Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 08/43/52. The contractual start date was in September 2009. The final report began editorial review in April 2013 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Wardlaw et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Stroke remains a major public health burden. In the UK, about 150,000 people have a stroke each year. About 30% die within 6 months and another 30% survive dependent on others for everyday activities, making stroke the commonest cause of dependency in adults and the third commonest cause of death in the world. Eighty per cent of strokes are ischaemic and most ischaemic strokes are due to a blocked (thrombosed) artery. Recombinant tissue plasminogen activator (rt-PA, alteplase) reopens blocked arteries and was first licensed for use in the USA following publication of the National Institute of Neurological Disorders and Stroke (NINDS) trial,1 but only for highly selected patients within 3 hours of acute ischaemic stroke in the USA. Cumulative evidence from other trials since then, summarised in the Cochrane Review of all data from randomised trials of rt-PA2 and individual patient data meta-analyses,3,4 plus data from an observational patient registry,5 have been published since, showing a reduction in poor functional outcome in spite of increased risk of symptomatic intracerebral haemorrhage (SICH). However, confidence intervals (CIs) for some outcomes remained wide with unexplained heterogeneity for primary outcomes, the licensing and guideline treatment criteria remained highly restrictive and usage of rt-PA was limited. 6,7
Against this background, the International Stroke Trial 3 (IST-3) started in May 2000, aiming to provide robust evidence on the use of rt-PA in a wider range of patients, including those aged over 80 years, at later time windows and with comorbidities such as prior stroke or diabetes. Practical questions also remained concerning how to reduce the major hazard (intracranial haemorrhage) and how to identify determinants of the latest time after stroke when thrombolysis might still be effective. Focusing treatment on patients with still-viable tissue or persistent arterial occlusion might help to reduce the risk of intracranial haemorrhage and death with thrombolysis, particularly at later time windows. 4,8 However, there were uncertainties about how to identify still-viable at-risk tissue and arterial occlusion, as well as about whether or not patients with these features were most likely to benefit from rt-PA treatment.
Brain imaging is essential prior to rt-PA to exclude intracranial haemorrhage (an absolute contraindication to rt-PA) and lesions that can mimic acute stroke (e.g. brain tumours). Patient assessment for rt-PA in most trials to date was based on a plain computed tomography (CT) brain scan. CT is very practical for use in patients with acute stroke and, in many ischaemic stroke patients, especially those with moderate to severe stroke symptoms, may show early ischaemic changes. 9–14 However, early ischaemic tissue changes that occur during the first few hours after stroke onset and that are thought to indicate irreversible injury, though frequent,9 are subtle;15 lack of confidence among clinicians in recognising these early signs is thought to be one factor that might contribute to the underuse of rt-PA, as many patients who might benefit from thrombolysis remain untreated. Magnetic resonance (MR) brain imaging with diffusion-weighted imaging (DWI) shows acute ischaemia very clearly, but is not widely available as an emergency investigation for stroke16,17 and is not well tolerated by hyperacute stroke patients. 18,19
Identifying the full extent of brain tissue where blood flow is reduced but tissue is still viable outside the non-viable ‘core’ of the infarct could help select patients for treatment with rt-PA – referred to as the ‘ischaemic penumbra’, ‘tissue at risk’ or ‘mismatch’. Imaging the perfusion defect with an intravenous (i.v.) injection of MR contrast agent had been available for MR imaging (MRI) for about 10 years, and became available for CT about 6 years prior to the start of the IST-3 substudy. 20,21 However, a consensus on how the perfusion data should be processed,22–24 or which thresholds distinguish tissue at risk,25 was still to be established. Thus, it had long been considered that advanced imaging methods with CT perfusion (CTP) or MR DWI and perfusion-weighted imaging (PWI) could help focus use of rt-PA on patients with large amounts of tissue at risk and avoid exposing those with little at-risk tissue to the risk of rt-PA. Although some stroke experts strongly advocate using this imaging approach,26 and some observational studies provided encouraging results,27 several randomised trials that used MR DWI/PWI mismatch had been inconclusive,28–30 or conflicting. 31 Indirect comparisons between randomised controlled trials (RCTs) which used plain CT and MR DWI/MR perfusion showed no clear improvement in functional outcome or in SICH risk according to MR DWI/MR perfusion (MRP) tissue status. 32,33 The few studies which included patients without MR DWI/PWI mismatch found that about half of those without mismatch also had infarct growth and, therefore, presumably might have benefited from treatment. 34,35 Similarly, some observational data suggest that CTP did not differentiate core from salvageable tissue. 36 There are no randomised rt-PA studies based on CT with CTP [although some patients were included in the Desmoteplase in Acute Stroke (DIAS) 2 trial with CT/CTP, these data are not available separately].
The other information that might guide the use of thrombolysis, derivable from CT or MRI, is the presence and location of an occluded artery as this determines the likely extent of the tissue affected by the stroke. 37 An occluded artery may be suspected by the presence of a hyperattenuated artery on plain CT or an absent flow void or a hypointense artery on T2/fluid attenuated inversion recovery (FLAIR) or T2* MR, respectively. Disappearance of the hyperattenuated artery/absent flow void (i.e. presumed recanalisation) is associated with improved clinical outcome with or without rt-PA38,39 and its persistence is associated with poor clinical outcome. 40 Arterial occlusion may be identified with CT angiography (CTA) or MR angiography (MRA) with an i.v. injection of contrast agent. The angiographic images are generally faster to acquire than perfusion imaging, and require some image reconstruction and careful interrogation but there is, in general, less scope for variation in acquisition, processing or interpretation, and the acquisition and image processing are faster than for perfusion imaging. However, there have been far fewer publications on angiographic imaging and the relationship to likely rt-PA response and clinical outcomes than on perfusion imaging. As with perfusion imaging, several factors need to be addressed before CTA or MRA can be used reliably to inform clinical practice.
It is clear that improved outcome after ischaemic stroke is associated with arterial recanalisation in observational studies whether spontaneous or rt-PA induced,41 but there is disagreement about how information from angiography should be used. Some consider that rt-PA may be effective only when a visible thrombus is present. Others consider that the absence of a visible occlusion may simply reflect lack of sensitivity of imaging to small peripheral thrombi or to occlusion at the origin of a proximal major branch point making that branch ‘invisible’ angiographically, that in any case the major arteries may be patent when the tissue arterioles/capillaries are not, and that patients without a visible arterial occlusion should not be denied thrombolytic treatment in the absence of further information from RCTs. The marginal benefit or hazard of rt-PA in the presence or absence of a visible arterial occlusion was unknown as there were no completed randomised trials of rt-PA where randomisation was on the basis of presence or absence of arterial occlusion. Previous, recently completed trials [e.g. Systemic versus Intra-arterial thrombolysis for Ischaemic Stroke (SYNTHESIS) Expansion42 and International Management of Stroke (IMS) 11143,44] and (still) ongoing trials have included only patients with angiography-confirmed arterial occlusion (e.g. DIAS 3 and 445). Angiographic interpretation is based on visual assessment. Multiple visual rating scores have been described, but all appear to conflate several items in one score and there was little information on observer reliability or which score was best when deciding whether or not to use rt-PA treatment. The very limited data on observer reliability of angiography scoring indicated poor agreement: the intraobserver agreement between nine neuroradiologists reading intra-arterial angiograms using the Thrombolysis in Cerebral Infarction (TICI) score was poor (к< 0.2) with little evidence of improvement with training, possibly because of the conflation of three concepts inherent in the score. 37,46 A detailed discussion of the scores and problems with their use was provided in the IST-3 perfusion and angiography imaging protocol paper. 47
Other factors derivable from angiographic imaging may help guide rt-PA therapy. Some thrombi may dissolve more easily with rt-PA. Thrombus composition influences its appearance on imaging. However, the reliability of the imaging appearance–composition relationship is unknown. Despite this, there is emerging (although conflicting) literature on thrombus attenuation, probable composition and likelihood of rt-PA responsiveness48–52 which required further testing prior to clinical use. Other angiographic features that may influence both tissue viability and rt-PA response are the burden of occlusive thrombus53 and the adequacy of collateral pathways. 54 Several scores exist to code the collateral circulation55,56 but these, in general, had undergone little independent validation.
The IST-3 Perfusion and Angiography Study was embedded in the IST-3 main trial and aimed to determine whether or not there is a differential benefit of rt-PA in patients with, compared with patients without, perfusion lesions or arterial occlusion. If, as suggested in recent studies, very high proportions of patients with large artery territory cortical ischaemic symptoms have MR DWI/MRP mismatch within 6 hours of stroke,30 and if rt-PA is effective in those with mismatch, then simply determining the clinical stroke syndrome and time lapsed since stroke may be almost as effective as complex imaging in guiding patient selection (as well as being quicker and less expensive). If, on the other hand, the benefits of rt-PA are confined to those either with imaging evidence of tissue at risk or with arterial occlusion, regardless of time lapsed since onset, and who cannot be identified by other means, then it will require substantial investment in imaging services to deliver effective thrombolysis. If the presence of perfusion-visible at-risk tissue has no impact on responsiveness to rt-PA treatment, then clinicians will have greater confidence to treat patients on the basis of plain CT (or MR DWI) and thorough clinical assessment alone, which would immediately improve access to rt-PA.
Chapter 2 Research objectives
The original research objectives of the IST-3 perfusion and angiography substudy were to determine:
-
whether acute ischaemic stroke patients with versus without imaging evidence of tissue at risk (perfusion lesion or mismatch), on either CT with CTP or MR DWI/PWI, have (a) less infarct growth and (b) better functional outcome if treated with rt-PA versus control?
-
which perfusion parameter [cerebral blood flow (CBF), cerebral blood volume (CBV) or mean transit time (MTT)], processing method (qualitative, quantitative) and threshold best predicts (a) infarct growth and (b) poor functional outcome at 6 months?
-
if patients with angiographic evidence of an occluded artery on either CT or MR angiography have (a) less infarct growth and (b) better clinical functional outcome if treated with rt-PA versus control?
Secondary questions included:
-
what is the threshold of reduced cerebral perfusion that can be tolerated, and for what period of time after stroke onset, which determines whether tissue ultimately survives or infarcts?
-
are there imaging features on plain CT or MR DWI that differentiate viable from non-viable tissue?
-
determining the interobserver reliability of perfusion and angiography scoring methods
-
determining the influence on the plain-scan rating of knowing what the perfusion or angiography imaging shows.
We also aimed to:
-
establish a core of interested physicians and radiologists in IST-3 to guide the proposed advanced imaging substudy, inform and participate in the analysis and prepare manuscripts for publication and presentation; and
-
contribute data to the Stroke Imaging Repository (STIR), an international, multicentre project which aims to standardise stroke perfusion imaging.
Chapter 3 Methods
We provide minimum details of the IST-3 main trial, followed by the specific methods in the perfusion and angiography substudy. The full IST-3 trial protocol, details of the patients’ baseline demographic variables, the statistical analysis plan and primary results57–60 and the protocol for the Perfusion and Angiography Substudy47 have all been published. The protocol was approved by the Multicentre Research Ethics Committees (MREC/99/0/78) and by local ethical committees. The trial was registered as ISRCTN25765518.
Main trial
The IST-3 was an international, prospective, randomised, open, blinded, end-point (PROBE) controlled trial of i.v. rt-PA within 6 hours of onset of acute ischaemic stroke (see www.ist3.com). 60 Plain CT brain scanning was the primary imaging modality for the main trial.
Participants
Patients with suspected acute ischaemic stroke who reached hospital, could be assessed and treated within 6 hours of stroke onset. Patients in whom rt-PA was ‘promising but unproven’ could be randomised in the trial after informed consent was obtained.
Inclusion criteria
(a) Symptoms and signs of clinically definite acute stroke, (b) time of stroke onset definitely < 6 hours previously, (c) CT or MR brain scanning has excluded intracranial haemorrhage and (d) treatment can be started within 6 hours of stroke. Patients with symptoms of large and medium-sized cortical, lacunar and posterior circulation stroke were all included, with no upper age limit. Patients with early visible infarct signs were also included (though not if established infarct signs were present, as these suggest a stroke onset of more than > 6 hours previously).
Exclusion criteria
Age < 18 years, imaging signs that the stroke might be older than 6 hours, and usual contraindications to rt-PA. 60
Interventions
Intravenous rt-PA (total dose 0.9 mg/kg to a maximum of 90 mg, 10% as bolus and the rest infused over 1 hour) compared with ‘open control’ (avoid rt-PA and receive stroke care in exactly the same clinical environment as those allocated ‘immediate rt-PA’).
Baseline assessment
All patients were assessed for stroke severity [National Institutes of Stroke Scale (NIHSS) score], stroke subtype [total anterior circulation infarct (TACI); partial anterior circulation infarct (PACI); lacunar infarct (LACI); or posterior circulation infarct (POCI), clinical syndrome], presence of atrial fibrillation (AF), systolic and diastolic blood pressure and blood glucose.
Objectives
To determine if rt-PA, given to a wider range of patients up to 6 hours after stroke, would improve functional outcome by 6 months net of any hazard.
Outcomes
The primary outcome was alive and independent [Oxford Handicap Score (OHS) 0–2,61 which is very similar to modified Rankin 0–262] at 6 months after stroke. Symptomatic and fatal intracranial haemorrhage, death and recurrent stroke within 7 days and death at 6 months were also assessed.
Brain scanning
All patients had a CT or MR brain scan before randomisation and a follow-up scan at 24–48 hours. A repeat brain scan was required if the patient deteriorated neurologically or intracranial haemorrhage was suspected for any reason. All scans were sent to the trial centre in Edinburgh for blinded central rating of any signs of visible early ischaemia (presence and extent of hypoattenuation, swelling, hyperattenuated artery), haemorrhage, and background brain changes (leukoaraiosis, atrophy, prior stroke lesions, non-stroke lesions) with validated rating tools. 15,63–67 Images were assessed blindly, and assessed via a secure web-based image viewing system by an international panel of expert radiologists.
Sample size
The IST-3 main trial was powered to detect a 4.7% absolute improvement with rt-PA compared with no rt-PA in the number alive and independent at 6 months with power 80% at p = 0.05 with 3100 patients. 57 This effect size was based on the Cochrane Thrombolysis Review in 2000,2 but remained unaltered following the update in 2008. 32
Randomisation
Randomisation was via a secure central telephone or web-based computer system, which recorded all of the baseline data and generated the treatment allocation. A minimisation algorithm was used to achieve optimum balance for key prognostic factors. 57,59
Follow-up
Follow-up of 6-month outcomes was by central office staff blinded to treatment allocation, by postal questionnaire or telephone for non-responders (by an experienced, blinded assessor).
Statistical methods59
The statistical analysis plan was published59 prior to unblinding to the data. To avoid complicating the estimation of the effect of treatment overall and in subgroups,57 the primary analysis was logistic regression for linear effects adjusted for the following covariates: age; NIHSS score; time from onset of stroke symptoms to randomisation; and presence (vs. absence) of ischaemic change on the pre-randomisation brain scan according to the expert read. Unadjusted analyses were also performed. 60 The statistical analysis plan writing committee, while still blinded, adopted the ordinal method, as it is statistically more efficient (effectively reducing the sample size required in stroke trials68). The OHS as a dependent variable had five levels: levels four, five and six were combined into a single level and levels zero, one, two and three were retained as distinct. In this model, the treatment odds ratios between one level and the next are assumed constant, so a single parameter summarises the shift in outcome distribution between treatment and control groups. Analyses were carried out with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Any changes to protocol
Two changes occurred. The first was the change from placebo-controlled to open-label treatment after the first 297 patients due to withdrawal of support for the trial by Boehringer Ingelheim (Bracknell, UK). The second was the revised sample size estimation and introduction of the ordinal analysis described above as a secondary outcome analysis.
The Third International Stroke Trial Perfusion and Angiography Substudy
In centres where perfusion and/or angiography imaging with CT or MR were performed routinely for acute stroke, data from these imaging modalities were collected centrally according to established IST-3 methods. In those centres, patients were randomised into IST-3 according to plain CT or MR criteria so that decisions were not influenced by knowledge of perfusion or angiography information.
Participants, interventions, clinical outcomes, randomisation and blinding were the same as for the main trial and detailed above except that, as per routine clinical practice, patients with definite renal impairment [estimated glomerular filtration rate (eGFR) < 30 ml/minute/1.73 m2] or on metformin were excluded from the perfusion/angiography study. Reduced eGFR is common on admission to hospital in patients with acute ischaemic stroke and usually normalises with rehydration;69 therefore, patients with an eGFR of 30–59 ml/minute/1.73 m2 could be included if there was no documented history of renal impairment and the low eGFR was considered likely to reflect dehydration, at the discretion of the recruiting physician. Low-risk MR contrast agents were to be used. Oxygen was continued in MR or CT where necessary.
Objectives
The basic questions to be addressed are ‘should “perfusion-structural imaging mismatch” or “arterial occlusion” influence whether or not patients receive rt-PA?’ Here, the key question was whether or not rt-PA is more effective in patients with imaging evidence of tissue at risk than in those without apparent tissue at risk. Tissue at risk was defined as:
-
the difference between the extent of core damaged tissue on MR DWI or plain CT and the extent of the MR or CT perfusion lesion (further details of perfusion lesion measurements and comparisons below); or
-
evidence of arterial occlusion on CT or MR angiography.
Imaging acquisition
Where possible, patients were to be examined on the same scanner at baseline and at follow-up, although combinations, for example CT pre randomisation and MR at 24-hour follow-up, were allowed as local clinical practice dictated. Basic minimum acquisition standards were required (see Appendix 1). We provided basic minimum acquisition standards to encourage best practice in perfusion or angiography imaging while allowing for the considerable variation that exists in available scanning technology. Thus, it would have been counterproductive to provide overly narrow acquisition criteria that only a proportion of centres might have been able to meet, as that would have further limited the sample size and generalisability of the data. Full details of the minimum acquisition criteria as sent to participating centres are given in Appendix 1. In addition, before a centre could participate in the Perfusion and Angiography Study, a test perfusion and/or angiogram image data set had to be sent to the IST-3 trial co-ordinating centre to ensure that the imaging met minimum standards and that the data could be processed centrally.
The trial image data were received at the IST-3 trial co-ordinating centre, linked with their demographic data and trial records, anonymised and transferred into the image-processing pipeline. Plain CT and MR images were read according to the IST-3 established structured image analysis protocol by a panel of experts via a web-based image reading system, the Systematic Image Review System (SIRS: see www.neuroimage.co.uk/) as detailed above.
Outcomes
The primary outcome measures were the same clinical measures as for the IST-3 main trial above: functional outcome (OHS 0–2), symptomatic and fatal intracranial haemorrhage, early and late death and massive infarct swelling.
The secondary outcomes were absolute infarct growth, defined qualitatively as a change in the extent of hypoattenuated tissue on CT or of hyperintense tissue on MR FLAIR between baseline and 24–48-hour follow-up, of one point or more on either the IST-3 scale score,64,70 in any arterial territory, or the Alberta Stroke Program Early CT score (ASPECTS)63 if in the middle cerebral artery (MCA) territory; defined quantitatively as the difference in measured lesion volume on plain CT or MR DWI between randomisation and follow-up scans.
Blinding
All image data were collected centrally in Digital Imaging and Communications in Medicine (DICOM) format, matched with the patient record, anonymised and identified only by the study identification number. All image data analyses were performed centrally, blind to treatment allocation, baseline demographic information and follow-up.
Perfusion analysis
We produced perfusion parameter maps for each patient for visual rating and measurement of lesion volume without any threshold applied (Figure 1; Table 1): quantitative (q) perfusion with deconvolution CBF quantitative (CBFq), CBV quantitative (CBVq), MTT quantitative (MTTq), time to maximum flow quantitative (time to peak of the residue function) (Tmaxq) and relative (r) perfusion, that is to say without deconvolution relative CBF (rCBF); relative arrival time fitted; relative time to peak; relative peak time fitted; relative maximum concentration peak; relative full width at half maximum. Full details of the perfusion processing are given in Appendix 2. We also produced a set of parameter maps with thresholds applied (see Table 1). These parameters and thresholds were based on literature values that had been proposed for identifying still-viable but at-risk tissue and core tissue, of which there were many, but none had been validated independently. 71 This was because the IST-3 perfusion substudy was not large enough to generate new thresholds in one half of the data set and validate these in the other half. Therefore, we focused on validating ones which had been reported previously.
| MR perfusion | CT perfusion | ||
|---|---|---|---|
| Visual score | Volume | Visual score | Volume |
| Raw data | Raw data | ||
| rCBF | rCBF | ||
| rCBV | rCBV | ||
| rMTT (first moment) | rMTT (1.45) | ||
| TTP (various thresholds) | TTP (1.4 wrt normal side) | ||
| Tmax plus 6 seconds | Tmax plus 6 seconds | ||
| ATF | ATF | ||
| CBFq | CBFq (including 12.7 ml/100 g/minute) | ||
| CBVq | CBVq (including < 2.2 ml/100 g) | ||
| MTTq | MTTq | ||
FIGURE 1.
Example of CT perfusion parameter maps. (a) CT with plain structural image at randomisation and post randomisation, with infarct outlined, perfusion maps and various thresholds below; (b) MR with acute DW1 and T2, follow-up T2, perfusion maps and various thresholds below. ROI, region of interest.
