Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 12/10/04. The contractual start date was in May 2014. The final report began editorial review in June 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Ruban et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Obesity
Epidemiology
Obesity has reached epidemic proportions, with the World Health Organization (WHO) estimating that approximately 2.3 billion adults worldwide are overweight and more than 700 million are obese. In 2015, 58% of the female population and 68% of the male population in the UK were overweight or obese, with obesity prevalence increasing from 15% in 1993 to 27% in 2015. 1 The Department of Health and Social Care estimates that obesity could cost society and the economy £50B by 2050 if obesity rates continue to increase. 2
Definition
Obesity is defined by WHO as ‘abnormal or excessive fat accumulation that presents a risk to health’. 3 The body mass index (BMI) one of the most widely adopted classifications to assess weight (Table 1) and is calculated by dividing weight in kilograms by height in metres squared (kg/m2).
| Classification | BMI (kg/m2) |
|---|---|
| Underweight | < 18.5 |
| Normal weight | 18.5–24.9 |
| Overweight | 25.0–29.9 |
| Obese class I | 30.0–34.9 |
| Obese class II | 35.0–39.9 |
| Obese class III | ≥ 40.0 |
Lifestyle modification
Weight loss hinges on the concept of kilocalories as units of energy quantification, and can be achieved by a net energy deficit as a result of reducing dietary calorie intake. There are various dietary methods of achieving a negative balance but so far no diet has emerged as the clear leader. Calorie restriction remains the common factor for weight loss, irrespective of macronutrient composition, but this is dependent on diet adherence, especially as dietary effects on weight loss plateau with time as a result of compensatory adaptation.
Pharmacological treatments are recommended for weight loss maintenance in addition to a reduced-calorie diet and optimal physical exercise, but the options currently available in the NHS are fairly limited. Only orlistat (Xenical, Roche Pharmaceuticals, Basel, Switzerland), a pancreatic lipase inhibitor, is licensed for weight loss maintenance in patients with a BMI of > 27 kg/m2 with associated risk factors or those with a BMI of ≥ 30 kg/m2. Treatment should be discontinued at 3 months if < 5% weight loss has been achieved while on the drug.
Type 2 diabetes mellitus
Definition
Diabetes mellitus is a chronic condition whereby the body is unable to produce or respond to insulin, which is a hormone that is crucial in the regulation of blood glucose levels. This results in hyperglycaemia, which can ultimately lead to deleterious effects on the body.
Diabetes can be categorised as type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). T1DM is an autoimmune condition in which the body’s immune system is overactive and destroys the insulin-producing cells located within the pancreas, resulting in absolute insulin deficiency. T2DM is the most prevalent form of diabetes; the pancreas is able to produce insulin, but the insulin is produced in insufficient quantities and/or the body is resistant to the effects of the hormone.
Diabetes UK estimates that currently there are 4.5 million people living with diabetes in the UK, with 90% of them having T2DM. 4 In the past, T2DM typically occurred in the older or middle-aged population but it is now increasingly being observed in the younger, overweight population.
Diagnosis and monitoring
The WHO has been producing guidelines for the diagnosis and classification of diabetes since 1965 and the current recommendations are summarised in Table 2. 5,6
| Diagnostic test | Glucose level |
|---|---|
| Random plasma glucose | ≥ 11.1 mmol/l |
| Fasting plasma glucose | ≥ 7.0 mmol/l |
| 2-hour plasma glucosea | ≥ 11.1 mmol/l |
| bHbA1c | ≥ 48 mmol/mol (6.5%) |
In the absence of diabetes symptoms such as polyuria or polydipsia, it is recommended that at least one additional glucose result is obtained on another day with a value in the diabetic range. 7
Glycated haemoglobin (HbA1c) is formed when glucose reacts non-enzymatically with the beta chain of haemoglobin, resulting in the formation of A1c. 8 This reaction is potentiated in patients with diabetes who have higher circulating levels of glucose. 9 As the life cycle of these red blood cells is 120 days, HbA1c is now utilised as a marker of long-term glycaemic control, giving an indication of average blood glucose levels over a 3-month period. 10 The International Diabetes Federation (IDF) guidelines11 state that patients with diabetes should aim to maintain a HbA1c level of < 53 mmol/mol (7.0%) to minimise the risk of developing complications. These recommendations are based on the findings of the UK Prospective Diabetes Study,12 in which intensive blood glucose control (maintaining a HbA1c level of < 7.0%) over a 10-year period was associated with a 10% reduction in any diabetes death and a 6% lower all-cause mortality when compared with the control group. The control group were managed with diet alone, with medication being added only if hyperglycaemic symptoms occurred or if fasting plasma glucose (FPG) levels reached 15 mmol/l. 12
Complications of type 2 diabetes mellitus
The complications of T2DM can be categorised into macrovascular complications (coronary artery disease, peripheral arterial disease and stroke) and microvascular complications (diabetic nephropathy, neuropathy and retinopathy). Cardiovascular disease (CVD) is the commonest cause of death among adults with T2DM, and the risk of cardiovascular complications is 2–2.5 times that of the general population. 13,14 Cardiovascular complications include angina, ischaemic heart disease and heart failure.
The risk of end-stage kidney disease is 4.5 times greater for people with T2DM than for the general population, and it is the leading cause of dialysis in the UK. 14 Diabetic neuropathy encompasses a wide range of disorders affecting the large and small nerve fibres primarily caused by axonal degeneration from metabolic factors, which include high circulating blood sugars. 15 It is the commonest complication of diabetes and is responsible for a large proportion of non-traumatic amputations. 16 Peripheral arterial disease is also a major risk factor for lower limb amputation, particularly in this cohort of patients, because abnormalities of endothelial function and vascular regulation occur with diabetes, which in turn accelerate atherosclerotic processes in the arterial vessels. 17,18 Strict glycaemic control is paramount in avoiding the long-term complications of this chronic condition.
Treatment
Type 2 diabetes mellitus remission, defined as the alleviation of diabetic symptoms and the requirement for medication to control diabetes, is possible through intensive lifestyle changes and with the advent of metabolic surgery. 19
Lifestyle modification
The majority (80–90%) of patients with T2DM are obese or overweight, so weight loss interventions are favourable in the management of this condition. 20,21 Intensive weight loss interventions have been shown to lead to 10–15% remission rates at 1-year follow-up. 22 However, sole reliance on lifestyle modification therapy may be successful only in a minority of patients in establishing good glycaemic control and ultimately this benefit may be short-lived. The Look Ahead trial23 showed remission rates of 7% at 4-year follow-up in the intensive medical therapy arm, and the Predimed study24 reported remission rates of 5% at 6-year follow-up in the lifestyle intervention arm, in which participants followed a Mediterranean diet. A recent meta-analysis of lifestyle weight loss interventions in overweight or obese adults with T2DM found that the majority of patients achieved a weight loss of < 5% and this did not result in beneficial metabolic outcomes. 25 These interventions included energy intake restriction, regular physical activity, education and support from health-care professionals. Lifestyle interventions have an important role in diabetes management, which complement pharmacotherapy and surgery.
Metabolic surgery
Diet, medication and exercise to control diabetes and obesity have limited long-term efficacy when compared with metabolic surgery. Fewer than half of the patients achieved glycaemic control using these approaches. 26,27
Metabolic surgery is the treatment of choice when all other interventions have failed. Regardless of the type of metabolic surgery performed, its effects on weight loss and associated comorbidities are superior when compared with non-surgical interventions. 28
The Swedish Obesity Study29 is one of the largest prospective studies to date providing observational data on the impact of metabolic surgery on obesity and long-term outcomes. The study reported a greater degree of weight loss in the surgical group (n = 2010) than in the control group (n = 2037), as well as major improvements in obesity-related comorbidities. In particular, there was a 72% remission rate of T2DM after 2 years, dropping to 36% at 10 years. More recent randomised controlled trials (RCTs) have shown metabolic surgery to have better long-term outcomes in terms of weight loss and diabetes resolution than medical treatment alone for obese patients with T2DM. 30,31 Based on estimates, the reduction in diabetes medications and inpatient stay from diabetes complications could lead to potential savings of approximately £18.1M over a 4-year period after surgery. 32 Indeed, surgery is emerging as the more cost-effective management option for patients with diabetes and other obesity-related comorbidities. 33
Following the 2nd Diabetes Surgery Summit in 2015, several national diabetes societies, such as the American Diabetes Association (ADA) and Diabetes UK, have recommended the use of metabolic surgery in obese patients with type 2 diabetes and have reported diabetes remission rates of between 30% and 60% following surgery. 34 The recently published STAMPEDE31 (Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently) randomised trial demonstrated that metabolic surgery (gastric bypass or sleeve gastrectomy) plus intensive medical therapy is superior to intensive medical therapy alone for the treatment of obese patients with type 2 diabetes. Of the 134 patients who completed the 5-year study, only 5% of patients in the medical therapy group achieved the primary end point (a HbA1c level of ≤ 6%), compared with 29% in the gastric bypass group and 23% in the sleeve gastrectomy group. Reductions in body weight and BMI were also greater in the surgical intervention arm than in the medical therapy group.
The common types of metabolic surgery performed are depicted in Figure 1, with Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy being the most popular types of surgery currently performed.
FIGURE 1.
Common types of metabolic surgery. Reproduced from Ruban A, Stoenchev K, Ashrafian H and Teare J. Current treatments for obesity. Clin Med 2019;19:205–12. 35 Copyright © Royal College of Physicians 2019. Reproduced with permission.

The exact mechanisms underpinning the clinical effects observed in weight loss and glycaemic improvement post metabolic surgery (in particular RYGB) remain a mystery. Various theories have been postulated, including the so-called BRAVE effects [i.e. bile flow alteration, reduction in energy intake, anatomical gastrointestinal (GI) rearrangement, vagal manipulation, enteric hormonal modulation]. 36 These BRAVE effects take place within minutes of RYGB surgery to induce multiple short- and long-term beneficial metabolic sequelae.
EndoBarrier®: duodenal–jejunal bypass liner
This section is reproduced from Ruban et al. 37 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Background
Endoscopic treatments are becoming increasingly popular among a cohort of patients who are unwilling to accept the potential complications associated with surgery or in whom surgery is contraindicated because pre-existing comorbidities make them a high anaesthetic risk. In recent years, we have seen the development of relatively non-invasive endoscopic therapies that manipulate anatomical and physiological mechanisms in the upper GI tract to achieve weight loss. 38 Often these devices attempt to mimic the effects of metabolic surgery on weight reduction. Endoscopic treatments may also be utilised as bridging therapy, inducing weight loss in the supermorbidly obese patients, who can then proceed to more definitive treatment such as metabolic surgery.
The EndoBarrier® is an endoluminal duodenal–jejunal bypass liner (DJBL) developed by GI Dynamics Inc. (Boston, MA, USA) for the treatment of obese patients with T2DM. 39 It consists of a single-use endoscopic implant with a removable nitinol stent anchor to affix to the wall of the duodenum and to which is attached an impermeable fluoropolymer sleeve that extends 60 cm into the small bowel (Figure 2). As a result, gastric contents bypass the proximal intestinal tract by travelling inside the sleeve, coming into contact with pancreatic juices and bile only when they exit the sleeve in the jejunum. This device is currently licensed for up to 12 months of treatment. It is envisaged that this device might mimic the effects of restrictive surgery such as gastric bypass but without the risks of undergoing surgery and the possible long-term complications associated with metabolic surgery.
FIGURE 2.
The EndoBarrier DJBL. Reproduced with permission from GI Dynamics Inc.

Clinical trial data
To date there have been five RCTs examining the efficacy of the endoluminal DJBL (Table 3), the largest of which was a multicentre trial performed in the Netherlands in which 73 patients were randomised to receive either endoluminal DJBL treatment in combination with dietary intervention or dietary intervention alone (control group). 41 A total of 35 patients successfully had the endoluminal DJBL implanted for a 6-month period. Mean BMI at baseline was 35 kg/m2 and 37 kg/m2 in the device arm and control arm, respectively, and reduced to 31 kg/m2 and 35 kg/m2, respectively, over the 6-month period. Mean HbA1c at baseline was 8.3% in both groups and reduced to 7.0% and 7.9% in the device arm and control arm, respectively. There was only one early device removal, due to blockage of the endoluminal DJBL with food. Patients were also followed up for 6 months following removal of the device, at which point BMI and HbA1c were measured. Mean BMI was 32 kg/m2 in the device group and 36 kg/m2 in the control group, that is it was slightly increased in the treatment arm. Mean HbA1c was 7.3% and 8.0% at the end of the follow-up period in the device group and control group, respectively, a mean reduction of 1% and 0.3%, respectively.
| Study | Number of patients | BMI (kg/m2) | Duration of device implantation (weeks) | Weight loss | Change in HbA1c | Stent removal rate (%) |
|---|---|---|---|---|---|---|
| Gersin et al.40 | 47; 21 in treatment arm | 46 | 12 | –8.2 kg ± 1.3 kg in treatment arm vs. 2 kg ± 1.1 kg in control arm | Not an end point | 38 |
| Koehestanie et al.41 | 73; 34 in treatment arm | 35 device; 37 control | 26 | 10.6 kg device; 5.3 kg control | –1.3% vs. 0.4% in control | 3 |
| Rodriguez-Grunert et al.42 | 18; 12 in treatment arm | 39 | 24 | –10.2 kg ± 1.3 kg in device arm vs. 7.1 ± 4.3 kg in control arm | –2.4% ± 0.7% vs. –0.8% ± 0.4% control | 25 |
| Schouten et al.43 | 41; 30 in treatment arm | 49 | 12 | 19% device; 6.9% control | –1.1% vs. 0.4% in control | 15 |
| Tarnoff et al.44 | 35; 29 in treatment arm | 42 device; 40 control | 12 | –10.3 kg ± 3.2 kg vs. 2.6 kg ± 3.5 kg in control group | Not an end point | 20 |
In another study of 41 patients, 26 patients in whom the device was implanted were compared with a control group of 11 patients on a low-calorie diet. There was a mean loss of excess weight of 19% in the device group compared with 6.9% in the control group. 43 Furthermore, out of eight patients in the device arm with T2DM at baseline, improvements were seen in glucose levels and HbA1c in all but one of them.
Betzel et al. 45 published the largest cohort of patients receiving the EndoBarrier with 185 patients from 2011 to 2014 who received the device for 1 year. Excess weight loss was 40.9% at 6 months and 46.3% at the time of explantation (p < 0.001). HbA1c reduced by 6 mmol/l from 67 mmol/l to 61 mmol/l at the time of explantation (p < 0.001); however, 31% of devices were removed prematurely because of intolerable side effects and adverse events (AEs). The main side effects reported were abdominal discomfort and nausea, with more serious side effects including GI bleeding, device migration or obstruction, and the development of a hepatic abscess.
Pilot study
Our research group at Imperial College conducted the first post-marketing clinical trial of the EndoBarrier in the UK, consisting of 45 patients recruited from three centres (i.e. St Mary’s Hospital, London; University Hospital Southampton NHS Foundation Trust; and Manchester University NHS Foundation Trust). 46 In this study, participants were aged 18–65 years with T2DM and a BMI of > 30 kg/m2 and received the implant for a period of 1 year. Mean HbA1c and BMI at baseline were 69 mmol (8.5%) and 39.9 kg/m2, respectively.
A summary of baseline characteristics and patient demographics is shown below (see Table 9). Of the 45 patients, 31 (69%) completed the 12-month study period. Average implantation time was 27 minutes and fluoroscopic time was 7 minutes [standard deviation (SD) 5.7 minutes]. There were no procedure-related complications during implant or explant. There were 14 early withdrawals from the study within the 12-month implant period, two of which were the result of premature explant owing to device-related AEs, in one case melaena and in the other device migration, both resulting in abdominal pain. The other reasons for withdrawal included the development of other medical complications precluding EndoBarrier implantation and patient choice for early removal.
At 1 year, the average reduction in HbA1c was 0.8%. A mean reduction in BMI of 4.9 kg/m2 was observed with a mean total body weight loss of 15 kg. These positive changes appeared to be maintained at the 6-month follow-up period with small but non-significant changes in these parameters after explantation.
Safety profile
By far the most commonly reported side effect of the device is GI upset, including abdominal pain and nausea. These symptoms usually resolve as the patient acclimatises to having the device in situ, but a minority of patients (2%) are unable to tolerate this, leading to early device removal. The other complications include GI bleeding (1.5%) and device migration (1.4%). Rarer complications include cholestasis and pancreatitis.
Liver abscesses pose the most serious complication associated with the EndoBarrier, with most cases reported late during the course of treatment, towards the time of explantation (9–12 months). The German DJBL registry reported one case in 66 patients who had received the EndoBarrier for 1 year, having previously reported four cases in 235 patient registries. 47 Three were documented at explantation, with the other one occurring following early removal for device dislocation. All were managed with antibiotics and/or drained with no permanent sequelae.
The ENDO trial48 was a multicentre, double-blind, randomised trial in the USA to evaluate the safety and efficacy of the EndoBarrier on glycaemic control. However, in March 2015, the Food and Drug Administration (FDA) halted the trial owing to the development of seven liver abscesses (3.5%) in participants, which was a much higher rate than anticipated. The cause of these liver abscesses is unclear, but the theory is that the EndoBarrier creates a nidus for infection that may spread to the liver bed.
Post-market surveillance data from GI Dynamics Inc., the device manufacturer, show an incidence of liver abscesses of 1%, which is also supported by data from a worldwide registry established in 2017 by the Association of British Clinical Diabetologists. 49 Among 492 EndoBarrier patients there were six reported cases of liver abscesses. The rate of early removal of the device because of GI bleed was 4%. Device migration occurred in 3%, and liner obstruction was rare, accounting for 0.3% of cases.
Potential mechanisms of action
The EndoBarrier mimics the bypass portion of the RYGB so it is thought to elicit its effects on weight loss and glycaemia by similar mechanisms, including:
-
reductions in energy intake
-
changes in food preferences
-
increases in insulin sensitivity but no increase in insulin secretion.
Potential mediators of these mechanisms are:
-
enhanced secretion of anorexigenic and/or incretic gut hormones
-
reduced brain reward responses to energy-dense food
-
altered jejunal nutrient sensing
-
enhanced plasma bile acid secretion
-
alterations in the gut microbiota (GM) and metabonomic profile of the host.
Currently there is a sparsity of data on how the endoluminal DJBL influences the potential pathways listed above. Some of the key mechanisms are explored in more detail below, and these are supported by the very few EndoBarrier studies that have reported on these outcomes.
Reduction in energy intake and food preferences
Eating behaviour describes any interaction between humans/animals with food and incorporates total food/energy intake and food preferences. Total energy intake and food preferences are regulated by two integrated brain systems, the homeostatic and non-homeostatic. 50 The homeostatic system controls total energy intake by increasing the motivation to eat in response to hunger or termination of an eating episode in response to satiation. The non-homeostatic system controls both total energy intake and food preferences, and is influenced by a number of factors, both internal (physiological), such as the pleasant and unpleasant post-ingestive effects of food, previous experience and learning, religion, emotional state, and external, such as the social and cultural context of the eating occasion. Additional external factors include cues such as the sight, smell or taste of food. 51 Obesity is a complex chronic disease of the brain, with the characteristic symptoms of elevated hunger, diminished satiation and possibly unhealthy food preferences.
Lifestyle modification and pharmacotherapy have mild to moderate efficacy for the treatment of obesity and, where this is so, they should be continued in the long term. Obesity surgery is the most effective treatment, resulting in durable weight loss and improvements in physical, functional and psychological health. 52 RYGB and vertical sleeve gastrectomy (VSG) are the most commonly performed procedures worldwide. 53 In terms of effects on eating behaviour, RYGB has been studied more than VSG. Most patients after RYGB report reduced hunger, increased satiation and some changes in food preferences; combined, these changes in eating behaviour contribute to weight loss and glycaemic improvements.
Hunger and satiation after Roux-en-Y gastric bypass
Reduced hunger and increased satiation have been reported after RYGB. 54 Le Roux et al. ,53 found that the increase in satiety gut hormones glucagon-like peptide 1 (GLP-1) and peptide YY3-36 [peptide tyrosine–tyrosine (PYY)] was associated with reduced hunger and increased satiety after RYGB, and the inhibition of these gut hormones resulted in a reversal effect, leading to increased hunger and reduced satiation. 7
Eating behaviour after Roux-en-Y gastric bypass
Changes in eating behaviour have been reported in patients after RYGB. Healthier eating behaviours including reduced restraint eating, external eating, and weight and shape concerns reduced hedonic hunger and reduced emotional and uncontrolled eating. 55
Food preferences after Roux-en-Y gastric bypass
Halmi et al. 56 were the first to report changes in food preferences after RYGB and a shift towards ‘healthier’ food choices. Several short-term57,58 and long-term studies59,60 have subsequently demonstrated a shift from high-fat, high-sugar to lower-fat and lower-sugar food preferences. However, there is substantial heterogeneity in the findings of these studies in terms of whether or not food preferences actually take place, the direction of change and its durability. These discrepant results suggest that not all patients respond similarly to RYGB and the possibility that those patients who change their food preferences may have superior weight loss to those who do not.
Taste function after Roux-en-Y gastric bypass
One of the determinants of food preferences is taste function. This can be heuristically broken down into three domains: the sensory domain, which incorporates taste detection and discrimination; the reward domain, which incorporates the appetitive (willingness to obtain a specific taste) and consummatory (reward elicited on exposure to the specific taste) subdomains; and the physiological domain, which incorporates the physiological responses on exposure to a specific taste (i.e. salivation). 58
Burge et al. 61 used the cornsweet staircase method (forced choice) for sweet and bitter and found that sweet taste detection thresholds decreased and sweet taste/food intensity increased after RYGB, potentially contributing to reduced sweet food intake. Similarly, Bueter et al. 59 found that sweet taste detection thresholds decreased but sweet taste intensity did not change after RYGB using the method of constant stimuli. 59 In contrast, Pepino et al. 60 used the two-alternative, forced-choice staircase method and found no changes in detection thresholds for any of the taste qualities after RYGB and laparoscopic adjustable gastric banding. The differences in these findings may be due to variation in methods, concentrations of taste stimuli or time of test administration but also the above-mentioned heterogeneity in responses after RYGB.
Miras et al. 62 studied patients before and after RYGB using the progressive ratio task (PRT). They found that surgery resulted in the selective reduction in the appetitive reward value of a sweet/fat tastant, but not of a vegetable tastant.
Pepino et al. 60 used the sweet taste palatability test, in which participants rate different sucrose concentrations using global label magnitude scales. These are considered to be superior to standard visual analogue scales (VASs) for the measurement of taste reward. They found that after RYGB, but not after laparoscopic adjustable gastric banding, patients experience a shift in the palatability of sweet taste from pleasant to unpleasant. 60 In contrast, Bueter et al. 59 did not find similar results when using the ‘Just About Right’ VASs.
Once the food is in contact with the mouth, a number of physiological responses occur, known as the cephalic phase response. Salivation is the most obvious cephalic response. A number of studies have suggested that weight can affect salivation, with higher salivation rates seen in people with obesity. This can be explained by the higher responses to food cues and higher food reinforcing values seen in patients with obesity. 63 Hauge and Baechle64 described a case of reduced salivary flow after 5 years of RYGB. Marsicano et al. 65 compared patients with obesity with patients who had undergone RYGB and found no differences in salivary flow between the two groups. Studies in this area are scarce and inconclusive.
Mediators underlying changes in eating behaviour after Roux-en-Y gastric bypass
Gut hormones have been implicated as likely mediators of the beneficial effects of RYGB on appetite and food intake. The gut hormones GLP-1 and PYY are secreted from the L cells present throughout the GI tract in response to food intake and have appetite-suppressing effects, leading to food intake and weight loss. 66 An exaggerated release of the gut hormones GLP-1 and PYY in response to a meal is seen after RYGB as a result of enhanced nutrient sensing by the L cells of the distal ileum, which may contribute to reduced hunger and increased satiation and, consequently, weight loss. 67
Insulin sensitivity
Current evidence would support an overall improvement in peripheral insulin sensitivity and glucose homeostasis through weight loss-dependent and -independent mechanisms following endoluminal DJBL placement. Similar to what is observed following surgical duodenal–jejunal bypass, murine models have confirmed early (within 1 week) improvements in insulin resistance following implantation of an endoluminal sleeve, as demonstrated by a 55% decrease in homeostatic model assessment to insulin resistance (HOMA-IR). 68,69 This was associated with a decrease in fasting insulin and plasma glucose concentrations and, as hepatic glucose output is the major determinant of FPG, it would be apparent that the improvements in glycaemic control are a consequence of improvements in hepatic insulin resistance. Improved oral glucose tolerance with a concurrent decrease in glucose-stimulated insulin levels also implies an overall improvement in peripheral insulin sensitivity with increased peripheral glucose utilisation and disposal. 63,68
Model assessments of insulin resistance have also been utilised in several human studies to demonstrate rapid improvements in insulin sensitivity and rapid reductions in hepatic glucose output following endoluminal DJBL implantation. 69–75 Cohen et al. 70 found convincing evidence of this in a prospective observational study of 16 patients (mean BMI 30 kg/m2, HbA1C 8.6%) implanted with a endoluminal DJBL for 1 year. In this cohort, HOMA-IR significantly decreased, and the Matsuda index significantly increased within 1 week of implantation and remained improved for the duration of the implantation period. Insulin secretion data suggested a decrease over time, but this was not significant when analysed for both fasting values (p = 0.051) and area under the curve analysis (p = 0.28), and there were non-significant changes in the insulinogenic index (a measure of first-phase insulin response). 70
Following an implantation period of 3–12 months, reductions in HbA1c have been reported in the range of 0.3–2.4% (3–27 mmol/mol). Most recently, a case–control study from the national German DJBL registry (DJBL, n = 111, vs. matched controls receiving standard treatment, n = 222) demonstrated superior reductions in HbA1C in the endoluminal DJBL group (–1.37% ± 1.54% vs. –0.51% ± 1.83%; p < 0.0001) associated with significantly greater reductions in glucose-lowering medications. In the largest RCT to date of which we are aware, conducted by Koehestanie et al. ,41 endoluminal DJBL implantation (n = 34) for 6 months resulted in a decrease in HbA1c of 1.3%, compared with 0.4% in the dietary control group (p < 0.05), and fasting glucose levels were reduced from 11.0 mmol/l to 8.5 mmol/l compared with from 11.0 mmol/l to 10.0 mmol/l, respectively (p = 0.10). In addition, 85.3% of endoluminal DJBL patients achieved a decrease in post-prandial glucose excursions, compared with 48.7% in the control group (p < 0.05), and daily insulin or sulfonylurea dosages were decreased or discontinued more often in the endoluminal DJBL group than in the control group (p < 0.05). De Moura et al. 76 demonstrated some of the most significant improvements in glycaemic control: endoluminal DJBL patients (n = 22), after a mean implantation period of 41.9 ± 3.2 weeks, reduced their HbA1c by 2.1% ± 0.3% and their FPG by –30.3 ± 10.2 mg/dl. Furthermore, 73% of patients reached a final HbA1c measurement of < 7%, indicative of adequate glycaemic control. 76 In the largest observational study of patients with T2DM of which we are aware, by Betzel et al. 45 (n = 185), both HbA1c and FPG decreased significantly, by 0.6% and 1.2 mmol/l, respectively (p = 0.001), following an implantation period of 1 year. Similar improvements have been reported in numerous other studies that have demonstrated significant reductions in HbA1c, FPG and post-prandial glucose excursions, as well as dose reductions or discontinuation of anti-diabetic medications. 70–74,76–84 In a 2015 systematic review, it was concluded that the relative risk of reducing or discontinuing antidiabetic medications was 3.28 and 1.13 in endoluminal DJBL groups and dietary control groups, respectively. 85
To date, only one study of which we are aware has evaluated glucose homeostasis following endoluminal DJBL implantation using hyperinsulinaemic–euglycaemic clamps. In this study, by Miras et al. ,86 seven obese patients (mean BMI 48.5 kg/m2) underwent three clamps in order to evaluate the early effects of endoluminal DJBL on insulin sensitivity and hepatic glucose production (HGP), while controlling for the effects of caloric restriction in the peri-implantation period. This study concluded that the endoluminal DJBL did not improve hepatic insulin sensitivity beyond the improvements achieved with caloric restriction. This study was, however, limited by its small number of participants and lack of a control group.
Mediators underlying mechanisms of action
Gut hormones
There is increasing evidence that alterations in enteric gut hormones significantly contribute to many of the beneficial effects observed following metabolic surgery. 53 Altering the GI anatomy following RYGB changes the flow of nutrients, leading to important changes in gut-derived hormones by foregut exclusion and modified hindgut signals. This in turn positively influences the metabolic changes seen following surgery, including improvement in glycaemic control and weight loss. De Jonge et al. 71 investigated the effects of the endoluminal DJBL on the incretin gut hormones GLP-1 and gastric inhibitory polypeptide (GIP) in addition to glucose, insulin and glucagon levels, in 17 obese patients with T2DM receiving the endoluminal DJBL implant for 6 months. Both fasting and post-prandial glucose levels were decreased in parallel with a reduction in glucagon levels but fasting insulin levels did not change. GLP-1 levels increased, but GIP levels were found to be decreased at 6 months. The authors postulate that these findings are similar to those seen post RYGB, suggesting that the device works in a similar fashion; however, in contrast to these findings, Koehestanie et al. 69 studied the effects of fasting GIP, GLP-1 and ghrelin levels at baseline, 1 week and 4 weeks in 12 obese patients with T2DM post implant and identified no significant changes in GIP; in fact, levels of GLP-1 appeared to decrease 1 week post implant, followed by an elevation back to baseline levels in the following 3 weeks. Ghrelin levels were found to rise in this study, particularly in the first week following EndoBarrier implantation. No correlation between gut hormone changes and reductions in body weight and BMI was identified.
Similarly, Vilarrassa et al. 82 investigated gut hormone changes in 21 patients with obesity and diabetes and found no differences in GLP-1 values at baseline and at 12 months, although PYY and ghrelin levels increased over this period. This suggests that GLP-1 may not account for the metabolic improvements seen in patients receiving the endoluminal DJBL. Furthermore, the increase in ghrelin seen in both these studies contradicts findings post RYGB, which suggest that ghrelin levels fall.
Rohde et al. 87 compared the effect of the EndoBarrier on post-prandial physiology in 10 obese patients with normal glucose tolerance and nine age-, body weight- and BMI-matched patients with T2DM. Parameters investigated included insulin, glucose, glucagon, gut hormone secretion, gall bladder emptying, appetite and food intake using a liquid mixed-meal test and a subsequent ad libitum meal test at baseline, 1 week and 26 weeks following endoluminal DJBL implantation. Basal plasma concentrations of GLP-1, GIP and PYY were similar in the two groups before endoluminal DJBL implantation and the device did not appear to affect basal concentrations significantly in any of the groups. Small but significant increases were observed in post-prandial levels of GLP-1 and PYY levels at weeks 1 and 26 in the patient group with T2DM but not in those with normal glucose tolerance and, overall, the endoluminal DJBL did not appear to have any impact on levels of insulin, glucose or glucagon following implantation although the numbers reported are very small. Clearly, larger numbers in RCTs are required in order to draw any firm conclusion on the effects of endoluminal DJBL on the gut hormones.
Bile flow modulation
Bile acid metabolism appears to vary between obese and lean individuals, with several studies demonstrating decreased circulating levels of bile acids in obese relative to lean individuals. 88,89 Bile acids are believed to play an integral role in regulating satiety as well as influencing lipid, cholesterol and glucose metabolism through complex interactions, which include stimulating the secretion of incretin hormones GLP-1 and PYY, growth factors and disruption of the GM. 90
Fibroblast growth factor-19 (FGF-19) is a potent stimulator of bile acid synthesis and in a small study of 30 obese patients with T2DM, levels were found to be markedly increased following endoluminal DJBL implantation for 10 months in these individuals. 73 The increase in bile acid signalling in the liver might provide a partial mechanism of how the device elicits its effects on improvements in glycaemic control. Free bile acids also interact closely with the microbiota found in the small intestine, so increased concentrations of these bile acids may influence not only the overall number of bacteria in this region but also their composition.
Metabonomics
Metabonomics was defined in 1999 as ‘the quantitative measurement of the dynamic multiparametric metabolic response of living systems to pathophysiological stimuli or genetic modification’. 91 Since then it is a field that has advanced rapidly, providing an unbiased method for quantitative and qualitative analyses of metabolites present in a biological sample such as urine, stool or plasma.
Metabolic profiling often utilises high-field 1H nuclear magnetic resonance (NMR) spectroscopic technique to characterise large sets of biological fluids. 92 This is an untargeted approach focusing on the global metabolic profile or ‘fingerprint’ of a sample. NMR spectra of biofluids generate vast numbers of data, which would be impossible to interpret manually. Using multivariate statistical data analysis methods can help in information extraction, noise reduction and fine-tuning spectral information. 93
Metabonomics in metabolic surgery
Metabolic surgery results in alterations in the metabolic profiling of individuals but only a limited number of studies to date have explored these changes. A major group of metabolites that appear to alter following metabolic procedures are amino acids such as alanine, glutamate and glycine. 94,95 Branched-chain amino acids such as isoleucine and valine are also affected, as well as peptides such as glutathione. 96 Following sleeve gastrectomy, serum concentrations of serine and glycine were found to be elevated, whereas RYGB surgery resulted in a decrease in methionine, alanine and lysine compared with pre-surgery samples. 97
Gralka et al. 98 explored the metabolic alterations occurring post metabolic surgery by analysing the serum of > 100 obese patients using 1H NMR spectroscopy. 98 In this longitudinal observational study, serum samples were collected prior to and in the 1-year follow-up period post metabolic surgery (sleeve gastrectomy and RYGB). In addition, serum samples were analysed from normal weight individuals and from 30 patients with BMIs that were matched with those achieved by the severely obese patients 12 months after their metabolic surgery. The study found that, once again, amino acids were altered significantly in samples taken pre and post surgery with an increase in arginine and glutamine regardless of the type of surgery performed. Markedly increased levels of isopropanol and methanol were also found in severely obese patients and the authors speculated that elevated concentrations of these metabolites in the blood may be as a consequence of altered GM composition as these metabolites are associated with bacterial metabolism. 98 Finally, increases were seen in dimethyl sulfone concentrations after all metabolic procedures; a compound barely seen in the baseline samples prior to surgery or in normal weight individuals. Dimethyl sulfone is an intermediate metabolite of methionine metabolism and, again, the authors postulate that this rise might be a consequence of the altered microbiome post surgery. 98
Gut microbiota
The GM has been implicated in numerous disease processes, and obesity is no different. Manipulation of the host gut microbiome using faecal transplantation has been shown to alter host phenotype, as evidenced by improvements in insulin resistance observed in obese individuals following transplantation with lean microbiota. 99 Conversely, transplantation of the GM from obese mice to normal-weight germ-free mice leads to increased weight gain in these recipients. 38 In another study, transplantation of the bacterium Akkermansia muciniphila into rats fed a high-fat diet led to an increase in GLP-1 secretion and improvements in insulin sensitivity. 100
Changes in dietary intake have also been shown to change microbiota composition significantly. In a RCT investigating the impact of dietary fat on the GM, faecal metabolites and cardiometabolic risk factors, lower-fat diets led to an increase in abundance of organisms assessed by the Shannon index. 101 Moderate- and high-fat diets decreased the ratio of Firmicutes to Bacteroidetes. Bacteroidetes species not only increased in abundance following a high-fat diet but were also associated with an increase in plasma lipid markers.
Following RYGB, the GM alters, with an increase in bacterial richness as a consequence of changes in pH levels in the proximal small bowel and alterations in gastric motility and nutrient flow. 102 As RYGB surgery delays glucose and amino acids absorption, the increase in simple sugars reaching the distal small bowel and colon may stimulate bacteria here to derive energy from these malabsorbed nutrients. 103
Increases in the abundance of bacterial species post RYGB surgery, in particular those in the class Gammaproteobacteria and phylum Firmicutes have been observed and may have a potential role in the positive metabolic changes seen following surgery. 104,105 Similar patterns in microbiota adaptation have been seen with duodenal exclusion devices, although research in this field remains in its infancy.
In a rodent model, implantation of a duodenal endoluminal sleeve stimulated an increase in the abundance of species in the class Gammaproteobacteria (e.g. Escherichia coli) and phylum Firmicutes (e.g. Clostridium). 106 C. perfringens was found to increase following duodenal exclusion, and reduced levels have previously been implicated in obesity. 107
To date, and to our knowledge, only one study has investigated the impact of the endoluminal DJBL on the GM and this was in a cohort of 17 patients who received endoluminal DJBL therapy for 6 months and were then followed up for 6 months. 108 Faecal microbiota appeared to be profoundly altered by endoluminal DJBL therapy, most notably being associated with an increase in abundance of species of the phyla Firmicutes and Proteobacteria. This included a 25-fold increase in the relative abundance of Lactobacillus gasseri et rel., an 11-fold increase in L. plantarum et rel. and a fivefold increase in Escherichia coli et rel. over the 6-month period. It is possible that alterations in the nutrient stream by bypassing the proximal intestine might lead to shifts in colonisation of typical small intestinal microbiota such as Proteobacteria into the distal small bowel and colon. The excess weight loss after 6 months of endoluminal DJBL therapy was 18.3% but, despite this significant weight loss being maintained at 6 months following device removal, the faecal microbiota composition at the same time point appeared similar to baseline samples (prior to endoluminal DJBL therapy). This may suggest either that the metabolic impact of endoluminal DJBL therapy is independent of changes in the microbiome profile or that GM alterations may initially influence the improvements seen in glycaemic control and weight loss, but that other mechanisms such as enteric gut hormonal changes may be chiefly responsible for the sustained impact of the device following DJBL removal. Certainly, larger studies involving a larger patient population in a randomised setting are required to investigate the impact of endoluminal DJBL on the GM and to determine which bacterial species may influence the metabolic improvements observed with endoluminal DJBL therapy.
Objectives
-
To compare the efficacy of intensive medical therapy with versus without the endoluminal DJBL on glycaemic control in patients with T2DM and obesity in both the short and medium terms.
-
To evaluate the safety of the endoluminal DJBL.
-
To investigate the mechanism of the effect of the endoluminal DJBL on eating behaviour and glucose metabolism.
-
To estimate the cost-effectiveness of the endoluminal DJBL device compared with conventional treatment over the trial period.
Chapter 2 Methods
Reproduced with permission from Glaysher et al. 109 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Study design
This study is a RCT of the EndoBarrier compared with a combination of conventional medical therapy, diet and exercise for the management of patients with both obesity and T2DM. Over a 2-year period (1 year of treatment and a 1-year follow-up period), the trial was performed over two investigational sites in the UK: Imperial College Healthcare NHS Trust in London and University Hospital Southampton NHS Foundation Trust. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial can be found in Figure 6. The trial protocol and schedule are summarised in Figure 3.
FIGURE 3.
Study interventions and follow-up schedule. A, weight, waist, blood pressure, routine bloods; AEs, changes in medication/medical history; B, dietary counselling; C, medical therapy (diabetologist/endocrinologist); D, gastroenterologist; E, dietitian follow-up; F, bioelectrical impedance; G, functional magnetic resonance imaging; H, gut hormones (fasting and post-meal profile); I, gut hormones; J, metabolomics; K, heath economics questionnaires; L, eating and behaviour questionnaires; M, insulin clamps; N, eating behaviour computerised tasks; O, cognitive assessment tasks; P, deoxyribonucleic acid sample; Q, food preference and taste assessment; R, telephone counselling. Reproduced with permission from Glaysher et al. 109 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original figure.

