano, Sally Springett, Hazel Wynn, Cassandra Baker, Vanessa Ferreira, Aleema Iqbal, Joana Leal, Rebecca Mills, Raj Soundarajan, Joe Terry, Swapna Thummala and Shobana Yoganayagam.
Christie NHS Foundation Trust: Ankhi Barua, Fiona Britton, Rachel Broadbent, Adam Gibb, Jane Gibson, Tiana Kordbacheh, Kim Linton, Bence Nagy, Elizabeth Phillips, Manssor Shaikh, Rohan Shotton, Ann Tivey and Laura Woodhouse, Chiara Dalla Torre, Joanna Dash, Caroline Hamer, Helen Harbord, Nicola Heazell, Megan Jones, Rob Miller, Andrea Whitmore, Juliet Harris, Sophie Price, Abbey Walker and Marie Woolley.
Mount Vernon Cancer Centre: Stephanie Sutherland, Helen Cladd, Elizabeth Lodge, Farhan Ahmed, Rachael Bowie, Nica Da Silva, Victoria Major, Michelle Pillay Achour, Elham Shadman Zanjans and Kirti Thakor.
Milton Keynes University Hospital: Diane Ritchie, Michele Burnham, Chloe Green, Rhiannon James, Amy Oakley, Cheryl Padilla-Harris, Diane Scaletta, Melanie Tucker, Lynn Wren, Cassandra Baker, Sam Bosompem, Snehal Shah, Jeannette Smith and Felicity Williams.
New Victoria Hospital: Louise Humphreys and Rebecca O’Neil.
Trial Manager: Dr Morag MacLean.
Edinburgh Clinical Trials Unit: Anna Foster, Garry Milne, Lynsey Milne, Hannah Rickman and Michelle Stevens.
Edinburgh Imaging and clinical MRI reporting: Suzanne Ashford, David Brian, Annette Cooper, Russell Everett, Alexander Fletcher, Alan Japp, Tom MacGillivray, Alexandra Nicolae, Giorgos Papanastasiou, Scott Semple, Nick Spath and Michelle Williams.
Edinburgh Imaging Core Analysis: Shruti Josh and Trish Singh.
Other: The co-applicants, Professor Nicholas Mills and Professor Dave Newby, provided valuable input throughout the trial.
Governance
Throughout the study oversight was provided from the Trial Steering Committee, Data Monitoring and Ethics Committee, sponsor and patient advisory group. We sincerely thank the following members of the Trial Steering Committee for their support and guidance during the trial. Their contribution was invaluable. We also thank the members of the Data Monitoring and Ethics Committee, for their helpful contribution and scrutiny of the data. The trial management group also thank the Patient Advisory Group for their involvement. Their feedback and input was not only helpful for improving trial documentation but also for reinforcing that patients are at the heart of research and their contribution is essential. This study was co-sponsored by the University of Edinburgh and NHS Lothian, through the ACCORD office, the Queen’s Medical Research Institute, 47 Little France Crescent, Edinburgh, EH16 4TJ. The sponsor reference number is AC16148.
Trial Steering Committee
Chairperson: Professor Stephen Evans.
Members: Professor Abigail Marks, Professor Torbjørn Omland and Professor Russell Petty until he withdrew from the committee and was replaced by Dr Gordon Urquhart.
Data Monitoring Committee
Chairperson: Professor Helena Earl until her retirement, when the chair was taken by Dr C Mark Francis.
Members: Dr Ellen Copson, Dr Dominic Culligan and Ms Evie Gardner until her retirement, when she was replaced by Ms Helen Mossop.
Patient Advisory Group
Julie Croft, Heather Goodare, Ailsa Martin and Lesley Stephen.
Equality, diversity and inclusion
The patient population would only have been inclusive of patients who met the protocol-defined inclusion criteria. All patients who met the pre-screening criteria were eligible to be invited by site staff to participate in the Cardiac CARE trial. As NHS employees the site staff followed the NHS policy on equality, diversity and inclusion when approaching potential patients. The patient information leaflet and patient questionnaire were available in a large font for sight-impaired patients. Standard NHS translation resources were available to discuss the study and if necessary to discuss the PIS and consent with anyone who did not have English as their first language. Details of protected characteristics such as ethnicity or sexual orientation were not collected from patients. All oversight committees comprised men and women with a range of expertise. Individuals were recruited from across the UK and Norway. When vacancies became available in committees we sought out younger individuals to provide them with a development opportunity but also to provide a different perspective for our study. A PPI representative was a member of the Trial Steering Committee and a patient advisory group was convened and consulted for their input.
Reporting patient and public involvement
Two Patient Advisory Group (PAG) meetings were convened early in the trial to provide advice on study set-up and patient-facing documentation. The PAG supported the idea of keeping patients informed of the study progress. A patient newsletter was subsequently disseminated to patients. Further PAG meetings were disrupted by the COVID-19 pandemic; however, the group had already highlighted to us the importance of feeding back results to patients. This was backed up by the PPI representative on the Trial Steering Committee. Therefore, the chief investigator held a webinar to which patients were invited in order to share the study results. A lay summary has been posted on the clinical trials website and is open to the public. The link to the trial website was provided in the patient information leaflet.
Protocol
The study protocol is published on protocols.io: www.protocols.io/private/A47518A1A5A011EC9B000A58A9FEAC02
It is also available on the ECTU website: www.ed.ac.uk/usher/edinburgh-clinical-trials/our-studies/ukcrc-studies/cardiac-care/cardiac-care-trial
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
Patient data have been managed to safeguard the confidentiality of patients and is consistent with the terms of consent signed by patients. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Any data sharing approved would have to ensure patient confidentiality.
Ethics statement
Ethics approval was received for our research on 19 June 2017 from the East of Scotland Research Ethics Service. The Research Ethics Committee reference number is 17/ES/0071.
Information governance statement
Edinburgh Clinical Trials Unit, University of Edinburgh is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EUGDPR) 2016/679 under Data Protection legislation. ECTU is the data processor, ACCORD (joint sponsor consisting of the University of Edinburgh and NHS Lothian) are the data controller, and ECTU processes personal data in accordance with their instructions. You can find out how we handle personal data and how to exercise your individual rights in the Cardiac CARE GDPR statement which can be found here: www.ed.ac.uk/usher/edinburgh-clinical-trials/our-studies/ukcrc-studies/cardiac-care/cardiac-care-trial.
Publications
Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2018;104:971–7.
Tzolos E, Adamson PD, Hall PS, Macpherson IR, Oikonomidou O, MacLean M, et al. Dynamic changes in high-sensitivity cardiac troponin I in response to anthracycline-based chemotherapy. Clin Oncol (R Coll Radiol) 2020;32:292–7.
Henriksen PA, Hall P, Oikonomidou O, MacPherson IR, Maclean M, Lewis S, et al. Rationale and Design of the Cardiac CARE Trial: a randomised trial of troponin-guided neurohormonal blockade for the prevention of anthracycline cardiotoxicity. Circ Heart Fail 2022;15:e009445.
Results Abstract: Late Breaking Clinical Trials Presentation; Heart Failure. European Society of Cardiology Congress August 2022.
Henriksen PA. Cardiac CARE – A Randomised Trial of Troponin-guided Neurohormonal Blockade for the Prevention of Anthracycline Cardiotoxicity. ESC Congress, Barcelona, Spain, 2022.
Medscape report on Cardiac CARE Trial results from ESC presentation in Barcelona, Spain, August 2022.
Davenport L. Does Cardio-Protection Work After Anthracycline Chemotherapy? Medscape UK Discussion, 5 September 2022.
Henriksen PA, Hall P, MacPherson IR, Joshi S, Singh T, Maclean M, et al. A multicenter prospective randomized controlled trial of high sensitivity cardiac troponin I-guided combination angiotensin receptor blockade and beta-blocker therapy to prevent anthracycline cardiotoxicity: the Cardiac CARE trial. Circulation 2023;148:1680–690.
Henriksen PA, Rankin S and Lang NN. Cardioprotection in patients at high risk of anthracycline-induced cardiotoxicity JACC: CardioOncology Primer. JACC CardioOncology 2023;5:292–7.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/APTU2400.
Primary conflicts of interest: Steff Lewis was a member of the National Institute for Health and Care Research Health Technology Assessment (HTA) Efficient Study Designs – 2 and HTA General Committee.
Disclaimers
This article presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75.
- Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol 2005;23:8597-605.
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2018;104:971-7.
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97:2869-79.
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. ESC Scientific Document Group . 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768-801.
- Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. ESMO Guidelines Committee . Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171-90.
- Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;37:1671-80.
- Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749-54.
- Shah AS, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8.
- Tzolos E, Adamson PD, Hall PS, Macpherson IR, Oikonomidou O, MacLean M, et al. Dynamic changes in high-sensitivity cardiac troponin I in response to anthracycline-based chemotherapy. Clin Oncol 2020;32:292-7.
- Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2017;13:270-5.
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229-361.
- Apple FS, Collinson PO. IFCC Task Force on Clinical Applications of Cardiac Biomarkers . Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61.
- Michel L, Mincu RI, Mrotzek SM, Korste S, Neudorf U, Rassaf T, et al. Cardiac biomarkers for the detection of cardiotoxicity in childhood cancer: a meta-analysis. ESC Heart Fail 2020;7:423-33.
- Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981-8.
- Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006;114:2474-81.
- Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline- induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213-20.
- Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN, Investigators S. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685-91.
- Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385-90.
- Heck SL, Mecinaj A, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): extended follow-up of a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation 2021;143:2431-40.
- Vaduganathan M, Hirji SA, Qamar A, Bajaj N, Gupta A, Zaha V, et al. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. JACC CardioOncol 2019;1:54-65.
- Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, et al. ICOS-ONE Study Investigators . Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril – the International CardioOncology Society-one trial. Eur J Cancer 2018;94:126-37.
- Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018;71:2281-90.
- Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J 2014;35:2312-21.
- Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350.
- Gulati G, Heck SL, Røsjø H, Ree AH, Hoffmann P, Hagve TA, et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study. J Am Heart Assoc 2017;6.
- Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006;2:65-7.
- Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003;42:736-42.
- Cicoira M, Rossi A, Chiampan A, Frigo G, Bergamini C, Rigolli M, et al. Identification of high-risk chronic heart failure patients in clinical practice: role of changes in left ventricular function. Clin Cardiol 2012;35:580-4.
- Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010;56:392-406.
- Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29-34.
- Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004;147:218-23.
- Henriksen PA, Hall P, Oikonomidou O, MacPherson IR, Maclean M, Lewis S, et al. Rationale and design of the cardiac CARE trial: a randomized trial of troponin-guided neurohormonal blockade for the prevention of anthracycline cardiotoxicity. Circ Heart Fail 2022;15.
- Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17.
- Heck SL, Gulati G, Ree AH, Schulz-Menger J, Gravdehaug B, Røsjø H, et al. Rationale and design of the prevention of cardiac dysfunction during an Adjuvant Breast Cancer Therapy (PRADA) Trial. Cardiology 2012;123:240-7.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 4 August 2022).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- EuroQol Research Foundation . EQ-5D-5L User Guide 2019. https://euroqol.org/wp-content/uploads/2021/01/EQ-5D-5LUserguide-08-0421.pdf (accessed 28 June 2023).
- van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Manca A, Hawkins N, Sculpher MJ. Estimating Mean QALYs in Trial-Based Cost-Effectiveness Analysis: The Importance of Controlling for Baseline Utility 2004. https://onlinelibrary.wiley.com/doi/10.1002/hec.944 (accessed 28 June 2023).
- AA . Fuel Price Report (January 2021) 2021. www.theaa.com/~/media/the-aa/pdf/motoring-advice/fuel-reports/january-2021.pdf?rev=add0b2ce0d5242ca8ca708fd411f13e4&hash=1803ADA66F7DB9110874799096F923E2 (accessed 28 June 2023).
- Office for National Statistics . Consumer Price Inflation Time Series 2022. www.ons.gov.uk/file?uri=/economy/inflationandpriceindices/datasets/consumerpriceindices/current/mm23.xlsx (accessed 28 June 2023).
- Department of Health and Social Care . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1020981/eMIT_NATIONAL_202106_WEBVERSION.ods (accessed 28 June 2023).
- NHS . National Cost Collection: National Schedule of NHS Costs – Year 2020–21: NHS Trust and NHS Foundation Trusts 2021. www.england.nhs.uk/wp-content/uploads/2022/07/2_National_schedule_of_NHS_costs_FY20-21.xlsx (accessed 28 June 2023).
- Jones K, Burns A. Unit Costs of Health and Social Care 2021. Personal Social Services Research Unit; 2021.
- National Institute for Health and Care Excellence . Candesartan Cilexetil 2022. https://bnf.nice.org.uk/medicinal-forms/candesartan-cilexetil.html (accessed 28 June 2023).
- NHS Lothian . Patient-Level Information and Costing Systems (PLICS 2019 20) 2022.
- National Institute for Health and Care Excellence . CARVEDILOL 2022. https://bnf.nice.org.uk/medicinal-forms/carvedilol.html (accessed 28 June 2023).
- Canadian Agency for Drugs and Technologies in Health . Point-of-Care Troponin Testing in Patients With Symptoms Suggestive of Acute Coronary Syndrome: A Health Technology Assessment PROSPERO Registration Number: CRD42015023442 Product Line: Optimal Use Report 2016. www.cadth.ca/sites/default/files/pdf/OP0519_POC_Troponin_Report.pdf (accessed 28 June 2023).
- xe . Historical Rates Tables n.d. www.xe.com/currencytables/ (accessed 28 June 2023).
- Office for National Statistics . Annual Survey of Hours and Earnings (ASHE) 2021. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/annualsurveyofhoursandearningsasheguidetotables (accessed 28 June 2023).
- Office for National Statistics . Average Hourly Pay 2020. www.ethnicity-facts-figures.service.gov.uk/work-pay-and-benefits/pay-and-income/average-hourly-pay/latest (accessed 28 June 2023).
- Public Health Scotland . Detailed Tables 2019. www.isdscotland.org/health-topics/finance/costs/Detailed-Tables/index.asp (accessed 28 June 2023).
- Sillitoe L. Request for Information 2014. www.whatdotheyknow.com/request/199758/response/498958/attach/3/FOI23023%20ResponseAttachment%20Deakin.pdf (accessed 28 June 2023).
- National Institute for Health and Care Excellence . Methylprednisolone 2022. https://bnf.nice.org.uk/drugs/methylprednisolone/medicinal-forms/ (accessed 28 June 2023).
- National Institute for Health and Care Excellence . Prednisolone 2022. https://bnf.nice.org.uk/drugs/prednisolone/medicinal-forms/ (accessed 28 June 2023).
- Yurday E. Average MPG for Cars UK 2022. NimbleFins; 2022.
- Stein RC, Dunn JA, Bartlett JMS, Campbell AF, Marshall A, Hall P, et al. OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer. Health Technol Assess 2016;20:1-202.
- Royal College of Radiologists . Radiotherapy Dose Fractionation Third Edition 2019. www.rcr.ac.uk/media/fezluddf/rcr-publications_radiotherapy-dose-fractionation-third-edition_march-2019.pdf (accessed 8 March 2024).
- York Health Economics Consortium . Perspective 2016. https://yhec.co.uk/glossary/perspective/ (accessed 28 June 2023).
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Pol 2004;9:197-204.
Appendix 1 Health economic trial analysis
Introduction
The purpose of this document, as outlined in the health economics analysis plan (HEAP), is to present the results of the Cardiac CARE within-trial analysis, including estimates of quality-adjusted life-years (QALYs), total costs and HRU for each trial arm (cardioprotection vs. standard care for breast cancer and lymphoma patients with elevated cTnI).
Summary of key variables and population groups
Summaries are provided in Tables 15 and 16.
| Patient gender | All patients (%) (n = 191) | Not randomised (%) (n = 134) | Standard care (%) (n = 28) | Intervention (%) (n = 29) |
|---|---|---|---|---|
| Female | 168 (88) | 123 (92) | 22 (79) | 23 (79) |
| Male | 23 (12) | 11 (8) | 6 (21) | 6 (21) |
| Patient status | All patients (n = 191) (%) | Not randomised (n = 134) (%) | Standard care (n = 28) (%) | Intervention (n = 29) (%) |
|---|---|---|---|---|
| Active | 162 (85) | 106 (79) | 28 (100) | 28 (97) |
| Deceased | 2 (1) | 2 (1) | 0 (0) | 0 (0) |
| Withdrawn | 11 (6) | 10 (7) | 0 (0) | 1 (3) |
| Screening failure | 16 (8) | 16 (12) | 0 (0) | 0 (0) |
Patient-reported outcome measure questionnaires
This section contains information about the observation period (in days) captured by the patient-reported outcome measures (PROMs). Baseline measurement was taken on day 1, corresponding to the first day of the first cycle of chemotherapy. Subsequent PROM measurements were conducted at the following time points:
-
fourth cycle of chemotherapy
-
post-anthracycline visit
-
sixth cycle of chemotherapy
-
follow-up (2 months)
-
follow-up (4 months)
-
follow-up (6 months).
The study period ended either on the date of the last available completed PROM form or on the day a patient’s status changes (e.g. from active to deceased or withdrawn). The planned study period captured by the study period captured by the questionnaires consisted of up to 15 weeks of chemotherapy and 6 months of follow-up. The actual period captured and the number of questionnaires completed at each time point are summarised in Tables 17 and 18.
| All patients (n = 191), mean (95% CI) | Standard care (n = 28), mean (95% CI) | Intervention (n = 29), mean (95% CI) | |
|---|---|---|---|
| PROM study period (days) | 273.95 (249.29 to 298.62) | 269 (255.23 to 282.77) | 308.07 (238.66 to 377.48) |
| All patients | Time point (n = 191) (%) | Not randomised (n = 134) (%) | Standard care (n = 28) (%) | Intervention (n = 29) (%) |
|---|---|---|---|---|
| Cycle 1 (baseline) | 174 (91) | 117 (87) | 28 (100) | 29 (100) |
| Cycle 4 | 117 (61) | 81 (60) | 21 (75) | 15 (52) |
| Post-anthracycline visit | 52 (27) | 32 (24) | 7 (25) | 13 (45) |
| Cycle 6 | 61 (32) | 34 (25) | 14 (50) | 13 (45) |
| Follow-up 2 months | 167 (87) | 111 (83) | 28 (100) | 28 (97) |
| Follow-up 4 months | 164 (86) | 108 (81) | 28 (100) | 28 (97) |
| Follow-up 6 months | 162 (85) | 106 (79) | 28 (100) | 28 (97) |
Data cleaning and inspection
Revisions
Revisions to the study population are outlined in ‘Analysis populations decision’ version 1.0. The document stipulates, ‘Patients 11,015 and 12,018 should be removed from all populations as these patients took prohibited medications before randomisation’.
Patient-reported outcome measure questionnaire errors
Patient 12,026 was removed from the QALY analysis due to two conflicting EQ-5D-5L observations recorded on the same day.
Health state utility and quality-adjusted life-years
The purpose of this section is to present 6-month and 1-year QALYs for each trial arm. QALYs are extrapolated from health state utility values (HSUVs), which in turn are derived from EQ-5D-5L profiles recorded in the PROM questionnaires.
Methods
The process of calculating 6-month and 1-year QALYs consists of the following steps and assumptions:
-
collection of EQ-5D-5L data, which consists of five domains (see EuroQoL’s EQ-5D-5L user guide):38
-
mobility
-
anxiety/depression
-
pain/discomfort
-
usual activities
-
self-care.
