Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 15/18/14. The contractual start date was in February 2017. The final report began editorial review in April 2021 and was accepted for publication in September 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Turner et al. This work was produced by Turner et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Turner et al.
Chapter 1 Introduction
Parts of this report have been reproduced from Turner et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and redistribute the material in any medium or format, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Childhood asthma
Asthma is the most common chronic condition in childhood and affects 1.1 million children in the UK. 2 Asthma is characterised by coughing, wheezing and shortness of breath. Symptoms can be brought on by many triggers, including an upper respiratory tract infection, exercise or exposure to inhaled allergens (e.g. cat dander and pollen) or chemicals (e.g. cigarette smoke). Physiological testing reveals that children with asthma have obstructed lung function compared with their non-asthmatic peers, although this obstruction is reversible. Pathologically, asthma is characterised by epithelial shedding, eosinophilic inflammation, proliferation of mucus-producing goblet cells, smooth muscle hypertrophy and basement membrane thickening. 3 Despite the high prevalence of asthma in the population of all countries worldwide, there is no universally accepted definition of asthma, nor is there a diagnostic test of sufficient sensitivity and specificity. 4
Asthma causation
Asthma is a typical complex condition in which genetic and environmental factors interact and result in disease. Twin studies suggest that up to 70% of causation can be attributed to heritable factors. 5 Many environmental factors are implicated in childhood asthma causation, including exposure to second-hand smoke, pollution and moulds, and dietary factors. 6 Asthma symptoms can develop at any stage of life, and thereafter follow a pattern of remission and relapse. Asthma is a major risk factor for chronic obstructive pulmonary disease in the seventh decade of life, a condition that is characterised by irreversible airway obstruction and is a leading cause of death in the Western world. 7 Asthma is known to be a syndrome in which a number of different mechanisms lead to common symptoms and pathology. 8 In adults, childhood-onset asthma is genetically distinct from other forms of asthma and is associated with allergic conditions, such as eczema, hay fever and food allergies. 9–12 Two multifaceted interventions10,11 that promoted breastfeeding and affected early-life exposures to smoking, ingested allergens and inhaled allergens were found to reduce asthma incidence in at-risk individuals, but many other interventions aimed at preventing asthma have not been successful. At the time of writing, there is no accepted intervention that prevents asthma development de novo. The current management approach to asthma is to control symptoms with inhaled and ingested medications that relieve acute symptoms and prevent chronic symptoms and adverse events (AEs), such as lung function decline.
Asthma control and exacerbations
Asthma control can be defined as ‘the extent to which the various manifestations of asthma are reduced or removed by treatment’. 13 A key goal of asthma treatment is to achieve asthma control. 14 There are several questionnaires that are used to objectively measure asthma control, and the Asthma Control Test (ACT) is currently the most commonly used test for older children and adults. 15 The Children’s Asthma Control Test (CACT) has been validated for use in children aged between 4 and 11 years. 16 Patients (or parents/carers) complete the ACT or CACT by answering questions about nocturnal and exertional asthma symptoms and how frequently they take asthma-reliever treatment. The maximum score on the ACT is 25, while the maximum score on the CACT is 27. A score of > 19 from the ACT or CACT indicates that an individual’s asthma is controlled; this means that, despite subtle differences in methodology, the definitions of ‘uncontrolled asthma’ within the ACT and CACT are equivalent. A second key goal of asthma management is to prevent asthma exacerbations. Persistent, poorly controlled asthma symptoms place a child at increased risk of an asthma exacerbation. 14,17
Asthma exacerbations (synonymous with asthma attacks) are a worsening of symptoms and may lead to coughing, wheezing and breathing difficulties. During an asthma exacerbation, the airways become swollen and inflamed, and the muscles around the airways contract. Asthma exacerbations are usually treated with oral corticosteroids (OCs) at home but can result in hospitalisation. They are associated with morbidity and occasionally mortality. The incidence of death from an asthma exacerbation in the UK is at least double that of other European countries. 18 In the UK, a child is admitted to hospital with an asthma exacerbation every 20 minutes. 2 The annual cost to the NHS that is attributable to asthma is £1.1B, with one-third of this incurred from unscheduled care2 and, on a pro rata basis, at least £150M as a result of asthma exacerbations in children. Parental absence from work is an additional cost to the economy that is attributable to asthma exacerbations; one parent will typically be absent from work for 4 days when a child has an asthma exacerbation. 19 Another important impact of asthma is that a child’s education suffers as a result of missed days of school. 20
Pharmacological management of childhood asthma
Medications that are used to manage asthma are considered to be relievers [short-acting beta-agonists (SABAs) taken on an ad hoc basis to relieve symptoms] or preventers (taken on a daily basis independent of symptoms). There is an extensive evidence base that shows that asthma symptoms can be reduced by preventer treatments (also known as maintenance therapy), such as inhaled corticosteroids (ICs), inhaled long-acting beta-agonists (LABAs) and oral leukotriene receptor antagonists (LTRAs). 14,17,21 Current UK guidelines for the management of asthma recommend that decision-making for asthma preventer treatment should be driven by very recent symptoms, for example symptoms over the last 2–4 weeks. 17,21 The initial asthma preventer treatment recommended by all guidelines is ICs, but there is uncertainty about what the best treatment option is when symptoms are not controlled by IC-preventer treatment alone. 14,17,21 The British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN) guideline17 suggests adding either LABA or LTRA, whereas the National Institute for Health and Care Excellence (NICE) guideline21 suggests adding LTRA and the Global Initiative for Asthma guideline suggests increasing IC dose before adding LABA or LTRA. 14 Disappointingly, 70% of children in the UK experience asthma symptoms despite treatment, and in the USA 50% of children have uncontrolled asthma symptoms. 22,23 The reasons for children’s symptoms being uncontrolled despite receiving preventer treatment are described elsewhere and include misdiagnosis, illness behaviour, poor inhaler technique and non-adherence to preventer treatment. 17
Adherence to asthma preventer treatment is important to achieving good asthma control and reducing the risk of exacerbations,24 but adherence to preventers is typically < 50% in children with asthma. 24 Interventions to improve adherence can be effective in improving asthma outcomes. Examples of interventions that address poor adherence include electronic logging devices that objectively record inhaler use and let the patient (or parent) know that the treatment is due, and direct observation of therapy. 25,26
There is a widely recognised need for objective tests to help guide asthma treatment decision-making in children to prevent asthma exacerbations and improve day-to-day control. Spirometry, which measures how fast children can breathe out and for how long, is recommended by some, but not all, asthma guidelines; however, its use in asthma management has not been formally evaluated. Other tests have been evaluated for use in guiding the management of childhood asthma, but investigations such as peak expiratory flow, bronchial hyperresponsiveness and exhaled breath analysis (e.g. exhaled breath temperature, exhaled breath condensate, exhaled air volatile organic compounds) have so far proven unsuccessful or not feasible in routine practice. 27–29 We all produce a gas called nitric oxide in our breath, and the concentration of nitric oxide in exhaled breath [called fractional exhaled nitric oxide (FeNO)] is elevated in children with asthma compared with children without asthma. FeNO is considered to be a surrogate for the allergic airway inflammation (also called airway eosinophilia) that is characteristic of childhood asthma.
The potential role of fractional exhaled nitric oxide in monitoring childhood asthma
The evidence that FeNO may be useful in guiding asthma treatment comes from studies that show that treatment with ICs and LTRAs leads to reduced FeNO, and that FeNO rises before an asthma exacerbation and in association with poor asthma control. 30–35 There are limitations to the application of FeNO in clinical practice, including the fact that some children with high FeNO but little or no eosinophilia fail to respond to treatment with ICs despite being known to be compliant. 36,37 A small clinical trial found no added benefit of sputum eosinophils to guide asthma treatment in children in addition to symptoms. 38
Fractional exhaled nitric oxide fulfils many of the criteria of a biomarker for allergic airway inflammation and is approved by the Federal Drug Authority for the management of asthma. 39 The measurements can be made in children as young as 5 years of age using hand-held and portable apparatus. Measurements of FeNO are reproducible, are available within a minute of testing and have been costed at £22.90 per test (2005 prices). 40
There have been eight published clinical trials that have used FeNO to guide asthma treatment in children and the results have been mixed. 41 A Cochrane review of the evidence concluded that there was evidence that FeNO-guided treatment was associated with reduced exacerbations, but not with improved asthma control. 41 These studies had different inclusion criteria for participants, had different primary outcomes and applied different treatment algorithms to (often) different changes in FeNO values. 42 Not all of the clinical trials found that the intervention reduced exacerbations or improved control, and there was evidence of an overall increase in IC treatment associated with FeNO-guided management. 43
The original data from seven of these eight trials have been obtained and combined to facilitate understanding of how FeNO might be used to guide asthma treatment in children. To date, three relevant papers have been published from this data set. 44–46 The first paper46 described the variability in repeated measurements of FeNO over time and suggested that concentrations in children with controlled asthma symptoms, whose treatment is classed as ‘stable’, may change by ≥ 50% over a 3-month period. These findings are consistent with earlier results from children without asthma. 35 The second paper44 described how percentage change in FeNO (but not absolute change in FeNO) over a 3-month period was weakly associated with loss of future asthma control (but not a future asthma exacerbation). The third paper45 reported that FeNO-guided treatment was associated with improved asthma outcomes in some subgroups. Overall, children who were not treated with LTRAs had better outcomes if their treatment was guided by FeNO than children treated with LTRAs. In addition, there was evidence that treatment guided by FeNO led to better outcomes in children who were not obese and who were allergic than in children who were obese or who were not allergic. 45
Current guidelines14,17,21 do not recommend that FeNO should be routinely used to guide asthma treatment in children, but the Cochrane review suggests that FeNO-guided asthma management may be useful in reducing asthma exacerbations in children. 41
The Reducing Asthma Attacks in Children using Exhaled Nitric Oxide (RAACENO) trial was designed to compare the efficacy of asthma treatment guided by symptoms and FeNO with asthma treatment guided by symptoms alone for the risk of asthma exacerbation (full details in Chapter 2). The RAACENO trial differed from previous FeNO trials in the following aspects:47
-
The change in FeNO was expressed as a percentage where previous values were available, based on recent evidence. 44 This recognised that some individuals have higher FeNO values than others.
-
A threshold of ≥ 50% change from the previous value was used to define a clinically meaningful change, based on recent evidence. 46
-
Within the FeNO group there were different treatment algorithms between those with symptoms and a meaningful change in FeNO and those experiencing symptoms and no meaningful change in FeNO. This strategy has proved effective in pregnant women but has not previously been tested in children. 48
Hypothesis
Our hypothesis is that the proportion of children with one or more asthma exacerbations over 12 months will be reduced when asthma treatment guided by FeNO plus symptoms is compared with treatment guided only by symptoms.
In Chapter 2, we describe the trial design and methods. Chapters 3 and 4 include the baseline characteristics and clinical results. In Chapters 5 and 6 we describe the health economic and qualitative process evaluations, respectively. Finally, in Chapter 7, we discuss the results of the trial and consider implications for practice and recommendations for research.
Chapter 2 Trial design and methods
Trial design
The study protocol has been published in an open access journal47 and is a standalone document.
RAACENO was a pragmatic, multicentre, parallel, randomised controlled trial (RCT) that aimed to compare the efficacy of asthma treatment guided by symptoms and FeNO with that of asthma treatment guided by symptoms alone for the risk of asthma exacerbation. The aim was to recruit 502 children with asthma who were treated with ICs and who had had an asthma exacerbation treated with OCs in the past year, with at least 50 participants being recruited in primary care.
The trial design is summarised in Figure 1. Participants were recruited to the trial and were followed up for 12 months post randomisation. Clinical assessments (including assessment of symptoms and measurement of FeNO) took place at recruitment and then at 3, 6, 9 and 12 months post randomisation. FeNO was measured in all participants at every assessment, but guided treatment decisions in the experimental group only (asthma treatment guided by symptoms and FeNO). In the control group (asthma treatment guided by symptoms alone), FeNO was also measured, but the results were not used for any treatment decisions. At each visit, a treatment recommendation was made in relation to recent asthma symptoms, FeNO measurements, treatment adherence and current asthma treatment.
FIGURE 1.
Trial design. FEV1, forced expiratory volume in 1 second.
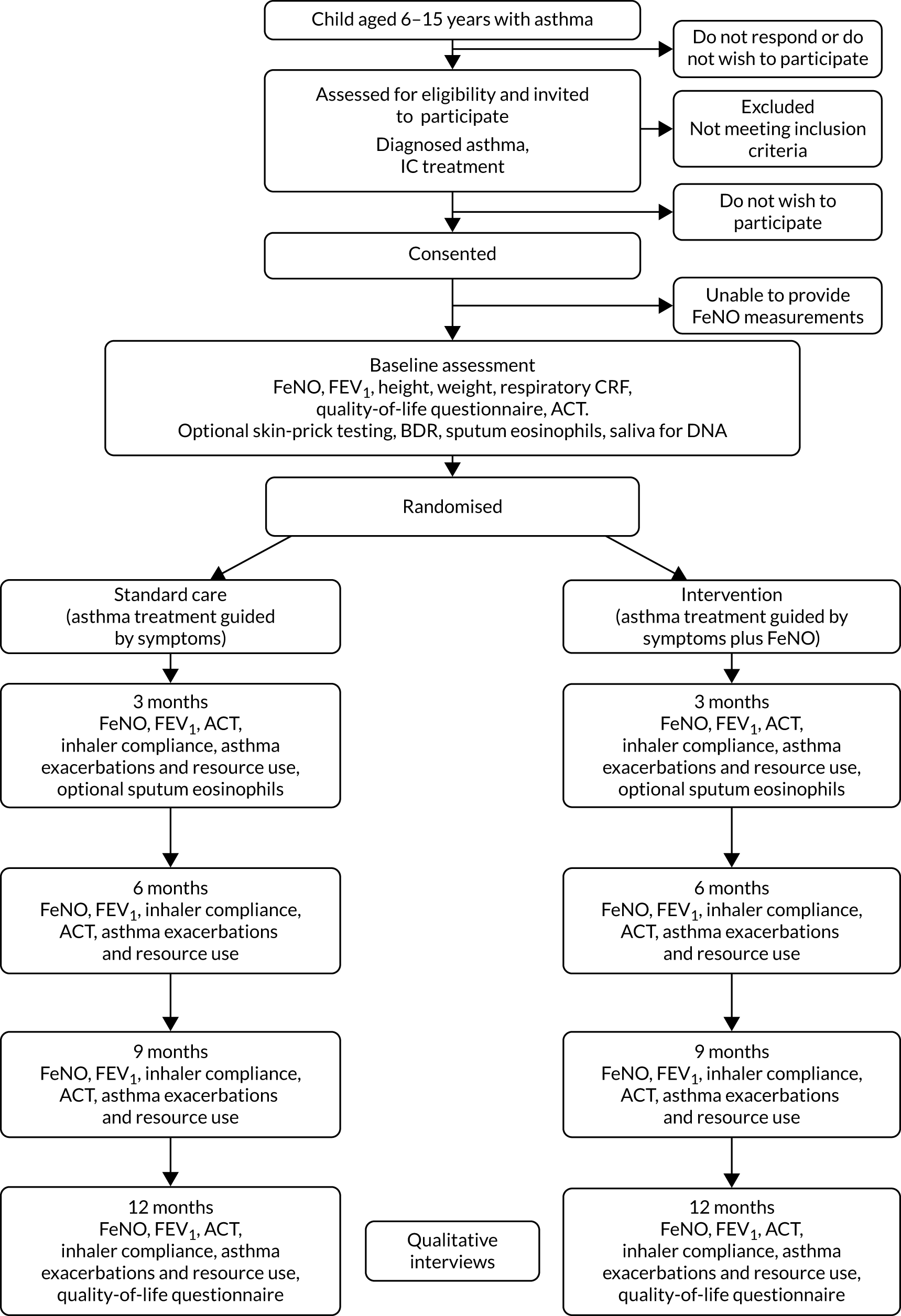
The primary outcome (one or more asthma exacerbations treated with OCs) was collected at each assessment and determined up to 12 months (or to the time at which the participant was lost to follow-up or withdrew from the trial).
Adherence to IC treatment was logged electronically where possible using a smart inhaler; some IC inhalers did not have a compatible smart inhaler device.
The research design also included an evaluation of health-care costs, which considered primary and secondary care contacts and asthma treatment. This information was collected at each assessment, supported by a patient-held diary. The inclusion of a health economic evaluation was in response to a recent Health Technology Assessment publication which noted that ‘little or no empirical evidence exists’ for economic evaluation of FeNO monitoring. 49 Since RAACENO was funded, a health economic evaluation of FeNO monitoring in children has been published. 50 It found no difference in cost or quality-adjusted life-years (QALYs) between the group whose asthma treatment was guided by FeNO plus symptoms and the group whose treatment was guided by symptoms alone.
A qualitative process evaluation was also incorporated into the study design to explore participant experiences and determine the acceptability of the intervention. A sample of up to 20 families (parent and child pairs) across both groups of the trial, as well as between 15 and 20 trial staff, were interviewed until data saturation was reached.
The University of Aberdeen and NHS Grampian co-sponsored the trial. The trial was approved by North of Scotland Research Ethics Committee A (16/NS/0106).
Participants
Participants were children aged 6–15 years with asthma. We aimed to recruit 502 children, with 452 recruited in secondary care sites across the UK and 50 recruited in primary care centres in the east of England. The following inclusion criteria were used to identify eligible participants.
Inclusion criteria
-
Asthma diagnosed or confirmed by consultant paediatrician or respiratory/asthma specialist nurse (or Read code for asthma if recruited in primary care).
-
Aged ≥ 6 years and not reached the date of their 16th birthday (generally children below the age of 6 find it difficult to provide FeNO measurements).
-
Currently prescribed ICs in a device that can be fitted with a smart inhaler [within the study the smart inhalers used were Adherium Haile® sensors (Adherium Ltd, Auckland, New Zealand)]. For the purposes of the study, the maximum dose for children aged < 12 years was 1000 µg of budesonide equivalent per day; the maximum dose for children aged ≥ 12 years was 2000 µg of budesonide equivalent per day.
-
Parent-/patient-reported asthma exacerbation treated with at least one course of OCs in the 12 months prior to recruitment.
Exclusion criteria
-
Unable to provide FeNO measurement at the baseline assessment (expected prevalence of < 5%).
-
Other chronic respiratory conditions that also have exacerbations and may be confused with an asthma exacerbation.
-
Current treatment with maintenance oral steroids, given that treatment cannot be increased any further.
Notes on the inclusion and exclusion criteria
-
There were children who wished to take part in the study but were using an IC inhaler device that could not be fitted with a smart inhaler device. During the course of the study, the criterion that participants could take part only with an IC inhaler compatible with a smart inhaler device was relaxed.
-
No more than one sibling per family could participate in RAACENO at a time (to avoid allocation of siblings to different groups of the trial). Once a sibling had completed 12-month follow-up, another sibling could be approached to take part in the study, provided that they were eligible.
-
Children who wished to participate but could not provide a FeNO measurement at baseline could be invited back for a second baseline assessment. Eligibility was rechecked at the second baseline assessment. At this second baseline assessment, if the parent-/patient-reported asthma exacerbation treated with at least one course of OCs was more than 12 months ago, these inclusion criteria were considered to have been met on the basis of the asthma exacerbation being within 12 months of the initial baseline assessment.
-
There was no minimum dosage of OCs for inclusion. There was no minimum time since the last dosage of OC; however, eligible patients on maintenance oral steroids required a 14-day minimum wash-out period before they could be randomised. Concurrent treatment with antihistamines, LABAs, LTRAs and biologics, such as omalizumab (Xolair; Novartis, Basel, Switzerland), was permitted. There were no minimum/maximum FeNO readings. Inhaler technique was checked at every assessment and education was given to improve technique where required.
Identification
Potential participants were recruited from both primary and secondary care sites across the UK. Recruitment strategies differed between centres depending on local NHS organisational factors and are described in the following sections.
Secondary care
The usual clinical team identified eligible children. The initial approach was in person (at a clinic appointment) by a member of the usual-care team [which may have also included embedded research nurses (RNs)] or by a letter/e-mail from the managing clinician or asthma specialist nurse. For those approached in clinic, the parent and age-appropriate child patient information leaflets (PILs) were given to the family by a member of the usual clinical team. At this time, the parent was asked whether or not they would be happy for a member of the study team to speak to them in the clinic or to contact them by telephone to answer any questions that they may have about the study. For those approached by post or e-mail, the parent and child PIL and letters of invitation were sent to the family by the managing clinician or asthma specialist nurse. A member of the usual care team (which included embedded RNs) contacted the parent by telephone around 2 weeks after the initial approach to answer any questions they had about the study.
In Scotland, the NHS Research Scotland Primary Care Network also identified potential participants in primary care for three secondary care sites. The initial approach was by letter from the primary care practice, which was sent with the short PIL, a reply slip and a pre-paid envelope. Those interested in taking part in the research were asked to return the reply slip or contact the research team based at the secondary care site. Interested families were then sent the parent and age-appropriate child PILs and arrangements were made for those who wished to take part to be seen at the secondary care site for a recruitment appointment.
Primary care
Primary care recruitment was conducted through a number of primary care practices in the east of England. Practices participated as independent research sites, supported by local Clinical Research Network (CRN) RNs. Staff at the primary care practice identified eligible children from electronic records. The initial approach was by a letter from a general practitioner (GP) at the practice, which was sent with the short PIL, a reply slip and a prepaid envelope. Those interested in taking part in the research were asked to return the reply slip or contact the research team. A member of the primary care team or CRN member of staff contacted the parent by telephone around 2 weeks after the initial approach to answer any questions that they had about the study. Interested families were then sent the parent and age-appropriate child PILs, and arrangements were made for those who wished to take part to be seen at the primary care practice for a recruitment appointment.
Recruitment
For families that were interested in participating, a baseline appointment was then arranged in either primary or secondary care. At this baseline appointment, consent was received in accordance with good clinical practice. Fully informed, written consent was received from parent(s)/carer(s) and (where appropriate) from the child. If the child did not provide written consent, they were asked to give verbal assent. At the baseline visit, the participant’s eligibility was confirmed by a medically qualified doctor. Baseline data were also collected (described in more detail in Baseline).
Some recruitment sites were able to offer one or more of the optional mechanistic components (listed below). In these sites, consent for each relevant component was received from participants who wished to take part in one or more of the components:
-
bronchodilator-derived response
-
skin-prick test
-
saliva collection for deoxyribonucleic acid (DNA) analysis
-
sputum collection for eosinophil count.
Children who turned 16 years of age during follow-up and who wished to re-consent to their continued participation in the study could do so at their next follow-up visit. They were given a PIL and were asked to sign a consent form. If they wished to continue with the study, but did not wish to complete a new consent form, the study team confirmed verbally that they were happy to continue taking part.
Randomisation/treatment allocation
Eligible and consenting participants were randomised to one of the two groups (1 : 1) [treatment decisions based on FeNO plus symptoms (intervention group) or treatment decisions based on symptoms alone (standard-care group)]. A member of the research team at the site randomised participants using a 24-hour web-based randomisation application (bespoke in-house system), which was hosted by the Centre for Healthcare Randomised Trials, University of Aberdeen. Random allocation used a minimisation algorithm, including a random element (20%). Stratification was by centre (primary care sites were considered as one centre for the purpose of minimisation), age (< 11 years, ≥ 11 years), sex and asthma severity [as evidenced by BTS/SIGN treatment step (BTS step 2, BTS step 3, BTS step 4)]. 51
The principal investigator (PI), or the individual at a site with delegated authority, accessed the web-based randomisation system. Minimisation characteristics (centre, age, sex and asthma severity) were entered into the web-based system, which returned the allocation. Randomisation was completed in the clinic at the baseline visit after consent had been given and baseline data collected. Participants were informed of their allocated treatment group following randomisation. The study teams at each site were also made aware of the allocated treatment group. Each participant’s GP was informed that the child was taking part in RAACENO and which treatment group they had been randomised to.
Treatment decisions
In this study, treatment decisions in the intervention group were guided by FeNO measurement and symptoms. Symptoms were assessed using the ACT or, if the participant was aged < 12 years, the CACT. In the standard-care group, treatment decisions were guided by symptoms alone (assessed using ACT/CACT), as per the BTS/SIGN national guideline. 51
Treatment decisions in both groups were protocolised. The relevant data for the treatment decision (current medication, any recent change to medication, adherence to IC medication, symptoms and, in the intervention group, the FeNO result) were entered into the study website. For participants in the standard-care group, the RN and clinician were blinded to the FeNO result until after the visit was complete and the treatment decision had been made. The treatment algorithm was incorporated into the study website and, therefore, this was applied without subjectivity. The treatment algorithm could recommend no change to current treatment, a reduction in treatment, an increase in treatment or, for participants who received the highest treatment via a dry powder inhaler, a change to receive the same treatment but by metered dose inhaler (MDI) via a spacer. Where the child was already receiving the maximum treatment for their age, the algorithm recommended referral to the asthma specialist. At each visit, the clinical team could follow the recommendation of the treatment algorithm or decide to make an alternative treatment decision in consultation with the family.
Where an alternative treatment decision was made, this was documented within the study case report forms (CRFs) and not considered to be a protocol deviation.
At each follow-up, the algorithm was applied from the treatment that they were currently taking.
For participants recruited in secondary care, the asthma team could either prescribe any change in treatment or write to the GP to request a change in treatment, depending on local prescribing practice. For participants recruited in primary care, the GP could prescribe any change in treatment.
Experimental intervention
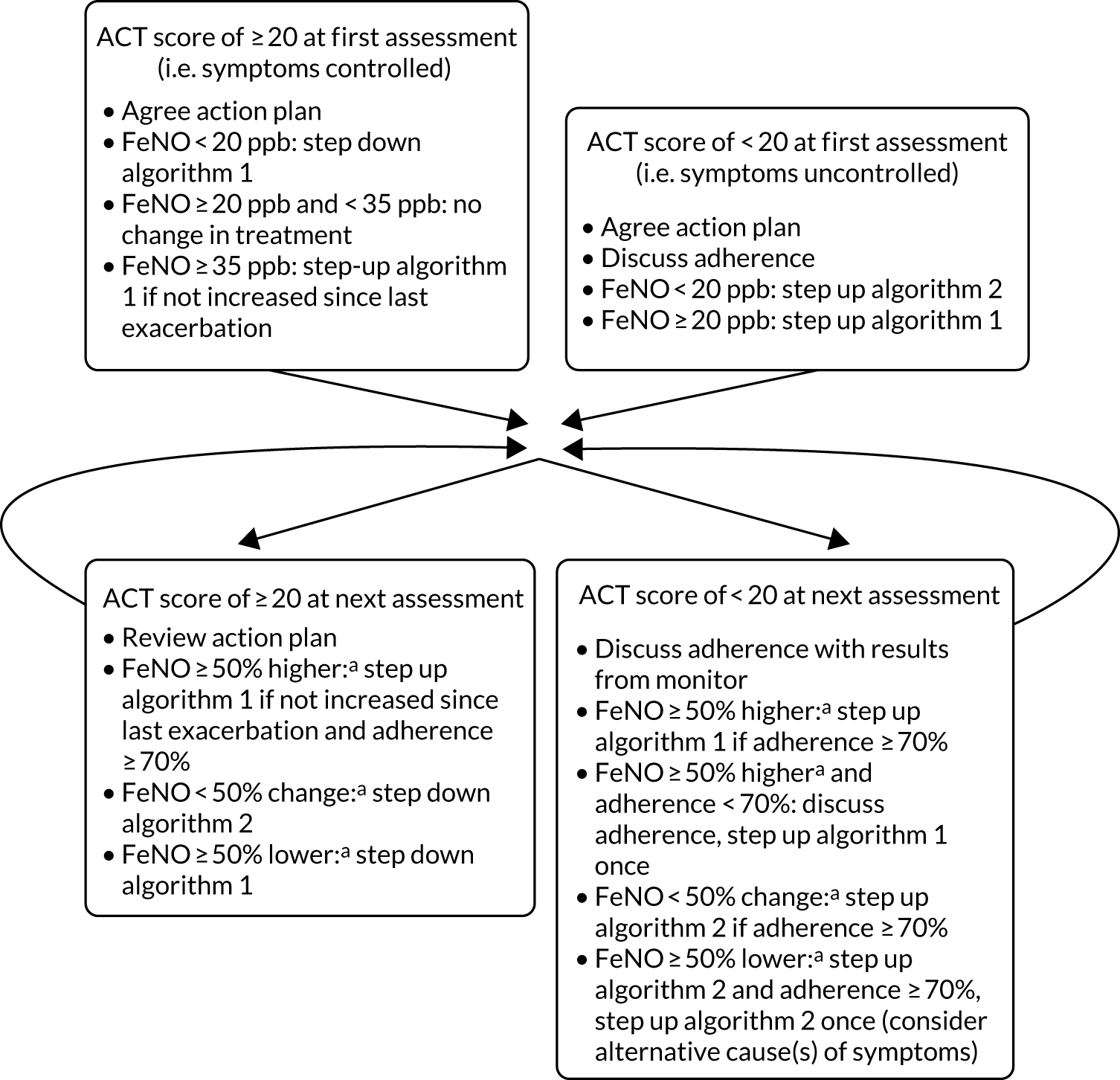
In the intervention group, asthma treatment decisions were guided by FeNO and symptoms. Figure 2 summarises the experimental intervention and Table 1 summarises the treatment steps. The experimental intervention and subsequent adjustment of treatment steps were applied at baseline and at each of the follow-up visits.
FIGURE 2.
Simplified schematic of the experimental intervention.
| Step | Algorithm 1 | Algorithm 2 |
|---|---|---|
| 1 | SABA (as required) only | SABA (as required) only |
| 2 | Budesonide (or beclomethasone) 200 µg daily plus SABA | Budesonide (or beclomethasone) 100 µg twice daily plus SABA |
| 3 | Budesonide (or beclomethasone) 400 µg microgram or fluticasone 200 µg daily plus SABA | Budesonide (or beclomethasone) 400 µg or fluticasone 200 µg daily plus SABA |
| 4 | Budesonide (or beclomethasone) 800 µg or fluticasone 500 µg daily plus SABA | Add LABA |
| 5 |
Budesonide (or beclomethasone) 1600 µg daily or fluticasone 1000 µg daily plus SABA (only for those aged ≥ 12 years) Go to step 6 for those aged < 12 years |
Add LTRA |
| 6 | Add LABA in fixed dose combination | Budesonide 800 µg or fluticasone 500 µg daily in fixed dose combination |
| 7 | Add LTRA |
Budesonide (or beclomethasone) 1600 µg daily or fluticasone 1000 µg daily plus SABA (only for those aged ≥ 12 years) Go to step 8 for those aged < 12 years |
| 8 | Refer for assessment by asthma specialist | Refer for assessment by asthma specialist |
When developing the experimental intervention, the following considerations were made:
-
FeNO-guided treatment escalated either anti-inflammatory medication (algorithm 1, elevated FeNO) or early intervention with bronchodilators (algorithm 2, FeNO not elevated). The response to increasing ICs or the addition of LABA or LTRA is known to be heterogeneous in children,52 and this algorithm uses FeNO to stratify treatment with early escalation of IC treatment or early addition of LABA and LTRA ‘add-on’ therapies, an approach that has been proven in a FeNO trial in pregnant mothers. 53
-
Inhaled corticosteroid was increased only once in controlled children with elevated FeNO, and only if their IC dose had not been increased since their asthma exacerbation and they were adherent.
-
In accordance with the BTS/SIGN 2016 guideline,51 poor adherence was also considered (and discussed) before escalating treatment. Elevated FeNO is a result of airway inflammation but does not identify the cause of airway inflammation, and poor adherence is an important mechanism for persisting airway inflammation; therefore, adherence was part of the algorithm. Adherence was a continuous measure of a complex outcome, and a single cut-off value to prompt a discussion about adherence was arbitrary but needed. Poor adherence was defined as < 70%; an audit of children attending the asthma clinic in Aberdeen found that 48% had collected ≥ 70% of their IC treatment from their GP over 12 months (data from unpublished NHS Grampian audit by Dr Steve Turner).
-
Interpretation of FeNO on the first visit relied on population norms, rather like lung function or height, but at subsequent visits it was interpreted as a percentage change from the previous measurement.
-
Asthma control was defined as ACT score of ≥ 20. We know from two previous FeNO intervention trials54,55 and one observational study of children attending secondary care asthma clinics56 that 75% of children with asthma have an ACT score of ≥ 20 at any one time.
More details of the treatment steps are provided in Appendix 1 and the detailed decision tree is given in Appendix 2.
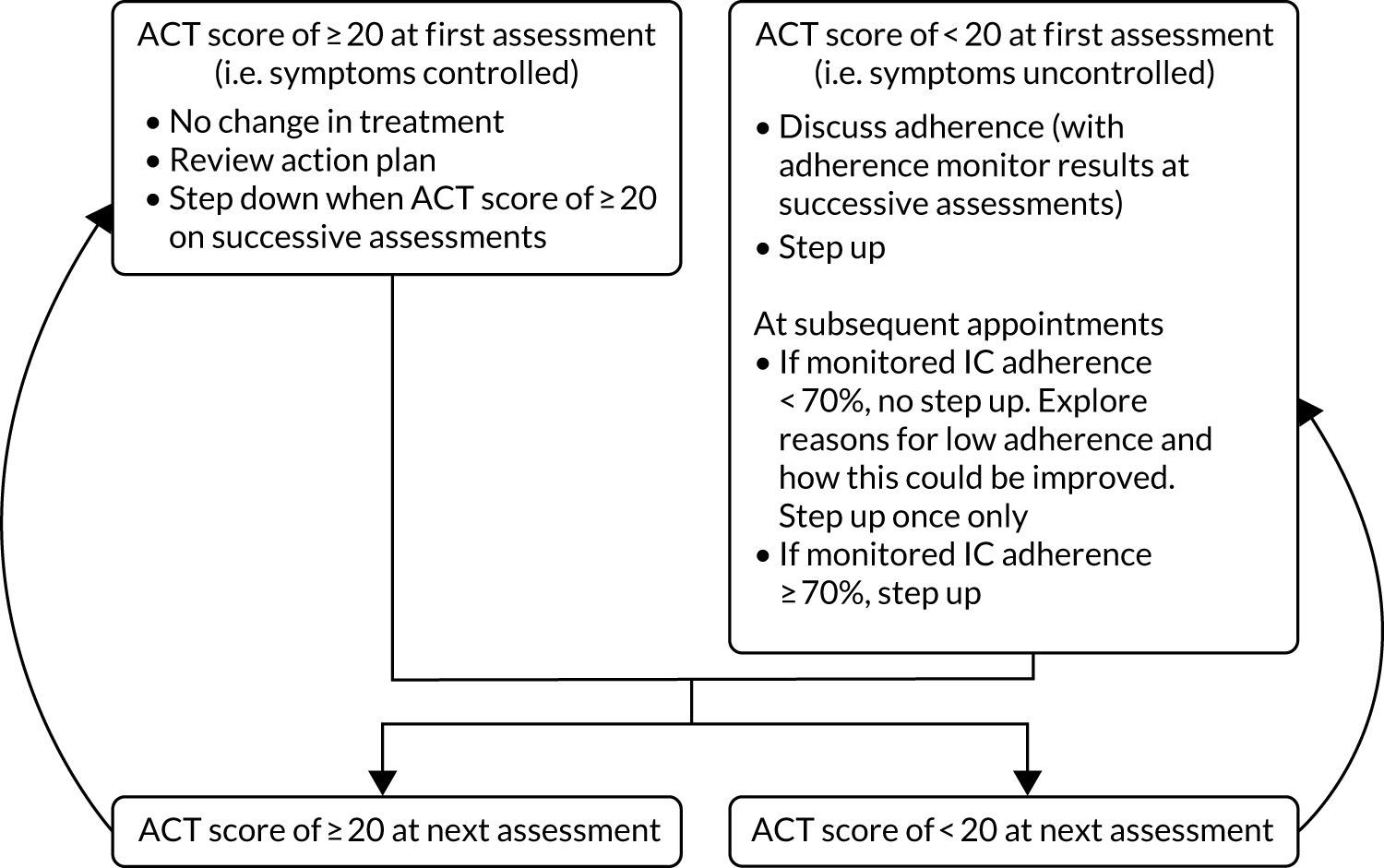
Control intervention (standard care)
In the standard-care group, asthma treatment was guided by symptoms alone.
Figure 3 summarises the control intervention and Table 2 summarises the treatment steps, which are in accordance with the national guideline that was in place when RAACENO started. 51 The control intervention and subsequent adjustment of treatment steps were applied at recruitment and at each of the follow-up visits (3, 6, 9 and 12 months).
FIGURE 3.
Simplified schematic of the control intervention.
| Treatment step | Daily IC dose of budesonide or equivalent (µg) | Name of treatment step | Dose and frequency of ICs inhaler |
|---|---|---|---|
| 1 | 0 | No ICs | SABA (as required) only |
| 2 | 200 | Very low dose of ICs | Budesonide (or equivalent) 100 µg twice daily plus SABA |
| 3 | 400 | Low dose of ICs | Budesonide (or equivalent) 200 µg twice daily plus SABA |
| 4 | 400 | IC plus LABA combination inhaler | Budesonide (or equivalent) 200 µg twice daily plus SABA and LABA (dose depending on IC molecule used) |
| 5 | 400 | Add on LTRA | Budesonide (or equivalent) 200 µg twice daily plus SABA, LABA and LTRA |
| 6 | 800 | High dose of ICs | Budesonide (or equivalent) 400 µg twice daily plus SABA, LABA (dose depending on IC molecule used) and LTRA |
| 7 for those aged 12–15 years (go to step 8 for those aged < 12 years) | 1600 | High dose of ICs | Budesonide (or equivalent) 800 µg twice daily plus SABA, LABA and LTRA |
| 8 | Refer for assessment by asthma specialist |
More details of the treatment steps are provided in Appendix 1 and the detailed decision tree is shown in Appendix 3.
Measurement of fractional exhaled nitric oxide
FeNO was measured prior to spirometry.
Although FeNO was measured in children in each of the groups at each of the follow-up visits that were attended, the results of FeNO were not used in treatment decisions in the standard-care group of the trial. In this group, the FeNO result was recorded after the assessment was completed and the child had left the assessment room. Participants in this group were blinded to the result and it was not available to the patient’s GP or paediatrician during the trial period.
In the intervention group (FeNO plus symptoms), it was not feasible to blind the participant and parent to FeNO; for example, in the scenario in which a child’s asthma is controlled and treatment stepped down, it will be clear that FeNO has fallen.
Code break/emergency unblinding procedures
There was no requirement for emergency unblinding procedures. This was because knowledge of whether a participant was in the standard-care group or intervention group would not affect any management decisions being taken if an AE occurred.
Study exit at 12-month follow-up for both the intervention and the control group
The treatment algorithm was applied to participants in both groups of the trial at the 12-month assessment. For participants who were recruited in secondary care, the letter that was sent to the GP confirmed that ongoing management of the child would revert to current standard practice.
Data collection
Baseline and safety data were collected during face-to-face assessments at baseline and then outcome and safety data were collected at the 3-, 6-, 9- and 12-month follow-ups. The schedule for data collection is outlined in Table 3.
| Details collected | Time point | ||||
|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | |
| FeNO | ✓ | ✓ | ✓ | ✓ | ✓ |
| Smart inhaler data | ✓ | ✓ | ✓ | ✓ | |
| Baseline data, asthma history | ✓ | ||||
| Current medication, inhaler technique | ✓ | ✓ | ✓ | ✓ | ✓ |
| ACT/CACT | ✓ | ✓ | ✓ | ✓ | ✓ |
| PAQLQ | ✓ | ✓ | |||
| Spirometry and height | ✓ | ✓ | ✓ | ✓ | ✓ |
| Weight | ✓ | ✓ | |||
| Asthma exacerbations | ✓ | ✓ | ✓ | ✓ | ✓ |
| Asthma-related health care and other related resource use | ✓ | ✓ | ✓ | ✓ | ✓ |
| Mechanistic studies (all optional) | |||||
| Bronchodilator response | ✓ | ||||
| Induced sputum | ✓ | ✓ | |||
| Skin-prick testinga | ✓ | ||||
| Saliva for DNA extractiona | ✓ | ||||
Baseline
At baseline, the web-based CRF recorded the participant’s details [date of birth, sex, ethnicity, information about their asthma (including current treatment, recent history), co-morbidities, family history of atopic disease, second-hand smoke exposure and other information about environmental exposure]. Spirometry was undertaken using standard methodology (described in Methodology for spirometry) to assess forced expiratory volume in 1 second (FEV1) inhaler technique, and height and weight were measured.
At the baseline assessment, children and their parent/carer completed asthma questionnaires: for children aged ≤ 11 years the CACT and the Paediatric Asthma Quality of Life Questionnaire (PAQLQ) were used, and for children aged ≥ 12 years the ACT and the PAQLQ were used.
If a smart inhaler device was available for the recommended IC inhaler, this was given to the family along with a charging wire.
At the end of the baseline assessment, a diary card was given to families to record any asthma exacerbations, asthma-related health care and other related resource use. Families were asked to bring it back to follow-up appointments as an aide-memoire to recollect outcome data.
Follow-up visits
Participants were followed up in clinic at 3, 6, 9 and 12 months. The web-based CRF captured current asthma treatment, height, spirometry data (as described for baseline) and FeNO (again as described for baseline). In addition, at the 12-month assessment weight was also measured. Patient-reported adherence to their IC medication was recorded (never, occasionally, about half of the time, most of the time, all of the time). Patients were classed as adherent to IC medication if they reported taking their inhaler ‘most of the time’ or ‘all of the time’. Where children had been provided with a smart inhaler at their previous visit and brought it back to the follow-up visit, adherence data (daily adherence, presented as a percentage) were downloaded from the smart inhaler. Using smart inhaler data, adherence was defined as ≥ 70% of the doses taken. Where there was a mismatch between patient-reported adherence and data from the smart inhaler, the research team explored potential explanations and, based on the discussion, recorded whether or not they thought that the child used the inhaler most/all of the time (adherence ≥ 70%). Similarly, if smart inhaler data were not available, the research team discussed the patient-reported adherence and recorded whether or not they thought that the child used the inhaler most/all of the time (adherence ≥ 70%).
Inhaler technique was also checked at each follow-up visit. Technique was categorised as satisfactory, satisfactory after training (which was provided during the visit) or not satisfactory. Technique was not incorporated into the treatment algorithm.
Information about any asthma exacerbations was recorded at each follow-up, including date of onset, treatment and associated resource use. Asthma exacerbations were not incorporated into the treatment algorithm. Other resource use associated with the child’s asthma was also recorded.
At each follow-up appointment, children and their parent/carer completed the ACT or CACT, depending on the age of the child. At the 12-month appointment, they also completed the PAQLQ.
At the end of the 3-, 6- and 9-month follow-up visits, a diary card (described above) was given to families. In addition, if a smart inhaler was previously not available but was now available, this was given to the family, along with a charging wire.
Those who did not attend for follow-up at 3, 6, 9 or 12 months were contacted by telephone and another appointment was offered. There was a 6-week visit-window before and after each assessment date for that assessment to take place.
Telephone follow-up with families was offered to those who could not attend a follow-up appointment and, on the instruction of the sponsor, all follow-up from 20 March 2020 (around the time of the first COVID-19 lockdown in the UK) was carried out by telephone.
Where the assessment was carried out by telephone, it was not possible to measure FeNO or download adherence data from the smart inhaler; family-reported adherence was recorded. In these cases, for both groups the treatment algorithm was applied without FeNO measurements or smart inhaler data, and in the FeNO group the symptom-only algorithm was applied. In these cases, the treatment algorithm recommendation would be discussed with the PI, particularly if the child had an ACT/CACT test that suggested that symptoms were poorly controlled (score of < 20). The local team was responsible for informing the GP and family if any changes to treatment were needed.
Those who did not attend for follow-up, and for whom telephone follow-up could not be arranged, were sent the CACT or ACT by post and were asked about any asthma exacerbations since their last visit. Those who did not attend the 12-month visit were also sent the PAQLQ.
At the end of the 12-month follow-up period, if primary outcome data had not been obtained from the participant for the full follow-up period, data on asthma exacerbations was sought from primary and secondary care medical records by the local or central trial office team.
Methodology for fractional exhaled nitric oxide measurement
Fractional exhaled nitric oxide was measured using the NIOX VERO® device (Circassia, Oxford, UK) and standard methodology. The NIOX VERO® was switched on and a clean mouthpiece was applied. When the machine indicated that it was ready, the participant breathed in slowly through the mouthpiece and then breathed out slowly (as directed by the visual incentive) for 10 seconds (6 seconds if aged ≤ 10 years). Only one technically acceptable measurement was required. Children could make multiple attempts until a technically acceptable measurement was achieved. If children aged > 10 years could not complete the 10-second measurement, they were asked to try the 6-second measurement. Wherever possible, the same apparatus was used for the same individual and the measurement took place at the same time (± 2 hours) at each assessment. The apparatus took 1 minute to produce the result and an acceptable result was indicated by a beep.
Fractional exhaled nitric oxide was measured before spirometry for all participants because FeNO is known to fall slightly after spirometry; therefore, measuring FeNO before spirometry more accurately reflects the value. 39,57 Furthermore, by consistently measuring FeNO before spirometry throughout the trial, intra-subject comparison of FeNO results would be valid.
Methodology for spirometry
The standard methodology was used. 58 Apparatus was calibrated in accordance with manufacturer recommendations. Apparatus varied between centres, but we recommended that the same apparatus was used for each individual throughout their follow-up. Incentive spirometry was used where appropriate. The participant was seated and a nose clip was used. After a full breath in, the participant exhaled as quickly as possible and for as long as possible. As many measurements were taken as were required to produce three technically acceptable measurements. FEV1 was the spirometric outcome.
Mechanistic components
Bronchodilator-derived response
Those who agreed to the optional bronchodilator response measurements were asked to withhold their bronchodilator (SABA) for 4 hours before their recruitment appointment. However, they could participate even if they had not withheld their bronchodilator prior to their appointment. At the recruitment appointment, the child was asked to take their bronchodilator (400 µg salbutamol or equivalent ideally via spacer device) and repeat the spirometry after 15 minutes using the methods described above.
Those not opting into the bronchodilator response component of the study did not need to withhold their bronchodilator before their recruitment appointment.