To investigate the mechanism of the effect of the endoluminal DJBL device, both treatment arms have been divided into three optional subgroups, which included the following additional assessments during the course of the trial:
-
subgroup 1 – functional magnetic resonance imaging (fMRI) of food reward and addictive behaviours
-
subgroup 2 – insulin sensitivity
-
subgroup 3 – taste preference and diet.
Table 4 summarises the visit schedule, the data that were collected across both study arms and supplementary data that were collected from the three optional mechanistic subgroups.
| Activity | Screening | Baseline | Treatment | Follow-up | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | T1 | V5 | V6 | T2 | V7 | T3 | V8 | T4 | V9 | T5 | V10 | V11 | T6 | V12 | T7 | V13 | T8 | V14 | V15 | |
| –4 weeks ± 7 days | –2 weeks ± 7 days | –0 weeks ± 3 days | +5 days ± 3 days | +10 days ± 3 days | +1 month ± 7 days | +2 months ± 7 days | +3 months ± 7 days | +4.5 months ± 7 days | +6 months ± 7 days | +7.5 months ± 7 days | +9 months ± 7 days | +10.5 months ± 7 days | +11.5 months ± 7 days | +12 months ± 7 days | +13.5 months ± 7 days | +15 months ± 7 days | +16.5 months ± 7 days | +18 months ± 7 days | +19.5 months ± 7 days | +23 months ± 7 days | +24 months ± 7 days | ||
| Informed consent | X | ||||||||||||||||||||||
| Inclusion and exclusion criteria | X | ||||||||||||||||||||||
| Demographics | X | ||||||||||||||||||||||
| Medical history (including medications) | X | ||||||||||||||||||||||
| Physical examination | X | ||||||||||||||||||||||
| ECG | X | ||||||||||||||||||||||
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Body weight | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Height | X | ||||||||||||||||||||||
| Waist circumference | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Routine blood tests | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Urine dipstick and female pregnancy test | X | ||||||||||||||||||||||
| Changes in medical history/medication | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Randomisation | X | ||||||||||||||||||||||
| Health economic questionnaires | X | X | X | X | X | X | |||||||||||||||||
| Dietary counselling | X | C | |||||||||||||||||||||
| Dietitian follow-up | X | X | X | X | X | X | X | ||||||||||||||||
| Urine albumin–creatinine ratio | X | X | X | X | X | ||||||||||||||||||
| Reporting of AEs | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| DNA and RNA sampling | X | X | X | X | X | X | |||||||||||||||||
| Telephone counselling | X | X | X | X | X | X | X | X | |||||||||||||||
| Diabetologist review | X | C | X | X | X | C | X | X | X | ||||||||||||||
| Metabolomics | X | X | X | X | X | ||||||||||||||||||
| Bioelectrical impedance | X | X | X | X | X | ||||||||||||||||||
| EndoBarrier group only | |||||||||||||||||||||||
| PPI and Helicobacter pylori test | X | ||||||||||||||||||||||
| Distribution of proton pump inhibitors | T | ||||||||||||||||||||||
| EndoBarrier implant | T | ||||||||||||||||||||||
| Preparation for EndoBarrier removal | T | ||||||||||||||||||||||
| EndoBarrier removal | T | ||||||||||||||||||||||
| Biopsies during implant and explant | T | T | |||||||||||||||||||||
| Gastroenterologist appointment | T | T | T | T | T | T | Ta | T | |||||||||||||||
| Subgroups | |||||||||||||||||||||||
| Fixed/test meal and post-meal gut hormones and metabolites (groups 1 and 3) | X | X | X | X | |||||||||||||||||||
| Gut hormones and metabolites (fasting only) (groups 1–3) | X | X | X | X | X | ||||||||||||||||||
| Food diaries (groups 1–3) | X | X | X | X | X | ||||||||||||||||||
| Eating and behaviour questionnaires (groups 1–3) | X | X | X | X | |||||||||||||||||||
| Appetite VASs (groups 1–3) | X | X | X | X | X | ||||||||||||||||||
| Eating behaviour computerised tasks (groups 1 and 3) | X | X | X | X | |||||||||||||||||||
| Metal check form (groups 1) | X | ||||||||||||||||||||||
| Handedness questionnaire (group 1) | X | ||||||||||||||||||||||
| Additional pregnancy tests | F | F | |||||||||||||||||||||
| DS-R questionnaire (group 1) | X | ||||||||||||||||||||||
| fMRI (group 1) | X | X | |||||||||||||||||||||
| Insulin clamps (group 2) | X | X | X | ||||||||||||||||||||
| Cognitive assessment tasks (group 1) | X | X | X | X | |||||||||||||||||||
| Food preference/taste assessment (group 3) | X | X | X | ||||||||||||||||||||
| 24-hour dietary recall (group 3) | X | X | X | X | X | ||||||||||||||||||
Study population
The study population comprised male and female patients aged 18–65 years with a BMI of 30–50 kg/m2 and confirmed diagnosis of T2DM for at least 1 year, who had inadequate glycaemic control and were on oral glucose-lowering medications. Box 1 shows a complete list of the inclusion and exclusion criteria.
Age 18–65 years (male or female).
T2DM duration ≥ 1 year.
HbA1c level of 7.7–11.0%, equivalent to 58–97 mmol/mol.
On oral hypoglycaemic medications.
BMI 30–50 kg/m2.
Exclusion criteriaLanguage barrier, mental incapacity, unwillingness or inability to understand and be able to complete questionnaires.
Non-compliance with eligibility criteria.
Females of childbearing potential who are pregnant, breastfeeding or intend to become pregnant or are not using adequate or reliable contraceptive methods.
Evidence of absolute insulin deficiency as indicated by clinical assessment, a long duration of T2DM and a fasting plasma C-peptide of < 333 pmol/l.
Current use of insulin.
Previous diagnosis with T1DM or a history of ketoacidosis.
Requirement for non-steroidal anti-inflammatory drugs or prescription of anticoagulation therapy during the implant period.
Current iron deficiency and/or iron deficiency anaemia.
Symptomatic gallstones or kidney stones at the time of screening.
History of coagulopathy, upper GI bleeding conditions such as oesophageal or gastric varices, congenital or acquired intestinal telangiectasia.
Previous GI surgery that could affect the ability to place the device or the function of the implant.
History or presence of active Helicobacter pylori (if patients are randomised into the EndoBarrier arm and have a history or presence of active Helicobacter pylori tested at study visit 2, they can receive appropriate treatment and then subsequently enrol in the study).
Family history of a known diagnosis or pre-existing symptoms of systemic lupus erythematosus, scleroderma or other autoimmune connective tissue disorder.
Severe liver impairment (i.e. aspartate aminotransferase, alanine aminotransferase or gamma-glutamyl transferase more than four times the upper limit of the reference range) or kidney impairment (i.e. estimated glomerular filtration rate < 45 ml/min/1.73 m2).
Severe depression, unstable emotional or psychological characteristics (including Beck Depression Inventory II score of > 28).
Poor dentition and inability to adequately chew food.
Planned holidays up to 3 months following the EndoBarrier implant.
Previous EndoBarrier implantation.
Study recruitment
Participants were identified from several areas across primary, secondary and tertiary health-care and community settings:
-
diabetes research registers [e.g. Diabetes Alliance for Research in England (DARE), Research Ethics Committee (REC) 2002/7/118]
-
hospital or general practice patient databases (Participant Identification Centres)
-
patients referred to diabetes and metabolic specialist clinics
-
other research studies within the Imperial College Healthcare NHS Trust and the Local Clinical Research Network
-
study websites
-
local and national media: websites, radio, newspaper articles and adverts
-
posters
-
diabetes, obesity and other support groups
-
social media websites.
Potential patients who, after reading a summary patient information sheet (PIS), were interested in entering the trial gave their verbal consent for preliminary telephone screening to check basic inclusion and exclusion criteria. Written consent was then taken from the patient to allow the study team to contact their general practitioner (GP) for the purpose of obtaining additional information on the patient’s medical history and current medical therapies, and to identify any other clinical reasons why the patient should not participate. Patients who appeared to meet the eligibility criteria were provided with a full trial PIS and were then invited to a formal screening visit at one of the study centres. At this stage the patient was fully informed of the nature of the study and given relevant information about the objectives of the research, the benefits and possible AEs, verbally and in writing. The patient had the opportunity to ask questions about the trial and formal written consent was taken for the patient to participate in the main study with or without additional consent for participation in one of the three optional mechanistic subgroups. The patients also had the option not to consent to participation in any of the three subgroups. Once consent had been obtained, the patient’s full eligibility was checked against all inclusion and exclusion criteria (see Box 1). Each patient was informed of their eligibility for the trial once all results were available (on average within 1 week from obtaining consent).
Randomisation
After the screening visit, all eligible patients for the trial were randomised into one of the two arms of the study via the InForm system [the electronic case report form (eCRF) database for the study]. A dummy randomisation list was created by an independent statistician and submitted to the InForm system, thus ensuring protection against bias in the randomisation process. Randomisation was allocated between treatment arms on a 1 : 1 basis and stratified by site and by BMI group (30–40 kg/m2 and 40–50 kg/m2). The final randomisation list was completed in Stata® version 13 (StataCorp LP, College Station, TX, USA) using randomly assigned block sizes of 2, 4 and 8. As the randomisation lists were designed to allow for additional patient recruitment [as standard Imperial Clinical Trials Unit (ICTU) procedure], no changes were made to the original randomisation list in order to incorporate additional patients to replace those who dropped out in the lead-in period from randomisation to intervention. At no point did the total number of patients starting treatment exceed 160.
Only patient number and patient initials were recorded in the case report form (CRF); and, if the patient’s name appeared on any other document (e.g. pathologist report) it was subsequently redacted. The investigator maintained a personal patient identification list (patient numbers with the corresponding patient names) to enable records to be identifiable, if required. Patients were informed about their allocated treatment arm on visit 2.
Trial interventions
EndoBarrier gastrointestinal liner
The EndoBarrier GI liner device received the Conformité Européenne (CE) mark for 12 months’ implant duration on 11 December 2009 as a single-use, minimally invasive device, used to achieve weight loss and improve T2DM status in patients who are obese.
At visit 2 (–4 weeks), participants who were randomised to receive the EndoBarrier device were tested for the presence of Helicobacter pylori, by either faecal antigen or urea breath testing. Those patients testing positive were offered 1 week of triple eradication therapy, as per guidance published in the British National Formulary (BNF),110 and were then retested after a further 4 weeks to confirm complete eradication before continuing with implantation of the EndoBarrier device. Subsequently, all patients were prescribed a proton pump inhibitor (PPI) [omeprazole (TEVA Pharmaceuticals, Petah Tikva, Israel) 40 mg twice daily] and instructed to commence this 3 days prior to the implant procedure. They continued this for the duration of the implant period (12 months) and for a further 2 weeks following device removal.
At visit 4 (0 weeks), after an 8-hour fast, patients had the EndoBarrier device implanted under a general anaesthetic. The implant was delivered endoscopically on a custom catheter and the anchor was sited in the duodenal bulb using a custom delivery system under fluoroscopic X-ray guidance (mean fluoroscopic X-ray time for insertion is 7 minutes, range 1–20 minutes). The 60-cm sleeve was then unfurled and the final positioning plus patency was confirmed by assessing for the free flow of radio-opaque contrast through the device. Videos and photos of the fluoroscopy images were recorded to help the investigators make treatment decisions. During implantation, eight gastric and small bowel biopsies were taken using standard biopsy forceps. Four biopsies were used for routine histology and four biopsies were used for ribonucleic acid (RNA) extraction to perform genome-wide expression analysis. Participants were discharged from hospital the same day with an implant information card, which described the implant and identified whom to call in the case of an emergency, and what symptoms to look for following the implant.
The device was removed at visit 11 (after 12 months) under sedation or general anaesthetic. The gastroscope was fitted with a foreign body retrieval hood and then used to locate the implant, and then a custom grasper was passed through the working channel of the gastroscope to grab a polypropylene tether located on the proximal portion of the anchor. Pulling on this tether collapsed the proximal end of the anchor, which could then be pulled into the foreign body hood and removed by withdrawing the gastroscope through the patient’s mouth. During this removal, eight further biopsies were taken for histology and RNA extraction. Following removal of the EndoBarrier device, patients were followed up for a further 12 months.
Diabetes management
Participants in both arms of the trial had their T2DM managed in accordance with the guidelines of the ADA. 58,59 Both treatment groups had a review of their T2DM by three consultant diabetologists at visits 2, 6, 7, 9, 12, 13 and 15. Furthermore, the standard care arm of the trial had an additional review at visits 4 and 11 in place of the endoluminal DJBL implant and removal. Adjustments to a patient’s oral glucose-lowering medication and escalation of therapy were at the investigator’s discretion and complied with the general recommendations laid out by the ADA. 59
Dietary counselling and physical activity
At visit 2, all patients’ historical and current eating behaviours were assessed by a qualified dietitian using the following information: anthropometry; biochemistry; comorbidities; activity levels; eating habits including previous diets; lifestyle including smoking and drug and alcohol misuse; weight history; psychiatric history; family history of obesity, diabetes, mental illness or eating disorders; available support network; work status; and readiness and motivation for change. Patients received dietary and physical activity counselling in accordance with local standards with the intention of providing each patient with lifestyle/behavioural modification information and good eating practices advice. In addition, patients in the endoluminal DJBL arm received written information on how their diet would change after implantation of the device and they received specialist guidance for eating with their endoluminal DJBL.
All patients were reviewed by a specialist dietitian at visits 2, 6, 7, 9, 12, 13 and 15. In addition, participants in the standard care arm of the trial had an additional review at visits 4 and 11 in place of the endoluminal DJBL implant and removal. During the course of the trial, participants were recommended to consume 600 kcal fewer every day, depending on their age, gender, activity levels and body weight. Guidelines for daily amounts were between 1200 and 1500 kcal for women and between 1500 and 1800 kcal for men. In accordance with standard dietary practice, patients were advised to eat regularly every day (five times per day); to control their portion sizes and intake of carbohydrates/starchy foods; to increase their intake of low glycaemic index and high-protein foods, as well as vegetables; and to reduce their intake of foods high in fat and sugar, and alcohol. Participants were advised to include more physical activity in their daily routine and encouraged to do more activity in their leisure time. Their goal was to include 150 minutes per week of moderate intensity, and 75 minutes per week of vigorous intensity, aerobic activity and muscle strengthening activities on more than 2 days per week. Changes in physical activity level were monitored using the International Physical Activity Questionnaire. 60
Liquid diet
To avoid disruption of the device in the immediate period following implantation, patients followed a liquid diet for the 7 days before and 13 days (± 3 days) after the intervention visit (visit 4). The liquid diet was guided by the specialist dietitian and comprised 125 ml of Fortisip Compact drinks (Nutricia, Trowbridge, UK), five times per day for males and four times per day for females, which contained the following per 100 ml: 240 kcal, 9.6 g protein (16% total energy), 29.7 g carbohydrate (49%), 15 g sugars and 9.3 g fat (35%). Patients were also allowed to consume sugar-free squashes, smooth/clear soup (one medium bowl per day), tea or coffee without sugar, or unsweetened purée. To standardise both therapy groups, all patients across both arms followed the liquid diet for this duration and period of the study.
Primary and secondary outcomes
Primary objective
To compare the endoluminal DJBL with a combination of conventional medical therapy, diet and exercise for obesity-related T2DM and its effectiveness on metabolic state as defined by the IDF as a HbA1c reduction of ≥ 20%.
Secondary objectives
To compare the endoluminal DJBL with a combination of conventional medical therapy, diet and exercise for obesity-related T2DM and its effect on:
-
metabolic state as defined by the IDF with a HbA1c level of < 6% (or < 42 mmol/mol)
-
blood pressure of < 135/85 mmHg
-
absolute weight loss.
To investigate the mechanism of the effect of the endoluminal DJBL via changes in:
-
gut hormones
-
microbiome
-
appetite, food hedonics and brain reward systems
-
body fat content
-
food preferences
-
hepatic or peripheral insulin sensitivity
-
bile acids
-
biomarkers such as genetic markers.
To estimate the cost-effectiveness of the endoluminal DJBL device compared with conventional treatment over the trial period (within-trial analysis).
To estimate the long-term cost-effectiveness (over 24 months) of the endoluminal DJBL device compared with conventional treatment and alternative surgical interventions.
Post hoc exploratory analysis of the changes in the number of diabetes medications in both treatment arms (over 24 months) was also analysed.
Assessment of primary objective
Each study participant had their International Federation of Clinical Chemistry HbA1c measured at screening and then subsequently at visits 5, 7, 8, 9, 10, 12, 13 and 15. Samples were processed at the laboratory local to each study centre using standard methods. Results were recorded on the InForm system.
Assessment of secondary objectives
Individuals in both study arms were invited for regular medical check-ups, which included routine anthropometric measurements (height, weight, waist circumference, pulse and blood pressure) and blood tests (Table 5). Any changes to the patients’ health or medications were documented on the CRF and all AEs were reported in detail in line with standard principles of good clinical practice.
| Blood test | V1 | V3 | V5 | V6 | V7 | V8 | V9 | V10 | V11 | V12 | V13 | V14 | V15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haematology (full blood count) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Routine biochemistry (including urea and electrolytes) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Liver function tests | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Fasting glucose | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Creatinine | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| HbA1c | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Fasting lipids (cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| C-peptide | ✗ | ||||||||||||
| Insulin (fasting) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Vitamin D | ✗ | ✗ | ✗ | ||||||||||
| Iron studies | ✗ | ✗ | ✗ | ||||||||||
| Vitamin B12 | ✗ | ✗ | ✗ | ||||||||||
| Serum folate | ✗ | ✗ | ✗ | ||||||||||
| Free thyroxine | ✗ | ✗ | ✗ | ||||||||||
| Thyroid-stimulating hormone | ✗ | ✗ | ✗ | ||||||||||
| Cortisol (subgroup 1 only) | ✗ | ✗ | |||||||||||
| Estradiol (subgroup 1 only) | ✗ | ✗ | |||||||||||
| Progesterone (subgroup 1 only) | ✗ | ✗ | |||||||||||
| Luteinising hormone (subgroup 1 only) | ✗ | ✗ | |||||||||||
| Follicle-stimulating hormone (subgroup 1 only) | ✗ | ✗ |
Mechanistic study methodology
Subgroup 1: functional magnetic resonance imaging of food reward and addictive behaviours
Study design
This section reports the findings from the fMRI mechanistic subgroup 1 (performed at Imperial College London only), including fMRI of food reward and addictive behaviours; dietary and appetite assessments including ad libitum test lunch meal; food preference; VAS ratings; and eating behaviour questionnaires.
Results for the eating behaviour questionnaires were combined with data from both the insulin clamp mechanistic subgroup 2 (Southampton only) and taste mechanistic subgroup 3 (Imperial College London and Southampton). Results for fasting VAS ratings and bloods, and Leeds Food Preference Questionnaire (LFPQ), were combined with data from the taste mechanistic subgroup 3 (Imperial College London and Southampton). The latter subgroup, subgroup 3 also included assessment of sweet taste thresholds, additional dietary assessments and measurement of post-prandial plasma appetitive gut hormones and glucose.
Inclusion and exclusion criteria
Full information regarding general inclusion and exclusion criteria is available. 109 Additional exclusion criteria for the fMRI mechanistic subgroup 1 were as follows: (1) metal implant and claustrophobia as contraindications to magnetic resonance imaging (2) vegetarianism, veganism or gluten or lactose intolerance, given food pictures used in fMRI food evaluation paradigm; (3) current smoker, or current or previous history of drug addiction or alcohol dependence, given assessment of addictive behaviours; and (4) history of moderate to severe traumatic brain injury, given need for neuroimaging.
Study outcome variables
The study outcome variables collected at different study visits for the fMRI mechanistic subgroup that are presented in this report are shown in Appendix 2, Table 22, together with those that could be combined from the other two mechanistic subgroups. All visits were attended after an overnight fast. Endoluminal DJBL was inserted at week 0 and removed at week 52.
Scanning visit protocol
The scanning visit protocol for the fMRI mechanistic subgroup is illustrated in Figure 4. Patients arrived at 09.00 at the National Institute for Health Research (NIHR) Imperial Clinical Research Facility, Hammersmith Hospital, London, having not eaten or drunk anything other than water since supper the day before. They were advised to avoid any alcohol or strenuous exercise the day before. They were asked for their verbal consent and general questions about their overall health and medical history, including AEs and medication changes.
FIGURE 4.
Schematic representation of study visits. a, Data combined with other mechanistic subgroups; only blood and VAS time points marked in red are presented in this report; b, done only once at baseline (visit 3). ASL, arterial spin labelling; DTI, diffusion tensor imaging; Food, food picture evaluation task; IPAQ, International Physical Activity Questionnaire; Kirby DD, Kirby delay discounting task; MID, monetary incentive delay task; Neg, negative; PANAS, Positive and Negative Affect Schedule; Rest, resting state; T1, T1-weighted magnetic resonance imaging; WTAR, Wechsler Test of Adult Reading.

