Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 10/27/01. The contractual start date was in April 2011. The final report began editorial review in January 2015 and was accepted for publication in July 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr McCann reports grants from Institut de Recherches Internationales Servier and Menarini International, outside the submitted work. Dr Curzen reports grants and personal fees from St Jude Medical and Haemonetics, grants from Medtronic, personal fees from Abbott Vascular and Heartflow, and non-financial support from Volcano Corporation, outside the submitted work. Dr Dalby reports providing consultancy services for Medtronic, Boston, AstraZeneca, Daichii Sankyo–Eli Lilly alliance and Sanofi and receiving grants from Abbott Vascular and Daichii Sankyo–Eli Lilly alliance, outside the submitted work. Professor Ring has received conference travel expenses from Boehringer Ingelheim, personal consultancy fees from Roche and research funding from Novartis outside the submitted work. Professor Flather reports serving on advisory and speaker panels for AstraZeneca and Menarini International outside the current work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by McCann et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Treatment of ST-segment elevation myocardial infarction
ST-segment elevation myocardial infarction (STEMI) results from complete occlusion or critically impaired flow in a main epicardial coronary artery or one of its side branches, deemed the infarct-related artery (IRA). There are more than 100,000 STEMI presentations in the UK each year. 1 The key goal of treatment is to open the blocked coronary artery, to limit myocardial necrosis and subsequent left ventricular (LV) dysfunction, which is a key determinant of prognosis. 2 The primary percutaneous coronary intervention (PPCI) is the preferred revascularisation strategy in STEMI. 3,4
Multivessel disease at ST-segment elevation myocardial infarction
Multivessel coronary artery disease (MVD) is typically defined as the presence of ≥ 70% stenosis in ≥ 1 non-IRA. MVD occurs in 28–67% of patients with STEMI and is an independent predictor (IP) of short- to medium-term prognosis, as summarised in Table 1. 5–10 The presence of a chronic total occlusion in the non-IRA confers a particularly poor prognosis and was an IP of mortality and reduction in LV ejection fraction (LVEF) post STEMI after correcting for baseline risk profile and cardiogenic shock. 11
| Study | Year | Number of participants in study | Main findings | Mean follow-up |
|---|---|---|---|---|
| Tarantini5 | 2010 | 288 | MVD IP of non-fatal MI (OR 5.7), combined death/MI (OR 4.8) and combined MACEs (OR 4.7). MVD also IP for LV remodelling (OR 2.2) | 32 months |
| Dziewierz6 | 2010 | 1598 | MVD IP (HR 1.58) for mortality at 12 months in a model including left anterior descending artery IRA, Killip class | 12 months |
| Corpus7 | 2004 | 820 | Significantly higher non-fatal MI, target vessel revascularisation, mortality, MACEs at 30 days in MVD. MVD IP of mortality at 12 months (OR 3.3) in model including age, renal function and LVEF | 12 months |
| Sorajja8 | 1997 | 2082 | Increasing composite MACEs at 12 months with number of diseased vessels. MVD strongest IP for MACEs (HR 1.9) and mortality (HR 2.6) | 12 months |
| Jaski9 | 1992 | 151 | MVD only IP for prediction of angioplasty success (MVD 75% vs. single vessel disease 92%; p < 0.005) on stepwise logistic regression | Inpatient |
| Muller10 | 1991 | 236 | Reduced LVEF and increased mortality in MVD group. MVD strongest IP of inpatient mortality in model including LVEF, age, TIMI post lysis | Inpatient |
Management of multivessel disease in patients presenting with ST-segment elevation myocardial infarction
Current guidelines
The guidelines from the European Society of Cardiology (ESC)3 and American Heart Association (AHA)4 recommend percutaneous coronary intervention (PCI) of the IRA only at the PPCI unless the patient is in cardiogenic shock, where complete revascularisation (CR) is permitted for critical (> 90% stenosis) or unstable lesions (ESC/AHA: class IIa, level of evidence B). The two alternative options at the PPCI are CR during the index admission (of all significant lesions, including in non-IRAs) and planned outpatient PCI of the non-IRA at a later date (usually < 6 weeks) as a staged procedure. 12 Where outpatient revascularisation to non-IRAs is being considered, this should be preceded by non-invasive ischaemia assessment [e.g. myocardial perfusion scintigraphy (MPS), stress cardiovascular magnetic resonance imaging (MRI)] (ESC: class I, level of evidence A; AHA: class IIa, level of evidence B). 3,4 Fractional flow reserve (FFR) assessment of non-IRAs at the PPCI is not currently undertaken as it is felt that potential microvascular dysfunction in non-IRA territories could render FFR inaccurate. However, one study has investigated this and showed that FFR at the PPCI was unchanged when reassessed 35 days later (FFR 0.77 at both time points), suggesting that non-IRA lesion severity may be accurately measured by FFR in acute STEMI. 13 The resulting lack of consensus regarding management of MVD at the PPCI was reflected by the US Cardiovascular Data Registry, demonstrating wide variation in practice, with 0–38% of MVD patients undergoing CR at the PPCI on registry studies. 14,15
Evidence base for revascularisation strategies for multivessel disease at primary percutaneous coronary infarction
At the time of this grant application (July 2010) the evidence base for the management of MVD at the PPCI was weak, largely based on retrospective analyses and registries. 3,4 There were only a small number of randomised clinical trials, with clinical outcomes that were of limited quality, lacking statistical power, and with varying design and outcomes (Table 2).
| Trial | Design | Year | Mean follow up | Favoured strategy | Findings | Comment |
|---|---|---|---|---|---|---|
| Di Mario16 | IRA only, n = 17; CR, n = 69 | 2002 | 1 year | ↔ | No MACE difference. Trend to less repeat revascularisation with CR (CR 17% vs. IRA-only 35%; p = 0.247). Equivalent costs | Study powered on basis of cost efficacy calculation; unequal randomisation |
| Politi17 | Immediate CR (n = 65), staged CR (n = 65) and IRA only (n = 84) | 2003–7 | 2.5 years | CR | MACE: 50.0% CR, 20.0% staged CR and immediate CR 23.1%; p < 0.001; HR ≈0.4. MACE driven by revascularisation | Unequal groups Low antiplatelet use in complete group |
| Ghani18 | CR (n = 80) and IRA only (n = 41) | 2012 | 3 years | ↔ | MACEs 35% in both groups | Reduced re-PCI in complete |
| Wald19 | CR (n = 234) and IRA only (n = 231) | 2013 | 23 months | CR | MACEs 9% in CR, 23% in IRA; HR 0.34 | Reduced MI and refractory angina |
By far the best-quality study to date is the Preventative Angioplasty In Myocardial Infarction (PRAMI) trial, the results of which were published in August 2013,19 at which time the Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial (CvLPRIT) was in follow-up. This trial demonstrated an extremely large reduction [hazard ratio (HR) 0.35, 95% confidence interval (CI) 0.21% to 0.58%; p < 0.001] in major adverse cardiovascular events [MACEs; death from cardiac causes, non-fatal myocardial infarction (MI) or refractory angina], which was driven by all components of the primary end point. 19 However, the trial could be criticised for randomising patients after the PPCI, potentially introducing selection bias, and an excess of anterior MI in the IRA-only arm and the inclusion of the ‘soft’ end point of refractory angina in the primary outcome. The benefits of a CR strategy remained in doubt, particularly with concern over the safety of such a strategy undertaken at same sitting as the PPCI.
Potential risks and benefits of a complete revascularisation strategy at primary percutaneous coronary intervention
Potential benefits
A CR strategy at the time of the PPCI in STEMI patients with MVD may:
-
Limit infarct size (IS) and increase the amount of salvaged myocardium by increasing collateral flow to the at-risk, but non-necrotic, peri-infarct zone. There are no specific data (either observational or from clinical trials) available to confirm such a benefit.
-
Reduce overall hospital stay and total cost of care.
-
Reduce ischaemic burden,20 which appears to be an important determinant of outcome following MI, at least in the era before the PPCI. 21
-
May reduce further PCI at a later date, either for symptoms or silent ischaemia as per current guidelines,3,4 reducing subsequent hospitalisation for the patients and with resultant economic benefits.
-
Reduce the risk of recurrent MI/death, as has been observed for non-STEMI,22 although this finding has not been replicated in chronic stable angina. 23
-
Reduce vascular complications by having all PCI performed during the index intervention through a single-access site.
Potential risks
Potential risks of a CR strategy are detailed below.
-
IS may be increased. Approximately one-third of patients undergoing even seemingly uncomplicated elective PCI experience a rise in troponin levels consistent with the diagnosis of myocardial necrosis. 24 The risk for unstable angina patients is higher, with 53% experiencing a post-PCI troponin elevation. 24 There is debate whether or not such troponin increases are of independent prognostic significance. 25 The mechanisms of injury are likely to include necrosis of myocardial tissue adjacent to stent insertion and microembolisation to the distal vasculature. 26 IS in the 30% of patients with new infarction was 5% of LV mass (LVM) and 1.3% of LVM for the entire cohort. 26 In a mixed cohort of patients undergoing elective PCI (n = 92) or coronary artery bypass grafting (n = 60), those who experienced new myocardial injury, detectable on cardiac magnetic resonance (CMR) imaging, had a threefold increase in MACEs. 27 There is also the not insignificant, but impossible to quantify, risk of complete no-reflow in the non-IRA which could have devastating consequences in a patient undergoing the PPCI. There are no data available that tell us the frequency of ‘new’ injury in the non-IRA with multivessel PPCI, but we can anticipate this will be significantly higher than in patients undergoing elective PCI. 24 Even without myocardial necrosis, resting perfusion28 and myocardial perfusion reserve29 are reduced following PCI, potentially impairing collateral flow to the area at risk (AAR) and decreasing the amount of salvaged myocardium.
-
Contrast-induced nephropathy, as a result of the increased volume load of contrast, could be increased.
-
Stenting of bystander lesions in the non-IRAs which are neither causing ischaemia nor symptoms may lead to no symptomatic or prognostic benefit to the patient and with increased costs to the NHS.
-
There may be an increased risk of both early, especially in the thrombogenic milieu of acute infarction, and late stent thrombosis and restenosis.
-
Non-IRA revascularisation may not reduce ischaemia more effectively than by intensive medical therapy following MI. 30
Rationale for Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial cardiac magnetic resonance imaging
Prognosis following acute myocardial infarction
Left ventricular systolic dysfunction has long been recognised as an important sequelae in survivors of MI. 2 In 605 male survivors of acute MI, LVEF, LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were all predictive of mortality during an average of 78 months’ follow-up, but only LVESV was an IP on multivariate analysis. 2 The importance of reductions in ejection fraction, IS and increases in LV volumes have been confirmed in over 2300 survivors of STEMI receiving reperfusion therapy. 31 Limiting IS with reperfusion leads to improved LV function and attenuation of subsequent cardiac remodelling (LV dilatation > 20%), and is a key aim of current treatment strategies in STEMI.
Cardiac magnetic resonance imaging assessment of myocardial injury following ST-segment elevation myocardial infarction
Cardiac MRI is the gold standard technique for the quantification of LV volumes and function and late gadolinium enhancement (LGE) imaging can detect and quantify MI with unique precision. 32,33 In a dog model of experimental reperfused MI, microvascular obstruction (MVO) and IS were strongly related to early LV remodelling (r = 0.89 and r = 0.81, respectively). 34 MVO was the best IP (r2 = 0.71; p < 0.001) of remodelling. 34 In patients, CMR-measured MVO correlates strongly with ST-segment resolution in patients undergoing the PPCI but relatively weakly with myocardial blush grade and not significantly with thrombolysis in MI (TIMI) flow. 35 However, CMR-measured MVO is not simply an oversensitive measure of small vessel obstruction. Larger infarcts on CMR are consistently associated with larger ventricular volumes, reduced ejection fraction and increased MVO, which occurs in 40–60% of patients treated by the PPCI. 35–37 IS and MVO have consistently been related to adverse ventricular remodelling. In all the PPCI studies in which MVO has been included in a multivariate model, MVO predicts remodelling independently of IS, ejection fraction and cardiac volumes. 35,36,38–40
Prognostic value of cardiac magnetic resonance imaging following primary percutaneous coronary intervention
Infarct size and MVO have been shown to be related to medium-term prognosis, even in relatively small studies. For example, in 122 patients with STEMI undergoing the PPCI, LVEF, LVEDV and LVESV were all associated with IS (r = –0.75, r = 0.42 and r = 0.69, respectively; all p < 0.001) and outcome. IS on CMR was the only IP of MACEs (one death, one MI and 16 heart failure admissions) at 2 years. 40 In 184 patients undergoing successful PPCI, the presence of MVO on CMR was independently predictive of MACEs (five deaths, 13 heart failure, 18 re-infarction and eight unstable angina) at 1 year. 40 Larger studies have consistently shown that IS and MVO are independently related to prognosis, even when other clinical variables, and LV volumes and ejection fraction are considered. 41–43
Myocardial salvage index
The extent of myocardial necrosis after an acute coronary occlusion is variable and dependent on a number of factors including the time to reperfusion, collateral blood flow, metabolic demand of the tissue and the total AAR, as determined by amount of tissue that is acutely hypoperfused at the time of coronary artery occlusion, probably being the most important. 44 The efficacy of reperfusion strategies can be assessed by calculating myocardial salvage index (MSI) (AAR IS/AAR), which may be an important measure of outcome. 45
Cardiac magnetic resonance imaging and myocardial salvage index
Cardiac MRI can accurately quantify MSI. During ischaemia or infarction, myocardial tissue develops oedema that can be detected as high signal intensity on T2-weighted (T2W) images, and the area of oedema is greater than the area of irreversibly damaged, necrotic myocardium. 46,47 Myocardium with high T2 signal closely correlates with the AAR, confirmed in experimental models of both reperfused48 and non-reperfused MI. 49 As expected, the size of salvaged myocardium decreases with increased IS, as measured by CMR imaging. 46 Two small clinical studies have validated the MSI with CMR imaging against single-photon emission computed tomography (SPECT). 50,51 A major advantage of CMR imaging-measured salvage index is that it can be measured during a single examination in addition to quantification of volumes, function, IS and MVO. Although black-blood T2W imaging has been prone to artefact, recent advances including increased slice thicknesses, use of coil signal intensity correction algorithms and motion correction have made the assessment of oedema much more robust. 45
Summary
Cardiac MRI offers a unique and robust assessment of the success of revascularisation for STEMI. CMR imaging infarct characteristics are the best proven surrogate markers of medium-term outcome in patients with STEMI treated by the PPCI. It was aimed, by embedding CMR imaging in the main CvLPRIT, to have a more robust assessment of the differences in the efficacy and safety of the revascularisation strategies being tested that could only be seen with a much larger population if there was reliance on clinical outcomes alone. Another aim was to obtain a greater understanding of the mechanisms by which differences in outcome between the two groups may result. The risk of new MI from PCI to the non-IRA’s during the PPCI and the effect on myocardial salvage and subsequent ventricular remodelling and medium-term outcome could be established for the first time through the use of CMR imaging in this trial population.
Chapter 2 Research objectives
The original research objectives of the CvLPRIT-CMR imaging substudy were to assess whether or not:
-
IS, MSI and the extent of MVO are different in the CR versus IRA-only strategies.
-
A CR strategy in STEMI patients with MVD results in altered LV volumes and function in the medium term (9 months post MI).
-
Reducing ischaemic burden post STEMI by CR is associated with altered medium-term outcome (death, MI, hospitalisation for heart failure/angina).
Primary hypothesis
-
IS (% LVM) will be increased in the CR versus IRA-only group.
Secondary hypotheses
-
MSI will be reduced in the CR versus IRA-only group.
-
The extent of MVO will be increased and ejection fraction will be decreased in complete versus IRA-only patients.
-
A CR strategy will reduce ischaemic burden more than an IRA-only strategy, but will not be associated with reduced MACEs at the 1-year follow-up.
-
New (post-index MI) myocardial injury (CMR imaging detected) will be increased in patients having further PCI compared with those managed with culprit-only PPCI and optimal medical therapy.
Chapter 3 Methods
Study overview
Study design
The CvLPRIT was a multicentre, open, randomised controlled clinical trial comparing inpatient IRA-only and CR for the management of MVD at the PPCI for STEMI. The trial was funded by the British Heart Foundation (BHF) in 2010, as a pilot study aiming to recruit 250 patients in four centres (Leicester, Leeds, Harefield and Southampton). The embedded CMR imaging substudy (CvLPRIT-CMR) was funded by the Efficacy and Mechanism Evaluation (EME) programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership, following a fast-track application in August 2010. The design was a pragmatic, multicentre, prospective, randomised controlled, open, clinical trial with blinded end-point (CMR imaging) analysis (PROBE design). 52 It was intended to complete recruitment, follow-up and data analysis within 2 years of study initiation.
Participants
Patients presenting to the participating centres with acute STEMI and MVD being treated by the PPCI.
Participating centres and recruitment dates
Recruitment started in May 2011 and was slower than anticipated. The study was rolled out to three additional centres with support from the NIHR Comprehensive Local Research Networks. The BHF awarded a 1-year extension in 2013 to allow the recruitment target to be extended (285 patients, ensuring) and the 12-month follow-up to be completed. The NIHR EME also awarded a 9-month time extension with a small cost extension. The participating hospitals and recruitment dates are given below.
The seven centres undertaking 24/7 PPCI in this multicentre study were:
-
Glenfield Hospital (recruited May 2011–April 2013).
-
Southampton General Hospital (recruited August 2011–April 2013).
-
Leeds General Hospital (recruited September 2011–April 2013).
-
Harefield Hospital (recruited November 2011–April 2013).
-
Kettering General Hospital (recruited July 2012–April 2013).
-
Royal Derby Hospital (recruited August 2012–April 2013).
-
Royal Bournemouth Hospital (recruited February 2013–April 2013).
Inclusion criteria
-
Suspected acute STEMI: ST-segment elevation (≥ 2 mm in two or more adjacent chest leads, ≥ 1 mm in two or more adjacent limb leads, ≥ 1 mm in leads V7–V9) or left bundle branch block (LBBB) on 12-lead electrocardiography (ECG).
