Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 15/178/09. The contractual start date was in August 2017. The final report began editorial review in December 2021 and was accepted for publication in April 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Lindsay et al. This work was produced by Lindsay et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Lindsay et al.
Chapter 1 Introduction
Crohn’s disease
Crohn’s disease (CD) affects at least 115,000 people in the UK. 1 It is characterised by chronic inflammation of the gastrointestinal tract resulting from an inappropriate and prolonged effector lymphocyte reactivity to luminal dietary and microbial antigen. In addition, there is evidence of impaired barrier function with increased permeability, microbial activation of aspects of the innate immune system and a failure of the normal process of restitution. Apart from rare cases of very early-onset CD, which can have a monogenic aetiology, CD patients have polygenic susceptibility, with each polymorphism contributing a small increased risk of disease. There are some well-described environmental risk factors for CD, including smoking, but the exact aetiology of CD is not completely defined. The peak incidence of CD is in adolescence and young adulthood, and therefore affected individuals may live with symptoms for many years. 2
Untreated active disease can progress, leading to stricturing/penetrating complications and severe and debilitating symptoms, recurrent hospitalisation and disability. 2 Disease complications are the most common indication for surgery to resect the affected intestine, which may result in a temporary or permanent stoma. In addition to the substantial impact of CD on patients’ quality of life, the economic burden of the condition is high: CD profoundly impairs work productivity and is associated with significant healthcare costs, the majority of which relate to biologic therapies such as the anti-tumour necrosis factor (TNF) agents ustekinumab and vedolizumab. 3 In the UK, CD accounts for 27,000 hospital admissions each year,4 and this figure is set to rise as the incidence of CD increases worldwide. Although the introduction of biosimilar medication has reduced anti-TNF therapy-related costs, the costs associated with medical therapy for CD continue to increase year on year, mainly because of an increasing number of patients starting biologic medications. 3,4
Current service provision
Traditional medical therapy consists of drugs that either have a broad impact on mucosal immune activity (corticosteroids) or are targeted to specific immune pathways {anti-TNF agents [infliximab/adalimumab], anti-integrins [vedolizumab (Entyvio, Takeda UK Limited, London, UK)] or antibodies that target the p40 subunit of interleukin (IL) 12 and IL-23 [ustekinumab (Stelara, Janssen Pharmaceuticals, New Brunswick, NJ, USA)]}. Early use of optimised conventional and biologic therapy is unable to deliver sustained clinical and mucosal remission in the majority of patients. In a UK trial5 of personalised anti-TNF therapy in CD, primary non-response was reported in 24% of patients at 14 weeks, and non-remission was reported in 63% of patients at 52 weeks after starting anti-TNF therapy. The National Institute for Health and Care Excellence approves second-line therapy with the monoclonal antibodies vedolizumab and ustekinumab for patients with refractory CD. However, biologic medications have reduced efficacy in sustaining remission in treatment-experienced patients. A recent meta-analysis6 showed that patients with primary non-response to anti-TNF therapy are less likely to achieve remission with ustekinumab as an induction therapy than those with secondary loss of response to anti-TNF therapy. In addition, vedolizumab as an induction therapy did not achieve a Crohn’s Disease Activity Index (CDAI)-100 response in patients previously exposed to anti-TNF in one phase III clinical trial7 and was no more effective than placebo in inducing clinical remission at week 6 in another phase III clinical trial, with approximately 30% of patients in remission at week 10. 8
Patients with treatment-refractory CD suffer from persistent physical symptoms, treatment-related morbidity associated with chronic steroid use and psychological morbidity, which in turn have an impact on work productivity and lifetime achievement. Although a single short ileo-caecal resection can result in enhanced quality of life in the short term, CD frequently recurs despite post-operative preventative medication. Repeated surgery or resections in patients with extensive small bowel disease may result in nutritional deficiency, necessitating enteral or parenteral supplementary feeding. Refractory colonic CD or complications at critical parts of the bowel such as the rectum will often result in the need for a permanent stoma.
Alternative treatment for refractory CD remains an unmet need. One potentially effective alternative is haematopoietic stem cell transplant (HSCT). Research has supported the role of HSCT in the treatment of other autoimmune diseases, including multiple sclerosis (MS). Clinical trials of HSCT in MS patients have demonstrated a halt in detectable inflammatory activity for a prolonged period following treatment,9 and prolonged time to disease progression when compared with disease-modifying therapies. 10 Previous non-randomised studies suggest remarkable benefits of HSCT for the treatment of CD, with long-term treatment-free remission in some patients,11 and, when treatment-free remission declines with time, subsequent disease control with previously ineffective treatments. 12,13
Theory of haematopoietic stem cell transplant in autoimmune disease
Haematopoietic stem cell transplant seeks to relieve symptoms by resetting the autoreactive immune system and ablating immune memory. Cyclophosphamide and granulocyte colony-stimulating factor (G-CSF) induce stem cell proliferation within the bone marrow and subsequent release of stem cells into the bloodstream (termed ‘mobilisation’), from where they can be removed (or ‘harvested’) from the body. Subsequent conditioning with high-dose chemotherapy depletes the autoreactive immunological memory, after which stem cells are reinfused to rebuild a naive immune system. 14 HSCT is believed to stimulate the renewal of regulatory T cells, increase the diversity of T-cell receptors and reduce autoantibodies. 15 However, the exact mechanisms of action and time course of response to HSCT in refractory CD remains unknown.
Haematopoietic stem cell transplant in Crohn’s disease: the ASTIC trial
Currently, HSCT is considered an experimental therapy and is rarely used for treatment of refractory CD in the UK. A randomised controlled trial (RCT) of autologous stem cell transplantation for Crohn’s disease (ASTIC) was designed under the European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP). 16 Patients in the ASTIC trial underwent high-dose cyclophosphamide (4 g/m2) and G-CSF mobilisation before being randomised to receive immediate HSCT or standard care for 1 year. No benefit of HSCT compared with standard care was found using the primary end point of medication-free clinical remission for 3 months, with no imaging or endoscopic evidence of disease activity. Furthermore, HSCT was associated with a high burden of adverse events (AEs) and serious adverse events (SAEs), including one patient death. 17
Although the ASTIC trial17 did not achieve its primary end point and was associated with significant toxicity, a modified HSCT regimen may have a role in the treatment of refractory CD for a number of reasons. 13,18-23 First, the primary end point used in the ASTIC trial was notably more ambitious than that used in any other published trial in CD. HSCT was significantly more effective than standard care in several secondary end points, including clinical remission and endoscopic disease activity, all of which are important both to patients and to the NHS. Notably, 6 out of 23 treated patients showed no evidence of active CD ulceration on endoscopic and radiological assessment, yet did not meet the primary end point owing to ongoing gastrointestinal symptoms. These symptoms were likely to reflect prior, irreversible bowel damage rather than ongoing active disease. On completion of the trial, usual-care arm patients also underwent HSCT. A subsequent analysis of the combined cohort reported regression of all endoscopic ulceration in 50% of patients at 1 year post transplantation,24 although no post-HSCT maintenance therapy was given to patients with recurrent disease. A single-centre cohort study13 of patients undergoing HSCT reported that, although treatment-free remission declined over time, 80% of patients responded to drugs to which they were previously refractory, such that the marked reduction in drug-free remission reported over the study’s 5-year period is reversed when assessing remission rates on therapy.
Despite the potential efficacy of HSCT, toxicity remains a significant barrier to the use of HSCT for CD in the UK. A large number of AEs were reported in the ASTIC trial, one resulting in death. 16 An analysis that comprised eight cohort studies and the ASTIC trial estimated a treatment-related mortality of 6.4%. 25 Specialist reviews16,18,19 of the ASTIC trial implicated the high-dose cyclophosphamide used in mobilisation and conditioning to the infectious AEs observed. Cyclophosphamide for mobilisation of peripheral blood stem cells (PBSCs) was given at a relatively high dose of 4 g/m2 to both the usual-care arm and the intervention arm patients in the ASTIC trial. The dose was chosen to ensure that patients in the usual-care arm received immune-suppressing therapy that would improve disease activity and to prevent a disease flare during G-CSF treatment. Cyclophosphamide at this dose had a significant short-term benefit on disease activity, but led to an increase in the number of SAEs. 13,26 Subsequent research13,20,21,27 has linked lower-intensity mobilisation and conditioning regimens to lower morbidity in autoimmune diseases. Furthermore, clinical factors such as smoking and active perianal disease that predict AEs22 have been identified, and the importance of supportive care in reducing SAEs has been demonstrated. 13,19,27
Rationale
Patients with refractory CD experience severe and lifelong morbidity, which arises from both the condition and its treatment. The current lack of an effective alternative means that patients with refractory CD are often prescribed successive courses of biologic and conventional immune suppression, which deliver a diminishing potential for benefit and an increased likelihood of inducing further harm. Ongoing active disease results in a significant impact on patient quality of life and productivity. Disease progression may mandate repeated surgery, with the risk of short bowel syndrome and the requirement for lifelong total parenteral nutrition. When CD affects critical areas of the intestine, surgical resection requires temporary or permanent stoma formation. Given this context, HSCT may be appropriate in a carefully selected group of treatment-refractory patients despite its many side effects. The previously reported risks associated with HSCT may be decreased using reduced-intensity regimens and increased supportive care (see Chapter 1, Introduction, Haematopoietic stem cell transplant in Crohn’s disease: the ASTIC trial). Considering the subsequent analysis of the ASTIC trial and other large single-centre series,16,24 further well-designed trials are needed to determine the level and duration of benefit.
Our Trial Steering Committee (TSC) patient representative stated the following:
The prospect of a potential respite from Crohn’s Disease will give most patients such hope that they will be more than willing to take a risk that this time something may actually work. The disease is so debilitating and affects your whole life, so that any slight hope will be grasped with both hands. Every single day is made miserable by Crohn’s and the idea that, by taking part in a trial such as ASTIC, could make your quality of life so much better, most people would think that the possible benefits are well worth the risk.
The ASTIClite trial was a multicentre, parallel-group RCT that sought to assess the clinical effectiveness, safety and long-term impact of low-dose cyclophosphamide/G-CSF mobilisation with reduced-intensity conditioning (HSCTlite) compared with standard care in inducing regression of intestinal ulceration in refractory CD patients at 1 year. Patients with evidence of disease activity after HSCT could be treated with biologic therapy from week 24. As the time course of response and mechanism of action of HSCT in CD is unknown, mechanistic substudies were embedded within the ASTIClite trial to evaluate the timeline of treatment response, post-HSCTlite immune reconstitution and the mechanisms via which HSCTlite improves CD and restores responsiveness to anti-TNF therapy in patients who were previously refractory. As part of the ASTIClite trial, participants were also invited to take part in a long-term follow-up through the EBMT, to assess the safety and efficacy of HSCTlite beyond the data collected as part of the ASTIClite clinical trial.
Given the incidence of AEs in the ASTIC trial, the transplant mobilisation and conditioning regimens were modified as follows: for mobilisation, the cyclophosphamide dose was reduced from 4 g/m2 to 1 g/m2 given prior to G-CSF, and for the conditioning regimen the dose of cyclophosphamide was reduced from 200 mg/kg to 120 mg/kg [given as 60 mg/kg on days –3 and –2, whereas the anti-thymocyte globulin (ATG) dose remained the same as in the ASTIC trial at 2.5 mg/kg for three days (days –3, –2 and –1; total 7.5 mg/kg)]. Given the anticipated relapse rate with lower doses of cyclophosphamide, additional immunosuppression was provided with fludarabine (25 mg/m2 on days –6, –5, –4, –3 and –2; total 125 mg/m2), which for many years has widely and routinely been used in combination with alkylating agent chemotherapy (including cyclophosphamide) and therapeutic antibodies (including ATG) in conditioning regimens for allogeneic HSCT. It can be also be used, in similar combinations, in conditioning regimens for patients with severe autoimmune diseases who are undergoing autologous HSCT. Otherwise the regimen included supportive care drugs, with accommodation for local protocols.
In addition, ASTIClite differed from the original ASTIC trial in its eligibility criteria, which were modified to exclude patients with penetrating intestinal and active undrained perianal disease based on the assessment of factors associated with SAEs in ASTIC.
Finally, the introduction of additional advanced and biologic means increased the range of drugs to which patients had been exposed prior to trial entry. These criteria were expected to have affected the nature of patients recruited with severe, resistant disease.
Chapter 2 Research objectives
The ASTIClite trial was a multicentre, parallel-group RCT designed to evaluate the efficacy, safety and long-term impact of low-dose cyclophosphamide and G-CSF mobilisation with a reduced intensity condition regimen (HSCTlite) in patients with refractory CD compared with usual care.
Primary objective
-
To assess the efficacy of HSCTlite compared with usual care at inducing regression of intestinal ulceration in patients with refractory CD at week 48.
Secondary objective
-
To assess the impact of HSCTlite on clinical disease activity and quality of life compared with usual care.
Safety objectives
-
Ongoing monitoring of AEs and SAEs continued throughout the trial using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE). The Data Monitoring and Ethics Committee (DMEC) initially assessed the safety of low-dose cyclophosphamide and G-CSF as part of an embedded pilot study.
Exploratory objectives
-
To assess the safety and efficacy of anti-TNF therapy (or alternative agents where contraindicated) in HSCTlite-treated patients with endoscopic disease recurrence at week 24.
Mechanistic objectives
-
To determine the early impact of HSCTlite on mucosal disease via intestinal magnetic resonance imaging (MRI) at week 4.
-
Through immune profiling of peripheral blood and transcriptional profiling of mucosal biopsies:
-
Characterise immune reconstitution after HSCTlite and assess the impact of HSCTlite.
-
Assess immunological events that precede disease recurrence after HSCTlite.
-
Assess the mechanism of restoration of response to anti-TNF therapy.
-
The last two mechanistic objectives could not be met because of small patient numbers. Week 4 MRIs were also not assessed for the same reason.
Chapter 3 Methods
Some text in this chapter has been reproduced from the ASTIClite trial protocol. 28
Some text in this chapter has been reproduced from Snowden et al. 29 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The final protocol for this study can be found on the National Institute for Health and Care Research website and has also been published. 29
Trial design
The ASTIClite trial was a multicentre, parallel-group RCT. Eligible patients were randomised in a 2 : 1 ratio to receive low-dose cyclophosphamide and G-CSF mobilisation and reduced-intensity conditioning (HSCTlite) or usual care.
An internal pilot study was incorporated to determine whether or not the mobilisation regimen of 1 g/m2 cyclophosphamide delivered effective stem cell harvest without a CD activity flare. The ability to recruit to target was assessed after 10 months of recruitment, with stop/go criteria set at 60% of the anticipated recruitment figure at that time.
The trial is reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement for individually randomised parallel group trials. 30
Important changes to methods after trial commencement
Throughout the trial, 11 substantial amendments and three non-substantial amendments were approved. Significant changes to patient care during the trial, approved via amendments, included the addition of genetic and/or functional tests during screening, to exclude monogenic causes of intestinal inflammation in patients with predictive clinical phenotype; allowing the possibility of a second attempt at mobilisation with reduced or no cyclophosphamide in patients who failed to mobilise the first time; an increase in the dose of methylprednisolone from 1 mg/kg/day to 2 mg/kg/day, with scope to increase further up to 500–1000 mg in the setting of an ATG reaction; allowing a computerised tomography (CT) scan at screening as an alternative to MRI if MRI was contraindicated, and to remove MRI from week 4, 24 and 48 visits where contraindicated; and clarification on reintroducing anti-TNF therapy at week 24 for patients with MS, where therapy was to be considered on a case-by-case basis if anti-TNF was contraindicated. Full details of each amendment can be found in Appendix 1.
Participants
Recruitment commenced on 9 May 2018. Patients with refractory CD were eligible for randomisation according to the following eligibility criteria.
Inclusion criteria
-
Any gender and aged between 18 and 60 years.
-
Willing and able to provide full informed consent.
-
Well nourished and of healthy body weight in the opinion of the principal investigator (PI) [typically a body mass index (BMI) score of > 18.5 kg/m2].
-
Diagnosis of CD using colonoscopy, histology and/or radiology.
-
Disease duration of at least 6 months.
-
Disease distribution accessible to endoscopic assessment (jejuno-ileal, ileo-caecal or colonic).
-
Active clinical CD activity with impaired quality of life at any time within 3 months prior to randomisation into the trial, as assessed by a gastroenterology clinician.
-
Refractory or intolerant to azathioprine, mercaptopurine or methotrexate.
-
Refractory or intolerant to at least two classes of biologic therapy (currently anti-TNF therapy, vedolizumab or ustekinumab) despite dose optimisation.
-
Surgery is considered not appropriate or has been declined.
-
Endoscopic evidence of active disease in screening [Simple Endoscopic Score for Crohn’s Disease (SES-CD) ulcer size subscore of ≥ 2 in at least one segment]. SES-CD will be used as standard for patients with disease in the ileum and/or colon. Should the disease only be proximal to the ileum, the SES-CD will still be used to score the relevant bowel segment.
-
Satisfactory EBMT ADWP-recommended screening assessment prior to HSCT.
-
Willingness to discontinue all immunosuppressant medication after randomisation if allocated to HSCT arm.
-
Are fit enough to undergo treatment, in the opinion of the Trial Management Group (TMG).
Exclusion criteria
-
Diagnosis of ulcerative colitis or indeterminate colitis.
-
No evidence of active CD on screening endoscopic assessment.
-
Inability to assess for endoscopic active disease owing to strictures.
-
Undrained perianal fistulae (patients with previous perianal disease or perianal disease adequately drained with a seton in situ are eligible).
-
Presence of undrained perianal sepsis on screening the pelvis by way of a MRI scan (or CT scan if MRI is contraindicated).
-
Evidence of intra-abdominal sepsis on abdominal MRI (or CT scan if MRI is contraindicated).
-
Active or latent mycobacterial infection.
-
Prior exposure to hepatitis B virus, hepatitis C virus or human immunodeficiency virus (HIV).
-
Evidence of an enteric or systemic infection.
-
Currently pregnant or breastfeeding, or planning pregnancy within the study duration. The possibility of current pregnancy was to be be ruled out with a pregnancy test at the screening assessment.
-
Unwilling to use adequate contraception (if appropriate) until at least 12 months after the last dose of study drug.
-
Contraindication to the use of cyclophosphamide, fludarabine, filgrastim or rabbit ATG.
-
Significant medical comorbidity that precludes HSCT, adjudicated by the TMG.
-
Significant psychiatric comorbidity.
-
Significant language barriers, which are likely to affect the participant’s understanding of the study, or ability to complete outcome questionnaires.
-
Concurrent participation in another interventional clinical trial.
-
Not considered medically fit for HSCT defined by any of the following:
-
Renal – creatinine clearance of < 40 ml/min (measured or estimated).
-
Cardiac – clinical evidence of refractory congestive heart failure, left ventricular ejection fraction of < 45% by multigated radionuclide angiography (MUGA) or echocardiography, uncontrolled ventricular arrhythmia or pericardial effusion with haemodynamic consequences as evaluated by an experienced echocardiographer.
-
Hepatic – aspartate transaminase (AST) levels ≥ 2 times the upper limit of normal.
-
Concurrent neoplasms or myelodysplasia.
-
Bone marrow insufficiency defined as neutropenia with an absolute neutrophil count of < 1 × 109/l, thrombocytopenia with a platelet count of < 50 × 109/l or anaemia with haemoglobin levels of < 80 g/l.
-
Uncontrolled hypertension, defined as resting systolic blood pressure of ≥ 140 mmHg and/or resting diastolic pressure of ≥ 90mmHg despite at least two anti-hypertensive agents (patients with elevated blood pressure not on medication can be included subject to discussion at TMG).
-
Uncontrolled acute or chronic infection with HIV, human T-lymphotropic virus 1 or 2, hepatitis viruses or any other infection the investigator or TMG consider a contraindication to participation.
-
Other chronic disease causing significant organ failure, including established cirrhosis with evidence of impaired synthetic function on biochemical testing and known respiratory disease causing resting arterial oxygen tension of < 8 kPa or carbon dioxide tension of > 6.7 kPa. Forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) of < 50%. Patients not known to have respiratory disease need not have blood gas measurements.
-
Recruitment and consent procedures
Participants were identified by PIs and co-investigators across eight hospital sites in the UK, or by way of referrals from neighbouring NHS trusts. The trial was publicised through press releases, on websites and in journals. Patients who enquired about participating were advised to seek a clinical referral via their usual inflammatory bowel disease (IBD) team to one of the participating sites for consideration for the trial.
Potential participants received an approved participant information sheet and were given the opportunity to speak with both the gastroenterology and the haematology specialist teams prior to giving consent.
All potentially eligible patients consented to the trial multidisciplinary team (MDT) panel discussing and determining their eligibility for participation. A minimum of two gastroenterology members and one haematology member of the panel were required to agree that a participant was potentially eligible for the patient to proceed to give full consent and undergo screening assessments.
Patients deemed ineligible by the MDT were not consented to the study, unless specific actions were requested to subsequently confirm eligibility, in which case the MDT met to discuss the patient’s eligibility further on completion of the required actions. In some cases, to avoid delays in recruitment, MDT discussions took place via e-mail rather than in monthly meetings. In all cases, communication was saved alongside the referral forms as evidence of discussion.
When the MDT agreed that a patient was eligible, the patient was invited to give full consent to the RCT and proceed through screening investigations. A medically qualified member of the trial team provided clinical oversight for the consent process. Each patient was given the opportunity to visit their local transplant centre and receive counselling from an independent clinician. On completion of the screening investigations, each patient’s case was discussed by the trial MDT for final confirmation of eligibility. If there were no concerns raised around the information gathered during screening, the MDT approved the patient for randomisation.
Screening
Consented patients underwent screening and baseline assessments to ensure eligibility. These included:
-
Standard pre-HSCT workup, including chest radiography and echocardiography or MUGA scan (as per EBMT ADWP guidelines. 20
-
Genetic/functional assessment for monogenic cause of disease in patients with very early-onset disease or with atypical phenotypes for whom a monogenic aetiology had not been excluded.
-
Assessment of clinical disease activity [CDAI and Harvey–Bradshaw index (HBI)].
-
Assessment of quality of life using patient-completed questionnaires [Inflammatory Bowel Disease Questionnaire (IBDQ), Inflammatory Bowel Disease Control Questionnaire (IBD-Control), EuroQol-5 Dimensions, five-level version (EQ-5D-5L), Work Productivity and Impairment (WPAI) and healthcare resource utilisation].
-
Endoscopic assessment of disease at screening (local PI endoscopic disease activity assessment scored using SES-CD).
-
MRI scan of the small bowel to record disease activity [magnetic resonance index of activity (MaRIA) score] (or CT if MRI was not clinically possible) during screening.
-
MRI scan of the pelvis in patients with previous perianal disease to exclude perianal sepsis.
-
Confirmation of eligibility by the MDT.
-
Criteria for fitness for HSCT, as per exclusion criterion 17. Participants who met one or more of the exclusion criteria but who in the opinion of the PI were medically fit enough to undergo HSCT were put forward to the MDT for discussion about eligibility.
Although an 8-week window prior to randomisation was given for the assessment of disease activity (MaRIA score, SES-CD and CDAI) and screening blood tests, this was not always achieved owing to delays in randomisation visits. The participants were also asked to complete a symptom diary for 1 week prior to the assessment of the CDAI; this was not done immediately preceding a colonoscopy and participants were asked to finish the diary prior to starting bowel preparation for colonoscopy.
Study settings
The trial was co-ordinated from the Clinical Trials Research Unit (CTRU) in Sheffield at the School of Health and Related Research. The study took place at nine UK hospitals: eight recruiting centres and one treatment-only centre. All HSCT procedures were undertaken at transplant centres accredited by Joint Accreditation Committee – ISCT Europe & EBMT (JACIE) for allogeneic transplants in adults, or for autologous transplants in adults, at sites with previous experience of administering autologous HSCT for CD.
Interventions
Intervention arm: HSCTlite
The mobilisation and conditioning regimens were extremely immunosuppressive, and any additional immunosuppression may have posed unnecessary risk to the participant. As such, participants randomised to receive HSCTlite were asked to discontinue all immunosuppressant medication before commencing treatment using standard wash-out periods. Infliximab, vedolizumab and ustekinumab were discontinued at least 4 weeks prior to mobilisation, and adalimumab, azathioprine and mercaptopurine at least 2 weeks before mobilisation. Immunosuppressive drugs (e.g. methotrexate, cyclosporin) were stopped at least 1 week before mobilisation.
Mobilisation
All participants in the intervention arm underwent PBSC mobilisation. Participants undertook mobilisation as either inpatients or outpatients, depending on the sites’ local practices. The dosing regimen for mobilisation was as follows, and is shown in Table 1:
-
Cyclophosphamide [investigational medicinal product (IMP)] – the mobilisation phase commenced with a 1-hour infusion of cyclophosphamide 1 g/m2 on day 1.
-
Mesna [non-investigational medicinal product (NIMP)] – to prevent haemorrhagic cystitis caused by the chemotherapy, mesna was given alongside cyclophosphamide in line with local trust procedures.
-
G-CSF (filgrastim) (IMP) – G-CSF (filgrastim) 5 μg/kg, rounded according to local practice, was given subcutaneously 4 days following the cyclophosphamide infusion until the day of stem cell harvest.
| IMP/NIMP | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Cyclophosphamide 1 g/m2 | ✓ | |||||||||
| G-CSF (filgrastim) 5 µg/kg | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Mesna (as per local practice) | ✓ | |||||||||
| CD34+ cell count | ✓a | ✓a | ✓a | |||||||
| Stem cell harvest | ✓b | |||||||||
Non-investigational medical products are defined as medicines given as part of an IMP regimen, to mitigate the side effects of an IMP. NIMPs are not subject to the same governance as IMPs as they are not the focus of the trial intervention.
Full blood count and CD34+ cell counts were monitored in accordance with local practice, although sites were informed that this was ideally from day 8. Once CD34+ cell levels exceeded 10 × 106/l, stem cells were harvested. Participants underwent leukapheresis according to local standard operating procedures until a minimum of 2.0 × 106/kg CD34+ cells were collected and cryopreserved, having allowed for 10% wastage. In cases of mobilisation failure, a second mobilisation regimen was permitted. Prophylactic antibiotics and other supportive care measures were given at the discretion of the treating physician.
Conditioning
Commencement of transplantation conditioning was separated by approximately 6 weeks from the administration of cyclophosphamide for mobilisation, with a minimum separation period of 3 weeks to avoid the risk of cumulative cardiac and other toxicities from cyclophosphamide. Participants were admitted to hospital and required to isolate during the conditioning and transplantation procedures. The dosing regimen for conditioning was as follows, and is shown in Table 2:
-
Fludarabine (IMP) – intravenous (IV) fludarabine 25 mg/m2 was given on days –6, –5, –4, –3 and –2. Reduced doses were permitted in the presence of impaired renal function.
-
Cyclophosphamide (IMP) – cyclophosphamide 60 mg/kg/day IV over 1 hour was given in 500 ml of normal saline on days –3 and –2.
-
Mesna (NIMP) – mesna was given as a continuous IV infusion on days –3 and –2 in line with local site procedures.
-
Supportive care – throughout cyclophosphamide administration, standard hydration was given. Diuretics were used and fluids decreased as necessary to maintain baseline weight. Any other supportive care was given as per local site procedures.
-
Rabbit ATG (IMP) – rabbit ATG (thymoglobulin; Genzyme) IV 2.5 mg/kg was given on days –3, –2 and –1. A test dose was given as per standard local practice.
-
Methylprednisolone (NIMP) – IV methylprednisolone 2 mg/kg/day was given for 3 days alongside ATG, then tapered in accordance with local practice. Additional doses of up to 500–1000 mg per day were permitted in the presence of an ATG reaction.
-
G-CSF – stem cells were reinfused at day 0. G-CSF 5 µg/kg/day (to the nearest vial) began on day +5 and continued until absolute neutrophil counts reached > 1.0 × 109/l for 2 consecutive days.
| IMP/NIMP | Day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| –6 | –5 | –4 | –3 | –2 | –1 | 0 | 1 | 2 | 3 | 4 | 5 | |
| Fludarabine 25 mg/m2/day | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Cyclophosphamide 60 mg/kg/day | ✓ | ✓ | ||||||||||
| Mesna (as per local practice) | ✓ | ✓ | ||||||||||
| Hydration (as per local practice) | ✓ | ✓ | ||||||||||
| Rabbit ATG 2.5 mg/kg/day | ✓ | ✓ | ✓ | |||||||||
| Methylprednisolone 2 mg/kg/day | ✓ | ✓ | ✓ | |||||||||
| Stem cell reinfusion | ✓ | |||||||||||
| G-CSF (filgrastim) 5 µg/kg/day | ✓a | |||||||||||
Usual-care arm: standard care
Participants randomised to the usual-care arm continued with conventional, biologic or nutritional therapy for the management of CD until the primary end point was assessed, as dictated by the trial site or the participant’s regular clinical team. There were no restrictions on the treatment and supportive care that usual-care arm participants could receive.
Outcomes
Primary outcome
Treatment success at week 48, defined as mucosal healing [no endoscopic ulceration (SES-CD ulcer subscore of zero, assessed by central readers blind to allocation and time of assessment)] without surgery or death. Patients who did not complete the week 48 endoscopic assessment were categorised as treatment failures.
Secondary outcomes
Clinical end points
-
Clinical remission (CDAI score of < 150).
-
Steroid-free clinical remission (CDAI score of < 150) at week 48.
-
Clinical remission (HBI score of ≤ 4).
-
Clinical remission [patient-reported outcomes (PROs): mean scores – abdominal pain, ≤ 1; stool frequency, ≤ 1.5].
-
Absolute CDAI at week 48.
-
Absolute SES-CD at week 48.
-
Change in CDAI and SES-CD between baseline and week 48.
-
Proportion of patients in complete endoscopic remission (SES-CD score of 0) at weeks 24 and 48.
-
Absolute MaRIA score at week 48.
Safety end points
-
Toxicity of chemotherapy using NCI CTCAE criteria version 4.03.
-
Adverse events and SAEs, including mortality.
Patient-reported end points
-
Disease-specific quality of life using the IBDQ.
-
Disease-specific quality of life using the IBD-Control.
-
Quality of life using the EQ-5D-5L.
-
Healthcare resource utilisation questionnaire.
Exploratory secondary end points
-
Efficacy of reintroduction of anti-TNF therapy in patients with disease recurrence post HSCT (change in CDAI scores at 6 weeks and change in SES-CD scores at 22 weeks after initiation).
-
Safety of reintroduction of anti-TNF therapy in patients with disease recurrence post HSCT.
-
Presence of any of the late side effects of HSCT, documented through AEs.
Assessments and procedures
The assessments required during the study are detailed within the Statistical Analysis Plan (SAP), available on the project web page (https://www.journalslibrary.nihr.ac.uk/eme/10.3310/CGLT7102). All participants underwent the assessments, irrespective of the treatment arm to which they were randomised. Follow-up visits were carried out at weeks 8, 14, 24, 32, 40 and 48, with day 0 the date on which stem cells were reinfused for participants in the HSCTlite arm. For usual-care arm participants, day 0 was calculated as 49 days after randomisation, to align the timescales for assessments in both trial arms as much as possible. This timescale was taken from the median time to transplantation in the ASTIC trial. 17 Although a visit window of ± 1 week was initially permitted, a wider visit window was considered in the light of the COVID-19 pandemic, which led to difficulties in arranging and carrying out research visits within the permitted time windows for safety reasons. In addition to the wider visit windows, as a result of the COVID-19 pandemic, some follow-up visits were conducted over the telephone rather than in person, where appropriate.
Procedures for assessing efficacy
Blood samples
Routine blood tests taken at the week 8, 14, 24, 32, 40 and 48 study visits were analysed in local laboratories. Additional serum and whole-blood samples were collected at each study visit and shipped by 01.00 via overnight delivery to the John van Geest Cancer Research Centre at Nottingham Trent University, Nottingham, UK, for the mechanistic analysis. Whole blood samples were collected in lithium heparin, Tempus and ethylenediaminetetraacetic acid (EDTA) tubes. Samples in lithium heparin tubes and Tempus™ blood ribonucleic acid (RNA) tubes (Thermo Fisher Scientific, Inchinnan, UK) were posted at an ambient temperature whereas samples in EDTA tubes were frozen at –80 °C and shipped on dry ice. Serum samples were allowed to clot for 15–30 minutes at room temperature before being centrifuged and frozen at –80 °C for shipment on dry ice.
Ileo-colonoscopy
Ileo-colonoscopy was performed according to local practices using standard bowel preparation and conscious sedation, at baseline, week 24 and week 48. Ileoscopy/enteroscopy was performed in patients in whom the disease was limited to the small intestine. Videos of withdrawal from all endoscopies were recorded. Local PI endoscopy assessment using SES-CD-determined eligibility for trial inclusion at baseline and the requirement for anti-TNF therapy at week 24 in HSCTlite arm patients. In patients in whom disease was proximal to the ileum, the SES-CD was used to score the present diseased bowel segment. For analysis of primary and secondary outcomes, all videos were centrally assessed using the SES-CD by investigators blinded to site, treatment assignation and timing of colonoscopy.
A web-based image sharing system was devised (EndoRead™; Exeter, UK), which allowed site staff to upload anonymised colonoscopy videos, which could then be allocated by the study manager to a member of the central reading team for measurement. Prior to measuring ASTIClite videos, a set of 10 training videos were assessed by all members of the central reading team to measure the intraclass correlation coefficient (ICC) between readers. Previous work31,32 suggested that an ICC with a lower 95% confidence interval (CI) exceeding 0.6 was acceptable, a score which was almost met with the first 10 test videos (ICC 0.73, 95% CI 0.54 to 0.9). A meeting was held to discuss and share guidance on difficult-to-read images. A second set of 10 test videos were scored, which produced an ICC within the target range (0.82, 95% CI 0.73 to 0.90). The ASTIClite videos were then allocated and scored centrally.
Biopsied tissue samples were embedded in formalin and sent to local laboratories for routine histology. Biopsies for mechanistic analysis were later placed into cryovials containing RNA and stored overnight at 4 °C, before being transferred into a clean, dry cryovial and stored at –80 °C, ready for shipment to the John van Geest Cancer Research Centre on dry ice.
Magnetic resonance imaging scans
Magnetic resonance imaging scans were conducted at baseline, week 24 and 48 according to standard clinical protocols using, at a minimum, a 1.5 -T scanner and gadolinium contrast. Local PI assessment determined eligibility for trial inclusion and the requirement for anti-TNF therapy at week 24 in HSCTlite group patients. Sequences were recorded to a disc for central scoring of the validated MaRIA scores by an investigator who was blinded to intervention allocation and the timing of assessment. Intervention arm participants also had a MRI scan at week 4, as part of the mechanistic analysis.
Stool and stem cell samples for future research
Stool samples were collected, frozen at –80 °C and shipped to the John van Geest Cancer Research Centre on dry ice, where they were stored for use in future studies.
With participants’ consent, a small sample of stem cells were stored for use in future research. Stem cells were mixed with dimethyl sulfoxide (DMSO) and transferred into a cryovial, which was stored in liquid nitrogen until it was shipped on dry ice to a human tissue authority-licensed storage facility.
Procedures for assessing safety
To assess the safety and efficacy of the HSCTlite regimen, a DMEC assessment was built into the ASTIClite trial, specifically after the first 10 participants in the intervention group completed treatment. However, the DMEC safety assessments were accelerated in the light of the suspected unexpected serious adverse reactions (SUSARs) that occurred in the trial.
All AEs, SAEs and SUSARs were captured from consent until study closure. AEs were recorded using the National Cancer Institute classification of toxicity for 100-day safety post HSCTlite for assessment of grade. SAEs and SUSARs were reported in accordance with the CTRU’s standard operating procedures. The summary of product characteristics (SmPC) for relevant products was used as the reference safety information (RSI) for reporting SAEs. SmPC were reviewed annually, and the RSI updated if required.
Sample size
Sample size calculations were based on the endoscopic assessment after HSCT reported in the ASTIC trial. 17,24 To detect a significant difference in the proportion of patients with no ulceration in endoscopy assessment of 35% based on 50% in the HSCTlite arm and no more than 15% in the usual-care arm, with 90% power at 5% significance level, 62 patients were required in the HSCTlite arm, and 31 in the usual-care arm.
Based on previous experience in the ASTIC trial, a 6% drop-out rate (after enrolment) was anticipated. The recruitment target was therefore set at 99 participants, using a 2 : 1 randomisation ratio (66 participants in the intervention arm and 33 participants in the usual-care arm).
Based on the ASTIC trial, it was anticipated that recruitment would take 36 months. Ability to recruit to time and target was assessed at month 10 of recruitment with the stop/go criterion being 60% of the anticipated recruitment figure at that time.
Recruitment to the ASTIClite trial was suspended in December 2019, and a formal decision to terminate recruitment was taken in June 2020 on safety grounds. Owing to the early closure of the trial, the recruitment target was not reached. The SAP was amended accordingly.
Randomisation
Participants were centrally randomised in a 2 : 1 ratio to either the HSCTlite arm or the usual-care arm. The trial statistician, blinded to treatment allocation, generated the randomisation schedule before the start of the study using the CTRU’s online randomisation system, SCRAM. To ensure that participants were allocated in the correct ratio within each site, randomisation was stratified by site using random permuted blocks, with block sizes of ‘3’ and ‘6’. The allocation sequence was concealed from all study staff except the statisticians who generated it. Delegated site staff were given access to SCRAM. Once eligibility was confirmed, patient identification and date of birth were entered, and the treatment allocation was returned.
Blinding
Owing to the nature of HSCT, neither patients nor their treating physicians were blinded to the treatment allocation. However, central readers blinded to both the timing of the procedure and the treatment allocation assessed and scored anonymised videos of all endoscopic procedures used to determine the primary end point and also provided a a central reading score for the baseline videos. Similarly, expert physicians blinded to the timing of investigation and intervention arm performed central MRI reviews for screening, week 4 (intervention arm participants) and week 48 scans, and calculated the MaRIA score using anonymised images. Only those scans from randomised participants who completed the study were centrally verified.
The senior statistician reviewed and approved the trial SAP prior to seeing any outcome data, but was subsequently unblinded to the treatment allocation throughout the trial.
The trial statistician remained blinded throughout the study until recruitment to the trial was suspended in December 2019 on safety grounds. The nature of the discussion (including the information discussed) around the rationale for early closure effectively led to the unblinding of the TSC and the trial statistician. The SAP addendum setting out the revised plans for analysis was written and signed off prior to the trial statistician accessing the trial data.
Statistical methods
General considerations
A comprehensive trial SAP was developed while the statistician was blinded to treatment allocation. In the light of the early trial closure, an addendum to the SAP was written to account for the reduced sample size and thus to scale back the originally planned analysis. Summaries of continuous variables comprised the number of observations used, mean, median, standard deviation (SD), interquartile range (IQR), and minimum and maximum as appropriate for the distributional form of the data. Summaries of categorical variables comprised the number of observations used, and the number and percentage of observations in each category.
Analysis populations
The reporting of efficacy outcomes followed intention-to-treat (ITT) principles. The ITT population included all randomised participants, including those who did not complete therapy. Exceptions to this were participants who had no recorded consent or who were found to be ineligible after randomisation. The primary outcome analysis was performed on the ITT population for participants who had valid primary outcome data. The presentation of primary outcome results was also repeated for a subset of the ITT: the per-protocol population. Participants in the HSCTlite arm were defined as per protocol if they received the stem cell transplant as intended. Participants in the usual-care arm were defined as per protocol if they did not receive the stem cell transplant.
The early closure of the trial resulted in a smaller data set than intended. For this reason, a third analysis population was included. This was an extended ITT population that included all randomised participants, including those found to be ineligible post randomisation. All safety summaries and mechanistic analyses were presented on the extended ITT population, to retain as much useful information as possible, particularly in relation to safety. Some of the mechanistic analyses were grouped by HSCTlite patients who experienced a SUSAR, HSCTlite patients who did not experience a SUSAR and patients in the usual-care arm.
Data completeness
A CONSORT flow diagram was produced to summarise the flow of participants through the trial, from screening and during follow-up to inclusion in the primary analysis. This also included reasons for any participant withdrawal(s) at each stage of the trial.
Primary outcome analysis methods
The primary outcome, absence of ulceration (ulcer subscore of zero in all segments examined) at the week 48 follow-up, was summarised by treatment group and overall. The original analysis plan was to use mixed-effects logistic regression to estimate the odds ratio for the absence of ulceration in the HSCTlite arm in comparison to usual care; however, this could not be performed owing to small numbers of participants. Therefore, only descriptive statistics of the primary outcome were produced.
The following three sensitivity analyses were conducted:
-
Participants who had undergone surgery for CD in the usual-care arm were removed.
-
Week 24 colonoscopy data were used in place of missing week 48 primary outcome data (last observation carried forward), owing to the large number of missed week 48 outcomes as a result of the COVID-19 pandemic.
-
Participants with week 48 colonoscopy data outside the extended visit window were removed (36–60 weeks).
An extended visit window (36–60 weeks) was considered for the week 48 data to allow for visits that were rescheduled because of the COVID-19 pandemic. The decision to extend the visit windows was made when the addendum to the SAP was written. Other planned sensitivity analyses were not conducted because of small numbers of participants; the details of these sensitivity analyses can be found in the trial SAP, relevant sections of which are in Appendices 2 and 3.
Analysis of secondary outcomes
Owing to the reduced size of the data set, no statistical models were fitted on any of the secondary outcomes. For this reason, no treatment differences or CIs were reported. Secondary outcome data were summarised by group using tables and spaghetti plots. Late effects were reported as part of the AE summaries (details available in Appendix 2, Table 21). Details regarding the reintroduction of anti-TNF and subsequent disease activity were reported descriptively in the text.
Safety and harms
All safety data were presented on the extended ITT population. Descriptive statistics of AEs and SAEs were produced. Summaries of both the AEs and SAEs were presented overall, by treatment group and by the following time periods:
-
Mobilisation phase (period from start of mobilisation to start of conditioning). This took place from randomisation to day 0 for usual-care participants; for usual-care participants, day 0 was the date of randomisation plus 49 days.
-
Transplantation phase (period from start of conditioning up to 100 days from day 0, i.e. day of autologous transplantation/infusion). This took place from day 0 to day 100 for usual-care participants.
-
Follow-up phase (from +100 days post-transplantation phase to 1-year assessment). This took place between day 100 and the 1-year assessment for usual-care participants.
The safety data were also summarised by treatment group and by Common Terminology Criteria for Adverse Events (CTCAE) grade. It was also deemed important to summarise details for those participants who experienced SUSARs against the primary outcome within each treatment arm, so this was also reported.
Ethics aspects
The ASTIClite trial received favourable opinion from the London-Chelsea Research Ethics Committee (REC reference number 17/LO/1690) and authorisation from the Medicines and Healthcare Products Regulatory Agency [(MHRA) reference number 14620/0051/001-0001]. The trial was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice guidelines33 and the Declaration of Helsinki. 34
Patient and public involvement
To ensure readability, format and ease of understanding, all patient-facing documents were reviewed by two patients who took part in the ASTIC trial. This review process allowed for any concerns with the study design to be addressed. The trial was also presented to the British Society of Gastroenterology IBD Clinical Research Group in collaboration with Crohn’s and Colitis UK charity patient engagement panel. The feedback received was incorporated into the trial design.
For ongoing patient involvement in the management of the trial, and to obtain patient perspectives on major decisions affecting trial processes or conduct, two patient representatives sat on the panel of the TSC throughout the study. These patient representatives were also consulted when writing Plain English summaries, and materials to disseminate results to participants and the wider patient population. One TSC patient and public representative provided a quotation specifically for this report, as noted in the Discussion.
Mechanistic substudy
Immune reconstitution
Peripheral blood immune population monitoring by flow cytometry
For peripheral blood immune monitoring by flow cytometry, blood was collected into lithium heparin anticoagulant and sent to the central analysis laboratory at Nottingham Trent University using overnight delivery at ambient temperature. Previous validation studies demonstrated that the expression of the relevant antigens was stable for up to 3 days when anticoagulated samples were stored at ambient/room temperature (Gemma Folds, Nottingham Trent University, 2017, personal communication). For each monoclonal antibody flow cytometry staining panel, blood (100 µl) was incubated with a FcR blocking reagent [provided by Miltenyi Biotec (Bergisch Gladbach, Germany) to prevent non-specific mAb binding], the relevant mAb cocktail (Table 3) and LIVE/DEAD™ fixable viability reagent (Thermo Fisher Scientific, Inchinnan, UK). Samples were washed and red cells were lysed, then washed again. Samples to be stained for the expression of intracellular antigens were fixed and permeabilised, after which they were incubated with the relevant mAbs before being washed. Samples were resuspended to a final volume of 350 µl, to which 50 µl of counting beads was added. Samples were analysed and data acquired using a three-laser, 10-fluorescent channel Beckman Coulter Gallios™ flow cytometer (Beckman Coulter UK Limited, High Wycombe, UK) and Beckman Coulter Kaluza™ software (v2.1) (Beckman Coulter UK Limited, High Wycombe, UK). Protocols for data acquisition and the relevant compensation parameters were established and validated in advance. Daily quality control procedures confirmed correct instrument operation.
| Panel | Antigen | Fluorochrome | Clone |
|---|---|---|---|
| T-cells | CCR7 | FITC | G043H7 |
| β7 | PE | FIB504 | |
| CD3 | PE/Dazzle594 | UCHT1 | |
| CCR9 | PerCP/Cy5.5 | L053E8 | |
| α4 | PE/Cy7 | 9F10 | |
| CD45RA | APC | HI100 | |
| CD8a | APC/Fire750 | RPA-T8 | |
| CD4 | BrilliantViolet421 | RPA-T4 | |
| Th | TCRγδ | FITC | B1 |
| CCR10 | PE | 6588-5 | |
| CD3 | PE/Dazzle594 | UCHT1 | |
| CXCR5 | PerCP/Cy5.5 | J252D4 | |
| CCR6 | PE/Cy7 | G034E3 | |
| CCR4 | AlexaFluor647 | L291H4 | |
| CXCR3 | AlexaFluor700 | G025H7 | |
| CD8a | APC/Fire750 | RPA-T8 | |
| CD4 | BrilliantViolet421 | RPA-T4 | |
| MDSC/DC | HLA-DR | FITC | LN3 |
| CD11b | PE | ICRF44 | |
| CD123 | PE/Dazzle594 | 6H6 | |
| CD124 | PE/Cy7 | G077F6 | |
| Lin (3/19/20/56) | APC | UCHT1, HIB19, 2H7, 5.1H11 | |
| CD14 | AlexaFluor700 | 63D3 | |
| CD15 | APC/Fire750 | W6D3 | |
| CD11c | BrilliantViolet421 | 3.9 | |
| ILC/MCP | Lin (3/14/19/20) | FITC | |
| CD117 | PE | A3C6E2 | |
| CD203c | PE/Dazzle594 | NP4D6 | |
| NKp44 | PE/Cy7 | P44-8 | |
| CD294 | AlexaFluor647 | BM16 | |
| CD127 | AlexaFluor700 | A019D5 | |
| FcεRIα | APC/Cy7 | AER-37 | |
| CD161 | BrilliantViolet421 | HP-3G10 | |
| NK/Mono/B | CD27 | FITC | O323 |
| CD38 | PE | HIT2 | |
| CD3 | PE/Dazzle594 | UCHT1 | |
| CD66b | PerCP/Cy5.5 | G10F5 | |
| CD16 | PE/Cy7 | 3G8 | |
| CD56 | AlexaFluor647 | HCD56 | |
| CD14 | AlexaFluor700 | 63D3 | |
| IgD | APC/Fire750 | IA6-2 | |
| CD19 | BrilliantViolet421 | HIB19 | |
| Breg | CD24 | FITC | ML5 |
| CD1d | PE | 51.1 | |
| CD3 | PE/Dazzle594 | UCHT1 | |
| CD66b | PerCP/Cy5.5 | G10F5 | |
| CD21 | PE/Cy7 | Bu32 | |
| CD56 | AlexaFluor647 | HCD56 | |
| CD14 | AlexaFluor700 | 63D3 | |
| CD5 | APC/Cy7 | UCHT2 | |
| CD19 | BrilliantViolet421 | HIB19 | |
| Treg | CD45RO | FITC | UCHL1 |
| FoxP3 * | PE | ICFC | |
| CD3 | PE/Dazzle594 | UCHT1 | |
| CD25 | PE/Cy7 | BC-96 | |
| CCR4 | AlexaFluor647 | L291H4 | |
| CD127 | AlexaFluor700 | A019D5 | |
| CD4 | BrilliantViolet421 | RPA-T4 |
Monitoring of immune responsiveness
Peripheral blood mononuclear cell stimulation: isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the remaining anticoagulated blood by density gradient centrifugation using Ficoll® Paque PLUS (GE HealthCare, Chicago, IL, USA). Harvested PBMCs were suspended in fetal bovine serum (FBS) containing 10% (v/v) DMSO, transferred to cryovials (107 cells/ml), and frozen overnight in a CoolCell™ freezing container (Appleton Woods Limited, Birmingham, UK) at –80 °C, before being transferred to liquid nitrogen for long-term storage.
Peripheral blood mononuclear cells were defrosted, washed, counted and resuspended in phosphate-buffered saline at a concentration of 2 × 106 cells/ml. For stimulation, cells (106) were incubated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA; final concentration of 50 ng/ml) and ionomycin (Sigma-Aldrich; final concentration of 1 µg/ml). Unstimulated cells were incubated with phosphate-buffered saline. Brefeldin A (BioLegend, San Diego, CA, USA; 10 µg/ml) was added to all samples and these were incubated at 37 °C for 5 hours, after which they were incubated with FcR blocking reagent (Miltenyi Biotec), the relevant mAb cocktail and LIVE/DEAD™ fixable viability reagent. Samples were fixed and permeabilised before being incubated with the mAb cocktail for intracellular antigens. Samples were washed, resuspended in 400 µl of phosphate-buffered saline and analysed using a three-laser, 10-colour Beckman Coulter Gallios™ flow cytometer an Beckman Coulter Kaluza™ software (v2.1). Protocols for data acquisition with relevant compensation were set up in advance. Daily quality control procedures confirmed correct instrument operation.
Analysis of recent thymic emigrants
T-cell receptor excision circle quantification
Deoxyribonucleic acid (DNA) was extracted from frozen EDTA blood samples using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) including RNase A (QIAGEN) and adhering to the manufacturer’s instructions. Extracted DNA was concentrated using DNA Clean & Concentrator kit (Zymo Research, Irvine, CA, USA) and quantified using a NanoDrop™ 8000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). T-cell receptor excision circle (TREC) quantification was undertaken using the MyTREC™ Sensi Duplex TREC/Beta Actin Human Assay RT-qPCR kit [genenplus: www.mytreckit.com (accessed May 27, 2021)]. Samples were run on a QIAGEN Rotor-Gene Q instrument (40 cycles). TREC counts were normalised using the beta-actin reference gene and reported as TREC counts per 106 cells. The flow cytometry staining panels for immune monitoring and PBMC stimulation are shown in Tables 3 and 4, respectively.
| Panel | Antigen | Fluorochrome | Clone |
|---|---|---|---|
| Tstim | IL-2 | FITC | MQ1-17H12 |
| IL4 | PE | 8D4-8 | |
| CD3 | PE/Dazzle594 | UCHT1 | |
| IL-17 | PerCP/Cy5.5 | BL168 | |
| TNFα | PE/Cy7 | Mab11 | |
| IL-10 | AlexaFluor647 | JES3-9D7 | |
| IFNγ | AlexaFluor700 | B27 | |
| CD8a | APC/Fire750 | RPA-T8 | |
| CD4 | BrilliantViolet421 | RPA-T4 |
European Society for Blood and Marrow Transplantation long-term follow-up study
A longitudinal, observational study following participants recruited to the ASTIClite trial over a further 4–7 years via annual follow-up visits was planned to assess the long-term safety and efficacy of HSCTlite. Patients who consented to the ASTIClite RCT were also invited to take part in the ASTIClite EBMT follow-up study, regardless of the treatment they went on to receive as part of the ASTIClite RCT. However, owing to the early closure of the ASTIClite clinical trial, the EBMT long-term follow-up study was terminated before data collection began.
Chapter 4 Results
Early trial closure
The ASTIClite trial was paused on 30 December 2019 while a number of suspected unexpected serious adverse reactions (SUSARs) were investigated, including the death of one patient. Two further SUSARs were reported in May 2020. In June 2020, the DMEC and TSC held a joint meeting to discuss the unexplained AEs, the outcomes of the trial team’s investigations and the impact of the coronavirus pandemic. The DMEC and TSC agreed that:
-
recruitment to the trial should cease
-
patients who were in screening or randomised to HSCTlite but had not yet received treatment should be withdrawn
-
patients that had completed the intervention or were randomised to usual care should continue to be followed up as normal.
The pandemic associated with SARS-CoV-2 was recognised as a global issue in early 2020. It had a marked impact on the delivery of health care across the UK, with the suspension of many routine services to allow focus of care for those affected by COVID-19. The impact on the delivery of clinical trials across the UK was immense, with many closing completely to recruitment. At that time the ASTIClite trial had already been halted and no additional patients were recruited after the onset of the pandemic. However, the recommendations on social distancing, travel and isolation prevented some face-to-face trial visits during the follow-up period of the trial. Pressure on endoscopy services and the British Society of Gastroenterology guidelines35 on service provision during the pandemic precluded or significantly delayed colonoscopy to assess the primary and secondary outcomes in some patients, and for others this could not be completed at all. In-person site monitoring was suspended. To our knowledge, no patient had symptomatic SARS-CoV-2 infection during the trial.
The last patient last visit was in November 2020 and the database was finalised in December 2020. The planned analyses as outlined in the study SAP were amended to account for the early trial closure and the significantly reduced sample size. These changes were documented in an addendum to the SAP and were approved by the TSC chairperson and statistician. The results presented in this report follow the analysis as stipulated in both the SAP and the addendum to the SAP.
Data completeness
In total, 23 participants were randomised between 18 October 2018 and 8 November 2019. The DMEC recommended that recruitment cease on 8 June 2020. Study follow-up concluded in November 2020. Figure 1 shows the CONSORT flow diagram of participants through the trial. Thirteen participants were randomised to receive HSCTlite (the intervention arm) and 10 were allocated to receive usual care (the usual-care arm). In the HSCTlite arm, 12 participants underwent mobilisation, of whom two withdrew pre transplantation; the reasons for these withdrawals are shown in Figure 1. Ten participants underwent transplantation and received both the conditioning regimen and stem cell infusion. In the HSCTlite arm, one patient died at 24 weeks; the remaining nine participants reached the 48-week follow-up and completed the study. In the usual-care arm, one participant was withdrawn from the study at week 8 owing to ineligibility, leaving nine participants, all of whom completed the study.
FIGURE 1.
The CONSORT flow diagram. a, Treatment for two patients was discontinued owing to early study close (one of these received mobilisation, one participant withdrew consent); b, patient was found to be ineligible and was withdrawn; c, patient died. A further participant died after their week 48 visit.
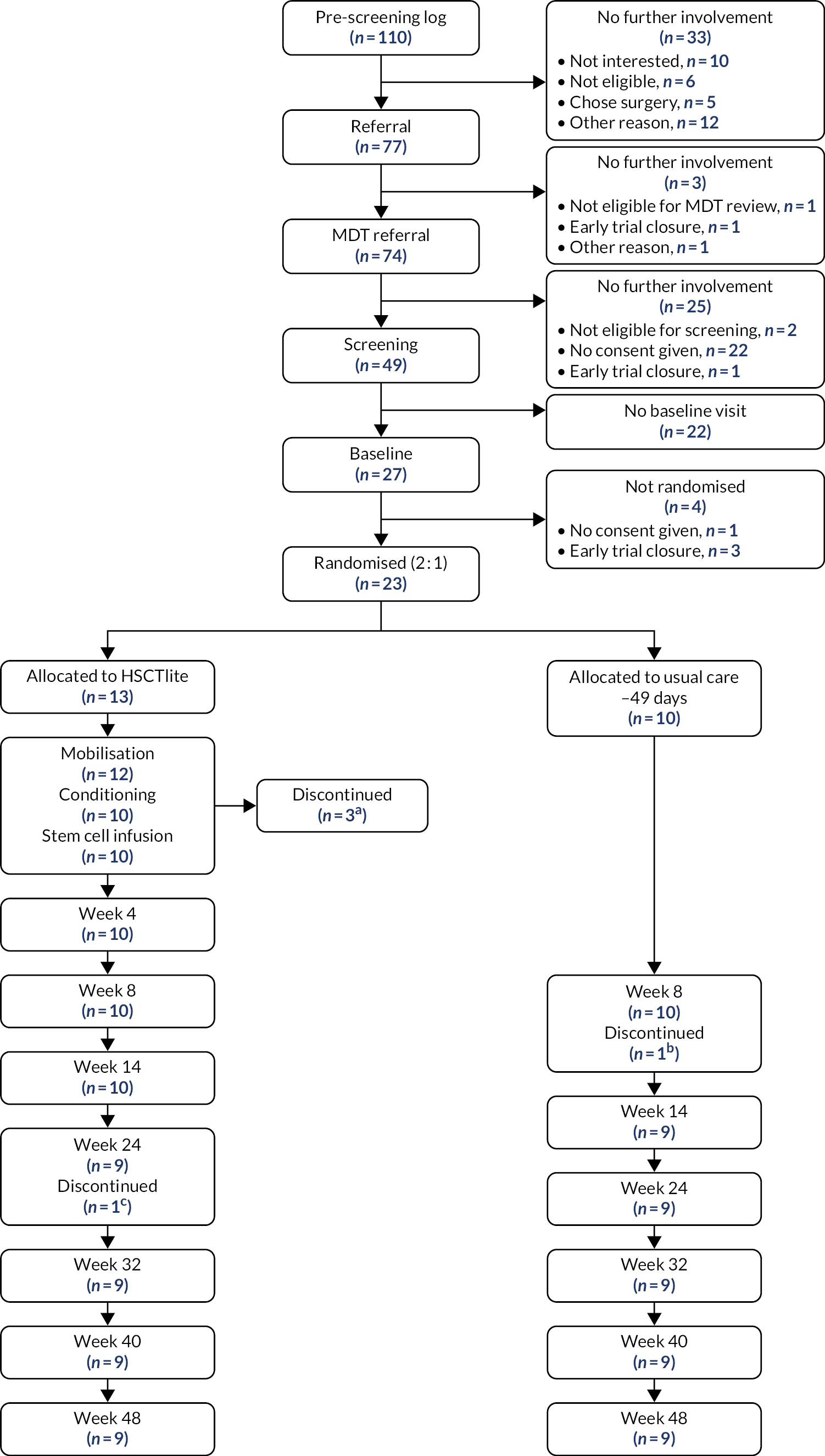
Of the nine participants followed up at week 48 in the intervention arm, one declined their week 48 colonoscopy and, for a further two patients, colonoscopies were not recorded, so could not be centrally scored. Therefore, we did not have valid primary outcome data for these patients although locally read endoscopic scores were available. The participant who died at week 24 was classified as a treatment failure according to the definition of the primary end point, which leaves seven participants in the intervention arm with valid week 48 primary outcome data. In the usual-care arm, five patients did not have valid central colonoscopy data. The reasons for missing data were as follows: two missing week 48 colonoscopies were due to the COVID-19 pandemic (one patient was shielding and one patient’s colonoscopy was cancelled due to changes to non-urgent appointment availability in the NHS at the time), one colonoscopy was not recorded and could not be centrally scored and two patients did not have their colonoscopies because of worsening disease/surgery. However, they were included in the analysis of the primary end point as treatment failures, as they did not meet the prespecified criteria. Therefore, six participants in the usual-care arm had valid data to contribute to the primary outcome. In total, 13 participants contributed week 48 primary outcome data. We followed up ongoing SAEs until resolution or database freeze, whichever came first, and from this it was recorded that one participant from the intervention arm died after the week 48 follow-up visit.
Baseline data
This section details the baseline data for the participants in the ITT population; this was defined as all randomised participants excluding those (n = 1) who were subsequently found to be ineligible post randomisation or with no recorded consent information. There were 22 participants who met these criteria, 13 in the HSCTlite arm and nine in the usual-care arm.
The baseline demographics of the ITT participants are shown in Table 5. Overall, seven centres recruited to the study, with Barts Health and Nottingham contributing over 60% of the total participants. The majority of participants were white British (82%), with the remaining participants being of Asian/Asian British ethnicity. The average age of the participants was 35 years, with participants in the usual-care arm having a slightly higher average age than those in the HSCTlite arm. Table 6 shows the baseline CD characteristics by treatment group.
| Variable | Treatment arm | Total (N = 22) | |
|---|---|---|---|
| HSCTlite (n = 13) | Usual care (n = 9) | ||
| Centre, n (%) | |||
| Barts Health | 5 (38) | 3 (33) | 8 (36) |
| Cambridge | 0 (0) | 1 (11) | 1 (5) |
| Edinburgh | 1 (8) | 2 (22) | 3 (14) |
| Liverpool | 1 (8) | 0 (0) | 1 (5) |
| Nottingham | 4 (31) | 2 (22) | 6 (27) |
| Oxford | 1 (8) | 0 (0) | 1 (5) |
| Sheffield | 1 (8) | 1 (11) | 2 (9) |
| Sex, n (%) | |||
| Male | 6 (46) | 4 (44) | 10 (45) |
| Female | 7 (54) | 5 (56) | 12 (55) |
| Age (years) | |||
| Mean (SD) | 34.5 (9.5) | 36.3 (10.1) | 35.3 (9.6) |
| Median (IQR) | 35.0 (26.0–43.0) | 30.0 (28.0–44.0) | 33.0 (28.0–44.0) |
| 19.0, 47.0 | 26.0, 53.0 | 19.0, 53.0 | |
| Ethnicity, n (%) | |||
| Whitea | 10 (77) | 8 (89) | 18 (82) |
| Asian/Asian Britishb | 3 (23) | 1 (11) | 4 (18) |
| BMI (kg/m2) | |||
| Mean (SD) | 26.2 (6.0) | 27.4 (6.2) | 26.7 (5.9) |
| Median (IQR) | 27.0 (20.3–30.5) | 26.4 (22.7–30.5) | 26.7 (22.5–30.5) |
| Minimum, maximum | 17.0, 37.8 | 20.4, 37.7 | 17.0, 37.8 |
| Smoking status,c n (%) | |||
| Never smoked | 7 (54) | 7 (78) | 14 (64) |
| Current smoker | 2 (15) | 1 (11) | 3 (14) |
| Previous smoker (stopped ≥ 5 years) | 3 (23) | 1 (11) | 4 (18) |
| Current tobacco intake in cigarettes per day equivalentd | |||
| Mean (SD) | 2.8 (5.8) | 6.7 (14.1) | 4.4 (10.0) |
| Cumulative tobacco intake (in pack-year equivalent)d | |||
| Mean (SD) | 17.5 (45.1) | 88.9 (176.4) | 46.7 (119.6) |
| Variable | Treatment arm | Total (N = 22) | |
|---|---|---|---|
| HSCTlite (n = 13) | Usual care (n = 9) | ||
| Perianal CD, n (%) | |||
| Yes | 3 (23) | 5 (56) | 8 (36) |
| No | 10 (77) | 4 (44) | 14 (64) |
| Family history of IBD, n (%) | |||
| Yes | 3 (23) | 3 (33) | 6 (27) |
| CD | 3 (100) | 1 (33) | 4 (67) |
| Ulcerative colitis | 1 (33) | 3 (100) | 4 (67) |
| No | 10 (77) | 6 (67) | 16 (73) |
| Stoma, n (%) | |||
| Yes | 7 (54) | 2 (22) | 9 (41) |
| Ileostomy | 3 (43) | 2 (100) | 5 (56) |
| End-ileostomy | 3 (43) | 0 (0) | 3 (33) |
| Loop colostomy | 1 (14) | 0 (0) | 1 (11) |
| No | 6 (46) | 7 (78) | 13 (59) |
| Behaviour of CD, n (%) | |||
| B1 non-stricturing, non-penetrating | 5 (38) | 0 (0) | 5 (23) |
| B2 stricturing | 7 (54) | 3 (33) | 10 (45) |
| B3 penetrating | 1 (8) | 6 (67) | 7 (32) |
| Disease location,a n (%) | |||
| L1 ileal | 3 (23) | 0 (0) | 3 (14) |
| L2 colonic | 1 (8) | 1 (11) | 2 (9) |
| L3 ileo-colonic | 5 (38) | 3 (33) | 8 (36) |
| L4 isolated upper disease | 0 (0) | 1 (11) | 1 (5) |
| L1 L4 | 3 (23) | 2 (22) | 5 (23) |
| L3 L4 | 1 (8) | 2 (22) | 3 (14) |
| Montreal age at onset classification, n (%) | |||
| A1 (below 16 years) | 5 (38) | 2 (22) | 7 (32) |
| A2 (between 17 and 40 years) | 8 (62) | 7 (78) | 15 (68) |
| Previous operations for CD, n (%) | |||
| Intestinal surgery | 12 (92) | 8 (89) | 20 (91) |
| Perianal surgery | 4 (31) | 4 (44) | 8 (36) |
| Other surgery for CD | 1 (8) | 0 (0) | 1 (5) |
| Extraintestinal manifestations, n (%) | |||
| Yes | 4 (31) | 2 (22) | 6 (27) |
| No | 9 (69) | 7 (78) | 16 (73) |
| Extraintestinal involvement, n (%) | |||
| Yes | 3 (75) | 1 (50) | 4 (67) |
| Joints | 3 (100) | 1 (100) | 4 (100) |
| Skin | 0 (0) | 0 (0) | 0 (0) |
| Eyes | 1 (33) | 0 (0) | 1 (25) |
| Other | 0 (0) | 0 (0) | 0 (0) |
| No | 1 (25) | 1 (50) | 2 (33) |
| Age at CD onset | |||
| Mean (SD) | 20.7 (7.7) | 22.6 (6.3) | 21.4 (7.0) |
| Median (IQR) | 20 (15.0–24.0) | 22 (19.0–25.0) | 22.0 (16.0–24.8) |
| Duration of CD (years) | |||
| Mean (SD) | 13.6 (6.7) | 14.1 (7.8) | 13.8 (7.0) |
| Median (IQR) | 11.0 (10.0–16.0) | 10.0 (10.0–19.0) | 11.0 (10.0–18.5) |
The medical and drug history of the participants is shown in Table 7. Specific comorbidities are shown by treatment arm, as well as exposure to immunosuppressants, steroids and biological therapy prior to baseline. Baseline laboratory tests, disease activity scores and PROs are summarised in Table 8. Those in the intervention arm had a notably worse health state (measured by EQ-5D-5L) and illness severity (measured by CDAI) than those in the usual-care arm, but conversely experienced a lower impact on work (measured by hours of work lost to CD and self-rated effect on work productivity).
| Comorbidities, medical and drug history | Treatment arm | Total (N = 22) | |
|---|---|---|---|
| HSCTlite (n = 13) | Usual care (n = 9) | ||
| Comorbidities, n (%)b | |||
| Hypertension | 2 (15) | 0 (0) | 2 (9) |
| Respiratory disease | 2 (15) | 4 (44) | 6 (27) |
| Established renal disease | 1 (8) | 1 (11) | 2 (9) |
| Psychiatric disease | 1 (8) | 1 (11) | 2 (9) |
| Other | 6 (46) | 4 (44) | 10 (45) |
| Previous immunosuppressants, n (%)b | |||
| Azathioprine | 11 (85) | 9 (100) | 20 (91) |
| Cyclosporin | 0 (0) | 1 (11) | 1 (5) |
| Mercaptopurine | 3 (23) | 3 (33) | 6 (27) |
| Methotrexate | 10 (77) | 6 (67) | 16 (73) |
| Mycophenolate | 1 (8) | 1 (11) | 2 (9) |
| Other immunosuppressants | 0 (0) | 1 (11) | 1 (5) |
| Number of immunosuppressants | |||
| Mean (SD) | 1.92 (0.76) | 2.44 (0.88) | 2.14 (0.83) |
| Median (IQR) | 2.00 (1.00–2.00) | 2.00 (2.00–3.00) | 2.00 (2.00–3.00) |
| Months used immunosuppressants, mean (SD)a | |||
| Azathioprine | 17.00 (20.54) | 6.20 (5.02) | 13.14 (17.21) |
| Cyclosporine | – (–) | 1.00 (–) | 1.00 (–) |
| Mercaptopurine | 0 (0) | 0 (0) | 0 (0) |
| Methotrexate | 18.12 (20.85) | 16.00 (12.46) | 17.21 (17.17) |
| Mycophenolate | 10.00 (–) | 12.00 (–) | 11.00 (1.41) |
| Previous steroid use, n (%) | |||
| Budesonide | 4 (31) | 5 (56) | 9 (41) |
| Methylprednisolone | 0 (0) | 3 (33) | 3 (14) |
| Prednisolone | 12 (92) | 7 (78) | 19 (86) |
| Previous biological therapy, n (%) | |||
| Adalimumab | 13 (100) | 7 (78) | 20 (91) |
| Infliximab | 12 (92) | 8 (89) | 20 (91) |
| Ustekinumab | 11 (85) | 8 (89) | 19 (86) |
| Vedolizumab | 8 (62) | 8 (89) | 16 (73) |
| Other biological therapy | 2 (15) | 1 (11) | 3 (14) |
| Variable | Treatment arm | Total (N = 22) | |
|---|---|---|---|
| HSCTlite (n = 13) | Usual care (n = 9) | ||
| Haemoglobin | |||
| Mean (SD) | 123.5 (16.9) | 127.9 (8.3) | 125.3 (14.0) |
| Median (IQR) | 122 (110.0–135.0) | 127 (120.0–131.0) | 124.0 (116.8–133.2) |
| Platelet count | |||
| Mean (SD) | 371.5 (141.5) | 407.7 (94.5) | 386.3 (123.2) |
| Median (IQR) | 356 (274.0–404.0) | 379 (342.0–460.0) | 361.0 (313.2–446.8) |
| Albumin | |||
| Mean (SD) | 40.7 (4.7) | 39.4 (4.3) | 40.2 (4.5) |
| Median (IQR) | 41 (40.0–44.0) | 40 (39.0–41.0) | 40.5 (39.0–43.8) |
| CRP | |||
| Mean (SD) | 15.1 (14.1) | 26.8 (27.1) | 19.7 (20.5) |
| Median (IQR) | 10.9 (7.5–14.2) | 20.0 (4.8–34.2) | 12.0 (5.8–24.0) |
| CDAI | |||
| Mean (SD) | 381.5 (209.1) | 271.5 (115.2) | 337.5 (182.4) |
| Median (IQR) | 354.5 (197.2–481.0) | 290.5 (174.2–352.2) | 327.5 (182.2–415.8) |
| PRO2 | |||
| Mean (SD) | 25.0 (20.0) | 18.2 (8.7) | 22.4 (16.7) |
| Median (IQR) | 23 (12.0–31.0) | 17.0 (12.2–25.8) | 19 (12.0–30.0) |
| HBI | |||
| Mean (SD) | 12.7 (10.8) | 12.6 (6.9) | 12.6 (9.2) |
| Median (IQR) | 10 (4.0–16.0) | 13 (8.0–18.0) | 11.5 (5.0–17.5) |
| Central SES-CD scorea | |||
| Mean (SD) | 10.8 (6.3) | 10.0 (6.1) | 10.4 (6.0) |
| Median (IQR) | 8.0 (7.0–15.0) | 8.5 (5.5–14.2) | 8.0 (6.0–15.0) |
| Local SES-CD score | |||
| Mean (SD) | 11.8 (8.7) | 10.1 (5.7) | 10.9 (7.2) |
| Median (IQR) | 8.0 (6.8–12.5) | 9.0 (7.2–11.0) | 8.5 (6.8–11.0) |
| Segments examined in colonoscopy,b n (%) | |||
| n | 10 | 8 | 18 |
| Ileum | 9 (90) | 8 (100) | 17 (94) |
| Right colon | 2 (15) | 2 (22) | 4 (18) |
| Transverse colon | 6 (46) | 6 (67) | 12 (55) |
| Left colon | 6 (46) | 6 (67) | 12 (55) |
| Rectum | 7 (54) | 7 (78) | 14 (64) |
| Number of segments examined in colonoscopy | |||
| Median (IQR) | 2 (1.0–4.0) | 4 (2.0–4.0) | 4 (1.0–4.0) |
| IBD-Control | |||
| Mean (SD) | 2.6 (2.6) | 0.7 (1.0) | 1.9 (2.3) |
| Median (IQR) | 2 (1.0–4.0) | 0 (0.0–1.5) | 1.5 (0.0–2.0) |
| EQ-5D-5L | |||
| Mean (SD) | 0.398 (0.431) | 0.507 (0.269) | 0.442 (0.370) |
| Median (IQR) | 0.434 (–0.023 to 0.768) | 0.582 (0.336–0.632) | 0.529 (0.126–0.691) |
| IBDQ | |||
| Mean (SD) | 105.2 (59.4) | 88.4 (11.9) | 95.4 (37.9) |
| Median (IQR) | 85 (54.0–166.0) | 89 (81.0–95.5) | 88.5 (73.5–98.2) |
| MaRIA score | |||
| Mean (SD) | 65.5 (24.2) | 62.5 (10.6) | 63.8 (15.9) |
| Median (IQR) | 67.2 (53.8–78.0) | 60.8 (53.9–69.4) | 67.2 (53.7–71.3) |
| WPAIc – Currently employed, n | 7 | 2 | 9 |
| Hours of work missed owing to CD (in the past 7 days) | |||
| Mean (SD) | 7.7 (9.7) | 15.0 (21.2) | 9.3 (11.7) |
| Median (IQR) | 0 (0.0–17.0) | 15.0 (7.5–22.5) | 0 (0.0–18.0) |
| Hours of work missed for other reasons (in the past 7 days) | |||
| Mean (SD) | 9.1 (14.6) | 18.5 (26.2) | 11.2 (16.2) |
| Median (IQR) | 2 (0.0–11.0) | 18.5 (9.2–27.8) | 2 (0.0–14.0) |
| Hours worked (in the past 7 days) | |||
| Mean (SD) | 26.9 (14.5) | 5.0 (7.1) | 22.0 (16.0) |
| Median (IQR) | 24 (16.0–39.0) | 5.0 (2.5–7.5) | 22 (10.0–38.0) |
| Effect of CD on work productivityd | |||
| Mean (SD) | 5.0 (2.6) | 8.0 (–) | 5.4 (2.6) |
| Median (IQR) | 4 (3.0–7.5) | 8 (8.0–8.0) | 5.5 (3.0–8.0) |
| Ability to do regular daily activitiesd | |||
| Mean (SD) | 6.5 (2.8) | 7.8 (1.4) | 7.0 (2.4) |
| Median (IQR) | 7 (4.0–8.0) | 8 (7.0–8.0) | 8.0 (5.5–8.0) |
The baseline data split by completion status are shown in Appendix 4, Tables 22 and 23. Completers were defined as those who reached the week 48 follow-up. Of the 22 participants who were in the ITT population, 18 completed the study, nine in the HSCTlite arm and nine in the usual-care arm. The usual-care arm had no non-completers other than one patient who was found to be ineligible post randomisation and was therefore excluded from the ITT population. The intervention arm had four non-completers: the reasons for non-completion are shown in Figure 1. The continuous and categorical characteristics measured at baseline are shown in Appendix 4, Tables 22 and 23, respectively; they are split by treatment group and completion status. The average CDAI score in the completer group was 333.3, compared with 354.2 in the non-completers group at baseline. The participants who completed the study had a mean SES-CD score of 10.6 at baseline. It is worth noting that the non-completer group comprised only four participants, so averages and summary statistics should be taken with caution.
Treatment summaries
Table 9 shows the treatment summaries for those in the HSCTlite arm (the intervention arm). In total, 13 participants were randomised to receive HSCTlite, 12 of whom reached mobilisation, with one participant receiving two cycles of mobilisation. On average, patients received 5 days of G-CSF. The disease activity after mobilisation is also shown in Table 9. Patients reported a wide range of disease burden, with HBI scores ranging from 2 to 51 (median 12.5). Ten participants went on to receive stem cell reinfusion, on average 43 days after stem cell harvest. All 10 participants who received stem cell infusion were considered to be per protocol based on blinded treatment review by the chief investigator. The median (IQR) number of stem cells reinfused was 4.6 × 106/kg (4.0–6.3). The reasons for the withdrawal participants in the HSCTlite arm are shown at the bottom of Table 9. Three of the participants withdrew pre transplantation, and one participant died at week 24.
| Variable | HSCTlite (n = 13) |
|---|---|
| Mobilisation | |
| Mobilisation successful (first cycle) | |
| Yes | 11 |
| No | 2a |
| Number of mobilisation cycles | |
| One | 11 |
| Two | 1 |
| Cyclophosphamide dose (g) | |
| Mean (SD) | 1.9 (0.2) |
| Median (IQR) min, max | 1.8 (1.8–2.0) 1.6, 2.3 |
| Number of days GCSF | |
| Mean (SD) | 5.0 (0.6) |
| Median (IQR) min, max | 5 (5.0–5.0) 4, 6 |
| Days between cyclophosphamide and harvest of stem cells | |
| Mean (SD) | 8.8 (0.6) |
| Median (IQR) min, max | 9.0 (9.0–9.0) 7, 9 |
| Number of cells harvested × 108/kg (total number of nucleated cells) | |
| Mean (SD) | 3.4 (2.5) |
| Median (IQR) min, max | 2.6 (2.0–4.4) 1, 9 |
| Number of cells harvested × 106/kg (CD34+) | |
| Mean (SD) | 5.5 (3.3) |
| Median (IQR) min, max | 5.4 (4.3–6.4) 1, 14 |
| Disease activity after mobilisation | |
| Karnofsky performance status | |
| Mean (SD) | 68.3 (11.1) |
| Median (IQR) min, max | 70.0 (60.0–72.5) 50, 90 |
| HBI | |
| Mean (SD) | 15.3 (12.5) |
| Median (IQR) min, max | 12.5 (8.0–17.0) 2, 51 |
| Haemoglobin (g/l) | |
| Mean (SD) | 109.5 (15.6) |
| Median (IQR) min, max | 111.5 (99.5–120.2) 87, 137 |
| Platelet count (× 109/l) | |
| Mean (SD) | 359.5 (104.3) |
| Median (IQR) min, max | 332.5 (274.8–420.8) 239, 552 |
| Albumin (g/l) | |
| Mean (SD) | 37.5 (4.5) |
| Median (IQR) min, max | 38.5 (35.5–40.2) 30, 43 |
| CRP (mg/l) | |
| Mean (SD) | 34.3 (50.5) |
| Median (IQR) min, max | 12.0 (4.4–28.4) 1, 141 |
| Conditioning and transplantation | |
| Days between harvest and stem cell reinfusion | |
| Mean (SD) | 42.5 (25.1) |
| Median (IQR) min, max | 36 (29.5–41.0) 27, 114 |
| Number of stem cells reinfused × 106/kg | |
| Mean (SD) | 5.6 (3.0) |
| Median (IQR) min, max | 4.6 (4.0–6.3) 3, 14 |
| Number of red cell transfusions | |
| Mean (SD) | 4.8 (5.9) |
| Median (IQR) min, max | 4.0 (1.2–4.0) 1, 21 |
| Number of platelet transfusions | |
| Mean (SD) | 7.7 (12.2) |
| Median (IQR) min, max | 3 (2.0–5.5) 1, 35 |
| Early termination of treatment | |
| Number of patients randomised to HSCTlite who withdrew from the study and from treatment, n (%) | |
| Participant withdrew consent | 1 (8) |
| Participant died | 1 (8) |
| Participant lost to follow-up | 0 (0) |
| Investigator decisionb | 2 (15) |
Of the nine participants in the usual-care arm, eight received medications specifically to treat CD and one participant underwent surgery for CD (small bowel resection). Two patients received IV nutrition (one of these following complications after small bowel resection). One patient required an examination under anaesthetic and seton insertion and another had a guided drainage of a collection. Eight patients continued biologic therapy (two infliximab, one certolizumab, three ustekinumab and two vedolizumab). This was combined with azathioprine in one and methotrexate in two patients. One patient received tacrolimus. Corticosteroids were prescribed for seven patients (four received budesonide and three prednisolone).
In the HSCTlite group, the mean number of days between randomisation and stem cell infusion (time to day 0) for the 11 participants who underwent stem cell reinfusion was 99 days, with a range of 71 to 185 days. This is considerably larger than the planned 49 days, which was the median number of days (from the ASTIC trial). This increase in days between randomisation and stem cell reinfusion in the ASTIClite trial was largely due to waiting lists for haematology wards for non-urgent stem cell transplantations.
Information relating to the reintroduction of anti-TNF treatment following HSCT is provided in Appendix 5.
Summary of the primary outcome
The primary outcome and treatment success at week 48 was defined as mucosal healing [no endoscopic ulceration (SES-CD ulcer subscore of zero, assessed by central readers blinded to allocation and time of assessment)] without surgery or death. Table 10 shows the proportion of participants in each group achieving the primary outcome. Although 18 of the 22 participants in the ITT population completed the study, only 10 (n = 6, HSCTlite arm; n = 4, usual-care arm) had centrally confirmed colonoscopy data to assess absence of ulceration. Of the eight participants who did not have valid week 48 colonoscopy data, three of the colonoscopies (n = 2, HSCTlite arm; n = 1 usual-care arm) were not recorded and could not be centrally scored (two were not recorded in error, and, for one, a technical issue meant that the recording was not saved); two patients (both in the usual-care arm) did not attend their colonoscopy visit because of the COVID-19 pandemic; two (both in the usual-care arm) did not attend their week 48 colonoscopy as they were too ill; and one participant (in the HSCTlite arm) declined to attend. The two participants who did not attend their colonoscopies because of worsening disease were included as treatment failures as per the primary outcome definition, as was the one HSCTlite patient who had died prior to week 48. The primary outcome is summarised in Table 11. Three out of the seven patients undergoing HSCTlite achieved absence of ulceration, whereas no responses were seen in the usual-care arm. Of the three participants without centrally assessed colonoscopy data, local scoring by the investigator reported that the two HSCTlite-arm participants achieved absence of ulceration in their colonoscopy but the participant in the usual-care arm was classified as a treatment failure. Therefore, using the local colonoscopy data alone, five out of the nine intervention participants achieved absence of ulceration. One of the HSCTlite participants who achieved absence of ulceration subsequently died after the week 48 follow-up (further details given in Suspected unexpected serious adverse reactions).
| Colonoscopy data status | Treatment arm, n | Total (N = 22), n | |
|---|---|---|---|
| HSCTlite (n = 13) | Usual care (n = 9) | ||
| Completed study | |||
| Colonoscopy performed | |||
| Centrally confirmed | 6 | 4 | 10 |
| Local notes onlya | 2 | 1 | 3 |
| Colonoscopy not performed | |||
| Owing to worsening diseaseb | 0 | 2 | 2 |
| Owing to COVID-19 restrictions | 0 | 2 | 2 |
| Declined | 1 | 0 | 1 |
| Did not complete study | |||
| Diedb | 1 | 0 | 1 |
| Discontinued without receiving HSCTlite | 3 | 0 | 3 |
| Analysis | Number n/N (%) without ulceration | |
|---|---|---|
| HSCTlite | Usual care | |
| Primary | 3/7 (43) | 0/6 (0) |
| Sensitivity | ||
| Removing participants who had surgery for CD in the usual-care arm | 3/7 (43) | 0/5 (0) |
| Imputation of missing data with week 24 colonoscopy data. | 4/10 (40) | 0/9 (0) |
| Removing week 48 colonoscopy data outside of the visit windowa | 3/6 (50) | 0/6 (0) |
Table 11 shows the three sensitivity analyses on the primary outcome: removing participants who had surgery for CD in the usual-care arm, imputation of missing data with week 24 colonoscopy data and removing week 48 colonoscopy data outside the extended visit window. One patient had surgery for CD in the usual-care arm. The overall proportion of participants achieving absence of ulceration following HSCTlite ranged between 40% and 50%, compared with 43% in the original analysis.
In total, six patients had missing week 48 data, but had valid week 24 data to use, three each in both the usual care and the HSCTlite arm. The results were similar to the primary analysis: the proportion of patients achieving absence of ulceration in the HSCTlite arm was 40%, and the number of treatment successes in the usual-care arm remained zero. One patient in the HSCTlite arm had their week 48 colonoscopy outside the extended visit window.
Secondary outcomes
Table 12 shows the categorical secondary outcomes, in particular several definitions of clinical remission, as well as complete endoscopic remission. In the usual-care arm, no participants achieved complete endoscopic remission, whereas two participants achieved it in the HSCTlite arm.
| Secondary categorical outcomes | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| HSCTlite | Usual care | ||
| Clinical remission (CDAI score of < 150) | 4/7 (57) | 1/6 (17) | 5/13 (39) |
| Steroid-free clinical remission (CDAI score of < 150) | 3/7 (43) | 1/6 (17) | 4/13 (31) |
| Clinical remission (HBI score of < 4) | 3/8 (38) | 1/8 (13) | 4/16 (25) |
| Clinical remission (PRO2- abdominal pain score of < 1 and stool frequency score of < 1.5) | 2/7 (29) | 0/7 (0) | 2/14 (14) |
| Complete endoscopic remission (SES–CD score of 0) | 3/6 (50) | 0/3 (0) | 3/9 (33) |
The continuous secondary outcomes at 48 weeks are shown in Table 13. Although 13 participants were eligible to be assessed for absence of ulceration, only eight had substantial valid colonoscopy data to calculate a SES-CD score. This was because two participants did not attend their week 48 colonoscopy because of illness and one participant died at week 24. Two additional patients had enough colonoscopy data to ascertain absence of ulceration, but they did not have centrally calculated SES-CD scores. The average SES-CD score in the HSCTlite arm was 2.8, whereas in the usual-care arm the average score was 18.7, suggesting more disease activity in the usual-care arm. This is again demonstrated in the change in SES-CD score from baseline, where the intervention arm on average had a significant decrease in SES-CD score, whereas the usual-care SES-CD score increased. The CDAI at week 48 and the change from baseline are also shown. The HSCTlite arm had a lower average CDAI score at 48 weeks, and had a larger decline in CDAI from baseline.
| Secondary continuous outcomes | Treatment arm | Total | |
|---|---|---|---|
| HSCTlite | Usual care | ||
| EQ-5D-5L | |||
| n | 9 | 7 | 16 |
| Mean (SD) | 0.496 (0.434) | 0.493 (0.315) | 0.495 (0.374) |
| Median (IQR) | 0.584 (0.516–0.720) | 0.585 (0.380–0.723) | 0.585 (0.400–0.722) |
| IBD-Control | |||
| n | 9 | 7 | 16 |
| Mean (SD) | 9.4 (5.3) | 3.4 (4.4) | 6.8 (5.7) |
| Median (IQR) | 10.0 (6.0–14.0) | 1.0 (0.5–5.5) | 6.5 (1.0–11.2) |
| IBDQa | |||
| Bowel systems | |||
| n | 9 | 7 | 16 |
| Mean (SD) | 5.0 (2.1) | 3.6 (1.4) | 4.4 (1.9) |
| Median (IQR) | 5.0 (4.0–7.0) | 3.0 (3.0–4.0) | 4.5 (3.0–6.0) |
| Emotion health | |||
| n | 9 | 7 | 16 |
| Mean (SD) | 4.7 (1.6) | 3.1 (0.9) | 4.0 (1.5) |
| Median (IQR) | 5.0 (4.0–6.0) | 3.0 (2.5–4.0) | 4.0 (3.0–5.0) |
| Systemic systems | |||
| n | 9 | 7 | 16 |
| Mean (SD) | 4.4 (1.8) | 2.6 (1.3) | 3.6 (1.8) |
| Median (IQR) | 5.0 (3.0–6.0) | 2.0 (2.0–3.0) | 3.0 (2.0–5.0) |
| Social function | |||
| n | 9 | 6 | 15 |
| Mean (SD) | 5.1 (2.4) | 3.5 (1.5) | 4.5 (2.2) |
| Median (IQR) | 6.0 (3.0–7.0) | 3.5 (3.0–4.8) | 5.0 (3.0–6.5) |
| Total score | |||
| n | 9 | 6 | 15 |
| Mean (SD) | 151.8 (53.2) | 103.3 (32.0) | 132.4 (50.8) |
| Median (IQR) | 167.0 (100.0–198.0) | 91.5 (81.0–124.5) | 135.0 (91.5–169.5) |
| Central SES-CD | |||
| n | 5 | 3 | 8 |
| Mean (SD) | 2.8 (2.9) | 18.7 (9.1) | 8.8 (9.8) |
| Median (IQR) | 3.0 (0.0–4.0) | 15.0 (13.5–22.0) | 5.5 (2.2–12.8) |
| Local SES-CD score | |||
| n | 5 | 4 | 9 |
| Mean (SD) | 1.6 (1.8) | 14.8 (11.0) | 7.4 (9.7) |
| Median (IQR) | 1.0 (0.0–3.0) | 10.5 (9.2–16.0) | 4.0 (1.0–10.0) |
| SES-CD change from baseline to week 48 | |||
| n | 5 | 2 | 7 |
| Mean (SD) | –6.4 (4.3) | 7.5 (3.5) | –2.4 (7.8) |
| Median (IQR) | –6.0 (–7.0 to –5.0) | 7.5 (6.2–8.8) | –5.0 (–6.5 to 2.0) |
| MaRIA score | |||
| n | 1 | 2 | 3 |
| Mean (SD) | 66.3 (–) | 58.8 (11.3) | 61.3 (9.1) |
| Median (IQR) | 66.3 (66.3–66.3) | 58.8 (54.8–62.8) | 66.3 (58.6–66.6) |
| CDAI | |||
| n | 7 | 6 | 13 |
| Mean (SD) | 299.9 (271.3) | 307.8 (156.4) | 303.5 (216.8) |
| Median (IQR) | 127.0 (108.5–422.0) | 319.9 (179.8–443.7) | 227.0 (118.0–454.0) |
| CDAI change from baseline to week 48 | |||
| n | 7 | 5 | 12 |
| Mean (SD) | –87.3 (100.3) | 14.8 (159.9) | –44.8 (132.4) |
| Median (IQR) | –82.0 (–149.0 to –19.0) | 28.0 (–28.0 to 64.0) | –28.0 (–116.5 to 29.8) |
| Patient Global Impression of Change | |||
| Activity change, n (%) | |||
| No change | 2 (22) | 4 (50) | 6 (35) |
| Almost the same or somewhat better | 0 (0) | 3 (37) | 3 (18) |
| Moderately better or a great deal better | 7 (78) | 1 (13) | 8 (47) |
| Degree of change | |||
| n | 9 | 8 | 17 |
| Mean (SD) | 3.2 (4.0) | 6.1 (2.1) | 4.6 (3.5) |
| Median (IQR) | 2.0 (0.0–3.0) | 6.5 (5.0–8.0) | 5.0 (2.0–8.0) |
The CDAI and SES-CD scores across all recorded time points are presented in Figures 2 and 3, respectively. The scores are split by treatment arm, with the average for each treatment arm shown by the dashed lines. The CDAI scores for both arms fluctuates over the follow-up period; however, the average CDAI score at 48 weeks in the usual-care arm is higher than that in the HSCTlite arm. The SES-CD score for the HSCTlite arm declines over time, and is lower than in the usual-care arm at 24 and 48 weeks.
FIGURE 2.
The CDAI score over time split by treatment arm, presented on the ITT population. Dashed lines show arm averages and solid lines show individual trajectories.
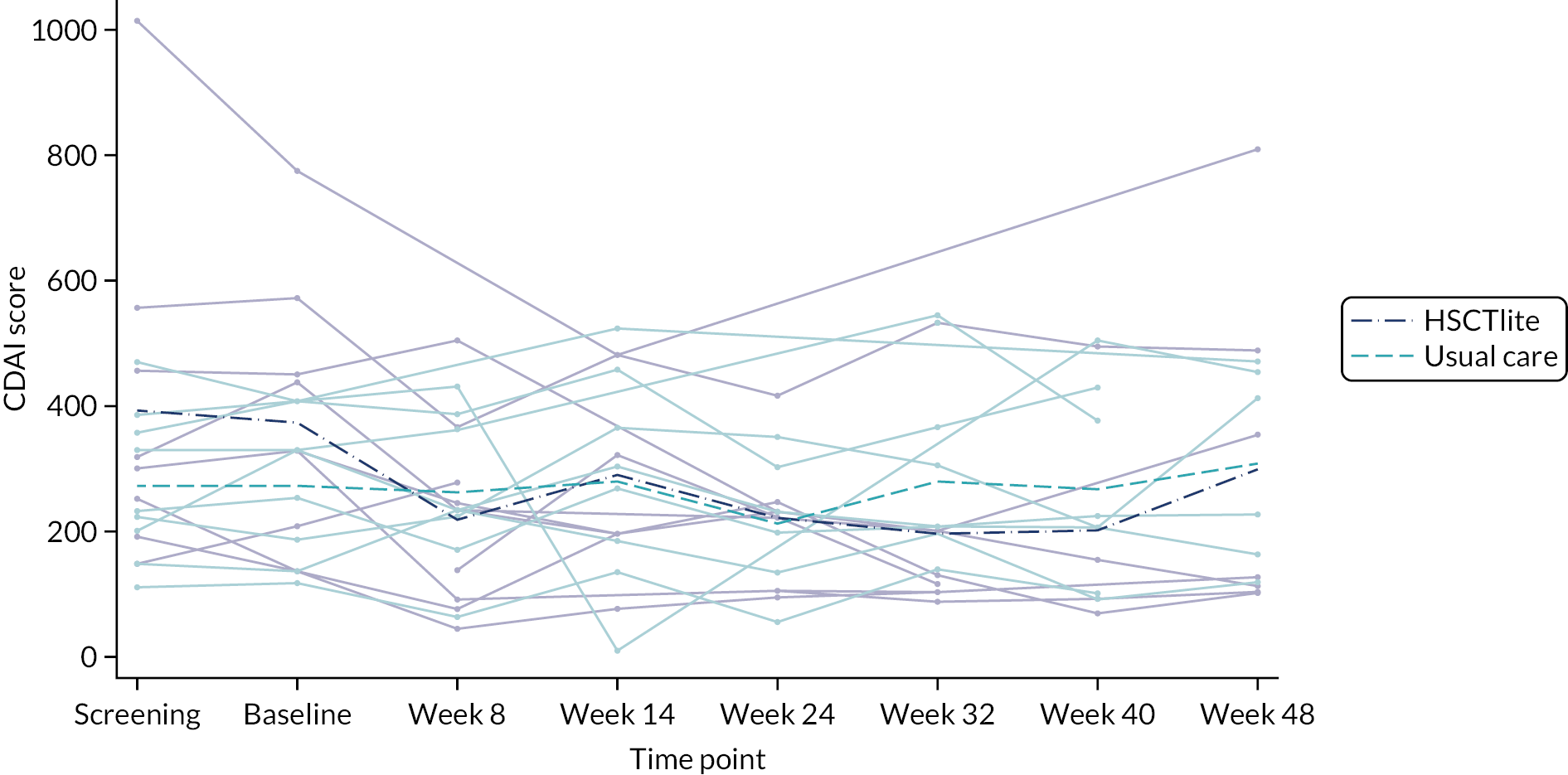
FIGURE 3.
The SES-CD score over time split by treatment arm, presented on the ITT population. Dashed lines show group averages and solid lines show individual trajectories.

