Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 11/20/05. The contractual start date was in September 2012. The final report began editorial review in September 2015 and was accepted for publication in March 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Stephen J Till reports personal fees and grants from ALK Abelló, and personal fees from Thermofisher Scientific, outside the submitted work. Mohamed H Shamji reports grants from BioTech Tools and Regeneron USA, outside the submitted work. David J Cousins reports grants from GlaxoSmithKline, Asthma UK, and the Medical Research Council, outside the submitted work. Stephen R Durham reports grants from ALK Abelló, grants and personal fees from Merck, grants from Regeneron USA, personal fees from Biomay Austria and personal fees from Circassia UK, outside the submitted work; in addition, Stephen R Durham has a patent pending. Emily Lam is a Health Technology Assessment Primary Care, Community and Preventive Interventions panel member.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Slovick et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Tables, figures and parts of the text of this report are reproduced or adapted from Slovick et al. 1 © 2016 The Authors. Published by Elsevier Inc. on behalf of American Academy of Allergy, Asthma & Immunology. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Allergic rhinitis caused by grass pollen affects one-quarter of the UK population. 2 Of these, around 5 million people suffer moderate or severe persistent symptoms that have an impact on quality of life, including disturbed sleep, disruption of leisure activities and impairment of performance at work or school. 3 Therefore, there is a substantial unmet need for both therapy and prophylaxis of seasonal allergic rhinitis. In the UK, subcutaneous and sublingual immunotherapy is indicated in patients with moderate or severe symptoms who fail to respond to conventional medications. 4 Immunotherapy, that is, prophylactic inoculation with grass pollen for treatment of seasonal allergic rhinitis, was first described in 1911. 5 The conventional approach involves the regular subcutaneous administration of allergen extracts at high doses (typically microgram quantities of group 5 grass pollen allergens). 4 The most commonly used form of grass pollen immunotherapy is that given by injections into the tissue beneath the skin (i.e. subcutaneously) over a period of 2–3 years, with increasing amounts of allergen administered weekly for 12–15 weeks followed by monthly maintenance injections. 4 A body of evidence, including a Cochrane meta-analysis,6 exists to support the clinical efficacy of high-dose subcutaneous immunotherapy. Grass pollen allergen may also be administered at a high dose as sublingual tablets or drops, an approach further supported by Cochrane meta-analysis. 7 Both subcutaneous and sublingual high-dose immunotherapy have limitations: the vaccine products are expensive and the need for repeated administration in a specialist clinic (subcutaneous immunotherapy) or daily at home (sublingual immunotherapy) is associated with additional expense and/or inconvenience.
Injections of relatively small quantities of allergen (nanograms of major allergen proteins) into the dermis leads to the development of local swelling within 6 hours, which persists for 24–36 hours. This ‘late-phase response’ is characterised by infiltration of inflammatory cells – notably activated T helper type 2 cells (Th2), eosinophils and basophils – and has been extensively used as a model for investigating mechanisms of chronic allergic inflammation. 8 We previously established that when these injections are repeated at 2-weekly intervals there is a progressive and significant decline in the size of cutaneous late-phase response that is antigen-specific and systemic. Administration of six intradermal injections of grass pollen containing only 7 ng of major allergen Phl p 5 resulted in a > 90% suppression in the cutaneous late-phase response measured after 24 hours in response to these injections. 9 The magnitude of inhibition was comparable to that seen with a conventional high-dose subcutaneous grass pollen vaccine10 despite equating to over 1000-fold less allergen over the same time period, and significantly exceeded the inhibition seen with sublingual immunotherapy given daily and containing 20,000-fold more group 5 allergen over a 10-week period. 11 This observation provided the rationale for progressing to a clinical trial of low-dose intradermal grass pollen immunotherapy as a treatment for allergic rhinitis. The concept of therapeutic intradermal allergen inoculation is not without precedent. In 1926, Phillips, a physician dworking in Arizona, published a preliminary account of his uncontrolled experiences with intradermal grass pollen immunotherapy in 29 patients,12 extended to 322 patients by 1933,13 > 90% of whom obtained ‘satisfactory relief’. However, no randomised controlled trial (RCT) has previously addressed the efficacy of this approach.
High-dose subcutaneous and sublingual immunotherapy is associated with induction of regulatory T cells (Tregs),14–16 probably through interaction of cluster of differentiation 4-positive (CD4+) T cells with protolerogenic dendritic cells (DCs). These cells are anti-inflammatory and also induce B-cell production of allergen-specific ‘blocking’ immunoglobulin G (IgG) antibodies. 17 Low-dose intradermal allergen desensitisation is biologically plausible: for example, intradermal injection of radiotracer in animal models results in 100-fold higher rates of drainage to regional lymph nodes than subcutaneous injection, potentially leading to more efficient pulsing of lymph node DCs. 15 In addition, the dermis is, itself, an immunologically active environment, rich in DCs and lymphatic vessels. 18,19 In contrast, conventional subcutaneous immunotherapy injections target a compartment consisting mostly of connective and adipose tissue but few DCs. Therefore, in this study we hypothesised that intradermal grass pollen immunotherapy would be a clinically effective treatment for seasonal allergic rhinitis, and that accompanying desensitisation of the late-phase response would be reflected in local suppression of proallergic Th2 responses. To test this hypothesis, we conducted a Phase II RCT, the Pollen Low dose Intradermal Therapy Evaluation (PollenLITE), with embedded mechanistic studies to evaluate the immunological response to treatment.
Chapter 2 Methods
Setting
This single-centre RCT was conducted in the Clinical Research Facility of the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s Hospital from September 2012. The final study visit was on 27 August 2014. The study was conducted in accordance with the principles of Good Medical Practice (GMP) for clinical trials, and approved by the National Research Ethics Service Committee (London–Harrow; 12/LO/0941), with oversight by King’s Health Partners Clinical Trial Office, together with an independent Trial Steering Committee and Data Monitoring and Ethics Committee. The clinical trial protocol was published20 and the statistical analysis plan finalised prior to randomisation. All participants provided written informed consent prior to participation.
Patient and public involvement
The recruitment campaign for this trial involved development of a dedicated advertising campaign and website (developed by Media with Impact Ltd, London, UK) (see Appendices 1 and 2). The website contained a number of online prescreening questions. With the assistance of Asthma UK, patient representatives reviewed the design and helped to ensure appropriate engagement with the target audience. Patient representatives also reviewed all advertisement materials, participant information sheets and consent forms. In response to this feedback, substantial changes were made to the branding of the trial website and advertising materials in particular, to ensure appropriate engagement with the target population. Patient representatives also reviewed materials prior to disseminating the results to study participants.
Primary objective
The primary objective was to determine if preseasonal low-dose intradermal grass pollen allergen immunotherapy [seven 2-weekly injections of 10 BU (33.3 SQ-U)] reduces symptoms and requirements for antiallergic drugs in seasonal allergic rhinitis during the 2013 grass pollen season compared with the control intervention (histamine only).
Secondary objectives
The secondary objectives were to:
-
determine if this intervention is associated with improvement in quality of life compared with the control intervention, as assessed during the 2013 grass pollen season
-
evaluate if this intervention is safe and well tolerated
-
investigate immunological changes in response to repeated intradermal allergen injections by examining humoral and cellular responses, both in peripheral blood and in tissue
-
explore if the intradermal late-phase response desensitisation effect is long-lived, that is, persists following cessation of intradermal injections.
Participants
Participants were identified via a recruitment campaign including advertisements in press, online and on public transport. Potential participants were invited to visit the trial website (www.pollenlite.co.uk) to answer seven prescreening questions before registering. Participants passing the prescreening on the trial website were contacted for further telephone screening, and, if considered potentially eligible, they were invited to attend the Clinical Research Facility at Guy’s Hospital for a formal screening visit. Full eligibility criteria were as follows.
Inclusion criteria
-
Adults aged 18–65 years.
-
A clinical history of grass pollen-induced allergic rhinoconjunctivitis for at least 2 years, with peak symptoms in May, June or July.
-
A clinical history of moderate or severe persistent rhinoconjunctivitis symptoms interfering with usual daily activities or with sleep.
-
A clinical history of rhinoconjunctivitis that remains troublesome despite treatment with either antihistamine drugs or nasal corticosteroid drugs during the grass pollen season.
-
Positive skin prick test (SPT) response, defined as wheal diameter ≥ 3 mm, to Phleum pratense.
-
Positive specific immunoglobulin E (IgE), defined as ≥ IgE class 2, against P. pratense.
-
For women of childbearing age, a willingness to use an effective form of contraception for the duration of intradermal injections.
-
The ability to give informed consent and comply with study procedures.
Exclusion criteria
-
Pre-bronchodilator forced expiratory volume in 1 second (FEV1) of < 70% of predicted value at screening visit.
-
A history of seasonal grass pollen-induced asthma requiring regular treatment with salbutamol or inhaled corticosteroids. Patients with mild seasonal grass pollen-induced asthma were included, provided that symptoms were satisfactorily controlled with occasional salbutamol only.
-
A clinical history of symptomatic seasonal allergic rhinitis and/or asthma due to tree pollen or weed pollen, near or overlapping the grass pollen season, although patients with mild intermittent symptoms requiring only occasional antihistamines were included.
-
A clinical history of symptomatic allergic rhinitis and/or asthma caused by a perennial allergen to which the participant is regularly exposed, although patients with mild intermittent symptoms requiring only occasional antihistamines were included.
-
Emergency department visit or hospital admission for asthma in the previous 12 months.
-
History of chronic obstructive pulmonary disease.
-
History of significant recurrent acute sinusitis, defined as two episodes per year for the last 2 years, all of which required antibiotic treatment.
-
History of chronic sinusitis, defined as a sinus symptoms lasting > 12 weeks outside the grass pollen season, that includes two or more major factors, or one major factor and two minor factors. Major factors are defined as facial pain or pressure, nasal obstruction or blockage, nasal discharge or purulence or discoloured postnasal discharge, purulence in nasal cavity, or impaired/loss of smell. Minor factors are defined as headache, fever, halitosis, fatigue, dental pain, cough, and ear pain, pressure or fullness.
-
At randomisation, current symptoms of, or treatment for, upper respiratory tract infection, acute sinusitis, acute otitis media, or other relevant infectious process; serous otitis media was not an exclusion criterion.
-
Current smokers or a history of ≥ 5 pack-years.
-
Previous treatment by immunotherapy with grass pollen allergen within the previous 5 years.
-
History of life-threatening anaphylaxis or angioedema.
-
Ongoing systemic immunosuppressive treatment.
-
History of intolerance of grass pollen immunotherapy, rescue medications or their excipients.
-
For females of childbearing age, a positive serum or urine pregnancy test with sensitivity of < 50 mIU/ml within 72 hours of first administration of study therapy.
-
Lactating females.
-
The use of any investigational drug within 30 days of the screening visit.
-
Ongoing treatment with leukotriene receptor antagonists, beta-blockers, calcium channel blockers, tricyclic antidepressants, monoamine oxidase inhibitors or anti-IgE monoclonal antibody (mAb).
-
The presence of any medical condition that the investigator deemed incompatible with participation in the trial.
-
Individuals with insufficient understanding of the trial.
Randomisation
Randomisation was performed by King’s Clinical Trials Unit (KCTU; UK Clinical Research Collaboration registered) at King’s College London (KCL) using a 24-hour, web-based randomisation system. Participants were randomised 1 : 1 to active intradermal immunotherapy or the control arm by the method of block randomisation with randomly varying block sizes, stratified by the size of skin test response to grass pollen at screening visit (the cut-off SPT size being the median value of all subjects to be randomised, ≥ 11 mm) and presence/absence of rhinitis symptoms outside the grass pollen season. Study medication was blinded. To minimise bias through accidental unblinding, as a result of common injection site reactions in the active trial arm, the control intervention consisted of a reducing dose of histamine to produce similar clinical effects as the active medication. All physicians, researchers, research nurses, outcome assessors and patients remained blinded to treatment allocation until the primary analysis was completed. The trial statistician was subgroup unblind only. Only the KCTU randomisation service provider and the manufacturing pharmacy had access to the blinding information for the study.
In August 2013, the KCTU also randomly selected participants to be approached to undergo skin biopsies. The first 40 participants who gave agreement then underwent biopsy after giving additional procedure-specific informed consent. Furthermore, in August 2013, the KCTU randomised all participants for a second time to one of three groups. These three groups then underwent repeat intradermal allergen injections, at 7, 10 or 13 months after the final intradermal immunotherapy or control injection, to assess if low-dose intradermal allergen immunotherapy was associated with prolonged suppression skin responses.
Trial medication
Each active intradermal allergen injection contained 10 BU (33.3 SQ-U) of P. pratense soluble grass pollen extract (Aquagen SQTM Timothy Grass Pollen extract, ALK Abelló, Reading, UK) contained in a 20-µl volume [i.e. 500 BU/ml (1666.7 SQ-U/ml)]. Individual vials for each participant and each visit were preprepared and prelabelled by Guy’s Hospital Pharmacy under GMP conditions. In brief, Aquagen SQ Timothy Grass Pollen extract was reconstituted in manufacturer-supplied diluent to the maximum recommended concentration [30,000 BU/ml (100,000 SQ-U/ml), i.e. 60 times the final working strength; shelf-life 6 months at 2–8 °C after reconstitution] and 0.15 ml was aliquoted into glass study vials. At each visit for intradermal injection the investigator added 8.85 ml of clinical grade 0.9% normal saline at ambient temperature to the vial corresponding with that participant’s visit, to achieve a 60-fold dilution. Then 20 µl was aspirated from this vial and administered directly. The allergen required dilution on the day of administration, as the recommended shelf-life of Aquagen SQ Timothy Grass Pollen extract at 500 BU/ml (1666.7 SQ-U/ml) is 14 days. The control drug was histamine only, administered at a concentration of 100 µg/ml for the first and second injections. To help preserve blinding, histamine concentrations were reduced to 30 µg/ml for the third and fourth injections, and 10 µg/ml for fifth, sixth and seventh injections. To match the grass pollen extract dilution and preserve blinding, histamine was also aliquoted into study vials at 60 times the final working strength in 0.15-ml volumes, for further dilution with 8.85 ml of clinical grade 0.9% normal saline immediately prior to injection. Active and control study medications appeared to be identical.
Following manufacture, vials were packed into individual dispensing packs and dispensed by Guy’s Hospital Pharmacy against a single study prescription for each study participant, covering all visits. At randomisation, an e-mail was sent from the randomisation system to the dispensing pharmacy. The blinded dispensed packs were thereafter stored in the Clinical Research Facility in temperature-monitored fridges, in a secure environment. Study drug accountability was assessed and documented by Guy’s Hospital Pharmacy. Study vials that had been reconstituted in saline for injection were stored separately at room temperature after use for return to pharmacy for drug accountability to be assessed.
Intervention
A series of seven intradermal active or control histamine injections was administered 2-weekly into the forearm before the 2013 grass pollen season (Figure 1). The first injection for each participant was administered between 18 February and 1 March 2013, with the seventh and final injection given between 13 May and 24 May 2013. The injection site was alternated between left and right arms at each visit. Intradermal injections were administered in a 20-µl volume using a 29 gauge insulin syringe (BD Micro-FineTM, Becton Dickinson, Oxford, UK). In the event of an injection being administered too deeply (i.e. into subcutaneous tissue) to elicit an immediate injection ‘bleb’ and subsequent characteristic wheal, the injection was repeated 1 cm from the original site. Most participants were not taking antihistamines at the time of intradermal injections, as these were performed before the grass pollen season. Nevertheless, all of the participants were asked to avoid taking antihistamines for 5 days before receiving an intradermal injection, so that the presence of a wheal could be confirmed. Following an intradermal injection, participants were able to take an antihistamine to reduce the local itching and swelling if they so wished.
FIGURE 1.
Study design.