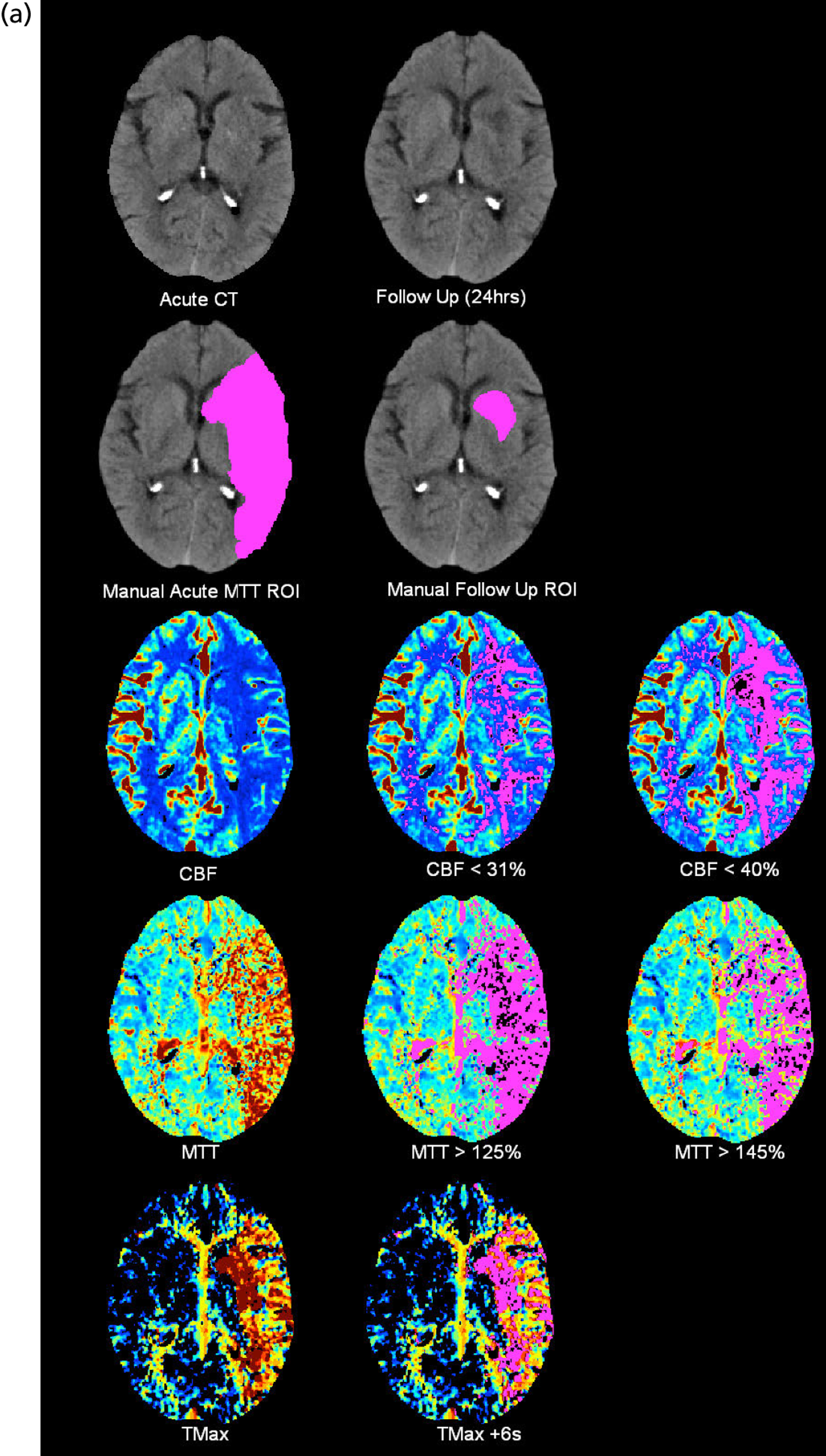
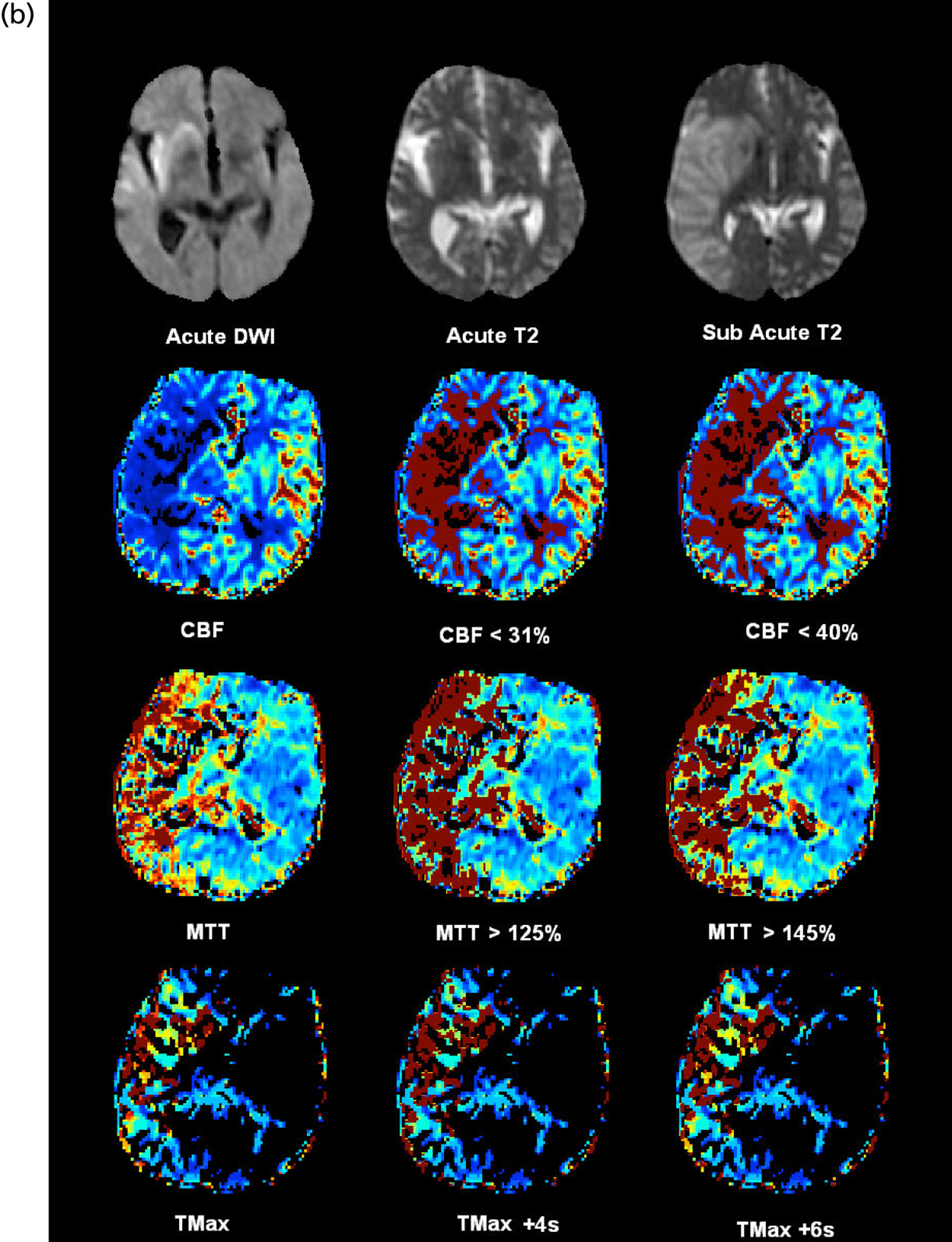
Maps of the following perfusion thresholds were produced for volumetric and visual measurement (details in Table 1):
-
Representing non-salvageable tissue:
-
Representing at-risk tissue:
These perfusion parameters reflected commonly applied thresholds and image types while keeping the total number of comparisons manageable and reducing the potential for false-positive results. Our systematic review had not identified a specific parameter or threshold that seemed optimum;71 different research groups had not identified an agreed perfusion parameter/threshold since the systematic review. We therefore tested several perfusion parameters/thresholds which covered the most easily available and most promising derived from the most recent research. Many of these thresholds have been defined for one modality only (mostly CTP) but could equally be applied to MR data and, therefore, were tested.
Perfusion lesion extent was quantified visually by one expert neuroradiologist, blind to all other data. We used the ASPECTS,63 subtracting one point from a total of 10 for each MCA ASPECTS region that is in part or wholly affected by the perfusion lesion, even where perfusion image does not cover the whole ASPECTS region. We also recorded if there was (a) no visible perfusion lesion, (b) a visible perfusion lesion that was < 80%, (c) about the same size as or (d) ≥ 20% larger than the structural ischaemic lesion by visually-estimated volume on plain CT or MR DWI/FLAIR. 27,30 ‘Mismatch’ was defined as a perfusion lesion > 20% larger than the structural lesion. We validated these methods in a separate three-centre study (Translational Medicine Research Collaboration Multicentre Acute Stroke Imaging Study47). Visual coding forms are available in Appendix 3. Perfusion lesion volume was measured by manual outlining the lesion by a trained observer blind to all other data on two of the unthresholded parameter maps from above (MTTq and rCBF perfusion lesions) to represent at-risk tissue and non-salvageable tissue respectively. The perfusion lesion volume was also measured on thresholded parameter maps listed in Table 1 using automated thresholding.
Angiography analysis
The randomisation CT angiography images were scored by a blinded neuroradiologist who also measured thrombus density on a workstation in Hounsfield Units (HU). The hyperattenuated artery sign (HAS) was scored on the available imaging, that is to say thin section if available or routine 5 mm section if not, depending on what imaging had been received. Separately, a panel of 11 experts also read all of the CT and MR angiograms using the web-based SIRS (SIRS2), which we modified so as to be able to see the plan scan and angiographic image on the same screen and record both the plain-scan findings and angiographic appearance (SIRS2, sirs2/neuroimage.co.uk/sirs2; Figure 2).
FIGURE 2.
Screen capture of web interface used to visually assess angiographic images.
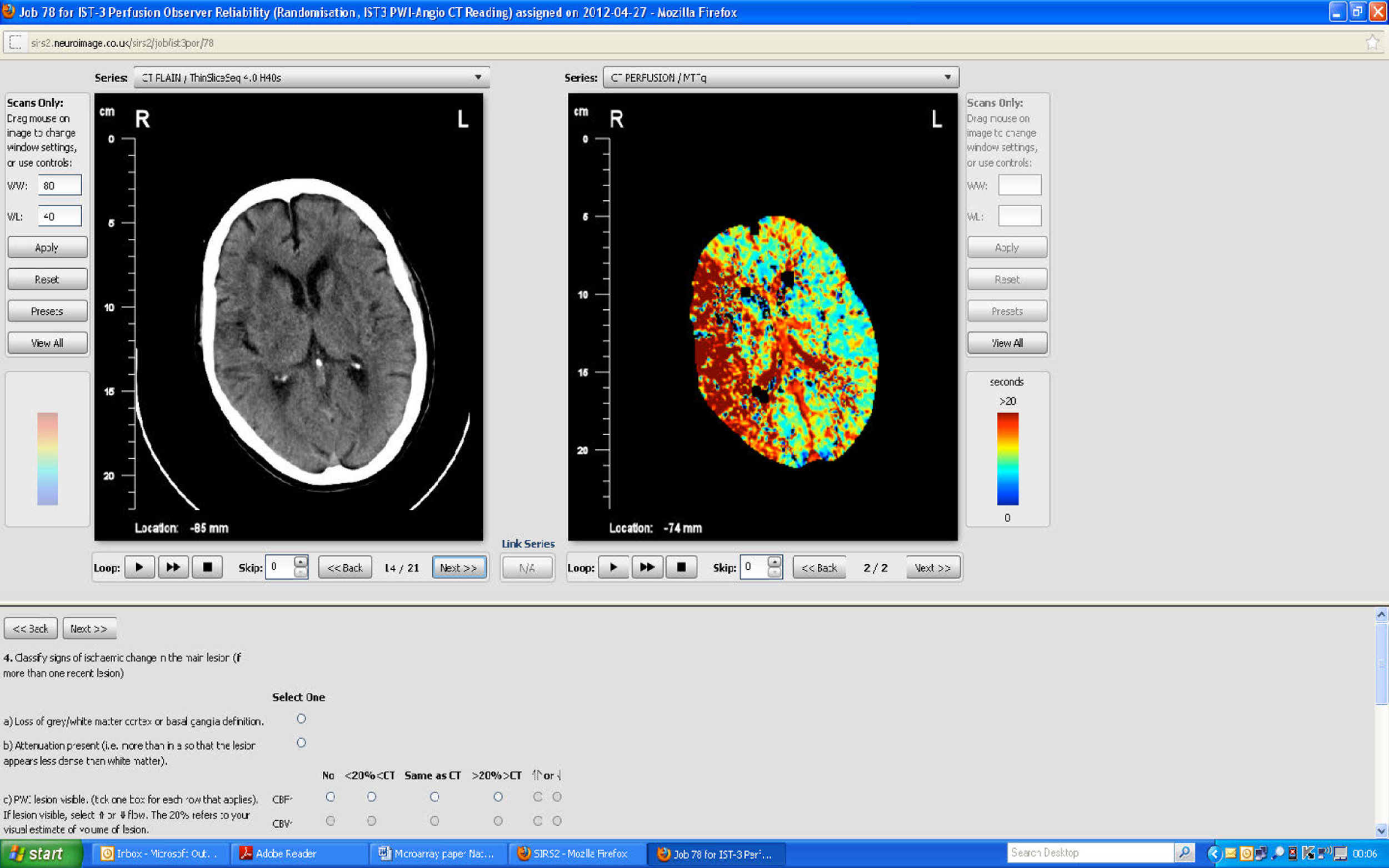
We assessed the location, extent of vessel affected and degree of obstruction to the lumen of any arterial occlusion, the presence of collateral pathways, the clot burden53 and the attenuation properties of the occluding thrombus. Location and extent of thrombus was coded in the internal carotid artery (ICA), MCA mainstem or sylvian branch, anterior cerebral artery (ACA), posterior cerebral artery (PCA), basilar artery, vertebral artery or combinations thereof. 15,38,40 We debated, at length, the best score to use. Several scores are available to classify the degree of major arterial obstruction (Table 2). These mostly conflate three different concepts – peripheral microvascular tissue perfusion, primary arterial patency, and recanalisation – in a single score, thereby mixing three separate and probably semi-independent entities. 87 This, no doubt, contributes to the poor observer reliability. We previously used the Mori82 and Thrombolysis in Myocardial Infarction (TIMI)83 scores purely to classify arterial patency at the primary point of obstruction on CTA and MRA and, separately, used CTP or MRP to classify tissue-level perfusion and reperfusion which worked well. Other scores (summarised in Table 2) mixed primary occlusion, perfusion and recanalisation. 37,56,84,85 Therefore, in IST-3 we used a score that combines the best elements of the TICI (including 2a and 2b) and arterial occlusive lesion (AOL) scores that only scored angiographic patency at the main point of occlusion and filling of immediate distal vessels, but not tissue perfusion or recanalisation. This score, used in DIAS 3 and 4 and IMS-3,43–45 is described in the protocol paper. 47
| TIMI score83 | Mori score82,86 |
|---|---|
| 0: No flow/patency | 0: No flow/patency |
| 1: Minimal flow/patency | 1: Minimal flow/patency |
| 2: Partial flow/patency | 2: Flow/patency of less than half of the territory of the occluded artery |
| 3: Flow/patency of more than half of the territory of the occluded artery | |
| 3: Complete flow/patency37 | 4: Complete flow/patency82,86 |
| AOL score37 | TIMI score, adapted for the intracranial circulation in ischaemic stroke37 |
| Grade 0: No recanalisation of the primary occlusive lesion | Grade 0: No perfusion |
| Grade 1: Incomplete or partial recanalisation of the primary occlusive lesion with no distal flow | Grade 1: Perfusion past the initial occlusion, but no distal branch filling |
| Grade 2: Incomplete or partial recanalisation of the primary occlusive lesion with any distal flow | Grade 2: Perfusion with incomplete or slow distal branch filling |
| Grade 3: Complete recanalisation of the primary occlusion with any distal flow | Grade 3: Full perfusion with filling of all distal branches, including M3, 4 |
| TICI score, adapted the TIMI score with further granularity for partial patency 56 | |
| Grade 0: No perfusion. No antegrade flow beyond the point of occlusion | |
| Grade 1: Penetration with minimal perfusion. The contrast material passes beyond the area of obstruction but fails to opacify the entire cerebral bed distal to the obstruction for the duration of the angiographic run | |
| Grade 2: Partial perfusion. The contrast material passes beyond the obstruction and opacifies the arterial bed distal to the obstruction. However, the rate of entry of contrast into the vessel distal to the obstruction and/or its rate of clearance from the distal bed are perceptibly slower than its entry into and/or clearance from comparable areas not perfused by the previously occluded vessel, e.g. the opposite cerebral artery or the arterial bed proximal to the obstruction | |
| Grade 2a: Only partial filling (less than two-thirds) of the entire vascular territory is visualised | |
| Grade 2b: Complete filling of all of the expected vascular territory is visualised, but the filling is slower than normal | |
| Grade 3: Complete perfusion. Antegrade flow into the bed distal to the obstruction occurs as promptly as into the obstruction and clearance of contrast material from the involved bed is as rapid as from an uninvolved other bed of the same vessel or the opposite cerebral artery | |
| Two further variations of the TICI score | |
| TICI grade of perfusion confuses arterial patency/recanalisation and perfusion including grades 0 to 3 and subscores 2a to 2c84 | TICI reperfusion (I): essentially the same as TIMI score applied in IMS 1 with grade 2 further divided into 2a, partial filling (less than half) of, and 2b, partial filling (half or more) of, for post hoc analysis85 |
| Score to be used in IST-3: TICI–AOL hybrid (see Figure 1) | |
| 0: No patency – artery completely blocked at main obstruction point | |
| 1: Minimal patency – some contrast penetrates main obstruction point but no/minimal opacification of artery or branches distally | |
2: Patency of less than half of the lumen at the point of obstruction and
|
|
| 3: Patency of more than half of the lumen at the point of obstruction and filling of most of the major branches of the affected artery | |
| 4: Complete patency – normal | |
Recanalisation was indicated by a change of one point or more on the scale between randomisation and follow-up scans.
We also coded thrombus burden using the clot burden score,53 where one or two points are subtracted from a normal score of 10 for each segment of the main intracranial arteries or their branches that is abnormal on angiography; thus, a score of zero indicates that all major intracranial arteries on one side of the head are thrombosed. We also scored visible HASs on CT and abnormal arteries on CTA whether the abnormality was in a proximal (internal carotid or basilar artery), middle (ACA, MCA or PCA) or distal (sylvian branches of MCA) artery. The resulting proximal-middle-distal (PMD) score value ranges from 1 to 6, where 1 = only distal vessel, 2 = only middle vessel, 3 = middle and distal vessels, 4 = proximal vessel, 5 = proximal and middle vessels and 6 = proximal, middle and distal vessels.
We scored the adequacy of the collateral pathways54 in patients with ICA/MCA main stem occlusion only using the Score for Collateral Status,55 a three-point scale of good, moderate or poor based on the number of opacified arteries visible in the peripheral parts of the affected tissue. Examples are provided for comparison.
The resulting coding forms are available in Appendix 3 and can be seen at www.bric.ed.ac.uk/research/imageanalysis.html#ais.
A neuroradiologist also measured the mean density of any HAS in HU (i.e. standard units used to assess tissue X-ray beam attenuation) using a region of interest cursor placed on the affected artery and also of the unaffected arteries (i.e. basilar, left or right middle cerebral arteries) on a personal computer workstation running Digital Jacket software (an in-house image server application allowing manipulation of DICOM data sets; DesAcc, Bellevue, WA, USA). Ovoid ‘regions of interest’ were applied to the HAS or normal artery and three measurements were taken from each artery at similar locations for each patient; natural anatomical and scan parameter variability meant that measurement location could not always be identically reproduced, though this was attempted as near as possible.
Observer reliability
We tested the interobserver reliability of angiographic image analysis by inviting the expert panel to read the same 10 angiograms using the SIRS2 blind to all other data including their initial analysis. We tested observer reliability of the perfusion imaging by inviting as many raters as possible to rate 20 perfusion images using the modified SIRS2 system that was able to handle colour images and to view two image modalities from the same acquisition time point (e.g. a perfusion and a structural CT image) side by side (SIRS2: sirs2/neuroimage.co.uk/sirs2). These analyses are ongoing at the time of writing.
Sample size
The IST-3 perfusion analysis aimed to examine primarily whether or not rt-PA improves functional outcome more in those with, than without, tissue at risk and secondarily reduced infarct growth. Based on systematic reviews of all available data34 and recent studies,27,30 we estimated that 60% will have mismatch overall;27 70% with mismatch will have infarct growth compared with 30% without mismatch; and rt-PA will reduce infarct growth by 20% in those with, but not those without, mismatch. 34 At 80% power and α = 0.05, a sample of 100 patients would detect a 27% difference in infarct growth, with rt-PA compared without rt-PA, in the presence of mismatch compared with the absence of mismatch; 160 patients would detect a 20% difference in infarct growth; and 400 patients would detect a 15% difference in infarct growth. We acknowledged that, with, at most, 300 patients in the perfusion study, the perfusion study would be underpowered to detect a ‘mismatch × treatment effect’ interaction on the primary clinical outcome. We therefore selected infarct growth as an outcome for the perfusion analysis (as in EPITHET)30 to increase statistical power.
The centres that were using perfusion imaging were among the most active in IST-3. Therefore, we estimated that in 3 years, in up to 15 active centres recruiting between four and eight patients per year each, a total of between 100 and 300 patients with baseline perfusion and or angiography data would be recruited. We estimated that approximately two patients would have CTA/CTP for every one with MRA/MRP. However, that may change as more centres are now acquiring CT perfusion equipment, and so the proportion may end up being nearer to four patients having CTA/CTP for every one with MRA/MRP.
Statistical methods
We first compared imaging variables with each other, then with clinical features and clinical outcomes, and then tested for interactions between imaging variables and rt-PA effects. Thus, we assessed:
-
variation in the size of perfusion lesions and proportion with mismatch for each perfusion parameter tested
-
associations between clinical and structural imaging variables at baseline, perfusion lesion extent and presence/absence of angiography lesions
-
associations between baseline perfusion or angiography imaging variables and subsequent infarct growth, swelling and haemorrhagic transformation on follow-up scanning
-
associations between baseline perfusion and angiography lesions and 6-month functional outcome
-
test for an interaction between treatment with rt-PA and perfusion lesion extent, presence or absence of mismatch, angiographic arterial occlusion and SICH and 6-month functional outcome.
All analyses were unadjusted and adjusted for key baseline variables using an established prognostic model determined in the IST-3 main trial analysis. 59 We also performed ordinal analysis as this increases the statistical power. 68,88
Secondly, we also compared quantitative perfusion lesion volume with qualitative visual perfusion lesion assessment as coded by the ASPECTS; different perfusion processing algorithms [in this case, the in-house software and MiStar (Apollo Medical Imaging Technology Pty. Ltd, Melbourne, VIC, Australia)]; and test if relative (i.e. to the contralateral hemisphere) parameters are more consistent than quantitative parameters between different software, by comparing (a) the measured volumes of different perfusion parameter lesions, that is mm3, and (b) also by taking account of geometric concordance.
Statistical analyses for the CTA data presented here were performed with Statistical Product and Service Solutions (SPSS) software (v. 20, IBM, New York, NY, USA). Chi-squared testing was used for comparisons between dichotomous data. Simple t-tests were employed to compare normally distributed continuous and dichotomous data, while Mann–Whitney U-testing was employed where continuous or ordinal data were skewed (ASPECT and clot burden scores, HAS length). Similarly, both Pearson and Spearman’s rank-order correlations were applied as appropriate. Significance was taken as p < 0.05.
Any changes to protocol
There were two minor changes to process rather than to fundamental study design.
-
We originally planned to analyse infarct growth as the primary outcome and functional status, with death and SICH as secondary outcomes. However, in view of the clinical importance of functional outcome, and because infarct growth is less clinically relevant to patients, we reordered the primary outcome to be clinical and the secondary outcome to be infarct growth. Additionally, infarct growth can be assessed only in patients with visible infarction – those without a visible infarct do not contribute to this analysis, leading to distorted and potentially misleading results. Hence we focused on the influence of baseline perfusion imaging on clinical outcomes.
-
At the time of original submission, perfusion imaging was thought to be the more important advanced imaging modality to test in stroke and, hence, the focus of planned analysis was on perfusion imaging, which draws heavily on centralised computational analysis. However, in the 4 years since the original submission, angiographic imaging has come into prominence in stroke, and indeed we received far more angiography images than originally expected, almost three times as many as we received of perfusion images. The original planned analysis had been set up for perfusion imaging; angiographic imaging analysis is largely visual and so required a completely different approach. In the event, in order to cope with the number of angiography data efficiently, we had to redesign a visual web-based image viewing and data recording tool (SIRS2) to handle plain scan and angiographic images and then identify several expert neuroradiologists to assist with reading the angiograms. This took extra time, and hence the completion of the angiographic imaging analysis has been delayed. We were, however, fortunate to attract a senior neuroradiology trainee to the project who has been assisting by reading the CT angiograms and measuring thrombus density on a workstation. The results of this latter analysis are included in the report. However, to avoid biasing the analyses, the observers are all still blinded to treatment allocation and the final unblinded analysis has not yet been performed. The unblinded analysis will be presented to the investigators before being presented in public or submitted for publication, as with the perfusion imaging results, and as is proper in clinical trials.
The costs of the programming to redesign the SIRS2 web-based scan-viewing system, the time of the additional neuroradiology expert readers and the neuroradiology trainee’s time to undertake the work on the angiography is all outside the Efficacy and Mechanism Evaluation (EME) funding provided for the original project. There were no other changes.
Patient and public involvement
The IST-3 trial was designed with input from focus group discussions with stroke patients and their carers in the late 1990s. 89 A lay representative was on the IST-3 steering committee and also contributed to the discussions on design of the perfusion and angiography substudy. The lay representative also contributed to the writing of the main trial primary results paper60 and accompanying systematic review. 8 Her input will be sought on all publications arising from the perfusion and angiography substudy.
Chapter 4 Results
Participant flow and recruitment
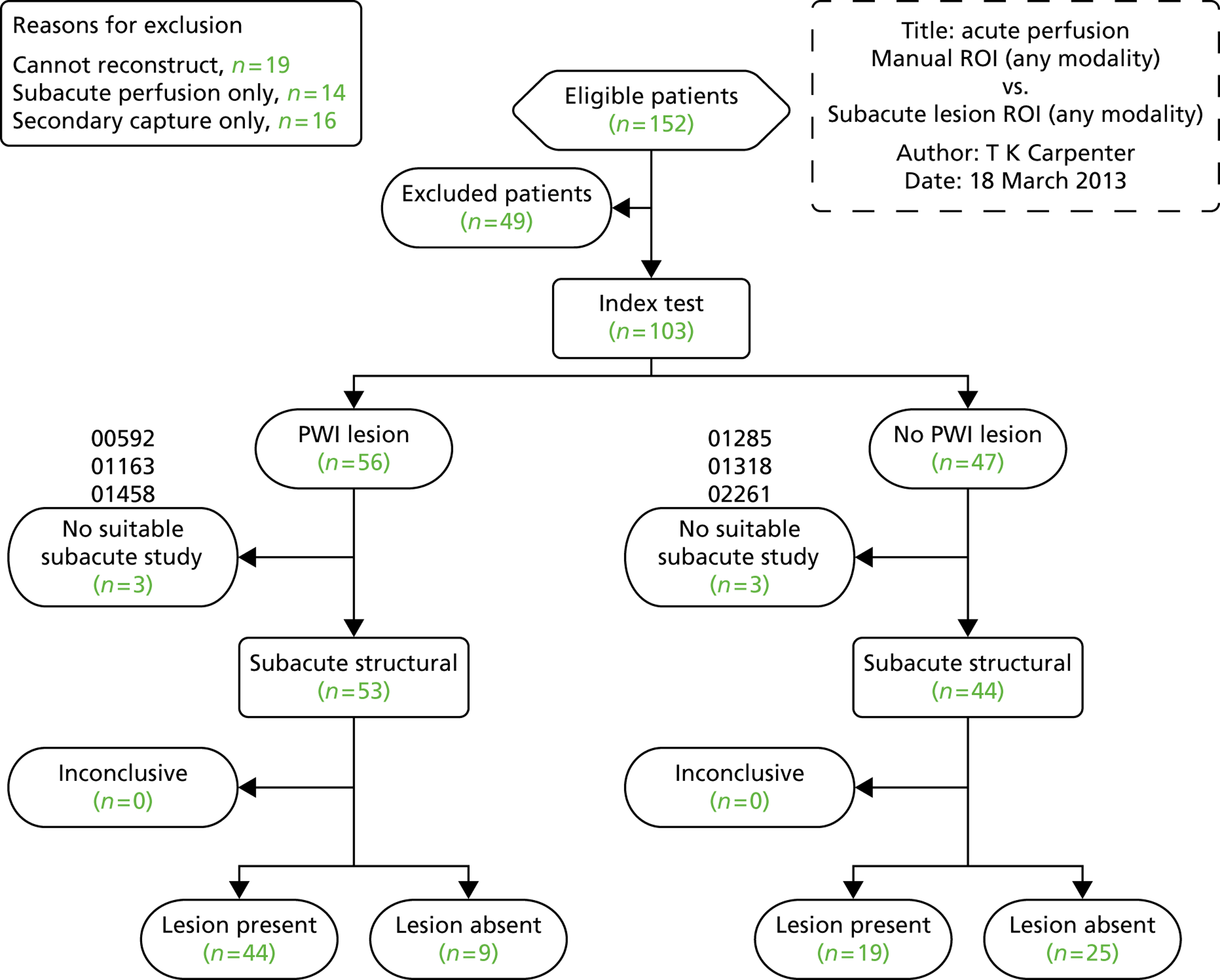
When randomisation ceased in IST-3 in July 2011, 3035 patients had been randomised to rt-PA or control in 156 centres in 12 countries in the main trial [Consolidated Standards of Reporting Trials (CONSORT) diagram; see Appendix 4]. 60 The total patient recruitment in the Perfusion and Angiography Study was 472 patients from 47 centres in eight countries performing CT perfusion and/or angiography and 36 centres in 11 countries performing MR perfusion and/or angiography (the flow diagram for perfusion and angiography patients is shown in Figure 3). The cumulative recruitment with perfusion and/or angiography is shown in Figure 4. The 472 total included 49 patients with only perfusion imaging, 321 patients with only angiography imaging and 102 patients with both perfusion and angiography imaging. At randomisation, 123 patients had perfusion and 265 patients had angiography imaging. At follow-up, 10 patients had perfusion and 116 patients had angiography imaging. A further 18 patients and 42 patients had perfusion and angiography imaging, respectively, at both randomisation and follow-up. Therefore, allowing for some patients having both randomisation and follow-up imaging, the total number of patients with perfusion imaging is 141 at randomisation and 28 at follow-up and with angiographic imaging is 307 at randomisation and 158 at follow-up. The cumulative recruitment according to whether MR or CT was used is shown in Figure 5. Participating centres are listed in Appendix 5.
