Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 10/90/22. The contractual start date was in June 2013. The final report began editorial review in October 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bryan Williams has received honoraria fees from C.H. Boehringer Sohn AG & Ko. KG (Ingelheim am Rhein, Germany), Daiichi Sankyo Company, Ltd (Tokyo, Japan), Pfizer Inc. (New York, NY, USA) and Servier Laboratories (Neuilly-sur-Seine, France). All other authors declare no competing interests in relation to this study. Support was also provided by the North Thames Local Clinical Research Network and the North Central and East London Comprehensive Research Network. Bryan Williams is a National Institute for Health Research Senior Investigator Emeritus and is supported by the National Institute for Health Research Biomedical Research Centre at University College London Hospitals and University College London.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Williams et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Hypertension and risk
High blood pressure (BP) is the leading preventable cause of premature morbidity and mortality worldwide. 1,2 In the UK, surveys suggest that at least 25% of the adult population is hypertensive (i.e. defined as having a seated clinic BP of ≥ 140/90 mmHg), rising to more than 50% in people aged over 65 years. 3 The benefits of treating hypertension has one of the largest evidence bases in medicine and is the cornerstone of national strategies to reduce cardiovascular disease (CVD) risk. Detection, treatment and monitoring of BP represents one of the commonest reasons for consultation in primary care and in the UK accounts for over £1B expenditure on drug costs alone. 4
The dilemma of when to treat hypertension in younger patients
The decision to treat hypertension is based not only on the level of BP but also on an individual’s risk of CVD4 as indicated by:
-
the presence of existing CVD
-
the presence of hypertension-mediated organ damage (HMOD) [e.g. left ventricular (LV) hypertrophy, markers of renal disease, the presence of hypertensive retinopathy] or
-
when cardiovascular risk is calculated to be high, based on formal estimation using cardiovascular risk calculators.
Age is a major determinant of cardiovascular risk;5 consequently, most research has focused on the treatment of older people, who are more likely to develop measurable clinical end points within the typical duration of clinical trials, such as morbidity and mortality resulting from stroke and heart disease. Consequently, most of the evidence used to formulate treatment guidelines has been acquired from people over 55 years of age and there is a paucity of research data on which to base treatment decisions for younger people with hypertension. 6–8 This is compounded by the fact that conventional clinical outcome randomised controlled trials (RCTs) are not feasible in younger patients with hypertension because the traditional outcome measures (e.g. myocardial infarction, heart failure, stroke and death) are unlikely to occur in sufficient frequency, if at all, over the typical RCT duration. This has created a major dilemma in formulating guidelines for treating high BP: how and when to treat younger people with hypertension.
In the UK, the National Institute for Health and Care Excellence (NICE)’s guidelines recommend treatment of hypertension in all patients when the brachial BP (BrBP) is ≥ 160/100 mmHg (i.e. grade 2 hypertension). 4 However, for younger people (i.e. those aged < 55 years) with less severe hypertension, that is, grade 1 hypertension (BrBP 140–159/90–99 mmHg), there is uncertainty about the benefits of treatment unless the patient already has evidence of overt HMOD, established CVD, chronic kidney disease or diabetes mellitus. 9–11 Moreover, the use of formal 10-year cardiovascular risk calculations has little validity in these younger age groups and is unlikely to accurately reflect their lifetime risk.
The dilemma of when to treat younger people with grade 1 hypertension is not insignificant. A study involving over 30 years of follow-up of over 1 million young male Swedish army recruits suggested that grade 1 hypertension in younger people is unlikely to be benign and is associated with an increased risk of cardiovascular mortality. 12,13 Other long-term follow-up studies of university students and other younger people with early, mild elevations in BP, have reported an increased incidence of CVD and mortality later in life. 14–17 Important questions arise from these observations:
-
whether or not all lower-risk younger patients with grade 1 hypertension would benefit from treatment of their hypertension
-
whether or not it is possible to identify a subset of low-risk younger people with grade 1 hypertension who might particularly benefit from early treatment
-
whether or not early treatment brings about significant clinical benefit in younger people.
Rationale for the TREAT CASP study
To evaluate a strategy to better identify younger people with grade 1 hypertension who might benefit from early treatment of their blood pressure
Although the routine diagnosis of hypertension relies on the long-established technique of measurement over the brachial artery using an inflatable cuff, it has long been recognised that pressure [particularly systolic and pulse pressure (PP)] is amplified as it moves from the aortic root to the peripheral circulation (i.e. the brachial artery). 18 Thus, pressure measured over the brachial artery may not always reflect pressure in the central aorta, which may be better related to HMOD. 19,20
We and others have shown that the magnitude in amplification of brachial systolic BP (BrSBP) from the central aorta to the brachial artery can vary markedly and is profoundly influenced by age, sex, vascular disease (especially aortic arteriosclerosis and stiffening), heart rate and drug therapies used to treat hypertension. 21–26 Importantly, we and others have shown that the variation in the relationship between central aortic systolic pressure (CASP) and BrSBP might be particularly marked in younger people. 22,27 The difference between CASP and BrSBP is typically around 10 mmHg in older people (i.e. those aged > 55 years), but can be as much as 30 mmHg in younger people. 28 What has remained unclear is the frequency of low CASP in patients with grade 1 hypertension. Nevertheless, it has been suggested that an elevated BrSBP in younger people may sometimes be spurious when it is associated with a much lower CASP value,29–40 implying that brachial BP measurements may not always be the best way to categorise hypertension in younger people or stratify their need for BP-lowering treatment. An inaccurate diagnosis of hypertension can have important implications for younger people, not only because of the anxiety and cost of treatment but also because of the impact of disease labelling on insurance weighting and some forms of employment. Therefore, it is particularly important to establish the correct diagnosis in younger people so as to avoid these consequences and exposure to lifelong treatment that may not be necessary.
Measurement of central aortic systolic pressure
Although CASP is best measured using a pressure sensor advanced into the ascending aorta (as is done in cardiac catheterisation), this is clearly not feasible for routine care and, consequently, techniques for the non-invasive assessment of CASP have been developed. These techniques involve the sampling of arterial pressure waveforms using either a pressure sensor applied over an accessible artery (applanation tonometry)41–44 or sampled using a non-occluding cuff. 45–47 The resultant waveforms are then calibrated to a contemporaneous measurement of BrBP and mathematically transformed using a Fourier transfer function, or similar mathematical filter, to reveal the unamplified aortic pressure at the aortic root. We previously described and validated a simple, non-invasive method to measure CASP. 103 The method used a pressure sensor mounted in the flexible strap of a wristwatch-type device. Accurate placement of the pressure sensor (tonometer) over the radial artery allows the capture of the arterial waveform. This waveform has no pressure indices but is calibrated to the brachial artery pressure to generate a pressure waveform. The pressure waveform is then processed using an n-point moving average to derive the central aortic pressure. This methodology has been shown by us and others to generate accurate assessments of CASP when validated versus invasive catheterisation studies. 48–50
Potential value in non-invasive central aortic systolic pressure measurement
A number of studies have evaluated the value of invasively and non-invasively acquired aortic pressures versus conventional brachial pressures with regard to predicting cardiovascular organ damage, differential effects of drug therapy and clinical outcomes. 26,51–57 Population-based studies have shown that aortic pressures are more strongly related than brachial pressures to markers of HMOD in people with hypertension, for example left ventricular hypertrophy (LVH), carotid intima/media thickness and albuminuria. 19,20,58 In addition, we and others have shown that, despite similar effects of BP-lowering drug therapies on brachial pressures, there are differential effects of different drug treatments on brachial and central aortic pressures. 26,59–63 Moreover, the treatments associated with more effective central aortic pressure lowering were also associated with more effective reduction of clinical outcomes, especially stroke. 26,51,53,54 Other population-based observational studies have shown that when central aortic and brachial pressures have been measured, central aortic pressures have been a better predictor of clinical outcomes. 56,64 Together, this provided proof of concept for the hypothesis that central aortic pressure measurement could provide a more accurate biomarker of HMOD and disease risk in younger people with hypertension and, thus, a more effective clinical tool to stratify risk and the need for treatment.
Defining high and low central aortic systolic pressure values in younger people with grade 1 hypertension
The TREAT CASP study was designed to stratify younger people into high CASP (higher risk) and low CASP (lower risk). Therefore, we needed to define the threshold value defining high and low CASP in people with grade 1 hypertension. A definitive analysis of all available studies was published in 2014,28 including 45,436 people who had measurements of both conventional BrBP and CASP. In this report,28 11,402 patients were hypertensive (i.e. had a BrBP of ≥ 140/90 mmHg), of whom 3288 had grade 1 hypertension (consistent with the target population for the TREAT CASP study). In total, 18,183 were normal healthy people.
Based on an analysis of these data, for the population we intended to study, that is, younger males aged 18–55 years with grade 1 hypertension, a high CASP value was reported to be ≥ 125 mmHg. In fact, according to these data, no normotensive male patients in any age grouping had a median CASP above 116 mmHg. Thus, a CASP value of ≥ 125 mmHg is certainly high relative to a normotensive population. We used this threshold for the definition of high CASP in the TREAT CASP study because this CASP level is well above normal CASP levels for a healthy normotensive population. Low CASP for the TREAT CASP population was defined as < 125 mmHg, creating a CASP threshold for consideration of drug treatment analogous to conventional brachial BP thresholds for treatment decisions.
Evaluating the benefit of treatment in young people with grade 1 hypertension
A key challenge in evaluating potential benefits of treatment in younger people with grade 1 hypertension is demonstrating clinical benefit in terms of risk reduction. As discussed in The dilemma of when to treat hypertension in younger patients, this is usually done through RCTs demonstrating a reduction in major cardiovascular events. This is not feasible in younger people over the time course of a typical clinical trial as such events rarely occur. An alternative strategy has been to use reliable surrogate markers of subsequent clinical events. A logical surrogate is one that can be unequivocally attributed to elevated BP and is known to be associated with an increased risk of cardiovascular morbidity and mortality. One of the earliest consequences of high BP is remodelling of the left ventricle, characterised by LVH. 65–71 LVH has been shown to have strong prognostic significance with regard to risk of future cardiovascular events and mortality. 72,73 These early BP-related structural changes are often subclinical, that is, they are not detected by routine screening in primary care. However, they are readily detectable by non-invasive imaging, typically by echocardiography, but also more recently by cardiac magnetic resonance imaging (cMRI) studies. 74–76
This study will use cMRI quantification of LV mass as a definitive indicator of elevated pressure, and to determine whether or not treatment provides benefit by regression of BP-related cardiac structural change. cMRI quantification of LV mass is considered more accurate than echocardiographic or electrocardiographic measures. cMRI also allows quantification of cardiovascular function, such as regional wall motion and diastolic function, using new analytical methods. 77–80
Only men will be recruited into this study because the inclusion of younger women could expose women who become pregnant during the study to medications that are contraindicated during pregnancy. This also avoids the confounding effects of the oral contraceptive pill on BP81 and well-recognised sex differences in LV mass, which could have introduced variation requiring a larger study. 82–84
Summary of the rationale for a randomised controlled trial of blood pressure lowering in younger patients with low-risk grade 1 hypertension and high central aortic systolic pressure
There is currently uncertainty about whether or not younger people with uncomplicated grade 1 hypertension should be treated with BP-lowering medications. This study examines the hypothesis that the non-invasive measurement of CASP in younger people (i.e. those aged < 55 years) with uncomplicated grade 1 hypertension will help stratify which of these people might benefit from treatment (1) by showing that those people with high CASP will have greater LV mass than those people with low CASP, and (2) that BP-lowering treatment in those people with high CASP will regress LV mass versus no treatment, thereby confirming that their elevated pressure was contributing to an increased LV mass even at this early stage of hypertension.
Chapter 2 Methods
Study setting
The TREAT CASP study was a single-centre study recruiting at multiple research sites. The co-ordinating centre and main recruiting site was at University College London (UCL) and its associated hospital, University College London Hospitals (UCLH). Study clinical procedures were carried out at a dedicated National Institute for Health Research (NIHR) clinical research facility at UCLH and/or in some primary care sites. cMRI was performed using a research-dedicated magnetic resonance imaging (MRI) scanner within UCL’s Institute of Cardiovascular Science, based at the Great Ormond Street Hospital. The study commenced on 1 November 2013 and the final study visit was on 7 February 2018. The study received ethics approval from the National Research Ethics Service London (Bloomsbury) committee in June 2013 and was conducted in accordance with the principles of good clinical practice (GCP). The UK Medicines and Healthcare products Research Authority (MHRA) judged that the TREAT CASP study was not a clinical trial of an investigational medicinal product (CTIMP). The study was run in association with the UCL Comprehensive Clinical Trials Unit (CCTU) and was overseen by an Independent Trial Steering Committee.
Study design
The TREAT CASP study used a two-stage design (Figure 1), incorporating:
-
stage 1 – a cross-sectional screening study to identify patients with grade 1 hypertension who were then invited to participate in stage 2
-
stage 2 – a 12-month RCT.
FIGURE 1.
Study design flow chart. ABPM, ambulatory BP monitoring; CKD, chronic kidney disease; LVMI, left ventricular mass index; ΔLVMI, change in left ventricular mass index. ABPM range represents waking (daytime) mean.

There was a stop–go checkpoint at the end of the screening study (see Stop–go: checkpoint for proceeding from stage 1 screening study to stage 2 randomised controlled trial and observational study). Participants with grade 1 hypertension completing the screening study were stratified into two groups according to their CASP status: participants with a low CASP (i.e. those with a CASP of < 125 mmHg) or participants with a high CASP (i.e. those with a CASP of ≥ 125 mmHg).
In the randomised controlled trial (RCT), participants were randomised (simple one-to-one randomisation; sealed envelope™; Sealed Envelope Ltd, London, UK; www.sealedenvelope.com; accessed 1 February 2017) to either BP lowering using a national guideline-directed treatment or a no-treatment group (i.e. usual care in the UK for participants with grade 1 hypertension). Follow-up was for 12 months with a cMRI scan at baseline and study closeout. The study utilised a prospective, randomised, open-label, blinded end-point (PROBE) design to evaluate whether or not people from the high CASP group benefit from BP-lowering drugs compared with no treatment, with regard to regression of their LV mass on cMRI. LV mass was indexed to body size, deriving the LV mass index (LVMI). The change in LVMI from baseline to 12 months, comparing the treatment versus no-treatment arms, constituted the primary outcome for the TREAT CASP study.
Alongside the RCT, the second stage of the study incorporated an observational follow-up study in those participants with grade 1 hypertension and low CASP, who remained untreated for BP over 12 months’ follow-up. The observational follow-up study was designed to evaluate whether or not there was any progression of cardiac structural change (as measured by the LVMI) in people with grade 1 hypertension and low CASP over a period of 12 months and to provide a reference value against which regression of cardiac structure in the RCT could be assessed.
Recruitment strategy: stage 1 screening study
Potential participants for the TREAT CASP study were identified from a number of sources:
-
patients referred to the BP outpatient clinic at UCLH
-
from general practice in association with liaison with the local North Central London Research Consortium (Noclor) or from searches of general practitioner (GP) databases within the greater London area by the NIHR-funded Clinical Practice Research Datalink (CPRD) organisation
-
responses to advertisements placed in various media or prominent local buildings in London, for example railway stations, restaurants and other buildings for social functions.
Participants
The TREAT CASP study recruited male patients aged between 18 and < 55 years, who had uncomplicated, untreated grade 1 hypertension [with a seated clinic BP of 140–159/90–99 mmHg and/or a 24-hour ambulatory BP monitoring (ABPM) daytime mean of ≥ 135/85 mmHg] and, if previously treated for hypertension, for whom BP-lowering treatment had been discontinued for at least 3 months prior to screening. Study participants were also required to be low-risk grade 1 hypertension, that is, that they had no pre-existing CVD or diabetes mellitus or chronic kidney disease and no evidence of HMOD [i.e. electrocardiography (ECG) LVH, grade 2 or more hypertensive retinopathy or renal impairment or an increased urinary albumin-to-creatinine ratio (UACR)].
Inclusion criteria
Inclusion criteria were men who:
-
were aged 18 to < 55 years
-
had a clinical diagnosis of grade 1 hypertension based on a seated clinic BP of 140–159/90–99 mmHg, and/or ABPM
-
were currently not being treated for hypertension (if previously treated with antihypertensive medication, patients will have received no treatment for > 3 months)
-
were without evidence of HMOD on routine clinical screening, established CVD, diabetes mellitus or chronic kidney disease
-
were willing and capable of giving informed consent.
Exclusion criteria
Exclusion criteria included:
-
women of any age
-
men –
-
with grade 2 hypertension on clinic BP
-
with ‘white coat hypertension’, that is, grade 1 hypertension according to clinic blood BP but a normal BP on ABPM (daytime mean BP of < 135/85 mmHg)
-
who had a prior diagnosis of a secondary cause for hypertension, for example renal artery stenosis, primary aldosteronism, phaeochromocytoma, aortic coarctation
-
who had grade 1 hypertension with evidence of HMOD on routine clinical testing (e.g. LVH, hypertensive retinopathy, renal impairment, proteinuria) or concurrent CVD (e.g. stroke, transient ischaemic attack, cardiac or peripheral vascular disease) and/or diabetes mellitus or chronic kidney disease – that is, risk factors that would mandate treatment of grade 1 hypertension according to current NICE guidance [NICE Clinical Guidance (CG) 1274]
-
in whom it was not possible to measure conventional BrBP
-
with atrial fibrillation or any other significant pulse rhythm irregularity, making BP measurement difficult and unreliable
-
who regularly consumed > 28 units of alcohol per week or used recreational drugs
-
who had chronic inflammatory diseases requiring regular concomitant steroids and/or non-steroidal anti-inflammatory drugs
-
who had severe hepatic impairment
-
who had previous hypersensitivity to the drugs to be used in the study
-
who had current malignancy
-
who were unwilling to undergo, or had a contraindication to, MRI scanning
-
who had currently or recently (i.e. in the last 6 weeks) participated in an interventional clinical trial
-
who had any clinical condition for which the investigator would consider the patient unsuitable for the trial
-
who had co-enrolled in any CTIMP or any other study of a non-investigational medicinal product (there was no prohibition on co-enrolment into observational studies).
-
Withdrawal criteria
Participants had the right to withdraw from the study at any time, for any reason and without giving a reason. The investigator also had the right to withdraw patients from the study in the event of intercurrent illness, adverse events (AEs), serious adverse events (SAEs), suspected unexpected serious adverse reactions (SUSARs), protocol violations or other reasons. If a patient decided to withdraw from the study, all efforts were made to report the reason for withdrawal. Participants randomised to study intervention who then declined to take any study medication were withdrawn from the study with no further study follow-up. For participants electing to withdraw, having commenced taking their study medication, efforts were made to continue to obtain follow-up data (with the permission of the patient).
All recruited participants were required to provide written informed consent after reviewing the detailed study information prior to the consent process.
Stage 1 screening study
In order to recruit men with uncomplicated grade 1 hypertension, the first stage of the TREAT CASP study comprised a screening study. Information about the study was disseminated widely via advertisements in local or national press. Participants with a history of elevated BP, who remained untreated, were sought through searches of local GP databases and through local specialist hypertension clinics. Men responding to study advertisements or who were identified via their GP records as previously having an elevated BP in the grade 1 hypertension range and no other CVD were invited to participate in the screening study.
The screenees were invited to attend for an initial BP check (i.e. a prescreen) where they underwent a measurement of seated clinic BP and CASP. Participants with elevated clinic BP were asked to undergo 24-hour ambulatory BP monitoring to confirm the diagnosis of hypertension and to exclude white coat hypertension. Prescreening was essential to filter out, at an early stage, participants who were clearly normotensive or those who had grade 2 or higher BP and would require treatment according to current national treatment guidelines. Participants identified with grade 1 hypertension and who met study inclusion criteria were then invited to undergo detailed cardiovascular screening.
Clinical assessments performed during the detailed cardiovascular screening included:
-
documentation of medical and lifestyle history
-
physical examination
-
measurement of seated BrBP and CASP
-
height and weight measurement
-
waist and hip circumference measurement
-
body fat composition using non-invasive bioimpedance
-
blood tests – including haematological, biochemical, lipid profile, glucose and renal function [i.e. estimated glomerular filtration rate (eGFR)] assessments
-
UACR analysis/urine dipstick for blood and protein
-
12-lead ECG.
The TREAT CASP screening study was designed to generate a cohort of participants with confirmed grade 1 hypertension who were then stratified according to their CASP measurement as high CASP (i.e. a CASP of ≥ 125 mmHg) or low CASP (i.e. a CASP of < 125 mmHg) prior to entry to stage 2 of the study, the high CASP RCT and the low CASP observational study.
Defining high and low central aortic systolic pressure
When the study was designed, there was no existing definition of high versus low CASP. The original plan was to screen approximately 500 patients in stage 1 of the study and then identify a high CASP cohort for the RCT from the upper half of the distribution and the low CASP cohort from the lower half of the distribution. This became unnecessary when population threshold values for the low and high CASP groups were defined following publication of a definitive analysis of all available studies worldwide, including 45,436 people who had measurements of both conventional BrBP and CASP. 28 From these data, a high CASP threshold of ≥ 125 mmHg was defined for the TREAT CASP study (see Chapter 1, Defining high and low central aortic systolic pressure values in younger people with grade 1 hypertension).
Stop–go: checkpoint for proceeding from stage 1 screening to stage 2 randomised controlled trial and observational study
At the time the study was designed, it was unknown whether or not CASP would be related to LVMI, although this seemed highly likely as CASP is mathematically derived from BrBP and CASP is the pressure closest to the left ventricle. To avoid futility in undertaking the RCT if high CASP was not associated with an increased LVMI, a stop–go checkpoint was requested by the funder and this was introduced into the study protocol. The stop–go checkpoint required confirmation (evidence) that there was a significant difference in LVMI between men in the low CASP and men in the high CASP groups prior to commencement of the RCT. This required all screenees considered suitable for the RCT and observational study (i.e. ≈ 160 participants) to undergo baseline cMRI. The cMRI scan would then be evaluated to confirm that the stop–go checkpoint had been satisfied, before any patient could be randomised into the RCT in the second stage of the study. The implications of this stop–go checkpoint soon became apparent. Patients screened and eligible for stage 2 of the study would have to wait until the entire stage 1 screening study had been completed before they could enter stage 2. Moreover, once screening had been completed, ≈ 160 cMRI scans would have to be performed and evaluated within a few weeks. This was logistically very difficult for the study team and the participants. Moreover, it meant that some of the baseline cMRIs for the RCT would have been performed a few weeks, or even months, before randomisation into the RCT, which was undesirable.
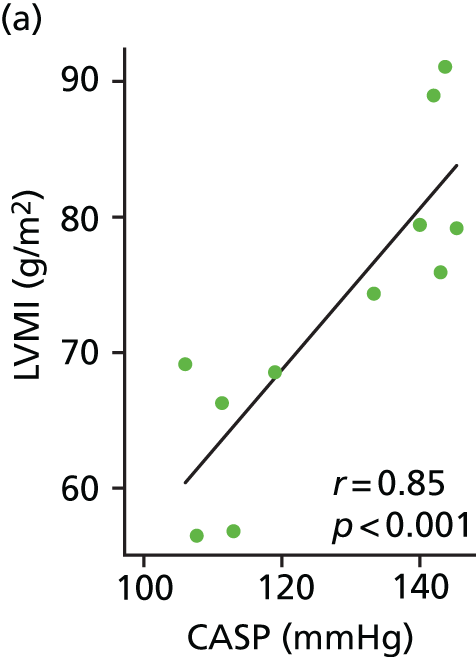
To evaluate the need for such an extensive and detailed cMRI study to confirm the stop–go criterion, we undertook an exploratory study in which we evaluated cMRI LVMI data on 11 patients from the high and low CASP groups we had identified during screening. The exploratory study showed a clear and significant association between high CASP and increased LVMI relative to the low CASP patients (Figure 2). It was agreed with the funder, based on this analysis, that it was highly likely that the stop–go criterion would be satisfied once all baseline cMRIs had been performed (which it was – see Chapter 3, Retrospective evaluation of the stop–go criterion based on baseline cardiac magnetic resonance imaging data from the stage 2 studies); to avoid further delay it was agreed that the study should proceed to stage 2.
FIGURE 2.
Relationship between LVMI and CASP (a) and LVMI and 24-hour ambulatory SBP (b) in a preliminary subset (n = 11) of stage 1 study participants undergoing cMRI.

