Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 08/43/61. The contractual start date was in January 2010. The final report began editorial review in November 2015 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Gary A Ford received personal fees from Lundbeck Ltd, Boehringer Ingelheim, Pfizer and AstraZeneca, and grants and personal fees from Athersys outside the submitted work. Alastair Cozens had equity in Skene Software Ltd and other financial activity outside the submitted work for SiLCK Clinical Solutions Ltd. David Meads is a member of the Health Technology Assessment (HTA) Elective and Emergency Specialist Care (EESC) Panel. Catherine M Sackley is a member of Health Services and Delivery Research researcher-led board. Amanda J Farrin is a member of the HTA Clinical Evaluation and Trials Board and the HTA Commissioning Strategy Group.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Ford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Up to one in five people in the UK will suffer a stroke,1 with approximately 110,000 new cases annually in England. 2 There are 900,000 stroke survivors in England, 300,000 of whom are moderately or severely disabled. 2 The cost of stroke care to the NHS is around £3B annually, with wider economic costs of £8B. 3 It is anticipated that the number of disabled stroke survivors will increase as a result of an ageing population. Physical therapy4 (PT) and occupational therapy5 (OT) have been shown to promote the recovery of function following stroke, and early access to multidisciplinary rehabilitation is recommended for all patients to improve function and quality of life. 6,7
What is ‘rehabilitation’?
The World Health Organization (WHO) definition of rehabilitation focuses on the enablement of people in order to reduce the impact that a disabling condition may have on a person’s life. 8
The cornerstone of rehabilitation is the WHO’s International Classification of Functioning, Disability and Health9 (ICF). This model postulates that an underlying health condition (such as stroke) may cause impairment in bodily structures and function, limitation of activities and restriction of participation. 10 Clinically important impairments following stroke may include changes in gait pattern and intellectual functions, inability to sequence complex movements, and alterations in muscle tone and power. 11 Activity limitations and participation restrictions may include difficulties in problem-solving, transferring oneself, maintaining family and personal relationships, and acquiring and keeping a job. 11 However, an individual’s functional abilities are also profoundly influenced by wider contextual factors,8 both environmental and personal. Environmental factors comprise not only the built environment but also legislation and societal attitudes. 8 Depending on circumstances, they may serve as barriers to, or facilitators of, individual function. 8 Personal factors are intrinsic to the individual but may not be directly related to the underlying health condition. 8 They include sex, age, race and physical fitness. 8
Therefore, in ICF terms, ‘disability’ is constructed as an interaction between an individual and their environment and personal factors. 10,12 ‘Rehabilitation’ typically comprises a complex package of measures targeting multiple levels of the ICF. The precise nature of the intervention required varies depending on the extent of cerebral damage caused by the stroke and the patient’s goals, and with the passage of time from the stroke. Within the first few hours, optimum acute care minimises the extent of tissue injury and secondary complications (impairment in structure) and, therefore, maximises preservation of function. 13 As planning for discharge progresses, environmental assessment and, if necessary, provision of assistive technologies (contextual factors) may help to enhance safety and personal independence on leaving hospital. In the long term, a combination of rehabilitation interventions at impairment, activities and environmental levels may be necessary to address specific goals such as return to work (participation). 14 Many stroke survivors require not only intermittent discrete periods of time-limited rehabilitation to address particular functional goals, but also longer-term monitoring and support to prevent long-term complications and to mitigate the effects of changing disability (such as deterioration in mobility due to accelerated joint ageing). 13
Rehabilitation interventions may be restorative (e.g. encouraging use of a hemiplegic limb) or compensatory (such as the teaching of new techniques or provision of orthoses or assistive technologies). 14 Since the 1950s, a variety of rehabilitation approaches have been developed, each based on differing theories about how patients recover from stroke. 4 National Institute for Health and Care Excellence (NICE) quality standards15 state that a minimum of 45 minutes of each active therapy should be offered to stroke patients for a minimum of 5 days per week. The dose of active therapy is such to allow patients to meet their rehabilitation goals, and active therapy should continue as long as the patient benefits and can tolerate the therapy. More recently, the provision by the therapist of active feedback on the quality of movements has been emphasised. 16 A recent Cochrane review17 has demonstrated no clear evidence in favour of any one approach. Although many rehabilitation strategies are in clinical use,18 most restorative interventions share one crucial commonality: their reliance on the patient’s ability to learn (or to relearn) motor skills.
Brain structures involved in motor learning
Motor learning is fundamental not only to rehabilitation but also to daily life,19 from a baby learning to walk to a musician practising a symphony. Becoming skilled in a motor task requires us not only to learn the correct order of movements but also to develop an awareness of the sensory input that guides decisions, such as timing of the movement, trajectory and what force should be applied. 20 Often, acquiring a skill also requires the learner to manipulate or interact with objects in his or her environment. 21 Although there is no universally accepted definition of ‘motor learning’, it has been conceptualised as ‘a change in motor behaviour, specifically referring to the increased use of novel, task-specific joint sequences and combinations, resulting from practice and/or repetition’. 22
Many studies have sought to define the brain structures involved in motor learning. Whether or not it is reasonable to extrapolate theoretical frameworks for motor learning derived in healthy individuals to those with stroke is debatable. However, stroke patients participating in rehabilitation demonstrate increased activation of the ipsilesional primary motor cortex, pre-motor cortex and supplementary motor areas. 23 This suggests that functional reconfiguration is possible after stroke, allowing lost functions to be recovered using partially spared pathways. 23 The cellular basis for this is axonal remodelling, changes in the number and morphology of dendrites,24–26 and long-term potentiation or depression of synaptic transmission both adjacent to and remote from the original stroke,24,25 a process termed ‘neuroplasticity’.
Magnetic resonance imaging (MRI) has been the mainstay of attempts to understand learning processes, both in healthy participants20,21,27,28 and in those recovering from stroke. 23 Attention has focused on the role of the basal ganglia. These contain the striatum (caudate, putamen and nucleus accumbens) and the globus pallidus. 29,30 They are now known to form part of wider corticobasal and corticocerebellar loop circuits. The striatum receives white matter projections from almost all cortical areas. It sends projections to the thalamus and cerebellum, which, in turn, project to the cortex29–31 (Figure 1). Although the precise function of these pathways remains opaque, it is clear that they are crucial to a variety of cognitive and motor processes. 32–34 Lesions at any point in these pathways may mimic deficits resulting from cortical injury:31 a phenomenon that has been termed ‘disconnection syndrome’. 31 For example, discrete lesions to the basal ganglia may result in a spectrum of clinical manifestations more typically associated with frontal lobe insult due to the extensive projections these structures receive from the prefrontal cortex. 31
FIGURE 1.
Connectional pathway and neurotransmitter systems of the corticobasal ganglia thalamic pathway. GABA, gamma-aminobutyric acid.

The indirect pathway (light-green arrow) projects from the cortex via a complex web of connections between the globus pallidus externa and the subthalamic nucleus, to the substantia nigra and globus pallidus interna and, thence, to the thalamus. The direct pathway (light-blue arrow) projects from the cortex to the striatum, and thence to the thalamus. The hyperdirect pathway (grey arrow) bypasses the striatum and projects directly to the substantia nigra and globus pallidus interna. Dopaminergic projections from the substantia nigra (black arrows) exert an inhibitory effect on GABAergic interneurons of the indirect pathway, and an excitatory effect on GABAergic interneurons of the direct pathway.
The role of dopamine in learning processes
There has recently been a growing appreciation of the central role that dopamine plays in learning. Dopamine is a key modulator of striatal function; through its selective action on striatal projection interneurones, dopamine is able to inhibit the indirect striato-thalamic pathway and excite the direct pathway. 35 By directly influencing the activity of these pathways, dopamine may contribute to the selection and termination of motor programmes for skilled movements. 35,36 In conditioned learning, dopamine may potentiate drive and arousal, encode the ‘value’ of a reward, or ‘stamp in’ associations between stimulus and response. 37 More recently, it has been proposed that phasic dopamine release dopamine acts as an ‘alerting signal’, prompting the orientation of conscious attention and cognitive processing towards salient environmental cues and increasing general arousal and motivation. 38 Together with other neurotransmitters (noradrenaline, serotonin and acetylcholine), dopamine up-regulates excitatory glutamatergic transmission, which in turn stimulates activation of N-methyl-D-aspartate receptors. 39 It is the activity of these receptors that promotes long-term potentiation or long-term depression of synaptic efficacy. 39 These long-term synaptic changes are thought to mediate consolidation of memory traces (e.g. of the association between stimulus and reward,29 or motor memories40).
‘Pharmacorehabilitation’: a new frontier in stroke rehabilitation?
Taken together, these disparate findings present an intriguing possibility: might pharmacological manipulation of neurotransmitter systems be used to enhance the reacquisition of motor skills after stroke? Several drugs influence directly the levels of key neurotransmitters. Dextroamphetamine and metamphetamine inhibit reuptake of dopamine, noradrenaline and serotonin into the presynaptic terminal, thereby increasing their bioavailability within the synaptic cleft. 39 Methylphenidate, similarly, blocks presynaptic reuptake of dopamine and noradrenaline. 39 As all of these neurotransmitters are known to enhance excitatory glutamatergic transmission in the corticostriatal pathways, using drugs that act on these systems may present an opportunity to manipulate neuroplasticity, thereby enhancing the effectiveness of conventional rehabilitation. 39 An early-phase trial41 in rats with a unilateral experimental lesion of the motor cortex reported an immediate improvement the animals’ ability to run along a rotating beam following a single dose of dextroamphetamine. This finding has been replicated in other trials in both rats42–46 and primates47 (albeit with some dissenting findings). 48 The treatment response appears to depend on repetitive task practice during the drug’s period of action. 42–44,46
Encouraged by these findings, there have been several trials of amphetamines to enhance recovery in human stroke survivors. 39,49 Although there is a trend towards improved motor outcomes in those treated with amphetamine,39 many trials are of small scale, with considerable heterogeneity in the dosing regimens, timing between dose and therapy, follow-up period and outcome measures. 39 Several showed differences in the baseline characteristics of the intervention and placebo groups (age, consciousness level), which may account for a trend towards higher mortality rates in those treated with amphetamine. 39 Therefore, positive findings must be interpreted with caution39 and clinical use of amphetamine has been limited as a result of the concern about sympathomimetic effects. 49
Drugs that promote dopaminergic activity directly may be more appropriate targets for pharmacological enhancement of rehabilitation. 50 Levodopa, an orally administered precursor of dopamine, crosses the blood–brain barrier before being metabolised to dopamine centrally resulting in a rise in brain dopamine levels. 39 Around 5% of the levodopa that enters the central nervous system is further metabolised to noradrenaline, raising the possibility that its effects may be mediated at least in part by noradrenaergic transmission. 51 Co-careldopa (Sinemet®, Merck Sharp & Dohme Ltd) is a combined preparation of 100 mg of levodopa with a peripheral DOPA decarboxylase inhibitor, carbidopa. Carbidopa reduces peripheral levodopa metabolism, thereby maximising the central bioavailability of levodopa. 51 Peak plasma levels of levodopa occur between 30 minutes and 2 hours after a single oral dose of co-careldopa, with a plasma half-life of 1–3 hours.
Only one systematic review has examined the use of dopamine agonists to enhance motor recovery from stroke in humans. Berends et al. 39 assessed the evidence for a number of candidate drug classes, including amphetamine, selective serotonin reuptake inhibitors (SSRIs) and levodopa. Two papers52,53 concerning the use of levodopa met their inclusion criteria. The evidence of a positive treatment effect with this agent was judged to be lacking. 39 A number of other trials54–62 that they did not cite also address this question. These are of variable quality and report mixed results. Many were limited by small sample sizes56,58 or comparatively short follow-up,58,60 or administered only single doses of co-careldopa. 58,59 Some recruited patients months or years after stroke. 56,58 Several demonstrated benefit with dopamine on motor outcomes including the Barthel index54 (BI), the National Institutes of Health Stroke Scale,54 the Functional Independence Measure (FIM),55 walking speed and manual dexterity,56 procedural motor learning,57 motor memory,59 the Fugel-Mayer Assessment60 and the Rivermead Motor Assessment. 62 However, others have found no improvement in length of stay,61 cognitive and motor domains of the FIM61 or upper limb function. 58
A systematic search was carried out to identify relevant studies and emerging data as the trial progressed. An overview of identified randomised controlled trials (RCTs) is given in Table 1 and the search strategy is in Appendix 1.
| Study | Participants (n) | Time post stroke that patients were enrolled | Treatment regimen | Outcome measures | Follow-up points | Results |
|---|---|---|---|---|---|---|
| Lokk et al., 201154 | 100 | – | PT + : methylphenydate, levodopa, levodopa + methylphenidate placebo | Fugl-Meyer Assessment score, BI, NIHSS | 15, 90 and 180 days | Slight, but significant, improvement in BI and NIHSS at 6 months for those on drug treatment vs. placebo |
| Cramer et al., 200963 | 33 | 1–12 months | 0.25–4 mg of ropinirole daily (titrated up) or placebo | Gait velocity | 12 weeks | Both treatment arms showed improvement; no significant effect with ropinirole |
| Acler et al., 200956 | 10 | 10–48 months | 100 mg of levodopa or placebo daily for 5 weeks | RMA, Nine-Hole Peg Test, 10-metre walk | 5 weeks | Significant improvement in walking speed and manual dexterity (p < 0.01) |
| Rösser et al., 200857 | 18 | 3.3 years (SD 2.1 years) | Three doses of 100 mg of levodopa + 25 mg of carbidopa/placebo | Motor learning: serial reaction time task performed with paretic hand | – | Improvement in procedural motor learning in levodopa state vs. placebo state |
| Restemeyer et al., 200758 | 10 | > 6 months | Two doses of 100 mg of levodopa or placebo in a random order, then 1 hour of PT | Nine-Hole Peg Test, grip strength, Action Research Arm Test | 2 weeks | No effect of levodopa on motor function |
| Sonde and Lökk, 200753 | 25 | 5–10 days | 10 PT sessions with placebo, 20 mg of dextroamphetamine, 100 mg of levodopa and 50 mg of levodopa + 10 mg of dextroamphetamine | Fugl-Meyer Assessment motor score, BI | 2 weeks | All patients improved. No additional benefit with drug treatment |
| Scheidtmann et al., 200152 | 53 | 3 weeks–6 months | 3 weeks of 100 mg of levodopa or placebo, then 3 weeks of PT only | RMA | 3 weeks | At end of study, RMA gain of 8.2 points for levodopa vs. 5.7 points for placebo (p = 0.020) |
These equivocal results may perhaps be explained by dopamine’s complex and multiple actions in vivo and the heterogeneous groups of patients involved in these trials. Any link between administration of dopamine and recovery of function may be attenuated by injury to pathways involved in motor learning and cognitive function, or by reduced motivation to participate in rehabilitation. However, it is clear that co-careldopa is well tolerated, with no serious adverse events (SAEs) reported with the dosing regimens used. Therefore, there is a need for a larger RCT to address the question of whether or not administering levodopa is beneficial in enhancing recovery from stroke.
The Dopamine Augmented Rehabilitation in Stroke (DARS) trial set out to evaluate the effect of administering co-careldopa for up to 6 weeks on patients’ ability to walk ≥ 10 metres independently at up to 8 weeks after stroke. 64
Chapter 2 Methods
Aim and objectives
The aim of this trial was to determine if combining co-careldopa with routine PT and OT during early rehabilitation in people with new stroke admitted to a stroke unit enhances the effect of conventional rehabilitation treatments in terms of physical functioning.
Primary objective
The primary objective related to physical functioning and was to compare the proportion of patients in both treatment groups who could walk independently (≥ 10 metres with an aid if necessary but with no standby help) at 8 weeks post randomisation.
Physical functioning was assessed using the Rivermead Mobility Index (RMI),65 which assesses functional mobility in gait, balance and transfers after stroke. It is a 15-item scale comprising 14 self-reported items and one direct observation item. Items increase in difficulty and the higher the score, the better the mobility. Scores range from 0 to 15 points, where 0 is given for a ‘no’ response and 1 is given for a ‘yes’ response.
To meet the primary end point in the DARS trial, participants had to have a RMI score of ≥ 7 points and had to answer ‘yes’ to item 7 (‘Do you walk 10 metres with an aid or furniture if necessary but with no standby help?’) to confirm that they could walk independently.
Secondary objectives
-
Assess the impact on physical functioning and mood at 8 weeks, 6 months and 12 months to:
-
compare the proportion of patients who are walking at 6 months and 12 months post randomisation in the two groups
-
compare activities of daily living, mobility and dependency [assessed using the RMI (continuous), BI,66 modified Rankin Scale (mRS),67 Nottingham Extended Activities of Daily Living (NEADL) scale,68 ABILHAND Manual Ability Measure (ABILHAND)69] between groups
-
compare psychological distress/mood between the two groups [assessed using the General Health Questionnaire 12-item version (GHQ-12)70]
-
compare carer burden between groups using the Caregiver Burden Scale (CBS)71
-
investigate cost-effectiveness of co-careldopa and conventional rehabilitation treatments [assessed using the EuroQol-5 Dimensions (EQ-5D) to quantify care costs].
-
-
Investigate potential moderators and mediators of effect at 8 weeks, to investigate whether or not:
-
baseline patient clinical characteristics and investigations [e.g. routine brain computerised tomography (CT)] help to predict those who might benefit from co-careldopa-augmented rehabilitation
-
key factors {e.g. fatigue [using the Fatigue Assessment Scale (FAS)72], concurrent musculoskeletal – symptoms/signs and pain (MSK-SSP) (using the MSK-SSP manikin73,74) and cognitive function [using the Montreal Cognitive Assessment (MoCA)75]} influence the short-term effect of co-careldopa on physical functioning.
-
-
Investigate the implementation within routine NHS services to:
-
assess the adverse event (AE) profile associated with the combination treatment (NHS stroke rehabilitation treatment linked with co-careldopa)
-
investigate the practical implications of delivering this intervention within routine NHS acute and early community care of people with stroke
-
assess the acceptability of co-careldopa treatment to stroke patients (study drug adherence will be measured and a semistructured interview will be undertaken with participants at the 8-week assessment).
-
-
Investigate the cost-effectiveness of co-careldopa-augmented rehabilitation for stroke compared with usual care within stroke services.
Trial design
The DARS trial was a multicentre, prospective, randomised, double-blinded, placebo-controlled trial of NHS PT and OT treatment alone compared with NHS PT and OT treatment in addition to up to 6 weeks of co-careldopa treatment for patients admitted to acute stroke services after new or recurrent stroke. 64 Outcome measures were obtained at 8 weeks, 6 months and 12 months following randomisation.
Changes to trial design
Recruitment into the trial was lower than anticipated. Screening logs were reviewed during the early stages of recruitment, and after approximately 11 months of recruitment the following relaxations were made to the eligibility criteria to increase the number of potential patients who could participate in the trial without compromising the scientific validity of the research: (1) inclusion of patients with new or recurrent stroke (previously new stroke only) and (2) extension of the recruitment window from 2 weeks to 42 days post stroke.
Trial timelines
The original project plan was to deliver the project in 45 months. However, the recruitment and implementation of the project were more challenging than anticipated and the funder granted a 21-month funded extension. The main reasons for the extension are outlined below:
-
The initial setup time was prolonged as a result of a worldwide lack of availability of the investigational medicinal product (IMP) (Sinemet), which delayed drug procurement for the recruiting sites. Thus, trial setup increased from 9 months to 16 months because of a global shortage of the active drug.
-
Challenges in identifying and recruiting suitable people with stroke led to much lower recruitment estimates per centre than those envisaged by the sites at trial setup. Thus, many more centres than planned were required in order to recruit 572 patients within a reasonable time frame.
-
Implementation of the DARS trial was challenging for the following reasons – the need to deliver seamless trial intervention across acute and community NHS trusts, the need to administer medication with therapy sessions in both hospital and community settings, and service reconfiguration during the recruitment period.
The impact of these challenges was not fully realised at the outset of the trial. These issues led to a delay in the start of the site setup and poor recruitment rates for the first few months.
Participants
Patient recruitment
Patients admitted to participating NHS stroke services after experiencing a new or recurrent stroke were screened for trial entry from admission up to 42 days post stroke. It is possible that a patient’s condition could improve during the 42 days post stroke and the patient was reviewed during this period to reassess eligibility. Screening logs were maintained for all patients admitted to stroke services after a new or recurrent stroke.
Potential patients were identified by local Stroke Research Network (SRN) staff in liaison with ward nurses and therapists, and were provided with verbal information about the trial. Patients interested in receiving further information were provided with a detailed patient information booklet and a patient digital versatile disc (DVD) that provided an overview of the trial and a visual aid to increase understanding of the implications of participating in the trial. The principal investigator (PI) (or medically qualified member of the trial team) obtained written informed consent. When the patient was able to comprehend but unable to sign or date the consent form, provision was made for completion of the consent form by an independent person.
Carer recruitment
Carers of eligible and consenting patients were approached and consented at the time of patient enrolment to provide information relating to carer burden.
Eligibility
Inclusion criteria
-
New or recurrent clinically diagnosed ischaemic or haemorrhagic (excluding subarachnoid haemorrhage) stroke within 5 to 42 days prior to randomisation.
-
Could not independently walk (that is, without the use of physical assistance) ≥ 10 metres indoors.
-
Achieved a score of < 7 points on the RMI, scored by a professional.
-
Were expected to need rehabilitation treatment.
-
Were ≥ 18 years of age.
-
Were able to give informed consent.
-
Were able to access continuity of rehabilitation treatment following discharge from hospital (i.e. continuity of rehabilitation available within 5 days following discharge).
-
Were expected to be able to comply with the treatment schedule.
-
Were expected to be in hospital for at least their first two doses of trial medication.
Coenrolment into another trial was permitted if it had been agreed with the chief investigator of the relevant studies and provided that it would not confound the results of the DARS trial nor overburden the patient, attribution of AEs was not compromised and there were no potential interactions.
Exclusion criteria
-
Not expected to survive for 2 months following stroke.
-
A diagnosis of Parkinson’s disease, severe medical or surgical illness, or severe psychosis.
-
Known hypersensitivity or contraindications to co-careldopa.
-
Symptomatic orthostatic hypotension.
-
Required physical assistance from at least one person to walk prior to stroke due to pre-existing comorbidities (e.g. heart failure or osteoarthritis).
-
Pregnancy, lactation or, in the case of women of child-bearing potential, unwillingness to use medically approved contraception during treatment and for 1 month after treatment had finished.
-
Participation in another interventional drug or treatment therapy trial.
-
Inability to walk ≥ 10 metres indoors prior to stroke [with a walking aid if necessary, but without physical assistance, which, in this context, means help from one or more person(s)].
Potential trials for coenrolment with the DARS trial were considered by the chief investigator and trial management team if (1) it was agreed with the chief investigator of the relevant studies, (2) it did not confound the results of the DARS trial, (3) it did not overburden the patient, (4) attribution of causality to AEs was not compromised and (5) there were no potential interactions of trial interventions.
Carer eligibility
A carer was defined as an individual identified by the patient as their main informal carer who provides the patient with practical support a minimum of once per week. Carers had to provide written informed consent to be eligible for participation. Presence of a carer was not a prerequisite for patient enrolment.
Trial settings
A total of 51 NHS stroke services across the UK with an acute inpatient stroke rehabilitation facility and a service allowing rehabilitation treatments within the community setting that could consist of early supported discharge or community stroke teams/services obtained NHS permissions to take part in the DARS trial. A further five stroke services participated as repatriation sites for participants recruited from other services.
Face-to-face follow-up was conducted by a blinded researcher in the participant’s home, at hospital or at a community facility. When it was not possible to complete a face-to-face assessment, telephone follow-up was conducted.
Interventions
Patients were randomised to receive either co-careldopa (Sinemet) or a matched placebo tablet in addition to routine NHS PT and OT. The initial two doses of co-careldopa were 62.5 mg (50 mg of levodopa and 12.5 mg of carbidopa) and the remaining doses were 125 mg (100 mg of levodopa and 25 mg of carbidopa).
Patients were required to take a single oral tablet 45–60 minutes before PT or OT sessions (this also includes programmed rehabilitation delivered by rehabilitation assistants). Rehabilitation treatment appropriate for drug administration within the DARS trial was defined as active physical treatment (i.e. most PT and OT directed at motor skills such as walking, transfers and dressing, but not psychological input sessions or speech and language therapy).
The dose and timing of the medication reflects current evidence on the use of co-careldopa in this context. 52,57,76,77 A pragmatic approach to taking the IMP was chosen; although the IMP should optimally be taken 45–60 minutes prior to the rehabilitation treatment session, it was recognised that, on occasions, the therapist was unable to contact the patient to remind them to take the tablet or the patient forgot and it was acceptable for the tablet to be taken 0–15 minutes before the start of therapy in these situations. The reasons for any deviations from the optimal timing were recorded. If the participant was scheduled to have two therapy sessions either one directly after the other or within 3 hours of a dose then a repeat dose was not given before the second therapy session. If the participant was scheduled to have more than two PT or OT sessions, the dose was not administered more than twice during any one 24-hour period.
Co-careldopa or placebo in addition to NHS PT or OT was given for a maximum of 6 weeks and assuming a maximum of two sessions of therapy per day for 30 days over a 6-week period. The duration of treatment could be < 6 weeks if the patient was clinically deemed to not require further rehabilitation treatment. The decision about need for rehabilitation intervention was made by the treating clinicians, therapists and nurses in consultation with patients and families.
A pack of 62 tablets (two 62.5-mg tablets and 60 125-mg tablets), each with a unique kit number, was dispensed to each participant by the hospital pharmacy on the day of randomisation. The first two co-careldopa or placebo doses were administered in the hospital setting, allowing the participant to be observed in case of early AEs. Blood pressure was checked before administering the first dose and, if the systolic blood pressure was < 90 mmHg, the decision whether or not to administer the IMP was discussed with the local PI. Subsequent doses were administered in the hospital setting by the attending nursing staff or within the home setting post discharge.
Following discharge into the community, telephone reminders were given to the participant approximately 1 hour prior to the therapy visit by the treating community rehabilitation staff to remind the patient to take the medication.
Figure 2 provides an overview of the treatment pathways permitted within the DARS trial.
FIGURE 2.
Treatment pathways. a, Rehabilitation treatment appropriate for drug administration within DARS is defined as an active physical treatment (i.e. most physical and occupational therapy directed at motor skills such as walking, transfers and dressing, but not psychological input sessions or speech and language therapy, swallowing, splinting).

Adherence to medication
Stroke survivors may have significant residual impairments that may affect their ability to comply with trial medication, and ensuring pharmaco-adherence in the community setting is a challenge. To maximise compliance, patients, clinicians, pharmacy staff and the manufacturer were consulted during the design of the IMP packaging to ensure that it was usable by people with hemiparesis and included clear instructions as to when the trial medication should be taken in relation to the NHS PT and OT provision. A DVD was developed for participants and carers to use as a visual aid to complement the other patient information. This content was presented in a manner accessible to patients with aphasia or hemisensory neglect and used graphics to illustrate abstract concepts. A telephone reminder was also implemented, whereby the treating community rehabilitation staff contacted the participant approximately 1 hour prior to their community-based therapy session to prompt them to take the trial medication. 73
Outcomes
Primary outcome
The primary outcome was the ability to walk independently at 8 weeks post randomisation, defined as a score of ≥ 7 points and an answer of ‘yes’ to item 7 on the RMI (‘Do you walk 10 metres with an aid or furniture if necessary but with no standby help?’). 65
Secondary outcomes
Patient outcomes (measured at 8 weeks, 6 months and 12 months)
-
Physical functioning was assessed using the RMI,65 BI,66 mRS,67 NEADL68 and ABILHAND. 69
-
Mood was assessed using the GHQ-12. 70
-
Fatigue (measured using the FAS72), concurrent musculoskeletal symptoms, signs and pain (using the MSK-SSP manikin74,78), activities of daily living (using the BI and NEADL), cognitive function (using the MoCA75), and number of therapy sessions and IMP doses were assessed for influence on the short-term effect of co-careldopa on physical functioning.
-
Cost-effectiveness was assessed using the EQ-5D79 and patient NHS resource use data (see Economic evaluation).
Carer outcomes (measured at 8 weeks, 6 months and 12 months)
-
Caregiver burden was assessed using the CBS. 71
Potential moderators and mediators of treatment effect (at 8 weeks)
-
Baseline clinical characteristics and investigations (e.g. routine brain neuroimaging) were recorded for use in later analysis to determine if they predict those who might benefit from co-careldopa-augmented rehabilitation.
Investigation of implementation within the NHS (including safety)
-
The AE profile associated with the combination treatment (NHS stroke rehabilitation treatment linked with co-careledopa) was recorded.
-
The practical implications of delivering the intervention within routine NHS acute and early community care of people with stroke were investigated.
-
The acceptability of co-careldopa treatment to stroke patients (study drug adherence) was measured at 8 weeks.
Data collection methods
Baseline data were collected by the clinical research team from clinical records and via face-to-face administration of the questionnaires, and participants also self-completed a baseline RMI measure. All baseline data were collected prior to randomisation.
Follow-up data were collected at 8 weeks, 6 months and 12 months via face-to-face administration by an independent blinded researcher in the participant’s home, at the hospital or at a community facility and documented on paper case report forms (CRFs) provided by the Clinical Trials Research Unit (CTRU). Completion of the primary outcome measure (RMI) was via telephone when it was not possible to arrange a face-to-face visit.
The researcher could choose to send the questionnaires to the patient in advance to allow time to prepare for the interview. Carer-completed outcomes were collected at the same time as the patient’s outcomes, when possible, and documented.
Assessment instruments
Table 2 summarises the measures used at each assessment time point and a summary of each instrument used is provided below:
-
The RMI assesses the ability to walk independently. It was completed by researchers prior to randomisation to inform the stratification of patients and by participants at baseline and follow-up to see how they assessed their own mobility. It has 15 items (score range of 0–15 points), with higher scores indicating an increased ability to walk independently.
-
The ABILHAND questionnaire measures upper limb impairment by asking participants to rate 23 items relating to their manual ability on a three-level scale (1, impossible; 2, difficult; and 3, easy). The raw scores are converted into a linear measure of ability using Rasch analysis. The scores are, thus, expressed as logits on an interval scale ranging from plus to minus with the centre of the scale set to zero; a higher number logit indicates greater patient-perceived ability. The baseline score in the DARS trial is based on participants’ own assessment of their manual ability in the month before their stroke and at the time of assessment at the follow-up.
-
Physical and social independence were assessed using the NEADL. This assesses aspects of physical and social independence performance across 22 items (score range of 0–66 points) grouped into four categories [(1) mobility, (2) kitchen, (3) domestic and (4) leisure activities]. A higher score indicates greater independence. At baseline, participants were asked to consider their independence before their stroke and at the follow-up they were asked to consider their indepencence in the previous month.
-
The GHQ-12 assesses emotional health. It contains 12 questions (score range of 0–36) addressing issues of decision-making, loss of sleep and confidence, feelings of strain, enjoyment of daily activities, confidence and happiness. A higher score indicates worse emotional health. The scores can also be categorised into no sign of psychological distress (score of ≤ 15 points), evidence of distress (score of > 15 points but ≤ 20 points), or severe problems and psychological distress (score of > 20 points).
-
Activities of daily living, disability and mobility were assessed using the BI. Ten items (score range of 0–20 points) evaluate the patient’s functional ability; higher scores indicate a greater degree of functional independence.
-
Joint, neck or back pain was measured using the MSK-SSP manikin. Combinations of locations of (1) pain in upper limbs, (2) pain in lower limbs, (3) central post-stroke pain, (4) thumb, hand, finger or wrist joint pain and (5) spinal pain were based on the previous work with MSK-SSP. 74,78
-
The FAS contains 10 items (score range of 10–50 points); higher scores indicate more severe fatigue.
-
The mRS is used to assess global outcome after stroke and is scored from 0 (no symptoms at all) to 5 (severe disability, bedridden); patients who die are given a score of 6.
-
The MoCA is a researcher-administered instrument that assesses cognitive function. It contains 10 items (score range of 0–30 points); a score of < 26 points indicates cognitive impairment.
-
The CBS is a 22-item scale (score range of 22–88 points) assessing various aspects of caregiver burden, including general strain, isolation, disappointment, emotional involvement and environment; higher scores indicates higher burden.
| Assessment | Baseline | Time (post randomisation) | ||
|---|---|---|---|---|
| 8 weeks (± 7 days) | 6 months (± 14 days) | 12 months (± 14 days) | ||
| Eligibility and consent | ✗ | |||
| Baseline data (researcher/nurse completed from routinely collected data and ward staff) | ||||
| RMI (professional perspective on patient’s ability for stratification) | ✗ | |||
| Past medical history | ✗ | |||
| Lesion location and type (CT) | ✗ | |||
| MoCA | ✗ | |||
| Randomisation (within 5–42 days post stroke) | ✗ | |||
| Patient questionnaires (completed via researcher interview with patient) | ||||
| RMI (patient’s perspective on ability) | ✗ | ✗ | ✗ | ✗ |
| ABILHAND | ✗a | ✗ | ✗ | ✗ |
| NEADL | ✗a | ✗ | ✗ | ✗ |
| GHQ-12 | ✗ | ✗ | ✗ | ✗ |
| EQ-5D | ✗ | ✗ | ✗ | ✗ |
| BI (postal version but collected face to face) | ✗ | ✗ | ✗ | ✗ |
| MSK-SSP manikin | ✗a | ✗ | ✗ | ✗ |
| FAS | ✗ | ✗ | ✗ | |
| Health economics resource use questionnaire | ✗ | ✗ | ✗ | ✗ |
| Carer questionnaires (carer completed) | ||||
| CBS | ✗ | ✗ | ✗ | |
| EQ-5D | ✗ | ✗ | ✗ | ✗ |
| Health economics resource use questionnaire | ✗ | ✗ | ✗ | ✗ |
| Qualitative follow-up | ||||
| Patient/therapist perspective regarding use of the IMP | ✗ | |||
| Clinical follow-up data (researcher/therapist/nurse completed) | ||||
| Treatment data (rehabilitation and drug compliance) | ✗ | |||
| mRS | ✗ | ✗ | ||
| MoCA | ✗ | ✗ | ✗ | |
| Serious and non-serious AE monitoring | Continuous reporting | |||
| New significant medical/surgical illness (e.g. for stroke, myocardial infarction, cancer, fracture, elective surgical procedures) | ✗ | ✗ | ||
Strategies to maximise data collection
Site achievement reports (SARs) were developed to provide sites with clear communication of accomplishments against trial-specific targets to assist sites in understanding their own performance. SAR benchmarks were based on recruitment, screening, baseline and consent data, intervention delivery monitoring and return of data essential for primary end-point reporting. RAG ratings indicated predefined target completion. Site-specific SARs were sent to site researchers (nurses and PIs); monthly and regional summaries were sent to the National Institute for Health Research (NIHR) SRN managers. 80
Several methods were implemented to allow flexibility in the collection of follow-up data to maximise return rate. This included a research nurse visiting the participant’s home to complete the questionnaires or completion at a hospital visit. The research nurse could also choose to send the questionnaires to the participant in advance of the scheduled appointment to allow the patient some time to prepare for the visit.
To maximise completion of the primary end-point data, the research nurse could complete the RMI questionnaire via telephone when it was not possible to arrange a face-to-face visit.
Sample size
The sample size calculations were based on the primary outcome of the proportion of people walking independently 8 weeks after randomisation. Independent walking is a robust and easily identifiable objective clinical outcome; according to Scheidtmann et al. ,52 42% of levodopa patients (11/26) were walking independently at 6 weeks, compared with 26% of placebo-group patients (7/27). Based on these published data, the sample size calculations indicated that 572 patients should be recruited in total. This provides 90% power at 5% significance to detect a 50% relative difference (< 15% absolute difference) between the placebo and active treatment groups in the proportion walking independently at 8 weeks post randomisation (as measured by an RMI score of ≥ 7 points and answer ‘yes’ to item number 7). This assumes a control rate of 26% independently walking by 8 weeks and ensures sufficient power to detect a rate of at least 39% in the active treatment group. This is slightly more conservative than the proportion improved in the Scheidtmann et al. 52 study. The calculations assume that the primary intention-to-treat (ITT) analysis includes all randomised patients and that patients who died or were lost to follow-up were unable to walk independently.
The DARS trial sample size also provides 80% power to detect a small to moderate effect size of 0.3 in key secondary outcomes (e.g. ABILHAND and NEADL). It was important that the trial had sufficient power to detect a real change in these secondary outcomes given that they are (1) important functional parameters in addition to walking and (2) likely to change if the treatment is effective. For all secondary analyses, loss to follow-up was estimated at 10% at 8 weeks, rising to 20% by 12 months, and accounted for intercurrent illness, late mortality and trial withdrawal. Loss to follow-up was minimised by collecting data via a face-to-face interview with a researcher.
Interim analyses and stopping guidelines
Outcome data were analysed once only, although statistical monitoring of safety data was conducted throughout the trial and reported at agreed intervals to the Data Monitoring and Ethics Committee (DMEC).
Strategies to maximise recruitment
Recruitment in to a pharmacorehabilitation stroke trial is challenging and the barriers to achieving target recruitment are multifaceted. A responsive approach was implemented to ensure issues in recruitment and implementation were identified in a timely manner so that the trial could adapt processes accordingly. Table 3 provides a summary of the strategies implemented and the perceived benefit.
| Strategy | Rationale |
|---|---|
| Liaison with sites and NIHR SRNs |
|
| Monitoring of screening/recruitment rates |
|
| Trial champions at local and national level |
|
| Central trial team |
|
| Newsletters/publicity |
|
| Regular trial oversight committee meetings |
|
| Addition of new sites |
|
Randomisation
Randomisation method
The randomisation sequence was created by the safety statistician using Stata® version 11.1 (StataCorp, College Station, TX, USA) statistical software. Random permuted block sizes of four were used to ensure that treatment groups were well balanced for the following stratification factors:
-
centre
-
type of stroke (primary intracranial haemorrhage or infarction)
-
RMI (score of 0–3 points, > 3 but < 7 points).
Eligible patients who had given written informed consent and completed baseline assessments were randomised on a 1 : 1 basis via the CTRU automated 24-hour telephone randomisation system to receive either co-careldopa or placebo.
Allocation concealment
A placebo tablet was manufactured to match the commercial co-careldopa (Sinemet) and the final assembly, packaging and labelling of the co-careldopa/placebo kit was undertaken by Sharp Clinical Services, UK. The composition of the placebo was approved by the Medicines and Healthcare products Regulatory Agency. Co-careldopa and the matching placebo were labelled with a unique random five-digit kit number, which was assigned to a participant on randomisation by the central randomisation system at the CTRU.
Two sets of code-break envelopes were provided by Sharp Clinical Services one set was shipped with the study medication to the participating site pharmacy and a second set was held securely at the CTRU for use in case of an emergency.
Blinding
The trial was double blind. Participants, clinicians, research staff and trial personnel at the CTRU involved in the day-to-day running of the trial were blinded to group allocation until the final database lock. Outcomes were collected by assessors masked to the treatment allocation. All analyses until the final analysis were undertaken blinded to treatment allocation. A trial safety team had access to the treatment allocation for the purposes of emergency unblinding and preparation of unblinded reports to the DMEC.
Emergency unblinding
Unblinding was permitted only when information about the participant’s trial treatment was clearly necessary and could alter the appropriate medical management of the patient.
