Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 09/100/05. The contractual start date was in November 2010. The final report began editorial review in July 2015 and was accepted for publication in February 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Derek J Hausenloy and Timothy Clayton report grants from the British Heart Foundation during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Hausenloy et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Coronary heart disease (CHD) is the leading cause of death in the UK and accounted for 155,000 deaths in 2014 [see www.heartstats.org (accessed 5 May 2016)]. It is estimated to cost the UK economy over £7.9B per year, of which 45% is attributed to direct health-care costs (the cost of hospital care and drugs), 40% is attributed to productivity losses (as a result of CHD mortality and morbidity) and 15% is attributed to the informal care of such patients [see www.heartstats.org (accessed 5 May 2016)]. Therefore, improving health outcomes in patients with CHD is a major priority of the NHS, as outlined in the National Service Framework for Coronary Heart Disease1 and embodied in several clinical guidelines and technology appraisals issued by the National Institute for Health and Care Excellence. 2
Coronary artery bypass graft (CABG) surgery remains the procedure of choice for coronary artery revascularisation in a large number of CHD patients, particularly in patients with triple vessel coronary artery disease (CAD), as highlighted in the recently published SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study, which demonstrated improved clinical outcomes with CABG surgery compared with percutaneous coronary intervention (PCI) in this patient group. 3 About 20,000 first-time CABG operations are performed in the UK each year, with an overall operative mortality risk of about 1.0% for elective CABG surgery. 4 Innovative treatment strategies are required to reduce myocardial injury and improve clinical outcomes in patients undergoing CABG surgery and, in this regard, one potential approach is remote ischaemic preconditioning (RIPC).
Remote ischaemic preconditioning
Remote ischaemic preconditioning describes the phenomenon in which the application of brief episodes of non-lethal ischaemia and reperfusion to an organ (such as the kidney, liver or small intestine) or tissue (such as skeletal muscle) protects the heart against a sustained episode of lethal ischaemia–reperfusion injury. 5,6 The discovery that the RIPC stimulus could be reproduced by applying brief episodes of ischaemia and reperfusion to the upper or lower limb7,8 has facilitated its recent translation from animal studies into the clinical arena.
MacAllister et al. 9–11 first demonstrated the concept of RIPC in human volunteers using a non-invasive RIPC stimulus that involved inflating a blood pressure cuff applied to the upper arm to 200 mmHg for 5 minutes (to induce brief ischaemia) and deflating the cuff for 5 minutes (to induce brief reperfusion), a cycle that was repeated two more times. 9 This RIPC stimulus attenuated ischaemia-induced endothelial dysfunction in the contralateral arm arising from a 20-minute episode of sustained cuff inflation. 9 Cheung et al. 12 were the first to apply this RIPC protocol in the clinical arena in a study in which four 5-minute cycles of lower-limb ischaemia and reperfusion reduced myocardial injury, improved airway resistance and decreased inotrope score in children undergoing cardiac surgery. We have demonstrated that three 5-minute cycles of upper-limb ischaemia and reperfusion had the potential to reduce myocardial injury (43% reduction in serum troponin T released over 72 hours) in adult patients undergoing elective CABG with or without valve surgery. 13,14 This last proof-of-concept clinical study forms the pilot data for the current study.
Remote ischaemic preconditioning using lower-limb ischaemia and reperfusion has also been reported to be beneficial in terms of cardiac, renal and neurological protection in the setting of elective surgery for abdominal aortic aneurysm (AAA)15,16 and surgery for cervical decompression. 17 Ali et al. 16 demonstrated that invasive lower-limb ischaemia using two 10-minute episodes of iliac artery occlusion reduced myocardial injury (as indicated by a 27% reduction in serum troponin I) and preserved renal function during elective AAA surgical repair. Hoole et al. 18 have reported that RIPC using brief ischaemia and reperfusion of the arm reduced the periprocedural myocardial injury associated with elective PCI for stable CAD. Finally, Bøtker et al. 19 have demonstrated that RIPC using four 5-minute cuff inflations/deflations administered in the ambulance reduced myocardial infarct (MI) size in ST elevation myocardial infarction (STEMI) patients undergoing primary PCI. In the current research protocol, we used this particular RIPC protocol consisting of four 5-minute cycles of cuff inflation and deflation.
Therefore, although several proof-of-concept studies have been published, whether or not RIPC can impact on clinical outcomes and improve patient health care in higher-risk patients undergoing CABG with or without valve surgery is unknown and was the subject of the current research study.
Rationale for the study
The risk profile of patients undergoing CABG surgery continues to change with factors such as (1) the ageing population (the proportion of patients aged > 75 years increased by more than 4.5-fold from 1990 to 1999, with the 5-year mortality rate in this age group being 35%) and (2) the increasing prevalence of diabetes (the proportion of diabetic patients has risen from 15% to 22%, with the operative mortality in this patient group being 2.6%), resulting in an increase in the number of higher-risk patients [we have defined ‘high risk’ as an additive euroSCORE (European System for Cardiac Operative Risk) of ≥ 5] being operated on and a corresponding increase in the overall operative risk to 5–6%. 20 It has been estimated that at least 50% of patients undergoing CABG surgery in our recruiting centres have an additive euroSCORE of ≥ 5.
These higher-risk patients are at a greater risk of sustaining periprocedural myocardial injury, requiring inotropic support post surgery, experiencing significant acute kidney injury (AKI) (up to 34% of patients)21 and experiencing a stroke (1–3%),22 resulting in worse clinical outcomes. Clearly, new treatment strategies are required to protect the heart, the brain and the kidney during higher-risk CABG with or without valve surgery, such that clinical outcomes can be improved in this patient group. This research study investigated a non-invasive, virtually cost-free intervention called RIPC that has the potential to improve clinical outcomes in CHD patients undergoing higher-risk CABG with or without valve surgery.
Perioperative myocardial injury (PMI), as measured by serum creatine kinase MB (CKMB),23 troponin T24–26 or troponin I27–29 during surgery, has been associated with worse clinical outcomes post surgery. Therefore, treatment interventions capable of reducing PMI in the setting of CABG with or without valve surgery may preserve left ventricular systolic function and improve clinical outcomes. The incidence of AKI following cardiac surgery can be as high as 30%, with up to 2% of patients going on to require dialysis. 30–32 Even after adjustment for patient comorbidities and surgical complications of the surgical procedure, the presence of AKI requiring dialysis increases the risk of death by 7.9 times compared with those patients not developing AKI. 33 Furthermore, it has been reported that changes in creatinine of > 0.5 mg/dl (44 mmol/l) after cardiac surgery also contribute to a significant increase in mortality at 30 days post surgery. 34
Remote ischaemic preconditioning prior to elective surgical repair of an AAA was reported to preserve renal function post surgery. 15,16 Whether or not RIPC is able to preserve renal function in the setting of CABG with or without valve surgery remains to be determined.
As previously described, RIPC using three 5-minute cycles of arm ischaemia and reperfusion was associated with a 43% reduction in total troponin T release in patients undergoing elective CABG with or without valve surgery at the University College London Hospitals (UCLH) Heart Hospital. 13 We have gone on to demonstrate that the beneficial effect of RIPC extends to CABG with or without valve surgery patients receiving cold-blood cardioplegia alone. 14
A similar RIPC stimulus can reduce myocardial injury in children undergoing cardiac surgery for congenital heart disease,12 reduce myocardial and renal injury in patients undergoing surgical repair of an AAA,15,16 reduce neurological injury during cervical decompression surgery17 and reduce myocardial injury in stable CHD patients undergoing elective PCI18 or STEMI patients undergoing primary PCI. 19,35–38
However, all of these clinical trials are proof-of-concept studies and whether or not RIPC can improve clinical outcomes is unclear. In this study we have investigated whether or not in a large multicentre randomised controlled clinical trial, RIPC using brief upper-limb ischaemia and reperfusion can impact on short-term and long-term clinical outcomes in higher-risk patients undergoing CABG with or without valve surgery.
Trial objectives
The single main research question in terms of PICO (population, intervention, comparator, outcome) was as follows: ‘In higher-risk adult patients undergoing CABG with or without valve surgery, does RIPC induced by brief arm ischaemia and reperfusion improve clinical outcomes at 1 year compared with a control protocol?’
Primary research objective
The primary research objective was to determine whether or not RIPC improves 1-year clinical outcomes in patients undergoing CABG with or without valve surgery.
Secondary research objectives
-
To determine if RIPC improves 30-day clinical outcomes in patients undergoing CABG with or without valve surgery.
-
To determine if RIPC has an effect on all-cause death at 12 months.
-
To determine if RIPC reduces PMI in higher-risk patients undergoing CABG with or without valve surgery (assessed by measuring serum troponin T over the 72-hour perioperative period).
-
To determine if RIPC reduces AKI and preserves renal function post CABG with or without valve surgery [assessed by measuring serum neutrophil gelatinase-associated lipocalin (NGAL) and serum creatinine and the AKI score].
-
To determine if RIPC improves patient morbidity as measured by duration of intensive therapy unit (ITU) stay, inotrope score, the 6-minute walk test (6MWT) and quality-of-life assessment.
-
In a substudy of patients recruited through selected hospitals to determine the effect of RIPC on left ventricular ejection fraction (LVEF) measured by echocardiography.
Chapter 2 Methods
Study design
This study was a multicentre, double-blind, randomised controlled trial (RCT) assessing the effect of RIPC on major adverse cardiac and cerebral events (MACCE) consisting of cardiovascular death, MI, coronary revascularisation and stroke 12 months following CABG with or without valve surgery, in 30 cardiac surgery centres in the UK. Patients were randomised in a 1 : 1 ratio to receive either RIPC or a sham protocol, which was carried out immediately prior to surgery while patients were anaesthetised.
Aim
The Effect of Remote Ischaemic preconditioning on Clinical outcomes in patients undergoing Coronary Artery bypass graft surgery (ERICCA) trial investigated whether or not RIPC improves long-term clinical outcomes after cardiac surgery. RIPC is a simple, non-invasive and virtually cost-free intervention and any reduction in MACCE would indicate an improved outcome following CABG. The findings will also have implications for the use of RIPC in other clinical ischaemic syndromes.
Participants
The trial intended to recruit 1610 higher-risk patients undergoing CABG from centres in the UK. Higher-risk patients undergoing CABG with or without valve surgery were invited to take part in the trial. The final inclusion and exclusion criteria were as follows:
-
inclusion criteria
-
patients undergoing CABG with or without valve surgery
-
patients aged ≥ 18 years
-
additive euroSCORE of ≥ 5
-
-
exclusion criteria
-
cardiogenic shock (the definition used for this was systolic blood pressure of < 90 mmHg for 30 minutes before inotrope/vasopressor administration or vasopressors or intra-aortic balloon pump are required to maintain systolic blood pressure of > 90 mmHg)
-
cardiac arrest on current admission
-
pregnancy
-
significant peripheral arterial disease affecting the upper limb
-
significant hepatic dysfunction (international normalised ratio of > 2)
-
significant pulmonary disease (forced expiratory volume in 1 second of < 40% predicted)
-
known renal failure with a glomerular filtration rate of < 30 ml/minute/1.73 m2
-
taking glibenclamide or nicorandil as these medications may interfere with RIPC
-
recruited into another study that may impact on the ERICCA study.
-
There were major protocol amendments that affected the inclusion and exclusion criteria. These were:
-
Peripheral vascular disease was changed to peripheral arterial disease as the former was felt to be vague and includes venous disease such as deep-vein thrombosis. Peripheral arterial disease was felt to be more accurate and specific to the type of patients to be included.
-
A positive troponin T or I at baseline was removed from the exclusion criteria. This was because such a result was not always known as this was not routinely measured at baseline.
-
The euroSCORE was lowered from ≥ 6 to ≥ 5 to aid with the recruitment of patients.
-
Patients with a bilirubin level of > 20 mmol/l were originally excluded but this was removed as an exclusion criterion to enhance recruitment.
A full list of protocol amendments can be found in Appendix 1.
Recruitment
The 30 centres included in the study were the Royal Brompton Hospital, London; Harefield Hospital, Middlesex; UCLH Heart Hospital, London; King’s College Hospital, London; Papworth Hospital, Cambridge; Hammersmith Hospital, London; St George’s Hospital, London; St Thomas’ Hospital, London; London Chest Hospital; Essex Cardiothoracic Centre, Basildon; Royal Sussex County Hospital, Brighton; Royal Wolverhampton Hospitals NHS Trust; Bristol Royal Infirmary; Golden Jubilee National Hospital, Clydebank; Edinburgh Royal Infirmary; Morriston Hospital, Swansea; Cardiff and Vale University Health Board; Manchester Royal Infirmary; Derriford Hospital, Plymouth; Northern General Hospital, Sheffield; Trent Cardiac Centre, Nottingham; Blackpool Victoria Hospital; Wythenshawe Hospital, Manchester; Glenfield Hospital, Leicester; Southampton General Hospital; Leeds General Infirmary; James Cook University Hospital, Middlesbrough; North Staffordshire University Hospital, Stoke-on-Trent; Castle Hill Hospital, Hull; and University Hospital Coventry.
Patients were recruited from two groups: outpatients on the waiting list for CABG surgery or inpatients awaiting CABG surgery.
Randomisation, concealment and blinding
Patients were randomly assigned in a 1 : 1 ratio to receive sham RIPC or RIPC with randomly permuted block sizes of four and six stratified by recruiting centre. Randomisation was conducted via a secure website (Sealed Envelope™; Clekenwell Workshops, London, UK) through the Clinical Trials Unit (CTU) at the London School of Hygiene & Tropical Medicine (LSHTM).
The enrolment and preconditioning procedures were performed by an unblinded research nurse or clinician who was not involved in sample or data collection. Cardiac anaesthetists, cardiac surgeons, ITU staff, ward staff and all other research personnel at each study site were blinded to the treatment allocation. The patients were also blinded to the allocation of their randomised intervention as they were anaesthetised at the time of the RIPC intervention. A limited number of staff at the CTU were unblinded to the allocations to enable them to enter data onto the electronic case report form (eCRF), which was a web-based data capture system provided by Sealed Envelope. The unblinded trial statistician supporting the Data Monitoring Committee (DMC) also had access to the randomisation codes during the study. However, no member of staff at the CTU who was involved in the collection of outcome data had access to the treatment allocation of the patients.
Interventions
The trial intervention was a physiological procedure and was performed on patients after induction of anaesthesia but prior to CABG surgery. There were no other interventions. The active RIPC procedure consisted of four 5-minute inflations of a standard blood pressure cuff on the upper arm to 200 mmHg separated by 5-minute periods when the cuff was deflated. For patients with systolic blood pressure of > 185 mmHg the cuff was inflated to at least 15 mmHg above their systolic blood pressure. The sham RIPC procedure, which also used a standard blood pressure cuff, consisted of four 5-minute simulated inflations of the blood pressure cuff on the upper arm. The simulated inflations involved opening the air valve on the blood pressure cuff such that the cuff did not inflate on squeezing the bulb. The bulb was then squeezed for a duration of 15 seconds to give the impression that the cuff was being inflated. After 5 minutes the air valve was closed again to give the impression that the cuff was being deflated. After 5 minutes the air valve was opened again and the bulb squeezed as before. This cycle was undertaken four times in total.
Data collection and management
Randomisation and collection of data on completion of the interventions were performed by the unblinded research staff. The intervention data were then faxed to the CTU where the data were entered onto the eCRF and then stored in locked cabinets. Paper copies were requested to ensure that the forms were signed by the person carrying out the intervention or sham procedure. Following this, all subsequent follow-up visits and data collection were completed by blinded research staff at the hospitals. A paper case report form (CRF) was provided to assist with data collection but the source data were considered to be those in the eCRF. A series of logic and range checks were built into the system to reduce the possibility of erroneous data being entered. The system also contained a log that detailed all notable events associated with the trial (including inserts, updates and deletions) and this provided a clear and complete audit trail throughout the trial. The data management processes were conducted following the principles of Good Clinical Practice39 and the Data Protection Act 1998. 40
Monitoring and site visits
The first site visit was a pre-recruitment visit for training (demonstration of the trial intervention and procedures) and to ensure that all relevant documentation was in place prior to recruitment commencing. After the first patient was recruited in each site, the senior data manager provided training on the eCRF. This training either took place as part of a visit to the site or was performed remotely using the standard operating procedure document.
On-site monitoring visits were not conducted routinely as this was a low-risk trial; they took place only if problems were identified (e.g. poor data collection or under-reporting of primary end point data) or when a site requested a visit to discuss specific issues (including data collection, screening patients, recruitment and staff training).
After the first five patients had been recruited and completed the 6-week follow-up, the CTU reviewed data from each site. If no problems were identified the sites were informed that there was no need for a site monitoring visit. Data validation was then conducted centrally by statistical monitoring across all sites. If problems were identified, the CTU would contact the relevant site to discuss the situation and arrange a site monitoring visit.