The study proceded to the stage 2 RCT and observational study. Patients considered eligible for the high CASP RCT and low CASP observational study then had to be re-contacted to undergo baseline cMRI scans and enter the observational study or the RCT. Inevitably, following the delay in starting stage 2 of the study, some of the patients deemed eligible when they were originally screened were either not contactable or no longer interested, or were no longer eligible because their BP status had changed or they had been treated. This meant that we had to continue to recruit further patients to ensure that a sufficient number of patients were entered into the RCT.
Stipulating a stop–go requirement for this study was not without consequences. It resulted in patients eligible for the study when originally screened waiting for up to 2 years before they could enter the RCT, rather than immediately transitioning from screening to the RCT. Moreover, the need to complete all screening before any patients could be entered into the RCT, and the subsequent need to undertake further screening, resulted in an overall delay in completion of the study by approximately 2 years. This resulted in 98 patients originally identified as eligible and consented for the study subsequently being unable to enter the RCT or observational study (lost contact or no longer willing or change in BP status or treated) as a result of the delays in completing the stop–go decision. This meant that we had to undertake further recruitment alongside the initiation of the second phase of the study. The stop–go checkpoint was retrospectively evaluated in participants who went on to complete the study and confirmed the prediction obtained from the exploratory cMRI scans (see Chapter 3, Retrospective evaluation of the stop–go criterion based on baseline cardiac magnetic resonance imaging data from the stage 2 studies).
It was originally planned to screen approximately 500 participants to yield at least 50 evaluable patients for each arm of the RCT (treatment vs. no treatment) and at least a further 50 participants with low CASP to enter the observational study. The first participant was recruited into the stage 1 screening study on 15 November 2013. The first participants for the observational study and RCT were recruited on 18 August 2015 and 21 October 2015, respectively. The final participant was recruited into stage 2 of the study on 8 February 2017 and the last patient visit for the RCT was on 14 February 2018 when trial follow-up had been completed. A total of 726 men were screened for the stage 1 study and 162 men entered the stage 2 RCT and observational studies.
Stage 2 randomised controlled trial and observational study
The second stage of the TREAT CASP study incorporated a RCT of BP lowering for men with grade 1 hypertension and high CASP. The RCT was designed to evaluate whether or not treatment regresses LVMI relative to the comparator, that is, no treatment, which represents usual clinical care for these people. Participants with grade 1 hypertension and low CASP were entered into an observational study and remained untreated. Participants in both the RCT and the observational study were followed up over the course of 12 months and underwent cMRI at both baseline and at the end of the study (i.e. study closeout).
The RCT used a prospective, randomised, open, blinded, end-point (PROBE) design85 to allow the BP-lowering treatment to be titrated to achieve a CASP reduction of at least 5 mmHg in the treatment arm participants. Study team members involved in the MRI measurement and/or analysis were blinded to treatment allocation.
Baseline visit procedures for the randomised controlled trial and observational study
Participants with grade 1 hypertension and high CASP underwent a baseline cardiovascular evaluation to reconfirm their eligibility for the study and to collect baseline data. The baseline examination included:
-
documentation of medical and lifestyle history
-
measurement of seated BrBP and CASP
-
24-hour ABPM
-
a physical examination
-
height and weight measurement
-
waist and hip circumference measurement
-
body fat composition evaluation using non-invasive bioimpedance
-
blood tests – including haematological, biochemical, lipid profile, glucose and renal function (i.e. eGFR) assessments
-
UACR analysis (analysed by an accredited external laboratory, specifically by The Doctors Laboratory, London, UK)/urine dipstick analysis for blood and protein
-
a 12-lead ECG
-
non-invasive measurement of arterial stiffness [as assessed via the cardio-ankle vascular index (CAVI)]
-
quality-of-life assessment using the Short Form questionnaire-36 items (SF-36)
-
non-mydriatic retinal photography
-
1.5-T cardiovascular MRI scan.
Randomisation
Participants in the RCT were randomised to BP-lowering treatment or no intervention. Randomisation (simple, one-to-one allocation ratio using the next available treatment in the randomisation list) was performed using a web-based randomisation service (Sealed Envelope Ltd).
Blood pressure-lowering intervention
The BP-lowering treatment used for this study was as recommended by NICE4 for this age group. BP-lowering treatment was up-titrated according to NICE4 to achieve a seated CASP value of < 120 mmHg and a minimum 5-mmHg reduction in CASP from baseline. The intervention comprised the angiotensin receptor blocker (ARB) losartan [50–100 mg once daily (o.d.)] plus the calcium channel blocker (CCB) amlodipine (5–10 mg o.d.) when required (Table 1). For men of black African ethnicity, consistent with NICE guidance,4 treatment commenced with the CCB to which an ARB was added if required. Participants allocated to treatment began taking their medication 1 day after their baseline cMRI scan. There was no wash-out or run-in period as the RCT was conducted in treatment-naive participants or previously treated participants who had not taken BP-lowering medication for at least 3 months prior to randomisation. For participants unable to tolerate a higher dose of medication, treatment could be back-titrated to achieve the best CASP reduction that could be tolerated.
| White or Asian ethnicity | Black African ethnicity | DDD |
|---|---|---|
| 50 mg o.d. losartan | 5 mg o.d. amlodipine | 1 |
| 100 mg o.d. losartan | 5 mg o.d. amlodipine + 50 mg o.d. losartan | 2 |
| 100 mg o.d. losartan + 5 mg o.d. amlodipine | 5 mg o.d. amlodipine + 100 mg o.d. losartan | 3 |
| 100 mg o.d. losartan + 10 mg o.d. amlodipine | 10 mg o.d. amlodipine + 100 mg o.d. losartan | 4 |
The comparator was no treatment (i.e. usual care); no placebo was used in this open-label study. The intervention was prescribed unblinded and was dispensed from the hospital pharmacy.
Concomitant medication was permitted with the exception of other BP-lowering medications.
Randomised controlled trial follow-up schedule and procedures
Follow-up visits for participants randomised to treatment were scheduled at 4, 8, 12, 16, 26 and 38 weeks to allow for study drug titrations. Participants randomised to no treatment attended follow-up visits at 12 and 26 weeks. Follow-up visits included measurements of seated brachial clinic BP and CASP, and documentation of AEs. Follow-up appointments were scheduled ± 2 weeks of the due date. Study closeout occurred after 12 months of study participation. This terminology (‘study closeout’) is used throughout this report to refer to the end of follow-up. Study closeout procedures, including the final cMRI, were identical to those at baseline; however, a physical examination was not performed unless indicated (Table 2).
| Study activities | Vist and visit name | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Baseline | MRI scan | 4-week FU | 8-week FU | 12-week FU | 16-week FU | 26-week FU | 38-week FU | Study closeout | MRI scan | |
| Brachial BP monitoring | ✗ | O | O | ✗ | O | ✗ | O | ✗ | ||
| CASP monitoring | ✗ | O | O | ✗ | O | ✗ | O | ✗ | ||
| ABPM | ✗ | ✗ | ||||||||
| SF-36 QoL questionnaire | ✗ | ✗ | ||||||||
| Anthropometric data collected | ✗ | ✗ | ||||||||
| ECG | ✗ | ✗ | ||||||||
| CAVI | ✗ | ✗ | ||||||||
| Urine tests | ✗ | ✗ | ||||||||
| Blood tests | ✗ | ✗ | ||||||||
| Retinal photography | ✗ | ✗ | ||||||||
| Physical examination | ✗ | |||||||||
| MRI scan | ✗ | ✗ | ||||||||
| AEs | ✗ | ✗ | O | O | ✗ | O | ✗ | O | ✗ | ✗ |
| Dispense medication | O | O | O | O | O | O | O | O | O | O |
Observational study
A 12-month observational follow-up study of participants with grade 1 hypertension and low CASP was performed in parallel with the RCT. Participants in the observational study received no intervention and the visit schedule was identical to that of the no-treatment group for the RCT. Concomitant medication (other than BP-lowering medication) was allowed.
Study outcomes
First phase of the study: relationship between central aortic systolic pressure and left ventricular mass index
The first objective of the study was to determine whether or not CASP was related to LVMI on cMRI, specifically whether or not high CASP was associated with greater LVMI than low CASP. This formed the stop–go checkpoint for the RCT. The first phase of the study also allowed evaluation of whether or not CASP was more strongly related to LVMI than conventional clinic BP or ABPM, which would suggest that CASP measurement may be a better means of stratifying younger patients with grade 1 hypertension for treatment.
Second phase of the study: randomised controlled trial to determine if reducing central aortic systolic pressure in patients with high central aortic systolic pressure leads to a reduction of left ventricular mass index versus no treatment
Primary outcome of the randomised controlled trial
The primary outcome for the TREAT CASP RCT was the change in LVMI evaluated by cMRI, between baseline and study closeout (i.e. 12 months following treatment initiation). The RCT compared active BP-lowering treatment with usual care (no treatment) in participants with high CASP. LVMI is expressed as left ventricular mass (in grams) indexed to body size (body surface area calculated using the method of Dubois86) and expressed as g/m2.
Secondary outcomes
A number of secondary outcomes were prespecified for the TREAT CASP RCT, which were designed to evaluate mechanisms and explore the relationship between CASP and conventional BrBP on extended markers of cardiac and vascular damage, both in cross-sectional evaluations at the end of stage 1 and in stage 2, at baseline and study closeout (i.e. after 12 months’ follow-up of the low CASP and the high CASP participant groups).
Prespecified secondary outcomes for the TREAT CASP study are as follows:
-
Un-indexed LV mass.
-
cMRI measurements of regional systolic and diastolic strain – early markers of hypertension-mediated damage.
-
cMRI assessments of cardiac output/stroke volume/peripheral resistance – markers of cardiac function and assessments of mechanisms for BP elevation.
-
cMRI measurement of central aortic pressure.
-
cMRI measurement of diastolic function using ventricular inlet/outlet flow measurements – sensitive markers of early pressure-mediated cardiac functional change.
-
cMRI motion vector analysis of systolic and diastolic function.
-
cMRI assessment of coronary perfusion reserve – markers of coronary perfusion.
-
cMRI-measured aortic distensibility and pulse wave velocity (PWV) at differing locations – sensitive indices of aortic stiffening. This tests our subsidiary hypothesis that increased aortic stiffness causes the detrimental increase in CASP relative to BrBP.
-
cMRI measurements of aortic blood flow and diameter – markers of aortic function.
-
LV mass-to-volume ratio on cMRI.
-
MRI measurement of body fat distribution – sensitive marker of early obesity-related changes, which may have an impact on BP and cardiovascular risk.
-
Non-mydriatic retinal photography for analysis of changes to retinal vasculature architecture.
-
Urinary albumin excretion rate measured as UACR – an index of early renal injury.
-
Electrocardiography-derived parameters including ECG LVH, Q–T dispersion, Cornell voltage, Sokolow–Lyon voltage, augmented vector left (aVL) voltage, etc. – ECG markers of cardiac structure/function.
-
CAVI and related parameters.
-
Blood pressure in relation to all study outcomes measured as a variety of parameters, including clinic-measured BP, central aortic BP, ambulatory BP, home-measured BP, MRI-derived central aortic BP, BP variability.
-
Quality-of-life assessments using the SF-36.
Statistical analysis plan
Sample size estimates
This study was planned to detect a mean change in LVMI (primary outcome) of 6.6 g/m2 with BP lowering over the course of 12 months of treatment. This was based on limited previously published data investigating the effects of BP-lowering treatment on cMRI-determined LVMI, usually in older people with more severe hypertension and existing CVD. Data from four individual treatment studies comprising seven active treatment arms in 627 patients, with a mean patient age of 60 ± 3.4 years,87–90 indicated an average difference in LVMI between treatments (treatment vs. no treatment) of 6.6 g/m2 [standard deviation (SD) 10.9 g/m2]. All treatments in the cited studies used inhibitors of the renin–angiotensin system and achieved moderate reductions in BP (average reduction with treatment –8.0/–4.5 mmHg). Based on an anticipated difference in LVMI between treatments of 6.6 g/m2 (SD 10.9 g/m2), power calculation indicated that a sample size of 58 people per treatment arm would be required to show a difference with 90% power at a p-value of 0.05 in LVMI between the treatment and no-treatment groups for the RCT.
Once the TREAT CASP RCT was under way, the change in BP for the study was estimated to be approximately –16/–10 mmHg. From the study power calculation, this suggested that the fall in LVMI might be as high as –14 g/m2. Using a conservative estimation of a fall in LVMI of 10 g/m2, a revised power calculation was undertaken and presented to the TREAT CASP Steering Committee. This power calculation indicated that a sample size of 44 study participants per treatment arm would show a difference in LVMI between treatment arms with 90% power at a p-value of 0.05. To allow for dropout or loss to follow-up, a revised sample size of 50 study participants per treatment arm was proposed for the study. This revised power calculation and sample size were approved by the Trial Steering Committee in April 2016 and a statement specifying that the objective to recruit at least 50 patients to each arm of the TREAT CASP RCT was added to the final protocol.
The duration of treatment in the TREAT CASP RCT was 12 months. The duration of 12 months was based on prior studies of LVMI change in response to BP-lowering therapy, which indicated that the majority of LVMI regression occurs within the first 12 months of treatment, with very little additional change with longer-term periods of follow-up (i.e. up to 5 years). 91,92 This is because LVMI regression is powerfully determined by the extent of the reduction in BP on treatment, and the BP change is maximal during the first year of therapy. The 12-month duration was chosen to maximise the change in LVMI within a reasonable time scale for the RCT.
In addition to powering the study primary end point, the inbuilt stop–go checkpoint was powered to detect a difference in LVMI of 10 g/m2. Previously published studies indicated that LVMI is directly related to BP and that the relationship between the change in systolic blood pressure (SBP) and the change in LVMI approximates to the order of 1 g/m2 LVMI per mmHg SBP. 93,94 This order of magnitude of change is similar to that seen for the regression in LVMI with BP-lowering treatment as shown above (i.e. an average reduction in SBP of –8 mmHg and an average reduction in LVMI with treatment of –6.6 g/m2). Preliminary data from our previous studies in men with grade 1 hypertension stratified by their CASP values had indicated that groups at the extremes of the highest and lowest CASP values are separated by a BrSBP of at least 10 mmHg. This suggested potential separation in LVMI of up to 10 g/m2 between men with high and low CASP. With regard to a starting value, a review of LVMI values by MRI in 1032 young men from 11 published studies yielded a mean LVMI value of 78.5 g/m2 (SD 11.8 g/m2). 74,95–102 Combining an estimated 10 g/m2 difference between the high and low CASP groups with the SD for LVMI in young men indicated that a sample size of 31 per group would be required to demonstrate a difference in LVMI with 90% power at a p-value of 0.05 between men with high and low CASP.
Statistical analysis
Statistical analyses were performed using Stata® (version 14.2; StataCorp LP, College Station, TX, USA) or RStudio (version 3.4.1; RStudio, Inc., Boston, MA, USA).
Univariate linear regression was used to evaluate whether or not CASP had a stronger correlation with LVMI than the other BP measurements (i.e. clinic BP and ABPM). Correlations between LVMI and the three BP measurement strategies (CASP, clinic BP and ABPM) were compared using Fisher’s r-to-z transformation. Regression slopes and intercepts were compared using a comparison of estimates from a seemingly unrelated regressions procedure, with testing using a Wald test. Data for the stop–go checkpoint, to evaluate whether or not there was a difference in LVMI between participants with low or high CASP, used a Student’s t-test.
Data for the TREAT CASP RCT are presented for the intention-to-treat population. The primary outcome for assessment was the change in LVMI over the 12-month follow-up period and was assessed in all participants with LVMI data at baseline and study closeout, as prespecified in the study protocol. The change in LVMI was compared between the two groups using a Student’s t-test without adjustment. The effect size was estimated using Cohen’s d statistic. Adjustment for potential confounders of the change in LVMI or other secondary outcomes were made using multivariable models. Additional variables included baseline LVMI (or the baseline value for the adjusted variable), baseline age, brachial artery BP, smoking history and measures of adiposity, such as body mass index (BMI). Other variables were added where appropriate. Careful attention to the adjusted R2 of the models was given to avoid overfitting. Thus, coefficients that only significantly improved the fit of the models or where a strong case for their known biological importance could be made were included. Missing data in multivariable models were imputed as the mean of data for that variable for each treatment arm.
Data at baseline or for the change (i.e. study closeout minus baseline) in various parameters between individual arms of the TREAT CASP RCT and the observational study were compared using one-way analysis of variance, with adjustment for multiple comparisons. Correlations between LVMI and other parameters were assessed using Pearson’s correlation coefficient.
Data distributions were assessed and, where plausible, parametric analyses were used with appropriate transformation to normality if necessary, for example log-transformation for right-skewed data.
Study procedures/experimental assessments
Clinic blood pressure measurement
Brachial clinic BP was evaluated at heart level over the upper arm with a suitably sized cuff, using a validated oscillometric monitor (OMRON 705CP-II; Omron Corporation, Kyoto, Japan). Measurements were taken in standardised conditions with the study participant seated comfortably and relaxed for at least 5 minutes, with their back and arm supported and the middle of the upper arm at heart level, legs uncrossed and feet flat on the floor. Participants were requested not to talk during BP measurement. A minimum of three measurements (up to a maximum of six measurements) were taken 1 minute apart, with measurements continued until three consecutive SBP and diastolic blood pressure (DBP) readings within 10 mmHg were achieved. The mean of the last two readings was used to define the BrBP. During the TREAT CASP RCT and observational study, measurements were taken over both arms at baseline, with the first arm to be measured chosen at random. The BP from the arm with the highest CASP value was taken as the BrBP and that same arm in each participant was used for successive measurements during follow-up.
Central aortic systolic pressure measurement
Non-invasive CASP measurement was measured using applanation tonometry with the BPro® device (Healthstats International Pte Ltd, Singapore). The technique uses a sensitive pressure sensor (i.e. a tonometer) to capture high-fidelity radial artery pulse waves at the wrist, which are calibrated to a contemporaneous measurement of BrBP. Mathematical processing using an n-point moving average103 generates a moving average array, from which CASP is derived. Pulse waves were sampled for 10 seconds immediately following complete cuff deflation for each individual BP measurement taken, as described in Clinic blood pressure measurement. The resulting ensemble-averaged pressure waves were calibrated to the previously measured oscillometric SBP and DBP. As with BrBP, CASP was calculated as the average from the last two BP measurements.
Ambulatory blood pressure monitoring
Twenty-four-hour ambulatory BP was measured using a validated oscillometric device (90207-30/90207-1Q/90217-1Q; Spacelabs Healthcare, Hertford, UK). Measurements were taken using a suitably sized cuff over the participant’s non-dominant arm every 30 minutes during waking hours and every 60 minutes during sleep, over the course of 24 hours. Participants were asked to complete a diary documenting the time at which they went to sleep and wakened and other activities that might have influenced their BP during the measurement period. Data were analysed as 24-hour, waking (daytime) and sleeping (night-time) averages based on data from the patient diary. A minimum of 14 waking and seven sleeping measurements were required for each 24-hour measurement to be analysed.
Twelve-lead electrocardiography
Study participants rested supine for ≈ 5 minutes prior to undergoing a 12-lead ECG (ASSY CAM-14 version 2; GE Medical Systems Information Technologies Inc., Wauwatosa, WI, USA). Data were acquired using a chart speed of 25 mm/second at a sampling frequency of 100 Hz. Data were collected and sorted on a dedicated laptop for the study using the GE Medical Systems Cardiosoft software (Cardiosoft version 6.73; GE Healthcare, Chicago, IL, USA). Data were exported from extensible markup language (XML) files directly into the study database.
Phlebotomy/urine sampling
Non-fasting venous blood samples were obtained by venepuncture from a forearm antecubital vein, dispensed into collection tubes [i.e. K2 EDTA or FX sodium fluoride/potassium oxalate or BD Vacutainer™ SST™ II Advance Tubes (BD Vacutainer Systems; Plymouth, UK)] and analysed by an NHS-accredited external laboratory (Health Services Laboratories, London, UK) for haematology and clinical chemistry.
Mid-stream urine samples were collected and dipstick assessment of blood, protein and glucose (Multistix® 10SG; Siemens Healthcare Diagnostics, Camberley, UK) was performed. Samples were also sent for analysis of sodium, potassium and UACR at an accredited external laboratory (Health Services Laboratories).
Height, weight and anthropometric data
The patients’ heights and weights were measured using a stadiometer (Seca 217; Seca Ltd, Birmingham, UK) and weighing scales (Seca 761; Seca Ltd). Hip and waist circumferences were evaluated with a measuring tape.
Body composition by bioelectrical impedance
Body composition was evaluated for weight, total body fat mass, trunk fat mass, total body impedance, total body water and trunk fat-free mass using a bioelectrical impedance device (BC-418 Body Composition Analyser; Tanita Europe BV, Amsterdam, the Netherlands).
Magnetic resonance imaging
Magnetic resonance imaging was carried out at the UCL Institute of Cardiovascular Science Imaging Centre at Great Ormond Street Hospital in London. All examinations were performed using a five-element phased-array coil set up on a 1.5-T magnetic resonance imager (MAGNETOM® Avanto; Siemens Healthineers AG, Erlangen, Germany). A vector electrocardiographic system was used for cardiac gating. All patients underwent a standard clinical cMRI examination. In all patients, following acquisition of scout images, this examination included ventricular volumetric assessment (using cardiac-gated breath-hold cine imaging of the ventricular short axis), ventricular volume assessment [using real-time imaging – radial k-t sensitivity-encoding (SENSE) imaging] and high-resolution imaging of aortic flow and diameter, which was performed during breath-hold together with phase-contrast imaging. In addition, diastolic function assessment (E/A ratio) was made using spiral real-time unfold SENSE phase-contrast imaging together with tissue phase mapping imaging in a short-axis mid-ventricular slice. Finally, T2-IDEAL (iterative decomposition of water and fat with echo asymmetry and least squares estimation) imaging of pericardial and whole-body fat was performed. Details of the imaging protocol are shown in Appendix 1.
For analysis of ventricular volumes and mass for the primary end point, epicardial and endocardial contours were drawn across a short-axis stack of 10-mm thick sections using the OsiriX DICOM imaging platform (version 8.5.1; Pixmeo Sàrl, Bernex, Switzerland). Image frames corresponding to end-systole and end-diastole were selected in a mid-ventricular slice and epicardial and endocardial contours drawn manually for each slice in the short-axis stack. Trabeculae and papillary muscle were not excluded. Volumes and mass at end-systole and end-diastole were calculated using a custom-written plug-in module. Stroke volume was cross-referenced to flow, as measured in the ascending aorta. All MRI scans were analysed by two independent observers blinded to treatment allocation of patients.
The intraclass correlation coefficient (ICC) was used to assess agreement for two observers. ICC at baseline was 0.90 [95% confidence interval (CI) 0.84 to 0.93] for LVMI at end-systole and 0.90 (95% CI 0.85 to 0.93) and for LVMI diastole. At study closeout, the ICC was 0.89 (95% CI 0.83 to 0.93) for LVMI at end-systole and 0.89 (95% CI 0.85 to 0.92) for LVMI at end-diastole. Therefore, the primary end point for LVMI and secondary end point for LV mass is displayed as averaged data for the two observers.
Quality-of-life questionnaire: Short Form questionnaire-36 items
Quality of life and changes to quality of life were assessed using the standard Short Form questionnaire-36 items (SF-36). 78 These questionnaires were administered by the study nurse or research assistant at baseline and study closeout and completed by participants in both the TREAT CASP RCT and the observational study.
Cardio-ankle vascular index
The CAVI is a non-invasive measurement of arterial stiffness and function104,105 and was measured non-invasively using simultaneous cuff-based oscillometric BP measurements over both arms and ankles, using a dedicated device (i.e. the vascular screening system VS-1500N; Fukuda Denshi Co., Ltd, Tokyo, Japan). CAVI reflects stiffness of the whole arterial segment composed of the aorta, femoral artery and tibial artery. CAVI is analogous to the established arterial parameter, stiffness index β and was calculated from the PWV over this arterial segment together with BP measured over the brachial artery. PWV was determined from the pulse transit time over various arterial segments and referenced to a cardiac phonogram. Measurement of the distance between sites was then used in the calculation of PWV. CAVI measurements were performed using the vascular profiling device (VS-1500N) at baseline and study closeout for the TREAT CASP RCT and the observational study.
Retinal photography
Fundal images were acquired using a dedicated retinal camera (Canon CR-DGi; Canon Inc., Tokyo, Japan). Images were taken in a darkened room without mydriasis acquiring a 45° view in each eye, centred between the optic nerve and the macula. Images were acquired in TREAT CASP RCT and observational study follow-up participants at baseline and study closeout.
Physical examination
A standard physical examination was carried out by the study doctor.
Adverse event reporting
The pharmacological intervention used in the RCT was medication routinely prescribed according to national guidelines for the treatment of hypertension in the UK both as monotherapy and combination therapy, and, for this reason, the intervention has a good safety profile. Moreover, the UK’s MHRA determined on more than one occasion that the RCT part of this study did not constitute a CTIMP. Accordingly, an independent data monitoring and ethics committee was not required for safety monitoring. Nevertheless, data on AEs, adverse reactions (ARs) and SAEs were routinely collected at study visits and recorded in the participant’s notes. In the event of ARs inconsistent with the known safety profile of the study medication, the intention was to report these via the Yellow Card Scheme through the MHRA website106 and to report such events to the study sponsor as SUSARS. By the end of the study, no such reports were deemed necessary to be made.
The severity of AEs was assessed by the study physician and the chief investigator, with reference to the study protocol and the World Health Organization (WHO)’s toxicity grading. 107 Expectedness and causality of AEs were also assessed by the study doctor and chief investigator with regard to clinical judgement and the known safety profile of the medications as set out in national texts, for example the British National Formulary. 108
Study monitoring/Comprehensive Clinical Trials Unit involvement
The quality assurance (QA) and quality control (QC) considerations for the TREAT CASP RCT were based on standard UCL CCTU quality management policy that included a formal risk assessment, and that acknowledged the risk associated with the conduct of the trial and proposals of how to mitigate them through appropriate QA and QC processes. Risks were defined in terms of their impact on the rights and safety of participants; project concept including trial design, reliability of results and institutional risk; project management; and other considerations.
Quality assurance was defined as all the planned and systematic actions established to ensure that the trial was performed and data generated, documented and/or recorded and reported in compliance with the approved protocol, the principles of GCP and applicable regulatory requirements. QC was defined as the operational techniques and activities performed within the QA system to verify that the requirements for quality of the trial-related activities were fulfilled. A risk assessment was completed for the TREAT CASP RCT and reviewed by the UCL CCTU Quality Management Group. UCL CCTU staff reviewed case report forms and electronic data for errors and missing key data points.
Trial oversight was intended to preserve the integrity of the trial by independently verifying a variety of processes and prompting corrective action where necessary. The processes reviewed related to participant enrolment, consent, eligibility and allocation to trial groups; adherence to trial interventions and policies to protect participants, including the reporting of harms; and completeness, accuracy and timeliness of data collection. Independent trial oversight complied with the UCL CCTU trial oversight policy.
Protocol amendments and changes to recruitment strategy
The stage 1 screening study began in November 2013. By September 2014, recruitment was behind schedule and it was clear that age was a constraint to recruitment (the study’s original design had been to recruit patients up to the age of 40 years). Moreover, many recruited participants who attended the study centre for a detailed cardiovascular investigation were not suitable for further study participation because their ambulatory BP monitoring, performed at the end of the study screening visit, revealed that their BP was normal despite a high-seated clinic BP.
To address these concerns, a substantial amendment was approved in February 2014 to raise the maximum age of study participants from 40 to 55 years. This change was made in order to increase study recruitment and to maintain consistency with NICE guidelines,4 which use a cut-off point of 55 years for defining treatment strategies for younger and older patients. In addition, an amendment was made to introduce a prescreening BP check (i.e. measurement of both clinic BP and ambulatory BP) to identify participants who were normotensive or had higher grade hypertension, prior to undergoing a detailed cardiovascular evaluation. This complemented changes to the study’s recruitment strategy, which became more focused on direct advertising to the general public because searches of GP databases for patients identified as having grade 1 hypertension did not provide a reliable source of study recruits, as many were normotensive on more formal testing of their BP.
We were encouraged to engage with the CPRD, which took considerable time and effort, only to find that the return from this method of recruitment was very disappointing because, unbeknown to us at the time, CPRD relied on data solely acquired from GP records using the VISION database system. The VISION database mainly covered outer London areas and not our immediate locality (i.e. inner London), where most GPs were using EMIS (Egton Medical Information Systems) Health, which was not accessible to the CPRD at the time. Moreover, even when letters were dispatched to potential participants via the CPRD, the response was very poor. It was therefore decided to stop using the CPRD and focus our recruitment effort on local advertising, which was the most effective strategy and more straightforward.
To further improve recruitment, in 2016 the study team worked with the local NIHR network, Noclor and recruitment sites were established in local general practice surgeries, where prescreening activities (recruitment, clinic and ambulatory BP measurement) were performed by Noclor research nurses after training by our study staff. Participants undergoing successful prescreening were then invited to attend for a detailed cardiovascular evaluation at the UCL/UCLH clinical research facility. Owing to the length of time required to recruit all the study participants, compounded by delays introduced by the stop–go process (see Stop–go: checkpoint for proceeding from stage 1 screening to stage 2 randomised controlled trial and observational study), a 1-year no-cost extension was granted to the study in 2016.
Chapter 3 Results
Study recruitment: stage 1 screening study
Participants were recruited into the stage 1 screening study between 15 November 2013 and 19 January 2017. The final patient was recruited into the stage 1 screening study in January 2017.
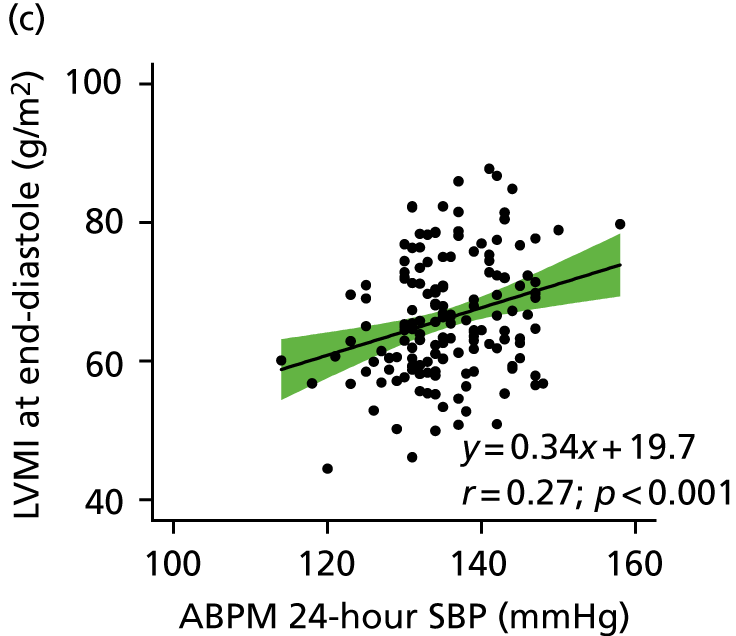
From a total of 726 participants recruited into the study, 424 (58%) were excluded either at their prescreening visit or following the detailed cardiovascular examination, predominantly because their BP was outside the grade 1 BP range. Thus, 302 participants were confirmed with grade 1 hypertension based on clinic-measured BP and/or ABPM criteria. Of these 302 participants, using a CASP cut-off point of ≥ 125 mmHg, 200 had high CASP, 100 had low CASP and CASP was not measurable in two participants. Figure 3 shows the normal distribution of CASP in the patients with grade 1 hypertension identified in the screening study. Figure 4 shows the study Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
FIGURE 3.
Distribution of CASP values from the screening study population with grade 1 hypertension (300 participants with grade 1 hypertension and measurable CASP values). The cut-off point for high and low CASP was ≥ 125 mmHg and is shown by the vertical line.
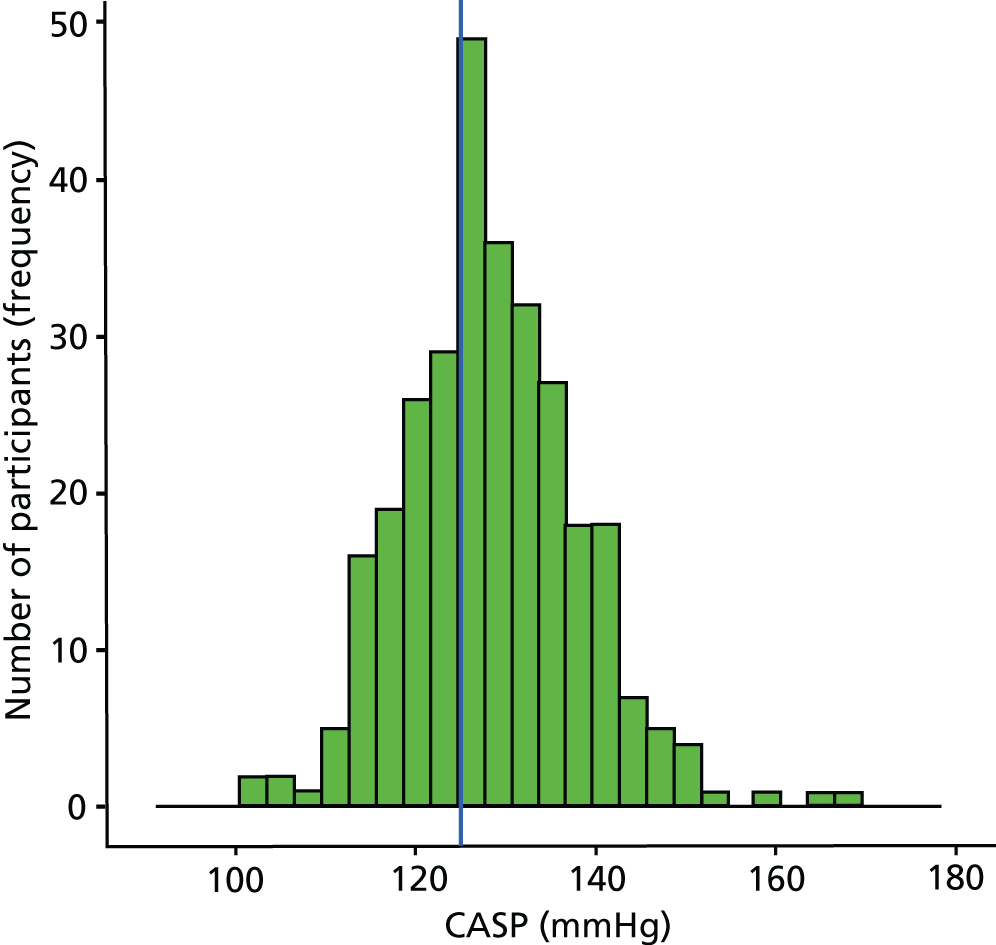
FIGURE 4.
The TREAT CASP RCT CONSORT flow diagram.