-
Scheduled for the PPCI.
-
Verbal assent followed by written informed consent. 53
-
MVD: defined as IRA plus ≥ 1 non-IRA with significant disease (> 70% stenosis in one plane or > 50% in two planes). The non-IRA must be a stentable epicardial coronary artery or a major branch of > 2 mm in diameter. 53
Exclusion criteria
-
Age < 18 years.
-
Clear indication for or against CR according to operator.
-
Previous Q-wave MI.
-
Previous coronary artery bypass graft (CABG).
-
Cardiogenic shock.
-
Ventricular septal defect or moderate/severe mitral regurgitation.
-
Severe chronic kidney disease [estimated glomerular filtration rate (eGFR) < 30 ml/minute].
-
Stent thrombosis.
-
The only significant non-IRA lesion is a chronic total occlusion.
-
Standard MRI contraindications (pacemaker, implantable cardiac defibrillator, intracranial implant incompatible with magnetic field, severe claustrophobia, weight > 200 kg).
Initial assessment and assent
The coronary care unit at each centre was alerted by paramedics of incoming STEMI patients. On arrival at hospital, the CvLPRIT research team discussed the study with patients once acute STEMI of < 12 hours’ duration was confirmed on history and ECG. Prior to the PPCI, an ethically approved, short study narrative was read to the patient (see Appendix 1). Where eligible patients provided verbal agreement (assent) to enter the randomised controlled trial, this was documented in the medical records. Assent allowed delivery of key information to patients within expected time constraints during STEMI and sufficient opportunity for patients to ask questions. Verbal information is understood and retained significantly better by patients compared with written information in acute MI trials. 54–56 The assent procedure was successfully used in the Strategic Reperfusion Early after Myocardial Infarction (STREAM)57 and the Reperfusion Facilitated by Local adjunctive therapy in STEMI (ReFLO-STEMI)58 multicentre acute STEMI studies. If patients met the inclusion criteria after angiography they were asked to give further verbal assent before randomisation.
Randomisation
Patients were randomised on-table, pre PCI, via a dedicated interactive voice recognition telephone service to either in-hospital IRA-only revascularisation or CR. Randomisation was concealed to all investigators and stratified using minimisation, by anterior or non-anterior STEMI (ECG-guided), and symptom time (time to reperfusion) less than or equal to, or greater than 3 hours, as these are strong prognostic indicators post STEMI. 59 Randomisation was run through an independent company (Sealed EnvelopeTM, London, UK) and took less than 90 seconds.
Consent
Randomised patients were given patient information leaflets within 24 hours, assuming they were medically fit, and asked to provide full written informed consent to continued study participation, including the optional CMR imaging substudy. At all times, patients were informed that they were under no obligation to continue study participation.
Interventions
Infarct-related artery-only revascularisation
The PPCI to the IRA only was regarded as the standard of care and was performed in accordance with the ESC3 and American College of Cardiology Foundation (ACCF)/AHA4 guidelines. Multiple angiographic views of the left and right coronary artery systems were acquired in standard radiographic projections using digital fluoroscopic angiography systems at 15 frames per second.
PeriPCI adjuncts were administered at the operator’s discretion: dual antiplatelet loading with aspirin plus clopidogrel (Plavix®; Sanofi-aventis Ltd, UK) or prasugrel (Efient®; Eli Lilly and Company Ltd, UK) or ticagrelor (Brilique®; AstraZeneca, UK) for P2Y12 inhibition pre angiography; heparin, bivalirudin (Angiox®; Medicines Company, USA), glycoprotein IIb/IIIa inhibitors [e.g. abciximab (ReoPro®; Eli Lilly & Co Ltd, UK)], thrombus aspiration devices (e.g. Export®; Medtronic, USA), vasodilators [e.g. adenosine (Adenoscan®; Sanofi-aventis Ltd, UK)] and isosorbide dinitrate (Isoket®; UCB Pharma Ltd, Belgium) during the PPCI. The choice of stent and stent implantation technique were at the operator’s discretion but drug-eluting stent (DES) use was strongly encouraged.
Complete revascularisation
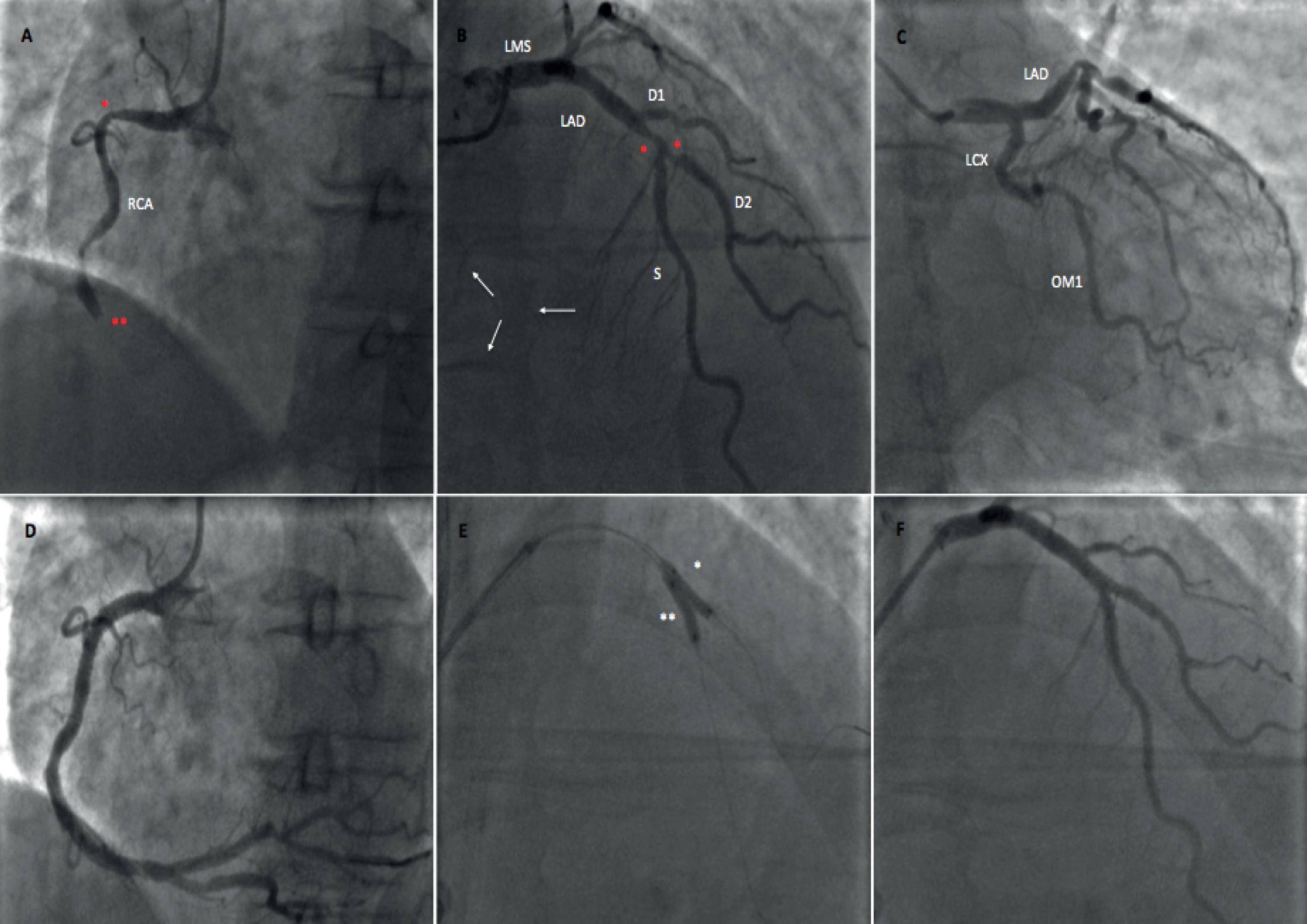
Complete revascularisation was the investigational intervention. It was recommended that revascularisation be completed during the index PPCI procedure (Figure 1). Where this was not possible (at the operator’s discretion), non-IRA PCI was performed during the index admission, within 36 hours of the PPCI and prior to CMR imaging.
FIGURE 1.
Multivessel coronary artery disease at the PPCI managed with CR. (a) RCA is IRA in this patient with inferior MI (two significant stenoses: *≈70% stenosis in proximal RCA and **complete occlusion); (b) angiogram of left coronary system, with two stenosis of > 70% (*) in the mid-LAD and ostium of the second diagonal branch of the LAD (D2) artery demonstrating non-IRA disease and confirming MVD (white arrows show collateral flow from septal branches of the LAD artery to the territory of the RCA); (c) no significant LCX disease; (d) RCA after the PPCI with both lesions successfully stented; (e) post stent dilatation (* in D2 and ** in mid-LAD); and (f) both lesions in LAD artery system successfully treated, confirming CR angiographic success. D2, second diagonal; LAD, left anterior descending artery; LCX, left circumflex artery; LMS, left main stem; OM1, obtuse marginal branch 1 of LCX; RCA, right coronary artery; S, septal branches of LAD.
All patients were treated with optimal medical treatment as per ESC and ACCF/AHA guidelines [dual antiplatelet therapy (DAPT), angiotensin-converting enzyme (ACE) inhibition, beta blockade, high-dose statin]. 3,4 Repeat coronary angiography was recommended only for (1) recurrent ischaemic symptoms with confirmation on non-invasive imaging (e.g. stress CMR imaging, SPECT) or (2) at the discretion of the local investigator following a positive non-invasive test at 6–8 weeks post PPCI.
Ethics
The study was conducted in accordance with the Fifth Declaration of Helsinki. 60 Trial protocols, patient information leaflets and consent forms were approved by the National Research Ethics Service and each site was granted site-specific approval from its NHS Research and Development department before trial commencement.
History-taking
Patients were interviewed once clinically stable post PPCI to ascertain their past medical history, cardiac risk factors and medications history. Particular attention was paid to determining the presence or absence of the following:
-
diabetes mellitus (DM)
-
hypercholesterolaemia
-
hypertension
-
prior MI or PCI
-
smoking history.
Investigations and analyses
Consenting patients were allocated an anonymised study number allowing blinded CMR imaging analysis. Investigations performed relevant to the CvLPRIT-CMR substudy are summarised in Table 3.
| Events | Order | Time point | Investigation |
|---|---|---|---|
| Inpatient | 1 | Immediately | Angiography and the PPCI |
| Inpatient | 2 | Pre PPCI | Biomarker assessment (creatine, eGFR, CK) |
| Inpatient | 3 | 90 minutes post PPCI | ECG |
| Inpatient | 4 | 12 hours post PPCI | Biomarker assessment (creatinine, eGFR, CK) |
| Inpatient | 5 | 24 hours post PPCI | Biomarker assessment (CK) |
| Inpatient | 6 | Pre-discharge | History-taking |
| Inpatient | 7 | Pre-discharge | Acute CMR scan |
| 9-month follow-up | 9 | 9 months post PPCI | Follow-up CMR scan |
| 12-month follow-up | 11 | 12 months post PPCI | History-taking/case note review |
Angiographic analysis
Pre- and post-PPCI epicardial coronary flow was assessed using TIMI scoring (Table 4). 61
| Perfusion analysed | Scoring system | Definition |
|---|---|---|
| Epicardial coronary flow | TIMI flow grade 0 | No perfusion: no antegrade flow beyond occlusion |
| Epicardial coronary flow | TIMI flow grade 1 | Penetration without perfusion: contrast passes beyond occlusion, but fails to opacify entire distal coronary bed |
| Epicardial coronary flow | TIMI flow grade 2 | Partial reperfusion: contrast passes occlusion and opacifies distal coronary bed, but rate of entry and exit of contrast slower than in unaffected vessels (non-IRAs) |
| Epicardial coronary flow | TIMI flow grade 3 | Complete reperfusion: contrast passes occlusion and opacifies distal coronary bed, and rate of entry and clearance of contrast same as in non-IRAs |
| Collateral flow to IRA territory (AAR) pre PPCI | Rentrop grade 0 | Absent visible collateral flow |
| Collateral flow to IRA territory (AAR) pre PPCI | Rentrop grade 1 | IRA side branches only filled |
| Collateral flow to IRA territory (AAR) pre PPCI | Rentrop grade 2 | Partial filling of main IRA vessel |
| Collateral flow to IRA territory (AAR) pre PPCI | Rentrop grade 3 | IRA completely filled by collaterals |
The degree of stenosis in each significant IRA and non-IRA lesion was graded visually by local investigators on a five-point scale (1, 1–49%; 2, 50–74%; 3, 75–94%; 4, 95–99%; and 5, 100%). Additionally, after CMR analysis had been completed and the database locked, core laboratory angiographic analysis was performed by a single operator (JNK) to determine (1) collateral flow to the IRA pre PPCI (graded using the Rentrop system);62 (2) the percentage diameter stenosis of lesions by two-dimensional (2D) quantitative coronary angiography (QCA) using QAngioXA version 1.0 (Medis, Leiden, the Netherlands) (Figure 2); and (3) the complexity and extent of coronary artery disease (CAD) using the validated SYNTAX score (sum of SYNTAX scores for each lesion) by two observers (JNK and SAN). 63
FIGURE 2.
Two-dimensional QCA (patient X642).
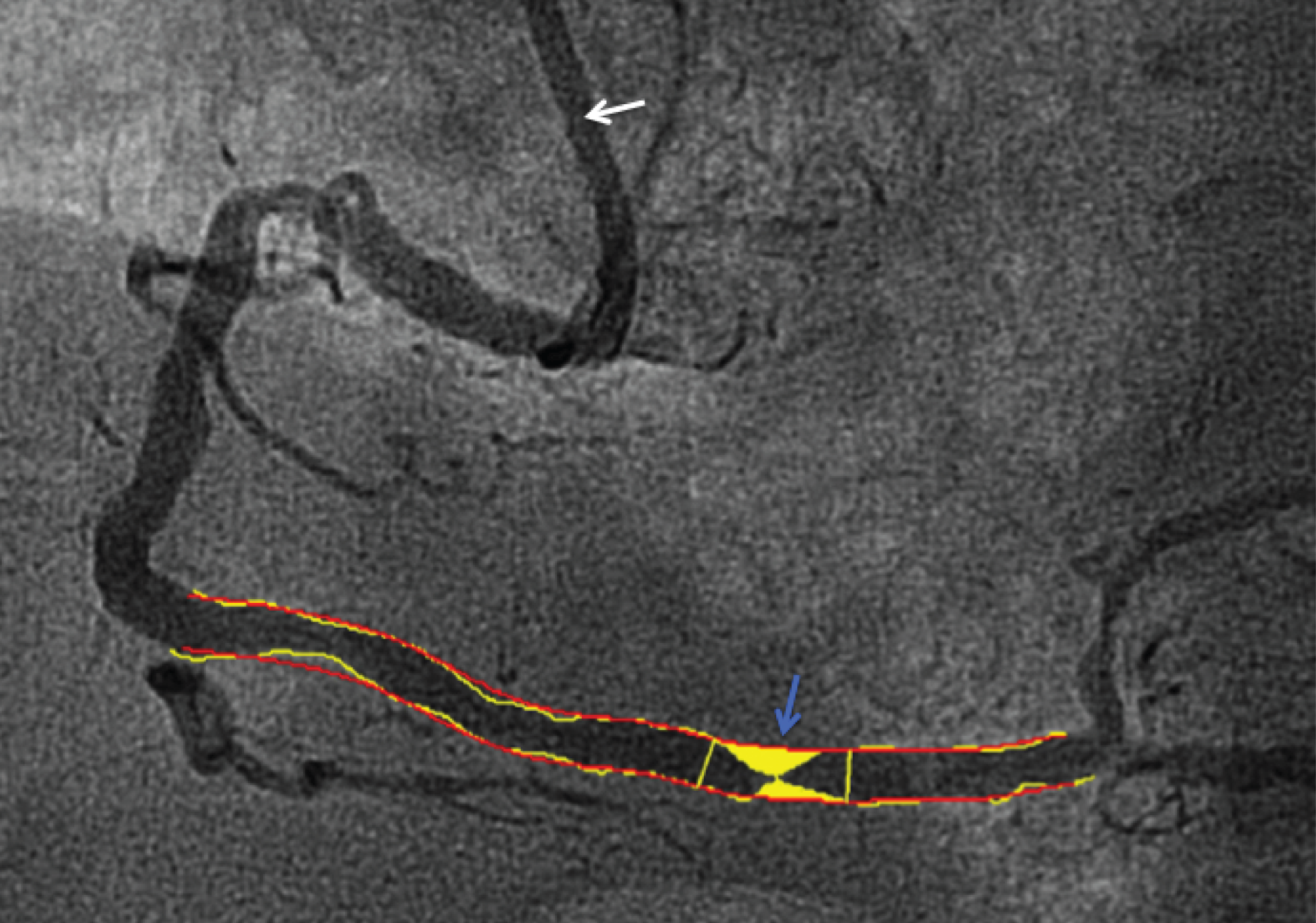
The percentage diameter stenosis of lesions was assessed by 2D QCA using QAngioXA version 1.0. The artery diameter is quantified by comparison with the reference catheter (white arrow, here 6 French, 2.0 mm). Loci proximal and distal to the lesion under assessment are manually identified. The software then automatically contours the artery and lesion. Manual adjustment of contours can be performed if needed. In Figure 2, the distal right coronary artery lesion (blue arrow) is of 80% stenosis in a segment of 2.4 mm diameter.
Blood sampling
Twenty millilitres of venous blood was collected with the patient lying semirecumbent. For the assessment of serum creatinine, eGFR and creatine kinase (CK), blood was collected in clot activator tubes (BD Diagnostics, Oxford, UK) as summarised in Table 3. These were routine clinical bloods analysed using the Clinical Pathology Accreditation Service (CPA)-accredited (United Kingdom Accreditation Service, Middlesex, UK) laboratories at each centre, which were to ISO 15189 standard (International Organisation for Standardisation, UK).