FIGURE 3.
Flow diagram of recruitment into the perfusion and angiography substudy, the image analysis and final numbers of sufficient quality for statistical analysis. R, randomisation; P, post randomisation.
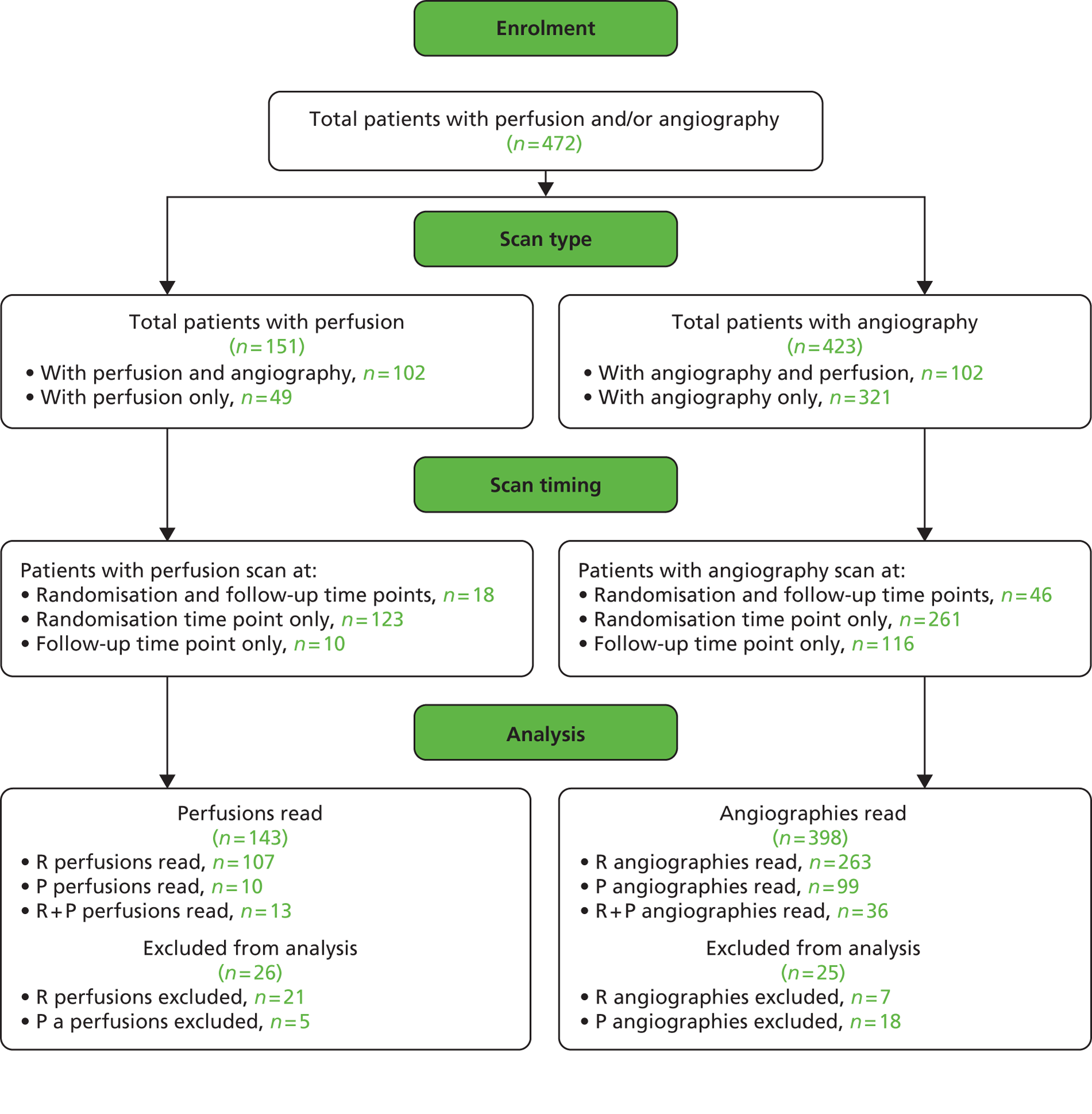
FIGURE 4.
Patient accrual in the (a) IST-3 perfusion study; and (b) IST-3 angiograph study against anticipated targets. Blue lines indicate projected target recruitments; green line indicates actual recruitment.
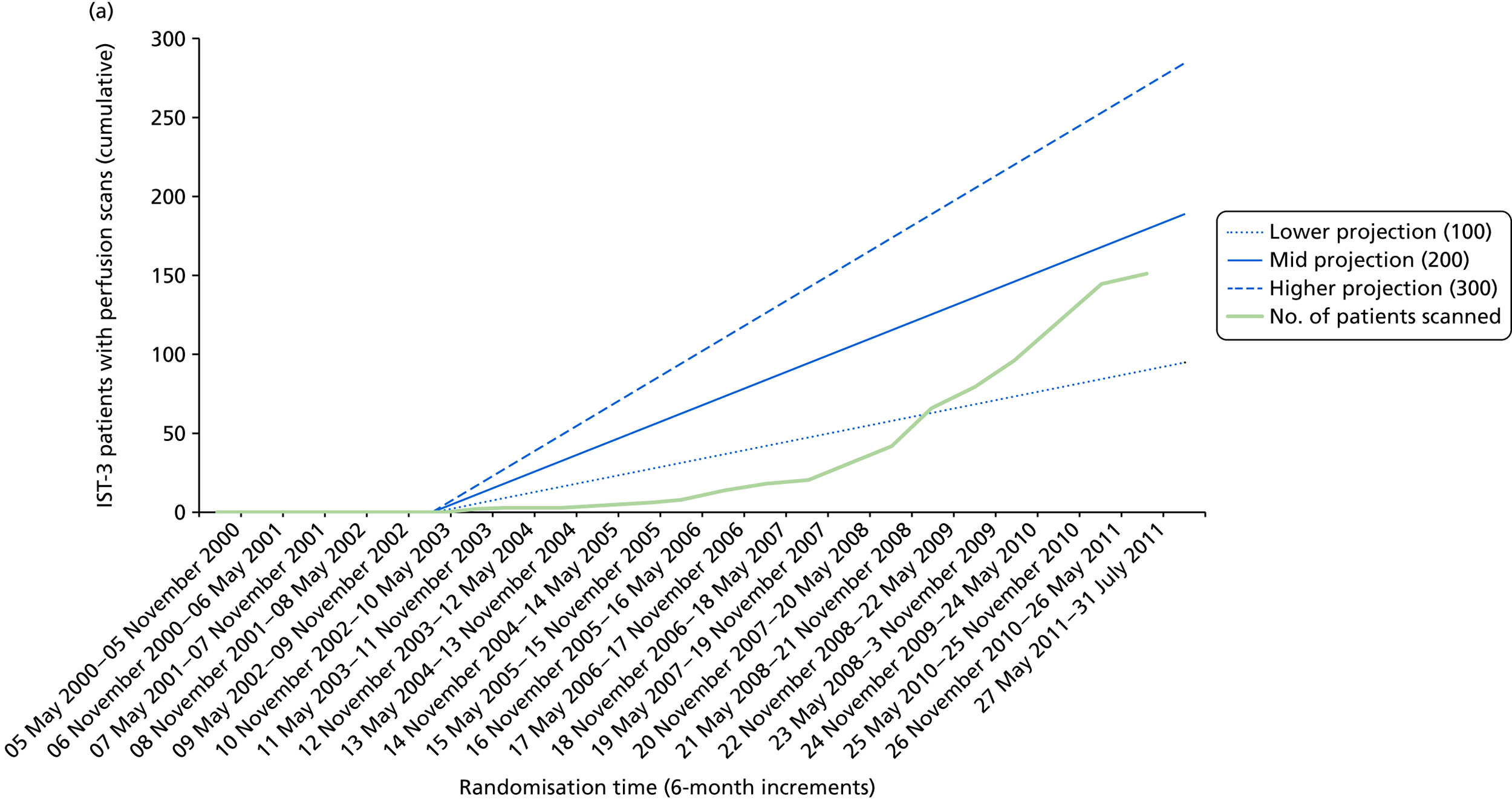
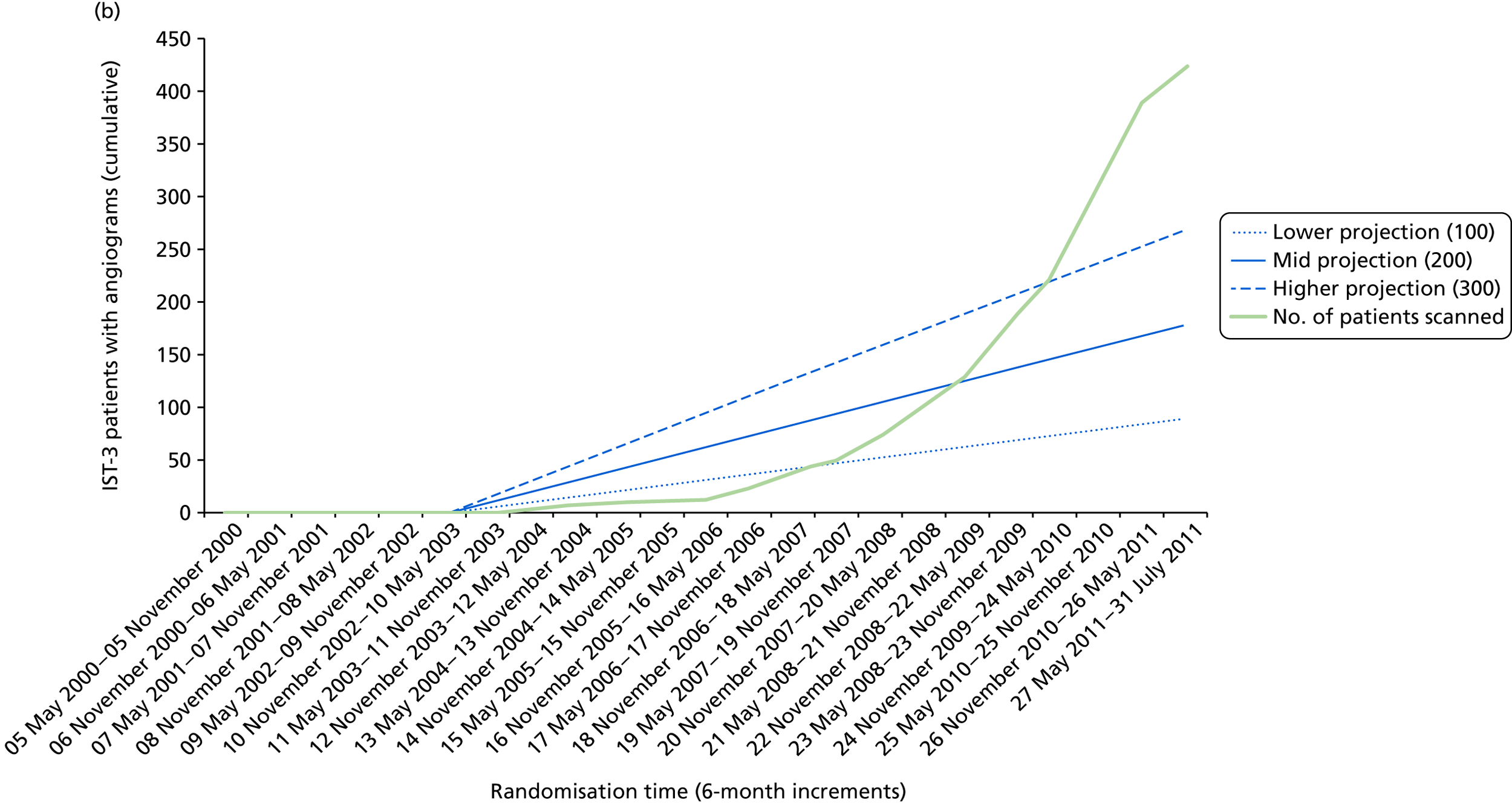
FIGURE 5.
Cumulative recruitment by CT and MR, perfusion and angiography usage.
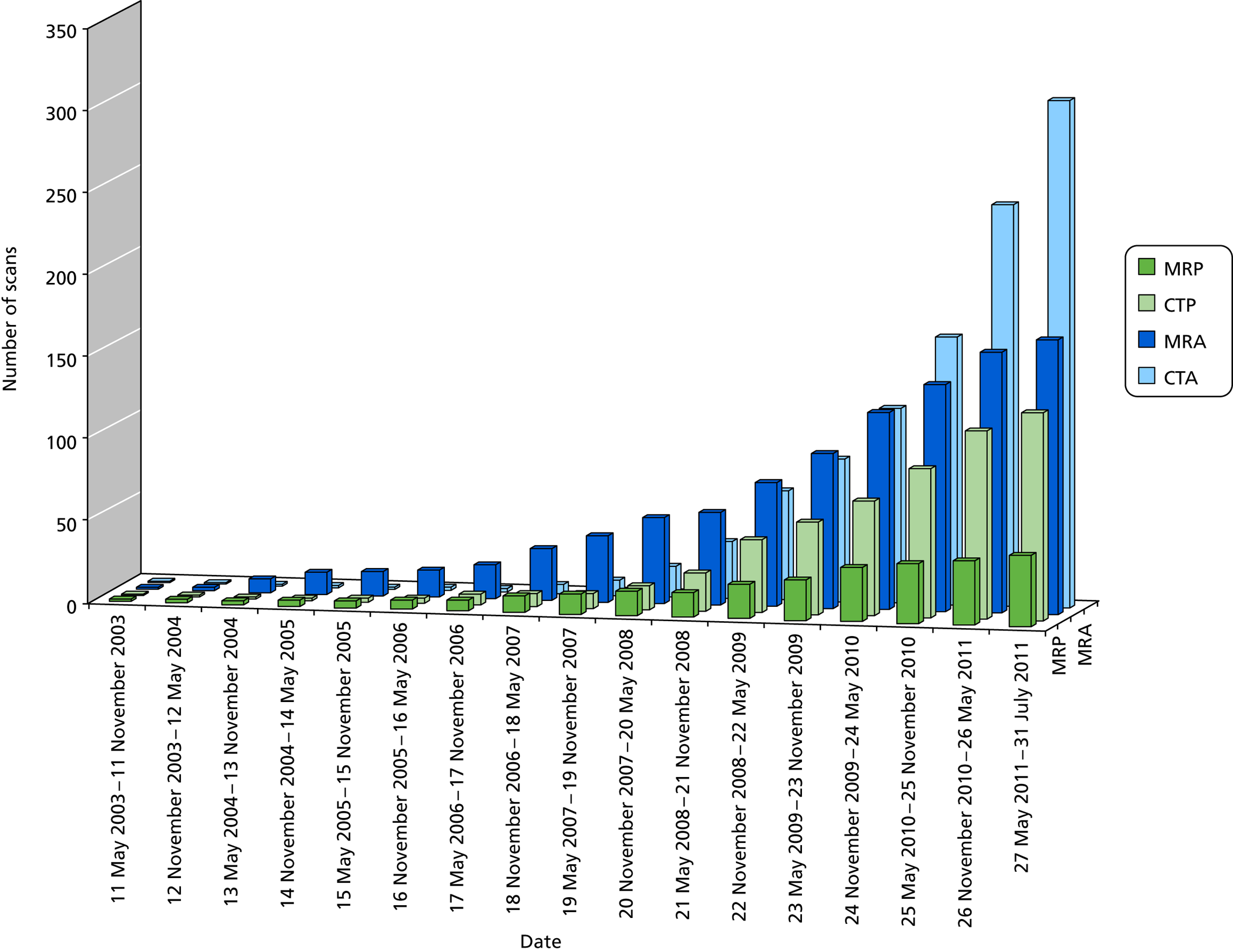
Most imaging at randomisation was with CT and at follow-up was with MR, a consistent pattern throughout the trial. At randomisation, more patients had CT (n = 125/141, 89% perfusion; n = 277/307, 88% angiography) with little MR (n = 16/141, 11% perfusion; n = 30/307, 10% angiography). The expected against actual recruitment is shown in Figure 4. We anticipated recruiting between four and eight patients per year per centre in up to 15 active centres (i.e. between 180 and 360 in total), most of which we expected to be with perfusion imaging. In fact, there were more centres that recruited to the substudy than expected, and angiography proved to be more accessible for acute stroke than perfusion imaging; therefore, we exceeded our overall target, with 472 patients.
Few patients met the prevailing rt-PA licence criteria at the time of recruitment. Only 3 of 121 patients randomised with perfusion imaging met the conditional rt-PA licence criteria as granted in 2003. Considering the period after publication of the European Cooperative Acute Stroke Study (ECASS) III in 2008,6 only eight of 121 patients (8.4%) met rt-PA licence criteria. Only three patients (1%) randomised with angiography imaging met the 2003 conditional licence criteria and only 12 patients (5%) met the criteria after publication of ECASS III in 2008.
Baseline clinical and plan scan data
The baseline demographic data of the patients recruited with perfusion or angiography imaging compared with patients recruited without perfusion or angiography imaging are given in Table 3.