Unblinding could be requested on the grounds of safety by the chief investigator, local PI or authorised delegate or treating physician. Requests for unblinding were first handled by the local PI, who explored the reason for the request and evaluated the importance of knowledge of treatment assignment for participant safety. In the event of a SAE, all patients were treated as though they were receiving the active medication.
Emergency unblinding was carried out using the code-break envelopes by the CTRU during office hours, and this was delegated to the local pharmacy department at the appropriate centre at all other times.
Withdrawal from treatment and data collection
Participants could withdraw from further trial treatment and/or data collection.
Participants did not continue to receive co-careldopa or the placebo after randomisation if they developed contraindications to the co-careldopa treatment, if the treating physician deemed that the patient was at a significant health risk from continued participation in the trial or if the participant decided that they no longer wished to take part in the trial.
If treatment was stopped, participants were still followed up unless they had withdrawn consent to follow-up. The primary reason for discontinuation was recorded.
Participants were made aware (through the information sheet and consent form) that, should they withdraw, safety data would still be collected after their last dose and all data collected prior to the withdrawal date would be used in the final analysis.
Patient and public involvement
We consulted with the Consumer Research Advisory Group (CRAG), which is a local Yorkshire-based group comprising stroke survivors and caregivers who give advice on research projects. Their advice was sought on the design of the IMP packaging and the proposal to invite patients to participate in more than one research project.
Mr Ossie Newell (Joint Chairperson of the Nottingham Stroke Research Consumer Group) reviewed the original research idea from a patient perspective and reviewed the patient information sheets and proposed outcome measures to ensure that they were appropriate for the patient population. Mr Michael Bonner, a member of the Trial Steering Committee (TSC), ensured that the patients’ perspective was considered during the progress of the trial. He also reviewed the patient information sheet and proposed outcome measures.
Statistical methods
The statistical analysis plan was finalised and approved by the Trial Management Group and TSC. Significance was tested at the 5% level for all analyses. Analyses were completed in SAS® version 9.2 (SAS Institute Inc., Cary, NC, USA). Analyses were pragmatic, based on an ITT sample.
Screening data
A flow diagram summarised the course of all patients through the screening process, including the number of patients approached, reasons for ineligibility, issues with consent and other reasons why the patient was not randomised. Age, sex and ethnicity data of the screening population were also summarised.
Baseline characteristics
Baseline characteristics of recruited participants were summarised by treatment group and overall. Baseline characteristics included stratification factors [centre, type of stroke (primary intracranial haemorrhage or infarction) and RMI (summarised as a continuous measure based on the researcher-completed questionnaire)], classification of stroke (if infarction) [total anterior circulation infarction (TACI), lacunar infarction (LACI), partial anterior circulation infarction (PACI) or posterior circulation infarction (POCI) classification], age, sex and ethnicity.
Baseline characteristics of carers were summarised by treatment group and overall, and included age, sex, ethnicity, relationship to the patient and whether or not they provided care before the patient’s stroke.
Continuous variables were reported as means and SDs, and categorical variables were presented as frequencies (n) and percentages. Only descriptive statistics were presented; no formal statistical testing was carried out on these data.
Primary end-point analysis
The primary end point was independent walking ability at 8 weeks post randomisation (defined by a RMI score of ≥ 7 points and answer ‘yes’ to item number 7 of the RMI).
For the ITT analysis of the primary outcome, it was assumed that patients who died or were lost to follow-up were unable to walk independently. The planned analysis for estimating differences in walking ability between treatment groups (co-careldopa and placebo) was to use a multilevel logistic regression model adjusted for stratification variables: sex, type of stroke, researcher-completed RMI baseline score (continuous) and centre (the last fitted as a random effect). However, inspection of the residuals, influence and model fit statistics and the diagnostic plots suggested poor model fit; therefore, stepwise regression was used to build a more robust model and included additional variables as agreed within the research team (age, baseline scores for BI, ABILHAND, NEADL, MoCA and GHQ-12, number of days between stroke and randomisation, and total number of sufficient motor therapy sessions). There were 13 missing values for baseline NEADL and BI; as the proportion of missing data was < 5%, missing data were imputed using mean imputation. 81 All stratifying variables were included in the stepwise model and a significance level of 0.05 was used to retain baseline variables. The final model contained, in addition to the stratifying variables, baseline NEADL and BI scores, age and the number of days from stroke to randomisation.
Parameter estimates or odds ratios (ORs) are reported, together with 95% confidence intervals (CIs) and p-values (fixed effects) or standard errors (SEs) (random effects).
Sensitivity and per-protocol analyses
A series of sensitivity analyses were conducted to test the robustness of the conclusions of the primary ITT analysis:
-
assuming that participants who died or were lost to follow-up were able to walk independently at 8 weeks
-
complete-case analysis
-
fitting centre as a fixed effect
-
using researcher-completed RMI score at baseline in place of score at 24-hour randomisation
-
using patient-completed RMI score at baseline instead of score at 24-hour randomisation
-
including only participants for whom the 8-week questionnaire was received by 12 weeks post randomisation
-
assuming that participants who had no primary outcome data because they were too unwell were unable to walk independently, and participants who had died, had withdrawn, were unwilling to be visited, refused to complete the questionnaire, had moved out of the area, those we could not get hold of and questionnaires that were lost at site or in the post were classed missing.
A per-protocol analysis was planned but could not be undertaken because of the small numbers remaining in the per-protocol population.
Patient secondary end points
Independent walking ability (primary outcome, defined by item 7 of the RMI and a RMI score of ≥ 7 points overall) at 6 months and 12 months post randomisation was analysed using a stepwise multilevel logistic regression model adjusted for stratifying variables. As with the primary end-point analysis, the planned analysis for other secondary end points including RMI (as a continuous measure), BI, ABILHAND, NEADL, MoCA and GHQ-12 at 8 weeks, 6 months and 12 months resulted in poor model fit and the same strategy of using stepwise multilevel regression (in this case linear) was employed. However, for secondary outcomes, the baseline outcome measurement was kept in the model with the stratifying variables, and the random effect was removed from the GHQ-12 model because the variance component was negative. mRS was analysed at 8 weeks and 6 months using a stepwise multilevel proportional odds logistic regression model adjusting for the same covariates; score test to check proportional odds assumption is reported. For dealing with missing items within individual outcome measures, the strategy was thus: mean imputation was used for the BI; NEADL, GHQ-12, FAS and CBS were prorated. No data were imputed for RMI as a continuous variable, MSK-SSP manikin or mRS.
Additionally, RMI at 12 months was analysed using repeated measures to investigate long-term trajectory using a random coefficients model to focus on difference between treatments in change in outcome over time. Only cases for which data were available at all time points were included in the model.
Parameter estimates, predicted mean values or ORs (ordinal logistic regression only) are reported with 95% CI and p-values.
Carer secondary end points
The carer burden using CBS was analysed at each follow-up by linear regression, adjusting for patient stratification factors (type of stroke and patient-completed RMI at baseline) and carer characteristics (age, sex and relationship to participant). Centre was not included in the model because of the small number of observations. Parameter estimates and predicted mean values with 95% CI and p-values are presented.
Safety
The number of AEs (reported up to the 8-week post-randomisation follow-up visit), the number of patients with AEs, the suspected relationship of all SAEs (reported up to 30 days beyond the last drug dose) to co-careldopa/placebo, the number of resolved SAEs and the proportion of patients with all SAEs resolved, the cause, Medical Dictionary for Regulatory Activities (MedDRA) System Organ Classification, seriousness criteria and outcome of SAEs are summarised by treatment group.
New, significant medical or surgical events from 8 weeks to 6 months and from 6 months to 12 months are summarised by treatment group.
The number, primary cause and timings of deaths are summarised by treatment group. The percentage of patients and carers who die from any cause between randomisation and 12 months follow-up are summarised by arm and centre.
The number of participants who vomited once or more often between taking the dose of protocol treatment and the end of therapy and the number of therapy sessions in which the participants vomited are presented by treatment arm.
Content of occupational and physical therapy
The number of participants being discharged to the community during treatment is summarised by treatment group. For patients discharged within 6 weeks of therapy, the time to discharge is also summarised by treatment group. The number of patients who commenced therapy > 5 days post discharge is summarised by treatment group.
The number of therapy sessions undertaken per patient from randomisation to the end of the 6-week treatment period, the average length of therapy sessions and therapist present is summarised by treatment group. The number of therapy sessions per week for each patient in hospital and in the community is summarised by treatment group.
The number of patients receiving sufficient motor therapy (i.e. those who received at least 20 minutes of motor therapy in at least 80% of therapy sessions) is summarised by treatment group.
Treatment compliance
The number of co-careldopa/placebo doses received during the 6 weeks of treatment is presented using summary statistics by treatment group as well as the proportion of patients receiving the same number of treatments as therapy sessions. If the numbers of therapy sessions and treatments are not consistent, a table summarises how much they differ by treatment group.
The proportion of all motor therapy sessions and the number of therapy sessions per patient in which the drug is taken as per protocol, in which the drug is taken but not 45–60 minutes before the therapy session and in which the drug is not taken at all are summarised. The timing of when each drug is taken is summarised, including those that are taken 45–60 minutes before the start of the therapy session. Reasons for not taking the drug at all are summarised. The proportion of times the drug was taken as per protocol (45–60 minutes before a motor therapy session) is summarised when the patient was still in hospital and when the patient received or did not receive a telephone call reminder for motor therapy sessions held in the community.
The number of patients with kit replacements and the total number of kit replacements are presented by treatment group and by centre.
Definitions of treatment compliance
The primary analysis was by ITT; subsequently, further analyses assessed the sensitivity of the conclusions of this analysis to non-compliance using a staged definition of ‘compliance’ (binary: yes or no). This definition was used for the purpose of complier-average causal effect (CACE) analyses. This was based on whether or not the drug was taken and the timing of this, the amount of motor therapy and the number of sessions:
-
Strict compliance – randomised drug taken 45–60 minutes before therapy, involving ≥ 20 minutes of motor therapy for ≥ 80% of the sessions.
-
Relaxed timing compliance – randomised drug taken 0–60 minutes or within 3 hours of a dose of drug (if patient had two therapy sessions directly one after the other) before therapy, involving ≥ 20 minutes of motor therapy for ≥ 80% of the sessions.
-
Relaxed timing and motor therapy compliance – randomised drug taken 0–60 minutes or within 3 hours of a dose of drug (if patient had two therapy sessions directly one after the other) before therapy for ≥ 80% of the motor sessions.
-
Drug intake compliance – missed ≤ 20% of drug within therapy sessions involving ≥ 20 minutes of motor therapy for ≥ 80% of the motor sessions.
If < 5 therapy sessions had been arranged, the definitions of compliance were set to missing. Treatment withdrawals were handled within the above definitions.
Patient perspective regarding the use of the investigational medicinal product with rehabilitation treatment
Responses to questions regarding the patient’s perspective of the use of the IMP measured at 8 weeks’ follow-up are summarised by treatment group, including how easy it was to take the tablet, how easy it was to remember to take it, how easy it was to get it out of the packet and how easy it was to understand the instructions on the packaging.
Unblinding requests
A summary of requests for unblinding was produced, including whether or not the patient was subsequently unblinded, reasons for this and whether or not the research team was unblinded.
Exit poll
An exit poll was completed by the patient and researcher at 12 months post randomisation. If patients had not completed an unblinding request, both the patient and the researcher were asked to guess which treatment the patient was randomised to.
This analysis was conducted on patient and researcher responses separately. A summary is included that looks at the proportion of patient and researcher guesses that are consistent by treatment group.
A blinding index has been calculated for each treatment group with 95% CI. 82 Reasons for the patient’s/researcher’s choice are summarised by treatment group and whether or not their choice was correct. Other reasons are listed.
Further analyses
The number of patients who watched the DARS trial information DVD is summarised by treatment group. Results from the questionnaire to establish whether or not the DVD was easy to understand and its usefulness for other trials are summarised by treatment group.
The correlations between watching the DVD, treatment compliance and questionnaire completion were calculated to explore if the DVD had a positive impact in the trial as well as for the patient. Logistic regression models are used while controlling for RMI at baseline, age, sex and type of stroke.
The level of assistance with patient-completed questionnaires and the person that provided assistance are summarised at baseline, 8 weeks, 6 months and 12 months, overall and by treatment group. The proportion of patients who received at least some help is compared between treatment groups using the chi-squared test. The difference between the two groups and corresponding 95% CI are reported.
Moderator and mediator analyses
Potential predictors of response to co-careldopa via moderators and mediators were explored.
Moderator analyses explored whether or not the size of the treatment effect depended on baseline characteristics of the patients. Moderator variables included baseline RMI score, depression (measured using the GHQ-12), pain (measured using the MSK-SSP manikin pre stroke), patient baseline medical history, neuroimaging and cognitive function (measured using the MoCA). Each moderator was tabulated by treatment and outcome, and analyses included moderator-by-treatment interaction effects one at a time into the final primary outcome multilevel model.
Mediator analyses explored the extent to which the treatment effect could be explained by an intermediate mechanistic outcome. Analyses focused on RMI at 8 weeks. Potential mediator variables related to a period prior to the outcome but post randomisation. At 8 weeks, these included the number of motor therapy sessions, number of IMP doses, fatigue (measured using the FAS), pain (measured using the MSK-SSP manikin), cognitive function (measured using the MoCA) and activities of daily living (measured using the BI and NEADL). Summary statistics of the mediators were tabulated by treatment and outcome, and the analyses followed the traditional approach of Baron and Kenny83 to establish mediation. 84 Each model was fitted as a multilevel model with centre as a random intercept and adjusting for the covariates included in the primary outcome model. The planned analyses also included investigation of departures from randomised treatment as a mediator through CACE analysis using the definitions of compliance as outlined in Definitions of treatment compliance. However, on inspection of the data, all patients received the treatment they were randomised to and, therefore, analysis focused on descriptive statistics of the different levels of compliance.
Radiological review
The influence of the stroke lesion itself (as seen via imaging) was explored as part of the secondary analysis. All available CT and MRI scans obtained during the index admission were obtained for participants who had provided informed consent. Participating sites copied the images to CD (compact disc) USB (universal serial bus) memory stick in Digital Imaging and Communication in Medicine (DICOM) format,85 and sent them to the CTRU for centralised review. Participating sites were instructed to remove patient-identifiable information (name, NHS number and treating clinician) from the scans before they were sent to the CTRU. If this was not possible, sites were required to encrypt the scan data. The possibility that scans would be incompletely anonymised was explained to participants in the patient information sheet and as part of the informed consent process.
The use of a standardised classification system for interpreting scans has been shown to minimise the risk of missing subtle changes. 86 A number of systems have been developed. 87–89 When selecting a system for the DARS trial, it was desirable to use an existing protocol with proven interobserver agreement that could be applied to both CT and MRI scans. As motor learning is thought to depend on the interaction of several cortical areas and the basal ganglia,20,21,28 the template was required to accurately code the location and extent of subcortical infarcts as well as the cortical lesions. The Acute Ischaemic Stroke Classification Template (AISCT)87 was developed by Professor Wardlaw et al. of the University of Edinburgh Brain Research Imaging Centre and is available to download from www.ed.ac.uk/files/imports/fileManager/CT%20and%20MR%20reading%20form.pdf (accessed 6 July 2018). It was used in DARS with permission of Professor Wardlaw (2015, personal communication).
The AISCT was developed in 1994 as a system that reliably codes both the site and size of an infarct, as well as the degree of swelling and any haemorrhagic transformation. 87 Following review of over 100 scans, Wardlaw90 constructed standardised templates to illustrate the different patterns of infarction and tissue swelling. The AISCT shows good interobserver agreement between experienced neuroradiologists for infarct size and type (K = 0.78), excellent agreement for infarct swelling (K = 0.8) and moderate agreement for haemorrhagic transformation of the infarct. 87 Among radiology trainees, it has moderate to good agreement for infarct size and site, fair to moderate agreement for infarct swelling and poor to fair agreement for haemorrhagic transformation. 87 The interobserver agreement of this system has subsequently been evaluated by the Acute Cerebral CT Evaluation of Stroke Study (ACCESS): a large online validation, which has now been ongoing for several years. 86,91 Neuroradiologists tended to spot more subtle signs of ischaemia than non-radiologists91 and took longer to read each scan than non-radiologists. 86 More severe ischaemia (hypodensity and swelling) was more reliably detected than subtle signs and a longer time from presentation to scan also improved detection rates for ischaemic change. 86 Detection of acute ischaemia was not influenced by scan quality or by the presence of an old ischaemic lesion. 86 The AISCT has been used in the Third International Stroke Trial (IST-3)92 and also a subsidiary study of IST-3 to determine if CT or MRI angiography might be used to guide administration of tissue plasminogen activator at up to 6 hours after stroke. 93
The AISCT begins with an overall subjective judgement on the quality of images (trichotomised as good, moderate or poor). The presence of any visible abnormality (an acute stroke lesion or any other pre-existing abnormality) is then documented. For those scans judged to be abnormal, the presence or absence and laterality of any ischaemic change is documented. Features of early ischaemia were classified on CT as loss of definition between the cortical grey matter and underlying white matter; loss of the outline of the basal ganglia; and frank hypodensity. 86,91,93 For MRI, signs of early ischaemic change were classified as a faint hyperintensity on diffusion-weighted imaging (DWI) sequences but no lesion visible on T2-weighted or fluid-attenuated inversion recovery (FLAIR) images; bright hyperintensity on DWI but no/pale lesion visible on T2 or FLAIR images; or a lesion clearly visible on T2 or FLAIR images as well as on DWI. 93 Acute swelling was classified using the AISCT framework and reference diagrams. 86 Middle cerebral artery (MCA) lesions were classified as involving less than or more than one-third of this territory. 86,88 Using the reference diagrams developed for the AISCT, MCA territory lesions were then further classified as small cortical; basal ganglia striatocapsular; lateral to ventricle striatocapsular; anterior cortical MCA territory; posterior cortical MCA territory; whole of cortical MCA territory; whole of cortical MCA territory with lateral part of basal ganglia; and whole MCA territory. 86 Lesions in the anterior cerebral artery and posterior cerebral artery territories were each defined as involving < 50% of that territory, > 50%, or complete. 86 Lacunar lesions were classified as involving the internal capsule or lentiform, internal border zone, centrum semiovale, or thalamus. 86 Infarcts involving the anterior and posterior border zones were noted. 86 Cerebellar lesions were classified as lacunar infarcts or as involving < 50% or > 50% of the hemisphere. 86 Similarly, brain stem lesions were classified as lacunar or as involving < 50% or > 50% of the brain stem. 86 The Alberta Stroke Proforma Early CT Score (ASPECTS)89 was recorded for all lesions involving the MCA territory. 86 Only the primary acute ischaemic lesion was coded in this way. When two acute ischaemic lesions were present, we utilised clinical judgement when deciding which was likely to be the most clinically significant. Any second (minor) acute ischaemic lesion was coded simply as present or absent. 93
The AISCT categorises the presence of haemorrhage as petechial, significant haemorrhagic transformation of an underlying infarct parenchymal haematoma with no infarct visible, parenchymal haematoma clearly remote from infarct, subdural haematoma, subarachnoid haemorrhage and extradural haemorrhage. 93 It also records the maximum diameter of the lesion (< 3 cm, 3–5 cm, 5–8 cm or > 8 cm). 93 If blood is present in more than one location (e.g. a primary parenchymal haematoma with rupture in the subarachnoid space), then the presence of both was recorded93 and a clinical judgement was exercised to place them in rank order of likely significance. 93 The following variables were documented for the DARS trial analysis: the location of haematoma (frontal, temporal, parietal, occipital, basal ganglia/thalamus, internal capsule, brain stem, cerebellum);94 the extent of midline shift (in millimetres); the presence or absence of hydrocephalus; and the presence or absence of intraventricular extension. Haematoma volume was calculated using the equation:
where A is the greatest diameter of the haemorrhage on axial imaging, B is the greatest diameter at 90° to A and C is the approximate number of axial imaging slices on which the haematoma is visible, multiplied by slice thickness. 95,96 Haematoma diameter and volume were calculated for confluent haematomas only and no attempt was made to assess these parameters for petechial haemorrhage or for subarachnoid blood.
The presence and extent of white matter lesions were documented using the same validated systems97,98 as were used by Wardlaw et al. 93 Old vascular lesions were classified as old cortical infarct(s), old striatocapsular infarct(s), old borderzone infarct(s), old lacunar infarct(s), old brain stem/cerebellar infarct(s) or probable old haemorrhage. 93 Finally, non-stroke lesions were classified as cerebral tumour, encephalitis, cerebral abscess and demyelination. 93 Brain atrophy was recorded as present or absent.
All scans were co-reported by the clinical research fellow and one of two experienced consultant neuroradiologists (Dr Jeremy Macmullen-Price and Dr Tufail Patankar) on a CRF using the standardised reporting system using AISCT or ASPECTS. Images were reviewed blinded to the original study report from the recruiting centre, clinical information (stroke laterality, administration of thrombolysis and Oxford Community Stroke Project clinical stroke syndrome),99 and trial treatment allocation. Images were reviewed in DICOM format. The time taken to review each case was not recorded; reviewers were free to take as long as they deemed necessary.
It was expected that all scans would be subjected to routine reporting by local radiologists in order to guide the clinical management of the patient. As the review of scans for the DARS trial could be delayed by several months from the point of a patient’s enrolment in the trial, the DARS trial analysis could not provide clinically significant information in a timely manner. It was also important that the trial team did not seek to influence or guide the clinical management of participants in any way. Clinical feedback was not provided to local sites. However, when clinically significant and unexpected findings arose, they were communicated to the local PI, who was then responsible for ensuring that they were noted and acted on if necessary. 100
Economic evaluation
Introduction
An economic evaluation was conducted to estimate the cost-effectiveness of DARS compared with placebo. To enable cross-analyses comparisons, decision-making and efficient distribution of scarce health-care resources, health economic analyses often follow prescribed methods. Thus, the proposed analyses followed the reference case set out by NICE in its guidance for technology appraisals. 101 The economic evaluation was conducted alongside the clinical trial analysing the data gathered therein; such economic evaluations are commonplace among stroke rehabilitation trials (e.g. Logan et al. 102 and Forster et al. 103).
The protocol stated that a decision-analytic model would be developed to model the costs and benefits of the interventions forward beyond the trial period. However, the decision model development was halted as the results from the statistical analysis indicated that co-careldopa did not confer incremental benefits over placebo at 8 weeks and, hence, there were no benefits to propagate forward. Despite these initial results, it was still considered necessary to conduct the economic evaluation based on the trial data.
Methods
End points, perspectives and discounting
The primary analysis was a cost–utility analysis with the primary end point of cost per quality-adjusted life-year (QALY) gained from a 6-week course of co-careldopa for rehabilitation post stroke versus placebo at 12 months. A secondary cost-effectiveness analysis (CEA) was conducted to establish the cost per incremental achievement of ≥ 7 points on the RMI. Although values from this may be less interpretable, it was incorporated to be consistent with the main trial statistical analysis, which employed this as the primary end point. 64
The cost perspective for the primary, base-case analysis was that of the Health and Personal Social Services (i.e. the health-care provider). However, a supplementary analysis considered a wider perspective encompassing costs to carers and patients. Given that the duration of the trial was 12 months, discounting of either costs or benefits was not required.
Measurement of effectiveness
The analysis used QALYs as the main outcome measure. QALYs represent a quality-weighted survival value where 1 QALY is the equivalent of 1 year of full health. The health-state utility values required for QALY calculation were captured using the EQ-5D, three-level version,104 at baseline, 2 months, 6 months and 12 months after randomisation. The EQ-5D is a commonly used generic measure of health-related quality of life (HRQoL) comprising five domains [(1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression], each with three response levels (1, no problems; 2, some problems; and 3, severe problems). The EQ-5D is NICE’s101 preferred measure of health benefit and has been validated in stroke105 and incorporated in many stroke trials (e.g. Palesch et al. 106 and Sackley et al. 107). Patient responses were converted to utility values using the UK time trade-off tariff. 108 Caregivers also reported on their own HRQoL using EQ-5D at the same time intervals. The RMI was completed via an interview with the patients at the same time as the EQ-5D. The RMI has 15 items that measure the ability of patients to make postural adjustments, transfer and walk and is scored from 0 to 15 points, with higher scores reflecting better mobility.
Measurement of costs
Health-care resource utilisation was captured using specially developed questionnaires covering primary care [e.g. general practitioner (GP) and nurse visits] and secondary care [e.g. hospital stays and accident and emergency (A&E) visits] use over the trial period. The questionnaires also included items capturing costs to the patient (e.g. travel, aid and home adaptation expenses) and impact on their income (i.e. earning losses). Caregivers responded separately with costs they had incurred (including earning losses) in the course of caring for the patient. The resource use questionnaires were completed alongside other outcome measures at 8 weeks, 6 months and 12 months after randomisation. The recall period for the measures was ‘Since you joined the study’ at 2 months and ‘In the last 3 months’ for the remaining two follow-ups. As the last two completions did not cover the full trial period, monthly resource counts were calculated and then multiplied by the number of months between the follow-up periods (i.e. by 4 and by 6 for the 6-month and 12-month follow-ups, respectively). Unit costs for health service staff and resources were obtained mainly from the Personal Social Services Research Unit (PSSRU)109 report and NHS Reference Cost databases. 110,111 The market price for co-careldopa was assigned using the electronic market information tool (eMit). 112 All costs were inflated to 2015 prices (Great British pounds) using an online inflator (see Appendix 2 for more details on unit costs). 113
Analysis
The primary analysis was a cost–utility analysis with a secondary CEA considering a RMI score of ≥ 7 points as the effect of interest. In line with the clinical efficacy analysis, an ITT analysis was the primary method for analysing and summarising the health economic trial data. A supplementary per-protocol analysis (using criteria set out by the clinical trial team) had been planned but, as very few patients met the strict compliance criteria, this analysis was not considered feasible. EQ-5D responses were converted to health-state utility values and multiplied by the proportion of a year the time period represented (baseline to 8 weeks = 0.167; 2–6 months = 0.333; 6–12 months = 0.5) to calculate QALYs. Average QALYs between adjacent time points were calculated to generate smoothed estimates between time points. If an individual died during the trial, their utility was assumed to be ‘0’ at that point and QALYs were calculated in the usual way.
Quality-adjusted life-years were calculated using an area under the curve approach:
Total costs and QALYs were calculated for each arm. For the supplementary analysis, the former included the costs to the patient and caregiver. If required, adjustments were made to account for differences in baseline utility.
In the event that one intervention was more costly and more effective or cheaper and less effective than the other, incremental cost-effectiveness ratios (ICERs) were calculated:
We used the NICE willingness to pay (WTP) per incremental QALY threshold [lambda (λ) = £20,000] to determine cost-effectiveness. Interventions with an ICER of < £20,000 per QALY are generally considered cost-effective. A non-parametric bootstrapping analysis (with replacement) was employed to determine the level of sampling uncertainty around the ICER by generating 10,000 estimates of incremental costs and benefits. The bootstrapped estimates were plotted on the cost-effectiveness plane. Estimates of net monetary benefit (NMB) were generated and used to estimate the probability that co-careldopa was cost-effective given a range of WTP per incremental QALY values (λ). This was presented as the cost-effectiveness acceptability curve (CEAC). 114,115 Therapies with average incremental NMB of > £0 should be adopted (assuming λ = £20,000). NMB was derived for each patient, thus:
We also ran a net benefit regression model to allow parametric analysis of the costs and benefits of the interventions. 116 Controlling for any baseline sample heterogeneity and significant baseline differences between groups, we determined whether or not treatment arm was a significant predictor of net benefit. This analysis was also used to determine whether or not the effects of co-careldopa were heterogeneous across different subgroups (including age, sex and stroke severity as indicated by 8-week mRS and infarct type). An additional NMB regression explored whether or not treatment compliance affected trial results.
Deterministic sensitivity analysis of the ICER was undertaken to test the robustness of the results. Outcomes and costs were altered by ± 20% (in both arms) to determine the effect of input parameter variation on the cost-effectiveness results. Multiple Imputation117 was used to generate estimates of missing cost and utility values. This approach is recommended for economic analyses conducted alongside clinical trials as it reflects the uncertainty inherent in replacing missing data. 118 Imputation was conducted using baseline characteristics (e.g. age and sex) and available health status measures, such as mRS, as these were considered to be important predictors of both EQ-5D and costs (for more details on the predictors see Chapter 4, Missing data). A complete-case sample was also presented, referring to those patients with no missing costs and no missing utility values over the trial. All analyses were conducted in Stata® version 13 (StataCorp LP, College Station, TX, USA) and Microsoft Excel® (2010; Microsoft Corporation, Redmond, WA, USA).
Chapter 3 Trial results
Screening and recruitment
During the recruitment period of May 2011 to March 2014, 19,509 patients were screened for eligibility. Of those screened, 1547 (7.9%) were deemed eligible, 599 (3.1%) were consented and 593 (3.0%) were randomised. Figure 3 shows the monthly, cumulative and target accrual and Figure 4 shows the flow of patients through the trial. The number of participants recruited per site ranged from 1 to 50.
FIGURE 3.
Cumulative recruitment over the DARS trial period (with revised target).
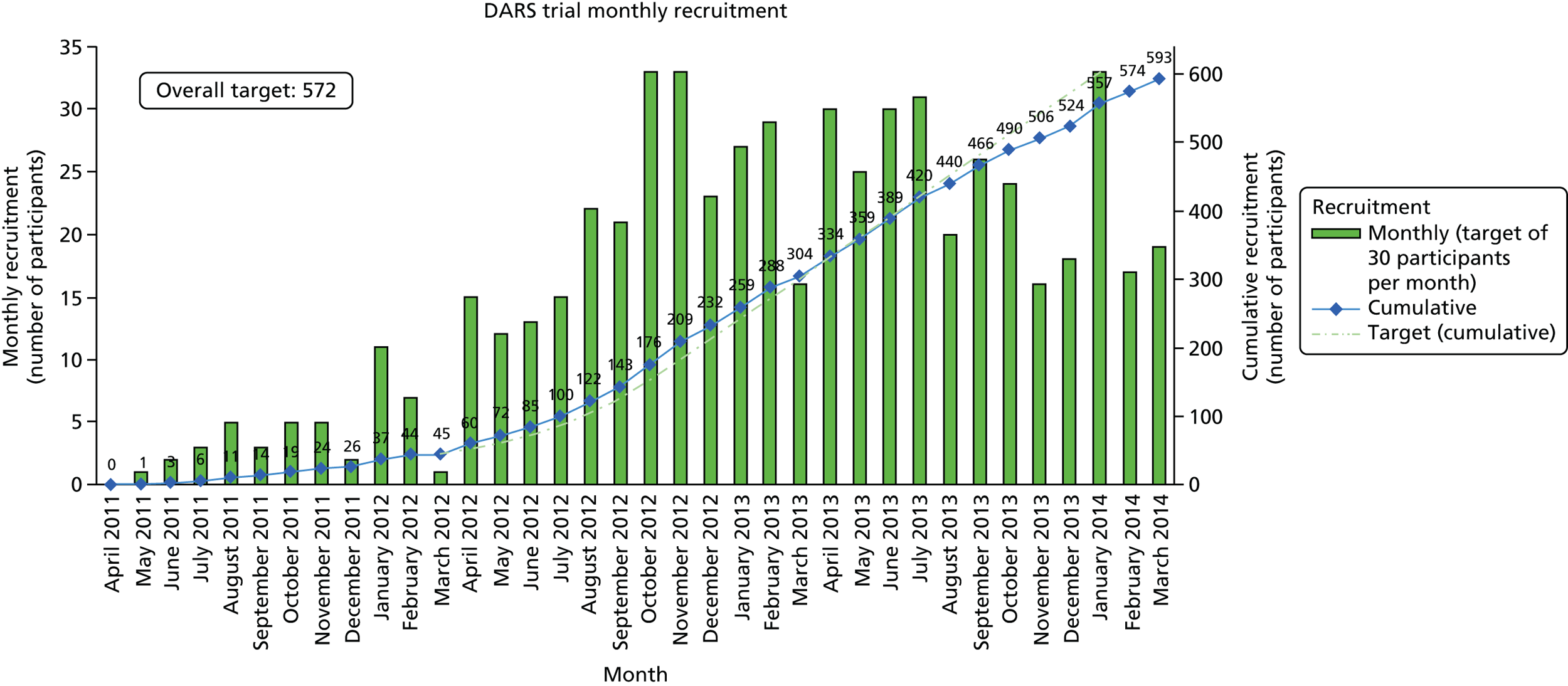
FIGURE 4.
Screening flow diagram for the DARS trial. a, Initial screening category. Reproduced with permission from Ford et al. 119 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
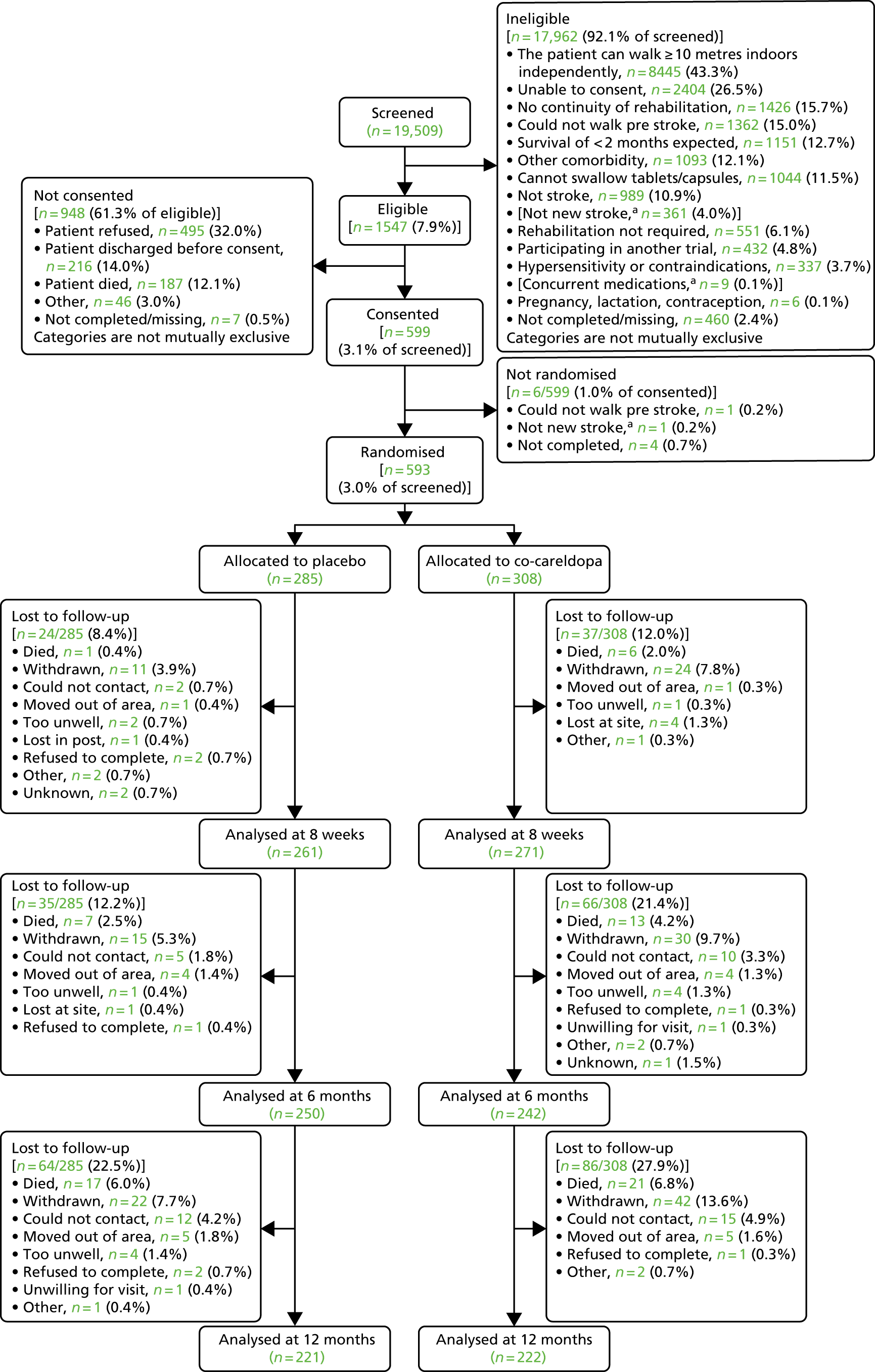
A comparison of screening data for randomised and non-randomised patients is presented in Table 4. Compared with screened patients, randomised patients were younger and more likely to be male, of white ethnicity and screened at a later date post stroke.
| Patient characteristic | Participant | |
|---|---|---|
| Screened (N = 19,509) | Randomised (N = 593) | |
| Days from stroke | ||
| Mean (SD), missing | 6.4 (9.45), 767 | 11.6 (10.36), 5 |
| Median (range) | 3 (0–180) | 8 (0–72) |
| Age (years) | ||
| Mean (SD), missing | 73.6 (14.28), 130 | 68.1 (13.17), 6 |
| Median (range) | 76 (18–105) | 70 (20–98) |
| Sex: male, n (%), missing (%) | 9297 (47.7), 90 (0.5) | 364 (61.4), 0 (0.0) |
| Ethnicity, n (%) | ||
| White | 17,197 (88.1) | 543 (91.6) |
| Other | 767 (3.9) | 32 (5.4) |
| Not stated or missing | 1545 (7.9) | 18 (3.0) |
Eligibility violations
Table 5 provides details of the 10 eligibility violations that were identified during the trial: five in each randomised group. In the placebo group, one participant was unable to comply with the treatment schedule and four were not randomised within the eligible time period post stroke.
| Randomisation arm | Eligibility breach | Protocol treatment received? | Number of | |
|---|---|---|---|---|
| Drug doses taken | Therapy sessions | |||
| Placebo | Unable to comply with treatment schedule | No | 0 | 0 |
| Not randomised within specified time period post stroke | Yes | 53 | 48 | |
| Not randomised within specified time period post stroke | Yes | 24 | 28 | |
| Not randomised within specified time period post stroke | Yes | 8 | 5 | |
| Not randomised within specified time period post stroke | Yes | 24 | 25 | |
| Co-careldopa | Incorrect diagnosis of stroke | Yes | 37 | 46 |
| Incorrect diagnosis of stroke | Yes | 2 | 2 | |
| Participating in another trial | Yes | 3 | 16 | |
| Known hypersensitivity or contraindications | Yes | 6 | 4 | |
| Known hypersensitivity or contraindications | Yes | 6 | 4 | |
Initially, the eligibility criteria stated that participants must be diagnosed with stroke within 2 weeks prior to randomisation, which was subsequently changed to 42 days. One participant violated eligibility early on in the trial by being diagnosed > 2 weeks before randomisation; the remaining were diagnosed > 42 days after randomisation.
In the co-careldopa group, one participant was taking part in another trial, two had known hypersensitivity or contraindications to co-careldopa and two had their diagnosis of stroke revised (one to demyelination and one to traumatic brain injury). With the exception of the participant who was unable to comply with treatment (because they were nil by mouth), all participants received some protocol treatment.
Participant baseline characteristics
Overall, patient baseline characteristics and questionnaire scores were balanced between the randomised groups, as shown by the demographic characteristics (see Table 6), hospital admission and current stroke data (see Table 7) and neuroimaging data (see Table 8).