Central statistical monitoring was performed by the unblinded statistician by reviewing event rates, unusual trends and data anomalies.
Baseline assessment
At baseline all patients had a medical history taken and a clinical examination as part of usual care. Baseline information collected included weight, height, sex, ethnicity, blood pressure, heart rate, electrocardiogram (ECG), date of birth, euroSCORE, medication, levels of creatinine, troponin T, NGAL and biomarkers, 6MWT distance and European Quality of Life-5 Dimensions (EQ-5D) questionnaire; patients were also given a patient diary to record health resource use and an echocardiogram was obtained if they were part of the substudy.
Follow-up
All patients were followed up approximately 6 weeks post CABG. They were reviewed in clinic as per normal standard of care. Investigations performed at this visit were the 6MWT, creatinine level, ECG and EQ-5D questionnaire; the patient diary was also checked for health resource use.
At 3, 6 and 9 months post CABG all patients were requested to complete an EQ-5D questionnaire, which was carried out by post, telephone or e-mail.
At 1 year post CABG all patients were reviewed, when possible, in a research outpatient clinic. If they were unable to attend in person the follow-up was completed by telephone. General practitioner and hospital notes were checked for any events. At this visit, when possible, the following information was collected: weight, heart rate, blood pressure, recording of primary end points, ECG, creatinine level, echocardiogram (if part of the substudy), 6MWT distance and EQ-5D questionnaire; the patient diary was also checked for resource use.
Safety assessments
All untoward occurrences were termed adverse events rather than adverse reactions. Adverse events were assessed for relatedness to the intervention and were reported to the sponsor and the DMC. Safety assessments were collected from the time of randomisation to the completion of follow-up. There was a list of expected adverse events (both serious and non-serious) for which information was not collected. A detailed listing of individual adverse events was supplied as part of the reports to the DMC.
Outcome measures
Primary outcome
The primary study end point was the rate of MACCE consisting of cardiovascular death, MI, coronary revascularisation and stroke within 12 months of surgery.
Prespecified secondary outcomes were:
-
30-day MACCE
-
components of 30-day and 12-month MACCE
-
12-month MACCE including definite MIs and strokes only
-
death from all causes
-
72-hour area under the curve (AUC) troponin T
-
AKI injury score after 72 hours
-
serum creatinine level at baseline, 6 weeks and 12 months
-
24-hour AUC NGAL
-
length of ITU stay
-
inotrope score after 72 hours
-
length of hospital stay
-
6MWT at 6 weeks and 12 months
-
quality of life (EQ-5D) at 6 weeks and 3, 6, 9 and 12 months
-
substudy: ejection fraction at 12 months.
An additional secondary outcome was postoperative atrial fibrillation (AF), which was selected for analysis after finalising the statistical analysis plan and unblinding the trial data.
Collection of outcome data
Cardiovascular death included death resulting from an acute MI, sudden cardiac death, death from heart failure, death from stroke and death from other cardiovascular causes. This also included those deaths in which there was no clear non-cardiovascular cause. MI included perioperative MI (occurring within 72 hours of surgery) and postoperative MI (occurring >72 hours following surgery). Perioperative MI was defined as (1) troponin T level > 10 times the 99th percentile of the normal reference range (≥ 14 ng/ml) during the first 72 hours following surgery associated with the appearance of new pathological Q waves or new left bundle branch block or angiographically documented new graft or native coronary artery occlusion or imaging evidence of new loss of viable myocardium or (2) a troponin T level > 100 times the 99th percentile of the normal reference range during the first 72 hours following surgery (≥ 140 ng/ml) or (3) ECG evidence of perioperative MI in the absence of postsurgery troponin T results. Postoperative MI was defined as a MI occurring > 72 hours following the operation (definition of MI according to recent guidelines41).
Repeat coronary revascularisation was defined as repeat PCI or CABG of any segment of the coronary arteries. Stroke was defined as a focal neurological deficit of cerebrovascular cause that persisted beyond 24 hours or was interrupted by death within 24 hours.
Collection of major adverse cardiac and cerebrovascular events
Data were collected on the eCRF in relation to any events. An initial evaluation was made by the principal investigator (PI), or someone delegated by the PI, at each of the recruiting centres.
An independent Event Validation Committee (EVC) was set up consisting of a cardiologist (chairperson), surgeon and neurologist. All members were blinded to treatment allocation. Every effort was made to collect as much information as possible for assessing the events. A LSHTM-hosted secure file-sharing system was used to load the event data. Access to this was password protected and could be accessed at a time that suited each of the committee members. Quarterly conference calls were arranged to discuss adjudication of events and agree a final classification. In cases in which a unanimous decision could not be made the majority classification was used.
The following was used to classify each category.
Death
All members of the EVC reviewed descriptions of the circumstances surrounding the death, the certified cause of death (if available) and autopsy information (if available). The primary cause of death was classified using one of the following:
-
cardiovascular death
-
non-cardiovascular death
-
unknown.
Myocardial infarction
All ECGs submitted were reviewed for the presence of new Q waves and left bundle branch block. Troponin T or I results and CKMB level were all reviewed when available. The MI was then classified using one of the following:
-
perioperative MI
-
definite MI, which included the following categories: STEMI and MIs that are not definitive STEMI
-
probable MI
-
no evidence of MI.
Stroke
All members of the EVC reviewed data on clinical features, an assessment from the hospital where the event occurred, written reports of computerised tomography scans [if any doubt the imaging was requested on compact disc (CD)] and any other relevant information.
The stroke was then classified into one of the following:
-
definite ischaemic stroke
-
probable ischaemic stroke
-
haemorrhagic stroke
-
no evidence of stroke.
Revascularisation
For each repeat CABG and PCI recorded on the eCRF, information was collected on whether or not the procedure was completed as intended. For each revascularisation the following classification was used:
-
completed
-
not completed.
A further independent committee with two members was set up to review ECGs for all ERICCA patients to identify unreported PMIs.
Perioperative troponin T
The 72-hour AUC troponin T was assessed by measuring the serum high-sensitivity troponin T (hsTnT) preoperatively and at 6, 12, 24, 48 and 72 hours after coming off bypass. Six samples were collected per patient in 5-ml serum-separating tubes (SSTs) or the blood bottle used in the local hospital for measuring troponin T.
Quantitative hsTnT measurement was performed using a one-step immunoassay based on electrochemiluminescence technology (Elecsys 2010; Roche, Basel, Switzerland). Internal quality control was performed on a daily basis with external quality control every 4 weeks. Each hsTnT blood sample was labelled, centrifuged, divided into two samples, aliquoted, frozen at –20 °C and stored locally. Every quarter throughtout the recruitment period batches of samples were couriered from the centres to The Doctors Laboratory in London for analysis.
Inotrope score
The inotrope score provided an objective measurement of the requirement for inotropes in the immediate postoperative period. Data were collected from the daily medical chart in the ITU. The inotrope score was calculated at 0 (time when coming off bypass), 24, 48 and 72 hours after surgery. For time 0, the inotrope score was calculated from the dose of the individual inotrope used at the time of coming off bypass. For the 24-, 48- and 72-hour time points, the inotrope score was calculated from the maximum dose of the individual inotropes used in the previous 24 hours:
Creatinine and acute kidney injury grade
Creatinine was measured at baseline, daily over the 3-day perioperative period and at 6 weeks and 1 year post CABG. The AKI grade was calculated from the creatinine level measured at baseline during the 3-day perioperative period. Grades 1–3 were defined as follows:
-
a post-surgery rise of > 26.4 µmol/l or 150–200% of baseline
-
a post-surgery rise of 200–300% of baseline
-
a post-surgery increase of > 300% or post-surgery creatinine > 354 µmol/l associated with a rise from baseline of at least 44 µmol/l.
Neutrophil gelatinase-associated lipocalin
Neutrophil gelatinase-associated lipocalin in plasma/serum was measured at 6, 12 and 24 hours after coming off bypass using the NGAL Rapid ELISA Kit (Caltag Medsystems Ltd, Buckingham, UK).
Each NGAL blood sample was labelled and centrifuged and the plasma divided into two samples; these were then frozen and stored locally at –20 °C. During the recruitment period batches of samples were couriered quarterly to Caltag Medsystems for analysis.
Duration of intensive therapy unit and hospital stay
This was recorded for the initial hospital admission for cardiac surgery.
Six-minute walk test
The 6MWT was performed at baseline (in the surgical pre-admission clinic 2 weeks before surgery), at 6 weeks (at the outpatient clinic follow-up appointment) and at 1 year (at the research outpatient clinic follow-up appointment). Patients were instructed to walk as far as possible along a straight, flat hospital corridor in 6 minutes.
Quality of life
The EQ-5D three-level health-related quality of life questionnaire was used to assess patient quality of life post CABG at baseline (in the surgical pre-admission clinic 2 weeks prior to surgery). If an inpatient, this was collected prior to undergoing CABG. Patient quality of life was assessed again at 6 weeks (at the surgical outpatient clinic follow-up appointment), 3, 6 and 9 months (by telephone, e-mail or post) and 1 year (at the research outpatient clinic follow-up appointment or by telephone, e-mail or post if unable to attend in person). Health states from the EQ-5D were converted into health-related quality of life using UK-based utility weights. 42
Genetic and biomarker analysis
A 5-ml SST sample (to obtain serum for biomarker testing) and a 5-ml ethylenediaminetetra-acetic acid (EDTA) sample (to obtain plasma for biomarker testing and blood for genomic testing) were taken before RIPC (or sham RIPC) and immediately after RIPC (or sham RIPC). In the biochemistry or pathology laboratory, once the SST blood bottles had clotted the blood sample was centrifuged at 1300 rpm for 15 minutes and the resultant serum aliquoted into tubes. In the biochemistry or pathology laboratory the EDTA blood bottles were centrifuged at 1300 rpm for 15 minutes and the resultant plasma aliquoted into aliquot tubes and frozen at –20 °C. The EDTA blood tubes containing the residual blood were then frozen at –20 °C.
Every quarter throughout the recruitment period batches of samples were couriered to University College London (UCL) for analysis.
Left ventricular ejection fraction: echo substudy
An echo substudy had been planned to investigate the effect of RIPC on LVEF at 1 year compared with the control. However, because of logistical issues with recruitment, only 10 patients completed the baseline and follow-up scan and so this substudy was not completed.
Sample size
The planned sample size was 1610 patients. This was informed by the SYNTAX study, in which 12.4% of patients experienced MACCE at 12 months following CABG surgery. 3 However, the patients recruited into the SYNTAX study included lower-risk patients with a mean euroSCORE of 3.8 [standard deviation (SD) 2.7], whereas the patients in the ERICCA trial were higher-risk patients with a euroSCORE of ≥ 5. In another study of higher-risk patients who all had left main stem coronary lesions, by 12 months 25% had MACCE (which included some additional neurological criteria). 43 Therefore, for the higher-risk CABG with or without valve surgery patients in the ERICCA trial, the estimated MACCE rate was taken as 20% at 1 year, which means that to detect a 27% relative reduction in this primary end point in the RIPC-treated group (from 20.0% to 14.6%), with a power of 80% and a significance level of 5%, a sample size of 770 patients was required for each trial arm (1540 in total). To allow for dropouts (4.5% in the SYNTAX study) the sample size was increased to 1610 patients in total (805 patients each arm).
Statistical analysis
The primary analysis was conducted on an intention-to-treat (ITT) basis with all patients, when information was available, considered in the groups to which they were randomised. A per-protocol (PP) analysis was also undertaken including those who received the randomised intervention as specified and in whom CABG surgery was completed (i.e. including patients for whom all cycles of RIPC or sham RIPC were fully completed according to protocol and who received CABG surgery).
Primary outcome
The primary analysis was a comparison of the MACCE rate 1 year after CABG with or without valve surgery between the RIPC arm and the sham arm of the trial. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards modelling and Kaplan–Meier curves were produced. The time scale used for survival analysis was time since intervention. This date was taken as the date of RIPC/sham RIPC if this was attempted, the date of surgery if the RIPC/sham RIPC was not carried out and the date of randomisation if neither RIPC/sham RIPC nor surgery was carried out. Participants who did not experience MACCE were censored on the date of non-cardiovascular death, loss to follow-up or withdrawal from the study or at 12 months. The proportional hazard assumption underlying the Cox model was assessed graphically and through global test of the scaled Schoenfeld residuals. 44
Secondary outcomes
Thirty-day major adverse cardiac and cerebral events, components of 30-day and 12-month major adverse cardiac and cerebral events, definite major adverse cardiac and cerebral events and all-cause death at 12 months
Cox proportional hazards models were used to evaluate 30-day MACCE; components of 30-day and 12-month MACCE; and all-cause death at 12 months. Definitions of start of follow-up and censoring were as described for the analysis of the primary outcome.
Seventy-two-hour area under the curve troponin T
Differences in mean PMI (72-hour AUC troponin T) were assessed using a simple linear regression model for the natural logarithm of the AUC, with results expressed as a ratio of the geometric means. A log transform was used to provide a better approximation to the normal distribution as this outcome showed substantial skew.
Acute kidney injury score after 72 hours
Acute kidney injury grade was compared between the two groups using a non-parametric test for trend.
Inotrope score after 72 hours
Inotrope score was compared between groups using the non-parametric Wilcoxon rank sum test.
Serum creatinine level at baseline, 6 weeks and 12 months
Analysis of covariance (ANCOVA), adjusting for baseline values, was used to compare the RIPC arm and the sham arm in terms of the natural logarithm of serum creatinine at 6 weeks and 12 months. Serum creatinine was log transformed for analysis because it showed substantial skew.
Twenty-four-hour area under the curve neutrophil gelatinase-associated lipocalin
Differences in mean 24-hour AUC NGAL were assessed using a simple linear regression model for the natural logarithm of the AUC, with the results expressed as a ratio of the geometric means. A log transform was used to provide a better approximation to the normal distribution because of substantial skew.
Length of intensive therapy unit stay and hospital stay
Competing-risk proportional hazards models45 were used to compare groups with regard to hospital stay and ITU stay. These models were used to account for censoring because of the competing risk of death before discharge or end of ITU, respectively.
Six-minute walk test
A linear mixed model was used to compare the mean distance walked in the 6MWT between the RIPC arm and the sham arm at 6 weeks and 12 months. Distance walked at baseline was treated as an additional time point in the outcome model with treatment effect constrained to zero, making this analysis essentially equivalent to an analysis of covariance, adjusting for baseline.
Health status and self-rated health
Analysis of covariance, adjusting for baseline values, was used to compare the RIPC and sham arms with regard to mean health status and self-rated health at 6 weeks and 3, 6, 9 and 12 months. Health status and self-rated health showed substantial departures from a normal distribution that were still apparent after transformation, so inference was based on non-parametric, bias-corrected and accelerated bootstrap CIs, which were calculated from 2000 replications stratified by treatment group.
Post-surgery atrial fibrillation
Logistic regression was used to compare the RIPC and sham groups with regard to the proportion with postoperative AF, which was a post hoc outcome.
Missing data
Initial models used complete case analysis and so included only participants with full data, which assumes that outcomes are missing at random given the treatment group. For analysis of AUC troponin T and AUC NGAL this required participants to have data available for all time points, and for analysis of serum creatinine this required participants to have data available at baseline and at the relevant follow-up visit. As a large number of participants were excluded from the complete case analysis of these outcomes, further imputation analysis was conducted to examine whether or not these missing data had an impact on the findings.
Multiple imputation was used to replace any missing values of troponin T, NGAL and serum creatinine. Data were imputed on the natural logarithm scale for each time point using Gaussian normal regression multiple imputation by chained equations. Twenty imputed data sets were generated using a separate imputation model for troponin T, NGAL and serum creatinine. Variables collected at baseline or during follow-up were included in the imputation model in which they were independent predictors of a participant having missing data (assessed using logistic regression models) or were predictive of the values of the outcome (assessed using linear regression).
The imputation model for natural logarithm of troponin T included log of troponin T at each time point (baseline and 6, 12, 24, 48 and 72 hours); treatment group; sex; baseline euroSCORE, New York Heart Association (NYHA) class, Canadian Cardiovascular Society angina class, LVEF class, smoking status, age, natural logarithm of creatinine, diastolic blood pressure and heart rate; previous use of aspirin, beta-blockers, nitrates, diuretics, clopidogrel and metformin; previous diagnosis of MI, peripheral arterial disease and hypercholesterolaemia; bypass duration; use of cardioplegia during surgery; use of nitrates during surgery; number of grafts; post-surgical outcomes of number of days in ITU, requirement for cardiac pacing, renal failure and AKI; and death from cardiovascular causes within 12 months of surgery.