The TREAT CASP stage 1 screening study population
Stage 1 screening study demographics
The demographics and clinical characteristics of the 302 participants identified in the stage 1 screening study with grade 1 hypertension, stratified by their CASP status, are shown in Tables 3 and 4. The mean age (± SD) of the participants was 44.0 ± 7.8 years, with a seated clinic SBP of 144.4 ± 10.0 mmHg and DBP of 87.2 ± 7.2 mmHg. Baseline characteristics were similar between the high and the low CASP groups, except that men in the high CASP group were slightly older and had higher BP measurements (both clinic and ambulatory). There was no difference in cardiovascular risk score [QRISK®-lifetime or QRISK 2 at age 60 years (ClinRisk Ltd, Leeds, UK)] between the groups. By design, CASP was higher in the high CASP group. As expected, pressure amplification (expressed as BrSBP minus CASP, SBP amplification and PP amplification) was significantly lower for men in the high CASP group than in the low CASP group (low CASP vs. high CASP – BrSBP minus CASP difference: 1.75 mmHg, 95% CI 0.3 to 3.2 mmHg; p = 0.01; SBP amplification difference 0.03, 95% CI 0.01 to 0.04; p < 0.001; PP amplification difference 0.11, 95% CI 0.08 to 0.15; p < 0.001).
| Characteristic | Group | All (n = 302) | Difference (low CASP – high CASP), mean (95% CI) | |
|---|---|---|---|---|
| Low CASP (n = 100) | High CASP (n = 200) | |||
| Age (years), mean ± SD | 41.0 ± 8.4 | 45.5 ± 7.0 | 44.0 ± 7.8 | –4.50 (–6.39 to –2.54) |
| Height (cm), mean ± SD | 177.4 ± 7.0 | 177.4 ± 7.6 | 177.4 ± 7.3 | 0.05 (–1.74 to 1.84) |
| Weight (kg), mean ± SD | 87.5 ± 14.2 | 88.6 ± 15.5 | 88.2 ± 15.0 | –1.11 (–4.76 to 2.54) |
| BMI (kg/m2), mean ± SD | 27.8 ± 4.0 | 28.1 ± 4.1 | 28.0 ± 4.0 | –0.32 (–1.30 to 0.65) |
| Waist-to-hip ratio, mean ± SD | 0.97 ± 0.1 | 0.99 ± 0.1 | 0.98 ± 0.1 | –0.01 (–0.03 to 0.00) |
| Trunk fat (kg), mean ± SD | 12.5 ± 4.5 | 12.9 ± 5.1 | 12.7 ± 4.9 | –0.42 (–1.65 to 0.80) |
| QRISK 2 at 60 years (%), mean ± SD | 8.0 ± 5.1 | 7.7 ± 4.7 | 7.8 ± 4.8 | 0.29 (–0.88 to 1.47) |
| QRISK-lifetime (%), mean ± SD | 45.5 ± 17.1 | 47.0 ± 17.3 | 46.5 ± 17.2 | –1.49 (–5.69 to 2.71) |
| Total-to-HDL cholesterol ratio, mean ± SD | 4.3 ± 1.4 | 4.2 ± 1.4 | 4.2 ± 1.4 | 0.10 (–0.25 to 0.45) |
| Current smoker, n (%) | 8 (8.0) | 5 (2.5) | 13 (4.3) | |
| Ethnicity, n (%) | ||||
| White | 69 (69.7) | 152 (78.8) | 223 (75.9) | |
| Mixed race | 2 (2.0) | 3 (1.6) | 5 (1.7) | |
| Asian | 17 (17.2) | 23 (11.9) | 40 (13.6) | |
| Black | 9 (9.1) | 13 (6.7) | 22 (7.5) | |
| Chinese | 2 (2.0) | 2 (1.0) | 4 (1.4) | |
| Characteristic | Group, mean ± SD | All (n = 302), mean ± SD | Difference (low CASP – high CASP), mean (95% CI) | |
|---|---|---|---|---|
| Low CASP (n = 100) | High CASP (n = 200) | |||
| Clinic SBP (mmHg) | 136.6 ± 7.8 | 148.4 ± 8.6 | 144.4 ± 10.0 | –11.85 (–13.85 to –9.84) |
| Clinic DBP (mmHg) | 82.9 ± 6.6 | 89.4 ± 6.3 | 87.2 ± 7.2 | –6.55 (–8.10 to –5.00) |
| Heart rate (beats per minute) | 73.0 ± 12.6 | 68.9 ± 11.4 | 70.3 ± 12.0 | 4.16 (1.31 to 7.02) |
| Clinic CASP (mmHg) | 118.6 ± 4.8 | 133.7 ± 7.4 | 128.6 ± 9.8 | –15.1 (–16.54 to –3.74) |
| SBP minus CASP (mmHg) | 14.5 ± 6.4 | 12.7 ± 4.9 | 13.3 ± 5.5 | 1.75 (0.31 to 3.19) |
| SBP amplification | 1.12 ± 0.05 | 1.10 ± 0.04 | 1.10 ± 0.05 | 0.03 (0.01 to 0.04) |
| PP amplification | 1.42 ± 0.16 | 1.31 ± 0.12 | 1.35 ± 0.15 | 0.11 (0.08 to 0.15) |
| ABPM 24-hour SBP (mmHg) | 132.4 ± 6.6 | 136.4 ± 6.2 | 135.0 ± 6.6 | –4.02 (–5.56 to –2.48) |
| ABPM 24-hour DBP (mmHg) | 83.8 ± 5.3 | 86.2 ± 5.4 | 85.3 ± 5.5 | –2.44 (–3.73 to –1.14) |
Transition to the stage 2 TREAT CASP randomised controlled trial and the observational cohort study
As indicated in Stage 1 screening study demographics, 302 patients were identified who were eligible to enter the TREAT CASP RCT and the observational study. However, the stop–go checkpoint between stage 1 and stage 2 of the study meant that the screening study had to be completed before any of these patients could transition to the stage 2 RCT and observational studies.
As a consequence, many patients had to be re-contacted and re-screened many months, and, in some cases, almost 2 years, after they were originally deemed eligible. As a result, 98 potential participants in stage 2 were not contactable, were not available or were no longer eligible for further study participation. This reduced the pool of people available for recruitment into the second stage to 204 (see Figure 4). Of these 204 participants, a further 42 were excluded on re-screening as a result of the delayed start of stage 2, predominantly because of a change in their BP status while awaiting the stop–go decision. The remaining 162 men entered stage 2 of the study. Of these, 57 with low CASP entered the observational study and 105 with high CASP were randomised into the TREAT CASP RCT. For the RCT, 51 men were randomised to receive BP-lowering treatment and 54 men were randomised to no treatment. During follow-up, 17 participants (seven randomised to treatment, five randomised to no treatment and five participants in the observational study) were excluded or lost to study follow-up. Reasons for exclusion or loss are shown in Figure 4. By the end of the study, 145 men had completed follow-up, with 44 in the treatment arm, 49 in the no-treatment arm and 52 in the observational study.
Retrospective evaluation of the stop–go criterion based on baseline cardiac magnetic resonance imaging data from the stage 2 studies
The stop–go decision was taken to proceed to the second phase of the study (i.e. the TREAT CASP RCT and the observational study) on the basis of a limited number of cMRI scans (see Figure 2) that demonstrated that patients with high CASP had a higher LVMI than patients with low CASP. This decision was then retrospectively evaluated from the much larger number of cMRI data collected at baseline for the stage 2 study in patients with high and low CASP. Comparison between participants with high CASP (i.e. TREAT CASP RCT participants) and low CASP (i.e. the observational study participants) demonstrated a significant difference in LVMI between groups [low CASP LVMI (end-systole): 64.0 ± 8.5 g/m2 (n = 54); high CASP LVMI (end-systole): 67.9 ± 8.8 g/m2 (n = 101); difference 4.0 g/m2, 95% CI 1.1 to 6.9 g/m2; p < 0.01; low CASP LVMI (end-diastole): 63.9 ± 8.3 g/m2 (n = 54); high CASP LVMI (end-diastole): 67.5 ± 8.7 g/m2 (n = 101); difference 3.7 g/m2, 95% CI 0.8 to 6.5 g/m2; p = 0.01]. This confirmed that the decision to proceed to the second-stage RCT was appropriate.
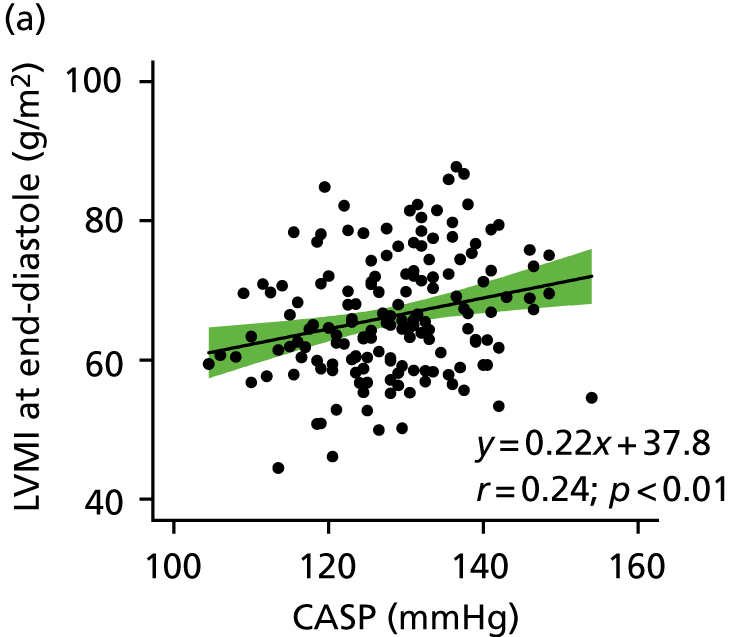
The relationship between CASP and LVMI by linear regression for cMRI scans performed at baseline is shown in Figures 5 and 6. A clear and positive relationship between LVMI and CASP was seen and a higher CASP was associated with a higher LVMI. The relationships with clinic-measured and ambulatory SBP and LVMI are also shown in Figures 5 and 6. Regression analysis of the relationship between LVMI and BP showed similar regression coefficients and slopes, irrespective of the modality of BP measurement (ABPM, clinic BP or CASP).
FIGURE 5.
Relationship between LVMI at end-systole and central aortic, clinic-measured and ambulatory SBP. The regression line is shown with 95% CI.
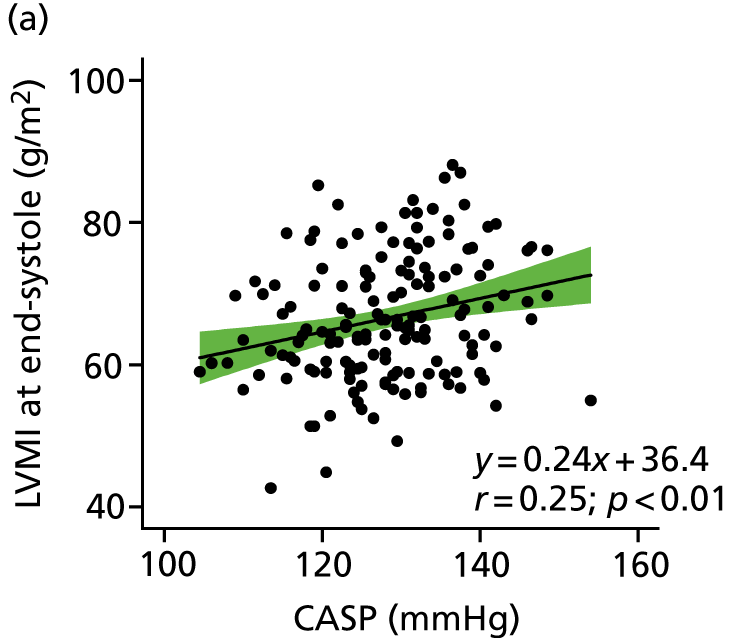

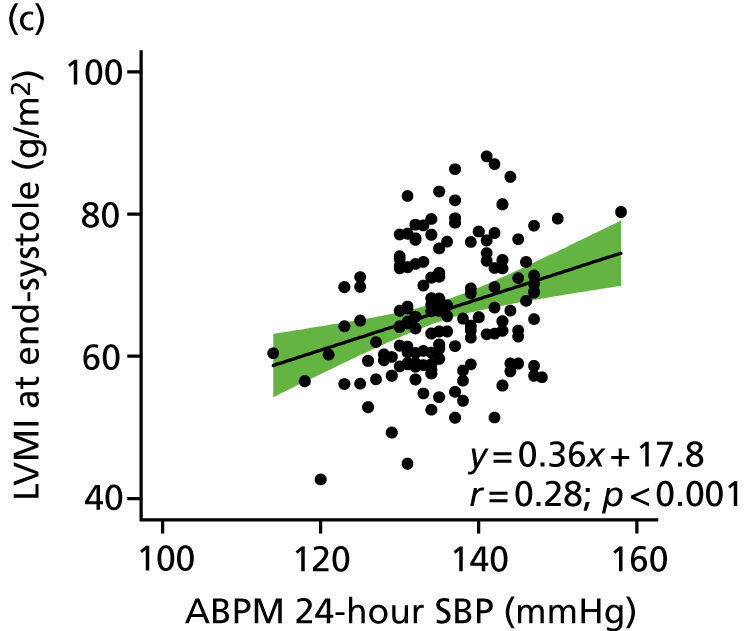

FIGURE 6.
Relationship between LVMI at end-diastole and central aortic, clinic-measured and ambulatory SBP. The regression line is shown with 95% CI.


Formal testing of regression coefficients (Fisher’s r-to-z transformation) showed no significant differences between BP modalities:
-
comparison of regression coefficients for LVMI at end-systole versus BP modality (format: regression 1: regression 2; comparison) –
-
LVMI versus CASP: LVMI versus brachial SBP; z = –0.19; p = 0.9
-
LVMI versus CASP: LVMI versus ABPM 24-hour SBP; z = –0.28; p = 0.8
-
LVMI versus CASP: LVMI versus ABPM daytime SBP; z = –0.66; p = 0.5.
-
-
comparison of regression coefficients for LVMI at end-diastole (format: regression 1; regression 2; comparison) –
-
LVMI versus CASP: LVMI versus brachial SBP; z = –0.09; p = 0.9
-
LVMI versus CASP: LVMI versus ABPM 24-hour SBP; z = –0.28; p = 0.8
-
LVMI versus CASP: LVMI versus ABPM daytime SBP; z = –0.66; p = 0.5.
-
Similarly, comparisons of regression slopes (seemingly unrelated regression estimates) showed no differences between BP modalities:
-
comparison of regression slopes for LVMI at end-systole versus BP modality (format: regression 1; regression 2; comparison) –
-
LVMI versus CASP: LVMI versus brachial SBP; χ2 = 0.20; p = 0.7
-
LVMI versus CASP: LVMI versus ABPM 24-hour SBP; χ2 = 1.62; p = 0.2
-
LVMI versus CASP: LVMI versus ABPM daytime SBP; χ2 = 2.96; p = 0.1.
-
-
comparison of regression slopes for LVMI at end-diastole (format: regression 1; regression 2; comparison) –
-
LVMI versus CASP: LVMI versus brachial SBP; χ2 = 0.15; p = 0.7
-
LVMI versus CASP: LVMI versus ABPM 24-hour SBP; χ2 = 1.65; p = 0.2
-
LVMI versus CASP: LVMI versus ABPM daytime SBP; χ2 = 2.96; p = 0.1.
-
Taken together, these data provide evidence to refute the hypothesis for there being a superior relationship for CASP with LVMI, compared with any other BP modality used in this study at baseline.
Baseline characteristics for the TREAT CASP randomised controlled trial and the observational study
A total of 162 participants were recruited into the stage 2 TREAT CASP RCT and the observational follow-up study. The baseline characteristics of these participants are shown in Tables 5–11 and see also Table 17. The distribution of CASP values at baseline for the TREAT CASP RCT and the observational study in relation to the cut-off point defining high and low CASP is shown in Figure 7 and was similar to that observed for participants with grade 1 hypertension in the screening cohort (see Figure 4).
| Demographic | TREAT CASP RCT group | Observational study (n = 57) | |
|---|---|---|---|
| Treatment (n = 51) | No treatment (n = 54) | ||
| Age (years), mean ± SD | 47.7 ± 6.3 | 46.6 ± 6.1 | 41.9 ± 8.7 |
| Height (cm), mean ± SD | 177.0 ± 7.1 | 176.0 ± 8.2 | 178.4 ± 7.5 |
| Weight (kg), mean ± SD | 90.4 ± 16.8 | 87.2 ± 17.3 | 88.8 ± 13.1 |
| BMI (kg/m2), mean ± SD | 28.8 ± 4.5 | 28.0 ± 4.1 | 27.9 ± 3.5 |
| Trunk fat (kg), mean ± SD | 13.8 ± 5.7 | 12.8 ± 5.4 | 12.9 ± 4.4 |
| Trunk fat-free mass (kg), mean ± SD | 36.8 ± 5.1 | 36.1 ± 4.9 | 36.5 ± 4.1 |
| QRISK-lifetime (%), mean ± SD | 45.8 ± 14.3 | 48.3 ± 17.5 | 47.9 ± 18.0 |
| QRISK 2 at 60 years (%), mean ± SD | 6.6 ± 3.5 | 7.7 ± 3.9 | 8.2 ± 4.9 |
| Waist-to-hip ratio, mean ± SD | 0.99 ± 0.08 | 0.98 ± 0.07 | 0.97 ± 0.06 |
| Current smoker, n (%) | 5 (9.8) | 1 (1.9) | 5 (8.8) |
| Ethnicity, n (%) | |||
| White | 38 (77.6) | 40 (75.5) | 42 (77.8) |
| Mixed race | 1 (2.0) | 0 (0) | 0 (0) |
| Asian | 5 (10.2) | 9 (17.0) | 10 (18.5) |
| Black | 4 (8.2) | 4 (7.5) | 2 (3.7) |
| Chinese | 1 (2.0) | 0 (0) | 0 (0) |
| Parameter | TREAT CASP RCT group, mean ± SD | Observational study (n = 57), mean ± SD | |
|---|---|---|---|
| Treatment (n = 51) | No treatment (n = 54) | ||
| Na+ (mmol/l) | 140.8 ± 2.0 | 141.1 ± 1.8 | 140.4 ± 1.5 |
| K+ (mmol/l) | 4.4 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 |
| Creatinine (mmol/l) | 82.8 ± 12.2 | 80.3 ± 11.1 | 81.8 ± 12.8 |
| eGFR (ml per minute per 1.73 m2) | 95.5 ± 15.9 | 99.3 ± 16.5 | 99.4 ± 18.5 |
| Albumin (mmol/l) | 46.5 ± 2.8 | 46.9 ± 2.5 | 47.4 ± 2.0 |
| ALP (mmol/l) | 64.8 ± 19.0 | 65.8 ± 16.5 | 65.5 ± 15.7 |
| ALT (mmol/l) | 32.4 ± 15.1 | 37.3 ± 21.3 | 34.5 ± 16.2 |
| Glucose (mmol/l) | 4.9 ± 0.5 | 5.0 ± 0.7 | 5.0 ± 0.8 |
| HbA1c (%) | 5.4 ± 0.3 | 5.3 ± 0.3 | 5.4 ± 0.4 |
| Total cholesterol (mmol/l) | 5.3 ± 1.0 | 5.2 ± 0.9 | 5.3 ± 0.9 |
| HDL cholesterol (mmol/l) | 1.4 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.3 |
| LDL cholesterol (mmol/l) | 3.2 ± 0.8 | 3.0 ± 0.9 | 3.1 ± 0.8 |
| Total-to-HDL cholesterol ratio | 4.1 ± 1.2 | 4.3 ± 1.5 | 4.6 ± 1.5 |
| Triglycerides (mmol/l) | 1.7 ± 1.0 | 1.9 ± 0.9 | 2.2 ± 1.3 |
| Parameter | TREAT CASP RCT group, mean ± SD | Observational study (n = 54), mean ± SD | |
|---|---|---|---|
| Treatment (n = 49) | No treatment (n = 53) | ||
| Haemoglobin (g/l) | 14.9 ± 0.8 | 15.0 ± 0.9 | 15.1 ± 0.9 |
| Haematocrit (l/l) | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.44 ± 0.02 |
| Mean cell volume (fl) | 88.7 ± 3.3 | 86.9 ± 4.1 | 87.3 ± 5.5 |
| Platelets (× 109/l) | 246.6 ± 44.2 | 220.7 ± 39.9 | 234.0 ± 43.8 |
| WCC (× 109/l) | 6.6 ± 2.0 | 6.3 ± 1.6 | 6.7 ± 1.4 |
| Neutrophils (× 109/l) | 3.7 ± 1.3 | 3.5 ± 1.1 | 3.9 ± 1.2 |
| Lymphocytes (× 109/l) | 2.2 ± 1.1 | 2.2 ± 0.7 | 2.1 ± 0.7 |
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| SBP (mmHg) | |||||
| Treatment | 146.0 ± 7.1 | 125.8 ± 9.1** | –20.00** | –23.30 to –16.60 | 44 |
| No treatment | 145.1 ± 6.7 | 135.3 ± 11.2 | –9.60 | –12.90 to –6.30 | 49 |
| Observational | 133.3 ± 8.2 | 132.0 ± 9.5 | –1.70 | –4.30 to 1.00 | 52 |
| DBP (mmHg) | |||||
| Treatment | 89.4 ± 6.4 | 76.0 ± 7.8** | –13.00** | –15.00 to –11.10 | 44 |
| No treatment | 90.0 ± 6.5 | 83.8 ± 8.5 | –5.80 | –7.90 to –3.70 | 49 |
| Observational | 80.4 ± 5.1 | 80.5 ± 6.9 | 0.10 | –1.80 to 1.90 | 52 |
| Heart rate (beats per minute) | |||||
| Treatment | 66.7 ± 11.0 | 68.2 ± 11.8 | 2.30 | –0.50 to 5.10 | 44 |
| No treatment | 66.7 ± 9.5 | 68.0 ± 12.1 | 2.20 | –0.70 to 5.10 | 49 |
| Observational | 74.9 ± 14.3 | 71.7 ± 13.5 | –2.20 | –5.70 to 1.30 | 52 |
| CASP (mmHg) | |||||
| Treatment | 134.0 ± 7.1 | 113.1 ± 8.2** | –21.10** | –24.40 to –17.90 | 44 |
| No treatment | 132.8 ± 5.6 | 122.6 ± 10.7 | –10.20 | –13.30 to –7.10 | 49 |
| Observational | 118.0 ± 5.4 | 117.1 ± 8.3 | –1.00 | –3.30 to 1.30 | 52 |
| SBP-CASP (mmHg) | |||||
| Treatment | 11.9 ± 3.4 | 12.7 ± 5.4 | 1.20 | –0.20 to 2.50 | 44 |
| No treatment | 12.3 ± 4.5 | 12.7 ± 3.6 | 0.60 | –0.40 to 1.60 | 49 |
| Observational | 15.3 ± 5.6 | 14.9 ± 4.9 | –0.70 | –1.90 to 0.50 | 52 |
| SBP amplification | |||||
| Treatment | 1.09 ± 0.03 | 1.11 ± 0.05 | 0.03 | 0.01 to 0.04 | 44 |
| No treatment | 1.09 ± 0.03 | 1.10 ± 0.03 | 0.01 | 0.00 to 0.02 | 49 |
| Observational | 1.13 ± 0.05 | 1.13 ± 0.04 | 0.00 | –0.01 to 0.01 | 52 |
| PP amplification | |||||
| Treatment | 1.28 ± 0.10 | 1.35 ± 0.15 | 0.08 | 0.04 to 0.12 | 44 |
| No treatment | 1.29 ± 0.11 | 1.34 ± 0.11 | 0.05 | 0.02 to 0.08 | 49 |
| Observational | 1.41 ± 0.16 | 1.41 ± 0.14 | –0.01 | –0.04 to 0.03 | 52 |
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| SBP (mmHg) | |||||
| Treatment | 137.0 ± 6.6 | 126.9 ± 8.0** | –10.34** | –12.79 to –7.91 | 43 |
| No treatment | 137.4 ± 6.9 | 137.3 ± 9.2 | –0.06 | –2.31 to 2.18 | 47 |
| Observational | 132.1 ± 6.5 | 131.7 ± 8.0 | –1.55 | –3.62 to 0.51 | 47 |
| DBP (mmHg) | |||||
| Treatment | 86.7 ± 5.7 | 79.5 ± 6.8** | –6.88** | –8.58 to –5.19 | 43 |
| No treatment | 87.4 ± 6.0 | 87.2 ± 7.9 | –0.28 | –1.66 to 1.11 | 47 |
| Observational | 83.0 ± 5.8 | 82.7 ± 6.8 | –0.28 | –1.67 to 1.12 | 47 |
| MAP (mmHg) | |||||
| Treatment | 102.8 ± 5.5 | 94.9 ± 6.6** | –7.86** | –9.64 to –6.08 | 42 |
| No treatment | 103.3 ± 5.7 | 103.1 ± 8.0 | –0.21 | –1.84 to 1.42 | 47 |
| Observational | 98.6 ± 4.6 | 98.3 ± 6.1 | –0.55 | –2.04 to 0.93 | 47 |
| PP (mmHg) | |||||
| Treatment | 50.5 ± 5.6 | 47.5 ± 6.0* | –3.50** | –5.07 to –1.93 | 42 |
| No treatment | 50.2 ± 5.8 | 50.1 ± 4.8 | –0.02 | –1.58 to 1.54 | 47 |
| Observational | 49.1 ± 8.5 | 49.1 ± 7.7 | –1.21 | –2.65 to 0.22 | 47 |
| Heart rate (beats per minute) | |||||
| Treatment | 66.9 ± 8.7 | 67.4 ± 8.9 | 1.60 | –0.08 to 3.27 | 42 |
| No treatment | 66.2 ± 8.3 | 67.3 ± 9.4 | 1.68 | –0.27 to 3.63 | 47 |
| Observational | 72.6 ± 8.3 | 71.0 ± 8.4 | 0.04 | –1.75 to 1.84 | 47 |
| SBP dip (%) | |||||
| Treatment | 14.4 ± 6.6 | 13.2 ± 5.0 | –1.45 | –3.74 to 0.84 | 40 |
| No treatment | 13.3 ± 4.5 | 13.6 ± 5.6 | 0.16 | –1.38 to 1.69 | 46 |
| Observational | 15.1 ± 4.7 | 15.1 ± 5.9 | –0.05 | –2.00 to 1.90 | 43 |
| DBP dip (%) | |||||
| Treatment | 18.4 ± 7.2 | 16.8 ± 7.4 | –1.60 | –4.26 to 1.06 | 40 |
| No treatment | 16.9 ± 6.8 | 16.9 ± 6.1 | –0.08 | –2.23 to 2.07 | 47 |
| Observational | 20.4 ± 6.4 | 20.7 ± 6.6 | 0.77 | –1.43 to 2.98 | 43 |
| MAP dip (%) | |||||
| Treatment | 16.7 ± 6.4 | 14.3 ± 6.1 | –2.25 | –4.70 to 0.19 | 40 |
| No treatment | 14.7 ± 5.6 | 14.8 ± 6.0 | 0.00 | –1.89 to 1.88 | 47 |
| Observational | 17.4 ± 5.4 | 17.4 ± 6.0 | 0.22 | –1.77 to 2.21 | 43 |
| Successful readings (%) | |||||
| Treatment | 88.9 ± 12 | 81.8 ± 18.6 | –6.58 | –13.05 to –0.10 | 40 |
| No treatment | 89.1 ± 10 | 86.3 ± 15.1 | –3.17 | –7.89 to 1.54 | 46 |
| Observational | 85.7 ± 16 | 84.2 ± 17.3 | –2.77 | –8.52 to 2.99 | 47 |
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| SBP (mmHg) | |||||
| Treatment | 141.0 ± 6.9 | 130.2 ± 7.5** | –11.09** | –13.65 to –8.54 | 43 |
| No treatment | 141.8 ± 6.8 | 141.3 ± 9.1 | –0.28 | –2.58 to 2.03 | 47 |
| Observational | 136.6 ± 6.5 | 135.8 ± 8.6 | –2.26 | –4.65 to 0.14 | 47 |
| DBP (mmHg) | |||||
| Treatment | 90.0 ± 5.7 | 82.1 ± 6.5** | –7.60** | –9.42 to –5.79 | 43 |
| No treatment | 90.9 ± 6.2 | 90.7 ± 8.1 | –0.17 | –1.67 to 1.33 | 47 |
| Observational | 86.9 ± 5.7 | 86.2 ± 6.8 | –0.83 | –2.39 to 0.73 | 47 |
| MAP (mmHg) | |||||
| Treatment | 106.6 ± 5.6 | 97.6 ± 6.5** | –8.88** | –10.85 to –6.91 | 42 |
| No treatment | 107.1 ± 6.1 | 106.6 ± 8.2 | –0.40 | –2.17 to 1.36 | 47 |
| Observational | 102.6 ± 4.3 | 101.9 ± 6.3 | –1.04 | –2.69 to 0.61 | 47 |
| PP (mmHg) | |||||
| Treatment | 51.2 ± 5.8 | 47.9 ± 5.7* | –3.88** | –5.47 to –2.29 | 42 |
| No treatment | 51.0 ± 6.1 | 50.8 ± 4.9 | –0.23 | –1.90 to 1.43 | 47 |
| Observational | 49.7 ± 8.9 | 49.7 ± 8.2 | –1.47 | –3.16 to 0.22 | 47 |
| Heart rate (beats per minute) | |||||
| Treatment | 68.6 ± 9.1 | 68.8 ± 9.3 | 1.50 | –0.14 to 3.14 | 42 |
| No treatment | 68.6 ± 9.5 | 69.3 ± 10.1 | 1.49 | –0.74 to 3.72 | 47 |
| Observational | 75.7 ± 8.8 | 73.4 ± 8.9 | –0.55 | –2.51 to 1.40 | 47 |
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| SBP (mmHg) | |||||
| Treatment | 121.0 ± 10.6 | 112.8 ± 9.4** | –8.17** | –11.37 to –4.97 | 41 |
| No treatment | 122.8 ± 8.6 | 122.2 ± 11.5 | –0.55 | –3.17 to 2.07 | 47 |
| Observational | 116.0 ± 7.5 | 115.6 ± 9.1 | –1.55 | –3.79 to 0.70 | 44 |
| DBP (mmHg) | |||||
| Treatment | 73.6 ± 8.3 | 68.1 ± 8.2** | –5.22** | –7.26 to –3.18 | 41 |
| No treatment | 75.4 ± 7.2 | 75.1 ± 8.0 | –0.21 | –2.15 to 1.72 | 47 |
| Observational | 69.2 ± 7.0 | 68.6 ± 7.9 | –0.95 | –2.65 to 0.75 | 44 |
| MAP (mmHg) | |||||
| Treatment | 88.9 ± 8.4 | 83.3 ± 7.7** | –5.33** | –7.51 to –3.1 | 40 |
| No treatment | 90.7 ± 6.8 | 90.8 ± 8.4 | 0.17 | –1.67 to 2.0 | 47 |
| Observational | 84.7 ± 6.1 | 84.3 ± 7.4 | –0.84 | –2.67 to 0.98 | 44 |
| PP (mmHg) | |||||
| Treatment | 47.2 ± 6.5 | 44.7 ± 6.9* | –2.85 | –4.97 to –0.73 | 40 |
| No treatment | 47.7 ± 5.7 | 47.6 ± 5.7 | –0.26 | –1.97 to 1.46 | 47 |
| Observational | 46.8 ± 7.9 | 47.0 ± 7.3 | –0.52 | –2.09 to 1.04 | 44 |
| Heart rate (beats per minute) | |||||
| Treatment | 60.2 ± 8.5 | 61.5 ± 8.2 | 1.85 | –0.53 to 4.23 | 40 |
| No treatment | 59.2 ± 7.5 | 59.3 ± 8.8 | 0.34 | –1.57 to 2.25 | 47 |
| Observational | 61.6 ± 7.5 | 61.9 ± 8.5 | 1.07 | –1.07 to 3.21 | 44 |
FIGURE 7.
Distribution of CASP values at baseline from the pooled TREAT CASP RCT (i.e. those participants with high CASP) and the observational study (i.e. those participants with low CASP) population with grade 1 hypertension (n = 162). The cut-off point for high and low CASP (≥ 125 mmHg) is shown by the vertical line.

