Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 09/100/10. The contractual start date was in June 2011. The final report began editorial review in June 2018 and was accepted for publication in November 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Dhillon-Smith et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Clinical background
Miscarriage, the loss of a pregnancy before 24 weeks of gestation, affects one in five women, making it the commonest complication of pregnancy. It substantially affects the physical and psychological well-being of women: research1 shows that the level of distress associated with miscarriage can be equivalent to that of a stillbirth of a term baby.
In addition, preterm birth, the delivery of a baby between 24 and 37 completed weeks of gestation, occurs in 6–10% of pregnancies. Preterm birth is responsible for up to 85% of newborn deaths. 2 Of those who survive, approximately 10% suffer long-term disability. The human cost of preterm birth is, therefore, enormous; the financial cost of preterm birth is estimated at £939M per year in the UK. 2 This includes health-care costs (including neonatal care), education and costs to the parents.
The prevalence of measurable circulating antithyroid autoantibodies to thyroglobulin or thyroid peroxidase (TPO) in women of childbearing age in the developed world is 5–15%; that of overt hypothyroidism is estimated to be 0.3–0.5% and that of subclinical hypothyroidism is estimated to be 2–3%. 3,4 Prevalence rates are similar during pregnancy. 4,5
Pregnancy may trigger progression to a relative hypothyroid state in women with thyroid peroxidase antibodies (TPOAbs). This is because of an increased demand for thyroid hormone during pregnancy and because women with thyroid autoimmune disease are less able to sustain this increased demand.
To understand the relationship between thyroid autoantibodies and adverse outcomes, systematic reviews of the literature were conducted.
Association between thyroid antibodies and miscarriages
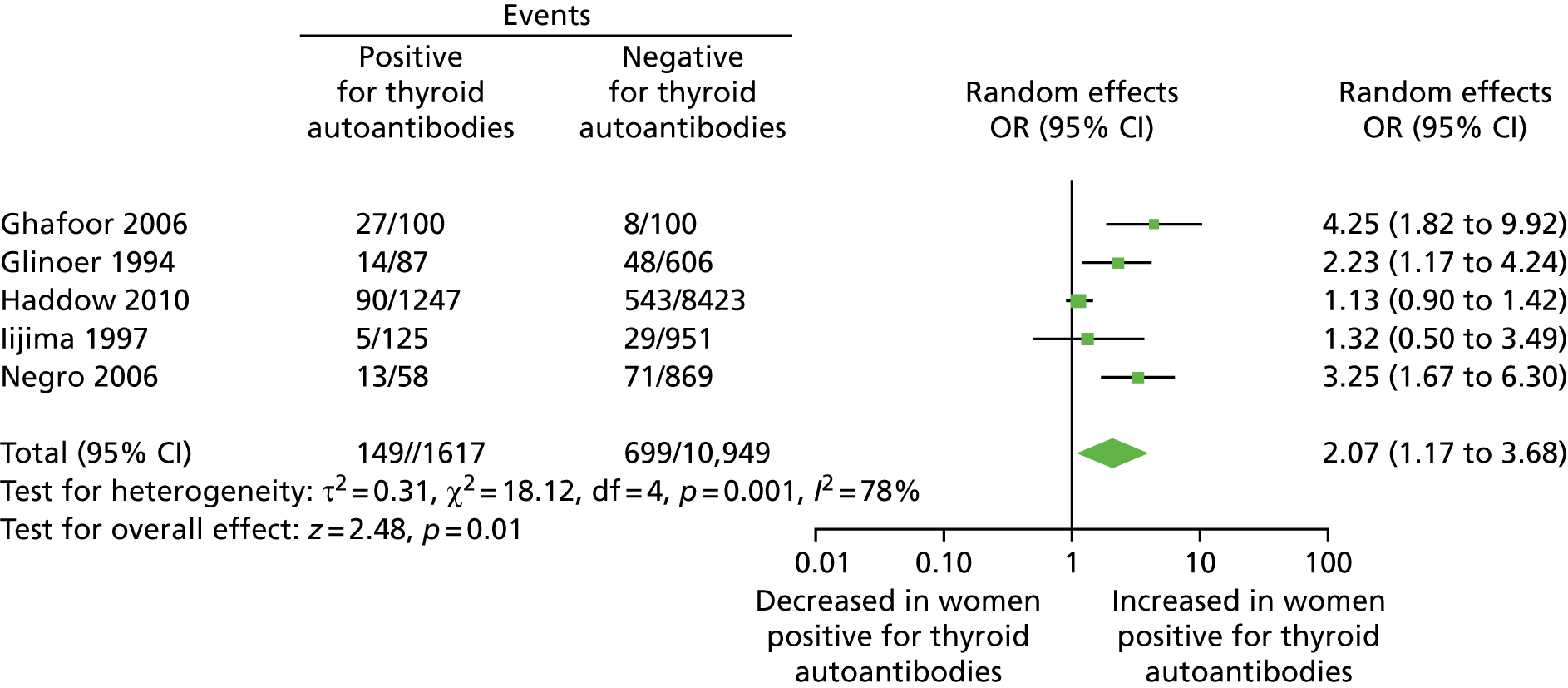
A systematic review, published in the British Medical Journal (BMJ), identified 31 studies, including a total of 12,126 women and three reviews. 6 Thirteen studies were in recurrent miscarriage populations, nine were in infertile populations and nine were in unselected or other populations. The quality of the studies was judged to be generally good on the Newcastle–Ottawa Scale,7 with most studies (22/29; 76%) establishing good comparability of the antibody-positive and -negative cohorts. Of the 31 studies, 28 showed a positive association between thyroid antibodies and miscarriage. A meta-analysis of the results from 19 cohort studies demonstrated more than a tripling in the odds of miscarriage in the presence of thyroid antibodies [odds ratio (OR) 3.9, 95% confidence interval (CI) 2.48 to 6.12] (Figure 1). This strong and statistically significant association between thyroid antibodies and miscarriage was observed in all three population subgroups. A ‘dose–response’ relationship between thyroid antibody positivity and the number of miscarriages was observed. There was also a similar magnitude of increased risk of miscarriage in each of the three subpopulations identified.
FIGURE 1.
Association between thyroid autoantibodies and miscarriage. Reproduced with permission from Thangaratinam et al. 6 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.

Association between thyroid antibodies and preterm birth
The same BMJ systematic review6 identified five studies examining the association between thyroid antibodies and preterm birth, including a total of 12,566 women and one review. All five were cohort studies, and all were judged to be of good quality on the Newcastle–Ottawa Scale. 7 All studies showed a positive association between the presence of thyroid antibodies and preterm births. A meta-analysis showed a more than twofold increase in the odds of preterm birth in the presence of thyroid antibodies (OR 2.07, 95% CI 1.17 to 3.68) (Figure 2).
FIGURE 2.
Association between thyroid autoantibodies and preterm births. Reproduced with permission from Thangaratinam et al. 6 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.
Effectiveness of levothyroxine treatment
Prior to commencing the Thyroid AntiBodies and LEvoThyroxine (TABLET) trial, only two randomised trials,8,9 comprising a total of 187 women, had examined levothyroxine treatment for women with TPOAbs. Both studies were in euthyroid women with thyroid autoantibodies; one was in unselected women8 and the other in women scheduled to have in vitro fertilisation (IVF) treatment. 9 One study9 used a dose of 1 µg/kg/day of levothyroxine and the other study8 used a titrated dose of levothyroxine. The quality of the studies was deemed to be satisfactory (Jadad Quality Scores of 5/5 and 3/5). Both studies showed a reduction in miscarriage rates (36% and 75% relative reductions) and, when the results were pooled, there was a statistically significant 52% reduction in miscarriages with levothyroxine treatment [relative risk (RR) 0.48, 95% CI 0.25 to 0.92]. One of the two studies reported on preterm birth;8 this study (n = 115) found a 69% reduction in preterm births with levothyroxine treatment (RR 0.31, 95% CI 0.11 to 0.90).
The full search terms used in the BMJ review can be found in Appendix 1. The search was updated to 2018 to include any further trials that had been published on this topic.
Since the initiation of the TABLET trial, a further two randomised trials10,11 have evaluated levothyroxine treatment for thyroid antibodies. Vissenberg et al. 10 have designed a double-blind, randomised controlled trial (T4-Life) evaluating the use of levothyroxine in TPOAb-positive euthyroid women who have had recurrent miscarriages (defined as two or more consecutive losses). This trial is still in the recruitment phase and so the results are not yet available. A further trial by Wang et al. 11 has evaluated euthyroid TPOAb-positive women undergoing IVF treatment. The details of this study, and its findings and interpretation, are reported in Chapter 5.
Levothyroxine is a commonly used drug in obstetric–endocrine clinics (and, indeed, general internal medicine clinics), and has a well-established safety profile. The three randomised studies8,9,11 did not find any safety concerns for the mother or the baby. Specifically, there were no instances of hyperthyroidism (from overtreatment with levothyroxine). However, as the total number of women across all three trials was 787 and follow-up was only to the end of pregnancy, these trials would not have been suitable for assessing rare or long-term adverse events (AEs). We therefore carried out a literature search to identify studies of potential harm of levothyroxine treatment in pregnancy by using medical subject heading (MeSH) terms and keywords to capture AEs and combined this with search terms to capture levothyroxine and pregnancy studies. This safety review identified 1026 studies, of which 191 were reviews. Most studies evaluated the use of levothyroxine in hypothyroid pregnant women, and found no clear or consistent evidence of serious adverse effects on the mother or the baby, provided there was appropriate monitoring and dose titration. 12,13 A comprehensive literature review, which was interpreted and graded by an international panel of endocrinologists, found that the potential risk of treating subclinical hypothyroidism with levothyroxine was limited to the development of subclinical hyperthyroidism. 14 Although this review may not directly apply to the euthyroid population, the absence of any serious side effects in this review provides reassurance on the safety of levothyroxine, particularly at the proposed dose of 50 µg per day.
The pathophysiological consequences of thyroid antibodies
The exact mechanisms to explain the observed associations between thyroid antibodies and miscarriages or preterm birth are largely unknown. Two mechanisms have been postulated:
-
It has been suggested that the presence of thyroid antibodies may reflect a generalised activation of the immune system and specifically, a dysregulated activity of the immune system at the fetal–maternal interface. The presence of TPOAbs in several non-thyroidal autoimmune diseases supports this hypothesis of global immune dysfunction. 15 Furthermore, there is evidence that there is an alteration in cytokine expression by peripheral T-lymphocytes in TPO-positive individuals outside pregnancy. 16
-
Alternatively, the presence of thyroid antibodies in euthyroid women could be associated with a subtle deficiency in thyroid hormone availability (a fall in circulating free thyroid hormones within the reference ranges) or a lower capacity of the thyroid gland to adequately rise to the increased demand for augmented synthesis of thyroid hormones required in pregnancy. Indeed, the mean serum thyroid-stimulating hormone (TSH) values, although being within normal range, are significantly higher in thyroid antibody-positive women than in women without thyroid antibodies (TSH in TPO-positive women, 2.14 ± 0.84 mlU/l; TSH in TPO-negative women, 1.33 ± 0.32 mlU/l). 17
How may levothyroxine alter the pathophysiology?
Higher concentrations of thyroid hormones within the normal reference range can directly enhance innate and adaptive immunity in normal, healthy individuals. 18 Pregnancy is an inflammatory process involving a shift in the regulation of cytokine networks within the local placental–decidual environment. Dysregulation of local inflammatory processes may be associated with miscarriage and premature delivery. 19 The main regulators of inflammation within the decidua are a whole host of cells of ‘bone marrow lineage’. 20 In particular, uterine natural killer cells, which are a major source of angiogenic growth factors and cytokines, have been shown to regulate vascular remodelling. 21 Thyroid hormones can potentially influence (1) angiogenic growth factor and cytokine production,22,23 as well as (2) trophoblast proliferation, survival and invasion. 24,25 Thus, thyroid hormones may influence the maternal immune regulation both in general and at the fetal–maternal interface, as well as specifically affect trophoblast and decidual cell behaviour.
Aims and objectives
The primary aim of the TABLET trial was to test the hypothesis that, in euthyroid women with TPOAbs, levothyroxine (a dose 50 µg taken orally once daily), started preconceptually and continued to the end of pregnancy, increases the proportion of women who attain a live birth at or beyond 34 completed weeks of gestation by at least 10% compared with placebo.
The secondary aims were to:
-
Test the hypothesis that levothyroxine improves secondary outcomes such as ongoing pregnancy at 12 weeks, gestation at delivery and survival at 28 days of neonatal life (the full list of outcomes is given in Chapter 2).
-
Explore subgroup effects of levothyroxine in prognostic subgroups (including maternal age, number of previous miscarriages, initial serum TSH concentration and women who were having infertility treatment).
-
Test the hypothesis that levothyroxine, compared with placebo, does not incur substantial adverse effects to the mother or the neonate.
The parallel mechanistic study had specific aims, which are detailed in Chapter 4.
Chapter 2 Methods for the randomised trial
This chapter reports the methods used to conduct the TABLET trial.
Trial design
The TABLET trial was a randomised, double-blind, placebo-controlled multicentre trial of levothyroxine in euthyroid women with TPOAbs, conducted to determine if levothyroxine can reduce miscarriage and premature births in women. The trial had a favourable ethics opinion from the South West 3 Multicentre Research Ethics Committee (reference number 11/SW/0036).
Eligibility (inclusion and exclusion)
Participants were recruited from early pregnancy units, recurrent miscarriage clinics and infertility clinics in the participating NHS hospitals across the UK. Participants had to meet the following eligibility criteria (see Recruitment for more details on the recruitment process):
-
women trying to conceive
-
a history of one or more miscarriage(s) or primary or secondary infertility
-
aged 16–40 years at randomisation
-
biochemically euthyroid [TSH 0.44–3.63 mlU/l; free thyroxine 4 (T4) 10.0–21.0 pmol/l using the appropriate analyser]
-
TPOAb positive according to local laboratory reference ranges
-
willing and able to give informed consent.
Participants could not be included if any of the following criteria were applicable:
-
current treatment for any thyroid disorder [past treatment was considered on an individual basis (see below)]
-
taking amiodarone or lithium therapy
-
contraindications to levothyroxine therapy – thyrotoxicosis, hypersensitivity to levothyroxine, or any of its excipients
-
participation in any other blinded, placebo-controlled trial of investigational medicinal products (IMPs) in pregnancy
-
previous or current diagnosis of cardiac disease.
Women who had previously been treated for thyroid disorders were considered on a case-by-case basis. It was left to the discretion of the principal investigator whether or not a woman with a history of thyroid disorder could be safely offered participation in the trial. The rationale for exercising this discretion was because it was agreed that women who may have received very short-term treatment a long time ago and whom have since had normal thyroid function and not required treatment long term should not be automatically discounted from participation in the trial. These women would have been classified as ‘euthyroid’ for some time and it was not deemed clinically unsafe for these women to participate if they were TPO positive.
Recruitment
The TABLET trial recruited from three main populations: those with a history of one or two miscarriages, those with recurrent miscarriage (defined as three or more consecutive losses) and those under investigation or treatment for infertility. Recruitment was via a two-step process. Women were initially invited to be screened for TPOAbs and thyroid function tests (TFTs) and then those who were found to be positive for TPOAbs, with normal thyroid function, were introduced to the TABLET randomised controlled trial. Further details are given in the following sections.
Screening of potential participants
Potential participants were identified and approached by clinic doctors, nurses and research staff. The routes of initial approach for screening are shown in a flow diagram (Figure 3). All participants were clearly advised that participation in the trial was entirely voluntary with the option of withdrawing from the trial at any stage, and that participation or non-participation would not affect their usual care. All women were approached by staff who were appropriately trained in Good Clinical Practice and specifically trained in taking consent for this trial. To be invited for screening, the woman must have been willing and able to give informed consent and to provide a blood sample (of 10 ml) for thyroid antibody and thyroid function testing (TPOAbs and measurement of serum TSH and free T4).
FIGURE 3.
Routes of initial approach for screening.
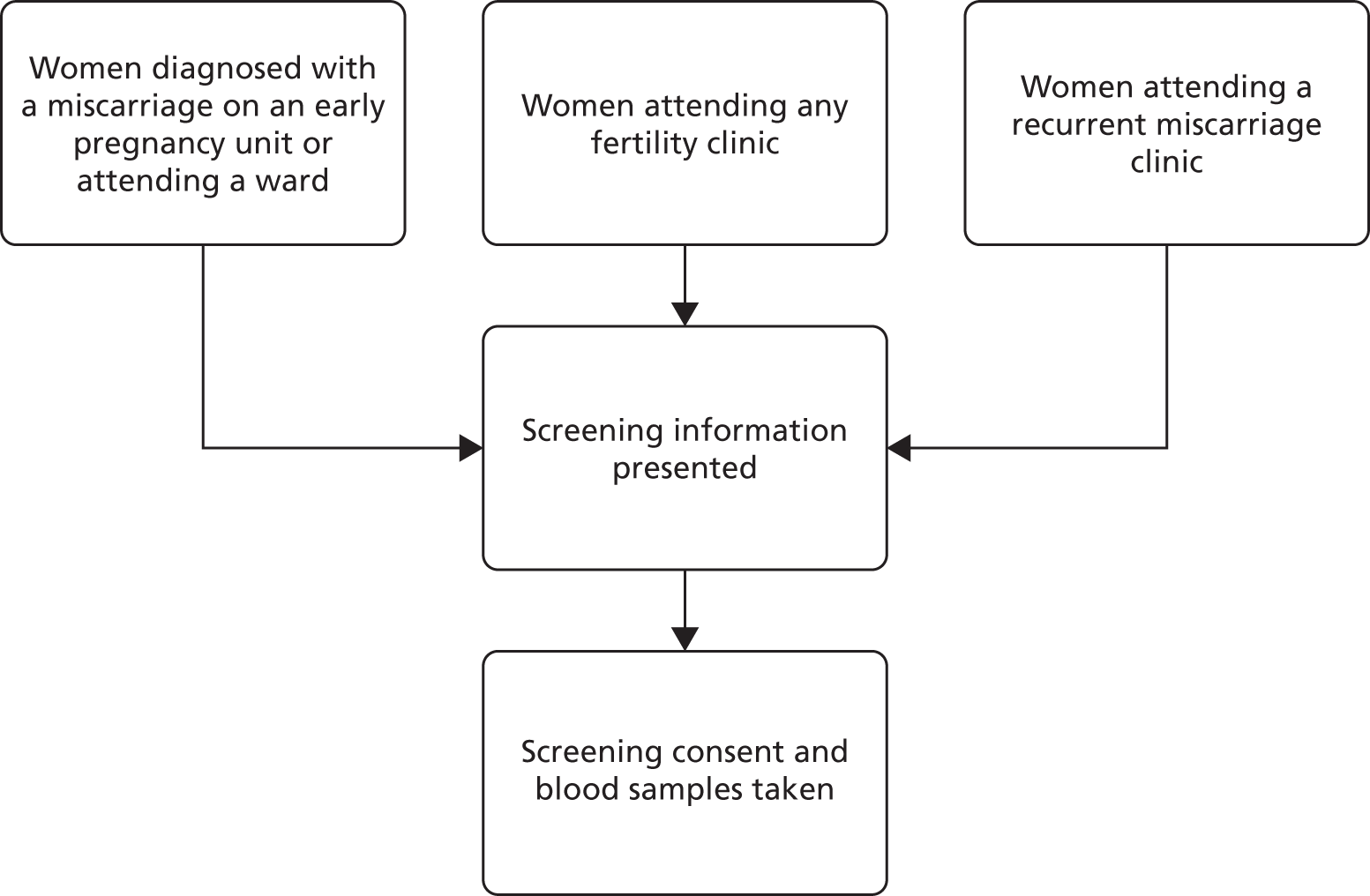
The aim was to approach women at the optimum point, before their subsequent conception. For women who had a recent miscarriage, the initial approach was carried out after the miscarriage had been confirmed on the early pregnancy unit or when they were admitted to the ward for management. For women who were under infertility services, the initial approach was made at a routine clinic appointment and women with recurrent miscarriage were approached in the recurrent miscarriage clinics.
Some women were screened on an early pregnancy unit following an acute pregnancy loss, when it was their third (or more) miscarriage. These women were categorised into the recurrent miscarriage population. Potential participants were provided with a short screening patient information sheet and given time to consider their involvement.
Figure 4 shows the potential pathways that were followed by all of the screened participants. The co-ordinating midwife/nurse at each centre was responsible for contacting TPO-negative women to inform them that they were ineligible for the TABLET trial and provide reassurance about normal TFTs. A small number of asymptomatic women were identified as having abnormal TFTs via the screening process. It was advised that the local principal investigator would make decisions on further investigations and/or treatment for these women based on the degree of thyroid abnormality and local guidelines. If TPOAbs were positive and TSH and free T4 concentrations were within the normal range for the trial [see Eligibility (inclusion and exclusion) for limits], the woman was sent a TABLET trial participant information sheet and an appointment was arranged to discuss participation at a subsequent clinic visit at which final eligibility checks could be performed. For those who had recently suffered a miscarriage, the woman’s desire to conceive again was explored and only those who indicated that they intended to try again, within the next 12 months, were invited to participate. It was made clear to participants that they could withdraw from the trial at any time. Consent was confirmed in writing. This included consent for future evaluation of themselves and the child and the health records of both through the Office for National Statistics or equivalent.
FIGURE 4.
Screening of potential participants. GP, general practitioner.

