Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 11/47/01. The contractual start date was in June 2013. The final report began editorial review in April 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Robert Howard reports membership of the Health Technology Assessment (HTA) Commissioning Board between 2013 and 2018. John O’Brien reports personal fees from TauRx Pharmaceuticals Ltd (Singapore) and GE Healthcare (Chicago, IL, USA), Eli Lilly and Company (Indianapolis, IN, USA) and Eisai Co., Ltd (Tokyo, Japan), outside the submitted work. Gill Livingston reports membership of the HTA Clinical Trials Board Associate from 2007 to 2010. Peter Bentham reports personal fees from TauRx Pharmaceuticals Ltd outside the submitted work. Craig Ritchie reports other payments from Roche Holding AG (Basel, Switzerland), Nutricia International B.V. (Zoetermeer, the Netherlands), Actinogen Medical (Sydney, NSW, Australia), Kyowa Hakko Kirin Co., Ltd (Singapore), Biogen Inc. (Cambridge, MA, USA) and Merck Sharp & Dohme Corp. (Kenilworth, NJ, USA) and grants from Janssen Pharmaceutica (Beerse, Belgium) outside the submitted work. Simon Lovestone reports other from Janssen-Cilag Ltd (High Wycombe, UK) and grants from AstraZeneca plc (Cambridge, UK) and European Federation of Pharmaceutical Industries and Associations (Brussels, Belgium) outside the submitted work. In addition, Simon Lovestone has patents issued and pending related to biomarkers for Alzheimer’s disease. He also reports membership of the Efficacy and Mechanism Evaluation Strategy Group from 2015 to 2019 and of the Medical Advisory Board of SomaLogic (Boulder, CO, USA) up to 2019 and other consultancy for Merck Sharp & Dohme Corp., Eli Lilly and Company and Optum, Inc. (Eden Prairie, MN, USA). Clive Ballard reports grants and personal fees from Acadia Pharmaceutical Company (San Diego, CA, USA) and Lundbeck A/S (Copenhagen, Denmark) and personal fees from Roche Holding AG, Otusaka Pharmaceutical Co., Ltd (Tokyo, Japan), Novartis International AG (Basel Switzerland), Eli Lilly and Company and Pfizer Inc. (New York, NY, USA) outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Howard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that currently affects 50 million people worldwide,1 with projected numbers of affected people reaching 135.5 million by 2050 and associated costs, just for the USA, at US$1.2T. 2 At the Dementia Summit in 2013, the G8 health ministers committed to identifying a cure or a disease-modifying therapy for dementia by 2025,2 but no treatments have so far been shown to delay the progression of cognitive and functional disability that characterises AD. Failure of treatment approaches directed at preventing the associated build-up of β-amyloid (Aβ) or tau protein deposits has stimulated investigation of alternative treatment approaches, including targeting inflammation in the brain.
The risk of developing AD is associated with immune-related and inflammatory genes, including genes coding for myeloid-specific sialic acid-binding receptor (CD33), triggering receptor expressed on myeloid cell 2 (TREM2), complement receptor 1 (CR1) and bridging integrator 1 (BIN1). 3 Microglial activation is increased in AD. 4 Aβ is a pro-inflammatory agent in AD5 and several microglial surface receptors are also Aβ receptors. 6 In the early stages of AD, microglia clear Aβ by phagocytosis and produce Aβ-degrading enzymes. 7 However, as AD pathology progresses, accumulation of Aβ stimulates the microglial production of pro-inflammatory agents, which drives further neurodegeneration. 7
Two independent systematic reviews of mechanisms, tolerability, brain penetration, epidemiology and early phase trial efficacy data on several classes of repositioned drugs have identified minocycline among the most promising of these agents to progress to clinical trials in patients with AD. 8,9 Minocycline is an anti-inflammatory tetracycline that crosses the blood–brain barrier and inhibits the pro-inflammatory functions of microglia, and there is a substantial body of evidence to indicate that minocycline may be neuroprotective in neurodegenerative diseases. Although the primary neuroprotective target of minocycline in the central nervous system is not known, the principal effects of minocycline include inhibition of microglial activation, attenuation of apoptosis and suppression of the production of reactive oxygen species. 10 In vitro, minocycline protects against Aβ-induced cell death and prevents fibrillisation of Aβ. 11 In transgenic mice, minocycline prevents Aβ deposition and neuronal death,12 reduces tau phosphorylation and insoluble tau aggregates,13 downregulates inducible nitric oxide synthetase, cyclo-oxygenase-2 and Aβ precursor protein-cleaving enzyme 1 (BACE1)14 and protects hippocampal neurogenesis in the presence of Aβ. 15 Minocycline reduces interleukin and tumour necrosis factor alpha (TNFα) levels in mice,16 and neuronal death and learning deficits in rats, following Aβ administration. 17 In stroke patients, open-label treatment with 200 mg per day of minocycline for 5 days after infarct has been reported to improve functional outcome. 18 In animal models of Parkinson’s disease, studies have reported both reduced microglial activation and neuronal death19,20 and reduced microglial activation and worsened neuronal death. 21,22 Pilot clinical trials in Parkinson’s disease at a dose of 200 mg per day of minocycline over 18 months have shown no effect on symptoms and no significant increase in adverse events. 23
Minocycline treatment in the superoxide dismutase 1 transgenic mouse model for amyotrophic lateral sclerosis (ALS) delayed the onset of neurodegeneration and muscle strength decline. 24 A completed Phase III trial in ALS, however, reported worse outcomes with minocycline in terms of faster decline in forced vital capacity and manual muscle strength. 25
Suggested explanations for this faster decline is that the dose of up to 400 mg per day of minocycline may have contributed to fatigue in a highly susceptible population, and that increased levels of glutamate receptor 1 phosphorylation may have promoted glutamate toxicity to motor neurons. 26 In AD, both in vitro and in vivo studies have shown reduced microglial activation, attenuated neuronal death, astrogliosis and improved behavioural performance. 11–13,17,27–31 However, to date, there have been no published clinical trials in AD patients and none is currently registered as recruiting.
The minimum daily dose of minocycline that offers neuroprotection in humans has not been established. A dose of 200 mg per day of minocycline is generally very well tolerated in the long-term treatment of acne32 and has been shown to be neuroprotective in acute stroke,18 spinal cord injury33 and multiple sclerosis. 34 However, 200 mg per day of minocycline, although well tolerated, did not improve outcomes in trials in Parkinson’s disease23 or Huntington’s disease. 35 Some authors have argued that one reason for the failure of some trials may be that such doses of minocycline are too low to be neuroprotective, pointing out that the typical effective dose in animal studies would be equivalent to 3–7 g per day in humans. 10 It would not be feasible or ethical to subject AD participants to such very high doses of minocycline, but the Minocycline in Alzheimer’s Disease Efficacy (MADE) trial includes a comparison of 400 mg per day with 200 mg per day to investigate the tolerability of 400 mg per day and whether or not the higher dose confers increased efficacy.
Alzheimer’s disease is a major public health issue and the imperative to discover and develop treatments that can stop or at least delay disease progression is clear. Symptomatic AD treatments in the form of cholinesterase inhibitors and memantine have been the mainstay of current treatment for > 10 years but do not slow progression of the disease. With a more detailed understanding of the basic biology of the AD process, a wide range of cellular and animal model systems have been developed within which several candidate disease-modifying treatments appear promising, though no such agent has performed successfully in Phase III trials. 36,37 Unfortunately, the development of treatments for AD is a complex and difficult process. The slowness of the neurodegenerative process and the substantial difficulties involved in demonstrating that the process has been changed by treatment are major contributors to this problem. Minocycline was arguably the most promising off-patent candidate for AD modification that was not yet in trials, and it was cheap and well tolerated. The time was known to be right for an adequately powered clinical trial, conducted for a sufficiently long period to demonstrate efficacy on simple cognitive and functional outcomes. The results, even if clearly negative, could be expected to move the field on a significant degree.
Based on this wealth of preclinical research suggesting neuroprotection, this trial investigated whether or not minocycline slows decline in cognitive and functional ability in people with mild AD over a 2-year treatment period. The safety and tolerability of minocycline was also compared at doses of 200 mg and 400 mg per day.
Chapter 2 Methods
Objectives
The MADE study was a multicentre randomised controlled trial with the following objectives.
The primary objective was to determine whether or not minocycline is superior to placebo in slowing the disease course of early AD, over a 2-year period, as measured by rate of decline in cognition [as measured by the standardised Mini Mental State Examination (sMMSE)] and function [as measured by the Bristol Activities of Daily Living Scale (BADLS)].
The secondary objectives of the MADE trial were to:
-
compare the safety and tolerability of minocycline at doses of 400 and 200 mg per day
-
determine whether or not 400 mg per day offers superior neuroprotection to 200 mg per day
-
investigate associated risks of side effects and serious adverse events
-
estimate the magnitude of any statistically significant positive treatment effects on cognitive and functional decline and, thereby, inform the design and powering of a future Phase III trial of definitive clinical effectiveness within the NHS.
Trial design
The MADE study was a multicentre, randomised, double-blind trial for patients with very mild AD. It had a semifactorial design in which participants were allocated to one of three treatment arms:
-
arm 1 – 400 mg per day of minocycline
-
arm 2 – 200 mg per day of minocycline
-
arm 3 – placebo.
Trial treatment continued for a period of up to 2 years.
A summary of all changes to the protocol, including amendments to the inclusion/exclusion criteria and safety monitoring protocol, are presented in Appendix 1.
Ethics approval and research governance
The MADE trial was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice guidelines and the Declaration of Helsinki. 38 The study protocol, patient and caregiver information sheets and informed consent forms were approved by East of England/Essex Research Ethics Committee (REC; reference number 13/EE/0063) and the Medicines and Healthcare Products Regulatory Agency (reference number 14523/0246/001-0005). Ethics approval for the trial was given by the National Research Ethics Service (NRES) Committee East of England on 1 May 2013 (reference number 13/EE/0063). Local REC approval and the appropriate site-specific assessments were obtained from each of the 32 participating NHS trusts (see Acknowledgements for a full list of participating trusts). The trial was registered with the International Standard Randomised Controlled Trial Register (ISRCTN) with the reference number ISRCTN16105064. Trial conduct was overseen by independent Data Monitoring and Trial Steering Committees. The protocol for the trial can be found on the National Institute for Health Research (NIHR)’s management information system portal.
Outcomes
Standardised Mini Mental State Examination
The first primary outcome measure was the sMMSE,39,40 a widely used clinician-rated instrument for assessing cognition. Scores range from 0 to 30 points, with higher scores indicating better cognitive function. 41 The original Mini Mental State Examination (MMSE) was designed as a brief test to detect organic brain disease and quantify the degree of cognitive impairment. It is still probably the most widely used cognitive test in the world42 and has good psychometric properties. 43 The sMMSE was developed to provide raters with explicit guidelines for administration and scoring, with the aim of improving the reliability of the instrument. The sMMSE differs from the MMSE in four main areas: serial sevens are omitted, the order of the time orientation questions is changed, for all questions a response time limit is imposed and for each item unambiguous scoring rules are given. The sMMSE score is considered to be of clinical relevance, with the minimum clinically important difference estimated to be 1.4 points. 44 The sMMSE has been shown to be sensitive to the effects of anti-dementia drug treatment in previous AD clinical trials. 45–47
Standardised Mini Mental State Examination data were collected at screening and at 6, 12, 18 and 24 months.
Bristol Activities of Daily Living Scale
The second outcome measure is the BADLS,48 used to assess functional ability (activities of daily living). Scores range from 0 to 60 points, with higher scores indicating greater impairment. The BADLS was specifically designed for use with dementia patients living in the community and participating in clinical trials. The BADLS is sensitive to change, correlates well with economic outcomes and, despite being a carer-rated instrument, appears to have good test–retest reliability. The levels of disability between which the scale aims to discriminate were also carer generated, giving some perspective on the value of change, with the minimum clinically important difference estimated to be 3.5 points. 44 The BADLS has also been shown to be sensitive to change across a wide range of functional disability in previous AD clinical trials. 45,49
Bristol Activities of Daily Living Scale data were collected at baseline and at 6, 12, 18 and 24 months.
Participants
Patients were identified from NHS Memory Services at 32 university and general mental health trusts in England and Scotland. The inclusion and exclusion criteria used to select patients are detailed in the next two sections.
Inclusion criteria
The inclusion criteria include patients:
-
with a diagnosis of possible or probable AD by National Institute on Aging–Alzheimer’s Association’s (NIA–AA) criteria50
-
with a sMMSE score of > 23 points, with no upper limit
-
giving informed consent to participate
-
aged ≥ 50 years
-
who have a potential informant who will assist in the administration of the BADLS.
Exclusion criteria
The exclusion criteria include patients:
-
with a known allergy to tetracycline antibiotics
-
of childbearing potential, that is, female patients who have not been surgically sterile (via hysterectomy, bilateral salpingectomy/oophorectomy) for a minimum of 6 months or undergone bilateral tubal occlusion/ligation at least 6 months prior or been postmenopausal for at least 1 year
-
who have an uncontrolled serious concomitant illness
-
who have a known chronic kidney disease stages 3b–5
-
with moderate liver disease (based on the Child–Pugh Classification for Severity of Liver Disease)
-
with abnormal serum chemistry laboratory values at screening, which are deemed to be clinically relevant by the chief investigator
-
who withhold consent for the study team to inform his/her general practitioner (GP)
-
having systemic lupus erythematosus (SLE)
-
participating in another clinical trial of an investigational medicinal product (IMP) in the previous 28 days. 41
Recruitment procedure
Patients with very mild (as defined by a sMMSE score of > 23 points) AD (as defined by NIA–AA criteria50) were identified from memory services, both within the participating NHS trusts (see below for a comprehensive list of all participating NHS sites) where the principal investigators practised and within the network of memory services supported by the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN). Potentially eligible patients were approached by a clinician who knew them and were provided with an opportunity to hear more about research. The Join Dementia Research recruitment tool was also used. Those patients interested met with a member of the research team, who provided further information about the study. Potentially interested individuals were given an opportunity to review the information, ask questions over the telephone and arrange a date for interview. Written informed consent was obtained prior to commencing the screening assessment for the trial and after the individuals had received the information sheet.
Screening
The diagnosis and provisional eligibility for the study was first confirmed using the inclusion/exclusion criteria listed in Inclusion criteria and Exclusion criteria. The sMMSE was performed and this score was also used as the baseline value. Blood was taken for full blood count and biochemical profile analysis. The screening blood analysis results and concomitant medicines were reviewed and recorded to confirm eligibility before randomisation.
Trial treatment
Trial treatment consisted of oral minocycline modified-release (MR) capsules or identically appearing placebo packed into treatment cartons sufficient for 13 weeks’ treatment (with a small overage). The dosing regimens for the three treatment arms were:
-
arm 1 – minocycline (400 mg): two MR 100-mg capsules of minocycline in the morning and two MR 100-mg capsules in the evening
-
arm 2 – minocycline (200 mg): one MR 100-mg capsule of minocycline plus one minocycline placebo capsule in the morning and one MR 100-mg capsule of minocycline plus one minocycline placebo capsule in the evening
-
arm 3 – placebo minocycline: two minocycline placebo capsules in the morning and two placebo minocycline capsules in the evening.
Treatment packs were supplied on a 3-monthly basis for a total treatment duration of 24 months. Participants, their carers, prescribing clinicians, outcome assessors and all MADE trial staff (except statisticians) were masked to trial arm assignment.
Treatment preparation
Modepharma Ltd (Beckenham, UK) was responsible for arranging the IMP’s manufacture, as well as project management and assistance relating to the IMP for the trial, including preparation of the IMP Dossier (IMPD). The actual manufacturing of placebo, all IMP packaging and labelling, and final qualified person (QP) release of the IMPs were undertaken by Piramal Healthcare UK Ltd (licence number 29595; Morpeth, UK).