-
-
conversion of EQ-5D-5L data into HSUVs using the Crosswalk Index Value Calculator39
-
calculation of unadjusted and adjusted 6-month and 1-year QALYs using the area-under-the-curve outlined by Manca. 40
Obtaining utility values
Utility values are obtained by converting EQ-5D-5L observation sets into HSUVs using the Crosswalk Index Value Calculator (CIVC). 39 The CIVC provides a ‘validated mapping function to derive utility values for the EQ-5D-5L from the existing EQ-5D (−3L)’, which matches specific 5L profiles with 3L-derived HSUV values and is the preferred method of EQ-5D-5L analysis by the NICE reference case. 36
Calculating quality-adjusted life-years
Daily QALY fractions (HSUV365.25) are calculated and summed for each patient from day 1 (first chemotherapy cycle) to the cut-off day based on the closest available utility observation; this ensures that calculations are consistent with the area-under-the-curve methodology. Furthermore, utility is set to 0 on and after the date of death and observations after the cut-off day (182 or 365) are ignored; utility between the last observation and the cut-off day is assumed not to change (last point carried forward). QALYs are calculated for each trial arm and summarised in Table 19.
| All patients (n = 191), mean (95% CI) | Standard care (n = 28), mean (95% CI) | Intervention (n = 29), mean (95% CI) | |
|---|---|---|---|
| 6 months | 0.4 (0.39 to 0.42) | 0.4 (0.37 to 0.42) | 0.43 (0.41 to 0.45) |
| 1 year | 0.78 (0.75 to 0.81) | 0.76 (0.67 to 0.85) | 0.82 (0.75 to 0.89) |
Adjusting quality-adjusted life-years
To estimate the difference in QALYs between trial arms, it is important to control for baseline utility. The regression adjustment methodology and justification used in this analysis are outlined by Manca. 40 The formula provided in the paper is as follows:
Where:
-
i= patient ID
-
β0 = intercept
-
β1 = adjusted differential QALY after controlling for imbalance in the mean utility at baseline
-
ti = treatment arm dummy variable
-
Qib = patient-specific baseline utility
EuroQol-5 Dimensions utility versus Visual Analogue Scale
As part of the EQ-5D-5L instrument, the Cardiac CARE study collected patient-reported health state scores using a Visual Analogue Scale (VAS), with results ranging from 0 (worst health) to 100 (perfect health).
The key difference between utilities derived from EQ-5D data and VAS scores in this study hinges on VAS scores being derived from patients’ subjective perceptions of health and quality of life (QoL), whereas EQ-5D utilities are based on general population preferences. Thus, the function of VAS is to provide ‘an alternative way to elicit an individual’s rating of their own overall current health’. 38
According to the NICE Reference Case, ‘EQ-5D is the preferred measure of health-related quality of life in adults’. 41 Therefore, VAS scores are not included in QALY calculations.
Results
EuroQol-5 Dimensions, five-level version profiles
Figures 4–8 depict the distribution of responses across EQ-5D-5L health domains, categorised by trial arm and time point. Each figure shows the percentage of responses at each level of the domain, which range from 5 (extreme problems) to 1 (no problems). Figures 4–8 show responses for ‘usual activities’, ‘anxiety/depression’, ‘mobility’, ‘pain/discomfort’ and ‘self-care’, respectively.
FIGURE 4.
EuroQol-5 Dimensions, five-level version profiles – usual activities, by time point and trial arm.
FIGURE 5.
EuroQol-5 Dimensions, five-level version profiles – anxiety/depression, by time point and trial arm.
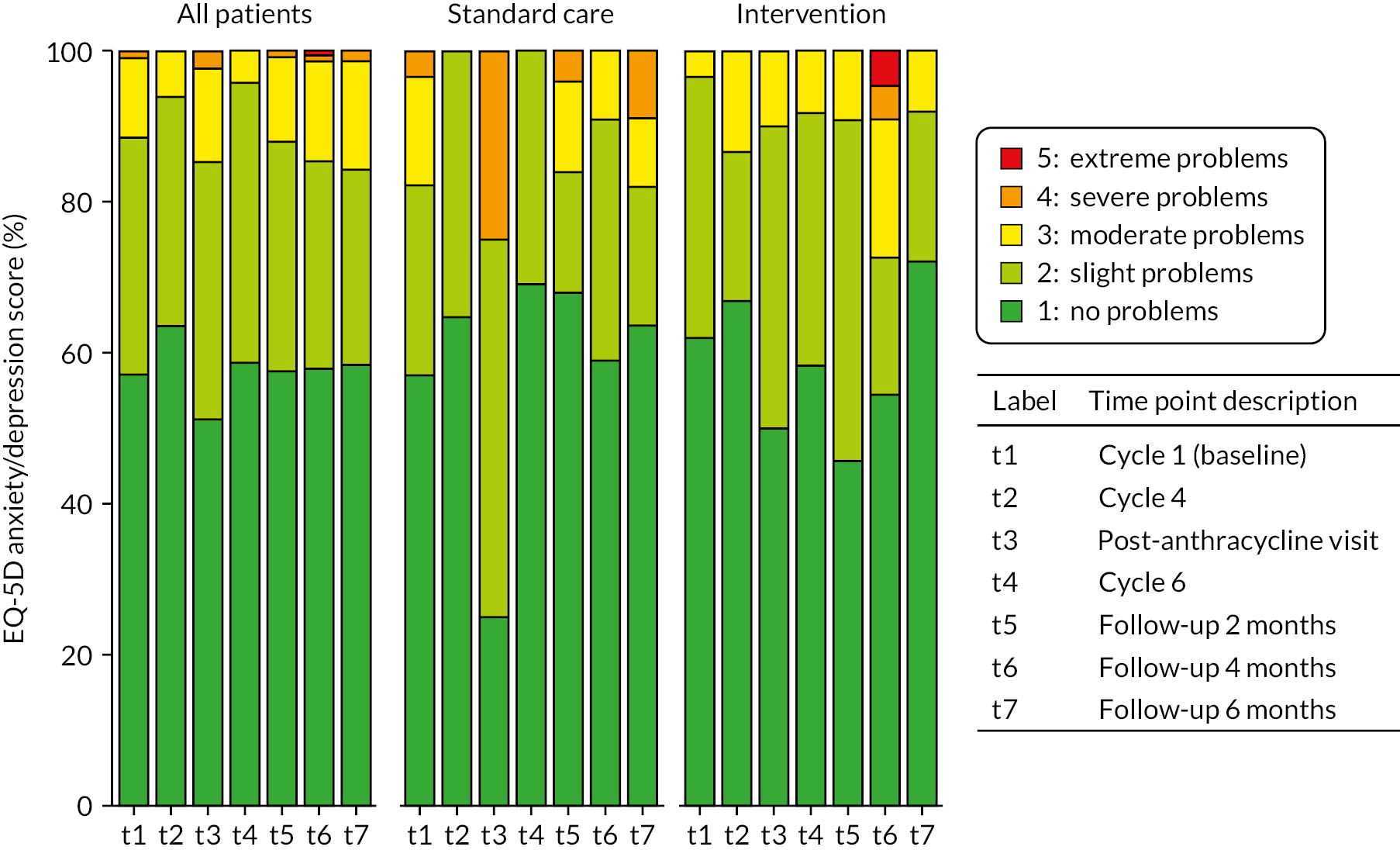
FIGURE 6.
EuroQol-5 Dimensions, five-level version profiles – mobility, by time point and trial arm.
FIGURE 7.
EuroQol-5 Dimensions, five-level version profiles – pain/discomfort, by time point and trial arm.
FIGURE 8.
EuroQol-5 Dimensions, five-level version profiles – self-care, by time point and trial arm.
EuroQol-5 Dimensions health state utility
Figure 9 shows the mean HSUVs at each time point, stratified by randomisation group.
FIGURE 9.
EQ-5D utility scores, by time point and trial arm.
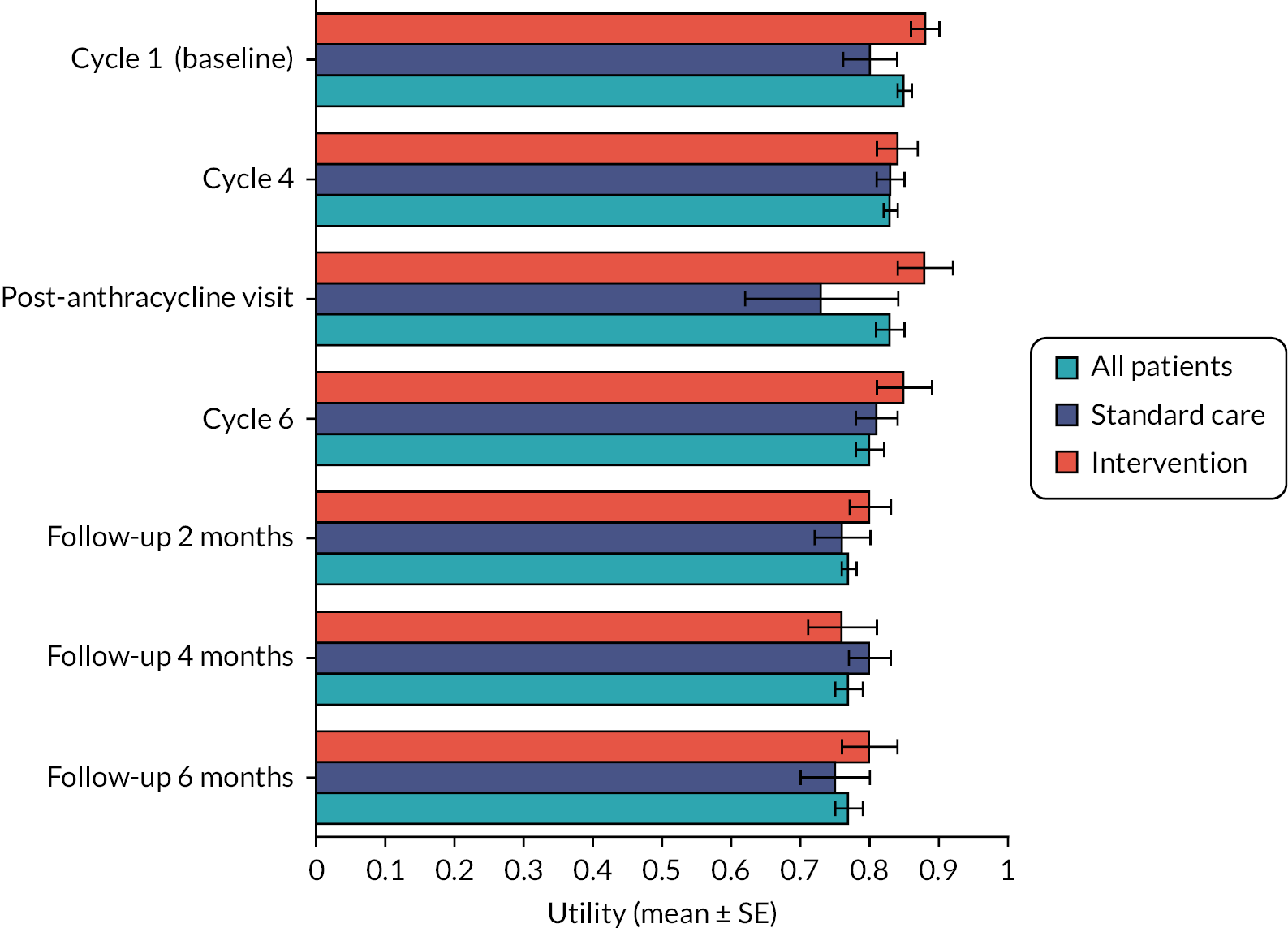
Visual Analogue Scale
Figure 10 shows the mean VAS scores at each time point, stratified by randomisation group.