Note on thresholds for thyroid function tests
Various assays for TPOAbs are available, each with different detection limits and thresholds for test positivity, which are pre-determined by the assay manufacturer. These variations are an accepted part of normal practice in the UK. Quality assurance for assays in the laboratories for all of the participating centres is provided by UK IMMQAS (Immunology Quality Services), which shows > 99% concordance in the classification of samples as either positive or negative for TPOAbs across all assays. Therefore, the TABLET trial protocol did not define a threshold for TPO positivity but, instead, accepted the classification provided by the laboratories servicing the participating centres. For TFT and free T4 testing, the participating site must have used an analyser approved by the Trial Management Group (TMG), and it must be routinely participating in the UK National External Quality Assessment Service (NEQAS) (Sheffield, UK) external quality assurance scheme.
The approved analysers were Elecsys®/Modular/Cobas® (F. Hoffmann-La Roche AG, Basel, Switzerland), Abbott ARCHITECT (Abbott Laboratories, Chicago, IL, USA) and Advia Centaur® (Siemens Healthineers AG, Munich, Germany). The euthyroid reference range of TSH 0.44–3.63 mlU/l and circulating free T4 10–21 pmol/l covered the central quartiles of all three assays and was in keeping with the non-pregnant reference range from the Roche manufacturer recommendations. 26
The main aim of monitoring TFTs was to ensure the safety of the participant and the pregnancy according to the available evidence at the time. A significantly elevated level of TSH or a significantly elevated level of free T4 have been associated with adverse pregnancy outcomes and warrant treatment. There was no evidence of harm and treatment benefit for subclinical hyperthyroidism (an isolated lowering of TSH accompanied by a normal free T4) or isolated hypothyroxinaemia (low free T4 accompanied by a normal TSH). Thus, only upper limits of TSH and free T4 had been set to ensure safety in this trial.
Non-pregnant
We defined a similar (but not identical) upper limit to the TSH upper limit for eligibility for the non-pregnant recruits. This was justified by factoring in a 10% allowance for intra-individual variation over time and for interassay variations. For example, if we recruited a woman with a TSH of 3.63 mlU/l, she would not be withdrawn if her follow-up TSH was 3.9 mlU/l because of variations in the assay and the normal fluctuations of TSH and not because of a real difference in her thyroid function. Therefore, the agreed TSH level at follow-up was < 4.0 mlU/l and for free T4 it was < 25 pmol/l.
Pregnant
It was difficult to define one set of limits for all three assays during pregnancy because of the apparent differential exaggerated bias associated with different assays in the assessment of pregnancy samples. The limits also had to be similar to the current limits in use by some of the centres, as there would have been conflict in the management of trial and non-trial women. Based on a review of literature for the three assays, certain limits were proposed (see Appendix 2).
Randomisation
Eligibility criteria were confirmed prior to obtaining consent, and demographic and prognostic factors on the Randomisation Notepad were gathered. Following this, the woman could be randomised into the trial. Randomisation was conducted through a secure online randomisation service provided by the University of Birmingham Clinical Trials Unit (BCTU). Following this, trial and bottle numbers were allocated.
Randomisation method and stratification variables
Participants were randomised individually in an equal (1 : 1) ratio of levothyroxine to placebo. A ‘minimisation’ procedure using a computer-based algorithm was used to avoid chance imbalances in important stratification variables. Strata used in the minimisation were:
-
maternal age (< 35 years, ≥ 35 years)
-
number of previous miscarriages (0, 1 or 2, ≥ 3)
-
initial TSH concentration (≤ 2.5 mlU/l, > 2.5 mlU/l)
-
women who were having infertility treatment (yes/no).
For logistical reasons, the randomisation was also minimised by centre.
Treatment allocations
Levothyroxine
The IMP was levothyroxine; 50 µg of levothyroxine sodium as an encapsulated tablet was taken once daily after randomisation and preconceptually, and continued to the end of any pregnancy or until 12 months post randomisation if pregnancy did not occur. It was assumed that, for the majority of participants, pregnancy would occur within 1 year of randomisation and that the pregnancy may continue to term up to 42 weeks. Thus, the treatment period ranged from 42–44 weeks to 94 weeks for term pregnancies.
It was advised that the IMP should be taken orally before breakfast and ingested with water (milk, iron supplements, calcium supplements and antacids can impair the absorption of levothyroxine and it was advised to not take these at the same time).
The choice of 50 µg per day was made after a careful review of the existing literature, an extensive survey of endocrinologists as well as obstetricians with an interest in maternal medicine, a review of the host organisation’s obstetric–endocrine practice database and a review of other related evidence.
Placebo
The placebo was a placebo tablet, encapsulated in the same format as the IMP to be identical in colour, shape and weight. The treatment regime was exactly the same as in the levothyroxine group.
Excluded medications
The use of amiodarone and lithium were included in the exclusion criteria for the study as these medications can independently affect thyroid function. Women were advised to stop taking the TABLET trial treatment if these drugs were indicated during the trial. Oral contraceptives may alter the pharmacodynamics of thyroxine, so women were also advised to stop trial treatment if these were taken.
Drug supply and dispensing
Interventions were supplied by Sharp Clinical Services (Rhymney, UK; formerly Bilcare UK Ltd), which procured the trial drug and manufactured the placebo tablet, overencapsulated the IMP and placebo, and dispensed them into containers accordingly. This company had no role in the design of the trial, the collection, analysis, interpretation of the data or the writing of the report.
A hospital pharmacist prepared the trial treatment bottle for dispensing. Each trial treatment bottle contained 13 weeks’ supply for use by one participant. Each subsequent trial treatment bottle contained a further 13 weeks’ supply (see Figure 6 and Scheduled trial appointments).
Blinding
Participants, investigators, research midwives/nurses and other attending clinicians all remained blind to the trial drug allocation for the duration of the trial.
In the case of any serious adverse events (SAEs), management and care of the women was initiated as though the woman was taking levothyroxine. Cases that were considered serious, unexpected and possibly, probably or definitely related [i.e. possible suspected unexpected serious adverse reactions (SUSARs)] were unblinded only at the trial office by the trial co-ordinator. The attending clinician and local principal investigator were not made aware of the actual trial drug. If a participant was withdrawn from the trial as a result of abnormal TFTs (see Thyroid hormone monitoring and criteria for stopping trial treatment) and if the drug allocation was required for the continued medical management of the withdrawn participant, clinicians were advised to contact the TABLET trial office or use the online access to gain unblinding information. Any instances of this were recorded on the trial database.
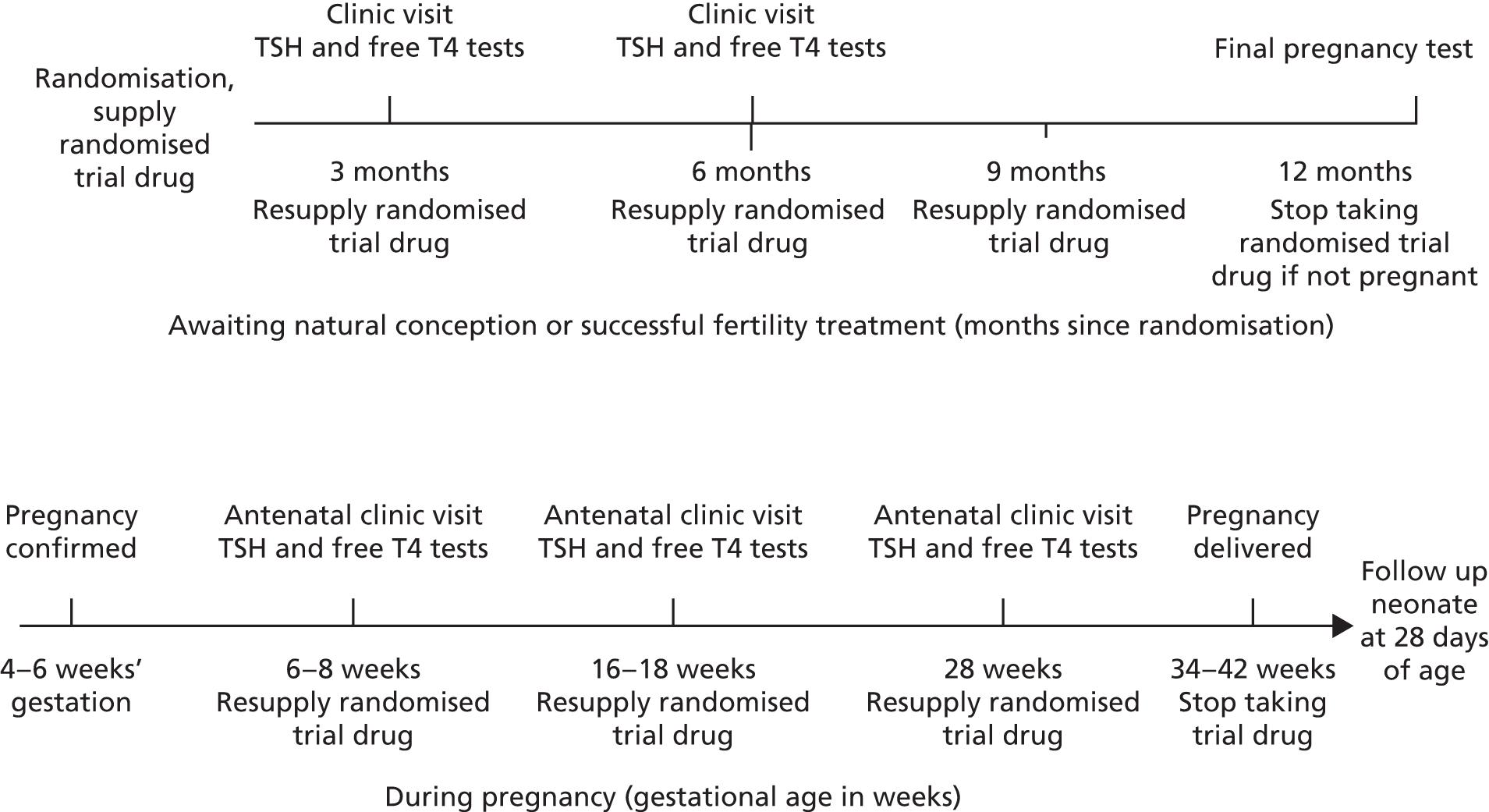
Scheduled trial appointments
Trial participants returned to the randomising hospital at two further intervals while trying to conceive and for routine antenatal appointments. At each visit, blood samples were taken for TSH and free T4 level (see Thyroid hormone monitoring and criteria for stopping trial treatment). If conception did not take place by the end of the 12th month, the woman was asked to perform a pregnancy test and ensure that it was negative prior to stopping trial medication (Figure 5).
FIGURE 5.
Thyroid drug supply and monitoring timelines.
Compliance and treatment withdrawal
Compliance monitoring
Compliance was evaluated by two methods, first by pill-counting. Women were asked to bring completed, partially used and unused treatment bottles to the trial centres at follow-up visits. The research nurse would receive the empty/partially used/unused treatment bottles at the local centres and document the number of remaining pills (if any) in the database for each trial participant. Second, the participant was asked how often they took the capsules at each monitoring and resupply visit, and asked again when they completed the trial. The categories of compliance were as follows: 0%, never; 1–24%, hardly any; 25–49%, some; 50–74%, most; 75–99%, almost always; 100%, every day. Good compliance was defined as ≥ 75% usage.
Participant withdrawal from treatment
A participant was considered for withdrawal from the trial treatment if, in the opinion of the investigator or the care providing clinician or clinical team, it was medically necessary to do so. Participants could also voluntarily withdraw from treatment at any time; however, given that withdrawn patients can bias clinical trial results, women were encouraged to allow data collection to continue even if trial treatment ceased.
Thyroid hormone monitoring and criteria for stopping trial treatment
If a woman developed overt or subclinical hypothyroidism with TSH concentrations above the decision limit for the specified analyser, or overt hyperthyroidism with a free T4 above the decision limit for the specified analyser, she was discontinued from trial medication and treated according to local clinical guidelines.
Withdrawal from trial
Participants could voluntarily withdraw their consent to trial participation at any time. If a participant did not return for a scheduled visit, attempts were made to contact her and, when possible, review compliance and AEs. We made an attempt to document all reasons for self-withdrawal. If a participant explicitly withdrew consent to have any further data recorded, their decision was respected and recorded on the electronic data capture system. All communication surrounding the withdrawal was noted in the patient’s records and no further data collected for that patient.
Outcomes and assessment
Primary outcome measures
The primary outcome was the proportion of women who had a live birth at or beyond 34 completed weeks of gestation. This proportion was calculated with the denominator totalling all women randomised, and the numerator totalling women who conceived within 1 year of randomisation and went on to give live birth at or beyond 34 weeks of gestation. Women who failed to conceive within 1 year, or who became pregnant but had a miscarriage, ectopic pregnancy, termination, or gave birth before 34 weeks or experienced a stillbirth were included in the denominator but not the numerator.
Secondary outcome measures
Secondary outcomes were as follows:
-
clinical pregnancy at 7 weeks
-
ongoing pregnancy at 12 weeks
-
miscarriage at < 24 weeks
-
stillbirth (intrauterine death at ≥ 24 weeks)
-
ectopic pregnancy
-
termination (and reasons)
-
live birth at < 34 weeks
-
time from conception to pregnancy end (any reason)
-
mode of initiation of labour (spontaneous/induced)
-
mode of delivery (vaginal/operative vaginal/caesarean)
-
gestation at delivery (weeks)
-
time from conception to live birth
-
gestation at delivery of < 28 weeks/< 34 weeks/< 37 weeks
-
birthweight (g)
-
birthweight adjusted for gestational age and sex (centiles)
-
birthweight adjusted for gestational age, sex, parity, maternal body mass index (BMI) and ethnicity (centiles)
-
small for gestational age and sex (proportion < 10th centile)
-
small for gestational age, sex, parity, maternal BMI and ethnicity (birthweight proportion < 10th centile)
-
large for gestational age and sex (proportion ≥ 90th centile)
-
large for gestational age, sex, parity, maternal BMI and ethnicity (birthweight proportion ≥ 90th centile)
-
Apgar (appearance, pulse, grimace, activity and respiration) score at 1 minute/5 minutes
-
serum TSH concentration (mlU/l; log-transformed) at each assessment time
-
serum free T4 level (pmol/l) at each assessment time
-
subclinical/overt hypothyroidism
-
subclinical/overt hyperthyroidism
-
maternal antenatal complications (hyperemesis gravidarum/gestational diabetes/pre-eclampsia or eclampsia/obstetric cholestasis/preterm pre-labour rupture of membranes/intrauterine growth restriction/others)
-
intrapartum complications (shoulder dystocia/others)
-
maternal postnatal complications [admission to a high-dependency unit (HDU) or intensive care unit/abnormal thyroid test within 4 weeks/referred to a psychiatrist or started on antidepressants/others]
-
neonatal complications (early neonatal death, defined as death within 7 days after delivery/late neonatal death, defined as death beyond 7 days and before 28 days post delivery/admission to neonatal unit or special care baby unit, or active resuscitation, within first 28 days/surfactant use/mechanical ventilation/intermittent positive pressure ventilation/continuous positive airway pressure/oxygen use/congenital abnormalities/hypoxic ischaemic encephalopathy/retinopathy of prematurity/respiratory distress syndrome/pneumothorax/intraventricular haemorrhage (grade 3 or 4)/necrotising enterocolitis/early infection/others)
-
reported symptoms that participant is concerned about at each assessment time
-
SAEs.
Outcome assessment details
The timing of scheduled hospital assessments is described in Scheduled trial appointments. Details of how outcome measures were generated are given in Table 1.
| Outcome assessed | When? | How? | By whom? |
|---|---|---|---|
| Biochemical pregnancy | Approximately 4 weeks of gestation | Urinary pregnancy test | Trial participant |
| Clinical pregnancy | 6–8 weeks | Ultrasonography | Ultrasonographer |
| Ongoing pregnancy | 11–13 weeks | Ultrasonography | Ultrasonographer |
| Antenatal outcomes | Any time in the antenatal period or afterwards |
|
Research nurse or doctor |
Final pregnancy outcomes, including:
|
At or after the end of pregnancy |
|
Research nurse or doctor |
| Neonatal outcomes | Up to 28 days of neonatal life |
|
Research nurse or doctor |
| TFTs |
|
Venous blood sample | Nurse or phlebotomist |
Relevant trial data were transcribed directly onto a secure web-based database. All personal information was treated as strictly confidential. Source data comprised the research clinic notes, hospital notes, hand-held pregnancy notes and laboratory results. Women were encouraged to report pregnancies, miscarriages or other pregnancy losses, deliveries and AEs that occurred between clinic visits or that were presented at non-participating hospitals to the research midwife. Self-reports were verified against clinical notes.
Definition of the end of the trial
The interventional phase of the trial ended when the last participant delivered her baby, suffered a pregnancy loss or completed 12 months of treatment without becoming pregnant. The observational phase of the trial ceased when the 28-day follow-up had been completed for the baby of the last participant recruited who became pregnant. The primary analysis was scheduled to occur after all randomised women had completed the primary and secondary outcomes (up to 28 days of neonatal life following a maximum of 12 months preconception, i.e. up to a maximum of approximately 2 years post randomisation) and the corresponding outcome data were entered into the trial database and validated as being ready for analysis.
Notes on adverse events and serious adverse events
All AEs, from the first administration of trial treatment until the end of the pregnancy or 12 months of trial participation without pregnancy (whichever was later), whether observed directly or reported by the participant, were collected and recorded. Trial participants were asked about the occurrence of AEs and SAEs at each trial visit. All SAEs were recorded and faxed to BCTU within 24 hours of the research staff becoming aware of the event. The local principal investigator (or other nominated clinician) had to assign seriousness, severity, causality and expectedness to the SAE before reporting. SAEs categorised by the local investigator as both suspected to be related to the trial drug and unexpected were classified as SUSARs, and were subject to expedited reporting.
All relevant trial documentation, including the screening information sheet, screening consent form, randomisation information sheet, randomisation consent form, trial schema and the SAE form, can be found in the trial protocol.
Statistical considerations
Sample size
We planned to randomise 900 women (450 in each arm). To detect a minimally important difference of 10% in live birth at or beyond 34 weeks of gestation (from 55% to 65%), at p = 0.05 and power of 80%, 380 women needed to be randomised to the levothyroxine arm and 380 women to the placebo arm (760 in total). Including a worse-case scenario attrition rate of 15%, the total number of participants required was 900.
The minimally important difference of 10% was defined following consultations with health-care practitioners, patients and representatives of patient bodies for the progesterone in recurrent miscarriages (PROMISE) trial. 27 However, it should be noted that this difference was smaller than that expected from the existing literature, which showed that the risk of miscarriage alone is halved with levothyroxine therapy (RR 0.48, 95% CI 0.25 to 0.92). Hence, assuming an expected absolute difference of 15% in live births beyond 34 weeks of gestation, 900 participants (after accounting for 15% attrition) would provide a power of 99%.
The 55% baseline live birth rate in the control group was based on the assumption that 10% of women would fail to conceive within 1 year28 and a further 35% would either miscarry or have a preterm birth. 2
Statistical analysis
A comprehensive statistical analysis plan was drawn up prior to any analysis and provided to the independent Data Monitoring Committee (DMC) and Trial Steering Committee (TSC) for review. Full details of the statistical analysis can be found in the statistical analysis plan.
In summary, categorical baseline data were summarised with frequencies and percentages. Normally distributed continuous variables were summarised as means with standard deviations (SDs); for continuous variables that were not normally distributed, medians with interquartile ranges (IQRs) were presented. In the first instance, participants were analysed in the treatment group to which they were randomised, irrespective of compliance with the treatment protocol. All estimates of differences between groups are presented with 95% two-sided CIs. p-values from two-sided tests at the 5% significance level are also included.
For the primary outcome (live birth at ≥ 34 weeks of gestation), the population was all randomised participants. A log-binomial model was used to generate RRs along with 95% CIs, adjusting for the minimisation parameters. Statistical significance of the treatment group parameter was determined through examination of the associated chi-squared statistic.
Analysis was performed as per the primary outcome for the other binary outcomes. For maternal pregnancy outcomes (such as miscarriage and stillbirth), the analysis population was all women who went on to achieve confirmed pregnancy. For all other secondary maternal and neonatal outcomes, the analysis population was those with live births at ≥ 24 weeks of gestation. For secondary neonatal outcomes and complication rates, twin babies were both counted in the analysis population. For time from conception to pregnancy end, and time from conception to birth, a Cox proportional hazards model was employed, adjusting for the minimisation variables. A chi-squared test was used to test the statistical significance of the treatment group parameter. For continuous outcomes [e.g. birthweight, birthweight centiles, TSH (following a log-transformation) and free T4 values], a linear regression model was used, adjusting for the same minimisation parameters. Here, a F-test was used to test the statistical significance of the estimated treatment group parameter generated from the restricted maximum likelihood estimates. The proportion and percentage of participants experiencing any SAE were presented by group. Statistical significance was determined by a chi-squared test.
A sensitivity analysis was performed on the primary outcome and the outcome of miscarriage at < 24 weeks of gestation to test the impact of any missing data. This assumed that all participants lost to follow-up had a negative outcome (i.e. preterm < 34 week birth or miscarriage). The number of missing data was very limited for these outcomes (< 1%), so no further sensitivity analysis was performed.