Acnamino™ (Dexel®-Pharma Ltd, Daventry, UK) MR 100-mg capsules were used as the active treatment. These capsules were procured and supplied to Piramal Healthcare for IMP packaging by Modepharma. Acnamino MR 100-mg capsules are hard gelatin capsules each containing one pink film-coated tablet and one peach enteric-coated tablet. Each capsule contained 100 mg of the active substance minocycline as minocycline hydrochloride. Placebo tablet intermediates were made using similar tablet tooling and film-coating colour to the tablets in the active Acnamino MR 100-mg capsule. The blinding of the placebo product was achieved by using the same capsule size and similar gelatin capsule body and cap colours as the Acnamino MR 100-mg capsule. Placebo blister strips and patient treatment packs matched those of the active substance and were labelled in the same way.
Packaging and labelling
Both active and placebo IMPs were packaged under QP control by Piramal Healthcare UK Ltd. Capsules were packed in blister strips of 27 capsules, with a colour-coded Annex 13-compliant label. 51 Seven blister strips (i.e. 189 capsules) were placed in a carton, each with its own individual randomisation number (or treatment pack number) and colour-coded Annex 13-compliant label. Patients were given two cartons, each containing seven blister strips, at randomisation and every 3 months subsequently. Two cartons made up a 3-month (13-week) supply. Patients were directed to take one capsule from one carton and one capsule from the other carton every morning and every evening. As the capsules and blisters looked the same, for patients’ ease, the labels used on the first and second cartons (and blisters) had two different colours.
Storage and dispensing
Batches of treatment packs were distributed to participating trial pharmacies by Polar Speed (Leighton Buzzard, UK). Drug supplies were kept in a secure, limited-access storage area, in their original packaging and under the authorised storage conditions for the Acnamino MR 100-mg capsules, and instructions stated ‘Store in the original package’. Trial participants were advised to store medication at ambient temperature and out of the reach of children. All unused medication was destroyed by the site pharmacy. Receipt, usage and destruction was monitored and documented throughout the trial on the respective forms. Account was given for discrepancies.
Unblinding
Investigators and patients remained blinded to the treatment allocation throughout the trial. Unblinding was not normally necessary as serious side effects were dealt with on the assumption that the patient was on active minocycline treatment. Study medication was omitted rather than unblinded. If considered urgently necessary for patient management, a 24-hour unblinding service was available (personal communication).
Concomitant medication
Patients could be randomised into the MADE study while taking a cholinesterase inhibitor or memantine. Cholinesterase inhibitors and memantine could also be commenced or discontinued during the course of the study at the discretion of the responsible consultant.
Other concomitant medications that may interact with minocycline [listed in the summary of product characteristics (SmPC) for minocycline and summarised below] were recorded at each visit and the prescriber informed:
-
angiotensin-converting enzyme (ACE) inhibitors – absorption of minocycline decreased by quinapril tablets
-
antacids, adsorbents and vitamin/mineral supplements – absorption of minocycline is impaired by the concomitant administration of antacids, iron, calcium, aluminium, magnesium and zinc salts (interactions with specified salts, antacids and kaolin) unless taken 3 hours apart; dosages should be maximally separated
-
antibacterials – minocycline can decrease the effectiveness of penicillins
-
anticoagulants – tetracyclines depress plasma prothrombin activity and reduced dosages of concomitant anticoagulants may be necessary
-
diuretics – may aggravate nephrotoxicity by volume depletion
-
ergotamine and ergometrine – increased risk of ergotism
-
retinoids – administration of isotretinoin should be avoided shortly before, during and shortly after minocycline therapy as the combined administration of the two drugs increases the risk of benign intracranial hypertension
-
ulcer-healing drugs – absorption of minocycline is decreased by sucralfate and bismuth salts.
Side effects
If side effects were reported their significance was discussed with the study doctor. Depending on severity, participants were asked to continue with the study drug if possible and a review by the study doctor arranged in 2 weeks. If, at the time of the review, the side effects were severe enough to warrant withdrawal from the study, participants were advised to omit the morning dose and a further review arranged in 2 weeks. If side effects persisted, participants were advised to take a temporary (e.g. 2-week) break from IMP treatment and were reminded to restart once the symptoms resolved. If side effects persisted, then participants were advised to stop taking the study drug.
Treatment compliance
Treatment compliance was monitored by capsule count at the 6-, 12-, 18- and 24-month visits and monitored over the telephone at week 2, and then at months 3, 9, 15 and 21. Participants were asked to bring any unused study medication at each follow-up visit and at the end of the trial. Unused study medication was obtained from the carer at these assessments. The local principal investigator (PI) or research worker kept a log of study medication returns, return date and amount of study medication returned and entered the information on the case report form. Once returned medication had been logged, it was destroyed by the local pharmacy. Carers were also questioned regarding study drug compliance at all interim assessments. The study-specific prescriptions were maintained in the pharmacy file for audit purposes.
Drug accountability
The study drug was dispensed by the local site pharmacy and full accountability of dispensing by the pharmacy and of returns (at each face-to-face visit) was undertaken by the PI or one of the research team. The pharmacy departments at each site maintained a study medication dispensing log, which included date dispensed, batch number, expiry date and number of capsules dispensed. In addition, the unique code numbers assigned to the treatment pack and trial patients were recorded. The study-specific prescriptions were maintained in the pharmacy file for audit purposes. Once the unused or partially used drug supplies were verified they were destroyed by the local pharmacy.
Assessments
Screening and randomisation were performed prior to the assessments described below.
Baseline
The baseline assessment took place within 28 days of screening. The BADLS was then administered to complete the primary outcome assessments. Three months’ study drug was supplied via the trial pharmacy.
2-week assessment
A telephone assessment of safety, tolerability, compliance and concomitant medicines was made.
3-, 9-, 15- and 21-month assessments
An assessment of safety, tolerability, compliance and concomitant medicines was made. Blood was taken for analysis at the clinic or patient’s home. Three months’ study drug was supplied via the trial pharmacy.
6-, 12- and 18-month assessments
Compliance and safety was assessed and concomitant medicines recorded. Primary outcomes were administered and blood was be taken for analysis at the clinic or patient’s home. Three months’ study drug was supplied via the trial pharmacy.
24-month assessment
Primary outcomes were administered. Compliance and safety were assessed and concomitant medicines recorded. See Table 1 for further details on the administration of the study assessments.
| Event | Time point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Baseline | Week 2 | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | Month 21 | Month 24 | |
| Diagnosis | ** | ||||||||||
| Inclusion | ** | ||||||||||
| Exclusion | ** | ||||||||||
| Concomitant medicines | ** | * | ** | ** | * | ** | * | ** | * | ** | |
| Consent | ** | ||||||||||
| Random | ** | ||||||||||
| sMMSE | ** | ** | ** | ** | ** | ||||||
| BADLS | ** | ** | ** | ** | ** | ||||||
| Dispense | ** | ** | ** | * | ** | * | ** | * | |||
| Compliance | * | * | ** | * | ** | * | ** | * | ** | ||
| Safety check | * | * | ** | * | ** | * | ** | * | ** | ||
| FBC/biochemistry | ** | ** | ** | ** | ** | ** | ** | ** | |||
Safety and tolerability
Our secondary objectives focused on the safety and tolerability of the treatment and, therefore, data on safety parameters [including blood monitoring of haematological, renal and hepatic function as well as documentation of skin reactions, gastrointestinal and neurological symptoms and concurrent infections (i.e. bacterial enteritis, Clostridium difficile and orogenital candidiasis)] were also assessed and recorded every 3 months. To monitor renal function, the Modification of Diet in Renal Disease (MDRD) formula was used to calculate the estimated glomerular filtration rate (eGFR) at baseline and changes in creatinine levels were used to monitor renal function post baseline. In particular, the following guidelines (which were generated with approval by the Trial Management Group and in consultation with renal physicians) were used:
Any patient with a follow-up creatinine [level] of ≥ 25% and < 50% higher than their baseline value can remain on treatment, but will have a repeat blood sample in 2–3 weeks. If creatinine [levels] remain the same or higher, then a further check will be required. Any patient with a follow-up creatinine [level] of ≥ 50% higher than baseline can remain on treatment but will have a repeat blood sample within 10 days. If creatinine [levels] remain the same or higher, then study treatment will be stopped (unless an obvious alternative cause is identified, e.g. NSAID [non-steroidal anti-inflammatory drug] use, other illness).
Randomisation and blinding
Once informed consent had been given, and baseline assessments completed, participants were randomly allocated, using a centralised randomisation service, to one of three trial arms: 400 mg of minocycline, 200 mg of minocycline or placebo. Treatment packs were allocated centrally by the MADE study office. The minimised randomisation procedure aimed to balance treatment allocation overall, and by four stratification variables: centre, duration of symptoms prior to randomisation (< 6 months, ≥ 6 months), baseline sMMSE score (24–26, 27–30 points) and age (< 65, 65–74, ≥ 75 years). Participants were enrolled by their clinicians or appropriately trained clinical study officers or research nurses. These staff also administered the outcome assessments. Patients, caregivers, clinicians, outcome assessors and investigators were blinded to treatment allocation.
The person randomising participants completed the MADE randomisation form and had to answer a set of prespecified questions over the telephone. Alternatively, completed randomisation forms were faxed – or scanned and e-mailed – to the MADE randomisation service, which provided a call-back service with a treatment allocation. After all the necessary details had been provided, a MADE patient trial number was assigned and two treatment pack numbers were allocated. Allocated treatment packs of 400 mg per day of minocycline, 200 mg per day of minocycline or matching placebo were dispensed to patients via a local pharmacy and/or researcher.
Ethics considerations
Any neuroprotective benefit from minocycline is likely to outweigh the risks. Minocycline is routinely used at doses of 200 mg per day in the long-term treatment of acne and is considered safe in this indication. 31 Rare side effects include acute renal failure, irreversible skin pigmentation and, very rarely, SLE.
The trial applied for multicentre research ethics approval and local research governance approval for the studies. The study personnel, co-investigators and collaborators [the management group and independent Trial Steering Committee (TSC)] ensured that the study was conducted within appropriate NHS and professional ethics guidelines. Information was kept strictly confidential and held in accordance with the Data Protection Act 1998. 52 Data are held on a secure database on a password-protected university computer. Access to data was restricted to the research team.
Participation in the MADE trial carried only a 1 in 3 risk of randomisation to placebo and patients did not have to forgo treatment with a cholinesterase inhibitor or memantine if their responsible clinician considered that they would benefit from such treatment.
Potentially eligible patients were approached by a clinician who knows them, and given the opportunity to hear more about research activities. The study was also advertised directly to patients (via posters, leaflets, etc.). Those individuals who were interested in learning more about research were referred to a member of the research team, who provided an information sheet with full study details, including possible benefits and risks. The potentially eligible patients were offered the opportunity to ask questions and discuss any queries with the carer/relative/doctor and make a date for the eligibility interview. Interested patients were sent the information sheet with the invitation to screening and written informed consent obtained prior to commencing the screening assessment.
All researchers were trained in gaining informed consent through DeNDRoN or the Mental Health Research Network training course. NRES guidance on the content and format of patient information and consent documentation was followed. Consumer representatives were involved to ensure that these documents and all other written trial materials were fit for purpose. Information about the study was mailed to potential participants and their caregivers.
The main potential ethics issue in dementia trials is that the disease may interfere with an individual’s ability to give informed consent. Because the trial was studying people with mild dementia, most of the participants had capacity to give informed consent for their involvement. Consequently, fully informed written consent was obtained from patients entering the MADE study. However, as patients remained in the study for up to 2 years it was likely that some may have lost capacity over this period. Patients were therefore also asked what they would want to happen in the event of them losing capacity during the course of the study. These patients were given the option of either withdrawing at this point or a decision being made on their behalf by their personal legal representative in line with the Medicines for Human Use (Clinical Trials) Regulations 2004. 53 This person was usually the patient’s main carer, who would have the best knowledge of the individual’s attitudes and stated preference to research and, consequently, was best placed to judge whether or not they would have wished to continue if they had capacity. In this situation the patient’s agreement to participate was still obtained to their best level of understanding and they did not remain in the study if they refused or showed significant distress.
Patient and public involvement
The study ensured that patients, carers and members of the general public were closely involved in the trial. During the design of the trial all relevant documentation, including participant information sheets and consent forms, were reviewed by our patient and public involvement (PPI) representatives. A representative was invited from the Alzheimer’s Society to sit on the TSC and they were involved in all ongoing trial matters including review of progress, group meetings, review of results and dissemination stages.
Statistical considerations
The study aimed to randomise 560 participants in a semifactorial (i.e. 2 x 1) design, that is, 1 : 1 : 1 between minocycline (400 or 200 mg) and placebo. Based on previous studies, it was estimated that 24-month assessments would be available on at least 80% of surviving participants (i.e. ≈ 390), which would provide 90% power at a p-value of < 0.05 to detect a small to moderate [0.35 standard deviation (SD)] effect size for minocycline (any dose) compared with placebo on the primary outcome measures. With outcome assessments on 130 patients allocated 400 mg of minocycline and 130 allocated 200 mg of minocycline, the study would have 80% power at a p-value of < 0.05 to detect a 0.35 SD treatment effect of 400 mg compared with 200 mg of minocycline at 24 months.
Only participants who received at least one capsule of the study treatment drug or placebo were to be included in the analyses of primary and secondary outcomes. The primary analyses of the effect of minocycline on rate of decline of sMMSE and BADLS scores, and subgroup analyses, used repeated measures regression methods, adjusted for baseline scores. These analyses use all available assessment data to maximise statistical power to detect any differences between treatments, and to minimise the impact of missing outcome data. For both primary outcomes, the difference in the rate of decline between minocycline (any dose) and placebo, and between patients allocated 400 mg and 200 mg of minocycline, was compared using a time-by-treatment interaction test, with time modelled as a continuous variable. Comparisons of time on trial medication over the 24-month follow-up period split by treatment arms are displayed in Kaplan–Meier curves, with statistical significance determined by log-rank tests. Participants who died were censored at the last assessment time point before death. Reasons for stopping trial medication and adverse events are tabulated by treatment arm. The study used SAS® software version 9.3 (SAS Institute Inc., Cary, NC, USA) for all statistical analyses. The independent data monitoring committee and REC reviewed the unblinded accumulating data and the safety of patients in the study at approximately yearly intervals.
Literature search
Prior to analysing the trial results, a literature search was carried out in 2018 to assess whether or not minocycline affects disease progression in AD, but no relevant studies were found at this stage (see Appendix 2, Table 6).
Chapter 3 Results
Between 23 May 2014 and 14 April 2016, 554 participants were entered from 32 NHS memory services in England and Scotland. Ten patients did not start trial medication and, as prespecified in the protocol, were excluded from all analyses (Figure 1); one participant had been allocated to 400 mg of minocycline, four to 200 mg of minocycline and five to placebo. Baseline characteristics of the 544 eligible participants were well balanced across the three treatment trial arms (Table 2).
FIGURE 1.
Flow chart of participants through the trial.

| Characteristic | Treatment arm | ||
|---|---|---|---|
| 400 mg of minocycline (N = 184) | 200 mg of minocycline (N = 181) | Placebo (N = 179) | |
| Age (years) | |||
| < 65, n (%) | 22 (12) | 22 (12) | 21 (12) |
| 65–74, n (%) | 68 (37) | 66 (36) | 66 (37) |
| ≥ 75, n (%) | 94 (51) | 93 (51) | 92 (51) |
| Mean (SD) | 74.3 (8.0) | 74.1 (8.4) | 74.6 (8.1) |
| Gender, n (%) | |||
| Male | 104 (57) | 100 (55) | 99 (55) |
| Female | 80 (43) | 81 (45) | 80 (45) |
| Home circumstance, n (%) | |||
| Living with spouse/partner/relative | 153 (83) | 153 (85) | 149 (83) |
| Alone | 31 (17) | 28 (15) | 29 (16) |
| Duration of symptoms | |||
| < 6 months, n (%) | 20 (11) | 20 (11) | 20 (11) |
| ≥ 6 months, n (%) | 164 (89) | 161 (89) | 159 (89) |
| Mean (SD) | 23.5 (18.3) | 23.1 (17.8) | 24.2 (18.0) |
| sMMSE score (points) | |||
| 24–26, n (%) | 100 (54) | 97 (54) | 96 (54) |
| 27–30, n (%) | 84 (46) | 84 (46) | 83 (46) |
| Mean score (SD) | 26.4 (1.9) | 26.5 (1.9) | 26.4 (1.8) |
The mean age of participants was 74.3 years, 57% (303/544) of whom were male and 84% (455/544) of whom were living with a spouse, partner or relative. The average duration of symptoms was 24 months and the average sMMSE score at baseline was 26.4 points.