FIGURE 10.
Visual Analogue Scale scores, by time point and trial arm.
Unadjusted quality-adjusted life-years
Table 19 shows the unadjusted QALYs accumulated by each trial arm during the study period.
Adjusted quality-adjusted life-years
Table 20 shows the regression coefficients, while Table 21 shows estimates of expected adjusted QALYs for each trial arm predicted by the regression model (assuming mean baseline utility). Intervention denotes a binary variable [0 = standard care, 1 = intervention (candesartan and carvedilol)]. Baseline utility refers to utility derived from the PROM questionnaires collected during cycle 1 of chemotherapy.
| Characteristic | Beta | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention | 6 months | 0.01 | –0.02 to 0.04 | 0.5 |
| 1 year | 0.004 | –0.10 to 0.11 | >0.9 | |
| Standard care | Intervention | |
|---|---|---|
| 6 months | 0.405 | 0.414 |
| 1 year | 0.796 | 0.800 |
The p-values in Table 20 indicate that the results are not statistically significant. However, the results confirm the feasibility of data capture, a key objective outlined in the Cardiac CARE protocol.
Healthcare resource use
This section presents 6-month and 1-year costs of all HRU items recorded in the Cardiac CARE clinical trial, as well as differences in resource use patterns across trial arms.
Methods
Patient-reported outcome measure items
The Cardiac CARE PROM data include patient-reported resource use split into three categories: hospital based, community based and employment related. Each PROM observation contains a record of all resources used by a patient since their last questionnaire.
Six-month and 1-year costs for each item were calculated by multiplying the number of units used in the period of interest by the corresponding unit cost (see Table 22). For patients whose last PROM observation fell prior to the 6-month or 1-year cut-off, imputation was conducted based on calculating the mean daily cost between the penultimate and final PROM date and assuming that it stayed constant until death or cut-off. All unit costs in Table 22 were inflated to 2022 GBP using Consumer Price Index weights specific to medical goods published by the Office for National Statistics. 42
| Item | Unit | Cost (£, 2022) | Sources and assumptions |
|---|---|---|---|
| Anthracycline therapy (drug costs) | 1 mg | 0.24 | Composed of the cost of 1 mg of epirubicin based on its 200 mg/100 ml vial (DHA026), as well as £0.07 worth of cyclophosphamide calculated based on its proportion of the EC100 chemotherapy regimen43 |
| Anthracycline therapy (non-drug costs) | Cycle | 587.03 | Chemotherapy cycle delivery costs (£381.97), specialist nurse review (£190.59) and blood tests (£13.93)44 |
| Bone scans | Scan | 147.66 | 2020/21 NHS cost reference: nuclear bone scan of two or three phases, 19 years and over44 |
| Breast cancer nurse telephone calls | Call | 7.72 | Assumed to be approximately equal to a nurse-led telephone triage with average time of 6.56 minutes45 |
| Breast cancer nurse visits | Visit | 90.49 | 2020/21 NHS cost reference: specialist nursing, cancer related, adult, face to face44 |
| Cancer treatment helpline calls | Call | 7.72 | Assumed to be approximately equal to a nurse-led telephone triage with average time of 6.56 minutes45 |
| Cancer treatment helpline visits | Visit | 7.72 | Assumed to be equal to the cost of a cancer treatment helpline call |
| Candesartan | 1 mg | 0.07 | Derived from the drug tariff price of £1.01 for 7 candesartan cilexetil 2-mg tablets46 |
| Cardiac MRIs | Scan | 404.42 | Obtained from NHS Lothian PLICS 2019/20 data47 |
| Carvedilol | 1 mg | 0.01 | Derived from the drug tariff price of £0.90 for 28 carvedilol 3.125-mg tablets48 |
| Community physiotherapist clinic visits | Visit | 118.88 | 2020/21 NHS cost reference: physiotherapy44 |
| Community physiotherapist home visits | Visit | 118.88 | Assumed to be approximately equal to clinic visit |
| Community physiotherapist telephone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| CT scans | Scan | 98.90 | 2020/21 NHS cost reference: computerised tomography scan of one area, without contrast, 19 years and over44 |
| cTnI test | 1 test | 5.53 | CA$10 (2016) converted to 2016 £4.89 using xe currency conversion tables from 1 January 201649,50 |
| ECGs | Scan | 61.21 | Nurse-led rapid-access ECG clinic visit, obtained from NHS Lothian PLICS 2019/2047 |
| GP surgery clinic visits | Visit | 39.75 | GP per-patient contact lasting 9.22 minutes including direct care staff costs with qualification costs45 |
| GP surgery home visits | Visit | 39.75 | Assumed to be approximately equal to clinic visit |
| GP surgery telephone calls | Call | 8.79 | Average cost per GP telephone consultation based on an assumed average time of 5 minutes45 |
| Help support hours | Hour | 12.70 | Average hourly pay in 2018 (all ethnicities)51 |
| Helper days off | Day | 75.69 | Mean weekly paid hours worked by women multiplied by average hourly pay and divided by 5 (assumed five working days per week)51,52 |
| Hospital doctor phone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| Hospital doctor visits | Visit | 217.22 | 2020/21 NHS cost reference: medical oncology44 |
| Hospital nurse telephone calls | Call | 7.72 | Based on the cost of a nurse-led telephone triage with an average time of 6.56 minutes45 |
| Hospital nurse visits | Visit | 90.49 | 2020/21 NHS cost reference: specialist nursing, cancer related, adult, face to face44 |
| Hospital physiotherapist telephone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| Hospital physiotherapist visits | Visit | 114 | PSSRU 2021: physiotherapy, one-to-one session45 |
| Inpatient days | Diem | 704.05 | Derived from average inpatient week cost in Scotland of £470253 |
| Mammograms | Scan | 8.79 | Assumed to be approximately equal to X-ray costs54 |
| Methylprednisolone | mg | 0.06 | Derived from NHS indicative price of £3.88 for 30 2-mg tablets55 |
| NHS direct calls | Call | 7.72 | Assumed to be approximately equal to a nurse-led telephone triage with average time of 6.56 minutes45 |
| NHS direct visits | Visit | 7.72 | Assumed to be approximately equal to the cost of a NHS Direct call |
| Nurse clinic visits | Visit | 51.84 | 2020/21 NHS cost reference: district nurse, adult, face to face44 |
| Nurse home visits | Visit | 51.84 | Assumed to be approximately equal to clinic visit |
| Nurse telephone calls | Call | 7.72 | Nurse-led telephone triage based on an average time of 6.56 minutes45 |
| Prednisolone | mg | 0.03 | Derived from NHS indicative price of 0.77 for 28 1-mg tablets56 |
| Psychiatrist clinic visits | Visit | 266.77 | 2020/21 NHS cost reference: liaison psychiatry44 |
| Psychiatrist home visits | Visit | 266.77 | Assumed to be approximately equal to clinic visit |
| Psychiatrist telephone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| Psychologist clinic visits | Visit | 221.52 | 2020/21 NHS cost reference: clinical psychology44 |
| Psychologist home visits | Visit | 221.52 | Assumed to be approximately equal to clinic visit |
| Psychologist telephone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| Psychotherapist clinic visits | Visit | 117.41 | 2020/21 NHS cost reference: psychotherapy44 |
| Psychotherapist home visits | Visit | 117.41 | Assumed to be approximately equal to clinic visit |
| Psychotherapist telephone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| Radiotherapy fraction | Fraction | 217.25 | 2020/21 NHS cost reference: deliver a fraction of complex treatment on a megavoltage machine44 |
| Radiotherapy preparation | Patient | 1308.29 | 2020/21 NHS cost reference: preparation for complex conformal radiotherapy, with technical support44 |
| Surgeon telephone calls | Call | 8.79 | Assumed to be approximately equal to the average cost per GP telephone consultation based on an average time of 5 minutes45 |
| Surgeon visits | Visit | 180.44 | 2020/21 NHS cost reference: general surgery44 |
| Travel (miles driven) | Mile | 0.13 | Calculated from January 2021 Fuel Price Report and average UK MPG figure41,57 |
| Ultrasounds | Scan | 176.60 | 2020/21 NHS cost reference: ultrasound elastography44 |
| X-rays | Scan | 29.41 | Freedom-of-information request to clinic54 |
Limitation
Unscheduled hospital assessments were recorded as a binary variable instead of a continuous one, giving no information on the number of units used. Thus, the costs of unscheduled assessments were not captured in this analysis.