Subgroup and moderator analysis
Table 14 shows those who achieved the primary outcome summarised by subgroups and treatment arms. The objective of an exploratory subgroup analysis was to explore heterogeneity in the intervention effects across the following predefined subgroups:
-
perianal CD
-
current smoker
-
disease location
-
current treatment at screening.
| Subgroup classification | Absence of ulceration, n (%) | |||||
|---|---|---|---|---|---|---|
| HSCTlite | Usual care | Total | ||||
| Yes (n = 3) | No (n = 4) | Yes (n = 0) | No (n = 6) | Yes (n = 3) | No (n = 10) | |
| Perianal | ||||||
| Yes | 1 (33) | 1 (25) | 0 (–) | 3 (50) | 1 (33) | 4 (40) |
| No | 2 (67) | 3 (75) | 0 (–) | 3 (50) | 2 (67) | 6 (60) |
| Current smoker | ||||||
| Yes | 0 (0) | 1 (25) | 0 (–) | 1 (17) | 0 (0) | 2 (20) |
| No | 3 (100) | 3 (75) | 0 (–) | 5 (83) | 3 (100) | 8 (80) |
| Disease locationa | ||||||
| Ileal | 1 (33) | 2 (50) | 0 (–) | 2 (33) | 1 (33) | 5 (50) |
| Colonic | 0 (0) | 0 (0) | 0 (–) | 0 (0) | 0 (0) | 0 (0) |
| Ileo-colonic | 2 (67) | 2 (50) | 0 (–) | 3 (50) | 2 (67) | 5 (50) |
| Isolated upper disease | 0 (0) | 0 (0) | 0 (–) | 1 (17) | 0 (0) | 1 (10) |
| Current CD treatment at screening | ||||||
| Yes | 3 (100) | 0 (0) | 0 (–) | 0 (0) | 3 (100) | 0 (0) |
These analyses were included within the SAP. However, numbers were small owing to the early closure of the trial, which significantly limits these analyses: the usual-care arm had no treatment successes, so the subgroup analysis affects only the HSCTlite arm. The proportion of participants in the HSCTlite arm who had perianal CD and achieved absence of ulceration was 33%. Notably, all three treatment successes in the intervention arm were non-smokers. The successfully treated patients did not have CD in the colonic or isolated upper regions. All three were receiving treatment for CD at screening. As stated above, limited conclusions can be made from this owing to the small numbers.
Safety and harms
The safety data are summarised for the extended ITT population, that is, all randomised patients including the participant found to be ineligible post randomisation. The AEs in Table 15 are split by time periods and treatment group. The time periods are as follows:
-
Mobilisation phase – period from start of mobilisation to start of conditioning, randomisation to day 0 for usual-care participants.
-
Transplant phase – period from start of conditioning up to 100 days from day 0 (day of autologous transplantation/infusion), day 0 to day 100 for usual-care participants.
-
Follow-up phase: from ≥ 100 days post-transplantation phase on-wards, day 100 on-wards in usual care participants.
| Safety outcomes: AEs | Time period and treatment arm, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mobilisation | Transplantation | Follow-up | Totala | ||||||
| HSCTlite (n = 13) | Usual care (n = 10) | HSCTlite (n = 13) | Usual care (n = 10) | HSCTlite (n = 13) | Usual care (n = 10) | HSCTlite (n = 13) | Usual care (n = 10) | Total (n = 23) | |
| Number (%) of participants who experienced one or more AE | 3 (23) | 3 (30) | 11 (85) | 4 (40) | 10 (77) | 4 (40) | 13 (100) | 4 (40) | 17 (74) |
| Number (%) of participants who experienced one or more AE by relation to interventionb | |||||||||
| Cyclophosphamide | 1 (33) | 0 (0) | 9 (81) | 0 (0) | 7 (70) | 0 (0) | 12 (92) | 0 (0) | 12 (71) |
| Filgrastim | 0 (0) | 0 (0) | 3 (27) | 0 (0) | 0 (0) | 0 (0) | 3 (23) | 0 (0) | 3 (18) |
| Fludarabine | 0 (0) | 0 (0) | 8 (73) | 0 (0) | 6 (60) | 0 (0) | 9 (69) | 0 (0) | 9 (53) |
| Rabbit ATG | 0 (0) | 0 (0) | 10 (91) | 0 (0) | 6 (60) | 0 (0) | 11 (85) | 0 (0) | 11 (65) |
| Mesna | 0 (0) | 0 (0) | 2 (18) | 0 (0) | 0 (0) | 0 (0) | 2 (15) | 0 (0) | 2 (12) |
| Methylprednisolone | 0 (0) | 0 (0) | 2 (18) | 0 (0) | 0 (0) | 0 (0) | 2 (15) | 0 (0) | 2 (12) |
| Another drug | 0 (0) | 0 (0) | 6 (55) | 0 (0) | 2 (20) | 0 (0) | 7 (54) | 0 (0) | 7 (41) |
| Number of all AEs (including repeated events for each patient) a | 5 | 3 | 61 | 5 | 31 | 18 | 100 | 27 | 127 |
| Relationship to intervention | |||||||||
| Cyclophosphamide | 3 (60) | 0 (0) | 32 (52) | 0 (0) | 15 (48) | 0 (0) | 50 (50) | 0 (0) | 50 (39) |
| Filgrastim | 0 (0) | 0 (0) | 4 (7) | 0 (0) | 0 (0) | 0 (0) | 4 (4) | 0 (0) | 4 (3) |
| Fludarabine | 0 (0) | 0 (0) | 27 (44) | 0 (0) | 14 (45) | 0 (0) | 41 (41) | 0 (0) | 41 (32) |
| Rabbit ATG | 1 (20) | 0 (0) | 33 (54) | 0 (0) | 14 (45) | 0 (0) | 48 (48) | 0 (0) | 48 (38) |
| Mesna | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| Methylprednisolone | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| Another drug | 0 (0) | 0 (0) | 15 (25) | 0 (0) | 2 (6) | 0 (0) | 17 (17) | 0 (0) | 17 (13) |
| NCI CTCAE category | |||||||||
| Blood and lymphatic system disorders | 0 (0) | 0 (0) | 4 (7) | 0 (0) | 4 (13) | 0 (0) | 8 (8) | 0 (0) | 8 (6) |
| Cardiac disorders | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Ear and labyrinth disorders | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Eye disorders | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Gastrointestinal disorders | 3 (60) | 0 (0) | 8 (13) | 1 (20) | 3 (10) | 6 (33) | 16 (16) | 11 (41) | 27 (21) |
| General disorders and administration site conditions | 0 (0) | 0 (0) | 5 (8) | 1 (20) | 1 (3) | 1 (6) | 6 (6) | 2 (7) | 8 (6) |
| Immune system disorders | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| Infections and infestations | 2 (40) | 0 (0) | 15 (25) | 2 (40) | 6 (19) | 10 (56) | 23 (23) | 12 (44) | 35 (28) |
| Investigations | 0 (0) | 0 (0) | 5 (8) | 0 (0) | 9 (29) | 0 (0) | 14 (14) | 0 (0) | 14 (11) |
| Metabolism and nutrition disorders | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Nervous system disorders | 0 (0) | 0 (0) | 5 (8) | 0 (0) | 0 (0) | 0 (0) | 5 (5) | 0 (0) | 5 (4) |
| Psychiatric disorders | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 2 (6) | 0 (0) | 3 (3) | 0 (0) | 3 (2) |
| Renal and urinary disorders | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 4 (13) | 0 (0) | 6 (6) | 0 (0) | 6 (5) |
| Respiratory, thoracic and mediastinal disorders | 0 (0) | 0 (0) | 5 (8) | 1 (20) | 0 (0) | 0 (0) | 5 (5) | 1 (4) | 6 (5) |
| Skin and subcutaneous tissue disorders | 0 (0) | 0 (0) | 5 (8) | 0 (0) | 0 (0) | 1 (6) | 5 (5) | 1 (4) | 6 (5) |
| Vascular disorders | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 3 (3) | 0 (0) | 3 (2) |
The time periods used for the intervention and usual-care arms are not exactly equivalent, but have been included so that the safety data can be compared across arms by the key stages of the intervention.
In the HSCTlite arm, all 13 participants experienced at least one AE. In total, 100 AEs occurred in the HSCTlite arm. In the usual-care arm, four people experienced at least one AE. In the HSCTlite arm, 61 AEs occurred during the transplantation phase, compared with five AEs in the usual-care arm during the same time period. A further 31 AEs occurred in the HSCTlite arm and a further 18 occurred in the usual care arm during the follow-up phase. In total, 127 AEs were recorded throughout the study.
Table 16 displays the SAEs by time point and treatment group. In total, 38 SAEs were experienced by 13 participants in the HSCTlite arm and 16 SAEs were experienced by four participants in the usual care arm. A large proportion of the SAEs occurred during the transplantation phase: 24 were recorded in the HSCTlite arm, compared with three in the usual-care arm. Four SAEs (experienced by two participants) resulted in the participants’ deaths, one during the study (at 24 weeks) and one post 48 weeks. One SAE resulted in treatment withdrawal, and the majority of SAEs required specific treatment or other action.
| Safety outcomes: SAEs | Time period and treatment arm, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mobilisation | Transplantation | Follow-up | Totala | Total (n= 23) | |||||
| HSCTlite (n = 13) | Usual care (n = 10) | HSCTlite (n = 13) | Usual care (n = 10) | HSCTlite (n = 13) | Usual care (n = 10) | HSCTlite (n = 13) | Usual care (n = 10) | ||
| Number (%) of participants who experienced one or more SAE | 2 (15.38) | 2 (20.00) | 11 (84.62) | 3 (30.00) | 6 (46.15) | 3 (30.00) | 13 (100.00) | 4 (40.00) | 17 (73.91) |
| Number of all SAEs (including repeated events) | 4 | 3 | 24 | 3 | 8 | 9 | 38 | 16 | 54 |
| SAEs by seriousness | |||||||||
| Death | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (2) |
| Life threatening | 1 (25) | 0 (0) | 3 (13) | 1 (33) | 0 (0) | 0 (0) | 4 (11) | 1 (6) | 5 (9) |
| Inpatient hospitalisation | 3 (75) | 3 (100) | 10 (42) | 2 (67) | 6 (75) | 9 (100) | 21 (55) | 14 (88) | 35 (65) |
| Prolonged hospitalisation | 0 (0) | 0 (0) | 3 (13) | 0 (0) | 2 (25) | 0 (0) | 5 (13) | 1 (6) | 6 (11) |
| Persistent or significant disability/incapacity | 0 (0) | 0 (0) | 3 (13) | 0 (0) | 0 (0) | 0 (0) | 3 (8) | 0 (0) | 3 (6) |
| Congenital abnormality/birth defect | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Another important medical event | 0 (0) | 0 (0) | 4 (17) | 0 (0) | 0 (0) | 0 (0) | 4 (11) | 0 (0) | 4 (7) |
| SAEs by outcome | |||||||||
| Recovered | 3 (75) | 1 (33) | 11 (46) | 2 (67) | 2 (25) | 2 (22) | 17 (45) | 6 (38) | 23 (43) |
| Improved | 1 (25) | 2 (67) | 7 (29) | 1 (33) | 3 (38) | 7 (78) | 12 (32) | 10 (63) | 22 (41) |
| Unchanged | 0 (0) | 0 (0) | 3 (13) | 0 (0) | 1 (13) | 0 (0) | 4 (11) | 0 (0) | 4 (7) |
| Deterioration | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Persisted | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 1 (3) | 0 (0) | 1 (2) |
| Death | 0 (0) | 0 (0) | 3 (13) | 0 (0) | 1 (13) | 0 (0) | 4 (11) | 0 (0) | 4 (7) |
| SAE by action taken | |||||||||
| None | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Treatment withdrawn | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (2) |
| NIMP dose alteration | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (2) |
| Conmed dose change | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (2) |
| Specific treatment | 4 (100) | 3 (100) | 15 (63) | 3 (100) | 4 (50) | 4 (44) | 24 (63) | 11 (69) | 35 (65) |
| Other | 0 (0) | 0 (0) | 6 (25) | 0 (0) | 4 (50) | 5 (56) | 11 (29) | 5 (31) | 16 (30) |
Table 17 details both AEs and SAEs by NCI CTCAE grade. The percentage of participants in the HSCTlite arm experiencing at least one grade 1–2 AE was 77%, compared with 30% in the usual-care arm. One participant in the usual-care arm experienced an AE above grades 1–2. Three participants experienced a grade 3 AE, but no grade 4 AEs were recorded. However, six participants in the HSCTlite arm experienced at least one grade 4 SAE, and 10 experienced one grade 3 SAE. In the HSCTlite arm, 59 AEs were grade 1–2, compared with 10 in the usual-care arm. The number of grade 3 SAEs was 27 in the HSCTlite arm and 16 in the usual-care arm, and there were eight grade 4 SAEs recorded, all of which were in the HSCTlite arm.
| CTCAE Grade: | Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Safety outcomes - Adverse Events | HSCTlite | Usual Care | HSCTlite | Usual Care | HSCTlite | Usual Care | HSCTlite | Usual Care |
| Number (%) of participants who experienced ≥ 1 AE | 10 (76.92) | 3 (30) | 2 (15.38) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Number (%) of participants who experienced ≥ 1 SAE | 3 (23.08) | 0 (0) | 10 (76.92) | 4 (40) | 5 (38.46) | 0 (0) | 1 (7.69) | 0 (0) |
| Number of all AEs (including repeated events) | 59 | 10 | 3 | 1 | 0 | 0 | 0 | 0 |
| Number of all SAEs (including repeated events) | 3 | 0 | 27 | 16 | 7 | 0 | 1 | 0 |
| AEs by NCI CTCAE Category, n (%) | ||||||||
| Blood and lymphatic system disorders | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cardiac disorders | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ear and labyrinth disorders | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Eye disorders | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal disorders | 11 (19) | 4 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| General disorders and administration site conditions | 1 (2) | 1 (10) | 1 (33) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Infections and infestations | 10 (17) | 4 (40) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Investigations | 12 (20) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Metabolism and nutrition disorders | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nervous system disorders | 4 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Psychiatric disorders | 3 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Renal and urinary disorders | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Skin and subcutaneous tissue disorders | 5 (8) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular disorders | 3 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SAEs by NCI CTCAE Category, n (%) | ||||||||
| Blood and lymphatic system disorders | 1 (33) | 0 (0) | 5 (19) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal disorders | 1 (33) | 0 (0) | 3 (11) | 7 (44) | 1 (14) | 0 (0) | 0 (0) | 0 (0) |
| Infections and infestations | 1 (33) | 0 (0) | 10 (37) | 8 (50) | 1 (14) | 0 (0) | 0 (0) | 0 (0) |
| General disorders and administration site conditions | 0 (0) | 0 (0) | 2 (7) | 0 (0) | 2 (29) | 0 (0) | 0 (0) | 0 (0) |
| Immune system disorders | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) |
| Investigations | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nervous system disorders | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Renal and urinary disorders | 0 (0) | 0 (0) | 3 (11) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | 0 (0) | 0 (0) | 1 (4) | 1 (6) | 1 (14) | 0 (0) | 1 (100) | 0 (0) |
Suspected unexpected serious adverse reactions
In total, nine SUSARs occurred across six patients (three patients had more than one SUSAR) in the HSCTlite arm. The SAEs and SUSARs for the HSCTlite arm and the timing from stem cell reinfusion are shown in Table 18. Two SUSARs occurred on the day of stem cell reinfusion. There were three occurrences of renal biopsy-proven thrombotic microangiopathy (TMA) between days 90 and 153.
| SAE | Time from stem cell reinfusion date (days) |
|---|---|
| Acute anaphylaxis | –4 |
| Shortness of breath | –4 |
| Adverse drug reaction | –3 |
| Adverse drug reaction | –2 |
| Anaphylaxis | –2 |
| Adverse reaction | –2 |
| Respiratory failure | 0 |
| Acute oliguric renal failure | 0 |
| Cytomegalovirus infection reactivation | 28 |
| Pneumocystis pneumonia | 28 |
| Vomiting | 31 |
| Optic neuritis | 46 |
| Perianal abscess | 56 |
| Pneumonia | 61 |
| Influenza | 69 |
| Renal failure | 74 |
| Pyrexia of unknown origin | 75 |
| Shortness of breath | 78 |
| TMA | 90 |
| Pulmonary veno-occlusive disease | 93 |
| TMA | 99 |
| Shingles | 100 |
| Methaemoglobinaemia | 133 |
| Acute kidney injury | 133 |
| Neutropenic sepsis | 146 |
| TMA | 153 |
| Anaemia | 162 |
| Acute kidney injury | 220 |
| Perianal abscess | 238 |
| hs-CRP abnormal | 281 |
Two HSCTlite patients died after having SUSARs. One patient died approximately 24 weeks after their stem cell reinfusion, having experienced one SUSAR (pulmonary veno-occlusive disease) which occurred on day 93 (12 weeks after stem cell reinfusion). A second patient died after their 48-week follow-up (at 60 weeks post stem cell transplantation); they experienced three SUSARs. Initially, their C-reactive protein (CRP) levels were abnormal, with no apparent underlying cause; later, further SUSARs of respiratory failure and acute oliguric renal failure occurred. Respiratory failure and acute oliguric renal failure occurred on the day of stem cell reinfusion, and the patient was hospitalised during this time.
Table 19 shows the primary outcome by treatment arm and by SUSARs experienced. In the HSCTlite arm, all three participants who achieved absence of ulceration experienced at least one SUSAR. Only two participants in the HSCTlite arm did not experience a SUSAR, neither of whom achieved absence of ulceration. No patients in the usual-care arm experienced a SUSAR. Table 20 shows a line listing of the SAEs and SUSARs experienced by patients in the HSCTlite arm who had valid primary outcome data at 48 weeks. The table shows the timing of the SAE and whether or not the patient died.
| Absence of ulceration, n (%) | Treatment arm, n (%) | |||||
|---|---|---|---|---|---|---|
| HSCTlite | Usual care | Total | ||||
| SUSAR (n = 5) | No SUSAR (n = 2) | SUSAR (n = 0) | No SUSAR (n = 6) | SUSAR (N = 5) | No SUSAR (N = 8) | |
| Yes | 3 (60) | 0 (0) | 0 (0) | 0 (0) | 3 (60) | 0 (0) |
| No | 2 (40) | 2 (100) | 0 (0) | 6 (100) | 2 (40) | 8 (100) |
| Patient | Absence of ulceration | SAE | Timing from stem cell reinfusion date (days) | Patient death |
|---|---|---|---|---|
| 1 | 0 | Anaphylaxis | –2 | No |
| 1 | 0 | Shingles | 100 | No |
| 2 | 0 | Vomiting | 31 | Yes |
| 2 | 0 | Pneumonia | 61 | Yes |
| 2 | 0 | Pneumonia | 78 | Yes |
| 2 | 0 | Pulmonary veno-occlusive disease | 93 | Yes |
| 3 | 1 | Shortness of breath | –4 | No |
| 3 | 1 | Cytomegalovirus infection reactivation | 28 | No |
| 3 | 1 | Pneumocystis pneumonia | 28 | No |
| 3 | 1 | Pyrexia of unknown origin | 75 | No |
| 3 | 1 | TMA | 153 | No |
| 3 | 1 | TMA | 153 | No |
| 4 | 0 | Optic neuritis | 46 | No |
| 4 | 0 | Neutropenic sepsis | 146 | No |
| 4 | 0 | Acute kidney injury | 133 | No |
| 5 | 1 | Respiratory failure | 0 | Yes |
| 5 | 1 | Acute oliguric renal failure | 0 | Yes |
| 5 | 1 | hs-CRP abnormal | 281 | Yes |
| 6 | 1 | TMA | 90 | No |
| 7 | 0 | Adverse drug reaction | -3 | No |
| 7 | 0 | Perianal abscess | 56 | No |
Mechanistic study
Clinical data
Clinical laboratory test data (treatment group over time)
The following box plots (Figure 4) report the median, IQR and range of clinically relevant routine laboratory data by clinical arm over time. As would be expected, after receiving HSCTlite, there is a marked difference in haematological parameters between groups. Patients in the HSCTlite arm experienced anaemia after mobilisation, which persisted despite appropriate transfusion throughout the study. In addition, patients undergoing HSCTlite experienced a marked leukopenia in all lineages as well as a thrombocytopenia after conditioning. There was near-complete recovery of lymphopenia by week 48, but neutrophil, monocyte and platelet counts did not show recovery by week 48. As outlined above, several renal SUSARs were reported in the intervention group. This was confirmed by the reduction in renal function seen (elevated urea and creatinine associated with fall in estimated glomerular filtration rate (eGFR) from week 14. There was partial recovery by week 48, but clinically meaningful differences from the usual-care arm persisted.
FIGURE 4.
Clinical laboratory test data, split by treatment group, presented on the ITT population. (a) Haemoglobin; (b) white cell count; (c) platelet count; (d) neutrophils; (e) lymphocytes; (f) monocytes; (g) creatinine; (h) urea; (i) bilirubin; (j) CRP; and (k) eGFR. Note: the neutrophils plot has had an outlier at week 48 removed (value 88.4). The bilirubin plot has had an outlier at week 48 removed (value 996).