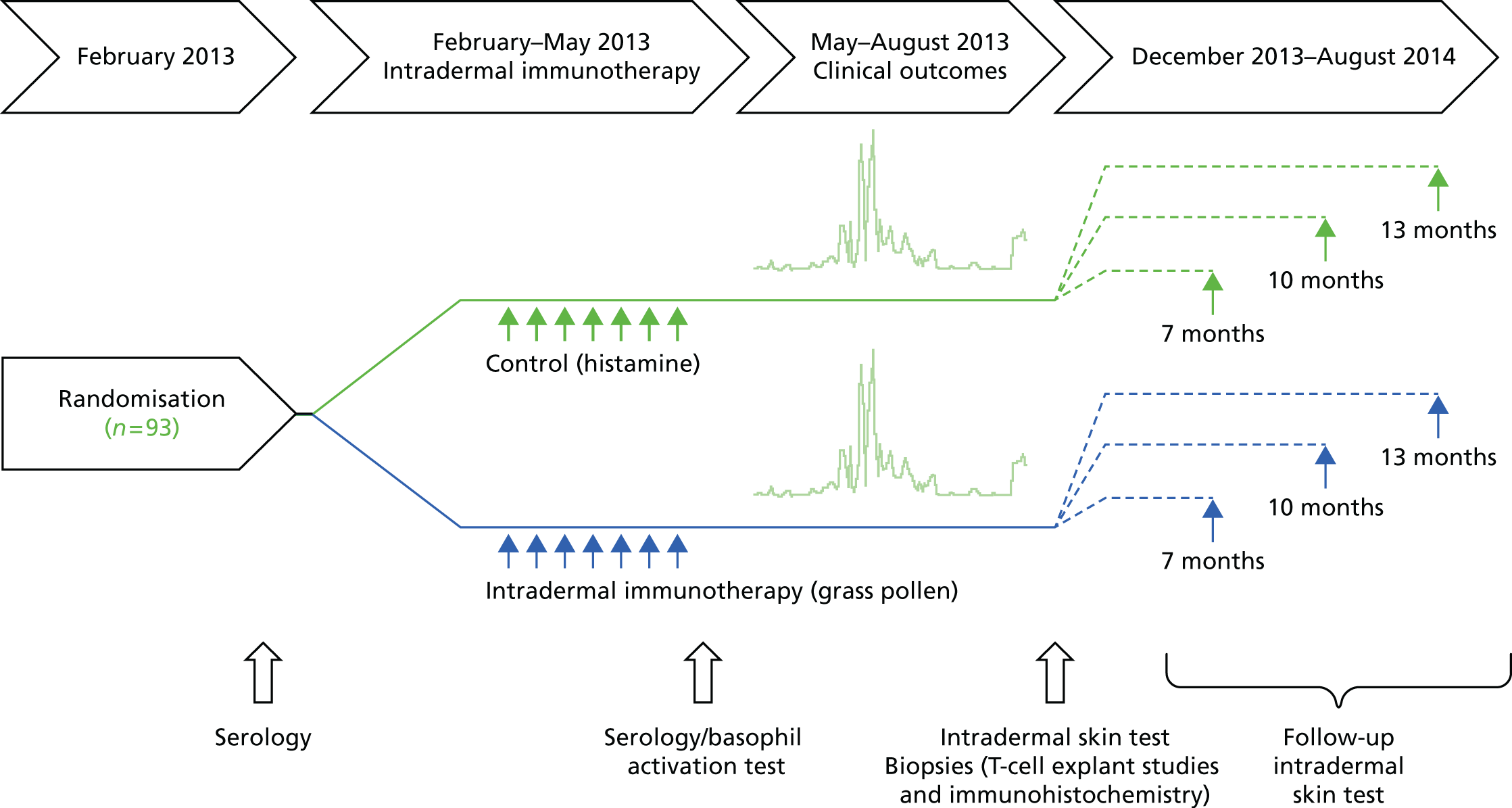
Assessment of efficacy
The primary end point was the area under curve (AUC) of the combined symptom and medication score (CSMS) during the grass pollen season period spanning 13 May to 31 August 2013 (111 days), the clinical end point recommended by World Allergy Organization (WAO) guidelines for clinical trials of immunotherapy for allergic rhinitis. 21 Participants were provided with daily diary cards (see Appendix 3) to record symptoms in the nose (sneezing, blockage and running), eyes (itching, redness, tears and swelling), mouth and throat (itching and dryness) and chest (breathlessness, cough, wheezing and tightness), on a scale of 0–3 (with a score of ‘0’ indicating no symptoms and ‘1’, ‘2’ and ‘3’ indicating mild, moderate and severe symptoms, respectively). Daily rescue medication was scored as follows: desloratadine (Merck Sharp and Dohme Ltd., Moddesdon, UK), 5 mg, up to one tablet daily (6 points per day); olopatadine eye drops, 1 mg/ml, up to one drop per eye twice daily (1.5 points per drop, up to 6 points per day); fluticasone proprionate nasal spray, 50 µg per spray, up to two sprays per nostril once daily (2 points per spray, up to 8 points per day); and prednisolone, 5 mg per tablet, up to six tablets per day (2 points per tablet, up to 12 points per day). Symptom and medication scores were expressed as AUC for the entire grass pollen season. As maximum scores for symptoms (39) and medications (32) were different in magnitude, these parameters were normalised as per WAO guidelines. 21
Secondary clinical end points were:
-
symptom scores (AUC) over entire pollen season
-
medication scores (AUC) over entire pollen season.
-
Mini Rhinitis Quality of Life Questionnaire (Mini-RQLQ) scores22 (overall score and domain scores), recorded three times during the pollen season (12 June, 26 June and 10 July) and once after the season in September 2013.
-
Health-related quality of life: evaluated using the European Quality of Life-5 Dimensions, 5-levels (EQ-5D-5L) questionnaire23 three times during the pollen season (12 June, 26 June and 10 July) and once after the season in September 2013.
-
Visual analogue scales (VASs) for nasal and eye symptoms (see Appendix 4). These were recorded every 2 weeks during the pollen season and AUC values calculated.
-
Global evaluation scores (see Appendix 5).
-
The number of primary care [i.e. general practitioner (GP)] visits for hay fever during summer 2013.
-
CSMSs during the peak of the 2013 grass pollen season.
-
Number of medication-free days covering the grass pollen season period of 13 May to 31 August 2013.
-
Number of symptom-free days covering the grass pollen season period of 13 May to 31 August 2013.
-
Individual symptoms scores (AUC) for each organ: nose, mouth, eyes and lungs.
-
Total number of days during which prednisolone was used between 13 May and 31 August 2013.
-
Frequency of adverse events (AEs).
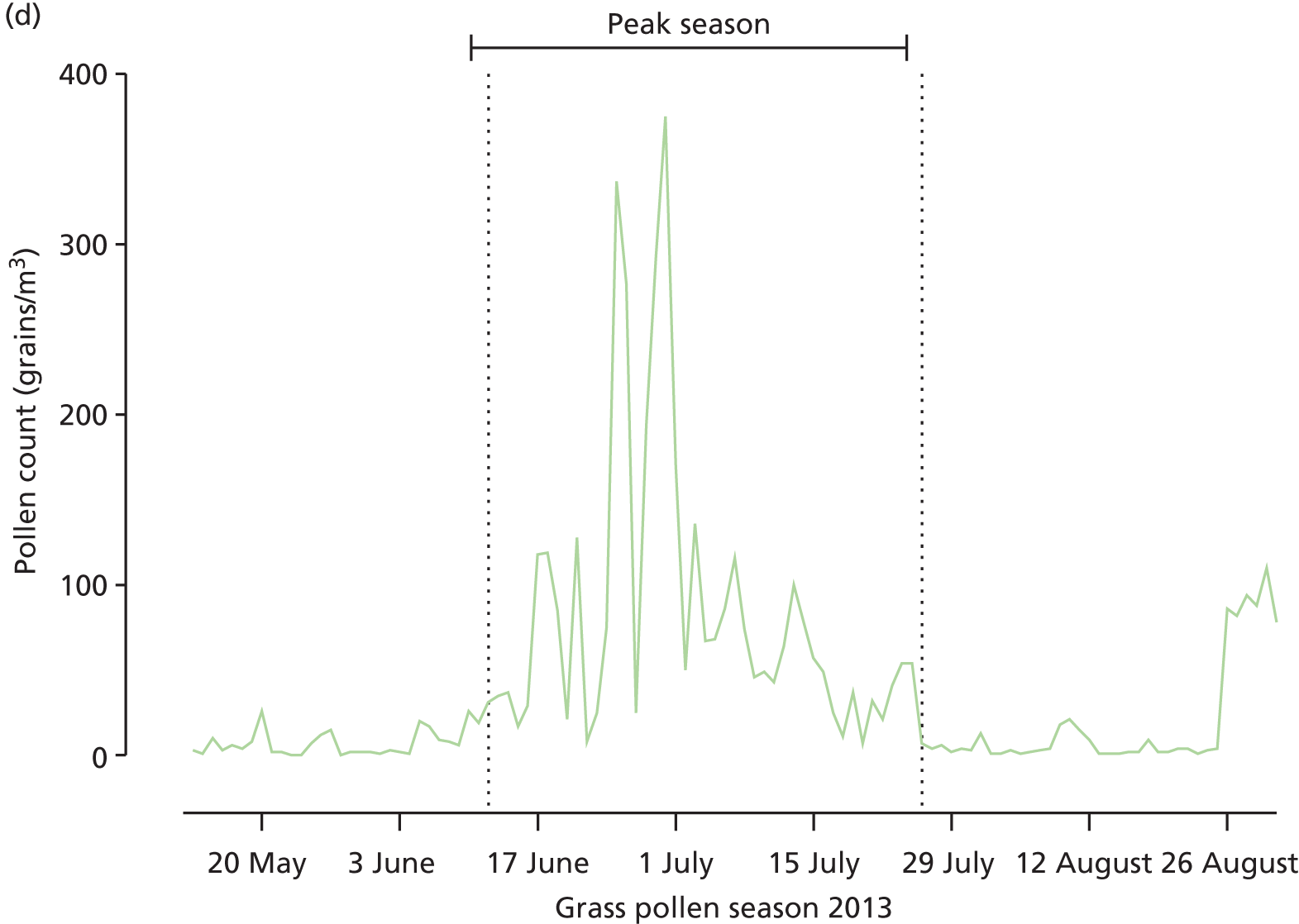
The peak of grass pollen season was prospectively defined as starting on the first three consecutive days between 13 May and 31 August 2013, when grass pollen counts in central London were ≥ 30 grains/cm3, using counts supplied by the UK Meteorological Office. The end of the peak season was defined as the first of 3 consecutive days when grass pollen counts were < 30 grains/cm3.
Data management
Data were managed using the regulatory compliant [GCP (Good Clinical Practice), 21CRF11, EC Clinical Trial Directive] InferMed MACRO database system (MACRO 4, Elsevier, Amsterdam, the Netherlands). An electronic case report form (eCRF) was created in collaboration with the trial statisticians and the chief investigator, and maintained by the KCTU. Data were hosted on a dedicated secure server within KCL, and all source data were entered into the eCRF by authorised staff with a full audit trail. Trial data may be obtained from the corresponding author on request.
Safety
Adverse events and side effects were recorded in the eCRF after randomisation and then throughout the study, regardless of their severity or relation to study participation. As a precaution against systemic allergic reactions, all participants were observed after the first intradermal injection for 1 hour and, if there was no systemic reaction, for 30 minutes after subsequent injections. In the event of a participant experiencing a grade 1 reaction, the clinical observation period for that individual was maintained at 1 hour after subsequent injections.
The following AEs were anticipated and not reported:
-
symptoms attributable to aeroallergen exposure: that is, nasal blockage, rhinorrhoea, itching or sneezing; itching, watering, redness or swelling of eyes; itching or dryness of mouth/throat; breathless, cough, wheeze and chest tightness
-
transient discomfort from intradermal injections
-
appearance of an itchy oedematous wheal, with surrounding erythema, after intradermal injection
-
appearance of swelling (oedema) within hours of intradermal injection
-
temporary discomfort, bleeding, bruising, swelling at the needle site following venesection
-
mild localised itching arising from skin prick testing during screening.
Withdrawal criteria and stopping rules
The prespecified criteria for discontinuation of the study therapy (active or control) were as follows.
-
Inability or failure to attend for intervention within 3 weeks of previous administration.
-
Inability or failure to receive seven or eight injections within the dates specified.
-
Two grade 2 systemic reactions, or a single systemic reaction of grade 3 or above after administration of study therapy. Systemic reactions were graded according to the WAO criteria:
-
Grade 1 Symptoms of 1 organ system (cutaneous, upper respiratory tract, conjunctival, gastrointestinal, other).
-
Grade 2 Symptoms of more than one organ system present or asthma symptoms/signs [cough, wheezing, shortness of breath but, < 40% drop in peak expiratory flow (PEF) or FEV1].
-
Grade 3 Asthma symptoms/signs (with ≥ 40% drop in PEF or FEV1), upper respiratory tract (laryngeal, uvula, tongue) oedema with or without stridor.
-
Grade 4 Respiratory failure or hypotension with or without loss of consciousness.
-
-
An AE that, in the judgement of the principal investigator or the medical monitor, presented an unacceptable consequence or risk to the participant.
-
An illness or infection not associated with the condition under study and which required treatment that was not consistent with protocol requirements or if a participant developed an intercurrent illness that, in the judgement of the principal investigator, in any way justified discontinuation.
-
An inability or unwillingness to comply with the study protocol, with the protocol deviations being sufficient to jeopardise the participant’s well-being or the integrity of the study.
-
Pregnancy occurring during study participation.
Predefined study-stopping rules included the occurrence of five grade 3 reactions or a single grade 4 reaction.
Concomitant medications
Rescue medications were provided to participants before and throughout the pollen season. These included: desloratadine (5 mg, up to one tablet daily), olopatadine eye drops (1.0 mg/ml, up to one drop per eye twice daily), fluticasone propionate nasal spray (50 µg per spray, up to two sprays per nostril once daily) and prednisolone (for use at 30 mg per day for up to 5 days). Participants were asked to use only these medications to treat their hay fever symptoms on an ‘as required’ basis. However, participants who were not experiencing hay fever symptoms were encouraged to try not to use these medications. Participants were asked to use only these medications. A short course of prednisolone was made available for severe symptoms, although participants were instructed to contact a trial doctor prior to starting this treatment. Concurrent treatment with beta-blockers, calcium channel blockers, tricyclic antidepressant drugs, monoamine oxidase inhibitors or anti-IgE monoclonal antibody (mAb) were not permitted.
Measurement of skin early- and late-phase responses
All participants underwent intradermal skin challenge testing 4 months after the final intradermal allergen immunotherapy or control injection (September 2013). Participants were then randomised to undergo a repeat follow-up test at either 7, 10 or 13 months later to assess persistence of late-response suppression by comparing late-phase response sizes in those who had received active intradermal immunotherapy with those who had received the control intervention. The procedure for the intradermal skin challenge testing and the dose of allergen used were identical to that for an active intradermal allergen immunotherapy injection. In brief, grass pollen extract (10 BU, equivalent to 33.3 SQ-U, of P. pratense Aquagen ALK Abelló) in a 20-µl volume of allergen diluent was injected intradermally into the extensor aspect of each forearm. A negative control injection of 20 µl of diluent was injected into the contralateral forearm. Although the trial was not unblinded at this stage, these intradermal injections were performed open label. Participants were asked to refrain from taking antihistamines or oral steroids for a minimum of 5 days and 2 weeks beforehand, respectively. Early phase responses were measured 15 minutes after the intradermal injection. The wheal outline was traced and transferred into the patient record. Late-phase responses were measured after 24 hours by palpating the outline of oedema. The areas of the late response was also traced and transferred to the patient record. A single clinician performed all measurements under double-blind conditions. The early- and late-phase response areas were calculated from scaled scanned images of the tracings with NIS Elements v4.2 software (Nikon Instruments, Surrey, UK). Early- and late-phase response areas were then compared in the intradermal immunotherapy and control arms at each time point.
Skin biopsy
Forty participants (20 in each trial arm) were randomised to undergo 3-mm skin punch biopsies immediately after measurement of late-phase responses (i.e. 24 hours after challenge), 4 months after the final treatment injection, in September 2013. Biopsies were collected from both allergen-challenged and diluent control sites. Local anaesthesia was achieved with 10 mg/ml of lidocaine hydrochloride with 5 µg/ml of adrenaline (1 in 200,000). In the first 20 subjects, biopsies were divided with a scalpel into two pieces and one half piece was fixed in 4% paraformaldehyde (Sigma-Aldrich, Poole, UK) for 2 hours. In the rest of the subjects, entire biopsies were processed for immunohistochemistry by fixation in 4% paraformaldehyde at room temperature for 4 hours. After washing twice in 15% sucrose, biopsies were mounted in Optimal Cutting Temperature compound (OCT) embedding medium (Bayer UK Ltd, Basingstoke, UK) and stored at –80 °C pending analysis. The remaining unfixed half-biopsy pieces were cultured directly for T-cell analysis.