The sMMSE assessments were obtained for 542 (99.6%) of the 544 participants at baseline, 498 (92%) of the 544 participants at 6 months, 453 (84%) of the 537 participants at 12 months, 420 (80%) of the 528 participants at 18 months, and 403 (78%) of the 517 participants at 24 months (see Appendix 4, Table 8). There were fewer BADLS than sMMSE assessments, because BADLS assessments are not valid for participants in residential care.
Minocycline at a daily dose of 400 mg was poorly tolerated by participants, with just 29% (53/184) of those allocated 400 mg of minocycline completing 2 years of treatment, significantly fewer participants than in the 200 mg of minocycline (62%; 112/181) or placebo arm (64%; 114/179) (p < 0.0001; see Figures 1 and 7). By contrast, 200 mg of minocycline was well tolerated by participants, with similar discontinuation rates with 200 mg of minocycline and placebo (p = 0.56). When reasons for stopping trial treatment were compared (Table 3), more participants allocated to minocycline than to placebo stopped because of gastrointestinal symptoms (p = 0.0008), dermatological side effects (p = 0.02) and dizziness (p = 0.01).
| Reasons for stopping | Treatment arm (n) | Total (n) | Minocycline vs. placebo p-value | ||
|---|---|---|---|---|---|
| 400 mg of minocycline (N = 184) | 200 mg of minocycline (N = 181) | Placebo (N = 179) | |||
| GI symptoms | 42 | 15 | 10 | 67 | 0.00080 |
| Dizziness | 14 | 3 | 1 | 18 | 0.01000 |
| Dermatological symptoms | 10 | 5 | 1 | 16 | 0.02000 |
| Haematological | 5 | 3 | 1 | 9 | 0.16000 |
| Impaired renal function | 2 | 5 | 4 | 11 | 0.81000 |
| Infection | 1 | 2 | 2 | 5 | 0.74000 |
| Shortness of breath | 6 | 0 | 0 | 6 | 0.08000 |
| Worsening dementia | 1 | 3 | 3 | 7 | 0.57000 |
| Depression or anxiety | 4 | 2 | 2 | 8 | 0.63000 |
| Joint or muscle pain | 2 | 0 | 2 | 4 | 0.47000 |
| Concomitant disease/illness | 9 | 6 | 7 | 22 | 0.91000 |
| General deterioration in physical health | 2 | 0 | 2 | 4 | 0.47000 |
| Unknown | 1 | 0 | 0 | 1 | 0.48000 |
| Unspecified side effect | 5 | 2 | 7 | 14 | 0.17000 |
| Patient or carer choice | 23 | 21 | 18 | 62 | 0.49000 |
| Total | 127 | 67 | 60 | 254 | 0.00002 |
As a consequence of the higher treatment withdrawal rate, fewer assessments were obtained for the 400 mg of minocycline treatment arm than for the 200 mg of minocycline and placebo arms (see Appendix 4, Table 8). For sMMSE assessments at 24 months, 68% of assessments (119 of the 174 expected) were received for the 400 mg of minocycline arm, 82% (144/176) for the 200 mg of minocycline arm and 84% (140/167) for the placebo arm. Return rates for BADLS assessments were similarly lower for the 400 mg of minocycline arm than for the 200 mg of minocycline and placebo arms (see Appendix 4, Table 8).
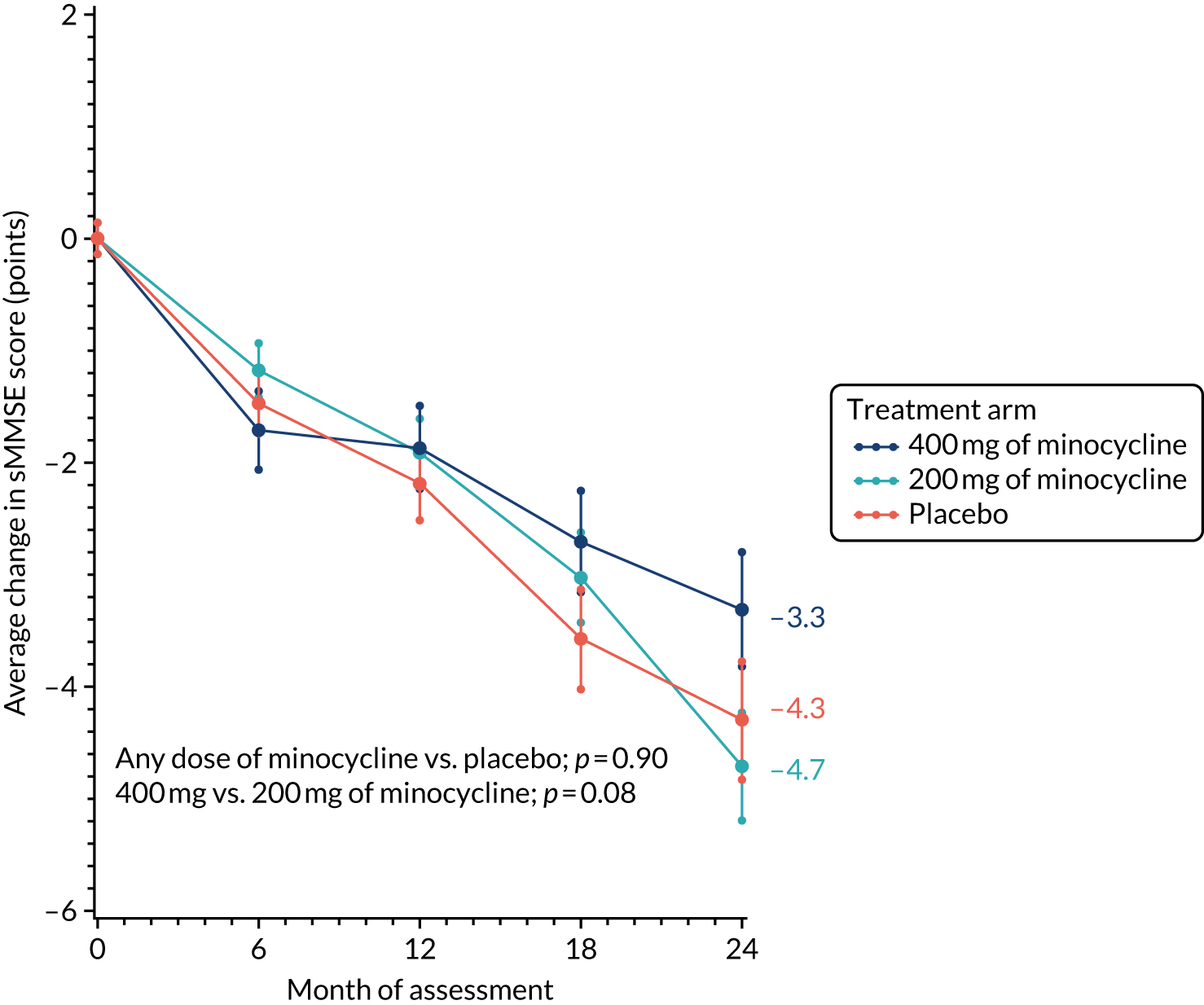
The change from baseline in sMMSE scores over time, with standard error bars, is shown in Figure 2. There was an average 4.1-point reduction in the combined minocycline arms compared with a 4.3-point reduction in the placebo arm over the 24-month study period (p = 0.90). The reduction in sMMSE score in the 400 mg of minocycline arm over 24 months was less than that observed in the 200 mg of minocycline arm (i.e. 3.3 vs. 4.7 points), but this difference was not significant (p = 0.08).
FIGURE 2.
Change in sMMSE scores: baseline to 24 months’ follow-up. The graph shows change in mean sMMSE score with standard errors; baseline scores are set to zero; p-values are from tests of the time-by-treatment interaction from repeated measures analyses.
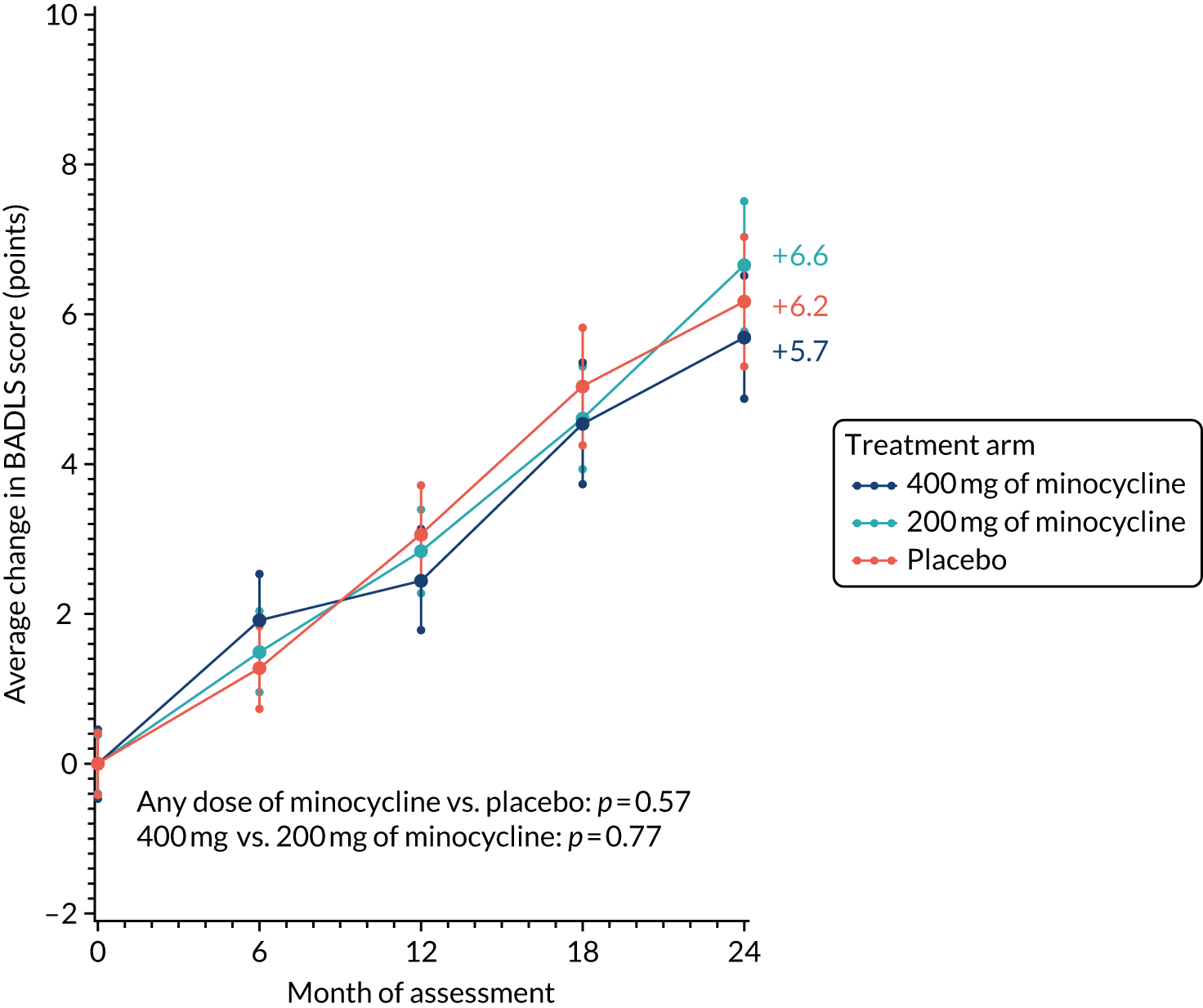
Likewise, the worsening of BADLS scores over 24 months was similar in all treatment arms: 5.7, 6.6 and 6.2 points in the 400 mg of minocycline, 200 mg of minocycline and placebo trial arms, respectively. There were no significant differences in BADLS scores between participants allocated minocycline and those allocated to the placebo arm (p = 0.57) or between those participants allocated 400 mg of minocycline and those allocated 200 mg of minocycline (p = 0.77; Figure 3).
FIGURE 3.
Change in BADLS scores: baseline to 24 months’ follow-up. The graph shows change in mean BADLS scores with standard errors; baseline scores are set to zero; p-values are from tests of the time-by-treatment interaction from repeated measures analyses.
To assess how the higher number of missing outcome assessments in the 400 mg of minocycline treatment arm than in the 200 mg of minocycline or placebo arms (see Appendix 4, Table 8) might have affected outcome comparisons, various sensitivity analyses were run to investigate potential bias from non-random dropout. In particular, there were 41 participants who had a baseline sMMSE score but no further assessments, so did not contribute any information to the primary analysis (see Appendix 4, Figure 8). Those participants who discontinue treatment in AD trials are often atypical, usually having worse cognitive and functional ability than those who continue. 20 This is evident from the scores of the 41 participants with a 6-month sMMSE score but no later assessments.
In total, there were 252 reported serious adverse events (SAEs), with the most common categories being neuropsychiatric and cardiocirculatory. The number of SAEs was somewhat higher in the placebo arm than in the 400- and 200-mg minocycline treatment arms (Table 4). Given that gastrointestinal symptoms were the main reason for stopping trial treatment, it is reassuring that the numbers of gastrointestinal SAEs in the minocycline arms were low, and no higher than in the placebo arm. Similarly, though more skin-related toxicities, particularly pigmentation, were reported with minocycline than with placebo [36% (130/365) vs. 21% (38/179); p = 0.0007], few participants stopped trial treatment because of such toxicities (see Table 3) and only six skin toxicities were considered severe (three in participants allocated to any dose of minocycline and three in participants to placebo; see Appendix 4, Table 9). There were no differences in the numbers of participants stopping treatment because of impaired renal function, which had been a prior concern, nor in numbers of renal SAEs. Twenty-eight patients died during the study: 10 allocated to the 400-mg minocycline treatment arm, six to the 200-mg minocycline treatment arm and 12 to the placebo arm (see Appendix 4, Table 10 and Figure 11a). Fifteen of these 28 patients had stopped trial treatment prior to dying. One additional patient died without starting trial treatment. Rates of care home admission were low in this mild AD population, with no difference in numbers between trial arms (see Appendix 4, Figures 11b and 11c).
| SAE class | Treatment arm, counts of SAEs reported | Total counts of SAEs reported | Minocycline vs. placebo p-value | ||
|---|---|---|---|---|---|
| 400 mg of minocycline (n = 184) | 200 mg of minocycline(n = 181) | Placebo (n = 179) | |||
| Gastrointestinal | 3 | 8 | 10 | 21 | 0.1400 |
| Respiratory | 8 | 8 | 10 | 26 | 0.5400 |
| Mechanical injury | 6 | 11 | 13 | 30 | 0.2100 |
| Endocrine and metabolic | 2 | 1 | 9 | 12 | 0.0020 |
| Cancer | 12 | 3 | 11 | 26 | 0.0200 |
| Haematological/thrombosis | 3 | 1 | 2 | 6 | 0.9800 |
| Dermatological | 0 | 1 | 0 | 1 | 0.4800 |
| Neuropsychiatric | 10 | 13 | 16 | 39 | 0.2600 |
| Cardiocirculatory | 14 | 9 | 11 | 34 | 0.9400 |
| Renal | 3 | 2 | 2 | 7 | 0.4000 |
| Infection | 10 | 1 | 19 | 30 | 0.0003 |
| Other | 7 | 11 | 2 | 20 | 0.0300 |
| Total | 78 | 69 | 105 | 252 | – |
The average decline in sMMSE score from baseline to 6 months in this subset of patients (i.e. those patients without any post-baseline assessments) was 3.9 points, a rate of decline three times higher than the 1.3-point average decline among the 498 patients who had a 6-month sMMSE assessment and went on to complete later assessments. It seems likely, therefore, that those patients without any post-baseline assessments, who do not contribute to the estimate of the rate of decline, also had a worse than average decline in cognitive and functional ability.
