Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 08/52/02. The contractual start date was in July 2009. The final report began editorial review in August 2014 and was accepted for publication in January 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by MacAllister et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Chronic kidney disease requiring renal replacement therapy affects approximately 50,000 adult patients in the UK. 1 Approximately 20,000 of these patients have undergone transplantation, leaving about 30,000 requiring dialysis. Of these, approximately 6000 are deemed best treated by a kidney transplant and are on the active transplant list at any given time. After an average waiting time of about 3 years they undergo transplantation, with approximately 2000 such transplants performed in 2012–13. 2 Increased transplant activity is just keeping pace with the increased demand for kidney transplants, not only from the increase in incident cases but also from those patients whose transplanted kidney has failed; this occurs in 3% of the transplant population per year, resulting in approximately 600 patients per year returning to the transplant list. The consequences for a patient deemed best treated by transplantation of remaining on or returning to the transplant list are substantial. Among these patients there is not only significant morbidity of dialysis but also appreciable annual mortality (approximately 3%) and a substantial impact on quality of life; dialysis is a time-consuming hospital-based treatment requiring three sessions per week, each occupying half a day, with substantial and permanent restrictions on lifestyle, including diet and fluid intake. This is in addition to the cost of dialysis (about £30,000 per patient per year, and greater than the costs of treating patients by transplantation), which consumes approximately 1–2% of the NHS budget. 2 Therefore, approaches that maximise the lifespan of each transplanted kidney will benefit patients directly, contribute to a reduction in the transplant list and moderate the costs of renal replacement therapy.
Renal injury is caused by ischaemia and reperfusion during transplantation
When an organ or tissue is rendered ischaemic there is inevitable cell death and tissue injury, the extent of which can be limited by timely reperfusion. However, paradoxically, an additional injury occurs on reperfusion that limits the amount of tissue that can be salvaged. This composite injury is termed ‘ischaemia–reperfusion (IR) injury’. IR injury underlies much of the tissue damage that occurs in stroke and myocardial infarction, two of the most common clinical IR syndromes, but it also plays a part in damage to all organs when they become ischaemic. In many renal transplants the kidney is exposed to warm ischaemia before organ recovery, cold ischaemia caused by the delay in transplanting the recovered organ and a further period of warm ischaemia during the transplantation procedure. 3 Cell death follows interruption of the blood supply to the kidney and successful reperfusion is mandatory for tissue salvage. Although reperfusion may be an integral part of the healing process, it may also contribute to tissue injury. 4 The degree of IR injury determines the speed of recovery of organ function in the short term. 5 In addition, it may modulate organ rejection in the longer term by priming the immune response early after transplantation. 6–8 Reduction in IR injury has the potential to improve the outcome of kidney (and other organ) transplantation, in both the short term and the long term. 9,10
Mechanisms of ischaemia–reperfusion injury
Ischaemia of the kidneys (like that of other tissues) deprives cells of adenosine triphosphate (ATP), as a result of which the cells are then unable to maintain essential homeostatic processes. This ultimately leads to cell death by apoptosis or necrosis if timely reperfusion does not occur. 11 Reperfusion injury is multifactorial and is partly attributable to rapid reoxygenation of hypoxic tissues, resulting in oxidative damage, and calcium overload because of loss of ion pump homeostasis. 12 Although any segment of the nephron may be affected, the cells most vulnerable to IR injury are in the renal proximal tubule and distal medullary thick ascending limb of the loop of Henle. This is because of the high metabolic rate required for ion transport in these cells and also because of a limited capacity for anaerobic metabolism. Additionally, there is marked microvascular congestion and hypoperfusion in this region that persists despite restoration of cortical blood flow, therefore contributing to prolonged ischaemic injury. 13 Endothelial cell injury and endothelial dysfunction are primarily responsible for this phenomenon, known as the extension phase of acute kidney injury. Ischaemic injury causes loss of the apical brush border of proximal tubular cells. Disrupted microvilli detach from the apical surface forming membrane-bound blebs that are released into the tubular lumen. The detachment and loss of tubular cells, in combination with brush-border vesicle remnants and cellular debris, results in tubular casts that may cause obstruction. 13
Strategies to limit clinical IR injury have mainly focused on timely reperfusion. These strategies include interventions such as primary coronary intervention, thrombolysis for stroke and reducing both warm and cold ischaemic times in transplantation. There has arguably been optimisation of therapeutic techniques and their timing (within the current framework of health-care delivery) to limit ischaemia times and attention has turned towards interventions that target IR injury, either to enhance resistance to ischaemia and/or to reduce reperfusion injury. One such strategy is ischaemic preconditioning (IPC).
Ischaemic preconditioning
Ischaemic preconditioning utilises sub-lethal ischaemia (preconditioning stimulus) to induce a state of protection against subsequent prolonged ischaemia. 14 There are two phases of protection. The early phase of IPC occurs within minutes of the preconditioning stimulus and lasts for up to 4 hours. 15 The mechanism of early IPC has been extensively studied in animals. It involves mediators that are generated during hypoxia (e.g. adenosine), which initiate the cascade of protection by activating G-protein-coupled receptors,15 promoting recruitment of protein kinase mediators [such as phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), protein kinase C (PKC) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT)]. 16 IPC activates at least three main salutatory pathways, the cyclic guanosine monophosphate (cGMP)-dependent protein kinase [cGMP/protein kinase G (PKG)] pathway,17 the reperfusion injury salvage kinase (RISK) pathway16 and the survivor activating factor enhancement (SAFE) pathway. 18 There is a degree of overlap, in particular where the pathways converge in mitochondria. 19 Here, although there is some uncertainty, the potassium-dependent ATP (KATP) channel is activated and leads to closure of the mitochondrial permeability transition pore (mPTP), preventing the influx of ions through this channel that would trigger mitochondrial rupture and cell death by apoptosis.
A late phase of IPC occurs 24 hours after the preconditioning stimulus and lasts for up to 72 hours; this is termed the ‘second window of protection’, distinguishing it from early IPC. 15 The prolonged (24-hour) interval between the preconditioning event and its renewed protection 1 day later is consistent with new protein synthesis. IPC initiates a complex genomic and proteomic response that is thought to underpin the late phase of protection. This includes regulation of anti-apoptotic and anti-inflammatory gene transcription, likely to be responsible for the second window of protection. 20 Later-phase protection requires synthesis of inducible nitric oxide synthase (iNOS), heat shock proteins (HSPs) or cyclo-oxygenase-2 (COX-2), secondary to induced upregulation of genes for these factors. These act locally through the mPTP or KATP channels to induce a state of protection. 15 Although the majority of studies to date have demonstrated protection by IPC against IR injury to the myocardium of animals and humans, a smaller number of studies have investigated the potential of IPC to protect other organs, including the kidney. In animal models IPC attenuates injury and preserves function following renal IR and after renal transplantation. 21
Remote ischaemic preconditioning
Nearly 30 years have elapsed since the first description of IPC but its therapeutic value in the clinical setting remains to be validated. This is largely because of the logistical difficulties of applying ischaemic stimuli to induce preconditioning in vital organs in humans. Nor has it yet been possible to induce IPC pharmacologically, a reflection of the incomplete understanding of the mechanisms and the likelihood that multiple biological targets need to be activated. Demonstrating that there is clinically relevant tissue protection would stimulate renewed interest in pharmacological approaches to modulate IPC. However, the realisation that IPC may protect tissues that are distant from those undergoing preconditioning has led to recent interest in the direct clinical application of IPC. 22 This facet of preconditioning (termed remote ischaemic preconditioning; RIPC) has been shown to be protective against IR injury to the myocardium23,24 and the kidney. 25 RIPC is mechanistically similar to IPC and causes a similar degree of tissue protection.
The mechanism by which the protective signal is transferred systemically from the area of index ischaemia has been the subject of some debate. Evidence for involvement of a humoral factor is supported by preclinical observations that protection can be transferred by the transfusion of serum from an animal that has undergone IPC to one that has not. 26,27 This factor is believed to be heat stable, dialysable and < 15 kDa in size. 28 In transplant studies in pigs, RIPC applied to the recipient animal conferred protection against IR injury to the denervated donor heart during transplantation, again supporting a humoral hypothesis. 29 Attempts to identify this circulating factor have proved challenging. However, recently, stromal cell-derived factor-1 alpha (SDF-1α; also known as C–X–C motif chemokine 12 or CXCL12), a cardioprotective chemokine of 10 kDa that is induced by hypoxia, has been demonstrated to be upregulated following RIPC in rats. The resultant cardioprotection was blocked in rats treated with AMD3100, a highly specific inhibitor of C–X–C chemokine receptor type 4 (CXCR4), the target receptor for SDF-1α. 30 Neurogenic mechanisms of signal transfer have also been suggested. In rats, Dong et al. 31 demonstrated that femoral nerve section abolished the effects of limb IPC. In a rat myocardial infarction model,24 hexamethonium (an autonomic antagonist) abolished protection by RIPC achieved by mesenteric artery occlusion. The autonomic ganglionic blocker trimetaphan has been shown to inhibit RIPC in a human model. 32 The humoral and neuronal pathways may work in series to spread protection systemically. Lim et al. 33 demonstrated that, in mice, femoral vein occlusion or femoral and sciatic nerve resection abolished the protective effects of RIPC, implicating both humoral and neural pathways.
Clinical studies of remote ischaemic preconditioning
Most human studies have used limb ischaemia to activate RIPC because of the inaccessibility of vital organs for IPC. The first clinical study demonstrated an effect of limb ischaemia on experimental IR injury to the endothelium23 and was rapidly followed by the first clinical trial of RIPC. In this small study, eight patients undergoing coronary artery bypass grafting (CABG) were randomised to receive either RIPC or a control condition. The study demonstrated an increase in blood lactate dehydrogenase (collected from the coronary perfusion catheter) in the preconditioned group, which the investigators attributed to an ability to maintain anaerobic metabolism in preconditioned cells.
In 2007, Hausenloy et al. 34 first demonstrated a reduction in troponin T levels in adults randomised to receive RIPC prior to CABG with cross-clamp fibrillation. In 2009, Venugopal et al. 35 also demonstrated a reduction in troponin T following RIPC in patients undergoing cold blood cardioplegia. However, in 2010, Rahman et al. 36 published the results of a larger single-centre, double-blind randomised controlled trial in which 162 patients undergoing CABG were randomised to receive either RIPC or placebo. In this study there was no difference in troponin release or any other clinical outcome between the two groups. Most recently, a larger single-centre study of 329 patients undergoing isolated CABG with cold blood cardioplegia and cardiopulmonary bypass who were randomised to RIPC or no RIPC demonstrated a reduction in postoperative troponin I in the preconditioned group. 37 The authors also attempted to address the question of whether a reduction in troponin equated to a measurable longer-term clinical benefit. They reported a reduction in all-cause mortality in the preconditioned group, which was sustained over > 4 years of follow-up.
In 2006, Iliodromitis et al. 38 first investigated whether RIPC would attenuate the inflammatory response in elective single-vessel percutaneous coronary intervention (PCI) for acute myocardial infarction with coronary stenting. They demonstrated an increase in cardiac enzymes and C-reactive protein in the preconditioned group (n = 41) and postulated that RIPC increased the inflammatory response. Subsequently, in 2009, Hoole et al. ,39 in a study of 242 patients undergoing elective PCI, demonstrated that RIPC prior to PCI attenuated procedure-related troponin release. Botker et al. 40 demonstrated that RIPC increased myocardial salvage in ST-segment elevation myocardial infarction. Increased interest in the clinical usefulness of RIPC in the setting of myocardial ischaemia (CABG or PCI) has led to the publication of many other small trials in recent years, all reporting differing outcomes. Other larger randomised controlled trials in cardioprotection are ongoing [Effect of Remote Ischaemic Preconditioning on Clinical Outcomes in CABG Surgery (ERICCA)41 and Remote Ischaemic Preconditioning for Heart Surgery (RIPHeart)42], recruiting over 3000 patients in total, and these are likely to eliminate some of the noise generated by the small studies and their attendant biases.
Evidence for a clinical benefit of remote ischaemic preconditioning in the kidney
Animal studies have demonstrated the therapeutic potential of RIPC in protecting against IR injury in the kidney;21 however, these benefits have yet to be established in clinical studies in humans. Although there have been several trials published, these have tended to be small, single-centre studies and they are likely to be affected by publication bias. Many report differing and short-term end points, making it difficult to compare outcomes or interpret the results. Additionally, the roles of coexistent comorbid states and polypharmacy in such patients are confounders, the degree to which cannot easily be ascertained.
A recent meta-analysis of randomised studies in cardiac/abdominal aortic aneurysm surgery suggested that there was a benefit of RIPC in reducing renal injury post surgery. 43 However, only five trials had absolute creatinine values documented and could be included and, of these, differing measures were reported and so the results were adjusted and reported as standardised mean values. Additionally, these trials were not powered individually for renal end points.
One other potential application that has been investigated in a clinical trial is the use of RIPC to protect against contrast-induced acute kidney injury. Patients with pre-existing renal dysfunction [serum creatinine > 1.4 mg/dl or estimated glomerular filtration rate (eGFR) < 60 ml/minute/1.73 m2] were randomised to receive RIPC (four times 5-minute arm cuff inflations) or sham treatment prior to elective coronary angioplasty. The authors reported a reduction in the rate of contrast-induced acute kidney injury, from 40% in the control group to 12% in the RIPC group (n = 100, p = 0.002). 44
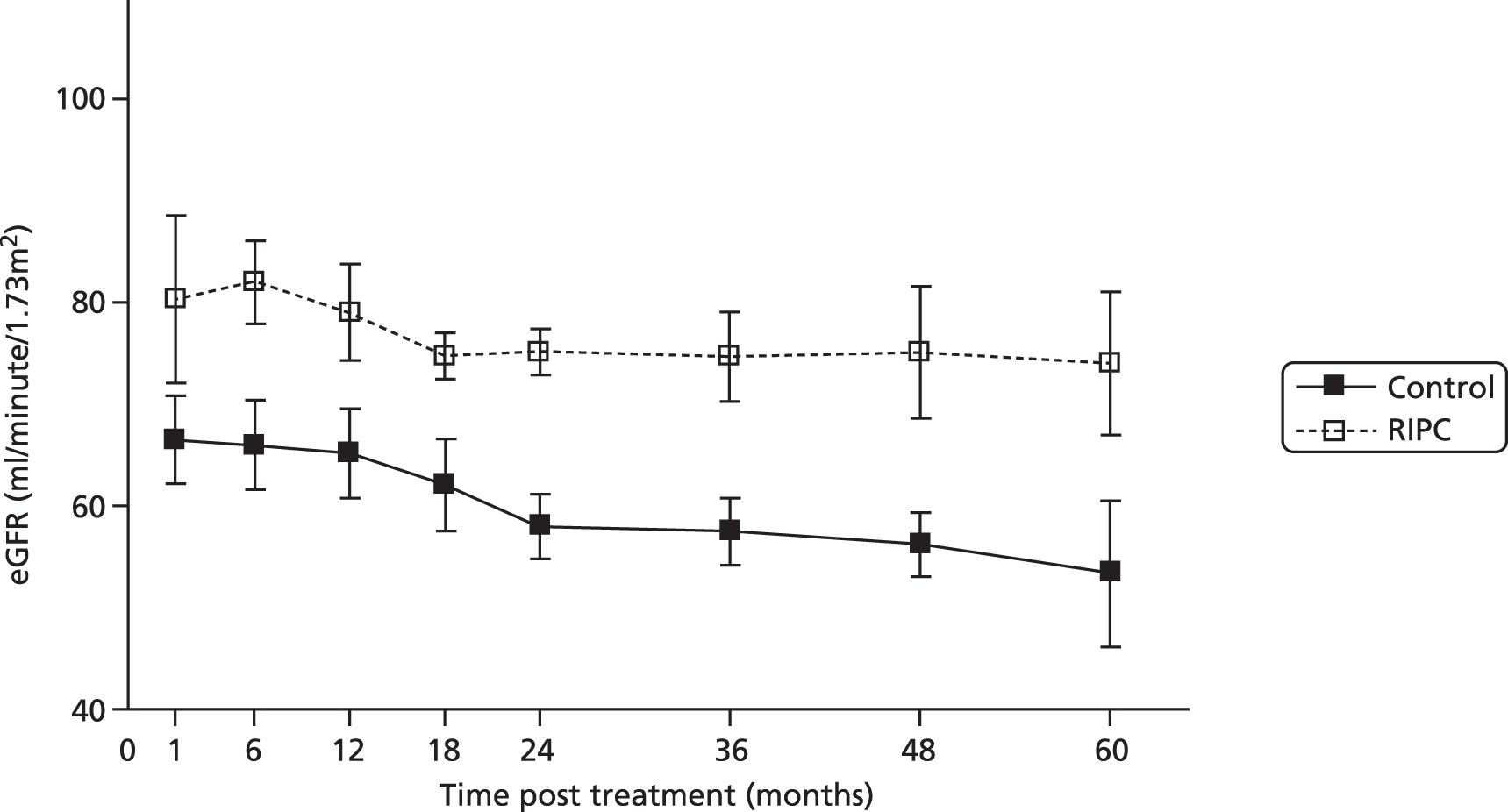
The use of direct IPC in transplantation (preconditioning of the donor organ at retrieval by repeated clamping/unclamping of the arterial supply) has been investigated in clinical trials in liver transplantation;45 however, no similar studies have yet been published in kidney transplantation. A pilot clinical trial carried out by our group in the setting of paediatric living-donor renal transplantation demonstrated the protective effects of late (‘second window’) RIPC. 46 A blood pressure cuff was used to produce 5-minute periods of limb ischaemia (three cycles; applied to the donor and recipient) 24 hours in advance of surgery. A prospective cohort of patients (n = 20) was randomised in a blinded fashion to sham RIPC or RIPC (n = 10 in each group). There was a beneficial effect of RIPC on long-term renal function (Figure 1).