Analysis of T cells cultured from skin biopsies
Skin biopsy tissue was finely dissected and suspended in complete medium [Roswell Park Memorial Institute Medium, Sigma-Aldrich® (RPMI) supplemented with 10% foetal calf serum, penicillin (100 U/ml), streptomycin (100 µg/ml) and l-glutamine (2 mM); all Life Technologies, Warrington, UK]. Tissue was then cultured at 37 °C in a humidified atmosphere containing 5% carbon dioxide in the presence of interleukin 2 (IL-2; 50 U/ml). After 3–4 days, cells were passed through a 0.2-µm cell strainer to obtain single cell suspensions, and restimulated with immobilised anti-cluster of differentiation 3 (CD3)/cluster of differentiation 28 antibodies for a further 3 days, followed by expansion for 4 days in the presence of IL-2. Expanded T cells were stained with the viability dye eFluor®780 (eBioscience, Vienna, Austria) prior to surface staining with anti-cluster of differentiation 4 (CD4) PerCP-Cy5.5 (BioLegend, London, UK), anti-cluster of differentiation 8 BV510 (BD Biosciences, Oxford, UK), anti-CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2 cells) PE (BioLegend), anti-CXCR3 [chemokine (C-X-C Motif) receptor 3] BV421 (BioLegend), anti-chemokine receptor 6 (CCR6) PE-Cy7 (BD Biosciences) and anti-interleukin 25 receptor AF647 (kind gift of Dr Andrew McKenzie). Samples were resuspended for flow cytometric analysis (FACSCalibur™, BD Biosciences). Data were analysed using FlowJoTM v7.6 software (Tree Star Inc., Ashland, OR, USA). For microarray studies, cells were activated for 4 hours with ionomycin (0.5 µg/ml) and phorbol 12-myristate 13-acetate (5 ng/ml) (both Sigma-Aldrich). Ribonucleic acid (RNA) was isolated from cell pellets using the miRNeasy Mini Kit and RNeasy MinElute Cleanup Kit (both Qiagen, Manchester, UK) in accordance with the manufacturer’s instructions. Complementary deoxyribonucleic acid (cDNA) synthesis and amplification were performed with the Ovation PicoSL WTA Systems V2 kit (NuGEN, Leek, the Netherlands) as per the manufacturer’s instructions. Purity and yield was then analysed using the Bioanalyzer Platform (Agilent, Stockport, UK) and NanoDrop 2000 spectrophotometer (Thermo Scientific, Loughborough, UK), respectively, before amplified cDNA was biotin-labelled with the NuGEN Encore BiotinIL Module according to the manufacturer’s instructions. Biotin-labelled cDNA was hybridised to an Illumina HumanHT-12 v4 Expression BeadChip (Illumina, Saffron Walden, UK) before scanning with the iScan System (Illumina) utilising GenomeStudio software. Data analysis was performed with the Partek Genomics Suite™ software (Partek Inc., Chesterfield, MO, USA).
Immunohistochemistry
Immunohistochemical staining of skin biopsies was performed using the modified alkaline phosphatase anti-alkaline phosphatase (APAAP) method to stain for eosinophils, neutrophils, CD4+ T cells and CD3-positive (CD3+) T cells. 24,25 In brief, 8- to 10-µm thickness tissue sections were air dried overnight on poly-l-lysine-coated slides. For immunostaining, slides were incubated at room temperature in a humidified chamber with the primary mouse mAb [neutrophil elastase (Dako, Ely, UK); eosinophil major basic protein (Abcam, Cambridge, UK); CD3 and CD4, both Dako] suspended in 5% human serum/phosphate-buffered saline (PBS) for predetermined optimised incubation times. Sections were then washed in PBS and incubated with rabbit anti-mouse immunoglobulin (Dako) for 30 minutes then washed again. Slides were then incubated with a third layer of soluble complexes of alkaline phosphatase (AP) and mouse anti-APAAP (Serotec, Kidlington, UK) for 30 minutes, washed and developed with Fast Red (Sigma-Aldrich) for a further 20 minutes. Sections were washed extensively in PBS before counterstaining with Harris haematoxylin (BDH, Poole, UK) and mounting in glycerol gel. For negative controls, each primary antibody was substituted with the appropriate isotype-matched irrelevant mAb. Slides were counted blind in random order by two observers. Allergen and diluent biopsy sections were evaluated from each subject. The total number of positive cells was expressed as the number of cells per square millimetre of biopsy. Interobserver variability was 7%, assessed on repeat counts of 19 slides. The difference between the two counts was plotted against the mean of the two counts; all but one of the differences fell within two standard deviations (SDs) of the mean difference, indicating satisfactory agreement between observers.
Serum antibody measurements
Sera were analysed for concentrations of pre- and post-treatment P. pratense-specific IgG, IgG4 and IgE, and IgE specific to the major allergens Phl p 5 and Phl p 1 using a commercial assay system (ImmunoCAP™, ThermoFisher Scientific, Horsham, UK) in accordance with the manufacturer’s instructions.
Basophil activation tests
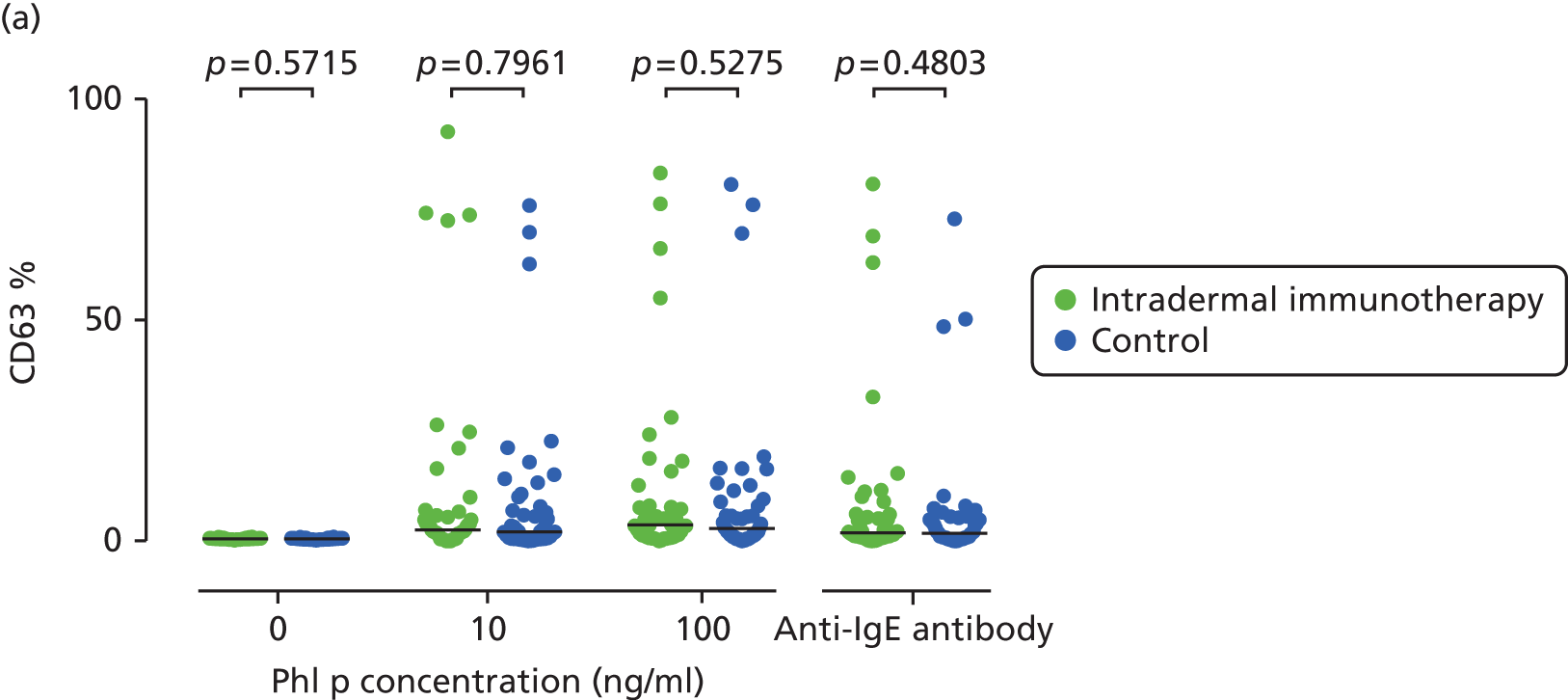
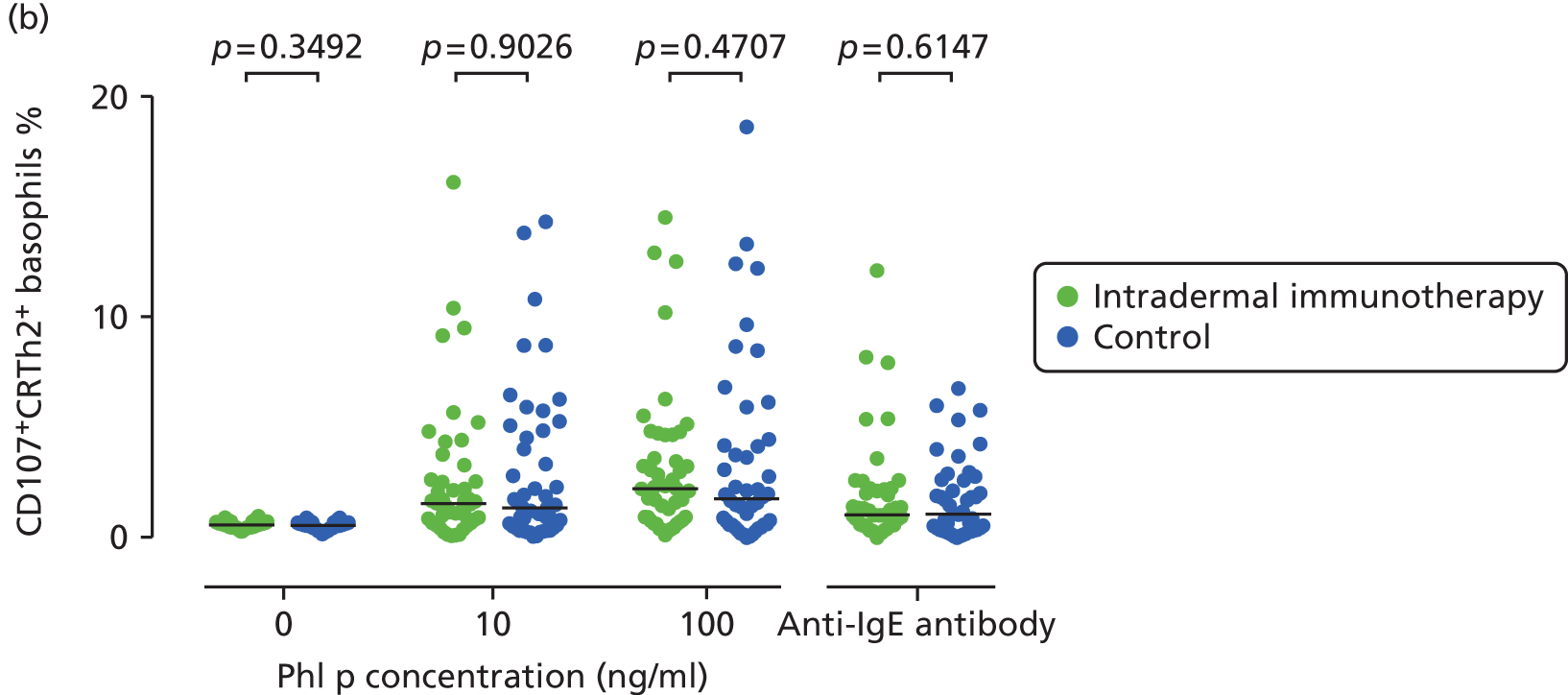
Basophil activation tests were performed in 92 participants following administration of the final intradermal allergen immunotherapy or control injection (May 2013). Whole blood was collected and tested within 2 hours of sampling under blinded conditions by a single investigator. Heparinised whole blood was immunostained with anti-human CD3 PE-Cy7 (BD Biosciences), CD294 PE (Miltenyi Biotec, Woking, UK), CD203c PerCP-Cy5.5 (BioLegend), CD303 APC (Miltenyi Biotec), CD107a Brilliant Violet 421 (BioLegend), CD63 FITC (BioLegend) and isotype controls. Basophils were then stimulated with anti-human IgE (1000 ng/ml, positive control; Abcam) or P. Pratense extract (ALK Abelló) at 10 ng/ml and 100 ng/ml for 15 minutes at 37 °C. Samples were then lysed (BD FACS Lysing Solution, BD Biosciences), washed and resuspended (CellFIX™, BD Biosciences) for flow cytometric analysis (FACSCalibur™, BD Biosciences). Data were analysed using FlowJoTM v7.6 software (Tree Star), gating on CD3–CD303–CD294+ basophils. Basophil activation was expression as the percentage of CD63+, CD203c+ or CD107a+ basophils of the entire basophil population, and compared between the two groups.
Statistical analysis
Sample size calculations for the primary outcome (CSMS) were performed, based on raw data from a previous clinical trial of subcutaneous grass pollen immunotherapy. 26 The power calculation was conservatively based on the detection of a clinical effect size of 80% of that reported in that trial. Using this method and a two-sided non-parametric test based on a Monte Carlo approach, group sample sizes of 35 and 35 achieved 90% power to detect such a difference in AUC of the CSMSs at a significance level of 0.05. To make allowance for the unknown distribution of the primary outcome, and based on the lower bound for the asymptotic relative efficiency of the Mann–Whitney U-test, the sample size was increased by a further 15% to 40 in each arm. Further accounting for a post-randomisation dropout rate of up to 10%, consistent with previous trials of grass pollen immunotherapy, a total sample size of 90 (45 each arm) was estimated as required.
The statistical analysis plan was finalised and agreed before any analysis was undertaken (see Appendix 6). Statistical analyses were performed on an intention-to-treat (ITT) basis, with data from all participants who could be assessed for the primary outcome. Summary measures for the baseline characteristics of each group were calculated as mean and SD for continuous (approximate) normally distributed variables, medians and interquartile ranges (IQRs) for non-normally distributed variables, and frequencies and percentages for categorical variables. The AUC of the CSMSs was plotted against time as a summary measure of the primary outcome. The primary efficacy analysis, that is, the difference between the two arms in AUC of the CSMSs, was analysed on randomised patients using a stratified Mann–Whitney U-test (van Elteren test), adjusted for the baseline stratification factors of size of the skin test to grass pollen, and presence or absence of rhinitis symptoms outside the grass pollen season. Median differences between the groups were calculated using the stratified Hodges–Lehmann method. Similar analyses were conducted for symptom scores, medication scores, symptoms in different organs and VAS scores. Linear mixed models were used to evaluate Mini-RQLQ and EQ-5D-5L scores in order to isolate the effect of the intervention on each arm after adjusting for stratification factors. Differences between the groups were reported with their 95% confidence intervals (CIs). All mechanistic between-group comparisons were performed by Mann–Whitney U-test, with the exception of serology and immunohistochemistry comparisons, which were analysed by analysis of covariance (ANCOVA). Comparisons of serology between pre and post treatment, and skin biopsy immunohistochemistry between diluent control and allergen challenge were made by Wilcoxon signed-rank test.
The primary outcome and secondary outcomes are reported in the ITT population without imputation of missing data. However, a sensitivity analysis was also performed, with missing data imputed for the primary outcome and secondary outcomes in the ITT population. A multiple imputation technique was applied, whereby missing data on a particular date were substituted with the mean CSMS on that date in the corresponding trial arm. Further sensitivity analyses were undertaken for the primary outcome and secondary outcomes in the predefined per-protocol population. Participants who were on holiday outside continental Europe during the daily collection period were considered as ‘missing data’ for the days concerned, in accordance with the Trial Steering Committee and statistical analysis plan. When > 50% of the data were missing, participants were excluded from the per-protocol analysis.
The principal software package was SAS/STAT® version 9.2 (SAS Institute Inc., Cary, NC, USA), with verification of results from syntax for selected analyses analysed in Stata® version 12.1 (StataCorp LP, College Station, TX, USA).
