Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 10/60/30. The contractual start date was in April 2012. The final report began editorial review in January 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Pomeroy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The trial reported here focused on a top 10 research priority identified by people who have had a stroke, namely upper limb recovery after stroke. 1 Upper limb recovery is a research priority because of the need to perform everyday tasks such as drinking from a cup, unscrewing the top from a bottle of water and fastening buttons/zips for independent living. Difficulty with these and other everyday tasks is a frequent consequence of stroke. Indeed, upper limb neuromuscular weakness (paresis) has been estimated as occurring in 77% of people after stroke. 2 Upper limb recovery is therefore a key target for rehabilitation, but at 6 months after stroke, only 38% of people have some dexterity. 2 ‘Better methods of upper limb rehabilitation’ are required. 1
The socioeconomic impact of residual upper limb disability is high. Indeed, stroke alone produces most of the adult disability across the globe. 3 In England alone, each year around 110,000 people have a stroke and the estimated annual cost is £9B. 4 Most of the cost is in ‘rehabilitation and life after stroke’ (reproduced with permission from the National Audit Office4). The impact is unlikely to lessen because most people who have a stroke are > 65 years, and the population is ageing. Improving the outcome of upper limb rehabilitation after stroke is a research priority for the NHS5 and, more widely, across Europe. 6
It is known that (1) upper limb recovery is enhanced by the provision of physical therapy based on repetitive task-specific training,7 and (2) the 3 months immediately after stroke is when recovery is most rapid8 and there is most potential for central nervous system reorganisation (neuroplasticity). 9 However, not everybody responds in the same way to particular forms of task-specific training. For example, constraint-induced movement therapy (CIMT) is suitable only for people with at least 10º of voluntary movement of the paretic thumb and two or more fingers10,11 who are between 3 and 9 months after stroke. 12 When used with people early after stroke, CIMT did not provide better recovery than an equal dose of usual therapy. 13 These and other findings highlight that not everybody has the same set of physiological deficits after stroke, recovers in the same way or responds to the same form of therapy. 14 Therefore, it is important to have a greater understanding of the mechanisms of recovery and whether a therapy is driving beneficial or maladaptive neuroplasticity. 9–16
Progress towards better methods of upper limb rehabilitation after stroke needs to consider that clinical phenotype may be insufficient for targeting forms of task-specific therapy to those people most likely to benefit,17 and that restoring physiological function probably requires the development and application of rehabilitation therapies that promote activity-driven reorganisation of neural networks spared by the stroke18 and consideration of the characteristics of the indivdual. 19 For example, non-primary cortical motor regions, including premotor and supplementary motor areas, show adaptation associated with improvement in movement performance. 18,20 This adaptation is most evident in people with greater damage to the corticospinal system. 21,22 In terms of predictive markers, there are experimental, non-trial indications that baseline, before therapy, brain activity in the primary motor cortex during movement23 and the amount of damage to descending motor white matter pathways24 at baseline (i.e. before therapy) may be related to recovery in response to therapy. Accordingly, rehabilitation trials need objective, sensitive neural measures to understand how a therapy produces benefit (mechanisms) and which people are likely to respond (predictive markers). 14,15,17,18,25 Proof of concept of this approach is provided by investigations into language recovery. 26 Gaining this greater understanding will identify the mechanisms that should be targets for therapy and add information to algorithms designed to aid therapists to provide the most appropriate therapy for individuals27 (www.viatherapy.org).
Realistically, if we are to incorporate mechanism and predictive marker data into accurate models to inform clinical decision-making then larger sample sizes are clearly required. Embedded in the trial reported here are objective neuroimaging and neurophysiological measures of participants’ residual motor neural network before and after a 6-week treatment period.
The specific forms of physical therapy employed in the trial reported here were functional strength training (FST) focused on improving ability to perform everyday functional tasks, and movement performance therapy (MPT) focused on enhancing quality of movement required for everyday functional tasks [called extra conventional physical therapy (CPT) in our previous early-phase trial]. 28 FST is based on evidence from experimental and clinical studies of benefit for people with substantial to moderate paresis early after stroke. 28–34 It forms part of the CPT provided in routine clinical practice. MPT is the component of routine CPT that is focused on enhancing the quality of the movements required for the performance of everyday functional tasks. 28,35–38 Such conceptually different physical therapies have been found in a meta-analysis to be no more or less effective than each other. 39 However, in both of our early-phase trials we found marked variation within people in their response to MPT and to FST. 28,35 Thus, a comparison of the clinical efficacy FST and MPT was used in the trial reported here as the context for investigating the predictors of response to and mechanisms of action to specific physical therapies.
Objectives
The scientific driver for this trial was that detailed understanding of the interaction between the treatment and each patient’s residual neural function will more likely enable physical therapies to be targeted at recovery mechanisms in those stroke survivors most likely to respond. The specific objectives were to:
-
determine if upper limb motor recovery is enhanced more by FST + CPT than an equal dose of MPT + CPT commenced early after stroke
-
identify the similarities and differences in the neural correlates of clinical improvement in upper limb motor function in response to (1) FST + CPT and (2) MPT + CPT
-
determine whether or not any pretreatment neural characteristics or combination of (1) anatomical location of infarction, (2) volume of the stroke lesion, (3) residual structural corticocortical connectivity, (4) residual corticospinal connectivity and (5) brain–muscle functional connectivity [derived from Transcranial Magnetic Stimulation (TMS)], are sufficiently predictive of upper limb recovery after stroke to enable physical therapy to be targeted at those people most likely to respond.
Achieving these objectives before undertaking a Phase III randomised controlled trial (RCT) conforms with the Medical Research Council (MRC) Framework for Design and Evaluation of Complex Interventions to Improve Health. 40
Chapter 2 Randomised controlled trial: methods
Design
A randomised, controlled, observer-blind, two-group, multicentre trial. The randomisation order was generated before the trial began and allocation was via an independent telephone interactive voice response randomisation service. Clinical efficacy measures were made before randomisation (baseline), the working day (± 7 days) after the 6-week intervention ended (outcome) and 6 calendar months (± 14 days) after the index stroke (follow-up). Neural measures were made at baseline and outcome within 10 days following the clinical efficacy measures. All assessors were blinded at baseline as randomisation had not yet occurred. At outcome and follow-up all assessors were, where exceptional events did not dictate, blinded to the randomisation. When at all possible, randomised participants were included in outcome and follow-up measures. The trial procedure is illustrated in Figure 1. The trial is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines and covers all the CONSORT checklist items.
FIGURE 1.
Flow chart to illustrate trial procedure.

Trial registration
The trial was registered on Current Controlled Trials with the unique identifier ISRCTN19090862 (www.controlled-trials.com). In addition, the protocol was published41 and the study was adopted by the UK Clinical Research Network and, therefore, appeared on the UK Clinical Research Network Study Portfolio.
Ethics and research governance approval
The Norfolk Research Ethics Service provided ethics approval on 22 February 2012 (reference number 11/EE/0524). All participants provided informed consent.
The trial was outside the scope of the Clinical Trials Directive defined by the Medicines and Healthcare Products Regulatory Agency as neither trial intervention included provision of a pharmacological compound.
The University of East Anglia (UEA) was the recipient of the funding. UEA together with the University of Glasgow Clinical Trials Unit [Robertson Centre for Biostatistics (RCB)] and the Norwich Clinical Trials Unit was responsible for the organisation and running of the trial. UEA had subcontracts with each partner university that laid out the delegated responsibilities of the partner and funding to be provided. Each partner university was responsible for procuring suitable management and governance provision with their local NHS trust centres. All members of the trial team had current good clinical practice (GCP) training. Those members of the trial who were actively involved in clinical contact with participants but were not employed by the NHS had either honorary clinical contracts or research passports.
All decisions regarding eligibility for entry, provision of written informed consent, inclusion, exclusion and attrition were documented as per the MRC Code of Good Practice in Clinical Trials and the CONSORT guidelines. Relevant trial documentation will be retained for a period of 10 years after the end of data collection to comply with the GCP regulations and to ensure availability of data for any subsequent systematic reviews and meta-analyses. The UEA will archive trial documents in a secure facility. The custodian will be Professor Pomeroy.
Participants
The combined inclusion and exclusion criteria for potential participants were people:
-
with a clinical diagnosis of stroke in the territory of the anterior cerebral circulation, cortical and/or subcortical as corroborated through routine clinical imaging
-
aged ≥ 18 years
-
who were between 2 and 60 days after stroke when providing informed consent
-
were able, before the index stroke, to use the paretic, contralesional, upper limb to lift and then drink from a cup
-
who were defined as medically stable as confirmed by the stroke service medical team responsible for the individual’s stroke care
-
with enough voluntary muscle contraction in the paretic upper limb to score at least 11 of the 33 points on the Motricity Index pinch section42
-
who were unable to complete the Nine-Hole Peg Test (9HPT) within 50 seconds41
-
with a score of 0 or 1 on the Extinction and Inattention subscale of the National Institutes of Health Stroke Scale (no obvious spatial neglect)
-
who could imitate action with the non-paretic (ipsilesional) upper limb. The research therapist sat alongside a potential participant to demonstrate five upper limb activities. The potential participant was asked to watch and then perform the activities. The accuracy of imitation was assessed on the 3-point scale used by Decety: 2 = correctly reproduced action, 1 = incorrectly reproduced action and 0 = not reproduced. 43 Those people scoring 8 out of 10 or above were considered as able to imitate and, therefore, eligible for inclusion in this trial.
Settings for recruitment, assessment and treatment
-
Birmingham (centre 1).
-
Moseley Hall Hospital, Birmingham Community Healthcare NHS Trust.
-
Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust.
-
University of Birmingham Imaging Centre.
-
Participants’ own homes.
-
-
Norfolk (centre 2).
-
Acute Stroke Unit, Norfolk and Norwich University Hospital NHS Trust.
-
Stroke Rehabilitation Ward, Mulberry Unit, and Early Supported Discharge Team, Norfolk Community Health and Care NHS Trust.
-
James Paget University Hospitals.
-
The Movement Analysis Laboratory at the UEA.
-
Participants’ own homes.
-
-
North Staffordshire (centre 3).
-
Haywood Hospital, Staffordshire and Stoke-on-Trent Partnership NHS Trust.
-
University Hospital of North Midlands NHS Trust.
-
Participants’ own homes.
-
Screening and recruitment of participants
Potential participants were screened from the three centres and followed up on the condition that research governance and ethics approvals were in place for that setting. A two-stage screening process was used because some study criteria required movement assessment expertise that was not always present in the Comprehensive Local Research Network (CLRN) screening teams.
Stage 1 of the screening process was conducted by CLRN research nurses in liaison with stroke service clinical nurses and therapists. They screened for study criteria (a)–(f) as described in Participants (Figure 2). For some potential participants, the screening happened more than once. This was because medical and functional characteristics change over time after stroke so if somebody was unsuitable on one occasion it was possible that they could change and meet the trial criteria later. An individual’s progress was monitored until eligibility for this trial was clear (Figure 3). At the end of stage 1, informed consent was taken by either CLRN nurses or research therapists (enrolment). All people screened were categorised as unsuitable, refused informed consent, or provided informed consent.
FIGURE 2.
Stage 1: initial screening.
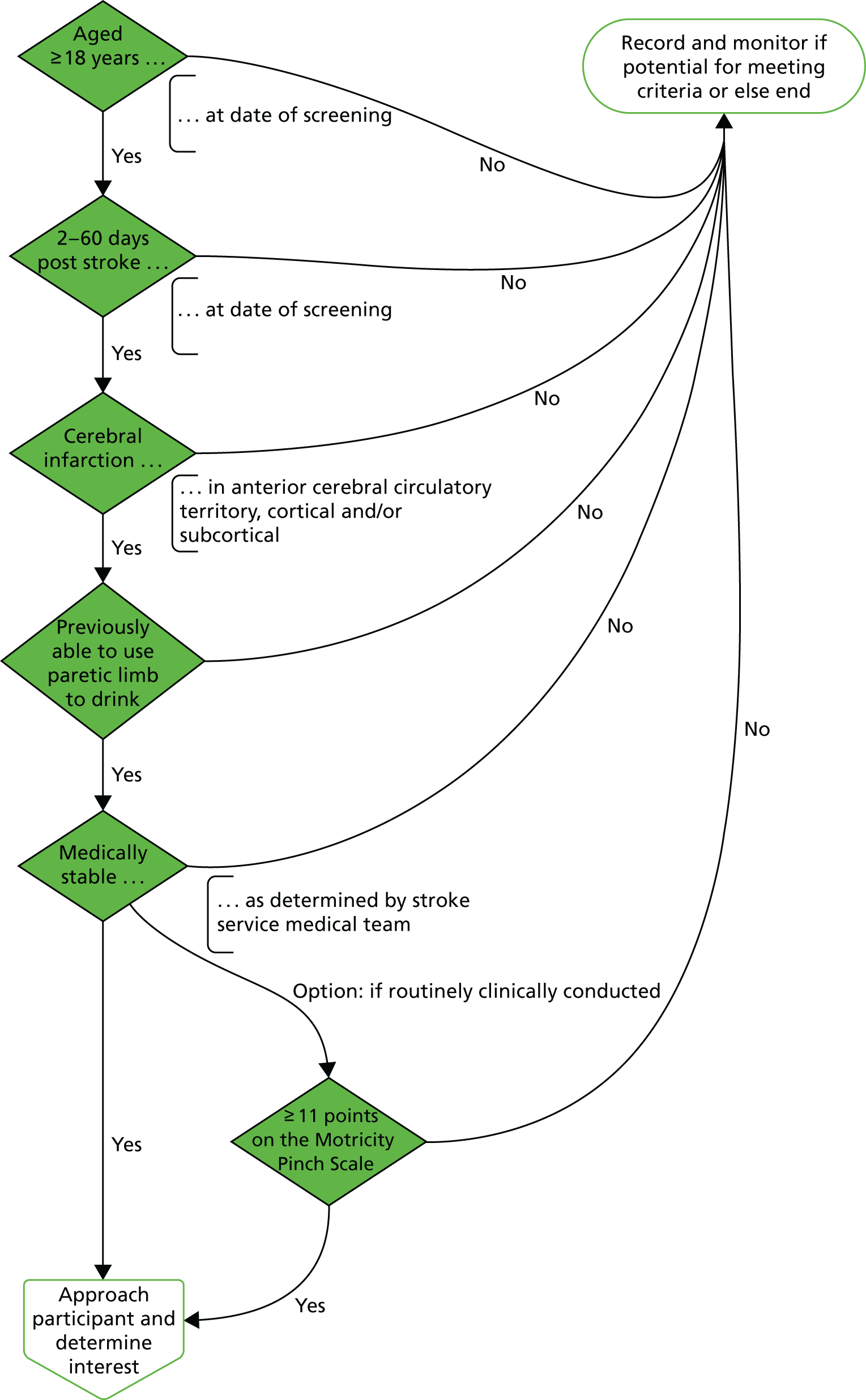
FIGURE 3.
Stage 1: participant eligibility pathway. Dark green indicates clinician or research network colleague, light green indicates research network colleague or researcher, dark blue indicates researcher and light blue indicates participant.

People providing informed consent undertook stage two of the screening process with a research therapist to ascertain concordance with study criteria (g), (h) and (i) (Figure 4). Those people who did not meet all three of the inclusion criteria were reassessed later if they agreed and the exclusion time limit had not been met. At the end of stage 2 individuals were categorised as unsuitable for the trial or as a recruited participant.
FIGURE 4.
Stage 2: post-consent suitability assessment. Dark green indicates researcher and light green indicates participant.

Randomisation
Before the trial began, the computer-generated randomisation sequence was created by an independent statistician at the Glasgow Clinical Trials Unit. Participants were allocated to FST + CPT or MPT + CPT in a 1 : 1 ratio, stratified by (1) time after stroke (up to 30 days or 31–60 days); (2) ability to use the paretic upper limb as assessed by the 9HPT, moving one peg or less in 50 seconds, or moving 2–8 pegs in 50 seconds; and (3) clinical centre. An independent telephone randomisation service was used to conceal group allocation order from investigators, research therapists and blinded assessors prior to the randomisation of a participant. The telephone randomisation service was contacted by the team member (research therapist or blinded assessor) immediately after they had completed the baseline measures with a participant.