The following clinical laboratory data are not presented in graphical form, as levels remained stable throughout the trial period and/or there was no clear trend or difference between treatment arms: eosinophils, basophils, sodium, potassium, calcium, albumin, alkaline phosphatase, magnesium, gamma-glutamyl transpeptidase, glucose, protein total, aspartate aminotransferase and alanine aminotransferase.
Clinical data
As seen in Figure 5, the anaemia experienced by patients in the HSCTlite arm was more marked in those who reported a renal SUSAR than in those who did not, but both subsets of patients seemed to improve by week 48. Likewise, the thrombocytopenia was more marked in intervention patients reporting a renal SUSAR and persisted to week 48, whereas the platelet count in those with no renal SUSAR increased after week 32. All intervention patients experienced marked lymphopenia, but in those without a renal SUSAR this resolved between week 32 and week 40. In contrast, intervention patients who reported a renal SUSAR had persistent lymphopenia.
FIGURE 5.
Clinical data, split by SUSAR group. (a) eGFR; (b) creatinine; (c) urea; (d) platelet count; (e) haemoglobin; (f) neutrophils; and (g) lymphocytes. Note: split by intervention arm participants who experienced a SUSAR, HSCTlite arm participants who did not experience a SUSAR, and those in the usual-care arm. Presented on the ITT population.