Candesartan and carvedilol
Candesartan and carvedilol costs were calculated by multiplying the cumulative dose taken of each drug during the study period (recorded in the Compliance SQL table) by its corresponding per-mg unit cost (see Table 22). For patients whose number of days prescribed exceeded the cut-off periods of 6 or 12 months, results were adjusted by the following formula, where unadjusted cost denotes the cost of the total dosage recorded over the study period:
Anthracycline therapy
As outlined in the Cardiac CARE protocol version 11.0, patients underwent 3, 4 or 6 anthracycline therapy cycles lasting 6, 9 or 15 weeks. Since the chemotherapy treatment lasted no longer than 15 weeks, 1-year costs were equal to 6-month costs, as all costs were incurred within the first 6 months.
Based on chemotherapy cost calculators used in the OPTIMA trial,58 all present calculations assumed:
-
Patients followed the EC100 regimen for adjuvant and metastatic breast cancer, composed of epirubicin and cyclophosphamide – this assumption was based on expert advice.
-
The AnthracyclineDose variable recorded in the SQL database corresponds to epirubicin (mg) but does not include cyclophosphamide.
-
The cost of cyclophosphamide was calculated as a function of the epirubicin dosage, based on the proportion of cyclophosphamide in EC100 in the OPTIMA chemotherapy cost template.
-
Per-cycle costs consist of:
-
delivery costs
-
specialist nurse review
-
blood tests.
-
-
The supportive medications of EC100 are not included in the costs of anthracycline therapy; these were captured in the Steroids SQL table and calculated separately.
Steroids
Steroid dosage observations in the SQL database are recorded in one of two units:
-
milligrams (mg)
-
milligrams per metre squared (m2) (of surface area).
The conversion of mg/m2 to mg assumes a surface area of 1.75 m2 and a relative dose intensity of 0.92, based on the values in the OPTIMA chemotherapy cost template. 58
Radiotherapy
The radiotherapy costs consist of two elements:
-
radiotherapy preparation costs
-
radiotherapy fraction administration costs, multiplied by the number of administered treatments.
In the Cardiac CARE SQL database, dates of the first and last doses (fractions) are recorded for each patient who received radiotherapy, but the number of fractions itself is not recorded.
Radiotherapy cost calculations are based on an estimate of the number of patients’ fractions, which assumes five fractions per week (on weekdays) between the dates of the first and last fractions recorded in the database. The calculation’s assumptions are based on treatments described in the Royal College of Radiologists’ Radiotherapy Dose Fractionation, Third Edition, most of which involve five fractions per week. 59
For two patients, the period between the first and last fraction exceeded 2 months. These patients were assumed to have received 25 fractions, the maximum amount described in the RCR’s document.
Limitations
The fraction administration costs could differ if patients’ radiotherapy regimes diverge from the assumptions based on the RCR’s document, for example by patients having received radiotherapy fractions:
-
on an irregular basis
-
at weekends
-
more or less often than five times a week.
Perspectives
All HRU items were aggregated into totals and stratified into NHS perspective and non-NHS perspective costs. The societal perspective consists of the sum of costs in both categories. Each item was grouped according to the following guide:
The NHS perspective includes treatment costs such as medicine costs, administration and monitoring, other health service resource use costs associated with the managing the disease (e.g. GP visits, hospital admissions), and costs of managing adverse events caused by treatment. It does not include patients’ costs of obtaining care such as transportation, over the-counter purchases, co-payments or time off work. 60
Reproduced with permission from York Health Economics Consortium
Regression
To assess the statistical significance of differences in total costs between trial arms, a simple linear regression of trial arm on costs was conducted.
Limitations
The model linear model is potentially sensitive to non-normally distributed cost data and confounding variables. In a future study, the model could be replaced with a multiple general linear model, as suggested by Barber and Thompson. 61
Unit costs
Where possible, costs were sourced from a published paper or database (e.g. NHS reference costs). Where published data were lacking or judged as inapplicable or unrealistic during a review by the Cardiac CARE chief investigator, price weights were supplied by a NHS Lothian finance manager using internal and unpublished data. Where no suitable data were identified, unit costs were based on assumptions (Table 22). All costs are reported in 2022 GBP.
Summary of resource units used
Table 23 summarises HRU units used per patient in each trial arm. Figures 11–14 show the mean per-patient HRU units used within various resource categories, split by trial arm. Figures 11–14 show on-site visits, home visits, telephone calls and scans, respectively. Figures 15 and 16 are histograms that show the distribution of the number of chemotherapy cycles (see Figure 15) and radiotherapy fractions (see Figure 16) per patient in each trial arm.
| Units used | All patients (n = 191), mean (95% CI) | Standard care (n = 28), mean (95% CI) | Intervention (n = 29), mean (95% CI) |
|---|---|---|---|
| Bone scans | 0.11 (0.06 to 0.16) | 0.04 (–0.03 to 0.11) | 0.07 (–0.02 to 0.16) |
| Breast cancer nurse telephone calls | 1.07 (0.77 to 1.38) | 0.68 (0.1 to 1.26) | 0.52 (0.2 to 0.83) |
| Breast cancer nurse visits | 0.85 (0.51 to 1.18) | 0.61 (–0.01 to 1.22) | 0.66 (0.14 to 1.17) |
| Cancer treatment helpline calls | 1.47 (1.18 to 1.76) | 1.54 (0.7 to 2.38) | 1.38 (0.73 to 2.03) |
| Cancer treatment helpline visits | 0.72 (0.48 to 0.96) | 0.93 (0.2 to 1.66) | 0.34 (–0.04 to 0.73) |
| Cardiac MRIs | 0.6 (0.47 to 0.73) | 0.5 (0.16 to 0.84) | 0.9 (0.54 to 1.25) |
| Community physiotherapist clinic visits | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Community physiotherapist home visits | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Community physiotherapist telephone calls | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| CT scans | 1.04 (0.8 to 1.28) | 0.96 (0.49 to 1.44) | 1.21 (0.77 to 1.65) |
| ECGs | 0.63 (0.45 to 0.81) | 0.68 (0.27 to 1.08) | 0.76 (0.22 to 1.3) |
| GP surgery clinic visits | 2.36 (1.91 to 2.81) | 3.79 (2.15 to 5.42) | 2.55 (1.35 to 3.75) |
| GP surgery home visits | 0.05 (0.01 to 0.1) | 0.18 (–0.05 to 0.41) | 0 (0 to 0) |
| GP surgery telephone calls | 1.61 (1.24 to 1.99) | 2.11 (0.88 to 3.33) | 2.17 (0.91 to 3.43) |
| Help support hours | 45.97 (35.74 to 56.19) | 101.32 (58.15 to 144.5) | 42.05 (22.37 to 61.73) |
| Helper days off | 20.09 (11.24 to 28.94) | 22.84 (–2.73 to 48.41) | 12.6 (–2.27 to 27.47) |
| Hospital doctor telephone calls | 0.62 (0.43 to 0.81) | 0.64 (0.29 to 1) | 0.79 (0.18 to 1.4) |
| Hospital doctor visits | 2.77 (2.25 to 3.29) | 3.11 (2.02 to 4.19) | 3.07 (1.83 to 4.31) |
| Hospital nurse telephone calls | 0.61 (0.4 to 0.82) | 0.61 (0.15 to 1.06) | 0.72 (0.16 to 1.29) |
| Hospital nurse visits | 2.59 (1.57 to 3.61) | 3.07 (0.67 to 5.48) | 4.69 (–0.75 to 10.13) |
| Hospital physiotherapist telephone calls | 0.08 (0.03 to 0.14) | 0 (0 to 0) | 0.07 (–0.02 to 0.16) |
| Hospital physiotherapist visits | 0.31 (0.17 to 0.44) | 0.5 (–0.02 to 1.02) | 0.48 (0.1 to 0.87) |
| Inpatient days | 3.62 (2.6 to 4.64) | 3.79 (1.52 to 6.05) | 5.03 (1.86 to 8.21) |
| Mammograms | 0.41 (0.28 to 0.55) | 0.36 (< 0.01 to 0.71) | 0.45 (0.05 to 0.84) |
| NHS direct calls | 0.09 (0.03 to 0.15) | 0.11 (–0.01 to 0.22) | 0.03 (–0.03 to 0.1) |
| NHS direct visits | 0.08 (0.01 to 0.16) | 0.11 (–0.01 to 0.22) | 0.03 (–0.03 to 0.1) |
| Nurse clinic visits | 3.44 (2.61 to 4.28) | 5.39 (1.41 to 9.38) | 3.45 (1.61 to 5.29) |
| Nurse home visits | 0.49 (0.07 to 0.92) | 0.25 (–0.24 to 0.74) | 0.79 (–0.38 to 1.97) |
| Nurse telephone calls | 0.2 (0.07 to 0.32) | 0.21 (<0.01 to 0.42) | 0.1 (–0.1 to 0.31) |
| Patient days off | 216.53 (175.35 to 257.71) | 236.68 (139.43 to 333.92) | 228.52 (88.78 to 368.25) |
| Psychiatrist clinic visits | 0.04 (–0.01 to 0.08) | 0.04 (–0.03 to 0.11) | 0.14 (–0.13 to 0.41) |
| Psychiatrist home visits | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Psychiatrist telephone calls | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Psychologist clinic visits | 0.1 (–0.01 to 0.21) | 0.32 (–0.24 to 0.88) | 0.14 (–0.02 to 0.3) |
| Psychologist home visits | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Psychologist telephone calls | 0.03 (<0.01 to 0.05) | 0.07 (–0.07 to 0.21) | 0 (0 to 0) |
| Psychotherapist clinic visits | 0.11 (–0.06 to 0.27) | 0.64 (–0.48 to 1.77) | 0.03 (–0.03 to 0.1) |
| Psychotherapist home visits | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Psychotherapist telephone calls | 0.01 (–0.01 to 0.03) | 0 (0 to 0) | 0 (0 to 0) |
| Surgeon telephone calls | 0.1 (0.01 to 0.18) | 0.04 (–0.03 to 0.11) | 0.03 (–0.03 to 0.1) |
| Surgeon visits | 0.75 (0.55 to 0.94) | 0.75 (0.17 to 1.33) | 0.52 (0.12 to 0.91) |
| Travel (miles driven) | 242.88 (181.12 to 304.64) | 132 (65.53 to 198.48) | 347.07 (185.61 to 508.53) |
| Ultrasounds | 0.66 (0.49 to 0.83) | 0.54 (0.14 to 0.93) | 0.86 (0.41 to 1.32) |
| X-rays | 6.1 (–4.49 to 16.69) | 1.04 (0.42 to 1.65) | 0.93 (0.53 to 1.33) |
FIGURE 11.