Sample size and power
The sample size calculation that was used accounted for the expected clustered data structure (patients within therapist within treatment group). 44 This sample size calculation was based on actual ARAT score data from our previous early-phase trial28 and the expectation that the intraclass correlation coefficient (ICC) would be somewhat lower than 0.05 for patient outcomes. 45 On the assumption of an ICC of 0.01 in both treatment arms and three centres with a separate therapist for each randomised arm, a sample size of 99 participants per group would provide 80% power to detect a clinically important mean difference of 6.2 points in ARAT score change for data analysis using a two-sample t-test, with Satterthwaite correction, applying a 5% two-sided significance level and allowing for potentially different standard deviations (SDs) in the CPT + MPT (SD 7.9) and CPT + FST (SD 19.3) groups.
To account for clustering in the design (participants within a therapist within randomised treatment at each study site), a sample size inflation factor 1 + (m – 1) × ICC was applied, where m is the cluster size and ICC uses SSC software version 1 (Health Services Research Unit, University of Aberdeen, Aberdeen, UK). On the assumption that recruitment would be distributed evenly across therapists, the sample size was therefore inflated to 129 evaluable participants per group. To allow for an attrition rate of 10% (7% in our previous single-centre trial)28 we recruited 144 participants per group. The total sample size was 288.
Description of interventions
Therapy as usual, conventional physical therapy
All participants continued to receive their usual CPT provided by the clinical therapists. The CPT as delivered in the centres for the trial combined hands-on techniques emphasising postural alignment and quality of movement (an aspect of MPT) with goal-orientated task-specific training (an aspect of FST). The content and amount of CPT delivered to participants was recorded by the clinical therapists each day on a standardised form (see Appendix 1)46 in accordance with the explanatory manual used in our earlier trials. 28,35 The clinical therapists providing CPT were trained before the trial began. In addition, researchers provided regular reminders of the rationale for, and importance of, recording CPT and worked with clinicians around using the treatment schedule focused on queries that arose during the trial. Clinical therapists chose the CPT that they considered appropriate for participants. Whenever possible, the completed CPT forms were collected each week by a member of the research team. When not possible, these forms were stored with the clinical records until completion of the intervention phase and then collected by a member of the research team.
Trial interventions
The trial interventions were MPT and FST as described in the succeeding subsections.
Research therapists were assigned and trained to provide either FST or MPT. Clinical staff were not told which research therapist was assigned to provide which trial intervention so that the potential for bias in the provision of clinician-delivered CPT was minimised. However, this possibility cannot be eliminated completely as allocation to different types of exercise therapy is not as concealable as, for example, allocation to an active or placebo pharmaceutical compound.
Both trial interventions were provided for participants by a research therapist for up to 90 minutes per day, up to 5 days a week, for up to 6 weeks. It was anticipated that the trial interventions would not be provided (1) when a participant was unwell, (2) on the day of a trial assessment (e.g. MR scan), (3) when a participant was unavailable because of holiday or other personal reasons or (4) when there was a UK public holiday. If a session was missed then the reason for this was recorded. Participants received therapy both with the therapist physically present (direct contact) and during practice of activities prescribed as ‘homework’ by the research therapist (non-direct contact).
Training in delivering MPT and FST was provided for the research therapists before the first participant was recruited (see Appendix 2). All research therapists attended this training. If a therapist started during the trial then their training was completed before their first participant was randomised. In addition, two training update days were held during the trial for those therapists employed at that point. Through these update days, the within-trial research therapist networking was maintained and enhanced. This network enabled research therapists to consult regularly with their peers from different centres providing the same trial intervention to talk through understandings and practice. The trial manager, Dr Sue Hunter, and Professor Valerie Pomeroy were also involved in these networking conversations. Fidelity to intervention protocols was also assessed when Dr Sue Hunter and Professor Valerie Pomeroy made visits at short notice to accompany research therapists during a treatment session. Notice had to be given because it was essential to gain agreement from participants concerned. The purpose of training and monitoring was to minimise potential deviation from intervention protocols and difference between centres.
Trial intervention: movement performance therapy
The hands-on and sensory stimulation components of CPT that focus on restoring movement quality (i.e. efficient, smooth, timely, co-ordinated movement with normal alignment or symmetry of body structures prior to retraining functional movement) can be termed MPT. 36 MPT is ‘therapist dependent’, particularly when there is limited voluntary muscle activation; the therapist monitors and provides intrinsic feedback on movement performance through skilled observation, handling and facilitation techniques. Therapist-led hands-on guidance and feedback assists practice of functional tasks. Trial MPT emphasised interventions provided by a therapist using facilitation and guiding movement (therapist dependent) to provide sensory input to optimise joint alignment in preparation for voluntary movement (see Appendix 3). Some iterative practice of functional tasks was included but without systematic progression in resistance to movement or repetition.
Trial intervention: functional strength training
Functional strength training was provided according to a treatment schedule consisting of standardised therapy activities. 47 An online supplement to the published paper provides full details47 in accordance with the TIDieR (Template for Intervention Description and Replication) guidelines. 48 In summary, FST is repetitive progressive resistive exercise during goal-directed functional activity, with the therapist providing verbal prompting and feedback. Hands-on intervention is not provided other than that required to maintain safety (e.g. prevent a fall). FST focuses on improving the power of shoulder/elbow muscles for appropriate placing of the hand for the task being practised, the production of appropriate force in the arm and hand muscles to achieve the specific grasp, and manipulation of everyday objects. Initially, the resistance level is the maximum load that permits five repetitions of the task being practised. Systematic progression uses repetition and increased resistance. Content of FST is divided into (1) specific movements for muscle groups (e.g. emphasis on elbow flexion/extension), (2) upper limb gross movement patterns underlying functional activity (e.g. shoulder flexion/external rotation and elbow extension to reach forward), (3) hand reaching/retrieval activity (e.g. reaching to grasp something on a shelf while seated), (4) hand grip activities, (5) hand manipulation involving entire everyday movements and (6) using objects such as screw top canisters, pegs, food items (e.g. bag of dried pasta), mugs and pens. These activities are extended into more complex everyday activities such as using the paretic upper limb to place different food items into a shopping bag and then lift the bag onto a shelf, and open a bottle and drink from it and pour tea from a pot. The therapist provides feedback and instructions that encourage an external focus of attention (e.g. whether or not the teapot has been lifted off the table) rather than focusing on the arm/hand (e.g. amount of shoulder movement when lifting the teapot) and informative verbal feedback on performance on at least 50%, but less than 100%, of attempts to encourage self-evaluation for motor learning.
Measurement battery
Clinical efficacy measures: objective 1
For the clinical efficacy measures (objective 1) participants were seated in an upright chair, except for some items of the Wolf Motor Function Test (WMFT), which allows for a posture in which knees, hips and ankles are maintained at 90°. A table was available so that, when appropriate for the measurements, the forearms were supported on the table with elbows directly below the glenohumeral joint at the start of an item of the WMFT. Measures pertain to the contralesional upper limb (paretic).
The primary outcome measure was:
-
The Action Research Arm Test (ARAT), which has four subsections (grasp, grip, pinch and gross movements). Within each subsection are three to six items scored from 0 (unable) to 3 (normal performance), giving a total possible score of 57. 49 The ARAT is a measure of the primary focus of both interventions (i.e. improved upper limb function).
The secondary outcome measures were:
-
The WMFT, which is a valid and reliable assessment with 15 items designed for use with stroke survivors and is complementary to the ARAT. It measures quality of movement during 15 functional tasks including both simple actions (e.g. placing forearm on table) and complex tasks (e.g. turning key in a lock). 50,51
-
Hand Grip and Pinch Grip Force tests using a myometer held securely on a stable surface. The upper limb position for both pinch and grip force was standardised52 and the myometer was set to ‘zero’ after the subject was positioned with their hand/digits around the bars, ‘at rest’. Force values were obtained during three trials for which participants were instructed to ‘squeeze as hard as you can’. The maximum value was used for data analysis.
Neural measures: objectives 2 and 3
Participants were studied twice: at baseline and at outcome (see Design). The trial team had systems in place to maintain consistency and data quality across sites and to treat multicentre data appropriately. Full training was given to all trial centre teams.
Once testing began, data from all sites were promptly sent to the University of Oxford team for rigorous quality control prior to processing to extract relevant values. Quality control assessments included manual checks (e.g. subject motion) and automated checks (e.g. signal-to-noise ratio, motion correction parameters and range checks).
To maximise recruitment to and minimise attrition from neural measures, we provided participants with full explanations and opportunities to ask questions and plenty of time to be made comfortable and to practice the tasks.
The neural measures were:
-
The anatomical overlap of the stroke lesion with the corticospinal tract [Montreal Neurological Institute (MNI) CST – yes/no]. This variable identifies whether or not the lesion overlaps with a mask approximating the CST. The CST mask was defined using tractography in a group of control brains in standard space to track between motor cortex and medullary pyramids. The lesion was delineated manually on each patient’s T1-weighted 1 × 1 × 1 mm brain image using FSL view, an image viewing tool available within the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain’s (FMRIB) Software Library (FSL). Overlap between the lesion volume and the CST was determined by overlaying the lesion mask with the CST mask.
-
The volume of the stroke lesion (lesion volume mm3). This variable was determined by calculating the volume of the manually defined stroke mask described for (1).
-
Corticocortical anatomical connectivity as per fractional anisotropy (FA) from corpus callosum midline (FA MNI corpus callosum midline – range = 0–1). Diffusion tensor imaging data were first corrected for head motion and eddy current using tools from FSL. A diffusion tensor model was then fitted to the pre-processed data to calculate voxel-wise values of FA. Mean FA from within a standard space region of interest, incorporating the whole corpus callosum on a midline brain slice, was calculated.
-
Corticospinal anatomical integrity as per asymmetry of CST FA (ipsilesional-to-contralesional ratio MNI CST – range = –1 to 1). Regions of interest on a single slice at the level of the internal capsule were created to provide masks estimating the location of the CST. Mean FA from within these masks was calculated. A ratio of ipsilesional-to-contralesional values was calculated.
-
Corticospinal functional connectivity as per presence or absence of a resting motor-evoked potential (MEP) for biceps brachii muscle in paretic upper limb (yes/no). Single pulses of TMS using a standard 70 mm figure-of-eight coil were given over the hand/arm area of primary motor cortex of the ipsilesional hemisphere and then the contralesional hemisphere. MEP data were recorded and processed using electromyography data collection software. Resting motor threshold (RMT) was defined as the stimulator output that produced > 50 µV in 5 out of 10 trials. 53
-
Corticospinal functional connectivity as per presence or absence of a resting MEP for the extensor carpi radialis muscle in the paretic (contralesional) upper limb (yes/no). Data were processed as for (5).
-
The RMT in paretic biceps brachii (pBB) when MEP was present (percentage). Value extracted was the lowest percentage output of the stimulator at which a MEP was present.
-
The RMT in paretic extensor carpi radialis when MEP was present (percentage). The value extracted was the lowest percentage output of the stimulator at which a MEP was present.
Adverse reactions and adverse events
Participation in FST and MPT is considered low risk for adverse events (AEs). However, there is a small possibility that either therapy could be associated with an overuse syndrome, expressed as experience of pain or fatigue. These were therefore classified as potential adverse reactions as follows:
-
Pain was considered an adverse reaction if (1) a participant reported onset or increase of paretic upper limb pain (verbally or behaviourally), (2) the pain was sustained over four consecutive therapy sessions and (3) the clinical team were unable to account for this in any other way than involvement in the trial. If pain occurred then the research therapist adjusted the trial therapy as appropriate or, if indicated, stopped it on either a permanent or temporary basis. The date of the adverse reaction was recorded as the date of the fourth therapy session.
-
Fatigue was considered to have occurred if (1) a participant demonstrated a decrease of two levels in the Motricity Index upper limb score on two consecutive therapy sessions and (2) the clinical team could not account for this in any other way than involvement in the trial. This was addressed by the therapist adjusting the trial therapy as appropriate or, if indicated, stopping the trial therapy on either a permanent or temporary basis. The date of the adverse reaction was recorded as the date of the second therapy session.
To report serious adverse events (SAEs), the trial team used the Norfolk and Norwich University Hospital and UEA joint standard operating procedure (SOP). The latest version can be found www.nnuh.nhs.uk/Dept.asp?ID%20=%20681 (see SOP 205). All SAEs were assessed by the local principal investigator, countersigned by the chief investigator, reported to the sponsor and reported to the Data Monitoring and Ethics Committee (DMEC) and Trial Steering Committee (TSC). All SAEs were followed up until a documented end date and resolution could be provided, or the participant ended the trial. Participants were considered to have reached the end of the trial when the first of the following occurred:
-
completion of assessment at 6 months after stroke
-
withdrawal of consent
-
SAE resulting in withdrawal of participant or death
-
loss to follow-up.
If a SAE was still ongoing at the time a participant reached a trial end point, their trial end date was also used to record their SAE end data.
Statistical analysis
The statistical analysis plan (SAP) was agreed, signed and dated prior to the database lock and unblinding of the treatment allocations (see Appendix 4).
There was a change to the original analysis plan from taking account of clustering by therapist to only adjusting for the study site. The change was made because, for practical reasons, the participants were not clustered within the same therapist for all their sessions.
Clinical efficacy (objective 1)
To address objective 1 the primary analysis compared the change in the efficacy parameters (baseline and outcome) between the treatment groups. Change in the efficacy parameters (ARAT, WMFT, and Hand Grip Force and Pinch Grip Force tests) at outcome were analysed using analysis of covariance (ANCOVA) models adjusted for the baseline value and randomisation strata (time after stroke, ability to use paretic upper limb, clinical centre). Adjusted least squares mean difference and 95% confidence intervals (CIs) were reported. When the outcome distribution deviated from a normal distribution, a log or other appropriate transformation was applied.
Neural correlates of clinical improvement (objective 2)
To answer the second objective, associations between the changes in neural variables were compared with the changes in clinical efficacy measures (baseline to outcome). Correlation coefficients were calculated for the two treatment groups separately and for the groups combined.
Predictive neural markers of clinical improvement (objective 3)
The third objective was answered through subgroup analysis of the change in ARAT score at outcome. For each baseline covariate being investigated as a potential predictive marker of clinical improvement, the treatment effect was calculated within each level of the subgroup (adjusted as for the first objective) and an interaction term between randomised treatment and baseline covariate was included in the model.
Trial management
Data collection
All data were collected in accordance with trial operating procedures. These were developed to the standards required in the SOPs of the Norwich Clinical Trial Unit.
Case report forms (CRFs) were developed to enable capture of primary and secondary outcome data at the time points of baseline, outcome and follow-up. In addition, the CRFs recorded demographic information such as sex and type of stroke. Throughout the trial, the completed CRFs were secured in a locked filing cabinet in a research office in the Moseley Hall Hospital (Birmingham), the Queen’s Building at the UEA (Norfolk), or the Haywood Hospital (North Staffordshire). Once the follow-up phase of the trial was completed, all CRFs were moved securely to the UEA for final data query resolution, in preparation for the final database lock. All CRFs were then securely stored and archived at the UEA.
Data entry and quality assurance
The study database was set up and managed by the Data Management Team at Glasgow Clinical Trials Unit. The data management plan is included as Appendix 5. Data management included:
-
design and specification of the database and data entry system
-
real-time validation of data entry for data types and ranges
-
provision of a double data entry and checking system at the end of the study
-
import of data
-
data queries for validation at end of study
-
database lock
-
data set extraction for analysis.