The baseline characteristics were well matched between the TREAT CASP RCT randomised groups. There were no significant differences in baseline demographics and characteristics between the treatment and no-treatment arms of the TREAT CASP RCT. However, men in the treatment arm of the RCT were slightly older (mean 47.7 vs. 46.6 years; p = 0.37) and slightly heavier (mean 90.4 vs. 87.2 kg; p = 0.33) than men in the no-treatment arm. There was no difference in cardiovascular risk score (i.e. QRISK 2 at age 60 years, QRISK-lifetime) between the randomised groups.
Baseline characteristics for the TREAT CASP randomised controlled trial and the observational study by high and low CASP status
The participants with low CASP in the observational study were younger than those with high CASP in the TREAT CASP RCT (age: observational study, 41.9 ± 8.7 years; TREAT CASP RCT, 47.1 ± 6.2 years; difference –5.3 years, 95% CI –7.9 to –2.7 years; p < 0.001) and had lower clinic BP (clinic SBP: observational study, 133.3 ± 8.2 mmHg; TREAT CASP RCT, 145.5 ± 6.9 mmHg; difference –12.3 mmHg, 95% CI –14.7 to –9.9 mmHg, p < 0.001; clinic DBP: observational study, 80.4 ± 5.1 mmHg; TREAT CASP RCT, 89.7 ± 6.4 mmHg; difference –9.3 mmHg, 95% CI –11.2 to –7.3 mmHg, p < 0.001) and CASP (CASP: observational study, 118.0 ± 5.4 mmHg; TREAT CASP RCT, 133.4 ± 6.3 mmHg; difference –15.4 mmHg, 95% CI –17.4 to –13.5 mmHg; p < 0.001).
The participants with low CASP in the observational study also had lower ambulatory BP (24-hour SBP: observational study, 132.1 ± 6.5 mmHg; TREAT CASP RCT, 137.2 ± 6.7 mmHg; difference –5.2 mmHg, 95% CI –7.3 to –3.0 mmHg, p < 0.001; 24-hour DBP: observational study, 83.0 ± 5.8 mmHg; TREAT CASP RCT, 87.1 ± 5.8 mmHg; difference –4.1 mmHg, 95% CI –6.0 to –2.2 mmHg, p < 0.001). Participants with low CASP in the observational study also had higher heart rates on ECG than those with high CASP in the TREAT CASP RCT (heart rate: observational study, 68.6 ± 12.9 beats per minute; TREAT CASP RCT, 61.3 ± 9.5 beats per minute; difference 7.3 beats per minute, 95% CI 3.4 to 11.2 beats per minute; p < 0.001).
Baseline blood pressure characteristics for the TREAT CASP randomised controlled trial and the observational study
Patients were entered into the study with grade 1 hypertension according to clinic BP and/or ABPM. No patient with grade 1 hypertension as determined by clinic BP had a normal ABPM (i.e. < 135/85 mmHg daytime mean). The majority of participants (81%) with low CASP had high–normal clinic BP (i.e. 130–139/80–89 mmHg) but qualified for the study because their ABPM was in the grade 1 hypertensive range. In the observation study group, 19% of participants had a clinic BP in the grade 1 hypertension range. In contrast, the majority of participants (85%) with high CASP were classified with grade 1 hypertension as determined by clinic BP. The remainder of the participants qualified because their ABPM was in the grade 1 hypertension range. There was no difference in baseline cardiovascular risk score between the observational group and TREAT CASP RCT participants (QRISK 2 at age 60 years: observational study, 8.2% ± 4.9%; TREAT CASP RCT, 7.2% ± 3.7%, difference 1.1%, 95% CI –0.4% to 2.5%; p = 0.16. QRISK-lifetime: observational study, 47.9% ± 18.0%; TREAT CASP RCT, 47.1% ± 16.0%, difference 0.8%, 95% CI –4.6% to 6.3%; p = 0.8).
Baseline pressure amplification characteristics for the TREAT CASP randomised controlled trial and the observational study
Pressure amplification at baseline differed, as expected, between men with low CASP in the observational study and men with high CASP in the TREAT CASP RCT (low CASP vs. high CASP: BrSBP minus CASP difference: 3.1 mmHg, 95% CI 1.5 to 4.8 mmHg, p < 0.001; SBP amplification difference: 0.04, 95% CI 0.02 to 0.05, p < 0.001; PP amplification difference: 0.13, 95% CI 0.08 to 0.18, p < 0.001; see Table 8).
Relationship between clinic blood pressure and central aortic systolic pressure in high and low central aortic systolic pressure patient groups
The relationship between CASP and BrSBP for 663 men recruited into the stage 1 screening study is shown in Figure 8. Figure 8 shows that there was a good relationship between CASP and BrSBP, with moderate scatter around the line of regression. This indicates that CASP and BrSBP track well with each other. It was also demonstrated that the median BrSBP for patients in the high CASP range was 148.5 mmHg and 40% of patients with high CASP had a BrSBP in the upper half of the grade 1 hypertension range (i.e. ≥ 150–159 mmHg). In this BrSBP range (i.e. ≥ 150–159 mmHg), low CASP (i.e. < 125 mmHg) was found in only seven people (≈ 1%). In fact, across the entire SBP range for grade 1 hypertension (i.e. ≥ 140–159 mmHg), low CASP was found in only 53 people (i.e. only 8% of the initial screening population). This indicates that the prevalence of high systolic pressure amplification resulting in low CASP in younger patients with grade 1 hypertension is actually quite low. This illustrates why matching the BrSBP in groups with high and low CASP was very difficult to achieve.
FIGURE 8.
The relationship between CASP and BrSBP for participants in the TREAT CASP screening study. (a) Linear regression between SBP and CASP showing the prevalence of SBP in the grade 1 hypertension range (140–159 mmHg) and low CASP (i.e. < 125 mmHg). Points in green show participants with a SBP of 140–149 mmHg and low CASP. Points in blue show participants with a SBP of 150–159 mmHg and low CASP. (b) Frequency distributions for SBP in people with low CASP (green) and people with high CASP (black shading). Blue and light-green areas show the overlap of SBP for people with low CASP and a SBP of 140–159 mmHg (light green) and 150–159 mmHg (blue).

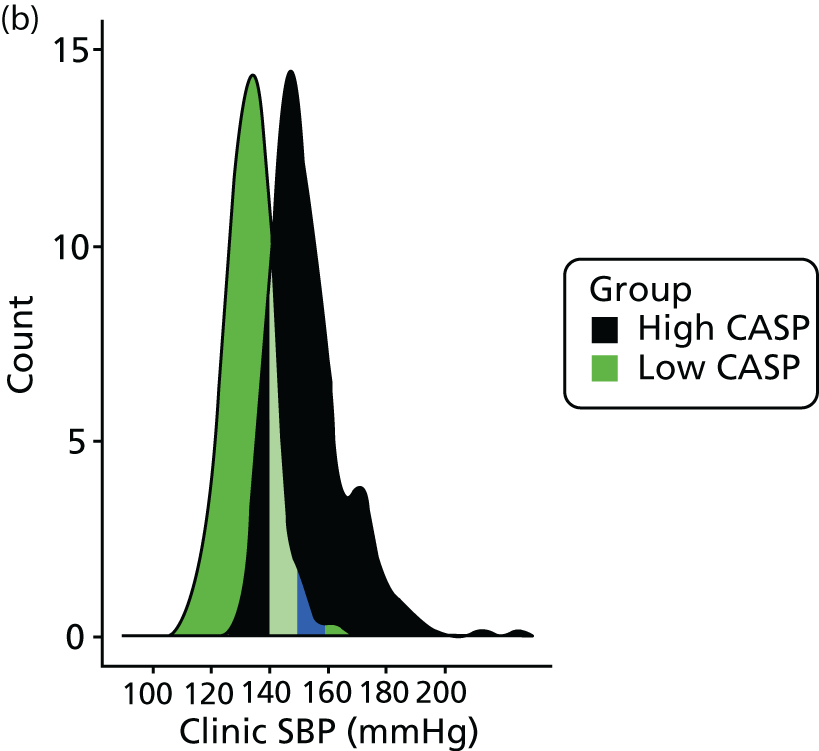
Interestingly, the data in Figure 8a also indicate that the line of regression between SBP and CASP intersects a CASP value of 125 mmHg for a SBP of 140 mmHg (the threshold BP for grade 1 hypertension). This suggests that the level of CASP used in this study to categorise high and low CASP values was appropriate for people with grade 1 hypertension.
Seated clinic blood pressure changes during randomised controlled trial and observational study follow-up
Seated clinic BP and CASP during study follow-up are shown in Figure 9 and Table 8.
FIGURE 9.
Clinic-measured SBP (a), DBP (b) and CASP (c) (mean ± SD) throughout the TREAT CASP RCT. The SBP, DBP and CASP values are presented as means ± SDs. The AUC data (d) are displayed as means and 95% CIs. **p < 0.01. AUC, area under the curve.

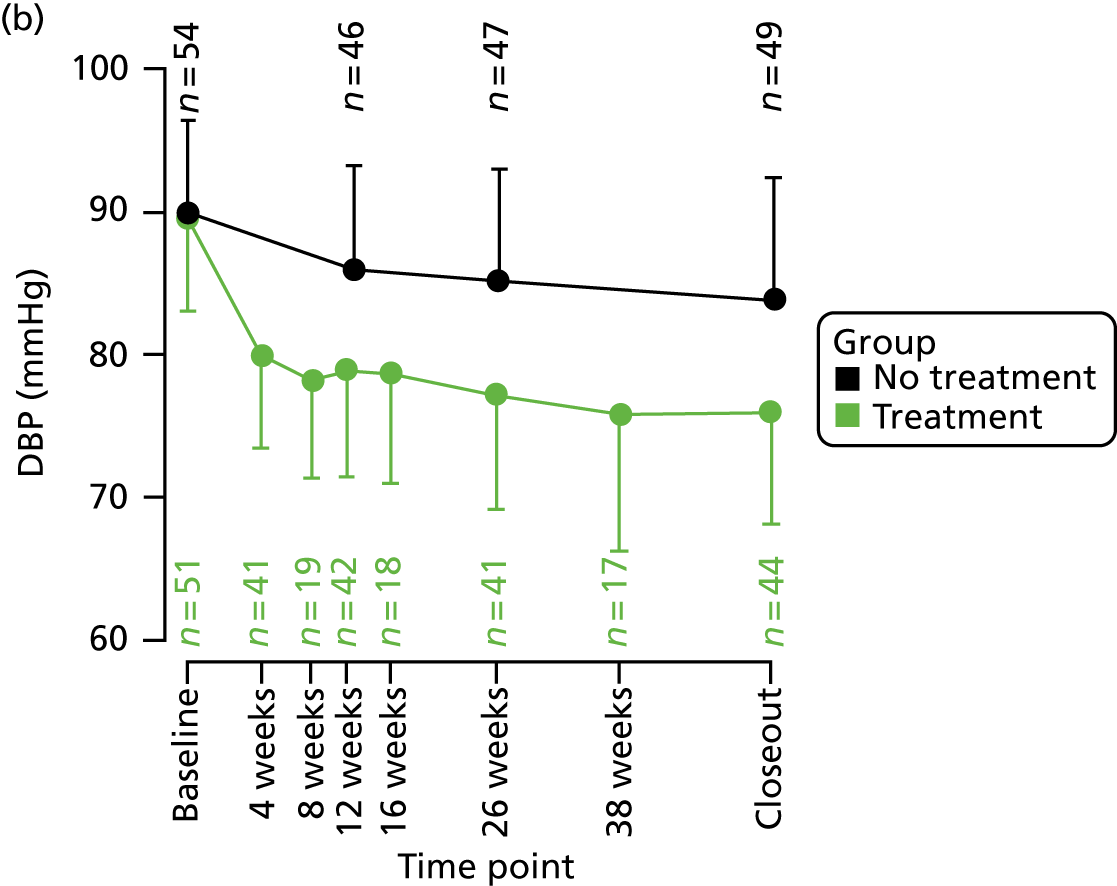
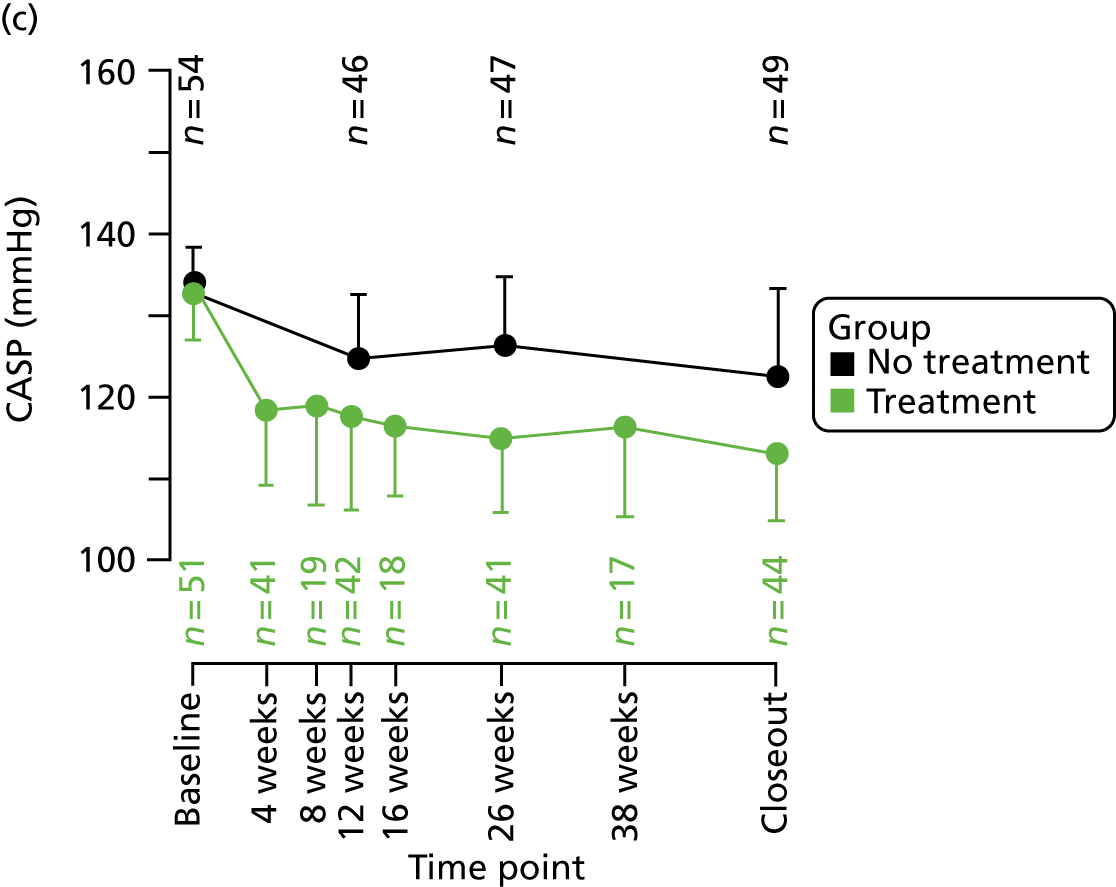

The average SBP, DBP and CASP were reduced from baseline in both the treatment and no-treatment groups and these reductions were sustained throughout the course of study follow-up. By the end of the study follow-up (i.e. study closeout), the changes from baseline were:
-
treatment –
-
BP change: –20.0 mmHg (95% CI –23.3 to –16.6 mmHg)/–13.0 mmHg (95% CI –15.0 to –11.1 mmHg)
-
CASP change: –21.1 mmHg (95% CI –24.4 to –17.9 mmHg).
-
-
no treatment –
-
BP change: –9.6 mmHg (95% CI –12.9 to –6.3 mmHg)/–5.8 mmHg (95% CI –7.9 to –3.7 mmHg)
-
CASP change: –10.2 mmHg (95% CI –13.3 to –7.1 mmHg).
-
Using area under the curve, the changes in SBP, DBP and CASP (baseline to study closeout) were significantly greater for the treatment arm than the no-treatment arm of the TREAT CASP RCT (all p < 0.001) (see Figure 9).
By contrast, clinic BP and CASP showed no significant change during follow-up for participants in the observational study [clinic BP change –1.7 mmHg (95% CI –4.3 to 1.0 mmHg)/0.1 mmHg (95% CI –1.8 to 1.9 mmHg); CASP change –1.0 mmHg (95% CI –3.3 to 1.3 mmHg); see Table 8 and Figure 10].
FIGURE 10.
Clinic-measured SBP and DBP (a) and CASP (b) (mean ± SD) throughout the observational study.

Ambulatory blood pressure changes during TREAT CASP RCT and observational study follow-up
Ambulatory BP was measured at baseline and study closeout in the TREAT CASP RCT and observational study. For participants in the treatment arm, ambulatory BP decreased significantly between baseline and study closeout [24-hour BP change –10.3 mmHg (95% CI –12.8 to –7.9 mmHg)/–6.9 mmHg (95% CI –8.6 to –5.2 mmHg); p < 0.001 for both]. By contrast, there was no significant change for participants in the no-treatment arm [24-hour BP change –0.1 mmHg (95% CI –2.3 to 2.2 mmHg; p = 0.95)/–0.3 mmHg (95% CI –1.7 to 1.1 mmHg; p = 0.7)]. No significant change in ambulatory BP was observed in the observational study [24-hour BP change: –1.6 mmHg (95% CI –3.6 to 0.5 mmHg; p = 0.1)/–0.3 mmHg (95% CI –1.7 to 1.1 mmHg; p = 0.7)]. Although there was little change in ambulatory BP during study follow-up for participants in the no-treatment arm and observational study, there was considerable variability in the range of values for ABPM change within individual participants. These data are shown in Tables 9–11 and Figures 11 and 12.
FIGURE 11.
Ambulatory SBP during RCT and observational study follow-up. Change in 24-hour ambulatory SBP between baseline and study closeout by randomised or allocated study group (a–c – upper panel) showing absolute number (and percentage) of participants with positive and negative changes. Absolute values for 24-hour ambulatory SBP at baseline and study closeout with means ± SDs also shown (d–f – lower panel). Significant differences (p < 0.001) within participants (overtime) are indicated with ***.