Although the patients experiencing a renal SUSAR accounted for the majority of the reported deterioration in renal function in the HSCTlite arm, it does appear that renal function was impaired more in intervention patients who did not report a renal SUSAR than in those in the usual-care arm. The following variables chosen from the clinical laboratory data were selected as those that were particularly important from a clinical point of view.
Immune reconstitution data
Peripheral blood immune cell subsets by flow cytometry (split by treatment arm)
The box plots in Figure 6 report the median, IQR and range of peripheral blood immune cell subsets by clinical group over time. As would be expected, there is a marked difference in the profile of all the immune cell subsets studied between the HSCTlite and usual-care arms. Of all the subsets studied, only the numbers of CD3+CD8+CD31+ recent thymic emigrant cytotoxic T helper cells, CD3+CD8+CD49d+α4integrin+CCR9+ gut-homing cytotoxic T cells, CD3+CD4+CD45RA–CCR7- effector memory T helper cells and CD8+ cytotoxic T cells had returned to equivalence with the usual-care arm at week 48, with levels of CD3+, CD4+, CD4+ recent thymic emigrants, CD3+CD4+CD45RA–CCR7+ central memory T helper cells, CD4+ effector T helper cells and CD3+CD4+CD45RA+CCR7+ naive T helper cells remaining lower in the HSCTlite arm.
FIGURE 6.
Peripheral blood immune cell subsets by flow cytometry, split by treatment group, presented on the ITT population. (a) Number of T cells (CD3+); (b) number of T helper cells (CD3+, CD4+); (c) number of cytotoxic T cells (CD3+, CD8+); (d) number of recent thymic emigrant T helper cells (CD3+, CD4+, CD31+); (e) number of central memory T helper cells (CD3+, CD4+, CD45RA–, CCR7+); (f) number of effector T helper cells (CD3+, CD4+, CD45RA+, CCR7–); (g) number of effector memory T helper cells (CD3+, CD4+, CD45RA–, CCR7–); (h) number of naive T helper cells (CD3+, CD4+, CD45RA+, CCR7+); (i) number of gut homing cytotoxic T cells (CD3+, CD8+ CD49d + α4integrin + CCR9+); and (j) number of recent thymic emigrant cytotoxic T helper cells (CD3+, CD8+, CD31+). Note: the number of CD3+, CD8+, CD49d + α4integrin + CCR9+ gut homing cytotoxic T cells per µl of peripheral blood plot has had an outlier at baseline removed (value = 425.07).
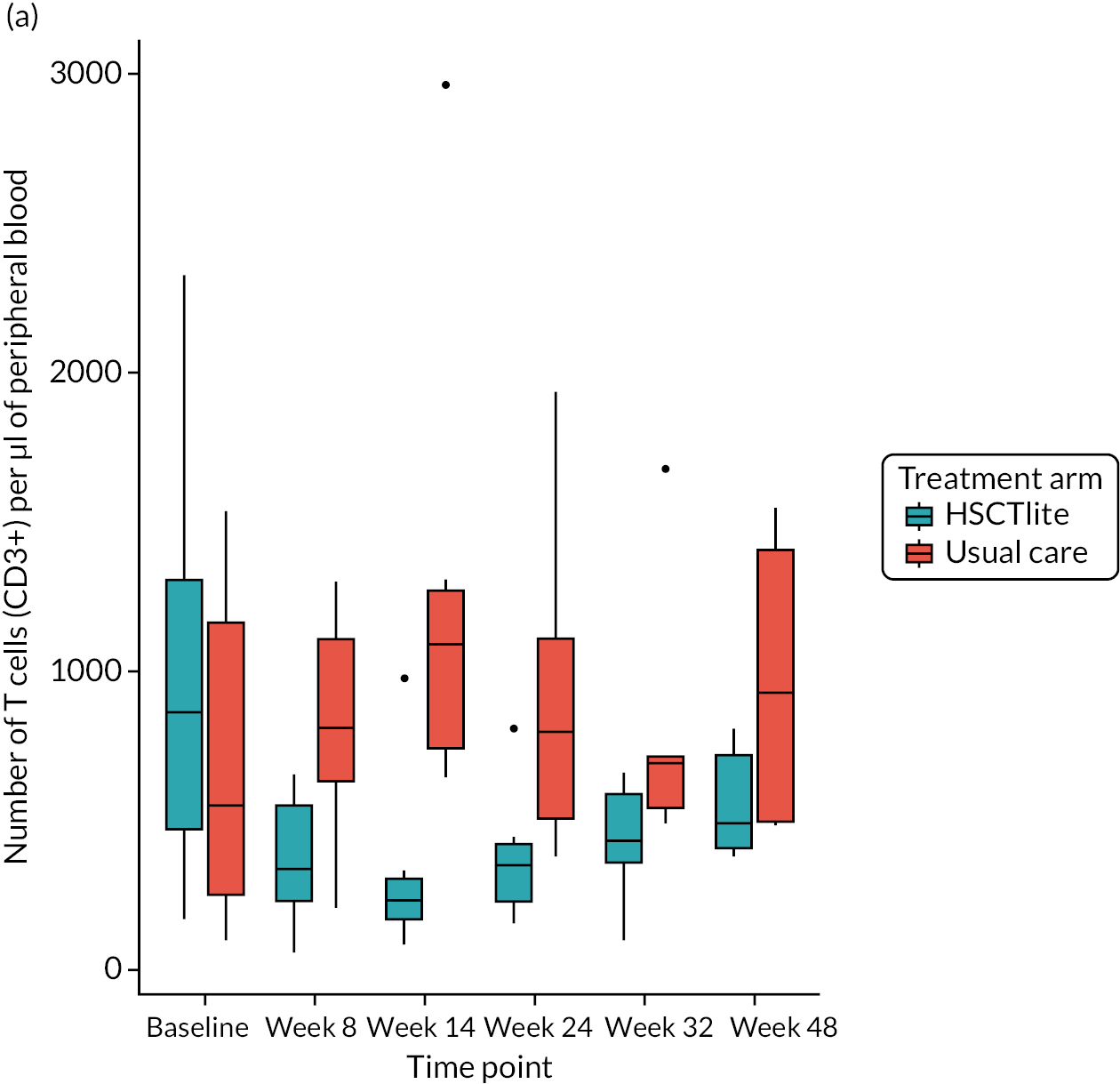
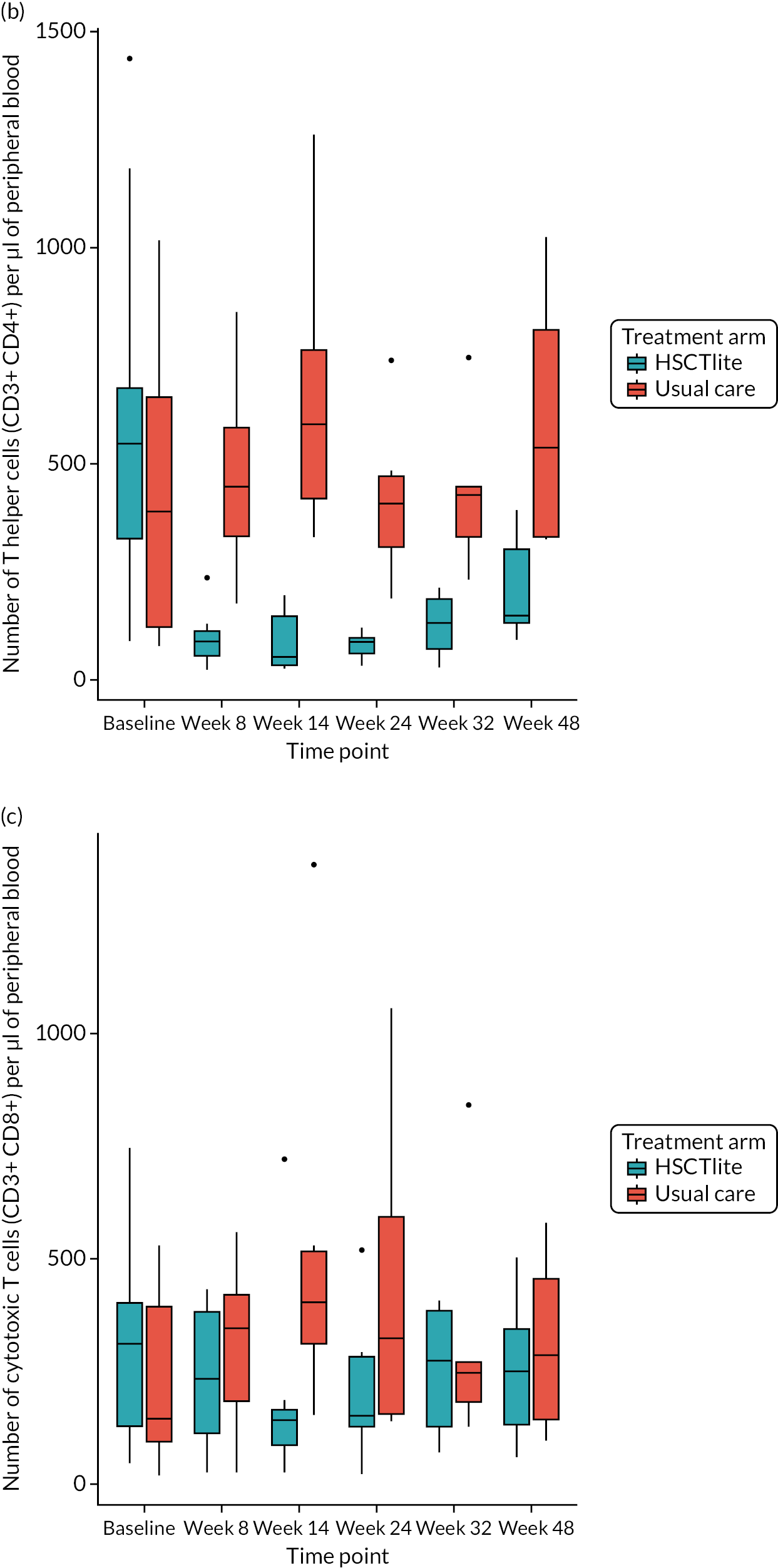
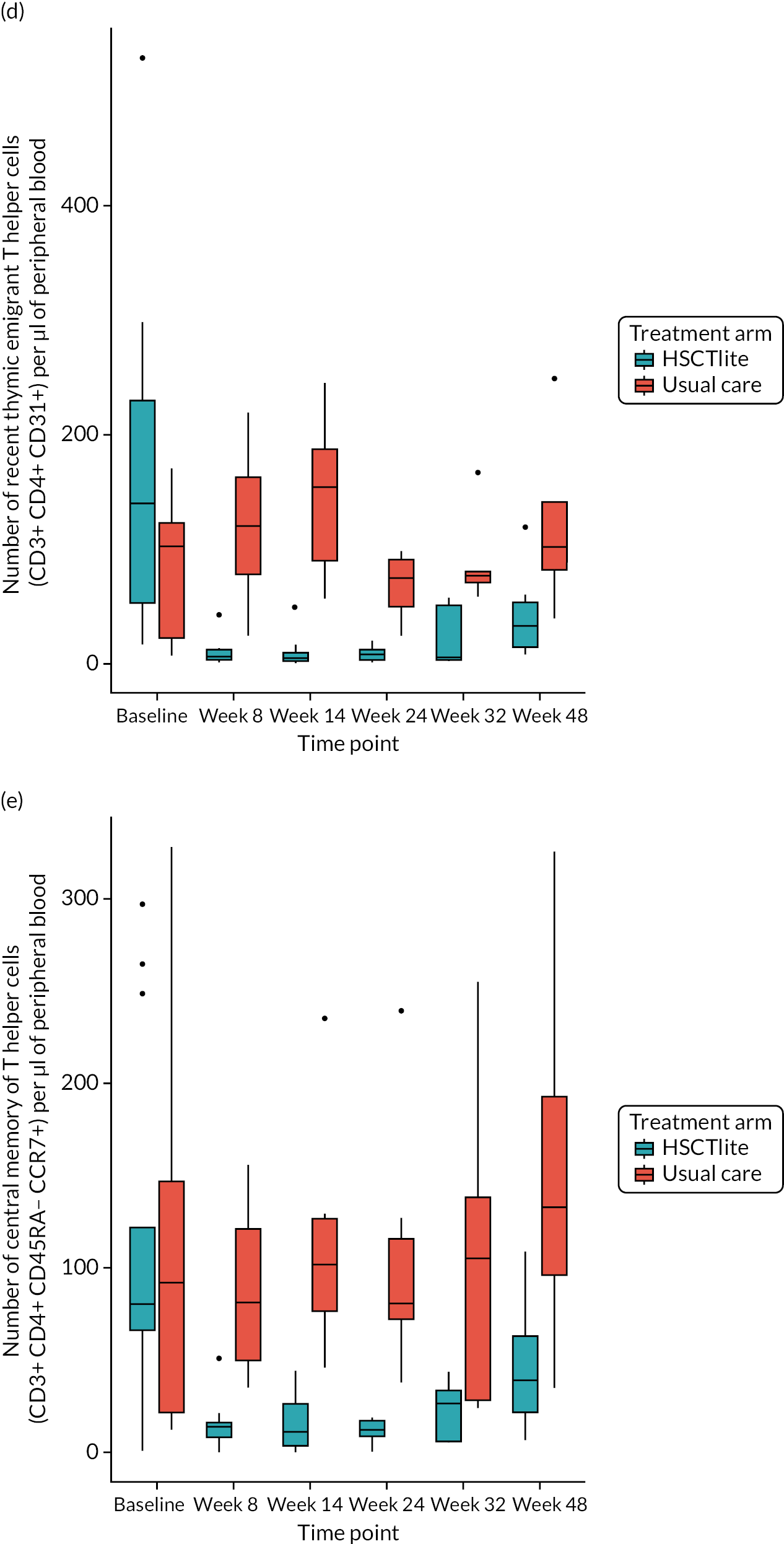