Procedures were developed, implemented and monitored to maintain blinding and limit access of members of the research team to data throughout the trial. Data were sent to the RCB for entry and quality control in a secure standardised manner in keeping with the Data Management Plan produced by the RCB (see Appendix 5). The RCB was responsible for accumulating, reviewing and reporting on data from several primary and secondary sources.
Primary data sources were from paper copies of study CRFs completed at study site.
Secondary data sources were from (1) database amendment requests and (2) responses to data queries.
External data sources included the results of the TMS and magnetic resonance imaging (MRI) tests/procedures.
Trial Steering Committee
A TSC was convened to provide overall supervision and ensure good conduct of the trial (e.g. adherence to the Declaration of Helsinki and good practice in user involvement). This was undertaken by nominating potential members to the Efficacy and Mechanism Evaluation (EME) programme board, which then appointed people to the TSC. Members of the TSC were:
-
Professor Anne Forster (independent chairperson)
-
Professor Christopher Weir (collaborator, statistician and methodologist)
-
Dr Sue Hunter (collaborator, principal investigator)
-
Professor Jon Marsden (independent member)
-
Professor John Rothwell (collaborator, TMS lead)
-
Professor Valerie Pomeroy (chief investigator)
-
Dr Ailie Turton (independent member)
-
Ms Emma Costello (clinical research administrator)
-
Mr Nick Leavey (clinical trial manager).
The TSC met on 22 November 2012, 23 May 2013, 27 August 2013, 11 December 2013, 6 February 2014, 3 July 2014, 27 February 2015, 11 April 2016 [a full meeting was not convened on request of independent chairperson (AF); however, an up-to-date trial progress report was distributed to the TSC and comments were subsequently shared and responded to by e-mail)] and 5 December 2016 (a final TSC meeting to review the final Robertson Centre Report and the current draft monograph).
Data Monitoring and Ethics Committee
The DMEC reported directly to the chair of the TSC. Members of the DMEC appointed by the EME programme were:
-
Professor Sally Singh (independent chairperson)
-
Professor Gert Kwakkel (independent member)
-
Dr Martyn Lewis (independent member)
-
Professor Valerie Pomeroy (chief investigator)
-
Dr Andrew Walker (until 31 December 2014) (clinical trial manager)
-
Nick Leavey (from 3 September 2014) (clinical trial manager).
The DMEC met on 16 July 2013, 29 January 2014, 16 July 2014, 2 February 2015, 2 September 2015 and 25 November 2016.
Public and Patient Involvement
At the beginning of the trial, the initial trial manager and Professor Pomeroy attempted to secure a Patient and Public Involvement (PPI) representative through the Patient and Public Involvement in Research (PPIRes)54 group. An early version of the protocol for the grant application was reviewed by PPIRes. Unfortunately, however, no one was identified by PPIRes to be the PPI representative for this trial.
Nonetheless, PPI has still taken various forms throughout the trial. From the outset, the trial featured at meetings of the Norfolk Community Health and Care NHS Trust patient and carer groups, in Norwich. The trial team therefore benefited from PPI comments and suggestions on a range of elements, particularly the development of participant information resources.
Further PPI was also explored by Dr Andrew Walker via a contact provided by the EME programme manager. This did not lead to further PPI. Therefore, a replacement PPI representative was not identified at this point in the trial.
As outlined in the progress report submitted on 1 April 2015, a PPI representative was identified from Headway Suffolk. The person identified was Helen Fairweather, the chief executive officer of Headway Suffolk, who kindly agreed to be a member of the TSC. Since April 2015, Helen has been invited to all TSC meetings and sent copies of the agendas and subsequent minutes and associated documents.
A participant thank-you event was held at the Norwich Community Hospital on 3 May 2016. The event was to celebrate the trial achieving its 288-participant recruitment target. Over 50 local participants attended, who were provided with a verbal report on the remaining ongoing trial activity and plans for dissemination of the trial results. All were appreciative of their involvement in the trial and comments were received in approval of the dissemination plan.
During the trial, and following a substantial amendment (eighth amendment), three editions of a FAST INdICATE participant newsletter were distributed to trial participants to keep them updated on trial progress and its planned dissemination.
Chapter 3 Results: clinical efficacy
Objective addressed
To determine if upper limb motor recovery is enhanced more by FST + CPT than an equal dose of MPT + CPT commenced early after stroke.
Dates of recruitment and follow-up periods
Screening for potential participants started on 1 April 2012 and ended on 25 January 2016. The first participant was randomised on 17 October 2012 and the last participant was randomised on 29 January 2016. The last follow-up assessment, at 6 months after stroke, was 6 July 2016.
Participant characteristics
The characteristics of participants are detailed in Table 1. In summary, the mean age (SD) of participants in this trial was 72.2 (12.5) years. The sex distribution was 64.6% male and 35.4% female. Informed consent was given within 30 days of stroke by 59% of participants and at ≥ 31 days by 41%. All participants had been diagnosed with stroke in the territory of the anterior cerebral circulation. The stroke was caused by a haemorrhage for 8.7% of participants and by ischaemia for 91.3%. The right-hand side of the body was more affected by the stroke than the left-hand side for 42% of participants.
| Characteristic | Treatment group | All (N = 288) | |
|---|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | ||
| Age (years), mean (SD) | 71.9 (12.7) | 72.4 (12.3) | 72.2 (12.5) |
| Sex, n (%) | |||
| Male | 96 (66.2) | 90 (62.9) | 186 (64.6) |
| Female | 49 (33.8) | 53 (37.1) | 102 (35.4) |
| Type of stroke, n (%) | |||
| Ischaemic | 131 (90.3) | 132 (92.3) | 263 (91.3) |
| Haemorrhagic | 14 (9.7) | 11 (7.7) | 25 (8.7) |
| Side of brain lesion, n (%) | |||
| Left | 63 (43.5) | 58 (40.6) | 121 (42.0) |
| Right | 82 (56.5) | 85 (59.4) | 167 (58.0) |
| 9HPT at consent, n (%) | |||
| ≤ 1 peg | 91 (62.8) | 90 (63.0) | 181 (62.9) |
| 2–8 pegs | 54 (37.2) | 53 (37.1) | 107 (37.2) |
| Days after stroke at consent, n (%) | |||
| ≤ 30 days | 86 (59.3) | 84 (58.7) | 170 (59.0) |
| ≥ 31 days | 59 (40.7) | 59 (41.3) | 118 (41.0) |
| ARAT total score – paretic, mean score (SD)a | 24.7 (18.9) | 26.2 (17.4) | 25.5 (18.2) |
| WMFT – performance, mean score (SD)b | 36.4 (20.25) | 37.6 (17.1) | 37.0 (18.8) |
| Grip force (kg), mean score (SD)c | 7.6 (8.7) | 6.9 (8.1) | 7.2 (8.4) |
| Pinch force (kg), mean score (SD)d | 2.2 (2.2) | 1.9 (2.3) | 2.1 (2.2) |
Flow of participants through the trial
The CONSORT flow chart for this trial in respect to the primary outcome (ARAT score) is provided in Figure 5. In summary, a total of 5064 stroke survivors were screened for eligibility for this trial. Of these, 2929 (58%) were excluded because they did not meet the initial study inclusion criteria, with a further 872 (17%) excluded for additional reasons, such as being outside the trial catchment area or having severe cognitive and/or language impairment. This left 1263 identifiable eligible people; however, 536 (42%) of these people declined to talk to the research team. The remaining 481 (38%) provided informed consent and undertook the trial suitability screening assessment; of those, 138 (29%) did not meet the eligibility criteria and a further 55 (11%) either withdrew consent or otherwise were not randomised. The remaining 288 were recruited as participants and randomised into the trial. Consequently, 288 participants undertook the baseline assessments and were subsequently allocated randomly to the MPT group (n = 143) or the FST group (n = 145).
FIGURE 5.
All centres: CONSORT 2010 flow diagram. a, Data are excluded from analysis if missing items prevent the ARAT total score from being calculated. UL, upper limb.
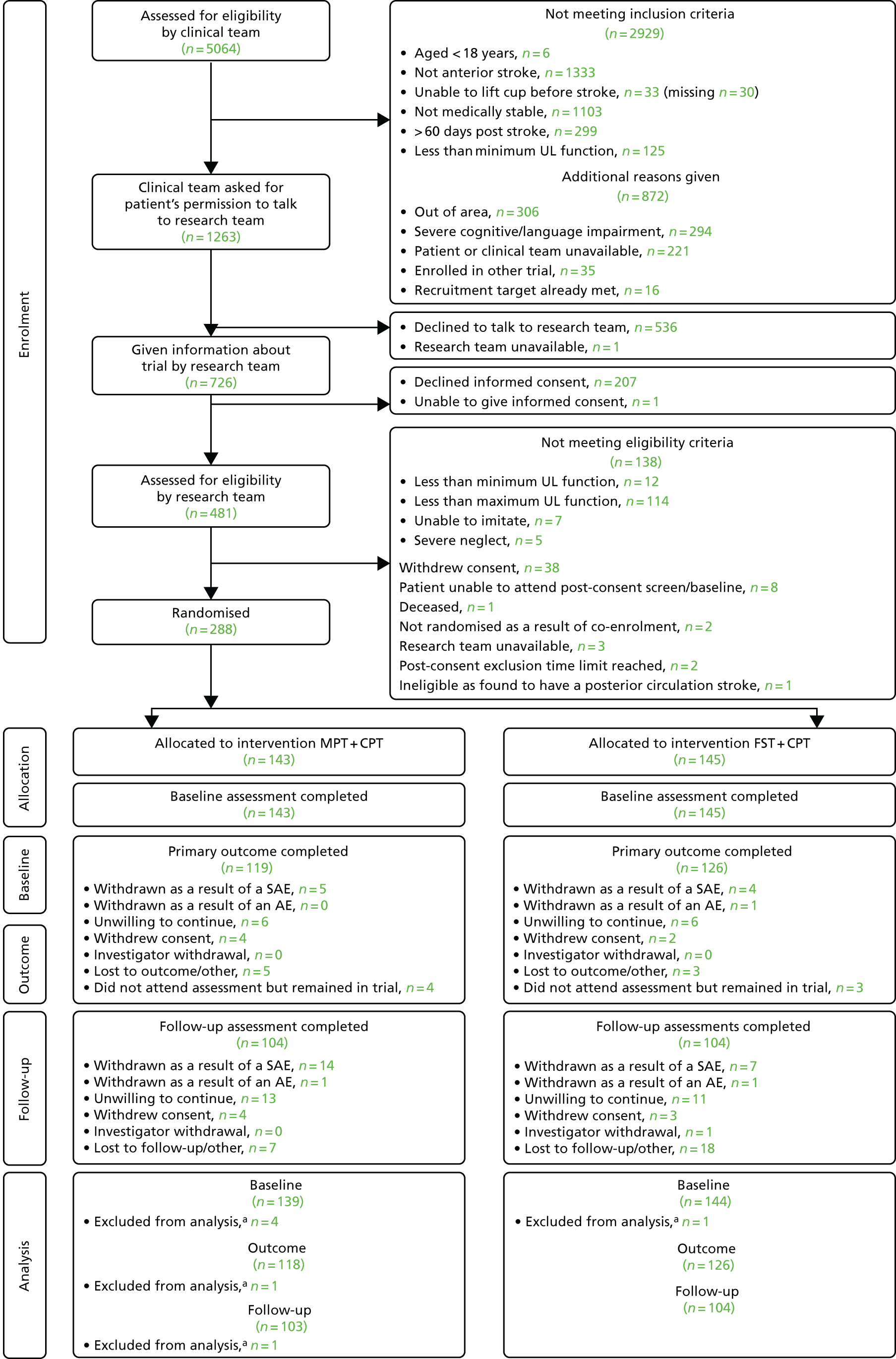
At the primary end point of the outcome (at the end of the 6-week intervention phase), 245 (85.1%) participants undertook the primary measure (ARAT score), of whom 119 had been allocated to MPT + CPT and 126 to FST + CPT. Attrition reasons were:
-
withdrew as a result of a SAE or AE (MPT + CPT, n = 5; FST + CPT, n = 5)
-
unwilling to continue (MPT + CPT, n = 6; FST + CPT, n = 6)
-
withdrew consent (MPT + CPT, n = 4; FST + CPT, n = 2)
-
lost to outcome/other (MPT + CPT, n = 5 FST + CPT, n = 3)
-
did not attend for assessment, but remained in trial (MPT + CPT, n = 4; FST + CPT, n = 3).
At 6 months after stroke, the follow-up time point, 208 (72.2%) participants undertook the primary measure (ARAT score), of whom 104 were allocated to MPT + CPT and 104 to FST + CPT. Attrition at the follow-up point had occurred for the following reasons:
-
withdrew as a result of a SAE or AE (MPT + CPT, n = 15; FST + CPT, n = 8)
-
unwilling to continue (MPT + CPT, n = 13; FST + CPT, n = 11)
-
withdrew consent (MPT + CPT, n = 4; FST + CPT, n = 3)
-
investigator withdrawal (MPT + CPT, n = 0; FST + CPT, n = 1)
-
lost to outcome/other (MPT + CPT, n = 7; FST + CPT, n = 18).
Adverse reactions, adverse events and serious adverse events
No SAEs met the criteria for reporting to the National Research Ethics Service. All SAEs were reviewed by the DMEC. Only four participants experienced an adverse reaction of pain or fatigue during the intervention phase (Table 2).
| Adverse reaction | Treatment group, n (%) | All (N = 288), n (%) | |
|---|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | ||
| Any adverse reaction of pain | 1 (0.69) | 1 (0.70) | 2 (0.69) |
| Any adverse reaction of fatigue | 3 (2.07) | 1 (0.70) | 4 (1.39) |
Adverse events, related AEs, SAEs and unexpected AEs during the intervention phase are detailed in Tables 3–6. No clinically important differences are detectable between the FST + CPT and MPT + CPT groups.
| Adverse event | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 56 (38.62) | 59 (41.26) |
| Injury, poisoning and procedural complications | 18 (12.41) | 19 (13.29) |
| Infections and infestations | 17 (11.72) | 7 (4.90) |
| Musculoskeletal and connective tissue disorders | 11 (7.59) | 11 (7.69) |
| General disorders and administration site conditions | 11 (7.59) | 8 (5.59) |
| Nervous system disorders | 8 (5.52) | 8 (5.59) |
| Gastrointestinal disorders | 4 (2.76) | 6 (4.20) |
| Vascular disorders | 1 (0.69) | 8 (5.59) |
| Psychiatric disorders | 5 (3.45) | 1 (0.70) |
| Respiratory, thoracic and mediastinal disorders | 1 (0.69) | 4 (2.80) |
| Cardiac disorders | 1 (0.69) | 3 (2.10) |
| Renal and urinary disorders | 2 (1.38) | 1 (0.70) |
| Eye disorders | 0 (0.00) | 2 (1.40) |
| Immune system disorders | 1 (0.69) | 1 (0.70) |
| Investigations | 0 (0.00) | 2 (1.40) |
| Ear and labyrinth disorders | 1 (0.69) | 0 (0.00) |
| Metabolism and nutrition disorders | 0 (0.00) | 1 (0.70) |
| Neoplasms benign, malignant and unspecified | 0 (0.00) | 1 (0.70) |
| Social circumstances | 1 (0.69) | 0 (0.00) |
| Surgical and medical procedures | 0 (0.00) | 1 (0.70) |
| Related AE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 5 (4.45) | 9 (6.29) |
| Injury, poisoning and procedural complications | 1 (0.69) | 1 (0.70) |
| Musculoskeletal and connective tissue disorders | 4 (2.76) | 3 (2.10) |
| General disorders and administration site condition | 1 (0.69) | 2 (1.40) |
| Nervous system disorders | 0 (0.00) | 2 (1.40) |
| Eye disorders | 0 (0.00) | 1 (0.70) |
| Metabolism and nutrition disorders | 0 (0.00) | 1 (0.70) |
| SAE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 17 (11.72) | 11 (7.69) |
| Injury, poisoning and procedural complications | 1 (0.69) | 2 (1.40) |
| Infections and infestations | 6 (4.14) | 0 (0.00) |
| Musculoskeletal and connective tissue disorders | 1 (0.69) | 0 (0.00) |
| General disorders and administration site conditions | 4 (2.76) | 0 (0.00) |
| Nervous system disorders | 4 (2.76) | 2 (1.40) |
| Gastrointestinal disorders | 1 (0.69) | 0 (0.00) |
| Psychiatric disorders | 1 (0.69) | 1 (0.70) |
| Respiratory, thoracic and mediastinal disorders | 0 (0.00) | 1 (0.70) |
| Cardiac disorders | 1 (0.69) | 3 (2.10) |
| Immune system disorders | 1 (0.69) | 0 (0.00) |
| Neoplasms benign, malignant and unspecified | 0 (0.00) | 1 (0.70) |
| Surgical and medical procedures | 0 (0.00) | 1 (0.70) |
| Unexpected SAE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 9 (6.21) | 4 (2.80) |
| Injury, poisoning and procedural complications | 0 (0.00) | 1 (0.70) |
| Infections and infestations | 4 (2.76) | 0 (0.00) |
| General disorders and administration site condition | 1 (0.69) | 0 (0.00) |
| Nervous system disorders | 2 (1.38) | 0 (0.00) |
| Gastrointestinal disorders | 1 (0.69) | 0 (0.00) |
| Psychiatric disorders | 0 (0.00) | 1 (0.70) |
| Respiratory, thoracic and mediastinal disorders | 0 (0.00) | 1 (0.70) |
| Cardiac disorders | 0 (0.00) | 1 (0.70) |
| Immune system disorders | 1 (0.69) | 0 (0.00) |
Those AEs, related AEs, SAEs and unexpected AEs that occurred during the follow-up phase are detailed in Tables 7–10. No clinically important differences are detectable between the FST + CPT and MPT + CPT groups.
