Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 11/14/34. The contractual start date was in July 2013. The final report began editorial review in January 2018 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sue Pavitt was a member of the National Institute for Health Research Efficacy and Mechanism Evaluation Board in 2012–18. Robert West holds membership of the Health Services and Delivery Research Researcher-led Panel and Public Health Research Research Funding Board. Sheena Lewis is chief executive officer of the University of Belfast spinout company, Examen Ltd (Belfast, UK) outside the submitted work. David Miller received a grant from Biocoat Inc. (Horsham, PA, USA) outside the submitted work. Jackson Kirkman Brown received support from Origio Inc. (Reigate, UK) to attend a meeting outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Kirkman-Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and background
Background
Gamete quality is now recognised as an important determinant of successful pregnancy outcome as donor eggs from younger women seem able to compensate for lower fertility in older women. 1 It is probable, however, that future advances in assisted reproduction technologies (ART) will benefit from procedures that target selection of higher-quality sperm regardless of parental age. Sperm selection has not really advanced since in vitro fertilisation (IVF) was introduced and, therefore, holds great promise. Although offering benefits to the fertility field overall, this approach would also offer particular promise for older couples (notably where the female is aged > 35 years) and whose oocytes are less efficient at repairing DNA damage in their partners’ sperm. These couples are hitherto challenging to treat with current fertility technologies and have the poorest live birth outcomes, but they are also the fastest-growing group requesting treatment. The relationship between sperm selection, integrity of deoxyribonucliec acid (DNA) and pregnancy outcome is what the Hyaluronic Acid Binding sperm selection (HABSelect) study was designed to evaluate. A successful conclusion of the study could help in the development of a more consistent, evidence-based procedure for intracytoplasmic sperm injection (ICSI) sperm selection that complies with and extends the National Institute for Health and Care Excellence’s 2013 clinical guidance. 2
In 2008 (2006 figures), almost 47,000 couples in the UK alone were treated with ART, comprising 62,000 treatment cycles, over half of which involved ICSI, a technique originally developed to treat male infertility. 3 At that time, live birth rates (LBRs) following ICSI treatment averaged ≈24% per treatment cycle started. The latest figures from 2014 for all IVF4 show an increase in this rate to ≈26%. Although it is estimated that one-third of naturally conceived pregnancies end in failure, we may not have reached the limit for improvements in LBR following ART. For all ART procedures, including ICSI, the embryologist seeks to use the best sperm available. Selection is aided by semen ‘washing’ techniques using density gradient centrifugation (DGC) that can enrich for sperm with high motility and good morphology. 5 In contrast to standard IVF, where the egg is the final arbiter of selection, ICSI is dependent on the relatively subjective judgement of the embryologist to choose the ‘right’ single sperm for each egg. Various studies have shown clear inverse relationships between the burden of DNA-damaged sperm in the ejaculate and clinical pregnancy rates (CPRs) or LBRs in standard IVF, but this relationship is less obvious with ICSI cycles. 6 Reductions in levels of sperm DNA fragmentation following density gradient washing of semen have been reported. 7 However, although the values from washed semen were reduced, they were still over twice as high in the non-pregnant (≈50%) as in the pregnant (≈23%) cohorts. These and other data suggest that sperm with poor DNA quality persist in washed sperm preparations from fertile and infertile men8–13 and unlike IVF, where there is a natural selection by the egg, ICSI could be particularly vulnerable to a poor choice of sperm. We and others have reported that sperm DNA fragmentation is a risk factor for miscarriage in ICSI treatment14,15 and this may result from an oocyte-mediated DNA repair process16–19 that provides adequate support from fertilisation to clinical pregnancy (CP) (hence the lack of an association between DNA fragmentation and CP in ICSI compared with IVF), but may be inadequate to sustain it beyond CP with resulting pregnancy loss. By eliminating abnormal sperm from the sample preparation for ICSI, success rates could theoretically be improved. Alternatively, there may be forms of genotoxic DNA damage in the sperm nucleus that are not detected by existing assays and do not prevent fertilisation by either standard IVF or ICSI-based procedures but can compromise embryo development and result in higher rates of miscarriages.
Summary of evidence leading up to and justification for the study
Prior to 2012, several key studies suggested that DNA packaging and fragmentation anomalies influenced by sperm DNA damage were strongly associated with CPR, LBR20–23 and pregnancy loss in IVF procedures. 24–26 For ICSI, the only clear association was with pregnancy loss,23 supporting the existence of genotoxic damage that is hidden from conventional tests. Hence, any improvement in ICSI that allows the selection of sperm with reduced damage is to be encouraged. Additional benefits of increasing success rates include a reduction in the potentially harmful ovarian hyperstimulation protocols that are an integral part of the ICSI cycle (fewer cycles) and a concomitant reduction in the associated costs of ART procedures. Based on a cost analysis average of 1.3 cycles per patient [Human Fertilisation and Embryology Authority (HFEA) data27 and Access Fertility Ltd28], we calculated that an 8% improvement in full-term live births per cycle started from 24% to 32% could lead to a corresponding improvement in successful live births overall to almost 42% in future. One effect of this could be to reduce cycles while maintaining current success rates (losing one cycle in five overall). Hence, with > 25,000 ICSI cycles performed in the UK in 2008, this would represent an annual NHS saving of > £17.5M (based on average costs of £3500 per ICSI cycle). In 2014, > 30,000 ICSI cycles were carried out4 and, assuming 50% of more recent IVF is ICSI, maintaining the current cycle average could see even greater longer-term savings in relation to the knock-on effects of pregnancy failure to NHS costs.
Work conducted in this and other laboratories suggests that the DNA in human and mouse sperm is carefully and systematically organised in the nucleus into distinct geographical domains (Figure 1). 30–32 These studies showed that some domains are enriched in histones,33 which can account for their hitherto unexplained persistence in sperm nuclei alongside the more abundant protamines. 34,35 Histone-bound sperm chromatin domains are enriched in developmental gene sequences expressed in early embryogenesis. 30,32,33 We hypothesised that damage to these domains was critically relevant for subsequent early embryonic development and could account for the early pregnancy failure observed after both IVF and ICSI-based procedures. 36 Failure of the embryo to thrive following successful implantation may be related to the fragmented or deranged paternal DNA resulting in sequences that are important for early embryological function remaining bound to histones. 30,32 We also had evidence that the form of paternal DNA damage responsible for such early pregnancy failure may involve nucleotide oxidation. 37 In this respect, an association between sperm DNA damage and early pregnancy failure can be revealed after treating the DNA with an enzyme that converts extant oxidised purines [such as 8-hydroxy-2’-deoxyguanosine (8-OHdG)] into DNA strand breaks. 38 Such damage is probably caused by reactive oxygen species (ROS) gaining access to chromatin domains that should normally be protected by proteins but are exposed owing to anomalous packaging defects at critically important locations.
FIGURE 1.
Ability of sperm to bind spots of hyaluronan on glass substrates. Fluorescence (a) and brightfield micrograph (b) of adherent sperm. Fluorescence micrograph of live–dead staining of sperm in contact with hyaluronan-coated ‘spot’ (c) before and (d) after washing to remove non-adherent cells. Note that the absence of dead sperm in (a) and the tip of the handling pipette in (b). Live–dead assay used cyber green (living) and propidium iodide (dead) staining in combination. Reprinted from Reproductive Biomedicine Online, vol. 14, Huszar et al. ,29 Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects, pp. 650–63, 2007, with permission from Elsevier.

Is deoxyribonucleic acid damage the link connecting sperm chromatin integrity and pregnancy failure?
It is likely that some important regions in sperm chromatin13,21,38–43 are sensitive to DNA-damaging agents. 30,32 In the most severe forms of DNA packaging defects, such as complete absence of protamine (e.g. as in mouse knockout models), embryo lethality is the norm. 21,44 Moreover, even small imbalances in the balance of DNA packaging proteins in sperm have deleterious effects on fertility. 40,44,45 Hence, there are clear connections between stoichiometric chromatin imbalance and DNA fragmentation, suggesting that problems with one are reflected by complementary problems in the other. 46–49 During spermiogenesis, when the paternal genome is being repackaged to fit a much smaller nucleus,50,51 any deficiencies in the packaging process are likely to leave some DNA sequences more exposed to damaging ROS than others. Although it may be the case that we cannot do anything about such types of DNA damage in standard IVF procedures, it may be possible to eliminate these damaged sperm from the pool prepared for ICSI-based procedures and by so doing, reducing pregnancy loss and correspondingly increasing LBRs.
Work leading up to the study
The potential for hyaluronan binding to discriminate and select for sperm with high chromatin integrity
In the clinic, whenever possible, ART makes use of sperm isolated through either DGC or swim-up processing (and occasionally both). This helps to obtain the better-quality sperm for subsequent IVF or ICSI,5 although even selected sperm are not entirely free of DNA fragmentation (Figure 2). 7,48 Hyaluronan (HA) is the major glycosaminoglycan secretion of the cervix and the cumulus–oophorus complex. 52 Sperm reaching these surfaces can bind to HA and subsequent hyperactivation facilitates their penetration to the zona pellucida of the egg. Work by Huszar et al. 53 showed that immature sperm with excessive cytoplasm had higher rates of aneuploidy, lowered cytoplasmic maturity and a dysfunctional ability to bind HA. 53,54 Pelleted sperm are more homogeneously normal in this critical respect. The cytoplasm-rich, poorly HA-binding sperm of DGC interface sperm also have poorer morphology and motility and exhibit higher rates of DNA damage. 29,48,55,56 Prinosilova et al. 55 obtained an over threefold greater number of strict Tygerberg sperm (a rigorous test for normal sperm morphology) following exposure of highly abnormal semen samples to a HA-coated substrate. Using a similar selection system, Sati et al. 56 showed that HA-binding sperm had more compact chromatin, lower decapacitation factor receptor and less residual cytoplasm than non-binding sperm.
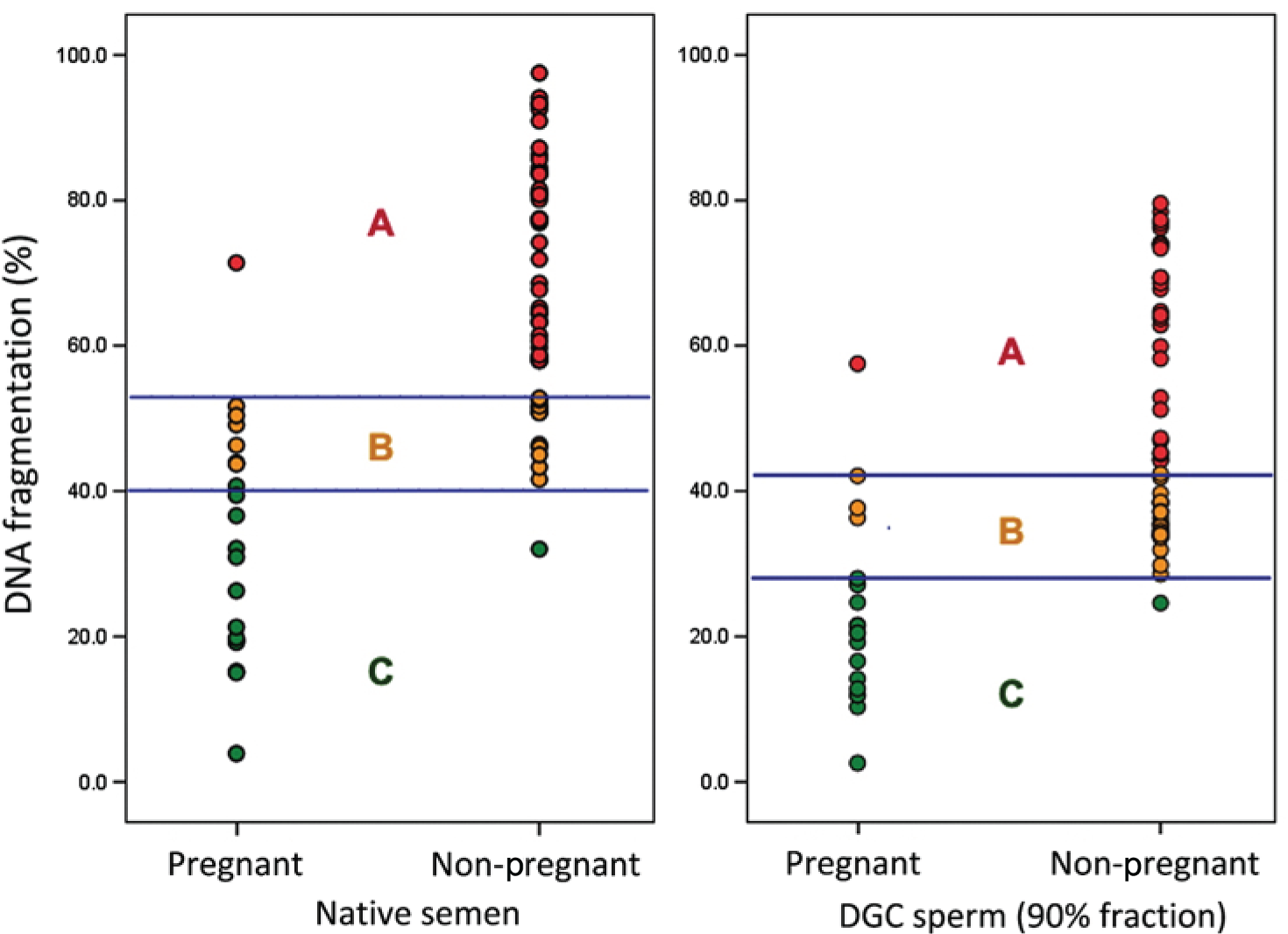
FIGURE 2.
Relationship between sperm DNA fragmentation and CPR in native semen and semen processed by DGC (90% fraction). Reprinted from Fertility and Sterility, vol. 95, Simon et al. 7 Sperm DNA damage measured by the alkaline comet assay as an independent predictor of male infertility and in vitro fertilization success, pp. 652–7, 2011, with permission from Elsevier.
Evidence of the beneficial effect of hyaluronan selection on pregnancy outcome
In many clinics, polyvinylpyrrolidone (PVP) is normally used to slow sperm down sufficiently for capture by the clinical embryologist. Two clinically relevant studies have reported on effects following a HA selection procedure for ICSI. Parmegiani et al. 48 obtained higher numbers of grade 1 embryos for transfer following ICSI with HA- rather than PVP-selected sperm (36% vs. 24%) and an improved LBRs (23% vs. 18%). A more recent and larger randomised study used a fully developed HA-based sperm selection [physiological intracytoplasmic sperm injection (PICSI)] versus PVP procedures in 802 ICSI cycles (Table 1 and Figure 3). 58 Worrilow et al. 57 showed a 13% increase in CPR (n = 121) using HA- versus PVP-selected sperm with a corresponding drop in miscarriage rate (14.1% vs. 3.8%; n = 168). Closer examination of the trial data indicated a more general 5–10% improvement in CPR if the data were stratified according to the DGC-washed HA binding score (obtained prior to PICSI selection), with lower scores (≤ 65%) giving the best results.
| HBS | Implantation rate (%) at 4 weeks | CPR (%) at 6–8 weeks | Miscarriage rates (%) based on CP with fetal sac (6 weeks) less fetal heartbeat (8 weeks) |
|---|---|---|---|
| All scores | 32.2/33.5 [482] | 47.8/47.3 [482] | 10.0/4.3 [247] |
| > 65% | 34.8/37.9 [357] | 51.1/46.2 [357] | 7.8/5.9 [188] |
| ≤ 65% | 30.7/37.4 [121] | 37.9/50.8 [121] | 18.5/0 [59] |
FIGURE 3.
Shift from nucleosomal (histone)- to toroidal (protamine)-based chromatin via transition proteins during spermatogenesis. Histones are acetylated (Ac) prior to their removal. Although it is not known whether the remaining histones are there by design or as a residue of this shift, their presence introduces a ‘weakness’ into the overall chromatin structure that may be more vulnerable to naturally or iatrogenically induced damage. Reproduced from Oliva,45 in accordance with the terms of the Creative Commons Attribution-NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/.

This may explain why CPRs in HA versus PVP arms were balanced before stratification according to the post-DGC washed HA binding score, while miscarriage rates fell by 6% (see Table 1). The US trial did not report LBR, but the trial data suggested that the main benefit of HA selection was a lowering of early pregnancy failure rates. 59 The current trial sought to confirm this as well as to contribute data on LBR, miscarriage rates and notably to understanding the basic underlying mechanistic action of HA sperm selection.
The study rationale
Mechanistic aspects
Evidence suggested that the less compact and, hence, more susceptible domains are enriched in regulatory sequences for genes that are important in early embryonic development. 30,32,33 As DNA is differentially packaged into domains that reflect a clear organisational framework, we hypothesised that sperm DNA fragmentation reflects alterations in the packaging of sperm chromatin that leaves some critical DNA sequences more exposed to oxidative damage than others. The aim was to test the hypothesis that PICSI more robustly selects for sperm with good chromatin integrity, and correspondingly low DNA damage than manual selection normally permits. Although this suggested that PICSI (or other HA-based selection procedures) may best be applied among semen samples that are of particularly low quality, there is no reason why it could not be applied more widely in IVF-ICSI if the evidence from this study supported its efficacy. Although not the purpose of this study, HA-based sperm selection could potentially be extended into standard IVF procedures if methods were developed to restore the fertilisation potential of pre-HA bound sperm.
Interventional aspects
The 2010 World Health Organization (WHO) manual5 on semen analysis has altered the definition of a ‘normal’ fertile sample because the relationship between sperm ‘normality’ and the ability to achieve a pregnancy following 12 months of unprotected intercourse is unclear. The emerging consensus based on some older observations that remain just as valid today is that the morphology of sperm recovered from the endocervix or zona pellucida is a better indicator of their functionality than morphology, based on raw semen analysis. 60–63 Hence, the emphasis now is on identifying those sperm in the ejaculate that can progress through the female genital tract to reach the endocervical mucus and beyond to the egg. Using the WHO guidelines, the range of percentage ‘normal’ values for both fertile and infertile men is likely to be between 0% and 30%, with few samples exceeding a level of 25% of normal spermatozoa. 64 Such low values inevitably produce low thresholds.
For example, limits and thresholds as low as 3–5% normal forms were found in studies of in vitro fertilisation,65 intrauterine insemination66 and in vivo fertility. 67 Similarly, the range of percentage motile sperm found in even ‘pristine’ spermatozoa in the ejaculates of fathers were very wide (8–25%). 68 Hence, none of the aforementioned parameters was particularly helpful in providing a useful definition of sperm ‘normality’. What seems to count most is the sperms’ ability to reach the egg’s zona pellucida, which supports the contention that a prior binding to the HA matrix of the cumulus is a prerequisite. This is why sperm selection for IVF in general, and ICSI in particular, needs improved standards that do not rely on, or at least minimise possible adverse effects of, subjective decisions. In clinical practice, PICSI processes make use of special chambers into which DGC or swim-up processed sperm are introduced (Figure 4c).
FIGURE 4.
(a) HYDAK® (Biocoat Inc., Horsham, PA, USA) HBS slide showing one of the two main HA-coated chambers and a magnified field of view showing sperm on the grid. (b) Stills of time-lapse movies from sperm samples with differing HBS. Binding is indicated with yellow squares and shows the results of high (left panel) vs. low (right panel) binding of samples at similar sperm concentrations. Note that the restricted motility of high-binding sample (fewer red motility tracks). (c) PICSI plate showing channels into which sperm suspensions are introduced. Sperm migrate towards the HA-coated areas at one end of each channel where they bind. (a) and (c) are courtesy of Rick Seiler, Biocoat Inc., Horsham, PA, USA, 2017, personal communication, and (b) is courtesy of Matt Tomlinson, Procreative Diagnostics Ltd, Nottingham, UK, 2017, personal communication.

Both the PVP and PICSI processes make use of media droplets within the ‘ICSI Dish’ into which DGC or swim-up processed sperm are introduced (see Figure 4c). Neither process is inherently any more difficult to perform than the other, and an embryologist used to PVP-based processing can be quickly trained to use PICSI either to augment selection or as an alternative to PVP.
Risks and benefits
Hyaluronan is a natural polymeric secretion of the cervical mucus and cumulus–oophorus complex and so poses no known risks to the egg or zygote. PICSI (a HA-based selection system) was CE approved for use and the manufacturer identified no risks. However, as a precaution against possible adverse effects of intervention, such as early pregnancy loss or preterm labour, we agreed to conduct a safety monitoring interim analysis.
First, sperm bind to HA, effectively immobilising them. Second, HA is thought to work by selectively binding sperm of a higher viability, allowing the embryologist to disregard non-adherent sperm before the choice of sperm for pick-up is made. Third, although the trained embryologist can be very good at selecting the ‘right’ sperm for injection, HA should remove any subjective operator selection and allow consistent objective selection of the ‘right’ sperm for injection.
The main benefits expected of including HA were a decrease in early pregnancy loss and a subsequent increase in LBR at normal term. We considered that HA selection would be beneficial to couples for whom semen quality is too poor for IVF and may also have a significant benefit for older women with poorer quality eggs that have a decreased potential to repair sperm DNA damage.
Justification
-
There was and remains a need to increase LBR at term for IVF and IVF-ICSI patients by reducing fertilisation failure and miscarriage rate.
-
Male fertility in the developed world is thought to be declining. 69
-
The number of IVF and ICSI procedures are rapidly expanding and ICSI in particular is being increasingly used for reasons other than treating male infertility (> 50% of all cycles); hence, the selection of high-quality sperm becomes a more urgent priority.
-
Average LBRs for IVF and IVF-ICSI have remained relatively static at 24%.
-
Lower rates of fertilisation and higher rates of pregnancy loss following ICSI procedures are likely to generate higher costs as the use of ICSI widens beyond treatment for male infertility. Wider use of ICSI without appropriate and adequate safeguards could lead to a future increase in the incidence of deleterious gene lesions in the wider population. 23
-
The largest clinical trial so far, involving nine US centres, showed efficacy for PICSI in increasing CPR (10%) and a corresponding reduction in miscarriage rate (10%). 57 A smaller Italian trial reported an encouraging 5% improvement in LBR following HA-based selection (using a non-optimised HA-containing solution). 70
-
Of the two commercially available HA-based selection systems, PICSI can be introduced into the ART procedure with minimal disruption or training and without any additional intervention.
-
HA-based selection overcomes the highly subjective assessment of sperm quality used by the practising embryologist to choose the ‘right’ sperm for injection.
Main objectives
The primary clinical objective was to determine whether or not a prior HA-binding step (PICSI) in an assisted reproduction setting could improve full-term LBRs over that achieved by conventional ICSI procedures. Secondary clinical objectives were to evaluate the effect of PICSI compared with ICSI on CPRs, miscarriage and preterm LBRs.
The mechanistic objective was to explore the relationships between sperm DNA integrity and HBS in the context of CPR, LBR and miscarriage.
Chapter 2 Trial design, materials and methods
The following is a shortened version of the final protocol that has been updated to reflect the end of the trial, its conduct and final outcomes, now known and being reported here in full. The full, finally approved, protocol including details relating to data handling, management, ethics and funding is available elsewhere. 71 Shorter versions are also available by Witt et al. 72 and in the International Standard Randomised Controlled Trial Number (ISRCTN) registry73 and a summary list of all protocol changes is provided in Appendix 1.