Pre-planned subgroup analyses (limited to the primary outcome measure and miscarriage rate) were completed in the following: (1) maternal age: (< 35 years, ≥ 35 years), (2) number of previous miscarriages (0, 1 or 2, ≥ 3), (3) initial TSH concentration (≤ 2.5 mlU/l, > 2.5 mlU/l), (4) women undergoing infertility treatment (yes, no), (5) ethnicity (black, white, Chinese, South Asian, other), (6) TPO baseline level (‘very high’, taken as ≥ 50th percentile, and ‘high’, taken as < 50th percentile) and (7) BMI (< 25 kg/m2, ≥ 25 kg/m2). The effects of these subgroups were examined by adding the subgroup by treatment group interaction parameters to the log-binomial model; a chi-squared test was used to test the statistical significance of this parameter.
Interim analyses of effectiveness and safety end points were performed on behalf of the Data and Safety Monitoring Committee on an approximately 6-monthly basis during the period of recruitment. These analyses were performed with the use of the Haybittle–Peto principle;29 hence, no adjustment was made in the final p-values to determine significance.
Trial oversight
Trial oversight was provided by a TSC (chaired by Professor Jane Norman, University of Edinburgh) and a DMC (chaired by Professor John Lazarus, University of Cardiff).
The TSC provided independent supervision for the trial, providing advice to the chief and co-investigators and the sponsor on all aspects of the trial throughout the trial. The DMC adopted the DAMOCLES charter30 to define its terms of reference and operation in relation to oversight of the TABLET trial. The DMC met on an approximately 6-monthly basis during the period of recruitment. The patient safety and treatment efficacy aspects were reviewed at each meeting and a decision to continue or stop trial recruitment was based on the criteria defined prior to the trial starting.
Chapter 3 Results of randomised trial
This chapter reports the results of the TABLET trial. It commences with a description of the flow of participants through the trial and is followed by demographic information and results of the primary and secondary outcome measures, including safety outcomes.
Participant flow
Participant flow through the trial is illustrated in Figure 6. A total of 19,556 women were screened for eligibility for the trial, including assessment of whether or not they were TPO antibody positive and biochemically euthyroid. The overall prevalence of TPOAb positivity was found to be 9.5%. Ultimately, 1420 (7%) women were eligible for randomisation. Of those who were ineligible, 16,162 (83%) were TPO antibody negative, 1492 (8%) were not euthyroid and were referred for treatment based on local guidelines and 482 (2%) did not complete screening for entry into the trial.
FIGURE 6.
Flow of participants through the trial. From the New England Journal of Medicine. Dhillon-Smith RK, et al. 31 Levothyroxine in women with thyroid peroxidase antibodies before conception. Vol. 380, pp. 1316–25. Copyright © 2019. Massachusetts Medical Society. Reprinted with permission.
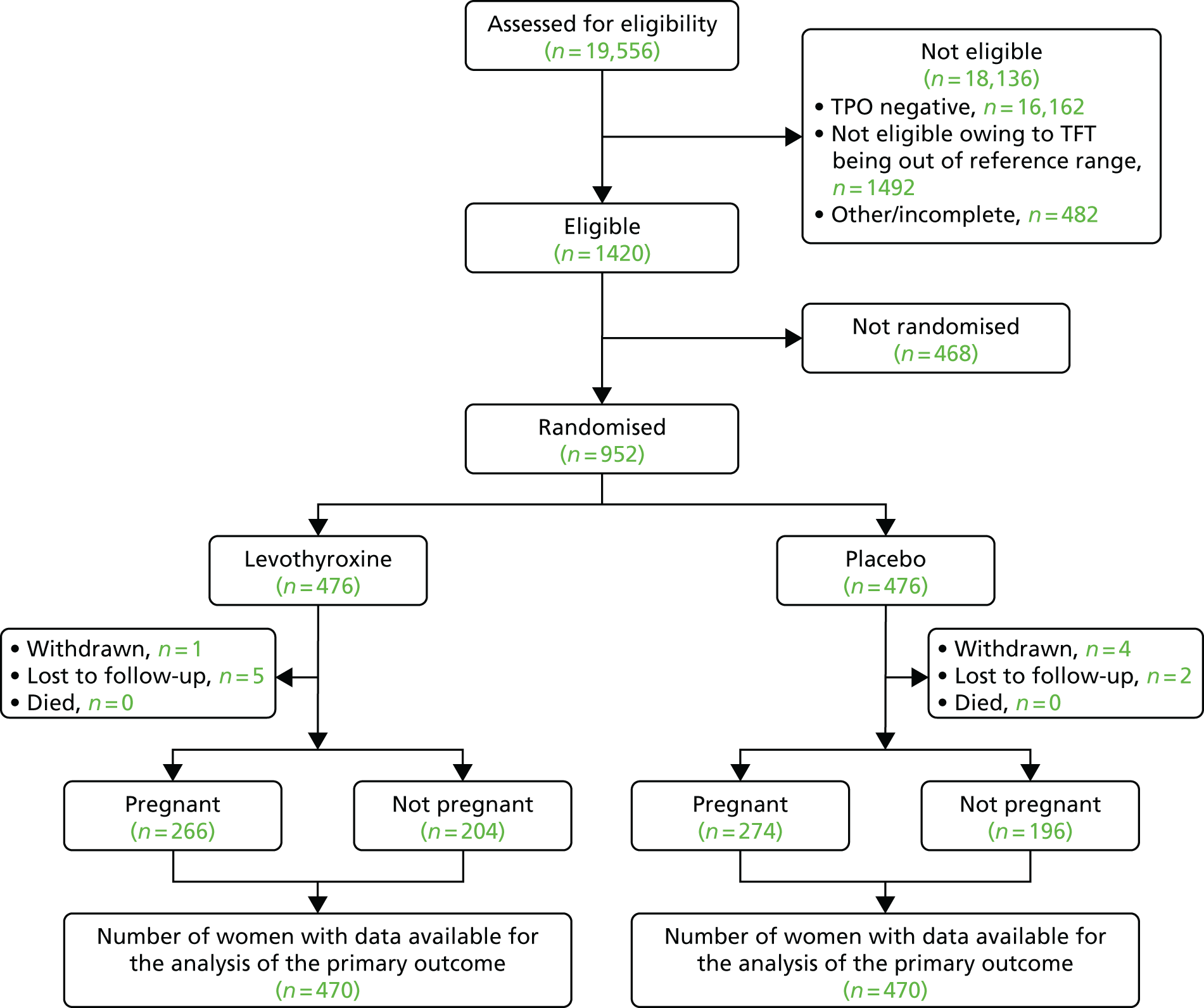
A total of 952 out of 1420 (67%) women proceeded to randomisation, with 476 allocated to levothyroxine and 476 to placebo. Six participants in each group were either withdrawn from the trial or lost to follow-up, meaning that 940 (98.7% of those randomised) participants were available for analysis of the primary outcome.
Recruitment
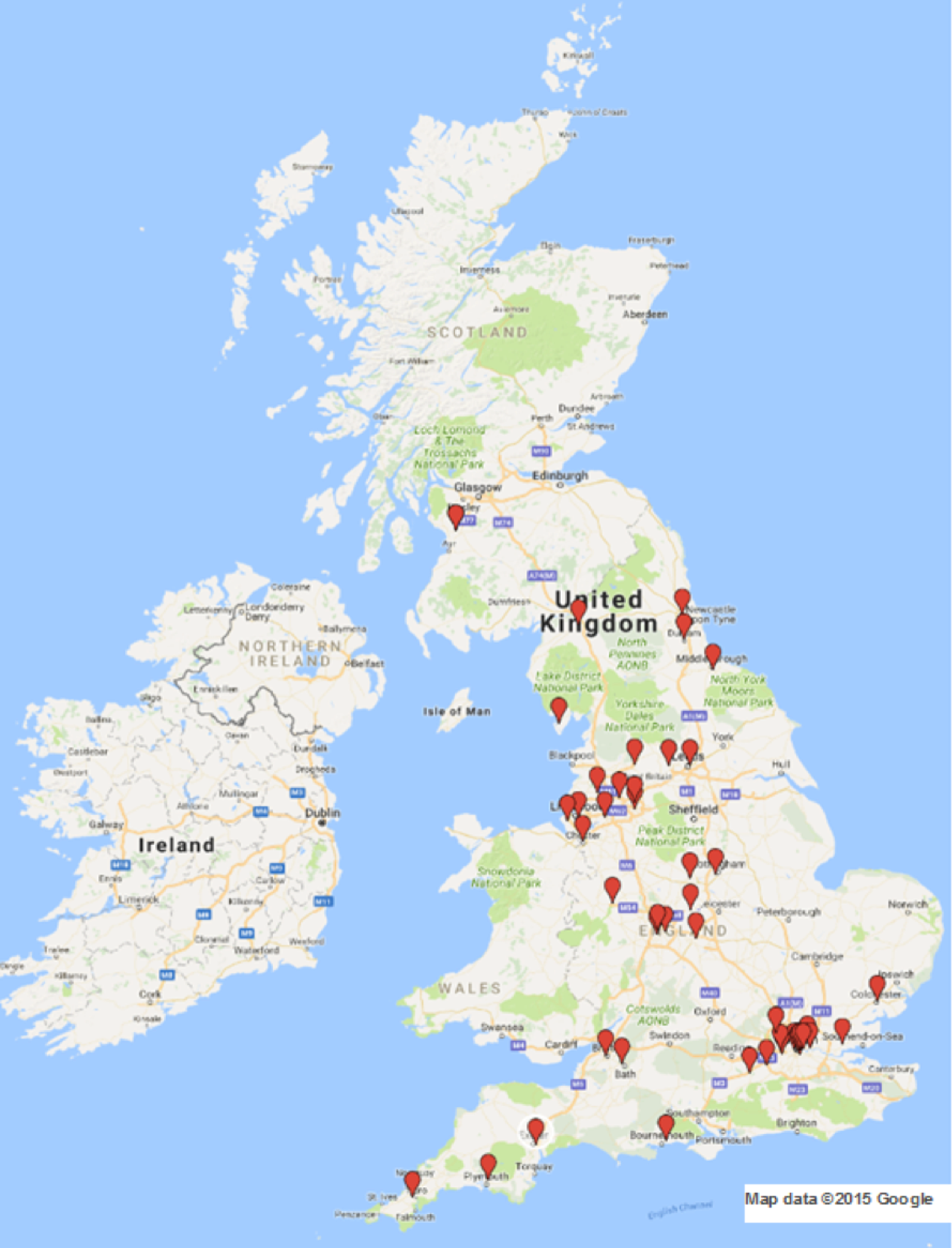
Recruitment took place over 51 months in 49 UK NHS hospitals (Figure 7) from November 2011 to January 2016 (see Appendix 3). With agreement from the TSC, recruitment finished slightly over the sample size target, at 952 recruits. This enabled us to maximise the available power to detect any differences between groups, should one exist, in the primary outcome. Given the lower than anticipated rate of loss to follow-up, 940 participants with available data meant that we had 89% power to detect a difference under the original assumptions set out in Chapter 2. The original sample size calculation assumed 80% power. Site contributions to recruitment are given in Table 2.
FIGURE 7.
Sites in the UK. This map data have been reproduced with the permission of Google (Google Inc., Mountain View, CA, USA) from Google Maps (2015) for non-commercial purposes. Map data: © 2015 Google. For more details, see: www.google.com/permissions/geoguidelines/.
| Site | Assessed for eligibility (n) | Assessed with screening results (n) | TPO positive and euthyroid, n (%) | Randomised (n) |
|---|---|---|---|---|
| Arrowe Park Hospital, Wirral | 261 | 261 | 19 (7) | 16 |
| Basildon Hospital | 114 | 114 | 10 (9) | 8 |
| Birmingham Heartlands Hospital | 780 | 780 | 59 (8) | 46 |
| Birmingham Women’s Hospital | 2630 | 2625 | 131 (5) | 114 |
| Bradford Royal Infirmary | 153 | 153 | 14 (9) | 9 |
| Burnley General Hospital | 699 | 682 | 55 (8) | 40 |
| City Hospital Birmingham | 508 | 505 | 34 (7) | 18 |
| Colchester General Hospital | 266 | 240 | 14 (6) | 8 |
| Countess of Chester Hospital | 358 | 351 | 23 (7) | 9 |
| Cumberland Infirmary | 111 | 110 | 9 (8) | 5 |
| Derriford Hospital, Plymouth | 117 | 116 | 15 (13) | 14 |
| Ealing Hospital | 18 | 18 | 2 (11) | 1 |
| Frimley Park Hospital, Surrey | 76 | 74 | 5 (7) | 3 |
| Furness General Hospital | 71 | 70 | 11 (16) | 9 |
| Guy’s Hospital, London | 1327 | 1303 | 101 (8) | 57 |
| King’s College Hospital, London | 944 | 940 | 100 (11) | 54 |
| Liverpool Women’s Hospital | 650 | 647 | 32 (5) | 27 |
| New Cross Hospital, Wolverhampton | 140 | 140 | 15 (11) | 11 |
| Newham General Hospital, London | 235 | 230 | 22 (10) | 15 |
| North Manchester General Hospital | 95 | 95 | 7 (7) | 5 |
| Ormskirk and District General Hospital | 21 | 21 | 0 (0) | 0 |
| Queen Charlotte’s and Chelsea Hospital | 18 | 18 | 0 (0) | 0 |
| Queens Medical Centre, Nottingham | 511 | 508 | 54 (11) | 26 |
| Royal Bournemouth General Hospital | 125 | 124 | 5 (4) | 4 |
| Royal Cornwall Hospital | 80 | 80 | 4 (5) | 4 |
| Royal Derby Hospital | 203 | 203 | 14 (7) | 7 |
| Royal Devon and Exeter Hospital | 39 | 37 | 2 (5) | 2 |
| Royal United Hospital, Bath | 45 | 35 | 3 (9) | 3 |
| St Bartholomew’s Hospital, London | 809 | 799 | 64 (8) | 46 |
| St James’ University Hospital, Leeds | 304 | 297 | 15 (5) | 11 |
| St Mary’s Hospital, Manchester | 1636 | 1627 | 77 (5) | 50 |
| St Mary’s Hospital, Paddington | 766 | 764 | 52 (7) | 30 |
| St Michael’s Hospital, Bristol | 399 | 398 | 34 (9) | 27 |
| St Peter’s Hospital, Chertsey | 145 | 142 | 8 (6) | 6 |
| St Thomas’ Hospital, London | 651 | 650 | 38 (6) | 21 |
| The James Cook University Hospital, Middlesbrough | 218 | 218 | 20 (9) | 13 |
| The Princess Royal Hospital, Telford | 193 | 188 | 38 (20) | 25 |
| The Royal Bolton Hospital | 394 | 392 | 53 (14) | 36 |
| The Royal London Hospital | 366 | 364 | 27 (7) | 19 |
| The Royal Victoria Infirmary, Newcastle | 180 | 178 | 5 (3) | 5 |
| University College Hospital, London | 403 | 393 | 18 (5) | 11 |
| University Hospital Coventry | 446 | 431 | 40 (9) | 30 |
| University Hospital Crosshouse, Kilmarnock | 542 | 542 | 44 (8) | 33 |
| University Hospital of North Durham | 64 | 61 | 12 (20) | 8 |
| Warrington Hospital | 320 | 317 | 27 (9) | 18 |
| Watford General Hospital | 304 | 302 | 13 (4) | 4 |
| West Middlesex University Hospital | 295 | 293 | 23 (8) | 15 |
| Whipps Cross University Hospital, London | 349 | 340 | 36 (11) | 18 |
| Wrightington Hospital | 177 | 174 | 16 (9) | 11 |
| Total sites = 49 | 19,556 | 19,350 | 1420 (7) | 952 |
Baseline data
The baseline demographic characteristics of participants in the two groups were comparable to the minimisation algorithm, ensuring balance for the factors indicated in Table 3.
| Characteristic | Levothyroxine (n = 476) | Placebo (n = 476) |
|---|---|---|
| General demographics | ||
| Maternal age, yearsa | ||
| < 35, n/N (%) | 306/476 (64) | 306/476 (64) |
| Mean (SD), n | 32.5 (4.9), 476 | 32.7 (4.9), 476 |
| BMI (kg/m2) | ||
| ≥ 25, n/N (%) | 240/462 (52) | 241/464 (52) |
| Mean (SD), n | 26.4 (5.6), 462 | 26.5 (5.5), 464 |
| Ethnic group, n/N (%) | ||
| White | 328/476 (69) | 337/476 (71) |
| Chinese | 4/476 (1) | 4/476 (1) |
| South Asian | 110/476 (23) | 94/476 (20) |
| Black | 16/476 (3) | 23/476 (5) |
| Other | 18/476 (4) | 18/476 (4) |
| Pregnancy history | ||
| Nulliparous, n/N (%) | 141/476 (30) | 131/473 (28) |
| Previous miscarriages,a n/N (%) | ||
| 0 | 166/476 (35) | 165/473 (35) |
| 1 or 2 | 219/476 (46) | 213/473 (45) |
| ≥ 3 | 91/476 (19) | 95/473 (20) |
| In those with one or more previous miscarriages | ||
| Previous miscarriages, median (IQR), n | 2 (1–3), 310 | 2 (1–3), 308 |
| Previous first-trimester losses (< 14 weeks gestation), median (IQR), n | 2 (1–3), 310 | 2 (1–3), 308 |
| Number of previous second-trimester losses (< 24 weeks gestation), median (IQR), n | 0 (0–0), 310 | 0 (0–0), 308 |
| Number of previous preterm births (< 34 weeks), n/N (%) | 11/476 (2) | 10/473 (2) |
| Past medical history, n/N (%) | ||
| Antiphospholipid syndrome | 2/473 (< 1) | 4/470 (1) |
| Systemic lupus erythematosus | 3/473 (1) | 2/471 (< 1) |
| Type 1 diabetes mellitus | 3/473 (1) | 2/471 (< 1) |
| Type 2 diabetes mellitus | 4/473 (1) | 6/471 (1) |
| Other thrombophilias | 6/473 (1) | 6/471 (1) |
| Other autoimmune disease | 6/473 (1) | 15/471 (3) |
| Chronic hypertension | 2/472 (< 1) | 7/471 (2) |
| Renal disease | 5/472 (1) | 5/471 (1) |
| Family/social history, n/N (%) | ||
| Family history of thyroid disease | 121/473 (26) | 120/471 (25) |
| Current smoker | 51/473 (11) | 46/470 (10) |
| Alcohol consumption | 167/473 (35) | 168/469 (36) |
| Recreational drug use | 1/473 (< 1) | 3/471 (1) |
| Pre-randomisation blood thyroid hormone concentrations | ||
| Serum TSH (mlU/l)a | ||
| ≤ 2.5, n/N (%) | 329/476 (69) | 330/476 (69) |
| > 2.5, n/N (%) | 147/476 (31) | 146/476 (31) |
| Median (IQR), n | 2.10 (1.51–2.74), 476 | 2.01 (1.45–2.70), 476 |
| Mean (SD), n (log scale) | 0.674 (0.422), 476 | 0.652 (0.418), 476 |
| Serum free T4 (pmol/l), mean (SD), n | 14.6 (1.9), 476 | 14.5 (2.0), 476 |
| Serum TPO antibody (IU/ml), median (IQR), n | 170 (83–428), 470 | 202 (94–417), 472 |
| Infertility cohort-specific characteristics | ||
| Currently treated for infertility,a n/N (%) | 216/476 (45) | 213/476 (45) |
| Duration (months) of infertility prior to entering trial, median (IQR), n | 36 (24–48), 165 | 34 (24–54), 156 |
| Previous treatment for infertility, n/N (%) | 67/216 (31) | 57/213 (27) |
| If yes, type of treatment, n/N (%) | ||
| Clomifene citrate | 2/67 (3) | 0/57 (0) |
| Intrauterine insemination | 0/67 (0) | 0/57 (0) |
| IVF | 2/67 (3) | 0/57 (0) |
| Intracytoplasmic sperm injection | 0/67 (0) | 0/57 (0) |
| FSH level, median (IQR), n | 6.1 (4.9–8.4), 158 | 6.5 (5.5–7.9), 157 |
Compliance to treatment
Given the long duration for which a participant could potentially be taking the trial medication (12 months to conceive, plus, potentially, an additional 9 months of pregnancy if conception was successful), as well as the known pharmacokinetics of levothyroxine,32 intermittently missing tablets would not affect the thyroxine levels in the body. Therefore, a pragmatic approach was taken, which defined pill-taking of > 75% as good compliance.
In those women for whom compliance data were reported, compliance was found to be good; however, compliance reporting overall was poor. There was a trend of compliance reducing in those trying for a pregnancy from 3 months to 9 months preconception, but then increasing in early pregnancy (at 6–8 weeks). A summary of the compliance to treatment allocation is shown in Table 4.
| Time point | Compliance (%) | Trial group, n (%) | |
|---|---|---|---|
| Levothyroxine | Placebo | ||
| 3 months pre pregnancy | ≥ 75 | 210 (89) | 227 (94) |
| < 75 | 26 (11) | 15 (6) | |
| 6 months pre pregnancy | ≥ 75 | 122 (86) | 147 (90) |
| < 75 | 20 (14) | 16 (10) | |
| 9 months pre pregnancy | ≥ 75 | 67 (81) | 88 (94) |
| < 75 | 16 (19) | 6 (6) | |
| 12 months pre pregnancy | ≥ 75 | 61 (82) | 68 (76) |
| < 75 | 13 (18) | 22 (24) | |
| 6–8 weeks’ gestation | ≥ 75 | 154 (93) | 151 (90) |
| < 75 | 11 (7) | 17 (10) | |
| 16–18 weeks’ gestation | ≥ 75 | 117 (83) | 116 (85) |
| < 75 | 24 (17) | 20 (15) | |
| 28 weeks’ gestation | ≥ 75 | 109 (84) | 104 (85) |
| < 75 | 21 (16) | 18 (15) | |
Results overview
The TABLET trial found no evidence of differences in the primary or any of the key secondary outcomes between the group randomised to receive levothyroxine and the group randomised to placebo. Differences were seen in serum TSH concentration (which was reduced with levothyroxine) and free T4 levels (which increased with levothyroxine) at every time point observed (suggesting the biological effect of levothyroxine), but this did not translate to a clinical benefit to the participants randomised to levothyroxine.
