Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 08/52/01. The contractual start date was in March 2010. The final report began editorial review in March 2018 and was accepted for publication in March 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Alessio Pigazzi is a consultant and proctor for Intuitive Surgical Inc. (Sunnyvale, CA, USA) and receives personal fees from Covidien plc (Medtronic plc; Dublin, Ireland) and Ethicon, Inc. (Somerville, NJ, USA) outside the submitted work. David Jayne is a proctor for Intuitive Surgical Inc. and was formerly a member of the Efficacy and Mechanism Evaluation (EME) Strategy Group and the EME Prioritisation Group, and was previously involved in an EME Intraoperative Imaging Review. Claire Hulme was formerly a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Commissioning Board. Julia Brown is a member of the HTA Clinical Evaluation and Trials Board, HTA Funding Board Policy Group, HTA Mental, Psychological and Occupational Health Methods Group, HTA Post-Board Funding Teleconference Group and NIHR Standing Advisory Committee.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Jayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Total mesorectal excision (TME) is the standard of care in rectal cancer surgery, involving complete removal of the tumour along with the draining lymphatics within an intact mesorectal envelope. 1 The feasibility and safety of laparoscopic surgery has been established for colon cancer. 2–4 The case for rectal cancer is less clear, and, of the reported multicentre trials at the time of study design in 2010, only the MRC CLASICC trial included an evaluation of laparoscopic rectal cancer surgery compared with open rectal cancer surgery. 5 Although both laparoscopic and open rectal cancer resection were associated with similar lymph node yields, concern was expressed at the higher rate of circumferential resection margin (CRM) involvement in the laparoscopic group (12.4%) than in the open group (6.3%) for patients undergoing anterior resection (AR). This, however, did not translate into a difference in local recurrence at either 3-year2 or 5-year follow-up. 6 The difference in CRM involvement was felt to reflect the increased technical difficulties associated with the laparoscopic technique in the rectal cancer subgroup. This was supported by the higher conversion rate in the laparoscopic rectal subgroup (34%) than the laparoscopic colon subgroup (25%). 5 Analysis of CLASICC data revealed higher morbidity and mortality rates associated with laparoscopic cases converted to open operation. Some of this increased morbidity may be related to more advanced cancers requiring conversion, but a proportion of it will inevitably have resulted from the increased operative time, increased technical difficulty and the need for a laparotomy wound in converted cases.
Since completion of the CLASICC trial, there have been several other large studies comparing laparoscopic with open surgery for rectal cancer. A large European randomised controlled trial, COLOR II, recruited 1103 participants to a non-inferiority study involving 30 centres in eight countries. 7 Laparoscopic surgery was reported to be advantageous in terms of short-term outcomes (quicker return of bowel function, reduced hospital stay), with similar morbidity and pathological outcomes to open surgery. The 3-year results from the same study were reported in 2015 and showed similar rates of locoregional recurrence and disease-free survival (DFS) and overall survival (OS) in both the laparoscopic and the open groups. 8 These findings were echoed by the results of the COREAN trial, which again reported better short-term outcomes following laparoscopic rectal cancer resection and similar pathological outcomes compared with open surgery. 9
In contrast, there have been two large randomised trials, ALaCaRT10 and ACOSOG,11 that have cast doubt on the benefits of laparoscopic rectal cancer surgery compared with open rectal cancer surgery. Both were non-inferiority studies and both used a novel composite primary outcome combining rates of negative circumferential and distal cancer margins with completeness of mesorectal excision as a measure of oncological clearance. Both studies failed to demonstrate the non-inferiority of the laparoscopic compared with the open surgery approach, concluding that the evidence was not sufficient to support the routine use of the laparoscopic technique.
Robotic-assisted laparoscopic surgery was introduced into clinical practice in the early 1990s with the promise to eliminate many of the technical difficulties inherent in laparoscopic surgery. The technical advantages associated with robotic-assisted surgery include intuitive manipulation of the laparoscopic instruments with 7 degrees of freedom of movement, a three-dimensional field of view, a stable camera platform with zoom magnification, dexterity enhancement and an ergonomic operating environment.
The feasibility of robotics for TME rectal cancer resection was established by Pigazzi et al. in a series of six low rectal cancers. 12 A subsequent follow-up study of 39 rectal cancers treated prospectively by robotic-assisted resection reported a zero rate of conversion with a mortality of 0% and morbidity of 12.8%. 13 The only randomised trial at the time of design of the ROLARR study compared 18 patients assigned to robotic-assisted resection with 18 patients assigned to standard laparoscopic resection. 14 No difference was observed in the operative times, the conversion rates (two laparoscopic, zero robotic), or the quality of mesorectal resection. The only difference was the length of hospital stay, which was significantly shorter following robotic-assisted laparoscopic surgery (robotic assisted: 6.9 ± 1.3 days; standard laparoscopic: 8.7 ± 1.3 days; p < 0.001) and attributed by the authors to a reduction in surgical trauma. Since the commencement of the ROLARR trial, there have been numerous reports from single centres, analyses of national databases,15 and several systematic reviews and meta-analyses,16–18 but no large randomised comparison with laparoscopic or open rectal cancer surgery. Results from the meta-analyses tell a broadly similar story, with no clear advantage for robotic over laparoscopic surgery in terms of short-term outcomes, with the exception of lower conversion rates and a suggestion of improved postoperative bladder and sexual function. 19 The disadvantage of robotic surgery, compared with laparoscopic surgery, appears to be the longer operating times and perhaps an increase in operative blood loss. Importantly, the hospital costs associated with the use of the robot are higher, which has fuelled the ongoing debate about whether or not robotic-assisted rectal cancer surgery can be justified in the absence of clear patient benefits and considering its higher hospital costs. 15,20,21
The ROLARR trial was designed with the above concerns in mind and with the primary objective to evaluate the short-term safety and efficacy of robotic-assisted surgery compared with laparoscopic surgery for rectal cancer resection. The primary end point chosen was conversion to open surgery, on the basis that if the robot offered a technical advantage over laparoscopic surgery it should be reflected in a reduced conversion rate. Secondary end points were chosen to reflect the oncological nature of the investigation and the compelling need for rigorous patient-reported outcomes and cost-effectiveness evaluation.
Chapter 2 Methods
Objectives
The purpose of the trial was to perform a rigorous evaluation of robotic-assisted rectal cancer surgery by means of a randomised controlled trial. The chosen comparator was standard laparoscopic rectal cancer resection, which is essentially the same procedure but without the use of the robotic device. The two operative interventions were evaluated for short- and longer-term outcomes. The key short-term outcomes included assessment of technical ease of the operation, as determined by the clinical indicator of low conversion rate to open operation, and clear pathological resection margins as an indicator of surgical accuracy and improved oncological outcome. In addition, quality-of-life (QoL) assessment and analysis of cost-effectiveness were performed to aid evidence-based knowledge to inform the NHS and other service providers and decision-makers. The short-term outcomes were analysed after the last randomised patient had had 6 months of follow-up, to provide a timely assessment of the new technology, and were made available to the public, clinicians and health-care providers to inform health-care decision-making. Longer-term outcomes concentrated on oncological aspects of the disease and its surgical treatment with analysis of DFS, OS and local recurrence rates at 3 years’ follow-up.
Trial design
The ROLARR trial was an international, multicentre, prospective, unblinded, parallel-group randomised controlled trial22 comparing robotic-assisted with laparoscopic surgery for the curative treatment of rectal cancer (defined as an adenocarcinoma whose distal extent was situated at or within 15 cm of the anal margin) by low anterior resection (LAR), high anterior resection (HAR) or abdominoperineal resection (APR). The trial design required that each participating surgeon had performed a minimum of 30 minimally invasive (laparoscopic or robotic) rectal cancer resections (at least 10 laparoscopic and at least 10 robotic). The trial received national ethics approval in the UK and either ethics committee or institutional review board (IRB) approval as was required at the location of each of the international centres; all participants gave written informed consent. The trial conduct was overseen by an independent Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC). The trial was registered on the International Standard Randomised Controlled Trial Number (ISRCTN) register (ISRCTN80500123).
Participants
The inclusion criteria were:
-
Aged ≥ 18 years.
-
Able to provide written informed consent.
-
Diagnosis of rectal cancer (defined as an adenocarcinoma for which distal extent is situated at or within 15 cm of the anal margin, as assessed by endoscopic examination or radiological contrast study) amenable to curative surgery by LAR, HAR or APR, for example, staged T1–3, N0–2, M0 by imaging as per local practice. Although not mandated, computed tomography (CT) imaging with either additional magnetic resonance imaging (MRI) or transrectal ultrasound is recommended to assess distant and local disease.
-
Rectal cancer suitable for resection by either standard laparoscopic procedure or robotic-assisted laparoscopic procedure.
-
Fit for robotic-assisted or standard laparoscopic rectal resection.
-
An American Society of Anesthesiologists (ASA) physical status of ≤ 3.
-
Capable of completing required questionnaires at time of consent (provided questionnaires were available in a language spoken fluently by the participant).
The exclusion criteria were:
-
benign lesions of the rectum
-
benign or malignant diseases of the anal canal
-
locally advanced cancers not amenable to curative surgery
-
locally advanced cancers requiring en bloc multivisceral resection
-
synchronous colorectal tumours requiring multisegment surgical resection (a benign lesion within the resection field in addition to the main cancer would not exclude a patient)
-
coexistent inflammatory bowel disease
-
clinical or radiological evidence of metastatic spread
-
concurrent or previous diagnosis of invasive cancer within 5 years that could confuse diagnosis (non-melanomatous skin cancer or superficial bladder cancer treated with curative intent were acceptable; other cases were individually discussed with the chief investigator)
-
history of psychiatric or addictive disorder or other medical condition that in the opinion of the investigator would preclude the patient from meeting the trial requirements
-
pregnancy
-
participation in another rectal cancer clinical trial relating to surgical technique.
Preoperative investigation and preparation was as per institutional protocol. Laparoscopic mesorectal resection was performed in accordance with each surgeon’s usual practice. Robotic surgery involved either a totally robotic approach or a hybrid approach; the only absolute requirement was that the robot had to be used for mesorectal resection. For the purposes of the trial, a totally robotic operation was defined as a resection of the entire surgical specimen with the use of robotic assistance. A hybrid operation was defined as use of laparoscopic techniques to mobilise the proximal colon, with robotic assistance employed to perform the rectal mesorectal dissection. It was permissible to perform a partial mesorectal excision with a suitable distal margin, rather than a TME.
The specifics of each operation were at the discretion of the operating surgeon (e.g. port-site placement, mobilisation of the splenic flexure, inferior mesenteric artery/vein division, high vs. low vascular division, etc.), as was the decision to convert to an open operation. Detailed guidance was provided to ensure consistent histopathological analysis and reporting of the rectal dissection specimens in accordance with internationally agreed criteria. 23 Digital photographs of the anterior and posterior of the specimen and sequential cross-sectional views of the surgical specimen, as well as close-ups of the front and back of the levator/anal sphincter (if appropriate), were collected (prior to dissection) to allow blinded assessment of the quality of the plane of surgery. To enable a central pathology review, the tissue slides (or high-quality digital slide images) were submitted.
Postoperative care was as per institutional protocol; however, the protocol required that patients underwent a clinical assessment at 30 days and at 6 months post operation. Any further visits were in accordance with local standard clinical practice. Follow-up data were collected on an annual basis until the last participant reached 3 years post randomisation.
Participants completed questionnaires prior to randomisation (baseline), and at 30 days and 6 months postoperatively. General QoL [Short Form questionnaire-36 items version 2 (SF-36v2)] and fatigue [Multidimensional Fatigue Inventory-20 (MFI-20)] data were collected at baseline and at the 30-day and 6-month postoperative visits. In addition, bladder and sexual function questionnaires [International Prostatic Symptom Score (I-PSS) and International Index of Erectile Function/Female Sexual Function Index (IIEF/FSFI)] were completed by patients at baseline and at 6 months post operation. Participants in the UK and USA also completed the EuroQol-5 Dimensions (EQ-5D) at baseline and at 30 days and at 6 months post operation, and a resource utilisation questionnaire at 30 days and at 6 months post operation for the health economic component of the trial.
The SF-36v2,24 a well-validated, multipurpose standard health-related QoL evaluation questionnaire, was used to assess generic QoL. It generates an eight-scale profile of functional health and well-being scores, as well as summary measures of physical and mental health. This information related to the previous 4-week time period.
The MFI-20 was used to assess fatigue;25 it is a 20-item self-report validated instrument designed to measure current fatigue. It creates a global score as well as individual scale scores that cover the following dimensions: general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue.
The I-PSS26 was used to assess bladder function. This questionnaire includes seven questions relating to lower urinary tract function, which form an overall symptom score that can be used to classify bladder dysfunction as mild, moderate or severe. To assess sexual function, the IIEF27 and FSFI28 were used. Both are brief male-/female-specific questionnaires developed to assess various domains of sexual function. The IIEF, FSFI and I-PSS questionnaires obtained information relating to the patient’s functioning over the previous 4 weeks.
For the health economic analysis, the EQ-5D questionnaire was used to assess self-reported utility. This is a standardised non-disease-specific instrument that describes and values health-related QoL and provides a single index value for a number of different health states. In addition, the resource utilisation questionnaire collected information on community-based medical resource usage [e.g. general practitioners (GPs), nurses, physiotherapists/occupational therapists, outpatients and medications]. Please refer to Appendix 12 for a summary of protocol changes.
End points
Primary end point
Rate of intraoperative conversion to open surgery
Conversion to open surgery was defined as the use of a laparotomy wound for any part of the mesorectal dissection. The use of a small abdominal wound to facilitate a low, stapled anastomosis and/or specimen extraction was permissible and not considered as a conversion to open surgery. The decision to convert to an open operation was at the discretion of the operating surgeon. Details relating to the planned and actual operation were collected on the baseline and operative case report forms (CRFs).
Key secondary end points
Pathological circumferential resection margin positivity
Pathological circumferential resection margin positivity (CRM+) was defined as a distance of ≤ 1 mm of the cancer from the CRM as recorded on the local histopathology review.
Three-year local recurrence
Local recurrence was defined as evidence of locoregional disease within the surgical field. Time to local recurrence was calculated from the date of randomisation to the date of local recurrence, defined as the date of the relevant assessment (i.e. clinical, radiological and pathological) that first detected the local recurrence.
Further secondary end points
Intraoperative complications
Defined as adverse events occurring during surgery related to the surgical or related procedures (e.g. anaesthetic).
Thirty-day postoperative complications
Defined as an adverse event occurring during the first 30 days postoperatively and related to surgery or related procedures (e.g. anaesthetic).
Six-month postoperative complications (after 30 days)
Defined as an adverse event occurring during the first 6 months (after 30 days) postoperatively and related to surgery or related procedures (e.g. anaesthetic).
Thirty-day postoperative mortality
Defined as death from any cause within 30 days postoperatively.
Patient self-reported bladder function
Assessed by the patient self-reported I-PSS.
Patient self-reported sexual function
Assessed in males by the patient self-reported IIEF questionnaire and in females by the patient self-reported FSFI questionnaire.
Patient self-reported generic health
Assessed by the patient self-reported SF-36v2 questionnaire.
Patient self-reported fatigue
Assessed by the patient self-reported MFI-20 questionnaire.
Quality of the plane of surgery
Defined by the grading criteria using the local histological review. For an AR there was only one criterion: the quality of the mesorectum. For APR, the quality of the plane of surgery was assessed by the grade for the mesorectum and a second grade for the anorectal dissection below the levators. The quality of resection of the mesorectum was assessed as muscularis propria plane (worst), intramesorectal plane (intermediate) and mesorectal plane (best). The quality of surgery of the anorectum below the levators was assessed as intrasphincteric/submucosal plane (worst), sphincteric plane (intermediate) and levator plane (best).
Three-year disease-free survival
Disease-free survival time was defined as the time from date of randomisation to date of death from any cause, recurrent disease (locoregional or distant recurrence) or occurrence of a second primary cancer.
Three-year overall survival
Overall survival time was calculated from the date of randomisation to the date of death from any cause.
-
Health economics evaluation (see Health economic evaluation).
Pathology central review
Local pathology data were used to carry out the analyses. A central blinded review of the local pathology data for all assessable patients was carried out. The agreement of local pathology and central pathology with respect to factors feeding into the analyses (e.g. T-staging) was assessed via summaries.
Sample size
Original sample size calculation and justification
The sample size calculation was based on ensuring that sufficient numbers of patients were recruited to address the primary end point of conversion to open rectal resection. A relative reduction of at least 50% (in absolute terms, 25% to 12.5% in the robotic-assisted laparoscopic group) was strongly believed to be achievable and also represented an extremely clinically important difference, not only in terms of outcomes for health-care providers but also in terms of patient-related outcomes, as it had been shown that patients who convert during surgery have worse outcomes. Therefore, using a conversion rate of 25% for standard laparoscopic surgery and a 50% relative reduction to be clinically relevant, with 80% power and a 5% (two-sided) significance level, 336 patients were required using a two-group continuity corrected chi-squared test of equal proportions (nQuery Advisor 6.01, Statistical Solutions, Saugus, MA, USA). Therefore, it was planned to recruit 400 patients (200 per group) to allow for early withdrawals, cross-over, protocol violations (e.g. benign tumours) and missing follow-up data.
Updated sample size
Recruitment to the original target sample size of 400 patients was completed 5 months earlier than planned and was under budget. Note that the original sample size of 400 patients aimed to achieve 80% power. Although this is conventionally considered to be sufficient, it is also commonly argued that 90% power is preferable. Given this, coupled with the fact that there was the opportunity to continue recruitment as a result of reaching the target of 400 patients early and under budget, we proposed to continue to recruit to the ROLARR trial until the date that was originally set to end recruitment. The aim of recruitment during this period was to recruit as many additional patients as possible to maximise power, up to a maximum of 520 patients (which, under the original sample size assumptions, would provide 90% power to detect a difference of at least 12.5% in conversion rates between the groups). This plan was endorsed by the EME programme, the DMEC and the TSC. This decision to continue recruitment was made before seeing any data or interim analyses. Consequently, a total of 471 patients had been randomised by the time the trial closed to recruitment. Under the original sample size assumptions, this provides around 86% power to detect a difference of at least 12.5% in conversion rates between the groups.
Randomisation
Randomisation took place as soon as possible after consent was obtained and after patients had completed their baseline patient-reported questionnaires (I-PSS, IIEF/FSFI, SF-36v2, MFI-20, EQ-5D). Randomisation took place as close to the date of surgery as possible. Surgeons were strongly encouraged to consent and randomise patients within 14 days of the planned surgery date whenever possible.
Following confirmation of written informed consent and eligibility, patients were randomised into the trial by authorised members of staff at the trial sites. Randomisation was performed centrally using the Clinical Trials Research Unit (CTRU) automated 24-hour telephone randomisation system. Authorisation codes and personal identification numbers (PINs), provided by the CTRU, were required to access the randomisation system.
Patients were randomised on a 1 : 1 basis to receive either robotic-assisted or standard laparoscopic rectal cancer surgery and were allocated a unique trial number. A computer-generated minimisation programme that incorporated a random element was used, with the following minimisation factors:
-
treating surgeon
-
patient sex (male or female)
-
neo-adjuvant therapy (yes or no)
-
nature of intended procedure (HAR, LAR or APR)
-
body mass index (BMI) [calculated automatically from height (cm) and weight (kg) provided at randomisation and classified according to World Health Organization (WHO) criteria29]:
-
underweight/normal
-
overweight
-
obese class I
-
obese class II
-
obese class III.
-
Participating research sites were required to complete a log of all patients screened for eligibility who were not randomised either because they were ineligible or because they declined participation. Anonymised information was collected including:
-
age
-
sex
-
date screened
-
reason not eligible for trial participation
-
eligible but declined and reason for this
-
other reason for non-randomisation.
Blinding
As the two surgical procedures create incisions that can allow the patient to be blinded to the operative procedure performed, it arguably would have been scientifically preferable to blind patients to their surgical procedure, particularly in respect of patient-reported outcomes. However, it was anticipated that in practice maintaining the blind would have been extremely problematic (e.g. in countries such as the USA where private health-care insurance companies require disclosure of surgery details). Furthermore, it was anticipated that patients would also be seen by many health-care professionals throughout their time in the trial, increasing the risk that the blind may be broken. As a consequence, the trial design did not involve blinding patients to the operative procedure.
It should be noted that the trial end points are mainly objective measures and a central blinded assessment of these measures was included when possible (e.g. blinded central assessment of the quality of the plane of surgery).
Statistical methods
Unless otherwise stated, all analyses were prespecified and conducted on the intention-to-treat population (i.e. all randomised patients were categorised into treatment groups based on their randomisation, regardless of what treatment they subsequently received). All hypothesis tests were two-sided and conducted at the 5% level of significance. Estimates and their corresponding 95% confidence intervals (CIs) and p-values are presented for fixed effects. For the (random) surgeon effect, the intracluster correlation coefficient (ICC), estimated via the analysis-of-variance method, and bias-corrected bootstrapped 95% CIs are reported.
For most end points there was only a small number of missing data, such that a complete-case analysis was appropriate. For end points with non-negligible numbers of missing data, exploratory analyses were performed to consider the potential impact of the missing data. All models were fitted using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
All analyses, unless otherwise stated, adjusted for the minimisation factors only (see Randomisation). For each end point, sensitivity analyses to include adjustment for treating centre and country were considered; Subgroup analyses gives further details of this.
Primary end point: conversion to open surgery
The primary analysis was a complete-case analysis. Multilevel logistic regression was used to estimate the odds ratios (ORs) between treatment groups for conversion to open surgery, adjusting for all minimisation factors. All minimisation factors were included as fixed effects except intended operating surgeon, which was included as a random effect. A random intercept model was fitted first, then a model with both a random intercept and a random slope (i.e. random treatment effect) was fitted (hereafter referred to as the ‘random slope’ model). The need for the random slope term was assessed via consideration of a likelihood ratio test and the Akaike information criterion (AIC). The models were fitted using SAS version 9.4 glimmix procedure.
A number of prespecified sensitivity analyses were also performed.
Additional covariates
Fixity of tumour, whether or not the tumour was an obstructing tumour, T-stage or N-stage, whether or not the patient had abdominal surgery prior to their ROLARR operation and the level of scarring, whether or not adhesions were identified and whether or not there was a tumour perforation (non-iatrogenic) or abscess were all considered for inclusion in the model via examination of their effect on the model fit.
Actual operating surgeon
The primary analysis adjusted for the minimisation factors (i.e. the values of those factors that were used in the minimisation, regardless of whether or not those values were correct). In some cases, patients may have been allocated treatment under incorrect minimisation factors. In particular, their intended operating surgeon (used for minimisation) may not have been their actual operating surgeon. A sensitivity analysis was performed that incorporated actual operating surgeon rather than intended operating surgeon as a random effect in the model.
Learning effects
For each surgeon, the number of robotic-assisted and laparoscopic rectal operations relevant to the ROLARR trial performed by that surgeon was collected at regular intervals throughout the trial. From this, the number of ROLARR-relevant robotic-assisted and laparoscopic operations previously performed by the operating surgeon before each patient’s operation was derived, assuming that the timings of all counted previous operations were uniformly distributed across the interval in which they occurred. These patient-level covariates (‘number of previous robotic operations’ and ‘number of previous laparoscopic operations’) were included in the multilevel model used in the primary analysis to explore potential associations between increased numbers of operations and patient outcomes. Interactions between the numbers of operations performed and the treatment effect were also considered.
Actual operation (post hoc)
The primary analysis adjusted for the minimisation factors (i.e. the values of those factors that were used in the minimisation, regardless of whether or not those values were correct). In some cases, patients may have been allocated treatment under incorrect minimisation factors. In particular, their intended procedure (used for minimisation) may not have been the actual procedure that they received. A sensitivity analysis was performed that incorporated actual procedure rather than intended procedure as a fixed effect in the model.
Key secondary end points
Circumferential resection margin positivity
The analysis of CRM+ was a complete-case analysis. Multilevel logistic regression was used to estimate the ORs between treatment groups for CRM+, adjusting for all minimisation factors. All minimisation factors were included as fixed effects except intended operating surgeon, which was included as a random effect. A random intercept model was fitted first, then a random slope model was fitted and the need for the random slope term was assessed via consideration of a likelihood ratio test and the AIC. The models were fitted using SAS version 9.4 glimmix procedure.
A number of prespecified sensitivity analyses were also performed.
Additional covariates
Fixity of tumour, T-stage and N-stage (post neo-adjuvant therapy) and whether or not there was a tumour perforation (non-iatrogenic)/abscess were all considered for inclusion in the model via examination of their effect on the model fit.
Three-year local recurrence
All patient follow-up, including follow-up beyond 3 years post randomisation, was incorporated into the analysis of local recurrence. Time to local recurrence was defined as the time from randomisation to the date of the relevant assessment (i.e. clinical, radiological and pathological) that first detected the local recurrence.
Differences in time to local recurrence between the treatment groups were estimated using a shared frailty model (Cox proportional hazards regression with mixed effects), including intended operating surgeon as a random effect. The models were fitted using SAS version 9.4 phreg procedure. The 3-year local recurrence rate was estimated using cumulative incidence functions for time to local recurrence, treating death as a competing risk.
Patients who were alive and without any local recurrence at the time of analysis were censored at the time they were last known to be alive and local recurrence free. If patients were lost to follow-up, they were also censored at the time they were last known to be alive and local recurrence free. Patients who died without any local recurrence were censored at date of death in analyses estimating treatment effects, but were classed as having a competing risk event in the analysis estimating incidence of local recurrence (as calculated using cumulative incidence functions) to avoid overestimation of cumulative incidence. 30 In certain non-standard circumstances (prespecified in the statistical analysis plan), patients were censored at time 0. Patients with non-standard circumstances who were censored at time 0 are summarised, and reasons are given in the results (see Chapter 3, Disease-free survival).
Further secondary end points
The analyses of further binary secondary end points – intraoperative complications, postoperative complications within 30 days, after 30 days and within 6 months, and quality of the plane of surgery (i.e. mesorectal plane Yes/No) – were complete-case analyses. Multilevel logistic regression was used to estimate the ORs between treatment groups for each end point, adjusting for all minimisation factors. All minimisation factors were included as fixed effects except intended operating surgeon, which was included as a random effect via a random intercept term. The models were fitted using SAS version 9.4 glimmix procedure.
For further continuous secondary end points [bladder function (I-PSS), sexual function in males (IIEF) and in females (FSFI), generic health-related QoL (SF-36v2) and fatigue (MFI-20)], multilevel generalised linear models were used to estimate the mean difference between treatment groups, adjusting for all minimisation factors and the baseline score. All minimisation factors were included as fixed effects except intended operating surgeon, which was included as a random effect via a random intercept term. The I-PSS, IIEF and FSFI were modelled using a two-level model: patients nested within surgeon. The SF-36v2 questionnaire and MFI-20 were modelled using a three-level model: repeated assessments within patient within surgeon. The models were fitted using the SAS version 9.4 glimmix procedure.
All patient follow-up, including follow-up beyond 3 years post randomisation, was incorporated into the analysis of DFS and OS. For DFS and OS, shared frailty models were used to estimate the hazard ratios (HRs) between treatment groups, adjusting for all minimisation factors. All minimisation factors were included as fixed effects except intended operating surgeon, which was included as a random effect via a random intercept term. In certain non-standard circumstances (prespecified in the statistical analysis plan), patients were censored at time 0. Patients with non-standard circumstances who were censored at time 0 are summarised and reasons are given in the results (see Chapter 3, Disease-free survival). The models were fitted using SAS version 9.4 phreg procedure.
Subgroup analyses
Subgroup analyses relating to the primary end point across sex, BMI class and procedure received, as well as relating to CRM+ across sex, BMI class and T-stage, were performed. Subgroup analyses relating to each of local recurrence, DFS and OS across type of operation, T-stage and neo-adjuvant therapy were also performed. All subgroup analyses tested heterogeneity of the treatment effect across the subgroups and also estimated the treatment effect within each subgroup, via the addition of an appropriate interaction term to the primary analysis model.
Model diagnostics
Multilevel logistic regression models
Model fit was assessed by examining the raw residuals on the probability scale outputted from SAS version 9.4 glimmix procedure, for example the residual for patient i:
in which:
and p^i is the predicted probability of the event (e.g. conversion to open surgery) for patient i [including empirical Bayes’ estimate (EBE) of the random effect]. Index plots (plots of the raw residuals vs. patient identification) were used to identify potential outliers. Empirical probability plots were also used to assess model fit and identify potential outliers. These plots plotted the observed, ordered Pearson residuals (outputted from SAS version 9.4 glimmix procedure) against expected, ordered Pearson residuals under the model assumptions – analogous to a normal Q–Q plot for normal-errors regression. The expected sampling distributions of Pearson residuals were determined empirically via simulations. Specifically, for each simulation each patient’s outcome was randomly drawn from a Bernoulli (p) distribution with:
for patient i.
The model was refitted to this simulated data set and the Pearson residuals recorded.
This was repeated 100 times to yield an empirical sampling distribution of Pearson residuals for each patient. In the empirical probability plot, the actual observed Pearson residuals for each patient were plotted against the median, 2.5th percentile and 97.5th percentile of the empirical sampling distribution. Observations were considered to be potential outliers if they lay outside the 2.5th percentile to 97.5th percentile range [analogous to considering Pearson residuals lying outside the interval (–2,2) to be potential outliers in a normal-errors regression].
Overly influential observations on the treatment effect regression coefficient were identified via the calculation of exponentiated delta-betas. The exponentiated delta-beta was calculated for each patient; for example, for patient i, the exponentiated delta-beta for the treatment effect regression coefficient was:
in which β1 is the regression coefficient for the treatment effect in the full model and β1(i) is the treatment effect regression coefficient in the model where patient i has been omitted. Note that this is the ratio of the estimated ORs from the two models; for example, an exponentiated delta-beta for the treatment effect for patient i of 1.05 would imply that the inclusion of patient i increases the treatment effect OR estimate by 5% compared with the omission of patient i. The exponentiated delta-betas were plotted against patient identifier (ID) in order to visually identify highly influential observations.
Shared frailty models
Models were refitted as Cox proportional hazards models with robust standard errors, without the random effect for operating surgeon. This gave the same point estimates as the shared frailty model, and broadly similar standard errors. Deviance residuals were used to identify any potential outliers. The proportional hazards (PH) assumption for the treatment effect was assessed via a plot of the standardised Schoenfeld residuals over time, as well as a plot of the observed standardised score process versus simulated standardised score processes under PH. The PH assumption was also tested via the Supremum test. Exponentiated delta-betas (as described in Multilevel logistic regression models) were used to identify overly influential observations.
Health economic evaluation
An economic evaluation was performed using a UK NHS perspective to aid the development of an evidence base to support NHS service providers and budget holders in their decision-making processes. Costs associated with robotic surgery excluded acquisition and maintenance costs. The evaluation estimated the expected incremental cost-effectiveness of robotic resection compared with laparoscopic resection at 6 months. It was planned that this would be extrapolated using a decision-analytic model to estimate lifetime cost-effectiveness.
The ROLARR trial collected information on the nature of all initial resection operations using trial CRFs. This included information on the type of operation and resources used within this operation, including instrumentation and times for operation theatres and staff. CRF data also captured information on postoperative and distal complications on all trial patients.
However, many types of resource utilisation were not collected for all patients in the ROLARR trial. In particular, given the challenges of conducting research within global trials, data on resource utilisation after the initial operation were collected only on patients from the UK and the USA. As the adjuvant chemotherapy is likely to both vary widely and be expensive, there is a danger that any small differences at this stage will be both unrelated to the surgery received and, given the cost of chemotherapy, outweigh any cost differences that are related to the intended type of surgery. For this reason, the cost data do not attempt to consider the chemotherapies received or antinausea drugs attached to these chemotherapies.
For patients in the UK and USA, information was collected alongside the trial-related questionnaires at approximately 30 days and at 6 months. It was expected that data collection might be poor and as a result it would be necessary to impute data for a substantial number of these patients.
Data were collected on both primary care and secondary care, including contact with GPs and primary care physicians (including the location of any contacts), nurse contacts (including at primary care, district/at home nursing, and stoma nursing) and outpatient visits.
The analysis considered costs in GBP with 2015 as the base year, from an NHS-payer perspective. Given the focus of this perspective, when clinical practice appeared to differ in the USA, particularly around pain medication, the approach taken was the one that appeared to apply in the UK. This perspective also means that unit costs are those costs that apply within the NHS.
Costing individual resource utilisation
Surgery
Surgical costs were computed by first identifying an overall global average for resection surgeries, for which a weighted sum of non-elective complex large intestine operative costs was used. Individualised surgical costs allowed for both excess bed-days (at £326.11 per day) and differences in the time and staffing within the theatre. For the laparoscopic group, costs were calculated based on data provided on the operative team and time, and it was assumed that on average this group would cost the same as the baseline surgical cost. Therefore, those patients receiving a laparoscopic operation might have had a cheaper or more extensive operation than ‘average’ but would be similar overall. Given that robotic surgeries were expected to require longer use of the operating theatre (e.g. including greater setup time), incorporating staffing/time costs modifies the resection surgery costs to reflect this. The instrumentation costs were included separately for those items that, in the opinion of the chief investigator, would not necessarily be considered an automatic inclusion within the operating theatre. (So, for instance, although data were collected on suction, this is not costed.)
As staff costs appear below (Table 1), and instrument costs are assessed, it is not appropriate to include these. Excluding these costs, the use of theatres costs £339 per hour.
| Surgical costs | Unit | Cost (£) | Source |
|---|---|---|---|
| Baseline resection surgery: operative cost | Per operation | 8307.78 | a NHS Reference Costs 2014 to 201531 |
| Baseline resection surgery: operative cost – excess bed-days | Per excess bed-dayb | 326.11 | a NHS Reference Costs 2014 to 201531 |
| Other surgeries for complications | Per operation | 8307.78 | c NHS Reference Costs 2014 to 201531 |
| Surgeon | Per hour | 138.00 | Personal Social Services Research Unit32 |
| Anaesthetist | Per hour | 60.74 | Personal Social Services Research Unit32 |
| First surgical assistant (band 7) | Per hour | 60.00 | Personal Social Services Research Unit32 (including qualifications) |
| Subsequent surgical assistants (band 5) | Per hour | 43.00 | Personal Social Services Research Unit32 (including qualifications) |
| Operating theatre (no staff or specialist instruments) | Per hour | 339.00 | dDerived from Information Services Division, NHS National Services Scotland33 |
Instrumentation costs were identified in discussion with the chief investigator who provided data on which of the instruments to specifically include (and which were more or less trivial given operating theatre costs) and unit costs for each instrument (Table 2).
| Robotic | Unit | Cost (£) |
|---|---|---|
| Aspirator | Each | 150 |
| Bipolar forceps | Each | 150 |
| Vessel sealer | Each | 500 |
| Graspers | Each | 150 |
| Haemolock | Initial | 150 |
| Per clip | 30 | |
| Hook | Each | 150 |
| Needle driver | Each | 150 |
| Scissors | Each | 150 |
| Stapler | Per firing | 150 |
| Laparoscopic and open | ||
| Disposable Babcock | Each | 150 |
| Graspers | Each | 100 |
| Stapler (including open staplers) | Initial | 300 |
| Per reload | 80 | |
| Scissors | Each | 100 |
| Vascular clips | Each | 80 |
| Wound protector | Each | 50 |
| Wound retractor | Each | 50 |
| Vessel sealers (e.g. Ligasure) | Each | 500 |
An overall in-theatre cost was calculated for each surgery (where data were complete on the fields above) and calculated, including the theatre, staff and instrumental costs. The mean of these costs among the group who received laparoscopic surgery (as opposed to allocated) was identified, and this was subtracted from all individual cost figures to indicate how costs would be likely to differ from a typical operation. As such, the average laparoscopic difference is zero, although the robotic difference could be positive (if more expensive) or negative (if less expensive). The difference in the cost figure was then added back onto the reference cost to give an estimated cost for each individual surgery.
It should be noted that the analysis presented here does not include the cost of the surgical robot (when applicable), in order to provide an optimistic case figure for the cost-effectiveness of robotic surgery.
Other inpatient visits
The main inpatient costs assessed after the initial surgery were for stoma reversal operations and for other related colorectal surgeries identified from the CRFs. When other major related surgery was indicated, this was coded as an average of complex large intestine surgeries at £7621.24. 31
Following approaches used elsewhere, stoma reversals are coded as elective intermediate procedures (FZ50Z, intermediate large intestine procedures, aged ≥ 19 years) at £1691.06 per case. 31
The unit costs of all other inpatient visits are taken from NHS Reference Costs 2014 to 201531 and are shown in Table 3.
| Procedure | Code | Unit costsa (£) |
|---|---|---|
| Non-reversal stoma operations | FZ50Z. Elective inpatient | 1691.06 |
| Transient ischaemic attack | AA29F. Transient ischaemic attack with a CC score of 0–4 (Non-elective) | 1252.81 |
| Deep-vein thrombosis | YQ51E. Deep-vein thrombosis with a CC score of 0–2 | 1361.97 |
| Pulmonary embolism | DZ09 K. Pulmonary embolus with interventions, with a CC score of 0–8 | 3509.12 |
| Renal failure | Acute kidney injury without interventions, with a CC score of 0–3 | 1785.63 |
| LA07 K. Acute kidney injury with interventions, with a CC score of 0–5 | 3784.72 | |
| Abdominal infections, anastomotic leak | FZ36L Gastrointestinal infections with single intervention, with a CC score of 0–1 | 3610.31 |
| Inpatient urinary tract infections | LA04S. Kidney or urinary tract infections, without interventions, with a CC score of 0–1 | 1502.55 |
| Haemorrhage | FZ38P. Gastrointestinal bleed without interventions, with a CC score of 0–4 | 1370.09 |
| Cardiac events | EB12C. Unspecified chest pain with a CC score of 0–4 | 1088.68 |
| Protracted ileus | FZ13C. Minor therapeutic or diagnostic, general abdominal procedures, ≥ 19 years. Non-elective | 3471.40 |
| Urinary retention | LA09Q. General renal disorders without interventions, with a CC score of 0–2 | 1399.13 |
| Gastrointestinal obstruction | FZ27G. Intermediate therapeutic general abdominal procedures, ≥ 19 years and over, with a CC score of 0 | 3335.27 |
| High stoma output (not coded as serious though) | FZ50Z Intermediate large intestine procedures, ≥ 19 years | 1836.19 |
| Respiratory inpatient (non-infection) | DZ19 N. Other respiratory disorders without interventions, with a CC score of 0–4 | 1163.67 |
| Not specified | Average of all elective inpatients, all sources | 3573.02 |
Primary care
When possible, unit costs for GPs were taken from the Personal Social Services Research Unit (PSSRU) 201532 using qualification costs and direct care staff costs, with similar figures (without direct care staff costs) used for nurse-based visits. For primary care, GP costs assumed a mean duration of surgery visits of 11.7 minutes (£44) and of 11.4 minutes for home visits (plus 10 minutes of travel) (£81), and a cost of £27 for telephone consultations. Nurse costs were assessed based on location, assuming 15.5 minutes of contact for each visit, with an additional 10 minutes of travel for visits away from surgeries (surgery £14.47, other £21.25).
Outpatient and other health professional visits
Outpatient visits costed using unit costs (consultant-led outpatient attendances) from NHS Reference Costs 2014 to 15,31 after grouping most visits into colorectal surgery, gastroenterology, medical oncology, medical ophthalmology, trauma and orthopaedics, nephrology, urology and others. Accident and emergency visits were costed using the overall average of all emergency medical attendances with the NHS Reference Costs 2014 to 2015. 31
Contacts for many of the remaining health professionals were taken from the PSSRU costs, including occupational therapy (PSSRU 201532) and counselling (PSSRU 201434), and chiropody/podiatry (PSSRU 201035), with costs inflated to 2015 figures using either the HCHS (Health and Community Health Service) price index for health or mid-point changes in Agenda for Change pay bands. 36
Stoma costs
The costs of ongoing stoma were calculated from Jones,37 who reported on figures from the Cwm Taf Health Board (NHS Wales) (Table 4). This assumes a monthly cost of £84 per 30 colostomy bags and £94 per 30 ileostomy bags. These 2011/12 figures were inflated to 2014/15 figures using the HCHS price index. 32 It should be noted that this is likely to underestimate the true costs of stoma, as this does not include items such as wipes, although there do not appear to be clear, published figures available that include such items.
| Stoma costs | Unit | Unit cost | Source |
|---|---|---|---|
| Colostomy costs @ two bags per day (2011/12) | Per 30 days | £84.00 | Cwm Taf Health Board (Jones, 201537) |
| Ileostomy costs @ one bag per day (2011/12) | Per 30 days | £94.00 | Cwm Taf Health Board (Jones, 201537) |
| Inflation between 2011/12 and 2014/15 using HCHS (293.1 vs. 282.5) | 3.75% | ||
| Stoma reversals | Per operation | £1691.06 | a NHS Reference Costs 2014 to 201531 |
| Colostomy costs @ two bags per day (2011/12) | Per 30 days | £84.00 | Cwm Taf Health Board (Jones, 201537) |
| Ileostomy costs @ one bag per day (2011/12) | Per 30 days | £94.00 | Cwm Taf Health Board (Jones, 201537) |
| Inflation between 2011/12 and 2014/15 using HCHS (293.1 vs. 282.5) | 3.75% | ||
| Stoma reversals | Per operation | £1691.06 | a NHS Reference Costs 2014 to 201531 |
Medication costs
Medication costs were found by coding responses from UK/US patients on a line-by-line basis initially and then across all responses for specified pharmaceuticals (Table 5). When possible, individual statements about frequency and duration of treatment were used to inform assumptions about utilisation. In cases where no statements were made to identify utilisation, the British National Formulary38 was used to identify an indicative strength/dosage. Unit costs are taken from eMIT (the drugs and pharmaceutical electronic market information tool),39 when possible, or the NHS Indicative Drug Tariff40 figures, when not.
| Symptom | Drug | Unit | Cost (£) |
|---|---|---|---|
| Cardiac/statins | Simvastatin | Per 28 units | 0.16 |
| Cardiac/statins | Atorvastatin | Per 28 units | 0.49 |
| Cardiac/statins | Rosuvastatin | Per 28 units | 18.03 |
| Cardiac/statins | Doxazosin | Per 28 units | 0.19 |
| Cardiac/statins | Candesartan | Per 28 units | 0.55 |
| Cardiac/statins | Losartan | Per 28 units | 0.30 |
| Cardiac/statins | Olmesartan (Benicar®) | Per 28 units | 10.95 |
| Cardiac/statins | Lisinopril | Per 28 units | 0.29 |
| Cardiac/statins | Perindopril | Per 30 units | 0.61 |
| Cardiac/statins | Ramipril | Per 28 units | 0.27 |
| Cardiac/statins | Bisprolol | Per 28 units | 0.25 |
| Cardiac/statins | Metaprolol (plus unspecified beta blocker) | Per 28 units | 0.55 |
| Cardiac/statins | Carvedilol | Per 28 units | 0.59 |
| Cardiac/statins | Propranolol | Per 56 units | 1.67 |
| Cardiac/statins | Atenolol | Per 28 units | 0.18 |
| Cardiac/statins | Amlodipine | Per 28 units | 0.16 |
| Cardiac/statins | Feoldipine | Per 28 units | 0.55 |
| Cardiac/statins | Lercanidipine | Per 28 units | 1.42 |
| Cardiac/statins | Nifedipine | Per 56 units | 21.00 |
| Cardiac/statins | Cartia | Per 56 units | 41.87 |
| Cardiac/statins | Furosemide | Per 28 units | 0.13 |
| Cardiac/statins | Indapamide | Per 28 units | 1.02 |
| Cardiac/statins | Esomeprazole | Daily | 2.22 |
| Cardiac/statins | Lansoprazole | Per 28 units | 0.98 |
| Cardiac/statins | Glytrin | Daily | 1.13 |
| Cardiac/statins | Amiodarone | Once | 13.17 |
| Pain | Tramadola | Per 14 units | 1.67 |
| Pain | Paracetamolb | Per 16 units | 0.13 |
| Pain | Ibuprofen | Per 16 units | 0.17 |
| Pain | Codeine phosphatec | Per 28 units | 0.37 |
| Pain | Gabapentin | Per 100 units | 1.30 |
| Pain | Cocodamol | Per 30 units | 0.65 |
| Pain | Codrydramol | Per 30 units | 0.47 |
| Pain | Oramorph | Single use | 1.89 |
| Pain | Oxycodone | Per 56 units | 6.06 |
| Pain | Indomethacin | Single pack | 0.55 |
| Pain | Meloxicam | Single pack | 0.43 |
| Pain | Solaraze | Single pack | 0.67 |
| Pain | Naproxen | Per 28 units | 0.70 |
| Anticoagulants | Aspirin | Per 28 units | 0.14 |
| Anticoagulants | Clopidogrel | Per 30 units | 4.58 |
| Anticoagulants | Heparin | Per 10 units | 16.62 |
| Anticoagulants | Delteparin | Per 10 units | 51.22 |
| Anticoagulants | Warfarin | Per 28 units | 0.25 |
| Anticoagulants | Tinzaparin | Per 10 vials | 105.66 |
| Anticoagulants | Enoxaparin | Per 10 units | 20.86 |
| Anticoagulants | Rivaroxaban | Per 28 units | 50.40 |
| Antibiotics and immunological | Amoxicillin (plus unspecified antibiotics) | Per 21 units | 0.45 |
| Antibiotics and immunological | Trimethoprim | Per 14 units | 0.89 |
| Antibiotics and immunological | Nitrofurantoin | Per 28 units | 3.57 |
| Antibiotics and immunological | Cephalexin | Per 28 tablets | 0.73 |
| Antibiotics and immunological | Lexofloxacin | Per 5 tablets | 0.92 |
| Antibiotics and immunological | Bactrim | Per 28 units | 3.03 |
| Antibiotics and immunological | Oxytetracycline | Per 28 units | 0.43 |
| Antibiotics and immunological | Vancomycin | Per 28 units | 32.90 |
| Antibiotics and immunological | Ciprofloxacin | Per 20 units | 0.42 |
| Antibiotics and immunological | Metronidazole | Per 21 tablets | 0.39 |
| Antibiotics and immunological | Fluconazole | Per unit | 0.22 |
| Stool thickeners | Loperamide | Per 30 units | 1.61 |
| Stool thickeners | Atropine diphenoxylate | Per 100 units | 10.74 |
| Stool softeners/laxatives | Domperidone | Per 100 units | 0.91 |
| Stool softeners/laxatives | Lactulose (laxative if not clearly stated) | Per bottle | 1.21 |
| Stool softeners/laxatives | Docusate | Per 30 units | 2.09 |
| Stool softeners/laxatives | Metamucil | Per 10 units | 4.22 |
| Stool softeners/laxatives | Movicol/Laxido | Per 30 units | 2.99 |
| Stool softeners/laxatives | Clorphenamine | Per 28 units | 0.84 |
| Stool softeners/laxatives | Fluticasone | Per bottle | 4.17 |
| Other stomach/digestive | Mebeverine | Per unit | 4.68 |
| Other stomach/digestive | Ranitidine | Per 12 units | 0.30 |
| Other stomach/digestive | Omeprazole | Per 28 units | 0.44 |
| Urinary | Bendoflumethiazide | Per 28 units | 0.11 |
| Urinary | Tamsulosin | Per 30 units | 0.73 |
| Urinary | Solifenacin succinate | Per 30 units | 27.62 |
| Antidepressants/anti-anxiety | Alprazolam | Per 60 units | 3.18 |
| Antidepressants/anti-anxiety | Citalopram | Per 28 units | 0.18 |
| Antidepressants/anti-anxiety | Diazepam | Per 28 units | 0.23 |
| Antidepressants/anti-anxiety | Lorazepam | Per 28 units | 1.19 |
| Antidepressants/anti-anxiety | Sertraline | Per 28 units | 0.48 |
| Antidepressants/anti-anxiety | Duloxetine | Per 28 units | 22.40 |
| Antidepressants/anti-anxiety | Amitripyline | Per 28 units | 0.14 |
| Sexual dysfunction | Tadalafil | Per 28 units | 54.99 |
| Sexual dysfunction | Sildenafil | Per 28 units | 0.92 |
| Sleeping pills | Zopiclone | Per 28 units | 0.41 |
Medications for unrelated events (e.g. shingles, thyroid conditions, chronic obstructive pulmonary disease, glaucoma) were ignored. Information on dietary supplementation (e.g. vitamins) was provided infrequently but was not included.
For some pain medications, differences in clinical practice mean that medications have been recoded. For example, although hydrocodone is widely used in the USA, it does not appear within the (UK) British National Formulary;38 codeine phosphate is used in preference to hydrocodone. Codeine phosphate is also used when several other medications (i.e. Norco®, codeine sulphate) are indicated.
Given the cost of chemotherapies and the relatively sparse information collected on these (and the danger that the costs involved mask all useful information), these have been ignored in the range of medications being considered. Furthermore, most of the stoma-related costs are removed, as the stoma unit costs specified in Table 4 include a range of costs that may overlap. Chemotherapies as adjunct therapies and anti-emetic/anti-nausea drugs (including anti-psychotics) are also ignored, since the cost of these drugs risks swamping any useful information provided.
Quality of life
The outcome measure for the economic evaluation was the quality-adjusted life-year (QALY). Health-related quality of life (HRQoL) was measured using the EQ-5D and valued using the standard UK tariff. 41 The EQ-5D data were obtained using English-language version questionnaires completed by patients recruited from UK and North American trial sites. The data were collected alongside resource data at baseline and at 30 days and at 6 months postoperatively. Multiple imputation methods were used to estimate HRQoL for those patients not completing this questionnaire. In this way, the analysis includes HRQoL for all patients in the trial, regardless of language.
Quality-of-life estimates were constructed using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), responses, valued using the standard UK tariff. Responses were in most cases valued as an area under the curve. The exception to this was when the data indicated that a stoma reversal operation occurred between 30 days and 6 months postoperatively; in this case, the 30-day figure was assumed to apply between 30 days and the reversal operation, and the 6-month figure was assumed to apply from the reversal date through to the end of follow-up.
As a sensitivity analysis (which has been run but is additional to those analyses displayed here), values were also inferred from the SF-36v2 data obtained from all patients in the ROLARR trial. Health-related quality-of-life figures were obtained as Short Form questionnaire-6 Dimensions (SF-6D) utilities by applying the algorithm developed by Brazier et al. 42
Imputing costs and quality of life
Given that not all UK and US patients answered the medical resource utilisation questionnaire, imputation was necessary within the trial. Values were multiply imputed by category of variable and timing, using 100 imputations for each incomplete observation. By using a large number of multiple imputations, we aimed to more accurately reflect uncertainty.
The first set of variables imputed as chained equations related to the original inpatient admission, being the duration of surgery, the number of assistants and length of stay. These figures were imputed based on the procedure reviewed, whether or not this was a low anterior operation, whether or not there was evidence of locoregional spread, whether or not there had been any CT staging and using age/sex as demographics. Once these figures for the incomplete variables were imputed (and so non-missing), then they were used to inform subsequent variables. These figures also allowed the calculation of a (complete) series of figures for operative costs.
Figures were then imputed to find both the number of other surgeries required and the number of days a stoma would be in place and the type of stoma.
Quality-of-life observations were imputed next, with the EQ-5D-3L and SF-6D data as chained equations using age/sex and information about baseline health conditions. For time periods after baseline, the previous observation for both the EQ-5D and the SF-6D were also used as predictors. With data now complete on these observations, and for the number of stoma days, QALYs could then be calculated.
Other costs were imputed based on the EQ-5D-3L utilities and observed complications, as represented by dummy variables representing common (n > 10) complications graded on the Clavien–Dindo classification to ≥ 3. As these equations were lengthy, each equation was examined and terms removed where p > 0.200. For all health professional and outpatient visits, the equations estimated the number of events. In order to turn these into costs, these utilisation figures were multiplied by the average cost of events observed within the data. There were no clear significant predictors of medication costs within the data. Medication costs were imputed by predicting first whether or not any medication costs existed for that patient, within the relevant period (i.e. post discharge within the first 30 days, between 30 days and 6 months), and then as random variables reflecting those patients with data in that period.
Analysis
Data for all cost items were combined together to form an estimate of total costs, alongside the estimated total number of QALYs within the first 6 months. Because this covers only a 6-month time period, the maximum number of QALYs that could be observed is 0.500 QALYs (or, more properly, 0.499, given that 182 days are used).
With these figures, total costs and costs within different cost categories are presented in terms of both tables and probabilistic sensitivity analyses. As missing data are multiply imputed, it is efficient to conduct the probabilistic sensitivity analysis by bootstrapping (sampling with replacement) among the relevant data set, selecting from the imputed data set until the number of patients in the initial sample has been reached. For example, if a laparoscopic group had 75 patients within a particular scenario, the multiply-imputed data set would have 75 × 100 = 7500 observations, and the procedure would choose one of these 7500 observations, 75 times, in order to obtain a resampled estimate. The results in terms of total costs and total QALYs for each group are then compared and assessed to identify the most cost-effective option. Repeating this resampling procedure 10,000 times provides an estimate of the distribution of incremental costs and incremental benefits between the two options under consideration. This also allows the calculation of cost-effectiveness acceptability curves, which display the probability of each of the options under consideration being cost-effective at different values of the cost-effectiveness threshold. By convention, the values of £20,000 and £30,000 per QALY are focused on,43 although the evidence is that the true cost-effectiveness threshold may be lower than these figures. 44
The primary analysis used imputed data for UK and US patients (n = 190). Secondary analyses were undertaken using the following:
-
complete data for all patients (n = 97)
-
imputed data for UK and US patients intended to receive low anterior surgery (n = 135)
-
imputed data for all observations (n = 471).
Chapter 3 Results
Recruitment
Between 7 January 2011 and 30 September 2014, 1276 patients were assessed for eligibility by 40 surgeons from 26 sites across 10 countries (i.e. UK, Italy, Denmark, USA, Finland, South Korea, Germany, France, Australia and Singapore). In total, 471 (36.9%) of these patients were randomised: 234 to laparoscopic and 237 to robotic surgery (Figure 1). Four patients withdrew from data collection before their operation and one patient did not undergo surgery because of a complete clinical response to neo-adjuvant therapy. The remaining 466 patients underwent an operation, with 456 (97.9%) undergoing the allocated treatment.
FIGURE 1.
The CONSORT flow diagram. ITT, intention to treat.