| AE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 22 (15.17) | 35 (24.48) |
| Injury, poisoning and procedural complications | 8 (5.52) | 10 (6.99) |
| Infections and infestations | 7 (4.83) | 6 (4.20) |
| Skin and subcutaneous tissue disorders | 1 (0.69) | 4 (2.80) |
| General disorders and administration site conditions | 2 (1.38) | 5 (3.50) |
| Nervous system disorders | 3 (2.07) | 7 (4.90) |
| Gastrointestinal disorders | 1 (0.69) | 3 (2.10) |
| Vascular disorders | 0 (0.00) | 1 (0.70) |
| Psychiatric disorders | 2 (1.38) | 1 (0.70) |
| Respiratory, thoracic and mediastinal disorders | 0 (0.00) | 3 (2.10) |
| Renal and urinary disorders | 1 (0.69) | 0 (0.00) |
| Eye disorders | 0 (0.00) | 1 (0.70) |
| Neoplasms benign, malignant and unspecified | 0 (0.00) | 1 (0.70) |
| Musculoskeletal and connective tissue disorders | 1 (0.69) | 1 (0.70) |
| Surgical and medical procedures | 3 (2.07) | 2 (1.40) |
| Related AE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 3 (2.07) | 0 (0.00) |
| Musculoskeletal and connective tissue disorders | 1 (0.69) | 0 (0.00) |
| General disorders and administration site condition | 1 (0.69) | 0 (0.00) |
| Nervous system disorders | 1 (0.69) | 0 (0.00) |
| SAE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 5 (3.45) | 13 (9.09) |
| Injury, poisoning and procedural complications | 1 (0.69) | 2 (1.40) |
| General disorders and administration site conditions | 1 (0.69) | 1 (0.70) |
| Nervous system disorders | 1 (0.69) | 6 (4.20) |
| Gastrointestinal disorders | 0 (0.00) | 1 (0.70) |
| Psychiatric disorders | 1 (0.69) | 2 (1.40) |
| Respiratory, thoracic and mediastinal disorders | 0 (0.00) | 2 (1.40) |
| Renal and urinary disorders | 1 (0.69) | 0 (0.00) |
| Neoplasms benign, malignant and unspecified | 0 (0.00) | 1 (0.70) |
| Surgical and medical procedures | 1 (0.69) | 0 (0.00) |
| Unexpected SAE | Treatment group, n (%) | |
|---|---|---|
| FST + CPT (N = 145) | MPT + CPT (N = 143) | |
| Any event | 2 (1.38) | 7 (4.90) |
| Injury, poisoning and procedural complications | 0 (0.00) | 1 (0.70) |
| Nervous system disorders | 0 (0.00) | 2 (1.40) |
| Gastrointestinal disorders | 0 (0.00) | 1 (0.70) |
| Psychiatric disorders | 0 (0.00) | 2 (1.40) |
| Respiratory, thoracic and mediastinal disorders | 0 (0.00) | 2 (1.40) |
| Renal and urinary disorders | 1 (0.69) | 0 (0.00) |
| Neoplasms benign, malignant and unspecified | 0 (0.00) | 1 (0.70) |
| Surgical and medical procedures | 1 (0.69) | 0 (0.00) |
Primary clinical efficacy analysis
The primary outcome measure was ARAT score. There were 126 participants for the FST + CPT group and 114 for the MPT + CPT group with data at both baseline and outcome (Table 11). At follow-up, the number of participants with data at baseline had reduced to 104 for the FST + CPT group and to 100 for the MPT + CPT group (Table 12). Both groups showed improvement (mean and SD) from baseline at outcome [FST + CPT = 9.70 (SD 11.72) and MPT + CPT = 7.90 (SD 9.18)], but there was little difference between the groups and this did not reach statistical significance (p = 0.298). Further improvements were evident at follow-up with mean (SD) changes from baseline of 11.10 (SD 14.68) for the FST + CPT group and 10.3 (SD 10.74) for the MPT + CPT group. Again, this difference was small and did not reach statistical significance (p = 0.743).
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at outcome | FST + CPT | 126 | 24.40 (18.45) | 34.10 (17.81) | 9.70 (11.72) | 9.80 (7.87 to 11.73) | 1.35 (–1.20 to 3.90); p = 0.298 |
| MPT + CPT | 114 | 26.50 (17.78) | 34.40 (18.68) | 7.90 (9.18) | 8.45 (6.41 to 10.49) | ||
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at follow-up | FST + CPT | 104 | 25.80 (18.21) | 36.80 (19.14) | 11.10 (14.68) | 10.90 (8.31 to 13.49) | 0.55 (–2.77 to 3.88); p = 0.743 |
| MPT + CPT | 100 | 27.10 (17.49) | 37.40 (17.50) | 10.3 (10.74) | 10.35 (7.66 to 13.03) | ||
Secondary clinical efficacy analysis
Secondary clinical efficacy outcome measures (Tables 13–18) showed a similar pattern to the ARAT scores (primary outcome) in terms of their mean improvements. SDs indicated more variability in response for grip and pinch force. Differences between groups were small and did not reach statistical significance.
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at outcome | FST + CPT | 122 | 7.60 (8.72) | 10.7 (9.99) | 3.1 (7.11) | 3.98 (2.74 to 5.21) | 0.47 (–1.16 to 2.09); p = 0.571 |
| MPT + CPT | 115 | 7.20 (8.19) | 9.90 (9.35) | 2.70 (6.25) | 3.51 (2.24 to 4.78) | ||
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at follow-up | FST + CPT | 101 | 7.40 (8.50) | 11.80 (10.20) | 4.4 (7.02) | 5.33 (3.70 to 6.95) | –0.29 (–2.37 to 1.79); p = 0.785 |
| MPT + CPT | 97 | 7.20 (8.35) | 12.00 (10.10) | 4.70 (8.71) | 5.62 (3.95 to 7.28) | ||
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at outcome | FST + CPT | 115 | 2.20 (2.20) | 3.00 (2.93) | 0.90 (2.13) | 0.91 (0.48 to 1.33) | 0.02 (–0.54 to 0.59); p = 0.934 |
| MPT + CPT | 109 | 2.00 (2.30) | 2.90 (2.88) | 0.90 (2.16) | 0.89 (0.45 to 1.32) | ||
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at follow-up | FST + CPT | 94 | 2.30 (2.22) | 3.30 (2.45) | 1.00 (2.19) | 1.16 (0.62 to 1.71) | –0.30 (–0.98 to 0.39); p = 0.395 |
| MPT + CPT | 95 | 2.10 (2.24) | 3.50 (3.09) | 1.40 (2.76) | 1.46 (0.92 to 2.01) | ||
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at outcome | FST + CPT | 117 | 36.60 (19.97) | 47.80 (19.70) | 11.20 (10.62) | 11.31 (0.35 to 13.27) | 0.65 (–1.91 to 3.21); p = 0.616 |
| MPT + CPT | 109 | 39.00 (16.84) | 49.00 (18.52) | 10.00 (9.61) | 10.65 (8.62 to 12.69) | ||
| Comparison | Treatment group | Number of participantsa | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Least squares | Least squares difference and p-value of change between group | |||
| Change at follow-up | FST + CPT | 98 | 38.30 (19.42) | 51.80 (19.83) | 13.50 (14.28) | 14.21 (11.53 to 16.88) | 0.01 (–3.46 to 3.47); p = 0.997 |
| MPT + CPT | 93 | 39.30 (16.96) | 52.60 (18.41) | 13.30 (11.55) | 14.20 (11.40 to 17.00) | ||
Chapter 4 Results: neural correlates of clinical improvement
Objective addressed
To identify the similarities and differences in the neural correlates of clinical improvement in upper limb motor function in response to (1) FST + CPT and (2) MPT + CPT. To reiterate, baseline measures were made immediately before randomisation and outcome measures made at the end of the 6-week intervention phase (see Chapter 2, Design).
Flow of participants through the correlates analysis
Magnetic resonance imaging
Structural MR scans were undertaken for those participants who had no contraindications, had provided separate informed consent for this part of the assessment and who could travel to the neuroimaging facility. MR scans were undertaken for 94 (32.6%) participants at baseline and 62 (25.3%) participants at outcome. Reasons for non-completion of MRI are shown in Table 19.
| Data | Time point, n (%) | |
|---|---|---|
| Baseline (N = 288)a | Outcome (N = 245)a,b | |
| MRI data acquired | ||
| Attended for neuroimaging | 94 (32.6) | 62 (25.3) |
| Reason for lack of data | ||
| Unable to attend | 31 (10.8) | 39 (15.9) |
| Did not consent | 44 (15.3) | 62 (25.3) |
| Other | 119 (41.3) | 106 (43.3) |
Transcranial magnetic stimulation
Transcranial magnetic stimulation was undertaken by those participants who had no contraindications. The TMS measures were made with 111 (38.5%) participants at baseline and 83 (33.9%) at outcome. Reasons for no TMS are shown in Table 20.
| Data | Time point, n (%) | |
|---|---|---|
| Baseline (N = 288)a | Outcome (N = 245)a,b | |
| TMS data acquired | ||
| Attended for TMS | 111 (38.5) | 83 (33.9) |
| Reason for lack of data | ||
| Did not consent for TMS | 30 (10.4) | 39 (15.9) |
| Unable to attend | 21 (7.3) | 39 (15.9) |
| Other | 126 (43.8) | 114 (46.5) |
Neural variables at baseline and outcome
The numbers of participants for whom neuroimaging variables were available at outcome and follow-up are shown in Tables 21–23. The number of participants varies from 45 for corticocortical and corticospinal anatomical connectivity at outcome (see Table 21) to 84 for volume of the stroke lesion at baseline (see Table 23).
| Statistic | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Outcome | |||||
| FST + CPT | MPT + CPT | All | FST + CPT | MPT + CPT | All | |
| Number of participants | 42 | 38 | 80 | 25 | 20 | 45 |
| Mean | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| SD | 0.09 | 0.08 | 0.08 | 0.09 | 0.08 | 0.08 |
| Median | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.4 |
| Minimum–maximum | 0.21–0.54 | 0.29–0.55 | 0.21–0.55 | 0.22–0.54 | 0.31–0.57 | 0.22–0.57 |
| IQR | 0.342–0.47 | 0.34–0.49 | 0.34–0.47 | 0.35–0.46 | 0.35–0.49 | 0.35–0.47 |
| Statistic | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Outcome | |||||
| FST + CPT | MPT + CPT | All | FST + CPT | MPT + CPT | All | |
| Number of participants | 42 | 38 | 80 | 25 | 20 | 45 |
| Mean | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| SD | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Minimum–maximum | –0.06 to 0.14 | –0.07 to 0.15 | –0.07 to 0.15 | –0.02 to 0.18 | –0.04 to 0.18 | –0.04 to 0.18 |
| IQR | 0.01 to 0.06 | –0.02 to 0.06 | 0.001 to 0.06 | 0.00 to 0.07 | –0.01 to 0.06 | –0.01 to 0.07 |
| Statistic | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Outcome | |||||
| FST + CPT | MPT + CPT | All | FST + CPT | MPT + CPT | All | |
| Number of participants | 44 | 40 | 84 | 29 | 23 | 52 |
| Mean | 23,847.89 | 25,476.38 | 24,623.36 | 28,129.97 | 32,398.22 | 30,017.85 |
| SD | 47,664.63 | 59,957.23 | 53,542.90 | 60,884.91 | 57,371.06 | 58,818.52 |
| Median | 6537.50 | 2486.00 | 4293.00 | 1753.00 | 2139.00 | 1946.00 |
| Minimum–maximum | 91.00–218,162.00 | 56.00–324,152.00 | 56.00–324,152.00 | 99.00–252,757.00 | 54.00–221,613.00 | 54.00–252,757.00 |
| IQR | 1067.50–19,551.50 | 474.00–14,046.50 | 845.50–17,160.50 | 919.00–13,945.00 | 788.00–45,496.00 | 822.00–28,184.50 |
Change in neural variables derived from neuroimaging between baseline and outcome for those participants with data at both visits and ARAT score data at both visits is shown in Table 24. There were no statistically significant differences between the two groups for change in any of these variables.
| Variable | Group | n | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| Lesion volume (mm3) | FST + CPT | 24 | 8.20 (2.13) | 8.10 (2.17) | –0.10 (0.45) | –0.11 (–0.31 to 0.09) | –0.12 (–0.36 to 0.13); p = 0.349 |
| MPT + CPT | 20 | 8.20 (2.49) | 8.10 (2.40) | –0.00 (0.32) | 0.01 (–0.21 to 0.22) | ||
| Corticocortical connectivitya | FST + CPT | 20 | 0.38 (0.09) | 0.38 (0.09) | –0.00 (0.02) | –0.01 (–0.02 to 0.01) | 0.01 (–0.01 to 0.03); p = 0.386 |
| MPT + CPT | 18 | 0.45 (0.07) | 0.43 (0.08) | –0.01 (0.03) | –0.02 (–0.03 to 0.00) | ||
| Corticospinal connectivityb | FST + CPT | 20 | 0.03 (0.05) | 0.03 (0.04) | 0.00 (0.01) | 0.00 (–0.01 to 0.02) | –0.01 (–0.02 to 0.01); p = 0.524 |
| MPT + CPT | 18 | 0.01 (0.05) | 0.031 (0.05) | 0.02 (0.04) | 0.01 (–0.01 to 0.03) | ||
The number of participants for whom neural variables derived from TMS were available at outcome and follow-up together are shown in Tables 25 and 26. The number of participants ranges between 72 for RMT for paretic extensor carpi radialis (pECR) at outcome (see Table 26) and 110 for presence of a MEP of pBB and pECR at baseline (see Table 25).