Anthropometric measures were taken, and a cannula inserted to collect a total of five blood samples across the visit: baseline, pre scan, pre meal after the magnetic resonance imaging (MRI) scan, and 1 and 2 hours after the ad libitum lunch meal presented at ≈ 13.00. The 90-minute MRI session was performed from ≈ 11.00 to 12.30. Appetite, nausea and other mood VASs ratings were obtained at time points 1, 2, 3, 5, 7 and 8; an abbreviated appetite VAS was collected at time point 4 during the scanning, and taste ratings of the foods presented at the ad libitum lunch meal were collected at time point 6.
Various computer-based tasks and online questionnaires were administered throughout the study visit (see Figure 4).
Anthropometry
Anthropometric measures were collected, including height, weight, waist and hip circumference. Height was measured using a wall-mounted stadiometer, and weight and body composition analysis (e.g. % body fat and trunk fat) was carried out using a bioelectrical impedance analysis machine (Tanita BC-418, Tanita Europe BV, Manchester, UK).
Blood sampling and assays
With a venous cannula inserted, basal fasting, serial fasting and post-prandial blood samples were assayed for plasma glucose, gut hormones, and serum insulin and cortisol. Blood samples for gut hormones were collected into chilled lithium heparin polypropylene tubes, containing 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) (A8456 Sigma-Aldrich, Dorset, UK) and aprotinin (Nordic Pharma Ltd, Reading, UK) protease inhibitor to give final concentrations of 1 mg/ml and 200 kIU/ml whole blood, respectively. Aliquots of separated plasma for acyl ghrelin assay were immediately mixed with hydrochloric acid (final concentration of 0.05 M). All plasma samples were stored at –80°C until assayed.
Samples were assayed in the Department of Chemical Pathology, Imperial College Healthcare NHS Trust, London, using standard clinical assays; plasma PYY, GLP-1 and FGF-19 were assayed in duplicate using commercial enzyme-linked immunosorbent assays (ELISAs) by Professor Carel le Roux, University of Dublin, Ireland,111 and plasma acyl and desacyl ghrelin in duplicate using an in-house two-site ELISA, by Bruce Gaylinn, University of Virginia, VA, USA. 112,113 Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the formula [glucose (mmol/l) × insulin (mU/l)]/22.5. 114
Appetite visual analogue scale rating
Visual analogue scale ratings, with a scale of 1–100 mm, were collected on an iPad (Apple Inc., Cupertino, CA, USA) to rate hunger, pleasantness to eat, amount able to eat, fullness, stress, anxiety, sickness (feeling nauseated) and sleepiness. 113 A composite ‘appetite’ score was calculated from the first four VAS scores, as follows [hunger + pleasantness to eat + amount to eat + (100 – fullness)]/4. 115
Functional magnetic resonance imaging
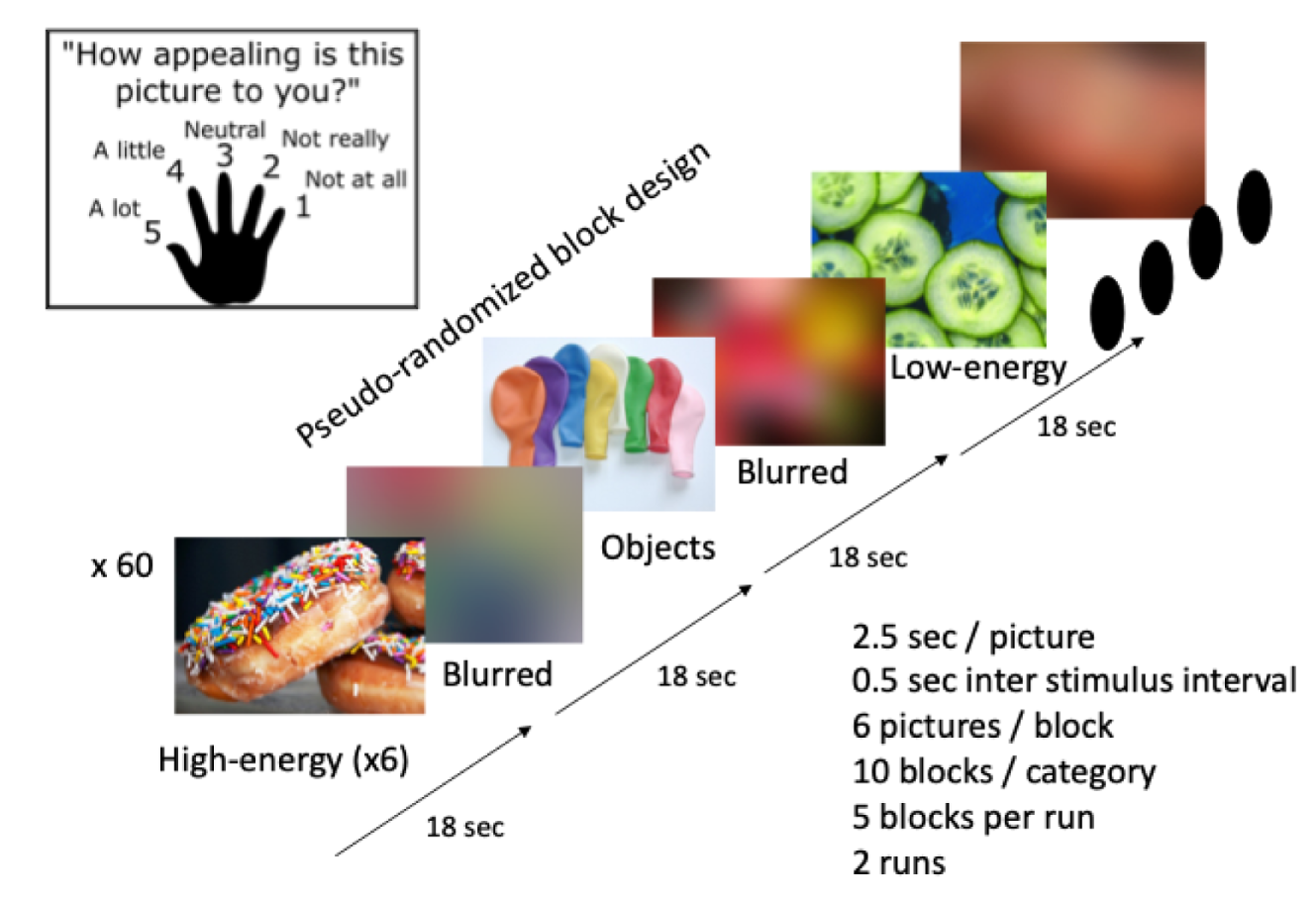
Participants had a 90-minute fMRI session in which they could respond to the display instructions and images seen on a computer screen via an angled mirror using a handheld five-button or single-button keypad (Figure 5). Tasks were programmed using E-Prime Professional v2.0 (Psychology Software Tools, Pittsburgh, PA, USA). This was used for the following task-related fMRI scans:
-
resting state fMRI (5 minutes) and arterial spin labelling (5 minutes)
-
food picture evaluation fMRI task (two runs, 20 minutes).
Magnetic resonance imaging acquisition parameters
Functional magnetic resonance images were acquired using a 3-T Siemens Verio MRI scanner (Siemens Healthineers AG, Erlangen, Germany) (at the Clinical Imaging Centre, Imperial College London, Hammersmith Hospital, London, UK) using a 32-channel radiofrequency head coil with T2*-weighted gradient-echo echoplanar imaging (EPI) with an automated higher-order shim procedure: 39 ascending interleaved contiguous 3.0-mm-thick slices, 3.0 × 3.0 mm voxels, generalized autocalibrating partial parallel acquisition (GRAPPA) acceleration factor 2, repetition time (TR) 2250 milliseconds, echo time (TE) 30 milliseconds, 80° flip angle, field of view 192 mm, with slice acquisition angle parallel to anterior commissure–posterior commissure line.
High-resolution T1-weighted magnetisation-prepared rapid acquisition with gradient echo (MPRAGE) structural scans were also acquired for image registration. Field maps were used to correct for geometric distortions caused by inhomogeneities in the magnetic field.
Food picture evaluation functional magnetic resonance imaging paradigm
The food picture evaluation task was performed as previously described. 54,111,113,116 Participants looked at colour food and non-food photographs and simultaneously rated their ‘appeal’ from 1 to 5, with 1 being ‘not at all’ appealing and 5 being ‘a lot’, to assess anticipatory food reward or food cue reactivity (see Figure 5). Participants briefly practised the appeal rating task in the scanner using pictures of animals at the start of the scanning session on each visit.
Four categories of pictures were shown in a block design: (1) 60 pictures of high-energy (HE) food, such as cake, chocolate, pizza and burgers (with an equal number of chocolate, non-chocolate sweet, and savoury categories per block); (2) 60 pictures of low-energy (LE) food, such as salads, fish and vegetables; (3) 60 pictures of non-food household objects, such as furniture and clothing; (4) 180 Gaussian-blurred versions of the previous pictures in blocks after every other block, to act as a low-level baseline (see Figure 5). The images shown were representative of a typical Western diet. Images used were taken from the International Affective Picture System (National Institute of Mental Health Center for the Study of Emotion and Attention, University of Florida, Gainesville, FL, USA) and publicly available websites. All pictures had a similar level of luminosity and resolution.
The sequence was split into two runs, each with 192 pictures running for 9 minutes 27 seconds. An individual run consists of five separate blocks (six pictures in 18 seconds) of each of the first three block designs (HE food, LE food or objects) interspersed with 31 blocks of the blurred images. The order of images within each block was randomised, whereas the overall sequence was one of two pseudorandomised arrangements. Each individual image was displayed for 2500 milliseconds with a 500-millisecond interval presenting a white cross on a black background to separate image stimuli.
A priori functional regions of interest (fROIs) were derived from a separate cohort of adults across a range of BMIs [n = 81; ≈ 1/3 lean (BMI < 25 kg/m2), ≈ 1/3 overweight (BMI 25–30 kg/m2) and ≈ 1/3 obese (BMI > 30 kg/m2)], who performed an identical fMRI task after an overnight fast, by masking the group activation map for HE food or LE food > object contrast in whole brain analysis (voxel-wise false discovery rate p < 0.05) with anatomical masks from the Harvard–Oxford cortical and subcortical atlases:117 amygdala, orbitofrontal cortex, anterior insula, nucleus accumbens, putamen and caudate. Median blood oxygen level-dependent (BOLD) signal within each fROI was extracted for individual participants at each visit separately for the HE food > object and LE food > object contrasts.
Functional magnetic resonance imaging processing and analysis
Functional magnetic resonance imaging data processing was performed using FSL (FMRIB Software Library) version 5.0.10 with fMRI Expert Analysis Tool (FEAT) version 6.00 (FMRIB Analysis Group, Oxford, UK; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; accessed 9 July 2020) with the following applied preprocessing: motion correction using MCFLIRT (Motion Correction FMRIB’s Linear Image Registration Tool);118 field map map-based EPI unwarping using PRELUDE (Phase Region Expanding Labeller for Unwrapping Discrete Estimates) and FUGUE (FMRIB’s Utility for Geometrically Unwarping EPIs);119 non-brain removal using BET (Brain Extraction Tool);120 spatial smoothing using a Gaussian kernel of full-width half-maximum 6.0 mm; grand-mean intensity normalisation of the entire four-dimensional data set by a single multiplicative factor; and high-pass temporal filtering (Gaussian-weighted least-squares straight 0 line fitting, with sigma 100.0 seconds for food task).
Time-series statistical analysis was done using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction including event onsets as explanatory variables within the context of the general linear model (GLM) on a voxel-by-voxel basis (stick functions convolved with the γ haemodynamic response function) for the relevant contrast. Motion parameters were included as part of the GLM. 121 For the food evaluation task, the GLM also included the temporal derivatives of the event onsets as covariates to correct for slice timing.
Registration to high-resolution T1 structural images was carried out using FLIRT (FMRIB’s Linear Image Registration Tool; FMRIB Analysis Group, Oxford, UK) including boundary-based registration. 119,122 Registration from high-resolution structural to standard space was then further refined using FNIRT (FMRIB’s Non-linear Image Registration Tool; FMRIB Analysis Group, Oxford, UK) non-linear registration. For the food evaluation paradigms, the two runs for each visit were averaged using higher-level fixed-effects analysis for each contrast.
Median BOLD signal was extracted from a priori fROIs, using Featquery software (FMRIB Analysis Group, Oxford, UK), to compare between visits and groups.
Leeds Food Preference Questionnaire
At the start of the study visit, having fasted overnight and before the MRI session, participants completed the LFPQ to examine explicit liking, explicit wanting and implicit wanting, using combinations of different low-fat (LF)/high-fat (HF) foods and savoury/sweet foods (in collaboration with Graham Finlayson, University of Leeds, UK). 123
Participants saw individual food photographs, or pairs of photos, on a computer for explicit rating of ‘liking’ and ‘wanting’ at that moment, and were asked to choose which they would prefer to eat right now for assessment of implicit wanting (determined from reaction time during forced choice adjusted for pooled SD and choice frequency). 124–126
Ad libitum test meal
At ≈ 40 minutes after the end of the MRI session at ≈ 13.10, participants were presented with an ad libitum lunch meal provided in excess quantities consisting of chicken broth [LF savoury (tinned Baxters Favourites; Baxters Food Group Limited, Fochabers, UK)], cream of chicken soup with added cream [HF savoury (tinned Baxters Favourites)], natural yoghurt [LF sweet (Yeo Valley; Yeo Valley Limited, Rhodyate, UK)], vanilla ice cream [HF sweet (Häagen-Dazs®; Minneapolis, MN, USA)]. If participants did not like chicken soups, they were instead given tomato broth and cream of tomato soup (Baxters Favourites). See Figure 20 for nutritional composition of the dishes served.
Taste ratings
Participants were first asked to rate the taste of a teaspoon of each presented dish, with emphasis on certain taste sensations:
-
creaminess intensity – all four dishes, LF and HF soup, yoghurt and ice cream
-
pleasantness – all four dishes
-
sweetness intensity – only the desserts (i.e. yoghurt and ice cream).
Ratings were performed using the Sussex Ingestion Pattern Monitor (SIPM) system version 2.0.14.0 general linear scale rating computer software (www.sipm.co.uk; accessed 9 July 2020; Martin Yeomans, University of Sussex, Brighton, UK).
Lunch energy intake
Participants were instructed to eat as much of the lunch meal as they wished while the investigators were outside the room. Each item was weighed before and after to determine total, dish and macronutrient (fat, carbohydrate, protein) energy intake expressed as absolute kilocalories, percentage of estimated 24-hour resting energy expenditure {calculated using the Cunningham equation: resting energy expenditure = 501 + [21.6 × lean body mass (kg)] in kilocalories per 24 hours, equating lean body mass with fat-free mass determined by bioelectrical impedance analysis127}, and as a percentage of total meal energy intake.
Progressive ratio task
To assess appetitive motivation for HE sweets (appetitive food reward), patients completed the PRT on a laptop, where they pressed a computer mouse in an exponentially increasing manner to receive each sweet. 62,111 This task evaluates the break point of effort to achieve the HE food. The task was performed ≈ 2–3 hours after the start of the ad libitum lunch meal in the satiated state. Although this task was also carried out by participants in the taste mechanistic subgroup, subgroup 3, the results were analysed separately as the task was performed in a different state (i.e. after they had tasted many solutions of different sweetness).
A plate of 20 M&M crispy sweets (Mars Inc., McLean, VA, USA) was placed in front of participants, who were given the same instruction: ‘Press as little or as much as you like. When you no longer want to continue, press the space bar.’ After a practice trial run, patients were left alone in the room to complete the exercise; for example, pressing 10 times would earn the patient a single M&M sweet. To earn another, they had to press 20 more times, then 40, and so on until they had pressed the space bar indicating completion.
The number of clicks was correlated with the amount of sweets eaten via the computer software, which calculates the last completed ratio of sweets consumed. Each M&M was only 4 kcal (43.7% sugar and 44.1% fat). The total number of clicks and the last completed ratio are included in the analysis.
Eating behaviour questionnaires
The following web-based questionnaires were completed by participants using an iPad at each mechanistic study visit for all three subgroups.
Eating behaviour
The Three Factor Eating Questionnaire (TFEQ) is used to evaluate current eating behaviour under subscales of (1) hunger: susceptibility to eating due to hunger cues; (2) disinhibited eating: loss of control during eating; and (3) restraint: cognitive control over daily intake of food. 128
The Dutch Eating Behaviour Questionnaire (DEBQ) is used to measure current dietary restraint, emotional eating and external influences (e.g. hedonic properties) on eating behaviour. 129
The Eating Disorder Examination Questionnaire (EDEQ) is used to assess dietary restraint, preoccupation with weight and shape, and binge eating in the last 4 weeks. 130
The Power of Food Scale (PFS) is used to assess the current psychological influence of the food environment, that is the appetite for, rather than the consumption of, food, using the 21-item version. 131
The Yale Food Addiction Scale (YFAS) is used to measure features of addiction towards particular foods in the last year. 132
The Binge Eating Scale (BES) is used to assess the current presence of binge eating behaviour (e.g. binging or purging food), as well as cognitive indicators of binging, such as fear, guilt and an inability to stop. 133
Dumping syndrome
Participants were asked about characteristic symptoms of dumping syndrome that they experienced 1 hour after a meal over the last 4 weeks.
Sigstad’s dumping score is a validated scoring system developed for the diagnosis of dumping syndrome in partial gastrectomy patients, with weighted scoring for the presence of post-prandial symptoms, including borborygmia (rumbling) +1, bloating +1, nausea +1, sweating +1, headache +1, dizziness +2, restlessness +2, palpitations +3, drowsiness +3, weakness/exhaustion +3, breathlessness +3, desire to lie or sit down +4, syncope +4, shock +5, with negative scores for the presence of vomiting –4 and eructation (belching) –1. 134 A clinical diagnostic index of +7 or above indicates dumping syndrome and indices of +4 or below indicate non-dumping.
Modified Arts dumping score is a validated questionnaire measuring the severity of dumping symptoms using a 4-point Likert scale, either early, that is, in the first hour (e.g. sweating, flushing, dizziness, palpitations, abdominal pain, diarrhoea, bloating, nausea) or late, that is, between the first and second hours (sweating, palpitations, hunger, drowsiness/unconsciousness, tremor, irritability) after food ingestion. 135 This modified version asked only about all these symptoms at 1 hour after eating.
Additional subgroup specific procedures and measurements
Across all three subgroups only, the following additional data were also collected during the mechanistic study visits:
-
Visual analogue scale ratings – to assess subjective feelings of hunger, nausea, fullness, sleepiness, stress and anxiety when fasted and during meal tests.
Subgroup 2: insulin sensitivity
Those participants who consented to take part in subgroup 2 of the EndoBarrier RCT underwent a hyperinsulinaemic–euglycaemic clamp at visits 3 (–2 weeks ± 7 days), 5 (+10 days ± 7 days) and 8 (+6 months ± 7 days). Patients were advised to take their usual oral diabetes medications the morning of the day before the study visit and then not again until after the clamp was completed. Participants were also told to avoid alcohol or strenuous exercise in the 24 hours prior to the visit, and on the evening prior to the clamp all participants consumed a fixed meal of 2 × 125 ml of Fortisip Compact drinks, containing per 100 ml: 240 kcal, 9.6 g protein (16% total energy), 29.7 g carbohydrate (49%) and 9.3 g fat (35%). They then remained fasted until after the clamp was completed.
Participants attended the Wellcome Trust Clinical Research Facility at Southampton General Hospital for their visit. On arrival in the morning, the patient had a clinical review in which their general well-being was assessed and any AEs or changes to concomitant medications were recorded. They then had their blood pressure, pulse rate, weight, waist circumference and body composition measured by bioelectrical impedance using a Seca medical body composition analyser (mBCA 515) (Seca, Germany). Each participant was asked to sit in an infusion chair in a semi-recumbent position with their arms placed horizontally on armrests at chest height. An 18- to 20-gauge cannula was placed into a large vein in each antecubital fossa, with one cannula being used for blood sampling and the other for administering multiple infusions via two three-way taps. During cannulation, baseline blood tests were taken (see Table 5). The participant was then started on a variable-rate insulin infusion: 50 international units (IU) of Actrapid® insulin (Novo Nordisk, Copenhagen, Denmark) diluted in 48 ml of 0.9% normal saline and 2 ml of the patient’s own blood in a 50 ml Luer lock syringe. The infusion rate was adjusted in response to the patient’s blood glucose level, which was measured at the bedside every 5–15 minutes by the glucose oxidase method using a YSI biochemistry analyser (YSI Life Sciences, Yellow Springs, OH, USA) until a stable blood glucose level of 4.0–6.0 mmol/l was achieved.
Once achieved, blood samples were taken for determination of baseline glucose enrichment (T = –120 minutes) followed immediately by a primed continuous infusion of [6,6-2H2]glucose (170-mg priming bolus followed by 1.7 mg/minute continuous infusion). Once a steady state of enrichment with the stable isotopes was achieved, five baseline samples were taken every 5 minutes between T = –20 and 0 minutes for measurement of the glucose enrichment.
At T = 0 minutes, the blood glucose level was recorded as the ‘clamped’ glucose and a two-step euglycaemic–hyperinsulinaemic clamp was initiated. For the first stage (T = 0 to + 120 minutes), Actrapid insulin was infused at a low dose (0.3–0.5 mU/kg/minute) to estimate HGP, which is predominantly a measure of hepatic insulin sensitivity. Blood glucose concentration was measured every 5–10 minutes using the YSI analyser and was maintained around the ‘clamped’ glucose level ± 0.5 mmol/l using a variable rate infusion of 20% dextrose. This exogenous glucose infusion was spiked with [6,6-2H2]glucose (8 mg/g) in order to prevent a fall in plasma tracer enrichment and an underestimation of endogenous glucose production rate. Blood samples were taken every 30 minutes for the first 90 minutes and then every 10 minutes for the final 30 minutes to measure the isotopic enrichment of glucose.
At T = + 120 minutes, the second stage of the clamp was commenced by infusing Actrapid insulin at a high dose of 1.5 mU/kg/minute. These high insulin concentrations suppress HGP and, therefore, measurements made during this stage of the clamp primarily reflect changes in peripheral insulin sensitivity. For this stage, the variable rate infusion of 20% dextrose was spiked with a further 2 mg/g [6,6-2H2]glucose and euglycaemia maintained.
Following completion of the clamp at + 240 minutes, the insulin and tracer infusions were stopped but the 20% dextrose infusion was continued for a further 30 minutes and the participant given a meal to avoid hypoglycaemia.
The isotopic enrichment of plasma glucose was determined by gas chromatography–mass spectrometry (MS) on an Agilent Technologies 5975C inert Xl EI/CI MSD system (Agilent Technologies Inc., Santa Clara, CA, USA) and glucose concentrations were measured on the Mira autoanalyser by way of the glucose PAP assay (Horiba ABX, Montpellier, France) at the Department of Clinical and Experimental Medicine, Postgraduate Medical School, University of Surrey, Guildford, UK. HGP [endogenous glucose appearance (Ra)] and glucose disposal rate [glucose disappearance (Rd)] were calculated using the Steel model, modified for the inclusion of stable isotopes. Both Ra and Rd were then corrected for prevailing insulin concentrations during the clamp.
Subgroup 3: taste preference and diet
On visits 3, 5, 8, 10 and 14, patients in each study arm, at both the London and the Southampton sites, attended the research facility after an overnight fast. The total duration of these visits was up to 7 hours (visits 3, 5, 8 and 10) and 5 hours (visit 14). A trained dietitian/nutritionist performed a detailed 24-hour dietary recall assessment and then participants were asked to complete an EPIC (European Prospective Investigation into Cancer and Nutrition) study food frequency questionnaire. Three-day food diaries, 24-hour recall and the EPIC questionnaire were used to quantify total caloric intake and macronutrient composition.
Sweet taste detection testing was performed at visits 3, 5 and 8, when seven ascending sucrose concentrations in solution were used to determine sweet detection thresholds. 82 At the same visits consummatory taste reward was assessed: five ascending sucrose solutions were used to test responses in intensity ratings and hedonic reward. Finally, a fixed mixed-meal tolerance test with measurement of post-meal hormones and metabolites was performed.
Metabonomics
Plasma, urine and faecal samples for metabolic profiling analysis were collected from all participants who were able to provide samples at:
-
visit 3 – pre implant, and therefore considered the baseline sample
-
visit 5 – up to 10 days following EndoBarrier implant
-
visit 8 – 6 months following EndoBarrier implant
-
visit 10 – 1 year following EndoBarrier implant, and the last visit prior to explantation
-
visit 14 – 1 year following EndoBarrier explant.
Plasma
Approximately 1.2 ml of whole blood was collected via venepuncture into 6-ml sodium heparinised vacutainers (BD Vacutainers® PST™ Lithium Heparin Tubes) from each participant at each visit listed above. Within 30 minutes of collection, these samples were centrifuged at 1600 g relative centrifugal force at 4 °C for 15 minutes. The plasma supernatant above the white blood cell layer was then transferred into Eppendorf tubes® (Eppendorf AG, Hamburg, Germany) and stored at –80 °C until analysis.
Urine
Following an overnight fast, patients provided a fresh urine sample on the morning of their visit, which was collected in a universal container. This was aliquoted immediately using a sterile pipette into cryotubes and stored at –80 °C until NMR analysis. The SOP for urine sample preparation for 1H-NMR spectroscopic analysis is available in the appendices.
Faeces
Stool samples were collected in sterile faecal containers. Patients were advised to provide a sample on the morning of their visit. These were allocated into cryotubes and stored in a freezer at –80 °C until the time of analysis.
Faecal water was extracted from the crude faecal samples for analysis by NMR. Metabolic profiles of faecal water samples have been shown to be more stable than those of crude samples. 136 The SOP is available in the study protocol.
Nuclear magnetic resonance protocol
All biological samples were run through 600-mHz 1H-NMR spectroscopy with a 5-mm tube NMR probe using the previously published protocol in Dona et al. 137 The processed NMR spectra data generated were modelled using the unsupervised method of principal component analysis using SIMCA 13.0.3 software (Umetrics, Umeå, Sweden) to generate an unbiased overview of the major metabolic differences between the EndoBarrier and the control group. To explore the class-related metabolic changes, an orthogonal partial least squares discriminant analysis (OPLS-DA) model was used to discriminate between the two groups, which was carried out in the program MATLAB (The MathWorks Inc., Natick, MA, USA).
Methods of the economic analysis
Analytical framework
The aim of the economic analysis is to estimate the cost-effectiveness of the DJBL intervention compared with the control of a combination of conventional medical therapy, diet and exercise alone. The analysis presented in this report is a within-trial economic evaluation, with the following objectives:
-
to compare mean costs between the intervention group and control group over the 2-year study period
-
to compare health-related quality of life [mean EuroQol-5 Dimensions, five-level version (EQ-5D-5L) utility scores] between the intervention group and control group at 10 days, 1 month, 3 months, 6 months, 1 year and 2 years
-
to estimate mean quality-adjusted life-years (QALYs) accrued over the 2-year study period for the intervention group compared with the control group (calculated as the area under the EQ-5D-5L utility curve)
-
to estimate the incremental cost per QALY gained within the trial period for the intervention group compared with the control group.
We also explore uncertainty surrounding the aforementioned estimates based on variation in trial observations with non-parametric bootstrapping to reflect joint uncertainty over costs and effects and deterministic sensitivity analysis to assess the impact of uncertainty over the cost of the DJBL device and consumables. Our preferred analysis includes multiple imputation (MI) of missing EuroQol-5 Dimensions (EQ-5D) utility and cost data, but we also present results without imputed data for comparison.
The study protocol included the additional aim of extrapolating cost and QALY estimates over a lifetime horizon, using a decision model. This would have incorporated cost savings and QALY gains beyond the 2-year follow-up attributable to lasting reductions in the risks of cardiovascular and other diabetes-related complications induced by the DJBL. However, given that the clinical results of the trial did not show that improvements in key risk factors persisted to 1 year, we do not anticipate that extrapolation of the cost-effectiveness analysis would be informative.
In conducting the economic analysis, we sought to follow principles recommended by the National Institute for Health and Care Excellence (NICE) in its reference case for technology appraisals. 138
-
All direct health effects expressed as QALYs.
-
EQ-5D as the preferred measure of health-related quality of life, reported directly by patients. Trial participants were asked to complete the five-level version of the questionnaire [EuroQol-5 Dimensions, five-level version (EQ-5D-5L)] at seven visits during the 2-year follow-up.
-
Preference data for valuation of health-related quality of life elicited from a representative sample of the UK population. Following a position statement from NICE, we used the van Hout crosswalk algorithm to map from the EQ-5D-5L to the three-level version [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)], and hence to utility values based on the UK Social Tariff. 139–141
-
Costs estimated from the perspective of the English NHS and, where relevant, Personal Social Services (PSS) as funded by local authorities.
-
Resources were valued using prices (in Great British pounds at 2018 prices) relevant to the NHS and PSS, obtained from standard national sources: NHS reference costs for inpatient stays, procedures, tests, and outpatient consultations with medical and allied professionals; NHS list prices for medicines; and the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2018 for primary and social care services. 142–144
-
Costs and QALYs discounted at a rate of 3.5% per year.
Quality-adjusted life-year calculations
Patients were asked to complete the EQ-5D-5L at visit 3, shortly prior to randomisation (–2 weeks), and at visit 5 (10 days), visit 6 (1 month), visit 7 (3 months), visit 8 (6 months), visit 10 (11.5 months) and visit 14 (23 months).
Index scores (utilities) were calculated for individuals at each time point. Following the position statement from NICE,141 we have not used the currently available value set developed from the general population survey for England;145 instead, we used the van Hout crosswalk algorithm to map from the EQ-5D-5L to EQ-5D-3L, and hence to utility values based on the UK social tariff. 139,140
We estimated QALYs for each participant over the 2-year trial period using an area under the curve approach. There are six time periods defined by the seven EQ-5D-5L observation time points. For each period, QALYs accrued were estimated by taking the mean of the utility scores at the adjacent time points and multiplying by the duration of the period (in years). To avoid bias due to any differences between the study arms in the precise timing of the follow-up visits, we assumed a fixed duration of each time period, as specified in the study protocol (Table 6). We also assumed that the observations at visit 3, visit 10 and visit 14 reflected utilities at 0, 12 and 24 months relative to randomisation.
| Time period | Adjacent study visits | Relative to randomisation | Assumed duration |
|---|---|---|---|
| P1 | Visit 3 to visit 5 | –14 to 10 days | 10 days (0.027 years) |
| P2 | Visit 5 to visit 6 | 10 to 30 days | 20 days (0.055 years) |
| P3 | Visit 6 to visit 7 | 1 to 3 months | 2 months (0.167 years) |
| P4 | Visit 7 to visit 8 | 3 to 6 months | 3 months (0.25 years) |
| P5 | Visit 8 to visit 10 | 6 to 11.5 months | 6 months (0.5 years) |
| P6 | Visit 10 to visit 14 | 11.5 to 23 months | 12 months (1 year) |
Cost calculations
We estimated costs incurred over 2 years from the date of visit 3 for each individual. Three broad categories of cost were collated: intervention costs, medication costs, and costs for other health services and personal social care.
Intervention costs
Costs directly related to the intervention were calculated excluding additional tests and follow-up related to outcome assessment for the trial. Included costs for the intervention group were:
-
Pre-implant: we assumed one GI outpatient visit for pre-operative assessment and H. pylori faecal antigen or urea breath test. Patients scheduled for an implant were also assumed to have one outpatient consultation with a dietitian. Patients who tested positive for H. pylori were prescribed 1 week of triple eradication therapy as per BNF guidance110 (costed under medications; see Medication costs), and given a repeat test after 4 weeks to confirm eradication (costed with other tests).
-
Implant procedure – the cost of the implant procedure comprised an assumed cost for consumables specific to the endoluminal DJBL procedure (the device itself and custom catheter and delivery system for endoscope), a day-case endoscopic procedure under general anaesthetic and an overnight stay, if required. The cost of the procedure was based on the current NHS reference cost144 for therapeutic upper GI endoscopy of intermediate complexity (FE03A). A price is not yet publicly available for the endoluminal DJBL device and delivery system so, for the purposes of this evaluation, we have assumed a cost of £1000 per implant. The sensitivity of the cost-effectiveness results to this assumption is tested in sensitivity analyses.
-
Routine follow-up: we assumed one GI outpatient attendance and one diabetes clinic review per patient after the implant procedure. The protocol specifies that patients receive gastro-protection with a PPI (omeprazole 40 mg twice daily) from 3 days prior to implant up to 2 weeks after device removal (included as prescribed under Medication costs).
-
Explant procedure: comprised endoscopic procedure under sedation or general anaesthetic, with an overnight stay if required. As for the implant, we assumed that the cost of the explant would be similar to the current reference cost for an intermediate upper GI therapeutic endoscopic procedure (FE03A).
-
AEs: additional treatments due to AEs classified as definitely, possibly or probably related to the intervention were individually costed. This included tests and medications for GI and other symptoms and repeated attempts at the explant procedure.
Medication costs
Information about prescribed diabetes and concomitant medications before and during the study period was collected for each individual – including dose, frequency and start and stop date. Costs were calculated for any medications that are potentially related to diabetes, obesity or GI symptoms. Where there was uncertainty, costs were included:
-
Unit costs (NHS net prices) were obtained online from the Monthly Index of Medical Specialties on 11 April 2019. 142
-
Costs were estimated for named brands, where specified, or otherwise for the cheapest available brand or generic formulation.
-
Individual specified doses were used for costing. If the dose was missing or ambiguous, we assumed the WHO-defined daily dose. 146
-
For partial dates of starting or ending medication, we made the following assumptions:
-
If the day was unknown, we assumed the first of the month.
-
If the month or year was unknown, we imputed a date of 1 January if it was unambiguous that the start date preceded the date of randomisation (baseline) or that the end date was after the end of follow-up (24 months).
-
Otherwise, we treated the observation as missing.
-
Other health and social services
Information about use of other health and PSSs was collected through a specially designed questionnaire, administered at the same visits as the EQ-5D-5L (see Table 6). The questionnaire asked participants whether or not they had used any of a range of resources since their last visit and, if so, how many times and why.
Resources specifically mentioned on the questionnaire included primary care and community services (including consultations with a GP, practice nurse, dietitian, podiatrist, physiotherapist and counsellor), outpatient consultations, hospital admissions and day-case procedures, and diagnostic tests. In addition, patients were asked about other services that they had used. In cases where the number of consultations was not specified, we assumed a single visit.
Costs were assigned to resources potentially related to diabetes, obesity or GI symptoms – where there was uncertainty, costs were included.
Unit costs were assigned based on NHS Reference Costs for 2017/18144 or the PSSRU Unit Costs of Health and Social Care 2018 report. 143
Costs were collated in four categories (primary and community care, outpatient, inpatient and day case, and tests) and over the six time periods defined in Table 6.
Analysis of utility and cost data
An intention-to-treat (ITT) principle was followed in the analysis of the EQ-5D-5L data and QALY and cost estimates. The difference in mean costs for the intervention group compared with the control group (incremental cost) was estimated with a gamma generalised linear model regression. The difference in mean QALYs between the intervention and control group (incremental effect) was estimated by linear regression with adjustment for baseline utility (at visit 3). We considered alternative model specifications including log-normal for costs and beta for QALYs, and the inclusion of additional baseline covariates in the cost and QALY models (including age, sex, BMI, HbA1c and systolic blood pressure and health-care costs prior to randomisation); however, results did not differ significantly. Results are presented as an incremental cost-effectiveness ratio (ICER): incremental costs divided by incremental effects. This is compared with the conventional upper limit for cost-effectiveness of £30,000 per QALY gained (the cost-effectiveness threshold).
Imputation for missing data
Missing data are a particular problem for trial-based economic evaluations, even in studies with good follow-up. The area under the curve approach to QALY estimation requires utility data from several time points. Similarly, cost estimates over the trial period are based on estimates of resource quantities used over several periods of time. The proportion of participants who can be included in a complete case analysis of costs and QALYs can therefore be greatly reduced, as each person with any missing data point must be excluded.
Multiple imputation was therefore used to reduce the potential for bias due to missing data. We used the Stata MI procedures with a chained predictive mean matching (PMM) procedure to impute missing utility and cost values. This approach has been recommended for cost data, which are constrained to be greater than or equal to zero, and usually has a large positive skew. 147 PMM may also be more appropriate than linear regression-based imputation for utilities, which are not normally distributed and are constrained between a lower and upper bound. Instead of using predicted outcome values from a parametric regression as the imputed values, PMM identifies a number of ‘near neighbours’ (five in our analysis) – cases with outcomes similar to the prediction for the missing case – and randomly selects one of these for the imputed value. This can yield imputed values with a distribution more like that of the real values; however, as PMM still uses a prediction equation, it does require the assumption that data are missing at random (MAR) (i.e. that the probability that data are missing does not depend on unobserved data but may depend on observed data included in the imputation equation).
A single imputation equation was used to impute individual-level missing EQ-5D utilities at all measured time points (visits 3, 5, 6, 7, 8, 10 and 14) and estimated medicine costs and other health-care costs for all time periods (P1 to P6; see Table 6). Intervention cost data were complete. We tested the impact of adding other covariates (age, sex, baseline BMI, HbA1c, systolic blood pressure and costs prior to randomisation) to the imputation equation. These variables were chosen as they had complete data and were potentially associated with utilities, costs and/or the probability of missing data; however, regression analysis did not confirm these potential associations, so they were not included in the final imputation equation.
Total costs and area under the curve QALYs were calculated at the individual patient level from the MI data sets. The mean costs, utilities and QALYs were then re-estimated using the Stata ‘mi estimate’ procedures to adjust coefficients and standard errors for the variability between imputations.
We conducted checks to assess the face validity and stability of the imputations. For cost data, we checked to see if the pre-imputation distribution differed from the post-imputation distribution. We plotted histograms of the total costs at randomly selected iterations of imputation (from a total of 40 imputations) and compared their distributions individually with the distribution of the total costs pre imputation. We found them to be similar for both data sets, suggesting that the post-imputation data were a good fit to the pre-imputation data. Similarly, we found that the histogram of the QALY distribution pre imputation was similar to those at randomly selected imputed iterations.
A choice of 40 imputations was reached after comparing imputation outputs for higher numbers of imputation (60 and 100). Model outputs at higher imputations were comparable to those at 40 imputations with insignificant differences.
Uncertainty analysis
Simple one-way sensitivity analysis was used to assess the impact of key uncertainties on the estimated ICER. In particular, we varied the incremental cost and incremental effect between lower and upper confidence limits. We also varied the assumed cost of the DJBL device and consumables from the baseline value of £1000 per implant up to £2500 and £5000, and down to a lower limit at which the ICER fell below the NICE threshold of £30,000 per QALY gained.
We also used non-parametric bootstrapping to estimate the joint distribution of costs and QALYs. This was conducted using the Stata ‘bsample’ command to draw a series of 1000 non-parametric bootstrap samples (random with replacement and stratified by treatment arm), each drawn from one imputed data set (a random sample from the set of ‘close neighbours’ in the MI PMM procedure described in Imputation for missing data). This approach combines uncertainty due to sampling variation with uncertainty over the imputed missing data; however, we note that other types of uncertainty are not accounted for, including uncertainty over unit costs and non-random missing data not adjusted for in the MI procedure.
The bootstrap results are summarised as 1000 pairs of incremental cost (IC) and incremental effect (IE) estimates. The extent of uncertainty is illustrated with a scatterplot of the 1000 pairs of incremental costs and effects. We also report a 95% confidence interval (CI) for the incremental net benefit (INB) at a ‘willingness-to-pay’ threshold (λ) of £30,000 per QALY gained:
Sample size estimation
The primary end point of a 20% reduction in HbA1c was chosen as the IDF produced new guidelines148 in June 2011 for the conduct of studies in diabetes using metabolic surgery or devices aiming to produce standardisation allowing comparison between studies. To date, there have been no published large patient group studies using this end point, so using the new end point in a well-designed and conducted study may be of scientific value in itself.
The Steno study149 was, to our knowledge, the best quality randomised study (80 patients in each arm) into the effect of best medical therapy published to date. It demonstrated, over an average of 7.8 years, significant improvements in HbA1c among those having intensive medical therapy, from 8.4 ± 1.6 mmol/mol to 7.7 ± 1.2 mmol/mol, but no change in HbA1c among those continuing with standard medical therapy. This study defines the very best that could realistically be achieved in the control arm but expects there to be very little, if any, change in this group. The reporting of HbA1c as an outcome measure was not in accordance with the newly defined IDF criteria, but considering the small average reduction achieved in the Steno study,149 it was assumed that a target of 15% of patients will reach the primary end point. This was a conservative figure and was likely to be an overestimate. Company data on the small number of patients who had reached a year with the device in place suggest that 40% would achieve this target.
According to our own past experience with the device in commercially sponsored studies, up to 30% of patients in the treatment group may have had the device removed early. Nevertheless, other commercially sponsored (unpublished) studies of this device have achieved lower explant rates [Jean-Claude Tetreault, GI Dynamics Inc. (Boston, MA, USA), personal communication, 2019]. We had, therefore, diluted the treatment effect in achieving the target of a 20% reduction in HbA1c for the treatment arm versus the standard arm, from 40% versus 15% to 35% versus 15%. Seventy-three patients per group will give 80% power to detect a significant effect. Adding a 10% loss to follow-up increases the sample size to 80 per group.
The above dilution was calculated starting from the assumption that 40% of patients with the device would reach the target (this estimate was based on company data on patients with T2DM in the same range of BMI as in the present proposal). If 30% of patients in the treatment group needed to remove the device early but remain available for follow-up, in the worst-case scenario the proportion reaching the target is the same as in the control group, bringing the estimated proportion of the treatment group to 32.5%; however, most patients in keeping the device for some time would obtain some benefit. Based on this, it was plausible to assume that the estimate is higher than 32.5%. Dividing the main effect of 15% versus 40% into three parts within the 30% of patients with removal, we estimated that one-third of patients will achieve the same effect as the control group (15% reaching the target), one-third of patients will achieve a marginally increased effect (23% reach the target) and the final one-third of patients will achieve a greater increase in effect (31% reach the target).
Overall, considering the dilution for the 30% of patients with removal, this provided a proportional estimate of 35% for the treatment group. For that reason, two arms of 80 patients should be sufficient to ensure potential demonstration of a significant effect and very conservatively allowed for explant rates of up to 30%, a higher level of benefit in the control arm than predicted and drop-out rates of 10%. Furthermore, the landmark Steno study (which in some ways may be considered similar to this study), which in all likelihood had a less effective intervention arm, was sufficiently powered with 80 patients in each arm.
To account for patients dropping out in the lead-in period from randomisation to intervention, it was considered appropriate, following discussion, to randomise additional patients at each study site (see Table 8).
Statistical analyses
Clinical outcomes
All statistical output was performed according to the ITT principle. Unless specified, any randomised patient was considered part of the analysis population. Patients lost to follow-up were subsequently approached at months 12 and 24 to provide data. Any data collected were included within the ITT analysis.
To account for any potential effect caused by missing HbA1c data within the primary analysis, sensitivity analysis was undertaken. Two methods were used. The first imputed missing data using multiple imputation by chained equations (MICE). Where applicable, we assumed that the probability of missing data was not dependent on the values of the unobserved data and that the data were MAR, conditional on treatment group and stratification factors (BMI groups and sites) as well as on HbA1c values at time points months 3, 6, 9 and 12. Tests based on 10 and 50 imputed data sets were drawn separately for each randomised group, replacing missing outcome values with simulated values from a set of imputation models containing BMI group, sites, HbA1c values at months 3, 6, 9 and 12. Using MICE, missing values for the binary outcome were imputed using a binary logistic model, including all other covariates. Missing values for any of the continuous interim HbA1c included in the imputation model were imputed using linear regression models.
The second method investigated the difference in the proportion of patients who achieved a 20% reduction in HbA1c among those missing compared with those observed in order to observe an alternative result (from that concluded from the ITT analysis). Four scenarios were tested:
-
proportion of patients required in missing data to obtain a significant superior treatment effect by increasing effect rate in treatment arm
-
proportion of patients required in missing data to obtain a significant superior treatment effect by reducing effect rate in control arm
-
proportion of patients required in missing data to obtain a significant superior control effect by increasing effect rate in control arm
-
proportion of patients required in missing data to obtain a significant superior control effect by reducing effect rate in treatment arm.
Analysis was carried out using SAS® software v9.4 (SAS Institute Inc., Cary, NC, USA) using proc logistic for the logistic regression model.
To investigate the primary objective, the difference between the two study groups in the proportion of patients achieving substantial improvement at 12 months was analysed using logistic regression. To take into consideration any potential effects the stratification variables of BMI group and study site may have on the analysis, these two variables were also added to the logistic regression model as shown below:
To investigate any difference in effect following the 12-month period of active intervention, additional analyses were run at 18 months (6 months post removal) and at 24 months (12 months post removal).
Additional secondary efficacy end points were the proportion of patients achieving HbA1c levels of < 6% (or 42 mmol/mol), the proportion of patients achieving blood pressure values of < 135/85 mmHg and the proportion of patients achieving absolute weight loss of > 15% body fat content, which were also assessed using the same logistic regression model defined above and in Recruitment results.
Mechanistic studies
Data from the mechanistic studies were assessed using a mixed-model approach:
In this model:
-
µ is the intercept of the model.
-
τi is the ith fixed treatment, i = 0 (standard therapy) or i = 1 (EndoBarrier).
-
πj is the fixed visit effect at j months where j = 1, . . ., 12.
-
CV1 + . . . + CVr is the fixed effect of covariates 1 to r.
-
bj(k) is the random visit effect at the jth visit month of the kth patient.
-
eijk is the random error associated with the kth patient receiving treatment i at visit j.
-
bj(k) and eijk are independent for i = 0, 1, j = 1, . . ., 5, and k = 1, . . ., n.
Covariates will include age and gender.
Owing to the small size of the subgroups, an additional random effect will not be included in order to keep the nested model as simple as possible. An unstructured covariate structure will be used. Even where convergence has not been met, an alternative structure will be used as appropriate.
Analyses were presented in the form of test results of fixed effects and estimates of model parameters. Post hoc tests were performed on any model parameters with a p-value of < 0.05.
It should be noted that no adjustments or corrections were made for multiple testing within the subgroup analysis. This decision was formed on the basis that subgroup testing was for generating new hypotheses and further discussion, and that any significant results should be considered with this in mind.
Mechanistic studies: additional functional magnetic resonance imaging analysis
Using the above mixed model, where variables are nested an additional fixed effect (and subsequent interaction terms) will be included to account for the nested variable as described below.
For each outcome variable, repeated-measures analyses included only participants who had data from baseline (visit 3) and from at least one subsequent follow-up visit up to week 100 (visit 14). Comparison between groups at baseline used the unpaired Student’s t-test (t-statistic), the Mann–Whitney U-test (z-statistic), if the data were not normally distributed, or the chi-squared test (γ-statistic) for frequencies, as appropriate.
Comparison of data available from only two MRI visits, baseline versus week 26 (visit 8), used repeated measures of analysis of variance (ANOVA), with post hoc Fisher’s least significance difference (LSD) test, including group as a between-patient factor, with time and relevant outcome characteristics (e.g. HF vs. LF, sweet vs. savoury, HE vs. LE food, macronutrients, i.e. carbohydrate, fat and protein content) as within-patient factors.
Comparison of data available from more than two visits used fixed-effects mixed-model repeated measures of ANOVA, with post hoc Fisher’s LSD test, including group as a between-patient factor, with time as a within-patient factor.
Analysis of variance looked for interaction effects for time*group, and main effects of time or group, together with any interactions with relevant outcome characteristics. Analysis was conducted using IBM SPSS Statistics version 24 (IBM Corporation, Armonk, NY, USA), and graphs made using Prism 8 (GraphPad Software Inc., CA, USA).
Interim analysis
Other than the reports prepared for the Data Monitoring and Ethics Committee (DMEC), no formal interim analysis was conducted in the study.
Trial management
The UK Clinical Research Collaboration-registered ICTU was responsible for trial management, quality assurance, trial statistics, development and maintenance of the trial database, and data management. The ICTU core staff and the InForm team were supported by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Trial sponsor
The sponsor of the trial was Imperial College London. Imperial College London signed a clinical trial agreement with each of the participating centres prior to the start of the trial.
Ethics considerations
The trial was conducted in accordance with the Declaration of Helsinki on research involving human patients. The study protocol, PIS and informed consent form were submitted to the REC prior to the start of the study and a favourable opinion was obtained on 10 July 2014 (REC #14/LO/0871).
Research governance
The trial was carried out in accordance with the NHS Research Governance Framework,150 and local NHS permission was granted by the research and development departments at each participating site prior to recruitment commencing.
Regulatory requirements
There was no need to obtain prior regulatory approval from the Medicines and Healthcare products Regulatory Agency for this trial as the trial devices were CE marked and used for their intended purpose.
Trial registration
The trial was registered on the International Standard Randomised Controlled Trial Number clinical trial database with reference ISRCTN30845205.
National Institute for Health Research Clinical Research Network portfolio
The EndoBarrier trial was adopted on the NIHR Clinical Research Network portfolio. Accrual data were uploaded to the NIHR Clinical Research Network database on a monthly basis.
Trial oversight
Steering Committee
A Trial Steering Committee (TSC) was established to oversee the conduct of the study. The TSC met 10 times over the course of the trial. The TSC approved the trial protocol prior to the start of the study and received regular recruitment reports throughout the duration of the trial.
The TSC membership:
-
Independent members –
-
Jonathan Brown (Chairperson)
-
Bu Hayee
-
Edward Fogden.
-
-
Investigators –
-
Julian Teare (Chief Investigator)
-
James Byrne (Principal Investigator).
-
-
Other members –
-
Mary Cross/Claire Smith/Hema Collappen/Natalia Klimowska-Nassar (ICTU Operations Managers)
-
Christina Prechtl (ICTU Trials Manager)
-
Nicholas Johnson (ICTU Trial Statistician)
-
Emmanuela Falaschetti (ICTU Senior Statistician)
-
Aruchuna Ruban (Independent Observer)
-
Michael Glaysher (Independent Observer)
-
Two public members.
-
Data Monitoring and Ethics Committee
An independent DMEC was established to review reports for AEs and protocol deviations, and the results of interim analyses. The DMEC meetings took place on 9 March 2015, 28 September 2015, 25 April 2016, 10 October 2016, 20 March 2017, 9 October 2017, 23 April 2018 and 11 February 2019. The first DMEC meeting was organised to agree the DMEC charter outlining operational details and responsibilities. The DMEC provided feedback reports for each meeting to the chairperson of the TSC and this was reviewed, as applicable, at subsequent TSC meetings.
The DMEC membership:
-
Main members –
-
Stephen Attwood (Chairperson)
-
Jonathan Cook (Independent Statistician)
-
Lorraine Albon (Independent Clinican)
-
Nicholas Johnson (ICTU Trial Statistician).
-
-
Other members (present during open report discussions) –
-
Julian Teare (Chief Investigator)
-
Christina Prechtl (ICTU Trials Manager)
-
Mary Cross/Claire Smith/Hema Collappen/Natalia Klimowska-Nassar (ICTU Operations Managers)
-
Emmanuela Falaschetti (ICTU Senior Statistician).
-
Trial Management Group
A Trial Management Group (TMG) was established to discuss ongoing trials’ issues and day-to-day management of the trial. The meetings took place, on average, every 2 months. The TMG consisted of the chief investigator, ICTU trials manager, operations manager and statisticians as well as staff members from each research site that were working on this trial.
Data collection
The study captured and processed data using the InForm electronic case report form (eCRF), which was built and tested by ICTU. InForm is a fully validated high-quality electronic data capture system, allowing a fully auditable data entry process and controlled level of access. Training was provided to all study staff on use of the database entry system. The patient and any biological material obtained from the patient were identifiable by patient number, trial site and trial identification number.
Appropriate measures such as encryption or deletion were enforced to protect the identity of human patients in all presentations and publications, as required by local, regional and national requirements.
Electronic laboratory data were considered source data such that, in cases where laboratory data were transferred via non-secure electronic networks, data were encrypted.
A summary of the data collected at each study visit can be found in Table 4.
Data and reports were extracted from the database throughout the study to monitor progress. Clinical monitoring was undertaken frequently and routinely to permit the timely collection of safety data. Any unforeseen risks arising during the course of the investigation were evaluated on occurrence and reported in accordance with local government regulations.
Annual safety reports were provided to the REC, in accordance with clinical trial regulations, on the anniversary of the clinical trial authorisation each year. A total of four annual safety reports were submitted over the course of the trial.
Data management
Predefined data ranges were included in the eCRF, which raised automated queries if data outside the expected range were entered. In addition to the automated queries, the trial data were reviewed on a regular basis by the study monitor to look for discrepancies and errors. Furthermore, the trial statistician also performed a series of checks on snapshots of data to look for inconsistencies. The checks performed by the study monitor and statistician were documented in a data management plan, which was updated over the course of the study as required.
Risk assessment and monitoring plan
A risk assessment was performed by the ICTU Quality Assurance Manager prior to the start of the trial. The result of the risk assessment indicated that the study was of low risk and that 20% of trial data, 100% of consent forms and 100% of serious adverse events (SAEs) should be source verified. A monitoring plan was prepared in accordance with the risk assessment to specify the frequency of monitoring visits and number of source data verifications required.
Monitoring visits
A site initiation visit was performed at all participating centres. Interim monitoring visits were carried out approximately four times per site in the first year and then twice annually, depending on site compliance and the recruitment rate. Closeout visits were carried out at all centres following the final follow-up visit for the last patient recruited. The monitoring visits were conducted by the trial manager.
Investigational medicinal product manufacturer
The devices for the EndoBarrier trial were manufactured by GI Dynamics Inc., Boston, MA, USA, and distributed in the UK via Elemental Healthcare, Hungerford, UK.
Patient and public involvement
The TSC membership included two patient representatives who were invited to attend all TSC meetings and were included in all relevant correspondence. The patient representatives were consulted during preparation of the PIS and contributed by suggesting changes to the PIS, including reducing the length and complexity of information.
Chapter 3 Clinical results
Recruitment results
Most patients at Imperial College London self-referred after hearing about the study from newspaper adverts. Over 1000 telephone calls were received from patients following the newspaper adverts. This compared with only 65 patient reply slips received from GP practices. In comparison, University Hospital Southampton NHS Foundation Trust received 397 patient reply slips from GP practices. The different sources of recruitment are shown in Table 7, and Figure 6 shows the CONSORT flow diagram for recruitment from initial enrolment into the study through to randomisation and follow-up over the 2-year period. Table 8 shows patient randomisation and subgroup allocation at each site. A detailed analysis of patient recruitment to the trial has been previously published. 151
| Sources of patient recruitment | Imperial College London | University Hospital Southampton NHS Foundation Trust | Total |
|---|---|---|---|
| GP | 65 | 397 | 462 |
| Newspaper adverts | 1004 | 102 | 1106 |
| Study website | 75 | 9 | 84 |
| DARE | 16 | 0 | 16 |
| Other bariatrics and diabetes clinics | 9 | 9 | 18 |
| Diabetes UK | 7 | 16 | 23 |
| Other: research/science museum | 7 | 0 | 7 |
| Poster | 4 | 3 | 7 |
| Telescreen outpatient clinics | 4 | 0 | 4 |
| Radio station interview | 0 | 2 | 2 |
| Social media [Facebook (Facebook, Inc., Menlo Park, CA, USA; https://en-gb.facebook.com) or Twitter (Twitter, San Francisco, CA, USA; www.twitter.com)] | 4 | 0 | 4 |
| Friend | 1 | 1 | 2 |
| Other/unknown | 14 | 28 | 42 |
| Total | 1210 | 567 | 1777 |
FIGURE 6.
Recruitment CONSORT flow diagram.
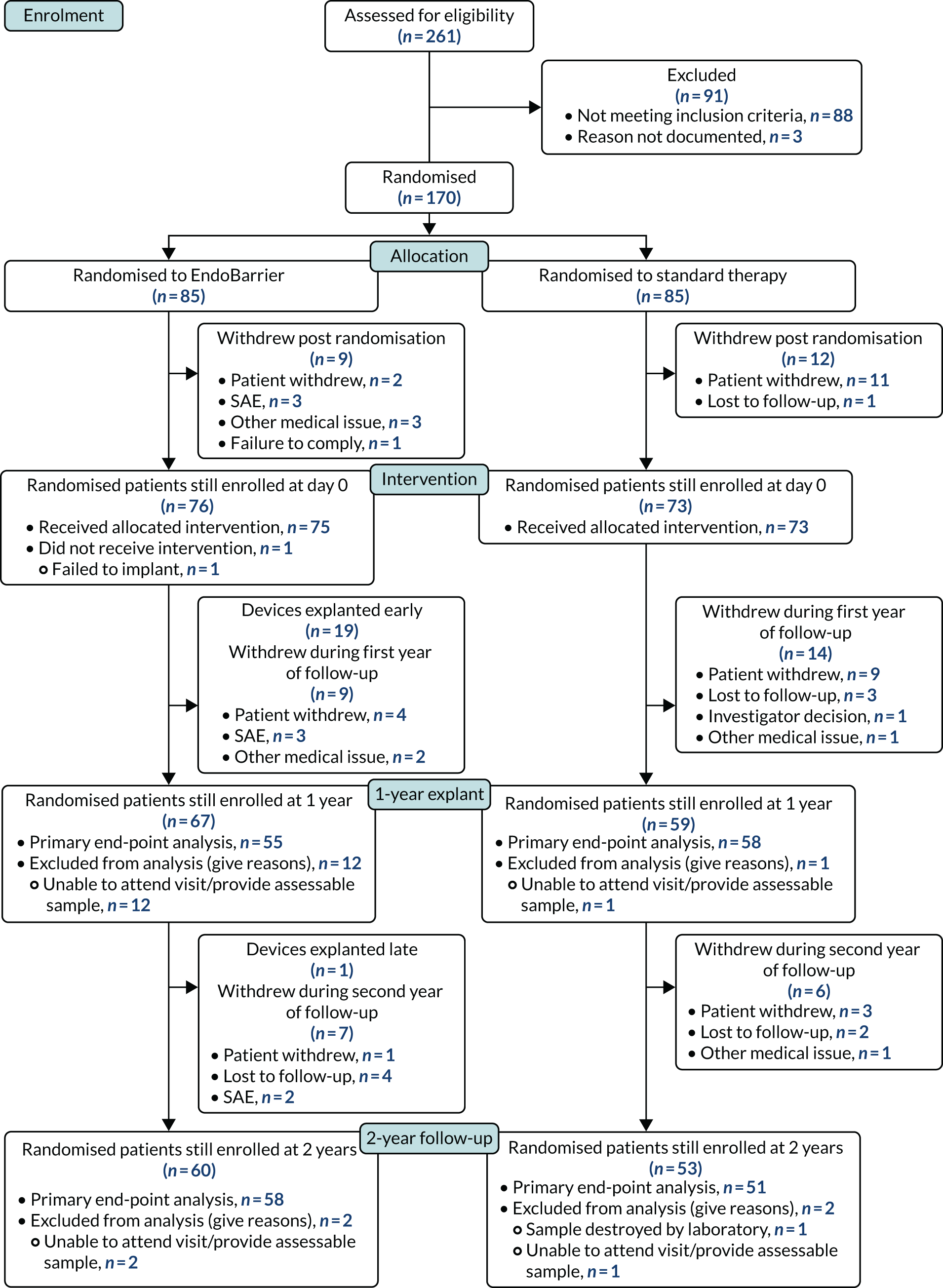
| Site | Standard therapy | EndoBarrier | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Main only | fMRI | Insulin clamp | Food preference | Total | Main only | fMRI | Insulin clamp | Food preference | Total | |
| Imperial College London | 5 | 20 | 0 | 18 | 43 | 5 | 17 | 0 | 20 | 42 |
| University Hospital Southampton NHS Foundation Trust | 8 | 0 | 24 | 10 | 42 | 10 | 0 | 20 | 13 | 43 |
| All sites | 13 | 20 | 24 | 28 | 85 | 15 | 17 | 20 | 33 | 85 |
Baseline demographics are presented in Table 9. Additional baseline output is presented in Appendix 2, Table 23, and shows baseline output based on subgroups split by those who were included in the primary analysis and those who were not. The additional output suggests that there is no difference in characteristics between subgroups such that the ITT population used in the primary analysis is generalisable in comparison with the study population.
| Variable | Statistics | Standard therapy (N = 85) | EndoBarrier (N = 85) | All patients (N = 170) |
|---|---|---|---|---|
| Age (years) | n | 85 | 85 | 170 |
| Mean | 51.9 | 51.6 | 51.8 | |
| SD | 8.46 | 7.94 | 8.18 | |
| Ethnicity (%) | Asian | 9 (10.6) | 11 (12.9) | 20 (11.8) |
| Black | 13 (15.3) | 3 (3.5) | 16 (9.4) | |
| Mixed | 1 (1.2) | 1 (1.2) | 2 (1.2) | |
| White | 62 (72.9) | 70 (82.4) | 132 (77.6) | |
| Gender (%) | Female | 39 (45.9) | 39 (45.9) | 78 (45.9) |
| Male | 46 (54.1) | 46 (54.1) | 92 (54.1) | |
| Height (cm) | n | 85 | 85 | 170 |
| Mean | 51.9 | 51.6 | 51.8 | |
| SD | 8.46 | 7.94 | 8.18 | |
| Weight (kg) | n | 85 | 85 | 170 |
| Mean | 104.24 | 107.89 | 106.07 | |
| SD | 14.914 | 17.059 | 16.079 | |
| BMI (kg/m2) | n | 85 | 85 | 170 |
| Mean | 35.82 | 36.82 | 36.32 | |
| SD | 4.222 | 4.955 | 4.617 | |
| Pulse (b.p.m.) | n | 85 | 85 | 170 |
| Mean | 77.2 | 77.2 | 77.2 | |
| SD | 10.28 | 10.68 | 10.45 | |
| Systolic blood pressure (mmHg) | n | 85 | 85 | 170 |
| Mean | 132.8 | 130.3 | 131.5 | |
| SD | 15.33 | 11.91 | 13.74 | |
| Diastolic blood pressure (mmHg) | n | 85 | 85 | 170 |
| Mean | 83.2 | 82.2 | 82.7 | |
| SD | 10.51 | 9.69 | 10.09 | |
| HbA1c (mmol/mol) | n | 85 | 85 | 170 |
| Mean | 71.19 | 73.66 | 72.42 | |
| SD | 9.697 | 10.284 | 10.042 | |
| BMI stratum, n (%) | 30–40 kg/m2 | 63 (74.1) | 67 (78.8) | 130 (76.5) |
| 40–50 kg/m2 | 22 (25.9) | 18 (21.2) | 40 (23.5) |
Primary end-point analysis
From samples taken just before the 12-month explant visit, 30 (54.5%) patients in the endoluminal DJBL arm achieved a 20% reduction in HbA1c levels, compared with 32 (55.2%) patients in the standard therapy arm. Using logistic regression, adjusting for stratification variables of site and BMI group, the odds ratio (OR) estimate for achieving the target in the endoluminal DJBL arm compared with the standard therapy arm is 0.93 (95% CI 0.44 to 1.98; p = 0.85).
Exploring later visits (Table 10), at the 15-month visit (3 months post explant), 32 (53.3%) patients in the endoluminal DJBL arm achieved a 20% reduction in HbA1c level, compared with 20 (37.7%) patients in the standard therapy arm. At the 18-month visit (6 months post explant), 31 (50.8%) patients in the endoluminal DJBL arm achieved a 20% reduction in HbA1c level, compared with 21 (40.4%) patients in the standard therapy arm. Finally, at the 24-month visit (1 year post explant), 23 (39.7%) patients in the endoluminal DJBL arm achieved a 20% reduction in HbA1c level, compared with 19 (36.5%) patients in the standard therapy arm. The corresponding OR output is 1.81 (95% CI 0.84 to 3.86; p = 0.13) for the 15-month visit, 1.50 (95% CI 0.70 to 3.18; p = 0.30) for the 18-month visit and 1.13 (95% CI 0.52 to 2.47; p = 0.75) for the 24-month visit.
| Time point | Standard therapy | EndoBarrier | ORc (95% CI) | p-valued | ||||
|---|---|---|---|---|---|---|---|---|
| n | Meana (SD) | 20% reduction, n (%)b | n | Mean (SD) | 20% reduction, n (%)b | |||
| Month 11.5 | 58 | 18.19 (18.799) | 32 (55.2%) | 55 | 21.50 (13.944) | 30 (54.5%) | 0.93 (0.44 to 1.98) | 0.85 |
| Month 15 | 53 | 13.92 (15.849) | 20 (37.7%) | 60 | 20.47 (16.673) | 32 (53.3%) | 1.81 (0.84 to 3.86) | 0.13 |
| Month 18 | 52 | 12.86 (16.990) | 21 (40.4%) | 61 | 18.58 (17.229) | 31 (50.8%) | 1.50 (0.70 to 3.18) | 0.30 |
| Month 24 | 52 | 10.70 (18.173) | 19 (36.5%) | 58 | 10.69 (23.092) | 23 (39.7%) | 1.13 (0.52 to 2.47) | 0.75 |
A per-protocol sensitivity analysis was run to investigate whether or not treatment compliance had any bearing on the analysis. Patients with early device removal were removed from the analysis population alongside patients in the control arm who failed to attend dietitian visits in the first year of follow-up. Results were unaffected by this analysis.
To investigate for any potential effect caused by missing data, two missing data analyses were run on the primary outcome measure. The first, a MI model, derived missing values for HbA1c at visit 10 based on HbA1c values obtained at earlier time points. The model adjusted for the stratification variables of site and BMI group. The primary analysis was re-run for the 10 and 50 imputed data sets that were created. Results were combined using Rubin’s rules and indicated conclusively that missing data would not have affected the findings of the primary ITT analysis for either the 10- or the 50-iteration tests.
A second missing data analysis investigated the proportion of patients required within the missing data to change the result of the analysis. In the scenario in which a proportional increase in the endoluminal DJBL arm resulted in a change in result (significant difference in proportions), all 30 patients required a positive result (20% reduction in HbA1c). As the existing proportion was 54.6%, the likelihood of the missing data having a 100% success rate was statistically significant, therefore indicating that such a change in rates was not feasible. The same result was concluded from the remaining three scenarios: proportional increase in standard therapy arm to obtain a significant result, proportional decrease in standard therapy arm to obtain a significant result and proportional decrease in treatment arm to obtain a significant result. Both sets of missing data analysis conclude that the extent of missing trial data would not have had any bearing of the primary analysis.
Secondary analysis: clinical end points
Reduction of HbA1c levels over time
Over time, both treatment arms displayed a reduction in absolute HbA1c levels (Figure 7 and Table 11), with the greatest levels of reduction seen at 3 months. This level of reduction remains consistent in the treatment arm across the whole of the 12-month treatment period. However, in the standard therapy arm the mean change from baseline starts to increase again, changing from –16.49 mmol/mol at 3 months to –13.29 mmol/mol in the visit prior to explant (month 11.5). This pattern continues in the second year of follow-up such that, by the end of the study period, the mean change from baseline is half that of its peak value (–8.02 mmol/mol at 24 months). The endoluminal DJBL treatment arm sustains the treatment effect for longer; at 18 months the mean change from baseline is still –13.98 mmol/mol. However, in the final 6 months of follow-up the treatment effect reduces, and by 24 months the mean change from baseline is similar to that found in the control arm (–8.6 mmol/mol). Despite the suggested difference, a post hoc analysis using a mixed-model approach did not indicate a significant difference in performance between the two treatment arms.
FIGURE 7.
Change in HbA1c levels over time.
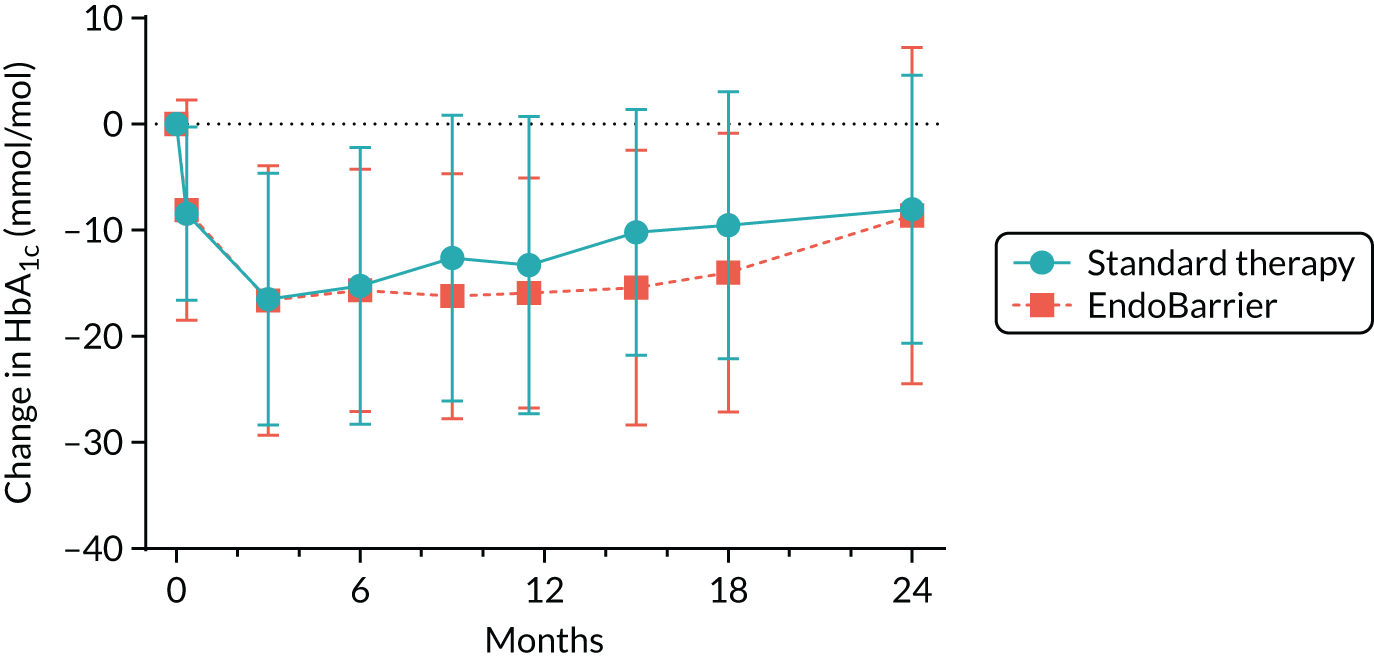
| Visit | Time point | Standard therapy | EndoBarrier | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| 5 | Day 10 | 68 | –8.44 (8.16) | 73 | –8.11 (10.364) |
| 7 | Month 3 | 63 | –16.49 (11.871) | 68 | –16.59 (12.716) |
| 8 | Month 6 | 61 | –15.23 (13.033) | 63 | –15.63 (11.44) |
| 9 | Month 9 | 57 | –12.61 (13.447) | 63 | –16.19 (11.562) |
| 10 | Month 11.5 | 58 | –13.29 (14.031) | 55 | –15.89 (10.847) |
| 12 | Month 15 | 53 | –10.17 (11.594) | 60 | –15.38 (12.994) |
| 13 | Month 18 | 50 | –9.54 (12.604) | 60 | –13.98 (13.114) |
| 15 | Month 24 | 51 | –8.02 (12.636) | 58 | –8.6 (15.817) |
Rates of patients achieving glycaemic targets
Investigating the secondary end point of patients with a HbA1c level of < 42 mmol/mol at 1 year, six (10.9%) patients achieved the required HbA1c level in the endoluminal DJBL arm, compared with four (6.9%) patients in the standard therapy arm. Using logistic regression, adjusting for the stratification variables of site and BMI group, the OR estimate for achieving the target in the endoluminal DJBL arm compared with the standard therapy arm is 2.15 (95% CI 0.54 to 8.55; p = 0.28). Post explant, at 15, 18 and 24 months, the numbers of patients who reach the remission level in the endoluminal DJBL arm and the standard therapy arm are three (5.0%) and two (3.8%), three (5.0%) and two (4.0%), and three (5.2%) and zero (0.0%), respectively.
Post hoc exploratory analysis of number of glucose-lowering medications
At the start of the treatment period (day zero), in the endoluminal DJBL arm, 20 (29.9%) patients were on one class of diabetes medication, 28 (41.8%) were on two classes, 15 (22.4%) were on three classes and four (6.0%) were on four classes. In comparison, the standard therapy arm had 18 (31.0%) patients on one class of diabetes medication; 27 (46.6%) on two classes, 12 (20.7%) on three classes and 1 (1.7%) on four classes. No significant difference between groups in the number of medications on the day of intervention was reported (Table 12).
| Medications at day zero | Standard therapy | EndoBarrier | Total |
|---|---|---|---|
| 1 | 18 (31.0%) | 20 (29.9%) | 38 |
| 2 | 27 (46.6%) | 28 (41.8%) | 55 |
| 3 | 12 (20.7%) | 15 (22.4%) | 27 |
| 4 | 1 (1.7%) | 4 (6.0%) | 5 |
| Total | 58 | 67 | 125 |
At 12 months in the endoluminal DJBL arm, 13 (19.4%) patients recorded a decrease in the number of medications taken, whereas 19 (28.4%) recorded an increase (Table 13). In comparison, in the standard therapy arm 10 (17.2%) patients recorded a decrease in the number of medications taken whereas another 10 (17.2%) patients recorded an increase. Thirty-four (50.8%) patients in the treatment arm recorded no difference in the number of medications taken, compared with 38 (65.5%) patients in the control arm. Chi-squared testing revealed no significant difference between the two treatment arms.
| Change at 12 months | Standard therapy | EndoBarrier | Total |
|---|---|---|---|
| Decrease | 10 (17.3%) | 13 (19.4%) | 23 |
| Increase | 10 (17.2%) | 19 (28.4%) | 29 |
| N/A | 0 (–) | 1 (1.5%) | 1 |
| None | 38 (65.5%) | 34 (50.8%) | 72 |
| Total | 58 | 67 | 125 |
At 24 months in the endoluminal DJBL arm, four (6.0%) patients recorded a decrease in the number of medications taken from 12 months whereas 14 (20.9%) patients recorded an increase (Table 14). In comparison, in the standard therapy arm, three (5.1%) patients recorded a decrease from 12 months whereas 16 (27.6%) patients recorded an increase. Thirty-nine (58.2%) patients in the treatment arm recorded no difference in the number of medications taken, compared with 33 (56.9%) in the standard therapy arm. Again, chi-squared testing revealed no significant difference between the two treatment arms.
| Change at 24 months | Standard therapy | EndoBarrier | Total |
|---|---|---|---|
| Decrease | 3 (5.1%) | 4 (6.0%) | 7 |
| Increase | 16 (27.6%) | 14 (20.9%) | 30 |
| N/A | 6 (10.3%) | 10 (14.9%) | 16 |
| None | 33 (56.9%) | 39 (58.2%) | 72 |
| Total | 58 | 67 | 125 |
A follow-up analysis incorporating ‘change in number of treatments at 12 months’ and ‘number of treatments at baseline’ as additional covariates in the primary end point analysis model does not affect the main outcome of treatment effect. Likewise, the corresponding interaction term between ‘change in number of treatments at 12 months’ (increase or decrease) and treatment group was non-significant.
Reduction of weight over time
Investigating the change in weight over time, both treatment groups display a loss in weight over the 2-year follow-up period. After an initial mean drop of 4.19 kg in the control arm, the reduction gradually increases over the first 6 months, peaking at 6.3 kg. This level of reduction appears to remain over the treatment period before steadily declining to 4.82 kg by 24 months. In contrast to the change from baseline in HbA1c here we see a pronounced difference in weight reduction when comparing the endoluminal DJBL arm. By 6 months, the mean weight reduction is at 10.82 kg, and weight loss continues, peaking at 11.74 kg. After the explant visit, the mean weight reduction decreases in a consistent manner to 5.4 kg at 24 months, just 0.6 kg greater than in the control arm. Table 15 and Figure 8 display the mean and SD values taken across the 2-year follow-up period.
| Time point | Standard therapy | EndoBarrier | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Day 10 | 70 | –4.19 (2.023) | 73 | –6.06 (2.77) |
| Month 1 | 69 | –4.75 (3.031) | 70 | –7.39 (2.912) |
| Month 3 | 64 | –5.61 (4.424) | 69 | –9.33 (4.983) |
| Month 6 | 61 | –6.3 (5.53) | 63 | –10.82 (5.393) |
| Month 9 | 57 | –5.82 (6.262) | 63 | –11.02 (6.127) |
| Month 11.5 | 58 | –6.17 (6.389) | 54 | –11.74 (6.841) |
| Month 12 (explant) | 54 | –5.57 (6.355) | 66 | –11.43 (7.401) |
| Month 15 | 53 | –5.14 (6.381) | 60 | –8.44 (6.732) |
| Month 18 | 52 | –4.51 (6.459) | 61 | –6.68 (5.695) |
| Month 21 | 48 | –5.31 (5.663) | 48 | –5.87 (5.739) |
| Month 24 | 51 | –4.82 (6.183) | 58 | –5.4 (5.849) |
FIGURE 8.
Change in weight levels over time.