Electrocardiography
A 12-lead surface ECG was taken on arrival of the patient to hospital to confirm STEMI. This was repeated at 90 minutes post PPCI to assess the degree of ST-segment resolution, and quantified as the sum of ST-segment elevation at 60 milliseconds after the J-point in the infarct-related leads. ST-segment resolution was defined as complete (> 70%), partial (30–70%) or absent (< 30%)64 compared with the initial ECG.
Cardiovascular magnetic resonance imaging
Cardiac MRI was performed on 1.5-T scanners (Table 5) as close to 72 hours post PPCI as possible (acute CMR imaging) during the index admission, and at 9 months (follow-up CMR imaging). CMR imaging was permitted at 24–48 hours in patients due for weekend discharge, and could be delayed if necessitated by the patient’s clinical condition and was always performed after additional PCI in CR patients who had staged procedure to treat the non-IRA(s). Prior to CMR imaging, patients completed a safety questionnaire. At the 9-month CMR imaging, an additional stress questionnaire was completed to ensure suitability for adenosine and caffeine abstinence.
| Recruitment centre | Centre where CMR imaging performed | 1.5-T scanner used |
|---|---|---|
| Bournemouth | Bournemouth | Siemens Avanto (Erlangen, Germany) |
| Derby, Glenfield, Kettering | Glenfield | Siemens Avanto (Erlangen, Germany) |
| Harefield | Harefield | Siemens Avanto (Erlangen, Germany) |
| Leeds | Leeds | Philips Intera (Best, the Netherlands) |
| Southampton | Southampton | Siemens Avanto (Erlangen, Germany) |
Acute cardiac magnetic resonance imaging
The detailed protocol for the acute CMR scan is summarised in Figure 3 and explained in subsequent sections. All imaging was performed with retrospective electrocardiographic gating using dedicated cardiac receiver coils, unless atrial fibrillation or frequent ectopy was present, or for tagging images, where prospective gating was used. Parallel imaging (factor 2) was used to shorten breath-holds for all imaging, except T2W short-tau inversion recovery (T2W-STIR).
FIGURE 3.
Magnetic resonance imaging protocol for the acute CMR scan. 4/3/2C, four-, three-, two-chamber long axis; FOV, field of view; FWHM, full width, half maximum; IMH, intramyocardial haemorrhage; RV, right ventricular; SAX, short axis; TE, echo time; TR, repetition time. Reproduced under Creative Commons CC BY license from McCann GP, Khan JN, Greenwood JP, Sheraz N, Dalby M, Curzen N, et al. Complete versus lesion-only primary PCI: the randomized cardiovascular MR CuLPRIT substudy. J Am Coll Cardiol 2015;66:2713–24.

Cine imaging
After the acquisition of localising images, balanced steady-state free precession cine imaging (bSSFP) was performed in four-, two- and three-chamber long-axis views. The field-of-view was optimised to achieve in-plane spatial resolution of ≈1.1–1.7 mm × 1.3–1.9 mm. The number of segments was adjusted according to heart rate [heart rate < 50 beats per minute (b.p.m.), 17 segments; heart rate 50–70 b.p.m., 15 segments; heart rate 71–90 b.p.m.; 13 segments; heart rate > 90 b.p.m., 11 segments] at the discretion of the supervising investigator.
Intravenous contrast was administered before short-axis cine stack acquisition to minimise scans time before acquiring the LGE images (primary end point). Cine imaging was performed in contiguous short-axis slices covering the entire left ventricle and right ventricle (Figure 4). The basal short-axis slice was planned at the mitral valve annulus perpendicular to the interventricular septum to minimise partial volume at the atrioventricular boundary.
FIGURE 4.
Steady-state free precession cine imaging in a contiguous short-axis stack. (a) In end-diastole; and (b) corresponding end-systolic image. Left to right images: basal to apical, complete left and right.

Oedema (area-at-risk) imaging
The AAR was assessed using black-blood T2W-STIR imaging. T2W-STIR imaging was performed using coil signal intensity correction in four-, two- and three-chamber long-axis views and contiguous short-axis slices covering the entire left ventricle (Figure 5). Slices 10-mm thick were acquired to optimise signal-to-noise ratio. The echo train length (ETL) was adjusted with heart rate (heart rate < 50 b.p.m., ETL 40; heart rate 50–70 b.p.m., ETL 30; heart rate 71–90 b.p.m., ETL 25; heart rate > 90 b.p.m., ETL 20).
FIGURE 5.
T2-weighted short-tau inversion recovery imaging showing myocardial oedema (AAR). Myocardial oedema seen as hyperenhancement on T2W-STIR imaging in the anteroseptal segments (left anterior descending artery IRA) as indicated by *. (a) Four-chamber long-axis view; (b) two-chamber long-axis view; (c) basal LV short-axis view; (d) mid-LV short-axis view; and (e) apical LV short-axis view.

Late gadolinium enhancement imaging
Late gadolinium enhancement imaging was commenced 10 minutes after intravenous administration of 0.2 mmol/kg gadolinium diethylenetriaminepentaacetate (Gd-DTPA; Magnevist, Bayer, Germany) using a segmented inversion-recovery gradient-echo sequence with a two-beat trigger. This was preceded by a bSSFP Look–Locker inversion time (TI) scout to determine the optimal TI to null unaffected myocardium. The TI was progressively adjusted to maintain nulling of unaffected myocardium. LGE imaging was performed in four-, two- and three-chamber long-axis views and contiguous short-axis slices covering the entire left ventricle (Figure 6). T2W-STIR, cine and LGE short-axis images were acquired at identical slice positions. The number of segments was adjusted with heart rate (heart rate < 50 b.p.m., 40 segments; heart rate 50–70 b.p.m., 30 segments; heart rate 71–90 b.p.m., 25 segments; heart rate > 90 b.p.m., 20 segments).
FIGURE 6.
Late gadolinium enhancement imaging showing infarction. MI seen as hyperenhancement on LGE imaging in the anteroseptal segments as indicated by * in the oedematous territory (AAR) (see Figure 5). (a) Four-chamber long-axis view; (b) two-chamber long-axis view; (c) basal LV short-axis view, (d) mid-LV short-axis view; and (e) apical LV short-axis view.

Follow-up cardiac magnetic resonance imaging
The protocol for follow-up CMR imaging was similar to the acute scan, but with oedema (T2W-STIR) imaging omitted and assessment of reversible ischaemia included with perfusion assessment included.
Perfusion imaging
Stress perfusion imaging was performed following pharmacological vasodilator stress using intravenous adenosine infusion at 140 µg/kg/minute for 3 minutes. Heart rate and blood pressure and symptoms were closely monitored during stress at 1-minute intervals. A radiographer was present within the scanner room with the patient during stress. First-pass perfusion imaging was performed following intravenous 0.1 mmol/kg Gd-DTPA using a breath-hold, saturation-recovery gradient-echo sequence at basal, mid-ventricular and apical short-axis LV slices, planned as per myocardial tagging. Acquisition was undertaken every heart beat to optimise visual assessment of contrast wash in. Where the heart rate was > 110 b.p.m., phase resolution was reduced to 70% to increase temporal resolution. In the rare situation where heart rate was > 125 b.p.m., acquisition was undertaken every other heart beat. Rest perfusion imaging was performed 10 minutes after stress perfusion imaging using identical parameters with further administration of 0.1 mmol/kg Gd-DTPA.
Cardiac magnetic resonance imaging analysis
All quantitative CMR imaging analysis was performed offline, blinded to all patient details and randomisation, by a single operator (JNK) and supervised by a CMR imaging expert (GPM, 10 years’ experience). Image quality was graded by two observers (JNK and GPM) as summarised in Table 6.
| Sequence | Grade | 1.5-T scanner used |
|---|---|---|
| All sequences | N/A | Sequence not performed |
| 0 | Non-analysable | |
| 1 | Minor artefact in ROI that may affect analysis; however, images analysable | |
| 2 | Minimal artefact, which does not affect images analysis | |
| 3 | Good quality, no artefact | |
| Oedema | No visible oedema | No artefact however no oedema seen (no CNR between oedema and unaffected myocardium) |
Volumetric analysis
Volumetric analysis was performed using QMass® v7.1 (Medis, Leiden, the Netherlands). LV endocardial and epicardial borders were manually contoured onto contiguous short-axis cine slices at end-diastole and end-systole, excluding papillary muscles, trabeculae and epicardial surfaces. This method has superior reproducibility65 compared with inclusion of papillary muscles and trabeculae in mass assessment. This allowed calculation of LVEDV, LVESV, LV stroke volume, LVEF and LVM. Volumes and LVM were indexed for body surface area.
Oedema (area at risk) quantification
Oedema (AAR) was quantified as hyperenhancement on T2W-STIR imaging using CMR imaging40 (Circle Cardiovascular Imaging, Calgary, AB, Canada) using Otsu’s automated threshold (OAT). 66 Endocardial and epicardial borders were manually contoured on contiguous LV short-axis slices, excluding papillary muscles, trabeculae, epicardial surfaces and blood pool artefact (Figure 7).
FIGURE 7.
Exclusion of blood pool artefact from oedema quantification. Blood pool artefact (hyperenhancement) is caused as stagnant blood in regions of severe hypokinesia receives all inversion pulses in T2W-STIR imaging, and needs to be excluded from LV myocardium during endocardial contouring. (a) T2W-STIR image showing oedema (hyperenhancement) in the anteroseptal segments (*); (b) hyperenhancement on the cavity side of the endocardial contour (red) is blood pool artefact; and (c) hyperenhancement is correctly excluded from LV myocardial contours.

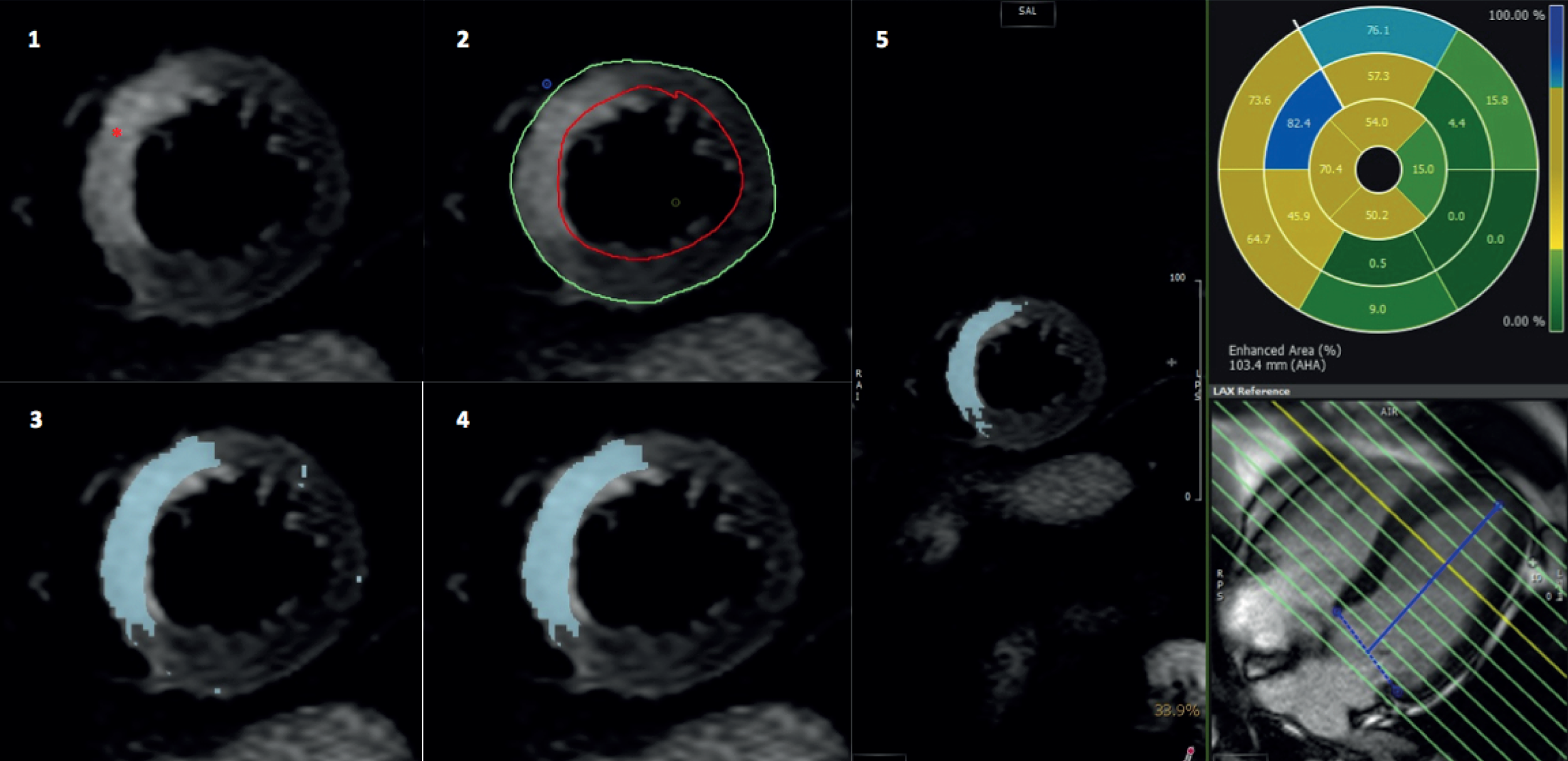
Otsu’s automated threshold automatically calculates a unique signal intensity threshold for each slice by dividing the greyscale signal intensity histogram into two groups (enhanced and normal) based on the threshold giving the least intraclass variance within each group,66 without the need for a user-defined region of interest (ROI). Oedema was calculated as a percentage area for each of the 16 AHA segments67 (Figure 8). Total AAR was expressed as percentage of LVM. The most apical T2W-STIR slice was excluded to minimise partial volume.
FIGURE 8.
Oedema quantification on T2W-STIR imaging. (1) T2W-STIR image showing oedema (*) in basal anteroseptum and anterior segments; (2) endocardial (green) and epicardial contours (red) drawn; (3) OAT automatically highlights enhanced myocardium (oedema) in blue; (4) final image after exclusion of noise artefact; and (5) percentage segmental area extent of oedema.
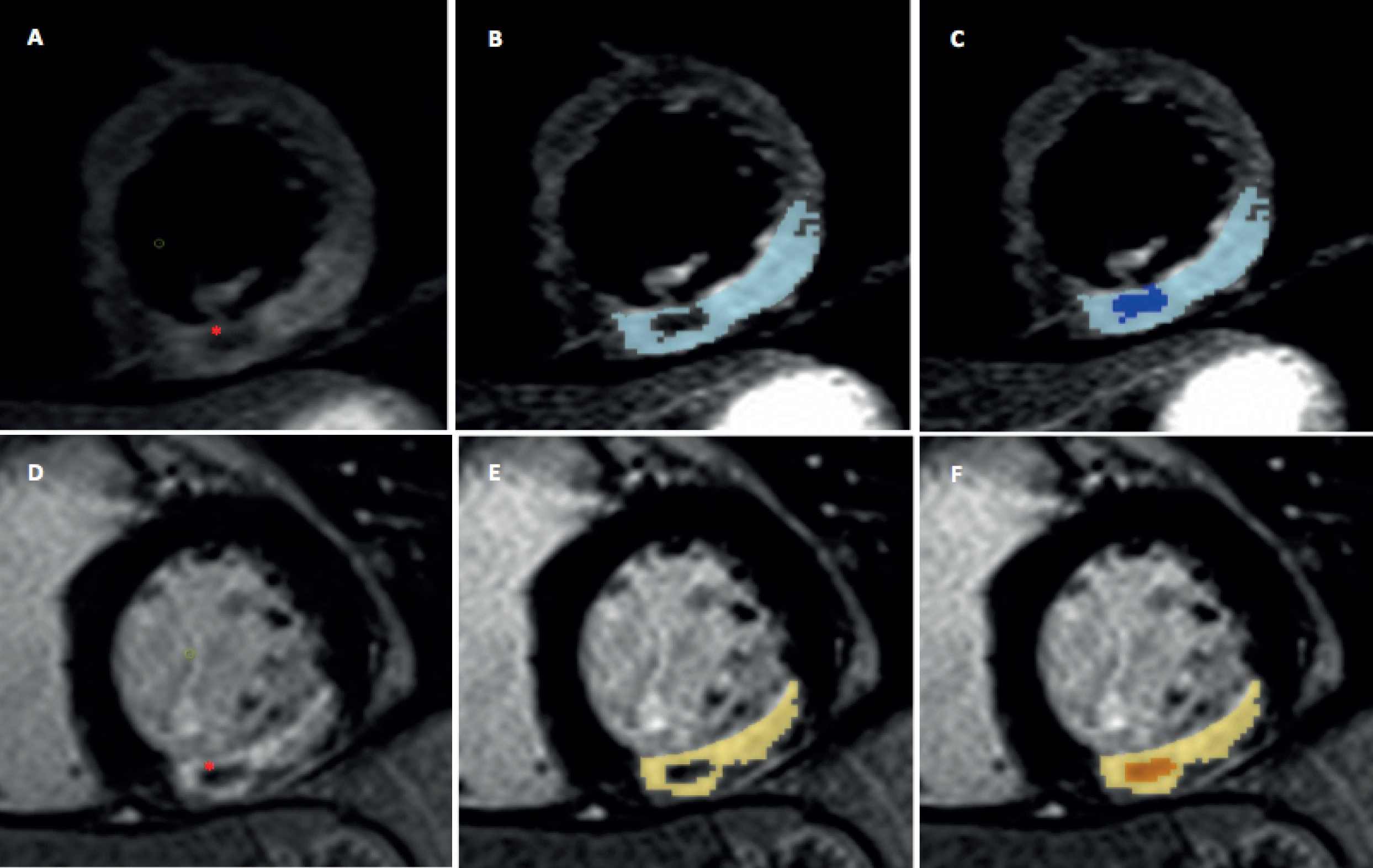
Two manual corrections were applied to AAR measurements: (1) inclusion of hypointensity within enhancement corresponding to intramyocardial haemorrhage (IMH);68 and (2) and exclusion of small, isolated enhanced regions without interslice continuity in non-IRA territories deemed noise artefact (Figure 9).