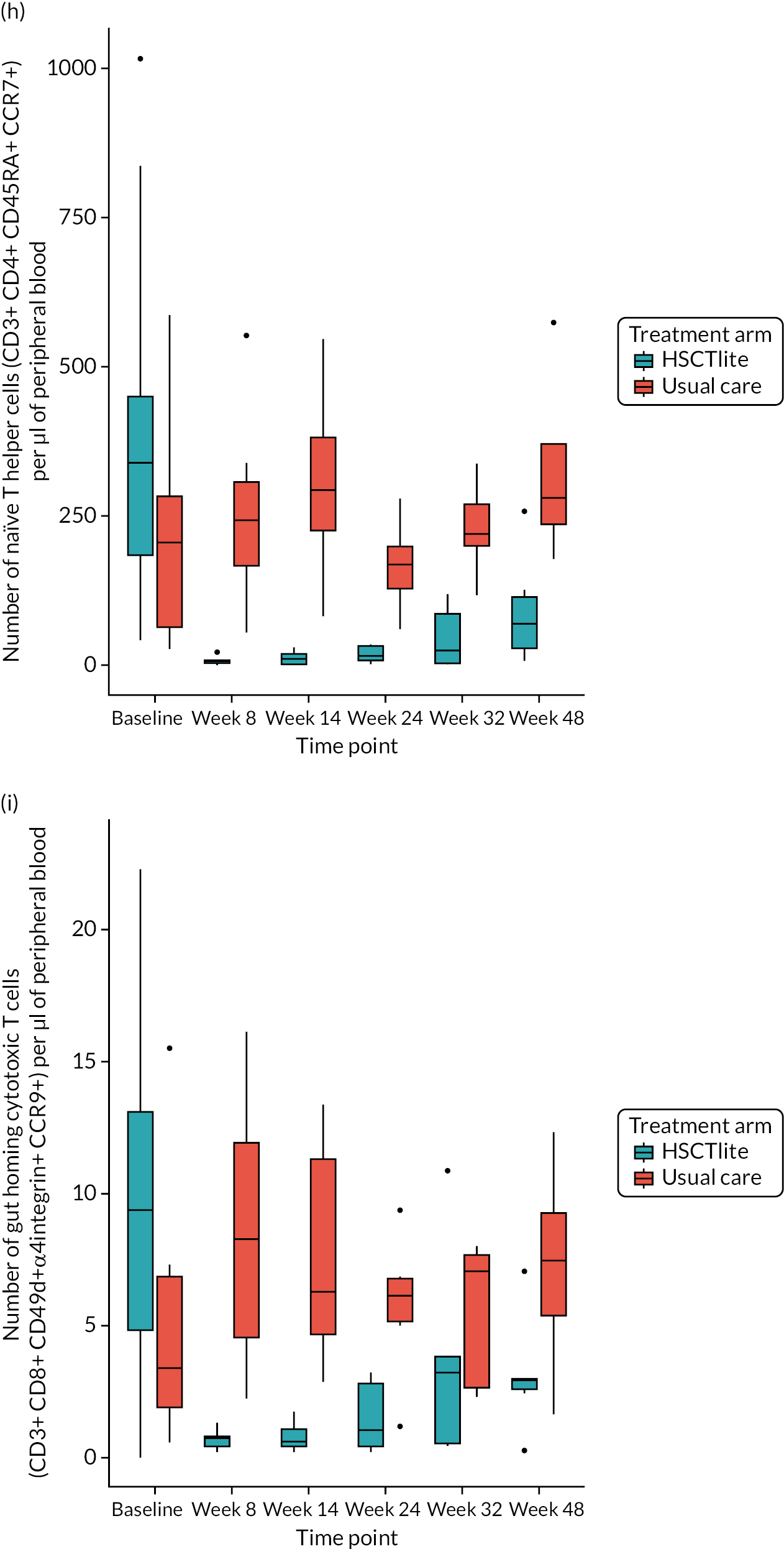

Not all patients provided samples for all time points for this analysis. The numbers of samples used are as follows:
-
baseline – n = 13, HSCTlite arm; n = 8, usual-care arm
-
week 8 – n = 9, HSCTlite arm; n = 7, usual-care arm
-
week 14 – n = 8, HSCTlite arm; n = 6, usual-care arm
-
week 24 – n = 7, HSCTlite arm; n = 7, usual-care arm
-
week 32 – n = 5, HSCTlite arm; n = 5, usual-care arm
-
week 48 – n = 6, HSCTlite arm; n = 5 usual-care arm.
Cytokine expression by peripheral blood CD4+ and CD8+ T cells after in vitro stimulation (treatment arm)
The following box plots (Figure 7) report the median, IQR and range of pro-inflammatory (IL-2, IL-17, TNF-α IFN-γ) and anti-inflammatory (IL-4, IL-10) cytokine production by CD4+ and CD8+ T cells after ex vivo activation by clinical group over time. The production of IL-2 and IL-10 by CD4+ and CD8+ T cells was similar in both the HSCTlite and the usual-care arms, whereas the production of IL-4, IL-17, TNFα and IFN-γ by CD4+ T cells was higher in the HSCTlite arm than in the usual-care arm. With regards to the CD8+ T cell population, the production of all cytokines was similar, apart from IL-4, which was higher in the HSCTlite arm than in the usual-care arm. Data on the cytokine generation by peripheral blood T cell subsets after stimulation according to treatment success/failure for the intervention arm for individual patients are provided in Appendix 6, Figures 10–21.
FIGURE 7.
Cytokine expression by peripheral blood CD4+ and CD8+ T cells after in vitro stimulation, split by treatment arm, presented on the ITT population. (a) CD4+ T cells expressing IL-2; (b) CD4+ T cells expressing IL-4; (c) CD4+ T cells expressing IL-17; (d) CD4+ T cells expressing TNFα; (e) CD4+ T cells expressing IL-10; (f) CD4+ T cells expressing IFNγ; (g) CD8+ T cells expressing IL-2; (h) CD8+ T cells expressing IL-4; (i) CD8+ T cells expressing IL-17; (j) CD8+ T cells expressing TNFα; (k) CD8+ T cells expressing IL-10; and (l) CD8+ T cells expressing IFNγ.