| Baseline characteristic | No perfusion scan, n (%) | Perfusion scan, n (%) |
|---|---|---|
| All | 2894 | 141 |
| Baseline variables collected before treatment allocation | ||
| Region | ||
| North-west Europe (UK, Austria, Belgium, Switzerland) | 1506 (52) | 83 (59) |
| Scandinavia (Norway, Sweden) | 485 (17) | 16 (11) |
| Australasia | 154 (5) | 25 (18) |
| Southern Europe (Italy, Portugal) | 394 (14) | 14 (10) |
| Eastern Europe (Poland) | 345 (12) | 2 (1) |
| Americas (Canada, Mexico) | 11 (0) | – |
| Age (years) | ||
| 18–50 | 118 (4) | 9 (6) |
| 51–60 | 195 (7) | 7 (5) |
| 61–70 | 350 (12) | 15 (11) |
| 71–80 | 693 (24) | 31 (22) |
| 81–90 | 1338 (46) | 69 (49) |
| > 90 | 201 (7) | 9 (6) |
| Sex | ||
| Female | 1497 (52) | 73 (52) |
| NIHSS | ||
| 0–5 | 587 (20) | 25 (18) |
| 6–10 | 810 (28) | 42 (30) |
| 11–15 | 581 (20) | 20 (14) |
| 16–20 | 507 (18) | 36 (26) |
| > 20 | 410 (14) | 17 (12) |
| Delay in randomisation | ||
| 0–3 hours | 806 (28) | 43 (31) |
| 3–4.5 hours | 1131 (39) | 46 (33) |
| 4.5–6 hours | 956 (33) | 51 (36) |
| > 6 hours | 2 (0) | – |
| AF | ||
| Number with AF | 865 (30) | 49 (35) |
| Systolic BP | ||
| ≤ 143 mmHg | 937 (32) | 42 (30) |
| 144–164 mmHg | 977 (34) | 39 (28) |
| ≥ 165 mmHg | 981 (34) | 59 (42) |
| Diastolic BP | ||
| ≤ 74 mmHg | 862 (30) | 45 (32) |
| 75–89 mmHg | 1074 (37) | 55 (39) |
| ≥ 90 mmHg | 940 (33) | 40 (29) |
| Blood glucose | ||
| ≤ 5 mmol/l | 515 (20) | 24 (18) |
| 6–7 mmol/l | 1239 (47) | 63 (46) |
| ≥ 8 mmol/l | 862 (33) | 49 (36) |
| Treatment with antiplatelet drugs in previous 48 hours | 1493 (52) | 69 (49) |
| Clinician’s assessment of pre-randomisation scan | ||
| No evidence of recent ischaemic change | 1739 (60) | 53 (38) |
| Possible evidence of recent ischaemic change | 675 (23) | 26 (19) |
| Definite evidence of recent ischaemic change | 481 (17) | 61 (44) |
| Predicted probability of poor outcome at 6 months | ||
| < 40% | 697 (24) | 32 (23) |
| 40–50% | 312 (11) | 17 (12) |
| 50–75% | 693 (24) | 25 (18) |
| ≥ 75% | 1193 (41) | 66 (47) |
| Stroke syndrome | ||
| TACI | 1249 (43) | 56 (40) |
| PACI | 1094 (38) | 53 (38) |
| LACI | 320 (11) | 12 (9) |
| POCI | 228 (8) | 18 (13) |
| Other | 4 (0) | 1 (1) |
| Baseline variables collected from blinded reading of pre-randomisation scan | ||
| Expert reader’s assessment of acute ischaemic change on initial scan | ||
| Scan completely normal | 255 (9) | 14 (10) |
| Scan not normal but no sign of acute ischaemic change | 1444 (50) | 77 (55) |
| Signs of acute ischaemic change | 1174 (41) | 49 (35) |
| Lesion territory | ||
| Indeterminate | 1704 (59) | 92 (66) |
| MCA or ACA or borderzone | 1098 (38) | 45 (32) |
| Posterior | 56 (2) | 2 (1) |
| Lacunar | 15 (1) | 1 (1) |
| Lesion size | ||
| 0 | 1704 (59) | 92 (66) |
| 1 | 199 (7) | 8 (6) |
| 2 | 470 (16) | 30 (21) |
| 3 | 254 (9) | 7 (5) |
| 4 | 246 (9) | 3 (2) |
| Depth of tissue damage | ||
| None | 1720 (60) | 91 (65) |
| Mild | 957 (33) | 37 (26) |
| Severe | 196 (7) | 12 (9) |
| Degree of swelling | ||
| None | 2207 (77) | 113 (81) |
| Mild sulcal | 525 (18) | 22 (16) |
| Mild ventricular | 139 (5) | 5 (4) |
| Moderate | 1 (0) | – |
| Severe | 1 (0) | – |
| Location of hyperdense arteries | ||
| None | 2164 (75) | 114 (81) |
| Anterior | 678 (24) | 24 (17) |
| Posterior | 31 (1) | 2 (1) |
| Evidence of atrophy | 2211 (77) | 112 (80) |
| Evidence of periventricular lucencies | 1476 (51) | 67 (48) |
| Evidence of old lesions | 1275 (44) | 58 (41) |
| Evidence of non-stroke lesions | 142 (5) | 8 (6) |
| Baseline variables collected from 7-day form | ||
| Pre-trial history of stroke | 670 (23) | 29 (21) |
| Pre-trial treatment with antiplatelet drugs | ||
| Pre-trial treatment with aspirin | 1253 (48) | 53 (39) |
| Pre-trial treatment with dipyridamole | 122 (5) | 3 (2) |
| Pre-trial treatment with clopidogrel | 131 (5) | 15 (11) |
| Pre-trial treatment with anticoagulants | ||
| Warfarin or other oral anticoagulant | 112 (4) | 6 (4) |
| Heparin (low dose) | 20 (1) | – |
| None of the above | 2470 (95) | 131 (96) |
| Pre-trial treatment for hypertension | 1856 (64) | 98 (71) |
| Pre-trial treatment for diabetes | 369 (13) | 19 (14) |
| Phase of trial in which patient recruited | ||
| Blinded | 272 (9) | 4 (3) |
| Open | 2623 (91) | 136 (97) |
| Patients recruited in centre with pre-trial experience of thrombolysis | 1071 (37) | 72 (51) |
| Baseline characteristic | No angiography scan, n (%) | Angiography scan, n (%) |
|---|---|---|
| All | 2728 | 307 |
| Baseline variables collected before treatment allocation | ||
| Region | ||
| North-west Europe (UK, Austria, Belgium, Switzerland) | 1434 (53) | 155 (51) |
| Scandinavia (Norway, Sweden) | 441 (16) | 60 (20) |
| Australasia | 148 (5) | 31 (10) |
| Southern Europe (Italy, Portugal) | 380 (14) | 28 (9) |
| Eastern Europe (Poland) | 315 (12) | 32 (10) |
| Americas (Canada, Mexico) | 11 (0) | – |
| Age (years) | ||
| 18–50 | 113 (4) | 14 (5) |
| 51–60 | 183 (7) | 19 (6) |
| 61–70 | 324 (12) | 41 (13) |
| 71–80 | 641 (23) | 83 (27) |
| 81–90 | 1279 (47) | 128 (42) |
| > 90 | 189 (7) | 21 (7) |
| Sex | ||
| Female | 1398 (51) | 172 (56) |
| NIHSS | ||
| 0–5 | 523 (19) | 89 (29) |
| 6–10 | 769 (28) | 83 (27) |
| 11–15 | 551 (20) | 50 (16) |
| 16–20 | 496 (18) | 47 (15) |
| > 20 | 390 (14) | 37 (12) |
| Delay in randomisation | ||
| 0–3 hours | 760 (28) | 89 (29) |
| 3–4.5 hours | 1092 (40) | 85 (28) |
| 4.5–6 hours | 875 (32) | 132 (43) |
| > 6 hours | 2 (0) | – |
| AF | ||
| Number with AF | 836 (31) | 78 (25) |
| Systolic BP | ||
| ≤ 143 mmHg | 890 (33) | 89 (29) |
| 144–164 mmHg | 916 (34) | 100 (33) |
| ≥ 165 mmHg | 923 (34) | 117 (38) |
| Diastolic BP | ||
| ≤ 74 mmHg | 803 (30) | 104 (34) |
| 75–89 mmHg | 1022 (38) | 107 (35) |
| ≥ 90 mmHg | 885 (33) | 95 (31) |
| Blood glucose | ||
| ≤ 5 mmol/l | 477 (19) | 62 (21) |
| 6–7 mmol/l | 1167 (48) | 135 (45) |
| ≥ 8 mmol/l | 807 (33) | 104 (35) |
| Treatment with antiplatelet drugs in previous 48 hours | 1403 (52) | 159 (52) |
| Clinician’s assessment of pre-randomisation scan | ||
| No evidence of recent ischaemic change | 1627 (60) | 165 (54) |
| Possible evidence of recent ischaemic change | 641 (23) | 60 (20) |
| Definite evidence of recent ischaemic change | 461 (17) | 81 (26) |
| Predicted probability of poor outcome at 6 months | ||
| < 40% | 632 (23) | 97 (32) |
| 40–50% | 290 (11) | 39 (13) |
| 50–75% | 660 (24) | 58 (19) |
| ≥ 75% | 1147 (42) | 112 (37) |
| Stroke syndrome | ||
| TACI | 1207 (44) | 98 (32) |
| PACI | 1010 (37) | 137 (45) |
| LACI | 307 (11) | 25 (8) |
| POCI | 201 (7) | 45 (15) |
| Other | 4 (0) | 1 (0) |
| Baseline variables collected from pre-randomisation scan | ||
| Expert reader’s assessment of acute ischaemic change on initial scan | ||
| Scan completely normal | 247 (9) | 22 (7) |
| Scan not normal but no sign of acute ischaemic change | 1346 (50) | 175 (58) |
| Signs of acute ischaemic change | 1117 (41) | 106 (35) |
| Lesion territory | ||
| Indeterminate | 1598 (59) | 198 (65) |
| MCA or ACA or borderzone | 1047 (39) | 96 (32) |
| Posterior | 50 (2) | 8 (3) |
| Lacunar | 15 (1) | 1 (0) |
| Lesion size | ||
| 0 | 1598 (59) | 198 (65) |
| 1 | 185 (7) | 22 (7) |
| 2 | 450 (17) | 50 (17) |
| 3 | 246 (9) | 15 (5) |
| 4 | 231 (9) | 18 (6) |
| Depth of tissue damage | ||
| None | 1613 (60) | 198 (65) |
| Mild | 912 (34) | 82 (27) |
| Severe | 185 (7) | 23 (8) |
| Degree of swelling | ||
| None | 2073 (76) | 247 (82) |
| Mild sulcal | 504 (19) | 43 (14) |
| Mild ventricular | 131 (5) | 13 (4) |
| Moderate | 1 (0) | – |
| Severe | 1 (0) | – |
| Location of hyperdense arteries | ||
| None | 2023 (75) | 255 (84) |
| Anterior | 658 (24) | 44 (15) |
| Posterior | 29 (1) | 4 (1) |
| Evidence of atrophy | 2082 (77) | 241 (80) |
| Evidence of periventricular lucencies | 1380 (51) | 163 (54) |
| Evidence of old lesions | 1198 (44) | 135 (45) |
| Evidence of non-stroke lesions | 132 (5) | 18 (6) |
| Baseline variables collected from 7-day form | ||
| Pre-trial history of stroke | 633 (23) | 66 (22) |
| Pre-trial treatment with antiplatelet drugs | ||
| Pre-trial treatment with aspirin | 1161 (48) | 145 (48) |
| Pre-trial treatment with dipyridamole | 113 (5) | 12 (4) |
| Pre-trial treatment with clopidogrel | 120 (5) | 26 (9) |
| Pre-trial treatment with anticoagulants | ||
| Warfarin or other oral anticoagulant | 107 (4) | 11 (4) |
| Heparin (low dose) | 17 (1) | 3 (1) |
| None of the above | 2315 (95) | 286 (95) |
| Pre-trial treatment for hypertension | 1731 (64) | 223 (73) |
| Pre-trial treatment for diabetes | 343 (13) | 45 (15) |
| Phase of trial in which patient recruited | ||
| Blinded | 270 (10) | 6 (2) |
| Open | 2459 (90) | 300 (98) |
| Patients recruited in centre with pre-trial experience of thrombolysis | 1014 (37) | 129 (42) |
The median age of the 141 patients randomised in IST-3 with perfusion imaging was 81.0 years, the NIHSS was 11.0, the median time to randomisation was 3.9 hours and 48% were male. These data were identical for the 2986 patients randomised in IST-3 without perfusion imaging.
Of the patients with angiography imaging, we will focus on those with CTA at randomisation as there were many more with CTA than with MRA. The median age of the 271 patients with CTA at randomisation was 81 years [interquartile range (IQR) 71–85 years], the youngest patient was 18 years and the oldest was 102 years; 41.5% were male. The stroke syndrome was TACI in 34%, PACI in 39%, LACI in 10% and POCI in 17%. The median time to pre-randomisation CT (taken from the CT scan) was 2.8 hours (IQR 1.8–4.2 hours), minimum 0.5 hours and maximum 5.4 hours. AF was noted in 61 out of 234 (26.1%) patients at admission.
We were concerned that patients randomised in IST-3 with perfusion or angiography imaging would be different to those randomised with a plain CT or MR scan. However, the only difference in patients with perfusion imaging (but not those with angiography) was that the randomising clinician thought that more patients had a possible or definite visible ischaemic lesion on structural imaging (63% vs. 40%, respectively; p < 0.0001) than patients without perfusion imaging. However, the blinded central expert panel image readings (which were performed without knowledge of the perfusion imaging) showed no difference in the proportion of patients with visible infarction at randomisation (41% vs. 35% had visible infarction on structural scanning with vs. without perfusion imaging; p = not significant). Among the patients randomised with angiography, there was no difference in the proportion with visible infarction according to either the randomising clinician (46% vs. 40%) or the expert panel. Otherwise, there was no difference in age, NIHSS, proportion with AF, predicted outcome, or in any other variables. The blinded expert scan readers did not have access to the perfusion and angiography imaging. This illustrates the importance of separating the perfusion/angiography images from the structural image interpretation when trying to determine the true additional contribution of the perfusion and angiography.
Perfusion imaging
Numbers analysed
We received perfusion imaging on 151 patients in total (see Figure 3). Of these, 10 had perfusion imaging performed post randomisation only. In 21 it was not possible to process the image data, mainly due to incomplete image acquisition, leaving 123 with perfusion imaging at randomisation and that were rated visually. Of these, in 16 patients we did not receive ‘raw’ perfusion data, only the ‘already processed’ screen capture images created on the scanner where the images were obtained, on which it was not possible to measure lesion volume, although it was possible to perform visual scoring. Hence there were more visual readings than volume measures. Additionally, the ‘already processed’ images tended to have fewer perfusion parameters and, therefore, the full list of perfusion parameters was incomplete for some patients. The majority were CT perfusion imaging. Details of the data available for volume measures are given in Figure 3.
Perfusion parameters variation in perfusion lesion size
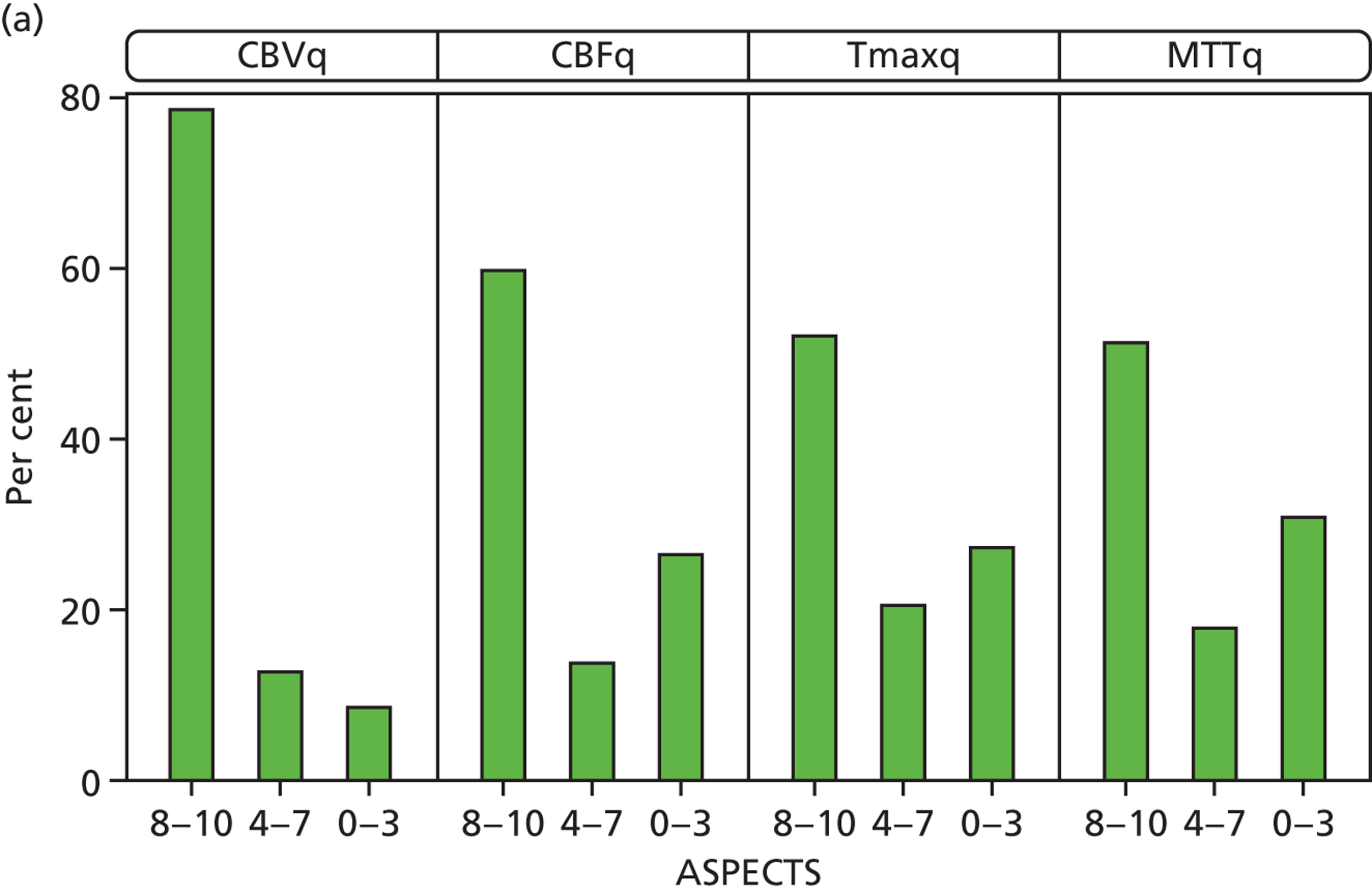
We compared CBVq, CBFq, Tmaxq and MTTq lesions at randomisation using ASPECTS and relative to the plain-scan acute ischaemic lesion. Lesion size was smallest for CBVq; CBVq was significantly smaller than CBFq (p < 0.000), which was the same as Tmaxq, which was significantly smaller than MTTq (p < 0.002) (Figure 6). We found similar lesion size variation when the perfusion lesion was expressed in terms of ‘mismatch’ with respect to the plain-scan lesion size. On Tmaxq, 53 out of 116 (46%) patients had mismatch (perfusion lesion 20% larger by visual estimation than the plain-scan lesion).
FIGURE 6.
Perfusion lesion size (a) on ASPECTSs scores by different parameters: 8–10 is no or small lesion, 0–3 is large lesion; (b) relative to the structural imaging lesion by different perfusion parameters.
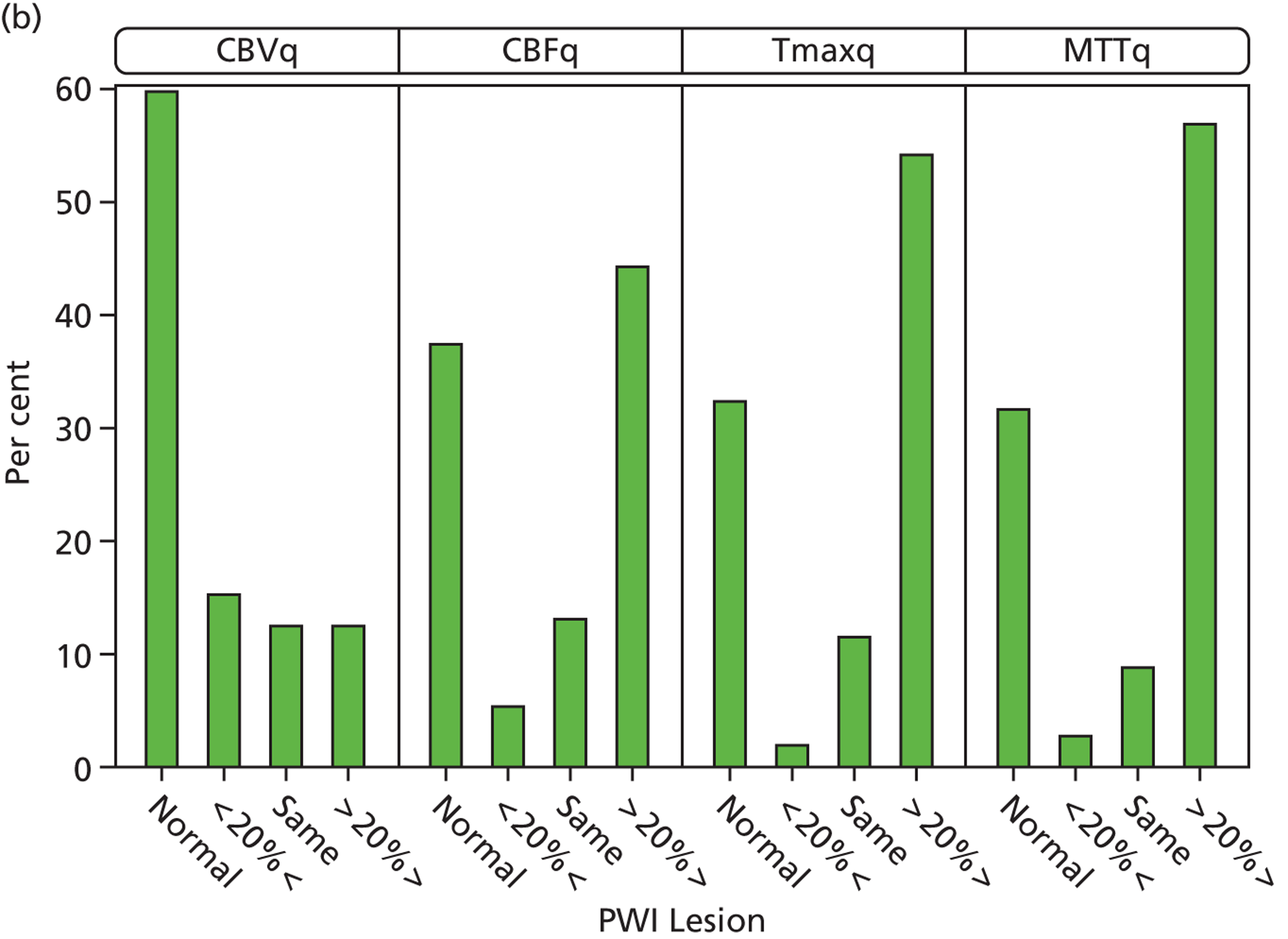
Perfusion parameters and plain scan findings
The acute ischaemic lesion on the plain scan was not significantly different in size to the CBVq perfusion lesion, but was significantly smaller than the CBFq, Tmaxq and MTTq lesion sizes (all p < 0.0000, t-tests). For example, the CBFq lesion had, on average, an ASPECTS that was 2.1 [standard deviation (SD) 3.4] points larger than the plain scan (p < 0.000); the MTTq lesion ASPECTS was 2.7 (SD 3.6) points larger than the plain-scan lesion (p < 0.000).
Perfusion parameters and baseline clinical features
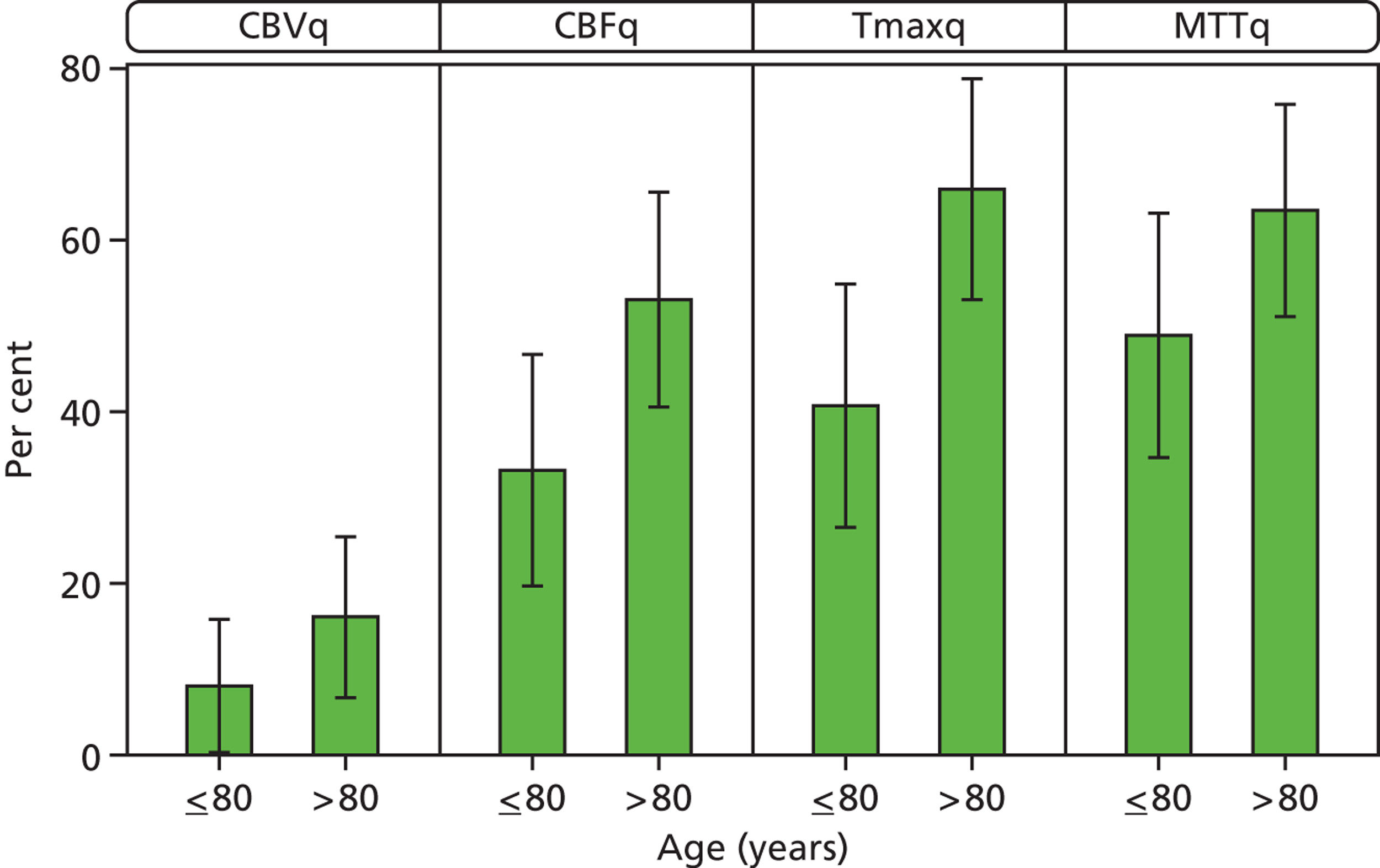
Older patients had larger perfusion lesions on all perfusion parameter maps, and more often had perfusion–plain scan lesion mismatch (Figure 7). For example, in patients aged > 80 years, 67% had mismatch on Tmaxq compared with 41% of patients aged ≤ 80 years (p < 0.04). ASPECTS were lower (i.e. larger lesion) for CBFq and Tmaxq in patients aged >80 years compared with ≤ 80 years (p < 0.05).
FIGURE 7.
Perfusion lesion and plain scan mismatch extent by age ≤ 80 years vs. > 80 years. Error bars represent 95% CIs. Older patients had larger perfusion lesions with more perfusion–plain scan mismatch: CBFq, Tmaxq; p = 0.04. They also had lower ASPECTS: CBFq, Tmaxq; p < 0.05.
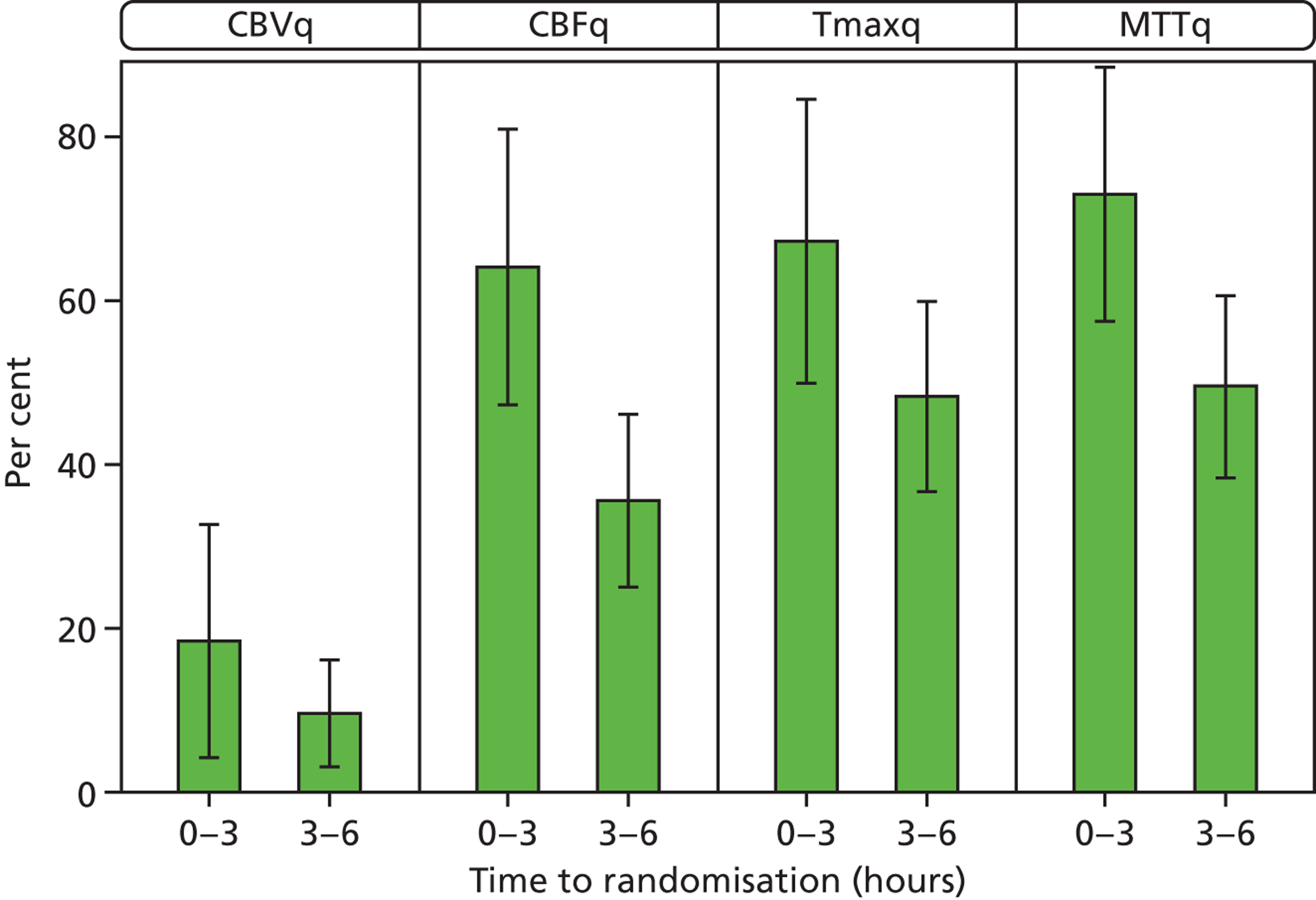
Patients scanned within 3 hours of stroke had larger perfusion lesions (lower ASPECTS) and more often had perfusion–plain-scan lesion mismatch than did patients scanned between 3 and 6 hours after stroke, which was significant for CBFq (ASPECTS < 3 hours, 5.4; 4.5–6 hours, 7.7; p < 0.05) (Figure 8).