Rates of patients achieving 15% weight loss
Investigating weight loss at explant, 16 (24.2%) patients achieved in the endoluminal DJBL arm achieved a 15% reduction, compared with two (3.7%) patients in the standard therapy arm. Using logistic regression, adjusting for the stratification variables of site and BMI group, the OR estimate for achieving the target in the endoluminal DJBL arm compared with the standard therapy arm is 8.33 (95% CI 1.78 to 39.0; p = 0.001; Table 16). Investigating time points post explant visit, the number of patients achieving a 15% reduction reduces to six (10.0%) in the endoluminal DJBL arm and one (1.9%) in the standard therapy arm. Logistic regression fails to return a significant result at 15 months (p = 0.12) and non-significant results are also found at 18 and 24 months (see Table 16).
| Time point | Standard therapy | EndoBarrier | OR (95% CI)c | p-valued | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD)a | 15% reduction, n (%)b | n | Mean (SD) | 15% reduction, n (%)b | |||
| Month 11.5 | 58 | 5.94 (5.844) | 2 (3.5%) | 55 | 10.88 (4.657) | 13 (23.6%) | 8.50 (1.77 to 41.0) | 0.008 |
| Month 12 | 54 | 5.38 (5.800) | 2 (3.7%) | 66 | 10.61 (6.160) | 16 (24.2%) | 8.33 (1.78 to 39.0) | 0.007 |
| Month 15 | 53 | 4.98 (5.809) | 1 (1.9%) | 60 | 7.84 (5.657) | 6 (10.0%) | 5.70 (0.65 to 49.66) | 0.12 |
| Month 18 | 52 | 4.33 (5.910) | 1 (1.9%) | 61 | 6.24 (5.079) | 3 (4.9%) | 2.82 (0.27 to 29.08) | 0.38 |
| Month 24 | 52 | 4.61 (5.702) | 1 (1.9%) | 58 | 5.08 (5.379) | 3 (5.2%) | 2.80 (0.27 to 28.54) | 0.39 |
Rates of patients achieving blood pressure targets
A potential treatment effect in reducing hypertension (defined as blood pressure of < 135/85 mmHg) was tested; at the visit just prior to explant, 39 (70.9%) patients in the endoluminal DJBL arm achieved a reading below the required level, compared with 35 (60.3%) in the standard therapy arm. Using logistic regression, adjusting for the stratification variables of site and BMI group, the OR estimate for achieving the target in the endoluminal DJBL arm compared with the standard therapy arm was 1.51 (95% CI 0.68 to 3.34; p = 0.31; Table 17). At 1 year, 45 (68.2%) patients in the endoluminal DJBL arm, compared with 24 (44.4%) in the standard therapy arm achieved a reading below the required level, providing an OR estimate of 8.33 (95% CI 1.78 to 8.33; p = 0.014; see Table 17).
| Time point | Standard therapy | EndoBarrier | OR (95% CI)b | p-valuec | ||
|---|---|---|---|---|---|---|
| N | Non-hypertensive, n (%)a | N | Non-hypertensive, n (%)a | |||
| Month 11.5 | 58 | 35 (60.3%) | 55 | 39 (70.9%) | 1.51 (0.68 to 3.34) | 0.31 |
| Month 12 | 54 | 24 (44.4%) | 66 | 45 (68.2%) | 2.57 (1.21 to 5.48) | 0.014 |
| Month 15 | 53 | 32 (60.4%) | 60 | 31 (51.7%) | 0.77 (0.36 to 1.66) | 0.50 |
| Month 18 | 52 | 32 (61.5%) | 61 | 33 (54.1%) | 0.81 (0.37 to 1.75) | 0.59 |
| Month 24 | 52 | 33 (63.5%) | 58 | 31 (53.5%) | 0.72 (0.33 to 1.59) | 0.42 |
Safety analysis
Safety: frequency in adverse events
In the EndoBarrier study, 857 AEs were reported among 151 (89%) randomised patients. Fifty of these were reported to be SAEs, which occurred in 40 (24%) patients. Of the SAEs, 26 were classified as unexpected, 5 in the standard therapy arm and 21 in the endoluminal DJBL arm.
Investigating the difference in frequency of events between groups shows a difference in terms of the overall number of events and a large difference in the number of SAEs. Breaking the figures down by site also indicates that a higher frequency of events was recorded in Southampton. More details on the frequency of AEs and SAEs across both sites can be found in Appendix 2, Tables 24–26.
Comparing the two arms shows that 59 (69%) patients in the endoluminal DJBL arm and 12 (14%) in the standard therapy arm reported an AE definitely related to the treatment.
Serious adverse events
Of the 50 SAEs, 45 (90%) were reported in the endoluminal DJBL arm, 35 of which were reported under the category ‘Required inpatient hospitalisation/prolongation of existing hospitalisation’. Eight events were reported under the category ‘Other medically important event’ and one event was reported as life-threatening. Twenty-six of the 45 SAEs (57.8%) were deemed to be definitely related to the study treatment. Of the five SAEs in the standard therapy arm, one was reported as life-threatening; the other four were recorded under ‘Required inpatient hospitalisation/prolongation of existing hospitalisation’. All five events were unrelated to the study treatment.
There were two procedure-related AEs; both related to failed explantation of the device. On one occasion the device could not be removed as food debris obscured views and as a result the patient had to book a repeat procedure under general anaesthetic, which was successful on this second attempt. Another device could not be removed endoscopically as the device appeared tethered to the duodenum and would not collapse down safely to be retrieved. This patient required laparoscopic removal under a general anaesthetic and stayed in hospital for 1 week for the procedure and post-operative recovery before being discharged with no permanent sequelae.
There was one reported liver abscess in a patient who presented to the University Hospital Southampton site 11 months after the initial implant with a 1-week history of malaise, fevers and arthralgia. Blood tests revealed raised inflammatory markers (white cell count 21.4 109/l, C-reactive protein 304 mg/l) and deranged liver function tests [bilirubin 35 mg/dl, alanine aminotransferase (ALT) 366 U/l, alkaline phosphatase 462 IU/l]. Abdominal computed tomography revealed a large liver abscess, which was treated with intravenous antibiotics, fluids and analgesia. Computerised tomography-guided drainage of this abscess was performed, and the device was removed under general anaesthetic. The patient required inpatient care for 11 days and received antibiotics for a further month but subsequently made a full recovery.
A total of eight torn devices were noted on explant in this study.
Other safety parameters
Analytes for clinical biochemistry, haematology and vital signs were assessed and reported to the DMEC throughout the study. The DMEC did not indicate any concern for patient safety based on the presented data and the study was allowed to complete its 2-year follow-up period.
Chapter 4 Mechanistic study results
Subgroup 1: functional magnetic resonance imaging
Participants in functional magnetic resonance imaging study visits
Baseline characteristics for those participants completing the fMRI tasks can be found in Appendix 2, Table 27.
Food evaluation functional magnetic resonance imaging task
The appeal of food pictures decreased at week 26 in both groups. Although the decrease over time was larger in the endoluminal DJBL group than in the standard treatment group, and the decrease over time for HE foods was greater than that found for LE foods across both groups, neither result proved significant (Figures 9 and 10). However, neither endoluminal DJBL insertion nor standard therapy changed the BOLD signal in a priori reward system fROIs during evaluation of any food pictures at week 26 (see Figure 11).
FIGURE 9.
Appeal rating of HE and LE foods from food evaluation fMRI task. *p = < 0.05.
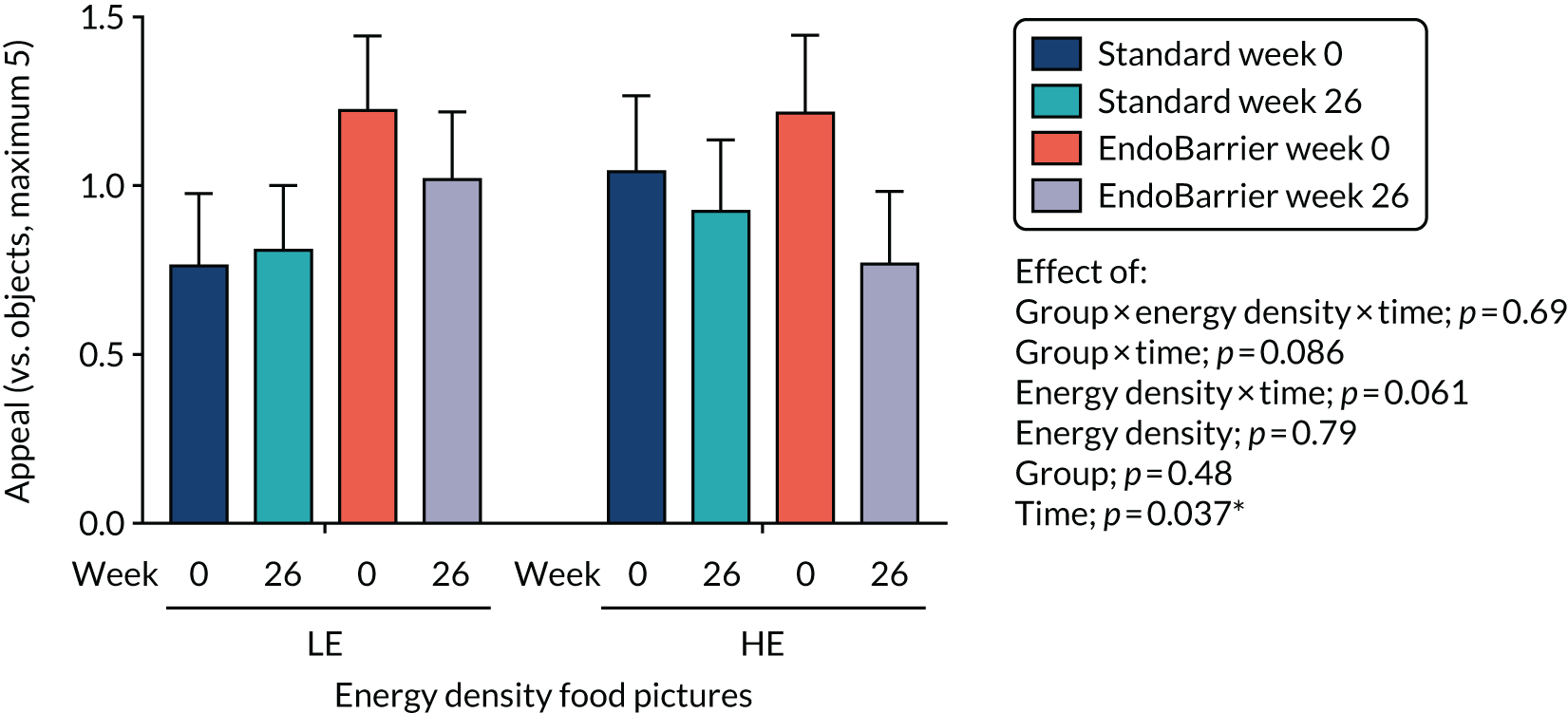
FIGURE 10.
The BOLD signal to HE and LE foods from food evaluation fMRI task. Average six fROIs.
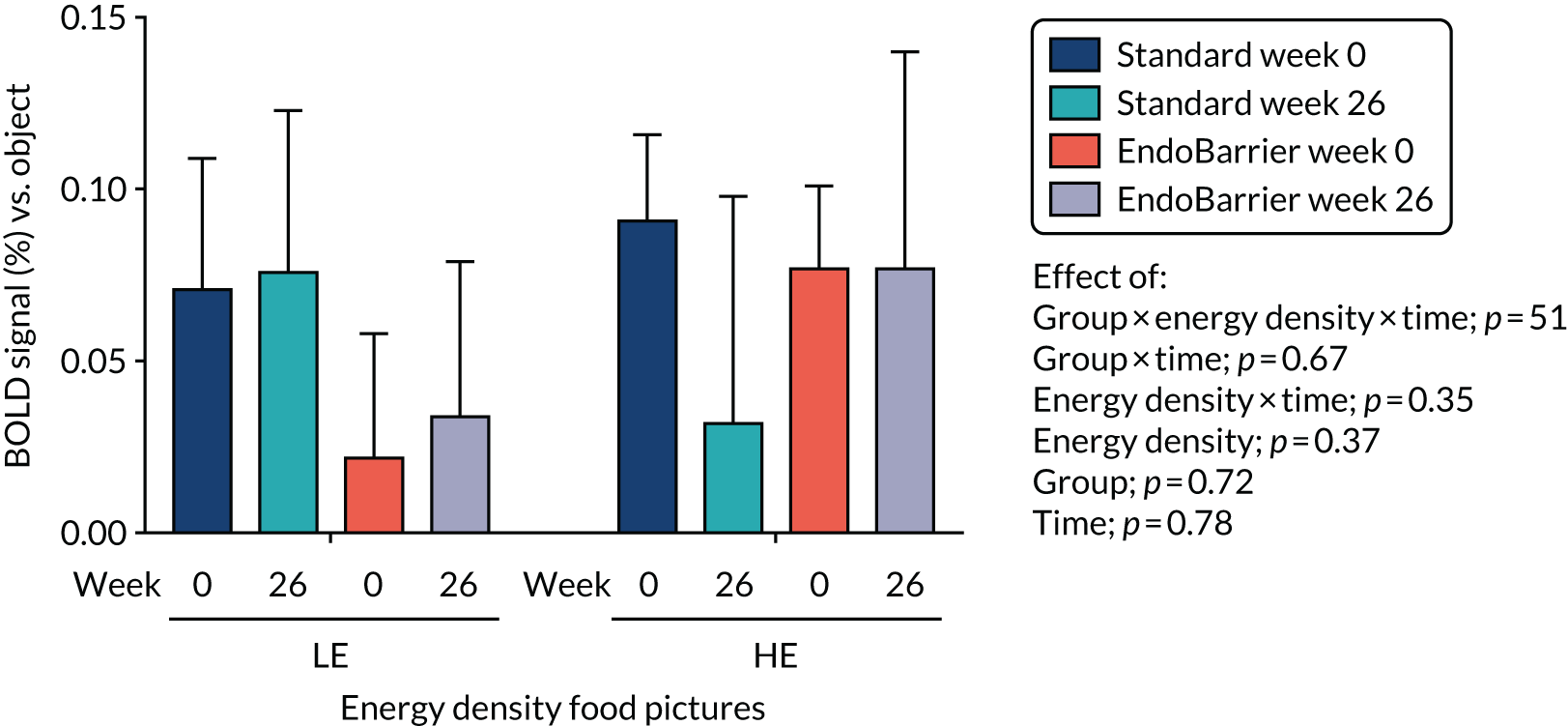
Useable data were available for 11–13 patients in the standard treatment group and 12 patients in the endoluminal DJBL group for analysis of behavioural outcomes (picture appeal rating) both at baseline (visit 3) and at 6 months (visit 8).
Comparison of appeal rating of foods (vs. objects) between standard treatment and EndoBarrier groups over time. Data are presented in Figure 9 as mean ± standard error of the mean (SEM) (n = 12–13) and the statistics are from repeated measures ANOVA, with group (standard and EndoBarrier) as a between-patient factor, and time (0 and 26 weeks), food category (LE, HE) and fat (LF, HF) as within-patient factors (p < 0.05).
The comparison of BOLD signal during the evaluation of HE or LE foods (vs. objects) was averaged across all fROIs (i.e. amygdala, anterior insula, orbitofrontal cortex, nucleus accumbens, putamen and caudate) between the standard treatment group and the EndoBarrier group over time. Data are presented in Figure 10 as mean ± SEM (n = 11–12) and statistics are from repeated measures ANOVA, with group (standard and EndoBarrier) as a between-patient factor, and time (0 and 26 weeks), food category (LE and HE) and fat (LF and HF) as within-patient factors (p < 0.05).
Leeds Food Preference Questionnaire
There were no differences in the effects of endoluminal DJBL insertion and standard therapy on measures of explicit liking and wanting, and implicit wanting, for sweet versus savoury or HF versus LF foods, although both explicit measures fell similarly in both groups at week 26 (see Appendix 2, Figure 18).
Participants in both the fMRI and the taste mechanistic subgroups completed the LFPQ after an overnight fast so that the results could be combined into a single analysis. Including participants who had LFPQ data from both a baseline visit and at least one subsequent visit, and omitting incomplete or corrupted data (e.g. only one of two picture runs done), resulted in LFPQ data being available at visit 3 (baseline, week 0) for 28 participants in the standard therapy group and 30 participants in the endoluminal DJBL group, at visit 8 (26 weeks post endoluminal DJBL insertion) for 25 and 30 participants, respectively, at visit 10 (50 weeks post endoluminal DJBL insertion) for 27 and 24 participants, respectively, and at visit 14 (100 weeks post endoluminal DJBL insertion, 50 weeks post endoluminal DJBL removal) for 22 and 21 participants, respectively. Baseline characteristics can be found in Appendix 2.
Explicit wanting of all foods fell slightly after both interventions at week 26, and by a similar degree, but returned to baseline by week 50 and week 100.
Participants had a greater implicit wanting for sweet over savoury foods, but not HF over LF foods, which was stable over time and did not differ between standard therapy and endoluminal DJBL interventions.
Ad libitum test lunch meal
For the fMRI mechanistic subgroup, data were available for the ad libitum test lunch meal at both visit 3 (baseline) and visit 8 (26 weeks post endoluminal DJBL insertion) for 13 participants in the standard therapy group and 12 participants in the endoluminal DJBL group. Two participants (one from each group) chose the tomato soups but the majority of participants had the chicken soups. See Appendix 2, Table 28, for participant demographics at baseline.
Taste ratings
The creaminess intensity of sweet foods (yoghurt and ice cream) and sweetness intensity of HF sweet foods (ice cream) both fell at week 26 in the endoluminal DJBL group, but not in the standard therapy group (see Appendix 2, Figure 19).
Creaminess intensity
Endoluminal DJBL insertion, but not standard therapy, preferentially reduced the creaminess intensity of tasted sweet foods (yoghurt and ice cream) at week 26.
Pleasantness
Neither endoluminal DJBL insertion nor standard therapy changed the pleasantness rating of any tasted individual dish or food category (sweet, savoury, LF, HF) at week 26 (see Appendix 2, Figure 19).
Sweetness intensity
Standard therapy had opposite effects to endoluminal DJBL insertion (increasing vs. decreasing) on the sweetness intensity rating of tasted sweet HF food (ice cream) at week 26 (see Appendix 2, Figure 19).
Lunch energy intake
Neither endoluminal DJBL insertion nor standard therapy changed total energy, dish or macronutrient intake at the ad libitum test meal at week 26 (see Appendix 2, Figure 20).
Progressive ratio task
Neither endoluminal DJBL insertion nor standard therapy changed the motivation to receive a sweet taste using the PRT break point at week 26, in similar post-prandial appetite states (Figure 11).
FIGURE 11.
Appetite VAS ratings and break points from the PRT. (a) Fullness VAS; (b) appetite VAS; (c) total clicks; and (d) last completed click. Data presented as mean ± SEM (n = 11–12). SEM, standard error of the mean.