The mean age was 67.5 years for co-careldopa participants and 69.6 years for placebo participants. More males than females were randomised: 187 (60.7%) in the co-careldopa group and 177 (62.1%) in the placebo group. The majority of participants were of white ethnic origin: 289 (93.8%) in the co-careldopa group and 270 (94.7%) in the placebo group. With regard to education, 134 co-careldopa participants (43.5%) and 139 placebo participants (48.8%) spent ≤ 12 years in formal, full-time education (Table 6).
| Baseline characteristic | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Patient age (years) at randomisation | |||
| Mean (SD) | 67.5 (13.65) | 69.6 (12.68) | 68.5 (13.22) |
| Median (range) | 69 (20–94) | 71 (31–98) | 70 (20–98) |
| Missing (n) | 0 | 0 | 0 |
| Patient sex, n (%) | |||
| Male | 187 (60.7) | 177 (62.1) | 364 (61.4) |
| Female | 121 (39.3) | 108 (37.9) | 229 (38.6) |
| Patient ethnicity, n (%) | |||
| White | 289 (93.8) | 270 (94.7) | 559 (94.3) |
| Mixed: white and black Caribbean | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Mixed: white and Asian | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Other mixed background | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Asian: Indian | 4 (1.3) | 3 (1.1) | 7 (1.2) |
| Asian: Pakistani | 2 (0.6) | 3 (1.1) | 5 (0.8) |
| Other Asian background | 3 (1.0) | 2 (0.7) | 5 (0.8) |
| Black: Caribbean | 1 (0.3) | 5 (1.8) | 6 (1.0) |
| Black: African | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Other black background | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Chinese | 2 (0.6) | 1 (0.4) | 3 (0.5) |
| Other ethnic group | 3 (1.0) | 0 (0.0) | 3 (0.5) |
| Education, n (%) | |||
| ≤ 12 years | 134 (43.5) | 139 (48.8) | 273 (46.0) |
| > 12 years | 168 (54.5) | 140 (49.1) | 308 (51.9) |
| Missing | 6 (1.9) | 6 (2.1) | 12 (2.0) |
Participants were randomised at around 2 weeks post stroke (median of 14 and 15 days in the co-careldopa and placebo groups, respectively). The cause of stroke was cerebral infarction in the majority of cases: in 270 participants (87.7%) in the co-careldopa group and in 238 participants (83.5%) in the placebo group. Thrombolysis was received by 62 co-careldopa participants (23.0%) and 59 placebo participants (24.8%) (Table 7). The proportion of patients with total anterior circulation infarcts was higher in the co-careldopa group than in the placebo group (36.3% vs. 26.5%, respectively; see Table 7), although baseline walking/mobility did not differ between the two groups (see Table 13). This difference was due to chance.
| Patient demographics | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Days from stroke to randomisation | |||
| Mean (SD) | 17.4 (9.91) | 18.0 (10.23) | 17.7 (10.06) |
| Median (range) | 14 (3–50) | 15 (3–59) | 15 (3–59) |
| Missing (n) | 0 | 0 | 0 |
| Days in hospital up to randomisation | |||
| Mean (SD) | 16.9 (10.10) | 17.4 (10.34) | 17.1 (10.21) |
| Median (range) | 14 (2–50) | 14 (3–59) | 14 (2–59) |
| Missing (n) | 0 | 0 | 0 |
| Days in stroke unit up to randomisation | |||
| Mean (SD) | 15.7 (9.89) | 16.5 (9.97) | 16.1 (9.93) |
| Median (range) | 13 (1–50) | 14 (1–55) | 13 (1–55) |
| Missing (n) | 0 | 0 | 0 |
| Type of stroke, n (%) | |||
| Infarction | 270 (87.7) | 238 (83.5) | 508 (85.7) |
| Primary haemorrhage | 38 (12.3) | 47 (16.5) | 85 (14.3) |
| If infarction, classification, n (% out of those with infarction) | |||
| TACI | 98 (36.3) | 63 (26.5) | 161 (31.7) |
| LACI | 58 (21.5) | 58 (24.4) | 116 (22.8) |
| PACI | 87 (32.2) | 91 (38.2) | 178 (35.0) |
| POCI | 27 (10.0) | 25 (10.5) | 52 (10.2) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Thrombolysis received, n (%) | |||
| Yes | 62 (23.0) | 59 (24.8) | 121 (23.8) |
| No | 208 (77.0) | 178 (74.8) | 386 (76.0) |
| Missing | 0 (0.0) | 1 (0.4) | 1 (0.2) |
The majority of participants underwent digital imaging [263 co-careldopa participants (88.0%) and 250 placebo participants (89%)] and more participants underwent CT than MRI [299 co-careldopa participants (97.1%) and 281 placebo participants (98.6%)] (Table 8).
| Neuroimaging details | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Days from stroke to imaging | |||
| Mean (SD) | 1.0 (3.44) | 0.6 (2.68) | 0.8 (3.10) |
| Median (range) | 0 (–2a to 32) | 0.0 (–36b to 13) | 0 (–36 to 32) |
| Missing (n) | 0 | 1 | 1 |
| Type of imaging, n (%) | |||
| CT brain | 299 (97.1) | 281 (98.6) | 580 (97.8) |
| Brain MRI | 9 (2.9) | 4 (1.4) | 13 (2.2) |
| Digital scans, n (%) | |||
| Yes | 263 (88.0) | 250 (89.0) | 513 (88.4) |
| No | 35 (11.7) | 31 (11.0) | 66 (11.4) |
| Missing (n) | 1 (0.3) | 0 (0.0) | 1 (0.2) |
Overall, 46 patients (7.8%) had also participated in another study: 27 in the co-careldopa group (8.8%) and 19 in the placebo group (6.7%).
The majority of participants had at least one current or ongoing medical condition: 284 in the co-careldopa group (92.2%) and 265 in the placebo group (93.0%), with a mean of 3.5 and 3.3 conditions, respectively.
Carer baseline characteristics
Eighty-four carers (27.3%) of participants in the co-careldopa group and 81 carers (28.4%) of participants in the placebo group consented to participate in the trial, and their baseline characteristics are presented in Table 9.
| Carer characteristics | Treatment group | Total (N = 165) | |
|---|---|---|---|
| Co-careldopa (N = 84) | Placebo (N = 81) | ||
| Carer age at randomisation (years) | |||
| Mean (SD) | 60.5 (15.33) | 58.9 (13.62) | 59.7 (14.49) |
| Median (range) | 62 (20–85) | 60 (21–87) | 61.5 (20.2–87) |
| Missing (n) | 2 | 1 | 3 |
| Carer sex, n (%) | |||
| Male | 23 (27.4) | 24 (29.6) | 47 (28.5) |
| Female | 61 (72.6) | 57 (70.4) | 118 (71.5) |
| Carer ethnicity, n (%) | |||
| White | 82 (97.6) | 77 (95.1) | 159 (96.4) |
| Asian: Indian | 1 (1.2) | 0 (0.0) | 1 (0.6) |
| Asian: Pakistani | 1 (1.2) | 1 (1.2) | 2 (1.2) |
| Other Asian background | 0 (0.0) | 1 (1.2) | 1 (0.6) |
| Black: Caribbean | 0 (0.0) | 2 (2.5) | 2 (1.2) |
| Carer-preferred language, n (%) | |||
| English | 84 (100.0) | 81 (100.0) | 165 (100.0) |
| Patient–caregiver relationship, n (%) | |||
| Partner (married/never married/divorced/separated) | 66 (78.6) | 56 (69.1) | 122 (73.9) |
| Daughter/son (including in-law, stepchild) | 15 (17.9) | 20 (24.7) | 35 (21.2) |
| Grandchild | 0 (0.0) | 1 (1.2) | 1 (0.6) |
| Parent | 2 (2.4) | 0 (0.0) | 2 (1.2) |
| Other relative | 0 (0.0) | 3 (3.7) | 3 (1.8) |
| Friend/neighbour | 1 (1.2) | 1 (1.2) | 2 (1.2) |
| Provided care pre stroke, n (%) | |||
| Yes | 36 (42.9) | 43 (53.1) | 79 (47.9) |
| No | 48 (57.1) | 38 (46.9) | 86 (52.1) |
Participant withdrawals
Ninety-one participants (51.3%) withdrew from the trial: 58 (18.8%) in the co-careldopa group and 33 (11.6%) in the placebo group (Table 10). The majority of withdrawals occurred within 8 weeks of randomisation. Patient withdrawal accounted for most of the withdrawals: 33 (10.7%) in the co-careldopa group and 12 (4.2%) in the placebo group. The PI withdrew 13 patients (4.2%) and nine patients in the co-careldopa (3.2%) and placebo groups, respectively. A participant’s health and/or an AE were the most common reasons given for withdrawal, although a large proportion did not provide a reason. Approximately half of the withdrawals from treatment were for a clinical reason, with a higher proportion in the placebo group (n = 7, 63.6%) than in the co-careldopa group (n = 8, 40.0%).
| Participant withdrawals | Treatment group, n (%) | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Withdrawals | 58 (18.8) | 33 (11.6) | 91 (15.3) |
| Time of withdrawal from randomisation | |||
| Up to 8 weeks | 40 (13.0) | 24 (8.4) | 64 (10.8) |
| From 8 weeks to 6 months | 6 (1.9) | 4 (1.4) | 10 (1.7) |
| From 6 months to 12 months | 12 (3.9) | 5 (1.8) | 17 (2.9) |
| Not withdrawn | 250 (81.2) | 252 (88.4) | 502 (84.7) |
| Person who withdrew consent for further trial treatment | |||
| Patient | 33 (10.7) | 12 (4.2) | 45 (7.6) |
| PI | 13 (4.2) | 9 (3.2) | 22 (3.7) |
| Multidisciplinary team | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| N/A – trial treatment completed | 12 (3.9) | 11 (3.9) | 23 (3.9) |
| If patient withdrew consent, is the patient still willing to be followed up? | |||
| Yes | 11 (33.3) | 6 (50.0) | 17 (37.8) |
| No | 22 (66.7) | 6 (50.0) | 28 (62.2) |
| If not, are they willing to have therapy forms collected? | |||
| Yes | 6 (27.3) | 0 (0.0) | 6 (21.4) |
| No | 7 (31.8) | 2 (33.3) | 9 (32.1) |
| NA – forms already collected | 9 (40.9) | 4 (66.7) | 13 (46.4) |
| Patient reason for withdrawal | |||
| Participant health/AE | 14 (42.4) | 8 (66.7) | 22 (48.9) |
| No continuity of rehabilitation | 0 (0.0) | 1 (8.3) | 1 (2.2) |
| Missing | 19 (57.6) | 3 (25.0) | 22 (48.9) |
| If PI withdrew consent, is the patient still willing to be followed up? | |||
| Yes | 6 (46.2) | 5 (55.6) | 11 (50.0) |
| No | 7 (53.8) | 4 (44.4) | 11 (50.0) |
| If not, are they willing to have therapy forms collected? | |||
| Yes | 0 (0.0) | 2 (50.0) | 2 (18.2) |
| No | 5 (71.4) | 1 (25.0) | 6 (54.5) |
| NA – forms already collected | 2 (28.6) | 1 (25.0) | 3 (27.3) |
| PI reason for withdrawal | |||
| Participant health/AE | 7 (53.8) | 5 (55.6) | 12 (54.5) |
| No continuity of rehabilitation | 1 (7.7) | 1 (11.1) | 2 (9.1) |
| Missing | 5 (38.5) | 3 (33.3) | 8 (36.4) |
| Clinically relateda | |||
| Yes | 8 (40.0) | 7 (63.6) | 15 (48.4) |
| No | 12 (60.0) | 4 (36.4) | 16 (51.6) |
Carer withdrawals
Ten carers (1.7%) withdrew: eight (2.6%) were carers of co-careldopa participants and two (0.7%) were carers of placebo participants. The majority of withdrawals occurred between 6 and 12 months following randomisation (Table 11).
| Carer withdrawals | Treatment group, n (%) | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Withdrawals | 8 (2.6) | 2 (0.7) | 10 (1.7) |
| Time of carer withdrawal from randomisation | |||
| Up to 8 weeks | 2 (0.6) | 0 (0.0) | 2 (0.3) |
| From 8 weeks to 6 months | 3 (1.0) | 0 (0.0) | 3 (0.5) |
| From 6 months to 12 months | 3 (1.0) | 2 (0.7) | 5 (0.8) |
Participant assessments
The time between randomisation and completion of questionnaires is shown in Figure 5. The median time between randomisation and follow-up assessments was 56 days for the 8-week primary outcome, 183 days for 6-month secondary outcomes and 370 days for 12-month secondary outcomes. At 8 weeks, six participants completed questionnaires later than the agreed timelines (five in the co-careldopa group and one in the placebo group). At 6 months, this increased to 16 participants (11 in the co-careldopa group and five in the placebo group). At 12 months, 25 questionnaires were completed late (16 in the co-careldopa group and nine in the placebo group).
FIGURE 5.
Time between randomisation and completion of RMI at each follow-up assessment.
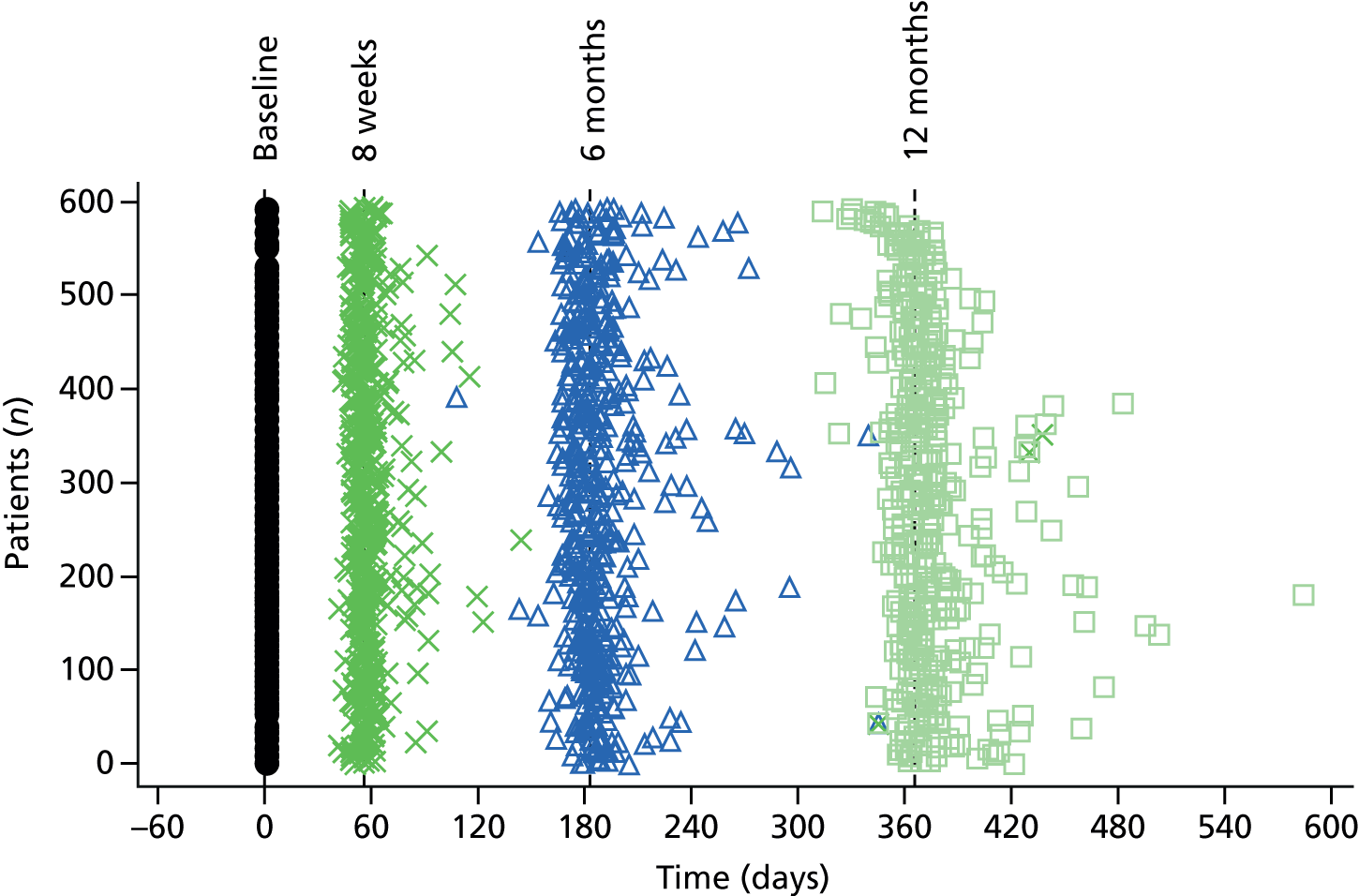
Table 12 shows the number of questionnaires completed at each follow-up assessment, together with reasons for non-completion. Loss to follow-up was balanced between treatment arms at 8 weeks and 12 months; however, at the secondary outcome assessment at 6 months, more participants in the co-careldopa group than in the placebo group did not complete the assessments (21.4% and 12.3%, respectively). A comparison between randomised groups of the characteristics of those who did not respond to the 6-month questionnaire revealed no differences in sex, age, stroke type, RMI score at baseline, ability to walk independently at 8 weeks or BI at baseline. However, among participants who did not respond to the 6-month questionnaire, those in the co-careldopa group had a statistically significantly higher NEADL mean score at baseline (59.6 points, 95% CI 57.4 to 61.7 points) than those in the placebo group (52.5 points, 95% CI 47.4 to 57.5 points; p = 0.012).
| Questionnaire completion at follow-up assessments | Time point, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | |||||||
| Co-careldopa (N = 308) | Placebo (N = 285) | Total (N = 593) | Co-careldopa (N = 308) | Placebo (N = 285) | Total (N = 593) | Co-careldopa (N = 308) | Placebo (N = 285) | Total (N = 593) | |
| Questionnaire completed | |||||||||
| Yes | 271 (88.0) | 261 (91.6) | 532 (89.7) | 242 (78.6) | 250 (87.7) | 492 (83.0) | 222 (72.1) | 221 (77.5) | 443 (74.7) |
| No | 37 (12.0) | 24 (8.4) | 61 (10.3) | 66 (21.4) | 35 (12.3) | 101 (17.0) | 86 (27.9) | 64 (22.5) | 150 (25.3) |
| Reason for non-completion | |||||||||
| Died | 6 (2.0) | 1 (0.4) | 7 (1.2) | 13 (4.2) | 7 (2.5) | 20 (3.4) | 21 (6.8) | 17 (6.0) | 38 (6.4) |
| Withdrawn | 24 (7.8) | 11 (3.9) | 35 (5.9) | 30 (9.7) | 15 (5.3) | 45 (7.6) | 42 (13.6) | 22 (7.7) | 64 (10.8) |
| Cannot get hold of participant | 0 (0.0) | 2 (0.7) | 2 (0.3) | 10 (3.3) | 5 (1.8) | 15 (2.5) | 15 (4.9) | 12 (4.2) | 27 (4.6) |
| Moved out of area | 1 (0.3) | 1 (0.4) | 2 (0.3) | 4 (1.3) | 4 (1.4) | 8 (1.3) | 5 (1.6) | 5 (1.8) | 10 (1.6) |
| Too unwell | 1 (0.3) | 2 (0.7) | 3 (0.5) | 4 (1.3) | 1 (0.4) | 5 (0.8) | 0 (0.0) | 4 (1.4) | 4 (0.7) |
| Lost in post | 0 (0.0) | 1 (0.4) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lost at site | 4 (1.3) | 0 (0.0) | 4 (0.7) | 0 (0.0) | 1 (0.4) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Refused to complete | 0 (0.0) | 2 (0.7) | 2 (0.3) | 1 (0.3) | 1 (0.4) | 2 (0.3) | 1 (0.3) | 2 (0.7) | 3 (0.5) |
| Unwilling for visit | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Other | 1 (0.3) | 2 (0.7) | 3 (0.5) | 2 (0.7) | 0 (0.0) | 2 (0.3) | 2 (0.7) | 1 (0.4) | 3 (0.5) |
| Unknown | 0 (0.0) | 2 (0.7) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Table 13 summarises the participant assessments at baseline and 8 weeks, 6 months and 12 months post randomisation. Further description is provided in the following sections.
| Assessment | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 8 weeks | 6 months | 12 months | |||||
| Co-careldopa | Placebo | Co-careldopa | Placebo | Co-careldopa | Placebo | Co-careldopa | Placebo | |
| Researcher-reported RMI score (points) | ||||||||
| Mean (SD) | 2.2 (1.8) | 2.3 (1.8) | ||||||
| 0–3, n (%) | 237 (76.9) | 223 (78.2) | – | – | – | – | – | – |
| > 3 but < 7, n (%) | 71 (23.1) | 62 (21.8) | – | – | – | – | – | – |
| Patient-reported RMI score (points) | ||||||||
| Mean (SD) | 2.4 (2.2) | 2.5 (2.2) | 6.8 (4.2) | 7.0 (4.2) | 8.3 (4.6) | 8.1 (4.5) | 8.7 (4.7) | 8.5 (4.6) |
| Able to walk independently, n (%) | 10 (3.2) | 7 (2.5) | 125 (40.6) | 127 (44.6) | 159 (51.6) | 152 (53.3) | 159 (51.6) | 162 (56.8) |
| NEADL score (points), mean (SD) | 59.0 (11.0) | 58.6 (12.4) | 21.0 (17.7) | 20.0 (15.8) | 27.2 (18.2) | 27.3 (18.1) | 30.4 (19.4) | 29.8 (18.9) |
| BI score (points), mean (SD) | 7.7 (3.8) | 7.8 (3.7) | 12.9 (5.1) | 13.2 (4.9) | 14.0 (5.1) | 14.4 (5.1) | 14.4 (5.4) | 14.6 (5.1) |
| ABILHAND, mean (SD) logits | 0.8 (3.9) | 0.3 (1.8) | 0.2 (2.3) | 0.4 (2.2) | 0.1 (2.4) | 0.3 (2.5) | 0.2 (2.6) | 0.4 (2.6) |
| MSK-SSP manikin, n (%) | ||||||||
| Joint, neck or back pain | 129 (41.9) | 107 (37.5) | 206 (66.9) | 191 (67.0) | 188 (61.0) | 187 (65.6) | 152 (49.4) | 146 (51.2) |
| Pain in upper limbs | 60 (19.5) | 54 (18.9) | 165 (53.6) | 159 (55.8) | 148 (48.1) | 138 (48.4) | 113 (36.7) | 108 (37.9) |
| Pain in lower limbs | 82 (26.6) | 72 (25.3) | 127 (41.2) | 113 (39.6) | 126 (40.9) | 130 (45.6) | 117 (38.0) | 100 (35.1) |
| Right central post-stroke pain | 6 (1.9) | 2 (0.7) | 14 (4.5) | 11 (3.9) | 10 (3.2) | 13 (4.6) | 11 (3.6) | 10 (3.5) |
| Left central post-stroke pain | 3 (1.0) | 1 (0.4) | 19 (6.2) | 22 (7.7) | 19 (6.2) | 17 (6.0) | 19 (6.2) | 14 (4.9) |
| Central post-stroke pain | 7 (2.3) | 3 (1.1) | 31 (10.1) | 32 (11.2) | 26 (8.4) | 30 (10.5) | 29 (9.4) | 22 (7.7) |
| Right thumb, hand, finger or wrist joint pain | 5 (1.6) | 3 (1.1) | 16 (5.2) | 20 (7.0) | 8 (2.6) | 13 (4.6) | 17 (5.5) | 6 (2.1) |
| Left thumb, hand, finger or wrist joint pain | 6 (1.9)a | 3 (1.1)a | 37 (12.0) | 33 (11.6) | 22 (7.1) | 18 (6.3) | 17 (5.5) | 13 (4.6) |
| Any thumb, hand, finger or wrist joint pain | 8 (2.6)a | 5 (1.8)a | 52 (16.9) | 48 (16.8) | 29 (9.4) | 31 (10.9) | 32 (10.4) | 18 (6.3) |
| Spinal pain | 10 (3.2)a | 5 (1.8)a | 3 (1.0) | 3 (1.1) | 2 (0.6) | 5 (1.8) | 6 (1.9) | 8 (2.8) |
| GHQ-12 score (points) | ||||||||
| Mean (SD) | 19.4 (6.7) | 19.3 (7.0) | 16.9 (7.2) | 16.4 (6.6) | 15.1 (7.00) | 16.3 (6.80) | 14.0 (6.77) | 14.4 (7.16) |
| No sign of psychological distress, n (%) | 91 (29.5) | 94 (33.0) | 128 (41.6) | 121 (42.5) | 139 (45.1) | 125 (43.9) | 152 (49.4) | 133 (46.7) |
| Evidence of distress, n (%) | 85 (27.6) | 68 (23.9) | 60 (19.5) | 72 (25.3) | 46 (14.9) | 58 (20.4) | 32 (10.4) | 46 (16.1) |
| Severe problems and psychological distress, n (%) | 117 (38.0) | 115 (40.4) | 79 (25.6) | 62 (21.8) | 56 (18.2) | 64 (22.5) | 37 (12.0) | 39 (13.7) |
| FAS score (points) mean (SD) | – | – | 25.1 (7.6) | 24.8 (7.4) | 25.9 (8.1) | 25.4 (7.6) | 24.9 (8.3) | 24.5 (8.2) |
| mRS score, n (%) | ||||||||
| 0 | – | – | 3 (1.0) | 1 (0.4) | 1 (0.3) | 2 (0.7) | – | – |
| 1 | – | – | 15 (4.9) | 11 (3.9) | 29 (9.4) | 25 (8.8) | – | – |
| 2 | – | – | 24 (7.8) | 30 (10.5) | 23 (7.5) | 30 (10.5) | – | – |
| 3 | – | – | 101 (32.8) | 114 (40.0) | 123 (39.9) | 128 (44.9) | – | – |
| 4 | – | – | 95 (30.8) | 79 (27.7) | 41 (13.3) | 47 (16.5) | – | – |
| 5 | – | – | 34 (11.0) | 34 (11.9) | 27 (8.8) | 16 (5.6) | – | – |
| 6 | – | – | 6 (1.9) | 1 (0.4) | 6 (1.9) | 4 (1.4) | – | – |
| MoCA score (points) | ||||||||
| Mean (SD) | 20.0 (6.6) | 20.5 (6.0) | 22.4 (6.3) | 22.9 (5.5) | 23.1 (6.2) | 23.6 (5.5) | 23.1 (5.9) | 23.5 (5.6) |
| Normal (≥ 26 points), n (%) | 57 (18.5) | 63 (22.1) | 106 (34.4) | 95 (33.3) | 110 (35.7) | 104 (36.5) | 95 (30.8) | 96 (33.7) |
| Cognitive impairment (< 26 points), n (%) | 242 (78.6) | 218 (76.5) | 160 (51.9) | 165 (57.9) | 132 (42.9) | 142 (49.8) | 124 (40.3) | 119 (41.8) |
Rivermead Mobility Index
The mean baseline researcher-completed RMI score was 2.2 points in the co-careldopa group and 2.3 points in the placebo group. Participant-completed baseline RMI scores were similar, although 10 participants (3.2%) in the co-careldopa group and seven (2.5%) in the placebo group considered themselves able to walk independently. At the 8-week follow-up, patient-reported RMI scores showed a considerable increase, compared with baseline, in the proportion of participants who considered themselves to be able to walk independently: 125 (40.6%) in the co-careldopa group and 127 (44.6%) in the placebo group. A further increase in the proportion of participants who were able to walk was observed at 6 months, reaching 159 (51.6%) in the co-careldopa group and 152 (53.3%) in the placebo group; however, these figures remained relatively stable at 12 months, with no increase in the co-careldopa group and only a slight increase in the placebo group [162 participants (56.8%)].
Nottingham Extended Activities of Daily Living
The mean baseline (pre-stroke) NEADL score was 59.0 points in the co-careldopa group and 58.6 points in the placebo group. As expected, NEADL scores at 8 weeks post randomisation were considerably worse than the pre-stroke scores; the mean score was 21.0 points in the co-careldopa group and 20.0 points in the placebo group. Scores were higher at 6 months: the mean score was 27.2 points in the co-careldopa group and 27.3 points in the placebo group. A further moderate increase was observed at 12-month follow-up: the mean score was 30.4 points in the co-careldopa group and 29.8 points in the placebo group.
Barthel Index
The mean BI score at baseline was 7.7 points in the co-careldopa group and 7.8 points in the placebo group. A greater degree of functional independence was indicated at the 8-week follow-up, when the mean BI score was 12.9 points in the co-careldopa group and 13.2 points in the placebo group. The mean BI score was higher at 6 months than at 8 weeks but levelled off at 12 months.
The ABILHAND Manual Ability Measure
The mean ABILHAND score (pre stroke) was 3.1 logits in the co-careldopa group and 3.2 logits in the placebo group. Scores at the 8-week follow-up were predictably worse than pre-stroke scores but improved at later follow-ups, particularly in the co-careldopa group.
Musculoskeletal – symptoms/signs and pain manikin
The proportion of participants reporting pre-stroke pain was approximately balanced between treatment arms. A total of 129 participants (41.9%) and 107 participants (37.5%) in the co-careldopa and placebo group, respectively, said that they had joint, neck or back pain in at least one area. Musculoskeletal pain was more prevalent in both randomised groups at the 8-week follow-up but was experienced by fewer participants at 6 months and 12 months.
General Health Questionnaire 12-item version
The mean GHQ-12 score at baseline was 19.4 points in the co-careldopa group and 19.3 points in the placebo group, with a large proportion of participants reporting severe problems and psychological stress: 117 (38.0%) in the co-careldopa group and 115 (40.4%) in the placebo group. At the 8-week follow-up, the mean GHQ-12 score was lower in both randomised groups and the proportion of participants reporting severe problems and psychological distress decreased to 26% in the co-careldopa group and to 22% in the placebo group. A mild improvement in mean GHQ-12 score continued in the co-careldopa group at 6 months and 12 months, whereas an improvement was not seen in the placebo group until 12 months.
Montreal Cognitive Assessment
The mean baseline MoCA score was 20.0 points in the co-careldopa group and 20.5 points in the placebo group, and the majority of participants were found to have cognitive impairment: 218 (78.6%) and 218 (76.5%) in the co-careldopa and placebo groups, respectively. Although the mean score stayed relatively stable for all of the follow-up assessments, in both randomised groups, the proportion of participants with cognitive impairment was lower at the 8-week, 6-month and 12-month follow-ups than at baseline.
Fatigue Assessment Scale
The mean FAS scores at 8 weeks indicated similar levels of fatigue in the co-careldopa and placebo groups (25.1 and 24.8 points, respectively) and remained relatively unchanged at the 6- and 12-month follow-ups.
Modified Rankin Scale
The mRS indicated good functional independence at 8 weeks (i.e. a score of ≤ 2) in 42 participants (13.7%) in the co-careldopa group and 42 participants (14.8%) in the placebo group. This improved to 53 participants (17.2%) in the co-careldopa group and 57 participants (20.0%) in the placebo group at the 6-month follow-up.
Carer assessments
Table 14 summarises the category and total scores for CBS at all time points. At the 8-week follow-up, carers of participants in both arms reported moderate to severe burden, although those in the placebo group reported higher mean total scores than those in in the co-careldopa group: 46.6 points compared with 43.0 points, respectively, with strain, emotional burden and disappointment accounting for the greatest differences between the groups. The score at 6 months increased slightly in both groups, to 44.6 points in the co-careldopa group and to 49.1 points in the placebo group, whereas, at 12 months, the mean score remained stable in the co-careldopa group (44.6 points) but increased slightly in the placebo group (51.8 points). The participant characteristics for the subsample with a consenting carer are shown in Table 15 and appear similar between arms.
| Assessment | Time point | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Co-careldopa (n = 74) | Placebo (n = 72) | Co-careldopa (n = 62) | Placebo (n = 65) | Co-careldopa (n = 50) | Placebo (n = 107) | |
| Total CBS score (points), mean (SD) | 43.0 (13.4) | 46.6 (13.9) | 44.6 (13.6) | 49.1 (14.7) | 44.6 (15.1) | 51.8 (15.3) |
| Strain, mean (SD) | 17.3 (6.1) | 18.8 (6.4) | 18.3 (5.9) | 19.7 (6.4) | 18.4 (6.7) | 21.1 (6.6) |
| Isolation, mean (SD) | 6.3 (2.5) | 6.9 (2.4) | 6.5 (2.4) | 7.1 (2.3) | 6.1 (2.4) | 7.4 (2.5) |
| Disappointment, mean (SD) | 10.4 (3.8) | 11.1 (3.6) | 10.5 (3.7) | 11.9 (3.9) | 10.6 (3.9) | 12.3 (4.2) |
| Emotional, median (IQR) | 10 (5–20) | 11 (5–20) | 4 (3–12) | 4.0 (3–12) | 4 (3–10) | 4 (3–12) |
| Environment, mean (SD) | 5.0 (2.3) | 5.3 (2.1) | 5.0 (2.2) | 5.6 (2.5) | 4.9 (2.0) | 6.1 (2.3) |
| Characteristic of patients with carers | Treatment group | Total (N = 165) | |
|---|---|---|---|
| Co-careldopa (N = 84) | Placebo (N = 81) | ||
| Patient sex: male, n (%) | 55 (65.5) | 55 (67.9) | 110 (66.7) |
| Patient ethnicity: white, n (%) | 82 (97.6) | 76 (93.8) | 158 (95.8) |
| Education, n (%) | |||
| ≤ 12 years | 41 (48.8) | 48 (59.3) | 89 (53.9) |
| > 12 years | 41 (48.8) | 30 (37.0) | 71 (43.0) |
| Missing | 2 (2.4) | 3 (3.7) | 5 (3.0) |
| Type of stroke, n (%) | |||
| Infarction | 73 (86.9) | 67 (82.7) | 140 (84.8) |
| Primary haemorrhage | 11 (13.1) | 14 (17.3) | 25 (15.2) |
| If infarction, then classification, n (%) | |||
| TACI | 29 (34.5) | 21 (25.9) | 50 (30.3) |
| LACI | 15 (17.9) | 13 (16.0) | 28 (17.0) |
| PACI | 23 (27.4) | 28 (34.6) | 51 (30.9) |
| POCI | 6 (7.1) | 5 (6.2) | 11 (6.7) |
| Patient walking independently (binary), n (%) | |||
| Baseline (yes) | 3 (3.6) | 3 (3.7) | 6 (3.6) |
| 8 weeks (yes) | 33 (39.3) | 33 (40.7) | 66 (40.0) |
| 6 months (yes) | 42 (50.0) | 39 (48.1) | 81 (49.1) |
| 12 months (yes) | 46 (54.8) | 46 (56.8) | 92 (55.8) |
| Patient-reported RMI score (points) (continuous), mean (SD), missing | |||
| Baseline | 2.3 (2.27), 3 | 2.4 (2.31), 2 | 2.3 (2.28), 5 |
| 8 weeks | 6.4 (3.96), 7 | 6.5 (4.19), 4 | 6.5 (4.07), 11 |
| 6 months | 8.0 (4.84), 14 | 7.6 (4.57), 5 | 7.8 (4.69), 19 |
| 12 months | 8.5 (4.76), 20 | 7.8 (4.56), 12 | 8.2 (4.65), 32 |
Primary end point
Intention-to-treat analysis
Table 16 presents the summary statistics by ability to walk independently at 8 weeks and presents the ORs and 95% CIs for the ITT analysis.
| Variable in the primary end-point analysis | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 252) | No (N = 341) | Total (N = 593) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 125 (40.6) | 183 (59.4) | 308 (100.0) |
| Placebo | 127 (44.6) | 158 (55.4) | 285 (100.0) |
| Patient sex, n (%) | |||
| Male | 169 (46.4) | 195 (53.6) | 364 (100.0) |
| Female | 83 (36.2) | 146 (63.8) | 229 (100.0) |
| Age (years) | |||
| Mean (SD) | 65.5 (14.12) | 70.7 (12.07) | 68.5 (13.22) |
| Median (range) | 66 (20–96) | 72 (21–98) | 70 (20–98) |
| Type of stroke, n (%) | |||
| Infarction | 206 (81.7) | 302 (88.6) | 508 (85.7) |
| Primary haemorrhage | 46 (18.3) | 39 (11.4) | 85 (14.3) |
| RMI score at baseline (points) | |||
| Mean (SD) | 3.1 (1.79) | 1.6 (1.49) | 2.3 (1.79) |
| Median (range) | 3 (0–6) | 1 (0–6) | 2 (0–6) |
| NEADL score at baseline (points) | |||
| Mean (SD) | 60.7 (10.00) | 57.4 (12.61) | 58.8 (11.68) |
| Median (range) | 66 (0–66) | 63 (0–66) | 63 (0–66) |
| Missing (n) | 7 | 6 | 13 |
| BI score at baseline (points) | |||
| Mean (SD) | 9.3 (3.44) | 6.5 (3.44) | 7.7 (3.70) |
| Median (range) | 9 (3–19) | 6 (0–20) | 7 (0–20) |
| Missing (n) | 5 | 8 | 13 |
| Days between stroke and randomisation | |||
| Mean (SD) | 15.0 (9.55) | 19.7 (9.98) | 17.7 (10.06) |
| Median (range) | 12 (3–59) | 17 (3–55) | 15 (3–59) |
There was no evidence of a statistically significant difference between treatment groups in the proportion of participants able to walk independently at 8 weeks [125 (40.6%) in the co-careldopa group vs. 127 (44.6%) in the placebo group; OR 0.780, 95% CI 0.528 to 1.153; see Table 16] (Table 17).
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.780 (0.528 to 1.153) | 0.212 |
| Sex: female vs. male | 0.900 (0.599 to 1.353) | 0.612 |
| Stroke type: infarction vs. primary haemorrhage | 0.382 (0.219 to 0.665) | 0.001 |
| RMI score at baseline | 1.522 (1.313 to 1.764) | 0.000 |
| Age (years) | 0.980 (0.965 to 0.995) | 0.010 |
| NEADL score at baseline | 1.026 (1.006 to 1.046) | 0.011 |
| BI score at baseline | 1.110 (1.034 to 1.191) | 0.004 |
| Days between stroke and randomisation | 0.945 (0.925 to 0.965) | 0.000 |
The ability to walk independently did not differ between males and females [169 (46.4%) vs. 83 (36.2%), respectively; OR 0.900, 95% CI 0.599 to 1.353]. Participants who had suffered an infarction were significantly less likely to be able to walk than those who had a primary haemorrhage [206 (40.6%) vs. 46 (54.1%), respectively; OR 0.382, 95% CI 0.219 to 0.667]. The ability to walk independently at 8 weeks was associated with higher baseline scores for RMI (OR 1.522, 95% CI 1.313 to 1.764), NEADL (OR 1.026, 95% CI 1.006 to 1.046) and BI (OR 1.110, 95% CI 1.034 to 1.191). There was an inverse relationship between walking at 8 weeks and age (OR 0.980, 95% CI 0.965 to 0.995), and number of days between stroke and randomisation (OR 0.945, 95% CI 0.925 to 0.965).
Per-protocol analysis
The per-protocol population excluded the following participants: (1) non-eligible patients; (2) patients not receiving the treatment they were randomised to including treatment crossovers; (3) those receiving no IMP prior to rehabilitation therapy; (4) patients not strictly complying with protocol (i.e. randomised drug taken 45–60 minutes before therapy, involving ≥ 20 minutes of motor therapy for ≥ 80% of the sessions); and (5) patients completing the 8-week follow-up primary end point outside 7–9 weeks. Figure 6 shows the number of exclusions at each step. The final sample size of the per-protocol population was 54 (9.1% of the ITT population), representing 9.7% of the co-careldopa group and 8.4% of the placebo group. Table 18 presents the descriptive statistics of the per-protocol population. Owing to the small sample size, no analyses were conducted on this population.