Mean clinic/on-site visits per patient during the study period, by trial arm.
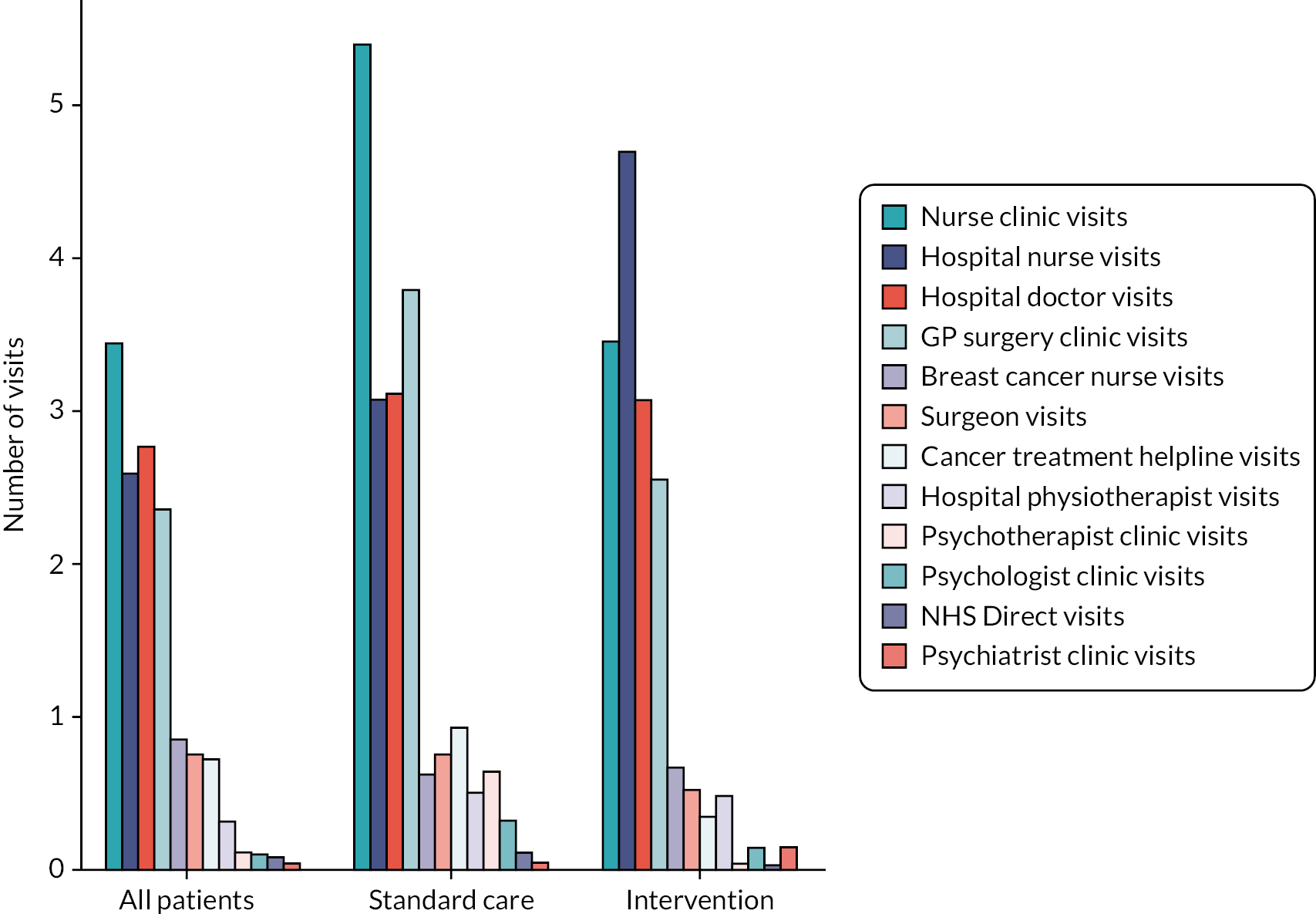
FIGURE 12.
Mean home visits per patient during the study period, by trial arm.
FIGURE 13.
Mean telephone calls per patient during the study period, by trial arm.
FIGURE 14.
Mean scans per patient during the study period, by trial arm.
FIGURE 15.
Histogram of chemotherapy cycles per patient, by trial arm.
FIGURE 16.
Histogram of radiotherapy fractions per patient, by trial arm.
Results
This section summarises the 6-month and 1-year costs of all HRU items included in this analysis, as well as total costs and regression outputs. Table 24 summarises the costs of all HRU items recorded in the PROM questionnaires incurred by patients in each trial arm, categorised by resource types that include hospital-based, community care and employment-related (productivity loss) costs. Costs of the trial drugs (candesartan and carvedilol) are presented in Table 25. Chemotherapy costs are presented in Table 26. Costs of concomitant medications (steroids) are presented in Table 27. Radiotherapy costs are presented in Table 28. A summary of total costs by trial arm is presented in Table 29.
| Cost type | Item | Time point | All patients (n = 191), mean (£) (95% CI) | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) |
|---|---|---|---|---|---|
| Hospital | Bone scans | 6 months | 17 (7 to 27) | 0 (0 to 0) | 7 (–4 to 17) |
| 1 year | 33 (13 to 53) | 17 (–16 to 50) | 22 (–12 to 56) | ||
| Breast cancer nurse telephone calls | 6 months | 8 (5 to 11) | 3 (< 1 to 7) | 2 (<1 to 3) | |
| 1 year | 16 (10 to 22) | 8 (1 to 16) | 8 (3 to 14) | ||
| Breast cancer nurse visits | 6 months | 68 (44 to 92) | 46 (1 to 90) | 51 (7 to 96) | |
| 1 year | 121 (76 to 166) | 5 (–8 to 157) | 73 (13 to 133) | ||
| Cancer treatment helpline calls | 6 months | 13 (10 to 15) | 12 (5 to 18) | 11 (6 to 16) | |
| 1 year | 14 (11 to 18) | 12 (5 to 18) | 11 (6 to 16) | ||
| Cancer treatment helpline visits | 6 months | 6 (4 to 8) | 7 (1 to 12) | 2 (<1 to 5) | |
| 1 year | 7 (5 to 10) | 7 (2 to 13) | 3 (<1 to 6) | ||
| Cardiac MRIs | 6 months | 172 (126 to 218) | 140 (32 to 249) | 160 (81 to 239) | |
| 1 year | 409 (317 to 500) | 335 (110 to 560) | 430 (257 to 604) | ||
| CT scans | 6 months | 84 (66 to 101) | 76 (27 to125) | 90 (55 to 125) | |
| 1 year | 146 (116 to 176) | 133 (76 to 189) | 155 (93 to 217) | ||
| ECGs | 6 months | 35 (25 to 45) | 38 (14 to 61) | 33 (10 to 56) | |
| 1 year | 55 (38 to 72) | 46 (18 to 75) | 59 (8 to 111) | ||
| Hospital doctor telephone calls | 6 months | 5 (3 to 6) | 4 (1 to 7) | 5 (1 to 9) | |
| 1 year | 8 (5 to 10) | 7 (3 to 11) | 7 (1 to 13) | ||
| Hospital doctor visits | 6 months | 528 (436 to 619) | 601 (379 to 824) | 523 (328 to 719) | |
| 1 year | 973 (700 to 1247) | 763 (513 to 1013) | 850 (435 to 1266) | ||
| Hospital nurse telephone calls | 6 months | 5 (3 to 6) | 4 (1 to 7) | 5 (1 to 10) | |
| 1 year | 6 (4 to 8) | 5 (1 to 9) | 5 (1 to 10) | ||
| Hospital nurse visits | 6 months | 209 (141 to 277) | 237 (38 to 435) | 271 (18 to 524) | |
| 1 year | 317 (185 to 448) | 330 (103 to 557) | 539 (–132 to 1210) | ||
| Hospital physiotherapist telephone calls | 6 months | 0 (<1 to 1) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 2 (<1 to 3) | 0 (0 to 0) | 1 (–1 to 3) | ||
| Hospital physiotherapist visits | 6 months | 29 (14 to 44) | 57 (–2 to 116) | 24 (<1 to 47) | |
| 1 year | 53 (30 to 76) | 57 (–2 to 116) | 84 (4 to 164) | ||
| Inpatient days | 6 months | 2167 (1549 to 2785) | 2450 (879 to 4021) | 2422 (661 to 4183) | |
| 1 year | 3549 (2529 to 4569) | 3097 (1312 to 4882) | 4505 (1078 to 7933) | ||
| Mammograms | 6 months | 4 (2 to 6) | 3 (<1 to 5) | 4 (<1 to 7) | |
| 1 year | 6 (3 to 9) | 3 (<1 to 6) | 5 (1 to 9) | ||
| NHS Direct calls | 6 months | 1 (<1 to 1) | 1 (<1 to 1) | <1 (<1 to 1) | |
| 1 year | 1 (<1 to 2) | 2 (–1 to 4) | <1 (<1 to 1) | ||
| NHS Direct visits | 6 months | 1 (<1 to 1) | 1 (<1 to 1) | <1 (<1 to 1) | |
| 1 year | 1 (<1 to 2) | 1 (<1 to 3) | <1 (<1 to 1) | ||
| Surgeon telephone calls | 6 months | 1 (<1 to 1) | <1 (<1 to 1) | 0 (0 to 0) | |
| 1 year | 1 (<1 to 3) | <1 (<1 to 1) | 1 (–1 to 2) | ||
| Surgeon visits | 6 months | 113 (82 to 144) | 96 (20 to 171) | 68 (15 to 120) | |
| 1 year | 242 (170 to 313) | 194 (26 to 362) | 176 (24 to 328) | ||
| Ultrasounds | 6 months | 103 (75 to 132) | 90 (24 to 157) | 116 (40 to 192) | |
| 1 year | 157 (107 to 207) | 95 (25 to 165) | 189 (85 to 293) | ||
| X-rays | 6 months | 194 (–153 to 540) | 28 (11 to 44) | 22 (12 to 32) | |
| 1 year | 205 (–141 to 552) | 32 (12 to 51) | 27 (15 to 39) | ||
| Employment | Help support hours | 6 months | 406 (298 to 514) | 862 (422 to 1302) | 347 (161 to 534) |
| 1 year | 690 (499 to 881) | 1192 (658 to 1725) | 563 (270 to 856) | ||
| Helper days off | 6 months | 1008 (571 to 1445) | 1238 (–386 to 2861) | 577 (–74 to 1228) | |
| 1 year | 2275 (1188 to 3363) | 2773 (–499 to 6045) | 1135 (–567 to 2837) | ||
| Lost earnings | 6 months | 3306 (1243 to 5369) | 3136 (1159 to 5113) | 1336 (–156 to 2827) | |
| 1 year | 6753 (3902 to 9604) | 5017 (1417 to 8617) | 2410 (184 to 4635) | ||
| Community | Community physiotherapist clinic visits | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| Community physiotherapist home visits | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| Community physiotherapist telephone calls | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| GP surgery clinic visits | 6 months | 80 (63 to 98) | 111 (54 to 169) | 76 (32 to 120) | |