Primary outcome results
Overall, 354 out of 940 participants (38%) experienced a live birth at ≥ 34 weeks of gestation. The live birth rate in the levothyroxine group was 37% (176/470) and the rate in placebo group was 38% (178/470), translating to a RR of 0.97 (95% CI 0.83 to 1.14; p = 0.74) and an absolute risk difference of –0.4% (95% CI –6.6% to 5.8%) (Table 5).
| Outcome | Trial group | Comparison, RRa or MDb (95% CI); p-value | |
|---|---|---|---|
| Levothyroxine | Placebo | ||
| Primary outcome | |||
| Live birth at ≥ 34 weeks’ gestation, n/N (%) | 176/470 (37) | 178/470 (38) | RR 0.97 (0.83 to 1.14); 0.74 |
| Secondary maternal outcomes: pregnancy outcomes | |||
| As a proportion of women who achieved pregnancy within 12 months | N = 266 | N = 274 | |
| Clinical pregnancy at 7 weeks,c n/N (%) | 237/266 (89) | 248/274 (91) | RR 0.98 (0.93 to 1.04); 0.59 |
| Ongoing pregnancy at 12 weeks,c n/N (%) | 194/266 (73) | 200/274 (73) | RR 1.00 (0.90 to 1.11); 0.99 |
| Miscarriage at < 24 weeks,d,e n/N (%) | 75/266 (28) | 81/274 (30) | RR 0.95 (0.73 to 1.23); 0.68 |
| Stillbirth (intrauterine death at ≥ 24 weeks), n/N (%) | 1/266 (< 1) | 0/274 (0) | – |
| Ectopic pregnancy, n/N (%) | 3/266 (1) | 6/274 (2) | RR 0.50 (0.13 to 1.99); 0.33 |
| Termination,f n/N (%) | 1/266 (< 1) | 0/274 (0) | – |
| Live birth at < 34 weeks, n/N (%) | 10/266 (4) | 10/274 (4) | RR 1.02 (0.43 to 2.42); 0.96 |
| Live birth at ≥ 34 weeks, n/N (%) | 176/266 (66) | 178/274 (65) | RR 1.02 (0.90 to 1.15); 0.77 |
| Secondary maternal outcomes: other outcomes | |||
| Singleton baby (in live births at ≥ 24 weeks of gestation)g | 177/186 (95) | 181/188 (96) | |
| Twins (in live births at ≥ 24 weeks of gestation)g | 9/186 (5) | 7/188 (4) | |
| Mode of initiation of labour (in live births at ≥ 24 weeks of gestation) | |||
| Spontaneous | 70/177 (40) | 83/178 (47) | |
| Induced | 63/177 (36) | 70/178 (39) | RR 0.93 (0.72 to 1.21); 0.60 |
| Pre-labour caesarean section | 44/177 (25) | 25/178 (14) | RR 1.75 (1.12 to 2.73); 0.01 |
| Not known | 9 | 10 | |
| Mode of delivery (in live births at ≥ 24 weeks of gestation) | |||
| Vaginal | 76/180 (42) | 81/183 (44) | |
| Operative vaginal | 28/180 (16) | 37/183 (20) | RR 0.75 (0.48 to 1.16); 0.19 |
| Caesarean | 76/180 (42) | 65/183 (36) | RR 1.13 (0.88 to 1.46); 0.34 |
| Not known | 6 | 5 | |
| Did not achieve pregnancy within 12 months, n/N (%) | 204/470 (43) | 196/470 (42) | |
| Failed to conceive | 178/204 (87) | 170/196 (87) | |
| Stopped trying to conceive | 26/204 (13) | 26/196 (13) | |
| Secondary neonatal outcomes (in live births at ≥ 24 weeks of gestation) | |||
| Gestation at delivery (weeks), mean (SD), n | 38+6 (2+3), 186 | 39+0 (2+4), 188 | MD –0+1 (–0+4 to 0+3); 0.65 |
| Gestation at delivery (weeks), n/N (%) | |||
| < 28 | 0/186 (0) | 1/188 (1) | – |
| < 34 | 10/186 (5) | 10/188 (5) | RR 1.01 (0.43 to 2.38); 0.98 |
| < 37 | 28/186 (15) | 34/188 (18) | RR 0.83 (0.53 to 1.31); 0.43 |
| Birthweight (g),h mean (SD), n | 3226 (660), 187 | 3262 (668), 188 | MD –35 (–168 to 97); 0.60 |
| Birthweight, adjusted for gestational age and sex (using INTERGROWTH33 standards) (centiles), mean (SD), n | 57.2 (30.6), 187 | 59.5 (28.7), 188 | MD –2.2 (–8.1 to 3.8); 0.47 |
| Birthweight, adjusted for gestational age, sex, parity, maternal BMI and ethnicity (using GROW34 standards) (centiles), mean (SD), n | 46.7 (30.9), 187 | 49.0 (29.8), 188 | MD –2.4 (–8.5 to 3.6); 0.43 |
| Small for gestational age and sex (using INTERGROWTH33 standards; proportion < 10th centile), n/N (%) | 14/187 (7) | 13/188 (7) | RR 1.02 (0.50 to 2.07); 0.95 |
| Small for gestational age, sex, parity, maternal BMI and ethnicity (using GROW34 standards; proportion < 10th centile), n/N (%) | 29/187 (16) | 22/188 (12) | RR 1.35 (0.81 to 2.26); 0.25 |
| Large for gestational age and sex (using INTERGROWTH33 standards; proportion ≥ 90th centile), n/N (%) | 34/187 (18) | 27/188 (14) | RR 1.25 (0.79 to 1.97); 0.34 |
| Large for gestational age, sex, parity, maternal BMI and ethnicity (using GROW34 standards; proportion ≥ 90th centile), n/N (%) | 20/187 (11) | 22/188 (12) | RR 0.92 (0.52 to 1.61); 0.76 |
| Apgar score at 1 minute, median (IQR), n | 9 (9–9), 179 | 9 (9–8), 178 | MD 0.1 (–0.2 to 0.4); 0.51 |
| Apgar score at 5 minutes, median (IQR), n | 9 (9–10), 178 | 9 (9–10), 178 | MD 0.0 (–0.2 to 0.2); 0.66 |
Secondary outcome results
Secondary maternal outcomes: pregnancy outcomes
Similar numbers of women in each group became pregnant: 266 out of 470 (57%) in the levothyroxine group and 274 out of 470 (58%) in the placebo group (see Table 5). In this population, the rate of miscarriage was similar in both groups: 75 out of 266 (28%) in the levothyroxine group and 81 out of 274 (30%) in the placebo group (RR 0.95, 95% CI 0.73 to 1.23). This difference was not statistically significant (p = 0.68). The median gestational age at the time of miscarriage was 8 weeks (IQR 6–10 weeks) in the levothyroxine group and 9 weeks in the placebo group (IQR 7–10 weeks). Ten women in each group delivered before 34 weeks’ gestation (RR 1.02, 95% CI 0.43 to 2.42; p = 0.96), meaning that the number of live births (at ≥ 24 weeks), overall, was 186 in the levothyroxine group and 188 in the placebo group. Overall, there was no evidence of any difference in the time pregnancy ended, through either miscarriage or successful delivery (Figures 8 and 9) (hazard ratio 1.03, 95% CI 0.87 to 1.23; p = 0.72). The results of other pregnancy outcomes appeared similar in both groups, with no significant differences.
FIGURE 8.
Kaplan–Meier curve: time from conception to pregnancy end. From the New England Journal of Medicine. Dhillon-Smith RK, et al. 31 Levothyroxine in women with thyroid peroxidase antibodies before conception. Vol. 380, pp. 1316–25. Copyright © 2019. Massachusetts Medical Society. Reprinted with permission.

FIGURE 9.
Kaplan–Meier curve: time from conception to birth (live birth at ≥ 24 weeks).

Other secondary maternal outcomes
Nine women in the levothyroxine group and seven women in the placebo group gave birth to twins (see Table 5). There was some evidence that women in the levothyroxine group were more likely to have their birth initiated through pre-labour caesarean section (25% vs. 14%; RR 1.75, 95% CI 1.12 to 2.73; p = 0.01), but this did not ultimately translate to higher rates of caesarean delivery (42% vs. 36%; RR 1.13, 95% CI 0.88 to 1.46; p = 0.34). The reasons for pre-labour caesarean section were not recorded, so we are unable to explain why there was a higher rate in the levothyroxine group; however, it is highly unlikely to have been attributable to the levothyroxine.
Neonatal outcomes
The distribution of gestational age at delivery in those women with a live birth was very similar in both groups overall (Figure 10) (hazard ratio 1.09, 95% CI 0.89 to 1.34; p = 0.43). Live births were delivered at 38+6 weeks and 39+0 weeks of gestation, on average, in the levothyroxine and placebo groups, respectively (mean difference: –1 day, 95% CI –4 to 3 days; p = 0.65). There were 62 (17%) preterm births (< 37 weeks) observed, but the numbers were very similar in both groups (15% vs. 18%; RR 0.83, 95% CI 0.53 to 1.31; p = 0.43). Birthweights appeared similar in both groups (mean difference: 35 g, 95% CI –168 to 97 g; p = 0.60), with no evidence of any differences in the numbers, large or small, for their gestational age (plus other covariates listed in Table 5). No differences were noted in Apgar scores.
FIGURE 10.
Thyroid-stimulating hormone over time by group. From the New England Journal of Medicine. Dhillon-Smith RK, et al. 31 Levothyroxine in women with thyroid peroxidase antibodies before conception. Vol. 380, pp. 1316–25. Copyright © 2019. Massachusetts Medical Society. Reprinted with permission.

Thyroid function data
As expected, differences were seen in levels of serum TSH concentration (reduced with levothyroxine) and free T4 levels (increased with levothyroxine) at every time point observed (Table 6 and Figures 10 and 11), demonstrating a biological effect of levothyroxine treatment. Levels of both TSH and free T4 dropped during pregnancy, but clear differences between groups remained throughout.
| Thyroid function pre-randomisation, pre-pregnancy and during pregnancy | Levothyroxine | Placebo | Mean difference,a 95% CI; p-value |
|---|---|---|---|
| Serum TSH concentration (mlU/l) | |||
| Pre-randomisation | |||
| Mean (SD), n (log-transformed) | 0.674 (0.422), 476 | 0.652 (0.418), 476 | |
| Median (IQR) | 2.10 (1.51–2.74) | 2.01 (1.45–2.70) | |
| Time (months) after randomisation pre pregnancy | |||
| 3 | |||
| Mean (SD), n (log-transformed) | –0.111 (1.389), 298 | 0.698 (0.687), 288 | –0.822 (–0.990 to –0.654); < 0.001 |
| Median (IQR) | 1.33 (0.74–2.00) | 2.11 (1.50–2.97) | |
| 6 | |||
| Mean (SD), n (log-transformed) | 0.250 (1.271), 172 | 0.716 (0.643), 172 | –0.514 (–0.721 to –0.308); < 0.001 |
| Median (IQR) | 1.64 (1.10–2.39) | 2.10 (1.50–2.60) | |
| 9 | |||
| Mean (SD), n (log-transformed) | 0.520 (0.705), 50 | 0.658 (0.343), 54 | –0.17 (–0.38 to –0.04); 0.102 |
| Median (IQR) | 1.73 (1.28–2.53) | 1.94 (1.62–2.45) | |
| Time (weeks) during pregnancy | |||
| 6–8 | |||
| Mean (SD), n (log-transformed) | 0.141 (0.967), 177 | 0.573 (0.766), 184 | –0.421 (–0.584 to –0.259); < 0.001 |
| Median (IQR) | 1.36 (0.85–1.98) | 2.05 (1.38–2.80) | |
| 16–18 | |||
| Mean (SD), n (log-transformed) | 0.177 (0.634), 141 | 0.385 (0.626), 142 | –0.216 (–0.346 to –0.085); 0.001 |
| Median (IQR) | 1.31 (0.94–1.70) | 1.60 (1.12–2.20) | |
| 28 | |||
| Mean (SD), n (log-transformed) | 0.123 (0.552), 134 | 0.327 (0.519), 130 | –0.203 (–0.319 to –0.088); 0.001 |
| Median (IQR) | 1.30 (0.81–1.61) | 1.50 (1.10–1.95) | |
| Serum free T4 level (pmol/l), mean (SD), n | |||
| Pre-randomisation | 14.6 (1.9), 476 | 14.5 (2.0), 476 | |
| Time (months) after randomisation pre pregnancy, mean (SD), n | |||
| 3 | 17.3 (3.9), 298 | 14.5 (2.6), 288 | 2.7 (2.2 to 3.2); < 0.0001 |
| 6 | 16.3 (3.5), 172 | 14.6 (2.2), 172 | 1.7 (1.2 to 2.3); < 0.0001 |
| 9 | 16.4 (4.1), 51 | 14.3 (1.9), 54 | 1.8 (0.7 to 2.9); 0.0012 |
| Time (weeks) during pregnancy, mean (SD), n | |||
| 6–8 | 16.6 (3.1), 177 | 14.9 (2.5), 184 | 1.7 (1.2 to 2.2); < 0.0001 |
| 16–18 | 14.2 (1.8), 140 | 13.3 (1.8), 142 | 1.0 (0.6 to 1.4); < 0.0001 |
| 28 | 13.0 (1.7), 134 | 12.3 (1.7), 130 | 0.8 (0.4 to 1.1); < 0.0001 |
FIGURE 11.
Free T4 over time by group. From the New England Journal of Medicine. Dhillon-Smith et al. 31 Levothyroxine in women with thyroid peroxidase antibodies before conception. Vol. 380, pp. 1316–25. Copyright © 2019. Massachusetts Medical Society. Reprinted with permission.

Complications
Rates of antenatal, intrapartum, postpartum and neonatal complications appeared to be similar in both groups (Table 7). The denominator was determined by the number of completed forms for the relevant outcome; this is why it differs across each outcome.
| Type of complication | Trial group, n/N (%) | |
|---|---|---|
| Levothyroxine | Placebo | |
| Maternal antenatal complications | ||
| Hyperemesis gravidarum | 2/178 (1) | 3/176 (2) |
| Gestational diabetes | 20/178 (11) | 16/176 (9) |
| Pre-eclampsia/eclampsia | 9/175 (5) | 5/175 (3) |
| Obstetric cholestasis | 3/177 (2) | 3/173 (2) |
| Preterm pre-labour rupture of membranes | 8/177 (5) | 7/177 (4) |
| Intrapartum complications | ||
| Shoulder dystocia | 4/176 (2) | 2/181 (1) |
| Maternal postnatal complications | ||
| Admission to HDU or intensive care unit | 10/176 (6) | 6/183 (3) |
| Abnormal thyroid test within 4 weeks (if performed because of clinical indication) | 1/181 (1) | 2/185 (1) |
| Referred to psychiatrist/started on antidepressants | 1/181 (1) | 0/185 (0) |
| Neonatal complications | ||
| Early neonatal death (death within 7 days after delivery) | 0/190 (0) | 0/187 (0) |
| Late neonatal death (death > 7 days and < 28 days after delivery) | 0/190 (0) | 0/187 (0) |
| Admission to neonatal unit | 27/192 (14) | 24/187 (13) |
| Active resuscitation within first 28 days | 8/191 (4) | 8/185 (4) |
| Surfactant use | 8/191 (4) | 8/185 (4) |
| Mechanical ventilation | 6/189 (3) | 10/184 (5) |
| Intermittent positive pressure ventilation | 8/191 (4) | 8/185 (4) |
| Continuous positive airway pressure | 6/189 (3) | 10/184 (5) |
| Oxygen use | 5/189 (3) | 10/184 (5) |
| Congenital abnormalitiesa | 9/129 (7) | 10/123 (8) |
| Hypoxic ischaemic encephalopathy | 1/190 (1) | 1/185 (1) |
| Retinopathy of prematurity | 0/188 (–) | 1/184 (1) |
| Respiratory distress syndrome | 2/190 (1) | 8/184 (4) |
| Pneumothorax | 1/190 (1) | 0/184 (–) |
| Intraventricular haemorrhage (grade 3 or 4) | 0/190 (–) | 1/184 (1) |
| Necrotising Enterocolitis | 0/190 (–) | 1/184 (1) |
| Early infection | 14/190 (7) | 20/186 (11) |
Safety data
The overall number of SAEs across both groups is presented in Table 8. Participant-reported symptoms were recorded at each scheduled appointment; rates appeared similar in both groups (see Appendix 4). Only one TABLET trial participant incurred a SUSAR; at her 6-month follow-up visit, the participant reported symptoms of tiredness and difficulty swallowing following cessation of the trial IMP at 3 months post randomisation. The IMP was stopped on the patient’s own accord. The event was reported to the regulatory authorities as appropriate.
| Serious adverse events: overall | Trial group, n (%) | p-value | |
|---|---|---|---|
| Levothyroxine | Placebo | ||
| Total number of participants experiencing a SAE (either maternal or neonatal) | 28/470 (6) | 18/470 (4) | 0.14 |
| Total number of SAEs | 28/470 (6) | 18/470 (4) | |
| Maternal SAEs | 28/470 (6) | 16/470 (3) | |
| Neonatal SAEs | 0/470 (0) | 2/470 (< 1) | |
Slightly more SAEs were recorded in the levothyroxine group than the placebo group (see Tables 8 and 9), 6% (28/470) versus 4% (18/470), but this difference was not statistically significant (p = 0.14). One of the SAEs in the placebo group was also classified as a SUSAR. This was because the participant reported a swollen neck. On investigation, she was found to have a slightly enlarged thyroid and cysts. This was reported to the necessary authorities; 1 month later, they symptoms had subsided. It was agreed to be improbable that this had been caused by the placebo capsule.