Baseline data
All minimisation factors except treating surgeon are summarised by treatment group across all randomised patients in Table 6. The minimisation factor intended operating surgeon is summarised by treatment group for all randomised patients in Table 7. Summaries of selected additional baseline fields are given in Table 8.
| Minimisation factor | Treatment group, n (%) | Total, n (%) (N = 471) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| Sex | |||
| Male | 159 (67.9) | 161 (67.9) | 320 (67.9) |
| Female | 75 (32.1) | 76 (32.1) | 151 (32.1) |
| BMI classification | |||
| Underweight/normal | 87 (37.2) | 93 (39.2) | 180 (38.2) |
| Overweight | 92 (39.3) | 90 (38.0) | 182 (38.6) |
| Obese class I | 38 (16.2) | 41 (17.3) | 79 (16.8) |
| Obese class II | 10 (4.3) | 9 (3.8) | 19 (4.0) |
| Obese class III | 7 (3.0) | 4 (1.7) | 11 (2.3) |
| Neo-adjuvant therapy | |||
| Yes | 103 (44.0) | 109 (46.0) | 212 (45.0) |
| No | 131 (56.0) | 128 (54.0) | 259 (55.0) |
| Intended procedure | |||
| HAR | 34 (14.5) | 35 (14.8) | 69 (14.6) |
| LAR | 158 (67.5) | 159 (67.1) | 317 (67.3) |
| APR | 42 (17.9) | 43 (18.1) | 85 (18.0) |
| Surgeon ID | Treatment group, n (%) | Total, n (%) (N = 471) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| 1 | 20 (47.6) | 22 (52.4) | 42 (8.9) |
| 2 | 20 (57.1) | 15 (42.9) | 35 (7.4) |
| 3 | 17 (51.5) | 16 (48.5) | 33 (7.0) |
| 4 | 17 (51.5) | 16 (48.5) | 33 (7.0) |
| 5 | 11 (40.7) | 16 (59.3) | 27 (5.7) |
| 6 | 12 (46.2) | 14 (53.8) | 26 (5.5) |
| 7 | 13 (52.0) | 12 (48.0) | 25 (5.3) |
| 8 | 11 (52.4) | 10 (47.6) | 21 (4.5) |
| 9 | 9 (50.0) | 9 (50.0) | 18 (3.8) |
| 10 | 10 (55.6) | 8 (44.4) | 18 (3.8) |
| 11 | 9 (50.0) | 9 (50.0) | 18 (3.8) |
| 12 | 9 (50.0) | 9 (50.0) | 18 (3.8) |
| 13 | 7 (43.8) | 9 (56.3) | 16 (3.4) |
| 14 | 6 (46.2) | 7 (53.8) | 13 (2.8) |
| 15 | 6 (46.2) | 7 (53.8) | 13 (2.8) |
| 16 | 5 (45.5) | 6 (54.5) | 11 (2.3) |
| 17 | 3 (30.0) | 7 (70.0) | 10 (2.1) |
| 18 | 5 (50.0) | 5 (50.0) | 10 (2.1) |
| 19 | 3 (33.3) | 6 (66.7) | 9 (1.9) |
| 20 | 4 (44.4) | 5 (55.6) | 9 (1.9) |
| 21 | 3 (42.9) | 4 (57.1) | 7 (1.5) |
| 22 | 5 (83.3) | 1 (16.7) | 6 (1.3) |
| 23 | 1 (20.0) | 4 (80.0) | 5 (1.1) |
| 24 | 2 (40.0) | 3 (60.0) | 5 (1.1) |
| 25 | 3 (60.0) | 2 (40.0) | 5 (1.1) |
| 26 | 3 (75.0) | 1 (25.0) | 4 (0.8) |
| 27 | 3 (75.0) | 1 (25.0) | 4 (0.8) |
| 28 | 0 (0.0) | 3 (100.0) | 3 (0.6) |
| 29 | 2 (66.7) | 1 (33.3) | 3 (0.6) |
| 30 | 2 (66.7) | 1 (33.3) | 3 (0.6) |
| 31 | 1 (33.3) | 2 (66.7) | 3 (0.6) |
| 32 | 3 (100.0) | 0 (0.0) | 3 (0.6) |
| 33 | 1 (33.3) | 2 (66.7) | 3 (0.6) |
| 34 | 1 (50.0) | 1 (50.0) | 2 (0.4) |
| 35 | 1 (50.0) | 1 (50.0) | 2 (0.4) |
| 36 | 2 (100) | 0 (0.0) | 2 (0.4) |
| 37 | 2 (100) | 0 (0.0) | 2 (0.4) |
| 38 | 1 (50.0) | 1 (50.0) | 2 (0.4) |
| 39 | 0 (0.0) | 1 (100.0) | 1 (0.2) |
| 40 | 1 (100) | 0 (0.0) | 1 (0.2) |
| Treatment group, n (%) | Total, n (%) (N = 471) | ||
|---|---|---|---|
| Laparoscopic surgery (N = 234) | Robotic surgery (N = 237) | ||
| Age (years), mean (SD) | 65.5 (11.93) | 64.4 (10.98) | 64.9 (11.01) |
| ASA classification | |||
| I: A normal healthy patient | 52 (22.2) | 39 (16.5) | 91 (19.3) |
| II: A patient with mild systemic disease | 124 (53.0) | 150 (63.3) | 274 (58.2) |
| III: A patient with severe systemic disease | 52 (22.2) | 46 (19.4) | 98 (20.8) |
| IV: A patient with severe systemic disease that is a constant threat to life | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Missing | 5 (2.1) | 2 (0.8) | 7 (1.5) |
| Prior abdominal surgery | |||
| Yes | 67 (28.6) | 62 (26.2) | 129 (27.4) |
| No | 162 (69.2) | 174 (73.4) | 336 (71.3) |
| Missing | 5 (2.2) | 1 (0.4) | 6 (1.3) |
Operative and pathology summaries
Crude summaries of operative and local pathology data fields are given in Table 9. The primary end point, conversion to open surgery, is summarised here but is considered in more detail in Primary end point: conversion to open surgery. The key secondary end point, CRM+, is also summarised here but is considered in more detail in Key secondary end point: circumferential resection margin positivity (CRM+).
| Operative | Treatment group, n (%) | Total, n (%) (N = 466) | |
|---|---|---|---|
| Laparoscopic surgery (N = 230) | Robotic surgery (N = 236) | ||
| Operation performed | |||
| HAR | 19 (8.3) | 28 (11.9) | 47 (10.1) |
| LAR | 165 (71.7) | 152 (64.4) | 317 (68.0) |
| APR | 45 (19.6) | 52 (22.0) | 97 (20.8) |
| Othera | 1 (0.4) | 4 (1.7) | 5 (1.1) |
| Operative time (minutes) | |||
| Mean (SD) | 261 (83.24) | 298.5 (88.71) | 280.0 (87.98) |
| Missing | 4 | 1 | 5 |
| Stoma formation | |||
| Temporary | 157 (68.3) | 142 (60.2) | 299 (64.2) |
| Permanent | 49 (21.3) | 53 (22.5) | 102 (21.9) |
| No | 24 (10.4) | 41 (17.4) | 65 (13.9) |
| Length of stay (days) | |||
| Mean (SD) | 8.2 (6.03) | 8.0 (5.85) | 8.1 (5.94) |
| Missing | 13 | 14 | 27 |
| Intraoperative conversion to open surgery | |||
| Yes | 28 (12.2) | 19 (8.1) | 47 (10.1) |
| No | 202 (87.8) | 217 (91.9) | 419 (89.9) |
| Pathologyb | |||
| T-stage | |||
| 0 | 24 (10.4) | 22 (9.3) | 46 (9.9) |
| 1 | 20 (8.7) | 24 (10.2) | 44 (9.4) |
| 2 | 61 (26.5) | 64 (27.1) | 125 (26.8) |
| 3 | 114 (49.6) | 117 (49.6) | 231 (49.6) |
| 4 | 8 (3.5) | 5 (2.1) | 13 (2.8) |
| Tx or missing | 3 (1.3) | 4 (1.7) | 7 (1.5) |
| N-stage | |||
| 0 | 150 (65.2) | 146 (61.9) | 296 (63.5) |
| 1 | 58 (25.2) | 63 (26.7) | 121 (26.0) |
| 2 | 21 (9.1) | 25 (10.6) | 46 (9.9) |
| Missing | 1 (0.4) | 2 (0.8) | 3 (0.6) |
| Lymph node yield | |||
| Mean (SD) | 24.1 (12.91) | 23.2 (11.97) | 23.6 (12.43) |
| Missing | 9 | 1 | 10 |
| Plane of surgery | |||
| Mesorectal area (all specimens) | |||
| Mesorectal plane | 173 (75.2) | 178 (75.4) | 351 (75.3) |
| Intramesorectal plane | 38 (16.5) | 33 (14.0) | 71 (15.2) |
| Muscularis propria plane | 12 (5.2) | 22 (9.3) | 34 (7.3) |
| Missing | 7 (3.1) | 3 (1.3) | 10 (2.1) |
| Sphincter area (APRs only) | (n = 45) | (n = 52) | (n = 97) |
| Levator plane | 18 (40.0) | 18 (34.6) | 36 (37.1) |
| Sphincteric plane | 19 (42.2) | 22 (42.3) | 41 (42.3) |
| Intrasphincteric/submucosal plane | 5 (11.0) | 9 (17.3) | 14 (14.4) |
| Missing | 3 (6.7) | 3 (5.8) | 6 (6.2) |
| CRM involvement | (n = 224) | (n = 235) | (n = 459) |
| Yes | 14 (6.3) | 12 (5.1) | 26 (5.7) |
| No | 210 (93.7) | 223 (94.9) | 433 (94.3) |
Table 10 presents the different pathways of intraoperative conversions between robotic, laparoscopic and open surgery. For example, the pathway ‘Laparoscopic → Robotic’ indicates that the patient’s operation began as a standard laparoscopic operation, but was converted to a robotic operation intraoperatively. A pathway with only one type of operation indicates no conversions, for example ‘Laparoscopic’ indicates that the patient’s operation began as a standard laparoscopic operation and was completed without conversion to robotic or open surgery.
| Intraoperative conversion pathway | Treatment group, n (%) | Total, n (%) (N = 471) | |
|---|---|---|---|
| Standard laparoscopic surgery, (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| Laparoscopic | 194 (82.9) | 3 (1.3) | 197 (41.8) |
| Laparoscopic → Open | 28 (12.0) | 0 (0.0) | 28 (5.9) |
| Laparoscopic → Robotic | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Robotic | 7 (3.0) | 209 (88.2) | 216 (45.9) |
| Robotic → Open | 0 (0.0) | 14 (5.9) | 14 (3.0) |
| Robotic → Laparoscopic | 0 (0.0) | 5 (2.1) | 5 (1.1) |
| Robotic → Laparoscopic → Open | 0 (0.0) | 5 (2.1) | 5 (1.1) |
| Did not receive surgery | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Missing | 3 (1.3) | 1 (0.4) | 4 (0.8) |
Primary end point: conversion to open surgery
The rate of conversion to open surgery was 47 out of 466 (10.1%) patients overall: 28 out of 230 (12.2%) in the laparoscopic group and 19 out of 236 (8.1%) in the robotic group (unadjusted difference in proportions 4.12%, 95% CI –1.35% to 9.59%). There was no statistically significant difference between robotic surgery and conventional laparoscopic surgery with respect to odds of conversion (adjusted OR 0.614, 95% CI 0.311 to 1.211; p = 0.16).
The random intercept model was preferred to the random slope model, because the random slope model did not offer sufficient improvement in model fit, which is clear from both the non-significant likelihood ratio test result and the increase in AIC (see Appendix 1, Table 51).
Table 11 presents adjusted estimates of ORs and 95% CIs from the random intercept model, as well as crude summaries and unadjusted risk difference estimates and 95% CIs for conversion to open surgery by treatment group and also by each of the minimisation factors. The model shows significantly increased odds of conversion in obese patients versus underweight/normal patients (adjusted OR 4.691, 95% CI 2.080 to 10.581; p < 0.01) and in males versus females (adjusted OR 2.444, 95% CI 1.047 to 5.708; p = 0.04). Patients whose intended procedure was a LAR had a significantly higher rate of conversion than patients whose intended procedure was APR (adjusted OR 5.435, 95% CI 1.595 to 18.519; p = 0.007). Operating surgeon had a mild to moderate effect on odds of conversion, as reflected by the ICC estimate of 0.056 (95% CI 0.007 to 0.056).
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 28/230 (12.2) | 19/236 (8.1) | 4.1 (–1.4 to 9.6) | 0.614 | 0.311 to 1.211 | 0.16 |
| Sex: male (vs. female) | 8/149 (5.4) | 39/317 (12.3) | –6.9 (–12.1 to –1.8) | 2.444 | 1.047 to 5.708 | 0.04 |
| BMI class: overweight (vs. underweight/normal) | 13/179 (7.3) | 9/180 (5.0) | 2.3 (–2.7 to 7.2) | 0.538 | 0.210 to 1.374 | 0.19 |
| BMI class: obese (vs. underweight/normal) | 13/179 (7.3) | 25/107 (23.4) | –16.1 (–25.0 to –7.2) | 4.691 | 2.080 to 10.581 | 0.0002 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 27/262 (10.3) | 20/204 (9.8) | 0.5 (–5.0 to 6.0) | 1.069 | 0.504 to 2.265 | 0.86 |
| Intended procedure: HAR (vs. LAR) | 37/312 (11.9) | 6/68 (8.8) | 3.0 (–4.6 to 10.7) | 0.551 | 0.194 to 1.563 | 0.26 |
| Intended procedure: APR (vs. LAR) | 37/312 (11.9) | 4/86 (4.7) | 7.2 (1.5 to 12.9) | 0.184 | 0.054 to 0.627 | 0.007 |
| Effect | Variance component | ICC | 95% CI for ICC | ||
|---|---|---|---|---|---|
| Estimate | Standard error | Lower limit | Upper limit | ||
| Operating surgeon (random effect) | 0.626 | 0.431 | 0.050 | 0.007 | 0.056 |
Subgroup analyses
Odds ratios presented in Tables 13–15 are derived from the linear combination of the estimated treatment (main effect) and treatment-by-subgroup interaction terms on the logit scale. The p-values are presented for the test of the treatment effect within each subgroup – this is the first column of p-values [e.g. in Table 13 the test that the treatment effect is null (OR = 1) within the male subgroup is 0.0429]. The p-values are also presented for the test of heterogeneity of treatment effect across subgroups, the details of which are given in the footnotes of the tables (Table 12).
| Effect | Surgery [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted 95% CI)a | p-value | ||
|---|---|---|---|---|---|---|
| Laparoscopic | Robotic | |||||
| Treatment in males: robotic surgery (vs. laparoscopic) | 25/156 (16.0) | 14/161 (8.7) | 7.3 (0.1 to 14.6) | 0.455 (0.209 to 0.987) | 0.0429 | 0.0939b |
| Treatment in females: robotic surgery (vs. laparoscopic) | 3/74 (4.1) | 5/75 (6.7) | –2.6 (–9.8 to 4.6) | 2.022 (0.425 to 9.621) | 0.3757 | |
| Effect | Surgery [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted 95% CI)a | p-value | ||
|---|---|---|---|---|---|---|
| Laparoscopic | Robotic | |||||
| Treatment in obese patients: robotic-assisted surgery (vs. laparoscopic) | 15/54 (27.8) | 10/53 (18.9) | 8.9 (–7.0 to 24.8) | 0.583 (0.212 to 1.602) | 0.2944 | 0.6862b |
| Treatment in overweight patients: robotic-assisted surgery (vs. laparoscopic) | 6/90 (6.7) | 3/90 (3.3) | 3.3 (–3.0 to 9.7) | 0.508 (0.117 to 2.213) | 0.3661 | 0.7509b |
| Treatment in underweight and normal patients: robotic-assisted surgery (vs. laparoscopic) | 7/86 (8.1) | 6/93 (6.5) | 1.7 (–6.0 to 9.3) | 0.751 (0.227 to 2.492) | 0.6396 | |
| Effect | Surgery [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted 95% CI)a | p-value | ||
|---|---|---|---|---|---|---|
| Laparoscopic | Robotic | |||||
| Treatment (HAR): robotic-assisted surgery (vs. laparoscopic) | 2/19 (10.5) | 2/28 (7.1) | 3.4 (–13.4 to 20.2) | 0.771 (0.078 to 7.617) | 0.8234 | 0.7106b |
| Treatment (APR): robotic-assisted surgery (vs. laparoscopic) | 4/45 (8.9) | 4/52 (7.7) | 1.2 (–9.8 to 12.2) | 0.705 (0.144 to 3.452) | 0.6656 | 0.6833b |
| Treatment (LAR): robotic-assisted surgery (vs. laparoscopic) | 22/165 (13.3) | 11/152 (7.2) | 6.1 (–0.5 to 12.7) | 0.486 (0.210 to 1.123) | 0.0909 | |
In the sex subgroup analysis, 39 out of 317 (12.3%) male patients underwent conversion to laparotomy: 25 out of 156 (16.0%) in the laparoscopic group and 14 out of 161 (8.7%) in the robotic group (unadjusted difference in proportions 7.3%, 95% CI 0.1% to 14.6%). There were 8 out of 149 (5.4%) female patients who underwent conversion to laparotomy: 3 out of 74 (4.1%) in the laparoscopic group and 5 out of 75 (6.7%) in the robotic group (unadjusted difference in proportions –2.6%, 95% CI –9.8% to 4.6%). A Wald test of interaction between treatment effect and sex in the adjusted model yielded p = 0.094. This acts as moderate evidence that the difference between treatment groups is different for males and females. Furthermore, the estimated adjusted OR for conversion to laparotomy (robotic vs. conventional laparoscopic) in males is 0.455 (95% CI 0.209 to 0.987; p = 0.043), suggesting that there may in fact be a significant benefit of robotic surgery compared with laparoscopic surgery in terms of odds of conversion in male patients.
No substantial interactions between treatment effect and BMI or type of operation were found. The treatment effect OR in patients who underwent LAR was 0.486 (95% CI 0.210 to 1.123; p = 0.091), which may warrant further investigation into a potential benefit of robotic surgery in this group of patients.
Sensitivity analysis: learning effects
For 464 out of 471 (98.5%) patients we had sufficient data to derive the level of experience of the operating surgeon, expressed in terms of ‘number of previous laparoscopic operations performed’ and ‘number of previous robotic operations performed’ by the operating surgeon, at the time of the patient’s operation. Table 16 presents the distribution across patients of the previous experience of the operating surgeon who performed their operation. The amount of previous laparoscopic and robotic experience varied widely between participating surgeons, and there was a clear disparity between laparoscopic and robotic experience.
| Statistic | Previous experience (number of patients) | |
|---|---|---|
| Laparoscopic (n = 464) | Robotic (n = 464) | |
| Mean (SD) | 152.5 (178.38) | 67.9 (48.75) |
| Median (range) | 91.4 (10.0–853.0) | 49.5 (10.3–183.0) |
| (Q1, Q3) | (44.9, 180.1) | (30.4, 101.3) |
The patient-level variables ‘number of previous laparoscopic operations performed by the operating surgeon, at the time of the patient’s operation’ and ‘number of previous robotic operations performed by the operating surgeon, at the time of the patient’s operation’ were added to the primary analysis model as centred, linear fixed effects terms. Interactions between each of these variables and the treatment allocation were also included. The resulting estimated treatment effect ORs at various levels of operating surgeon laparoscopic and robotic experience are presented in Table 17. The model suggests that increasing operating surgeon robotic experience notably affects the treatment effect OR in favour of robotic surgery, regardless of the level of laparoscopic experience. The full fitted model is given in Tables 18 and 19, with untransformed estimates (i.e. on the log-odds scale).
| Effect | Surgeon’s experience level (number of previous operations) | OR (robotic vs. laparoscopic) | 95% CI for OR | |
|---|---|---|---|---|
| Laparoscopic | Robotic | |||
|
Primary analysis model Treatment: robotic surgery (vs. laparoscopic) |
– | – | 0.614 | 0.311 to 1.211 |
|
Learning effects model Treatment: robotic surgery (vs. laparoscopic) |
45 | 30 | 0.961 | 0.336 to 2.747 |
| 50 | 0.691 | 0.277 to 1.721 | ||
| 100 | 0.303 | 0.090 to 1.018 | ||
| 91 | 30 | 0.963 | 0.383 to 2.424 | |
| 50 | 0.692 | 0.317 to 1.513 | ||
| 100 | 0.303 | 0.096 to 0.959 | ||
| 180 | 30 | 0.966 | 0.416 to 2.245 | |
| 50 | 0.694 | 0.336 to 1.437 | ||
| 100 | 0.304 | 0.094 to 0.989 | ||
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | –3.0056 | 0.5794 | < 0.0001 | –4.1787 to –1.8326 |
| Treatment: robotic surgery (vs. laparoscopic) | –0.6618 | 0.3893 | 0.0899 | –1.4271 to 0.1035 |
| Sex: male (vs. female) | 0.8688 | 0.4355 | 0.0467 | 0.01283 to 1.7248 |
| BMI class: overweight (vs. underweight/normal) | –0.6763 | 0.4832 | 0.1623 | –1.6262 to 0.2735 |
| BMI class: obese (vs. underweight/normal) | 1.4781 | 0.4257 | 0.0006 | 0.6413 to 2.3149 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 0.1537 | 0.3924 | 0.6956 | –0.6177 to 0.9250 |
| Intended procedure: HAR (vs. LAR) | –0.5234 | 0.5342 | 0.3278 | –1.5734 to 0.5267 |
| Intended procedure: APR (vs. LAR) | –1.7021 | 0.6267 | 0.0069 | –2.9340 to –0.4702 |
| Surgeon’s laparoscopic experience level (number of previous operations) | –0.00038 | 0.001759 | 0.8307 | –0.00383 to 0.003082 |
| Surgeon’s robotic experience level (number of previous operations) | –0.00232 | 0.006330 | 0.7141 | –0.01476 to 0.01012 |
| Interaction term: treatment × surgeon’s laparoscopic experience level | 0.000037 | 0.002728 | 0.9891 | –0.00533 to 0.005399 |
| Interaction term: treatment × surgeon’s robotic experience level | –0.01651 | 0.009887 | 0.0958 | –0.03594 to 0.002929 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.640 | 0.429 |
Sensitivity analysis: actual operating surgeon
The overall proportion of patients who were operated on by a surgeon other than their intended operating surgeon was low [42/466 (9.0%)] and occurred mainly at high recruiting sites, so the discrepancies were not influential on the results.
Adjusting for actual operating surgeon instead of intended operating surgeon made little difference to the model estimates. In particular, the treatment effect estimate (OR) adjusting for actual operating surgeon was 0.612 (95% CI 0.310 to 1.207), a negligible change from the primary analysis model.
Further details are given in Appendix 1, Sensitivity analysis: actual operating surgeon – further details.
Sensitivity analysis: actual procedure
The numbers of patients whose actual procedure was different from their intended procedure (the stratification factor) are summarised in Table 20. There were 65 out of 466 patients (13.9%) who had a procedure other than their ‘intended procedure’. The most common discrepancy was for patients whose intended procedure was a HAR to actually undergo a LAR.
| Actual procedure performed | Intended procedure, n (%) | |||
|---|---|---|---|---|
| HAR (N = 68) | LAR (N = 312) | APR (N = 86) | Total (N = 466) | |
| HAR | 37 (54.4) | 10 (3.2) | 0 (0.0) | 47 (10.1) |
| LAR | 29 (42.6) | 283 (90.7) | 5 (5.8) | 317 (68.0) |
| APR | 1 (1.5) | 15 (4.8) | 81 (94.2) | 97 (20.8) |
| Other | 1 (1.5) | 4 (1.3) | 0 (0.0) | 5 (1.1) |
Adjusting for actual procedure instead of intended procedure had a minor effect on model estimates. The treatment effect estimate (OR) adjusting for actual procedure was 0.572 (95% CI 0.289 to 1.132), which was a minor change from the primary analysis model, although it still points to the same conclusion.
Further details are given in Appendix 2.
Key secondary end point: circumferential resection margin positivity (CRM+)
A total of 459 (98.5%) patients of the 466 who had an operation had complete pathology data available (Table 21). In that group, 26 out of 459 (5.7%) patients were CRM+, 14 out of 224 (6.25%) patients in the laparoscopic group and 12 out of 235 (5.11%) patients in the robotic group (unadjusted difference in proportions 1.14%, 95% CI –3.10% to 5.38%). There was no statistically significant difference in the odds of CRM+ between the groups (adjusted OR 0.785, 95% CI 0.350 to 1.762; p = 0.56). It should be noted that the variance component estimate for operating surgeon is 0, and consequently there is not a valid standard error estimate for this. This indicates that the variation of odds of CRM+ between surgeons was negligible (Table 22).
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 14/224 (6.3) | 12/235 (5.1) | 1.1 (–3.1 to 5.4) | 0.785 | 0.350 to 1.762 | 0.5566 |
| Sex: male (vs. female) | 4/148 (2.7) | 22/311 (7.1) | –4.4 (–8.2 to –0.5) | 2.770 | 0.928 to 8.270 | 0.0679 |
| BMI class: overweight (vs. underweight/normal) | 12/178 (6.7) | 12/176 (6.8) | –0.1 (–5.3 to 5.2) | 1.099 | 0.471 to 2.563 | 0.8272 |
| BMI class: obese (vs. underweight/normal) | 12/178 (6.7) | 2/105 (1.9) | 4.8 (0.3 to 9.4) | 0.263 | 0.057 to 1.216 | 0.0872 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 13/258 (5.0) | 13/201 (6.5) | –1.4 (–5.8 to 2.9) | 1.136 | 0.491 to 2.628 | 0.7647 |
| Intended procedure: HAR (vs. LAR) | 16/308 (5.2) | 2/68 (2.9) | 2.3 (–7.0 to 2.5) | 0.593 | 0.129 to 2.736 | 0.5022 |
| Intended procedure: APR (vs. LAR) | 16/308 (5.2) | 8/83 (9.6) | –4.4 (–11.3 to 2.4) | 2.010 | 0.799 to 5.056 | 0.1377 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.000 | N/A |
Subgroup analyses
As a result of the low frequency of CRM+, many subgroups had insufficient numbers to yield meaningful estimates of the treatment effect.
Further details are given in Appendix 2.
Key secondary end point: 3-year local recurrence
Follow-up times are summarised in Table 23. Median follow-up time from randomisation was 3.1 years. The seven patients with missing data in Table 23 had non-standard circumstances; three patients had benign disease (two laparoscopic, one robotic), one patient did not undergo surgery (laparoscopic) and three patients had palliative surgery only (one laparoscopic, two robotic). These seven patients were censored at time 0.
| Length of follow-up from randomisation (years) | Treatment group | Total (n = 471) | |
|---|---|---|---|
| Standard laparoscopic surgery (n = 234) | Robotic-assisted laparoscopic surgery (n = 237) | ||
| Mean (SD) | 3.1 (1.07) | 3.2 (1.12) | 3.2 (1.10) |
| Median (range) | 3.1 (0.0–6.1) | 3.1 (0.0–6.0) | 3.1 (0.0–6.1) |
| (Q1, Q3) | (3.0, 4.0) | (3.0, 3.9) | (3.0, 4.0) |
| Missing | 4 | 3 | 7 |
A local recurrence was observed in 30 out of 471 (6.4%) patients, 14 out of 234 (6.0%) in the laparoscopic group and 16 out of 237 (6.8%) in the robotic group. The date of local recurrence was defined as the date of the relevant assessment (i.e. clinical, radiological and pathological) that first detected the local recurrence.
The estimated cumulative incidence of local recurrence in each treatment group is presented in Figure 2 (note that the y-axis is truncated to 0–0.1). At 3 years, the estimated difference (robotic minus laparoscopic) in cumulative incidence of local recurrence is 0.002 (95% CI –0.041 to 0.046).
FIGURE 2.
Estimated cumulative incidence of local recurrence, by treatment group.