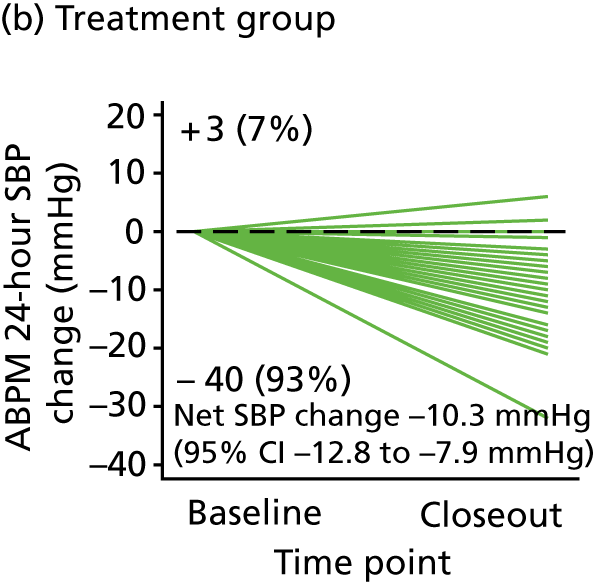
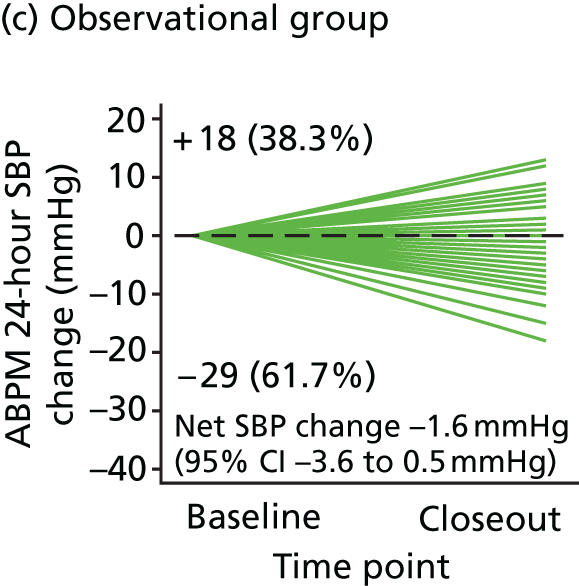

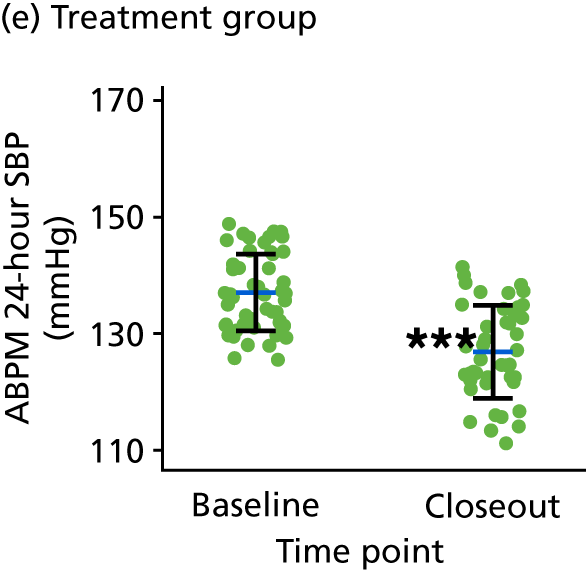

FIGURE 12.
Ambulatory DBP during RCT and observational study follow-up. Change in 24-hour ambulatory DBP between baseline and study closeout by randomised or allocated study group (a–c – upper panel) showing absolute number (and percentage) of participants with positive and negative changes. Absolute values for 24-hour ambulatory SBP at baseline and study closeout with means ± SDs also shown (d–f – lower panel). Significant differences (p < 0.001) within the participants (overtime) are indicated with ***.
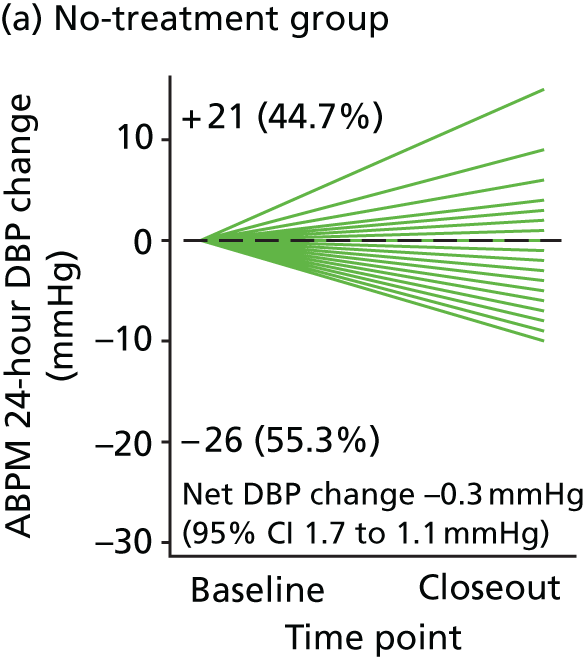
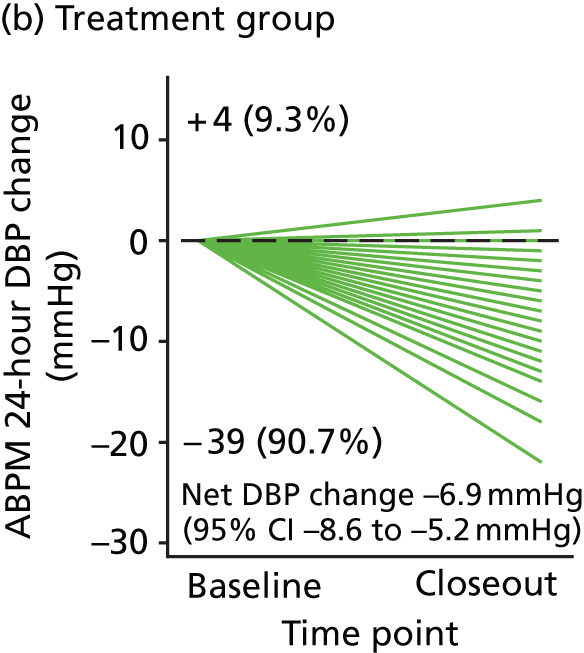


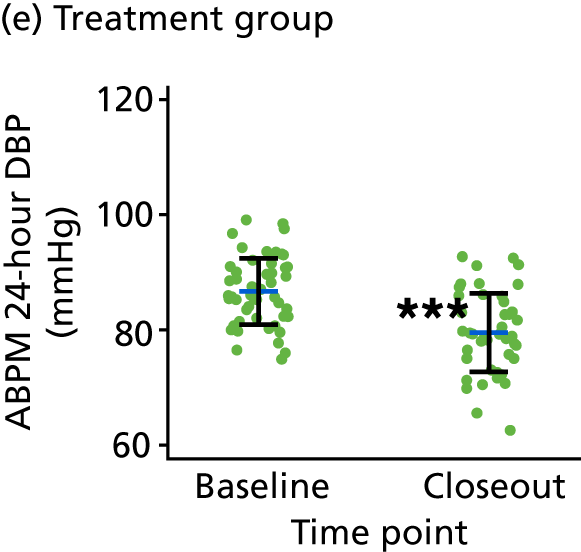

Medication time course
The treatment dose and number of treatments are described as a defined daily dose (DDD) in accordance with the WHO’s classification index. 109 According to the DDD method, the starting dose of any drug (i.e. 50 mg of losartan o.d. or 5 mg of amlodipine o.d. is defined as 1 DDD). One hundred mg of losartan o.d. or 10 mg of amlodipine o.d. each represent 2 DDDs, or a combination of 50 mg of losartan and 5 mg of amlodipine o.d. is 2 DDDs (see Table 4). Participants randomised to treatment, received treatment for, an average of, 373 ± 16 days. The majority of participants in the treatment arm completed the study on 2 DDDs (n = 26). Ten participants completed the study on 3 DDDs, whereas seven participants were treated with 1 DDD throughout the trial. One participant discontinued taking study medication shortly after randomisation, but was followed up to study closeout according to intention to treat. For those participants receiving more than one DDD, mean time to first up-titration of treatment was 63 ± 45 days and 136 ± 72 days to the second up-titration. For all study participants, the average time spent on 1 DDD was 118 ± 125 days, 2 DDDs was 243 ± 114 days and 3 DDDs was 244 ± 69 days. The total time spent on each DDD for participants completing the study on 1, 2 or 3 DDDs is shown in Table 12. None of the study participants allocated to treatment had their medication down-titrated by the study physician during follow-up. Those participants randomised to no treatment were followed up for, an average of, 378 ± 36 days.
| Time | Completed on | ||
|---|---|---|---|
| 1 DDD (n = 7) | 2 DDDs (n = 26) | 3 DDDs (n = 10) | |
| Number of days on: | |||
| 1 DDD | 368 | 66 | 55 |
| 2 DDDs | N/A | 306 | 80 |
| 3 DDDs | N/A | N/A | 244 |
Outcomes
Primary end point: change in left ventricular mass index
The primary outcome for the TREAT CASP RCT was the change in LVMI (i.e. baseline to study closeout), comparing the treatment versus the no-treatment arms. Analysis was performed according to original randomised group and LVMI was evaluated at both end-systole and end-diastole. Baseline LVMI was similar between the treatment arms [treatment vs. no treatment, baseline LVMI (end-systole): 67.9 ± 8.9 vs. 67.9 ± 8.7 g/m2; baseline LVMI (end-diastole): 67.6 ± 8.7 vs. 67.5 ± 8.8 g/m2].
Unadjusted analysis (Student’s t-test) for the primary end point showed a significantly greater reduction in LVMI with treatment than with no treatment [treatment vs. no treatment: LVMI (end-systole) change (baseline to study closeout) –3.3 g/m2 (95% CI –4.5 to –2.2 g/m2) vs. –0.9 g/m2 (95% CI –1.7 to –0.2 g/m2); difference –2.4 g/m2 (95% CI –3.8 to –1.0 g/m2); p < 0.01] (Table 13 and Figure 13). A similar effect of treatment was observed with the evaluation of the primary end point at end-diastole [treatment vs. no treatment: LVMI (end-diastole) change (baseline to study closeout) –3.4 g/m2 (95% CI –4.5 to –2.2 g/m2) vs. –1.0 g/m2 (95% CI –1.7 to –0.3 g/m2); difference –2.4 g/m2 (95% CI –3.7 to –1.0 g/m2; p < 0.01] (Table 13 and Figure 13). Effect sizes calculated using Cohen’s d statistic were –0.74 (LVMI end-systole) and –0.75 (LVMI end-diastole), indicating a medium-to-large effect of treatment.
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| LVMI systole (g/m2) | |||||
| Treatment | 67.9 ± 8.9 | 65.1 ± 8.6 | –3.34** | –4.53 to –2.15 | 44 |
| No treatment | 67.9 ± 8.7 | 67.5 ± 8.7 | –0.94 | –1.66 to –0.22 | 49 |
| Observational | 64.0 ± 8.5 | 63.9 ± 8.1 | –0.49 | –1.22 to 0.23 | 52 |
| LVMI diastole (g/m2) | |||||
| Treatment | 67.6 ± 8.7 | 64.8 ± 8.4 | –3.37** | –4.52 to –2.23 | 44 |
| No treatment | 67.5 ± 8.8 | 67.0 ± 8.7 | –1.00 | –1.73 to –0.27 | 49 |
| Observational | 63.9 ± 8.3 | 63.6 ± 7.9 | –0.66 | –1.40 to 0.09 | 52 |
FIGURE 13.
Primary end point for the TREAT CASP RCT showing the change in LVMI (baseline to study closeout), by randomised group. Data show individual values with means ± 95% CIs at end-systole (a) and end-diastole (b). Significant differences (i.e. p-values < 0.01) between groups are indicated with **. The effect sizes calculated using Cohen’s d statistic were –0.74 for LVMI evaluated at end-systole and –0.75 for LVMI evaluated at end-diastole.

The primary end point was then analysed using an analysis of covariance model. The change in LVMI (baseline to study closeout) at end-systole was adjusted for baseline covariates, including age, LVMI, clinic BP, heart rate, smoking status, waist-to-hip ratio and trunk fat-free mass. Correlations within the model were found for baseline LVMI (p = 0.06), heart rate (p < 0.01), smoking status (p = 0.06) and trunk fat-free mass (p < 0.01). The significant effect of treatment on the change in LVMI (baseline to study closeout) was maintained after adjustment [treatment: adjusted LVMI change (end-systole) –3.2 g/m2, 95% CI –4.1 to –2.3 g/m2; no treatment: –1.1 g/m2, 95% CI –2.0 to –0.2 g/m2; difference –2.1 g/m2, 95% CI –0.8 to –3.4 g/m2]. Similar results were obtained for the change in LVMI at end-diastole [treatment: adjusted LVMI change (end-diastole) –3.2 g/m2, 95% CI –4.1 to –2.3 g/m2; no treatment: –1.1 g/m2, 95% CI –2.0 to –0.3 g/m2; difference –2.1 g/m2, 95% CI –3.3 to –0.8 g/m2].
Secondary end point: change in unindexed left ventricular mass
Unindexed LV mass was specified as a secondary outcome. Baseline values for LV mass were:
-
140.8 ± 23.5 g for the treatment arm (end-systole)
-
138.1 ± 24.1 g for the no-treatment arm (end-systole)
-
140.2 ± 23.2 g for the treatment arm (end-diastole)
-
137.2 ± 24.3 g for the no-treatment arm (end-diastole).
There was a greater reduction in LV mass at end-systole with treatment [treatment vs. no treatment: LV mass (end-systole) change (baseline to study closeout) –6.3 g (95% CI –8.7 to –4.0 g) vs. –1.5 g (95% CI –3.0 to 0.0 g); difference –4.9 g (95% CI –7.6 to –2.2 g); p < 0.001]. Similar results were seen for LV mass evaluated at end-diastole [treatment vs. no treatment: LV mass (end-diastole) change –6.4 g (95% CI –8.6 to –4.2 g) vs. –1.6 g (95% CI –3.1 to –0.1 g); difference –4.8 g (95% CI –7.5 to –2.1 g); p < 0.001)] (Table 14 and see Figure 14). The effect sizes were –0.75 for LV mass at end-systole and –0.76 for LV mass at end-diastole, suggesting a medium-to-large effect of treatment. Differences were maintained after adjustment for baseline covariates, as used for analysis of the primary outcome [treatment-adjusted LV mass change (end-systole): –5.9 g, 95% CI –7.8 to –4.0 g; no-treatment-adjusted LV mass change (end-systole): –1.9 g, 95% CI –3.7 to 0.0 g; difference –4.0 g, 95% CI –6.8 to –1.4 g; treatment-adjusted LV mass change (end-diastole): –6.0 g, 95% CI –7.9 to –4.1 g; no-treatment-adjusted LV mass change (end-diastole): –2.0 g, 95% CI –3.7 to –0.2 g; difference –4.0 g, 95% CI –6.7 to –1.4 g].
| Outcome | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| LVM end-systole (g) | |||||
| Treatment | 140.8 ± 23.5 | 135.7 ± 23.2 | –6.34** | –8.68 to –4.01 | 44 |
| No treatment | 138.1 ± 24.1 | 137.7 ± 24.6 | –1.48 | –3.01 to 0.04 | 49 |
| Observational | 132.6 ± 23.8 | 132.4 ± 22.9 | –1.16 | –2.60 to 0.28 | 52 |
| LVM end-diastole (g) | |||||
| Treatment | 140.2 ± 23.2 | 135.0 ± 23.1 | –6.39** | –8.63 to –4.16 | 44 |
| No treatment | 137.2 ± 24.3 | 136.7 ± 24.6 | –1.60 | –3.11 to –0.09 | 49 |
| Observational | 132.4 ± 23.3 | 131.8 ± 22.4 | –1.47 | –2.94 to 0.00 | 52 |
Secondary end point: left ventricular mass index in the observational study
In the observational study, the baseline LVMI was 64.0 ± 8.5 g/m2 at end-systole and 63.9 ± 8.3 g/m2 at end-diastole. These values are significantly lower than the corresponding baseline LVMI in TREAT CASP RCT participants [67.9 ± 8.8 g/m2 (end-systole); 67.5 ± 8.7 g/m2 (end-diastole); p < 0.01 and p = 0.01, respectively; see Table 13].
There was a small non-significant reduction in LVMI in observational study participants (baseline to study closeout) after 12 months’ follow-up [LVMI change (end-systole): –0.5 g/m2, 95% CI –1.2 to 0.2 g/m2, p = 0.18; LVMI change (end-diastole): –0.7 g/m2, 95% CI –1.4 to 0.1 g/m2, p = 0.08].
Secondary end point: comparison of change in left ventricular mass indices across observational study and TREAT CASP randomised controlled trial participants
Comparing the changes in LVMI and unindexed LV mass after 12 months, for all three groups [treatment group in the TREAT CASP RCT (i.e. high CASP), no-treatment group in the TREAT CASP RCT (i.e. high CASP) and observational study (low CASP)], showed that the reduction in LVMI and unindexed LV mass was significantly greater with treatment than for either of the other two groups (i.e. no treatment and observational study; see Tables 13 and 14 and Figure 14). BP-lowering treatment in people with high CASP reduced their LVMI to values approaching those seen in the observational study group [treatment vs. observational: LVMI (end-systole) at study closeout, 65.1 ± 8.6 vs. 63.9 ± 8.1 g/m2; difference –1.2 g/m2, 95% CI –4.6 to 2.2 g/m2, p = 0.47; treatment vs. observational: LV mass (end-systole) at study closeout, 135.7 ± 23.2 vs. 132.4 ± 22.9 g; difference –3.3 g, 95% CI –12.7 to 6.1 g, p = 0.48]; see Table 14 and Figure 13. By contrast, at study closeout, LVMI was higher in the TREAT CASP RCT no-treatment group than in the observational group [no treatment vs. observational: LVMI (end-systole), 67.5 ± 8.7 vs. 63.9 ± 8.1 g/m2; difference –3.6 g/m2, 95% CI –6.9 to –0.3 g/m2; p = 0.04].
FIGURE 14.
Secondary end point showing the change in LVMI (a and b) and unindexed LV mass (c and d) between baseline and study closeout, by study group, for the TREAT CASP RCT and the observational study. Data show individual values with means ± 95% CIs at end-systole (a and c) and end-diastole (b and d). Significant differences (i.e. p-values < 0.01) between the treatment and no-treatment groups are indicated with **.
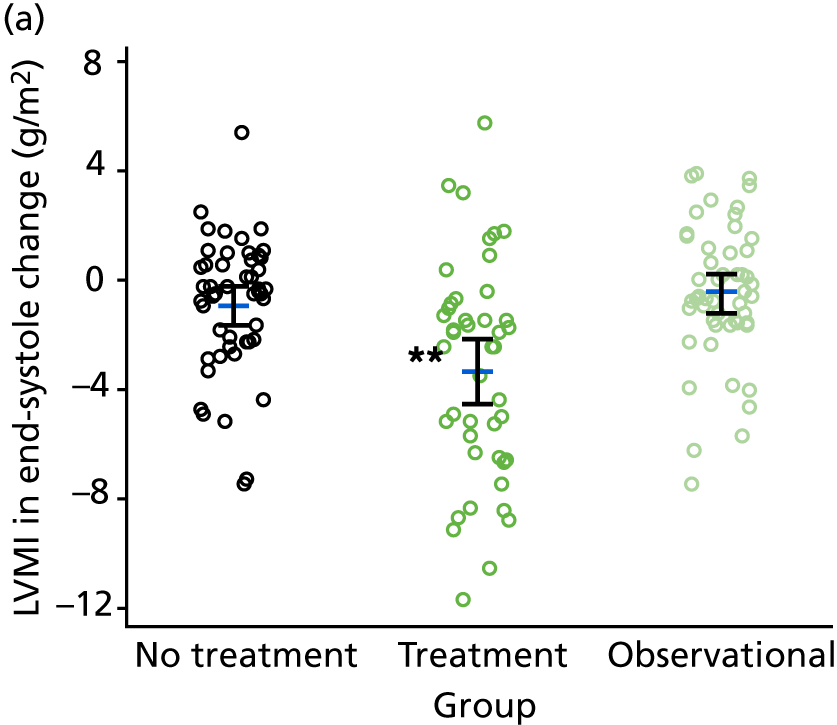