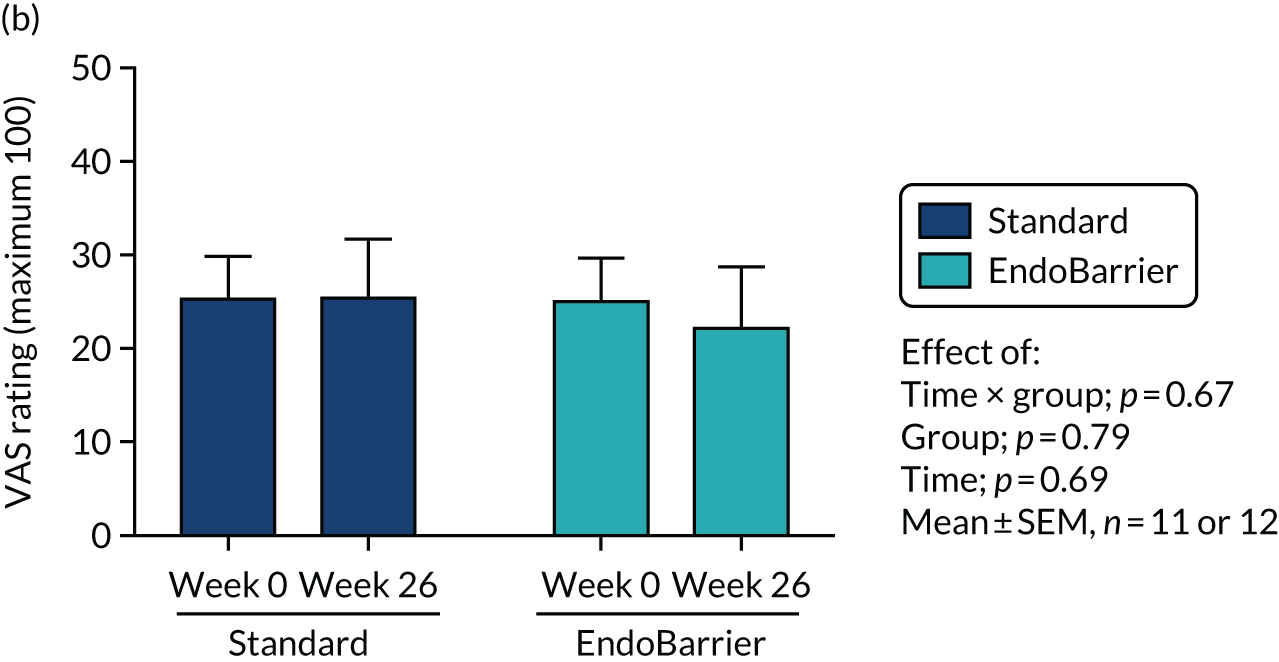

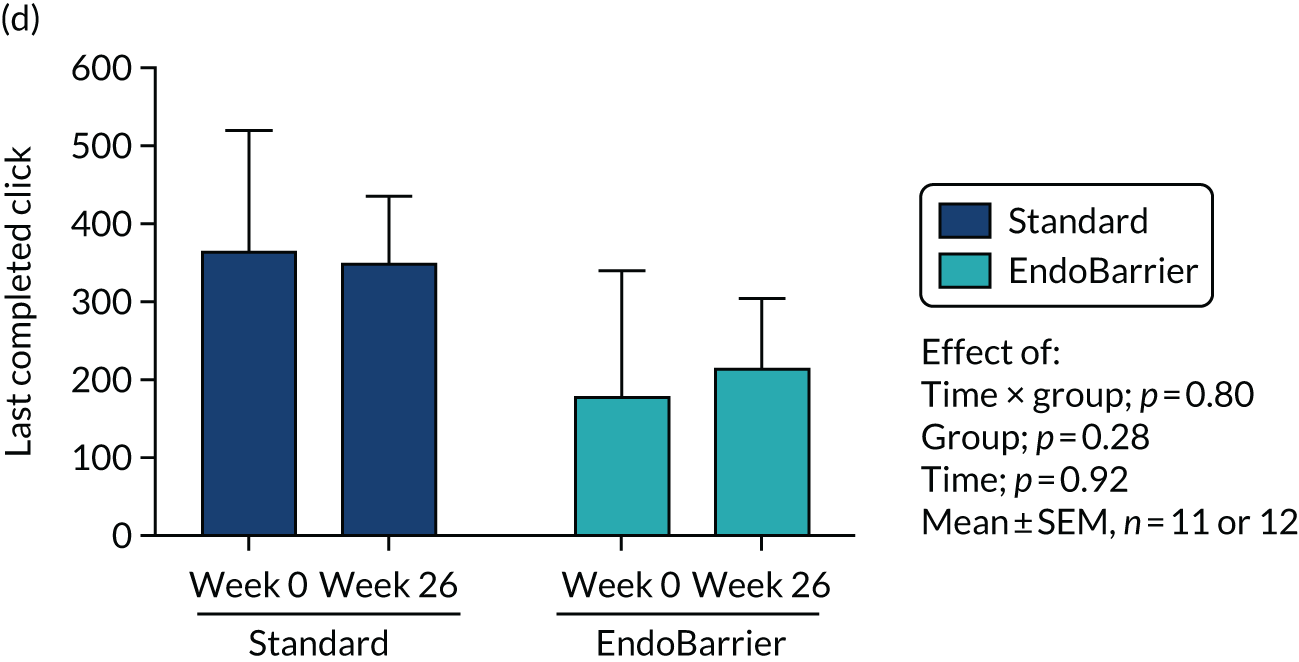
For the fMRI mechanistic subgroup, useable data were available for the PRT at both visit 3 (baseline) and visit 8 (26 weeks post endoluminal DJBL insertion) for 11 standard therapy and 11 endoluminal DJBL patients. On average, the PRT was performed at 2.58 ± 0.25 hours (mean ± SD) (range 2.12–3.10 hours) after the start of the ad libitum meal.
Figure 11(a) and (b) show VAS ratings for fullness and composite appetite just before the task, and Figure 11(c) and (d) show outcome measures from the PRT, performed 2–3 hours after the ad libitum lunch meal. Figure 11(c) shows total clicks completed and Figure 11(d) shows last completed click (break point) to receive an M&M sweet between the standard treatment group and the EndoBarrier group over time at baseline (week 0) or at 26 weeks. The statistics were from two-way repeated measures ANOVA.
Fasting appetite visual analogue scale ratings and sleep
When fasted overnight, hunger and appetite fell similarly after both interventions at week 2 (although this was also after a period of reduced energy intake with a liquid diet), but returned towards baseline from week 26 onwards (Figure 12). Sleep was unchanged after both interventions from weeks 2 to 100.
FIGURE 12.
Appetite VAS ratings and sleep. Comparison of VAS ratings after an overnight fast of (a) hunger, (b) composite appetite, (c) sleepiness and (d) Pittsburgh Sleep Quality Index (PSQI) score, between standard treatment (blue unfilled circles, solid line) and EndoBarrier (orange filled circles, dotted line) groups over time (week 0 at baseline, and 2, 26, 50 and 100 weeks after EndoBarrier insertion; black bar indicates period when EndoBarrier in situ). Data presented as mean ± SEM, numbers at each time point are given beneath graph. Fixed-effects mixed-model ANOVA with post hoc Fisher’s LSD test: p-value vs. week 0. For ANOVA results see Appendix 2, Table 30.
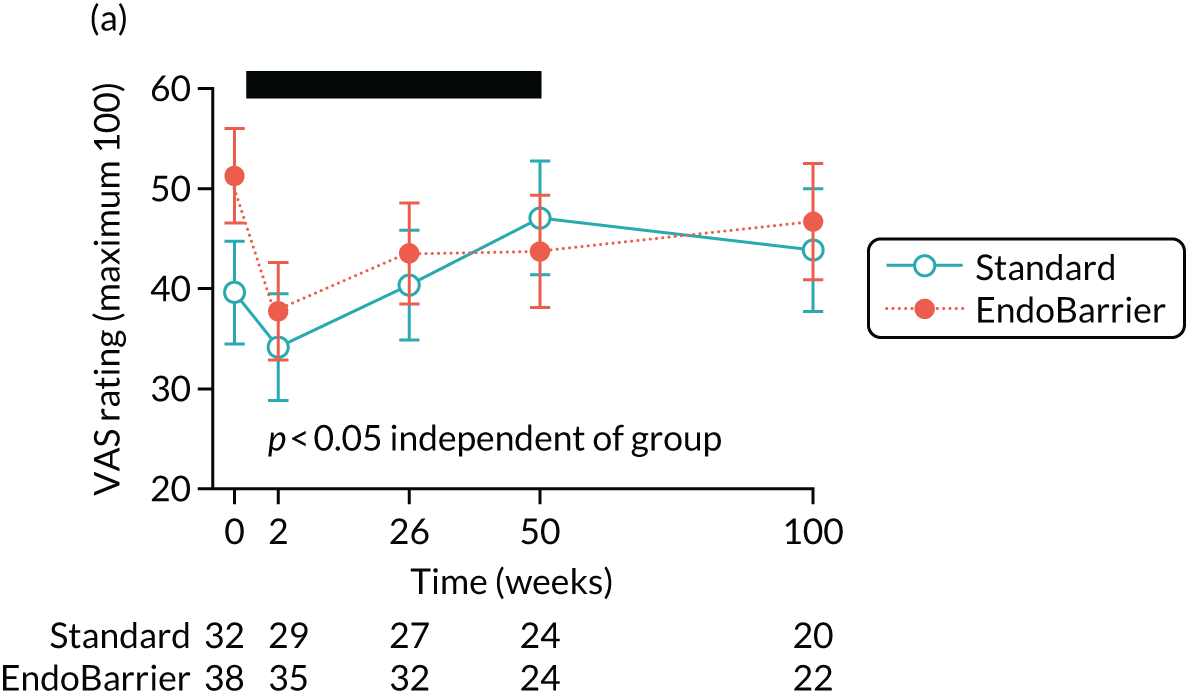


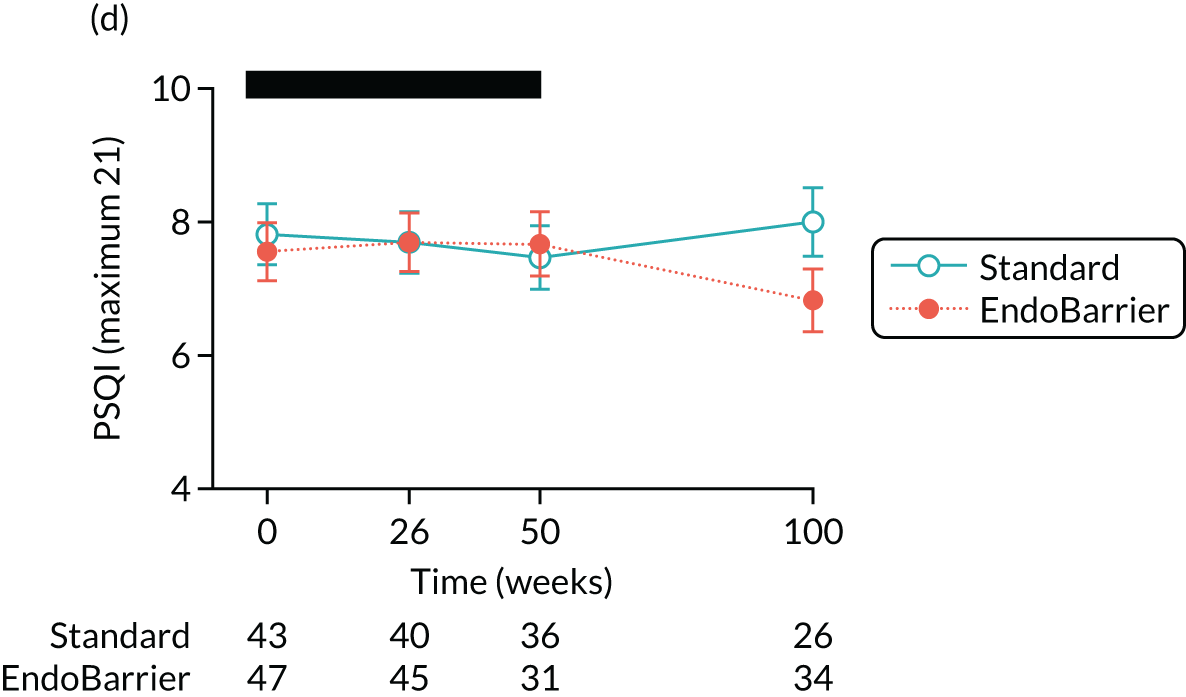
Eating behaviour questionnaires
By week 26, and lasting to week 50 or 100, both the endoluminal DJBL insertion group and the standard therapy group had healthier eating behaviours, with similar increases in dietary restraint, and similar decreases in food hedonics (external eating, binge eating, ‘food addiction’) (see Appendix 2, Figure 21), hunger-related eating, disinhibited eating (see Appendix 2, Figure 22), and symptoms of dumping syndrome (see Appendix 2, Figure 23). They also had similar improvements in general and weight-related quality of life, but neither intervention had any noticeable effect on emotional eating, anxiety or stress.
Although overnight fasting nausea increased more in the endoluminal DJBL group than in the standard therapy group at week 2 (although this was also after a period of reduced energy intake with a liquid diet in both groups), this subsequently settled, and, in fact, nausea was lower in the endoluminal DJBL group than in the standard therapy group from week 26 onwards (see Appendix 2, Figure 23).
Subgroup 2: insulin sensitivity
Thirty-five patients provided additional consent to take part in the insulin clamp subgroup of the EndoBarrier trial. Two patients in the endoluminal DJBL arm and one patient in the control arm of the study withdrew from the subgroup following the first clamp and were not included in the final analysis. The baseline characteristics of the two groups are presented in Appendix 2, Table 31. Of note, the control group in this substudy had a significantly lower baseline BMI than the endoluminal DJBL group (33.9 ± 3.3 vs. 36.8 ± 5.0; p = 0.029). Groups were otherwise comparable at their baseline visit.
Anthropometric outcomes
Anthropometric outcomes are summarised in Appendix 2, Table 32. Both groups significantly reduced their weight between their baseline clamp visit and their second visit at 10 days. Between 10 days and 6 months, the weight in the control group plateaued whereas weight in the endoluminal DJBL group continued to reduce significantly. There was no significant difference in weight between arms at each of the three study visits but absolute and total percentage weight loss at 10 days and 6 months were significantly greater in the endoluminal DJBL group than in the control group.
Glycaemic control
Glycaemic control outcomes are summarised in Appendix 2, Table 33. In the endoluminal DJBL arm there was a significant reduction in fasting glucose and insulin levels that was maintained at 6 months. In the control group, there was also a significant reduction in fasting glucose and insulin levels at 10 days, but these had increased significantly again by 6 months. There was no significant difference in fasting glucose values between groups at baseline, 10 days or 6 months. Differences in fasting insulin levels between the endoluminal DJBL group and the control group failed to reach statistical significance (p = 0.101). Overall glycaemic control, as demonstrated by a decrease in HbA1c, significantly improved in both groups at 10 days. In the endoluminal DJBL group, HbA1c significantly decreased further between 10 days and 6 months, whereas levels plateaued in the control group by 6 months. There was no significant difference in HbA1c between groups at baseline, 10 days or 6 months. There was a reduction in the median number of glucose-lowering medications taken by patients in the endoluminal DJBL group, but this was not statistically significant when compared with the control group.
Insulin resistance outcomes
Insulin resistance outcomes are summarised in Appendix 2, Table 34. There were significant reductions in Ra within both groups at 10 days compared with baseline. This was maintained at 6 months in the endoluminal DJBL group but returned to levels similar to those at baseline in the control group. There were no differences between groups (Figure 13). There were significant increases in Rd at 10 days in both groups but no differences between the groups. Rd continued to increase significantly only in the endoluminal DJBL group at 6 months but returned to levels similar to baseline in the control group. Rd was significantly higher in the endoluminal DJBL group than in the control group at 6 months (Figure 14).
FIGURE 13.
Change in HGP (Ra) during hyperinsulinaemic–euglycaemic clamp. (a) Low-dose Ra; and (b) corrected low-dose Ra.


FIGURE 14.
Change in peripheral glucose disposal (Rd) during hyperinsulinaemic–euglycaemic clamp. (a) High-dose Rd; and (b) corrected high-dose Rd.


Subgroup 3: eating behaviour
Key clinical measurements of the cohort
Out of the 170 patients taking part in the entire EndoBarrier RCT, a subgroup of 47 took part in food preference mechanistic studies. There were no significant differences in baseline characteristics between the groups.
There was a significant reduction in weight in both groups at 10 days and at 6, 12 and 24 months compared with baseline. There were no significant differences in weight between groups except at 24 months, when the weight of the control group was significantly lower than the weight of the endoluminal DJBL group. There was significant percentage weight loss within each group at 10 days and at 6 and 12 months compared with baseline, but no significant differences between the groups (see Appendix 2, Table 35).
Mechanistic study results
Total energy intake using 24-hour recall and food diaries
Total energy intake per day obtained from both the 24-hour recall and 3-day food diaries was significantly reduced in both groups at all time points except 24 months compared with baseline, but there were no significant differences between the groups (see Appendix 2, Table 36).
Food preferences using 24-hour recall and food diaries
There were no consistent reductions in the percentage contribution of total calories per day from carbohydrates, protein or fat within groups or any differences between groups (see Appendix 2, Tables 37 and 38).
Assessment of taste function
Sweet taste detection threshold using the method of constant stimuli
There was no significant change in sweet taste detection threshold within groups or any differences between groups at any time point (see Appendix 2, Figure 24).
Sweet taste intensity using the global label magnitude scales
There was no significant change in sweet taste intensity within groups or any differences between groups at any time point (see Appendix 2, Figure 25).
Consummatory reward value of sweet taste using the Just About Right and pleasantness visual analogue scales
There was no significant change in the consummatory reward value of sweet taste within groups or any differences between groups at any time point (see Appendix 2, Figures 26 and 27).
Fasting and post-prandial appetite ratings and concentrations of glucagon-like peptide 1, peptide tyrosine tyrosine and fibroblast growth factor-19 during the mixed-meal tolerance test
There was no significant change in appetite ratings (hunger, fullness, pleasantness to eat, amount to eat, sickness) within groups or any differences between groups at any time point (see Appendix 2, Figure 28). There were no significant changes in plasma total GLP-1 and PYY concentrations in either group. There were no consistent differences in plasma total GLP-1 and PYY concentrations between the groups (Figures 15 and 16). There were no significant changes in FGF-19 concentrations within groups or any differences between groups.
FIGURE 15.
Results of absolute values of fasting and post-prandial PYY after 180 minutes MMT.
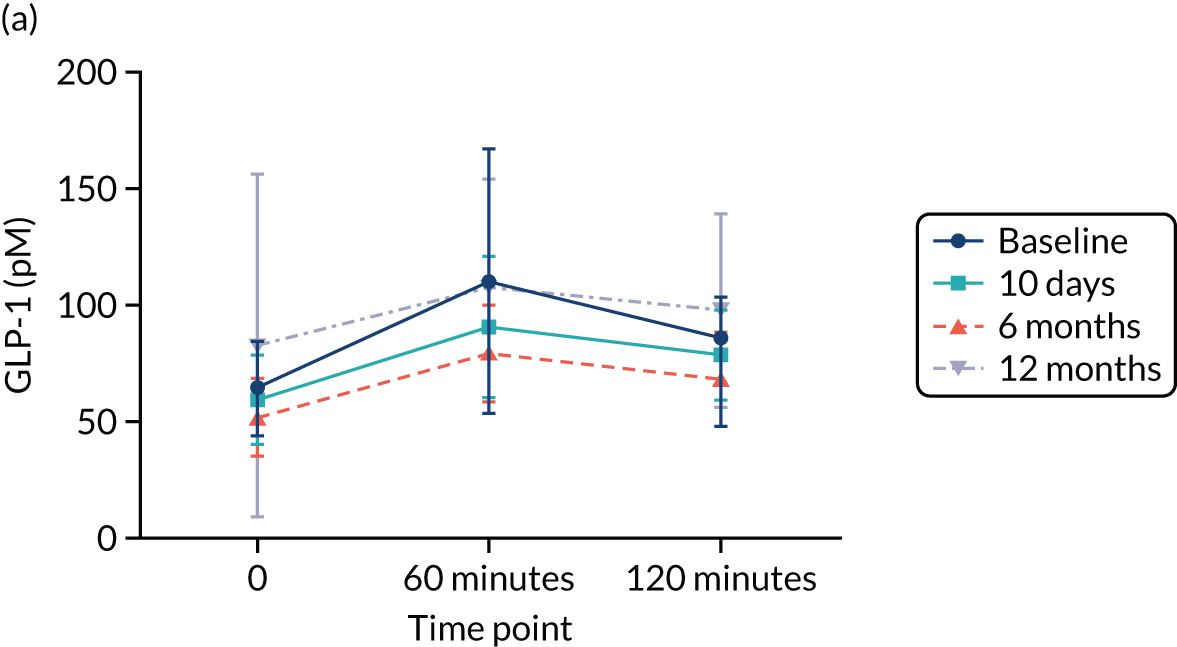
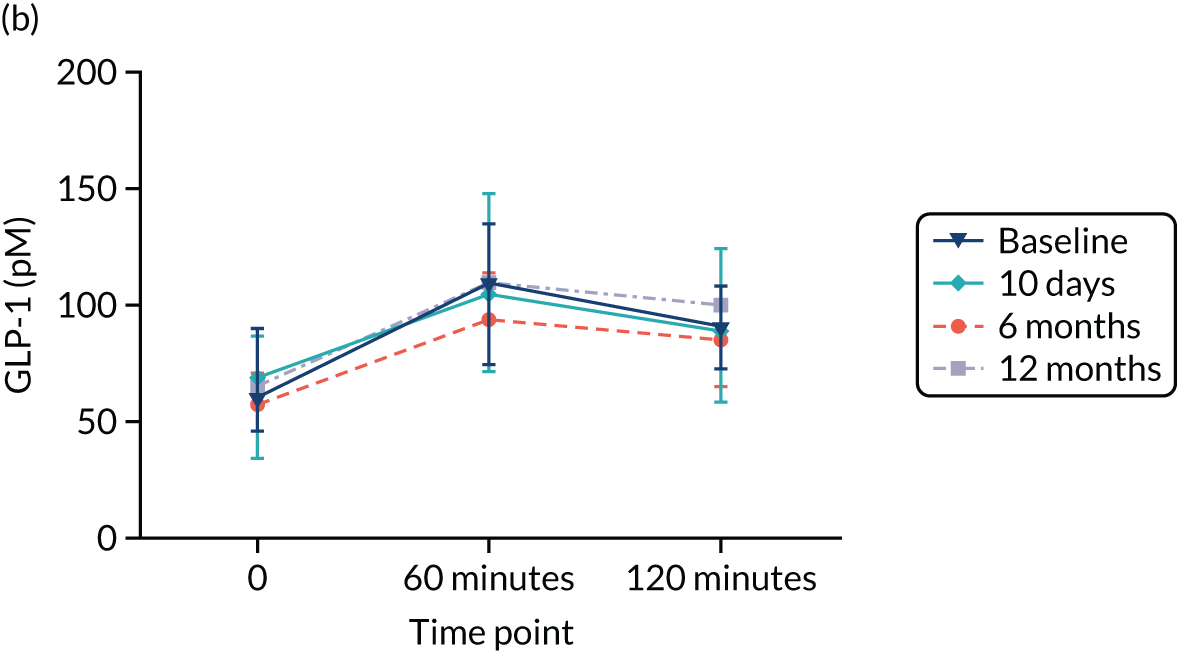
FIGURE 16.
Results of absolute values of fasting and post-prandial GLP-1 after 180 minutes MMT.