Saliva collection for deoxyribonucleic acid analysis
Participants who opted into this optional mechanistic component of the study had saliva collection carried out after completion of questionnaires and FeNO measurement. If Oragene 500 saliva testing kits (DNA Genotek, Ottawa, ON, Canada) were available at the site, the manufacturer’s protocol for collection of saliva would be followed. If commercial saliva testing kits were not available at the site, the participant would rinse their mouth for 20 seconds with 10 ml of tap water and spit the fluid into a universal container that was labelled and stored at –20 °C or below. If saliva was not collected at baseline, it could be collected at the subsequent follow-up visits. At the time of writing, the DNA analysis has not been completed, and did not form part of the original grant funding for this project. These data will be analysed and reported in a future publication.
Skin-prick test
Participants who opted into this optional mechanistic component of the study had the skin-prick test carried out after completion of the questionnaires and FeNO measurement. The method described by Pepys59 was used to determine skin-prick reactivity to cat dander, house dust mite, hen’s egg and Timothy/mixed grass. Positive (histamine 10 mg/ml) and negative controls (0.9% saline) were used. A drop of each of the six agents was placed on the volar surface of the non-dominant forearm. The skin under the drop was pricked with a lancet. The response to the positive control was measured as the greatest diameter 10 minutes after the skin was broken and any response to the other agents was measured after 15 minutes. Atopy was defined as at least one wheal that measured ≥ 3 mm in longest diameter or, in cases of dermatographism, a wheal greater than the negative control. There was no requirement to withhold antihistamines or skin corticosteroids or other medication prior to skin-prick testing.
Skin-prick testing, if not carried out at baseline, could be carried out at any of the study visits. If skin-prick testing had been carried out as part of routine clinical care in the previous 6 months, results from this test could be entered into the CRF with the consent of the family.
If the allergen agents used routinely at a site differed to those described above, then the routine allergens were used in the study skin-prick testing.
Sputum induction
Participants who opted into this optional mechanistic component of the study had sputum collection carried out after completion of questionnaires and FeNO measurement, and after saliva collection and the skin-prick test (if carried out). The participant was asked not to eat anything for 1 hour prior to sputum induction and to rinse out their mouth prior to testing. A total of 200 µg of salbutamol (via a MDI/spacer or dry powder device) was given and FEV1 was determined 15 minutes later. Induction was not carried out if FEV1 was < 50% of that predicted after salbutamol. The participant inhaled 4% saline for 5 minutes via a new nebuliser mouthpiece driven by air from a wall-mounted gas supply or portable nebuliser, and the participant asked to expectorate. If no sputum was brought up, then 5% saline was inhaled by nebuliser for another 5 minutes and the participant asked to expectorate again. If no sputum was brought up, no further attempt was made to obtain sputum. (Where 4% and 5% saline were not available at a recruitment site, saline of other concentrations could be used in line with local routine practice.)
Those who agreed to participate in this optional mechanistic component of the study were asked to provide sputum at the recruitment and 3-month study visits. If they were unable to produce a sputum sample at the recruitment visit, they were not asked to provide one at the 3-month visit.
The sample was processed in accordance with the standard operating procedure in the local laboratory (either NHS or university, depending on local expertise). The sample was centrifuged and the cell pellet was resuspended in a standard volume before staining. A slide was created and 400 non-squamous cells were counted by a blinded investigator. The percentages were then averaged to give a final eosinophil count. The remainder of the fluid sample was frozen at –20 °C or below, and we had planned to transport these to Aberdeen for storage and future microbiome analysis (if funding could be secured). Consent for this was sought at the outset of the study.
However, very few sites had the laboratory facilities or capacity to undertake sputum collection and eosinophil count. In addition, the process of collecting sputum is not pleasant for children. This optional mechanistic component was abandoned with the prior approval of the Trial Steering Committee (TSC) and funder in December 2018.
Data processing
Research nurses at each centre entered data into the study website. Given that the treatment decisions were protocolised as part of the study website, the data were added (ideally) in real time to allow the treatment algorithm to be applied and any treatment step-up/step-down decisions to be communicated with participants and their families during the visit.
Staff in the trial office worked closely with local RNs to ensure that the data were as complete and as accurate as possible. Follow-up questionnaires to participants unable to attend follow-up appointments were sent from and returned to the trial office in Aberdeen (or, if sites preferred, sent from and returned to the site team). Data from questionnaires returned to the trial office were entered into the study website by trial office staff.
As part of the trial’s monitoring plan, the trial office carried out accuracy checks on a sample of questionnaires entered by each site.
Based on the free-text information that research teams added to record why they were not complying with the recommendation of the treatment algorithm, we coded reasons for algorithm non-compliance into seven overarching themes (knowledge, beliefs, behaviour, emotion, environment, social and technical) and 28 subthemes. The reason could be coded into up to four subthemes. We differentiated between knowledge and beliefs on the basis of whether underlying knowledge was suggested in the free text (e.g. it was known that there had been a recent change in treatment) or the decision-making was based on a belief (e.g. that no change in treatment was needed). Where free-text information was provided about who had made the decision, this information was categorised (clinical team, family, joint decision).
Primary outcome
The primary outcome was one or more asthma exacerbations treated with OCs in the 12 months after randomisation (yes/no). This outcome was identified in a meta-analysis43 published before a Cochrane analysis,41 which reached the same conclusions. The outcome is approved as an exacerbation outcome by the American Thoracic Society and European Respiratory Society task force on asthma exacerbations. 13 The decision to prescribe OCs is usually made by clinicians independent of the research team and working in accordance with the national guideline. 17 Some children may have had ‘rescue’ packs of OCs at home and use of such rescue packs for an asthma exacerbation met the criteria for definition as an asthma exacerbation. Asthma exacerbations were determined from the child/parent or carer and supplemented with information from primary and secondary care medical records where appropriate.
Secondary outcomes
There were a number of secondary outcomes:
-
time to first exacerbation
-
number of exacerbations during follow-up, based on prescribed OCs
-
need for unscheduled health-care assessment during follow-up (yes/no)
-
number of unscheduled health-care assessments
-
asthma control during follow-up (i.e. age-appropriate ACT score of ≥ 20), as used in our observational study and other FeNO studies16,35,54,55
-
spirometry during the 12-month follow-up (i.e. %FEV1, a standard objective index of asthma severity)
-
FeNO at the 12-month follow-up
-
dose of IC at the 12-month follow-up (i.e. daily dose of budesonide equivalent averaged over 3 months)
-
PAQLQ score at 12-month follow-up60
-
qualitative outcomes from interviews
-
health economic evaluation (derived from participant-reported data, supplemented with information from primary and secondary care medical records where appropriate). 60
Adverse events
The NIOX VERO® is CE (Conformité Européenne) certified and known to be safe for use in this age group. In RAACENO, we recorded only AEs and serious adverse events (SAEs) that were related to the use of the NIOX VERO® or other study assessments.
An asthma exacerbation (defined as an increase in asthma symptoms requiring treatment with OCs) was the primary outcome and was not classified as an AE or SAE.
An AE was defined as any untoward medical event affecting a clinical trial participant. Each initial AE was considered for severity, causality or expectedness and was reclassified as a SAE, related or not related to the use of the NIOX VERO® or other study assessments, based on prevailing circumstances.
A SAE was defined as any AE that:
-
resulted in death
-
was life-threatening (i.e. the subject was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe)
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Hospitalisations for treatment planned prior to randomisation and hospitalisation for elective treatment of a pre-existing condition were not considered to be AEs or SAEs. Emergency hospital admissions that did not relate to the use of the NIOX VERO® or other study assessments were not considered to be SAEs.
In this trial, the following events were potentially expected:
-
feeling faint following any of the study interventions
-
itching (following skin-prick testing)
-
coughing/wheezing (following spirometry or sputum collection).
All AEs and SAEs meeting the criteria for recording within RAACENO were recorded from the time that a participant consented to join the trial to the last trial visit.
Participant withdrawal
Participants remained in the trial unless they chose to withdraw consent or they were unable to continue for a clinical reason. All changes in status (with the exception of complete withdrawal of consent) meant that the participant was followed up for all trial outcomes wherever possible. All data collected up to the point of complete withdrawal were retained and used in the analysis. If participants withdrew from the intervention or standard care, they were encouraged to remain in the trial and be followed up as per trial schedule. Participants who wished to withdraw from study follow-up were encouraged to allow routine follow-up data from hospital or GP records to be used for trial purposes. Patients who did not attend for follow-up assessment but for whom any outcome data were available were included in the analysis. Participants who had their asthma diagnosis overturned while participating in the trial were no longer managed through the intervention or control treatment algorithm; data collected up to this point were included in the analysis.
Sample size
We knew from an audit of GP records of children attending the asthma clinic at the Royal Aberdeen Children’s Hospital that 55% of children had received one or more course of OCs in the past year. An exacerbation incidence of 55% is consistent with results from a study of children in secondary care asthma clinics across Scotland; however, we anticipated a lower exacerbation rate of 44% in the control group given that outcomes in children with asthma participating in clinical trials are often better than in observational studies. 54,56 Our meta-analysis found a relative 33% reduction in the proportion of participants with one or more exacerbation receiving FeNO-guided treatment. 43 Assuming an exacerbation proportion of 44% for the symptom-guided treatment group and using a 33% relative reduction as the target difference implies 29.5% exacerbation in the intervention group [equating to an odds ratio (OR) of 0.52]. We had 90% power with 5% significance (two sided) to detect this absolute 14.5% reduction if we recruited 238 children per group. Allowing for 5% incomplete follow-up, we planned to recruit 502 children (i.e. 251 per group).
Statistical analysis of clinical outcomes
All analyses were prespecified in the statistical analysis plan, which was approved by both the TSC and the Data Monitoring Committee (DMC) in advance of analysis. The statistical analysis plan is a stand-alone document. 47 There was no interim analysis or stopping rules. A 5% two-sided significance level was used to denote statistical significance, and estimates are presented alongside their 95% confidence intervals (CIs). No adjustments were made for multiple testing. Analysis was in accordance with the intention-to-treat (ITT) principle and was undertaken in Stata® version 15 (StataCorp LP, College Station, TX, USA).
We describe categorical variables with number and percentage in each category. We describe normally distributed continuous variables with mean and standard deviation (SD) and skewed continuous variables with median and interquartile range. We report the number of missing data for each variable.
Primary outcome
A logistic regression model was used to determine whether or not the intervention was associated with the primary outcome, that is any exacerbation within 12 months of randomisation. The primary analysis was adjusted for the factors used in the minimisation process to allocate participants to groups: centre, age (< 11 years, ≥ 11 years), sex and asthma severity (BTS step 2, 3 or 4). An unadjusted analysis was also carried out. Odds ratios and their 95% CIs were used as the main measure of effect with an absolute measure (difference in percentages) also reported. This analysis was on those with available primary outcome data (complete-case analysis).
Sensitivity analysis (primary outcome only)
The primary analysis was by ITT, but we also conducted a post hoc per-protocol analysis according to whether or not the treatment algorithm was followed (including individuals in both groups of the trial who complied with the treatment algorithm). For this analysis, a definition of compliance is required. A participant was considered to be compliant with the trial if the treatment decision was based on the algorithm recommendation at three or four out of the four scheduled visits (from baseline, 3, 6 and 9 months). A treatment decision could be made only if a participant attended a visit (or, during the COVID-19 pandemic, this was carried out by telephone). Our definition of compliance meant that children who missed two or more visits could not be classified as compliant in the trial.
A complier-average causal effect (CACE) analysis was also undertaken for the primary outcome. For the CACE analysis, the treatment effect calculated for the primary outcome was divided by the proportion of compliers in the FeNO group (the same definition of compliance was used as described above for the per protocol analysis). The Stata command ‘bpbounds’ was used to obtain the 95% CI. In this analysis, all participants in the standard-care group were assumed to be compliant, irrespective of whether or not the algorithm was followed.
We planned to assess the influence of any missing data on the robustness of the findings and to conduct sensitivity analyses using pattern mixture models or multiple imputation, if appropriate. 61,62
Prespecified subgroup analyses
The analysis for the primary outcome was repeated for eight subgroups. The primary outcome model included a randomised group-by-subgroup interaction term, with the estimates of treatment effect estimated in each group separately, with an interaction p-value reported. Results of the subgroup analyses were presented graphically. The subgroups are as follows:
-
baseline treatment (includes LTRA, does not include LTRA)
-
age at baseline (< 11 years, ≥ 11 years)
-
sex (male, female)
-
severity (BTS step 2, BTS step 3, BTS step 4)
-
wheeze only with colds (yes, no)
-
obesity at baseline (obese, non-obese)
-
current eczema in last 12 months (yes, no)
-
skin-prick testing (positive for any of cat, house dust mites, hen’s egg, Timothy/mixed grass; yes, no).
Secondary outcomes
The total number of asthma exacerbations, based on prescribed OCs, during the 12-month follow-up period was analysed using negative binomial regression, adjusting for the minimisation factors and presented using an incidence rate ratio (IRR) and its 95% CI. Time to first exacerbation was compared between groups using Cox proportional hazards regression and reported using a hazard ratio (HR) and 95% CI. Four secondary outcomes were collected at baseline and at each of the four study visits: ACT (combined ACT/CACT), FeNO, FEV1 and dose of IC. These outcomes were analysed using linear mixed-effects models with unstructured covariance to account for the correlation between repeated measures, using Stata’s ‘xtmixed’ command. Centre and participant were included as random effects. Design covariates, treatment and nominal time were included as fixed effects, and a treatment-by-time interaction was included. The primary effect of interest was the between-treatment group marginal difference over all time points. The benefit of this approach is the ability to include all individuals who have had at least one clinical assessment. Unscheduled health-care contacts (yes or no) were compared between treatment groups using logistic regression and the total number of unscheduled health-care contacts were compared using negative binomial regression. Comparison of quality of life (using the total PAQLQ score and its subscales) at the final assessment (12 months) was assessed using linear regression.
Both adjusted and unadjusted analyses were performed for the secondary outcomes. Unadjusted analyses were controlled for the baseline score where possible. All adjusted analyses were controlled for the minimisation factors and, where appropriate, the baseline score.
Exploratory analysis
We carried out a prespecified exploratory analysis, using both randomised groups of the study pooled together. We assessed whether or not changes in FeNO over a 3-month period affected exacerbation (yes/no) in (1) the same 3-month period or (2) the subsequent 3-month period. For this analysis, we used a multilevel model that allowed data from more than one time period for each trial participant to be included.
Post hoc analysis
A post hoc analysis was performed to further examine the relationship between baseline FeNO and future asthma exacerbation. This analysis was restricted to the standard-care group only and included asthma exacerbations for the total follow-up period.
Additional post hoc analyses investigated the impact of COVID-19 (where face-to-face visits were replaced with telephone follow-up) and the impact on algorithm treatment recommendations if children had been allocated to the alternative treatment group.
Health economic evaluation
An economic evaluation compared treatment guided by FeNO plus symptoms with treatment guided by symptoms alone in terms of asthma-related NHS costs, number of asthma exacerbations and expected QALYs over a 12-month follow-up period. The analysis relied on resource use data collected at 3, 6, 9 and 12 months post randomisation, and estimated incidence rates for exacerbations by treatment group applied in a decision analytic modelling framework. The mean difference in costs was estimated for major cost categories of medication use, exacerbation-related costs and background asthma-related health service costs. The model-based cost-effectiveness analysis results were expressed in terms of both the incremental cost per exacerbation avoided and the incremental cost per QALY gained for FeNO plus symptom-guided treatment compared with standard symptom-guided treatment. A further secondary analysis also quantified direct travel costs incurred by participants and their parents in attending any unscheduled asthma-related health-care appointments, and indirect costs associated with time lost from productive activities owing to asthma and associated health-care contacts. Full details of the methods and results of the economic analysis are provided in Chapter 5.
Qualitative assessment
A qualitative process evaluation63,64 was carried out as part of the trial. This was to explore experiences and ascertain acceptability of the intervention and to solicit in-depth feedback on the process of taking part in the trial from both participant and trial staff perspectives. Interviews with families (child and parent pairs) in both the intervention and the control group, and with a range of trial staff representing a number of roles and across different sites, were planned. The full qualitative process evaluation assessment including methodology and rationale is provided in Chapter 6.
Patient and public involvement
A mother of a child with asthma who is also an asthma nurse was an independent voting member of the TSC.
Early versions of the PILs and consent forms were reviewed by the Young Persons’ Advocacy Group (part of the NHS Scottish Children’s Research Network), which provided valuable feedback for the development of these documents. The Young Persons’ Advocacy Group and patient and public involvement (PPI) member of the TSC were asked to comment on the Plain English summary.
We anticipate that the PPI member of the TSC will comment on the results letter to be sent to trial participants and GPs and we also anticipate liaising with the Young Persons’ Advocacy Group for this activity. It is anticipated that the publication of the trial results will be co-ordinated with press releases from Asthma UK and British Lung Foundation Partnership (London, UK).
Protocol amendments
There were six protocol amendments, which are summarised in Appendix 4, Table 46. Most were minor clarifications within the protocol. Of note, we refined the definition of the primary outcome to also include asthma exacerbations treated for < 3 days (e.g. with one dose of dexamethasone, which became common practice after the study started). We also revised details with respect to the qualitative process evaluation and the mechanistic components of the study.
Trial oversight
A TSC, with independent members including a PPI representative, oversaw the conduct and progress of the trial. The TSC met approximately every 6 months. An independent DMC oversaw the safety, rights and well-being of subjects within the trial. The DMC met approximately every 6 months and reviewed accruing data. The DMC did not have any statistical stopping rules.
Breaches
There was one breach recorded within the study: two siblings were recruited into the study at the same time. These siblings remained in the study and are included in the ITT analysis. The breach was assessed by the chief investigator and the sponsor as non-serious.
Chapter 3 Description of the population and baseline characteristics
Recruitment
Participants were recruited to the trial between June 2017 and August 2019, at which point the sample size was achieved (with follow-up completed in August 2020). During this 26-month period, 52 UK sites were opened for recruitment: 35 secondary care sites and 17 primary care sites. Ten primary care sites, all of which opened for recruitment in 2019, did not recruit any patients to the study.
In total, 515 participants were recruited from 42 sites, comprising 499 participants recruited from secondary care and 16 from primary care sites (Table 4). A detailed summary of recruitment by site is given in Appendix 5, Table 47.
| Recruitment site based in | Sites (n) | Participants (n) |
|---|---|---|
| Secondary care | 35 | 499 |
| Primary care | 7 | 16 |
The initial protocol included a 24-month recruitment period. There were delays in opening sites as a result of several factors, including the complexity of contract arrangements and staff capacity. Once recruitment started, the accrual of participants persistently rose 6–8 weeks behind the projected recruitment numbers (Figure 4) and recruitment was completed 2 months after the planned end date (without additional funding).
FIGURE 4.
Cumulative recruitment (vs. target recruitment).

Permission was obtained to over-recruit beyond our original target of 502 participants, given that some families had already been approached about the study and had indicated a willingness to be recruited.
Six participants were recruited but did not meet the inclusion criteria and were excluded after randomisation (two in the intervention group and four in the standard-care group). These were children aged < 12 years who were on a dose of ICs higher than the protocol allowed for children of that age. Permission was also obtained to over-recruit to replace these six post-randomisation exclusions.
Baseline characteristics
Baseline characteristics are presented for the 509 included participants (after exclusion of the six post-randomisation exclusions); the two randomised groups were well balanced in terms of demographic and disease characteristics at baseline (Tables 5–9).
| Characteristic | Intervention group | Standard-care group | Overall |
|---|---|---|---|
| Sex, N, n (%) | |||
| Male | 255, 156 (61.2) | 254, 152 (59.8) | 509, 308 (60.5) |
| Age (years) | |||
| n, mean (SD) | 255, 10.0 (2.6) | 254, 10.1 (2.5) | 509, 10.1 (2.6) |
| Median (p25, p75) | 10 (8, 12) | 10 (8, 12) | 10 (8, 12) |
| Age group, N, n (%) | |||
| < 11 years | 255, 146 (57.3) | 254, 142 (55.9) | 509, 288 (56.6) |
| ≥ 11 years | 255, 109 (42.7) | 254, 112 (44.1) | 509, 221 (43.4) |
| Height centile (cm), N, mean (SD) | 253, 50.1 (29.1) | 252, 47.1 (28.7) | 505, 48.6 (29.0) |
| Weight centile (kg), N, mean (SD) | 253, 61.5 (31.7) | 252, 60.0 (31.6) | 505, 60.7 (31.7) |
| BMI centile, N, mean (SD) | 253, 62.9 (32.0) | 252, 63.7 (31.6) | 505, 63.3 (31.7) |
| BMI groups,a N, n (%) | |||
| Thin | 253, 17 (6.7) | 252, 24 (9.5) | 505, 41 (8.1) |
| Healthy weight | 253, 161 (63.6) | 252, 151 (59.9) | 505, 312 (61.8) |
| Overweight | 253, 51 (20.2) | 252, 55 (21.8) | 505, 106 (21.0) |
| Obese | 253, 24 (9.5) | 252, 22 (8.7) | 505, 46 (9.1) |
| Ethnic group, N, n (%) | |||
| White | 255, 187 (73.3) | 254, 198 (78.0) | 509, 385 (75.6) |
| Mixed/multiple ethnic groups | 255, 6 (2.4) | 254, 4 (1.6) | 509, 10 (2.0) |
| Asian/Asian British | 255, 28 (11.0) | 254, 31 (12.2) | 509, 59 (11.6) |
| Black/African/Caribbean/black British | 255, 25 (9.8) | 254, 20 (7.9) | 509, 45 (8.8) |
| Other | 255, 9 (3.5) | 254, 1 (0.4) | 509, 10 (2.0) |
| Age mother left education (years), n, median (p25, p75) | 248, 18 (16, 21) | 247, 18 (16, 21) | 495, 18 (16, 21) |
| Age father left education (years), n, median (p25, p75) | 224, 18 (16, 21) | 230, 17 (16, 19) | 454, 17 (16, 20) |
| Characteristic | Intervention group (N = 255) | Standard-care group (N = 254) | Overall |
|---|---|---|---|
| Age of asthma diagnosis (years) | |||
| n, mean (SD) | 253, 3.94 (2.6) | 253, 3.72 (2.5) | 506, 3.83 (2.6) |
| Median (p25, p75) | 3 (2, 5) | 3 (2, 5) | 3 (2, 5) |
| Premature birth (before 36 weeks), N, n (%) | 255, 43 (16.9) | 254, 26 (10.2) | 509, 69 (13.6) |
| Hospital admission for asthma, n, median (p25, p75) | |||
| Last 6 months | 255, 0 (0, 1) | 254, 0 (0, 1) | 509, 0 (0, 1) |
| Last year | 255, 1 (0, 2) | 254, 1 (0, 2) | 509, 1 (0, 2) |
| Number of hospital admissions for asthma in a lifetime, N, n (%) | |||
| Never | 255, 30 (11.8) | 254, 34 (13.4) | 509, 64 (12.6) |
| 1–5 times | 255, 114 (44.7) | 254, 97 (38.2) | 509, 211 (41.5) |
| > 5 times | 255, 111 (43.5) | 254, 123 (48.4) | 509, 234 (46.0) |
| Asthma-related school absences (days), n, median (p25, p75) | |||
| Last 6 months | 255, 5 (2, 10) | 252, 5 (0, 10) | 507, 5 (1, 10) |
| Last year | 255, 10 (5, 20) | 252, 10 (4, 20) | 507, 10 (5, 20) |
| Number of courses of OC tablets, n, median (p25, p75) | |||
| Last 6 months | 255, 1 (1, 2) | 254, 1 (1, 2) | 509, 1 (1, 2) |
| Last year | 255, 3 (1, 4) | 254, 3 (1, 5) | 509, 3 (1, 5) |
| Wheeze only with a cold, N, n (%) | 255, 100 (39.2) | 253, 90 (35.6) | 508, 190 (37.4) |
| Daily dose of IC (budesonide equivalent) (µg), N, median (p25, p75) | 255, 400 (400, 1000) | 254, 400 (400, 1000) | 509, 400 (400, 1000) |
| Daily dose of IC (budesonide equivalent), N, n (%) | |||
| ≤ 400 µg | 255, 143 (56.1) | 254, 143 (56.3) | 509, 286 (56.2) |
| 401–800 µg | 255, 27 (10.6) | 254, 27 (10.6) | 509, 54 (10.6) |
| > 800 µg | 255, 85 (33.3) | 254, 84 (33.0) | 509, 169 (33.2) |
| Current LABA use, N, n (%) | 255, 196 (76.9) | 254, 190 (74.8) | 509, 386 (75.8) |
| Current LTRA use, N, n (%) | 255, 150 (58.8) | 254, 152 (59.8) | 509, 302 (59.3) |
| BTS step,a N, n (%) | |||
| 2 | 255, 39 (15.3) | 254, 38 (15.0) | 509, 77 (15.1) |
| 3 | 255, 109 (42.7) | 254, 110 (43.3) | 509, 219 (43.0) |
| 4 | 255, 107 (42.0) | 254, 106 (41.7) | 509, 213 (41.8) |
| Asthma control score in children, n, median (p25, p75) | |||
| < 12 years (CACT) | 179, 19 (15, 22) | 171, 18 (14, 21) | 350, 18.5 (14, 21) |
| ≥ 12 years (ACT) | 76, 19 (14, 22) | 83, 18 (14, 21) | 159, 19 (14, 21) |
| Controlled asthma,b N, n (%) | 255, 132 (51.8) | 254, 124 (48.8) | 509, 256 (50.3) |
| Per cent predicted FEV1, n, mean (SD) | 234, 89.79 (17.76) | 221, 89.29 (18.22) | 455, 89.55 (17.97) |
| FeNO (ppb), n, median (p25, p75) | 255, 20 (11, 45) | 254, 22.5 (10, 51) | 509, 21 (10, 48) |
| FeNO, N, n (%) | |||
| Low FeNOc | 255, 185 (72.6) | 254, 167 (65.8) | 509, 352 (69.2) |
| High FeNOc | 255, 70 (27.4) | 254, 87 (34.2) | 509, 157 (30.8) |
| Asthma inhaler technique, N, n (%) | |||
| Satisfactory | 255, 218 (85.5) | 253, 215 (85.0) | 508, 433 (85.2) |
| Satisfactory after training | 255, 36 (14.1) | 253, 38 (15.0) | 508, 74 (14.6) |
| Not satisfactory | 255, 1 (0.4) | 253, 0 (0.0) | 508, 1 (0.2) |
| Frequency of blue reliever inhaler use in the last 6 months, N, n (%) | |||
| Never | 255, 3 (1.2) | 254, 4 (1.6) | 509, 7 (1.4) |
| Occasionally | 255, 133 (52.2) | 254, 136 (53.5) | 509, 269 (52.8) |
| Once per day | 255, 48 (18.8) | 254, 43 (16.9) | 509, 91 (17.9) |
| More than once per day | 255, 71 (27.8) | 254, 71 (28.0) | 509, 142 (27.9) |
| Characteristic | Overall | Intervention group | Standard-care group |
|---|---|---|---|
| Exposure to smoking | |||
| Child exposed to smoking, N, n (%) | 255, 45 (17.6) | 254, 49 (19.3) | 509, 94 (18.5) |
| Number of smokers in the household, N, median (p25, p75) | 246, 0 (0, 0) | 247, 0 (0, 0) | 493, 0 (0, 0) |
| Other conditions linked to asthma, N, n (%) | |||
| Eczema diagnosed by a doctor, ever | 255, 141 (55.3) | 254, 152 (59.8) | 509, 293 (57.6) |
| Eczema in the last 12 months (among those with eczema, ever) | 141, 91 (64.5) | 152, 92 (60.5) | 293, 183 (62.5) |
| Rhinitis, ever | 255, 151 (59.2) | 254, 153 (60.2) | 509, 304 (59.7) |
| Rhinitis in the last 12 months (among those with rhinitis, ever) | 151, 139 (92.1) | 153, 149 (97.4) | 304, 288 (94.7) |
| Food allergy, ever | 254, 76 (29.9) | 254, 63 (24.8) | 508, 139 (27.4) |
| Family history within immediate family (parent, sibling, half-sibling), N, n (%) | |||
| Asthma | 254, 165 (65.0) | 254, 151 (59.4) | 508, 316 (62.2) |
| Eczema | 254, 126 (49.6) | 254, 137 (53.9) | 508, 263 (51.8) |
| 254, 99 (39.0) | 254, 89 (35.0) | 508, 188 (37.0) | |
| PAQLQ | Intervention group | Standard-care group | Overall |
|---|---|---|---|
| Overall score | 254, 5.7 (4.3, 6.6) | 254, 5.5 (4.2, 6.4) | 508, 5.6 (4.3, 6.5) |
| Symptoms domain score | 254, 5.5 (4.1, 6.5) | 254, 5.5 (4.0, 6.4) | 508, 5.5 (4.1, 6.4) |
| Limitations domain score | 254, 5.4 (4.2, 6.4) | 254, 5.3 (4.2, 6.4) | 508, 5.4 (4.2, 6.4) |
| Emotional domain score | 254, 6.0 (4.5, 6.9) | 254, 6.0 (4.5, 6.8) | 508, 6.0 (4.5, 6.8) |
| Characteristics measured | Intervention group | Standard-care group | Overall |
|---|---|---|---|
| Bronchodilator response (% change), N, mean (SD) | 82, 10.2 (9.5) | 78, 9.6 (8.8) | 160, 9.8 (9.1) |
| Skin-prick allergy test results, N, n (%a) | |||
| Positive | 255, 54 (77.1) | 254, 48 (68.6) | 509, 102 (75.9) |
| Negative | 255, 16 (22.9) | 254, 22 (31.4) | 509, 38 (27.1) |
| Not carried out | 255, 185 | 254, 184 | 509, 369 |
The mean age of the participants was 10.1 years and 60.5% were male. The majority of children (61.8%) were a healthy weight, while 21.0% were overweight, 9.1% were obese and 8.1% were thin. The majority (75.6%) of participants identified themselves as being of white ethnicity. The median age that participants’ mothers left education was 18 years. A total of 18.5% of participants were exposed to smoking at home.
The mean age at asthma diagnosis was 3.8 years, and 13.6% of participants were born early (before 36 weeks’ gestation). The median number of admissions to hospital because of asthma in the previous year was one. With regard to previous hospital admissions, 12.6% of participants had never been admitted to hospital for their asthma, 41.5% had been admitted between one and five times during their lifetime and 46.0% had been admitted more than five times. The median days of absence from school in the previous year was 10 days. The median number of courses of steroid tablets for an asthma exacerbation in the previous year was three courses.
At baseline, the median daily dose of IC was 400 µg of budesonide equivalent. In total, 75.8% of participants were prescribed a LABA (usually combined with their IC or in a separate inhaler) and 59.3% were prescribed a LTRA. A total of 52.8% used their rescue inhaler occasionally, while 17.9% used it once per day, 27.9% used it more than once per day and 1.4% reported never using their rescue inhaler. Inhaler technique was satisfactory at the first time of asking in 85.2% of participants and was satisfactory after training in 14.6%; in only one participant (0.2% of participants) was it not considered satisfactory at either stage. Using recognised cut-off points from the ACT/CACT, asthma was poorly controlled in 49.7% of participants and was partly or fully controlled in 50.3% of participants.
The median baseline FeNO was 21 parts per billion (ppb). Lung function was measured at baseline in 455 participants and the mean per cent predicted FEV1 in children was 90%. It was not possible to measure lung function in the remaining patients owing to a lack of equipment or trained personnel to make these assessments.
The proportions of children reported to have ever had eczema, rhinitis and food allergy were 57.6%, 59.7% and 27.4% respectively.
A total of 62% of participants had a first-degree family member (parent, sibling or half-sibling) with asthma, 51.8% had a first-degree family member with eczema and 37.0% had a first-degree family member with rhinitis.
The median score for the PAQLQ was 5.6 points. The median scores on the individual domains were as follows: symptoms domain 5.5 points, limitations domain 5.4 points and emotional domain 6.0 points.
Bronchodilator response was determined in 160 participants and the mean change was + 10%. Skin-prick testing was carried out in 140 participants, of whom 102 (73%) had at least one positive response.
Chapter 4 Clinical results
In this chapter, we compare the results for children with asthma who were treated for 1 year with treatment guided by FeNO plus symptoms (intervention group) with children who received treatment guided by symptoms alone (standard-care group).
A total of 515 participants were randomised; after six post-randomisation exclusions there were 509 participants who were eligible and whose baseline characteristics are reported (see Chapter 3). Participants were excluded post randomisation if they were on a dose of IC higher than the protocol allowed for children of that age (all six participants were aged < 12 years). Follow-up data for the primary outcome were unavailable for three (0.1%) participants (all in the standard-care group); the ITT analysis is, therefore, based on 506 participants (intervention group, n = 255; standard-care group, n = 251). Nineteen children (seven in the intervention group and 12 in the standard-care group) declined to have their treatment guided by the algorithm during follow-up. Reasons for this included the length and frequency of appointments. The children who withdrew from having their treatment guided by the algorithm remained in the study for outcome assessment (apart from two children who also requested no further outcome data collection). Figure 5 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the ITT analysis.
FIGURE 5.
The CONSORT flow diagram for the ITT analysis. a, Participants were excluded post randomisation if they were on a dose of IC higher than the protocol allowed for children of that age (all six participants were aged < 12 years).

Primary outcome: asthma exacerbations
Intention-to-treat analysis
There were 255 children in the intervention group and 251 in the standard-care group who were included in the ITT analysis. The primary outcome (one or more exacerbation treated with OCs in the 12 months following randomisation) was reported in 123 out of 255 (48.2%) participants allocated to the intervention group and in 129 out of 251 (51.4%) participants allocated to the standard-care group (Table 10). In the adjusted model, the OR for the primary outcome was 0.88 (95% CI 0.61 to 1.27) for participants allocated to the intervention group compared with the standard-care group. The corresponding unadjusted OR was 0.88 (95% CI 0.62 to 1.25).
| Outcome | Intervention group | Standard-care group | Unadjusted OR, effect size (95% CI); p-value | Adjusted OR,a effect size (95% CI); p-value |
|---|---|---|---|---|
| Intention-to-treat analysis | ||||
| Number with at least one exacerbation, n/N | 123/255 | 129/251 | 0.88 (0.62 to 1.25); 0.477 | |
| Percentage with at least one exacerbation (%) | 48.2 | 51.4 | 0.88 (0.61 to 1.27); 0.486 | |
| Per-protocol analysisb | ||||
| Number with at least one exacerbation, n/N | 84/165 | 79/153 | 0.97 (0.62 to 1.51); 0.897 | |
| Percentage with at least one exacerbation (%) | 50.9 | 51.6 | 0.98 (0.61 to 1.55); 0.918 | |
For the primary outcome, we also calculated the effect size using an absolute measure of effect. The adjusted difference in percentage of participants having at least one exacerbation for the intervention group compared with the standard-care group was –3.13% (95% CI –11.9% to 5.6%).
Sensitivity analyses
The post hoc per-protocol analysis for the primary outcome provided a similar interpretation. In the intervention group and standard-care group, respectively, 165 out of 255 (64.7%) and 153 out of 251 (61.0%) participants were considered ‘compliant’ with the algorithm (i.e. the algorithm was followed in at least three out of four visits: baseline, 3 months, 6 months and 9 months). After including only those considered ‘compliant’ with the algorithm, 84 out of 165 (50.9%) participants in the intervention group and 79 out of 153 (51.6%) participants in the standard-care group had at least one exacerbation (adjusted OR 0.98, 95% CI 0.61 to 1.55) (see Table 10).
There was also no change in the interpretation after accounting for compliance with the algorithm (CACE OR 0.82, 95% CI 0.48 to 1.41).
Primary outcome data were available for all participants except for three in the standard-care group. Therefore, it was not considered necessary to conduct sensitivity analyses to account for missing data.
Prespecified subgroup analyses
The interaction terms for the subgroup by treatment group interactions were also not statistically significant. Table 11 and Figure 6 detail the results of the analyses for the prespecified subgroups for the primary outcome: any exacerbation within 12 months of randomisation. Results are presented using adjusted ORs and 95% CIs. There was no evidence at the 5% level of statistical significance for any subgroup differences by baseline treatment, age, sex, wheezing status with cold, obesity, eczema in the last year, asthma severity or allergy skin-prick test.
| Subgroup factors | N, n (%) | Adjusted OR (95% CI) | p-value | p-value for interaction | |
|---|---|---|---|---|---|
| Intervention group | Standard-care group | ||||
| All participants | 255, 123 (48.2) | 251, 129 (51.4) | 0.88 (0.61 to 1.27) | 0.486 | |
| Treatment at baseline | |||||
| Includes LTRA | 150, 83 (55.3) | 151, 86 (57.0) | 0.95 (0.56 to 1.60) | 0.842 | 0.154 |
| Does not include LTRA | 105, 40 (38.1) | 100, 43 (43.0) | 0.81 (0.46 to 1.44) | 0.476 | |
| Age at baseline | |||||
| < 11 years | 146, 72 (49.3) | 139, 70 (50.3) | 0.95 (0.62 to 1.46) | 0.823 | 0.630 |
| ≥ 11 years | 109, 51 (46.8) | 112, 59 (52.7) | 0.79 (0.42 to 1.49) | 0.469 | |
| Sex | |||||
| Male | 156, 78 (50.0) | 150, 82 (54.7) | 0.86 (0.57 to 1.29) | 0.457 | 0.949 |
| Female | 99, 45 (45.4) | 101, 47 (46.5) | 0.86 (0.49 to 1.52) | 0.607 | |
| Wheeze only with cold | |||||
| Yes | 100, 41 (41.0) | 88, 44 (50.0) | 0.67 (0.38 to 1.20) | 0.179 | 0.214 |
| No | 155, 82 (52.9) | 162, 84 (51.9) | 1.03 (0.69 to 1.56) | 0.872 | |
| Obesity | |||||
| Obese | 24, 16 (66.7) | 22, 11 (50.0) | 1.91 (0.53 to 6.86) | 0.324 | 0.246 |
| Non-obese | 229, 106 (46.3) | 227, 117 (51.5) | 0.81 (0.56 to 1.18) | 0.278 | |
| Eczema in the last year | |||||
| Yes | 91, 46 (50.65) | 90, 44 (48.9) | 1.03 (0.59 to 1.81) | 0.913 | 0.655 |
| No | 164, 77 (47.0) | 161, 85 (52.8) | 0.79 (0.50 to 1.25) | 0.315 | |
| Asthma severitya | |||||
| BTS step 2 | 39, 14 (35.9) | 37, 9 (24.3) | 1.41 (0.45 to 4.37) | 0.553 | 0.559 |
| BTS step 3 | 109, 50 (45.9) | 108, 58 (53.7) | 0.74 (0.39 to 1.43) | 0.376 | |
| BTS step 4 | 107, 59 (55.1) | 106, 62 (58.5) | 0.86 (0.53 to 1.40) | 0.552 | |
| Allergy skin-prick test | |||||
| Positive | 54, 22 (40.7) | 48, 19 (39.6) | 1.02 (0.51 to 2.04) | 0.959 | 0.244 |
| Negative | 16, 7 (43.8) | 22, 12 (54.6) | 0.56 (0.16 to 2.01) | 0.378 | |
| Not carried out | 185, 94 (50.8) | 181, 98 (54.1) | 0.89 (0.55 to 1.42) | 0.618 | |
FIGURE 6.
Forest plot showing subgroup analyses of the primary outcome.
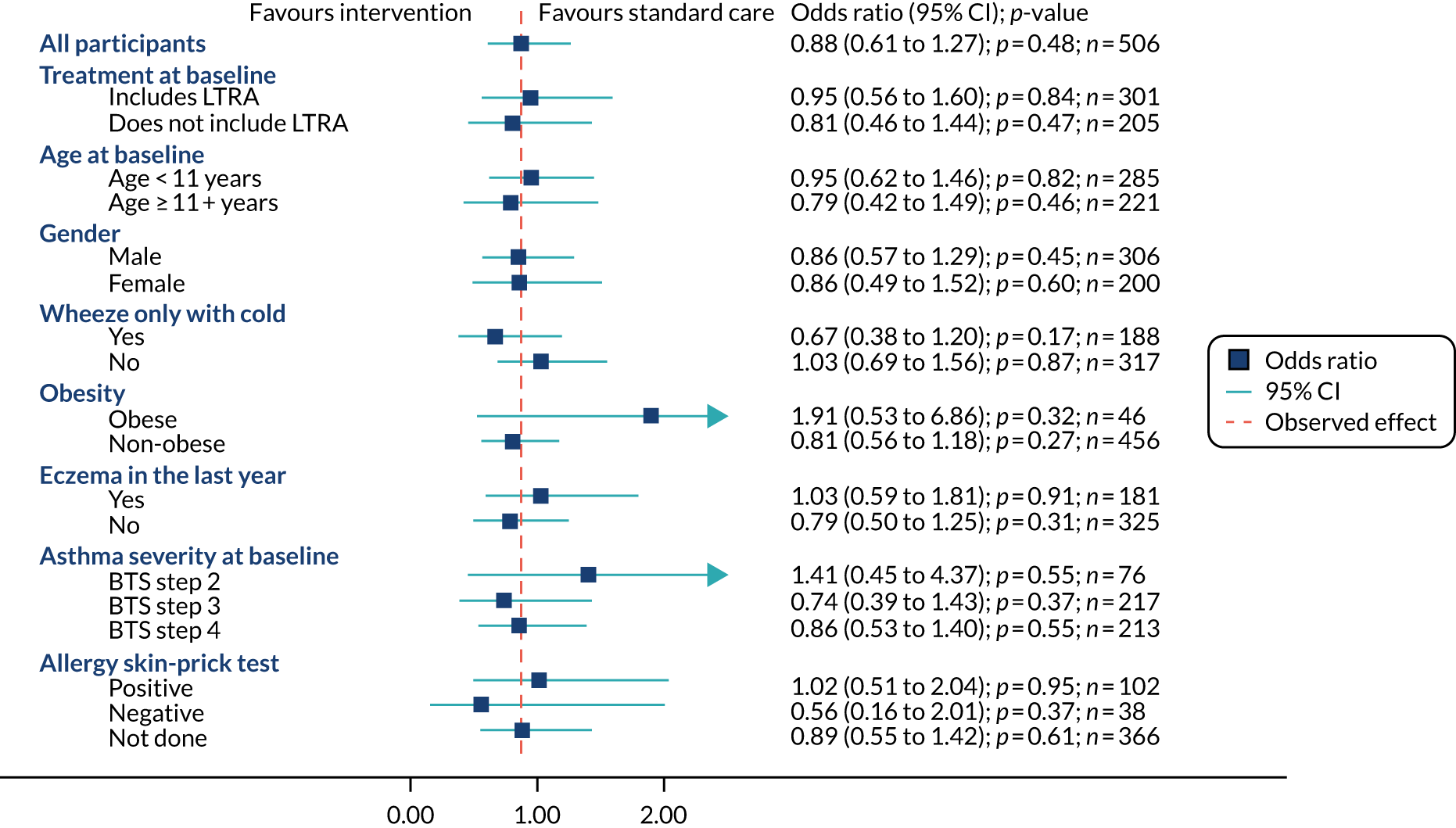
Additional post hoc subgroup analyses on the primary outcome
Three additional post hoc subgroup analyses were carried out on the primary outcome (any exacerbation in the 12 months post randomisation). There was no evidence at the 5% level of statistical significance for any subgroup differences by any LTRA use during follow-up, FeNO at baseline or asthma phenotype (see Appendix 6, Table 48).
Secondary outcomes
Time to first exacerbation
Given that more than half of the participants in the intervention group did not have an exacerbation, it was not possible to estimate the median time to first event, although the lower quartile (25th percentile) value was 137 days. The median time to first exacerbation for participants in the standard-care group was 344 days (p25, 112 days) (Figure 7 and Table 12). In the Cox regression analysis, the adjusted HR for the time to first exacerbation was 0.92 (95% CI 0.71 to 1.18) for participants in the intervention group compared with participants in the standard-care group.
FIGURE 7.
Kaplan–Meier plot for time to first exacerbation.