FIGURE 1.
Effect of RIPC on long-term graft function following transplantation. The graph shows eGFR against time (1–60 months post transplantation) in the control and RIPC groups (mean ± standard error). The eGFR against time curves differed between the control group and the RIPC group (p < 0.001, two-way analysis of variance).
A second randomised controlled study of RIPC in renal transplantation was published (as a letter to the editor) in 2013. 47 In this study of 40 patients, live-donor kidney transplant recipients and their donors were randomised in pairs to receive either donor RIPC, recipient RIPC or no RIPC. In this small study the authors did not observe any differences between the three groups in urine volume, plasma creatinine level, acute kidney injury biomarkers, length of hospital stay or cost.
Rationale for the REmote preconditioning for Protection Against Ischaemia–Reperfusion in renal transplantation trial
The REmote preconditioning for Protection Against Ischaemia–Reperfusion in renal transplantation (REPAIR) trial was designed to provide definitive evidence for a benefit of RIPC in renal IR injury. The REPAIR trial measured the effects of early and late RIPC on renal IR injury using a 2 × 2 factorial design. Early RIPC activates an immediate but non-sustained protective effect. Identifying a protective effect of the early phase of RIPC has implications for future studies in deceased-donor transplantation, in which the unpredictable availability of organs precludes the scheduling of a preconditioning protocol 24 hours in advance of surgery. In this clinical setting (and currently the majority of transplants) the only feasible preconditioning stimulus will be early RIPC. Late RIPC had demonstrable benefits in a pilot study of renal transplantation,46 which we hypothesised were secondary to its prolonged and sustained phenotype. This profile might reduce IR injury and blunt the secondary inflammatory response to tissue injury. Applying the RIPC stimulus 24 hours before surgery enabled the late and sustained effects of RIPC on renal function (primary end point) to be assessed and was considered to establish aspects of the mechanism in humans. Moreover, the factorial design of the REPAIR trial allowed an assessment of the combination of early and late RIPC. Lastly, we sought to investigate the mechanism of RIPC in human tissue samples, recovered perioperatively from donor and recipient. These were sections of renal graft artery and vein that are trimmed from vessels to facilitate anastomosis and redundant biopsy material from protocol biopsies.
Chapter 2 Methods
Study design
The REPAIR trial was a multicentre, double-blind, European-based randomised controlled trial assessing the impact of RIPC on kidney function following renal transplantation. It used a 2 × 2 factorial design in which the recipients and their donors were randomised to RIPC or a sham procedure both 24 hours before surgery (late RIPC) and immediately pre surgery (early RIPC). Therefore, there were four arms in total: (1) a sham procedure both 24 hours before surgery and immediately pre surgery, (2) early RIPC and a sham procedure 24 hours before surgery, (3) late RIPC and a sham procedure immediately pre surgery and (4) late RIPC and early RIPC. Both donor and recipient were randomised to the same intervention group.
Aim
The REPAIR trial investigated whether RIPC improved kidney function and other clinical outcomes following renal transplantation. RIPC is a simple, non-invasive and virtually cost-free intervention, and any improvement in graft function might ultimately lead to prolonged allograft life in these patients, with resultant economic and quality of life benefits. The findings might also have implications for the use of RIPC in other clinical ischaemic syndromes.
Participants
The study intended to recruit 400 pairs of transplant recipients and their living donors from centres in the UK, the Netherlands, Belgium and France. Patients undergoing living-donor transplantation aged ≥ 18 years from 13 tertiary care hospitals in the UK, the Netherlands, Belgium and France were invited to take part in the study.
Final inclusion and exclusion criteria
Inclusion criteria
-
Patients undergoing living-donor transplantation.
-
Patients aged ≥ 18 years.
Exclusion criteria
-
Patients on KATP channel-opening or -blocking drugs.
-
Patients on ciclosporin.
-
Patients with a known iodine sensitivity (who cannot undergo iohexol clearance studies).
-
Patients with ABO incompatibility.
-
Any patient requiring human leucocyte antigen (HLA) antibody removal therapy.
There were two major protocol amendments that affected the inclusion and exclusion criteria. In December 2009, the decision was made to exclude patients who require HLA antibody removal therapy as they required a different immunosuppression regime that may have an effect on preconditioning. In September 2010, the decision was made to include patients who had had a previous transplant, who were originally excluded from the trial. It had been considered that RIPC may not be as effective in patients who have undergone a previous transplant; however, there was subsequently a change in opinions and evidence.
A full list of protocol amendments can be found in Appendix 1.
Recruitment
The 13 centres from which patients were recruited were Guy’s and St Thomas’ NHS Foundation Trust, London, UK; Leiden University Medical Centre, Leiden, the Netherlands; North Bristol NHS Trust, Bristol, UK; Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; Royal Free London NHS Foundation Trust, London, UK; Queen Elizabeth Hospital, Birmingham, UK; Vrije Universiteit (VU) University Medical Centre, Amsterdam, the Netherlands; St George’s Hospital, London, UK; Royal London Hospital, London, UK; Western Infirmary, Glasgow, UK; Centre Hospitalier Universitaire (CHU) Erasme, Brussels, Belgium; CHU de Liège, Belgium; and Centre Hospitalier Régional Universitaire (CHRU) de Lille, France. The recruitment of patients was initiated in the outpatient setting. Both the recipient and the donor were given the information sheet prior to giving consent.
Randomisation, concealment and blinding
Patients were allocated at random in a 1 : 1 : 1 : 1 ratio to the control condition (sham RIPC), early RIPC alone (immediately pre surgery), late RIPC alone (24 hours pre surgery) and dual RIPC (RIPC 24 hours before surgery and immediately pre surgery). This was performed using a web-based service provided by Sealed Envelope™ (Sealed Envelope Ltd, London, UK) through the Clinical Trials Unit (CTU) at the London School of Hygiene and Tropical Medicine. The method of randomisation was random permuted blocks of size four and eight stratified by recruiting centre. During the study only the unblinded statistician supporting the Data Monitoring Committee (DMC) had access to the randomisation codes.
The enrolment and preconditioning procedures were performed by an unblinded research nurse who was not involved in sample collection or data analysis. All other research personnel at each study site, including those responsible for assessing outcomes, remained blinded to the allocation of patients to either real or sham RIPC. The patients and donors were also blinded to the allocation of their randomised intervention by the use of sham procedures in those not allocated to RIPC. A limited number of staff at the CTU were unblinded to the allocations, in order to enter data onto the electronic case report forms (eCRFs), a web-based data capture system provided by Sealed Envelope. However, no member of staff at the CTU had involvement or influence in any outcome measures.
Treatment group allocation
The trial intervention was a physiological procedure and was performed on both the donor and recipient at two time points before transplantation (24 hours before surgery and immediately before surgery). At the time point immediately before surgery, the active or sham RIPC sequences were initiated before induction of anaesthesia and were completed in advance of the initiation of surgery. There were no other interventions. The interventions consisted of different combinations of the active RIPC intervention and sham RIPC intervention as described below. The active RIPC procedure consisted of four 5-minute inflations of a blood pressure cuff on the upper arm to 40 mmHg above systolic blood pressure separated by 5-minute periods when the cuff was deflated. The sham RIPC procedure consisted of four 5-minute inflations of a blood pressure cuff on the upper arm to a pressure that would not impede blood flow (40 mmHg) separated by 5-minute periods when the cuff was deflated. All patients and donors underwent either the sham or the active RIPC procedure at both time points and so were randomised to one of the following groups:
-
control group: the control group underwent sham RIPC both 24 hours before surgery and immediately before surgery
-
early RIPC group: the early RIPC group underwent sham RIPC 24 hours before surgery and active RIPC immediately before surgery
-
late RIPC group: the late RIPC group underwent active RIPC 24 hours before surgery and sham RIPC immediately before surgery
-
dual RIPC: the dual RIPC group underwent active RIPC both 24 hours before surgery and immediately before surgery.
Postintervention treatment regimens
Patients followed the same immunosuppressive protocol, which was agreed by all participating centres. Patients received methylprednisolone and/or prednisolone according to local practice and anti-CD25 antibody [basiliximab (Simulect®, Novartis)] according to the manufacturer’s recommendations (20 mg intravenously pre transplant followed by 20 mg intravenously on day 4). Patients received mycophenolate or azathioprine according to local practice. Mycophenolate was administered as mycophenolate mofetil (CellCept®; Roche), starting at a dose of at least 1 g/day, or as mycophenolate sodium (Myfortic®; Novartis), starting at a dose of at least 720 mg/day. Azathioprine was administered at a starting dose of 2 mg/kg. Patients received tacrolimus with a target concentration according to local practice. Antimicrobial and antithrombotic prophylaxis was administered in accordance with local practice. It was anticipated that patients would receive prophylaxis against Pneumocystis carinii pneumonia, oral Candida albicans and cytomegalovirus (donor positive, recipient negative). There were no alterations to routine treatment and no changes from routine practice for anaesthesia.
Data collection and management
Data management
Randomisation and completion of the interventions were performed by the unblinded research staff. The intervention data were then faxed to the CTU, where the data were entered onto the eCRFs and then stored in locked cabinets. Following this, all subsequent follow-up visits and data collection were completed by blinded research staff. A paper case report from (CRF) was provided to assist with data collection but the source data were considered to be those on the eCRF. A series of logic and range checks were built into the system to reduce the possibility of erroneous data being entered. The system also contained a log that detailed all notable events associated with the trial (including inserts, updates and deletions) and this provided a clear and complete audit trail throughout the trial. The data management processes were conducted following the principles of good clinical practice (GCP) (see www.ich.org; accessed 12 March 2015) and the Data Protection Act 1998. 48
Monitoring and site visits
The first site visit was a prerecruitment visit for training (trial interventions and procedures) and ensuring that all relevant documentation was in place before the start of recruitment. After the first patient had been recruited in each site, the senior data manager provided training on the eCRF. This training was either carried out as part of a visit to the site or performed remotely using the standard operating procedure document. A further site visit occurred at each centre after five patients had been recruited. At this visit the CRF, consent forms, source data, sample storage and the site file were monitored and the data verified. Following the visit, a report was sent to the principal investigator (PI) and any other relevant research staff involved in the trial, which included recommendations for changes if any issues were raised at the visit. Regular monitoring of the data was also performed by the trial statistician and senior data manager. Further visits could be arranged at the different sites if any problems arose or if the statistical monitoring highlighted any concerns.
Baseline assessment
All recipients had a medical history taken and a clinical examination as part of usual care. The following information was recorded at baseline: weight, height, gender, ethnicity, systolic blood pressure, creatinine, urea and albumin levels, comorbidities, date of birth, date when started dialysis and type of dialysis at time of admission. All donors filled out either a UK Transplant or a Eurotransplant organ donor form, depending on location. The following information was collected from this form at baseline: weight, height, gender, ethnicity, systolic blood pressure, creatinine, urea and albumin levels, age and glomerular filtration rate (GFR).
Follow-up
Donors were followed up immediately after RIPC and at day 1 (the day of the transplant), day 2 and day 3. Recipients underwent follow-up immediately after RIPC, perioperatively and at day 1, day 2, day 3, day 5, month 3 and month 12. The 3-month and 1-year follow-ups were carried out primarily at clinic visits. If a patient was not able to attend, information was obtained directly from the patient or from the patient’s general practitioner. In addition, recipients are being followed up annually to 5 years using clinic visits, telephone calls and renal registry data to assess eGFR. Recipients at the Leiden University Medical Centre were also followed up at 6 months post transplant for renal fibrosis assessment.
Safety assessments
This was not a trial of an investigational medicinal product; therefore, by definition, all untoward occurrences were adverse events rather than adverse reactions. Safety assessments were collected from the time of randomisation to the completion of follow-up for recipients and from the time of intervention to discharge for donors. There was a list of expected adverse events (both serious and non-serious) for which information was not collected. A detailed listing of individual adverse events was supplied as part of the reports to the DMC.
Outcome measures
Primary outcome
The primary end point of the study was GFR at 12 months after transplantation as measured by iohexol clearance.
Secondary outcomes
The secondary outcomes were:
-
Time for serum creatinine to fall by 50%. For each recipient the time for serum creatinine to fall by 50% from the value in recovery was derived. Creatinine was measured up to 24 hours post surgery.
-
eGFR 3 months after transplantation, derived from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. 49
-
eGFR 12 months after transplantation, derived from the CKD-EPI equation.
-
Plasma interleukin 6 (IL-6), interleukin 1 beta (IL-1β), interferon gamma (IFN-γ) and tumour necrosis factor alpha (TNF-α) before and 1–3 days after surgery for donors and 1–5 days after surgery for recipients.
-
RIPC-induced protein expression changes in renal tissue [analysis in biopsy material; protein kinase C (epsilon isoform; activated/membrane-bound fraction), manganese superoxide dismutase (MnSOD), COX-2, iNOS, HSPs 27/72, reperfusion injury salvage kinases [PI3K–protein kinase B (Akt) and mitogen/extracellular signal-regulated kinases (MEK1/2)–ERK)].
-
Renal graft cortical tubulointerstitial fibrosis at 6 months (digital analysis of Sirius red staining in biopsy material).
-
Incidence of delayed graft function (either the need for dialysis in the first 7 days after transplantation or serum creatinine levels increase, remain unchanged or decrease by < 10% per day in 3 consecutive days in the first week after transplantation).
-
Incidence of acute rejection during the first 12 months after transplantation. Rejection was defined as biopsy-proven rejection, clinical acute rejection or steroid-resistant rejection. Biopsy-proven rejection was defined as any rejection grade according to the Banff criteria,50 based on histopathological appearance of a needle core biopsy of the transplant kidney. Clinical acute rejection was defined as any biopsy-proven or biochemical rejection that is treated with pulsed methylprednisolone. Steroid-resistant rejection was defined as a rejection episode that did not respond to a 3-day course of pulsed methylprednisolone and which required antithymocyte globulin (Thymoglobuline®; Sanofi-Aventis) or muromonab-CD3 therapy.