FIGURE 8.
Perfusion lesion: mismatch and ASPECTS by time to randomisation 0–3 hours vs. 3–6 hours. Error bars represent 95% CIs. Larger perfusion imaging lesions at 0–3 hours than at 4.5–6 hours: more mismatch; lower ASPECTSs e.g. for CBFq < 3 hours, 5.4; 4.5–6 hours, 7.7; p < 0.05.
There was a strong correlation between increasing stroke severity as measured by NIHSS score and larger perfusion lesions on all perfusion parameters, according to both the ASPECTS and perfusion–plain scan mismatch (Table 4). The Spearman rank-order correlation coefficients ranged from 0.54 to 0.58 (all p < 0.000) for ASPECTS and 0.38 to 0.48 (all p < 0.000) for mismatch.
| Perfusion parameter | NIHSS | |||
|---|---|---|---|---|
| 0 to 5 | 6 to 14 | 15 to 24 | ≥ 25 | |
| CBFq | ||||
| n | 23 | 45 | 39 | 6 |
| Mean ASPECTS | 9.4 | 7.9 | 4.3 | 3.2 |
| CBVq | ||||
| n | 23 | 45 | 39 | 6 |
| Mean ASPECTS | 10.0 | 9.2 | 7.4 | 4.3 |
| MTTq | ||||
| n | 23 | 45 | 39 | 6 |
| Mean ASPECTS | 8.6 | 7.3 | 3.7 | 2.5 |
| Tmaxq | ||||
| n | 23 | 45 | 39 | 6 |
| Mean ASPECTS | 9.0 | 8.0 | 4.1 | 2.8 |
| Perfusion parameter | NIHSS score | |||
|---|---|---|---|---|
| 0 to 5, n (%) | 6 to 14, n (%) | 15 to 24, n (%) | ≥ 25, n (%) | |
| PWI_CBFq | ||||
| Missing | 10 (43.5) | 15 (33.3) | 8 (20.5) | 1 (16.7) |
| Normal | 7 (30.4) | 6 (13.3) | 2 (5.1) | |
| < 20% < (CT or DWI) | 1 (4.3) | 2 (4.4) | 2 (5.1) | |
| Same as (CT or DWI) | 1 (4.3) | 6 (13.3) | 4 (10.3) | 1 (16.7) |
| > 20% > (CT or DWI) | 4 (17.4) | 16 (35.6) | 23 (59.0) | 4 (66.7) |
| PWI_CBVq | ||||
| Missing | 10 (43.5) | 16 (35.6) | 8 (20.5) | 1 (16.7) |
| Normal | 11 (47.8) | 16 (35.6) | 9 (23.1) | |
| < 20% < (CT or DWI) | 1 (4.3) | 5 (11.1) | 10 (25.6) | |
| Same as (CT or DWI) | 2 (4.4) | 9 (23.1) | 2 (33.3) | |
| > 20% > (CT or DWI) | 1 (4.3) | 6 (13.3) | 3 (7.7) | 3 (50.0) |
| PWI_MTTq | ||||
| Missing | 10 (43.5) | 15 (33.3) | 8 (20.5) | 1 (16.7) |
| Normal | 4 (17.4) | 6 (13.3) | 1 (2.6) | |
| < 20% < (CT or DWI) | 1 (4.3) | 1 (2.2) | ||
| Same as (CT or DWI) | 4 (8.9) | 3 (7.7) | 1 (16.7) | |
| > 20% > (CT or DWI) | 8 (34.8) | 19 (42.2) | 27 (69.2) | 4 (66.7) |
| PWI_Tmaxq | ||||
| Missing | 10 (43.5) | 15 (33.3) | 9 (23.1) | 1 (16.7) |
| Normal | 4 (17.4) | 8 (17.8) | ||
| < 20% < (CT or DWI) | 1 (2.2) | 1 (2.6) | ||
| Same as (CT or DWI) | 6 (13.3) | 5 (12.8) | 1 (16.7) | |
| > 20% > (CT or DWI) | 9 (39.1) | 15 (33.3) | 24 (61.5) | 4 (66.7) |
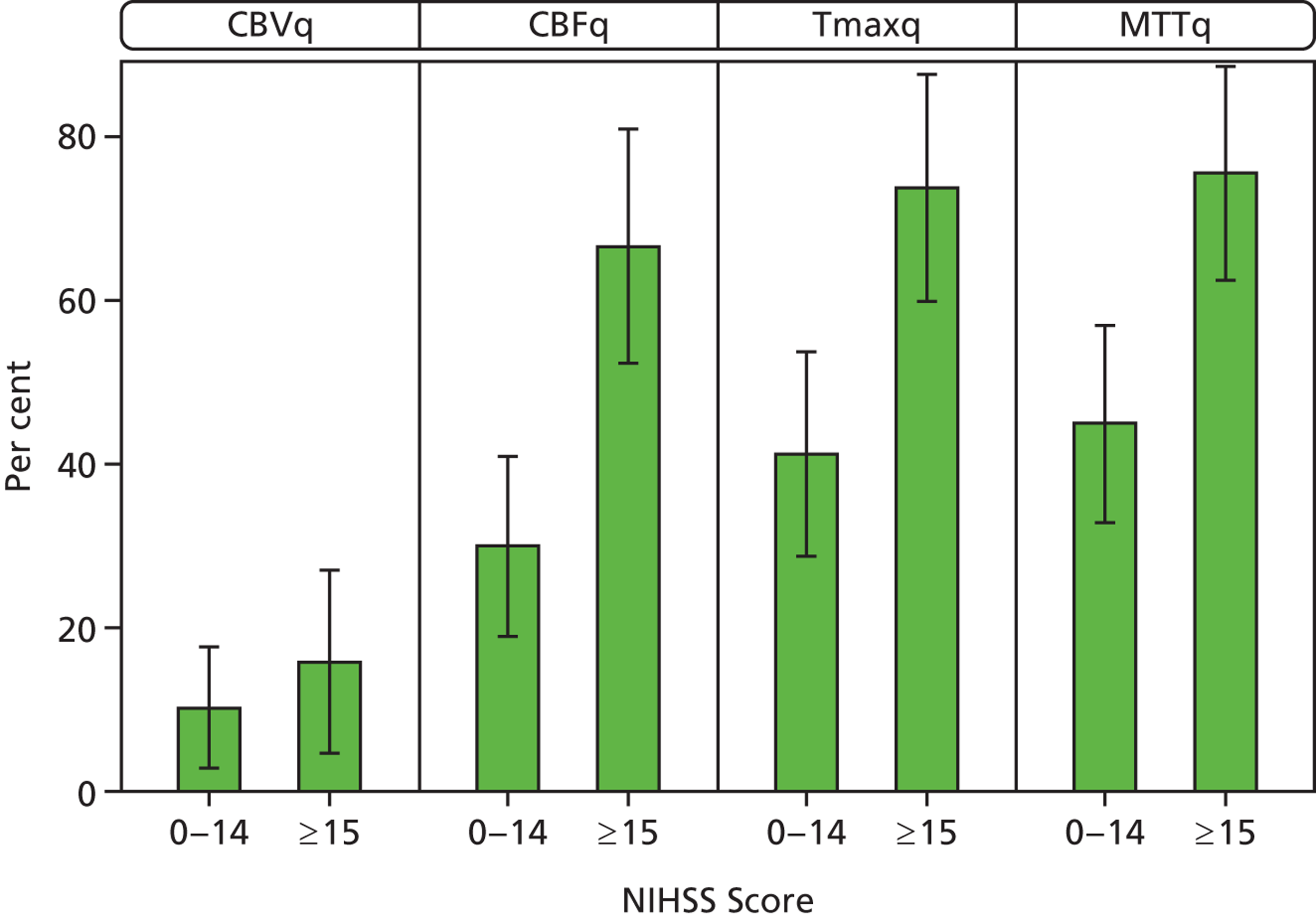
Patients with higher NIHSS scores were also more likely to have mismatch (Figure 9); 72% of patients with NIHSS ≥ 15 had mismatch on Tmaxq or MTTq compared with about 45% of those with NIHSS < 15.
FIGURE 9.
Proportion of patients with perfusion–plain scan mismatch by NIHSS score. Error bars represent 95% CIs.
Perfusion parameters and symptomatic intracerebral haemorrhage, death and functional outcome
Larger perfusion lesions were associated with worse functional outcomes (Table 5). Patients with perfusion lesion–plain-scan mismatch on CBFq, Tmaxq or MTTq were less likely to be alive and independent at 6 months, all significant at the p < 0.05 level on unadjusted analyses; after adjusting for baseline prognosis, only CBFq and MTTq mismatch was significantly associated with worse outcome. Similarly, larger perfusion lesion ASPECTSs were associated significantly with poor functional outcome, for CBVq, CBFq, Tmaxq and MTTq on unadjusted analyses (all p < 0.001); on adjusted analyses, all parameters except MTTq retained their significance. The odds of being alive and independent decreased by about 20% for every point worsening of the ASPECTS, significant for CBVq and Tmaxq.
| Perfusion parameter | n | Dead ≤ 7 days (%) | Symptomatic ICH in 7 days (%) | Dead by 6 months (%) | OHS 0–2 at 6 months (%) | OHS 0–1 at 6 months (%) |
|---|---|---|---|---|---|---|
| PWI_CBFq | ||||||
| Normal | 35 | 2.9 | 2.9 | 5.7 | 65.7 | 31.4 |
| < 20% < (CT or DWI) | 5 | 20.0 | 0.0 | 40.0 | 40.0 | 20.0 |
| Same as (CT or DWI) | 12 | 16.7 | 8.3 | 33.3 | 50.0 | 25.0 |
| > 20% > (CT or DWI) | 49 | 8.2 | 6.1 | 28.6 | 22.4 | 10.2 |
| PWI_CBVq | ||||||
| Normal | 58 | 3.4 | 5.2 | 12.1 | 55.2 | 27.6 |
| < 20% < (CT or DWI) | 16 | 12.5 | 0.0 | 50.0 | 18.8 | 0.0 |
| Same as (CT or DWI) | 13 | 15.4 | 15.4 | 30.8 | 23.1 | 7.7 |
| > 20% > (CT or DWI) | 13 | 15.4 | 0.0 | 23.1 | 23.1 | 15.4 |
| PWI_MTTq | ||||||
| Normal | 30 | 3.3 | 0.0 | 6.7 | 66.7 | 23.3 |
| < 20% < (CT or DWI) | 2 | 0.0 | 0.0 | 0.0 | 50.0 | 50.0 |
| Same as (CT or DWI) | 8 | 25.0 | 0.0 | 50.0 | 50.0 | 25.0 |
| > 20% > (CT or DWI) | 61 | 8.2 | 8.2 | 26.2 | 27.9 | 16.4 |
| PWI_Tmaxq | ||||||
| Normal | 31 | 3.2 | 0.0 | 3.2 | 67.7 | 25.8 |
| < 20% < (CT or DWI) | 2 | 0.0 | 0.0 | 50.0 | 0.0 | 0.0 |
| Same as (CT or DWI) | 12 | 16.7 | 16.7 | 50.0 | 33.3 | 16.7 |
| > 20% > (CT or DWI) | 55 | 9.1 | 5.5 | 25.5 | 29.1 | 18.2 |
| Perfusion parameter | n | Dead ≤ 7 days (%) | Sympt ICH in 7 days (%) | Dead by 6 months (%) | OHS 0–2 at 6 months (%) | OHS 0–1 at 6 months (%) |
|---|---|---|---|---|---|---|
| ASPECTS for CBFq | ||||||
| 0–3 | 31 | 12.9 | 6.5 | 35.5 | 16.1 | 6.5 |
| 4–7 | 16 | 12.5 | 6.3 | 25.0 | 43.8 | 25.0 |
| 8–10 | 66 | 6.1 | 3.0 | 18.2 | 53.0 | 27.3 |
| ASPECTS for CBVq | ||||||
| 0–3 | 10 | 30.0 | 10.0 | 60.0 | 0.0 | 0.0 |
| 4–7 | 15 | 13.3 | 6.7 | 26.7 | 20.0 | 6.7 |
| 8–10 | 88 | 5.7 | 3.4 | 19.3 | 50.0 | 26.1 |
| ASPECTS for MTTq | ||||||
| 0–3 | 36 | 11.1 | 5.6 | 38.9 | 16.7 | 8.3 |
| 4–7 | 21 | 14.3 | 4.8 | 19.0 | 52.4 | 28.6 |
| 8–10 | 56 | 5.4 | 3.6 | 16.1 | 53.6 | 26.8 |
| ASPECTS for Tmaxq | ||||||
| 0–3 | 32 | 12.5 | 6.3 | 34.4 | 15.6 | 9.4 |
| 4–7 | 24 | 12.5 | 4.2 | 33.3 | 37.5 | 25.0 |
| 8–10 | 57 | 5.3 | 3.5 | 14.0 | 57.9 | 26.3 |
More patients with larger perfusion lesion ASPECTSs on all parameters except CBFq were dead at 6 months (p < 0.05 unadjusted), although not on adjusted analyses. There were more patients with SICH and who died within the first 7 days after stroke with larger perfusion lesions on all parameters but these differences were not statistically significant. Similar associations were seen for perfusion lesion size expressed as the perfusion–plain-scan mismatch.
Treatment interaction
We examined for any evidence that the effect of rt-PA was different in the presence of a perfusion lesion mismatch or with increasing perfusion lesion size by testing for an interaction between the perfusion lesion size and rt-PA on the proportion of patients with SICH within 7 days, who had died, or who were alive and independent at 6 months on ordinal analysis (Table 6). We found no evidence that rt-PA effects differed in patients with or without mismatch, or who had large or small perfusion lesions by ASPECTSs on any perfusion parameter. This was true for OHS 0–1, 0–2, SICH, death within 7 days or death within 6 months.
| Perfusion parameter | OHS 0–2 rate | OHS 0–2 risk (%) | Odds | rt-PA effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | rtPA | Control | rtPA | Control | rtPA | Control | Risk ratio | Odds ratio | p-value for interactiona | |
| CBFq | No mismatch (normal/less/same) | 14/27 | 22/37 | 51.9 | 59.5 | 1.077 | 1.467 | 0.872 | 0.734 | 0.701 |
| CBFq | Mismatch (> 20%) | 5/23 | 6/28 | 21.7 | 21.4 | 0.278 | 0.273 | 1.014 | 1.019 | * |
| CBVq | No mismatch (normal/less/same) | 16/43 | 26/55 | 37.2 | 47.3 | 0.593 | 0.897 | 0.787 | 0.661 | 0.246 |
| CBVq | Mismatch (> 20%) | 2/6 | 1/8 | 33.3 | 12.5 | 0.500 | 0.143 | 2.667 | 3.500 | * |
| MTTq | No mismatch (normal/less/same) | 10/21 | 18/28 | 47.6 | 64.3 | 0.909 | 1.800 | 0.741 | 0.505 | 0.225 |
| MTTq | Mismatch (> 20%) | 9/29 | 9/36 | 31.0 | 25.0 | 0.450 | 0.333 | 1.241 | 1.350 | * |
| Tmaxq | No mismatch (normal/less/same) | 9/19 | 17/29 | 47.4 | 58.6 | 0.900 | 1.417 | 0.808 | 0.635 | 0.519 |
| Tmaxq | Mismatch (> 20%) | 8/26 | 9/31 | 30.8 | 29.0 | 0.444 | 0.409 | 1.060 | 1.086 | * |
Ancillary analyses
Lesion volumes were measurable in 56 out of 103 patients with perfusion data that could be processed centrally. Additionally, infarct size was measured on the post-randomisation follow-up scan in 63 patients with a visible lesion. Figure 10 shows the breakdown of patients by lesion presence for analysis. These data are still being analysed.
FIGURE 10.
Perfusion imaging flow chart showing numbers for computational analysis, lesion presence or absence. ROI, region of interest.
Angiography imaging
Analysis by the blinded expert panel is complete but still under analysis at the time of submission of this report. There are 11 raters who have read all 423 scans. The total number of angiograms received is documented in Figure 3.
Of the 307 randomisation CT or MR angiograms received, 277 were CT and 30 were MR; seven of the CT and one MR angiogram were not readable due to incomplete, inadequate (missed correct area of brain) or corrupted data; therefore, analysis is based on 271 CT and 29 MR angiograms at randomisation. Analysis of the 271 patients with CT angiography at randomisation by the neuroradiologist, including measurement of thrombus density scores and interaction with rt-PA, is provided here.
Hyperdense artery sign and clinical findings
Among these 271 patients with CTA at randomisation, a recent acute ischaemic lesion was visible in 74 (27%, Table 7), most in the MCA territory (67 out of 74, 91%, Table 8). The median ASPECTS on these initial scans was 10 (IQR 9–10).
| Ischaemia visible | Initial scan, n (%) | Follow-up scan, n (%) |
|---|---|---|
| Whole group (n = 271) | 74 (27) | 192 (71) |
| HAS (n = 69) | 45 (65) | 62 (90) |
| χ2 | 67; p < 0.001 | 31; p < 0.001 |
| Abnormal CTA (n = 113) | 58 (5) | 103 (91) |
| χ2 | 56; p < 0.001 | 49.0; p < 0.001 |
| Hyperdense artery, n (%) | Abnormal CTA, n (%) | |
|---|---|---|
| ICA | 4 (5.8) | 5 (4.4) |
| ICA and MCA main stem | 15 (13.3) | |
| ICA, MCA main stem, sylvian branch | 7 (6.2) | |
| ACA | 1 (0.9) | |
| MCA main stem | 42 (60.9) | 29 (25.7) |
| MCA main stem and sylvian branch | 2 (2.9) | 26 (23.0) |
| Sylvian branch | 19 (27.5) | 19 (16.8) |
| Vertebral | 5 (4.4) | |
| Basilar | 2 (2.9) | 4 (1.8) |
| PCA | 2 (1.8) | |
| Totals | 69 | 113 |
On plain CT, 69 out of 271 patients (25.5%) had a HAS indicating arterial thrombus. Hyperdensity involved a MCA main stem in 44 cases (64%) and had a mean length of 17.5 mm (SD 8.9 mm). The next most frequent site was the MCA sylvian branch (27%). The mean density within these hyperdense vessels was 51.0 HU (SD 8 HU). This compares with mean densities of non-hyperdense arteries of 40.1 HU (SD 5.6 HU), 40.3 HU (SD 7.0 HU) and 39.2 HU (SD 7.1 HU) for the basilar, left and right MCAs, respectively (these differences are significant, with p < 0.001 in all cases). The density ratio of normal to abnormal vessel was 1.4 in those with a HAS in one artery compared with 1.0 in those without any HAS (p < 0.001).
Patients with a HAS were more likely to have acute ischaemia on the plain CT scan (χ2 = 67; p < 0.001); acute ischaemia was found at the higher-than-background rate of 65.2%.
Patients with a HAS were significantly more likely to be female (χ2 = 4.9; p = 0.028) and have a higher NIHSS score at presentation (mean difference 7.2 points; p < 0.001) than those without a HAS. In addition, patients with a HAS were more likely to have a TACI or PACI syndrome (χ2 = 33.3; p < 0.001) with nearly two-thirds of all TACI being associated with a HAS. There was no difference in age, presence of AF, hypertension, previous stroke or time to CT between those with or without a HAS. Neither HAS length, PMD score (or a combination of the two) nor HAS density were related to NIHSS, time to CT, or the presence of AF.
Computed tomography angiography abnormalities, plain computed tomography and clinical findings
There were 113 out of 253 patients (41.7%) with an abnormality on CTA at randomisation (see Table 8). The MCA main stem was most frequently affected (53%), followed by a MCA main stem plus sylvian branch (30%) or a sylvian branch alone (17%). Among patients with an abnormal artery, the TIMI scores were equally spread across degrees of obstruction from complete to sight. Collateral status was defined as ‘good’ (32.5%), ‘moderate’ (37.3%) or ‘poor’ (30.1%). A ‘poor’ collateral supply was associated with lower initial ASPECT scores (p = 0.014) and an increased likelihood of acute ischaemia on the initial scan (χ2 = 4.8; p = 0.028). Similar trends were demonstrated for ischaemic change on follow-up scans but these changes were not significant.
Patients with an abnormal CTA were more likely to have a visible infarct on plain CT at randomisation (47%, χ2 = 50.6; p < 0.001). Patients with an abnormal CTA were significantly more likely to be female (χ2 = 7.0; p = 0.008), older (median 82 years vs. 78 years without abnormal CTA, mean difference 4.0 years; p < 0.001), have a higher NIHSS score (16 vs. 6, mean difference 10; p < 0.001) and be scanned earlier after stroke (mean difference 0.49 hours; p = 0.005) than those with a normal CTA. Patients with abnormal CTA were more likely to have a TACI or PACI clinical syndrome (χ2 = 58.0; p < 0.001), 59% of all TACIs being associated with an abnormal CTA. AF was associated with abnormal CTA: 50% of patients in AF (32 out of 61) had an abnormal CTA, compared with around 36% (62 out of 173) of those in sinus rhythm (χ2 = 3.8; p = 0.05).
Clot burden score (where 10 indicates no thrombus and 0 indicates all major intracranial arteries and their branches are thrombosed) showed a strong inverse relationship with NIHSS (–0.62; p < 0.001). Clot burden score was also associated with faster time to CT (0.18; p = 0.008). There was a trend towards a similar association with CTA PMD score but this was not significant. Patients with AF compared with those without had more thrombus (lower clot burden score; mean difference −0.72; p = 0.007). Collateral status was not related to NIHSS score.
Hyperdense artery sign compared with computed tomography angiography
The presence of a HAS was strongly and significantly related to the finding of an abnormal CTA (χ2 = 80.3; p < 0.001) (see Table 8). The location of the HAS and CTA abnormality was the same in 63 patients (major vessel involved matched but CTA was more sensitive at detecting thrombus extending distally into small branches); this represents 96.3% of those with a HAS (high specificity) and 69.3% of the CTA abnormalities identified (moderate sensitivity). There were six false-positive HASs (i.e. HAS not associated with any CTA abnormality); despite the appearance of increased attenuation, the measured arterial attenuation in these segments was not significantly different to that within normal vessels (mean 38.3, SD 10.2). A significant inverse relationship of −0.41 (p = 0.001) was demonstrated between the length of the HAS and the clot burden score as calculated from angiography.
Abnormal arteries and follow-up plain scan findings
Follow-up imaging demonstrated a recent infarct in 192 patients (71%), most commonly in the MCA territory (80%), more than double the visible infarction rate on the initial scans. The median ASPECTS on follow-up imaging was 9 (IQR 6–10). Patients with a HAS on the randomisation CT scan were more likely to have ischaemia on follow-up CT (χ2 = 31; p < 0.001), as were those with an abnormal CTA at randomisation (χ2 = 49.0; p < 0.001). All patients with a HAS at randomisation had a visible infarct on the follow-up CT; in 9% of patients, an abnormal CTA was not related to ischaemia on follow-up. Those with a HAS had significantly different IST-3 and ASPECTs on both pre randomisation and follow-up imaging. The median IST-3 scores were 1 at pre randomisation and 3 on follow-up imaging for those with a HAS compared with 0 and 1 in those patients without a HAS (p < 0.001 in both cases). Similarly, the median ASPECTs for those with a HAS were 9 at pre randomisation and 5 at follow-up, compared with 10 and 9 for those without a HAS (p < 0.001 in both cases). Those with a HAS were more likely to undergo a change in IST-3 ischaemia score between pre randomisation and follow-up CT and for that change to be greater than in those without a HAS. Similar results were obtained if this analysis was performed using change in ASPECTS.
There was complete clearance of HAS in 14 cases (23.0%) at follow-up, while three patients developed a new HAS between randomisation and follow-up imaging. Taking all patients with a HAS at either time point, the relationship between time and density of HAS was highly significant, with a correlation coefficient of −0.36 (p < 0.001) (Figure 11).
FIGURE 11.