Table 24 presents the estimated adjusted HRs and corresponding 95% CIs and Wald test p-values from the shared frailty model. There is not a statistically significant difference between the treatment groups. The estimated adjusted HR suggests that a patient undergoing robotic surgery is 1.137 (95% CI 0.554, 2.335; p = 0.756) times more likely to experience local recurrence than a patient undergoing laparoscopic surgery, all else being equal.
| Parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Treatment allocation: robotic (vs. laparoscopic) | 1.137 | 0.554 to 2.335 | 0.7257 |
| Sex: male (vs. female) | 3.184 | 1.109 to 9.174 | 0.0314 |
| Neo-adjuvant therapy: yes (vs. no) | 1.083 | 0.510 to 2.299 | 0.8361 |
| BMI classification obese (vs. underweight/normal) | 0.954 | 0.345 to 2.634 | 0.927 |
| BMI classification overweight (vs. underweight/normal) | 1.366 | 0.603 to 3.095 | 0.4545 |
| Intended procedure HAR (vs. LAR) | 0.645 | 0.187 to 2.224 | 0.4873 |
| Intended procedure APR (vs. LAR) | 1.07 | 0.423 to 2.707 | 0.886 |
There appears to be a substantial difference in probability of local recurrence between males and females, with males much more likely to experience the event (adjusted HR 3.184, 95% CI 1.109 to 9.174; p = 0.031). This is reflected in the plot of cumulative incidence by sex in Figure 3 (note that the y-axis is truncated to 0–0.1).
FIGURE 3.
Estimated cumulative incidence of local recurrence by sex.
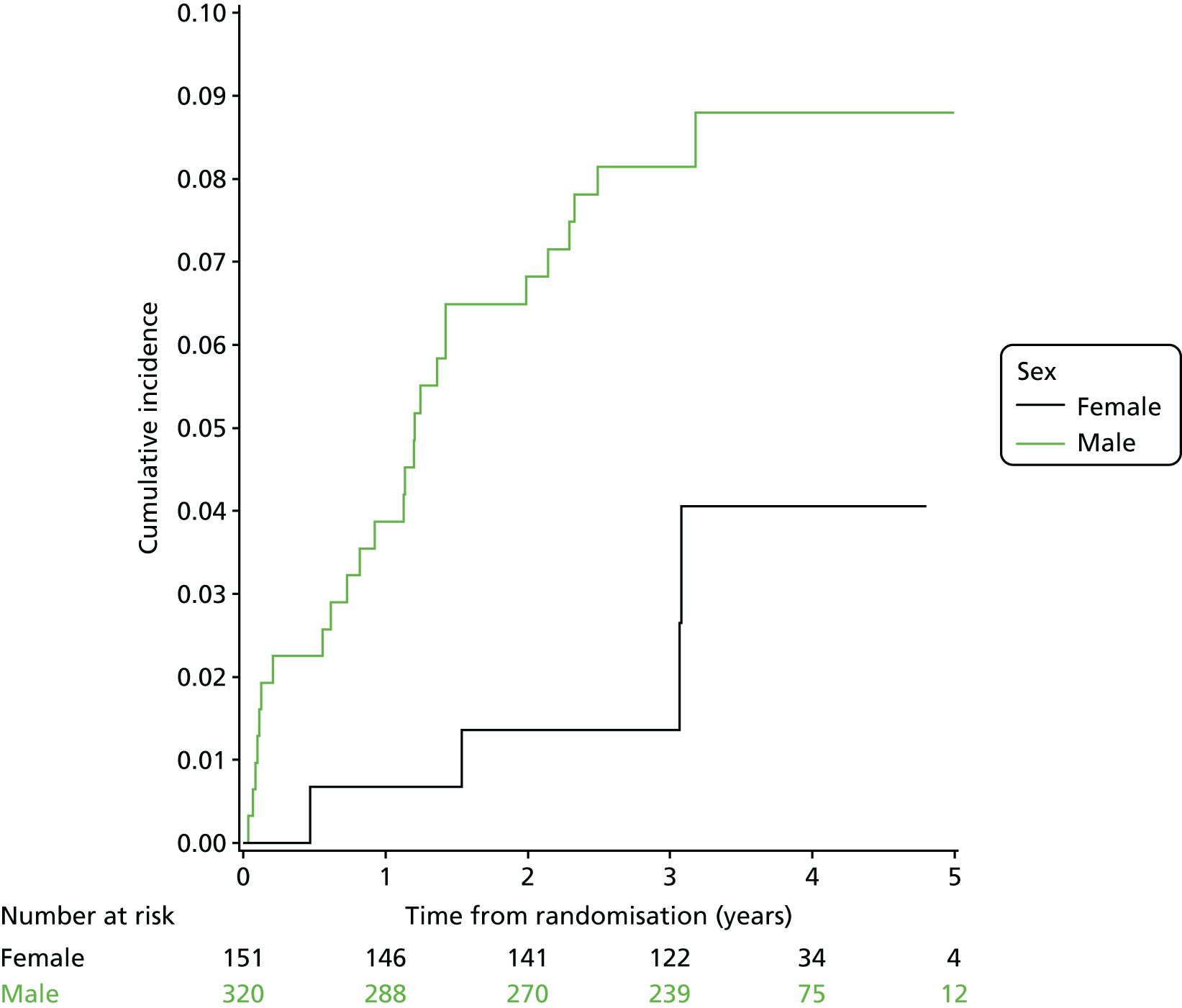
Subgroup analyses
None of the prespecified subgroup analyses yielded meaningful evidence of an interaction between treatment effect and subgroup, or evidence of a treatment effect within any individual subgroup. Given the clear (main) effect of sex on local recurrence, and the clinical plausibility of a potential difference of treatment effect by sex, an ad-hoc sex subgroup analysis was performed. Similarly, this subgroup analysis showed no evidence of a subgroup by treatment interaction and no significant treatment effect within either subgroup.
Intraoperative complications
A total of 70 out of 466 (15.0%) patients had an intraoperative complication, 34 out of 230 (14.8%) in the laparoscopic group and 36 out of 236 (15.3%) in the robotic group (unadjusted risk difference 0.5%, 95% CI –6.0% to 7.0%). Table 25 presents the numbers of patients experiencing different types of intraoperative complications. The most common intraoperative complications were damage to an organ/structure, significant haemorrhage and surgical equipment failure. Table 26 presents the multilevel logistic regression model. There was no significant difference between the groups (adjusted OR 1.020, 95% CI 0.599 to 1.736; p = 0.94). There is significant evidence of a difference in the odds of having an intraoperative complication between males and females (adjusted OR 3.083, 95% CI 1.543 to 6.158; p = 0.0015). Note that the variance component estimate for operating surgeon is 0 and consequently there is not a valid standard error estimate for this. This indicates that the variation of odds of CRM+ between surgeons was negligible (Table 27).
| Intraoperative complications | Treatment group, n (%) | |
|---|---|---|
| Laparoscopic surgery (N = 230) | Robotic surgery (N = 236) | |
| Damage to organ/structure | 5 (2.2) | 11 (4.7) |
| Significant haemorrhage | 11 (4.8) | 4 (1.7) |
| Equipment failure | 6 (2.6) | 8 (3.4) |
| Faecal contamination | 6 (2.6) | 7 (3.0) |
| Anastomotic complication | 6 (2.6) | 7 (3.0) |
| Iatrogenic tumour perforation | 3 (1.3) | 2 (0.8) |
| Inadequate tumour localisation/clearance | 2 (0.9) | 2 (0.8) |
| Respiratory event | 2 (0.9) | 1 (0.4) |
| Cardiac event | 1 (0.4) | 1 (0.4) |
| Overall | 34 (14.8) | 36 (15.3) |
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 34/230 (14.8) | 36/236 (15.3) | –0.5 (–6.0 to 7.0) | 1.020 | 0.599 to 1.736 | 0.9426 |
| Sex: male (vs. female) | 11/149 (7.4) | 59/317 (18.6) | –11.2 (–17.2 to –5.2) | 3.083 | 1.543 to 6.158 | 0.0015 |
| BMI class: overweight (vs. underweight/normal) | 25/179 (14.0) | 30/180 (16.7) | –2.7 (–10.2 to 4.7) | 1.280 | 0.699 to 2.344 | 0.4222 |
| BMI class: obese (vs. underweight/normal) | 25/179 (14.0) | 15/107 (14.0) | –0.1 (–8.4 to 8.3) | 0.939 | 0.456 to 1.931 | 0.8634 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 24/262 (9.2) | 46/204 (22.6) | –13.4 (–20.1 to –6.7) | 3.480 | 1.955 to 6.192 | < 0.0001 |
| Intended procedure: HAR (vs. LAR) | 53/312 (17.0) | 9/68 (13.2) | 3.8 (–5.3 to 12.8) | 1.143 | 0.502 to 2.601 | 0.7504 |
| Intended procedure: APR (vs. LAR) | 53/312 (17.0) | 8/86 (9.3) | 7.7 (0.3 to 15.1) | 0.403 | 0.179 to 0.908 | 0.0284 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.0 | N/A |
Thirty-day postoperative complications
A total of 151 out of 466 (32.4%) patients had a postoperative complication within 30 days of their operation, 73 out of 230 (31.7%) in the laparoscopic group and 78 out of 236 (33.1%) in the robotic group (unadjusted risk difference –1.3%, 95% CI –9.8% to 7.2%). Table 28 presents the numbers of patients who experienced different types of postoperative complications within 30 days of their operation. The most common were gastrointestinal complications (including anastomotic leak), surgical site infections and urinary complications. Tables 29 and 30 present the multilevel logistic regression model. There was no significant difference between the groups (adjusted OR 1.043, 95% CI 0.689 to 1.581; p = 0.84). There is significant evidence of a difference in the odds of having a postoperative complication within 30 days of an operation between males and females (adjusted OR 3.083, 95% CI 1.573 to 4.183; p = 0.0002).
| 30-day complications | Treatment group, n (%) | |
|---|---|---|
| Laparoscopic surgery (N = 230) | Robotic surgery (N = 236) | |
| Gastrointestinal complication | 40 (17.4) | 35 (14.8) |
| Surgical site infection | 19 (8.3) | 21 (8.9) |
| Urinary complication | 14 (6.1) | 17 (7.2) |
| Respiratory complication | 6 (2.6) | 4 (1.7) |
| Cardiac complication | 6 (2.6) | 3 (1.3) |
| Other | 12 (5.2) | 17 (7.2) |
| Overall | 73 (31.7) | 78 (33.1) |
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 73/230 (31.7) | 78/236 (33.1) | –1.3 (–9.8 to 7.2) | 1.043 | 0.689 to 1.581 | 0.8407 |
| Sex: male (vs. female) | 30/149 (20.1) | 121/317 (38.2) | –18.0 (–26.4 to –9.7) | 2.565 | 1.573 to 4.183 | 0.0002 |
| BMI class: overweight (vs. underweight/normal) | 53/179 (29.6) | 52/180 (28.9) | 0.1 (–8.7 to 10.1) | 0.946 | 0.578 to 1.548 | 0.8236 |
| BMI class: obese (vs. underweight/normal) | 53/179 (29.6) | 46/107 (43.0) | –13.4 (–24.9 to –1.9) | 1.758 | 1.022 to 3.024 | 0.0417 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 75/262 (28.6) | 76/204 (37.3) | –8.6 (–17.2 to –0.3) | 1.432 | 0.906 to 2.264 | 0.1241 |
| Intended procedure: HAR (vs. LAR) | 101/312 (32.4) | 15/68 (22.1) | 10.3 (–21.5 to 0.8) | 0.599 | 0.304 to 1.180 | 0.1383 |
| Intended procedure: APR (vs. LAR) | 101/312 (32.4) | 35/86 (40.7) | –8.3 (–19.9 to 3.3) | 1.278 | 0.740 to 2.209 | 0.3778 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.286 | 0.213 |
Six-month postoperative complications (after 30 days)
A total of 72 out of 466 (15.5%) patients had a postoperative complication after 30 days and within 6 months of their operation, 38 out of 230 (16.5%) in the laparoscopic group and 34 out of 236 (14.4%) in the robotic group (unadjusted risk difference 2.1%, 95% CI –4.5% to 8.7%). Table 31 presents the numbers of patients to experience different types of postoperative complications after 30 days and within 6 months of their operation. The most common was gastrointestinal complication (including anastomotic leak). Tables 32 and 33 present the multilevel logistic regression model. There was no significant difference between the groups (adjusted OR 0.719, 95% CI 0.411 to 1.258; p = 0.25).
| 6-month complications (after 30 days) | Treatment group, n (%) | |
|---|---|---|
| Laparoscopic surgery (N = 230) | Robotic surgery (N = 236) | |
| Gastrointestinal complication | 18 (7.8) | 20 (8.5) |
| Urinary complication | 6 (2.6) | 7 (3.0) |
| Surgical site infection | 8 (3.5) | 4 (1.7) |
| Respiratory complication | 3 (1.3) | 2 (0.8) |
| Cardiac complication | 1 (0.4) | 0 (0.0) |
| Cerebrovascular complication | 1 (0.4) | 0 (0.0) |
| Other | 12 (5.2) | 7 (3.0) |
| Overall | 38 (16.5) | 34 (14.4) |
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 38/230 (16.5) | 34/236 (14.4) | 2.1 (–4.5 to 8.7) | 0.719 | 0.411 to 1.258 | 0.2468 |
| Sex: male (vs. female) | 19/149 (12.8) | 53/317 (16.7) | –4.0 (–10.7 to 2.8) | 1.230 | 0.654 to 2.313 | 0.5197 |
| BMI class: overweight (vs. underweight/normal) | 31/179 (17.3) | 24/180 (13.3) | 4.0 (–3.5 to 11.4) | 0.715 | 0.371 to 1.378 | 0.3156 |
| BMI class: obese (vs. underweight/normal) | 31/179 (17.3) | 17/107 (15.9) | 1.4 (–7.4 to 10.3) | 0.663 | 0.316 to 1.390 | 0.2754 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 31/262 (11.8) | 41/204 (20.1) | –8.3 (–15.0 to –1.5) | 1.704 | 0.906 to 3.206 | 0.0979 |
| Intended procedure: HAR (vs. LAR) | 50/312 (16.0) | 6/68 (8.8) | 7.2 (–0.7 to 15.1) | 0.620 | 0.228 to 1.686 | 0.3479 |
| Intended procedure: APR (vs. LAR) | 50/312 (16.0) | 16/86 (18.6) | –2.6 (–11.8 to 6.6) | 1.166 | 0.561 to 2.423 | 0.6794 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 1.343 | 0.708 |
Thirty-day operative mortality
Death within 30 days of operation was a rare event, with 2 out of 230 (0.87%) and 2 out of 236 (0.85%) events in the standard laparoscopic and robotic groups, respectively. All deaths involved a septic complication and were related to the surgical intervention. Owing to the small number of events, sophisticated statistical models were not fitted.
Patient self-reported bladder function
Higher I-PSS indicates worse bladder function and is measured on a scale of 0–35.
Baseline characteristics of the population of patients with complete I-PSS data, and a comparison with those patients with missing I-PSS data, are given in Appendix 4.
Figure 4 visualises the distribution of I-PSS scores at 6 months post operation in the two treatment groups. The distribution of scores is very similar between the groups.
FIGURE 4.
Box plot of observed I-PSS values at baseline and at 6 months post randomisation, by treatment group.

Tables 34 and 35 present the multilevel generalised linear model. Normal errors were assumed, so the estimates represent differences in the mean I-PSS. The estimated difference in mean I-PSS (robotic minus standard) is –0.7426 (95% CI –2.0722 to 0.5870; p = 0.2726). On the 35-point scale, this is a very small effect size that is also not statistically significant. The baseline score is highly prognostic of the 6-month score. The estimated difference in mean I-PSS between patients with a difference in baseline score of 10 points, all else being equal, is 4.20 (95% CI 3.23 to 5.17; p < 0.0001).
| Effect | Estimate | Standard error | p-value | 95% CI |
|---|---|---|---|---|
| Intercept | 3.8249 | 0.9557 | 0.0003 | 1.8867 to 5.7631 |
| Treatment: robotic-assisted surgery (vs. standard) | –0.7426 | 0.6757 | 0.2726 | –2.0722 to 0.5870 |
| Sex: male (vs. female) | 1.7798 | 0.7425 | 0.0171 | 0.3188 to 3.2407 |
| BMI class: overweight (vs. underweight/normal) | 0.3740 | 0.7741 | 0.6293 | –1.1493 to 1.8973 |
| BMI class: obese (vs. underweight/normal) | 0.2473 | 0.9268 | 0.7898 | –1.5764 to 2.0710 |
| Previous neo-adjuvant therapy: yes (vs. no) | –1.1450 | 0.7345 | 0.1201 | –2.5903 to 0.3003 |
| Intended procedure: HAR (vs. LAR) | –1.0208 | 1.0117 | 0.3138 | –3.0116 to 0.9699 |
| Intended procedure: APR (vs. LAR) | 2.7760 | 0.9326 | 0.0031 | 0.9410 to 4.6111 |
| Baseline I-PSS (1-unit increase) | 0.4198 | 0.04933 | < 0001 | 0.3228 to 0.5169 |
| Parameter | Subject | Estimate | Standard error |
|---|---|---|---|
| Intercept | Surgeon | 1.2834 | 1.4209 |
| Residual | 38.9462 | 3.1275 |
Patient self-reported sexual function: males
A higher IIEF score indicates better sexual function; the score is measured on a scale of 5–75.
Baseline characteristics of the population of patients with complete IIEF data, and a comparison with those patients with missing IIEF data, are given in Appendix 5.
Figure 5 visualises the distribution of IIEF scores at 6 months post operation in the two treatment groups. The distribution of scores is very similar between the treatment groups. Median IIEF scores at 6 months were notably lower than at baseline in both groups.
FIGURE 5.
Box plot of observed IIEF values at baseline and at 6 months post randomisation, by treatment group.

Tables 36 and 37 present the multilevel generalised linear model. Normal errors were assumed, so the estimates represent differences in the mean IIEF score. The estimated difference in mean IIEF (robotic minus standard) is –0.8020 (95% CI –5.7039 to 4.1000; p = 0.7468). On the 70-point scale, this is a very small effect size that is also not statistically significant.
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 9.7690 | 3.6018 | 0.0104 | 2.4493 to 17.0887 |
| Treatment: robotic-assisted surgery (vs. standard) | –0.8020 | 2.4793 | 0.7468 | –5.7039 to 4.1000 |
| BMI class: overweight (vs. underweight/normal) | –0.7386 | 2.9556 | 0.8030 | –6.5823 to 5.1051 |
| BMI class: obese (vs. underweight/normal) | 3.0106 | 3.3590 | 0.3717 | –3.6307 to 9.6519 |
| Previous neo-adjuvant therapy: yes (vs. no) | –5.1767 | 3.0462 | 0.0915 | –11.1996 to 0.8462 |
| Intended procedure: HAR (vs. LAR) | 7.2280 | 3.7064 | 0.0532 | –0.1001 to 14.5562 |
| Intended procedure: APR (vs. LAR) | –0.7213 | 3.6837 | 0.8450 | –8.0046 to 6.5620 |
| Baseline total IIEF score (1-unit increase) | 0.5171 | 0.05045 | < 0001 | 0.4174 to 0.6169 |
| Parameter | Subject | Estimate | Standard error |
|---|---|---|---|
| Intercept | Surgeon | 51.9161 | 29.1957 |
| Residual | 250.47 | 29.2417 |
Patient self-reported sexual function: females
A higher FSFI score indicates better sexual function; the score is measured on a scale of 2–36.
Baseline characteristics of the population of patients with complete FSFI data, and a comparison with those patients with missing FSFI data, are given in Appendix 6.
Figure 6 visualises the distribution of FSFI scores at 6 months post operation in the two treatment groups. The distribution of scores is very similar between the treatment groups.
FIGURE 6.
Box plot of observed FSFI values at baseline and at 6 months post randomisation, by treatment group.

Tables 38 and 39 present the multilevel generalised linear model. Normal errors were assumed, so the estimates represent differences in the mean FSFI score. The estimated difference in the mean FSFI score (robotic minus standard) is –1.2309 (95% CI –6.0030 to 3.5413; p = 0.6010). On the 34-point scale, this is a small effect size that is also not statistically significant.
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 9.0710 | 3.2464 | 0.0116 | 2.2762 to 15.8657 |
| Treatment: robotic-assisted surgery (vs. standard) | –1.2309 | 2.3258 | 0.6010 | –6.0030 to 3.5413 |
| BMI class: overweight (vs. underweight/normal) | 4.1518 | 2.7584 | 0.1439 | –1.5079 to 9.8116 |
| BMI class: obese (vs. underweight/normal) | –0.9541 | 3.2873 | 0.7738 | –7.6992 to 5.7909 |
| Previous neo-adjuvant therapy: yes (vs. no) | –0.8097 | 2.7129 | 0.7676 | –6.3761 to 4.7567 |
| Intended procedure: HAR (vs. LAR) | –0.7669 | 3.2401 | 0.8147 | –7.4151 to 5.8813 |
| Intended procedure: APR (vs. LAR) | –4.9505 | 3.1579 | 0.1286 | –11.4300 to 1.5289 |
| Baseline FSFI score (1-unit increase) | 0.4629 | 0.1147 | 0.0004 | 0.2275 to 0.6982 |
| Parameter | Subject | Estimate | Standard error |
|---|---|---|---|
| Intercept | Surgeon | 0.1703 | 10.2019 |
| Residual | 70.5888 | 16.9383 |
Patient self-reported generic health
The SF-36 is a multipurpose, short-form health survey with 36 questions. It has eight scales of functional health: physical functioning, social functioning, role limitation physical, role limitation emotional, mental health, vitality, pain and general health that are scored on a 0–100 scale. It also provides a physical component score (PCS) and a mental component score (MCS), which are combinations of the eight scales.
The SF-36 was collected at baseline, and at 30 days and at 6 months post operation.
A higher score indicates a better QoL.
Baseline characteristics of the population of patients with complete generic QoL data, and a comparison with those patients with missing generic QoL data, are given in Appendix 7.
Tables 40 and 41 show the multilevel linear model for the PCS and MCS, respectively. Figures 7 and 8 illustrate the model estimates and 95% CIs at baseline and at 1 month and 6 months post randomisation of the average PCS and MCS respectively, split by treatment group. The estimated average difference in PCS between the groups at baseline is negligible: –0.1220 (95% CI –1.6281 to 1.3840; p = 0.8737). This is also the case at 1 month and 6 months post randomisation, as shown by the small magnitude and large p-values for the estimates of interaction between treatment effect and time (see Table 40). The estimated average difference in MCS between the groups at baseline is also negligible, –0.4875 (95% CI –2.6008 to 1.6258; p = 0.6508). Again this does not change notably over time, as seen in Figure 8 and by the small magnitude and large p-values of the estimates of interaction between time and treatment effect in Table 32.
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 54.5476 | 1.6592 | ||
| 30 days | –7.3421 | 1.7918 | < 0.0001 | –10.8595 to –3.8247 |
| 6 months | –2.1738 | 1.8099 | 0.2301 | –5.7266 to 1.3791 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | –0.1220 | 0.7672 | 0.8737 | –1.6281 to 1.3840 |
| Sex: female (vs. male) | –1.4117 | 0.6549 | 0.0314 | –2.6974 to –0.1260 |
| Neo-adjuvant therapy: no (vs. yes) | 3.0683 | 0.8419 | 0.0003 | 1.4156 to 4.7210 |
| Intended procedure: APR (vs. HAR) | –2.8637 | 1.4321 | 0.0459 | –5.6750 to –0.05246 |
| Intended procedure: LAR (vs. HAR) | –0.2736 | 1.1468 | 0.8115 | –2.5249 to 1.9776 |
| BMI class: obese (vs. underweight/normal) | –0.2153 | 1.0415 | 0.8363 | –2.2597 to 1.8292 |
| BMI class: overweight (vs. underweight/normal) | 0.9225 | 0.8818 | 0.2958 | –0.8085 to 2.6535 |
| ASA grade: (II vs. I) | –3.8012 | 1.0400 | 0.0003 | –5.8427 to –1.7597 |
| ASA grade: (III vs. I) | –6.6250 | 1.2870 | < 0001 | –9.1513 to –4.0986 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | 0.4651 | 0.8664 | 0.5916 | –1.2357 to 2.1659 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | 0.6086 | 0.8777 | 0.4882 | –1.1143 to 2.3315 |
| ASA grade II and 30-day interaction | 2.4549 | 1.1467 | 0.0326 | 0.2039 to 4.7058 |
| ASA grade III and 30-day interaction | 3.4690 | 1.3853 | 0.0125 | 0.7495 to 6.1884 |
| ASA grade II and 6-month interaction | 0.5546 | 1.1382 | 0.6262 | –1.6797 to 2.7889 |
| ASA grade III and 6-month interaction | 2.7739 | 1.3844 | 0.0455 | 0.05625 to 5.4916 |
| No neo-adjuvant therapy and 30-day interaction | –3.4295 | 0.9152 | 0.0002 | –5.2262 to –1.6329 |
| No neo-adjuvant therapy and 6-month interaction | –2.9066 | 0.9271 | 0.0018 | –4.7266 to –1.0867 |
| APR and 30-day interaction | –2.6589 | 1.5995 | 0.0969 | –5.7989 to 0.4810 |
| APR and 6-month interaction | 0.5959 | 1.6210 | 0.7133 | –2.5862 to 3.7780 |
| LAR and 30-day interaction | –1.9226 | 1.2971 | 0.1387 | –4.4688 to 0.6237 |
| LAR and 6-month interaction | –0.2858 | 1.3196 | 0.8286 | –2.8764 to 2.3047 |
| Obese and 30-day interaction | –2.6187 | 1.1657 | 0.0250 | –4.9071 to –0.3303 |
| Obese and 6-month interaction | –1.5758 | 1.1784 | 0.1816 | –3.8891 to 0.7375 |
| Overweight and 30-day interaction | –0.5450 | 0.9780 | 0.5775 | –2.4647 to 1.3748 |
| Overweight and 6-month interaction | –0.3320 | 0.9915 | 0.7378 | –2.2784 to 1.6143 |
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 40.4101 | 3.3900 | ||
| 30 days | –2.3060 | 2.3656 | 0.3300 | –6.9498 to 2.3378 |
| 6 months | 4.4232 | 2.3888 | 0.0645 | –0.2661 to 9.1125 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | –0.4875 | 1.0766 | 0.6508 | –2.6008 to 1.6258 |
| Sex: female (vs. male) | –3.0157 | 0.9511 | 0.0016 | –4.8828 to –1.1486 |
| Neo-adjuvant therapy: no (vs. yes) | 1.6362 | 1.1905 | 0.1697 | –0.7008 to 3.9733 |
| Intended procedure: APR (vs. HAR) | –2.8133 | 2.0057 | 0.1611 | –6.7505 to 1.1240 |
| Intended procedure: LAR (vs. HAR) | –0.7372 | 1.6047 | 0.6461 | –3.8873 to 2.4130 |
| BMI class: obese (vs. underweight/normal) | 0.8777 | 1.4551 | 0.5466 | –1.9786 to 3.7340 |
| BMI class: overweight (vs. underweight/normal) | –0.2983 | 1.2335 | 0.8090 | –2.7198 to 2.1232 |
| Age | 0.1339 | 0.04350 | 0.0022 | 0.04845 to 0.2193 |
| ASA grade: (II vs. I) | 0.2121 | 1.4829 | 0.8863 | –2.6988 to 3.1230 |
| ASA grade: (III vs. I) | –0.6905 | 1.8218 | 0.7048 | –4.2668 to 2.8859 |
| ASA grade: (IV vs. I) | –13.0801 | 11.6604 | 0.2623 | –35.9699 to 9.8097 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | 2.0753 | 1.1435 | 0.0699 | –0.1694 to 4.3200 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | 0.2681 | 1.1576 | 0.8169 | –2.0042 to 2.5404 |
| ASA grade II and 30-day interaction | 0.5556 | 1.5142 | 0.7137 | –2.4168 to 3.5281 |
| ASA grade III and 30-day interaction | 0.1340 | 1.8290 | 0.9416 | –3.4564 to 3.7243 |
| ASA grade II and 6-month interaction | –2.4933 | 1.5015 | 0.0972 | –5.4408 to 0.4541 |
| ASA grade III and 6-month interaction | –1.5419 | 1.8273 | 0.3990 | –5.1289 to 2.0451 |
| No neo-adjuvant therapy and 30-day interaction | –1.1184 | 1.2080 | 0.3548 | –3.4897 to 1.2530 |
| No neo-adjuvant therapy and 6-month interaction | –1.0979 | 1.2230 | 0.3696 | –3.4987 to 1.3029 |
| APR and 30-day interaction | –0.4158 | 2.1120 | 0.8440 | –4.5618 to 3.7302 |
| APR and 6-month interaction | –1.0937 | 2.1408 | 0.6096 | –5.2960 to 3.1087 |
| LAR and 30-day interaction | –1.4976 | 1.7126 | 0.3822 | –4.8595 to 1.8644 |
| LAR and 6-month interaction | –1.9039 | 1.7421 | 0.2748 | –5.3237 to 1.5159 |
| Obese and 30-day interaction | –1.7664 | 1.5395 | 0.2516 | –4.7885 to 1.2557 |
| Obese and 6-month interaction | 0.4705 | 1.5564 | 0.7625 | –2.5849 to 3.5258 |
| Overweight and 30-day interaction | 0.4266 | 1.2903 | 0.7410 | –2.1063 to 2.9596 |
| Overweight and 6-month interaction | 1.2397 | 1.3073 | 0.3433 | –1.3266 to 3.8059 |
FIGURE 7.
Adjusted estimates and 95% CIs of mean SF-36v2 PCS values at baseline, at 1 month and at 6 months post randomisation, by treatment group.

FIGURE 8.
Adjusted estimates and 95% CIs of mean SF-36v2 MCS values at baseline, at 1 month and at 6 months post randomisation, by treatment group.