The imputation model for natural logarithm of NGAL included log of NGAL at each time point (baseline and 6, 12 and 24 hours); treatment group; sex; baseline euroSCORE, natural logarithm of creatinine, age and diastolic blood pressure; previous use of aspirin, nitrates, cholesterol-lowering medication, angiotensin-converting enzyme inhibitors/angiotensin receptor antagonists; previous diagnosis of diabetes; previous PCI; bypass duration; use of cardioplegia during surgery; valve repair/replacement; post-surgical outcomes of number of days in ITU, AF, renal failure and AKI; and death from cardiovascular causes within 12 months of surgery.
The imputation model for natural logarithm of serum creatinine included log of creatinine at each time point (baseline, 6 weeks and 12 months); treatment group; sex; baseline euroSCORE, NYHA class, LVEF class, age, heart rate, diastolic blood pressure and body mass index; previous use of aspirin, nitrates, diuretics, sulphonylureas, metformin and insulin; previous diagnosis of stroke; and post-surgical outcomes of AF and AKI.
The multiple imputation analysis for all outcomes excluded 15 participants who did not have any baseline data and one patient who was randomised but following enrolment was found not to meet the eligibility criteria for the trial. Analysis additionally excluded any patients who died before the outcome could have been collected: 16 patients for 72-hour AUC hsTnT; 11 patients for 24-hour AUC NGAL; 57 patients for creatinine at 6 weeks; and 125 patients for creatinine at 12 months. All other patients were included in the imputation analyses.
Subgroup analyses
Subgroup analysis were conducted to assess if the effect of RIPC on MACCE differed according to age, baseline euroSCORE, baseline LVEF, aortic cross-clamp time, cardiac bypass time, method of cardioplegia and presence/absence of diabetes. Each subgroup was assessed through a Wald test of the interaction between the subgroup variable and treatment group in the Cox proportional hazards model. The subgroup variable was entered as a continuous variable for age, euroSCORE, clamp time and bypass time and as a categorical variable for LVEF class (normal/good vs. moderate vs. poor) and diabetes (binary: yes vs. no). Two post hoc subgroup analyses were carried out for the time interval between the start of RIPC and the end of bypass (continuous) and the type of anaesthetic used during surgery (binary: volatiles with or without propofol vs. propofol with no use of volatiles). When subgroup variables were continuous we provide the numbers with events and HRs and 95% CIs for RIPC treatment among those above and those below the median level of the subgroup variable. Otherwise, results are given for each category of the subgroup.
Ethical considerations
Ethical approval for the study in the UK was given in October 2010 by the East London 3 Research Ethics Committee (reference number 10/H0701/111). The trial was registered with a National Clinical Trial number of NCT01247545. The trial had two committees overseeing its conduct: the Trial Steering Committee (TSC) and the Project Management Group. In addition, there was an independent DMC to ensure the safety of patients in the trial and review operational issues such as recruitment. The DMC was the only group to review interim analyses broken down by treatment groups during recruitment and follow-up of patients in the trial. The DMC performed interim safety analyses annually. The interim reports contained details of patient recruitment, demographic and baseline characteristics, the CABG surgery and intervention, primary safety end points, the primary efficacy end point and other end points identified by the DMC including adverse and serious adverse events (SAEs).
The TSC had overall responsibility for the scientific integrity and quality of the trial. This involved conduction of the trial to the standards set out in the guidelines for Good Clinical Practice,39 adherence to the protocol as far as possible and responsibility for overall patient safety as well as consideration of new relevant information arising throughout the duration of the trial. The TSC was also responsible for considering any recommendations made by the DMC. The TSC met annually throughout the ERICCA trial to monitor the progress and quality of the trial (review the recruitment rate and consider protocol amendments). The Project Management Group was responsible for the day-to-day running of the trial, meeting fortnightly during the setting up of the ERICCA trial and the early stages of recruitment and then approximately monthly for the remainder of the trial.
Patient and consumer involvement
Three consumers were actively involved as members of the TSC. The consumers were recruited from the rehabilitation group that was run at UCL for patients who had undergone CABG surgery. Although not all of them were able to attend all of the TSC meetings, there was always one representative of the three present. They all, however, contributed to and commented on any ERICCA literature, including the patient information sheet or newsletters.
One of the consumers was very actively involved in the trial and helped produce a training video on how to administer RIPC [see www.youtube.com/watch?v = SsX3atcc08M (accessed 5 June 2016)]. This was also available on digital versatile disc. This consumer was invited to the investigator meetings and was very active in discussing the trial.
Chapter 3 Results
Between April 2011 and March 2014, 1612 patients undergoing on-pump CABG (with or without valve) surgery were recruited to the ERICCA trial from 30 study sites. Figure 1 shows the number of patients randomised to each treatment arm along with the numbers completing the intervention and losses to follow-up. Detailed screening logs of 1 month’s duration were obtained for 3 separate months out of the 36-month recruitment period. Out of 1869 patients screened during the 3 months, 414 (22.2%) patients were found to be eligible for inclusion in the study, of whom 195 (47.1%) were randomised into the study. A euroSCORE of < 5 was the main reason for patient ineligibility.
FIGURE 1.
Consolidated Standards of Reporting Trial (CONSORT) diagram.
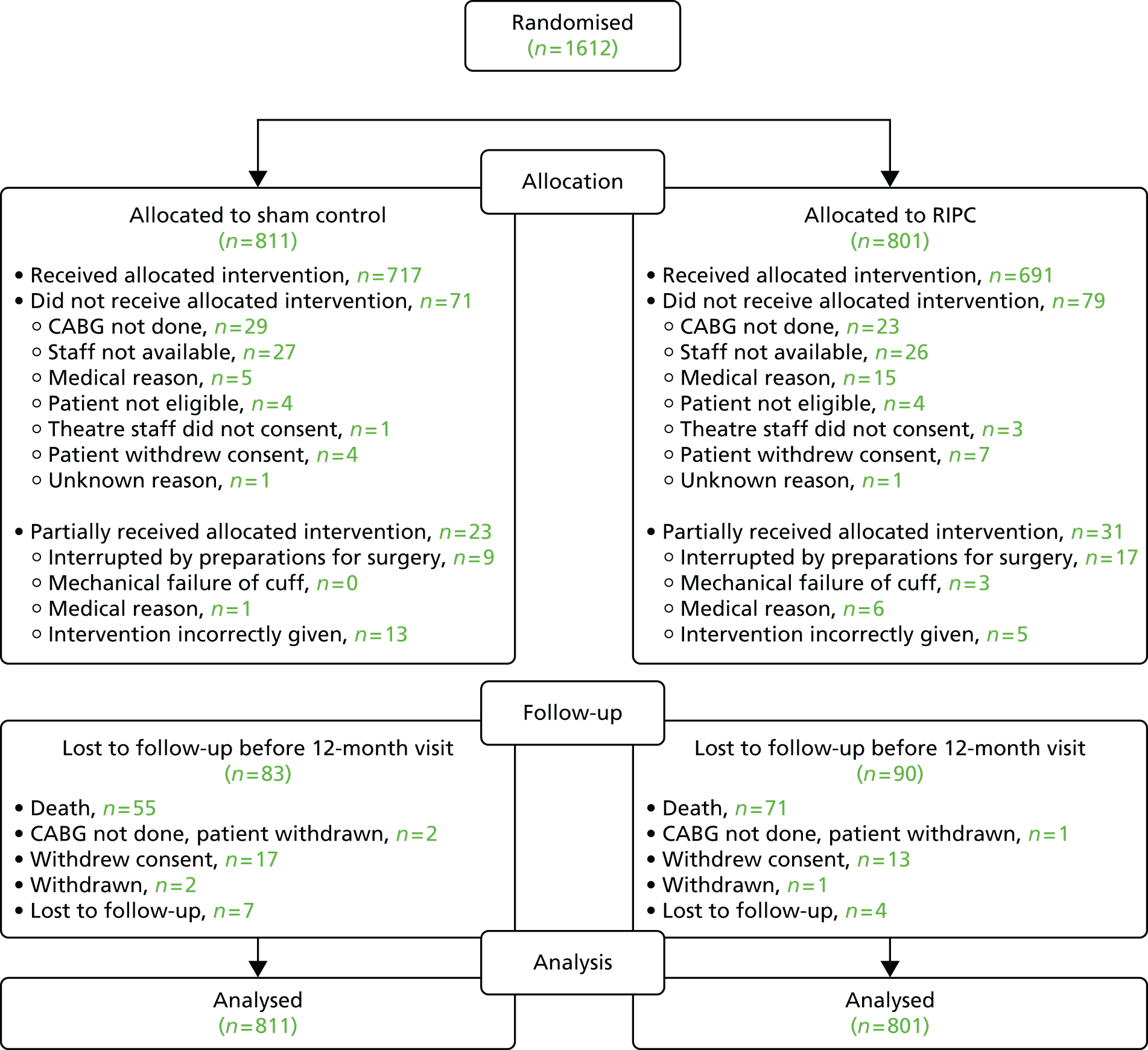
Patients attended a clinic visit at the time of randomisation and at 6 weeks and 12 months following surgery. Information on the primary outcome MACCE was collected during the first 12 months following surgery, with the last follow-up in May 2015.
The treatment groups were well balanced in respect of both patient baseline characteristics (Table 1) and surgical details (Table 2). Table 1 does not include data for all patients randomised in the study as some patients were withdrawn from the study; some patients did not have CABG surgery or the intervention performed; and some patients’ notes were unavailable or it was not possible to track the notes down or data were not recorded in the notes. Use of cardiovascular medications was similar in the two treatment groups at baseline, on discharge from hospital and at the 6-week and 12-month follow-up visits (Table 3).
| Characteristic | Sham control (N = 811) | RIPC (N = 801) |
|---|---|---|
| Male, n (%) | 586 (72.7) | 556 (70.4) |
| Age (years), mean (SD)b | 76.3 (7.0) | 76.1 (6.1) |
| euroSCORE, median (minimum–maximum) | 6 (5–16) | 6 (5–17) |
| BMI (kg/m2), mean (SD) | 27.5 (4.3) | 27.7 (4.6) |
| Smoking status (ex/current), n (%) | 525 (65.1) | 504 (63.8) |
| Creatinine (µmol/l), mean (SD) | 94.8 (35.2) | 94.3 (27.2) |
| Previous diagnoses, n (%)c | ||
| Diabetes | 211 (26.1) | 203 (25.7) |
| Hypercholesterolaemia | 555 (68.8) | 570 (72.2) |
| Hypertension | 599 (74.2) | 602 (76.2) |
| MI | 309 (38.3) | 328 (41.5) |
| Stroke | 89 (11.0) | 95 (12.0) |
| AF | 145 (18.0) | 117 (14.8) |
| Peripheral arterial disease | 62 (7.7) | 59 (7.5) |
| Previous PCI | 121 (15.0) | 115 (14.6) |
| Previous CABG | 19 (2.4) | 27 (3.4) |
| LVEF, n/N (%) | ||
| Good (> 50%) | 515/767 (67.1) | 497/741 (67.1) |
| Moderate (31–50%) | 158/767 (20.6) | 163/741 (22.0) |
| Poor (< 30%) | 94/767 (12.3) | 81/741 (10.9) |
| NYHA class, n (%) | ||
| No symptoms | 118 (14.6) | 121 (15.3) |
| I | 108 (13.4) | 108 (13.7) |
| II | 327 (40.6) | 336 (42.5) |
| III | 241 (29.9) | 208 (26.3) |
| IV | 12 (1.5) | 17 (2.2) |
| CCS angina class, n (%) | ||
| No symptoms | 262 (32.5) | 212 (26.8) |
| I | 142 (17.6) | 118 (14.9) |
| II | 223 (27.7) | 270 (34.2) |
| III | 128 (15.9) | 119 (15.1) |
| IV | 51 (6.3) | 71 (9.0) |
| Surgery | Sham control (N = 811) | RIPC (N = 801) |
|---|---|---|
| CABG surgery completed, n/N (%) | 776/805 (96.4) | 772/789 (97.8) |
| One graft | 178/776 (22.9) | 148/772 (19.2) |
| Two grafts | 193/776 (24.9) | 180/772 (23.3) |
| Three grafts | 295/776 (38.0) | 328/772 (42.5) |
| Four or more grafts | 109/776 (14.0) | 116/772 (15.0) |
| Valve surgery, n/N (%) | 406/775 (52.4) | 371/772 (48.1) |
| Cross-clamp time (minutes), median (minimum–maximum)a | 71 (15 to 292) | 69 (18 to 324) |
| CPB time (minutes), median (minimum–maximum)b | 107 (29 to 422) | 105 (34 to 585) |
| Time between start of RIPC and initiation of bypass (hours), mean (SD)c | 1.75 (0.64) | 1.72 (0.65) |
| Anaesthetic type, n (%)d | ||
| Volatile, without propofol | 11 (1.4) | 12 (1.6) |
| Volatile, with propofol | 312 (40.7) | 313 (40.7) |
| Propofol, without volatile | 397 (51.8) | 409 (53.2) |
| Other: no propofol or volatile | 47 (6.1) | 35 (4.6) |
| Perioperative agents, n (%)d | ||
| Atracurium | 23 (3.0) | 28 (3.6) |
| Cisatracurium | 3 (0.4) | 2 (0.3) |
| Desflurane | 1 (0.1) | 0 (0.0) |
| Diazepam | 11 (1.4) | 20 (2.6) |
| Lorazepam | 0 (0.0) | 1 (0.1) |
| Ketamine | 1 (0.1) | 0 (0.0) |
| Lignocaine | 0 (0.0) | 1 (0.1) |
| Nitrous oxide | 2 (0.3) | 1 (0.1) |
| Oxycodone | 1 (0.1) | 1 (0.1) |
| Pethidine | 2 (0.3) | 0 (0.0) |
| Remifentanil | 77 (10.0) | 82 (10.7) |
| Suxamethonium | 3 (0.4) | 2 (0.3) |
| Thiopentone | 20 (2.6) | 21 (2.7) |
| Tramadol | 1 (0.1) | 0 (0.0) |
| Fentanyl | 674 (87.9) | 668 (86.9) |
| Midazolam | 494 (64.4) | 494 (64.2) |
| Isoflurane | 310 (40.4) | 312 (40.6) |
| Morphine | 236 (30.8) | 241 (31.3) |
| Etomidate | 84 (11.0) | 85 (11.1) |
| Propofol | 709 (92.4) | 722 (93.9) |
| Pancuronium | 241 (31.4) | 252 (32.8) |
| Vecuronium | 46 (6.0) | 52 (6.8) |
| Glycopyrrolate | 29 (3.8) | 27 (3.5) |
| Paracurium | 10 (1.3) | 6 (0.8) |
| Rocuronium | 420 (54.8) | 410 (53.3) |
| Bevicuronium | 7 (0.9) | 1 (0.1) |
| Alfentanil | 52 (6.8) | 49 (6.4) |
| Sevoflurane | 18 (2.3) | 17 (2.2) |
| IV nitrates, n/N (%) | 230/775 (29.7) | 233/772 (30.2) |
| Medication | Sham control (N = 811) | RIPC (N = 801) |
|---|---|---|
| Baseline | ||
| n | 807 | 791 |
| Aspirin | 635 (78.7) | 629 (79.5) |
| Beta-blocker | 516 (63.9) | 515 (65.1) |
| Calcium channel blocker | 222 (27.5) | 239 (30.2) |
| Nitrates | 253 (31.4) | 255 (32.2) |
| Cholesterol-lowering agents | 698 (86.5) | 670 (84.7) |
| Statins | 652 (80.8) | 633 (80.0) |
| Other cholesterol-lowering agents | 7 (0.9) | 5 (0.6) |
| Both statins and other cholesterol-lowering agents | 6 (0.7) | 2 (0.3) |
| Unspecified | 33 (4.1) | 30 (3.8) |
| ACE inhibitor/AT2 antagonist | 473 (58.6) | 499 (63.1) |
| Insulin | 59 (7.3) | 50 (6.3) |
| Sulphonylurea | 49 (6.1) | 42 (5.3) |
| Metformin | 137 (17.0) | 135 (17.1) |
| Clopidogrel | 177 (21.9) | 208 (26.3) |
| Warfarin | 98 (12.1) | 89 (11.3) |
| Diuretics | 327 (40.5) | 290 (36.7) |
| At discharge from hospital | ||
| n | 784 | 766 |
| Aspirin | 698 (89.0) | 671 (87.6) |
| Beta-blocker | 592 (75.5) | 579 (75.6) |
| Calcium channel blocker | 103 (13.1) | 87 (11.4) |
| Nitrates | 7 (0.9) | 9 (1.2) |
| Cholesterol-lowering agents | 678 (86.5) | 677 (88.4) |
| Statins | 659 (84.1) | 667 (87.1) |
| Other cholesterol-lowering agents | 11 (1.4) | 5 (0.7) |
| Both statins and other cholesterol-lowering agents | 7 (0.9) | 5 (0.7) |
| Unspecified | 1 (0.1) | 0 (0.0) |
| ACE inhibitor/AT2 antagonist | 383 (48.9) | 380 (49.6) |
| Insulin | 50 (6.4) | 52 (6.8) |
| Sulphonylurea | 40 (5.1) | 31 (4.0) |
| Metformin | 118 (15.1) | 128 (16.7) |
| Clopidogrel | 168 (21.4) | 180 (23.5) |
| Warfarin | 168 (21.4) | 161 (21.0) |
| Diuretics | 492 (62.8) | 523 (68.3) |
| 6 weeks’ follow-up | ||
| n | 776 | 758 |
| Aspirin | 647 (83.4) | 629 (83.0) |
| Beta-blocker | 584 (75.3) | 559 (73.7) |
| Calcium channel blocker | 101 (13.0) | 92 (12.1) |
| Nitrates | 12 (1.5) | 19 (2.5) |
| Cholesterol-lowering agents | 658 (84.8) | 656 (86.5) |
| Statins | 643 (82.9) | 648 (85.5) |
| Other cholesterol-lowering agents | 9 (1.2) | 3 (0.4) |
| Both statins and other cholesterol-lowering agents | 6 (0.8) | 5 (0.7) |
| Unspecified | 0 (0.0) | 0 (0.0) |
| ACE inhibitor/AT2 antagonist | 398 (51.3) | 397 (52.4) |
| Insulin | 41 (5.3) | 48 (6.3) |
| Sulphonylurea | 36 (4.6) | 33 (4.4) |
| Metformin | 112 (14.4) | 121 (16.0) |
| Clopidogrel | 146 (18.8) | 154 (20.3) |
| Warfarin | 168 (21.6) | 162 (21.4) |
| Diuretics | 394 (50.8) | 414 (54.6) |
| 12 months’ follow-up | ||
| n | 742 | 719 |
| Aspirin | 566 (76.3) | 556 (77.3) |
| Beta-blocker | 531 (71.6) | 525 (73.0) |
| Calcium channel blocker | 119 (16.0) | 103 (14.3) |
| Nitrates | 28 (3.8) | 37 (5.1) |
| Cholesterol-lowering agents | 625 (84.2) | 615 (85.5) |
| Statins | 614 (82.7) | 598 (83.2) |
| Other cholesterol-lowering agents | 8 (1.1) | 8 (1.1) |
| Both statins and other cholesterol-lowering agents | 3 (0.4) | 5 (0.7) |
| Unspecified | 0 (0.0) | 4 (0.6) |
| ACE inhibitor/AT2 antagonist | 437 (58.9) | 422 (58.7) |
| Insulin | 40 (5.4) | 40 (5.6) |
| Sulphonylurea | 29 (3.9) | 28 (3.9) |
| Metformin | 107 (14.4) | 116 (16.1) |
| Clopidogrel | 102 (13.7) | 112 (15.6) |
| Warfarin | 141 (19.0) | 121 (16.8) |
| Diuretics | 283 (38.1) | 255 (35.5) |
The intervention was completed according to protocol for 716 (88%) of the sham control participants and 691 (86%) of the RIPC participants, with reasons for incomplete intervention given in Figure 1. The mean (SD) time between the start of RIPC and initiation of bypass was 1.75 hours (0.64 hours) in the sham control group and 1.72 hours (0.65 hours) in the RIPC group. There were very few participants lost to follow-up before 12 months: 28 (3%) losses to follow-up for reasons other than death in the sham control group and 19 (2%) in the RIPC group (see Figure 1). All 1612 patients were included in the analysis of the primary outcome.