FIGURE 6.
Flow chart of exclusions to obtain per-protocol population.

| Participant characteristics | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 24) | No (N = 30) | Total (N = 54) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 14 (46.7) | 16 (53.3) | 30 (100.0) |
| Placebo | 10 (41.7) | 14 (58.3) | 24 (100.0) |
| Patient sex, n (%) | |||
| Male | 13 (48.1) | 14 (51.9) | 27 (100.0) |
| Female | 11 (40.1) | 16 (59.9) | 27 (100.0) |
| Type of stroke, n (%) | |||
| Infarction | 22 (44.9) | 27 (55.1) | 49 (100.0) |
| Primary haemorrhage | 2 (40.0) | 3 (60.0) | 5 (100.0) |
| RMI score at baseline (points) | |||
| Mean (SD) | 2.8 (1.58) | 1.8 (1.24) | 2.3 (1.48) |
| Median (range) | 3.0 (0.0–6.0) | 2.0 (0.0–4.0) | 2.0 (0.0–6.0) |
| Q1, Q3 (points) | 1.5, 4.0 | 1.0, 3.0 | 1.0, 3.0 |
A number of sensitivity analyses were conducted to test the robustness of the assumption that patients who died or were lost to follow-up were categorised as unable to walk independently. Descriptive statistics and estimates with 95% CIs and p-values are presented in Appendix 3 for the following analyses:
-
Analysis with the assumption that patients who died, were lost to follow-up or their RMI score could not be imputed were able to walk independently at 8 weeks (see Tables 69 and 70).
-
Using patient-completed RMI score at baseline: descriptive statistics as for sensitivity analysis (see Table 73).
-
Using researcher-completed RMI score at baseline in place of score at 24-hour randomisation (see Tables 74 and 75).
-
Including only participants for whom the 8-week questionnaire was received by 12 weeks post randomisation (see Tables 76 and 77).
-
Assuming participants with no primary outcome data because they were too unwell were unable to walk independently, and participants who had died, withdrawn, were unwilling to be visited, refused to completed the questionnaire, had moved out of the area, those we could not get hold of and questionnaires that were lost at site or in the post were classed missing (see Tables 78–80).
The results for a sensitivity analysis when centre was included as a fixed effect in the model is not presented as the model fit was poor.
None of the sensitivity analyses affected the estimates: the ORs were not statistically different from those in the primary ITT analysis.
Patient secondary end points
Estimates and 95% CIs for the patient secondary end points are presented in Tables 19–24. At all follow-up assessments, there was no evidence of statistically significant differences between the randomised groups in patient secondary end points, with the exception of GHQ-12 score. Participants in the co-careldopa group had a significantly lower GHQ-12 score than those in the placebo group at the 6-month follow-up.
Rivermead Mobility Index
Results of the stepwise multilevel linear regression analysis suggest that there was no evidence of statistically significant differences in RMI scores (continuous scores) between treatment arms at any follow-up time points; RMI scores were higher (not statistically) at 8 weeks and lower (not statistically) at 6 and 12 months in the placebo group compared with intervention. Model was adjusted for stratification variables and additionally for age, baseline NEADL, BI, MoCA scores and days between stroke and randomisation (Table 19). Infarction was associated with lower MoCA score at 8 weeks and 12 months than primary haemorrhage, higher age and number of days between stroke and randomisation were associated with lower RMI scores at all time points.
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | –0.354 (–0.894 to 0.186) | 0.198 | 0.144 (–0.504 to 0.791) | 0.662 | 0.170 (–0.540 to 0.881) | 0.637 |
| Sex: female vs. male | –0.371 (–0.943 to 0.201) | 0.203 | –0.470 (–1.160 to 0.220) | 0.182 | –0.302 (–1.052 to 0.448) | 0.429 |
| Stroke type: infarction vs. primary haemorrhage | –1.617 (–2.429 to –0.806) | 0.000 | –0.788 (–1.755 to 0.179) | 0.110 | –1.132 (–2.184 to –0.081) | 0.035 |
| RMI score on 24-hour randomisation system (points) | 0.834 (0.626 to 1.043) | 0.000 | 0.665 (0.412 to 0.918) | 0.000 | 1.019 (0.812 to 1.227) | 0.000 |
| Age (years) | –0.048 (–0.070 to –0.026) | 0.000 | –0.080 (–0.106 to –0.053) | 0.000 | –0.076 (–0.105 to –0.047) | 0.000 |
| Baseline NEADL score (points) | 0.043 (0.019 to 0.067) | 0.001 | 0.068 (0.040 to 0.096) | 0.000 | 0.060 (0.029 to 0.092) | 0.000 |
| Baseline BI score (points) | 0.228 (0.125 to 0.331) | 0.000 | 0.244 (0.119 to 0.369) | 0.000 | NSa | |
| Baseline MoCA score (points) | NSa | NSa | 0.084 (0.012 to 0.144) | 0.007 | ||
| Days between stroke and randomisation | –0.094 (–0.122 to –0.067) | 0.000 | –0.087 (–0.119 to –0.054) | 0.000 | –0.110 (–0.143 to –0.073) | 0.000 |
Analysis of the long-term trajectory of RMI over the follow-up period revealed no differences between randomised groups in change of outcome over time [adjusted mean difference (MD) for intervention vs. placebo 0.274, 95% CI –0.039 to 0.586; p = 0.086].
Montreal Cognitive Assessment
Results of the stepwise multilevel regression analysis suggest that there was no evidence of statistically significant differences in MoCA scores between treatments at any follow-up time points; MoCA scores were higher (not statistically) in the placebo group in all time points. Model was adjusted for stratification variables and additionally for age, baseline NEADL, ABILHAND, MoCA and number of days between stroke and randomisation (Table 20). Infarction, higher age and number of days between stroke and randomisation were associated with lower MoCA scores. Baseline MoCA and NEADL scores were positively associated with MoCA scores at all time points.
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | –0.160 (–0.747 to 0.427) | 0.592 | –0.269 (–0.959 to 0.419) | 0.445 | –0.194 (–0.949 to 0.561) | 0.613 |
| Sex: female vs. male | 0.172 (–0.444 to 0.787) | 0.584 | –0.147 (–0.871 to 0.577) | 0.690 | –0.148 (–0.648 to 0.945) | 0.714 |
| Stroke type: infarction vs. primary haemorrhage | –0.899 (–1.757 to –0.041) | 0.040 | –1.021 (–2.041 to –0.001) | 0.050 | –1.205 (–2.317 to –0.094) | 0.034 |
| RMI score on 24-hour randomisation system (points) | 0.090 (–0.082 to 0.262) | 0.306 | 0.065 (–0.134 to 0.264) | 0.521 | 0.148 (–0.067 to 0.363) | 0.177 |
| Baseline MoCA score (points) | 0.674 (0.625 to 0.724) | 0.000 | 0.596 (0.538 to 0.653) | 0.000 | 0.574 (0.508 to 0.634) | 0.000 |
| Age (years) | –0.055 (–0.078 to –0.031) | 0.000 | –0.066 (–0.094 to –0.038) | 0.000 | –0.073 (–0.103 to –0.041) | 0.000 |
| Baseline NEADL score (points) | 0.046 (0.019 to 0.072) | 0.001 | 0.048 (0.018 to 0.079) | 0.002 | 0.048 (0.015 to 0.082) | 0.004 |
| Baseline ABILHAND score (logits) | NSa | NSa | 0.162 (0.036 to 0.288) | 0.012 | ||
| Days between stroke and randomisation | –0.06 (–0.093 to –0.033) | 0.000 | –0.069 (–0.104 to –0.034) | 0.000 | –0.061 (–0.099 to –0.022) | 0.002 |
Barthel Index
There was no evidence of statistically significant differences in BI scores between treatment arms at any follow-up time points; BI scores were higher (not statistically) in the placebo group at all time points. Stepwise multilevel linear regression model was adjusted for stratification variables and additionally for age, baseline BI, MoCA and NEADL scores and number of days between stroke and randomisation (Table 21). Infarction was associated with lower MoCA scores at 8 weeks and 12 months, age and number of days between stroke and randomisation were associated with lower BI scores; RMI score, baseline BI, MoCA (except for 6 months) and NEADL scores were positively associated with MoCA scores.
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | –0.218 (–0.868 to 0.433) | 0.511 | –0.334 (–1.079 to 0.410) | 0.378 | –0.224 (–1.042 to 0.594) | 0.591 |
| Sex: female vs. male | 0.112 (–0.578 to 0.801) | 0.751 | 0.016 (–0.775 to 0.807) | 0.969 | 0.175 (–0.692 to 1.042) | 0.692 |
| Stroke type: infarction vs. primary haemorrhage | –1.813 (–2.799 to –0.827) | 0.000 | –1.050 (–2.164 to 0.063) | 0.064 | –1.216 (–2.430 to –0.002) | 0.050 |
| RMI score on 24-hour randomisation system (points) | 0.883 (0.634 to 1.134) | 0.000 | 0.675 (0.390 to 0.961) | 0.000 | 0.997 (0.760 to 1.234) | 0.000 |
| Age (years) | –0.042 (–0.069 to –0.016) | 0.002 | –0.070 (–0.100 to –0.039) | 0.000 | –0.079 (–0.112 to –0.045) | 0.000 |
| Baseline BI score (points) | 0.312 (0.186 to 0.438) | 0.000 | 0.304 (0.165 to 0.443) | 0.000 | NSa | |
| Baseline MoCA score (points) | 0.066 (0.001 to 0.121) | 0.021 | NSa | 0.115 (0.045 to 0.185) | 0.001 | |
| Baseline NEADL score (points) | 0.051 (0.022 to 0.080) | 0.001 | 0.065 (0.031 to 0.098) | 0.000 | 0.071 (0.034 to 0.107) | 0.000 |
| Days between stroke and randomisation | –0.117 (–0.151 to –0.083) | 0.000 | –0.117 (–0.155 to –0.079) | 0.000 | –0.122 (–0.145 to –0.080) | 0.000 |
ABILHAND Manual Ability Measure
There was no evidence of statistically significant differences in ABILHAND logit scores between treatment arms at any follow-up time points; ABILHAND scores were higher (not statistically) in the placebo group in all time points. Stepwise multilevel linear regression model contained stratification variables and baseline ABILHAND, BI, MoCA and NEADL scores and number of days between stroke and randomisation (Table 22). Infarction and baseline BI score were associated with lower ABILHAND score at all time points.
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | –0.100 (–0.458 to 0.259) | 0.585 | –0.152 (–0.573 to 0.269) | 0.478 | –0.157 (–0.592 to 0.278) | 0.479 |
| Sex: female vs. male | 0.022 (–0.357 to 0.401) | 0.910 | 0.098 (–0.345 to 0.541) | 0.664 | –0.107 (–0.573 to 0.359) | 0.651 |
| Stroke type: infarction vs. primary haemorrhage | –0.516 (–1.065 to 0.032) | 0.065 | –0.330 (–0.951 to 0.291) | 0.296 | –0.756 (–1.409 to –0.104) | 0.023 |
| RMI score on 24-hour randomisation system (points) | 0.070 (–0.068 to 0.206) | 0.315 | 0.092 (–0.071 to 0.254) | 0.267 | 0.251 (0.086 to 0.415) | 0.003 |
| Baseline ABILHAND score (logits) | 0.035 (–0.027 to 0.096) | 0.269 | 0.032 (–0.040 to 0.104) | 0.383 | 0.008 (–0.067 to 0.083) | 0.834 |
| Baseline BI score (points) | 0.142 (0.074 to 0.211) | 0.000 | 0.185 (0.105 to 0.265) | 0.000 | 0.134 (0.052 to 0.216) | 0.001 |
| Baseline MoCA score (points) | NSa | 0.044 (0.009 to 0.079) | 0.015 | NSa | ||
| Baseline NEADL score (points) | NSa | NSa | 0.022 (0.003 to 0.042) | 0.026 | ||
| Days between stroke and randomisation | –0.024 (–0.042 to –0.005) | 0.012 | NSa | –0.044 (–0.066 to –0.021) | 0.000 | |
Nottingham Extended Activities of Daily Living
There was no evidence of statistically significant differences in NEADL scores between treatment arms at any follow-up time points. Stepwise multilevel linear regression model was adjusted for stratification variables and age, baseline NEADL, MoCA and BI scores and days between stroke and randomisation (Table 23). Baseline scores were positively associated with NEADL scores at all time points; age and days between stroke were negatively associated with NEADL scores.
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | 1.018 (–1.268 to 3.303) | 0.382 | 0.027 (–2.724 to 2.777) | 0.985 | 1.036 (–1.564 to 3.636) | 0.434 |
| Sex: female vs. male | –0.114 (–2.531 to 2.303) | 0.926 | –0.018 (–2.931 to 2.894) | 0.990 | –0.257 (–3.003 to 2.489) | 0.854 |
| Stroke type: infarction vs. primary haemorrhage | –5.328 (–8.795 to –1.860) | 0.003 | –1.980 (–6.091 to –2.131) | 0.344 | –5.595 (–9.493 to –1.697) | 0.005 |
| RMI score on 24-hour randomisation system (points) | 2.453 (1.575 to 3.331) | 0.000 | 2.767 (1.718 to 3.816) | 0.000 | 2.345 (1.371 to 3.319) | 0.000 |
| Age (years) | –0.010 (–0.192 to –0.007) | 0.036 | –0.206 (–0.321 to –0.092) | 0.000 | NSa | |
| Baseline NEADL score (points) | 0.156 (0.053 to 0.258) | 0.003 | 0.184 (0.063 to 0.305) | 0.003 | 0.180 (0.065 to 0.294) | 0.002 |
| Baseline MoCA score (points) | 0.250 (0.054 to 0.446) | 0.013 | 0.263 (0.025 to 0.500) | 0.030 | 0.288 (0.066 to 0.511) | 0.011 |
| Baseline BI score (points) | 1.182 (0.739 to 1.624) | 0.000 | 0.679 (0.156 to 1.202) | 0.011 | 1.199 (0.706 to 1.693) | 0.000 |
| Days between stroke and randomisation | –0.269 (–0.388 to –0.149) | 0.000 | –0.314 (–0.456 to –0.172) | 0.000 | –0.285 (–0.421 to –0.149) | 0.000 |
General Health Questionnaire 12-item version
There were no statistically significant differences in GHQ-12 scores between treatment arms at 8 weeks [adjusted score was higher (not statistically) in intervention than placebo] and 12 months follow-up [adjusted score was higher (not statistically) in placebo than intervention]. There was evidence of statistically significant difference in GHQ-12 scores at 6 months (difference of 1.33 points, higher in placebo than intervention, p-value of 0.035). This requires careful interpretation due to multiple testing. Baseline GHQ-12 score was positively associated with GHQ-12 scores at all time points (Table 24).
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | Adjusted MD (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | 0.238 (–0.884 to 1.361) | 0.677 | –1.332 (–2.569 to –0.096) | 0.035 | –0.769 (–2.006 to 0.518) | 0.241 |
| Sex: female vs. male | 0.146 (–1.015 to 1.306) | 0.805 | –0.094 (–1.372 to 1.183) | 0.885 | –0.159 (–1.503 to 1.156) | 0.817 |
| Stroke type: infarction vs. primary haemorrhage | 1.109 (–0.556 to 2.773) | 0.191 | –0.127 (–1.956 to 1.705) | 0.892 | –0.418 (–2.348 to 1.512) | 0.670 |
| RMI score on 24-hour randomisation system (points) | 0.077 (–0.246 to 0.400) | 0.640 | 0.338 (–0.127 to 0.803) | 0.153 | –0.275 (–0.649 to 0.100) | 0.150 |
| Baseline GHQ-12 score (points) | 0.377 (0.294 to 0.459) | 0.000 | 0.215 (0.125 to 0.306) | 0.000 | 0.256 (0.164 to 0.349) | 0.000 |
| Baseline NEADL score (points) | –0.071 (–0.119 to –0.022) | 0.004 | NSa | –0.097 (–0.153 to –0.041) | 0.001 | |
| Baseline BI score (points) | NSa | –0.230 (–0.456 to –0.003) | 0.047 | NSa | ||
Modified Rankin Scale
There was no significant difference between treatment arms at either follow-up assessment (Table 25). Although the test score to check the proportional odds assumption of the mRS analysis was significant at 8 weeks and 6 months, this is a liberal test that tends to reject the null hypothesis more than it should. A graphical assessment of the proportional odds assumption was acceptable (see Appendix 4, Figures 15 and 16) and mRS was analysed as an ordinal variable using a stepwise regression model.
| Model parameter | Follow-up | |||
|---|---|---|---|---|
| 8 weeks | 6 months | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Treatment group: co-careldopa vs. placebo | 0.871 (0.629 to 1.206) | 0.404 | 0.808 (0.571 to 1.142) | 0.226 |
| Sex: female vs. male | 1.053 (0.746 to 1.486) | 0.769 | 1.122 (0.777 to 1.621) | 0.539 |
| Stroke type: infarction vs. primary haemorrhage | 1.601 (1.403 to 1.828) | 0.001 | 1.313 (1.145 to 1.506) | 0.638 |
| RMI score on 24-hour randomisation system (points) | 1.018 (1.003 to 1.033) | 0.000 | 0.976 (0.962 to 0.990) | 0.000 |
| Age (years) | NSa | 1.020 (1.005 to 1.035) | 0.001 | |
| Baseline NEADL score (points) | 1.164 (1.093 to 1.239) | 0.018 | 1.176 (1.100 to 1.257) | 0.011 |
| Baseline BI score (points) | 0.952 (0.936 to 0.969) | 0.000 | 0.958 (0.941 to 0.975) | 0.000 |
| Days between stroke and randomisation | 0.429 (0.263 to 0.700) | 0.000 | 0.883 (0.526 to 1.483) | 0.000 |
Carer secondary end points
Owing to small numbers, a single-level model was conducted for carer secondary end points (i.e. centre was not fitted as a random effect). Adjusted MDs in CBS scores were statistically significantly higher in carers of those in the placebo group than in carers of those in the co-careldopa group (Table 26), indicating higher levels of burden in the placebo group. Female carers reported greater burden than males at each follow-up time and older carers had lower scores at 12 months.
| Model parameter | Follow-up | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| MD (95% CI) | p-value | MD (95% CI) | p-value | MD (95% CI) | p-value | |
| Participant treatment group: co-careldopa vs. placebo | –4.547 (0.139 to 8.955) | 0.043 | –4.991 (0.173 to 9.811) | 0.042 | –7.171 (1.698 to 12.644) | 0.011 |
| Sex: female vs. male | 5.716 (–1.084 to 12.516) | 0.099 | 7.108 (0.027 to 14.188) | 0.049 | 9.561 (1.617 to 17.505) | 0.019 |
| Participant stroke type: infarction vs. primary haemorrhage | –4.738 (–11.460 to 1.985) | 0.166 | 0.355 (–6.168 to 6.878) | 0.914 | 4.569 (–3.391 to 12.578) | 0.258 |
| RMI score on 24-hour randomisation system (points) | 0.351 (–0.937 to 1.639) | 0.591 | –0.395 (–1.758 to 0.967) | 0.567 | –0.308 (–1.962 to 1.347) | 0.713 |
| Carer sex: female vs. male | 9.809 (2.859 to 16.758) | 0.006 | 14.080 (6.766 to 21.394) | 0.000 | 17.513 (9.374 to 25.651) | 0.000 |
| Baseline GHQ-12 score (points) | 0.460 (0.145 to 0.775) | 0.005 | NSa | NSa | ||
Safety
Adverse events
Overall, 365 participants reported a total of 1299 AEs. There was no evidence of a difference in levels of AEs reported between the two groups: 195 participants (63.3%) in the co-careldopa group had an average of 3.5 AEs each and 170 participants (59.6%) in the placebo group had an average of 3.6 AEs each (Table 27).
| Adverse event characteristic | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Number of AEs reported | 688 | 611 | 1299 |
| Participants reporting AEs, n (%) | 195 (63.3) | 170 (59.6) | 365 (61.6) |
| Number of AEs per participant | |||
| Mean (SD) | 3.5 (3.06) | 3.6 (3.11) | 3.6 (3.08) |
| Median (range) | 2 (1–15) | 2 (1–15) | 2 (1–15) |
Serious adverse events
A total of 57 participants (18.5%) reported 74 SAEs in the co-careldopa group and 50 participants (17.5%) reported 58 SAEs in the placebo group, with a mean of just over 1 SAE per participant occurring in each group. The majority of SAEs reported in both the co-careldopa group and the placebo group were not suspected to be related to the IMP (Table 28). Table 29 provides details of those SAEs that were attributable to the trial medication and Table 30 summarises the MedDRA System Organ Classification of SAEs. The majority were categorised as vascular disorders, nervous system disorders, respiratory disorders and infections/infestations, with those in the co-careldopa group experiencing proportionally more vascular disorders and infections and those in the placebo group experiencing more nervous system and respiratory disorders.
| Serious adverse event characteristic | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Number of SAEs reported | 74 | 58 | 132 |
| Participants with one or more SAEs, n (%) | 57 (18.5) | 50 (17.5) | 107 (18.0) |
| Number of SAEs per participant | |||
| Mean (SD) | 1.3 (0.68) | 1.2 (0.42) | 1.2 (0.58) |
| Median (range) | 1 (1–4) | 1 (1–3) | 1 (1–4) |
| Suspected to be related to trial medication, n (%) | |||
| Yes | 2 (2.7) | 1 (1.7) | 3 (2.3) |
| No | 72 (97.3) | 57 (98.3) | 129 (97.7) |
| Randomised allocation | Sex | Age (years) | SAE | MedDRA System Organ Classification | Outcome | |
|---|---|---|---|---|---|---|
| Description | In medical terms | |||||
| Co-careldopa | Male | 71 | During PT, patient became sweaty/clammy/faint. Therapist called an ambulance. Symptoms resolved after 10–15 minutes. Seen by PI in A&E. Discharged home from A&E New Cross | Faint, clammy, unwell | Vascular disorders | Recovered |
| Placebo | Male | 59 | During PT, patient became pale then his body was rigid, eyes rolled, lips blue, lasted 2–3 minutes. BP 96/71 mmHg. Oxygen saturation 96% on air. HR 82 beats/min | Tonic–clonic event | Infections and infestations | Recovered |
| Co-careldopa | Female | 66 | Symptoms commenced within approximately 1 hour of trial medication (half-dose): dizziness and nausea | Severe nausea and dizziness | Vascular disorders | Recovered |
| Serious adverse events as per MedDRA System Organ Classification | Treatment group, n (%) | Total (N = 132), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 74) | Placebo (N = 58) | ||
| Blood and lymphatic system disorders | 1 (1.4) | 0 (0.0) | 1 (0.8) |
| Cardiac disorders | 6 (8.1) | 2 (3.4) | 8 (6.1) |
| Gastrointestinal disorders | 4 (5.4) | 3 (5.2) | 7 (5.3) |
| General disorders and administration site conditions | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| Hepatobiliary disorders | 1 (1.4) | 2 (3.4) | 3 (2.3) |
| Infections and infestations | 10 (13.5) | 6 (10.3) | 16 (12.1) |
| Injury, poisoning and procedural complications | 4 (5.4) | 3 (5.2) | 7 (5.3) |
| Metabolism and nutrition disorders | 2 (2.7) | 1 (1.7) | 3 (2.3) |
| Musculoskeletal and connective tissue disorders | 1 (1.4) | 0 (0.0) | 1 (0.8) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 2 (2.7) | 3 (5.2) | 5 (3.8) |
| Nervous system disorders | 10 (13.5) | 11 (19.0) | 21 (15.9) |
| Psychiatric disorders | 2 (2.7) | 0 (0.0) | 2 (1.5) |
| Renal and urinary disorders | 2 (2.7) | 1 (1.7) | 3 (2.3) |
| Reproductive system and breast disorders | 1 (1.4) | 3 (5.2) | 4 (3.0) |
| Respiratory, thoracic and mediastinal disorders | 8 (10.8) | 12 (20.7) | 20 (15.2) |
| Social circumstances | 4 (5.4) | 0 (0.0) | 4 (3.0) |
| Vascular disorders | 16 (21.6) | 10 (17.2) | 26 (19.7) |
‘Other’ illnesses were the biggest cause of SAEs, accounting for around 50% in both the co-careldopa group and the placebo group (Table 31). Stroke accounted for a greater proportion of SAEs in the co-careldopa group than the placebo group.
| Serious adverse event causes | Treatment group, n (%) | Total (N = 132), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 74) | Placebo (N = 58) | ||
| Treatment medication | 2 (2.7) | 1 (1.7) | 3 (2.3) |
| Concomitant medications | 4 (5.4) | 3 (5.2) | 7 (5.3) |
| Ischaemic or haemorrhagic stroke | 20 (27.0) | 10 (17.2) | 30 (22.7) |
| Ischaemic or haemorrhagic stroke and other illness | 5 (6.8) | 4 (6.9) | 9 (6.8) |
| Other illness | 37 (50.0) | 28 (48.3) | 65 (49.2) |
| Missing | 6 (8.1) | 12 (20.7) | 18 (13.6) |
With regard to the seriousness criteria of the reported SAEs, the largest proportion required hospitalisation; 10 SAEs (13.5%) in the co-careldopa group and six (10.3%) in the placebo groups resulted in death (Table 32). However, the majority of participants who experienced a SAE recovered by the time of final follow-up: 77% of co-careldopa group participants and 81% of placebo group participants (Table 33).
| Serious adverse events seriousness criteria | Treatment group, n (%) | Total, n (%) | |
|---|---|---|---|
| Co-careldopa | Placebo | ||
| Death | 10 (13.5) | 6 (10.3) | 16 (12.1) |
| Life-threatening illness | 13 (17.6) | 9 (15.5) | 22 (16.7) |
| Required or prolonged hospitalisation | 58 (78.4) | 43 (74.1) | 101 (76.5) |
| Persistent or significant disability/incapacity | 4 (5.4) | 3 (5.2) | 7 (5.3) |
| Patient jeopardised (i.e. intervention required to prevent one of the above) | 10 (13.5) | 12 (20.7) | 22 (16.7) |
| Total number of events | 74 (100.0) | 58 (100.0) | 132 (100.0) |
| Serious adverse event outcome | Treatment group, n (%) | Total (N = 132), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 74) | Placebo (N = 58) | ||
| Recovered | 57 (77.0) | 47 (81.0) | 104 (78.8) |
| Recovered with sequelae | 6 (8.1) | 5 (8.6) | 11 (8.3) |
| Condition improving | 1 (1.4) | 0 (0.0) | 1 (0.8) |
| Death | 10 (13.5) | 6 (10.3) | 16 (12.1) |
No SAEs satisfied the criteria of being suspected unexpected serious adverse reactions.
Mortality
A total of 39 participants (6.6%) died within 12 months of randomisation: 22 (7.1%) in the co-careldopa group and 17 (6.0%) in the placebo group. More participants in the co-careldopa group died within 8 weeks than in the placebo group: six (1.9%) and one (0.4%), respectively. The highest proportion of deaths occurred between 6 and 12 months [nine (2.9%) in the co-careldopa group and nine (3.2%) in the placebo group], and the majority occurred in hospital [14 (4.5%) in the co-careldopa group and 12 (4.2%) in the placebo group] (Table 34). The cause of death of the seven participants who died during treatment is presented in Table 35 and a graphical representation of the time between randomisation and death is presented in Figure 7. There were no carer deaths.
| Participants deaths | Treatment group, n (%) | Total (N = 593), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Number of deaths | 22 (7.1) | 17 (6.0) | 39 (6.6) |
| Time death occurred | |||
| Up to 8 weeks | 6 (1.9) | 1 (0.4) | 7 (1.2) |
| From 8 weeks to 6 months | 7 (2.3) | 7 (2.5) | 14 (2.4) |
| From 6 months to 12 months | 9 (2.9) | 9 (3.2) | 18 (3.0) |
| Place of death | |||
| Home | 2 (0.6) | 4 (1.4) | 6 (1.0) |
| Hospital | 14 (4.5) | 12 (4.2) | 26 (4.4) |
| Institutional care | 5 (1.6) | 2 (0.7) | 7 (1.2) |
| Unknown | 3 (1.0) | 2 (0.7) | 5 (0.8) |
| Randomised allocation | Cause of death |
|---|---|
| Co-careldopa | Cause of death for participants who died during treatment |
| NSTEMI, respiratory arrest | |
| Pneumonia | |
| Deterioration from initial stroke | |
| Re-admitted to acute stroke unit from community rehabilitation unit with having had another stroke | |
| Peritonitis, bowel perforation | |
| Placebo | Aspiration pneumonia |
FIGURE 7.
Time between randomisation and death.

New significant medical or surgical events
The most frequently occurring new significant medical or surgical events from 8 weeks to 6 months and from 6 to 12 months were falls, infection, elective/pre-planned treatment, cardiac problems and gastrointestinal problems. The proportion of participants experiencing new events was similar between randomisation arms, with the exception of falls, of which there was a higher proportion in the placebo group at both time points; gastrointestinal problems, which were experienced by a greater proportion of those in the placebo group between 8 weeks and 6 months; and cardiac problems, which were experienced by a significantly greater number of participants in the co-careldopa group. The median time from randomisation to event was 27 days in both groups for those that occurred between 8 weeks and 6 months, and 53 days for events between 6 and 12 months (Table 36).
| New significant medical or surgical events | Time period | |||||
|---|---|---|---|---|---|---|
| 8 weeks to 6 months | 6 months to 12 months | |||||
| Co-careldopa (N = 308) | Placebo (N = 285) | Total (N = 593) | Co-careldopa (N = 308) | Placebo (N = 285) | Total (N = 593) | |
| Elective/pre-planned treatment, n (%) | ||||||
| Yes | 22 (7.1) | 24 (8.4) | 46 (7.8) | 21 (6.8) | 20 (7.0) | 41 (6.9) |
| No | 224 (72.7) | 225 (78.9) | 449 (75.7) | 204 (66.2) | 204 (71.6) | 408 (68.8) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| New stroke, n (%) | ||||||
| Yes | 4 (1.3) | 1 (0.4) | 5 (0.8) | 3 (1.0) | 3 (1.1) | 6 (1.0) |
| No | 242 (78.6) | 248 (87.0) | 490 (82.6) | 222 (72.1) | 221 (77.5) | 443 (74.7) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Fits, n (%) | ||||||
| Yes | 9 (2.9) | 6 (2.1) | 15 (2.5) | 14 (4.5) | 8 (2.8) | 22 (3.7) |
| No | 237 (76.9) | 243 (85.3) | 480 (80.9) | 211 (68.5) | 216 (75.8) | 427 (72.0) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Infection, n (%) | ||||||
| Yes | 52 (16.9) | 43 (15.1) | 95 (16.0) | 44 (14.3) | 43 (15.1) | 87 (14.7) |
| No | 194 (63.0) | 206 (72.3) | 400 (67.5) | 181 (58.8) | 181 (63.5) | 362 (61.0) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Falls, n (%) | ||||||
| Yes | 58 (18.8) | 65 (22.8) | 123 (20.7) | 59 (19.2) | 73 (25.6) | 132 (22.3) |
| No | 188 (61.0) | 184 (64.6) | 372 (62.7) | 166 (53.9) | 151 (53.0) | 317 (53.5) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Cardiac problem, n (%) | ||||||
| Yes | 17 (5.5) | 6 (2.1) | 23 (3.9) | 16 (5.2) | 3 (1.1) | 19 (3.2) |
| No | 229 (74.4) | 243 (85.3) | 472 (79.6) | 209 (67.9) | 221 (77.5) | 430 (72.5) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Orthopaedic surgery, n (%) | ||||||
| Yes | 4 (1.3) | 4 (1.4) | 8 (1.3) | 3 (1.0) | 1 (0.4) | 4 (0.7) |
| No | 242 (78.6) | 245 (86.0) | 487 (82.1) | 222 (72.1) | 223 (78.2) | 445 (75.0) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Diabetes mellitus related, n (%) | ||||||
| Yes | 6 (1.9) | 5 (1.8) | 11 (1.9) | 6 (1.9) | 4 (1.4) | 10 (1.7) |
| No | 240 (77.9) | 244 (85.6) | 484 (81.6) | 219 (71.1) | 220 (77.2) | 439 (74.0) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Gastrointestinal problem, n (%) | ||||||
| Yes | 16 (5.2) | 28 (9.8) | 44 (7.4) | 19 (6.2) | 18 (6.3) | 37 (6.2) |
| No | 230 (74.7) | 221 (77.5) | 451 (76.1) | 206 (66.9) | 206 (72.3) | 412 (69.5) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Other, n (%) | ||||||
| Yes | 30 (9.7) | 31 (10.9) | 61 (10.3) | 29 (9.4) | 23 (8.1) | 52 (8.8) |
| No | 216 (70.1) | 218 (76.5) | 434 (73.2) | 196 (63.6) | 201 (70.5) | 397 (66.9) |
| Missing | 62 (20.1) | 36 (12.6) | 98 (16.5) | 83 (26.9) | 61 (21.4) | 144 (24.3) |
| Weeks to event from randomisation | ||||||
| Mean (SD) | 27.0 (3.70) | 26.8 (2.56) | 26.9 (3.18) | 53.9 (4.83) | 53.2 (3.68) | 53.6 (4.30) |
| Median (range) | 26 (15–49) | 26 (20–41) | 26 (15–49) | 53 (45–84) | 53 (45–83) | 53 (45–84) |
| Missing, (n) | 62 | 36 | 98 | 83 | 61 | 144 |
Vomiting between the investigational medicinal product dose and end of therapy
During 12,078 motor therapy sessions, only 27 participants (4.6%, 27/593) in 37 motor therapy sessions (0.3%) vomited between taking the IMP dose and the end of therapy: 19 participants in 28 sessions (0.5%) in the co-careldopa group and nine participants in nine sessions (0.1%) in the placebo group (see Appendix 5, Table 81). Of the 28 participants who vomited, eight withdrew (seven in the co-careldopa group and one in the placebo group). In the co-careldopa group, two withdrawals were directly related to nausea and vomiting; one withdrawal was related to ‘. . . postural drop in line with taking medications (for trial)’.
Pregnancy
Notification of pregnancy was provided for one participant 48 days after they were randomised; a medical termination was carried out 14 days later.