Secondary end point: additional analyses of cardiac magnetic resonance imaging parameters
Data for cardiac volumes, aortic flow and cardiac output are shown in Tables 15 and 16. A substantial number of additional data were acquired from the cMRI scans, including cardiac diastolic functional parameters (including tissue-phase mapping) and aortic stiffness and compliance. These data are the subject of ongoing analyses for subsequent reports.
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Study closeout | Mean | 95% CI | ||
| EDV (ml) | |||||
| Treatment | 145.3 ± 26.0 | 145.0 ± 25.9 | –0.32 | –4.79 to 4.15 | 44 |
| No treatment | 139.1 ± 27.1 | 139.8 ± 26.8 | 0.09 | –2.90 to 3.08 | 49 |
| Observational | 144.2 ± 30.5 | 143.9 ± 31.7 | –1.22 | –4.41 to 1.97 | 52 |
| ESV (ml) | |||||
| Treatment | 50.8 ± 14.8 | 48.4 ± 13.6 | –2.34 | –5.39 to 0.71 | 44 |
| No treatment | 47.1 ± 13.8 | 48.2 ± 15.3 | 0.81 | –1.74 to 3.35 | 49 |
| Observational | 52.0 ± 15.7 | 51.6 ± 15.6 | –0.45 | –2.80 to 1.89 | 52 |
| SV (ml) | |||||
| Treatment | 94.6 ± 17.7 | 96.6 ± 18.2 | 1.98 | –2.17 to 6.12 | 44 |
| No treatment | 92.1 ± 18.0 | 91.6 ± 17.2 | –0.85 | –3.39 to 1.69 | 49 |
| Observational | 92.1 ± 18.9 | 92.3 ± 20.6 | –0.81 | –3.33 to 1.72 | 52 |
| EF (%) | |||||
| Treatment | 65.4 ± 6.9 | 66.9 ± 6.4 | 1.44 | –0.38 to 3.27 | 44 |
| No treatment | 66.5 ± 6.0 | 66.0 ± 6.7 | –0.50 | –2.02 to 1.02 | 49 |
| Observational | 64.3 ± 5.6 | 64.4 ± 5.9 | –0.18 | –1.43 to 1.07 | 52 |
| CO (l/minute) | |||||
| Treatment | 6.1 ± 1.3 | 6.1 ± 1.2 | 0.02 | –0.41 to 0.46 | 44 |
| No treatment | 6.1 ± 1.4 | 5.8 ± 1.3 | –0.31 | –0.63 to 0.02 | 49 |
| Observational | 6.5 ± 1.4 | 6.3 ± 1.3 | –0.21 | –0.53 to 0.11 | 51 |
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| FF (ml) | |||||
| Treatment | 96.5 ± 18.1 | 98.3 ± 20.0 | 1.86 | –2.61 to 6.34 | 44 |
| No treatment | 94.3 ± 18.8 | 93.0 ± 16.9 | –1.64 | –4.38 to 1.09 | 49 |
| Observational | 94.1 ± 19.6 | 93.5 ± 20.6 | –1.50 | –4.10 to 1.10 | 52 |
| BF (%) | |||||
| Treatment | 2.2 ± 1.1 | 2.2 ± 1.3 | –0.10 | –0.52 to 0.32 | 44 |
| No treatment | 2.5 ± 1.6 | 2.2 ± 1.2 | –0.19 | –0.60 to 0.21 | 49 |
| Observational | 2.2 ± 1.2 | 2.0 ± 1.2 | –0.22 | –0.69 to 0.25 | 52 |
| Net FF (ml) | |||||
| Treatment | 94.2 ± 18.3 | 96.1 ± 20.0 | 1.97 | –2.56 to 6.49 | 44 |
| No treatment | 91.8 ± 18.5 | 90.7 ± 16.9 | –1.51 | –4.25 to 1.23 | 49 |
| Observational | 92.0 ± 19.4 | 91.5 ± 21.0 | –1.49 | –4.23 to 1.24 | 52 |
| RF (%) | |||||
| Treatment | 2.4 ± 1.4 | 2.3 ± 1.4 | –0.12 | –0.64 to 0.39 | 44 |
| No treatment | 2.7 ± 1.8 | 2.5 ± 1.3 | –0.08 | –0.56 to 0.40 | 49 |
| Observational | 2.3 ± 1.3 | 2.2 ± 1.5 | –0.10 | –0.63 to 0.44 | 52 |
| CO (l/minute) | |||||
| Treatment | 6.1 ± 1.4 | 6.0 ± 1.2 | –0.01 | –0.41 to 0.39 | 44 |
| No treatment | 6.1 ± 1.5 | 5.7 ± 1.3 | –0.39 | –0.72 to –0.06 | 49 |
| Observational | 6.4 ± 1.4 | 6.2 ± 1.2 | –0.29 | –0.58 to 0.01 | 52 |
Secondary end point: 12-lead electrocardiogram voltages at baseline and changes during follow-up
Electrocardiography voltage criteria are used clinically as a marker of LVH110 and were evaluated in this study as a prespecified secondary end point. ECG voltage was assessed by three common criteria to approximate LV mass:
-
Cornell voltage
-
voltage in lead aVL
-
Sokolow–Lyon voltage. 110
At baseline, voltage in lead aVL was significantly higher for participants with high CASP versus low CASP (high CASP vs. low CASP: aVL voltage 662.9 ± 398.6 µV vs. 501.1 ± 357.6 µV; p = 0.01). Cornell voltage was also higher at baseline for participants with high CASP, but the difference did not achieve significance (high CASP vs. low CASP: Cornell voltage 1.7 ± 0.6 mV vs. 1.6 ± 0.6 mV; p = 0.4).
In the TREAT CASP RCT, BP lowering was associated with a reduction in both Cornell and aVL voltages, although this did not quite achieve significance for voltage in lead aVL (treatment: Cornell voltage change (baseline to study closeout) –0.2 mV, 95% CI –0.2 to –0.1 mV, p < 0.01; aVL voltage change –51.5 µV, 95% CI –104.6 to 1.6 µV, p = 0.06). By contrast, Cornell and aVL voltage both increased after 12 months’ follow-up for the no-treatment group (no treatment: Cornell voltage change 0.1 mV, 95% CI 0.0 to 0.2 mV, p < 0.01; aVL voltage change 68.7 µV, 95% CI 5.0 to 132.4 µV, p = 0.035); Table 17 and Figure 15. Taken together, a highly significant difference was seen for the change in voltage for these parameters between TREAT CASP RCT groups (i.e. treatment vs. no treatment; difference in Cornell voltage change: –0.3 mV, 95% CI –0.4 to –0.2 mV, p < 0.001; difference in aVL voltage change: –120.2 µV, 95% CI –202.1 to –37.8 µV, p < 0.01). Effect sizes using Cohen’s d statistic were –0.97 for Cornell voltage change and –0.62 for aVL voltage change, thus suggesting large and medium effects of treatment, respectively. Differences were maintained after adjustment for baseline covariates (treatment: adjusted Cornell voltage change –0.1 mV, 95% CI –0.2 to –0.1 mV; no treatment: adjusted Cornell voltage change 0.1 mV, 95% CI 0.0 to 0.2 mV; difference –0.2 mV, 95% CI –0.4 to –0.1 mV; treatment-adjusted aVL voltage change –49.1 µV, 95% CI –109.2 to 11.0 µV; no treatment-adjusted aVL voltage change 66.4 µV, 95% CI 8.4 to 124.4 µV; difference –115.5 µV, 95% CI –201.0 to –30.0 µV).
| Parameter | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| ECG rate (beats per minute) | |||||
| Treatment | 60.8 ± 10.3 | 62.9 ± 10.6 | 2.22 | –0.86 to 5.30 | 44 |
| No treatment | 61.7 ± 8.7 | 63.8 ± 10.3 | 2.34 | –0.41 to 5.09 | 47 |
| Observational | 68.6 ± 12.9 | 65.3 ± 11.5 | –2.13 | –5.18 to 0.93 | 51 |
| PR interval (milliseconds) | |||||
| Treatment | 169.0 ± 26.1 | 169.4 ± 24.8 | –0.05* | –3.73 to 3.64 | 44 |
| No treatment | 164.8 ± 18.8 | 167.7 ± 18.6 | 4.04 | 0.57 to 7.52 | 47 |
| Observational | 161.2 ± 21.6 | 164.4 ± 21.9 | 1.14 | –1.72 to 3.99 | 51 |
| QRS duration (milliseconds) | |||||
| Treatment | 96.0 ± 10.7 | 95.4 ± 12.0 | –0.45 | –2.55 to 1.64 | 44 |
| No treatment | 93.8 ± 7.1 | 93.6 ± 6.9 | –0.04 | –1.15 to 1.06 | 47 |
| Observational | 99.0 ± 13.9 | 99.5 ± 14.5 | 0.59 | –0.57 to 1.74 | 51 |
| QTc (milliseconds) | |||||
| Treatment | 405.5 ± 23.6 | 407.0 ± 24.8 | 2.23 | –4.26 to 8.71 | 44 |
| No treatment | 404.2 ± 19.0 | 407.8 ± 21.4 | 2.72 | –2.29 to 7.73 | 47 |
| Observational | 407.0 ± 23.3 | 405.3 ± 17.5 | –0.86 | –6.34 to 4.61 | 51 |
| Cornell voltage (mV) | |||||
| Treatment | 1.7 ± 0.6 | 1.5 ± 0.4 | –0.15** | –0.24 to –0.06 | 43 |
| No treatment | 1.6 ± 0.5 | 1.7 ± 0.5 | 0.12 | 0.04 to 0.21 | 46 |
| Observational | 1.6 ± 0.6 | 1.6 ± 0.7 | –0.02 | –0.10 to 0.06 | 50 |
| aVL voltage (µV) | |||||
| Treatment | 679.7 ± 380.5 | 644.9 ± 356.5 | –51.51** | –104.59 to 1.57 | 43 |
| No treatment | 646.8 ± 418.2 | 652.4 ± 422.5 | 68.70 | 4.99 to 132.41 | 46 |
| Observational | 501.1 ± 357.6 | 518.8 ± 340.0 | 21.18 | –23.37 to 65.73 | 51 |
| Sokolow–Lyon voltage (mV) | |||||
| Treatment | 2.4 ± 0.7 | 2.3 ± 0.7 | –0.08 | –0.20 to 0.05 | 38 |
| No treatment | 2.5 ± 0.7 | 2.5 ± 0.8 | –0.11 | –0.23 to 0.00 | 42 |
| Observational | 2.4 ± 0.8 | 2.4 ± 0.9 | –0.05 | –0.17 to 0.08 | 49 |
FIGURE 15.
Change in ECG Cornell voltage (a), voltage in lead aVL (b) and Sokolow–Lyon voltage (c) between baseline and study closeout for the TREAT CASP RCT and the observational study, by study group. Significant differences (i.e. p-values of < 0.01) between the treatment and no-treatment groups are indicated with **.
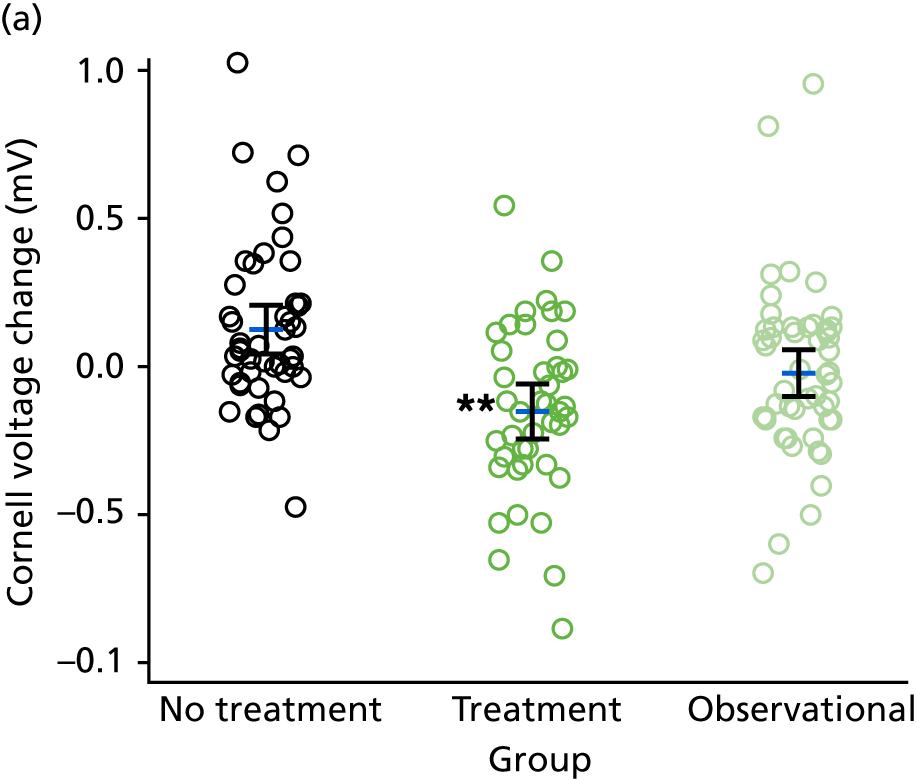
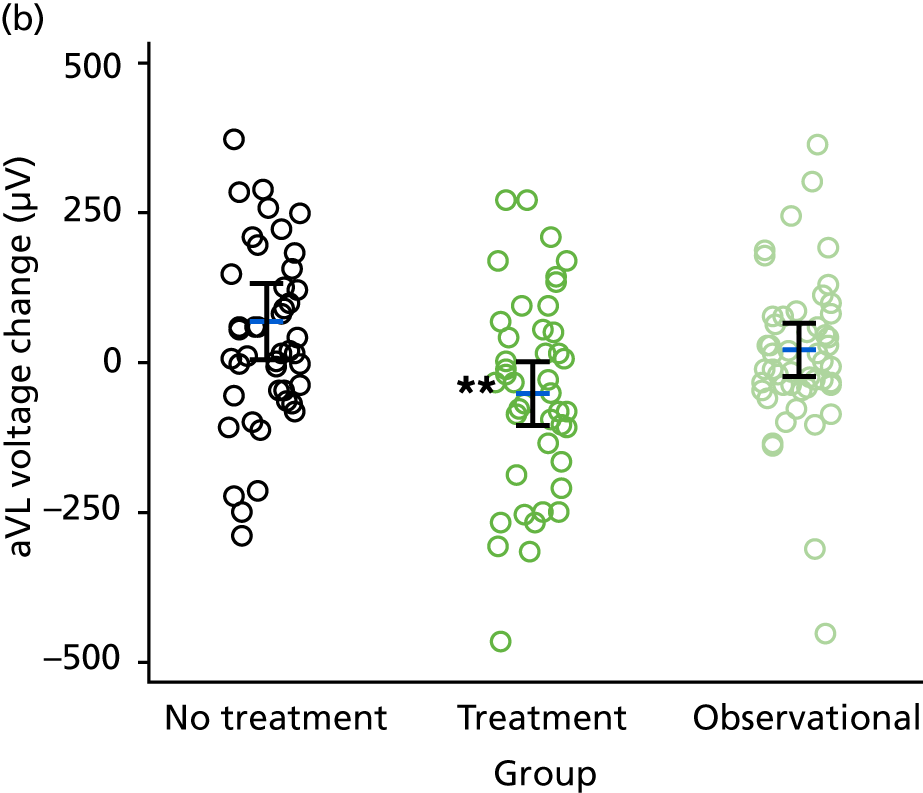
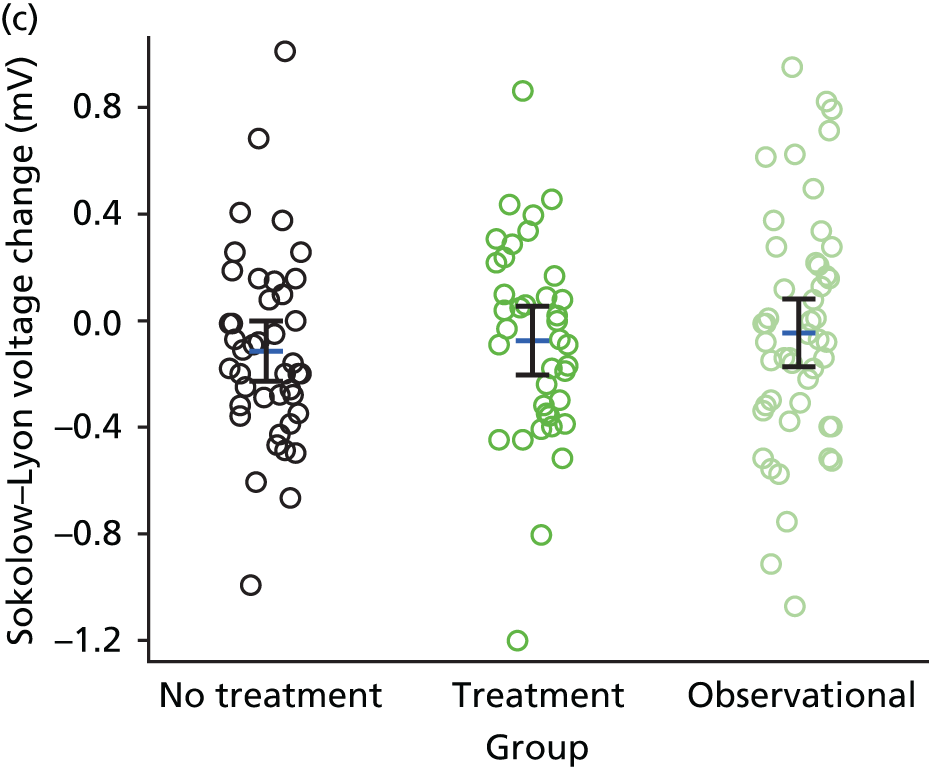
In the observational study of low CASP participants, the Cornell and aVL voltages were unchanged after 12 months’ follow-up [Cornell voltage change (baseline to study closeout): 0.0 mV, 95% CI –0.1 to 0.1 mV; aVL change: 21.2 µV, 95% CI –23.4 to 65.7 µV; see Table 17 and Figure 15].
The Sokolow–Lyon voltage criteria showed no significant differences between groups (low CASP vs. high CASP) at baseline or study closeout. Furthermore, there was no change in Sokolow–Lyon voltage at study closeout after treatment for 12 months. There was, however, a small reduction in Sokolow–Lyon voltage after 12 months’ follow-up for the no-treatment arm [no treatment Sokolow–Lyon voltage change (baseline to study closeout): –0.1 mV, 95% CI –0.2 to 0.0 mV, p = 0.05; see Table 17 and Figure 15].
Urinary albumin-to-creatinine ratio
The UACR was measured at baseline and study closeout. As this study was in a relatively healthy study population without evidence of HMOD, diabetes mellitus or CKD, as expected, many urine samples were returned with undetectable albumin levels. A total of 55 participants (treatment, n = 15; no treatment, n = 19; observational, n = 21) were identified with detectable urine albumin at both baseline and study closeout. Comparison of the change in UACR in the TREAT CASP RCT showed a modest reduction with treatment relative to that seen in the no-treatment group [treatment: UACR change (baseline to study closeout) –0.5, 95% CI –1.1 to 0.1; no treatment: UACR change 0.1, 95% CI –0.2 to 0.4; difference –0.6, 95% CI –1.2 to 0.0, p < 0.05; Figure 16]. However, this change may have been influenced by one outlying data point in the treatment group, as exclusion of these data points yielded a slightly smaller change (treatment: UACR change (baseline to study closeout) –0.3, 95% CI –0.7 to 0.1; no treatment: UACR change 0.1, 95% CI –0.2 to 0.4; difference –0.4, 95% CI –0.9 to 0.1; p = 0.09). The effect size, calculated using Cohen’s d statistic, was –0.74, suggesting a medium-to-large effect of treatment. However, the difference for the change in UACR (baseline to study closeout) between treatment arms for the TREAT CASP RCT (including the outlier described above) was attenuated after adjustment for baseline variables [treatment: adjusted UACR change (baseline to study closeout) –0.5, 95% CI –1.0 to 0.0; no treatment: UACR change 0.1, 95% CI –0.4 to 0.6; difference –0.6, 95% CI –1.4 to 0.2].
FIGURE 16.
Change in UACR across the TREAT CASP RCT, by randomised group. Absolute values with means and 95% CIs for change in UACR in the TREAT CASP RCT are shown. Significant differences (i.e. p-values of < 0.05) between the treatment and no-treatment groups are indicated with *.

Eleven (73.3%) of those participants with detectable urine albumin, at both baseline and study closeout, in the high CASP group who received BP-lowering treatment showed a reduction in their UACR, whereas four (26.7%) showed an increase in UACR from baseline after 12 months. In the high CASP group, which did not receive treatment, nine (47.4%) participants showed a reduction in UACR, whereas 10 (52.6%) showed an increase in UACR from baseline after 12 months (Figure 17).
FIGURE 17.
Change in UACR across the TREAT CASP RCT and the observational study, by study group. Individual changes (a–c) and absolute values with means ± SDs (d–f) for UACR at baseline and study closeout across all study groups.

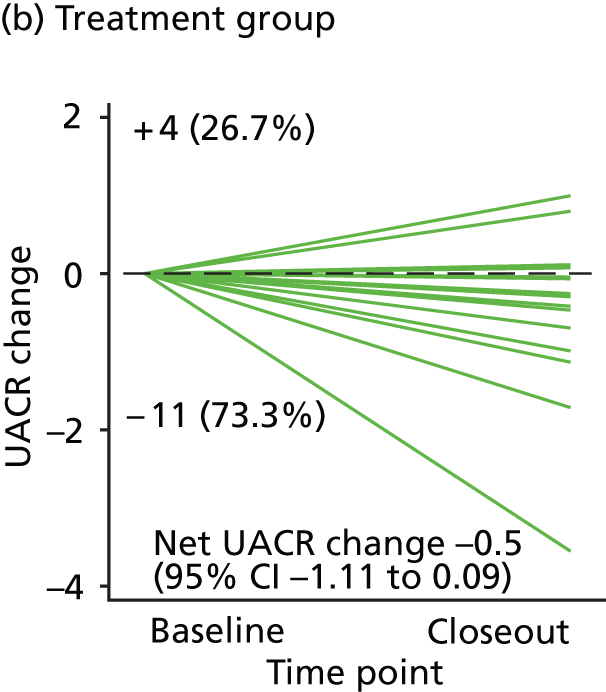
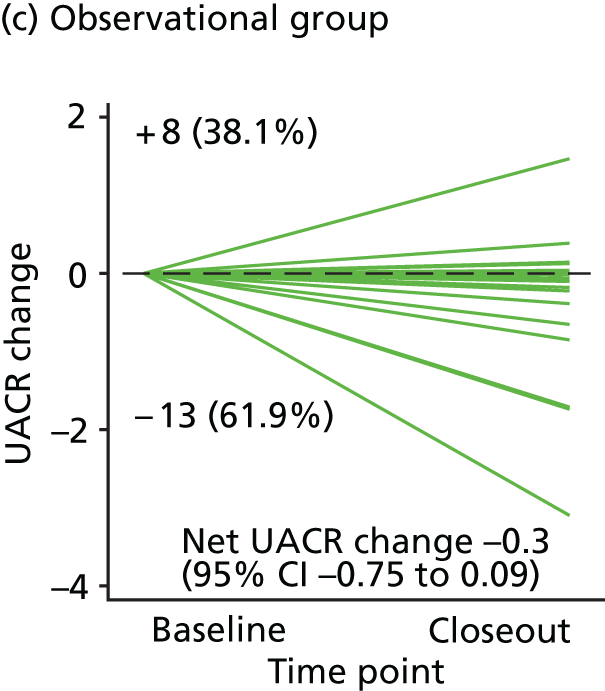