Relationship between time of scan after stroke and mean density in HU of the hyperattenuated artery sign (HAS).
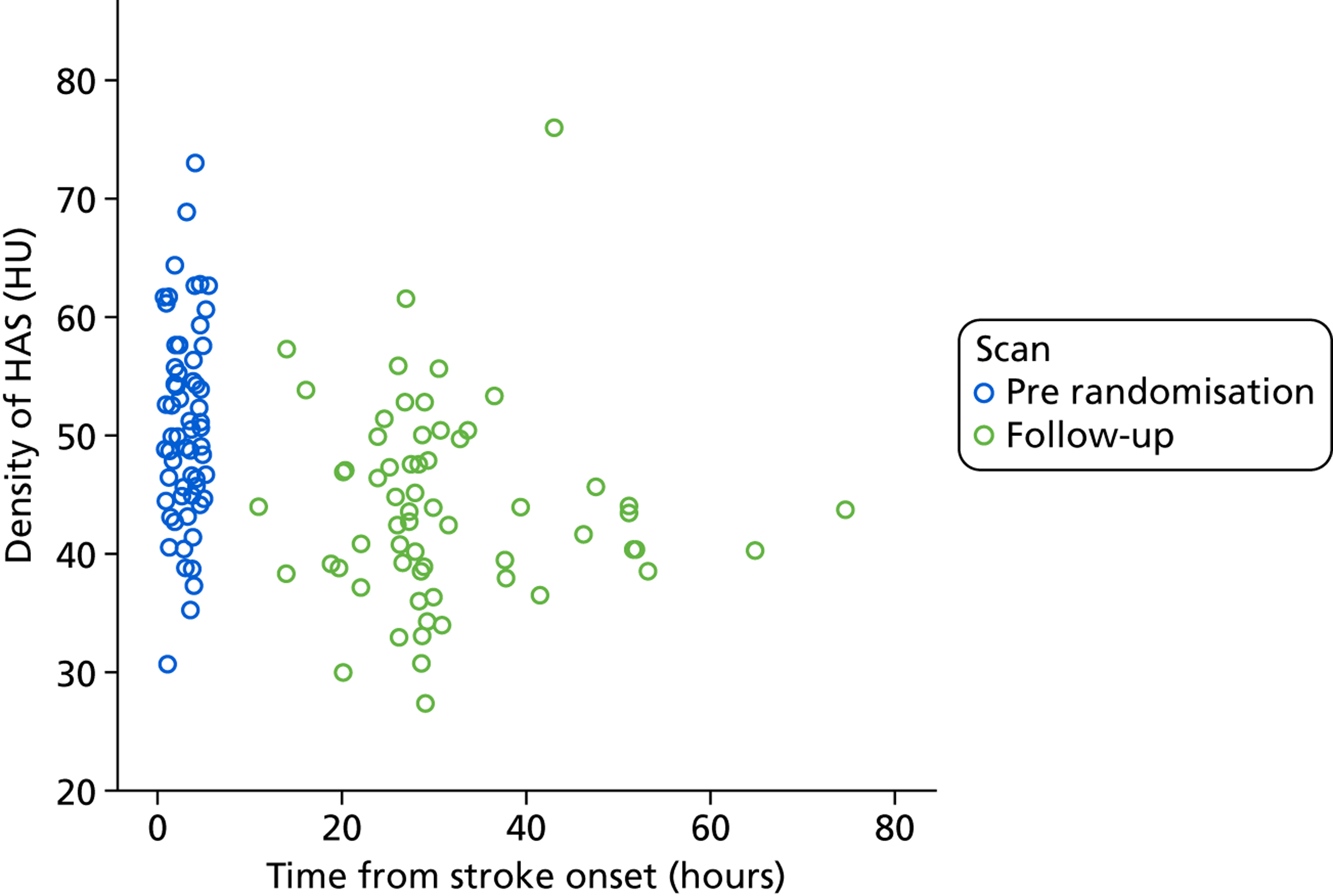
Haemorrhagic transformation was present in 46 out of 271 patients (17%), mostly small areas of petechial haemorrhage (64%) unlikely to be associated with neurological deterioration. More pronounced haemorrhagic transformation (larger haematoma in infarct) was less common (12 patients, 26%).
The presence of a HAS was not associated with SICH. HAS length was, however, found to be greater in those patients with SICH (mean difference 10.2 mm; p = 0.048). This relationship was improved with the inclusion of PMD data (mean difference 33.9; p = 0.012). The density of HAS or the hourly change in this density were not related to SICH. Neither an abnormal CTA, the clot burden score or collateral status was associated with SICH.
We tested the effect of plain CT HAS + visible ischaemic change and CTA (individually or in combination: either, or, both) in predicting a visible infarct on follow-up CT in the 271 patients. The randomisation CT was abnormal (acute ischaemia or HAS) in 37% (95% CI 31% to 43%); CTA was abnormal in 40% (95% CI 34% to 47%); either randomisation CT or CTA was abnormal in 50% (95% CI 43% to 56%); both were abnormal in 27% (95% CI 22% to 33%). The sensitivity and specificity for infarct on follow-up CT were (respectively) of abnormal randomisation CT alone, 57% and 96%; of abnormal CTA alone, 55% and 91%; of both pre-randomisation CT and CTA, 44% and 100% (compared with pre-randomisation CT alone χ2 = 22; p < 0.001); and of either pre-randomisation CT or CTA, 71% and 87% (compared with pre-randomisation CT alone χ2 = 27; p < 0.001) (Figure 12). Thus, combining pre-randomisation CT with CTA in acute stroke significantly increases sensitivity (if either non-contrast CT or CTA are abnormal) or specificity (if both are abnormal) for predicting infarct on 48-hour follow-up CT.
FIGURE 12.
Graph comparing tests with differing sensitivities and specificities. The value of an abnormal non-contrast (plain) CT in predicting infarct on follow-up CT is plotted (dark green marker). Solid lines connect this marker to points 1,1 and 0,0, thereby creating likelihood ratios (represented by the slope of the lines) for the sensitivity and specificity of plain CT. The addition of CT angiography to plain CT in the acute setting improves either sensitivity or specificity but not both. NCCT, non-contrast (i.e. plain) CT.
Hyperdense artery, computed tomography angiography and clinical outcomes
At the end of 6 months, 55 out of 271 patients had died (20%), 93 were dependent (34.3%) and 123 (45.5%) were alive and independent (OHS 0–2). Patients with visible ischaemic change on the randomisation CT were less likely to be alive and independent (mean difference in OHS 1.3; p < 0.001). Similarly, significant inverse correlations were identified between 6-month OHS and ASPECTS (r = −0.29; p < 0.001).
The presence of a HAS was significantly associated with death at 6 months (χ2 = 15.96; p < 0.001). There was no significant association between length of HAS or density of HAS and death. An abnormal CT angiogram was significantly associated with death at 6 months (χ2 = 28.79; p < 0.001). Clot burden scores were significantly lower in those patients who died (mean difference 1.9; p < 0.001). Collateral supply score was not associated with death.
A hyperdense artery was associated with worsening OHS (mean difference 1.7; p < 0.001). There was no significant association between length of HAS or density of HAS and OHS. An abnormal CTA was associated with an increase in OHS (mean difference 2.1; p < 0.001). The clot burden score was significantly inversely correlated with OHS (r = −0.53; p < 0.001). Collateral status was not related to OHS.
Multiple linear regression modelling to assess the predictive value of acute CT signs including visible ischaemic change, the HAS and an abnormal CTA on death and dependency showed that age and either a HAS or an abnormal CTA were independently predictive of outcome (Table 9) but not visible acute ischaemic change on CT. There was no evidence of collinearity. Abnormal pre-randomisation CT in isolation related to worse NIHSS score (seven points higher; p < 0.001) and a greater likelihood of OHS 3–6 (χ2 = 20; p < 0.001). The combined effect of abnormal pre-randomisation CT ± CTA provided very similar results: NIHSS score was eight points higher (p < 0.001) with an increased rate of OHS 3–6 (χ2 = 29; p < 0.001). Neither NIHSS score nor rate of OHS 3–6 was significantly different between the pre-randomisation CT and pre-randomisation CT ± CTA groups (p = 1.000 in both cases). Thus, including CTA in the imaging assessment of acute stroke improves diagnosis by identifying more patients with changes of the acute ischaemic stroke on imaging but does not translate into better prediction of prognosis over simple clinical variables (age, NIHSS score) and plain CT findings alone.
| Model | Partial correlations | p-value |
|---|---|---|
| Death within 6 months R2 = 0.12; p < 0.001 | ||
| Age | –0.19 | 0.003 |
| Sex | 0.08 | NS |
| Acute ischaemia | –0.08 | NS |
| Hyperdense artery | –0.18 | 0.007 |
| Model | Partial correlations | p-value |
|---|---|---|
| Death within 6 months R2 = 0.16; p < 0.001 | ||
| Age | –0.17 | 0.011 |
| Sex | 0.07 | NS |
| Acute ischaemia | –0.05 | NS |
| Abnormal CTA | –0.26 | < 0.001 |
| Model | Partial correlations | p-value |
|---|---|---|
| OHS R2 = 0.24; p < 0.001 | ||
| Age | 0.32 | < 0.001 |
| Sex | –0.08 | NS |
| Acute ischaemia | 0.13 | NS |
| Hyperdense artery | 0.25 | < 0.001 |
| Model | Partial correlations | p-value |
|---|---|---|
| OHS R2 = 0.30; p < 0.001 | ||
| Age | 0.28 | < 0.001 |
| Sex | –0.07 | NS |
| Acute ischaemia | 0.09 | NS |
| Abnormal CTA | 0.37 | < 0.001 |
Interaction between computed tomography angiography findings and recombinant tissue plasminogen activator treatment effect
Of the 271 patients with pre-randomisation CTA, 142 patients were randomly assigned rt-PA and 129 to control. The odds ratios (ORs) for the effect of rt-PA on early and late outcomes, in the presence compared with absence of occlusion on CTA, were symptomatic haemorrhage 1.41 (95% CI 0.86 to 2.31), death 1.23 (95% CI 0.70 to 2.17), and independent survival (OHS 0–2) 1.39 (95% CI 0.59 to 3.27). This indicates no significant interaction between rt-PA and the presence or absence of occlusion on CTA.
Additional analysis of hyperdense artery sign and recombinant tissue plasminogen activator effect
We assessed the effect of rt-PA in patients with or without the HAS in all patients in IST-3 who had a plain CT scan at randomisation and for follow-up (n = 2730). Some patients who had MR at one or other time instead of CT were excluded from this analysis and randomisation CT scans for 19 out of 3035 patients were not received in the central trials office. There were 674 patients randomised in IST-3 with a HAS (24.7% of 2730) and 2056 without a HAS (75.3% of 2730). The clinical and imaging features and associations with SICH and 6-month outcomes were the same for the whole trial as for the subset with angiography imaging reported above.
We will, therefore, not repeat those findings here. Patients allocated to rt-PA were more likely to have the HAS shrink or disappear between randomisation and follow-up scans (OR 1.53; p = 0.011 and OR 1.49; p = 0.010 respectively) and a trend towards lower likelihood of new HAS formation (OR 1.25; p = 0.141). An analysis of the interaction between rt-PA and presence or absence of a HAS on 6-month functional outcome in all IST-3 patients using adjusted ordinal regression analysis is ongoing.
Ongoing analyses
Further analyses pending are as follows.
Perfusion
We plan to compare the:
-
volume of the perfusion lesions as seen on the different perfusion parameters (similar analysis to the comparison of perfusion lesion size using the visual assessment)
-
visual with volume assessment of perfusion lesion size
-
perfusion lesion volume with baseline plain-scan early infarct signs, with clinical parameters (age, NIHSS score, time), with imaging (infarct extent at follow-up) and clinical outcomes (SICH, death within 7 days, death and OHS at 6 months).
Finally, we will determine whether or not perfusion lesion volume interacts with rt-PA effects. However, as the sample size will be less than for the analysis using visually scored perfusion lesions, we think it unlikely that use of volumes will alter the conclusions.
We will also examine the effect of perfusion imaging on early infarct diagnosis by comparing the interpretation of the plain scan performed with knowledge of the perfusion lesion with the plain scan interpretation performed without knowledge of the perfusion lesion by the expert panel of readers.
A large study of observer reliability of perfusion imaging interpretation is being established. Twenty representative cases have been identified showing a range of perfusion abnormalities and plain-scan findings. This will be made available over the web using the SIRS2 tool and we will invite as many interested radiologists, neurologists and stroke physicians as possible to participate as we did when testing observer reliability for plain scan findings in the Acute Cerebral CT Evaluation Stroke Study (ACCESS). 15,90
Angiography
We will assess whether or not knowledge of angiography influences the plain-scan interpretation (i.e. increases the detection of early ischaemic changes) by comparing the plain-scan interpretation performed by the central expert panel without knowledge of angiography with the plain scan interpretation performed by the (a) expert panel using the SIRS2 tool and (b) the single neuroradiologist on the workstation with knowledge of angiography.
Chapter 5 Discussion
The Third International Stroke Trial is the largest RCT of rt-PA compared with control in acute ischaemic stroke. It confirmed the benefit of rt-PA in a wide range of patients, most of whom did not meet the prevailing licence criteria at the time. 60 Most IST-3 patients (> 95%) did not meet the prevailing licence criteria because they were outside the time window, older than the upper age limit, or had comorbidities or contraindications to rt-PA. IST-3 is also the largest RCT of rt-PA compared with control with perfusion imaging or angiography at randomisation and, therefore, provides valuable and reliable evidence on the role of perfusion or angiography imaging in the assessment of patients prior to rt-PA.
The IST-3 perfusion and angiography study demonstrates, by the numbers recruited with each imaging modality, that CT is the easiest acute stroke imaging tool, much easier or more available/accessible than MR, and that angiography imaging is easier to obtain than perfusion imaging. Although we had few perfusion data from the same patients, obtained closely enough in time, to compare CT and MR perfusion directly, the large proportion of perfusion imaging at randomisation that was based on CT suggests that CT is much more accessible and practical for use in acute stroke than is MR. 91
We also demonstrate that visual lesion assessment allows use of more data, and therefore provides a larger sample size, than does computational lesion volume measurement because the human observer can interpret more data than can be analysed by computational lesion volume methods: this includes ‘screen capture’ data (i.e. where the centre sent the perfusion image created by local processing but was not able to send the raw perfusion image data for central processing) and scans that are of quality that still produces a readable image but that are of insufficient quality for computational processing (e.g. where the patient has moved). As in other spheres of image interpretation and analysis,92 visual assessment and computational analysis are complementary.
We show that visual assessment of perfusion lesion size and of mismatch between the perfusion lesion and plain scan lesion is associated with early clinical features, early and late clinical and early imaging outcomes. We found that perfusion lesion visibility, size and mismatch differed widely depending on which perfusion parameter was used – this has been demonstrated previously22,76 but not with visual lesion assessment (only volumes).
We demonstrate other novel findings, notably that perfusion lesions were larger (and mismatch more frequent) in older patients, in patients scanned early after stroke and (demonstrated previously) in patients with worse NIHSS scores. Most studies using perfusion imaging in acute stroke have included predominantly younger patients than were in IST-3. 71 They have also mostly used perfusion imaging to identify patients with persistent ‘tissue at risk’ at later times after stroke. An important consequence of the IST-3 findings is that studies which aim to extend the time window to thrombolysis by using perfusion lesions or perfusion–plain-scan lesion mismatch as a way of identifying potentially salvageable tissue at late times after stroke, and which also exclude older patients, are likely to be seeking a very small target population that is not representative of most patients. A second consequence is that most patients with a moderate or severe stroke will have a perfusion lesion/mismatch within the first few hours after stroke, leading one to question the value of performing perfusion imaging soon after stroke to look for mismatch if there is a high likelihood that all patients will have it based on clinical grounds. 30,93 In other words, most patients with a moderate or severe stroke are likely to have salvageable tissue in the first few hours after stroke.
As noted in previous studies, larger perfusion lesions were associated with worse functional outcome. 27,35 However, we found no evidence of a perfusion lesion/mismatch–rt-PA interaction, that is to say no evidence of a different effect of rt-PA in patients with perfusion lesions or mismatch with compared with those without. This may be because the perfusion study did not have a large enough sample size, or because the patients were older in IST-3, or that any differential effect of rt-PA in patients with perfusion imaging compared with those without is small, or that if most patients have a perfusion lesion, and rt-PA works a bit in most patients, then we should not expect there to be much difference between those with and without a perfusion lesion. A further possible explanation is that the benefit of rt-PA may primarily arise from reperfusion of the ischaemic lesion core and that CT perfusion may overestimate the core or alternatively underestimates penumbra. However, rt-PA is generally thought to rescue penumbra from progressing to infarction rather than rescuing core, which is thought already to be dead. Regarding patient age, we found no evidence of lack of benefit of rt-PA at older ages in primary IST-3 analyses. It is possible that perfusion imaging provides different information or performs differently in older patients but there is no information on that as most previous studies excluded patients over the age of 80 years. However, we have no good reason to think that perfusion imaging provides different information in patients aged over 80 years. As these older patients are more likely to have atrophy, old infarcts and white matter changes which adversely affect identification of acute ischaemic changes, perfusion imaging might (a) increase diagnostic certainty that the patient is having a stroke and (b) show altered perfusion in periventricular white matter indicating underlying leukoaraiosis (noted anecdotally in some of our patients). This would be a point for further study. We also cannot exclude the possibility that rt-PA effect differs in patients with mismatch imaged very early after stroke.
How did the perfusion substudy compare with other data?
Our findings are consistent with systematic reviews of previous thrombolysis observational studies which provided information on perfusion imaging and mismatch34 or trials and mismatch33 which did not find that outcomes were materially different in patients with mismatch who received rt-PA compared with those without mismatch who received rt-PA, that is to say they did not find a rt-PA–mismatch interaction.
The EPITHET trial is the only other randomised trial of rt-PA compared with control with perfusion imaging at randomisation and it included 98 patients. 30 It also only recruited between 3 and 6 hours after stroke, and although it did not have an upper age limit, it mainly included patients younger than 80 years. The primary analysis did not show benefit for rt-PA on functional outcome or on reduction in infarct growth, although numerous secondary publications have suggested that rt-PA reduced infarct growth if calculated in other ways. We have avoided performing these additional analyses on subsets of data: at best, this might be useful exploratory work. An individual patient data meta-analysis of all perfusion–diffusion imaging from randomised trials, with further central standardised analysis of imaging data, might provide useful information on effects of age, time to treatment, background brain changes and perfusion imaging and rt-PA response if a large enough sample size could be generated.
The series of trials testing desmoteplase, a novel recombinant plasminogen activator derived from vampire bat saliva which is thought to have better clot specificity (DIAS trials28,29,31) given between 3 and 9 hours after acute ischaemic stroke, only included patients with diffusion–perfusion mismatch (and therefore did not assess the effect of thrombolysis in patients without a perfusion lesion or mismatch) or with angiographic large artery occlusion (and therefore did not test the effect of thrombolysis in patients without visible arterial occlusion). The ongoing DIAS 3 and 4 trials are testing desmoteplase in patients with angiographic occlusion.
The tenecteplase in acute stroke trial was a RCT of rt-PA compared with tenecteplase (Metalyse®, Boehringer Ingelheim) in 75 patients with a perfusion lesion–plain CT lesion mismatch of at least 20% and an arterial occlusion on CTA. 94 Tenecteplase reduced infarct growth compared with rt-PA but there was no difference in functional outcome.
The Mechanical Retrieval and Recanalisation of Stroke Clots Using Embolectomy (MR Rescue) study95 randomised 118 patients within 8 hours of large artery anterior circulation stroke to mechanical embolectomy or standard care, stratified according to whether or not the patient had perfusion: plain-scan mismatch suggesting a large area of salvageable tissue (favourable penumbral pattern) or not. The mean age was 65.5 years, mean time to enrolment was 5.5 hours and 58% had a favourable penumbral pattern. There was no evidence that the favourable penumbral pattern identified patients who would differentially benefit from endovascular therapy and endovascular therapy was not superior to standard care. This latter result was consistent with the IMS trial,43 published simultaneously with MR Rescue, that also showed no benefit for mechanical thrombus extraction devices over i.v. rt-PA alone.
Other randomised trials involving perfusion or angiography imaging are ongoing. The ECASS 496 and EXTEND (EXtending the time for Thrombolysis in Emergency Neurological Deficits)97 trials plan to use perfusion imaging in patient selection. However, the ECASS 4 trial steering committee have just decided to proceed with the trial without implementing specific perfusion software80 owing to difficulties in implementing the software in participating hospitals.
Many observational cases series and multicentre studies have described associations between early clinical features or clinical outcomes in patients with CT or MR perfusion lesions or mismatch (by a variety of definitions). Theory suggests that patients with mismatch or visible arterial occlusion ‘have a lesion to treat’ and therefore should respond more to thrombolysis and, therefore, that thrombolysis use should be restricted to patients with mismatch or a perfusion lesion or arterial occlusion. However, it is important to note that this theory can only be tested in a trial where patients with a range of perfusion or angiographic imaging findings are randomly allocated to rt-PA or placebo. The following studies (on which much of current thinking about use of advanced imaging technologies is based) are observational and do not provide data on whether or not rt-PA is more effective in patients with mismatch or occluded arteries. The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE)27 and DEFUSE 298 observational studies of rt-PA in patients, all of whom had perfusion–diffusion imaging mismatch, suggested that rt-PA was most effective in patients with ‘target mismatch’ where the perfusion lesion exceeded the diffusion lesion by more than 20% by estimated volume. However, we have shown that rt-PA is effective in a wide range of patients, not just those with mismatch, and others have shown that mismatch does not identify a group of patients who benefit more or less from either rt-PA30 or mechanical thrombus extraction devices95 (as above). The evidence from RCTs is likely to be more reliable than that from observational studies. The use of mismatch delays treatment. It is possible that the loss of viable tissue while waiting for the perfusion scan outweighs any benefit from the additional information, or that any differential effect in patients with mismatch compared with those without is very marginal due to the dynamic nature of the stroke lesion. Worse, recent evidence from the USA suggests that confusion over whether or not or when to use perfusion or angiography imaging is actually hampering use of rt-PA, preventing treatment rates from reaching many patients who would otherwise be eligible. 99
Visual rating of perfusion lesions with ASPECTS or a simple ‘mismatch’ rating shows relevant imaging and clinical associations and is practical in the acute situation. We will be able to compare the visual ratings with lesion volume measurements and also to test the observer reliability of the visual ratings in the next phase of analysis. The only previous study comparing visual and computational assessment found good agreement between the two. 100 Local investigators saw more plain-scan early ischaemic signs in the patients with perfusion imaging than in those without, although the blinded central expert adjudication saw no such difference. It may be, as others have suggested, that perfusion imaging increases confidence in the diagnosis of acute ischaemic change among less expert scan readers. 101
Angiography is an easier technique to use in acute stroke. For CT angiography, the HAS is highly specific (96%) and moderately sensitive (63%) for arterial occlusion. The density of the HAS decreases with time consistent with clot lysis and about 25% have completely cleared by 24–48 hours. The extent of the HAS correlates well with the extent of occlusion on CTA. Both HAS and abnormal CTA are significantly associated with CT identifiable infarct developing at follow-up (parenchymal hypodensity). HAS and abnormal CTA both related to clinical stroke severity (independent of age, sex and extent of parenchymal hypodensity). The presence of a HAS and abnormal CTA both independently increase the risk of poor functional outcome. Including CTA in the acute assessment of stroke improves the detection rate of abnormal scans but neither extent of deficit nor outcome is different in these patients. Apart from confirming the previously described association between HAS and AF, we did not find any association between likely thrombus source and thrombus density. Further analyses are required to see if rt-PA effect differs with HAS density and, therefore, HAS density should influence rt-PA treatment decisions.
Angiography delays time to treatment, although by less than perfusion imaging. Angiography is less complex to interpret than perfusion imaging, although there are few data on observer reliability, especially among non-experts (for either technique). Perfusion imaging influenced the local investigators to see more acute ischaemic changes on plain imaging, but there was no actual difference in plain scan findings as the expert panel saw no difference in plain scan findings between patients with and without perfusion imaging. The local investigators were less influenced by the angiography imaging in their interpretation of the plain scans as there was no difference in their detection rate of plain scan abnormalities between patients with angiography and without. This may be because they did not see any angiographic abnormality or because the HAS (which is one of the easier acute ischaemic signs for less experienced observers to identify15) closely mirrors the CTA findings. Whether or not there is any interaction between CTA abnormalities and rt-PA effects is the subject of ongoing analyses.