To estimate what impact the missing outcome data from the 41 participants with no post-baseline assessments might have had on the trial results, the study’s sensitivity analyses made two different assumptions:
-
It was assumed that for the first 6 months the scores declined at a rate of 3.9 points (as did those scores for participants who had a 6-month sMMSE but no further assessments) and then declined at the average rate of 1.1 points every 6 months for the rest of the trial.
-
It was assumed that for patients who had no postbaseline assessments the scores declined at the average rate of those participants with assessments, that is, 1.3 sMMSE points for the first 6 months and then 1.1 points every 6 months subsequently.
The results from imputation methods 1 and 2 are shown in Appendix 4, Figures 9a and 9c. The results are not qualitatively different from those of the primary analyses. The only borderline-significant (p = 0.06) differences seen in these sensitivity analyses were between 400 and 200 mg of minocycline. However, with 400 mg of minocycline a little better and 200 mg of minocycline a little worse than placebo, and no difference between any dose of minocycline and placebo, this is probably a chance finding.
Because return rates for BADLS assessments were also lower for the 400 mg of minocycline arm than the 200 mg of minocycline and placebo arms, similar sensitivity analyses were run. There were 39 participants with no BADLS assessment post baseline who did not contribute to the primary analysis. Imputation method 1 assumed that their BADLS score worsened (i.e. increased) by 3.7 points over the first 6 months and then by 1.9 points every 6 months for the rest of the trial. Method 2 assumed that their BADLS score worsened by 1.5 points over the first 6 months and then by 1.9 points subsequently. Because BADLS is valid only for community-resident patients, BADLS scores for those who went into residential care were imputed only up until the last time point before moving into care. Results for imputation methods 1 and 2 are shown in Appendix 4, Figures 9b and 9d. Again, results were not qualitatively different from those of the primary analyses of BADLS assessment scores.
To investigate whether or not the efficacy of minocycline varied by baseline characteristics, subgroup analyses of change in sMMSE over 24 months for minocycline (any dose) versus placebo by duration of symptoms, baseline sMMSE, age and gender were conducted (see Appendix 4, Figure 10). There were no indications of any benefit from minocycline treatment in those participants with shorter or longer durations of symptoms, lower or higher baseline sMMSE scores, or in men or women. There was a borderline-significant (i.e. p = 0.04) trend towards greater efficacy in younger than older patients, but this unanticipated finding could be a chance occurrence given the number of subgroup investigations.
Chapter 4 Discussion
The MADE trial has shown that, in patients with mild AD, 24 months of minocycline treatment at the doses tested does not delay the progress of cognitive or functional impairment, as measured by the well-validated and widely used sMMSE and BADLS clinical rating scales. The trial has also established that minocycline at a dose of 400 mg is poorly tolerated in this population, with fewer than one-third of participants completing 24 months of treatment. By contrast, 200 mg per day of minocycline is well tolerated, with participants allocated this treatment being no more likely to withdraw from trial medication than those taking placebo.
The failure of minocycline to slow the progression of cognitive and functional decline in mild AD is disappointing given the evidence suggesting that neuroinflammation is instrumental in AD progression,7 minocycline’s established anti-inflammatory and neuroprotective effects and the positive data from several experimental animal models of AD. 11–17 NSAIDs have similarly failed to slow disease progression in clinical trials,54 despite long-term use being associated with a lower risk of developing AD in observational studies55 and promising data from transgenic animal models. 56 The study’s findings also parallel those of clinical trials of minocycline in other neurodegenerative disorders in which, despite preclinical research suggesting neuroprotection, minocycline worsened outcomes in ALS,25 had no effect in Huntington’s disease57 and multiple system atrophy,58 had negative symptoms in schizophrenia59 and had only short-term benefits in multiple sclerosis. 60
Therefore, there could be three broad potential explanations for the negative results of this trial. First, although there is good evidence for neuroinflammation in AD,7 this may be more a reaction to pathology than an important driver of progressive neurodegeneration, particularly in patients who are still at the mild stage of dementia. Second, even if neurodegeneration is accelerated by neuroinflammation, minocycline at the doses administered in the MADE study may not have had sufficient activity against these processes to show efficacy. Animal studies, from which much of the evidence for minocycline’s activity as an anti-inflammatory and anti-AD agent has come, have generally used much higher doses of minocycline than used in the MADE trial (i.e. typically equivalent to 3–7 g per day in humans)10 and, so, it could be that trial participants were not exposed to a sufficiently high dose for efficacy. However, if doses of 200–400 mg per day are insufficient for neuroprotection, the difficulties with tolerability experienced by participants allocated 400 mg of minocycline indicate that use of higher doses in this patient population is not feasible.
Minocycline is potentially neuroprotective through several anti-inflammatory processes (suppression of microglial proliferation and activation, reduced release of interleukins 1β and 6 and of tumour necrosis factor alpha, decreased chemokine expression and decreased activity of metalloproteases) as well as anti-apoptotic and anti-oxidant effects. 11–17 A study of 15 patients with traumatic brain injury found reduced microglial activation, as visualised with 11C-PBR28 positron emission tomography (PET),61 following 12 weeks of treatment with 200 mg of minocycline per day, indicating that the dose ranges used in the MADE trial can have a measurable effect on anti-inflammatory targets. The relationship between minocycline-sensitive microglial activation and neurodegeneration may, however, be complicated. Minocycline treatment in the traumatic brain injury study61 was also associated with increased plasma levels of neurofilament light, considered a marker of neurodegeneration. The faster progression seen with minocycline in ALS25 also suggests that some activated microglia might have a reparative function so that their inhibition could accelerate neurodegeneration. This study’s results do not suggest that reduced microglial activation with minocycline worsens neurodegeneration in AD.
A third plausible explanation for the negative results of the MADE study could be that minocycline did have some efficacy against progression of AD but treatment effects were too small to be detectable in the trial. It is difficult to discount this possibility. The MADE trial was, however, powered to detect minimal clinically important differences between minocycline and placebo, so smaller differences might not be considered of clinical relevance.
This pragmatic trial had a number of strengths. It was based in a broad network of academic and NHS memory services and the wide eligibility criteria facilitated the recruitment of participants who were representative of patients with very mild AD diagnosed within the NHS. Outcome measures were limited in number, practical and easy to administer reliably by trial staff and chosen because any differences between minocycline and placebo treatment would have unambiguous clinical relevance. The trial recruited to target and was sufficiently large to detect a clinically meaningful treatment effect, and the trial arms were well matched on potentially important variables at baseline. This streamlined trial design could usefully be applied to test other putative disease-modifying therapies.
Potential limitations of the study include that biomarkers were not used to confirm AD diagnosis, because these are not routinely available within the NHS and, therefore, there was no access to these data. Nonetheless, no diagnoses were revised during the study and rates of decline were as expected in a mild AD population. Compliance was also problematic, with few patients in the 400-mg arm completing 2 years of treatment and only moderate compliance in the 200-mg and placebo arms. A recommendation to take trial medication once rather than twice daily in the event of perceived side effects helped improve compliance but was introduced only late in the trial when the problem with 400-mg compliance was identified.
Although the trial protocol specified that outcome assessments should be obtained irrespective of treatment compliance, this could not always be achieved despite the vigorous efforts of the trial team. Consequently, differential follow-up rates could have biased the study’s results. However, despite the large number of treatment withdrawals in the 400-mg arm, and consequent loss to follow-up of some participants, results were essentially unchanged in sensitivity analyses investigating potential bias from missing data. Thus, the study’s conclusion that 2 years of minocycline treatment for patients with mild AD does not result in any clinically meaningful difference in the rate of decline of cognitive and functional ability is disappointing but robust.
Acknowledgements
The study team would like to thank the following people and organisations for their contributions:
-
Rebecca Gathercole for helping to manage the trial
-
Keith Anderson for his input in designing and programming the study database
-
Nicholas Woodthorpe, Ajay Macharouthu, Anilkumar Pillai, Vandana Mate, Demi Onalaja, Simon Thacker, Richard Brown, Anna Green, Santanu Chakrabarti, Latha Velayudhan, Abdul Patel, Ban Al-Kaissy, Iracema Leroi, Judy Rubinsztein, Vandana Menon, Paul Koranteng, Robert Barber, Rob Jones, Sujata Das, Rohan Van Der Putt, Ejaz Nazir, Jeremy Isaacs, Paul Loughlin, Divya Tiwari, Vanessa Raymont, Tarun Kuruvilla, Rosalind Ward, Marisa Wray, Wendy Neil, Robert Lawrence, Farhad Huwez, Bryan Corridan, Tarik Qassem, Vijayendra Waykar and Aparna Prasanna for recruiting participants.
The trial was supported by a grant from the Efficacy Mechanisms and Evaluation Board funded by NIHR and the Medical Research Council following external peer and board review. MODEPHARMA Limited manufactured the IMP and placebo that was distributed to participating cites by Polar Speed.
MADE Triallist Group
Writing committee
Robert Howard (University College London, London, UK); Olga Zubko (King’s College London, London, UK); Rosie Bradley [Nuffield Department of Population Health (NDPH), University of Oxford, Oxford, UK); Emma Harper (NDPH, University of Oxford, Oxford, UK); Linda Kelly (NDPH, University of Oxford, Oxford, UK); Lynn Pank (NDPH, University of Oxford, Oxford, UK); John O’Brien (University of Cambridge, Cambridge, UK); Chris Fox (University of East Anglia, Norwich, UK); Naji Tabet (University of Sussex, Brighton, UK); Gill Livingston (University College London, London, UK); Peter Bentham (Birmingham and Solihull Mental Health NHS Foundation Trust, Birmingham, UK); Rupert McShane (University of Oxford, Oxford, UK); Alistair Burns (University of Manchester, Manchester, UK); Craig Ritchie (University of Edinburgh, Edinburgh, UK); Suzanne Reeves (University College London, London, UK); Simon Lovestone (University of Oxford, Oxford, UK); Clive Ballard (University of Exeter, Exeter, UK); Wendy Noble (King’s College London, London, UK); Gordon Wilcock (Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK); and Richard Gray (NDPH, University of Oxford, Oxford, UK).
Data monitoring committee
Peter Crome (chairperson), Jeremy Brown and Sarah Walker.
Trial Steering Committee
Gordon Wilcock (chairperson), Peter Dyte, Declan McLoughlin, Rosie Bradley, Richard Gray and Robert Howard.
Trial management
Olga Zubko, Rebecca Gathercole (King’s College, University of London, London, UK); Emma Harper, Lynn Pank, Linda Kelly, Natalie Lam and Keith Anderson (NDPH, University of Oxford, Oxford, UK).
Trial monitoring
King’s Health Partner’s Clinical Trials Office (London, UK).
Pharmacist
Nigel Barnes.
Participating centres and MADE Collaborative Group members
An asterisk (*) indicates PI at that centre.
Sadly, Rob Jones (†) and Selina Paul (‡) died unexpectedly during the course of the MADE trial.
Avon and Wiltshire Mental Health Partnership NHS Trust (number of patients, 20)
Rosalind Ward,* Hayley Dash, Joanna Morris-Bone, Catherine Roiz De S’a, Tanya Atapattu, Emma McNeill, Peter Arthure and Aiste Baltramaityte.
Berkshire Healthcare NHS Foundation Trust (number of patients, 26)
Nicholas Woodthorpe* and Lynn Rigby.
Birmingham and Solihull Mental Health NHS Foundation Trust (number of patients, 31)
Peter Bentham,* Jane Dyer, Di Baines, Abdul Patel,* Jan Wright, Akram Ali and Nigel Barnes.
Black Country Partnership NHS Foundation Trust (number of patients, three)
Tarik Qassem,* Aparna Prasanna,* Joanne Sawyer, Neeti Gupta, Laura Lord, Aparna Prasanna, Amy Shipman, Darren Weaver, Gurj Bhella, Danielle Cornford, Susan Horton, Tim Kingscote-Davies, Amanda Nicklin, Carolyn Ogden and Sukvinder Sandhar.
Bradford District Care NHS Foundation Trust (number of patients, 10)
Anilkumar Pillai,* Deepa George, Alison Barraclough, John Hiley, Sarah Poll, Jyoti Rana, Gregor Russell, Angus Sturrock, Sade Abiola, Jaspreet Sohal and Edward Sykes.
Cambridgeshire and Peterborough NHS Foundation Trust (number of patients, 23)
John O’Brien,* Siobhan Rust, Julie Philps, Annabel Price, Ben Underwood, Steven Albery, Lorraine Carter, Rachel Wade, Lauren Dawson, Bernice Gregory, George Griffiths, Christopher Jenkins, Clare Mundell, Christine Rowe and Sara Williamson.
Camden and Islington NHS Foundation Trust (number of patients, 16)
Gill Livingston,* Jonathan Flor Mary-Jo Doyle, Yvonne Foreshaw, Kate O’Connor, Selina Paul,‡ Poureya Aghakhani, Silvia Ceci and Adam Heffer.
Cornwall Partnership NHS Foundation Trust (number of patients, 15)
Vandana Mate,* Sharon Hudson, Suzanne Dean, Jackie Kerr, Joanna Ledger, Sadir Altaan, Emma O’Shaughnessy, Linda Allsop, Carolyn Brodie, Kimberley Moore and Johanna Skewes.
Coventry and Warwickshire Partnership NHS Trust (number of patients, 16)
Demi Onalaja,* Emily Benson, Wendy Roughan and David Tait.
Cumbria Partnership NHS Foundation Trust (number of patients, 15)
Marisa Wray* and Yumna Masood.
Derbyshire Healthcare NHS Foundation Trust (number of patients, 23)
Simon Thacker,* Smita Saxena, John Sykes, Gemma Harrison, Audrey Williamson, Caroline Cheetham, Graham Spencer, Victoria Baron, Russell Cooper, Megan Harman and Sandra Kimberlin.
Gloucestershire 2gether NHS Foundation Trust (number of patients, 11)
Tarun Kuruvilla,* Bethan Cartwright, Jenny Romer, Marelle Harvey.
Kent and Medway NHS and Social Care Partnership Trust (number of patients, 8)
Richard Brown,* Amy Hammond, Alison Welfare-Wilson, Vilma Gilis, Sam Manktelow and Agostina Secchi.
Leeds and York Partnership NHS Foundation Trust (number of patients, 20)
Anna Green,* Lisa Hackney, Wendy Neil,* Aishia Perkis, Damian Reynolds, Jenny Sweetman, Danielle Varley, Francesca Williams, Amanda Taffinder, Michael Dixon, David Braun and Lucy Allender.
Leicestershire Partnership NHS Trust (number of patients, 17)
Sarah Baillon, Latha Velayudhan,* Santanu Chakrabarti,* Sarah Thomason, Howard Fairey, Tom Pringle, Robyn McAskill and Lynne Hartwell.
Lincolnshire Partnership NHS Foundation Trust (number of patients, six)
Ban Al-Kaissy,* Diane Brennan, Lizwi Nyathi, Vijayendra Waykar, Kerry Evans, Dimple Oza and Aliya Turk Richard Lewis.
Manchester Mental Health and Social Care Trust (number of patients, 23)
Iracema Leroi,* Lewis Harpin, Alistair Burns, Christopher Broughton, Hannah Goldup, Sharon Hall, Lewis Harpin, Adam Kennedy, Sally-Anne Heasman, Javier Torres Martin, Andrew Peers, Jane Smithies, Maxine Syme and Michelle Thorpe.