FIGURE 9.
Manual inclusion of IMH and MVO in AAR and IS. Top row: T2W-STIR images demonstrating oedema in inferolateral segments. Region of hypoenhancement within oedema corresponding to IMH (*): (a) without oedema detection; (b) with automated oedema detection; and (c) IMH manually included in total AAR and labelled as IMH. Corresponding images demonstrating MVO on LGE imaging in the same patient: (d) no detection; (e) automated detection; and (f) manually included MVO.
Late gadolinium enhancement
Infarct was defined semi-automatically on magnitude LGE images using CMR imaging. 40 Endocardial and epicardial borders were manually contoured on contiguous short-axis LV slices, excluding papillary muscles, trabeculae and epicardial surfaces and the full-width half-maximum (FWHM) technique69 applied. Here, a 2-cm2 ROI was manually drawn in the infarct core and enhancement calculated as pixels of > 50% of the automatically determined maximum signal intensity in the ROI (Figure 10). Total IS was expressed as a percentage of LVM and segmental area extent of LGE was calculated. 67 The apical LGE slice was excluded to minimise partial volume effect. Total IS was manually corrected by including hypointensity within enhancement (MVO) to total IS, and exclusion of noise artefact as per AAR quantification.
FIGURE 10.
Full-width half-maximum infarct quantification method on LGE. (1) LGE image showing infarct (*) in basal anteroseptum and anterior segments; (2) endocardial (green) and epicardial contours (red) drawn; (3) a 2-cm2 ROI (pink) drawn in infarct core; (4) FWHM enhancement with signal intensity threshold > 50% of maximum in infarct core; and (5) percentage area of each myocardial segment with infarct.

Myocardial salvage index quantification
Myocardial salvage index70 was expressed as ‘baseline MSI’ using total IS at acute CMR imaging and ‘final MSI’ using final total IS at follow-up CMR imaging:
Perfusion analysis
Perfusion images were visually, semiquantitatively assessed for perfusion defects (visible defect for five or more heartbeats) by the consensus of two experienced observers (JNK and GPM). Stress perfusion, rest perfusion and LGE images were coregistered to allow accurate assessment based on all available data. Three perfusion patterns were possible: (1) no perfusion defect – normal perfusion of myocardium during stress and rest; (2) reversible perfusion defect – perfusion defect seen only during stress perfusion, in viable, non-infarcted myocardium; and (3) matched perfusion defect – stress perfusion defect in infarcted myocardium (Figure 11). Perfusion defects and areas of infarction were graded as subendocardial (≤ 50% transmurality) or transmural (> 50% transmurality) and given a score of 1 or 2, respectively, per segment, whereas normal myocardium was scored 0. A modified summed difference score was calculated (maximum score 32),71 defined as the difference between the sum of segmental stress perfusion defects and LGE. 23 The summed difference score was expressed as percentage of the maximum possible to give an estimate of ischaemic burden (% LVM). 72 Examples of no perfusion defect, reversible perfusion defect and matched defect to IS are shown in Figure 11.
FIGURE 11.
The range of perfusion patterns possible. (a) Stress; (b) rest perfusion imaging, no perfusion defect (SDS = 0 for segments in image); (c) stress and (d) rest perfusion imaging, with reversible transmural defect in anterolateral and inferolateral segments (*SDS = 2 for both segments); and (e) and (f) matched subendocardial perfusion defect in infarcted basal inferior/inferolateral segments (*SDS = 0 for segment). SDS, summed difference score.

Study outcomes
Primary outcome
The primary outcome of the CMR imaging substudy was total IS (% LVM) on acute CMR imaging (pre discharge).
Secondary cardiac magnetic resonance imaging outcomes
The following outcomes were compared in the treatment arms at both CMR scans, except for those underlined [at acute CMR scan only (pre discharge)] or in italic (at follow-up scan only):
-
IS (% LVM) at 9 months
-
number of discrete infarcts on CMR scan
-
new MI (CMR imaging detected) at 9 months compared with acute CMR imaging
-
LV volumes, LVEF and right ventricular (RV) ejection fraction
-
IMH and MVO (% LVM)
-
AAR (% LVM)
-
baseline and final MSI
-
proportion of patients with ischaemia and global ischaemic burden (% LV)
-
visual presence of RV infarction, LV thrombus.
Clinical outcomes
The following clinical end points were recorded (time points in brackets) and definitions are detailed in Appendix 2:
-
contrast-induced nephropathy (inpatient)
-
vascular access injury requiring surgical repair (inpatient)
-
all-cause mortality (all: inpatient, 6 week, 6 month, 12 month)
-
MI (all)
-
planned or repeat revascularisation (CABG or PCI) (all)
-
heart failure admission (all)
-
transient ischaemic attack/cerebrovascular event (all)
-
major bleed (TIMI)73 (all).
The primary clinical outcome for the main CvLPRIT was first combined MACE at 12 months (all-cause mortality, MI, planned or repeat revascularisation, heart failure admission) and this was assessed for all patients in the CMR imaging substudy. Secondary outcomes included individual clinical end points at 12 months and inpatient events (safety analysis).
Data handling
Cardiac MRI data were recorded in a lockable, validated74 Research Electronic Data Capture version 5.0 (REDCap) database (Vanderbilt University, Nashville, TN, USA). No clinical data were released to the CMR imaging core laboratory until the database was complete, checked for errors and a locked copy provided to the Clinical Trials and Evaluation Unit (CTEU). The CMR imaging database was locked on 13 June 2014 (see Appendix 3). Data entry into the REDCap database was automated, using data transposition from automatically produced data files from CMR imaging analysis software. Complete data sets for 5% of randomly selected patients were manually checked and 100% of these data were correct compared with raw data files from CMR imaging software.
Patient and public involvement
As this grant application went through a fast-track application there was limited time to involve service users. However, the study was presented, before initiation, to the patient and public involvement (PPI) group of the NIHR Leicester Cardiovascular Biomedical Research Unit, and was welcomed. One patient with a history of MI and previous PCI volunteered to join the Trial Steering Committee (TSC) and regularly attended these meetings. The study progress was presented to the PPI group on two further occasions and the chief investigator spoke to regional PPI meetings on active CMR imaging studies and heart disease, including CvLPRIT-CMR imaging.
The plain English summary was forwarded to our patient representative, our PPI officer and the PPI representatives. No specific concerns or suggestions for improvement were raised. Once the results have been published the study will be presented at our local and regional PPI meetings to help disseminate the findings.
Protocol changes
Original protocol (version 1.1) is dated 30 September 2010 and the final version of the protocol is available online. 75
Protocol (version 2) dated 30 March 2011
Summary of changes
-
On page 15, section 12.1, Inclusion criteria: we have clarified that patients with LBBB with angiographic confirmation of the occlusion of the IRA can also be included as guidelines also recommend primary PCI for patients with clinical evidence of MI and LBBB.
-
On page 15, section 12.1: Guidance for classification of multivessel coronary disease. We have simplified the classification as follows:Gershlick et al. 75
For this study MVD is considered to be the IRA plus at least one non-infarct related epicardial artery (N-IRA) with at least one lesion deemed angiographically ‘significant’ (i.e. > 70% diameter stenosis observed in at least one plane). The non-IRA should be a major (> 2 mm) epicardial coronary artery or branch (> 2 mm) and be suitable for stent implantation.
In the original protocol, patients with > 50% coronary artery stenosis could be entered, which the TSC felt was not sufficiently narrow. In addition, the original protocol states that non-IRA vessels ≥ 2 mm could be treated, whereas the TSC wanted to ensure that vessels are > 2 mm (not equal to 2 mm as was stated in the original protocol).
-
On page 15, section 12.2, Exclusion criteria: we have reworded the current exclusion criterion number 8 from ‘STEMI thought to be due to occlusion of a coronary artery bypass graft’ to ‘Patients with previous coronary artery bypass graft (CABG)’. TSC members agreed that these patients often need a different revascularisation strategy from patients without prior CABG and, therefore, would be best excluded from the study. For clarity we have also removed the text ‘Clear indication for or’ from exclusion criterion 3.
-
We have also clarified that patients with stent thrombosis be excluded from the study by adding the exclusion criterion: ‘Suspected or confirmed thrombosis of a previously stented artery’.
-
On page 17, Table 3, Summary of baseline, randomisation and follow-up procedures: we have included CK blood test. CK is a routine blood test to assess myocardial damage in STEMI patients. Also, post-procedural ECG (at 90 minutes after the procedure) is also usual clinical practice in STEMI patients. Therefore, the TSC members decided to collect this useful data.
-
On page 18, section 14, In-hospital management: we have clarified current in-hospital management regarding use of stents. The TSC members recommended using DESs, as the stents of choice as there is growing evidence and acceptance that these are the ‘standard of care’ in comparison to bare-metal stents.
-
On page 19, section 14, In-hospital management: we clarified the in-hospital management of patients with renal impairment. Specifically, we recommend that patients found to have significant renal impairment after randomisation should be treated according to the best clinical practice.
-
On page 20, section 15.1, Follow-up: we have changed the ischaemic burden threshold from 15% to 20% to disclose MPS results to the clinicians in charge of the patients. The TSC members agreed that there was some evidence of improved prognosis when patients were managed more aggressively for ischaemic burden > 20% by MPS (although this evidence is generated from observational studies), but little or no evidence of improved outcomes with more aggressive treatment when the ischaemic threshold is < 20%.
Protocol (version 2.1) dated 15 December 2011
Summary of changes
-
The research team have identified that assent for some patients may not be appropriate if they already know they will not be eligible for randomised part of the trial and it would therefore be more appropriate to gain written consent to participate in the registry after the procedure. Therefore, the inclusion criterion for the registry patient was changed in the protocol from ‘provision verbal assent followed by written informed consent’ to ‘provision of written informed consent’.
Protocol (version 2.2) dated 1 February 2013
Summary of changes
-
In the last CvLPRIT TSC meeting the members agreed that CvLPRIT should recruit 300 patients to the randomised part of the trial. This allows at least 200 patients to be included in the CMR scans. We had anticipated that the CMR scans would be 80% of all patients randomised, so our initial aim was for 250 patients to provide the 200 CMR scans. The current CMR scan rate is 75%, but has been as low as 70%. Therefore, based on a CMR scans range of 70–75%, we will require between 268 and 285 patients in the main study to provide the 200 CMR scans. The recruitment of 300 is based on the worst-case scenario of 70%.
-
Three more investigator sites (Kettering, Derby and Bournemouth) have been added to the original four sites and a total of seven sites are currently recruiting.
-
There is also a clarification added to the trial MI definition in page 32 of the protocol.
Statistical methods
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 20; IBM Corporation, Armonk, NY, USA). Primary and secondary outcomes were analysed according to an intention-to-treat basis. As the primary CMR imaging outcome (IS) was expected to be right skewed, it was pre-specified to be log-transformed for all analyses in order to obtain approximately normally distributed data. Linear regression was undertaken to assess baseline characteristics associated with IS. The results for IS were adjusted for univariate predictors of IS (p < 0.1) and other covariates known to affect IS (DM and sex) using generalised linear models. As AAR was only available in 74% of patients, adjustments were made with and without this included in the model. To test whether or not the results were affected by the distribution of the variables, each analysis was rerun as a generalised estimating equation (GEE), making use of the robust standard error. The GEEs produced very similar results to our primary analyses and, hence, only the original univariate results are reported here.
For other outcome variables, normality was assessed using Q–Q plots, Kolmogorov–Smirnov and Shapiro–Wilk tests. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and non-normally distributed data as medians (25–75th quartiles). Comparison of normally distributed continuous outcomes in the study arms was performed with independent t-testing. Variables that could not be normalised despite progressive transformation attempts were analysed using non-parametric testing (Mann–Whitney U-tests) and have ‘§’ after the p-value. Categorical variables were summarised with the number and proportion of participants in each category and compared using chi-squared testing or Fisher’s exact test as appropriate.
Interobserver and intraobserver variability
Interobserver and intraobserver agreement were assessed on 10 randomly selected acute CMR scans using two-way mixed-effect intraclass correlation coefficients (ICCs) for absolute agreement76 and Bland–Altman analysis. 77 The ICC agreement was defined as excellent (ICC ≥ 0.75), good (ICC 0.60–0.74), fair (ICC 0.40–0.59) or poor (ICC < 0.40). 78 Intraobserver agreement was assessed using requantification by a single observer (JNK) after a 2-month interval and interobserver agreement was assessed by comparing observations of two independent observers (JNK and SN).
Assessment of clinical outcomes
Inpatient safety outcomes were compared in the two treatment arms using chi-squared testing. Results were presented as odds ratios (ORs) with 95% CIs. The 12-month clinical outcomes were compared in the two treatment arms in CMR imaging substudy patients. These analyses were performed for time to first event with survival analysis using the log-rank test (Cox regression) with right censoring. Results were presented as HRs with their 95% CIs. The Schoenfeld residuals output test was used to confirm the validity of the proportional hazards model. Kaplan–Meier survival curves were produced for the subgroups.
Sample size
There are no published CMR data comparing the revascularisation strategies. There are however numerous data on unselected patients undergoing the PPCI and CMR imaging IS,37,40,79 which are similar to that seen in our centre. A total of 100 patients in each arm had 81% power to detect a 4% absolute difference in IS, assuming a IS ≈20% of LVM, a SD of 10%, α = 0.05 and two-tailed given that either strategy may be associated with a larger IS. A new IS of 4% of LVM is associated with adverse prognosis in CAD patients with revascularisation-related injury. 27
Study organisation
Trial management and governance
The CvLPRIT was sponsored by University Hospitals of Leicester NHS Trust. The University of Leicester was the co-ordinating centre responsible for CMR imaging substudy management, including production of final protocols, case record forms, standard operating procedures, data handling, quality assurance and statistical reporting. Regular progress reports were provided to relevant parties. Close liaison with the Royal Brompton CTEU, that co-ordinated the main CvLPRIT, occurred throughout the study.
The main trial and CMR imaging substudy were overseen by a TSC, with the chairperson and two members being independent of the investigators. There was an independent Data and Safety Monitoring Board (DSMB) that constituted reviewed clinical outcomes during the study. An interim data review performed by the DSMB in October 2012 (16 months after recruitment started, at which point 147 patients had been recruited into the CMR imaging substudy and 36 had undergone 9-month follow-up CMR imaging) was satisfied with progress to date and for the trial to continue (see Appendix 4).
This CMR imaging substudy was funded by the MRC through the EME programmed (project number 09/150/28) and managed by the NIHR on behalf of the MRC–NIHR partnership. The main trial was funded by the BHF.
Chapter 4 Results
The main Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial
The main trial screened 850 patients presenting with STEMI, of whom 296 were randomised. The main results of the trial were presented at the ESC Annual congress in Barcelona, Spain, in August 2014 and were published in the Journal of the American College of Cardiology. 80 Patient groups were well matched for baseline clinical characteristics. The primary end point (MACEs) occurred in 10.0% of the CR group, compared with 21.2% in the IRA-only revascularisation group (HR 0.45, 95% CI 0.24 to 0.84; p = 0.009). A trend towards benefit was seen early following CR (p = 0.055 at 30 days). Although there was no significant reduction in death or MI, a non-significant reduction in all primary end-point components was seen. There was no reduction in ischaemic burden on MPS or in the safety end points of major bleeding, contrast-induced nephropathy or stroke between the groups. 80
Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial cardiac magnetic resonance substudy
The proportion of patients randomised and completing study aspects are shown in Figure 12. A total of 91% (n = 269) of the 296 randomised patients consented to ongoing participation in CvLPRIT, of whom 76% (n = 205) entered the CMR imaging substudy. LGE images from one patient were unanalysable and one patient did not complete the acute CMR scan, resulting in 203 acute CMR scans analysable for the primary CMR imaging outcome. The recruitment target of 200 was exceeded, as four patients were recruited on the final day.
FIGURE 12.
Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial cardiac magnetic resonance substudy recruitment: CONSORT (Consolidated Standards of Reporting Trials) diagram illustrating recruitment into main CvLPRIT and CMR imaging substudy. Reproduced under Creative Commons CC BY license from McCann GP, Khan JN, Greenwood JP, Sheraz N, Dalby M, Curzen N, et al. Complete versus lesion-only primary PCI: the randomized cardiovascular MR CuLPRIT substudy. J Am Coll Cardiol 2015;66:2713–24.

The reasons for non-participation in the CMR imaging substudy and drop-out from the second CMR scan are shown in Table 7. A total of 81% (164/203) of eligible patients had follow-up CMR scans, as per Figure 12. Those who did not have a second CMR scan had similar baseline characteristics to those who completed both scans.
| Reason | Number of participants |
|---|---|
| Reasons for patients consenting to enter CvLPRIT but not entering the CMR imaging substudy | |
| Patient declined consent to enter the CMR imaging substudy | 9 |
| Claustrophobia | 14 |
| Renal failure | 2 |
| CMR imaging contraindicated | 4 |
| Too unwell for CMR imaging/death | 7 |
| After CMR imaging substudy | 13 |
| No CMR imaging available at centre at time of consent into CvLPRIT | 10 |
| Other | 2 |
| Repatriated to district general hospital | 3 |
| Total | 64 |
| Reasons for patients in the CMR imaging substudy but not returning for follow-up CMR scan | |
| Patient withdrawn from CvLPRIT | 3 |
| Patient declined follow-up CMR scan | 26 |
| Death | 3 |
| Implantable cardioverter defibrillator | 1 |
| Other severe illness | 1 |
| Follow-up CMR scan due after end of CMR imaging substudy period | 3 |
| Claustrophobia | 2 |
| Total | 39 |
The CMR imaging substudy completed recruitment 1 month before the main trial and 13 patients randomised were not approached to participate in the CMR imaging substudy.