Effect of remote ischaemic preconditioning on major adverse cardiac and cerebral events
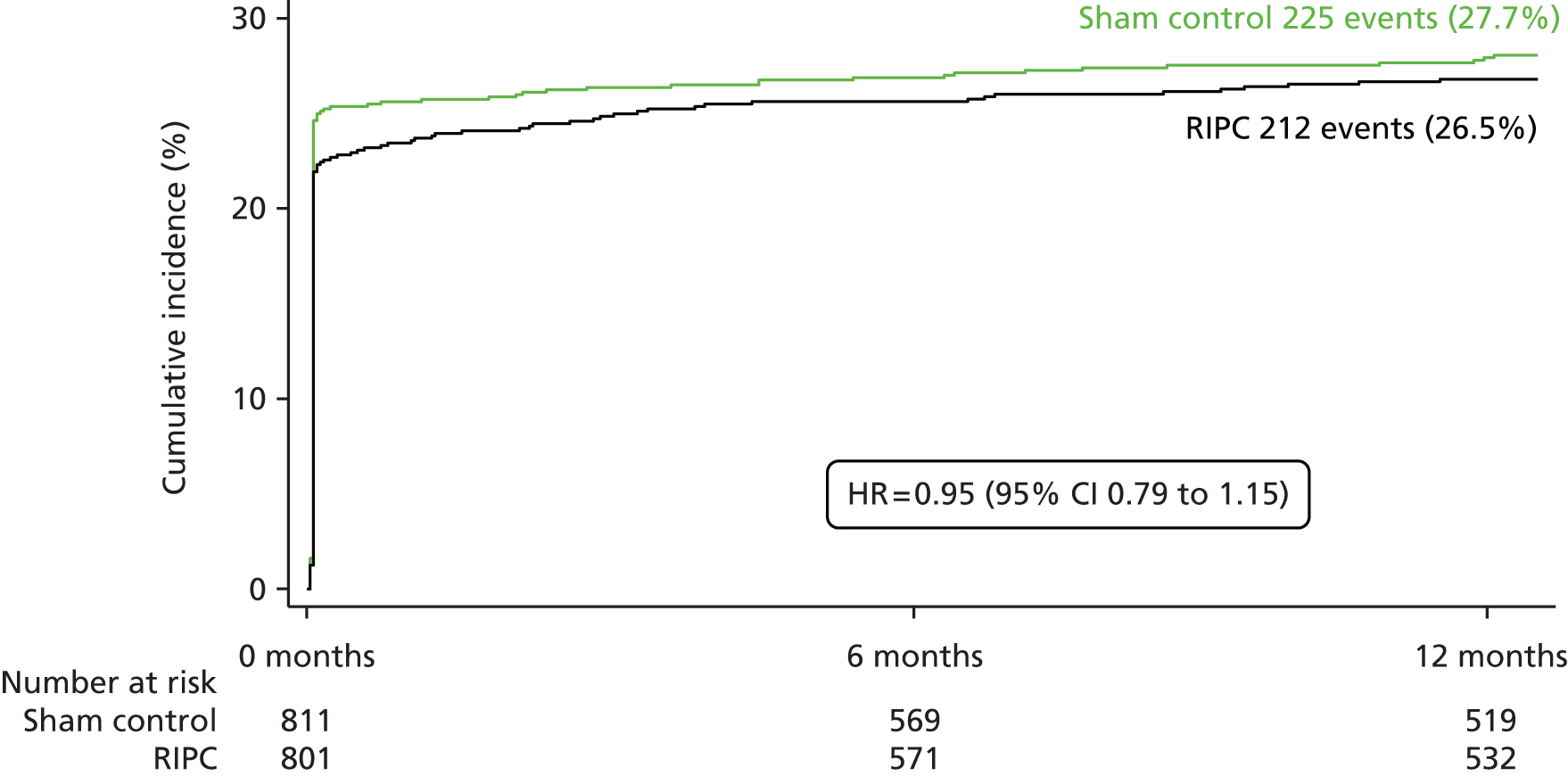
The proportion of participants with the MACCE primary end point within 12 months was similar between the groups [RIPC group 26.5% (n = 212) vs. control group 27.7% (n = 225); HR 0.95, 95% CI 0.79 to 1.15; p = 0.58)] (Table 4 and Figure 2). There was no evidence of a difference between the sham control group and the RIPC group in any of the individual components of MACCE (cardiovascular death, MI, stroke and coronary revascularisation) (see Table 4). The results of the PP analysis were very similar to those of the ITT analysis and showed little difference in the incidence of MACCE between the RIPC group and the sham control group. In the PP analysis 27.2% (n = 188/691) of participants in the RIPC group experienced MACCE within 12 months compared with 28.5% (n = 204/717) of participants in the sham control group (HR 0.95, 95% CI 0.78 to 1.16; p = 0.64) (Table 5).
| Outcome | n (%) with event in sham control group (N = 811) | n (%) with event in RIPC group (N = 801) | HR (sham control vs. RIPC) (95% CI) | p-value |
|---|---|---|---|---|
| Primary end point | ||||
| MACCE within 12 months | 225 (27.7) | 212 (26.5) | 0.95 (0.79 to 1.15) | 0.58 |
| Cardiovascular death | 32 (3.9) | 47 (5.9) | 1.50 (0.96 to 2.35) | 0.08 |
| MI | 191 (23.6) | 173 (21.6) | 0.91 (0.74 to 1.12) | 0.39 |
| Stroke | 16 (2.0) | 17 (2.1) | 1.08 (0.55 to 2.14) | 0.82 |
| Coronary revascularisation | 3 (0.4) | 2 (0.2) | 0.68 (0.11 to 4.09) | 0.68 |
| Secondary end points | ||||
| Definite MACCE within 12 monthsa | 93 (11.5) | 104 (13.0) | 1.13 (0.86 to 1.50) | 0.38 |
| MACCE within 30 days | 206 (25.4) | 186 (23.2) | 0.91 (0.75 to 1.11) | 0.36 |
| Cardiovascular death | 20 (2.5) | 24 (3.0) | 1.22 (0.67 to 2.20) | 0.52 |
| MI | 188 (23.2) | 168 (21.0) | 0.90 (0.73 to 1.11) | 0.34 |
| Stroke | 10 (1.2) | 10 (1.2) | 1.01 (0.42 to 2.44) | 0.98 |
| Coronary revascularisation | 0 (0) | 0 (0) | ||
| Death within 12 months | 54 (6.7) | 69 (8.6) | 1.31 (0.92 to 1.87) | 0.14 |
FIGURE 2.
Cumulative incidence of MACCE up to 12 months.
| Outcome | n (%) with event in sham control group (N = 717) | n (%) with event in RIPC group (N = 691) | HR (sham vs. RIPC) (95% CI) | p-value |
|---|---|---|---|---|
| Primary end point | ||||
| MACCE within 12 months | 204 (28.5) | 188 (27.2) | 0.95 (0.78 to 1.16) | 0.64 |
| Cardiovascular death | 26 (3.6) | 40 (5.8) | 1.61 (0.98 to 2.64) | 0.06 |
| MI | 177 (24.7) | 156 (22.6) | 0.91 (0.74 to 1.13) | 0.41 |
| Stroke | 14 (2.0) | 15 (2.2) | 1.12 (0.54 to 2.32) | 0.76 |
| Coronary revascularisation | 3 (0.4) | 2 (0.3) | 0.70 (0.12 to 4.18) | 0.70 |
| Secondary end points | ||||
| Definite MACCE within 12 monthsa | 83 (11.6) | 94 (13.6) | 1.18 (0.88 to 1.58) | 0.28 |
| MACCE within 30 days | 187 (26.1) | 168 (24.3) | 0.93 (0.76 to 1.15) | 0.50 |
| Cardiovascular death | 16 (2.2) | 22 (3.2) | 1.43 (0.75 to 2.73) | 0.27 |
| MI | 174 (24.3) | 153 (22.1) | 0.91 (0.73 to 1.13) | 0.41 |
| Stroke | 8 (1.1) | 9 (1.3) | 1.17 (0.45 to 3.04) | 0.74 |
| Coronary revascularisation | 0 (0.0) | 0 (0.0) | ||
Subgroup analysis for major adverse cardiac and cerebral events
There was no evidence that the effect of the RIPC intervention differed between any of the prespecified subgroup analyses for the incidence of MACCE at 12 months (Figure 3). Although a subgroup analysis was planned by type of cardioplegia, only 19 participants had retrograde cardioplegia and so this subgroup analysis was not conducted. The other post hoc subgroup analyses found no evidence that the effect of RIPC on MACCE differed by the type of anaesthetic used during surgery (p = 0.17 interaction test) or by the duration between the start of RIPC and the initiation of bypass (p = 0.66 interaction test).
FIGURE 3.
Prespecified subgroup analyses for the incidence of MACCE at 12 months. HRs are shown for subgroups of participants above/below the median value for each factor.

Effect of remote ischaemic preconditioning on secondary outcomes
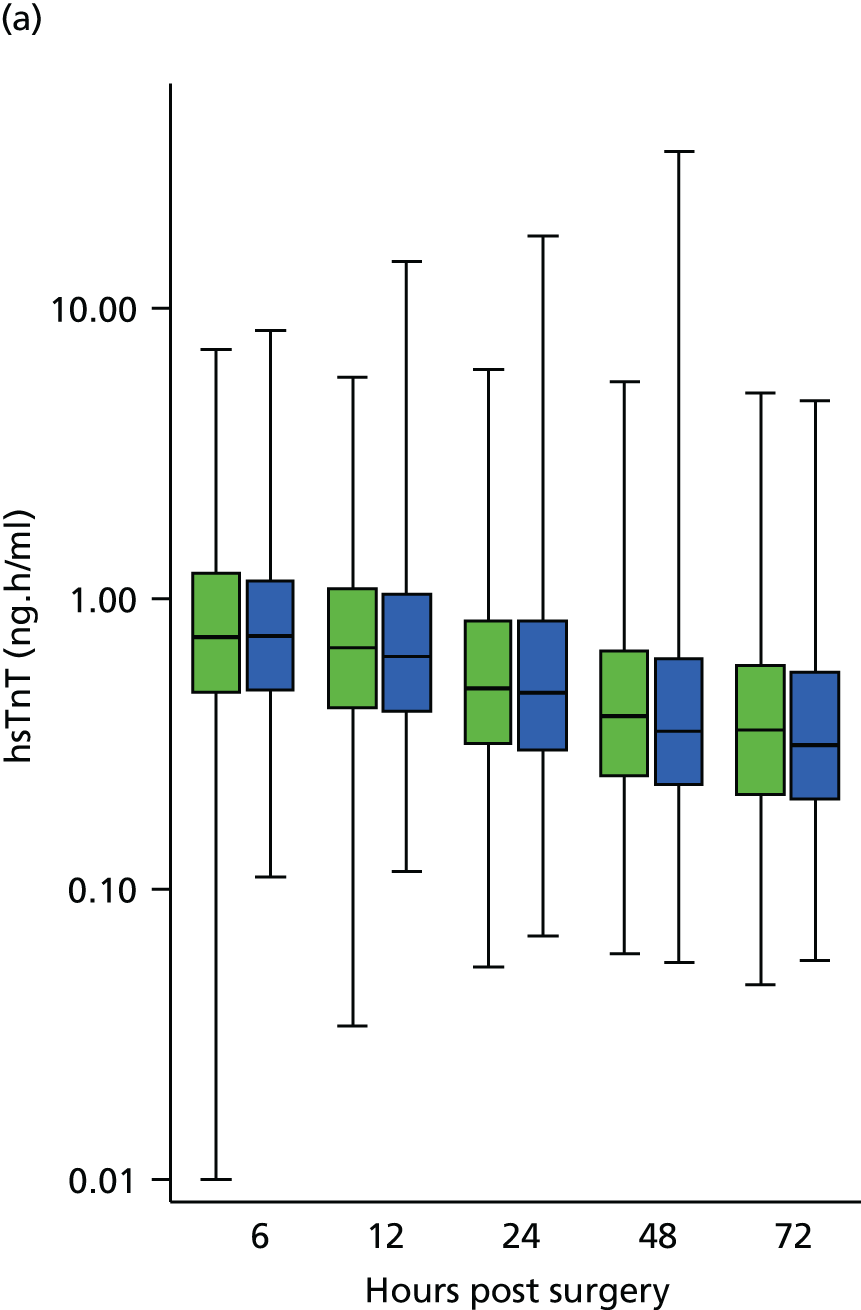
Complete case analysis of the 728 of 1612 patients (45%) with full data on PMI suggested that there was a 10% lower AUC hsTnT in patients undergoing RIPC than in sham control patients (Table 6). This effect largely disappeared when multiple imputation analyses were undertaken (2% reduction, 95% CI 9% reduction to 6% increase; p = 0.63). This lack of effect was supported by examination of the data for 1282 of 1612 patients (80%) who had at least one perioperative hsTnT result, which showed little difference at any time point between the RIPC group and the sham control group (Figure 4). Post hoc subgroup analyses found no evidence that the effect of RIPC on PMI differed by the type of anaesthetic used during surgery or by the duration between the start of RIPC and the initiation of bypass (Table 7).
| Outcome | Sham control (N = 811) | RIPC (N = 801) | Ratio of geometric means (sham control vs. RIPC) (95% CI) | p-value |
|---|---|---|---|---|
| 72-hour AUC hsTnT (ng.h/ml) | ||||
| Median (IQR) | 35.7 (22.8–57.3) | 30.1 (20.3–53.9) | ||
| Complete cases, n | 367 | 361 | 0.90 (0.81 to 0.99) | 0.031 |
| Multiple imputation, n | 798 | 782 | 0.98 (0.91 to 1.06) | 0.63 |
| Serum creatinine 6 weeks (µmol/l) | ||||
| Median (IQR) | 90 (76–109) | 92 (75–111) | ||
| Complete cases, n | 397 | 368 | 1.01 (0.98 to 1.04) | 0.64 |
| Multiple imputation, n | 782 | 757 | 1.01 (0.98 to 1.04) | 0.67 |
| Serum creatinine 12 months (µmol/l) | ||||
| Median (IQR) | 93 (81–113) | 91 (79–111) | ||
| Complete cases, n | 320 | 320 | 0.98 (0.95 to 1.01) | 0.26 |
| Multiple imputation, n | 752 | 719 | 0.97 (0.95 to 1.00) | 0.06 |
| 24-hour AUC NGAL (ng.h/ml) | ||||
| Median (IQR) | 7293 (5310–10,436) | 7148 (5318–10,389) | ||
| Complete cases, n | 541 | 544 | 0.99 (0.93 to 1.05) | 0.652 |
| Multiple imputation, n | 800 | 785 | 0.99 (0.94 to 1.05) | 0.785 |
FIGURE 4.