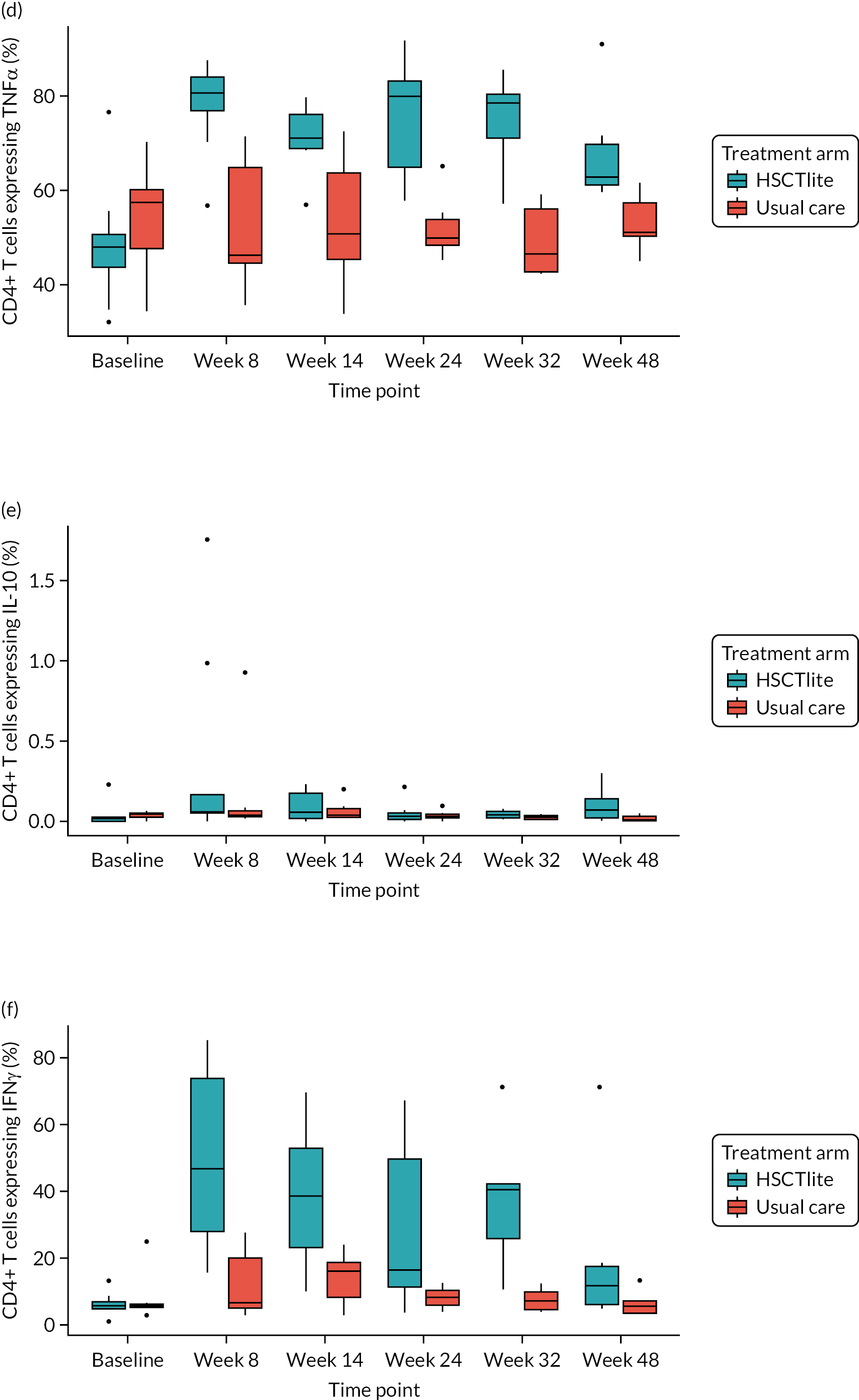
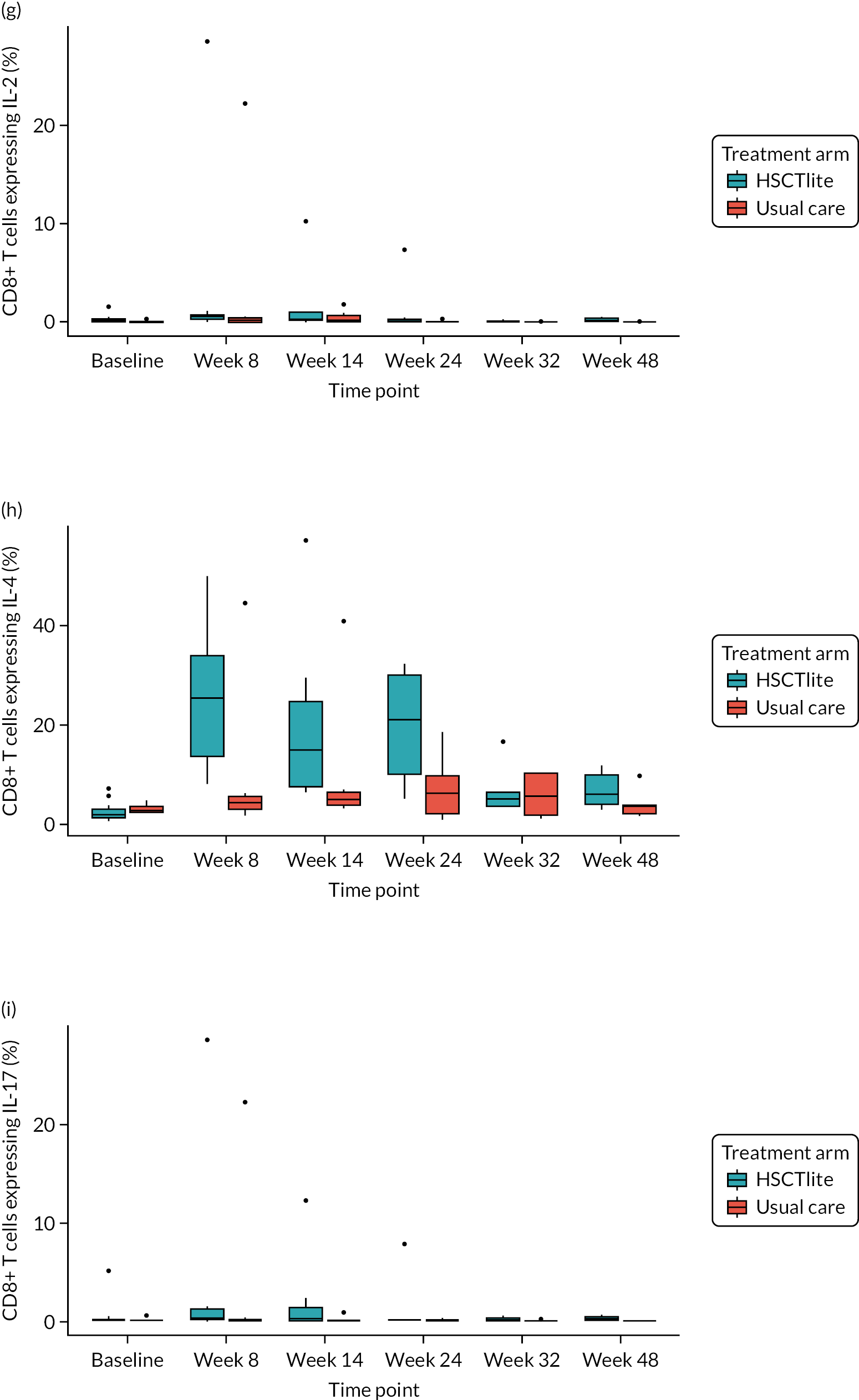
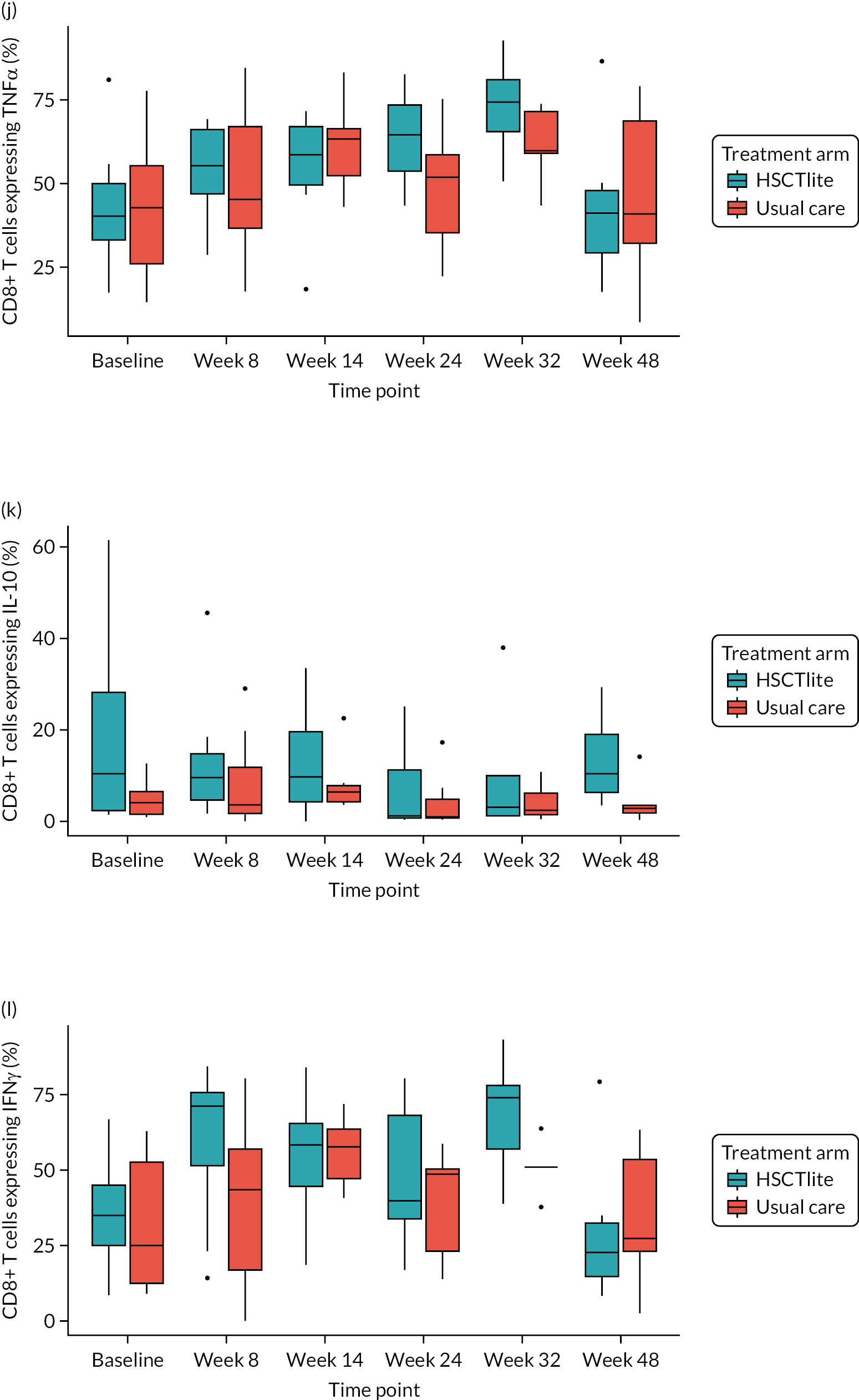
A comparison of week 32 creatinine against week 8 T cell subsets after stimulation is provided in Appendix 7, Figures 22–33.
Not all patients provided samples for all time points for this analysis. The numbers of samples used are as follows:
-
baseline – n = 13, HSCTlite arm; n = 8, usual-care arm
-
week 8 – n = 9, HSCTlite arm; n = 6, usual-care arm
-
week 14 – n = 8, HSCTlite arm; n = 6, usual-care arm
-
week 24 – n = 7, HSCTlite arm; n = 7, usual-care arm
-
week 32 – n = 5, HSCTlite arm; n = 5, usual-care arm
-
week 48 – n = 6, HSCTlite arm; n = 5, usual-care arm.
Analysis of recent thymic emigrants: T-cell receptor excision circle quantitation using reverse transcription polymerase chain reaction (treatment arm)
The following box plot (Figure 8) reports the median, IQR and range for the presence of recent thymic emigrants (TRECs) by clinical group over time. As would be expected, the number of TRECs was lower in the HSCTlite arm than in the usual-care arm, but had recovered to approximately those numbers in the usual-care arm by week 48.
FIGURE 8.
TREC per 106 cells, split by treatment arm. Presented on the ITT population.
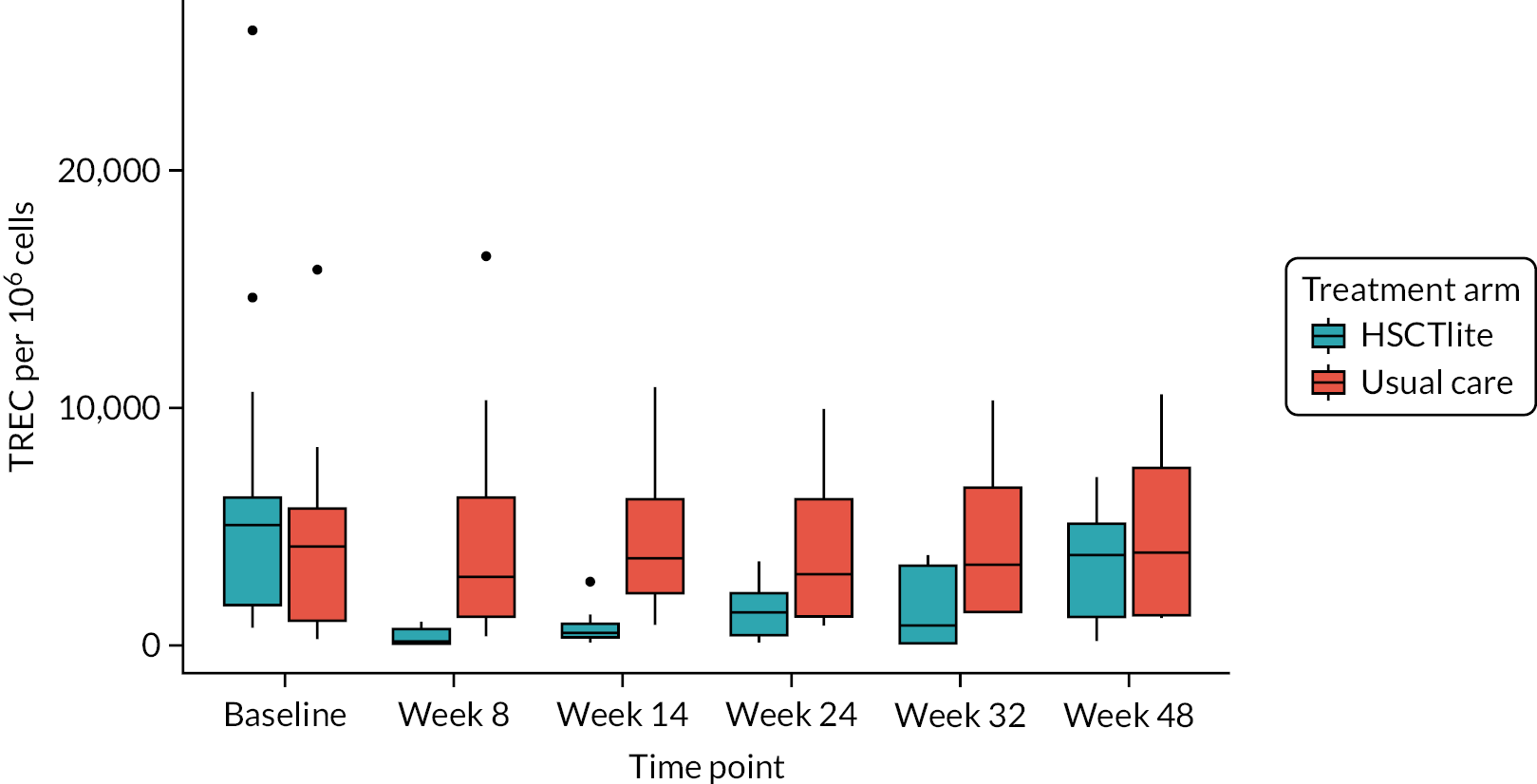
Not all patients provided samples for all time points for this analysis. The numbers of samples used are as follows:
-
baseline – n = 13, HSCTlite arm; n = 9, usual-care arm
-
week 8 – n = 9, HSCTlite arm; n = 8, usual-care arm
-
week 14 – n = 8, HSCTlite arm; n = 7, usual-care arm
-
week 24 – n = 8, HSCTlite arm; n = 7, usual-care arm
-
week 32 – n = 5, HSCTlite arm; n = 5, usual-care arm
-
week 48 – n = 6, HSCTlite arm, n = 4, usual-care arm.
Chapter 5 Discussion
Some text in this section has been reproduced from Lindsay et al. 36
Summary of findings
The ASTIClite trial was designed to determine if autologous stem cell transplantation using a lower-intensity regimen than previously trialled would, compared with usual care, lead to a significantly greater number of patients with treatment-refractory CD achieving endoscopic remission at 1 year with an acceptable safety profile. The trial was stopped before full recruitment on the advice of the DMEC and TSC owing to a high incidence of suspected unexpected SAEs and one patient death.
In total, 23 patients with advanced refractory disease were randomised (n = 13, HSCTlite arm; n = 10, usual-care arm). The efficacy analysis comprised 10 patients who completed HSCTlite treatment and nine patients who received usual care. Data on the primary outcome of death, confirmed colonoscopy or non-attendance due to progressive disease were available for 7 out of 10 HSCTlite and six out of nine usual-care patients at week 48. Absence of endoscopic ulceration without surgery or death was reported in three out of seven (43%) HSCTlite patients compared with zero out of six (0%) usual-care patients.
The endoscopic disease activity measured using the SES-CD [mean (SD)] was 10.8 (6.3) and 10.0 (6.1) at baseline, compared with 2.8 (2.9) and 18.7 (9.1) at week 48 in the HSCTlite and usual-care arms, respectively. Clinical remission (CDAI score < 150) occurred in 57% and 17% of patients in the HSCTlite and usual-care arms, respectively, at week 48.
There was a marked difference in the profile of all the immune cell subsets studied between the HSCTlite and usual-care arms after HSCTlite. Of all the subsets studied, only the numbers of recent thymic emigrant cytotoxic T helper cells, gut-homing cytotoxic T cells, effector memory T helper cells and CD8+ cytotoxic T cells had returned to equivalence with the usual-care arm at week 48, with levels of CD3+, CD4+, CD4+ recent thymic emigrants, central memory T helper cells, CD4+ effector T helper cells and naive T helper cells remaining lower in the HSCTlite arm. After in vitro stimulation, the CD4+ and CD8+ lymphocytes from HSCT patients expressed higher levels of Th1 and Th17 cytokines, that is, IFN-gamma, TNF and IL-17, as well as Th2 cytokines, that is, IL-4, compared with usual care at most time points.
Serious adverse events were more frequent in patients undergoing HSCTlite treatment [38 in 13 (100%) patients] than in patients undergoing usual care [16 in 4 (40%) patients]. Importantly, nine SUSARs were reported in six HSCTlite patients, including three cases of delayed renal failure due to proven TMA. Two patients in the HSCTlite arm died: one from pulmonary veno-occlusive disease at week 24 and one from infection and renal failure (not proven to be TMA) at week 60 after having completed the trial. All patients undergoing HSCTlite treatment showed evidence of re-engraftment with immune recovery during the first year.
Despite being designed as low intensity with lower doses of cyclophosphamide than used previously, the mobilisation and conditioning regimen used for HSCTlite in this trial was associated with an unacceptable rate of AEs. There was evidence of efficacy, with some patients experiencing a meaningful reduction in endoscopic disease activity. However, the observed benefit does not mitigate the AEs, and HSCTlite using this regimen should not be used as a therapy even in patients with severely active treatment-refractory CD.
ASTIClite trial design
The inclusion and exclusion criteria were designed to ensure that only patients with significantly active treatment-refractory disease for which surgery was inappropriate or declined were recruited. Patients at high risk of AEs from HSCTlite such as active perianal disease or penetrating disease were excluded, as were patients who had comorbidity that might increase AEs. All patients were discussed in a MDT meeting prior to being consented into the trial, which had to include at least two gastroenterologists and a haematologist independent of their clinical case. Autologous HSCTlite would be ineffective and inappropriate in patients with a monogenic cause of intestinal inflammation. After a substantial amendment, genotyping for a monogenic cause of disease was suggested in patients with early-onset disease or with a relevant disease phenotype. In view of the AEs reported in the original ASTIC trial, all participating transplant units had to have JACIE accreditation for allogeneic HSCTlite or have participated in the ASTIC trial. Patients randomised to receive HSCTlite stopped all immune-suppressing therapies apart from corticosteroids, using standard wash-out periods prior to mobilisation.
Patients were randomised 2 : 1 to receive HSCTlite versus usual care as all patients were refractory to currently available therapy and considered for trial entry only if they were prepared and keen to undergo HSCTlite treatment. The patient representatives on the TSC highlighted that patients with refractory disease would be prepared to be exposed to the risks associated with HSCTlite. The usual-care arm was considered essential to ensure that any observed benefit of HSCTlite did not simply reflect the natural history of disease. At the end of the trial the intention was to offer patients in the usual-care arm out-of-trial autologous HSCTlite. This did not occur as (1) the trial was halted owing to SUSARs and (2) there was a general suspension of activity of autologous HSCT for autoimmune diseases because of the COVID-19 pandemic (based on guidelines37 supporting its use in only exceptional cases in which the risk–benefit ratio could be justified during the pandemic).
The trial was designed and powered using an endoscopic end point, which is now considered as standard in randomised controlled therapeutic trials in CD. This ensures that there is (1) an objective assessment of therapeutic benefit and (2) a reduced placebo effect. Patients may experience significant non-inflammatory symptoms due to prior intestinal damage even when in complete endoscopic remission, which reduces the ability of disease activity scores that rely heavily on a subjective assessment of symptoms to assess therapeutic benefit. Assessment of endoscopic disease activity by central readers blinded to treatment allocation or time of assessment is considered to be the gold standard in CD clinical trials, as it removes bias associated with local scoring. This was particularly important as it was not possible to blind either patients or investigators to the treatment allocation in this trial. To our knowledge, this was the first UK investigator-led trial to utilise central reading for its primary end point. As such, a bespoke web-based platform was developed to share anonymised videos. After a period of training in reporting the SES-CD scores, the reliability and validity of the pool of central readers was verified using sets of 10 independent videos. This ensured robust assessment of the primary end point.
ASTIClite trial outcome
Recruitment
There was a significant delay in opening all eight recruitment sites, in part owing to changes in the approval processes required for radiation exposure certification. However, the pre-specified early interim analysis confirmed that (1) the reduced 1 g/m2 dose of cyclophosphamide was sufficient for stem cell mobilisation and harvest; (2) the new mobilisation regimen did not precipitate CD flare; (3) it was associated with fewer SAEs than were reported in the ASTIC trial, which used 4 g/m2 cyclophosphamide; and (4) recruitment to time and target was feasible. Indeed, there was a robust ‘pipeline’ of potentially eligible patients flowing through the MDT and subsequent consent process. There were some delays in screening due to (1) a delay in obtaining the genotyping of applicable patients to exclude a monogenic cause of their disease; (2) delays in local referral for fertility assessment and subsequent appointments; and (3) limited capacity for autologous HSCTlite at some sites, such that randomisation was delayed until an available slot was identified if required.
Trial progress
The trial was temporarily halted in December 2019 after one patient developed respiratory failure due to pulmonary artery hypertension. Despite intensive care and extracorporeal membrane oxygenation (ECMO) support at the National Centre for Excellence, the patient died of suspected pulmonary veno-occlusive disease that was subsequently proven on autopsy. In addition, several cases of renal failure developed a significant time after HSCTlite treatment. Three patients had biopsy-proven TMA. These were all fully investigated by tertiary renal centres in collaboration with the National Renal Complement Therapeutic Centre in Newcastle. Although this is a recognised complication of HSCT, it is very rarely reported in patients undergoing autologous HSCT. It is more commonly seen after allogeneic HSCT and has been linked to atypical viral infections, cyclosporin use and endothelial damage. No unifying precipitant for these cases was identified despite exhaustive investigation. Taking into consideration these SUSARs, the delayed presentation and the uncertain aetiology, the DMEC recommended to the TSC that the trial be permanently halted. Patients in screening were withdrawn from the trial, as were those who had been randomised to receive HSCTlite and undergone mobilisation. Patients who had been randomised to receive usual care or had already undergone HSCTlite prior to December 2019 continued in the trial and underwent follow-up and assessment as described in the protocol. This decision was taken for the following reasons: (1) to ensure the safety of all patients recruited to the trial, (2) to acquire as many data as possible to assess the aetiology of TMA in the relevant patients and (3) to assess as many of the objectives of the trial within the limitation of the significantly reduced sample size.
Patients recruited
The assessment of baseline demographics confirm that a population of treatment-refractory patients with a heavy burden of endoscopically active disease were recruited. Almost all had had at least one operation and many had a stoma (predominantly an end-ileostomy after colectomy). Therefore, the total SES-CD score was calculated using a median of four rather than five segments. Patients with an ileostomy only had one segment contributing to their assessed endoscopic disease activity.
Efficacy outcomes
The primary end point of absence of endoscopic ulceration (SES-CD ulcer size subscore of zero) using central reading without surgery or death was achieved in 43% of patients undergoing HSCTlite, compared with 0% of patients in the usual-care arm, although it is recognised that numbers are small. Several sensitivity analyses were performed to impute missing data, including using a centrally read week 24 endoscopy score and excluding those in whom the outcome assessment was delayed outside the protocol-defined window. In addition, there were cases in which local reads of the endoscopy score were available despite technical issues preventing central reading. The most generous efficacy assessment using local disease assessment when available suggested that five out of nine patients (56%) achieved the primary end point. The range of 40–55% patients with refractory CD achieving endoscopic healing at 1 year is in line with our a priori assumptions for the power calculation. In all analyses no patients in the usual-care arm achieved the primary end point, reflecting the severe and refractory nature of the population studied.
In addition, there were clinically meaningful differences between HSCTlite and usual care in many of the secondary endoscopic assessments, including complete endoscopic remission (total SES-CD score of zero), absolute SES-CD score at week 48 and change in SES-CD score between baseline and week 48. Insufficient numbers of MRI scans were available for a meaningful assessment of transmural healing. Analysis of factors that predicted response was limited by the small number of patients, although it is noteworthy that no patients who smoked achieved the primary end point.
Although clinical remission (CDAI score < 150) was achieved in more patients undergoing HSCTlite treatment than usual care, the absolute CDAI at week 48 was not that different between groups. This perhaps reflects the fact that previous intestinal damage and surgery drives symptoms in these refractory patients, which may not correlate with disease activity. Importantly, patient-assessed IBD-Control and both total and disease-specific quality of life as assessed by the IBDQ were greater in the HSCTlite arm than in the usual-care arm.
Safety analysis
It is clear that the modified HSCT regimen used in this trial was not associated with a reduced burden of side effects compared with previously reported regimens. All patients undergoing HSCTlite experienced at least one SAE, and there were a total of 100 AEs, including 38 SAEs, in the HSCTlite arm. Importantly, the only NCI CTCAE grade 4 AEs occurred it the HSCTlite arm. There were nine SUSARs reported in six patients in the HSCTlite arm, including the cases of TMA and pulmonary veno-occlusive disease. It is important to note that patients in the usual-care arm also experienced SAEs related to disease activity, with complications from surgery, admission for disease flare and thromboembolic disease reported. These life-threatening AEs highlight the significant impact of ongoing active disease on patient safety and the importance of effective therapy to control intestinal inflammation.
Aetiology of thrombotic microangiopathy
Thrombotic microangiopathy and associated veno-occlusive disease are extremely rare in the setting of autologous transplantation. Comprehensive analysis of the patients who experienced TMA did not suggest that background comorbidity, disease phenotype or prior drug history were relevant. Many patients undergoing HSCTlite experienced post-transplantation viral reactivation, and this was not restricted to patients who subsequently developed TMA. All patients who experienced TMA received ATG from the same batch; however, working together with the manufacturer, the MHRA and European Medicines Agency, no issues with this drug batch were identified. In addition, reviewing patients undergoing autologous HSCT in other autoimmune disease who received drug from this batch of ATG did not identify any renal AEs. The fact that all patients undergoing HSCT (even those without reported TMA) had a mild deterioration in renal function suggests that aspects of the drug regimen used in this trial affect kidney function. The key difference between this regimen and that used in ASTIC17 and other series in patients with CD is the addition of fludarabine. This has not been associated with reports of TMA after autologous HSCT, but has been associated with endothelial damage and subsequent TMA in allogeneic HSCT. 38,39 Alternatively, it is plausible that the unexpected incidence of TMA renal failure and pulmonary veno-occlusive disease, which has not been reported after autologous HSCT in other autoimmune indications, relates to aspects of CD pathology such as bacterial translocation or to the range of advanced therapies to which patients are exposed.
Overview of the mechanistic data
In the HSCTlite arm, there was a significant/consistent reduction in haemoglobin concentration, platelet count and renal function (as reflected by creatinine, GFR and urea) at all time points to week 48 compared with the usual-care arm. This was most pronounced in the patients in whom a SUSAR was reported (i.e. in those patients who develop renal failure, including TMA). Renal failure (including TMA) is a plausible clinical explanation to account for these differences between the groups. Clinically, this was a serious unexpected finding and was related to the decision to discontinue the trial.
Total lymphocyte count, but not total white cell count or other subsets such as neutrophils or monocytes, was significantly and consistently lower in the HSCTlite arm at all time points. Given the immunosuppression expected with the conditioning regimen,40 this is an expected finding.
The immune phenotyping flow cytometric data indicate that the reduced total lymphocyte count (i.e. CD3+) reflected a low CD4+ cell count (and associated subsets emigrants, central memory T cells, effector memory T cells, naive) at all time points. In contrast, CD8+ and CD8+ subsets recovered rapidly after transplantation, returning back to levels similar to those in the usual-care arm. This picture has been previously reported41 and is consistent with repopulation of CD8+ cells from the peripheral regeneration, with limitations of the CD4+ cell compartment to regenerate in the year after transplantation. Along with the TREC analysis, which was performed on the total lymphocyte population (not specifically CD4+ cells), the picture supports limitations in regeneration via thymic pathways42 in the first 48 months after transplantation. Further analysis to complete the picture could examine NK cell regeneration and specific CD4+/CD8+ TRECs.
There are several exploratory observations related to cytokine expression. Generally, the CD4+ and CD8+ lymphocytes from HSCTlite patients expressed higher levels of Th1 and Th17 cytokines, that is, IFN-gamma, TNF and IL-17, as well as Th2, that is, IL-4, after stimulation than lymphocytes from usual-care patients at most time points. The data on IL-2 and IL-10 expression after stimulation were considered too low level to be interpretable. The clinical and scientific significance of this is uncertain, except to support the concept that HSCTlite is associated with a generalised, non-specific and persistently up-regulated capacity to generate certain cytokines after stimulation. 41,43 The small numbers of patients studied means that it is not possible to associate this with clinical improvements, including remission of CD in some patients. However, it is possible that up-regulated or dysregulated cytokine expression capacity, either in an acute setting as a ‘cytokine storm’ or in a more chronic inflammatory setting, may have an association with the significantly greater incidence of AEs in the HSCT arm, including SAEs and SUSARs such as renal injury/TMA and pulmonary veno-occlusive disease, for which there is ‘mechanistic evidence’ from other studies to support endothelial damage related to cytokines and other biological mediators. 44,45 However, whether the cytokine dysregulation is a ‘cause’ or ‘effect’ of the AEs is not clear from the data and is a point for discussion and further investigation. Although there are reasonable explanations for effects on lower haemoglobin levels (i.e. anaemia of chronic disease),46 hypothetical associations with renal injury and specific SUSARs (especially TMA and pulmonary veno-occlusive disease, which are endothelial damage syndromes) are areas of further consideration.
Strengths and limitations
This trial was designed to be the largest ASTIC RCT to date, taking into account issues identified in the previous ASTIC trial and case series as outlined above. The inclusion criteria allowed a wide population of patients with refractory disease to be recruited, including patients with multiple previous surgeries or with a stoma. These patients are rarely eligible for clinical trials of novel therapies in CD. Patients with active perianal or penetrating disease were excluded, as previous studies24 had shown higher levels of SAEs in these patients. An endoscopic primary end point was chosen with central reading by investigators blind to treatment assignation and time of assessment to counter the fact that neither patient nor investigators were blind to treatment group. Multiple endoscopic, clinical and quality-of-life assessments were included. The key limitation of this study, as reported, was that recruitment was halted owing to safety concerns of the HSCTlite regimen after only 23 patients had been recruited. In addition, the COVID-19 pandemic prevented a number of patients undergoing some of the scheduled study visits and outcome assessments. Therefore, the total number of patients in whom the primary and secondary end points could be analysed was markedly reduced, and hence the power to detect benefit from HSCTlite was reduced. In addition, three primary end point colonoscopies were not recorded, either through error or because of technical issues, further reducing the available data on which to make conclusions. However, given the number of SAEs and SUSARs reported in the HSCTlite arm, it is clear that this HSCT regimen does not have an acceptable safety profile.
Implications for policy makers, health professionals and patients
Despite the meaningful benefits in terms of endoscopic disease regression in some patients undergoing HSCTlite, it is clear that the regimen assessed in this clinical trial is not appropriate for use in clinical practice because of the adverse safety profile. The study highlights that there are many patients with treatment-refractory CD in the UK who suffer significant morbidity related to chronic intestinal inflammation. Further studies to identify treatments that result in long-term resolution of intestinal inflammation with an acceptable safety profile for this refractory patient group are urgently required. Advanced therapies with either novel or more selective mechanisms of action are in phase III clinical trials. Although preliminary studies suggest enhanced benefit, their impact on refractory CD still requires study. It may be that combinations of advanced therapy are required to induce remission in this patient group and that studying the efficacy and, importantly, safety of these strategies is required.
Further research
There have now been two RCTs of autologous stem cell transplantation in patients with treatment-refractory CD. Both were halted on the advice of the DMEC prior to complete recruitment after a patient death. The aetiology of the TMA seen in ASTIClite has not been determined. Without modifiable cause being ascertained, a further large clinical trial of HSCTlite in patients with refractory CD is not considered appropriate. However, there may be individual situations in which HSCTlite for refractory CD is considered in expert centres, particularly those that are usually excluded from clinical trials. A prospective database of patients undergoing HSCTlite that documents baseline characteristics, HSCTlite regimen, clinical/endoscopic outcome and adverse effects could be created for such patients.
The reduced-intensity conditioning regimen (with fludarabine, cyclophosphamide and ATG) could be adapted further to deliver autologous HSCTlite, or even allogeneic HSCTlite, which is likely to be highly curative despite having a number of additional risks. Recent developments in the broader HSCT field, such as the use of antibody-targeted conditioning regimens and post-transplantation cyclophosphamide or other immunosuppressive post-transplantation therapy, could also be incorporated into both autologous and allogeneic HSCTlite regimens.
The mobilisation regimen with reduced-dose cyclophosphamide (1 g/m2) and G-CSF used in ASTIClite was safe and effective for autologous HSCTlite, and could be used in future patients, although other mobilisation regimens, including chemotherapy-free and novel antibody-targeted mobilisation regimens, could be trialled.
The prospective database could be used to compare HSCTlite interventions with other advanced and biologic therapies, both as single agents and in combination with an endoscopic primary end point.
The research priorities moving forward are to determine the optimal treatment strategy for the significant number of patients with refractory active CD who fail to respond to currently available therapy. This should include patients who have a stoma or have had multiple previous resections, who are currently excluded from most clinical trials. The intervention will likely be a combination of advanced and biologic therapies compared with a single agent. Given the population and likelihood of prior intestinal damage, an endoscopic primary end point is appropriate.
Conclusions
The ASTIClite trial was designed to assess the efficacy and safety of autologous stem cell transplantation in patients with treatment-refractory CD using a mobilisation and conditioning regimen with lower doses of cyclophosphamide than previously assessed in the hope that this would reduce the number of significant serious infections that had been observed. 17 The trial was halted early because of several SUSARs and one patient death. Although several patients undergoing HSCTlite experienced marked improvement in endoscopic disease activity, the large number of AEs (including serious reactions) preclude the use of this regimen in its current form. Patients with treatment-refractory CD nevertheless experience severe and sustained poor quality of life, and in the absence of therapeutic alternatives HSCTlite may remain a potential future treatment if a more tolerable regimen can be identified. Further research is required to identify the optimal treatment for this population with refractory disease, which is associated with poor quality of life and significant morbidity.
Acknowledgements
We gratefully acknowledge the hard work, support and advice from the following: Marie-Claire Good and Robert Hughes, GCP and Governance Managers (Barts Health NHS Trust) as Research Sponsor; research nurses; and research support staff and clinical teams in the nine participating NHS trusts for participant screening, delivering the intervention, data collection and patient follow up. We thank the 23 patients who took part in the study, as well as their families for supporting their choice.
We acknowledge the support and guidance from Miranda Clark (ASTIC Trial Manager) and Sarah Bowden and colleagues (Birmingham Cancer Research Clinical Trials Unit).
We acknowledge advice and oversight from members of the TSC – Dr John Mansfield (Chairperson, Consultant Gastroenterologist), Dr Kim Orchard (Consultant Haematologist), Professor Ailsa Hart (Consultant Gastroenterologist), Dr Victoria Cornelius (Statistician), Dr Elena Ricart (Consultant Gastroenterologist), Ms Helen Bartlett (Patient Representative), Ms Charlotte Howe (Patient Representative) – and the independent DMEC – Dr Tariq Iqbal (Consultant Gastroenterologist), Professor David Marks (Consultant Haematologist), Professor Siobhan Creanor (Statistician), Professor Matthieu Allez (Consultant Gastroenterologist), Professor Dominique Farge-Bancel (Professor of Autoimmune Diseases and Vascular Pathology).
Contributions of authors
James O Lindsay (https://orcid.org/0000-0003-3353-9590) (Chief Investigator, Consultant Gastroenterologist) was involved in producing the first draft of the report, conceiving and designing the work, the acquisition, analysis and interpretation of data for the work, and drafting the monograph.
Daniel Hind (https://orcid.org/0000-0002-6409-4793) was involved in conceiving and designing the work.
Lizzie Swaby (https://orcid.org/0000-0001-9443-7681) (Study Manager) was involved in producing the first draft of the report, conceiving and designing the work, the acquisition, analysis and interpretation of data for the work, and drafting the monograph.
Hannah Berntsson (https://orcid.org/0000-0002-6285-6985) (Research Assistant) was involved in producing the first draft of the report, the acquisition, analysis and interpretation of data for the work, and drafting the monograph.
Mike Bradburn (https://orcid.org/0000-0002-3783-9761) (Senior Statistician) was involved in producing the first draft of the report, conceiving and designing the work, the analysis and interpretation of data for the work, and drafting the monograph.
Uday Bannur C (https://orcid.org/0000-0003-0330-0327) was involved in the acquisition of data for the work.
Jennifer Byrne (https://orcid.org/0000-0002-9381-1851) was involved in reviewing and amending the transplant and supportive care regimen in the protocol and the acquisition of data for the work.
Christopher Clarke (https://orcid.org/0000-0002-8092-9877) was involved in the acquisition of data for the work.
Lauren Desoysa (https://orcid.org/0000-0002-6151-836X) (Statistician) was involved in producing the first draft of the report, the analysis and interpretation of data for the work, and drafting the monograph.
Shahida Din (https://orcid.org/0000-0003-2855-3400) was involved in the acquisition of data for the work.
Richard Emsley (https://orcid.org/0000-0002-1218-675X) was involved in conceiving and designing the work and the analysis of data for the work.
Gemma A Foulds (https://orcid.org/0000-0002-2053-7580) (Research Fellow and Mechanistic Analytical Project Manager) was involved in producing the first draft of the report, the acquisition, analysis and interpretation of data for the work, and drafting the monograph.
John Gribben (https://orcid.org/0000-0002-8505-7430) was involved in conceiving and designing the work, reviewing and amending the transplant and supportive care regimen in the protocol, and the acquisition of data for the work.
Christopher Hawkey (https://orcid.org/0000-0002-6031-1017) was involved in conceiving and designing the work.
Peter M Irving (https://orcid.org/0000-0003-0972-8148) was involved in acquisition of data for the work.
Peter Johnson (https://orcid.org/0000-0002-8849-0790) was involved in reviewing and amending the transplant and supportive care regimen in the protocol and the acquisition of data for the work.
Majid Kazmi (https://orcid.org/0000-0002-6863-6297) was involved in reviewing and amending the transplant and supportive care regimen in the protocol.
Ellen Lee (https://orcid.org/0000-0003-4529-7410) (Statistician) was involved in producing the first draft of the report, analysis and interpretation of data for the work, and drafting the monograph.
Amanda Loban (https://orcid.org/0000-0001-7089-1580) was involved in the acquisition and analysis of data for the work.
Alan Lobo (https://orcid.org/0000-0001-8037-793X) was involved in conceiving and designing the work and acquisition of data for the work.
Yashwant Mahida (https://orcid.org/0000-0001-8666-7815) was involved in conceiving and designing the work.
Gordon Moran (https://orcid.org/0000-0002-8906-6908) was involved in conceiving and designing the work and acquisition of data for the work.
Diana Papaioannou (https://orcid.org/0000-0002-6259-0822) was involved in conceiving and designing the work.
Miles Parkes (https://orcid.org/0000-0002-6467-0631) was involved in conceiving and designing the work and acquisition of data for the work.
Andrew Peniket (https://orcid.org/0000-0002-6833-9882) was involved in acquisition of data for the work.
A Graham Pockley (https://orcid.org/0000-0001-9593-6431) (Co-Applicant, Lead for Mechanistic Analysis) was involved in producing the first draft of the report, conceiving and designing the work, analysis and interpretation of the data for the work, and drafting the monograph.
Jack Satsangi (https://orcid.org/0000-0002-6357-9684) was involved in conceiving and designing the work.
Sreedhar Subramanian (https://orcid.org/0000-0002-6483-1730) was involved in acquisition of data for the work.
Simon Travis (https://orcid.org/0000-0002-2690-4361) was involved in conceiving and designing the work and acquisition of data for the work.
Emily Turton (https://orcid.org/0000-0001-5763-9604) was involved in acquisition and analysis of data for the work.
Ben Uttenthal (https://orcid.org/0000-0002-5340-2527) was involved in reviewing and amending the transplant and supportive care regimen in the protocol and acquisition of data for the work.
Sergio Rutella (https://orcid.org/0000-0003-1970-7375) was involved in analysis and interpretation of data for the work.
John A Snowden (https://orcid.org/0000-0001-6819-3476) (Lead Consultant Haematologist) was involved in producing the first draft of the report, conceiving and designing the work, reviewing and amending the transplant and supportive care regimen in the protocol, acquisition, analysis and interpretation of data for the work, and drafting the monograph.
Amit Patel was involved in reviewing and amending the transplant and supportive care regimen in the protocol and the acquisition of data for the work.
All named authors revised the work critically for important intellectual content.
All named authors were involved in the final approval of the version to be published.
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publications
Snowden JA, Sharrack B, Akil M, Kiely DG, Lobo A, Kazmi M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases – a guide for the generalist. Clin Med 2018;18:329–34.
Pockley AG, Lindsay JO, Foulds GA, Rutella S, Gribben JG, Alexander T, Snowden JA. Immune reconstitution after autologous hematopoietic stem cell transplantation in Crohn’s disease: current status and future directions. A review on behalf of the EBMT Autoimmune Diseases Working Party and the Autologous Stem Cell Transplantation In Refractory CD-Low Intensity Therapy Evaluation study investigators. Front Immunol 2018;9:646.
Snowden JA, Hawkey C, Hind D, Swaby L, Mellor K, Emsley R, et al. Autologous stem cell transplantation in refractory Crohn’s disease – low intensity therapy evaluation (ASTIClite): study protocols for a multicentre, randomised controlled trial and observational follow up study. BMC Gastroenterol 2019;19:82.
Lindsay JO, Hind D, Swaby L, Berntsson H, Bradburn M, Bannur CU, et al. Safety and efficacy of autologous haematopoietic stem-cell transplantation with low-dose cyclophosphamide mobilisation and reduced intensity conditioning versus standard of care in refractory Crohn’s disease (ASTIClite): an open-label, multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol 2024; in press. https://doi.org/10.1016/S2468-1253(23)00460-0
Data-sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Tun GSZ, Cripps S, Lobo AJ. Crohn's disease: management in adults, children and young people–concise guidance. Clin Med (Lond) 2018;18:231-6. https://doi.org/10.7861/clinmedicine.18-3-231.
- Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741-55. https://doi.org/10.1016/S0140-6736(16)31711-1.
- van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014;63:72-9. https://doi.org/10.1136/gutjnl-2012-303376.
- van der Valk ME, Mangen MJ, Severs M, van der Have M, Dijkstra G, van Bodegraven AA, et al. Evolution of costs of inflammatory bowel disease over two years of follow-up. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0142481.
- Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341-53. https://doi.org/10.1016/S2468-1253(19)30012-3.
- Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis 2018;12:635-43. https://doi.org/10.1093/ecco-jcc/jjy004.
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711-21. https://doi.org/10.1056/NEJMoa1215739.
- Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618-27. https://doi.org/10.1053/j.gastro.2014.05.008.
- Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 2016;388:576-85. https://doi.org/10.1016/S0140-6736(16)30169-6.
- Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA 2019;321:165-74. https://doi.org/10.1001/jama.2018.18743.
- Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood 2010;116:6123-32. https://doi.org/10.1182/blood-2010-06-292391.
- Snowden JA, Ansari A, Sachchithanantham S, Jackson G, Thompson N, Lobo A, et al. Autologous stem cell transplantation in severe treatment-resistant Crohn’s disease: long-term follow-up of UK patients treated on compassionate basis. QJM 2014;107:871-7. https://doi.org/10.1093/qjmed/hcu095.
- López-García A, Rovira M, Jauregui-Amezaga A, Marín P, Barastegui R, Salas A, et al. Autologous haematopoietic stem cell transplantation for refractory Crohn’s disease: efficacy in a single-centre cohort. J Crohns Colitis 2017;11:1161-8. https://doi.org/10.1093/ecco-jcc/jjx054.
- Swart JF, Delemarre EM, van Wijk F, Boelens JJ, Kuball J, van Laar JM, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol 2017;13:244-56. https://doi.org/10.1038/nrrheum.2017.7.
- Alexander T, Arnold R, Hiepe F, Radbruch A. Resetting the immune system with immunoablation and autologous haematopoietic stem cell transplantation in autoimmune diseases. Clin Exp Rheumatol 2016;34:53-7.
- Brierley CK, Castilla-Llorente C, Labopin M, Badoglio M, Rovira M, Ricart E, et al. Autologous haematopoietic stem cell transplantation for Crohn’s disease: a retrospective survey of long-term outcomes from the European Society for Blood and Marrow Transplantation. J Crohns Colitis 2018;12:1097-103. https://doi.org/10.1093/ecco-jcc/jjy069.
- Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA 2015;314:2524-34. https://doi.org/10.1001/jama.2015.16700.
- Hawkey CJ, Lindsay J, Gribben J. Stem cell transplantation for refractory Crohn disease – reply. JAMA 2016;315:2620-1. https://doi.org/10.1001/jama.2016.4033.
- Jauregui-Amezaga A, Rovira M, Marín P, Salas A, Pinó-Donnay S, Feu F, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut 2016;65:1456-62. https://doi.org/10.1136/gutjnl-2015-309836.
- Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2012;47:770-90. https://doi.org/10.1038/bmt.2011.185.
- Karanth M, Chakrabarti S, Lovell RA, Harvey C, Holder K, McConkey CC, et al. A randomised study comparing peripheral blood progenitor mobilisation using intermediate-dose cyclophosphamide plus lenograstim with lenograstim alone. Bone Marrow Transplant 2004;34:399-403. https://doi.org/10.1038/sj.bmt.1704598.
- Burt RK, Ruiz MA, Kaiser RL. Stem cell transplantation for refractory Crohn disease. JAMA 2016;315. https://doi.org/10.1001/jama.2016.4030.
- Kim H, Lee JH, Joo YD, Bae SH, Hyun MS, Lee JH, et al. A randomized comparison of cyclophosphamide vs. reduced dose cyclophosphamide plus fludarabine for allogeneic hematopoietic cell transplantation in patients with aplastic anemia and hypoplastic myelodysplastic syndrome. Ann Hematol 2012;91:1459-69. https://doi.org/10.1007/s00277-012-1462-x.
- Lindsay JO, Allez M, Clark M, Labopin M, Ricart E, Rogler G, et al. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol Hepatol 2017;2:399-406.
- Qiu X, Feng JR, Chen LP, Liu S, Zhang M, Zhou Z, et al. Efficacy and safety of autologous hematopoietic stem cell therapy for refractory Crohn’s disease: a systematic review and meta-analysis. Medicine 2017;96. https://doi.org/10.1097/MD.0000000000007381.
- Cosma G, Acampora G, Brown D, Rees RC, Khan M, Pockley AG. Prediction of pathological stage in patients with prostate cancer: a neuro-fuzzy model. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0155856.
- Burt RK, Fassas A, Snowden J, van Laar JM, Kozak T, Wulffraat NM, et al. Collection of hematopoietic stem cells from patients with autoimmune diseases. Bone Marrow Transplant 2001;28:1-12. https://doi.org/10.1038/sj.bmt.1703081.
- Lindsay J, Snowden J, Hind D, Papaioannou D, Swaby L, Berntsson H, et al. ASTIClite Trial n.d. www.sheffield.ac.uk/scharr/research/centres/ctru/asticlite (accessed 24 March 2022).
- Snowden JA, Hawkey C, Hind D, Swaby L, Mellor K, Emsley R, et al. Autologous stem cell transplantation in refractory Crohn’s disease – low intensity therapy evaluation (ASTIClite): study protocols for a multicentre, randomised controlled trial and observational follow up study. BMC Gastroenterol 2019;19. https://doi.org/10.1186/s12876-019-0992-2.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063-70. https://doi.org/10.1097/AOG.0b013e3181d9d421.
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6:284-90. https://doi.org/10.1037/1040-3590.6.4.284.
- Khanna R, Zou G, D’Haens G, Rutgeerts P, McDonald JW, Daperno M, et al. Reliability among central readers in the evaluation of endoscopic findings from patients with Crohn’s disease. Gut 2016;65:1119-25. https://doi.org/10.1136/gutjnl-2014-308973.
- European Medicines Agency . Guideline for Good Clinical Practice E6(R2) 2018. www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf (accessed 14 March 2023).
- British Medical Journal (BMJ) . Declaration of Helsinki (1964). BMJ 1996;313.
- Kennedy NA, Jones G-R, Lamb CA, Appleby R, Arnott I, Beattie RM, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020;69:984-90. https://doi.org/10.1136/gutjnl-2020-321244.
- Lindsay JO, Berntsson H, Bradburn M, Desoysa L, Din S, Gribben J, et al. OP192 A randomised controlled clinical trial of autologous stem cell transplantation (HSCT) in patients with treatment refractory Crohn’s disease (low intensity therapy evaluation): ASTIClite. United Eur Gastoenterol 2021;9. https://doi.org/10.1136/gutjnl-2021-BSG.6.
- Greco R, Alexander T, Burman J, Del Papa N, de Vries-Bouwstra J, Farge D, et al. Hematopoietic stem cell transplantation for autoimmune diseases in the time of COVID–19: EBMT guidelines and recommendations. Bone Marrow Transplant 2021;56:1493-508. https://doi.org/10.1038/s41409-021-01326-6.
- Shimoni A, Yeshurun M, Hardan I, Avigdor A, Ben-Bassat I, Nagler A. Thrombotic microangiopathy after allogeneic stem cell transplantation in the era of reduced-intensity conditioning: The incidence is not reduced. Biol Blood Marrow Transplant 2004;10:484-93. https://doi.org/10.1016/j.bbmt.2004.03.002.
- Nakamae H, Yamane T, Hasegawa T, Nakamae M, Terada Y, Hagihara K, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol 2006;81:525-31. https://doi.org/10.1002/ajh.20648.
- Nagler A, Shimoni A. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham: Springer Open; 2019.
- Tsukamoto H, Nagafuji K, Horiuchi T, Mitoma H, Niiro H, Arinobu Y, et al. Analysis of immune reconstitution after autologous CD34+ stem/progenitor cell transplantation for systemic sclerosis: predominant reconstitution of Th1 CD4+ T cells. Rheumatology 2011;50:944-52. https://doi.org/10.1093/rheumatology/keq414.
- Arruda LCM, Malmegrim KCR, Lima-Júnior JR, Clave E, Dias JBE, Moraes DA, et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv 2018;2:126-41. https://doi.org/10.1182/bloodadvances.2017011072.
- Schlenke P, Sheikhzadeh S, Weber K, Wagner T, Kirchner H. Immune reconstitution and production of intracellular cytokines in T lymphocyte populations following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 2001;28:251-7. https://doi.org/10.1038/sj.bmt.1703121.
- Carreras E, Cooke KR. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham: Springer Open; 2019.
- Carreras E, Diaz-Ricart M. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham: Springer Open; 2019.
- de Las Cuevas Allende R, Díaz de Entresotos L, Conde Díez S. Anaemia of chronic diseases: pathophysiology, diagnosis and treatment. Med Clin 2021;156:235-42. https://doi.org/10.1016/j.medcli.2020.07.035.
- Wolford B. What Is GDPR, the EU'S New Data Protection Law? n.d. https://gdpr.eu/what-is-gdpr/ (accessed 14 March 2023).
Appendix 1 Summary of amendments
| Amendment | Date implemented | Summary of changes |
|---|---|---|
| Substantial amendment 1 (Protocol v4.0) | 27 February 2018 |
|
| Substantial amendment 2 (Protocol v5.0) | 1 June 2018 |
|
| Substantial amendment 3 (Protocol v5.1) | 31 July 2018 |
|
| Substantial amendment 4 | 6 August 2018 |
|
| Substantial amendment 5 (Protocol v5.2) | 10 October 2018 |
|
| Substantial amendment 6 (Protocol v6.0) | 16 November 2018 |
|
| Non-substantial amendment 1 (Protocol v6.0) | 15 November 2018 |
|
| Non-substantial amendment 2 (Protocol v6.1) | 7 March 2019 |
|
| Substantial amendment 7 (Protocol v7.0) | 29 August 2019 |
|
| Substantial amendment 8 (Protocol v7.1) | 23 September 2019 |
|
| Substantial amendment 9 (Protocol v8.0) | 4 May 2020 |
|
| Substantial amendment 10 (Protocol v8.0) | 30 December 2019 |
|
| Non-substantial amendment 3 (Protocol v8.0) | 23 March 2020 |
|
| Substantial amendment 11 (Protocol v8.0) | 28 October 2020 |
|
| Non-substantial amendment 4 (Protocol v8.0) | 9 December 2020 |
|
Appendix 2 Table of late effects after haematopoietic stem cell transplant
| Effects | Recommended timing of assessment (corresponding study visit) | ||
|---|---|---|---|
| 3 months (week 14) | 6 months (week 24) | 1 year (week 48) | |
| General | |||
| Weight | 1 | 1 | 1 |
| Blood pressure | 1 | 1 | 1 |
| Performance status (Karnofsky/Lansky) | 1 | 1 | 1 |
| Haematology | |||
| FBC | 1 | 1 | 1 |
| Renal | |||
| Renal function | 1 | 1 | 1 |
| Urine protein (dipstick) | 1 | 1 | 1 |
| Liver | |||
| Liver function | 1 | 1 | 1 |
| Iron studies | 1 | 1 | |
| Endocrine | |||
| Thyroid function | |||
| TSH, free T4 | 1 | 1 | 1 |
| Gonadal function | |||
| FSH, LH, oestradiol, progesterone (women aged ≤ 50 years) | |||
| FSH, LH, testosterone (men) | 1 | 1 | 1 |
| Sexual function assessment (as per patient report) | 1 | 1 | |
| Bone | |||
| Bone profile | 1 | 1 | 1 |
| Bone density scan | |||
| Women and men with evidence of hypogonadism | |||
| Patients on prolonged corticosteroids or calcineurin inhibitors | 1 | ||
| Respiratory | |||
| Clinical assessment | 1 | 1 | 1 |
| Pulmonary function test | 1 | ||
| Chest radiograph | a | a | |
| Counselling re: smoking cessation | 1 | 1 | 1 |
| Nervous system | |||
| Neurological assessment | 1 | ||
| Vascular | |||
| Cardiovascular risk factors | 1 | ||
| Echocardiogram | 1 | ||
| HbA1c | 1 | 1 | |
| Lipid profile and abdominal girth | 1 | 1 | |
| Immune system | |||
| CD4 subsets | 1 | 1 | 1 |
| Immunoglobulin levels | 1 | 1 | 1 |
| Antimicrobial prophylaxis as per local protocol | 1 | 1 | 1 |
| Immunisation and antibody levels as per local protocol | 1 | ||
| Oral complications | |||
| Dental assessment | 1 | 1 | |
| Ocular | |||
| Cataracts assessment | 1 | 1 | 1 |
| Second cancers | |||
| Mammograms (women aged > 40 years) | 1 | ||
| Vigilance and self-examination | 1 | 1 | |
| Second autoimmune diseases | |||
| Second autoimmune diseases | 1 | 1 | |
| Psychosocial | |||
| Psychosocial/psychosexual issues, by standard holistic needs assessment | 1 | 1 | 1 |
Appendix 3 Scoring summary
FIGURE 9.
Scoring summary table.