Chapter 3 Results
Study population
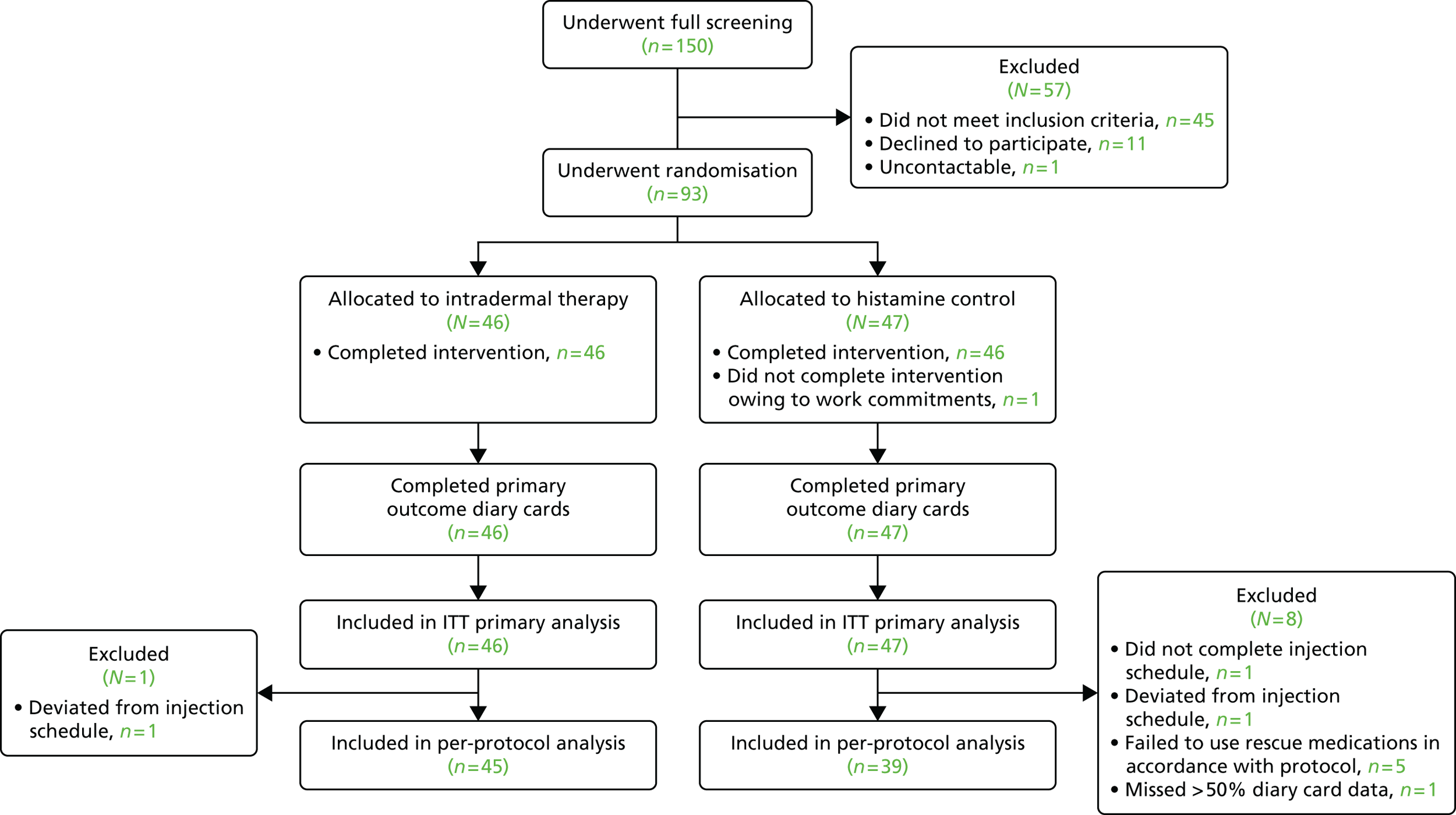
From 1660 people who completed initial online prescreening, 150 potential participants attended the Clinical Research Facility for full screening. Of these, 93 were enrolled and randomised to receive intradermal allergen or histamine control injections between 18 February and 1 March 2013 (Figure 2). Study arms were well balanced for baseline characteristics (Table 1). All 46 participants assigned to intradermal allergen immunotherapy completed the seven-injection treatment course, although one participant deviated from the administration schedule by 1 day for one injection. Of the 47 participants who were assigned to control injections, one did not complete the treatment course, withdrawing after the second injection because of work commitments, and another participant deviated from the administration schedule by 4 days because of an unrelated upper respiratory tract infection that necessitated postponement of the injection. There was a high rate of diary card data collection for the primary outcome: 99% of participants supplied > 50% of data for all days; 96% of participants supplied > 75% of daily data; and 94% of participants supplied > 90% of daily data. One patient holidayed outside continental Europe for 52% of the data collection period, so was excluded from the per-protocol analysis, in accordance with the predefined statistical analysis plan. Five participants, all in the control arm, significantly deviated from use of the rescue medications that were specified in the trial protocol according to criteria specified prior to unblinding. Participants were unable to identify if they had received active intradermal allergen treatment or histamine control (Table 2).
FIGURE 2.
Consolidated Standards of Reporting Trials diagram. All randomised participants were included in the ITT analysis. Only participants who adequately adhered to treatment and rescue medications were included in the per-protocol analysis.
| Baseline characteristic | Intradermal immunotherapy (N = 46) | Control (N = 47) |
|---|---|---|
| Age at screening (years), mean (SD) | 32 (9.9) | 35 (10.8) |
| Female sex, n (%) | 19 (41) | 12 (26) |
| Ethnicity, n (%) | ||
| White | 37 (80) | 37 (79) |
| Mixed | 3 (7) | 2 (4) |
| Asian | 4 (9) | 3 (6) |
| Black | 0 (0) | 3 (6) |
| Other | 2 (4) | 2 (4) |
| Allergy symptoms outside grass pollen season, n (%) | 16 (35) | 18 (38) |
| Total IgE (kUc/l), median (IQR) | 160 (80–263) | 121 (64–255) |
| P. pratense-specific IgE (kUA/l), median (IQR) | 22 (9–49) | 27 (10–54) |
| P. pratense SPT wheal diameter (mm), mean (SD) | 11 (5.0) | 12 (4.2) |
| SPT positive, n (%) | ||
| Timothy grass | 46 (100) | 47 (100) |
| Mixed grass | 46 (100) | 47 (100) |
| Silver birch | 24 (52) | 19 (40) |
| Mugwort | 9 (20) | 11 (23) |
| House dust mite | 24 (52) | 28 (60) |
| Cat | 18 (39) | 24 (51) |
| Dog | 36 (78) | 41 (87) |
| Horse | 6 (13) | 4 (9) |
| Aspergillus | 2 (4) | 1 (2) |
| Alternaria | 7 (15) | 6 (13) |
| Cladosporium | 2 (4) | 2 (4) |
| Vital signs | ||
| Pulse rate (b.p.m.), mean (SD) | 72 (10.9) | 69 (9.6) |
| Blood pressure – systolic (mmHg), mean (SD) | 133 (15.5) | 137 (12.5) |
| Blood pressure – diastolic (mmHg), mean (SD) | 80 (9.6) | 81 (9.4) |
| Spirometry | ||
| FEV1 (l), mean (SD) | 4 (0.9) | 4 (0.7) |
| FVC (l), mean (SD) | 5 (1.2) | 5 (1.0) |
| FEV1% predicted spirometry, mean (SD) | 101 (10.8) | 101 (11.2) |
| Allergy history, n (%) | ||
| Asthma (controlled with salbutamol) | 15 (33) | 17 (36) |
| Urticaria | 13 (28) | 16 (34) |
| Eczema | 14 (30) | 7 (15) |
| Food allergy | 6 (13) | 5 (11) |
| Drug allergy | 5 (11) | 5 (11) |
| Insect allergy | 2 (4) | 3 (6) |
| Medical history, n (%) | ||
| Respiratory | 10 (22) | 10 (21) |
| Dermatology | 9 (20) | 11 (23) |
| Musculoskeletal | 3 (7) | 9 (19) |
| Gastrointestinal | 6 (13) | 3 (6) |
| Genitourinary | 5 (11) | 4 (9) |
| Neurological | 1 (2) | 6 (13) |
| ENT | 4 (9) | 3 (6) |
| Psychiatric | 3 (7) | 2 (4) |
| Haematological | 1 (2) | 3 (6) |
| Cardiovascular | 2 (4) | 1 (2) |
| Hepatic | 1 (2) | 1 (2) |
| Endocrine | 1 (2) | 1 (2) |
| Neoplasia | 2 (4) | 0 (0) |
| Immunological | 1 (2) | 0 (0) |
| Infection | 1 (2) | 0 (0) |
| Other | 3 (7) | 2 (4) |
| Patient guess trial arm | Trial arm | |
|---|---|---|
| Intradermal immunotherapy (N = 44) | Control (N = 43) | |
| Intradermal immunotherapy | 22 | 22 |
| Control | 22 | 21 |
Clinical outcomes
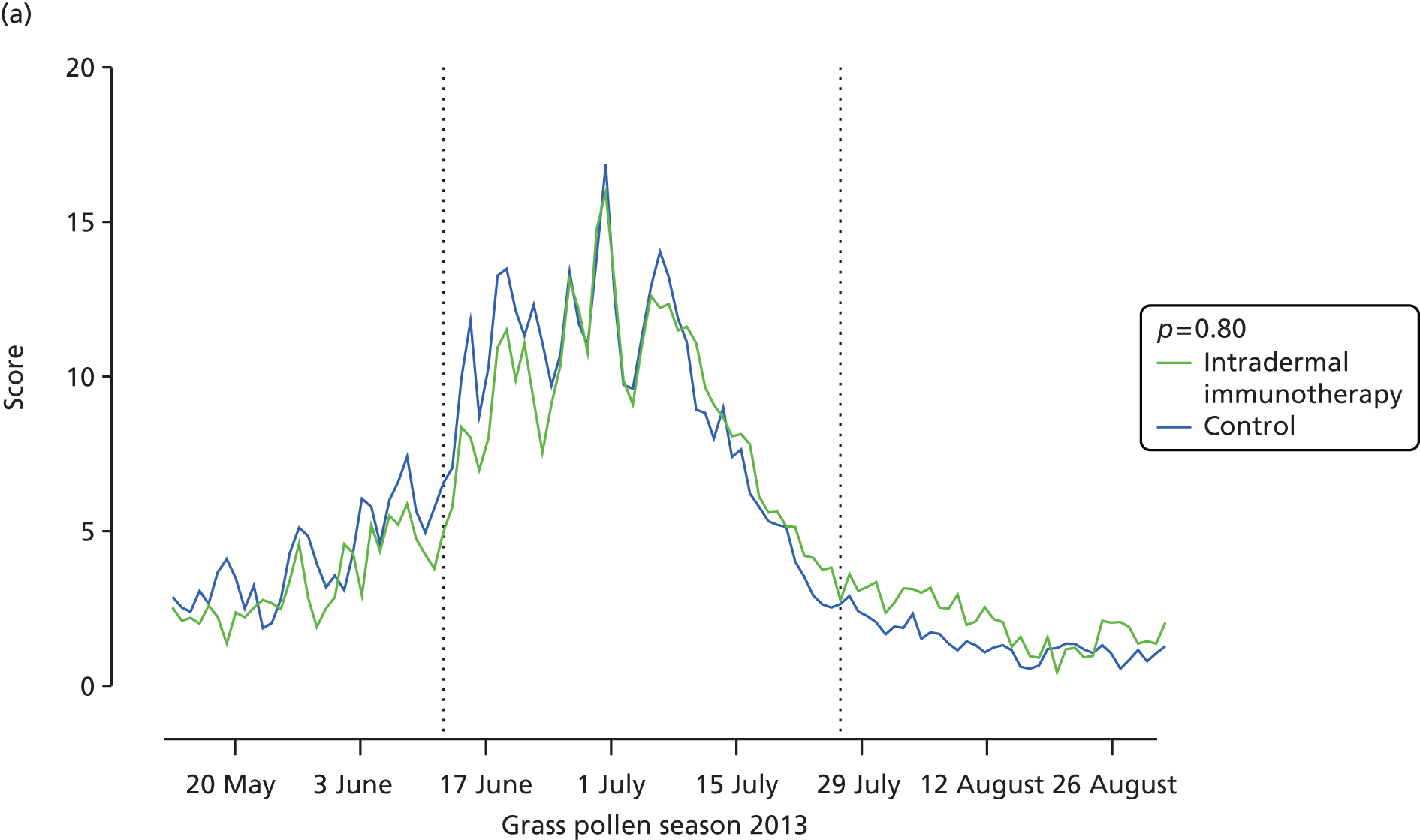
All 93 randomised participants were evaluated for the primary outcome and were included in the ITT analysis. The CSMS showed a clear correlation with daily pollen counts in London (Figure 3), which peaked at levels in the above-average range. However, intradermal immunotherapy did not significantly affect the primary end point, that is, the CSMS over the whole grass pollen season (difference in median AUC = 14; 95% CI –172.5 to 215.1; p = 0.80) (see Figure 3 and Table 3). Furthermore, significant differences were not seen between the trial arms in the secondary end points of overall symptom scores or rescue medication use (p = 0.44) during the whole season, or the CSMSs during peak season (12 June to 26 July 2013) (p = 0.99; see Table 3).
FIGURE 3.
Primary outcome and daily symptom and daily medication scores in the primary intention-to-treat analysis. (a) Mean daily combined symptom and medication; and (b) median daily symptom scores (sum of scores for nose, eyes, lungs, mouth according to treatment arm. The p-values are based on (c) mean medication scores; and (d) daily grass pollen counts in central London over the 2013 grass pollen season. AUC values for each participant were compared Mann–Whitney U-tests. Broken vertical lines indicate the beginning and end of the peak pollen season (12 June to 26 July 2013).
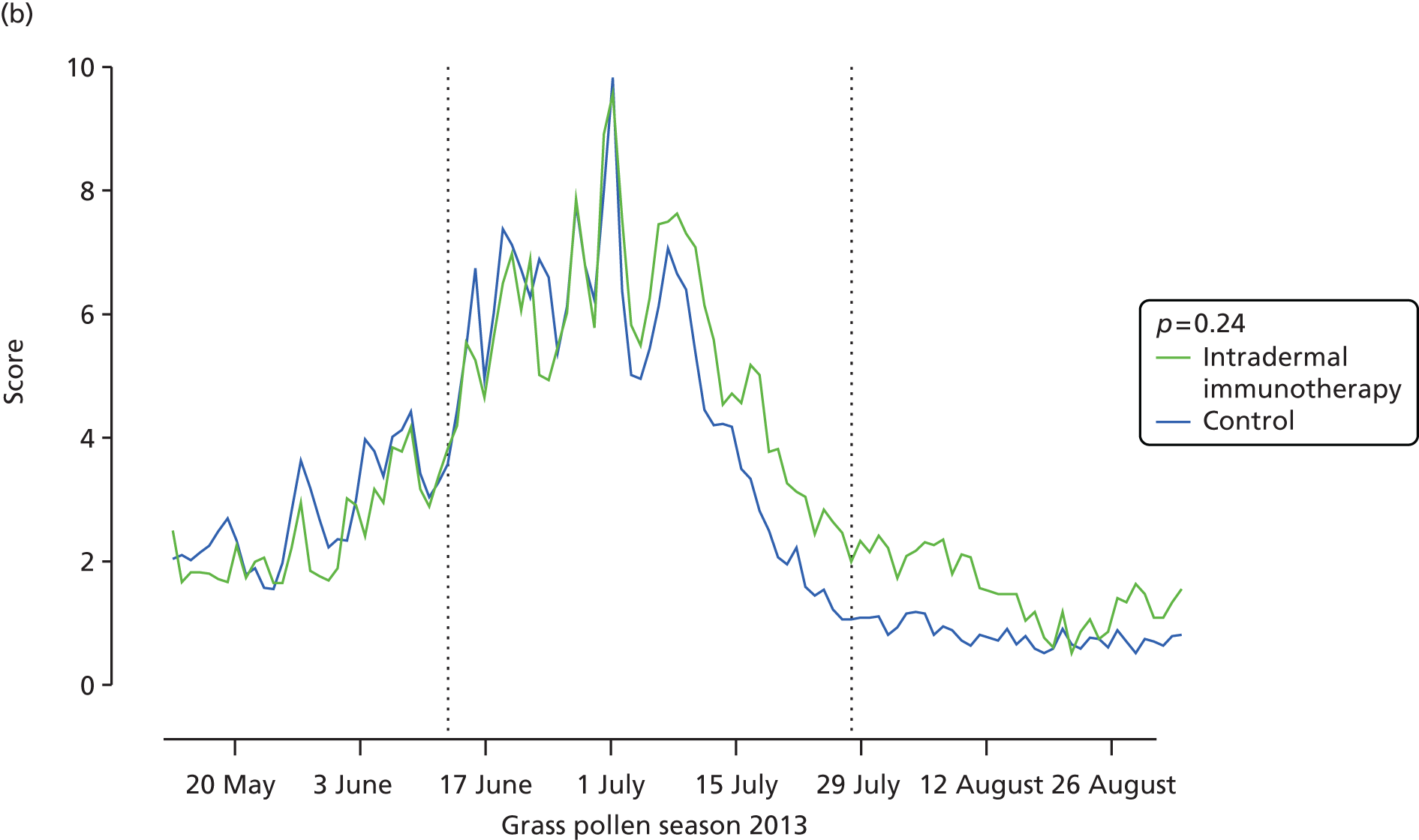

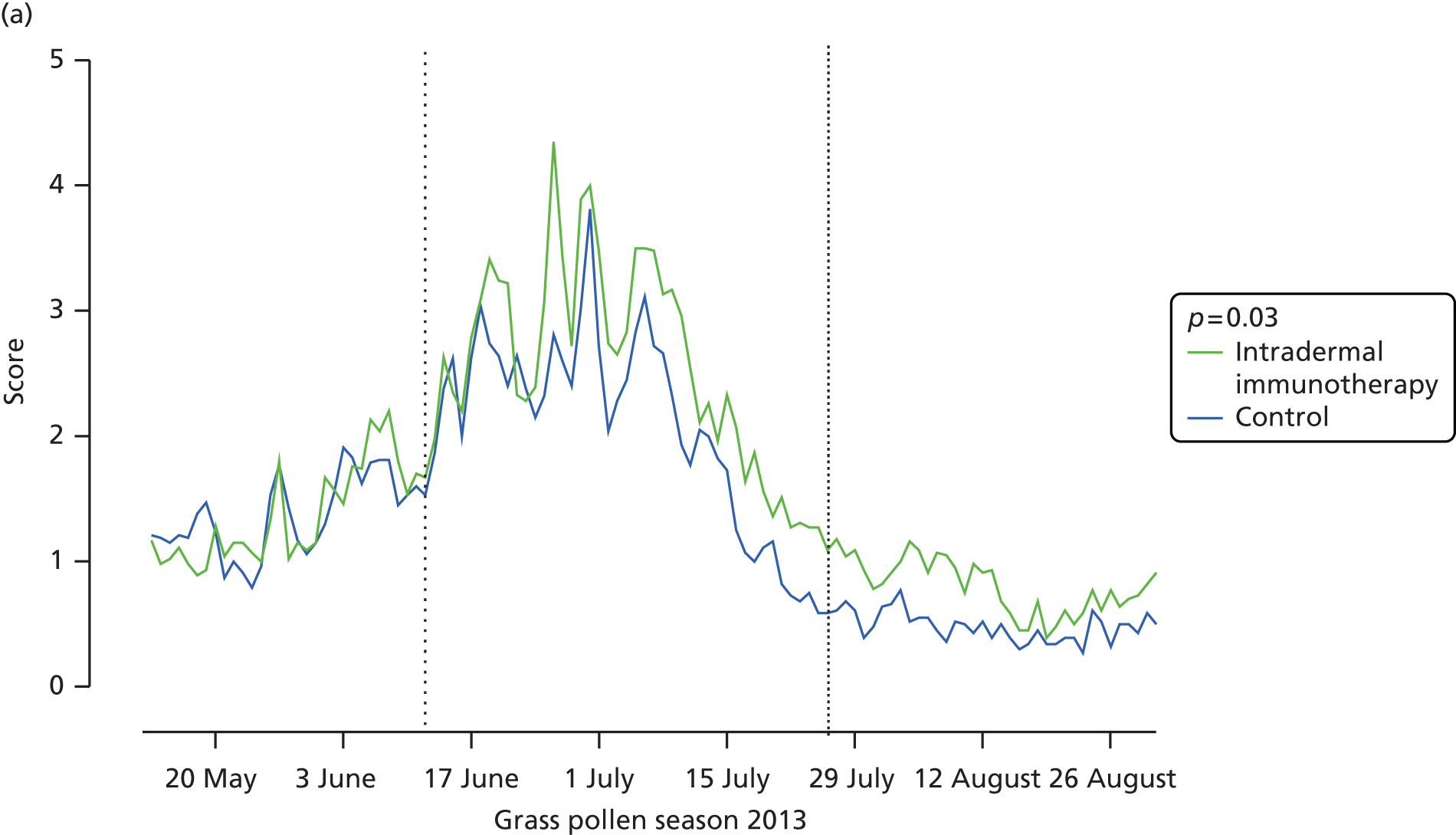
Among other prespecified secondary end points, allergic rhinitis symptoms, measured by daily nasal symptom scores, were significantly higher in the intradermal allergen immunotherapy group than in the histamine control group, with a difference in median AUC of 35 (95% CI 4.0 to 67.5; p = 0.03) (see Table 3 and Figure 4). Furthermore, there was a trend for rhinitis symptoms measured by VAS to be higher in the arm that received intradermal immunotherapy group, with a difference in median AUC of 53 (95% CI –11.6 to 125.2; p = 0.05) (see Table 3 and Figure 4). No significant differences were seen between groups in daily eye or lung symptoms (see Table 3), although there was a trend for mouth symptoms to be higher in the intradermal allergen group (difference in median AUC 10.0; 95% CI –3.8 to 24; p = 0.05). No significant group differences were observed in eye symptoms measured by VASs, Mini-RQLQ scores, EQ-5D-5L scores, global evaluation of symptoms scores, numbers of symptom-free or medication-free days or number of days during which prednisolone was used as rescue medication (see Table 3). Analysis of the ITT population after imputation of missing data values gave results that were consistent with the main ITT analysis (see Appendix 7). The per-protocol analysis included 45 participants who received intradermal allergen immunotherapy and 39 who received the histamine control treatment (see Appendix 8). In this population, daily individual nasal (p = 0.05) and mouth symptoms (p = 0.02) were also higher in the actively treated group, with a trend for worse lung symptoms (p = 0.05). Participants in the intradermal allergen group in this population also had significantly worse nasal symptoms measured by VAS (p = 0.01) and recorded fewer symptom-free days than subjects in the control group (p = 0.04).