Content of occupational and physical therapy
Discharge from hospital
Table 37 summarises all participant discharges from hospital. The median number of days from randomisation to discharge was 25 days in the co-careldopa group and 27 days in the placebo group, and median time from stroke to discharge was 44 days in the co-careldopa group and 47 days in the placebo group. The majority of participants were discharged to their own home or a relative’s home [174 (56.5%) in the co-careldopa group and 170 (59.6%) in the placebo group], mostly to live with identified carers or others as opposed to living alone.
| Discharge characteristic | Co-careldopa (N = 308) | Placebo (N = 285) | Total (N = 593) |
|---|---|---|---|
| Days from randomisation to discharge | |||
| Mean (SD) | 35.2 (33.59) | 34.5 (28.17) | 34.8 (31.00) |
| Median (range) | 25 (–5 to 209)a | 27 (–1 to 166)a | 26 (–5 to 209) |
| Date of discharge unknown (n) | 68 | 50 | 118 |
| Days from stroke to discharge | |||
| Mean (SD) | 52.6 (36.21) | 51.7 (31.25) | 52.1 (33.82) |
| Median (range) | 44 (6–227) | 47 (9–186) | 45.0 (6–227) |
| Date of discharge unknown (n) | 68 | 50 | 118 |
| Discharge location, n (%) | |||
| Home | 174 (56.5) | 170 (59.6) | 344 (58.0) |
| Lives alone | 26 (14.9) | 43 (25.3) | 69 (20.1) |
| Cohabits with identified carer | 76 (43.7) | 66 (38.8) | 142 (41.3) |
| Cohabits with other | 72 (41.4) | 60 (35.3) | 132 (38.4) |
| Missing | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| Nursing/residential/care home | 23 (7.5) | 19 (6.7) | 42 (7.1) |
| Community hospital/rehabilitation centre/intermediate care | 34 (11.0) | 32 (11.2) | 66 (11.1) |
| Other | 0 (0.0) | 2 (0.7) | 2 (0.3) |
| Missing/not applicable | 77 (25.0) | 62 (21.8) | 139 (23.4) |
Table 38 summarises the details of those who were discharged to the community during treatment: 115 (37.3%) in the co-careldopa group and 122 (42.8%) in the placebo group (median 13 and 16 days from randomisation to discharge in the co-careldopa and placebo groups, respectively). The majority of participants were discharged to their own home or to a relative’s home: 88 (76.5%) in the co-careldopa group and 95 (77.9%) in the placebo group. Two participants were discharged before randomisation (one on the day before randomisation and the other 5 days before randomisation). The delay in randomising the latter participant was due to the therapist working off-site, trying to fit in with the participant’s other activities and the weekend. One participant randomised to the co-careldopa group commenced therapy 6 days post discharge.
| Participant discharge characteristic | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Discharged during treatment, n (%) | |||
| Yes | 115 (37.3) | 122 (42.8) | 237 (40.0) |
| No | 122 (39.6) | 110 (38.6) | 232 (39.1) |
| Died before end of treatment | 1 (0.3) | 1 (0.4) | 2 (0.3) |
| Withdrew before end of treatment | 10 (3.2) | 5 (1.8) | 15 (2.5) |
| No treatment received | 11 (3.6) | 4 (1.4) | 15 (2.5) |
| Unknown | 49 (15.9) | 43 (15.1) | 92 (15.5) |
| Days from randomisation to dischargea,b | |||
| Mean (SD) | 15.1 (9.99) | 17.9 (10.14) | 16.6 (10.14) |
| Median (range) | 13 (–5 to 42) | 16 (–1 to 43) | 15 (–5 to 43) |
| Missing, (n) | 0 | 0 | 0 |
| Discharge location,a n (%) | |||
| Home | 88 (76.5) | 95 (77.9) | 183 (77.2) |
| Nursing/residential/care home | 5 (4.3) | 4 (3.3) | 9 (3.8) |
| Community hospital/rehabilitation centre/intermediate care | 18 (15.7) | 18 (14.8) | 36 (15.2) |
| Missing | 4 (3.5) | 5 (4.1) | 9 (3.8) |
| Commenced therapy > 5 days post discharge,a n (%) | |||
| Yes | 1 (0.9) | 0 (0.0) | 1 (0.4) |
| No | 114 (99.1) | 122 (100.0) | 236 (99.6) |
Therapy sessions
A total of 14,551 therapy sessions were delivered during the trial. The mean number of therapy sessions provided was 23.2 and 24.8 per patient with an average length of 42.8 and 43.1 minutes in the co-careldopa and placebo groups, respectively (Table 39). The mean number of minutes spent on motor/non-motor activities was 40.8/15.8 in the co-careldopa group and 40.8/17.3 in the placebo group.
| Therapy session characteristic | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Number of therapy sessions | |||
| Mean (SD) | 23.2 (14.36) | 24.8 (12.50) | 24.0 (13.51) |
| Median (range) | 21 (0–73) | 25 (0–68) | 24 (0–73) |
| Q1, Q3 (points) | 13, 31 | 17, 32 | 15, 32 |
| Length of therapy session (minutes) | |||
| Mean (SD) | 42.8 (15.10) | 43.1 (16.04) | 43.0 (15.58) |
| Median (range) | 45 (5–180) | 45 (5–210) | 45 (5–210) |
| Q1, Q3 (points) | 30, 50 | 30, 50 | 30, 50 |
| Minutes of motor therapy | |||
| Mean (SD) | 40.8 (14.91) | 40.8 (15.27) | 40.8 (15.09) |
| Median (range) | 40 (1–180) | 40 (4–180) | 40 (1–180) |
| Q1, Q3 (points) | 30, 50 | 30, 50 | 30, 50 |
| Minutes of non-motor therapy | |||
| Mean (SD) | 15.8 (14.24) | 17.3 (15.68) | 16.6 (15.02) |
| Median (range) | 10 (1–120) | 10 (1–165) | 10 (1–165) |
| Q1, Q3 (points) | 5, 20 | 10, 20 | 5, 20 |
The proportion of participants who received sufficient motor therapy (at least 20 minutes of motor therapy in at least 80% of therapy sessions) was higher in the placebo group than in the co-careldopa group: 257 (90.2%) and 259 (84.1%), respectively (Table 40).
| Sufficient therapy for 80% of sessions | Treatment group, n (%) | Total (N = 593), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Yes | 259 (84.1) | 257 (90.2) | 516 (87.0) |
| No | 12 (3.9) | 8 (2.8) | 20 (3.4) |
| NA, fewer than five therapy sessions | 37 (12.0) | 20 (7.0) | 57 (9.6) |
Therapists present at therapy sessions
Table 41 summarises the number of sessions in which occupational therapists, physiotherapists and rehabilitation assistants were present. At least one occupational therapist was present in 24.7% of sessions in the co-careldopa group and and 25.5% of sessions in the placebo group. At least one physiotherapist was present in 61.4% and 61.0% of sessions in the co-careldopa and placebo group, respectively, and at least one rehabilitation assistant was present in 43.4% and 42.1% of sessions, respectively.
| Therapist presence | Treatment group, n (%) | Total (N = 14,551), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 7319) | Placebo (N = 7232) | ||
| Occupational therapists present | |||
| None | 5508 (75.3) | 5390 (74.5) | 10,898 (74.9) |
| 1 | 1513 (20.7) | 1492 (20.6) | 3005 (20.7) |
| 2 | 208 (2.8) | 224 (3.1) | 432 (3.0) |
| ≥ 3 | 6 (0.1) | 6 (0.1) | 12 (0.1) |
| Number unknown | 84 (1.1) | 120 (1.6) | 204 (1.4) |
| Physical therapists present | |||
| None | 2825 (38.6) | 2824 (39.0) | 5649 (38.8) |
| 1 | 3079 (42.1) | 3064 (42.4) | 6143 (42.2) |
| 2 | 1144 (15.6) | 1078 (14.9) | 2222 (15.3) |
| ≥ 3 | 112 (1.5) | 121 (1.7) | 233 (1.6) |
| Number unknown | 159 (2.2) | 145 (2.0) | 304 (2.1) |
| Rehabilitation assistants present | |||
| None | 4146 (56.6) | 4185 (57.9) | 8331 (57.3) |
| 1 | 2726 (37.2) | 2648 (36.6) | 5374 (36.9) |
| 2 | 339 (4.6) | 275 (3.8) | 614 (4.2) |
| ≥ 3 | 4 (0.1) | 2 (0.0) | 6 (0.0) |
| Number unknown | 104 (1.4) | 122 (1.7) | 226 (1.5) |
Intensity of therapy sessions by location
The total mean length of the therapy session was 41.5 minutes in the hospital setting and 45.4 minutes in the community. The mean time of motor and non-motor therapy was 40.1 and 16.4, respectively, in the hospital setting, and 41.9 and 16.7, respectively, in the community setting (Table 42).
| Length of therapy sessions (minutes) | Location | Total (n = 14,551) | |
|---|---|---|---|
| Hospital (n = 7319) | Community (n = 7232) | ||
| Length of therapy session | |||
| Mean (SD) | 41.5 (15.78) | 45.4 (14.90) | 43.0 (15.58) |
| Median (range) | 40 (5–210) | 45 (5–180) | 45 (5–210) |
| Q1, Q3 (points) | 30, 50 | 35, 55 | 30, 50 |
| Length of motor therapy | |||
| Mean (SD) | 40.1 (15.41) | 41.9 (14.47) | 40.8 (15.09) |
| Median (range) | 40 (1–180) | 43.0 (5–130) | 40 (1–180) |
| Q1, Q3 (points) | 30, 45 | 30, 50 | 30, 50 |
| Length of non-motor therapy | |||
| Mean (SD) | 16.4 (16.19) | 16.7 (13.93) | 16.6 (15.02) |
| Median (range) | 10 (1–165) | 10 (2–105) | 10 (1–165) |
| Q1, Q3 (points) | 5, 20 | 10, 20 | 5, 20 |
Treatment compliance
Investigational medicinal product doses and motor therapy sessions
Table 43 summarises the number of IMP doses received compared with the number of motor therapy sessions undertaken. The mean number of therapy sessions that included motor activities was 23.2 in the co-careldopa group and 24.8 in the placebo group, and the mean number of IMP doses received by participants was 20.7 in the co-careldopa group and 22.4 in the placebo group. The co-careldopa group received an average of 2.6 more therapy sessions than IMP doses and the placebo group received 2.4 more sessions than IMP doses. Around one-fifth of participants received the same number of IMP doses as therapy sessions: 58 (18.8%) in the co-careldopa group and 61 (21.4%) in the placebo group. Fourteen participants did not receive any IMP doses or therapy; this was mainly attributable to participants withdrawing before starting therapy, but in a few cases therapy forms were unobtainable and it was, therefore, unknown if any therapy took place.
| IMP dose and motor therapy session characteristics | Treatment group | Total (N = 593) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Number of motor therapy sessions | |||
| Mean (SD) | 23.2 (14.36) | 24.8 (12.50) | 24.0 (13.51) |
| Median (range) | 21 (0–73) | 25 (0–68) | 24 (0–73) |
| Q1, Q3 (points) | 13, 31 | 17, 32 | 15, 32 |
| Number of IMP doses taken | |||
| Mean (SD) | 20.6 (13.07) | 22.4 (11.10) | 21.5 (12.18) |
| Median (range) | 19 (0–61) | 23 (0–62) | 22 (0–62) |
| Q1, Q3 (points) | 12, 29 | 15, 29 | 13, 29 |
| Difference in IMP doses and motor therapy sessions (categorical), n (%) | |||
| Same number of IMP doses and therapy sessions | 58 (18.8) | 61 (21.4) | 119 (20.1) |
| More therapy sessions than IMP doses | 194 (63.0) | 186 (65.3) | 380 (64.1) |
| More IMP doses than therapy sessions | 46 (14.9) | 34 (11.9) | 80 (13.5) |
| No IMP doses or therapy sessions | 10 (3.2) | 4 (1.4) | 14 (2.4) |
| Difference in IMP doses and motor therapy sessions (continuous) | |||
| Mean (SD) | 2.6 (5.39) | 2.4 (4.40) | 2.5 (4.93) |
| Median (range)a | 1 (–33 to 31) | 1 (–25 to 23) | 1 (–33 to 31) |
| Q1, Q3 (points) | 0, 4 | 0, 4 | 0, 4 |
Table 44 summarises the time frame of the IMP dose with respect to the motor therapy sessions. The IMP was taken as per protocol (45–60 minutes before the therapy session) in 55% of therapy sessions in both treatment arms.
| Timing of IMP dose | Treatment group, n (%) | Total (N = 14,551), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 7319) | Placebo (N = 7232) | ||
| IMP taken 45–60 minutes before motor therapy session | 4030 (55.1) | 3976 (55.0) | 8006 (55.0) |
| IMP taken > 60 minutes before motor therapy session | 1079 (14.7) | 1074 (14.9) | 2153 (14.8) |
| IMP taken < 45 minutes before motor therapy session | 805 (11.0) | 885 (12.2) | 1690 (11.6) |
| IMP taken after start of motor therapy session | 100 (1.4) | 154 (2.1) | 254 (1.7) |
| IMP taken at unknown time before/after motor therapy session | 77 (1.1) | 73 (1.0) | 150 (1.0) |
| IMP not taken before motor therapy session | 1205 (16.5) | 1031 (14.3) | 2236 (15.4) |
| Missing IMP or therapy data | 23 (0.3) | 39 (0.5) | 62 (0.4) |
Patient refusal, ill health, cancelled or rearranged therapy session or no planned therapy session were the main reasons for not receiving therapy (Table 45). The majority of IMP doses were not taken because a previous dose had been taken within 3 hours or staff forgot to dispense it (Table 46).
| Reasons | Treatment group, n (%) | Total (N = 474), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 263) | Placebo (N = 211) | ||
| Patient refused/too ill | 61 (23.2) | 44 (20.9) | 105 (22.2) |
| Unscheduled/cancelled/rearranged therapy session | 60 (22.8) | 60 (28.4) | 120 (25.3) |
| No planned therapy session | 62 (23.6) | 47 (22.3) | 109 (23.0) |
| Not appropriate to give therapy | 14 (5.3) | 8 (3.8) | 22 (4.6) |
| No evidence that therapy was given | 7 (2.7) | 16 (7.6) | 23 (4.9) |
| Non-motor activity session | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Patient not available | 4 (1.5) | 5 (2.4) | 9 (1.9) |
| Timing issues | 11 (4.2) | 1 (0.5) | 12 (2.5) |
| Staff error | 14 (5.3) | 2 (0.9) | 16 (3.4) |
| Unknown | 11 (4.2) | 4 (1.9) | 15 (3.2) |
| Other | 17 (6.5) | 17 (8.1) | 34 (7.2) |
| Missing | 1 (0.4) | 7 (3.3) | 8 (1.7) |
| Reasons | Treatment group, n (%) | Total (N = 2236), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 1205) | Placebo (N = 1031) | ||
| Patient refused/too ill | 39 (3.2) | 29 (2.8) | 68 (3.0) |
| Unscheduled/rearranged therapy session | 54 (4.5) | 46 (4.5) | 100 (4.5) |
| Previous dose taken within 3 hours | 386 (32.0) | 387 (37.5) | 773 (34.6) |
| Not appropriate to give drug | 80 (6.6) | 16 (1.6) | 96 (4.3) |
| No evidence that drug was taken | 2 (0.2) | 8 (0.8) | 10 (0.4) |
| Temporarily stopped drug | 2 (0.2) | 1 (0.1) | 3 (0.1) |
| Permanently stopped drug | 0 (0.0) | 2 (0.2) | 2 (0.1) |
| Not aware of motor therapy | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Non-motor activity session | 14 (1.2) | 7 (0.7) | 21 (0.9) |
| Patient not available | 10 (0.8) | 2 (0.2) | 12 (0.5) |
| Not aware patient on trial | 33 (2.7) | 16 (1.6) | 49 (2.2) |
| Prescribing issues | 37 (3.1) | 33 (3.2) | 70 (3.1) |
| Timing issues | 57 (4.7) | 75 (7.3) | 132 (5.9) |
| Communication issues | 11 (0.9) | 12 (1.2) | 23 (1.0) |
| Staff forgot | 297 (24.6) | 231 (22.4) | 528 (23.6) |
| Patient forgot | 105 (8.7) | 83 (8.1) | 188 (8.4) |
| Unknown | 51 (4.2) | 23 (2.2) | 74 (3.3) |
| Other | 26 (2.2) | 46 (4.5) | 72 (3.2) |
| Missing | 1 (0.1) | 13 (1.3) | 14 (0.6) |
Therapy in the community
Participants who were discharged to the community (their own or a relative’s/carer’s home, or to a nursing, residential or care home) should have received a telephone call to remind them to take their IMP before they received therapy. To explore the effectiveness of these reminders, the proportion of times the drug was taken as per protocol when the participant was still in hospital and when they did or did not receive a reminder in the community is summarised in Table 47. Approximately half of participants in both treatment arms received the IMP as per protocol while in hospital. For those participants in the community, receiving a reminder was effective in ensuring that the IMP was taken as per protocol: 69.9% of co-careldopa group participants and 75.8% of placebo group participants. When a reminder was not received, 64.9% of the co-careldopa group and 52.2% of the placebo group took the IMP as per protocol.
| Summary | Treatment group, n (%) | Total (N = 9537), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 4691) | Placebo (N = 4846) | ||
| Was IMP taken per protocol when motor therapy was received in hospital? | |||
| Yes | 2327 (49.6) | 2527 (52.1) | 4854 (50.9) |
| No | 2364 (50.4) | 2319 (47.9) | 4683 (49.1) |
| N = 452 | N = 418 | N = 870 | |
| Was IMP taken per protocol when motor therapy was received in the community and a telephone reminder was received? | |||
| Yes | 316 (69.9) | 317 (75.8) | 633 (72.8) |
| No | 136 (30.1) | 101 (24.2) | 237 (27.2) |
| N = 530 | N = 500 | N = 1030 | |
| Was IMP taken per protocol when motor therapy was received in the community and a telephone reminder was not received? | |||
| Yes | 344 (64.9) | 261 (52.2) | 605 (58.7) |
| No | 186 (35.1) | 239 (47.8) | 425 (41.3) |
| N = 12 | N = 30 | N = 42 | |
| Was IMP taken per protocol when motor therapy was received in the community and it is unknown if a telephone reminder was received? | |||
| Yes | 4 (33.3) | 6 (20.0) | 10 (23.8) |
| No | 8 (66.7) | 24 (80.0) | 32 (76.2) |
Drug (kit) replacement
Four participants lost their drug kits and required a replacement: three in the co-careldopa group and one in the placebo group. No participant received the wrong drug.
Patient perspective regarding the use of the investigational medicinal product with rehabilitation treatment
Although many participants did not answer the questions relating to patient perspective regarding the use of IMP, those who did answer generally found it easy to remember to take the drug and thought that the package instructions were clear. In total, 21% of participants in both groups found it difficult to remove the IMP from the packaging and had to ask for help, but the majority did not have problems taking the drug (Table 48).
| Patient perspective | Treatment group, n (%) | Total (N = 593), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Remembering to take drug | |||
| Easy | 147 (47.7) | 142 (49.8) | 289 (48.7) |
| Managed most of the time | 37 (12.0) | 39 (13.7) | 76 (12.8) |
| Often forgot | 11 (3.6) | 11 (3.9) | 22 (3.7) |
| Missing | 113 (36.7) | 93 (32.6) | 206 (34.7) |
| Removal from packaging | |||
| Easy | 82 (26.6) | 92 (32.3) | 174 (29.3) |
| Difficult but could do it myself | 29 (9.4) | 28 (9.8) | 57 (9.6) |
| Difficult and had to ask for help | 66 (21.4) | 61 (21.4) | 127 (21.4) |
| Missing | 131 (42.5) | 104 (36.5) | 235 (39.6) |
| Clear instructions | |||
| Yes | 122 (39.6) | 112 (39.3) | 234 (39.5) |
| Mostly | 25 (8.1) | 37 (13.0) | 62 (10.5) |
| No, had to ask for help | 34 (11.0) | 29 (10.2) | 63 (10.6) |
| Missing | 127 (41.2) | 107 (37.5) | 234 (39.5) |
| Problems taking tablet | |||
| None | 199 (64.6) | 198 (69.5) | 397 (66.9) |
| Occasionally | 13 (4.2) | 5 (1.8) | 18 (3.0) |
| All the time | 5 (1.6) | 3 (1.1) | 8 (1.3) |
| Missing | 91 (29.5) | 79 (27.7) | 170 (28.7) |
Blinding
Unblinding requests and errors
A request to unblind one participant was received because the PI had concerns regarding the participant’s blood pressure, which was low when measured using an electronic device but within range when repeated manually. The participant was not experiencing any symptoms and felt well, and it transpired that the telephone call was to seek advice on whether or not the site could stop treatment for the participant without knowing the treatment allocation. The research nurse was happy that no unblinding would occur for this participant and the participant was withdrawn from trial treatment.
Exit poll
The aim of the exit poll undertaken by the local stroke service research nurse/researcher at 12 months (patients and therapists), using the blinding index, was to ascertain the level of masking to active drug. A blinding index was calculated to assess whether or not blinding was successful in participants and researchers. The blinding index has a range between –1 and 1, where a blinding index of 0 represents complete blinding, a blinding index of –1 indicates that all guesses were incorrect and a blinding index of 1 indicates that all guesses were correct. The certainty of guesses was rated by researchers and participants on a continuous scale (0–10), where 0 = ‘not at all’ and 10 = ‘completely sure’, and was summarised according to the randomised arm the participant had been allocated to and by the treatment group guessed.
Blinding was successful from both the researcher perspective and the participant perspective. The blinding index for researchers was 0.05 (95% CI –0.01 to 0.12) in the co-careldopa group and –0.06 (95% CI –0.13 to 0.00) in the placebo group, suggesting that blinding was maintained. For participants, the blinding index was 0.05 (95% CI –0.02 to 0.13) for those in the co-careldopa group and –0.04 (95% CI –0.13 to 0.03) for those in the placebo group (Table 49), suggesting that blinding was maintained.
| Researcher and patient group | Blinding index (95% CI) | p-value |
|---|---|---|
| Researcher treatment group | ||
| Co-careldopa | 0.05 (–0.01 to 0.12) | 0.098 |
| Placebo | –0.06 (–0.13 to 0.00) | 0.942 |
| Participant treatment group | ||
| Co-careldopa | 0.05 (–0.02 to 0.13) | 0.136 |
| Placebo | –0.05 (–0.13 to 0.03) | 0.835 |
Table 50 summarises the results of the exit poll from the researcher perspective. Approximately half of the researchers made their choice of treatment because they thought that the treatment benefited the participant, although one-third chose a treatment arm because they thought that the treatment had no benefit. Similar proportions were observed for reason of choice when the correct treatment group was chosen. The certainty of guess was similar between randomised arms and by treatment group chosen.
| Reason for choice, by randomised group | Co-careldopa (N = 88), n (%) | Placebo (N = 80), n (%) | Total (N = 168), n (%) |
|---|---|---|---|
| Treatment had no benefit | 30 (34.1) | 24 (30.0) | 54 (32.1) |
| Treatment benefited patient | 42 (47.7) | 40 (50.0) | 82 (48.8) |
| AE | 4 (4.5) | 4 (5.0) | 8 (4.8) |
| Other reason | 12 (13.6) | 12 (15.0) | 24 (14.3) |
| Reason for choice, by correct choice | Yes (N = 82), n (%) | No (N = 86), n (%) | Total (N = 168), n (%) |
| Treatment had no benefit | 26 (31.7) | 28 (32.6) | 54 (32.1) |
| Treatment benefited patient | 43 (52.4) | 39 (45.3) | 82 (48.8) |
| AE | 4 (4.9) | 4 (4.7) | 8 (4.8) |
| Other reason | 9 (11.0) | 15 (17.4) | 24 (14.3) |
| Certainty of choice, by randomised group | Co-careldopa (N = 88) | Placebo (N = 80) | Total (N = 168) |
| Mean (SD) | 6.3 (2.07) | 5.9 (2.59) | 6.1 (2.34) |
| Median (range) | 6.8 (0.0–10.0) | 6.5 (0.0–10.0) | 6.8 (0.0–10.0) |
| Q1, Q3 (points) | 5.0, 8.0 | 5.0, 8.0 | 5.0, 8.0 |
| Missing (n) | 0 | 0 | 0 |
| Certainty of choice, by treatment group chosen | Co-careldopa (N = 100) | Placebo (N = 68) | Total (N = 168) |
| Mean (SD) | 6.2 (2.25) | 6.0 (2.47) | 6.1 (2.34) |
| Median (range) | 7.0 (0.0–10.0) | 6.0 (0.0–10.0) | 6.8 (0.0–10.0) |
| Q1, Q3 (points) | 5.0, 8.0 | 5.0, 8.0 | 5.0, 8.0 |
| Missing (n) | 0 | 0 | 0 |
| Certainty of choice, by reason | Mean (SD) | Median (range) | |
| Treatment had no benefit (n = 54) | 5.8 (2.48) | 6.0 (0.0–9.0) | |
| Treatment benefited patient (n = 82) | 6.3 (1.95) | 6.5 (0.0–10.0) | |
| Total (N = 168) | 6.1 (2.34) | 6.8 (0.0–10.0) | |
Answers from a participant perspective are summarised in Table 51. Forty-five participants (39.1%) in the co-careldopa group made their choice because the treatment did not work and 38 participants (33.0%) made their choice because the treatment did work. Of the participants in the placebo group, 31 (31.0%) chose the treatment because it did not work, whereas 41 (41.0%) chose it because it did work. Similarly, the reasons for choice for those who answered correctly and incorrectly were mainly because the treatment did not work [correct choice 33 (32.4%), incorrect choice 43 (38.1%)] or because the treatment did work [correct choice 39 (38.2%), incorrect choice 40 (35.4%)]. The certainty of guesses by participants was similar by randomised arms, treatment chosen and reason [overall mean approximately 7.0, SD 2.3)].
| Reason for choice, by randomised group | Co-careldopa (N = 115), n (%) | Placebo (N = 100), n (%) | Total (N = 215), n (%) |
|---|---|---|---|
| NHS staff told me the name of the drug | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Treatment did not work | 45 (39.1) | 31 (31.0) | 76 (35.3) |
| Treatment worked | 38 (33.0) | 41 (41.0) | 79 (36.7) |
| I had a side effect | 8 (7.0) | 7 (7.0) | 15 (7.0) |
| Appearance or taste of the pill | 3 (2.6) | 4 (4.0) | 7 (3.3) |
| Other | 21 (18.3) | 15 (15.0) | 36 (16.7) |
| Missing | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Reason for choice, by correct choice | Yes (N = 102), n (%) | No (N = 113), n (%) | Total (N = 215), n (%) |
| NHS staff told me the name of the drug | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Treatment did not work | 33 (32.4) | 43 (38.1) | 76 (35.3) |
| Treatment worked | 39 (38.2) | 40 (35.4) | 79 (36.7) |
| I had a side effect | 8 (7.8) | 7 (6.2) | 15 (7.0) |
| Appearance or taste of the pill | 5 (4.9) | 2 (1.8) | 7 (3.3) |
| Other | 17 (16.7) | 19 (16.8) | 36 (16.7) |
| Missing | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Certainty of choice, by randomised group | Co-careldopa (N = 115) | Placebo (N = 99) | Total (N = 214) |
| Mean (SD) | 7.3 (2.31) | 7.1 (2.40) | 7.2 (2.35) |
| Median (range) | 8.0 (0.0–10.0) | 8.0 (0.0–10.0) | 8.0 (0.0–10.0) |
| Q1, Q3 (points) | 6.0, 9.0 | 5.0, 9.0 | 5.0, 9.0 |
| Certainty of choice, by treatment group chosen | Co-careldopa (N = 119) | Placebo (N = 95) | Total (N = 214) |
| Mean (SD) | 7.4 (2.09) | 7.0 (2.62) | 7.2 (2.35) |
| Median (range) | 8.0 (0.0–10.0) | 7.5 (0.0–10.0) | 8.0 (0.0–10.0) |
| Q1, Q3 (points) | 6.0, 9.0 | 5.0, 9.0 | 5.0, 9.0 |
| Certainty of choice, by reason | Mean (SD) | Median (range) | |
| NHS staff told me the name of the drug (n = 1) | 7.0 (0.0) | 7.0 (7.0–7.0) | |
| Treatment did not work (n = 76) | 6.9 (2.74) | 7.8 (0.0–10.0) | |
| Total (N = 214) | 7.2 (2.35) | 8.0 (0.0–10.0) | |
Overall, the blinding index results and summaries from the exit poll suggest that the trial blinding was maintained.
Further analysis
The DARS trial information DVD
Only 21 participants (6.8%) in the co-careldopa group and 25 participants (8.8%) in the placebo group watched the DARS trial information DVD. Of these, the majority said that it helped them understand the project (Table 52). Owing to the low number of responses, planned analyses exploring correlation between DVD viewing and both treatment compliance and patient questionnaire completion were not conducted.
| Participant responses to information via DVD | Treatment group, n (%) | Total (N = 593), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 308) | Placebo (N = 285) | ||
| Watched DVD | |||
| Yes | 21 (6.8) | 25 (8.8) | 46 (7.8) |
| No | 255 (82.8) | 241 (84.6) | 496 (83.6) |
| Missing | 32 (10.4) | 19 (6.7) | 51 (8.6) |
| Understood research project | |||
| Yes, a lot | 9 (2.9) | 9 (3.2) | 18 (3.0) |
| Yes, a little | 9 (2.9) | 12 (4.2) | 21 (3.5) |
| No | 2 (0.6) | 3 (1.1) | 5 (0.8) |
| Missing | 288 (93.5) | 261 (91.6) | 549 (92.6) |
| DVDs for future research projects | |||
| Yes | 13 (4.2) | 17 (6.0) | 30 (5.1) |
| No | 2 (0.6) | 3 (1.1) | 5 (0.8) |
| Not sure | 6 (1.9) | 4 (1.4) | 10 (1.7) |
| Missing | 287 (93.2) | 261 (91.6) | 548 (92.4) |
Level of assistance with patient-completed questionnaires
Help with patient questionnaire completion was monitored at each time point. At baseline, 38 co-careldopa participants (12.3%) required no help, 200 (64.9%) required some help and 53 (17.2%) required a lot of help, whereas 22 placebo participants (7.7%) required no help, 210 (73.7%) required some help and 43 (15.1%) required a lot of help. Help completing the questionnaires at the 8-week follow-up was more balanced than at baseline. More participants in the co-careldopa group were able to complete the questionnaires with no help at 6 months than at 8 weeks [56 (18.2%)], but fewer were able to in the placebo group [40 (14.0%)]. At 12 months, the ability to complete the questionnaire with no help was similar to the 8-week follow-up. There were borderline statistically significant differences between arms at baseline and 6 months, with a greater proportion of participants in the co-careldopa than in the placebo group needing no help (p = 0.051 and p = 0.052 at baseline and 6 months, respectively) (Table 53).
| Help with questionnaire completion | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 8 weeks | 6 months | 12 months | |||||
| Co-careldopa (N = 308) | Placebo (N = 285) | Co-careldopa (N = 308) | Placebo (N = 285) | Co-careldopa (N = 308) | Placebo (N = 285) | Co-careldopa (N = 308) | Placebo (N = 285) | |
| Questionnaire help, n (%) | ||||||||
| No help | 38 (12.3) | 22 (7.7) | 49 (15.9) | 48 (16.8) | 56 (18.2) | 40 (14.0) | 46 (14.9) | 51 (17.9) |
| Some help | 200 (64.9) | 210 (73.7) | 165 (53.6) | 160 (56.1) | 141 (45.8) | 161 (56.5) | 139 (45.1) | 130 (45.6) |
| A lot of help | 53 (17.2) | 43 (15.1) | 46 (14.9) | 43 (15.1) | 40 (13.0) | 41 (14.4) | 31 (10.1) | 35 (12.3) |
| Missing | 17 (5.5) | 10 (3.5) | 48 (15.6) | 34 (11.9) | 71 (23.1) | 43 (15.1) | 92 (29.9) | 69 (24.2) |
| p-valuea | 0.051 | 0.936 | 0.052 | 0.564 | ||||
| If help received, person who helped, n (%) | ||||||||
| DARS trial researcher | 215 (85.0) | 223 (88.1) | 161 (76.3) | 169 (83.3) | 138 (76.2) | 146 (72.3) | 134 (78.8) | 129 (78.2) |
| Relative, carer or friend | 29 (11.5) | 25 (9.9) | 37 (17.5) | 28 (13.8) | 36 (19.9) | 44 (21.8) | 24 (14.1) | 31 (18.8) |
| Therapist | 1 (0.4) | 0 (0.0) | 3 (1.4) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 12 (7.1) | 5 (3.0) |
| Nurse | 2 (0.8) | 1 (0.4) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (2.4) | 4 (1.6) | 9 (4.3) | 4 (2.0) | 7 (3.9) | 12 (5.9) | 12 (7.1) | 5 (3.0) |
Moderator analysis
The moderator analysis explored whether or not the size of the treatment effect (irrespective of whether or not the effect is significant) was influenced by baseline characteristics of patients. The following results are based on exploratory analysis and should be treated with caution.
Each moderator variable was tabulated by primary outcome category and treatment group (see Appendix 6, Tables 82 and 83). Mean baseline RMI score (regardless of reporting status) and the proportion of patients with no lower limb pain was higher among those walking independently at 8 weeks. There was no difference in baseline RMI score or lower limb pain between the treatment groups. There was a suggestion that both site of MCA lesions and haemorrhage location were associated with the primary outcome and treatment group. A higher proportion of those with no MCA lesion and those with a (subcortical) haemorrhage walked independently at 8 weeks and belonged to the placebo group, which fits with the ITT analysis that found that those in the placebo group have a higher odds of walking independently at 8 weeks (though not statistically higher than those in the co-careldopa group). Scan data were available for 472 out of 593 patients (79.6%) and patients with haemorrhage are those who had a primary haemorrhage stroke (rather than infarction). Those without scan data were more likely to be in the co-careldopa group (58.7% without scan data vs. 50.2% with scan data) and have fewer (≤ 12) years of education (53.7% without scan data vs. 45.2% with scan data). Hence, by not including those with scan data, the numbers in the co-careldopa arm reduces and the ITT analysis of the primary outcome shows an OR closer to 1 (OR 0.806, 95% CI 0.521 to 1.249).
With the exception of baseline RMI score, no other baseline characteristics showed potential moderation when accounting for covariates included in the primary analysis multilevel model (see Appendix 6, Table 84). There was weak evidence (p = 0.069) that baseline RMI score moderates the effect of treatment on walking independently at 8 weeks, such that there is a significant difference in the association of baseline RMI score with the primary outcome for placebo and co-careldopa. The effect of treatment on walking independently at 8 weeks changes as baseline RMI score increases (Figure 8). When the baseline RMI score is 0 points, those in the placebo arm have a significantly higher chance of walking independently at 8 weeks than those in the co-careldopa arm, but there is no evidence of a significant difference between the treatment groups at the mean baseline RMI score or as the baseline RMI increases towards a score of 6 points (Figure 9).
FIGURE 8.
Linear prediction plot for treatment group, by baseline RMI score.

FIGURE 9.
Predictive margins of treatment group with 95% CI.

Departures from randomised treatment as a mediator
All patients received their treatment in accordance with their randomised allocation. Of the 593 patients with primary outcome data, 536 had five or more therapy sessions and were included in descriptive compliance statistics. With the exception of drug intake compliance, there was no suggestion of a difference in the distribution of the other categories of compliance between the treatment groups (Table 54) or of a difference between the treatment groups in the outcomes of ‘non-compliers’, where ‘non-compliers’ are defined as those not satisfying the criteria for the compliance category (Table 55). Within the relaxed drug intake compliance category, the proportion of compliant patients was higher in the placebo group than in the co-careldopa group (76% vs. 68%, respectively) and the proportion of non-compliant patients walking independently at 8 weeks was higher in the placebo group than in the co-careldopa group (48% vs. 38%, respectively).
| Category of compliance | Treatment group, n (%) | Overall, n (%) | |
|---|---|---|---|
| Placebo | Co-careldopa | ||
| Strict compliance | |||
| No | 233 (87.9) | 232 (85.6) | 465 (86.8) |
| Yes | 32 (12.1) | 39 (14.4) | 71 (13.2) |
| Relaxed timing compliance | |||
| No | 167 (63.0) | 180 (66.4) | 347 (64.7) |
| Yes | 98 (37.0) | 91 (33.6) | 189 (35.3) |
| Relaxed timing and therapy compliance | |||
| No | 149 (56.2) | 156 (57.6) | 305 (56.9) |
| Yes | 116 (43.8) | 115 (42.4) | 231 (43.1) |
| Relaxed drug intake compliance | |||
| No | 63 (23.8) | 86 (31.7) | 149 (27.8) |
| Yes | 202 (76.2) | 185 (68.3) | 387 (72.2) |
| Stage of compliance | Treatment group | |||
|---|---|---|---|---|
| Placebo | Co-careldopa | |||
| N | Walking independently at 8 weeks, n (%) | N | Walking independently at 8 weeks, n (%) | |
| Strict compliance | ||||
| No | 233 | 108 (46.3) | 232 | 95 (41.0) |
| Yes | 32 | 12 (37.5) | 39 | 19 (48.7) |
| Relaxed timing compliance | ||||
| No | 167 | 71 (42.5) | 180 | 68 (37.8) |
| Yes | 98 | 49 (50.0) | 91 | 46 (50.6) |
| Relaxed timing and therapy compliance | ||||
| No | 149 | 62 (41.6) | 156 | 57 (36.5) |
| Yes | 116 | 58 (50.0) | 115 | 57 (49.8) |
| Relaxed drug intake compliance | ||||
| No | 63 | 30 (47.6) | 86 | 33 (38.4) |
| Yes | 202 | 90 (44.6) | 185 | 81 (43.8) |
Mediator analysis
The details of the mediator analyses are shown in Appendix 7 (see Tables 85–89). In summary, there was no evidence that any of the variables investigated mediated the effect of treatment on walking independently at 8 weeks.
Chapter 4 Economic evaluation
Sample
A total of 593 patients were recruited to the trial (308 patients to the co-careldopa group and 285 to the placebo group). Of these, 1% died between baseline and 8 weeks, 2% died between 8 weeks and 6 months and 3% died between 6 months and 12 months. Two-thirds of those who died before the end of the trial were from the co-careldopa group.
Missing data
A total of 122 patients had complete EQ-5D scores and costs at baseline and all follow-up periods. For the remaining 471 participants (53% from the co-careldopa group and 47% from the placebo group), either EQ-5D scores and/or cost data were missing for at least one of the follow-up periods. Indicatively, and as shown in Figure 10, 99 patients from the co-careldopa group had missing EQ-5D scores at 12 months, compared with only 19 patients at baseline; of these, 13 patients had all EQ-5D items missing at 12 months compared with 93 at baseline. The number of patients with missing EQ-5D items increased over time, and a majority of those with missing data did not complete the measure. Figure 11 shows the number of patients with missing resource use items and, hence, missing costs. For example, 180 patients in the placebo group had missing costs at baseline, compared with 228 patients at 12 months. The number of patients with missing costs increased over time and co-careldopa patients were more likely to have missing cost data. In addition, most of the missing costs were the result of at least one missing item rather than non-completion of the resource use questionnaire.
FIGURE 10.
Total number of patients with missing EQ-5D scores, by treatment group.

FIGURE 11.
Total number of patients with missing total resource use costs, by treatment group.

Missing utility and cost data were imputed in each period using, as predictors, health status measures (BI),120 mRS121 and GHQ-12,122 and baseline characteristics such as age, sex, ethnicity and type of stroke. Imputation was possible for 313 patients but not for 158 patients. The latter was due to missing scores over time for at least one of the predictor variables (BI, mRS and GHQ-12). Therefore, a total of 435 patients (216 placebo and 219 co-careldopa) were included in the primary base-case analysis.