Recommendations for future research
The effect of perfusion and angiography imaging on physician confidence in making a diagnosis of acute ischaemic stroke, and the effect that that has on decisions to treat with rt-PA, should be tested in a future trial where patients are randomised to receive or not to receive perfusion imaging or angiography prior to rt-PA. Such a trial (PRACTICE) is now funded by the Health Technology Assessment panel of the National Institute for Health Research (NIHR) and is being initiated. Several groups have tested whether or not different thresholds of perfusion parameters can identify core compared with penumbra more precisely. However, independent testing in a separate cohort (e.g. IST-3) should be performed prior to any testing in further trials or clinical practice. At the time of writing, the ECASS 4 trial has just decided not to use perfusion image processing software at the point of acquisition due to problems with implementation. In future, studies should evaluate the effects of age and background brain changes (e.g. leukoaraiosis) on perfusion lesion visualisation and lesion growth. Perfusion and angiography imaging technologies continue to advance and significant advances may require further testing in new trials. Further attempts should be directed to better standardisation of perfusion imaging and to understand sources of variability of perfusion values in the perfusion image. Further research is required to reduce the observer variability of angiography interpretation. Recent publications from the STIR group should help this process. 102,103
Recommendations for practice
There is no indication from these results that perfusion or angiography imaging, however processed, interact with rt-PA. In other words, there was no group of patients identified by perfusion or angiography criteria who benefited more or were harmed more by rt-PA. Hence there is no indication at present for routine use of perfusion or angiography imaging prior to decisions on use of rt-PA. Both perfusion and angiography imaging identified patients with more severe stroke, and perfusion-imaging lesions were larger in older patients and in those scanned soon after stroke. Thus, both perfusion and angiography imaging provide powerful prognostic information but do not provide information that indicates if some patients are more or less likely to benefit from rt-PA. The large variation in perfusion lesion size depending on which parameter is being displayed means that anyone wishing to use perfusion imaging in clinical practice should be very careful that they understand which parameter they are looking at and that they understand what it shows. Doctors should be aware of the limited information on observer reliability on interpretation or perfusion or angiographic imaging when interpreting such images or using the information to influence treatment decisions.
Chapter 6 Conclusions
At present, there is no argument for routine use of perfusion imaging prior to rt-PA. A plain CT (or MR) brain scan to exclude haemorrhage and non-stroke causes of symptoms should be followed as quickly as possible by i.v. rt-PA. The presence of a perfusion lesion may increase confidence in the diagnosis of acute ischaemia among less expert scan readers but there was no evidence that the presence or absence of a perfusion lesion, on any perfusion parameter, significantly altered the hazard or benefit of rt-PA. Although the sample for the perfusion imaging study was small, it was larger than most other trials to date and the angiography sample was larger. The lack of interaction with rt-PA was in spite of finding strong associations between perfusion or angiography imaging and stroke severity, age and time to treatment, suggesting that the lack of significant interaction with rt-PA is not simply a methodological error. Arguments against routine perfusion imaging include that it delays the time to treatment, that there is no standard for data processing, that multiple perfusion parameters can be derived, and that there is as yet no agreement as to which of these is the most informative. Confusion about use of perfusion or angiography imaging may actually be a barrier to greater implementation of rt-PA. Patients with renal impairment may be harmed by contrast agents and CT perfusion exposes the patient to extra radiation dose.
The CTA analyses show that the plain scan HAS is a very specific and moderately sensitive sign of angiographically confirmed large artery occlusion. Patients with visible large artery occlusion have more severe stroke and worse outcomes than those without. CTA abnormalities predict early imaging outcomes (visible infarction at follow-up) more than plain CT alone, but did not alter the prediction of functional outcome. We did not find a significant interaction between CTA findings and rt-PA hazards or benefits. The substantial number of angiograms required development of a different analysis pathway from that planned originally, as explained in Chapter 3 (see Any changes to protocol).
Further subgroup analyses are ongoing to investigate whether the presence of collateral arterial flow improves outcome or alters rt-PA effect; to investigate whether or not thin slice CT improves detection of HAS; to investigate if any of the perfusion lesion thresholds identifies a group with more hazard or benefit from rt-PA; to test observer reliability for perfusion and angiography interpretation and to examine the effect of knowledge of perfusion or angiography on plain scan interpretation.
Further research could address whether or not use of perfusion or angiography imaging, by increasing confidence in the diagnosis of acute ischaemic stroke,101 might increase use of rt-PA – this could be a benefit if these imaging technologies encourage greater use of this highly effective treatment. Once several ongoing randomised trials of thrombolysis, which include perfusion or angiography imaging, are completed, an individual patient data meta-analysis should be performed, on a larger sample size, to determine for certain whether or not these imaging methods should be used in patient selection for thrombolytic treatment.
Acknowledgements
We are grateful to Calum Grey, Wellcome Trust Clinical Research Facility Edinburgh, for manually outlining all infarcts on follow-up imaging. We thank the IST-3 centres that participated in the perfusion and angiography substudy. We are grateful to the extended steering committee who advised on data collection and the analysis plan (particularly Rudiger von Kummer, Adam Kobayashi, Mark Parsons and Veronica Murray) and Professor Richard Lindley, co-chief investigator on IST-3.
Contributions of authors
Joanna M Wardlaw (Professor, Neuroradiology): study conception, design, obtaining funds, management and co-ordination; data analysis, interpretation, and writing the report.
Trevor Carpenter (Research Fellow, image analysis): establishment of perfusion imaging processing pipeline, and processing of all perfusion imaging data to produce perfusion lesion maps including analysis of lesion volumes.
Eleni Sakka (Digital Imaging Manger, Neuroimaging): co-ordination with IST-3 participating centres, advice on imaging, receipt of images, housekeeping, curation, distribution to processing areas; and measurement of perfusion lesion volumes and intracranial volume.
Grant Mair (Neuroradiology Fellow): reading all CT angiograms, and analysis of CTA data.
Geoff Cohen (Statistician, Medical): analysis of perfusion data.
Kirsten Shuler (Project Administrator, Scientific Administration): data entry, data checking, assistance with report writing, and financial reconciliation.
Jeb M Palmer (Programmer, Web Applications): implementation of web based image reading system for angiography, and co-ordinating angiography imaging reading.
Karen Innes (Trials Manager, IST-3): assistance with centre co-ordination and overall management of IST-3 main trial.
Peter A Sandercock (Professor, Neurology and Stroke): IST-3 chief investigator, centre liaison and IST-3 trial main administration.
Funding
Third International Stroke Trial Perfusion and Angiography Imaging Study
The EME programme is funded by the Medical Research Council (MRC) and NIHR, with contributions from the Chief Scientist Office (CSO) in Scotland, National Institute for Social Care and Health Research (NISCHR) in Wales and the Health and Social Care Research and Development (HSC R&D), Public Health Agency in Northern Ireland, and is managed by the NIHR. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the funding agencies or UK Department of Health.
Third International Stroke Trial: main trial
The start-up phase was supported by a grant from the Stroke Association, UK. The expansion phase was funded by the Health Foundation UK. The main phase of the trial is funded by UK MRC (grant numbers G0400069 and EME 09–800–15) and managed by the NIHR on behalf of the MRC–NIHR partnership; the Research Council of Norway; Arbetsmarknadens Partners Forsakringsbolag (AFA) Insurances Sweden; the Swedish Heart Lung Fund; The Foundation of Marianne and Marcus Wallenberg, Stockholm County Council; Karolinska Institute Joint ALF-project grants Sweden, the Polish Ministry of Science and Education (grant number 2PO5B10928); the Australian Heart Foundation; Australian National Health and Medical Research Council; the Swiss National Research Foundation; the Swiss Heart Foundation; the Foundation for Health and Cardio-/Neurovascular Research, Basel, Switzerland; the Assessorato alla Sanita, Regione dell’Umbria, Italy; and Danube University, Krems, Austria. Boehringer-Ingelheim GmbH donated drug and placebo for the 300 patients in the double-blind phase, but thereafter had no role whatsoever in the trial. The UK Stroke Research Network (SRN study ID 2135) adopted the trial on 1 May 2006, supported the initiation of new UK sites, and in some centres, and, after that date, data collection was undertaken by staff funded by the network or working for associated NHS organisations. IST-3 gratefully acknowledges the extensive support of the NIHR Stroke Research Network, NHS Research Scotland, through the Scottish SRN, and the National Institute for Social Care and Health Research Clinical Research Centre. The central imaging work was undertaken at the Brain Imaging Research Centre (www.bric.ed.ac.uk), a member of the Scottish Imaging Network – A Platform for Scientific Excellence (SINAPSE) collaboration (www.sinapse.ac.uk), at the Division of Clinical Neurosciences, University of Edinburgh. SINAPSE is funded by the Scottish Funding Council and the CSO of the Scottish Executive. Additional support was received from Chest Heart and Stroke Scotland, DesAcc, University of Edinburgh, Danderyd Hospital Research and Development Department, Karolinska Institutet, Oslo University Hospital, and the Dalhousie University Internal Medicine Research Fund.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NETSCC, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health.
References
- The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med 1995;333:1581-7.
- Wardlaw JM, del Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2003;3.
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768-74. http://dx.doi.org/10.1016/S0140-6736(04)15692-4.
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS and EPITHET trials. Lancet 2010;375:1695-703. http://dx.doi.org/10.1016/S0140-6736(10)60491-6.
- Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275-82. http://dx.doi.org/10.1016/S0140-6736(07)60149-4.
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med 2008;359:1317-29. http://dx.doi.org/10.1056/NEJMoa0804656.
- Wardlaw JM, Murray V, Sandercock PAG. Thrombolysis for acute ischaemic stroke. An update of the Cochrane thrombolysis meta-analysis. Int J Stroke 2008;3.
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012;379:2364-72. http://dx.doi.org/10.1016/S0140-6736(12)60738-7.
- Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment – systematic review. Radiology 2005;235:444-53. http://dx.doi.org/10.1148/radiol.2352040262.
- Wardlaw JM, West TM, Sandercock PA, Lewis SC, Mielke O. Visible infarction on computed tomography is an independent predictor of poor functional outcome after stroke, and not of haemorrhagic transformation. J Neurol Neurosurg Psychiatry 2003;74:452-8. http://dx.doi.org/10.1136/jnnp.74.4.452.
- von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol 1994;15:9-15.
- von Kummer R, Nolte PN, Schnittger H, Thron A, Ringelstein EB. Detectability of cerebral hemisphere ischaemic infarcts by CT within 6 h of stroke. Neuroradiology 1996;38:31-3. http://dx.doi.org/10.1007/BF00593212.
- von Kummer R, Allen KL, Holle R, Bozzao L, Bastianello S, Manelfe C, et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology 1997;205:327-33.
- von Kummer R, Bourquain H, Bastianello S, Bozzao L, Manelfe C, Meier D, et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 2001;219:95-100. http://dx.doi.org/10.1148/radiology.219.1.r01ap0695.
- Wardlaw JM, Farrall AJ, Perry D, von Kummer R, Mielke O, Moulin T, et al. Factors influencing the detection of early computed tomography signs of cerebral ischemia. An internet-based, international multiobserver study. Stroke 2007;38:1250-6. http://dx.doi.org/10.1161/01.STR.0000259715.53166.25.
- Leys D, Ringelstein EB, Kaste M, Hacke W. Facilities available in European hospitals treating stroke patients. Stroke 2007;38:2985-91. http://dx.doi.org/10.1161/STROKEAHA.107.487967.
- Kane I, Whiteley WN, Sandercock PA, Wardlaw JM. Availability of CT and MR for assessing patients with acute stroke. Cerebrovasc Dis 2008;25:375-7. http://dx.doi.org/10.1159/000120688.
- Barber PA, Hill MD, Eliasziw M, Demchuk AM, Pexman JH, Hudon ME, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 2005;76:1528-33. http://dx.doi.org/10.1136/jnnp.2004.059261.
- Hand PJ, Wardlaw JM, Rowat AM, Haisma JA, Lindley RI, Dennis MS. Magnetic resonance brain imaging in patients with acute stroke: feasibility and patient-related difficulties. J Neurol Neurosurg Psychiatry 2005;76:1525-7. http://dx.doi.org/10.1136/jnnp.2005.062539.
- Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-CT assessment of infarct core and penumbra. Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979-85. http://dx.doi.org/10.1161/01.STR.0000209238.61459.39.
- Provenzale JM, Shah K, Patel U, McCrory DC. Systematic review of CT and MR perfusion imaging for assessment of acute cerebrovascular disease. AJNR Am J Neuroradiol 2008;29:1476-82. http://dx.doi.org/10.3174/ajnr.A1161.
- Kane I, Carpenter T, Chappell F, Rivers C, Armitage P, Sandercock P, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke. Effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke 2007;38:3158-64. http://dx.doi.org/10.1161/STROKEAHA.107.483842.
- Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Rother J, et al. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke 2005;36:388-97. http://dx.doi.org/10.1161/01.STR.0000152268.47919.be.
- Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, et al. Acute stroke imaging research roadmap. Stroke 2008;39:1621-8. http://dx.doi.org/10.1161/STROKEAHA.107.512319.
- Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke. A systematic review. Stroke 2006;37:1334-9. http://dx.doi.org/10.1161/01.STR.0000217418.29609.22.
- Schellinger PD. EPITHET: failed chance or new hope?. Lancet Neurol 2008;7:286-7. http://dx.doi.org/10.1016/S1474-4422(08)70045-0.
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and perfusion imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol 2006;60:508-17. http://dx.doi.org/10.1002/ana.20976.
- Hacke W, Albers G, Al Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a Phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66-73. http://dx.doi.org/10.1161/01.STR.0000149938.08731.2c.
- Furlan AJ, Eyding D, Albers GW, Al Rawi Y, Lees KR, Rowley HA, et al. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS). Evidence of safety and efficacy 3 to 9 h after stroke onset. Stroke 2006;37:1227-31. http://dx.doi.org/10.1161/01.STR.0000217403.66996.6d.
- Davis SM, Donnan G, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled radomised trial. Lancet Neurol 2008;7:299-30. http://dx.doi.org/10.1016/S1474-4422(08)70044-9.
- Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol 2008;8:141-50. http://dx.doi.org/10.1016/S1474-4422(08)70267-9.
- Wardlaw JM, Murray VE, Berge E, del Zoppo GJ. Thrombolysis in acute ischaemic stroke. Cochrane Database Syst Rev 2009;4.
- Mishra NK, Albers GW, Davis SM, Donnan GA, Furlan AJ, Hacke W, et al. Mismatch-based delayed thrombolysis. A meta-analysis. Stroke 2010;41:e25-33. http://dx.doi.org/10.1161/STROKEAHA.109.566869.
- Kane I, Sandercock P, Wardlaw J. Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry 2007;78:485-91. http://dx.doi.org/10.1136/jnnp.2006.100347.
- Rivers CS, Wardlaw JM, Armitage P, Bastin ME, Carpenter TK, Cvoro V, et al. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke?. Stroke 2006;37:98-104. http://dx.doi.org/10.1161/01.STR.0000195197.66606.bb.
- Zhao L, Barlinn K, Bag AK, Kesani M, Cava LF, Balucani C, et al. Computed tomography perfusion prognostic maps do not predict reversible and irreversible neurological dysfunction following reperfusion therapies. Int J Stroke 2011;6:544-6. http://dx.doi.org/10.1111/j.1747-4949.2011.00681.x.
- Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke 2005;36:2400-3. http://dx.doi.org/10.1161/01.STR.0000185698.45720.58.
- Kharitonova T, Ahmed N, Thorén M, Wardlaw JM, von Kummer R, Glahn J, et al. Hyperdense middle cerebral artery sign on admission CT scan – prognostic significance for ischaemic stroke patients treated with intravenous thrombolysis in the Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Register. Cerebrovasc Dis 2009;27:51-9. http://dx.doi.org/10.1159/000172634.
- Kobayashi A, Wardlaw JM, Lindley RI, Lewis SC, Sandercock PAG, Czlonkowska A. Oxfordshire Community Stroke Project clinical stroke syndrome and appearances of tissue and vascular lesions on pre-treatment CT in hyperacute ischaemic stroke among the first 510 patients in the Third International Stroke Trial (IST-3). Stroke 2009;40:743-8. http://dx.doi.org/10.1161/STROKEAHA.108.526772.
- Kharitonova T, Thoren M, Ahmed N, Wardlaw J, von Kummer R, Thomassen L, et al. Disappearing hyperdense middle cerebral artery sign in ischemic stroke patients treated with intravenous thrombolysis – clinical course and prognostic significance. J Neurol Neurosurg Psychiatry 2008;80:273-8. http://dx.doi.org/10.1136/jnnp.2008.150185.
- Rha J, Saver JL. The impact of recanalization on ischemic stroke outcome. A meta-analysis. Stroke 2007;38:967-73. http://dx.doi.org/10.1161/01.STR.0000258112.14918.24.
- Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368:904-13. http://dx.doi.org/10.1056/NEJMoa1213701.
- Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893-90. http://dx.doi.org/10.1056/NEJMoa1214300.
- Broderick J. Interventional Management of Stroke (IMS) III Trial (IMSIII). Washington, DC: National Institutes of Health; 2006.
- Lundbeck. Efficacy and Safety Study of Desmoteplase to Treat Acute Ischemic Stroke (DIAS-4). Washington, DC: National Institutes of Health; 2009.
- Arnold M, Nedeltchev K, Remonda L, Fischer U, Brekenfeld C, Keserue B, et al. Recanalisation of middle cerebral artery occlusion after intra-arterial thrombolysis: different recanalisation grading systems and clinical functional outcome. J Neurol Neurosurg Psychiatry 2005;76:1373-6. http://dx.doi.org/10.1136/jnnp.2004.055160.
- Wardlaw JM, von Kummer R, Carpenter T, Parsons M, Lindley R, Cohen G, et al. Protocol for the perfusion and angiography imaging sub-study of the Third International Stroke Trial (IST-3) of alteplase treatment within six hours of acute ischemic stroke. Int J Stroke 2013.
- Kirchhof K, Welzel T, Zoubaa S, Lichy C, Sikinger M, de Ruiz HL, et al. New method of embolus preparation for standardized embolic stroke in rabbits. Stroke 2002;33:2329-33. http://dx.doi.org/10.1161/01.STR.0000027436.82700.73.
- Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237-43. http://dx.doi.org/10.1161/STROKEAHA.110.605576.
- Kimura K, Iguchi Y, Shibazaki K, Watanabe M, Iwanaga T, Aoki J. M1 susceptibility vessel sign on T2* as a strong predictor for no early recanalization after IV-t-PA in acute ischemic stroke. Stroke 2009;40:3130-2. http://dx.doi.org/10.1161/STROKEAHA.109.552588.
- Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost 2009;102:1169-75.
- Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006;37:2086-93. http://dx.doi.org/10.1161/01.STR.0000230307.03438.94.
- Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009;30:525-31. http://dx.doi.org/10.3174/ajnr.A1408.
- Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 2011;10:909-21. http://dx.doi.org/10.1016/S1474-4422(11)70195-8.
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009;132:2231-8. http://dx.doi.org/10.1093/brain/awp155.
- Higashida RT, Furlan AJ. Trial design and reporting standards for intra-aterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:1923-4. http://dx.doi.org/10.1161/01.STR.0000082720.85129.0A.
- Sandercock P, Lindley R, Wardlaw J, Dennis M, Lewis S, Venables G, et al. Third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke. Trials 2008;9. http://dx.doi.org/10.1186/1745-6215-9-37.
- Sandercock P, Lindley R, Wardlaw J, Dennis M, Innes K, Cohen G, et al. Update on the Third International Stroke Trial (IST-3) of thrombolysis for acute ischaemic stroke and baseline features of the 3035 patients recruited. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-252.
- Sandercock P, Lindley R, Wardlaw J, Whiteley W, Murray G. IST3 collaborative group . Statistical analysis plan for the third International Stroke Trial (IST-3): part of a ‘thread’ of reports of the trial. Int J Stroke 2012;7:186-7.
- The IST-3 collaborative group . The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352-63. http://dx.doi.org/10.1016/S0140-6736(12)60768-5.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project – 1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53:16-22. http://dx.doi.org/10.1136/jnnp.53.1.16.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. http://dx.doi.org/10.1161/01.STR.19.5.604.
- Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670-4. http://dx.doi.org/10.1016/S0140-6736(00)02237-6.
- Wardlaw JM, Sellar RJ. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. AJNR Am J Neuroradiol 1994;15:1933-9.
- van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. J Neurol Neurosurg Psychiatry 1990;53:1080-3. http://dx.doi.org/10.1136/jnnp.53.12.1080.
- Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CLM, et al. Improving interrater agreement about brain microbleeds. Development of the Brain Observer MicroBleed Scales (BOMBS). Stroke 2009;49:94-9. http://dx.doi.org/10.1161/STROKEAHA.108.526996.
- Farrell C, Chappell F, Armitage PA, Keston P, MacLullich A, Shenkin S, et al. Development and initial testing of normal reference MR images for the brain at ages 65–70 and 75–80 years. Eur Radiol 2008;19:177-83. http://dx.doi.org/10.1007/s00330-008-1119-2.
- The Optimising Analysis of Stroke Trials (OAST) collaboration . Can we improve the statistical analysis of stroke trials?: statistical reanalysis of function outcomes in stroke trials. Stroke 2007;38:1911-15.
- Rowat A, Graham C, Dennis M. Dehydration in hospital-admitted stroke patients: detection, frequency, and association. Stroke 2011;43:857-9. http://dx.doi.org/10.1161/STROKEAHA.111.640821.
- Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation Of Stroke Study (ACCESS). PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0015757.
- Dani KA, Thomas RGR, Chappell FM, Shuler K, MacLeod MJ, Muir KW, et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann Neurol 2011;70:384-401. http://dx.doi.org/10.1002/ana.22500.
- Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011;42:3435-40. http://dx.doi.org/10.1161/STROKEAHA.111.618355.
- Bivard A, McElduff P, Spratt N, Levi C, Parsons M. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc Dis 2011;31:238-45. http://dx.doi.org/10.1159/000321897.
- McVerry F. Int J Stroke 2009;4.
- Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke 2010;41:1169-74. http://dx.doi.org/10.1161/STROKEAHA.110.580670.
- Christensen S, Mouridsen K, Wu O, Hjort N, Karstoft H, Thomalla G, et al. Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke 2009;40:2055-61. http://dx.doi.org/10.1161/STROKEAHA.108.546069.
- Takasawa M, Jones PS, Guadagno JV, Christensen S, Fryer TD, Harding S, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke 2008;39:870-7. http://dx.doi.org/10.1161/STROKEAHA.107.500090.
- Olivot J-M, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke 2009;40:469-75. http://dx.doi.org/10.1161/STROKEAHA.108.526954.
- Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke 2010;41:2817-21. http://dx.doi.org/10.1161/STROKEAHA.110.594432.
- Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010;32:1024-37. http://dx.doi.org/10.1002/jmri.22338.
- Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Stroke 2011;42:1608-14. http://dx.doi.org/10.1161/STROKEAHA.110.609008.
- Mori E, Tabuchi M, Yoshida T, Yamadori A. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988;19:802-12. http://dx.doi.org/10.1161/01.STR.19.7.802.
- TIMI Study Group . The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med 1985;312:932-6. http://dx.doi.org/10.1056/NEJM198504043121437.
- Noser EA, Shaltoni HM, Hall CE, Alexandrov AV, Garami Z, Cacayorin ED, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic theraphy in acute stroke?. Stroke 2005;36:292-6. http://dx.doi.org/10.1161/01.STR.0000152331.93770.18.
- Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008;29:582-7. http://dx.doi.org/10.3174/ajnr.A0843.
- Mori E, Yoneda Y, Tabuchi M, Yoshida T, Ohkawa S, Ohsumi Y, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992;42:976-82. http://dx.doi.org/10.1212/WNL.42.5.976.
- Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol 2007;28:382-4.
- The Optimising Analysis of Stroke Trials (OAST) collaboration . Calculation of sample size for stroke trials assessing functional outcome: comparison of binary and ordinal approaches. Int J Stroke 2008;3:78-84. http://dx.doi.org/10.1111/j.1747-4949.2008.00184.x.
- Koops L, Lindley RI. Thrombolysis for acute ischaemic stroke: consumer involvement in design of new randomised controlled trial. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7361.415.
- Wardlaw J, Farrall A, Chappell F, von Kummer R, Perry D. Comparison of CT rating scales in hyperacute ischaemic stroke in the ACCESS study, a large, multireader, web-based observer reliability study. Cerebrovasc Dis 2009;27.
- Wardlaw JM, Muir KW, MacLeod MJ, Weir C, McVerry F, Carpenter T, et al. Clinical relevance and practical implications for trials of perfusion and angiographic imaging in patients with acute ischaemic stroke: a multicentre cohort imaging study. J Neurol Neurosurg Psychiatry 2013;84:1001-7. http://dx.doi.org/10.1136/jnnp-2012-304807.
- Valdes Hernandez MC, Morris Z, Dickie DA, Royle NA, Munoz MS, Aribisala BS, et al. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology 2012;40:13-22. http://dx.doi.org/10.1159/000341859.
- Wardlaw JM. Surrogate outcomes. A cautionary note. Stroke 2009;40:1029-31. http://dx.doi.org/10.1161/STROKEAHA.108.540641.
- Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 2012;366:1099-107. http://dx.doi.org/10.1056/NEJMoa1109842.
- Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914-23. http://dx.doi.org/10.1056/NEJMoa1212793.
- Hacke W. European Cooperative Acute Stroke Study-4: Extending the Time for Thrombolysis in Emergency Neurological Deficits – a Double-Blind, Placebo-Controlled Randomized Study 2013. http://controlled-trials.com/ISRCTN71616222?close=1.
- Donnan G, Davis S. Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND) 2013. http://clinicaltrials.gov/show/NCT00887328 (accessed 7 July 2014).
- Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860-7. http://dx.doi.org/10.1016/S1474-4422(12)70203-X.
- Shamy MC, Jaigobin CS. The complexities of acute stroke decision-making: a survey of neurologists. Neurology 2013;81:1-4. http://dx.doi.org/10.1212/WNL.0b013e3182a55ec7.
- Luby M, Ku KD, Latour LL, Merino JG, Hsia AW, Lynch JK, et al. Visual perfusion-diffusion mismatch is equivalent to quantitative mismatch. Stroke 2011;42:1010-14. http://dx.doi.org/10.1161/STROKEAHA.110.603290.
- Campbell BCV, Weir L, Desmond PM, Tu HTH, Hand PJ, Yan B, et al. CT perfusion improves diagnostic accuracy and confidence in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2013;84:613-18. http://dx.doi.org/10.1136/jnnp-2012-303752.
- Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Acute Stroke Imaging Research Roadmap II. Stroke 2013;44:2628-39. http://dx.doi.org/10.1161/STROKEAHA.113.002015.
- Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke. A consensus statement. Stroke 2013;44:2650-63. http://dx.doi.org/10.1161/STROKEAHA.113.001972.
- Gonzalez DR, Carpenter T, van Hemert JI, Wardlaw J. An open source toolkit for medical imaging de-identification. Eur Radiol 2010;20:1896-904. http://dx.doi.org/10.1007/s00330-010-1745-3.
- Wu O, Ostergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 2003;50:164-74. http://dx.doi.org/10.1002/mrm.10522.
- Sasaki M, Kudo K, Ogasawara K, Fujiwara S. Tracer delay-insensitive algorithm can improve reliability of CT perfusion imaging for cerebrovascular steno-occlusive disease: comparison with quantitative single-photon emission CT. AJNR Am J Neuroradiol 2009;30:188-93. http://dx.doi.org/10.3174/ajnr.A1274.
- Bivard A, Spratt N, Levi C, Parsons M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain 2011;134:3408-16. http://dx.doi.org/10.1093/brain/awr257.
- Kjolby BF, Mikkelsen IK, Pedersen M, Ostergaard L, Kiselev VG. Analysis of partial volume effects on arterial input functions using gradient echo: a simulation study. Magn Reson Med 2009;61:1300-9. http://dx.doi.org/10.1002/mrm.21849.
- Kiselev VG. On the theoretical basis of perfusion measurements by dynamic susceptibility contrast MRI. Magn Reson Med 2001;46:1113-22. http://dx.doi.org/10.1002/mrm.1307.
- Madsen MT. A simplified formulation of the gamma variate function. Phys Med Biol 1992;37:1597-600. http://dx.doi.org/10.1088/0031-9155/37/7/010.
Appendix 1 Advisory minimum standards for (a) magnetic resonance and computed tomography perfusion acquisition and (b) magnetic resonance and computed tomography angiography acquisition
Advisory minimum standards for (i) magnetic resonance and (ii) computed tomography perfusion acquisition
(i) Magnetic resonance perfusion
| MRP | |
|---|---|
| Sequence | Single-shot gradient-echo echoplanar imaging |
| TR | TR = 1500–2000 ms |
| TE | TE = 35–45 ms @ 1.5 T |
| TE= 2 5–30 ms @ 3 T | |
| Flip angle | Flip angle α = 60–90° @ 1.5 T, 60° @ 3.0 T |
| Baseline | At least 10–12 baseline images (please note the first few images prior to steady state are discarded) |
| Coverage | At least 12 slices, with same slice thickness and gap as DWI, increase TR and slice gap to achieve reasonable coverage |
(ii) Computed tomography perfusion
| CTP | |
|---|---|
| Acquisition rate | One image per second (ideally at one source rotation per second) |
| Total acquisition time | 40–60 seconds |
| Baseline period | 5–10 volumes should be acquired prior to contrast arrival |
| kVp and | 80 kVp (not 120 kVp) |
| mAs | 100 mAs or higher |
| Contrast volume | 35–50 ml (with saline flush) |
| Delivery rate | 4–6 ml per second |
| Coverage | As dictated by configuration of hardware |
Advisory minimum standards for (i) magnetic resonance and (ii) computed tomography angiography acquisition
(i) Magnetic resonance angiography
| MRA | |
|---|---|
| Sequence | 3D TOF 2 slab HR |
| TR (ms) | 23 |
| TE (ms) | 2.7 |
| Flip angle | 20° |
| Location/slab | 32 |
| Slice thickness | 1.6 |
| Slice gap | 0 |
| Matrix | 320 × 224 |
| Φ FOV | 1 |
| FOV | 16 |
| Slice orientation | Straight axial |
| Tscan | 5:46 |
(ii) Computed tomography angiography
| CTA | |
|---|---|
| kVp | 100 |
| mAs | 120 |
| Contrast (volume/type/rate) | 50 ml omnipaque 300 at 4 ml/second |
| Flush (volume/type/rate) | 40 ml saline at 4 ml/second |
| Delay | 15 seconds |
| Coverage | Circle of Willis (upwards) |
| Slice collimation | 0.75 mm |
| Pitch | 1.25 |
Appendix 2 Perfusion image processing
Basic perfusion processing
Digital Imaging and Communications in Medicine storage and conversion
Subjects in the main arm of IST-3 who have been identified as having perfusion images are transferred to the perfusion substudy file system by DICOM sent to a receiver running on a server attached to a high-performance computing facility. The receiver is implemented using DICOM confidential104 and stores the received images in standard DICOM format as well as cataloguing the images in a database. The cataloguing process records the unique identifiers which reference the image data, the dates and times of the imaging studies as well as information specific to the series modality such as echo time or tube voltage and current. The catalogue makes this information available to other processing steps; it is also used to identify structural and perfusion-imaging series in the studies labelled as R (randomisation) and P (post) time points.
The next step in the initial conversion of the DICOM data is reconstruction. After relevant structural and perfusion series have been identified, each image in the series is composited into a 3D volume; in the case of perfusion series the individual 3D volumes are joined in acquisition order to create a 4D ‘volume’. This composition is carried out by the widely used utility DCM2NII and the final data are stored in NIFTI format for subsequent processing and analysis. In all of the data reconstructed, the actual acquisition order of the data is only represented at the volume level and not the individual slice level, often referred to as slice timing. The slice timing has been shown to be important in the processing of MR perfusion data, especially in the case of the parameter Tmax;75 however, no account could be taken of this in such a chronologically and geographically diverse data set.
Perfusion post-processing
The perfusion post-processing is used to estimate cerebral blood flow, volume, transit time and other parameters, and the following paragraphs describe the important points of each step. Overall, it is based upon the block circulant method developed by Ona Wu105 and this approach can now be considered a de facto standard as it has been independently implemented and applied by several different groups. 106,107
Contrast concentration estimation
The first step in the processing is to convert the signal time series within each voxel into a time series proportional to contrast concentration using the relevant relationship between signal and contrast concentration for either MR or CT. Additionally, in MR, the relationship between signal and concentration is non-linear108,109 and a modulation transfer function is applied, as described in Straka et al. ,80 after applying the usual formula. In both cases, this conversion relies upon estimating the mean signal in each voxel prior to the arrival of contrast, and in MR data the first three time points are disposed of to ensure the signal has reached a steady state.
Discretisation
Discretisation is the process used to convert the equations describing the distribution of contrast (perfusion) into a form suitable for solving using the quantities observed in the contrast concentration time series. The first step is defining a quantity referred to as the arterial input function (AIF). Owing to the diverse sources of data, the different perfusion series had no standard anatomical coverage, and therefore the AIF could not be placed in a consistent location. Consequently, the AIF was placed manually in a location adjacent to a vessel with early enhancement where possible in the case of MR, and in CT series which included it, this was in the contralateral middle cerebral artery; in CT series with limited coverage, the anterior cerebral artery was chosen. Locations for two additional time series were also defined, one for the venous out flow (VOF) which was placed in the superior sagittal sinus and another in a region of what was assumed to be normal white matter in the contralateral hemisphere often close to the anterior horns of the ventricles. To mitigate for possible partial volume effects in estimating the AIF, the area under the AIF as adjusted to match that of the VOF by multiplication with a scalar. The normal white matter time series was used as a means to compare data from different sources.
As previously stated, the method used to convert the AIF and voxel time series values into a form suitable for numerical solution was first applied to perfusion imaging by Wu et al. 105 The method uses a set of simultaneous equations expressed as a matrix equation to represent the convolution integral, deconvolution is achieved by inverting the matrix and solving for the vector of unknown quantities. The discretisation is different from other alternative schemes in that the signals are treated as being periodic; this is achieved by using a matrix with a special structure referred to as a circulant Toeplitz matrix.
The individual values of the AIF are used to provide the elements of this matrix, where from left to right each column is a shifted copy of the previous column. Being circulant means that as elements drop off the last row of the matrix they reappear in the first row of the next column. When populated in this way, the first column of the circulant matrix contains the unshifted vector of AIF values in the correct temporal order, whereas in the last row the temporal order is reversed. As copies of a deterministic signal corrupted by noise, the individual elements of a matrix populated in this way are very far from independent and their true values are unknown. Consequently, it can be expect that obtaining the inverse of such a matrix will be problematic. It is for this reason that regularisation is applied to the deconvolution used to obtain the parameter estimates. The advantage of the block circulant method is that the estimated quantities are less sensitive to delay between the AIF and the voxel concentration time series.
Parameter estimation
The parameters of interest were recovered, as follows, from the observed quantities and the residue function obtained from deconvolution of the arterial and voxel time series on a per-voxel basis. The CBV was defined as the ratio of the areas under the voxel and arterial concentration time courses. The CBF and Tmax were defined as the peak value of the residue function and the time at which it occurred. The MTT was defined as the ratio of CBV to CBF.
The bolus arrival time (AT), peak time (PT) and the difference of the two, time to peak (TTP) from bolus arrival as well as the maximum value of contrast concentration (Cmax), etc., were obtained for each voxel as follows. A reconstructed contrast concentration time series was formed by convolving the AIF with the estimated residue function on a per-voxel basis. Owing to the regularisation applied in the deconvolution, the time series formed in this way is much smoother than the original data, and, therefore, initial estimates of parameters such as AT, Cmax, etc., obtained directly from it, are less affected by noise than would be the case if they were taken from the original time series. These initial estimates are then used to obtain the starting parameters for fitting a heuristic model to the reconstructed time series. 110 The parameters of the fitted model are then used to provide the estimates of AT, PT, TTP, etc., used to create the parametric maps.
Parametric map storage
The parametric maps were stored in NIFTI format as single precision floating point values, with each voxel value equal to the parameter value for that voxel, that is to say with out any scaling. The CBV was stored in units of ml/100 g by assuming a fixed value for the density of brain tissue; the CBF was stored in units of ml/100 g/minute and the MTT in seconds. The other parameters either have units of seconds or arbitrary units (e.g. Cmax and rCBF).
Appendix 3 Visual coding forms for plain computed tomography or magnetic resonance imaging, perfusion and angiography imaging
Third International Stroke Trial Perfusion and Angiography studies
Computed tomography image interpretation form
Magnetic resonance image interpretation form
Appendix 4 Consolidated Standards of Reporting Trials 2010 flow diagram for Third International Stroke Trial main trial
Consolidated Standards of Reporting Trials diagram of the IST-3 main trial recruitment of 3035 patients. tPA, tissue plasminogen activator.
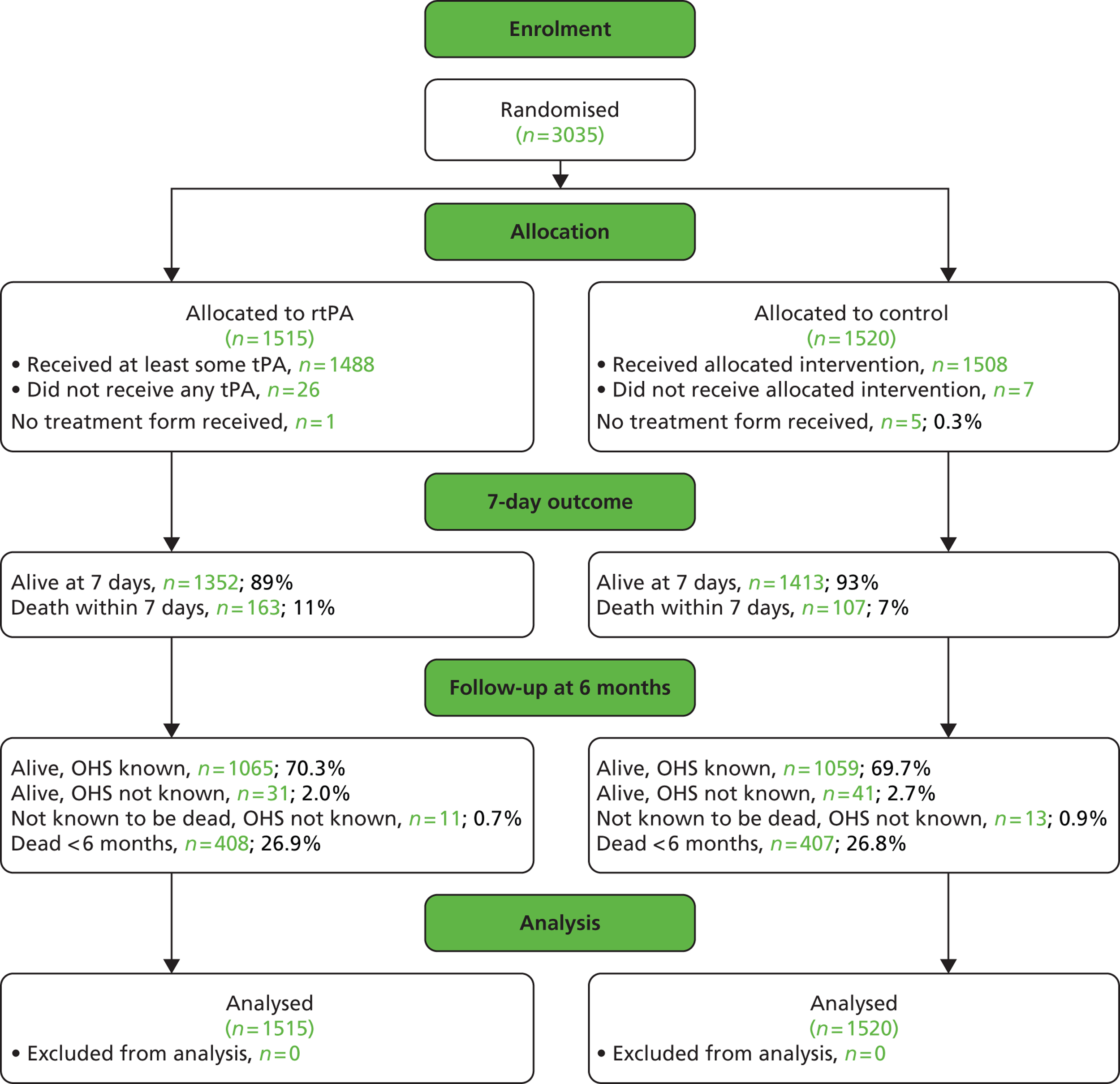
Appendix 5 Participating countries and centres
| Centre no. | Total patients (perfusion/angiography) | Country | Centre |
|---|---|---|---|
| 1 | 6 | UK | Western General Hospital |
| 5 | 1 | UK | Nottingham City Hospital |
| 27 | 1 | Italy | Ospedale di Cattinara – Trieste |
| 29 | 4 | Australia | Royal Perth Hospital |
| 42 | 2 | UK | Countess of Chester Hospital |
| 69 | 2 | UK | University Hospital Aintree |
| 79 | 1 | UK | Leeds General Infirmary |
| 98 | 1 | UK | King’s College Hospital |
| 124 | 13 | UK | Addenbrookes Hospital |
| 127 | 5 | UK | Royal Hallamshire Hospital |
| 155 | 14 | Norway | St Olavs Hospital, University Hospital of Trondheim |
| 157 | 3 | Norway | Ullevål University Hospital |
| 158 | 32 | Belgium | Cliniques Universitaires St Luc |
| 169 | 2 | UK | Queen Elizabeth the Queen Mother Hospital |
| 171 | 2 | UK | York Hospital, York NHS Foundation Trust |
| 172 | 12 | UK | University Hospital of North Staffordshire |
| 173 | 3 | Sweden | Danderyds Sjukhus |
| 176 | 9 | Italy | Ospedale Citta di Castello |
| 180 | 2 | Canada | QEII Health Sciences Centre |
| 182 | 31 | Poland | 2nd Department of Neurology, Institute of Psychiatry & Neurology |
| 184 | 1 | Norway | Harstad Sykehus |
| 188 | 25 | Australia | John Hunter Hospital |
| 191 | 8 | UK | William Harvey Hospital |
| 196 | 1 | UK | Norfolk and Norwich University Hospital NHS Trust |
| 200 | 1 | Poland | Military Medical Institute |
| 203 | 1 | UK | University Hospitals Coventry & Warwickshire NHS Trust |
| 207 | 12 | Norway | University Hospital Northern Norway |
| 208 | 3 | Australia | Royal Brisbane and Women’s Hospital |
| 210 | 5 | Australia | Nambour General Hospital |
| 211 | 3 | UK | The Royal London Hospital, Barts and The London NHS Trust |
| 213 | 1 | Poland | Institute of Psychiatry & Neurology – 1st Dept |
| 221 | 12 | Austria | Landesklinikum Donauregion Tulln |
| 224 | 3 | Sweden | Falu Hospital |
| 225 | 4 | Italy | Ospedale di Branca (Ospedale di Gubbio) |
| 226 | 5 | UK | Guy’s & St Thomas’ Hospital |
| 232 | 13 | UK | Gosford Hospital |
| 233 | 4 | Australia | Box Hill Hospital (Monash University) |
| 236 | 26 | Sweden | Uppsala University Hospital |
| 246 | 1 | Poland | Central University Hospital |
| 248 | 10 | UK | Hammersmith Hospitals & Imperial College |
| 249 | 2 | Poland | Medical University of Gdansk |
| 260 | 3 | Australia | Austin Health – Repatriation Campus |
| 264 | 74 | UK | The National Hospital for Neurology & Neurosurgery |
| 267 | 12 | Italy | Ospedale Maggiore |
| 269 | 1 | Norway | Aalesund Sjukehus |
| 281 | 1 | Sweden | University Hospital of Northern Sweden |
| 284 | 14 | Sweden | Hassleholm Hospital |
| 292 | 2 | Italy | Universita degli Studi di Genova, Dipartimento di Neuroscienze Oftalmologia e Genetica |
| 298 | 19 | UK | Southend University Hospital |
| 308 | 20 | Italy | Nuovo Ospedale Civile |
| 312 | 21 | Switzerland | Universitätsspital Basel |
| 319 | 2 | Poland | SPZZOZ w Sandomierzu |
| 321 | 5 | Italy | Ospedale Valduce di Como |
| 324 | 1 | Portugal | Centro Hospitalar de Trás-os-Montes e Alto Douro |
| 333 | 6 | UK | St George’s Healthcare NHS Trust |
| 340 | 1 | UK | Darent Valley Hospital |
| 342 | 1 | UK | City Hospital, Sandwell & West Birmingham Hospitals NHS Trust |
| 375 | 1 | UK | Queen’s Hospital, Barking, Havering & Redbridge Hospitals NHS Trust |
| 378 | 1 | Switzerland | Universitätsspital Zürich |
Appendix 6 Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist
| Item name | Item no. | Recommendation |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract (p. v) |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found (p. v–vi) | ||
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported (p. 1, paragraph 2 + p. 2, paragraph 2) |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses (p. 3, paragraph 2 + p. 5) |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper (pp. 7–9) |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection (pp. 7–9) |
| Participants | 6 | (a) Cohort study – Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case–control study – Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study – Give the eligibility criteria, and the sources and methods of selection of participants (p. 7) |
| (b) Cohort study – For matched studies, give matching criteria and number of exposed and unexposed Case–control study – For matched studies, give matching criteria and the number of controls per case (pp. 7–9, p. 19) |
||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable (clinical: pp. 9–11; imaging: pp. 10–11 + p. 13) |
| Data sources/measurement | 8a | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group (pp. 9–16) |
| Bias | 9 | Describe any efforts to address potential sources of bias (p. 7) |
| Study size | 10 | Explain how the study size was arrived at (p. 16) |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why (pp. 9–16) |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding (p. 16) |
| (b) Describe any methods used to examine subgroups and interactions (p. 16) | ||
| (c) Explain how missing data were addressed (p. 19 + Figure 3 flow diagram) | ||
| (d) Cohort study – If applicable, explain how loss to follow-up was addressed Case–control study – If applicable, explain how matching of cases and controls was addressed Cross-sectional study – If applicable, describe analytical methods taking account of sampling strategy (N/A) |
||
| (e) Describe any sensitivity analyses (p. 16) | ||
| Results | ||
| Participants | 13a | (a) Report numbers of individuals at each stage of study, e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed (p. 19 + Figure 3 flow diagram) |
| (b) Give reasons for non-participation at each stage (pp. 19–23 + pp. 30–2) | ||
| (c) Consider use of a flow diagram (Figure 3 flow diagram) | ||
| Descriptive data | 14a | (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders (Table 3) |
| (b) Indicate number of participants with missing data for each variable of interest (Table 3, Table 4, Figure 11, pp. 30–2) | ||
| (c) Cohort study – Summarise follow-up time (e.g. average and total amount) (all 6/12) | ||
| Outcome data | 15a | Cohort study – Report numbers of outcome events or summary measures over time (pp. 30–2, Table 5) |
| Case–control study – Report numbers in each exposure category, or summary measures of exposure (Figure 3, pp. 36–41) | ||
| Cross-sectional study – Report numbers of outcome events or summary measures (pp. 30–32, Table 5, pp. 36–41) | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g. 95% confidence interval). Make clear which confounders were adjusted for and why they were included (Table 5, p. 36) |
| (b) Report category boundaries when continuous variables were categorised (N/A) | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 17 | Report other analyses done, e.g. analyses of subgroups and interactions, and sensitivity analyses (p. 43) |
| Discussion | ||
| Key results | 18 | Summarise key results with reference to study objectives (p. 45 + p. 49) |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias (pp. 45–6) |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence (pp. 46–9) |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results (p. 49) |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based (pp. 51–2) |
List of abbreviations
- ACA
- anterior cerebral artery
- ACCESS
- Acute Cerebral CT Evaluation Stroke Study
- AF
- atrial fibrillation
- AOL
- arterial occlusive lesion
- ASPECTS
- Alberta Stroke Program Early CT score
- AT
- arrival time
- CBF
- cerebral blood flow
- CBFq
- cerebral blood flow quantitative
- CBV
- cerebral blood volume
- CBVq
- cerebral blood volume quantitative
- CI
- confidence interval
- Cmax
- maximum value of contrast concentration
- CSO
- Chief Scientist Office
- CT
- computed tomography
- CTA
- computed tomography angiography
- CTP
- computed tomography perfusion
- DEFUSE
- Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution
- DIAS
- Desmoteplase in Acute Stroke Trial
- DICOM
- Digital Imaging and Communications in Medicine
- DWI
- diffusion-weighted imaging
- ECASS
- European Cooperative Acute Stroke Study
- eGFR
- estimated glomerular filtration rate
- EME
- Efficacy and Mechanism Evaluation
- EPITHET
- Echoplanar Imaging Thrombolytic Evaluation Trial
- FLAIR
- fluid attenuated inversion recovery
- HAS
- hyperattenuated artery sign
- HU
- Hounsfield Units
- ICA
- internal carotid artery
- IMS
- Interventional Management of Stroke
- IQR
- interquartile range
- IST-3
- Third International Stroke Trial
- i.v.
- intravenous
- LACI
- lacunar infarct
- MCA
- middle cerebral artery
- MR
- magnetic resonance
- MRA
- magnetic resonance angiography
- MRC
- Medical Research Council
- MRI
- magnetic resonance imaging
- MRP
- magnetic resonance perfusion imaging
- MR Rescue
- Mechanical Retrieval and Recanalisation of Stroke Clots Using Embolectomy
- MTT
- mean transit time
- MTTq
- meant transit time quantitative
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- OHS
- Oxford Handicap Score
- OR
- odds ratio
- PACI
- partial anterior circulation infarct
- PCA
- posterior cerebral artery
- PMD
- proximal-middle-distal
- POCI
- posterior circulation infarct
- PT
- peak time
- PWI
- perfusion-weighted imaging
- rCBF
- relative cerebral blood flow
- RCT
- randomised controlled trial
- rMTT
- relative mean transit time
- rt-PA
- recombinant tissue plasminogen activator
- SAP
- statistical analysis plan
- SD
- standard deviation
- SICH
- symptomatic intracerebral haemorrhage
- SINAPSE
- Scottish Imaging Network – A Platform for Scientific Excellence
- SIRS
- Systematic Image Review System
- SRN
- Stroke Research Network
- STIR
- Stroke Imaging Repository
- TACI
- total anterior circulation infarct
- TICI
- thrombolysis in cerebral infarction
- TIMI
- thrombolysis in myocardial infarction
- Tmax
- time to maximum flow
- Tmaxq
- time to maximum flow quantitative
- TTP
- time to peak