Trial design
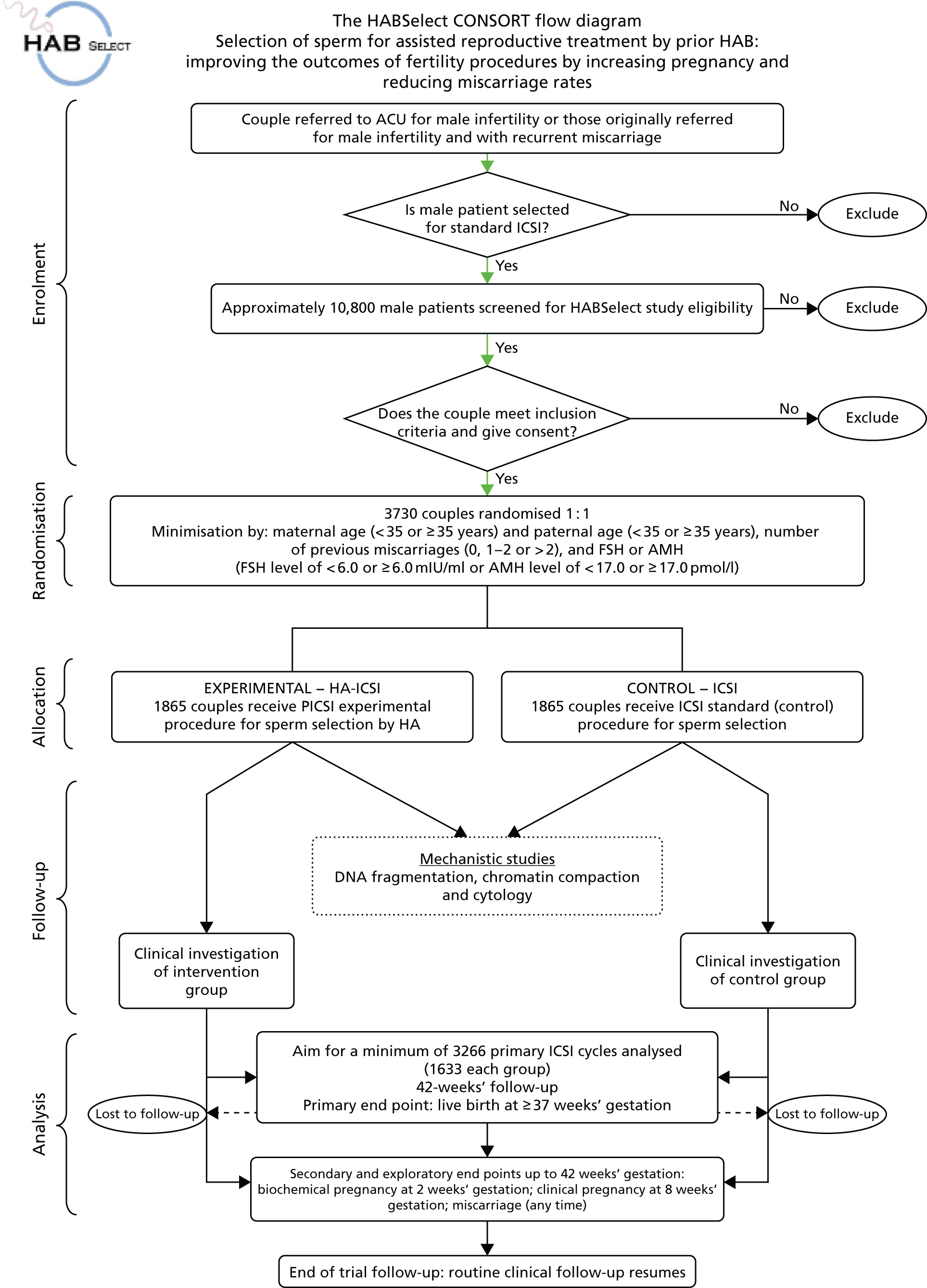
A parallel-group, two-arm, multicentre, blinded efficacy randomised clinical trial with mechanistic evaluation. The original Consolidated Standards of Reporting Trials (CONSORT) flow chart for HABSelect study is shown in Figure 5.
FIGURE 5.
Original CONSORT flow chart for the HABSelect study, which combines the clinical trial and associated mechanistic work. ACU, Assisted Conception Unit.
Setting
Assisted Conception or Reproductive Medicine Units where IVF-ICSI and other clinically relevant services are practised.
Participants and centre eligibility
Participating centres were IVF-licensed hospitals or clinics able to provide appointments in a dedicated clinic. There were originally 10 planned participating centres, which was increased to 16 to improve recruitment rate.
Patient public involvement
A team of patient public advisors was identified through consultation with the national charity (Fertility UK; www.fertilityuk.org) led by Kate Brian and also locally with people who had undergone ART at Leeds Fertility (represented by Mrs Bonnie Bermann). Collectively, these people brought the ‘lived experience’ to the HABSelect management team meetings and ensured that a patient-centric approach was adopted. Our patient advisors contributed to the design stage of our recruitment and ethics review strategy throughout, helping to ensure that the trial was presented to prospective participating couples in an accurate and considerate manner. This helped ensure that couples who may have been potentially vulnerable to coercion at the point of their consideration of IVF treatment were adequately protected. We adopted a patient-centric approach from the outset and during all subsequent stages of the HABSelect trial, including the study design, enrolment and delivery. Kate Brian is an acknowledged expert in patient advocacy, working with the UK’s leading patient support group in reproduction medicine (Fertility UK). She became an active contributor to the Trial Steering Committee and its oversight of HABSelect trial governance.
Inclusion criteria for randomisation
-
Couples able to provide informed consent.
-
Couples undergoing ICSI.
-
Women:
-
with a body mass index (BMI) of 19.0–35.0 kg/m2
-
with a follicle-stimulating hormone (FSH) level of 3.0–20.0 mIU/ml and/or with a anti-Müllerian hormone (AMH) level of ≥ 1.5 pmol/l
-
aged 18–43 years.
-
-
Men:
-
aged 18–55 years
-
who were able to produce freshly ejaculated sperm for the treatment cycle.
-
Exclusion criteria
-
Couples not consenting prior to ICSI were ineligible.
-
Couples using non-ejaculated sperm.
-
Couples using donor gametes.
-
Men with vasectomy reversal, cancer treatment involving any chemotherapy and/or radiotherapy in the previous 2 years.
-
Previous participation in the HABSelect trial.
-
Split IVF/ICSI procedures.
-
If both FSH and AMH were tested and either of them fell outside the accepted range.
Eligibility and informed consent process
The process of identifying potential participants and inviting them to the study was individualised for each participating centre and adapted to their routine practice. Potential trial participants were identified in several ways:
-
Approached during standard IVF fertility centre visits, either during individual appointment with a clinician or at a patient evening/meeting.
-
From waiting lists, registries or review of case records. Participants identified by these means were normally sent the personalised HABSelect invitation letter inviting them to take part. This letter included a brief introduction to the study and also a copy of the couple information sheet and informed consent form. Patients were invited to contact their local research clinician to find out more information and to make an appointment to discuss the study further.
-
Self-referral after accessing information from the study website, which we linked to other similarly themed websites or from the posters displayed in each participating centre.
Couples were identified as candidates for the HABSelect study by local IVF-ICSI-licensed fertility centre staff if they had opted for or been advised to make use of ICSI-based procedures. Normally, routine NHS assessment of ejaculate semen quality was sufficient for men to be selected for ICSI procedures over IVF. The clinical team checked that the couple met the inclusion and exclusion criteria (see Inclusion criteria for randomisation and Exclusion criteria). Only couples meeting these criteria were approached to provide consent to participate. Details were recorded on the trial screening log.
Informed consent procedures
Assessment of eligibility and the informed consent process was undertaken by the principal investigator or other suitably qualified member of the trial team who had received appropriate training and was approved by the principal investigator as detailed on the delegation of responsibilities log. All staff involved in taking informed consent to the study had a thorough knowledge and experience of good clinical practice and issues around consent and were fully conversant and trained in the study protocol. Informed, written consent for entry into the trial was obtained prior to participant enrolment to the study.
Consent for the donation of residual semen samples for biomedical research
Patients who were eligible to take part in the trial were also eligible to have any residual semen samples remaining after the ICSI procedure and mechanistic evaluations donated to the Human Biomaterials Resource Centre (HBRC) Biobank, University of Birmingham. Participation within the HBRC Biobank was discussed with couples at the same time as discussing their participation in the HABSelect trial. Verbal and written details (the Donation of Human Tissue for Research Patient Information Sheet) were provided to patients. Following information provision, patients were given as long as needed to consider participation (a minimum of 24 hours is recommended) and were given the opportunity to discuss the biobanking of any residual semen sample after all the HABSelect procedures were completed with their family and other healthcare professionals.
Enrolment
After written informed consent was obtained, participants were enrolled into the study by a delegated member of staff at the trial research site. At the point of enrolment, the couple was issued a unique identifier (ID) number and recorded on the trial enrolment log. Participants were enrolled into the trial only by an authorised member of staff at the trial research site, as detailed on the Site Research Staff Delegation Log. A unique ID number consisted of the trial site code (site ID) followed by the consecutive screening number starting with 001 was also used (see appendix 2 of the protocol71).
Trial interventions
Physiological intracytoplasmic sperm injection dishes and HYDAK slides
The investigational instruments were the PICSI™ sperm selection dish (Origio, Måløv, Denmark) and the HYDAK, HBS slides, both marketed (in the UK) by Origio (Cooper Surgical, London, UK). Both products were CE marked and approved for clinical use. Regardless of the randomised allocation, HA binding assay scores (henceforth referred to as HBS) were obtained from ≈66% of semen samples from both the interventional (PICSI) and the non-interventional (ICSI) arms using the HYDAK slide. Our original goal of obtaining scores for all samples was prevented by the manufacturer’s temporary withdrawal of the slides (see Chapter 3). Only the interventional arm made use of the PICSI plates.
Application
The protocol made no additional demands on couples undergoing IVF-ICSI treatment. Normally, density gradient (DGC) washed and prepared motile sperm were selected for ICSI after adding a suspension to PVP under an inverted microscope. Sperm motility is slowed sufficiently to allow capture by the experienced embryologist, who then immobilised the sperm by breaking its tail with the injection pipette. The sperm was then taken up into the injection pipette and injected directly into the egg. In the interventional arm, exactly the same procedure was carried out except that the washed and prepared motile sperm were allowed to interact with the PICSI substrate beforehand. There were no other interventions.
Outcomes
Clinical outcomes
All clinical outcomes were defined as a proportion of all women randomised, excluding losses to follow-up.
Primary outcome
-
A live birth at ≥ 37 weeks’ gestation after PICSI or ICSI procedure with first fresh embryo transfer.
Secondary outcomes
-
Clinical pregnancy based on detection of fetal heartbeat or presence of fetal sac at 6–9 weeks’ gestation.
-
Miscarriage rate defined as pregnancy loss any time after confirmation of CP.
-
Live birth at < 37 weeks’ gestation.
Mechanistic outcomes
Relationships between clinical outcomes and tests of sperm DNA integrity were assessed by a combination of structural equation modelling (SEM), classification tree analysis and linear regression. All analyses were undertaken in the R (The R Foundation for Statistical Computing, Vienna, Austria) environment. 74 Mechanistic outcomes are reported solely for the purpose of hypothesis generation.
Sample size
From the 2008 UK national average for ICSI success,3 the LBR at ≥ 37 weeks’ gestation in the control group was estimated to be 24%. To detect a 5% increase (i.e. from 24% to 29%) with 90% power at the 5% significance level required 1633 participants in the analysis for each group. We aimed to recruit at least 3700 couples into the trial over 24 months, allowing for 10% loss to follow-up. Because of poorer than expected recruitment at an interim assessment (albeit with loss to follow-up well below 10%), the funder and Trial Steering Committee recommended an extension of the recruitment period to 30 months, with a revised target for power of 80% requiring 1222 couples in the primary analysis from each group (see Report Supplementary Material 1 for more details).
Randomisation sequence
Following screening and formal enrolment in the study, confirmation of eligibility and completion of baseline assessments, the female participant commenced ovarian stimulation and the couple entered the clinical care pathway. Couples were randomised into the trial on the day of the ICSI/PICSI and no more than 24 hours beforehand. The time interval between enrolment and randomisation was centre dependent as it followed local practice for down-regulation and egg simulation, which precedes IVF. Using a secure web-based 24-hour automated randomisation engine developed by the Pragmatic Clinical Trials Unit (PCTU), an authorised member of staff at the research site performed randomisation. Couples’ treatment group allocation was known only to the person performing the randomisation (usually the embryologist carrying out the procedure).
Couples were randomised in a 1 : 1 ratio using minimisation with a random component to the interventional (PICSI) or the non-interventional (ICSI) arm. Minimisation was stratified by site. Minimisation variables were:
-
maternal age (< 35 and ≥ 35 years)
-
paternal age (< 35 and ≥ 35 years)
-
number of previous miscarriages (0, 1–2 or > 2)
-
hormonal indicator of ovarian reserve – FSH level (< 6.0 or ≥ 6.0 mIU/ml) or AMH level (< 17.0 or ≥ 17.0 pmol/l) when FSH was not available.
Informed consent procedures
The principal investigator or another suitably qualified member of the trial team undertook assessment of eligibility and the informed consent process. Informed, written consent for entry into the trial was obtained prior to participant enrolment.
Withdrawal
Couples consented to the initial baseline screening for eligibility, trial intervention, follow-up and data collection. Couples or individual partner participants were also able to withdraw from the trial at any time without explanation but, unless specifically requesting otherwise, data collected up to the point of withdrawal could be included in any subsequent analysis.
Withdrawal before randomisation
Post enrolment and prior to randomisation, couples who withdrew and did not receive the trial intervention resumed standard treatment/care. No further data collection occurred for couples who withdrew prior to randomisation and they were not included in the trial analysis.
Withdrawal after randomisation
Participant withdrawal post randomisation was categorised as follows:
-
Withdrawal of consent but the participant was willing for clinical data to be collected on pregnancy outcome but not for any further mechanistic assessments to be undertaken. Data collected to this point could be used.
-
Withdrawal of consent for the trial follow-up schedule but the participant was willing to have any information already collected to be utilised.
-
Withdrawal of consent for follow-up information to be used and refusal of data already collected to be utilised.
Study personnel made every effort to obtain and record information about the reasons for discontinuation and to follow up the women for all safety and efficacy outcomes, as appropriate. To make a clear distinction as to exact participants’ preferences, we used a withdrawal of consent form. All communication surrounding the withdrawal was noted in the participant’s records and no further case report forms were completed for that participant.
Blinding
All participants, clinical care providers in IVF-licensed units and maternity and neonatal wards, research nurses responsible for participants’ recruitment and follow-up, the trial chief investigator, trial manager and statisticians for the clinical arm were all blinded. Unblinding occurred only after all data collection was completed and the (clinical) statistical analysis plan was signed off. The only unblinded group were the embryologists, who were also responsible for couple randomisation and who performed ICSI/PICSI and HBS procedures at study sites. The sole exception to this rule was for the mechanistic statistician (RW), who was unblinded after randomisation ended. Study data managers were also unblinded to allocations. To monitor blinding, an independent statistician, not otherwise involved in the trial, prepared reports for the Data Monitoring and Ethics Committee.
Mechanistic assessments
Sample selection
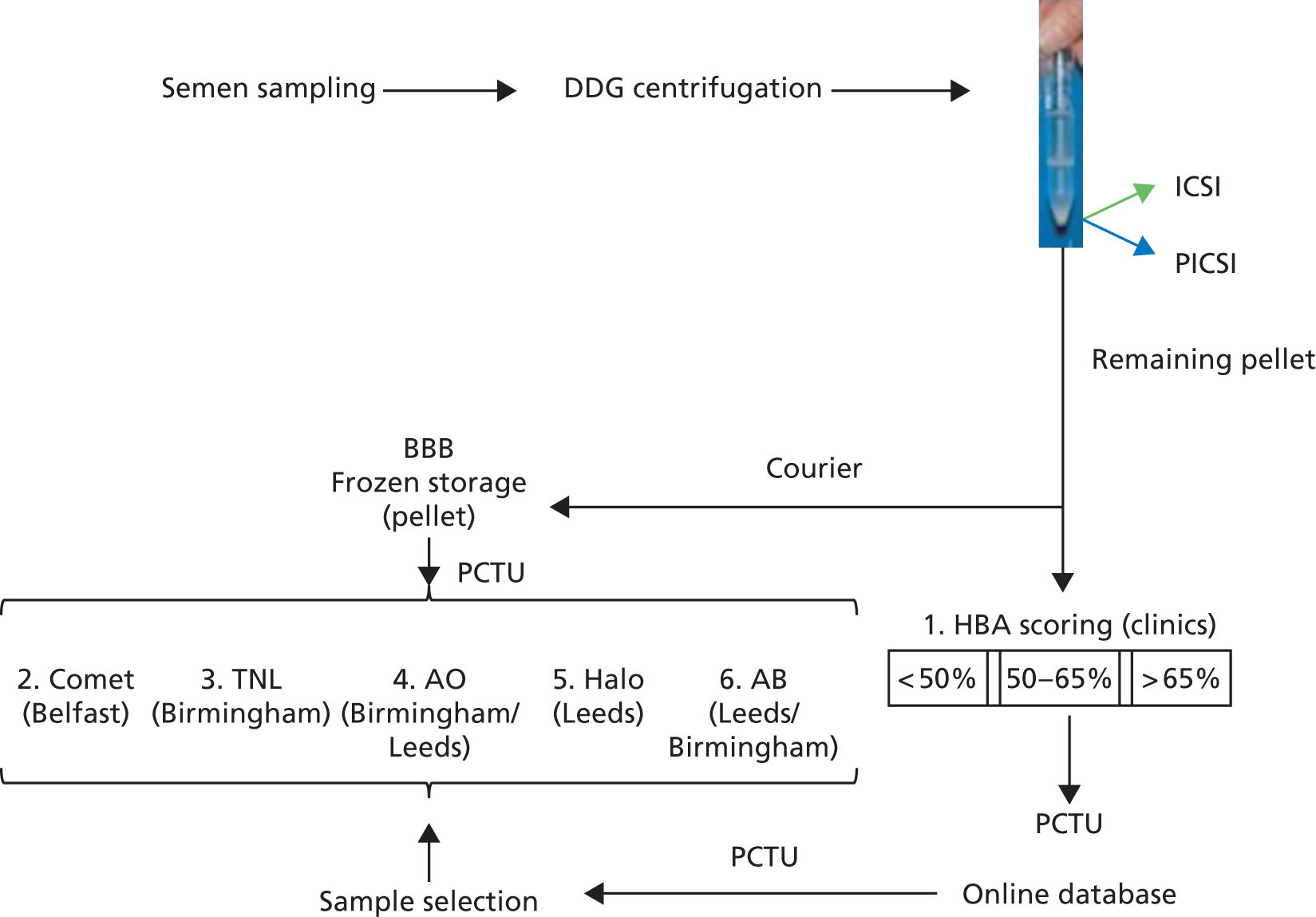
It proved logistically impossible to thaw out sample aliquots and undertake cytology on all or as many of the trial samples as possible as originally intended (see Chapter 5, Limitations and Mechanistic summary). In addition, midway through the mechanistic work, we recognised that because of serious time constraints, we would be unable to accommodate the sample coverage we had originally anticipated. To compensate and following PCTU and Trial Steering Committee approval, the mechanistic statistician (RW) was unblinded, permitting a more enriched, balanced selection of samples for analysis from both arms based on miscarriage. All sample selections based on this screening process were communicated to the HBRC central sample repository, which then co-ordinated their delivery to the mechanistic laboratories for the assays as described in Figure 6 and see also Table 9. To summarise, in conjunction with the mechanistic statistician, the PCTU selected samples to be used for mechanistic analysis and communicated this information to the HBRC that arranged shipment to the Mechanistic Laboratories. This process was reiterated until ≈1300 samples were finally tested overall by one or more assay. With this dynamic monitoring of sample selection for analysis in place, high-quality information for each test in each HBS stratum was expected even if only a minority of samples were amenable to global examination by all tests. The mechanistic statistician was confident that the information could be integrated adequately.
FIGURE 6.
Schematic of the sampling for mechanisms. The reduction from four to three centres made a hierarchical priority of testing more important, with the number of tests carried out depending on the number of available sample aliquots after HBA scoring. The testing priority was in the numerical order shown, with neutral comet included at a later stage. All tests could in principle be carried out across the three research centres and all samples were stored centrally in Birmingham HBRC prior to distribution. Routine cytology, however, was abandoned when it was recognised that the information provided did not justify the time it took to carry out. Instead, the trials unit assisted in maximising the use of available samples by all mechanistic labs. Owing to its disruptive effect on the priority clinical practice, collection of sperm from the differentially centrifuged interface fractions of selected samples could not be undertaken.
Sample processing
Three (originally four) basic science laboratories performed mechanistic evaluation of the collected residual prepared sperm samples for sperm DNA integrity as follows:
-
Birmingham – terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL)75 and aniline blue (AB)76 assays.
-
Belfast – alkaline and neutral comet assays. 77
-
Sheffield withdrew by agreement to permit release of additional funds for recruitment.
-
Standard operating procedures for these assays are available in Report Supplementary Material 3.
Outputs of the mechanistic studies were recorded as follows:
-
Initial observations recorded in paper-based laboratory notebooks according to standard practice.
-
Digital images associated with experimental outputs held locally on portable, encrypted solid-state hard drives.
Digital images and experimental outputs uploaded on to a secure web page shared between the three mechanistic labs. Times of data uploads were logged automatically.
Statistical methods
Clinical trial
Analyses of clinical effectiveness were by intention to treat. The primary analysis included only those couples with non-missing outcomes, which was an unbiased approach if the outcome is missing at random (i.e. if ‘missingness’ for the outcome is related only to observed covariates79). In the event that > 5% of primary outcomes were missing, our analysis plan prespecified a sensitivity analysis to explore the impact on conclusions of deviations from the missing at random assumption. Numbers lost to follow-up or with missing baseline assessments are summarised in Chapter 3.
Differences between trial arms for the primary and secondary clinical outcomes are presented as odds ratios with 95% confidence intervals, obtained using mixed-effects logistic regression adjusting for the minimisation factors (maternal age, paternal age, number of previous miscarriages and hormonal indicator of ovarian reserve), with a random intercept to account for variation between recruitment centres. Maternal age and paternal age were adjusted for using restricted cubic splines with three knots (knot locations based on Harrell’s recommendations80). Number of previous miscarriages and hormonal indicator of ovarian reserve were adjusted for as categorical variables. Number of previous miscarriages had three categories 0, 1–2 or > 2. Hormonal indicator of ovarian reserve had two categories: FSH level of < 6.0 or ≥ 6.0 mIU/ml, or AMH level of < 17.0 or ≥ 17.0 pmol/l when FSH is not available. Absolute risk differences with 95% confidence intervals were also calculated from unadjusted logistic regression models using the delta method.
Prespecified subgroup analyses for the primary outcome were performed for the following factors:
-
HBS [high (> 65%) vs. low (≤ 65%)]
-
maternal age (< 35 vs. ≥ 35 years)
-
number of previous miscarriages (0 vs. > 0)
-
FSH level (< 6.0 vs. ≥ 6.0 mIU/ml) or AMH level (< 17 vs. ≥ 17 pmol/l) when FSH testing is not available
-
sperm concentration (< 15 vs. ≥ 15 mml).
For each factor, the subgroup analysis investigated possible modification of the treatment effect using a mixed-effects logistic regression model with the addition of an interaction between treatment and effect modifier. Once the results of the prespecified clinical effectiveness analysis had been reviewed, it was decided to repeat the subgroup analysis carried out for the primary outcome for the miscarriage following CP outcome. These analyses were carried out using the same methods as the subgroup analysis for the primary outcome.
Mechanisms
The flow of residual samples from the clinics to the mechanistic labs is shown in Figure 5. Changes from the protocol included the agreed withdrawal of Sheffield, the omission of chromomycin A3 (CMA3), which was not ready for inclusion by the time analysis began, and the omission of tests on the differentially centrifuged 45:90 (40:80) interface sperm of selected samples. The latter could not be accommodated by the clinics.
Structural equation modelling integrating DNA integrity assays remained a key step along the logical data analysis path described herein. However, the SEM was redirected to focus on the relationship between DNA integrity and HBS (see Figure 12). Alternative methods, including decision (classification) trees and generalised regression models, were employed to help refine the model and integrate it with the clinical outcomes.
Chapter 3 Results (clinical trial)
Recruitment and participant flow (based on the CONSORT flow chart)
Figure 7 shows the flow of participants through the clinical trial during the period February 2014 to August 2016 with follow-up to July 2017. We screened 6700 couples at 16 centres (Figure 8) for trial eligibility and randomised, 2772 (PICSI, n = 1387 and ICSI, n = 1385), but 6 (PICSI, n = 1 and ICSI, n = 5) were excluded post-randomisation as they were subsequently found not to have met eligibility criteria. Of those excluded, 1323 screened couples did not meet the inclusion criteria, 795 declined to participate, 484 consented but were not randomised, there was no contact from 626 couples and the remaining 700 were not included for other reasons, such as decision to split cycle, inability to produce a fresh semen sample on the day of treatment, decision not to transfer fresh embryo(s) and conversion to IVF treatment. The final number available for the primary clinical analysis was 2752. Mechanistic analysis is covered in Chapter 4.
FIGURE 7.
The CONSORT flow chart for the clinical trial, showing patient flow.

FIGURE 8.