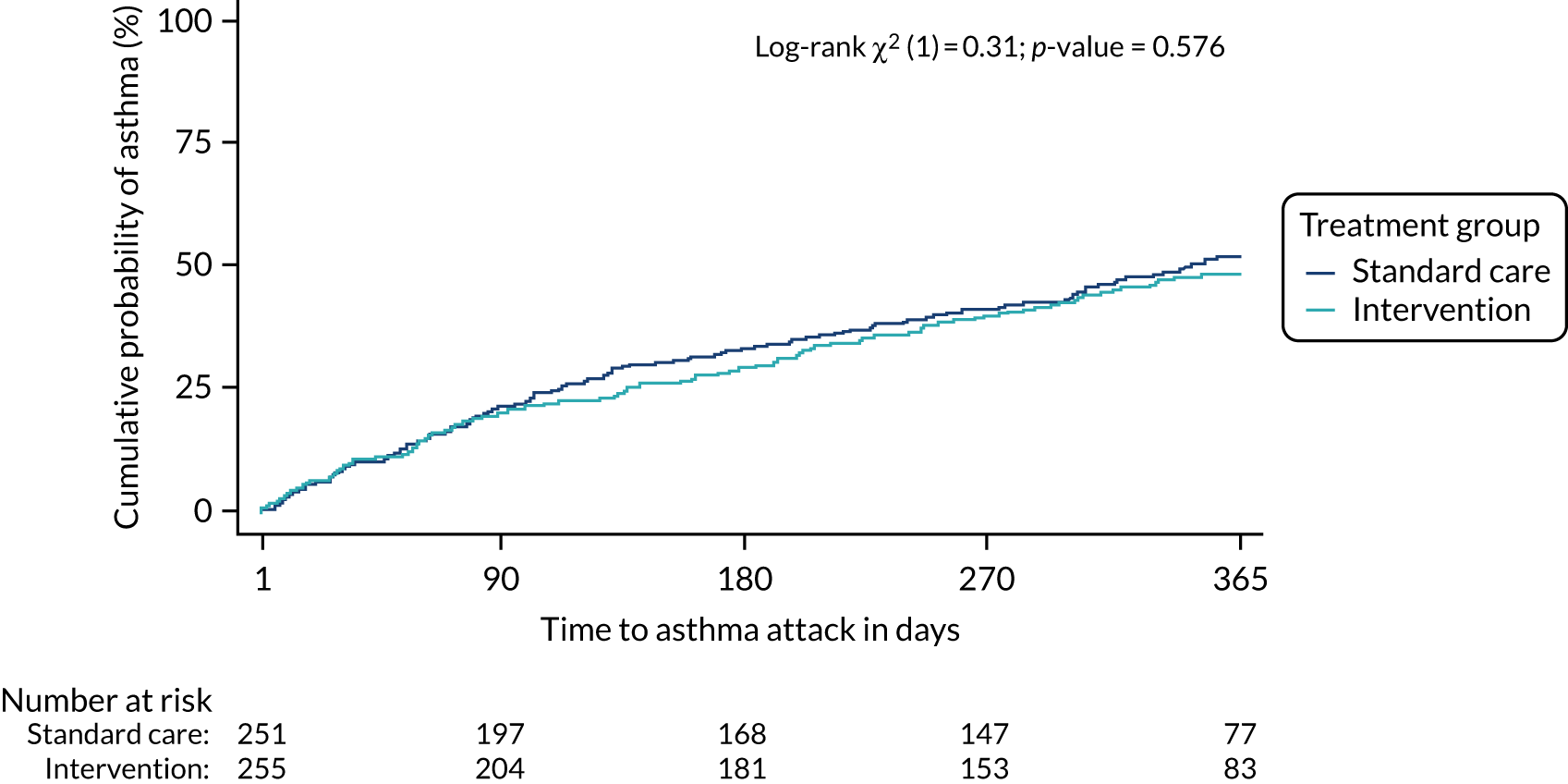
| Outcome | Intervention group (N = 255) | Standard-care group (N = 251) | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| Time to first exacerbation (from randomisation) | ||||
| Number with at least one exacerbation | 123 | 129 | ||
| Median time to first exacerbation (days) | NR | 344 | Unadjusted HR: 0.92 (0.72 to 1.17); 0.489 | |
| 25th percentile (time to first exacerbation) (days) | 137 | 112 | Adjusted HR:a 0.92 (0.71 to 1.18); 0.497 | |
| 75th percentile (time to first exacerbation) (days) | NR | NR | ||
| Number of exacerbations | ||||
| Total number of exacerbations | 254 | 255 | ||
| Mean number of exacerbations per participant | 0.99 | 1.01 | ||
| Median number of exacerbations | 0 | 1 | Unadjusted IRR: 0.98 (0.77 to 1.26); 0.893 | |
| Number of exacerbations, p25, p75 | 0, 2 | 0, 2 | Adjusted IRR:a 0.99 (0.82 to 1.18); 0.873 | |
Number of exacerbations during follow-up
There were a total of 254 exacerbations among those allocated to the intervention group and 255 among those allocated to the standard-care group (Figure 8; see Table 12). The median (p25, p75) number of exacerbations was 0 (0, 2) in the intervention group and 1 (0, 2) in the standard-care group. The adjusted IRR for the number of exacerbations for those in the intervention group relative to the standard-care group was 0.99 (95% CI 0.82 to 1.18).
FIGURE 8.
Distribution of total number of exacerbations during follow-up.
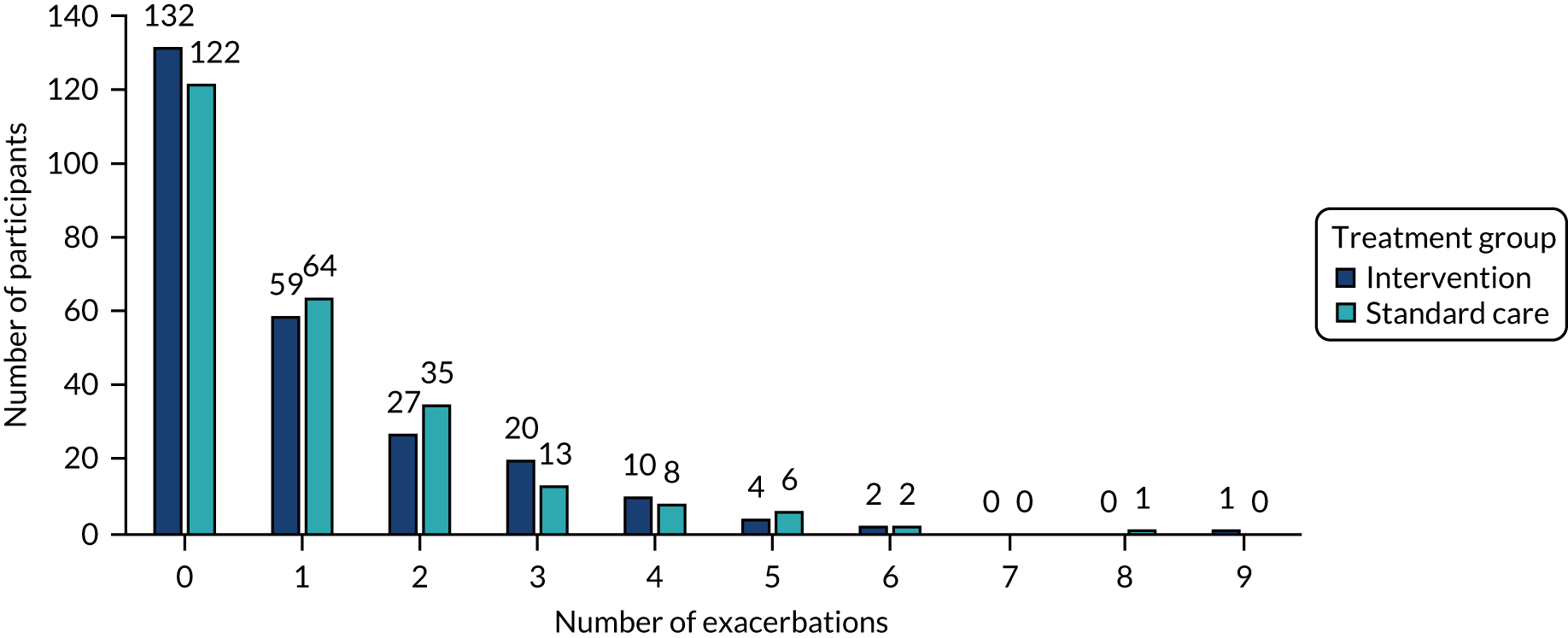
Unscheduled health-care contacts
There were 133 (52.2%) participants in the intervention group and 139 (55.4%) participants in the standard-care group with at least one unscheduled health-care contact. The adjusted OR was 0.88 (95% CI 0.65 to 1.17) for participants in the intervention group relative to the standard-care group (Table 13).
| Intervention group (N = 255) | Standard-care group (N = 251) | Unadjusted OR (95% CI); p-value | Adjusted ORa (95% CI); p-value | Unadjusted IRR (95% CI); p-value | Adjusted IRRa (95% CI); p-value | |
|---|---|---|---|---|---|---|
| Unscheduled health-care contacts, n (%) | 133 (52.2) | 139 (55.4) | 0.88 (0.62 to 1.25); 0.467 | 0.88 (0.65 to 1.17); 0.374 | ||
| Number of unscheduled health-care contacts, median (p25, p75) | 1 (0, 2) | 1 (0, 3) | 0.92 (0.70 to 1.21); 0.544 | 0.91 (0.72 to 1.13); 0.389 |
Total number of unscheduled health-care contacts
The median (p25, p75) total number of unscheduled contacts was 1 (0, 2) for those in the intervention group and 1 (0, 3) for those in the standard-care group. The adjusted IRR was 0.91 (95% CI 0.72 to 1.13) for the number of unscheduled contacts for participants in the intervention group relative to the standard-care group (see Table 13).
Percentage forced expiratory volume in 1 second
The mean (SD) percentage predicted FEV1 at the baseline assessment was 90% (18%) (n = 234) for participants in the intervention group and 89% (18%) (n = 219) for participants in the standard-care group (Figure 9 and Table 14). At the 12-month assessment, the corresponding values were 92% (16%) (n = 156) and 91% (17%) (n = 137). In the repeated-measures model, the overall adjusted mean difference in percentage predicted FEV1 across all four follow-up time points was 0.24% (95% CI –1.68% to 2.16%).
FIGURE 9.
Mean (error bars denote SD) percentage predicted FEV1 at each time point.
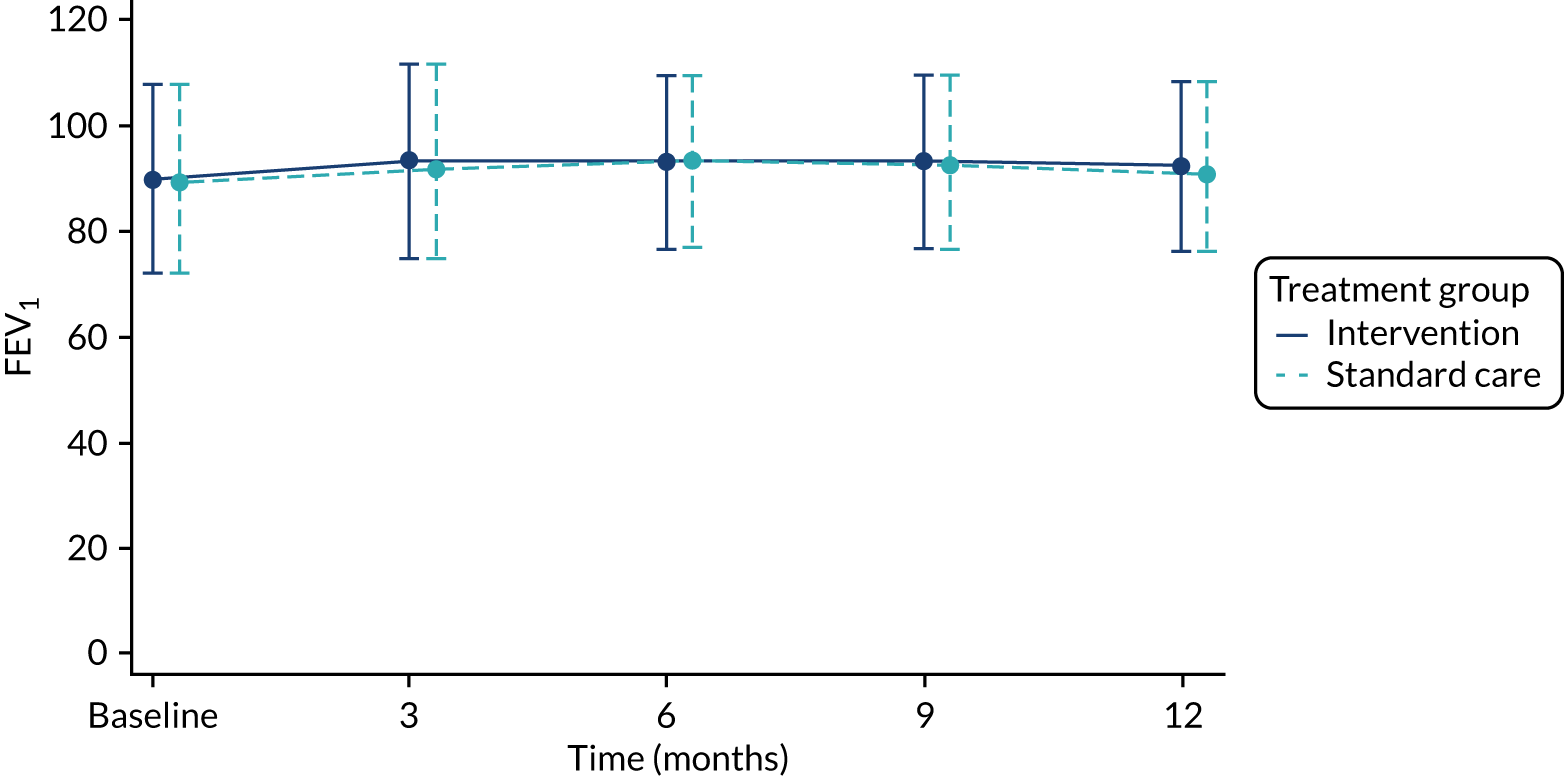
| Time point | Intervention group | Standard-care group | Overall mean difference (95% CI); p-value |
|---|---|---|---|
| Baseline | |||
| Total (n) | 234 | 219 | |
| Mean (SD) | 89.8 (17.8) | 89.3 (18.3) | |
| 3 months | |||
| Total (n) | 210 | 188 | |
| Mean (SD) | 93.3 (18.4) | 91.9 (16.8) | |
| 6 months | |||
| Total (n) | 193 | 183 | |
| Mean (SD) | 93.0 (16.3) | 93.4 (16.8) | |
| 9 months | |||
| Total (n) | 179 | 162 | |
| Mean (SD) | 93.2 (16.5) | 92.7 (16.3) | |
| 12 months | |||
| Total (n) | 156 | 137 | |
| Mean (SD) | 92.2 (16.0) | 91.0 (17.4) | |
| Unadjusted | 0.37 (–1.60 to 2.34); 0.713 | ||
| Adjusteda | 0.24 (–1.68 to 2.16); 0.807 | ||
Fractional exhaled nitric oxide
The median (p25, p75) FeNO at the baseline assessment was 20 ppb (11, 45 ppb) (n = 255) for participants in the intervention group and 23 ppb (10, 51 ppb) (n = 254) for participants in the standard-care group. At the 12-month assessment, the corresponding values were 19 ppb (10, 47 ppb) (n = 163) and 22 ppb (11, 39 ppb) (n = 147) (Figure 10 and Table 15). Across the four follow-up time points, the overall mean group difference in FeNO was 2.75 ppb (95% CI –0.97 to 6.47 ppb).
FIGURE 10.
Median (error bars denote p25 and p75) FeNO at each time point (ppb).

| Time point | Intervention group | Standard-care group | Overall mean difference (95% CI); p-value |
|---|---|---|---|
| Baseline | |||
| Total (n) | 255 | 254 | |
| Median (p25, p75) | 20.0 (11, 45) | 22.5 (10, 51) | |
| 3 months | |||
| Total (n) | 219 | 209 | |
| Median (p25, p75) | 17.0 (10, 34) | 15.0 (9, 33) | |
| 6 months | |||
| Total (n) | 207 | 202 | |
| Median (p25, p75) | 17.0 (9, 40) | 17.0 (9, 30) | |
| 9 months | |||
| Total (n) | 190 | 181 | |
| Median (p25, p75) | 19.0 (10, 44) | 17.0 (11, 37) | |
| 12 months | |||
| Total (n) | 163 | 147 | |
| Median (p25, p75) | 19.0 (10, 47) | 22.0 (11, 39) | |
| Unadjusted | 2.59 (–1.16 to 6.35); 0.176 | ||
| Adjusteda | 2.75 (–0.97 to 6.47); 0.147 | ||
Daily dose of inhaled corticosteroid
At baseline, the median (p25, p75) daily dose of IC was 400 µg (400 µg, 1000 µg) (n = 255) of budesonide equivalent for participants in the intervention group and 400 µg (400 µg, 1000 µg) (n = 251) of budesonide equivalent for participants in the standard-care group. At the 12-month follow-up, the corresponding figures were 500 µg (400 µg, 1000 µg) (n = 224) of budesonide equivalent and 400 µg (400 µg, 1000 µg) (n = 208) of budesonide equivalent (Figure 11 and Table 16). The overall adjusted mean group difference in IC was –10.88 µg (95% CI –70.76 µg to 49.01 µg).
FIGURE 11.
Daily IC dose (budesonide equivalent) (µg) (median, error bars denote p25, p75 at each time point).

| Time point | Intervention group | Standard-care group | Overall mean difference (95% CI); p-value |
|---|---|---|---|
| Baseline | |||
| Total (n) | 255 | 251 | |
| Median (p25, p75) | 400.0 (400, 1000) | 400.0 (400, 1000) | |
| 3 months | |||
| Total (n) | 228 | 216 | |
| Median (p25, p75) | 800.0 (400, 1000) | 500.0 (400, 1000) | |
| 6 months | |||
| Total (n) | 211 | 210 | |
| Median (p25, p75) | 500.0 (400, 1000) | 400.0 (400, 1000) | |
| 9 months | |||
| Total (n) | 209 | 198 | |
| Median (p25, p75) | 500.0 (400, 1000) | 400.0 (400, 1000) | |
| 12 months | |||
| Total (n) | 224 | 208 | |
| Median (p25, p75) | 500.0 (400, 1000) | 400.0 (400, 1000) | |
| Unadjusted | –10.62 (–71.12 to 49.88); 0.731 | ||
| Adjusteda | –10.88 (–70.76 to 49.01); 0.722 | ||
Asthma control
The ACT (score range of 0–25, definition of partial or full control is a score of > 19) and CACT (score range of 0–27, definition of partial or full control is a score of > 19) were combined and presented as a single measure. The median (p25, p75) ACT/CACT score was 19 (14, 22) (n = 255) for participants allocated to the intervention group and 18 (14, 21) (n = 251) for participants allocated to the standard-care group (Figure 12 and Table 17). At the 12-month follow-up, the corresponding scores were 22 (18, 24) (n = 222) and 21 (18, 24) (n = 208). The overall adjusted mean group difference in ACT/CACT across the 12-month follow-up was 0.05 (95% CI –0.47 to 0.58).
FIGURE 12.
Asthma control (ACT/CACT) (median, error bars denote p25, p75) at each time point.
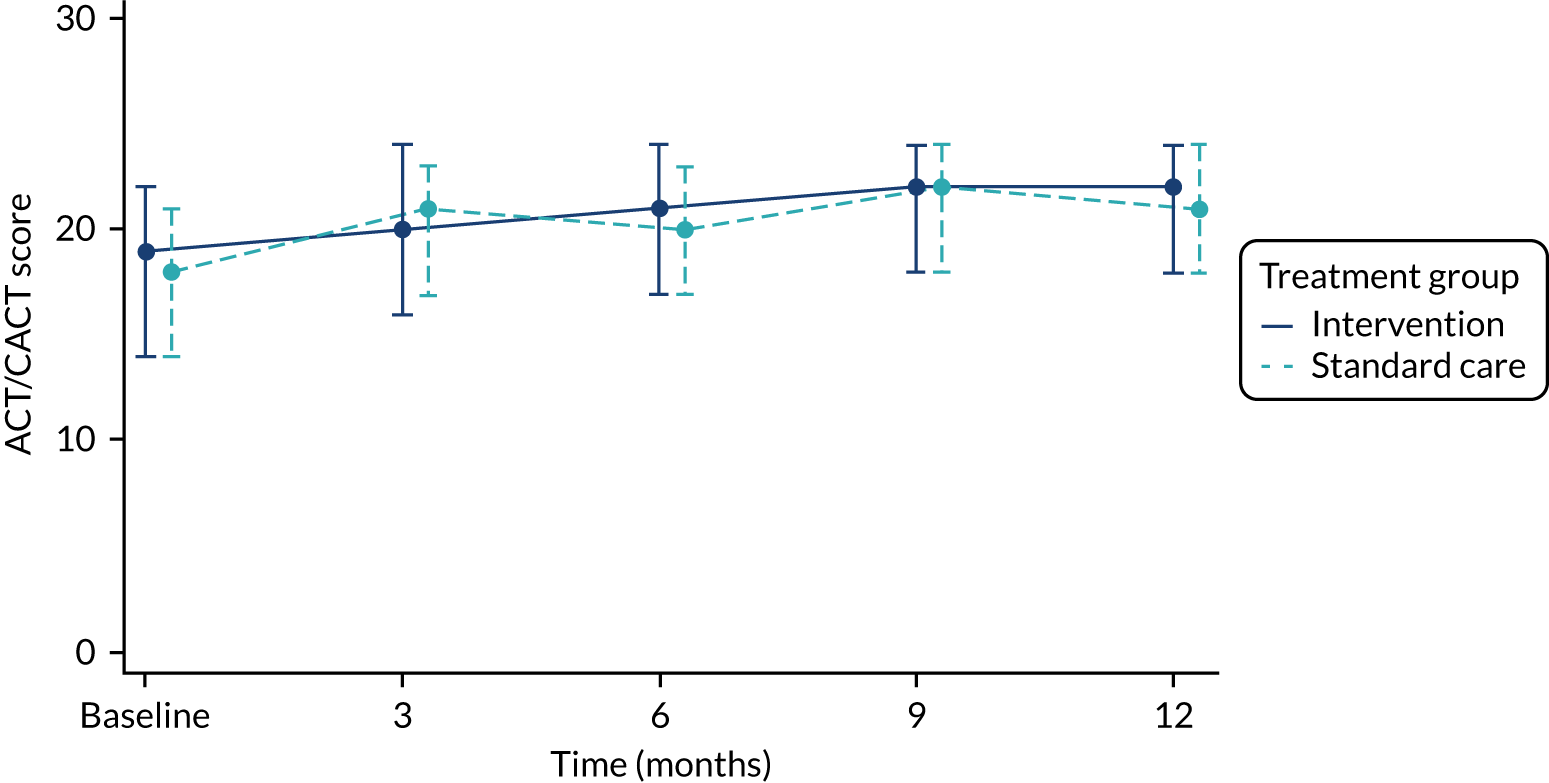
| Time point | Intervention group | Standard-care group | Overall mean difference (95% CI); p-value |
|---|---|---|---|
| Baseline | |||
| Total (n) | 255 | 251 | |
| Median (p25, p75) | 19.0 (14, 22) | 18.0 (14, 21) | |
| 3 months | |||
| Total (n) | 231 | 224 | |
| Median (p25, p75) | 20.0 (16, 24) | 21.0 (17, 23) | |
| 6 months | |||
| Total (n) | 214 | 215 | |
| Median (p25, p75) | 21.0 (17, 24) | 20.0 (17, 23) | |
| 9 months | |||
| Total (n) | 213 | 204 | |
| Median (p25, p75) | 22.0 (18, 24) | 22.0 (18, 24) | |
| 12 months | |||
| Total (n) | 222 | 208 | |
| Median (p25, p75) | 22.0 (18, 24) | 21.0 (18, 24) | |
| Unadjusted | 0.04 (–0.50 to 0.57); 0.895 | ||
| Adjusteda | 0.05 (–0.47 to 0.58); 0.840 | ||
Quality of life
Quality of life was measured using the PAQLQ. The overall PAQLQ scores (and scores for the individual domains of the PAQLQ) range from 1 (severely affected) to 7 (unaffected). At baseline, the median (p25, p75) overall PAQLQ score was 5.7 (4.3, 6.6) (n = 254) in the intervention group and 5.5 (4.2, 6.4) (n = 254) in the standard-care group (Figure 13). At the 12-month follow-up the corresponding figures were 6.4 (5.5, 6.9) (n = 215) and 6.2 (5.4, 6.8) (n = 199) (Table 18). The adjusted mean difference in total PAQLQ scores at the 12-month follow-up was 0.098 (95% CI –0.088 to 0.283) between the groups.
FIGURE 13.
Quality of life (PAQLQ) (median, error bars denote p25, p75) at baseline and 12 months. (a) Overall score, (b) symptom domain score, (c) activity limitation domain score and (d) emotional function domain score.
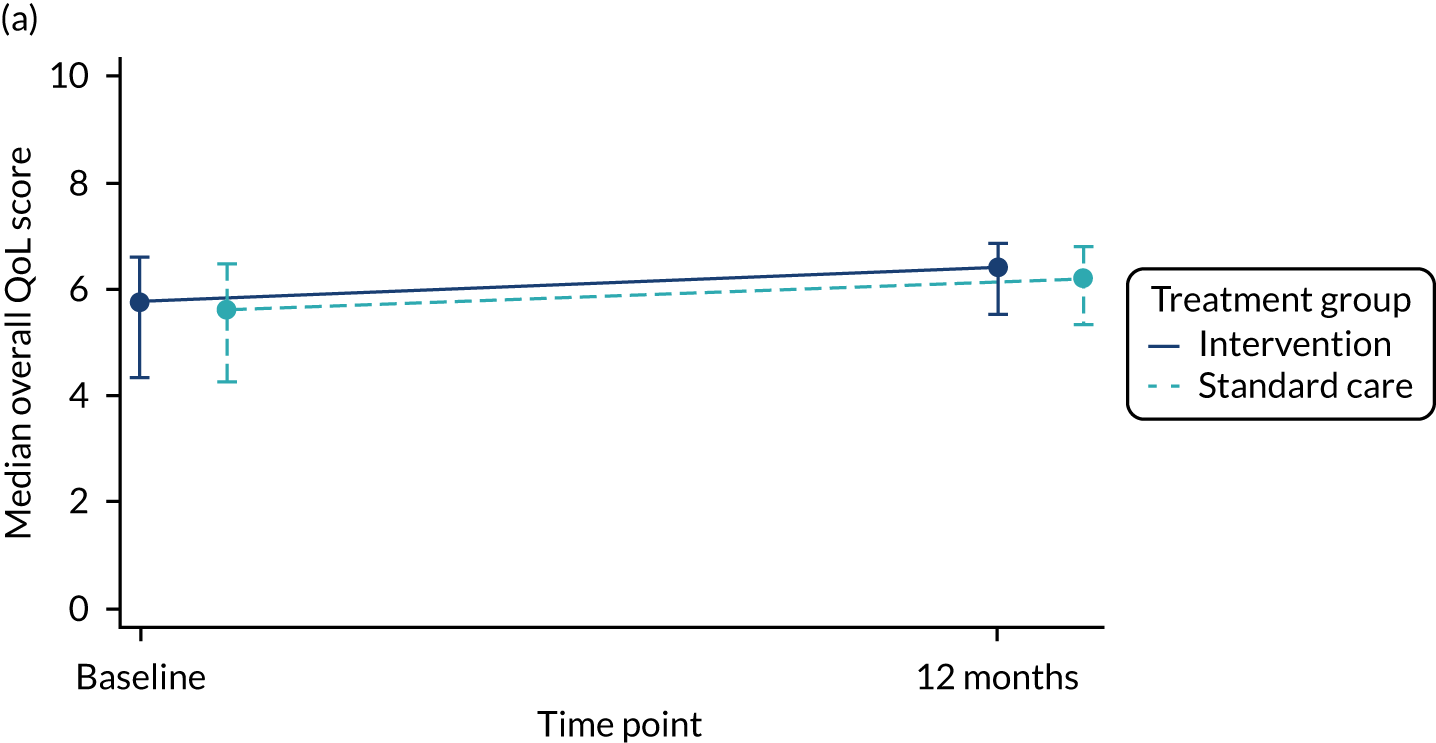

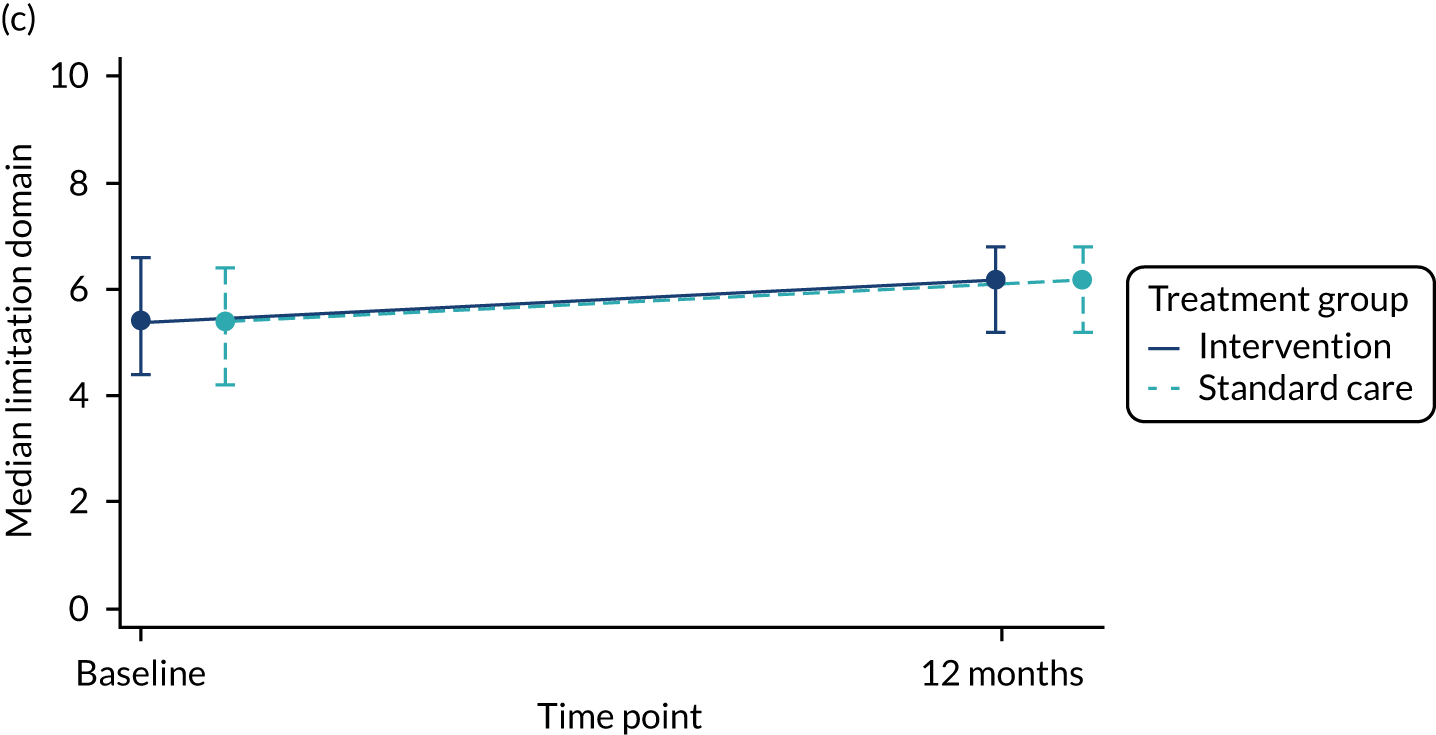
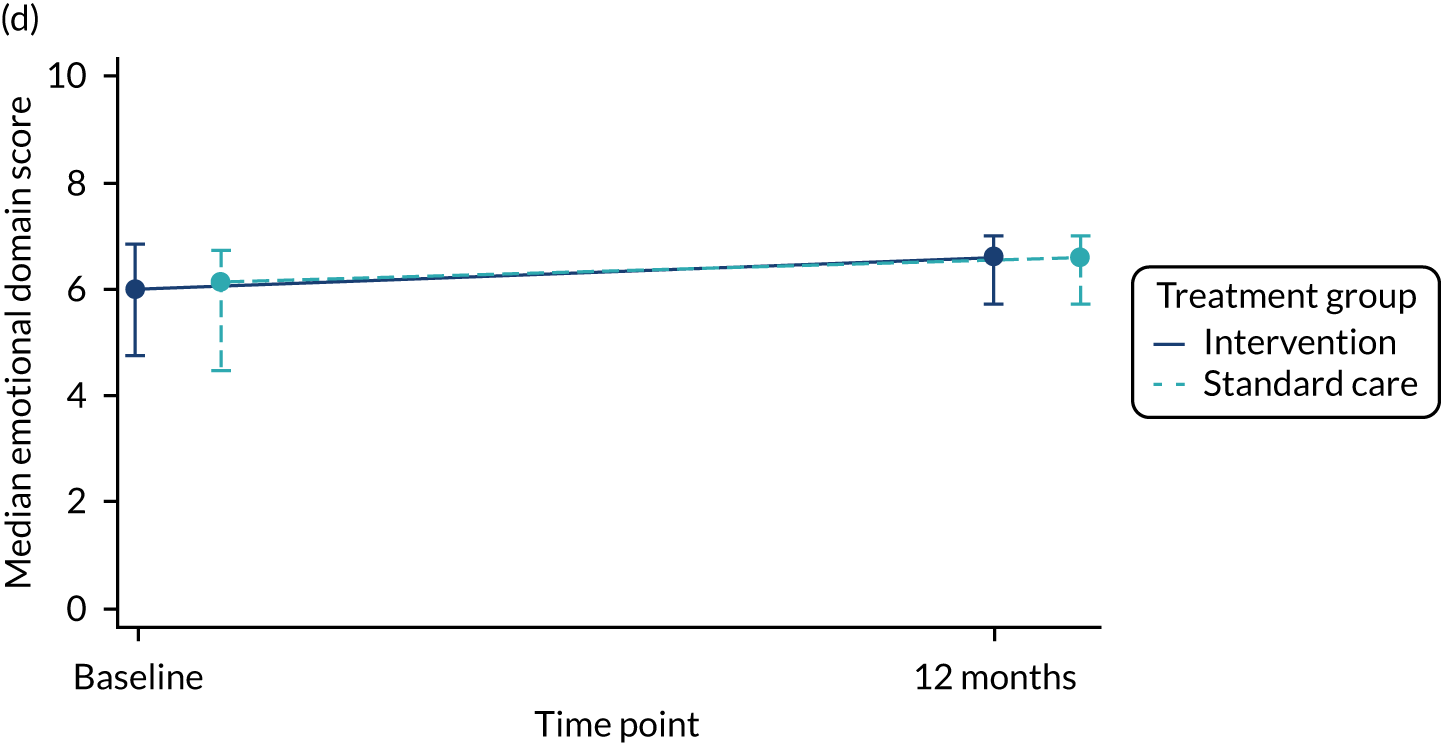
| Outcome | Intervention group (N = 215) | Standard-care group (N = 199) | Mean difference (95% CI); p-value |
|---|---|---|---|
| PAQLQ score at 12 months: overall quality of life | |||
| Median (p25, p75) | 6.39 (5.52, 6.86) | 6.22 (5.35, 6.78) | |
| Unadjusted | 0.092 (–0.095 to 0.279); 0.333 | ||
| Adjusteda | 0.098 (–0.088 to 0.283); 0.302 | ||
| PAQLQ score at 12 months: symptoms domain | |||
| Median (p25, p75) | 6.33 (5.33, 6.89) | 6.11 (5.22, 6.67) | |
| Unadjusted | 0.162 (–0.050 to 0.374); 0.135 | ||
| Adjusteda | 0.169 (–0.042 to 0.379); 0.115 | ||
| PAQLQ score at 12 months: limitations domain | |||
| Median (p25, p75) | 6.20 (5.20, 6.80) | 6.20 (5.20, 6.80) | |
| Unadjusted | –0.012 (–0.216 to 0.192); 0.908 | ||
| Adjusteda | –0.007 (–0.209 to 0.196); 0.947 | ||
| PAQLQ score at 12 months: emotional domain | |||
| Median (p25, p75) | 6.63 (5.75, 7.00) | 6.63 (5.75, 7.00) | |
| Unadjusted | 0.074 (–0.116 to 0.264); 0.445 | ||
| Adjusteda | 0.079 (–0.111 to 0.268); 0.413 | ||
There were no differences between treatment groups during follow-up with respect to the symptoms, limitations and emotional domains of the quality-of-life score (see Table 18).
Algorithm recommendation at each clinic attendance
At baseline, the majority of recommendations from the treatment algorithm in both groups was a step up in treatment (68.2% in the intervention group and 59.8% in the standard-care group; Table 19). A step down in treatment was possible at baseline in the intervention group; 22.4% of recommendations were for a step down in treatment at that time point. Such a step down was not possible at baseline in the standard-care group. At subsequent time points, the proportion of step-up recommendations from the treatment algorithm was lower than that at baseline. At baseline and 3, 6 and 9 months, the proportion of recommendations involving no change in treatment was higher in the standard-care group than in the intervention group (3 months: 31.5% vs. 7.1%; 6 months: 20.6% vs. 13.7%; and 9 months: 26.1% vs. 19.0%, respectively).
| Algorithm recommendation | Number (%) of participants | Overall, n (%) | |
|---|---|---|---|
| Intervention group | Standard-care group | ||
| Baseline | |||
| Total | 255 | 251 | 506 |
| Step down | 57 (22.4) | 0a (0.0) | 57 (11.3) |
| Remain the same | 24 (9.4) | 101 (40.2) | 125 (24.7) |
| Step up | 174 (68.2) | 150 (59.8) | 324 (64.0) |
| At 3 months | |||
| Total | 226 | 216 | 442 |
| Step down | 105 (46.5) | 69 (31.9) | 174 (39.4) |
| Remain the same | 16 (7.1) | 68 (31.5) | 84 (19.0) |
| Step up | 105 (46.5) | 79 (36.6) | 184 (41.6) |
| At 6 months | |||
| Total | 211 | 209 | 420 |
| Step down | 87 (41.2) | 84 (40.2) | 171 (40.7) |
| Remain the same | 29 (13.7) | 43 (20.6) | 72 (17.1) |
| Step up | 95 (45.0) | 82 (39.2) | 177 (42.1) |
| At 9 months | |||
| Total | 205 | 199 | 404 |
| Step down | 92 (44.9) | 92 (46.2) | 184 (45.5) |
| Remain the same | 39 (19.0) | 52 (26.1) | 91 (22.5) |
| Step up | 74 (36.1) | 54 (27.1) | 128 (31.7) |
| Missing | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| At 12 months | |||
| Total | 219 | 206 | 425 |
| Step down | 107 (48.9) | 108 (52.4) | 215 (50.6) |
| Remain the same | 47 (21.5) | 39 (18.9) | 86 (20.2) |
| Step up | 63 (28.8) | 57 (27.7) | 120 (28.2) |
| Missing | 2 (0.9) | 2 (1.0) | 4 (0.9) |
Compliance with the recommendation of the treatment algorithm
At each time point (baseline and the 3-, 6- and 9-month follow-ups), between 20.0% and 24.0% of treatment recommendations made by the algorithm were not followed (Table 20). Overall non-compliance with the algorithm took place in 200 out of 887 (23%) assessments of participants in the intervention group and 177 out of 874 (20%) assessments of participants receiving standard care (see Table 20). The proportion of assessments in which the algorithm recommendation was not followed was similar in both treatment groups (see Table 20). Where the treatment recommendation was ‘remain the same’, compliance with the treatment recommendation was much more common than if the recommendation was to step up or step down treatment.
| Algorithm recommendation | Number (%) of participants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3-month follow-up | 6-month follow-up | 9-month follow-up | |||||||||
| Intervention group | Standard-care group | Total | Intervention group | Standard-care group | Total | Intervention group | Standard-care group | Total | Intervention group | Standard-care group | Total | |
| Step up | ||||||||||||
| Total (n) | 174 | 150 | 324 | 105 | 79 | 184 | 95 | 82 | 177 | 74 | 54 | 128 |
| Not followed | 42 (24.1) | 34 (22.7) | 76 (23.5) | 24 (22.9) | 22 (27.8) | 46 (25.0) | 25 (26.3) | 24 (29.3) | 49 (27.7) | 19 (25.7) | 12 (22.2) | 31 (24.2) |
| Step down | ||||||||||||
| Total (n) | 57 | NA | 57 | 105 | 69 | 174 | 87 | 84 | 171 | 92 | 92 | 184 |
| Not followed | 19 (33.3) | NA | 19 (33.3) | 24 (22.9) | 16 (23.2) | 40 (23.0) | 24 (27.6) | 27 (32.1) | 51 (29.8) | 19 (20.7) | 30 (32.6) | 49 (26.6) |
| Remain the same | ||||||||||||
| Total (n) | 24 | 101 | 125 | 16 | 68 | 84 | 29 | 43 | 72 | 39 | 52 | 91 |
| Not followed | 2 (8.3) | 4 (4.0) | 6 (4.8) | 0 (0.0) | 7 (10.3) | 7 (8.3) | 0 (0.0) | 1 (2.3) | 1 (1.4) | 2 (5.1) | 0 (0.0) | 2 (2.2) |
| All algorithm recommendations | ||||||||||||
| Total (n) | 255 | 251 | 506 | 226 | 216 | 442 | 211 | 209 | 420 | 205 | 198 | 403 |
| Not followed | 63 (24.7) | 38 (15.1) | 101 (20.0) | 48 (21.2) | 45 (20.8) | 93 (21.0) | 49 (23.2) | 52 (24.9) | 101 (24.0) | 40 (19.5) | 42 (21.2) | 82 (20.3) |
Research teams added free text that recorded why they were not complying with the recommendation of the treatment algorithm, which was coded into overarching themes and subthemes (see Chapter 2).
The majority of reasons for non-compliance with the treatment algorithm were based on beliefs, most frequently that no step up or step down in treatment was required (Table 21). The second most frequently cited reason for non-compliance was knowledge, including knowledge of previous side effects of LTRA (when the treatment algorithm recommended a step up in treatment to include LTRA). Other frequently cited reasons included non-adherence to current treatment (within the behaviour theme) and the cold season (within the environment theme). The pattern of reasons was similar across all time points (Table 22). The decision-maker in the majority of the decisions was the clinical team (Table 23).
| Overarching theme | Subthemea | Number of participants | ||
|---|---|---|---|---|
| Intervention group | Standard-care group | Total | ||
| Knowledge | LTRA beneficial (previous trial of stopping LTRA) | 2 | 1 | 3 |
| LTRA ineffective | 3 | 2 | 5 | |
| LTRA side effects | 6 | 20 | 26 | |
| Other conditions | 12 | 11 | 23 | |
| Recent change to treatment | 1 | 0 | 1 | |
| Recent step up in treatment | 11 | 6 | 17 | |
| Other specialist knowledge of child or condition | 5 | 4 | 9 | |
| Beliefs | No step down in treatment needed | 39 | 23 | 62 |
| No step up in treatment needed | 29 | 36 | 65 | |
| Other change to treatment made | 23 | 28 | 51 | |
| Want step down | 1 | 3 | 4 | |
| Current treatment OK | 1 | 0 | 1 | |
| Failure of treatment | 10 | 5 | 15 | |
| Lung function results considered in decision-making | 14 | 15 | 29 | |
| Other causes of symptoms | 5 | 6 | 11 | |
| Perceived side effects associated with recommended treatment | 2 | 6 | 8 | |
| No further information | 0 | 2 | 2 | |
| Behaviour | Inhaler technique | 5 | 2 | 7 |
| Non-adherence to current treatment | 30 | 8 | 38 | |
| Emotion | Concern over device change | 1 | 1 | 2 |
| Do not wish to increase steroid | 5 | 4 | 9 | |
| Stress of new treatment | 1 | 1 | 2 | |
| Environment | Cold season | 19 | 21 | 40 |
| Summer | 1 | 1 | 2 | |
| COVID-19 | 2 | 3 | 5 | |
| No further information | 1 | 0 | 1 | |
| Social | Social reasons: no further information | 1 | 1 | 2 |
| Technical | Technical issues, including data entry | 3 | 6 | 9 |
| Reason | Number of participants | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | |||||
| Intervention group | Standard-care group | Intervention group | Standard-care group | Intervention group | Standard-care group | Intervention group | Standard-care group | |
| Knowledge | 17 | 15 | 8 | 13 | 5 | 9 | 10 | 7 |
| Beliefs | 37 | 21 | 34 | 34 | 33 | 38 | 20 | 31 |
| Behaviour | 6 | 3 | 11 | 4 | 12 | 1 | 6 | 2 |
| Emotion | 4 | 2 | 3 | 2 | 0 | 0 | 0 | 2 |
| Environment | 4 | 0 | 6 | 2 | 6 | 9 | 7 | 14 |
| Social | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Technical | 1 | 4 | 1 | 1 | 1 | 1 | 0 | 0 |
| Decision-maker | Number of participants | ||
|---|---|---|---|
| Intervention group | Standard-care group | Total | |
| Clinical team | 155 | 126 | 281 |
| Family | 11 | 13 | 24 |
| Joint: clinical team and family | 11 | 11 | 22 |
| Unclear | 23 | 29 | 52 |
Safety
Safety reporting within the study was limited to events related to the use of the NIOX VERO® or other study assessments. There were no SAEs or deaths in the trial. In total, 28 participants experienced at least one adverse reaction during the trial: 12 in the intervention group and 15 in the standard-care group (Table 24).
| Adverse reaction | Intervention group (n = 255) | Standard-care group (n = 254) | |
|---|---|---|---|
| Any adverse reaction | |||
| Total with at least one adverse reaction | 12a | 15b | |
| Per cent (95% CIc) with at least one adverse reaction | 4.7 (2.7 to 8.0) | 5.9 (3.6 to 9.5) | |
| Itching | |||
| Total with at least one adverse reaction of this type | 8 | 8 | |
| Per cent (95% CIc) with at least one adverse reaction of this type | 3.1 (1.6 to 6.1) | 3.1 (1.6 to 6.1) | |
| Coughing | |||
| Total with at least one adverse reaction of this type | 1 | 5b | |
| Per cent (95% CIc) with at least one adverse reaction of this type | 0.4 (0.0 to 2.2) | 2.0 (0.8 to 4.5) | |
| Feeling faint | |||
| Total with at least one adverse reaction of this type | 1 | 0 | |
| Per cent (95% CIc) with at least one adverse reaction of this type | 0.4 (0.0 to 2.2) | 0 | |
| Other | |||
| Total with at least one adverse reaction of this type | 3 | 3 | |
| Per cent (95% CIc) with at least one adverse reaction of this type | 1.2 (0.4 to 3.4) | 1.2 (0.4 to 3.4) | |
Exploratory analysis: relationship between change in fractional exhaled nitric oxide and asthma exacerbation
Table 25 explores whether or not the change in FeNO from the previous FeNO measurement to the current follow-up was associated with an exacerbation in the same 3-month follow-up period. The adjusted OR for asthma exacerbation was 1.02 (95% CI 0.97 to 1.08) for a 10-unit (ppb) change in FeNO. Similarly, it was investigated whether or not the change in FeNO in a given 3-month period was associated with an asthma exacerbation during the subsequent 3-month follow-up period. The adjusted OR for asthma exacerbation was 1.05 (95% CI 0.99 to 1.12) for a 10-unit (ppb) change in FeNO.
| OR (95% CI); p-value | |
|---|---|
| Asthma exacerbation in the same 3-month period | |
| Unadjusted | 1.02 (0.97 to 1.07); 0.466 |
| Adjusteda | 1.02 (0.97 to 1.08); 0.435 |
| Asthma exacerbation in the subsequent 3-month period | |
| Unadjusted | 1.04 (0.98 to 1.11); 0.234 |
| Adjusteda | 1.05 (0.99 to 1.12); 0.106 |
Post hoc analysis
Baseline fractional exhaled nitric oxide and subsequent asthma exacerbation
In those randomised to the standard-care group, there was no association between baseline FeNO measurement (log-transformed) and future asthma exacerbation (adjusted OR 0.95, 95% CI 0.59 to 1.51, p = 0.816; adjusted for age, sex, asthma severity and centre).