-
Serum creatinine levels and eGFR 2–5 years after transplantation.
-
Patient survival (at 12 months and 2–5 years) and graft survival (at 3 months, 12 months and 2–5 years). These were measured from the date of transplant and were reported for all deaths and graft failures because of rejection and from all causes.
Laboratory techniques
Glomerular filtration rate assessment at 12 months
Omnipaque 240 (5 ml; GE Healthcare Ltd) was administered intravenously and blood samples were taken at 5, 120, 180 and 240 minutes after dosing. Heparinised blood (5 ml) was centrifuged at 400 g for 10 minutes and a 2-ml sample of supernatant was aspirated and collected in a labelled Eppendorf tube. Heparinised plasma was stored at –70°C/–80°C until analysis, with GFR calculated using the iohexol clearance rate between 120 and 240 minutes. Results were corrected for body surface area.
Enzyme-linked immunosorbent assay assessments
Enzyme-linked immunosorbent assays (ELISAs) were used to measure IL-6, IL-1β, IFN-γ and TNF-α in plasma (donors and recipients) and urine (recipients) before (plasma) and 1–2 days after surgery. Urine and heparinised blood (5 ml) were centrifuged at 400 g for 10 minutes and the supernatant was aspirated and collected in labelled Eppendorf tubes.
Immunoblotting
Vascular tissue was homogenised in buffer containing peptidase inhibitors, electrophoresed on a sodium dodecyl sulphate-polyacrylamide gel and transferred to a nitrocellulose membrane. Antibodies (Calbiochem, Invitrogen and Dako) were used to probe membranes for proteins activated by early and late RIPC. Analysis is ongoing.
Immunohistochemistry
Cross-sections were obtained from formalin-fixed, paraffin-embedded renal biopsy tissues. Analysis is ongoing but sections will be prepared for immunohistochemistry by dewaxing and rehydrating using xylene and alcohol. Antibodies for use in immunohistochemistry will be determined from the results of the immunoblotting analysis.
Kidney graft fibrosis
Cortical tubulointerstitial collagen deposition was assessed by Sirius red staining of tissue slices, using digital analysis software. Analysis is in progress and will be performed at baseline and at 6 months following transplantation; graft fibrosis at 6 months will be expressed relative to baseline graft fibrosis.
Sample size
Primary analyses
There were two main analyses to reflect the factorial design of the trial, using mean GFR in the first year after transplantation. These were: (1) the two arms receiving early RIPC compared with the two arms not receiving early RIPC and (2) the two arms receiving late RIPC compared with the two arms not receiving late RIPC.
Mean GFR in the first year after transplantation was estimated to be 47.3 ml/minute/1.73 m2 in those not receiving RIPC, with a standard deviation (SD) of 13.9 (data from the Cambridge transplantation programme). The calculations in Table 1 were based on either early or late RIPC increasing GFR by 10% (or 4.73 ml/minute/1.73 m2). A trial of 80 patients in each of the four arms (160 for each comparison group, 320 in total) gives 80% power (with a 5% type 1 error) to detect this difference in GFR at 12 months for either comparison allowing for a 15% dropout rate.
| SD | Treatment group, n | Total, n | |||
|---|---|---|---|---|---|
| Control early, control late | Early RIPC, control late | Control early, late RIPC | Early RIPC, late RIPC | ||
| 12.9 | 69 | 69 | 69 | 69 | 276 |
| 13.9 | 80 | 80 | 80 | 80 | 320 |
| 14.9 | 92 | 92 | 92 | 92 | 368 |
This sample size provided reasonable power for the primary end point while retaining useful power for secondary analyses. The trial would provide > 80% power if the difference was > 4.73 ml/minute/1.73 m2 (as might be expected if the effects of early and late RIPC combine multiplicatively, i.e. a 21% increase in GFR compared with no RIPC), the SD was lower than anticipated or the dropout rate was < 15%.
Further, some allowance was made for the possibility of a moderate interaction between early and late RIPC whereby the impact might be to lessen the anticipated effect when comparing: (1) the arms receiving late RIPC and the arms not receiving late RIPC and (2) the arms receiving early RIPC and the arms not receiving early RIPC. To allow for this possibility the aim was to recruit 100 pairs of patients in each of the four arms (400 in total).
Statistical analysis
The primary analysis was conducted on an intention-to-treat (ITT) basis with all patients and donors, when information was available, considered in the groups to which they were randomised. A per-protocol (PP) analysis was undertaken including those who received the randomised intervention as specified [i.e. excluding those pairs in which the intervention was not undertaken or in which the intervention was incomplete (whether RIPC or sham)]. All p-values are two-sided.
Primary outcome
Primary analysis
The primary analysis included the comparison of mean GFR at 1 year after transplantation (1) between the two arms receiving early RIPC and the two arms not receiving early RIPC and (2) between the two arms receiving late RIPC and the two arms not receiving late RIPC.
The model used to complete the primary analysis was a two-way analysis of covariance (ANCOVA). Specifically, this was a regression model adjusted for the donor’s baseline eGFR with indicator variables for the two treatment schedules (early and late RIPC). The distribution of GFR was examined to assess whether transformations were necessary to adhere to the assumptions of the ANCOVA model.
Secondary analysis
A large interaction between early and late RIPC on mean GFR was not expected and the trial was not powered to detect small interactions. However, any interaction was formally assessed by inclusion of an interaction term between the two treatment types in the ANCOVA model. Irrespective of the result of this interaction test a secondary analysis was conducted combining all RIPC arms together compared with the control arm receiving no RIPC, that is, to address the question of whether giving early and/or late RIPC confers a benefit compared with no RIPC. In addition, for patients in whom an iohexol measurement was not available at 12 months, eGFR at 12 months was used when available.
Secondary outcomes
Estimated glomerular filtration rate 3 months and 12 months after transplantation
The ANCOVA models described for the primary analysis were also used for the analysis of these secondary outcomes.
Time for serum creatinine to fall by 50% from the value in recovery
A Cox proportional hazards regression model for time to event (fall in serum creatinine of 50%) was used with indicator variables for the two treatment types. The interaction between early and late RIPC was assessed by inclusion of an interaction term in the Cox regression model, along with indicator variables for early and late RIPC. As with the primary outcome, a secondary analysis combined all RIPC arms compared with the arm receiving no RIPC.
Plasma interleukin 6, interleukin 1 beta, interferon gamma and tumour necrosis factor alpha before and up to 3 days after surgery for donors and 5 days after surgery for recipients
The ANCOVA model described for the primary analysis was used for the analysis of these secondary end points. Values on the second day after surgery were compared between treatment groups with adjustment for baseline values of the outcome measure. As values of these outcomes showed positive skew, data were transformed into natural log before analysis. Results for IL-1β and INF-γ used bias-corrected and accelerated bootstrapped confidence intervals (CIs) based on 2000 replications, as even after transformation it was apparent that the parametric assumptions of the linear model were violated. The use of bootstrapping means that there is no p-value for these analyses, but inference of statistical significance at p < 0.05 can be made from whether the 95% CI for treatment effects cross zero.
Incidence of delayed graft function
The proportion of participants with delayed graft function during the first 7 days was analysed using a logistic regression model with indicator variables for the two treatment types. Because of the small number of events it was not possible to test formally for an interaction between treatments. As with the primary outcome, a secondary analysis combined all RIPC arms compared with the arm receiving no RIPC.
Incidence of acute rejection during the first 12 months after transplantation
A Cox proportional hazards regression model for time to event (acute rejection) was used with indicator variables for the two treatment types. The interaction between early and late RIPC was assessed by inclusion of an interaction term in the Cox regression model, along with indicator variables for early and late RIPC. As with the primary outcome, a secondary analysis combined all RIPC arms compared with the arm receiving no RIPC. Participants were censored on the date at which the outcome occurred, if they died, if they were lost to follow-up or at 12 months after transplantation.
Three-month and 12-month graft survival
Graft survival was analysed using a time-to-event framework. The log-rank test was used to evaluate difference in graft loss between: (1) the two arms receiving late RIPC and the the two arms not receiving late RIPC and (2) the two arms receiving early RIPC and the two arms not receiving early RIPC. Participants were censored on the date at which the outcome occurred, if they died, if they were lost to follow-up or at 12 months after transplantation
Twelve-month survival
Patient survival was analysed using a time-to-event framework, as described for evaluation of the incidence of graft loss.
Missing data
An analysis was undertaken by imputing the eGFR for those patients who did not have a GFR determined by iohexol clearance measured at 12 months. All other analyses were conducted on a complete case basis, excluding any participants who had missing data for the outcome or predictors of interest in that particular model.
Subgroup analyses
The main subgroup analysis was to assess whether any effect of RIPC differed according to a patient’s underlying risk of low GFR at 12 months. This was assessed using a linear regression model to predict GFR at 12 months from baseline explanatory variables. Potential predictors of GFR at 12 months were identified from the published literature. 51–54 For each potential predictor, a linear regression model was used to assess the independent association between the value at baseline and GFR measured by iohexol clearance at 12 months. Those factors that showed evidence of association with GFR were entered into a multiple linear regression model, starting with factors with the strongest association. The final model included all factors that were associated with GFR (p < 0.10) together with terms for early and late RIPC. From this model, a risk score was calculated for each patient as the predicted GFR at 12 months excluding the estimated effects of early and late RIPC (i.e. the prediction for that participant if they had received sham early and late RIPC). An interaction term was fitted between risk and treatment to establish if there was a difference in benefit according to the underlying risk. Patients were categorised into quartiles of baseline risk and the data were presented as the mean (SD) GFR by treatment group in each baseline risk group.
Two other specific subgroup analyses were conducted:
-
to compare patients for whom this was their first transplant with patients who had had a previous transplant
-
to compare patients with a ‘000’ mismatch (i.e. those with no mismatches at any of the major HLA loci) type with patients without a ‘000’ mismatch type.
These two subgroup analyses were chosen because they represent changes to the inclusion criteria of the study after it had begun. The mean difference in the treatment groups between each subgroup and 95% CIs were calculated and subgroups compared using an interaction test.
Ethical considerations
Ethical approval for the study in the UK was given by the Joint University College London (UCL)/University College London Hospital (UCLH) Committees on the Ethics of Human Research in June 2009 (reference number 09/H0715/48). Outside the UK, local research ethics committee approvals were gained at all recruiting sites and all hospitals that were involved in trial follow-up. The trial was registered with the International Standard Randomised Controlled Trial Register (reference number ISRCTN30083294). The trial had two committees overseeing its conduct: the Trial Steering Committee (TSC) and the Project Management Group (PMG). In addition, there was an independent DMC to ensure the safety of patients in the trial and to review operational issues such as recruitment. The DMC was the only group to review interim analyses broken down by treatment group during recruitment and follow-up of patients in the trial. The DMC performed interim safety analyses annually. The interim reports contained details of patient recruitment, demographic and baseline characteristics, transplant and intervention details, primary safety end points, the primary efficacy end point and other end points identified by the DMC, including adverse and serious adverse events. At the 18-month DMC meeting, a review of the assumptions on which the sample size calculations were based was carried out as requested by the Efficacy and Mechanism Evaluation (EME) programme board.
The TSC had overall responsibility for the scientific integrity and quality of the trial. This involved ensuring that the trial was conducted to the standards set out in the guidelines for GCP and that the protocol was adhered to as far as possible and having responsibility for overall patient safety as well as considering new relevant information arising throughout the duration of the trial. The TSC also had responsibility for considering any recommendations made by the DMC. The TSC met annually throughout the trial to monitor the progress and quality of the trial, to review the recruitment rate and consider protocol amendments. The PMG was responsible for the day-to-day running of the trial, meeting fortnightly during the setting up of the trial and the early stages of recruitment and then approximately monthly for the remainder of the trial.
Patient and public involvement
There were two consumer representatives on the TSC who were actively involved in all TSC activities. The consumers were recruited from the Royal Free Hospital, London, where there is a very active support network for patients who have undergone kidney transplantation. In particular, they were key in developing both the participant information sheet and consent forms. Although the consumers did not always attend the meetings, they were always willing to contribute and comment on any REPAIR trial literature. Their contribution was extremely valuable, in particular in relation to raising the profile of the trial within the kidney transplant community. Both the Royal Free Hospital Kidney Patients Association (RFHKPA) and the National Kidney Federation published articles on the REPAIR trial in their newsletters, which helped to raise further awareness of the REPAIR trial within the kidney transplant community.
Chapter 3 Results
Figure 2 shows the numbers of pairs of participants who were screened and randomised and who underwent measurement of the primary outcome. Between 4 January 2010 and 29 April 2013, 406 donor–recipient pairs were randomised: 99 to sham RIPC, 102 to early RIPC, 103 to late RIPC and 102 to dual RIPC. Donors and recipients attended a clinic visit at the time of randomisation (baseline). Recipients attended follow-up visits at 3 and 12 months post surgery. Recipients gave blood samples for assessment of the primary outcome, GFR at 12 months by iohexol clearance, between 7 January 2011 and 22 May 2014.
FIGURE 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram.

Tables 2–4 show the baseline characteristics of the donors and recipients, which were reasonably well balanced across the four treatment arms.