The SAEs were categorised in the body systems outlined in Table 9.
| Category | Trial group (n) | |
|---|---|---|
| Levothyroxine | Placebo | |
| Maternal | ||
| Fetal anomaly | 2 | 0 |
| Obstetric/gynaecologicala | 3 | 8 |
| Infection/sepsis | 6 | 2 |
| Thyroid/endocrine | 1 | 1 |
| Gastrointestinal/surgical | 2 | 2 |
| Respiratory | 3 | 1 |
| Neurological | 4 | 0 |
| Urological | 1 | 1 |
| Psychological | 2 | 0 |
| Miscellaneousb | 5 | 1 |
| Total | 29c | 16 |
| Neonatal | ||
| Neonatal | 0 | 2 |
| Total | 0 | 2 |
Ancillary analyses
Sensitivity analyses
A sensitivity analysis making a worst-case scenario (negative outcome) assumption on the small number of missing data had no impact on the analysis of live birth at ≥ 34 weeks and miscarriage (Table 10). There was a very low rate of missing data for the primary outcome and miscarriage (1% in each group), so this was not unexpected.
| Primary outcome and miscarriage rate sensitivity analyses | Trial group, n/N (%) | RRa (95% CI); p-value | |
|---|---|---|---|
| Levothyroxine | Placebo | ||
| Live birth at ≥ 34 weeks | |||
| Sensitivity analysis 1: assuming all missing responses are treatment failures | 176/476 (37) | 178/476 (3) | 0.97 (0.83 to 1.14); 0.74 |
| Sensitivity analysis 2: simulate missing responses with multiple imputation | – | – | Not attempted as the number missing is small (1% in each group) |
| Miscarriage at < 24 weeks | |||
| Sensitivity analysis 1: assuming all missing responses are treatment failures | 81/272 (30) | 87/280 (31) | 0.94 (0.71 to 1.23); 0.63 |
| Sensitivity analysis 2: simulate missing responses with multiple imputation | – | – | Not attempted as the number missing is small (1% in each group) |
Subgroup analyses
All subgroup analyses were planned a priori (see Chapter 2) and are displayed in Table 11. There were no subgroup effects for the primary outcome of live birth at ≥ 34 weeks of gestation. When we evaluated TPOAbs [divided into ‘very high’ (≥ 50th percentile) and ‘high’ (< 50th percentile) groupings], there was no subgroup effect for live birth at ≥ 34 weeks, but we observed some evidence of interaction for the miscarriage at < 24 weeks outcome (result of test for interaction: p = 0.009). In those participants with ‘very high’ TPOAb values, there was evidence (p = 0.04) that miscarriage was reduced in the levothyroxine group compared with the placebo group (RR 0.66, 95% CI 0.44 to 0.98), but the opposite appeared to be the case in the ‘high’ subgroup, suggesting that there was a higher miscarriage rate in the levothyroxine group than in the placebo group (RR 1.39, 95% CI 0.94 to 2.04; p = 0.10).
| Subgroup | Trial group, n/N (%) | RRa (95% CI); p-value | Test for interaction p-value | |
|---|---|---|---|---|
| Levothyroxine | Placebo | |||
| Live birth at ≥ 34 weeks | ||||
| Maternal age (years) | ||||
| < 35 | 123/302 (41) | 124/301 (41) | 0.96 (0.80 to 1.15); 0.65 | 0.74 |
| ≥ 35 | 53/168 (32) | 54/169 (32) | 1.02 (0.75 to 1.38); 0.92 | |
| Number of previous miscarriages | ||||
| 0 | 44/157 (28) | 51/151 (34) | 0.83 (0.59 to 1.16); 0.27 | 0.55 |
| 1 or 2 | 98/219 (45) | 91/217 (42) | 1.02 (0.83 to 1.25); 0.87 | |
| ≥ 3 | 34/94 (36) | 36/102 (35) | 1.04 (0.72 to 1.51); 0.84 | |
| TSH concentration (mlU/l) at baseline | ||||
| ≤ 2.5 | 121/325 (37) | 120/327 (37) | 1.00 (0.83 to 1.22); 0.98 | 0.59 |
| > 2.5 | 55/145 (38) | 58/143 (41) | 0.91 (0.69 to 1.20); 0.52 | |
| Having infertility treatment at the time of randomisation | ||||
| Yes | 61/215 (28) | 58/209 (28) | 1.03 (0.76 to 1.40); 0.85 | 0.67 |
| No | 115/255 (45) | 120/261 (46) | 0.95 (0.79 to 1.15); 0.61 | |
| TPO level at baseline | ||||
| Very high (≥ 50th percentile) | 79/217 (36) | 82/247 (33) | 1.08 (0.84 to 1.38); 0.55 | 0.16 |
| High (< 50th percentile) | 93/247 (38) | 95/219 (43) | 0.85 (0.69 to 1.05); 0.14 | |
| Ethnicity | ||||
| White | 121/324 (37) | 132/334 (40) | 0.91 (0.76 to 1.10); 0.35 | 0.40 |
| South Asian | 37/108 (34) | 30/92 (33) | 1.11 (0.77 to 1.62); 0.60 | |
| Chinese, black or other | 18/38 (47) | 16/44 (36) | 1.25 (0.78 to 2.03); 0.37 | |
| BMI (kg/m2) | ||||
| ≥ 25 | 87/237 (37) | 88/239 (37) | 1.01 (0.81 to 1.27); 0.92 | 0.78 |
| < 25 | 87/219 (40) | 88/221 (40) | 0.97 (0.78 to 1.21); 0.77 | |
| Miscarriage at < 24 weeks | ||||
| Maternal age (years) | ||||
| < 35 | 44/177 (25) | 46/181 (25) | 0.94 (0.66 to 1.33); 0.72 | 0.86 |
| ≥ 35 | 31/89 (35) | 35/93 (38) | 0.98 (0.67 to 1.44); 0.92 | |
| Number of previous miscarriages | ||||
| 0 | 16/63 (25) | 13/66 (20) | 1.22 (0.64 to 2.34); 0.54 | 0.23 |
| 1 or 2 | 40/146 (27) | 34/135 (25) | 1.10 (0.75 to 1.63); 0.62 | |
| ≥ 3 | 19/57 (33) | 34/73 (47) | 0.70 (0.45 to 1.09); 0.12 | |
| TSH concentration (mlU/l) at baseline | ||||
| ≤ 2.5 | 54/188 (29) | 55/186 (30) | 0.94 (0.69 to 1.28); 0.69 | 0.94 |
| > 2.5 | 21/78 (27) | 26/88 (30) | 0.96 (0.59 to 1.57); 0.87 | |
| Having infertility treatment at the time of randomisation | ||||
| Yes | 24/88 (27) | 26/91 (29) | 0.85 (0.54 to 1.32); 0.47 | 0.56 |
| No | 51/178 (29) | 55/183 (30) | 1.00 (0.73 to 1.37); 0.99 | |
| TPO level at baseline | ||||
| Very high (≥ 50th percentile) | 26/110 (24) | 52/143 (36) | 0.66 (0.44 to 0.98); 0.04 | 0.009 |
| High (< 50th percentile) | 48/151 (32) | 29/130 (22) | 1.39 (0.94 to 2.04); 0.10 | |
| Ethnicity | ||||
| White | 60/195 (31) | 59/200 (30) | 1.03 (0.77 to 1.38); 0.83 | 0.49 |
| South Asian | 9/47 (19) | 14/49 (29) | 0.66 (0.32 to 1.39); 0.28 | |
| Chinese, black or other | 6/24 (25) | 8/25 (32) | 0.77 (0.32 to 1.88); 0.57 | |
| BMI (kg/m2) | ||||
| ≥ 25 | 41/132 (31) | 39/133 (29) | 1.09 (0.76 to 1.56); 0.64 | 0.14 |
| < 25 | 29/125 (23) | 41/137 (30) | 0.73 (0.49 to 1.09); 0.12 | |
Chapter 4 Mechanistic study
Introduction
A widely held view is that TPOAb positivity identifies women who display altered immune responses towards a pregnancy and, hence, are vulnerable to adverse pregnancy outcomes. Even in the non-pregnant state, TPOAb positivity has been associated with autoimmune diseases and peripheral T-lymphocytes show a significantly increased Th1 (cell-mediated immunity) to Th2 (humoral immunity) ratio of immune responses,16 particularly in women with a history of reproductive failure. 35 During pregnancy, the maternal immune system plays a key role in normal placentation by allowing the implantation of a ‘foreign body’ while balancing adequate trophoblast invasion with protection of the maternal tissues. 21 Normal pregnancy involves a shift to a Th2-predominant phenomenon, whereas Th1 predominance is associated with miscarriage and premature delivery. 19 It is therefore conceivable that, with TPOAb positivity, an unfavourable pre-pregnancy immune profile or an inappropriate immune response during early pregnancy could lead to an adverse pregnancy outcome.
It has also been postulated that TPOAb positivity induces mild maternal thyroid dysfunction leading to relative thyroid insufficiency for a given individual, despite circulating thyroid hormone levels being within the normal population range. Pregnant euthyroid women with TPOAb have been found to have TSH levels nearer the upper end of the normal range. 36 Thyroid function variations have been associated with changes in immune function even within normal physiological ranges. Higher concentrations of T3 and T4 within physiological ranges could directly enhance innate and adaptive immunity in normal, healthy individuals. 18 At the same time, subclinical hypothyroidism has been associated with increased risk of miscarriage37 and preterm birth. 38 Whether these clinical outcomes are mediated mainly by a direct effect of thyroid hormone on uteroplacental tissue or also indirectly through changes in maternal immune responses that could affect the pregnancy is not known.
The evidence thus far suggests that pregnancy is sensitive to mild changes in both immune function and thyroid function. In this trial, we have tested whether or not levothyroxine treatment can improve pregnancy outcomes in TPOAb-positive women. In this mechanistic study, we explored if levothyroxine exerts its effects on pregnancy outcome through changes in immune responses.
We hypothesised that (1) women with TPOAb positivity display a different composition of circulatory chemocytokines in the non-pregnant state and are unable to mount an appropriate balance of Th1/Th2-type immune responses during pregnancy, which increases their risk of an adverse pregnancy outcome and (2) treatment with levothyroxine can normalise chemocytokine concentrations before conception and promote the ability to mount an appropriate immune response during pregnancy, which increases the chance of a successful pregnancy outcome.
The study aimed to address these research questions:
-
Is there any evidence of an altered immune status systemically (as found in the maternal circulation) in TPOAb-positive women compared with TPOAb-negative women?
-
Does levothyroxine treatment alter serum chemocytokine concentrations in TPOAb-positive women before and during pregnancy?
-
Are the changes in circulatory chemocytokines brought about by levothyroxine treatment associated with an improvement in the chance of a livebirth outcome?
Methods
Subjects
Only women recruited in Birmingham were offered participation into this mechanistic study, with separate ethics approval and consent administered through the Human Biomaterials Resource Centre at the University of Birmingham. A generic Human Biomaterials Resource Centre form was signed by participants, which included a one-off consent for serial blood collections. Women were approached once they had agreed to participate in the main TABLET trial. A total of 49 trial participants consented to participation in the mechanistic study. In addition, six non-trial euthyroid TPOAb-negative non-pregnant women were recruited as controls. The control group all had TPOAb concentrations of < 33 IU/ml and TSH values of between 1 and 2 mlU/l. The mean age was 32 years and the mean BMI was 27.2 kg/m2, comparable with those of participants in the trial.
Blood collection and Multiplex Luminex® assays
Blood was obtained at recruitment into the trial (pre treatment) and at 3 and 6 months after recruitment if participants were not already pregnant (non-pregnant), then in pregnancy at 6–8 weeks (early gestation, trimester 1), 16–18 weeks (mid-gestation, trimester 2) and 28 weeks (late gestation, trimester 3) of gestation.
Venous blood was obtained in plain collection tubes and left to clot for at least 30 minutes at room temperature before centrifugation at 2200 rpm for 5 minutes at 4 °C. Serum was aliquoted for storage at –80 °C until batch analyses.
Multiplex Luminex® (Thermo Fisher Scientific, Waltham, MA, USA) assays were carried out according to the manufacturer’s guidelines for the overnight (16-hour) incubation protocol, to assess the concentrations of 17 specific cytokines and chemokines of interest. These were selected based on existing knowledge about inflammatory changes in TPOAb-positive populations and in miscarriage and preterm births. 16,19,21,35,39–41 Samples were batch-assayed in duplicate. Analytes were distributed across four individual plates performed on separate occasions. Plate 1 included granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), interleukin (IL)-1 beta (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL-17A, macrophage inflammatory protein (MIP)-1α and tumour necrosis factor alpha (TNF-α) (Milliplex® Human High Sensitivity T-Cell Panel; Merck Group, Darmstadt, Germany; catalogue number HSTCMAG-28SK). Plate 2 included epithelial-derived neutrophil-activating peptide 78 (ENA-78) and thrombopoietin (Milliplex Human Cytokine/Chemokine Panel II, Merck Group; catalogue number HCYP2MAG-62 K). Plate 3 included RANTES (Regulated on Activation, Normal T cell Expressed and Secreted) (Milliplex Human Cytokine/Chemokine Panel I, Merck Group, Cat No HCYTOMAG-60 K). Plate 4 included chemokine ligand 2 (CCL2), granulocyte colony-stimulating factor (G-CSF) and vascular endothelial growth factor A (VEGF-A) (Milliplex Human Cytokine/Chemokine Panel I, Merck Millipore, catalogue number HCYTOMAG-60 K). Plates were read on a Luminex® (Luminex Corporation, Austin, TX, USA) plate reader (Bio-Plex® 200; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the results were interpreted by Bio-Plex Manager 6.1 software (Bio-Rad Laboratories, Inc.). Absolute concentrations of chemocytokines in pg/ml were calculated from standard curves, derived from accompanying series of standards and were run with each batch.
Statistical analysis
Four samples were excluded because of the lack of a complete chemocytokine data set, which is required for principal component analysis (PCA). Chemocytokine concentrations below the minimum detectable limit provided by the manufacturer were replaced by an arbitrary value of half of the detection limit. All concentrations were log10-transformed to correct for skewness.
Cross-sectional univariate analyses with t-tests and linear regression were performed for each of the 17 cytokines at each of the six time points to assess differences between TPOAb-positive and TPOAb-negative status, between treatment groups and between pregnancy outcomes, using Stata® version 14.1 (StataCorp LP, College Station, TX, USA). If a participant had two non-pregnant results post treatment (i.e. at 3 and 6 months), the later one was used, as this would be most reflective of the periconception state. To account for multiple comparisons, the p-values were false-discovery rate (FDR), corrected using the Benjamini–Hochberg42 approach, with a false discovery rate set at 0.15.
Ratios between specific cytokines were calculated using absolute concentrations. When two cytokines were considered together in the same ratio, the geometric means of absolute concentrations were used. Calculated ratios were then compared between groups using the non-parametric Mann–Whitney U-test. Statistical significance was taken as p < 0.05.
Principal component analysis was performed using R version 3.33 (The R Foundation for Statistical Computing, Vienna, Austria) with log10-transformed chemocytokine concentrations from a total of 106 samples from 55 subjects (49 trial subjects and six controls) over six time points, alongside the clinical variables of TPOAb status, history of miscarriage or infertility, treatment with placebo or levothyroxine, outcomes of live birth, miscarriage, did not conceive, withdrawn for non-thyroid-related issues or withdrawn because of development of abnormal TFTs.
Results
Of the 49 trial subjects who participated in this mechanistic study, 16 provided samples at recruitment (baseline), of whom 10 had a history of infertility and six had a history of miscarriage. The remaining 33 subjects provided their first sample only after treatment had commenced. The subject numbers and characteristics of those in the mechanistic study are shown in Table 12. Overall, the subjects were fairly distributed across the two treatment groups (levothyroxine, n = 26; placebo, n = 23); 60% of subjects had a history of infertility and, overall, 40% had a live birth outcome (Table 13).
| Initial history | Samples at recruitment (n) | Treatment group | Total (n) | Time point post treatment | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before pregnancy (months) | Pregnancy (trimester)a | Livebirth (n) | Did not conceive/withdrawn (n) | |||||||
| 3 | 6 | 1 | 2 | 3 | ||||||
| History of infertility | 10 | Placebo | 12 | 5 | 4 | 4 | 3 | 2 | 5 | 8 |
| Levothyroxine | 16 | 9 | 7 | 5 | 4 | 3 | 6 | 9 | ||
| History of miscarriage | 6 | Placebo | 9 | 5 | 3 | 3 | 2 | 3 | 3 | 4 |
| Levothyroxine | 8 | 3 | 4 | 5 | 5 | 5 | 6 | 1 | ||
| TPOAb-negative controls | 6 | |||||||||
| Chemocytokine ratio | Median (IQRa) | p-value | |
|---|---|---|---|
| TPOAb negative (n = 6) | TPOAb positive (n = 16) | ||
| TNF-α : IL-10 | 5.86 (0.82–14.29) | 1.72 (0.40–5.04) | 0.1613 |
| TNF-α : IL-4 | 4.68 (0.40–7.14) | 0.67 (0.53–6.06) | 0.4174 |
| IFN-γ : IL-10 | 11.64 (5.41–29.36) | 8.94 (2.41–16.72) | 0.4610 |
| IFN-γ : IL-4 | 11.29 (1.97–17.55) | 3.58 (2.12–16.19) | 0.5553 |
| TNF-α + IFN-γ : IL-4 + IL-10 | 7.29 (1.27–15.38) | 2.49 (1.50–6.30) | 0.3020 |
| History of miscarriage (n = 6) | History of infertility (n = 10) | ||
| TNF-α : IL-10 | 3.20 (1.66–6.61) | 0.52 (0.34–3.47) | 0.1037 |
| TNF-α : IL-4 | 0.67 (0.61–0.70) | 2.18 (0.50–6.96) | 0.8283 |
| IFN-γ : IL-10 | 12.12 (11.47–32.48) | 3.57 (1.63–9.21) | 0.0301 |
| IFN-γ : IL-4 | 2.77 (1.79–4.91) | 8.21 (2.45–19.07) | 0.5152 |
| TNF-α + IFN-γ : IL-4 + IL-10 | 2.85(1.72–4.45) | 2.11 (0.95–8.14) | 0.4477 |
Data were not available at every time point for all subjects. Different sets of subjects were included at each time point. Of those that provided a baseline sample, only seven provided a non-pregnant sample post treatment commencement and only four participants provided a sample in the first trimester of pregnancy; thus, the study had limited power to examine the longitudinal effects of levothyroxine treatment. If conception was achieved prior to the 3-month follow-up, a pre-pregnancy sample would not have been taken. Subjects who did not achieve conception after 1 year post recruitment or who developed abnormal thyroid function were withdrawn from the trial, so no pregnancy samples were obtained. Those who miscarried after the first pregnancy visit did not have any samples taken at later pregnancy time points.
Principal components analysis
Physiologically, chemocytokines work in concert to exert their effects and each one cannot be considered in isolation. Previous studies have compared ratios of specific Th1 cytokines to specific Th2 cytokines, but even these would not be able to capture a comprehensive picture. We have, thus, used PCA, which assesses the combined contribution of groups of chemocytokines using weighted averages.
Three main clusters of chemocytokines emerged as drivers for the variation in the data. The first three principal components (PCs) cumulatively explained 52% of the variation in the data (PC1: 29%, PC2: 13% and PC3: 10%; Figure 12). The chemocytokines IL-1β, IL-2, IFN-γ, IL-17A, IL-10, IL-6 and IL-4 contributed most heavily to the first PC. TNF-α, RANTES and MIP-1α contributed most to the second PC. VEGF and ENA-78 contributed most negatively, whereas IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) contributed most positively, to the third PC. These data are shown in Figure 12.
FIGURE 12.
Principal component analysis. (a) The relative contributions of the various chemocytokines to the variation in data; and (b) biplot depicting PC1 and PC2 axes. Green font and arrows show the contribution of various cytokines to the axis.


Results for research question 1
Research question 1: is there any evidence of an altered immune status systemically (as found in the maternal circulation) in TPOAb-positive women compared with TPOAb-negative women?
Univariate analysis
Comparisons of TPOAb-positive subjects at recruitment (n = 16) with TPOAb-negative controls (n = 6) showed no statistically significant differences in any of the chemocytokines (data are not shown). Among TPOAb-positive women, those with a history of miscarriage had a trend of a lower RANTES concentration than those with a history of infertility, but this was not statistically significant after FDR-correction.
To address the postulation that there could be an imbalance in Th1 and Th2 immunity in TPOAb-positive women, we examined the ratio in the concentrations of the classical Th1 cytokines, TNF-α and IFN-γ, to the concentrations of the Th2 cytokines IL-10 and IL-4. There were no significant differences in the ratio of Th1 : Th2 cytokines between TPOAb-positive and TPOAb-negative groups (Table 14), although there was a general tendency for a lowering of Th1 cytokines relative to Th2 cytokines with TPOAb positivity, contrary to previous reports. When we stratified by history of miscarriage or infertility, the ratio of IFN-γ/IL-10 was significantly lower in those with a history of infertility than in those with a history of miscarriage (see Table 14).
| Chemocytokine | Unadjusted | Adjusted for history of miscarriage or infertility | ||||
|---|---|---|---|---|---|---|
| βa (95% CI) | Raw p-value | FDR-corrected p-value | βa (95% CI) | Raw p-value | FDR-corrected p-value | |
| ENA-78 | 0.29 (0.00 to 0.59) | 0.051 | NS | 0.35 (0.05 to 0.64) | 0.024 | NS |
| IL-8 | –0.41 (–0.81 to –0.01) | 0.043 | NS | –0.47 (–0.88 to –0.06) | 0.027 | NS |
Principal components analysis
At recruitment, there was no significant difference or separation between TPOAb-negative women, TPOAb-positive women with a history of miscarriage and TPOAb-positive women with a history of infertility (Figure 13).
FIGURE 13.
Principal component analysis plot showing the weighted averages of all 17 chemocytokines [in TPOAb-negative controls (n = 6), TPOAb-positive women with a history of miscarriage (n = 6) and TPOAb-positive women with a history of infertility (n = 10)].

Results for research question 2
Research question 2: does levothyroxine treatment alter serum chemocytokine concentrations in TPOAb positive women?
Univariate analysis and logistic regression
Non-pregnant cross-sectional assessment
Following the treatment of TPOAb-positive women, non-pregnant women (results at 3 or 6 months) demonstrated no significant differences in any of the chemocytokine concentrations in those who received levothyroxine treatment compared with those who received placebo, although a non-statistically significant trend of an increase in ENA-78 and a reduction in IL-8 was observed. Because our analysis of baseline samples was suggestive of possible differences between women with a history of miscarriage and those with a history of infertility, we also performed linear regression to adjust for this factor, but this did not change the results (Table 15).
| Chemocytokine | Unadjusted | Adjusted for history of miscarriage or infertility | ||||
|---|---|---|---|---|---|---|
| βa (95% CI) | Raw p-value | FDR-corrected p-value | βa (95% CI) | Raw p-value | FDR-corrected p-value | |
| MIP-1α | 0.58 (–0.70 to 1.87) | 0.297 | 0.46 | 0.92 (0.42 to 1.41) | 0.007b | 0.12b |
| IL-1β | 0.25 (0.13 to 0.38) | 0.004b | 0.07b | 0.26 (0.10 to 0.41) | 0.01b | 0.09b |
| IL-4 | 0.12 (0.04 to 0.19) | 0.009b | 0.08b | 0.12 (0.04 to 0.20) | 0.013b | 0.07b |
| IL-8 | 0.95 (0.23 to 1.67) | 0.019b | 0.11b | 1.07 (0.33 to 1.80) | 0.016b | 0.07b |
| G-CSF | 0.53 (–0.13 to 1.18) | 0.094 | 0.32 | 0.66 (0.11 to 1.21) | 0.029b | 0.10b |
| IFN-γ | 0.30 (0.05 to 0.54) | 0.026b | 0.11b | 0.32 (0.02 to 0.61) | 0.04b | 0.11b |
The ratio of the Th1 cytokines to Th2 cytokines was also not significantly different between those treated with levothyroxine and those on placebo (data are not shown).