The impact of COVID-19
The impact of COVID-19 was that follow-up visits scheduled on or after 20 March 2020 could not be carried out face to face and had to be rearranged as a telephone follow-up. All of the 6-month follow-up visits were undertaken before 20 March 2020. In 13 children in the intervention group, the 9-month visit was carried out by telephone after 20 March 2020; given that FeNO could not be measured at this follow-up, these children received the treatment recommendation for the standard-care group. In 12 children in the standard-care group, the 9-month assessment was carried out by telephone after 20 March 2020; they received the recommendation for the standard-care group (as they would have if the visit had been face to face).
Algorithm treatment recommendations if participants had been allocated to the other treatment group
For this analysis, we considered the maximum number of algorithm recommendations for all participants, had all assessments been attended (i.e. 2545). There were 327 (13%) assessments for which data were not captured because the participant did not attend the visit and an additional 31 assessments for which the algorithm was not applied for reasons including FeNO not being available (predominantly where COVID-19 restrictions prevented face-to-face assessment), the child withdrawing from having their treatment managed via the algorithm and technical issues with the algorithm. Removing these 358 assessments gives 2187 assessments for which the algorithm was applied.
The recommendation ‘refer for specialist opinion’ was made during 642 assessments and, had participants been allocated to the alternate treatment group, this recommendation would have been made for 631 (98%) of these 642 participants.
After removing assessments for which ‘refer specialist opinion’ was recommended and for which the algorithm was not run, the remaining 1556 assessments were considered and were stratified by treatment group and by recommendation. Had participants in the standard-care group been randomised to the intervention group, the addition of FeNO would have meant that the treatment recommendations would have been the same in only 28% of assessments in which the standard-care algorithm recommendation was ‘no change’ (Table 26). By contrast, for those randomised to the standard-care group, the algorithm treatment recommendations to step up and step down were the same in 82% and 62% of assessments, respectively. When considering participants randomised to the FeNO-guided treatment group, had they been allocated to the other treatment group, the algorithm treatment recommendations would have been the same in 62–67% of assessments.
| Algorithm recommendation if participants had been allocated to the other treatment group | Actual allocation (%), n/N | |
|---|---|---|
| Intervention | Standard care | |
| No change | 66 (81/122) | 28 (70/250) |
| Step upa | 67 (193/288) | 82 (183/224) |
| Step downb | 62 (225/365) | 62 (208/297) |
Chapter 5 Health economics
Introduction
This chapter reports the methods and results of the economic analysis that was carried out as part of the RAACENO trial and includes a discussion of the modelling. The economic evaluation compares treatment guided by FeNO and symptoms (intervention) with treatment guided by symptoms alone (standard care) in terms of asthma-related NHS costs, the number of asthma exacerbations and total QALYs over a 12-month follow-up period. Costs falling directly on participants and indirect costs associated with time lost from productive activities are also considered in a separate analysis. The methods for measuring and valuing the resource use are summarised and the results are presented by a participant’s treatment allocation. These analyses are then incorporated into a simple Markov model to assess cost-effectiveness.
Objectives of the economic evaluation
The primary economic objective, as stated in the trial protocol, was to undertake an economic evaluation to assess the asthma-related health-care costs, direct and indirect costs to participants, and quality-of-life effects (QALYs) of the intervention compared with routine care over the 12-month trial follow-up period to identify an optimal treatment regime in terms of cost-effectiveness. The analysis relied on medication and asthma-related health-care resource use data collected at 3, 6, 9 and 12 months post randomisation, and estimated incidence rates of exacerbations by treatment allocation group applied in a decision-analytic modelling framework. Cost-effectiveness analysis results are presented as the cost per exacerbation avoided and the incremental cost per QALY gained. The perspective is that of the UK NHS. The health economics analysis plan is a standalone document. 47
Methods
Study design and participants
Details of the study design are provided in the study protocol47 and in Chapter 2. The economic analysis included the 506 participants in the statistical analysis and utilised the same ITT principles. Participants were followed up in the trial for 12 months. Clinical assessments took place at recruitment and at 3, 6, 9 and 12 months.
Cost and outcome assessment
Total resource use, outcomes and time displaced for families, friends and relatives were assessed via the CRFs collected at clinical assessment visits. Other sources (including GP records or a short-form CRF completed by telephone or postal questionnaire) were used to collect data on the number of asthma exacerbations if a participant did not attend a follow-up appointment. Health-care utilisation reported in the CRFs collected during visits was used to quantify health-care resource use associated with asthma, such as treatment and contacts with health-care professionals. National unit costs were used to value all elements of resource use. The CRFs also collected information on time displaced from usual activities as a result of the child’s asthma over the follow-up period. Travel costs for a series of most recent health-care contacts were collected during follow-up and were used to estimate the cost of travel associated with contacts during the follow-up period.
The calculated costs were summed over the 12-month follow-up period for each participant and were aggregated by treatment group following ITT principles. Follow-up appointments at 3, 6, 9 and 12 months were not included in the calculation of costs over the 12-month follow-up period. The mean difference in costs was estimated for major cost categories: medication use, exacerbation-related costs and background asthma-related health service costs.
Health-care resource use measurement and valuation
Cost of the primary intervention
The measurement of FeNO requires little time (typically < 5 minutes); therefore, additional staff time required for training, conducting and interpreting the test was assumed in the analysis to be negligible. The intervention incurs the cost of only the NIOX VERO® device, breathing handle and a ‘test kit’. The accompanying ‘test kit’ included all consumables and a sensor that contains a set amount of uses with a maximum lifespan of 1 year. The breathing handle is recommended to be replaced after 1 year. The cost of the device is estimated per individual test based on the equivalent annual cost (annuitised over the expected useful lifespan) divided by the throughput. Given that all participating centres at the end of the trial period ordered the maximum test kit size (n = 300 tests), the annual throughput for the equipment was assumed to be 300 tests. Based on the above assumptions, the estimated cost per test was £6.35. As an alternative scenario, a lower annual throughput of 60 per annum was considered, giving a cost per test of £21.73. Further details of the components of these costs are found in Appendix 7, Table 49.
Cost of medication (treatment)
Asthma medication use, including types and doses of inhalers, oral steroids, LTRA, theophylline (Slo-phyllin; Merck, Darmstadt, Germany) antihistamines, cyclosporin and omalizumab injections, was recorded in the CRFs collected at 3, 6, 9 and 12 months. These data were combined with NHS unit cost data sourced from the British National Formulary for Children66 and the Personal Social Service Research Unit (PSSRU) cost per hour of staff time (if administration was required) to estimate the cost of preventative medication use and exacerbation-related medication use per participant (or exacerbation) over the follow-up period. Table 27 reports the types of medication reported and the cost per unit.
| Drug | Drug name | NHS indicative pricea | Size (dose) | Description |
|---|---|---|---|---|
| Oral steroid | Prednisolone | £1.45 | 28 | Prednisolone 5-mg tablets (AAH Pharmaceuticals Ltd, Coventry, UK) |
| Dexamethasone | £7.62 | 50 | Dexamethasone 2-mg tablets (AAH Pharmaceuticals Ltd) | |
| Antihistamine | Cetirizine | £4.42 | 14 | Benadryl Allergy One-A-Day 10-mg tablets (McNeil Products Ltd, High Wycombe, UK) |
| Antibiotics | Amoxicillin | £1.75 | 21 | Amoxicillin 500-mg capsules (AAH Pharmaceuticals Ltd) |
| Painkillers/anti-inflammatory | Paracetamol | £4.40 | 200 | 200 ml Calpol Six Plus 250 mg/5 ml oral suspension sugar free (McNeil Products Ltd) |
| £1.19 | 16 | Anadin Paracetamol 500-mg tablets (Pfizer Consumer Healthcare Ltd, Sandwich, UK) | ||
| Ibuprofen | £1.57 | 12 | Nurofen 200-mg tablets (Reckitt Benckiser Healthcare UK Ltd, Kingston upon Hull, UK) | |
| Omalizumab | Administration | £14.00 | N/A | 30 minutes of band 5 hospital-based nurse time: per hour of participant contact for subcutaneous injection (£46)67 |
| Injection | £128.07 | 1 | Xolair 75 mg/0.5 ml solution for injection pre-filled syringes (Novartis Pharmaceuticals UK Ltd, London, UK) | |
| £256.15 | 1 | Xolair 150 mg/1 ml solution for injection pre-filled syringes (Novartis Pharmaceuticals UK Ltd) | ||
| Xanthines | Theophylline | £2.96 | 56 | Uniphyllin Continus 200-mg tablets (Napp Pharmaceuticals Ltd, Cambridge, UK) |
| Calcineurin inhibitors | Ciclosporin | £48.50 | 30 | Capimune 100-mg capsules (Mylan, Canonsburg, PA, USA) |
| Preventer inhaler | ||||
| Beclomethasone | £3.70 | 200 | Beclomethasone (Clenil) 50 µg MDI/spacer | |
| £7.42 | 200 | Beclomethasone (Clenil) 100 µg MDI/spacer | ||
| £16.17 | 200 | Beclomethasone (Clenil) 200 µg microgram MDI/spacer | ||
| Budesonide | £14.25 | 200 | Pulmicort 100 Turbohaler (AstraZeneca UK Ltd, Luton, UK) | |
| £14.25 | 100 | Pulmicort 200 Turbohaler (AstraZeneca UK Ltd) | ||
| £14.25 | 50 | Pulmicort 400 Turbohaler (AstraZeneca UK Ltd) | ||
| Budesonide with formoterol | £28.00 | 120 | Symbicort 100/6 Turbohaler (AstraZeneca UK Ltd) | |
| £28.00 | 120 | Symbicort 200/6 Turbohaler (AstraZeneca UK Ltd) | ||
| £28.00 | 60 | Symbicort 400/12 Turbohaler (AstraZeneca UK Ltd) | ||
| Fluticasone | £4.00 | 60 | Flixotide 50 µg/dose Accuhaler (GlaxoSmithKline UK Ltd, London, UK) | |
| £8.00 | 60 | Flixotide 100 µg/dose Accuhaler (GlaxoSmithKline UK Ltd) | ||
| £6.53 | 120 | Flixotide 50 µg/dose Evohaler (GlaxoSmithKline UK Ltd) | ||
| £21.26 | 120 | Flixotide 125 µg/dose Evohaler (GlaxoSmithKline UK Ltd) | ||
| Fluticasone with vilanterol | £22.00 | 30 | Relvar Ellipta 92 µg/dose dry powder inhaler (GlaxoSmithKline UK Ltd) | |
| £29.50 | 30 | Relvar Ellipta 184 µg/dose/22 µg/dose dry powder inhaler (GlaxoSmithKline UK Ltd) | ||
| Fluticasone with formoterol | £14.40 | 120 | Flutiform 50 µg/dose/5 µg/dose inhaler (Napp Pharmaceuticals Ltd) | |
| Fluticasone with salmeterol | £22.45 | 120 | Aloflute 25 µg/dose/125 µg/dose inhaler (Mylan) | |
| £17.46 | 60 | Seretide 100 Accuhaler (GlaxoSmithKline UK Ltd) | ||
| £33.95 | 60 | Seretide 250 Accuhaler (GlaxoSmithKline UK Ltd) | ||
| £32.74 | 60 | Seretide 500 Accuhaler (GlaxoSmithKline UK Ltd) | ||
| Fluticasone with salmeterol | £17.46 | 120 | Seretide 50 Evohaler (GlaxoSmithKline UK Ltd) | |
| £23.45 | 120 | Seretide 125 Evohaler (GlaxoSmithKline UK Ltd) | ||
| £29.32 | 120 | Seretide 250 Evohaler (GlaxoSmithKline UK Ltd) | ||
| Ciclesonide | £38.62 | 120 | Alvesco 160 inhaler (AstraZeneca UK Ltd) | |
| Reliever inhaler | ||||
| Salbutamol | £1.50 | 200 | Ventolin 100 µg/dose Evohaler (GlaxoSmithKline UK Ltd) | |
| £3.60 | 60 | Ventolin 200 µg/dose Accuhaler (GlaxoSmithKline UK Ltd) | ||
| £3.31 | 200 | Easyhaler salbutamol sulfate 100 µg/dose dry powder inhaler (Orion Pharma UK Ltd, Reading, UK) | ||
| £6.30 | 200 | Salamol 100 µg/dose Easi-Breathe inhaler (Teva UK Ltd, Castleford, UK) | ||
| £3.60 | 60 | Ventolin 200 µg/dose Accuhaler (GlaxoSmithKline UK Ltd) | ||
| Terbutaline sulfate | £8.30 | 120 | Bricanyl 500 µg/dose Turbohaler (AstraZeneca UK Ltd) | |
| Ipratropium bromide | £4.14 | 20 | Atrovent 250 µg/1-ml nebuliser liquid UDVs (Boehringer Ingelheim Ltd, Bracknell, UK) | |
| LABA inhaler | ||||
| Salmeterol | £29.26 | 120 | Serevent 25 µg/dose Evohaler (GlaxoSmithKline UK Ltd) | |
| £35.11 | 60 | Serevent 50 µg/dose Accuhaler (GlaxoSmithKline UK Ltd) | ||
| LTRA | ||||
| Montelukast | £2.25 | 28 | Montelukast 5-mg chewable tablets sugar free (AAH Pharmaceuticals Ltd) | |
| £2.88 | 28 | Montelukast 10-mg tablets (AAH Pharmaceuticals Ltd) | ||
If medication data were not available at a follow-up time point, they were assumed to be the same as those reported at the last follow-up appointment. If medication use data were missing at time point 4, they were considered to be missing, meaning that a complete medication cost could not be calculated for the year. The total cost of medication was expressed in terms of the total cost required should a participant have 100% adherence because this represents what would have been prescribed by a health-care professional. As an alternative scenario, medication use was quantified on a per-dose basis adjusted for a participant’s adherence.
The smart inhaler recorded a participant’s adherence; however, this information was not always in line with the family’s reported adherence or the research team’s opinion. Therefore, a participant’s adherence was determined using the decision rules detailed in Table 28. If adherence information was missing for some time points, a participant’s average adherence across the other time points was applied.
| Does clinical team think the child is adherent? | Smart inhaler adherence score | ||
|---|---|---|---|
| < 70% | ≥ 70% | Not available | |
| Clinical team thinks child is adherent |
|
|
|
| Clinical team thinks child is not adherent |
|
|
|
Health service costs
The frequency of health-care resource use associated with asthma exacerbations was collected at each assessment (3, 6, 9 and 12 months) and was supported by a patient-held diary. The child’s parent reported information for the RNs to capture retrospectively in the follow-up CRF any contact with health-care professionals associated with asthma, breathing problems and asthma exacerbations in the previous period.
The costing approach assigned unit costs to each individual component of health-care resource use to capture participant-level variation in costs. Unit costs applied to different types of health service contact are provided in Table 29. All costs are reported in 2018/19 Great British pounds and adjustments for inflation have been made, where necessary, using the Hospital & Community Health Services Index and the new Health Services Index. 68 Further details of unit costs for hospital contacts and emergency admissions are found in Appendix 8, Tables 50–52.
| Resource use and type of contact | Unit cost (£) | Source |
|---|---|---|
| GP | ||
| Visit to the surgery | 39.65 | PSSRU 2019 (£4.30 per minute of staff time at average consultation length of 9.22 minutes)68,69 |
| Telephone | 23.22 | PSSRU 2019 (£4.30 per minute of staff time at average telephone consultation length of 5.4 minutes)68,69 |
| Community asthma nurse | ||
| Visit to the surgery | 28.00 | PSSRU 201968 (£84 per hour of participant contact of band 6 GP nurse at average nurse consultation visit length of 20 minutes) |
| Home visit | 23.14 | PSSRU 2010 (inflated to 2019 prices)67 |
| Telephone | 7.97 | PSSRU 2019 (£84 per hour of participant contact of band 6 GP nurse at average nurse telephone consultation length of 5.69 minutes)68,69 |
| NHS 24/111 service | 12.96 | Pope et al.70 £12.26 inflated to 2019 prices using the PSSRU inflation indices68 |
| Out-of-hours GP service | 74.02 | Weighted average of T03 A and T03NA (excluding emergency dental), NHS reference costs 2018/1971 |
| Walk-in centre | 45.71 | Weighted average of T04 A and T04NA (excluding emergency dental), NHS reference costs 2018/1971 |
| Pharmacist | 6.82 | 9.22 minutes of band 6 community-based scientific and professional staff68,70 |
| Emergency department (non-admitted) | 133 | VB09Z Emergency medicine, category 1 investigation with category 1–2 treatment (type 1 non-admitted)71 |
| Emergency department (admitted) | 264 | Weighted average of VB06Z and VB04Z72 by severity of admission (see Appendix 8, Table 53) |
| Asthma clinic | ||
| Visit | ||
| Consultant | 204 | CL WF01C, Non-admitted face-to-face attendance, Follow-up. Paediatric Respiratory Medicine71 |
| Nurse | 133 | CHS NURS N08CF F2F, Child, Specialist Nursing, Asthma and Respiratory Nursing/Liaison, Child, Face to face71 |
| Telephone | ||
| Consultant | 105 | CL WF01C, Non-admitted non-face-to-face attendance, Follow-up. Paediatric Respiratory Medicine71 |
| Nurse | 24 | CHS NURS N08CF, Child. Specialist Nursing, Asthma and Respiratory Nursing/Liaison, Child, Non-face to face71 |
| Hospital short stay (admitted for ≤ 1 night) | 594 | NES, Weighted average of PD12 Paediatric, Asthma or Wheezing71 |
| Hospital long stay (admitted for ≤ 4 days and > 1 night) | 1913 | NEL, Weighted average of PD12 Paediatric, Asthma or Wheezing. Inflated to 2019 prices68,72 |
| Excess bed-days | 575 | NEL excess bed-day, Weighted average of PD12 Paediatric, Asthma or Wheezing. Inflated to 2019 prices68,72 |
| Hospital day case | 394 | DC, Weighted average of PD12 Paediatric Asthma or Wheezing71 |
| Bronchoscopy | 952 | DZ69B Diagnostic Bronchoscopy, 18 years and under, combined day case/ordinary elective spell tariff. Admitted participant care & outpatient procedure prices 2018/19. Annex A73 |
| Ambulance | ||
| See and treat | 209 | AMB ASS0172 |
| See and convey | 257 | AMB ASS0272 |
| Clinical psychologist | 54 | 1 hour of a clinical psychologist’s time. Band 7 scientific and professional staff68 |
| Physiotherapist | 57 | 1 hour of a specialist (respiratory problems) physiotherapist’s time. Band 7 hospital-based scientific and professional staff68 |
| Speech and language therapist | 34 | 1 hour of a speech therapist’s time. Band 5 scientific and professional staff68 |
For primary care contacts, such as GP visits and community or practice nurse visits, the unit costs were sourced from the Unit Costs of Health and Social Care. 67 The unit costs do not distinguish between planned and unplanned use of services, such as visits to the GP; therefore, the cost of a contact associated with an exacerbation is not different from those contacts associated with general asthma or breathing problems.
For secondary care contacts, each resource use item was mapped to an appropriate Healthcare Resource Group (HRG), where available, and costed using the relevant NHS reference cost. 71–73 The core HRG codes related to paediatric asthma care recorded in the CRF are PD12 (Paediatric, Asthma or Wheezing), T01 (Type 1, Emergency medicine), T03NA (Type 3 non-admitted, Emergency medicine), T04NA (Type 4 non-admitted, Emergency medicine), ASS01 (See treat or refer, Ambulance) and N08C (CHS, Specialist Nursing, Asthma and Respiratory Nursing/Liaison, Child, Face to face/Non-face to face). Where a HRG code was not available, costs were applied as the cost per working hour sourced from the Unit Costs of Health and Social Care. 68
All resource use data were provided retrospectively by participants for the period since their last clinical assessment visit. For example, if a participant missed their 6-month clinical assessment visit, costs incurred from 3 to 9 months were collected at the 9-month visit. If a participant missed their final 12-month clinical assessment visit, their health-care resource use and direct and indirect costs were considered missing since their last observed visit. If a participant attended a study follow-up visit and provided no data for resource use but did provide data for preventative treatment use, health-care resource use and direct and indirect costs were assumed to be not applicable (zero) for the relevant time interval rather than missing.
Data collected in the CRF that are not associated with an exacerbation do not incorporate the date of that event. Therefore, we cannot distinguish whether the costs incurred occurred before or after 365 days post randomisation, as we could for the primary outcome of the trial. For the purpose of this analysis, it was assumed that all resource use and direct and indirect participant costs were accrued within the 365 days post randomisation.
Health service costs associated with an exacerbation
The CRF was used to record information about the date, health-care contacts, prescribed medicines and number of resource use events for each reported asthma exacerbation. This information was used to calculate the cost of all health-care resource use associated with each asthma exacerbation treated with OCs occurring within 365 days of randomisation. If a participant reported an exacerbation within 14 days following another reported exacerbation, this was defined as a continued exacerbation. Therefore, all resource use associated with continuing exacerbations was counted as resource use attributable to the initial exacerbation, even if the continuation occurred outside the follow-up period of 365 days.
Health service costs not associated with an exacerbation (background cost of asthma)
The CRF included a section for capturing any further health-care contacts not associated with an exacerbation. The background cost of asthma includes the cost of all contacts, with health-care professionals and prescribed medications not captured in the preventative treatment section of the CRF.
Direct costs to families (travel and over-the-counter medications)
The participant time and travel questionnaire recorded the time and travel data for a participant’s most recent emergency hospital in-participant admission, accident and emergency visit, outpatient appointment, GP appointment or out-of-hours or walk-in appointment. If any of these appointments had occurred since the previous study visit, the time and travel questionnaire was completed at the next study follow-up visit. Travel costs were estimated from these data based on the reported mode of transport and/or any reported fares/charges incurred. Private car journeys were costed using the rate per mile of £0.45 set by HM Revenue and Customs. 74 The participant travel costs were assigned on a participant-level basis to all health-care contacts associated with each asthma exacerbation over the trial period. Where individual travel cost data were not available, the average travel cost for that appointment type, across all other participants, was applied to each contact reported by the individual.
In addition, the CRF captured family-reported out-of-pocket expenditure on over-the-counter medications for asthma or breathing problems (excluding exacerbations). Resource use data from this source were summed over the follow-up period and included in the analysis as a component of direct participant costs.
Indirect costs (time lost from productive activities)
The total time displaced from usual activities owing to asthma, such as days missed from school or work, was recorded in the CRF for children, parents and friends or relatives. To account for the time lost from productive activities, the indirect cost to society was calculated based on the activity displaced.
Information was collected for parents, friends and relatives for hours displaced from paid work, unpaid work, leisure time and study as a result of the participant’s asthma. Given that we did not collect the age and sex of the parents, friends or relatives, a weighted average using the distribution of parents of primary school-aged children,75 adjusted for the age demographic of the children in the data set, was used against the gross average wage rates obtained from the Annual Survey of Hours and Earnings (ASHE) published by the Office for National Statistics76 to value the time lost from paid employment. The time lost from unpaid work for adults was estimated using the average cost per hour of housework, volunteering and informal care published by the Office for National Statistics. 77 The value of forgone leisure time was estimated by multiplying time losses by the current value of non-working time available from the Department of Transport. 78 For participants, displaced time was reported as days missed from paid work or school. The time lost from work was calculated using the national minimum wage for under-18-year-olds,79 assuming an 8-hour workday. The unit costs per hour are presented in Table 30.
| Category | Cost per unit (£) | Source |
|---|---|---|
| Travel costs (per mile) | ||
| Car | 0.45 | HM Revenue and Customs. Expenses and Benefits: Business Travel Mileage for Employees’ Own Vehicles74 |
| Displaced time (per hour) | ||
| Work | ||
| Participant | 4.35 | National minimum wage for under-18-year-olds79 |
| Adult | 19.86 | Weighted average of ASHE table 6 using the estimates of the age distribution of parents of primary school-aged children adjusted for the mean age of RAACENO participants75,76 |
| Unpaid work (volunteering, housework and informal care) | 12.01 | Average of per hour values for housework, child care, adult care and volunteering77 |
| Leisure | 5.03 | Transport Analysis Guidance table A 1.3.2 – Non-working other perceived cost 201978 |
Given that there is no generally accepted way of placing a monetary value on the time lost from full-time schooling or study for children or adults, this outcome is reported separately in unvalued units of time.
Outcome measures for cost-effectiveness
For cost-effectiveness, the total number of exacerbations was used as the unit of effectiveness. This represents a secondary clinical effectiveness outcome of the trial, but is more suited to the cost-effectiveness analysis, which should consider the cost and health impact of all asthma-related exacerbations over a defined time horizon. A secondary economic analysis also estimated the expected difference in QALYs based on the assumption that exacerbations are associated with a health state utility decrement, as informed by external literature. 80
Quality-of-life weights
Quality-of-life weights for the participants were sourced from the external literature. Asthma is characterised by periods of good health interrupted by short-lived exacerbations. It is assumed that the quality of life associated with periods of good health is not affected by the number or duration of exacerbations that a participant has within a year. The quality-of-life weight of 0.96 (SD 0.07) was applied for all of the cohort. This was sourced from the baseline EuroQoL-5 Dimensions (EQ-5D) utility values of children with mild to moderate asthma in the control arm of a previous RCT (n = 27). 81 Owing to a lack of sufficient data relating to the impact of exacerbations on a child’s health-related quality of life, it is assumed that children experience the same quality-of-life decrement as adults, which is consistent with the method used in the PLEASANT trial. 82 The EQ-5D quality-of-life decrement associated with an exacerbation was 0.1 for exacerbations treated with OCs and 0.2 for exacerbations necessitating hospitalisation. This was sourced from a study of UK asthma participants (n = 112), who had an average age of 40.5 years and a severity of BTS step 4 or 5. 80 Julious et al. 82 recognise that evidence from these sources may underestimate the utility losses associated with an exacerbation requiring hospitalisation because the utility values were not recorded during the exacerbation period itself. However, in the absence of reliable, high-quality data for a representative cohort, this acknowledgement is uncertain in itself.
Modelling of cost-effectiveness
The cost-effectiveness analysis utilised a simple decision model (Figure 14) to determine the incremental cost per exacerbation avoided and the incremental cost per QALY gained with the intervention compared with standard care. The model utilised a 1-year time horizon, in line with the trial follow-up period, but we also assessed the impact of extending this to 5 years. The model took the form of a simple Markov model with a 2-weekly cycle length in line with the exacerbation definition utilised in the trial, which was informed through clinical consultation. The model was informed by the trial resource use data (translated into background costs, treatment costs per participant per year and the cost per exacerbation), the exacerbation rate by treatment group and published evidence on the health-related quality-of-life impact of asthma exacerbations. 80,81 Furthermore, the cost of an outpatient appointment with a consultant (see Table 29) was applied at 3-month intervals for both treatment groups in the model. This is consistent with the trial protocol to reflect the resource use required to achieve the effectiveness outcomes utilised.
FIGURE 14.
Schematic of the decision model.
The utility decrements associated with asthma exacerbations were applied to the expected number of asthma exacerbations for each cycle of the model. The utility decrements for asthma exacerbations reported in the literature for those requiring inpatient hospitalisation differ from those for patients managed without inpatient admission; therefore, a weighted average of QALY decrements was applied based on the proportion of exacerbations requiring hospitalisation from the trial. No death events occurred during the trial; therefore, it was assumed that the intervention has no effect on survival and that any difference in QALYs between groups is wholly driven by differences in the number of asthma exacerbations over the follow-up period. This approach is in line with a previous National Institute for Health and Care Research-funded economic evaluation, in which additional asthma-related mortality was not considered in the analysis. 49
The model was used to estimate the expected costs, numbers of exacerbations and QALYs over the observed time horizon of the trial, assuming a constant incidence rate for exacerbations by treatment group. Given the time horizon of the model, no discount rate was applied to QALYs or costs. Furthermore, it was assumed that there is no additional risk of death over the UK age-specific risk for participants in the cohort because no death events occurred in the trial. All results are presented using probabilistic analysis, in terms of both the incremental cost per exacerbation avoided and the incremental cost per QALY gained. These were calculated using the modelled expected differences in costs, exacerbations and QALYs between treatment groups. Input parameters are assigned probability distributions to reflect uncertainty in their mean estimates. Probability distributions were determined by the nature of the data that they describe. Utility decrement and total cost parameters were assigned gamma distributions. The incremental costs of the intervention and rate parameters were applied normal distributions and log-normal distributions, respectively. The variance for each cost input parameter was derived from the analysis of the trial data described in the following section. Where the standard error was not known, a value of 10% was assumed. A Monte Carlo simulation of 10,000 draws was used to characterise the uncertainty surrounding the model-based estimates of cost-effectiveness, with mean costs, QALYs and exacerbations obtained by taking the average over 10,000 simulations. Results are displayed graphically in the form of the cost-effectiveness plane and cost-effectiveness acceptability curve. 82,83
Statistical analysis of trial economic data
Aggregating and summarising costs
Resource use, costs and health outcome data were summarised and tabulated for comparison by treatment group, following the principles of ITT analysis. Continuous and count variables are presented as means (± SD), and dichotomous and categorical variables are presented as absolute numbers and percentages. All asthma-related health service cost elements were summed over the follow-up period to estimate a total health service cost per participant. Costs to participants and their families were summarised separately by ITT.
Missing data
Missing cost data are a common challenge associated with trial-based economic evaluations of participant-level data. To estimate the total costs, several assumptions were made depending on the category of cost data, as described in the previous sections. Based on these assumptions, the estimation of total costs was established for > 80% of the trial sample in each cost category (Table 31). Missing data with respect to exacerbations were minimal; 497 of the 509 observed exacerbations had complete resource use data.
| Cost category | Number (%) of observations | ||
|---|---|---|---|
| Intervention group (n = 255) | Standard-care group (n = 251) | Total (n = 506) | |
| Treatment | 225 (88) | 207 (82) | 432 (85) |
| Backgrounda | 230 (90) | 213 (85) | 443 (88) |
| Direct family | 230 (90) | 213 (85) | 441 (87) |
| Indirect family | 228 (89) | 213 (85) | 443 (88) |
It is recommended that, if total health service costs are missing for > 10% of participants, missing data methods, such as multiple imputation, should be implemented to fit plausible values to the missing elements. 84 However, given that the data used to inform the model (treatment, background and exacerbation cost) were at least 80% complete (98% for observed exacerbations), and no statistically significant between-group differences were observed for the primary or secondary clinical effectiveness outcomes based on almost complete data, it is argued that the complete cost data are sufficient for making an unbiased comparison of the alternatives in the economic model.
Analysis of cost and outcome data
To inform the inputs for the model-based assessment of cost-effectiveness, an analysis of costs was carried out on an ITT basis using individual participant-level cost data. All analyses were performed using Stata® statistical software for data science (StataCorp LLC, College Station, TX, USA). For 12-month aggregated costs, generalised linear models (GLMs) with appropriate variance and link functions were used to estimate the difference in total health service, participant and indirect costs between the treatment groups. 84,85 The models were adjusted for the following minimisation factors: age band (< 11 years and ≥ 11 years), sex and asthma severity (BTS step 2, BTS step 3 and BTS step 4). Robust standard errors were used, clustered by centre, to account for within-centre correlation. The modified Park test was implemented to select the appropriate family and the Box–Cox test of functional form and Pregibon link test were applied to determine the appropriate link function. 84,85 Where necessary, costs with a high proportion of zeros were modelled using a two-part model with a probit and GLM form. Details of these can be found in Appendix 9. The adjusted means and mean differences between treatment groups were tabulated for each cost outcome by ITT.
For the cost per exacerbation, we estimated the average cost of health-care resource use across all exacerbations that met the definition used for exacerbation in the trial (requiring treatment with OCs). The difference in cost per exacerbation between the treatment groups was calculated using a multilevel mixed-effects model with minimisation covariates, robust standard errors clustered by centre and a random effect for participant, as participants could experience several exacerbations over the study period.
Results
Comparison of costs at 12 months
Table 32 summarises the costs associated with resource use accrued over the 12-month study period by ITT from an NHS perspective. Prescribed treatment costs were £718.16 (95% CI £525.70 to £910.63) and £732.71 (95% CI £502.03 to £963.40) for the standard-care group and intervention group, respectively. Mean treatment costs adjusted for adherence were £556.96 (95% CI £376.39 to £737.54) and £561.73 (95% CI £345.12 to £778.34) for the standard-care group and intervention group, respectively. The total resource use over 12 months reported to be associated with exacerbations translated into a mean cost of £294.87 (95% CI £187.65 to £402.09) and £297.30 (95% CI £169.24 to £425.35) for the standard-care group and intervention group, respectively; the large SD indicates the skewed nature of the health-care resource use data for exacerbation across the sample. Background health-care costs, which include all health-care contacts not associated with an exacerbation and other prescribed medications, were £176.92 (95% CI £90.39 to £263.45) and £115.74 (95% CI £83.70 to £147.77) for the standard-care group and intervention group, respectively. Further information on the components of these costs are provided in Table 32. For most secondary care and treatment cost categories, there is a large SD, which indicates a heavily right-skewed distribution to the cost data; this is common for this type of data.
| Resource use | Total (n) | Intervention group, mean (£) (SD) | Standard-care group, mean (£) (SD) |
|---|---|---|---|
| Prescribed treatment cost | 732.71 (1755.97) | 718.16 (1404.53) | 432 |
| Adherence adjusted treatment cost | 561.73 (1648.80) | 556.96 (1317.77) | 432 |
| Exacerbation-related health-care resource utilisation costs | 297.30 (1032.17) | 294.87 (853.80) | 498 |
| Exacerbation-related primary care costs | 43.79 (92.91) | 39.38 (66.44) | 498 |
| GP | 31.98 (65.92) | 30.19 (54.53) | 498 |
| Nurse | 2.10 (11.98) | 1.96 (8.09) | 498 |
| NHS 24/111 | 0.93 (5.09) | 1.21 (5.42) | 498 |
| Out-of-hours GP service | 7.64 (50.61) | 3.61 (19.73) | 498 |
| Walk-in | 1.09 (8.09) | 2.41 (12.50) | 498 |
| Pharmacist | 0.05 (0.61) | 0 (0) | 498 |
| Exacerbation-related secondary care costs | 252.00 (1017.24) | 253.69 (844.71) | 498 |
| Emergency department | 19.00 (56.23) | 26.38 (70.58) | 498 |
| Hospital outpatient | 4.30 (26.40) | 4.09 (26.16) | 498 |
| Hospital inpatient | 226.12 (1011.86) | 213.35 (821.47) | 498 |
| Day case | 1.56 (24.81) | 1.60 (25.11) | 498 |
| Ambulance | 1.02 (16.19) | 8.16 (54.55) | 498 |
| Exacerbation-related medication costs | 1.51 (3.79) | 1.79 (3.26) | 498 |
| Total background health-care resource utilisation costs | 115.74 (246.59) | 176.92 (640.67) | 443 |
| Total 12-month health service costs | 1158.94 (2113.23) | 1168.78 (1885.18) | 430 |
Background cost
The primary component of health-care resource use that was not associated with exacerbations was secondary care (Table 33). Higher inpatient hospitalisation costs were generated by participants in the standard-care group than in the intervention group; this was due to there being five long inpatient stays in the standard-care group compared with two short inpatient stays and a bronchoscopy procedure in the intervention group. The majority of the alternative contacts within secondary care were very similar, apart from ‘Other’. ‘Other’ secondary care use describes services including psychiatrist, psychologist and speech and language therapist. Given that participants will attend such services either weekly or fortnightly, this accrues a high cost over the period for the participants; of those referred, one participant received standard care and three participants received the intervention. Appendix 10, Table 55, details the health-care contacts associated with the costs presented in Table 33.
| Individual health-care resource | Mean (SD) value | |
|---|---|---|
| Intervention group | Standard-care group | |
| Total cost (£) | 115.74 (246.60) | 176.92 (640.66) |
| Total (n) | 230 | 213 |
| Primary care (£) | 33.86 (68.96) | 37.20 (74.45) |
| GP | 25.23 (57.69) | 27.70 (60.58) |
| Nurse | 4.40 (23.92) | 3.29 (12.38) |
| NHS 24/111 | 0.34 (2.07) | 1.22 (6.55) |
| Out-of-hours GP service | 2.90 (15.96) | 2.78 (15.83) |
| Walk-in | 0.99 (6.68) | 2.15 (12.37) |
| Pharmacist | 0 (0) | 0.06 (0.66) |
| Secondary care (£) | 81.86 (224.10) | 139.70 (635.48) |
| Emergency department | 12.72 (62.08) | 11.86 (42.16) |
| Hospital outpatient | 37.78 (90.39) | 43.10 (159.76) |
| Hospital inpatient | 8.90 (78.85) | 71.64 (581.75) |
| Day case | 3.42 (36.65) | 0 (0) |
| Bronchoscopy | 8.28 (88.58) | 8.94 (92.03) |
| Ambulance | 2.23 (23.91) | 3.62 (30.36) |
| Other (physiotherapist/psycholog ist/speech and language therapist) | 8.52 (88.60) | 0.54 (7.81) |
| Medication | 0.02 (0.16) | 0.02 (0.17) |
Preventative treatment cost
Treatment costs included the cost of inhalers, LTRA, theophylline, antihistamines, cyclosporin and prescribed omalizumab injections. The components of the 12-month treatment costs are summarised in Table 34. No notable difference between treatment groups was observed for any of the categories. The total cost refers to the total amount of medication required to fulfil the participant’s prescription, giving £718.16 (95% CI £525.70 to £910.63) and £732.72 (95% CI £502.03 to £963.40) in the standard-care group and intervention group, respectively. After adjusting for participants’ adherence, the estimated cost of utilised medication was £556.96 (95% CI £376.39 to £737.54) and £561.37 (95% CI £344.74 to £777.99) for the standard-care group and intervention group, respectively. It is worth acknowledging that a confidential participant access scheme exists for omalizumab injections for this indication. However, only seven participants received this treatment, with a maximum 12-month treatment cost of £18,078.44 in this study. Given a broadly similar distribution and mean cost between treatment groups, applying the discounted cost would have little impact on the between-group difference.
| Treatment type | Mean (SD) cost (£) | |
|---|---|---|
| Intervention group | Standard-care group | |
| Preventer | 289.53 (130.37) | 285.59 (135.27) |
| Reliever | 4.15 (3.98) | 3.58 (2.13) |
| LABAa | 0.31 (4.68) | 1.78 (25.62) |
| LTRA | 19.72 (16.94) | 19.68 (16.57) |
| Theophylline | 4.32 (13.05) | 4.00 (12.02) |
| Antihistamines | 229.25 (159.38) | 245.77 (156.45) |
| Cyclosporin | 4.53 (39.03) | 4.92 (40.67) |
| Omalizumab | 180.78 (1725.25) | 152.84 (1364.02) |
| Prescribed cost | 732.72 (1755.97) | 718.16 (1404.53) |
| Adherence adjusted | 561.37 (1648.91) | 556.96 (1317.77) |
Direct and indirect participant costs and outcomes
Table 35 summarises the direct and indirect productivity costs incurred by participants, family and friends over the 12-month follow-up period. Direct costs, which include travel and over-the-counter medication, were small, with a mean cost of £10.41 (95% CI £8.35 to £12.47) and £9.09 (95% CI £6.72 to £11.46) in the standard-care group and the intervention group, respectively. On average, each participant missed 24.96 and 23.48 days of school, and those older participants who had a part-time job missed 1.16 and 0.94 days of work, in the standard-care group and intervention group, respectively. The greatest source of indirect cost was that of paid employment, resulting from parents’/carers’ and friends’ or relatives’ absenteeism from paid work owing to the participants’ asthma. Total indirect costs were £1587.27 (95% CI £1173.92 to £2000.62) and £1707.10 (95% CI £1200.93 to £2213.28) for the standard-care group and intervention group, respectively.
| Item with cost | Mean (SD) value | Total (n) | |
|---|---|---|---|
| Intervention group | Standard-care group | ||
| Direct family costs (£) | 9.09 (18.25) | 10.41 (15.23) | 443 |
| Travel costs | 5.68 (14.55) | 7.03 (13.90) | 443 |
| Out-of-pocket costs | 3.41 (9.16) | 3.38 (6.73) | 443 |
| Days displaced (participant) | 23.48 (37.05) | 26.12 (32.38) | 443 |
| School | 22.54 (36.35) | 24.96 (31.65) | 443 |
| Paid employment | 0.94 (4.21) | 1.16 (4.31) | 443 |
| Hours displaced (adults) | 116.82 (306.32) | 110.61 (216.66) | 443 |
| Paid employment | 53.51 (128.65) | 50.10 (115.92) | 443 |
| Unpaid work | 45.13 (189.42) | 38.78 (111.13) | 443 |
| Leisure | 13.84 (80.98) | 17.09 (86.36) | 443 |
| Study | 4.33 (27.56) | 4.64 (37.33) | 443 |
| Indirect costs (£) (participant) | 32.68 (146.55) | 40.52 (149.93) | 443 |
| Paid employment | 32.68 (146.55) | 40.52 (149.93) | 443 |
| Indirect costs (£) (parentsa) | 1674.42 (3877.35) | 1546.75 (3012.49) | 443 |
| Paid employment | 1062.77 (2555.00) | 995.05 (2302.22) | 443 |
| Unpaid work | 542.02 (2274.93) | 465.74 (1334.72) | 443 |
| Leisure | 69.63 (406.92) | 85.96 (434.38) | 443 |
| Total indirect costs | 1707.10 (3895.97) | 1587.27 (3060.39) | 443 |
Exacerbation cost
The mean cost per exacerbation and health-care contact was comparable between the treatment groups: £291.32 (95% CI £207.07 to £375.57) and £302.26 (95% CI £187.19 to £417.32) for the standard-care group and intervention group, respectively. The mean cost per exacerbation pooled across treatment groups was £296.78 (95% CI £225.76 to £367.79). The majority of the cost was attributable to hospital inpatient attendances, followed by GP contacts. Full information on the components of this cost can be found in Table 36.
| Item with cost | Intervention group | Standard-care group |
|---|---|---|
| Cost category | ||
| Number of children with at least one exacerbation (n) | 123 | 129 |
| Number of exacerbations per participant among those having at least one exacerbation, mean (SD) | 2.07 (1.39) | 1.98 (1.34) |
| Mean cost per exacerbation (£) (SD) | 302.26 (920.00) | 291.32 (675.00) |
| Total exacerbations (n) | 248 | 249 |
| Primary care (£) | 44.66 (62.89) | 38.91 (35.48) |
| GP | 32.66 (35.04) | 29.82 (£31.34) |
| Nurse | 2.14 (8.87) | 1.93 (7.47) |
| NHS 24/111 | 0.94 (4.42) | 1.20 (5.39) |
| Out-of-hours GP service | 7.76 (50.13) | 3.57 (17.22) |
| Walk in | 1.11 (8.15) | 2.39 (10.98) |
| Pharmacist | 0.06 (0.61) | 0 (0) |
| Secondary care (£) | 256.07 (924.53) | 250.64 (682.49) |
| Emergency department | 19.31 (46.95) | 26.17 (59.33) |
| Hospital outpatient | 4.37 (26.61) | 4.04 (26.01) |
| Hospital inpatient | 229.77 (928.79) | 210.78 (684.05) |
| Day case | 1.59 (25.01) | 1.58 (24.96) |
| Ambulance | 1.04 (16.32) | 8.06 (49.07) |
| Medication (£) | 1.53 (1.93) | 1.77 (1.98) |
The mean number of health service contacts associated with an exacerbation was 1.49 (SD 1.26) and 1.47 (SD 1.34) in the standard-care group and the intervention group, respectively. Table 37 shows a further breakdown of the components of health service contacts associated with exacerbations. Just 46 exacerbations were treated with OCs in the home using treatment provided for domestic use and did not require health service contact. Most participants required the care of their GP; the mean number of GP contacts was 0.79 (SD 0.87) and 0.84 (SD 0.89) in the standard-care group and intervention group, respectively. The mean number of secondary care contacts was 0.40 (SD 0.61) and 0.31 (SD 0.49) in the standard-care group and the intervention group, respectively, the majority of which comprised emergency department visits [0.20 (SD: 0.45) and 0.15 (SD: 0.35), respectively].
| Individual cost item | Intervention group | Standard-care group |
|---|---|---|
| Total exacerbationsa (n) | 248 | 249 |
| Exacerbations with no health-care contact (n) | 26 | 20 |
| Number of contacts with the health service, mean (SD) | 1.47 (1.34) | 1.49 (1.26) |
| Primary care | 1.16 (1.30) | 1.09 (1.16) |
| GP | 0.84 (0.89) | 0.79 (0.87) |
| Nurse | 0.11 (0.48) | 0.11 (0.43) |
| NHS 24/111 | 0.07 (0.34) | 0.09 (0.42) |
| Out-of-hours GP service | 0.10 (0.68) | 0.05 (0.23) |
| Walk in | 0.02 (0.18) | 0.05 (0.24) |
| Pharmacist | 0.01 (0.09) | 0.00 (0.00) |
| Secondary care | 0.31 (0.49) | 0.40 (0.61) |
| Emergency department | 0.15 (0.35) | 0.20 (0.45) |
| Hospital outpatient | 0.04 (0.19) | 0.03 (0.17) |
| Hospital inpatient | 0.12 (0.33) | 0.14 (0.38) |
| Day case | 0.00 (0.06) | 0.00 (0.06) |
| Ambulance | 0.00 (0.06) | 0.03 (0.20) |
The multilevel random effect model found no statistically significant difference between the treatment groups. After adjusting for minimisation covariates, the estimated difference in cost per exacerbation for the intervention compared with standard care was –£9.39 (95% CI–£200.60 to £181.82; p = 0.92).