| Characteristic | Control (n = 99) | Early RIPC (n = 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| Male | 95 | 58 | 61.1 | 98 | 71 | 72.4 | 99 | 65 | 65.7 | 99 | 73 | 73.7 |
| Female | 37 | 39.0 | 27 | 27.6 | 34 | 34.3 | 26 | 26.3 | ||||
| Ethnicity | 95 | 98 | 99 | 99 | ||||||||
| White | 76 | 80.0 | 81 | 82.7 | 86 | 86.9 | 78 | 78.8 | ||||
| Asian | 5 | 5.3 | 8 | 8.2 | 4 | 4.0 | 6 | 6.1 | ||||
| Black | 5 | 5.3 | 5 | 5.1 | 5 | 5.1 | 11 | 11.1 | ||||
| Other | 6 | 6.3 | 3 | 3.1 | 4 | 4.0 | 2 | 2.0 | ||||
| Not stated | 3 | 3.2 | 1 | 1.0 | 0 | 0.0 | 2 | 2.0 | ||||
| Totala | Mean | SD | Totala | Mean | SD | Totala | Mean | SD | Totala | Mean | SD | |
| Age (years) | 95 | 46.8 | 15.1 | 98 | 47.6 | 15.1 | 99 | 45.9 | 14.2 | 99 | 45.3 | 15.3 |
| Height (cm) | 95 | 172.0 | 10.0 | 98 | 173.4 | 10.4 | 99 | 173.0 | 10.1 | 99 | 175.2 | 9.2 |
| Weight (kg) | 95 | 78.1 | 17.4 | 98 | 79.0 | 16.9 | 97 | 76.8 | 15.8 | 97 | 76.6 | 16.4 |
| BMI (kg/m2) | 95 | 26.3 | 4.9 | 98 | 26.2 | 4.7 | 97 | 25.5 | 4.2 | 97 | 24.8 | 4.4 |
| Creatinine (µmol/l) | 95 | 635.4 | 292.6 | 98 | 606.9 | 256.1 | 99 | 622.2 | 296.7 | 99 | 643.1 | 261.4 |
| Urea (mmol/l) | 94 | 21.5 | 7.9 | 94 | 21.3 | 8.2 | 99 | 21.9 | 8.3 | 99 | 20.8 | 8.2 |
| Plasma albumin (g/l) | 90 | 39.4 | 6.2 | 93 | 40.0 | 5.5 | 92 | 40.0 | 5.4 | 88 | 39.6 | 5.3 |
| Systolic blood pressure (mmHg) | 94 | 132.3 | 19.0 | 98 | 137.6 | 19.0 | 98 | 132.9 | 19.7 | 99 | 132.6 | 15.3 |
| Characteristic | Control (n = 99) | Early RIPC (n = 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| Previous transplant | 99 | 8 | 8.1 | 102 | 5 | 4.9 | 103 | 6 | 5.8 | 102 | 10 | 9.8 |
| Glomerulonephritis | 95 | 19 | 20.0 | 98 | 18 | 18.4 | 99 | 13 | 13.1 | 99 | 17 | 17.2 |
| Biopsy proven | 95 | 13 | 13.7 | 98 | 13 | 13.3 | 99 | 10 | 10.1 | 99 | 16 | 16.2 |
| Pyelonephritis | 95 | 3 | 3.2 | 98 | 2 | 2.0 | 99 | 4 | 4.0 | 99 | 2 | 2.0 |
| Diabetes | 95 | 11 | 11.6 | 98 | 13 | 13.3 | 99 | 6 | 6.1 | 99 | 7 | 7.1 |
| Polycystic kidney | 95 | 13 | 13.7 | 98 | 12 | 12.2 | 99 | 18 | 18.2 | 99 | 13 | 13.1 |
| Hypertension | 95 | 31 | 32.6 | 98 | 41 | 41.8 | 99 | 38 | 38.4 | 99 | 48 | 48.5 |
| Renal vascular disease | 95 | 1 | 1.1 | 98 | 2 | 2.0 | 99 | 4 | 4.0 | 99 | 2 | 2.0 |
| Aetiology uncertain | 95 | 7 | 7.4 | 98 | 6 | 6.1 | 99 | 7 | 7.1 | 99 | 7 | 7.1 |
| Other | 95 | 47 | 49.5 | 98 | 49 | 50.0 | 99 | 54 | 54.5 | 99 | 53 | 53.5 |
| Dialysis prior to transplant | 95 | 51 | 53.7 | 98 | 51 | 52.0 | 99 | 46 | 46.5 | 99 | 57 | 57.6 |
| Years of dialysis at transplant (total, median, range) | 50 | 1.2 | 0–9.5 | 51 | 1.1 | 0–27.7 | 46 | 1.0 | 0–9.5 | 57 | 1.1 | 0–11.9 |
| Characteristic | Control (n = 99) | Early RIPC (n= 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| Male | 95 | 41 | 43.2 | 98 | 47 | 48.0 | 99 | 47 | 47.5 | 99 | 41 | 41.4 |
| Female | 54 | 56.8 | 51 | 52.0 | 52 | 52.5 | 58 | 58.6 | ||||
| Ethnicity | 95 | 98 | 99 | 99 | ||||||||
| White | 78 | 82.1 | 75 | 76.5 | 85 | 85.9 | 81 | 81.8 | ||||
| Asian | 3 | 3.2 | 10 | 10.2 | 4 | 4.0 | 4 | 4.0 | ||||
| Black | 5 | 5.3 | 5 | 5.1 | 4 | 4.0 | 10 | 10.1 | ||||
| Other | 5 | 5.3 | 4 | 4.1 | 4 | 4.0 | 3 | 3.0 | ||||
| Not stated | 4 | 4.2 | 4 | 4.1 | 2 | 2.0 | 1 | 1.0 | ||||
| Totala | Mean | SD | Totala | Mean | SD | Totala | Mean | SD | Totala | Mean | SD | |
| Age (years) | 95 | 50.2 | 11.8 | 98 | 50.8 | 12.9 | 99 | 49.1 | 12.2 | 99 | 49.2 | 12.5 |
| Height (cm) | 87 | 170.2 | 9.3 | 89 | 171.2 | 10.4 | 90 | 172.0 | 9.6 | 91 | 171.3 | 10.1 |
| Weight (kg) | 92 | 75.4 | 11.9 | 94 | 78.1 | 15.9 | 92 | 75.1 | 15.1 | 95 | 77.5 | 14.3 |
| BMI (kg/m2) | 87 | 25.9 | 3.2 | 89 | 26.8 | 4.0 | 89 | 25.2 | 4.0 | 91 | 26.5 | 3.9 |
| Creatinine (µmol/l) | 95 | 71.5 | 13.2 | 98 | 72.6 | 15.9 | 99 | 73.2 | 16.9 | 99 | 75.0 | 16.5 |
| Urea (mmol/l) | 90 | 5.4 | 3.3 | 94 | 5.1 | 1.4 | 95 | 5.9 | 5.5 | 93 | 5.1 | 1.2 |
| Serum albumin (g/l) | 85 | 43.1 | 3.8 | 82 | 43.5 | 3.8 | 86 | 43.2 | 4.8 | 81 | 43.6 | 4.4 |
| eGFR (ml/minute/1.73 m2) | 95 | 96.3 | 13.0 | 98 | 95.1 | 16.4 | 99 | 95.5 | 15.5 | 99 | 93.3 | 16.8 |
| Systolic blood pressure (mmHg) | 94 | 121.7 | 13.3 | 98 | 126.3 | 15.6 | 97 | 125.8 | 15.5 | 99 | 124.3 | 15.3 |
Tables 5 and 6 give details of the intervention and transplant procedure, respectively. As shown in Table 5, 362 of the 406 pairs (89%) received the full sham or RIPC intervention according to protocol. The reasons for incomplete intervention were human error (six pairs), such as accidental lowering of the pressure on the cuff; mechanical failure of the cuff (one pair); medical reasons (one pair); not eligible for the REPAIR trial (12 pairs); operational reasons (nine pairs), such as surgery time being moved; donor or recipient unable to tolerate the intervention (nine pairs); donor or recipient declined the intervention (three pairs); or the pair withdrew from the trial before surgery (three pairs).
| RIPC cycles | Control (n = 99) | Early RIPC (n = 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| All 16 cycles completed PP | 88 | 88.9 | 89 | 87.3 | 92 | 89.3 | 93 | 91.2 |
| All 16 cycles completed, duration or pressure not PP | 1 | 1.0 | 6 | 5.9 | 3 | 2.9 | 2 | 2.0 |
| Fewer than 16 cycles completed | 10 | 10.1 | 7 | 6.9 | 8 | 7.8 | 7 | 6.9 |
| 0 | 5 | 5.1 | 4 | 3.9 | 5 | 4.9 | 3 | 2.9 |
| 8 | 2 | 2.0 | 0 | 0.0 | 2 | 1.9 | 0 | 0.0 |
| 12 | 2 | 2.0 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 |
| 13 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 |
| 14 | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 | 1 | 1.0 |
| 15 | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 |
| Surgery details | Control (n = 99) | Early RIPC (n= 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| Nephrectomy left side | 93 | 71 | 76.3 | 98 | 78 | 79.6 | 99 | 80 | 80.8 | 99 | 80 | 80.8 |
| Laparoscopic | 93 | 73 | 78.5 | 98 | 75 | 76.5 | 99 | 83 | 83.8 | 99 | 76 | 76.8 |
| Preservation solution | 90 | 94 | 96 | 96 | ||||||||
| Custodiol | 25 | 27.7 | 27 | 28.7 | 27 | 28.1 | 29 | 30.2 | ||||
| Marshall’s HOC | 58 | 64.4 | 63 | 67.0 | 64 | 66.7 | 61 | 63.5 | ||||
| UW (ViaSpan) | 7 | 7.8 | 4 | 4.3 | 5 | 5.2 | 6 | 6.3 | ||||
| HLA A mismatches | 89 | 96 | 96 | 92 | ||||||||
| 0 | 17 | 19.1 | 25 | 26.0 | 27 | 28.1 | 25 | 27.2 | ||||
| 1 | 49 | 55.1 | 52 | 54.2 | 45 | 46.9 | 52 | 56.5 | ||||
| 2 | 23 | 25.8 | 19 | 19.8 | 24 | 25.0 | 15 | 16.3 | ||||
| HLA B mismatches | 89 | 96 | 96 | 92 | ||||||||
| 0 | 18 | 20.2 | 20 | 20.8 | 15 | 15.6 | 15 | 16.3 | ||||
| 1 | 45 | 50.6 | 49 | 51.0 | 51 | 53.1 | 55 | 59.8 | ||||
| 2 | 26 | 29.2 | 27 | 28.1 | 30 | 31.3 | 22 | 23.9 | ||||
| HLA DR mismatches | 89 | 96 | 96 | 92 | ||||||||
| 0 | 24 | 27.0 | 23 | 24.0 | 15 | 15.6 | 22 | 23.9 | ||||
| 1 | 52 | 58.4 | 56 | 58.3 | 58 | 60.4 | 48 | 52.2 | ||||
| 2 | 13 | 14.6 | 17 | 17.7 | 23 | 24.0 | 22 | 23.9 | ||||
| Totala | Mean | SD | Totala | Mean | SD | Totala | Mean | SD | Totala | Mean | SD | |
| Warm ischaemia time (minutes) | 69 | 25.1 | 23.2 | 73 | 26.6 | 22.3 | 75 | 26.1 | 21.9 | 75 | 24.5 | 20.8 |
| Cold ischaemia time (minutes) | 67 | 140.6 | 76.7 | 70 | 129.8 | 71.4 | 71 | 139.9 | 74.2 | 67 | 133.7 | 74.8 |
Table 7 provides information on the postsurgery hospital stay by treatment group and Table 8 provides information on the immunosuppressive regimes used at surgery, 3 months and 12 months by treatment group.
| Post-transplant events | Control (n = 99) | Early RIPC (n= 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Range | n | Median | Range | n | Median | Range | n | Median | Range | |
| Duration of hospital stay (days) | 92 | 7 | 3–54 | 98 | 6 | 2–6 | 99 | 6 | 4–28 | 99 | 7 | 3–57 |
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| Renal artery/vein thrombosis | 93 | 2 | 2.2 | 98 | 0 | 0.0 | 99 | 2 | 2.0 | 99 | 0 | 0.0 |
| Sepsis | 93 | 7 | 7.5 | 98 | 2 | 2.0 | 99 | 1 | 1.0 | 99 | 4 | 4.0 |
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| Admitted to ITU | 93 | 18 | 19.4 | 98 | 19 | 19.4 | 99 | 18 | 18.2 | 99 | 21 | 21.2 |
| n | Median | Range | n | Median | Range | n | Median | Range | n | Median | Range | |
| Duration of ITU stay (days) | 18.0 | 1 | 1–6 | 19.0 | 1 | 1–33 | 18 | 1 | 1–2 | 21 | 1 | 1–3 |
| Medication | Control (n = 99) | Early RIPC (n= 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| At surgery | 93 | 98 | 99 | 99 | ||||||||
| Prednisolone | 58 | 62.4 | 67 | 68.4 | 61 | 61.6 | 67 | 67.7 | ||||
| Methylprednisolone | 56 | 60.2 | 58 | 59.2 | 65 | 65.7 | 60 | 60.6 | ||||
| Basiliximab | 89 | 95.7 | 92 | 93.9 | 95 | 96.0 | 96 | 97.0 | ||||
| Mycophenolate mofetil | 67 | 72.0 | 68 | 69.4 | 68 | 68.7 | 69 | 69.7 | ||||
| Mycophenolate sodium | 4 | 4.3 | 5 | 5.1 | 4 | 4.0 | 7 | 7.1 | ||||
| Tacrolimus | 90 | 96.8 | 97 | 99.0 | 97 | 98.0 | 98 | 99.0 | ||||
| Alemtuzumab (Lemtrada®, Genzyme) | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | ||||
| Antithymocyte globulin | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | ||||
| Azathioprine | 9 | 9.7 | 12 | 12.2 | 13 | 13.1 | 11 | 11.1 | ||||
| Ciclosporin | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | ||||
| Intravenous immunoglobulin | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||||
| Sirolimus (Rapamune®, Pfizer) | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | ||||
| 3 months | 93 | 98 | 99 | 99 | ||||||||
| Prednisolone | 76 | 81.7 | 77 | 78.6 | 78 | 78.8 | 83 | 83.8 | ||||
| Methylprednisolone | 10 | 10.8 | 8 | 8.2 | 18 | 18.2 | 12 | 12.1 | ||||
| Mycophenolate mofetil | 65 | 69.9 | 68 | 69.4 | 68 | 68.7 | 65 | 65.7 | ||||
| Mycophenolate sodium | 13 | 14.0 | 14 | 14.3 | 15 | 15.2 | 16 | 16.2 | ||||
| Tacrolimus | 89 | 95.7 | 96 | 98.0 | 97 | 98.0 | 99 | 100.0 | ||||
| Azathioprine | 11 | 11.8 | 11 | 11.2 | 17 | 17.2 | 12 | 12.1 | ||||
| Ciclosporin | 4 | 4.3 | 1 | 1.0 | 0 | 0.0 | 4 | 4.0 | ||||
| Everolimus (Votubia®, Afinitor®, Novartis) | 1 | 1.1 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | ||||
| Sirolimus | 2 | 2.2 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 | ||||
| Antithymocyte globulin | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | ||||
| Alemtuzumab | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 2.0 | ||||
| Basiliximab | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | ||||
| Co-trimoxazole | 2 | 2.2 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 | ||||
| Tocilizumab | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | ||||
| 12 months | 95 | 97 | 97 | 98 | ||||||||
| Prednisolone | 76 | 80.0 | 75 | 77.3 | 73 | 75.3 | 80 | 81.6 | ||||
| Methylprednisolone | 10 | 10.5 | 6 | 6.2 | 12 | 12.4 | 7 | 7.1 | ||||
| Mycophenolate mofetil | 59 | 62.1 | 61 | 62.9 | 57 | 58.8 | 60 | 61.2 | ||||
| Mycophenolate sodium | 13 | 13.7 | 10 | 10.3 | 16 | 16.5 | 12 | 12.2 | ||||
| Tacrolimus | 86 | 90.5 | 90 | 92.8 | 92 | 94.8 | 94 | 95.9 | ||||
| Azathioprine | 15 | 15.8 | 15 | 15.5 | 17 | 17.5 | 14 | 14.3 | ||||
| Ciclosporin | 5 | 5.3 | 1 | 1.0 | 1 | 1.0 | 6 | 6.1 | ||||
| Everolimus | 1 | 1.1 | 1 | 1.0 | 0 | 0.0 | 1 | 1.0 | ||||
| Sirolimus | 5 | 5.3 | 1 | 1.0 | 2 | 2.1 | 7 | 7.1 | ||||
| Alemtuzumab | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | ||||
| Basiliximab | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | ||||
| Belatacept (Nulojix®, Bristol-Myers-Squibb) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | ||||
| Co-trimoxazole | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | ||||
| Leflunomide | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||||
Effect of remote ischaemic preconditioning on glomerular filtration rate
Table 9 and Figure 3 show GFR measured by iohexol clearance at 12 months and eGFR at 3 months and 12 months for each of the four groups in the 2 × 2 factorial design. Although there was substantial variability in GFR within each treatment group, mean GFR and eGFR were higher among those who were randomised to early or dual RIPC.
| GFR | Control (n = 99) | Early RIPC (n= 102) | Late RIPC (n = 103) | Dual RIPC (n = 102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| GFR by iohexol (ml/minute/1.73 m2) | 80 | 54.9 | 20.1 | 86 | 58.6 | 21.1 | 79 | 57.0 | 17.0 | 86 | 58.1 | 17.4 |
| GFR by iohexol, with imputed eGFR (ml/minute/1.73 m2) | 95 | 54.5 | 19.7 | 97 | 58.7 | 21.0 | 98 | 57.6 | 17.3 | 99 | 59.1 | 17.3 |
| eGFR 12 months (ml/minute/1.73 m2) | 95 | 59.2 | 20.2 | 97 | 64.9 | 22.6 | 97 | 62.1 | 19.1 | 98 | 64.7 | 20.6 |
| eGFR 3 months (ml/minute/1.73 m2) | 93 | 54.2 | 17.2 | 98 | 57.3 | 17.5 | 99 | 54.3 | 17.2 | 99 | 59.6 | 19.3 |
FIGURE 3.
Glomerular filtration rate at 3 and 12 months after transplantation by treatment group. (a) GFR by iohexol, 12 months; (b) GFR by iohexol and CKD-EPI equation, 12 months; (c) GFR by CKD-EPI equation, 12 months; (d) GFR by CKD-EPI equation, 3 months.