| MEP | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Outcome | |||||
| FST + CPT | MPT + CPT | All | FST + CPT | MPT + CPT | All | |
| pBB | ||||||
| Number | 53 | 57 | 110 | 43 | 39 | 82 |
| Yes, n (%) | 37 (69.81) | 43 (75.44) | 80 (72.73) | 39 (90.70) | 34 (87.18) | 73 (89.02) |
| No, n (%) | 16 (30.19) | 14 (24.56) | 30 (27.27) | 4 (9.30) | 5 (12.82) | 9 (10.98) |
| pECR | ||||||
| Number | 53 | 57 | 110 | 42 | 39 | 81 |
| Yes, n (%) | 39 (73.58) | 45 (78.95) | 84 (76.36) | 37 (88.10) | 35 (89.74) | 72 (88.89) |
| No, n (%) | 14 (26.42) | 12 (21.05) | 26 (23.64) | 5 (11.90) | 4 (10.26) | 9 (11.11) |
| MEP | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Outcome | |||||
| FST + CPT | MPT + CPT | All | FST + CPT | MPT + CPT | All | |
| pBB | ||||||
| Number | 37 | 43 | 80 | 39 | 34 | 73 |
| Mean | 61.1 | 63.0 | 62.1 | 65.9 | 69.9 | 67.8 |
| SD | 11.49 | 14.39 | 13.08 | 18.12 | 16.59 | 17.42 |
| Median | 60.0 | 66.0 | 60.0 | 63.0 | 71.5 | 65.0 |
| Minimum–maximum | 38–98 | 27–85 | 27–98 | 34–96 | 39–98 | 34–98 |
| IQR | 54–66 | 53–74 | 54–70 | 51–76 | 55–85 | 55–85 |
| pECR | ||||||
| Number | 39 | 44 | 83 | 37 | 35 | 72 |
| Mean | 52.4 | 54.5 | 53.5 | 57.4 | 57.9 | 57.6 |
| SD | 12.30 | 15.27 | 13.91 | 18.58 | 16.40 | 17.43 |
| Median | 50.0 | 52.5 | 51.0 | 55.0 | 53.0 | 54.0 |
| Minimum–maximum | 24–90 | 27–94 | 24–94 | 27–95 | 33–95 | 27–95 |
| IQR | 46–58 | 44–61 | 45–60 | 47–63 | 44–70 | 44–68 |
Change between baseline and outcome for RMT for pBB and pECR muscles for those participants with data at both visits and ARAT score data at both visits is shown in Table 27. There are no statistically significant differences between the two groups for change in either of these variables.
| Variable | Group | n | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| pBB | FST + CPT | 28 | 61.6 (12.07) | 60.6 (13.61) | –1.0 (14.27) | –0.82 (–6.32 to 4.68) | –2.87 (–8.91 to 3.16); p = 0.343 |
| MPT + CPT | 27 | 62.5 (13.83) | 65.0 (14.90) | 2.5 (10.06) | 2.06 (–3.86 to 7.97) | ||
| pECR | FST + CPT | 28 | 53.1 (13.15) | 52.0 (11.62) | –1.1 (11.46) | –3.29 (–7.68 to 1.09) | 0.98 (–4.31 to 6.28); p = 0.710 |
| MPT + CPT | 27 | 57.4 (17.16) | 53.2 (12.93) | –4.2 (12.98) | –4.28 (–9.19 to 0.63) | ||
The correlations between change, from baseline to outcome, between neural variables and ARAT score ranged from r = –0.147 (p = 0.385) for all participants’ corticospinal connectivity to r = 0.199 (p = 0.320) for the MPT + CPT group for RMT pBB (Table 28). Consequently, the neural correlates of improvement in ARAT score were similar for the two groups.
| Neural variable | Treatment group | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FST + CPT | MPT + CPT | ||||||||
| Number of participants | r-value | p-value | Number of participants | r-value | p-value | Number of participants | r-value | p-value | |
| Volume of the stroke lesion | 24 | –0.021 | 0.921 | 19 | –0.043 | 0.863 | 43 | –0.042 | 0.787 |
| Corticocortical anatomical connectivity | 20 | 0.092 | 0.699 | 17 | –0.029 | 0.913 | 37 | 0.069 | 0.684 |
| Corticospinal anatomical connectivity | 20 | –0.031 | 0.897 | 17 | –0.306 | 0.232 | 37 | –0.147 | 0.385 |
| RMT – pBB | 28 | 0.095 | 0.631 | 27 | 0.199 | 0.320 | 55 | 0.093 | 0.501 |
| RMT – pECR | 28 | –0.080 | 0.635 | 27 | 0.043 | 0.831 | 55 | –0.001 | 0.996 |
Chapter 5 Results: predictive markers of clinical improvement
Objective addressed
To determine if any pretreatment parameters or any combination of pretreatment parameters [(a) anatomical location of infarction, (b) volume of the stroke lesion, (c) residual structural corticocortical connectivity, (d) residual corticospinal connectivity and (e) brain–muscle functional connectivity (derived from TMS)] are sufficiently predictive of upper limb recovery after stroke to enable physical therapy to be targeted at those people most likely to respond.
Participants with neural variables data at baseline
Data on the number of participants at baseline and the values of the neural variables are presented in Tables 21–28.
Change at outcome from baseline for Action Research Arm Test score
For those people with both ARAT score and neural variable data at baseline and outcome there were no statistically significant interaction effects (Table 29). The data for change in ARAT score underlying these interaction effects are provided in Tables 30–34. There are no statistically significant differences between the subgroups for any of the baseline characteristics.
| Baseline characteristic | Interaction p-value |
|---|---|
| Anatomical location of stroke lesion: MNI CST affected | 0.384 |
| Volume of the stroke lesion (logged) | 0.762 |
| Corticocortical anatomical connectivity (FA MNI corpus callosum midline) | 0.723 |
| Corticocortical anatomical connectivity (asymmetry ipsilesional : contralesional MNI CSTS) | 0.553 |
| Presence of MEP pBB | 0.237 |
| pBB RMT | 0.697 |
| Presence of MEP pECR | 0.193 |
| pECR RMT | 0.503 |
| Variable | Group | Number of participants | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| Corticocortical connectivity value below median | FST + CPT | 18 | 27.4 (20.29) | 35.6 (17.54) | 8.2 (9.47) | 11.92 (6.74 to 17.10) | 3.83 (–2.41 to 10.07); p = 0.218 |
| MPT + CPT | 14 | 27.4 (17.53) | 34.0 (20.04) | 6.6 (6.87) | 8.09 (3.12 to 13.06) | ||
| Corticocortical connectivity value equal to or above median | FST + CPT | 18 | 22.3 (18.22) | 39.3 (14.58) | 17.1 (13.45) | 16.95 (11.22 to 22.68) | 4.29 (–2.99 to 11.58); p = 0.238 |
| MPT + CPT | 18 | 24.6 (17.74) | 33.8 (19.65) | 9.2 (6.78) | 12.66 (5.40 to 19.92) | ||
| Variable | Group | Number of participants | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| Lesion volume value below median | FST + CPT | 17 | 25.1 (18.64) | 41.2 (14.91) | 16.1 (11.99) | 16.05 (10.71 to 21.38) | 5.3 (–1.09 to 11.70) p = 0.101 |
| MPT + CPT | 20 | 28.7 (18.96) | 38.3 (18.26) | 9.7 (8.03) | 10.74 (5.62 to 15.87) | ||
| Lesion volume value equal to or above median | FST + CPT | 21 | 22.8 (19.99) | 32.5 (17.44) | 9.8 (12.36) | 12.59 (6.22 to 18.96) | 4.44 (–2.89 to 11.76) p = 0.225 |
| MPT + CPT | 14 | 22.4 (16.98) | 27.7 (19.57) | 5.4 (5.39) | 8.15 (0.02 to 16.29) | ||
| Variable | Group | Number of participants | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| CST lesioned = yes | FST + CPT | 33 | 23.3 (19.67) | 35.2 (17.35) | 11.9 (12.32) | 13.9 (9.55 to 18.29) | 2.88 (–2.29 to 8.04); p = 0.269 |
| MPT + CPT | 27 | 25.7 (17.04) | 34.0 (18.77) | 8.30 (7.40) | 11.0 (6.48 to 15.60) | ||
| CST lesioned = no | FST + CPT | 5 | 27.0 (17.07) | 44.2 (9.42) | 17.2 (13.65) | 26.1 (–9.59 to 61.82) | 20.64 (–14.07 to 55.36); p = 0.187 |
| MPT + CPT | 7 | 27.6 (23.59) | 33.7 (22.63) | 6.1 (7.13) | 5.5 (–12.28 to 23.22) | ||
| Variable | Group | Number of participants | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| Ratio value below median | FST + CPT | 19 | 28.6 (16.69) | 43.2 (11.54) | 14.6 (11.90) | 14.90 (8.84 to 20.96) | 2.42 (–3.95 to 8.79) p = 0.443 |
| MPT + CPT | 16 | 31.9 (15.80) | 42.4 (16.15) | 10.4 (6.65) | 12.48 (6.55 to 18.42) | ||
| Ratio value equal to or above median | FST + CPT | 17 | 20.6 (21.36) | 31.1 (18.11) | 10.4 (12.73) | 15.38 (7.81 to 22.95) | 6.62 (–0.33 to 13.56) p = 0.061 |
| MPT + CPT | 16 | 19.7 (17.26) | 25.4 (19.26) | 5.7 (6.35) | 8.76 (1.51 to 16.02) | ||
| Variable | Group | Number of participants | Mean (SD) | Mean (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Change | Least squares | Least squares difference; p-value | |||
| MEP pBB = yes | FST + CPT | 34 | 32.2 (16.69) | 43.0 (12.76) | 10.9 (11.85) | 11.68 (8.24 to 15.12) | 3.19 (–0.71 to 7.09); p = 0.107 |
| MPT + CPT | 36 | 34.0 (15.05) | 40.3 (14.34) | 6.2 (7.95) | 8.49 (5.04 to 11.93) | ||
| MEP pBB = no | FST + CPT | 16 | 12.6 (15.34) | 22.1 (21.30) | 9.4 (11.43) | 11.62 (3.68 to 19.56) | –0.60 (–7.38 to 6.18); p = 0.856 |
| MPT + CPT | 13 | 12.7 (13.31) | 22.2 (16.60) | 9.5 (7.41) | 12.22 (3.07 to 21.37) | ||
| MEP pECR = yes | FST + CPT | 36 | 31.9 (16.87) | 43.6 (12.87) | 11.7 (12.37) | 12.67 (9.31 to 16.03) | 3.41 (–0.53 to 7.34); p = 0.089 |
| MPT + CPT | 38 | 33.8 (14.60) | 40.6 (13.43) | 6.8 (7.52) | 9.26 (5.89 to 12.64) | ||
| MEP pECR = no | FST + CPT | 14 | 10.4 (13.15) | 17.5 (18.15) | 7.1 (8.98) | 14.97 (–2.04 to 31.98) | –1.74 (–9.54 to 6.07); p = 0.646 |
| MPT + CPT | 11 | 9.7 (12.62) | 17.8 (15.87) | 8.1 (9.31) | 16.71 (–1.18 to 34.60) | ||
Chapter 6 Discussion
The present trial combined investigation of the clinical efficacy of MPT and FST together with (1) identification of the neural correlates of response to therapy as a basis for identifying precise therapy target(s) and (2) understanding whether or not neural characteristics at baseline could indicate response to MPT and FST and thereby identify which stroke survivors should receive which of these therapies. Hence, investigating efficacy and mechanisms together in the present trial provides robust information for subsequent definitive trials to investigate the effectiveness of MPT and FST targeted at the underlying central nervous system mechanisms of their means of action in those people most likely to respond. The need for such research has been highlighted by the UK Academy of Medical Sciences,55 the US National Institutes of Health16 and an international consensus group. 15
Summary of main results
Clinical efficacy: summary of main results
This study found little difference between the groups in upper limb recovery in response to the two forms of physical therapy. Differences did not reach statistical significance. Importantly, the variability in response within groups was considerable, especially for (hand) grip force and (finger) pinch force.
Neural correlates of clinical improvement: summary of main results
This study found no clinically important association between clinical improvement and change in the neural measures in response to either trial intervention. However, the sample sizes available for analysis of neural therapy interactions were small. Therefore, there was little chance of detecting any significant probability.
Predictive markers of clinical improvement: summary of main results
This study found no interaction effects between baseline neural variables and change in ARAT total score (primary outcome measure) for the paretic upper limb between baseline and outcome (primary time point). As for the neural correlates of clinical improvement analysis, the sample sizes available for analysis of neural therapy interactions were small, which gave little chance of detecting significant probability.
Strengths and limitations
Bias protection for this trial was provided by the blinding of assessors to group allocation, concealment of randomisation order, group allocation via independent telephone randomisation service, adhering to the intention-to-treat principle and reporting all planned outcomes. This trial evaluated a behavioural intervention and, therefore, it was not possible to blind research therapists, participants or clinical staff to the allocated intervention. Although this is perceived as a distinct limitation in drug trials, it is recognised that double blinding is not possible in trials of many stroke rehabilitation interventions. Because of staffing challenges in centres at some points in the trial, it was not always possible to have blinded assessment of the behavioural outcomes.
The sample size for this trial was estimated by a power calculation informed by data from our early-phase trial. 28 The estimated sample size allowed for an attrition rate of 10% at the primary end point of outcome. In the event our attrition rate at outcome was 12.5%, which is substantially lower than the 25% reported by the well-regarded EXCITE (Extremity Constraint-Induced Therapy Evaluation) trial of CIMT. 56
This trial was congruent with the requirements for stroke rehabilitation trials. 40 A strength for all aspects of this trial is that the interventions, both MPT and FST, were described in sufficient detail to enable both research replication of the trial and transferability of results to clinical practice. 48,57,58 Thus, this trial has followed the recommendations for improving rehabilitation research. 57,59
The experimental FST + CPT in this trial was consistent with key principles of neural plasticity9,60 and was therefore well placed to (1) exhibit clinical efficacy and (2) form a useful probe of correlates of, and indicators for, upper limb recovery.
Clinical efficacy: strengths and limitations
An important strength of the trial reported here is that 59% of participants were recruited within 30 days of stroke. Early rehabilitation is recommended because the brain has most potential for reorganisation particularly in the first month after stroke but also in the following 2 months. 9 In clinical practice, most rehabilitation is provided in this early period and yet most rehabilitation trials are conducted later in recovery. A recent systematic review found that only 5.6% of identified RCTs met a required quality threshold and were conducted within the first month after stroke. 61 Therefore, the present trial is likely to be important for stroke rehabilitation research and practice.
Another strength of the trial is that the sample did not just include stroke survivors with mild paresis after stroke who were therefore expected to make a good recovery. The participants in this trial are representative of those who receive upper limb rehabilitation in clinical stroke services. The results are therefore directly relevant to clinical practice.
The power calculation that informed the sample size for this trial accounted for the clustering of participants within therapists. In the event, staff turnover and securing of additional resource in the form of additional therapists meant that many more therapists than anticipated initially provided either MPT or FST to participants in this trial. Although this is a limitation in terms of not allowing for the clustering analysis, it is also a strength as a greater number of therapists indicates higher generalisability to the clinical environment.
Neural correlates of clinical improvement: strengths and limitations
A key strength of this mechanistic study is that it was embedded within a robust clinical trial. Thus, unlike many mechanistic studies, the one reported here guarded against potential risk of bias and allowed for sufficient description of the interventions provided. Although the number of participants in this mechanistic study was lower than anticipated, it still recruited many more participants than many such studies.
This analysis was restricted by the low proportion of participants who had both (1) full sets of clinical data and (2) neural measures at the key time points of baseline and outcome. This ranged from 37 participants for corticocortical anatomical connectivity to 55 participants for RMT in pBB and pECR muscles.
Predictive markers of clinical improvement: strengths and limitations
Again, as for the neural correlates study, being embedded in a RCT provides strength to this mechanistic study. However, it was disappointing that a greater number of participants were unable to be included in the analysis. On the other hand, we have shown that it is possible to conduct mechanistic neural studies in pragmatic clinical trials. Furthermore, we have shown that these highly specialised neural measures can be made in clinical settings that are not associated with specialist neurological centres and have not previously conducted these mechanistic investigations. However, the challenges to this approach are evident in the number of measures not undertaken for ‘other’ reasons (see Tables 19 and 20).