| 1 year | 155 (123 to 187) | 215 (119 to 310) | 139 (72 to 207) | ||
| GP surgery home visits | 6 months | 3 (<1 to 6) | 6 (–3 to 14) | 0 (0 to 0) | |
| 1 year | 5 (–1 to 10) | 7 (–2 to 16) | 0 (0 to 0) | ||
| GP surgery telephone calls | 6 months | 12 (9 to 15) | 14 (5 to 23) | 13 (4 to 23) | |
| 1 year | 23 (18 to 29) | 25 (11 to 39) | 28 (13 to 43) | ||
| Nurse clinic visits | 6 months | 177 (131 to 223) | 235 (28 to 442) | 161 (68 to 254) | |
| 1 year | 256 (198 to 313) | 318 (111 to 525) | 219 (106 to 333) | ||
| Nurse home visits | 6 months | 22 (2 to 42) | 13 (–12 to 38) | 24 (–5 to 54) | |
| 1 year | 33 (1 to 66) | 13 (–12 to 38) | 92 (–66 to 250) | ||
| Nurse telephone calls | 6 months | 1 (<1 to 2) | 1 (<1 to 2) | 1 (–1 to 2) | |
| 1 year | 2 (1 to 4) | 2 (<1 to 3) | 1 (–1 to 2) | ||
| Psychiatrist clinic visits | 6 months | 10 (–3 to 23) | 10 (–9 to 28) | 38 (–37 to 113) | |
| 1 year | 13 (–2 to 28) | 10 (–9 to 28) | 38 (–37 to 113) | ||
| Psychiatrist home visits | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| Psychiatrist telephone calls | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| Psychologist clinic visits | 6 months | 15 (–2 to 31) | 36 (–21 to 94) | 13 (–5 to 30) | |
| 1 year | 35 (–8 to 78) | 126 (–106 to 358) | 24 (–10 to 58) | ||
| Psychologist home visits | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| Psychologist telephone calls | 6 months | <1 (<1 to <1) | <1 (<1 to <1) | 0 (0 to 0) | |
| 1 year | 1 (<1 to 2) | 2 (–2 to 7) | 0 (0 to 0) | ||
| Psychotherapist clinic visits | 6 months | 11 (–6 to 27) | 60 (42 to 162) | 1 (–1 to 3) | |
| 1 year | 17 (–5 to 40) | 75 (–56 to 207) | 13 (–13 to 39) | ||
| Psychotherapist home visits | 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | ||
| Psychotherapist telephone calls | 6 months | <1 (<1 to <1) | 0 (0 to 0) | 0 (0 to 0) | |
| 1 year | <1 (<1 to 1) | 0 (0 to 0) | 0 (0 to 0) | ||
| Travel (miles driven) | 6 months | 29 (22 to 35) | 13 (6 to 19) | 37 (19 to 55) | |
| 1 year | 56 (38 to 74) | 30 (9 to 51) | 70 (33 to 106) | ||
| Travel (parking) | 6 months | 2 (<1 to 5) | 9 (–6 to 24) | <1 (<1 to 1) | |
| 1 year | 3 (<1 to 6) | 11 (–6 to 28) | 1 (<1 to 1) | ||
| Travel (public transport) | 6 months | 26 (16 to 36) | 29 (5 to 54) | 31 (5 to 56) | |
| 1 year | 33 (21 to 45) | 38 (6 to 69) | 38 (10 to 67) |
| All patients (n = 191), mean (£) (95% CI) | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) | ||
|---|---|---|---|---|
| Candesartan | 6 months | 158.08 (49.22 to 266.93) | 0 (0 to 0) | 1030.22 (401.56 to 1658.88) |
| 1 year | 185.25 (54.34 to 316.16) | 0 (0 to 0) | 1207.34 (445.37 to 1969.32) | |
| Carvedilol | 6 months | 226.03 (71.09 to 380.98) | 0 (0 to 0) | 1473.12 (579.52 to 2366.71) |
| 1 year | 261.75 (80.39 to 443.11) | 0 (0 to 0) | 1705.86 (656.43 to 2755.29) | |
| Item | Time point | All patients (n = 191), mean (£) (95% CI) | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) |
|---|---|---|---|---|
| Anthracycline drugs | 6 months | 134 (126 to 142) | 156 (144 to 167) | 145 (121 to 168) |
| 1 year | 134 (126 to 142) | 156 (144 to 167) | 145 (121 to 168) | |
| Chemotherapy cycle delivery | 6 months | 2305 (2159 to 2450) | 2788 (2501 to 3076) | 2571 (2246 to 2896) |
| 1 year | 2305 (2159 to 2450) | 2788 (2501 to 3076) | 2571 (2246 to 2896) | |
| Total chemotherapya | 6 months | 2439 (2287 to 2590) | 2944 (2652 to 3236) | 2716 (2381 to 3050) |
| 1 year | 2439 (2287 to 2590) | 2944 (2652 to 3236) | 2716 (2381 to 3050) |
| All patients (n = 191), mean (£) (95% CI) | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) | |
|---|---|---|---|
| Prednisolone | |||
| 6 months | 5.09 (2.59 to 7.60) | 9.23 (1.10 to 17.37) | 10.11 (1.02 to 19.20) |
| 1 year | 6.07 (3.39 to 8.74) | 10.31 (2.22 to 18.41) | 12.81 (2.66 to 22.97) |
| Methylprednisolone | |||
| 6 months | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| 1 year | 0.05 (–0.04 to 0.14) | 0 (0 to 0) | 0 (0 to 0) |
| All patients (n = 191), mean (£) (95% CI) | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) | |
|---|---|---|---|
| 6 months | 1924 (1587 to 2261) | 1810 (941 to 2678) | 2077 (1138 to 3016) |
| 1 year | 2683 (2337 to 3030) | 2485 (1593 to 3378) | 2789 (1818 to 3761) |
| Cost type | Time point | All patients (n = 191), mean (£) (95% CI) | Standard care (n = 28), mean (£) (95% CI) | Intervention (n = 29), mean (£) (95% CI) |
|---|---|---|---|---|
| Total NHS-perspective costsa | 6 months | 8934 (8048 to 9820) | 9142 (6906 to 11,377) | 9074 (6658 to 11,491) |
| 1 year | 12,550 (11,207 to 13,893) | 11,451 (8749 to 14,154) | 13,378 (8873 to 17,882) | |
| Total non-NHS-perspective costsb | 6 months | 4777 (2645 to 6908) | 5287 (2406 to 8168) | 2329 (745 to 3913) |
| 1 year | 9811 (6609 to 13,013) | 9060 (3547 to 14,574) | 4216 (1463 to 6969) | |
| Total societal costs | 6 months | 13,711 (11,328 to 16,093) | 14,428 (10,196 to 18,660) | 11,403 (8055 to 14,752) |
| 1 year | 22,361 (18,744 to 25,978) | 20,511 (13,019 to 28,004) | 17,594 (12,162 to 23,026) |
Healthcare resource use item costs
Total costs
Total costs are summarised in Table 29. Figure 17 shows a breakdown of the largest components of the total costs for each trial arm.
FIGURE 17.
Mean 1-year HRU item cost breakdown, by trial arm.