Patient self-reported fatigue
The MFI-20 is a self-report instrument. It contains 20 statements that cover different aspects of fatigue.
These 20 items are organised in five scales: general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue.
The scores per item run from 1 to 5. For each scale, consisting of four items, a total score is calculated by summation of the scores of the individual items. Scores can range from the minimum of 4 to the maximum of 20. The use of a total score over all 20 items is not recommended.
The MFI-20 was collected at baseline, at 30 days post operation and at 6 months post operation. A higher score indicates more fatigue.
Baseline characteristics of the population of patients with complete MFI-20 data, and a comparison with those patients with missing MFI-20 data, are given in Appendix 8.
Figure 9 illustrates the model estimates and 95% CIs at baseline, at 1 month and at 6 months post randomisation for each of the five scales, split by treatment group. The estimated differences between the treatment groups in scores at baseline were as follows: general fatigue –0.2517 (95% CI –0.5965 to 1.0999; p = 0.5603), physical fatigue 0.3964 (95% CI –0.4404 to 1.2332; p = 0.3527), reduced activity –0.1634 (95% CI –0.9777 to 0.6510; p = 0.6938), reduced motivation –0.03917 (95% CI –0.7324 to 0.6540; p = 0.9117) and mental fatigue 0.1374 (95% CI –0.6626 to 0.9374; p = 0.7360). All of these differences are small and none are statistically significant. Furthermore, this lack of a notable difference between the groups persists over time, as seen in Figure 9 and in the non–significant interaction terms in the model; further details of the models are given in Appendix 8.
FIGURE 9.
Adjusted estimates and 95% CIs of mean values of each of the five scales of the MFI-20 at baseline, at 1 month and at 6 months post randomisation, by treatment group. (a) General fatigue; (b) physical fatigue; (c) reduced activity; (d) reduced motivation; and (e) mental fatigue.
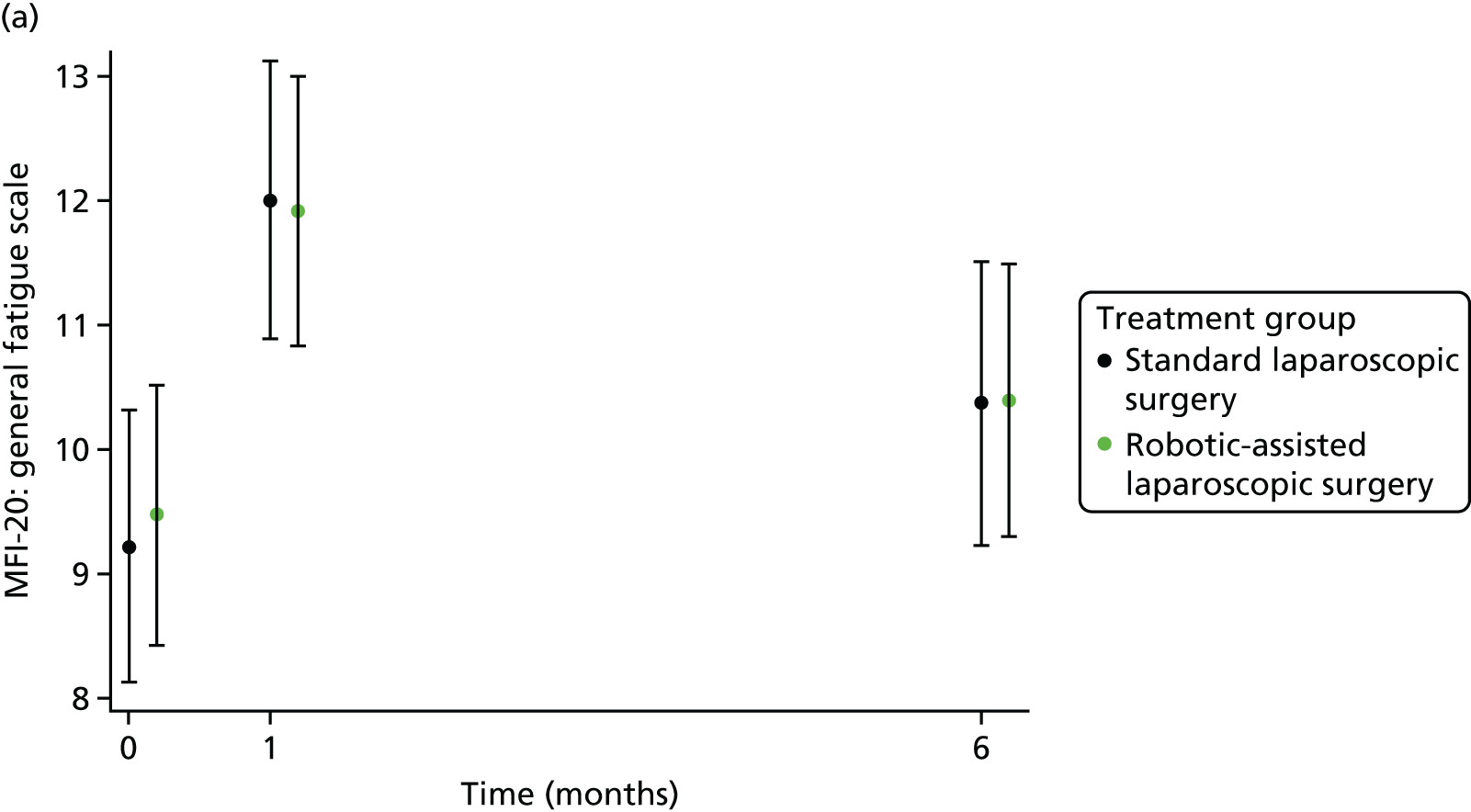
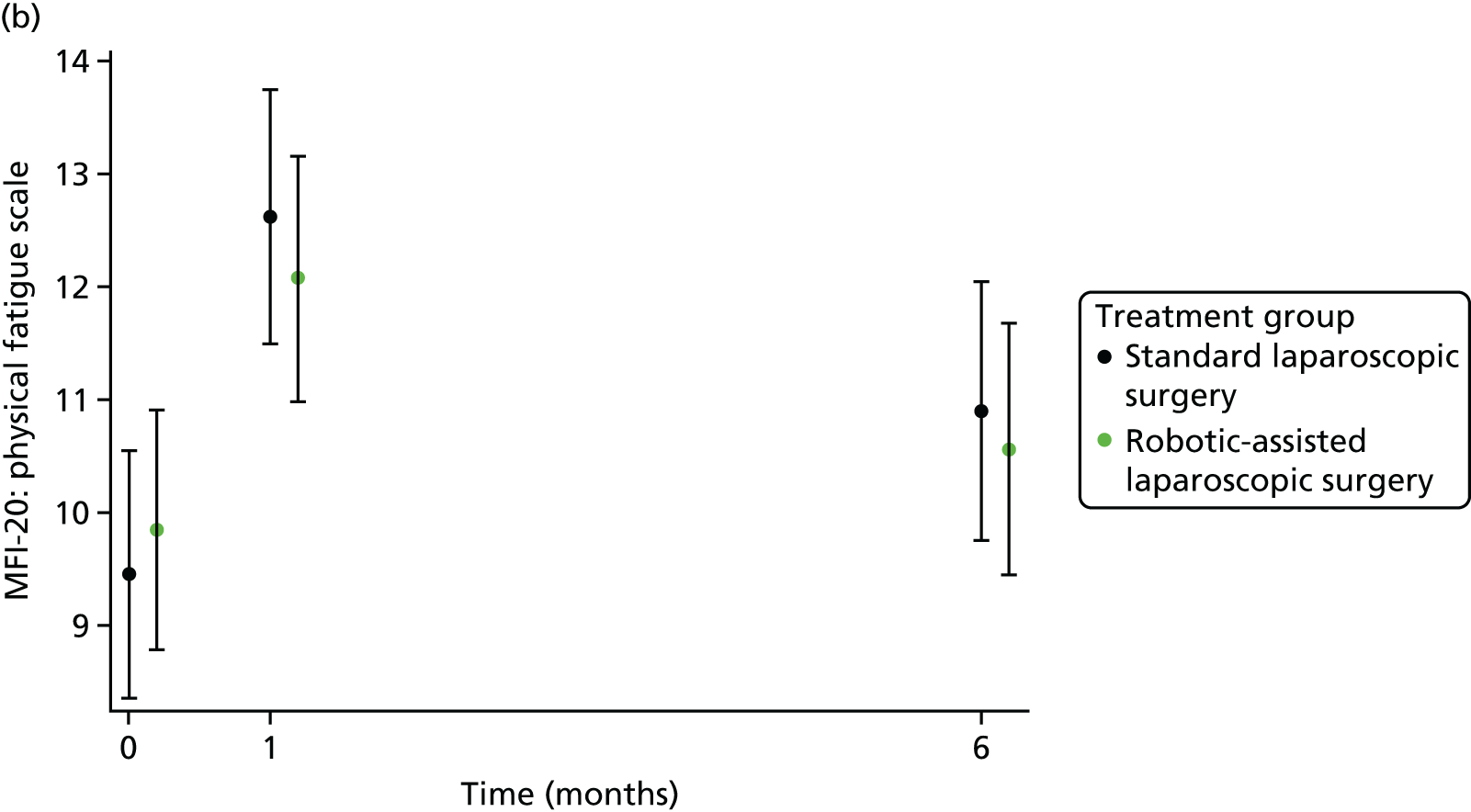
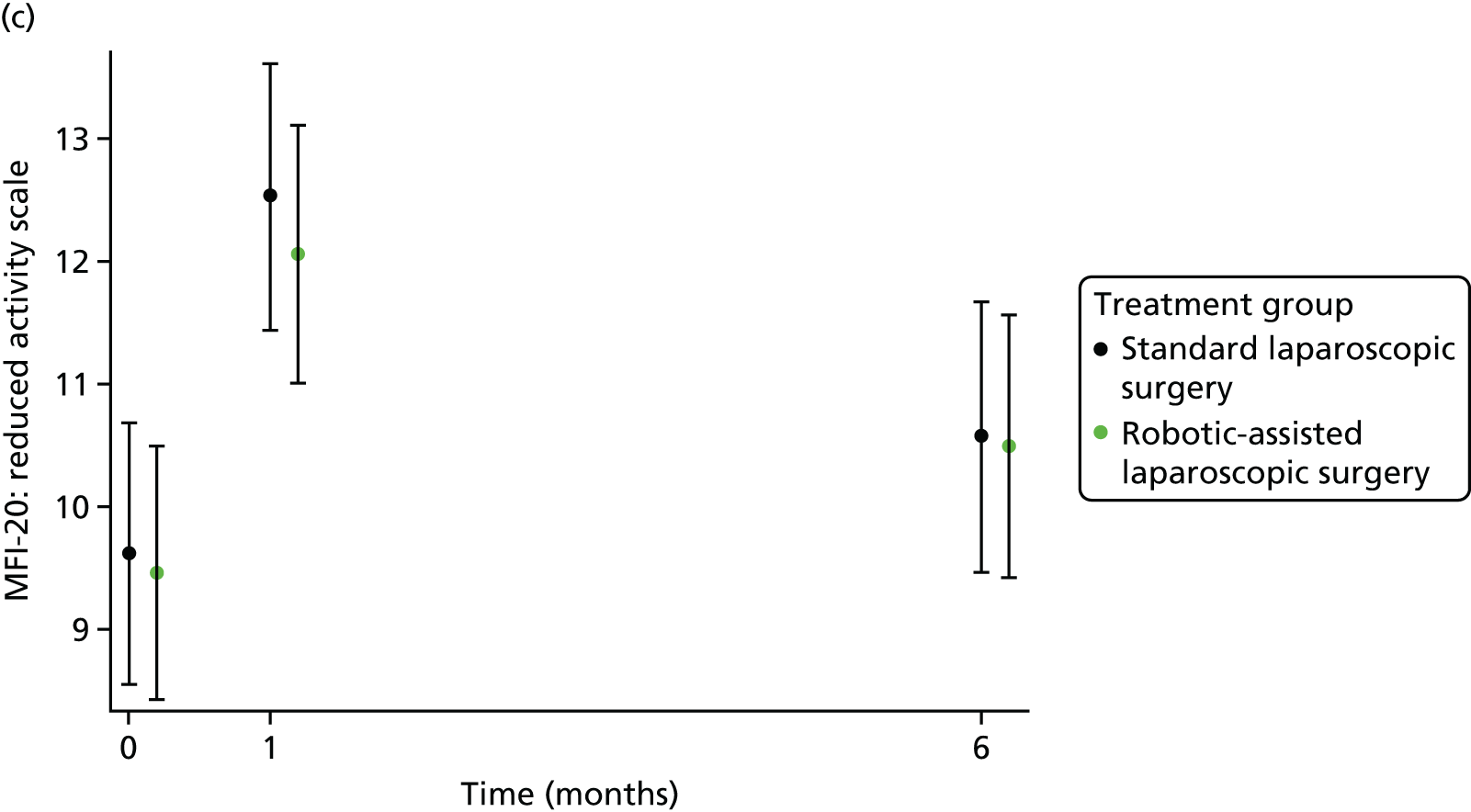
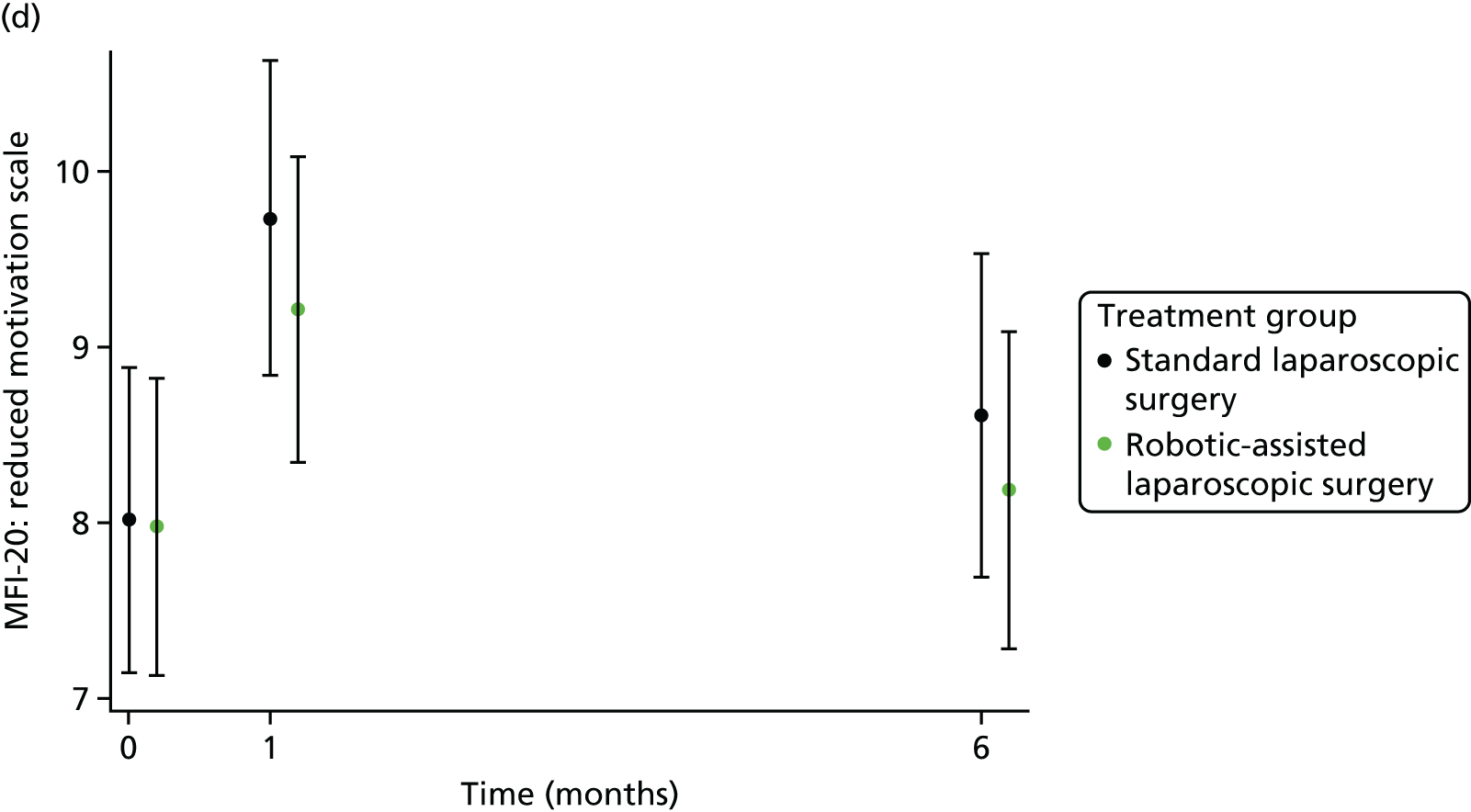

Plane of surgery
A total of 456 out of 471 (96.8%) patients had a returned pathology report with data for the mesorectal plane assessment. There were 351 out of 456 (77.0%) patients’ specimens graded as mesorectal plane in the pathology report, 178 out of 233 (76.4%) in the laparoscopic group and 173 out of 223 (77.6%) in the robotic group (unadjusted risk difference 1.2%, 95% CI –6.5% to 8.9%). Table 42 presents the crude summary of plane of resection (mesorectum) between the treatment groups. Tables 43 and 44 present the multilevel logistic regression model. There was no significant difference of the odds of ‘mesorectal plane’ between the groups (adjusted OR 0.943, 95% CI 0.565 to 1.572; p = 0.821). Patients undergoing APR have notably lower odds of a mesorectal plane grading [adjusted OR (vs. LAR) 0.358, 95% CI 0.185 to 0.694; p = 0.0024].
| Mesorectum plane | Treatment group, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| Mesorectal fascial plane | 173 (73.9) | 178 (75.1) | 351 (74.5) |
| Intramesorectal plane | 38 (16.2) | 33 (13.9) | 71 (15.1) |
| Muscularis propria plane | 12 (5.1) | 22 (9.3) | 34 (7.2) |
| Missing | 11 (4.7) | 4 (1.7) | 15 (3.2) |
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 173/223 (77.6) | 178/233 (76.4) | 1.2 (–6.5 to 8.9) | 0.943 | 0.565 to 1.572 | 0.8211 |
| Sex: male (vs. female) | 122/149 (81.9) | 229/307 (74.6) | 7.3 (–0.6 to 15.2) | 0.729 | 0.411 to 1.295 | 0.2808 |
| BMI class: overweight (vs. underweight/normal) | 142/177 (80.2) | 134/175 (76.6) | 3.7 (–4.9 to 12.3) | 0.851 | 0.458 to 1.580 | 0.6086 |
| BMI class: obese (vs. underweight/normal) | 142/177 (80.2) | 75/104 (72.1) | 8.1 (–2.3 to 18.5) | 0.905 | 0.457 to 1.795 | 0.7757 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 197/256 (77.0) | 154/200 (77.0) | –0.1 (–7.8 to 7.7) | 0.796 | 0.435 to 1.454 | 0.4569 |
| Intended procedure: HAR (vs. LAR) | 251/308 (81.5) | 55/68 (80.9) | 0.6 (–9.7 to 10.9) | 0.901 | 0.411 to 1.977 | 0.7943 |
| Intended procedure: APR (vs. LAR) | 251/308 (81.5) | 45/80 (56.3) | 25.2 (13.5 to 37.0) | 0.358 | 0.185 to 0.694 | 0.0024 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 2.236 | 1.021 |
Disease-free survival
A recurrence was observed in 73 out of 471 (15.5%) patients, 38 out of 234 (16.2%) in the laparoscopic group and 35 out of 237 (14.8%) in the robotic group.
The date of recurrence was defined as the date of the relevant assessment (i.e. clinical, radiological and pathological) that first detected the recurrence.
Kaplan–Meier estimates of DFS in each treatment group are presented in Figure 10.
FIGURE 10.
Kaplan–Meier plot of disease-free survival, by treatment group. Product-limit survival estimates with number of patients at risk. Note that the y-axis is truncated to 0.4–1.0.

Tables 45 and 46 present the estimated HRs and corresponding 95% CIs and Wald test p-values from the shared frailty model. There is no statistically significant difference between the treatment groups. The estimated adjusted HR suggests that a patient undergoing robotic surgery is 1.030 (95% CI 0.713 to 1.489; p = 0.874) times more likely to experience a recurrence or a new primary cancer or to die than a patient undergoing laparoscopic surgery, all else being equal.
| Parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Treatment allocation: robotic (vs. laparoscopic) | 1.030 | 0.713 to 1.489 | 0.8736 |
| Sex: male (vs. female) | 1.487 | 0.973 to 2.272 | 0.0665 |
| Neo-adjuvant therapy: yes (vs. no) | 1.259 | 0.857 to 1.848 | 0.2401 |
| BMI classification: obese (vs. underweight/normal) | 0.875 | 0.523 to 1.462 | 0.6095 |
| BMI classification: overweight (vs. underweight/normal) | 1.274 | 0.840 to 1.934 | 0.2542 |
| Intended procedure: APR (vs. LAR) | 1.602 | 1.035 to 2.479 | 0.0344 |
| Intended procedure: HAR (vs. LAR) | 0.421 | 0.191 to 0.925 | 0.0313 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.0271 | 0.0745 |
There appears to be a substantial difference in DFS between patients undergoing different types of operation. All else being equal, those patients undergoing an APR are most likely to have a recurrence, a new primary cancer or to die (adjusted HR vs. LAR: 1.602, 95% CI 1.035 to 2.479; p = 0.034), and those patients undergoing HAR are least likely to have a recurrence, a new primary cancer or to die (adjusted HR vs. LAR 0.421, 95% CI 0.191 to 0.925; p = 0.031).
Subgroup analyses
None of the prespecified subgroup analyses yielded meaningful evidence of an interaction between treatment effect and subgroup, or evidence of a treatment effect within any individual subgroup. Given the clear (main) effect of sex on DFS, and the clinical plausibility of a potential difference of treatment effect by sex, an ad hoc sex subgroup analysis was performed. Similarly, this subgroup analysis showed no evidence of a subgroup by treatment interaction and no significant treatment effect within either subgroup.
Further details are given in Appendix 9.
Overall survival
Death was observed for 46 out of 471 (9.8%) patients, 23 out of 234 (9.8%) in the laparoscopic group and 23 out of 237 (9.7%) in the robotic group.
Kaplan–Meier estimates of OS in each treatment group are presented in Figure 11.
FIGURE 11.
Kaplan–Meier plot of OS, by treatment group. Product-limit survival estimates with number of patients at risk. Note that the y-axis is truncated to 0.7–1.0.

Tables 47 and 48 present the estimated HRs and corresponding 95% CIs and Wald test p-values from the shared frailty model. There is not a statistically significant difference between the treatment groups. The estimated adjusted HR suggests that a patient undergoing robotic surgery is 0.945 (95% CI 0.530 to 1.686; p = 0.848) times more likely to die than a patient undergoing laparoscopic surgery, all else being equal.
| Parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Treatment allocation: robotic (vs. laparoscopic) | 0.945 | 0.530 to 1.686 | 0.8483 |
| Sex: male (vs. female) | 2.187 | 1.017 to 4.700 | 0.0450 |
| Neo-adjuvant therapy: yes (vs. no) | 1.380 | 0.756 to 2.522 | 0.2945 |
| BMI classification: obese (vs. underweight/normal) | 0.577 | 0.258 to 1.290 | 0.1804 |
| BMI classification: overweight (vs. underweight/normal) | 0.652 | 0.339 to 1.255 | 0.2007 |
| Intended procedure: APR (vs. LAR) | 1.442 | 0.741 to 2.804 | 0.2815 |
| Intended procedure: HAR (vs. LAR) | 0.520 | 0.155 to 1.739 | 0.2881 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | < 0.001 | 0.1649 |
There appears to be a notable difference in the risk of death between males and females. All else being equal, the probability of death in males is 2.187 (95% CI 1.017 to 4.700; p = 0.045) times greater than in females.
Subgroup analyses
None of the prespecified subgroup analyses yielded meaningful evidence of an interaction between treatment effect and subgroup, or evidence of a treatment effect within any individual subgroup. Given the clear (main) effect of sex on OS, and the clinical plausibility of a potential difference of treatment effect by sex, an ad-hoc sex subgroup analysis was performed. Similarly, this subgroup analysis showed no evidence of a subgroup by treatment interaction and no significant treatment effect within either subgroup.
Further details are given in Appendix 9.
Health economics
The results of the primary analysis are reported in Table 49. There are very similar QoL figures across the two groups, with a difference of 0.014 QALYs across the first 6 months of treatment. Across both groups, the pattern is of baseline EQ-5D utilities being highest pre-surgery (0.810 vs. 0.828) and noticeably lower at 30 days (0.680 vs. 0.700). The utilities are much closer to their pre-surgery values by 6 months (0.774 vs. 0.798).
| Parameter | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| Laparoscopic surgery (n = 95) | Robotic surgery (n = 95) | |||||||
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | |
| Overall figures | ||||||||
| QALY | 0.364 | 0.097 | –0.029 | 0.499 | 0.378 | 0.089 | 0.023 | 0.499 |
| Total costs (£) | 10,874 | 2676 | 6245 | 26,969 | 11,853 | 2940 | 7330 | 22,872 |
| Health and QoL | ||||||||
| Baseline | 0.810 | 0.192 | 0.085 | 1.000 | 0.828 | 0.181 | 0.131 | 1.000 |
| 30 days | 0.680 | 0.229 | –0.458 | 1.000 | 0.700 | 0.244 | –0.116 | 1.000 |
| 6 months | 0.774 | 0.221 | –0.287 | 1.000 | 0.798 | 0.201 | –0.248 | 1.000 |
| Stoma formed (%) | 91 | 28 | 0 | 100 | 81 | 39 | 0 | 100 |
| Days with stoma | 146.35 | 58.71 | – | 182.00 | 132.92 | 69.30 | – | 182.00 |
| Cost breakdown (£) | ||||||||
| Total costs | 10,874 | 2676 | 6245 | 26,969 | 11,853 | 2940 | 7330 | 22,872 |
| Initial surgery | 8423 | 1443 | 5249 | 12,466 | 9508 | 1219 | 6591 | 14,328 |
| Stoma reversals | 585 | 805 | – | 1691 | 481 | 763 | – | 1691 |
| Stoma supplies | 547 | 292 | – | 1057 | 486 | 351 | – | 1057 |
| Other surgery | 729 | 2245 | – | 15,242 | 723 | 2233 | – | 7621 |
| All other costs | 590 | 494 | – | 5178 | 656 | 468 | – | 3606 |
There is an overall cost difference of £980 across the two groups of the trial over the first 6 months of treatment. Across the different categories of costs in the model, robotic surgery is around £1085 more expensive than laparoscopic surgery. The main drivers of the higher operative costs for robotic surgery are (1) the duration of surgery (357 minutes and 408 minutes, respectively), which has a knock-on effect on the cost of theatre time and staff, and (2) the use of surgical instruments. There is very little difference in the number of staff in attendance (mean number of assistants 1.7 and 1.63). As more stomas are formed with laparoscopic surgery, the mean costs of both reversal surgeries (£585 vs. £481; £104) and stoma supplies (£547 vs. £486; £61) are slightly higher for this form of surgery. Mean costs are marginally higher for the robotic group in terms of medications and other health professional contacts (e.g. outpatients, GPs, nurses, etc.), although these differences are small (£590 vs. £656; –£66).
Across this scenario, the expected costs for robotic surgery are higher (£980) and robotic surgery provides marginally more health (QALY gain 0.014). These figures combine for an estimated incremental cost-effectiveness ratio (ICER) of £69,837 per QALY. This figure is well in excess of a standard threshold of £20,000–30,000 per QALY and suggests that robotic surgery, even when not including the sizeable cost of the robot, is not cost-effective. The implication for this is that, even if this means that the robot is lying idle, it is less cost-effective to use the robot for rectal resections.
The cost-effectiveness acceptability curve (Figure 12) for this scenario provides an estimate of the likelihood that this is cost-effective at a cost-effectiveness threshold of £20,000 or £30,000 per QALY. Across the cases calculated, robotic surgery is cost-effective only 10% of the time when the threshold is taken to be £20,000 per QALY and only 20% of the time when the threshold is £30,000 per QALY.
FIGURE 12.
Cost-effectiveness acceptability curve for the primary analysis.