Box plot of the median, lower quartile, upper quartile, minimum and maximum for hsTnT at each time point for (a) all participants; and (b) participants with complete data. Sham control shown in green, RIPC in blue. Number of observations: (a) 1186 at 6 hours, 1171 at 12 hours, 1209 at 24 hours, 1082 at 48 hours and 937 at 72 hours; and (b) 728 at all time points.

| Subgroup | Sham control (N = 811) | RIPC (N = 801) | Ratio of geometric means (95% CI) | Interaction p-value |
|---|---|---|---|---|
| Anaesthetics, median (IQR) | ||||
| Volatile, with or without propofol | 35.7 (23.0 to 54.7) | 29.2 (19.1 to 51.5) | ||
| Propofol, without volatile | 35.9 (22.0 to 63.9) | 32.8 (22.4 to 58.3) | ||
| Complete case analysis, n | ||||
| Volatile, with or without propofol | 178 | 183 | 0.88 (0.77 to 1.02) | 0.728 |
| Propofol, without volatile | 156 | 161 | 0.92 (0.79 to 1.07) | |
| Multiple imputation analysis, na | ||||
| Volatile, with or without propofol | 321 | 320 | 0.94 (0.84 to 1.06) | 0.453 |
| Propofol, without volatile | 393 | 405 | 1.01 (0.90 to 1.13) | |
| Interval between start of RIPC and end of bypass (hours), median (IQR) | ||||
| 0.4 to < 1.7 | 36.0 (24.4–57.2) | 31.6 (21.7–53.9) | ||
| 1.7 to 8.4 | 35.7 (22.1–58.9) | 28.9 (19.5–55.2) | ||
| Complete case analysis, n | ||||
| 0.4 to < 1.7 | 169 | 177 | 0.98 (0.79 to 1.04) | 0.633 |
| 1.7 to 8.4 | 174 | 170 | 0.87 (0.76 to 1.01) | |
| Multiple imputation analysis, na | ||||
| 0.4 to < 1.7 | 355 | 359 | 1.01 (0.90 to 1.13) | 0.303 |
| 1.7 to 8.4 | 346 | 336 | 0.95 (0.85 to 1.06) | |
Participants in the RIPC arm had a further walk distance than sham control participants on the 6MWT at 12 months, although this finding should be interpreted with caution, as only 785 participants completed the 6MWT on one or more occasions (baseline, 6 weeks or 12 months) and, of these, only 360 participants completed the 6MWT at 12 months (Table 8). There was no evidence of any effect of RIPC on any of the other secondary end points (Tables 8–10). Although an echocardiography substudy had been planned, because of logistical issues very few patients were included in the study and so no meaningful data on LVEF were available for analysis.
| Outcome | Sham control (N = 811) | RIPC (N = 801) | Difference in means (95% CI) | p-value |
|---|---|---|---|---|
| 6MWT distance (metres), mean (SD) | ||||
| 6 weeks | 335 (125) | 332 (109) | –3.8 (–24.4 to 16.8) | 0.72 |
| 12 months | 365 (128) | 386 (116) | 23.3 (2.2 to 44.4) | 0.031 |
| n | 402 | 383 | ||
| EQ-5D health status, mean (SD) | NAa | |||
| 6 weeks | 0.74 (0.27) | 0.73 (0.29) | –0.02 (–0.053 to 0.006) | |
| n | 689 | 682 | ||
| 3 months | 0.78 (0.26) | 0.78 (0.27) | –0.01 (–0.037 to 0.015) | |
| n | 698 | 693 | ||
| 6 months | 0.78 (0.28) | 0.78 (0.30) | –0.01 (–0.036 to 0.021) | |
| n | 704 | 685 | ||
| 9 months | 0.78 (0.28) | 0.78 (0.30) | –0.01 (–0.035 to 0.023) | |
| n | 694 | 690 | ||
| 12 months | 0.77 (0.29) | 0.76 (0.31) | –0.03 (–0.056 to 0.004) | |
| n | 703 | 706 | ||
| EQ-5D thermometer self-rated health index, mean (SD) | NAa | |||
| 6 weeks | 72 (17) | 73 (17) | 0.6 (–1.1 to 2.3) | |
| n | 662 | 647 | ||
| 3 months | 76 (16) | 76 (16) | –0.3 (–1.8 to 1.5) | |
| n | 673 | 650 | ||
| 6 months | 78 (16) | 79 (16) | 0.9 (–0.6 to 2.6) | |
| n | 662 | 631 | ||
| 9 months | 79 (16) | 80 (15) | 0.6 (–1.1 to 2.2) | |
| n | 646 | 625 | ||
| 12 months | 80 (16) | 80 (16) | –0.4 (–2.1 to 1.3) | |
| Outcome | Sham control (N = 811) | RIPC (N = 801) | p-value |
|---|---|---|---|
| Inotrope score | |||
| Median (IQR) | 6 (0–16) | 6 (0–15) | 0.917 |
| n | 794 | 775 | |
| Hospital stay (days) | |||
| Median (IQR) | 10 (7–17) | 10 (7–16) | 0.363 |
| n | 775 | 758 | |
| ITU stay (days) | |||
| Median (IQR) | 3 (1–5) | 2 (1–4) | 0.346 |
| n | 775 | 758 | |
| AKI, n/N (%) | 293/772 (38.0) | 287/749 (38.3) | 0.975 |
| Grade 1 | 226/772 (29.3) | 230/749 (30.7) | |
| Grade 2 | 44/772 (5.7) | 38/749 (5.1) | |
| Grade 3 | 23/772 (3.0) | 19/749 (2.5) | |
| Outcome | n/N (%) with event in sham control group (N = 811) | n/N (%) with event in RIPC group (N = 801) | Odds ratio (sham vs. RIPC) (95% CI) | p-value |
|---|---|---|---|---|
| Postoperative AFa | 314/794 (39.5) | 305/779 (39.2) | 0.98 (0.80 to 1.20) | 0.873 |
A total of 1085 out of 1612 (67%) patients with full data on NGAL were included in the complete case analysis; of these, 1411 out of 1612 (88%) patients had at least one NGAL result, including pre-operative samples. The majority of patients had at least one perioperative result (1318/1612, 82%). The proportions with data were balanced between the treatment arms: 669 out of 811 (82%) patients in the sham control arm and 649 out of 801 (81%) in the RIPC arm. The number of results available at individual time points was between 1206 out of 1612 (75%) at 12 hours and 1356 out of 1612 (84%) preoperatively, with fairly similar numbers of patients with data in the two treatment arms at any given time point. Of the 779 participants with creatinine recorded at 6 weeks, nearly all (765/779, 98%) also had baseline data and so were included in the complete case analysis. Similarly, there were 651 participants with creatinine recorded at 12 months, and 640 out of 1612 (40%) had data available at both baseline and 12 months and so were included in the complete case analysis. As most patients with data at a given time point were included in the complete case analysis for creatinine, it is not meaningful to compare serum creatinine between those who were included and those who were excluded from this analysis. However, it can be seen that the patterns of creatinine at other time points was generally similar in those with and without any missing data. Values at 12 months were slightly higher in the control than RIPC arm in both the participants included in and those excluded from the 6 weeks’ complete case analysis. There was little difference between the RIPC and control arm in values at 6 weeks in both participants who were included and participants who were excluded from the 12 months’ analysis: 90 µmol/l control versus 89 µmol/l RIPC in those excluded from the complete case and 93 µmol/l control versus 93 µmol/l RIPC in those included in the complete case analysis.
Adverse events
The number of adverse events was similar between the RIPC group and the sham control group [364/801 (45%) vs. 354/811 (44%), respectively] (Table 11). More participants in the RIPC group than in the sham control group experienced skin petechiae at the time of the intervention [35/801 (4.4%) vs. 2/811 (0.2%), respectively], with no long-term consequences. Three unexpected adverse events occurred at the time of the RIPC/sham control intervention. Only one of these was thought to be related to the intervention – the blood pressure cuff used in the RIPC intervention remained inflated during surgery, but this had no long-term consequences. A similar proportion in the RIPC and sham control groups experienced adverse events at times other than during the RIPC/sham control intervention [349/801 (44%) vs. 353/811 (44%), respectively], but none of these was thought to be related to the intervention. Although there was a trend towards an increase in the rate of cardiovascular death in the RIPC group compared with the sham control group, this difference was not significant and the study was not powered to detect this individual end point.
| Adverse event | Sham control (n = 811) | RIPC (n = 801) |
|---|---|---|
| Any adverse event | 354 (43.6) | 364 (45.4) |
| Adverse event at time of RIPC/sham intervention | 3 (0.4) | 37 (4.6) |
| Unexpected adverse event | 1 (0.1) | 2 (0.2) |
| Skin petechiae | 2 (0.2) | 35 (4.4) |
| Adverse event during follow-up | 353 (43.5) | 349 (43.6) |
| Death | 55 (6.8) | 71 (8.9) |
| Hospital admission | 267 (32.9) | 257 (32.1) |
| Other reported adverse event | 88 (10.9) | 80 (10.0) |
Chapter 4 Discussion
In higher-risk patients undergoing CABG (with or without valve) surgery with blood cardioplegia, RIPC with transient arm ischaemia–reperfusion did not reduce MACCE (cardiovascular death, MI, revascularisation and stroke) at 12 months following surgery compared with a sham control procedure. Furthermore, RIPC had no effect on any of the major secondary end points.
Perioperative myocardial injury
Following cardiac surgery, the release of cardiac enzymes, including CKMB,23 troponin T24–26 and troponin I,27–29 has been associated with worse short- and long-term clinical outcomes with a subsequent impact on patient morbidity and mortality. One of the potential mechanisms underlying PMI during cardiac surgery is represented by acute ischaemia–reperfusion injury secondary to intermittent aortic cross-clamp, intermittent cross-clamp fibrillation or intermittent or continuous administration of cardioplegia. 46 In this regard, RIPC, describing the protection provided to an organ/tissue by a stimulus generated in a remote or distant organ/tissue subjected to transient ischaemia–reperfusion prior to prolonged ischaemia, offers a non-invasive strategy capable of reducing PMI in patients undergoing cardiac surgery and therefore potentially improving their morbidity and mortality.
Remote ischaemic preconditioning
The concept of RIPC was first introduced by Przyklenk et al. ,5 who found a significant reduction in MI size in dogs subjected to four 5-minute cycles of circumflex occlusion prior to 1 hour of sustained left anterior descending artery ischaemia. From this ‘intramyocardial’ application of ischaemic preconditioning (IPC), Birnbaum et al. 7 went on to demonstrate that ‘remote’ transient ischaemia in the hindlimb, applied with a partial occlusion of the femoral artery in conjunction with rapid pacing of the gastrocnemius muscle, could reduce MI size in rabbits. Subsequently, Kharbanda et al. 9 were the first to apply the concept of RIPC to healthy human volunteers by inducing transient non-invasive limb ischaemia with a simple blood pressure cuff applied to one arm and demonstrating improved endothelial function in the contralateral arm.
Numerous small randomised controlled trials (RCTs) have followed this pioneering discovery with often discordant outcomes: Cheung et al. 12 were the first to apply RIPC in a clinical setting, randomising 37 children undergoing elective corrective surgery for a congenital heart defect to either a control treatment or RIPC (four cycles of lower-limb ischaemia–reperfusion with a simple blood pressure cuff) and demonstrating decreased PMI, inotropic requirements and airway resistance in the preconditioned group. Similarly, Zhou et al. 47 showed that, in children undergoing surgical repair of a simple ventricular septal defect, RIPC (three 5-minute cycles of left upper-arm ischaemia–reperfusion 24 hours and 1 hour prior to surgery) attenuated the systemic inflammatory response as well as myocardial and pulmonary injury. Additionally, Pavione et al. 48 failed to demonstrate enhanced cardioprotection or a reduced postoperative inflammatory response with four 5-minute cycles of lower-limb ischaemia–reperfusion applied 24 hours prior to corrective paediatric surgery.
However, it was in the setting of adult CABG surgery that understandably RIPC found an extensive application.
Remote ischaemic preconditioning in coronary artery bypass graft surgery
Our research group was the first to demonstrate that RIPC had the potential to reduce PMI in adult patients undergoing elective CABG surgery. 13 In a pioneering single-blinded RCT13 involving 57 patients undergoing elective CABG surgery with either cardioplegia or intermittent cross clamp fibrillation and randomised to RIPC (three 5-minute cycles of inflation and deflation of a blood pressure cuff placed on the upper arm) or a control treatment (an uninflated blood pressure cuff placed on the upper arm for 30 minutes) we found that preconditioned subjects had a 43% reduction in troponin T release over the 72-hour perioperative period compared with control subjects. These findings were confirmed in a further study involving 45 non-diabetic patients undergoing elective CABG with or without valve surgery and receiving cold-blood cardioplegia alone,14 with RIPC (three 5-minute cycles of upper-arm ischaemia–reperfusion) significantly reducing the 72-hour AUC of troponin T by 42.4%. The same preconditioning stimulus was applied by Ali et al. 49 in a study including 100 patients undergoing elective CABG for two- or three-vessel CAD and similarly led to a significant reduction in postoperative CKMB levels.
The concept of RIPC in the context of elective CABG surgery was then extended to patients receiving antegrade cold crystalloid cardioplegia in two seminal studies by Thielmann et al. 50,51 In one of the studies50 non-diabetic patients with triple-vessel CAD subjected to three cycles of 5-minute transient upper-arm ischaemia sustained a significantly lessened PMI than control subjects, with a 44.5% reduction in the total 72-hour AUC of cardiac troponin I. In the other study,51 the largest proof-of-concept RCT on RIPC in cardiac surgery so far, the same preconditioning stimulus improved myocardial protection (ratio of RIPC to control for cardiac troponin I AUC was 0.83) and reduced the combined end point of all-cause mortality, major adverse cardiac and cerebrovascular events and repeat revascularisation.
However, recently, a number of studies have failed to demonstrate significant RIPC-induced cardioprotection. Within the context of crystalloid cardioplegia, Wagner et al. 52 showed only a small beneficial effect of RIPC applied 18 hours prior to elective CABG surgery with or without aortic valve replacement. Similarly, Lomivorotov et al. 53 did not find any statistically significant benefit of RIPC for PMI in patients undergoing CABG surgery with cold crystalloid cardioplegia. In a trial involving 162 patients undergoing CABG surgery, Rahman et al. 54 found no statistically significant difference in troponin T release, ECG changes, cardiac index, inotrope and vasoconstrictor requirements, renal impairment and lung injury between patients receiving a sham protocol and patients receiving a RIPC protocol (three 5-minute cycles of upper-limb ischaemia–reperfusion). However, importantly, the study included patients undergoing elective or urgent (post-acute coronary syndrome) CABG surgery and it is therefore possible that the beneficial effects of RIPC might have been attenuated by the previous acute event, which could have already ‘preconditioned’ the patients. Moreover, in this double-blinded study, patients were prepared and draped to obscure the visibility of both the blood pressure cuff placed around the upper arm and the one placed around a ‘dummy arm’ and, although the correct inflation was verified though the disappearance of a pulsatile signal on a pulse oximeter, it is still possible that during the inflation and deflation phases the cuff might have moved and the RIPC stimulus might not have been delivered correctly. Third, and importantly, a significant proportion of these patients received glyceryl trinitrate (GTN) intraoperatively, which might have interfered with the cardioprotection provided by RIPC. Subsequently, Young et al. 55 failed to demonstrate that a standard preconditioning stimulus could improve PMI, AKI incidence or inotrope requirement in a study enrolling 96 patients undergoing high-risk cardiac surgery, including combined CABG and valve surgery, CABG surgery with a LVEF of < 50%, ‘redo-operation’, mitral valve surgery and double or triple valve surgery.