Recruitment in the main trial and substudy at each centre is shown in Table 8.
| Centre | Number of patients randomised (% of total) | Number of patients in CMR imaging substudy (%) |
|---|---|---|
| Glenfield | 99 (33.4) | 78 (78.8) |
| Southampton | 35 (11.8) | 26 (77.1)a |
| Leeds | 57 (19.3) | 32 (56.1) |
| Harefield | 38 (12.8) | 26 (68.4) |
| Kettering | 32 (10.8) | 26 (81.3) |
| Derby | 20 (6.8) | 12 (60.0) |
| Bournemouth | 15 (5.1) | 3 (26.7)b |
| Total | 296 | 203 diagnostic |
Baseline characteristics of the cardiac magnetic resonance substudy cohort
The CMR imaging substudy cohort closely represented the overall CvLPRIT group, with similar baseline characteristics, comorbidities and important prognostic predictors including symptom to the PPCI time [time to revascularisation (TTR)], infarct location and Killip class (Table 9).
| Variable | Overall CvLPRIT group (n = 296) | CMR imaging substudy cohort (n = 203) | p-value | 95% CI of difference |
|---|---|---|---|---|
| Age (years), mean (SD) | 64.9 ± 11.6 | 63.6 ± 11.0 | 0.21 | –0.7 to 3.3 |
| Male sex, n/N (%) | 240/296 (81.1) | 172/205 (83.9) | 0.42 | N/Aa |
| BME n/N (%) | 33/293 (11.3) | 22/200 (11.0) | 0.93 | N/Aa |
| BMI (kg/m2), median (IQR) | 27.3 (24.4–30.2) | 27.5 (24.7–30.1) | 0.62 | –0.02 to 0.01b |
| SBP (mmHg), mean (SD) | 137.6 (27.1) | 137.5 (27.7) | 0.96 | –4.8 to 5.1 |
| Hypertension, n/N (%) | 105/287 (36.6) | 73/203 (36.0) | 0.89 | N/Aa |
| Hypercholesterolaemia, n/N (%) | 75/287 (26.1) | 56/203 (27.6) | 0.72 | N/Aa |
| DM, n/N (%) | 39/287 (13.6) | 28/203 (13.8) | 0.95 | N/Aa |
| Current smoker, n/N (%) | 87/285 (30.5) | 66/204 (32.4) | 0.67 | N/Aa |
| Previous MI, n/N (%) | 12/287 (4.2) | 8/203 (3.9) | 0.90 | N/Aa |
| Previous PCI, n/N (%) | 9/287 (3.1) | 7/203 (3.4) | 0.85 | N/Aa |
| Symptom PCI time (TTR, minutes), median (IQR) | 184 (131–304) | 177 (130–292) | 0.49 | –0.03 to 0.06b |
| Peak CK (IU/l), mean (IQR) | 1010 (423.3–1740) | 997 (429.8–1740) | 0.98 | –0.09 to 0.08b |
| Anterior infarct, n/N (%) | 106/296 (35.8) | 72/203 (35.5) | 0.94 | N/Aa |
| Killip class II or III on arrival, n/N (%) | 24/286 (8.4) | 16/203 (7.9) | 0.84 | N/Aa |
Anthropometrics and demographics
Baseline characteristics and comorbidities were closely matched in the IRA and CR treatment arms of the CMR imaging substudy cohort (Table 10). There were slightly more males in the CR arm but the difference was not significant (CR 88.8% vs. IRA-only 79.0%; p = 0.06). The proportion of anterior infarcts in each arm was closely matched.
| Variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Anthropometrics | ||||
| Age (years), mean (SD) | 64.1 ± 10.8 | 63.1 ± 11.3 | 0.53 | –0.9 to 6.8 |
| Male sex, n/N (%) | 83/105 (79.0) | 87/98 (88.8) | 0.06 | N/Aa |
| BME, n/N (%) | 9/103 (8.7) | 13/97 (13.4) | 0.29 | N/Aa |
| BMI (kg/m2), median (IQR) | 27.5 (24.7–30.6) | 27.5 (24.6–29.7) | 0.36 | 0.02 to 0.01b |
| SBP (mmHg), mean (SD) | 140.0 ± 28.0 | 134.7 ± 27.3 | 0.18 | –8.4 to 11.3 |
| Anterior infarct, n/N (%) | 37/105 (37.2) | 35/98 (35.7) | 0.94 | N/Aa |
| Killip class II or III on arrival, n/N (%) | 10/105 (9.5) | 6/98 (6.1) | 0.37 | N/Aa |
| Biochemical | ||||
| eGFR (ml/minute/1.73 m2), mean (SD) | 93.49 (30.7) | 98.2 (34.3) | 0.36 | –15.8 to 7.4 |
| Peak CK (IU/l), median (IQR) | 1057 (614–1834) | 1025 (628–1660) | 0.37 | –0.16 to 0.14b |
| Past medical history | ||||
| Hypertension, n/N (%) | 37/105 (35.2) | 36/98 (36.7) | 0.82 | N/Aa |
| Hypercholesterolaemia, n/N (%) | 28/105 (26.7) | 28/98 (28.6) | 0.76 | N/Aa |
| DM, n/N (%) | 13/105 (12.4) | 15/98 (15.3) | 0.55 | N/Aa |
| Current smoker, n/N (%) | 28/105 (28.0) | 36/98 (36.7) | 0.12 | N/Aa |
| Previous MI, n/N (%) | 4/105 (3.8) | 4/98 (4.1) | 0.92 | N/Aa |
| Previous PCI, n/N (%) | 3/105 (2.9) | 4/98 (4.1) | 0.63 | N/Aa |
Antiplatelet and discharge medication
Discharge medications were similar in the treatment arms. All patients received DAPT. Two-thirds of patients in each arm received newer DAPT agents, prasugrel or ticagrelor (Table 11).
| Discharge medications | Treatment arm | p-value | |
|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | ||
| Aspirin, n/N (%) | 105/105 (100) | 97/98 (99.0) | 0.30 |
| Dual antiplatelet agent, n/N (%) | 105/105 (100) | 98/98 (100) | 1.00 |
| Clopidogrel | 36/105 (34.3) | 34/98 (34.7) | 0.95 |
| Prasugrel | 53/104 (51.0) | 49/98 (50.0) | 0.89 |
| Ticagrelor | 16/105 (14.3) | 15/98 (15.3) | 0.91 |
| Warfarin, n/N (%) | 0/105 (0.0) | 2/98 (2.0) | 0.14 |
| Beta-blocker, n/N (%) | 97/105 (92.4) | 93/98 (94.9) | 0.46 |
| ACE or ARB2 inhibitor, n/N (%) | 101/105 (96.2) | 95/98 (96.9) | 0.77 |
| Lipid-lowering agent, n/N (%) | 104/105 (99.1) | 98/98 (100) | 0.33 |
| Loop diuretic, n/N (%) | 13/105 (12.4) | 9/98 (9.2) | 0.46 |
| Aldosterone inhibitor, n/N (%) | 5/105 (4.8) | 5/98 (5.1) | 0.91 |
| Oral diabetic drug, n/N (%) | 8/105 (7.6) | 8/98 (8.2) | 0.89 |
| Insulin, n/N (%) | 7/105 (6.7) | 4/98 (4.1) | 0.42 |
Angiographic markers
Radial artery access was the preferred technique in both treatment arms. Coronary disease complexity, severity and IRA at baseline angiography were similar in the groups. There was a greater proportion of CR patients with well-collateralised IRA territory, defined as Rentrop grade 2 or 3 (Table 12).
| Variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Radial access, n/N (%) | 82/105 (78.1) | 81/97 (83.5) | 0.33 | N/Aa |
| Visible thrombus, n/N (%) | 71/105 (67.6) | 60/97 (61.9) | 0.39 | N/Aa |
| Vessels > 75% stenosis (n), mean (SD) | 1.5 (0.6) | 1.5 (0.6) | 0.39 | b |
| Total lesions > 75% stenosis (n), mean (SD) | 1.6 (0.7) | 1.6 (0.7) | 0.58 | b |
| Non-IRA lesions > 75% stenosis (n), mean (SD) | 0.6 (0.7) | 0.6 (0.7) | 0.58 | b |
| Vessels > 70% QCA stenosis (n), mean (SD) | 1.8 (0.6) | 1.7 (0.6) | 0.82 | b |
| Total lesions > 70% QCA stenosis (n), mean (SD) | 1.9 (0.8) | 1.9 (0.8) | 0.95 | b |
| Non-IRA lesions > 70% QCA stenosis (n), mean (SD) | 0.9 (0.8) | 0.9 (0.8) | 0.86 | b |
| Left anterior descending IRA, n/N (%) | 38/105 (36.2) | 34/98 (34.7) | 0.82 | N/Aa |
| Left circumflex artery IRA, n/N (%) | 18/105 (17.1) | 20/98 (20.4) | 0.55 | N/Aa |
| Right coronary artery IRA, n/N (%) | 48/105 (45.7) | 44/98 (44.9) | 0.91 | N/Aa |
| Rentrop grade, median (IQR) | 0 (0–1) | 0 (0–1) | 0.14 | –0.11 to 0.02c |
| Rentrop grade 2 or 3 pre PCI, n/N (%) | 3/105 (2.9) | 10/98 (10.2) | 0.033 | N/Aa |
| TIMI grade pre PCI, median (IQR) | 0 (0–1) | 0 (0–1) | 0.56 | –0.15 to 0.08c |
| TIMI pre-PCI grades 0–2, n/N (%) | 97/105 (92.4) | 89/98 (90.8) | 0.69 | N/Aa |
| SYNTAX score (total), median (IQR) | 18 (14–22) | 17.3 (13–23.5) | 0.81 | –2.3 to 1.8 |
| SYNTAX score (IRA), median (IQR) | 9 (6–14.5) | 8 (6–11.5) | 0.75 | –1.3 to 1.8 |
| SYNTAX score (non-IRAs), median (IQR) | 7 (3–11) | 7 (4–10) | 0.51 | –0.12 to 0.06 |
Percutaneous coronary intervention details
In the main trial, 42 out of the 139 CR patients who received the allocated treatment had a staged PCI to the non-IRA and, in the CMR imaging substudy, 30 non-IRA PCI patients were staged. Total screening time, contrast dose, procedure length and number of implanted stents were greater in CR patients. The majority of patients in both arms received DESs, although this proportion was slightly higher in CR patients (see Table 13). Symptom to balloon times (TTR), peri-PCI adjunct usage and post-PPCI CK were similar in both arms (Table 13). No reflow in the IRA patients was more common in the CR group.
| Variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Glycoprotein inhibitor use, n/N (%) | 36/104 (34.6) | 34/97 (35.1) | 0.95 | N/Aa |
| Bivalirudin use, n/N (%) | 43/94 (45.7) | 52/92 (56.5) | 0.14 | N/Aa |
| Thrombectomy catheter use, n/N (%) | 79/105 (75.2) | 67/97 (69.1) | 0.33 | N/Aa |
| Contrast dose (ml), median (IQR) | 190 (150–230) | 300 (220–400) | < 0.001 | –0.26 to –0.16b |
| Screening time (minutes), median (IQR) | 9 (7–13) | 17 (12–23) | < 0.001 | –0.35 to –0.22b |
| Procedure length (minutes), median (IQR) | 42 (30–55) | 66 (43–84) | < 0.001 | –0.25 to –0.13b |
| Symptom PCI time (TTR, minutes), median (IQR) | 171 (127–268) | 192 (131–302) | 0.20 | –0.13 to –0.12 |
| TIMI grade post PCI, median (IQR) | 3 (3–3) | 3 (3–3) | 0.31 | –0.5 to –0.2 |
| TIMI post PCI, grade 3, n/N (%) | 100/105 (95.2) | 89/98 (90.8) | 0.21 | N/A |
| Successful IRA PCI, n/N (%) | 101/105 (96.2) | 90/98 (91.8) | 0.19 | N/A |
| IRA no reflow, n/N (%) | 2/105 (1.9) | 8/98 (8.2) | 0.039 | N/A |
| IRA PCI complication, n/N (%) | 14/105 (13.3) | 8/98 (8.2) | 0.24 | N/A |
| DES use, n/N (%) | 96/105 (91.4) | 97/98 (99) | 0.013 | N/A |
| Total number of stents (n), median (IQR) | 1 (1–2) | 3 (2–4) | < 0.001 | c |
| Peak CK (IU/l), median (IQR) | 1057 (614–1834) | 1025 (628–1660) | 0.37 | –0.17 to 0.06b |
| Time at peak CK (hours), median (IQR) | 12 (12–12) | 12 (12–12) | 0.98 | –0.05 to 0.52b |
Acute cardiac magnetic resonance
Cardiac magnetic resonance image quality
Acute CMR imaging was undertaken at approximately 3 days post PPCI in both treatment arms. One hundred per cent of cine and LGE images in the final 203 CMR imaging substudy subjects were of very good quality (Table 14). Fifty-two patients’ (26%) short-tau inversion recovery (STIR) data sets were non-diagnostic [no artefact but no oedema discernible (n = 33); STIR not performed because of arrhythmia or suboptimal breath-holding (n = 14); and severe artefact (n = 5)]. Image quality was similar in both treatment arms.
| Variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Time to acute CMR scan (days), median (IQR) | 2.8 (1.8–3.4) | 3.0 (2.0–4.3) | 0.13 | –0.13 to 0.02 |
| Cine imaging quality score, mean (SD) | 2.4 (0.8) | 2.5 ± 0.7 | 0.31 | –0.30 to 0.10 |
| Oedema imaging diagnostic, n/N (%) | 76/105 (72.4) | 75/98 (76.5) | 0.50 | N/Aa |
| Oedema image quality score, mean (SD) | 1.3 (0.9) | 1.4 ± 0.9 | 0.53 | –0.34 to 0.17 |
| LGE image quality score, mean (SD) | 1.9 (0.7) | 2.0 ± 0.6 | 0.13 | –0.33 to 0.04 |
Observer variability
Intra- and interobserver variability were excellent for CMR imaging volumetric and tissue characterisation (AAR and IS). Results are displayed in Table 15.
| CMR imaging variable | Intraobserver agreement | Interobserver agreement | ||||
|---|---|---|---|---|---|---|
| ICC | Mean bias | ± 95%, LoA | ICC | Mean bias | ± 95%, LoA | |
| Volumetric analysis | ||||||
| LVM index | 0.986 | –0.3 | +6.3, –6.8 | 0.995 | + 0.5 | +4.4, –3.5 |
| LV end-diastolic volume index | 0.996 | +1.2 | +6.4, –4.0 | 0.995 | + 1.3 | + 7.9, –5.3 |
| LVESV index | 0.988 | –0.9 | +4.6, –6.4 | 0.996 | + 0.8 | + 6.4, –4.8 |
| LVEF (%) | 0.976 | +1.0 | +3.8, –1.8 | 0.996 | –0.1 | + 1.4, –1.6 |
| Tissue characterisation | ||||||
| IS | 0.988 | +0.2 | +1.5, –1.1 | 0.990 | –0.5 | + 1.7, –2.7 |
| AAR | 0.948 | +2.8 | +7.3, –1.7 | 0.908 | +3.4 | + 9.1, –2.4 |
Cardiac magnetic resonance outcomes
Predictors of infarct size
Univariate predictors of IS are shown in Table 16. The primary outcome was adjusted for variables with p < 0.1 (age, anterior MI, TIMI grade prior to the PPCI, TTR, male sex, SYNTAX score plus DM and sex). Given that anterior infarct location (ECG based) is closely related to left anterior descending artery IRA, only anterior infarct location was used.
| Variable | Baseline IS, r (r2) | p-value |
|---|---|---|
| Anthropometrics | ||
| Age | 0.15 (0.22) | 0.04 |
| Male sex | –0.02 (0.00) | 0.98 |
| SBP | –0.02 (0.00) | 0.79 |
| Anterior infarct | 0.27 (0.07) | < 0.001 |
| Past medical history | ||
| Treated hypertension | –0.06 (< 0.01) | 0.42 |
| Treated hypercholesterolaemia | –0.09 (< 0.01) | 0.22 |
| DM | 0.06 (< 0.01) | 0.41 |
| Coronary angiography | ||
| Killip class II or III on arrival | 0.11 (0.13) | 0.12 |
| Visible thrombus | < 0.01 (< 0.01) | 0.90 |
| Symptom to PCI time (TTR, minute) | 0.11 (0.01) | 0.14 |
| LAD IRA | 0.21 (0.05) | 0.003 |
| Rentrop grade | 0.05 (< 0.01) | 0.71 |
| Rentrop grade 2 or 3 pre PCI | 0.04 (< 0.01) | 0.64 |
| TIMI grade pre PCI | –0.27 (0.07) | 0.06 |
| SYNTAX score (total) | 0.31 (0.10) | < 0.001 |
| Number of affected vessels > 75% (CRF) | 0.10 (0.01) | 0.15 |
| Number of total lesions > 75% (CRF) | 0.06 (< 0.01) | 0.39 |
| Number of non-IRA lesions > 75% (CRF) | 0.06 (< 0.01) | 0.39 |
| Number of affected vessels > 75% (QCA) | 0.16 (0.02) | 0.03 |
| Number of total lesions > 75% (QCA) | 0.12 (0.02) | 0.09 |
| Number of non-IRA lesions > 75% (QCA) | 0.12 (0.02) | 0.09 |
| Baseline CMR imaging parameters (CMR1) | ||
| AAR (% LVM) | 0.54 (0.29) | < 0.001 |
Myocardial and microvascular injury and salvage
There was no difference in the primary CMR imaging outcome of median IS {IRA 13.5% [interquartile range (IQR) 6.2–21.9%] vs. CR 12.6% (IQR 7.2–22.6%); 95% CI –4.09% to 31.17%; p = 0.57}. Adjustment for important covariates including AAR (p = 0.347) or without AAR included (p = 0.501) did not significantly change the results. There was also no difference in IRA IS or number of transmurally infarcted segments in the treatment arms (Table 17). LGE was absent in 13 patients, of whom eight were confirmed aborted infarcts (oedema present but no LGE), with a trend towards fewer aborted infarcts (p = 0.06) with CR. There was a significantly higher prevalence of multiple infarcts and multiple acute infarcts with CR.