Incremental adjusted costs
Table 38 shows the results of the GLM, in which the costs have been adjusted for the minimisation covariates (age group, sex, asthma severity and centre number) and in which the base case is the symptom-only treatment group. Models for the background cost, direct participant cost and indirect productivity costs all required a two-part model because the data included a significant proportion of zero values. In these cases, a probit model was used for the first part. Columns 2 and 3 of Table 38 describe the family and link functions used in the GLM regression. After adjusting for minimisation covariates, all 12-month incremental costs used in the cost-effectiveness model were not statistically significant at the 5% level. Appendix 10, Table 56, presents the 12-month mean and incremental costs of all cost categories associated with the per-protocol subgroup; consistent with Table 38, incremental costs were not statistically significant at the 5% level.
| Cost category | GLM specification | Incremental cost (£) (intervention vs. standard care) | 95% CI (£) | p-value | |
|---|---|---|---|---|---|
| Family | Link | ||||
| Background cost | Gamma | Log | –23.43 | –82.29 to 35.43 | 0.44 |
| Direct participant cost | Gamma | Log | –1.34 | –3.99 to 1.31 | 0.32 |
| Indirect productivity | Gamma | Log | 87.54 | –458.70 to 633.77 | 0.75 |
| Prescribed treatment | Gamma | Log | 18.23 | –103.64 to 140.09 | 0.77 |
| Adherence adjusted treatment | Gamma | Log | 5.37 | –100.65 to 111.40 | 0.92 |
Cost-effectiveness analysis results
Table 39 presents the probabilistic results of the base-case cost-effectiveness analysis. Although the expected cost and QALYs are slightly higher in the intervention group, the differences are very small (close to zero). Considering the uncertainty surrounding the expected differences, Figure 15 shows the joint difference in cost and effect from 10,000 probabilistic simulations plotted on the incremental cost-effectiveness plane. It can be noted that the probabilistic points are scattered quite equally around the origin of the plane, representing zero difference in costs (y-axis) and zero difference in effects (x-axis). Thus, the probability of the intervention offering a cost-effective approach compared with standard symptom-guided treatment never rises higher than 49%, irrespective of the monetary value placed on a QALY (Figure 16). The potential viability of the intervention is negatively influenced by the cost of FeNO testing. Where the throughput of FeNO testing was assumed to be between 60 and 300 tests per year, the probability of the intervention being cost-effective decreases to 34% (see Appendix 10, Table 57). Should the pooled cost of asthma exacerbations be utilised in the model, the probability of the intervention being cost-effective reduces to 41% (see Appendix 10, Table 57).
| Strategya | Cost (£) | Δ Cost (£) | Number of exacerbations (n) | Δ Exacerbations (n) | QALYs | Δ QALYs | Incremental cost per exacerbation avoided (£) | Incremental cost per QALY gained (£) | Probability cost-effective at | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| £13,000 | £20,000 | £30,000 | |||||||||
| Standard care | 2007 | – | 1.03 | – | 0.95 | – | – | – | 0.52 | 0.52 | 0.53 |
| Intervention | 2014 | 7 | 1.02 | –0.01 | 0.95 | 0.00 | –709 | 165,239 | 0.49 | 0.49 | 0.49 |
FIGURE 15.
Incremental cost-effectiveness scatterplot (intervention vs. standard care).
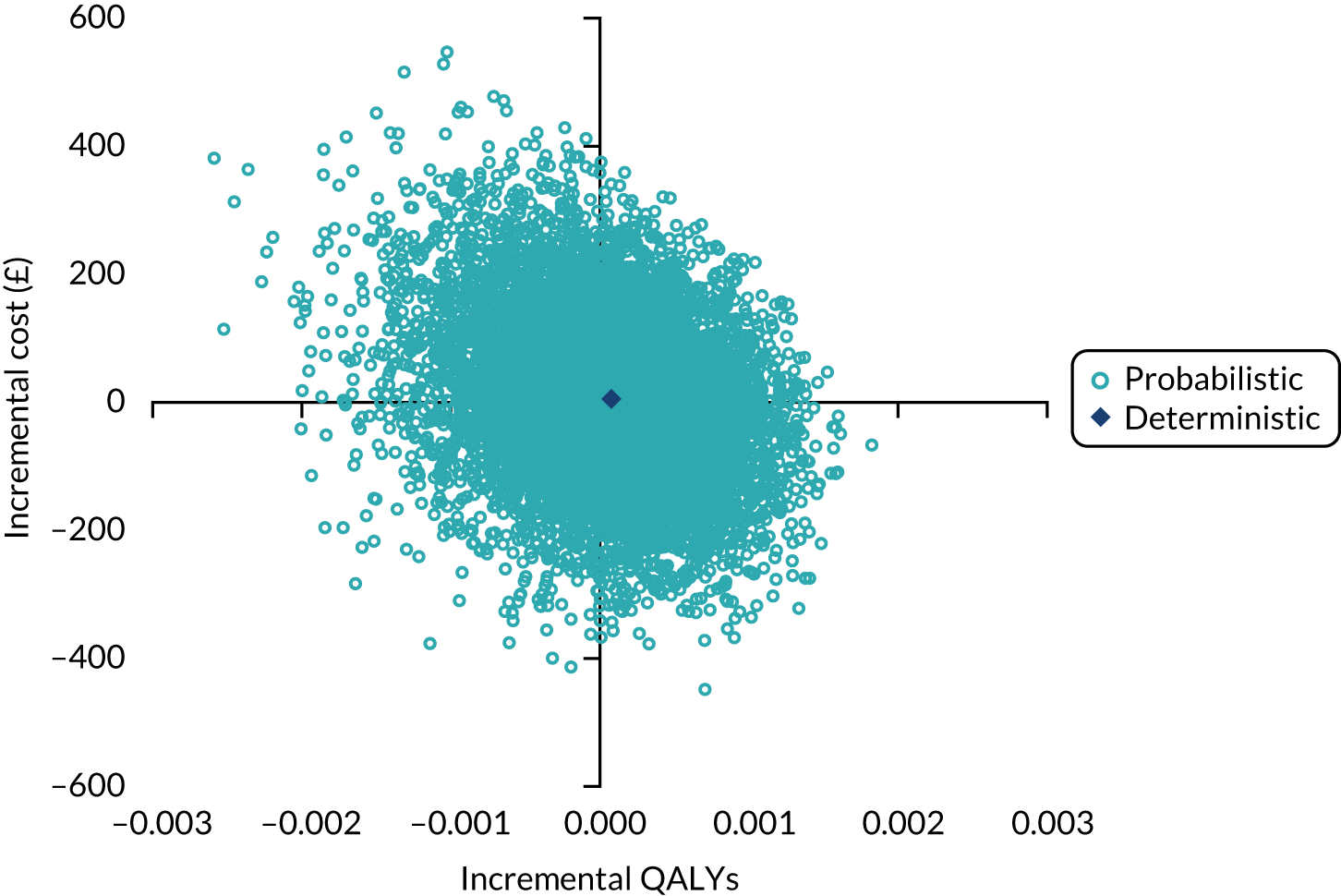
FIGURE 16.
Cost-effectiveness acceptability curve (intervention vs. standard care).

Discussion
The results of the economic analysis indicate no statistically significant difference in any category of cost over the follow-up period of the trial, which is consistent with the lack of effect on asthma exacerbations. Correspondingly, the model-based cost-effectiveness analysis found no meaningful difference in expected costs or QALYs between the treatment groups, with the simulated differences close to zero. Considering the uncertainty around the estimated differences, the probability of FeNO plus symptom-guided treatment (intervention) being cost-effective compared with standard care remained below 50%, regardless of willingness to pay per QALY gained. Furthermore, while there was no statistically significant difference in treatment, background or exacerbation-related costs, FeNO testing would result in a small increase in monitoring costs per participant, without a demonstrable benefit. Therefore, our cost-effectiveness findings based on data from RAACENO do not support the widespread adoption of FeNO testing for guiding treatment in children.
A strength of this economic analysis is that it is based on data from a large multicentre pragmatic RCT of participants from England and Scotland. Therefore, it can be assumed that the results have high internal validity and are generalisable across the NHS. The approach of RAACENO was detailed with respect to the collection of health-care utilisation data; for the observed exacerbations, a large number of complete resource use data were collected. This analysis considered only the short-term health-care costs of exacerbations treated with OCs. However, there is evidence that treatment with OCs, such as prednisolone, carries long-term health risks, such as growth impairment, pubertal disorders, hirsutism, diabetes, hypertension and many more. 86–89
A limitation of the economic analysis is the lack of health-related quality-of-life data for children experiencing exacerbations. Furthermore, previous evidence has suggested that hospitalisations may result in greater quality-of-life loss in children than in adults. 82 However, the exacerbation rate and the proportion of exacerbations requiring hospitalisation were not found to be different between the treatment groups. Therefore, it is unlikely that the uncertainties surrounding these point estimates of utility decrements would have an impact on the cost-effectiveness results. Another potential weakness relates to the fact that no direct data were collected on the severity or duration of exacerbations in RAACENO, and these factors may affect the extent of QALY losses associated with exacerbations. 82,90 Nevertheless, it may be assumed that there was no meaningful difference in the severity of exacerbations between treatment groups in RAACENO, given that there was no meaningful or statistically significant difference in the resource use required to manage them. Another limitation to be considered is that the frequency of monitoring appointments within the RAACENO trial was greater than in NHS clinical practice: four appointments per year compared with two appointments per year. Therefore, there is a risk that we have captured protocol-induced costs and effects in this analysis. However, given that all results were not statistically significant, we are confident that it would not affect our conclusions. Finally, we did not incorporate asthma-related mortality risk into our analysis. Although asthma exacerbations can be potentially life-threating, no death events occurred during trial follow-up. Furthermore, given the short model time horizon of 1 year, it was determined that such an inclusion would have a negligible, if any, impact on the results.
A previous Health Technology Assessment report,49 which included a systematic review and economic modelling study to assess the clinical effectiveness and cost-effectiveness of using FeNO for the diagnosis and management of asthma in children, indicated the need for further cost-effectiveness evidence using data with a follow-up of at least 1 year. This highlighted the importance of the RAACENO trial. Based on a review of efficacy data from eight earlier studies, the report49 found that asthma management using FeNO and symptoms could be expected to produce a modest reduction in exacerbations in children over a lifetime horizon, compared with treatment guided by symptoms alone. Correspondingly, the health economic modelling suggested that FeNO testing would be expected to produce a small QALY gain compared with standard management for an increased cost, which is mostly due to an increase in the use of ICs. The RAACENO trial data did not provide support for a meaningful reduction in exacerbations or the use of preventative treatments, and consequently our economic evaluation results are not consistent with those of this previous Health Technology Assessment report. 49 Owing to the flat findings at 12 months, we did not extend the time horizon of the health economic model for RAACENO beyond the time horizon of the trial.
Another more recent economic analysis,50 which was carried out alongside a RCT of 268 children aged between 4 and 18 years in the Netherlands, also compared FeNO plus standard care with standard care alone over a period of 12 months. The standard care was based on the BTS guidelines for a step-up or step-down treatment approach based on the child’s symptoms. 17,55,91 Similar to RAACENO, this study found no statistically significant differences in QALYs or total health-care costs between the standard-care group and the FeNO plus standard-care group. 50
In conclusion, the economic evaluation based on RAACENO participant-level data does not provide evidence to support the cost-effectiveness of treatment guided by FeNO plus symptoms compared with treatment guided by symptoms alone in children with asthma who are at risk of an asthma exacerbation. No statistically significant or meaningful differences in health service costs, direct participant costs or indirect costs to society were identified.
Chapter 6 Qualitative process evaluation
Introduction
The UK Medical Research Council outlines several recommendations for the evaluation of complex interventions, which include the use of both quantitative methods, to assess effectiveness and cost-effectiveness, and qualitative methods, to understand processes of change. 92 The document offers guidance on how these should be applied before, during and after clinical trials. Qualitative research is usually conducted prior to the commencement of a trial to assess the feasibility and acceptability of the intervention and protocol. This pre-trial approach provides an opportunity to develop solutions designed to address potential barriers to and facilitators of various aspects of the trial before its implementation. Qualitative research can also be used in a process evaluation during and after a trial to understand change processes through exploring the experiences of those delivering and participating in a study, as well as the acceptability of the intervention. 64 Process evaluations are usually aligned with a ‘theory of change’ or ‘programme theory’, or use a logic model. 64
Prior to the RAACENO trial, no qualitative work was undertaken by the study team to establish the feasibility and acceptability of the intervention. Although the FeNO aspect of the intervention has a strong evidence base,43 the introduction of algorithms and adherence monitors into childhood asthma management was novel; however, some studies had indicated their feasibility and acceptability. 53–55 Given that the combination of the intervention ingredients was new in this population and condition, we undertook qualitative research during the trial with those delivering and participating in our study. Our ‘theory of change’ was underpinned by the hypothesis that a reduction in asthma exacerbations in children could be achieved by using FeNO as a biomarker to inform treatment strategy, as per the main trial protocol. 47
The qualitative process evaluation described in this chapter was conducted concurrently with the RAACENO trial to explore key aspects of staff and patient experiences, as well as the acceptability of the intervention ingredients together and in context. We explicitly focused on perspectives around the use of (1) FeNO, together with (2) algorithm-generated treatment recommendations plus clinical decision-making for medication prescription, and (3) the smart inhalers utilised to monitor medication adherence. We interviewed trial staff to explore health-care decision-making based on the intervention components and beliefs about their benefits for asthma management among children. We interviewed trial participants (patients and their parents) to elicit their experiences and views of the intervention components in determining and monitoring their treatment, including perceptions of effectiveness.
Aim and objectives
The overarching aim of this component of the project, outlined within the grant application and published protocol,47 was to undertake a qualitative process evaluation to explore staff and family experiences and the acceptability of the intervention.
The specific objectives were to:
-
garner views on the RAACENO intervention from a range of trial staff representing a number of roles and different sites to understand intervention delivery from provider perspectives and to access any additional observations made around acceptability from their perspectives and their impressions of their patients’ views
-
solicit in-depth feedback on the process of taking part in this trial from the perspectives of the parents and children who participated, as well as their views on the RAACENO intervention
-
synthesise our findings and report them as a process evaluation alongside the main trial outcomes.
Methods
Process evaluation design
This was a primary qualitative process evaluation that used semistructured interviews93 with trial staff and families (children with their parents).
Two separate topic guides were used for interviews with trial staff and families (see Appendix 11). These were designed and developed by two researchers (HMM and DB). Prior to conducting the interviews, one researcher (LL) conducted practice face-to-face interviews with the chief investigator (ST) and a RN from the Aberdeen site to elicit feedback regarding the clarity of the questions presented in the topic guide for trial staff. A ‘role play’ practice interview with the trial manager (JW) was also conducted via Microsoft Teams® (Microsoft Corporation, Redmond, WA, USA) to gain feedback on the topic guide designed for use in the family interviews. Audio-recordings of the practice interviews were assessed by two researchers (LL and HMM) who adjusted the order of the questions presented within both topic guides based on the content of the audio-recordings and in response to feedback received from the (practice) interviewees. An iterative approach was adopted to revising the topic guides throughout the qualitative process evaluation and the staff topic guide was subsequently refined in response to review of non-compliance data (see Chapter 4).
Research questions
Our key research questions were:
-
What were the experiences of staff and families delivering and taking part in the RAACENO trial?
-
How acceptable was the RAACENO intervention for staff and families?
Specific sub-questions were:
-
What were staff and families’ experiences of and views on the use of FeNO measurements?
-
What were staff and families’ experiences of and views on the use of algorithm-generated treatment recommendations plus clinical decision-making for medication prescription?
-
We were especially interested in probing staff views about not applying the algorithm’s recommendation, particularly around confidence in the algorithm, especially over time, and ‘intelligent non-concordance’ to the FeNO-informed algorithm – that is, a reluctance to increase treatment if the participant was already prescribed a high level of treatment or a reluctance to decrease treatment if the participant was on a low level of treatment (or stopping treatment was recommended). 47
-
-
What are staff and families’ experiences of and views on the smart inhalers utilised to monitor medication adherence?
-
An additional question was:
-
Could and should the RAACENO intervention apply to usual childhood asthma care in the future?
Table 40 summarises the topics and areas of exploration.
| Interview type | Topic | Areas of exploration | Mapped Research Question |
|---|---|---|---|
| Staff | Hosting the RAACENO trial | Aspects of the site’s people and culture that facilitated the conduct of the trial, such as experience, teamwork and materials, as well as interactions with the trial office | 1 |
| Recruitment/randomisation/retention | Factors that influenced trial recruitment and modes of undertaking/approaches to recruitment, including randomisation and retention | 1 | |
| Intervention | Experiences and acceptability of using FeNO, and the algorithm and smart inhalers in childhood asthma management | 1/2 | |
| Future | The feasibility of implementing RAACENO treatment procedures into standard care | 3 | |
| Families | Taking part in the RAACENO trial | General experiences of and views on participation | 1 |
| Recruitment/randomisation/retention | Factors that influenced the decision to participate in the RAACENO trial, including recruitment, understandings of the concept of randomisation in relation to participation in the trial, family perceptions of ‘burden’ of clinic appointments and the requirements of taking part | 1 | |
| Intervention | Experiences and acceptability of using FeNO, and the algorithm and smart inhalers in childhood asthma management | 1/2 | |
| Future | The feasibility of implementing RAACENO treatment procedures into standard care | 3 |
Sampling and recruitment
Staff
We initially aimed to sample around five trial staff for the qualitative process evaluation, which was based on recommendations from the literature94 and our judgement and experience of conducting qualitative health research. Once the study began, however, we increased this in response to informal feedback and conversations with sites to include between 15 and 20 trial staff in our sample, as we considered that there was a diversity and richness of experiences and insights to be gleaned from staff at different sites and performing different roles. Those with and without previous expertise in paediatric or respiratory medicine and/or delivering clinical trials were included to maximise variety in the sample and gain insights into whether respiratory and/or research experience affected perspectives. Prior to the COVID-19 pandemic,95 e-mail invites were distributed by the trial manager (JW) to a purposive sample of staff members who occupied various roles within the trial (e.g. RNs, consultants and PIs) across 10 sites: Brighton, Edinburgh, Harrogate, King’s College London, Leicester, Nottingham, St George’s London, Stoke-on-Trent, Walsall and Wolverhampton. These sites were selected to obtain interview data from staff who worked with different levels of recruitment to the RAACENO trial (smaller vs. larger numbers) and based on trends that demonstrated the sites associated with low/high compliance to algorithm recommendations. However, owing to the COVID-19 pandemic and the resulting low uptake of individuals responding to our initial invite at the time, we elected to adopt a convenience sampling method to improve recruitment figures. Reminder invites were subsequently distributed along with new invites to the following sites: Barts Health London, Exeter and Shropshire. Interviews with trial staff who agreed to participate were arranged and conducted by a researcher (LL) who had no previous correspondence with them and who introduced herself as a qualitative researcher with no involvement in clinical data collection.
Families
We aimed to sample up to 20 families for the qualitative process evaluation, within both the intervention group and the control group, based on recommendations in the qualitative literature94 and our judgement and experience of conducting qualitative health research. Families were first approached and invited by RNs across seven trial sites, located in Aberdeen, Edinburgh, Leicester, Nottingham, Stoke-on-Trent, Walsall and Wolverhampton. Site selection was initially based on recruitment levels at sites and the convenience of accessing the hospitals for face-to-face interviews based on geographical location (prior to the requirement of remote working owing to the COVID-19 pandemic). Thereafter, potential interviewees identified by RNs as interested in being interviewed were followed up by one researcher (HMM) by telephone during the course of the study and were then approached again by the RNs at their 6-, 9- or 12-month assessments to ask whether or not they would still be happy to be interviewed following their 9-month (penultimate) or 12-month (final) assessment. Qualitative interviews with families who still agreed to participate were arranged and conducted by a researcher (LL) who had no previous correspondence with them and who introduced herself as a qualitative researcher with no involvement in clinical data collection.
Recruitment was planned to continue for both trial staff and families until data saturation was reached and no new data would be collected.
Data collection
Study settings
One-off interviews were initially designed to take place either face to face or by telephone. Owing to COVID-19 restrictions, one researcher (LL) conducted all interviews via telephone, with one parent and child pair or one staff member being interviewed at a time. The researcher (LL) contacted and interviewed the first two staff interviewees from an office within the University of Aberdeen, whereas the remaining staff were contacted and subsequently interviewed from the researcher’s home. Staff interviewees’ settings varied: trial staff were interviewed from either their workplace or their home environment on a one-to-one basis, whereas all families were interviewed while they were at home. Family interviews involved parents and their children participating together, as agreed with the interviewer (LL) in advance.
Written consent was obtained for the first three interviews conducted with trial staff. Verbal consent was obtained for the remaining interviews with trial staff and all interviews with families (recorded and transcribed) because the trial team agreed that this would be the most appropriate method for recording consent during the COVID-19 pandemic. The sponsor reviewed this change on 26 March 2020 and indicated that it was a minor amendment to safeguard participants during the outbreak of COVID-19 at that time, and that it did not need to be reviewed by other review bodies and could be implemented immediately.
All interviews were recorded using an Olympus DS-9000 audio-recorder (Olympus, Tokyo, Japan) with an encryption function, and the audio files were transcribed verbatim by a professional, approved external service. All file sharing with the external service and between members was undertaken using secure and password-protected data transfer software.
Data analysis
A thematic approach was used to analyse transcripts from the qualitative interviews within and across cases, and data management was assisted by Microsoft Excel® and Microsoft Word®. 96 Initially, one researcher (LL) identified key themes and categories by listening to the interview audio files and reading the associated transcripts. A second researcher (HMM) independently conducted a more granular analysis by reviewing the transcripts line-by-line and generating common themes representing topical ideas that were identified within the data. Both researchers (LL and HMM) met to discuss the themes that resulted from these independent analyses and agreed on the key findings from within the data. Key themes are summarised in Table 40. One researcher (HMM) led the write-up of the results with input from a second researcher (LL) to ensure that the interpretation of the findings aligned with their combined analysis. Notes taken during interviews by the interviewer (LL) were referred to during analysis. Transcripts were not returned to participants for checking. Consistency between the data presented and the findings was verified by the wider RAACENO team and advisory group.
The reporting of this qualitative process evaluation complies with the Consolidated Criteria for Reporting Qualitative Studies checklist. 97
Findings
Sample
Staff
We interviewed 15 trial staff members in total after 18 had initially responded, with three lost to follow-up (one due to clinical pandemic response pressures and two due to receiving no reply). Data saturation became evident following the first six interviews. 98 However, we elected to continue data collection to ensure that we obtained a more representative sample of staff occupying various roles within the trial and at different sites, and, therefore, to achieve the understanding required for this study. 99,100 Sample characteristics are provided in Table 41. These interviews lasted between 18 and 34 minutes.
| Site ID | Interviewed (n)a | Roles | Staff identifiersb |
|---|---|---|---|
| 1 | 2 |
|
1CT1, 1RN2 |
| 2 | 2 |
|
2RN1, 2RN2 |
| 3 | 1 |
|
3CT1 |
| 4 | 2 |
|
4RN1, 4CT2 |
| 5 | 2 |
|
5RN1, 5RN2 |
| 6 | 3 |
|
6RN1, 6CT2, 6RN3 |
| 7 | 1 |
|
7RN1 |
| 8 | 1 |
|
8RN1 |
| 9 | 1 |
|
9RN1 |
Where direct quotes are presented below, we have used the staff identifier noted above to attribute data to the individuals who took part in the qualitative process evaluation, while preserving their anonymity.
Families
In total, six families were interviewed, involving 12 people (six parent and child pairs, with all parents being the child’s mother; Table 42). Seventeen families initially volunteered across six sites, but 11 did not respond to subsequent follow-up contacts that took place during the COVID-19 pandemic. All families who participated in the qualitative process evaluation were participants who had been assigned to the intervention group of the trial (no interviews were conducted with families randomised to the control group). After four interviews, no new themes emerged. Subsequent interviews confirmed these themes and it was agreed that data saturation had been reached. 98 The children’s and young adults’ ages ranged from 12 to 15 years. The parents provided more in-depth responses to interview questions overall compared with the children/young adults. However, children/young adults provided more input in response to the questions that were explicitly tailored to them (e.g. ‘What’s it like for you to have asthma?’). These interviews lasted between 28 and 45 minutes.
| Site ID | Interviewed (n) | Study numbers |
|---|---|---|
| 3 | 2 | 26–1, 26–2 |
| 8 | 1 | 23–1 |
| 10 | 1 | 11–1 |
| 11 | 1 | 22–1 |
| 12 | 1 | 17–1 |
Where direct quotes are presented below, we have used the study number of the trial participant to attribute data to the respective families who took part in the qualitative process evaluation, while preserving their anonymity.
Data
Staff
Levels of experience: trials/research
We sampled staff across trial sites who represented different levels of experience of running trials with children and on childhood asthma, including those with previous or no experience of using FeNO in clinical practice. For example, our sample included a children’s RN doing most of the work around the trial (9RN1); someone who described themselves as quite inexperienced and completely new to asthma research trials (6RN1); someone who considered themselves to be experienced in trials research, but who had not previously conducted trials with children (5RN1); someone who was experienced in research with adults and children, from observational through to RCTs (9RN1); and someone with years of FeNO research and whose opinion had not changed: they considered it to be a helpful measure, but not the ultimate measure in children (6CT2).
Some sites were well established and experienced in running trials like RAACENO and interviewees reflected on how having an established team working together was conducive to running the trial. Having an established team locally was perceived to facilitate both recruitment and retention, for instance:
I think the collaboration between myself and [5RN2], who’s the respiratory asthma nurse, well, I think it’s worked really well. Yeah, I think it’s been really good. You know, we’ve been a good [inaudible] and I think the families have appreciated the collaboration there. Might have been more difficult for me to have done the respiratory side of it without her input because they already have a relationship with her, so I think that’s what has helped.
5RN1
Hosting the RAACENO trial
Trial staff were asked about their experiences of hosting the trial and running it at their sites. Feedback was positive in terms of setting up and the support and engagement received from the Aberdeen trial office.
The staff liked the setting-up phase and support of the trial team (9RN1, 1CT1, 8RN1, 7RN1, 6RN1, 6RN3, 5RN1, 2RN1, 2RN2) and having images of staff on newsletters (8RN1), and the involvement of the chief investigator (ST) in meetings was valued (8RN1, 4RN1). In addition, the technical and support aspects and doing everything online was considered useful (9RN1), although sometimes did not work (6RN3).
One interviewee commented on problems within previous asthma research teams ‘. . . where they’ve . . . had a poor understanding of the real world and felt unrealistic’ and said that this ‘was a very difficult team to try and work with’. In contrast, the RAACENO team was not difficult. The interviewee said ‘I’ve had no issues, I thought they were very good. It was a good study’ (5RN1).
Staff reported other positive experiences relating to the effectiveness of the study materials:
The information sheets were really good and the one for the different age groups of children were very good. So, we would make sure they’d had that, and they’d read it fully, and if they hadn’t read it, we would read it with them when they came so that as we went through it all we just covered that part of it and everything else so that they could ask questions.
4RN1
One staff interviewee indicated that they liked doing something with patients, not just collecting data on them but following the patient’s journey and establishing a rapport with the family:
When I see the clinic list and I know they’re about, I’m like, sometimes, I’m like, I might just pop and say hello anyway, because you know, you’ve spent that time with them and you feel like you’ve been on that journey with them a bit, so it’s really nice.
9RN1
One PI saw their role as very technical in implementing RAACENO, but this staff member also said that the work was not too onerous compared with the usual trial workload:
I enjoyed it more because I didn’t have to run it in the first instance. I think it’s been very fluid; I think it’s been very well supported both from the central trial management team and also from the . . . from our own local team. Yeah, and that’s really all that I would say. I’m looking forward to the results.
1CT1
Recruitment, randomisation and retention
Recruitment
As part of understanding the acceptability of the intervention, we were keen to explicitly explore staff experiences of recruiting to the RAACENO study. Staff commented on several aspects.
Staff perceived that good teamwork influenced recruitment success. It was felt that the information was clear and easy to explain (9RN1), and staff perceived that they were able to explain the study well as a result of the parental/patient response (9RN1, 1CT1):
Generally, yes, with regards to the study, the information sheets are quite clear and it’s not a difficult study to explain what we’re trying to look at and what it actually does. In terms of questions about the nature of the study, they’ve been quite limited really, or maybe we’ve just explained it quite well.
Yes, could be, it sounds like it, for sure. The information leaflets were quite clear, were they?
Yes, they were very clear. They were absolutely fine. Yes. Like I say, most of the people were . . . I mean, we didn’t have any that didn’t want to go in it of the people that we approached that were suitable, so that must speak volumes about the information sheet really, because it gave clear and concise information.
9RN1
It was considered to be easy to take part in the RAACENO study because there were not too many more appointments than usual (although there were some parental concerns, but these were mostly about missing school) (8RN1, 4CT2, 2RN1). Positive feedback was reported from parents about taking part/making a difference (9RN1).
The main concerns expressed by parents to staff were related to the smart inhaler (6CT2), technique (9RN1) and individual measurements:
Sometimes, they weren’t even related to the study questions as such? They’d be asking things like what the height is; what the weight is; what the spirometry score was. They wanted to know about the smart inhaler percentage. But relating to when we did the recruitment of it, they’re asking more things like, how many visits they’ll be attending and how long the visits would actually take. That was important to them. Parents are wanting to come in school holidays because of the time off school; they didn’t want them to be taking a lot of time off school.
8RN1
One member of staff did question whether or not inclusion was broad enough to capture ‘deviant’ cases:
In terms of the practicalities of it, it’s very easy to recruit for because the inclusion and exclusion criteria were not too stringent. The flip side of that is I genuinely believe that there’s an awful lot of ‘asthma’ that is not complex in that and I don’t think that the inclusion criteria, or exclusion criteria, are sufficiently robust that excludes some of those kids that will throw your data, potentially throw your data out, but that’s just me.
1CT1
Randomisation
We were interested in asking questions about how randomisation was explained to potential trial participants, and for staff to reflect on how this may have affected recruitment and the acceptability of the trial.
Some staff reported that there were no questions about randomisation (8RN1). However, the intervention and control allocations were both deemed acceptable and easy to explain, especially because there was no placebo and both sets of outcomes were considered important (5RN1). Others noted parental concerns around randomisation or what the numbers meant:
I’d say maybe half had a clear understanding of what randomisation meant, and maybe the other half didn’t understand or didn’t, hadn’t actually thought about the process and the concept of that. I kind of broke it down as it’s random and that’s the key word from randomisation. It’s completely random and we don’t know, and I think the computer doesn’t know until the last minute, because it’s random, it doesn’t take anything into account, really, it just allocates something to you and that was the only way I could really explain it in very basic terms.
9RN1
One site provided some thorough descriptions of how they explained the randomisation process:
I would explain to them as they might be . . . will be randomised to two or might be one column, might be randomised to one of the two arms, the control or the experimental one. The experimental one is the FeNO-guided one. Where the doctor might use the FeNO score alongside his assessment and the other one is the controlled group when the doctor will be blinded to the FeNO score so the doctor will just give the treatment or prescription based on his assessment.
6RN1
Like with most randomisations we would always say to the patients that it would be done by computer program if it was and it would basically, we wouldn’t be able to choose what group you’d go into. And it would be up to chance and that we wouldn’t know.
6RN3
Another site also described the process in detail:
We go through the program on the computer with the family, so she always says . . . Right, this is the moment where we’re going to find out what arm of the trial you’d be on, and they do get full instructions at the beginning and the leaflets are sent out with lots of e-mails backwards and forwards . . . They do understand that from the beginning and then we do, she does make a point when they come in for their reviews, what . . . because I get confused which arm each one’s on, so whether it’s FeNO guided, or not. So, yes, yes . . . We’ve been lucky because some of the ones that were FeNO guided, we wanted them to be FeNO guided, that just was luck, really, at the beginning.
5RN2
Some sites paid specific attention to explaining the study and randomisation to children:
The kids it would be . . . most, actually, were very, really quite onboard with the study and completely understood it when you explained it at a very child-friendly level. A bit like you would do a science experiment at school like where you’d pick groups, and it would be done fairly and randomly. The children I’d pitch it at that level, and then to parents, obviously at a higher level and talking about not being biased and that’s why it needs to be done randomly and usually by a computer.
2RN2
One staff participant reported that there seemed to be an understanding of randomisation (6CT2) and the concept of ‘tossing a coin’ (3CT1) was used.
Some sites experienced issues with misunderstanding later along the line:
Control group participants wanting to know what scores were even though they had agreed to be randomised to control and having to explain that over again.
9RN1
One site found that it was hard to explain the randomisation process:
I think that was a little bit hard to explain the fact that we weren’t using the FeNO, even though we were measuring it. So, in a way I think most families forget that it was a randomisation to be honest, because it didn’t make any difference.
3CT1
Retention
Although we did not capture specific data on retention to ascertain acceptability, one site commented on this and suggested that the main issues regarding withdrawals were family related rather than trial or intervention related, for example separated parents or parental illness:
Then any of the follow-ups that haven’t been able to attend have been due to either family issues, if we’ve tried to rebook it and they haven’t made it, so I’ve done it by telephone. I did get the data that was needed over the phone, and that was only two of them. Both were family issues really, one’s dad’s got terminal cancer and [inaudible] been up and down to [location] so it’s been really difficult for her. But we got to see them at their last appointment but we didn’t get to see them at 9 months.
5RN1
Intervention
Staff feedback was positive in terms of the overall intervention package and one described a feeling of working towards something that is of real benefit to patients (9RN1).
Staff felt that families who agreed to take part liked the extra contact and attention that they received through participating in the study (5RN1, 4RN1, 2RN2 and 1CT1). They described additional benefits for families, which were educational and confidence building (4RN1, 3CT1 and 4CT2), and the feeling that families had of being special as recipients of additional medical attention:
Families, they don’t like it when it comes to an end because they enjoy when they come for a visit, they get to have one to one care. They get a longer time with the clinician than they would do otherwise, so they get more of their questions answered and I think it makes them feel sort of quite special, so I think in that sense it’s been a popular study and it’s been easy to run, I think the kind of data entry side of things seems to be working well.
3CT1
Fractional exhaled nitric oxide
We probed about the experiences and acceptability of the FeNO measure within the context of the RAACENO intervention. We discovered a range of experiences and views among our trial staff. Some had not heard of FeNO before the trial (9RN1 and 2RN1) or had not used it because they had previously worked in another country (6RN1). Some were familiar with FeNO (5RN1) or even used FeNO regularly (7RN1).
Another trial staff member had heard of FeNO, but thought that it was well researched and accepted that FeNO is helpful to identify cases where other measures look normal but where things are not (1CT1). Another suggested that it should give definitive evidence (8RN1), while another said it was helpful for building a history and picture of children (9RN1) and another saw it as an additional tool, adding weight and power (9RN1). Other trial staff said that it was useful to see families every 3 months and to consider the season and how this may be affecting asthma (7RN1 and 4RN1).
Some expressed a strong belief in the value of FeNO:
There was one little boy who came to clinic recently and he’d completed research and his FeNO had always been very low during research and we’d reduced his treatment, but he was still on obviously some treatment, and then his mum went and stopped all his treatment after seeing the paediatrician, and then he came back for a normal review and his FeNO was significantly higher.
5RN1
The other thing that’s quite good about it is it’s not looking at individual’s absolute values, it’s looking at changes in the value in individuals, or the extent of change which is I think an appropriate approach.
4CT2
A perceived gap in current guidance was identified as a reason why FeNO (research) was needed:
Utility of FeNO measurements in the management of asthma and it’s needed because it explores the inconsistency between the BTS/SIGN and NICE asthma guidance, which do have significant gaps or differences that need to be addressed.
4CT2
Some sites’ staff reflected on how they communicated FeNO to parents:
I think what they just don’t understand mostly is the significance of FeNO and what does it do. So, I tell them that FeNO is will detect . . . if the score is high then perhaps it could be a sign of an impending exacerbation or an attack that we want to treat right away.
6RN1
I think it’s something that’s much more concrete, I think it does back-up what we would . . . we would hope it would anticipate that the FeNO does [inaudible] that the children are heading towards an exacerbation or they’re actually in a difficult time, more than I think if they don’t take their medication regularly.
5RN1
Several staff commented on how it was good to be able to show patients their data:
When they did come, I found that having the data and the algorithm output was useful, you could show the patient how much they’d been having the medicine and things like that, it was like having something tangible to show them, that was quite good.
6RN3
And I think, you know, the use of the statistics of what your physiology actually is saying to influence treatment . . . yeah, I think they were quite happy with that.
5RN1
Well, whatever you’ve said, here’s what the test shows, so this is what we’ve got to work on and here’s a number that you can improve on.
3CT1
One staff participant believed that the FeNO measure was lower because of better inhaler adherence and, therefore, thought that it was less useful as a biomarker because the change could be explained by the child’s adapted behaviour rather than a medical difference:
I think overall the FeNO has been less useful if you know what I mean? Because they’re taking their inhaler therefore their asthma has been better, I think almost all the patients have been better during the study, therefore the FeNO is less useful because it’s low and even if it’s raised if their asthma is better, there’s no advantage to increasing their treatment.
3CT1
Taking the FeNO measure was considered difficult with some age groups (8RN1), but this was not a commonly reported problem.
Algorithm
In exploring the experiences and acceptability of the algorithm’s role in the diagnostic and decision-making process, there were a range of considerations.
Familiarity with using algorithms in treatment was a factor: one staff member said that they had never used an algorithm before to determine treatment (4RN1). Another expressed a very clear interpretation of what should happen, suggesting that a ‘purist’ would follow the algorithm to the letter (9RN1). Staff seemed to express an open mind to using such an approach in clinical practice. Nevertheless, there were some caveats. For example, as long as it was considered ‘reasonable’ (8RN1) or ‘appropriate’ (6CT2), staff said that they would accept the recommendation; however, if not, staff explained how they would use it:
If you were stepping down as you went into the school return in September it made you more anxious if you knew that in historical years that they’d had asthma attacks at that time. So that did influence a minority of patients in terms of whether the algorithm was followed or not.
4CT2
You know, the algorithm is set, but obviously the algorithm is set but it doesn’t account [for] what time of year it is, what the child’s weaknesses are, whether they’ve got loads of allergies, if it’s spring time and the algorithm says, ‘Let’s step down’, and we all know that that’s going to be their trigger. So yes, you’re randomised, we have no control over it. Your algorithm will be influenced by the FeNO results as well as everything else, but in the end, the child’s well-being will always become paramount.
5RN1
There was especially concern if a step down was recommended in the context of continuing A&E visits and needing to look more closely (1CT1), or causing A&E visits through a step down that led to exacerbation (6CT2). Conversely, the use of the algorithm was considered to be beneficial when a step down meant that a patient could be returned to community care (7RN1). Others articulated a need for balance, for instance where being too cautious could be replaced with being less cautious through needing to trust, while also being safe (1CT1).
Internal ‘missing data’ problems and external contextual factors were also considered; for example, one staff interviewee who said that there may be a problem if the algorithm was not operating on a full data set owing to missing data (6RN1) also highlighted that data were missing elsewhere because a child was staying with their other parent and forgetting their smart inhaler (6CT2).
There were perceptions that people, both staff and families, are not accepting of ‘technology’ on its own:
It’s almost like the clinicians have in their mind what they’re going to do before I tell them what the algorithm wants them to do. Sometimes they’ll follow it and sometimes they don’t want to follow it, because they want . . . they’ve just done something and they want to see how that responds before they change it, or . . . it’s really difficult, because yes, I have found that with the clinicians, getting them to follow it can be a bit of a challenge.
9RN1
I think most families who were accepting of the algorithm but only with the proviso that it’s never just the technology and the algorithm, and the doctor can always over-ride that if necessary.
2RN2
Reassuring them that the computer’s not dictating to them what’s going to happen, that the doctor will overall decide what’s safe for them.
2RN1
Clinician ‘gut instinct’ was considered to be critical to applying the algorithm’s recommendation (6RN1) and the notion that understanding among trial participants was that the algorithm recommends, and the clinician decides, was highlighted (7RN1). Trust in the consultant and families accepting clinical judgement, whether or not this involved complying with the algorithm (9RN1), was raised. The notion of ‘computer says no’ and that algorithms are limited was raised, alongside the idea that patients and their families come to see an expert in whom they trust and with whom they have a personal relationship, which cannot be replaced by a computer and algorithm (3CT1).
Staff perceived that parents were sometimes reluctant to go ‘too low’ or alarmed about ‘how high’ (8RN1) the algorithm-recommended treatment seemed and, if a parent was unhappy about a step down, staff believed that their feelings should be taken into account (6CT2). One staff member said:
Yes, actually fairly frequently, we haven’t agreed with the algorithm. Well, for various reasons. Probably more often that parents have felt a bit more cautious when a step down is suggested.
2RN1
Research nurses checking compliance with the algorithm rather than consultants was noted by one site (1CT1) and not using the word ‘algorithm’ with families was highlighted at another (7RN1).
Ultimately, providing the best care was the main factor:
To them it was just like, at the end of the day, we’re going to give you the best care we can and the best treatment. As much as we’re going to, we want to stick to the algorithm when we can, so it was PI and [RN] who were the respiratory specialists knowing the children, so actually, no, we don’t want to step down, we’re heading into pollen season and this kid’s just going to be a nightmare. We had the flexibility with that.
5RN1
In terms of rates of agreement, sites indicated that they had different experiences. For example, at one site, there was an estimate of disagreeing about 20% of the time owing to the time of year and triggers (apprehensive about stepping down) (5RN1), whereas at another site it was suggested that there was disagreement about half of the time (4RN1).
One site perceived that the algorithm was ‘chopping and changing’ and preferred more settled treatment (9RN1), while another site considered that there were ‘too big jumps’ (8RN1). However, sites did express that they really liked having data:
I think what really I like about RAACENO is the smart data, there’s data on compliance with therapy, it’s really helpful and we can use that sometimes in our consultations with families to try and help improve compliance and also help us with decision making, so I think that’s helpful, yeah.
6CT2
One of our consultants, in particular, struggled with that a little bit, because you could have a child that would have . . . that would score very well on asthma control test because that’s obviously self-reported symptoms and actually we had a couple of children that are very used to living life feeling quite breathless with those symptoms, so and they [inaudible] they would always score quite high on that and their adherence would actually be excellent, so step downs were frequently suggested and when actually their lung function was decreasing, with those patients we had to override the algorithm.
2RN1
The need for GPs to have a better tool for use in primary care, that is using the algorithm, was noted (1CT1).
Smart inhalers
Overall, smart inhalers were perceived to be generally good, but with some technical problems in relation to recording/uploading data (9RN1, 8RN1, 7RN1, 6CT2, 5RN1, 4RN1 and 3CT1). Accuracy was questioned by one staff interviewee (3CT1). Disappointment was also reported if a score had to be disregarded:
In the beginning a few seemed to work, but towards the end I was disappointed not to be able to show the parents good results if they said their child was being compliant.
Oh, I see.
So we had to you know, to ignore the score even though the child . . . the child wanted to beat their last score and it could be disappointing . . .
2RN1
Smart inhalers were considered to be good for monitoring adherence (8RN1) and telling the truth or not (6RN1), as well as for setting targets:
There were more ideas associated with the smart devices, with the inhalers. They were really intrigued with these and really liked the idea of them, and then of course, whether it was me or I don’t know, but they seemed to struggle with some of them and the downloads, so they were a bit disappointed in that, as I was as well. I’m thinking, you know, it’s such a great idea and the concept of it for children, it’s quite visual. To me, it was really good, but unfortunately, I don’t know whether I just . . . but the ones that worked, worked really well. The ones that were downloaded, they were really happy with them and it gave them a focus. One of my early recruits, hers always worked amazing and her mum was really good at encouraging her and saying, ‘Right, we want the number higher next time, come on, we want better there’, you know? So really used it as a tool to encourage them. So, it was a bit disappointing probably for the families where I just couldn’t get the thing to download!
5RN1
The data were useful for facilitating ‘difficult conversations’: for example, helping to have conversations and improve adherence to see asthma under better control (3CT1); discussing when adherence had drifted off, for example during holidays (8RN1); and uncovering when a child had not been taking their medication with parents assuming that they were (7RN1). For some, improved adherence was reported initially, but this was not always sustained (6RN3, 4CT2), although the smart inhalers could be used as an incentive to get a score (5RN1, 4CT2 and 2RN1). For example:
I think the children enjoyed that game you know, they always tried to guess what it was going to be and then they tried to beat their score the next time, and they understood how to achieve that and what the significance that inflammation can mean in combination with their lung function test. It helped them to understand it a little bit better and the importance of using their inhaler as per what the doctor said.
2RN1
So, they like the fact that they think most people need help with remembering their treatment, so it’s kind of a help that’s not nagging, it’s not shouting up the stairs every morning from their parents, it’s a help that’s positively reinforcing because they can have a little bit of a target for a number to achieve and so on. It won’t work with everyone, but I think for a lot of young people they feel that benefit.
3CT1
Some were excited to see the results and their performance (6RN1), but it was identified as a potential reason for drop out of children who were not complying (7RN1). However, even when adherence was low, families did not seem to mind being monitored (6CT2), although one clinician did mention ‘big brother’:
I think there is a big brother factor, they get a bit anxious about us scrutinising how well they take their medicines. But having something that brings it to the forefront so that you can have an honest discussion I think is very, very valuable.
4CT2
Staff believed that adherence improved with smart inhalers (6CT2) and thought that smart inhalers for non-compliant children (9RN1) were ‘a bit of a game’, with technology to monitor adherence (7RN1) and to manage some patients (6RN3). However, it was acknowledged that some children would probably not take their inhalers properly even if monitored (6RN3); for example:
What you don’t want to be doing is to continually escalate doses down an algorithm when in fact the reality is, is they’re not taking their medicine at the required frequency.
4CT2
Staff perceived that parents wanted access to their children’s data so that they could monitor them (8RN1) and suggested that it would be better if there was an opportunity for instant download at home rather than in clinic (2RN1).
Factors that concerned staff around data capture, which are also highlighted above in terms of missing data affecting the algorithm’s recommendation, were weekends when children of separated parents go to the other parent and forget the smart device (6CT2 and 2RN1) and cut-off points/timings automated within the device:
The other thing that I found wasn’t so good is the way that it printed out the compliance data. So, if you’ve got a teenager that’s getting up at 1.00 in the afternoon, they’ll get up, they’ll take their inhaler and then they’ll go and do whatever, and then they’ll take it again, but that was saying ‘No’ for the morning dose because it had a cut off of 12.00 [noon]. So, what I would tend to do is go through it, even though the printout would say 50% adherence on that day, sometimes they were 100% adherence on that day, but it had picked up as because it was after 12.00 [noon].
6CT2
Otherwise, positive experiences were reported:
I think it’s quite nifty, it’s not heavy, it’s not bulky, it’s quite easy. So, I think yeah, it’s not too bad to use, it’s quite easy, yeah.
6CT2
In addition, the smart inhaler was also considered to be helpful in wider aspects of inhaler care, for example the expiry date and counter (8RN1).
Future
We asked staff to reflect on the potential and future of the RAACENO intervention after completing the trial. This was before the outcome results were known or shared. It was perceived that the intervention did make a difference and so could fit into the existing clinical model very easily (5RN1) and that the algorithm could be incorporated into usual care, with any decisions not to comply recorded, that is taking into account contextual factors (9RN1 and 1CT1) and with a safety net regarding seasonal changes included (8RN1). However, time was considered to be a factor in one clinic, as well as costs, for example, of lost equipment (6RN3). It was suggested that the intervention could be implemented in nurse-led clinics (1CT1) or as an additional step, which would be helpful in the future (6RN1).