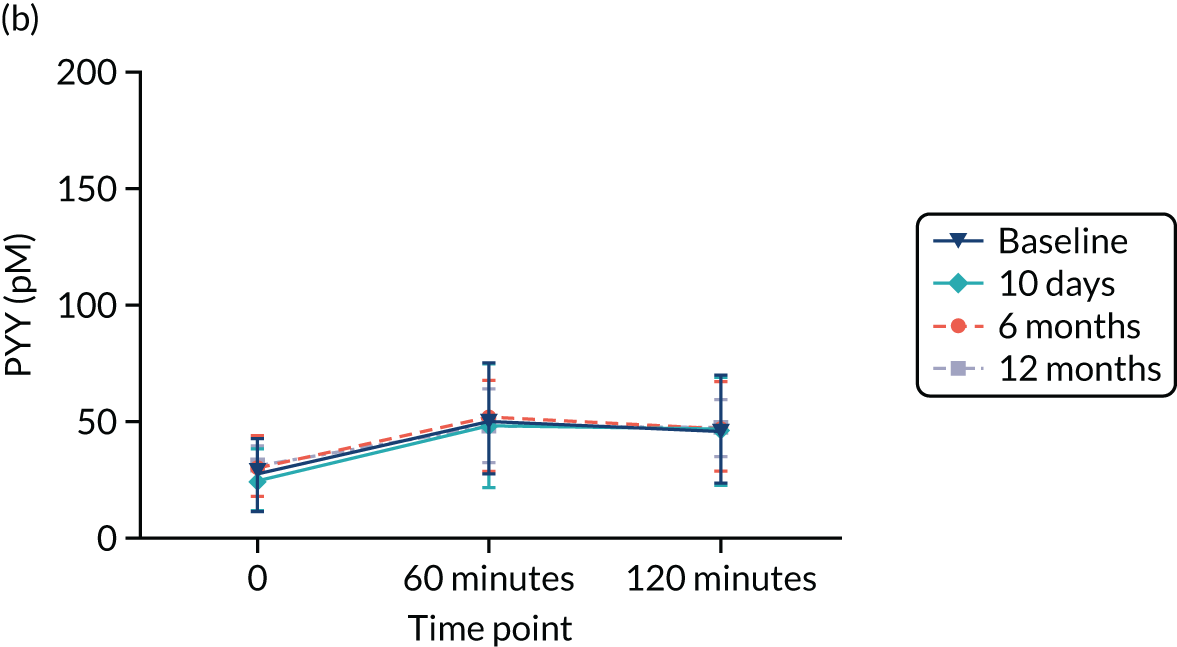
Metabonomics
A preliminary analysis was performed on samples collected from the first year of the trial. This included plasma, urine and faecal samples collected from patients at:
-
visit 3 – pre implant, therefore considered the baseline sample
-
visit 8 – 6 months post EndoBarrier implant
-
visit 10 – 1 year post EndoBarrier implant and the last visit prior to explantation.
In total, 810 samples were processed and then analysed. These consisted of the following:
-
309 plasma samples
-
255 urinary samples
-
246 faecal samples.
In the final analysis, all samples collected from the trial were analysed using 1H-NMR spectroscopy, as well as MS.
The 1H-NMR spectral analysis of urine, plasma and faecal water identified a variety of metabolites. A list of selectively assigned metabolites can be found in Table 18, and the typical 1H-NMR spectra for plasma, urine and faeces as well as the significant OPLS-DA models can be found in Appendix 2, Figures 29–35.
| Metabolite | Chemical formula | Selected δ1H (multiplicity) | Biofluids |
|---|---|---|---|
| 2-Aminoisobutyrate | C4H9NO2 | 1.48(s) | f |
| 3-Indoxylsulfate | C8H7NO4S | 7.7(d), 7.5(d), 7.3(s), 7.27(t), 7.19(t) | u |
| 4-Cresylsulfate | C7H8O4S | 2.35(s) | u |
| 5-Aminopentanoate | C5H11NO2 | 3.00(t), 2.24(t), 1.65(m) | f |
| Ascorbic acid | C6H7O6– | 4.50(d) | p |
| Creatinine | C4H7N3O | 3.05(s), 4.05(s) | u |
| Fumaric acid | C4H4O4 | 6.53(s) | f |
| Lactate | C3H6O3 | 4.11(q), 1.32(d) | p, f |
| Malic acid | C4H6O5 | 4.31(dd) | f |
| Phenylacetylglutamine | C13H16N2O4 | 1.95(m), 2.1(m), 2.25(m), 3.67(d), 4.19(m), 7.36(t), 7.43(t) | u |
| Propylene glycol | C3H8O2 | 1.12(d) | p |
| Trigonelline | C7H7NO2 | 4.42(s) | f |
| Trimethylamine N-oxide | C3H9NO | 3.28(s) | p |
| Tyramine | C8H11NO | 7.21(d), 6.90(d), 3.23(t), 2.92(t) | f |
| Tyrosine | C9H11NO3 | 7.18(d), 6.88(d), 3.94(dd), 3.20(dd), 3.10(dd) | f, u |
| α-Glucose | C6H12O6 | 5.22(d), 3.54(dd), 3.71(t), 3.42(t), 3.83(ddd), 3.84(m), 3.76(m) | f, p |
| β-Glucose | C6H12O6 | 4.65(d), 3.24(dd), 3.48(t), 3.40(t), 3.47(ddd), 3.72(dd), 3.90(dd) | f, p |
Plasma
Significant differences were observed between the control group and the EndoBarrier group at 6 and 12 months. Levels of the metabolites including trimethylamine N-oxide and ascorbic acid were found to be lower in the EndoBarrier group at 6 months than in the control group. Two other unknown compounds at proton chemical shift 3.355 p.p.m. and 3.346 p.p.m. were also found to decrease as well as another metabolite at chemical shift 1.12 p.p.m., which was putatively assigned as propylene glycol.
In the EndoBarrier arm, there were significant changes in plasma metabolic profiles of patients at 6 or 12 months post EndoBarrier implantation in comparison with the baseline profiles. No significant difference was observed between 6 and 12 months in the EndoBarrier group. In the control arm, a significant OPLS-DA model based on samples from baseline and 12 months was also observed. There were no significant metabolic differences between the control group and EndoBarrier group at baseline.
Urine
No significant metabolic differences between the control group and EndoBarrier group at baseline were observed, but, as observed in plasma analysis, significant differences were seen between the control group and EndoBarrier group at 6 and 12 months, respectively, in the urinary spectra. Key metabolic differences between the EndoBarrier group and the control group at 6 and 12 months include an increase in both phenylacetylglutamine and 3-indoxylsulfate. The urinary concentration of creatinine was found to be lower in the EndoBarrier group than in the control group at 6 and 12 months. A similar picture was seen when comparing the EndoBarrier patient cohort at baseline and at 6 months, with a reduction in creatinine at 6 months. At 12 months, phenylacetylglutamine, 3-indoxylsulfate, tyrosine and 4-cresylsulfate were significantly different from baseline in EndoBarrier patients but no significant differences in creatinine levels were observed.
In the EndoBarrier arm, there were significant changes in the urine metabolic profiles of patients at 6 or 12 months post EndoBarrier implantation in comparison with the baseline profiles. Again, no significant difference was observed between 6 and 12 months in the EndoBarrier group. In the control arm, there were no significant differences in the metabolic profile between samples at baseline and 12 months.
Faeces
There was a clear separation of metabolic profiles between the EndoBarrier patients and the control patients at 6 and 12 months, and a separation between the baseline and 6- or 12-month time points in the EndoBarrier cohort. Higher concentrations of faecal metabolites including lactate, 5-aminopentanoic acid and tyramine were observed in the EndoBarrier group than in the control group at 6 months, whereas glucose levels were lower. At 12 months, in addition to an increase in the metabolites lactate and tyramine seen at 6 months in the EndoBarrier group, there was also an increase in 2-aminoisobutyrate. At 12 months there was also a decrease in tyrosine, malic acid, fumaric acid, glucose and oligosaccharides in the EndoBarrier group compared with the control group. Analysis of the EndoBarrier cohort of patients at 6 and 12 months showed increased levels of lactate and tyramine in the stool compared with baseline, but a decrease in glucose. Another metabolite, trigonelline, was found to be lower in EndoBarrier patients at both 6 and 12 months than their baseline samples.
In the EndoBarrier arm, there were significant changes in the faeces metabolic profiles of patients at 6 and 12 months post EndoBarrier implantation in comparison with the baseline profiles. No significant differences were observed between 6 and 12 months in the EndoBarrier group. In the control arm, there were no significant differences in faecal samples from baseline and 12 months.
Gut microbiome
Deoxyribonucleic acid (DNA) sequencing of bacteria from stool samples obtained from participants is currently ongoing and the results will be available in due course.
Utility
Estimated utility scores at each measured time point and area under the curve QALYs with imputation or without imputation for missing data are available in Appendix 2, Figures 36 and 37 and Tables 39 and 40. Mean QALYs are slightly higher for the treatment group than for the control group, but with overlapping CIs: 1.660 (95% CI 1.596 to 1.723) for the intervention group and 1.643 (95% CI 1.581 to 1.705) for the control group (with imputation). The utility results show an initial dip in mean utility for the intervention group at day 10, but by day 30 the groups have similar means. In both groups, mean utility is lower at year 1 than at baseline, and lower at year 2 than at year 1; however, the CIs illustrate that none of the differences between the groups or changes over time is statistically significant.
Costs
Base-case cost estimates are shown in Appendix 2, Tables 41 and 42 (without imputation and with imputation, respectively). Overall costs over the 2-year period were significantly higher in the treatment group than in the control group: a mean of £5445 (95% CI £4921 to £5968) compared with £2225 (95% CI £1853 to £2596), respectively, with imputation. The mean difference of £3220 was mostly attributable to the direct cost of the intervention (mean £2489; 95% CI £2257 to £2721), but the intervention group also incurred excess medication costs of: £1802 (95% CI £1621 to £1984), compared with £189 (95% CI £900 to £1279) in the control group. This difference was mostly due to the use of prophylaxis and treatment for GI complaints (see Appendix 2, Table 43). This included H. pylori eradication, if needed, prior to implant and gastroprotection with a PPI while the implant was in place. The mean cost of diabetes medication was also slightly higher in the intervention group. The distribution of costs across the six periods of follow-up is shown in Appendix 2, Tables 44 and 45, without imputation and with imputation, respectively. As might be expected, a large proportion of the additional costs in the treatment group were incurred in the first month, when most of the implant procedures were performed, or around 12 months, when explants were planned; however, mean costs in the treatment group exceeded those in the control group in each of the costing periods.
Cost-effectiveness
The results of the base-case incremental cost-effectiveness analysis are shown in Tables 19 and 20. Without imputation, the intervention is associated with a mean gain of 0.042 QALYs for an additional cost of £3507, yielding an ICER of £83,775. With MI for missing data (our preferred analysis), the estimated QALY gain is 0.022 for an additional cost of £3220, giving a higher ICER of £147,408. Both estimates are well in excess of the usual NICE upper threshold of £30,000 per QALY gained.
Uncertainty analysis
The impact of uncertainty over the additional cost and QALY gain associated with the intervention on the ICER, adjusted for baseline utility, is illustrated by one-way simple sensitivity analysis in Appendix 2, Tables 46 and 47 (without and with imputation for missing data, respectively). The result is robust to variation of the incremental cost from lower to upper 95% confidence limits, as the ICER remains well above the threshold of £30,000 per QALY gained. However, the results are sensitive to uncertainty over incremental effects: at the lower limit, QALYs are estimated to be higher in the control group than in the treatment group, so that the intervention would be dominated (with fewer QALYs at higher cost), whereas, at the upper limit, the QALY gain with the intervention is higher than in the base case, yielding a lower ICER (£26,930 per QALY gained without imputation, and £35,700 with imputation).
The results of the threshold analysis used to explore the impact of uncertainty over the price of the DJBL device and consumables are shown in Appendix 2, Tables 48 and 49 (without imputation and with imputation of missing data, respectively). When the price of the device and consumables is increased above the base-case value of £1000, the ICER increases, reducing the estimated cost-effectiveness of the intervention. Conversely, the ICER falls with a lower device and consumables cost, but still remains above the threshold of £30,000 per QALY gained even at zero price.
Non-parametric bootstrap
Estimates of incremental costs and effects from the non-parametric bootstrap analysis with imputation (1000 replications) are shown in Figure 17 and Table 21.
FIGURE 17.
Cost-effectiveness scatterplot: non-parametric bootstrap with imputation.