Randomisation broken down by recruitment centre. The contributing proportion per centre of the total randomisations (n = 2766) discounting post-randomisation exclusions is shown. Reproduced with permission from Miller et al. 81 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.

Baseline characteristics
All participants
The baseline characteristics of all participants in the trial were well balanced between the arms for both males and females (Table 2). Age, BMI, ethnicity, smoking status, alcohol consumption and records of recreational drug use (all with potential effects and impacts on fertility) did not differ between the PICSI (n = 1386) and ICSI (n = 1380) arms in male or female participants, although this was in agreement with other data, a males reported higher levels of smoking, alcohol consumption and recreational drug use. 82
| Characteristic | Summary | Missing data, n (%) | ||
|---|---|---|---|---|
| PICSI (N = 1386) | ICSI (N = 1380) | PICSI | ICSI | |
| Male partner | ||||
| Average age (years) | 36.1 (5.5) | 35.9 (5.4) | ||
| Aged ≥ 35, n (%) | 812 (58.6) | 803 (58.2) | ||
| BMI (kg/m2) | 27.3 (4.6) | 27.0 (4.2) | 816 (58.9) | 831 (60.2) |
| Ethnicity, n (%) | ||||
| White | 1047 (75.5) | 1078 (78.1) | ||
| Asian | 193 (13.9) | 166 (12.0) | ||
| Black | 49 (3.5) | 45 (3.3) | ||
| Other | 36 (2.6) | 45 (3.3) | ||
| Not stated | 61 (4.4) | 46 (3.3) | ||
| Current smoker, n (%) | 68 (5.0) | 65 (4.8) | 21 (1.5) | 27 (2.0) |
| If yes, how many cigarettes/day, n (%) | 8.0 (5.5) | 8.5 (5.2) | 5 (0.4) | 6 (0.4) |
| Drink alcohol, n (%) | 771 (59.1) | 791 (60.8) | 82 (5.9) | 80 (5.8) |
| If yes, how many units/week, n (%) | 7.7 (6.3) | 7.7 (6.8) | 47 (3.4) | 51 (3.7) |
| Recreational drug use, n (%) | 7 (0.5) | 6 (0.5) | 83 (6.0) | 94 (6.8) |
| Semen assessment | ||||
| Sperm concentration (× 106/ml), median (IQR) | 11.0 (3.5–29.5) | 11.0 (3.6–31.0) | 51 (3.7) | 42 (3.0) |
| Based on semen assessment ICSI recommended, n (%) | 1268 (96.1) | 1245 (95.0) | 66 (4.8) | 70 (5.1) |
| Female partner | ||||
| Average age (years) | 33.6 (4.4) | 33.7 (4.3) | ||
| Aged ≥ 35, n (%) | 618 (44.6) | 617 (44.7) | ||
| BMI (kg/m2) | 24.7 (3.5) | 24.4 (3.5) | 18 (1.3) | 20 (1.4) |
| Ethnicity, n (%) | ||||
| White | 1029 (74.2) | 1049 (76.0) | ||
| Asian | 214 (15.4) | 189 (13.7) | ||
| Black | 45 (3.2) | 46 (3.3) | ||
| Other | 52 (3.8) | 55 (4.0) | ||
| Not stated | 46 (3.3) | 41 (3.0) | ||
| Current smoker, n (%) | 31 (2.3) | 20 (1.5) | 11 (0.8) | 12 (0.9) |
| If yes, how many cigarettes/day, n (%) | 6.4 (3.3) | 6.3 (3.6) | 3 (0.2) | 0 (0.0) |
| Drink alcohol, n (%) | 646 (48.2) | 673 (50.7) | 46 (3.3) | 52 (3.8) |
| If yes, how many units/week, n (%) | 5.1 (4.3) | 5.1 (4.7) | 32 (2.3) | 39 (2.8) |
| Recreational drug use, n (%) | 1 (0.1) | 1 (0.1) | 69 (5.0) | 78 (5.7) |
| Pre-treatment hormonal assessment | ||||
| FSH level (mIU/l), mean (SD) | 7.1 (2.3) | 7.1 (2.3) | 477 (34.4) | 458 (33.2) |
| AMH level pmol/l, mean (SD) | 22.6 (18.7) | 22.0 (18.5) | 571 (41.2) | 585 (42.4) |
| FSH level of < 6.0 mIU/ml or AMH level of < 17.0 pmol/l, when FSH testing is not available, n (%) | 292 (21.1) | 274 (19.9) | ||
| Length of menstrual cycle (days), mean (SD) | 30.3 (11.0) | 30.7 (12.9) | 97 (7.0) | 79 (5.7) |
| Type of menstrual cycle, n (%) | 12 (0.9) | 8 (0.6) | ||
| Regular | 1176 (85.6) | 1170 (85.3) | ||
| Irregular | 187 (13.6) | 189 (13.8) | ||
| Not known | 11 (0.8) | 13 (0.9) | ||
| Previous fertility and pregnancy history, n (%) | ||||
| Previous natural pregnancy | 302 (21.8) | 313 (22.7) | ||
| Live birth following natural pregnancy | 47 (3.4) | 57 (4.2) | 14 (1.0) | 19 (1.4) |
| Previous IVF/ICSI fertility treatment cycle | 411 (29.7) | 401 (29.1) | ||
| Live birth following previous IVF/ICSI fertility treatment | 82 (6.0) | 74 (5.4) | 4 (1.0) | 13 (0.9) |
| Previous miscarriage, n (%) | ||||
| 0 | 1190 (85.9) | 1174 (85.1) | ||
| 1–2 | 187 (13.5) | 193 (14.0) | ||
| > 2 | 9 (0.6) | 13 (0.9) | ||
| Gynaecological disorders, n (%) | ||||
| Polycystic ovaries | 216 (15.6) | 208 (15.1) | ||
| Fibroids | 60 (4.3) | 80 (5.8) | ||
| Endometriosis | 98 (7.1) | 109 (7.9) | ||
| Other | 109 (7.9) | 122 (8.8) | ||
| Pelvic surgery, n (%) | ||||
| Myomectomy | 15 (1.1) | 18 (1.3) | ||
| Endometriosis surgery | 52 (3.8) | 48 (3.5) | ||
| Salpingectomy | 45 (3.2) | 37 (2.7) | ||
| Caesarean | 24 (1.7) | 22 (1.6) | ||
| Other | 180 (13.0) | 201 (14.6) | ||
| Hormonal treatment, n (%) | ||||
| Type of hormonal cycle | 2 (0.1) | 1 (0.1) | ||
| Long agonist | 697 (50.4) | 692 (50.2) | ||
| Short agonist | 147 (10.6) | 122 (8.8) | ||
| Antagonist | 533 (38.5) | 550 (39.9) | ||
| Other | 7 (0.5) | 15 (1.1) | ||
Female participants
Pre-treatment levels of serum FSH and AMH levels were similar in both arms (see Table 2), as were the duration and quality of menstrual cycles (see also primary outcome subgroup analyses in Table 6). Before moving over to using AMH as an indicator of ovarian reserve, clinical IVF services were reliant initially on FSH levels. In total, 563 women tested overall had FSH scores of < 6.0 mIU/l and/or AMH scores of < 17.0 pmol/l with the remaining 2189 women having FSH scores of ≥ 6.0 mIU/l and/or AMH scores of ≥ 17.0 pmol/l, a ratio of almost 1 : 4, indicating a relatively fertile female population.
Male participants
Original semen assessment was similar across both arms, with mean sperm concentrations (11.0 × 106/ml) lying below the WHO lower reference limit (5th centile; 15 × 106/ml) in both arms, and thus consistent with a strong clinical recommendation for ICSI treatment (see Table 2). The average pre-preparation (for treatment) sperm concentration measured on the day of treatment (Table 3) rose slightly to just within the lower reference limit (≈15 × 106/ml). The positive effect of sample processing for treatment, however, was clearly obvious with the pre-preparation assessment of forward progressive motility (≈40%), rising to 69% overall post preparation (see Table 3). Forward progressive motility, therefore, was above the 5th centile lower reference limit (32%) to start with, but rose to within the 90th centile (69%). As the way semen samples are processed prior to treatment could have an important effect on the outcome of the final treatment cycle, we were reassured to note that these measures were also well balanced between arms, with differential DGC being the most frequently used process, followed by direct centrifugation and swim-up (see Table 3). A small proportion of samples prepared by ‘other’ methods were equally balanced between arms, with ‘other’ typically referring to those samples with too few sperm to process through a gradient or to swim-up. Such samples were generally centrifuged directly (no density gradient) to pellet the sperm. In this regard, stratified HBS were obtained from sperm only following sample preparation and were balanced between the two arms, with the highest proportion having scores > 65%, followed by the intermediate (25% to ≤ 65%) and lowest (< 25%) scoring categories. More samples with low scores in the PICSI arm (see Table 3) could have led to an improved outcome, according to Worrilow et al. ,57 raising the possibility of a selection bias.
| Characteristic | Summary | Missing data, n (%) | ||
|---|---|---|---|---|
| PICSI (N = 1386) | ICSI (N = 1380) | PICSI | ICSI | |
| Male partner semen pre-preparation assessment | ||||
| Semen volume (ml), mean (SD) | 2.9 (1.4) | 3.0 (1.5) | 48 (3.5) | 48 (3.5) |
| Sperm concentration (× 106/ml), median (IQR) | 14.7 (4.0–35.0) | 16.0 (5.0–36.4) | 150 (10.8) | 157 (11.4) |
| % of forward progressive motility, mean (SD) | 39.5 (20.1) | 40.8 (20.3) | 170 (12.3) | 182 (13.2) |
| Sperm concentration mean (× 106/ml), mean (SD) | 23.9 | 24.1 | ||
| Male partner semen post-preparation assessment, mean (SD) | ||||
| Sample processing | 43 (3.1) | 43 (3.1) | ||
| Swim-up | 18 (1.3) | 19 (1.4) | ||
| Density gradient | 1044 (77.7) | 1028 (76.9) | ||
| Direct centrifugation | 191 (14.2) | 198 (14.8) | ||
| Other form of processing | 89 (6.6) | 90 (6.7) | ||
| Sample not processed | 1 (0.1) | 2 (0.1) | ||
| Forward motility (%) | 68.6 (28.1) | 69.5 (27.5) | 225 (16.2) | 240 (17.4) |
| HBS, mean (SD) | ||||
| HBS | 423 (30.5) | 433 (31.4) | ||
| ≤ 25% | 86 (8.9) | 74 (7.8) | ||
| > 25% and ≤ 65% | 188 (19.5) | 181 (19.1) | ||
| > 65% | 689 (71.5) | 692 (73.1) | ||
| Female partner oocytes collection, mean (SD) | ||||
| Number of eggs collected (per couple) | 10.9 (6.3) | 10.8 (6.3) | 41 (3.0) | 43 (3.1) |
| Number of metaphase II oocytes injected with sperm | 8.7 (5.1) | 8.5 (5.1) | 45 (3.2) | 49 (3.6) |
There were no differences between participants in either arm with respect to female fertility and pregnancy history (including previous pregnancy losses). Histories of gynaecological disorders and reasons for pelvic surgery (if recorded) across the arms were well balanced. The types of down-regulation protocol also did not differ between arms, with long agonist being the most frequently used protocol, followed by antagonist and short agonist. All choices of down-regulation protocol were balanced between both trial arms. There were twice as many ‘other’ protocols reported in the PICSI (n = 15; 1.1%) versus ICSI (n = 7; 0.5%) arms; (see Table 2).
Primary outcome by arm allocation (physiological intracytoplasmic sperm injection vs. intracytoplasmic sperm injection)
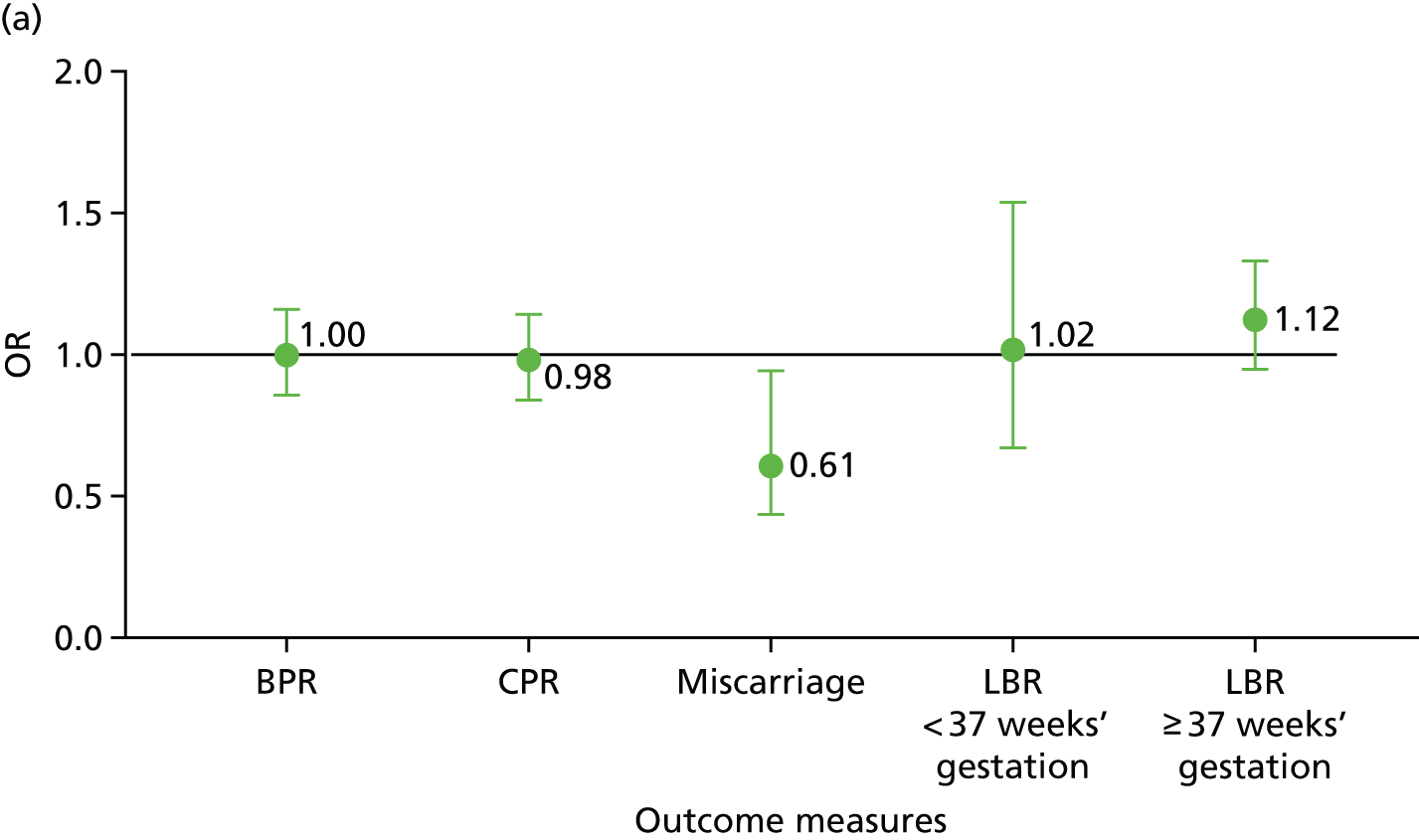
The analysis (Figure 9 and see Report Supplementary Material 1) showed the primary outcome (LBR at ≥ 37 weeks’ gestation) was not significantly different between the two arms of the trial [odds ratio (OR) 1.12, 95% confidence interval (CI) 0.94 to 1.34; p = 0.18] where 379 (27.4%) and 346 (25.2%) couples who were randomised, respectively, into the PICSI and ICSI arms, successfully achieved a full-term live birth. The absolute risk difference was 2.2% (95% CI –1.1% to 5.5%).
FIGURE 9.
Summary of main clinical outcome data. (a) Plots of all clinical outcome measures showing ORs and 95% CIs; and (b) outcomes expressed as the proportion of cycles between the two trial arms. Absolute numbers are shown above the bars. Reproduced with permission from Miller et al. 81 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.

A sensitivity analysis of the primary outcome measure that adjusted for additional baseline covariates (female partner BMI, female partner ethnicity, history of previous pregnancy, female partner hormonal status and hormonal treatment) provided near-identical outputs for live birth with PICSI (379/1379, 27.4%) and ICSI (346/1370, 25.2%) with an OR of 1.13 (95% CI 0.95 to 1.34; p = 0.17).
Subgroup analysis of the primary outcome
Subgroup analysis of the primary outcome (Table 4 and Figure 10) showed no evidence of modification of the treatment effect by HBS, maternal age, previous miscarriage, maternal FSH or AMH levels and paternal sperm concentrations. Fertility declines in older women and HA-based sperm selection may therefore benefit them more if their eggs have a reduced capacity to accommodate sperm DNA fragmentation. In this regard, compared with women < 35 years, a small improvement in LBR at > 37 weeks’ gestation for women ≥ 35 years was noted for the PICSI group (22.8%) versus the ICSI group (18.7%), although the difference was not significant (p-value for interaction = 0.22; see Table 4). HBS, which is thought to be a useful indicator of male fertility when males with high scores are more fertile than those with lower scores, did not differ across the two arms. Although because of a withdrawal of the scoring slides early on the trial, scores were available for only 69% (n = 961) and 68% (n = 944) of men in the PICSI and ICSI arms, respectively.
| Characteristic | Number included in the analysis | Summary, n (%) | OR (95% CI) | p-value (interaction between treatment and subgrouping factor) | ||
|---|---|---|---|---|---|---|
| PICSI (n) | ICSI (n) | PICSI | ICSI | |||
| HBS | ||||||
| ≤ 65% | 273 | 254 | 80 (29.3) | 72 (28.3) | 1.10 (0.75 to 1.61) | 0.67 |
| > 65% | 688 | 690 | 178 (25.9) | 180 (26.1) | 0.99 (0.78 to 1.27) | |
| ≤ 25% | 85 | 74 | 23 (27.1) | 24 (32.4) | 0.79 (0.40 to 1.58) | 0.50 |
| > 25% and ≤ 65% | 188 | 180 | 57 (30.3) | 48 (26.7) | 1.26 (0.80 to 2.01) | |
| > 65% | 688 | 690 | 178 (25.9) | 180 (26.1) | 0.99 (0.78 to 1.27) | |
| Maternal age (years) | ||||||
| < 35 | 766 | 755 | 239 (31.2) | 231 (30.6) | 1.03 (0.83 to 1.29) | 0.22 |
| ≥ 35 | 615 | 616 | 140 (22.8) | 115 (18.7) | 1.29 (0.98 to 1.71) | |
| Previous miscarriage | ||||||
| 0 | 1186 | 1165 | 327 (27.6) | 296 (25.4) | 1.13 (0.94 to 1.36) | 0.86 |
| > 0 | 195 | 206 | 52 (26.7) | 50 (24.3) | 1.08 (0.69 to 1.71) | |
| FSH level or AMH level (when FSH not tested) | ||||||
| < 6.0 mIU/l (< 17.0 pmol/l for AMH) | 291 | 272 | 78 (26.8) | 68 (25.0) | 1.08 (0.74 to 1.59) | 0.82 |
| ≥ 6.0 mIU/l (≥ 17.0 pmol/l for AMH) | 1090 | 1099 | 301 (27.6) | 278 (25.3) | 1.14 (0.94 to 1.38) | |
| Sperm concentration | ||||||
| < 15 × 106/ml | 777 | 763 | 225 (29.0) | 196 (25.7) | 1.16 (0.92 to 1.46) | 0.71 |
| ≥ 15 × 106/ml | 553 | 566 | 141 (25.5) | 140 (24.7) | 1.08 (0.82 to 1.42) | |
FIGURE 10.
Summary of (a) primary and (b) miscarriage outcome subgroup analysis for maternal age (p = 0.22/0.11); FSH level (p = 0.82/0.12); HBS (p = 0.67/0.43); previous miscarriage (p = 0.86/0.42); and semen concentration (p = 0.71/0.33). a, AMH < 17.0 pmol/l when FSH not measured; b, AMH ≥ 17.0 pmol/l when FSH not measured. p-values are for the interaction term between the subgroup variable and the treatment variable. ORs are indicated. Subgroup analysis for miscarriage was carried out post hoc. Reproduced with permission from Miller et al. 81 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.


Secondary outcomes by arm allocation (physiological intracytoplasmic sperm injection vs. intracytoplasmic sperm injection)
Clinical pregnancy rate at 6–9 weeks’ gestation
There was no difference in CPR at 6–9 weeks’ gestation (OR 0.98, 95% CI 0.84 to 1.15; p = 0.80) with 487 (35.2%) and 491 (35.7%) clinical pregnancies, respectively, in the PICSI and ICSI arms of the trial (absolute risk difference –0.5%, 95% CI –4.0% to 3.1%) (Table 5).
| Outcome | Number included in analysis (n) | Summary, n (%) | OR (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| PICSI | ICSI | PICSI | ICSI | |||
| CP at 6–9 weeks’ gestation | 1382 | 1375 | 487 (35.2) | 491 (35.7) | 0.98 (0.84 to 1.15) | 0.80 |
| Miscarriage following CP | 1381 | 1371 | 60 (4.3) | 96 (7.0) | 0.61 (0.43 to 0.84) | 0.003 |
| Live birth < 37 weeks’ gestation | 1381 | 1371 | 46 (3.3) | 45 (3.3) | 1.02 (0.67 to 1.55) | 0.94 |
Preterm live birth rate at ≤ 37 weeks’ gestation
There was no difference in preterm birth rate < 37 weeks’ gestation (OR 1.02, 95% CI 0.67 to 1.55; p = 0.94), with 46 (3.3%) and 45 (3.3%) preterm births, respectively, in the PICSI and ICSI arms of the trial (absolute risk difference 0.0%, 95% CI –1.3% to 1.4%) (Table 5).
Miscarriage following clinical pregnancy
There was a significant difference in the CP loss rate (OR 0.61, 95% CI 0.43 to 0.84; p = 0.003), with 60 (4.3%) and 96 (7.0%) CP losses, respectively, in the PICSI and ICSI arms of the trial (absolute risk difference –2.7%, 95% CI –4.4% to –0.9%) (Table 5).
Subgroup analysis of miscarriage
Post hoc subgroup analysis showed that the statistically significant difference between miscarriage in the PICSI and ICSI arms was not associated with HBS, maternal age, previous miscarriage, FSH or AMH levels (when FSH was not tested) or sperm concentration (at least for the clinical analysis). These analyses were performed in the same way as subgroup analyses for the primary outcome (Table 6 and see Figure 9) by including a treatment subgroup interaction.
| Characteristic | Number included in the analysis (n) | Summary, n (%) | OR (95% CI) | p-value (interaction between treatment and subgrouping factor) | ||
|---|---|---|---|---|---|---|
| PICSI | ICSI | PICSI | ICSI | |||
| HBS | ||||||
| ≤ 65% | 273 | 254 | 8 (2.9) | 16 (6.3) | 0.44 (0.18 to 1.05) | 0.43 |
| > 65% | 688 | 690 | 35 (5.1) | 52 (7.5) | 0.65 (0.42 to 1.01) | |
| ≤ 25% | 85 | 74 | 1 (1.2) | 2 (2.7) | 0.42 (0.04 to 4.71) | 0.75 |
| > 25% and ≤ 65% | 188 | 180 | 7 (3.7) | 14 (7.8) | 0.45 (0.18 to 1.15) | |
| > 65% | 688 | 690 | 35 (5.1) | 52 (7.5) | 0.65 (0.42 to 1.01) | |
| Maternal age (years) | ||||||
| < 35 | 766 | 755 | 31 (4.0) | 38 (5.0) | 0.81 (0.50 to 1.32) | 0.11 |
| ≥ 35 | 615 | 616 | 29 (4.7) | 58 (9.4) | 0.47 (0.30 to 0.75) | |
| Previous miscarriage | ||||||
| 0 | 1186 | 1165 | 55 (4.6) | 83 (7.1) | 0.63 (0.45 to 0.90) | 0.42 |
| > 0 | 195 | 206 | 5 (2.6) | 13 (6.3) | 0.40 (0.14 to 1.15) | |
| FSH level or AMH level (when FSH level not tested) | ||||||
| < 6.0 mIU/l (< 17.0 pmol/l for AMH) | 291 | 272 | 15 (5.2) | 14 (5.1) | 1.04 (0.49 to 2.20) | 0.12 |
| ≥ 6.0 mIU/l (≥ 17.0 pmol/l for AMH) | 1090 | 1099 | 45 (4.1) | 82 (7.5) | 0.53 (0.36 to 0.77) | |
| Sperm concentration | ||||||
| < 15 × 106/ml | 777 | 763 | 28 (3.6) | 53 (6.9) | 0.52 (0.32 to 0.83) | 0.33 |
| ≥ 15 × 106/ml | 553 | 566 | 29 (5.2) | 39 (6.9) | 0.73 (0.44 to 1.19) | |
Missing data
Levels of missing data were extremely low for both primary and secondary outcomes, with 14 out of 2766 (0.05%) eligible couples randomised with missing data for the primary outcome, and it was not considered necessary for sensitivity analysis to be performed for missing data. Information about baseline BMI (see Table 2) was unavailable for over half of all males (≈59%), compared with more modest omissions for females (19%), with information on smoking, alcohol consumption and recreational drug use unavailable from fewer participants. Additional data for semen assessment (see Table 3) were missing from a similar small proportion of participants in each arm. A high proportion of participants in both arms had no record for FSH (≈34%) and/or AMH (≈42%) and information on previous fertility and pregnancy history was not available for a similar proportion of women in both arms (≈16.5% for live birth following natural pregnancy and 13.5% for live birth following IVF/ICSI). There were also missing original (see Table 2) and pre- and post-preparation (see Table 3) assessment data for various aspects of semen profiles. A HBS could not be obtained for almost one-third (≈31%) of all samples (see Table 3). This was due to a temporary and unavoidable withdrawal of Medicines and Healthcare products Regulatory Agency (MHRA) approval for the scoring slides early on in the trial.