Two suggestions were made by one staff member about how, specifically, the intervention might be used by more junior colleagues as a support and improved through the addition of an additional measure:
But if you have more junior respiratory trainees, I could see that an algorithm that supports what they are thinking might be of use.
2RN2
I mean I think if it could include lung function, I think that would be well worth it. That’s definitely been the biggest thing I think is missing from it.
2RN2
Scale up was perceived to be possible, but staff were unsure of the costs of this (6RN1, 5RN2 and 4CT2) and suggested that the intervention might be better targeted with selected families (5RN2 and 4CT2). Others considered that it might have the potential to reduce health-care demands and costs (3CT1 and 1RN2).
Overall, it was felt that it was really good to be able to use data in clinical care (6RN3) and some said that they would continue to use FeNO and other RAACENO measures, such as skin prick and quality of life (8RN1), in their clinics.
Regarding the technological possibilities, live harvesting of data [via Bluetooth® (Bluetooth Special Interest Group, Kirkland, WA, USA)] to see within-day adherence was discussed and was seen to be potentially useful (3CT1), and using technology with this age group was also seen to be useful:
Definitely, I think the way forward would be technology, apps, smart inhalers, that kind of thing. I know there are other ones out on the market . . . yeah, I think the way forward, especially for those children who are early teens and trying to gain their independence, smart technology is obviously the way to go.
7RN1
Yeah, I think so, especially the younger generation nowadays, they’re more adapted to new technologies so I think it is the way forward in terms of treatment and assessing their condition, it’s a very useful tool and most patients are well adapted to it now. It’s the age and date of technology also, I think it’s going to be a new standard of care.
1RN2
Before revealing the outcomes of the trial, we had some indications that trial staff considered that patient health outcomes would be better due to being in the study (9RN1) and thought that patients’ health had improved (6RN1), possibly through the intervention or perhaps owing to adherence to medication:
Actually, we cannot tell though, because some have never actually improved, some of them have been following the algorithm religiously and then a lot of them have improved actually. And then they’re very happy with the results.
6RN1
So yeah, once . . . if I was convinced that it was effective in a study trial setting that it’s effective then I will be convinced, but I suppose I’d have to see the data for that.
6CT2
In addition, one member of staff said that the parents were really happy with improvements that they had seen in their child through participation in the study (8RN1).
Families
Taking part in the RAACENO trial
Parents described being motivated to take part in the trial because of their experiences of having a child with asthma and wanting to get something more for their child (11022, 26–1); for instance:
He had mentioned that he would give us information and asked if we wanted to be part of it, and of course we just said yes, because why wouldn’t you? It’s going to either help [daughter’s name] or help asthma, the study. So yeah, we just think it was a bit of a no-brainer.
26–1
Some expressed a willingness to be doing something for others and the future of childhood asthma (11022, 26–2) as their main motivation:
I’m really happy if there’s any research that could help another child in the future, because we really benefited from somebody else’s research.
26–2
Perceptions of contributing to medical knowledge were also mentioned (17–1, 23–1); for example:
Just thought it was important to, you know, thought it was something that wasn’t [several inaudible words] and we thought it might improve doctor’s knowledge and understanding of how to control asthma and after what she’d just been through, I thought it was really important.
17–1
One parent–child pair said that taking asthma seriously and trying to quantify it was another reason for taking part (26–1). Another family said that their interest in how technology might help (26–2) was a factor. Others related their choice to take part to a recent hospital admission (17–1, 23–1).
Recruitment, randomisation and retention
Information about the study was described as being easy to understand by one parent, who explicitly said that they did not feel pushed (17–1). A decision to take part was influenced by the perception that there was no risk involved (17–1, 26–1) and that participation was still couched within a safety net of NHS services:
Research is important, as long as you know you’re not going to come to any harm in taking part, then it’s a good thing to take part.
26–1
One child wished to take part because of an interest in research and a career in medical sciences:
Well, I’m kind of into studies and I’d quite like to go into microbiology and things like that.
22–1
It was considered to be easy to take part, with not many more appointments than normal (17–1), although others said that they liked the extra appointments (22–1 and 26–2). The timings were experienced differently by different families: some found them convenient (26–2) and some found them less convenient (26–1). One family described liking the extra appointments and insights:
Well, I think it was quite good because obviously we were going . . . although we do go regular to see the consultant anyway, this was like an in between appointment and I think having the data analysed the way it was, it was proven that actually you do need to keep going up a step and down a step on your medication rather than just going you know, once in a blue moon lung function tests. I think doing it over a period of time when we did with the trial . . .
22–1
Yeah. It’s almost, in a way it’s almost like wishing that you could do it like that all the time, so we know that it’s . . . your symptoms and everything’s being monitored . . .
22–1
Despite staff perceptions (reported above), there was a mixed understanding of the randomisation process. Most trial participants’ parents were familiar with the word and some knew that it was carried out by a computer (11022). One parent understood that studies cannot be carried out on individual people and, therefore, was happy for their child to be randomised to take part in the trial:
Yeah, and how did you feel about that after being told about [randomisation]? Did you have any particular strong feelings, or did you have views about . . .?
No, not really because I understood that’s how it works, you can’t just do it on individual people because everybody’s different. So, I didn’t really worry about that.
23–1
Some parents said that they did not know the results yet (11022), whereas another knew that they would be getting them and which group they were in (26–2). Two parents described the process of waiting for the outcome but not knowing or remembering potential outcomes (17–1, 22–1), although one knew that there were ‘trial arms’ (22–1).
One parent reported that they did not understand the concept of randomisation, but suggested that they trusted the allocation decisions and understood that the process would help other asthma sufferers:
Honestly I don’t know because they just . . . they always ask if we want to participate and they invite to hospital and give us some paperwork and ask some questions. So, we think we agree because you want to do something for people suffering from asthma.
11022
Intervention
Fractional exhaled nitric oxide
The breathing technique for FeNO measurement was tricky for someone new to it to begin with:
Yeah, the first time you did it, it was quite hard to do, trying to keep it in the middle was quite hard but you soon got the hang of it . . .
Yeah, so it wasn’t the easiest to use.
No, it was a little bit tricky to start with because she had to try and balance, yeah, you had to keep the balance right, which wasn’t an easy thing to do herself, but . . .
22–1
Having a ‘whole picture’ or ‘full picture’ of the child’s asthma condition was useful (17–1, 23–1) and this also applied to having additional feedback from FeNO results (17–1 and 26–1). When asked about the perceived effectiveness of FeNO in informing treatment decisions, one parent responded:
Well, I think it was good for [daughter’s name] because it meant she was on the absolute minimum amount of steroids to keep her healthy. I think . . . but then who knows, because maybe that’s what . . . we used to see [consultant’s name] in clinic as well, so they maybe recommended that anyway. But personally, I liked the fact that they were very happy to reduce her steroid when the computer thought that they should, so that was good.
26–1
Algorithm
One parent felt that the algorithm made good decisions (17–1) and another mostly agreed with both the step ups and the step downs (22–1); this was linked to their perception about how the condition was experienced by their child at the time. Another liked the close monitoring with recommendations about stepping up and down and ‘being on the right amount’:
No, the doctor always just did what the computer recommended. But he always explained it and always said, you know, he always kind of justified it because her asthma had been fine, or because, you know, she hadn’t been on steroids for the previous three months or whatever. So, he always talked it through.
26–1
The pattern of asthma symptoms (22–1 and 26–1) and that asthma is a fluctuating condition (26–2) were identified as relevant to how the algorithm was used. One family ignored the study for a month, after consulting a clinician, because of travel plans to an area of poor air quality (26–1). One parent cited that they respected decisions based on the algorithm because they trusted the clinical staff:
It’s a programme that works out information. I don’t know anything about that, very technology behind, but yeah, they did explain it to me. I just trusted that they knew what they were doing.
23–1
Some parents remembered the word ‘algorithm’ (17–1) and one said that it was used a lot (22–1), whereas others said that they did not remember the word but did not feel that they had been treated as stupid (26–1).
In response to questions designed to elicit perceptions of the role of the algorithm in treatment decisions, one parent said that it was initially daunting but that this seemed to be acceptable for making recommendations about treatment and was described as ‘clever’:
. . . we were just like, ‘Oh, okay, this is a bit bizarre’, but then logically when you think about it the technology behind it is very, very clever isn’t it, so I think you know, when we realised that lung function had dropped slightly and things like that and different symptoms, the computer seemed to pick up on all of that, which I think was . . . I think it was really, really clever. But obviously having . . . knowing that there was a doctor overseeing it as well was that little bit of reassurance.
22–1
Most parents reported that the algorithm had suggested changes, one on three occasions (11022). Some liked that a step down was recommended because this meant taking less medication (or had been taking more than required) (11022); however, others did not and were anxious about a step down, but also wanted less steroid (26–2) or a maintained level of treatment based on discussion between parents and clinicians, even where the algorithm suggested no treatment (17–1). One parent considered it to be reliable because it seemed to recommend a step up when the asthma had felt bad but suggested that it would have never recommended no treatment and, for this reason, was reliable:
I think I liked knowing that she was being very well taken care of, to that [inaudible], you know? I quite liked knowing that there were lots of people looking after her, that’s always quite a nice feeling. And she started the study on 250 mg, whatever that is, micrograms, milligrams, whatever, of Seretide [combination inhaler containing fluticasone and salmeterol (Seretide; GlaxoSmithKline, London, UK)] every day, morning and night. Now she’s down to 150 mg, [inaudible]. So, I like the fact that her steroids reduced significantly. That might have happened without being on the study, who knows. But I mean, it’s a good thing. She did have a bad asthma year the year of the study, but not particularly worse than it would have been anyway, I don’t think. She’s just got pretty bad asthma. But I liked that they were very responsive to everything.
26–1
Regarding a step-up decision, one parent liked to be able to have a conversation about the computer recommendation and was reassured that their clinician would not follow the algorithm if everyone perceived that a step up was too big a step (22–1). Another felt similarly confident that, should they want to over-ride it, for example because the child was not keen to increase their usage of steroids (and the resultant potential consequences of stunted growth), their consultant would step in:
You didn’t like that did you? [daughter’s name] has this huge hang up of steroid treatment because she’s very short for her age and we’d read somewhere how much steroids you can have can inhibit growth. So, she was really paranoid about the more steroids that she took, the shorter she would end up. So, every time the computer said she needed to increase her steroid dose she said, ‘Oh I don’t think so’.
23–1
The majority of trial participants’ parents reported feeling comforted by the idea that they could ultimately depend on a doctor’s interpretation and ability to over-ride the algorithm’s recommendation based on knowledge of the case and adapting to the season (11022, 17–1) or knowledge of a previous bad reaction (26–2). The fact that the algorithm was being used as a guide and not on its own (17–1, 22–1, 26–2) was identified as being important to the acceptability of the algorithm:
I think . . . I am intrigued to see how technology can help, but actually, even . . . there were two times when the technology advised to take Montelukast, and because we’ve had negative reactions to that in the past, the doctor agreed to not go with what the technology said. Then her symptoms have since improved, you know, it’s not been detrimental that she’s not taken that Montelukast, so yes, you know, I’m fine with technology helping advise the doctors, but ultimately the doctor made the call, using the technology and the finding.
26–2
Smart inhalers
Smart inhalers were considered to be useful, but many encountered problems with their accuracy (11022, 23–1) and reported that the battery required regular charging (22–1, 26–1). One parent said that it was difficult to use, even for adults:
OK, I’m not sure on this, I think the [inaudible] is better with spacer because we can hear it, we can control it better. Obviously from my experience, I work as support worker and when I give my residents the smart inhaler, they don’t know what to do with it, so some . . . even adult people don’t know what to do with this. So, I think this one with spacer is better.
11022
There was a suggestion that a battery indicator could be helpful (17–1) and that children are more tech savvy and would manage to charge it better than their parents (22–1).
One parent suggested that having the option to download data at home, perhaps weekly, to monitor their child would have been useful (26–1). One parent wanted to buy a smart inhaler:
I have asked if I could buy one either from the NHS or online. I think it’s fantastic that I’m not having to nag, as a mum, because ultimately [daughter’s name] is now a teenager and she’ll be leaving home soon and just for her to know that there is somebody checking up on it, even if it was just an app, you know, beyond the study, even if we had a device and she was checking on an app and going, ‘Oh right, no wonder my symptoms are getting worse, I’ve been taking it less’, it would just have been fantastic. As a parent, it’s been unquestionably my favourite thing about the study. I really echo what she said, I wish that we could buy it. I mean, I said I would buy it for somebody else in the study that maybe couldn’t afford it, I have no idea how much they are, but I know they said it would be difficult to get the software or something on a phone. Just for something . . . I hope that that’s where the future goes with that treatment, that it means that people would be able to have . . . they did say it would be tricky if she changed inhaler, then the smart metre would need to be changed.
26–2
Families reported that they liked being monitored (22–1) and the computer being able to report adherence after issues were fixed. Most families specifically mentioned that the monitoring was a motivator to comply, even when medication was being well managed (17–1, 22–1, 23–1, 26–1, 26–2). One parent said:
I think it’s good to use actually because it makes, in my opinion it makes the child a little bit more conscious about making sure they take it knowing that it’s being recorded. It’s very rarely that we missed the dose anyway, but it definitely made you . . . made sure that you had it at roughly the same time each day. And I think you made that little bit more of a conscious effort didn’t you [daughter’s name] . . .
22–1
Two parents described that a child feeling more responsible was a good thing (17–1, 26–2). One parent said that their child had learned to manage their asthma better as a result of being in the study (17–1).
Future
One family said that they would like to do this all of the time, knowing that everything is being monitored (22–1). Another said that they wanted to see an NHS-endorsed monitoring technology/app and that they saw this kind of monitoring with data, for example, as the future of asthma treatment – really tailoring treatment:
I think there could be a possibility of a nicer, better one that had been adopted by the NHS, that ideally would feed into something like a smart meter in future. I really hope that smart meters in the future become more mainstream and affordable for people that have . . . certainly the first one, people that have had [inaudible] and then ideally, hopefully, for everybody. Surely everybody’s asthma would be better if you actually knew they were controlling it because then you could compare the facts. ‘Okay, you’re more ill this month, well look here, you’re reducing it 60%’ say, ‘You should be taking much higher’.
26–2
One parent recommended that the recommendation from the algorithm could be made more child-friendly with smiley faces and consolidated instructions with a key message for child to take away and act on:
I don’t know whether you could give some information following the appointment like you’re leaving with this today. You know they discuss what the decision is with you, but you know, it’s just a little game or something child-friendly like smiley faces or something. Important information that you can take away that would help appreciate the results and things that the clinician could pick that would show like ‘We suggested you do this today’ like ‘Try and ensure you take your inhalers’, I don’t know [several inaudible words] I just think that because the child part of it and like little reminders like ‘Remember if you need your inhaler more than four times when you’re at school to tell somebody’, I don’t know.
17–1
One family reported that their GP was not happy with all of the changes from the hospital (11022) and another said that their GP did not know enough about the study and medication changes (26–1). Another family said that they recognised that GPs could not be experts in everything:
I think also because GPs don’t always know enough about asthma, that any studies that are being done in the hospital that could help future GPs have more information to decide whether or not to up or reduce somebody’s medication or when to seek more help, I think would be an excellent thing.
26–2
COVID-19
Through the interviews, which took place during the COVID-19 pandemic, one parent commented on the need for better guidance in general for children with asthma, but that they were happy to have been in the trial during this time:
I worry about really asthma attack what we should do, should we call emergency and what to tell them. She is at home all the time, she goes just for short walk with doggy, so yeah, hopefully she has [inaudible] of increase of any asthma attack, I don’t know really what to do if she . . .
So a bit more guidance about what . . . ‘If this happens what should I do?’ kind of thing?
Yeah, we’ve got blue inhaler of course but sometimes it doesn’t work, we have to go to emergency, it happens. First time it was one year ago just around this time of the year, so we really worry about it . . .
11022
Another said that they had been concerned about their child and, although they understood that they were of no greater likelihood of contracting coronavirus, they knew that their child would have severe problems if they tested positive (23–1). Another parent reported feeling terrified:
I just think it’s very scary if you’ve got a child like [daughter’s name] that’s got severe asthma, it is very, very scary knowing . . . I mean I spoke to the consultant because she had an appointment like at the beginning of March, and I asked about it and he said, ‘Whilst she’s no more likely to get it than anybody else, if she does get it, she’s likely to have some quite severe problems’. So, we’ve been really, really worried about it, I mean at the moment we’re shielding because she got the letter from the government.
26–2
Discussion
Principal findings
Through this qualitative process evaluation, we discovered that experiences of taking part in the RAACENO trial were mostly positive in the views of our volunteer staff and family participants. Some concerns around strictly following the algorithm’s treatment recommendations were raised owing to contextual factors not being taken into consideration beyond the child’s data. However, the use of FeNO to facilitate conversations and inform treatment were valued by staff and families alike. Non-compliance is discussed further in Chapter 4. In terms of time commitment, both staff and families valued the extra contacts and relationships fostered through the study and it was not considered burdensome to take part. Some technical issues were reported, but the benefits of using novel technology in managing and treating asthma were recognised. Overall, the RAACENO intervention was considered to be acceptable by both staff and families, with the caveat that, ultimately, clinical judgement around safety prevented any harm, although it was emphasised that both intervention and control groups were ‘no harm’ and this offered reassurance and facilitated recruitment to the study. We should also note that, despite public health guidance from early on in the pandemic being that children with asthma were not at greater risk of COVID-19, participating in the RAACENO trial offered added benefits to parents concerned about the COVID-19 pandemic and their children’s asthma because they felt able to access more information and support as a result of being involved.
Strengths
The main strengths of this qualitative process evaluation are that it was conducted by a team of qualitative researchers with backgrounds in a range of disciplines and undertaken over time in phases, including developing materials and collecting data, with two researchers independently coding and analysing all data. We were also able to draw on the wider team and TSC for comment on the qualitative findings in relation to other aspects of the trial research, especially the non-compliance data, which we reviewed in the light of the qualitative findings to help us to better interpret their meaning and impact. That we were able to collect data during a pandemic95 was also a strength. In addition, not all effectiveness trials include qualitative components101 and, therefore, having this element within the study has helped us to explain the main trial findings.
Limitations
Our sample was adapted to include more staff members, but we were unable to achieve our planned recruitment among families. However, we found that saturation was achieved among both staff and the heterogeneous family samples102 within six interviews for each group, which we considered to be early. We wondered whether or not this was because of sample bias, especially among families, as a result of having managed to recruit participants who were from the intervention group only; however, randomisation was not considered to have affected group decisions because there was no placebo, potential harm or withholding of treatment in the RAACENO trial.
The next steps on potential future research can be found in Chapter 7.
Conclusions
In this qualitative process evaluation, trial staff representing a number of roles and across different sites were interviewed to understand their experiences of delivering RAACENO from provider perspectives and to explore the acceptability of the intervention from their points of view. A number of families (mother and child pairs) were also interviewed about their experiences and views. Overall, experiences within both groups were positive. Key was that the RAACENO trial impacted positively on staff–family relationships and communication around asthma management and treatment among children, and that the use of technology and individual data within clinical appointments was considered useful: closer monitoring and the educational impacts were especially highlighted. We also ascertained that the intervention was broadly acceptable, with caveats around clinicians using the algorithm recommendation as a guide (rather than being dictated by it) and wariness around extreme step ups/downs in the light of contextual factors not taken into account by the algorithm.
Chapter 7 Discussion
Summary of the main findings
There is evidence that using FeNO to guide asthma treatment in patients already receiving maintenance therapy may reduce the risk of asthma exacerbations in children, but not other outcomes. This study was designed to determine whether or not intervention with an asthma treatment algorithm, which considered FeNO, current symptoms, current treatment and adherence to treatment among children, was more effective in reducing asthma exacerbations than ‘standard care’, which differed by not including FeNO. We recruited children whose characteristics were typical for those seen in a secondary care asthma clinic in the UK, with a high burden of exacerbation and symptoms and for whom an objective measurement, such as FeNO, to guide asthma treatment decisions would be welcome. The desired sample size was achieved, the participant characteristics were consistent with children who had more troublesome asthma and the primary outcome had the expected incidence. There were no SAEs or deaths. The primary outcome was determined in 99% of participants. The intervention was not associated with reduced odds for the primary outcome, that is one or more exacerbation, or for secondary outcomes between participants in the two treatment groups. In the adjusted model, the OR for the primary outcome was 0.88 (95% CI 0.61 to 1.27) for participants allocated to the intervention group compared with the standard-care group. The CI for the main result includes the possibility of a 33% reduction in the odds of having an exacerbation (as specified in the sample size calculation). Intentional non-adherence to the algorithm-generated treatment recommendations occurred and the analysis found that the primary outcome did not differ between randomised groups where the algorithm recommendations were followed. Interviews with staff, trial participants and their parents gave insights into the acceptability of algorithm-generated treatment recommendations and the use of electric logging devices, which objectively measure treatment adherence. The health economic evaluation gave insights into the educational and financial implications for children with asthma and their families. However, we conclude that, for children attending secondary care asthma clinics already receiving maintenance therapy, the addition of FeNO to standard care does not improve any of the outcomes captured in the RAACENO study.
Relevance to the existing literature
RAACENO trial results in the context of national guidelines and the Cochrane review
There is currently uncertainty around the role of FeNO in guiding asthma treatment in children in the UK and around the world. The BTS/SIGN guidelines17 and NICE guidelines21 do not recommend that FeNO should be used routinely for people with asthma of any age. The BTS/SIGN guideline17 does permit the use of FeNO in specialist asthma clinics to monitor asthma in adults or children. A Cochrane systematic review41 found that FeNO-guided asthma treatment is associated with reduced odds for an asthma exacerbation. 41 Previous studies103,104 have also shown that FeNO may also be useful to monitor adherence to IC treatment105 and predict the risk of future asthma exacerbation in children. The findings from the RAACENO trial, which do not support the use of FeNO in guiding asthma treatment, will benefit clinicians and colleagues writing clinical guidelines, and the NHS.
An earlier review,43 which included data from 1077 children and looked at the seven studies featured in the Cochrane review,41 concluded that FeNO-guided treatment was associated with an OR for any exacerbation of 0.67 (95% CI 0.51 to 0.88) compared with standard care. This review also found that FeNO-guided treatment was associated with a mean increase in daily IC dose of 106 µg (95% CI 75 µg to 138 µg). In the RAACENO trial population, there was no difference in the odds of an exacerbation requiring OCs or ICs between participants in the intervention group and participants in the standard-care group.
The previously mentioned Cochrane review41 identified nine studies that had used FeNO as part of an algorithm to guide asthma treatment. Only one study of 63 participants used exacerbation as the single primary outcome measure. 106 The mean age of the 1329 participants from the eight studies included in the analysis ranged from 10 to 14 years. The authors concluded that the intervention was associated with an OR for any exacerbation of 0.62 (95% CI 0.49 to 0.80) and an OR for an exacerbation requiring OCs of 0.63 (95% CI 0.48 to 0.83). 41 The Cochrane review found no association between FeNO-guided treatment and changes in asthma symptom control, spirometry, FeNO or dose of ICs. 41 The only result from RAACENO that differs from the results of the Cochrane review41 is that there was no difference in odds for an exacerbation requiring OCs between participants in the two RAACENO treatment groups.
The current BTS/SIGN guideline17 and a second international guideline (from the Global Initiative for Asthma)14 consider individuals aged over 12 years as adults and, given that the RAACENO participants were aged up to 15 years, our findings have some, albeit limited, relevance to ‘adult’ populations. The NICE guideline21 considers adults as those aged ≥ 17 years, for which the RAACENO findings cannot be extrapolated. A Cochrane review41 of trials completed in adults found a reduced OR for exacerbation when asthma treatment is guided by FeNO, compared with standard care (0.60, 95% CI 0.43 to 0.84). A recent review suggests that FeNO may be useful for adults with severe asthma,107 although a recent clinical trial, which used FeNO and two other biomarkers to guide treatment,108 did not find that the biomarker-guided treatment was associated with a reduced IC dose. In RAACENO, the OR for the primary outcome in the FeNO-guided treatment group compared with the standard-care group was 0.95 (95% CI 0.62 to 1.46) for those aged < 11 years and 0.79 (95% CI 0.42 to 1.49) for those aged 11–16 years, which possibly indicates that the intervention might work in older individuals, but the analyses for the older age groups remain clearly not statistically significant.
Comparison of methodologies used in RAACENO and similar trials
The many differences in the study design between the trials that have used FeNO to guide asthma treatment in children, including RAACENO, may explain the heterogenous findings between studies. These differences include the chosen primary outcome, sample size, inclusion criteria and follow-up periods (Table 43). In addition, the trials applied different algorithms; for example, different FeNO cut-off points were used, some used spirometry in the treatment algorithm, and, some54,55,109 used the CACT (also used in RAACENO), although many did not and all employed a symptom score in the algorithm. Furthermore, medication schedules were different between studies, with only some including LTRA, and the number of treatment steps ranging between four110 and seven. 106 Finally, the baseline characteristics of the participants differed between the trials. The RAACENO participants were notable for being among the populations with the greatest obesity and highest LABA and LTRA use and the lowest FeNO concentrations, mean predicted FEV1 and proportion with controlled symptoms (Table 44).
| Study (first author) | Primary outcome(s) | Mean age (years) (SD) | Participants (n) | Atopy as inclusion criteria? | FEV1 < 80% also used in treatment algorithm? | FeNO cut-off point(s) used (ppb) | Follow-up periods after baseline | What did the trial find? (FeNO treatment compared with standard care) |
|---|---|---|---|---|---|---|---|---|
| RAACENO | Exacerbation | 10.1 (2.6) | 509 | No | No | > 50% change | 3, 6, 9 and 12 months | |
| Fritsch110 | FEV1 | 11.5 (3.1) | 47 | Yes | Yes | 20 | 6, 12, 18 and 24 weeks | Higher mid-expiratory flow, higher dose of ICs |
| Peirsman109 | Symptom-free days | 10.7 (2.1) | 99 | Yes | Yes | 20 | 3, 6, 9 and 12 months | Reduced exacerbations, increased LTRA and IC dose. No difference in the primary outcome |
| Petsky41 | Exacerbations | 10.0 (3.2) | 63 | No | No | 10 for non-atopic, 12 with one PSPT, 20 for > 1 PSPT | 1, 2, 3, 4, 6, 8, 10 and 12 months | Reduced exacerbation, increased IC dose |
| Pijnenburg111 | Cumulative IC dose | 12.3 (2.8) | 85 | No | No | 30 | 3, 6, 9 and 12 months | Reduced FeNO and bronchial hyper-responsiveness. No increase in IC dose |
| Pike112 | IC dose and exacerbation frequency | 10.9 (2.6) | 90 | No | No | ≤ 15 and ≥ 25 | 2, 4, 6, 8, 10 and 12 months | No differences in outcomes |
| Szefler54 | Days with asthma symptoms | 14.4 (2.1) | 546 | Yes | Yes | 20, 30 and 40 | 6, 14, 22, 30, 38 and 46 weeks | Reduced exacerbations, increased ICs dose. No difference in the primary outcome |
| Voorend-van Bergen55 | Proportion of symptom-free days | 10.2 (3.0) | 181a | Yes | No | 20 and 50 | 4, 8 and 12 months | Increased asthma control but not the primary outcome |
| Study (first author) | Male, % (n) | Median FeNO (ppb) (p25, p75); n | Mean predicted FEV1 (%) (SD); n | Per cent with allergya (n/N) | Per cent obese (n/N) | Per cent prescribed LTRA (n/N) | Per cent prescribed LABA (n/N) | Median dose of IC (µg) (p25, p75) | Per cent > 400 µg budesonide (n/N) | Per cent white ethnic group (n/N) | Per cent controlled (n/N) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAACENO | 61 (308) | 21 (10–48) | 89.6 (17.8); 445 | Not determined | 9 (46/505) | 59 (302/509) | 76 (386/509) | 400 (400–1000) | 44 (223/509) | 76 (385) | 50 (256/509) |
| Fritsch110 | 60 (28) | 34 (19–59); 46 | 93.5 (15.7); 47 | 100 | 8 (4/47) | 28 (13/47) | 38 (18/47) | 400 (0–800) | 30 (14/47) | Not stated | 49 (23/47) |
| Peirsman109 | 67 (66) | 31 (14–69); 49 | 91.4 (15.7); 98 | 100 | 1 (1/99) | 60 (59/99) | 32 (32/99) | 320 (200–400) | 15 (15/99) | 82 (69/84) | 75 (49/65) |
| Petsky41 | 49 (31) | 26 (12–48); 61 | 90.7 (15.6); 54 | 38 (24/63) | 2 (1/58) | 10 (6/58) | 67 (39/58) | 400 (250–500) | 49 (31/63) | Not stated | 72 (41/57) |
| Pijnenburg111 | 65 (56) | 32 (17–53); 85 | 97.5 (17.5); 85 | 100 | 4 (4/85) | 0 (0/85) | 39 (33/85) | 800 (400–1000) | 67 (57/85) | Not stated | 57 (44/77) |
| Pike112 | 57 (51) | 26 (10–48); 90 | 89.2 (14.3); 90 | 76 (68/90) | 8 (7/89) | 51 (46/90) | 76 (68/90) | 800 (400–1000) | 59 (53/90) | 92 (83/90) | 97 (87/90) |
| Szefler54 | 53 (288) | 20 (11–41); 546 | 90.9 (16.6); 546 | 88 (467/531) | 31 (165/526) | 15 (80/546) | 66 (360/546) | 1000 (400–2000) | 53 (287/546) | 0 (0/526) | 80 (421/528) |
| Voorend-van Bergen55 | 68 (123) | 18 (10–30); 179 | 93.8 (13.0); 157 | 100 | 3 (5/181) | 13 (23/181) | 46 (84/181) | 400 (400–800) | 33 (59/181) | 89 (160/179) | 67 (122/181) |
The differences in study design, algorithms, treatment schedules and baseline characteristics will increase the risk of the findings being heterogeneous. The differences between the RAACENO population and the other populations may explain why the apparent benefit of FeNO-guided treatment over standard care, in the context of exacerbations, was not clearly seen in the RAACENO population. Given that we recruited an adequately powered population, typical of secondary care asthma clinics in the UK and with 99% follow-up, we are confident that, in this population, the routine addition of FeNO to standard care will not reduce the OR for an asthma exacerbation. Given that we did not recruit a meaningful number of participants from primary care, it remains possible that the addition of FeNO to standard care in primary care may be beneficial to patients. However, when we considered the subgroup on BTS treatment step 2, and also likely to be seen in primary care, the OR for exacerbation in the FeNO-guided treatment group compared with the standard-care group was 1.41 (95% CI 0.45 to 4.37).
Possible reasons why the RAACENO intervention did not work
Our hypothesis was that the proportion of children with an asthma exacerbation would be reduced when asthma treatment guided by FeNO plus symptoms was compared with treatment guided only by symptoms. The proposed mechanism was that increasing anti-inflammatory treatment for those with elevated FeNO would lead to a fall in allergic airway inflammation (as evidenced by FeNO) and, thus, a reduced risk of exacerbation; however, we found no difference in IC dose, FeNO or exacerbation between participants in the two treatment groups. There are several potential reasons for the intervention not to have changed any outcome for participants in the FeNO-guided treatment group of RAACENO, which will now be discussed.
First, the treatment recommendations for participants in the intervention group and standard-care group of the trial were often similar and this will reduce the likelihood of the outcome differing between the treatment groups. Our treatment algorithm included 61 potential treatment steps (see Appendix 1). When the treatment algorithm was composed, we recognised that 31 (51%) of these steps had identical step-up/step-down recommendations in both treatment groups. This concurrence of options is explained by the lack of alternative treatments at the bottom and the top of the treatment ‘ladder’. Treatment options to reduce the risk for asthma exacerbations include increasing ICs and the addition of LABA and/or LTRA, and at baseline 77% of participants were on LABA treatment, 59% were prescribed LTRA and 33% were already on high-dose ICs (i.e. > 800 µg of budesonide equivalent). The polypharmacy received by many participants at baseline meant that treatment could be escalated only by increasing IC dose, regardless of the study group to which they were randomised. Our participants were predominantly recruited from a secondary care setting. The subgroup analysis included stratification by treatment and the 76 participants on only low-dose ICs at baseline (BTS step 2, see Table 11), who might usually be seen in primary care settings, gained no benefit from the intervention. This suggests that FeNO may not be effective in the primary care setting.
In a post hoc exploratory analysis we considered how treatment decisions from the algorithm would have differed in the event that participants in the standard-care group had been randomised to the FeNO-guided treatment group. Given the known correlation between increasing symptoms and rising FeNO, and the primacy of symptoms over FeNO in our algorithm, it is not surprising that algorithm recommendations were more often similar than different for an increase or reduction in treatment. On those occasions when standard care recommended no change to treatment, the addition of FeNO to usual care would have led to a change in treatment in 72% of cases; this proportion was similar for those participants on higher levels of treatment. This observation shows that the addition of FeNO to symptom-guided treatment was driving different treatment recommendations, in particular for RAACENO participants with controlled symptoms, but our results show that these FeNO-guided changes did not yield improved outcomes. In this exploratory analysis, the addition of FeNO to standard care would have led to different treatment decisions in 18% of the assessments for which the algorithm recommended step up and in 38% for which the algorithm recommended step down. We conclude that the addition of FeNO to standard care led to substantially different treatment recommendations, especially where standard care would be to not change treatment. Despite these FeNO-guided differences in treatment, these changes did not affect the primary outcome and were not necessary.
Second, the participants were predominantly under secondary care in which their treatment had been optimised, thus placing them at the lowest possible risk for future exacerbations and where no further improvement was possible. Given that many participants were on two and sometimes three asthma preventer treatments at baseline, treatment options were limited, meaning that treatment options guided by FeNO could be similar to those guided by symptoms. In addition, it is possible that taking part in the trial improved awareness of symptoms, adherence with treatment and inhaler technique, for example, and that this could reduce the likelihood of the intervention further reducing risk for exacerbation.
Third, consistent with the paradigm that participation in a trial improves outcome, there were marked improvements in outcomes for participants in the standard-care group, which made it difficult to detect differences between treatment groups. For example, the median ACT/CACT scores for RAACENO participants in the intervention group rose from 19 to 22 and in the standard-care group from 18 to 21 (an increased score of 3 is associated with a 33% reduced risk for exacerbation). 113 Quality of life improved in both groups: the PAQLQ score for participants in the RAACENO trial increased from 5.8 to 6.4 in the intervention group and from 5.6 to 6.2 in the standard-care group (a clinically meaningful difference is 0.5). 114 Finally, at randomisation, participants had experienced a median of three exacerbations in the previous year, but over follow-up this number fell to one. A trial that evaluated the benefit of FeNO added to standard care has shown that the proportion with well-controlled asthma symptoms improved from 24% at screening to 71% at randomisation. 115
Another reason for the intervention not being effective is that the algorithm recommendation was not followed in ≈ 25% of cases for participants in both groups of the RAACENO trial. In addition, in ≈ 25% of cases, the algorithm recommended ‘refer for specialist opinion’ in contexts that included the participant already being on maximal therapy and where symptoms were uncontrolled but FeNO was very low. The CACE analysis found the same magnitude of difference for the primary outcome when participants from the subgroup for whom the algorithm was always applied were compared with the whole population, and this provided evidence that intentional non-adherence did not substantially affect the RAACENO outcome.
Adherence to algorithm and treatment
Intentional non-adherence to the algorithm recommendation in clinical trials in which FeNO is included is not uncommon. One trial reported that physician recommendations over-rode protocol recommendations on a mean of 0.57 occasions per individual in the FeNO-guided treatment group and on 0.36 occasions per individual in the standard-care group in a trial that had three assessments. 55 In a second RCT, there were 42 occasions out of 543 (8%) in which physicians’ recommendations were not adherent to the protocol-recommended treatment. 111 Personal communications from clinicians in other FeNO trials found that physician-led or family-led non-adherence to the algorithm recommendation was not captured in one study and simply appeared not to have happened in two others. One trial reduced physician intentional non-adherence to 5% by having a blinded physician review the symptom score, lung function and (in the intervention group) FeNO, and the final regimen was often based on the blinded physician’s recommendation. 54 In childhood asthma studies, the proportion of assessments for which the child’s physician disagrees with the algorithm-recommended treatment is between 5% and 10%;115 of note, the previously mentioned trial still had 5% physician disagreement. 54
One potential mechanism for FeNO-guided treatment leading to reduced exacerbation is that knowledge of FeNO improves adherence to the preventer treatment. 105 In RAACENO, only participants in the FeNO-guided group knew their FeNO results, but participants in both groups had the smart inhaler device and were given the adherence figures from this at follow-up appointments. Knowledge about adherence may have improved adherence in both groups and negated any mechanism in which knowledge of FeNO improved adherence, with resulting improved asthma outcomes.
In RAACENO, the majority (63%) of intentional non-adherence decision episodes were made solely by the physician and a minority (5%) were made solely by the family. By contrast, a clinical trial in adults that used FeNO and two other biomarkers to guide treatment in adults with severe asthma found that it was patients who decided not to follow the recommended therapy. 108 In this study,108 treatment recommended by the algorithm was not followed in 15% of assessments and the risk of exacerbations was higher among this group than among participants who followed recommended treatment changes.
The proportion of assessments in which the algorithm advice was not followed in RAACENO was, therefore, higher than previous studies, and this is expected given that our trial had a pragmatic design, which could (if required) be rolled out into routine practice. Decision-making about asthma treatment is extremely subjective (e.g. the two current UK asthma guidelines17,21 recommend different treatment options in the same clinical setting) and, therefore, intentional non-adherence is not unexpected. The intentional non-adherence may have been a result of hesitancy about using the algorithm, but did not become less common over time; this suggests that centres did not learn to be more accepting of treatment recommendations. Evidence of hesitancy to change is supported by our observation that intentional non-adherence occurred over follow-up between 4–12% when ‘no change’ was recommended and 25–39% for ‘step up’ and 26–35% for ‘step down’ recommendations (see Table 19). The most common explanation for intentional non-adherence (explaining 40% of episodes) was that the physician disagreed with the recommendation (mostly in the case of patients who were close to the very top of the treatment schedule and whose asthma control score was just below the threshold for control and for patients for whom the recommendation was to stop treatment). Other common reasons for intentional non-adherence, which could be considered and minimised in future algorithms, were winter cold season (e.g. not stepping down treatment in the autumn, 9%), patient considered non-adherent (8%), LTRA treatment (e.g. previous side effects, medication previously found not to be effective, 7%) and lung function results considered (6%).
Qualitative insights (including smart inhaler technology)
The qualitative interviews gave useful insights into how staff and parents perceived the algorithm decisions and when they were wary of the treatment recommendations that were made. Parents who were interviewed included those who ‘mostly agreed’ with the algorithm decisions, saw the algorithm as a guide and not the definitive decision, and were comforted by knowing that the local clinical team might over-ride the algorithm decision. The mother who ‘mostly agreed’ with the algorithm (22–1) was anxious about recommendations to increased the dose of ICs. By contrast, one member of a research team (2RN1) commented that parents were often anxious about reducing treatment, and a member of another research team (5RN1) was anxious about stepping down treatment in the autumn. One parent noted that their GP was apparently unhappy with the treatment recommendations, which is likely to have reduced the parent’s trust in the algorithm. These observations highlight the challenges of implementing algorithm-based treatment in a clinical service and suggest that any future validated algorithm that recommends treatment changes in asthma will need to be delivered with clinical supervision. Training the clinical team to manage the scenario in which parent/carers are uncertain of algorithm recommendations may help in the delivery of such algorithms, for example training in the evidence base behind algorithm recommendations. Future RCTs that use an algorithm might benefit from having a full description of the rationale behind the algorithm in the information leaflets for participants, parents/carers and GPs to prepare and reassure individuals.
Good adherence to asthma prevention treatment is associated with reduced risk of exacerbation;24 adherence is known to be variable within and between individuals. RAACENO used an objective electronic metre to record adherence (smart inhaler) to objectively measure adherence to ICs. The smart inhaler system was sometimes problematic; for example, the device battery sometimes had to be recharged by the participant/their family, the device was sometimes incorrectly applied to the inhaler and occasionally (rarely) the device stopped working. These events created periods of time in which there was apparently zero adherence; these were easy to identify and the clinical and/or research team was reliant on participant/parent report. In addition, there were RAACENO assessments at which the adherence measured by the smart inhaler was below 70% and was challenged by the participant and/or their parent/carer as being erroneous; the apparent inconsistency between reported and measured adherence was managed on a case-by-case basis by local investigators. The possibility cannot be excluded that some genuine use of the inhaler was not recorded (indeed we experienced this among the trial team when piloting the use of smart inhalers). One reason for the smart inhaler to apparently under-record treatment adherence was that some participants’ parents do not live together and there were IC inhalers at each parent’s house, but only one smart inhaler. Other reasons for adherence to be under-recorded include forgetting to take the inhaler on holiday and teenage participants not taking their first dose before noon during school holidays (ie. they may have taken two doses each day, but have taken both between noon and midnight, which would be categorised as treatment once per day). Additional problems with smart inhalers include the introduction of new inhalers that did not have a compatible smart inhaler, for example Relvar [combination inhaler containing fluticasone furoate and vilanterol (Relvar; GlaxoSmithKline, London, UK)], and NHS firewalls that stopped some centres using the smart inhaler software. More recently, inhaler logging devices have been developed that include a microphone that can objectively determine whether or not the inhaler was used as intended (and not actuated into open space) and whether or not the patient inhaled correctly. The smart inhaler devices used within the study cannot indicate whether the medicine was inhaled or whether it was inhaled correctly. These smart inhaler-related limitations applied equally to both groups of the RAACENO trial and, therefore, did not preferentially influence the primary outcome in one treatment group and could not affect the outcome.
With regard to the smart inhaler, the interviews of research teams yielded contrasting perspectives on the application of monitoring to asthma management. One clinician felt that the monitoring may be seen as in the style of ‘Big Brother’ (4CT2), whereas others felt that parents were keen to learn of their child’s adherence and have it downloaded in real time (8RN1 and 2RN1). There was consensus that the smart inhaler facilitated the sometimes difficult discussions around adherence. Technical challenges to using the smart inhaler were recognised by several centres.
Health economic analysis
The health economic analysis provided unique insights into how asthma affected the lives of children with asthma and their families. There have been relatively few health economic analyses of children attending hospital asthma clinics. The analysis presented in Chapter 5 shows the financial implication to families and the NHS. For example, the annual cost for preventer treatment was £920–935 per individual in the two treatment groups, and this may be a useful sum to cite when describing the current cost of asthma in the NHS, although £229–246 of this is for antihistamine use (not an asthma preventer). The annual cost to families of asthma has been estimated at £1587–1707; this is 5–6% of the typical UK household income (median household disposable income per annum £29,400 in the financial year ending 2019)116 and is likely to have a relatively greater impact on poorer families, who are also at increased risk of hospitalisation. 117 A further notable aspect arising from the health economic analysis was the observation that the mean number of unscheduled contacts per participant per annum was approximately 1.5, but only 50% of participants had an exacerbation. This apparent discrepancy may be explained by some encounters not resulting in OC treatment. It remains to be determined whether or not, for this population, an exacerbation presenting to unscheduled services should be treated with OCs by default.
Methodological issues
Given that FeNO was used to guide treatment in only one group of the trial, it is possible that methodological issues relating to FeNO measurements may have reduced the precision of FeNO measurements and resulted in the intervention not reducing the odds for the primary outcome. International guidelines recommend that the mean of three FeNO measurements that are within 10% of each other, or two measurements that are within 10% of each other, should be reported,118 but only one measurement was used in RAACENO. The decision to use only one measurement was based on manufacturer’s advice and our pragmatic study design; each measurement costs approximately £8 and takes up to 5 minutes, and the time and cost implications of doing more than one measurement would make the intervention unattractive for clinical practice. There is diurnal variation in FeNO concentration, being approximately 14% higher in the evening than in the morning,119 and we could not ensure that measurements were made at exactly the same time of day; however, diurnal variation is much less than the threshold of 50% change in FeNO that was used in RAACENO to trigger treatment change and, therefore, is not likely to have affected the RAACENO results. A 3-month interval between FeNO measurements was chosen because this reflects current hospital asthma clinic practice; it is possible that a different outcome from RAACENO might have arisen if FeNO measurements were made on more frequent occasions. A previous trial found no benefit of daily FeNO measurements over standard care120 and other trials, which have reported that FeNO-guided treatment was associated with reduced exacerbations compared with standard care, had protocols in which FeNO was measured at intervals of between 6 weeks and 3 months. 54,106,109 These observations suggest that the 3-month interval used in RAACENO did not affect the outcome of the intervention. We do not believe that FeNO methodological issues have substantially affected the RAACENO results.
The follow-up period for RAACENO ended in August 2020, which was 5 months after the first lockdown of the COVID-19 pandemic. Owing to COVID-19 restrictions, there were 25 participants who missed their 9-month RAACENO assessment. Twelve of these individuals were in the standard-care group and received a treatment recommendation from the algorithm, albeit delivered over the telephone. There were 13 individuals in the FeNO-guided treatment group who could not have FeNO measured, and these individuals received treatment recommendations as if they were in the standard-care group. An additional challenge presented by the COVID-19 pandemic to RAACENO was that face-to-face qualitative interviews could not take place; however with the support of the sponsor, we were able to swiftly move to remote interviews and recruited the prespecified number of participants. Asthma exacerbations reduced during lockdown and afterwards;121 however, given that this period occurred late in the RAACENO time course, COVID-19 did not substantially affect the RAACENO outcome and would have affected both groups equally. In summary, COVID-19 did not make a meaningful impact on any aspect of the RAACENO trial.