Non-pregnant longitudinal assessment
In those who had provided a baseline sample as well as a non-pregnant sample post treatment commencement, we were able to calculate the longitudinal change in each chemocytokine concentration in individuals between the two time points. We compared the differences in these longitudinal changes in those who had received levothyroxine treatment (n = 5) with the changes in those who had received placebo (n = 2). There was a greater increase in the concentrations of the chemokines MIP-1α; G-CSF; the Th1 cytokines IL-1β, IL-8, IFN-γ; and the Th2 cytokine IL-4 with levothyroxine treatment than with placebo treatment, after adjustment for history of miscarriage or infertility (Table 16). We were unable to assess the implications of these ‘preconception’ changes on pregnancy outcome as two cases from the levothyroxine arm and both cases from the placebo arm did not conceive.
| Chemocytokine | All pregnancies | Only pregnancies resulting in live birth | ||||
|---|---|---|---|---|---|---|
| βa (95% CI) | Raw p-value | FDR-corrected p-value | βa (95% CI) | Raw p-value | FDR-corrected p-value | |
| Thrombopoietin | 0.73 (0.24 to 1.22) | 0.007b | 0.12b | 0.62 (0.07 to 1.17) | 0.03 | NS |
| IL-1β | 0.56 (0.17 to 0.95) | 0.008b | 0.07b | 0.43 (0.04 to 0.82) | 0.034 | NS |
Early first trimester (6–8 weeks’ gestation) cross-sectional assessment
There were higher thrombopoietin and IL-1β concentrations in the levothyroxine group than in the placebo group, with adjustment for history of infertility or miscarriage (Table 17). On excluding the three cases that subsequently miscarried, thrombopoietin and IL-1β concentrations showed a similar direction of change with levothyroxine treatment, but the changes were no longer significantly different, probably because of the lack of statistical power and the removal of slightly more exaggerated changes occurring in the miscarriage cases.
| Chemocytokine ratio | All pregnancies | Only pregnancies resulting in live birth | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 7), median (IQR) | Levothyroxine (n = 10), median (IQR) | p-value | Placebo (n = 6), median (IQR) | Levothyroxine (n = 8), median (IQR) | p-value | |
| TNF-α/IL-10 | 9.93 (0.25–13.54) | 1.30 (0.50–1.70) | 0.558 | 6.86 (0.25–13.54) | 1.50 (0.70–2.57) | 0.897 |
| TNF-α/IL-4 | 4.96 (0.12–6.77) | 0.53 (0.16–7.61) | 0.770 | 3.57 (0.12–6.77) | 0.67 (0.27–8.56) | 0.796 |
| IFN-γ/IL-10 | 30.32 (2.01–37.89) | 4.83 (3.61–11.44) | 0.558 | 20.49 (2.01–37.89) | 5.52 (3.37–17.41) | 0.897 |
| IFN-γ/IL-4 | 15.16 (1.63–18.95) | 2.56 (1.42–34.75) | 0.696 | 11.14 (1.63–18.95) | 3.14 (1.97–36.88) | 0.796 |
| TNF-α + IFN-γ/IL-4 + IL-10 | 12.27 (0.49–17.62) | 2.04 (0.60–6.73) | 0.558 | 8.73 (0.49–17.62) | 2.81 (1.30–6.91) | 0.897 |
| Thrombopoietin/G-CSF | 0.57 (0.28–3.01) | 4.86 (2.72–8.48) | 0.019 | 1.30 (0.44–3.01) | 5.37 (3.63–8.56) | 0.01 |
To address the hypothesis that levothyroxine could promote the mounting of a more favourable immune response in pregnancy, characterised by a reduced Th1 response and increased Th2 response, we examined the ratios of the Th1 cytokines TNF-α and IFN-γ to the Th2 cytokines IL-10 and IL-4. Because the elevation of thrombopoietin with concomitant decrease in G-CSF has previously been associated with miscarriage,39 we also examined the ratio of thrombopoietin to G-CSF. Although, overall, there was a tendency for a lowering of Th1 cytokines relative to Th2 cytokines in early pregnancy with levothyroxine treatment, there was wide interindividual variation and no significant differences were found between the levothyroxine-treated group and the placebo group (Table 18). There were higher levels of thrombopoietin when compared with G-CSF with levothyroxine treatment, which has previously been reported to be associated with a higher risk of miscarriage. More importantly, the same trends in the ratios were also found when analyses were confined to only those who had a live birth outcome, suggesting that changes in chemocytokine concentrations are not major determinants of pregnancy outcome in this subcohort.
| Chemocytokine | Unadjusted | Adjusted for levothyroxine treatment | ||||
|---|---|---|---|---|---|---|
| βa (95% CI) | Raw p-value | FDR-corrected p-value | βa (95% CI) | Raw p-value | FDR-corrected p-value | |
| MIP-1α | –0.86 (–1.31 to –0.41) | 0.001 | 0.017b | –0.84 (–1.28 to –0.40) | 0.001 | 0.017b |
| IFN-γ | –0.22 (–0.45 to 0.02) | 0.065 | NS | –0.23 (–0.46 to 0.01) | 0.055 | NS |
Second and third trimesters cross-sectional assessment
All cases resulted in a live birth beyond 34 weeks of gestation. There were no significant differences in chemocytokine concentrations between those treated with levothyroxine (n = 8) and those who received placebo (n = 5), with and without adjustment for history of miscarriage/infertility (data are not shown). Similarly, there were no differences in the Th1 : Th2 ratios with levothyroxine treatment, apart from the thrombopoietin : G-CSF ratio in the third trimester, which was significantly higher in the levothyroxine-treated women [median 6.16 (IQR 2.15–12.57)] than in those on placebo [median 1.31 (IQR 1.19–1.59)].
Principal components analysis
Levothyroxine treatment did not significantly change the levels of the three major PCs of chemocytokines, either in the non-pregnant participants or during pregnancy (data are not shown). However, an initial history of miscarriage or infertility could potentially modify the relationship between treatment group and chemocytokine concentrations (Figure 14), especially for placebo during pregnancy. Hence, we subsequently stratified our analyses by history of miscarriage or infertility to investigate the effects of levothyroxine.
FIGURE 14.
Principal component analysis showing separation in the weighted averages of chemocytokine concentrations between various subgroups.

Changes in the levels of PCs with treatment following stratification by history of miscarriage or infertility are shown in Figure 15. There were no significant differences in PC1, PC2 and PC3 at baseline (recruitment), and in non-pregnant women (3 months or 6 months) between treatment groups in both women with a history of miscarriage or women with a history of infertility. During pregnancy, there were no significant differences with levothyroxine treatment in PC1 and PC3, but PC2 showed a consistent trend of being higher with levothyroxine treatment in the women with a history of miscarriage but an opposite trend of being lower with levothyroxine in women with a history of infertility, although differences were not statistically significant. The differences in PC2 during pregnancy with levothyroxine treatment may simply be reflective of trends which were already present prior to any intervention, as suggested by similar PC2 trends observed at recruitment. This needs to be interpreted with caution because different cases are represented at recruitment and in pregnancy, so longitudinal trends cannot be assessed.
FIGURE 15.
Differences in the three main principal components between levothyroxine treatment and placebo, when stratified by history of miscarriage or infertility at each time point. Baseline (recruitment), non-pregnant (3 months or 6 months post recruitment) and during pregnancy (trimester 1: 6–8 weeks, trimester 2: 16–18 weeks and trimester 3: 28 weeks). Different women are included at each time point according to availability of samples for analysis. Box plots indicate the IQR and whiskers indicate 1.5 × IQR. The number of subjects in each group and p-values are given at the bottom of the figure. Tri1, trimester 1; Tri2, trimester 2; Tri3, trimester 3.
Results for research question 3
Research question 3: are the changes in circulatory chemocytokines brought about by levothyroxine treatment associated with an improvement in the chance of a live birth outcome?
Univariate analysis and logistic regression
At the 3- and 6-month time points (before pregnancy), there were no significant differences in chemocytokine concentrations between those who subsequently had a live birth and those who subsequently miscarried (data are not shown).
However, at 6–8 weeks of pregnancy, those who subsequently miscarried demonstrated significantly reduced MIP-1α concentration compared with those who subsequently had a live birth. Adjustment for treatment group did not change the results, indicating that levothyroxine treatment had no influence on the relationship between the chemocytokine difference and pregnancy outcome (see Table 18).
Furthermore, the chemocytokines, thrombopoietin and IL-1β, that were significantly changed by levothyroxine treatment at 6–8 weeks’ gestation (see Table 17) were not the ones that were significantly associated with differential pregnancy outcome. Similarly, the ratios of Th1 to Th2 cytokine concentrations were not significantly different between those who had a live birth and those who miscarried, regardless of whether or not they were on levothyroxine treatment (data are not shown).
Principal components analysis
There were similar chemocytokine concentrations observed between those who subsequently miscarried and those who subsequently had a live birth, regardless of treatment with levothyroxine or placebo.
Discussion and conclusions
This trial has demonstrated that serum chemocytokine concentrations of TPOAb-positive women do not differ from the serum chemocytokine concentrations of TPOAb-negative women outside pregnancy. Although levothyroxine treatment of TPOAb-positive women resulted in some changes in serum chemocytokine concentrations, both in the non-pregnant state and in early pregnancy, these changes did not influence the chance of a subsequent live birth outcome.
This trial has measured only chemocytokines in the serum, which comprise secretions from peripheral blood mononuclear cells (PBMCs), adipose tissue and other tissues. In uteroplacental tissues during pregnancy, local T cells are major producers of cytokines, and non-lymphoid decidual cells and trophoblast are also significant contributors. 19,21,43 Thus, serum chemocytokine changes in this trial may not reflect changes in cytokine expression by PBMCs or changes occurring locally at the maternal–fetal interface, and this may account for differences in the findings of this trial from previously reported studies.
The finding of no difference in individual serum chemocytokines between TPOAb-positive and TPOAb-negative status, and a general tendency for lower Th1 : Th2 ratios with TPOAb positivity, is in contrast to the findings previously reported in CD3+/CD4+ T cells obtained from a similar population of women with a history of infertility or recurrent miscarriage. 35 They reported an increase in the ratio of T cells expressing the Th1 cytokine TNF-α to T cells expressing the Th2 cytokine IL-10 in the circulation of TPOAb-positive women compared with TPOAb-negative women. 35 However, we did find a lower IFN-λ : IL-10 ratio in TPOAb-positive women with a history of infertility than in those with a history of miscarriage. This could represent a phenotypic difference in immune responses, especially in TPOAb-positive women with a history of infertility, that has not been distinguished before between women with a history of infertility and those without such a history.
In a different population of euthyroid TPOAb-positive patients with chronic idiopathic urticaria serum concentrations of the Th1 cytokines, IFN-γ and TNF-α were increased by levothyroxine treatment which the authors thought was because of TSH suppression. 44 In this trial, no changes in IFN-γ and TNF-α were observed in the non-pregnant state in cross-sectional analyses, but an increase in IFN-γ (with no change in TNF-α) and other chemocytokine changes were observed in a longitudinal analysis with levothyroxine treatment. Differences in trial findings may be accounted for by little or no TSH reduction in the levothyroxine-treated subcohort [the mean reduction in TSH concentration between recruitment and preconception follow-up was 0.0195 mlU/l (SD ± 1.88) in the levothyroxine-treated group and 0.287 mlU/l (SD ± 0.96) in the placebo group] and being limited to only a young adult female population in this trial.
We had found that, in early pregnancy, there was a suggestion of increased serum thrombopoietin and IL-1β with levothyroxine treatment, but no differences were observed in the second or third trimesters between treatment groups. Another study45 similarly reported a lack of change in a range of serum cytokines with levothyroxine treatment of TPOAb-positive women during the first and second trimesters of pregnancy; however, this study45 did not measure thrombopoietin and IL-1β. Furthermore, we observed an elevated thrombopoietin : G-CSF ratio in the first trimester with levothyroxine treatment, a change that has previously been associated with miscarriage,39 yet in this trial there was no correlation with pregnancy outcome. We have also previously reported that tri-iodothyronine treatment in vitro of isolated decidual cells from the first trimester changed the secretion of a range of chemocytokines,40 but this has not been reflected by the systemic serum chemocytokine measures in vivo with levothyroxine treatment in this trial.
Overall, the findings of a lower MIP-1α concentration in women who subsequently miscarried than in those who had a live birth, and no change in all other chemocytokines or in Th1 : Th2 cytokine ratios evaluated, are in contrast to the findings reported in other studies. 40 The difference between this trial and the others is that this trial population is confined to only TPOAb-positive women, who may display a different immune response to pregnancy compared with TPOAb-negative women. The PCA findings show that alterations occur across a range of chemocytokines with levothyroxine treatment in a way that is influenced by a history of miscarriage or infertility. However, these alterations showed no association with subsequent pregnancy outcome. All of this suggests that, in women with TPOAb positivity, differences in the specific chemocytokines that we have measured are not major determinants of a live birth outcome.
The main strength of this mechanistic trial is that it is embedded in the context of a large randomised controlled trial that has the power to provide definitive clinical outcomes, which cannot be derived from smaller sample sizes that are typical of studies analysing a range of chemocytokines in this field. Complementarity of the trial results provides greater confidence in drawing conclusions from the chemocytokine data based on this limited subset. A major limitation is the small sample size and missing time points, which limits statistical power and the ability to conduct longitudinal analyses. Additional assessments of cytokine expression in PBMCs and in cells at the maternal–fetal interface would have provided a more comprehensive picture of the changes in immune responses occurring with levothyroxine treatment.
In conclusion, treatment of TPOAb-positive women with levothyroxine resulted in some changes in chemocytokine concentrations in the non-pregnant state and in very early pregnancy, but these changes had no bearing on whether or not the pregnancy resulted in a live birth outcome.
Chapter 5 Discussion
Trial strengths
To our knowledge, this trial is the largest ever randomised placebo-controlled clinical trial to report on the treatment effects of pre- and post-conception levothyroxine for women with a history of miscarriage or infertility and thyroid autoantibodies. The trial design ensured internal validity, enabling the results to be interpreted with confidence.
Randomisation and minimisation were effective in achieving balanced treatment allocations at baseline. A computer-generated allocation sequence, allocation concealment and blinding prevented investigators from knowing the allocation of the next participant based on prior treatment assignments. Possible confounding factors such as number of previous miscarriages, maternal age and TSH levels were similarly distributed between treatment groups. The TABLET trial also avoided performance bias by blinding the participants and care providers to treatments.
To determine the minimally important clinical difference to prompt a change in practice, health-care professionals, patients and representatives of the Miscarriage Association were surveyed. A consensus of a 10% increase in live birth rates beyond 34 weeks’ gestation evolved from this consultation, and this was used to derive a target sample size for the trial of 760 participants with primary outcome data. The evidence for levothyroxine in the literature at the start of the trial suggested that a much greater effect could be anticipated, with a halving of the risk of miscarriage rate estimated in a meta-analysis of two comparable trials8,9 (RR 0.48, 95% CI 0.25 to 0.92). 6 If all other assumptions were correct, then the trial would have > 99% power to detect differences of ≥ 15%.
The sample size assumed a rate of live births at ≥ 34 weeks of gestation of 55% in the placebo group, acknowledging that not all women would become pregnant within 12 months of randomisation. We planned to randomise 900 women in total (450 participants to each group) and we actually exceeded this number, accruing 952 women. Furthermore, we anticipated that we would fail to capture the primary outcome in 15% of women, because of loss to follow-up or withdrawal. Ultimately, we had an outcome for 98.7% women, equally reported in each treatment group; hence, we consider the findings of this trial to be methodologically and statistically robust.
We originally restricted the population to solely women who had experienced a miscarriage. We expanded the eligible population to include women undergoing fertility treatment, as there was comparable evidence from cohort studies that there was an association between miscarriage and thyroid autoantibodies. 6 This allows the results to be generalisable to both populations. It was anticipated that the broadening of the criteria might result in a lower rate of the primary outcome, as a greater proportion of the fertility population might fail to conceive within 1 year than we had originally factored into the rationale in the sample size calculation. Once we had considered the low apparent loss to follow-up rate and the potential power from a range of live births at ≥ 34 weeks in the control group, we considered the original target of 900 women would be sufficient and did not adjust the target sample size. Ultimately, we observed an overall rate of 38% (354/940) for the primary outcome, with only a small absolute difference of 0.4% between groups. Estimated uncertainty around this estimate was, at most, 6.6%, allowing us to rule out missing a clinically meaningful difference, which had been defined as 10% at the outset of the trial.
The trial design offered a number of other strengths with respect to data collection and analysis. The treatment of participants in a large number of trial centres and by a large number of practitioners allowed intervention impact to be evaluated without confounding by individual variance in clinical practice and local reference ranges for thyroid function. The outcome measures selected were routine variables widely used by clinicians who are familiar with early pregnancy care. This ensured that the outcomes were well understood and easy to record. Almost all of the outcome data recorded during the TABLET trial were objective outcomes (rather than subjective descriptions), and the trial was blinded, so there was no risk of incurring assessor bias.
The trial intervention was deliverable in the context of customary care without major impacts on health service structure. The mode of administration of the IMP was designed to reflect the preferences expressed by patients, and most of the data collection could be performed during routine antenatal and postnatal appointments of the trial participants.
Limitations and critique
We consider the trial to have been designed and conducted in order to be methodologically robust. Nevertheless, there are some limitations that could affect the results observed.
The potential criticisms of this trial include (1) variation in the threshold of abnormality of TPOAbs at time of randomisation, (2) a possibly suboptimal dose, (3) a possibly inadequate duration of treatment prior to conception, (4) dilution of the treatment effect by factors such as breaches of protocol and (5) inappropriate thresholds for cessation of treatment in each trimester. We examine these issues individually in the following sections.
Variation in the threshold of abnormality of thyroid peroxidase antibodies
Various assays for TPOAbs are available, each with different detection limits and thresholds for test positivity, which are predetermined by the assay manufacturer. These variations are an accepted part of normal practice in the UK. Quality assurance for assays in the laboratories for all the participating centres is provided by UK Immunology, Immunochemistry and Allergy NEQAS, which shows > 99% concordance in the classification of samples as either positive or negative for TPOAbs across all assays. With this assurance, the TABLET trial protocol did not define a single threshold for TPOAb positivity but accepted the classification of abnormality provided by the laboratories servicing the participating centres. Not all laboratories provided discrete measurements, resulting in fewer participants entering the subgroup analysis that examined whether or not TPOAb titres at baseline of less than and greater than the 50th centile for the assay exhibited differential effects in relation to the primary outcome.
Possibly suboptimal dose
The dosage of levothyroxine that was adopted by the TABLET trial (50 µg per day) sits at the lower end of the suggested dosing for hypothyroidism, according to British National Formulary (BNF),46 recalling that these women are euthyroid according to local reference ranges for TSH and free T4. The choice of dose was made after a careful review of the existing trials using levothyroxine for the prevention of miscarriage,8,9 an extensive survey of endocrinologists as well as obstetricians with an interest in maternal medicine, a review of the host organisation’s obstetric–endocrine practice database and a review of other related evidence.
Unlike the T4-LIFE study,10 we did not titrate the dose to the baseline TSH or women’s weight. The BNF suggests that thyroxine doses for hypothyroidism may need to be increased in pregnancy, but does not suggest a dosing algorithm. We believed that dose titration, and adjustment once pregnant, could potentially compromise adherence and blinding, and instead chose a fixed dose with a change in the definitions of overt hyperthyroidism and overt or subclinical hypothyroidism once pregnant. We therefore believe that the outcome of the trial is unlikely to be affected by the possibility of suboptimal dosage and did not subject the trial participants to undue risks from the treatment. This trial has demonstrated evidence of a lower TSH and higher free T4 concentration in the levothyroxine-treated group than in the placebo group over the duration of trial participation. There was no evidence of over- or undertreatment with the dose of thyroxine; this confirms that the dose was adequate to effect change in TFT within safe methods.
Possibly inadequate duration of treatment prior to conception
We postulated that exogenous levothyroxine treatment may correct any relative deficiency of thyroid hormones, and affect both systemic immune regulation and the local placental–decidual environment. Establishing a favourable environment prior to conception was thought to be preferable to pre-empt the rapid increase in demand for thyroid hormone synthesis from very early pregnancy. It was also thought to help achieve a more rapid optimisation of the inflammatory processes required for a successful pregnancy. The optimal duration of preconception treatment is unknown, and the time taken to become pregnant will vary between women according to their history and circumstances; therefore, the trial adopted a pragmatic duration of preconception treatment of 12 months.
Dilution of the treatment effect
Trial drugs were dispensed in quantities sufficient for 13 weeks of treatment, with subsequent prescriptions dispensed once thyroid hormone levels were checked for normality. We attempted to count the number of pills taken by each participant, but because of the poor return of pill bottles at all time points we opted to use an additional measure of compliance estimation. This was in the form of a simple question regarding the proportion of pills taken, reported by the participant. Return of the pill bottles was the favoured method of compliance.
Inappropriate thresholds for cessation of treatment
There are variations in absolute TSH and free T4 measurements between assay platforms, with NEQAS Immunology, Immunochemistry and Allergy (IIA) assessing the extent to which the assay under- or overestimates a control sample for non-pregnant women of reproductive age and those who are pregnant. We restricted hospital participation to those sites that used a Roche, Abbot Architect or Siemens Advia Centaur analytic platform. The trial reference ranges for abnormality that defined euthyroidism and the eligibility criteria were identical across all platforms, to ensure that only strictly euthyroid women were included in the TABLET trial. We increased the upper limit of TSH normality to 4.0 mlU/l for monitoring women prior to becoming pregnant to allow for intraindividual variation over time, noting that this may not align with manufacturers’ reference ranges.