NHS Ayrshire and Arran (number of patients, four)
Ajay Macharouthu,* Mark Wilson, Jacqui Kerr, Colin Grant, Elma Norwood, Alistair Rennie, Karen Greig and Lynne McNeil.
Norfolk and Suffolk NHS Foundation Trust (number of patients, 15)
Judy Rubinsztein,* Vandana B Menon,* Chris Fox, Zoe Inman, Louise McCarthy, Juniper West, Bonnie Teague, James Curtis, Valerie Dixon and Dennis Liew.
Northamptonshire Healthcare NHS Foundation Trust (number of patients, 15)
Paul Koranteng,* Kim Burke and Helen Reboul.
Northumberland, Tyne and Wear NHS Foundation Trust (number of patients, 22)
Robert Barber,* Victoria Hetherington, Jill Davison, Nichola Duffelen, Caroline Gerrard and Matthew Haggerty.
Nottinghamshire Healthcare NHS Foundation Trust (number of patients, 21)
Rob Jones,*† Sujata Das,* David Trevor, Craig Beecroft, Kehinde Junaid, David Kelly, John Lawton and Effie Pitsillides.
Oxford Health NHS Foundation Trust (number of patients, 26)
Rohan Van Der Putt,* Jenny McCleery,* Deborah Cooper, Jemima Hume, Justine Adams, Hazel Eaton, Rupert McShane, Claire Merritt, Christine Parker, Gordon Wilcock, Marilyn Arnold, Ioana Fodor, Orla Macdonald and David Sharma.
Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust (number of patients, two)
Divya Tiwari,* Jo Bell, Chantel Cox, Owen David, Emma Gunter, Gail Hann, Becky Jupp, Catherine Ovington, James Page, Andrew Williams, Rachel Bower, Alison Hogan and Sam Lloyd.
Southend University Hospital NHS Foundation Trust (number of patients, five)
John Whitear* and Paula Harman.
South London and Maudsley NHS Foundation Trust (number of patients, 54)
Robert Howard,* Olga Zubko, Leeza Almedom, Lauren Armstrong, Ana Bajo, Rebecca Brendell, Jack Cahill, Hannah Grocott, Siobhan Hurley, James Rackie, Arann Rowe, Clive Ballard, Jenny Bousfield, Elizabeth Highton-Williamson, Zainab Al Noor, Suzanne Reeves, Melody Smith, Ola Dada, Martin Heasman, Glynis Ivin, Ian Osborne, Sophie Ward and Michael Welds.
South Staffordshire and Shropshire Healthcare NHS Foundation Trust (number of patients, 33)
Ejaz Nazir,* Paula Dolby, Lucy Hamilton, Yvette Lycett, Andy Taylor, Ayesha Bangash, Liz Glaves, Sarah Johnson, Susan Lavender, Prince Nwaubani, Richard Heys, Felicity Massey, Ruth Mills, Allison Newman, Sacheev Patel, Lindsay Rose, Carla Silva, Mark Stallard, Tamir Latif, Farzad Khalkhali, Sudhakar Anumanchi, Eva Kabir, Adnan Sharaf, Sajeev Kshemendran and Rashi Negi.
South West London and St George’s Mental Health NHS Trust (number of patients, nine)
Robert Lawrence,* Enitan Eboda, Ashes Howson, Mustabshira Qayyum, Philip Woodgate, Laura Dalrymple, Jess Lee, Felicity Mayer, Carl Holvey and Aiste Navickaite.
St George’s University Hospitals NHS Foundation Trust (number of patients, 20)
Jeremy Isaacs,* Sally Goff, Servious Dube, Peter Garrard and Jennifer Tulloch.
Surrey and Borders Partnership NHS Foundation Trust (number of patients, 30)
Ramin Nilforooshan,* Ruth Charig, Jane Gregg, Caroline Khurana, Helen Adams, Jack Holland, Brian Parsons, Emily Williams, Samantha Francis, Richard Johnson, Fiona Lockwood, Ailsa McKay and Jane Wenham.
Sussex Partnership NHS Foundation Trust (number of patients, 12)
Naji Tabet,* Samuel Holden, Gail Chandler, Andrew Risbridger, Gail Chandler and Gus Fernandez.
West London Mental Health NHS Trust (number of patients, three)
Sarah Gregory, Merrie Manalo, Vanessa Raymont,* Bryan Corridan,* Craig Ritchie,* Tahira Arshad, Sharon Linsell and Laura McKee.
Contributions of authors
Robert Howard (https://orcid.org/0000-0002-3071-2338), Richard Gray (https://orcid.org/0000-0003-4440-574X), John O’Brien (https://orcid.org/0000-0002-0837-5080), Peter Bentham (https://orcid.org/0000-0002-6443-3353), Simon Lovestone (https://orcid.org/0000-0003-0473-4565), Alistair Burns (https://orcid.org/0000-0002-9837-0645) and Suzanne Reeves (https://orcid.org/0000-0001-8053-7024) designed the trial.
Olga Zubko (https://orcid.org/0000-0002-3520-624X), Emma Harper (https://orcid.org/0000-0001-5651-6258), Linda Kelly (https://orcid.org/0000-0003-1936-0842), Lynn Pank (https://orcid.org/0000-0001-6398-6565), Robert Howard and Richard Gray ran the trial.
Rosie Bradley (https://orcid.org/0000-0002-0758-4905) and Richard Gray analysed the data.
Robert Howard, Peter Bentham, John O’Brien, Gill Livingston (https://orcid.org/0000-0001-6741-5516), Ramin Nilforooshan (https://orcid.org/0000-0001-9801-183X) and Naji Tabet (https://orcid.org/0000-0003-4629-6196) recruited participants.
Robert Howard, Olga Zubko, Rosie Bradley and Richard Gray wrote the initial paper draft.
Chris Fox (https://orcid.org/0000-0001-9480-5704), Rupert McShane (https://orcid.org/0000-0002-3272-5984), Craig Ritchie (https://orcid.org/0000-0002-6202-6906), Clive Ballard (https://orcid.org/0000-0003-0022-5632), Wendy Noble (https://orcid.org/0000-0002-7898-4295) and Gordon Wilcock (https://orcid.org/0000-0003-0163-3021) contributed to writing and reviewing the paper.
All authors contributed to writing the paper and assume responsibility for the accuracy and completeness of the data and for the overall content and integrity of the paper.
Publication
Howard R, Zubko O, Bradley R, Harper E, Pank L, O’Brien J, et al. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial [published online ahead of print November 18 2019]. JAMA Neurol 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Alzheimer’s Disease International . World Alzheimer Report 2015: The Global Impact of Dementia 2015.
- Vradenburg G. A pivotal moment in Alzheimer’s disease and dementia: how global unity of purpose and action can beat the disease by 2025. Expert Rev Neurother 2015;15:73-82. https://doi.org/10.1586/14737175.2015.995638.
- Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 2015;77:43-51. https://doi.org/10.1016/j.biopsych.2014.05.006.
- Edison P, Donat CK, Sastre M. In vivo imaging of glial activation in Alzheimer’s disease. Front Neurol 2018;9. https://doi.org/10.3389/fneur.2018.00625.
- VanItallie TB. Alzheimer’s disease: innate immunity gone awry?. Metab Clin Exp 2017;69S:S41-9. https://doi.org/10.1016/j.metabol.2017.01.014.
- Yu Y, Ye RD. Microglial Aβ receptors in Alzheimer’s disease. Cell Mol Neurobiol 2015;35:71-83. https://doi.org/10.1007/s10571-014-0101-6.
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015;14:388-405. https://doi.org/10.1016/S1474-4422(15)70016-5.
- Corbett A, Pickett J, Burns A, Corcoran J, Dunnett SB, Edison P, et al. Drug repositioning for Alzheimer’s disease. Nature Revi Drug Discovery 2012;11:833-46. https://doi.org/10.1038/nrd3869.
- Appleby BS, Cummings JL. Discovering new treatments for Alzheimer’s disease by repurposing approved medications. Curr Top Med Chem 2013;13:2306-27. https://doi.org/10.2174/15680266113136660162.
- Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol 2010;67:1442-8. https://doi.org/10.1001/archneurol.2010.191.
- Familian A, Boshuizen RS, Eikelenboom P, Veerhuis R. Inhibitory effect of minocycline on amyloid beta fibril formation and human microglial activation. Glia 2006;53:233-40. https://doi.org/10.1002/glia.20268.
- Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 2006;53:776-82. https://doi.org/10.1002/glia.20338.
- Noble W, Garwood C, Stephenson J, Kinsey AM, Hanger DP, Anderton BH. Minocycline reduces the development of abnormal tau species in models of Alzheimer’s disease. FASEB J 2009;23:739-50. https://doi.org/10.1096/fj.08-113795.
- Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer’s disease-like amyloid pathology. J Neuroinflammation 2012;9. https://doi.org/10.1186/1742-2094-9-62.
- Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis 2012;9:187-98. https://doi.org/10.1159/000330363.
- Garcez ML, Mina F, Bellettini-Santos T, Carneiro FG, Luz AP, Schiavo GL, et al. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid β (1-42) in mice. Prog Neuropsychopharmacol Biol Psychiatry 2017;77:23-31. https://doi.org/10.1016/j.pnpbp.2017.03.010.
- Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 2007;32:2393-404. https://doi.org/10.1038/sj.npp.1301377.
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 2007;69:1404-10. https://doi.org/10.1212/01.wnl.0000277487.04281.db.
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA 2001;98:14669-74. https://doi.org/10.1073/pnas.251341998.
- Radad K, Moldzio R, Rausch WD. Minocycline protects dopaminergic neurons against long-term rotenone toxicity. Can J Neurol Sci 2010;37:81-5. https://doi.org/10.1017/s0317167100009690.
- Diguet E, Fernagut PO, Wei X, Du Y, Rouland R, Gross C, et al. Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci 2004;19:3266-76. https://doi.org/10.1111/j.0953-816X.2004.03372.x.
- Yang L, Sugama S, Chirichigno JW, Gregorio J, Lorenzl S, Shin DH, et al. Minocycline enhances MPTP toxicity to dopaminergic neurons. J Neurosci Res 2003;74:278-85. https://doi.org/10.1002/jnr.10709.
- NINDS NET-PD Investigators . A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol 2008;31:141-50. https://doi.org/10.1097/WNF.0b013e3181342f32.
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002;417:74-8. https://doi.org/10.1038/417074a.
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045-53. https://doi.org/10.1016/S1474-4422(07)70270-3.
- Huntington Study Group . Minocycline safety and tolerability in Huntington disease. Neurology 2004;63:547-9. https://doi.org/10.1212/01.WNL.0000133403.30559.FF.
- Hunter CL, Quintero EM, Gilstrap L, Bhat NR, Granholm AC. Minocycline protects basal forebrain cholinergic neurons from mu p75-saporin immunotoxic lesioning. Eur J Neurosci 2004;19:3305-16. https://doi.org/10.1111/j.0953-816X.2004.03439.x.
- Familian A, Eikelenboom P, Veerhuis R. Minocycline does not affect amyloid beta phagocytosis by human microglial cells. Neurosci Lett 2007;416:87-91. https://doi.org/10.1016/j.neulet.2007.01.052.
- Cuello AC, Ferretti MT, Leon WC, Iulita MF, Melis T, Ducatenzeiler A, et al. Early-stage inflammation and experimental therapy in transgenic models of the Alzheimer-like amyloid pathology. Neurodegener Dis 2010;7:96-8. https://doi.org/10.1159/000285514.
- Ryu JK, McLarnon JG. Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood-brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus. Exp Neurol 2006;198:552-7. https://doi.org/10.1016/j.expneurol.2005.12.016.
- Parachikova A, Vasilevko V, Cribbs DH, LaFerla FM, Green KN. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J Alzheimers Dis 2010;21:527-42. https://doi.org/10.3233/JAD-2010-100204.
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol 1996;134:693-5. https://doi.org/10.1111/j.1365-2133.1996.tb06972.x.
- Casha S, Zygun D, McGowan D, Yong VW, Hurlbert RJ. Neuroprotection with minocycline after spinal cord injury: results of a double blind, randomized, controlled pilot study. Neurosurgery 2009;65:410-11. https://doi.org/10.1227/01.neu.0000358700.03703.a5.
- Metz LM, Li D, Traboulsee A, Myles ML, Duquette P, Godin J, et al. Glatiramer acetate in combination with minocycline in patients with relapsing–remitting multiple sclerosis: results of a Canadian, multicenter, double-blind, placebo-controlled trial. Mult Scler 2009;15:1183-94. https://doi.org/10.1177/1352458509106779.
- Bonelli RM, Hodl AK, Hofman P, Kapfhammer HP. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol 2004;19:337-42. https://doi.org/10.1097/00004850-200411000-00004.
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Effect of Tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer’s disease. A randomised controlled trial. JAMA 2009;302:2557-64. https://doi.org/10.1001/jama.2009.1866.
- Aisen PS, Gauthier S, Ferris SH, Saumier D, Haine D, Garceau D, et al. Tramiprosate in mild-to-moderate Alzheimer’s disease – a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci 2011;7:102-11. https://doi.org/10.5114/aoms.2011.20612.
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Molloy DW, Alemayehu E, Roberts R. Reliability of a Standardized Mini Mental State Examination compared with the traditional Mini Mental State Examination. Am J Psychiatry 1991;148:102-5. https://doi.org/10.1176/ajp.148.1.102.
- Molloy DW, Standish TI. A guide to the standardized Mini Mental State Examination. Int Psychogeriatr 1997;9:87-94. https://doi.org/10.1017/s1041610297004754.
- South London and Maudsley NHS Foundation Trust . Minocycline in Alzheimer’s Disease Efficacy Trial: The MADE Trial 2013. https://gtr.ukri.org/projects?ref=MC_PC_13091.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Tombaugh TN, McIntyre NJ. The Mini Mental State Examination: a comprehensive review. J Am Geriatr Soc 1992;40:922-35. https://doi.org/10.1111/j.1532-5415.1992.tb01992.x.
- Howard R, Phillips P, Johnson T, O’Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int J Geriatr Psychiatry 2011;26:812-17. https://doi.org/10.1002/gps.2607.
- Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004;363:2105-15. https://doi.org/10.1016/S0140-6736(04)16499-4.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. Donepezil MSAD Study Investigators Group . A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology 2001;57:613-20. https://doi.org/10.1212/wnl.57.4.613.
- Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007;357:1382-92. https://doi.org/10.1056/NEJMoa066583.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. https://doi.org/10.1093/ageing/25.2.113.
- Lavori PW. Clinical trials in psychiatry: should protocol deviation censor patient data?. Neuropsychopharmacology 1992;6:39-48.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263-9. https://doi.org/10.1016/j.jalz.2011.03.005.
- European Commission . EU Guidelines to Good Manufacturing Practice: Medicinal Products for Human and Veterinary Use – Annex 13: Investigational Medicinal Products 2010.
- Great Britain . Data Protection Act 1998 1998.
- Great Britain . Medicines for Human Use (Clinical Trials) Regulations 2004 2004.
- Miguel-Álvarez M, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Garatachea N, et al. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging 2015;32:139-47. https://doi.org/10.1007/s40266-015-0239-z.
- Breitner JC, Gau BA, Welsh KA, Plassman BL, McDonald WM, Helms MJ, et al. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology 1994;44:227-32. https://doi.org/10.1212/wnl.44.2.227.
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging 2007;28:639-47. https://doi.org/10.1016/j.neurobiolaging.2006.03.013.
- Huntington Study Group DOMINO Investigators . A futility study of minocycline in Huntington’s disease. Mov Disord 2010;25:2219-24. https://doi.org/10.1002/mds.23236.
- Dodel R, Spottke A, Gerhard A, Reuss A, Reinecker S, Schimke N, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord 2010;25:97-107. https://doi.org/10.1002/mds.22732.