The results of the ITT analysis of the primary and secondary GFR outcomes are provided in Tables 10 and 11 respectively. In total, 331 patients were included in the analysis of the primary outcome, GFR measured by iohexol clearance at 12 months: 321 patients evaluated at 12 months’ follow-up (between 7 January 2011 and 22 May 2014) and 10 patients whose GFR was imputed as zero because they had died or experienced graft loss before 12 months. Reasons for missing data for this outcome are shown in the CONSORT diagram (see Figure 2).
| GFR | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| GFR by iohexol | 159 | 55.9 | 18.6 | 172 | 58.3 | 19.3 | 166 | 56.8 | 20.7 | 165 | 57.6 | 17.2 |
| Adjusted differencea | 3.08 (–0.89 to 7.04) | 1.19 (–2.77 to 5.15) | ||||||||||
| p-value | 0.128 | 0.555 | ||||||||||
| GFR by iohexol, with imputed eGFR | 193 | 56.0 | 18.6 | 196 | 58.9 | 19.2 | 192 | 56.6 | 20.5 | 197 | 58.3 | 17.3 |
| Adjusted differencea | 3.41 (–0.21 to 7.04) | 2.18 (–1.45 to 5.80) | ||||||||||
| p-value | 0.065 | 0.239 | ||||||||||
| eGFR 12 months | 192 | 60.7 | 19.7 | 195 | 64.8 | 21.6 | 192 | 62.1 | 21.6 | 195 | 63.4 | 19.9 |
| Adjusted differencea | 4.98 (1.13 to 8.83) | 1.97 (–1.87 to 5.81) | ||||||||||
| p-value | 0.011 | 0.314 | ||||||||||
| eGFR 3 months | 192 | 54.2 | 17.1 | 197 | 58.5 | 18.4 | 191 | 55.8 | 17.4 | 198 | 57.0 | 18.4 |
| Adjusted differencea | 4.99 (1.69 to 8.29) | 1.84 (–1.46 to 5.14) | ||||||||||
| p-value | 0.003 | 0.273 | ||||||||||
| GFR | Control (n = 99) | RIPC (n = 307) | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| GFR by iohexol | 80 | 54.9 | 20.1 | 251 | 57.9 | 18.6 |
| Adjusted differencea | 3.88 (–0.74 to 8.50) | |||||
| p-value | 0.099 | |||||
| GFR by iohexol, with imputed eGFR | 95 | 54.5 | 19.7 | 294 | 58.5 | 18.6 |
| Adjusted differencea | 4.56 (0.34 to 8.77) | |||||
| p-value | 0.034 | |||||
| eGFR 12 months | 95 | 59.2 | 20.2 | 292 | 63.9 | 20.8 |
| Adjusted differencea | 5.51 (1.04 to 9.98) | |||||
| p-value | 0.016 | |||||
| eGFR 3 months | 93 | 54.2 | 17.2 | 296 | 57.1 | 18.1 |
| Adjusted differencea | 3.68 (–0.22 to 7.58) | |||||
| p-value | 0.064 | |||||
In the primary ITT analysis, shown in Table 10, there was no strong evidence for a difference in mean GFR (ml/minute/1.73 m2) measured by iohexol clearance between those receiving early RIPC and those in the control group, although there was an observed increase in GFR after early RIPC (adjusted difference 3.08, 95% CI –0.89 to 7.04; p = 0.13). There was no evidence of a difference between those receiving late RIPC and those in the control group (adjusted difference 1.19, 95% CI –2.77 to 5.15; p = 0.56). In a formal assessment of whether any benefit of early or late RIPC depended on whether the other period of RIPC was given, there was no evidence of an interaction between early and late RIPC (p = 0.47). Of note are the higher than expected SDs in each group for the GFR at 12 months, which in turn impacted on the width of the CIs and hence the precision of the results.
When missing values of GFR by iohexol clearance were imputed using the eGFR at 12 months, the GFR (ml/minute/1.73 m2) was again numerically greater in those receiving early RIPC than in those in the control group (adjusted difference 3.41, 95% CI –0.21 to 7.04; p = 0.065). There was little evidence that GFR differed between those receiving late RIPC and those in the control group (adjusted difference 2.18, 95% CI –1.45 to 5.80; p = 0.24). At 3 and 12 months there was stronger evidence of a beneficial effect of early RIPC on eGFR compared with the control group, but there was little evidence that eGFR differed between the late RIPC group and the control group. There was no evidence of an interaction between early and late RIPC for any secondary GFR outcomes.
The secondary analysis, comparing the combined early and late RIPC groups with the control group, shown in Table 11, suggested a trend towards a higher GFR (ml/minute/1.73 m2) measured by iohexol clearance in the RIPC treatment groups than in the control group (adjusted difference 3.88, 95% CI –0.74 to 8.50; p = 0.099). There was evidence that, compared with the control group, the combined RIPC group had a higher GFR when missing values of GFR measured by iohexol clearance were imputed using the eGFR at 12 months (adjusted difference 4.56, 95% CI 0.34 to 8.77; p = 0.034); the combined RIPC group had a higher eGFR at 12 months (adjusted difference 5.51, 95% CI 1.04 to 9.98; p = 0.016); and there was a trend towards a higher eGFR at 3 months in the combined RIPC group (adjusted difference 3.68, 95% CI –0.22 to 7.58; p = 0.064).
The PP analyses for GFR outcomes are shown in Tables 12 and 13. These analyses includes outcomes for recipients when both donor and recipient received the full 16 cycles of sham or active RIPC according to protocol. The results were very similar to those of the ITT analysis. Compared with the control group, among those who received early RIPC there was a trend towards a higher GFR measured by iohexol clearance (p = 0.061), which was similar after missing values were imputed with the eGFR (p = 0.055). There was also evidence of a higher eGFR at 12 and 3 months among those who received early RIPC (p = 0.022 and p = 0.002, respectively). In contrast, there was no evidence that GFR measured by iohexol clearance at 12 months or eGFR at 12 or 3 months differed between the late RIPC group and the control group. There was no evidence of an interaction between early and late RIPC for any of the GFR or eGFR outcomes. When the early and late RIPC groups were combined (see Table 13), there was a trend towards a higher GFR measured by iohexol clearance in the RIPC groups than in the control group (p = 0.077), and evidence became stronger after missing values were imputed using eGFR (p = 0.029). Recipients who received RIPC had a higher eGFR at 12 months than those in the control group (p = 0.034) and there was a trend towards a higher eGFR at 3 months (p = 0.097).
| GFR | Control (n = 180) | Early RIPC (n = 182) | Control (n = 177) | Late RIPC (n = 185) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| GFR | 149 | 55.5 | 19.0 | 159 | 58.6 | 18.6 | 154 | 56.6 | 20.8 | 154 | 57.6 | 16.6 |
| Adjusted differencea | 3.89 (–0.18 to 7.96) | 1.30 (–2.76 to 5.35) | ||||||||||
| p-value | 0.061 | 0.530 | ||||||||||
| GFR, with imputed eGFR | 178 | 55.9 | 18.9 | 180 | 58.8 | 18.4 | 175 | 56.0 | 20.4 | 183 | 58.6 | 16.9 |
| Adjusted differencea | 3.66 (–0.08 to 7.40) | 2.78 (–0.96 to 6.51) | ||||||||||
| p-value | 0.055 | 0.145 | ||||||||||
| eGFR 12 months | 177 | 60.7 | 19.9 | 180 | 64.3 | 21.1 | 175 | 61.8 | 21.5 | 182 | 63.2 | 19.8 |
| Adjusted differencea | 4.69 (0.69 to 8.69) | 1.73 (–2.26 to 5.73) | ||||||||||
| p-value | 0.022 | 0.394 | ||||||||||
| eGFR 3 months | 179 | 53.9 | 17.0 | 181 | 58.3 | 18.2 | 176 | 55.5 | 17.0 | 184 | 56.8 | 18.4 |
| Adjusted differencea | 5.32 (1.90 to 8.75) | 1.59 (–1.83 to 5.01) | ||||||||||
| p-value | 0.002 | 0.362 | ||||||||||
| GFR | Control (n = 88) | RIPC (n = 274) | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| GFR | 75 | 54.5 | 20.5 | 233 | 57.9 | 18.2 |
| Adjusted differencea | 4.27 (–0.46 to 9.00) | |||||
| p-value | 0.077 | |||||
| GFR, with imputed eGFR | 87 | 54.2 | 20.1 | 271 | 58.4 | 18.1 |
| Adjusted differencea | 4.84 (0.49 to 9.20) | |||||
| p-value | 0.029 | |||||
| eGFR 12 months | 87 | 59.4 | 20.7 | 270 | 63.5 | 20.5 |
| Adjusted differencea | 5.05 (0.39 to 9.71) | |||||
| p-value | 0.034 | |||||
| eGFR 3 months | 87 | 54.1 | 17.2 | 273 | 56.8 | 17.9 |
| Adjusted differencea | 3.41 (–0.62 to 7.44) | |||||
| p-value | 0.097 | |||||
Subgroup analysis
The main prespecified subgroup analysis was according to underlying risk of low GFR. Table 14 gives those baseline factors selected for the risk model used to predict GFR by iohexol clearance at 12 months. The strongest predictors of kidney function were donor age, donor gender, donor baseline creatinine level, donor body surface area and donor being recipient of a previous kidney transplant. Using this model a risk score was calculated for each patient as the predicted GFR at 12 months had he or she been randomised to the control arm, therefore excluding the estimated effects of early and late RIPC.
| Predictor | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Donor age (years) | –0.56 | –0.71 to –0.41 | < 0.001 |
| Female donor | –4.62 | –9.73 to 0.49 | 0.076 |
| Donor creatinine (µmol/l) | –0.15 | –0.30 to –0.01 | 0.039 |
| Donor body surface area (m2) | 16.29 | 5.16 to 27.43 | 0.004 |
| Recipient of previous kidney transplant | –8.51 | –15.90 to –1.13 | 0.024 |
| Late RIPCa | 1.48 | –2.25 to 5.20 | 0.437 |
| Early RIPCa | 3.24 | –0.53 to 7.01 | 0.092 |
An interaction between this risk score and treatment was fitted to establish whether there was any difference in benefit according to the underlying risk of poor kidney function. The interaction between risk score and early RIPC and between risk score and late RIPC was examined in turn and the results are provided in Table 15 for GFR measured by iohexol clearance at 12 months and for GFR with missing values imputed using eGFR and in Table 16 for eGFR at 3 and 12 months. There was little evidence of an interaction between underlying risk of poor kidney function and benefit from early RIPC. Analysis of GFR by iohexol clearance at 12 months suggested that there was a trend towards a greater benefit of late RIPC for those at greatest risk of poor kidney function (p = 0.133 for interaction). For the eGFR outcomes, similar trends were seen, with a greater benefit of late RIPC with worse predicted kidney function, although the evidence from the interaction test was again weak.
| GFR | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| GFR | ||||||||||||
| Quartile 1 | 30 | 42.9 | 20.1 | 40 | 47.5 | 13.0 | 35 | 42.3 | 19.1 | 35 | 48.8 | 12.7 |
| Quartile 2 | 38 | 55.2 | 14.6 | 40 | 54.0 | 20.5 | 41 | 51.0 | 20.6 | 37 | 58.6 | 13.2 |
| Quartile 3 | 38 | 59.3 | 19.1 | 39 | 63.9 | 16.5 | 41 | 64.5 | 17.2 | 36 | 58.3 | 18.2 |
| Quartile 4 | 38 | 63.0 | 14.0 | 39 | 71.6 | 12.9 | 35 | 68.3 | 14.4 | 42 | 66.6 | 13.9 |
| Risk interactiona | 0.08 (–0.38 to 0.53) | –0.33 (–0.77 to 0.10) | ||||||||||
| p-value | 0.735 | 0.133 | ||||||||||
| GFR, with imputed eGFR | ||||||||||||
| Quartile 1 | 42 | 47.4 | 20.5 | 47 | 48.6 | 12.7 | 45 | 45.4 | 19.4 | 44 | 50.8 | 13.1 |
| Quartile 2 | 43 | 55.3 | 14.9 | 45 | 54.9 | 20.3 | 46 | 51.7 | 20.5 | 42 | 58.8 | 13.5 |
| Quartile 3 | 46 | 57.3 | 18.4 | 43 | 63.8 | 17.7 | 45 | 62.9 | 17.7 | 44 | 57.9 | 18.7 |
| Quartile 4 | 44 | 63.6 | 15.6 | 44 | 71.1 | 12.9 | 39 | 66.9 | 15.0 | 49 | 67.7 | 14.7 |
| Risk interactiona | 0.15 (–0.26 to 0.57) | –0.25 (–0.66 to 0.16) | ||||||||||
| p-value | 0.471 | 0.232 | ||||||||||
| GFR | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| eGFR 12 months | ||||||||||||
| Quartile 1 | 41 | 52.1 | 20.8 | 47 | 52.1 | 14.0 | 45 | 48.7 | 18.7 | 43 | 55.6 | 15.3 |
| Quartile 2 | 43 | 58.2 | 14.0 | 45 | 60.9 | 22.8 | 46 | 58.7 | 20.9 | 42 | 60.6 | 16.9 |
| Quartile 3 | 46 | 59.4 | 17.0 | 43 | 70.1 | 20.2 | 45 | 66.8 | 18.9 | 44 | 62.3 | 19.6 |
| Quartile 4 | 44 | 74.2 | 19.1 | 44 | 79.3 | 16.8 | 39 | 76.9 | 18.6 | 49 | 76.7 | 17.8 |
| Risk interactiona | 0.11 (–0.35 to 0.57) | –0.21 (–0.66 to 0.24) | ||||||||||
| p-value | 0.630 | 0.353 | ||||||||||
| eGFR 3 months | ||||||||||||
| Quartile 1 | 41 | 48.0 | 17.3 | 47 | 46.6 | 13.2 | 44 | 43.9 | 15.5 | 44 | 50.6 | 14.2 |
| Quartile 2 | 43 | 53.4 | 14.2 | 46 | 60.4 | 20.7 | 47 | 55.1 | 15.7 | 42 | 59.1 | 20.5 |
| Quartile 3 | 46 | 53.3 | 16.5 | 43 | 60.7 | 16.2 | 45 | 57.8 | 14.8 | 44 | 55.9 | 18.5 |
| Quartile 4 | 44 | 64.9 | 15.9 | 44 | 69.6 | 15.0 | 38 | 68.9 | 14.5 | 50 | 66.0 | 16.3 |
| Risk interactiona | 0.11 (–0.30 to 0.52) | –0.31 (–0.71 to 0.09) | ||||||||||
| p-value | 0.590 | 0.131 | ||||||||||
Table 17 shows the results of the two other prespecified subgroup analyses, which examined differences in the effect of treatment on GFR measured by iohexol clearance at 12 months between patients who were having their first transplant and patients who had had a previous transplant and between patients with a ‘000’ mismatch type (i.e. those with no mismatches at any of the major HLA loci) and those without a ‘000’ mismatch type. These two subgroups were chosen because they represent changes made to the inclusion criteria of the study after it had begun. In addition, Table 17 shows the results of a post hoc subgroup analysis comparing treatment effects between recipients with diabetes at baseline and recipients without diabetes at baseline.
| Subgroup | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| No ‘000’ mismatch | 148 | 56.9 | 17.2 | 158 | 57.7 | 19.4 | 149 | 56.8 | 19.9 | 157 | 57.8 | 16.8 |
| ‘000’ mismatch | 11 | 42.9 | 30.9 | 14 | 65.9 | 16.9 | 17 | 56.7 | 27.6 | 8 | 53.8 | 24.7 |
| Risk interactiona | 26.1 (6.1 to 46.2) | 1.0 (–22.1 to 24.2) | ||||||||||
| p-value | 0.011 | 0.931 | ||||||||||
| No previous transplant | 149 | 56.4 | 18.4 | 160 | 58.5 | 19.5 | 155 | 56.9 | 20.5 | 154 | 58.1 | 17.3 |
| Previous transplant | 10 | 49.1 | 21.9 | 12 | 56.1 | 16.5 | 11 | 55.8 | 23.8 | 11 | 50.0 | 13.1 |
| Risk interactiona | 5.9 (–10.7 to 22.5) | –1.7 (–18.1 to 14.8) | ||||||||||
| p-value | 0.485 | 0.839 | ||||||||||
| No diabetes | 145 | 57.1 | 19.1 | 153 | 58.6 | 19.0 | 144 | 57.7 | 21.4 | 154 | 57.9 | 16.4 |
| Diabetes | 14 | 44.4 | 6.6 | 19 | 56.5 | 22.3 | 22 | 50.9 | 13.5 | 11 | 52.3 | 26.1 |
| Risk interactiona | 9.9 (–1.3 to 21.0) | 0.4 (–16.2 to 16.9) | ||||||||||
| p-value | 0.082 | 0.963 | ||||||||||
There was evidence of an interaction between early RIPC and ‘000’ mismatch status (p = 0.011). Among those without a ‘000’ mismatch type the adjusted mean GFR in the RIPC group was 1.2 ml/minute/1.73 m2 higher than in the control group (95% CI –2.8 to 5.1 ml/minute/1.73 m2, p = 0.548), whereas among those with a ‘000’ mismatch type the adjusted mean GFR in the RIPC group was 27.3 ml/minute/1.73 m2 higher than in the control group (95% CI 7.6 to 47.0, p = 0.007). However, some caution is advised in consideration of this interaction given the relatively small number of patients with a ‘000’ mismatch (n = 25). There was little evidence of an interaction between early RIPC and either previous transplant status or recipient diabetes at baseline, although the numbers with a previous transplant and diabetes were small. There was little evidence that the effect of late RIPC differed between any of the subgroups examined in Table 17.