FIGURE 4.
Nasal symptoms. (a) Mean daily nasal symptom scores (sum of scores for sneezing, blockage and running); and (b) mean nasal symptoms measured by VAS (total of blockage, running, itching and sneezing). AUC values for each participant were compared according to treatment arm. The p-values are based on Mann–Whitney U-tests.

| Trial outcomes | Intradermal immunotherapy (n = 46), median (IQR) | Control (n = 47), median (IQR) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Primary outcome | ||||
| CSMS during entire season | 502 (333–841) | 487 (365–717) | 14 (–172.5 to 215.1) | 0.80 |
| Secondary outcomes | ||||
| Symptom score during entire season | 335 (183–503) | 264 (156–398) | 59 (–1.3 to 110.9) | 0.24 |
| Medication score during entire season | 242 (116–405) | 263 (129–482) | –19 (–153.0 to 100.2) | 0.44 |
| CSMS score during peak season | 356 (232–521) | 365 (278–508) | –8 (–75.8 to 66.3) | 0.90 |
| Nasal symptom score during entire season | 174 (120–207) | 121 (81–200) | 35 (4.0 to 67.5) | 0.03 |
| Mouth symptom score during entire season | 34 (8–90) | 14 (5–45) | 10 (3.8 to 24) | 0.05 |
| Eye symptom score during entire season | 79 (41–153) | 78 (52–180) | –7 (–18.5 to 2.9) | 0.54 |
| Lung symptom score during entire season | 17 (3–32) | 12 (0–34) | 4 (–1 to 15) | 0.17 |
| Nasal allergic symptoms measured by VAS | 156 (104–275) | 122 (54–184) | 53 (–11.6 to 125.2) | 0.05 |
| Eye allergic symptoms measured by VAS | 84 (32–197) | 144 (41–176) | –3 (–46.0 to 35.8) | 0.40 |
| Global evaluation of symptom scores | 3 (2–4) | 3 (1–4) | 0 (0 to 1) | 0.48 |
| Symptom-free days | 35 (19–53) | 41 (23–61) | –6 (–17 to 3) | 0.15 |
| Number of days prednisolone used during entire season | 0 (0–0) | 0 (0–0) | 0 (0 to 0) | 0.36 |
| Medication-free days | 81 (65–93) | 76 (65–94) | 4 (–11 to 21) | 0.22 |
| Mini-RQLQ | 16 (13–23) | 18 (10–25) | –0.3 (–4.2 to 3.7) | 0.89 |
| EQ-5D-5L | 87 (83–94) | 88 (81– 94) | 9 (–24.8 to 43.6) | 0.59 |
Given the unexpected observation that allergic rhinitis nasal symptom scores were higher in participants who had received intradermal allergen immunotherapy, a post hoc analysis was performed to compare the daily data for each individual allergic symptom in the two trial arms (see Appendix 9). Sneezing (p = 0.01) and cough scores (p = 0.03) were both significantly higher in the intradermal immunotherapy group, with non-significant trends for greater chest tightness (p = 0.08) and mouth itching (p = 0.06). In contrast, eye swelling was lower in the intradermal immunotherapy group (p = 0.03). Further post hoc analysis of individual nasal symptoms measured by VAS also revealed higher scores after intradermal immunotherapy for running (p = 0.006), sneezing (p = 0.006) and itching (p = 0.003) (see Appendix 10).
Safety
There was a low rate of AEs that were related to treatment (Table 4). There were three serious AEs, although all were unrelated to treatment. One participant in the intradermal allergen immunotherapy group was hospitalised for severe tonsillitis. One control arm participant was admitted for overnight polysomnography, and another control participant required treatment to remove an infected dental plate. There were no deaths during the study. Three participants in the intradermal immunotherapy group and six in the control group were recorded with treatment-related AEs – all mild grade 1 systematic reactions. These reactions manifested as generalised pruritus without wheals, except for one intradermal allergen participant who developed erythema, which tracked from the injection site in a lymphatic distribution (IgE-mediated lymphangitis) approximately 20 minutes after every intradermal injection.
| Adverse events/reactions | Intradermal immunotherapy (n = 46) | Control (n = 47) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants with ≥ 1 AEs | % participants | Number of events | % event rate | Number of participants with ≥ 1 AEs | % participants | Number of events | % event rate | Number of participants | Number of events | |
| Any AEs | 40 | 87.0 | 148 | 42 | 89.4 | 145 | 0.76 | |||
| Serious AEs | 1 | 2.2 | 1 | 0.7 | 2 | 4.3 | 2 | 1.4 | 1.00 | 0.62 |
| Tonsillitis | 1 | 2.2 | 1 | 0.7 | 0 | 0.0 | 0 | 0.0 | 0.49 | 1.00 |
| Overnight stay – polysomnography | 0 | 0.0 | 0 | 0.0 | 1 | 2.1 | 1 | 0.7 | 1.00 | 0.49 |
| Extraction of infected dental plate | 0 | 0.0 | 0 | 0.0 | 1 | 2.1 | 1 | 0.7 | 1.00 | 0.49 |
| Relation of AE to treatment | ||||||||||
| Definite/probable | 3 | 6.5 | 15 | 10 | 6 | 12.8 | 14 | 9.7 | 0.49 | 0.89 |
| Possible | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.00 | 1.00 |
| Remote | 30 | 65.2 | 68 | 46 | 34 | 72.3 | 70 | 48.3 | 0.46 | 0.69 |
| None | 32 | 69.6 | 65 | 44 | 34 | 72.3 | 61 | 42.1 | 0.77 | 0.75 |
| AE withdrawals | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.00 | 1.00 |
| Systemic adverse reactions | 3 | 6.5 | 15 | 10.0 | 6 | 12.8 | 13 | 9.0 | 0.49 | 0.73 |
| Generalised pruritus | 2 | 4.3 | 8 | 5.4 | 4 | 8.5 | 9 | 6.2 | 0.68 | 0.77 |
| IgE-mediated lymphangitis | 1 | 2.2 | 7 | 4.7 | 0 | 0.0 | 0 | 0.0 | 0.49 | 0.01 |
| Light-headedness | 0 | 0.0 | 0 | 0.0 | 2 | 4.3 | 2 | 1.4 | 0.49 | 0.24 |
| Facial flushing/feeling hot | 0 | 0.0 | 0 | 0.0 | 2 | 4.3 | 3 | 2.1 | 0.49 | 0.12 |
| Systemic adverse reactionsa | ||||||||||
| Grade 1 | 3 | 6.5 | 15 | 10 | 6 | 12.8 | 12 | 8.3 | 0.49 | 0.58 |
| Grade 2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.00 | 1.00 |
| Grade 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.00 | 1.00 |
| Grade 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.00 | 1.00 |
Immunological end points
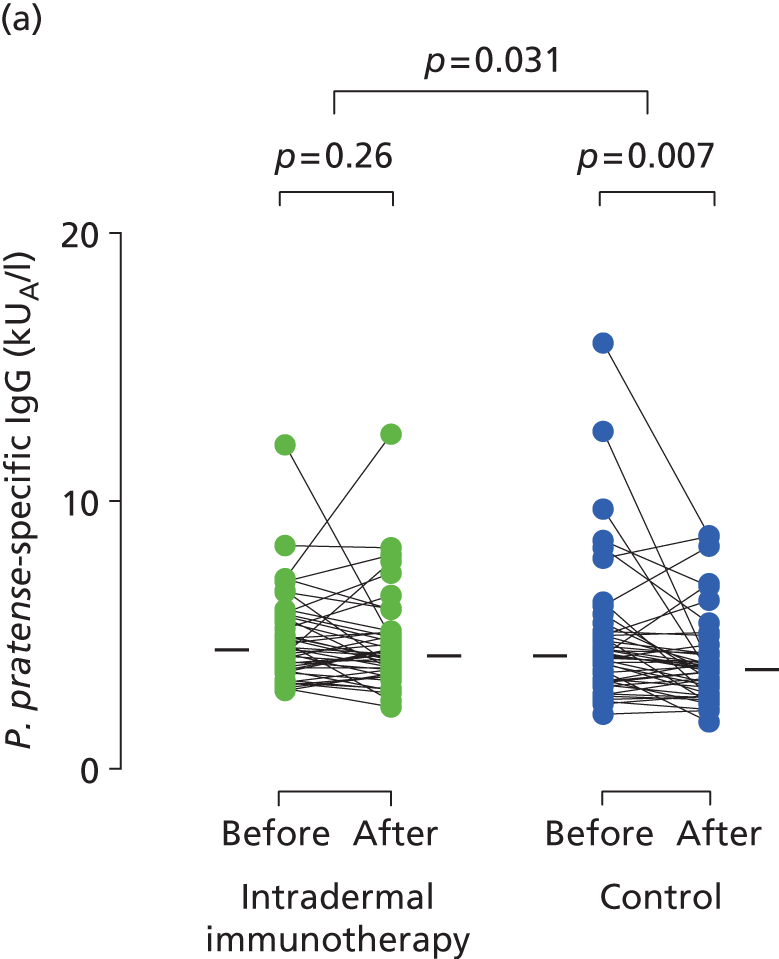
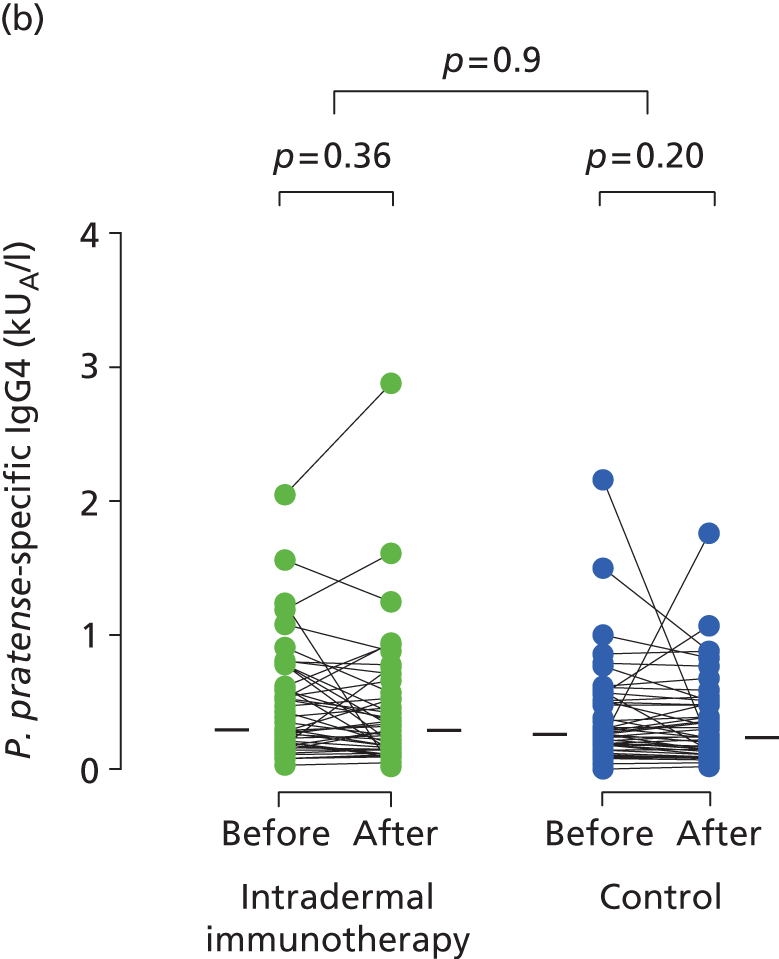
Serum immunoglobulins specific for whole P. pratense (Timothy grass) and major Timothy grass allergens Phl p 1 and Phl p 5 were compared before (between October 2012 and January 2013) and after (May 2013) intradermal allergen or control injection therapy. In the histamine control arm, there was a typical small seasonal decline in allergen-specific IgE antibodies (all p < 0.001; Figure 5). This seasonal decline in IgE was, however, significantly less in the intradermal allergen immunotherapy group than the control group (all p = 0.001), indicating that intradermal allergen treatment stimulated allergen-specific IgE production. A treatment effect was also seen on P. pratense-specific IgG titres, which fell in the control but not the intradermal allergen group over the same period (p = 0.03; Figure 6), although no effect was seen on IgG4 responses.
FIGURE 5.
Immunological outcomes. Levels of (a) P. pratense-specific IgE; (b) Phl p5-specific IgE; and (c) Phl p1-specific IgE before and after completion of seven intradermal allergen or histamine control injections. Solid bars represent median values. The p-values for pre- and post-treatment serology comparisons are based on the Wilcoxon signed-rank test. The p-values for between-group IgE comparisons are based on ANCOVA.