| Tissue characterisation variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Infarct present on LGE, n/N (%) | 95/105 (90.5) | 95/98 (96.9) | 0.06 | N/Aa |
| MVO present, n/N (%) | 54/105 (51.4) | 57/98 (58.2) | 0.34 | N/Aa |
| IMH present in diagnostic STIR sets, n/N (%) | 17/77 (22.1) | 22/75 (29.3) | 0.31 | N/Aa |
| Total IS (% LVM)b | ||||
| Median (IQR) | 13.5 (6.2–21.9) | 12.6 (7.2–22.6) | 0.57 | –4.09 to 31.17 |
| Mean (SD) | 15.9 (13.2) | 16.3 (13.0) | ||
| Patients with > 1 infarct, n/N (%) | 11/105 (10.5) | 22/98 (22.4) | 0.02 | N/Aa |
| Patients > 1 acute infarct, n/N (%) | 5/105 (4.8) | 17/98 (17.1) | 0.004 | N/Aa |
| IRA IS (main acute infarct, % LVM) | ||||
| Median (IQR) | 12.2 (6.2–21.2) | 12.1 (7.0–21.4) | 0.68 | –0.16 to 0.10c |
| Mean (SD)b | 15.3 (13.2) | 15.2 (12.1) | ||
| Non-IRA IS (acute non-IRA infarcts, % LVM) | ||||
| Median (IQR) | 2.1 (0.81–4.5) | 2.5 (0.54–4.5) | 0.004 | 3940 to 21229c |
| Mean (SD)a | 2.5 (1.9) | 3.2 (3.3) | ||
| Acute non-IRA IS > 4% LVM, n/N (%) | 2/105 (1.9) | 6/98 (6.1) | 0.12 | N/Aa |
| AAR (% LVM), mean (SD) | 36.0 (12.9) | 32.2 (11.8) | 0.06 | –0.12 to 7.9 |
| MVO (% LVM),d mean (SD) | 0.08 (0.00–1.05) | 0.19 (0.00–2.00) | 0.63 | 0.58 to 0.67 |
| IMH (% LVM), median (IQR) | 0.00 (0.0–0.20) | 0.00 (0.0–0.34) | 0.96 | 0.50 to 0.54 |
| Acute MSI (%), median (IQR) | 60.5 (40.6–81.9) | 58.5 (32.8–74.9) | 0.14 | –2.2 to 16.3 |
| Final MSI (%), median (IQR) | 79.4 (71.6–93.3) | 82.1 (63.0–90.3) | 0.20 | –3.1 to 15.2 |
| Patients with > 1 infarct, n/N (%) | 11/105 (10.5) | 22/98 (22.4) | 0.02 | N/Aa |
| Patients > 1 acute infarct, n/N (%) | 5/105 (4.8) | 17/98 (17.3) | 0.004 | N/Aa |
| Number of infarcts per patient, mean (SD) | 1.02 (0.4) | 1.22 (0.5) | < 0.001 | –0.0752 to –0.0753c |
| Number of acute infarcts per patient, mean (SD) | 1.05 (0.2) | 1.19 (0.4) | < 0.001 | –0.0687 to –0.0693c |
| RV infarction, n/N (%) | 4/105 (3.8) | 7/98 (7.1) | 0.29 | N/A |
| LV thrombus, n/N (%) | 3/105 (2.9) | 2/98 (2.0) | 0.71 | N/A |
There was a trend towards a higher prevalence of acute non-IRA infarcts > 4% LVM with CR. Acute non-IRA infarcts correlated with non-IRA PCI territories in 15 out of 17 CR patients. There was a non-significant trend towards smaller AAR with CR, but no difference in the MSI. Microvascular and RV injury, and LV thrombus were similarly prevalent in both arms.
Ventricular volumes and function
Left ventricular volumes, mass and systolic function (LVEF), were similar in the treatment arms (Table 18). LV function was similarly mildly impaired on LVEF.
| Volumetric variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Number of dysfunctional segments, median (IQR) | 5.0 (3–7) | 5.0 (3–7) | 0.64 | –0.64 to 1.1 |
| Wall motion score, median (IQR) | 23.0 (19–26) | 22.0 (19–26) | 0.71 | –1.2 to 1.8 |
| LVMI (g/m2), median (IQR) | 52.2 (44.7–59.2) | 52.3 (46.8–62.0) | 0.33 | –0.39 to 0.13a |
| LVEDVI (ml/m2), median (IQR) | 90.7 (80.4–102.0) | 89.7 (80.7–101.8) | 0.64 | –0.19 to 0.03a |
| LVESVI (ml/m2), median (IQR) | 49.8 (39.7–62.1) | 47.0 (38.0–58.4) | 0.56 | –0.03 to 0.05a |
| LVEF (%), mean (SD) | 45.1 (9.5) | 45.9 (9.9) | 0.60 | –3.4 to 2.0 |
Follow-up cardiac magnetic resonance imaging
Cardiac magnetic resonance image quality
Follow-up CMR imaging was undertaken approximately 9.4 months post PPCI in both treatment arms and all scans were analysable. Image quality for all sequences was very good (Table 19). Three patients were unable to undertake adenosine stress perfusion because of airways disease (two in the IRA-only group and one in the CR group). Perfusion imaging was unanalysable in two patients because of severe dark-rim artefact (one in the IRA-only group and one in the CR group).
| Variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 80) | CR (n = 84) | |||
| Time to follow-up CMR scan (months), median (IQR) | 9.3 (8.9–9.9) | 9.4 (9.0–10) | 0.20 | –0.51 to 0.11 |
| Cine imaging quality score, mean (SD) | 2.8 (0.6) | 2.7 (0.6) | 0.58 | –0.12 to 0.22 |
| LGE image quality score, mean (SD) | 2.2 (0.7) | 2.3 (0.7) | 0.74 | –0.25 to 0.18 |
| Stress perfusion diagnostic, n/N (%) | 76/79 (96.2) | 82/84 (97.6) | 0.60 | N/Aa |
| Stress perfusion quality score, mean (SD) | 2.4 (0.7) | 2.4 (0.8) | 0.70 | –0.29 to 0.19 |
| Rest perfusion diagnostic, n/N (%) | 76/79 (96.2) | 82/84 (97.6) | 0.60 | N/Aa |
| Rest perfusion quality score, mean (SD) | 2.5 (0.6) | 2.5 (0.7) | 0.82 | –0.23 to 0.18 |
Cardiac magnetic resonance imaging outcomes
Ventricular volumes and function
Left ventricular volumes, mass and systolic function (LVEF) were similar in the groups at the follow-up CMR scan (Table 20). LV function was mildly impaired in both treatment groups.
| Volumetric variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 80) | CR (n = 84) | |||
| LVMI (g/m2), median (IQR) | 43.4 (38.0–49.3) | 47.4 (40–52.6) | 0.33 | –0.04 to 0.14a |
| LVEDVI (ml/m2), median (IQR) | 95.0 (82.7–107) | 93.3 (82.2–110) | 0.63 | –0.03 to 0.02a |
| LVESVI (ml/m2), median (IQR) | 43.6 (34.8–57.9) | 45.1 (37.8–58) | 0.33 | –0.04 to 0.14a |
| LVEF (%), mean (SD) | 50.8 (8.7) | 49.7 (9.4) | 0.42 | –1.6 to 3.9 |
Myocardial injury
As per acute CMR imaging, a greater proportion of CR patients had LGE and multiple infarcts. Total IS was significantly different in the treatment arms (Table 21). There was a greater prevalence of residual RV infarction in the CR group.
| Tissue characterisation variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 80) | CR (n = 84) | |||
| Infarct present on LGE, n/N (%) | 71/80 (88.8) | 82/84 (97.6) | 0.023 | N/Aa |
| IS (% LVM), median (IQR) | 7.6 (3.2–15.1) | 7.3 (3.0–14.4) | 0.41 | –0.08 to 0.20b |
| Transmural LGE area extent > 50% (segments), median (IQR) | 0 (0–1) | 0 (0–1) | 0.96 | c |
| Patients with > 1 infarct, n/N (%) | 9/80 (11.2) | 20/84 (23.8) | 0.035 | N/Aa |
| Non-IRA IS (% LVM) in patients with > 1 infarct, median (IQR) | 4.1 (2.3–8.1) | 4.2 (1.9–6.1) | 0.92 | –0.48 to 0.54b |
| Number of infarcts per patient, mean (SD) | 1.03 (0.5) | 1.24 (0.5) | < 0.001 | –0.780 to –0.773b |
| RV infarction, n/N (%) | 0/80 (0.0) | 5/84 (6.0) | 0.027 | N/Aa |
| LV thrombus, n/N (%) | 1/80 (1.2) | 1/84 (1.2) | 0.97 | N/Aa |
Perfusion analysis
One hundred and fifty-nine patients underwent adenosine and rest myocardial perfusion. Results are shown in Table 22. Stress-induced perfusion defects were present in 21% of patients in each treatment arm, with a similar ischaemic burden. Only three patients (two in the IRA arm and one in the CR arm), who had a subsequent revascularisation after the index procedure, had residual ischaemia; however, the ischaemic burden in these patients was very small. Twelve patients (six in the IRA arm and six in the CR arm; p = 0.91) had an ischaemic burden on CMR scan > 20%.
| Variable | Treatment arm | p-value | 95% CI of difference | |
|---|---|---|---|---|
| IRA (n = 80) | CR (n = 84) | |||
| Perfusion analysis | ||||
| Presence of ischaemia, n/N (%) | 16/77 (20.8) | 17/82 (20.7) | 0.99 | N/Aa |
| Ischaemic burden | ||||
| Median (IQR) | 0.0 (0–0) | 0.0 (0–0) | 0.81 | –6.1 to 15.9b |
| Mean (SD) | 4.3 (11.3) | 3.4 (8.9) | 0.37 | |
| In those with ischaemia, mean (SD) | 20.4 (17.1) | 15.5 (13.7) | ||
| Patients, n/N (%) with ischaemic burden > 20% | 6/77 (7.8) | 6/82 (7.3) | 0.91 | N/Aa |
Clinical outcomes
Follow-up
Median follow-up length was 372 days (IRA 378 days vs. CR 366 days; p = 0.37). One hundred and ninety-eight (98%) patients attended the 12-month clinical follow-up (three patients died before this and two patients withdrew consent for follow-up at days 7 and 220).
Safety end points
Length of inpatient stay and incidence of in-hospital clinical events were similar in the treatment arms. There were no adverse effects on safety with CR (Table 23).
| Safety profile: inpatient clinical events | Treatment arm | OR (95% CI) | p-value | |
|---|---|---|---|---|
| IRA (n = 105) | CR (n = 98) | |||
| Length of inpatient stay (days) | ||||
| Median (IQR) | 3 (2–4) | 3 (2–4) | 0.13 | |
| Mean (SD) | 3.9 ± 2.8 | 3.5 ± 2.6 | ||
| Contrast nephropathy, n/N (%) | 0/105 (0.0) | 1/98 (1.0) | a | 0.30 |
| Vascular access injury needing repair, n/N (%) | 0/105 (0.0) | 0/98 (0.0) | a | 1.00 |
| Death, n/N (%) | 1/105 (0.9) | 1/98 (1.0) | 1.07 (0.07 to 17.4) | 0.96 |
| Recurrent MI, n/N (%) | 1/05 (0.9) | 0/98 (0.0) | a | 0.33 |
| CVA/TIA, n/N (%) | 0/105 (0.0) | 0/98 (0.0) | a | 1.00 |
| Heart failure, n/N (%) | 1/105 (1.0) | 2/98 (2.0) | 2.17 (0.19 to 24.3) | 0.52 |
| Repeat revascularisation, n/N (%) | 3/105 (2.9) | 2/98 (2.0) | 0.71 (0.12 to 4.3) | 0.71 |
| Major bleed, n/N (%) | 1/105 (1.0) | 1/98 (1.0) | 1.09 (0.07 to 7.7) | 0.90 |
The primary clinical outcome of the first combined MACE at 12 months was borderline significantly reduced in patients undergoing CR (see Table 24 and Figure 13). This was driven primarily by reduced revascularisation events (4.1% vs. 9.5%; p = 0.13) and recurrent MI (0% vs. 2.9%; p = 0.09). There was one death in each arm (both cardiovascular) and two-thirds of MIs were type 1 non-STEMI.
| Variable | Treatment arm | HR (95% CI) | p-value | |
|---|---|---|---|---|
| CR (n = 98) | IRA (n = 105) | |||
| Time to first event (MACEs) | ||||
| MACEs, n/N (%) | 8/98 (8.2) | 18/105 (17.1) | 0.43 (0.18 to 1.04) | 0.055 |
| All-cause mortality, n/N (%) | 1/98 (1.0) | 1/105 (1.0) | 1.07 (0.07 to 17.4) | 0.96 |
| CV mortality | 1/98 (1.0) | 1/105 (1.0) | 1.07 (0.07 to 17.4) | 0.96 |
| Non-CV mortality | 0/98 (0.0) | 0/105 (0.0) | a | 1.00 |
| Recurrent MI, n/N (%) | 0/98 (0.0) | 3/105 (2.9) | a | 0.09 |
| Type 1 | 0/98 (0.0) | 2/105 (1.9) | a | 0.17 |
| Type 4b (ST) | 0/98 (0.0) | 1/105 (1.0) | a | 0.33 |
| Heart failure, n/N (%) | 3/98 (3.1) | 4/105 (3.8) | 0.80 (0.17 to 3.7) | 0.77 |
| Repeat revascularisation, n/N (%) | 4/98 (4.1) | 10/105 (9.5) | 0.40 (0.12 to 1.3) | 0.13 |
FIGURE 13.
Kaplan–Meier survival curves for 12-month clinical outcomes.

Clinical event rates (time to first event) for patients in the CMR imaging substudy versus those who did not take part are shown in Table 25. There was a higher event rate in non-CMR imaging patients that was largely driven by an increase in non-cardiovascular mortality and acute stent thrombosis.
| Variable | CMR imaging substudy (n = 203) | Non-CMR imaging substudy (n = 93) | HR (95% CI) (CMR imaging substudy vs. non-CMR imaging substudy) | p-value |
|---|---|---|---|---|
| Time to first event (MACEs) | ||||
| MACEs, n/N (%) | 26/203 (13.3) | 22/93 (23.7) | 0.50 (0.27 to 0.93) | 0.026 |
| All-cause mortality, n/N (%) | 2/203 (1.0) | 6/93 (5.4) | 0.14 (0.03 to 0.73) | 0.007 |
| CV mortality | 2/203 (1.0) | 3/93 (3.2) | 0.30 (0.05 to 1.82) | 0.17 |
| Non-CV mortality | 0/203 (0.0) | 3/93 (3.2) | a | 0.03 |
| Recurrent MI, n/N (%) | 3/203 (1.5) | 4/93 (4.3) | 0.33 (0.07 to 1.52) | 0.14 |
| Type 1 | 2/105 (1.0) | 1/93 (1.1) | 0.92 (0.08 to 10.2) | 0.94 |
| Type 4b (ST) | 1/203 (0.5) | 3/93 (3.2) | 0.15 (0.02 to 1.45) | 0.06 |
| Heart failure, n/N (%) | 7/203 (3.5) | 5/93 (5.4) | 0.63 (0.19 to 2.04) | 0.44 |
| Repeat revascularisation, n/N (%) | 14/203 (7.0) | 7/93 (7.5) | 0.97 (0.39 to 2.5) | 0.98 |
Chapter 5 Discussion
This is the first detailed study of acute and follow-up CMR imaging outcomes in a randomised study of IRA only versus CR in patients with multivessel coronary disease at the PPCI. The data have confirmed that non-IRA PCI is associated with additional (type 4a) infarctions. However, these type 4a MIs are relatively infrequent, generally small and did not result in an increase in total IS. 81 There is mounting evidence from randomised trials that treating multivessel disease with CR leads to a reduction in MACEs after the PPCI than with an IRA-only strategy. 19,80 The current results provide reassurance that CR does not lead to increased total IS or reduced myocardial salvage.
The patients in the substudy had similar baseline characteristics to those in the main trial. Time to revascularisation82 and anterior MI83,84 are strongly associated with IS and, therefore, randomisation was stratified by these variables. The clinical event rates in CMR imaging participants was inevitably lower than that in those not participating in the CMR imaging substudy, largely because of the fact that some patients died or were too ill to take part. However, there was a similar reduction in the HR for MACEs in the CR CMR imaging subgroup as that seen in the main study compared with IRA-only revascularisation. These findings lead us to believe that there was no systematic bias in more sick patients in the IRA-only arm not participating in the CMR imaging substudy.