Appendix 4 Baseline data and completion status
| Variable | Completers | Non-completers | ||||
|---|---|---|---|---|---|---|
| HSCTlite (n = 9) | Usual care (n = 9) | Total (n = 9) | HSCTlite (n = 4) | Usual care (n = 0) | Total (n = 4) | |
| Age (years) | ||||||
| Mean (SD) | 34.6 (8.6) | 36.3 (10.1) | 35.4 (9.1) | 34.5 (12.9) | – (–) | 34.5 (12.9) |
| Median (IQR) | 35 (26.0–42.0) | 30 (28.0–44.0) | 33.0 (28.0–44.0) | 36.0 (26.5–44.0) | – (–) | 36.0 (26.5–44.0) |
| BMI (kg/m2) | ||||||
| Mean (SD) | 26.4 (6.2) | 27.4 (6.2) | 26.9 (6.1) | 25.7 (6.1) | – (–) | 25.7 (6.1) |
| Median (IQR) | 27.0 (20.3–30.5) | 26.4 (22.7–30.5) | 26.7 (22.5–30.5) | 27.2 (23.8–29.1) | – (–) | 27.2 (23.8–29.1) |
| Age at CD onset (years) | ||||||
| Mean (SD) | 18.7 (5.9) | 22.6 (6.3) | 20.6 (6.3) | 25.2 (10.1) | – (–) | 25.2 (10.1) |
| Median (IQR) | 20 (15.0–23.0) | 22 (19.0–25.0) | 22.0 (16.0–24.0) | 24.5 (18.0–31.8) | – (–) | 24.5 (18.0–31.8) |
| Duration of CD | ||||||
| Mean (SD) | 15.3 (7.0) | 14.1 (7.8) | 14.7 (7.2) | 9.8 (4.2) | – (–) | 9.8 (4.2) |
| Median (IQR) | 14 (11.0–20.0) | 10 (10.0–19.0) | 12.5 (10.0–19.8) | 10.5 (8.5–11.8) | – (–) | 10.5 (8.5–11.8) |
| Haemoglobin | ||||||
| Mean (SD) | 122.3 (15.2) | 127.9 (8.3) | 125.1 (12.3) | 126.0 (22.6) | – (–) | 126.0 (22.6) |
| Median (IQR) | 116 (110.0–135.0) | 127 (120.0–131.0) | 124.0 (116.8–133.2) | 125.5 (116.2–135.2) | – (–) | 125.5 (116.2–135.2) |
| Platelet count | ||||||
| Mean (SD) | 343.6 (112.4) | 407.7 (94.5) | 375.6 (106.0) | 434.5 (196.8) | – (–) | 434.5 (196.8) |
| Median (IQR) | 314 (274.0–367.0) | 379 (342.0–460.0) | 353.5 (313.2–406.2) | 439.5 (324.5–549.5) | – (–) | 439.5 (324.5–549.5) |
| Albumin | ||||||
| Mean (SD) | 41.7 (3.9) | 39.4 (4.3) | 40.6 (4.1) | 38.5 (6.4) | – (–) | 38.5 (6.4) |
| Median (IQR) | 43 (40.0–44.0) | 40 (39.0–41.0) | 40.5 (39.2–43.8) | 39.5 (36.0–42.0) | – (–) | 39.5 (36.0–42.0) |
| CRP | ||||||
| Mean (SD) | 16.7 (16.0) | 26.8 (27.1) | 21.4 (21.8) | 10.3 (5.0) | – (–) | 10.3 (5.0) |
| Median (IQR) | 11.0 (8.0–14.0) | 20.0 (4.8–34.2) | 13.0 (6.0–24.0) | 10.9 (8.0–12.9) | – (–) | 10.9 (8.0–12.9) |
| CDAI score | ||||||
| Mean (SD) | 395.1 (215.4) | 271.5 (115.2) | 333.3 (178.7) | 354.2 (224.9) | – (–) | 354.2 (224.9) |
| Median (IQR) | 384.0 (278.8–481.0) | 290.5 (174.2–352.2) | 327.5 (174.5–415.8) | 293.5 (197.2–450.5) | – (–) | 293.5 (197.2–450.5) |
| PRO2 | ||||||
| Mean (SD) | 25.4 (20.4) | 18.2 (8.7) | 22.1 (16.0) | 24.0 (22.1) | – (–) | 24.0 (22.1) |
| Median (IQR) | 23 (12.0–31.0) | 17.0 (12.2–25.8) | 19 (12.0–30.0) | 18.5 (11.5–31.0) | – (–) | 18.5 (11.5–31.0) |
| HBI | ||||||
| Mean (SD) | 11.4 (11.5) | 12.6 (6.9) | 12.0 (9.2) | 15.5 (10.2) | – (–) | 15.5 (10.2) |
| Median (IQR) | 5 (4.0–16.0) | 13 (8.0–18.0) | 11.0 (4.2–17.5) | 12.5 (9.2–18.8) | – (–) | 12.5 (9.2–18.8) |
| SES-CD scorea | ||||||
| Mean (SD) | 11.2 (6.5) | 10.0 (6.1) | 10.6 (6.2) | 7.0 (–) | – (–) | 7.0 (–) |
| Median (IQR) | 8.5 (6.8–15.2) | 8.5 (5.5–14.2) | 8.5 (6.0–15.2) | 7.0 (–) | – (–) | 7.0 (–) |
| IBD-Control | ||||||
| Mean (SD) | 2.9 (3.2) | 0.7 (1.0) | 1.8 (2.5) | 2.2 (1.3) | – (–) | 2.2 (1.3) |
| Median (IQR) | 2 (0.5–4.0) | 0 (0.0–1.5) | 1.0 (0.0–2.0) | 2.0 (1.8–2.5) | – (–) | 2.0 (1.8–2.5) |
| EQ-5D-5L | ||||||
| Mean (SD) | 0.348 (0.457) | 0.507 (0.269) | 0.428 (0.373) | 0.508 (0.403) | – (–) | 0.508 (0.403) |
| Median (IQR) | 0.340 (–0.023 to 0.768) | 0.582 (0.336–0.632) | 0.456 (0.126–0.649) | 0.678 (0.468–0.719) | – (–) | 0.678 (0.468–0.719) |
| IBDQ | ||||||
| Mean (SD) | 100.3 (59.5) | 88.4 (11.9) | 92.0 (30.2) | 112.5 (82.7) | – (–) | 112.5 (82.7) |
| Median (IQR) | 85 (67.5–125.5) | 89 (81.0–95.5) | 88.5 (76.8–95.8) | 112.5 (83.2–141.8) | – (–) | 112.5 (83.2–141.8) |
| MaRIA score | ||||||
| Mean (SD) | 78.0 (15.3) | 62.5 (10.6) | 67.7 (13.3) | 40.4 (–) | – (–) | 40.4 (–) |
| Median (IQR) | 78.0 (72.6–83.4) | 60.8 (53.9–69.4) | 67.3 (57.3–73.1) | 40.4 (40.4–40.4) | – (–) | 40.4 (40.4–40.4) |
| Variable | Completers, n (%) | Non-completers, n (%) | ||||
|---|---|---|---|---|---|---|
| HSCTlite (n = 9) | Usual care (n = 9) | Total (N = 18) | HSCTlite (n = 4) | Usual care (n = 0) | Total (N = 4) | |
| Sex | ||||||
| Male | 5 (56) | 4 (44) | 9 (50) | 1 (25) | – (–) | 1 (25) |
| Female | 4 (44) | 5 (56) | 9 (50) | 3 (75) | – (–) | 3 (75) |
| Perianal CD | ||||||
| Yes | 3 (33) | 5 (56) | 8 (44) | 0 (0) | – (–) | 0 (0) |
| No | 6 (67) | 4 (44) | 10 (56) | 4 (100) | – (–) | 4 (100) |
| Family history of IBD | ||||||
| Yes | 2 (22) | 3 (33) | 5 (28) | 1 (25) | – (–) | 1 (25) |
| CD | 2 (100) | 1 (33) | 3 (60) | 1 (100) | – (–) | 1 (100) |
| Ulcerative colitis | 1 (50) | 3 (100) | 4 (80) | – (–) | – (–) | – (–) |
| No | 7 (78) | 6 (67) | 13 (72) | 3 (75) | – (–) | 3 (75) |
| Stoma | ||||||
| Yes | 5 (56) | 2 (22) | 7 (39) | 2 (50) | – (–) | 2 (50) |
| Ileostomy | 2 (40) | 2 (100) | 4 (57) | 1 (50) | – (–) | 1 (50) |
| End ileostomy | 2 (40) | 0 (0) | 2 (29) | 1 (50) | – (–) | 1 (50) |
| Loop colostomy | 1 (20) | 0 (0) | 1 (14) | 0 (0) | – (–) | 0 (0) |
| No | 4 (44) | 7 (78) | 11 (61) | 2 (50) | – (–) | 2 (50) |
| Ethnicitya | ||||||
| White | 7 (78) | 8 (89) | 15 (83) | 3 (75) | – (–) | 3 (75) |
| Asian/Asian British | 2 (22) | 1 (11) | 3 (14) | 1 (0.25) | – (–) | 1 (25) |
| Smoking statusb | ||||||
| Never smoked | 6 (67) | 7 (78) | 13 (72) | 2 (50) | – (–) | 2 (50) |
| Current smoker | 1 (11) | 1 (11) | 2 (11) | 1 (25) | – (–) | 1 (25) |
| Previous smoker (stopped ≥ 5 years) | 2 (22) | 1 (11) | 3 (14) | 1 (25) | – (–) | 1 (25) |
| Behaviour of CD | ||||||
| B1 non-stricturing, non-penetrating | 2 (22) | 0 (0) | 2 (11) | 3 (75) | – (–) | 3 (75) |
| B2 stricturing | 6 (67) | 3 (33) | 9 (50) | 1 (25) | – (–) | 1 (25) |
| B3 penetrating | 1 (11) | 6 (67) | 7 (39) | 0 (0) | – (–) | 0 (0) |
| Disease locationc | ||||||
| L1 Ileal | 2 (22) | 0 (0) | 2 (11) | 1 (25) | – (–) | 1 (25) |
| L2 Colonic | 0 (0) | 1 (11) | 1 (6) | 1 (25) | – (–) | 1 (25) |
| L3 Ileo-colonic | 5 (56) | 3 (33) | 8 (44) | 0 (0) | – (–) | 0 (0) |
| L4 Isolated upper disease | 0 (0) | 1 (11) | 1 (6) | 0 (0) | – (–) | 0 (0) |
| L1 L4 | 1 (11) | 2 (22) | 3 (14) | 2 (50) | – (–) | 2 (50) |
| L3 L4 | 1 (11) | 2 (22) | 3 (14) | 0 (0) | – (–) | 0 (0) |
| Extraintestinal manifestations | ||||||
| Yes | 3 (33) | 2 (22) | 5 (28) | 1 (25) | – (–) | 1 (25) |
| No | 6 (67) | 7 (78) | 13 (72) | 3 (75) | – (–) | 3 (75) |
| Extraintestinal involvement, n (%) | ||||||
| Yes | 2 (67) | 1 (50) | 3 (60) | 1 (100) | – (–) | 1 (100) |
| Joints | 2 (100) | 1 (100) | 3 (100) | 1 (100) | – (–) | 1 (100) |
| Skin | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – (–) | 0 (0) |
| Eyes | 0 (0) | 0 (0) | 0 (0) | 1 (100) | – (–) | 1 (100) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – (–) | 0 (0) |
| No | 1 (33) | 1 (50) | 2 (40) | 0 (0) | – (–) | 0 (0) |
| Montreal age at onset classification (years) | ||||||
| A1 (< 16) | 4 (44) | 2 (22) | 6 (33) | 1 (25) | – (–) | 1 (25) |
| A2 (between 17 and 40) | 5 (56) | 7 (78) | 12 (67) | 3 (75) | – (–) | 3 (75) |
| Number of previous operations for CD | ||||||
| Intestinal surgery | 8 (89) | 8 (89) | 16 (89) | 4 (100) | – (–) | 4 (100) |
| Perianal surgery | 4 (44) | 4 (44) | 8 (44) | 0 (0) | – (–) | 0 (0) |
| Other surgery for CD | 1 (11) | 0 (0) | 1 (6) | 0 (0) | – (–) | 0 (0) |
| Segments examined in colonoscopyd | ||||||
| Ileum | 8 (89) | 8 (100) | 16 (94) | 1 (100) | – (–) | 1 (100) |
| Right colon | 2 (22) | 2 (22) | 4 (22) | 0 (0) | – (–) | 0 (0) |
| Transverse colon | 5 (56) | 6 (67) | 11 (61) | 1 (25) | – (–) | 1 (25) |
| Left colon | 5 (56) | 6 (67) | 11 (61) | 1 (25) | – (–) | 1 (25) |
| Rectum | 6 (67) | 7 (78) | 13 (72) | 1 (25) | – (–) | 1 (25) |
Appendix 5 Reintroduction of anti-tumour necrosis factor therapy
Patients who were in the HSCTlite arm with evidence of disease activity at the scheduled week 24 colonoscopy or MRI received induction and maintenance anti-TNF therapy. Owing to the small number of patients receiving reintroduction of anti-TNF therapy, any details regarding the reintroduction of anti-TNF therapy and subsequent disease activity are reported descriptively rather than as quantitative summaries.
Of the nine participants in the HSCTlite arm who reached the 24-week follow-up visit, four patients were considered to have evidence of disease activity from the colonoscopy. Only one of these patients then went on to receive anti-TNF therapy.
This patient experienced one AE and one SAE at 32 weeks. The SAE was acute kidney injury and the patient was admitted to hospital; simultaneously, an AE was recorded for abnormal liver function tests (which were also noted as abnormal at week 40). The patient experienced one further AE around 41 weeks after randomisation.
Appendix 6 Cytokine expression by peripheral blood CD4+ and CD8+ T cells after in vitro stimulation
FIGURE 10.
CD4+ T cells expressing IL-2 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.