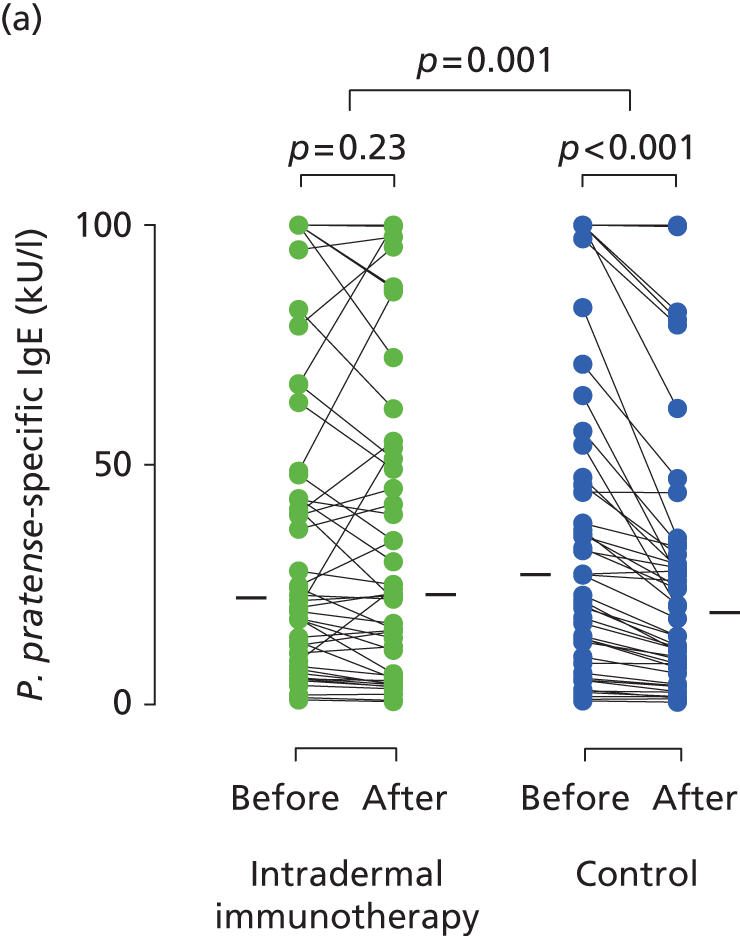
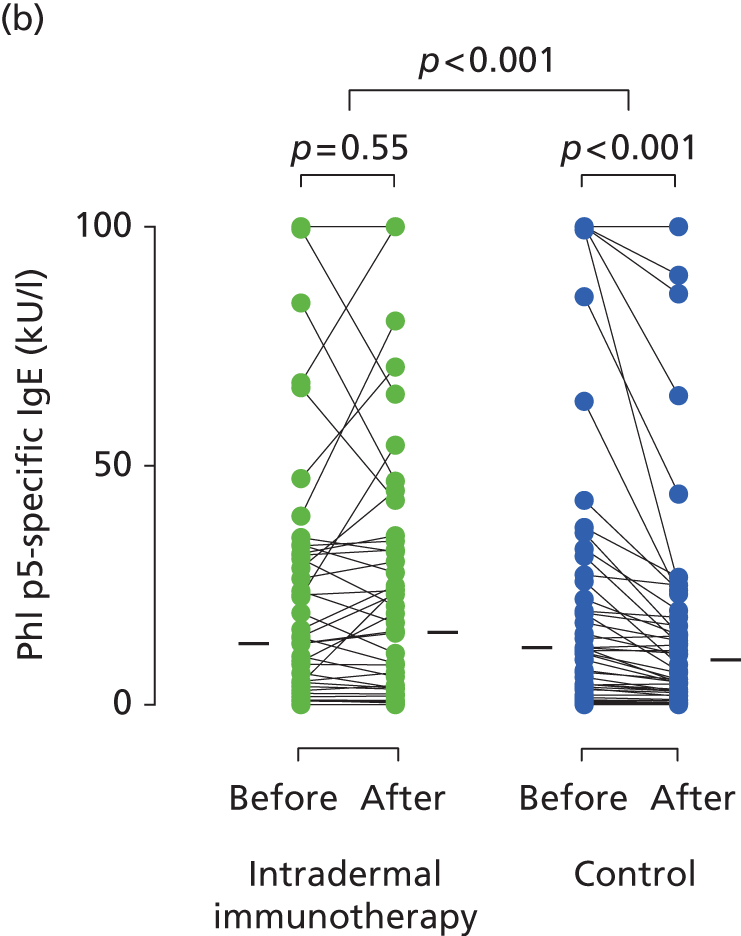

FIGURE 6.
Immunological outcomes. Levels of (a) P. pratense-specific IgG; and (b) P. pratense-specific IgG4 before and after completion of seven intradermal allergen or histamine control injections. The p-values for pre- and post-treatment serology comparisons are based on the Wilcoxon signed-rank test. The p-values for between-group IgG comparisons are based on ANCOVA.
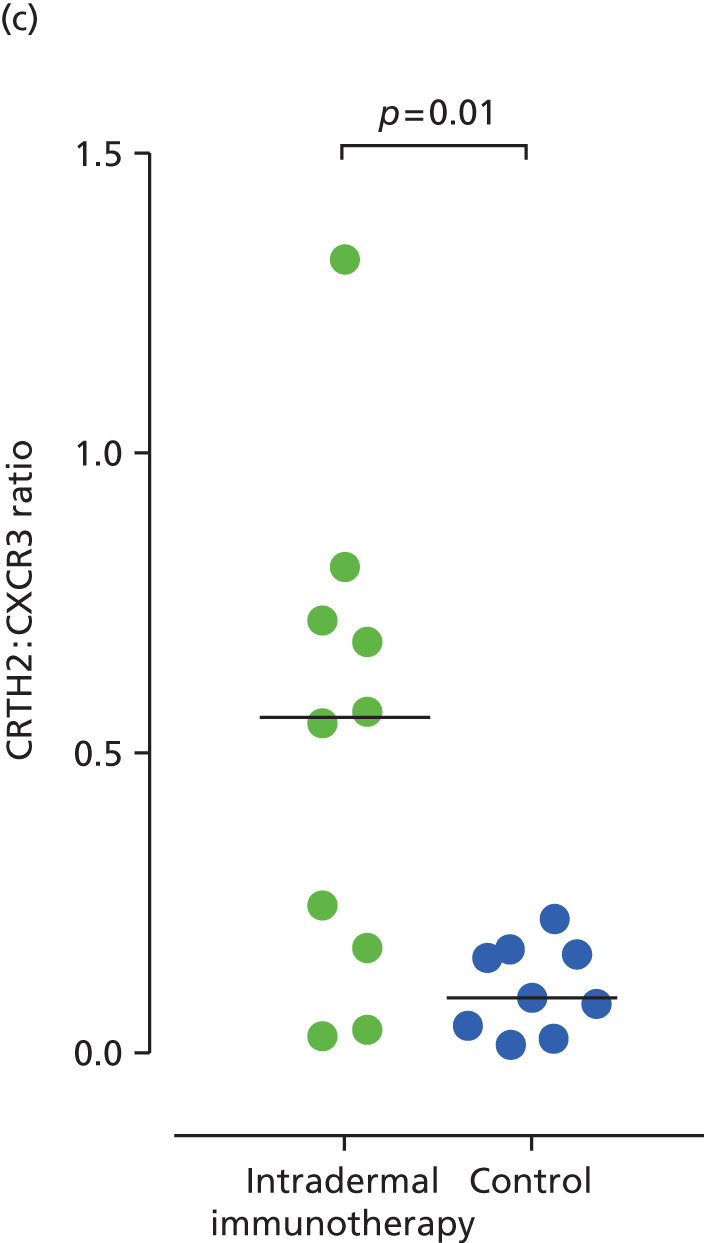
For surface phenotype analysis, CD4+ T cells were successfully expanded from 19 of 20 skin biopsies (10 from the intradermal immunotherapy group and nine from the control group) collected 24 hours after an intradermal grass pollen challenge, at the end of the 2013 grass pollen season. Cutaneous CD4+ T cells derived from grass pollen challenged sites showed higher expression of Th2 surface marker CRTH2 in the intradermal allergen immunotherapy group (median 13.4%, IQR 6.3–25.4) than the control group (median 6.3%, IQR 1.9–7.6; p = 0.04), whereas expression of the T helper type 1 cell (Th1) marker CXCR3 was lower in the intradermal allergen immunotherapy group [median 33.5 (IQR 24.7–47.3) vs. median 56 (IQR 45.8–63.8); p = 0.01] (Figure 7). No differences were seen in the expression of T helper type 17 cell marker CCR6 or the interleukin 25 receptor (not shown). Insufficient T cells could be expanded from diluent-challenged skin biopsies for analysis. Microarray transcriptional profiling was performed on cultured T cells that were derived from 15 allergen-challenged skin biopsies (seven intradermal allergen treatment and eight control arm subjects). Only 14 genes were significantly overexpressed by skin T cells in the intradermal allergen immunotherapy group [defined as > 1.5-fold higher expression than in control group and p < 0.05 using a three-way analysis of variance (ANOVA) model] including the Th2 cytokine interleukin 5 (IL-5) (p = 0.03) (Table 5; Microarray Gene Expression Omnibus accession number GSE72324).
FIGURE 7.
Expression of (a) CRTH2 (Th2 marker); (b) CXCR3 (Th1 marker); and (c) ratio of CRTH2 to CXCR3 expression on CD4+ cells expanded from skin biopsies (24 hours post-skin challenge). The p-values are based on Mann–Whitney U-tests.


| Gene | p-value | Fold difference |
|---|---|---|
| Intradermal immunotherapy ‘down’ vs. control group | ||
| LOC100133042 | 0.02 | –1.80 |
| CEP55 | 0.03 | –1.78 |
| GFOD1 | 0.00 | –1.77 |
| HIST2H2AB | 0.04 | –1.62 |
| H2AFZ | 0.02 | –1.61 |
| LOC730534 | 0.01 | –1.57 |
| HSD17B4 | 0.02 | –1.57 |
| HIST1H2AD | 0.03 | –1.56 |
| HDAC1 | 0.01 | –1.55 |
| CCL3L1 | 0.03 | –1.53 |
| CALR | 0.02 | –1.52 |
| CDCA5 | 0.01 | –1.52 |
| PRDX5 | 0.01 | –1.51 |
| FEN1 | 0.02 | –1.50 |
| Intradermal immunotherapy ‘up’ vs. control group | ||
| EPS15 | 0.02 | 1.51 |
| MYB | 0.01 | 1.52 |
| GK | 0.03 | 1.53 |
| RNASET2 | 0.03 | 1.55 |
| LOC729383 | 0.02 | 1.56 |
| GPR171 | 0.00 | 1.59 |
| LOC729387 | 0.04 | 1.60 |
| SLC11A2 | 0.02 | 1.60 |
| HS.508682 | 0.04 | 1.68 |
| IL5 | 0.03 | 1.71 |
| GBP5 | 0.05 | 1.79 |
| TNFSF8 | 0.01 | 1.79 |
| TNIP3 | 0.03 | 1.87 |
| CENTA1 | 0.05 | 2.11 |
Immunohistochemistry performed on the entire 40 diluent- and 40 allergen-challenged skin biopsies (20 intradermal allergen treatment and 20 control arm subjects) showed grass pollen-induced recruitment of eosinophils, neutrophils, CD3+ T cells and CD4+ T cells, but no significant treatment effect (Figure 8). Furthermore, no significant treatment effect was seen on surface expression of peripheral blood basophil activation markers (Figure 9).
FIGURE 8.
Immunohistochemistry analysis of skin biopsies. Comparison of allergen-induced inflammatory cell numbers in skin biopsies from intradermal immunotherapy and control arm participants. Data shown indicate numbers of (a) neutrophils; (b) eosinophils; (c) CD3 + T cells; and (d) CD4+ T cells in skin biopsies taken after diluent and P. pratense intradermal skin challenges in September 2013. Cells were stained using the APAAP method. Solid bars represent median values. The p-values comparing diluent- and allergen-challenged biopsies are based on the Wilcoxon signed-rank test. The p-values for between-group comparisons are based on ANCOVA.



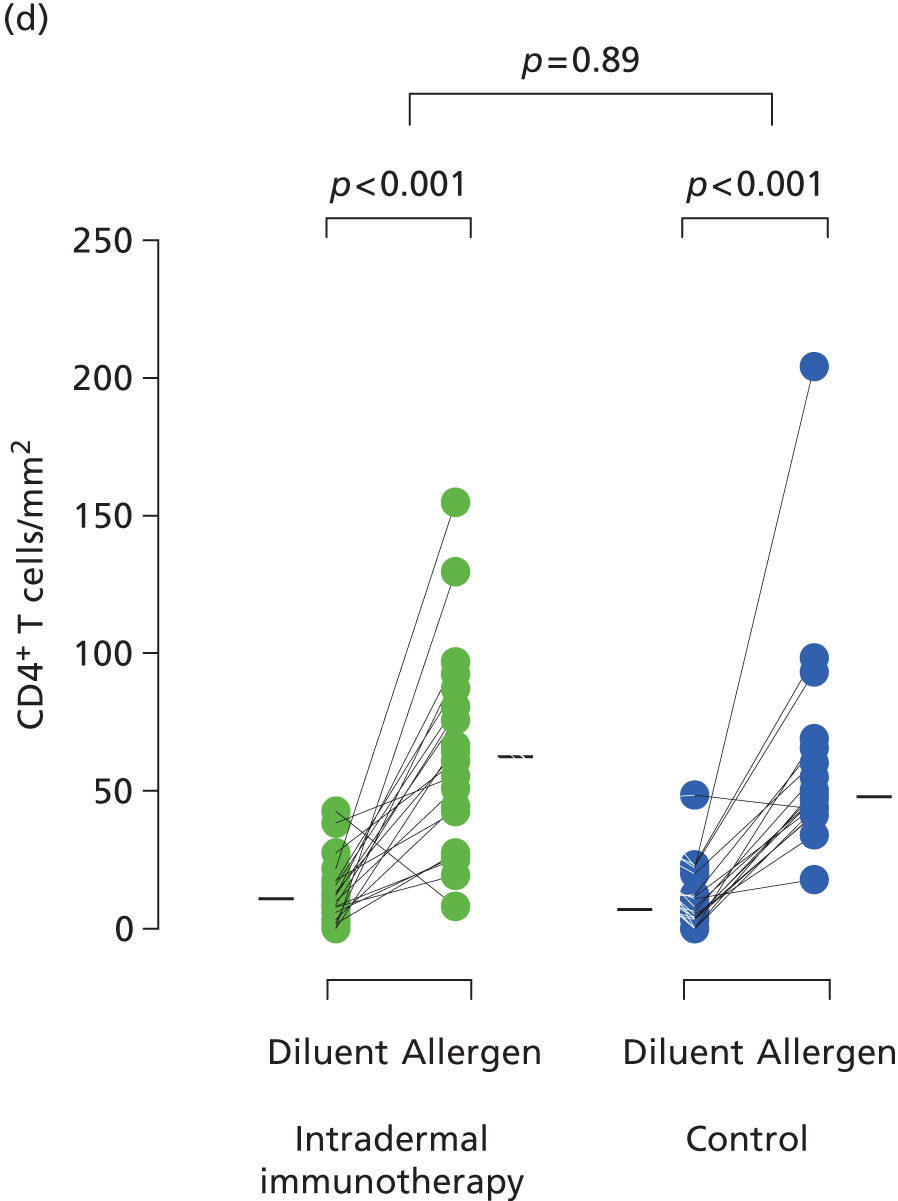
FIGURE 9.
Basophil activation tests. Percentage of basophils staining positive for activation markers. (a) CD63; (b) CD107a; and (c) CD203c. Whole blood was stimulated under the conditions described. The p-values are based on Mann–Whitney U-tests.