| Variable | Base-case analysis | Bootstrap estimates (1000 iterations) | ||
|---|---|---|---|---|
| Mean | Lower limita | Upper limita | ||
| Incremental costb | £3220 | £3185 | £2533 | £3808 |
| Incremental QALYsc | 0.022 | 0.020 | –0.046 | 0.091 |
| Incremental net benefitd | –£2560 | –£2573 | –£4688 | –£302 |
The means of the bootstrap estimates of incremental costs and QALYs are similar to the base-case results obtained by regression methods. The bootstrap confidence range for incremental costs (£2533–3808) indicates low uncertainty over the conclusion that mean costs were higher for DJBL than for standard care alone, whereas the range for incremental QALYs (–0.046 to 0.09) indicates high uncertainty over relative treatment effects. Valuing QALYs at a threshold value of £30,000, the incremental net benefit of DJBL compared with standard care is –£2560 (95% bootstrap uncertainty range of –£4688 to –£302). A negative incremental net benefit indicates that the intervention is not cost-effective at the defined threshold value.
Chapter 5 Discussion
In this trial, we have demonstrated that the addition of the endoluminal DJBL to intensive medical therapy was not associated with higher rates of participants achieving a ≥ 20% reduction in HbA1c. Participants in the endoluminal DJBL group lost significantly more weight than patients in the control group at 12 months but this weight loss benefit was not sustained at 24 months. The percentage of ‘excellent responders’ (i.e. participants achieving a clinically meaningful reduction in weight of 15%) was six times higher in the endoluminal DJBL group than in the control group at 12 months. Participants in the endoluminal DJBL group also experienced superior reductions in blood pressure, total cholesterol, ALT and aspartate aminotransferase (AST) at 12 months. The beneficial effects of the endoluminal DJBL on weight and cardiometabolic markers dissipated following explantation, with only marginal differences between the groups at 24 months. We were nevertheless encouraged from the observation that both groups sustained part of their achievements in terms of HbA1c and weight loss reductions at 24 months, thus demonstrating the effectiveness of a truly intensive behavioural modification programme. Both groups experienced clinically relevant reductions in blood pressure, but at 12 months the endoluminal DJBL group provided a significantly greater proportion of non-hypertensive patients.
We were surprised to observe no differences in glycaemic control between the two groups. This finding is in line with the first meta-analysis on the endoluminal DJBL3 but contradicts the findings of the most recent one,4 in which the endoluminal DJBL was superior to behavioural modification in terms of both glycaemia and weight loss. Indeed, the endoluminal DJBL was originally conceived as a metabolic rather than an obesity intervention. This was based on the weight loss-independent effects on glucose regulation observed after intestinal bypass surgical procedures like RYGB, biliopancreatic diversion and the duodenal–jejunal bypass. 12,13 An explanation of our findings could be the rapid improvements in the modern management of T2DM, which has been revolutionised in the last few years through the use of agents such as DPP-4 (Dipeptidyl peptidase-4) inhibitors, SGLT-2 (Sodium-glucose co-transporter-2) inhibitors and GLP-1 receptor agonists. The combination of the intensive medical therapy with intensive pharmacotherapy might have achieved a glucose-lowering ‘ceiling effect’, thus limiting our ability to detect any beneficial effects of the endoluminal DJBL. This combination of impactful interventions was not available when previous studies were conducted.
Participants in the endoluminal DJBL group experienced statistically superior and clinically relevant improvements in cardiometabolic risk factors including blood pressure and plasma lipid concentrations, but also markers of non-alcoholic fatty liver disease. These took place predominantly while the device was in situ and then gradually disappeared after explantation. Despite the deep phenotyping of participants in terms of eating behaviour, we were unable to identify the mechanisms through which the endoluminal DJBL reduces energy intake. Even though we did not measure energy expenditure, the available literature does not provide any indication that this may be altered after the endoluminal DJBL. There were also no reports of diarrhoea or steatorrhoea to suggest clinically relevant calorie malabsorption.
The endoluminal DJBL implant procedure is a straightforward one; the average duration was 41 minutes to complete the procedure in this trial with no related complications. The commonest reason for unsuccessful implantation is an anatomical variation, most commonly a short duodenal bulb preventing the device from being anchored securely. Although explantation of the device is in fact considered easier to perform than implantation (on average, the duration was 10 minutes shorter in this trial), it is associated with a higher risk of procedure-related complications. First, there is an increased risk of perforation and bleeding from localised trauma caused by the barbs, so it is imperative that all barbs are contained within the protective hood on removal and remain there throughout the retrieval process. Second, findings at endoscopy on explant can be more unpredictable with regard to the location of the device, which may well have migrated more proximally or distally from the duodenal bulb as a result of peristalsis, which may make its removal trickier. Last, the degree of the device’s adherence to the duodenal wall can vary, as some devices can become tethered or the strings that collapse the device can get tangled within inflammatory tissue caused by localised trauma and irritation of the duodenal lining by the device. Despite all these factors, the vast majority of devices are removed endoscopically, rarely requiring any surgical intervention (1 in 1000), although patients are informed of this risk during the consenting process. In this trial we did encounter this issue, whereby the device was tethered to the duodenal wall and we were unable to collapse the device and retrieve it endoscopically; in this case, laparoscopic removal was required.
Overall, the side effect profile from this study was similar to that reported previously published studies of the endoluminal DJBL. 3,4 Most of the AEs associated with the endoluminal DJBL were classified as mild to moderate, and most frequently occurred within the first few weeks of receiving the implant. The most common were abdominal pain and nausea as the participants acclimatised to having the device. All participants made a full recovery, including those who experienced SAEs. The early explant rate of 25% in the trial is in keeping with previously conducted clinical trials on the endoluminal DJBL such as Forner et al. (25%)152 and Betzel et al. (31%). 45 There was one case of a liver abscess in the 75 successful implantations performed (1.3%). This complication rate is similar to post-market surveillance data for the device and substantially lower than the 3.5% rate of liver abscesses that led to the discontinuation of the ENDO trial48 in 2015. GI bleeding is less likely if the patient is prescribed a high-dose PPI. In nearly all cases where SAEs have occurred there have been no permanent sequelae and the patient has made a full recovery.
Endoluminal DJBL therapy was also shown to lead to significant improvements in liver function tests, particularly ALT and AST levels. Researchers may not wish to ignore these important findings as non-alcoholic fatty liver disease is probably the commonest cause of elevated ALT levels, and current treatments for this condition are fairly limited. 153 Furthermore, higher levels of ALT have been associated with subsequent increased mortality risk in a large population-based study. 154 Compared with men with an ALT < 20 U/l, men with an ALT > 100 U/l were three times more at risk of death from CVD and 59 times more at risk of a death from liver disease. Average baseline ALT levels were 39 U/l in the endoluminal DJBL group but reduced to 22 U/l at 1 year, which would suggest that the endoluminal DJBL might be an effective therapy for patients with a diagnosis of non-alcoholic fatty liver disease. Further research in this field would be helpful, such as matching the biochemical changes with radiological changes by performing serial liver ultrasounds pre and post implant and monitoring for improvements. However, caution should be taken in deploying the device in any patients with a history of liver disease because of the real risk of liver abscesses, and the device should be avoided in patients with liver disease from other causes such as viral hepatitis or autoimmune disease.
The analysis of outcomes from the fMRI mechanistic subgroup was not able to find any definitive mechanistic changes in behaviour underlying the greater weight loss achieved in the endoluminal DJBL intervention than in standard therapy interventions. Interpretation of the findings is limited by the small number of participants who entered into the fMRI subgroup (limited by exclusion criteria, reduced consent to enter this mechanistic substudy combined with visit dropout).
However, analysis of outcomes into the expanded cohort, including the other mechanistic subgroups, although finding beneficial changes in eating behaviour over time (probably contributing to and related to weight loss and improvements in glycaemic control), found that there were no differences between the interventions.
Analysis in the food evaluation fMRI task did not find a between-group difference in the decrease in appeal of food pictures at 26 weeks. However, this may be due to the lack of power in the subgroup analysis. There was a trend for a greater decrease over time for HE than LE foods across both groups. This potential reduction in food cue appeal might contribute to greater weight loss but interpretation is hindered by the small numbers, lack of any associated change in reward system BOLD signal (although underpowered for small effect sizes), lack of any confirmatory findings from the LFPQ outcome measures looking at implicit and explicit food liking and wanting (despite expanded numbers), and energy intake at the ad libitum test lunch. Assessment of the 3-day food diaries at home might be helpful in finding differences in food intake outside the laboratory setting but they have their own limitations in accuracy and estimation of portion size in particular.
Furthermore, the study was performed in the fasted state, so we cannot conclude that a different pattern might be seen in the post-prandial fed state between interventions. However, surprisingly, a previous fMRI study of food cue reactivity after RYGB surgery found greater effects when participants were fasted than when they were fed. 155
By contrast, changes in food cue reactivity (both BOLD signal and appeal rating) away from HE food and towards LE food have been seen with similar numbers of participants when fasted at ≈ 3 months after weight loss from gastric bypass (RYGB) surgery using an identical paradigm at Imperial College London. 156 This suggests that exclusion of food from the duodenum–jejunum is unlikely to be responsible for the reduction in food cue reactivity to HE food relative to LE food after RYGB, at least when fasted. Indeed, our other studies have suggested that this healthier food cue reactivity after RYGB surgery is related to long-term effects of the elevations in post-prandial and fasting plasma satiety hormones PYY and GLP-1, which are not seen after endoluminal DJBL. 39,54,111,157
Furthermore, whole-brain analysis of the fMRI data is still ongoing, which may reveal additional brain regions showing changes in the BOLD signal over time between the two interventions, although, again, small numbers will reduce power to detect such changes.
There were some subtle changes in the taste ratings from the ad libitum lunch that may be of relevance, with the creaminess intensity of sweet foods (yoghurt and ice cream) and the sweetness intensity of HF sweet foods (ice cream) both falling at week 26 in the endoluminal DJBL group but not in the standard therapy group. This change might affect preference and intake of these foods, contributing to greater weight loss in the endoluminal DJBL group, but interpretation is complicated by the small numbers, and the lack of any changes in the actual amount of food eaten in the ad libitum meal, or appetitive reward and motivation in the PRT.
Similarly, reductions in fasting appetite ratings were observed only at week 2 (although this was also after a period of reduced energy intake with a liquid diet), and fasting appetite rating returned towards baseline from week 26 onwards with no difference between interventions; neither intervention changed fasting plasma satiety gut hormones (PYY, GLP-1, FGF-19). Interpretation of these results is not so limited by low numbers as this included data from the expanded subgroups, but looks only at the fasted state.
By week 26, and lasting to week 50 or 100, both endoluminal DJBL insertion and standard therapy groups had healthier eating behaviours, with similar increases in dietary restraint and similar decreases in food hedonics (external eating, binge eating, ‘food addiction’), hunger-related eating and disinhibited eating. As there was no difference between the intervention groups, these findings probably related to the dietary and psychological support offered to study participants rather than to any specific effect of the endoluminal DJBL.
There was no evidence for any aversive symptoms contributing to long-term weight loss after endoluminal DJBL insertion. Although overnight fasting nausea increased more in the DJBL group than in the standard therapy group at week 2, this subsequently settled and, in fact, nausea was lower in the endoluminal DJBL group than in the standard therapy group from week 26 onwards, whereas dumping syndrome scores were not higher in the endoluminal DJBL group than in the standard therapy group.
In both the fMRI and eating behaviour subgroups, the major limitation was that we used predominantly verbal reports and not direct measurements. There are inherent limitations to the use of verbal reports, especially in a trial that is not double blinded. It would have been preferable to measure these aspects of eating behaviour using a buffet meal or a 24-hour residential stay; however, we did use direct measures of taste function, which increases the validity of our findings. The length of the visit with the consecutive taste tasks might have contributed to sensory fatigue, which would be best avoided by having the tasks done on consecutive days; however, this would conflict with the patients’ working schedules and commitments.
In this insulin clamp subgroup of patients, the endoluminal DJBL group achieved early (10 days) and significant reductions in fasting glucose concentrations and in HbA1c but these were not significantly greater than what was achieved through calorie restriction alone. This is only the second study to investigate the metabolic changes following endoluminal DJBL implantation using the gold standard of hyperinsulinaemic–euglycaemic clamps. Through this technique we have been able to demonstrate that early reductions in FPG may be a consequence of reductions in hepatic glucose output (Ra significantly reduced at 10 days post implantation) but that these changes were not superior to those achieved in the control group. It is hypothesised that bypass of the foregut may result in early improvements in glycaemic indices through weight loss-independent mechanisms, as has been observed followed RYGB. These data, however, do not support this notion and we have demonstrated that duodenal–jejunal bypass sleeves provide no additional improvements to early changes in glucose homeostasis above that achieved through calorie restriction alone. This is consistent with the previously published clamp study by Miras et al. 86
Patients implanted with the endoluminal DJBL device lost significantly more weight than those patients receiving standard medical therapy, which was maintained at 6 months (percentage total weight loss 12.3% vs. 5.2%; p < 0.001). This is consistent with the 6-month weight loss outcomes of previously published studies. 41,77,81,152 Probably as a result of this superior weight loss, we observed a significantly higher rate of peripheral glucose disposal (Rd) in the endoluminal DJBL cohort of patients at 6 months than in the control group.
Our data demonstrate superior increases in peripheral but not hepatic insulin sensitivity in patients implanted with the endoluminal DJBL device compared with standard medical therapy.
This is the first study of its kind to explore the metabolic profiles of patients receiving the endoluminal DJBL. In addition to this, the data have been collected longitudinally in a randomised setting, allowing comparisons to be made over time with a control group of patients. 1H-NMR spectroscopic analysis of plasma, urine and faeces has revealed a number of distinct metabolic perturbations between the control arm and endoluminal DJBL arm occurring at 6 and 12 months compared with baseline. Similarly, variation in the metabolic profiles of patients in the endoluminal DJBL group over time is seen when compared with baseline samples, and this change was not observed in the control group in which the metabolic profiles observed did not alter significantly over time, apart from the comparison in plasma samples between the baseline and 12 months.
Furthermore, significant metabolic differences were observed between baseline and 6 or 12 months in the endoluminal DJBL arm but not between 6 months and 1 year, which suggests that the key metabolic changes primarily occur in the first 6 months of having the device in situ. This appears to correlate well with device efficacy, as it is usually in the first 3–6 months that the greatest weight loss occurs.
The observed metabolic changes in all biofluids are related to the host–microbial co-metabolism. This suggested that the endoluminal DJBL induces the metabolic disturbances in the GM.
A major limitation in this analysis is the potential confounders that will undoubtedly have had an influence on the metabolic profile of both treatment arms, but which were not controlled for in this study. Examples of these include dietary consumption, medications and physical activity, which would all have varied prior to study visits, thus having an impact on the samples being collected. However, integrating the metabolic data with other mechanistic studies included in this trial will improve the robustness of the metabolic findings.
In addition, NMR spectroscopic analysis gives a global overview of the metabolic changes. This can direct us to select appropriate MS-based assays for further metabolic investigation. These further key metabolites can be discovered and correlated with the clinical outcomes.
Utility scores were calculated from the EQ-5D-5L data using the NICE-recommended van Hout crosswalk method with ‘UK tariff’ general population valuations. For the intervention arm, after an initial dip at day 10 (which is probably related to the implant procedure and adverse effects), mean utility had recovered to a similar level to that in the control group by day 30. Thereafter, mean utility deteriorated in both study groups, with a slightly more pronounced decline in the control arm. This left a small, non-significant, utility advantage for the intervention group at month 24. This advantage, net of the adverse effect observed in the immediate post-implant period, resulted in a net QALY gain for the intervention group relative to the control group. It is unclear whether the utility difference or QALY again will persist into the third year post implant or beyond. It is also important to emphasise that, at all time points, between-group differences and within-group changes in mean utility and QALYs were small in magnitude with largely overlapping CIs.
There were significant cost differences between the intervention group and control group. The main drivers of these differences were intervention costs (which are, by definition, nil in the control arm), as well as medication costs. The latter are primarily a result of a greater use of GI medications in the intervention group, which might well be expected as GI complications and AEs were more common among individuals receiving the implant. Patients in the treatment arm also received diagnostic tests and medications for H. pylori and ulcers.
Putting together the cost and QALY results, we estimated an ICER of £83,775 without imputation for missing data, and £147,408 with imputation (our preferred analysis). Both values lie well above the threshold of £30,000 per QALY gained, commonly applied in the UK NHS.
The large difference between these figures arises because the denominator of the ICER (the incremental QALY) has a small absolute value. The high level of uncertainty over the QALY gain and the need to impute missing EQ-5D values causes uncertainty over the cost-effectiveness result. At a maximum willingness-to-pay threshold of £30,000 per QALY gained, we estimate that the additional costs of DJBL outweigh the value of the health benefits by £2560 per patient treated (95% CI –£4688 to –£302); however, these estimates do not incorporate all sources of uncertainty.
Another source of uncertainty over the ICER is the price of the endoluminal DJBL and consumables required for the implant and explant procedures. In our base case, we assumed a price of £1000. This is likely to be an underestimate. At prices of £2500 and £5000, our ICER estimates rise above £200,000 and £300,000 per QALY gained, respectively.
The other major uncertainty relates to persistence of cost or health effects after 24 months. Given the small and non-significant differences in weight loss and measures of blood glucose control, as well as other risk factors for micro- or macrovascular complications, we do not consider that extrapolation of the health economic results is warranted. However, we note that a cost-effectiveness analysis based on the REVISE [Randomisation to EndoBarrier alone Versus with Incretin analogue in SustainEd Diabesity (REVISE-Diabesity)] trial158 has reported more favourable BMI and HbA1c reductions and QALY gains over 2 years for a comparison of endoluminal DJBL plus liraglutide with liraglutide alone. The abstract reports that estimating cost per QALY gained over 2 years from the trial and then up to 50 years with the CORE Diabetes Model, the combination treatment dominated endoluminal DJBL alone and liraglutide alone (producing more QALYs at lower cost). It is difficult to draw direct comparisons with our results because of the different comparators and smaller sample size (n = 70 in total) of the REVISE trial. It is interesting that the total QALY per patient over 2 years in the REVISE EndoBarrier alone arm was 1.68, similar to our base case of 1.66. However, our cost estimates were quite different: £12,941 in the REVISE EndoBarrier alone arm compared with £5445 in our base case. It is not possible to understand the reasons for this difference, as the REVISE cost-effectiveness analysis has been published only in abstract form.
The strengths of the trial include the randomised design, short- and medium-term follow-up for 2 years, multidisciplinary care and delivery of a truly intensive medical therapy programme, use of two trial sites, study management by the ICTU, comprehensive profiling of patients in terms of their eating behaviour, glucose regulation and metabolic responses, and a detailed health economic analysis. The main limitation of the trial is the open-label design and this could be a source of bias (coming from the participants who knew what group they were allocated to). It is possible that those randomised to the endoluminal DJBL group could have made more of an effort to adhere to the dietary intervention. This limits the conclusions that can be drawn with regard to weight loss and eating behaviour outcomes. A limitation that was not anticipated when the trial was designed included the rapid improvement and widespread use of T2DM pharmacotherapy, which now includes agents that can improve glycaemia alongside weight loss. Another unanticipated limitation was the loss of the device CE mark that took place during the trial. Additional limitations include:
-
Interpretation of the fMRI subgroup findings owing to the small number of participants who entered the fMRI subgroup (limited by exclusion criteria, reduced consent to enter this mechanistic substudy combined with visit dropout).
-
Assessment of eating behaviour and neural responses to food in the fasting state.
-
Use of direct but also many indirect measures of eating behaviour.
-
Assessment of insulin sensitivity without a period of medication washout. The third clamp was also performed at 6 months but not at matched weight loss between the groups.
The external validity of this trial is high as we recruited patients with moderately advanced T2DM, a group that is highly representative of the type of patients who would be willing to have this intervention in real life in an attempt to avoid the use of insulin therapy. The factors that reduce external validity include the intensive lifestyle modification that was offered as part of this trial and challenging to find in the NHS, and the inherent nature of this group of participants, who by virtue of taking part in this demanding trial may be different from other patients in the real-life setting.
Future directions
The device manufacturers advocate that the endoluminal DJBL should remain in place for 1 year and then be removed. An issue that arises is that the vast majority of patients may then lose the beneficial effects on glycaemic control and weight loss that the device may have been exerting while in situ, resulting in a worsening in their diabetes and an increase in their BMI. An Australian study found that, of 30 patients who were followed up in the 6-month period immediately post removal of the EndoBarrier, 72% gained weight, with only five patients maintaining their weight loss and four patients losing further weight. 152 In the same study, 51 patients were followed up for a period of > 6 months following explant, with 69% regaining their weight and only five patients maintaining their weight, with seven patients losing further weight. The study did not report on how these particular patients managed to maintain their weight loss or lose further weight.
GI Dynamics Inc. has previously reported data demonstrating the feasibility and safety of re-implantation of the endoluminal DJBL in five patients who initially completed 12 months of EndoBarrier treatment but then proceeded to have the device re-implanted after 4 months for another 12 months. HbA1c fell from a baseline of 9.1% to 6.7% after the first explant, and from 7.8% on second implantation to 7.1% at explant with no reported complications. 159 Although these are small numbers, re-implantation of the EndoBarrier might be another treatment option to maintain the effect of the device.
A second-generation endoluminal DJBL device with a 1-mm increase in barb length was trialled in 80 patients in Chile. The patients initially consented to the implant for 1 year but were then given the opportunity to keep the device in for up to 3 years if tolerated. 74 The percentage (SD) of excess weight loss in the completer population at 52 weeks (71 patients), 104 weeks (40 patients) and 156 weeks (11 patients) was 44% ± 16%, 40% ± 22%, and 39% ± 20%, respectively (p < 0.001). There were 17 T2DM patients enrolled in the study with a baseline HbA1c level of 7.1% ± 1.6% which significantly decreased to 6% ± 0.9% and 5.7% ± 0.7% after 12 and 24 months, respectively. Two diabetic participants managed to complete 36 months of follow-up, and both maintained an HbA1c < 6%.
The endoluminal DJBL is currently unavailable both commercially and in the clinical trial setting. Following the closure of the ENDO trial48 in the USA by the FDA in 2015, the device was withdrawn from the US market, and in 2017 the device manufacturer GI Dynamics Inc. suffered further setbacks by losing its European market. An increase in the number of device tears recently reported (probably due to a manufacturing defect) culminated in the EndoBarrier losing its CE mark in November 2017 for non-compliance related to quality control issues.
From a mechanistic perspective, the device offers unique opportunities to investigate the signalling between the duodenum to the pancreas and brain. The finding of superior weight loss in the endoluminal DJBL group has rather unexpectedly moved the focus to understanding the elusive mechanisms through which the device reduces energy intake. Future studies with larger sample size and direct measurements of both energy intake and energy expenditure could provide answers to the questions this trial has generated. The aim would be to identify the mediators of this signalling and use the knowledge to develop targets for obesity pharmacotherapy.
Conclusion
In conclusion, this trial has demonstrated that the addition of the endoluminal DJBL to an intensive lifestyle intervention was not superior to intensive lifestyle interventions alone in improving glycaemic control in obese patients with T2DM, and was associated with more AEs. The safety profile of the EndoBarrier was similar to that provided in previous publications. Evaluation of secondary outcomes revealed that therapy with the endoluminal DJBL was associated with significantly greater weight loss and improvements in several cardiometabolic parameters (including blood pressure, total cholesterol, ALT and AST) at 12 months but not at 24 months. Superior reductions in peripheral insulin sensitivity also occurred, probably as a result of greater weight loss. Despite a comprehensive profiling of patients in terms of eating behaviour, we did not identify the mechanisms underlying weight loss. Future studies could address this gap in knowledge. Economic evaluation showed that the bypass liner was not cost-effective for glycaemic control or for weight loss.
Acknowledgements
The research was funded by the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
This report presents independent research funded by the EME programme and supported by the NIHR Clinical Research Facility and Biomedical Research Centre at Imperial College Healthcare NHS Trust and University Hospital Southampton NHS Foundation Trust.
This study is being executed with the support of GI Dynamics Inc. and with the kind support of Nutricia Advanced Medical Nutrition for providing oral nutritional supplements.
The following people are thanked for participating in the trial:
-
trial participants
-
ICTU
-
Professor Margot Umpleby for the insulin clamps analysis
-
Dr Bruce Gaylinn for the ghrelin analysis
-
GI Dynamics Inc. for the supply of the EndoBarrier devices
-
TSC members – Professor Jonathan Brown, Dr Edward Fogden, Dr Bu Hayee, Dr Tim Saunders and Mr Robert Thompson
-
DMEC members – Professor Stephen Attwood, Dr Lorraine Albon and Dr Jonathan Cook
-
participating sites – Imperial College Healthcare NHS Trust and University Hospital Southampton NHS Foundation Trust.
Contributions of authors
Dr Aruchuna Ruban (https://orcid.org/0000-0002-9105-4025) (Research Fellow in Gastroenterology, Imperial College London) helped to run (consenting and seeing participants for their clinical study visits, data collection and storage) the trial at the London research site. He designed the study, analysed the metabonomics study results, interpreted the data, and drafted, revised and approved the report.
Mr Michael A Glaysher (https://orcid.org/0000-0002-1746-2100) (Research Fellow in Surgery, University Hospital Southampton NHS Foundation Trust) helped to run (consenting and seeing participants for their clinical study visits, data collection and storage) the trial at the Southampton research site. He designed the study, analysed the insulin clamps study results, interpreted the data, and drafted, revised and approved the report.
Dr Alexander D Miras (https://orcid.org/0000-0003-3830-3173) (Consultant Endocrinologist at Imperial College London) designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Dr Anthony P Goldstone (https://orcid.org/0000-0001-8179-7071) (Consultant Endocrinologist at Imperial College London) designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Dr Christina G Prechtl (https://orcid.org/0000-0003-4122-8899) (Clinical Trials Manager, Imperial College London, Department of Public Health, ICTU) designed and managed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Mr Nicholas Johnson (https://orcid.org/0000-0002-3702-5530) (Trials Statistician, Imperial College London, Department of Public Health, ICTU) designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Dr Jia Li (https://orcid.org/0000-0002-5763-6670) (Lecturer in Human Development and Microbial Signalling, Imperial College London) designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Dr Madhawi Aldhwayan (https://orcid.org/0000-0002-9228-8712) (Dietitian at Imperial College London) analysed the food preference study results, interpreted the data, and drafted, revised and approved the report.
Miss Ghadah Aldubaikhi (https://orcid.org/0000-0002-1186-4379) (Dietitian at Imperial College London) analysed the fMRI and metabonomics study results, interpreted the data, and drafted, revised and approved the report.
Dr Ben Glover (https://orcid.org/0000-0003-3043-0012) (Research Fellow in Gastroenterology, Imperial College London) helped to run (seeing participants for their clinical study visits, data collection and storage) the trial at the London research site. He analysed the metabonomics study results, interpreted the data, and drafted, revised and approved the report.
Dr Joanne Lord (https://orcid.org/0000-0003-1086-1624) (Director Southampton Health Technology Assessments Centre, Health Economics) designed the study, conducted the analysis of economic effectiveness, interpreted the data, and drafted, revised and approved the report.
Mr Olu Onyimadu (https://orcid.org/0000-0002-1724-3485) (Health Economist at Southampton Health Technology Assessments Centre, University of Southampton) assisted in the analysis of economic effectiveness, interpreted the data, and drafted, revised and approved the report.
Dr Emmanuela Falaschetti (https://orcid.org/0000-0001-6964-2042) (Senior Trials Statistician, Imperial College London, Department of Public Health, ICTU) designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Mrs Natalia Klimowska-Nassar (https://orcid.org/0000-0003-3655-7436) (Operations Manager, Imperial College London, Department of Public Health, ICTU) helped manage the trial, co-ordinated, reviewed and approved the final report.
Mr Hutan Ashrafian (https://orcid.org/0000-0003-1668-0672) (Chief Scientific Advisor, Institute of Global Health Innovation, Imperial College London) helped to advise on the study, and revised and approved the report.
Mr James Byrne (https://orcid.org/0000-0003-3517-5110) (General Surgeon, University Hospital Southampton NHS Foundation Trust) acted in the capacity of co-investigator on this trial. He further led the research at the study site in Southampton in his role as local Principal Investigator. He attended the TSC and TMG. He designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Professor Julian P Teare (https://orcid.org/0000-0003-3551-9139) (Professor of Gastroenterology, Imperial College London) acted in the capacity of Chief Investigator for this trial. He designed the study, analysed and interpreted the data, and drafted, revised and approved the report.
Publications
Glaysher MA, Mohanaruban A, Prechtl CG, Goldstone AP, Miras AD, Lordet J, et al. A randomised controlled trial of a duodenal-jejunal bypass sleeve device (EndoBarrier) compared with standard medical therapy for the management of obese subjects with T2DM. BMJ Open 2017;7:e018598.
Ruban A, Prechtl CG, Glaysher MA, et al. Effectiveness of different recruitment strategies in an RCT of a surgical device: experience from the Endobarrier trial. BMJ Open 2019;9:e032439.
Data-sharing statement
All available data can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- NHS Digital Statistics Team . Statistics on Obesity, Physical Activity and Diet 2020. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2020 (accessed 18 August 2020).
- Department of Health and Social Care . Tackling Obesity: Empowering Adults and Children to Live Healthier Lives 2011. www.gov.uk/government/publications/tackling-obesity-empowering-adults-and-children-to-live-healthier-lives (accessed 18 August 2020).
- World Health Organization (WHO) . Obesity and Overweight Factsheet 2018 n.d. www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 6 December 2019).
- Diabetes UK. Us, Diabetes and a Lot of Facts and Stats 2019. www.diabetes.org.uk/resources-s3/2019-11/facts-stats-update-oct-2019.pdf (accessed 6 December 2019).
- World Health Organization (WHO) . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia 2006.
- World Health Organization (WHO) . Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation 2011.
- Diabetes UK. Diagnostic Criteria for Diabetes n.d. www.diabetes.org.uk/professionals/position-statements-reports/diagnosis-ongoing-management-monitoring/new_diagnostic_criteria_for_diabetes (accessed 6 December 2019).
- Bunn HF, Gabbay KH, Gallop PM. The glycosylation of haemoglobin: relevance to diabetes mellitus. Science 1978;200:21-7. https://doi.org/10.1126/science.635569.
- Gabbay KH, Sosenko JM, Banuchi GA, Mininsohn MJ, Flückiger R. Glycosylated hemoglobins: increased glycosylation of hemoglobin A in diabetic patients. Diabetes 1979;28:337-40. https://doi.org/10.2337/diab.28.4.337.
- Inada M, Oishi M, Nishikawa M, Kurata S, Imura H. Clinical evaluation of measuring glycosylated hemoglobin levels for assessing the long-term blood glucose control in diabetics. Endocrinol Jpn 1980;27:411-15. https://doi.org/10.1507/endocrj1954.27.411.
- International Diabetes Federation (IDF) . IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care 2018. https://idf.org/e-library/guidelines.html (accessed 29 September).
- UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. https://doi.org/10.1016/S0140-6736(98)07019-6.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation 2013;127:143-52. https://doi.org/10.1161/CIR.0b013e318282ab8f.
- Healthcare Quality Improvement Partnership . National Diabetes Audit, 2015–16 Report 2a: Complications and Mortality 2017.
- Martin CL, Albers JW, Pop-Busui R. DCCT/EDIC Research Group . Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:31-8. https://doi.org/10.2337/dc13-2114.
- Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am 2013;42:747-87. https://doi.org/10.1016/j.ecl.2013.06.001.
- Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998;47:457-63. https://doi.org/10.2337/diabetes.47.3.457.
- American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333-41. https://doi.org/10.2337/diacare.26.12.3333.
- Diabetes UK. Diabetes UK Interim Position Statement on Remission in Adults with Type 2 Diabetes. London: Diabetes UK; 2018.
- Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014;370:233-44. https://doi.org/10.1056/NEJMoa1304501.
- Public Health England . Adult Obesity and Type 2 Diabetes 2014.
- Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. Remission of type 2 diabetes: is bariatric surgery ready for prime time?. Endocrine 2015;48:417-21. https://doi.org/10.1007/s12020-014-0463-z.
- Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489-96. https://doi.org/10.1001/jama.2012.67929.
- Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: follow-up of a randomized trial. Diabetes Care 2014;37:1824-30. https://doi.org/10.2337/dc13-2899.
- Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 2015;115:1447-63. https://doi.org/10.1016/j.jand.2015.02.031.
- Müller-Stich BP, Senft JD, Warschkow R, Kenngott HG, Billeter AT, Vit G, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg 2015;261:421-9. https://doi.org/10.1097/SLA.0000000000001014.
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335-42. https://doi.org/10.1001/jama.291.3.335.
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;8. https://doi.org/10.1002/14651858.CD003641.pub4.
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-52. https://doi.org/10.1056/NEJMoa066254.
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964-73. https://doi.org/10.1016/S0140-6736(15)00075-6.
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med 2017;376:641-51. https://doi.org/10.1056/NEJMoa1600869.
- National Institute for Health and Care Excellence (NICE) . Costing Report: Obesity Implementing the NICE Guideline on Obesity (CG189) 2014.
- NHS England . Guidance for Clinical Commissioning Groups (CCGs): Clinical Guidance: Surgery for Severe and Complex Obesity 2016.
- Cohen RV, Shikora S, Petry T, Caravatto PP, Le Roux CW. The Diabetes Surgery Summit II Guidelines: a disease-based clinical recommendation. Obes Surg 2016;26:1989-91. https://doi.org/10.1007/s11695-016-2237-6.
- Ruban A, Stoenchev K, Ashrafian H, Teare J. Current treatments for obesity. Clin Med 2019;19:205-12. https://doi.org/10.7861/clinmedicine.19-3-205.
- Ashrafian H, Athanasiou T, Li JV, Bueter M, Ahmed K, Nagpal K, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev 2011;12:e257-72. https://doi.org/10.1111/j.1467–789X.2010.00802.x.
- Ruban A, Ashrafian H, Teare JP. The EndoBarrier: duodenal-jejunal bypass liner for diabetes and weight loss. Best Pract Res Clin Gastroenterol 2018. https://doi.org/10.1155/2018/7823182.
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1. https://doi.org/10.1126/scitranslmed.3000322.
- De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 2011;14:700-6. https://doi.org/10.1016/j.cmet.2011.09.010.
- Gersin KS, Rothstein RI, Rosenthal RJ, Stefanidis D, Deal SE, Kuwada TS, et al. Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest Endosc 2010;71:976-82. https://doi.org/10.1016/j.gie.2009.11.051.
- Koehestanie P, de Jonge C, Berends FJ, Janssen IM, Bouvy ND, Greve JW. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann Surg 2014;260:984-92. https://doi.org/10.1097/SLA.0000000000000794.
- Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, Tarnoff M. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis 2008;4:55-9. https://doi.org/10.1016/j.soard.2007.07.012.
- Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg 2010;251:236-43. https://doi.org/10.1097/SLA.0b013e3181bdfbff.
- Tarnoff M, Rodriguez L, Escalona A, Ramos A, Neto M, Alamo M, et al. Open label, prospective, randomized controlled trial of an endoscopic duodenal-jejunal bypass sleeve versus low calorie diet for pre-operative weight loss in bariatric surgery. Surg Endosc 2009;23:650-6. https://doi.org/10.1007/s00464-008-0125-4.
- Betzel B, Homan J, Aarts EO, Janssen IMC, de Boer H, Wahab PJ, et al. Weight reduction and improvement in diabetes by the duodenal-jejunal bypass liner: a 198 patient cohort study. Surg Endosc 2017;31:2881-91. https://doi.org/10.1007/s00464-016-5299-6.
- Patel N, Mohanaruban A, Ashrafian H, Le Roux C, Byrne J, Mason J, et al. EndoBarrier®: a safe and effective novel treatment for obesity and type 2 diabetes?. Obes Surg 2018;28:1980-9. https://doi.org/10.1007/s11695-018-3123-1.
- Riedel N, Laubner K, Lautenbach A, Schön G, Schlensak M, Stengel R, et al. Longitudinal evaluation of efficacy, safety and nutritional status during one-year treatment with the duodenal-jejunal bypass liner. Surg Obes Relat Dis 2018;14:769-79. https://doi.org/10.1016/j.soard.2018.02.029.
- ClinicalTrials.gov . Safety and Efficacy of EndoBarrier in Subjects With Type 2 Diabetes Who Are Obese (ENDO) Trial n.d. https://clinicaltrials.gov/ct2/show/NCT01728116 (accessed 29 September 2020).
- Ryder REJ, Munro L, McMaster JJ, Bessell J, Bascomb JM, Collins JE, et al. First risk–benefit data from the Worldwide Endobarrier Registry. Diabetes 2018;67:2097-P.
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 2002;36:199-211. https://doi.org/10.1016/S0896-6273(02)00969-8.
- Mattes RD. Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc 1997;97:406-13. https://doi.org/10.1016/S0002-8223(97)00101-6.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822-32. https://doi.org/10.1007/s11695-015-1657-z.
- le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007;246:780-5. https://doi.org/10.1097/SLA.0b013e3180caa3e3.
- Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014;63:891-902. https://doi.org/10.1136/gutjnl-2013-305008.
- Ullrich J, Ernst B, Wilms B, Thurnheer M, Schultes B. Roux-en Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes Surg 2013;23:50-5. https://doi.org/10.1007/s11695-012-0754-5.
- Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes 1981;5:457-64.
- Molin Netto BD, Earthman CP, Farias G, Landi Masquio DC, Grotti Clemente AP, Peixoto P, et al. Eating patterns and food choice as determinant of weight loss and improvement of metabolic profile after RYGB. Nutrition 2017;33:125-31. https://doi.org/10.1016/j.nut.2016.05.007.
- Spector AC, Glendinning JI. Linking peripheral taste processes to behavior. Curr Opin Neurobiol 2009;19:370-7. https://doi.org/10.1016/j.conb.2009.07.014.
- Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 2011;104:709-21. https://doi.org/10.1016/j.physbeh.2011.07.025.
- Pepino MY, Bradley D, Eagon JC, Sullivan, Abumrad NA, Klein S. Changes in taste perception and eating behaviour after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 2014;22:E13-20. https://doi.org/10.1002/oby.20649.
- Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 1995;95:666-70. https://doi.org/10.1016/S0002-8223(95)00182-4.
- Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr 2012;96:467-73. https://doi.org/10.3945/ajcn.112.036921.
- Muñoz R, Carmody JS, Stylopoulos N, Davis P, Kaplan LM. Isolated duodenal exclusion increases energy expenditure and improves glucose homeostasis in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 2012;303:R985-93. https://doi.org/10.1152/ajpregu.00262.2012.
- Hague AL, Baechle M. Advanced caries in a patient with a history of bariatric surgery. J Dent Hyg 2008;82.
- Marsicano JA, Sales-Peres A, Ceneviva R, de C, Sales-Peres SH. Evaluation of oral health status and salivary flow rate in obese patients after bariatric surgery. Eur J Dent 2012;6:191-7. https://doi.org/10.1055/s-0039-1698950.
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 2003;349:941-8. https://doi.org/10.1056/NEJMoa030204.
- le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243:108-14. https://doi.org/10.1097/01.sla.0000183349.16877.84.
- Aguirre V, Stylopoulos N, Grinbaum R, Kaplan LM. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity 2008;16:2585-92. https://doi.org/10.1038/oby.2008.502.
- Koehestanie P, Dogan K, Berends F, Janssen I, Wahab P, Groenen M, et al. Duodenal-jejunal bypass liner implantation provokes rapid weight loss and improved glycemic control, accompanied by elevated fasting ghrelin levels. Endosc Int Open 2014;2:E21-7. https://doi.org/10.1055/s-0034-1365222.
- Cohen R, le Roux CW, Papamargaritis D, Salles JE, Petry T, Correa JL, et al. Role of proximal gut exclusion from food on glucose homeostasis in patients with type 2 diabetes. Diabet Med 2013;30:1482-6. https://doi.org/10.1111/dme.12268.
- de Jonge C, Rensen SS, Verdam FJ, Vincent RP, Bloom SR, Buurman WA, et al. Endoscopic duodenal–jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg 2013;23:1354-60. https://doi.org/10.1007/s11695–013–0921–3.
- Escalona A, Pimentel F, Sharp A, Becerra P, Slako M, Turiel D, et al. Weight loss and metabolic improvement in morbidly obese subjects implanted for 1 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg 2012;255:1080-5. https://doi.org/10.1097/SLA.0b013e31825498c4.
- Kaválková P, Mráz M, Trachta P, Kloučková J, Cinkajzlová A, Lacinová Z, et al. Endocrine effects of duodenal–jejunal exclusion in obese patients with type 2 diabetes mellitus. J Endocrinol 2016;231:11-22. https://doi.org/10.1530/JOE-16-0206.
- Quezada N, Muñoz R, Morelli C, Turiel D, Hernández J, Pimentel F, et al. Safety and efficacy of the endoscopic duodenal–jejunal bypass liner prototype in severe or morbidly obese subjects implanted for up to 3 years. Surg Endosc 2018;32:260-7. https://doi.org/10.1007/s00464-017-5672-0.
- Stratmann B, Krepak Y, Schiffer E, Jarick I, Hauber M, Lee-Barkey YH, et al. Beneficial metabolic effects of duodenal jejunal bypass liner for the treatment of adipose patients with type 2 diabetes mellitus: analysis of responders and non-responders. Horm Metab Res 2016;48:630-7. https://doi.org/10.1055/s-0042–115175.
- de Moura EG, Martins BC, Lopes GS, Orso IR, de Oliveira SL, Galvão Neto MP, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal–jejunal bypass liner. Diabetes Technol Ther 2012;14:183-9. https://doi.org/10.1089/dia.2011.0152.
- de Moura EG, Orso IR, Martins BC, Lopes GS, de Oliveira SL, Galvão-Neto Mdos P, et al. Improvement of insulin resistance and reduction of cardiovascular risk among obese patients with type 2 diabetes with the duodenojejunal bypass liner. Obes Surg 2011;21:941-7. https://doi.org/10.1007/s11695–011–0387–0.
- Cohen RV, Neto MG, Correa JL, Sakai P, Martins B, Schiavon CA, et al. A pilot study of the duodenal–jejunal bypass liner in low body mass index type 2 diabetes. J Clin Endocrinol Metab 2013;98:E279-82. https://doi.org/10.1210/jc.2012-2814.
- Betzel B, Koehestanie P, Aarts EO, Dogan K, Homan J, Janssen IM, et al. Safety experience with the duodenal-jejunal bypass liner: an endoscopic treatment for diabetes and obesity. Gastrointest Endosc 2015;82:845-52. https://doi.org/10.1016/j.gie.2015.03.1911.
- de Moura EG, Lopes GS, Martins BC, Orso IR, Coutinho AM, de Oliveira SL, et al. Effects of duodenal-jejunal bypass liner (EndoBarrier®) on gastric emptying in obese and type 2 diabetic patients. Obes Surg 2015;25:1618-25. https://doi.org/10.1007/s11695-015-1594-x.
- Betzel B, Koehestanie P, Homan J, Aarts EO, Janssen IM, de Boer H, et al. Changes in glycemic control and body weight after explantation of the duodenal-jejunal bypass liner. Gastrointest Endosc 2017;85:409-15. https://doi.org/10.1016/j.gie.2016.07.027.
- Vilarrasa N, de Gordejuela AG, Casajoana A, Duran X, Toro S, Espinet E, et al. Endobarrier® in grade I obese patients with long-standing type 2 diabetes: role of gastrointestinal hormones in glucose metabolism. Obes Surg 2017;27:569-77. https://doi.org/10.1007/s11695-016-2311-0.
- Rodriguez L, Reyes E, Fagalde P, Oltra MS, Saba J, Aylwin CG, et al. Pilot clinical study of an endoscopic, removable duodenal-jejunal bypass liner for the treatment of type 2 diabetes. Diabetes Technol Ther 2009;11:725-32. https://doi.org/10.1089/dia.2009.0063.
- Segal-Lieberman G, Lang A, Lahav M, Lieberman N, Paster A, Konvalina N, et al. Acute and sub-acute effects of the endobarrier on glucose homeostasis and appetite in obese uncontrolled type 2 diabetes mellitus patients n.d.
- Rohde U, Hedbäck N, Gluud LL, Vilsbøll T, Knop FK. Effect of the EndoBarrier gastrointestinal liner on obesity and type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2016;18:300-5. https://doi.org/10.1111/dom.12603.
- Miras AD, Herring R, Vusirikala A, Shojaee-Moradi F, Jackson NC, Chandaria S, et al. Measurement of hepatic insulin sensitivity early after the bypass of the proximal small bowel in humans. Obes Sci Pract 2017;3:95-8. https://doi.org/10.1002/osp4.76.
- Rohde U, Federspiel CA, Vilmann P, Langholz E, Friis SU, Krakauer M, et al. The impact of EndoBarrier gastrointestinal liner in obese patients with normal glucose tolerance and in patients with type 2 diabetes. Diabetes Obes Metab 2017;19:189-99. https://doi.org/10.1111/dom.12800.
- Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab 2015;100:E1225-33. https://doi.org/10.1210/jc.2015-2467.
- Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes 2013;37:1553-9. https://doi.org/10.1038/ijo.2013.38.
- Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes 2015;39:1565-74. https://doi.org/10.1038/ijo.2015.115.
- Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999;29:1181-9. https://doi.org/10.1080/004982599238047.
- Barding GA, Salditos R, Larive CK. Quantitative NMR for bioanalysis and metabolomics. Anal Bioanal Chem 2012;404:1165-79. https://doi.org/10.1007/s00216-012-6188-z.
- Edlund U, Grahn H. Multivariate data analysis of NMR data. J Pharm Biomed Anal 1991;9:655-8. https://doi.org/10.1016/0731-7085(91)80191-B.
- Nicoletti CF, Morandi Junqueira-Franco MV, dos Santos JE, Marchini JS, Salgado W, Nonino CB. Protein and amino acid status before and after bariatric surgery: a 12-month follow-up study. Surg Obes Relat Dis 2013;9:1008-12. https://doi.org/10.1016/j.soard.2013.07.004.
- Tan HC, Khoo CM, Tan MZ, Kovalik JP, Ng AC, Eng AK, et al. The effects of sleeve gastrectomy and gastric bypass on branched-chain amino acid metabolism 1 year after bariatric surgery. Obes Surg 2016;26:1830-5. https://doi.org/10.1007/s11695-015-2023-x.
- Sarosiek K, Pappan KL, Gandhi AV, Saxena S, Kang CY, McMahon H, et al. Conserved metabolic changes in nondiabetic and type 2 diabetic bariatric surgery patients: global metabolomic pilot study. J Diabetes Res 2016;2016. https://doi.org/10.1155/2016/3467403.
- Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017;23:859-68. https://doi.org/10.1038/nm.4358.
- Gralka E, Luchinat C, Tenori L, Ernst B, Thurnheer M, Schultes B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am J Clin Nutr 2015;102:1313-22. https://doi.org/10.3945/ajcn.115.110536.
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913-16.e7. https://doi.org/10.1053/j.gastro.2012.06.031.
- Yan M, Song MM, Bai RX, Cheng S, Yan WM. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World J Gastrointest Surg 2016;8:301-7. https://doi.org/10.4240/wjgs.v8.i4.301.
- Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019;68:1417-29. https://doi.org/10.1136/gutjnl-2018-317609.
- Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011;60:1214-23. https://doi.org/10.1136/gut.2010.234708.
- Chen CY, Chen SW, Wang HT. Effect of supplementation of yeast with bacteriocin and Lactobacillus culture on growth performance, caecal fermentation, microbiota composition, and blood characteristics in broiler chickens. Asian-Australas J Anim Sci 2017;30:211-20. https://doi.org/10.5713/ajas.16.0203.
- Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010;59:3049-57. https://doi.org/10.2337/db10–0253.
- Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015;22:228-38. https://doi.org/10.1016/j.cmet.2015.07.009.
- Kim T, Holleman CL, Ptacek T, Morrow CD, Habegger KM. Duodenal endoluminal barrier sleeve alters gut microbiota of ZDF rats. Int J Obes 2017;41:381-9. https://doi.org/10.1038/ijo.2016.224.
- Zuo HJ, Xie ZM, Zhang WW, Li YR, Wang W, Ding XB, et al. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J Gastroenterol 2011;17:1076-81. https://doi.org/10.3748/wjg.v17.i8.1076.
- de Jonge C, Fuentes S, Zoetendal EG, Bouvy ND, Nelissen R, Buurman WA, et al. Metabolic improvement in obese patients after duodenal–jejunal exclusion is associated with intestinal microbiota composition changes. Int J Obes 2019;43:2509-17. https://doi.org/10.1038/s41366-019-0336-x.
- Glaysher MA, Mohanaruban A, Prechtl CG, Goldstone AP, Miras AD, Lord J, et al. A randomised controlled trial of a duodenal-jejunal bypass sleeve device (EndoBarrier) compared with standard medical therapy for the management of obese subjects with type 2 diabetes mellitus. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017–018598.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 9 June 2020).
- Goldstone AP, Miras AD, Scholtz S, Jackson S, Neff KJ, Penicaud L, et al. Link between increased satiety gut hormones and reduced food reward following gastric bypass surgery for obesity. J Clin Endocrinol Metab 2016;101:599-60. https://doi.org/10.1210/jc.2015-2665.
- Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 2008;93:1980-7. https://doi.org/10.1210/jc.2007-2235.
- Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 2014;99:1319-30. https://doi.org/10.3945/ajcn.113.075291.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-19. https://doi.org/10.1007/bf00280883.
- Anderson GH, Catherine NL, Woodend DM, Wolever TM. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr 2002;76:1023-30. https://doi.org/10.1093/ajcn/76.5.1023.
- Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr 2016;104:5-14. https://doi.org/10.3945/ajcn.115.126706.
- FSL . Atlases n.d. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases (accessed 9 July 2020).
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825-41. https://doi.org/10.1006/nimg.2002.1132.
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med 2003;49:193-7. https://doi.org/10.1002/mrm.10354.
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143-55. https://doi.org/10.1002/hbm.10062.
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 2004;21:1732-47. https://doi.org/10.1016/j.neuroimage.2003.12.023.
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143-56. https://doi.org/10.1016/S1361-8415(01)00036-6.
- Finlayson G, King N, Blundell JE. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol Behav 2007;90:36-42. https://doi.org/10.1016/j.physbeh.2006.08.020.
- Griffioen-Roose S, Finlayson G, Mars M, Blundell JE, de Graaf C. Measuring food reward and the transfer effect of sensory specific satiety. Appetite 2010;55:648-55. https://doi.org/10.1016/j.appet.2010.09.018.
- Miguet M, Fillon A, Khammassi M, Masurier J, Julian V, Pereira B, et al. Appetite, energy intake and food reward responses to an acute high intensity interval exercise in adolescents with obesity. Physiol Behav 2018;195:90-7. https://doi.org/10.1016/j.physbeh.2018.07.018.
- Carvalho-Ferreira JP, Finlayson G, da Cunha DT, Caldas G, Bandoni D, de Rosso VV. Adiposity and binge eating are related to liking and wanting for food in Brazil: a cultural adaptation of the Leeds food preference questionnaire. Appetite 2019;133:174-83. https://doi.org/10.1016/j.appet.2018.10.034.
- Cunningham JJ. An individualization of dietary requirements for energy in adults. J Am Diet Assoc 1982;80:335-8.
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71-83. https://doi.org/10.1016/0022-3999(85)90010-8.
- Wardle J. Eating style: a validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. J Psychosom Res 1987;31:161-9. https://doi.org/10.1016/0022-3999(87)90072-9.
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire?. Int J Eat Disord 1994;16:363-70.
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 2009;53:114-18. https://doi.org/10.1016/j.appet.2009.05.016.
- Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009;52:430-6. https://doi.org/10.1016/j.appet.2008.12.003.
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47-55. https://doi.org/10.1016/0306-4603(82)90024-7.
- Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand 1970;188:479-86. https://doi.org/10.1111/j.0954-6820.1970.tb08072.x.
- Arts J, Caenepeel P, Bisschops R, Dewulf D, Holvoet L, Piessevaux H, et al. Efficacy of the long-acting repeatable formulation of the somatostatin analogue octreotide in postoperative dumping. Clin Gastroenterol Hepatol 2009;7:432-7. https://doi.org/10.1016/j.cgh.2008.11.025.
- Gratton J, Phetcharaburanin J, Mullish BH, Williams HR, Thursz M, Nicholson JK, et al. Optimized sample handling strategy for metabolic profiling of human feces. Anal Chem 2016;88:4661-8. https://doi.org/10.1021/acs.analchem.5b04159.
- Dona AC, Jiménez B, Schäfer H, Humpfer E, Spraul M, Lewis MR, et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem 2014;86:9887-94. https://doi.org/10.1021/ac5025039.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. University of York, Centre for Health Economics; 1995.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Valuation Set for England 2018 n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 6 December 2019).
- Haymarket Media Group . Monthly Index of Medical Specialities (MIMS) 2019 n.d. www.mims.co.uk/ (accessed 6 December 2019).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit (PSSRU), University of Kent; 2018.
- NHS Improvement . National Schedule of Reference Costs – Year 2017–18 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 6 December 2019).
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- World Health Organization . Anatomic Therapeutic Chemical (ATC) and Daily Defined Dose (DDD) Toolkit 2020. www.who.int/medicines/regulation/medicines-safety/toolkit/en/ (accessed 29 September 2020).
- Vroomen JM, Eekhout I, Dijkgraaf MG, van Hout H, de Rooij SE, Heymans MW, et al. Multiple imputation strategies for zero-inflated cost data in economic evaluations: which method works best?. Eur J Health Econ 2016;17:939-50. https://doi.org/10.1007/s10198-015-0734-5.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Nauck M, . Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care 2012;35:1364-79. https://doi.org/10.2337/dc12-0413.
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383-93. https://doi.org/10.1056/NEJMoa021778.
- Department of Health and Social Care (DHSC) . Research Governance Framework for Health and Social Care 2005. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf (accessed 6 July 2020).
- Ruban A, Prechtl CG, Glaysher MA, Chhina N, Al-Najim W, Miras AD, et al. Effectiveness of different recruitment strategies in an RCT of a surgical device: experience from the Endobarrier trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-032439.
- Forner PM, Ramacciotti T, Farey JE, Lord RV. Safety and effectiveness of an endoscopically placed duodenal-jejunal bypass device (EndoBarrier®): outcomes in 114 patients. Obes Surg 2017;27:3306-13. https://doi.org/10.1007/s11695-017-2939-4.
- Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Public Policy Committee of the American Association for the Study of Liver Disease . Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008;47:1363-70. https://doi.org/10.1002/hep.22109.
- Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004;328. https://doi.org/10.1136/bmj.38050.593634.63.
- Ochner CN, Laferrère B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci Res 2012;74:138-43. https://doi.org/10.1016/j.neures.2012.08.002.
- Chhina N, Coxon C, Onowaki N, Scholtz S, Purayastha S, Moorthy K, et al. Healthier Food Hedonics and Emotional Eating Link with Orbitofrontal Cortex and Amygdala Responses to Food and Unpleasant Images after Gastric Bypass Surgery. New Orleans: Abstracts, The Obesity Society; 2016.
- Flores L, Chhina N, Parastika T, Zaki B, Onokwai N, Goldstone AP. Healthier Food Reward-Hedonic Responses After Gastric Bypass Surgery Correlate With Weight Loss But Not Decreases in Insulin Resistance n.d.
- ClinicalTrials.gov . Randomisation to Endobarrier Alone Versus With Incretin Analogue in SustainEd Diabesity (REVISE-Diabesity) n.d. https://clinicaltrials.gov/ct2/show/NCT02055014 (accessed 14 October 2020).
- Densford F. GI Dynamics Touts No Complications, Lowered Insulin in EndoBarrier Reimplantation Trial n.d. www.massdevice.com/gi-dynamics-touts-no-complications-lowered-insulin-in-reimplantation-trial/2017 (accessed 6 December 2019).
Appendix 1 Summary of protocol amendments
The ethics committee approved the following amendments to the trial protocol following approval of the first version of the document.
Protocol version 2.0 (23 September 2014):
-
Liquid diet increased from 14 to 20 days.
-
Swedish Obesity Study questionnaire exchanged with EPIC Food Frequency Questionnaire.
-
Blood tests changed (zinc and SHBG removed; number of vitamin D tests reduced; iron, serum folate and vitamin B12 added).
-
Bioelectrical impedance during mechanistic visits for all patients added.
-
Patients instructed not to take their diabetes medication in the morning of trial visits.
-
Urine dipstick added to screening visit.
-
H. pylori breath test changed to study visit 2.
-
Female patients asked for last day of their menstrual period (length of cycle and length of menstruation).
-
Potential overnight stay added to insulin clamps depending on blood sugar reading days prior to visit.
-
Patients instructed to eat a standardised meal or meal replacement prior to insulin clamp visit.
-
Number of DNA and RNA samples increased to all mechanistic visits.
-
Gastric and small bowel biopsies taken during device implant and explant.
-
Additional patient dietary information leaflets developed and given to patients by dietitians during consultations.
-
Other administrative changes, such as wording and typos.
Protocol version 3.0 (16 March 2015):
-
Wording of withdrawal criteria changed.
-
New recruitment strategies and material used for recruitment, such as business cards, news stories, adverts and posters added.
-
Changed stratification criteria for randomisation.
-
The AE section of the protocol has been restructured to distinguish between AEs in different treatment groups, and to clarify definitions of adverse device effects (ADEs).
-
A new ADE has been added to the EndoBarrier group (1% incidence of liver abscess during implant period).
-
Clarification of anticipated serious adverse device effects in the EndoBarrier group.
-
Frequency of some blood tests reduced (cortisol/luteinising hormone/follicle-stimulating hormone/oestradiol/progesterone only in subgroup 1 at visit 3 and 8).
Protocol version 3.1 (3 August 2015):
-
Correction of administrative error. Inclusion criterion for C-peptide has been changed from 1665 pmol/l to 166.5 pmol/l.
Protocol version 4.0 (10 August 2015):
-
HbA1c inclusion criterion 86 mmol/mol (10%) has been changed to 97 mmol/mol (11%).
-
Inclusion criterion ‘BMI 30–50 kg/m2 with adequate insulin reserve as indicated with insulin C-peptide levels > 166.5 pmol/l’ has been changed to ‘BMI 30–50 kg/m2’ only. Instead a new exclusion criterion has been added as follows: ‘Evidence of absolute insulin deficiency as indicated by clinical assessment, a long duration of T2DM and a fasting plasma cpeptide of < 333 pmol/l’.
-
Exclusion criteria ‘History of iron deficiency and/or iron deficiency anaemia’ has been changed to ‘Current iron deficiency and/or iron deficiency anaemia’.
-
Exclusion criterion ‘Severe liver (AST, ALT or gGT > 4 times upper limit) or kidney failure (serum creatinine > 180 mmol/l), estimated Glomerular Filtration Rate (GFR) cut-off 60’, has been changed to ‘Severe liver impairment i.e. AST, ALT or gGT > 4 times upper limit of the reference range or kidney impairment i.e. estimated Glomerular Filtration Rate (GFR) < 45 ml/min/1.73m2’.
-
‘C13 Urea Breath test’ has been removed from the screening visit for all patients and ‘PPI and H. Pylori test’ has been added to visit 2 for the EndoBarrier group only.
Protocol version 4.1 (29 September 2016):
-
The number of randomised patients has been increased from 80 to 85 per research site.
Protocol version 5.0 (5 March 2018):
-
Changes in safety reporting such as more specific outline of timelines for safety reporting and the role and responsibility of the manufacturer in terms of reporting SAEs.
-
Changes in the follow-up schedule for patients in the control arm and all patients who withdrew consent prior to treatment start but after randomisation.
Appendix 2 Tables and figures
| Outcome | Available mechanistic group | Visit | ||||
|---|---|---|---|---|---|---|
| 3 (baseline) | 5 | 8 | 10 | 14 | ||
| Time from EndoBarrier insertion (weeks) | –2 | 2 | 26 | 50 | 100 | |
| EndoBarrier in situ | ✗ | ✗ | ✗ | |||
| fMRI scan | 1 | ✗ | ✗ | |||
| LFPQ | 1, 3 | ✗ | ✗ | |||
| Food taste ratings | 1 | ✗ | ✗ | |||
| Ad libitum food intake | 1 | ✗ | ✗ | |||
| PRT | 1 | ✗ | ✗ | |||
| Fasting bloods | 1, 3 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Fasting VAS ratings | 1, 3 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Questionnaires | ||||||
| Eating behaviour | 1, 2, 3 | ✗ | ✗ | ✗ | ✗ | |
| Dumping syndrome | 1, 2, 3 | ✗ | ✗ | ✗ | ✗ | |
| Weight, bio-impedance analysis | 1, 2, 3 | ✗ | ✗ | ✗ | ✗ | |
| Variable | Statistics | Primary analysis population | Not analysed | ||
|---|---|---|---|---|---|
| Standard therapy | EndoBarrier | Standard therapy | EndoBarrier | ||
| Age (years) | n | 58 | 55 | 27 | 30 |
| Mean | 52.9 | 52.1 | 49.7 | 50.7 | |
| SD | 7.67 | 7.26 | 9.75 | 9.12 | |
| Ethnicity (%) | Asian | 5 (8.6) | 8 (14.5) | 4 (14.8) | 3 (10.0) |
| Black | 6 (10.3) | 3 (5.5) | 7 (25.9) | 0 | |
| Mixed | 1 (1.7) | 0 | 0 | 1 (3.3) | |
| White | 46 (79.3) | 44 (80.0) | 16 (59.3) | 26 (86.7) | |
| Gender (%) | Female | 26 (44.8) | 24 (43.6) | 13 (48.1) | 15 (50.0) |
| Male | 32 (55.2) | 31 (56.4) | 14 (51.9) | 15 (50.0) | |
| Height (cm) | n | 58 | 55 | 27 | 30 |
| Mean | 170.5 | 170.6 | 170.5 | 172.2 | |
| SD | 9.20 | 9.58 | 8.62 | 8.57 | |
| Weight (kg) | n | 58 | 55 | 27 | 30 |
| Mean | 102.55 | 107.99 | 107.88 | 107.70 | |
| SD | 14.130 | 16.755 | 16.146 | 17.893 | |
| BMI (kg/m2) | n | 58 | 55 | 27 | 30 |
| Mean | 35.22 | 37.10 | 37.12 | 36.30 | |
| SD | 3.706 | 4.804 | 4.994 | 5.265 | |
| Pulse (b.p.m.) | n | 58 | 55 | 27 | 30 |
| Mean | 76.8 | 77.0 | 78.2 | 77.6 | |
| SD | 11.68 | 10.81 | 6.40 | 10.63 | |
| Systolic blood pressure (mmHg) | n | 58 | 55 | 27 | 30 |
| Mean | 133.3 | 131.3 | 131.6 | 128.4 | |
| SD | 14.89 | 10.77 | 16.45 | 13.76 | |
| Diastolic blood pressure (mmHg) | n | 58 | 55 | 27 | 30 |
| Mean | 83.7 | 81.1 | 82.3 | 84.2 | |
| SD | 10.76 | 9.35 | 10.10 | 10.14 | |
| HbA1c (mmol/mol) | n | 58 | 55 | 27 | 30 |
| Mean | 70.55 | 73.27 | 72.56 | 74.37 | |
| SD | 9.627 | 10.645 | 9.889 | 9.722 | |
| Waist (cm) | n | 58 | 55 | 27 | 30 |
| Mean | 117.7 | 119.1 | 118.0 | 117.8 | |
| SD | 17.15 | 11.74 | 13.29 | 13.47 | |
| BMI stratum, n(%) | 30–40 kg/m2 | 50 (86.2) | 41 (74.5) | 17 (63.0) | 26 (86.7) |
| 40–50 kg/m2 | 8 (13.8) | 14 (25.5) | 10 (37.0) | 4 (13.3) | |
Adverse events
| Treatment | Site name | Total patients with AEs, n (%) | Number of AEs | Total patients with SAEs, n (%) | Number of SAEs |
|---|---|---|---|---|---|
| EndoBarrier | Imperial College London (N = 42) | 40 (95.2%) | 206 | 11 (26.2%) | 13 |
| University Hospital Southampton NHS Foundation Trust (N = 43) | 40 (93.0%) | 313 | 24 (55.8%) | 32 | |
| All sites (N = 85) | 80 (94.1%) | 519 | 35 (41.2%) | 45 | |
| Standard therapy | Imperial College London (N = 43) | 32 (74.4%) | 104 | – | – |
| University Hospital Southampton NHS Foundation Trust (N = 42) | 39 (92.9%) | 234 | 5 (11.9%) | 5 | |
| All sites (N = 85) | 71 (83.5%) | 338 | 5 (5.9%) | 5 | |
| All patients | Imperial College London (N = 85) | 72 (84.7%) | 310 | 11 (12.9%) | 13 |
| University Hospital Southampton NHS Foundation Trust (N = 85) | 79 (92.9%) | 547 | 29 (34.1%) | 37 | |
| All sites (N = 170) | 151 (88.8%) | 857 | 40 (23.5%) | 50 |
| Treatment group | All AEs | |||||
|---|---|---|---|---|---|---|
| Unrelated | Unlikely | Possible | Probable | Definite | Total | |
| EndoBarrier | 181 | 42 | 87 | 90 | 119 | 519 |
| Standard therapy | 280 | 14 | 13 | 18 | 13 | 338 |
| All patients | 461 | 56 | 100 | 108 | 132 | 857 |
| Patients with at least one AEa | ||||||
| EndoBarrier | 6 | 3 | 5 | 7 | 59 | 80 |
| Standard therapy | 40 | 7 | 3 | 9 | 12 | 71 |
| All patients | 46 | 10 | 8 | 16 | 71 | 151 |
| Site | Category | Treatment arm | ||
|---|---|---|---|---|
| Standard therapy | EndoBarrier | Total | ||
| Imperial College London | Other medically important event | – | 4 | 4 |
| Required inpatient hospitalisation/prolongation of existing hospitalisation | – | 9 | 9 | |
| Site total | – | 13 | 13 | |
| University Hospital Southampton NHS Foundation Trust | Life-threatening | 1 | 1 | 2 |
| Other medically important event | – | 5 | 5 | |
| Required inpatient hospitalisation/prolongation of existing hospitalisation | 4 | 26 | 30 | |
| Site total | 5 | 32 | 37 | |
| All | Life-threatening | 1 | 1 | 2 |
| Other medically important event | – | 9 | 9 | |
| Required inpatient hospitalisation/prolongation of existing hospitalisation | 4 | 35 | 39 | |
| Site total | 5 | 45 | 50 | |
Functional magnetic resonance imaging
| Characteristic | Standard therapy | EndoBarrier | Statistic | p-value |
|---|---|---|---|---|
| N | 13 | 12 | – | – |
| Age (years) | 50.6 ± 9.6 (31–64) | 51.9 ± 7.1 (33–59) | t –0.38 | 0.70 |
| Female | 4 (30.8%) | 8 (66.7%) | γ 3.22 | 0.073 |
| Caucasian | 6 (46.2%) | 8 (66.7%) | γ 1.07 | 0.30 |
| BMI (kg/m2) | 34.7 ± 3.8 (30.6–42.0) | 38.9 ± 5.2 (31.4–50.6) | t –2.31 | 0.030 |
| Body fat (%) | 32.8 [27.9–44.4] (26.6–47.5) | 46.4 [33.5–50.6] (27.2–56.3) | Z –2.23 | 0.026 |
| Characteristic | Standard therapy | EndoBarrier | Statistic | p-value |
|---|---|---|---|---|
| N | 28 | 30 | – | – |
| Age (years) | 52.9 ± 7.5 (31–64) | 52.0 ± 7.7 (33–64) | t 0.43 | 0.67 |
| Female | 12 (42.9%) | 13 (43.3%) | γ 0.001 | 0.97 |
| Caucasian | 18 (64.3%) | 21 (70.0%) | γ 0.22 | 0.64 |
| BMI (kg/m2) | 35.2 ± 3.7 (30.6–42.9) | 37.4 ± 5.7 (29.9–50.6) | t –1.74 | 0.087 |
| Body fat (%) | 36.0 [30.1–46.2] (26.6–52.5) | 39.5 [33.9–46.1] (26.7–56.3) | Z –0.71 | 0.48 |
| Characteristic | Standard therapy | EndoBarrier | Statistic | p-value |
|---|---|---|---|---|
| N | 44 | 48 | – | – |
| Age (years) | 52.4 ± 7.9 (31–64) | 52.3 ± 6.9 (33–64) | t 0.05 | 0.96 |
| Female | 16 (36.4%) | 23 (47.9%) | γ 1.26 | 0.26 |
| Caucasian | 33 (75.0%) | 39 (81.3%) | γ 0.53 | 0.47 |
| BMI (kg/m2) | 34.8 [31.6–36.4] (29.2–42.9) | 35.9 [32.7–40.6] (29.9–50.6) | Z –1.94 | 0.052 |
| Body fat (%) | 38.0 ± 7.2 (26.6–52.5) | 41.5 ± 7.7 (26.7–56.3) | t –2.23 | 0.028 |
FIGURE 18.
Explicit liking and wanting, and implicit wanting from LFPQ. Comparison of (a) explicit liking; (b) explicit wanting of any food category (averaged across HF, LF, sweet, savoury); (c) implicit wanting (adjusted for choice frequency) for sweet vs. savoury foods; and (d) implicit wanting (adjusted for choice frequency) for HF vs. LF foods, between standard treatment (blue unfilled circles, solid line) and EndoBarrier (red filled circles, dotted line) groups over time (baseline, and 26, 50 and 100 weeks after EndoBarrier insertion; black bar indicates period when EndoBarrier in situ). Data presented as mean ± SEM, numbers at each time point are given beneath graph, n = 24–30. Fixed-effects mixed-model analysis with post hoc Fisher’s exact LSD test: p-value vs. week 0 or week 100. LSD, least significant difference.
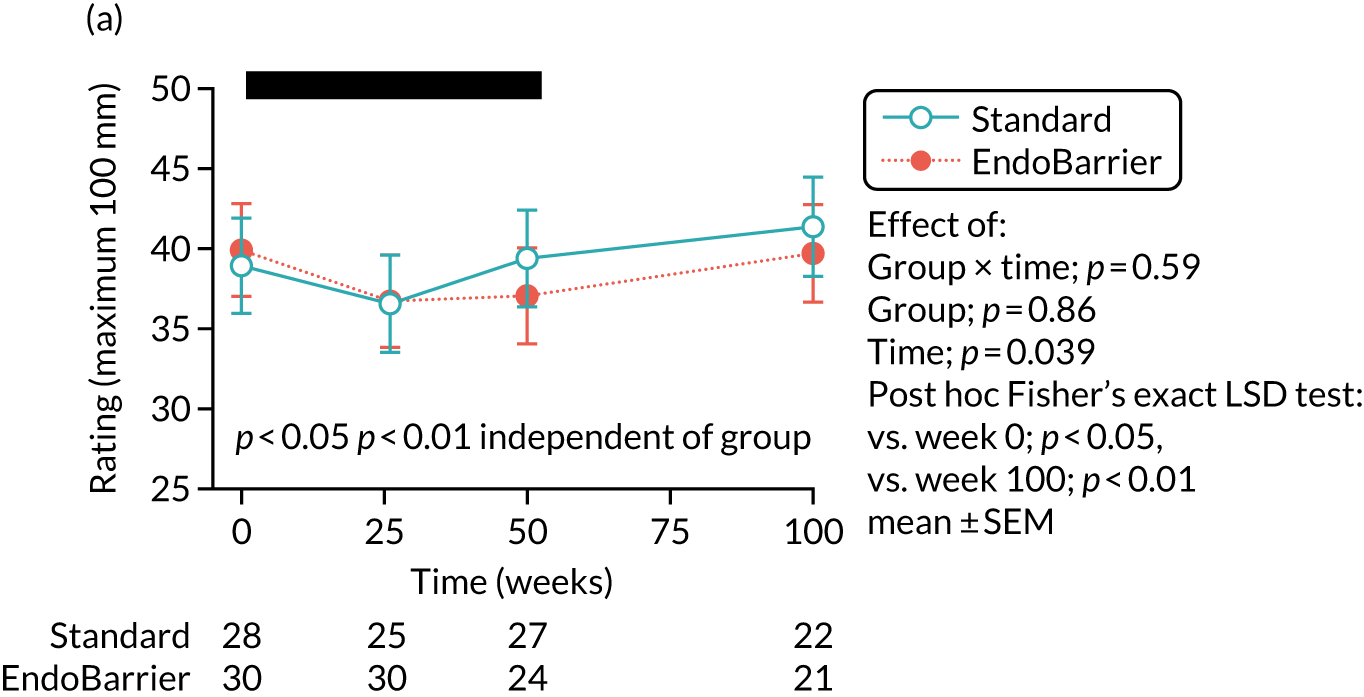
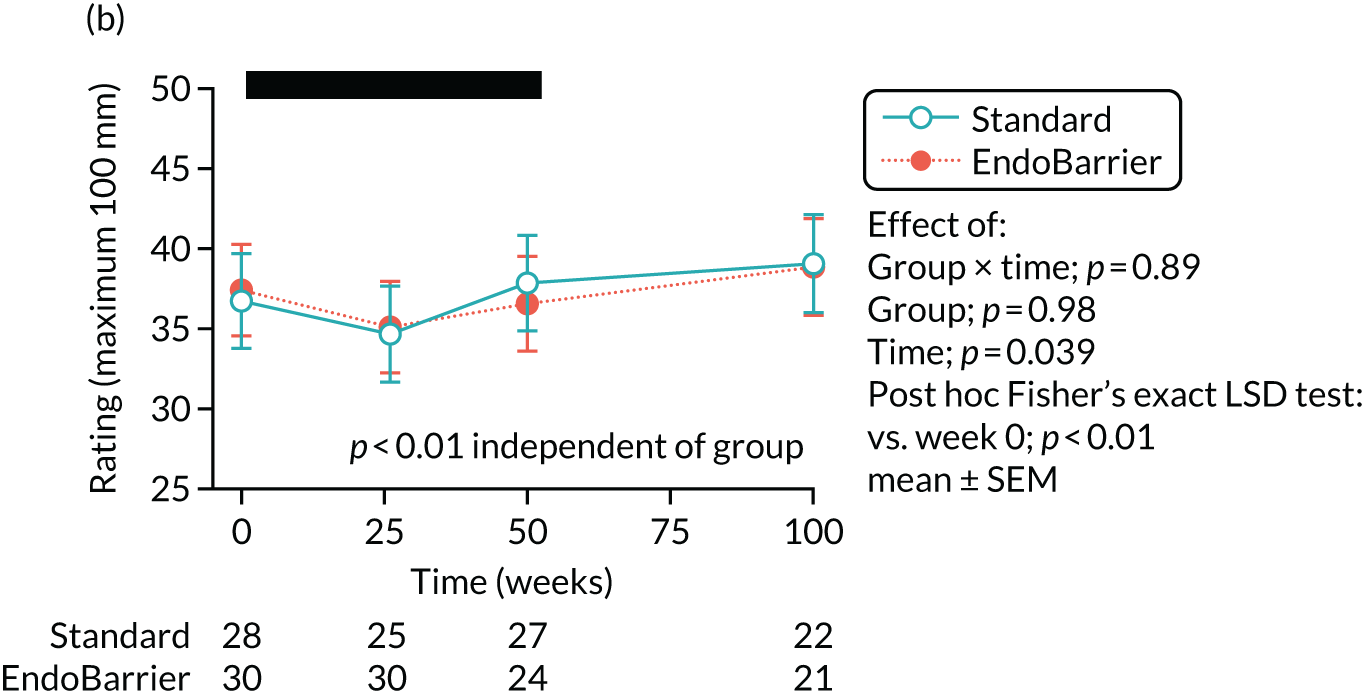