FIGURE 11.
CD4+ T cells expressing IL-4 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
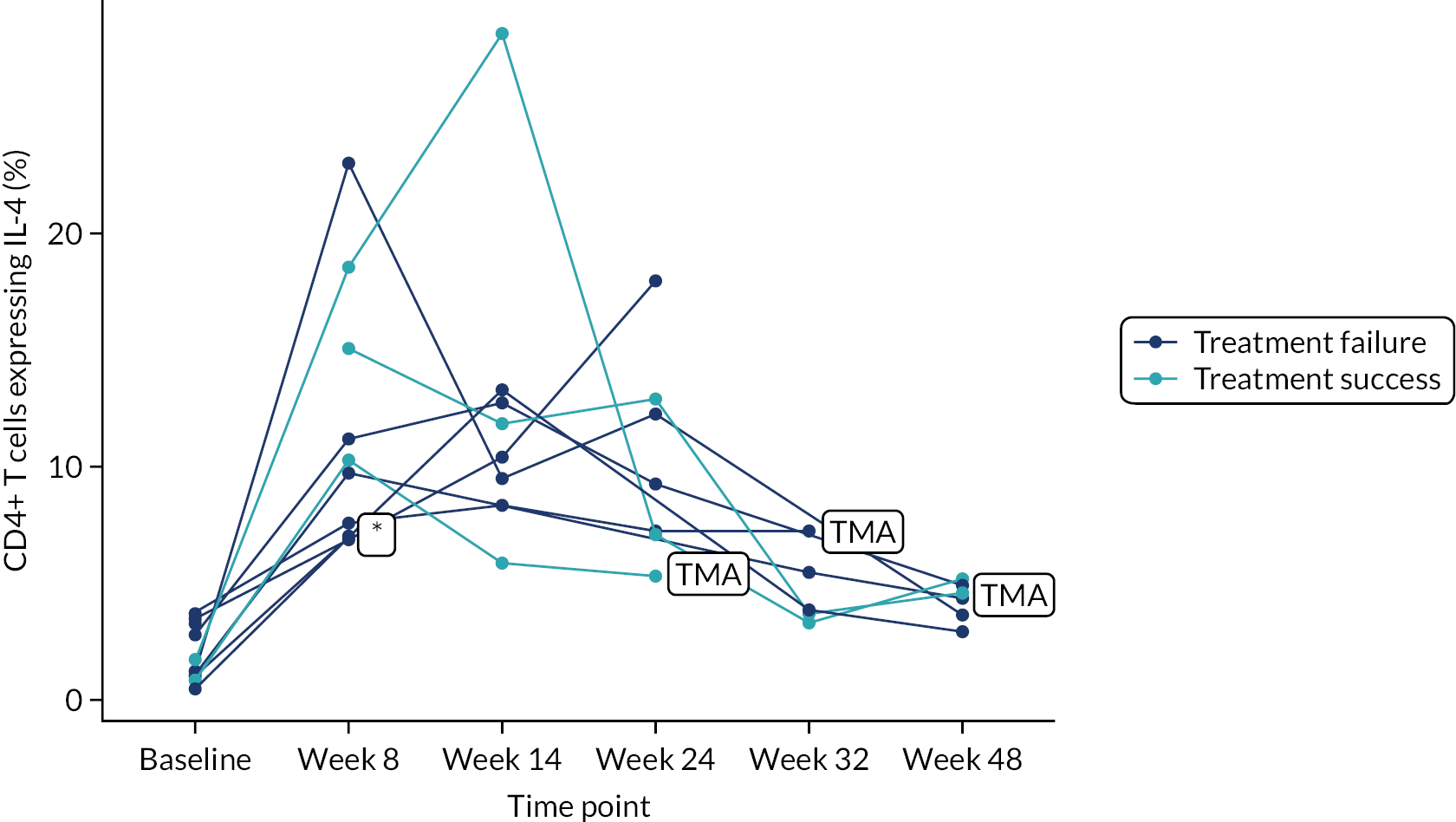
FIGURE 12.
CD4+ T cells expressing IL-17 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
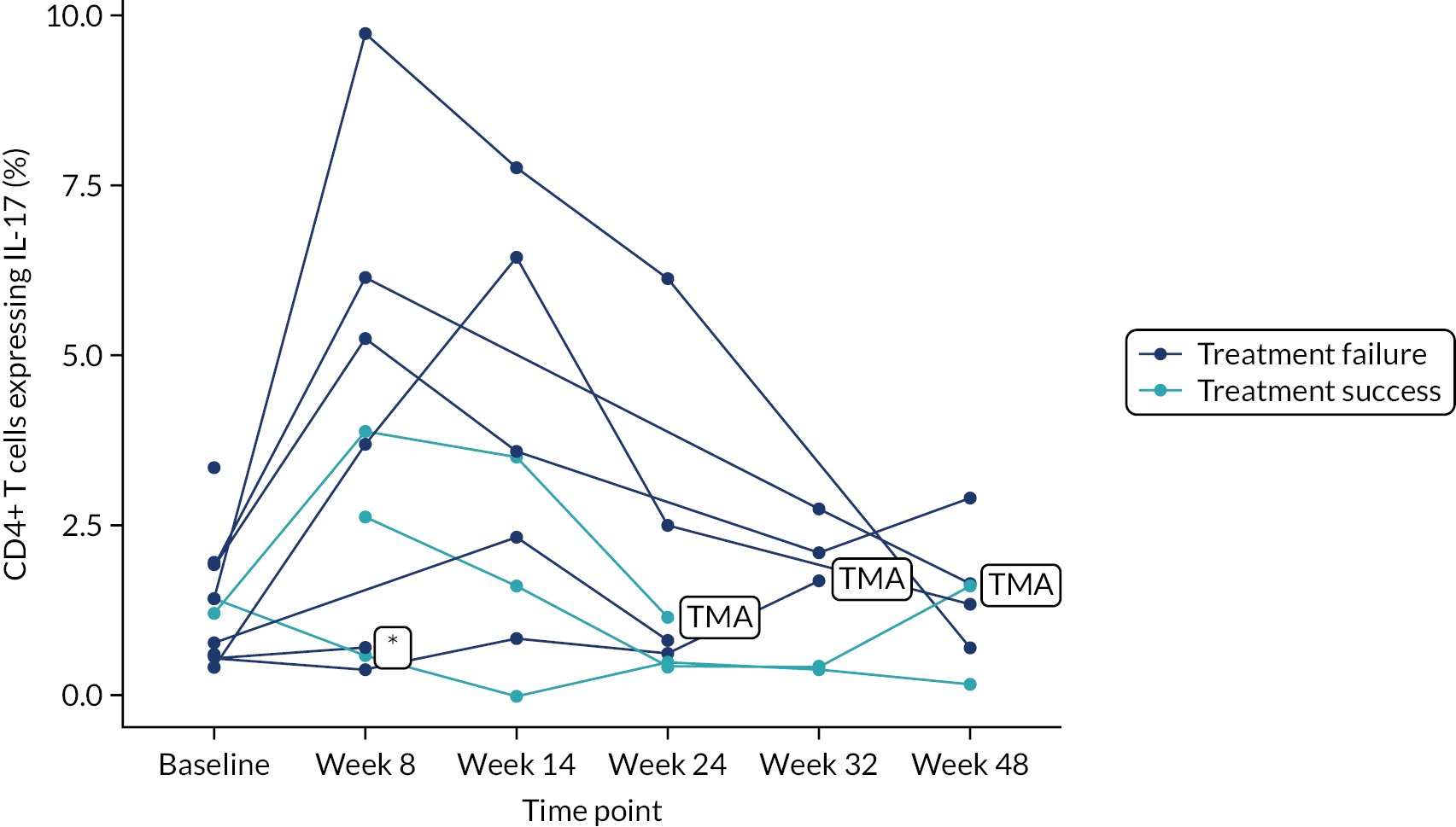
FIGURE 13.
CD4+ T cells expressing TNFα (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
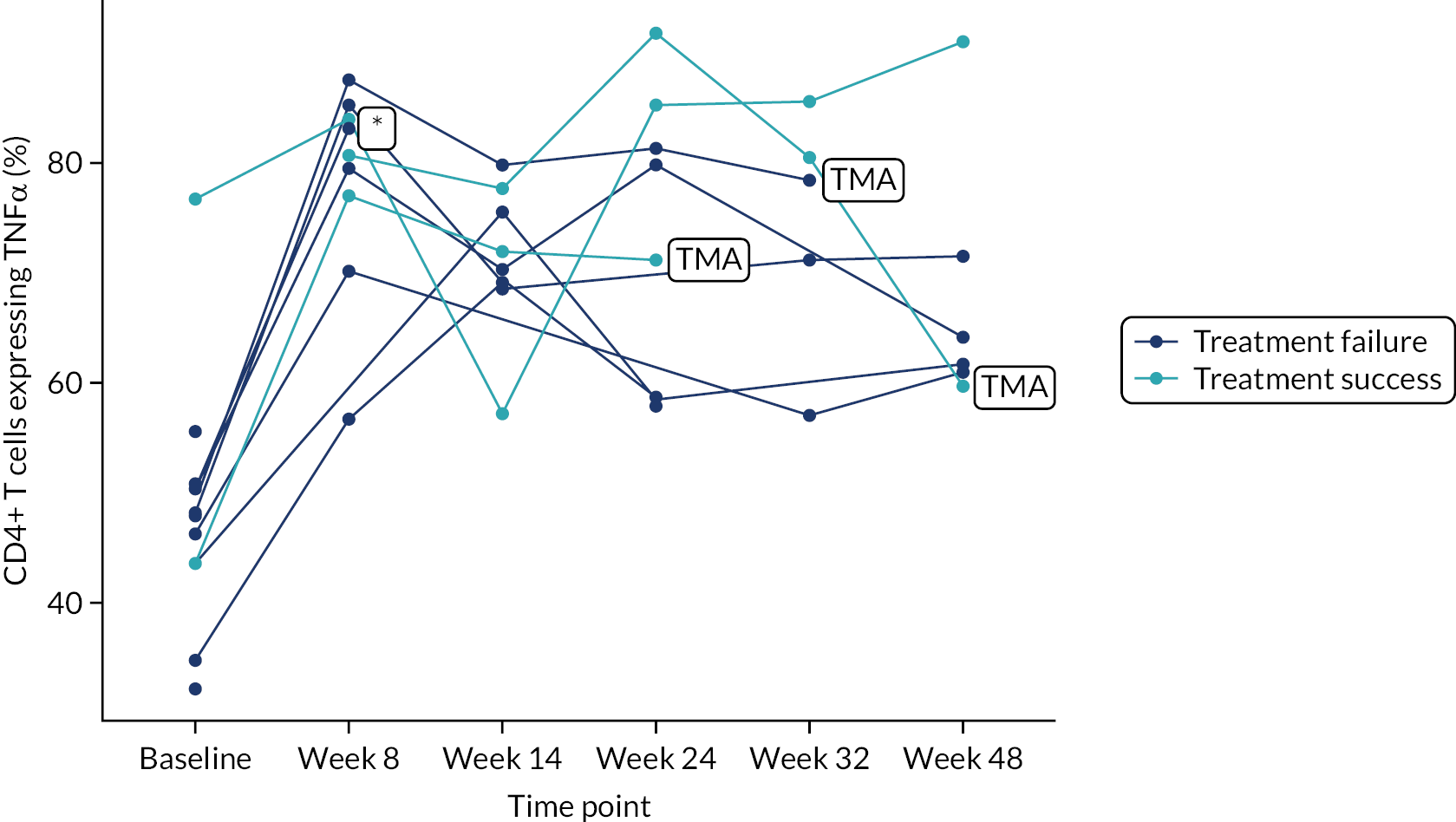
FIGURE 14.
CD4+ T cells expressing IL-10 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.

FIGURE 15.
CD4+ T cells expressing IFNγ (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
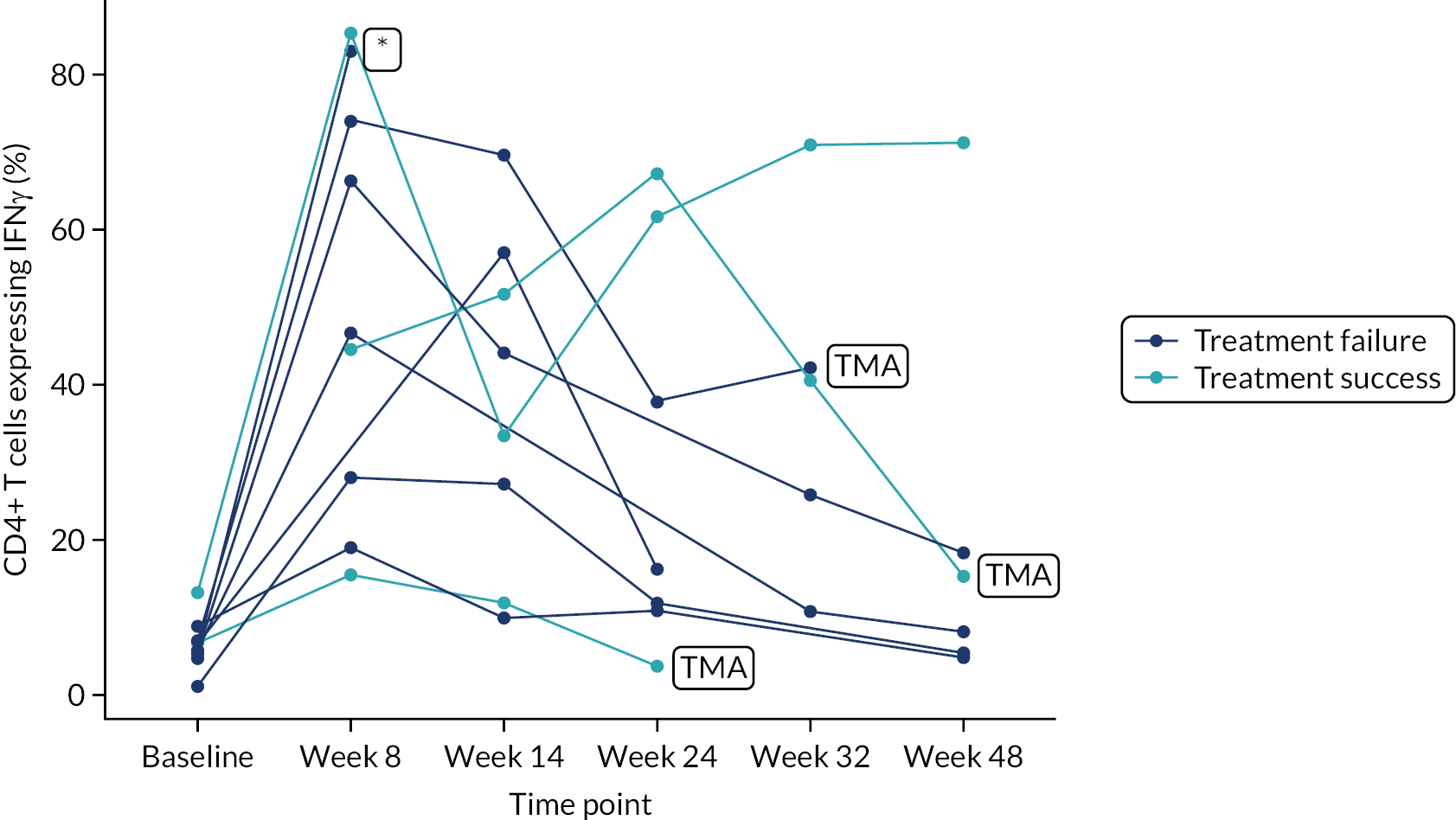
FIGURE 16.
CD8+ T cells expressing IL-2 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
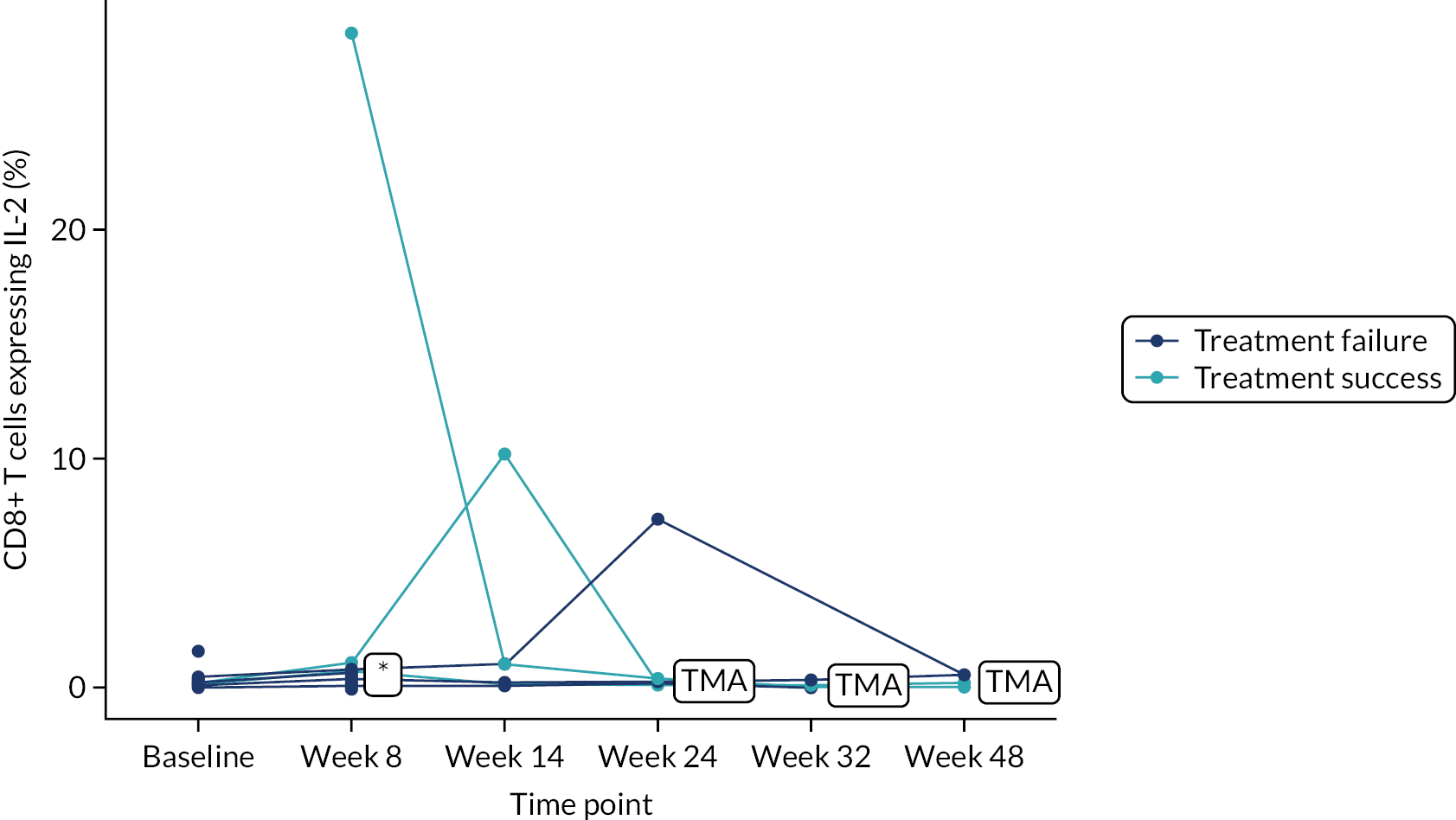
FIGURE 17.
CD8+ T cells expressing IL-4 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
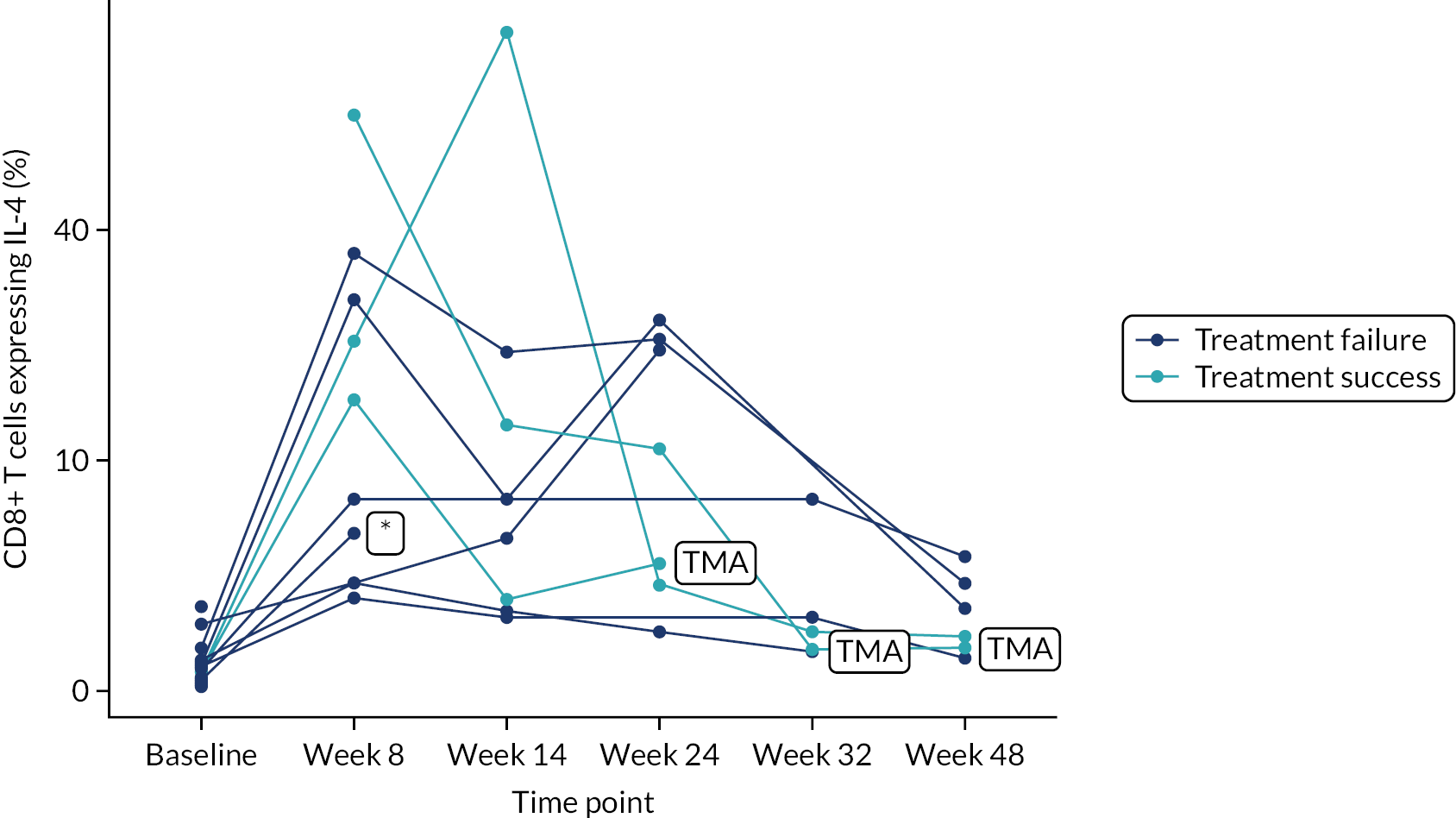
FIGURE 18.
CD8+ T cells expressing IL-17 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
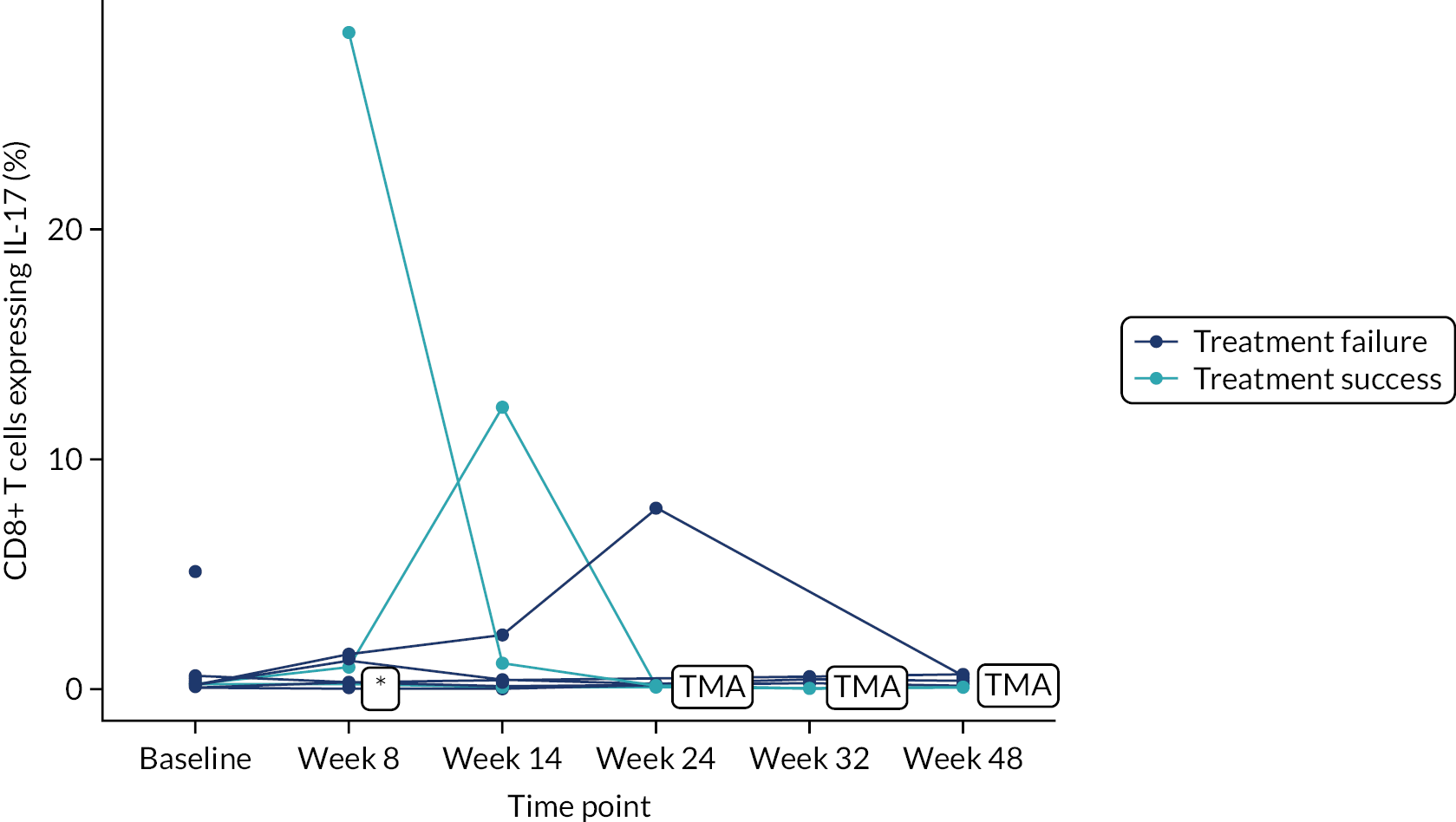
FIGURE 19.
CD8+ T cells expressing TNFα (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.
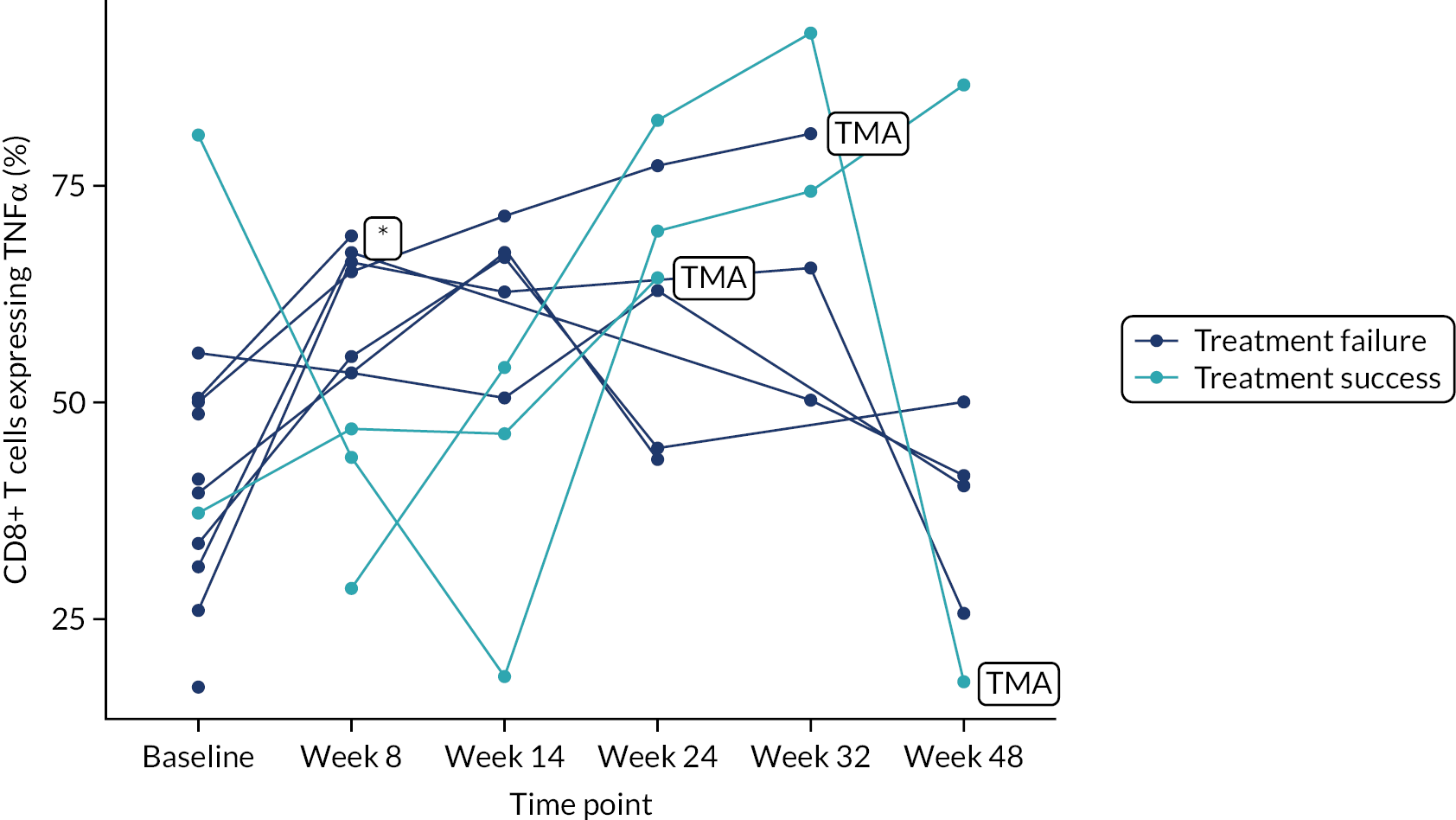
FIGURE 20.
CD8+ T cells expressing IL-10 (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.

FIGURE 21.
CD8+ T cells expressing IFNγ (%) split by treatment success/failure, presented on the HSCTlite arm only from the ITT population.

Appendix 7 Week 32 creatinine against week 8 T-stim data
FIGURE 22.
HSCTlite arm week 32 creatinine vs. week 8 %IL2 (CD4). Presented on the ITT population.
FIGURE 23.
HSCTlite arm week 32 creatinine vs. week 8 %IL-4 (CD4). Presented on the ITT population.

FIGURE 24.
HSCTlite arm week 32 creatinine vs. week 8 %IL-17a (CD4). Presented on the ITT population.
FIGURE 25.
HSCTlite arm week 32 creatinine vs. week 8 %TNF (CD4). Presented on the ITT population.

FIGURE 26.
HSCTlite arm week 32 creatinine vs. week 8 %IL10 (CD4). Presented on the ITT population.

FIGURE 27.
HSCTlite arm week 32 creatinine vs. week 8 %IFNg (CD4). Presented on the ITT population.
FIGURE 28.
Usual-care arm week 32 creatinine vs. week 8 %IL-2 (CD4). Presented on the ITT population.
FIGURE 29.
Usual-care arm week 32 creatinine vs. week 8 %IL-4 (CD4). Presented on the ITT population.
FIGURE 30.
Usual-care arm week 32 creatinine vs. week 8 %IL-17a (CD4). Presented on the ITT population.

FIGURE 31.
Usual-care arm week 32 creatinine vs. week 8 %TNF (CD4). Presented on the ITT population.

FIGURE 32.
Usual-care arm week 32 creatinine vs. week 8 %IL-10 (CD4). Presented on the ITT population.
FIGURE 33.
Usual-care arm week 32 creatinine vs. week 8 %IFN (CD4). Presented on the ITT population.

List of abbreviations
- ADWP
- Autoimmune Diseases Working Party
- AE
- adverse event
- ASTIC
- autologous stem cell transplantation for Crohn’s disease
- ATG
- anti-thymocyte globulin
- BMI
- body mass index
- CD
- Crohn’s disease
- CDAI
- Crohn’s Disease Activity Index
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRP
- C-reactive protein
- CT
- computerised tomography
- CTCAE
- Common Terminology Criteria for Adverse Events
- CTRU
- clinical trials research unit
- DMEC
- Data Monitoring and Ethics Committee
- DMSO
- dimethyl sulfoxide
- DNA
- deoxyribonucleic acid
- EBMT
- European Society for Blood and Marrow Transplantation
- EDTA
- ethylenediaminetetraacetic acid
- eGFR
- estimated glomerular filtration rate
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- G-CSF
- granulocyte colony-stimulating factor
- HBI
- Harvey–Bradshaw Index
- HIV
- human immunodeficiency virus
- HSCT
- haematopoietic stem cell transplant
- HSCTlite
- haematopoietic stem cell transplant with low-dose chemotherapy
- IBD
- inflammatory bowel disease
- IBD-Control
- Inflammatory Bowel Disease Control Questionnaire
- IBDQ
- Inflammatory Bowel Disease Questionnaire
- ICC
- intraclass correlation coefficient
- IL
- interleukin
- IMP
- investigational medicinal product
- IQR
- interquartile range
- ITT
- intention-to-treat
- IV
- intravenous
- JACIE
- Joint Accreditation Committee-ISCT & EBMT
- MaRIA
- Magnetic Resonance Index of Activity
- MDT
- multidisciplinary team
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MRI
- magnetic resonance imaging
- MS
- multiple sclerosis
- MUGA
- multigated radionuclide angiography
- NCI CTCAE
- National Cancer Institute Common Terminology Criteria for Adverse Events
- NIMP
- non-investigational medicinal product
- PBMC
- peripheral blood mononuclear cell
- PBSC
- peripheral blood stem cell
- PI
- principal investigator
- PRO
- patient-reported outcome
- RCT
- randomised controlled trial
- RNA
- ribonucleic acid
- RSI
- reference safety information
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SES-CD
- Simple Endoscopic Score for Crohn’s Disease
- SmPC
- Summary of Product Characteristics
- SUSAR
- suspected unexpected serious adverse reaction
- TMA
- thrombotic microangiopathy
- TMG
- Trial Management Group
- TNF
- tumour necrosis factor
- TSC
- Trial Steering Committee
- WPAI
- work productivity and impairment