A small reduction in UACR was also observed in the observational group (change in UACR baseline to study closeout –0.3, 95% CI –0.8 to 0.1). Three-way comparison of the change in UACR showed no difference between groups.
Secondary end point: quality-of-life questionnaire
Quality of life during the TREAT CASP RCT and the observational study was assessed using the SF-36 questionnaire at both baseline and study closeout. Summary data from the SF-36 are shown in Table 18. There were no differences in averaged responses to any category for those participants who completed questionnaires at both baseline and study closeout.
| SF-36 domain | Time point, mean ± SD | Δ from baseline | Number of participants | ||
|---|---|---|---|---|---|
| Baseline | Closeout | Mean | 95% CI | ||
| General health | |||||
| Treatment | 72.9 ± 16.1 | 73.2 ± 15.2 | 0.62 | –4.33 to 5.58 | 40 |
| No treatment | 71.0 ± 16.6 | 71.4 ± 14.5 | 2.13 | –2.01 to 6.27 | 47 |
| Observational | 69.0 ± 15.7 | 66.2 ± 16.1 | –1.73 | –5.26 to 1.79 | 49 |
| Limitations due to emotional health | |||||
| Treatment | 91.9 ± 22.6 | 91.7 ± 23.6 | –0.83 | –10.93 to 9.26 | 40 |
| No treatment | 93.6 ± 18.7 | 85.8 ± 33.1 | –7.80 | –17.63 to 2.03 | 47 |
| Observational | 82.1 ± 35.3 | 85.0 ± 32.0 | 4.76 | –7.29 to 16.81 | 49 |
| Emotional well-being | |||||
| Treatment | 72.4 ± 9.8 | 68.8 ± 11.6 | –4.10 | –7.96 to –0.24 | 40 |
| No treatment | 70.1 ± 9.7 | 68.0 ± 11.6 | –1.57 | –4.54 to 1.41 | 46 |
| Observational | 68.1 ± 13.4 | 68.7 ± 12.0 | 0.42 | –2.91 to 3.75 | 48 |
| Energy | |||||
| Treatment | 65.4 ± 12.7 | 63.8 ± 13.5 | –1.50 | –5.23 to 2.23 | 40 |
| No treatment | 65.0 ± 12.6 | 61.6 ± 13.0 | –2.87 | –6.78 to 1.03 | 47 |
| Observational | 59.5 ± 13.6 | 56.9 ± 12.6 | –2.76 | –6.37 to 0.86 | 49 |
| Pain | |||||
| Treatment | 88.6 ± 17.1 | 89.5 ± 15.7 | 0.75 | –5.33 to 6.83 | 40 |
| No treatment | 87.2 ± 13.6 | 85.6 ± 18.5 | –2.50 | –8.57 to 3.57 | 47 |
| Observational | 87.6 ± 15.9 | 85.5 ± 18.4 | –2.86 | –8.97 to 3.25 | 49 |
| Physical health | |||||
| Treatment | 92.0 ± 17.0 | 89.0 ± 19.4 | –2.25 | –10.68 to 6.18 | 40 |
| No treatment | 93.8 ± 13.8 | 94.0 ± 13.5 | 0.53 | –4.59 to 5.60 | 47 |
| Observational | 87.2 ± 23.8 | 91.1 ± 18.8 | 2.24 | –5.70 to 10.19 | 49 |
| Limitations due to physical health | |||||
| Treatment | 93.3 ± 21.6 | 93.8 ± 22.5 | 1.25 | –7.42 to 9.92 | 40 |
| No treatment | 93.3 ± 21.1 | 90.4 ± 25.8 | –2.66 | –13.18 to 7.86 | 47 |
| Observational | 90.7 ± 23.9 | 92.3 ± 23.5 | 2.55 | –6.98 to 12.08 | 49 |
| Social functioning | |||||
| Treatment | 92.2 ± 14.4 | 86.6 ± 20.7 | –6.88 | –12.88 to –0.87 | 40 |
| No treatment | 91.6 ± 15.4 | 87.0 ± 19.3 | –4.52 | –10.99 to 1.95 | 47 |
| Observational | 88.2 ± 20.5 | 86.7 ± 20.9 | –0.77 | –7.30 to 5.77 | 49 |
Secondary end points: cardio-ankle vascular index and retinal imaging
Measurements of arterial stiffness using the BP-independent parameter CAVI, together with a macula-centred image of the optic fundus were taken at baseline and study closeout during the TREAT CASP RCT and the observational study. Data from these measurements will be the subject of further reports.
Safety analyses
Adverse events and serious adverse events
During the study, a total of 41 participants reported 47 AEs. Thirty-one participants in the TREAT CASP RCT reported 36 AEs, with 19 participants in the treatment arm reporting 22 AEs, and 12 participants reporting 14 AEs in the no-treatment arm (see Appendix 2).
In the TREAT CASP RCT, seven adverse events were considered to be related to treatment. Of these, five participants reported dizziness on standing (in all cases minor orthostatic symptoms), one participant reported ankle oedema and one participant reported decreased libido. A small number of cardiovascular-related AEs and SAEs were reported and all were related to incidental ECG findings (treatment, n = 2; no treatment, n = 1; observational, n = 2). Non-specific (other) AEs were the most frequently reported in all study groups, but were uncommon.
Serious adverse events were experienced by 18 participants during the study, with no participant reporting more than one SAE. There was an equal distribution of SAEs in the TREAT CASP RCT (six participants randomised to treatment reported SAEs vs. seven participants randomised to no treatment reported SAEs). Four SAEs were reported in the observational follow-up group. The most commonly reported SAEs were incidental findings on MRI. No SAEs were reported as causally related to intervention and there were no SUSARs reported.
A summary of SAEs and AEs by category is shown in Table 19. Details of individual adverse events are reported in Appendix 1.
| Category | Number of events (number of patients experiencing events) | ||
|---|---|---|---|
| TREAT CASP RCT, by group | Observational study (n = 52) | ||
| Treatment (n = 44) | No treatment (n = 49) | ||
| SAEs | |||
| Totala | 6 (6) | 7 (7) | 4 (4) |
| Cardiovascular disorders | 1 (1) | ||
| Infections | 1 (1) | 1 (1) | |
| Injury | 1 (1) | 1 (1) | |
| Renal and urinary disorders | 1 (1) | ||
| Incidental MRI findings | 3 (3) | 2 (2) | |
| Hepatic | 1 (1) | ||
| Cancer | 1 (1) | 1 (1) | |
| Other | 3 (3) | ||
| AEs | |||
| Total | 22 (19) | 14 (12) | 11 (10) |
| Cardiovascular disorders | 1 (1) | 1 (1) | 2 (2) |
| Gastrointestinal disorders | 2 (2) | ||
| Hepatic | 1 (1) | 2 (2) | |
| Incidental MRI findings | 2 (2) | 1 (1) | 2 (2) |
| Infections | 2 (2) | 1 (1) | |
| Injury | 1 (1) | ||
| Musculoskeletal disorders | 4 (4) | 1 (1) | |
| Orthostatic disorders | 4 (4) | 1 (1) | |
| Renal and urinary disorders | 1 (1) | 1 (1) | |
| Other | 9 (9) | 5 (4) | 3 (3) |
Chapter 4 Discussion
There has been considerable uncertainty about whether or not to treat low-risk younger patients with grade 1 hypertension. The TREAT CASP RCT demonstrated that in younger men with grade 1 hypertension and low risk (i.e. no evidence of CVD, HMOD, diabetes mellitus or CKD) stratification, in accordance with their CASP, generated groups with significantly different LVMIs. Treatment to lower BP for 12 months in those with high CASP levels (i.e. a CASP of ≥ 125 mmHg) significantly reduced LVMI compared with no treatment, with a medium-to-large effect size. Treatment was well tolerated and was associated with a similar number of SAEs compared with no treatment and only a modest excess in AEs, most of which were non-specific and did not result in treatment withdrawal. Moreover, quality-of-life assessment (using the SF-36) did not differ between the treatment and no-treatment groups. ECG voltage parameters also showed treatment-related reductions in LV mass, with a large effect size relative to no treatment. These data suggest that grade 1 hypertension in low-risk, younger men with high CASP levels is not benign, and is associated with an increased LVMI that is reversible by BP-lowering treatment.
The TREAT CASP was a study in two stages. The first stage comprised a screening study to identify men with grade 1 hypertension and to stratify them as high or low CASP. The threshold for high CASP (i.e. ≥ 125 mmHg) for this stratification was derived from published data analysing all studies reporting both CASP and BrBP. 28 The selection of the high CASP threshold value of ≥ 125 mmHg was validated in this study because this CASP value corresponded to a BrSBP of 140 mmHg (i.e. the threshold for the diagnosis of grade 1 hypertension in current NICE guidelines4) (see Figure 8). Moreover, the high CASP threshold used for this study clearly identified men with early cardiac structural change, as evidenced by an increased LVMI in a positive relationship between CASP and LVMI (see Figure 5). However, CASP was not unique in this context because similar relationships were seen between BrSBP (both clinic and ambulatory) and LVMI (see Figure 5). This prompts the question of whether or not it was necessary to measure CASP to identify patients with grade 1 hypertension who had early evidence of hypertension-mediated changes in cardiac structure. What became clear as the study progressed is that although patients with high CASP did have reduced pressure amplification from the aorta to brachial artery, they also tended to have higher BrBP levels than those patients with low CASP. Matching low versus high CASP for BrBP was impossible because it was shown that only 8% of patients with low CASP have a BrSBP equivalent to those seen in patients with high CASP, the latter having higher BrBP. This suggests that stratification of the patients into those more likely to have increased LVMI and thus benefit from treatment could be obtained from conventional BrBP measurement and that the measurement of CASP provided no added value for treatment stratification. The lower clinic BrSBP in the patients with low CASP reflects the fact that grade 1 hypertension was defined as a clinic SBP of ≥ 140 mmHg and/or grade 1 hypertension according to ABPM. Thus, this study suggests that patients with grade 1 hypertension confirmed on both clinic BP and ABPM are those most likely to have a high CASP and an increased LVMI and thus benefit from treatment in terms of regression of their LVMI. This finding has important implications because it provides support for the recommendation by NICE4 that an elevated clinic BP should be confirmed by ABPM to establish the diagnosis of grade 1 hypertension. However, our study extends this by suggesting that such patients are likely to benefit from treatment to regress their LVMI.
Left ventricular mass index is the most appropriate end point to evaluate potential treatment benefits in younger people because:
-
LVMI is known to be a potent biomarker of chronic arterial pressure overload. 69,111,112
-
Increased LVMI is a strong predictor of future CVD events, stroke and premature mortality. 113,114
-
Recent data suggest a correlation between mid-life LV mass and brain white matter lesions and reduced hippocampal volume on MRI, and cognitive decline. 115
-
BP-lowering treatment has been shown to regress LVMI in hypertensive patients. 116,117
-
Regression of LVMI by BP-lowering treatment in older patients has been shown to be associated with a reduced risk of cardiovascular events and mortality. 89,118
Most previous studies examining LVMI have used echocardiography, usually in older patients with more severe hypertension than studied in this study. 119–121 In this study, cMRI was used because this is now considered the gold standard for LV mass measurement. 122,123 cMRI also allows the assessment of many functional parameters, including systolic and diastolic function and the impact of treatment, which will be the subject of further reports.
The measurement of ECG voltage criteria for approximating LV mass confirmed the primary outcome findings on cMRI. This was interesting because, traditionally, ECG voltage criteria have been considered insensitive markers of increased LV mass and response to treatment,110 although in some studies ECG LV mass regression has been associated with improved clinical outcomes. 96,124–127 Using two of the recommended criteria110 for approximating LV mass with ECG (the Cornell voltage and aVL voltage), our study showed that both of these ECG voltage criteria indicated a reduction of LV mass with treatment in the patients with high CASP, which is consistent with the cMRI findings. Effect sizes comparing the effects of treatment with those of no treatment were large (Cornell voltage) and medium (aVL voltage). A third ECG voltage criterion for approximating LV mass (Sokolow–Lyon voltage) was less useful. These findings are of practical importance because of the simplicity in performing a 12-lead ECG to evaluate treatment effects on LV mass.
The BP-lowering treatment used in this study was consistent with NICE recommendations4 and was very well tolerated, with no treatment-related AEs prompting treatment withdrawal. The treatment was also remarkably effective at reducing BP in this population, reducing clinic SBP by 20 mmHg, versus a 10-mmHg reduction in the no-treatment group, with similar changes and differences between groups for CASP. The majority of participants completed the study on at least two DDDs of medication, having received their initial up-titration after an average of 63 days. Interestingly, a reduction in clinic, but not ambulatory, BP was observed in the no-treatment group in the TREAT CASP RCT. As there was no placebo used, this most probably reflects an effect of acclimatisation to clinic BP measurement or a regression to the mean, effects not seen with ambulatory BP measurement. There was no change in clinic BP across follow-up for the untreated participants with low CASP enrolled into the observational follow-up study. This group of participants showed an overall consistency in their clinic BP throughout follow-up. This may be explained by the lower clinic BP at baseline in the group of participants with low CASP (see Chapter 3, Seated clinic blood pressure changes during study follow-up).
Blood pressure-lowering treatment significantly reduced LVMI in the TREAT CASP RCT versus no treatment. This implies a potentially beneficial effect of treatment and, in real terms, LVMI was reduced to levels similar to that in the group of participants with low CASP at baseline and was maintained after adjustment for baseline factors. Previous studies in patients with BP in the high–normal/grade 1 hypertensive range have reported reductions in LVMI with treatment of BP; however, the patients in these studies were all receiving antihypertensive medications prior to further reducing their BP to the high–normal BP range. Therefore, these patients were most likely to have had much more significant hypertension and a higher LV mass prior to their original treatment. 89,118 To our knowledge, the present study is the first study to evaluate the impact of treating BP on LVMI in younger, low-risk, grade 1 hypertension patients.
The reduction in LVMI in this study (LVMI change with treatment –3.3 g/m2, 4.9%) was modest compared with that seen in some other studies of hypertensive patients. In the Pathway 1 study, which compared combination therapy to lower BP in people with BP > 150/90 mmHg over the course of 1 year, a change in LVMI of 10.4% was achieved. 128 Similarly, in a study comparing treatment with the combination of sacubitril and valsartan versus olmesartan alone, in patients with hypertension and elevated PP, a reduction in LVMI of –6.8 g/m2 was achieved with the two-drug combination therapy over the course of 1 year. 129 However, treatment with olmesartan monotherapy in this study was associated with a modest reduction in LVMI of –3.6 g/m2, which was more consistent with the effects of, predominantly, monotherapy in our study. Differences between the magnitude of LVMI reduction in our study and other published reports most probably reflects the fact that other studies have recruited older patients with established (grade 2) hypertension or higher, and have predominantly used multidrug combination therapy for treatment. The change in LVMI with treatment in our study was consistent with the relationship between BP and LVMI at baseline and also consistent with the magnitude of LVMI regression with further BP-lowering treatment in patients whose treated BP was in the high–normal range prior to further BP lowering. 89,118,126
Finally, there was also a small reduction in the UACR for participants receiving treatment. This was surprising because all patients had a normal UACR and many had undetectable levels of urinary albumin, consistent with our strategy to recruit low-risk patients. Thus, our analysis of change in UACR was confined to only those with urinary albumin detectable at both baseline and study closeout. This reduced the power of this analysis and the interpretation of these data is cautious. Nevertheless, increased UACR in hypertensive patients is associated with increased risk of renal disease and CVD, and treatment has been shown to reduce UACR in people with higher levels of UACR and this reduction has been associated with reduced future risk. 110 Thus, in addition to an effect of treatment on LVMI, there is a suggestion that treatment also reduces another early marker of HMOD, namely UACR.
There were some significant challenges associated with recruitment of younger people with early-stage hypertension for this study. First, identification of potentially suitable participants via searches of GP databases proved problematic as evaluation of men with a diagnosis of grade 1 hypertension based on GP records revealed that many were normotensive with more formal measurement of their BP. Ironically, a more reliable source for our study proved to be self-referral in response to advertisements placed in local and national media. A total of 424 (58%) recruited participants were excluded during screening as a result of having BP levels outside the range for the study. This low yield of suitable participants prolonged study recruitment time. Second, once identified, retention of participants for participation in the second-stage RCT was problematic because of the requirements of the stop–go checkpoint based around assessment of LVMI by cMRI to confirm that there was the predicted relationship between CASP and LVMI. This prevented many suitable patients (i.e. n = 98) transitioning immediately into the TREAT CASP RCT. With hindsight, a more successful strategy would have been to enrol participants directly into the stage 2 RCT and observational studies on identification at screening, particularly as our original power calculation for the stop–go checkpoint proved to be reliable.
Public engagement and involvement were essential for the success of this study and we found self-referral of participants responding to advertising more successful than traditional recruitment through searching medical records or databases. In this, public engagement for our study was facilitated through resources provided by the UCL UCLH Biomedical Research Centre (BRC)’s patient and public involvement (PPI) group. The PPI group provided support for recruitment and advice regarding the clarity and relevance of study information from a potential participant’s point of view. Once the study was completed, we held a public engagement event for study participants and family members. The event took place on the evening of 10 April 2019 at UCL and was attended by 80 study participants. A formal presentation by the chief investigator provided the context for the study, the importance of hypertension as a risk factor, the main results of the study and the potential implications. This stimulated an enthusiastic discussion lasting over 30 minutes and continued into the evening at the subsequent reception. During the reception, posters were displayed to provide further detail on the main points from the study and these generated a lot of additional interest. The feedback from study participants suggested that this was a very successful and informative event that was greatly appreciated by study participants and their family members, many of whom requested to be kept informed regarding future opportunities to participate in research. Details, feedback, the programme and photographs of the event are provided in Appendix 3. Our suggestion is that an end-of-study presentation to study participants and family members should become a regular feature for public engagement in clinical trials.
Study strengths and weaknesses
This study had a number of strengths, the most important of which is that it addressed the paucity of data on the impact of BP-lowering treatment in younger, low-risk people with hypertension. Our study clearly shows that such people with CASP levels that corresponded to a conventional clinic BP level in the grade 1 hypertensive range (i.e. 140–159/90–99 mmHg), and confirmed by an elevated ABPM, have early evidence of cardiac remodelling and increased LV mass that is most probably attributable to increased BP because it was reduced by BP-lowering treatment. An additional strength of the study was the comprehensive characterisation of clinical phenotype of participants with grade 1 hypertension. In this, BP and changes in BP were measured using both clinic and ambulatory methods. Few other studies have reported both parameters across a clinical trial. Use of cMRI as a robust primary end point is also a study strength, with a wealth of parameters regarding cardiac structure and function being collected in a single measurement. Many of these parameters remain to be analysed and will generate future reports from this study. We also performed additional phenotypic measurements at baseline and study closeout, such as CAVI, to examine large artery function, and non-mydriatic retinal photography, to examine small artery changes, which will be the subject of future reports. Our finding that a standard ECG was able to detect beneficial changes in LV mass with treatment in younger people is also of interest with regard to implementation in routine clinical practice. With regard to practice, although our hypothesis was proven (i.e. that high CASP predicted a higher LVMI and that treatment of high CASP reduced LVMI back towards levels seen in patients with low CASP), we also discovered that the use of CASP offered no additional predictive value over and above the careful characterisation of BrBP in the clinic, complemented by ABPM, suggesting that conventional BP measurement would be sufficient to stratify these patients for treatment.
Our study has some weaknesses. We discovered that the prevalence of low CASP in patients with grade 1 hypertension was very low (≈ 8%) and ≈ 1% in those with a BrSBP in the upper range of men in the high CASP cohort, making it very difficult to match BrBP levels for those with high and low CASP and, thus, impossible to dissect the independent effect, if any, of CASP on LVMI. In so doing, we confirmed that CASP has no added or practical value in stratifying treatment decisions for younger patients with grade 1 hypertension.
We recruited exclusively men because of the complexity of recruiting and exposing women of childbearing potential to treatment. However, there is no evidence that BP-lowering treatment is any less effective at reducing LV mass and preventing subsequent cardiovascular events in women. On the contrary, increased LVMI is more strongly predictive of adverse cardiovascular outcomes in women versus men126 and BP lowering has been shown to regress LV mass to a greater extent in women than in men, after adjusting for differences in baseline LV mass. 130 We were unable to use a traditional double-blind study design or a placebo in the no-treatment arm because of the need to up-titrate treatment to achieve a predefined reduction in BP. Instead we used a PROBE design in which randomised treatment was open label, but the evaluation of end points was blind to treatment allocation. In reality, the clinic and ABPM BP responses to no treatment in the TREAT CASP RCT had little difference in magnitude of change to those seen in placebo-controlled trials of BP-lowering treatment. Another consideration is whether or not studies of this kind are too small to influence clinical practice. We used cMRI to record changes in LV mass and such studies have sufficient power with the number of patients we studied. Very much larger studies would be required to evaluate major cardiovascular events and mortality in this patient group but, as discussed in Chapter 1, Evaluating the benefit of treatment in young people with grade 1 hypertension, these are unlikely to happen because of the low rates of such end points in younger people. Thus, well-established portents of future cardiovascular risk, such as LV mass, are the only feasible option to evaluate potential benefits of treatment in this age group.
Clinical implications
There is a paucity of clinical trial data on the potential benefits of BP-lowering treatment in younger people (i.e. those aged < 55 years) with grade 1 hypertension and uncertainty about whether or not BP-lowering treatment is indicated. We show that the non-invasive measurement of CASP provided no added value over carefully measured clinic BP or ABPM in stratifying the risk of an increased LVMI in these patients. However, we also show that younger men with grade 1 hypertension and high CASP (corresponding to a higher BrBP in the grade 1 hypertensive range) but otherwise defined as low risk are already developing cardiac changes (increased LVMI) due to increased BP. We also show that the increase in LVMI is reversible with BP-lowering treatment and that treatment was well tolerated. As an increase in LV mass is the most well-characterised BP-related biomarker of future risk of CVD and premature mortality, these findings suggest that younger people with grade 1 hypertension, even if otherwise characterised as low risk, are more likely to have an increased LV mass and that this increase can be regressed by BP-lowering treatment.
Research implications
Previous studies have suggested that CASP is highly variable in patients with similar BrBP measurements and may be a better predictor of HMOD, such as increased LV mass. We found that CASP was variable, but that this variability was mainly a function of the BrBP from which it was derived and only rarely resulted in grade 1 hypertensive patients who had a low CASP level. Moreover, higher CASP values corresponded to higher BrBP levels and the high CASP threshold of ≥ 125 mmHg used in this study corresponds to the current guideline-recommended threshold for grade 1 hypertension, if the clinic BP elevation was also confirmed by ABPM. We found grade 1 hypertensive patients rarely had a low CASP level. In this regard, the measurement of CASP provided no incremental value in predicting LVMI over carefully measured clinic BP. This suggests that future studies evaluating BP-lowering treatment effects in younger patients should be based on carefully measured clinic BrBP, complemented by ABPM, rather than clinic BP alone, to more accurately define the hypertensive phenotype.
A key question generated by this study is whether or not younger patients with grade 1 hypertension, as well as having cardiac structural changes (increased LVMI), also have LV functional changes (i.e. early evidence of impaired systolic function or diastolic relaxation) and, if so, is this reversible with treatment? The cMRI data we have acquired in this study will allow us to address these questions. Whether or not impaired diastolic function is already manifested in younger patients with grade 1 hypertension is unknown. This question is of particular interest because diastolic dysfunction and, ultimately, heart failure with preserved ejection fraction (HFpEF) are closely linked to the development of LVH in hypertensive patients. Future analyses from cMRI data acquired in our study will examine conventional measures of diastolic function, such as E/A ratios, as well as more contemporary measures using tissue-phase mapping, to determine if diastolic function is already impaired in younger people with grade 1 hypertension and, if so, whether or not this has improved with BP-lowering treatment. The impact of BP-lowering treatment has never been assessed before in younger hypertensive patients and is particularly important because it is well established that hypertension is a major risk factor for diastolic dysfunction leading to HFpEF in later life. Furthermore, in these older patients, HFpEF does not appear to be reversible with treatment. Thus, it will be intriguing to determine if early signs of diastolic dysfunction are present in the participants in this study and, if so, whether or not it can be reversed by early intervention with BP-lowering treatment. This study also highlights the urgent need for more research in younger people with grade 1 hypertension and deemed to be at low short-term risk to determine if there is a way to better stratify those who might benefit from early initiation of BP-lowering drug therapy.
Acknowledgements
We acknowledge and thank the many volunteers who gave up their time and participated in our study. We also thank the Trial Steering Committee for their invaluable advice. We thank the NIHR UCLH BRC’s PPI group for their advice throughout the study.
We also acknowledge that the following staff made important contributions to the study:
-
Donna Moskal Fitzpatrick – Study Administrator
-
Dr Alexander Jones – Clinical Research Fellow
-
Dr Vivek Muthurangu – Reader in Cardiovascular Imaging and Physics
-
Dr Geraint Brown – Clinical Research Fellow
-
Dr Megan Borkum – Clinical Research Fellow
-
Amanda Wilson – Research Nurse
-
Kristen Mellander – Research Nurse
-
Wendy Norman – Radiographer
-
Rod Jones – Radiographer
-
Zainib Shabir – Clinical Trial Unit Study Co-ordinator
-
staff at the UCL/UCLH Joint Research Office
-
staff at the NIHR UCLH Clinical Research Facility
-
staff at Noclor
-
staff at the UCL Institute of Cardiovascular Science.
Trial Steering Committee
The Trial Steering Committee comprised:
-
Professor Tony Heagerty (chairperson), Division of Cardiovascular Sciences, University of Manchester, UK
-
Professor Paul Leeson, Medical Sciences Division, University of Oxford, UK
-
Professor John Potter, Foundation Chair in Ageing and Stroke Medicine, University of East Anglia, UK
-
Professor Bryan Williams, Institute of Cardiovascular Science, UCL, UK
-
Professor Clive Osmond, Department of Human Development and Health, University of Southampton, UK.
Contributions of authors
Bryan Williams (https://orcid.org/0000-0002-8094-1841) (Professor of Medicine, UCL, Institute of Cardiovascular Science, Director of Research, UCLH, NHS Foundation Trust, Director of the UCL/UCLH NIHR BRC) was the chief investigator. He devised the study, obtained funding, oversaw the design and conduct of the project and the interpretation and reporting of the results and drafted the manuscript with Peter S Lacy.
Ewan McFarlane (https://orcid.org/0000-0002-5183-8386) (Research Assistant, UCL, Institute of Cardiovascular Science) was involved in patient recruitment and data collection and processing and MRI data analysis. He was involved in the drafting and reviewing of the manuscript.
Dawid Jedrzejewski (https://orcid.org/0000-0001-5111-4807) (Research Assistant, UCL, Institute of Cardiovascular Science) was involved in patient recruitment and data collection and processing. He was involved in the drafting and reviewing of the manuscript.
Peter S Lacy (https://orcid.org/0000-0002-5916-6465) (Senior Research Associate, UCL, Institute of Cardiovascular Science) was involved in the design and acquisition of funding for the study. He contributed substantially to protocol design and revision, and the processing and interpretation of the study data, and drafted the manuscript.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by participants and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’ s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives you can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension . Global burden of blood-pressure-related disease, 2001. Lancet 2008;371:1513-18. https://doi.org/10.1016/S0140-6736(08)60655-8.
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
- Falaschetti E, Chaudhury M, Mindell J, Poulter N. Continued improvement in hypertension management in England: results from the Health Survey for England 2006. Hypertension 2009;53:480-6. https://doi.org/10.1161/HYPERTENSIONAHA.108.125617.
- National Institute for Health and Care Excellence (NICE) . Hypertension: Clinical Management of Primary Hypertension in Adults (Update). Clinical Guideline 127 (CG 127) 2011. http://guidance.nice.org.uk/cg127 (accessed 17 March 2013).
- Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol 1976;38:46-51. https://doi.org/10.1016/0002-9149(76)90061-8.
- The Stroke Association . Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;10:1-67. https://doi.org/10.1136/heartjnl-2014-305693.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
- Mansia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007;16:135-22. https://doi.org/10.1080/08037050701461084.
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD006742.pub2.
- Martin SA, Boucher M, Wright JM, Saini V. Mild hypertension in people at low risk. BMJ 2014;349. https://doi.org/10.1136/bmj.g5432.
- Zanchetti A. Do we over treat mild hypertension?. Expert Opin Pharmacother 2015;16:1121-6. https://doi.org/10.1517/14656566.2015.1040761.
- Sundström J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ 2011;342. https://doi.org/10.1136/bmj.d643.
- Williams B. High blood pressure in young people and premature death. BMJ 2011;342. https://doi.org/10.1136/bmj.d1104.
- McCarron P, Smith GD, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet 2000;355:1430-1. https://doi.org/10.1016/S0140-6736(00)02146-2.
- Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol 2011;58:2396-403. https://doi.org/10.1016/j.jacc.2011.07.045.
- Paffenbarger RS, Wing AL. Chronic disease in former college students. XI. Early precursors of nonfatal stroke. Am J Epidemiol 1971;94:524-30. https://doi.org/10.1093/oxfordjournals.aje.a121351.
- Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, et al. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J Am Coll Cardiol 2015;65:327-35. https://doi.org/10.1016/j.jacc.2014.10.060.
- Vlachopoulos C, O’Rourke M, Nichols WW. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Boca Raton, FL: CRC Press; 2005.
- Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of Central Versus Brachial Blood Pressure With Target-Organ Damage: systematic review and meta-analysis. Hypertension 2016;67:183-90. https://doi.org/10.1161/HYPERTENSIONAHA.115.06066.
- Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality?. J Hypertens 2009;27:461-7. https://doi.org/10.1097/HJH.0b013e3283220ea4.
- Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, et al. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension 2009;54:375-83. https://doi.org/10.1161/HYPERTENSIONAHA.109.134379.
- McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753-60. https://doi.org/10.1016/j.jacc.2005.07.037.
- McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, . Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension 2008;51:1476-82. https://doi.org/10.1161/hypertensionaha.107.105445.
- Williams B, Lacy PS. CAFE and the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) Investigators . Impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE-Heart Rate. J Am Coll Cardiol 2009;54:705-13. https://doi.org/10.1016/j.jacc.2009.02.088.
- Williams B, Lacy PS, Cruickshank JK, Collier D, Hughes AD, Stanton A, et al. Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation-Lipid-Lowering Arm (CAFE-LLA) study. Circulation 2009;119:53-61. https://doi.org/10.1161/CIRCULATIONAHA.108.785915.
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006;113:1213-25. https://doi.org/10.1161/CIRCULATIONAHA.105.595496.
- Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension 2001;38:1461-6. https://doi.org/10.1161/hy1201.097723.
- Herbert A, Cruickshank JK, Laurent S, Boutouyrie P. Reference Values for Arterial Measurements Collaboration . Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J 2014;35:3122-33. https://doi.org/10.1093/eurheartj/ehu293.
- Hulsen HT, Nijdam ME, Bos WJ, Uiterwaal CS, Oren A, Grobbee DE, et al. Spurious systolic hypertension in young adults; prevalence of high brachial systolic blood pressure and low central pressure and its determinants. J Hypertens 2006;24:1027-32. https://doi.org/10.1097/01.hjh.0000226191.36558.9c.
- Krzesinski JM, Saint-Remy A. Spurious systolic hypertension in youth: what does it really mean in clinical practice?. J Hypertens 2006;24:999-1001. https://doi.org/10.1097/01.hjh.0000226184.98439.7d.
- Lurbe E, Redon J. Isolated systolic hypertension in young people is not spurious and should be treated: con side of the argument. Hypertension 2016;68:276-80. https://doi.org/10.1161/hypertensionaha.116.06548.
- Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens 2003;16:229-32. https://doi.org/10.1016/S0895-7061(02)03255-7.
- McEniery CM, Franklin SS, Cockcroft JR, Wilkinson IB. Isolated systolic hypertension in young people is not spurious and should be treated: pro side of the argument. Hypertension 2016;68:269-75. https://doi.org/10.1161/hypertensionaha.116.06547.
- McEniery CM, Wilkinson IB, Cockcroft JR. Systolic hypertension in young adults: spurious definition of a genuine condition. J Hypertens 2006;24:2316-17. https://doi.org/10.1097/01.hjh.0000249715.51640.38.
- O’Rourke MF, Adji A. Guidelines on guidelines: focus on isolated systolic hypertension in youth. J Hypertens 2013;31:649-54. https://doi.org/10.1097/HJH.0b013e32835d8230.
- O’Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med 2000;5:141-5. https://doi.org/10.1177/1358836X0000500303.
- Protogerou AD, Blacher J, Safar ME. Isolated systolic hypertension: ‘to treat or not to treat’ and the role of central haemodynamics. J Hypertens 2013;31:655-8. https://doi.org/10.1097/HJH.0b013e32835f7e2b.
- Saladini F, Dorigatti F, Santonastaso M, Mos L, Ragazzo F, Bortolazzi A, et al. Natural history of hypertension subtypes in young and middle-age adults. Am J Hypertens 2009;22:531-7. https://doi.org/10.1038/ajh.2009.21.
- Saladini F, Santonastaso M, Mos L, Benetti E, Zanatta N, Maraglino G, et al. HARVEST Study Group . Isolated systolic hypertension of young-to-middle-age individuals implies a relatively low risk of developing hypertension needing treatment when central blood pressure is low. J Hypertens 2011;29:1311-19. https://doi.org/10.1097/HJH.0b013e3283481a32.
- Stepień M, Banach M, Jankowski P, Rysz J. Clinical implications of non-invasive measurement of central aortic blood pressure. Curr Vasc Pharmacol 2010;8:747-52. https://doi.org/10.2174/157016110793563852.
- Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform?. Mayo Clin Proc 2010;85:460-72. https://doi.org/10.4065/mcp.2009.0336.
- O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990;15:339-47. https://doi.org/10.1161/01.HYP.15.4.339.
- O’Rourke MF, Adji A. Basis for use of central blood pressure measurement in office clinical practice. J Am Soc Hypertens 2008;2:28-3. https://doi.org/10.1016/j.jash.2007.08.006.
- O’Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl 1996;14:S147-57.
- Cheng HM, Sung SH, Shih YT, Chuang SY, Yu WC, Chen CH. Measurement accuracy of a stand-alone oscillometric central blood pressure monitor: a validation report for Microlife WatchBP Office Central. Am J Hypertens 2013;26:42-50. https://doi.org/10.1093/ajh/hps021.
- Endes S, Bachler M, Li Y, Mayer C, Hanssen H, Hametner B, et al. Feasibility of oscillometric aortic pressure and stiffness assessment using the VaSera VS-1500: comparison with a common tonometric method. Blood Press Monit 2015;20:273-9. https://doi.org/10.1097/MBP.0000000000000137.
- Shoji T, Nakagomi A, Okada S, Ohno Y, Kobayashi Y. Invasive validation of a novel brachial cuff-based oscillometric device (SphygmoCor XCEL) for measuring central blood pressure. J Hypertens 2017;35:69-75. https://doi.org/10.1097/HJH.0000000000001135.
- Ott C, Haetinger S, Schneider MP, Pauschinger M, Schmieder RE. Comparison of two noninvasive devices for measurement of central systolic blood pressure with invasive measurement during cardiac catheterization. J Clin Hypertens 2012;14:575-9. https://doi.org/10.1111/j.1751-7176.2012.00682.x.
- Williams B, Lacy PS, Baschiera F, Brunel P, Düsing R. Novel description of the 24-hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: The Ambulatory Central Aortic Pressure (AmCAP) Study. Hypertension 2013;61:1168-76. https://doi.org/10.1161/HYPERTENSIONAHA.111.00763.
- Shih YT, Cheng HM, Sung SH, Hu WC, Chen CH. Application of the N-point moving average method for brachial pressure waveform-derived estimation of central aortic systolic pressure. Hypertension 2014;63:865-70. https://doi.org/10.1161/HYPERTENSIONAHA.113.02229.
- Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, et al. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens 2011;29:454-9. https://doi.org/10.1097/HJH.0b013e3283424b4d.
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, et al. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension 2008;51:848-55. https://doi.org/10.1161/HYPERTENSIONAHA.107.101725.
- Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol 2008;51:2432-9. https://doi.org/10.1016/j.jacc.2008.03.031.
- Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007;50:197-203. https://doi.org/10.1161/HYPERTENSIONAHA.107.089078.
- Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension 2002;39:735-8. https://doi.org/10.1161/hy0202.098325.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318-27. https://doi.org/10.1016/j.jacc.2009.10.061.
- Williams B, Lacy PS. Central haemodynamics and clinical outcomes: going beyond brachial blood pressure?. Eur Heart J 2010;31:1819-22. https://doi.org/10.1093/eurheartj/ehq125.
- Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens 2010;28:384-8. https://doi.org/10.1097/HJH.0b013e328333d228.
- Asmar RG, London GM, O’Rourke ME, Safar ME. REASON Project Coordinators and Investigators . Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension 2001;38:922-6. https://doi.org/10.1161/hy1001.095774.
- Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. American J Hypertens 2006;19:214-9. https://doi.org/10.1016/j.amjhyper.2005.08.007.
- Hirata K, Vlachopoulos C, Adji A, O’Rourke MF. Benefits from angiotensin-converting enzyme inhibitor ‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery?. J Hypertens 2005;23:551-6. https://doi.org/10.1097/01.hjh.0000160211.56103.48.
- Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension 2009;54:409-13. https://doi.org/10.1161/HYPERTENSIONAHA.109.133801.
- Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens 2004;17:118-23. https://doi.org/10.1016/j.amjhyper.2003.09.012.
- Williams B, Lacy PS. Central aortic pressure and clinical outcomes. J Hypertens 2009;27:1123-5. https://doi.org/10.1097/HJH.0b013e32832b6566.
- Bhuiyan AR, Srinivasan SR, Chen W, Paul TK, Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis 2006;189:1-7. https://doi.org/10.1016/j.atherosclerosis.2006.02.011.
- Devereux RB, Okin PM, Roman MJ. Left ventricular hypertrophy as a surrogate end-point in hypertension. Clin Exp Hypertens 1999;21:583-93. https://doi.org/10.3109/10641969909060991.
- Korner PI, Jennings GL. Assessment of prevalence of left ventricular hypertrophy in hypertension. J Hypertens 1998;16:715-23. https://doi.org/10.1097/00004872-199816060-00001.
- Mancia G, Giannattasio C, Failla M, Sega R, Parati G. Systolic blood pressure and pulse pressure: role of 24-h mean values and variability in the determination of organ damage. J Hypertens Suppl 1999;17:S55-61.
- Mansoor GA, Massie BM. Left ventricular hypertrophy: a potent cardiovascular risk factor and its relationship to office and ambulatory blood pressure. Blood Press Monit 1999;4:19-22. https://doi.org/10.1097/00126097-199912001-00005.
- Otterstad JE, Smiseth O, Kjeldsen SE. Hypertensive left ventricular hypertrophy: pathophysiology, assessment and treatment. Blood Press 1996;5:5-15. https://doi.org/10.3109/08037059609062101.
- Rizzoni D, Agabiti-Rosei E. Relationships of cardiac function and structure to blood pressure rhythms. Ann N Y Acad Sci 1996;783:159-71. https://doi.org/10.1111/j.1749-6632.1996.tb26714.x.
- Krauser DG, Devereux RB. Ventricular hypertrophy and hypertension: prognostic elements and implications for management. Herz 2006;31:305-16. https://doi.org/10.1007/s00059-006-2819-5.
- Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 2008;21:500-8. https://doi.org/10.1038/ajh.2008.16.
- Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2009;2:191-8. https://doi.org/10.1161/CIRCIMAGING.108.819938.
- Gosse P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl 2005;23:S27-33. https://doi.org/10.1097/01.hjh.0000165625.79933.9a.
- Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol 2006;48:2285-92. https://doi.org/10.1016/j.jacc.2006.03.072.
- Gupta A, Schiros CG, Gaddam KK, Aban I, Denney TS, Lloyd SG, et al. Effect of spironolactone on diastolic function in hypertensive left ventricular hypertrophy. J Hum Hypertens 2015;29:241-6. https://doi.org/10.1038/jhh.2014.83.
- Muthurangu V, Lurz P, Critchely JD, Deanfield JE, Taylor AM, Hansen MS. Real-time assessment of right and left ventricular volumes and function in patients with congenital heart disease by using high spatiotemporal resolution radial k-t SENSE. Radiology 2008;248:782-91. https://doi.org/10.1148/radiol.2482071717.
- Parikh JD, Hollingsworth KG, Wallace D, Blamire AM, MacGowan GA. Left ventricular functional, structural and energetic effects of normal aging: comparison with hypertension. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0177404.
- Wu V, Chyou JY, Chung S, Bhagavatula S, Axel L. Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: comparison with tissue Doppler imaging. J Cardiovasc Magn Reson 2014;16. https://doi.org/10.1186/s12968-014-0071-3.
- Stokes GS. Drug-induced hypertension: pathogenesis and management. Drugs 1976;12:222-30. https://doi.org/10.2165/00003495-197612030-00005.
- Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 2003;17:323-9. https://doi.org/10.1002/jmri.10262.
- Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 2005;7:775-82. https://doi.org/10.1080/10976640500295516.
- Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 1999;1:7-21. https://doi.org/10.3109/10976649909080829.
- Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press 1992;1:113-19. https://doi.org/10.3109/08037059209077502.
- Dubois DDE. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med 1916;17:863-71. https://doi.org/10.1001/archinte.1916.00080130010002.
- Johnson DB, Foster RE, Barilla F, Blackwell GG, Roney M, Stanley AW, et al. Angiotensin-converting enzyme inhibitor therapy affects left ventricular mass in patients with ejection fraction > 40% after acute myocardial infarction. J Am Coll Cardiol 1997;29:49-54. https://doi.org/10.1016/S0735-1097(96)00451-2.
- Reichek N, Devereux RB, Rocha RA, Hilkert R, Hall D, Purkayastha D, et al. Magnetic resonance imaging left ventricular mass reduction with fixed-dose angiotensin-converting enzyme inhibitor-based regimens in patients with high-risk hypertension. Hypertension 2009;54:731-7. https://doi.org/10.1161/HYPERTENSIONAHA.109.130641.
- Simpson HJ, Gandy SJ, Houston JG, Rajendra NS, Davies JI, Struthers AD. Left ventricular hypertrophy: reduction of blood pressure already in the normal range further regresses left ventricular mass. Heart 2010;96:148-52. https://doi.org/10.1136/hrt.2009.177238.
- Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, et al. Effect of the direct Renin inhibitor aliskiren, the Angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation 2009;119:530-7. https://doi.org/10.1161/CIRCULATIONAHA.108.826214.
- Devereux RB, Palmieri V, Liu JE, Wachtell K, Bella JN, Boman K, et al. Progressive hypertrophy regression with sustained pressure reduction in hypertension: the Losartan Intervention For Endpoint Reduction study. J Hypertens 2002;20:1445-50. https://doi.org/10.1097/00004872-200207000-00033.
- Wachtell K, Dahlöf B, Rokkedal J, Papademetriou V, Nieminen MS, Smith G, et al. Change of left ventricular geometric pattern after 1 year of antihypertensive treatment: the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. Am Heart J 2002;144:1057-64. https://doi.org/10.1067/mhj.2002.126113.
- Devereux RB, Pickering TG, Harshfield GA, Kleinert HD, Denby L, Clark L, et al. Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation 1983;68:470-6. https://doi.org/10.1161/01.CIR.68.3.470.
- Nyström F, Malmqvist K, Lind L, Kahan T. Nurse-recorded clinic and ambulatory blood pressures correlate equally well with left ventricular mass and carotid intima-media thickness. J Intern Med 2005;257:514-22. https://doi.org/10.1111/j.1365-2796.2005.01489.x.
- Cain PA, Ahl R, Hedstrom E, Ugander M, Allansdotter-Johnsson A, Friberg P, et al. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging 2009;9. https://doi.org/10.1186/1471-2342-9-2.
- Carlsson MB, Trägårdh E, Engblom H, Hedström E, Wagner G, Pahlm O, et al. Left ventricular mass by 12-lead electrocardiogram in healthy subjects: comparison to cardiac magnetic resonance imaging. J Electrocardiol 2006;39:67-72. https://doi.org/10.1016/j.jelectrocard.2005.07.005.
- Clay S, Alfakih K, Radjenovic A, Jones T, Ridgway JP, Sinvananthan MU. Normal range of human left ventricular volumes and mass using steady state free precession MRI in the radial long axis orientation. MAGMA 2006;19:41-5. https://doi.org/10.1007/s10334-005-0025-8.
- Maceira AM, Prasad SK, Pennell DJ, Mohiaddin RH. Integrated evaluation of hypertensive patients with cardiovascular magnetic resonance. Int J Cardiol 2008;125:383-90. https://doi.org/10.1016/j.ijcard.2007.03.002.
- Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:357-65. https://doi.org/10.2214/AJR.04.1868.
- Prakken NH, Velthuis BK, Teske AJ, Mosterd A, Mali WP, Cramer MJ. Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur J Cardiovasc Prev Rehabil 2010;17:198-203. https://doi.org/10.1097/HJR.0b013e3283347fdb.
- Salton CJ, Chuang ML, O’Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol 2002;39:1055-60. https://doi.org/10.1016/S0735-1097(02)01712-6.
- Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 2002;40:1856-63. https://doi.org/10.1016/S0735-1097(02)02478-6.
- Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an n-point moving average method. J Am Coll Cardiol 2011;57:951-61. https://doi.org/10.1016/j.jacc.2010.09.054.
- Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006;13:101-7. https://doi.org/10.5551/jat.13.101.
- Williams B. Arterial ageing: from the shadows to centre stage in cardiovascular medicine. Eur Heart J Suppl 2017;19:B1-3. https://doi.org/10.1093/eurheartj/suw057.
- UK MHRA . The Yellow Card Scheme n.d. https://yellowcard.mhra.gov.uk/ (accessed 17 March 2013).
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207-14. https://doi.org/10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2017.
- World Health Organization (WHO) . ATC Classification Index With DDDs n.d. www.whocc.no/atc_ddd_index/ (accessed 3 September 2018).
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. https://doi.org/10.1093/eurheartj/ehy339.
- Bülow R, Ittermann T, Dörr M, Poesch A, Langner S, Völzke H, et al. Reference ranges of left ventricular structure and function assessed by contrast-enhanced cardiac MR and changes related to ageing and hypertension in a population-based study. Eur Radiol 2018;28:3996-4005. https://doi.org/10.1007/s00330-018-5345-y.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000;35:580-6. https://doi.org/10.1161/01.HYP.35.2.580.
- Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148-55. https://doi.org/10.1016/j.jacc.2008.09.014.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561-6. https://doi.org/10.1056/NEJM199005313222203.
- Haring B, Omidpanah A, Suchy-Dicey AM, Best LG, Verney SP, Shibata DK, et al. Left ventricular mass, brain magnetic resonance imaging, and cognitive performance: results from the Strong Heart Study. Hypertension 2017;70:964-71. https://doi.org/10.1161/HYPERTENSIONAHA.117.09807.
- Bruder O, Jensen CJ, Bell M, Rummel R, Boehm G, Klebs S, et al. Effects of the combinations of amlodipine/valsartan versus losartan/hydrochlorothiazide on left ventricular hypertrophy as determined with magnetic resonance imaging in patients with hypertension. J Drug Assess 2012;1:1-10. https://doi.org/10.3109/21556660.2011.639418.
- Miller AB, Reichek N, St John Sutton M, Iyengar M, Henderson LS, Tarka EA, et al. Importance of blood pressure control in left ventricular mass regression. J Am Soc Hypertens 2010;4:302-10. https://doi.org/10.1016/j.jash.2010.09.003.
- Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol 2009;54:505-12. https://doi.org/10.1016/j.jacc.2009.03.066.
- Desai CS, Bartz TM, Gottdiener JS, Lloyd-Jones DM, Gardin JM. Usefulness of left ventricular mass and geometry for determining 10-year prediction of cardiovascular disease in adults aged > 65 years (from the Cardiovascular Health Study). Am J Cardiol 2016;118:684-90. https://doi.org/10.1016/j.amjcard.2016.06.016.
- Hoang K, Zhao Y, Gardin JM, Carnethon M, Mukamal K, Yanez D, et al. LV Mass as a predictor of CVD events in older adults with and without metabolic syndrome and diabetes. JACC Cardiovasc Imaging 2015;8:1007-15. https://doi.org/10.1016/j.jcmg.2015.04.019.
- Leigh JA, O’Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged ≥ 65 years. Am J Cardiol 2016;117:1831-5. https://doi.org/10.1016/j.amjcard.2016.03.020.
- Saeed M, Liu H, Liang CH, Wilson MW. Magnetic resonance imaging for characterizing myocardial diseases. Int J Cardiovasc Imaging 2017;33:1395-414. https://doi.org/10.1007/s10554-017-1127-x.
- Saeed M, Van TA, Krug R, Hetts SW, Wilson MW. Cardiac MR imaging: current status and future direction. Cardiovasc Diagn Ther 2015;5:290-31. https://doi.org/10.3978/j.issn.2223-3652.2015.06.07.
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995-1003. https://doi.org/10.1016/S0140-6736(02)08089-3.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004;292:2343-9. https://doi.org/10.1001/jama.292.19.2343.
- Pokharel PJB. Regression of left ventricular hypertrophy: lessons from clinical trials. Evid Based Med 2013;1:13-21. https://doi.org/10.13172/2053-2636-1-2-1110.
- Larstorp AC, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlöf B, et al. Regression of ECG-LVH is associated with lower risk of new-onset heart failure and mortality in patients with isolated systolic hypertension; the LIFE study. Am J Hypertens 2012;25:1101-9. https://doi.org/10.1038/ajh.2012.86.
- MacDonald TM, Williams B, Webb DJ, Morant S, Caulfield M, Cruickshank JK, et al. Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double-blind randomized controlled trial. J Am Heart Assoc 2017;6. https://doi.org/10.1161/JAHA.117.006986.
- Schmieder RE, Wagner F, Mayr M, Delles C, Ott C, Keicher C, et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Heart J 2017;38:3308-17. https://doi.org/10.1093/eurheartj/ehx525.
- Bella JN, Palmieri V, Wachtell K, Liu JE, Gerdts E, Nieminen MS, et al. Sex-related difference in regression of left ventricular hypertrophy with antihypertensive treatment: the LIFE study. J Hum Hypertens 2004;18:411-16. https://doi.org/10.1038/sj.jhh.1001708.
Appendix 1 Cardiac magnetic resonance imaging study protocol for data acquisition
| Activity/magnetic resonance sequence |
|---|
|
Appendix 2 Individual description of adverse events and serious adverse events in the TREAT CASP randomised controlled trial and the observational study
| Study ID | Nature of event | Group | Relevance | Status |
|---|---|---|---|---|
| 1033 (incidental MRI findings) | Incidental MRI finding: ARVC, trivial TR, mild–moderate RV function, some focal dyskinesis, low–normal LV function and no reciprocal changes | Observational group | SAE – not study related | Continued study participation |
| 1068 (incidental MRI findings) | Incidental MRI finding: right hydronephrosis and normal creatinine levels | Withdrew consent before study randomisation | SAE – not study related | Withdrew consent |
| 1074 (incidental MRI findings) | Incidental MRI findings: simple cysts in liver and spleen – benign looking and trivial AR | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1074 (renal and urinary disorders) | Asymptomatic microscopic haematuria | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1079 (incidental MRI findings) | Incidental MRI finding: left-upper pole kidney cyst (1.5–2 cm in size) | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1085 (cardiovascular disorders) | Asymptomatic incidental ECG findings: LBBB, sinus bradycardia, shortened QTc and borderline first-degree AVB (change from baseline ECG) | Observational study | AE – not study related | Continued study participation |
| 1115 (cardiovascular disorders) | Incidental ECG finding: WPW syndrome | RCT – high CASP (treatment) | SAE – not study related | Continued study participation |
| 1117 (musculoskeletal disorders) | Lower back pain | Observational study | AE – not study related | Continued study participation |
| 1125 (hepatic) | Incidental finding: persistent lymphocytosis – WCC of 13.98 × 109/l (baseline), WCC of 17.66 × 109/l (at study closeout) and of 17.67 × 109/l (at 5 months after study closeout) | RCT – high CASP (treatment) | SAE – not study related | Continued study participation |
| 1130 (musculoskeletal disorders) | Lower back pain | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1133 (incidental MRI findings) | Incidental MRI finding: liver cyst – likely benign | Observational study | AE – not study related | Continued study participation |
| 1134 (other) | Migraine | Observational study | AE – not study related | Continued study participation |
| 1138 (other) | Typical chest pain | Observational study | AE – not study related | Continued study participation |
| 1144 (cancer) | Recently diagnosed with squamous cell cancer of tonsils | RCT – high CASP (treatment) | SAE – not study related | Continued study participation |
| 1146 (injury) | Hospitalised – arthroscopy of right knee | Observational study | SAE – not study related | Continued study participation |
| 1148 (gastrointestinal disorders) | Episodes of GERD, under GP started proton pump inhibitor | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1186 (hepatic) | Incidental finding: chronic elevation in bilirubin levels, possible Gilbert syndrome | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1186 (other) | Atypical chest pain | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1197 (infections) | Upper respiratory tract infection | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1198 (orthostatic disorders) | Light-headedness with postural changes | RCT – high CASP (treatment) | AE – medication related (causality undetermined) | Continued study participation |
| 1198 (musculoskeletal disorders) | Lower back pain | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1227 (infections) | Upper respiratory tract infection | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1233 (cardiovascular disorders) | Prolonged QTc | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1236 (other) | Decreased libido | RCT – high CASP (treatment) | AE – medication related (causality undetermined) | Continued study participation |
| 1242 (other) | Atypical chest pain | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1242 (other) | Balanitis | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1247 (infections) | Lower respiratory tract infection | Observational study | AE – not study related | Continued study participation |
| 1247 (injury) | Bike accident | Observational | AE – not study related | Continued study participation |
| 1288 (cardiovascular disorders) | Incidental ECG finding: first-degree and incomplete RBBB | Observational study | AE – not study related | Continued study participation |
| 1312 (orthostatic disorders) | Vertigo | Observational study | AE – not study related | Continued study participation |
| 1318 (injury) | Tibial plateau fracture requiring hospitalisation and surgery | RCT – high CASP (treatment) | SAE – not study related | Continued study participation |
| 1334 (hepatic) | Probable Gilbert syndrome | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1340 (other) | Atypical chest pain | Observational study | AE – not study related | Continued study participation |
| 1354 (other) | Sinus problem | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1359 (other) | Right exophthalmos | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1367 (other) | Skin rash | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1367 (musculoskeletal disorders) | Knee pain | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1387 (musculoskeletal disorders) | Gout recurrence | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1414 (renal and urinary disorders) | Incidental finding: eGFR – 65 µmol/l (adjusted for ethnicity), no urinary abnormalities | RCT – high CASP (treatment) | SAE – not study related | Continued study participation |
| 1414 (other) | Mild ankle oedema | RCT – high CASP (treatment) | AE – medication related (causality undetermined) | Continued study participation |
| 1425 (other) | Cough | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1469 (incidental MRI findings) | Incidental MRI finding: left-sided simple renal cyst | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1483 (orthostatic disorders) | Light-headedness with postural changes | RCT – high CASP (treatment) | AE – medication related (causality undetermined) | Continued study participation |
| 1504 (other) | Hand swelling with exercise and heat | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1515 (incidental MRI findings) | Incidental MRI finding: dilated aortic root | RCT – high CASP (no treatment) | SAE – not study related | Continued study participation |
| 1528 (renal and urinary disorders) | Incidental asymptomatic microscopic haematuria | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1535 (other) | Flu | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1536 (other) | New diagnosis of sleep apnoea, seen by a specialist privately | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1544 (other) | Hospitalised for 1 day and off work for 1 month, discharged on antihistamines | RCT – high CASP (no treatment) | SAE – not study related | Continued study participation |
| 1567 (orthostatic disorders) | Light-headedness with postural changes | RCT – high CASP (treatment) | AE – medication related (causality undetermined) | Continued study participation |
| 1608 (incidental MRI findings) | Incidental MRI finding: congenital single kidney, normal renal function | RCT – high CASP (no treatment) | SAE – not study related | Continued study participation |
| 1608 (gastrointestinal disorders) | Traveller’s diarrhoea after visit to Mexico, saw GP – stool sample sent (fully resolved) | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1617 (incidental MRI findings) | Incidental MRI finding: bicuspid aortic valve | RCT – high CASP (no treatment) | SAE – not study related | Continued study participation |
| 1660 (infections) | Hospitalised for perianal abscesses, with probable ascending UTI | RCT – high CASP (treatment) | SAE – not study related | Continued study participation |
| 1666 (cancer) | Acute leukaemia | RCT – high CASP (no treatment) | SAE – not study related | Withdrew |
| 1682 (other) | Symptomatic hypogonadism – low levels of testosterone | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1684 (other) | Nausea, dizziness, lightheaded after taking first dose of 50 mg of losartan – treatment discontinued | RCT – high CASP (treatment) | AE – medication related (probably not causal) | Continued study participation (intention to treat) |
| 1689 (hepatic) | Biliary colic – under outpatient care of a general surgeon | RCT – high CASP (no treatment) | AE – not study related | Continued study participation |
| 1693 (infections) | Insect bite to right leg with subsequent Staphylococcus infection requiring hospitalisation, multiple antibiotic courses | Observational study | SAE – not study related | Continued study participation |
| 1694 (cardiovascular disorders) | Incidental ECG finding: three asymptomatic PVCs noted on 12-lead ECG | RCT – high CASP (treatment) | AE – not study related | Continued study participation |
| 1701 (incidental MRI findings) | Incidental MRI finding: suspected right hydronephrosis. Subsequently diagnosed as renal stone disease – managed as an outpatient | Observational study | SAE – not study related | Continued study participation |
| 1717 (other) | Hypertensive crisis at altitude while travelling in Chile | RCT – high CASP (no treatment) | SAE – not study related | Continued study participation |
| 1749 (other) | Possible sarcoidosis flare – previously in remission | RCT – high CASP (no treatment) | SAE – not study related | Continued study participation |
| 8118 (orthostatic disorders) | Light-headedness with postural changes | RCT – high CASP (treatment) | AE – medication related (causality undetermined) | Continued study participation |
| 8235 (incidental MRI findings) | Incidental MRI finding: eventually benign renal cyst on ultrasound | Observational study | AE – not study related | Continued study participation |
Appendix 3 The TREAT CASP post-trial patient and public involvement event: 10 April 2019
Account of the event reported to be published in the University College London Hospitals Biomedical Research Centre’s annual report and website
Blood pressure TREAT CASP trial participants hear trial results first at the innovative event
Patients who took part in a clinical trial which looked at a new way of measuring BP and whether people with mild hypertension (high BP) could benefit from BP-lowering medication got to hear the trial results last week before the findings are published later in the year.
Chief Investigator and UCLH BRC Director Professor Bryan Williams presented results and implications of the TREAT CASP study to men who took part in the study before answering questions from participants at the event at UCL.
Trial participants do not usually hear about findings directly and before they are published in a journal, but Professor Williams said that it was important for researchers to let participants – who have given up their time and made a real effort to take part in research – know what the results are and what they mean, adding that research participants want to know trial results.
‘The amazing thing about tonight is that all the people in the audience are the patients who participated in the study and it’s absolutely fantastic,’ he said. ‘They have stayed behind, they are chatting with all the investigators, and they are really interested in the findings of the study. And I really think we need to do this more often.’
Martin Marmoy-Haynes, who took part in the trial and attended the event, said: ‘Tonight’s been great. There’s been an excellent presentation and interesting results. So I’m pleased to take part.’.
Another attendee, Paul May, said he felt incredibly informed as a result of attending the event and felt privileged to be invited to the event.
The TREAT CASP study involved 120 participants under 55 and included only men, due to the complexity of carrying out a drug treatment study in women of childbearing age. But researchers said the results applied equally to women.
A major rationale for the study was uncertainly over whether patients under 55 [years] with grade 1 hypertension (BP measured in the clinic of 140–159/90–99 mmHg) should be treated. Countries around the world, including the United States and in Europe, recommend treatment for this group of patients. Currently, UK guidelines advise against treatment in this group of treatment.
Study results will be announced later in 2019.
Photographs taken during the TREAT CASP trial’s patient and public involvement event