Additionally, Karuppasamy et al. 56 showed no beneficial effects of standard RIPC in patients undergoing elective CABG surgery and receiving the volatile anaesthetic isoflurane before cardiopulmonary bypass and the intravenous anaesthetic propofol thereafter until the completion of surgery. Two other major clinical studies used a strict anaesthetic regime with similarly no significant impact of RIPC on PMI. 57,58 Furthermore, in a proof-of-concept study involving off-pump CABG surgery,59 RIPC induced by four cycles of 5-minute upper-limb ischaemia–reperfusion reduced the total cardiac troponin I AUC by 26%, which did not reach statistical significance. However, the same group found that the combination of RIPC with remote ischaemic postconditioning (RIPostC) in an analogous surgical setting [the same stimulus was applied twice, immediately after anaesthesia induction (RIPC) and just after completion of anastomoses (RIPostC)] could lead to a significant cardiac troponin I AUC reduction in the preconditioned group. 60 Crucially, our research group has more recently been able to demonstrate that an enhanced preconditioning stimulus, consisting of simultaneous multilimb RIPC, was able to reduce PMI and improve short-term clinical outcomes in patients undergoing elective cardiac surgery. Additionally, a number of systematic reviews investigating the effects of RIPC on PMI with or without clinical outcomes in patients undergoing cardiac or vascular surgery or elective PCI have been conducted,61,62 concluding that RIPC reduces post-procedure myocardial damage in these subjects but does not impact on their clinical outcomes.
It is also crucial to note that a significant part of the studies investigating the effects of RIPC on clinical outcomes in patients undergoing elective cardiac surgery included surrogate end points such as biochemical assessments of PMI through serial evaluation of serum levels of cardiac enzymes, yet very few data are available in the literature with regard to the potential beneficial effects of RIPC on clinical outcomes. The very first study to describe the impact of RIPC on patient morbidity and mortality in the context of cardiac surgery reported no postoperative deaths in either the preconditioned group or the control group 30 days after elective aortic valve replacement, mitral valve surgery or double valve replacement. 63 Similarly, no significant difference in MACCE was found 30 days postoperatively in two small studies involving patients undergoing elective CABG surgery with crystalloid cardioplegia. 50,53 Interestingly, in a study including high-risk patients,55 again no difference in mortality rate was found at 30 days post surgery; however, an improvement in NYHA functional status and mean LVEF at 3 months postoperatively was found in preconditioned patients undergoing elective valve replacement. 64 Additionally, in patients undergoing CABG surgery under a strict anaesthetic regime,58 RIPC was associated with a higher perioperative composite end point of new arrhythmias and new MI, yet no significant difference was found at 6 months’ follow-up.
Crucially, in the largest proof-of-concept clinical trial to date investigating the effects of RIPC in the context of elective CABG surgery, unpublished at the time of the initiation of the ERICCA trial, Thielmann et al. 65 reported a statistically significant improvement in all-cause mortality and MACCE rate in preconditioned patients at 1 year and at completion of follow-up (1.54 years ± 1.22 years), which was mainly driven by the reduced incidence of new MIs, whereas no significant difference was found in the occurrence of cardiac death, stroke and repeat revascularisation. Interestingly, of the 329 patients randomised and included in the ITT analysis, 71 were excluded in the final PP analysis, of whom 61 had known diabetes and, therefore, a total of 258 subjects were included in the PP analysis. However, the study was a single-centre trial and adequately powered for the primary end point of PMI but not for secondary end points, including clinical outcomes, and, crucially, despite still lower, the rate of all-cause mortality became non-statistically significant when deaths from sepsis were excluded. In our single-centre RCT, we could not demonstrate that simultaneous multilimb preconditioning reduces the rate of death, MI, revascularisation and stroke at 6 weeks post cardiac surgery, a finding that was also confirmed in the subsequent subgroup analyses; however, once again the study was not powered for this type of evaluation. 66 In addition, a significant number of systematic reviews and meta-analyses in patients undergoing cardiac or vascular surgery or elective PCI have been conducted;61,62,67–76 the overall conclusions confirmed the beneficial effects of RIPC on PMI reduction, but no statistically significant improvements in clinical outcomes were observed, including rate of death, perioperative MI, renal failure, stroke, mesenteric ischaemia or hospital or ITU stay.
Two recently published and adequately powered clinical trials in cardiac surgery failed to demonstrate any beneficial effect of RIPC on inpatient clinical outcomes in 299 paediatric patients (primary end point of postoperative hospital stay)77 and 1280 adult patients (combined primary end point of death, MI, arrhythmia, stroke, coma, renal failure, respiratory failure, cardiogenic shock, gastrointestinal complication and multiorgan failure),78 although the latter study used a combined remote pre- and post-conditioning stimulus.
In our ERICCA trial we demonstrated that four 5-minute cycles of upper-arm ischaemia–reperfusion were unable to improve clinical outcomes at 1 year in higher-risk patients undergoing elective CABG with or without valve surgery using blood cardioplegia. Multiple potential factors can be identified in this regard, which can be divided into patient characteristics, clinical settings, anaesthetic regimes and other agents administered in the perioperative period.
Patient characteristics involve baseline factors such as age and presence of comorbidities. More recently, the risk profile of patients undergoing cardiac surgery has substantially changed because of the ageing population. 79 The response of ageing myocardium to cardioprotection provided by IPC, RIPC and RIPostC, as well as by pharmacological agents including opioids, remains controversial. 80 Moreover, it has been established that the presence of comorbidities such as diabetes, hypertension and dyslipidaemia may interfere with cardioprotection. Experimental studies have shown that the diabetic myocardium may have an increased resistance to ischaemia–reperfusion injury compared with the non-diabetic heart, although significant differences in results have been obtained from different animal models and with different techniques of diabetes induction (reviewed by Galagudza et al. 81). Intriguingly, cardioprotection provided by IPC may be lost in ageing hypertensive hearts and discordant results have been obtained from experimental and human studies evaluating the effects of dyslipidaemia on myocardial ischaemia–reperfusion injury and more importantly on its impact on IPC, RIPC and RIPostC. 82
In addition, pharmacological agents administered concomitantly with cardiac surgery may have a significant impact on cardioprotection achieved with RIPC, in particular anaesthetic drugs and intravenous nitrates. GTN, a nitric oxide donor widely used in the context of cardiac surgery to achieve rapid blood pressure control and coronary vasodilatation,83 has been demonstrated to interfere with IPC and RIPC in experimental studies; however, its role in the clinical setting is yet to be clarified. 84–95 Inhaled anaesthetics have been shown to provide myocardial protection in patients undergoing cardiac surgery,58,96–98 either used alone or in combination with propofol. 58,59 However, the use of propofol alone does not lead to cardioprotection57 and this is probably because of the lack of action on adenosine triphosphate (ATP)-sensitive potassium channels and its interference with reactive oxygen species, which have both been implicated in mechanisms underlying RIPC. 57 It is therefore possible that inhaled anaesthetics, either alone or in combination with propofol, are capable of reaching the necessary threshold to induce cardioprotection and that the addition of RIPC may not provide any further benefit. In our trial, > 90% of patients received propofol at some point during surgery.
The clinical setting is another crucial aspect potentially able to interfere with cardioprotection. Patients undergoing elective CABG surgery sustain an overall small magnitude of PMI compared with that observed in patients presenting with STEMI. 99 Moreover, preclinical investigations of RIPC have been largely confined to experimental models of acute coronary artery occlusion/reflow (which represent the in vitro model of STEMI), rather than more clinically relevant animal models of cardiopulmonary bypass surgery, highlighting the discordancy between experimental and clinical cardioprotection studies. 57,80,100
Crucially, given the recent developments of treatment options in CAD and, more importantly, the advances in operative methods of myocardial preservation, surgical techniques and anaesthetic agents, it is possible that the additional benefit provided to these patients by RIPC might not be significant or identifiable with the current strategies.
It is also important to note that in our trial there was a suggestion of an increased risk of cardiovascular death with RIPC. However, our study was not powered to detect a difference in this individual end point and this finding should therefore be interpreted with caution. Crucially, the overwhelming majority of previously published studies51,77 and meta-analyses75,101,102 have failed to demonstrate any harmful effects of RIPC during cardiac bypass surgery in terms of mortality and, in fact, one recent study showed a lower all-cause mortality rate with RIPC. 51
Our multicentre study had several potential limitations.
-
We did not standardise pre-/perioperative anaesthesia and medication, although this was because we wanted to reflect current clinical practice in cardiac bypass surgery as much as possible.
-
The number of missing and incomplete data for some of the secondary end points.
-
Although an echocardiography substudy had been planned, because of logistical issues very few patients were included in the study and so no meaningful data on left ventricular function were available for analysis.
Chapter 5 Conclusions
We conducted a multicentre, double-blind, randomised sham controlled trial in higher-risk adult patients undergoing on-pump CABG with or without valve surgery with blood cardioplegia. RIPC, consisting of four 5-minute cycles of ischaemia–reperfusion of the upper arm, did not provide evidence of any beneficial effects on the combined primary end point of cardiovascular death, MI, coronary revascularisation and stroke at 12 months. We also found no difference in any of the secondary end points or in any subgroup analyses.
However, RIPC may still be beneficial in other settings of acute myocardial ischaemia–reperfusion injury such as in STEMI patients treated with primary PCI, in whom the magnitude of myocardial injury is substantially greater than for cardiac surgery. The CONDI2 (Effect of RIC on Clinical Outcomes in STEMI Patients Undergoing pPCI)/ERIC-PPCI (Effect of Remote Ischaemic Conditioning on Clinical Outcomes in STEMI Patients Undergoing PPCI) trial will investigate the effect of RIPC on major clinical outcomes in this patient group. Furthermore, RIPC may have a role to play in organ transplantation, another setting of acute ischaemia–reperfusion injury – the recently completed REPAIR (REmote preconditioning for Protection Against Ischaemia–Reperfusion in renal transplantation) trial found that RIPC using transient arm ischaemia–reperfusion preserved renal graft function at 12 months following renal transplantation.
It is therefore important that studies continue to investigate the potential mechanisms underlying RIPC, as this may facilitate the translation of this simple, non-invasive, low-cost intervention into patient benefit.
Acknowledgements
Part of this work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, of which Derek M Yellon is a senior investigator.
Trial Steering Committee: David Taggart (chairperson), Derek Hausenloy (chief investigator), Derek M Yellon, John Pepper, Gudrun Kunst (investigators), Richard Dunker, Derek Diamond, Peter Blackburn (consumers), Mike Marber, Chris Laing, Pablo Perel (independents), Tim Clayton (CTU), Luciano Candilio, Rosemary Knight and Richard Evans (observers).
Data Monitoring Committee: Rajesh Kharbanda (chairperson), Adrian Marchbank, Joan Morris and Jennifer Nicholas (CTU statistician; previous statistical support provided by Cono Ariti).
Project Management Group: Derek Hausenloy (chief investigator), Luciano Candilio, Rosemary Knight, Tim Clayton, Steven Robertson and Richard Evans.
Study design and support: David Jenkins, Shyam Kolvekar and Maria Xenou.
Statistician: Jennifer Nicholas.
Randomisation service: Sealed Envelope.
Electrocardiogram analysis: Dr Heerajnarain Bulluck and Dr Stefania Rosmini, UCLH Heart Hospital, London.
Blood sample analysis: Caltag Medsystems, The Doctors Laboratory.
Monograph preparation: Rebecca Chu.
Contributions of authors
Derek J Hausenloy was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Luciano Candilio was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Richard Evans was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
David P Jenkins was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Shyamsunder Kolvekar was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Rosemary Knight was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Gudrun Kunst was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Christopher Laing was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Jennifer M Nicholas was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
John Pepper was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Steven Robertson was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Maria Xenou was responsible for the conception or design of the study, data collection, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Timothy Clayton was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Derek M Yellon was responsible for the conception or design of the study, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published.
Contributing sites
Royal Brompton Hospital, London (118 patients, recruitment started 26 September 2011): John Pepper (PI), Jean Booth, Neel Chawla, Anthony Desouza, Jackie Donovan, Rob Gonella, Alison Goss, Hannah Morgan, Trevor Morgan, Carolina Relvas, Viola Roesler, Ian Saunders, Rebecca Savoury, Manju Shah, Olaf Stanger, Richard Trimlett, ShuFang Wang and Mark Yates.
University College London Hospitals Heart Hospital, London (157 patients, recruitment started 12 April 2011): Shyam Kolvekar (PI), Mathew Barnard, Luciano Candilio, Alvin Cortez, Carmelo Di Salvo, Claire Jarvis, David Lawrence, Abdul Malik, Bruce Martin, Myra O’Donovan, Neil Roberts, Natasha Shand, Vivek Sivaraman, Andy Smith, Ajay Suri, Shana Tehrani, Louise Warren, Steven White and Maria Xenou.
King’s College Hospital, London (68 patients, recruitment started 25 May 2011): Jatin Desai (PI), Michelle Andrews, Rhonda Bristol, Jonathan Breeze, Tracy Dew, Anna Eka, Vera Hogervorst, Joanne Jessup, Minija Joseph, Gudrun Kunst, Sheetal Patale, Tracey Preston, Kiran Salaunkey, Ava Williams and Rachel Williams.
Papworth Hospital, Cambridge (182 patients, recruitment started 19 September 2011): David Jenkins (PI), Yasir Abuomar, Mark Bennett, Pedro Catarino, Max Codispoti, Theresa Dean, Suzy Deeley, John Dunning, Stephen Fakelman, Lorraine Fingleton, Carol Freeman, Alan Hacking, Thomas Howlett, Christine Kelly, Annette Kenny, Emma King, Steve Large, Rachel Lowry, Karen Marston, Louise Myszko, Suku Nair, Sam Nashef, Choo Ng, Martin Pooley, Dawn Salisbury, Catherine Sudarshan and Sarah Woods.
Hammersmith Hospital, London (33 patients, recruitment started 3 August 2011): Prakash Punjabi (PI), KM John Chan, Thilanga Iddamalgoda, Antanas Macys, Marco Moscarelli, Imelda Munro, Clare Pengelley, Ahmed Abdel Salam, Jennifer Scriven and Joanna Smee.
St George’s Hospital, London (four patients, recruitment started 24 July 2012): Marjan Jahangiri (PI), Lisa Clutterbuck, Emma Cousins, Sandra Day, Beverly Edwards, Hazel Nyamajiyah, Michelle Rajab and Melody Smith.
St Thomas’ Hospital, London (73 patients, recruitment started 22 June 2011): Chris Blauth (PI), Lucy Clack, Susan Clark, Jayne Damm, Sophie Jones, Victoria Russell, Nicola Straiton, Yvonne Taylor and Karen Wilson.
London Chest Hospital (70 patients, recruitment started 6 February 2012): Rakesh Uppal (PI), Mervyn Andiapen, Kamran Baig, Catherine Barrett, Pat Brookman, Sheik Dowlut, Harrietta Habada, Julia Lungley, Claire Martin, Mary Prossora and Jessry Veerapen.
Basildon and Thurrock University Hospitals NHS Foundation Trust, Basildon (124 patients, recruitment started 3 June 2011) Inderpaul Birdi (PI), Rashidat Adeniba, Kezia Allen, Carol Alves, Daniel Alves, Marco Bondoc, Georgina Butt, Jean Byrne, Hazel Chitty, Natasha Christmas, Lesley Cruse, Kerry Goodsell, Jesusa Guinto, Bernard Hadebe, Ashraf Hamarneh, James Hampton-Till, Pille Harding, Karen Hayden, Raiji Koothoor, Claire McCormick, Maxwell Masuku, Nhlanhla Mguni, Sukoluhle Moyo, Stacey Pepper, Alice Pilcher, Emily Redman, Andrew Ritchie, Annaliza Sevillano, Amanda Solesbury, Romona Theodule, Joseph Tidey, Sifelani Tshumi and Irene Udeozor.
Royal Sussex County Hospital, Brighton (33 patients, recruitment started 4 October 2011): Uday Trivedi (PI), Lorraine Bennett, Andrew Cohen, Lucy Cooper, Nina Cooter, Cheryl Cowling, Fraser Duncan, John Duncliffe, Andrew Hill, Nevil Hutchinson, Jonathan Hyde, Elaine Joyce, Robert Kong, Michael Lewis, Marco Maccario, Ailie MacKenzie, Andrew McGregor, Mark Millar, Emily Peasgood, Emlyn Roberts, Lisa Roberts, Nicola Skipper and Emma Wickham.