Intradermal skin challenge responses
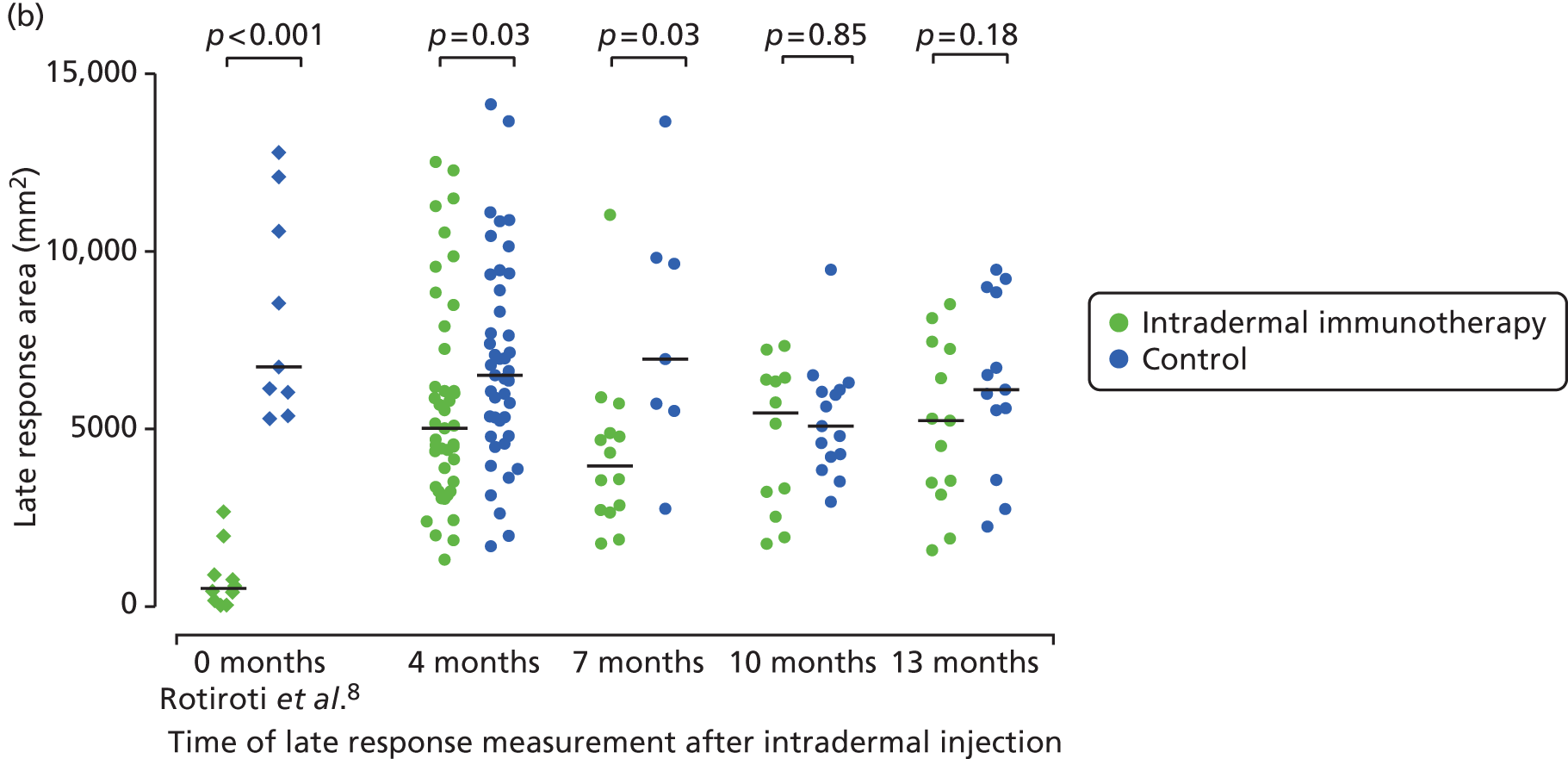
Early- (15 minutes) and late-phase (24 hours) skin responses could be measured in 86 participants 4 months after the final treatment injection in September 2013, and the measurements were repeated at either 7, 10 or 13 months (Figure 10a). The size of late-phase responses in the control group was consistent with that previously reported under the same conditions9 (shown for comparison in Figure 10). Late-phase responses remained significantly suppressed in the group that had received intradermal immunotherapy at both 4 and 7 months (both p = 0.03), although the degree of suppression at these time points was clearly less than that which we previously reported immediately after completion of six injections. Late responses were not suppressed at 10 or 13 months. These data suggest that the suppressive effect of intradermal immunotherapy on late-phase responses was wearing off within 4 months. In contrast with the late-phase response, no significant differences between treatment arms were seen in early-phase responses at 4-, 7-, 10- or 13-month time points (see Figure 10b).
FIGURE 10.
Late-phase skin responses. Areas of cutaneous (a) early-; and (b) late-phase responses (15 minutes and 24 hours after intradermal skin challenge, respectively), performed 4 months and either 7, 10 or 13 months post treatment (September 2013). Late response suppression is shown from our previous study (Rotiroti et al. 6) immediately after six 2-weekly intradermal injections. Solid bars represent median values. The p-values are based on Mann–Whitney U-tests.

Chapter 4 Discussion and conclusions
In this RCT, we have conclusively demonstrated that preseasonal treatment with seven intradermal grass pollen injections containing 7 ng of major allergen Phl p 5 is not a clinically effective treatment for seasonal allergic rhinitis. Furthermore, no benefit of this treatment approach was evident from analysis of secondary end points. In contrast, analysis of certain prespecified secondary end points showed that intradermal allergen immunotherapy was associated with a modest and unexpected worsening of allergic rhinitis nasal symptoms, as measured by daily symptom scores and 2-weekly VAS scores. Furthermore, in a per-protocol analysis we also found evidence for worsening of both lung and mouth symptoms in the group that received intradermal allergen treatment, together with fewer symptom-free days. In mechanistic studies we also observed evidence for a degree of immunological priming to allergen, manifest as a small relative increase in allergen-specific IgE responses and skewing of skin CD4+ T-cell surface markers in favour of a Th2 response. The study also confirmed our earlier observations that repeated intradermal allergen injections inhibits allergen-induced late-phase skin responses. However, this effect appeared to have dissipated when assessed 10 months after stopping intradermal immunotherapy, suggesting that the immunological effect of this intervention was transient.
No serious AEs occurred that were attributable to grass pollen intradermal allergen immunotherapy, and 92 of the 93 participants completed the seven-injection course. The one participant who withdrew during the treatment period did so for unrelated reasons. Five participants deviated significantly from the protocol in use of rescue medication, mainly in excessive use of antihistamines or topical nasal steroid or eye drops. Two participants also used prednisolone without reference to a study physician. We were unable to identify an explanation for why these five participants were all in the histamine control arm, but their exclusion in the per-protocol analysis did not affect the main outcome of the study.
Strengths of this study include the stringent selection of participants in accordance with clinical criteria specified in guidelines for grass pollen immunotherapy,4 the high rate of daily diary card data collection and the successful blinding of the active treatment. In addition, we used the clinical end point recommended by the WAO for trials of immunotherapy in allergic rhinitis. A possible limitation of this study is that the dose of grass pollen was not increased during the treatment course. We did not do this because of our previous observation that repeating the same dose was sufficient to achieve almost complete suppression of the late-phase response. In addition, our goal was to develop a treatment regimen that had the potential to be widely adopted, and dose escalations would probably increase the risk of systemic reactions. Another possible weakness is that injections were not continued throughout the grass pollen season, although previous RCTs of subcutaneous grass pollen immunotherapy have demonstrated efficacy for preseasonal regimens. 28 An allergen dose equivalent to 7 ng of the major Timothy grass pollen allergen Phl p 5 was selected for several reasons. First, we previously reported in our proof-of-concept study conducted in a similar population that six 2-weekly injections at this dose led to almost complete inhibition of the cutaneous allergen-induced late-phase response induced by these injections. This is similar to the effect of clinically effective cutaneous late-phase responses seen following high-dose subcutaneous immunotherapy10 and markedly exceeds that following treatment with sublingual grass pollen vaccines. 11 Second, the late-phase response induced by this dose corresponds to an average diameter of approximately 10 cm, a size that we considered to be at the limits of tolerability for patients for a routine treatment, especially as the response can be much larger in a proportion of subjects. Although it is impossible to equate this dosage precisely with that used in the uncontrolled historical studies of Phillips,12,13 it is notable that he described his treatment as inducing ‘a local reaction about the size of the patient’s palm’,12 which is similar to the response size we induced.
In this study, we measured only late-phase skin responses at the end of the 2013 grass pollen season, by which time some 4 months had passed since the completion of the intradermal allergen/control immunotherapy injection regimen. We did not perform these measurements earlier for two reasons. First, this would have necessitated giving an intradermal allergen challenge to the control arm participants, and we were concerned that this, in itself, might exert a biological effect and alter clinical outcomes in this group during the pollen season. Second, measuring the late-phase response sizes could have compromised blinding before collection of the clinical outcome data, as our previous data suggested that these responses would be > 90% suppressed at this time. Therefore, the first late-phase response measurements were obtained 4 months after the final preseasonal injection. Late-phase responses were still significantly lower in the intradermal allergen immunotherapy group than in the control group at this time point, and also at the subsequent 7-month time point. This difference was, however, significantly less than we previously observed immediately after six intradermal injections in the proof-of-concept study, suggesting that late-phase response suppression is transient and mostly reversed within 4 months, and completely reversed by 10 months. The lack of clinical benefit and potential worsening of allergic symptoms despite suppression of the late skin response in this study may indicate that the late-phase skin response is not relevant to expression of grass pollen allergic disease. An alternative explanation, which is more likely in our view, is that the consistent suppression of the late-phase response following subcutaneous and sublingual immunotherapy10,11 may be necessary, but not sufficient alone, to account for associated clinical improvement.
Allergen-specific IgE concentrations were measured in serum samples that were collected at initial screening, that is, between October 2012 and January 2013, and again immediately after completion of the intradermal allergen or control injections (May 2013). In the control arm, there was a consistent but small decline in the IgE levels that were specific for whole grass allergen and the Phl p 1 and Phl p 5 major allergens over this period. This seasonal variation in IgE levels is well described29 and can be explained by the proximity of the first time point to the 2012 grass pollen season, with recent environmental grass pollen exposure presumed to have stimulated memory B-cell responses. However, the ensuing fall in allergen-specific IgE was not observed in the active arm, indicating that intradermal allergen immunotherapy continued to stimulate IgE synthesis. This ‘priming’ effect on IgE responses also occurs with subcutaneous immunotherapy10 but is further evidence that the intradermal allergen injections were biologically active and exerted a systemic immunological effect. Similarly, like conventional subcutaneous and sublingual grass pollen immunotherapy,11,29,30 intradermal allergen treatment also stimulated allergen-specific IgG responses. Allergen-specific IgG responses to grass pollen immunotherapy block IgE-dependent histamine release from basophils and IgE-mediated facilitated antigen presentation to T cells. 10,31 Persistence of this effect has been associated with long-term efficacy. 32 In this study, we did not observe a treatment effect on basophil activation in response to allergen stimulation in vitro. It is therefore possible that the lack of efficacy of intradermal allergen immunotherapy stemmed from the failure of the dermal route to sufficiently stimulate a protective allergen-specific IgG response.
Immunohistochemistry was performed on skin punch biopsies from 20 active and 20 control participants. Biopsies were collected immediately after the late-phase response was measured, that is, at the 4-month time point and 24 hours after intradermal allergen challenge. Although late-phase responses were still partially inhibited at this time point, we observed no significant inhibition of allergen-induced infiltration of eosinophils, neutrophils, CD3+ T cells or CD4+ T cells by intradermal allergen immunotherapy. Biopsies were also examined for Fox p3+ Tregs, but no immunostaining could be observed despite successful staining of positive control nasal polyp tissue. In half of the participants who underwent biopsy, the biopsy was divided into two fragments, and one piece was immediately cultured for T-cell expansion. Only T cells from allergen-challenged skin (not diluent-challenged skin) could be expanded in sufficient numbers for analysis. This is consistent with the immunohistochemistry findings showing that only small numbers of T cells were present within diluent-challenged skin but that these numbers increased significantly after intradermal allergen challenge. Cultured skin CD4+ T cells in the active arm showed higher surface expression of the prostaglandin-D2 receptor CRTH2, a specific marker of Th2 cells. 33 Conversely, in the active treatment arm these T cells showed lower levels of surface Th1 marker CXCR3. In samples where sufficient cells were expanded, T cells were also stimulated and subjected to transcriptional profiling by microarray. Only 13 genes were found to be significantly overexpressed in the active intradermal immunotherapy group compared with the control arm. This relatively small number probably reflects a high degree of biological variability, but, significantly, one of the overexpressed genes encoded the Th2 cytokine IL-5. Collectively, these findings therefore suggest that intradermal allergen immunotherapy resulted in local priming of cutaneous Th2 responses, and suggest a mechanism for how this intervention may have facilitated IgE synthesis. This priming effect could also account for why intradermal immunotherapy may have acted to potentiate certain symptoms when participants were subsequently exposed to grass pollen naturally during the 2013 season.
Previous non-interventional human studies have linked cutaneous allergen exposure to IgE responses and development or exacerbation of allergy, albeit in the context of atopic eczema when skin barrier function is compromised. For example, in children with atopic eczema, exposure to peanut protein via the dermal route has been associated with development of peanut allergy. 34,35 It is plausible that grass pollen intradermal allergen injections may have acted similarly to target the dermis. Our findings also raise the possibility that repeated intracutaneous exposure to aeroallergens, for example in patients with eczema who have disrupted skin barrier function, could have the potential to exacerbate respiratory allergic disease.
There is considerable current interest in the concept of administering immunotherapy as allergen applied epicutaneously in patches to non-eczematous skin. 36 Preliminary clinical trials have provided evidence that this may be effective for treatment of grass pollen allergy and similar patches are also under investigation for peanut allergy. 37,38 Unlike the intradermal allergen immunotherapy tested in this study, epicutaneous treatment targets the epidermis rather than the dermis directly. However, recent studies have investigated methods that enhance keratinocyte activation and skin penetration by epicutaneous allergen, such as use of microneedles39 or skin stripping with tape. 40 Such methods are likely to promote dermal allergen exposure40 and in at least one animal model the application of allergen to stripped skin potentiated systemic Th2 responses and the in vivo response to allergen. 41 The findings from our trial provide the first human evidence that novel immunotherapy approaches that facilitate exposure of the dermis to allergen also have the potential to worsen symptoms, even if local macroscopic responses appear to be suppressed by the vaccine.
Conclusions
The results of this study provide evidence that low-dose intradermal allergen injection immunotherapy is not clinically effective, even if it is able to suppress late-phase skin responses. Furthermore, we found evidence that this intervention resulted in immunological priming in certain assays in parallel with worsening of certain symptoms during the grass pollen season. These findings support the concept that dermal allergen exposure has the potential to exacerbate, rather than ameliorate, allergic responses. We conclude that novel immunotherapy strategies that promote dermal allergen exposure have the potential to be deleterious, even if local macroscopic responses appear to be suppressed by this approach.
Acknowledgements
This work was supported by a Medical Research Council (MRC) and NIHR partnership. The King’s Health Partnership Challenge Fund provided additional research funding for this project. This research was also supported by the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and KCL. Dr Till was supported a Higher Education Funding Council for England (HEFCE) Clinical Senior Lectureship Award. Dr Lam was funded by a MRC-Asthma UK-funded PhD studentship. Dr Slovick received funding from Athena SWAN and the Royal College of Surgeons (England). Professor Cousins acknowledges support from NIHR Leicester Respiratory Biomedical Research Unit.
We are indebted to the members of the public who provided PPI input to project: Bernard Chan for assistance with data entry; James Dobbyn, John Brooks, Sharon Jones and Gerry Trillana of the NIHR Clinical Research Facility at Guy’s Hospital; Dr Alina Dumitru for assistance in setting up the recruitment campaign; Dr Elena Ortiz-Zapater for assistance with mechanistic studies; Paul Tunstell of Guy’s Hospital Pharmacy for GMP manufacture of grass pollen and histamine solutions for use in the trial; the UK Meteorological Office for managing the UK pollen network; and Bhopal Pandey, Kris Chan, Natalia Acero Martinez, Dr Trevor Blackall and Dr Robert Francis for collection and provision of pollen count data. The authors also gratefully acknowledge the contributions of the Trial Steering Committee (chairperson: Dr Samantha Walker, Asthma UK) and the Data Monitoring and Ethics Committee (chairperson: Professor Peter Burney, Imperial College London).
Contributions of authors
Ms Anna Slovick (Clinical Fellow, KCL, and Ear, Nose and Throat trainee) was overall trial co-ordinator and participated in the set-up of the trial, recruitment, administration of intradermal injections and collection of clinical outcome data; performed mechanistic assays; and participated in preparation of the first draft of this manuscript.
Dr Abdel Douiri (Senior Lecturer in Medical Statistics, KCL) participated in the design of the trial, preparation of the manuscript and was the trial statistician.
Dr Rachel Muir (Research Matron) participated in the set-up of the trial, recruitment, administration of intradermal injections and collection of clinical outcome data.
Dr Andrea Guerra (Clinical Fellow, KCL) participated in the set-up of the trial, recruitment, administration of intradermal injections and collection of clinical outcome data, and performed mechanistic assays.
Mr Konstantinos Tsioulos (Clinical Fellow, KCL) participated in the set-up of the trial, recruitment, and administration of intradermal injections.
Ms Evie Haye (Research technician) performed mechanistic assays.
Dr Emily PS Lam (PhD student, degree now awarded) performed mechanistic assays.
Dr Ms Joanna Kelly (Strategic Data Management Lead, KCTU) participated in the design and set-up of the trial.
Professor Janet L Peacock (Professor of Medical Statistics, KCL) participated in the statistical analysis and data interpretation.
Dr Sun Ying (Reader in Allergy, KCL) participated in design, supervision and co-ordination of mechanistic assays.
Dr Mohamed H Shamji (Research Fellow, Imperial College London) participated in design, supervision and coordination of mechanistic assays.
Professor David J Cousins (Professor Respiratory Science, University of Leicester) supervised mechanistic assays.
Professor Stephen R Durham (Professor of Allergy & Respiratory Medicine, Imperial College London; Consultant Allergist, Royal Brompton & Harefield NHS Trust) participated in the study conception, design, interpretation of the results and manuscript preparation.
Dr Stephen J Till (Reader in Allergy, KCL; Consultant Allergist, Guy’s and St Thomas’ NHS Foundation Trust) was chief investigator of the PollenLITE trial and conceived of the study, participated in its design and co-ordination, and prepared the first draft of this manuscript with the assistance of AS.