Strengths and limitations
Our study has a number of strengths. First, RAACENO recruited the desired number of participants. Second, the primary outcome was determined for 99% of participants (exceeding the expected 95%) and the expected and actual incidence of the primary outcome among participants randomised to receive standard care were 50% and 51.4%, respectively, which collectively means that RAACENO was adequately powered to conclude that the intervention did not achieve the predicted reduction in the primary outcome. Third, we recruited the population of children with troublesome asthma as intended. For example, participants typically had three exacerbations in the year prior to randomisation and the majority required add-on preventer therapy; any intervention to reduce morbidity would be welcome for this population compared with a population with mild asthma. A fourth strength is that the computer-delivered treatment algorithm ensured that standardised care was recommended to all participants in all trial centres. A fifth strength is that RAACENO was designed on an evidence base built from data collected in previous trials in which FeNO was used to guide asthma treatment in children; for example, we used percentage change in FeNO (and not absolute change) to guide treatment,45 we applied a more clinically meaningful and liberal threshold than in previous trials when defining a change in FeNO,46 and the analysis explored whether or not the intervention may have been effective in previously described subgroups. 45 Further strengths are the design of the study, which would enable easy roll-out into clinical practice and an objective marker of adherence to ICs, and that health economic and qualitative evaluations were included.
There are a number of limitations to our study, which should be considered. First, it is possible that the assumptions that were made in the algorithm were wrong. For example:
-
A well-recognised cut-off value from a validated symptom score was used to define uncontrolled asthma,15 but control is a continuum and an algorithm that included more than two categories of control (e.g. controlled, partly controlled and uncontrolled) may have yielded different results. However, this would have affected both groups of the trial and, therefore, would have been unlikely to affect the outcome.
-
The change in FeNO, which triggered a change in treatment, was based on the best evidence available,35,46 but this threshold might not have been correct. There are many factors that affect FeNO independent of asthma43 and a lower threshold would have lower sensitivity for detecting risk of future exacerbations. A higher threshold may have lacked specificity and cases at increased risk for future exacerbation would not have been detected. An alternative FeNO strategy would be to have censored all values below 20 ppb as normal;39 however, the median FeNO value at baseline was 21 ppb and such a strategy would probably have made treatment options between the two treatment groups more similar. RAACENO is one of a number of trials to have applied different FeNO criteria to changes in asthma treatment and it seems increasingly likely that there is no one-size-fits-all method to interpreting changes in FeNO.
-
Adherence to IC medication may have been over-reported. Participants with unrecognised poor adherence will have raised FeNO. 105 In the case of participants in FeNO-guided treatment group, this would lead to treatment escalation, but this would not be effective in reducing exacerbations owing to non-adherence, thus reducing the efficacy of the intervention. However, our algorithm permitted only one step up in treatment for asymptomatic individuals with raised FeNO (whose raised FeNO may reflect poor adherence), and the risk of participants being non-adherent to IC treatment was reduced by using the smart inhaler in many participants.
-
The definition of adherence that was applied, < 70% by objective measurement and neither ‘all of the time’ or ‘most of the time’ by report, may have been too liberal. Other RCTs that have evaluated the added benefit of FeNO to standard care applied a cut-off point of 50% for satisfactory adherence (more generous than RAACENO),54 excluded participants who were poorly adherent106,112,122 and reported a median adherence of 97% (range 55–124), as evidenced by a counter on the inhaler. 104 Our study was designed such that the intervention, if effective, could be rolled out throughout the NHS. For this reason, it did not incorporate elements used in other trials that may have reduced non-adherence, for example, a run-in period to identify non-adherent participants, who would subsequently be excluded. The threshold was applied to both treatment groups and, therefore, is unlikely to have affected the outcome in one group more than the other.
-
The treatment options given to participants in the two treatment groups may not have been sufficiently different to create a difference in outcomes. When the algorithm decisions were modelled after artificially allocating participants in the standard treatment group to the FeNO-guided treatment group, we found that treatment advice would have differed in 40% of participants. Two previous FeNO trials, which found no reduction in exacerbations between study groups, reported that, for 62%55 and 64%111 of assessments, the treatment advice would have been different if participants had been allocated to the other study group. A third FeNO trial,109 which reported reduced risk for exacerbation associated with FeNO-guided treatment, found that the ratio of discordant to concordant decisions, had participants been allocated to the other study group, was 1 : 1.05.
-
Our pragmatic study design used an ‘open-label’ design, given that participants, researchers and the clinical team knew which group participants were randomised to. Although the study could have had a double-blind design, this would have made the study less pragmatic and ‘real world’, and would have added to the complexity, cost and, potentially, ethics considerations. Therefore, we believe that the open-label design is a relative limitation to RAACENO, but one that is unlikely to have meaningfully affected the outcome.
Our initial application to the Efficacy and Mechanism Evaluation programme included a mechanistic component that was designed to explore the complex mechanistic relationship between sputum eosinophilia, FeNO, asthma control and exacerbation. Obtaining sputum for analysis on two occasions proved extremely challenging. Samples need to be processed immediately, and most centres did not have a local laboratory that was able to do this. Data from a study that used sputum eosinophilia to guide asthma treatment36 were shared with the RAACENO CI pre publication, and analysis demonstrated that there was a positive intra-subject correlation between percentage change in FeNO and sputum eosinophilia. Given the challenge in obtaining sputum samples and the presence of data that answered the research question, collection of sputum samples for measurement of eosinophils was stopped with the approval of the TSC and funder.
Generalisability
The RAACENO trial participants were representative of children attending asthma clinics in the UK. For example, the observational Paediatric Asthma Gene Environment Study (PAGES)56 recruited children from hospital asthma clinics across Scotland in 2008–9. The characteristics of the PAGES participants, who were aged 6–16 years, and the RAACENO participants are compared in Table 45. The RAACENO participants were selected for having had a recent asthma exacerbation and receiving higher IC treatment, and had lower %FEV1 and bronchodilator-derived response, but were very similar to the PAGES participants on other outcomes. Every assessment included a structured history, enquiring about treatment adherence, checking inhaler technique and in many cases taking spirometry measurements; all of these aspects are recommend by UK national guidelines for the management of asthma. 17 The RAACENO results are, therefore, generalisable to the population of children managed in secondary care asthma clinics across the UK and, therefore, the subset of children across the UK who have the greatest burden of morbidity from asthma.
| Characteristic | RAACENO | PAGES |
|---|---|---|
| Age (years), mean (SD); n | 10.1 (2.6); 509 | 10.7 (2.8); 483 |
| Male sex, n/N (%) | 308/509 (61) | 266/447 (60) |
| Eczema, n/N (%) | 183/509 (36) | 184/462 (38) |
| White ethnic group, n/N (%) | 385/509 (76) | 362/377 (96) |
| CACT or ACT score of > 19, n/N (%) | 256/509 (50) | 96/3010 (32) |
| Overall PAQLQ score, median (p25, p75); n | 5.7 (4.4–6.5); 508 | 5.5 (4.0–6.6); 252 |
| Any exacerbation in the last year | 100% | 240/440 (50%) |
| Mean FEV1 (%) (SD); n | 90 (18); 455 | 94 (16); 173 |
| Median FeNO (p25, p75); n | 21 (10–48); 509 | 21 (12–51); 184 |
| Mean bronchodilator response (SD); n | 10 (9.1); 160 | 4 (7.7); 150 |
| Median IC budesonide equivalent (p25, p75) (µg) | 400 (400–1000); 509 | 200 (200–500); 415 |
| Treated with LABA, n/N (%) | 386/509 (76) | 294/462 (61) |
| BTS treatment step 2, n/N (%) | 77/509 (15.1) | 87/457 (19) |
| BTS treatment step 3, n/N (%) | 219/509 (43.0) | 268/457 (59) |
| BTS treatment step 4, n/N (%) | 213/509 (41.8) | 66/457 (14) |
Public and patient involvement
The TSC included a lay member. The study documentation was created with the input of the Young Persons’ Group (a PPI group). The summary of the results that will be provided to participants and their families will be reviewed by the Young Persons’ Group.
Conclusions
In children with asthma being cared for in secondary care asthma clinics, the addition of FeNO to symptom-guided treatment did not reduce the OR for an asthma exacerbation.
Implications for practice
The RAACENO findings do not support the routine use of FeNO measurements as part of asthma management in a secondary care setting.
Recommendations for research
The role of FeNO in the diagnosis of asthma is increasingly recognised, and this work should continue. There may be some settings in which the addition of FeNO to standard care may improve patient care and future research might explore scenarios that include primary care and suspected poor adherence to IC treatment. In the RAACENO trial and other previous trials in children, FeNO was used in addition to symptoms, but in adults FeNO has been used to guide treatment independent of symptoms, either as a single biomarker123 or in combination with other biomarkers. 108 Future research in children could explore whether FeNO could be used to guide asthma treatment independent of symptoms.
RAACENO identified how some clinicians and parents/carers can be reticent to accommodate algorithm-recommended treatment and our qualitative interviews identified some of the reasons for this. Future trials could consider individualising algorithms to consider the time of year, the individual’s past exacerbation history and their future plans (e.g. holiday or sporting event). In addition, in the absence of a single ‘gold-standard’ test for monitoring asthma, two or more objective tests could be used to help to support stepping down treatment decisions, as has been previously described,103 and an algorithm based on these tests could give treatment decisions independently of symptoms.
The intervention delivered in the RAACENO trial was the addition of FeNO to an already complex intervention, which included regular standardised assessments, spirometry and an objective index of adherence. Future research could seek to understand which (if any) single component of a management intervention is associated with improved asthma outcomes. Such trials could consider whether or not different facets of intervention are more effective in different phenotypes/endotypes of asthma.
A further issue identified by trials, such as RAACENO, in which algorithms are used to generate treatment decisions is that patients and clinicians can be reluctant to follow algorithm recommendations. Future research could explore if/how algorithm recommendations could be made more acceptable.
Compared with many non-communicable diseases in children and young people (e.g. diabetes, epilepsy and inflammatory bowel disease), the management of asthma has not changed substantially over the last 25 years. Anticipating no novel therapies to cure or manage asthma, future research could evaluate the benefit of a standardised approach to treatment changes, for example using a standardised web-based algorithm to recommend a treatment regime and incorporating electronic logging devices to routine care, in addition to asthma action plans and 3- to 4-monthly assessments. Spirometry is used in many asthma clinics but its clinical utility is not well understood; future research could evaluate the benefit of adding spirometry to symptom-guided asthma treatment.
Acknowledgements
We would like to thank all the children who took part in the study and their families. We are grateful to all the staff at recruitment sites who facilitated identification, recruitment and follow-up of study participants (listed below). We could not have completed the study without the ongoing support of local and primary care research networks. We are grateful for the primary care practices that acted as participant identification centres and to the National Research Scotland Primary Care Network for facilitating this activity. The authors express their thanks to the staff in the primary care centres who provided primary outcome data.
We are grateful to Andrea Fraser for her secretarial and data co-ordination support. We are also grateful to Ruth Thomas for her help and advice in developing the grant proposal. We thank the programming team at the Centre for Healthcare Randomised Trials for developing and maintaining the study website and David Cooper for his statistical analysis advice. We thank Juliette Snow and Rachael West for their help with contracting, and Louise Cotterell, Helen Strachan, Blair Annadale and Anne Buckle for their help in managing the budget. We also thank the research governance team (Louise King, Stacey Dawson and Lynn McKay) at the University of Aberdeen, Aberdeen, for their advice and support during the study.
We would also like to thank Aileen Neilson, who initially led the health economic aspects of the study until 2018; Dan Brunsdon, who contributed to the development of the qualitative aspects of the study during 2018–19; and Laura Kruitbos, who provided temporary assistant trial manager support for the trial during 2018.
We would like to thank the Young Persons’ Advocacy Group, co-ordinated by Pamela Dicks from the NHS Scottish Children’s Research Network, for their invaluable input and feedback on the participant information leaflets.
We are grateful for the guidance and support of the TSC (chairperson: Michael Shields; independent members: Iain Small, Claire Snowdon and Susan Harley) and the DMC (chairperson: Colin Powell; independent members: Chris Griffiths and Catrin Tudur Smith).
We are grateful to Circassia Ltd (Oxford, UK) for the loan of 16 NIOX VERO® machines to measure nitric oxide in study participants and the supply of associated consumables, at no cost, in support of the study. Circassia Ltd have not had access to the data or had input into the analysis or interpretation of study data.
The Health Services Research Unit and the Health Economics Research Unit are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Staff at recruitment sites
Secondary care sites:
-
NHS Grampian (Royal Aberdeen Children’s Hospital) – Steve Turner (PI), Richard Brooker, Margaret Connon, Patricia Flanagan, Susan MacFarlane, Marie McWIlliam, Stephen Main, Mustafa Osman and Guy Wilson.
-
NHS Tayside (Ninewells Hospital) – Jonathan McCormick (PI), Richard Brooker, Susan MacFarlane, Anne MacLeod, Stephanie Menzies, Debbie Rice and Susie Roberts.
-
NHS Greater Glasgow & Clyde (Queen Elizabeth University Hospital) – James Paton (PI), Elizabeth Adair, Elise Buchan, Paul Burns, Katharine Duffy, Sheryl Goddard, Kathleen McLaughlin, Barry Milligan, Leanne Milne, Lynne Murray, Ashleigh Neil, Natalie Rodden, Katrina Watts and Liz Waxman.
-
University Hospitals of Leicester NHS Trust (Leicester Royal Infirmary) – Erol Gaillard (PI), Anisa Ahmed, Victor Jerome Ambrose, Clare Foxon, Teresa McNally, Sarita Makam, Deepa Patel and Simal Patel.
-
Barnsley Hospital NHS Foundation Trust (Barnsley Hospital) – Diarmuid Kerrin (PI), Rikki Crawley, Abigail Crew, Ezzedin Gouta, Sian Jones, Nicola Lancaster, Jamie Matthews, Linda Schofield, Janet Shackleton, Zena Thomas and Gemma Wray.
-
Sheffield Children’s NHS Foundation Trust (Sheffield Children’s Hospital) – Sonal Kansra (PI), Sara Ali, Alice Carey, Anita Critchlow, Mihaela Diaconu, Rachel Harrison, Jane Kirkby, Hemant Kulkarni and Janet Shackleton.
-
University Hospitals of North Midlands Trust (Royal Stoke University Hospital) – Will Carroll (PI), Emma Addison, Martin Booth, Sadie Clayton, Angelene Cope, Francis Gilchrist, Sheng-Ang Ho, Ruth Jones, Gillian Lockett, Holly Minchin, Emily Obhrai, Helen Parker, Jane Peach, Rachel Pringle, Victoria Riches, Eric Roe, Jayne Swift and Joanne Tomlinson.
-
The Royal Wolverhampton NHS Trust – Sally Edwards (PI), Lyndsay Bibb, Charlotte Busby, Rebecca Denyer, Ajay Gupta and Katherine Jones.
-
NHS Ayrshire & Arran (University Hospital Crosshouse) – Tim Adams (PI) and Claire Bell.
-
Royal Devon & Exeter NHS Foundation Trust – Patrick Oades (PI), Cassie Brady, Emma Chamberlain, Beth Enderby, Caroline Harrill, Nicola Jones, Megan Knight and Suzanne Wilkins.
-
Plymouth Hospitals NHS Trust (Derriford Hospital) – Denise Ullmann (PI), Julie Alderton, Jane Hallam, Cho Hsint, Jemma Inches, Jennifer Merrison-Hort, Kathiresan Natesan, Alice Phillips, Mala Raman, Sarah-Jane Sharman, Alison Stolton and Emma Storr.
-
Birmingham Women’s and Children’s NHS Foundation Trust (Birmingham Women’s and Children’s Hospital) – Priti Kenia (PI), Rehana Bi, Amy Burrill, Benjamin Davies, Susan Frost, Anas Ghivalla, Brinderjit Heyeer, Juliet Hopkins, Kiran Hunjan, Ciaran McArdle, Monica Mitchell, Prasad Nagakumar, George Preece, Satish Rao, Fiona Robinson and Ian Surplice.
-
Nottingham University Hospitals NHS Trust (Nottingham Children’s Hospital) – Jayesh Bhatt (PI), Carol Bertenshaw, Lindsay Crate, Rebecca Devaney, Emma Douglas, Debra Forster, Matthew Hurley, Laura Looby, Helen Navarra, Andrew Prayle, Alan Smyth, Stefanie Stafford, Rachel Wiffen and Caroline Youle.
-
Bradford Teaching Hospitals NHS Foundation Trust (Bradford Royal Infirmary) – Anil Shenoy (PI), Louise Akeroyd, Trudy Booth, Liz Ingram, Neelam Majid, Rachel Swingler, Joanna Urso, Rachel Wane, Aimee Watson and Dawn Woodward.
-
University Hospital Southampton NHS Foundation Trust – Graham Roberts (PI), Cherry Alviani, Rebecca Cartwright, Amber Cook, Jillian Doherty, Lisa Fairhead, Matt Harvey, Anne Magee, Jane Martin and Cilla Snape.
-
NHS Lothian (Royal Hospital for Sick Children) – Kenneth Macleod (PI), Julie Baggott, Sarah Blacklock, Naomi Matos, Debbie Miller, Maxine Ramsay, Stefan Unger and Julie Westwood.
-
Derby Teaching Hospitals NHS Foundation Trust (Royal Derby Hospital) – Donna Traces (PI), Jacey Allen, Claire Baldwin, Melanie Hayman, Nigel Ruggins, Rebecca Salloway, Coral Smith and Samantha West.
-
Walsall Healthcare NHS Trust (Walsall Manor Hospital) – Hesham Abdalla (PI), Joanne Butler, Sarah Freeth, Najma Iqbal, Sharon Kempson, Katharine Lewney, Joanne Mason, Priscilla Mhembere, Marie Phipps and Lisa Richardson.
-
University Hospitals Bristol (Bristol Children’s Hospital) – Simon Langton Hewer (PI), Karen Coy, Rachel Helyer, Myriam Kanchanatheera, Gurnie Kaur, Deborah Marriage, Huw Thomas and Lisa Tucker.
-
Leeds Teaching Hospitals NHS Trust (Leeds General Infirmary) – Emma Guy (PI), Alex Adams, Kate Baker, Nicola Balatoni, Louise Blackburn, Lai Wah Jenny Chung-Toole, Janet Clark, Lucy Fletcher, Saikiran Gopalakaje, Neil Hall and Heather Rostron.
-
King’s College Hospital Foundation Trust (King’s College Hospital) – Atul Gupta (PI), Rania Abusamra, Adedamola Adebayo, Cara Bossley, Ivan Caro, Holly Dickson, Katie Leigh-Ellis, Kathleen McLaughlin, Eniola Nsirim, Sormeh Salehian and Katie Tupper.
-
Barts Health NHS Trust – Chinedu Nwokoro (PI), Rasheda Choudhury, Bessie Cipriano, Emmily Clark, Mumtaz Idris, Hafiza Khatun, Ramiya Kirupananthan, Ivone Lancoma-Malcolm, Hina Mir, Tara Murray, Victoria Oyenuga, Elise Pabriaga, Caroline Pao, John Rae, Prita Rughani, Kevin Samuels, Gemma Sedgwick, Theresa Simangan, Surendan Thavagnanam and Natasha Thorn.
-
Newcastle Upon Tyne Hospitals NHS Foundation Trust (Royal Victoria Infirmary) – Matthew Thomas (PI), Karen Allen, Kathryn Bell, Malcolm Brodlie, Stephen Crulley, Charlotte Reigan, Evelyn Thomson, Jennifer Townshend and Jessica Williams.
-
Alder Hey Children’s NHS Foundation Trust (Alder Hey Children’s Hospital) – Ian Sinha (PI), Lynsey Brown, Debra Evans, Debbie Large, Phillip Lawrence, Nicola Rimmer, Joanne Shakeshaft, Elinor Thomason and Vicky Wheeler.
-
Manchester University NHS Foundation Trust (Wythenshawe Hospital) – Naveen Rao (PI), Sandra Bothwell, Anthony Brothwood, Claire Holliday, Kate Ransom, Karen Rhodes and Louise Woodhead.
-
St George’s University Hospital NHS Foundation Trust (St Georges University of London) – Richard Chavasse (PI from September 2018), Fran Mabesa, Young Ng, Jennifer Pearce, Anu Shankar (PI to September 2018) and Elia Vitale.
-
Shrewsbury & Telford Hospital NHS Trust (The Royal Shrewsbury Hospital) – Kumar Sethuraman (PI), Lynette Charles, James Jones, Helen Moore, Martyn Rees and Rebecca Wilcox.
-
Brighton & Sussex University Hospitals NHS Trust (Royal Alexandra Children’s Hospital) – Akshat Kapur (PI), Krishne Chetty, Kevin Donnelly, Paul Frattaroli, Sonia Sobowiec Kouman, Christine Laycock, Jason Lenton, Carolynn Lorimer, Kate Moscovici, Somnath Mukhopadhyay, Catherine Olden, Rebecca Ramsay, Vivien Richmond, Tom Ruffles, Paul Seddon, Emma Tagliavini, Cathy Warde and Edwina Wooler.
-
Countess of Chester Hospital NHS Foundation Trust (Countess of Chester Hospital) – Ravi Jayaram (PI), Caroline Burchett, Sarah De-Beger, Usha Downie, Stuart Eccles and Caroline Lonsdale.
-
Harrogate and District Foundation Trust (Harrogate District Hospital) – Alexandra Hardisty (PI), Sarah-Jane Foxton, Christine Morgan, Tracy Nixon, Rosalind Parkinson and Louise Wills.
-
The Dudley Group NHS Foundation Trust (Russells Hall Hospital) – Sohail Nassir (PI), Daljit Kaur, Katharine Lewney, Rebecca Marks and Abigail Twiss.
-
NHS Lanarkshire (Wishaw General Hospital) – Carol Dryden (PI), Angela Brown, Donna Corrigan, Karen Leitch, Jackie Quigley and Thin Thin Saing.
-
The Pennine Acute Hospitals NHS Trust (Royal Oldham Hospital) – Prakash Kamath (PI), Sunil Bagewadi, Bernadette Holt, Rachel Newport, Grainne O’Connor, Jennifer Philbin, Chloe Rishton and Zainab Sarwar Yasin.
-
Lancashire Teaching Hospitals – Karnam Sugumar (PI), Julie Abernethy, Andy Lancaster, Claire Lodge and Natalie Mattos-Harris.
-
Southport & Ormskirk NHS Trust (Ormskirk Hospital) – Sharryn Gardner (PI), Irene Gardner, Zena Haslam, Sudhaker Kandasamy, Emily McDonald, Moira Morrison and Laura Thomas.
-
Wirral University Hospital NHS Foundation Trust (Arrowe Park Hospital) – Sudeshna Bhowmik (PI), Sharon Hughes, David Lacy, Lucy Lewis and Joanne Mullen.
Primary care sites:
-
Barrack Lane Medical Centre – Debasish Banerjee (PI), Karen Drury and Beverly Kennell.
-
Castle Partnership – Joanne Walsh (PI), Jason Ladbrooke and Belinda Sewell.
-
Hanscombe House Surgery – Funlayo Subar (PI).
-
Ixworth Surgery – Vijay Chandraraj (PI), Deirdre Siddaway and Joshua Williams.
-
Lakenham Surgery – Emore Echuko (PI).
-
Parsonage Surgery – Jagjit Takhar (PI).
-
Roundwell Medical Centre – Keeva Rogers (PI), Chaminda Dooldeniya (PI), Caiman Hardiman, Alison Mortlock and Denise Steward.
Primary care clinical RNs:
-
CRN Eastern – Donna Clements, Phillipa Lincoln, Ruth Osborne and Suzanne Skinner.
Contributions of authors
Steve Turner (https://orcid.org/0000-0001-8393-5060) (Chief Investigator) contributed to the conception and design of the trial, the conduct of the trial, the recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Seonaidh Cotton (https://orcid.org/0000-0002-7883-0608) (Trial Manager) contributed to the design and conduct of the trial, the development of the treatment algorithm, the interpretation of results and writing/editing the report, and was responsible for the day-to-day management of the trial.
Jessica Wood (https://orcid.org/0000-0002-4233-811X) (Trial Manager) was responsible for the day-to-day management of the trial, and contributed to the interpretation of results and writing/editing the report.
Victoria Bell (https://orcid.org/0000-0001-9518-9296) (Assistant Trial Manager) was responsible for aspects of the day-to-day management of the trial and contributed to the interpretation of results and writing/editing the report.
Edwin-Amalraj Raja (https://orcid.org/0000-0003-0853-9724) (Statistician) contributed to the statistical analysis, the interpretation of results and writing/editing the report.
Neil W Scott (https://orcid.org/0000-0002-8067-9660) (Statistician) was responsible for the statistical analysis and contributed to the interpretation of results and writing/editing the report.
Heather Morgan (https://orcid.org/0000-0002-6118-8911) (Qualitative Researcher) was responsible for the day-to-day management of the qualitative process evaluation and contributed to the conception and design of the qualitative process evaluation, the interpretation of results and the writing/editing of the relevant parts of the report.
Louisa Lawrie (https://orcid.org/0000-0002-9867-2184) (Qualitative Researcher) was responsible for the day-to-day management of the study, including interviewing participants, and contributed to the design of the qualitative process evaluation, the interpretation of results and the writing/editing of the relevant parts of the report.
David Emele (https://orcid.org/0000-0003-1060-9963) (Senior Programmer) contributed to the design of the trial, was responsible for the development and computerisation of the treatment algorithm, and edited the report.
Charlotte Kennedy (https://orcid.org/0000-0002-1974-6318) (Health Economist) was responsible for the health economic analysis and contributed to the interpretation of results and writing the report.
Graham Scotland (https://orcid.org/0000-0001-5539-8819) (Health Economist) contributed to the design of the trial, oversaw the health economic analysis and contributed to the interpretation of results and writing/editing the report.
Shona Fielding (https://orcid.org/0000-0001-5363-8188) (Lecturer in Statistics) provided statistical expertise during the design and conduct of the trial and contributed to the statistical analysis and editing the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (CHaRT Director) contributed to the statistical analysis, the interpretation of results and writing/editing the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Director of Edinburgh Clinical Trials Unit) contributed to the conception and design of the trial and writing/editing the report.
Mark Forrest (https://orcid.org/0000-0002-2395-8823) (Senior IT Development Manager) contributed to the development and computerisation of the treatment algorithm and writing/editing the report.
Erol Gaillard (https://orcid.org/0000-0003-0996-8133) (Clinical Senior Lecturer) contributed to the conception and design of the trial, the conduct of the trial, the recruitment and follow-up of participants, the interpretation of results and writing/editing the report.
Johan de Jongeste (https://orcid.org/0000-0003-1321-5943) (Professor, Paediatrics) contributed to the conception and the design of the trial, the interpretation of results and writing/editing the report.
Marielle Pijnenburg (https://orcid.org/0000-0003-0902-0860) (Paediatric Pulmonologist) contributed to the conception and design of the trial, the interpretation of results and writing/editing the report.
Mike Thomas (https://orcid.org/0000-0001-5939-1155) (Professor of Primary Care Research) contributed to the conception and design of the trial, the interpretation of results and writing/editing the report.
David Price (https://orcid.org/0000-0002-9728-9992) (Professor of Primary Care Respiratory Medicine) contributed to the conception and design of the trial, the interpretation of results and writing/editing the report.
Publication
Turner S, Cotton S, Wood J, Bell V, Raja E-A, Scott N, et al. Reducing asthma attacks in children using exhaled nitric oxide (RAACENO) as a biomarker to inform treatment strategy: a multicentre, parallel, randomised, controlled, phase 3 trial [published online ahead of print January 28 2022]. Lancet Respir Med 2022.
Data-sharing statement
All data will be made available and can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Turner S, Cotton S, Wood J, Bell V, Raja E-A, Scott N, et al. Reducing asthma attacks in children using exhaled nitric oxide (RAACENO) as a biomarker to inform treatment strategy: a multicentre, parallel, randomised, controlled, phase 3 trial. Lancet Respir Med 2022. https://doi.org/10.1183/13993003.congress-2021.RCT2899.
- Asthma UK . Asthma Facts and FAQs 2020. www.asthma.org.uk/about/media/facts-and-statistics/ (accessed 12 April 2020).
- National Asthma Education and Prevention Program . Expert Panel Report 3—Guidelines for the Diagnosis and Management of Asthma 2007.
- Gaillard EA, Kuehni CE, Turner SW, Goutaki M, Holden KA, de Jong CCM, et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children 5 to 16 years. Eur Respir J 2021;58. https://doi.org/10.1183/13993003.04173-2020.
- Skadhauge LR, Christensen K, Kyvik KO, Sigsgaard T. Genetic and environmental influence on asthma: a population-based study of 11,688 Danish twin pairs. Eur Respir J 1999;13:8-14. https://doi.org/10.1183/09031936.99.13100899.
- Dick S, Friend A, Dynes K, Al Kandari F, Doust E, Cowie H, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to nine years. Brit Med J Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006554.
- Tagiyeva N, Devereux G, Fielding S, Turner S, Douglas G. Outcomes of childhood asthma and wheezy bronchitis. A 50-year cohort study. Am J Respir Crit Care Med 2016;193:23-30. https://doi.org/10.1164/rccm.201505-0870OC.
- Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet 2018;391:350-40. https://doi.org/10.1016/S0140-6736(17)30879-6.
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 2010;363:1211-21. https://doi.org/10.1056/NEJMoa0906312.
- Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax 2012;67:1046-51. https://doi.org/10.1136/thoraxjnl-2012-202150.
- Chan-Yeung M, Ferguson A, Watson W, Dimich-Ward H, Rousseau R, Lilley M, et al. The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol 2005;116:49-55. https://doi.org/10.1016/j.jaci.2005.03.029.
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18:716-25. https://doi.org/10.1038/nm.2678.
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59-9. https://doi.org/10.1164/rccm.200801-060ST.
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention 2019. https://ginasthma.org/gina-reports/ (accessed 26 July 2019).
- Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117:549-56. https://doi.org/10.1016/j.jaci.2006.01.011.
- Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007;119:817-25. https://doi.org/10.1016/j.jaci.2006.12.662.
- British Thoracic Society and Scottish Intercollegiate Guidelines Network . British Guideline on the Management of Asthma 2019. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed 27 July 2019).
- Ahmed H, Turner S. Severe asthma in children-a review of definitions, epidemiology, and treatment options in 2019. Pediatr Pulmonol 2019;54:778-87. https://doi.org/10.1002/ppul.24317.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract 2017;3. https://doi.org/10.1186/s40733-016-0029-3.
- Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A, Heaven M, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0657-8.
- National Institute for Health and Care Excellence (NICE) . Asthma: Diagnosis, Monitoring and Chronic Asthma Management 2017.
- NHS Digital . Health Survey for England 2018 – Asthma 2019. http://healthsurvey.hscic.gov.uk/media/81643/HSE18-Asthma-rep.pdf (accessed 16 June 2020).
- Centres for Disease Control and Prevention . AsthmaStats. Uncontrolled Asthma Among Children, 2012–2014 2019. www.cdc.gov/asthma/asthma_stats/documents/AsthmaStats_Asthma_Uncontrolled_Child_PDF_Cleared_H.pdf (accessed 16 June 2020).
- Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J 2015;45:396-407. https://doi.org/10.1183/09031936.00075614.
- Chan AH, Stewart AW, Harrison J, Camargo CA, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med 2015;3:210-19. https://doi.org/10.1016/S2213-2600(15)00008-9.
- Shields M, Alqahtani F, Rivey M, McElnay J. Using remote directly observed therapy (R-DOT) for optimising asthma therapy. Eur Respir J 2017;50. https://doi.org/10.1183/1393003.congress-2017.OA3448.
- Wensley D, Silverman M. Peak flow monitoring for guided self-management in childhood asthma: a randomized controlled trial. Am J Respir Crit Care Med 2004;170:606-12. https://doi.org/10.1164/rccm.200307-1025OC.
- Nuijsink M, Hop WC, Sterk PJ, Duiverman EJ, Hiemstra PS, de Jongste JC. Urinary eosinophil protein X in children with atopic asthma. Mediators Inflamm 2007;2007. https://doi.org/10.1155/2007/49240.
- Ferraro V, Carraro S, Bozzetto S, Zanconato S, Baraldi E. Exhaled biomarkers in childhood asthma: old and new approaches. Asthma Res Pract 2018;4. https://doi.org/10.1186/s40733-018-0045-6.
- Stern G, de Jongste J, van der Valk R, Baraldi E, Carraro S, Thamrin C, et al. Fluctuation phenotyping based on daily fraction of exhaled nitric oxide values in asthmatic children. J Allergy Clin Immunol 2011;128:293-300. https://doi.org/10.1016/j.jaci.2011.03.010.
- Beck-Ripp J, Griese M, Arenz S, Köring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002;19:1015-19. https://doi.org/10.1183/09031936.02.01582001.
- Montuschi P, Mondino C, Koch P, Barnes PJ, Ciabattoni G. Effects of a leukotriene receptor antagonist on exhaled leukotriene E4 and prostanoids in children with asthma. J Allergy Clin Immunol 2006;118:347-53. https://doi.org/10.1016/j.jaci.2006.04.010.
- Roberts G, Hurley C, Bush A, Lack G. Longitudinal study of grass pollen exposure, symptoms, and exhaled nitric oxide in childhood seasonal allergic asthma. Thorax 2004;59:752-6. https://doi.org/10.1136/thx.2003.008722.
- Piacentini GL, Peroni DG, Bodini A, Bonafiglia E, Rigotti E, Baraldi E, et al. Childhood Asthma Control Test and airway inflammation evaluation in asthmatic children. Allergy 2009;64:1753-7. https://doi.org/10.1111/j.1398-9995.2009.02068.x.
- Cutts R, Turner S. Longitudinal measurements of exhaled nitric oxide in children–what is a significant change in FE(NO)?. Pediatr Allergy Immunol 2013;24:540-8. https://doi.org/10.1111/pai.12101.
- Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Longitudinal relationship between sputum eosinophils and exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med 2013;188:400-2. https://doi.org/10.1164/rccm.201212-2156LE.
- Buchvald F, Eiberg H, Bisgaard H. Heterogeneity of FeNO response to inhaled steroid in asthmatic children. Clin Exp Allergy 2003;33:1735-40. https://doi.org/10.1111/j.1365-2222.2003.01822.x.
- Fleming L, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax 2012;67:1015-16. https://doi.org/10.1136/thoraxjnl-2012-201930.
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. https://doi.org/10.1164/rccm.9120-11ST.
- Price D, Berg J, Lindgren P. An economic evaluation of NIOX MINO airway inflammation monitor in the United Kingdom. Allergy 2009;64:431-8. https://doi.org/10.1111/j.1398-9995.2008.01855.x.
- Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD011439.pub2.
- Turner SW, Chang AB, Yang IA. Clinical utility of exhaled nitric oxide fraction in the management of asthma and COPD. Breathe 2019;15:306-16. https://doi.org/10.1183/20734735.0268-2019.
- Turner S. Exhaled nitric oxide and the management of childhood asthma – yet another promising biomarker ‘has been’ or a misunderstood gem. Paediatr Respir Rev 2015;16:88-96. https://doi.org/10.1016/j.prrv.2014.07.005.
- Fielding S, Pijnenburg M, de Jongste JC, Pike KC, Roberts G, Petsky H, et al. Change in FEV1 and Feno measurements as predictors of future asthma outcomes in children. Chest 2019;155:331-41. https://doi.org/10.1016/j.chest.2018.10.009.
- Fielding S, Pijnenburg MW, de Jongste JC, Pike K, Roberts GC, Petsky HL, et al. Does treatment guided by fractional exhaled nitric oxide improve outcomes in subgroups of children with asthma?. Eur Respir J 2020;55. https://doi.org/10.1183/13993003.01879-2019.
- Fielding S, Pijnenburg M, de Jongste J, Pike K, Roberts G, Petsky H, et al. What is a clinically meaningful change in exhaled nitric oxide for children with asthma?. Pediatr Pulmonol 2020;55:599-606. https://doi.org/10.1002/ppul.24630.
- Turner S, Cotton SC, Emele CD, Thomas R, Fielding S, Gaillard EA, et al. Reducing Asthma Attacks in Children using Exhaled Nitric Oxide as a biomarker to inform treatment strategy: a randomised trial (RAACENO). Trials 2019;20. https://doi.org/10.1186/s13063-019-3500-7.
- Powell H, Murphy VE, Taylor DR, Hensley MJ, McCaffery K, Giles W, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011;378:983-90. https://doi.org/10.1016/S0140-6736(11)60971-9.
- Harnan SE, Tappenden P, Essat M, Gomersall T, Minton J, Wong R, et al. Measurement of exhaled nitric oxide concentration in asthma: a systematic review and economic analysis of NIOX MINO, NIOX VERO and NObreath. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19820.
- Beerthuizen T, Voorend-van Bergen S, van den Hout WB, Vaessen-Verberne AA, Brackel HJ, Landstra AM, et al. Cost-effectiveness of FENO-based and web-based monitoring in paediatric asthma management: a randomised controlled trial. Thorax 2016;71:607-13. https://doi.org/10.1136/thoraxjnl-2015-207593.
- British Thoracic Society and Scottish Intercollegiate Guidelines Network . British Guideline on the Management of Asthma 2016. www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/ (accessed 31 August 2017).
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev 2010;5. https://doi.org/10.1002/14651858.CD005535.pub2.
- Gibson PG. Using fractional exhaled nitric oxide to guide asthma therapy: design and methodological issues for ASthma TReatment ALgorithm studies. Clin Exp Allergy 2009;39:478-90. https://doi.org/10.1111/j.1365-2222.2009.03226.x.
- Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet 2008;372:1065-72. https://doi.org/10.1016/S0140-6736(08)61448-8.
- Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, Landstra AM, van den Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax 2015;70:543-50. https://doi.org/10.1136/thoraxjnl-2014-206161.
- Turner SW, Ayres JG, Macfarlane TV, Mehta A, Mehta G, Palmer CN, et al. A methodology to establish a database to study gene environment interactions for childhood asthma. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-107.
- Gabriele C, Pijnenburg MW, Monti F, Hop W, Bakker ME, de Jongste JC. The effect of spirometry and exercise on exhaled nitric oxide in asthmatic children. Pediatr Allergy Immunol 2005;16:243-7. https://doi.org/10.1111/j.1399-3038.2005.00255.x.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. https://doi.org/10.1183/09031936.05.00034805.
- Pepys J. Skin tests for immediate, type I, allergic reactions. Proc R Soc Med 1972;65:271-2. https://doi.org/10.1177/003591577206500320.
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res 1996;5:35-46. https://doi.org/10.1007/BF00435967.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Cheng KKF, Metcalfe A. Qualitative methods and process evaluation in clinical trials context: where to head to?. Int J Qual Methods 2018;17:1-4. https://doi.org/10.1177/1609406918774212.
- Public Health England . Process Evaluation 2018. www.gov.uk/government/publications/evaluation-in-health-and-well-being-overview/process-evaluation (accessed 9 February 2021).
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240-3. https://doi.org/10.1136/bmj.320.7244.1240.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 11 March 2022).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30. https://doi.org/10.1016/S0140-6736(16)00620-6.
- Pope C, Turnbull J, Jones J, Prichard J, Rowsell A, Halford S. Has the NHS 111 urgent care telephone service been a success? Case study and secondary data analysis in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014815.
- NHS Improvement . National Schedule of Reference Costs: 2018–19 2019. https://improvement.nhs.uk/resources/national-cost-collection/#ncc1819 (accessed 24 August 2020).
- NHS Improvement . National Schedule of Reference Costs: 2017–18 2018. https://webarchive.nationalarchives.gov.uk/20200501111106/https://improvement.nhs.uk/documents/6468/201718_reference_costs_data_and_guidance.zip (accessed 24 August 2020).
- NHS Improvement . National Tariff: 2017–19 2019. https://improvement.nhs.uk/documents/6789/2019-20_NTPS.zip (accessed 26 January 2021).
- HM Revenue and Customs . Expenses and Benefits: Business Travel Mileage for Employess’ Own Vehicles n.d. www.gov.uk/expenses-and-benefits-business-travel-mileage/rules-for-tax (accessed 26 January 2021).
- Office for National Statistics . Estimated of the Age Distribution of Parents of Primary School Aged Children: England, Oct to Dec 2019 2020. www.ons.gov.uk/file?uri=/peoplepopulationandcommunity/educationandchildcare/adhocs/11865estimatesoftheagedistributionofparentsofprimaryschoolagedchildrenenglandocttodec2019/populationestimatesofprimaryparents.xlsx (accessed 4 February 2021).
- Office for National Statistics . Earnings and Hours Worked, Age Group: AHSE Table 6 2019. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/agegroupashetable6 (accessed 26 January 2021).
- Office for National Statistics . Annual Survey of Hours and Earnings: Unpaid Work Calculator 2016. www.ons.gov.uk/visualisations/dvc376/index.html (accessed 26 January 2021).
- Department for Transport . Transport Analysis Guidance (TAG) Data Book n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/816195/tag-data-book.xlsm (accessed 9 January 2020).
- GOV.UK . National Minimum Wage and National Living Wage Rates 2021. www.gov.uk/national-minimum-wage-rates (accessed 26 January 2021).
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7. https://doi.org/10.3132/pcrj.2007.00002.
- Willems DC, Joore MA, Hendriks JJ, Wouters EF, Severens JL. Cost-effectiveness of a nurse-led telemonitoring intervention based on peak expiratory flow measurements in asthmatics: results of a randomised controlled trial. Cost Eff Resour Alloc 2007;5. https://doi.org/10.1186/1478-7547-5-10.
- Julious SA, Horspool MJ, Davis S, Bradburn M, Norman P, Shephard N, et al. PLEASANT: Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term – a cluster randomised controlled trial and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20930.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Handbooks in Health Economic Evaluation. Oxford: Oxford University Press; 2007.
- Deb P, Norton EC, Manning WG. Health Econometrics Using Stata. College Station, TX: Stata Press; 2017.
- Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016;71:339-46. https://doi.org/10.1136/thoraxjnl-2015-207630.
- Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev 2020;29. https://doi.org/10.1183/16000617.0151-2019.
- De Filippo M, Clark E, Fillard A, Diaferio L, Caimmi D. Oral corticosteroids and asthma in children: practical considerations. Pediatr Allergy Immunol 2020;31:43-5. https://doi.org/10.1111/pai.13151.
- Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018;11:193-204. https://doi.org/10.2147/JAA.S176026.
- Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17520.
- Arabkhazaeli A, Vijverberg SJH, van der Ent CK, Raaijmakers JAM, Maitland-van der Zee AH. Asthma treatment patterns in Dutch children using medication dispensing data. Pediatr Allergy Immunol 2017;28:606-8. https://doi.org/10.1111/pai.12751.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions. BMJ 2006;337.
- Ayers L, Given LM. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: SAGE Publications, Inc.; 2008.
- Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179-83. https://doi.org/10.1002/nur.4770180211.
- World Health Organization . Coronavirus Disease (COVID-19) Pandemic 2021. www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 12 February 2021).
- Braun V, Clarke V. What can ‘thematic analysis’ offer health and wellbeing researchers?. Int J Qual Stud Health Well-Being 2014;9. https://doi.org/10.3402/qhw.v9.26152.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907. https://doi.org/10.1007/s11135-017-0574-8.
- Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough?. Qual Health Res 2017;27:591-608. https://doi.org/10.1177/1049732316665344.
- Braun V, Clarke V. To saturate or not to saturate? Questioning data saturation as a useful concept for thematic analysis and sample-size rationales. Qual Res Sport Exerc Health 2019;13:201-16. https://doi.org/10.1080/2159676X.2019.1704846.
- Rapport F, Storey M, Porter A, Snooks H, Jones K, Peconi J, et al. Qualitative research within trials: developing a standard operating procedure for a clinical trials unit. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-54.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60. https://doi.org/10.1177/1049732315617444.
- Zacharasiewicz A, Wilson N, Lex C, Erin EM, Li AM, Hansel T, et al. Clinical use of non-invasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med 2005;171:1077-82. https://doi.org/10.1164/rccm.200409-1242OC.
- Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax 2005;60:215-18. https://doi.org/10.1136/thx.2004.023374.
- Klok T, Kaptein A, Brand P. FeNO in children with asthma is related to inhaled corticosteroids adherence, not to asthma control. Eur Respir J 2014;44.
- Petsky HL, Li AM, Au CT, Kynaston JA, Turner C, Chang AB. Management based on exhaled nitric oxide levels adjusted for atopy reduces asthma exacerbations in children: a dual centre randomized controlled trial. Pediatr Pulmonol 2015;50:535-43. https://doi.org/10.1002/ppul.23064.
- Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J 2020;55. https://doi.org/10.1183/13993003.01633-2019.
- Heaney LG, Busby J, Hanratty CE, Djukanovic R, Woodcock A, Walker SM, et al. Composite type-2 biomarker strategy versus a symptom-risk-based algorithm to adjust corticosteroid dose in patients with severe asthma: a multicentre, single-blind, parallel group, randomised controlled trial. Lancet Respir Med 2021;9:57-68. https://doi.org/10.1016/S2213-2600(20)30397-0.
- Peirsman EJ, Carvelli TJ, Hage PY, Hanssens LS, Pattyn L, Raes MM, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol 2014;49:624-31. https://doi.org/10.1002/ppul.22873.
- Fritsch M, Uxa S, Horak F, Putschoegl B, Dehlink E, Szepfalusi Z, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol 2006;41:855-62. https://doi.org/10.1002/ppul.20455.
- Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2005;172:831-6. https://doi.org/10.1164/rccm.200503-458OC.
- Pike K, Selby A, Price S, Warner J, Connett G, Legg J, et al. Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J 2013;7:204-13. https://doi.org/10.1111/j.1752-699X.2012.00306.x.
- Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009;124:719-23. https://doi.org/10.1016/j.jaci.2009.06.053.
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994;47:81-7. https://doi.org/10.1016/0895-4356(94)90036-1.
- Kercsmar CM, Sorkness CA, Calatroni A, Gergen PJ, Bloomberg GR, Gruchalla RS, et al. A computerized decision support tool to implement asthma guidelines for children and adolescents. J Allergy Clin Immunol 2019;143:1760-8. https://doi.org/10.1016/j.jaci.2018.10.060.
- Office for National Statistics . Average Household Income, UK: Financial Year Ending 2019 2019. www.ons.gov.uk/peoplepopulationandcommunity/personalandhouseholdfinances/incomeandwealth/bulletins/householddisposableincomeandinequality/financialyearending2019provisional (accessed 1 March 2021).
- RCPCH . State of Child Health in the 2021. https://stateofchildhealth.rcpch.ac.uk/ (accessed 8 March 2021).
- American Thoracic Society, European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. https://doi.org/10.1164/rccm.200406-710ST.
- Pijnenburg MW, Floor SE, Hop WC, De Jongste JC. Daily ambulatory exhaled nitric oxide measurements in asthma. Pediatr Allergy Immunol 2006;17:189-93. https://doi.org/10.1111/j.1399-3038.2006.00394.x.