Effect of remote ischaemic preconditioning on secondary outcomes
The results of the ITT analysis for the main secondary outcomes are shown in Table 18. There was no difference in the time for a fall in serum creatinine of 50% following surgery between the early RIPC group and the control group (p = 0.75) or between the late RIPC group and the control group (p = 0.64). As shown in Figure 4, the median time for a fall in serum creatinine of 50% was around 48 hours in all treatment groups. There was also little evidence of a difference in the rate of acute rejection between the early RIPC group and the control group (p = 0.86) or between the late RIPC group and the control group (p = 0.17). Just over 10% of participants experienced acute rejection during the trial (Figure 5). Only 12 patients experienced delayed graft function and so substantial uncertainty remains with regard to the effects of early and late RIPC on this outcome. There was little evidence that the incidence of delayed graft function differed between the early RIPC group and the control group (p = 0.61) but the incidence was lower among the late RIPC group than among the control group (1.0% vs. 5.2%, p = 0.031).
| Secondary outcome | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| 50% fall in creatinine at | 136 | 144 | 135 | 145 | ||||||||
| 4 hours | 1 | 0.7 | 3 | 2.1 | 2 | 1.5 | 2 | 1.4 | ||||
| 8 hours | 1 | 0.7 | 2 | 1.4 | 1 | 0.7 | 2 | 1.4 | ||||
| 24 hours | 40 | 29.4 | 44 | 30.6 | 42 | 31.1 | 42 | 29.0 | ||||
| 48 hours | 69 | 50.7 | 67 | 46.5 | 60 | 44.4 | 76 | 52.4 | ||||
| 72 hours | 8 | 5.9 | 11 | 7.6 | 11 | 8.1 | 8 | 5.5 | ||||
| No fall by 50% within 72 hours | 17 | 12.5 | 17 | 11.8 | 19 | 14.1 | 15 | 10.3 | ||||
| Hazard ratio (95% CI) | 1.04 (0.81 to 1.34) | 1.06 (0.83 to 1.37) | ||||||||||
| p-value | 0.748 | 0.638 | ||||||||||
| Delayed graft function, first 7 days | 192 | 5 | 2.6 | 197 | 7 | 3.5 | 191 | 10 | 5.2 | 198 | 2 | 1.0 |
| Odds ratio (95% CI) | 1.36 (0.42 to 4.39) | 0.19 (0.04 to 0.86) | ||||||||||
| p-value | 0.609 | 0.031 | ||||||||||
| Acute rejection, first 12 months | 202 | 24 | 11.9 | 204 | 23 | 11.3 | 201 | 19 | 9.5 | 205 | 28 | 13.7 |
| Hazard ratio (95% CI) | 0.95 (0.54 to 1.68) | 1.50 (0.84 to 2.68) | ||||||||||
| p-value | 0.856 | 0.173 | ||||||||||
| Graft loss, first 3 months | 202 | 5 | 2.5 | 204 | 2 | 1.0 | 201 | 4 | 2.0 | 205 | 3 | 1.5 |
| p-value (log-rank) | 0.225 | 0.655 | ||||||||||
| Graft loss, first 12 months | 202 | 6 | 3.0 | 204 | 3 | 1.5 | 201 | 6 | 3.0 | 205 | 3 | 1.5 |
| p-value (log-rank) | 0.301 | 0.296 | ||||||||||
| Death, first 12 months | 202 | 1 | 0.5 | 204 | 1 | 0.5 | 201 | 1 | 0.5 | 205 | 1 | 0.5 |
| p-value (log-rank) | 0.982 | 0.997 | ||||||||||
FIGURE 4.
Time for serum creatinine to fall by 50% by treatment group.

FIGURE 5.
Incidence of acute rejection, by treatment group.

Nine recipients experienced graft loss and only two recipients died during the initial 12 months following transplant. There was little evidence of any differences between those receiving RIPC and those in the control group but the small number of events means that there is substantial uncertainty around the effect of treatment on these outcomes.
There was little evidence of any differences between the control group and the combined early and late RIPC group for any of the secondary outcomes (Table 19).
| Secondary outcome | Control (n = 99) | Any RIPC (n = 307) | ||||
|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | |
| 50% fall in creatinine at | 62 | 218 | ||||
| 4 hours | 0 | 0.0 | 4 | 1.8 | ||
| 8 hours | 1 | 1.6 | 2 | 0.9 | ||
| 24 hours | 21 | 33.9 | 63 | 28.9 | ||
| 48 hours | 26 | 41.9 | 110 | 50.5 | ||
| 72 hours | 6 | 9.7 | 13 | 6.0 | ||
| No fall by 50% within 72 hours | 8 | 12.9 | 26 | 11.9 | ||
| Hazard ratio (95% CI) | 1.00 (0.74 to 1.36) | |||||
| p-value | 0.984 | |||||
| Delayed graft function, first 7 days | 93 | 5 | 5.4 | 296 | 7 | 2.4 |
| Odds ratio (95% CI) | 0.43 (0.13 to 1.38) | |||||
| p-value | 0.609 | |||||
| Acute rejection, first 12 months | 99 | 11 | 11.1 | 307 | 36 | 11.7 |
| Hazard ratio (95% CI) | 1.08 (0.55 to 2.12) | |||||
| p-value | 0.856 | |||||
| Graft loss, first 3 months | 99 | 2 | 2.0 | 307 | 5 | 1.6 |
| p-value (log-rank) | 0.755 | |||||
| Graft loss, first 12 months | 99 | 3 | 3.0 | 307 | 6 | 2.0 |
| p-value (log-rank) | 0.522 | |||||
| Death, first 12 months | 99 | 1 | 1.0 | 307 | 1 | 0.3 |
| p-value (log-rank) | 0.401 | |||||
The results of the PP analysis for the main secondary outcomes are shown in Table 20. This analysis includes outcomes for recipients when both donor and recipient received the full 16 cycles of sham or active RIPC according to protocol. The results were very similar to those of the ITT analysis for the secondary outcomes.
| Secondary outcome | Control (n = 180) | Early RIPC (n = 182) | Control (n = 177) | Late RIPC (n = 185) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | n | % | Totala | n | % | Totala | n | % | Totala | n | % | |
| 50% fall in creatinine at | 136 | 144 | 135 | 145 | ||||||||
| 4 hours | 1 | 0.7 | 3 | 2.1 | 2 | 1.5 | 2 | 1.4 | ||||
| 8 hours | 1 | 0.7 | 2 | 1.4 | 1 | 0.7 | 2 | 1.4 | ||||
| 24 hours | 40 | 29.4 | 44 | 30.6 | 42 | 31.1 | 42 | 29.0 | ||||
| 48 hours | 69 | 50.7 | 67 | 46.5 | 60 | 44.4 | 76 | 52.4 | ||||
| 72 hours | 8 | 5.9 | 11 | 7.6 | 11 | 8.1 | 8 | 5.5 | ||||
| No fall by 50% within 72 hours | 17 | 12.5 | 17 | 11.8 | 19 | 14.1 | 15 | 10.3 | ||||
| Hazard ratio (95% CI) | 1.06 (0.82 to 1.37) | 1.06 (0.82 to 1.37) | ||||||||||
| p-value | 0.632 | 0.654 | ||||||||||
| Delayed graft function, first 7 days | 179 | 5 | 2.8 | 181 | 5 | 2.8 | 176 | 9 | 5.1 | 184 | 1 | 0.5 |
| Odds ratio (95% CI) | 0.98 (0.28 to 3.48) | 0.10 (0.01 to 0.81) | ||||||||||
| p-value | 0.974 | 0.031 | ||||||||||
| Acute rejection, first 12 months | 180 | 22 | 12.2 | 182 | 21 | 11.5 | 177 | 18 | 10.2 | 185 | 25 | 13.5 |
| Hazard ratio (95% CI) | 0.94 (0.52 to 1.71) | 1.38 (0.75 to 2.53) | ||||||||||
| p-value | 0.856 | 0.173 | ||||||||||
| Graft loss, first 3 months | 180 | 5 | 2.8 | 182 | 2 | 1.1 | 177 | 4 | 2.3 | 185 | 3 | 1.6 |
| p-value (log-rank) | 0.225 | 0.607 | ||||||||||
| Graft loss, first 12 months | 180 | 6 | 3.3 | 182 | 3 | 1.6 | 177 | 6 | 3.4 | 185 | 3 | 1.6 |
| p-value (log-rank) | 0.301 | 0.280 | ||||||||||
| Death, first 12 months | 180 | 1 | 0.6 | 182 | 0 | 0.0 | 177 | 1 | 0.6 | 185 | 0 | 0.0 |
| p-value (log-rank) | 0.304 | 0.315 | ||||||||||
Adverse events
Table 21 shows the types and numbers of adverse events by randomised group. Adverse events were more commonly experienced by donors and recipients in the RIPC arms than in the control arm. In particular, and as expected, far more donors and recipients in the RIPC arms than in the control arm experienced pain/paraesthesia (33–45% RIPC vs. 2–7% control) or skin petechiae (8–20% RIPC vs. 1% control). Although pain/paraesthesia was experienced by at least one-third of donors and recipients in the RIPC groups, this was only severe enough to prevent completion of the RIPC intervention in four pairs in the early RIPC arm, two pairs in the late RIPC arm and three pairs in the dual RIPC arm. Similar proportions of recipients experienced adverse events during follow-up (between 52% and 60% depending on study arm), with very few adverse events reported in donors during follow-up.
| Adverse event | Control (n = 99), n (%) | Early RIPC (n = 102), n (%) | Late RIPC (n = 103), n (%) | Dual RIPC (n = 102), n (%) |
|---|---|---|---|---|
| Recipients | ||||
| Any adverse event | 59 (59.6) | 75 (73.5) | 71 (68.9) | 81 (79.4) |
| Adverse event at RIPC/sham RIPC | 2 (2.0) | 47 (46.1) | 36 (35.0) | 49 (48.0) |
| Unexpected adverse event | 0 (0) | 2 (2.0) | 0 (0) | 0 (0) |
| Pain/paraesthesia | 2 (2.0) | 43 (42.2) | 34 (33.0) | 45 (44.1) |
| Pain/paraesthesia preventing completion of RIPC | 0 (0) | 4 (3.9) | 2 (1.9) | 3 (2.9) |
| Skin petechiae | 1 (1.0) | 11 (10.8) | 9 (8.7) | 20 (19.6) |
| Adverse event during follow-up | 59 (59.6) | 57 (55.9) | 54 (52.4) | 60 (58.8) |
| Death | 2 (2.0) | 1 (1.0) | 1 (1.0) | 2 (2.0) |
| Hospital admission | 59 (59.6) | 57 (55.9) | 54 (52.4) | 60 (58.8) |
| Other reported adverse event | 0 (0) | 2 (2.0) | 4 (3.9) | 2 (2.0) |
| Donors | ||||
| Any adverse event | 7 (7.1) | 42 (41.2) | 40 (38.8) | 48 (47.1) |
| Adverse event at RIPC/sham RIPC | 7 (7.1) | 42 (41.2) | 40 (38.8) | 48 (47.1) |
| Unexpected adverse event | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pain/paraesthesia | 7 (7.1) | 41 (40.2) | 38 (36.9) | 46 (45.1) |
| Skin petechiae | 1 (1.0) | 9 (8.8) | 8 (7.8) | 18 (17.6) |
| Adverse event during follow-up | 0 (0) | 2 (2.0) | 0 (0) | 0 (0) |
| Hospital admission | 0 (0) | 1 (1.0) | 0 (0) | 0 (0) |
| Other reported adverse event | 0 (0) | 1 (1.0) | 0 (0) | 0 (0) |
There were two reported unexpected adverse events that occurred during or immediately after administration of the early RIPC intervention. The first event was an episode of hypotension in a recipient following administration of the early RIPC intervention, which was rated as a non-serious adverse event that was possibly related to the RIPC intervention. The second event was an anaphylactoid reaction to basiliximab in a recipient during administration of the early RIPC intervention. This event was rated as a serious adverse event that was unrelated to the RIPC intervention.