Across the other scenarios (Table 50), there was no strong suggestion of cost-effectiveness. For those patients who intended to receive low anterior surgery at randomisation, the QoL benefit for robotic surgery is very close to zero; there is a benefit of 0.002 QALYs and the ICER for this scenario is nearly £700,000 per QALY. Although there is still some small uncertainty about cost-effectiveness (there is a 10% chance that robotic surgery is cost-effective at £30,000 per QALY), there is no clear case for cost-effectiveness in this subgroup. Although the corresponding cost-effectiveness would be higher among the other intended surgical groups at baseline, there is no clinical reason to justify the consideration of these subgroups.
| Scenario | Incremental cost (£) | Incremental QALY | Incremental cost-effectiveness ratio (£ per QALY) | Likelihood of cost-effectiveness (%) | |
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| Baseline (UK, USA) (n = 190) | 980 | 0.014 | 69,837 | 9.8 | 19.5 |
| Low anterior surgery (UK, USA) (n = 135) | 1096 | 0.002 | 698,000 | 5.9 | 9.8 |
| Complete cases (n = 97) | 1241 | 0.028 | 43,844 | 16.1 | 29.7 |
| All patients (n = 471) | 743 | 0.004 | 172,943 | 2.7 | 5.6 |
Across the two remaining sensitivity analyses, the ICER varied greatly according to the group of patients considered. Only 51% of UK and US patients in the subgroup had complete data on all costing categories and all QoL values. Data on QoL were more complete, with 133 patients providing enough information to allow QALYs to be computed. In contrast, only 99 patients provided full cost data. (Two patients provided cost data but did not provide full data for QALYs.)
In the subgroup with complete data, the ICER was lower than the baseline value, although still above the standard values for thresholds, at around £44,000 per QALY. It is worth noting that, here, the chance of cost-effectiveness at £20,000 and £30,000 per QALY is still only 16% and 30%, respectively.
However, the complete-case data appear slightly misleading. Across the 133 patients observed to have sufficient data to calculate QALYs, there is a 0.017 benefit (vs. 0.014 in the base case and 0.028 in the complete case). Across the 99 patients with full cost data, the cost difference observed (£1169) is much more similar to those in both the complete case (£1241) and the base case (£980). This suggests that the base-case results are broadly consistent with the overall data set and more representative than the complete-case example.
In contrast, the imputation across the pan-world trial (as opposed to the USA and the UK) produces quite different numbers. In this case, the benefits estimated are much lower than in the baseline case, leading to an ICER of > £170,000 per QALY.
Chapter 4 Discussion
In this study, which, to our knowledge, is the largest randomised trial of robotic-assisted surgery for patients with rectal adenocarcinoma suitable for curative resection, there were no statistically significant differences in the rates of conversion to laparotomy for robotic-assisted laparoscopic surgery compared with conventional laparoscopic surgery (8.1% vs. 12.2%, respectively), and there were no statistically significant differences in resection margin positivity, complication rates or QoL at 6 months. There is insufficient evidence to conclude that robotic-assisted laparoscopic surgery, compared with conventional laparoscopic surgery, reduces the risk of conversion to laparotomy when performed by surgeons of varying experience with robotic surgery.
The primary outcome measure was conversion to open surgery, based on the hypothesis that the technological advantages of the robot should facilitate rectal cancer resection and avoid the need to convert to an open operation. The sample size calculations were based on best available evidence in 2009 and included the largest randomised clinical trial of laparoscopic rectal cancer surgery, the MRC CLASICC trial, which reported a 34% conversion rate to open surgery. 5 A 25% conversion rate from laparoscopic to open surgery was assumed, giving a sample size of 400 patients to demonstrate a 50% relative reduction in conversion rate with robotic surgery. The actual overall conversion rate turned out to be much lower, at 10.1%. A similar reduction in conversion rates with time has been reported in other laparoscopic rectal cancer trials: COLOUR II 16%,7 ACOSOG Z6501 11%,11 ALaCaRT 9%. 10 In our trial, a difference in conversion rate between laparoscopic (12.2%) and robotic (8.1%) surgery was not statistically significant. The higher overall conversion in patients undergoing LAR than those undergoing APR probably reflects the fact that the majority of the oncological component of the operation is performed from the perineum in the abdominoperineal approach and is less affected by the laparoscopic approach. Similarly, the higher overall conversion rates for males than females, and obese patients than underweight/normal patients probably reflects the increasing technical difficulty of carrying out these procedures on these patients.
The sensitivity analysis exploring learning effects suggested a potential benefit of robotic surgery when performed by surgeons with substantial prior robotic experience, regardless of their level of laparoscopic experience. This suggests that the majority of participating surgeons were experts in laparoscopic surgery, but still in their learning phases for robotic surgery, and that at the higher end of the spectrum of experience in robotic surgery there is evidence of a benefit (in terms of conversion rate) over standard laparoscopic surgery.
In almost all of the subgroup analyses, there were insufficient numbers of patients to produce statistically meaningful comparisons between the groups regarding the need to convert to an open operation. However, differences were apparent in the conversion rates for the laparoscopic and robotic groups in males, with robotic surgery appearing to offer a benefit. Although results yielded by a subgroup analysis must be interpreted with caution, the moderate evidence of interaction between sex and treatment effect, the evidence of a difference between treatments in males, and the clinical plausibility of the robot facilitating dissections in the narrower male pelvis with more operator-controlled retraction, better optics and instrument precision, all warrant further investigation into the potential benefit of robotic surgery in this subgroup of technically challenging patients.
The experience of the participating surgeons was also evident in the low CRM+ rate (overall 5.7%), which was lower than in previous laparoscopic rectal cancer trials: COLOR II 10%, ACOSOG Z6501 12.1%, ALaCaRT 7%. Pathological grading of the plane of surgery showed a good standard, with mesorectal plane surgery observed in 76% overall. This is lower than that reported in COLOR II (88%) and ALaCaRT (87%), but similar to ACOSOG Z5601 (72.9%), and is probably because of the recognised variation in reporting between pathologists. In our trial, reporting of pathological plane of surgery was standardised to the method described by Nagtegaal and Quirke. 23 Despite this, there was considerable variation between local reporting of the plane of surgery and that reported on central review, illustrating the subjectivity in assessment and the need to interpret the results of other trials with caution.
The complication rates following laparoscopic and robotic surgery were similar and there were no safety issues attributable to the robotic system. Overall 30-day mortality was low at 0.9%, in keeping with the results of meta-analyses. 16,17 The leading causes of intraoperative morbidity were iatrogenic damage to an organ/structure and significant haemorrhage. In contrast to other studies, haemorrhage was not more frequently associated with robotic surgery. 13 Rectal cancer surgery is a high-risk intervention with 32.4% of patients experiencing a complication within 30 days and, after that, 15.5% of the patients experiencing complications between 30 days and 6 months. Complications related to the gastrointestinal tract, including anastomotic leak, were not surprisingly the most common cause of morbidity at both time points. Despite advances in operative technique and perioperative care, the high morbidity associated with rectal cancer surgery has not declined over the past few decades. There is a need for more sophisticated preoperative stratification systems to enable surgeons to predict patients at risk of complications, to stratify surgery accordingly and to put pathways in place to prevent complications from occurring and minimise the consequences should they occur.
Previous studies have shown that both laparoscopic and robotic rectal cancer surgery can result in bladder and sexual dysfunction, but suggest that recovery is earlier following robotic surgery. 19 The analysis of bladder and sexual function present in the ROLARR study was undertaken at the same time points and using the same research questionnaires as the previously reported studies. The findings do not support the published data; there was no significant benefit to postoperative bladder or sexual function from the use of the robot. Notably, there was little change in any of the I-PSS, IIEF and FSFI scores between baseline and 6 months, suggesting that the ROLARR surgeons were accomplished in autonomic nerve preservation and that clinically relevant bladder and sexual dysfunction were an infrequent event.
The case for laparoscopic surgery, rather than open surgery, for colorectal cancer is well established with proven benefits in terms of a shorter stay in hospital and a faster return to normal function. However, it has been difficult to demonstrate an advantage for the laparoscopic technique in terms of improvements in QoL. Similarly, in the ROLARR study, we have not been able to show an obvious advantage for robotic surgery over laparoscopic surgery in terms of QoL. A small benefit for robotic surgery was seen in QoL using the EQ-5D score, but no advantage over laparoscopic surgery was seen in either the physical components or the mental components in the SF-36v2 QoL analysis. Similarly, there was no difference in recovery following robotic surgery or laparoscopic surgery, as measured by the MFI-20 questionnaire. This is probably not surprising given that the extent of the surgery performed is not influenced by the surgical approach, with the robot serving as an alternative tool to enable a laparoscopic operation to be performed. Similar trends were noted using both the SF-36v2 questionnaire and the MFI-20 questionnaire, with the predictable deterioration in QoL at the 1-month follow-up time point and a period of 6 months being required before QoL returned towards baseline. This has implications for patients being scheduled for rectal cancer surgery who should be counselled about a prolonged recovery period, which extends far beyond the immediate postoperative period. Women, in particular, appear to be more likely to experience a protracted recovery than males.
Results from the health economics analysis suggest that robotic rectal cancer surgery is unlikely to be cost saving. The mean difference per operation, excluding the acquisition and maintenance costs, was £980 and was driven by longer operating theatre time and increased costs for robotic instruments. This concurs with previously reported data, which consistently report longer operating times associated with the robot. 21 When considering robotic surgery as a whole, rather than just rectal cancer surgery, one has to consider the cost of the purchase and maintenance of the system, the operational life and the total utilisation of the robot per year for all robotic procedures. Estimates of acquisition costs in 2017 varied between £0.43M and £1.8M with maintenance costs between £0.06M and £0.12M per year. 45 Assuming a mid-point acquisition cost of £1.12M and a mid-point maintenance cost of £0.896M per year, with an operational life/amortisation period of 7 years,46,47 the total cost of a robot would be around £1.738M. Estimates for total utilisation of the robot per year in 2017 varied between 819,000 and 843,000 procedures across 3919 installed systems, or 1505 procedures per robot over 7 years. 45 This gives the total fixed costs of around £1155 per procedure, in addition to the variable costs. Alternatively stated, the net benefits (excluding fixed costs) of any robotic procedure included in a set of cost-effective procedures needs to be positive, and the whole set of cost-effective procedures needs to have an average net benefit of at least £1155. On average, all robot procedures combined must exceed this figure, with all procedures making at least some positive contribution. On the basis of the evidence presented here, robotic rectal cancer surgery does not appear to provide a positive contribution and does not appear to be justified given the extra costs and the equivalency of clinical outcomes.
Analysis of the long-term outcomes, local recurrence, and DFS and OS, failed to show a difference between the treatment groups and conformed to the recognised patterns seen following rectal cancer surgery. Interestingly, local recurrence was more common in males than in females, which might reflect the increased technical difficulty in operating in the narrower male pelvis, although there was no benefit from robotic surgery compared with laparoscopic surgery in male patients. Although there was no difference between the treatment groups in DFS, those patients undergoing APR were most at risk of disease recurrence, and those undergoing HAR were least at risk. Males fared worse in terms of OS, which might be related to the higher risk of local recurrence, but will also be influenced by the generally shorter life expectancy for males, with no difference whether or not the operation was performed with the robot or by standard laparoscopy.
Limitations
The ROLARR study had several limitations. The much lower than anticipated rate of conversion to laparotomy limits the ability to provide conclusive evidence about our primary question of how robotic surgery compares with conventional laparoscopic surgery in terms of the odds of conversion. However, the fact that no statistically significant differences between the treatment groups were seen in any of the end points does suggest that robotic surgery, when performed by surgeons with varying robotic experience, does not confer a clinically important benefit over laparoscopic surgery in the short term.
No blinding to treatment allocation was incorporated into this trial. Our primary end point and the measure of mortality will certainly be unaffected by this, as an objective end point. However, there is the potential for end points that are not completely objective to have been affected. In our pathology end points, including CRM+, we have guarded against this by carrying out a blinded central review of pathology assessments.
Despite enforcing a mandatory minimum experience level for surgeon participation, operations in this trial were performed, on average, by a surgeon considered to be an expert in conventional laparoscopic surgery and who may still have been in their learning phase for robotic surgery. The prespecified sensitivity analysis of learning effects addresses this, by extending the primary analysis model to analyse the interaction between operating surgeon experience and the treatment effect.
The primary analysis adjusted only for stratification factors (including operating surgeon) and thus it did not include an adjustment for treating site in particular. A (prespecified) adjustment for treating site was considered in a sensitivity analysis, but model estimation issues were caused by the small sizes of the resulting strata, resulting in no meaningful output.
Chapter 5 Conclusions
The ROLARR study provides the first rigorous evaluation of robotic-assisted surgery compared with laparoscopic surgery for rectal cancer. It has failed to show an advantage for the robotic technique, although interesting trends have been noted. In particular, the trend to reduced conversions in males, and perhaps those patients undergoing LAR, deserves further investigation. A registry is currently being designed under the auspices of the European Society of Coloproctology that should enable data on several hundred robotic rectal cancer operations to be collected in a relatively short time frame and might provide further insight into the trends observed in the ROLARR study.
The health economic evaluation performed in the ROLARR study concludes that robotic rectal cancer surgery is not cost-effective compared with laparoscopic surgery. Although it is tempting to generalise this to wider surgical practice and the health-care provision of future robotic services, it should be borne in mind that the ROLARR study investigated only a single robotic system, the da Vinci surgical robot, which was the only system that was commercially available at the time. There have subsequently been rapid developments in other surgical robotic platforms, with several expected to come to market within the next couple of years. Future systems promise to be more competitive in terms of costs, with per-procedure costs challenging those of laparoscopic surgery. The health economic data from the ROLARR study will be beneficial to commercial companies developing robotic systems, in particular highlighting the need to bring the cost of robotic instruments down in order to be competitive with laparoscopic surgery.
Any judgement about the future of robotic surgery based on the ROLARR study should be tempered with future developments borne in mind. The situation is further complicated by the recent debate about the benefits of laparoscopic surgery that has been stirred by the recent publication of two large randomised trials comparing laparoscopic with open surgery for rectal cancer: the ALaCarte and ACOSOG trials. Both these studies failed to show the non-inferiority of laparoscopic surgery compared with open surgery for rectal cancer in terms of a short-term composite pathological outcome. It is therefore not clear whether or not any future analysis of robotic rectal cancer surgery should include an assessment of open surgery as well as laparoscopic techniques. In the UK’s NHS, the adoption of laparoscopic rectal cancer surgery is probably too advanced to countenance reverting back to open surgery, unless there is hard long-term evidence to suggest otherwise.
Acknowledgements
We are indebted to the patients who participated in this trial. We would also like to thank the TSC (Michael Parker, Lynn Faulds Wood, Gareth Griffiths and Hamish Macdonald), the DMEC (Azad Najmaldin, Nigel Scott and Craig Ramsay), additional members of the Trial Management Group (Jennifer Barrie, Sarah Perry, Christopher Garbett, Helen Howard and Fiona Collinson), and the CTRU Project Team for their important contributions.
Patient and public involvement
Patients were involved in all stages of the design and delivery of the ROLARR trial. Christopher Garbett provided valuable input into all aspects of the study and served on the Trial Management Committee, and Lynn Faulds Wood served on the Trail Steering Committee.
The following institutions and surgeons participated in the trial.
Aarhus University Hospital (Denmark): Soren Laurberg, Niels Thomassen and Lene H Iversen; Aria Health (USA): Luca Giordano; Augusta Kranken Anstalt (Germany): Benno Mann; Azienda Ospedaliera SS Antonio e Biagio (Italy): Giuseppe Spinoglio; Barnet & Chase Farm Hospitals NHS Trust (UK): Daren Francis; Centre Oscar Lambret (France): Mehrdad Jafari; Christie Hospital (UK): Chelliah Selvasekar; Duke University (USA): Linda Farkas and Michael Hopkins; European Institute of Oncology: Unit of Integrated Abdominal Surgery (Italy) – Fabrizio Luca and Roberto Biffi; European Institute of Oncology: Unit of Minimally Invasive Surgery (Italy) – Paolo Pietro Bianchi and Roberto Biffi; Frimley Park (UK): Henry Tilney and Mark Gudgeon; Gangnam Severance Hospital (South Korea): Kang Young Lee; Hospital Herlev, Copenhagen University (Denmark): Jacob Rosenburg, Henrik Loft Jakobsen, Mads Bundgaard, Jens Ravn Eriksen, Jesper Olsen and Thomas Bent Harvald; Jackson South Community Hospital (USA): Gustavo Plasencia and Henry J Lujan; John Muir Medical Center (USA): Samuel Oommen; Leeds Teaching Hospital Trust (UK): David Jayne, Richard Baker and Julian Hance; National University Hospital (Singapore): Charles Tsang; Oulu University Hospital (Finland): Tero Rautio; Peter MacCallum Cancer Centre (Australia): Andrew Craig Lynch; Roskilde Hospital (Denmark): Per Jess, Michael Seiersen, Ole Roikjaer and Steffen Brisling; Royal Surrey County Hospital (UK): Tim Rockall; San Pio X Hospital (Italy): Jacques Megevand; Torbay Hospital (UK): Stephen Mitchell; University of California, Irvine Medical Center (USA): Alessio Pigazzi, Joseph C. Carmichael and Steven Mills; University of Pisa (Italy): Luca Morelli; Washington University in St. Louis School of Medicine (USA): Elisa Birnbaum and Paul Wise.
The following people were local pathologists for the trial:
Aarhus University Hospital (Denmark): Rikke Hagemann-Madsen and Katrine Stribolt; Aria Health (USA): Peter Farano, Thomas Rizzo Jr and Behnaz Toorkey; Augusta Kranken Anstalt (Germany): Stathis Philippou and Konrad Morgenroth; Azienda Ospedaliera SS Antonio e Biagio (Italy): Narciso Mariani, Paola Barbieri and Paola Re; Barnet & Chase Farm Hospitals NHS Trust (UK): Khurram Chaudhary and Anupam Joshi; Centre Oscar Lambret (France): Charles André; Christie Hospital (UK): Bipasha Chakrabarty, Guy Betts, Igor Racu-Amoasii and Khalifa Sawalem; Duke University (USA): Carol Filomena; European Institute of Oncology: Unit of Integrated Abdominal Pelvic Surgery (Italy) – Angelica Sonzogni and Luca Bottiglieri; European Institute of Oncology: Unit of Minimally Invasive Surgery (Italy) – Angelica Sonzogni and Luca Bottiglieri; Frimley Park (UK): Salome Beeslaar and George Kousparos; Gangnam Severance Hospital (South Korea): Soon Won Hong; Hospital Herlev, Copenhagen University (Denmark): Jill Levin Langhoff and Peter Ingeholm; Jackson South Community Hospital (USA): Rebeca Porto; John Muir Medical Center (USA): Barry Latner; Leeds Teaching Hospital Trust (UK): Padmini Prasad and Heike Grabsch; National University Hospital (Singapore): Teh Ming, Fredrik Petersson and Wang Shi; Oulu University Hospital (Finland): Markus Mäkinen and Tuomo Karttunen; Peter MacCallum Cancer Centre (Australia): Phillip Moss and Catherine Mitchell; Roskilde Hospital (Denmark): Peter Engel, Susanne Eiholm, Matteo Biagini and Anne Mellon Mogensen; Royal Surrey County Hospital (UK): Izhar Bagwan; San Pio X Hospital (Italy): Claudio Clemente, Anna Maria Ferrari, Stefania Rao and Cristina Campidelli; Torbay Hospital (UK): Nick Ryley, Ian Buley and Maria Consuelo Garrido; University of California, Irvine Medical Center (USA): Mark Li-Cheng Wu, Roberta Edwards and Philip Carpenter; University of Pisa (Italy): Daniela Campani and Luca Emanuele Pollina; Washington University in St. Louis School of Medicine (USA): Ilke Nalbantoglu.
Contributions of authors
David Jayne (Professor of Surgery) contributed to the design of the study, patient recruitment, data interpretation and manuscript writing.
Alessio Pigazzi (Consultant Surgeon) contributed to the design of the study, patient recruitment, data interpretation and manuscript writing.
Helen Marshall (Senior Statistician) contributed to the study design and oversight, data analysis and interpretation, and manuscript writing.
Julie Croft (Senior Clinical Triallist) contributed to the study design, study coordination and manuscript writing.
Neil Corrigan (Senior Statistician) contributed to the study design and oversight, data analysis and interpretation, and manuscript writing.
Joanne Copeland (Clinical Triallist) contributed to the study coordination and manuscript writing.
Philip Quirke (Professor of Pathology) designed the pathology protocol, provided training to other centres, reviewed the pathology and interpreted the pathology data.
Nicholas West (Academic Consultant Histopathologist) contributed to the design of the pathology protocol, reviewed the pathology and interpreted the pathology data.
Richard Edlin (Health Economist) contributed to the design of the cost-effectiveness study, data analysis and manuscript writing.
Claire Hulme (Professor of Health Economics) contributed to the design of the cost-effectiveness study, data analysis and manuscript writing.
Julia Brown (Professor of Statistics and Director of the Leeds Clinical Trial and Research Unit) contributed to the study design and oversight, data analysis and interpretation, and manuscript writing. All authors reviewed the final manuscript.
Publications
Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 2012;27:233–41.
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, et al. Effect of robotic-assisted vs. conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 2017;318:1569–80.
Corrigan N, Marshall H, Croft J, Copeland J, Jayne D, Brown J. Exploring and adjusting for potential learning curve effects in ROLARR: a randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials 2018;19:339–49.
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993;341:457-60. https://doi.org/10.1016/0140-6736(93)90207-W.
- Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061-8. https://doi.org/10.1200/JCO.2006.09.7758.
- Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. https://doi.org/10.1056/NEJMoa032651.
- Tang CL, Eu KW, Tai BC, Soh JG, MacHin D, Seow-Choen F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg 2001;88:801-7. https://doi.org/10.1046/j.1365-2168.2001.01781.x.
- Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. https://doi.org/10.1016/S0140-6736(05)66545-2.
- Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 2010;97:1638-45. https://doi.org/10.1002/bjs.7160.
- van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group . Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-18. https://doi.org/10.1016/S1470-2045(13)70016-0.
- Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32. https://doi.org/10.1056/NEJMoa1414882.
- Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. https://doi.org/10.1016/S1470-2045(10)70131-5.
- Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 2015;314:1356-63. https://doi.org/10.1001/jama.2015.12009.
- Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 2015;314:1346-55. https://doi.org/10.1001/jama.2015.10529.
- Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 2006;20:1521-5. https://doi.org/10.1007/s00464-005-0855-5.
- Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol 2007;14:3168-73. https://doi.org/10.1245/s10434-007-9544-z.
- Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc 2008;22:1601-8. https://doi.org/10.1007/s00464-008-9752-z.
- Keller DS, Hashemi L, Lu M, Delaney CP. Short-term outcomes for robotic colorectal surgery by provider volume. J Am Coll Surg 2013;217:1063-9.e1. https://doi.org/10.1016/j.jamcollsurg.2013.07.390.
- Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ. Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot 2012;8:360-70. https://doi.org/10.1002/rcs.1426.
- Mirnezami AH, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ. Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis 2010;12:1084-93. https://doi.org/10.1111/j.1463-1318.2009.01999.x.
- Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis of eight studies. J Gastrointest Surg 2015;19:516-26. https://doi.org/10.1007/s11605-014-2697-8.
- Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 2012;19:2485-93. https://doi.org/10.1245/s10434-012-2262-1.
- Kim CW, Baik SH, Roh YH, Kang J, Hur H, Min BS, et al. Cost-effectiveness of robotic surgery for rectal cancer focusing on short-term outcomes: a propensity score-matching analysis. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000000823.
- Ramji KM, Cleghorn MC, Josse JM, MacNeill A, O’Brien C, Urbach D, et al. Comparison of clinical and economic outcomes between robotic, laparoscopic, and open rectal cancer surgery: early experience at a tertiary care center. Surg Endosc 2016;30:1337-43. https://doi.org/10.1007/s00464-015-4390-8.
- Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 2012;27:233-41. https://doi.org/10.1007/s00384-011-1313-6.
- Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer?. J Clin Oncol 2008;26:303-12. https://doi.org/10.1200/JCO.2007.12.7027.
- Keller DS, McGee MF, Goyal S, Nobel T, O’Brien Ermlich B, Cheruvu VK, et al. Construct validation and comparison of a novel postoperative quality-of-life metric and the Short Form-36 in colorectal surgery patients. Surgery 2013;154:690-5. https://doi.org/10.1016/j.surg.2013.06.037.
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39:315-25. https://doi.org/10.1016/0022-3999(94)00125-O.
- Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 1992;148:1549-57. https://doi.org/10.1016/S0022-5347(17)36966-5.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. https://doi.org/10.1016/S0090-4295(97)00238-0.
- Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191-208. https://doi.org/10.1080/009262300278597.
- World Health Organization . Body Mass Index (BMI) Classification n.d. www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed 11 August 2019).
- Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer 2004;91:1229-35. https://doi.org/10.1038/sj.bjc.6602102.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 n.d. https://gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 17 October 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Information Services Division, NHS National Services Scotland . Theatres n.d. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- NHS Employers . Agenda for Change Pay Bands and Points from 1 April 2015 (England) n.d. http://nhsemployers.org/-/media/Employers/Documents/Pay-and-reward/AfC-pay-bands-from-1-April-2015.pdf (accessed 17 October 2018).
- Jones S. Value of the Nurse Led Stoma Care Clinic. Royal Glamorgan &Amp; Ysbyty Cwm Rhondda Hospitals 2015. www.rcn.org.uk/professional-development/research-and-innovation/innovation-in-nursing/case-studies-demonstrating-the-value-of-nursing/sheila-jones (accessed 11 August 2019).
- Joint Formulary Committee . British National Formulary n.d. https://bnf.org/products/bnf-online/ (accessed 17 October 2018).
- Department of Health and Social Care . Drugs and Pharmaceutical Electronic Market Information Tool n.d. https://gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 17 October 2018).
- NHS Business Services Authority . Drug Tariff n.d. https://nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed 17 October 2018).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 11 August 2019).
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the NICE cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Intuitive Surgical, Inc . Annual Report 2018 n.d. https://isrg.gcs-web.com/static-files/31b5c428-1d95-4c01-9c85-a7293bac5e05 (accessed 11 August 2019).
- Iavazzo C, Papadopoulou EK, Gkegkes ID. Cost assessment of robotics in gynecologic surgery: a systematic review. J Obstet Gynaecol Res 2014;40:2125-34. https://doi.org/10.1111/jog.12507.
- Coronado PJ, Fasero M, Magrina JF, Herraiz MA, Vidart JA. Comparison of perioperative outcomes and cost between robotic-assisted and conventional laparoscopy for transperitoneal infrarenal para-aortic lymphadenectomy (TIPAL). J Minim Invasive Gynecol 2014;21:674-81. https://doi.org/10.1016/j.jmig.2014.01.023.
Appendix 1 Primary end point (conversion to open surgery) analysis: further details
| Model | AIC | Deviance | Difference in deviance | p-value (likelihood ratio test) |
|---|---|---|---|---|
| Random intercept | 276.94 | 258.94 | 1.22 | 0.269 |
| Random slope | 277.72 | 257.72 |
Model diagnostics
Residual index plot
Figure 13 presents the index plot of raw residuals (including EBEs of random effects) on the probability scales (y-axis) versus patient ID (x-axis). Residual ri for patient i is calculated as:
where
and p^i is the predicted probability of conversion to open surgery for patient i (including EBE of the random effect). Residuals with larger absolute values indicate poorer model fit. Patient 374 (labelled in Figure 13) stands out as an instance of poor model fit, with the model yielding a predicted probability of conversion of 0.676, but they were not converted. Many of the residuals for patients who did convert to open surgery (shown in green in Figure 13) are large, indicating that the model fitted low probabilities of conversion for those patients, but this is reasonable and perhaps expected because conversion to open surgery was an infrequent event. The empirical probability plot helps us to objectively determine what magnitude of residual is ‘expected’.
FIGURE 13.
Conversion to open surgery: index plot of raw residuals of the random intercept model. Scatterplot: raw residuals on the probability scale (including EBEs of random effects) by observed outcome.

Figure 14 presents the empirical probability plot for the primary analysis model, which can be used to compare actual Pearson residuals with expected Pearson residuals. The y-axis is the actual Pearson residual value, the x-axis is the empirical median Pearson residual expected under our fitted model assumptions. The dots represent patients. If the model was a perfect fit, then we would expect all of the dots to lie on the reference line. The band in Figure 14 represents the interval between the empirical 2.5th percentile and 97.5th percentile empirical Pearson residual. No values lie outside this region, indicating that we do not have any substantial outliers.
FIGURE 14.
Conversion to open surgery: empirical probability plot (including simulated envelope of 2.5th–97.5th percentile empirical Pearson residual) of raw residuals of the random intercept model.
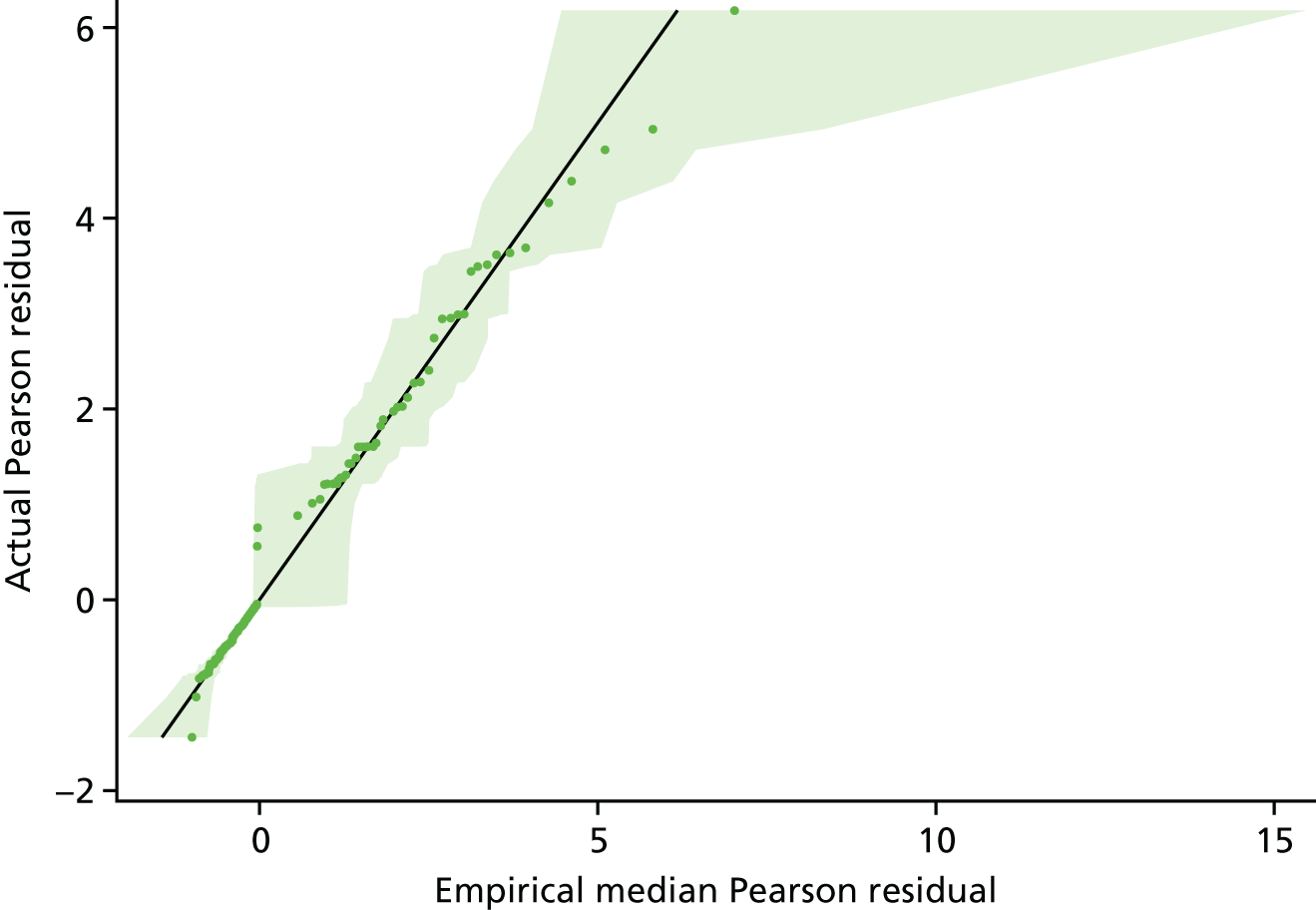
Delta-betas
Figure 15 presents the plot of exponentiated delta-betas (y-axis) versus patient ID. Exponentiated delta-betas further from 1 indicate greater influence of the observation on the estimated treatment effect. Conversions to open surgery demonstrably have greater influence on the estimated treatment effect, which is perhaps expected given that conversion to open surgery was an infrequent event. Patient 374 stands out as having high influence for a non-conversion to open surgery. Patient 374 was in the robotic treatment group and their exponentiated delta-beta is 1.051, indicating that the estimated OR for conversion to open surgery (robotic vs. laparoscopic) increases by a factor of 1.051 when they are removed from the model, from the original 0.614 (95% CI 0.311 to 1.211; p = 0.16) to 0.645 (95% CI 0.325 to 1.280; p = 0.21).
FIGURE 15.
Conversion to open surgery: plot of exponentiated delta-betas from the random intercept model. Scatterplot: random intercept model delta-betas by observed outcome.

Further investigation of outliers
The observation for patient 374 is genuine. It is just a relatively unexpected outcome, rather than erroneous data. Patient 374 was male, obese and underwent a LAR, all indicating a higher risk of conversion to open surgery according to the model. Furthermore, their operating surgeon converted 10 out of 33 (30.3%) of their ROLARR patients to open surgery: a much higher rate than average. The model therefore estimated a 67.6% probability of conversion to open surgery for patient 374 but they were not converted, hence the large magnitude of the residual. The EBE of this surgeon’s effect on the odds of conversion was that they increased the odds by a factor of around 5 (i.e. a patient being operated on by this surgeon was estimated to be five times more likely to convert to open surgery than a patient operated on by the average surgeon, according to this model). This is a larger effect than any of the other patient factors. However, patient 374 was this surgeon’s last patient in the ROLARR study, and their level of robotic experience changed substantially throughout their participation in the study, from 35 previous robotic operations before their first patient to 121 before patient 374. Most of the surgeon’s conversions came during their earlier ROLARR cases. The learning effects analysis (see Chapter 3, Sensitivity analysis: learning effects) accounts for this improvement over time and gives a predicted probability of conversion of 42.3% for patient 374, yielding a smaller residual that lies more comfortably within the expected range of residual values. It seems that the large residual in this model is therefore mainly because of poor fit as a consequence of assuming no learning effects.
Sensitivity analysis: actual operating surgeon – further details
| Surgeon other than randomised | Treatment group, n (%) | Total (N = 466), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 230) | Robotic-assisted laparoscopic surgery (N = 236) | ||
| Yes | 16 (7.0) | 26 (11.0) | 42 (9.0) |
| No | 214 (93.0) | 210 (89.0) | 424 (91.0) |
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 28/230 (12.2) | 19/236 (8.1) | 4.1 (–1.4 to 9.6) | 0.612 | 0.310 to 1.207 | 0.16 |
| Sex: male (vs. female) | 8/149 (5.4) | 39/317 (12.3) | –6.9 (–12.1 to 1.8) | 2.449 | 1.046 to 5.734 | 0.04 |
| BMI class: overweight (vs. underweight/normal) | 13/179 (7.3) | 9/180 (5.0) | 2.3 (–2.7 to 7.2) | 0.547 | 0.215 to 1.396 | 0.21 |
| BMI class: obese (vs. underweight/normal) | 13/179 (7.3) | 25/107 (23.4) | –16.1 (–25.0 to –7.2) | 4.649 | 2.069 to 10.448 | 0.0002 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 27/262 (10.3) | 20/204 (9.8) | 0.5 (–5.0 to 6.0) | 1.094 | 0.512 to 2.338 | 0.82 |
| Intended procedure: HAR (vs. LAR) | 37/312 (11.9) | 6/68 (8.8) | 3.0 (–4.6 to 10.7) | 0.564 | 0.199 to 1.598 | 0.28 |
| Intended procedure: APR (vs. LAR) | 37/312 (11.9) | 4/86 (4.7) | 7.2 (1.5 to 12.9) | 0.185 | 0.054 to 0.631 | 0.007 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.612 | 0.412 |
Sensitivity analysis: actual procedure – further details
The most notable effect that adjusting for actual procedure had on the model estimates was on the effect of APR (vs. LAR), which is attenuated compared with the primary analysis model. Specifically, in this model (Table 57) the OR is 0.433 (95% CI 0.165 to 1.134; p = 0.088) compared with 0.184 (95% CI 0.054 to 0.627; p = 0.007) in the primary analysis model.
| Procedure performed | Treatment group, n (%) | Total (N = 466), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 230) | Robotic- assisted laparoscopic surgery (N = 236) | ||
| HAR | 19 (8.3) | 28 (11.9) | 47 (10.1) |
| LAR | 165 (71.7) | 152 (64.4) | 317 (68.0) |
| APR | 45 (19.6) | 52 (22.0) | 97 (20.8) |
| Other | 1 (0.4) | 4 (1.7) | 5 (1.1) |
| Patient ID | Treatment allocation | Intended procedure | Details of procedure |
|---|---|---|---|
| 113 | Robotic | LAR | Dorsal pelvic exenteration, ureter resection distally right sided |
| 139 | Robotic | LAR | Hartmann’s procedure |
| 143 | Standard | LAR | Laparoscopic biopsy of peritoneum |
| 183 | Robotic | LAR | HAR + subtotal colectomy |
| 429 | Robotic | HAR | Hartmann’s procedure |
| Effect: comparator group (vs. reference group) | Group [number of conversions/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted) | 95% CI for OR (adjusted) | p-value | |
|---|---|---|---|---|---|---|
| Reference | Comparator | |||||
| Treatment: robotic surgery (vs. laparoscopic) | 28/230 (12.2) | 19/236 (8.1) | 4.1 (–1.4 to 9.6) | 0.572 | 0.289 to 1.132 | 0.1083 |
| Sex: male (vs. female) | 8/149 (5.4) | 39/317 (12.3) | –6.9 (–12.1 to 1.8) | 2.401 | 1.034 to 5.573 | 0.0416 |
| BMI class: overweight (vs. underweight/normal) | 13/179 (7.3) | 9/180 (5.0) | 2.3 (–2.7 to 7.2) | 0.562 | 0.221 to 1.432 | 0.2264 |
| BMI class: obese (vs. underweight/normal) | 13/179 (7.3) | 25/107 (23.4) | –16.1 (–25.0 to –7.2) | 4.374 | 1.972 to 9.700 | 0.0003 |
| Previous radiotherapy or chemoradiotherapy: yes (vs. no) | 27/262 (10.3) | 20/204 (9.8) | 0.5 (–5.0 to 6.0) | 1.050 | 0.509 to 2.166 | 0.8956 |
| Procedure: HAR (vs. LAR) | 33/317 (10.4) | 4/47 (8.5) | 1.9 (–6.8 to 10.6) | 0.718 | 0.209 to 2.464 | 0.5975 |
| Procedure: APR (vs. LAR) | 33/317 (10.4) | 8/97 (8.3) | 2.2 (–4.3 to 8.6) | 0.433 | 0.165 to 1.134 | 0.0883 |
| Procedure: other (vs. LAR) | 33/317 (10.4) | 2/5 (40.0) | –29.6 (–72.7 to 13.5) | 5.934 | 0.689 to 51.079 | 0.1047 |
| Effect | Variance component | |
|---|---|---|
| Estimate | Standard error | |
| Operating surgeon (random effect) | 0.535 | 0.389 |
Appendix 2 Key secondary end point: circumferential resection margin positivity (CRM+)
Model diagnostics
Residual index plot
Figure 16 presents the index plot of raw residuals (including EBEs of random effects) on the probability scales (y-axis) versus patient ID (x-axis). Residual ri for patient i is calculated as:
in which:
and p^i is the predicted probability of CRM+ for patient i (including EBE of the random effect). Residuals with larger absolute values indicate poorer model fit. Many of the residuals for patients who did have CRM+ (shown in green in Figure 16) are large, indicating that the model fitted low probabilities of CRM+ for those patients, but this is reasonable and perhaps expected because CRM+ was a rare event. There are no clear outliers in Figure 16. The empirical probability plot helps us to objectively determine what magnitude of residual is ‘expected’.
FIGURE 16.
Circumferential resection margin positivity: index plot of raw residuals of the random intercept model. Scatterplot: raw residuals on the probability scale (including EBEs of random effects) by observed outcome.

Figure 17 presents the empirical probability plot for the primary analysis model, which can be used to compare actual Pearson residuals with expected Pearson residuals. The y-axis is the actual Pearson residual value and the x-axis is the empirical median Pearson residual expected under our fitted model assumptions. The dots represent patients. If the model was a perfect fit, then we would expect all of the dots to lie on the reference line. The band in Figure 17b represents the interval between the empirical 2.5th percentile and 97.5th percentile empirical Pearson residual. No values lie outside this region, indicating that we do not have any substantial outliers.
FIGURE 17.
Circumferential resection margin positivity: empirical probability plot (including simulated envelope of 2.5th–97.5th percentile empirical Pearson residual) of raw residuals of the random intercept model.


There are two plots:
-
empirical probability plot with confidence region
-
same as plot 1 except restricted to only negative residuals (this has been added because the negative residuals are difficult to read on the other plot).
Delta-betas
Figure 18 presents the plot of exponentiated delta-betas (y-axis) versus patient ID. Exponentiated delta-betas further from 1 indicate greater influence of the observation on the estimated treatment effect. The CRM+ observations demonstrably have greater influence on the estimated treatment effect, which is expected given that CRM+ was a rare event. There are no clear overly influential observations.
FIGURE 18.
Circumferential resection margin positivity: plot of exponentiated delta-betas from the random intercept model. Scatterplot: random intercept model delta-betas by observed outcome.
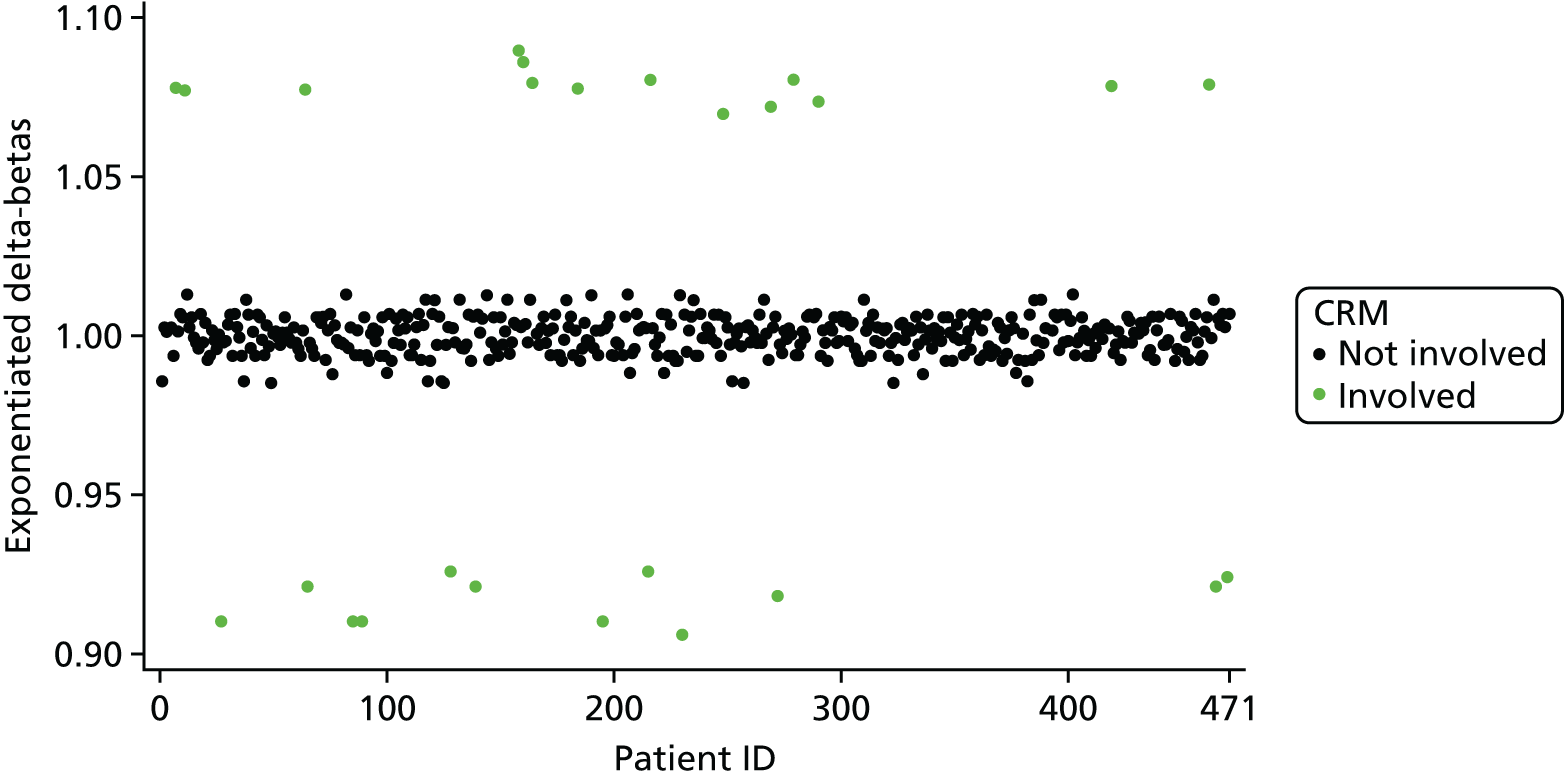
Subgroup analyses
| Effect | Treatment group [number of CRM+/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted 95% CI)a | p-value | ||
|---|---|---|---|---|---|---|
| Laparoscopic surgery | Robotic surgery | |||||
| Treatment in males: robotic surgery (vs. laparoscopic) | 10/151 (6.6) | 12/160 (7.5) | –0.9 (–6.6 to 4.8) | 1.118 (0.462 to 2.705) | 0.9961 | 0.9961b |
| Treatment in females: robotic surgery (vs. laparoscopic) | 4/73 (5.5) | 0/75 (0.0) | 5.5 (0.3 to 10.7) | < 0.001 (0 to ∞) | 1.000 | |
| Effect | Treatment group [number of CRM+/number of patients (%)] | Risk difference (unadjusted 95% CI) | OR (adjusted 95% CI)a | p-value | ||
|---|---|---|---|---|---|---|
| Laparoscopic surgery | Robotic surgery | |||||
| Treatment in obese patients: robotic-assisted surgery (vs. laparoscopic) | 1/52 (1.9) | 1/53 (1.9) | 0.0 (–5.2 to 5.3) | 0.989 (0.302 to 3.244) | 0.9493 | 0.5673b |
| Treatment in overweight patients: robotic-assisted surgery (vs. laparoscopic) | 6/87 (6.9) | 6/89 (6.7) | 0.2 (–7.3 to 7.6) | 0.914 (0.055 to 15.293) | 0.9856 | 0.7909b |
| Treatment in underweight and normal patients: robotic-assisted surgery (vs. laparoscopic) | 7/85 (8.2) | 5/93 (5.4) | 2.9 (–4.6 to, 10.3) | 0.604 (0.180 to 2.021) | 0.4123 | |
| Effect | Treatment group [number of CRM+/number of patients (%)] | Risk difference and (unadjusted 95% CI) | |
|---|---|---|---|
| Laparoscopic surgery | Robotic surgery | ||
| Treatment in T0 patients: robotic-assisted surgery (vs. laparoscopic) | 0/25 (0.0) | 0/26 (0.0) | . |
| Treatment in T1 patients: robotic-assisted surgery (vs. laparoscopic) | 0/24 (0.0) | 0/24 (0.0) | . |
| Treatment in T2 patients: robotic-assisted surgery (vs. laparoscopic) | 1/62 (1.6) | 2/68 (2.9) | –1.3 (–6.4 to 3.8) |
| Treatment in T3 patients: robotic-assisted surgery (vs. laparoscopic) | 11/106 (10.4) | 8/111 (7.2) | 3.2 (–4.4 to 10.7) |
| Treatment in T4 patients: robotic-assisted surgery (vs. laparoscopic) | 2/7 (28.6) | 2/5 (40.0) | –11.4 (–65.9 to 43.0) |
Appendix 3 Key secondary end point: 3-year local recurrence – further details
Local recurrences and censorings, including the reason for censoring, are summarised in Table 62.
| Nature of the end of follow-up for local recurrence analysis (by treatment group) | Treatment group, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| Event: local recurrence | 14 (6.0) | 16 (6.8) | 30 (6.4) |
| Censor: last known to be alive | 195 (83.3) | 199 (84.0) | 394 (83.7) |
| Censora: death | 16 (6.8) | 15 (6.3) | 31 (6.6) |
| Censor: withdrawal from further data collection | 5 (2.1) | 4 (1.7) | 9 (1.9) |
| Censor: non-standard circumstanceb | 4 (1.7) | 3 (1.3) | 7 (1.5) |
The methods of confirmation are summarised in Table 63.
| Method of confirmation | Treatment group, n (%) | Total (N = 30), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 14) | Robotic-assisted laparoscopic surgery (N = 16) | ||
| Clinical | 2 (14.3) | 2 (12.5) | 4 (13.3) |
| Radiological | 5 (35.7) | 6 (37.5) | 11 (36.7) |
| Pathological | 7 (50.0) | 8 (50.0) | 15 (50.0) |
Table 64 shows the estimated cumulative incidence of local recurrence by treatment group at several time points (1–5 years post randomisation).
| Time post randomisation (years) | Treatment group | |||
|---|---|---|---|---|
| Laparoscopic surgery | Robotic surgery | |||
| Probability of local recurrence | 95% CI | Probability of local recurrence | 95% CI | |
| 1 | 0.022 | 0.003 to 0.041 | 0.034 | 0.011 to 0.058 |
| 2 | 0.049 | 0.040 to 0.058 | 0.052 | 0.023 to 0.080 |
| 3 | 0.058 | 0.028 to 0.089 | 0.061 | 0.030 to 0.091 |
| 4 | 0.065 | 0.055 to 0.076 | 0.078 | 0.039 to 0.116 |
| 5 | 0.065 | 0.032 to 0.099 | 0.078 | 0.039 to 0.116 |
Model diagnostics
Deviance residuals
As a result of heavy censoring, the deviance residuals form a bimodal distribution, as seen in Figure 19. There are no clear outliers.
FIGURE 19.
Three-year local recurrence. (a) Histogram of deviance residuals; and (b) scatterplot of deviance residuals.