The baseline characteristics of the patients included in the analysis sample are presented in Table 56. Patients in the placebo group were marginally younger than those in the co-careldopa group. In both arms, more than half of the patients were male. More than two-thirds of the patients in both arms were admitted with an infarction stroke at baseline but did not receive thrombolysis. These results seem to be consistent across samples (i.e. base case compared with complete case) with the exception that, in the complete case, patients in the co-careldopa arm were slightly older than those in the placebo arm.
| Patient characteristics | Case | |||
|---|---|---|---|---|
| Base | Completea | |||
| Placebo (N = 216) | Co-careldopa (N = 219) | Placebo (N = 54) | Co-careldopa (N = 48) | |
| Age (years) | ||||
| Mean (SD) | 71.69 (12.59) | 69.91 (12.95) | 68.65 (14.35) | 69.60 (12.60) |
| Minimum | 33 | 27 | 33 | 41 |
| Maximum | 98 | 97 | 95 | 90 |
| Sex, n (%) | ||||
| Male | 144 (67) | 127 (58) | 40 (74) | 26 (54) |
| Female | 72 (33) | 92 (42) | 14 (26) | 22 (46) |
| Ethnicity, n (%) | ||||
| White | 209 (97) | 211 (96) | 52 (96) | 46 (96) |
| Non-white | 7 (3) | 8 (4) | 2 (4) | 2 (4) |
| Type of stroke, n (%) | ||||
| Infarction | 184 (85) | 195 (89) | 48 (89) | 43 (90) |
| Primary haemorrhage | 32 (15) | 24 (11) | 6 (11) | 5 (10) |
| Classification of stroke,b n (%) | ||||
| TACI | 48 (26) | 72 (37) | 10 (21) | 14 (33) |
| LACI | 45 (25) | 44 (23) | 10 (21) | 10 (23) |
| PACI | 71 (39) | 61(31) | 25 (52) | 14 (33) |
| POCI | 19 (10) | 18 (9) | 3 (6) | 5 (12) |
| Probability thrombolysis receivedc | ||||
| Mean (SD) | 0.214 (0.411) | 0.224 (0.418) | 0.167 (0.376) | 0.188 (0.394) |
Resource use and costs
As resource use questions for the last follow-up periods (6 months and 12 months) referred to the last 3 months, monthly face-to-face and telephone/e-mail contacts were calculated and then multiplied by 4 and 6, respectively, to cover the period between the follow-ups. Table 57 shows the average resource use per patient in each trial arm in the observed data only (i.e. complete case). Patients in the co-careldopa group were more likely than those in the placebo group to use the majority of services, and that pattern persisted over time. Any other conclusions should be tempered given the little reported resource use within the trial.
| Primary care and community health and social services | Time point | |||||
|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||
| Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 54) | Co-careldopa (n = 48) | |
| GP surgery visits (face-to-face contacts) | ||||||
| Mean (SD) | 0.407 (1.000) | 0.934 (2.971) | 1.975 (2.454) | 1.667 (2.674) | 2.148 (3.293) | 2.292 (3.892) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 5 | 20 | 9 | 15 | 16 | 24 |
| GP surgery visits (telephone/e-mail) | ||||||
| Mean (SD) | 0.019 (0.136) | 0.188 (0.762) | 0.667 (1.505) | 1.778 (8.669) | 0.519 (1.563) | 0.875 (2.017) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 1 | 5 | 7 | 60 | 8 | 8 |
| GP home visits | ||||||
| Mean (SD) | 0.222 (0.572) | 0.271 (0.574) | 0.469 (1.104) | 0.722 (1.289) | 0.889 (2.793) | 0.958 (1.798) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 3 | 2 | 4 | 4 | 14 | 6 |
| District nurse (face-to-face contacts) | ||||||
| Mean (SD) | 2.167 (4.546) | 3.750 (12.39) | 9.358 (22.10) | 6.861 (22.09) | 2.593 (8.831) | 2.458 (7.161) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 22 | 84 | 133 | 133 | 60 | 48 |
| District nurse (telephone/e-mail contacts) | ||||||
| Mean (SD) | 0.481 (2.238) | 0.521 (1.868) | 0.049 (0.254) | 0.056 (0.385) | 0.296 (1.667) | 0.708 (2.163) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 13 | 10 | 1 | 3 | 12 | 12 |
| Physiotherapist (face-to-face contacts)a | ||||||
| Mean (SD) | 0.185 (1.361) | 0.250 (1.466) | 1.333 (5.408) | 0.750 (3.086) | 1.889 (7.677) | 0.000 (0.000) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 10 | 10 | 33 | 15 | 44 | 0 |
| Hospital and residential care services | ||||||
| Hospital inpatient stay (days) | ||||||
| Mean (SD) | 22.54 (25.56) | 23.90 (26.70) | 6.519 (24.09) | 4.361 (15.80) | 0.296 (2.177) | 1.292 (4.237) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 89 | 84 | 120 | 80 | 16 | 22 |
| Hospital outpatient visits | ||||||
| Mean (SD) | 0.259 (0.732) | 0.542 (1.352) | 2.519 (6.551) | 2.694 (6.097) | 2.074 (4.774) | 2.333 (5.540) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 4 | 8 | 33 | 32 | 32 | 34 |
| Hospital A&E visits | ||||||
| Mean (SD) | 0.093 (0.446) | 0.125 (0.334) | 0.049 (0.254) | 0.083 (0.326) | 0.259 (0.873) | 0.417 (0.919) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 3 | 1 | 1 | 1 | 4 | 4 |
In terms of the total NHS resource use cost, the co-careldopa group used the highest number of resources and also had the highest cost. Mean total costs from the use of primary care and community health services (before imputation) were £5095.70 (SD £6555.99) for the placebo group and £7937.92 (SD £12,247.55) for the co-careldopa group. Mean total costs from the use of hospital and residential care services (before imputation) were also higher for the co-careldopa group [£15,595.72 (SD £20,182.44)] than for the placebo group [£13,619.56 (SD £14,950.22)]. There were no significant differences in costs between the two arms using Mann–Whitney U-tests. This was the case for both the complete-case sample and the sample after imputing for missing data. For this reason, no adjustments in costs for baseline differences were considered necessary. Table 58 reports resource use for complete cases only as the imputation generated total cost values rather than individual resource counts.
| Cost | Time point | Total costs | ||||||
|---|---|---|---|---|---|---|---|---|
| 8 weeks | 6 months | 12 months | ||||||
| Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 54) | Co-careldopa (n = 48) | |
| Total community costs (before imputation) | ||||||||
| Mean (SD) | 603.69 (912.71) | 617.33 (976.85) | 1980.38 (3010.07) | 2815.22 (4118.89) | 2511.64 (3876.52) | 4505.37 (8994.27) | 5095.70 (6555.99) | 7937.92 (12,247.55) |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 3460.82 | 3938.94 | 12,341.55 | 14,630.92 | 13,683.60 | 36,656.74 | 24,547.00 | 52,519.37 |
| Total hospital costs (before imputation) | ||||||||
| Mean (SD) | 8459.35 (9305.28) | 8904.18 (9603.46) | 4579.20 (10,939.52) | 3013.02 (6319.81) | 2557.17 (5931.34) | 1702.36 (4593.52) | 15,595.72 (20,182.44) | 13,619.56 (14,950.22) |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 32,392.44 | 30,572.64 | 45,045.60 | 29,116.80 | 20,531.64 | 24,020.70 | 81,291.59 | 53,591.24 |
| Total NHS costs (before imputation)a | ||||||||
| Mean (SD) | 9063.04 (9184.53) | 9538.50 (9260.10) | 6559.58 (11,539.69) | 5828.24 (8303.13) | 5068.80 (6603.01) | 6207.73 (9913.83) | 20,691.42 (20,675.24) | 21,574.47 (21,948.31) |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 33,208.08 | 30,589.62 | 56,462.85 | 34,042.12 | 24,972.18 | 37,948.82 | 85,957.30 | 75,063.11 |
| Cost | Placebo (n = 216) | Co-careldopa (n = 219) | Placebo (n = 216) | Co-careldopa (n = 219) | Placebo (n = 216) | Co-careldopa (n = 219) | Placebo (n = 216) | Co-careldopa (n = 219) |
| Total NHS costs (after imputation)a,b | ||||||||
| Mean (SD) | 5723.60 (8264.14) | 6048.49 (8363.46) | 4280.49 (8044.10) | 5405.00 (9619.64) | 4540.43 (7642.65) | 5638.45 (9666.25) | 14,544.52 (16,756.76) | 17,091.93 (20,507.22) |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 34,449.23 | 35,093.59 | 56,462.88 | 59,932.10 | 53,600.57 | 59,067.19 | 85,957.29 | 122,602.5 |
Quality-of-life data
Table 59 shows the mean EQ-5D scores for each period for the two arms of the trial when no scores are imputed (i.e. complete case) and when values are imputed (i.e. base case) (Figure 12 provides a graphical representation). In both arms, there was a considerable increase in EQ-5D scores from baseline to 8 weeks. There are small fluctuations in the utility values between 8 weeks and 12 months for both placebo and co-careldopa, but the improvement in utility scores is more or less maintained until the last follow-up period (12 months).
| Time point | Case, mean (SD) | |||
|---|---|---|---|---|
| Complete | Base | |||
| Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 216) | Co-careldopa (n = 219) | |
| Baseline | 0.186 (0.351) | 0.178 (0.297) | 0.215 (0.327) | 0.154 (0.287) |
| 8 weeks | 0.519 (0.273) | 0.471 (0.320) | 0.465 (0.306) | 0.412 (0.317) |
| 6 months | 0.506 (0.268) | 0.483 (0.339) | 0.448 (0.300) | 0.412 (0.342) |
| 12 months | 0.534 (0.321) | 0.467 (0.377) | 0.449 (0.341) | 0.395 (0.369) |
FIGURE 12.
Mean (unadjusted) EQ-5D scores by treatment group (base case).
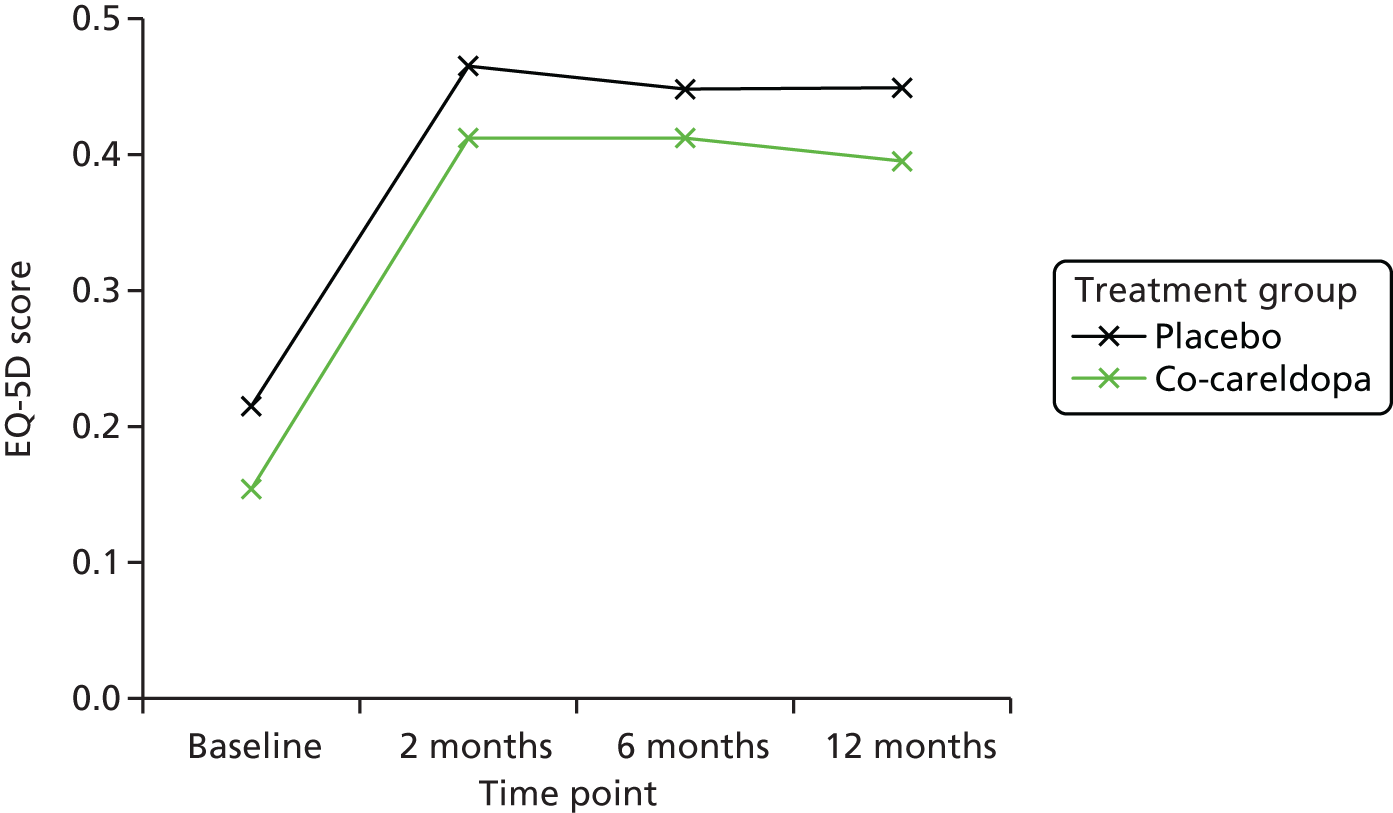
Table 60 provides the mean EQ-5D change scores between baseline and follow-up points for the base case. All of the observed changes are above the estimated minimally important difference (range 0.08–0.12) for the EQ-5D. 123 Independent-sample t-tests showed that there were statistically significant differences over time in EQ-5D scores in both trial arms. Differences in EQ-5D scores between the placebo and co-careldopa arms were significant (at the 1% level) at baseline and at 8 weeks but not at any of the other follow-up times. This indicated that baseline adjustments are necessary and, thus, adjustments were made using an ordinary least squares (OLS) regression of QALYs on the treatment indicator and baseline EQ-5D. 124
| Time point | Case, mean (p-value) | |||
|---|---|---|---|---|
| Complete | Base | |||
| Placebo (n = 54) | Co-careldopa (n = 48) | Placebo (n = 216) | Co-careldopa (n = 219) | |
| Baseline to 8 weeks | 0.333 (< 0.001) | 0.293 (< 0.001) | 0.250 (< 0.001) | 0.258 (< 0.001) |
| Baseline to 6 months | 0.321 (< 0.001) | 0.305 (< 0.001) | 0.233 (< 0.001) | 0.258 (< 0.001) |
| Baseline to 12 months | 0.349 (< 0.001) | 0.289 (< 0.001) | 0.235 (< 0.001) | 0.241 (< 0.001) |
Cost-effectiveness results
Table 61 shows the costs and QALYs for each of the two trial arms for the primary analysis (multiple imputation for missing data and adjustment for baseline EQ-5D differences). It also provides the incremental cost and benefit (expressed as QALY gains). On average, co-careldopa patients incurred higher costs and gained fewer QALYs than placebo patients. Therefore, co-careldopa is ‘dominated’ by placebo and, hence, the ICER was not calculated. The results indicate that co-careldopa is not cost-effective.
| Treatment group | Cost (£) | QALYsa | ICER (£/QALY) |
|---|---|---|---|
| Placebo, mean (SD) | 14,544.52 (16,756.76) | 0.420 (0.029) | |
| Co-careldopa, mean (SD) | 17,091.93 (20,507.22) | 0.397 (0.031) | |
| Treatment group comparison | Incremental cost (£) | Incremental QALYs | |
| Co-careldopa vs. placebo | 2547.41 | –0.023 | Co-careldopa dominated |
Figure 13 shows the cost-effectiveness plane for co-careldopa compared with placebo, based on bootstrapped estimates of costs and QALYs. The average costs from the bootstrapped estimates were £14,531.73 (SD £1130.00) and £16,640.91 (SD £1294.21) for the placebo and co-careldopa groups, respectively. The mean number of QALYs was 0.420 (SD 0.002) for the placebo group and 0.397 (SD 0.002) for the co-careldopa group. The simulation estimates are spread in the north-west and south-west quadrants, suggesting that co-careldopa is unlikely to lead to better health outcomes. They also indicate that placebo is less costly but more effective in terms of improving HRQoL. A small proportion of simulations is beneath the diagonal cost-effectiveness threshold (£20,000 per QALY) indicating cost-effectiveness. However, in these cases, co-careldopa is still less effective but less costly.
FIGURE 13.
Cost-effectiveness plane for co-careldopa compared with placebo (outcome measure: QALY, health-care provider perspective).

A CEAC is presented in Figure 14. At a threshold of £20,000 per QALY, co-careldopa has a 7% chance of being cost-effective, decreasing to 5% when the threshold λ = £30,000. There is a negative relationship between λ and co-careldopa’s chance of being cost-effective as placebo is more effective on average.
FIGURE 14.
Cost-effectiveness acceptability curve.
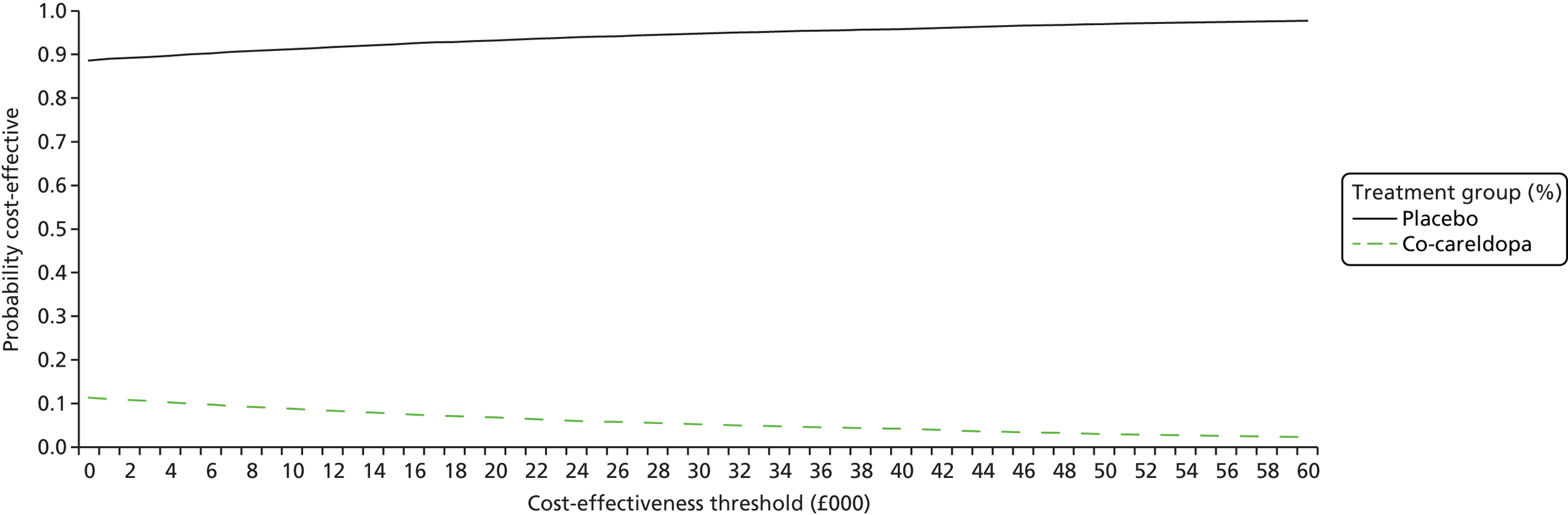
Net benefit regression
We calculated the NMB for each patient assuming a threshold λ = £20,000 and accounted for differences in EQ-5D scores between trial arms at baseline. The average NMB for both groups was negative. In detail, the NMB for the placebo group was –£6138.90 (SD £16,841.91) and for the co-careldopa group was –£9146.19 (SD £20,543.19). In Table 62 we present the results from the net benefit regression. We ran OLS regressions of the NMB on the treatment indicator and other controls such as age, sex, type of stroke, classification of stroke and mRS score at 8 weeks. Some of the models included also showed interactions between treatment and mRS, treatment and type of stroke, and treatment and stroke classification. In the majority of the models, treatment was not found to be a significant predictor of net benefit. On two occasions, the treatment coefficient was significant at the 10% level and negative, suggesting that co-careldopa was associated with lower net benefit than placebo.
| Explanatory variables | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Treatment | –3007.28* (1800.07) | –2.889.08 (1756.57) | –2813.66* (1667.20) | –2662.88 (1677.31) | –1968.36 (1790.62) | 2582.97 (4972.92) | –905.53 (5739.20) | –9287.27* (4953.46) |
| Age | – | –156.74** (72.83) | –154.26** (68.28) | –146.35** (68.59) | –210.06*** (79.02) | –144.31** (68.74) | –144.92** (68.58) | –213.18*** (78.77) |
| Female | – | –4572.18** (2052.77) | –3371.09* (1893.34) | –3400.62* (1891.84) | –3717.88* (2049.40) | –3507.93* (1919.19) | –3432.49* (1885.81) | –3828.01* (2046.12) |
| mRS | – | – | –6577.10*** (815.06) | –6536.22*** (2305.36) | –6096.89*** (916.99) | –5682.21*** (1118.82) | –6523.30*** (816.54) | –6173.53*** (924.87) |
| Type of strokea | ||||||||
| Primary haemorrhage | – | – | – | 3137.66 (2305.36) | – | 3287.49 (2322.50) | 3813.84 (2615.65) | – |
| Classification of strokea | ||||||||
| LACI | – | – | – | – | 5044.46* (2594.40) | – | – | 2974.26 (2719.27) |
| PACI | – | – | – | – | 4869.38** (2360.39) | – | – | 1273.39 (2878.82) |
| POCI | – | – | – | – | 6606.16** (3083.63) | – | – | 1279.90 (4624.86) |
| Treatment × mRS | – | – | – | – | – | –1573.99 (1677.89) | – | – |
| Treatment × stroke type | – | – | – | – | – | – | –1556.32 (4817.93) | – |
| Treatment × stroke classification | – | – | – | – | – | – | – | 3266.26* (1876.04) |
| Constant | –6138.90*** (1145.93) | 1194.24** (5033.67) | 30,871.25*** (5419.66) | 26,600.50*** (6217.28) | 29,294.66*** (6429.09) | 23,614.60*** (6660.48) | 25,719.84*** (6555.01) | 32,380.97*** (6985.13) |
| R 2 | 0.006 | 0.036 | 0.164 | 0.167 | 0.183 | 0.169 | 0.168 | 0.190 |
| F-statistic (p-value) | 2.79 (0.096) | 5.17 (0.002) | 18.35 (0.000) | 15.15 (0.000) | 9.50 (0.000) | 12.87 (0.000) | 12.84 (0.000) | 8.47 (0.000) |
| n | 435 | 435 | 427 | 427 | 371 | 427 | 427 | 371 |
As mentioned in the results section, a per-protocol analysis was not considered feasible because only a few patients met the strict compliance criteria (i.e. patient received tablet 45–60 minutes before therapy for ≥ 80% of the sessions) set by the trial team. However, as a sensitivity analysis, and to see whether or not patient non-compliance was a driver of results, we relaxed the strict compliance criterion (i.e. 80%). In particular, we looked at 75%, 65%, 55%, 50%, 45% and 40% compliance and ran OLS regressions of NMB on treatment and other controls, each time on the population that met the specific compliance percentage. The results are presented in Table 63. In all cases, except when compliance is 40%, the treatment coefficient is not significant, suggesting that the lower monetary benefits of co-careldopa than placebo were not due to the non-compliance of the patients.
| Explanatory variables | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Treatment | –536.73 (2721.70) | –830.62 (2514.26) | –1722.68 (2283.85) | –2066.38 (2138.10) | –2050.24 (2023.29) | –2749.86 (1929.16) | –3144.67 (1916.60) | –3462.73* (1881.52) |
| Other controlsa | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| n | 190 | 225 | 255 | 288 | 309 | 349 | 364 | 386 |
| Compliance | Strict compliance for ≥ 75% of the sessions | Strict compliance for ≥ 70% of the sessions | Strict compliance for ≥ 65% of the sessions | Strict compliance for ≥ 60% of the sessions | Strict compliance for ≥ 55% of the sessions | Strict compliance for ≥ 50% of the sessions | Strict compliance for ≥ 45% of the sessions | Strict compliance for ≥ 40% of the sessions |
Sensitivity analyses
To account for uncertainty around mean incremental costs and effectiveness, we conducted sensitivity analyses and non-parametric bootstrapping (Table 64). We added and subtracted 20% from the costs in both arms and assessed the subsequent impact on the ICER. We also looked at the case of no adjustment for baseline EQ-5D differences as well as only the complete cases. Furthermore, we examined the case of adjusting for baseline costs and the use of a different resource use count for PT and OT sessions. In all cases except one, the conclusion was unchanged and co-careldopa was dominated by placebo. Furthermore, the average incremental cost and QALY estimates from the bootstrapping were similar to those of the deterministic base-case scenario.
| Co-careldopa vs. placebo | Incremental cost (£) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|
| Without adjusting for baseline EQ-5D differences | 2547.41 | –0.046 | Co-careldopa dominated |
| Adjusting for baseline cost differencesa | 1903.90 | –0.023 | Co-careldopa dominated |
| Alternative PT and OT countsa,b | –166.44 | –0.023 | 7211.06 |
| Complete case (without imputation)a | 883.04 | –0.029 | Co-careldopa dominated |
| 20% increase in costs (in both arms)a | 3056.89 | –0.023 | Co-careldopa dominated |
| 20% decrease in costs (in both arms)a | 2037.93 | –0.023 | Co-careldopa dominated |
| Costs are normally distributed in imputation (adjusting for baseline differences)a | 3398.69 | –0.024 | Co-careldopa dominated |
| Bootstrapped average (10,000 replications)a | 2109.18 | –0.023 | Co-careldopa dominated |
Secondary analysis
Table 65 shows the costs and the proportion (in percentages) of those who achieved a score of ≥ 7 points on the RMI at 8 weeks. It also shows the incremental cost and proportion (expressed as increase in percentage of population), as well as the ICER. More patients in the placebo group than in the co-careldopa group achieved a score of ≥ 7 points on the RMI at 8 weeks. In addition, the cost to the health-care provider at 8 weeks is higher for the placebo group, which yields an ICER of £307.81. As co-careldopa is less effective and cheaper than placebo, this is interpreted as the costs saved by one person not achieving a RMI score of ≥ 7 points.
| Treatment group | Costs at 6 weeks (£),a mean (SD) | RMI at 8 weeks, proportion (%) with RMI ≥ 7 points | ICER (£/RMI) |
|---|---|---|---|
| Placebo | 4447.59 (7368.05) | 54.76 | |
| Co-careldopa | 3197.89 (4993.56) | 50.70 | |
| Incremental cost (£) | Incremental RMI | ||
| Co-careldopa vs. placebo | –1249.70 | –4.06 | 307.81 |
Supplementary analyses
Supplementary analyses using a wider perspective for costs were also conducted. Costs were considered to be the sum of the costs to the health-care provider and patients’ costs (Table 66). In this case, co-careldopa was still more expensive than placebo and less effective in terms of QALY gains.
| Treatment group | Costs (£), mean (SD) | QALYs,a mean (SD) | ICER (£/QALY) |
|---|---|---|---|
| Placebo | 15,266.73 (16,987.16) | 0.421 (0.030) | |
| Co-careldopa | 17,484.66 (18,579.82) | 0.407 (0.281) | |
| Incremental cost (£) | Incremental QALYs | ||
| Co-careldopa vs. placebo | 2217.93 | –0.022 | Co-careldopa dominated |
Table 67 shows the cost-effectiveness results when the wider cost perspective also encompassed caregiver costs. As before, co-careldopa was less effective (i.e. led to worse health outcomes) and more expensive than placebo. However, we should be cautious about any interpretations of the latter result given the small number of carers in the trial and questionable data quality.
| Treatment group | Costs (£), mean (SD) | QALYs,a mean (SD) | ICER (£/QALY) |
|---|---|---|---|
| Placebo | 57,740.67 (59,077.52) | 0.422 (0.026) | |
| Co-careldopa | 60,965.02 (66,242.36) | 0.398 (0.027) | |
| Incremental cost | Incremental QALYs | ||
| Co-careldopa vs. placebob | 3224.35 | –0.024 | Co-careldopa dominated |
Discussion
The primary CEAs indicated that co-careldopa was not cost-effective compared with placebo. Indeed, co-careldopa was more expensive, and less effective in terms of QALY gains, than placebo.
Both trial arms showed an increased mean EQ-5D score over 12 months, and the increase was significant at the 1% level. EQ-5D score differences between groups were significant at baseline and at 8 weeks. The former may be explained by the fact that the proportions of different stroke infarct types were not equally distributed across arms. For example, more patients in the co-careldopa group suffered a total anterior infarct. We adjusted for these baseline differences in the CEAs using simple linear regression techniques.
In the primary analysis, we used a health and social services perspective for costs. Costs from this perspective were higher in the co-careldopa group than in the placebo group because the former reported a higher use of resources such as GP visits, face-to-face contacts with the district nurse and hospital inpatient stays. That said, differences in costs were not significant between groups or over time. Higher costs were also incurred by the co-careldopa group when adopting a wider perspective (costs to the patients or caregivers) and after controlling for baseline differences between arms.
A limitation of this analysis was the high proportion of missing data. Even though missing EQ-5D scores were present in the data set, of greater concern was that about two-thirds of the patients in the trial had missing resource use data. This was primarily driven by missing items in the resource use questionnaire rather than by failure to attempt to complete the questionnaire. Some of the missing values can perhaps be explained by patient morbidity and the fact that the average age of participants in the trial was 71 years. To deal with the missing utility and cost data, we had to impute the values using the multiple imputation method. Although an accepted method, it is clearly suboptimal and less reliable the greater the proportion of data to be imputed. Analysis of complete-case data, although limited by the small sample, yielded the same conclusion as the primary analysis. Economic evaluations in trials of patients who may struggle to complete questionnaires as a result of failing recall, frailty or other morbidity should strive to capture health-care resource use data from central records such as Hospital Episode Statistics whenever possible. This would minimise missing data, allow greater confidence in results and reduce responder burden on patients.
Despite the missing data, we can be relatively confident in the QALY results, and these were not in favour of co-careldopa. However, there was some suggestion that the results may be influenced by the degree to which the protocol was followed in intervention delivery. The net benefit regression is also of interest, and a significant interaction was found between treatment arm and infarct type. The trial was not powered for such subgroup analysis, but further investigation may be warranted to determine whether this finding is spurious or highlights a real differential treatment effect by stroke type.
Conclusions
On the basis of the DARS trial, the conclusion is that co-careldopa is not cost-effective for improving rehabilitation after stroke. Indeed, the results indicate that it is more expensive and less effective than placebo alone. This was the conclusion after conducting several sensitivity and supplementary analyses. Although there were missing data for resource use and strict treatment non-compliance in many patients, this conclusion is robust given the lack of treatment effect of co-careldopa. Further analysis is required to explore the impact of trial arm imbalance, treatment compliance and missing data on the trial results.
Chapter 5 Discussion
Overview of results
The DARS trial found no benefit of combining dopaminergic therapy with occupational and physical motor therapy during early rehabilitation following acute stroke in improving walking ability or other motor function.
Just over 10% of patients were lost to follow-up at 8 weeks and < 10% of patients met the strict per-protocol analysis criteria. Despite this, the findings are robust and generalisable to patients with limited mobility in the first few weeks after stroke.
The proportion of patients in the placebo arm who achieved the primary outcome of independent walking at 8 weeks was greater than was anticipated from results of the Scheidtmann et al. 52 study, used to determine the DARS trial sample size (44% in DARS vs. 26%52). This is most likely attributable to the recruitment of participants at an earlier time point after stroke in the DARS trial than in the Scheidtmann et al. 52 study. Patients in the Scheidtmann et al. 52 study were randomised, on average, 43 days after stroke onset, compared with 18 days after stroke onset in the DARS trial. In any recovery study in stroke, the extent of potential recovery is greater the earlier patients are recruited after becoming medically stable after the acute management stage.
Analysis of the moderators of recovery in the DARS trial found that only baseline RMI was weakly associated with recovery of independent walking, which is an expected finding as patients with less impairment would be more likely to achieve independent walking.
A range of secondary outcomes in the DARS trial showed no suggestion of benefit on arm function, disability, activities of daily living and cognition. Cognition showed a recovery pattern seen in other studies, with significant early improvement and continuing improvement in cognitive functioning during the 12 months following stroke. Carers of patients in the co-careldopa group reported less burden at 6 months and 12 months. We consider that this may reflect an imbalance between the two carer groups prior to stroke, which we were unable to measure, rather than a treatment effect of co-careldopa on trial participants that was not apparent in the other outcome measures. Carers were identified opportunistically by research teams in discussion with patients after they had experienced a stroke at the time of trial consent. Carer input prior to, and after, stroke was not measured in the trial; therefore, change in carer burden could not be analysed in relation to care delivered. One possible contributory factor to carer burden may have been the better general health reported by patients in the co-careldopa group at 6 months. However, no difference in general health status was seen between groups at 8 weeks and 12 months, and the finding at 6 months is unlikely to be due to an effect of co-careldopa therapy and more likely to be due to the play of chance. The health economic analysis of the DARS trial demonstrated that dopaminergic therapy with co-careldopa is associated with increased health-care resource utilisation with lower QALY gain and is, therefore, clearly not cost-effective.
Strengths and weaknesses
In designing the DARS trial, levodopa in the form of co-careldopa was chosen from a number of drug therapies that were considered, including amphetamines and SSRIs. Levodopa was chosen because it has a good safety profile and is generally well tolerated by an older population with Parkinson’s disease. The previously published small trials also suggested an efficacy signal. 52–54,56–58,63 In contrast, other potential therapies, such as amphetamines, were known to have significant safety problems, such as hypertension and delirium, and, with other agents such as cholinesterase inhibitors, the pre-existing clinical trial database was much weaker.
The strengths of the trial are the double-blind, placebo-controlled design (and the results suggest that the blinding was maintained), the recruitment of a large of number of participants from multiple NHS stroke services and good adherence to study treatment and therapy sessions, with > 80% of participants receiving at least 20 minutes of motor therapy in > 80% of therapy sessions. However, < 10% of patients met the per-protocol analysis criteria, and this analysis was consequently severely underpowered. The main reason for lack of compliance with the per-protocol analysis was failure of participants to take the IMP 45–60 minutes prior to therapy. This time frame was chosen with the aim of achieving peak serum and brain levodopa concentrations at the time of therapy but may have been unnecessarily narrow. These criteria were, in retrospect, too strict for a multicentre trial involving multiple therapy teams, often in individual patients. We cannot absolutely exclude the possibility that, had high levels of strict compliance with optimal timing of therapy following drug administration been achieved, a benefit of co-careldopa may have been shown. It is also possible that the trial included too many patients who were going to make a good recovery; although, because fewer than half of participants in the placebo group achieved the primary mobility end point and there was no suggestion of benefit in patients with more impairment at trial baseline, this seems unlikely. Future trials of combined timed drug and therapy might consider using less strict criteria for per-protocol analyses.
Participant characteristics appear reasonably representative of a typical inpatient stroke population able to engage with routine stroke therapy who were independently mobile prior to stroke and able to consent to participate in research. Patients who had reduced mobility prior to their stroke or significant cognitive impairment were not included in the DARS trial. It is unlikely that this group would have shown a different therapeutic response, but they may have been more vulnerable to AEs of co-careldopa.
A weakness of the trial is the loss to follow-up at 8 weeks of just over 10% of participants. Excluding deaths, 8.1% of placebo participants and 10.3% of co-careldopa participants had no outcome at 8 weeks, with participant withdrawal from the trial being the main cause. The potential for incomplete follow-up in all randomised patients to bias the results of RCTs is well recognised, and patients with less good outcomes may be more likely to withdraw from follow-up. However, the primary analysis made the assumption that those participants with missing outcome data did not achieve a positive primary outcome (i.e. they were assumed to be unable to walk independently), and sensitivity analyses testing the robustness of this assumption did not contradict the primary ITT conclusion. It is possible that the differential loss to follow-up between groups may have led to bias, and this would probably have been in favour of placebo if participants who were lost to follow-up were less well. Considerable efforts were made by the trial management team and sites to reduce loss to follow-up, but some patients found the follow-up requirements of the trial demanding. This emphasises the importance of designing recovery trials with robust mechanisms to capture follow-up outcomes that minimise demands on patients and their carers.
Generalisability
Overall, despite these limitations, the main finding of the DARS trial of no benefit on independent walking at 8 weeks appears robust and generalisable to the typical stroke population in the UK and other countries with developed health-care systems.
Safety
Treatment with co-careldopa after stroke had an acceptable safety profile. Twelve-month mortality was, as expected, low for a stroke population recruited after acute phase and was not significantly increased with co-careldopa treatment. More patients in the co-careldopa group died within the first 8 weeks of the trial than in the placebo group when the study IMP was administered (n = 6, co-careldopa group; n = 1 placebo group). The cause of death in these patients was reveiwed and was not considered to be due to co-careldopa treatment. The rate of reported SAEs was not increased in the co-careldopa group and SAEs were not typical AEs of co-careldopa but mostly considered to be related to the underlying stroke or complications. Vomiting during therapy sessions after study drug administration was uncommon but more frequent in the co-careldopa group than in the placebo group (0.5% vs. 0.1%, respectively) and may have been drug related.
Intensity of therapy
The findings of the mediator analysis suggested that both the amount of therapy and the number of IMP doses, placebo or co-careldopa, received were associated with walking independently at 8 weeks. As IMP administration and therapy were highly correlated through trial design, this observation suggests that the amount of therapy received may be an important determinant of recovery. However, interpretation of these associations when the amount of therapy was not subject to randomisation is complex, as intercurrent illness, mood problems or clinical deterioration may prevent patients participating in therapy and impaired recovery may be related to these factors rather than the amount of received therapy alone. Further research is required to identify optimal therapy input in the acute and recovery stages of stroke. The lack of current knowledge on optimal type and intensity of therapy in different populations of stroke patients presents challenges in the design of combined drug therapy trials.
Stroke subtype
No difference was seen in the response of patients’ subcortical and cortical stroke to co-careldopa therapy. However, categorisation of patients had to be undertaken on the basis of imaging obtained in routine clinical care, which was obtained at different times after stroke onset, and was generally with CT brain imaging rather than MRI. There are theoretical reasons why patients whose motor deficit is due to basal ganglia lesions might respond differently to dopaminergic therapy to those with cortical strokes. Initially, the researchers had intended to undertake functional MRI (fMRI) studies to examine changes in functional brain responses during motor therapy in a subset of patients at sites where this imaging was available. However, this proved impractical to establish within a multicentre trial. Given the overall lack of response seen in a heterogeneous stroke population to dopaminergic therapy, it would be appropriate to undertake studies that examine in well-defined subgroups of stroke patients whether or not dopaminergic or other drug therapies can modify motor cortex activation following stroke, before undertaking clinical studies utilising impairment and disability measures.
Intermittent versus sustained dosing
Another possible explanation for the lack of response to co-careldopa is the use of intermittent dosing of the IMP rather than sustained daily dosing, as was used in some previous trials including the Scheidtmann et al. trial52 of 3 weeks’ levodopa therapy. An intermittent dosing strategy was chosen with the intention of maximising brain dopamine concentrations during therapy by administering the IMP 45–60 minutes prior to therapy so that peak brain concentrations of dopamine would be achieved during therapy. This dosing strategy was developed from the timing of clinical experience of response to dopaminergic therapy in Parkinson’s disease. A strategy of daily dosing for 6 weeks may have more consistently achieved elevated brain dopaminergic activity at the time of therapy, but would probably have resulted in an increase in AEs and patient withdrawal, and, unless all therapy sessions had been administered in the first 2–3 hours after daily administration of the IMP, would have resulted in therapy sessions being undertaken with relatively low levels of dopaminergic activity. It is possible that higher doses of co-careldopa might have been beneficial. However, this would probably have produced more AEs and been less well tolerated. The dose used in the trial produces chemical benefits in Parkinson’s disease. Future Phase II trials of recovery-enhancing drugs might usefully compare intermittent versus daily dosing in terms of tolerability and clinical and/or biomarker response of recovery.
Intensity of therapy received
A possible explanation for the lack of response to co-careldopa seen in the DARS trial is that the intensity of therapy delivered in the trial was insufficient. The therapy delivered to patients was at the discretion of the hospital and early supported discharge therapy teams. The average number of therapy sessions delivered to each patient was 23, with an average duration of 40 minutes of motor activities. This amount of therapy matches that recommended in guidance produced for stroke rehabilitation by the Royal College of Physicians. 125 The study authors intended that patients would receive at least 16 hours of therapy, and the study was based on the results of the 2004 meta-analysis of the effects of therapy time on recovery. 126 This level of therapy was achieved and, although it is possible that more intensive therapy might have been needed to see benefit from dopaminergic therapy, this would prove challenging to NHS services to provide, and many patients might be unable to tolerate higher levels of therapy. Further interpretation is limited within such a large multicentre trial conducted across many sites, as it was infeasible to collect further detailed information on the content or delivery of therapy.
The DARS trial and other trials
The findings of the DARS trial appear to be consistent with those of smaller studies of dopaminergic therapy. A formal meta-analysis of trials of dopaminergic therapy in stroke recovery has not been undertaken and, given the wide range of different outcome measures in populations of stroke patients recruited at different times after stroke, might not be justified. Of the seven reported randomised trials, three showed no benefit on motor function (n = 33,63 n = 1058 and n = 25 participants53), two showed improvements in walking speed (n = 10 participants56) or procedural motor learning (n = 18 participants57) and one, slightly larger, study showed a slight improvement in disability (n = 100 participants54). The one trial that showed a clinically significant effect of levodopa therapy was the Scheidtmann et al. 52 study n = 53 participants), which found a significant difference in RMI score gain of 2.3 points after 3 weeks in those on 100 mg of levodopa compared with those receiving placebo therapy.
Practical issues
The DARS trial was a complex stroke trial and is the largest multicentre stroke rehabilitation trial to combine timed administration of a blinded IMP with therapy sessions. The DARS trial was designed to optimise efficacy of dopaminergic therapy and minimise AEs by using a strategy of administering oral levodopa prior to motor therapy. This approach required a high degree of co-ordination of drug administration with planned therapy and differs from that used in most clinical trials. This novel approach of co-ordinating drug administration with motor therapy in a multicentre trial was successfully delivered with support from NIHR Stroke Research Network research teams. Recruitment to trial target was achieved and would not have been possible without the infrastructure and support provided by the network with site co-ordinators and the eight local stroke research network leads. Strict per-protocol adherence was achieved in 55% of therapy sessions and the IMP was administered before therapy in 85% of therapy sessions. Transfer of patients from hospital to community rehabilitation settings was a further challenge. The DARS trial has demonstrated that it is feasible to deliver multicentre trials of pharmacotherapy-enhanced rehabilitation in NHS stroke services. This learning and experience is of value in informing the design and conduct of future rehabilitation multicentre trials investigating the effect of drugs to enhance recovery.
Future trials
The DARS trial demonstrates no additional benefit of dopaminergic therapy to routine motor PT and OT in improving walking and motor function after stroke. Valuable experience has been gained in the design and conduct of combined studies of drug therapy and motor therapy that are complex and challenging in terms of deciding the timing of the start of the intervention, timing of drug/cellular therapy with motor therapy and intensity of motor therapy, which is highly relevant to the design of future trials of combined studies of drug/cellular therapy and motor therapy to improve outcomes from stroke.
Chapter 6 Conclusion
Implications for health care
The results of the DARS trial indicate that there is no role for the use of dopaminergic therapy in conjunction with PT and OT in improving walking after stroke.
Implications for research
The results of the DARS trial suggest that further clinical trials of levodopa to improve motor function in the first few weeks after stroke should not be undertaken.