- Deakin B, Suckling J, Barnes TRE, Byrne K, Chaudhry IB, Dazzan P, et al. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 2018;5:885-94. https://doi.org/10.1016/S2215-0366(18)30345-6.
- Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M, Cerchiaro G, et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med 2017;376:2122-33. https://doi.org/10.1056/NEJMoa1608889.
- Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain 2018;141:459-71. https://doi.org/10.1093/brain/awx339.
Appendix 1 Protocol changes
Table 5 is a summary of the changes made to the original MADE trial protocol, which have been approved by the REC and the Medicines and Healthcare products Regulatory Agency (where relevant).
| Change to protocol | Date |
|---|---|
| Increased leeway window for final assessments from ± 14 days to ± 30 days | 22 March 2018 |
| Guidance on restarting IMP added, with participants able to restart IMP at any time after break | 29 July 2016 |
| End-of-study participant letter added | 29 July 2016 |
| End-of-study GP letter added | 29 July 2016 |
| Increase recruitment from 480 to 560 participants by March 2016 | 30 October 2015 |
| Adding JDR as additional recruitment tool | 21 January 2015 |
| Inclusion criterion ‘severe liver disease’ modified to ‘moderate liver disease’ | 4 February 2015 |
| Added a trial leaflet | 3 September 2014 |
| Added a trial recruitment advertisement poster | 3 September 2014 |
| Added a GP letter of invitation | 3 September 2014 |
| Add inclusion criterion: participants must have a potential informant to assist with administration of the BADLS assessment | 9 January 2014 |
| Exclusion criterion ‘Patients with creatinine clearance < 50 ml/minute at screening, according to the Cockcroft and Gault equation must be excluded’ was added | 7 August 2014 |
| Exclusion criterion ‘Known chronic kidney disease stages 3–5′ was amended to ‘Known chronic kidney disease stages 3b-5’ | 7 August 2014 |
| Guidance for renal functioning monitoring updated | 9 January 2014 |
| Further guidance for monitoring side effects and loss to follow-up added | 9 January 2014 |
| Additional exclusion criterion added: uncontrolled serious concomitant illness | 9 January 2014 |
| Removed from exclusion criteria: pregnancy and lactation; diagnosis of MCI; lacks capacity to give informed consent; and severe liver disease | 9 January 2014 |
| Broadened recruitment strategy by allowing members of the research team to get patient consent after patients had been initially approached by the study doctor | 14 October 2013 |
| Guidance on reporting SAEs/SUSARs added | 28 August 2013 |
| Changed frequency of safety checks from every 6 months to every 3 months | 28 August 2013 |
| Broadened recruitment strategy by advertising the study and allowing potentially interested individuals to contact the research team directly | 28 August 2013 |
| Added age range (≥ 50 years) to inclusion criterion | 28 August 2013 |
Appendix 2 Literature search
Table 6 presents a summary of the number of articles identified by each search engine.
| Search engine | Number of articles identified |
|---|---|
| PubMed | 1549 |
| The Cochrane Library | 19 |
| Web of Science | 121 |
| Ovid (including EMBASE, PsycARTICLES and PsycINFO) | 1471 |
The following URL provides a link to the PROSPERO record for the search:
www.crd.york.ac.uk/prospero/display_record.php?RecordID = 90377 9 (accessed April 2018).
The following search terms were used in March 2018 to perform the search:
minocycline AND (AD OR alzheimer OR MCI OR “mild cognitive impairment” OR dementia).
Appendix 3 Outcome measure response sheets and sample participant responses
| Question | Time allowed | Score | |
|---|---|---|---|
| 1 | What year is this? | 10 seconds | |
| Which season is this? | 10 seconds | ||
| What month is this? | 10 seconds | ||
| What is today’s date? | 10 seconds | ||
| What day of the week is this? | 10 seconds | ||
| 2 | What country are we in? | 10 seconds | |
| What province are we in? | 10 seconds | ||
| What city/town are we in? | 10 seconds | ||
|
IN HOME – What is the street address of this house? IN FACILITY – What is the name of this building? |
10 seconds | ||
|
IN HOME – What room are we in? IN FACILITY – What floor are we on? |
10 seconds | ||
| 3 | SAY: I am going to name three objects. When I am finished, I want you to repeat them. Remember what they are because I am going to ask you to name them again in a few minutes. Say the following words slowly at 1-second intervals – ball/car/man | 20 seconds | |
| 4 | Spell the word WORLD. Now spell it backwards. | 30 seconds | |
| 5 | Now what were the three objects I asked you to remember? | 10 seconds | |
| 6 | SHOW wristwatch. ASK: What is this called? | 10 seconds | |
| 7 | SHOW pencil. ASK: What is this called? | 10 seconds | |
| 8 | SAY: I would like you to repeat this phrase after me: No ifs, ands or buts. | 10 seconds | |
| 9 | SAY: Read the words on the page and then do what it says. Then hand the person the sheet with CLOSE YOUR EYES on it. If the subject reads and does not close their eyes, repeat up to three times. Score only if subject closes eyes | 10 seconds | |
| 10 | HAND the person a pencil and paper. SAY: Write any complete sentence on that piece of paper. (Note: The sentence must make sense. Ignore spelling errors) | 30 seconds | |
| 11 |
PLACE design, eraser and pencil in front of the person. SAY: Copy this design please Allow multiple tries. Wait until person is finished and hands it back. Score only for a correctly copied diagram with a 4-sided figure between two 5-sided figures |
1 minute | |
| 12 | ASK the person if they are right- or left-handed. Take a piece of paper and hold it up in front of the person. SAY: Take this paper in your right/left hand (whichever is non-dominant), fold the paper in half once with both hands and put the paper down on the floor. Score 1 point for each instruction executed correctly:
|
30 seconds | |
| Total test score | /30 | ||
FIGURE 4.
Bristol Activities of Daily Living Scale: outcome measure responses sheet.



FIGURE 5.
Sample participant responses: BADLS responses for 12 months’ follow-up.


FIGURE 6.
Sample participant responses: sMMSE responses for 12 months’ follow-up.

Appendix 4 Additional figures and tables
| Time point | Treatment arm | Assessment | |||||
|---|---|---|---|---|---|---|---|
| sMMSE | BADLS | ||||||
| Received (n) | Expecteda (n) | % | Received (n) | Expectedb (n) | % | ||
| Screening | 400 mg of minocycline | 183 | 184 | 99.5 | 183 | 184 | 99.5 |
| 200 mg of minocycline | 181 | 181 | 100 | 181 | 181 | 100 | |
| Placebo | 178 | 179 | 99.4 | 177 | 178 | 99.4 | |
| Total | 542 | 544 | 99.6 | 541 | 543 | 99.6 | |
| 6 months | 400 mg of minocycline | 159 | 184 | 86 | 159 | 184 | 86 |
| 200 mg of minocycline | 172 | 181 | 95 | 172 | 181 | 95 | |
| Placebo | 167 | 179 | 93 | 164 | 176 | 93 | |
| Total | 498 | 544 | 92 | 495 | 541 | 91 | |
| 12 months | 400 mg of minocycline | 139 | 181 | 77 | 140 | 180 | 78 |
| 200 mg of minocycline | 158 | 180 | 88 | 157 | 178 | 88 | |
| Placebo | 156 | 176 | 89 | 155 | 171 | 91 | |
| Total | 453 | 537 | 84 | 452 | 529 | 85 | |
| 18 months | 400 mg of minocycline | 127 | 179 | 71 | 128 | 178 | 72 |
| 200 mg of minocycline | 146 | 177 | 82 | 146 | 169 | 86 | |
| Placebo | 147 | 172 | 85 | 148 | 167 | 89 | |
| Total | 420 | 528 | 80 | 422 | 514 | 82 | |
| 24 months | 400 mg of minocycline | 119 | 174 | 68 | 118 | 170 | 69 |
| 200 mg of minocycline | 144 | 176 | 82 | 142 | 167 | 85 | |
| Placebo | 140 | 167 | 84 | 137 | 154 | 89 | |
| Total | 403 | 517 | 78 | 397 | 491 | 81 | |
| Treatment arm | Toxicity rating | Number of patients |
|---|---|---|
| 400 mg of minocycline | Mild | 33 |
| Moderate | 27 | |
| Severe | 1 | |
| Subtotal | 61 | |
| 200 mg of minocycline | Mild | 38 |
| Moderate | 29 | |
| Severe | 2 | |
| Subtotal | 69 | |
| Placebo | Mild | 22 |
| Moderate | 13 | |
| Severe | 3 | |
| Subtotal | 38 |
| Treatment arm | Cause of death | Weeks until death | Stopped treatment ≥ 28 days previously? |
|---|---|---|---|
| Infection | |||
| Placebo | Infection | 64 | Yes – 17 weeks |
| Placebo | Pneumonia | 36 | No |
| Placebo | Pneumonia and pulmonary oedema | 28 | Yes – 23 weeks |
| Placebo | Pneumonia | 66 | No |
| Placebo | Chest infection | 83 | No |
| 200 mg of minocycline | Pneumonia | 56 | No |
| 400 mg of minocycline | Pneumonia | 86 | Yes – 2 weeks |
| Neuropsychiatric | |||
| Placebo | Dementia | 95 | No |
| Placebo | AD/Lewy body dementia | 92 | Yes – 87 weeks |
| 400 mg of minocycline | Progression of AD | 58 | Yes – 7 weeks |
| Cardiovascular | |||
| Placebo | Myocardial infarction | 102 | No |
| Placebo | Myocardial infarction | 72 | No |
| Placebo | Heart attack | 64 | No |
| 200 mg of minocycline | Cardiac event | 50 | No |
| 200 mg of minocycline | Heart attack | 58 | Yes – 51 weeks |
| 400 mg of minocycline | Heart attack | 37 | No |
| 400 mg of minocycline | Heart failure | 100 | Yes – 88 weeks |
| 400 mg of minocycline | Heart attack | 91 | No |
| Cerebrovascular | |||
| 200 mg of minocycline | Unknown (stroke on 21 March 2017) | 103 | Yes – 84 weeks |
| 400 mg of minocycline | CVA | 42 | Yes – 3 weeks |
| 400 mg of minocycline | Stroke | 36 | No |
| Renal failure | |||
| Placebo | Chronic renal failure | 32 | Yes – 12 weeks |
| 400 mg of minocycline | Lung and kidney failure | 103 | Yes – 1 week |
| Other cause | |||
| Placebo | Complications after bowel surgery | 89 | Yes – 44 weeks |
| 200 mg of minocycline | General health decline | 56 | Yes – 29 weeks |
| 200 mg of minocycline | Large abdominal tumour causing kidney failure | 28 | Never started |
| 400 mg of minocycline | COPD | 57 | Yes – 11 weeks |
| Unknown | |||
| 400 mg of minocycline | Unknown | 77 | Yes – 17 weeks |
FIGURE 7.
Proportion of participants taking trial treatment over time: Kaplan–Meier plot.

FIGURE 8.
Flow chart showing the completeness over time of participant follow-up. Colour coding to show assessments split by treatment arm: navy font is 400 mg of minocycline, light blue font is 200 mg of minocycline and red font is placebo.
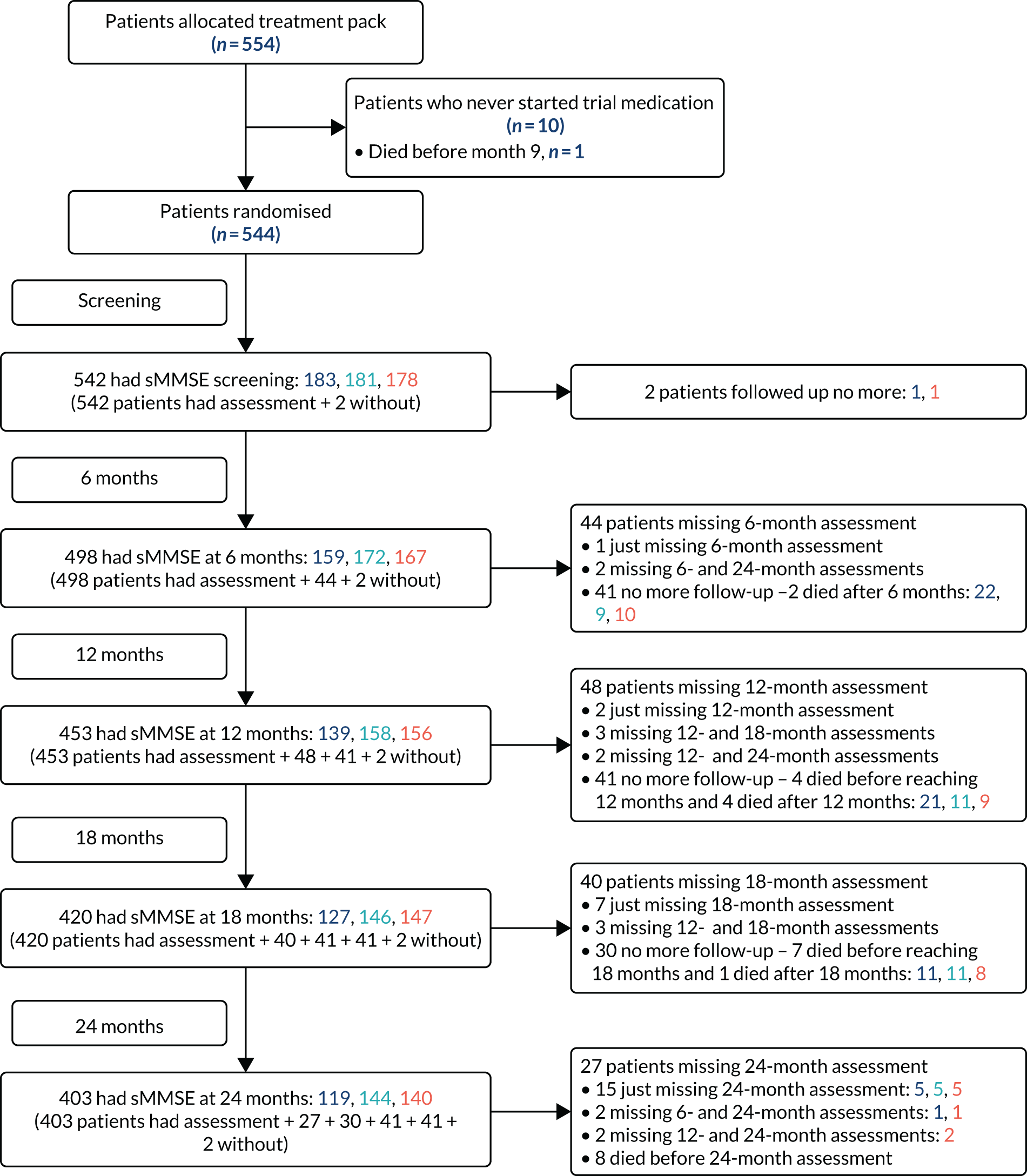
FIGURE 9.
Change in sMMSE and BADLS scores from baseline to month 24 using imputation methods 1 (a and b) and 2 (c and d). A to estimate scores for patients with no follow-up past baseline. Graph shows change in mean sMMSE and BADLS scores with standard errors. Baseline scores are set to zero and p-values are from tests for time-by-treatment interaction from repeated measures analyses. (a) Average change in sMMSE score from baseline to month 24, using imputation (baseline scores are 26.3 points for 400 mg of minocycline, 26.5 points for 200 mg of minocycline and 26.4 points for placebo). (b) Average change in BADLS score from baseline to month 24, using imputation (baseline scores are 5.6 points for 400 mg of minocycline, 4.9 points for 200 mg of minocycline and 5.5 points for placebo). (c) Average change in sMMSE score from baseline to month 24 using imputation. (d) Average change in BADLS score from baseline to month 24, using imputation.