Relationship to previous studies
Progress in stroke rehabilitation has been hampered by a paucity of large-scale projects in which neuroscientists and clinicians have been able to work together and so inform each other’s approach to a single clinical problem, such as the treatment of upper limb weakness. 55 For this trial, we combined neuroscience and clinical science expertise in the largest study of its kind to investigate underlying mechanisms of motor recovery in a representative sample of patients early after stroke with substantial to moderate upper limb motor impairment using well-characterised physical therapies.
Clinical efficacy: relationship to previous studies
The results of the trial reported here build on early-phase findings that suggest a trend towards better upper limb recovery, as assessed by ARAT score, in response to FST + CPT compared with MPT + CPT early after stroke. 28 However, the findings of the present trial, with a larger sample size, showed no difference between FST + CPT and MPT + CPT in respect of improvement of ARAT score over the 6-week intervention phase.
That MPT + CPT was found to produce equivalent benefit to FST + CPT is interesting, as FST is based on the findings that task-specific training drives recovery after stroke7 and that the largest impact on upper limb improvement is loss of muscle strength. 18,19 In contrast, MPT concentrates on enhancing the quality of movement during whole or part functional tasks and not on repetitive progressive training of those everyday tasks. Although there is some evidence of effect of such impairment-based therapy,38 a meta-analysis found that conceptually different physical therapies have equal efficacy. 39
Noticeable from these results is that there is substantial variation around the mean change from baseline for both FST + CPT and MPT + CPT (see Tables 11–18). Therefore, the present findings support the concept underlying the scientific driver for this trial, namely that stroke survivors respond differently to different physical therapies. 19
Neural correlates of clinical improvement: relationship to previous studies
Published studies report that there are different neural changes for physical therapies found to have more benefit than the comparator intervention. 60,62–64 However, the trial reported here found that the mean changes of ARAT score (primary outcome) in response to both interventions were above the clinical important difference of six points (see Table 11), although there was not a statistically significant difference between the groups, and there were no differences between the groups for the potential mechanisms of recovery of corticocortical connectivity, corticospinal connectivity or RMT of corticospinal functional connectivity for both muscles of interest (see Tables 24 and 27). Earlier studies have been smaller in scale (e.g. n = 13,62 n = 12,60 n = 1463 and n = 2364) than the present trial, but the trial reported here had larger sample sizes for the MRI variable (n = 38) and the TMS variables (n = 55). Another important difference to the present trial is that earlier studies show a potential risk of bias in the method of randomisation60,62,63 and the use of blinded assessors60,62 and selective recruitment,60,63 and one study was a post hoc analysis of a single-centre RCT. 64
Predictive markers of clinical improvement: relationship to previous studies
Although identification of clinical prediction rules for physical therapy interventions is a research priority in physical rehabilitation, a systematic review identified only six RCTs. 65 None of the identified publications was concerned with stroke rehabilitation. 65 It follows that the trial reported here is most likely one of the first trials designed to identify predictive markers of response to specific physical therapies early after stroke. Other investigations have been reported but are limited for use with people early after stroke because of lack of a comparator group, lack of specification of the therapy and/or lack of participants in the chronic phase after stroke. 24,66,67
Generalisability
The generalisability of this trial is considered in the wide inclusion criteria with direct relevance to clinical practice and the conduct of the trial in three different clinical centres.
Chapter 7 Conclusions
Objective 1
The trial reported here found small differences in the clinical efficacy of upper limb recovery between FST + CPT and MPT + CPT, but these did not reach statistical significance. Both groups showed increases in ARAT score (primary outcome measure) above the clinically important change. However, variation around the mean change from baseline scores was substantial in both groups.
Objective 2
The neural correlates of change were similar for the two forms of physical therapy. There were no statistically significant correlations between change in the neural correlates and change in clinical efficacy measures.
Objective 3
The trial reported here found that none of the pretreatment neural characteristics of interest predicted response to either FST + CPT or MPT + CPT.
Implications for health care
The findings of the trial reported here confirm clinical impressions and emerging research evidence of variation in response to specific therapies among people early after stroke.
Research recommendations
There is still an urgent need for evidence to guide decisions about (1) appropriate prescription of physical therapy for individuals and (2) the recovery mechanisms at which physical therapy should be targeted.
Acknowledgements
Thanks go to all the stroke survivors who agreed to participate in this research. Professor Weir was also supported in this work by NHS Lothian via the Edinburgh Clinical Trials Unit.
Grant co-applicants
-
Jane Burridge contributed to production of protocol and reviewed the final version of the report.
-
Roger Lemon contributed to production of protocol and reviewed the final version of the report.
-
Paulette vanVliet contributed to production of protocol and reviewed the final version of the report.
Research colleagues
-
Andrew Walker, formerly School of Health Sciences, UEA. Trial manager who set up the trial and oversaw the initial data collection stage.
-
Karla Miller, University of Oxford. MR physicist who supervised the setting up of the neuroimaging protocol across all sites.
-
James Kolasinski, University of Oxford. Neuroscientist who carried out the analysis of the neuroimaging data.
Clinical research colleagues
-
All of the research support staff in the NHS trusts in which this research was located, with particular thanks to Sue Thompson, Barker Unit, Haywood Hospital (Staffordshire and Stoke-on-Trent Partnership Trust), Stoke-on-Trent, and Pel Fordham at the UEA, who managed and supported the administration and quality of the trial documentation and supported participant liaison for imaging appointments.
-
The CLRN staff for support given to trial recruitment, especially Lesley Maloney, Research Manager for the Norfolk Community Health and Care NHS Trust.
-
The Norwich Clinical Trials Unit for clinical trial support and guidance.
-
The RCB for data handling and statistical analysis.
Trial research therapists and assessors
-
Carlo Areddu, Research Therapist.
-
Alison Aries, Blinded Assessor.
-
Deepa Barnabas, Blinded Assessor.
-
Scott Brown, Research Therapist.
-
Zoe Carmichael, Research Therapist.
-
Elisabeth Cato, Research Therapist.
-
Elizabeth Chandler, Research Therapist.
-
Kathryn Cogman, Research Therapist.
-
Kathryn Collins, Blinded Assessor.
-
Ellen Colton, Research Therapist.
-
Francine Cox, Research Therapist.
-
Hayley Crane, Research Therapist.
-
Claire Grocott, Blinded Assessor.
-
Jane Hargreaves, Blinded Assessor.
-
Claire Havis, Research Therapist.
-
Rachel Hodder/Austin, Research Therapist.
-
Claire Laxton, Research Therapist.
-
Laura Mason, Research Therapist.
-
Emma Mccoll, Research Therapist.
-
Stephanie Mills, Research Therapist.
-
Hannah Morgan, Research Therapist.
-
Jen Pearson, Research Therapist.
-
Francesca Quinn-Thomas, Blinded Assessor.
-
Rosie Salmon, Research Therapist.
-
Alyssa Snelson, Research Therapist.
-
Jessica Swart, Blinded Assessor.
-
Rachel Warren, Research Therapist.
-
Laurence Wood, Research Therapist.
-
Catherine Worth, Research Therapist.
-
Katie Young, Research Therapist.
Clinical colleagues
-
Principal investigators not listed elsewhere:
-
Bruce Jarvest, Principal Investigator, Imaging Department, University Hospital of North Midlands (Royal Stoke Hospital).
-
Dr Paul Malcolm, Principal Investigator, Radiology Department, Norfolk and Norwich University Hospital NHS Foundation Trust.
-
Dr Claire Sutton, Principal Investigator, Queen Elizabeth Hospital Birmingham and Moseley Hall Hospital Birmingham.
-
Ingrid Watmough, Principal Investigator, Community Rehabilitation, Norfolk Community Health and Care NHS Trust.
-
Dr Kneale Metcalf, Principal Investigator, Stroke Medicine, Norfolk and Norwich University Hospital NHS Foundation Trust.
-
-
The rehabilitation teams in Staffordshire and Stoke-on-Trent Partnership Trust; Haywood Hospital, Stoke-on-Trent (in particular Sneyd Ward, Broadfield Ward and the Stroke Early Supported Discharge Team; and Janet Butler for providing admission and discharge date information); Moseley Hall Hospital, Birmingham Community Healthcare NHS Foundation Trust; Norfolk Community Health and Care NHS Trust (in particular Beech Ward and Early Supported Discharge); James Paget University Hospital NHS Trust and the Norfolk and Norwich University Hospital NHS Foundation Trust.
-
The MRI teams in the Norfolk and Norwich University Hospital NHS Foundation Trust (in particular Richard Greenwood); University Hospital of North Midlands NHS Trust (in particular Dr Peter Wright, MRI and Non-ionising Medical Physicist; Sarah Prescott, Lead MRI Clinical Scientist, Medical Physics; and Harry Poole, Clinical Scientist, MRI Physics); and University of Birmingham Imaging Centre (in particular Nina Salman, Chief Radiographer and Dr Andrew Bagshaw, Scientific Director).
Other acknowledgements
We are grateful to the many other people who made this research possible.
-
Pel Fordham, for organising participant appointments, assisting with trial meetings and assisting with writing this report.
-
Lynn Aylett, for supporting data processing and returns to the RCB.
-
The Research and Enterprise and Finance Departments, UEA and Keele University.
-
All the research support staff in the NHS trusts in which this research took place.
Contributions of authors
Valerie M Pomeroy (Professor of Neurorehabilitation, UEA) was the chief investigator and was the principal investigator at the UEA, Norfolk Community Health and Care, and Norfolk and Norwich University Hospitals NHS Foundation Trust. She undertook the overall design of the study, supervised trial conduct, supervised conduct of trial in Norfolk, contributed to recruitment, wrote drafts and undertook the final editing of the report.
Susan M Hunter (Senior Lecturer, Institute for Applied Clinical Sciences, School of Health and Rehabilitation, Keele University) was the principal investigator at Keele University and NHS Staffordshire and Stoke-on-Trent Partnership Trust. She supported design of the study, provided training in intervention delivery to research therapists across all sites, supervised the conduct of the trial in North Staffordshire, contributed to recruitment of participants, provided intervention in the (long-term) absence of research therapists, conducted baseline and blinded outcome assessments in the (long-term) absence of research assessors, collected TMS data locally, contributed to the report and agreed the final version.
Heidi Johansen-Berg (Professor of Cognitive Neuroscience, University of Oxford) contributed to the overall study design, jointly supervised design conduct and analysis of neuroimaging aspects of the study, contributed to the report and agreed the final version.
Nick S Ward (Professor of Clinical Neurology and Neurorehabilitation, University College London Institute of Neurology) led neuroimaging aspects of the production of the protocol and conduct of the trial, contributed to the report and agreed the final version of the report.
Niamh Kennedy (Lecturer in Rehabilitation Neuroscience, UEA) led neurophysiological aspects of the production of the protocol and conduct of the trial, contributed to the report and agreed the final version of the report.
Elizabeth Chandler (Research Therapist, UEA) contributed to all trial operating procedures and development of CRFs, managed day-to-day conduct of the trial in Norfolk, produced recruitment data during the interim period between full-time trial managers, contributed to recruitment and provided experimental intervention, contributed to the report and agreed the final version of the report.
Christopher J Weir (Professor of Medical Statistics and Clinical Trials, University of Edinburgh) contributed to the design of the study, advised on the SAP, commented on drafts of the report and agreed the final copy version of the report.
John Rothwell (Professor of Human Neurophysiology, University College London Institute of Neurology) contributed to neurophysiological aspects of the protocol and oversaw the quality of data collection contributed to the report and agreed the final version of the report.
Alan Wing (Professor of Human Movement, School of Psychology, University of Birmingham) contributed to the production of the protocol, oversaw one of the clinical centres, contributed to the report and agreed the final version of the report.
Michael Grey (formerly Reader in Motor Neuroscience, University of Birmingham; currently Reader in Rehabilitation Neuroscience, UEA) led the conduct of neurophysiological measures in a key centre for this trial and is leading aspects of the analysis, contributed to the report and agreed final version of the report.
Garry Barton (Professor of Health Economics, UEA) led all aspects of the health economics investigation embedded in this trial, contributed to the report and agreed the final version of the report.
Nick Leavey (Clinical Trial Manager, UEA) managed the conduct of the trial across the three recruiting centres, contributed to the report and agreed the final copy version of the report.
Publications
Hunter SM, Johansen-Berg H, Ward N, Kennedy NC, Chandler E, Weir CJ, et al. Functional strength training and movement performance therapy for upper limb recovery early poststroke–efficacy, neural correlates, predictive markers, and cost-effectiveness: FAST INdiCATE Trial. Front Neurol 2018;8:733.
Pomeroy VM, Ward NS, Johansen-Berg H, van Vliet P, Burridge J, Hunter SM, et al. FAST INdiCATE Trial protocol. Clinical efficacy of functional strength training for upper limb motor recovery early after stroke: neural correlates and prognostic indicators. Int J Stroke 2014;9:240–5.
Van Vliet P, Hunter SM, Donaldson C, Pomeroy V. Using the TIDieR checklist to standardize the description of a functional strength training intervention for the upper limb after stroke. J Neurol Phys Ther 2016;40:203–8.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Patient Data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurol 2012;11. https://doi.org/10.1016/S1474-4422(12)70029-7.
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181-6. https://doi.org/10.1161/01.STR.0000087172.16305.CD.
- Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345-54. https://doi.org/10.1016/S1474-4422(09)70023-7.
- Reducing Brain Damage: Faster Access to Better Stroke Care. London: The Stationery Office; 2005.
- National Stroke Strategy. DHSC: London; 2007.
- Kjellström T, Norrving B, Shatchkute A. Helsingborg Declaration 2006 on European stroke strategies. Cerebrovasc Dis 2007;23:231-41. https://doi.org/10.1159/000097646.
- French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD006073.pub3.
- Verheyden G, Nieuwboer A, De Wit L, Thijs V, Dobbelaere J, Devos H, et al. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Repair 2008;22:173-9. https://doi.org/10.1177/1545968307305456.
- Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Numan Neurosci 2013;7. https://doi.org/10.3389/fnhum.2013.00887.
- Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke 2005;36:1172-7. https://doi.org/10.1161/01.STR.0000165922.96430.d0.
- Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair 2007;21:14-2. https://doi.org/10.1177/1545968306291858.
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006;296:2095-104. https://doi.org/10.1001/jama.296.17.2095.
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, et al. Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS). Neurology 2009;73:195-201. https://doi.org/10.1212/WNL.0b013e3181ab2b27.
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right. Neurorehabil Neural Repair 2012;26:923-31. https://doi.org/10.1177/1545968312440745.
- Cheeran B, Cohen L, Dobkin BH, Ford G, Greenwood R, . Cumberland Consensus Working Group . The future of restorative neurosciences in stroke: driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabil Neural Repair 2009;23:97-107. https://doi.org/10.1177/1545968308326636.
- Frontera WR, Bean JF, Damiano D, Ehrlich-Jones L, Fried-Oken M, Jette A, et al. Rehabilitation research at the National Institutes of Health: moving the field forward (executive summary). Arch Phys Med Rehabil 2017;98:795-803. https://doi.org/10.1016/j.apmr.2017.02.001.
- Cramer SC. Brain repair after stroke. N Engl J Med 2010;362:1827-9. https://doi.org/10.1056/NEJMe1003399.
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 2008;63:272-87. https://doi.org/10.1002/ana.21393.
- Wahl A, Schwab ME. Finding an optimal rehabilitation paradigm after stroke: enhancing fiber growth and training of the brain at the right moment. Front Hum Neurosci 2014;8:1-13. https://doi.org/10.3389/fnhum.2014.00381.
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003;126:2476-96. https://doi.org/10.1093/brain/awg245.
- Newton JM, Ward NS, Parker GJM, Deichmann R, Alexander DC, Friston KJ, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas – relevance to stroke recovery. Brain 2006;129:1844-58. https://doi.org/10.1093/brain/awl106.