Future clinical trials of other pharmacotherapies that act on motor learning should consider comparing strategies of continuous dosing and intermittent dosing prior to motor therapy and different doses of drug therapy.
The DARS trial has shown that multicentre trials of pharmacotherapy and standardised rehabilitation are feasible. Future trials should collect data on time and content of rehabilitation.
Clinical trials of pharmacotherapy to improve stroke recovery may need to consider using a greater intensity of therapy than was used in the DARS trial.
Future clinical rehabilitation trials should arrange follow-up assessments that minimise the burden on trial participants and carers and loss to follow-up at primary outcome assessment.
Future research should consider incorporation of emerging imaging markers, such as fMRI, as proof-of-concept biomarkers into early-phase trials of pharmacotherapy to improve recovery from stroke.
Future research is needed into the development of more sensitive clinical markers of motor recovery that would demonstrate proof-of-concept efficacy on neurological impairment in early-phase trials before undertaking large pragmatic trials using disability measures as the primary trial outcome.
Acknowledgements
Trial Steering Committee
Professor Helen Rodgers (Independent Chairperson), Professor Peter Langhorne, Dr Gerry Richardson, Mr Michael Bonner (Patient Representative) and Dr Christopher Weir.
Data Monitoring and Ethics Committee
Dr John Bamford (Independent Chairperson), Dr Steff Lewis and Dr Pippa Tyrell.
Leeds Institute of Clinical Trials Research
Miss Lorna Barnard, Mr Andrew Carter, Mr Colin Everett, Miss Alison Fergusson, Mr Simon Jones and Mrs Amanda Lilley-Kelly.
Academic Department of Rehabilitation Medicine Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds
Mrs Karen Lawson.
Academic Unit of Health Economics, Leeds Institute of Health Sciences, University of Leeds
Professor Claire Hulme and Miss Zenia Ferreira.
Independent imaging review
Dr Jeremy Macmullen-Price and Dr Tufail Patankar.
Patient and public involvement
Mr Ossie Newell, Mr Michael Bonner and the CRAG.
Participating sites
With thanks to all research staff at the participating centres who provided and cared for trial participants and collected trial data:
Aintree University Hospital NHS Foundation Trust (Dr Claire Cullen), Northern Health & Social Care Trust (Dr Jamil Vhidassr), Heart of England NHS Foundation Trust (Dr David Sandler), Blackpool Teaching Hospitals NHS Foundation Trust (Dr James McIlmoyle), Bradford Teaching Hospitals NHS Foundation Trust (Dr Chris Patterson), University Health Board, NHS Wales (Dr Phil Jones), Chelsea & Westminster Hospital NHS Foundation Trust (Dr Michael Pelly), Croydon Health Services NHS Trust (Dr Enas Lawrence), East Sussex Healthcare NHS Trust (Dr Mudali Conrad), Pennine Acute Hospitals NHS Trust (Dr Narayanamoorti Saravanan), NHS Grampian (Dr Alastair Cozens), NHS Greater Glasgow & Clyde (Dr Christine McAlpine), Gloucestershire Hospitals NHS Foundation Trust (Dr Katharina Nehrig), Harrogate and District NHS Foundation Trust (Dr Sean Brotheridge), Staffordshire and Stoke-on-Trent Partnership NHS Trust (Professor Tony Ward), Leeds Teaching Hospitals NHS Trust (Dr Peter Wanklyn), Mid Cheshire Hospitals NHS Foundation Trust (Dr Maqsud Salehin), Mid Yorkshire NHS Trust (Dr Prabal K Datta), The Royal Wolverhampton NHS Trust (Dr Kenneth Fotherby), NHS Tayside (Dr Ronald S MacWalter), University Hospital of North Staffordshire NHS Trust (Professor Christine Roffe), Northampton General Hospital NHS Trust (Dr Melanie Blake), Portsmouth Hospitals NHS Trust (Dr Ugnius Sukys), Burton Hospitals NHS Foundation Trust (Dr Partha Das), Rotherham Doncaster & South Humber NHS Foundation Trust (Dr James Okwera), East Lancashire Hospitals NHS Trust (Dr Mahiswar Goorah), Royal Liverpool & Broadgreen University Hospitals NHS Trust (Dr Shnakar Loharuka), Barts Health NHS Trust (Dr Rob Simister), Belfast Health & Social Care Trust (Dr Ken Fullerton), The Newcastle upon Tyne Hospitals NHS Foundation Trust (Dr Anand Dixit), North Lincolnshire & Goole NHS Foundation Trust (Dr Amit Banerjee), Heart of England NHS Foundation Trust (Dr Uzma Khan), University Hospital Southampton NHS Foundation Trust (Dr Nic Weir), St George’s Healthcare NHS Trust (Dr Geoffrey Cloud), Epsom & St Helier NHS Trust (Dr Paul Omahony), Guy’s & St Thomas’ NHS Foundation Trust (Dr Ajah Bhalla), South Eastern Health & Social Care Trust (Dr Kevin Dynan), Walsall Healthcare NHS Trust (Dr Elliot Epstein), Weston Area Health NHS Trust (Dr Michael Haley), St Helens & Knowesley Hospitals NHS Trust (Dr Tom Smith), York Teaching Hospital NHS Foundation Trust (Dr Paul Willcoxson), NHS Fife (Dr Vera Cvoro), Northumbria NHS Trust (Dr Chris Price), Southern Health and Social Care Trust (Dr Patricia McCaffrey), County Durham and Darlington NHS Trust (Dr Ali Mehrzad) and Imperial Trust, London (Dr Paul Bentley).
Participants
Thank you to all trial participants for their essential contribution to the trial.
Contributions of authors
Gary A Ford (Consultant Stroke Physician and Visiting Professor of Clinical Pharmacology) designed the DARS trial and took over overall responsibility for the trial when Bipin B Bhakta retired in January 2013.
Bipin B Bhakta (Emeritus Professor, former Charterhouse Professor of Rehabilitation Medicine, Leeds Institute of Rheumatic and Musculoskeletal Medicine) conceived and designed the DARS trial, and had overall responsibility until retirement in January 2013.
Alastair Cozens (Consultant in Rehabilitation Medicine) designed the DARS trial.
Bonnie Cundill (Principal Statistician) provided statistical input into the trial design, implementation and statistical analysis plan, under the supervision of Amanda J Farrin.
Suzanne Hartley (Head of Trial Management) was delivery lead for the implementation of the trial, acquisition of trial data, trial monitoring, Good Clinical Practice and regulatory and reporting requirements.
Ivana Holloway (Senior Medical Statistician) provided statistical input into the trial design, implementation and statistical analysis plan, under the supervision of Amanda J Farrin.
David Meads (Lecturer in Health Economics) had oversight of the economic evaluation.
John Pearn (Clinical Research Fellow in Rehabilitation Medicine, Academic Department of Rehabilitation Medicine, Leeds Institute of Rheumatic and Musculoskeletal Medicine; Registrar and Clinical Research Fellow in Rehabilitation Medicine) was the clinical research fellow and contributed to the trial implementation, data acquisition and led on the radiological review.
Sharon Ruddock (Senior Trial Co-ordinator) contributed to the protocol development and implementation and co-ordination of the data acquisition and trial reporting.
Catherine M Sackley (Professor of Rehabilitation) designed the DARS trial.
Eirini-Christina Saloniki (Research Fellow in Health Economics) refined the methods and undertook the analysis.
Gillian Santorelli (Medical Statistician) provided statistical input into the trial design, implementation and statistical analysis plan, under the supervision of Amanda J Farrin.
Marion F Walker (Professor of Stroke Rehabilitation) designed the DARS trial.
Amanda J Farrin (Professor of Clinical Trials & Evaluation of Complex Interventions and Director of Complex Interventions Division) designed the DARS trial.
With the exception of Bipin B Bhakta, all authors contributed to the writing of the report and had the opportunity to revise prior to submission.
Publications
Hartley S, Ruddock S, Bhakta BB, Pearn J, Barnard L, Fergusson A, et al. Maximising adherence to study protocol within pharmaco-rehabilitation clinical trials. Trials 2011;12(Suppl. 1):A133.
Ruddock S, Bhakta BB, Lilley-Kelly A, Hartley S, Barnard L. Rapid set up of research centres in a trial in an evolving research governance world. Trials 2013;14(Suppl. 1):132.
Bhakta BB, Hartley S, Holloway I, Cozens A, Ford GA, Meads D, et al. The DARS (Dopamine Augmented Rehabilitation in Stroke) trial: protocol for a randomised controlled trial of co-careldopa treatment in addition to routine NHS occupational and physical therapy after stroke. Trials 2014;15:316.
Holloway I, Farrin AJ, Hartley S, Lilley-Kelly A, Bhakta BB, Ford GA. From RAGs (Red-Amber-Green) to Riches: Site Achievement Report – An Efficient Way to Provide Feeedback to Improve Sites’ Performance. Oral presentation at the Society of Clinical Trial Conference, Philadelphia, PA; 18–21 May 2014.
Ford GA, Farrin A, Hartley S, Cozens A, Holloway I, Meads D, et al. DARS (Dopamine Augmented Rehabilitation in Stroke): results of a randomized controlled trial of co-careldopa treatment in addition to routine occupational and physical therapy after stroke. Int J Stroke 2015;10(Suppl. 2):12.
Ford G, Bhakta BB, Cozens A, Hartley S, Holloway I, Meads D, et al. DARS (Dopamine Augmented Rehabilitation in Stroke). Longer-term results for a randomised controlled trial of co-careldopa in addition to routine occupational and physical therapy after stroke. Int J Stroke 2015;10(Suppl. 5):6–7.
Ford GA, Bhakta BB, Cozens A, Hartley S, Holloway I, Meads D, et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2019;18:530–38.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006;37:345-50. https://doi.org/10.1161/01.STR.0000199613.38911.b2.
- Reducing Brain Damage: Faster Access to Better Stroke Care. London: DHSC; 2005.
- Progress in Improving Stroke Care. London: DHSC; 2010.
- Pollock A, Baer G, Langhorne P, Pomeroy V. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke: a systematic review. Clin Rehabil 2007;21:395-410. https://doi.org/10.1177/0269215507073438.
- Legg LA, Drummond AE, Langhorne P. Occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database Syst Rev 2006;4. https://doi.org/10.1002/14651858.CD003585.pub2.
- Stroke Quality Standards. London: NICE; 2009.
- National Stroke Strategy. London: DHSC; 2007.
- Ward AB, Gutenbrunner C, Chamberlain MA. White book on physical and rehabilitation medicine in Europe – foreword. J Rehabil Med 2007;39.
- International Classification of Functioning, Disability and Health. Geneva: WHO; 2001.
- World Report on Disability. Geneva: WHO; 2011.
- Geyh S, Cieza A, Schouten J, Dickson H, Frommelt P, Omar Z, et al. ICF Core Sets for stroke. J Rehabil Med 2004;36:135-41. https://doi.org/10.1080/16501960410016776.
- Dahl TH. International classification of functioning, disability and health: an introduction and discussion of its potential impact on rehabilitation services and research. J Rehabil Med 2002;34:201-4. https://doi.org/10.1080/165019702760279170.
- Medical Rehabilitation in 2011 and Beyond. London: RCP; 2010.
- Stroke Rehabilitation: Long-term Rehabilitation after Stroke. London: NICE; 2013.
- NICE Quality Standard [QS2]: Stroke and Transient Ischaemic Attack. London: NICE; 2010.
- Carr J, Sheperd R. A Motor Relearning Programme for Stroke. London: Heinemann Medical; 1982.
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Data Syst Rev 2014;4.
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009;8:741-54. https://doi.org/10.1016/S1474-4422(09)70150-4.
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 2011;72:443-54. https://doi.org/10.1016/j.neuron.2011.10.008.
- Penhune VB, Steele CJ. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res 2012;226:579-91. https://doi.org/10.1016/j.bbr.2011.09.044.
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 2009;199:61-75. https://doi.org/10.1016/j.bbr.2008.11.012.
- Nudo R, Byrne JH. Learning and Memory: A Comprehensive Reference. Cambridge MA: Academic Press; 2008.
- Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Arch Phys Med Rehabil 2006;87:S36-42. https://doi.org/10.1016/j.apmr.2006.09.005.
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 2011;7:76-85. https://doi.org/10.1038/nrneurol.2010.200.
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol 2004;61:1844-8. https://doi.org/10.1001/archneur.61.12.1844.
- Gillick BT, Zirpel L. Neuroplasticity: an appreciation from synapse to system. Arch Phys Med Rehabil 2012;93:1846-55. https://doi.org/10.1016/j.apmr.2012.04.026.
- Thomas C, Baker CI. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage 2013;73:225-36. https://doi.org/10.1016/j.neuroimage.2012.03.069.
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol 2002;12:217-22. https://doi.org/10.1016/S0959-4388(02)00307-0.
- Da Cunha C, Wietzikoski EC, Dombrowski P, Bortolanza M, Santos LM, Boschen SL, et al. Learning processing in the basal ganglia: a mosaic of broken mirrors. Behav Brain Res 2009;199:157-70. https://doi.org/10.1016/j.bbr.2008.10.001.
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat 2000;196:527-42. https://doi.org/10.1046/j.1469-7580.2000.19640527.x.
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008;44:1037-66. https://doi.org/10.1016/j.cortex.2008.04.004.
- Balleine BW, Lijeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res 2009;199:43-52. https://doi.org/10.1016/j.bbr.2008.10.034.
- Shiflett MW, Balleine BW. Prog Neurobiol 2011;95:1-13. https://doi.org/10.1016/j.pneurobio.2011.05.007.
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature 2011;469:53-7. https://doi.org/10.1038/nature09588.
- Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol 2008;18:595-604. https://doi.org/10.1016/j.conb.2008.11.001.
- Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J Neurosci 2006;26:3567-83. https://doi.org/10.1523/JNEUROSCI.5050-05.2006.
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004;5:483-94. https://doi.org/10.1038/nrn1406.
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010;68:815-34. https://doi.org/10.1016/j.neuron.2010.11.022.
- Berends HI, Nijlant JM, Movig KL, Van Putten MJ, Jannink MJ, Ijzerman MJ. The clinical use of drugs influencing neurotransmitters in the brain to promote motor recovery after stroke; a systematic review. Eur J Phys Rehabil Med 2009;45:621-30.
- Surmeier DJ, Carrillo-Reid L, Bargas J. Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience 2011;198:3-18. https://doi.org/10.1016/j.neuroscience.2011.08.051.
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 1982;217:855-7. https://doi.org/10.1126/science.7100929.
- Hovda DA, Fenney DM. Amphetamine with experience promotes recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res 1984;298:358-61. https://doi.org/10.1016/0006-8993(84)91437-9.
- Goldstein LB. Stroke 2009;40:133-5. https://doi.org/10.1161/STROKEAHA.108.533703.
- Ramic M, Emerick AJ, Bollnow MR, O’Brien TE, Tsai S-Y, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res 2006;1111:176-86. https://doi.org/10.1016/j.brainres.2006.06.063.
- Adkins DL, Jones TA. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci Lett 2005;380:214-18. https://doi.org/10.1016/j.neulet.2005.01.036.
- Gilmour G, Iversen SD, O’Neill MF, O’Neill MJ, Ward MA, Bannerman DM. Amphetamine promotes task-dependent recovery following focal cortical ischaemic lesions in the rat. Behav Brain Res 2005;165:98-109. https://doi.org/10.1016/j.bbr.2005.06.027.
- Barbay S, Zoubina EV, Dancause N, Frost SB, Eisner-Janowicz I, Stowe AM, et al. A single injection of D-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair 2006;20:455-8. https://doi.org/10.1177/1545968306290773.
- Auriat AM, Colbourne F. Influence of amphetamine on recovery after intracerebral hemorrhage in rats. Behav Brain Res 2008;186:222-9. https://doi.org/10.1016/j.bbr.2007.08.010.
- Martinsson L, Hardemark H, Eksborg S. Amphetamines for improving recovery after stroke. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD002090.pub2.
- Breitenstein C, Flöel A, Korsukewitz C, Wailke S, Bushuven S, Knecht S. A shift of paradigm: from noradrenergic to dopaminergic modulation of learning?. J Neurol Sci 2006;248:42-7. https://doi.org/10.1016/j.jns.2006.05.012.
- Nutt JG, Fellman JH. Pharmacokinetics of levodopa. Clin Neuropharmacol 1984;7:35-49. https://doi.org/10.1097/00002826-198403000-00002.
- Scheidtmann K, Fries W, Müller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001;358:787-90. https://doi.org/10.1016/S0140-6736(01)05966-9.
- Sonde L, Lökk J. Effects of amphetamine and/or L-DOPA and physiotherapy after stroke – a blinded randomized study. Acta Neurol Scand 2007;115:55-9. https://doi.org/10.1111/j.1600-0404.2006.00728.x.
- Lokk J, Salman Roghani R, Delbari A. Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke – a randomized, double-blind, placebo-controlled trial. Acta Neurol Scand 2011;123:266-73. https://doi.org/10.1111/j.1600-0404.2010.01395.x.
- Engelter ST, Frank M, Lyrer PA, Conzelmann M. Safety of pharmacological augmentation of stroke rehabilitation. Eur Neurol 2010;64:325-30. https://doi.org/10.1159/000322134.
- Acler M, Fiaschi A, Manganotti P. Long-term levodopa admnistration in chronic stroke patients. A clinical and neurophysiologic single-blind placebo-controlled cross-over pilot study. Restor Neurol Neurosci 2009;27:277-83.
- Rösser N, Heuschmann P, Wersching H, Breitenstein C, Knecht S, Flöel A. Levodopa improves procedural motor learning in chronic stroke patients. Arch Phys Med Rehabil 2008;89:1633-41. https://doi.org/10.1016/j.apmr.2008.02.030.
- Restemeyer C, Weiller C, Liepert J. No effect of a levodopa single dose on motor performance and motor excitability in chronic stroke. A double-blind placebo-controlled cross-over pilot study. Restor Neurol Neurosci 2007;25:143-50.
- Floel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology 2005;65:472-4. https://doi.org/10.1212/01.wnl.0000172340.56307.5e.
- Ge L-T, Lin L, Zhang J. Effect of levodopa and benserazide hydrochloride, mecobalamin in combination with acupuncture and functional training on motor dysfunction after acute cerebral infarction: a randomized, double-blind controlled study. Chin J Clin Rehabil 2005;9:128-30.
- Zorowitz RD, Smout RJ, Gassaway JA, Horn SD. Neurostimulant medication usage during stroke rehabilitation: the Post-Stroke Rehabilitation Outcomes Project (PSROP). Top Stroke Rehabil 2005;12:28-36. https://doi.org/10.1310/2403-B0CY-1UDN-4B6D.
- Celik C, Uzun M, Karaoglan B. The effect of levodopa in combination with rehabilitation programme on functional motor recovery in stroke patients. Turk Fiz Tip Rehab D 2004;50:18-20.
- Cramer SC, Dobkin BH, Noser EA, Rodriguez RW, Enney LA. Randomized, placebo-controlled, double-blind study of ropinirole in chronic stroke. Stroke 2009;40:3034-8. https://doi.org/10.1161/STROKEAHA.109.552075.
- Bhakta BB, Hartley S, Holloway I, Couzens JA, Ford GA, Meads D, et al. The DARS (Dopamine Augmented Rehabilitation in Stroke) trial: protocol for a randomised controlled trial of co-careldopa treatment in addition to routine NHS occupational and physical therapy after stroke. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-316.
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud 1991;13:50-4. https://doi.org/10.3109/03790799109166684.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. https://doi.org/10.1161/01.STR.19.5.604.
- Nouri F, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. https://doi.org/10.1177/026921558700100409.
- Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke 2001;32:1627-34. https://doi.org/10.1161/01.STR.32.7.1627.
- Golderberg D, Williams P. User’s Guide to the General Health Questionnaire. Windsor, UK: NFER-Nelson; 1988.
- Elmståhl S, Malmberg B, Annerstedt L. Caregiver’s burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Arch Phys Med Rehabil 1996;77:177-82. https://doi.org/10.1016/S0003-9993(96)90164-1.
- Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res 2003;54:345-52. https://doi.org/10.1016/S0022-3999(02)00392-6.
- Hartley S, Ruddock S, Bhakta BB, Pearn J, Barnard L, Fergusson A, et al. Maximising adherence to study protocol within pharmaco-rehabilitation clinical trials. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-S1-A133.
- Chakravarty K, Durkin CJ, al-Hillawi AH, Bodley R, Webley M. The incidence of acute arthritis in stroke patients, and its impact on rehabilitation. Q J Med 1993;86:819-23.
- Pendlebury ST, Cuthbertson FC, Welch SJV, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by mini-mental state examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke 2010;41:1290-3. https://doi.org/10.1161/STROKEAHA.110.579888.
- Kobari M, Fukuuchi Y, Shinohara T, Obara K, Nogawa S. Levodopa-induced local cerebral blood flow changes in Parkinson’s disease and related disorders. J Neurol Sci 1995;128:212-18. https://doi.org/10.1016/0022-510X(94)00237-I.
- Salgado-Pineda P, Delaveau P, Falcon C, Blin O. Brain T1 intensity changes after levodopa administration in healthy subjects: a voxel-based morphometry study. Br J Clin Pharmacol 2006;62:546-51. https://doi.org/10.1111/j.1365-2125.2006.02695.x.
- Keenan AM, Tennant A, Fear J, Emery P, Conaghan PG. Impact of multiple joint problems on daily living tasks in people in the community over age fifty-five. Arthritis Rheum 2006;55:757-64. https://doi.org/10.1002/art.22239.
- Krabbe P, Weijnen T, Brooks R, Rabin R, Charro F. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht: Springer Netherlands; 2003.
- Holloway I, Farrin A, Hartley S, Lilley-Kelly A, Bhakta BB, Ford GA. From RAGs (Red-Amber-Green) to Riches: Site Achievement Report – An Efficient Way to Provide Feedback to Improve Sites’ Performance. Philadelphia, PA: Society of Clinical Trials Conference; n.d.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials 2004;25:143-56. https://doi.org/10.1016/j.cct.2003.10.016.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. https://doi.org/10.1037/0022-3514.51.6.1173.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2010;19:237-70. https://doi.org/10.1177/0962280209105014.
- Medicine NEMADCaIi . National Electrical Manufacturers Association: Digital Communications and Imaging in Medicine (DICOM). PS 3.1 2011. http://dicom.nema.org/ (accessed 22 November 2016).
- Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0015757.
- Wardlaw JM, Sellar R. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. AJNR Am J Neuroradiol 1994;15:1933-9.
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, Vonkummer R, et al. Intravenous thrombolysis with recombinant tissue-plasminogen activator for acute hemispheric stroke – The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017-25. https://doi.org/10.1001/jama.1995.03530130023023.
- Barber PA, Demchuk AM, Zhang JJ, Buchan AM. ASPECTS Study Group . Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 2000;355:1670-4. https://doi.org/10.1016/S0140-6736(00)02237-6.
- Wardlaw J. Acute Ischaemic Stroke Classification Template. Edinburgh: Brain Research Imaging Centre, University of Edinburgh; 2009.
- Wardlaw JM, Farrall AJ, Perry D, von Kummer R, Mielke O, Moulin T, et al. Factors influencing the detection of early CT signs of cerebral ischemia – an internet-based, international multiobserver study. Stroke 2007;38:1250-6. https://doi.org/10.1161/01.STR.0000259715.53166.25.
- Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352-63. https://doi.org/10.1016/S0140-6736(12)60768-5.
- Wardlaw J, Carpenter T, Sakka E, Mair G, Cohen G, Shuler K, et al. Imaging perfusion deficits, arterial patency and thrombolysis safety and efficacy in acute ischaemic stroke. An observational study of the effect of advanced imaging methods in the Third International Stroke Trial (IST-3), a randomised controlled trial. Efficacy Mech Eval 2014;1.
- Bhattathiri PS, Gregson B, Prasad KSM, Mendelow AD, Hoff JT, Keep RF, et al. Brain Edema XIII. 2006.
- Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke 1983;14:493-500. https://doi.org/10.1161/01.STR.14.4.493.
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304-5. https://doi.org/10.1161/01.STR.27.8.1304.
- van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. J Neurol Neurosurg Psychiatr 1990;53:1080-3. https://doi.org/10.1136/jnnp.53.12.1080.
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987;149:351-6. https://doi.org/10.2214/ajr.149.2.351.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. https://doi.org/10.1016/0140-6736(91)93206-O.
- Management of incidental findings detected during research imaging. London: RCR; 2011.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Logan PA, Armstrong S, Avery TJ, Barer D, Barton GR, Darby J, et al. Rehabilitation aimed at improving outdoor mobility for people after stroke: a multicentre randomised controlled study (the Getting out of the House Study). Health Technol Assess 2014;18. https://doi.org/10.3310/hta18290.
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17460.
- Brooks R. EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Hunger M, Sabariego C, Stollenwerk B, Cieza A, Leidl R. Validity, reliability and responsiveness of the EQ-5D in German stroke patients undergoing rehabilitation. Qual Life Res 2012;21:1205-16. https://doi.org/10.1007/s11136-011-0024-3.
- Palesch YY, Yeatts SD, Tomsick TA, Foster LD, Demchuk AM, Khatri P, et al. Twelve-month clinical and quality-of-life outcomes in the Interventional Management of Stroke III Trial. Stroke 2015;46:1321-7. https://doi.org/10.1161/STROKEAHA.115.009180.
- Sackley CM, Walker MF, Burton CR, Watkins CL, Mant J, Roalfe AK, et al. An Occupational Therapy intervention for residents with stroke related disabilities in UK Care Homes (OTCH): cluster randomised controlled trial. BMJ 2015;350. https://doi.org/10.1136/bmj.h468.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- NHS Reference Costs 2012–2013. London: DHSC; 2012.
- NHS Reference Costs 2013–2014. London: DHSC; 2014.
- Drugs and Pharmaceutical Electronic Market Information (eMit). London: DHSC; 2011.
- Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre) . CCEMG – EPPI-Centre Cost Convertor n.d. https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 12 June 2015).
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: The ISPOR RCT-CEA task force report. Value Health 2005;8:521-33. https://doi.org/10.1111/j.1524-4733.2005.00045.x.
- Ford GA, Bhakta BB, Cozens A, Hartley S, Holloway I, Meads D, et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2019;18:530-38. https://doi.org/10.1016/S1474-4422(19)30147-4.
- Gompertz P, Pound P, Ebrahim S. A postal version of the Barthel Index. Clin Rehabil 1994;8:233-9. https://doi.org/10.1177/026921559400800308.
- Sulter G, Steen C, De Keyser J. Use of the Barthel Index and Modified Rankin scale in acute stroke trials. Stroke 1999;30:1538-41. https://doi.org/10.1161/01.STR.30.8.1538.
- Goldberg D, Williams P. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Kim SK, Kim SH, Jo MW, Lee SI. Estimation of minimally important differences in the EQ-5D and SF-6D indices and their utility in stroke. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0227-3.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Royal College of Physicians Guidance n.d. https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines (accessed May 2018).
- Kwakkel G, van Peppen R, Wagenaar RC, Dauphinee SW, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke – a meta-analysis. Stroke 2004;35:2529-36. https://doi.org/10.1161/01.STR.0000143153.76460.7d.
Appendix 1 Search strategy
Search strategy for Ovid MEDLINE (1946 to 25 September 2015)
| 1 | exp Stroke/ | 28 | exp Dihydroxyphenylalanine/ | 55 | recover$.mp. |
| 2 | exp Stroke, Lacunar/ | 29 | $dopa$.mp. | 56 | (motor adj3 function$).mp. |
| 3 | exp Cerebrovascular Disorders/ | 30 | sinemet.mp. | 57 | (motor adj3 perform$).mp. |
| 4 | exp Cerebral Infarction/ | 31 | $careldopa.mp. | 58 | (motor adj3 skill$).mp. |
| 5 | exp Brain Infarction/ | 32 | madopar.mp. | 59 | (upper adj3 function$).mp. |
| 6 | exp Cerebrovascular Circulation/ | 33 | 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 60 | (arm adj3 function$).mp. |
| 7 | exp Arterial Occlusive Diseases/ | 34 | exp Rehabilitation/ | 61 | (upper adj3 move$).mp. |
| 8 | exp Cerebral Hemorrhage/ | 35 | exp “Recovery of Function”/ | 62 | (hand adj3 function$).mp. |
| 9 | exp Brain Ischemia/ | 36 | exp Physical Therapy Modalities/ | 63 | (hand adj3 move$).mp. |
| 10 | exp Hemiplegia/ | 37 | exp Walking/ | 64 | (hand adj3 dexter$).mp. |
| 11 | exp Paresis/ | 38 | exp Mobility Limitation/ | 65 | (upper adj3 limb$).mp. |
| 12 | stroke.mp. | 39 | exp “Activities of Daily Living”/ | 66 | (upper adj3 extrem$).mp. |
| 13 | (cerebr*vascular adj3 accident).mp. | 40 | exp Arm/ | 67 | (leg adj3 function$).mp. |
| 14 | (cerebrovascular adj3 accident).mp. | 41 | exp Upper Extremity/ | 68 | (leg adj3 move$).mp. |
| 15 | (cerebral adj2 vascular adj2 accident).mp. | 42 | exp Hand/ | 69 | (lower adj3 limb).mp. |
| 16 | CVA.mp. | 43 | exp Hand Strength/ | 70 | (lower adj3 extrem$).mp. |
| 17 | hemipleg*.mp. | 44 | exp Leg/ | 71 | physi$ therapy.mp. |
| 18 | hemipar*.mp. | 45 | exp Lower Extremity/ | 72 | physiotherapy.mp. |
| 19 | cereb* isch?emi*.mp. | 46 | exp Muscle Strength/ | 73 | occupat$ therapy.mp. |
| 20 | cerebral h?emorrhage.mp. | 47 | exp Locomotion/ | 74 | walk$.mp |
| 21 | intracerebral h?emorrhage.mp. | 48 | exp Gait/ | 75 | gait.mp. |
| 22 | parenchymal h?emorrhage.mp. | 49 | exp Gait Disorders, Neurologic/ | 76 | ambulat$.mp. |
| 23 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 | 50 | exp Gait Apraxia/ | 77 | mobil$.mp. |
| 24 | exp Dopamine/ | 51 | exp Motor Skills/ | 78 | transfer$.mp. |
| 25 | exp Dopamine Agents/ | 52 | exp Treatment Outcome/ | 79 | 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 |
| 26 | exp Dopamine Agonists/ | 53 | exp Social Participation/ | 80 | 23 and 33 and 79 |
| 27 | exp Levodopa/ | 54 | rehabilitat$.mp. |
Search strategy for EMBASE (1996 to week 42, 2014) and EMBASE Classic (1947 to 25 September 2015)
| 1 | exp cerebrovascular accident/ | 32 | 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 63 | exp social participation/ |
| 2 | exp cerebrovascular disease/ | 33 | exp rehabilitation/ | 64 | exp social adaptation/ |
| 3 | exp brain infarction/ | 34 | exp convalescence/ | 65 | exp social interaction/ |
| 4 | exp brain hemorrhage/ | 35 | exp physiotherapy/ | 66 | exp occupational therapy/ |
| 5 | exp hemiplegia/ | 36 | exp home physiotherapy/ | 67 | exp physical disability/ |
| 6 | exp hemiparesis/ | 37 | exp walking/ | 68 | exp outcome assessment/ |
| 7 | exp lacunar stroke/ | 38 | exp walking difficulty/ | 69 | exp treatment outcome/ |
| 8 | exp brain ischemia/ | 39 | exp walking speed/ | 70 | exp outcomes research/ |
| 9 | exp paresis/ | 40 | exp locomotion/ | 71 | exp rating scale/ |
| 10 | stroke.mp. | 41 | exp gait/ | 72 | rehabilitat$.mp. |
| 11 | lacun$.mp. | 42 | exp gait disorder/ | 73 | recover$.mp. |
| 12 | ($vascular adj3 accident$).mp. | 43 | gait/ or exp neurologic gait disorder/ | 74 | arm.mp. |
| 13 | cerebrovascular accident.mp. | 44 | exp gait apraxia/ | 75 | leg.mp. |
| 14 | cerebral vascular accident.mp. | 45 | exp hemiplegic gait/ | 76 | (upper adj3 limb).mp. |
| 15 | hemipleg$.mp. | 46 | gait/ or exp unsteady gait/ | 77 | (upper adj3 extremit$).mp. |
| 16 | hemipar$.mp. | 47 | gait/ or exp spastic gait/ | 78 | (lower adj3 limb$).mp. |
| 17 | isch?emi$.mp. | 48 | exp limited mobility/ | 79 | (lower adj3 extremit$).mp. |
| 18 | CVA.mp. | 49 | exp patient mobility/ | 80 | mobil$.mp. |
| 19 | h?emorrhag$.mp. | 50 | exp physical mobility/ | 81 | walk$.mp. |
| 20 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 | 51 | exp daily life activity/ | 82 | ambulat$.mp. |
| 21 | exp dopamine/ | 52 | exp arm/ | 83 | $therapy.mp. |
| 22 | exp dopamine receptor stimulating agent/ | 53 | exp arm movement/ | 84 | outcome.mp. |
| 23 | exp levodopa/ | 54 | exp arm exercise/ | 85 | (arm adj3 function$).mp. |
| 24 | exp carbidopa plus levodopa/ | 55 | arm/ or exp arm weakness/ | 86 | (hand adj3 function$).mp. |
| 25 | exp carbidopa plus entacapone plus levodopa/ | 56 | exp hand/ | 87 | (leg adj3 function$).mp. |
| 26 | exp benserazide plus levodopa/ | 57 | exp hand grip/ | 88 | (upper limb adj3 function$).mp. |
| 27 | exp DOPA/ | 58 | exp hand movement/ | 89 | (lower limb adj3 function$).mp. |
| 28 | $dopa$.mp. | 59 | exp hand function/ | 90 | 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 |
| 29 | sinemet.mp. | 60 | exp hand strength/ or hand/ | 91 | 20 and 32 and 90 |
| 30 | $careldopa.mp. | 61 | exp leg/ | ||
| 31 | madopar.mp. | 62 | exp leg movement/ |
Search strategy for Ovid PsycINFO (1806 to 25 September 2015)
| 1 | exp Cerebrovascular Accidents/ | 23 | madopar.mp. | 45 | exp Physical Strength/ |
| 2 | exp Cerebral Ischemia/ | 24 | levodopa.mp. | 46 | exp Exercise/ |
| 3 | exp Cerebrovascular Disorders/ | 25 | L-dopa.mp. | 47 | exp Treatment Outcomes/ |
| 4 | exp Cerebral Hemorrhage/ | 26 | 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 48 | exp Rating Scales/ |
| 5 | exp Hemiplegia/ | 27 | exp Rehabilitation/ | 49 | exp Measurement/ |
| 6 | exp Hemiparesis/ | 28 | exp Motor Processes/ | 50 | rehabilitat$.mp. |
| 7 | Stroke.mp. | 29 | exp Motor Performance/ | 51 | recover$.mp. |
| 8 | lacun$.mp. | 30 | exp Physical Therapy/ | 52 | arm.mp. |
| 9 | cerebrovascular accident$.mp. | 31 | exp Occupational Therapy/ | 53 | leg.mp. |
| 10 | cerebral vascular accident$.mp. | 32 | exp “Activities of Daily Living”/ | 54 | (upper adj3 limb).mp. |
| 11 | $vascular accident$.mp. | 33 | exp Walking/ | 55 | (lower adj3 limb).mp. |
| 12 | CVA.mp. | 34 | exp Gait/ | 56 | (lower adj3 extremit$).mp. |
| 13 | hemipleg$.mp. | 35 | exp Physical Mobility/ | 57 | (upper adj3 extremit$).mp. |
| 14 | hemipare$.mp. | 36 | exp Disabilities/ | 58 | hand.mp. |
| 15 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 | 37 | exp Social Interaction/ | 59 | mobil$.mp. |
| 16 | exp Dopamine/ | 38 | exp Participation/ | 60 | walk$.mp. |
| 17 | exp Dopamine Agonists/ | 39 | exp Learning/ | 61 | ambulat$.mp. |
| 18 | exp Levodopa/ | 40 | exp Motor Coordination/ | 62 | $therapy.mp. |
| 19 | exp DOPA/ | 41 | exp “Arm (Anatomy)”/ | 63 | outcome.mp. |
| 20 | $dopa$.mp. | 42 | exp “Hand (Anatomy)”/ | 64 | 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 |
| 21 | sinemet.mp. | 43 | exp Grasping/ | 65 | 15 and 26 and 64 |
| 22 | $careldopa.mp. | 44 | exp “Leg (Anatomy)”/ |