Publications
Slovick A, Douiri A, Muir R, Guerra A, Tsioulos K, Hay E, et al. Intradermal grass pollen immunotherapy increases TH2 and IgE responses and worsens respiratory allergic symptoms [published online ahead of print October 20 2016]. J Allergy Clin Immunol 2016.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health.
References
- Slovick A, Douiri A, Muir R, Guerra A, Tsioulos K, Hay E, et al. Intradermal grass pollen immunotherapy increases TH2 and IgE responses and worsens respiratory allergic symptoms. J Allergy Clin Immunol 2016. http://dx.doi.org/10.1016/j.jaci.2016.09.024.
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004;24:758-64. http://dx.doi.org/10.1183/09031936.04.00013904.
- Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy 2005;60:350-3. http://dx.doi.org/10.1111/j.1398-9995.2005.00751.x.
- Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy 2011;41:1177-200. http://dx.doi.org/10.1111/j.1365-2222.2011.03794.x.
- Noon L. Prophylactic inoculation against hay fever (historical document). Ann Allergy 1955;13:713-16.
- Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.cd001936.pub2.
- Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010;12. http://dx.doi.org/10.1002/14651858.cd002893.pub2.
- Kay AB, Ying S, Varney V, Gaga M, Durham SR, Moqbel R, et al. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med 1991;173:775-8. http://dx.doi.org/10.1084/jem.173.3.775.
- Rotiroti G, Shamji M, Durham SR, Till SJ. Repeated low-dose intradermal allergen injection suppresses allergen-induced cutaneous late responses. J Allergy Clin Immunol 2012;130:918-24. http://dx.doi.org/10.1016/j.jaci.2012.06.052.
- Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 2008;121:1120-5. http://dx.doi.org/10.1016/j.jaci.2008.01.072.
- Lima MT, Wilson D, Pitkin L, Roberts A, Nouri-Aria K, Jacobson M, et al. Grass pollen sublingual immunotherapy for seasonal rhinoconjunctivitis: a randomized controlled trial. Clin Exp Allergy 2002;32:507-14. http://dx.doi.org/10.1046/j.0954-7894.2002.01327.x.
- Phillips E. Relief of hay-fever by intradermal injections of pollen extract. JAMA 1926;86:182-4. http://dx.doi.org/10.1001/jama.1926.02670290022008.
- Phillips E. Intradermal pollen therapy during the attack. J Allergy 1933;5:29-36. http://dx.doi.org/10.1016/S0021-8707(33)90167-7.
- Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol 2008;121:1467-72. http://dx.doi.org/10.1016/j.jaci.2008.03.013.
- Kersey TW, Van Eyk J, Lannin DR, Chua AN, Tafra L. Comparison of intradermal and subcutaneous injections in lymphatic mapping. J Surg Res 2001;96:255-9. http://dx.doi.org/10.1006/jsre.2000.6075.
- Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol 2003;111:1255-61. http://dx.doi.org/10.1067/mai.2003.1570.
- Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy 2008;63:1455-63. http://dx.doi.org/10.1111/j.1398-9995.2008.01774.x.
- Senti G, von Moos S, Kündig TM. Epicutaneous allergen administration: is this the future of allergen-specific immunotherapy?. Allergy 2011;66:798-809. http://dx.doi.org/10.1111/j.1398-9995.2011.02560.x.
- Romani N, Flacher V, Tripp CH, Sparber F, Ebner S, Stoitzner P. Targeting skin dendritic cells to improve intradermal vaccination. Curr Top Microbiol Immunol 2012;351:113-38. http://dx.doi.org/10.1007/82_2010_118.
- Slovick A, Douiri A, Kelly J, Guerra A, Muir R, Tsioulos K, et al. Protocol for a double-blind randomised controlled trial of low dose intradermal grass pollen immunotherapy versus a histamine control on symptoms and medication use in adults with seasonal allergic rhinitis (PollenLITE). Clin Transl Allergy 2013;3. http://dx.doi.org/10.1186/2045-7022-3-27.
- Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy 2007;62:317-24. http://dx.doi.org/10.1111/j.1398-9995.2006.01312.x.
- Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy 2000;30:132-40. http://dx.doi.org/10.1046/j.1365-2222.2000.00668.x.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- Frew AJ, Kay AB. The relationship between infiltrating CD4+ lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J Immunol 1988;141:4158-64.
- Gaga M, Frew AJ, Varney VA, Kay AB. Eosinophil activation and T lymphocyte infiltration in allergen-induced late phase skin reactions and classical delayed-type hypersensitivity. J Immunol 1991;147:816-22.
- Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ 1991;302:265-9. http://dx.doi.org/10.1136/bmj.302.6771.265.
- Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 2010;125:569-74.
- Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Study Group . Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy 2005;60:801-7. http://dx.doi.org/10.1111/j.1398-9995.2005.00790.x.
- Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol 2012;129:717-25. http://dx.doi.org/10.1016/j.jaci.2011.12.973.
- Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol 1982;70:261-71. http://dx.doi.org/10.1016/0091-6749(82)90062-8.
- Shamji MH, Ljørring C, Francis JN, Calderon MA, Larché M, Kimber I, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012;67:217-26. http://dx.doi.org/10.1111/j.1398-9995.2011.02745.x.
- James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol 2011;127:509-.
- Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol 2000;30:2972-9. http://dx.doi.org/10.1002/1521-4141(200010)30:10%3C2972::AID-IMMU2972%3E3.0.CO;2-%23.
- Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol 2015;135:164-70. http://dx.doi.org/10.1016/j.jaci.2014.10.007.
- Brough HA, Santos AF, Makinson K, Penagos M, Stephens AC, Douiri A, et al. Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol 2013;132:630-8. http://dx.doi.org/10.1016/j.jaci.2013.02.034.
- Senti G, von Moos S, Tay F, Graf N, Sonderegger T, Johansen P, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol 2012;129:128-35. http://dx.doi.org/10.1016/j.jaci.2011.08.036.
- Agbotounou W, Martin L, Dupont B, Pascal I, Vauléon C, Benhamou PH. Epicutaneous immunotherapy (EPIT) is safe for the treatment of peanut allergy in allergic patients. J Allergy Clin Immunol 2013;2. http://dx.doi.org/10.1016/j.jaci.2012.12.992.
- Dupont C. Peanut Epicutaneous Immunotherapy (EPIT) in Peanut-Allergic Children: 18 Months Treatment in the ARACHILD Study n.d.
- Spina L, Weisskopf M, von Moos S, Graf N, Kündig TM, Senti G. Comparison of microneedles and adhesive-tape stripping in skin preparation for epicutaneous allergen delivery. Int Arch Allergy Immunol 2015;167:103-9. http://dx.doi.org/10.1159/000434681.
- von Moos S, Johansen P, Tay F, Graf N, Kündig TM, Senti G. Comparing safety of abrasion and tape-stripping as skin preparation in allergen-specific epicutaneous immunotherapy. J Allergy Clin Immunol 2014;134:965-7. http://dx.doi.org/10.1016/j.jaci.2014.07.037.
- Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Letourneur F, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy 2012;2. http://dx.doi.org/10.1186/2045-7022-2-22.
Appendix 1 PollenLITE trial website with prescreening questions
Appendix 2 PollenLITE recruitment advertisement panel used on public transport
Appendix 3 Example of daily symptom and medication-use diary card
Appendix 5 Global Evaluation scores (completed September 2013)
Appendix 6 Statistical analysis plan
Appendix 7 Effect of intradermal immunotherapy on primary and secondary outcomes (intention to treat): missing data imputed
| Trial outcomes | Intradermal immunotherapy (n = 46), median (IQR) | Control (n = 47), median (IQR) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Primary outcome | ||||
| CSMS during entire season | 502 (333–841) | 509 (365–738) | 8 (–174.7 to 210.9) | 0.91 |
| Secondary outcomes (median (IQR) | ||||
| Symptom score during entire season | 335 (183–525) | 264 (156–434) | 61 (–7.8 to 123.2) | 0.22 |
| Medication score during entire season | 242 (116–405) | 263 (129–482) | –24 (–173.1 to 107.5) | 0.39 |
| CSMS score during peak season | 363 (232–570) | 370 (292–573) | –11 (–95.8 to 77.5) | 0.80 |
| Nasal symptom score during entire season | 178 (120–218) | 131 (80–200) | 33 (0.3 to 68.5) | 0.03 |
| Mouth symptom score during entire season | 39 (8–90) | 14 (6–45) | 11 (3.1 to 26.1) | 0.05 |
| Eye symptom score during entire season | 79 (41–158) | 78 (52–180) | –7 (–20.0 to 3.0) | 0.51 |
| Lung symptom score during entire season | 20 (3–32) | 12 (0–40) | 4 (–1.0 to 15.3) | 0.17 |
| Nasal allergic symptoms measured by VAS | 162 (107–275) | 124 (66–166) | 59 (–3.7 to 133.2) | 0.02 |
| Eye allergic symptoms measured by VAS | 97 (37–197) | 112 (42–169) | 2 (–45.6 to 49.0) | 0.56 |
| Global Evaluation of symptom scores | 3 (2–4) | 3 (1–3) | 0 (0.0 to 1.0) | 0.43 |
| Symptom-free days | 35 (19–53) | 41 (23–61) | –6 (–17.0 to 3.0) | 0.15 |
| Number of days prednisolone used during entire season | 0 (0–0) | 0 (0–0) | 0 (0 to 0) | 0.36 |
| Medication-free days | 81 (65–93) | 76 (56–94) | 4 (–11.0 to 21.0) | 0.22 |
| Mini-RQLQ | 16 (13–23) | 18 (10–25) | –0.3 (–4.2 to 3.7) | 0.89 |
| EQ-5D-5L | 87 (83–94) | 88 (81–94) | 9 (–24.8 to 43.6) | 0.59 |
Appendix 8 Effect of intradermal immunotherapy on primary and secondary outcomes (per-protocol analysis)
| Trial outcomes | Intradermal immunotherapy (n = 45), median (IQR) | Control (n = 39), median (IQR) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Primary outcome | ||||
| CSMS during entire season | 517 (344–841) | 453 (279–685) | 82 (–121.8 to 280.1) | 0.23 |
| Secondary outcomes | ||||
| Symptom score during entire season | 340 (189–503) | 241 (150–398) | 76 (25.9 to 133.5) | 0.09 |
| Medication score during entire season | 255 (119–405) | 254 (113–358) | 21 (–125.0 to 157.0) | 0.83 |
| CSMS score during peak season | 363 (242–546) | 342 (242–476) | 18 (–73.2 to 127.5) | 0.51 |
| Nasal symptom score during entire season | 173 (123–207) | 119 (80–205) | 40 (13.3 to 71.5) | 0.02 |
| Mouth symptom score during entire season | 38 (8–90) | 14 (4–43) | 14 (4.9 to 32.0) | 0.02 |
| Eye symptom score during entire season | 80 (41–153) | 72 (48–145) | 0 (–16.0 to 17.6) | 0.85 |
| Lung symptom score during entire season | 17 (3–32) | 11 (0–21) | 9 (1.0 to 17.0) | 0.05 |
| Nasal allergic symptoms measured by VAS | 162 (105–275) | 118 (50–154) | 68 (8.3 to 134.6) | 0.01 |
| Eye allergic symptoms measured by VAS | 90 (32–197) | 114 (42–159) | 1 (–52.8 to 62.0) | 0.49 |
| Global Evaluation of symptom scores | 3 (2–4) | 3 (1–3) | 1 (0.0 to 1.0) | 0.25 |
| Symptom-free days | 34 (19–47) | 44 (25–67) | –12 (–22.0 to –2.0) | 0.04 |
| Number days prednisolone used during entire season | 0 (0–0) | 0 (0–0) | 0 (0 to 0) | 0.33 |
| Medication-free days | 80 (65–92) | 78 (66–98) | –1 (–20.0 to 17.0) | 0.87 |
| Mini-RQLQ | 16 (13–23) | 17 (10–22) | –2.0 (–5.89 to 1.88) | 0.31 |
| EQ-5D-5L | 88 (83–94) | 88 (84–94) | 3 (–28.4 to 35.2) | 0.83 |
Appendix 9 Effect of intradermal immunotherapy on daily organ symptom scores (intention to treat)
| Trial outcomes | Intradermal immunotherapy (n = 46), median (IQR) | Control (n = 47), median (IQR) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Nose | ||||
| Sneezing | 76 (43.3–103.0) | 55 (35.0–71.0) | 21 (7.0 to 34.0) | 0.01 |
| Blockage | 41 (14.0–74.5) | 36 (12.5–61.0) | 6 (–2.5 to 13.5) | 0.33 |
| Running | 51 (30.0–81.5) | 46 (22.5–65.4) | 10 (–3.0 to 22.8) | 0.17 |
| Mouth | ||||
| Itching | 19 (4.0–52.3) | 8 (1.0–25.0) | 4 (1.8 to 6.8) | 0.06 |
| Drying | 7 (0.0–40.0) | 3 (0.0–15.0) | 3 (0.0 to 9.6) | 0.18 |
| Eyes | ||||
| Itching | 48 (21.0–68.0) | 44 (26.0–72.5) | –1 (–5.0 to 2.0) | 0.99 |
| Redness/sore | 17 (4.0–42.0) | 14 (7.0–45.0) | –1 (–6.0 to 3.0) | 0.55 |
| Streaming | 11 (2.0–19.0) | 14 (2.0–24.0) | 0 (–4.0 to 3.0) | 0.69 |
| Swelling | 2 (0.0–9.0) | 5 (0.0–14.0) | –2 (–4.0 to 0.0) | 0.03 |
| Lungs | ||||
| Breathlessness | 0 (0.0–4.0) | 0 (0.0–8.1) | 0 (0.0 to 2.0) | 0.27 |
| Cough | 8 (1.0–23.3) | 1 (0.0–12.1) | 2 (0.0 to 6.0) | 0.02 |
| Wheezing | 3 (0.0–7.0) | 0 (0.0–8.0) | 0 (0.0 to 2.0) | 0.25 |
| Tightness | 2 (0.0–4.0) | 0 (0.0–4.0) | 0 (0.0 to 2.0) | 0.08 |
Appendix 10 Effect of intradermal immunotherapy on individual visual analogue scale scores (intention to treat)
| Trial outcomes | Intradermal immunotherapy (n = 46), median (IQR) | Control (n = 47), median (IQR) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Nose | ||||
| Blockage | 152 (71.4–238.7) | 118 (39.1–178.8) | 39 (1.6 to 82.8) | 0.12 |
| Running | 169 (96.0–265.6) | 117 (62.0–162.7) | 58 (–8.2 to 124.5) | 0.006 |
| Itching | 138 (93.2–281.7) | 81 (41.9–141.6) | 64 (–16.3 to 165.4) | 0.003 |
| Sneezing | 187 (133.1–295.3) | 125 (46.1–182.4) | 77 (–1.6 to 150.9) | 0.006 |
| Eyes | ||||
| Itching | 120 (53.7–248.3) | 135 (41.9–217.8) | 4 (–35.3 to 46.1) | 0.97 |
| Watering | 69 (21.0–129.5) | 71 (33.6–119.4) | 1 (–40.5 to 55.5) | 0.792 |
List of abbreviations
- AE
- adverse event
- ANCOVA
- analysis of covariance
- ANOVA
- analysis of variance
- AP
- alkaline phosphatase
- APAAP
- alkaline phosphatase anti-alkaline phosphatase
- AUC
- area under curve
- CCR6
- chemokine (C-C motif) receptor 6
- CD3
- cluster of differentiation 3
- CD3+
- cluster of differentiation 3-positive
- CD4
- cluster of differentiation 4
- CD4+
- cluster of differentiation 4-positive
- cDNA
- complementary deoxyribonucleic acid
- CI
- confidence interval
- CRTH2
- chemoattractant receptor-homologous molecule expressed on Th2 cells
- CSMS
- combined symptom and medication score
- CXCR3
- chemokine (C-X-C Motif) receptor 3
- DC
- dendritic cell
- eCRF
- electronic case report form
- EQ-5D-5L
- European Quality of Life-5 Dimensions, 5-levels
- FEV1
- forced expiratory volume in 1 second
- GCP
- Good Clinical Practice
- GMP
- Good Medical Practice
- GP
- general practitioner
- IgE
- immunoglobulin E
- IgG
- immunoglobulin G
- IL-2
- interleukin 2
- IL-5
- interleukin 5
- IQR
- interquartile range
- ITT
- intention to treat
- KCL
- King’s College London
- KCTU
- King’s Clinical Trials Unit
- mAb
- monoclonal antibody
- Mini-RQLQ
- Mini Rhinitis Quality of Life Questionnaire
- MRC
- Medical Research Council
- mRNA
- messenger ribonucleic acid
- NIHR
- National Institute for Health Research
- PBS
- phosphate-buffered saline
- PEF
- peak expiratory flow
- PollenLITE
- Pollen Low dose Intradermal Therapy Evaluation
- RCT
- randomised controlled trial
- RNA
- ribonucleic acid
- SD
- standard deviation
- SPT
- skin prick test
- Th1
- T helper type 1 cell
- Th2
- T helper type 2 cell
- Treg
- regulatory T cell
- VAS
- visual analogue scale
- WAO
- World Allergy Organization