Mechanism of action of remote ischaemic preconditioning
Tables 22–24 present the analysis of TNF-α, IL-6, IL-1β and INF-γ levels in serum in donors and in serum and urine in recipients, respectively. There was little evidence of a difference in these outcomes between early RIPC and control and between late RIPC and control. Immunoblotting, immunohistochemistry and renal fibrosis analyses are in progress at the time of submission of this report.
| Serum cytokine | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| TNF-α (pg/ml) | 94 | 1.21 | 0.62 | 95 | 1.17 | 0.82 | 92 | 1.19 | 0.74 | 97 | 1.19 | 0.71 |
| Adjusted differencea | –0.03 (–0.23 to 0.17) | –0.01 (–0.21 to 0.19) | ||||||||||
| p-value | 0.80 | 0.92 | ||||||||||
| IL-6 (pg/ml) | 94 | 2.98 | 1.09 | 94 | 3.03 | 0.99 | 92 | 2.92 | 1.04 | 96 | 3.08 | 1.04 |
| Adjusted differencea | 0.03 (–0.27 to 0.32) | 0.20 (–0.09 to 0.49) | ||||||||||
| p-value | 0.85 | 0.18 | ||||||||||
| IL-1β (pg/ml) | 53 | –0.24 | 0.96 | 49 | –0.19 | 1.19 | 44 | –0.37 | 0.94 | 58 | –0.11 | 1.16 |
| Adjusted differencea | 0.004 (–0.31 to 0.45) | 0.29 (–0.05 to 0.69) | ||||||||||
| p-value | N/A | N/A | ||||||||||
| INF-γ (pg/ml) | 69 | –0.20 | 0.84 | 73 | 0.06 | 0.86 | 66 | –0.07 | 0.83 | 76 | –0.07 | 0.88 |
| Adjusted differencea | 0.27 (–0.01 to 0.50) | –0.02 (–0.27 to 0.24) | ||||||||||
| p-value | N/A | N/A | ||||||||||
| Serum cytokine | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| TNF-α (pg/ml) | 95 | 1.12 | 0.76 | 95 | 1.07 | 0.84 | 93 | 1.18 | 0.71 | 97 | 1.01 | 0.87 |
| Adjusted differencea | –0.05 (–0.26 to 0.16) | –0.14 (–0.35 to 0.07) | ||||||||||
| p-value | 0.63 | 0.18 | ||||||||||
| IL-6 (pg/ml) | 96 | 2.18 | 1.18 | 93 | 2.05 | 0.96 | 92 | 2.24 | 0.93 | 97 | 2.00 | 1.19 |
| Adjusted differencea | –0.17 (–0.48 to 0.14) | –0.24 (–0.54 to 0.07) | ||||||||||
| p-value | 0.27 | 0.13 | ||||||||||
| IL-1β (pg/ml) | 52 | –0.49 | 0.41 | 50 | –0.29 | 0.76 | 53 | –0.37 | 0.57 | 49 | –0.40 | 0.67 |
| Adjusted differencea | 0.18 (–0.03 to 0.44) | –0.02 (–0.23 to 0.22) | ||||||||||
| p-value | N/A | N/A | ||||||||||
| INF-γ (pg/ml) | 68 | –0.22 | 0.76 | 60 | –0.29 | 0.89 | 61 | –0.24 | 0.78 | 67 | –0.27 | 0.87 |
| Adjusted differencea | –0.08 (–0.33 to 0.26) | –0.03 (–0.32 to 0.26) | ||||||||||
| p-value | N/A | N/A | ||||||||||
| Serum cytokine | Control (n = 202) | Early RIPC (n = 204) | Control (n = 201) | Late RIPC (n = 205) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| TNF-α (pg/ml) | 78 | 0.10 | 0.88 | 66 | 0.33 | 0.93 | 74 | 0.16 | 0.83 | 70 | 0.26 | 0.99 |
| Adjusted differencea | 0.14 (–0.15 to 0.44) | 0.14 (–0.14 to 0.43) | ||||||||||
| p-value | 0.34 | 0.32 | ||||||||||
| IL-6 (pg/ml) | 86 | 2.65 | 1.30 | 72 | 2.51 | 1.52 | 81 | 2.43 | 1.45 | 77 | 2.75 | 1.33 |
| Adjusted differencea | –0.08 (–0.52 to 0.36) | 0.26 (–0.18 to –0.70) | ||||||||||
| p-value | 0.72 | 0.24 | ||||||||||
| IL-1β (pg/ml) | 74 | 0.70 | 1.23 | 61 | 0.36 | 1.11 | 70 | 0.49 | 1.27 | 65 | 0.61 | 1.10 |
| Adjusted differencea | –0.21 (–0.59 to 0.14) | 0.10 (–0.29 to 0.45) | ||||||||||
| p-value | N/A | N/A | ||||||||||
| INF-γ (pg/ml) | 40 | –0.13 | 0.65 | 38 | –0.39 | 0.74 | 38 | –0.08 | 0.81 | 40 | –0.42 | 0.54 |
| Adjusted differencea | –0.26 (–0.55 to 0.03) | –0.33 (–0.70 to –0.08) | ||||||||||
| p-value | N/A | N/A | ||||||||||
Chapter 4 Discussion
The REPAIR trial is the largest clinical trial of IPC to measure the effect of this procedure on a clinically relevant (real) end point. The REPAIR trial examined the early and delayed effects of RIPC on kidney function after transplantation. Although an effect of early RIPC on iohexol clearance (primary end point) did not reach conventional statistical significance, we observed a beneficial effect of early preconditioning on eGFR (secondary end point) 3 months and 1 year after living-donor transplantation, which was mirrored by an increase in GFR measured by iohexol clearance. There was little evidence of an effect of delayed preconditioning on kidney function. RIPC was tolerated by > 90% of patients, had minimal morbidity, had a very low cost and caused minimal inconvenience to patients. Regardless of the uncertainty that arises from interpreting a single Phase III clinical trial, and the larger than expected variability in the iohexol measurements, the benefit–risk ratio of RIPC is sufficiently large to recommend it for living-donor kidney transplantation.
Design of the REPAIR trial
Remote ischaemic preconditioning is a whole-body systemic reflex activated by localised transient ischaemia that is itself non-injurious. The discovery that limb ischaemia could activate this reflex, and limit tissue injury to vital organs in animals and humans, has stimulated a large number of clinical trials to detect protective effects in patients. Most of these have been too small to measure real clinical end points and the small sample sizes have doubtless contributed to heterogeneity and uncertainty in the trial findings. We chose living-donor kidney transplantation as a model for testing whether RIPC had more than a biological effect in patients. Kidney transplantation in this setting is carefully scheduled to facilitate the application of a preconditioning stimulus before surgery. It was possible to arrange for a preconditioning stimulus to be applied to the donor and recipient 24 hours before surgery and immediately before surgery to test for early and late effects of preconditioning. Lastly, the careful planning of surgery meant that kidney ischaemia times would be expected to be consistent, reducing the variability in ischaemia times, kidney injury and kidney function after surgery.
The RIPC stimulus that was used was based on that which had induced cardioprotection in a number of previous clinical trials, namely 5-minute cycles of arm ischaemia and reperfusion. Our previous work had indicated that at least three cycles were needed to induce systemic protection and, to build in a safety margin to ensure that the stimulus was sufficient, we designed the REPAIR trial to use a four-cycle preconditioning stimulus. Approximately 10% of patients did not receive the full preconditioning stimulus as planned, most commonly because arm ischaemia was poorly tolerated by a small number of donors or recipients. We planned a PP analysis of the effect of RIPC in those undergoing the full intervention cycle because it seemed unlikely that an inability to tolerate arm ischaemia would significantly bias the outcome of the trial.
We designed the REPAIR trial to have a large enough sample size to enable us to measure kidney function 12 months after transplantation. The usual measure of kidney function is the eGFR, calculated from the serum creatinine level, taking into account certain phenotypic features of the patient. In the REPAIR trial we elected to measure GFR directly from the rate of excretion of iohexol, taking the view that the increased precision of this assessment over the eGFR would be an advantage. We were mindful of the theoretical disadvantages of iohexol GFR, including its greater complexity requiring an additional outpatient visit and the greater potential for human error in administering the correct dose and in the timing of blood sampling. By setting iohexol GFR as our primary end point we attempted to optimise the possibility of capturing data on renal function using this direct measure.
The factorial design of the REPAIR trial was chosen to allow separate assessments of the immediate and delayed protection that RIPC might stimulate. In the early phase a window of protection of about 4 hours is provided immediately on completing a threshold preconditioning stimulus. Approximately 24 hours after preconditioning, a second more prolonged phase of protection is provided, mediated by a complex of anti-inflammatory mediators. The factorial design allowed us to investigate these two phases separately and, uniquely for a preconditioning study, to examine whether additional protection was provided by a combination of early and delayed protection stimulated by sequential application of preconditioning stimuli. To maximise the chances of inducing tissue protection during ischaemia and reperfusion, donors and recipients underwent the same preconditioning protocol. In this way the donor kidney underwent preconditioning in advance of its ischaemic insult, and reperfusion injury in the recipient might also be modulated by preconditioning.
Effects of remote ischaemic preconditioning on glomerular filtration rate
The major finding from the REPAIR trial was the effect of early RIPC on GFR. When measured by iohexol clearance the group randomised to early RIPC had a higher GFR at 12 months, although the evidence for an effect was weak. When measured by eGFR at 3 and 12 months, the evidence for a beneficial effect was stronger, with an increased eGFR in the early RIPC group, amounting to an approximate 5 ml/minute/1.73 m2 difference at 12 months. When missing values of iohexol GFR were imputed using the eGFR, the strength of evidence for an effect of RIPC also increased. These conclusions were supported by PP analyses, which increased the size of the effect of early RIPC and increased confidence in the findings. Given a mean annual rate of decline of eGFR after living-donor transplantation of 1.5 ml/minute/1.73 m2, a patient starting out after transplantation with a 5 ml/minute/1.73 m2 advantage might reasonably expect a 2- to 3-year extension to the lifespan of the transplant. In contrast to these findings were the largely null effects of delayed preconditioning. In a pilot trial in paediatric transplantation, we had observed a protective effect of late RIPC on eGFR, but this was not apparent in the REPAIR trial. Indeed, using the iohexol GFR, eGFR at 3 and 12 months and iohexol GFR with imputed values for eGFR, and in the PP analyses, there was no evidence of a clinical effect of delayed preconditioning. The most likely explanation for the difference in the strength of evidence between the primary and secondary GFR end points is the greater variability in the more complex and less standardised measure of GFR and the attrition of data for this measure. This conclusion is supported by the consistency between the effects of early and delayed RIPC when assessed by either estimate of GFR.
Effects of remote ischaemic preconditioning on short-term secondary end points
There was no evidence of an effect of RIPC on the short-term secondary end points. The time taken for creatinine to fall by 50% following transplantation was similar between early RIPC and the control (p = 0.75) and between late RIPC and the control (p = 0.64), with a median time of 48 hours in all treatment groups. However, it is possible that there were insufficient sample analyses for creatinine in the first 24 hours after transplantation to detect very early effects of RIPC or to assess accurately the time taken for creatinine to fall by 50%. There was little evidence of a difference in the rate of acute rejection between early RIPC and the control (p = 0.86) or between late RIPC and the control (p = 0.17); however, only 10% of participants experienced acute rejection during the trial. There was little evidence that the incidence of delayed graft function differed between the early RIPC group and the control group (p = 0.61) but the incidence was lower among the late RIPC group than among the control group (1.0% vs. 5.3%, p = 0.031). However, only 12 patients experienced delayed graft function and so substantial uncertainty remains with regard to the effects of early and late RIPC on this outcome. The median length of hospital stay was 6 days in all groups. Nine recipients experienced graft loss and only two recipients died during the initial 12 months following transplantation. There was little evidence of any differences between those receiving RIPC and those in the control group. The results of the PP analysis for the main secondary outcomes were similar to those of the ITT analysis.
Remote ischaemic preconditioning had no effect on the systemic inflammatory response to surgery in the donor or the recipient, with similar profiles of TNF-α, IL-1β, INF-γ and IL-6. The lack of any anti-inflammatory effect of RIPC is consistent with the null effects of delayed RIPC. Other mechanistic analyses are ongoing.
Safety of remote ischaemic preconditioning
There were no major safety concerns around RIPC. As expected, RIPC caused some discomfort to approximately 40% of donors and recipients alike. However, RIPC was discontinued in only nine pairs because they were unable to tolerate the intervention. Direct pressure effects of the cuff used to occlude blood flow in the arm resulted in a small proportion experiencing asymptomatic petechiae. However, during follow-up there were no differences between the groups in the adverse events recorded, the majority of which were unscheduled hospital admissions unrelated to preconditioning.
Limitations of the REPAIR trial
The length of follow-up in the REPAIR trial was set at 12 months. Longer-term follow-up is needed to determine whether differences in GFR are maintained in subsequent years, to such an extent that one can have greater confidence that RIPC will alter the lifespan of the transplant. As only about one-third of screened patients were recruited, the generalisability of our findings can be legitimately questioned. RIPC might have exerted its action by inducing ischaemic protection in the donor or limiting reperfusion injury in the recipient; as the donor and the recipient underwent RIPC, our data do not inform on the phase of IR injury influenced by RIPC. The lack of any effect of late RIPC on kidney function contrasts with that seen in our pilot data in children. Possible explanations for this include small study bias, differences in preconditioning pathways between children and adults and loss of the precision of the intervention when moving from a single-centre to a multicentre study.
Research recommendations
Further studies will, of course, help define the role of RIPC in renal transplantation, as one trial rarely changes practice and it would be necessary to confirm the effects of early RIPC on kidney function after living-donor transplantation. The REPAIR trial also refines the optimal design for a trial of RIPC in deceased-donor transplantation, which should test the effects of early RIPC, applied to the recipient only, on eGFR.
Chapter 5 Conclusions
Apart from an immediate unpleasant sensation that is short-lived, RIPC is a safe intervention that can be used with little added cost in living-donor transplantation. Its tolerability will be determined by the perception of benefit and, in the REPAIR trial, the totality of evidence points to an effect on renal function that is worthwhile. Although our primary measure of GFR provided evidence for an effect of early RIPC on GFR that was less strong than would be the accepted convention, the standard clinical measures of GFR (our secondary analysis) provided persuasive evidence of a clinically meaningful improvement in kidney function after transplantation. Further studies will, of course, help define the role of RIPC in renal transplantation, particularly its effect in deceased-donor transplantation. The REPAIR trial clearly identifies the optimal design for such a follow-on study: it should investigate the effects of early RIPC applied to the recipient and it should measure eGFR. In the interim, RIPC has the seductive appeal of the ideal intervention in living-donor transplantation: it is free at the point of delivery, it causes no harm, there is a very good chance that it will add life to kidneys, and kidneys to life, and it is not too good to be true.
Acknowledgements
Thank you to all of the kidney donors and recipients who took the time to be part of the REPAIR trial. Without their commitment this trial would not have been possible.
We would also like to thank:
-
the Trial Steering Committee: Tom Meade (chairperson); Adam McLean, John Forsyth and Lisa Silas (externals); Mark Harber and Chris Watson (investigators); Neil Woodnick and Francesca Crozier (consumers); and Rosemary Knight and Tim Clayton (CTU)
-
the Data Monitoring Committee: Rajesh Kharbanda (chairperson), Alan Jardine and Joan Morris
-
the Project Management Group: Raymond MacAllister, Rosemary Knight, Tim Clayton, Steven Robertson, Kristin Veighey, Stavros Loukogeorgakis, Jennifer Nicholas, Cono Ariti, Madhur Motwani, Will Jenner and Mike Okorie
-
the randomisation service Sealed Envelope
-
Neil Dalton for the iohexol analysis
-
Rebecca Matthews for monograph preparation.
Contributing sites
-
Leiden University Medical Centre (65 patients, recruitment started 4 January 2010): Professor Hans de Fijter (principal investigator), J Dubbeld, Krista Glas, Ada Haasnoot, Sabrina Hendriksen, Clarisca Montero, Sonja van Berkel and Ruth van Dam.
-
St George’s Hospital, London (83 patients, recruitment started 30 March 2010): Sarah Heap (principal investigator), Sharirose Abat, Rene Chang, Liz Cording, Mr Jiri Fronek, Helen Gregson, Mr Nicos Kessaris, Iain MacPhee, Joyce Popoola, Rajeshwar Ramkhelawon and Rhia Ventura.
-
Royal Free Hospital, London (64 patients, recruitment started 21 February 2010): Dr Mark Harber (principal investigator), Obaayaa Buahin, Vash Deelchand, Peter Dupont, Dr Nadia Godigamuwe, Ruth Kinyanjui, Aisling O’Riordan, Alison Richardson, Alan Salama, David Wheele and Ruth Yang.
-
Guy’s Hospital, London (23 patients, recruitment started 27 October 2010): Mr Jonathon Olsburgh (principal investigator), Golda-Grace Azanu, Karen Ignatian, Lisa Silas, Jane Watkins and all surgical, medical and nursing staff who helped with the patients.
-
Southmead Hospital, Bristol (27 patients, recruitment started 30 September 2010): Mr Najib Kadi (principal investigator), Helen Andrew, Susan Dawson, Vicki Elnagar, Kate Humphries, Dominic Janssen, Dr Chris Johnson, Mr Paul Lear, Stacey McGary, Helen McNally, Ronelle Mouton, Freya Murch, Louise Pearse, Joana Vaz, Joao Vicente, Mr Andy Weale, Nicola Woollven and Dr Karine Zander.
-
Queen Elizabeth Hospital, Birmingham (eight patients, recruitment started 13 October 2010): Mr Steve Mellor (principal investigator), Dr Chris Counsell, Mary Dutton, Jonathan Ellis, Melanie Field, Lesley B Fifer, Okdeep Kaur, Adnan Sharif and Chantelle Waite.