Appendix 2 Resource use: unit costs
| Resource type | Unit cost at 2015 prices, (£) | Source | Comments |
|---|---|---|---|
| GP, surgery visit | |||
| FTF | 46.75 | PSSRU 2013, p. 191109 | Lasting 11.7 minutes (with qualifications – including direct care staff costs) |
| T/E | 28.05 | PSSRU 2013, p. 191109 | Lasting 7.1 minutes (with qualifications – including direct care staff costs) |
| GP, home visit | |||
| FTF | 118.44 | PSSRU 2013, p. 191109 | Lasting 23.4 minutes (with qualifications – including direct care staff costs) |
| T/E | 70.69 | Assume 23.4 minutes × 0.6 = 14.04 minutes | |
| District nurse, health visitor or member of community team | |||
| FTF | 39.79 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, community health services – nursing, code: N02AF | |
| T/E | 24.03 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, community health services – nursing, code: N02AN | |
| Social worker | |||
| FTF | 234.80 | PSSRU 2013, p. 198109 | Assume 60 minutes |
| T/E | 78.26 | Assume 20 minutes | |
| Counsellor | |||
| FTF | 60.00 | PSSRU 2013, p. 54109 | 55 minutes |
| T/E | 20.00 | Assume 18.3 minutes | |
| Home help or care worker | 24.93 | PSSRU 2013, p. 202109 | Based on the price multipliers for independent sector home care provided for social services |
| Speech and language therapist | |||
| FTF | 35.32 | PSSRU 2013, p. 177109 | Assume 60 minutes |
| T/E | 11.77 | Assume 20 minutes | |
| Psychiatrist or psychologist | |||
| FTF | 139.22 | PSSRU 2013, p. 179109 | Assume 60 minutes |
| T/E | 46.41 | Assume 20 minutes | |
| Day centre | 213.97 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, community health services – day care facilities, code: DCF10 | |
| Lunch or social club | 39.48 | PSSRU 2013, p. 40109 | |
| Food, medicine or laundry delivery service | 36.75 | PSSRU 2013, p. 129109 | |
| Family or patient support or self-help groups | |||
| FTF | 52.99 | PSSRU 2013, p. 152109 | Ongoing support from the family support worker (London costs) |
| T/E | 17.66 | Assume 20 minutes | |
| Dentist | 119.33 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, community health services – medical and dental, code: M01 | |
| Nurse, general practice | |||
| FTF | 13.95 | PSSRU 2013, p. 188109 | 15.5 minutes |
| T/E | 4.69 | Assume 5.2 minutes | |
| Occupational therapist | |||
| FTF | 79.06 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, allied health professionals, code: A06A1 | |
| T/E | 26.36 | Assume one-third of FTF | |
| Paramedics | 239.03 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, ambulance services, code: ASS02 | |
| Pharmacist | |||
| FTF | 36.36 | PSSRU 2013, p. 180109 | Assume 30 minutes |
| T/E | 12.12 | Assume one-third of FTF | |
| Physiotherapist | |||
| FTF | 52.40 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, allied health professionals, code: A08A1 | |
| T/E | 17.46 | Assume one-third of FTF | |
| Re-ablement | 43.63 | PSSRU 2013, p. 114109 | |
| Rehabilitation centre | 71.71 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, allied health professionals, code: CRT1 | |
| Wheelchair service | 92.46 | PSSRU 2013, p. 108109 | |
| Citizens Advice | |||
| FTF | 15.58 | PSSRU 2013, p. 200109 | Assume 30 minutes |
| T/E | 5.19 | Assume one-third of FTF | |
| Optician | 25.97 | www.boots.com/en/Opticians/Opticians-offers/Latest-glasses-eye-test-offers/ (accessed 27 October 2015) | |
| Chiropodist | |||
| FTF | 43.55 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, allied health professionals, code: A09 | |
| T/E | 14.51 | Assume one-third of FTF | |
| Psychotherapy | |||
| FTF | 60.00 | Same as ‘Councellor’ | |
| T/E | 20.00 | Same as ‘Councellor’ | |
| Leisure facilities | 5.30 | www.leeds.gov.uk/sports/Documents/30.01.2014.pdf (accessed 27 October 2015) | |
| Leisure facility access | 10.39 | www.leeds.gov.uk/sports/Documents/30.01.2014.pdf (accessed 27 October 2015) | |
| Clinic incontinent | |||
| FTF | 87.82 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, community health services, code: N14AF | |
| Non-FTF | 39.92 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, community health services, code: N14AN | |
| Hypnotherapy | |||
| FTF | 60.00 | Same as ‘Counsellor’ | |
| T/E | 20.00 | Same as ‘Counsellor’ | |
| Dietitian | |||
| FTF | 73.49 | National Schedule of Reference Costs 2012–13,110 NHS trusts and NHS foundation trusts, allied health professionals, code: A03 | |
| T/E | 24.50 | Assume one-third of FTF | |
| Hospital inpatient stay | 363.96 | National Schedule of Reference Costs 2013–2014,111 NHS trusts and NHS foundation trusts, elective inpatient excess bed-days, code: AA35C | Stroke with CC score of 10–12 |
| Hospital day centre | 375.38 | National Schedule of Reference Costs 2013–2014,111 NHS trusts and NHS foundation trusts, day cases, code: AA35C | Stroke with CC score of 10–12 |
| Hospital outpatient clinic | 135.33 | National Schedule of Reference Costs 2013–2014,111 NHS trusts and NHS foundation trusts, non-consultant led, rehabilitation service, code: WF01C (314) | |
| Hospital A&E department | 112.59 | National Schedule of Reference Costs 2012–2013,110 NHS trusts and NHS foundation trusts, accident and emergency services, code: T01A | Category 1 investigation with category 1–2 treatment |
| Nursing/residential home | 111.31 | PSSRU 2013, p. 37109 | £750 per week |
Appendix 3 Sensitivity analyses tables
| Variables used in the sensitivity analysis | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 311) | No (N = 269) | Total (N = 580) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 159 (53.3) | 139 (46.7) | 298 (100.0) |
| Placebo | 152 (53.9) | 130 (46.1) | 282 (100.0) |
| Patient sex, n (%) | |||
| Male | 202 (57.2) | 151 (42.8) | 353 (100.0) |
| Female | 109 (48.0) | 118 (52.0) | 227 (100.0) |
| Type of stroke, n (%) | |||
| Infarction | 252 (50.7) | 245 (49.3) | 497 (100.0) |
| Primary haemorrhage | 59 (71.1) | 24 (28.9) | 83 (100.0) |
| RMI 24-hour randomisation system score | |||
| Mean (SD) | 2.9 (1.83) | 1.5 (1.38) | 2.2 (1.78) |
| Median (range) | 3.0 (0.0–6.0) | 1.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Age (years) | |||
| Mean (SD) | 66.5 (14.06) | 70.8 (11.61) | 68.5 (13.14) |
| Median (range) | 67.3 (20.2–95.6) | 72.1 (41.8–98.2) | 69.9 (20.2–98.2) |
| BI score at baseline | |||
| Mean (SD) | 8.9 (3.71) | 6.3 (3.19) | 7.7 (3.70) |
| Median (range) | 8.0 (1.0–19.0) | 6.0 (0.0–20.0) | 7.0 (0.0–20.0) |
| Days between stroke and randomisation | |||
| Mean (SD) | 15.8 (9.54) | 19.8 (10.26) | 17.7 (10.08) |
| Median (range) | 13.0 (3.0–59.0) | 17.0 (5.0–55.0) | 15.0 (3.0–59.0) |
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.915 (0.617 to 1.358) | 0.660 |
| Sex: female vs. male | 0.770 (0.509 to 1.166) | 0.217 |
| Stroke type: infarction vs. primary haemorrhage | 0.277 (0.152 to 0.505) | 0.000 |
| RMI score at baseline | 1.504 (1.283 to 1.763) | 0.000 |
| Age (years) | 0.978 (0.962 to 0.994) | 0.007 |
| BI score at baseline | 1.094 (1.016 to 1.177) | 0.017 |
| Days between stroke and randomisation | 0.958 (0.938 to 0.978) | 0.000 |
| Total number of sessions with sufficient motor therapy | 0.968 (0.951 to 0.985) | 0.000 |
| Variables used in the complete-case sensitivity analysis | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 238) | No (N = 256) | Total (N = 494) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 116 (46.8) | 132 (53.2) | 248 (100.0) |
| Placebo | 122 (49.6) | 124 (50.4) | 246 (100.0) |
| Patient sex, n (%) | |||
| Male | 161 (53.0) | 143 (47.0) | 304 (100.0) |
| Female | 77 (40.5) | 113 (59.5) | 190 (100.0) |
| Type of stroke, n (%) | |||
| Infarction | 195 (45.7) | 232 (54.3) | 427 (100.0) |
| Primary haemorrhage | 43 (64.2) | 24 (45.8) | 67 (100.0) |
| RMI 24-hour randomisation system score (points) | |||
| Mean (SD) | 3.1 (1.78) | 1.5 (1.39) | 2.3 (1.79) |
| Median (range) | 3.0 (0.0–6.0) | 1.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Age (years) | |||
| Mean (SD) | 65.3 (14.14) | 71.0 (11.44) | 68.3 (13.11) |
| Median (range) | 65.9 (20.2–95.6) | 72.5 (41.8–98.2) | 69.6 (20.2–98.2) |
| NEADL score at baseline (points) | |||
| Mean (SD) | 60.7 (10.13) | 57.6 (12.77) | 59.1 (11.67) |
| Median (range) | 66.0 (0.0–66.0) | 63.0 (0.0–66.0) | 63.0 (0.0–66.0) |
| Missing (n) | 7 | 2 | 9 |
| BI score at baseline (points) | |||
| Mean (SD) | 9.3 (3.39) | 6.3 (3.23) | 7.7 (3.63) |
| Median (range) | 9.0 (3.0–19.0) | 6.0 (0.0–20.0) | 7.0 (0.0–20.0) |
| Missing (n) | 5 | 3 | 8 |
| Days between stroke and randomisation | |||
| Mean (SD) | 15.1 (9.66) | 19.9 (10.29) | 17.6 (10.26) |
| Median (range) | 13.0 (3.0–59.0) | 17.0 (5.0–55.0) | 14.0 (3.0–59.0) |
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.809 (0.517 to 1.266) | 0.352 |
| Sex: female vs. male | 0.917 (0.574 to 1.463) | 0.714 |
| Stroke type: infarction vs. primary haemorrhage | 0.283 (0.143 to 0.559) | 0.000 |
| RMI score at baseline | 1.684 (1.400 to 2.025) | 0.000 |
| Age (years) | 0.976 (0.959 to 0.994) | 0.010 |
| NEADL score at baseline | 1.024 (1.003 to 1.046) | 0.027 |
| BI score at baseline | 1.116 (1.023 to 1.217) | 0.013 |
| Days from stroke to randomisation | 0.945 (0.922 to 0.967) | 0.000 |
| Variables used in the sensitivity analysis | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 244) | No (N = 332) | Total (N = 576) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 120 (40.5) | 176 (59.5) | 296 (100.0) |
| Placebo | 124 (44.3) | 156 (55.7) | 280 (100.0) |
| Patient sex, n (%) | |||
| Male | 164 (46.7) | 187 (53.3) | 351 (100.0) |
| Female | 80 (35.6) | 145 (64.4) | 225 (100.0) |
| Type of stroke, n (%) | |||
| Infarction | 199 (40.46) | 294 (59.6) | 493 (100.0) |
| Primary haemorrhage | 45 (54.2) | 38 (45.8) | 83 (100.0) |
| RMI 24-hour randomisation system score (points) | |||
| Mean (SD) | 3.1 (1.78) | 1.6 (1.46) | 2.2 (1.77) |
| Median (range) | 3.0 (0.0–6.0) | 1.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Age (years) | |||
| Mean (SD) | 65.5 (14.10) | 70.7 (12.02) | 68.5 (13.18) |
| Median (range) | 66.0 (20.2–95.6) | 72.2 (20.8–98.2) | 69.9 (20.2–98.2) |
| NEADL score at baseline (points) | |||
| Mean (SD) | 60.7 (10.01) | 57.6 (12.51) | 58.9 (11.61) |
| Median (range) | 66.0 (0.0–66.0) | 63.0 (0.0–66.0) | 63.0 (0.0–66.0) |
| BI score at baseline (points) | |||
| Mean (SD) | 9.3 (3.44) | 6.5 (3.44) | 7.7 (3.70) |
| Median (range) | 9.0 (3.0–19.0) | 6.0 (0.0–20.0) | 7.0 (0.0–20.0) |
| Days between stroke and randomisation | |||
| Mean (SD) | 15.0 (9.59) | 19.6 (10.01) | 17.7 (10.08) |
| Median (range) | 12.0 (3.0–59.0) | 17.0 (3.0–55.0) | 15.0 (3.0–59.0) |
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.784 (0.528 to 1.165) | 0.229 |
| Sex: female vs. male | 0.875 (0.580 to 1.321) | 0.525 |
| Stroke type: infarction vs. primary haemorrhage | 0.384 (0.219 to 0.672) | 0.001 |
| Baseline researcher RMI score | 1.545 (1.323 to 1.803) | 0.000 |
| Age (years) | 0.979 (0.964 to 0.994) | 0.008 |
| Baseline NEADL score | 1.024 (1.004 to 1.045) | 0.017 |
| Baseline BI score | 1.106 (1.030 to 1.187) | 0.006 |
| Days between stroke and randomisation | 0.947 (0.927 to 0.968) | 0.000 |
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.769 (0.518 to 1.142) | 0.193 |
| Sex: female vs. male | 0.886 (0.587 to 1.337) | 0.562 |
| Stroke type: infarction vs. primary haemorrhage | 0.426 (0.243 to 0.745) | 0.003 |
| RMI score on 24-hour randomisation system | 1.289 (1.132 to 1.468) | 0.000 |
| Age (years) | 0.978 (0.963 to 0.993) | 0.005 |
| Baseline NEADL score | 1.023 (1.004 to 1.042) | 0.019 |
| Baseline BI score | 1.140 (1.058 to 1.228) | 0.001 |
| Days between stroke and randomisation | 0.946 (0.925 to 0.966) | 0.000 |
| Variables used in the sensitivity analysis | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 230) | No (N = 261) | Total (N = 491) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 110 (44.9) | 135 (55.1) | 245 (100.0) |
| Placebo | 120 (48.8) | 126 (51.2) | 246 (100.0) |
| Patient sex, n (%) | |||
| Male | 156 (51.7) | 146 (48.3) | 302 (100.0) |
| Female | 74 (39.2) | 115 (60.8) | 189 (100.0) |
| Type of stroke, n (%) | |||
| Infarction | 187 (43.9) | 239 (56.1) | 426 (100.0) |
| Primary haemorrhage | 43 (66.2) | 22 (33.8) | 65 (100.0) |
| RMI 24-hour randomisation system score (points) | |||
| Mean (SD) | 3.0 (1.80) | 1.5 (1.37) | 2.2 (1.76) |
| Median (range) | 3.0 (0.0–6.0) | 1.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Age (years) | |||
| Mean (SD) | 65.7 (14.24) | 70.7 (11.61) | 68.4 (13.14) |
| Median (range) | 66.0 (20.2–95.6) | 72.0 (41.8–98.2) | 69.7 (20.2–98.2) |
| NEADL score at baseline (points) | |||
| Mean (SD) | 60.6 (10.20) | 57.9 (12.52) | 59.1 (11.56) |
| Median (range) | 66.0 (0.0–66.0) | 63.0 (0.0–66.0) | 63.0 (0.0–66.0) |
| BI score at baseline (points) | |||
| Mean (SD) | 9.2 (3.42) | 6.4 (3.16) | 7.7 (3.57) |
| Median (range) | 9.0 (3.0–19.0) | 6.0 (0.0–20.0) | 7.0 (0.0–20.0) |
| Days between stroke and randomisation | |||
| Mean (SD) | 14.8 (9.61) | 19.5 (10.06) | 17.3 (10.11) |
| Median (range) | 12.0 (3.0–59.0) | 17.0 (5.0–50.0) | 14.0 (3.0–59.0) |
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.790 (0.508 to 1.229) | 0.294 |
| Sex: female vs. male | 0.924 (0.582 to 1.468) | 0.737 |
| Stroke type: infarction vs. primary haemorrhage | 0.231 (0.117 to 0.457) | 0.000 |
| RMI score on 24-hour randomisation system | 1.631 (1.366 to 1.947) | 0.000 |
| Age (years) | 0.978 (0.960 to 0.995) | 0.013 |
| Baseline NEADL score | 1.024 (1.002 to 1.046) | 0.032 |
| Baseline BI score | 1.134 (1.043 to 1.233) | 0.003 |
| Days between stroke and randomisation | 0.943 (0.920 to 0.966) | 0.000 |
| Variables used in the sensitivity analysis | Able to walk independently at 8 weeks | ||
|---|---|---|---|
| Yes (N = 243) | No (N = 295) | Total (N = 538) | |
| Randomised allocation, n (%) | |||
| Co-careldopa | 120 (43.8) | 154 (56.2) | 274 (100.0) |
| Placebo | 123 (46.6) | 141 (53.4) | 264 (100.0) |
| Patient sex, n (%) | |||
| Male | 163 (49.4) | 167 (50.6) | 330 (100.0) |
| Female | 80 (38.5) | 128 (61.5) | 208 (100.0) |
| Type of stroke, n (%) | |||
| Infarction | 199 (42.5) | 269 (57.5) | 468 (100.0) |
| Primary haemorrhage | 44 (62.9) | 26 (37.1) | 70 (100.0) |
| RMI 24-hour randomisation system score (points) | |||
| Mean (SD) | 3.1 (1.78) | 1.5 (1.41) | 2.2 (1.78) |
| Median (range) | 3.0 (0.0–6.0) | 1.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Age (years) | |||
| Mean (SD) | 65.5 (14.12) | 70.8 (11.78) | 68.4 (13.15) |
| Median (range) | 66.0 (20.2–95.6) | 72.1 (41.8–98.2) | 69.7 (20.2–98.2) |
| NEADL score at baseline (points) | |||
| Mean (SD) | 60.7 (10.03) | 57.7 (12.57) | 59.0 (11.58) |
| Median (range) | 66.0 (0.0–66.0) | 63.0 (0.0–66.0) | 63.0 (0.0–66.0) |
| BI score at baseline (points) | |||
| Mean (SD) | 9.3 (3.43) | 6.3 (3.24) | 7.7 (3.64) |
| Median (range) | 9.0 (3.0–19.0) | 6.0 (0.0–20.0) | 7.0 (0.0–20.0) |
| Days between stroke and randomisation | |||
| Mean (SD) | 15.1 (9.60) | 19.5 (10.19) | 17.5 (10.17) |
| Median (range) | 12.0 (3.0–59.0) | 17.0 (5.0–55.0) | 14.0 (3.0–59.0) |
| Reasons | Treatment group, n (%) | Total (N = 45), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 26) | Placebo (N = 19) | ||
| Patient died | 1 (0.3) | 1 (0.4) | 2 (0.3) |
| Patient withdrew | 11 (3.2) | 5 (1.8) | 16 (2.5) |
| Cannot get hold of participant | 1 (0.3) | 2 (0.7) | 3 (0.5) |
| Moved out of area | 1 (0.3) | 1 (0.4) | 2 (0.3) |
| Too unwell | 1 (0.3) | 3 (1.1) | 4 (0.7) |
| Lost in post | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Lost at site | 4 (1.3) | 0 (0.0) | 4 (0.7) |
| Withdrew after follow-up period started | 6 (1.9) | 2 (0.7) | 8 (1.3) |
| Participant refused to complete | 0 (0.0) | 2 (0.7) | 2 (0.3) |
| Other | 1 (0.3) | 2 (0.7) | 3 (0.5) |
| Model parameter | OR (95% CI) | p-value |
|---|---|---|
| Treatment group: co-careldopa vs. placebo | 0.858 (0.566 to 1.302) | 0.471 |
| Sex: female vs. male | 0.948 (0.613 to 1.467) | 0.811 |
| Stroke type: infarction vs. primary haemorrhage | 0.265 (0.141 to 0.498) | 0.000 |
| RMI score on 24-hour randomisation system | 1.590 (1.347 to 1.876) | 0.000 |
| Age (years) | 0.981 (0.965 to 0.998) | 0.026 |
| Baseline NEADL score | 1.025 (1.004 to 1.047) | 0.018 |
| Baseline BI score | 1.137 (1.052 to 1.230) | 0.001 |
| Days between stroke and randomisation | 0.948 (0.927 to 0.970) | 0.000 |
Appendix 4 Model checks
FIGURE 15.
Modified Rankin Scale proportional odds assumptions at the 8-week follow-up (plot of four empirical logits for five mRS response levels).
FIGURE 16.
Modified Rankin Scale proportional odds assumptions at the 6-month follow-up.

Appendix 5 Vomiting between the investigational medicinal product dose and end of therapy
| Vomited between IMP dose and end of therapy | Treatment group, n (%) | Total (N = 11,987), n (%) | |
|---|---|---|---|
| Co-careldopa (N = 5967) | Placebo (N = 6020) | ||
| Yes | 27 (0.5) | 9 (0.1) | 36 (0.3) |
| No | 5829 (97.7) | 5861 (97.4) | 11,690 (97.5) |
| Unknown | 89 (1.5) | 134 (2.2) | 223 (1.9) |
| Missing | 22 (0.4) | 16 (0.3) | 38 (0.3) |
Appendix 6 Moderator analysis
| Moderator variable | Walking independently at 8 weeks | |
|---|---|---|
| No (N = 341) | Yes (N = 252) | |
| Baseline RMI score (points), n; mean (SD) | ||
| 24-hour randomisation | 341; 1.60 (1.49) | 252; 3.13 (1.79) |
| Researcher-reported | 341; 1.60 (1.48) | 252; 3.11 (1.77) |
| Patient-reported | 322; 1.74 (1.85) | 244; 3.43 (2.29) |
| GHQ-12 total score (points), n; mean (SD) | 331; 19.68 (6.56) | 239; 18.90 (7.22) |
| MoCA total score (points), n; mean (SD) | 331; 19.11 (6.58) | 249; 21.71 (5.60) |
| Joint, neck or back pain, n (%) | ||
| No | 195 (58.0) | 152 (61.5) |
| Yes | 141 (42.0) | 95 (38.5) |
| Pain in upper limbs, n (%) | ||
| No | 270 (80.4) | 199 (80.6) |
| Yes | 66 (19.6) | 48 (19.4) |
| Pain in lower limbs, n (%) | ||
| No | 235 (69.9) | 194 (78.5) |
| Yes | 101 (30.1) | 53 (21.5) |
| Central post-stroke pain, n (%) | ||
| No | 331 (98.5) | 242 (98.0) |
| Yes | 5 (1.5) | 5 (2.0) |
| Any thumb, hand, finger or wrist joint pain, n (%) | ||
| No | 326 (97.0) | 244 (98.8) |
| Yes | 10 (3.0) | 3 (1.2) |
| Spinal pain, n (%) | ||
| No | 330 (98.2) | 238 (96.4) |
| Yes | 6 (1.8) | 9 (3.6) |
| Patient medical history, n (%) | ||
| Cardiovascular and limiting mobility conditions (with/without other conditions) | 282 (82.7) | 195 (77.4) |
| Other conditions only | 40 (11.7) | 28 (11.1) |
| No medical conditions reported | 19 (5.6) | 29 (11.5) |
| Ischaemic change in MCA territory, n (%) | ||
| Not affected | 158 (59.4) | 135 (68.2) |
| < 33% of MCA territory | 72 (27.1) | 43 (21.7) |
| > 33% of MCA territory | 36 (13.5) | 20 (10.1) |
| Site of MCA territory, n (%) | ||
| None | 158 (59.4) | 135 (68.2) |
| Cortical | 40 (15.0) | 28 (14.1) |
| Subcortical | 41 (15.4) | 22 (11.1) |
| Both (cortical and subcortical) | 27 (10.2) | 13 (6.6) |
| Any haemorrhage, n (%) | ||
| No | 233 (86.9) | 156 (79.2) |
| Yes | 35 (13.1) | 40 (20.8) |
| Haemorrhage location, n (%) | ||
| None | 233 (88.9) | 156 (80.0) |
| Cortical | 11 (4.2) | 10 (5.1) |
| Subcortical | 18 (6.9) | 28 (14.9) |
| Periventricular lucencies, n (%) | ||
| No | 153 (57.1) | 118 (58.7) |
| Yes | 115 (42.9) | 83 (41.3) |
| White matter periventricular lucency, n (%) | ||
| No lucency | 153 (57.1) | 118 (58.7) |
| Restricted to region adjoining ventricles | 60 (22.4) | 49 (24.4) |
| Entire region from lateral ventricle to cortex | 55 (20.5) | 34 (16.9) |
| Old vascular lesions, n (%) | ||
| No | 200 (74.9) | 145 (73.2) |
| Yes | 67 (25.1) | 52 (26.8) |
| Moderator variable | Treatment group | |
|---|---|---|
| Placebo (N = 285) | Co-careldopa (N = 308) | |
| Baseline RMI score (points), n; mean (SD) | ||
| 24-hour randomisation | 285; 2.28 (1.79) | 308; 2.22 (1.79) |
| Researcher-reported | 285; 2.26 (1.76) | 308; 2.23 (1.78) |
| Patient-reported | 275; 2.50 (2.23) | 291; 2.45 (2.20) |
| GHQ-12 total score (points), n; mean (SD) | 277; 19.31 (7.02) | 293; 19.40 (6.69) |
| MoCA total score (points), n; mean (SD) | 281; 20.46 (5.96) | 299; 20.01 (6.62) |
| Joint, neck or back pain, n (%) | ||
| No | 177 (62.3) | 170 (56.9) |
| Yes | 107 (37.7) | 129 (43.1) |
| Pain in upper limbs, n (%) | ||
| No | 230 (81.0) | 239 (79.9) |
| Yes | 54 (19.0) | 60 (20.1) |
| Pain in lower limbs, n (%) | ||
| No | 212 (74.7) | 217 (72.6) |
| Yes | 72 (25.3) | 82 (27.4) |
| Central post-stroke pain, n (%) | ||
| No | 281 (98.9) | 292 (97.7) |
| Yes | 3 (1.1) | 7 (2.3) |
| Any thumb, hand, finger or wrist joint pain, n (%) | ||
| No | 279 (98.2) | 291 (97.3) |
| Yes | 5 (1.8) | 8 (2.7) |
| Spinal pain, n (%) | ||
| No | 279 (98.2) | 289 (96.7) |
| Yes | 5 (1.8) | 10 (3.3) |
| Patient medical history (category 3), n (%) | ||
| Cardiovascular and/or limiting mobility conditions (with/without other conditions) | 229 (80.3) | 248 (80.5) |
| Other conditions only | 33 (11.6) | 35 (11.4) |
| No medical conditions reported | 23 (8.1) | 25 (8.1) |
| Ischaemic change in MCA territory, n (%) | ||
| Not affected | 155 (67.1) | 138 (59.2) |
| < 33% of MCA territory | 49 (21.2) | 66 (28.3) |
| > 33% of MCA territory | 27 (11.7) | 29 (12.5) |
| Site and size of MCA territory, n (%) | ||
| None | 155 (67.1) | 138 (59.2) |
| Cortical | 28 (12.1) | 40 (17.2) |
| Subcortical | 29 (12.6) | 34 (14.6) |
| Both (cortical and subcortical) | 19 (8.2) | 21 (9.0) |
| Any haemorrhage, n (%) | ||
| No | 187 (81.0) | 202 (86.3) |
| Yes | 44 (19.0) | 32 (13.7) |
| Haemorrhage location, n (%) | ||
| None | 187 (82.4) | 202 (87.8) |
| Cortical | 13 (5.7) | 8 (3.5) |
| Subcortical | 27 (11.9) | 20 (8.7) |
| Periventricular lucencies, n (%) | ||
| No | 137 (58.6) | 134 (57.0) |
| Yes | 97 (41.4) | 101 (43.0) |
| White matter periventricular lucency, n (%) | ||
| No lucency | 137 (58.6) | 134 (57.0) |
| Restricted to region adjoining ventricles | 54 (23.1) | 55 (23.4) |
| Entire region from lateral ventricle to cortex | 43 (18.4) | 46 (19.6) |
| Old vascular lesions, n (%) | ||
| No | 170 (73.6) | 175 (74.8) |
| Yes | 61 (26.4) | 59 (25.2) |
| Model parameter | OR for treatment (95% CI) | OR for interaction (95% CI) | p-value for interaction |
|---|---|---|---|
| Model 1: baseline RMI score | 0.462 (0.233 to 0.918) | 1.251 (0.982 to 1.594) | 0.069 |
| Model 2: baseline RMI (patient-reported) score | 0.547 (0.287 to 1.042) | 1.133 (0.927 to 1.384) | 0.222 |
| Model 3: GHQ-12 score | 1.278 (0.388 to 4.203) | 0.975 (0.912 to 1.033) | 0.388 |
| Model 4: MoCA score | 0.976 (0.237 to 4.013) | 0.989 (0.926 to 1.056) | 0.735 |
| Model 5: joint, neck or back pain (yes vs. no) | 0.884 (0.528 to 1.480) | 0.778 (0.346 to 1.749) | 0.544 |
| Model 6: pain in upper limbs (yes vs. no) | 0.857 (0.550 to 1.337) | 0.695 (0.260 to 1.860) | 0.469 |
| Model 7: pain in lower limbs (yes vs. no) | 0.841 (0.530 to 1.335) | 0.813 (0.326 to 2.028) | 0.658 |
| Model 8: central post-stroke pain (yes vs. no) | 0.795 (0.533 to 1.185) | 1.109 (0.045 to 27.164)a | 0.949 |
| Model 9: thumb/hand/wrist joint pain (yes vs. no) | 0.819 (0.548 to 1.224) | 0.360 (0.018 to 7.307)a | 0.506 |
| Model 10: spinal pain (yes vs. no) | 0.852 (0.570 to 1.273) | –a | – |
| Model 11: patient medical historyb | 0.702 (0.452 to 1.092) | 1.663 (0.482 to 5.743) | 0.615 |
| 1.627 (0.380 to 6.961) | |||
| Model 12: site of MCA territoryc | 0.829 (0.477 to 1.44) | 0.730 (0.198 to 2.689) | 0.817 |
| 0.853 (0.231 to 3.141) | |||
| 1.916 (0.336 to 10.938) | |||
| Model 13: haemorrhage (yes vs. no) | 0.838 (0.517 to 1.357) | 0.815 (0.253 to 2.625) | 0.732 |
| Model 14: periventricular lucencyd | 0.737 (0.410 to 1.323) | 1.993 (0.686 to 5.791) | 0.214 |
| 0.612 (0.192 to 1.951) | |||
| Model 15: old vascular lesion (yes vs. no) | 0.9997 (0.598 to 1.670) | 0.462 (0.168 to 1.277) | 0.137 |
Appendix 7 Mediator analysis
The mediator analysis explored the extent to which the treatment effects can be explained by an intermediate mechanistic outcome. The first step in the Baron and Kenny83 approach to establishing mediation is to establish whether or not there is an effect that may be mediated. Since there was no evidence of a statistically significant difference in walking independently at 8 weeks between the treatment groups in the ITT analysis, the results below are based on exploratory analysis and should be treated with caution.
Summary statistics of the variables being considered for mediation were tabulated by treatment group and primary outcome category (Tables 85 and 86). There was no suggestion of any differences by treatment group but, as might be expected, those walking independently at 8 weeks also had higher BI and NEADL scores. There were a number of patients with missing questionnaire data at 8 weeks and examination of the baseline and treatment characteristics of those with and without questionnaire data showed that those missing data were more likely to be in the co-careldopa group (51% among those not missing vs. 60–63% among those missing, depending on the questionnaire missing data), to have attended fewer therapy sessions and to have taken fewer IMP doses (Table 87). With the exception of MoCA score, a higher proportion of patients without questionnaire data at 8 weeks had a primary haemorrhage (87% among those not missing vs. 76–77% among those missing, depending on the questionnaire missing data).
| Mediator variable | Walking independently at 8 weeks | |
|---|---|---|
| No (N = 341) | Yes (N = 252) | |
| Number of (motor) therapy sessions, n; mean (SD) | 341; 23.94 (14.05) | 252; 23.97 (12.77) |
| Number of IMP doses, n; mean (SD) | 341; 21.52 (13.04) | 252; 21.40 (10.94) |
| FAS score (points), n; mean (SD) | 274; 26.00 (7.67) | 249; 23.84 (7.11) |
| MoCA score (points), n; mean (SD) | 280; 21.23 (6.33) | 246; 24.28 (4.90) |
| BI score (points), n; mean (SD) | 276; 9.46 (3.88) | 251; 16.94 (2.56) |
| NEADL score (points), n; mean (SD) | 276; 10.47 (9.05) | 251; 31.49 (16.52) |
| Joint, neck or back pain, n (%) | ||
| No | 66 (23.7) | 64 (25.7) |
| Yes | 212 (76.3) | 185 (74.3) |
| Pain in upper limbs, n (%) | ||
| No | 108 (38.9) | 95 (38.1) |
| Yes | 170 (61.1) | 154 (61.5) |
| Pain in lower limbs, n (%) | ||
| No | 144 (51.8) | 143 (57.4) |
| Yes | 134 (48.2) | 106 (42.6) |
| Central post-stroke pain, n (%) | ||
| No | 240 (86.3) | 242 (90.0) |
| Yes | 38 (13.7) | 5 (10.0) |
| Any thumb, hand, finger or wrist joint pain, n (%) | ||
| No | 224 (80.6) | 244 (81.5) |
| Yes | 54 (19.4) | 3 (18.5) |
| Spinal pain, n (%) | ||
| No | 275 (98.9) | 246 (98.8) |
| Yes | 3 (1.1) | 3 (1.2) |
| Mediator variable | Treatment group | |
|---|---|---|
| Placebo (N = 285) | Co-careldopa (N = 308) | |
| Number of (motor) therapy sessions, n; mean (SD) | 285; 24.75 (12.50) | 308; 23.21 (14.36) |
| Number of IMP doses, n; mean (SD) | 285; 22.36 (11.10) | 308; 20.64 (13.07) |
| FAS score (points), n; mean (SD) | 257; 24.85 (7.39) | 266; 25.09 (7.57) |
| MoCA score (points), n; mean (SD) | 260; 22.87 (5.51) | 266; 22.44 (6.26) |
| BI score (points), n; mean (SD) | 259; 13.17 (4.90) | 268; 12.88 (5.09) |
| NEADL score (points), n; mean (SD) | 259; 19.98 (15.83) | 268; 20.97 (17.74) |
| Joint, neck or back pain, n (%) | ||
| No | 69 (26.5) | 61 (22.9) |
| Yes | 191 (73.5) | 206 (77.1) |
| Pain in upper limbs, n (%) | ||
| No | 101 (38.9) | 102 (38.2) |
| Yes | 159 (61.1) | 165 (61.8) |
| Pain in lower limbs, n (%) | ||
| No | 147 (56.5) | 140 (52.4) |
| Yes | 113 (43.5) | 127 (47.6) |
| Central post-stroke pain, n (%) | ||
| No | 228 (87.7) | 236 (88.4) |
| Yes | 32 (12.3) | 31 (11.6) |
| Any thumb, hand, finger or wrist joint pain, n (%) | ||
| No | 212 (81.5) | 215 (80.5) |
| Yes | 48 (18.5) | 52 (19.5) |
| Spinal pain, n (%) | ||
| No | 257 (98.8) | 264 (98.9) |
| Yes | 3 (1.2) | 3 (1.1) |
| Mediator variable | Missing/not missing at 8 weeks | Therapy sessions | IMP doses | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| FAS score | Missing | 70 | 12.54 (12.53) | 70 | 10.60 (11.53) |
| Not missing | 523 | 25.48 (12.90) | 523 | 22.93 (11.52) | |
| MoCA score | Missing | 67 | 11.78 (11.92) | 67 | 10.21 (11.33) |
| Not missing | 526 | 25.50 (12.91) | 526 | 22.90 (11.53) | |
| BI score | Missing | 66 | 11.38 (11.26) | 66 | 9.26 (9.79) |
| Not missing | 527 | 25.53 (12.94) | 527 | 23.0 (11.58) | |
| NEADL score | Missing | 66 | 11.79 (11.58) | 66 | 9.56 (10.04) |
| Not missing | 527 | 25.48 (12.96) | 527 | 22.96 (11.60) | |
Following the Baron and Kenny83 steps in establishing mediation, there was no evidence that any of the variables investigated potentially mediated the effect of treatment on walking independently at 8 weeks (Table 88). There was weak evidence that treatment group was associated with the amount of therapy (path a) but no evidence that the amount of therapy affects walking independently at 8 weeks. Similarly, there was also some evidence that treatment group was associated with the number of IMP doses (path a) but not that drug dose affects the primary outcome.
| Mediator variables | Path | |||||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Number of therapy sessions | –1.846 (–3.743 to 0.051) | 0.057 | 0.009 (–0.007 to 0.025) | 0.267 | –0.242 (–0.637 to 0.152) | 0.229 |
| Number of IMP doses | –1.968 (–3.698 to –0.238) | 0.026 | 0.008 (–0.009 to 0.025) | 0.331 | –0.241 (–0.636 to 0.153) | 0.231 |
| FAS score | 0.155 (–1.099 to 1.409) | 0.808 | –0.051 (–0.083 to –0.019) | 0.002 | –0.175 (–0.614 to 0.265) | 0.436 |
| MoCA score | –0.675 (–1.589 to 0.239) | 0.148 | 0.042 (–0.001 to 0.084) | 0.056 | –0.204 (–0.635 to 0.227) | 0.354 |
| BI score | –0.365 (–1.026 to 0.295) | 0.278 | 0.656 (0.521 to 0.791) | < 0.001 | –0.283 (–0.891 to 0.325) | 0.362 |
| NEADL score | 0.971 (–1.322 to 3.263) | 0.407 | 0.112 (0.086 to 0.138) | < 0.001 | –0.318 (–0.814 to 0.178) | 0.209 |
| Joint, neck or back pain | 0.169 (–0.252 to 0.590) | 0.431 | –0.133 (–0.650 to 0.385) | 0.616 | –0.188 (–0.622 to 0.246) | 0.396 |
| Model | OR (95% CI) | p-value |
|---|---|---|
| ITT primary analysis | 0.777 (0.524 to 1.151) | 0.208 |
| Strict compliance | 0.868 (0.282 to 2.676) | 0.806 |
| Relaxed timing compliance | 0.701 (0.354 to 1.391) | 0.310 |
| Relaxed timing and motor therapy compliance | 0.769 (0.412 to 1.437) | 0.411 |
| Relaxed drug intake compliance | 0.882 (0.540 to 1.440) | 0.615 |
Glossary
- Carer
- An individual identified by the patient as their main informal carer who provides the patient with practical support a minimum of once per week.
- Code-break envelopes
- Contain coded documents that enable unblinding of the treatment allocation of an individual patient. They are held by the Clinical Trials Research Unit safety team and the NHS trust pharmacy responsible for the backup of this process as per the protocol.
- Investigational medicinal product
- Defined in the Directive 2001/20/EC, Article 2 (d) as: . . . a pharmaceutical form of an active substance or placebo being tested or used as a reference in a clinical trial, including products already with a marketing authorization but used or assembled (formulated or packaged) in a way different from the authorised form, or when used for an unauthorised indication, or when used to gain further information about the authorised form.Reproduced with permission from Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001. URL: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/dir_2001_20/dir_2001_20_en.pdf (accessed July 2018); © European Union, 1995–201819952018© European Union
- Kit number
- A random code used to identify each treatment code allocation and container of investigational medicinal product (e.g. vial, box, bottle).
- Rehabilitation
- Occupational/physical therapy that addresses physical functioning (e.g. sitting practice, standing, dressing, kitchen skills).
- Researcher
- The research nurse/therapist at each trust who is responsible for the outcome assessment. This person may be a Dopamine Augmented Rehabilitation in Stroke researcher or be employed by a local National Institute for Health Research Stroke Research Network.
List of abbreviations
- A&E
- accident and emergency
- ABILHAND
- ABILHAND Manual Ability Measure
- AE
- adverse event
- AISCT
- Acute Ischaemic Stroke Classification Template
- BI
- Barthel Index
- CACE
- complier-average causal effect
- CBS
- Caregiver Burden Scale
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CRAG
- Consumer Research Advisory Group
- CRF
- case report form
- CT
- computerised tomography
- CTRU
- Clinical Trials Research Unit
- DARS
- Dopamine Augmented Rehabilitation in Stroke
- DICOM
- Digital Imaging and Communication in Medicine
- DMEC
- Data Monitoring and Ethics Committee
- DVD
- digital versatile disc
- DWI
- diffusion-weighted imaging
- EQ-5D
- EuroQol-5 Dimensions
- FAS
- Fatigue Assessment Scale
- FIM
- Functional Independence Measure
- FLAIR
- fluid-attenuated inversion recovery
- fMRI
- functional magnetic resonance imaging
- GHQ-12
- General Health Questionnaire 12-item version
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning, Disability and Health
- IMP
- investigational medicinal product
- IST-3
- Third International Stroke Trial
- ITT
- intention to treat
- LACI
- lacunar infarction
- MCA
- middle cerebral artery
- MD
- mean difference
- MedDRA
- Medical Dictionary for Regulatory Activities
- MoCA
- Montreal Cognitive Assessment
- MRI
- magnetic resonance imaging
- mRS
- Modified Rankin Scale
- MSK-SSP
- musculoskeletal – symptoms/signs and pain
- NEADL
- Nottingham Extended Activities of Daily Living
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OLS
- ordinary least squares
- OR
- odds ratio
- OT
- occupational therapy
- PACI
- partial anterior circulation infarction
- PI
- principal investigator
- POCI
- posterior circulation infarction
- PSSRU
- Personal Social Services Research Unit
- PT
- physical therapy
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RMI
- Rivermead Mobility Index
- SAE
- serious adverse event
- SAR
- site achievement report
- SD
- standard deviation
- SE
- standard error
- SRN
- Stroke Research Network
- SSRI
- selective serotonin reuptake inhibitor
- TACI
- total anterior circulation infarction
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
- WTP
- willingness to pay