It is well recognised that elective PCI can cause a rise in troponin levels is approximately 30% of patients and in approximately 50% undergoing PCI for unstable angina. 24 Such type 4a MI81 can be detected on CMR scans and have been associated with adverse prognosis. 27 In this substudy of the CvLPRIT, the prevalence of > 1 CMR imaging-detected infarct in patients receiving CR was double that in the IRA-only arm (23.8% vs. 11.2% respectively), and more than threefold for the acute non-IRA infarcts (17.1% CR vs. 4.8% IRA only). Previous Q-wave MI was an exclusion criterion in this study, but 4% of the CR group and 3% in the IRA-only group had a history of previous non-STEMI. These data suggest that for 100 patients with multivessel disease who were randomised to receive CR at the time of the PPCI, 12% will have evidence of additional CMR imaging-detectable infarctions compared with IRA-only revascularisation. However, this proportion is less than that might have been expected from previous studies in elective PCI. 24,27 The extent of acute non-IRA infarction was generally small (median 2.5% of LVM). Importantly, total IS was not increased at baseline or at follow-up, and there were no significant differences in LV volumes or ejection fraction between the treatment groups. Peak CK was also similar in the two groups. The mean IS was slightly lower than expected. This finding means that the power of the study to detect a 4% difference in IS was reduced. This is most likely because of the use of the FWHM technique to quantify IS, which gives lower ISs than commonly used thresholding techniques. 85,86
These findings confirm that the non-IRA intervention at the time of the PPCI does not lead to increased total IS. In the main CvLPRIT,80 CR resulted in a significantly reduced HR for the 12-month combined MACEs, despite the greater prevalence of CMR imaging-detected type 4a MI shown in the current results. There are limited data on whether or not revascularisation-induced myocardial injury detected by CMR imaging is linked to prognosis27 and no data in patients presenting with STEMI. In an observational study of 152 patients undergoing elective revascularisation, 32% had evidence of new LGE which averaged 5 g (≈4% of LVM), but half of these patients were treated with a CABG. 27 In that study, patients with new infarction following revascularisation had reduced ejection fraction, increased LV volumes, increased total IS and a threefold increase in MACEs at a median of 2.9 years’ follow-up compared with those without new LGE. 27 Although it cannot be completely discounted, given that the CR group in the current study group had no increase in total IS, LV volumes or reduced ejection fraction, it seems unlikely that the short- to medium-term clinical benefits of CR will be offset in the long term by increased heart failure or sudden cardiac deaths. 19,80 Long-term follow-up of the CvLPRIT patients will help answer this question, but larger studies are also needed to provide further reassurance on the safety of a CR strategy at the time of the PPCI.
No significant differences in myocardial salvage were observed between the treatment groups in this study. There was a trend for the AAR to be higher in IRA-only arm and it is a limitation that a large proportion of the patients did not have discernible oedema on T2W-STIR despite good image quality. This does reduce the power to detect differences in myocardial salvage between the treatment arms. Adjusting the primary outcome for AAR did not alter the conclusions. Additionally, as for IS, the fact that there was also no difference in LV volumes or ejection fraction makes it unlikely that this would have been significant with increased sample size. Non-IRA revascularisation at the time of the PPCI could increase perfusion by relieving flow-limiting stenoses to watershed areas resulting in increased myocardial salvage. 29 Alternatively, resting myocardial perfusion and flow reserve following PCI may actually be reduced, as has been shown in elective patients as a result of distal embolisation, particularly when the PCI is associated with new LGE. 28,29
Unexpectedly, we also observed no difference in ischaemic burden between the groups undergoing follow-up stress perfusion CMR imaging. There are several potential explanations for this finding. First, it is well recognised that even severe angiographic stenoses may not cause ischaemia. 87,88 Second, 11 patients in the IRA-only arm had further PCI before the stress CMR imaging, which is likely to have reduced ischaemic burden in this group. Third, the small number of crossovers from randomisation is likely to have diminished the differences in ischaemia between the groups. Finally, the stress CMR imaging was undertaken in patients on optimal medical therapy, which may dramatically reduce post-MI ischaemia30 making it more difficult to detect differences between the groups. This fact may also explain why the overall ischaemic burden in our study was small (3–4%) and is consistent with the MPS results that did not demonstrate any patients to have an ischaemic burden > 20%. 80 These results challenge the assumption that ischaemia is the major driver of adverse clinical events following STEMI30 for which there is an absence of data in the modern PPCI era. Although the numbers are small, we did see a trend to a reduction in spontaneous MI in the CR group, so plaque pacification may be one mechanism that contributes to the reduction in MACEs with CR.
Limitations
Seven patients died or were too ill to enter the CMR imaging substudy and inevitably the clinical event rate was lower in CMR imaging participants than the main study that was driven by non-cardiovascular death and recurrent MI. However, it is felt that it is unlikely any bias has arisen given the very similar reduction in HR in the CMR imaging substudy to the main trial. IS was lower than expected and this has decreased the power of the study to detect a 4% difference between treatment groups, The optimal timing to assess CMR imaging IS post STEMI is uncertain. 89 An early time point was chosen to enhance participation in the CMR imaging substudy as it was felt there could have been a higher dropout rate scanning patients after discharge from hospital. Finally, MSI was only reliably measured in ≈75% of patients and the use of novel T1 or T2 mapping techniques for future studies may lead to a more robust assessment. Longer-term follow-up of participants is required to assess whether or not the small increase in non-IRA MI seen with CR is clinically significant. Thirty patients in the CMR imaging substudy underwent a staged non-IRA PCI. As these numbers are small and the patients were not randomised it cannot be stated whether CR at the index procedure or staged during initial hospitalisation results in the same myocardial injury.
Chapter 6 Conclusions
An in-hospital CR strategy in patients with multivessel disease at the time of the PPCI does not lead to increased total IS compared with an IRA-only strategy, but is associated with a small increase in type 4a MI in non-IRA territories. These findings provide further reassurance that non-IRA intervention can be considered at the time of the PPCI, but larger studies with long-term follow-up data for safety are required.
Recommendations for research
Larger clinical trials with longer follow-up in patients with multivessel disease presenting for the PPCI are required to assess (1) whether or not death and MI are reduced by a CR strategy (this is particularly important given the findings of the current study, which have confirmed that CR is associated with a small increase in non-IRA MI); (2) whether or not functional assessment of non-IRA lesions results in similar outcomes to a pragmatic angiographic-based revascularisation strategy; (3) the optimal timing of in-hospital versus staged outpatient CR; and (4) the cost-effectiveness of various CR strategies (immediate, staged and FFR guided) versus an IRA-only strategy. Additionally, long-term follow-up of participants in the CvLPRIT-CMR imaging substudy is required to assess whether or not the small increase in non-IRA MI seen with CR is clinically significant.
Summary
The CvLPRIT-CMR imaging included 203 patients with inpatient CMR scans after being randomised to either a CR strategy (n = 98) or an IRA-only strategy (n = 105) for the treatment of multivessel disease at the time of the PPCI. A small, but statistically significant, increase in non-IRA infarction was detected in the CR group, but the primary outcome of IS as a proportion of the LVM was not significantly different with the two treatment strategies. There were no differences in MVO, LV volumes, ejection fraction or MSI between the groups. These data provide reassurance that in-hospital non-IRA related revascularisation does not lead to an increase in total IS in patients undergoing the PPCI for STEMI.
Acknowledgements
The authors would like to thank all the investigators and staff who contributed to the main CvLPRIT. The committee members and investigational team for CvLPRIT were as detailed below.
Trial Steering Committee
H Swanton (independent chairperson), P Schofield (independent member), M Gunning (independent member), AH Gershlick, N Curzen, D Blackman, J Greenwood, M Dalby, G McCann, A Kelion, D Kelly, S Hetherington, S Talwar, M Flather, T Sasikaran, D Hetmanski, K Fairbrother, S Amoils (BHF) and G Thompson (lay member).
Data Safety and Monitoring Board
R Hall (chairperson), T Gilbert and M Roughton.
Event adjudicators
AH Gershlick, M Gunning and S Hetherington.
Co-ordinating Centre
T Sasikaran, M Yanez-Lopez, W Aslam, D Babalis, E Matesanz, E Zbrzeska, N Lago, J Booth and F Nugara.
Glenfield Hospital, Leicester
Nurses
Lorraine Shipley, Kathryn Fairbrother, Gemma Turland, Emma Parker, Joanna Hughes, Victoria Meynell and Amanda Swinnerton.
Interventional cardiologists
Ian Hudson, Elved Roberts, David Adlam, Doug Skehan, Nilesh Samani, Jan Kovac, Gail Richardson, Raj Rajendra and Albert Alahmar.
Others
Jamal Khan, Sheraz Nazir, David Monk, Mini Pakal, Anna-Marie Marsh and John McAdam.
Harefield Hospital
Nurse
Paula Rogers.
Interventional cardiologists
Charles Ilsley, Rebecca Lane, Piers Clifford, Tito Kabir and Robert Smith.
Other
Wala Mattar.
Kettering General Hospital
Nurses
Charmaine Beirnes, Amanda Chapman, Howard Fairey and Michelle Bilson.
Interventional cardiologists
Kai Hogrefe, Martin Sluka, Mohsin Farooq, Naeem Shaukat, Javed Ehtisham and Salman Nishtar.
Leeds General Infirmary
Nurses
Kathryn Somers, Michelle Anderson, Charlotte Harland and Natalie Burton-Wood.
Interventional cardiologists
C Malkin, JM Blaxill, SB Wheatcroft and UM Sivananthan.
Royal Bournemouth Hospital
Nurses
Sarah Orr and Nicki Lakeman.
Royal Derby Hospital
Nurses
Fiona Robertson, Marie Appleby and Carmen Lisbey.
Interventional cardiologists
Tariq Azeem, Julia Baron, Manoj Bhandari, Kamal Chitkara and Alastair McCance.
Others
Jacqui McCance, Anne Bebbington, Teresa Grieve and Richard Donnelly.
Southampton General Hospital
Nurse
Zoe Nicholas.
Interventional Cardiologists
Huon Gray, Iain Simpson, Alison Calver, Simon Corbetta and James Wilkinson.
Contributions of authors
Gerry P McCann (NIHR Career Development Fellow and Reader in Cardiovascular Imaging) was the chief investigator for CvLPRIT-CMR imaging. He developed the study concept, wrote the grant, contributed to the protocol development, supervised and conducted CMR scan analyses, contributed to the statistical analysis plan, interpreted the results, and wrote the manuscript (with JNK). He is guarantor (with JNK) for the data accuracy.
Jamal N Khan (Clinical Research Fellow and Specialist Registrar in Cardiology) performed all of the blinded CMR imaging analyses, wrote the first draft of the statistical analysis plan, conducted the statistical analyses (under supervision of AR), interpreted the results and wrote the manuscript (with GPM).
John P Greenwood (Professor of Cardiology) helped developed the study concept and wrote the grant, contributed to the protocol development, recruited patients and contributed to the statistical analysis plan, interpreted the results and critically edited the manuscript.
Sheraz Nazir (Clinical Research Fellow and Specialist Registrar in Cardiology) helped recruit patients, supervised CMR scans, undertook CMR imaging analysis for interobserver variability and critically reviewed the manuscript for intellectual content.
Miles Dalby (Consultant Cardiologist) helped developed the study concept and wrote the grant, contributed to the protocol development, recruited patients, interpreted the results and critically reviewed the manuscript for intellectual content.
Nick Curzen (Professor of Interventional Cardiology) helped developed the study concept and wrote the grant, contributed to the protocol development, recruited patients, interpreted the results and critically edited the manuscript for important intellectual content.
Simon Hetherington (Consultant Cardiologist) recruited patients, interpreted the results and critically reviewed the manuscript for important intellectual content.
Damian J Kelly (Consultant Cardiologist) helped developed the study concept, contributed to the protocol development, recruited patients, interpreted the results and critically edited the manuscript for important intellectual content.
Daniel J Blackman (Consultant Cardiologist) helped developed the study concept and wrote the grant, contributed to the protocol development, recruited patients, interpreted the results and critically reviewed the manuscript for intellectual content.
Arne Ring (Professor of Statistics) oversaw the development of the statistical analysis plan and statistical analyses, interpreted the results and critically edited the manuscript for important intellectual content.
Charles Peebles (Consultant Radiologist) supervised the acquisition of CMR scans, interpreted the results and critically reviewed the manuscript for important intellectual content.
Joyce Wong (Consultant Cardiologist) supervised the acquisition of CMR scans, interpreted the results and critically reviewed the manuscript for important intellectual content.
Thiagarajah Sasikaran (Trial Manager and Co-ordinator) helped develop the protocol and ethics application, patient information leaflets, interpreted results and reviewed the manuscript for important intellectual content.
Marcus Flather [Professor of Medicine (Clinical Trials)], helped developed the study concept and write the grant, contributed to the protocol development and contributed to the statistical analysis plan, interpreted the results and critically edited the manuscript.
Howard Swanton (Consultant Cardiologist) was the independent chairperson of the TSC, interpreted results and reviewed the manuscript for important intellectual content.
Anthony H Gershlick (Professor of Interventional Cardiology) was the chief investigator for the main CvLPRIT, helped developed the study concept and wrote the grant, contributed to the protocol development, recruited patients and contributed to the statistical analysis plan, interpreted the results and critically edited the manuscript.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health.
References
- BHF . British Heart Foundation Statistics Website 2009. www.heartstats.org/topic.asp?id=17 (accessed 19 December 2009).
- White HD, Norris R, Brown M, Brandt P, Whitlock R, Wild C. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76:44-51. http://dx.doi.org/10.1161/01.CIR.76.1.44.
- Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, . Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) . ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619.
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol 2013;61:e78-140. http://dx.doi.org/10.1016/j.jacc.2012.11.019.
- Tarantini G, Napodano M, Gasparetto N, Favaretto E, Marra MP, Cacciavillani L, et al. Impact of multivessel coronary artery disease on early ischemic injury, late clinical outcome, and remodeling in patients with acute myocardial infarction treated by primary coronary angioplasty. Cor Artery Dis 2010;21:78-86. http://dx.doi.org/10.1097/MCA.0b013e328335a074.
- Dziewierz A, Siudak Z, Rakowski T, Zasada W, Dubiel JS, Dudek D. Impact of multivessel coronary artery disease and noninfarct-related artery revascularization on outcome of patients with ST elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER Registry). Am J Cardiol 2010;106:342-7. http://dx.doi.org/10.1016/j.amjcard.2010.03.029.
- Corpus RA, House JA, Marso SP, Grantham JA, Huber KC, Laster SB, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J 2004;148:493-500. http://dx.doi.org/10.1016/j.ahj.2004.03.051.
- Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007;28:1709-16. http://dx.doi.org/10.1093/eurheartj/ehm184.
- Jaski BE, Cohen JD, Trausch J, Marsh DG, Bail GR, Overlie PA, et al. Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: Comparison of single-vessel versus multivessel coronary artery disease. Am Heart J 1992;124:1427-33. http://dx.doi.org/10.1016/0002-8703(92)90053-X.
- Muller DW, Topol EJ, Ellis SG, Sigmon KN, Lee K, Califf RM. Multivessel coronary artery disease: a key predictor of short-term prognosis after reperfusion therapy for acute myocardial infarction. Thrombolysis and angioplasty in myocardial infarction (TAMI) study group. Am Heart J 1991;121:1042-9. http://dx.doi.org/10.1016/0002-8703(91)90661-Z.
- Claessen BE, van der Schaaf RJ, Verouden NJ, Stegenga NK, Engstrom AE, Sjauw KD, et al. Evaluation of the effect of a concurrent chronic total occlusion on long-term mortality and left ventricular function in patients after primary percutaneous coronary intervention. JACC Cardiovasc Interv 2009;2:1128-34. http://dx.doi.org/10.1016/j.jcin.2009.08.024.
- Widimsky P, Holmes DR. How to treat patients with ST elevation acute myocardial infarction and multi-vessel disease?. Eur Heart J 2011;32:396-403. http://dx.doi.org/10.1093/eurheartj/ehq410.
- Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv 2010;3:1274-81. http://dx.doi.org/10.1016/j.jcin.2010.08.025.
- Hannan EL, Samadashvili Z, Walford G, Holmes DR, Jacobs AK, Stamato NJ, et al. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv 2010;3:22-31. http://dx.doi.org/10.1016/j.jcin.2009.10.017.
- Vlaar PJ, Mahmoud KD, Holmes DR, van Valkenhoef G, Hillege HL, van der Horst ICC, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol 2011;58:692-703. http://dx.doi.org/10.1016/j.jacc.2011.03.046.
- Di Mario C, Mara S, Flavio A, Imad S, Antonio M, Anna P, et al. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int J Cardiovasc Intervent 2004;6:128-33.
- Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Leuzzi C, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart 2010;96:662-7. http://dx.doi.org/10.1136/hrt.2009.177162.
- Ghani A, Dambrink JH, van ’t Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC. Treatment of non-culprit lesions detected during primary PCI: long-term follow-up of a randomised clinical trial. Neth Heart J 2012;20:347-53. http://dx.doi.org/10.1007/s12471-012-0281-y.
- Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369:1115-23. http://dx.doi.org/10.1056/NEJMoa1305520.
- Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283-91. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.743963.
- Erne P, Schoenenberger AW, Burckhardt D, Zuber M, Kiowski W, Buser PT, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007;297:1985-91. http://dx.doi.org/10.1001/jama.297.18.1985.
- Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Rev Port Cardiol 2008;27:1063-143.
- Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16. http://dx.doi.org/10.1056/NEJMoa070829.
- Alcock RF, Roy P, Adorini K, Lau GT, Kritharides L, Lowe HC, et al. Incidence and determinants of myocardial infarction following percutaneous coronary interventions according to the revised joint task force definition of troponin T elevation. Int J Cardiol 2010;140:66-72. http://dx.doi.org/10.1016/j.ijcard.2008.11.005.
- Prasad A, Rihal CS, Lennon RJ, Singh M, Jaffe AS, Holmes DR. Significance of periprocedural myonecrosis on outcomes after percutaneous coronary intervention: an analysis of preintervention and postintervention troponin T levels in 5487 patients. Circ Cardiovasc Interv 2008;1:10-9. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.108.765610.
- Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng ASH, Petersen SE, et al. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation 2005;112:3289-96. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.549170.
- Rahimi K, Banning AP, Cheng ASH, Pegg TJ, Karamitsos TD, Channon KM, et al. Prognostic value of coronary revascularisation-related myocardial injury: a cardiac magnetic resonance imaging study. Heart 2009;95:1937-43. http://dx.doi.org/10.1136/hrt.2009.173302.
- Taylor AJ, Al-Saadi N, Abdel-Aty H, Schulz-Menger J, Messroghli DR, Gross M, et al. Elective percutaneous coronary intervention immediately impairs resting microvascular perfusion assessed by cardiac magnetic resonance imaging. Am Heart J 2006;151:e891-897. http://dx.doi.org/10.1016/j.ahj.2005.09.021.
- Selvanayagam JB, Cheng aSH, Jerosch-Herold M, Rahimi K, Porto I, van Gaal W, et al. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation 2007;116:1458-64. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.671909.