Other outcomes
Fertilisation (≈67.5%) and biochemical pregnancy rates (≈39%) in the two arms were similar (Table 7). There were higher numbers of multiple clinical pregnancies in the PICSI (n = 68) versus ICSI (n = 54) arms of the trial, although the similar frequencies in each arm (5.0% and 4.0%, respectively) led to a correspondingly higher frequency of multiple births in the PICSI arm (3.8% vs. 2.1% in the ICSI arm). There were slightly higher numbers of cycles with two embryo transfers in the ICSI (535, 39.5%) versus PICSI (510, 37.4%) arms. There were slightly more biochemical pregnancies in the PICSI arm failing to convert to clinical pregnancies (58, 4.2%) than in the ICSI arm (51, 3.7%).
| Characteristic | Summary | Missing data, n (%) | ||
|---|---|---|---|---|
| PICSI (N = 1386) | ICSI (N = 1380) | PICSI | ICSI | |
| Fertilisation, mean (SD) | ||||
| Fertilisation rate (number of two pro-nuclei stage eggs per injected egg), mean (SD) | 0.66 (0.24) | 0.69 (0.24) | 64 (4.6) | 68 (4.9) |
| Number of fresh embryos transferred, mean (SD) | 21 (1.5) | 24 (1.7) | ||
| 0 | 131 (9.6) | 116 (8.6) | ||
| 1 | 712 (52.2) | 691 (51.0) | ||
| 2 | 510 (37.4) | 535 (39.5) | ||
| 3 | 12 (0.9) | 14 (1.0) | ||
| Biochemical pregnancy, mean (SD) | ||||
| Positive biochemical pregnancy (bHGC test), mean (SD) | 546 (39.48) | 544 (39.51) | 3 (0.2) | 3 (0.2) |
| Biochemical pregnancy loss (PICSI, n = 1382; ICSI, n = 1375), mean (SD) | 58 (4.2) | 51 (3.7) | ||
| Multiple clinical pregnancies | 68 (5.0) | 54 (4.0) | 16 (1.2) | 19 (1.4) |
| Multiple births | 52 (3.8) | 29 (2.1) | 5 (0.4) | 9 (0.7) |
Frozen embryos
The number of embryos frozen among women receiving one or more fresh embryo transfers were similar (see Table 7). It should, at some future date, be possible to determine whether or not cumulative outcomes following frozen–thawed embryo transfer will be higher in the intervention group owing to higher cryosurvival rates relative to the control arm.
Adverse events
Serious adverse events (SAEs) related or unrelated to the study were balanced between arms, indicating no untoward events of the intervention. The single related suspected unexpected serious adverse reactions (SUSARs) were reported as a case of hypospadias in the PICSI and achondroplasia in the ICSI arms, respectively (Table 8).
| SAEs | Trial arm (n) | |
|---|---|---|
| PICSI (N = 1386) | ICSI (N = 1380) | |
| Number of SAEs | 29 | 27 |
| Number of related SUSAR | 1 | 1 |
| Number of unrelated SUSAR | 28 | 26 |
Conclusions
Outcomes are graphically summarised in Figures 8 and 9, with miscarriage rate being the only variable with a significant difference between the two trial arms. The primary outcome demonstrated a 2.2% improvement in LBR at ≥ 37 weeks’ gestation favouring PICSI, which was insufficient to show significant efficacy overall (OR 1.12, 95% CI 0.95 to 1.34).
Miscarriage rates between the two arms and favouring PICSI were significantly different (OR 0.61; p = 0.003). Subgroup analysis did not find evidence that this treatment effect was modified by HBS, maternal age, previous miscarriage, FSH (or AMH when FSH was not tested) or sperm concentration (see Table 6 and Figure 10).
Baseline data suggested that the likelihood of miscarriage increased with age. Analysis of the clinical trial outcomes can be concluded, however, by stating that PICSI offered no clear advantage with regard to LBR. These issues are dealt with more comprehensively in Chapter 5.
Chapter 4 Results (mechanisms)
General considerations (including changes to the original statistical analysis plan)
The mechanistic statistical analysis plan (SAPm) (see Report Supplementary Material 2) for HABSelect was intended as guidance only. It permitted exploration of the clinical and mechanistic data and enabled us to consider alternative plans once the clinical analysis was completed and the trial outcomes known.
The SAPm suggested that SEM would form the backbone of the analysis and SEM remains a key step along the logical analysis path described herein but, with fewer assays undertaken, its role was downgraded. Results were better identified by other methods including classification trees and generalised regression models. Although the analysis was of data from an observational study without the benefit of randomisation, the mechanistic cohort (1247 couples) was sampled from couples participating within the randomised controlled trial (RCT) (2766 couples). This subset constituted residual sperm samples originally selected from storage on the basis of a balanced set of HBS (≤ 25%, > 25%, ≤ 65%, > 65%) and later stratified by known outcome of miscarriage, which, once the trial had determined that there was no statistical significance to the primary outcome, became the main focus. It should be stated at the outset that unlike the hypothesis testing and analysis completion central to the clinical trial, the mechanistic analysis is hypothesis generating and is a continuing effort.
Baseline features
The results reported herein follow the underlying biological pathway of the treatment provided to participating couples (Figure 11). Owing to time and cost limitations, not all couples could have samples processed. In addition, some residual samples from fertility treatment that had used most of the original ejaculate had too few or no sperm available on which to perform any of the assays. In total, 905 comet assays, 889 TUNEL assays, 593 AO assays, 549 AB assays and 431 halo assays were carried out, of which 131 samples were tested by all five assays (see Appendix 1). Assay values were arbitrary in relation to DNA integrity (fragmentation and compaction) that is, we chose not to apply cut-off points in relation to reporting subgroups within the data (above or below a certain predetermined value indicative of low or high fragmentation for example). This was the most appropriate course of action given that there is no consensus over what the cut-off values giving rise to an informative DNA fragmentation index should be83–86 and assay calibration was unresolved. Instead, comparisons between means and quartiles for all data grouped into outcomes (e.g. clinically pregnant vs. not pregnant) were used throughout to indicate relative levels of DNA fragmentation (AO, comet and TUNEL assays) and/or compaction (AB) in our SEM. In our analyses, higher relative values for the TUNEL, AO and comet assays, indicated higher levels of DNA fragmentation. Larger halo areas (and higher ratios) indicated correspondingly lower DNA fragmentation. Lower AB (alongside, halo area and ratio) values indicated higher compaction.
FIGURE 11.
Flow chart for the mechanistic cohort. The sample size of 1247 was drawn from the main trial cohort and was enriched for couples experiencing miscarriage (balanced for couples experiencing full-term live birth with similar baseline characteristics).
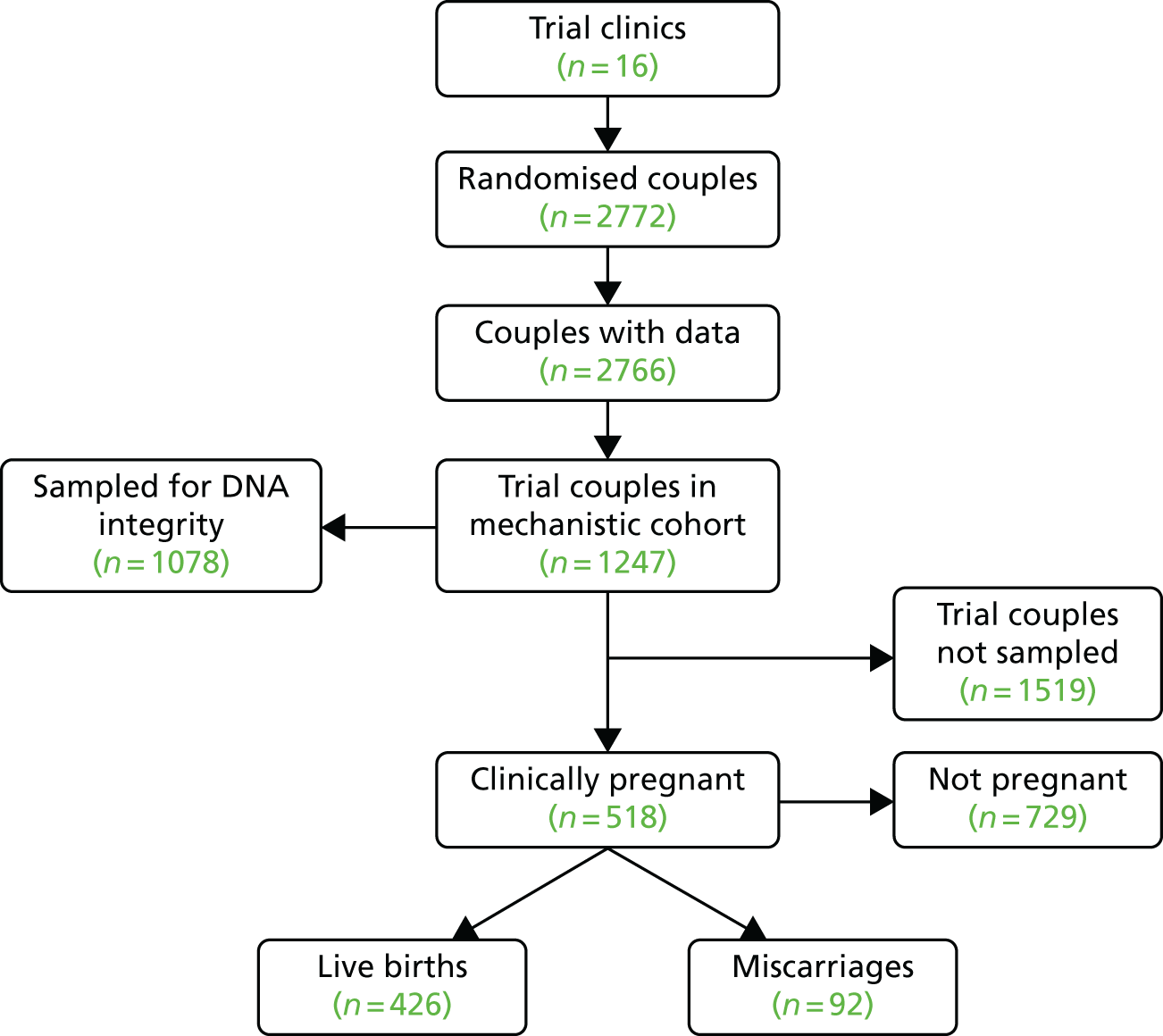
Being a subset of the trial cohort, mechanistic baseline data sets should have been balanced by randomisation and, indeed, this proved to be so, with Table 9 listing these data in relation to CP. A total of 518 (41%) couples in the mechanistic cohort successfully conceived and in terms of baseline p-values, parental age (female and male) was the only significant difference noted between pregnant and non-pregnant, although AMH level, sperm halo area and AB staining came close to significance. Neither HBS nor any of the assays of sperm DNA integrity predicted CP (however, see Chapter 5, Mechanistic outcomes). A total of 426 (82%) couples enjoyed a live birth outcome, with the remainder (18%) ending in miscarriage (Table 10). Here, female age and PICSI were the only significant factors distinguishing between the two outcomes (miscarriage or live birth).
| Characteristic | Pregnancy status | p-value | |
|---|---|---|---|
| Not pregnant | Clinically pregnant | ||
| Number | 729 | 518 | |
| Male partner | |||
| Age (years), mean (SD) | 36.42 (5.62) | 35.59 (5.39) | 0.010 |
| White, n (%) | 581 (79.7) | 413 (79.7) | 0.999 |
| BMI (kg/m2), mean (SD) | 26.80 (4.33) | 27.42 (4.38) | 0.214 |
| Alcohol units, mean (SD) | 7.78 (6.18) | 8.06 (7.39) | 0.600 |
| Sperm concentration (× 106/ml), mean (SD) | 26.91 (35.74) | 25.78 (34.24) | 0.579 |
| Sperm volume (ml), mean (SD) | 2.95 (1.48) | 2.98 (1.54) | 0.674 |
| Motility (%), mean (SD) | 41.43 (19.55) | 41.48 (19.45) | 0.966 |
| Female partner | |||
| Age (years), mean (SD) | 34.06 (4.36) | 33.39 (4.10) | 0.006 |
| White, n (%) | 564 (77.4) | 407 (78.6) | 0.555 |
| BMI (kg/m2), mean (SD) | 24.33 (3.53) | 24.45 (3.51) | 0.551 |
| Alcohol units, mean (SD) | 5.32 (4.21) | 5.00 (4.29) | 0.371 |
| Previous fertility treatment, n (%) | 259 (35.5) | 168 (32.4) | 0.283 |
| Previous natural pregnancy, n (%) | 166 (22.8) | 119 (23.0) | 0.988 |
| Previous miscarriage, n (%) | 126 (17.3) | 72 (13.9) | 0.125 |
| FSH level (mlU/l), mean (SD) | 7.14 (2.20) | 6.95 (2.09) | 0.216 |
| AMH level (pmol/l), mean (SD) | 20.62 (19.06) | 23.32 (16.12) | 0.057 |
| Assays | |||
| HBS, mean (SD) | 74.47 (24.29) | 73.38 (24.45) | 0.476 |
| Allocated PICSI, n (%) | 365 (50.1) | 261 (50.4) | 0.958 |
| TUNEL, mean (SD) | 12.42 (15.60) | 12.18 (13.81) | 0.812 |
| AO, mean (SD) | 45.30 (15.48) | 45.68 (15.73) | 0.773 |
| Comet, mean (SD) | 19.02 (9,38) | 18.77 (9.79) | 0.698 |
| Halo area, mean (SD) | 168.40 (64.09) | 179.66 (61.31) | 0.067 |
| Halo ratio, mean (SD) | 3.67 (1.55) | 3.76 (1.66) | 0.555 |
| AB, mean (SD) | 64.76 (21.54) | 61.19 (23.00) | 0.066 |
| Characteristic | Outcome | p-value | |
|---|---|---|---|
| Miscarriage | Live birth | ||
| Number | 92 | 426 | |
| Male partner | |||
| Age (years), mean (SD) | 36.54 (5.85) | 35.39 (5.27) | 0.063 |
| White, n (%) | 72 (78.3) | 341 (80.0) | 0.808 |
| BMI (kg/m2), mean (SD) | 26.77 (3.59) | 27.57 (4.54) | 0.271 |
| Alcohol units, mean (SD) | 8.12 (7.20) | 8.04 (7.46) | 0.942 |
| Sperm concentration (× 106/ml), mean (SD) | 25.53 (31.87) | 25.83 (34.76) | 0.938 |
| Sperm volume (ml), mean (SD) | 2.74 (1.76) | 3.04 (1.49) | 0.092 |
| Motility (%), mean (SD) | 40.62 (18.28) | 41.66 (19.70) | 0.655 |
| Female partner | |||
| Age (years), mean (SD) | 34.65 (4.20) | 33.12 (4.03) | 0.001 |
| White, n (%) | 68 (73.9) | 339 (79.6) | 0.289 |
| BMI (kg/m2), mean (SD) | 24.77 (3.73) | 24.38 (3.46) | 0.331 |
| Alcohol units, mean (SD) | 4.59 (3.32) | 5.09 (4.50) | 0.464 |
| Previous fertility treatment, n (%) | 32 (34.8) | 136 (31.9) | 0.683 |
| Previous natural pregnancy, n (%) | 20 (21.7) | 99 (23.2) | 0.862 |
| Previous miscarriage, n (%) | 10 (10.9) | 62 (14.6) | 0.447 |
| FSH level (mlU/l), mean (SD) | 7.16 (2.64) | 6.91 (1.94) | 0.381 |
| AMH level (pmol/l), mean (SD) | 22.67 (14.93) | 23.46 (16.41) | 0.753 |
| Assays | |||
| HBS, mean (SD) | 75.79 (21.06) | 72.89 (25.08) | 0.357 |
| Allocated PICSI, n (%) | 32 (34.8) | 229 (53.8) | 0.001 |
| TUNEL, mean (SD) | 10.24 (10.34) | 12.57 (14.39) | 0.235 |
| AO, mean (SD) | 49.60 (14.12) | 44.86 (15.96) | 0.076 |
| Comet, mean (SD) | 20.68 (10.11) | 18.36 (9.68) | 0.087 |
| Halo area, mean (SD) | 178.93 (56.22) | 179.80 (62.40) | 0.945 |
| Halo ratio, mean (SD) | 3.41 (1.30) | 3.83 (1.72) | 0.220 |
| AB, mean (SD) | 63.89 (23.62) | 60.55 (22.87) | 0.404 |
Inspection of baseline values
The weakness of the relationship between sperm DNA integrity and clinical outcome was unexpected and led to examination of the relationship with HBS (Table 11). Following logistic regression, all assays were shown to correlate with HBS, with TUNEL showing the strongest association, followed by AO, halo (area and ratio), comet and AB (see Table 11 and Figure 10 for examples).
| Association | n | t-value | p-value |
|---|---|---|---|
| TUNEL | 810 | –6.99 | < 0.001 |
| AO | 555 | –3.84 | < 0.001 |
| Comet | 854 | –2.45 | 0.015 |
| Halo area | 406 | 6.05 | < 0.001 |
| Halo ratio | 406 | 4.29 | < 0.001 |
| AB | 514 | 1.98 | 0.049 |
Correlations between the assays themselves, however, were weak (Table 12), with the strongest between AB and AO (R = 0.26). There were some weak correlations between assays (e.g. the expected positive correlations between the TUNEL, AO and comet assays and, correspondingly, negative correlations with the halo area/ratio). The weakness, however, suggests that the assays were qualitatively measuring different aspects of DNA fragmentation or compaction. Turning to the relationship between the assays and HBS, Table 13 shows the results for the coefficients for the single regression of HBS on assay values.
| Assay | Assay | |||||
|---|---|---|---|---|---|---|
| TUNEL | AO | Comet | Halo | AB | ||
| Area | Ratio | |||||
| TUNEL | 1 | |||||
| AO | 0.01 | 1 | ||||
| Comet | 0.05 | 0.01 | 1 | |||
| Halo area | –0.17 | 0.10 | –0.03 | 1 | ||
| Halo ratio | –0.10 | –0.03 | 0.17 | 0.54 | 1 | |
| AB | 0.14 | 0.26 | 0.06 | 0.02 | –0.13 | 1 |
| Assay | Estimate | Standard error | t-value | p-value |
|---|---|---|---|---|
| (Intercept) | 95.240 | 5.681 | 16.77 | < 0.001 |
| Tunel | –0.165 | 0.060 | –2.75 | 0.007 |
| AO | –0.129 | 0.070 | –1.84 | 0.068 |
| Comet | –0.160 | 0.091 | –1.77 | 0.079 |
| Halo area | 0.027 | 0.020 | 1.34 | 0.184 |
| AB | –0.002 | 0.042 | –0.041 | 0.967 |
| Halo ratio | 0.020 | 0.776 | 0.025 | 0.980 |
Here, the TUNEL assay was the strongest predictor of HBS, although, taken together, the results summarised in Tables 10–13 indicated that integrating data from the TUNEL, AO and comet assays had the best potential for predicting HBS and outcomes downstream in the biological process relating to DNA integrity.
Structural equation modelling
Structural equation modelling was the primary analysis described in the SAPm and it was intended that measurement models were built for fragmentation (as measured by the TUNEL, AO and comet assays) and for compaction (measured by AB and halo) (Table 14). The regression modelling above supports the work with TUNEL, AO, and comet measuring an underlying latent variable (arbitrarily named fragmentation) with the halo and AB assays measuring compaction (again, arbitrarily named in our model). The modelling is graphically represented in Figure 12. The package ‘lavaan’, version 0.5, was used within the statistical environment R, version 3.3.2.
| Coefficient | Estimate | Standard error | z-value | p-value |
|---|---|---|---|---|
| Fragmentation | ||||
| TUNEL | 1.000 | |||
| AO | 0.650 | 0.313 | 2.073 | 0.038 |
| Comet | 0.370 | 0.215 | 1.723 | 0.085 |
| Compaction | ||||
| AB | 1.000 | |||
| Halo area | –0.951 | 0.908 | –1.048 | 0.295 |
| Regressions | ||||
| HBS | 1.000 | |||
| Fragmentation | –0.477 | 0.301 | –1.586 | 0.113 |
| Compaction | –0.633 | 0.259 | –2.450 | 0.014 |
FIGURE 12.
Structural equation modelling showing relationships between measured quantities (boxes) and latent variables (ovals). Fragmentation is the latent variable for DNA fragmentation and compaction is the latent variable for chromatin compaction. In the model, halo (area and ratio) was associated with the compaction variable.

These results suggested that the set of theoretical relationships for the SEM illustrated in Figure 12 was viable. The research question being asked from this point was whether or not useful expressions could be derived from the assays to represent the aspects of DNA fragmentation and compaction within the sperm samples. More directly, the latent variables of fragmentation and compaction were defined as follows:
The arbitrary constant 7.5 and some rescaling were applied in the compaction equation to provide a scale similar to that of fragmentation. Fragmentation now ranged from 10.2 to 66.8 and compaction from 1.4 to 67.4. As scales and centrality of the assay values were arbitrary, it was not possible to interpret the size of the coefficients. With 131 complete observations with all assays (see Appendix 3 and Table 20), it was also not anticipated that statistical significance of all coefficients would be seen. The significance values provided, however, encouraged further investigation with a larger data set after the model viability was confirmed.
With the above definitions for fragmentation and compaction the regression predicting HBS became:
Note that the compaction term was dropped here as it was not statistically significant and contributed little. HBS, however, decreased with fragmentation, and for the remainder of the analysis the two dimensions (fragmentation and compaction) of the information from the five assays were used to inform sperm motility, count and concentration, predicted from the following models:
Such that motility increased with compaction and HBS and sperm count and concentration decreased with fragmentation. Terms that were not statistically significant were dropped from the models.
In the fitted models for each of the relationships in the four graphs (Figures 13), it was decided that a linear relationship would be a reasonable approximation and that more complex non-linear relationships be regarded as overfitting. The predictive models, therefore, were taken as satisfactory.
FIGURE 13.
Significant correlations in SEM. In the fitted models, each of the relationships was assumed to be linear. They show (a) HBS (% binding) against sperm % motile; (b) sperm % motile against DNA compaction variable; (c) sperm count against DNA fragmentation variable; and (d) sperm concentration against DNA fragmentation variable. The regression lines have been plotted within the 95% CI envelope (shaded area).