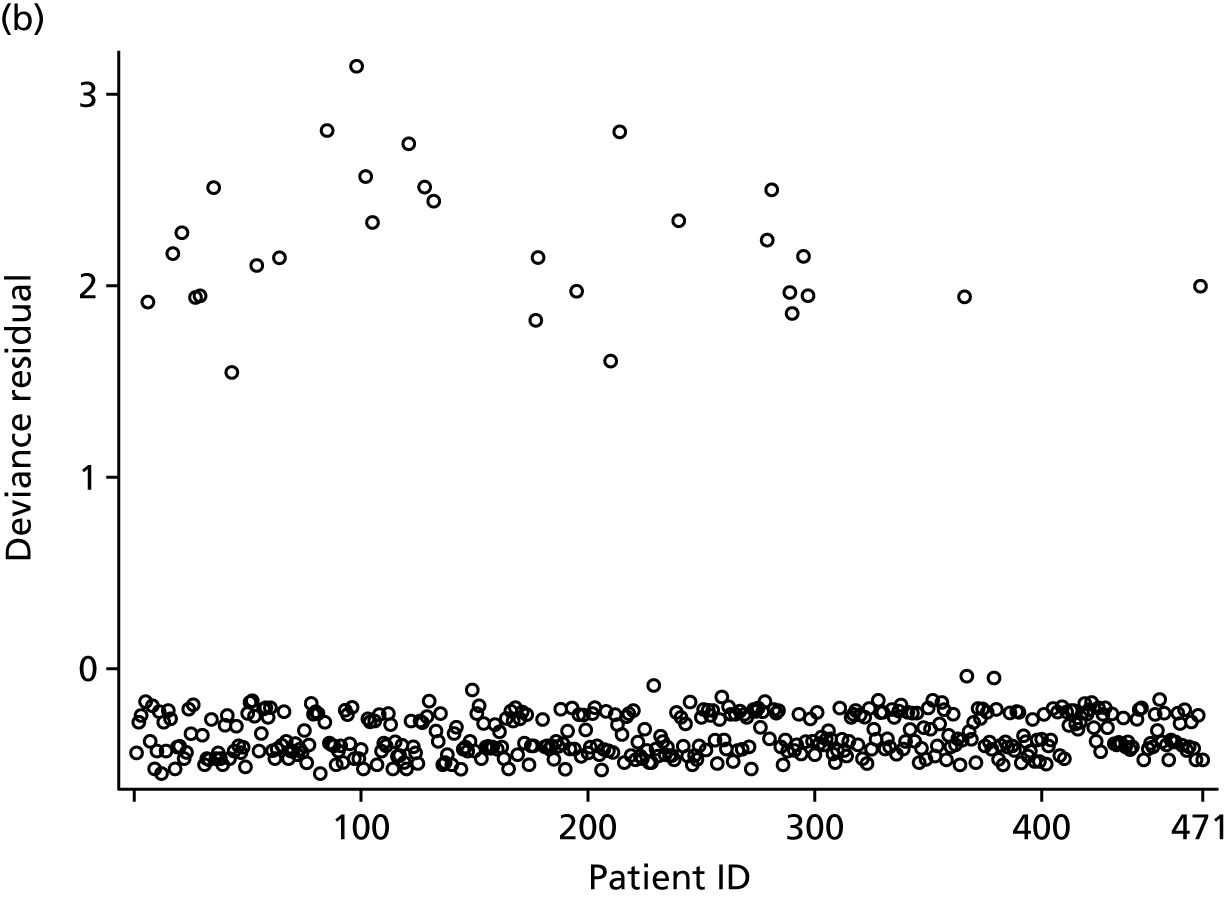
Figure 20 shows the standardised Schoenfeld residuals versus time. If the proportional hazards assumption was being violated, then we would expect the relationship between these residuals and time to deviate from a flat line in at least one of the treatment groups, but it does not. This is also reflected in Figure 21 (standardised score process), as the observed path lies within the simulated paths. Finally, the supremum test of the PH assumption returned a p-value of 0.265 relating to the treatment effect. All of this suggests that the proportional hazards assumption is viable for the treatment groups.
FIGURE 20.
Three-year local recurrence: Loess plot of standardised Schoenfeld residuals (by treatment group) for the shared frailty model.

FIGURE 21.
Three-year local recurrence: random sample of standardised score process simulated paths.
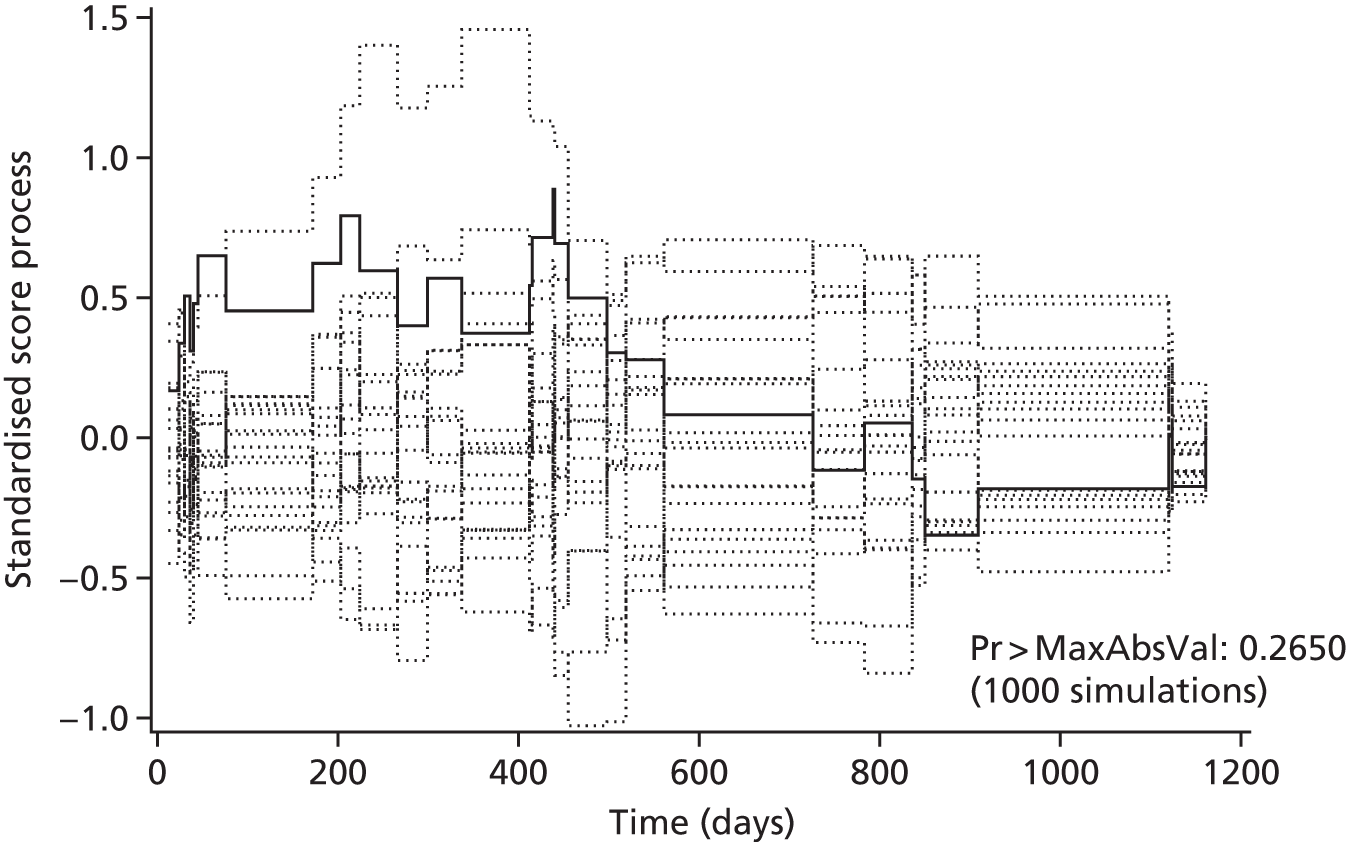
Delta-betas
Figure 22 presents the exponentiated delta-betas. As one might expect, given the low incidence of local recurrence, the influence of all observed events of local recurrence is notably greater than the influence of censored observations. There is, however, no clear overly influential observations.
FIGURE 22.
Three-year local recurrence: plot of exponentiated delta-betas from the random intercept model.
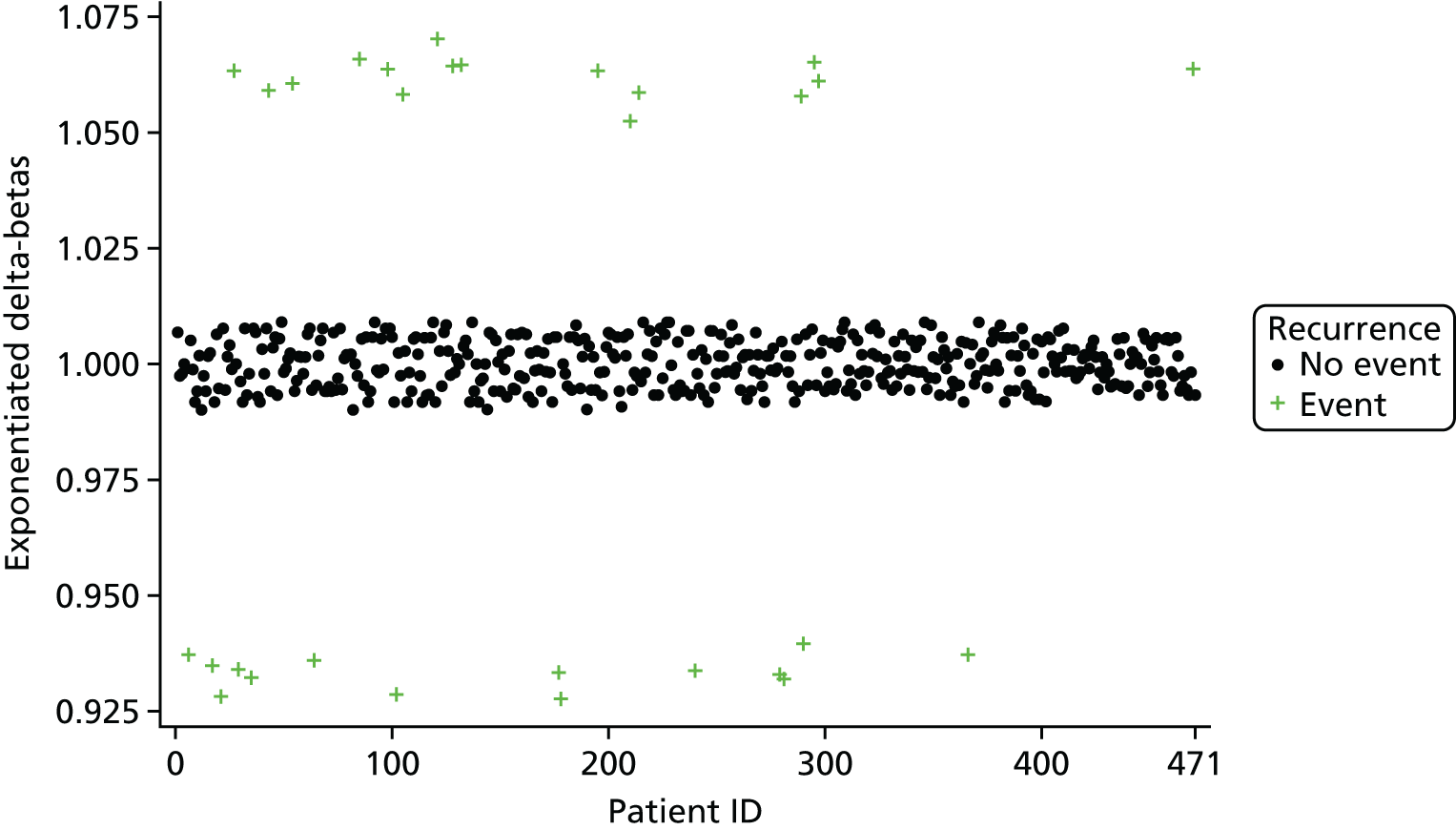
Subgroup analyses
Odds ratios presented in Tables 65 and 66 are derived from the linear combination of the estimated treatment (main effect) and treatment-by-subgroup interaction terms on the logit scale. The p-values are presented for the test of the treatment effect within each subgroup; this is the first column of p-values. For example, in Table 68 the test that the treatment effect is null (OR = 1) within the female subgroup is 1.000. The p-values are also presented for the test of heterogeneity of treatment effect across subgroups, the details of which are given in the footnotes of the tables. Note that a full model was not fitted to test T-stage subgroup analyses, because the small sample sizes and event rates within T-stage groups caused model convergence issues and so crude summaries are given.
Figures 23–26 display the Kaplan–Meier graphs for the effect of neo-adjuvant therapy, operation type, T-stage and sex, on 3-year local recurrence.
FIGURE 23.
Three-year local recurrence by neo-adjuvant therapy. (a) No neo-adjuvant therapy; and (b) neo-adjuvant therapy.

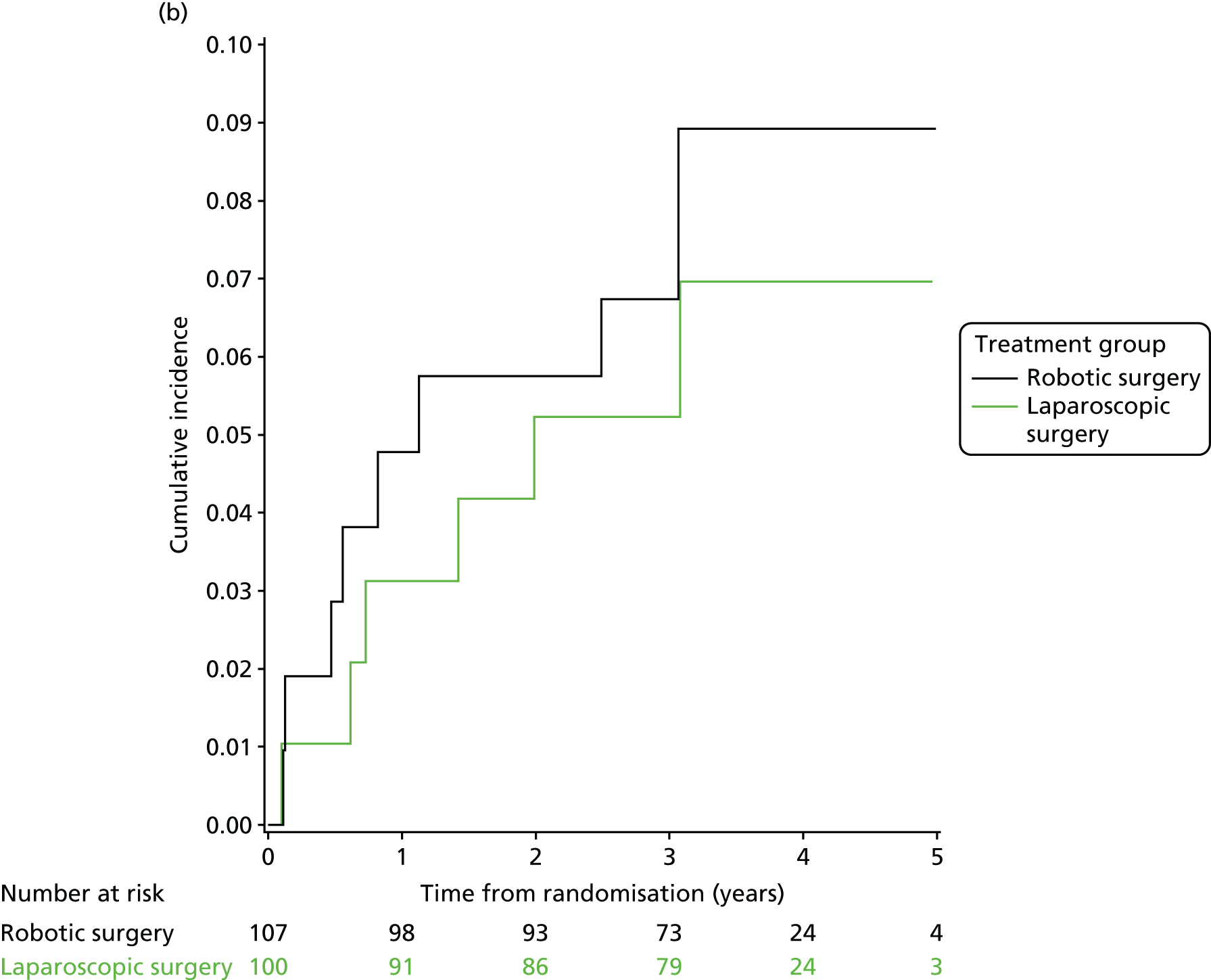
FIGURE 24.
Three-year local recurrence by operation type. (a) Cumulative incidence of local recurrence (operation = HAR); (b) cumulative incidence of local recurrence (operation = LAR); and (c) cumulative incidence of local recurrence (operation = APR).



FIGURE 25.
Three-year local recurrence by T-stage. (a) T1; (b) T2; and (c) T3.
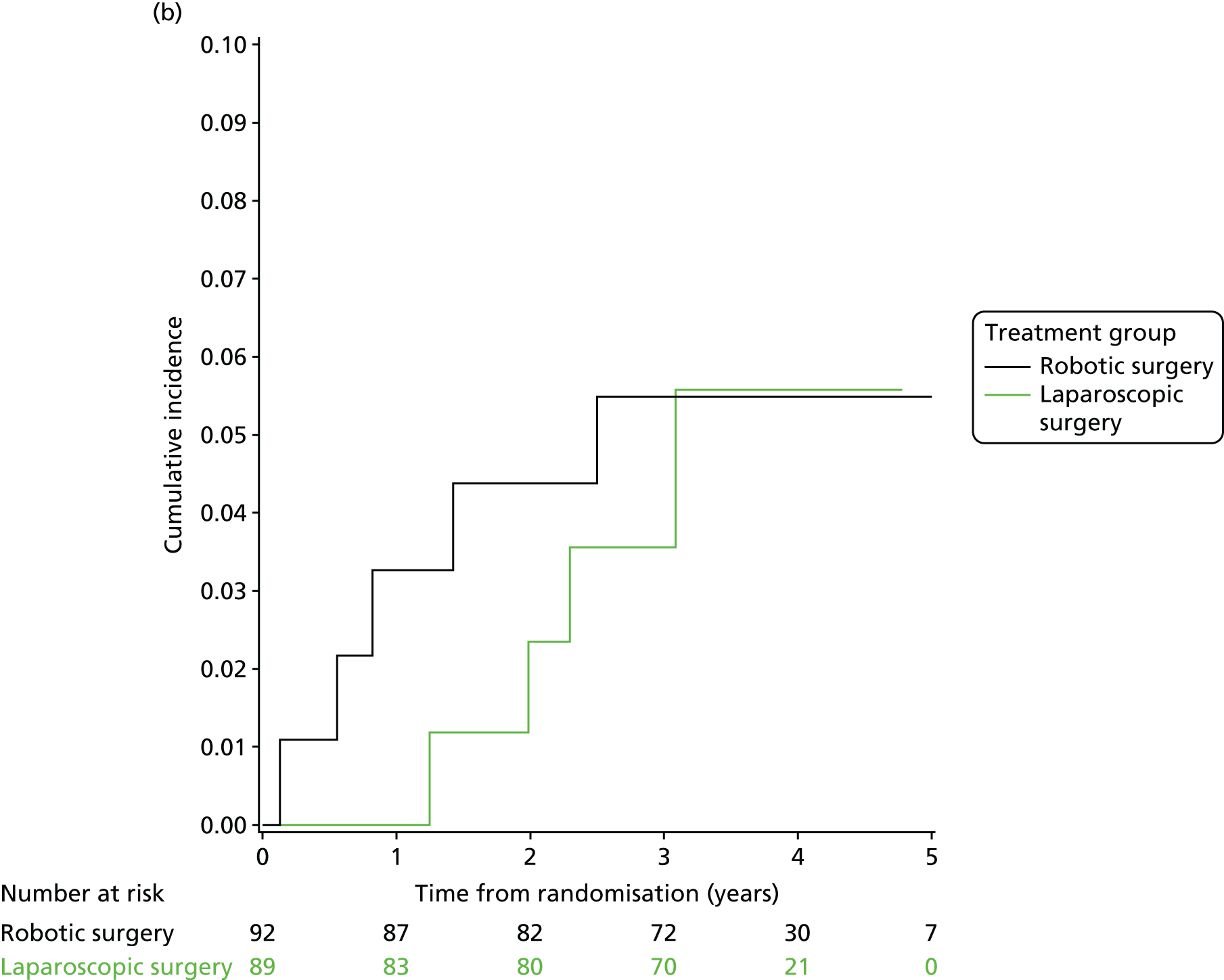