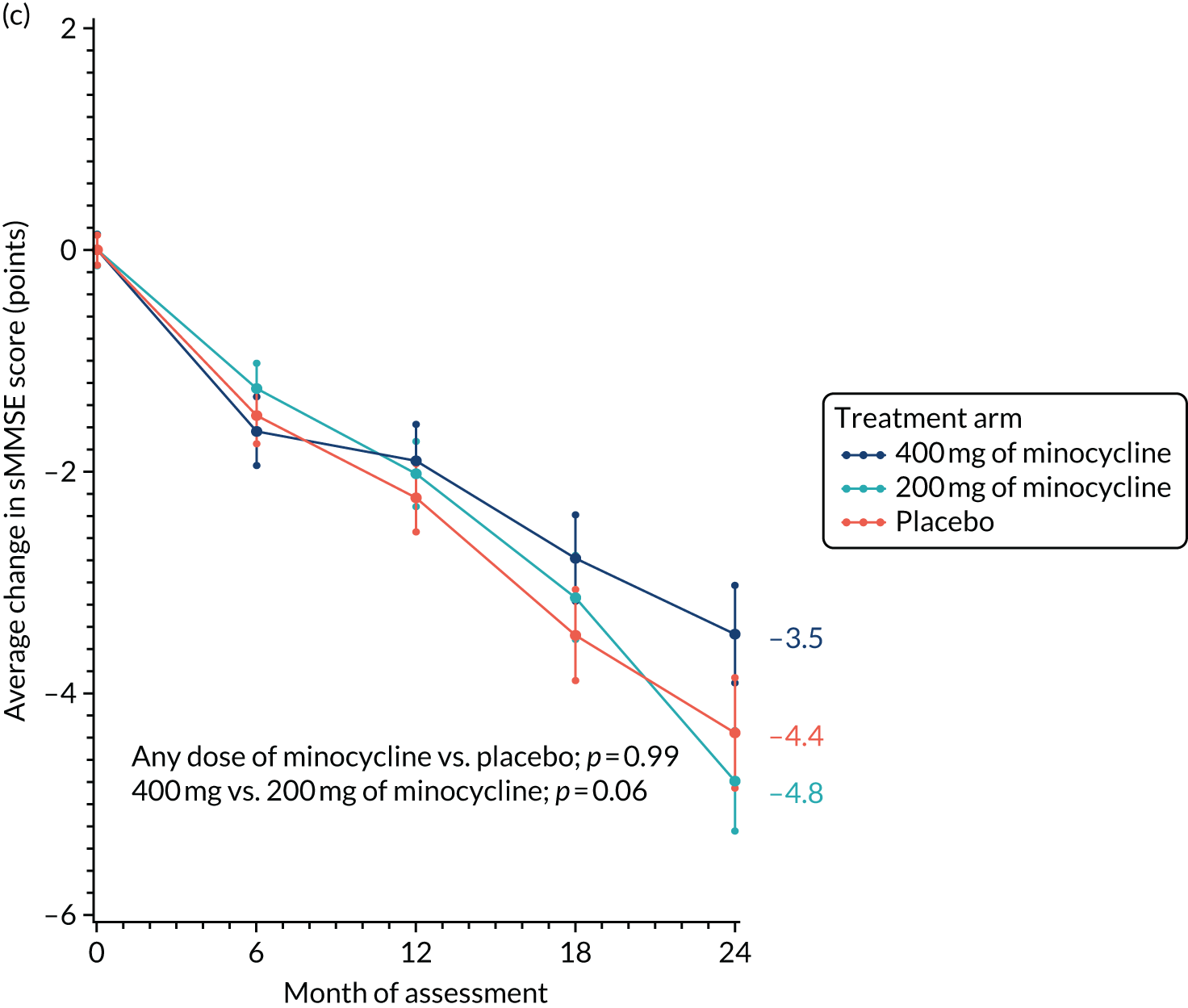
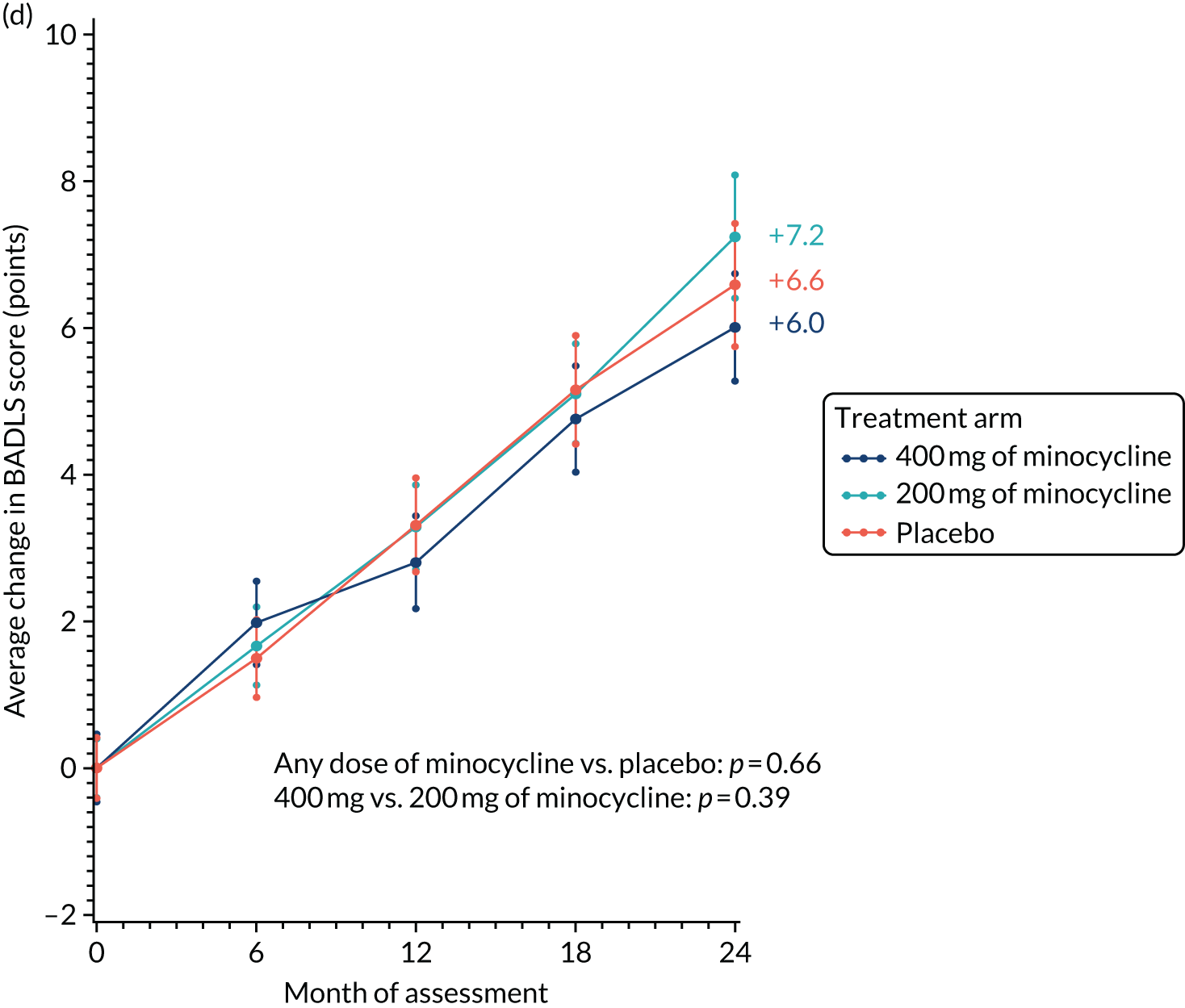
FIGURE 10.
Subgroup analyses of change in sMMSE score over 24 months for minocycline (any dose) vs. placebo by baseline characteristics: duration of symptoms, baseline sMMSE score, age and gender. Results are derived from a repeated measures model, with p-values from tests for interaction between treatment and the selected subgroup. Min., minimum. The p-value is from the test statistic for testing the interaction between the treatment and any subgroup variable.
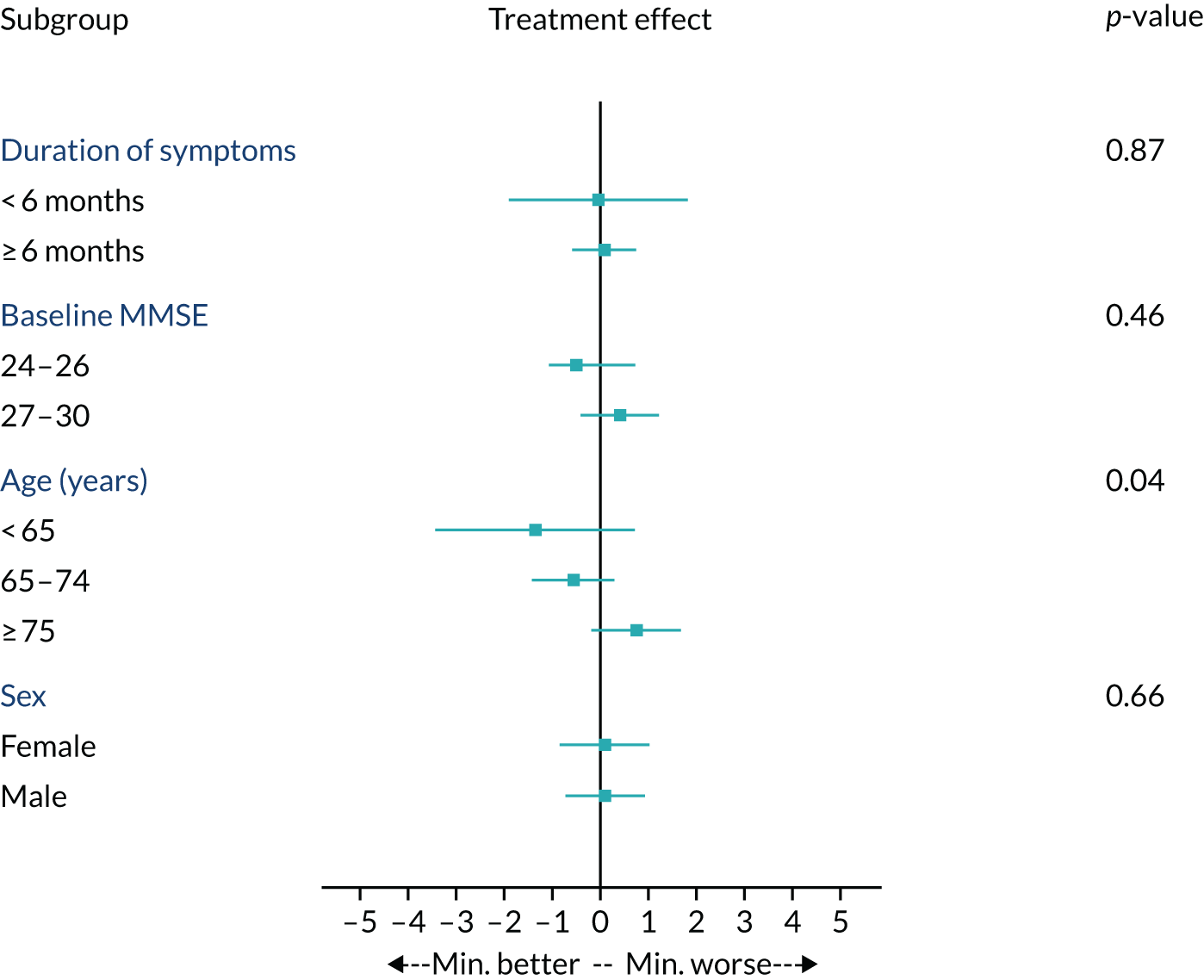
FIGURE 11.
Probability of (a) overall survival, (b) institutionalisation and (c) time to death or institutionalisation by treatment arm: Kaplan–Meier survival plots.



FIGURE 12.
Average decline of sMMSE score split by baseline sMMSE score (i.e. a score of 24–26 or 27–30a points). a, Eight patients start with a sMMSE score of 30 points and have a 24-month sMMSE score of 30 points.