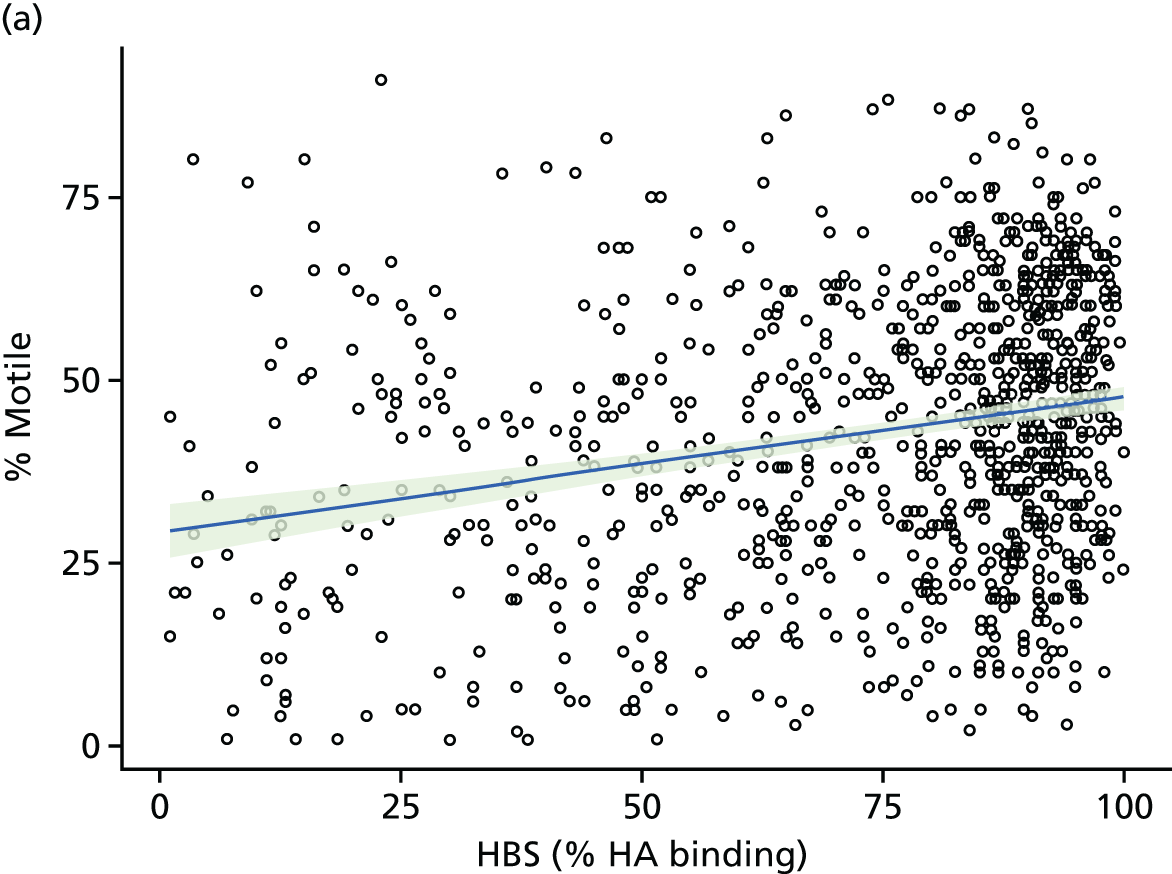



Fertilisation
It was next asked whether or not fertilisation rates were associated with the allocation to treatment, HBS, DNA integrity or combinations thereof. From records kept of the number of eggs fertilised and the successful development of those eggs into embryos, the number of eggs fertilised per couple varied from 1 to 35 and the number of embryos ranged from 0 to 23. A binomial regression with a log-link function of the fertilisation rate based on successful and failed fertilisation was undertaken. As the only statistically significant term was for HBS, the binomial regression was run without the other terms, permitting data from 1888 couples to be included. The coefficients from that regression are given in Table 15. Conversion of the coefficients to relative risk (RR) is shown in Table 16.
| Coefficient | Estimate | Standard error | z-value | p-value |
|---|---|---|---|---|
| Intercept | –0.327 | 0.044 | –7.455 | < 0.001 |
| PICSI allocation | –0.0378 | 0.011 | –3.601 | < 0.001 |
| HBS | 0.0010 | 0.0002 | 4.775 | < 0.001 |
| Female age | –0.0029 | 0.0012 | –2.346 | 0.019 |
| Coefficient | RR | 95% confidence interval |
|---|---|---|
| PICSI allocation | 0.9630 | 0.9434 to 0.9829 |
| HBS | 1.0010 | 1.0006 to 1.0015 |
| Female age | 0.9971 | 0.9947 to 0.9995 |
Clinical pregnancy
We next considered factors associated with CP, in particular whether HBS, DNA fragmentation or compaction was associated with CPRs.
The question was approached by classification tree and logistic regression. The classification tree had the advantage of being able to handle highly correlated covariates, such as maternal and paternal age. Logistic regression, on the other hand, is more familiar to researchers. By taking both approaches, a more thorough exploration of the relationship between these factors and CP was provided.
The results from fitting a classification tree, where the outcome was CP (CPR ‘yes’ or ‘no’), are shown in Figure 14, with the elliptical nodes representing branching by the variable within and the square nodes are terminal nodes where couples have been classified by chance of CP. Note that the classification tree first predicted the CPR (34.1%) for women aged > 35 years, but for younger women (≤ 35 years), the CPR was more dependent on DNA compaction with the predicted rate being 64.5% for couples with a higher compaction (> 40 arbitrary units), reducing to 44.1% for couples with a lower compaction ≤ 40 of arbitrary units.
FIGURE 14.
Classification tree for achieving CP based on the mechanistic cohort (n = 1247). Note the primary determinant was female age followed by sperm DNA compaction. Comp, DNA compaction.
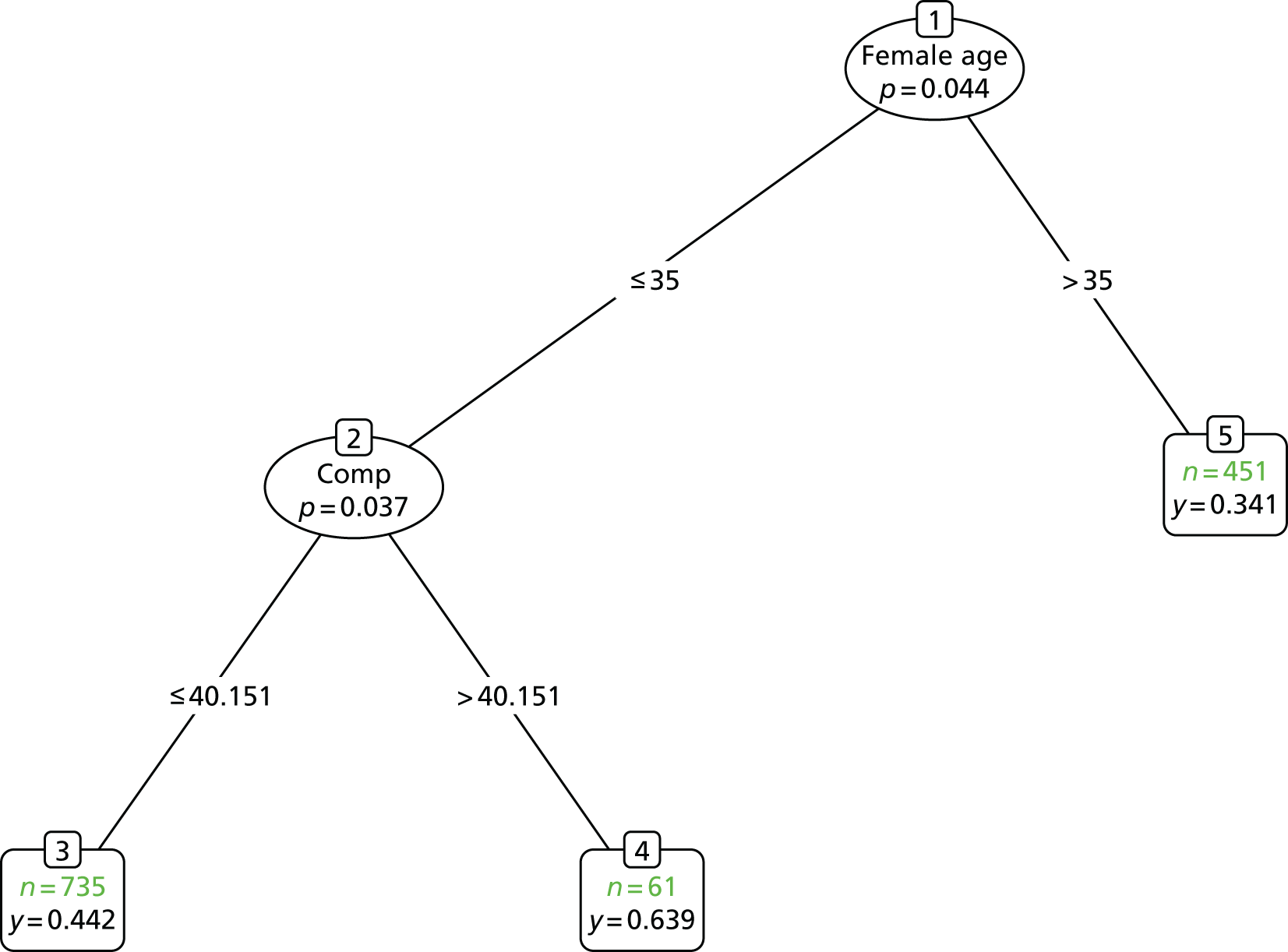
For those couples for whom DNA compaction was not determined, surrogate variables were used instead. This enabled use of data from all 1247 couples rather than just the 228 for which compaction was measured, although confidence in this result was limited because of the high number of surrogate splits that were performed. Fitting a logistic regression model instead of a classification tree provided the fitted model coefficients shown in Table 17, where female ethnicity (white) and age were the strongest predictors.
| Coefficient | Estimate | Standard error | z-value | p-value |
|---|---|---|---|---|
| (Intercept) | 1.965 | 1.288 | 1.526 | 0.127 |
| Female (white) | 1.454 | 0.439 | 3.313 | 0.001 |
| Compaction | 0.023 | 0.010 | 2.297 | 0.022 |
| Female age | –0.128 | 0.038 | –3.396 | 0.001 |
Miscarriage
As the clinical trial had determined that there was insufficient evidence for an increase in LBR following PICSI, the focus of the mechanistic analysis turned to miscarriage, which was significantly different between the trial arms. Single embryo transfer was not always practised in HABSelect and some patients who had a live birth also miscarried one or more of the transferred embryos, recorded as a miscarriage. The focus here, however, was where all of the transferred blastocysts had miscarried. That is, we compared the outcomes where there was a live birth or not following CP. For convenience, for the work undertaken within the mechanistic cohort, we define a miscarriage occurring when CP does not result in a live birth (including preterm and excluding stillbirth).
As shown in Table 18 and following similar trends for CP, age (male and female), the PICSI allocation and HBS all predicted miscarriage risk. Modelling of the outcome (miscarriage or live birth) was then undertaken first using a classification tree and then by logistic regression (Figure 15). In both cases, the clinically pregnant subset formed the population of interest.
| Coefficient | Estimate | Standard error | z-value | p-value |
|---|---|---|---|---|
| (Intercept) | 7.089 | 1.423 | 4.981 | < 0.001 |
| PICSI | –5.514 | 2.092 | –2.636 | 0.008 |
| Female age | –0.173 | 0.041 | –4.251 | < 0.001 |
| PICSI × age | 0.185 | 0.061 | 3.019 | 0.003 |
FIGURE 15.
Classification tree for live birth outcome among clinically pregnant women in the mechanistic cohort (n = 506). Note that female age was the primary discriminator with the allocation and then by allocation in older women.

Note that although these are made available, the classification tree did not use all variables. In particular, Table 9 shows that, in terms of a univariable analysis only, there was a significant difference between the miscarriage and live birth groups according to the number of embryos transferred. Having accounted for the age of the female partner and the allocation treatment (ICSI or PICSI), however, the number of embryos transferred discriminated no further.
The classification tree shown in Figure 16 indicates that for the oldest (> 37 years) female partners, the rate of miscarriage was 31.0% [(1 – 0.69) × 100]. More specifically, 48 out of the 155 clinical pregnancies ended with miscarriage and 107 with a live birth. For couples for whom the age of the female partner was ≤ 37 years and PICSI was given, 42 out of 409 miscarried, a rate of 10.3%. For those couples for whom the female age was 35–37 years and ICSI was given, the miscarriage rate was 29 out of 108 (26.9%). The miscarriage rate for couples for whom the female age was < 35 years was 37 out of 300 (12.3%). The conclusion was that PICSI augmented treatment for those couples for whom the female was aged 35–37 years by reducing the miscarriage rate from 26.9% of all clinical pregnancies in the ICSI group, to 12.3% in the PICSI group.
FIGURE 16.
Classification tree for live birth outcome among clinically pregnant women in the HABSelect cohort (n = 972).
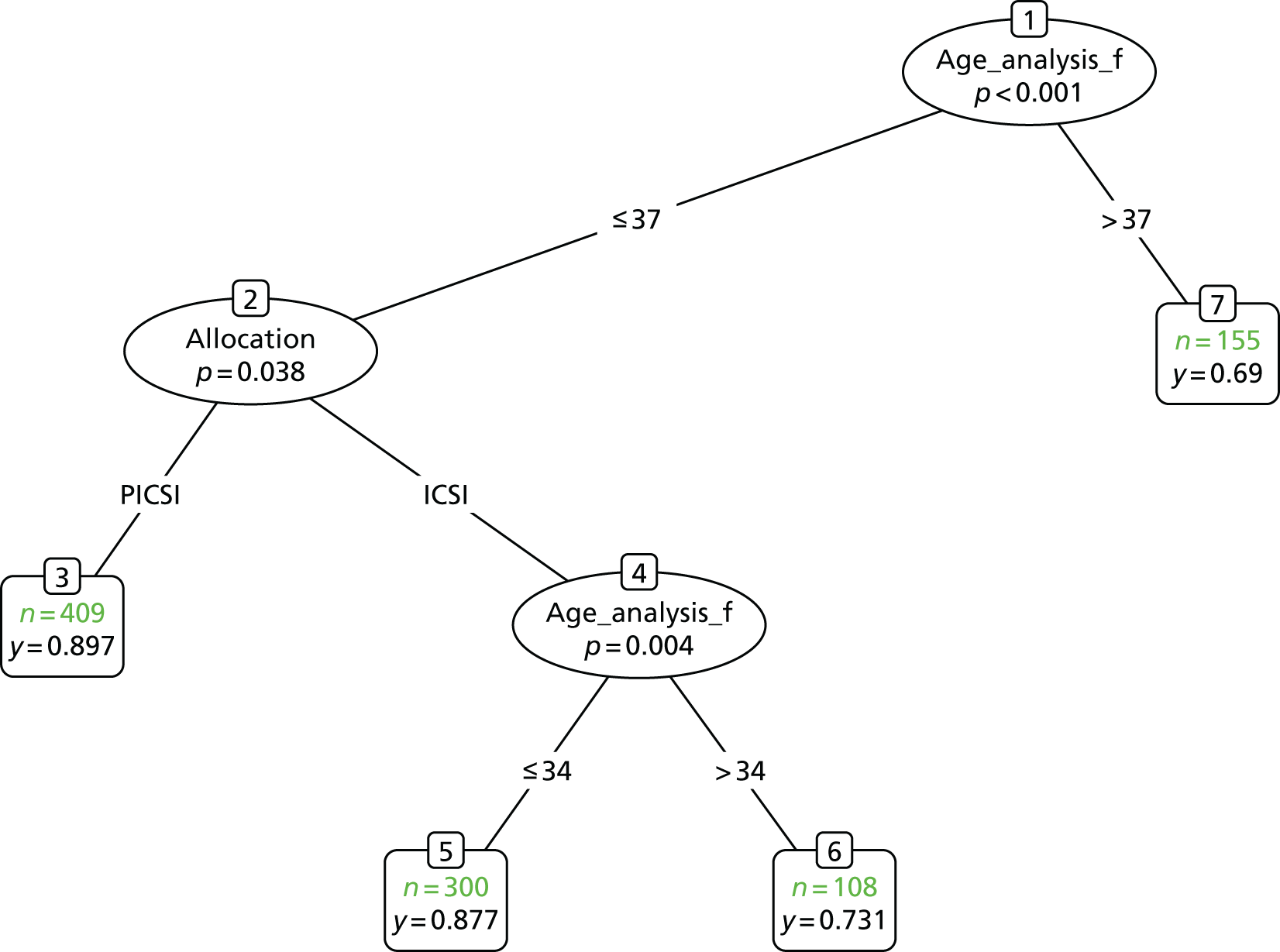
Although 35–37 years appears a restrictive range, this accounted for almost one-quarter (23.0%) of all couples recruited to the HABSelect trial, and 34.3% of those that achieved CP. Fitting a logistic regression achieved similar, albeit not identical, results. There was a significant interaction between age of the female partner and the treatment allocated.
Table 18 gives the table of coefficients for the final model in which non-significant terms were dropped. Individually, female age followed by PICSI + female age were the strongest predictors for miscarriage and PICSI alone was also highly predictive.
With the interaction term it is difficult to interpret the coefficients of the logistic regression specified by Table 17. Therefore, Table 19 gives examples for couples for whom the female partner is either 30 or 37 years of age and the treatment is either ICSI or PICSI.
| Trial arm | Female aged (%) | |
|---|---|---|
| 30 years | 37 years | |
| ICSI | 13.0 | 33.5 |
| PICSI | 12.7 | 11.8 |
Note from Table 19 the clear advantage of PICSI for older women once CP had been established.
The fitted models within the mechanistic cohort for miscarriage dropped all terms relating to the assays. As a consequence, it was possible to use the full trial data set to provide greater power for the models by using more couples. Characteristics of the 2766 HABSelect trial participants are provided in the trial report and are not replicated here. However, the characteristics of couples achieving CP are shown in Table 19.
A classification tree and a logistic regression were fitted as before and the final models are presented in Figure 16 and Table 20, based on 972 clinically pregnant couples. The classification tree and the logistic regression with the trial data reflect the findings from the mechanisic study. Among those who achieved CP, there was a statistically significant interaction between the allocation to treatment and the age of the female partner. The coefficients from Table 11 can be interpreted more easily by first considering the treatments, ICSI and PICSI. With ICSI, there was a decrease in the probability of live birth with age given by the coefficient –0.165. When PICSI was the treatment, the interaction coefficient 0.125 to the age coefficient giving a decline with age as only –0.040.
| Characteristic | Outcome | p-value | |
|---|---|---|---|
| Miscarriage | Live birth | ||
| Number | 156 | 816 | |
| Male partner | |||
| Age (years), mean (SD) | 36.85 (5.63) | 35.37 (5.18) | 0.001 |
| White, n (%) | 121 (77.6) | 642 (78.7) | 0.839 |
| BMI, mean (SD) | 26.62 (3.41) | 27.39 (4.48) | 0.195 |
| Alcohol units, mean (SD) | 7.66 (6.43) | 7.93 (6.74) | 0.725 |
| Sperm concentration (× 106/ml), mean (SD) | 25.82 (31.71) | 23.08 (32.60) | 0.107 |
| Sperm volume (ml), mean (SD) | 2.86 (1.60) | 2.97 (1.53) | 0.416 |
| Motility (%), mean (SD) | 40.22 (18.45) | 39.79 (19.98) | 0.816 |
| Female partner | |||
| Age (years), mean (SD) | 34.63 (4.39) | 32.88 (4.02) | 0.001 |
| White, n (%) | 115 (73.7) | 652 (79.9) | 0.104 |
| BMI, mean (SD) | 24.43 (3.43) | 24.57 (3.47) | 0.641 |
| Alcohol units, mean (SD) | 4.85 (3.27) | 5.08 (4.39) | 0.652 |
| Previous fertility treatment, n (%) | 52 (33.3) | 215 (26.3) | 0.090 |
| Previous natural pregnancy, n (%) | 34 (21.8) | 194 (23.8) | 0.666 |
| Previous miscarriage, n (%) | 20 (12.8) | 111 (13.6) | 0.893 |
| FSH level, mean (SD) | 7.14 (2.73) | 7.02 (2.02) | 0.596 |
| AMH level, mean (SD) | 25.80 (20.82) | 24.49 (17.49) | 0.523 |
| Number of blastocysts, mean (SD) | 1.45 (0.51) | 1.40 (0.53) | 0.368 |
| Assays | |||
| HBS, mean (SD) | 78.59 (20.69) | 72.58 (25.86) | 0.021 |
| Allocated PICSI, n (%) | 60 (38.5) | 425 (52.1) | 0.002 |
Mechanistic conclusions
In the original design of the mechanistic work, HBS linked the assays of DNA integrity and sperm physiology with the clinical trial outcomes using SEM. Hence, the finding that HBS was strongly associated with several indicators of male fertility, including sperm concentration and motility, was reassuring as were the associations between HBS and DNA fragmentation, compaction and fertilisation rate. HBS, however, alongside all measures of DNA integrity, was uninformative with regard to miscarriage or any other clinical outcome except CP, where compaction was a discriminator, particularly among younger women. Like the clinical analysis, the mechanistic analysis indicated that PICSI was protective against miscarriage and that female age was the strongest indication for PICSI efficacy in this regard. The data also showed that PICSI had a small, but significantly negative, impact on fertilisation rates (see Chapter 5, General summary). Although DNA compaction had an influence on CPR, the data were inconclusive in establishing the modus operandi for the beneficial effect of PICSI. The exploratory finding in relation to female age should also be treated with caution but could be studied further as it may have implications for service delivery in future.
Chapter 5 General discussion and conclusions
General summary
HABSelect was designed first and foremost to test whether or not a HA-based sperm selection system embodied by the PICSI dish could improve live birth outcomes (its primary outcome measure). The study included both clinical and mechanistic components that returned overlapping results. The trial itself demonstrated little evidence for an increase in term LBR with PICSI. The 95% CI for the difference in the proportions of couples with a full-term live birth between the PICSI and ICSI arms was –1.1% to 5.5%. For secondary outcomes, the miscarriage rate was significantly reduced in the PICSI arm but no other outcome measure was associated with the intervention.
Mechanistic analysis showed that PICSI had a negative effect on fertilisation rates, which, despite being negated by the expediency of producing multiple embryos, warrants further attention. With regard to the mechanistic underpinning of these outcomes and their progressive developmental context, it was found that higher levels of DNA compaction were associated with conversion of a chemical pregnancy to a CP (regardless of trial arm). The mechanistic analysis showed that female age was the main driver of subsequent miscarriage risk, with PICSI affording some measure of protection. However, although the results of subgroup analysis, carried out as part of the clinical analysis, did not rule out differences in treatment effect by female age, it did not find conclusive evidence that the effect of PICSI on miscarriage differed by female age. Causes of the miscarriages were undetermined but may have arisen from already understood complications of development, including fetal aneuploidy. 87,88 We could not establish a link between DNA fragmentation and pregnancy failure. This failure may have been due to the use of processed sperm throughout the study. However, higher sperm DNA compaction, as determined by AB staining, was weakly associated with CP and HBS was strongly predictive of fertilisation rates.
Clinical trial outcomes
HABSelect found no evidence of differences in full-term LBRs or CPRs, but the same data paradoxically indicated that PICSI helped avoid miscarriage. These seemingly contradictory findings are reconcilable. Absolute risk differences between PICSI and ICSI (per couple randomised) for full-term live birth and miscarriage were more or less equal and opposite (2.2% for full-term live birth; –2.7% for miscarriage). Miscarriage was a relatively uncommon outcome (7.0% in the ICSI group), so the risk difference is more precisely determined (the CI was narrower) for miscarriage than for full-term live birth, thus accounting for its greater statistical significance. Within the limits of confidence observed in this trial, it is possible, for example, that even with a reduction in the number of miscarriages, PICSI led to fewer clinical pregnancies and, therefore, no improvement in LBR.
The HABSelect clinical trial should be seen in the context of its time. Success rates for combined IVF/ICSI procedures in the UK in 2008 (the most recent data available at the time of trial planning were from 2006) averaged 24% per treatment cycle started (all ages3). The most recent data for all UK IVF (HFEA 2014 data4) reported an increase in LBR per cycle started (fresh eggs) to 26.5%. A 22.2% LBR per embryo transfer was also reported4 (includes IVF and IVF/ICSI). These are modest improvements since HABSelect commenced work. The equivalent figures for LBR from HABSelect were 26.3% per couple (PICSI 27.4%, ICSI 25.2%) and 20.3% per embryo transfer (PICSI 21.4%, ICSI 19.2%). The miscarriage rate (per CP) is estimated at 12–15%89 and in HABSelect, the rate overall was 15.9% (PICSI 12.3%, ICSI 19.6%). With respect to LBR, therefore, HABSelect reported similar outcomes to the current UK service as a whole. 4 The fertilisation rate (number of 2PN zygotes per injected egg) in HABSelect was 67.8%, also reflecting the UK service although rates overall were lower with PICSI (66.6%) than with ICSI (69.0%). See Chapter 4, Mechanistic conclusions, for more details.