FIGURE 26.
Three-year local recurrence by sex. (a) Male; and (b) female.
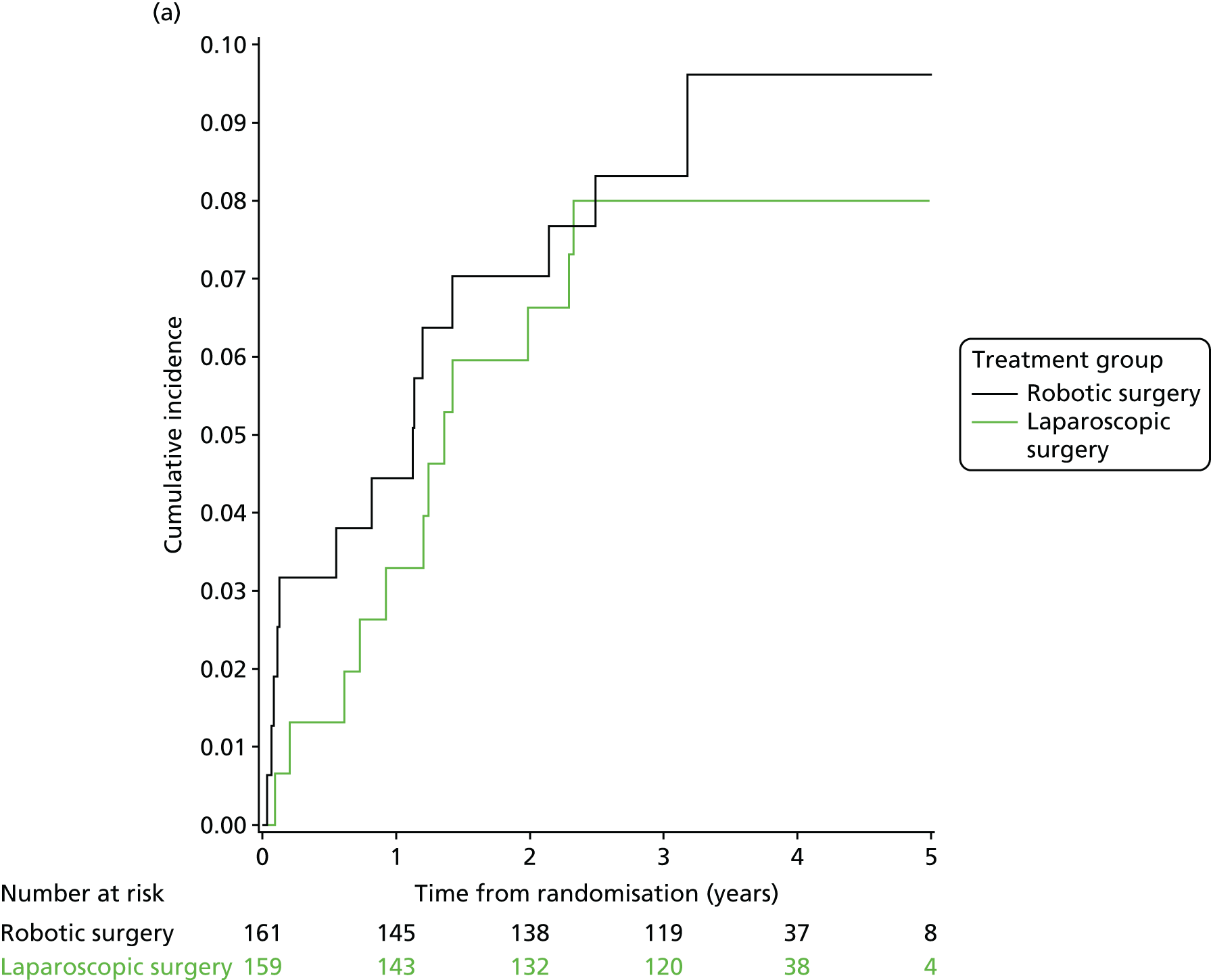
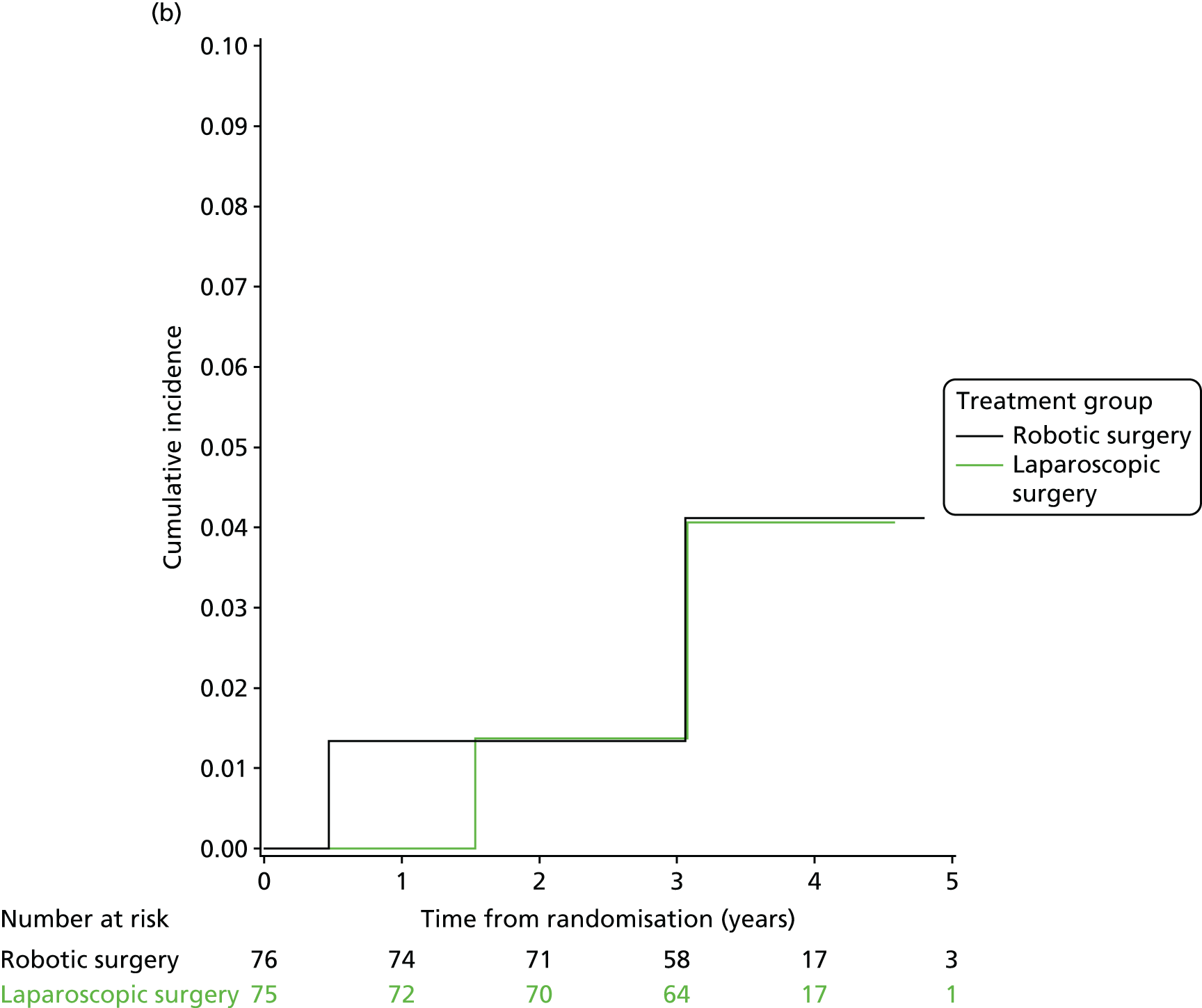
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in patients who underwent neo-adjuvant therapy: robotic surgery (vs. laparoscopic) | 1.233 (0.426 to 3.566) | 0.6990 | 0.8390 |
| Treatment in patients who did not undergo neo-adjuvant therapy: robotic surgery (vs. laparoscopic) | 1.061 (0.397 to 2.838) | 0.9062 |
Type of operation
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in patients who underwent HAR: robotic surgery (vs. laparoscopic) | 1.344 (0.121 to 14.957) | 0.8089 | 0.7737 |
| Treatment in patients who underwent LAR: robotic surgery (vs. laparoscopic) | 0.975 (0.413 to 2.301) | 0.9536 | |
| Treatment in patients who underwent APR: robotic surgery (vs. laparoscopic) | 1.924 (0.351 to 10.558) | 0.4518 |
T-stage
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in T0 patients: robotic surgery (vs. laparoscopic)b | 0.9832 | ||
| Treatment in T1 and T2 patients: robotic surgery (vs. laparoscopic) | 1.128 (0.301 to 4.224) | 0.8576 | |
| Treatment in T3 and T4 patients: robotic surgery (vs. laparoscopic) | 1.019 (0.423 to 2.453) | 0.9670 |
Sex
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in males: robotic surgery (vs. laparoscopic) | 1.166 (0.535 to 2.542) | 0.6999 | 0.8794 |
| Treatment in females: robotic surgery (vs. laparoscopic) | 0.991 (0.142 to 6.935) | 0.9927 |
Appendix 4 Patient self-reported bladder function: further information
Table 69 presents the baseline characteristics of the 351 out of 471 (74.5%) patients who returned questionnaires with sufficient data to derive an I-PSS score. Stratification factors of these 351 patients with complete data are well balanced between the groups, with distributions very similar to the group of patients with missing data (Table 70). The I-PSS score at baseline was similar between the groups in these patients, although there was a slightly more positive skew in the robotic group, with a marginally higher mean score indicating slightly higher severity of symptoms at baseline on average.
| Characteristics | Treatment group, n (%) | Total (N = 351), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 176) | Robotic-assisted laparoscopic surgery (N = 175) | ||
| Sex | |||
| Male | 116 (65.9) | 121 (69.1) | 237 (67.5) |
| Female | 60 (34.1) | 54 (30.9) | 114 (32.5) |
| BMI classification | |||
| Underweight/normal | 69 (39.2) | 74 (42.3) | 143 (40.7) |
| Overweight | 67 (38.1) | 67 (38.3) | 134 (38.2) |
| Obese | 40 (22.7) | 34 (19.4) | 74 (21.1) |
| Neo-adjuvant therapy | |||
| Yes | 81 (46.0) | 76 (43.4) | 157 (44.7) |
| No | 95 (54.0) | 99 (56.6) | 194 (55.3) |
| Intended procedure | |||
| HAR | 26 (14.8) | 25 (14.3) | 51 (14.5) |
| LAR | 119 (67.6) | 117 (66.9) | 236 (67.2) |
| APR | 31 (17.6) | 33 (18.9) | 64 (18.2) |
| Total I-PSS score (baseline) | |||
| Mean (SD) | 6.9 (6.91) | 8.5 (7.28) | 7.7 (7.13) |
| Median (range) | 5.0 (0.0–33.0) | 6.0 (0.0–32.0) | 6.0 (0.0–33.0) |
| (Q1, Q3) | (2.0, 9.0) | (3.0, 12.0) | (2.0, 11.0) |
| Missing | 0 | 0 | 0 |
| Categorical I-PSS score (baseline) | |||
| Mild | 112 (63.6) | 101 (57.7) | 213 (60.7) |
| Moderate | 50 (28.4) | 58 (33.1) | 108 (30.8) |
| Severe | 14 (8.0) | 16 (9.1) | 30 (8.5) |
| Characteristics | Complete-case analysis, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Excluded (N = 120) | Included (N = 351) | ||
| Sex | |||
| Male | 83 (69.2) | 237 (67.5) | 320 (67.9) |
| Female | 37 (30.8) | 114 (32.5) | 151 (32.1) |
| BMI classification | |||
| Underweight/normal | 37 (30.8) | 143 (40.7) | 180 (38.2) |
| Overweight | 49 (40.8) | 134 (38.2) | 183 (38.9) |
| Obese | 34 (28.3) | 74 (21.1) | 108 (22.9) |
| Neo-adjuvant therapy | |||
| Yes | 50 (41.7) | 157 (44.7) | 207 (43.9) |
| No | 70 (58.3) | 194 (55.3) | 264 (56.1) |
| Intended procedure | |||
| HAR | 17 (14.2) | 51 (14.5) | 68 (14.4) |
| LAR | 80 (66.7) | 236 (67.2) | 316 (67.1) |
| APR | 23 (19.2) | 64 (18.2) | 87 (18.5) |
Appendix 5 Patient self-reported sexualfunction: males
Table 71 presents the baseline characteristics of the 181 out of 320 (56.6%) male patients who returned questionnaires with sufficient data to derive an IIEF score. Stratification factors of these 181 patients with complete data are well balanced between the groups, with distributions very similar to the group of male patients with missing data (Table 72). The IIEF score at baseline was similar between the groups in these patients, although there was a slightly more positive skew in the robotic group, with a marginally higher mean score indicating slightly higher severity of symptoms at baseline on average.
| Characteristics | Treatment group, n (%) | Total (N = 181), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 84) | Robotic-assisted laparoscopic surgery (N = 97) | ||
| BMI classification | |||
| Underweight/normal | 34 (40.5) | 34 (35.1) | 68 (37.6) |
| Overweight | 30 (35.7) | 43 (44.3) | 73 (40.3) |
| Obese | 20 (23.8) | 20 (20.6) | 40 (22.1) |
| Neo-adjuvant therapy | |||
| Yes | 33 (39.3) | 45 (46.4) | 78 (43.1) |
| No | 51 (60.7) | 52 (53.6) | 103 (56.9) |
| Intended procedure | |||
| HAR | 15 (17.9) | 11 (11.3) | 26 (14.4) |
| LAR | 57 (67.9) | 68 (70.1) | 125 (69.1) |
| APR | 12 (14.3) | 18 (18.6) | 30 (16.6) |
| Total IIEF score (baseline) | |||
| Mean (SD) | 37.7 (23.85) | 40.1 (24.93) | 39.0 (24.40) |
| Median (range) | 37.0 (5.0–72.0) | 45.0 (5.0–72.0) | 43.0 (5.0–72.0) |
| (Q1, Q3) | (13.0, 62.0) | (13.0, 65.0) | (13.0, 63.0) |
| Characteristics | Complete-case analysis, n (%) | Total (N = 20), n (%) | |
|---|---|---|---|
| Excluded (N = 139) | Included (N = 181) | ||
| BMI classification | |||
| Underweight/normal | 53 (38.1) | 68 (37.6) | 121 (37.8) |
| Overweight | 51 (36.7) | 73 (40.3) | 124 (38.8) |
| Obese | 35 (25.2) | 40 (22.1) | 75 (23.4) |
| Neo-adjuvant therapy | |||
| Yes | 62 (44.6) | 78 (43.1) | 140 (43.8) |
| No | 77 (55.4) | 103 (56.9) | 180 (56.3) |
| Intended procedure | |||
| HAR | 20 (14.4) | 26 (14.4) | 46 (14.4) |
| LAR | 87 (62.6) | 125 (69.1) | 212 (66.3) |
| APR | 32 (23.0) | 30 (16.6) | 62 (19.4) |
| Treatment group, n (%) | Total (N = 351), n (%) | ||
|---|---|---|---|
| Standard laparoscopic surgery (N = 176) | Robotic-assisted laparoscopic surgery (N = 175) | ||
| Total I-PSS score (6 months) | |||
| Mean (SD) | 8.0 (7.69) | 8.0 (6.81) | 8.0 (7.26) |
| Median (range) | 5.0 (0.0–34.0) | 7.0 (0.0–34.0) | 6.0 (0.0–34.0) |
| (Q1, Q3) | (2.0, 12.5) | (3.0, 12.0) | (2.0, 12.0) |
| Categorical I-PSS score (6 months) | |||
| Mild | 109 (61.9) | 99 (56.6) | 208 (59.3) |
| Moderate | 50 (28.4) | 67 (38.3) | 117 (33.3) |
| Severe | 17 (9.7) | 9 (5.1) | 26 (7.4) |
Appendix 6 Patient self-reported sexual function: females
Table 74 presents the baseline characteristics of the 54 out of 151 (35.8%) female patients who returned questionnaires with sufficient data to derive a FSFI score. Stratification factors of these 54 patients with complete data are well balanced between the groups, with distributions similar to the group of female patients with missing data, with the exception of the higher rate of neo-adjuvant therapy in the patients with complete data and the higher proportion of underweight/normal patients (Table 73). The FSFI score at baseline was marginally lower in the robotic group, indicating slightly worse function at baseline for female patients in the robotic group who were included in the analysis.
| Charateristics | Treatment group, n (%) | Total (N = 54), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 29) | Robotic-assisted laparoscopic surgery (N = 25) | ||
| BMI classification | |||
| Underweight/normal | 15 (51.7) | 12 (48.0) | 27 (50.0) |
| Overweight | 10 (34.5) | 8 (32.0) | 18 (33.3) |
| Obese | 4 (13.8) | 5 (20.0) | 9 (16.7) |
| Neo-adjuvant therapy | |||
| Yes | 15 (51.7) | 16 (64.0) | 31 (57.4) |
| No | 14 (48.3) | 9 (36.0) | 23 (42.6) |
| Intended procedure | |||
| HAR | 6 (20.7) | 5 (20.0) | 11 (20.4) |
| LAR | 18 (62.1) | 15 (60.0) | 33 (61.1) |
| APR | 5 (17.2) | 5 (20.0) | 10 (18.5) |
| FSFI (baseline) | |||
| Mean (SD) | 16.7 (11.74) | 14.8 (9.96) | 15.8 (10.90) |
| Median (range) | 19.1 (2.0–34.2) | 14.8 (2.8–30.1) | 16.5 (2.0–34.2) |
| (Q1, Q3) | (4.4, 28.2) | (5.4, 22.7) | (4.7, 27.3) |
| Missing | 0 | 0 | 0 |
| Charateristics | Complete-case analysis, n (%) | Total (N = 54), n (%) | |
|---|---|---|---|
| Excluded (N = 97) | Included (N = 54) | ||
| BMI classification | |||
| Underweight/normal | 32 (33.0) | 27 (50.0) | 59 (39.1) |
| Overweight | 41 (42.3) | 18 (33.3) | 59 (39.1) |
| Obese | 24 (24.7) | 9 (16.7) | 33 (21.9) |
| Neo-adjuvant therapy | |||
| Yes | 36 (37.1) | 31 (57.4) | 67 (44.4) |
| No | 61 (62.9) | 23 (42.6) | 84 (55.6) |
| Intended procedure | |||
| HAR | 11 (11.3) | 11 (20.4) | 22 (14.6) |
| LAR | 71 (73.2) | 33 (61.1) | 104 (68.9) |
| APR | 15 (15.5) | 10 (18.5) | 25 (16.6) |
| FSFI (6 months) | Treatment group, n (%) | Total (N = 54), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 29) | Robotic- assisted laparoscopic surgery (N = 25) | ||
| Mean (SD) | 16.7 (11.25) | 14.2 (10.34) | 15.5 (10.81) |
| Median (range) | 16.7 (2.0–33.6) | 8.5 (2.9–31.4) | 16.1 (2.0–33.6) |
| (Q1, Q3) | (4.5, 28.3) | (5.2, 24.4) | (5.1, 25.9) |
| Missing | 0 | 0 | 0 |
Appendix 7 Patient-reported generic health
Table 77 presents the baseline characteristics of the 459 out of 471 (97.5%) patients who returned at least one questionnaire with sufficient data to derive a PCS/MCS score. Stratification factors and ASA grades of these 459 patients are well balanced between the groups.
| Characteristics | Treatment group, n (%) | Total (N = 459), n (%) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 232) | Standard laparoscopic surgery (N = 227) | ||
| Sex | |||
| Male | 157 (67.7) | 153 (67.4) | 310 (67.5) |
| Female | 75 (32.3) | 74 (32.6) | 149 (32.5) |
| Neo-adjuvant therapy | |||
| Yes | 106 (45.7) | 100 (44.1) | 206 (44.9) |
| No | 126 (54.3) | 127 (55.9) | 253 (55.1) |
| Intended procedure | |||
| HAR | 34 (14.7) | 33 (14.5) | 67 (14.6) |
| LAR | 155 (66.8) | 154 (67.8) | 309 (67.3) |
| APR | 43 (18.5) | 40 (17.6) | 83 (18.1) |
| BMI classification | |||
| Underweight/normal | 92 (39.7) | 85 (37.4) | 177 (38.6) |
| Overweight | 89 (38.4) | 88 (38.8) | 177 (38.6) |
| Obese | 51 (22.0) | 54 (23.8) | 105 (22.9) |
| ASA classification | |||
| A normal healthy patient | 39 (16.8) | 51 (22.5) | 90 (19.6) |
| A patient with mild systemic disease | 147 (63.4) | 123 (54.2) | 270 (58.8) |
| A patient with severe systemic disease | 46 (19.8) | 52 (22.9) | 98 (21.4) |
| A patient with severe systemic disease that is a constant threat to life | 0 (0.0) | 1 (0.4) | 1 (0.2) |
Tables 78 and 79 show the PCS/MCS at baseline, at 30 days post surgery and at 6 months post surgery in the two groups. The baseline PCS and MCS were similar in the two treatment groups.
| PCS | Treatment group, n (%) | Total (N = 459), n (%) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 232) | Standard laparoscopic surgery (N = 227) | ||
| Baseline | |||
| Mean (SD) | 51.4 (8.90) | 51.6 (8.79) | 51.5 (8.84) |
| Median (range) | 53.7 (24.8–67.4) | 53.7 (24.2–67.4) | 53.7 (24.2–67.4) |
| Missing | 6 | 6 | 12 |
| Number | 226 | 221 | 447 |
| 30 days | |||
| Mean (SD) | 42.4 (8.55) | 42.0 (8.42) | 42.2 (8.48) |
| Median (range) | 42.3 (22.8–61.7) | 42.1 (24.3–63.3) | 42.2 (22.8–63.3) |
| Missing | 19 | 29 | 48 |
| Number | 213 | 198 | 411 |
| 6 months | |||
| Mean (SD) | 48.7 (7.95) | 48.3 (8.90) | 48.5 (8.43) |
| Median (range) | 49.7 (27.4–61.2) | 50.2 (18.9–63.2) | 50.0 (18.9–63.2) |
| Missing | 33 | 32 | 65 |
| Number | 199 | 195 | 394 |
| MCS | Treatment group, n (%) | Total (N = 459), n (%) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 232) | Standard laparoscopic surgery (N = 227) | ||
| Baseline | |||
| Mean (SD) | 47.3 (11.82) | 48.1 (11.48) | 47.7 (11.65) |
| Median (range) | 50.5 (13.6–67.0) | 50.9 (10.3–65.8) | 50.8 (10.3–67.0) |
| Missing | 6 | 6 | 12 |
| Number | 226 | 221 | 447 |
| 30 days | |||
| Mean (SD) | 45.6 (11.73) | 44.1 (12.86) | 44.8 (12.30) |
| Median (range) | 46.4 (12.9–64.5) | 46.3 (7.2–68.0) | 46.4 (7.2–68.0) |
| Missing | 19 | 29 | 48 |
| Number | 213 | 198 | 411 |
| 6 months | |||
| Mean (SD) | 48.9 (11.62) | 49.6 (10.04) | 49.2 (10.85) |
| Median (range) | 52.3 (10.8–66.3) | 51.9 (20.1–67.2) | 52.1 (10.8–67.2) |
| Missing | 33 | 31 | 64 |
| Number | 199 | 196 | 395 |
| Characteristics | Inclusion, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Not included (N = 12) | Included (N = 459) | ||
| Sex | |||
| Male | 10 (83.3) | 310 (67.5) | 320 (67.9) |
| Female | 2 (16.7) | 149 (32.5) | 151 (32.1) |
| Neo-adjuvant therapy | |||
| Yes | 6 (50.0) | 206 (44.9) | 212 (45.0) |
| No | 6 (50.0) | 253 (55.1) | 259 (55.0) |
| Intended procedure | |||
| HAR | 2 (16.7) | 67 (14.6) | 69 (14.6) |
| LAR | 8 (66.7) | 309 (67.3) | 317 (67.3) |
| APR | 2 (16.7) | 83 (18.1) | 85 (18.0) |
| BMI classification | |||
| Underweight/normal | 3 (25.0) | 177 (38.6) | 180 (38.2) |
| Overweight | 6 (50.0) | 177 (38.6) | 183 (38.9) |
| Obese | 3 (25.0) | 105 (22.9) | 108 (22.9) |
| ASA classification | |||
| A normal healthy patient | 1 (8.3) | 90 (19.6) | 91 (19.3) |
| A patient with mild systemic disease | 4 (33.3) | 270 (58.8) | 274 (58.2) |
| A patient with severe systemic disease | 0 (0.0) | 98 (21.4) | 98 (20.8) |
| A patient with severe systemic disease that is a constant threat to life | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Missing | 7a (58.3) | 0 (0.0) | 7 (1.5) |
Appendix 8 Patient self-reported fatigue
Table 81 presents the baseline characteristics of the 440 out of 471 (93.4%) patients who returned at least one questionnaire with sufficient data to derive a score for at least one of the scales. Stratification factors and ASA grades for these 440 patients are well balanced between the groups.
| Characteristics | Treatment group, n (%) | Total (N = 440), n (%) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 222) | Standard laparoscopic surgery (N = 218) | ||
| Sex | |||
| Male | 150 (67.6) | 146 (67.0) | 296 (67.3) |
| Female | 72 (32.4) | 72 (33.0) | 144 (32.7) |
| Neo-adjuvant therapy | |||
| Yes | 104 (46.8) | 94 (43.1) | 198 (45.0) |
| No | 118 (53.2) | 124 (56.9) | 242 (55.0) |
| Intended procedure | |||
| HAR | 34 (15.3) | 33 (15.1) | 67 (15.2) |
| LAR | 145 (65.3) | 145 (66.5) | 290 (65.9) |
| APR | 43 (19.4) | 40 (18.3) | 83 (18.9) |
| BMI classification | |||
| Underweight/normal | 85 (38.3) | 77 (35.3) | 162 (36.8) |
| Overweight | 86 (38.7) | 87 (39.9) | 173 (39.3) |
| Obese | 51 (23.0) | 54 (24.8) | 105 (23.9) |
| ASA classification | |||
| A normal healthy patient | 36 (16.2) | 47 (21.6) | 83 (18.9) |
| A patient with mild systemic disease | 143 (64.4) | 121 (55.5) | 264 (60.0) |
| A patient with severe systemic disease | 43 (19.4) | 49 (22.5) | 92 (20.9) |
| A patient with severe systemic disease that is a constant threat to life | 0 (0.0) | 1 (0.5) | 1 (0.2) |
Tables 82–86 show each of the scales at baseline, at 30 days post surgery and at 6 months post surgery in the two groups. The baseline scores were similar between the two treatment groups for all five scales.
| General fatigue | Treatment group | Total (N = 440) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 222) | Standard laparoscopic surgery (N = 218) | ||
| Baseline | |||
| Mean (SD) | 10.4 (4.73) | 10.1 (4.49) | 10.3 (4.61) |
| Median (range) | 10.0 (4.0–20.0) | 10.0 (4.0–20.0) | 10.0 (4.0–20.0) |
| Missing | 13 | 11 | 24 |
| Number | 209 | 207 | 416 |
| 30 days | |||
| Mean (SD) | 12.5 (4.31) | 12.6 (4.36) | 12.6 (4.33) |
| Median (range) | 12.0 (4.0–20.0) | 13.0 (4.0–20.0) | 13.0 (4.0–20.0) |
| Missing | 31 | 37 | 68 |
| Number | 191 | 181 | 372 |
| 6 months | |||
| Mean (SD) | 11.0 (4.62) | 10.8 (4.41) | 10.9 (4.51) |
| Median (range) | 11.0 (4.0–20.0) | 11.0 (4.0–20.0) | 11.0 (4.0–20.0) |
| Missing | 36 | 41 | 77 |
| Number | 186 | 177 | 363 |
| Physical fatigue | Treatment group | Total (N = 440) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 222) | Standard laparoscopic surgery (N = 218) | ||
| Baseline | |||
| Mean (SD) | 10.1 (4.64) | 9.5 (4.52) | 9.8 (4.58) |
| Median (range) | 9.0 (4.0–20.0) | 9.0 (4.0–20.0) | 9.0 (4.0–20.0) |
| Missing | 12 | 12 | 24 |
| Number | 210 | 206 | 416 |
| 30 days | |||
| Mean (SD) | 12.5 (4.42) | 13.1 (4.44) | 12.8 (4.43) |
| Median (range) | 12.5 (4.0–20.0) | 13.0 (4.0–20.0) | 13.0 (4.0–20.0) |
| Missing | 28 | 31 | 59 |
| Number | 194 | 187 | 381 |
| 6 months | |||
| Mean (SD) | 10.7 (4.09) | 10.9 (4.55) | 10.8 (4.32) |
| Median (range) | 11.0 (4.0–20.0) | 10.5 (4.0–20.0) | 11.0 (4.0–20.0) |
| Missing | 39 | 36 | 75 |
| Number | 183 | 182 | 365 |
| Reduced activity | Treatment group | Total (N = 440) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 222) | Standard laparoscopic surgery (N = 218) | ||
| Baseline | |||
| Mean (SD) | 9.9 (4.44) | 9.9 (4.40) | 9.9 (4.42) |
| Median (range) | 9.5 (4.0–20.0) | 9.0 (4.0–20.0) | 9.0 (4.0–20.0) |
| Missing | 14 | 17 | 31 |
| Number | 208 | 201 | 409 |
| 30 days | |||
| Mean (SD) | 12.7 (4.31) | 13.1 (4.33) | 12.9 (4.32) |
| Median (range) | 13.0 (4.0–20.0) | 13.0 (4.0–20.0) | 13.0 (4.0–20.0) |
| Missing | 30 | 37 | 67 |
| Number | 192 | 181 | 373 |
| 6 months | |||
| Mean (SD) | 10.6 (4.23) | 10.5 (4.20) | 10.6 (4.21) |
| Median (range) | 10.0 (4.0–20.0) | 11.0 (4.0–20.0) | 10.0 (4.0–20.0) |
| Missing | 38 | 32 | 70 |
| Number | 184 | 186 | 370 |
| Reduced motivation | Treatment group | Total (N = 440) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 222) | Standard laparoscopic surgery (N = 218) | ||
| Baseline | |||
| Mean (SD) | 8.5 (3.56) | 8.5 (3.58) | 8.5 (3.56) |
| Median (range) | 8.0 (4.0–18.0) | 8.0 (4.0–20.0) | 8.0 (4.0–20.0) |
| Missing | 14 | 14 | 28 |
| Number | 208 | 204 | 412 |
| 30 days | |||
| Mean (SD) | 9.7 (3.89) | 10.2 (3.75) | 9.9 (3.82) |
| Median (range) | 9.0 (4.0–20.0) | 10.0 (4.0–20.0) | 10.0 (4.0–20.0) |
| Missing | 33 | 34 | 67 |
| Number | 189 | 184 | 373 |
| 6 months | |||
| Mean (SD) | 8.5 (3.39) | 8.7 (3.47) | 8.6 (3.43) |
| Median (range) | 8.0 (4.0–18.0) | 9.0 (4.0–20.0) | 8.0 (4.0–20.0) |
| Missing | 45 | 40 | 85 |
| Number | 177 | 178 | 355 |
| Mental fatigue | Treatment group | Total (N = 440) | |
|---|---|---|---|
| Robotic-assisted laparoscopic surgery (N = 222) | Standard laparoscopic surgery (N = 218) | ||
| Baseline | |||
| Mean (SD) | 8.5 (4.12) | 8.4 (4.38) | 8.4 (4.25) |
| Median (range) | 8.0 (4.0–20.0) | 7.0 (4.0–20.0) | 8.0 (4.0–20.0) |
| Missing | 14 | 12 | 26 |
| Number | 208 | 206 | 414 |
| 30 days | |||
| Mean (SD) | 9.0 (4.33) | 9.4 (4.37) | 9.2 (4.35) |
| Median (range) | 9.0 (4.0–20.0) | 9.0 (4.0–20.0) | 9.0 (4.0–20.0) |
| Missing | 30 | 37 | 67 |
| Number | 192 | 181 | 373 |
| 6 months | |||
| Mean (SD) | 8.3 (3.90) | 8.5 (3.84) | 8.4 (3.87) |
| Median (range) | 8.0 (4.0–20.0) | 8.0 (4.0–19.0) | 8.0 (4.0–20.0) |
| Missing | 37 | 34 | 71 |
| Number | 185 | 184 | 369 |
| Characteristics | Inclusion, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Not included (N = 31) | Included (N = 440) | ||
| Sex | |||
| Male | 24 (77.4) | 296 (67.3) | 320 (67.9) |
| Female | 7 (22.6) | 144 (32.7) | 151 (32.1) |
| Neo-adjuvant therapy | |||
| Yes | 14 (45.2) | 198 (45.0) | 212 (45.0) |
| No | 17 (54.8) | 242 (55.0) | 259 (55.0) |
| Intended procedure | |||
| HAR | 2 (6.5) | 67 (15.2) | 69 (14.6) |
| LAR | 27 (87.1) | 290 (65.9) | 317 (67.3) |
| APR | 2 (6.5) | 83 (18.9) | 85 (18.0) |
| BMI classification | |||
| Underweight/normal | 18 (58.1) | 162 (36.8) | 180 (38.2) |
| Overweight | 10 (32.3) | 173 (39.3) | 183 (38.9) |
| Obese | 3 (9.7) | 105 (23.9) | 108 (22.9) |
| ASA classification | |||
| A normal healthy patient | 8 (25.8) | 83 (18.9) | 91 (19.3) |
| A patient with mild systemic disease | 10 (32.3) | 264 (60.0) | 274 (58.2) |
| A patient with severe systemic disease | 6 (19.4) | 92 (20.9) | 98 (20.8) |
| A patient with severe systemic disease that is a constant threat to life | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Missing | 7 (22.6) | 0 (0.0) | 7 (1.5) |
Tables 88–92 show the fitted model estimates for each of the five scales.
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 9.5014 | 0.8549 | ||
| 30 days | 1.5634 | 0.7257 | 0.0316 | 0.1385 to 2.9882 |
| 6 months | –0.2130 | 0.7108 | 0.7645 | –1.6085 to 1.1824 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | 0.2517 | 0.4320 | 0.5603 | –0.5965 to 1.0999 |
| Sex: female (vs. male) | 1.0912 | 0.3728 | 0.0035 | 0.3592 to 1.8232 |
| Neo-adjuvant therapy: no (vs. yes) | –1.0316 | 0.4638 | 0.0264 | –1.9421 to –0.1210 |
| Intended procedure: APR (vs. HAR) | 1.4207 | 0.6392 | 0.0266 | 0.1657 to 2.6758 |
| Intended procedure: LAR (vs. HAR) | 0.6583 | 0.5147 | 0.2013 | –0.3522 to 1.6689 |
| BMI class: obese (vs. underweight/normal) | –0.1142 | 0.5732 | 0.8421 | –1.2397 to 1.0113 |
| BMI class: overweight (vs. underweight/normal) | –0.5285 | 0.4972 | 0.2882 | –1.5047 to 0.4477 |
| ASA grade: (II vs. I) | 0.09524 | 0.5837 | 0.8704 | –1.0509 to 1.2413 |
| ASA grade: (III vs. I) | 1.4558 | 0.7238 | 0.0447 | 0.03480 to 2.8769 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | –0.3385 | 0.4715 | 0.4730 | –1.2642 to 0.5872 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | –0.2278 | 0.4758 | 0.6322 | –1.1619 to 0.7063 |
| ASA grade II and 30-day interaction | –0.2271 | 0.6349 | 0.7207 | –1.4736 to 1.0194 |
| ASA grade III and 30-day interaction | –0.9397 | 0.7672 | 0.2211 | –2.4461 to 0.5666 |
| ASA grade II and 6-month interaction | 0.5650 | 0.6239 | 0.3655 | –0.6599 to 1.7899 |
| ASA grade III and 6-month interaction | –1.0492 | 0.7621 | 0.1691 | –2.5455 to 0.4472 |
| No neo-adjuvant therapy and 30-day interaction | 1.4474 | 0.4810 | 0.0027 | 0.5030 to 2.3917 |
| No neo-adjuvant therapy and 6-month interaction | 0.8010 | 0.4859 | 0.0997 | –0.1531 to 1.7551 |
| Obese and 30-day interaction | 1.4919 | 0.6187 | 0.0162 | 0.2771 to 2.7067 |
| Obese and 6-month interaction | 0.9778 | 0.6335 | 0.1232 | –0.2661 to 2.2216 |
| Overweight and 30-day interaction | 0.4738 | 0.5378 | 0.3787 | –0.5822 to 1.5297 |
| Overweight and 6-month interaction | 0.8162 | 0.5383 | 0.1299 | –0.2408 to 1.8731 |
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 7.8866 | 0.8527 | ||
| 30 days | 3.8465 | 0.7294 | < 0001 | 2.4146 to 5.2785 |
| 6 months | 0.6665 | 0.7218 | 0.3561 | –0.7505 to 2.0836 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | 0.3964 | 0.4262 | 0.3527 | –0.4404 to 1.2332 |
| Sex: female (vs. male) | 0.7560 | 0.3635 | 0.0379 | 0.04242 to 1.4696 |
| Neo-adjuvant therapy: no (vs. yes) | –0.5301 | 0.4614 | 0.2510 | –1.4359 to 0.3758 |
| Intended procedure: APR (vs. HAR) | 1.6339 | 0.6251 | 0.0091 | 0.4066 to 2.8611 |
| Intended procedure: LAR (vs. HAR) | 1.1461 | 0.5021 | 0.0228 | 0.1603 to 2.1320 |
| BMI class: obese (vs. underweight/normal) | –0.2618 | 0.5675 | 0.6447 | –1.3761 to 0.8524 |
| BMI class: overweight (vs. underweight/normal) | –0.8362 | 0.4923 | 0.0898 | –1.8027 to 0.1302 |
| ASA grade: (II vs. I) | 0.9504 | 0.5846 | 0.1045 | –0.1974 to 2.0983 |
| ASA grade: (III vs. I) | 2.0824 | 0.7252 | 0.0042 | 0.6586 to 3.5062 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | –0.9425 | 0.4749 | 0.0476 | –1.8749 to –0.01009 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | –0.7351 | 0.4815 | 0.1273 | –1.6804 to 0.2102 |
| ASA grade II and 30-day interaction | –1.7434 | 0.6408 | 0.0067 | –3.0015 to –0.4853 |
| ASA grade III and 30-day interaction | 0.003981 | 0.6358 | 0.9950 | –1.2443 to 1.2522 |
| ASA grade II and 6-month interaction | –1.6193 | 0.7721 | 0.0363 | –3.1353 to –0.1034 |
| ASA grade III and 6-month interaction | –1.1335 | 0.7782 | 0.1457 | –2.6613 to 0.3943 |
| Obese and 30-day interaction | 0.9738 | 0.6242 | 0.1192 | –0.2517 to 2.1993 |
| Obese and 6-month interaction | 1.4096 | 0.6396 | 0.0279 | 0.1539 to 2.6654 |
| Overweight and 30-day interaction | 0.6666 | 0.5417 | 0.2189 | –0.3970 to 1.7302 |
| Overweight and 6-month interaction | 0.8577 | 0.5480 | 0.1180 | –0.2182 to 1.9336 |
| No neo-adjuvant therapy and 30-day interaction | 1.0634 | 0.4833 | 0.0281 | 0.1146 to 2.0123 |
| No neo-adjuvant therapy and 6-month interaction | 0.7799 | 0.4919 | 0.1133 | –0.1858 to 1.7455 |
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 8.4917 | 0.8260 | ||
| 30 days | 3.0550 | 0.7251 | < 0001 | 1.6314 to 4.4786 |
| 6 months | –0.4520 | 0.7075 | 0.5231 | –1.8410 to 0.9370 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | –0.1634 | 0.4148 | 0.6938 | –0.9777 to 0.6510 |
| Sex: female (vs. male) | 0.7244 | 0.3482 | 0.0378 | 0.04081 to 1.4080 |
| Neo-adjuvant therapy: no (vs. yes) | –0.7325 | 0.4477 | 0.1022 | –1.6115 to 0.1465 |
| Intended procedure: APR (vs. HAR) | 2.0437 | 0.6001 | 0.0007 | 0.8654 to 3.2221 |
| Intended procedure: LAR (vs. HAR) | 1.0258 | 0.4829 | 0.0340 | 0.07763 to 1.9739 |
| BMI class: obese (vs. underweight/normal) | –0.4832 | 0.5515 | 0.3813 | –1.5659 to 0.5996 |
| BMI class: overweight (vs. underweight/normal) | –0.5767 | 0.4777 | 0.2277 | –1.5146 to 0.3611 |
| ASA grade: (II vs. I) | 0.8395 | 0.5703 | 0.1415 | –0.2802 to 1.9591 |
| ASA grade: (III vs. I) | 1.7121 | 0.7019 | 0.0150 | 0.3341 to 3.0901 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | –0.3080 | 0.4725 | 0.5147 | –1.2356 to 0.6196 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | 0.08269 | 0.4743 | 0.8616 | –0.8485 to 1.0139 |
| ASA grade II and 30-day interaction | –1.0403 | 0.6383 | 0.1036 | –2.2935 to 0.2130 |
| ASA grade III and 30-day interaction | 0.3085 | 0.6239 | 0.6211 | –0.9164 to 1.5334 |
| ASA grade II and 6-month interaction | –0.7610 | 0.7664 | 0.3211 | –2.2658 to 0.7437 |
| ASA grade III and 6-month interaction | –0.4147 | 0.7528 | 0.5819 | –1.8927 to 1.0633 |
| Obese and 30-day interaction | 1.5630 | 0.6212 | 0.0121 | 0.3434 to 2.7825 |
| Obese and 6-month interaction | 1.1287 | 0.6275 | 0.0725 | –0.1033 to 2.3608 |
| Overweight and 30-day interaction | 0.4547 | 0.5363 | 0.3969 | –0.5983 to 1.5077 |
| Overweight and 6-month interaction | 0.4901 | 0.5386 | 0.3631 | –0.5673 to 1.5476 |
| No neo-adjuvant therapy and 30-day interaction | 0.9029 | 0.4786 | 0.0596 | –0.03670 to 1.8424 |
| No neo-adjuvant therapy and 6-month interaction | 1.1007 | 0.4822 | 0.0228 | 0.1539 to 2.0475 |
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 8.1637 | 0.6844 | ||
| 30 days | 0.9350 | 0.5761 | 0.1051 | –0.1961 to 2.0662 |
| 6 months | –0.3237 | 0.5658 | 0.5675 | –1.4347 to 0.7873 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | –0.03917 | 0.3531 | 0.9117 | –0.7324 to 0.6540 |
| Sex: female (vs. male) | 0.7819 | 0.3053 | 0.0107 | 0.1824 to 1.3813 |
| Neo-adjuvant therapy: no (vs. yes) | –0.4394 | 0.3716 | 0.2374 | –1.1689 to 0.2902 |
| Intended procedure: APR (vs. HAR) | 1.0636 | 0.5227 | 0.0422 | 0.03734 to 2.0898 |
| Intended procedure: LAR (vs. HAR) | 0.4422 | 0.4242 | 0.2976 | –0.3907 to 1.2750 |
| BMI class: obese (vs. underweight/normal) | –0.03125 | 0.4627 | 0.9462 | –0.9398 to 0.8773 |
| BMI class: overweight (vs. underweight/normal) | –0.1524 | 0.4059 | 0.7075 | –0.9493 to 0.6445 |
| ASA grade: (II vs. I) | –0.1479 | 0.4712 | 0.7536 | –1.0731 to 0.7773 |
| ASA grade: (III vs. I) | 0.03344 | 0.5745 | 0.9536 | –1.0945 to 1.1614 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | –0.4805 | 0.3794 | 0.2057 | –1.2253 to 0.2644 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | –0.3817 | 0.3853 | 0.3222 | –1.1381 to 0.3748 |
| ASA grade II and 30-day interaction | –0.08389 | 0.5096 | 0.8693 | –1.0845 to 0.9167 |
| ASA grade II and 6-month interaction | 0.8070 | 0.5026 | 0.1088 | –0.1799 to 1.7939 |
| ASA grade III and 30-day interaction | 0.3101 | 0.6122 | 0.6127 | –0.8920 to 1.5121 |
| ASA grade III and 6-month interaction | 0.5378 | 0.6112 | 0.3792 | –0.6622 to 1.7379 |
| Obese and 30-day interaction | 0.9059 | 0.4994 | 0.0701 | –0.07469 to 1.8864 |
| Obese and 6-month interaction | 0.4964 | 0.5084 | 0.3292 | –0.5018 to 1.4945 |
| Overweight and 30-day interaction | 0.1694 | 0.4309 | 0.6944 | –0.6768 to 1.0155 |
| Overweight and 6-month interaction | –0.09029 | 0.4392 | 0.8372 | –0.9526 to 0.7720 |
| No neo-adjuvant therapy and 30-day interaction | 0.8648 | 0.3857 | 0.0253 | 0.1075 to 1.6221 |
| No neo-adjuvant therapy and 6-month interaction | 0.1111 | 0.3935 | 0.7777 | –0.6614 to 0.8836 |
| Effect | Estimate | Standard error | Pr > |t| | 95% CI |
|---|---|---|---|---|
| Intercept | 8.3964 | 0.7944 | ||
| 30 days | 0.8814 | 0.5758 | 0.1263 | –0.2491 to 2.0118 |
| 6 months | –0.7408 | 0.5652 | 0.1904 | –1.8506 to 0.3689 |
| Treatment: robotic-assisted laparoscopic surgery (vs. standard) | 0.1374 | 0.4075 | 0.7360 | –0.6626 to 0.9374 |
| Sex: female (vs. male) | 0.8899 | 0.3613 | 0.0140 | 0.1807 to 1.5992 |
| Neo-adjuvant therapy: no (vs. yes) | –0.1726 | 0.3656 | 0.6370 | –0.8905 to 0.5453 |
| Intended procedure: APR (vs. HAR) | 1.1727 | 0.6192 | 0.0586 | –0.04292 to 2.3884 |
| Intended procedure: LAR (vs. HAR) | 0.6569 | 0.5025 | 0.1915 | –0.3296 to 1.6435 |
| BMI class: obese (vs. underweight/normal) | –0.6885 | 0.5392 | 0.2021 | –1.7471 to 0.3702 |
| BMI class: overweight (vs. underweight/normal) | –0.1299 | 0.4685 | 0.7817 | –1.0497 to 0.7899 |
| ASA grade: (II vs. I) | –0.8621 | 0.5458 | 0.1147 | –1.9336 to 0.2095 |
| ASA grade: (III vs. I) | –0.5135 | 0.6657 | 0.4407 | –1.8205 to 0.7935 |
| Robotic-assisted laparoscopic surgery and 30-day interaction | –0.4997 | 0.4152 | 0.2291 | –1.3148 to 0.3154 |
| Robotic-assisted laparoscopic surgery and 6-month interaction | –0.5284 | 0.4171 | 0.2056 | –1.3473 to 0.2905 |
| ASA grade II and 30-day interaction | –0.06827 | 0.5569 | 0.9025 | –1.1615 to 1.0250 |
| ASA grade II and 6-month interaction | 0.9765 | 0.5473 | 0.0748 | –0.09799 to 2.0510 |
| ASA grade III and 30-day interaction | –0.4344 | 0.6625 | 0.5123 | –1.7351 to 0.8664 |
| ASA grade III and 6-month interaction | 0.1242 | 0.6547 | 0.8496 | –1.1612 to 1.4096 |
| Obese and 30-day interaction | 1.0461 | 0.5460 | 0.0558 | –0.02591 to 2.1181 |
| Obese and 6-month interaction | 1.1519 | 0.5494 | 0.0364 | 0.07317 to 2.2307 |
| Overweight and 30-day interaction | 0.1229 | 0.4735 | 0.7952 | –0.8068 to 1.0527 |
| Overweight and 6-month interaction | 0.5304 | 0.4753 | 0.2648 | –0.4027 to 1.4636 |
Appendix 9 Disease-free survival: further information
Disease-free survival events and censorings, including reason for censoring, are summarised in Table 93.
| Nature of the end of follow-up for DFS analysis | Treatment group, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| Event | 56 (23.9) | 58 (24.5) | 114 (24.2) |
| Censor: last known to be alive and disease-free | 169 (72.2) | 172 (72.6) | 341 (72.4) |
| Censor: withdrawal from further data collection | 5 (2.1) | 4 (1.7) | 9 (1.9) |
| Censor: non-standard circumstancea | 4 (1.7) | 3 (1.3) | 7 (1.5) |
Types of events (i.e. what event contributed to the DFS event) are summarised in Table 94.
| Type of (first) recurrence or death (by treatment group) | Treatment group, n (%) | Total (N = 114), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 56) | Robotic-assisted laparoscopic surgery (N = 58) | ||
| Locoregional spread | 13 (23.2) | 14 (24.1) | 27 (23.7) |
| Liver metastasis | 15 (26.8) | 6 (10.3) | 21 (18.4) |
| Lung metastasis | 10 (17.9) | 15 (25.9) | 25 (21.9) |
| New primary cancer | 8 (14.3) | 10 (17.2) | 18 (15.8) |
| Death | 9 (16.1) | 8 (13.8) | 17 (14.9) |
| Othera | 1 (1.8) | 5 (8.6) | 6 (5.3) |
The methods of confirmation are summarised in Table 95.
| Method of confirmation of recurrences | Treatment group, n (%) | Total (N = 73), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 38) | Robotic-assisted laparoscopic surgery (N = 35) | ||
| Clinical | 1 (2.6) | 3 (8.6) | 4 (5.5) |
| Radiological | 26 (68.4) | 24 (68.6) | 50 (68.5) |
| Pathological | 10 (26.3) | 8 (22.9) | 18 (24.7) |
| Positron emission tomography scan | 1 (2.6) | 0 (0.0) | 1 (1.4) |
Table 96 shows Kaplan–Meier estimates of DFS by treatment group at several time points (1–5 years post randomisation).
| Time (years) | Treatment group | |||
|---|---|---|---|---|
| Laparoscopic surgery | Robotic surgery | |||
| Probability of a DFS event | 95% CI | Probability of a DFS event | 95% CI | |
| 1 | 0.084 | 0.048 to 0.121 | 0.116 | 0.075 to 0.157 |
| 2 | 0.183 | 0.132 to 0.234 | 0.186 | 0.136 to 0.236 |
| 3 | 0.220 | 0.165 to 0.274 | 0.225 | 0.171 to 0.279 |
| 4 | 0.259 | 0.193 to 0.324 | 0.274 | 0.210 to 0.338 |
| 5 | 0.390 | 0.192 to 0.588 | 0.274 | 0.210 to 0.338 |
Subgroup analyses: further information
Figures 27–30 display the Kaplan-Meier graphs for the effect of neo-adjuvant therapy, operation type, T-stage and sex, on 3-year DFS.
FIGURE 27.
Disease-free survival by neo-adjuvant therapy. Product-limit survival estimates with number of patients at risk. (a) No neo-adjuvant therapy; and (b) neo-adjuvant therapy.
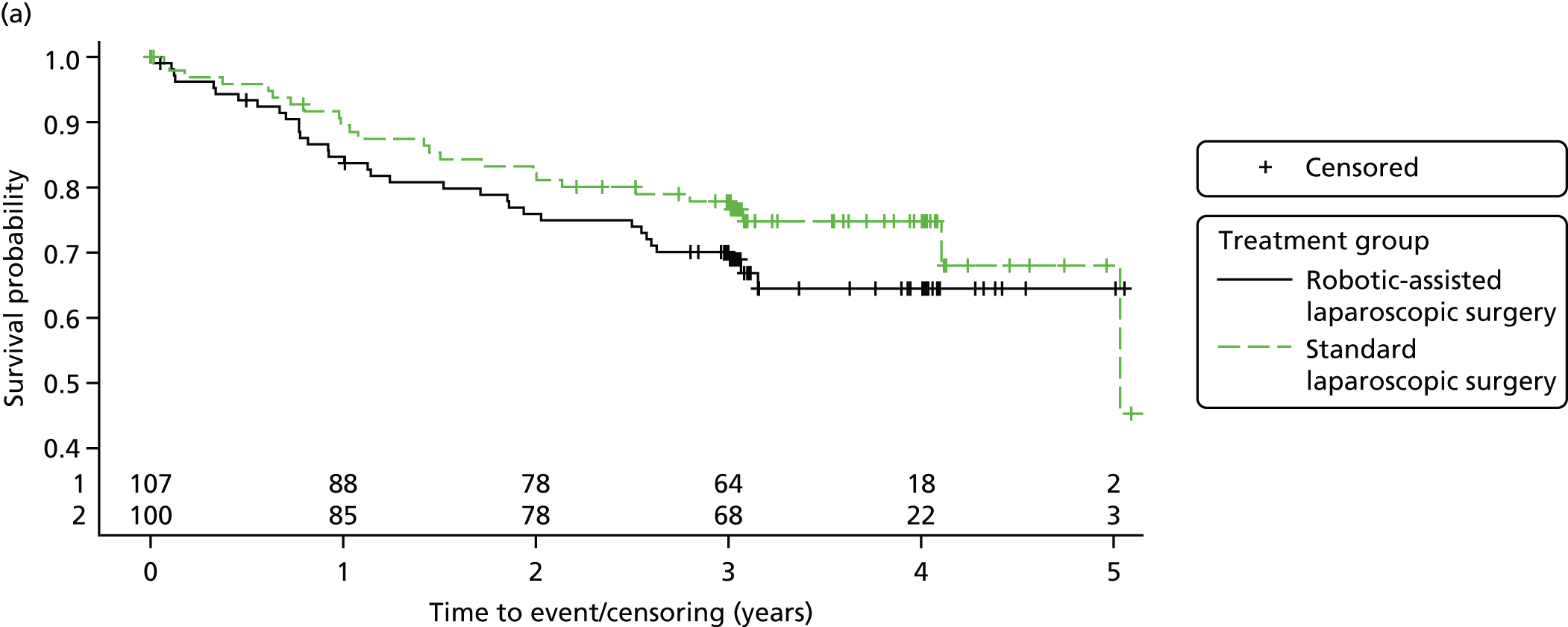

FIGURE 28.
Disease-free survival by operation type. (a) HAR; (b) LAR; and (c) APR. Product-limit survival estimates with number of patients at risk.


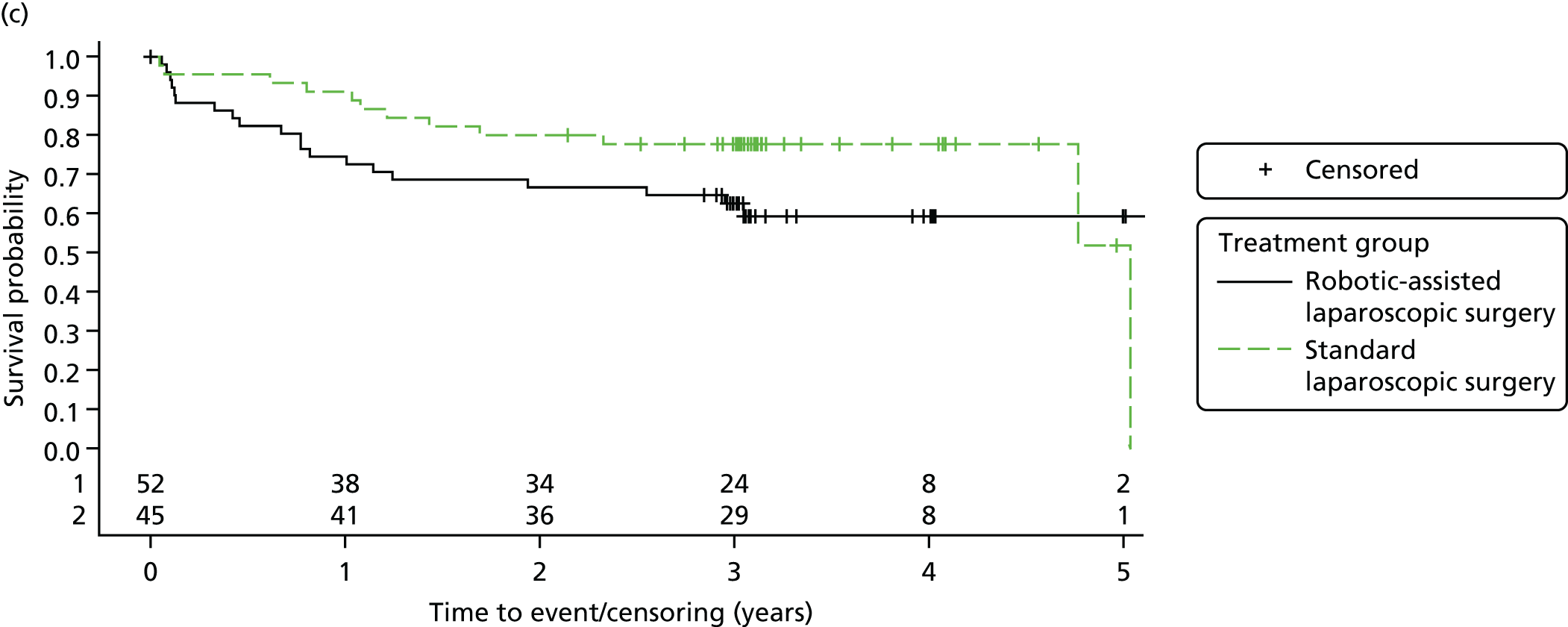
FIGURE 29.
Disease-free survival by T-stage. (a) T0; (b) T1 and T2; and (c) T3 and T4. Product-limit survival estimates with number of patients at risk.