- de Jongste JC, Carraro S, Hop WC, Baraldi E. CHARISM Study Group . Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009;179:93-7. https://doi.org/10.1164/rccm.200807-1010OC.
- Williams TC, MacRae C, Swann OV, Haseeb H, Cunningham S, Davies P, et al. Indirect effects of the COVID-19 pandemic on paediatric healthcare use and severe disease: a retrospective national cohort study. Arch Dis Child 2021;106:911-17. https://doi.org/10.1136/archdischild-2020-321008.
- Voorend-van Bergen S, Vaessen-Verberne AA, Landstra AM, Brackel HJ, van den Berg NJ, Merkus PJ, et al. Fractional exhaled nitric oxide monitoring does not improve asthma management in children with concordant and discordant asthma phenotypes. Am J Respir Crit Care Med 2015;192:1016-18. https://doi.org/10.1164/rccm.201506-1103LE.
- Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005;352:2163-73. https://doi.org/10.1056/NEJMoa043596.
- Stata Press . Stata Multiple-Imputation Reference Manual 2013.
Appendix 1 Treatment steps
| Baseline IC | Budesonide equivalent (µg); L | BTS step | Drug numbera | Control: symptom only | FeNO algorithm 1 | FeNO algorithm 2 | D/Sb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 12 years | ≥ 12 years | < 12 years | ≥ 12 years | < 12 years | ≥ 12 years | |||||||||||
| Step up | Step down | Step up | Step down | Step up | Step down | Step up | Step down | Step up | Step down | Step up | Step down | |||||
| SABA only (no IC) | 0 | NA | 0 | 1 | NA | 1 | NA | 1 | NA | 1 | NA | 1 | NA | 1 | NA | S |
| Beclomethasone (Clenil) | ||||||||||||||||
| 50 µg MDI/spacer, two puffs twice daily | 200 | Step 2 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | S |
| 100 µg MDI/spacer, two puffs twice daily | 400 | Step 2 | 2 | 25 | 1 | 25 | 1 | 14 | 1 | 14 | 1 | 25 | 1 | 25 | 1 | D |
| Budesonide (Pulmicort Turbohaler) | ||||||||||||||||
| 100 µg DPI, one dose twice daily | 200 | Step 2 | 3 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | S |
| 200 µg DPI, one dose twice daily | 400 | Step 2 | 4 | 28 | 3 | 28 | 3 | 5 | 3 | 5 | 3 | 28 | 3 | 28 | 3 | D |
| 400 µg DPI, one dose twice daily | 800 | Step 3/4c | 5 | 29 | 4 | 29 | 4 | 29 | 4 | 15 | 4 | 29 | 4 | 29 | 4 | D(o) |
| Fluticasone (Flixotide Accuhaler) | ||||||||||||||||
| 50 µg DPI one dose twice daily | 200 | Step 2 | 8 | 9 | 0 | 9 | 0 | 9 | 0 | 9 | 0 | 9 | 0 | 9 | 0 | S |
| 100 µg DPI one dose twice daily | 400 | Step 2 | 9 | 22 | 8 | 22 | 8 | 10 | 8 | 10 | 8 | 22 | 8 | 22 | 8 | D |
| 250 µg DPI, one dose twice daily | 1000 | Step 3/4c | 10 | 23 | 9 | 23 | 9 | 23 | 9 | 11 | 9 | 23 | 9 | 23 | 9 | D(o) |
| 500 µg DPI, one dose twice daily | 2000 | Step 4 | 11 | d | d | 24 | 10 | d | d | 24 | 10 | d | d | 24 | 10 | S |
| Fluticasone (Flixotide Evohaler) | ||||||||||||||||
| 50 µg MDI/spacer, one puff twice daily | 200 | Step 2 | 12 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | S |
| 50 µg MDI/spacer, two puffs twice daily | 400 | Step 2 | 13 | 25 | 12 | 25 | 12 | 14 | 12 | 14 | 12 | 25 | 12 | 25 | 12 | D |
| 125 µg MDI/spacer two puffs twice daily | 1000 | Step 3/4c | 14 | 26 | 13 | 26 | 13 | 26 | 13 | 15 | 13 | 26 | 13 | 26 | 13 | D(o) |
| 250 µg MDI/spacer, two puffs twice daily | 2000 | Step 4 | 15 | d | d | 27 | 14 | d | d | 27 | 14 | d | d | 27 | 14 | S |
| Flutiform | ||||||||||||||||
| 50/5, two puffs twice dailye | 400 L | Step 3 | 16 | 30 | 2 | 30 | 2 | 17 | 2 | 17 | 2 | 30 | 2 | 30 | 2 | D |
| 125/5, two puffs twice dailye | 1000 L | Step 4 | 17 | 31 | 16 | 31 | 16 | 31 | 16 | 18 | 16 | 31 | 16 | 31 | 16 | D(o) |
| 250/10, two puffs twice dailye | 2000 L | Step 4 | 18 | d | d | 32 | 17 | d | d | 32 | 17 | d | d | 32 | 17 | S |
| Mometasone (Twisthaler) | ||||||||||||||||
| 200 µg DPI, one dose once dailye | 400 | Step 2 | 19 | 25 | 1 | 25 | 1 | 14 | 1 | 14 | 1 | 25 | 1 | 25 | 1 | D |
| Qvar (Easi-Breathe) | ||||||||||||||||
| 50 µg, one dose twice dailye | 200 | Step 2 | 20 | 21 | 0 | 21 | 0 | 21 | 0 | 21 | 0 | 21 | 0 | 21 | 0 | S |
| 50 µg, two doses twice dailye | 400 | Step 2 | 21 | 25 | 20 | 25 | 20 | 14 | 20 | 14 | 20 | 25 | 20 | 25 | 20 | D |
| Seretide Accuhaler | ||||||||||||||||
| 100 µg, one dose twice daily | 400 L | Step 3 | 22 | 36 | 9 | 36 | 9 | 23 | 9 | 23 | 9 | 36 | 9 | 36 | 9 | D |
| 250 µg, one dose twice daily | 1000 L | Step 4 | 23 | 37 | 22 | 37 | 22 | 37 | 22 | 24 | 22 | 37 | 22 | 37 | 22 | D(o) |
| 500 µg, one dose twice daily | 2000 L | Step 4 | 24 | d | d | 38 | 23 | d | d | 38 | 23 | d | d | 38 | 23 | S |
| Seretide Evohaler | ||||||||||||||||
| 50/25 MDI/spacer, two puffs twice daily | 400 L | Step 3 | 25 | 39 | 13 | 39 | 13 | 26 | 13 | 26 | 13 | 39 | 13 | 39 | 13 | D |
| 125/25 MDI/spacer, two puffs twice daily | 1000 L | Step 4 | 26 | 40 | 25 | 40 | 25 | 40 | 25 | 27 | 25 | 40 | 25 | 40 | 25 | D(o) |
| 250/25 MDI/spacer, two puffs twice daily | 2000 L | Step 4 | 27 | d | d | 41 | 26 | d | d | 41 | 26 | d | d | 41 | 26 | S |
| Symbicort Turbohaler | ||||||||||||||||
| 200/6 DPI, one dose twice daily | 400 L | Step 3 | 28 | 42 | 4 | 42 | 4 | 29 | 4 | 29 | 4 | 42 | 4 | 42 | 4 | D |
| 400/12 DPI, one dose twice daily | 800 L | Step 4 | 29 | 43 | 28 | 43 | 28 | 43 | 28 | 27 | 28 | 43 | 28 | 43 | 28 | D(o) |
| Flutiform | ||||||||||||||||
| 50/5, two puffs twice daily plus LTRA | 400 L | Step 3 | 30 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | 31 | 16 | S |
| 125/5, two puffs twice daily plus LTRA | 1000 L | Step 4 | 31 | RSOf | 30 | 32 | 30 | RSOf | 30 | 32 | 30 | RSOf | 30 | 32 | 30 | S |
| 250/10, two puffs twice daily plus LTRA | 2000 L | Step 4 | 32 | d | d | RSOf | 31 | d | d | RSOf | 31 | d | d | RSOf | 31 | S |
| Mometasone (Twisthaler) | ||||||||||||||||
| 200 µg, DPI one dose once daily plus LTRA | 400 | Step 3 | 33 | 39 | 19 | 39 | 19 | 44 | 19 | 44 | 19 | 39 | 19 | 39 | 19 | D |
| Qvar (Easi-Breathe) | ||||||||||||||||
| 50 µg, one dose twice daily plus LTRA | 200 | Step 3 | 34 | 35 | 20 | 35 | 20 | 35 | 20 | 35 | 20 | 35 | 20 | 35 | 20 | S |
| 50 µg, two doses twice daily plus LTRA | 400 | Step 3 | 35 | 39 | 21 | 39 | 21 | 44 | 21 | 44 | 21 | 39 | 21 | 39 | 21 | D |
| Seretide Accuhaler | ||||||||||||||||
| 100 µg, one dose twice daily plus LTRA | 400 L | Step 3 | 36 | 37 | 22 | 37 | 22 | 37 | 22 | 37 | 22 | 37 | 22 | 37 | 22 | S |
| 250 µg, one dose twice daily plus LTRA | 1000 L | Step 4 | 37 | 40 | 36 | 38 | 36 | 40 | 36 | 38 | 36 | 40 | 36 | 38 | 36 | S |
| 500 µg, one dose twice daily plus LTRA | 2000 L | Step 4 | 38 | d | d | 41 | 37 | d | d | 41 | 37 | d | d | 41 | 37 | S |
| Seretide Evohaler | ||||||||||||||||
| 50/25 MDI/spacer, two puffs twice daily plus LTRA | 400 L | Step 3 | 39 | 40 | 25 | 40 | 25 | 40 | 25 | 40 | 25 | 40 | 25 | 40 | 25 | S |
| 125/25 MDI/spacer, two puffs twice daily plus LTRA | 1000 L | Step 4 | 40 | RSOf | 39 | 41 | 39 | RSOf | 39 | 41 | 39 | RSOf | 39 | 41 | 39 | S |
| 250/25 MDI/spacer, two puffs twice daily plus LTRA | 2000 L | Step 4 | 41 | d | d | RSOf | 40 | d | d | RSOf | 40 | d | d | RSOf | 40 | S |
| Symbicort Turbohaler | ||||||||||||||||
| 200/6 DPI, one dose twice daily plus LTRA | 400 L | Step 3 | 42 | 43 | 28 | 43 | 28 | 43 | 28 | 43 | 28 | 43 | 28 | 43 | 28 | S |
| 400/12 DPI, one dose twice daily plus LTRA | 800 L | Step 4 | 43 | 40 | 42 | 41 | 42 | 40 | 42 | 41 | 42 | 40 | 42 | 41 | 42 | S |
| Fluticasone (Flixotide Evohaler) | ||||||||||||||||
| 50 µg MDI/spacer, two puffs twice daily plus LTRA | 400 | Step 3 | 45 | 39 | 13 | 39 | 13 | 44 | 13 | 44 | 13 | 39 | 13 | 39 | 13 | D |
| 125 µg MDI/spacer, two puffs twice daily plus LTRA | 1000 | Step 4 | 44 | 40 | 45 | 40 | 45 | 40 | 45 | 46 | 45 | 40 | 45 | 40 | 45 | D(o) |
| 250 µg MDI/spacer, two puffs twice daily plus LTRA | 2000 | Step 4 | 46 | d | d | 41 | 44 | d | d | 41 | 44 | d | d | 41 | 44 | S |
| Beclomethasone (Clenil) | ||||||||||||||||
| 50 µg MDI/spacer two puffs twice daily plus LTRA | 200 | Step 3 | 47 | 48 | 1 | 48 | 1 | 48 | 1 | 48 | 1 | 48 | 1 | 48 | 1 | S |
| 100 µg MDI/spacer two puffs twice daily plus LTRA | 400 | Step 3 | 48 | 39 | 2 | 39 | 2 | 44 | 2 | 44 | 2 | 39 | 2 | 39 | 2 | D |
| Budesonide (Pulmicort Turbohaler) | ||||||||||||||||
| 100 µg DPI one dose twice daily plus LTRA | 200 | Step 3 | 49 | 50 | 3 | 50 | 3 | 50 | 3 | 50 | 3 | 50 | 3 | 50 | 3 | S |
| 200 µg DPI, one dose twice daily plus LTRA | 400 | Step 3 | 50 | 42 | 4 | 42 | 4 | 51 | 4 | 51 | 4 | 42 | 4 | 42 | 4 | D |
| 400 µg DPI, one dose twice daily plus LTRA | 800 | Step 4 | 51 | 43 | 50 | 43 | 50 | 43 | 50 | 46 | 50 | 43 | 50 | 43 | 50 | D(o) |
| Fluticasone (Flixotide Accuhaler) | ||||||||||||||||
| 50 µg DPI, one dose twice daily plus LTRA | 200 | Step 3 | 52 | 53 | 8 | 53 | 8 | 53 | 8 | 53 | 8 | 53 | 8 | 53 | 8 | S |
| 100 µg DPI, one dose twice daily plus LTRA | 400 | Step 3 | 53 | 36 | 9 | 36 | 9 | 54 | 9 | 54 | 9 | 36 | 9 | 36 | 9 | D |
| 250 µg DPI, one dose twice daily plus LTRA | 1000 | Step 4 | 54 | 37 | 53 | 37 | 53 | 37 | 53 | 55 | 53 | 37 | 53 | 37 | 53 | D(o) |
| 500 µg DPI, one dose twice daily plus LTRA | 2000 | Step 4 | 55 | d | d | 38 | 54 | d | d | 38 | 54 | d | d | 38 | 54 | S |
| Fluticasone (Flixotide Evohaler) | ||||||||||||||||
| 50 µg MDI/spacer, one puff twice daily plus LTRA | 200 | Step 3 | 56 | 45 | 12 | 45 | 12 | 45 | 12 | 45 | 12 | 45 | 12 | 45 | 12 | S |
| Symbicort Turbohaler | ||||||||||||||||
| 100/6, one dose twice daily | 200 L | Step 3 | 58 | 28 | 3 | 28 | 3 | 28 | 3 | 28 | 3 | 28 | 3 | 28 | 3 | S |
| 100/6, one dose twice daily plus LTRA | 200 L | Step 3 | 59 | 42 | 58 | 42 | 58 | 42 | 58 | 42 | 58 | 42 | 58 | 42 | 58 | S |
| Qvar (Easi-Breathe) | ||||||||||||||||
| 100 µg DPI, one dose twice daily | 400 | Step 2 | 60 | 25 | 20 | 25 | 20 | 61 | 20 | 61 | 20 | 25 | 20 | 25 | 20 | D |
| 100 µg DPI, two doses twice daily | 800 | Step 3/4c | 61 | 26 | 60 | 26 | 60 | 26 | 60 | 15 | 60 | 26 | 60 | 26 | 60 | D(o) |
| 100 µg DPI, one dose twice daily plus LTRA | 400 | Step 2 | 62 | 39 | 60 | 39 | 60 | 63 | 60 | 63 | 60 | 39 | 60 | 39 | 60 | D |
| 100 µg DPI, two doses twice daily plus LTRA | 800 | Step 3/4c | 63 | 40 | 61 | 40 | 61 | 40 | 61 | 46 | 61 | 40 | 61 | 40 | 61 | D(o) |
Rationale for step up/step down treatments
| Control: symptom only (see note 1 below) | FeNO algorithm 1 | FeNO algorithm 2 | |||
|---|---|---|---|---|---|
| Children aged < 12 years | Children aged ≥ 12 years | Children aged < 12 years | Children aged ≥ 12 years | Children aged < 12 years | Children aged ≥ 12 years |
Step up:
|
Step up:
|
Step up:
|
Step up:
|
Step up:
|
Step up:
|
Additional rules:
-
When increasing/decreasing IC, the steps are 200 µg budesonide equivalent per day, 400 µg budesonide equivalent per day, 800/1000 µg budesonide equivalent per day, 2000 µg budesonide equivalent per day. Children who step down from 200 µg budesonide equivalent per day will be on SABA only.
-
Where possible, delivery device should be maintained. In general, step up with the same inhaler device. If the maximum dose is reached on a dry powder device and step up is still required, switch to MDI/spacer. Therefore, when the maximum (for age) dose of IC is reached on the Accuhaler device and both LABA and LTRA have been added, the delivery device should be changed to an Evohaler device (with spacer) at the same dose. Younger children on Seretide Accuhaler 250, one dose twice daily, plus LTRA will therefore step up to Seretide Evohaler 125/25 MDI/spacer, two puffs twice daily. Older children on Seretide Accuhaler 500, one dose twice daily, plus LTRA will therefore step up to Seretide Evohaler 250/25 MDI/spacer, two puffs twice daily.
-
Children aged ≥ 12 years who step up from Symbicort Turbohaler 400/12 DPI one dose twice daily (800 µg budesonide equivalent) will step up to Seretide Evohaler 250/25 MDI/spacer two puffs twice daily (2000 µg budesonide equivalent).
General notes:
-
Note 1. The treatment steps for the symptom-only group are based on the BTS guidelines (2014). These guidelines are similar to the updated guidelines in 2016; however, the 2016 guidelines have recommendations for which there is not an appropriate inhaler device.
-
Note 2. If the FeNO responds to the increased IC and at the next appointment has not increased again by more than 50%, FeNO algorithm 2 will be applied; therefore, doses of IC above 400 µg budesonide equivalent would be supplemented with LABA/LRTA before increasing further.
-
Note 3. The drugs included on the treatment algorithm are based on Table 9 of the current BTS guidelines. A number of the drugs listed in the guideline are not included on the algorithm because (1) they are not licensed for use in children (Fostair, DuoResp Spiromax), (2) a suitable smart inhaler device is not available (Qvar autohaler, Asmabec, Budelin Novolizer, Asmanex Twisthaler, Relvar) or (3) children on these medications would not be eligible for the study [Alvesco Aerosol inhaler (ciclesonide)].
-
Note 4. If new drugs/devices become available during the study period, these will be considered for inclusion on the treatment algorithm if (1) they are licensed for children, (2) a suitable smart inhaler device is available and (3) they are appropriate given the step-up/-down rules listed above.
-
Note 5. There are differences in algorithms for symptom only/FeNO 2 and FeNO 1 at the lower and upper ends of the treatment spectrum. The algorithms for symptom only/FeNO 2 and FeNO 1 are as follows:
-
The same when the starting treatment step is 200 µg budesonide equivalent, 200 µg budesonide equivalent plus LTRA, 400 µg budesonide equivalent plus LABA and LTRA, 800–1000 µg budesonide equivalent plus LABA and LTRA, and 2000 µg budesonide equivalent ± LABA/LTRA.
-
Different for children of all ages when the starting treatment step is 400 µg budesonide equivalent, 400 µg budesonide equivalent plus LABA and 400 µg budesonide equivalent plus LTRA.
-
Different for children ≥ 12 years when the starting treatment step is 800–1000 µg budesonide equivalent, 800–1000 µg budesonide equivalent plus LABA and 800–1000 µg budesonide equivalent plus LTRA.
-
Appendix 2 Decision tree for fractional exhaled nitric oxide intervention

a At baseline, information about any recent step up is captured in the baseline CRF, question 13B. If the response to 13B is ‘yes’, then there has been a recent step up. If the response to 13B is ‘no’ or ‘unsure’ or ‘missing’, then we assume that there has been no recent step up. Recent = last 12 months. b, Children who are asymptomatic, but who have elevated FeNO (shown in navy at each visit), can step up more than once on the navy route during the 12-month follow-up period. However, they cannot step up treatment more than once unless they have stepped down after the first step up. For example, a child who steps up through the navy route at 3 months and steps down at 6 months could step up again at 9 months through the navy route, but a child who steps up through the navy route at 3 months, and has either no change at 6 months or a step up through coral or aqua routes, cannot step up again through the navy route at 9 months. c, At the 3-month assessment, the change in FeNO is calculated with respect to baseline FeNO value. d, At each of the 3-, 6-, 9- and 12-month assessments, ‘recent’ step up is since joining the study (i.e. a step up since baseline). e, Children who are poorly adherent can have only one step up through the coral route over the 12-month follow-up period. For example, any children who are poorly adherent at 6 months and who have previously stepped up through the coral route at 3 months will have no change to their treatment step at the 6-month appointment. Similarly, children who are poorly adherent at 9 months and who have previously stepped up through the coral route at 3 or 6 months will have no change to their treatment step at the 9-month appointment, and children who are poorly adherent at 12 months and who have previously stepped up through the coral route at 3 or 6 or 9 months will have no change to their treatment step at the 12-month appointment. f, At the 6-month assessment, change in FeNO is calculated with respect to the 3-month FeNO value. If the participant did not attend at the 3-month assessment, the change is based on the most recent FeNO value (in this case baseline). g, At the 9-month assessment, change in FeNO is calculated with respect to 6-month FeNO value. If the participant did not attend at the 6-month assessment, the change is based on the most recent FeNO value (in this case baseline or 3 months, whichever is most recent). h, At the 12-month assessment, change in FeNO is calculated with respect to 9-month FeNO value. If the participant did not attend at the 9-month assessment, the change is based on the most recent FeNO value (in this case baseline or 3 or 6 months, whichever is most recent). i, If at the most recent visit, the child has stepped up through the navy route, there will be no change to the treatment step. j, At consecutive visits, if the participant has an ACT score of < 20, FeNO ≥ 50% lower, adherence ≥ 70%, refer specialist opinion, the decision tree should be followed (i.e. step up, A2); however, the PI should be alerted for discussion of alternative diagnoses (e.g. whooping cough).
Appendix 3 Decision trees for standard care
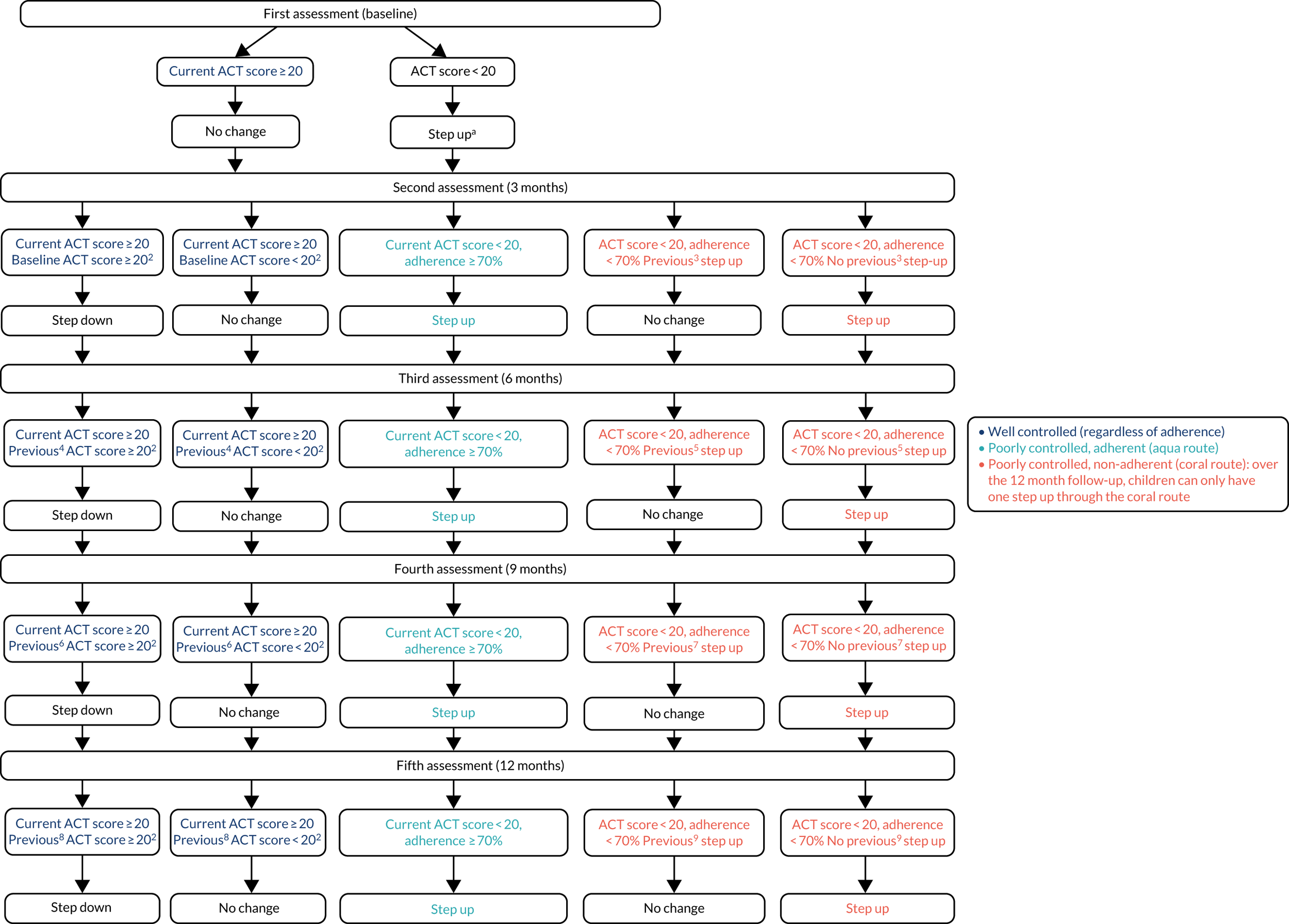
The ‘current’ ACT/CACT is the one completed at this assessment. a, Step up regardless of whether or not they were stepped up after their last asthma exacerbation (pre baseline). b, At 3, 6, 9 and 12 months, if the asthma is well controlled (i.e. current ACT score ≥ 20) adherence does not need to be considered in the decision tree. The decision to step down or make no change is based on current and previous ACT only. c, At the 3-month time point, when deciding whether or not there was a previous step up, we are thinking about previous step up through the coral route, (i.e. poor adherence) at baseline. Any step up through the aqua route, i.e. when adherence was good, is not relevant in making this decision. Over the 12-month period, patients can step up through the coral route only once. d, At the 6-month time point, the previous ACT will be at 3 months. If the participant did not attend for the 3-month visit, the previous ACT will be the baseline ACT. e, At the 6-month time point, when deciding whether or not there was a previous step up, we are thinking about previous step up through the coral route (i.e. poor adherence) at baseline or at 3 months. Any step up through the aqua route at 3 months, i.e. when adherence was good, is not relevant in making this decision. Over the 12-month period, patients can step up through the coral route only once. f, At the 9-month time point, the previous ACT will be at 6 months. If the participant did not attend for the 6-month visit, the previous ACT will be the 3-month ACT. If the participant did not attend either the 3 or the 6-month visit, the previous ACT will be the baseline ACT. g, At the 9-month time point, when deciding whether or not there was a previous step up, we are thinking about previous step up through the coral route (i.e. poor adherence) at baseline or at 3 months or at 6 months. Any step up through the aqua route at 3 months or at 6 months, i.e. when adherence was good, is not relevant in making this decision. Over the 12-month period, patients can step up through the coral route only once. h, At the 12-month time point, the previous ACT will be at 9 months. If the participant did not attend for the 9-month visit, the previous ACT will be the 6-month ACT. If the participant did not attend either the 6- or the 9-month visit, the previous ACT will be the baseline ACT. If the participant did not attend either the 3-, 6- or 9-month visit, the previous ACT will be the baseline ACT. i, At the 12-month time point, when deciding whether or not there was a previous step up, we are thinking about previous step up through the coral route, (i.e. poor adherence) at baseline or at 3, 6 or 9 months. Any step up through the aqua route at 3, 6 or 9 months, i.e. when adherence was good, is not relevant in making this decision. Over the 12-month period, patients can step up through the coral route only once.
Appendix 4 Summary of amendments to the RAACENO trial
| Protocol version, date | Summary of revisions |
|---|---|
| 1, 8 September 2016 | New document |
| 2, March 2017 | Amendments to:
|
| 3, 31 August 2017 | Amendments to:
|
| 4, 9 May 2018 | Amendments to:
|
| 5, 8 March 2019 | Amendments to:
|
| 6, 19 March 2020 | Amendments to:
|
Appendix 5 Recruitment, by site
| Site | Participants (n) |
|---|---|
| Secondary care | |
| NHS Grampian (Royal Aberdeen Children’s Hospital) | 20 |
| NHS Tayside (Ninewells Hospital, Dundee) | 10 |
| NHS Greater Glasgow & Clyde (Queen Elizabeth University Hospital, Glasgow) | 13 |
| University Hospitals of Leicester NHS Trust (Leicester Royal Infirmary) | 49 |
| Barnsley Hospital NHS Foundation Trust (Barnsley Hospital) | 14 |
| Sheffield Children’s NHS Foundation Trust (Sheffield Children’s Hospital) | 9 |
| University Hospitals of North Midlands Trust (Royal Stoke University Hospital) | 29 |
| The Royal Wolverhampton NHS Trust | 23 |
| NHS Ayrshire & Arran (University Hospital Crosshouse) | 1 |
| Royal Devon & Exeter NHS Foundation Trust | 20 |
| Plymouth Hospitals NHS Trust (Derriford Hospital) | 7 |
| Birmingham Women’s and Children’s NHS Foundation Trust (Birmingham Women’s and Children’s Hospital) | 9 |
| Nottingham University Hospitals NHS Trust (Nottingham Children’s Hospital) | 19 |
| Bradford Teaching Hospitals NHS Foundation Trust (Bradford Royal Infirmary) | 14 |
| University Hospital Southampton NHS Foundation Trust | 2 |
| NHS Lothian (Royal Hospital for Sick Children) | 19 |
| Derby Teaching Hospitals NHS Foundation Trust (Royal Derby Hospital) | 15 |
| Walsall Healthcare NHS Trust (Walsall Manor Hospital) | 21 |
| University Hospitals Bristol (Bristol Children’s Hospital) | 18 |
| Leeds Teaching Hospitals NHS Trust (Leeds General Infirmary) | 16 |
| Kings College Hospital Foundation Trust (Kings College Hospital) | 12 |
| Barts Health NHS Trust | 16 |
| Newcastle Upon Tyne Hospitals NHS Foundation Trust (Royal Victoria Infirmary) | 7 |
| Alder Hey Children’s NHS Foundation Trust (Alder Hey Children’s Hospital) | 11 |
| Manchester University NHS Foundation Trust (Wythenshawe Hospital) | 13 |
| St George’s University Hospital NHS Foundation Trust (St George’s University of London) | 7 |
| Shrewsbury & Telford Hospital NHS Trust (The Royal Shrewsbury Hospital) | 8 |
| Brighton & Sussex University Hospitals NHS Trust (Royal Alexandra Children’s Hospital) | 12 |
| Countess of Chester Hospital NHS Foundation Trust (Countess of Chester Hospital) | 11 |
| Harrogate and District Foundation Trust (Harrogate District Hospital) | 19 |
| The Dudley Group NHS Foundation Trust (Russells Hall Hospital) | 1 |
| NHS Lanarkshire (Wishaw General Hospital) | 13 |
| The Pennine Acute Hospitals NHS Trust (Royal Oldham Hospital) | 20 |
| Lancashire Teaching Hospitals (Royal Preston Hospital) | 3 |
| Southport & Ormskirk NHS Trust (Ormskirk Hospital) | 5 |
| Wirral University Hospital NHS Foundation Trust (Arrowe Park Hospital) | 13 |
| Primary care | |
| Hanscombe House Surgery | 5 |
| Parsonage Surgery | 2 |
| Barrack Lane Medical Centre | 2 |
| Roundwell Medical Centre | 4 |
| Ixworth Surgery | 1 |
| Lakenham Surgery | 1 |
| Castle Partnership | 1 |
| Total recruitment | 515 |
Appendix 6 Post hoc subgroup analysis
| Subgroup factors | Intervention group, n/N (%) | Standard-care group, n/N (%) | Adjusted ORa (95% CI) | p-value | p-value for interaction |
|---|---|---|---|---|---|
| LTRA use during follow upb | |||||
| LTRA taken ever | 94/164 (57.32) | 89/160 (55.63) | 1.07 (0.69 to 1.67) | 0.764 | 0.141 |
| LTRA never taken | 25/81 (30.86) | 32/76 (42.11) | 0.60 (0.31 to 1.18) | 0.138 | |
| High_FeNOc | |||||
| Low FeNO | 87/185 (47.0) | 79/166 (47.6) | 0.99 (0.61 to 1.58) | 0.959 | 0.412 |
| High FeNO | 36/70 (51.4) | 50/85 (58.8) | 0.71 (0.39 to 1.29) | 0.260 | |
| Asthma phenotyped | |||||
| Stable concordant | 21/44 (47.7) | 21/45 (46.7) | 0.93 (0.39 to 2.18) | 0.865 | 0.738 |
| Stable discordant | 20/33 (60.6) | 17/30 (56.7) | 1.10 (0.36 to 3.40) | 0.866 | |
| Unstable | 25/61 (41.0) | 39/69 (56.5) | 0.55 (0.24 to 1.26) | 0.160 | |
FIGURE 17.
Asthma phenotype at baseline and 3 months.
Appendix 7 Cost of the primary intervention (measurement of fractional exhaled nitric oxide)
| Type | Lifespan (years) | Cost (£) |
|---|---|---|
| NIOX VERO® cost | 5 | 2500.00 |
| Cartridge: 60 | 1 | 600.00 |
| Cartridge: 100 | 1 | 800.00 |
| Cartridge: 300 | 1 | 1200.00 |
| Breathing handle | 1 | 150.00 |
| Equivalent annual cost | 553.70 | |
| Total cost per test (annual throughput = 300) | 6.35 | |
| Total cost per test (annual throughput = 60) | 21.73 | |
Appendix 8 Tables of unit costs for valuation of health-care use
| Currency code | Currency description | Number of FCSs | National average unit cost (£) |
|---|---|---|---|
| PD12A | Paediatric, asthma or wheezing, with CC score of 4+ | 828 | 740 |
| PD12B | Paediatric, asthma or wheezing, with CC score of 1–3 | 9791 | 620 |
| PD12C | Paediatric, asthma or wheezing, with CC score of 0 | 11,697 | 562 |
| Weighted average across all non-elective short stay admissions | 594 | ||
| Currency code | Currency description | Number of FCSs | National average unit cost (£) |
|---|---|---|---|
| PD12A | Paediatric, asthma or wheezing, with CC score of 4+ | 416 | 2507 |
| PD12B | Paediatric, asthma or wheezing, with CC score of 1–3 | 3427 | 1913 |
| PD12C | Paediatric, asthma or wheezing, with CC score of 0 | 2623 | 1696 |
| Weighted average across all non-elective long stay admissions | 1863 | ||
| Inflated to 2019/19 prices using PSSRU inflation indices | 1913 | ||
| Currency code | Currency description | Number of FCSs | National average unit cost (£) |
|---|---|---|---|
| PD12A | Paediatric, asthma or wheezing, with CC score of 4+ | 271 | 428 |
| PD12B | Paediatric, asthma or wheezing, with CC score of 1–3 | 1186 | 372 |
| PD12C | Paediatric, asthma or wheezing, with CC score of 0 | 913 | 413 |
| Weighted average across all day case admissions | 394 | ||
| Currency code | Currency description | Severity of asthma exacerbationa | Treatmentb | National average unit cost (£) |
|---|---|---|---|---|
| VB06Z | Emergency medicine, category 1 investigation with category 3–4 treatment (type 1 admitted) | Severe (62.5%)c | Inhaler via spacer for SABAs (salbutamol), supplemental oxygen, oral prednisolone | 232 |
| VB04Z | Emergency medicine, category 2 investigation with category 4 treatment (type 1 admitted) | Life-threatening (37.5%)c | IV cannulation, nebulisation, guidance advice, vital signs monitoring, supplemental oxygen, administration of infusion | 318 |
| Weighted average of all A&E attendances using estimated proportions of asthma severity presenting to hospital | 264 | |||
Appendix 9 Model selection
Model specifications tests were conducted on the 12-month costs of health-care resource use not associated with an exacerbation, treatment (both the amount prescribed and the amount controlled for adherence) and the cost incurred on the family [direct (over-the-counter medications and travel) and indirect (displaced time)]. For GLMs, a link function and family must be specified. The link function describes the function that relates the mean of the outcome to the linear index of covariates. 124 The family refers to the relationship of the variance to the predicted outcome, which is a distribution of the exponential family. 84,85,124 Tests were conducted sequentially on the link function then the family, with the log-link and gamma family as a base case.
The link function was tested and chosen using the Box–Cox approach, where a coefficient of 1, 0.5 and 0 suggests the linear, square root and natural log-functions, respectively. 85 The results of these are shown in column 2 of Table 54. The Pregibon link test coefficient should be close to zero and not significant; in all cases this was true. 85 For all cost models, the log-link was deemed appropriate for both tests.
The family was chosen using a modified Park test, where the relationship between the variance of the error term and the mean is quantified;85 see column 4 of Table 54 for the results. The coefficient indicates which distributional family is preferred. Should the coefficient equal 1, the Poisson is appropriate. If the coefficient equals 2, the gamma is appropriate. In addition, if the coefficient is 3, the inverse Gaussian is appropriate. The gamma distribution was chosen in all cases.
| Cost category | Link | Family | First part | Second part | ||
|---|---|---|---|---|---|---|
| Box–Cox | Pregibon | Modified Park test | Link | Family | ||
| Background | 0.00 | 0.18 | 2.29 | Probit | Log | Gamma |
| Treatment (prescribed) | 0.14 | 0.08 | 2.32 | NA | Log | Gamma |
| Treatment (adherence) | 0.09 | 0.36 | 2.44 | NA | Log | Gamma |
| Direct family | 0.02 | 0.17 | 1.65 | Probit | Log | Gamma |
| Indirect family | 0.10 | –0.03 | 1.85 | Probit | Log | Gamma |
Appendix 10 Additional health economic results
| Individual health-care resource | Mean (SD) number of contacts | |
|---|---|---|
| Intervention group (n = 230) | Standard-care group (n = 213) | |
| Total | 1.49 (3.34) | 1.49 (2.79) |
| Primary care | 0.97 (2.31) | 1.09 (2.23) |
| GP | 0.64 (1.47) | 0.75 (1.65) |
| Nurse | 0.24 (1.59) | 0.15 (0.54) |
| NHS 24/111 | 0.03 (0.16) | 0.09 (0.51) |
| Out-of-hours GP service | 0.04 (0.22) | 0.04 (0.21) |
| Walk in | 0.02 (0.15) | 0.05 (0.27) |
| Pharmacist | 0.00 (0.00) | 0.01 (0.10) |
| Secondary care | 0.52 (2.28) | 0.40 (1.01) |
| Emergency department | 0.10 (0.47) | 0.09 (0.32) |
| Hospital outpatient | 0.20 (0.49) | 0.25 (0.92) |
| Hospital inpatient | 0.01 (0.11) | 0.02 (0.15) |
| Day case | 0.01 (0.09) | 0.00 (0.00) |
| Bronchoscopy | 0.01 (0.09) | 0.01 (0.10) |
| Ambulance | 0.01 (0.09) | 0.01 (0.11) |
| Other (physiotherapist/psychologist/speech and language therapist/psychologist) | 0.18 (1.95) | 0.01 (0.14) |
| Cost category | Mean (SD) cost (£) | GLM specification | Incremental cost (£) (intervention vs. control) (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Intervention group | Standard-care group | Family | Link | |||
| Background cost | 116.93 (246.42) | 190.54 (740.49) | Gamma | Log | –28.16 (–105.13 to 48.81) | 0.47 |
| Direct participant cost | 10.46 (1.60) | 9.17 (11.52) | Gamma | Log | 0.92 (–2.30 to 4.13) | 0.58 |
| Indirect productivity | 1773.51 (300.11) | 1593.49 (3185.11) | Gamma | Log | 41.20 (–649.43 to 731.83) | 0.91 |
| Prescribed treatment | 805.42 (2088.16) | 772.17 (1641.66) | Gamma | Log | 30.19 (–122.30 to 182.68) | 0.70 |
| Adherence adjusted treatment | 630.90 (1961.65) | 604.97 (1541.54) | Gamma | Log | 18.08 (–118.83 to 154.99) | 0.80 |
| Strategya | Cost (£) | Δ Cost (£) | Number of exacerbations | Δ Exacerbations | QALYs | Δ QALYs | Incremental cost per exacerbation avoided (£) | Incremental cost per QALY gained (£) | Probability cost-effective | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| £13,000 | £20,000 | £30,000 | |||||||||
| Throughput of FeNO testing of 60 per annum | |||||||||||
| Standard care | 2008 | – | 1.03 | – | 0.95 | – | – | – | 0.68 | 0.67 | 0.66 |
| Intervention | 2078 | 70 | 1.02 | –0.01 | 0.95 | 0.00 | –8544 | 1,943,759 | 0.32 | 0.33 | 0.34 |
| Pooled cost of asthma exacerbations | |||||||||||
| Standard care | 2011 | – | 1.03 | – | 0.95 | – | – | – | 0.59 | 0.59 | 0.59 |
| Intervention | 2027 | 17 | 1.02 | –0.01 | 0.95 | 0.00 | –1750 | 409,072 | 0.41 | 0.41 | 0.41 |
Appendix 11 Topic guides
Family/child
-
Greetings and introductions. Confirm OK to be audio-recorded.
-
[Begin audio-recording]. Go through study aim, participant information sheet and consent form, explain purpose and use of data, and reassure anonymity (use of pseudonyms) in knowledge/publications that should result. Reminder of inclusion criteria.
-
With consent – if no, stop recording and delete at earliest opportunity; take handwritten notes if alternative allowed.
-
Purpose of the study is to explore experiences and ascertain acceptability of the intervention.
-
We’re aiming to solicit in-depth feedback on process of taking part in nitric oxide measurement-based treatment/clinical trial (whether possible to deliver) and to access any additional observations made around acceptability/process.
-
It’s important to point out there are no right or wrong answers; we just want to hear about your experience(s).
-
Your name and what we discuss will remain confidential, though your contributions will be used (anonymously) in publications. The interview will take around 30–45 minutes (maximum 60 minutes).
-
Before we start, do you have any questions or concerns?
| 1. Recruitment process: can you reflect on how you came to take part? |
|
|
| 2. Trial randomisation: what did you think about/understand of this? |
|
|
|
|
|
| 3. Appointments: how did these go (smart inhaler, algorithm, distance/time, etc.)? |
|
|
| 4. Algorithm – in a bit more depth – what did this mean to you re treatment, etc.? |
|
|
| 5. Effectiveness/difference: overall, what impact has RAACENO had on X’s asthma experience/management? |
|
|
| 6. Future: looking forward, what aspects of the trial would you like to see continue, etc.? |
|
|
ENDING
-
Is there anything else you would like to say about your experience, or questions you have?
-
Anything we haven’t covered?
Thank, switch off recording, reassure about confidentiality, farewells.
Research staff/clinicians
-
Go through participant information sheet and consent form, explain purpose/use of data and reassure anonymity (use of pseudonyms).
-
Begin recording (with consent; notes otherwise), greetings and introductions.
-
Purpose of the study is to explore experiences and ascertain acceptability of the intervention.
-
We’re aiming to solicit in-depth feedback on process of taking part in trial. Research staff interviewed to understand feasibility of intervention delivery from provider perspectives and to access any additional observations made around acceptability/process.
-
It’s important to point out there are no right or wrong answers; we just want to hear about your experience.
-
Your name and what we discuss will remain confidential, though your contributions will be used in publications (anonymised), and it will take around 30 minutes (maximum 45 minutes). Can be face to face or by telephone.
-
Before we start do you have any questions or concerns?
| Topic area | Specific questions (to guide conversation) | Range of prompts (non-verbatim conversation cues) |
|---|---|---|
| Research culture | 1. How do you feel the trial is going so far? |
|
| 2. What do you think about the merit of this trial? |
|
|
| 3. Have you worked with or been involved in trials before? |
|
|
| 4. Do you feel you received enough help when you started the trial? |
|
|
| Recruitment Process | 5. Can you explain to me what your role is and what involvement you have with potential patients? |
|
| 6. What are the common questions you get asked and how do you answer them? |
|
|
| 7. Can you explain to me how randomisation works the way you explain it to patients? |
|
|
| Intervention and algorithm | 8. How would you say patients are reacting to the study? |
|
| 9. Do people seem open to accepting the role of technology and the algorithm? |
|
|
| 10. Do you ever disagree with the algorithm, and if so how does this change in decision-making affect your preparation for appointments? |
|
|
| 11. What do you tell people about the algorithm? |
|
|
| 12. What are your thoughts on the use of smart inhalers? |
|
|
| 13. What do patients think about smart inhalers? |
|
|
| Future | 14. Do you think this could be easily scaled up as usual care? |
|
| 15. What would you say is the impact this intervention has had on patients and on the clinic? |
|
|
| 16. Do you feel this technique of smart inhalers could or should be used more often? |
|
|
| 17. Overall, how would you describe your experience? |
|
ENDING
-
Is there anything else you would like to say about your experience, or questions you have?
-
Thank, switch off recording, reassure about confidentiality, farewells.
List of abbreviations
- ACT
- Asthma Control Test
- AE
- adverse event
- ASHE
- Annual Survey of Hours and Earnings
- BTS
- British Thoracic Society
- CACE
- complier-average causal effect
- CACT
- Children’s Asthma Control Test
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRN
- Clinical Research Network
- DMC
- Data Monitoring Committee
- DNA
- deoxyribonucleic acid
- EQ-5D
- EuroQol-5 Dimensions
- FeNO
- fractional exhaled nitric oxide
- FEV1
- forced expiratory volume in 1 second
- GLM
- generalised linear model
- GP
- general practitioner
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- IC
- inhaled corticosteroid
- IRR
- incidence rate ratio
- ITT
- intention to treat
- LABA
- long-acting beta-agonist
- LTRA
- leukotriene receptor antagonist
- MDI
- metered dose inhaler
- NICE
- National Institute for Health and Care Excellence
- OC
- oral corticosteroid
- PAGES
- Paediatric Asthma Gene Environment Study
- PAQLQ
- Paediatric Asthma Quality of Life Questionnaire
- PI
- principal investigator
- PIL
- patient information leaflet
- PPI
- patient and public involvement
- PSSRU
- Personal Social Service Research Unit
- QALY
- quality-adjusted life-year
- RAACENO
- Reducing Asthma Attacks in Children using Exhaled Nitric Oxide
- RCT
- randomised controlled trial
- RN
- research nurse
- SABA
- short-acting beta-agonist
- SAE
- serious adverse event
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- TSC
- Trial Steering Committee