HABSelect was a study focusing on full-term LBRs. It did not restrict its recruitment strategy to just male infertility, which, with respect to discriminating between the female and male factors in the ART context, remains challenging. We can be reasonably confident that 20–40% of human infertility is attributable to a male factor that is either identifiable (such as obstructive or non-obstructive azoospermia) or in an estimated 30% of all cases, has no obvious cause (idiopathic90). Of the 74% or so of cycles started that fail to produce a live birth outcome, a significant proportion will arise from pregnancies that are non-viable because of a female and/or male genomic factor, such as aneuploidy, estimated from a recent review of 390 German cases in which the frequency of aneuploidy was 61%. 91 Furthermore, HABSelect’s relatively permissive inclusion criteria for females would be unlikely to rule out female factors altogether, indicating that a female effect on trial outcomes was also possible. ICSI itself was serendipitously introduced to treat male infertility92 and its overall success has paradoxically led to far wider adoption such that, in 2017, many clinics located in regions of the world where regulation of the industry is less developed are reducing IVF and expanding ICSI cycles for treatment that does not necessarily require it. 93,94
Of course, ICSI bypasses natural barriers to fertilisation that might otherwise prevent a defective sperm from fertilising the egg, placing some considerable responsibility on the embryologist charged with the task of selecting sperm for injection. In this regard, most clinics, wherever practical, will process raw semen samples to remove (as far as possible) immature, abnormal or damaged sperm and then subjectively use their learned understanding of sperm viability to make the right choice. Preparation, however, often leads to some enrichment of a viable population but may not necessarily exclude damaged sperm altogether. 95,96 As indicated in Chapter 1, damaged sperm includes cells where the paternal genome may be compromised because of poor DNA integrity arising iatrogenically during sample processing (increased fragmentation) or because of intrinsic abnormalities with chromatin packaging that originate within the reproductive tract (abnormal compaction97–99). Such sperm may behave normally in density gradients and appear ‘normal’ by the usual measures of progressive motility and morphology, and so may not be disregarded by the practising embryologist. Non-subjective sperm selection for ICSI, therefore, is of considerable interest to ART practitioners if it can be shown to improve outcomes over and above that routinely achieved by the clinic. In this regard, sperm selection based on HAB had garnered sufficient interest in the ART community to be introduced, and/or experimentally tested in a number of settings including trials of efficacy that led ultimately to HABSelect. 48,100–103
Two commercially developed and available products with different modalities, but both employing HA, have been reported to date. The simpler, liquid state, process uses a highly viscous, soluble form of HA [Sperm Catch (Nidacon, Gothenburg, Sweden) and Sperm Slow (Origio)], which slows mature, motile sperm sufficiently for capture. These products are marketed as a viable and more physiological alternative to PVP although Sperm Slow is not considered as simply a passive retardant of sperm motility. 70 The other product (PICSI) is a solid-state platform formed from HA bonded physically to a plastic surface. PICSI utilises the physiological interaction and binding of motile sperm to the HA with the embryologist choosing only interacting sperm for subsequent injection; as such, PICSI cannot currently be considered as equivalent to or interchangeable with the viscous liquid HA methods. The binding conditions help to ensure that only motile sperm with high vitality are used for ICSI and the available research data on the properties of HA-binding sperm support this modus operandi. 49,104–107
HABSelect had to make use of a UK- and MHRA-registered product/procedure in the clinical context, and although solid or liquid-based products could have been used, PICSI was chosen because it was thought to be more physiologically relevant to the natural sperm–HA solid-state interaction that takes place in the female reproductive tract and because it was more straightforward to train operatives in its use to a uniform standard among multiple clinics. PICSI had also been more widely reported in the context of CPR and miscarriage. 57,72,108 In any event, the effects being investigated and reported were from sperm binding to HA (in the intervention arm). It was not a test of the product itself.
The clinical aspects of HABSelect constituted the largest trial of a HA-based sperm selection process conducted to date, with 2772 couples randomised equally into either the intervention (PICSI) or standard control (ICSI) arm. Clinical practice over 16 independent participating centres, alongside the need to make participation as straightforward as possible, led us to permit clinics to continue using their usual (normally PVP) sperm-holding procedure for the control and intervention arms. This strategy at first sight is at variance to the stated intention of our original objective 1 (see main protocol71), which described PICSI as a substitute for PVP. In practice, however, embryologists routinely transfer the sperm they collect under the microscope into ‘holding’ PVP prior to tail breakage and are more comfortable doing so. In hindsight, although there is no good evidence for PVP toxicity, this concession eliminated a potentially selective advantage in just one arm of excluding PVP that may have confounded interpretation of the outcomes.
In the final analysis, PICSI registered a small but not statistically significant increase in LBR at ≥ 37 weeks’ gestation of 2.2%, from 25.2% to 27.4% of cycles (OR 1.12, 95% CI 0.95 to 1.34; p = 0.18). The only other trial of similar size57 did not report LBR; however, in a trial of PICSI with 200 couples, Mokánszki et al. 108 reported an overall 3% improvement in LBR of 45% (PICSI) versus 42% (ICSI) cohorts, which was similar to our outcome and also not significant. However, following stratification of couples by HBS, a wider and statistically significant increase in LBR from 27% (ICSI) to 49% (PICSI) was reported in their ≤ 60% HBS subgroup. 108 A substantial but non-significant obverse rise in LBR from 42% (PICSI) to 58% (ICSI) was also noted in the > 60% subgroup, suggesting that HA was beneficial to couples if the male had a poorer initial semen profile but unhelpful or possibly even detrimental to those with a more normal semen profile (also suggested by Worrilow et al. 57).
The data presented here from this current trial on the reduction in miscarriage in relation to HBS within the PICSI arm agree with the Worrilow et al. 57 findings. The significantly raised LBR reported by Mokánszki et al. 108 was likely to have been caused by the mode of reporting as a percentage of clinical pregnancies rather than cycles and in this regard, unlike HABSelect, the CPR rate for this study was significantly higher in the PICSI cohort, irrespective of HBS. The studies by Worrilow et al. 57 and Mokánszki et al. 108 used HBS stratification to help determine how couples were treated, with the latter including only couples with an initial lower HBS in the PICSI arm and not randomising to treat. Both studies reported significant decreases in miscarriage.
HABSelect neither stratified couples by HBS before randomisation nor allocated them for randomisation by HBS. The idea was to avoid introducing a potential bias arising from preselection of couples on this basis. HABSelect also scored prepared (processed) rather than unprepared (liquefied ejaculate) samples. Despite processing, where better populations of sperm would be expected, samples could still be stratified by HBS with a score of < 25% binding forming the smallest subcategory, > 65% binding, by far the largest, with an intermediate subcategory (> 25% and ≤ 65% binding) having an intermediate number of couples. Unlike Worrilow et al. 57 and Mokánszki et al. ,108 with equivalent subcategories, where benefits of PICSI seemed to lie with couples having a lower HBS, subgroup analysis of our primary outcome found no significant differences between PICSI and ICSI arms for any of the HBS subcategories. There was also no statistically significant association between HBS and miscarriage rates in HABSelect, although numbers in the intermediate and low scoring groups were too low for statistical accuracy. As with Worrilow et al. ,57 a trend for PICSI favouring those with low HBS was also apparent.
A more recent study randomising 156 couples into PICSI or ICSI arms109 reported no differences in CPR or LBR but a statistically non-significant reduction in miscarriage in the PICSI cohort was reported. The relatively small sample size may have hindered statistical testing. HABSelect, together with the Worrilow et al. 57 and Mokánszki et al. 108 studies, and the smaller studies described above,70,108–110 are therefore either unsupportive or ambiguous with regard to a beneficial effect of PICSI on live birth outcomes, but they are in greater agreement on the reported reduction in miscarriage, one of the most devastating outcomes for an expectant couple. With the exception of HABSelect and Worrilow et al. ,57 sample sizes were too low for good confidence and/or randomisation was not part of the protocol. The number-needed-to-treat111 value for preventing miscarriage in HABSelect was 37 participants, which was low enough to consider PICSI under certain circumstances even with the attendant reduction in fertilisation rates.
Mechanistic outcomes
Introduction
HABSelect was designed to shed light on the relationships between clinical outcomes and indicators of sperm quality including HBS and DNA integrity, the latter measured by assays of two key variables of DNA integrity, namely fragmentation [AO, alkaline comet (AC) and TUNEL] and compaction (halo and AB). As with the comet assay, halo is based on decondensation of sperm chromatin78 but retains the architectural relationship between the condensation or compaction state by reference to the halo area and the ratio of the halo to nucleoid area. Hence, halo can be argued as overlapping and linking the two variables. We made this assumption with our SEM (see Figure 14), which was used to integrate the associations between our clinical and mechanistic outcomes followed by classification tree and linear regression analysis aimed at identifying specific effects. HABSelect was unique in bringing together multiple assays in a single study and attempting to link them with clinical outcomes in this way. We found that HBS was associated with both variables, with physiological aspects of sperm vitality and with fertilisation rates, but only DNA compaction showed a weak association with a clinical outcome (CPR). PICSI allocation reducing and older females increasing the risk of miscarriage were by far the strongest outputs of the classification tree and regression analyses. Hitherto, inferences on these relationships relied on small distinct studies and cohorts or on their systematic review and meta-analysis. 14,15,36,112 In the original SEM, HBS linked DNA integrity with clinical outcomes and, as HBS is thought to be a useful pre-treatment screening test, it is worth further consideration in its own right.
As indicated by the increase in forward progressive sperm motility to within WHO reference values following sample preparation,5 HABSelect confirmed that processed samples contained a more homogeneously viable population of sperm than would be expected in raw semen. Indeed, both HABSelect and Worrilow et al. 57 reported similar proportions of HBS (≥ 65%) at 72% and 74%, respectively, in their processed samples. These and data from elsewhere104 indicate that sample preparation by DGC enriched for a ‘better’ sperm population that is more able to bind HA. Indeed, the anticipated correspondence between HBS and markers of sample/sperm quality was confirmed for both original sample sperm concentration and for prepared sample progressive forward motility. At an individual assay level, HBS correlated with some measures of sperm DNA integrity, with TUNEL providing the highest level of correspondence, followed by AO and comet assays. Our findings on the relationships between HBS and sperm concentration and motility agreed with those of Mokánszki et al. 108 and Worrilow et al. 57 and we can reasonably assume, therefore, that, despite the limited sample size with data for all assays (n = 131), the associations we uncovered between sperm vitality and DNA integrity could be extended to these and other studies that examined their relationship with HBS. 49,70,104,113 In the spirit of the SAPm (see Report Supplementary Material 2), which sought to explore mechanistic outcomes in relation to clinical outcomes, results are discussed in developmental order and context.
Fertilisation
Having shown that HBS correlated (albeit weakly) with the assays of DNA integrity, their poor correlation with each other was noteworthy. The most likely explanation is that the assays measured different forms of DNA integrity from double-stranded DNA breaks only (TUNEL114) to both single (AO49) and single/double-stranded breaks (comet115) and histone retention (DNA compaction, AB116). Few samples, perhaps, display more than one predominant anomaly, hence the poor interassay correlations. Nevertheless, although HBS predicted both DNA integrity and sperm viability, regression showed that fertilisation rates were significantly associated with HBS (increasing), female age (decreasing) and the intervention (decreasing). Considering that sperm were being directly injected into eggs, it is likely that the main arbiter of successful fertilisation was gamete quality, which HBS has been shown to predict for sperm relative to their concentration and motility. 104,106,108 There are disagreements, however, on the assay’s ability to screen for improved clinical outcomes. 117 Fertilisation rates for the trial cohort were similar to the rates reported by Majumdar and Majumdar109 but lower than those reported by Worrilow et al. 57 and by Parmegiani et al. ,102 and was significantly lower in the PICSI arm than the ICSI arm (RR 0.963; p < 0.001). This unwanted PICSI effect was not restricted to the mechanistic subgroup as it applied to the trial cohort as a whole. There are several potential explanations. One possibility is that the mechanical act of lifting adherent sperm from the PICSI plate risked damaging them, perhaps compounded by a toxic effect of PVP. 118 However, as sperm tails are frequently crushed in PVP, this explanation seems unlikely. The longer time taken to complete the PICSI intervention is also a possible cause. There is certainly no indication that HA is itself toxic to eggs although the possibility cannot be excluded. When reported,57,108,109 fertilisation rates were not significantly different between trial arms. It would be interesting to return to those published studies and look again at their raw data, if available. Our data showed that PICSI selection offered no advantage so early in the developmental process. Its small but significant inhibitory effect on fertilisation had no bearing on outcomes overall as it did not affect the numbers of embryo transfers between the two arms.
Clinical pregnancy
HABSelect data broken down by classification tree analysis returned female age as the most significant discriminator for CP, which was perhaps not surprising in view of the widely acknowledged adverse effect of female age on reproductive success. Sperm DNA compaction was the second discriminator with a significant effect on pregnancy rates in the younger age group (44% for poor vs. 64.5% for good compaction).
Human sperm DNA packaging and compaction is achieved predominantly by protamines, although a small fraction of histones remain, which are thought to package regions of developmental importance. 31,33 Several reports have linked excess histones (which AB stains) associated with poor DNA compaction to poor ART outcomes, related possibly to abnormal relative abundances of the two main protamines found in human sperm. 40,119–121 In a study on 165 semen samples from 90 infertile patients with a high proportion of oligozoospermia and low motility compared with 75 control donors, Hammadeh et al. 122 reported significantly poorer compaction in the infertile group. Ovári et al. 76 reported higher rates of aneuploidy in poorly compacted sperm. These and other reports have been linked to low sperm maturity in relation to high cytoplasmic retention, a feature of sperm abnormality. 46,123,124 AB was also shown to discriminate between sperm isolated from native semen (mixed compaction) and sperm pelleted following DGC (good compaction), similar to the processed sperm used in HABSelect125 (and see Sakkas et al. 43 and Torabi et al. 104). In relation to achieving a CP, compaction defects, when relevant, seemed not to be offset by PICSI, which offered no advantage. With age being the most important discriminator, the most prosaic explanation for the CPR outcome is that PICSI did not discriminate sperm with packaging defects that were picked equally between the trial arms. HABSelect is the only prospective study to date reporting on the relationship between sperm DNA compaction and a clinical outcome in ART.
Miscarriage
Classification tree analysis revealed that female age was the primary predictor of miscarriage (31% in the > 37 years age group). After age, the allocation was most predicative with PICSI having the lowest rate of miscarriage (10%). Interestingly, with ICSI, female age was again predictive with a 27% miscarriage rate in the older (34–37 years) age group compared with 12% in the younger (≤ 34 years) age group. Regression analysis supported these results. The PICSI effect could not be explained by differences in DNA compaction or fragmentation at this stage, as these were broadly uninformative and so excluded from the analysis. Even a wider examination of the miscarriage subgroup within the mechanistic cohort compared with full-term live births failed to find a significant difference with the assays used. HBS could not discriminate unambiguously between these later clinical outcomes and measures of sperm DNA integrity results reflected this finding.
The failure to establish a link between the trial’s clinical outcomes and sperm DNA integrity (with the possible exception of CP) begs explanation. HABSelect chose to assay processed, pelleted sperm from post-centrifuged samples, rather than sperm from the original raw semen as this is one of the most common semen-processing procedures undertaken by ART clinics worldwide and these sperm represented the population used for ICSI/PICSI. Pelleted sperm, however, are rarely representative of the original ejaculate population, which contains a mixed population of viable and non-viable cells including non-germ cells. 5 It is not straightforward to compare our results for DNA integrity assays with those reported by others as they differ greatly with respect to sperm processing (often not disclosed), assay deployment and even implementation. For these reasons, systematic reviews with data meta-analyses can be more helpful, although distinguishing clearly between processed and unprocessed semen samples remains a problem36 and/or data from processed ejaculates are excluded. 126 The most recent and informative meta-analysis, by Cissen et al.,127 of 30 studies that included sperm chromatin structure assay (comparable with but not equivalent to our slide-based AO assay), TUNEL, halo and comet assays, reported a poor prediction for CP after IVF or ICSI regardless of sperm processing and the assay used. An earlier report of Collins et al. 128 drew a similar conclusion with reports using TUNEL and sperm chromatin structure assay.
In retrospect and in relation to the differing miscarriage rates, it might have been helpful, as originally planned, to have examined a selection of samples taken from the interface regions of post-centrifuged samples where immature sperm and other non-germ cells accumulate. 95 However, owing to time constraints associated with the clinical imperative, which always (and correctly) came first, it proved logistically impractical to do so. What the data suggest is that sperm processed for ART by recommended procedures5 are unlikely to be informative with regard to male fertility by way of sperm DNA fragmentation, probably because processing effectively removes most of the ‘informative’ sperm from the population. Quid pro quo, HABSelect’s data appear to confirm the efficacy of semen washing by differential DGC. 43,104,129 The data also inform the debate on the utility of measuring sperm DNA fragmentation in the clinical context, in which disagreements are common and the issue of the relative merits of different assays as well as their differing reporting criteria are all contentious. 36,83,130–132 The American Society for Reproductive Medicine’s 2013 position paper was generally not supportive of their introduction to the clinic,133 but others argued that this recommendation was short-sighted134 or took a softer position, indicating that further research was needed. 135,136 The many meta-analyses of efficacy, some supporting and some not supporting the use of DNA fragmentation assays, also demonstrate the continuing disagreements and general lack of consensus. 15,126,127,137
Limitations
Complete-case analysis
There is a major limitation concerning complete-case analysis. 138 For the analyses within the mechanistic study to be valid, the assumption of missing (data) completely at random needs to hold. Then the subset of couples for which full data are available will be representative of the whole study. Judgement was required here. The selection of samples that were sent for assay was a matter of convenience and, although they came from a randomised cohort balanced (as far as possible) between trial arms, it might not be regarded as completely random. There were a few samples for which the available volume was either too small or had too few sperm, restricting the number of assays that could be carried out. In these cases, assay choice, as far as practicable, followed the hierarchy of sampling (see Figure 5) but was somewhat haphazard and might be regarded as non-random. There does, however, remain the issue that some bias might have been introduced by using complete-case analysis only because it limited the sample size for the mechanistic analysis. No attempt was made to use imputation (i.e. multiple imputation), as the proportion of missing assay values in the mechanistic analysis was too high (see Appendix 2). When the full trial data were used for the analysis of live birth versus miscarriage, 972 out of 978 clinical pregnancies were examined so that the complete-case analysis would not have been influenced by the small number missing.
Linearity
For the structural equation modelling, which defined fragmentation and compaction as latent constructs, the linearity of the assay results was assumed. That is each of the assays was assumed to increase linearly with fragmentation and/or with compaction. Graphical plots revealed that this assumption was justified, and exploration with splines revealed that linear terms alone were sufficient given the marked scatter in the relationships. Had the assays been less noisy, then inclusion of non-linear terms might have changed how fragmentation and compaction were defined in all subsequent analyses. The regressions for fertilisation, CP and miscarriage/live birth also assumed linearity of the terms for age, HBS, fragmentation and compaction. The classification trees offer a way to check the validity of these assumptions. As there was close agreement from two separate analytical approaches, the linearity assumptions were supported.
Mechanistic summary
The mechanistic analysis explored different stages of the developmental process outside a formal hypothesis-testing framework and found potential contributions of sperm DNA compaction, HBS, allocation and female age to clinical outcomes. There could have been bias, but, from the complete-case analysis and from the assumptions of linearity, there were differences in the populations studied at each developmental stage of the process (the trial itself considered only the process as a whole), and different outcomes were used. In agreement with two earlier studies,57,108 significantly and substantially reduced miscarriage rates were reported. One small study reported no protective effect110 and another reported no efficacy with any clinical outcome. 109 Sperm HBS has been reported to be associated with markers of sperm viability, including concentration and motility,108 as was the case with HABSelect. Similarly, most reports indicate that HA-selected sperm are more viable in terms of lower levels of DNA fragmentation, higher levels of compaction and normal morphology,29,49,55,56,102,139 with one study reporting a lack of association with morphology examined by high-powered magnification. 108 HABSelect was the first sufficiently substantial study to attempt a linkage between the clinical outcomes reported previously, with the improved DNA integrity status reported of the sperm in several of those same studies. 48,102,139 We were, however, unable to detect a significant difference between clinical outcomes (including miscarriage) and any aspect of sperm DNA integrity, except DNA compaction, which was weakly predictive of CP. However, work on HABSelect sample mechanistic analysis is still ongoing and future updates can be expected. Our compaction results accord with several studies using or reporting AB staining. 116,125,140–142 In a recent meta-analysis, which included many of the original studies reported here, Beck-Fruchter et al. 112 concluded that routine application of a HA binding step in ICSI was not warranted as it did not influence clinical outcomes. Miscarriage, however, was not considered.
The influence of sperm DNA fragmentation on miscarriage rates in ART is controversial, with a consensus for a deleterious effect on IVF but less so for ICSI. 14,15,36,126,137 The positive effect of PICSI may have been related to sperm DNA integrity, but the residual samples used to test this possibility were generally uninformative, a factor that alongside the use of swim-up sperm, which also have improved measures of DNA integrity,143 may have affected other studies reporting similar findings with ICSI. 36,137 We can speculate that if miscarriage-related DNA integrity was indeed the compromising feature reduced or removed by PICSI selection and the processed samples were incompletely cleared of these compromised sperm, they may have been present, but in insufficient numbers to affect the DNA integrity scoring overall. Moreover, samples tested for mechanisms may not have included the clinically poorest, as these would have been entirely spent by the treatment and so unavailable for subsequent analysis. Unfortunately, it is not currently possible to test for DNA integrity the same sperm that are used for ICSI. Another consideration is that the freezing protocol used in HABSelect was non-optimal in that the cryoprotectant (SpermFreeze, Origio) was added to prepared rather than neat semen. Considering the wide range of values we obtained with most assays in HABSelect, we believe that any iatrogenic damage caused by a freeze–thaw cycle had only a minimal effect on DNA integrity. 144 Nonetheless, we think future studies should consider examining original, unprocessed ejaculates (fresh or frozen–thawed).
HABSelect final conclusions
The HABSelect trial found little evidence for an effect of PICSI on full-term LBRs, strong evidence for reduced miscarriage rates and no differences in either CPRs or preterm live births. The clinical analysis also included a predefined interaction term designed to investigate differences in treatment effect according to age. There was little evidence, however, supporting a difference in treatment effect on full-term live birth by age group.
Based on the results of HABSelect, where there was insufficient evidence to conclude an improvement in the primary outcome, further studies are required to confirm the differences in miscarriages found in this trial and to determine whether or not they can lead to corresponding improvements in LBRs. The mechanistic analysis indicated that PICSI preferentially benefited older women (≥ 35 years), who are a rising demographic in ART and made up 45% of HABSelect’s study population. In order to provide more conclusive evidence for this effect, future work might be advised to more formally explore the interaction between age and treatment in clinical outcomes of ART among older couples.
Acknowledgements
Clinical embryology
On behalf of the HABSelect grant holders and associated coinvestigators, we would like to thank the following for supporting the HABSelect study.
Dr Karen Thompson, for Leeds Fertility, Seacroft Hospital, Leeds Teaching Hospitals NHS Trust.
Dr David Wells for the Aberdeen Fertility Centre, The Aberdeen Maternity Hospital.
Dr Gregory Horne for the Department of Reproductive Medicine, Old St Mary’s Hospital, Central Manchester University Hospitals NHS Foundation Trust.
Dr Bonnie Collins for the Centre for Reproductive Medicine at Barts and the London NHS Trust.
Dr Kathryn Whalley and Mrs Ellen Drew for The Assisted Conception Unit, Ninewells Hospital, NHS Tayside, Dundee.
Dr Jane Blower, for the Leicester Fertility Centre, Leicester Royal Infirmary.
Dr Arasaratnam Srikantharajah, for the Homerton Fertility Centre, Homerton University Hospital NHS Trust, London.
Professor Geraldine Hartshorne and Mr Ben Lavender for the Centre for Reproductive Medicine, University Hospitals Coventry and Warwickshire NHS Trust, Coventry.
Ms Rebecca Lunt for The Hewitt fertility Centre, Liverpool Women’s Hospital NHS Foundation Trust.
Dr Marta Jansa-Perez for the Wolfson Fertility Centre, Hammersmith Hospital, London.
Dr Karen Turner and Dr Aysha Bevan for the Oxford Fertility Unit.
Mr Kevin McEleney for the Newcastle Fertility Centre at Life.