FIGURE 30.
Disease-free survival by sex. (a) Males; and (b) females. Product-limit survival estimates with number of patients at risk.
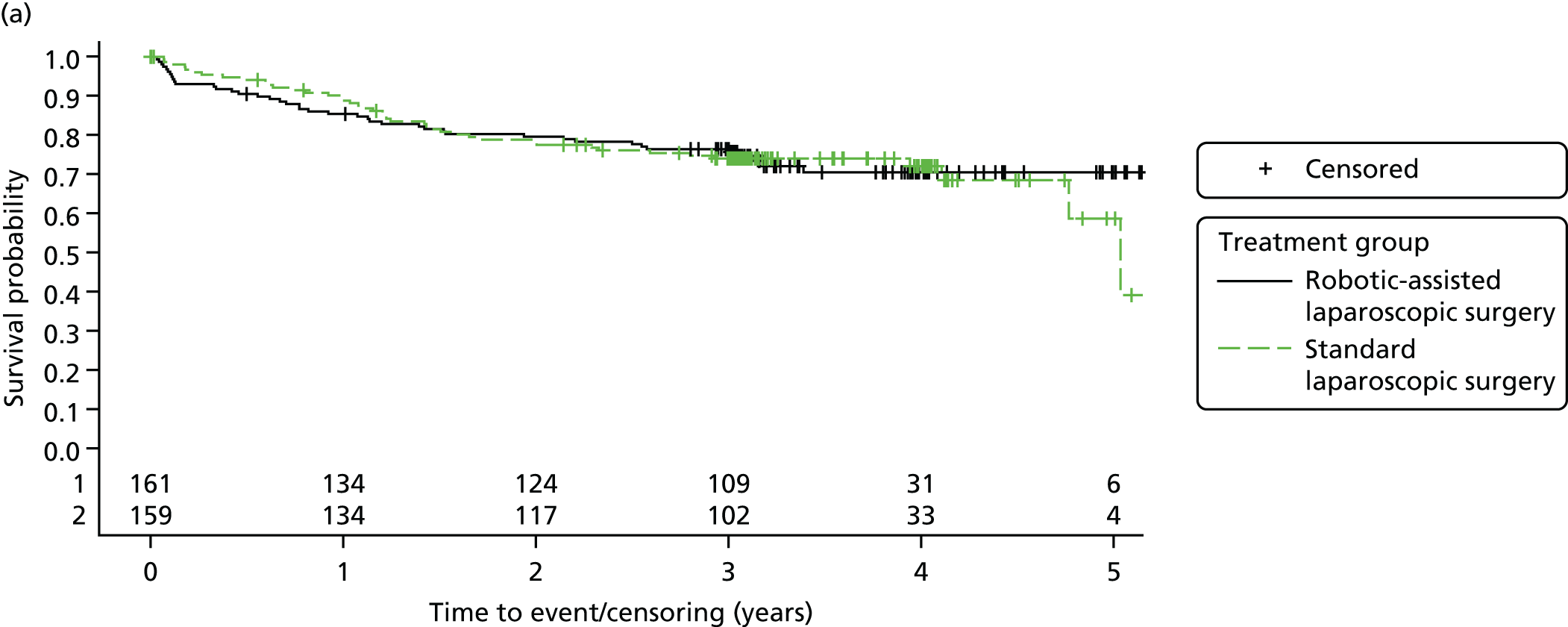
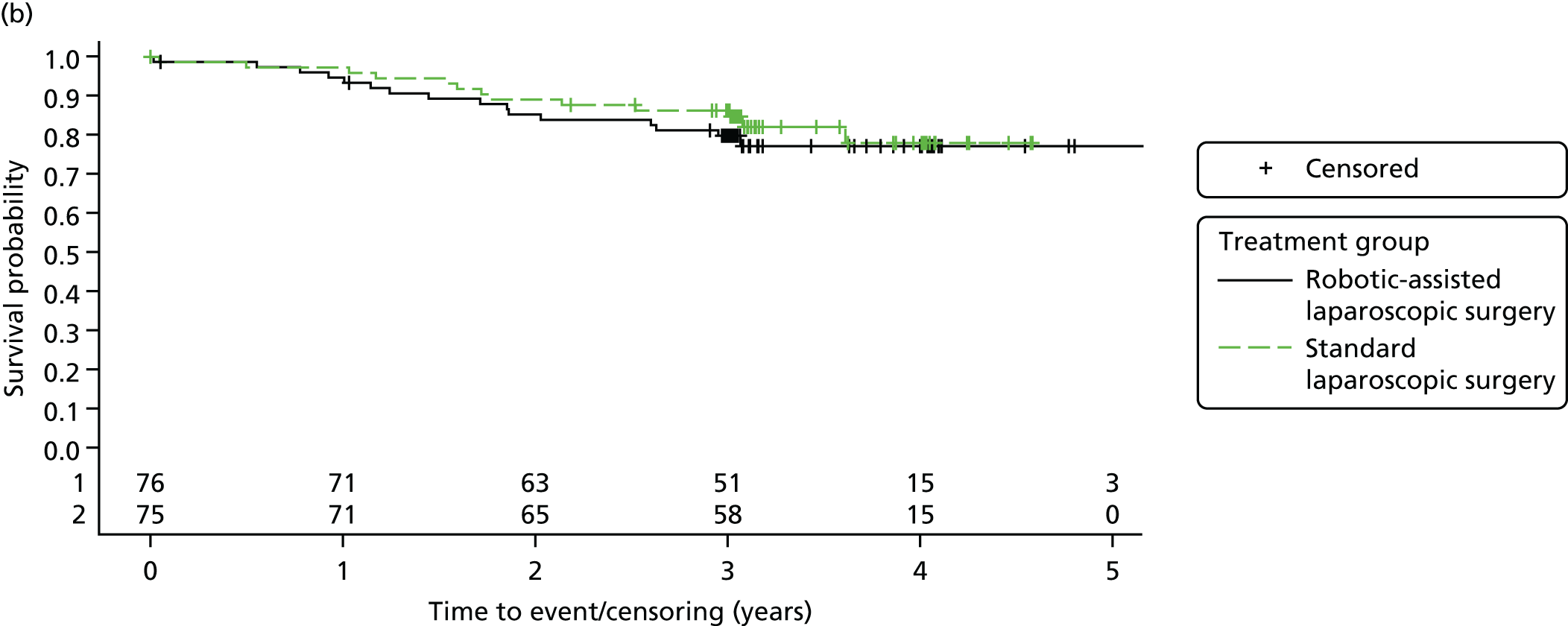
Neo-adjuvant therapy
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in patients who underwent neo-adjuvant therapy: robotic surgery (vs. laparoscopic) | 1.338 (0.795 to 2.251) | 0.2728 | 0.1653 |
| Treatment in patients who did not undergo neo-adjuvant therapy: robotic surgery (vs. laparoscopic) | 0.787 (0.459 to 1.350) | 0.3843 | |
Type of operation
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in patients who underwent HAR: robotic surgery (vs. laparoscopic) | 0.437 (0.097 to 1.957) | 0.2825 | 0.1818 |
| Treatment in patients who underwent LAR: robotic surgery (vs. laparoscopic) | 0.914 (0.580 to 1.440) | 0.6985 | |
| Treatment in patients who underwent APR: robotic surgery (vs. laparoscopic) | 1.703 (0.832 to 3.487) | 0.1450 | |
T-stage
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in T0 patients: robotic surgery (vs. laparoscopic) | 1.878 (0.309 to 11.391) | 0.4934 | 0.6226 |
| Treatment in T1 and T2 patients: robotic surgery (vs. laparoscopic) | 1.252 (0.623 to 2.516) | 0.5287 | |
| Treatment in T3 and T4 patients: robotic surgery (vs. laparoscopic) | 0.925 (0.585 to 1.463) | 0.7402 | |
Sex
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in males: robotic surgery (vs. laparoscopic) | 0.971 (0.633 to 1.490) | 0.8925 | 0.5570 |
| Treatment in females: robotic surgery (vs. laparoscopic) | 1.251 (0.599 to 2.613) | 0.5520 | |
Appendix 10 Overall survival: further information
Overall survival events and censorings, including reason for censoring, are summarised in Table 101.
| Nature of the end of follow-up | Treatment group, n (%) | Total (N = 471), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 234) | Robotic-assisted laparoscopic surgery (N = 237) | ||
| Event | 23 (9.8) | 23 (9.7) | 46 (9.8) |
| Censor: last known to be alive | 205 (87.6) | 210 (88.6) | 415 (88.1) |
| Censor: withdrawal from further data collection | 6 (2.6) | 4 (1.7) | 10 (2.1) |
Table 102 shows Kaplan–Meier estimates of DFS by treatment group at several time points (1–5 years post randomisation).
| Time (years) | Treatment group | |||
|---|---|---|---|---|
| Laparoscopic surgery | Robotic surgery | |||
| Probability of survival | 95% CI | Probability of survival | 95% CI | |
| 1 | 0.970 | 0.947 to 0.992 | 0.970 | 0.949 to 0.992 |
| 2 | 0.939 | 0.908 to 0.970 | 0.940 | 0.909 to 0.971 |
| 3 | 0.925 | 0.891 to 0.959 | 0.914 | 0.878 to 0.950 |
| 4 | 0.891 | 0.844 to 0.939 | 0.887 | 0.840 to 0.933 |
| 5 | 0.786 | 0.626 to 0.945 | 0.887 | 0.840 to 0.933 |
Subgroup analyses: further information
Figures 31–34 display the Kaplan-Meier graphs for the effect of neo-adjuvant therapy, operation type, T-stage and sex, on 3-year OS.
FIGURE 31.
Overall survival by neo-adjuvant therapy. (a) Neo-adjuvant therapy; and (b) no neo-adjuvant therapy.

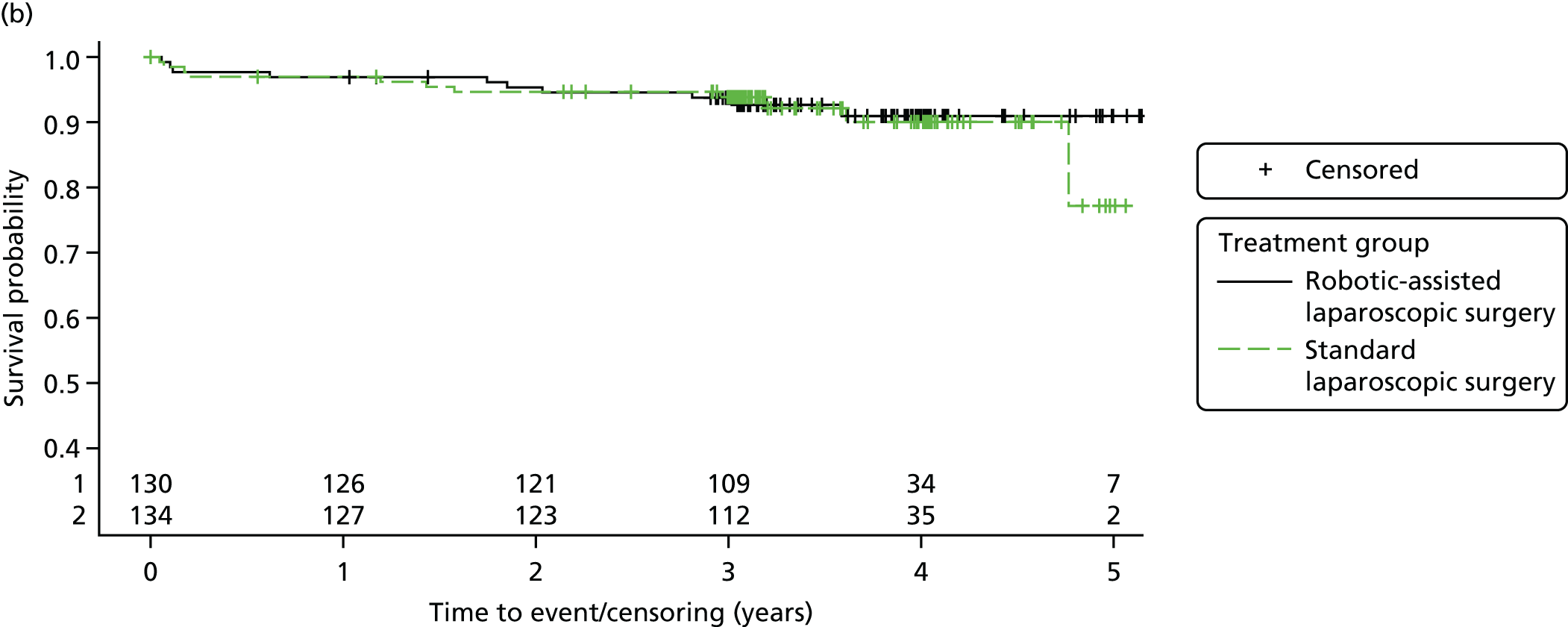
FIGURE 32.
Overall survival by operation type. (a) HAR; (b) LAR; and (c) APR. Product-limit survival estimates with number of patients at risk.

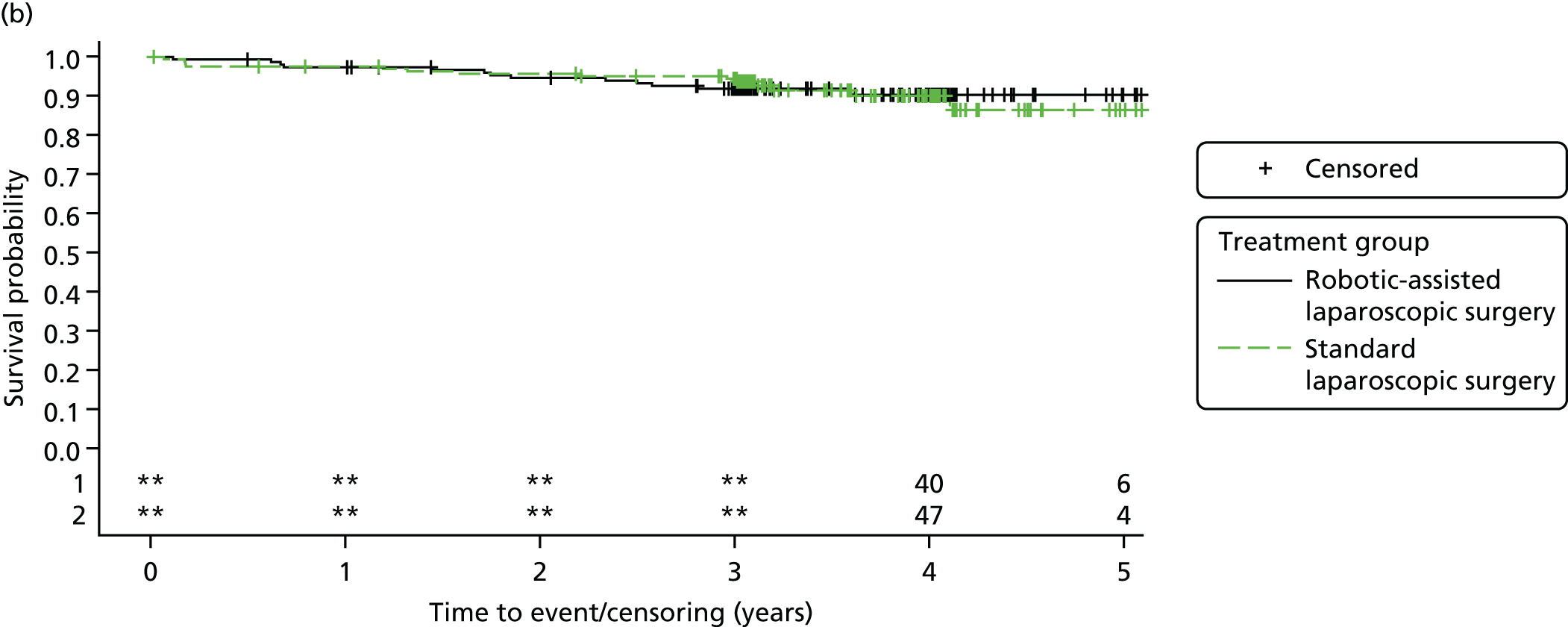
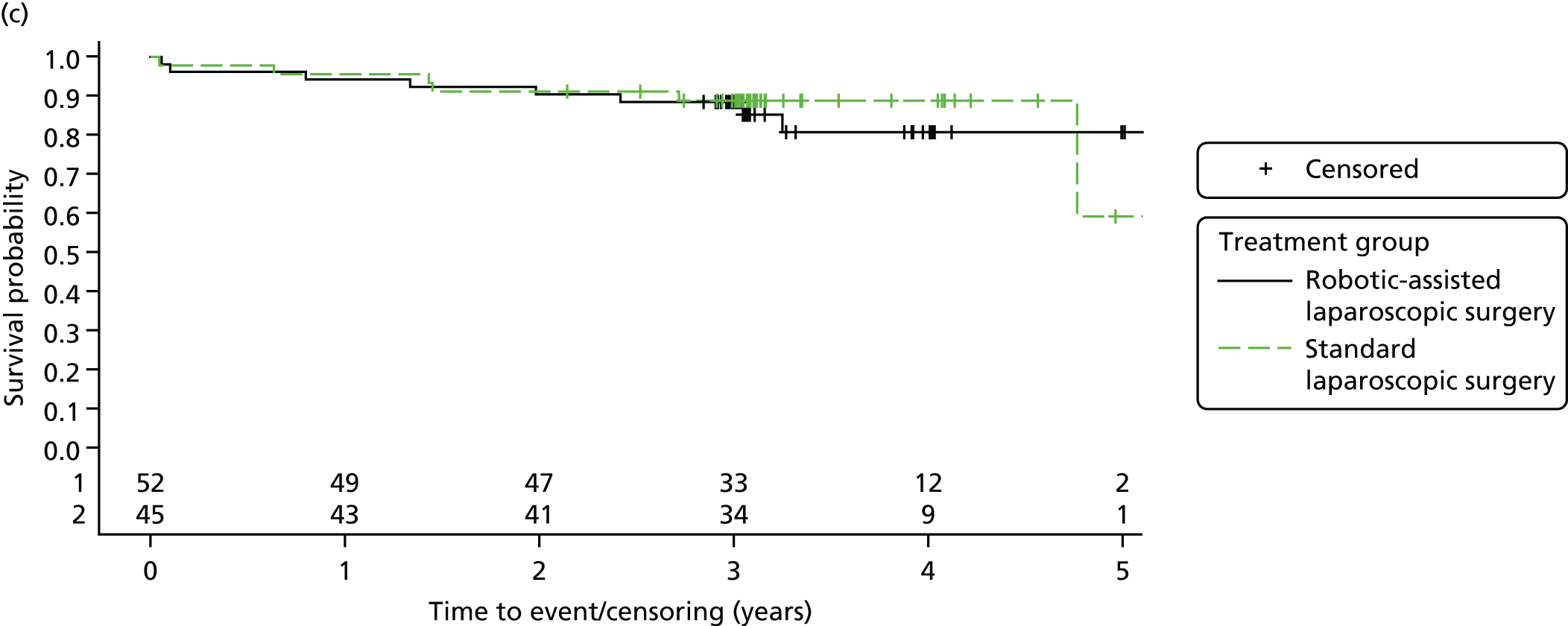
FIGURE 33.
Overall survival by T-stage. (a) T0; (b) T1 and T2; and (c) T3 and T4. Product-limit survival estimates with number of patients at risk.

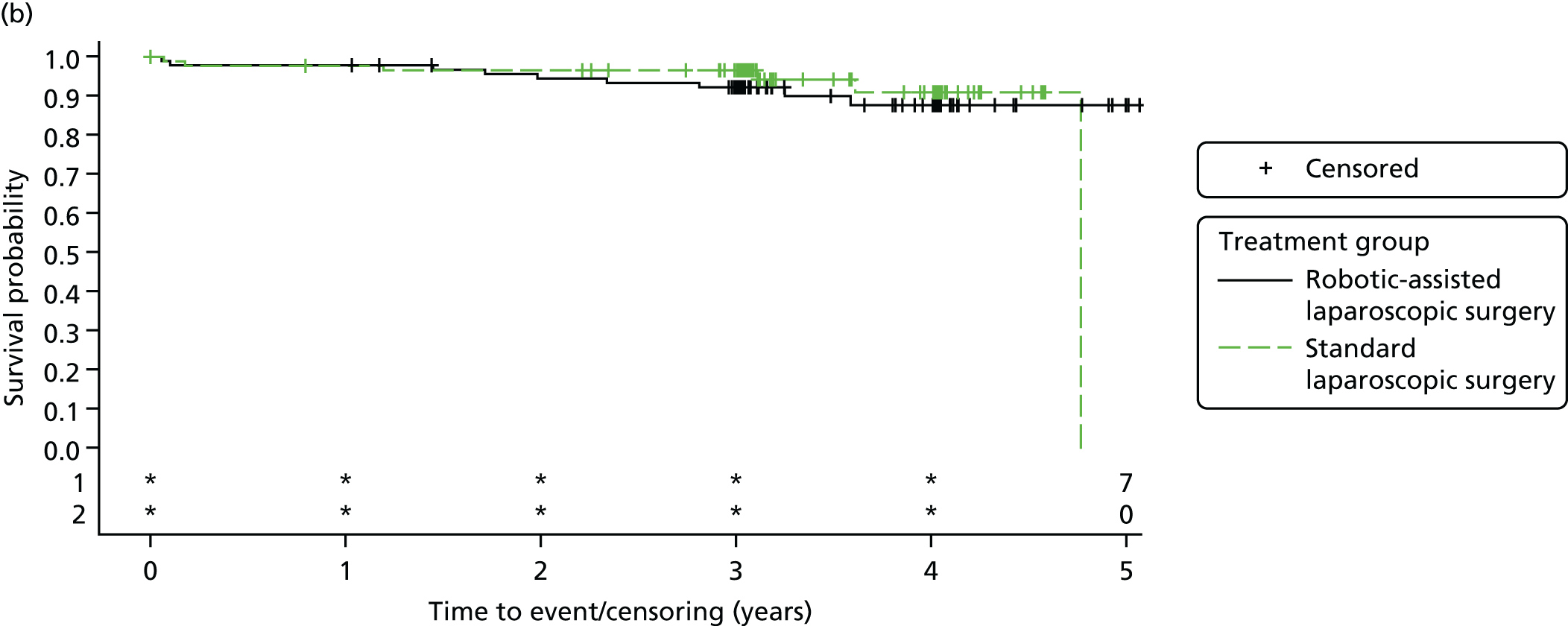
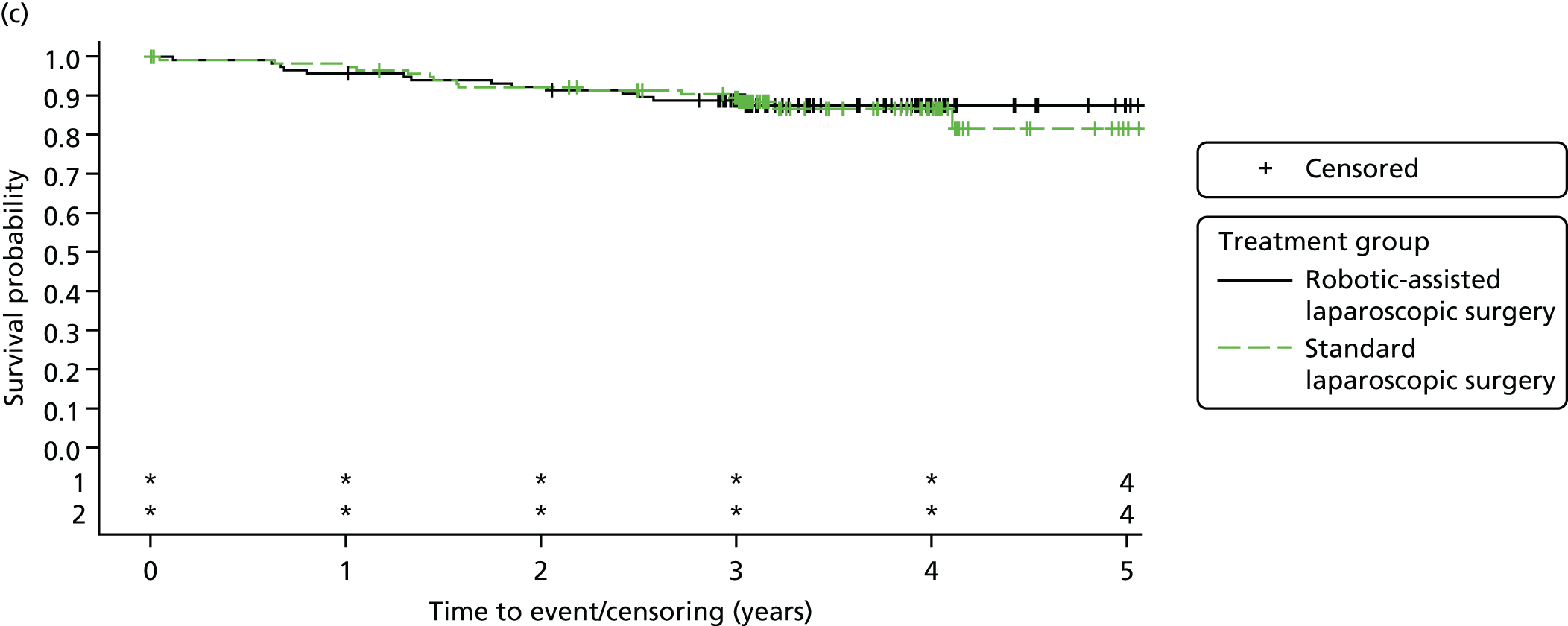
FIGURE 34.
Overall survival by sex. (a) Males; and (b) females. Product-limit survival estimates with number of patients at risk.
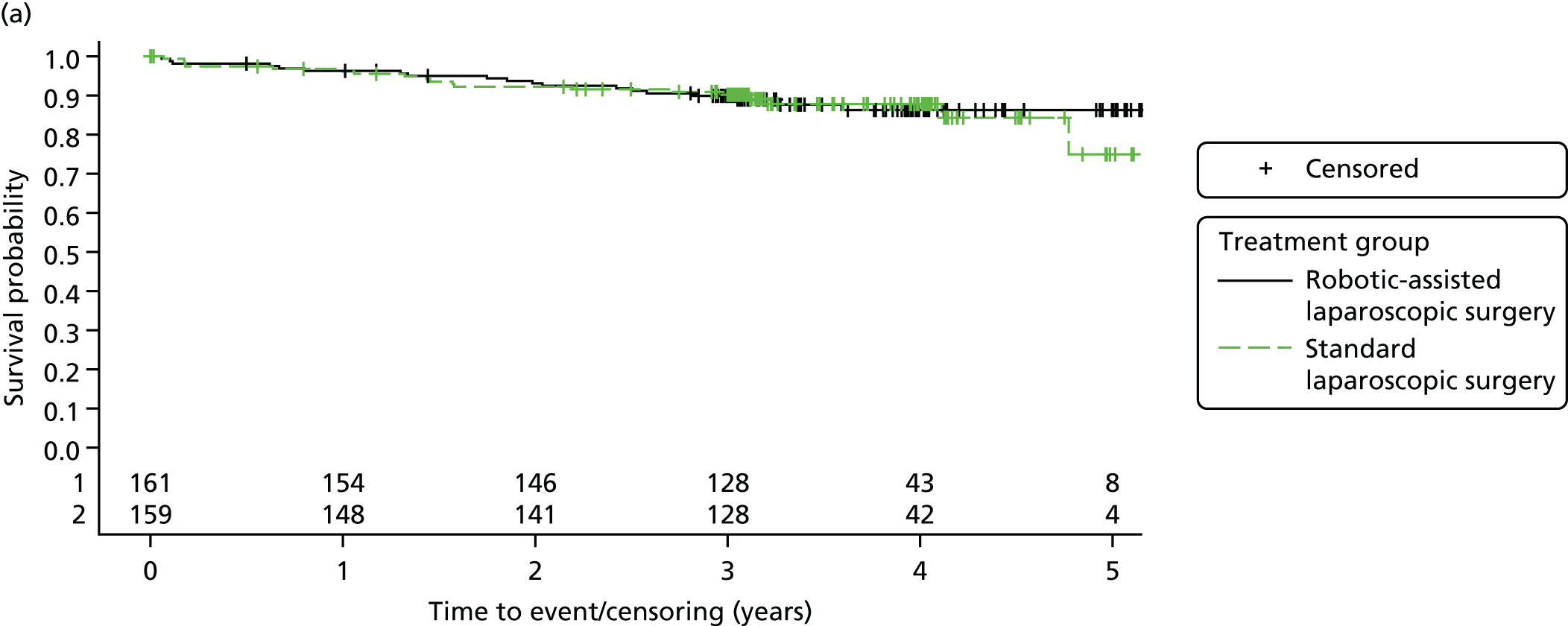

Neo-adjuvant therapy
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in patients who underwent neo-adjuvant therapy: robotic surgery (vs. laparoscopic) | 0.977 (0.448 to 2.133) | 0.9544 | 0.9018 |
| Treatment in patients who did not undergo neo-adjuvant therapy: robotic surgery (vs. laparoscopic) | 0.908 (0.379 to 2.172) | 0.8279 | |
Type of operation
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in patients who underwent HAR: robotic surgery (vs. laparoscopic) | 0.275 (0.025 to 3.053) | 0.2933 | 0.5713 |
| Treatment in patients who underwent LAR: robotic surgery (vs. laparoscopic) | 0.980 (0.460 to 2.088) | 0.9591 | |
| Treatment in patients who underwent APR: robotic surgery (vs. laparoscopic) | 1.122 (0.389 to 3.238) | 0.8312 | |
T-stage
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in T0 patients: robotic surgery (vs. laparoscopic)b | 0.8240 | ||
| Treatment in T1 and T2 patients: robotic surgery (vs. laparoscopic) | 1.310 (0.464 to 3.701) | 0.6106 | |
| Treatment in T3 and T4 patients: robotic surgery (vs. laparoscopic) | 0.875 (0.421 to 1.819) | 0.7215 | |
Sex
| Effect | HR (adjusted 95% CI)a | p-value | |
|---|---|---|---|
| Treatment in males: robotic surgery (vs. laparoscopic) | 0.930 (0.491 to 1.763) | 0.8248 | 0.9092 |
| Treatment in females: robotic surgery (vs. laparoscopic) | 1.018 (0.251 to 4.128) | 0.9801 | |
Appendix 11 Pathology central review
The agreement of the local pathology fields with the central review was assessed for pathology fields that fed into analyses. Summaries of agreement are presented below.
Circumferential resection margin positivity (CRM+)
A total of 359 out of 471 (76.2%) patients had both local pathology data and central review data for the CRM+ field, allowing for the evaluation of agreement. Agreement was non-evaluable for the remaining 112 patients, with reasons summarised in Table 107.
| Reasons | Treatment group, n (%) | Total (N = 112), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 60) | Robotic-assisted laparoscopic surgery (N = 52) | ||
| Central review data for CRM+ missing or non-evaluable | 50 (83.3) | 50 (96.2) | 100 (89.3) |
| Local pathology data for CRM+ missing | 1 (1.7) | 1 (1.9) | 2 (1.8) |
| Local pathology data and central review data for CRM+ missing or non-evaluable | 9 (15.0) | 1 (1.9) | 10 (8.9) |
There was agreement between local and central pathology in 343 out of 359 (95.5%) cases. The local and central evaluation of CRM+ is cross-tabulated in Table 108. In these 359 patients, the central review yields a CRM+ rate of 29 out of 359 (8.1%), which is greater than the rate of 21 out of 359 (5.8%) yielded by local pathology.
| CRM+ involvement | Agreement, n (%) | Total (N = 359), n (%) | |
|---|---|---|---|
| Yes (N = 21) | No (N = 338) | ||
| Yes | 17 (81.0) | 12 (3.6) | 29 (8.1) |
| No | 4 (19.0) | 326 (96.4) | 330 (91.9) |
Plane of surgery
A total of 420 out of 471 (89.2%) patients had both local pathology data and central review data for the plane of surgery field, allowing for the evaluation of agreement. Agreement was non-evaluable for the remaining 51 patients, with reasons summarised in Table 109.
| Reasons | Treatment group, n (%) | Total (N = 51), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 26) | Robotic-assisted laparoscopic surgery (N = 25) | ||
| Central review data for plane of surgery missing or non-evaluable | 15 (57.7) | 21 (84.0) | 36 (70.6) |
| Local pathology data for plane of surgery missing | 3 (11.5) | 2 (8.0) | 5 (9.8) |
| Local pathology data and central review data for plane of surgery missing or non-evaluable | 8 (30.8) | 2 (8.0) | 10 (19.6) |
There was agreement between local and central pathology in 262 out of 420 (62.4%) cases. The local and central evaluation of plane of surgery is cross-tabulated in Table 110. In these 420 patients, the central review yields a rate of mesorectal fascial plane of 179 out of 420 (42.6%), which is notably less than the rate of 319 out of 420 (76.0%) yielded by local pathology. The large majority of the disagreement between local and central pathology is seen when local pathology considered the plane of surgery to be mesorectal fascial, which is then downgraded to intramesorectal or muscularis propria plane by the central review.
| Mesorectum plane | Mesorectum plane, n (%) | Total (N = 420), n (%) | ||
|---|---|---|---|---|
| Mesorectal fascial plane (N = 319) | Intramesorectal plane (N = 67) | Muscularis propria plane (N = 34) | ||
| Mesorectal fascial plane | 174 (54.5) | 4 (6.0) | 1 (2.9) | 179 (42.6) |
| Intramesorectal plane | 124 (38.9) | 58 (86.6) | 3 (8.8) | 185 (44.0) |
| Muscularis propria plane | 21 (6.6) | 5 (7.5) | 30 (88.2) | 56 (13.3) |
Pathological T-stage
A total of 456 out of 471 (96.8%) patients had both local pathology data and central review data for the pT-stage field, allowing for the evaluation of agreement. Agreement was non-evaluable for the remaining 15 patients, with reasons summarised in Table 111.
| Reasons for non-evaluable agreement for pT-stage (by group) | Treatment group, n (%) | Total (N = 15), n (%) | |
|---|---|---|---|
| Standard laparoscopic surgery (N = 10) | Robotic-assisted laparoscopic surgery (N = 5) | ||
| Central review data for T-stage missing or non-evaluable | 2 (20.0) | 1 (20.0) | 3 (20.0) |
| Local pathology data for T-stage missing | 1 (10.0) | 2 (40.0) | 3 (20.0) |
| Local pathology data and central review data for T-stage missing or non-evaluable | 7 (70.0) | 2 (40.0) | 9 (60.0) |
There was agreement between local and central pathology in 424 out of 456 (93.0%) cases. The local and central evaluation of pT-stage is cross-tabulated in Table 112.
| pT-stage | pT stage, n (%) | Total (N = 456), n (%) | ||||
|---|---|---|---|---|---|---|
| 0 (N = 52) | 1 (N = 46) | 2 (N = 129) | 3 (N = 217) | 4 (N = 12) | ||
| 0 | 46 (88.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 47 (10.3) |
| 1 | 4 (7.7) | 46 (100.0) | 2 (1.6) | 1 (0.5) | 0 (0.0) | 53 (11.6) |
| 2 | 2 (3.8) | 0 (0.0) | 116 (89.9) | 6 (2.8) | 0 (0.0) | 124 (27.2) |
| 3 | 0 (0.0) | 0 (0.0) | 11 (8.5) | 205 (94.5) | 1 (8.3) | 217 (47.6) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.8) | 11 (91.7) | 15 (3.3) |
Appendix 12 Summary of protocol changes
| Version and date | Summary of changes |
|---|---|
| V1.0 dated 17 February 2010 | N/A – original protocol submitted for ethical review |
| V2.0 dated 25 March 2010 | Changes were required to the PIS/ICF document following ethical review, protocol was upversioned to match revised version of PIS/ICF (no changes made to the protocol) |
| V3.0 dated 2 August 2010 |
|
| V4.0 dated 1 March 2011 |
|
| V5.0 dated 19 March 2014 |
|
| V6.0 dated 1 July 2015 | This amendment to the protocol included an additional one-off questionnaire as a supplementary study to the ROLARR trial to determine the incidence and severity of Low Anterior Resection Syndrome (LARS) within participants of the ROLARR trial. It was felt that the results would have important consequences when counselling future patients with rectal cancer on the likely functional outcomes of surgery. The protocol was amended to include an additional appendix (Appendix 4) to cover the Low Anterior Resection Syndrome (LARS) supplementary study. Eligible ROLARR participants from Denmark, Germany, Italy, the USA and the UK were invited to complete a one-off postal survey |
List of abbreviations
- AIC
- Akaike information criterion
- APR
- abdominoperineal resection
- ASA
- American Society of Anesthesiologists
- BMI
- body mass index
- CI
- confidence interval
- CRF
- case report form
- CRM
- circumferential resection margin
- CRM+
- circumferential resection margin positivity
- CT
- computed tomography
- CTRU
- Clinical Trials Research Unit
- DFS
- disease-free survival
- DMEC
- Data Monitoring and Ethics Committee
- EBE
- empirical Bayes’ estimate
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FSFI
- Female Sexual Function Index
- GP
- general practitioner
- HAR
- high anterior resection
- HCHS
- Health and Community Health Service
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- IIEF
- International Index of Erectile Function
- I-PSS
- International Prostatic Symptom Score
- IQR
- interquartile range
- IRB
- institutional review board
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LAR
- low anterior resection
- MCS
- mental component score
- MFI-20
- Multidimensional Fatigue Inventory-20
- OR
- odds ratio
- OS
- overall survival
- PCS
- physical component score
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36v2
- Short Form questionnaire-36 items version 2
- TME
- total mesorectal excision
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