It would be difficult to define one set of limits for all three assay types during pregnancy because of the apparent bias reported by NEQAS IIA, and we would face criticism if we used seemingly very different values for each assay. The upper thresholds could not be too different from the existing limits set by laboratories servicing trial centres, as there would have been conflict in the management of trial and non-trial women. We chose the upper limits for pregnant women, at each gestational age range and each platform, based on a thorough search of the literature (as detailed in Chapter 2, Note on thresholds for thyroid function tests).
Finally, it is worth noting that, for some of the secondary outcomes, the ability to detect differences would have been limited by the size of the sample. The trial was powered to detect only a significant difference in live birth rate.
Findings in the context of existing literature
Since the initiation of TABLET trial, a study by Wang et al. 11 has explored the effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing IVF. They randomised 600 women to receive either levothyroxine or placebo. The levothyroxine dose was either 25 µg or 50 µg and then titrated according to TSH levels during pregnancy. They found no difference in miscarriage rates or live birth rates between the groups. A further randomised study by Nazarpour et al. 47 explored the effects of levothyroxine in TPOAb-positive women who were euthyroid or had subclinical thyroid disease. They found that treatment with levothyroxine did result in a lower preterm birth rate. This study was much smaller than the TABLET trial, with only 131 TPOAb-positive women. Given that the euthyroid and subclinical hypothyroid women (with TSH concentrations of up to 10 mlU/l) were combined in the same group, we felt that the data were not comparable with those in this trial.
When adding the results of this trial and the trial by Wang et al. 11 to those of the Negro et al. 8,9 trials, the pooled results show no significant reduction in miscarriage with levothyroxine treatment compared with control (Figure 16).
FIGURE 16.
Effect of levothyroxine treatment in reducing miscarriage in euthyroid women with TPOAbs. M–H, Mantel–Haenszel.
The slightly higher miscarriage rate observed in this trial (28% in the levothyroxine group and 30% in the placebo group) further confirms that women with TPOAbs are at a higher risk of miscarriage than the general population.
Patient and public involvement
In the TABLET trial, patient and public involvement was utilised at several stages of the trial design, development and monitoring. This included questionnaires for patients to assess the acceptability of the intervention, and engagement in the development of patient-facing literature for participants. The TSC included a representative of the Miscarriage Association. We believe that these roles were important to ensure appropriate communication with trial participants and project oversight throughout the duration of the research. Dissemination of the results will be supported by the Miscarriage Association.
Interpretation
These findings show that women with a normal thyroid function who are positive for TPOAbs do not benefit from levothyroxine treatment commenced preconceptually for any of the key clinical outcomes that we observed. There were no significant differences between levothyroxine and placebo for neonatal or maternal secondary outcomes. These findings are not consistent with the findings of two smaller and poorer-quality controlled studies,8,9 that reported benefit from levothyroxine in the same population. However, they are in keeping with more recent findings seen in the larger randomised controlled trial by Wang et al. 11 The TABLET trial is, to our knowledge, by far the largest of all randomised controlled trials conducted in this field; therefore, it has conclusively answered the question of whether or not levothyroxine is beneficial for women with TPOAb.
The TABLET trial has added to the available safety data regarding the use of levothyroxine during pregnancy, with some suggestion that there may be harm associated with its use in euthyroid women. This is discussed in more detail in Chapter 6, Implications for health care.
Generalisability
Centres participating in the trial were geographically spread across the UK, improving the generalisability of the results for euthyroid TPOAb-positive women actively trying for a pregnancy.
All women in the trial belonged to a ‘selected population’, whether it was history of miscarriage or infertility. Therefore, the results of this trial may not represent that of true unselected ‘low-risk’ women with no gynaecological or obstetric risk factors. Given that it would be impractical to test all women preconceptually for thyroid function and TPOAbs, we feel that the pragmatic approach to this trial (testing women who access health-care preconception) makes the results generalisable to all euthyroid women with TPOAb of reproductive age.
The exclusion criteria were kept to a minimum and the heterogeneity of the population was well reflected by trial participants.
Chapter 6 Conclusions
Implications for health care
The key findings of the TABLET trial are clear and sufficiently generalisable to inform clinical practice. On the basis of the results of this trial, levothyroxine treatment commenced preconceptually does not have clinically significant benefits in euthyroid women with TPOAbs.
Levothyroxine is a widely used drug in the treatment of overt hypothyroidism and has not been found to have harmful effects on mother or fetus in this group. 13 There is evidence to suggest that women with TPOAbs have higher levels of TSH and are at higher risk of progression to subclinical hypothyroidisim in pregnancy and in later life. 48 Subclinical hypothyroidism itself is linked to adverse obstetric outcomes such as miscarriage and preterm birth. For this reason, clinicians are moving towards empirically treating women with subclinical hypothyroidism with levothyroxine. This is most evident in the fertility setting where women with TSH levels of > 2.5 mIU/l, with or without TPOAbs, are being commenced on levothyroxine preconceptually. These approaches are based on the rationale that, despite the lack of convincing evidence of efficacy of levothyroxine in reducing obstetric risks, the potential benefits are considered to outweigh any potential risks from the medication itself. This belief, however, has recently been contested by a large retrospective cohort study of > 5000 women with subclinical hypothyroidism published in BMJ by Maraka et al. 49 This study found that, although treatment with levothyroxine contributed to a significantly lower odds of pregnancy loss than in untreated women, there were higher odds of preterm delivery, gestational diabetes and pre-eclampsia. 49 Similarly, for our trial we noted higher rates of gestational diabetes (11% vs. 9%; p = 0.62) and pre-eclampsia (5% vs. 3%; p = 0.28) in the levothyroxine group, although these were not statistically significant.
There was also a higher number of SAEs reported in the levothyroxine group than in the placebo group (28 vs. 16 cases, respectively), as well as a higher rate of maternal admissions to HDUs (6% vs. 3%, respectively; p = 0.27), but none of these were attributed to the trial medication.
The subset of women recruited into the mechanistic study demonstrated that treatment with levothyroxine resulted in some changes in chemocytokine concentrations in the non-pregnant state and in very early pregnancy, but these changes had no bearing on whether or not the pregnancy resulted in a live birth outcome.
Recommendations for research
In our opinion, no further research is required to evaluate the role of levothyroxine therapy in reducing miscarriage and preterm birth rates for euthyroid women with TPOAbs.
Questions that remain unaddressed relate to the effects of levothyroxine in women who have subclinical hypothyroidism. The authors of the existing published trials that evaluated euthyroid TPOAb-positive women8,9,11 and the authors of T4-LIFE10 (yet to be published) have verbally agreed to share their data to allow for an individual patient data meta-analysis to be conducted. This means that all existing trial data on the topic of subclinical hypothyroidism and TPOAbs will be collated. External funding will be sought for this work. The treatment of mild subclinical hypothyroidism (TSH levels of 2.5–4.5 mIU/l) is a highly controversial area, particularly in the fertility setting, and so collating high-quality data for women with/without TPOAbs in this group will be invaluable to shaping clinical practice.
The mechanistic study was unable to demonstrate any clinically relevant changes in serum chemocytokine concentrations in the non-pregnant state or in pregnancy.
Acknowledgements
The following list of people significantly contributed to recruitment; without them, the trial would not have been possible.
Principal investigators
Ash Alam (Arrowe Park Hospital, Wirral), Amaju Ikomi (Basildon Hospital), Derek Tuffnell (Bradford Royal Infirmary), Ayman Ewies (City Hospital, Birmingham), Fadi Alfhaily (Colchester General Hospital), Simon Wood (Countess of Chester Hospital), Laura Hipple (Cumberland Infirmary), Rukma Bhattacharya (Derriford Hospital, Plymouth), Seema Sen (University Hospital North Durham), Sridevi Sankaran (Frimley Park Hospital, Surrey), Vincent Bamigboye (Furness General Hospital), Padma Manda (James Cook Hospital, Middlesbrough), Richard Russell (Liverpool Womens Hospital), Jag Samra (New Cross Hospital, Wolverhampton), Alpa Shah (Newham General Hospital), Sachchidananda Maiti (North Manchester General Hospital), Judith Moore (Queens Medical Centre, Nottingham), Karen Watkins (Royal Cornwall Hospital), Kannamannadiar Jayaprakasan (Royal Derby Hospital), Lisa Joels (Royal Devon and Exeter Hospital), Jo Fiquet (Royal United Hospital, Bath), Zoey Robinson (Royal United Hospital, Bath), Jane Shillito (St James’ Hospital Leeds), Nigel Simpson (St James’ Hospital Leeds), Catherine Bass (St Peter’s Hospital, Chertsey), Saikat Banerjee (St Peter’s Hospital, Chertsey), Judith Hamilton (St Thomas’ Hospital, London), Tom Holland (St Thomas’ Hospital, London), Tristan Richardson (The Royal Bournemouth Hospital), Meenakshi Choudhary (The Royal Victoria Infirmary, Newcastle), Rita Arya (Warrington Hospital), Colin Johnston (Watford General Hospital), Ismail Wong (Whipps Cross University Hospital, London) and Jagadeeswari Karuppaswamy (Wrightington Hospital).
Research fellows, research nurses and clinical assistants
Justin Chu (Academic Clinical Lecturer, Birmingham Womens Hospital), Hoda Harb (Academic Clinical Lecturer, Birmingham Womens Hospital), Oonagh Pickering (Research Nurse, Birmingham Womens Hospital), Bev Hammond (Research Nurse, Burnley General Hospital), Matthew Milner (Research Nurse, Burnley General Hospital), Fiona Kinney (Research Nurse, City Hospital, Birmingham), Aine Turner (Research Nurse, Colchester General Hospital), Jean Bvumbe (Research Nurse, Guy’s Hospital, London), Charlotte Yearwood-Martin (Research Nurse, Guy’s Hospital, London), Sinead Morgan (Research Fellow, Kings College Hospital, London), Pamela Corlett (Research Nurse, Liverpool Womens Hospital), Elizabeth Kane (Research Nurse, Liverpool Womens Hospital), Lesley Harris (Research Nurse, Liverpool Womens Hospital), Rebecca Denyer (Research Clinical Assistant, New Cross Hospital, Wolverhampton), Karen Evans (Research Nurse, New Cross Hospital, Wolverhampton), Sharon Kempson (Research Nurse, New Cross Hospital, Wolverhampton), Joanne Hutchinson (Research Nurse, Newham Hospital), Helen Millward (Research Nurse, Princess Royal Hospital, Telford), Anna Molnar (Research Nurse, Queens Medical Centre, Nottingham), Liz Barnes (Research Nurse, Queens Medical Centre, Nottingham), Lucinda Wilson (Research Nurse, Queens Medical Centre, Nottingham), Kat Rhead (Research Nurse, Royal Bolton Hospital), Alice Rossi (Research Nurse, Royal London Hospital, London), Lilith Haynes (Research Nurse, St Bartholomew’s Hospital, London), Sharon Nettleton (Research Nurse, St James’ Hospital, Leeds), Jayne Wagstaff (Research Nurse, St James’ Hospital, Leeds), Jane Budd (Research Nurse, St Mary’s Hospital, Manchester), Lucy Dwyer (Research Manager, St Mary’s Hospital, Manchester), Christine Hughes (Research practitioner, St Mary’s Hospital, Manchester) Hannah Phillips (Research Nurse, St Mary’s Hospital, Manchester), Colleen Hunt (Research Assistant, St Michael’s Hospital, Bristol), Carole Shahin (Research Nurse, St Michael’s Hospital, Bristol), Alison Kirby (Research Midwife, St Michael’s Hospital, Bristol), Jennifer Hatton (Research Nurse, St Thomas’ Hospital, London), Debbie Finucane (Research Midwife, St Thomas’ Hospital, London), Victoria Murtha (Research Nurse, The Royal Victoria Infirmary, Newcastle), Michelle Perkins (Research Nurse, The Royal Victoria Infirmary, Newcastle), Debbie Bullen (University Hospital, Coventry), Lindsay Prue (University Hospital, Coventry), Rachel Crone (Research Nurse, Warrington Hospital), Lindsay Roughley (Research Midwife, Warrington Hospital) and Zandile Maseko (Research Nurse, Whipps Cross University Hospital, London).
We would like to acknowledge Adaikalavn Ramasamy (Singapore Institute for Clinical Sciences) and Hsin Fang Chang (National University Health System, Singapore) for their contribution to the mechanistic element of the trial. Their role involved assisting with statistical analyses.
Institutional support
We acknowledge, with thanks, the trial funder: the National Institute for Health Research Efficacy and Mechanism Evaluation programme in the UK.
We thank all those not otherwise mentioned who have contributed to the TABLET trial.
Contributions of authors
Dr Rima K Dhillon-Smith (Academic Clinical Lecturer) co-ordinated the practical conduct of the trial, including leading recruitment at Birmingham Women’s Hospital. She also co-ordinated the management of follow-up and contributed to the TMG. She was responsible for completion of data gathering, providing data quality assurance, co-ordination of the analysis and the writing groups, and produced the final report.
Mr Lee J Middleton (Statistics Lead) designed and conducted the statistical analysis of the primary and secondary trial outcomes, produced reports for the DMC and contributed to the TMG. He was also responsible for writing the methods and results section of the final report.
Mrs Kirandeep K Sunner (Senior Trial Manager) co-ordinated the practical conduct of the trial, including the management of follow-up, and was responsible for completion of data gathering. She also attended meetings of the TSC and contributed to the TMG and preparation of the final report.
Mrs Versha Cheed (Medical Statistician) helped with the design and conducted the statistical analysis of the primary and secondary trial outcomes, produced reports for the DMC and contributed to the TMG. She was also responsible for writing the methods and results section of the draft report.
Mrs Krys Baker (Unit Research Co-ordinator) co-ordinated the practical conduct of the trial and also attended meetings of the TSC. She also contributed to the TMG and preparation of the draft report.
Ms Samantha Farrell-Carver (Trials Assistant) co-ordinated the practical conduct of the trial, including the management of follow-up, and was responsible for completion of data gathering. She also attended meetings of the TSC and contributed to the TMG.
Ms Ruth Bender-Atik (National Director of the Miscarriage Association) provided advice and oversight to conduct patient and public involvement activities and contributed to the TSC.
Dr Rina Agrawal (Consultant Obstetrician and Gynaecologist, Specialist in Reproductive Medicine) was jointly responsible for the TABLET trial recruitment and data collection at University Hospital Coventry Assisted Conception Unit.
Dr Kalsang Bhatia (Consultant Obstetrician and Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at Burnley General Hospital.
Mr Edmond Edi-Osagie (Consultant Gynaecologist and Specialist in Reproductive Medicine) was responsible for leading the TABLET trial recruitment and data collection at St Mary’s Hospital, Manchester.
Mr Tarek Ghobara (Consultant Obstetrician and Gynaecologist, Specialist in Reproductive Medicine) was jointly responsible for the TABLET trial recruitment and data collection at University Hospital Coventry Assisted Conception Unit.
Dr Pratima Gupta (Consultant in Obstetrics and Gynaecology) was responsible for co-ordinating the TABLET trial recruitment and data collection at Birmingham Heartlands Hospital.
Mr Davor Jurkovic (Consultant Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at University College Hospital, London.
Mr Yacoub Khalaf (Consultant Gynaecologist and Specialist in Reproductive Medicine) was responsible for leading the TABLET trial recruitment and data collection at Guy’s Hospital, London.
Dr Marjory MacLean (Consultant Obstetrician) was responsible for leading the TABLET trial recruitment and data collection at Ayrshire Maternity Unit.
Professor Chris McCabe (Professor of Molecular Endocrinology) was involved with the design of the mechanistic study and reviewed the draft mechanistic report.
Dr Khashia Mulbagal (Consultant Obstetrician and Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at Royal Bolton Hospital.
Miss Natalie Nunes (Consultant Obstetrician and Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at West Middlesex University Hospital.
Dr Caroline Overton (Consultant Obstetrician and Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at St Michael’s Hospital, Bristol.
Professor Siobhan Quenby (Professor of Obstetrics) was responsible for leading the TABLET trial recruitment and data collection at University Hospital Coventry.
Mr Rajendra Rai (Consultant Obstetrician and Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at St Mary’s Hospital, London.
Mr Nick Raine-Fenning (Associate Professor of Reproductive Medicine) was responsible for leading the TABLET trial recruitment and data collection at Queen’s Medical Centre, Nottingham.
Dr Lynne Robinson (Consultant in Obstetrics and Gynaecology, Specialist in Reproductive Medicine) was responsible for co-ordinating the TABLET trial recruitment and data collection at Birmingham Women’s Hospital.
Dr Jackie Ross (Consultant Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at King’s College Hospital, London.
Mr Andrew Sizer (Consultant in Reproductive Medicine) was responsible for the TABLET trial recruitment and data collection at Princess Royal Hospital, Assisted Conception Unit, Telford.
Ms Rachel Small (Lead Midwife and Nurse for Early Pregnancy Care) conducted the TABLET trial recruitment and data collection at Birmingham Heartlands Hospital.
Dr Alex Tan (Clinical Research Fellow) was involved with the systematic review and design of the trial.
Mr Martyn Underwood (Consultant Obstetrician and Gynaecologist) was responsible for leading the TABLET trial recruitment and data collection at Princess Royal Hospital, Telford.
Professor Mark D Kilby (Professor of Fetal Medicine) was involved with trial design, contributed to the TMG and reviewed the draft report.
Dr Kristien Boelaert (Consultant Endocrinologist) contributed to the TMG and reviewed the draft report. She also provided oversight of the management of any abnormal test results.
Professor Jane Daniels (Professor of Clinical Trials) was involved with the design and conduct of the trial, contributed to the TMG and was part of the core writing group for the draft report.
Professor Shakila Thangaratinam (Professor of Maternal and Perinatal Health) was involved with the systematic review and the design and conduct of the main trial, contributed to the TMG and reviewed the draft report.
Dr Shiao-Yng Chan (Consultant in Obstetrics and Clinician Scientist) was involved with the design and conduct of the main trial and contributed to the TMG. She also designed and performed the data analysis and write-up for the mechanistic study, as well as reviewing the draft report.
Professor Arri Coomarasamy (Professor of Gynaecology and Chief Investigator of the TABLET trial) designed the trial, chaired the TMG, contributed to the TSC and took overall responsibility for the project. He also edited the final report.
All authors contributed substantially to the development of the research question and trial design, implementation, analysis and/or interpretation of data, and submission of the final report.
Publications
Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011;342:d2616.
Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med 2019;380:1316–25.
Data-sharing statement
All data requests should be submitted to the corresponding authors for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Frost J, Bradley H, Levitas R, Smith L, Garcia J. The loss of possibility: scientisation of death and the special case of early miscarriage. Sociol Health Illn 2007;29:1003-22. https://doi.org/10.1111/j.1467-9566.2007.01019.x.
- Tommy’s . Miscarriage Statistics n.d. www.tommys.org/our-organisation/charity-research/pregnancy-statistics/miscarriage (accessed 20 May 2018).
- Pedersen IB, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol 2003;58:36-42. https://doi.org/10.1046/j.1365-2265.2003.01633.x.
- Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol 1991;35:41-6. https://doi.org/10.1111/j.1365-2265.1991.tb03494.x.
- Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 1997;18:404-33. https://doi.org/10.1210/edrv.18.3.0300.
- Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011;342. https://doi.org/10.1136/bmj.d2616.
- Wells GA, Shea B, O’Connell D, Petersen J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Ottawa, ON: The Ottawa Hospital, Department of Epidemiology and Community Medicine, University of Ottawa; n.d.
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006;91:2587-91. https://doi.org/10.1210/jc.2005-1603.
- Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod 2005;20:1529-33. https://doi.org/10.1093/humrep/deh843.
- Vissenberg R, van Dijk MM, Fliers E, van der Post JAM, van Wely M, Bloemenkamp KWM, et al. Effect of levothyroxine on live birth rate in euthyroid women with recurrent miscarriage and TPO antibodies (T4-LIFE study). Contemp Clin Trials 2015;44:134-8. https://doi.org/10.1016/j.cct.2015.08.005.
- Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA 2017;318:2190-8. https://doi.org/10.1001/jama.2017.18249.
- Gyamfi C, Wapner RJ, D’Alton ME. Thyroid dysfunction in pregnancy: the basic science and clinical evidence surrounding the controversy in management. Obstet Gynecol 2009;113:702-7. https://doi.org/10.1097/AOG.0b013e3181996fe5.
- Okosieme OE, Marx H, Lazarus JH. Medical management of thyroid dysfunction in pregnancy and the postpartum. Expert Opin Pharmacother 2008;9:2281-93. https://doi.org/10.1517/14656566.9.13.2281.
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004;291:228-38. https://doi.org/10.1001/jama.291.2.228.
- Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao K, Kawasaki E, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest 2008;31:861-5. https://doi.org/10.1007/BF03346432.
- Colin IM, Isaac J, Dupret P, Ledant T, D’Hautcourt JL. Functional lymphocyte subset assessment of the Th1/Th2 profile in patients with autoimmune thyroiditis by flowcytometric analysis of peripheral lymphocytes. J Biol Regul Homeost Agents 2004;18:72-6.
- Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol 2004;150:751-5. https://doi.org/10.1530/eje.0.1500751.