Dr Sue Pickering for the Edinburgh Fertility and Reproductive Endocrine Centre, Edinburgh Royal Infirmary.
The Embryology team at Sheffield Hallam.
The Embryology team at Birmingham Women’s Fertility Clinic.
Virgina Bolton at the Guy’s and St Thomas’ NHS Trust.
The technical support teams: Leeds, Birmingham and Belfast
University of Leeds Institute of Cardiovascular and Metabolic Medicine, Leeds, UK. Riitta Partanen (halo), Forough Torabi (AO), Alex Hargreaves (AB).
Birmingham Women’s Fertility Centre, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, UK. Lorraine Frew (TUNEL), Sofia Tsagdi (TUNEL).
Queen’s University, Belfast and Examen Ltd, Belfast, UK. Rachael Hutton (comet and AB), Martin Lawlor (comet and AB).
Recruitment and consenting
Pauline McBeath for the Aberdeen Fertility Centre.
Alli Rossie and Lilith Loncke for the Centre for Reproductive Medicine, Barts and the London NHS Trust.
Chloe O’Hara, Paula Trinham and Fiona Beale for the Birmingham Women’s Hospital.
Debbie Bullen and Fiona Oldfield for the Coventry Centre for Reproductive Medicine.
Evelyn Barrett for Assisted Conception Unit, Ninewells Hospital, Dundee.
Sara Barnett for the IVF Unit at Hammersmith Hospital, London.
Merve Dilgil for the Homerton Fertility Centre, Homerton Hospital, London.
Julie Glanville for the Leeds Centre for Reproductive Medicine.
Catherine Clarkson and Caroline Bushby for the Leicester Fertility Centre.
Deborah Coppin (Stephenson), Cathy Bowles and Viv Sutton for the Hewitt Fertility Centre, Liverpool.
Claudette Wright, Lucy Dwyer and Stephanie Bateman for The Department of Reproductive Medicine, Manchester.
Elizabeth Taylor, Fiona Newton, Kirstin Johnson for the Centre for Reproductive Medicine and Fertility, Sheffield.
Ginny Mounce for the Oxford Fertility Unit.
Kayleigh Lennox, Maria Nesbitt and Allison Simpson for the Newcastle Fertility Centre.
Jane Clarke for the Edinburgh Fertility and Reproductive Endocrine Centre.
The HABSelect team would also like to thank Maggie Shergill at the National Institute for Health Research (NIHR) for her enthusiastic and ever-helpful support of our efforts while she was our project and liaison officer with EME.
Contributions of authors
Dr Jackson Kirkman-Brown (Mechanistic Lead) was instrumental in helping to draft the original proposal to NIHR EME, instrumental to the study design and conduct of the mechanistic work at Birmingham and to the HABSelect trial overall and he also contributed to the final report.
Professor Sue Pavitt (Trials Support Lead) was closely involved in the trial design and helped to write the protocol as well as bringing the HABSelect team and health service professionals together. She also contributed to and commented on the final report.
Mr Yacoub Khalaf (Clinical Lead) provided invaluable advice on all clinical aspects of the HABSelect trial design, including continual advice and feedback as the trial progressed and during the writing up of its key clinical outcomes, and also contributed to the protocol design and the final report.
Professor Sheena Lewis (Mechanistic Lead) was instrumental in helping to draft the original proposal to NIHR EME, instrumental to the study design and conduct of the mechanistic work at Belfast and to the HABSelect trial overall. She also contributed to the final report.
Dr Richard Hooper (Lead Clinical Statistician) was involved in the statistical planning and analysis for clinical trial aspects of HABSelect and also contributed to the final report.
Professor Siladitya Bhattacharya (Clinical Lead) provided invaluable advice on all clinical aspects of the HABSelect trial design, including continual advice and feedback as the trial progressed and during the writing up of its key clinical outcomes, and also contributed to the protocol design and the final report.
Mr Arri Coomarasamy (Clinical Lead) provided invaluable advice on all clinical aspects of the HABSelect trial design, including continual advice and feedback as the trial progressed and during the writing up of its key clinical outcomes, and also contributed to the protocol design and the final report.
Mrs Vinay Sharma (Clinical Lead) assisted with the protocol development for HABSelect and commented on the final report.
Professor Daniel Brison (Science Advisor) as a senior advisor on clinical embryology helped draft the original proposal to NIHR EME programme and was involved in the design of the follow-up protocol and to the final report.
Dr Gordon Forbes (Clinical Statistician) was involved in the statistical planning and analysis for clinical trial aspects of HABSelect and also contributed to the final report.
Professor Robert West (Mechanistic Statistician) was involved in the statistical planning and analysis for the mechanistic aspects of HABSelect and also contributed to the final report.
Professor Allan Pacey (Scientific Advisor) provided professional and media-level support to HABSelect from the outset, including trial and protocol design, and offered critical reading of the final report.
Mrs Kate Brian (Patient Public Involvement Representative) helped with the initial trial design for the study as both a patient participant and key interface with Infertility UK, one of the main advocates representing patients’ interest in fertility treatment. Mrs Brian also worked closely with and fed back to the Trial Steering Committee.
Ms Rachel Cutting (Embryology Advisor) was involved in designing key aspects of the trial protocol, including case reports and standard operating procedures. Dr Cutting represents the main body of embryologists working with HABSelect and without whom the study would not have been possible.
Dr Virginia Bolton (Consultant Embryologist) made a substantial contribution to the clinical research effort with over one-quarter of all couples treated under her guidance and jurisdiction.
Dr David Miller (Chief Investigator) was responsible for the original idea behind HABSelect and for drafting the final report and associated materials and was responsible overall for the conduct and management of HABSelect.
Publications
Torabi F, Binduraihem A, Miller D. Sedimentation properties in density gradients correspond with levels of sperm DNA fragmentation, chromatin compaction and binding affinity to hyaluronic acid. Reprod Biomed Online 2017;34:298–311.
Torabi F, Bogle OA, Estanyol JM, Olivia R, Miller D. Zona pellucida-binding protein 2 (ZPBP2) and several proteins containing BX7B motifs in human sperm may have hyaluronic acid binding or recognition properties. Mol Hum Reprod 2017;23:803–16.
Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S, et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial. Lancet 2019;393:416–22.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril 2011;95:124-8. https://doi.org/10.1016/j.fertnstert.2010.05.055.
- National Institute for Health and Care Excellence (NICE) . Fertility Problems: Assessment and Treatment 2017. www.nice.org.uk/guidance/cg156 (accessed 2017).
- A Long Term Analysis of the HFEA Register Data 1991–2006. London: HFEA; 2008.
- Fertility Treatment 2014: Trends and Figures. London: HFEA; 2016.
- WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: WHO Press; 2010.
- Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril 2001;75:674-7. https://doi.org/10.1016/S0015-0282(00)01796-9.
- Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril 2011;95:652-7. https://doi.org/10.1016/j.fertnstert.2010.08.019.
- Gu LJ, Chen ZW, Chen ZJ, Xu JF, Li M. Sperm chromatin anomalies have an adverse effect on the outcome of conventional in vitro fertilization: a study with strictly controlled external factors. Fertil Steril 2009;92:1344-6. https://doi.org/10.1016/j.fertnstert.2009.03.031.
- Lewis SE, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res 2005;322:33-41. https://doi.org/10.1007/s00441-005-1097-5.
- Miciński P, Pawlicki K, Wielgus E, Bochenek M, Tworkowska I. The sperm chromatin structure assay (SCSA) as prognostic factor in IVF/ICSI program. Reprod Biol 2009;9:65-70. https://doi.org/10.1016/S1642-431X(12)60095-3.
- Simon L, Lewis SE. Sperm DNA damage or progressive motility: which one is the better predictor of fertilization in vitro?. Syst Biol Reprod Med 2011;57:133-8. https://doi.org/10.3109/19396368.2011.553984.
- Sousa AP, Tavares RS, Velez de la Calle JF, Figueiredo H, Almeida V, Almeida-Santos T, et al. Dual use of Diff-Quik-like stains for the simultaneous evaluation of human sperm morphology and chromatin status. Hum Reprod 2009;24:28-36. https://doi.org/10.1093/humrep/den365.
- Zini A, Libman J. Sperm DNA damage: importance in the era of assisted reproduction. Curr Opin Urol 2006;16:428-34. https://doi.org/10.1097/01.mou.0000250283.75484.dd.
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. https://doi.org/10.1093/humrep/des261.
- Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998-1005.e8. https://doi.org/10.1016/j.fertnstert.2014.06.033.
- Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod 1996;2:46-51. https://doi.org/10.1093/molehr/2.1.46.
- Fulka H, Langerova A, Barnetova I, Novakova Z, Mosko T, Fulka J. How to repair the oocyte and zygote?. J Reprod Dev 2009;55:583-7. https://doi.org/10.1262/jrd.09-085H.
- Hirst R, Gosden R, Miller D. The cyclin-like uracil DNA glycosylase (UDG) of murine oocytes and its relationship to human and chimpanzee homologues. Gene 2006;375:95-102. https://doi.org/10.1016/j.gene.2006.02.030.
- Jaroudi S, SenGupta S. DNA repair in mammalian embryos. Mutat Res 2007;635:53-77. https://doi.org/10.1016/j.mrrev.2006.09.002.
- Aoki VW, Moskovtsev SI, Willis J, Liu L, Mullen JB, Carrell DT. DNA integrity is compromised in protamine-deficient human sperm. J Androl 2005;26:741-8. https://doi.org/10.2164/jandrol.05063.
- Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, et al. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod 2003;69:211-17. https://doi.org/10.1095/biolreprod.102.015115.
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. https://doi.org/10.1016/j.fertnstert.2009.10.046.
- Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod 2008;23:2663-8. https://doi.org/10.1093/humrep/den321.
- Bhattacharya C, Aggarwal S, Kumar M, Ali A, Matin A. Mouse apolipoprotein B editing complex 3 (APOBEC3) is expressed in germ cells and interacts with dead-end (DND1). PLOS ONE 2008;3. https://doi.org/10.1371/journal.pone.0002315.
- Carrell DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, Erickson L, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl 2003;49:49-55. https://doi.org/10.1080/01485010290099390.
- Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SE. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod 2010;25:1594-608. https://doi.org/10.1093/humrep/deq103.
- A long-term analysis of the HFEA Register data (1991–2006). 2008.
- Access Fertility . IVF Cost Calculator n.d. www.accessfertility.co.uk/ivf-cost-calculator/ (accessed November 2018).
- Huszar G, Jakab A, Sakkas D, Ozenci CC, Cayli S, Delpiano E, et al. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod Biomed Online 2007;14:650-63. https://doi.org/10.1016/S1472-6483(10)61060-7.
- Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, et al. Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Res 2009;19:1338-49. https://doi.org/10.1101/gr.094953.109.
- Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010;139:287-301. https://doi.org/10.1530/REP-09-0281.
- Saida M, Iles D, Elnefati A, Brinkworth M, Miller D. Key gene regulatory sequences with distinctive ontological signatures associate with differentially endonuclease-accessible mouse sperm chromatin. Reproduction 2011;142:73-86. https://doi.org/10.1530/REP-10-0536.
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature 2009;460:473-8. https://doi.org/10.1038/nature08162.
- Gardiner-Garden M, Ballesteros M, Gordon M, Tam PP. Histone- and protamine-DNA association: conservation of different patterns within the beta-globin domain in human sperm. Mol Cell Biol 1998;18:3350-6. https://doi.org/10.1128/MCB.18.6.3350.
- Gatewood JM, Cook GR, Balhorn R, Schmid CW, Bradbury EM. Isolation of four core histones from human sperm chromatin representing a minor subset of somatic histones. J Biol Chem 1990;265:20662-6.
- Zini A. Are sperm chromatin and DNA defects relevant in the clinic?. Syst Biol Reprod Med 2011;57:78-85. https://doi.org/10.3109/19396368.2010.515704.
- Boiteux S, O’Connor TR, Lederer F, Gouyette A, Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem 1990;265:3916-22.
- Andrabi SM. Mammalian sperm chromatin structure and assessment of DNA fragmentation. J Assist Reprod Genet 2007;24:561-9. https://doi.org/10.1007/s10815-007-9177-y.
- Aoki VW, Emery BR, Liu L, Carrell DT. Protamine levels vary between individual sperm cells of infertile human males and correlate with viability and DNA integrity. J Androl 2006;27:890-8. https://doi.org/10.2164/jandrol.106.000703.
- Castillo J, Simon L, de Mateo S, Lewis S, Oliva R. Protamine/DNA ratios and DNA damage in native and density gradient centrifuged sperm from infertile patients. J Androl 2011;32:324-32. https://doi.org/10.2164/jandrol.110.011015.
- Kierszenbaum AL. Transition nuclear proteins during spermiogenesis: unrepaired DNA breaks not allowed. Mol Reprod Dev 2001;58:357-8. https://doi.org/10.1002/1098-2795(20010401)58:4<357::AID-MRD1>3.0.CO;2-T.
- Nasr-Esfahani MH, Salehi M, Razavi S, Anjomshoa M, Rozbahani S, Moulavi F, et al. Effect of sperm DNA damage and sperm protamine deficiency on fertilization and embryo development post-ICSI. Reprod Biomed Online 2005;11:198-205. https://doi.org/10.1016/S1472-6483(10)60959-5.
- Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, et al. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod 2000;15:1112-16. https://doi.org/10.1093/humrep/15.5.1112.
- Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, et al. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat Genet 2001;28:82-6. https://doi.org/10.1038/88313.
- Oliva R. Protamines and male infertility. Hum Reprod Update 2006;12:417-35. https://doi.org/10.1093/humupd/dml009.
- Huszar G, Ozenci CC, Cayli S, Zavaczki Z, Hansch E, Vigue L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil Steril 2003;79:1616-24. https://doi.org/10.1016/S0015-0282(03)00402-3.
- Park CY, Uhm SJ, Song SJ, Kim KS, Hong SB, Chung KS, et al. Increase of ICSI efficiency with hyaluronic acid binding sperm for low aneuploidy frequency in pig. Theriogenology 2005;64:1158-69. https://doi.org/10.1016/j.theriogenology.2005.01.010.
- Parmegiani L, Cognigni GE, Ciampaglia W, Pocognoli P, Marchi F, Filicori M. Efficiency of hyaluronic acid (HA) sperm selection. J Assist Reprod Genet 2010;27:13-6. https://doi.org/10.1007/s10815-009-9380-0.
- Yagci A, Murk W, Stronk J, Huszar G. Spermatozoa bound to solid state hyaluronic acid show chromatin structure with high DNA chain integrity: an acridine orange fluorescence study. J Androl 2010;31:566-72. https://doi.org/10.2164/jandrol.109.008912.
- Brewer LR, Corzett M, Balhorn R. Protamine-induced condensation and decondensation of the same DNA molecule. Science 1999;286:120-3. https://doi.org/10.1126/science.286.5437.120.
- Corzett M, Mazrimas J, Balhorn R. Protamine 1: protamine 2 stoichiometry in the sperm of eutherian mammals. Mol Reprod Dev 2002;61:519-27. https://doi.org/10.1002/mrd.10105.
- Kim E, Yamashita M, Kimura M, Honda A, Kashiwabara S, Baba T. Sperm penetration through cumulus mass and zona pellucida. Int J Dev Biol 2008;52:677-82. https://doi.org/10.1387/ijdb.072528ek.
- Huszar G, Patrizio P, Vigue L, Willets M, Wilker C, Adhoot D, et al. Cytoplasmic extrusion and the switch from creatine kinase B to M isoform are completed by the commencement of epididymal transport in human and stallion spermatozoa. J Androl 1998;19:11-20.
- Moretti E, Gergely A, Zeyneloglu HB, Ward P, Ward D, Baccetti B, et al. Relationship among head size, morphology and chromosome structure in human spermatozoa. Fert Stert 1997;68. https://doi.org/10.1016/S0015-0282(97)90953-5.
- Prinosilova P, Kruger T, Sati L, Ozkavukcu S, Vigue L, Kovanci E, et al. Selectivity of hyaluronic acid binding for spermatozoa with normal Tygerberg strict morphology. Reprod Biomed Online 2009;18:177-83. https://doi.org/10.1016/S1472-6483(10)60253-2.
- Sati L, Ovari L, Bennett D, Simon SD, Demir R, Huszar G. Double probing of human spermatozoa for persistent histones, surplus cytoplasm, apoptosis and DNA fragmentation. Reprod Biomed Online 2008;16:570-9. https://doi.org/10.1016/S1472-6483(10)60464-6.
- Worrilow KC, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes – multicenter, double-blinded and randomized controlled trial. Hum Reprod 2013;28:306-14. https://doi.org/10.1093/humrep/des417.
- Worrilow K, Eid S, Matthews J, Pelts E, Khoury J. Multi-site clinical trial evaluating PICSI®, a method for selection of hyaluronan bound sperm (HBS) for use in ICSI: improved clinical outcomes. Human Reprod 2010;25:6-9.
- ClinicalTrials.gov . Effectiveness of the Use of the PICSI Dish (Hyaluronan Microdot) in the Selection of Sperm for Intracytoplasmic Sperm Injection (ICSI) n.d. https://clinicaltrials.gov/ct2/show/NCT00741494 (accessed September 2018).
- Fredricsson B, Björk G. Morphology of postcoital spermatozoa in the cervical secretion and its clinical significance. Fertil Steril 1977;28:841-5. https://doi.org/10.1016/S0015-0282(16)42738-X.
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990;5:586-92. https://doi.org/10.1093/oxfordjournals.humrep.a137150.
- Menkveld R, Franken DR, Kruger TF, Oehninger S, Hodgen GD. Sperm selection capacity of the human zona pellucida. Mol Reprod Dev 1991;30:346-52. https://doi.org/10.1002/mrd.1080300409.
- Liu DY, Baker HW. Morphology of spermatozoa bound to the zona pellucida of human oocytes that failed to fertilize in vitro. J Reprod Fertil 1992;94:71-84. https://doi.org/10.1530/jrf.0.0940071.
- Menkveld R, Wong WY, Lombard CJ, Wetzels AM, Thomas CM, Merkus HM, et al. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: an effort towards standardization of in-vivo thresholds. Hum Reprod 2001;16:1165-71. https://doi.org/10.1093/humrep/16.6.1165.
- Coetzee K, Kruge TF, Lombard CJ. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update 1998;4:73-82. https://doi.org/10.1093/humupd/4.1.73.
- Van Waart J, Kruger TF, Lombard CJ, Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update 2001;7:495-500. https://doi.org/10.1093/humupd/7.5.495.
- van der Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest 2005;59:86-91. https://doi.org/10.1159/000082368.
- Liu DY, Garrett C, Baker HW. Low proportions of sperm can bind to the zona pellucida of human oocytes. Hum Reprod 2003;18:2382-9. https://doi.org/10.1093/humrep/deg456.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646-59. https://doi.org/10.1093/humupd/dmx022.
- Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Taraborrelli S, Arnone A, et al. Comparison of two ready-to-use systems designed for sperm-hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: a prospective, randomized trial. Fertil Steril 2012;98:632-7. https://doi.org/10.1016/j.fertnstert.2012.05.043.
- Miller D, Brison D, Kirkman-Brown J, Hooper R, Khalaf Y, Cutting R, et al. Selection of Sperm for Assisted Reproductive Treatment by Prior Hyaluronic Acid Binding: Increasing Live Birth Outcomes and Reducing Miscarriage Rates – Multicentre Randomised Controlled, Blinded Trial n.d. https://njl-admin.nihr.ac.uk/document/download/2005824 (accessed 2017).
- Witt K, Beresford L, Bhattacharya S, Brian K, Coomarasamy A, Hooper R, et al. Hyaluronic Acid Binding Sperm Selection for assisted reproduction treatment (HABSelect): study protocol for a multicentre randomised controlled trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012609.
- HABSelect: A New Sperm Selection Process for ICSI (Intracytoplasmic Sperm Injection) Aimed at Increasing Live Birth Outcomes and Reducing Miscarriage Rates. BMC: Port of Springer Nature; 2013.
- R Development Core Team . R: A Language and Environment for Statistical Computing 2008. www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed 2017).
- De Sanctis V, Perera D, Katz M, Fortini M, Gamberini MR. Spermatozoal DNA damage in patients with B thalassaemia syndromes. Pediatr Endocrinol Rev 2008;6:185-9.
- Ovári L, Sati L, Stronk J, Borsos A, Ward DC, Huszar G. Double probing individual human spermatozoa: aniline blue staining for persistent histones and fluorescence in situ hybridization for aneuploidies. Fertil Steril 2010;93:2255-61. https://doi.org/10.1016/j.fertnstert.2009.05.033.
- Lewis SE, Agbaje I, Alvarez J. Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med 2008;54:111-25. https://doi.org/10.1080/19396360801957739.
- Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 2003;24:59-66.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Kahan BC, Rushton H, Morris TP, Daniel RM. A comparison of methods to adjust for continuous covariates in the analysis of randomised trials. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0141-3.
- Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S, et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial. Lancet 2019;393:416-22. https://doi.org/10.1016/S0140-6736(18)32989-1.
- Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioural Health Statistics and Quality; 2014.
- Basar MM, Kahraman S. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2017;6:574-6. https://doi.org/10.21037/tau.2017.04.40.
- Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online 2013;27:325-37. https://doi.org/10.1016/j.rbmo.2013.06.014.
- Lewis SEM. The place of sperm DNA fragmentation testing in current day fertility management. Middle East Fertility Society Journal 2013;18:78-83. https://doi.org/10.1016/j.mefs.2013.01.010.
- Barratt C, Mansell S. Andrology is desperate for a new assay – let us make sure we get it right this time. Middle East Fertil Soc J 2013;18:82-3. https://doi.org/10.1016/j.mefs.2013.01.008.
- Kajii T, Ferrier A, Niikawa N, Takahara H, Ohama K, Avirachan S. Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Hum Genet 1980;55:87-98. https://doi.org/10.1007/BF00329132.
- Simpson JL. Causes of fetal wastage. Clin Obstet Gynecol 2007;50:10-3. https://doi.org/10.1097/GRF.0b013e31802f11f6.
- García-Enguídanos A, Calle ME, Valero J, Luna S, Domínguez-Rojas V. Risk factors in miscarriage: a review. Eur J Obstet Gynecol Reprod Biol 2002;102:111-19. https://doi.org/10.1016/S0301-2115(01)00613-3.
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology Working Group on Male Infertility . European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol 2012;62:324-32. https://doi.org/10.1016/j.eururo.2012.04.048.
- Jenderny J. Chromosome aberrations in a large series of spontaneous miscarriages in the German population and review of the literature. Mol Cytogenet 2014;7. https://doi.org/10.1186/1755-8166-7-38.
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17-8. https://doi.org/10.1016/0140-6736(92)92425-F.
- Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 2015;313:255-63. https://doi.org/10.1001/jama.2014.17985.
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod 2016;31:1588-609. https://doi.org/10.1093/humrep/dew082.
- Mortimer D. Sperm recovery techniques to maximize fertilizing capacity. Reprod Fertil Dev 1994;6:25-31. https://doi.org/10.1071/RD9940025.
- Twigg J, Irvine DS, Houston P, Fulton N, Michael L, Aitken RJ. Iatrogenic DNA damage induced in human spermatozoa during sperm preparation: protective significance of seminal plasma. Mol Hum Reprod 1998;4:439-45. https://doi.org/10.1093/molehr/4.5.439.
- Alvarez JG. DNA fragmentation in human spermatozoa: significance in the diagnosis and treatment of infertility. Minerva Ginecol 2003;55:233-9.
- Chabory E, Damon C, Lenoir A, Kauselmann G, Kern H, Zevnik B, et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 2009;119:2074-85. https://doi.org/10.1172/JCI38940.
- Henkel R, Bastiaan HS, Schüller S, Hoppe I, Starker W, Menkveld R. Leucocytes and intrinsic ROS production may be factors compromising sperm chromatin condensation status. Andrologia 2010;42:69-75. https://doi.org/10.1111/j.1439-0272.2009.00967.x.
- Choe SA, Tae JC, Shin MY, Kim HJ, Kim CH, Lee JY, et al. Application of sperm selection using hyaluronic acid binding in intracytoplasmic sperm injection cycles: a sibling oocyte study. J Korean Med Sci 2012;27:1569-73. https://doi.org/10.3346/jkms.2012.27.12.1569.
- Ciray HN, Coban O, Bayram A, Kizilkanat A, Bahçeci M. Preliminary study of embryo development following assessment of male and female gametes. Reprod Biomed Online 2008;16:875-80. https://doi.org/10.1016/S1472-6483(10)60155-1.
- Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Ciampaglia W, Filicori M. ‘Physiologic ICSI’: hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril 2010;93:598-604. https://doi.org/10.1016/j.fertnstert.2009.03.033.
- Van Den Bergh MJ, Fahy-Deshe M, Hohl MK. Pronuclear zygote score following intracytoplasmic injection of hyaluronan-bound spermatozoa: a prospective randomized study. Reprod Biomed Online 2009;19:796-801. https://doi.org/10.1016/j.rbmo.2009.09.022.
- Torabi F, Binduraihem A, Miller D. Sedimentation properties in density gradients correspond with levels of sperm DNA fragmentation, chromatin compaction and binding affinity to hyaluronic acid. Reprod Biomed Online 2017;34:298-311. https://doi.org/10.1016/j.rbmo.2016.11.011.
- Nijs M, Creemers E, Cox A, Franssen K, Janssen M, Vanheusden E, et al. Chromomycin A3 staining, sperm chromatin structure assay and hyaluronic acid binding assay as predictors for assisted reproductive outcome. Reprod Biomed Online 2009;19:671-84. https://doi.org/10.1016/j.rbmo.2009.07.002.
- Huszar G, Ozkavukcu S, Jakab A, Celik-Ozenci C, Sati GL, Cayli S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol 2006;18:260-7. https://doi.org/10.1097/01.gco.0000193018.98061.2f.
- Jakab A, Sakkas D, Delpiano E, Cayli S, Kovanci E, Ward D, et al. Intracytoplasmic sperm injection: a novel selection method for sperm with normal frequency of chromosomal aneuploidies. Fertil Steril 2005;84:1665-73. https://doi.org/10.1016/j.fertnstert.2005.05.068.
- Mokánszki A, Tóthné EV, Bodnár B, Tándor Z, Molnár Z, Jakab A, et al. Is sperm hyaluronic acid binding ability predictive for clinical success of intracytoplasmic sperm injection: PICSI vs. ICSI?. Syst Biol Reprod Med 2014;60:348-54. https://doi.org/10.3109/19396368.2014.948102.
- Majumdar G, Majumdar A. A prospective randomized study to evaluate the effect of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. J Assist Reprod Genet 2013;30:1471-5. https://doi.org/10.1007/s10815-013-0108-9.
- Erberelli RF, Salgado RM, Pereira DH, Wolff P. Hyaluronan-binding system for sperm selection enhances pregnancy rates in ICSI cycles associated with male factor infertility. JBRA Assist Reprod 2017;21:2-6. https://doi.org/10.5935/1518-0557.20170002.
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452-4. https://doi.org/10.1136/bmj.310.6977.452.
- Beck-Fruchter R, Shalev E, Weiss A. Clinical benefit using sperm hyaluronic acid binding technique in ICSI cycles: a systematic review and meta-analysis. Reprod Biomed Online 2016;32:286-98. https://doi.org/10.1016/j.rbmo.2015.12.001.
- Nijs M, Creemers E, Cox A, Janssen M, Vanheusden E, Van der Elst J, et al. Relationship between hyaluronic acid binding assay and outcome in ART: a pilot study. Andrologia 2010;42:291-6. https://doi.org/10.1111/j.1439-0272.2009.00992.x.
- Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod 2002;66:1061-7. https://doi.org/10.1095/biolreprod66.4.1061.
- Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod 1996;2:613-19. https://doi.org/10.1093/molehr/2.8.613.
- Hammadeh ME, al-Hasani S, Stieber M, Rosenbaum P, Küpker D, Diedrich K, et al. The effect of chromatin condensation (aniline blue staining) and morphology (strict criteria) of human spermatozoa on fertilization, cleavage and pregnancy rates in an intracytoplasmic sperm injection programme. Hum Reprod 1996;11:2468-71. https://doi.org/10.1093/oxfordjournals.humrep.a019139.
- Kovacs P, Kovats T, Sajgo A, Szollosi J, Matyas S, Kaali SG. The role of hyaluronic acid binding assay in choosing the fertilization method for patients undergoing IVF for unexplained infertility. J Assist Reprod Genet 2011;28:49-54. https://doi.org/10.1007/s10815-010-9479-3.
- Kato Y, Nagao Y. Effect of PVP on sperm capacitation status and embryonic development in cattle. Theriogenology 2009;72:624-35. https://doi.org/10.1016/j.theriogenology.2009.04.018.
- Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod 2010;16:3-13. https://doi.org/10.1093/molehr/gap059.
- Muratori M, Tamburrino L, Marchiani S, Cambi M, Olivito B, Azzari C, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med 2015;21:109-22. https://doi.org/10.2119/molmed.2014.00158.
- Björndahl L, Kvist U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst Biol Reprod Med 2011;57:86-92. https://doi.org/10.3109/19396368.2010.516306.
- Hammadeh ME, Zeginiadov T, Rosenbaum P, Georg T, Schmidt W, Strehler E. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch Androl 2001;46:99-104. https://doi.org/10.1080/01485010117363.
- Schlicker M, Schnülle V, Schneppel L, Vorob’ev VI, Engel W. Disturbances of nuclear condensation in human spermatozoa: search for mutations in the genes for protamine 1, protamine 2 and transition protein 1. Hum Reprod 1994;9:2313-17. https://doi.org/10.1093/oxfordjournals.humrep.a138444.
- Steger K, Wilhelm J, Konrad L, Stalf T, Greb R, Diemer T, et al. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod 2008;23:11-6. https://doi.org/10.1093/humrep/dem363.
- Sellami A, Chakroun N, Ben Zarrouk S, Sellami H, Kebaili S, Rebai T, et al. Assessment of chromatin maturity in human spermatozoa: useful aniline blue assay for routine diagnosis of male infertility. Adv Urol 2013;2013. https://doi.org/10.1155/2013/578631.
- Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl 2017;19:80-9. https://doi.org/10.4103/1008-682X.182822.
- Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, Mol BW, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0165125.
- Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization?. Fertil Steril 2008;89:823-31. https://doi.org/10.1016/j.fertnstert.2007.04.055.
- Paasch U, Grunewald S, Glander HJ. Sperm selection in assisted reproductive techniques. Soc Reprod Fertil Suppl 2007;65:515-25.
- Agarwal A, Cho CL, Majzoub A, Esteves SC. The society for translational medicine: clinical practice guidelines for sperm DNA fragmentation testing in male infertility. Transl Androl Urol 2017;6:720-33. https://doi.org/10.21037/tau.2017.08.06.
- Ahmad G. Clinical utility of sperm DNA fragmentation testing: a requisite to infertility practice. Transl Androl Urol 2017;6:685-7. https://doi.org/10.21037/tau.2017.03.74.
- Bach PV, Schlegel PN. Sperm DNA damage and its role in IVF and ICSI. Basic Clin Androl 2016;26. https://doi.org/10.1186/s12610-016-0043-6.
- Practice Committee of the American Society for Reproductive Medicine . The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril 2013;99:673-7. https://doi.org/10.1016/j.fertnstert.2012.12.049.
- Lewis SE. Should sperm DNA fragmentation testing be included in the male infertility work-up?. Reprod Biomed Online 2015;31:134-7. https://doi.org/10.1016/j.rbmo.2015.05.006.
- Tomlinson M, Lewis S, Morroll D. British Fertility Society . Sperm quality and its relationship to natural and assisted conception: British Fertility Society guidelines for practice. Hum Fertil 2013;16:175-93. https://doi.org/10.3109/14647273.2013.807522.
- Barratt CL, Aitken RJ, Björndahl L, Carrell DT, de Boer P, Kvist U, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications – a position report. Hum Reprod 2010;25:824-38. https://doi.org/10.1093/humrep/dep465.
- Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. https://doi.org/10.1016/j.rbmo.2014.10.018.
- Pigott TD. Missing predictors in models of effect size. Eval Health Prof 2001;24:277-30. https://doi.org/10.1177/01632780122034920.
- Nasr-Esfahani MH, Razavi S, Vahdati AA, Fathi F, Tavalaee M. Evaluation of sperm selection procedure based on hyaluronic acid binding ability on ICSI outcome. J Assist Reprod Genet 2008;25:197-203. https://doi.org/10.1007/s10815-008-9223-4.
- Kim HS, Kang MJ, Kim SA, Oh SK, Kim H, Ku SY, et al. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin Exp Reprod Med 2013;40:23-8. https://doi.org/10.5653/cerm.2013.40.1.23.
- Simon L, Liu L, Murphy K, Ge S, Hotaling J, Aston KI, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod 2014;29:904-17. https://doi.org/10.1093/humrep/deu040.
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. https://doi.org/10.1016/j.fertnstert.2015.09.041.
- Gosálvez J, Núñez R, Fernández JL, López-Fernández C, Caballero P. Dynamics of sperm DNA damage in fresh versus frozen-thawed and gradient processed ejaculates in human donors. Andrologia 2011;43:373-7. https://doi.org/10.1111/j.1439-0272.2010.01022.x.
- Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 2001;76:892-900. https://doi.org/10.1016/S0015-0282(01)02834-5.
- ISRCTN Registry . E-Freeze: Freezing of Embryos in Assisted Conception n.d. www.isrctn.com/ISRCTN61225414 (accessed September 2018).
- ISRCTN Registry . Endometrial Scratch Trial n.d. www.isrctn.com/ISRCTN23800982 (accessed September 2018).
- Delbes G, Herrero MB, Troeung ET, Chan PT. The use of complimentary assays to evaluate the enrichment of human sperm quality in asthenoteratozoospermic and teratozoospermic samples processed with Annexin-V magnetic activated cell sorting. Andrology 2013;1:698-706. https://doi.org/10.1111/j.2047-2927.2013.00106.x.
- Sakkas D. Novel technologies for selecting the best sperm for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril 2013;99:1023-9. https://doi.org/10.1016/j.fertnstert.2012.12.025.
- McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev 2014;10. https://doi.org/10.1002/14651858.CD010461.pub2.
Appendix 1 Changes to protocol
| Document date | Version number | Details of amendment (section of the document) | Amendment number/type (minor/substantial) | Date approved by | Date implemented | |
|---|---|---|---|---|---|---|
| REC | R&D | |||||
| 24 June 2013 | 1.1 | NA | NA | NA | NA | Study start |
| 4 February 2014 | 2.0 | Main eligibility criteria:
|
Substantial amendment/4 February 2014 | 14 March 2014 | 21 April 2014 | 21 April 2014 |
| 25 March 2014 | 3.0 | Main eligibility criterion:
|
Substantial amendment 2.0 | 29 April 2014 | 7 May 2014 | 2 June 2014 |
| 10 October 2014 | 3.1 | Collection and storage of the samples from patients with chronic viral infections | Non-substantial amendment/4 November 2014 | 4 November 2014 | Accepted 5 November 2014 (no approval required) | 24 November 2014 |
| 29 June 2016 | 4.0 | Change of trial co-ordinator details | Non-substantial amendment 8 | 7 July 2016 | No approval required | Not implemented as superseded by version 5.0 |
| 7 September 2016 | 5.0 | Change of PI at the Dundee site | Non-substantial amendment 9 | 15 September 2016 | No approval required | 14 December 2016 |
| 8 June 2017 | 6.0 | Addition of sites, change of contact details and staff members | Non-substantial amendment 10 | 29 June 2017 | No approval required | 8 August 2017 |
Appendix 2 Mechanistic assays of deoxyribonucleic acid integrity
Mechanistic assays of DNA integrity for latent (SEM) variable DNA fragmentation (TUNEL, comet AO) and compaction (AB). Halo area and ratio overlap the two variables. The table indicates the relationship between samples and the numbers of assays they were investigated with. It shows, for example, that 131 samples reported data for all available assays and 169 samples reported no data.
| Number of samples assayed | Assay | Number of missing assays | |||||
|---|---|---|---|---|---|---|---|
| Comet (fragmentation) | TUNEL (fragmentation) | AO (fragmentation) | AB (compaction) | Halo | |||
| Area (fragmentation/compaction) | Ratio (fragmentation/compaction) | ||||||
| 131 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 76 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| 12 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 74 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 137 | 1 | 1 | 1 | 1 | 0 | 0 | 2 |
| 9 | 1 | 0 | 0 | 1 | 1 | 1 | 2 |
| 62 | 1 | 1 | 0 | 0 | 1 | 1 | 2 |
| 26 | 0 | 1 | 1 | 0 | 1 | 1 | 2 |
| 10 | 1 | 0 | 1 | 0 | 1 | 1 | 2 |
| 109 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| 3 | 0 | 1 | 1 | 1 | 0 | 0 | 3 |
| 22 | 1 | 0 | 1 | 1 | 0 | 0 | 3 |
| 16 | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| 113 | 1 | 1 | 1 | 0 | 0 | 0 | 3 |
| 9 | 1 | 0 | 0 | 0 | 1 | 1 | 3 |
| 3 | 0 | 0 | 1 | 0 | 1 | 1 | 3 |
| 14 | 0 | 1 | 0 | 1 | 0 | 0 | 4 |
| 23 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| 1 | 0 | 0 | 1 | 1 | 0 | 0 | 4 |
| 43 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| 22 | 0 | 1 | 1 | 0 | 0 | 0 | 4 |
| 3 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| 29 | 1 | 0 | 1 | 0 | 0 | 0 | 4 |
| 12 | 0 | 0 | 0 | 1 | 0 | 0 | 5 |
| 63 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| 46 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| 10 | 0 | 0 | 1 | 0 | 0 | 0 | 5 |
| 169 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Total | 342 | 358 | 654 | 698 | 816 | 816 | 3684 |
Appendix 3 Recruitment and randomisation chart
FIGURE 17.
Projected recruitment is shown from June 2015, which was ≈12 months in to the trial and shows the original (green), revised (dark blue) and revised projected adjusted (green dashed), based on extension from February 2016 to December 2016. The adjustment took account of competing trials [E-Freeze (ISRCTN61225414)145 and Scratch (ISRCTN23800982)146]. HABSelect randomised 2772 couples (light blue), reaching ≈85% power at alpha 5%.
Trial recruitment timelines from June 2015 through to December 2016. The original recruitment end date was 28 February 2016 but was extended to 31 December 2016.
Appendix 4 Data Monitoring and Ethics Committee and Trial Steering Committee composition (independent members)
Data Monitoring and Ethics Committee
Independent chairperson: Professor Jenny Kurinczuk.
Independent statistician: Mr Paul T Seed.
Independent clinician: Dr Nigel Simpson.
Independent scientist: Darren Griffin.
Trial Steering Committee
Independent chairperson: Professor Nick S. Macklon.
Independent vice chairperson: Ying Cheong.
Independent scientist: Dr Michael Carroll.
Independent statistician: Dr Andrew Povey.
Independent statistician: Dr Stephen Roberts.
Independent embryologist: Jane Cuthbert.
Patient representative: Kate Brian.
Appendix 5 HABSelect final extension justification
HABSelect EME Project 11/14/3. Miller et al. – Further justification for a fully funded extension considering the impact on the mechanistic evaluations.
Preamble
In the extension request to the NIHR EME Board, the focus was on presenting the case for achieving 90% power for the clinical outcome. It became apparent during our recent site visit that the mechanistic components were likely at greatest risk of not achieving power if the extension was not awarded. The Board was asked to consider this additional document.
Context
The mechanistic aspects of HABSelect are hypothesis driven and seek to deliver a paradigm shift in advancing scientific understanding of DNA fragmentation, chromatin compaction and ICSI outcome; that collectively will yield vital new knowledge that will impact on improving future IVF/ICSI outcomes for male infertility. Success rate for IVF/ICSI remains at an overall average of 24%, a figure that has not increased since its wider clinical adoption. HABSelect is the largest world-wide trial undertaken in a regulated assisted conception setting that incorporates mechanistic evaluation. Both the clinical and scientific communities in the speciality are aware of the scale of knowledge HABSelect will generate where theories of the impact of DNA fragmentation will finally and unequivocally be resolved and the knowledge used to guide further innovations for sperm selection (Figure 18).
FIGURE 18.
Integrating clinical with mechanistic outcomes. How HABSelect seeks to link the trial’s clinical outcomes (blue shading) with the quality of the paternal genome (pale green shading), in relation to the levels of sperm DNA fragmentation and the compaction of sperm chromatin.

The hypothesis under test is that paternal genome integrity (PGI), which is an integration of data on DNA fragmentation and compaction, is closely linked to clinical outcomes by association. Unlike IVF, in which sperm with DNA damage ultimately fail to reach or fertilise the egg, the association between PGI and ICSI LBR outcomes is highly contentious. 14,137 This is most likely because of the differing assays used to measure PGI that vary greatly in both the forms of damage that they detect, their detection sensitivity, specificity and the relatively small numbers of clinical samples in the studies concerned. HABSelect if fully recruited will be sufficiently powered to allow, for the first time (to the author’s knowledge), the assessment of many hundreds of samples using up to six independent measures (dark green shading) and is not hindered by reliance on any one assay of PGI. HABSelect is therefore the first rigorous study ever conducted that can test the hypothesis that sperm from samples with high genome integrity will have better ICSI clinical outcomes and vice versa. The HBS provides a bridge between the clinical intervention, which uses a MHRA-approved and CE-certified HA binding platform (PICSI) to select sperm for injection and the outcomes of both the clinical and mechanistic investigations. The HBS is obtained regardless of intervention arm and it allows us to make certain predictions that can be confirmed or refuted only by final data analysis.
HABSelect Mechanistic Evaluations on paternal genomic integrity remain highly pertinent to the IVF scientific community:
-
Sperm DNA fragmentation will be lower and/or chromatin compaction will be greater in samples from men with higher HBS (> 65% sperm binding).
-
Sperm DNA fragmentation will be lower and/or chromatin compaction will be higher among couples with live birth at ≥ 37 weeks’ gestation (the primary clinical outcome measure) regardless of the intervention arm.
-
Live birth at ≥ 37 weeks’ gestation (the primary clinical outcome measure) will not differ for men with higher HBA scores regardless of intervention arm (PICSI or ICSI).
-
live birth at ≥ 37 weeks’ gestation will be higher for men with lower HBS (< 50% binding) in the PICSI arm. This may hold true for LBR at earlier gestational ages and for men with intermediate HBS (50–65% sperm binding).
These predictions assume that paternal genomic integrity is related to HBS. 108,147,148 Men with a high HBS have a greater proportion of sperm in their ejaculate with high genomic integrity and, conversely, men with a low HBS will have a lower proportion of sperm with high genomic integrity. The clinical trial will test the hypothesis that prior selection of sperm by HA binding will improve LBR outcomes because PICSI ‘selects’ for those sperm within the ejaculate that have high genomic integrity. The linkage between PGI and ICSI outcomes should become apparent because of the high number of samples (> 900) that will be assayed from both trial arms.
Mechanistic evaluation sample size considerations
The original sample size that we need to prove the link between genomic integrity and HBS and, hence, reveal unequivocally why prior selection of sperm with HA will improve LBR outcomes was originally based on a logistic regression analysis of the likely relationship between HBS and clinical outcomes and was set at 900 samples or 28% of the minimum sample size for clinical randomisation (3266 couples). The sample size (informed by Worrilow et al. 57) assumed that, at most, one-third of the samples available for analysis overall would have a lower (≤ 65%) HBS. The Worrilow et al. 57 trial stratified only into normal (> 65%) and low (≤ 65%) sperm binding but demonstrated a statistically significant reduction in miscarriage rate among the 59 patients in the group with the lower scores. There was no significant reduction in the group with the higher scores. HABSelect will significantly advance scientific knowledge beyond the Worrilow et al. 57 study by its scale permitting the first linking of mechanistic and clinical outcome, notably the mechanism behind HA efficacy in improving live birth outcomes. HABSelect is powered to sufficiently generate a n = 900 stratified 1 : 1 : 1 into samples of low (< 50%) to intermediate (50–59%) to high (≥ 60%) sperm binding. We recognised that the lower (< 50%) HBS stratum would be the most challenging to populate and it therefore acts as the driver for sample acquisition; this is the group with the poorest PGI and, hence, the most likely to benefit from the PICSI intervention. HABSelect’s ≥ 900 assayed samples will be sufficient to determine the effect of PGI on ICSI clinical outcomes (samples leading to or not leading to live birth) with a > 95% confidence level at ≥ 85% power. It should be borne in mind, however, that, as is the case with the clinical trial itself, the sample size and power of the mechanistic study depends entirely on the magnitude of the differences we expect to observe. As this is currently uncertain, it had to be estimated and the chosen sample size in our case was based on a modest difference of 7% in PGI between successful and unsuccessful cycles (half that within the DNA fragmentation zone expected for success after multiple rounds of IVF7). How those data relate to the 1 : 1 : 1 split between low, intermediate and high HBS remains to be determined in the final data analysis.
The benefits of supporting and risks of not supporting an extension
As of September 2015, there were 1163 sperm samples in storage, of which 14% (n = 162) had a HBS of < 50%. Based on our projected randomisation accrual, among ≈2250 samples in storage by December 2016, ≈320 of these will have a HBS of < 50%, meeting our requirements. If recruitment ceases in February 2016 we anticipate receiving only ≈220 with a HBS of < 50%, and will fail to reach the necessary n = 300 required in this stratum to reach power. In meeting the demands of a constantly shifting clinical environment with regard to recruitment and sample accrual, we chose to commence some mechanistic development at Birmingham earlier than anticipated and to extend its remit. This was because of the need to establish and normalise the assays for rapid adoption in our other laboratories when sample availability permitted. We recognised early in the trial that this preparedness would be necessary in the event that time to process became limiting in view of the delay in sample accrual. We also recognised that, as many samples would be unsuitable for comprehensive analysis, we could concentrate the effort in three rather than four centres (Sheffield is no longer a mechanistic centre). The extension therefore covers Birmingham, Leeds and Belfast and for 10 months each of additional technical support time. Our dynamic response will also meet the need to obtain HBS for almost 200 samples that were stored without values when the supplier withdrew the HYDAK slides temporarily. Only the technical support team can obtain the HBS as the samples are already in storage. In short, if our technical support time is not extended beyond December 2016 (when it is was originally due to end), we will be unable to process and analyse sufficient numbers of stored samples to unequivocally link paternal genome integrity with HBS and clinical outcomes.
The appeal to extend based on wider scientific rationale
The broader link between DNA fragmentation and ICSI outcome remains controversial and unresolved,14,137,149 precisely because there has been no study to date with the power to test it unequivocally. Any study of this nature inevitably has to compromise between power and cost, but alongside answering the clinical question relating to the efficacy of HA sperm selection for ICSI. However, the technical effort needed to deliver the answers that the broader scientific community seeks can be realistically achieved only if, as we have outlined to the Board, a fully funded extension is granted. We appreciate that an extension to achieve mechanistic outcomes is likely to be unprecedented; but the advancement to the scientific community that this work will achieve goes well beyond its clinical impact and cannot be understated. The combined IVF and broader scientific communities eagerly await the HABSelect results.
Appendix 6 The HABSelect Gantt chart
List of abbreviations
- AB
- aniline blue
- AMH
- anti-Müllerian hormone
- AO
- acridine orange
- ART
- assisted reproduction technologies
- BMI
- body mass index
- CE
- Conformité Européenne
- CI
- confidence interval
- CMA3
- chromomycin A3
- CONSORT
- Consolidated Standards of Reporting Trials
- CP
- clinical pregnancy
- CPR
- clinical pregnancy rate
- DGC
- density gradient centrifugation
- DNA
- deoxyribonucleic acid
- FSH
- follicle-stimulating hormone
- HA
- hyaluronan
- HABSelect
- Hyaluronic Acid Binding sperm selection
- HBA
- hyaluronan binding assay
- HBRC
- Human Biomaterials Resource Centre
- HBS
- hyaluronan binding score
- HFEA
- Human Fertilisation and Embryology Authority
- ICSI
- intracytoplasmic sperm injection
- ID
- identifier
- ISRCTN
- International Standard Randomised Controlled Trial Number
- IVF
- in vitro fertilisation
- LBR
- live birth rate
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCTU
- Pragmatic Clinical Trials Unit
- PGI
- paternal genome integrity
- PICSI
- physiological intracytoplasmic sperm injection
- PVP
- polyvinylpyrrolidone
- RCT
- randomised controlled trial
- ROS
- reactive oxygen species
- RR
- relative risk
- SAE
- serious adverse event
- SAPm
- mechanistic statistical analysis plan
- SD
- standard deviation
- SEM
- structural equation modelling
- SUSAR
- suspected unexpected serious adverse reaction
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling
- WHO
- World Health Organization