| Reference number of SAE | SAE | On treatment? |
|---|---|---|
| Gastrointestinal | ||
| SAE031 | Gastroenteritis | Yes |
| SAE067 | Constipation. Was taken to hospital, patient described medication | Yes |
| SAE064 | Gastroenteritis | Yes |
| SAE077 | Sigmoid volvulus with faecal impaction | Stopped > 28 days ago |
| SAE075 | Diverticulitis and impacted bowel | Yes |
| SAE087 | Deranged LFTs/stomach ulcer with gastrointestinal bleed | Yes (stopped same time) |
| SAE149 | Abdominal distension pain | Stopped > 28 days ago |
| SAE091 | Constipation. Admitted to hospital with sickness/stomach pains | Stopped < 28 days ago |
| SAE095 | Gradual internal bleeding of the stomach lining | Yes |
| SAE092 | Diarrhoea, vomiting and weight loss. Ambulance called and patient was admitted to hospital overnight. Hospital-requested RNI scan | Yes |
| SAE106 | Under investigation – severe diarrhoea. Bowels going into spasms | Yes |
| SAE112 | Patient admitted to hospital with severe abdominal pains | Stopped > 28 days ago |
| SAE120 | Undiagnosed stomach pains. Investigations into possible stomach ulcer or recurrence of bowel cancer | Yes (stopped same time) |
| SAE138 | Diverticulitis | Yes |
| SAE181 | Gastroenteritis | Yes |
| SAE164 | Appendicitis | Yes |
| SAE187 | Death from complications after bowel surgery | Stopped > 28 days ago |
| SAE209 | Secondary, adhesion bowel obstruction – resulted in death | Stopped > 28 days ago |
| SAE194 | Bowel obstruction | Yes |
| SAE182 | Cyst on small intestine updated diagnosis previously bowel obstruction | Yes |
| SAE228 | Obstruction of the common bile duct | Yes |
| Respiratory | ||
| SAE001 | Pneumonia | Yes (stopped same time) |
| SAE002 | Wheezing and shortness of breath | Yes (stopped same time) |
| SAE012 | COPD | Yes |
| SAE020 | Pneumonia | Yes |
| SAE018 | Pneumonia – resulted in death | Yes (stopped same time) |
| SAE022 | Suicide attempt and subsequent aspiration pneumonia and pulmonary oedema – resulted in death | Stopped > 28 days ago |
| SAE025 | Community-acquired pneumonia | Yes (stopped same time) |
| SAE048 | Sepsis secondary to community-acquired pneumonia | Yes (stopped same time) |
| SAE043 | Pneumonia | Yes |
| SAE047 | Suspected pneumonia/further investigation | Yes |
| SAE079 | Pneumonia and pleural effusion – resulted in death | Yes |
| SAE085 | Pneumonia | Yes (stopped same time) |
| SAE116 | Died – COPD | Stopped > 28 days ago |
| SAE114 | Pneumonia – died in hospital | Yes (stopped same time) |
| SAE117 | Pneumonia preceded by declining neutrophil count which then rose before ceasing IMP | Yes |
| SAE136 | Community-acquired pneumonia | Yes |
| SAE122 | Pneumonia | Yes |
| SAE156 | Increased shortness of breath | Stopped < 28 days ago |
| SAE163 | Breathlessness and extreme thirst | Yes |
| SAE184 | Pneumonia | Yes |
| SAE200 | COPD | Yes |
| SAE226 | Admitted to hospital after fall and contracted pneumonia while in hospital | Yes |
| SAE266 | Admitted to hospital after fall and contracted pneumonia while in hospital. Had fractured a rib | Yes |
| SAE243 | Pneumonia | Stopped > 28 days ago |
| SAE270 | Pulmonary fibrosis | Yes |
| SAE215 | Pneumonia – resulted in death | Stopped > 28 days ago |
| Mechanical injury | ||
| SAE004 | Fall resulting in skull fracture | Yes |
| SAE015 | Fall and closed fracture of rib | Yes (stopped same time) |
| SAE021 | Fall out of bed resulting in head and neck injury | Stopped < 28 days ago |
| SAE034 | Fall sustaining cuts, bruising and reduced mobility | Yes |
| SAE053 | Unwitnessed fall out of bed | Yes |
| SAE068 | Fractured pubic rami from a fall | Yes |
| SAE046 | Fracture of right femur from a fall | Yes (stopped same time) |
| SAE054 | Fractured wrist from a fall | Yes |
| SAE080 | Patient fell and fractured pelvis, related to increased dizziness, less steady on feet since starting MADE trial treatment | Stopped < 28 days ago |
| SAE063 | Possible bruised, cracked or broken ribs following a fall as a result of tripping | Yes |
| SAE073 | Shoulder surgery following fall and dislocation | Yes |
| SAE065 | Fractured right humerus from a fall | Yes |
| SAE093 | Cerebral concussion and cut to head from a fall | Yes |
| SAE124 | Fractured right neck of femur | Yes (stopped same time) |
| SAE155 | Road traffic accident – patient hit by car | Stopped > 28 days ago |
| SAE119 | Fall and admission to hospital overnight | Yes |
| SAE139 | Fractured left femur and underwent left hemiarthroplasty | Yes |
| SAE175 | Fractured ribs from a fall | Yes |
| SAE117 | Broken left hip | Yes |
| SAE196 | Fracture of left neck of femur | Yes |
| SAE199 | Knee replacement operation | Yes |
| SAE222 | Fall | Yes |
| SAE232 | Fracture of metacarpal | Yes |
| SAE235 | Facial injury from a fall | Stopped > 28 days ago |
| SAE237 | Collapse and facial injury and nasal fracture | Yes |
| SAE254 | Fractured hip | Yes |
| SAE255 | Fall causing pubic ramus and wrist fracture | Yes |
| SAE258 | Vertigo/dizziness – leading to head injury from a fall | Stopped > 28 days ago |
| SAE256 | Fracture to middle finger and left hand | Yes |
| SAE263 | Mechanical fall and back pain | Stopped > 28 days ago |
| Endocrine and metabolic | ||
| SAE008 | Diabetes mellitus management impairment – resolved | Yes |
| SAE007 | New medical diagnosis of type 2 diabetes mellitus | Yes |
| SAE060 | Low sodium levels | Yes |
| SAE061 | Pituitary adenoma | Stopped > 28 days ago |
| SAE056 | Diabetic ketoacidosis | Stopped > 28 days ago |
| SAE072 | Hypoglycaemia | Stopped > 28 days ago |
| SAE118 | Hypoglycaemia | Yes |
| SAE132 | Syndrome of inappropriate anti-diuretic hormone | Yes |
| SAE135 | Low potassium levels as a result of bowel preparation for CT bowel scan | Yes |
| SAE189 | Admitted to hospital after feeling weak and faint. Diagnosed with low sodium levels | Yes |
| SAE190 | Admitted to hospital following low sodium levels and generally feeling weak | Yes |
| SAE249 | Inflammatory arthropathy – probably due to gout | Yes |
| Cancer | ||
| SAE005 | Recurrence of bladder cancer | Yes |
| SAE032 | Tumour on kidney – right kidney/part of liver removed | Yes |
| SAE024 | Colon cancer | Yes |
| SAE040 | Colon cancer (open anterior resection surgery) | Yes |
| SAE066 | Chronic lymphocytic leukaemia | Yes |
| SAE096 | Diagnosis of myeloproliferative neoplasm JAK1 | Yes |
| SAE078 | Working diagnosis – colon cancer. Patient feels well so patient/family do not want further tests | Yes |
| SAE083 | Bowel cancer – resulted in death | Yes |
| SAE011 | Cancer of oesophagus | Stopped > 28 days ago |
| SAE123 | Suspected kidney cancer, diagnosis of left renal tumour | Stopped > 28 days ago |
| SAE229 | Chronic lymphoid leukaemia | Stopped > 28 days ago |
| SAE102 | Recent lung cancer diagnosis. Further investigation of cancer shows it to be terminal with secondary cancers in the liver | Yes |
| SAE109 | Bowen’s disease | Yes |
| SAE183 | MDS | Yes |
| SAE147 | Bowel cancer | Stopped > 28 days ago |
| SAE130 | Probable lung cancer, will not undergo treatment for cancer | Stopped > 28 days ago |
| SAE142 | Patient diagnosed with prostate cancer | Yes |
| SAE161 | Prostate cancer with pelvic metastasis | Yes |
| SAE191 | Colonic primary tumour, with extensive liver metastasis | Stopped < 28 days ago |
| SAE193 | Complex atypical hyperplasia | Yes (stopped same time) |
| SAE206 | Lung tumour and secondary cancers | Yes |
| SAE212 | Appearances consistent with lung malignancy. Given comorbid condition for best supportive/palliative care | Yes |
| SAE217 | Vulvar cancer | Yes |
| SAE238 | Tonsillectomy as a result of cancer | Yes |
| SAE251 | Prostate cancer | Stopped > 28 days ago |
| SAE253 | Basal cell carcinoma | Yes |
| Haematological/thrombosis | ||
| SAE038 | Low levels of platelets | Yes (stopped same time) |
| SAE045 | Blood transfusion for suspected bleed, following low levels of haemoglobin | Yes |
| SAE110 | DVT | Yes (stopped same time) |
| SAE129 | Neutropenia | Yes |
| SAE145 | Anaemia | Yes |
| SAE236 | Blood clot | Yes |
| Dermatological | ||
| SAE245 | Minocycline type 2 pigmentation on face | Yes |
| Neuropsychiatric | ||
| SAE009 | Hospitalisation following seizures | Yes |
| SAE019 | Psychosis secondary to dementia. Also mild UTI | Yes (stopped same time) |
| SAE029 | Admitted to psychiatric unit following relapse in psychotic symptoms with agitation | Stopped > 28 days ago |
| SAE128 | Patient confused, lacked co-ordination and had been experiencing more falls for a few weeks | Yes |
| SAE160 | Hospital admission with severe Alzheimer’s dementia, with significant behavioural disturbance | Stopped > 28 days ago |
| SAE050 | Admission to hospital as a result of loss of consciousness | Yes |
| SAE158 | Death as a result of dementia | Yes |
| SAE016 | Subdural haematoma | Yes (stopped same time) |
| SAE152 | AD/Lewy body disease – resulted in death | Stopped < 28 days ago |
| SAE143 | Stroke | Yes (stopped same time) |
| SAE105 | Probable stroke. Also receiving treatment for chest infection | Yes (stopped same time) |
| SAE055 | Minor stroke | Yes |
| SAE146 | Seizure (known epilepsy), cracked bone in ankle | Yes |
| SAE086 | CVA – resulted in death | Stopped > 28 days ago |
| SAE170 | AD | Yes |
| SAE076 | AD | Yes |
| SAE203 | Minor stroke non-haemorrhagic | Yes |
| SAE069 | Stroke | Yes |
| SAE059 | Left intracranial bleed | Yes (stopped same time) |
| SAE154 | AD | Yes |
| SAE166 | Funny turns followed by suspected TIA. Patient hospitalised | Stopped > 28 days ago |
| SAE150 | Stroke | Yes |
| SAE167 | Bleeding on brain | Yes |
| SAE159 | Mini stroke (TIA) | Yes |
| SAE169 | Suspected stroke/seizure | Yes |
| SAE172 | Stroke – resulted in death | Yes |
| SAE088 | Small left frontal lobe cortical haemorrhage | Yes |
| SAE153 | Subdural haemorrhage/blood clot | Yes |
| SAE121 | Admitted following falls, is due to being discharged home with end-of-life/full-time carers. MRI scanning showed chronic subdural haematoma | Yes (stopped same time) |
| SAE134 | Seizure, no diagnosis given | Stopped < 28 days ago |
| SAE218 | Delirium | Yes (stopped same time) |
| SAE219 | Dementia in AD | Yes |
| SAE198 | Confusion, slurred speech, unsteady on feet. Admitted to hospital | Yes |
| SAE230 | Patient in acute psychiatric ward on section 2 | Yes |
| SAE231 | Suspected stroke. Patient died | Stopped > 28 days ago |
| SAE239 | Possible TIA | Yes |
| SAE247 | Delirium | Yes |
| SAE252 | Progression of AD – resulted in death | Yes (stopped same time) |
| SAE265 | CVA | Yes (stopped same time) |
| Cardio-circulatory | ||
| SAE036 | Suspected myocardial infarction | Yes |
| SAE030 | Shortness of breath and suspected MI | Yes |
| SAE010 | Cardiac abnormalities: long QT on ECG and impaired left ventricular function | Yes |
| SAE062 | Cardiac event – resulted in death | Yes (stopped same time) |
| SAE035 | Myocardial infarction | Yes |
| SAE099 | Syncope attributed to GTN spray overdose (accidental) | Yes |
| SAE090 | Swollen ankles, shortness of breath, ambulance called. Admitted to hospital for 11 days. Diagnosis fluid on lungs and heart murmur | Yes |
| SAE104 | Cardiogenic syncope | Yes |
| SAE094 | Collapsed, thought to be as a result of low blood pressure | Yes |
| SAE098 | Postural hypotension | Yes |
| SAE201 | Out-of-hospital ventricular fibrillation arrest as a result of anterior myocardial infarction – resulted in death | Yes (stopped same time) |
| SAE211 | Recurrent gradual-onset syncope, junctional bradycardia on implantable loop recorder | Yes |
| SAE208 | Postural hypotension | Yes |
| SAE039 | Hospitalisation – low blood pressure/pulse rate | Yes |
| SAE157 | Hypotension | Yes |
| SAE125 | Chest pain | Yes |
| SAE049 | Suspected heart attack resulting in death | Yes (stopped same time) |
| SAE137 | Severe mitral valve regurgitation, which resolved on rate-limiting control of AF and LV improvement in function | Yes |
| SAE144 | Currently unknown – heart-related problems | Yes |
| SAE151 | Death from MI | Yes (stopped same time) |
| SAE168 | Death – coronary atherosclerosis hypertension | Yes (stopped same time) |
| SAE171 | Cardiac arrest | Yes |
| SAE207 | Heart problems. Cardiac monitor had revealed that heart had stopped for short time | Yes |
| SAE202 | Heart failure | Stopped < 28 days ago |
| SAE205 | Heart failure | Stopped < 28 days ago |
| SAE176 | Aortic stenosis | Yes |
| SAE103 | Deterioration in cardiac failure plus syncopal episode that led to hospital admission | Yes (stopped same time) |
| SAE224 | Heart failure – resulted in death | Stopped > 28 days ago |
| SAE241 | Heart attack – resulted in death | Stopped > 28 days ago |
| SAE240 | Labile blood pressure/hypertension | Yes |
| SAE246 | Heart failure | Yes |
| SAE248 | Heart attack – resulted in death | Yes (stopped same time) |
| SAE564 | Syncope (probably as a result of bradycardia) | Stopped > 28 days ago |
| SAE268 | Atrial fibrillation | Stopped > 28 days ago |
| Renal | ||
| SAE057 | Kidney stones | Yes |
| SAE058 | Large abdominal tumour causing kidney failure – resulted in death | Never started |
| SAE084 | Chronic renal failure – resulted in death | Stopped > 28 days ago |
| SAE101 | No evidence of bladder cancer. Patient was having tests from a bladder biopsy | Yes |
| SAE113 | Radical left nephrectomy laparoscopy | Stopped > 28 days ago |
| SAE204 | Acute kidney injury | Yes |
| SAE267 | Lung and kidney failure – resulted in death | Stopped > 28 days ago |
| Infection | ||
| SAE014 | Hospitalisation – chest infection | Yes |
| SAE017 | Hospitalisation – delirium as a result of dehydration and urine infection | Yes |
| SAE044 | Probable UTI, symptoms of confusion, weakness, low mobility | Yes |
| SAE082 | Infection following a foreign body in arm | Yes |
| SAE051 | Chest infection | Yes (stopped same time) |
| SAE052 | Urinary tract infection | Yes (stopped same time) |
| SAE081 | Catheter-associated UTI | Yes (stopped same time) |
| SAE070 | Chest infection | Yes |
| SAE097 | Infection – resulted in death | Stopped > 28 days ago |
| SAE100 | Pruritic rash in the context or urosepsis | Yes |
| SAE107 | Urosepsis | Yes |
| SAE115 | Sepsis, possibly related to gall bladder problems | Stopped > 28 days ago |
| SAE131 | Progressive decline post chest infection | Stopped > 28 days ago |
| SAE127 | Urinary infection | Stopped < 28 days ago |
| SAE141 | Admitted with lower respiratory infection | Yes (stopped same time) |
| SAE165 | Admitted to hospital with very sore throat later diagnosed as thrush | Yes |
| SAE173 | Shortness of breath and chest infection | Yes |
| SAE174 | Shortness of breath and chest infection | Yes |
| SAE180 | Taken to hospital with very low blood pressure and infection | Yes |
| SAE188 | Sepsis | Stopped > 28 days ago |
| SAE195 | On 11 November 2016 wife reports participant has chest infection – resulted in death | Yes (stopped same time) |
| SAE197 | Urinary tract infection | Yes |
| SAE133 | Updated from discharge summary: cellulitis | Stopped > 28 days ago |
| SAE233 | UTI | Stopped > 28 days ago |
| SAE234 | UTI | Stopped > 28 days ago |
| SAE210 | Urinary tract infection, confusion | Yes |
| SAE216 | Oesophageal candidiasis | Yes |
| SAE213 | Admitted with lower respiratory tract infection | Stopped > 28 days ago |
| SAE22 | UTI | Yes |
| SAE223 | UTI | Yes |
| Other | ||
| SAE006 | Hospitalisation – collapsed in street | Yes |
| SAE028 | Suspected blood clot in legs. Swollen legs/painful. No blood clot found | Yes |
| SAE033 | Suspected thrombosis – investigations complete no diagnosis of thrombosis. Symptoms of swollen legs have been associated with previously known water condition. Symptoms reduced after treatment | Yes |
| SAE042 | Participant drank white spirit in error | Yes |
| SAE071 | Replacement of left knee | Stopped > 28 days ago |
| SAE111 | Sensitivity and tenderness around left nipple | Yes |
| SAE074 | IMP overdose | Yes |
| SAE089 | Patient collapsed following accidental overdose of ranitidine | Yes (stopped same time) |
| SAE108 | General health decline – resulted in death. Patient was in respite care | Stopped > 28 days ago |
| SAE148 | Osteoarthritis | Yes |
| SAE179 | Jaw, back and neck pain – no diagnosis – cardiac problems ruled out | Stopped > 28 days ago |
| SAE162 | Prolonged hospital stay after planned hernia operation | Yes |
| SAE186 | Admitted for elective abdominal hysterectomy and bilateral salpingo-oophorectomy | Yes |
| SAE178 | No acute medical problem identified. Patient felt as if she had severe indigestion. Investigations into whether or not it was a mild heart attack in A&E (stayed overnight). No explanation found | Stopped > 28 days ago |
| SAE185 | TURP | Yes |
| SAE214 | Hip screw removal | Yes |
| SAE225 | Yes | |
| SAE244 | Postoperative scrotal oedema | Yes |
| SAE242 | Swollen legs | Yes |
| SAE250 | Unknown – resulted in death | > 28 days ago |
List of abbreviations
- Aβ
- β-amyloid
- AD
- Alzheimer's disease
- ALS
- amyotrophic lateral sclerosis
- BADLS
- Bristol Activities of Daily Living Scale
- DeNDRoN
- Dementias and Neurodegenerative Diseases Research Network
- GP
- general practitioner
- IMP
- investigational medicinal product
- MADE
- Minocycline in Alzheimer's Disease Efficacy
- MMSE
- Mini Mental State Examination
- MR
- modified release
- NDPH
- Nuffield Department of Population Health
- NIA–AA
- National Institute on Aging–Alzheimer’s Association
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- NSAID
- non-steroidal anti-inflammatory drug
- PI
- principal investigator
- QP
- qualified person
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SLE
- systemic lupus erythematosus
- sMMSE
- standardised Mini Mental State Examination
- TSC
- Trial Steering Committee