- Hodkinson CF, Simpson EE, Beattie JH, O’Connor JM, Campbell DJ, Strain JJ, et al. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55-70 years. J Endocrinol 2009;202:55-63. https://doi.org/10.1677/JOE-08-0488.
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206-15. https://doi.org/10.1177/1933719108329095.
- Bulmer JN, Hollings D, Ritson A. Immunocytochemical evidence that endometrial stromal granulocytes are granulated lymphocytes. J Pathol 1987;153:281-8. https://doi.org/10.1002/path.1711530313.
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol 2006;6:584-94. https://doi.org/10.1038/nri1897.
- Mascanfroni I, Montesinos Mdel M, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, et al. Control of dendritic cell maturation and function by triiodothyronine. FASEB J 2008;22:1032-42. https://doi.org/10.1096/fj.07-8652com.
- Matsuo H, Maruo T, Murata K, Mochizuki M. Human early placental trophoblasts produce an epidermal growth factor-like substance in synergy with thyroid hormone. Acta Endocrinol 1993;128:225-9. https://doi.org/10.1530/acta.0.1280225.
- Barber KJ, Franklyn JA, McCabe CJ, Khanim FL, Bulmer JN, Whitley GS, et al. The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab 2005;90:1655-61. https://doi.org/10.1210/jc.2004-0785.
- Oki N, Matsuo H, Nakago S, Murakoshi H, Laoag-Fernandez JB, Maruo T. Effects of 3,5,3’-triiodothyronine on the invasive potential and the expression of integrins and matrix metalloproteinases in cultured early placental extravillous trophoblasts. J Clin Endocrinol Metab 2004;89:5213-21. https://doi.org/10.1210/jc.2004-0352.
- Roche Diagnostics GmbH . Reference Intervals for Children and Adults. Elecsys Thyroid Tests 2009.
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med 2015;373:2141-8. https://doi.org/10.1056/NEJMoa1504927.
- National Institute for Health and Care Excellence (NICE) . Assessment and Treatment for People With Fertility Problems 2013.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)70939-9.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med 2019;380:1316-25. https://doi.org/10.1056/NEJMoa1812537.
- Electronic Medicines Compendium (eMC) . Levothyroxine 25 Micrograms Tablets. Summary of Product Characteristics n.d. www.medicines.org.uk/emc/medicine/21395 (accessed 4 June 2018).
- Chatfield A, Caglia JM, Dhillon S, Hirst J, Cheikh Ismail L, Abawi K, et al. Translating research into practice: the introduction of the INTERGROWTH-21st package of clinical standards, tools and guidelines into policies, programmes and services. BJOG 2013;120:139-42. https://doi.org/10.1111/1471-0528.12416.
- Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol 1995;6:168-74. https://doi.org/10.1046/j.1469-0705.1995.06030168.x.
- Kim NY, Cho HJ, Kim HY, Yang KM, Ahn HK, Thornton S, et al. Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am J Reprod Immunol 2011;65:78-87. https://doi.org/10.1111/j.1600-0897.2010.00911.x.
- Debiève F, Dulière S, Bernard P, Hubinont C, De Nayer P, Daumerie C. To treat or not to treat euthyroid autoimmune disorder during pregnancy?. Gynecol Obstet Invest 2009;67:178-82. https://doi.org/10.1159/000185689.
- LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes?. Thyroid 2005;15:60-71. https://doi.org/10.1089/thy.2005.15.60.
- Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol 2006;107:337-41. https://doi.org/10.1097/01.AOG.0000197991.64246.9a.
- Whitcomb BW, Schisterman EF, Klebanoff MA, Baumgarten M, Luo X, Chegini N. Circulating levels of cytokines during pregnancy: thrombopoietin is elevated in miscarriage. Fertil Steril 2008;89:1795-802. https://doi.org/10.1016/j.fertnstert.2007.05.046.
- Whitcomb BW, Schisterman EF, Klebanoff MA, Baumgarten M, Rhoton-Vlasak A, Luo X, et al. Circulating chemokine levels and miscarriage. Am J Epidemiol 2007;166:323-31. https://doi.org/10.1093/aje/kwm084.
- Vasilopoulou E, Loubiere LS, Lash GE, Ohizua O, McCabe CJ, Franklyn JA, et al. Triiodothyronine regulates angiogenic growth factor and cytokine secretion by isolated human decidual cells in a cell-type specific and gestational age-dependent manner. Hum Reprod 2014;29:1161-72. https://doi.org/10.1093/humrep/deu046.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B 1995;57:289-300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
- Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med 2006;11:302-8. https://doi.org/10.1016/j.siny.2006.03.001.
- Kiyici S, Gul OO, Baskan EB, Hacioglu S, Budak F, Erturk E, et al. Effect of levothyroxine treatment on clinical symptoms and serum cytokine levels in euthyroid patients with chronic idiopathic urticaria and thyroid autoimmunity. Clin Exp Dermatol 2010;35:603-7. https://doi.org/10.1111/j.1365-2230.2009.03642.x.
- Turhan Iyidir O, Konca Degertekin C, Sonmez C, Atak Yucel A, Erdem M, Akturk M, et al. The effect of thyroid autoimmunity on T-cell responses in early pregnancy. J Reprod Immunol 2015;110:61-6. https://doi.org/10.1016/j.jri.2015.04.002.
- Joint Formulary Committee . British National Formulary 2017.
- Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Alavi Majd H, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 2017;176:253-65. https://doi.org/10.1530/EJE-16-0548.
- Col NF, Surks MI, Daniels GH. Subclinical thyroid disease: clinical applications. JAMA 2004;291:239-43. https://doi.org/10.1001/jama.291.2.239.
- Maraka S, Mwangi R, McCoy RG, Yao X, Sangaralingham LR, Singh Ospina NM, et al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ 2017;356. https://doi.org/10.1136/bmj.i6865.
Appendix 1 Search strategy
The search strategy used was taken directly from the systematic review performed prior to the trial. Reproduced with permission from Thangaratinam et al. 6 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.
We searched MEDLINE (1951–2011), EMBASE (1974–2011), The Cochrane Library (2011) and SciSearch (1974–2011) for relevant citations and examined the reference lists of all known primary and review articles to identify cited articles not captured by the electronic searches. Language restrictions were not applied. We used a combination of MeSH and text words to generate two subsets of citations: one indexing thyroid autoantibodies (‘thyroid autoimmune antibodies’, exp thyroid/AND exp antibodies, thyroid AND autoimmune AND antibodies) and the second indexing outcomes (‘miscarriage’, ‘abortion’, ‘pregnancy loss’, ‘preterm’, ‘premature’, ‘early labo(u)r’, ‘pret$’). These subsets were combined with ‘AND’ to generate a subset of citations relevant to our research question.
Full citation: Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011;342:d2616. 6
The search was continually updated throughout the trial to ensure that any new trials or evidence were included. The search was updated to February 2018 but including only RCTs. This resulted in new evidence from Wang et al. 11
Appendix 2 Proposed limits for thyroid function test results in the trial
| TSH (mlU/l) | Free T4 (pmol/l) | ||
|---|---|---|---|
| All trimesters | First and second trimesters | Third trimester | |
| Roche | < 4 | < 25 | < 20 |
| Abbott Architect | < 3.5 | < 22 | < 18 |
| Siemens | < 4 | < 22 | < 18 |
Appendix 3 Recruitment over time
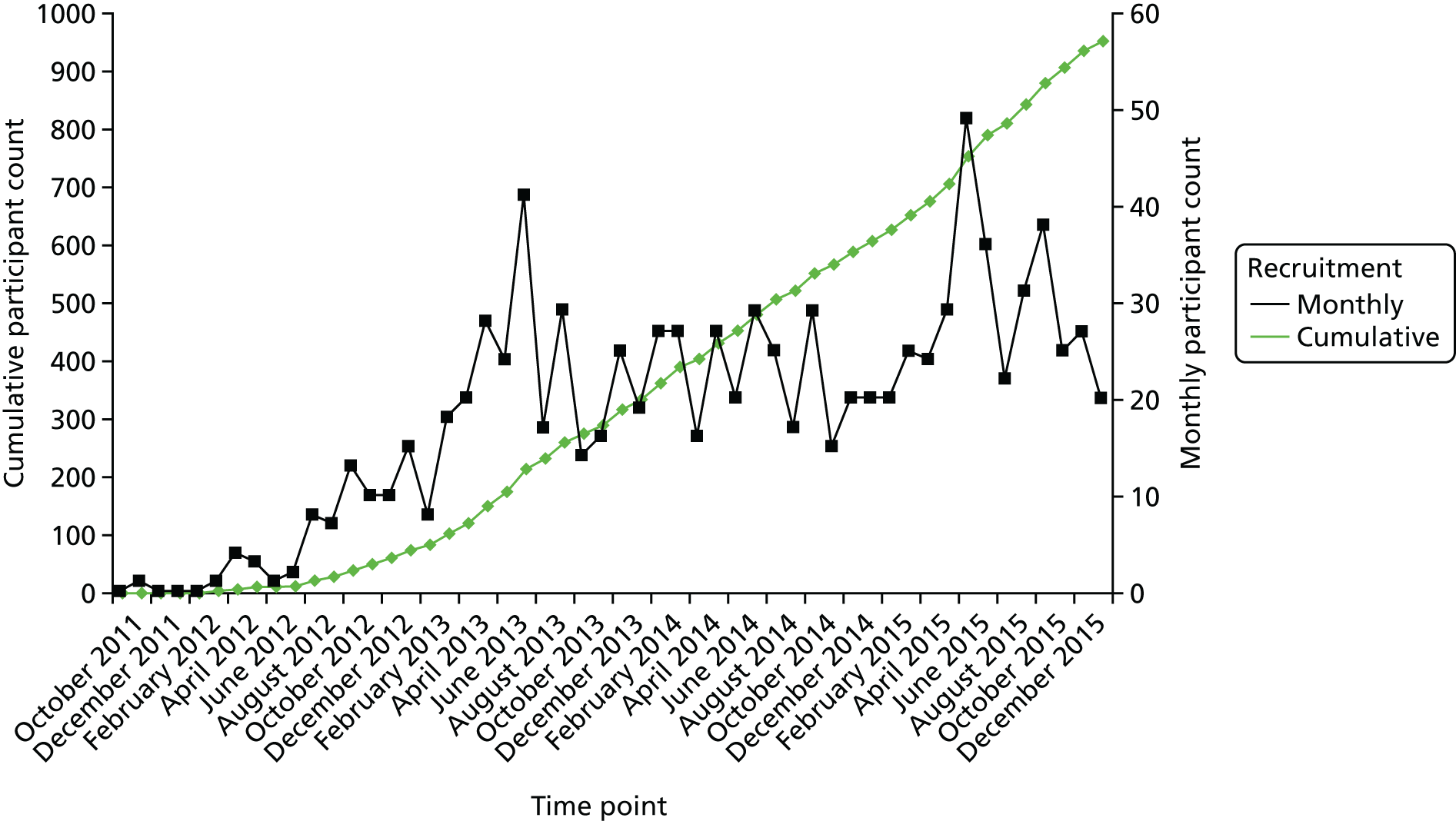
Appendix 4 Reported symptoms at each follow-up visit
| Type of symptom | Trial group, n/N (%) | |
|---|---|---|
| Levothyroxine | Placebo | |
| 3 months pre pregnancy | ||
| Participants with symptoms they are concerned about | 34/315 (11) | 45/306 (15) |
| Details | ||
| Anginal pain | 0/315 (0) | 0/306 (0) |
| Cardiac arrhythmias | 1/315 (< 1) | 0/306 (0) |
| Palpitations | 3/315 (< 1) | 4/306 (1) |
| Cramps in skeletal muscles | 0/315 (0) | 7/306 (2) |
| Tachycardia | 2/315 (< 1) | 1/306 (< 1) |
| Diarrhoea | 3/315 (1) | 1/306 (< 1) |
| Vomiting | 0/315 (0) | 1/306 (< 1) |
| Tremors | 0/315 (0) | 0/306 (0) |
| Insomnia | 3/315 (1) | 4/306 (1) |
| Headache | 7/315 (2) | 11/306 (4) |
| Flushing | 4/315 (1) | 8/306 (2) |
| Sweating | 3/315 (1) | 6/306 (1) |
| Excessive weight loss | 1/315 (< 1) | 1/306 (< 1) |
| Muscular weakness | 0/315 (0) | 1/306 (< 1) |
| Excitability | 0/45 (0) | 0/306 (0) |
| Restlessness | 1/315 (< 1) | 2/306 (1) |
| Other | 25/315 (8) | 37/306 (11) |
| 6 months pre pregnancy | ||
| Participants with symptoms they are concerned about | 17/180 (9) | 20/181 (12) |
| Details | ||
| Anginal pain | 1/180 (1) | 0/181 (0) |
| Cardiac arrhythmias | 0/180 (0) | 0/181 (0) |
| Palpitations | 2/180 (1) | 4/181 (2) |
| Cramps in skeletal muscles | 2/180 (1) | 1/181 (1) |
| Tachycardia | 0/180 (0) | 1/181 (1) |
| Diarrhoea | 1/180 (1) | 0/181 (0) |
| Vomiting | 1/180 (1) | 0/181 (0) |
| Tremors | 0/180 (0) | 1/181 (1) |
| Insomnia | 3/180 (2) | 0/181 (0) |
| Headache | 5/180 (3) | 2/181 (1) |
| Flushing | 0/180 (0) | 1/181 (1) |
| Sweating | 1/180 (1) | 1/181 (1) |
| Excessive weight loss | 0/180 (0) | 0/181 (0) |
| Muscular weakness | 0/180 (0) | 0/181 (0) |
| Excitability | 0/180 (0) | 0/181 (0) |
| Restlessness | 0/180 (0) | 1/181 (1) |
| Other | 14/180 (8) | 18/181 (10) |
| 9 months pre pregnancy | ||
| Participants with symptoms they are concerned about | 8/102 (8) | 5/126 (4) |
| Details | ||
| Anginal pain | 0/102 (0) | 0/126 (0) |
| Cardiac arrhythmias | 0/102 (0) | 0/126 (0) |
| Palpitations | 0/102 (0) | 1/126 (1) |
| Cramps in skeletal muscles | 0/102 (0) | 0/126 (0) |
| Tachycardia | 0/102 (0) | 0/126 (0) |
| Diarrhoea | 1/102 (1) | 0/126 (0) |
| Vomiting | 0/102 (0) | 0/126 (0) |
| Tremors | 0/102 (0) | 0/126 (0) |
| Insomnia | 1/102 (2) | 0/126 (0) |
| Headache | 2/102 (2) | 0/126 (0) |
| Flushing | 1/102 (1) | 0/126 (0) |
| Sweating | 1/102 (1) | 0/126 (0) |
| Excessive weight loss | 0/102 (0) | 0/126 (0) |
| Muscular weakness | 0/102 (0) | 0/126 (0) |
| Excitability | 0/102 (0) | 0/126 (0) |
| Restlessness | 0/102 (0) | 1/126 (1) |
| Other | 6/102 (6) | 4/126 (3) |
| 12 months pre pregnancy | ||
| Participants with symptoms they are concerned about | 3/86 (4) | 2/107 (2) |
| Details | ||
| Anginal pain | 0/86 (0) | 0/107 (0) |
| Cardiac arrhythmias | 0/86 (0) | 0/107 (0) |
| Palpitations | 0/86 (0) | 1/107 (1) |
| Cramps in skeletal muscles | 0/86 (0) | 0/107 (0) |
| Tachycardia | 0/86 (0) | 0/107 (0) |
| Diarrhoea | 0/86 (0) | 0/107 (0) |
| Vomiting | 0/86 (0) | 0/107 (0) |
| Tremors | 0/86 (0) | 0/107 (0) |
| Insomnia | 0/86 (0) | 0/107 (0) |
| Headache | 0/86 (0) | 0/107 (0) |
| Flushing | 0/86 (0) | 0/107 (0) |
| Sweating | 0/86 (0) | 0/107 (0) |
| Excessive weight loss | 1/86 (1) | 0/107 (0) |
| Muscular weakness | 0/86 (0) | 0/107 (0) |
| Excitability | 0/86 (0) | 0/107 (0) |
| Restlessness | 0/86 (0) | 0/107 (0) |
| Other | 2/86 (2) | 2/107 (1) |
| 6–8 weeks’ gestation | ||
| Participants with symptoms they are concerned about | 23/186 (12) | 21/193 (11) |
| Details | ||
| Anginal pain | 0/186 (0) | 0/193 (0) |
| Cardiac arrhythmias | 0/186 (0) | 0/193 (0) |
| Palpitations | 1/186 (< 1) | 1/193 (< 1) |
| Cramps in skeletal muscles | 0/186 (0) | 1/193 (< 1) |
| Tachycardia | 1/186 (< 1) | 0/193 (0) |
| Diarrhoea | 3/186 (2) | 2/193 (1) |
| Vomiting | 5/186 (3) | 1/193 (< 1) |
| Tremors | 0/186 (0) | 0/193 (0) |
| Insomnia | 1/186 (< 1) | 2/193 (1) |
| Headache | 4/186 (2) | 4/193 (2) |
| Flushing | 0/186 (0) | 5/193 (2) |
| Sweating | 0/186 (0) | 3/193 (2) |
| Excessive weight loss | 0/186 (0) | 0/193 (0) |
| Muscular weakness | 0/186 (0) | 0/193 (0) |
| Excitability | 0/186 (0) | 0/193 (0) |
| Restlessness | 0/186 (0) | 2/193 (1) |
| Other | 21/186 (11) | 10/193 (5) |
| 16–18 weeks’ gestation | ||
| Participants with symptoms they are concerned about | 12/143 (8) | 14/146 (10) |
| Details | ||
| Anginal pain | 0/143 (0) | 0/146 (0) |
| Cardiac arrhythmias | 0/143 (0) | 0/146 (0) |
| Palpitations | 1/143 (1) | 2/146 (1) |
| Cramps in skeletal muscles | 0/143 (0) | 0/146 (0) |
| Tachycardia | 0/143 (0) | 0/146 (0) |
| Diarrhoea | 1/143 (1) | 1/146 (1) |
| Vomiting | 2/143 (1) | 2/146 (1) |
| Tremors | 0/143 (0) | 0/146 (0) |
| Insomnia | 1/143 (1) | 2/146 (1) |
| Headache | 4/143 (3) | 6/146 (4) |
| Flushing | 0/143 (0) | 0/146 (0) |
| Sweating | 0/143 (0) | 0/146 (0) |
| Excessive weight loss | 0/143 (0) | 0/146 (0) |
| Muscular weakness | 0/143 (0) | 0/146 (0) |
| Excitability | 0/143 (0) | 0/146 (0) |
| Restlessness | 0/143 (0) | 1/146 (1) |
| Other | 9/143 (6) | 6/146 (4) |
| 28 weeks’ gestation | ||
| Participants with symptoms they are concerned about | 20/137 (15) | 9/133 (7) |
| Details | ||
| Anginal pain | 0/137 (0) | 0/133 (0) |
| Cardiac arrhythmias | 0/137 (0) | 0/133 (0) |
| Palpitations | 0/137 (0) | 2/133 (2) |
| Cramps in skeletal muscles | 1/137 (1) | 1/133 (1) |
| Tachycardia | 0/137 (0) | 1/133 (1) |
| Diarrhoea | 0/137 (0) | 0/133 (0) |
| Vomiting | 0/137 (0) | 0/133 (0) |
| Tremors | 0/137 (0) | 0/133 (0) |
| Insomnia | 3/137 (2) | 1/133 (1) |
| Headache | 2/137 (1) | 1/133 (1) |
| Flushing | 0/137 (0) | 0/133 (0) |
| Sweating | 1/137 (1) | 0/133 (0) |
| Excessive weight loss | 0/137 (0) | 0/133 (0) |
| Muscular weakness | 0/137 (0) | 0/133 (0) |
| Excitability | 0/137 (0) | 0/133 (0) |
| Restlessness | 0/137 (0) | 0/133 (0) |
| Other | 20/137 (15) | 6/133 (5) |
List of abbreviations
- AE
- adverse event
- Apgar
- appearance, pulse, grimace, activity and respiration
- BCTU
- University of Birmingham Clinical Trials Unit
- BMI
- body mass index
- BMJ
- British Medical Journal
- BNF
- British National Formulary
- CI
- confidence interval
- DMC
- Data Monitoring Committee
- ENA-78
- epithelial-derived neutrophil-activating peptide 78
- FDR
- false-discovery rate
- HDU
- high-dependency unit
- IFN-γ
- interferon gamma
- IIA
- Immunology, Immunochemistry and Allergy
- IL
- interleukin
- IMMQAS
- Immunology Quality Services
- IMP
- investigational medicinal product
- IQR
- interquartile range
- IVF
- in vitro fertilisation
- MeSH
- medical subject heading
- MIP
- macrophage inflammatory protein
- NEQAS
- National External Quality Assessment Service
- OR
- odds ratio
- PBMC
- peripheral blood mononuclear cell
- PC
- principal component
- PCA
- principal component analysis
- RANTES
- Regulated on Activation, Normal T cell Expressed and Secreted
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- T4
- thyroxine 4
- TABLET
- Thyroid AntiBodies and LEvoThyroxine
- TFT
- thyroid function test
- TMG
- Trial Management Group
- TNF-α
- tumour necrosis factor alpha
- TPO
- thyroid peroxidase
- TPOAb
- thyroid peroxidase antibody
- TSC
- Trial Steering Committee
- TSH
- thyroid-stimulating hormone
- VEGF
- vascular endothelial growth factor