Programme schedule for the TREAT CASP trial’s patient and public involvement event
The TREAT CASP trial’s patient and public involvement participant questionnaire and summary of responses from 25 attendees
| Question number | Question | Response, n (%) | |||
|---|---|---|---|---|---|
| Yes | No | Unsure | No response | ||
| 1 | Did you find tonight’s event helpful? | 25 (100) | 0 | 0 | 0 |
| 2 | Did you feel the results you heard tonight help your understanding of hypertension? | 25 (100) | 0 | 0 | 0 |
| 3 | Did you find participating for the TREAT CASP study of benefit to you? In what way? | 25 (100) | 0 | 0 | 0 |
| 4 | Based on your experience volunteering for this study, would you consider taking part in other studies that UCL/UCLH offer? | 24 (96) | 0 | 1 (4) | 0 |
| 5 | Do you feel the NHS should conduct more research like this? | 25 (100) | 0 | 0 | 0 |
| 6 | What motivated you to take part in the TREAT CASP study? | ||||
| 6.1 | Information and advice about high BP | 25 (100) | 0 | 0 | 0 |
| 6.2 | A new device (watch) to measure your BP | 3 (12) | 13 (52) | 5 (20) | 4 (16) |
| 6.3 | Health check | 22 (88) | 2 (8) | 0 | 1 (4) |
| 6.4 | Wanting information about lifestyle changes | 11 (44) | 8 (32) | 2 (8) | 4 (16) |
| 6.5 | Tips on weight management | 4 (16) | 15 (60) | 1 (4) | 5 (20) |
| 6.6 | Interested in the science and helping out | 22 (88) | 2 (8) | 0 | 1 (4) |
| 6.7 | Other – please describe | ||||
| 7 | Do you have any other comments or suggestions you would like to make regarding your participation in the TREAT CASP study? | ||||
| 8 | Do you have any other comments or suggestions you would like to make regarding tonight’s event? | ||||
Individual comments and question answers provided anonymously by study participants at the TREAT CASP trial’s patient and public involvement event
Questions
| Question number | Question |
|---|---|
| 1 | Did you find tonight’s event helpful? |
| 2 | Did you feel the results you heard tonight help your understanding of hypertension? |
| 3 | Did you find participating for the TREAT CASP study of benefit to you? In what way? |
| 4 | Based on your experience volunteering for this study would you consider taking part in other studies that UCL/UCLH offer? |
| 5 | Do you feel the NHS should conduct more research like this? |
| 6 | What motivated you to take part in the TREAT CASP study? |
| 6.1 | Information and advice about high BP |
| 6.2 | A new device (watch) to measure your BP |
| 6.3 | Health check |
| 6.4 | Wanting information about lifestyle changes |
| 6.5 | Tips on weight management |
| 6.6 | Interested in the science and helping out |
| 6.7 | Other – please describe |
| 7 | Do you have any other comments or suggestions you would like to make regarding your participation in the TREAT CASP study? |
| 8 | Do you have any other comments or suggestions you would like to make regarding tonight’s event? |
Anonymous responses
| Respondent | Question | Yes | No | Unsure | Comments |
|---|---|---|---|---|---|
| 1 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | I asked my GP for losartan, have seen great results, thank you | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | |||||
| 6.3 | 1 | ||||
| 6.4 | |||||
| 6.5 | |||||
| 6.6 | 1 | ||||
| 6.7 | Family history of stroke | ||||
| 7 | |||||
| 8 | Fantastic | ||||
| 2 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | MRI discloses I have bicuspid valve | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | |||||
| 6.3 | 1 | ||||
| 6.4 | |||||
| 6.5 | |||||
| 6.6 | Youngest of 12 and many older siblings having CV [cardiovascular] issues | ||||
| 6.7 | It was great to take part, and everyone involved were very caring and genuine | ||||
| 7 | |||||
| 8 | |||||
| 3 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Understanding of high BP and ways to manage it | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 4 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Having a MRI scan was interesting. Having discussions about lifestyle and hypertension | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | Supply participants with images of their heart as a keepsake. Provide educational materials for schools for GCSE and A level science, graph skills, statistics, correlations, etc. | ||||
| 8 | Perfect pitch – at right level for all the audience. Can a copy of the PowerPoint be provided? Provide pens | ||||
| 5 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Better understanding of BP and its effects | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | My doctor seemed to have a lack of information with regards to my treatment due to the study (I was randomised to 50 [mg] then 100 mg losartan) | ||||
| 8 | No | ||||
| 6 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Made me more aware of the condition | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | More confident approaching GP | ||||
| 7 | Any future studies I would be interested | ||||
| 8 | Thank-you to the team | ||||
| 7 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | ||||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 8 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Understanding access to resources – testing | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | Very grateful thanks | ||||
| 8 | See above | ||||
| 9 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | ||||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | Excellent event – very informative and well presented | ||||
| 10 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Helped me with saving my life! I had a triple bypass in Oct 2017 | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | |||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 11 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | ||||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 12 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | A better understanding | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 13 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | ||||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 14 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Educational and had improved my lifestyle choices, e.g. exercise and diet | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 15 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Helped me look at controlling and monitoring my BP | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 16 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Early warning of danger signs allowing corrective action/medication | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | Staff/team were excellent, professional, pleasant, thoughtful, fun. Prof Williams is a brilliant communicator | ||||
| 8 | None. It was very well done. Very clear, very thoughtful, actually inspiring me to assist in more trials. Look forward to getting the letter from Prof Williams | ||||
| 17 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Gave me a better understanding of the importance of grade 1 HTN and risk factors | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | |||||
| 6.3 | 1 | ||||
| 6.4 | |||||
| 6.5 | |||||
| 6.6 | 1 | ||||
| 6.7 | Help with further information in the study of HTN | ||||
| 7 | Been very useful and interesting in taking part in the study | ||||
| 8 | |||||
| 18 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | I am now prescribed losartan on the back of this study | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 19 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Very useful to explore in more detail my BP and get two MRI scans. Good to assist the study of an important health issue | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | For me the administration of the study worked very well. The times I needed to participate were efficiently organised and run. Very Impressive | ||||
| 8 | An excellent event | ||||
| 20 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | I am continuing the treatment and would not have had the treatment unless I had taken part. Thank-you on behalf of my family | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 21 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Greatly expanded my knowledge of BP and got me on medication | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | |||||
| 8 | |||||
| 22 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Understanding of risk factors the need for treatment to live long! | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | My BP has been borderline for a long time | ||||
| 7 | |||||
| 8 | |||||
| 23 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Very informative | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | The importance of clinical trials | ||||
| 7 | |||||
| 24 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | Data and detailed explanation | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | |||||
| 6.3 | |||||
| 6.4 | |||||
| 6.5 | |||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | Negotiate a swap between participants who did and did not want treatment. If it satisfied the stratification of the groups | ||||
| 8 | Can we have the slides? | ||||
| 25 | 1 | 1 | |||
| 2 | 1 | ||||
| 3 | 1 | You have sent me to GP | |||
| 4 | 1 | ||||
| 5 | 1 | ||||
| 6.1 | 1 | ||||
| 6.2 | 1 | ||||
| 6.3 | 1 | ||||
| 6.4 | 1 | ||||
| 6.5 | 1 | ||||
| 6.6 | 1 | ||||
| 6.7 | |||||
| 7 | Could you have the study for longer with a closer look at triggers, please? | ||||
| 8 |
Sample copy of the TREAT CASP trial’s patient and public involvement event questionnaire
List of abbreviations
- ABPM
- ambulatory blood pressure monitoring
- AE
- adverse event
- AR
- adverse reaction
- ARB
- angiotensin receptor blocker
- aVL
- augmented vector left
- BMI
- body mass index
- BP
- blood pressure
- BrBP
- brachial blood pressure
- BRC
- Biomedical Research Centre
- BrSBP
- brachial systolic blood pressure
- CASP
- central aortic systolic pressure
- CAVI
- cardio-ankle vascular index
- CCB
- calcium channel blocker
- CCTU
- Comprehensive Clinical Trials Unit
- CG
- Clinical Guidance
- CI
- confidence interval
- CKD
- chronic kidney disease
- cMRI
- cardiac magnetic resonance imaging
- CONSORT
- Consolidated Standards of Reporting Trials
- CPRD
- Clinical Practice Research Datalink
- CTIMP
- clinical trial of an investigational medicinal product
- CVD
- cardiovascular disease
- DBP
- diastolic blood pressure
- DDD
- defined daily dose
- ECG
- electrocardiography
- eGFR
- estimated glomerular filtration rate
- GCP
- good clinical practice
- GP
- general practitioner
- HFpEF
- heart failure with preserved ejection fraction
- HMOD
- hypertension-mediated organ damage
- ICC
- intraclass correlation coefficient
- IDEAL
- iterative decomposition of water and fat with echo asymmetry and least squares estimation
- LV
- left ventricular
- LVH
- left ventricular hypertrophy
- LVMI
- left ventricular mass index
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NOCLOR
- North Central London Research Consortium
- o.d.
- once daily
- PP
- pulse pressure
- PPI
- patient and public involvement
- PROBE
- prospective, randomised, open-label blinded end point
- PWV
- pulse wave velocity
- QA
- quality assurance
- QC
- quality control
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SBP
- systolic blood pressure
- SD
- standard deviation
- SENSE
- sensitivity encoding
- SF-36
- Short Form questionnaire-36 items
- SUSAR
- suspected unexpected serious adverse reaction
- UACR
- urinary albumin-to-creatinine ratio
- UCL
- University College London
- UCLH
- University College London Hospitals
- WHO
- World Health Organization