- Ward NS, Newton JM, Swayne OBC, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006;129:809-19. https://doi.org/10.1093/brain/awl002.
- Burke E, Dobkin B, Noser E, Enney LA, Cramer SC. Predictors and biomarkers of treatment gains in a clinical stroke trial targeting the lower extremity. Stroke 2013;10:54-6.
- Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, et al. Anatomy of stroke injury predicts gains from therapy. Stroke 2011;42:421-6. https://doi.org/10.1161/STROKEAHA.110.599340.
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170-80. https://doi.org/10.1093/brain/awl333.
- Price CJ, Seghier ML, Leff AP. Predicting language outcome and recovery after stroke: the PLORAS system. Nat Rev Neurol 2010;6:202-10. https://doi.org/10.1038/nrneurol.2010.15.
- Wolf SL, Kwakkel G, Bayley M, McDonnell MN. Upper Extremity Stroke Algorithm Working Group . Best practice for arm recovery post stroke: an international application. Physiotherapy 2016;102:1-4. https://doi.org/10.1016/j.physio.2015.08.007.
- Donaldson C, Tallis R, Miller S, Sunderland A, Lemon R, Pomeroy V. Effects of conventional physical therapy and functional strength training on upper limb motor recovery after stroke: a randomized phase II study. Neurorehabil Neural Repair 2009;23:389-97. https://doi.org/10.1177/1545968308326635.
- Kamper DG, Fischer HC, Cruz EG, Rymer WZ. Weakness is the primary contributor to finger impairment in chronic stroke. Arch Phys Med Rehabil 2006;87:1262-9. https://doi.org/10.1016/j.apmr.2006.05.013.
- Harris JE, Eng JJ. Paretic upper-limb strength best explains arm activity in people with stroke. Phys Ther 2007;87:88-97. https://doi.org/10.2522/ptj.20060065.
- Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother 2006;52:241-8. https://doi.org/10.1016/S0004-9514(06)70003-4.
- Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke. A randomized controlled trial. Stroke 1997;28:722-8. https://doi.org/10.1161/01.STR.28.4.722.
- Winstein CJ, Rose D, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil 2004;85:620-8. https://doi.org/10.1016/j.apmr.2003.06.027.
- Graef P, Michaelsen SM, Dadalt MLR, Rodrigues DAMS, Pereira F, Pagnussat AS. Effects of functional and analytical strength training on upper-extremity activity after stroke: a randomized controlled trial. Brazilian J Phys Ther 2016;20:543-52. https://doi.org/10.1590/bjpt-rbf.2014.0187.
- Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: phase I randomized controlled trial. Neurorehabil Neural Repair 2010;24:88-96. https://doi.org/10.1177/1545968309343216.
- Kerr A, Clark A, Cooke EV, Rowe P, Pomeroy VM. Functional strength training and movement performance therapy produce analogous improvement in sit-to-stand early after stroke: early-phase randomised controlled trial. Physiother 2017;103:259-65. https://doi.org/10.1016/j.physio.2015.12.006.
- Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, et al. Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS trial. Neurorehabil Neural Repair 2013;27:370-80. https://doi.org/10.1177/1545968313481284.
- Platz T, van Kaick S, Mehrholz J, Leidner O, Eickhof C, Pohl M. Best conventional therapy versus modular impairment-oriented training for arm paresis after stroke: a single-blind, multicenter randomized controlled trial. Neurorehabil Neural Repair 2009;23:706-16. https://doi.org/10.1177/1545968309335974.
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD001920.pub3.
- Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979-83. https://doi.org/10.1136/bmj.a1655.
- Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine-hole finger peg test of dexterity. Occup Ther J Res 1985;5:24-37. https://doi.org/10.1177/153944928500500102.
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980;19:382-9. https://doi.org/10.1159/000115178.
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, et al. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain 1997;120:1763-77. https://doi.org/10.1093/brain/120.10.1763.
- Roberts C. The implications of variation in outcome between health professionals for the design and analysis of randomized controlled trials. Stat Med 1999;18:2605-15. https://doi.org/10.1002/(SICI)1097-0258(19991015)18:19<2605::AID-SIM237>3.0.CO;2-N.
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). J Health Serv Res Policy 2000;5:12-6. https://doi.org/10.1177/135581960000500105.
- Donaldson C, Tallis RC, Pomeroy VM. A treatment schedule of conventional physical therapy provided to enhance upper limb sensorimotor recovery after stroke: expert criterion validity and intra-rater reliability. Physiotherapy 2009;95:110-19. https://doi.org/10.1016/j.physio.2008.11.005.
- van Vliet P, Hunter SM, Donaldson C, Pomeroy V. Using the TIDieR checklist to standardize the description of a functional strength training intervention for the upper limb after stroke. J Neurol Phys Ther 2016;40:203-8. https://doi.org/10.1097/NPT.0000000000000133.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair 2008;22:78-90. https://doi.org/10.1177/1545968307305353.
- Morris DM, Uswatte G, Crago JE, Cook EW, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity functiona after stroke. Arch Phys Med Rehabil 2001;82:750-5. https://doi.org/10.1053/apmr.2001.23183.
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001;32:1635-9. https://doi.org/10.1161/01.STR.32.7.1635.
- Fasoli SE, Trombly CA, Tickle-Degnen L, Verfaellie MH. Effect of instructions on functional reach in persons with and without cerebrovascular accident. Am J Occup Ther 2002;56:380-90. https://doi.org/10.5014/ajot.56.4.380.
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electoencephalography Clin Neurophysiol 1994;91:79-92. https://doi.org/10.1016/0013-4694(94)90029-9.
- Norfolk and Suffolk Primary and Community Care Research Office . Public and Patient Involvement in Research (PPIRes) n.d. http://nspccro.nihr.ac.uk/publicand-patient-involvement-in-research (accessed 5 June 2018).
- Restoring Neurological Function. London: The Academy of Medical Sciences; 2004.
- Blanton S, Morris DM, Prettyman MG, McCulloch K, Redmond S, Light KE, et al. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys Ther 2006;86:1520-33. https://doi.org/10.2522/ptj.20060091.
- Pomeroy VM, Tallis RC. Need to focus research in stroke rehabilitation. Lancet 2000;355:836-7. https://doi.org/10.1016/S0140-6736(99)08143-X.
- Glasziou P, Chalmers I, Altman DG, Bastian H, Boutron I, Brice A, et al. Taking healthcare interventions from trial to practice. BMJ 2010;341. https://doi.org/10.1136/bmj.c3852.
- Dobkin BH. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil Neural Repair 2009;23:197-206. https://doi.org/10.1177/1545968309331863.
- de Araújo RC, Junior FL, Rocha DN, Sono TS, Pinotti M. Effects of intensive arm training with an electromechanical orthosis in chronic stroke patients: a preliminary study. Arch Phys Med Rehabil 2011;92:1746-53. https://doi.org/10.1016/j.apmr.2011.05.021.
- Stinear C, Ackerley S, Byblow W. Rehabilitation is initiated early after stroke, but most motor rehabilitation trials are not: a systematic review. Stroke 2013;44:2039-45. https://doi.org/10.1161/STROKEAHA.113.000968.
- Lin KC, Chung HY, Wu CY, Liu HL, Hsieh YW, Chen IH, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehabil 2010;89:177-85. https://doi.org/10.1097/PHM.0b013e3181cf1c78.
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair 2011;25:819-29. https://doi.org/10.1177/1545968311411056.
- Globas C, Lam JM, Zhang W, Imanbayev A, Hertler B, Becker C, et al. Mesencephalic corticospinal atrophy predicts baseline deficit but not response to unilateral or bilateral arm training in chronic stroke. Neurorehabil Neural Repair 2011;25:81-7. https://doi.org/10.1177/1545968310382001.
- Lubetzky-Vilnai A, Ciol M, McCoy SW. Statistical analysis of clinical prediction rules for rehabilitation interventions: current state of the literature. Arch Phys Med Rehabil 2014;95:188-96. https://doi.org/10.1016/j.apmr.2013.08.242.
- Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology 2011;77:1076-83. https://doi.org/10.1212/WNL.0b013e31822e1482.
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910-5. https://doi.org/10.1161/STROKEAHA.109.577023.
- Hunter SM, Crome P, Sim J, Donaldson C, Pomeroy VM. Development of treatment schedules for research: a structured review to identify methodologies used and a worked example of ‘mobilisation and tactile stimulation’ for stroke patients. Physiotherapy 2006;92:195-207. https://doi.org/10.1016/j.physio.2006.01.001.
- National Clinical Guideline for Stroke. London: Royal College of Physicians; 2016.
- Schmidt RA, Lee TD. Motor Control and Learning; A Behavioural Emphasis. Champaign, IL: Human Kinetics; 1999.
- Magil RA. Motor Learning: Concepts and Applications. New York, NY: McGraw-Hill; 2001.
- Wulf G, Schmidt R, Deubel H. Reduced feedback frequency enhances generalised motor program learning but not parameterisation learning. J Experiment Psychol 1993;19:1134-50.
- Wulf G, Prinz W. Directing attention to movement effects enhances learning: a review. Phychon Bull Rev 2001;8:648-60. https://doi.org/10.3758/BF03196201.
- McNevin NH, Shae CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychol Res 2003;67:22-9.
Appendix 1 The conventional physical therapy, therapy as usual, intervention
Appendix 2 Training update days for research therapists
Training in delivering each of the trial interventions, MPT and FST, was provided for all research therapists employed to deliver the trial intervention; this was initially completed before the first participant was recruited at each site. Each therapist was ultimately allocated to one intervention only and undertook specific training in the allocated intervention. However, it was important that each therapist also had an awareness of the non-allocated intervention in order to fully understand the differences and similarities between each intervention/approach (MPT and FST) and, therefore, the parameters of each of the trial interventions.
During the trial there was a turnover in research therapist staffing, and as each new research therapist came into post during the trial, this initial training was also provided on an individual basis with subsequent and ongoing peer mentorship and networking from the research therapists delivering trial intervention at the other sites.
In addition to initial training, two training update days were held during the recruitment phase of the trial. All research therapists employed to deliver trial intervention at that time attended these sessions. Both training update days were held in London over a period of 2 consecutive days. Through these update days, the within-trial research therapist networking was maintained and enhanced, enabling the research therapists to consult regularly with their peers providing the same trial intervention, but in different centres, to talk through understandings and practice. The trial manager and two of the other authors, Susan Hunter and Valerie Pomeroy, were also involved in these networking conversations.
Training update dates: 7 November 2013 and 6–7 July 2015.
Venue: both training update days were held in London, one at UEA London (November 2013) and another at the Premier Inn Hotel, London Victoria (July 2015).
Facilitators/trainers: Valerie Pomeroy and Susan Hunter.
Programme/content of the day: see programmes to follow.
Format: open structured discussion, small-group work with whole-group feedback and discussion (see following for summary of discussion and key actions/decisions agreed by the team).
FAST-INdICATE training: delivering trial intervention – November 2013, UEA, London
Day 1
Session 1: 11.15–13.30
-
Plan for the day.
-
Experiences of delivering therapy so far – whole-group discussion.
-
Identify specific topics for the 2 days – whole-group discussion.
-
MPT and FST groups – discussion around therapy for various patient presentations and problems:
-
low/high tone
-
shoulder subluxation/pain
-
low/high ability
-
cognitive/perceptual problems
-
somatosensory loss.
-
-
Feedback from groups on therapy delivered by each team for a patient with low tone/low ability; discussion of differences between the two approaches (e.g. hands-on/hands-off; starting position/postural control).
Lunch 13.30–14.15
Session 2: 14.15–16.15
-
Feedback and discussion about therapy for the high-ability patient, particularly issues related to dexterity and progression for both MPT and FST.
-
Discussion/clarification around fundamental differences between MPT and FST, and how they might appear to be similar but remain different (e.g. progressive strengthening vs. task practice, strength vs. endurance, feedback, facilitation vs. guidance vs. active-assisted exercise) and what is a ‘middle therapy’ (i.e. neither MPT nor FST) (e.g. immersion of limb in water bath/’hydrotherapy’/use of hydrotherapy principles such as buoyancy/effect of temperature on tone and pain/stiffness).
Day 2
Session 3: 9.00–10.30
-
Self-directed therapy – what is involved, how is it monitored, how are instructions communicated to participants, how is it recorded, what is appropriate content, overcoming cognitive/memory difficulties, leaving equipment with patients and related issues of blinding, etc.
Session 4: 11.00–12.30
-
Documentation of therapy – inclusion of number of ‘reps’?
-
Adverse event reporting.
-
‘Middle therapies’ that are neither MPT or FST, for example hydrotherapy, pulleys, FES.
-
Feedback/evaluation.
12.30 Lunch and close
Topics identified for discussion and actions agreed during the training
-
Therapist-directed and participant-administered therapy.
-
Specific equipment that was required for FST would be provided by the research therapist. However, written instructions would not be provided by the therapist, but the participant or carer could make notes to remind them of what exercises they should do, and when/how often they should do them.
-
It was noted that hand dominance and side of hemiplegia potentially impacted on the ability to complete this participant-administered therapy.
-
The presence/involvement of a carer or spouse was likely to be helpful in participants administering their own therapy/exercises.
-
Variability and non-standardisation of this directed therapy was important because of personalised care.
-
-
Equipment left at participant’s house for directed therapy.
-
There is a potential threat to blinding if equipment that is obviously specific to FST is left at the participant’s home and seen by the clinical team. Its presence may confound the content of CPT, as the clinical team might be tempted to use this equipment in their CPT programme with that participant, thus increasing the dose of FST and changing the nature of actual CPT.
-
There was a suggestion that the content of CPT should be analysed for both groups to see if it is affected by group allocation (i.e. whether or not there is more strength training activity for the FST group), which might be a result of the presence of strengthening equipment in the home.
-
-
Documenting therapy.
-
It was agreed that the number of repetitions of an exercise/activity in the FST group would not be reported.
-
-
Progression of dexterity activities in high-functioning participants.
-
This was discussed in the context of generating ideas for progression exercises to improve dexterity in high-functioning participants and the difficulty this presents was acknowledged.
-
-
Difference between strength and endurance training/activities in FST.
-
Discussed and number of repetitions for strengthening to be based on six repetition maximum, whereas number of repetitions for endurance to be based on three sets of 10–12 repetitions.
-
-
Use of feedback and the difference between knowledge of performance and knowledge of results.
-
Knowledge of performance is characterised by a focus on movement (body part, direction/plane, e.g. bend, straighten elbow), whereas knowledge of results is characterised by a focus on quantitative measure of goal achievement (e.g. moving an object a set distance).
-
-
Use of active-assisted exercise/movement and facilitation in FST.
-
Neither active-assisted nor facilitated movement is acceptable as part of FST; however, providing support to a body part in order to minimise the effect of gravity as part of a progressive strengthening regime is acceptable as long as the support is simply supporting the weight of the limb and not assisting with movement.
-
-
Adverse event reporting.
-
Pain and fatigue are to be monitored at each therapy visit to identify adverse reactions to the therapy:
-
A change in two levels on the Motricity Index that occurs on four consecutive occasions is to be considered an adverse reaction of fatigue (AE on the CRFs).
-
The presence of new pain over four consecutive occasions is to be considered an adverse reaction of pain (AE on CRFs) only if the pain cannot be explained by the clinical team as resulting from a different cause. If a participant reports new pain, the research therapist or PI will contact the clinical team to determine whether the new pain is likely to be cause by the trial intervention or if there is another explanation for that pain. If that new pain is present on four consecutive visits by the research therapist is considered to be attributable to the trial therapy, then an AE form should be completed. If the pain is not considered to be attributable to the trial intervention, then it is not necessary to complete an AE form but an e-mail trail of the discussion with the clinician should be kept and stored.
-
If there is pre-existing pain at the start of the trial, this should not be recorded on the pain and fatigue form; a record should be made of this pain only if it changes in intensity or nature.
-
-
-
Use of ‘special’ equipment and ‘middle therapies’ that could constitute a different intervention.