-
Royal London Hospital (33 patients, recruitment started 14 October 2010): Dr Raj Thuraisingham (principal investigator), Belinda Englebright, Wancheung Li, Lilibeth Piso, Caroline Rolfe, Jamie Smith, Ray Trevitt, Clare Whittaker, Karen Williams and Magdi Yaqoob.
-
Cambridge University Hospitals NHS Foundation Trust (36 patients, recruitment started 24 September 2010): Mr Chris Watson (principal investigator), Roberto Cayado-Lopez, Sylvia Cottee, Nirvana Croft, Julia Ertner, Faye Forsyth, Dorothee Koscielny-Lemaire, Sue Miles, Ann-Marie O’Sullivan, Lucy Randle, Annie Rivera and Myrna Udarbe.
-
VU University Medical Centre, Amsterdam (42 patients, recruitment started 15 March 2010): Dr Azam Nurmohamed (principal investigator), Yvonne de Koter, Carla Schrauwers, Marjon van Vliet and Hiske Wellink.
-
Western Infirmary, Glasgow (10 patients, recruitment started 28 September 2011): Marc Clancy (principal investigator), Emma Aitken, Laura Buist, Julie Glen, Cheryl Keenan, Donna Kelly, Louise Maxwell, Lorraine McGregor, Barbara McLaren and Maria Nicoletti.
-
CHU Erasme, Brussels (seven patients, recruitment started 10 July 2012): Dr Annick Massart (principal investigator), Professor Daniel Abramowicz, Cherubine Addino, Brigitte Borre and Nicole Lietaer.
-
CHU de Liège (three patients, recruitment started 18 September 2012): Laurent Weekers (principal investigator), Catherine Bonvoisin, Arnaud Borsu, Michèle Focan and Stephanie Grosch.
-
CHRU de Lille (five patients, recruitment started 27 January 2013): Marc Hazzan (principal investigator), Celine Beaussart, Priscilla Couillet, Sophie Dessau, Christine Devlaminck, Laetitia Erichot, Valérie Fontaine, Marie Fruleux, Professor Gilles Lebuffe, Benedicte Mignot, Christian Noël, Ornella Savarino and Carole Zini.
-
St Helier Hospital, Carshalton (1-year and longer-term follow-up for 49 patients from St George’s Hospital; 18 underwent iohexol clearance at St Helier): Shamshersingh Jeetun, Dr David Makanjuola, Aileen Moore, Dr Mysore Phanish and Gillian Thomas.
-
Royal Sussex County Hospital, Brighton (1-year and longer-term follow-up for 10 patients from St George’s Hospital; all 10 underwent iohexol clearance at Brighton): Mary Flowerdew, Ed Kingdon and Richard Rye.
Contribution of authors
Raymond MacAllister (Clinical Pharmacologist) was the Chief Investigator on the REPAIR trial and was involved in the design and conduct of the trial, was a member of the TSC and PMG and provided input into the manuscript.
Tim Clayton (Medical Statistician) was a co-applicant on the REPAIR trial and was involved in the design and conduct of the trial (including the statistical analysis plan), was a member of the TSC and PMG and provided input into the manuscript.
Rosemary Knight (Senior Trial Manager) had overall responsibility for the REPAIR trial, ensuring that all relevant approvals were in place, organising the setting up and training of sites and ensuring that the project was delivered on time and to budget.
Steven Robertson (Senior Data Manager) had overall responsibility for all aspects of data management including involvement in the design and development of the CRF and eCRF. He was also responsible for data cleaning and preparation for the final analyses and was a member of the PMG.
Jennifer Nicholas (Medical Statistician) was the unblinded statistician supporting the DMC, was a member of the PMG and provided support in preparation of the statistical analysis plan. She conducted the statistical analysis and provided input into the manuscript.
Madhur Motwani (PhD Student) performed the ELISA experiments and organised the cytokine data for statistical analysis. He also co-ordinated the transfer of samples from the trial sites to UCL and was a member of the PMG.
Kristin Veighey (Clinical Research Fellow) was a member of the PMG and TSC. She carried out site induction and training and co-ordinated sample collection and transfer for storage and analysis at UCL. She also organised and performed the analysis of samples collected during the trial.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NETSCC, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health.
References
- NHS Blood and Transplant . Activity Report 2012–2013 n.d. www.organdonation.nhs.uk/statistics/transplant_activity_report/current_activity_reports/ukt/activity_report_2012_13.pdf (accessed 28 June 2014).
- Sharif A, Baboolal K. Complications associated with new-onset diabetes after kidney transplantation. Nat Rev Nephrology 2012;8:34-42. http://dx.doi.org/10.1038/nrneph.2011.174.
- Mehrabi A, Mood Zh A, Sadeghi M, Schmied BM, Müller SA, Welsch T, et al. Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation. Nephrol Dial Transplant 2007;22:viii54-60. http://dx.doi.org/10.1093/ndt/gfm651.
- Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 1960;70:68-7.
- Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 1997;64:945-7. http://dx.doi.org/10.1097/00007890-199710150-00001.
- Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 2005;79:505-14. http://dx.doi.org/10.1097/01.TP.0000153160.82975.86.
- Shackleton CR, Ettinger SL, McLoughlin MG, Scudamore CH, Miller RR, Keown PA. Effect of recovery from ischemic injury on class I and class II MHC antigen expression. Transplantation 1990;49:641-4. http://dx.doi.org/10.1097/00007890-199003000-00032.
- Lu CY, Penfield JG, Kielar ML, Vazquez MA, Jeyarajah DR. Hypothesis: is renal allograft rejection initiated by the response to injury sustained during the transplant process?. Kidney Int 1999;55:2157-68. http://dx.doi.org/10.1046/j.1523-1755.1999.00491.x.
- Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997;63:968-74. http://dx.doi.org/10.1097/00007890-199704150-00011.
- Halloran PF, Aprile MA, Farewell V, Ludwin D, Smith EK, Tsai SY, et al. Early function as the principal correlate of graft survival. A multivariate analysis of 200 cadaveric renal transplants treated with a protocol incorporating antilymphocyte globulin and cyclosporine. Transplantation 1988;46:223-8. http://dx.doi.org/10.1097/00007890-198808000-00007.
- Lien YH, Lai LW, Silva AL. Pathogenesis of renal ischemia/reperfusion injury: lessons from knockout mice. Life Sci 2003;74:543-52. http://dx.doi.org/10.1016/j.lfs.2003.08.001.
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrology 2011;7:189-200. http://dx.doi.org/10.1038/nrneph.2011.16.
- Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int 2004;66:496-9. http://dx.doi.org/10.1111/j.1523-1755.2004.761_5.x.
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-36. http://dx.doi.org/10.1161/01.CIR.74.5.1124.
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 2003;83:1113-51. http://dx.doi.org/10.1152/physrev.00009.2003.
- Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 2006;70:240-53. http://dx.doi.org/10.1016/j.cardiores.2006.01.017.
- Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia–reperfusion: opportunities and obstacles for survival signalling. Br J Pharmacol 2007;152:855-69. http://dx.doi.org/10.1038/sj.bjp.0707409.
- Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway?. J Mol Cell Cardiol 2009;47:32-40. http://dx.doi.org/10.1016/j.yjmcc.2009.03.019.
- Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 2010;105:151-4. http://dx.doi.org/10.1007/s00395-009-0080-9.
- Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 2004;19:143-50. http://dx.doi.org/10.1152/physiolgenomics.00046.2004.
- Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0032296.
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893-9. http://dx.doi.org/10.1161/01.CIR.87.3.893.
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002;106:2881-3. http://dx.doi.org/10.1161/01.CIR.0000043806.51912.9B.
- Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 1996;94:2193-200. http://dx.doi.org/10.1161/01.CIR.94.9.2193.
- Ateş E, Genç E, Erkasap N, Erkasap S, Akman S, Firat P, et al. Renal protection by brief liver ischemia in rats. Transplantation 2002;74:1247-51. http://dx.doi.org/10.1097/00007890-200211150-00009.
- Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis 1999;8:123-9. http://dx.doi.org/10.1023/A:1008911101951.
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci 2009;117:191-200. http://dx.doi.org/10.1042/CS20080523.
- Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 2002;34:1317-23. http://dx.doi.org/10.1006/jmcc.2002.2072.
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia–reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation 2005;79:1691-5. http://dx.doi.org/10.1097/01.TP.0000159137.76400.5D.
- Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, et al. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol 2013;108. http://dx.doi.org/10.1007/s00395-013-0377-6.
- Dong JH, Liu YX, Ji ES, He RR. Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng Li Xue Bao 2004;56:41-6.
- Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia–reperfusion injury in humans: role of the autonomic nervous system. J Am College Cardiol 2005;46:450-6. http://dx.doi.org/10.1016/j.jacc.2005.04.044.
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 2010;105:651-5. http://dx.doi.org/10.1007/s00395-010-0099-y.
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575-9. http://dx.doi.org/10.1016/S0140-6736(07)61296-3.
- Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 2009;95:1567-71. http://dx.doi.org/10.1136/hrt.2008.155770.
- Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischaemic preconditioning in human coronary artery bypass surgery: from promise to disappointment?. Circulation 2010;122:53-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.926667.
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 2013;382:597-604. http://dx.doi.org/10.1016/S0140-6736(13)61450-6.
- Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, Kolocassides KG, Adamopoulos S, Karavolias G, et al. Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning?. Heart 2006;92:1821-6. http://dx.doi.org/10.1136/hrt.2006.089060.
- Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation 2009;119:820-7. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.809723.
- Botker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010;375:727-34. http://dx.doi.org/10.1016/S0140-6736(09)62001-8.
- Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, et al. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol 2012;10:339-48. http://dx.doi.org/10.1007/s00392-011-0397-x.
- Meybohm P, Zacharowski K, Cremer J, Roesner J, Kletzin F, Schaelte G, et al. RIP Heart-Study Investigator Group . Remote ischaemic preconditioning for heart surgery. The study design for a multi-center randomized double-blinded controlled clinical trial – the RIPHeart-Study. Eur Heart J 2012;33:1423-6.
- Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol 2012;24:42-8.
- Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012;126:296-303. http://dx.doi.org/10.1161/CIRCULATIONAHA.112.096370.
- Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Ischaemic preconditioning for liver transplantation. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.CD006315.pub2.
- Loukogeorgakis SP, Rees L, Dalton NR, MacAllister RJ, Deanfield JE. Remote ischemic preconditioning protects against ischemia reperfusion injury in pediatric renal transplantation. J Am Soc Nephrol 2006;17.
- Chen Y, Zheng H, Wang X, Zhou Z, Luo A, Tian Y. Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation: a randomized controlled trial. Transplantation 2013;95:e4-6. http://dx.doi.org/10.1097/TP.0b013e3182782f3a.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI. Chronic kidney disease epidemiology collaboration. Ann Intern Med 2009;150:604-12.
- Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993;44:411-22. http://dx.doi.org/10.1038/ki.1993.259.
- Marcén R, Morales JM, Fernández-Rodriguez A, Capdevila L, Pallardó L, Plaza JJ, et al. Long-term graft function changes in kidney transplant recipients. NDT Plus 2010;3:ii2-8.
- Hawley CM, Kearsley J, Campbell SB, Mudge DW, Isbel NM, Johnson DW, et al. Estimated donor glomerular filtration rate is the most important donor characteristic predicting graft function in recipients of kidneys from live donors. Transpl Int 2007;20:62-7. http://dx.doi.org/10.1111/j.1432-2277.2006.00400.x.
- Chang SH, Russ, GR, Chadban SJ, Campbell SB, McDonald SP. Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation 2007;84:611-18. http://dx.doi.org/10.1097/01.tp.0000280553.23898.ef.
- Douverny JB, Baptista-Silva JC, Pestana JO, Sesso R. Importance of renal mass on graft function outcome after 12 months of living donor kidney transplantation. Nephrol Dial Transplant 2007;22:3646-51. http://dx.doi.org/10.1093/ndt/gfm487.
Appendix 1 Protocol amendments
Full approval for the trial was given in June 2009.
October 2009
Following approval further amendments were made to the trial protocol and information used for potential participants:
-
The immunosuppression protocol was changed to reflect the standard protocols in use at each of the centres.
-
RIPC/sham RIPC will be carried out before induction of anaesthesia to prevent any delays to transplantation.
-
Patient follow-up was changed to reflect clinical practice.
-
The timing of the collection of blood and urine samples from the donor was changed to 3 days postoperatively. Originally, samples were to be collected up to 5 days postoperatively but patients would likely have been discharged at this point.
-
The blood sampling procedures were clarified.
-
The arrangements for reporting adverse events were clarified.
-
Changes were made to the consent form and participant information sheet and two versions (one for the recipient and one for the donor) were submitted. Treatment of the donor and recipient is different and having separate information sheets highlighted this fact.
December 2009
Patients who require HLA antibody removal therapy were excluded as they require a different immunosuppression regime that may have an effect on preconditioning.
May 2010
-
Mycophenolate/azathiaprine were added to the immunosuppression regime as used by some of the recruiting hospitals. This would be beneficial in terms of increasing recruitment.
-
All patients will receive tacrolimus, titrated according to local practice.
-
Some of the centres wanted to send out an invitation letter to potential patients with a copy of the participant information sheet.
September 2010
Patients who have had a previous transplant, who were originally excluded from the trial, are now to be included. It had been considered that RIPC may not be as effective in patients who had undergone a previous transplant; however, there was a change of opinion and evidence and they could now be included.
December 2010
-
Changes were made to the protocol to include more flexibility in the immunosuppression protocol and increase recruitment.
-
Patients with a ‘000’ mismatch type are now to be included. These patients were originally excluded because their immunosuppression regime was different from that in other transplants. However, because of changes to the immunosuppression protocol since the original description, these patients can now be included.
January 2011
A letter was approved to remind patients to attend their 1-year appointment as this is the primary outcome. Patients were aware of this at the time of consent and it was included in the participant information sheet. Patients were followed up at 3 months and again at 1 year and, as there was a gap of 9 months between the two follow-ups, it was recommended that patients be reminded of the importance of attending for iohexol testing.
May 2011
-
There was a change to the PI at St Georges’ Hospital.
-
Further analysis was performed on the kidney tissue samples taken from the patients at the Royal Free Hospital. The patients were already aware that tissue samples were being gifted and had signed a consent form to agree to this. The analysis is in line with updated information.
List of abbreviations
- ANCOVA
- analysis of covariance
- ATP
- adenosine triphosphate
- CABG
- coronary artery bypass grafting
- CHRU
- Centre Hospitalier Régional Universitaire (France)
- CHU
- Centre Hospitalier Universitaire (Belgium)
- CI
- confidence interval
- CKD-EPI
- Chronic Kidney Disease Epidemiology Collaboration
- COX-2
- cyclo-oxygenase-2
- CRF
- case report form
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- eCRF
- electronic case report form
- eGFR
- estimated glomerular filtration rate
- ELISA
- enzyme-linked immunosorbent assay
- ERK
- extracellular signal-regulated kinase
- GCP
- good clinical practice
- GFR
- glomerular filtration rate
- HLA
- human leucocyte antigen
- HSP
- heat shock protein
- IL-1β
- interleukin 1 beta
- IL-6
- interleukin 6
- INF-γ
- interferon gamma
- iNOS
- inducible nitric oxide synthase
- IPC
- ischaemic preconditioning
- IR
- ischaemia–reperfusion
- ITT
- intention to treat
- KATP channel
- ATP-sensitive potassium channel
- mPTP
- mitochondrial permeability transition pore
- PCI
- percutaneous coronary intervention
- PI
- principal investigator
- PI3K
- phosphatidylinositol-4,5-bisphosphate 3-kinase
- PMG
- Project Management Group
- PP
- per protocol
- RIPC
- remote ischaemic preconditioning
- SD
- standard deviation
- SDF-1α
- stromal cell-derived factor 1 alpha
- TNF-α
- tumour necrosis factor alpha
- TSC
- Trial Steering Committee
- UCL
- University College London
- UCLH
- University College London Hospital
- VU
- Vrije Universiteit