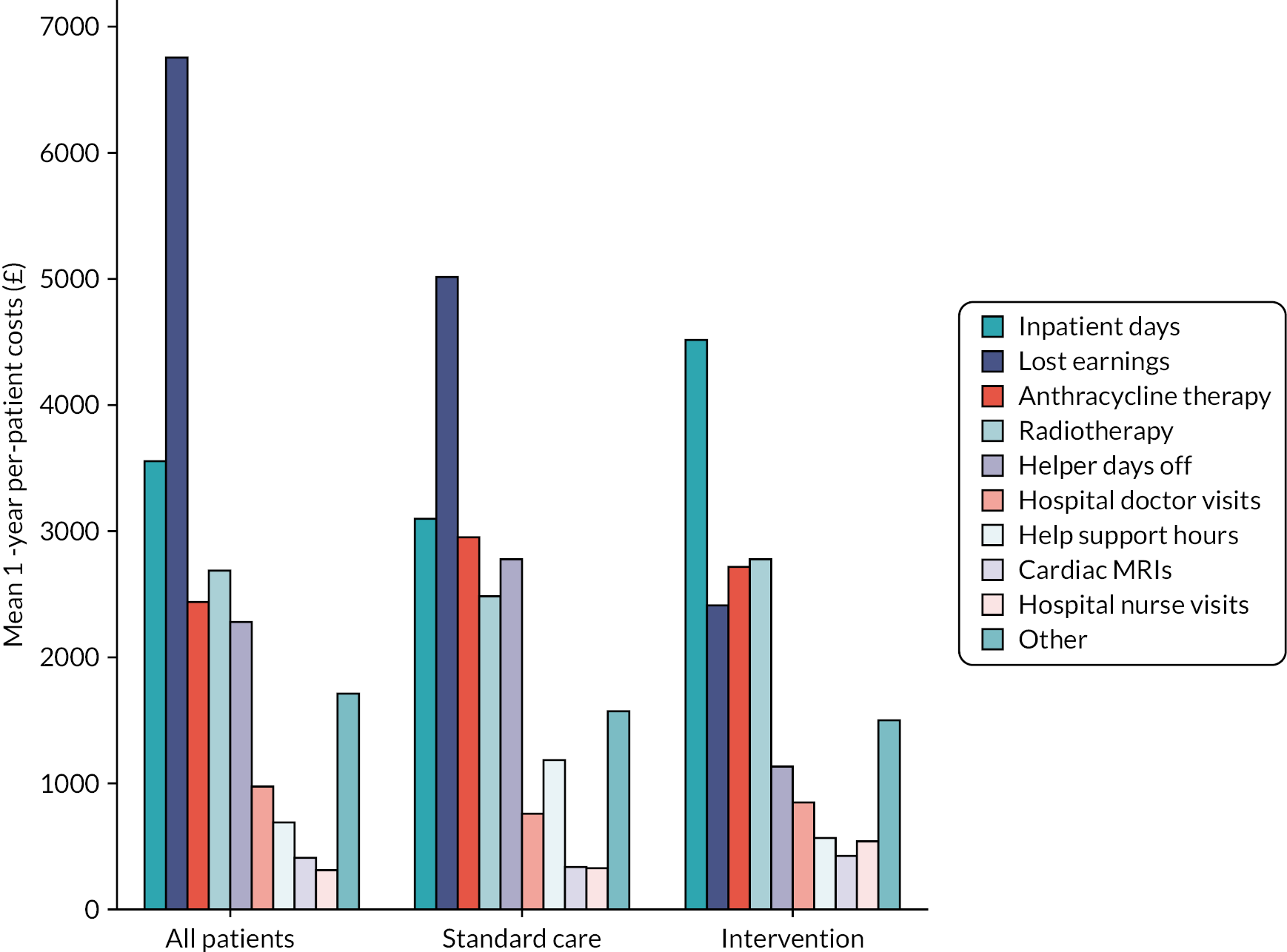
Regression
In the regression analysis, Intervention is a binary variable where 0 represents standard care and 1 represents the intervention. Thus, the coefficient (Beta) shows the estimated difference in the mean total NHS-perspective per-patient costs (2021 GBP) between the intervention and standard care arms.
Table 30 shows that 6-month costs are estimated to be £67 lower for the intervention arm than the standard care arm by the regression model, while 1-year costs are estimated to be £1926 higher.
| Characteristic | Beta (£) (95% CI) | p-value | |
|---|---|---|---|
| 6 months | –67 (–£3434 to £3300) | > 0.9 | |
| Intervention | 1 year | 1926 (–£3447 to £7300) | 0.5 |
Summary
The p-values in Table 30 indicate that differences in costs between trial arms are underpowered for health economic analysis and not statistically significant. However, the HRU analysis confirms the feasibility of data capture and shows inpatient stays, anthracycline therapy and radiotherapy to be key drivers of NHS perspective costs in the Cardiac CARE study population.
Incremental cost-effectiveness ratio
The ICER is a ‘ratio of the difference in the mean costs of a technology compared with the next best alternative to the differences in the mean outcomes’. 36 The formula for calculating the ICER for the candesartan and carvedilol intervention compared with standard care is:
Where:
-
C denotes total NHS perspective costs
-
E denotes total QALYs
-
I denotes the intervention arm (candesartan and carvedilol)
-
S denotes the standard care arm.
Estimates of ∆E and ∆C are given by the β1 regression coefficients for the intervention in Tables 20 and 30, respectively, yielding a 1-year ICER of:
However, Tables 20 and 30 also show that estimates of ∆E and ∆C are not statistically significant, yielding an inconclusive ICER result. Even if the results are indeed accurate and the ICER calculated in this section is a good approximation of the intervention’s cost-utility, then it far exceeds the willingness-to-pay threshold (WTP) of £20,000–30,000 per QALY recommended by NICE. 36
Discussion
Patient-reported outcome measure period
As shown in Table 17, the mean number of days tracked by PROM questionnaires (i.e. days between the first chemotherapy cycle and the last completed form) differs between trial arms: 269 days for standard care and 308.07 days for the intervention. Two patients in the intervention have over double the PROM days of the standard care arm’s maximum length of 362 days. As the 95% CIs for PROM days overlap (see Table 17), this difference may be due to chance. A future study could further investigate differing PROM response patterns between randomisation groups, which could also explain discrepancies in response rates at different study time points (see Table 18).
EuroQol-5 Dimensions utility and Visual Analogue Scale
In Figure 9, general population perception of health states (EQ-5D) is consistently higher for the intervention arm than for standard care across study time points, whereas the opposite is true for patient-perceived health state VAS scores in Figure 10. In both figures, 95% CIs overlap, suggesting that the differences could be due to random variation. However, one possible explanation for this discrepancy could be the presence of more AEs or serious adverse events (SAEs) in the intervention group, which, although not captured by the EQ-5D, may be perceived as a reduction in life quality by patients themselves.
Nurse visits
Figure 11 shows that, for patients in the standard care arm, the mean number of drop-in nurse clinic visits recorded over the study period (5.39) is greater than the mean number of hospital nurse visits (3.07). For the intervention arm, however, the opposite is true (3.45 drop-in nurse visits vs. 4.69 hospital nurse visits). However, as shown in Table 23, the 95% CIs for mean estimates of hospital versus drop-in nurse visits overlap, so variation between visit types and trial arms could be due to randomness. Nevertheless, a future study could further investigate differences between trial arms of nursing resource requirements.
Patient-reported outcome measure questionnaire design and recommendations
Overall, the results of this analysis confirm data capture feasibility. However, several elements of the PROM questionnaire could be amended to simplify data collection and remove some of the limitations in the current analysis.
The PROM questionnaire data contains information on a wide variety of cost variables, many of which are associated with negligible costs (e.g. non-GP telephone calls). A more streamlined questionnaire could reduce the burden on patients and improve the data collection process.
Another challenge to calculating costs from the Cardiac CARE PROM data stems from unscheduled assessments being recorded as binary variables. To estimate the costs, a questionnaire should collect the number of assessments in addition to binary information on whether or not a given patient had an unscheduled assessment.
References
See References.
Appendix 2 Protocol amendments
| V1.0 (14 March 2017) | Initial submission to Research Ethics Committee |
|---|---|
| V2.0 (14 June 2017) |
|
| V3.0 (28 August 2017) |
|
| V4.0 (28 September 2017) |
|
| V5.0 (31 January 2018) |
|
| V6.0 (21 February 2018) |
|
| V7.0 (8 October 2018) |
|
|
|
| V8.0 (19 June 2019) |
|
| V9.0 (29 April 2020) |
|
| V10.0 (17 May 2021) |
|
| V11.0 (27 July 2021) |
|
Glossary
- hs-cTnI
- cardiac troponin I concentration quantified using the ARCHITECTSTAT or ALINITY high-sensitivity cardiac troponin I assay (Abbott Laboratories, Chicago, IL, USA).
List of abbreviations
- ACEi
- angiotensin-converting enzyme inhibitor
- AE
- adverse event
- ARB
- angiotensin II type I receptor antagonist (e.g. candesartan)
- B-blocker
- B-adrenoceptor blocker
- Cardiac CARE
- High-Sensitivity Cardiac Troponin I-Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy
- CECCY
- Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity
- CI
- confidence interval
- CTIMP
- Clinical Trial of Investigational Medicinal Product
- CTRCD
- cancer-therapy related cardiac dysfunction
- cTnI
- cardiac troponin I
- ECTU
- Edinburgh Clinical Trials Unit
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCS
- global circumferential strain
- GLS
- global longitudinal strain
- HER2
- human epidermal growth factor receptor-2
- HRU
- healthcare resource use
- HSUV
- health state utility value
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- LAA
- left atrial area
- LVEDV
- left ventricular end-diastolic volume
- LVEF
- left ventricular ejection fraction
- LVEF%
- left ventricular ejection fraction (expressed as a percentage)
- LVM
- left ventricular mass
- MRI
- magnetic resonance imaging
- NHL
- non-Hodgkin lymphoma
- PRADA
- Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy
- PROBE
- prospective randomised open-label blinded end point
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- VAS
- Visual Analogue Scale
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/APTU2400).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