-
It was agreed that neither pulleys, nor FES, nor water bath/hydrotherapy should not be used for MPT or FST exercises.
-
TheraBand® (TheraBand, Akron, OH, USA) is acceptable for strength training as part of FST.
-
Throwing and catching a ball is an acceptable activity for FST or MPT; however, the use of the ball and progression of exercise would be subtly different for each intervention.
-
Training evaluation: research therapists, UEA, 7 November 2013
After the training had been completed in November 2013, the research therapists and trial manager were asked to summarise what they had gained most from the training. The following were the verbal responses received:
-
Greater understanding of the trial interventions – able to now differentiate between MPT and FST. This was reassuring and insightful.
-
Astounded by the quality of the team.
-
Reaffirming similarities of therapy between centres.
-
Team building – knowing everyone better.
-
Getting to know people; links for further discussions.
-
Discussion at length to clarify treatment – clinically reason choices.
-
Clarification re MPT and function and scope of what can be done.
-
New ideas for new members of the team.
-
Insight into MPT.
-
Increased scope of what can be done in MPT and FST.
-
Clarification re paperwork, especially AEs.
-
Focus on feedback given to patients.
-
Increased treatment options.
FAST-INdICATE training: London, 6–7 July 2015
Day 1: Monday 6 July 2015
-
Plan for the day.
-
Experiences of delivering therapy so far: good and not so good – whole-group discussion.
-
Identify specific topics for the 2 days – whole-group discussion.
-
MPT and FST groups – discussion around therapy you would deliver for:
-
Patient with low tone
-
Patient with high tone
-
Patient with shoulder subluxation/pain
-
Low-ability patient
-
High-ability patient
-
Patient with cognitive/perceptual problems
-
Patient with somatosensory loss.
-
-
Feedback from groups on therapy delivered by each team for patient with low tone/low ability; discussion of differences between the two approaches (e.g. hands-on/hands-off); starting position/postural control.
-
Feedback and discussion about therapy for the high-ability patient, particularly issues related to dexterity and progression for both MPT and FST.
Day 2: Tuesday 7 July 2015
-
Val Pomeroy to present an update on the mechanisms of recovery and predictors of stroke rehabilitation response.
-
Discussion/clarification around fundamental differences between MPT and FST, and how they might appear to be similar but remain different (e.g. progressive strengthening vs. task practice, strength vs. endurance, feedback, facilitation vs. guidance vs. active-assisted exercise); and what is a ‘middle therapy’ (i.e. neither MPT nor FST) (e.g. immersion of limb in water bath/’hydrotherapy’/use of hydrotherapy principles such as buoyancy/effect of temperature on tone and pain/stiffness).
-
Self-directed therapy – what is involved, how is it monitored, how are instructions communicated to participants, how is it recorded, appropriate content, overcoming cognitive/memory difficulties, leaving equipment with pts and issues of blinding, etc.
-
Claire and Liz to lead a discussion on trial assessment procedures.
-
Feedback/evaluation.
Close
Appendix 3 Description of movement performance therapy
The trial intervention MPT was formerly referred to as the control intervention CPT in our earlier trials and publications, and the content of MPT remains the same as for CPT described earlier in this paper. This intervention is intended to reflect a typical UK physiotherapy treatment for stroke. MPT emphasises therapy interventions provided by a therapist using predominantly hands-on facilitation and guiding movement (therapist dependent) to provide somatosensory stimulation and to optimise joint and body part alignment in preparation for voluntary movement. Some iterative practice of functional tasks is included but without systematic progression in resistance to movement or repetition. A manual was produced to support therapists delivering MPT, which clarifies terminology, etc. This has been appended in this section. The TIDier checklist format has been used to summarise and describe MPT (Table 35).
| Brief name | MPT |
|---|---|
|
Why? Describe any rationale, theory or goal of the elements essential to the intervention |
MPT is a part of established CPT, which is supported by a very limited research evidence base. MPT therapy content was first developed through literature reviews and iteratively with senior, experienced neuro-physiotherapists, who described in detail the therapy techniques and interventions they used in routine UK clinical practice to retrain sensorimotor function and movement in the hemiplegic upper limb following stroke; from this description, a schedule of treatment was developed and validated to provide a way of recording the content and amount of MPT provided for people presenting with upper limb motor impairment after stroke (Hunter et al., 2006;68 Donaldson et al., 200946). MPT is focused on:
|
|
What? Materials: describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL) Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support |
Quality of movement is emphasised during performance of a task. Participants are encouraged to practice activities such as grasp/release, reaching to objects, pointing in space, but not always as part of a specific functional task. During these activities, the therapist monitors and regulates quality of postural alignment and voluntary movement using hands-on techniques Before any voluntary movement is attempted by the participant, the therapist uses appropriate techniques to ensure that participants are in the optimum gross body posture for the movement, and that the joints and segments of the upper limb are in the correct alignment. During the voluntary production of movement, the therapist will use ‘hands-on’ techniques to maintain the optimal posture and alignment for the specific activity. Moreover, treatment activities are used in combination; for example, a participant might perform voluntary reaching for a cube, and the therapist may have provided some stroking to the skin overlying the forearm extensor muscles, and passive movement of the elbow joint, as a precursor to the voluntary movement. This would have been to provide sensory stimulation to the limb and increase the participant’s awareness of/attention to the limb segments and joints that need to be controlled during that movement. The therapist might also provide some active assistance during the reaching movement to ensure that the movement was smooth. Thus, the treatment activities are designed to be used in different combinations for different individuals depending on their ability to produce high-quality voluntary movement Treatment progression is determined by the therapist using clinical reasoning to inform how to change within and between activities to improve the production of quality movement. The process of clinical reasoning incorporates a range of variables including participant motivation, cognition, mood, response to previous activity and long term goals. Resultant clinical decisions may include changing the objects used, the spatial-temporal framework for movement, the focus of the activity and the activities themselves. There is no systematic progression in terms of objects, treatment activities or number of repetitions of an activity. Indeed, the emphasis is the production of quality movement underpinning normal performance of everyday functional tasks which need to be performed in different environmental contexts Motivation through goal-setting It is essential that participants feel motivated to engage in the intervention. Goal-setting can enhance motivation and clinical guidelines stress that it is an integral part of stroke rehabilitation (RCP, 201669). Therefore, the therapist and participant will discuss the activities that will be prioritised and document these on the intervention record sheets Engaging in therapeutic activities The therapist assesses the participant’s ability to perform a movement, such as reaching for a therapeutic object (ball/block/cone) and notes the movement components that are difficulty to perform with normal quality. The therapist will also note the blocks to quality movement. This assessment is based on expert knowledge of normal movement sequences and postural control. Clinical reasoning is employed to determine which components/elements are deficient for individual participants at different times in their recovery after stroke Practice Assessment would start with whole practice of a functional task with a therapeutic object to enable the therapist and participant to identify which components (if any) can be performed normally (with quality). Whole practice is only used if the participant is able to produce quality movement throughout. This may enhance motivation especially if used to emphasise how much improvement has been made. However, it will often be the case that hands-on therapy techniques are required to produce quality movement and, in this case, activity should focus upon the components that are problematic. When possible, the activity session should ideally finish with whole practice to enable the participant to put the components into context, and practice the dynamics of a task as a whole (and thus the entire motor programme) Constant practice involves the repetition of identical movement; variable practice involves practice of different variations of the same category of movement. Movement patterns underlying everyday functional activities are inherently variable, so MPT will encourage variable practice by, for example, using different start and end points (e.g. reaching in different directions), movement from different postures (e.g. sitting/standing) and using different therapeutic handling techniques Feedback Augmented feedback (i.e. feedback provided by the therapist) is one of the most important influences in the process of acquiring movement skill and, therefore, its application needs to be carefully considered (Schmidt and Lee, 1999;70 Magil, 2001;71 Wulf et al. , 199372). Augmented feedback pertains either to the quality (pattern) or the outcome of the movement: MPT feedback emphasises knowledge of performance. This emphasis will encourage an internal focus on the body parts involved in producing quality movement. The therapist will give prominence to the provision of information for movement and feedback during its performance through tactile input during therapeutic handling. For example, during active-assisted movement the therapist may guide the path taken by the hand during a reaching activity and thus provide sensory input to the participant about how the speed and direction required for quality movement. Any verbal feedback provided will direct the participant’s attention to the guidance from the therapist’s hands. Emphasis will also be given to encouraging participants to focus on how the movement feels and how individual joints/segments of the body are moving. For example, during reaching forwards, feedback could be given on movement of the elbow rather than the success of grasping the target (e.g. ‘next time keep you elbow closer to your body’). As the content of feedback depends on the performance of each individual participant, no attempt is made to standardise this. It is a strong requirement, however, that the research therapist is an expert in therapeutic handling with extensive knowledge of, and clinical experience in, the science of normal movement Amount and frequency of feedback A faded feedback schedule is recommended, whereby feedback is given frequently in the early stage of learning, and less frequently as the participant progresses (Schmidt and Lee, 1999;70 Magil, 200171). As participants benefit from self-learning, progressively decreasing feedback enables independent problem-solving to enhance motor learning; for MPT, participants will be aware of when their movement quality is improving as the therapist will reduce the amount of time during a session where tactile contact and guidance and provided. Another strategy of fading feedback is by providing bandwidth feedback; this refers to feedback provided only when performance falls outside the specified criteria. For example, a person with stroke may use their trunk to reach forward for a cup. The therapist may want to discourage this compensatory movement and stimulate active shoulder flexion instead. By touching the participant’s trunk if this moves forward, the participant will realise their mistake. Note that they only receive this feedback if they do something wrong, otherwise practice continues with the participant knowing their performance is correct. This type of feedback promotes consistency of performance Periodically, it is recommended that a summary of feedback or an average feedback score is provided to the participant, formulated in constructive terms. An example of summary feedback is the number of times out of five attempts that the movement was performed correctly. An example of average feedback is the average number of times that the participant has their elbow by their side while lifting an object five times. Both of these methods mean that feedback is not provided after each attempt, but only after a certain number have been completed. Both summary and average feedback are postulated to be more effective than feedback provide on every attempt by providing the learner with an opportunity to process their intrinsic feedback and reduce reliance on augmented feedback Focus on feedback Feedback can induce an internal focus of attention in the learner (about the body and body parts) or an external focus of attention (about effects of the movement on the environment). Although an external focus feedback may be more effective than internal focus feedback in healthy people (Wulf and Prinz, 2001;73 McNevin et al. , 200374), it remains uncertain whether or not this is generalisable to stroke survivors. Participants can be provided with some essential external focus information in order to ensure they understand the purpose of the activity, but the emphasis is on an internal focus of attention Order of activities in each intervention session Assessment should commence with the whole practice of a functional movement, using a therapeutic object, to enable the therapist and participant to identify which components (if any) can be performed normally (with quality). Activities and exercises are chosen from the selection contained in the Treatment Activities section of the Manual (see following), using clinical reasoning to determine the content of the intervention session. There is no predefined order for treatment activities within MPT Specific therapy activities Examples of equipment used: |
|
Who provided? For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, background and any specific training given |
MPT is delivered by therapists, providing a strong emphasis on ‘hands-on’ techniques (therapeutic handling). The therapy is therefore therapist dependent, with an emphasis on preparation and joint alignment via tactile/proprioceptive input. For the FAST-INdICATE trial, MPT was provided by qualified senior (UK band 6) physiotherapist or occupational therapist, or by a junior (UK band 5) therapist under the supervision of a band 6 therapist. UK ‘band 6′ is an indication of some specialised experience post qualification. Specific training was given to all trial therapists in understanding the philosophy behind the intervention, the content of the intervention and how to deliver the intervention. Training was provided for the group of therapists, and was also provided on an individual basis. Two follow-up training events were provided for therapists employed to work in the trial |
|
How? Describe the modes of delivery (e.g. face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group |
Therapy was delivered face to face with the trial participants; for the majority of treatments, this was on an individual basis. A small number of treatments were provided in a group situation but the therapy content was personalised. In addition to face-to-face therapy, some therapist-directed but participant-administered therapy was delivered; the therapist advised the participant about which exercises or activities should be done, how they should be done, and for how long (intensity, frequency and duration). For example, this might include massage and manipulation of the hand, and practice moving joints and digits in the arm and hand in a controlled manner |
|
Where? Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features |
MPT intervention was provided in the hospital ward, hospital out-patient facility or in the participant’s own home |
|
When and how much? Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose |
The MPT intervention was delivered for up to 120 minutes per day, Monday to Friday, for 6 weeks; this did not include bank holidays. This amounted to a maximum of 30 sessions of treatment. When the participant was not able to tolerate 120 minutes of therapy in one block of treatment, he or she was provided with directed therapy that he or she could self-administer later in the day to achieve the 120 minutes of therapy. An MPT treatment schedule was provided for the therapists to record the content and intensity (number of minutes) of treatment each day. A trial operating procedure or treatment manual was provided for therapists to refer to for clarification and guidance. The treatment schedule and manual are appended (see Appendices 4 and 5) |
|
Tailoring If the intervention was planned to be personalised, titrated or adapted, then describe what, why, when and how |
The intervention was personalised according to the clinical presentation of the participant each day; clinical decisions were made according to the participant’s sensorimotor performance/ability and the most-appropriate interventions from the MPT treatment schedule were selected in an appropriate combination determined by the skilled therapist. Choice of activities by the therapist is informed by expert assessment of how much quality voluntary movement can be performed by a participant, where this becomes difficulty during a functional task, and why this happens. Delivery of treatment activities is targeted at reducing the blocks to, and enhancing the performance of, quality movement. Choices are based on a process of clinical reasoning and problem-solving in relation to the identified movement problem. This process is undertaken in consultation with individual participants to ensure adherence to the principles of goal-setting |
|
Modifications If the intervention was modified during the course of the study, describe the changes (what, why, when and how) |
Initially, MPT was referred to as CPT (as detailed in the trial protocol); however, following further discussion with clinical colleagues, the trial intervention was renamed MPT because it did not represent all aspects of CPT but focused on movement performance in the upper limb |
| How well? | Intervention adherence/fidelity was assessed by two members of the Trial Management Group (VMP and SMH) who observed both trial interventions being provided to participants at two of the three sites during the trial. In order to maintain fidelity, training events were organised for all therapists providing trial intervention, and an internal network for the therapists was set up so that they could seek peer support and mentorship regarding intervention fidelity |
|
|
|
Appendix 4 Statistical analysis plan
Appendix 5 Data management plan
List of abbreviations
- 9HPT
- Nine-Hole Peg Test
- AE
- adverse event
- ANCOVA
- analysis of covariance
- ARAT
- Action Research Arm Test
- CI
- confidence interval
- CIMT
- constraint-induced movement therapy
- CLRN
- Comprehensive Local Research Network
- CONSORT
- Consolidated Standards of Reporting Trials
- CPT
- conventional physical therapy
- CRF
- case report form
- CST
- corticospinal tract
- DMEC
- Data Monitoring and Ethics Committee
- EME
- Efficacy and Mechanism Evaluation
- FA
- fractional anisotropy
- FSL
- FMRIB’s Software Library
- FST
- functional strength training
- GCP
- good clinical practice
- ICC
- intraclass correlation coefficient
- MEP
- motor-evoked potential
- MNI
- Montreal Neurological Institute
- MPT
- movement performance therapy
- MR
- magnetic resonance
- MRC
- Medical Research Council
- MRI
- magnetic resonance imaging
- pBB
- paretic biceps brachii
- pECR
- paretic extensor carpi radialis
- PPI
- Patient and Public Involvement
- PPIRes
- Patient and Public Involvement in Research
- RAT
- robot-assisted therapy
- RCB
- Robertson Centre for Biostatistics
- RCT
- randomised controlled trial
- RMT
- resting motor threshold
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SOP
- standard operating procedure
- TMS
- transcranial magnetic stimulation
- TSC
- Trial Steering Committee
- UEA
- University of East Anglia
- WMFT
- Wolf Motor Function Test