- Mahmarian JJ, Dakik HA, Filipchuk NG, Shaw LJ, Iskander SS, Ruddy TD, et al. An initial strategy of intensive medical therapy is comparable to that of coronary revascularization for suppression of scintigraphic ischemia in high-risk but stable survivors of acute myocardial infarction. J Am Coll Cardiol 2006;48:2458-67. http://dx.doi.org/10.1016/j.jacc.2006.07.068.
- Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to 6-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002;39:30-6. http://dx.doi.org/10.1016/S0735-1097(01)01711-9.
- Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992-200. http://dx.doi.org/10.1161/01.CIR.100.19.1992.
- Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374-9. http://dx.doi.org/10.1016/S0140-6736(03)12389-6.
- Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke A, Wu KC, et al. Microvascular obstruction and left ventricular remodelling early after acute myocardial infarction. Circulation 2000;101:2734-41. http://dx.doi.org/10.1161/01.CIR.101.23.2734.
- Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, et al. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 2008;52:181-9. http://dx.doi.org/10.1016/j.jacc.2008.04.006.
- Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 2005;26:549-57. http://dx.doi.org/10.1093/eurheartj/ehi147.
- Lund GK, Stork A, Muellerleile K, Bansmann MP, Schlichting U, Mu M, et al. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology 2007;245:95-104. http://dx.doi.org/10.1148/radiol.2451061219.
- Beek AM, Nijveldt R, van Rossum AC. Intramyocardial hemorrhage and microvascular obstruction after primary percutaneous coronary intervention. Int J Cardiovasc Imaging 2010;26:49-55. http://dx.doi.org/10.1007/s10554-009-9499-1.
- Ørn S, Manhenke C, Greve OJ, Larsen AI, Bonarjee VVS, Edvardsen T, et al. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J 2009;30:1978-85. http://dx.doi.org/10.1093/eurheartj/ehp219.
- Wu E, Ortiz JT, Tejedor P, Lee DC, Kansal P, Carr JC, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study infarct size by contrast enhanced cardiac magnetic resonance. Heart 2008;94:730-6. http://dx.doi.org/10.1136/hrt.2007.122622.
- Larose E, Rodés-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol 2010;55:2459-69. http://dx.doi.org/10.1016/j.jacc.2010.02.033.
- de Waha S, Desch S, Eitel I, Fuernau G. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on longterm outcome after ST elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 2010;31:2660-8. http://dx.doi.org/10.1093/eurheartj/ehq247.
- Cochet AA, Lorgis L, Lalande A, Zeller M, Beer JC, Walker PM, et al. Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol 2009;19:2117-26.
- Ibanez B, Prat-González S, Speidl WS, Vilahur G, Pinero A, Cimmino G, et al. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 2007;115:2909-16. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.679639.
- Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51:1581-7. http://dx.doi.org/10.1016/j.jacc.2008.01.019.
- Stork A, Lund GK, Muellerleile K, Bansmann PM, Nolte-Ernsting C, Kemper J, et al. Characterization of the peri-infarction zone using T2-weighted MRI and delayed-enhancement MRI in patients with acute myocardial infarction. Eur Radiol 2006;16:2350-7. http://dx.doi.org/10.1007/s00330-006-0232-3.
- Miller S, Helber U, Brechtel K, Nägele T, Hahn U, Kramer U, et al. MR imaging at rest early after myocardial infarction: Detection of preserved function in regions with evidence for ischemic injury and non-transmural myocardial infarction. Eur Radiol 2003;13:498-506.
- Aletras AH, Tilak GS, Natanzon A, Hsu L-Y, Gonzalez FM, Hoyt RF, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006;113:1865-70. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.576025.
- Tilak GS, Hsu LY, Hoyt RF Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for. Invest Radiol 2008;43:7-15. http://dx.doi.org/10.1097/RLI.0b013e3181558822.
- Carlsson M, Ubachs JFA, Hedström E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging 2009;2:569-76. http://dx.doi.org/10.1016/j.jcmg.2008.11.018.
- Hedström E, Engblom H, Frogner F, Åström-olsson K, Öhlin H, Jovinge S, et al. Infarct evolution in man studied in patients with first-time coronary occlusion in comparison to different species – implications for assessment of myocardial salvage. J Cardiovasc Magn Reson 2009;10:1-10. http://dx.doi.org/10.1186/1532-429x-11-38.
- Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end-point (probe) study. A novel design for intervention trials. Prospective randomized open blinded end-point. Blood Press 1992;1:113-19. http://dx.doi.org/10.3109/08037059209077502.
- RITA2-Investigators . Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA-2) trial. RITA-2 trial participants. Lancet 1997;350:461-8. http://dx.doi.org/10.1016/S0140-6736(97)07298-X.
- Agard A, Hermeren G, Herlitz J. Patients’ experiences of intervention trials on the treatment of myocardial infarction: is it time to adjust the informed consent procedure to the patient’s capacity?. Heart 2001;86:632-7. http://dx.doi.org/10.1136/heart.86.6.632.
- Yuval R, Halon DA, Merdler A, Khader N, Karkabi B, Uziel K, et al. Patient comprehension and reaction to participating in a double-blind randomized clinical trial (ISIS-4) in acute myocardial infarction. Arch Intern Med 2000;160:1142-6. http://dx.doi.org/10.1001/archinte.160.8.1142.
- Gammelgaard A. Informed consent in acute myocardial infarction research. J Med Philos 2004;29:417-34. http://dx.doi.org/10.1080/03605310490503533.
- Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2013;368:1379-87. http://dx.doi.org/10.1056/NEJMoa1301092.
- Gershlick AH. MVO-Collaborators . Randomized Controlled Trial Comparing Intracoronary Administration of Adenosine or Sodium Nitroprusside to Control for Attenuation of Microvascular Obstruction During Primary Percutaneous Coronary Intervention n.d. http://public.Ukcrn.Org.Uk/search/studydetail.Aspx?Studyid=12168 (accessed 5 September 2013).
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13-20. http://dx.doi.org/10.1016/S0140-6736(03)12113-7.
- World Medical Association . World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects (Revision 48) n.d. www.wma.net/en/30publications/10policies/b3/17c.pdf (accessed 5 September 2013).
- TIMI-Collaborators . The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI study group. N Engl J Med 1985;312:932-6. http://dx.doi.org/10.1056/NEJM198504043121437.
- Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587-92. http://dx.doi.org/10.1016/S0735-1097(85)80380-6.
- Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The syntax score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219-27.
- Dizon JM, Brener SJ, Maehara A, Witzenbichler B, Biviano A, Godlewski J, et al. Relationship between ST-segment resolution and anterior infarct size after primary percutaneous coronary intervention: Analysis from the INFUSE-AMI trial. Eur Heart J Acute Cardiovasc Care 2014;3:78-83. http://dx.doi.org/10.1177/2048872613508658.
- Papavassiliu T, Kuhl HP, Schroder M, Suselbeck T, Bondarenko O, Bohm CK, et al. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 2005;236:57-64. http://dx.doi.org/10.1148/radiol.2353040601.
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 1979;5MC–9:62-6.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation 2002;105:539-42. http://dx.doi.org/10.1161/hc0402.102975.
- Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, et al. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart 2011;97:2038-45. http://dx.doi.org/10.1136/heartjnl-2011-300098.
- Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 2004;44:2383-9. http://dx.doi.org/10.1016/j.jacc.2004.09.020.
- Masci PG, Andreini D, Francone M, Bertella E, De Luca L, Coceani M, et al. Prodromal angina is associated with myocardial salvage in acute ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging 2013;14:1041-8. http://dx.doi.org/10.1093/ehjci/jet063.
- Schuster A, Morton G, Hussain ST, Jogiya R, Kutty S, Asrress KN, et al. The intra-observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol 2013;82:296-301. http://dx.doi.org/10.1016/j.ejrad.2012.11.012.
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Stress myocardial perfusion single-photon emission computed tomography is clinically effective and cost effective in risk stratification of patients with a high likelihood of coronary artery disease (CAD) but no known CAD. J Am Coll Cardiol 2004;43:200-8. http://dx.doi.org/10.1016/j.jacc.2003.07.043.
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation 2011;123:2736-47. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.009449.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. http://dx.doi.org/10.1016/j.jbi.2008.08.010.
- Gershlick AH, Flather M, McCann GP, Sasikaran T, Dalby M, Curzen N, et al. Complete Versus Lesion-Only PRimary PCI Pilot 2013. www.nets.nihr.ac.uk/__data/assets/pdf_file/0009/55359/PRO-10-27-01.pdf (accessed 4 September 2015).
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1:30-46. http://dx.doi.org/10.1037/1082-989X.1.1.30.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. http://dx.doi.org/10.1016/S0140-6736(86)90837-8.
- Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson 2005;7:783-91. http://dx.doi.org/10.1080/10976640500295417.
- Hahn J-Y, Song YB, Gwon H-C, Choe YH, Kim JH, Sung J, et al. Relation of left ventricular infarct transmurality and infarct size after primary percutaneous coronary angioplasty to time from symptom onset to balloon inflation. Am J Cardiol 2008;102:1163-9. http://dx.doi.org/10.1016/j.amjcard.2008.06.042.
- Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol 2015;65:963-72. http://dx.doi.org/10.1016/j.jacc.2014.12.038.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. http://dx.doi.org/10.1093/eurheartj/ehs184.
- Khan JN, Razvi N, Nazir SA, Singh A, Masca NG, Gershlick AH, et al. Prevalence and extent of infarct and microvascular obstruction following different reperfusion therapies in ST-elevation myocardial infarction. J Cardiovasc Magn Reson 2014;16. http://dx.doi.org/10.1186/1532-429X-16-38.
- Lonborg J, Vejlstrup N, Kelbaek H, Holmvang L, Jorgensen E, Helqvist S, et al. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: an observational study. Eur Heart J Cardiovasc Imaging 2013;14:387-95. http://dx.doi.org/10.1093/ehjci/jes271.
- Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, et al. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J 2011;32:1640-8. http://dx.doi.org/10.1093/eurheartj/ehr064.
- Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, et al. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 2011;4:150-6. http://dx.doi.org/10.1016/j.jcmg.2010.11.015.
- Khan JN, Nazir SA, Horsfield MA, Singh A, Kanagala P, Greenwood JP, et al. Comparison of semi-automated methods to quantify infarct size and area at risk by cardiovascular magnetic resonance imaging at 1.5T and 3.0T field strengths. BMC Res Notes 2015;8. http://dx.doi.org/10.1186/s13104-015-1007-1.
- Dambrink JH, Debrauwere JP, van ’t Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC, et al. Non-culprit lesions detected during primary PCI: treat invasively or follow the guidelines?. EuroIntervention 2010;5:968-75. http://dx.doi.org/10.4244/EIJV5I8A162.
- De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. http://dx.doi.org/10.1056/NEJMoa1205361.
- Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Timing of cardiovascular MR imaging after acute myocardial infarction: Effect on estimates of infarct characteristics and prediction of late ventricular remodeling. Radiology 2011;261:116-26. http://dx.doi.org/10.1148/radiol.11110228.
Appendix 1 Assent form
Appendix 2 Clinical outcome definitions
Contrast-induced nephropathy
A rise in creatinine levels of > 25% or 44.2 µmol/l within 48 hours after angiography and persisting for at least 48 hours.
Death
Death from any cause classified as cardiovascular or non-cardiovascular.
Causes of cardiovascular deaths include, but are not limited to, deaths resulting from atherosclerotic vascular disease (excluding coronary), congestive heart failure, cardiogenic shock, during or immediately following a CABG procedure, during or immediately following a PCI procedure, dysrhythmia, pulmonary embolism, MI, sudden cardiac death, intracranial haemorrhage, non-haemorrhagic stroke and other cardiovascular causes. Cardiovascular death includes any cardiac causes, or other vascular causes (e.g. pulmonary embolism, aortic dissection).
Non-cardiovascular death includes accidental death, trauma, haemorrhage (not intracranial), infection, malignancy, suicide and other.
Myocardial infarction (new)
Hospital admission (or in hospital) with:
-
Type 1: spontaneous re-MI: recurrent angina symptoms or new ECG changes occurring before PCI or < 48 hours from PCI that is compatible with re-MI associated with an elevation of creatine kinase MB isoenzyme (CK-MB), troponin, or total CK levels beyond the upper limit of normal (ULN) and 20% or more above the previous value.
-
Type 4b: stent thrombosis documented by coronary angiography and/or autopsy AND fulfilling the criteria of spontaneous MI (type 1).
-
Type 4a: CK-MB or total CK levels more than three times the ULN within 48 hours following PCI. If the pre-PCI CK-MB or total CK level is higher than the ULN, there also needs to be:
-
either the demonstration of a falling CK-MB or total CK level prior to the onset of the suspected event
-
or a subsequent peak of the cardiac biomarker of at least 20% above the previous value obtained prior to the onset of the suspected event
-
with either an appropriate clinical presentation or new ischaemic ECG changes (ST-segment depression or ST-segment elevation or development of new pathological Q-waves/LBBB).
-
Transient ischaemic attack/cerebrovascular event
Defined as the presence of a new focal neurological deficit thought to be vascular in origin with signs or symptoms lasting more than 24 hours. It is strongly recommended (but not required) that an imaging procedure, such as a computed tomography scan or MRI, be performed. Stroke will be further classified as ischaemic, haemorrhagic or type uncertain.
Major bleed
-
Cumulative occurrence of intracranial or intraocular bleeding.
-
Haemorrhage at the vascular access site requiring intervention.
-
Reduction in haemoglobin levels of at least 5 g/dl.
-
Reoperation for bleeding or transfusion of a blood product (at least 2 units).
-
Bleeding causing substantial hypotension requiring the use of inotropic agents.
-
All other bleeding events were considered as minor (i.e. epistaxis, blood traces in the stool, etc.).
Planned or repeat coronary artery bypass graft or percutaneous coronary intervention
-
IRA target lesion re-interventions (TLRs) inside the implanted stent or within 5-mm proximally or distally or repeated interventions in the same vessel (target vessel revascularisation; TVR) PCIs or by CABG surgery.
-
Non-IRA TVR.
-
IRA TLR (i.e. lesions within the index IRA, but not the IRA culprit lesion).
-
Non-IRA TLR.
-
PCI to lesions not identified previously.
-
CABG for new symptoms or complications of PCI. Discussions in a cardiosurgical multidisciplinary team (MDT) forum must be recorded (MDT yes/no with result of discussion).
Heart failure
Any hospital admission with any of the following symptoms and signs:
-
worsening breathlessness
-
fatigue
-
fluid overload
-
pulmonary oedema
-
elevated venous pressure
-
elevated B-type natriuretic peptide levels.
Confirmation of heart failure according to local expert judgement and evidence of impaired LV function will be required for the event to be classified as heart failure.
Other serious adverse events
Any event requiring hospitalisation or prolonging length of stay during hospitalisation at time of index procedure.
Appendix 3 Cardiac magnetic imaging scans database lock
Appendix 4 Data and Safety Monitoring Board interim report
List of abbreviations
- 2D
- two-dimensional
- AAR
- area at risk
- ACCF
- American College of Cardiology Foundation
- ACE
- angiotensin-converting enzyme
- AHA
- American Heart Association
- b.p.m.
- beats per minute
- BHF
- British Heart Foundation
- bSSFP
- balanced steady-state free precession cine imaging
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CI
- confidence interval
- CK
- creatine kinase
- CK-MB
- creatine kinase MB isoenzyme
- CMR
- cardiac magnetic resonance
- CR
- complete revascularisation
- CTEU
- Clinical Trials and Evaluation Unit
- CvLPRIT
- Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial
- CvLPRIT-CMR
- Complete versus Lesion-only PRimary percutaneous coronary Intervention Trial – the embedded cardiac magnetic resonance imaging substudy
- DAPT
- dual antiplatelet therapy
- DES
- drug-eluting stent
- DM
- diabetes mellitus
- DSMB
- Data and Safety Monitoring Board
- ECG
- electrocardiography
- eGFR
- estimated glomerular filtration rate
- EME
- Efficacy and Mechanism Evaluation
- ESC
- European Society of Cardiology
- ETL
- echo train length
- FFR
- fractional flow reserve
- FWHM
- full-width half-maximum
- Gd-DTPA
- gadolinium diethylenetriaminepentaacetate
- GEE
- generalised estimating equation
- HR
- hazard ratio
- ICC
- intraclass correlation coefficient
- IMH
- intramyocardial haemorrhage
- IP
- independent predictor
- IQR
- interquartile range
- IRA
- infarct-related artery
- IS
- infarct size
- LBBB
- left bundle branch block
- LGE
- late gadolinium enhancement
- LV
- left ventricular
- LVEDV
- left ventricular end-diastolic volume
- LVEF
- left ventricular ejection fraction
- LVESV
- left ventricular end-systolic volume
- LVM
- left ventricular mass
- MACE
- major adverse cardiovascular event
- MDT
- multidisciplinary team
- MI
- myocardial infarction
- MPS
- myocardial perfusion scintigraphy
- MRC
- Medical Research Council
- MRI
- magnetic resonance imaging
- MSI
- myocardial salvage index
- MVD
- multivessel coronary artery disease
- MVO
- microvascular obstruction
- NIHR
- National Institute for Health Research
- OAT
- Otsu’s automated threshold
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- PPCI
- primary percutaneous coronary intervention
- PPI
- patient and public involvement
- QCA
- quantitative coronary angiography
- REDCap
- Research Electronic Data Capture database
- ROI
- region of interest
- RV
- right ventricular
- SD
- standard deviation
- SPECT
- single-photon emission computed tomography
- STEMI
- ST-segment elevation myocardial infarction
- STIR
- short-tau inversion recovery
- T2W
- T2 weighted
- T2W-STIR
- T2-weighted short-tau inversion recovery
- TI
- inversion time
- TIMI
- thrombolysis in myocardial infarction
- TLR
- target lesion re-intervention
- TSC
- Trial Steering Committee
- TTR
- time to revascularisation
- TVR
- target vessel revascularisation
- ULN
- upper limit of normal