Golden Jubilee National Hospital, Clydebank (76 patients, recruitment started 13 October 2011): Geoff Berg (PI), Christine Aitken, Elizabeth Boyd, Joanne Kelly, Maureen Mason and Rachel Small.
Harefield Hospital, Middlesex (two patients, recruitment started 6 February 2012): Andre Simon (PI), Neal Dolphin, Mel Gowen, Craig Hughes and Suad Warsama.
Edinburgh Royal Infirmary (35 patients, recruitment started 19 January 2012): Renzo Pessotto (PI), Jane Crowe, Lisa Derr, Sam Donaldson, Molly De Haan, Amanda Fairbairn, Antonella Ferrara, Liz Fraser, Vincenzo Giordano, Margaret Laird, Vikki Leslie, Lucy Marshall, Mary Morrissey, Hilary Nailon and Maria Van Dalen.
Royal Wolverhampton Hospitals NHS Trust (24 patients, recruitment started 22 March 2012): Heyman Luckraz (PI), Moninder Bhabra, Felicity Dean-Goodridge, Emma Greatbatch, Emma Jarvis, Sharon Kempson, Stella Metherell, Lawrence Phiri and Andrew Smallwood.
Bristol Royal Infirmary (19 patients, recruitment started 29 July 2013): Giovanni Angelini (PI), Lucy Culliford, Jonathan Evans, Athanasia Gravani, Emma Hopkins, Penny Lambert, Kayla Painter, Kate Rajakaruna, Amy Treagus, Wendy Underwood, Maria Wahab, Jenny Wilcox, Kim Wright and Rachel Wyatt.
Manchester Royal Infirmary (63 patients, recruitment started 7 December 2011): Daniel Keenan (PI), Gillian Cummings-Fosong, Lesley Doyle, Suzanne Elwood, Sarah Evans, Heather Iles-Smith, Shylet Kanyama, Kirsty Maciver, Seamus McLoughlin, Niall O’Keeffe, Wendy Osborne, Karen Palmer, Andreas Paschalis, Hannah Phillips (née Swift), Bradley Tallon and Sharon Williams.
Abertawe Bro Morgannwg University Health Board, Morriston Hospital, Swansea (25 patients, recruitment started 6 February 2012): Aprim Youhana (PI), Natalie Blytt-Jordens, Alun Scott Davies, Claire Fagan, Jane Griffiths, Christine Jones, Rosemarie Morgan, Jillian Scott and Rebeccah Thomas.
Cardiff and Vale University Health Board, Cardiff (seven patients, recruitment started 24 September 2012): Dheeraj Mehta (PI), Delyth Braim, Sian James, Anish John, Ana Lopez Marco, Anna Luen, Sita Rania Rao Podila, Rajani Rajnish, Jitendra Rathod, Tracey Roberts and Abby Waters.
Derriford Hospital, Plymouth (76 patients, recruitment started 23 November 2011): Jonathan Unsworth-White (PI), Julie Alderton, Louise Barrett, Paula Brockman, Claire Brown, Wendy Colwell, Clinton Lloyd, Sue Olver, Maxine Pearse, Nikki Persad, Tania Riches, Carolyn Stewart, Kate Tantam, Darren Waugh and Rob Wosley.
Northern General Hospital, Sheffield (22 patients, recruitment started 31 July 2012): Norman Briffa (PI), Michael Agyemang, Abiola Alli, Cheryl Bailey, Peter Braidley, Michelle Deighton, Joyce Fofie, Laura Hill, John Humphreys, Yvonne Jackson, Alison Jenkins, Sharon Kerrison, Craig King, Jay Lindley, Felicity Mackenzie, Gail Mills, Faith Okhuoya, Julie Sorrell, Rachel Walker, Rebecca Warren and Alison Weedon.
Trent Cardiac Centre, Nottingham (44 patients, recruitment started 28 June 2012): David Richens (PI), Rahul Basu, Karim Dakkak, Raj Jutley, Amr Mahmoud, Ganapathy Muthuswammy, Justin Richards, Prashanth Sadhahalli, Matloob Shajar, Arvind Singh, Henry Skinner, Julian Skoyles, Adam Szafranek, Gabor Ther and Louise Wyllie.
Blackpool Victoria Hospital (72 patients, recruitment started 31 December 2012): Amal Bose (PI), Kirsty Angus, Emma Brennan, Melanie Caswell, Laura Flannery, Gemma Hatton, Lesley Helliwell, Lisa Lane, Barbara Mitchell, Sherron Pickervance, Manij Puronit, Vivek Srivastava, Laura Ullyott, Vasanthi Vasudevan and Charlotte Waterhouse.
Wythenshawe Hospital, Manchester (40 patients, recruitment started 3 May 2012): Nizar Yonan (PI), Diane Daniel, Susan Ferguson, Emma Flook, Faisal Hashmi, Marie Kirwan, Deirdre Leonard, Teresa Mcnamara, Amanda Moran, Anu Oommen and Heather Perks.
Glenfield Hospital, Leicester (17 patients, recruitment started 10 December 2012): Tom Spyt (PI), Donna Alexander, Mark Hickey, Shiji Legi, Katrina Maxfield, Jacek Szostek, Martina Williams and Sarah Worthy.
Southampton General Hospital (92 patients, recruitment started 14 September 2012): Sunil Ohri (PI), Mark Apaya, Wendy Bannister, Gemma Beckett, Clare Bolger, Hannah Collins, Jasmin Crockett, Andy Curry, Wei Deng, Paul Diprose, Emma Ekins, Ravi Gill, Kim Golder, Edward Grinyer, Angela Jarca, Alexa King, Jessica Piper, Karen Salmon, Leanne Seaward, Natasha Tantony, Bryony Tyrell, Beverley Wadams and Kirstin Wilkinson.
Leeds General Infirmary (65 patients, recruitment started 28 November 2012): David O’Regan (PI), Zoe Beardow, Sian Birch, Stuart Elliot, Karen Griffiths and Elizabeth Wilby.
James Cook University Hospital, Middlesbrough (41 patients, recruitment started 3 September 2012): Andrew Goodwin (PI), Bev Atkinson, Ian Brown, Suzanne Cormack, Maggie Finlayson, Peter Hill, Elaine Morley, Cath Richardson, Heather Robinson, Sue Simmons and Laura Thompson.
North Staffordshire University Hospital, Stoke-on-Trent (10 patients, recruitment started 28 May 2013): Qamar Abid (PI), Loretta Barnett, Krys Castro-Foskett, Jane Delaney, Melanie Griffiths, Sue Gallagher, Julie Machin, Michael Martin, Elizabeth Sellars and Jill Wain.
University Hospital Coventry (18 patients, recruitment started 19 December 2012): Sunil Bhudia (PI), Emily Archer, Steven Clay, Stacey Gibbons-Smith, Catherine Gibson, Denise Gocher, Neil Hawthorne, Jill Lindsay, Marie McCauley, Rosemary Musanhu, Pamela O’Meara, Jeff Ting and Geraldine Ward.
Publications
Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass surgery (ERICCA study): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol 2012;101:339–48.
Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 2015;373:1408–17.
Data sharing statement
Research data will be made available to the scientific community with as few restrictions as possible to maximise the value of the data for research and for eventual patient and public benefit. These data must be shared in a timely and responsible manner.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health.
References
- National Service Framework for Coronary Artery Disease: Modern Standards and Service Models. London: Department of Health; 2000.
- Cardiovascular Disease Prevention. London: NICE; 2010.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. http://dx.doi.org/10.1056/NEJMoa0804626.
- Sixth National Adult Cardiac Surgical Database Report 2008. Demonstrating Quality. Henley-on-Thames: Dendrite Clinical Systems; 2009.
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893-9. http://dx.doi.org/10.1161/01.CIR.87.3.893.
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008;79:377-86. http://dx.doi.org/10.1093/cvr/cvn114.
- Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 1997;96:1641-6. http://dx.doi.org/10.1161/01.CIR.96.5.1641.
- Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 2008;151:1099-103. http://dx.doi.org/10.1016/j.neuroscience.2007.11.056.
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002;106:2881-3. http://dx.doi.org/10.1161/01.CIR.0000043806.51912.9B.
- Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia–reperfusion injury in the human forearm. Circulation 2006;113:1015-19. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.590398.
- Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a KATP-channel dependent mechanism. Circulation 2007;116:1386-95. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.653782.
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 2006;47:2277-82. http://dx.doi.org/10.1016/j.jacc.2006.01.066.
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575-9. http://dx.doi.org/10.1016/S0140-6736(07)61296-3.
- Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 2009;95:1567-71. http://dx.doi.org/10.1136/hrt.2008.155770.
- Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther 2009;16:680-9. http://dx.doi.org/10.1583/09-2817.1.
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 2007;116:I98-105. http://dx.doi.org/10.1161/circulationaha.106.679167.
- Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res 2012;1459:81-90. http://dx.doi.org/10.1016/j.brainres.2012.04.017.
- Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation 2009;119:820-7. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.809723.
- Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010;375:727-34. http://dx.doi.org/10.1016/S0140-6736(09)62001-8.
- Ferguson TB, Hammill BG, Peterson ED, DeLong ER, Grover FL. A decade of change: risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg 2002;73:480-9. http://dx.doi.org/10.1016/S0003-4975(01)03339-2.
- Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009;119:495-502. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.786913.
- Whitaker DC, Stygall J, Newman SP. Neuroprotection during cardiac surgery: strategies to reduce cognitive decline. Perfusion 2002;17:69-75. http://dx.doi.org/10.1191/0267659102pf572oa.
- Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ. Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J Am Coll Cardiol 2002;40:1961-7. http://dx.doi.org/10.1016/S0735-1097(02)02538-X.
- Kathiresan S, Servoss SJ, Newell JB, Trani D, MacGillivray TE, Lewandrowski K, et al. Cardiac troponin T elevation after coronary artery bypass grafting is associated with increased one-year mortality. Am J Cardiol 2004;94:879-81. http://dx.doi.org/10.1016/j.amjcard.2004.06.022.
- Lehrke S, Steen H, Sievers HH, Peters H, Opitz A, Müller-Bardorff M, et al. Cardiac troponin T for prediction of short- and long-term morbidity and mortality after elective open heart surgery. Clin Chem 2004;50:1560-7. http://dx.doi.org/10.1373/clinchem.2004.031468.
- Mohammed AA, Agnihotri AK, van Kimmenade RR, Martinez-Rumayor A, Green SM, Quiroz R, et al. Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation 2009;120:843-50. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.837278.
- Fellahi JL, Gue X, Richomme X, Monier E, Guillou L, Riou B. Short- and long-term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology 2003;99:270-4. http://dx.doi.org/10.1097/00000542-200308000-00007.
- Croal BL, Hillis GS, Gibson PH, Fazal MT, El-Shafei H, Gibson G, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 2006;114:1468-75. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.602370.
- Muehlschlegel JD, Perry TE, Liu KY, Nascimben L, Fox AA, Collard CD, et al. Troponin is superior to electrocardiogram and creatinine kinase MB for predicting clinically significant myocardial injury after coronary artery bypass grafting. Eur Heart J 2009;30:1574-83. http://dx.doi.org/10.1093/eurheartj/ehp134.
- Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 1998;128:194-203. http://dx.doi.org/10.7326/0003-4819-128-3-199802010-00005.
- Suen WS, Mok CK, Chiu SW, Cheung KL, Lee WT, Cheung D, et al. Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology 1998;49:789-800. http://dx.doi.org/10.1177/000331979804900902.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. http://dx.doi.org/10.2215/CJN.00240605.
- Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104:343-8. http://dx.doi.org/10.1016/S0002-9343(98)00058-8.
- Fiorina C, Vizzardi E, Lorusso R, Maggio M, De Cicco G, Nodari S, et al. The 6-min walking test early after cardiac surgery. Reference values and the effects of rehabilitation programme. Eur J Cardiothorac Surg 2007;32:724-9. http://dx.doi.org/10.1016/j.ejcts.2007.08.013.
- Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv 2010;3:49-55. http://dx.doi.org/10.1016/j.jcin.2009.10.015.
- Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv 2013;6:1055-63. http://dx.doi.org/10.1016/j.jcin.2013.05.011.
- White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2015;8:178-88. http://dx.doi.org/10.1016/j.jcin.2014.05.015.
- Prunier F, Angoulvant D, Saint Etienne C, Vermes E, Gilard M, Piot C, et al. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol 2014;109. http://dx.doi.org/10.1007/s00395-013-0400-y.
- Dixon JR. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur 1998;6:65-74. http://dx.doi.org/10.1080/105294199277860.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98. http://dx.doi.org/10.1016/j.jacc.2012.08.001.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; n.d.
- Lee MS, Kapoor N, Jamal F, Czer L, Aragon J, Forrester J, et al. Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol 2006;47:864-70. http://dx.doi.org/10.1016/j.jacc.2005.09.072.
- Grambsch PM TT. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515-16. http://dx.doi.org/10.1093/biomet/81.3.515.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. http://dx.doi.org/10.1080/01621459.1999.10474144.
- Wheatley DJ. Protecting the damaged heart during coronary surgery. Heart 2003;89:367-8. http://dx.doi.org/10.1136/heart.89.4.367.
- Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu P, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol 2010;31:22-9. http://dx.doi.org/10.1007/s00246-009-9536-9.
- Pavione MA, Carmona F, de Castro M, Carlotti AP. Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg 2012;144:178-83. http://dx.doi.org/10.1016/j.jtcvs.2011.12.029.
- Ali N, Rizwi F, Iqbal A, Rashid A. Induced remote ischemic pre-conditioning on ischemia–reperfusion injury in patients undergoing coronary artery bypass. J Coll Physicians Surg Pak 2010;20:427-31.
- Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 2010;105:657-64. http://dx.doi.org/10.1007/s00395-010-0104-5.
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 2013;382:597-604. http://dx.doi.org/10.1016/S0140-6736(13)61450-6.
- Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg 2010;11:758-62. http://dx.doi.org/10.1510/icvts.2010.243600.
- Lomivorotov VV, Shmyrev VA, Nepomnyaschih VA, Ponomarev DN, Knyazkova LG, Lomivorotov VN, et al. Remote ischaemic preconditioning does not protect the heart in patients undergoing coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2012;15:18-22. http://dx.doi.org/10.1093/icvts/ivs118.
- Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment?. Circulation 2010;122:S53-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.926667.
- Young PJ, Dalley P, Garden A, Horrocks C, La Flamme A, Mahon B, et al. A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol 2012;107. http://dx.doi.org/10.1007/s00395-012-0256-6.
- Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, et al. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation?. Basic Res Cardiol 2011;106:511-19. http://dx.doi.org/10.1007/s00395-011-0185-9.
- Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol – a clinical trial. Acta Anaesthesiol Scand 2012;56:30-8. http://dx.doi.org/10.1111/j.1399-6576.2011.02585.x.
- Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, et al. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection?. Anesthesiology 2012;116:296-310. http://dx.doi.org/10.1097/ALN.0b013e318242349a.
- Hong DM, Mint JJ, Kim JH, Sohn IS, Lim TW, Lim YJ, et al. The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off-pump coronary artery bypass graft surgery. Anaesth Intensive Care 2010;38:924-9.
- Hong DM, Jeon Y, Lee CS, Kim HJ, Lee JM, Bahk JH, et al. Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary artery bypass surgery – randomized controlled trial. Circ J 2012;76:884-90. http://dx.doi.org/10.1253/circj.CJ-11-1068.
- Haji Mohd Yasin NA, Herbison P, Saxena P, Praporski S, Konstantinov IE. The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta-analysis. J Surg Res 2014;186:207-16. http://dx.doi.org/10.1016/j.jss.2013.09.006.
- Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, . Remote Preconditioning Trialists Group . Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol 2014;176:20-31. http://dx.doi.org/10.1016/j.ijcard.2014.06.018.
- Li G, Chen S, Lu E, Luo W. Cardiac ischemic preconditioning improves lung preservation in valve replacement operations. Ann Thorac Surg 2001;71:631-5. http://dx.doi.org/10.1016/S0003-4975(00)02015-4.
- Xie JJ, Liao XL, Chen WG, Huang DD, Chang FJ, Chen W, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart 2012;98:384-8. http://dx.doi.org/10.1136/heartjnl-2011-300860.
- Thielmann M, Wendt D, Tsagakis K, Price V, Dohle DS, Pasa S, et al. Remote ischemic preconditioning: the surgeon’s perspective. J Cardiovasc Med 2013;14:187-92. http://dx.doi.org/10.2459/JCM.0b013e3283590df6.
- Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart 2015;101:185-92. http://dx.doi.org/10.1136/heartjnl-2014-306178.
- Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet 2013;381:166-75. http://dx.doi.org/10.1016/S0140-6736(12)60916-7.
- Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Review and meta-analysis of randomized controlled clinical trials of remote ischemic preconditioning in cardiovascular surgery. Am J Cardiol 2008;102:1487-8. http://dx.doi.org/10.1016/j.amjcard.2008.07.036.
- Takagi H, Umemoto T. Remote ischemic preconditioning for cardiovascular surgery: an updated meta-analysis of randomized trials. Vasc Endovascular Surg 2011;45:511-13. http://dx.doi.org/10.1177/1538574410379654.
- Yetgin T, Manintveld OC, Boersma E, Kappetein AP, van Geuns RJ, Zijlstra F, et al. Remote ischemic conditioning in percutaneous coronary intervention and coronary artery bypass grafting. Circ J 2012;76:2392-404. http://dx.doi.org/10.1253/circj.CJ-12-0518.
- Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol 2012;24:42-8.
- Pilcher JM, Young P, Weatherall M, Rahman I, Bonser RS, Beasley RW. A systematic review and meta-analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. J R Soc Med 2012;105:436-45. http://dx.doi.org/10.1258/jrsm.2012.120049.
- Zhou C, Liu Y, Yao Y, Zhou S, Fang N, Wang W, et al. Beta-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: a meta-analysis of 15 randomized trials. J Cardiothorac Vasc Anesth 2013;27:305-11. http://dx.doi.org/10.1053/j.jvca.2012.09.028.
- Brevoord D, Kranke P, Kuijpers M, Weber N, Hollmann M, Preckel B. Remote ischemic conditioning to protect against ischemia–reperfusion injury: a systematic review and meta-analysis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0042179.
- D’Ascenzo F, Cavallero E, Moretti C, Omedé P, Sciuto F, Rahman IA, et al. Remote ischaemic preconditioning in coronary artery bypass surgery: a meta-analysis. Heart 2012;98:1267-71. http://dx.doi.org/10.1136/heartjnl-2011-301551.
- Yang L, Wang G, Du Y, Ji B, Zheng Z. Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth 2014;28:682-9. http://dx.doi.org/10.1053/j.jvca.2013.05.035.
- McCrindle BW, Clarizia NA, Khaikin S, Holtby HM, Manlhiot C, Schwartz SM, et al. Remote ischemic preconditioning in children undergoing cardiac surgery with cardiopulmonary bypass: a single-center double-blinded randomized trial. J Am Heart Assoc 2014;3. http://dx.doi.org/10.1161/JAHA.114.000964.
- Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J 2014;35:176-83. http://dx.doi.org/10.1093/eurheartj/eht346.
- Biancari F, Kangasniemi OP, Aliasim Mahar M, Rasinaho E, Satomaa A, Tiozzo V, et al. Changing risk of patients undergoing coronary artery bypass surgery. Interact Cardiovasc Thorac Surg 2009;8:40-4. http://dx.doi.org/10.1510/icvts.2007.173922.
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 2007;59:418-58. http://dx.doi.org/10.1124/pr.107.06002.
- Galagudza MM, Nekrasova MK, Syrenskii AV, Nifontov EM. Resistance of the myocardium to ischemia and the efficacy of ischemic preconditioning in experimental diabetes mellitus. Neurosci Behav Physiol 2007;37:489-93. http://dx.doi.org/10.1007/s11055-007-0040-5.
- Ebrahim Z, Yellon DM, Baxter GF. Ischemic preconditioning is lost in aging hypertensive rat heart: independent effects of aging and longstanding hypertension. Exp Gerontol 2007;42:807-14. http://dx.doi.org/10.1016/j.exger.2007.04.005.
- Sogo N, Campanella C, Webb DJ, Megson IL. S-nitrosothiols cause prolonged, nitric oxide-mediated relaxation in human saphenous vein and internal mammary artery: therapeutic potential in bypass surgery. Br J Pharmacol 2000;131:1236-44. http://dx.doi.org/10.1038/sj.bjp.0703700.
- Heusch G, Post H, Michel MC, Kelm M, Schulz R. Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res 2000;87:146-52. http://dx.doi.org/10.1161/01.RES.87.2.146.
- Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, et al. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res 1997;81:1094-107. http://dx.doi.org/10.1161/01.RES.81.6.1094.
- Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res 2004;61:402-13. http://dx.doi.org/10.1016/j.cardiores.2003.09.019.
- Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, et al. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res 1997;81:42-5. http://dx.doi.org/10.1161/01.RES.81.1.42.
- Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, et al. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol 1998;93:325-38. http://dx.doi.org/10.1007/s003950050101.
- Hill M, Takano H, Tang XL, Kodani E, Shirk G, Bolli R. Nitroglycerin induces late preconditioning against myocardial infarction in conscious rabbits despite development of nitrate tolerance. Circulation 2001;104:694-9. http://dx.doi.org/10.1161/hc3201.092218.
- Bolli R, Li QH, Tang XL, Guo Y, Xuan YT, Rokosh G, et al. The late phase of preconditioning and its natural clinical application – gene therapy. Heart Fail Rev 2007;12:189-99. http://dx.doi.org/10.1007/s10741-007-9031-4.
- West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, et al. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 2008;118:1970-8. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.791533.
- Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, et al. Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol 2006;101:168-79. http://dx.doi.org/10.1007/s00395-005-0543-6.
- Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, et al. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 2010;299:H1598-603. http://dx.doi.org/10.1152/ajpheart.00396.2010.
- Foryś J, Peruga J, Plewka M, Lipiec P, Jasińska A, Krzemińska-Pakula M, et al. Nitroglycerin infusion after percutaneous coronary intervention does not influence short- and long-term outcome – a prospective NAPI (Nitroglycerin Administration after Percutaneous Intervention) study. Kardiol Pol 2007;65:789-803.
- Kleinbongard P, Thielmann M, Jakob H, Peters J, Heusch G, Kottenberg E. Nitroglycerin does not interfere with protection by remote ischemic preconditioning in patients with surgical coronary revascularization under isoflurane anesthesia. Cardiovasc Drugs Ther 2013;27:359-61. http://dx.doi.org/10.1007/s10557-013-6451-3.
- Lucchinetti E, Hofer C, Bestmann L, Hersberger M, Feng J, Zhu M, et al. Gene regulatory control of myocardial energy metabolism predicts postoperative cardiac function in patients undergoing off-pump coronary artery bypass graft surgery: inhalational versus intravenous anesthetics. Anesthesiology 2007;106:444-57. http://dx.doi.org/10.1097/00000542-200703000-00008.
- Symons JA, Myles PS. Myocardial protection with volatile anaesthetic agents during coronary artery bypass surgery: a meta-analysis. Br J Anaesth 2006;97:127-36. http://dx.doi.org/10.1093/bja/ael149.
- Yu CH, Beattie WS. The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anaesth 2006;53:906-18. http://dx.doi.org/10.1007/BF03022834.
- Candilio L, Hausenloy DJ, Yellon DM. Remote ischemic conditioning: a clinical trial’s update. J Cardiovasc Pharmacol Ther 2011;16:304-12. http://dx.doi.org/10.1177/1074248411411711.
- Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, et al. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2013;98:7-27. http://dx.doi.org/10.1093/cvr/cvt004.
- Healy DA, Clarke Moloney M, McHugh SM, Grace PA, Walsh SR. Remote ischaemic preconditioning as a method for perioperative cardioprotection: concepts, applications and future directions. Int J Surg 2014;12:1093-9. http://dx.doi.org/10.1016/j.ijsu.2014.08.352.
- Zhang B, Zhou J, Li H, Zhou M, Chen A, Zhao Q. Remote ischemic preconditioning does not improve the clinical outcomes in patients undergoing coronary artery bypass grafting: a meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:e36-8. http://dx.doi.org/10.1016/j.ijcard.2013.12.086.
Appendix 1
Summary of changes to the ERICCA protocol
Note on version numbers
Protocol version 5 (6 September 2010) was the first ethically approved version of the protocol and so is considered the original protocol; versions 1–4 were drafts and have not been included in this document.
The following changes were made to version 6 (28 October 2010):
-
Further information has been added with regard to the definition of the primary objective, and the study end point has been clarified as cardiovascular death.
-
Treatment period changed – the RIPC protocol has been changed from three 5-minute cycles to four cycles of ischaemia and reperfusion applied to the upper arm. This is because of a recent publication19 which has shown that RIPC using four 5-minute cycles reduces infarct size in patients presenting with an acute MI.
-
Changed from peripheral vascular disease to peripheral arterial disease, as the former is vague and includes venous disease such as deep-vein thrombosis. Peripheral arterial disease is more accurate and specific for the patients we would like to include.
-
The laboratory where we plan to undertake the troponin T assays for all of the sites has switched to hsTnT, which has different cut-off values.
-
Further information included on calculating inotrope score.
-
Further details included with regard to ejection fraction protocol.
-
Clinical outcome confirmed as death and not cardiovascular death. Information will be collected on all deaths. The definition of cardiovascular death was improved.
The following changes were made to version 7 (14 January 2011):
-
The laboratory where the samples are to be collected has been changed and, therefore, the size of the tube in which blood is collected has changed.
The following changes were made to version 8 (1 March 2011):
-
Further centres have been added. It was originally felt that 11 centres would be adequate for recruitment for this trial. However, following an investigator meeting it was decided to increase the number of centres to ensure that the trial is recruiting on target and on time.
-
The total time for the intervention has been changed from 40 minutes to 35 minutes. This is because the last 5 minutes is when the cuff is deflated and so it can be removed after the final inflation.
-
Recruiting centres will be advised to avoid the routine use of intravenous GTN during surgery, as this agent may interfere with RIPC. The use of any GTN will be recorded on the patient’s CRF.
-
The following exclusion criterion has been removed: positive troponin T or I at baseline. This has been removed as this status may not always be known as troponin is not measured routinely in these patients.
-
A definition of cardiogenic shock has been included as requested by investigators. This is a standard definition.
-
The Doctors Laboratory will now analyse all troponin samples.
-
Information will be collected on the day of surgery on any angiograms that have been performed during the last 5 days. During this procedure, dye is used and this may affect some of the blood tests.
-
As we have removed a positive troponin T or I at baseline as an exclusion criterion but are still measuring troponin T and troponin I as part of the trial procedures pre and post surgery, they will be reviewed further as part of the statistical analysis plan.
-
Richard Evans has now been added as the assistant trial manager.
The following changes were made to version 9 (4 May 2011):
-
An earlier time point for assessment of MACCE was agreed to be of benefit. It has therefore been added as a secondary end point at 6 weeks (which coincides with the first follow-up appointment of the patients). In addition, all-cause death will be noted as a secondary end point.
-
The original plan was for the control group not to have the blood pressure cuff inflated. However, the reviewers were concerned that this may unblind more staff. In light of this we have changed the control group to a sham control group. When patients are randomised to the sham control group they will undergo 5 minutes of simulated inflations of the blood pressure cuff followed by deflation.
-
As centres use different types of blood bottles, EDTA has been removed to avoid confusion at sites. Blood samples can be stored at –20 °C and not –70 °C as originally thought.
-
Three-dimensional techniques have been added to measurement of the LVEF.
-
Samples for genetic analysis have now changed slightly following on from the advice of a biochemist.
-
Luciano Candilio has been added as a clinical fellow.
-
It has been decided that an independent EVC will be convened to adjudicate all of the events that make up the primary end point.
-
The membership for the TSC has been updated.
-
Changes to inclusion criteria – recruitment to the trial has now started and all patients who are undergoing CABG are screened. Many patients are not meeting the inclusion criteria and so we are modifying these to maximise recruitment to the trial. There are two main changes: the euroSCORE is being lowered from ≥ 6 to ≥ 5 and the word ‘cold’ has been removed from point 1.
-
Changes to exclusion criteria – bilirubin of > 20 mmol/l has been deleted as this will also maximise our recruitment and centres have been updated: Mr Aprim Youhana, Swansea Morriston Hospital, has been added and Bristol Royal Infirmary has been removed.
-
In relation to the inotrope score some centres use milrinone and so this has been added.
The following changes were made to version 10 (12 January 2012):
-
The PI at Harefield Hospital has been changed from Professor John Pepper to Dr Andre Simon. Nine other sites have been added to increase and accelerate recruitment.
-
The number of sites has increased from 16 to 28. The recruitment rates have been updated to reflect the involvement of extra hospitals and the timetable has been updated to reflect the training of 28 sites.
-
St Thomas’ Hospital has now also agreed to take part in the echo substudy.
-
Cardiogenic shock and cardiac arrest are listed on separate lines on the CRF. Hepatic dysfunction has been changed from using the prothrombin ratio definition to using the international normalised ratio to reflect current practice.
-
The inotrope score has been adjusted slightly to incorporate alternative inotropes.
-
Dry ice is now being provided by the courier company and not locally to simplify transfer procedures.
-
The study timetable has been corrected to remove creatinine and urine volume at baseline as these need to be recorded preoperatively only.
-
The reporting of events has been adjusted as many centres are submitting SAEs relating to complications of CABG. Listed are the most common complications of surgery, which we hope will lead to the reporting of fewer SAEs.
-
Wording changed in the consent procedure for inpatients recommending that they are given 24 hours to consider the trial.
-
Trial unblinded statistician updated from Cono Ariti to Jennifer Nicholas.
The following changes were made to version 11 (7 December 2012):
-
The number of recruiting sites has been increased from 28 to 30 to expedite recruitment.
-
The echo substudy has been opened to other sites to increase recruitment.
-
The wording for randomisation timing has been amended as some sites need to randomise earlier than the morning before surgery for logistical reasons.
-
The recruitment targets have been amended to reflect expected recruitment numbers.
-
The requirement to record a screenshot of the euroSCORE has been removed to reflect the different methods that sites use to calculate it. The screenshot of the euroSCORE calculator has been removed from the appendix.
-
Wording has been changed to reflect the fact that the EQ-5D questionnaire can be completed over the telephone.
-
Wording has been changed to reflect the fact that if a patient is unable to attend the 1-year follow-up it may be conducted over the telephone.
-
Infection of donor site has been added to the list of expected SAEs.
-
The job descriptions of Richard Evans, Steven Robertson and Rosemary Knight have been updated to reflect their current roles.
-
The details for the EVC have been added.
-
The primary end point definitions have been clarified after input from the EVC.
-
The list of local investigators has been finalised and updated.
The following changes were made to version 12 (27 November 2014):
-
Update to Derek Hausenloy’s title (Professor).
-
Update to remove troponin I from the list of blood samples as only hsTnT was collected for the trial.
-
Update to perioperative MI definition to match the current universal definition of MI. Updated to include probable MI as a category to allow for cases in which sufficient evidence to consider definite MI is not available.
-
Update to make clear that both definite and probable MIs will be included in the MACCE end point.
-
Update to stroke definition to allow probable stroke to be recorded when there is insufficient evidence to classify as definite stroke.
-
Update to make clear that both definite and probable strokes will be included in the MACCE end point.
-
EVC updated to reflect membership changes.
-
Reference added supporting change to MI definition.
List of abbreviations
- 6MWT
- 6-minute walk test
- AAA
- abdominal aortic aneurysm
- AF
- atrial fibrillation
- AKI
- acute kidney injury
- AUC
- area under the curve
- BMI
- body mass index
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CHD
- coronary heart disease
- CI
- confidence interval
- CKMB
- creatine kinase MB
- CRF
- case report form
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- ECG
- electrocardiogram
- eCRF
- electronic case report form
- EDTA
- ethylenediaminetetra-acetic acid
- EQ-5D
- European Quality of Life-5 Dimensions
- ERICCA
- Effect of Remote Ischaemic preconditioning on Clinical outcomes in patients undergoing Coronary Artery bypass graft surgery
- euroSCORE
- European System for Cardiac Operative Risk
- EVC
- Event Validation Committee
- GTN
- glyceryl trinitrate
- HR
- hazard ratio
- hsTnT
- high-sensitivity troponin T
- IPC
- ischaemic preconditioning
- ITT
- intention to treat
- ITU
- intensive therapy unit
- LVEF
- left ventricular ejection fraction
- MACCE
- major adverse cardiac and cerebral events
- MI
- myocardial infarction
- NGAL
- neutrophil gelatinase-associated lipocalin
- NYHA
- New York Heart Association
- PCI
- percutaneous coronary intervention
- PI
- principal investigator
- PMI
- perioperative myocardial injury
- PP
- per protocol
- RCT
- randomised controlled trial
- RIPC
- remote ischaemic preconditioning
- RIPostC
- remote ischaemic postconditioning
- SAE
- serious adverse event
- SD
- standard deviation
- SST
- serum-separating tube
- STEMI
- ST elevation myocardial infarction
- SYNTAX
- SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery
- TSC
- Trial Steering Committee
- UCL
- University College London
- UCLH
- University College London Hospitals