Figure 19 shows the comparison of ratings of creaminess intensity (Figure 19a and b), pleasantness (Figure 19c and d) and sweet intensity (Figure 19e) for savoury versus sweet (Figure 19a and c) and HF versus LF foods (Figure 19b, d and e) tasted at the start of the ad libitum test lunch, across all dishes (Figure 19a–d) (savoury LF broth, savoury HF cream soup, sweet LF yoghurt, sweet HF ice cream), or just sweet dishes (Figure 19e) (yoghurt, ice cream), between standard treatment and EndoBarrier groups over time at baseline (week 0) or at 26 weeks.
FIGURE 19.
Taste ratings at ad libitum test lunch. (a) Creaminess intensity sweet vs. savoury; (b) creaminess intensity HF vs. LF; (c) taste pleasantness sweet vs. savoury; (d) taste pleasantness HF vs. LF; and (e) sweet intensity HF vs. LF. Data presented as mean ± SEM (n = 12–13). Statistics from repeated measures ANOVA, with group (standard, EndoBarrier) as a between-patient factor and time (0, 26 weeks) and sweetness (savoury, sweet) [parts (a) and (c)] or fat (LF, HF) [parts (b), (d) and (e)] as within-patient factors, with post hoc Fisher’s exact LSD test: *p < 0.05. LSD, least significant difference.
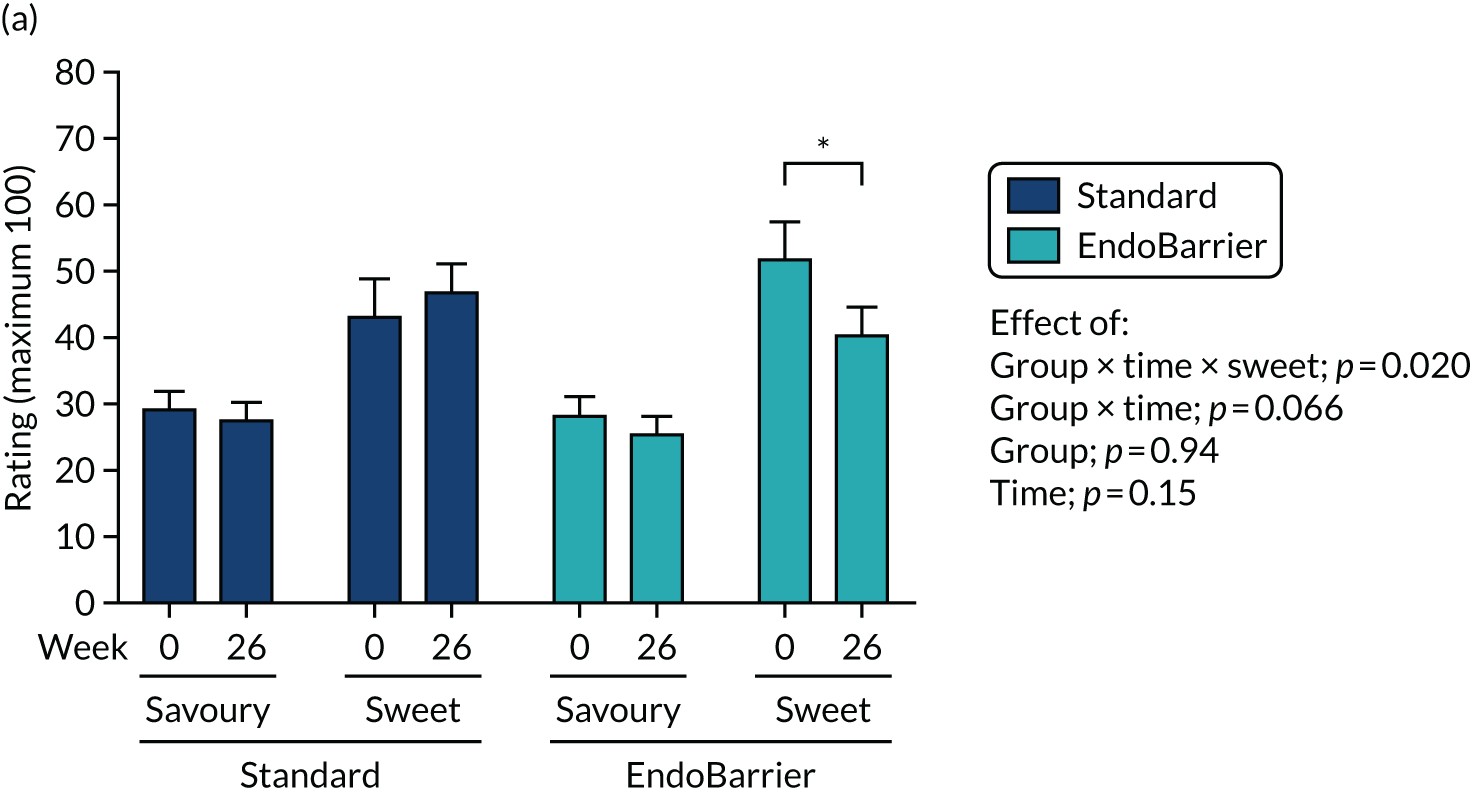
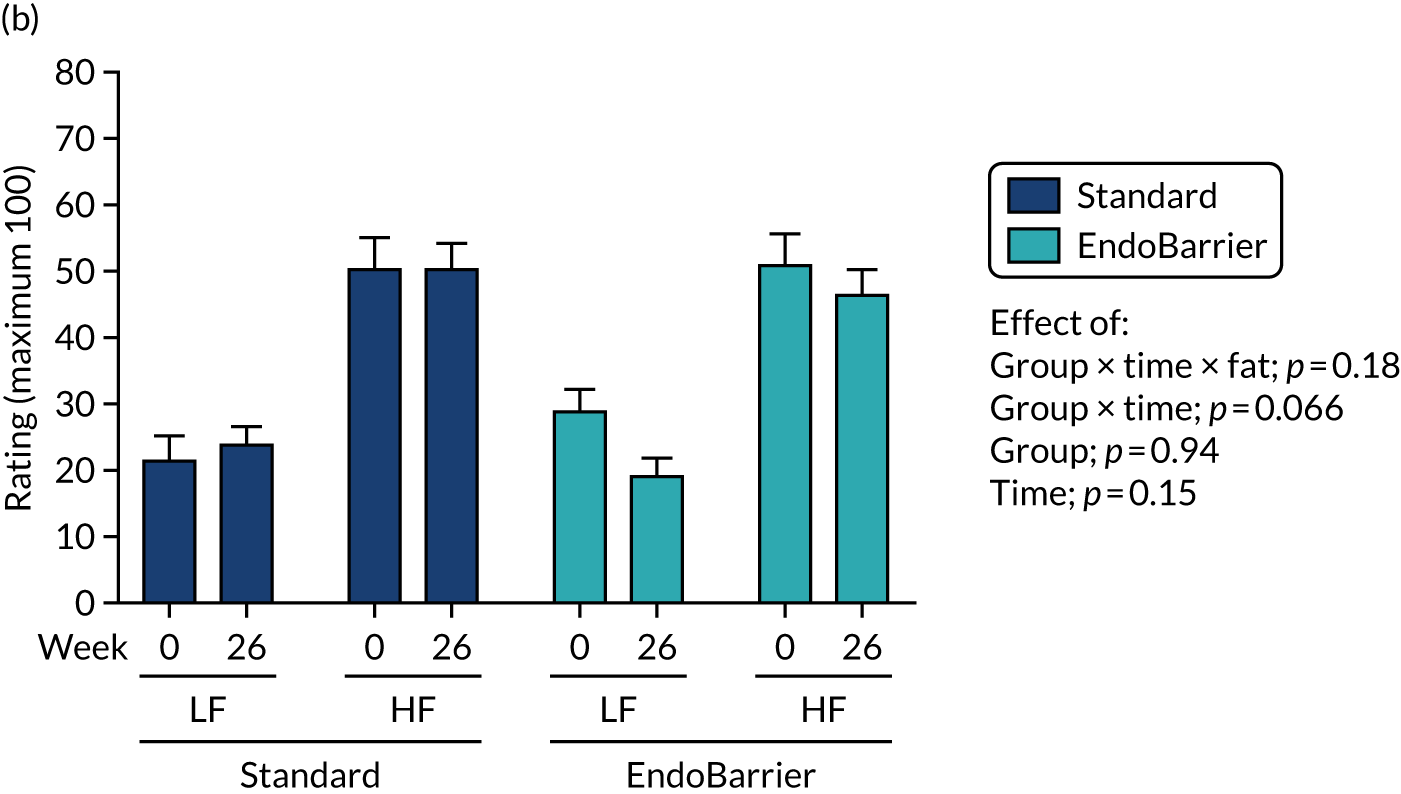
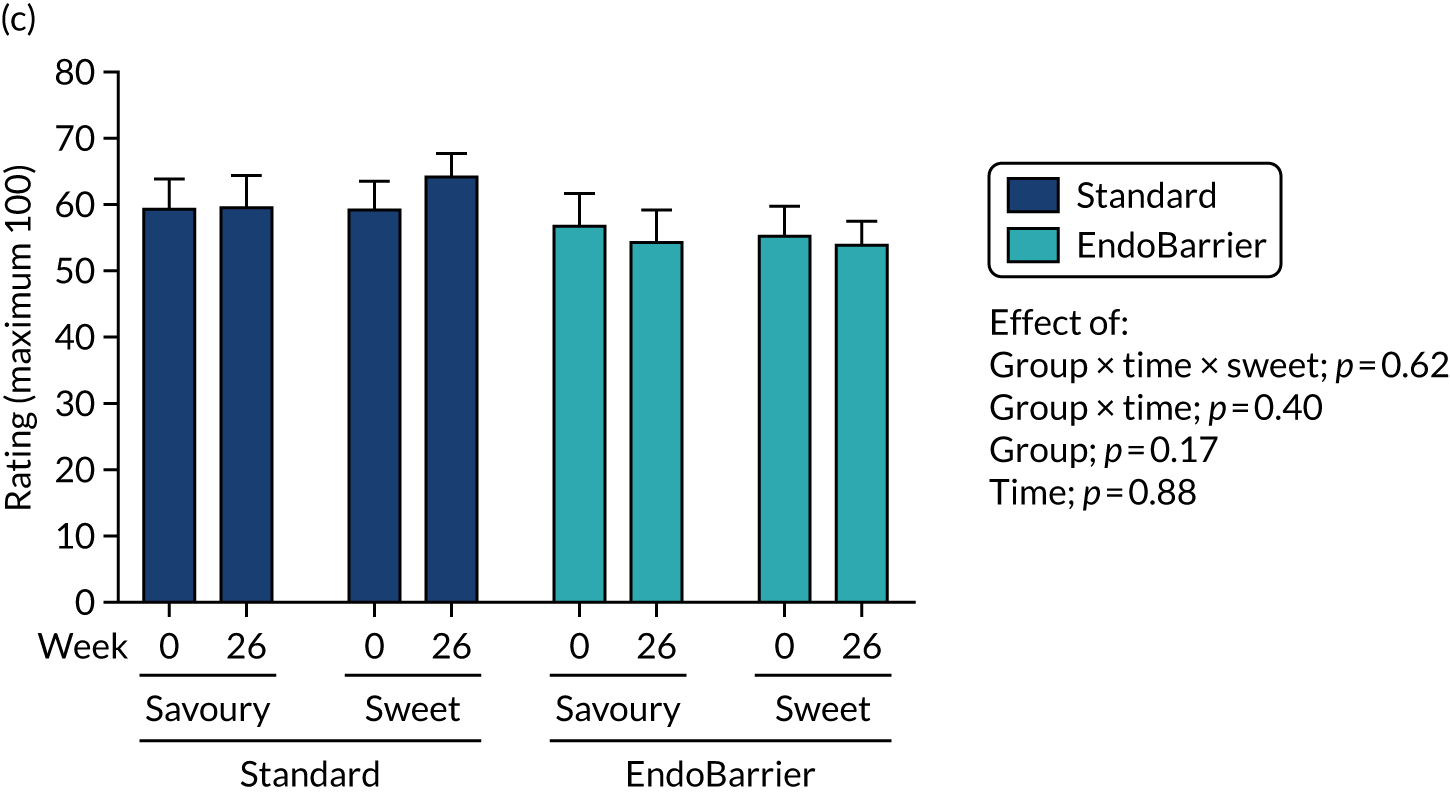
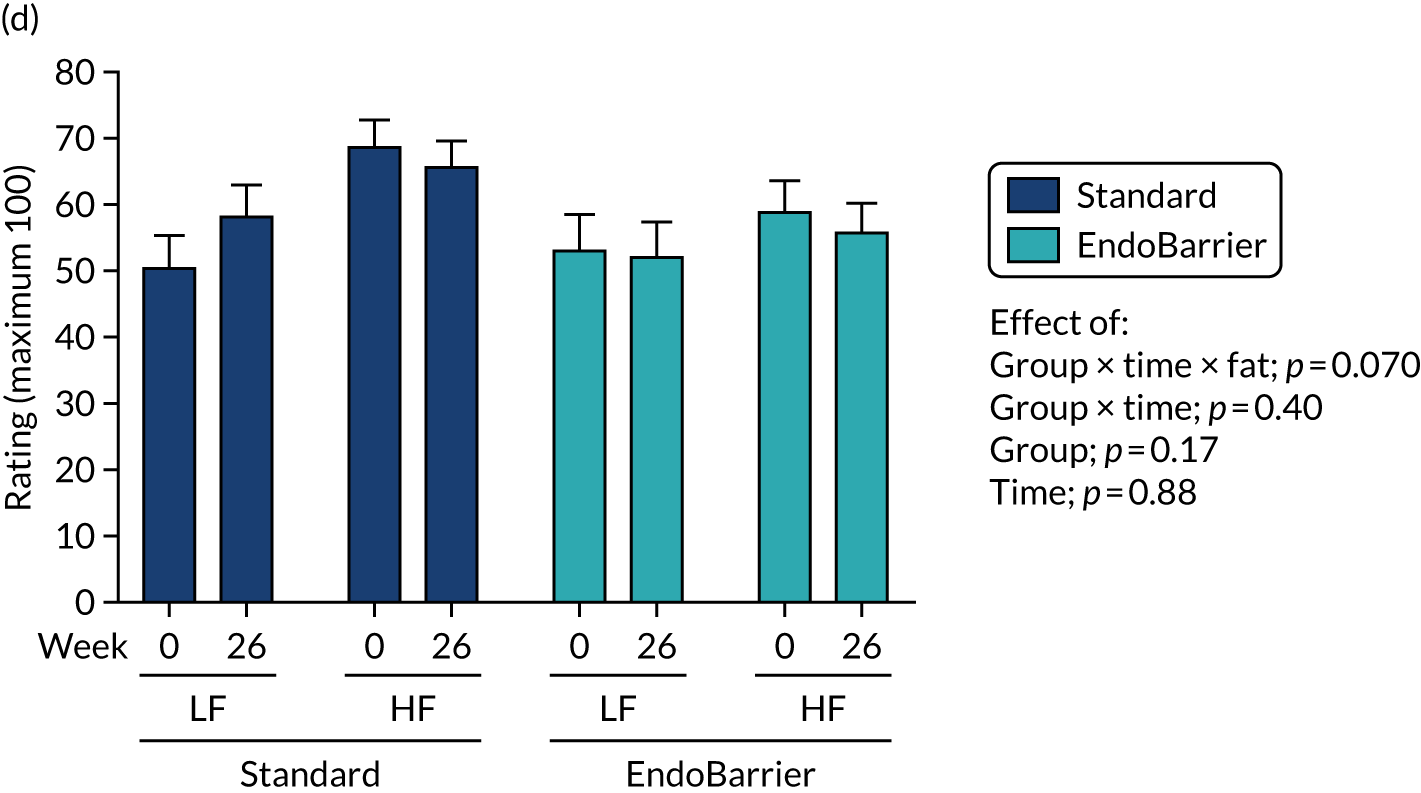
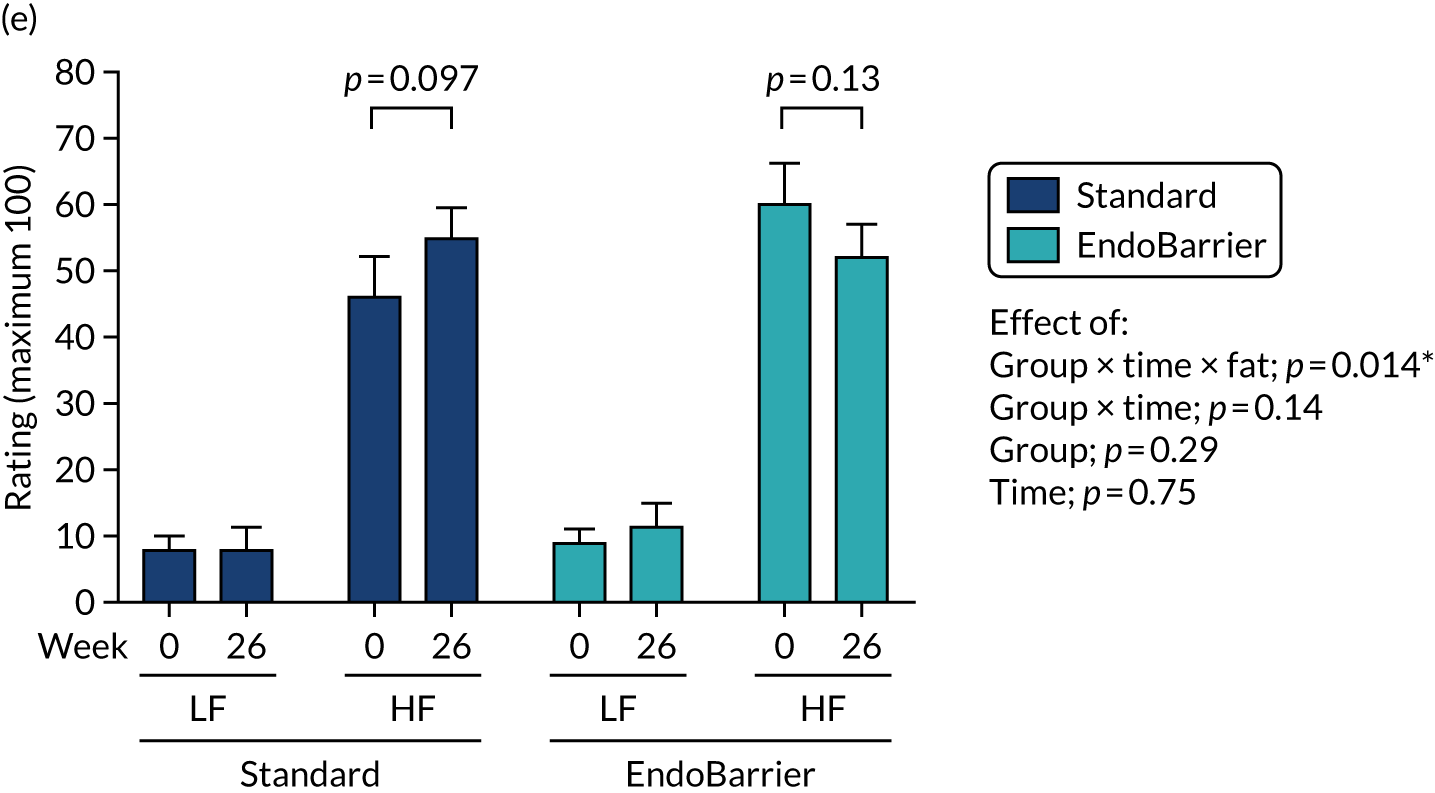
The comparison of energy intake for total food and individual dishes (LF savoury broth, HF savoury cream soup, LF sweet yoghurt, HF sweet ice cream) as absolute kilocalories is shown in Figure 20a, and for macronutrients (fat, carbohydrate, protein) is shown in Figures 20b and c between the standard treatment group and the EndoBarrier group over time at baseline (week 0) or at 26 weeks. This is expressed as absolute kilocalories in Figure 20b and as the percentage of total energy intake at the meal in Figure 20c.
FIGURE 20.
Energy intake at ad libitum test lunch. Ad libitum lunch intake by (a) dishes (absolute); (b) macronutrients (absolute); and (c) macronutrient (percentage). Data presented as mean ± SEM (n = 12–13). Statistics from repeated measures ANOVA, with group (standard, EndoBarrier) as a between-patient factor, and time (0, 26 weeks) and sweetness (savoury, sweet) [part (a)] and fat (LF, HF) or macronutrient (fat, carbohydrate, protein) [parts (b) and (c)] as within-patient factors. *p < 0.05. CHO, carbohydrate.



Figures 21a–c show the comparison of scores for dietary restraint using DEBQ, TFEQ and EDEQ, respectively; Figures 21d–g show the comparison of scores for food hedonics using DEBQ-external eating, PFS, YFAS and BES, respectively, between the standard treatment group (blue unfilled circles, solid line) and the EndoBarrier group (red filled circles, dotted line) over time (week 0 at baseline, and 2, 26, 50 and 100 weeks after EndoBarrier insertion; the black bar indicates the period when EndoBarrier in situ).
FIGURE 21.
Eating behaviour questionnaires measuring dietary restraint and food hedonics. (a) DEBQ-restraint; (b) TFEQ-restraint; (c) EDEQ-restraint; (d) DEBQ-external; (e) PFS; (f) YFAS; and (g) BES. Data presented as mean ± SEM, numbers at each time point are given beneath graph. Fixed-effects mixed-model ANOVA with post hoc Fisher’s LSD test: p-value vs. week 0.


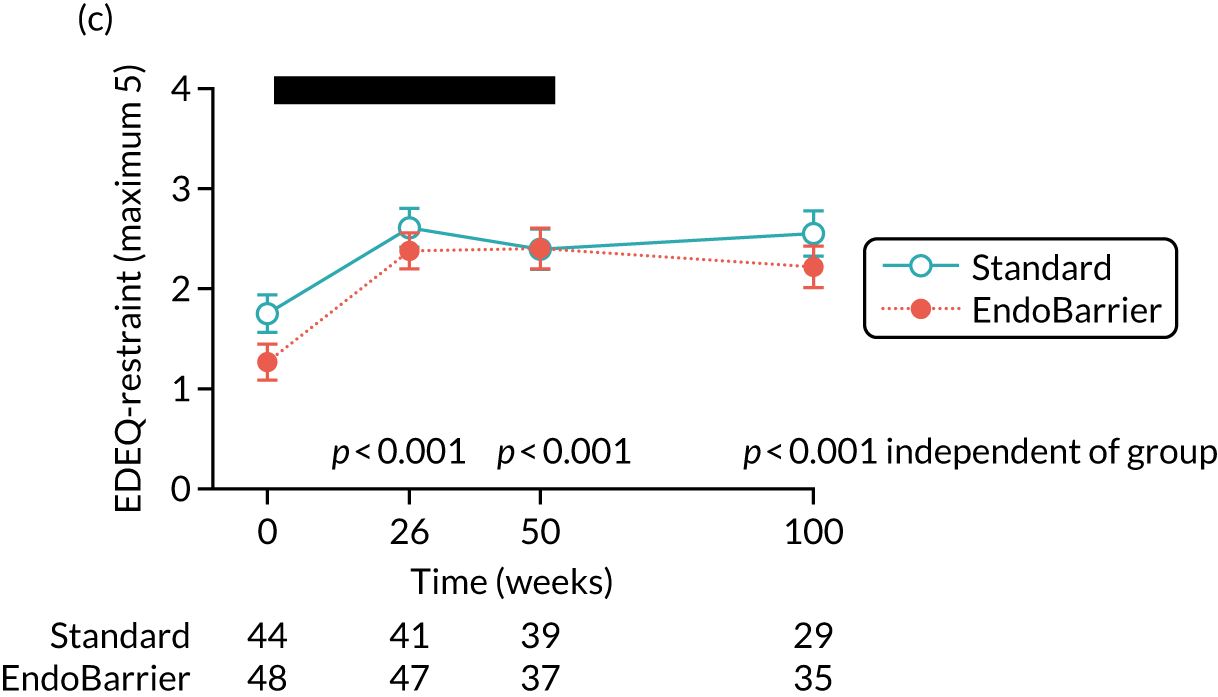

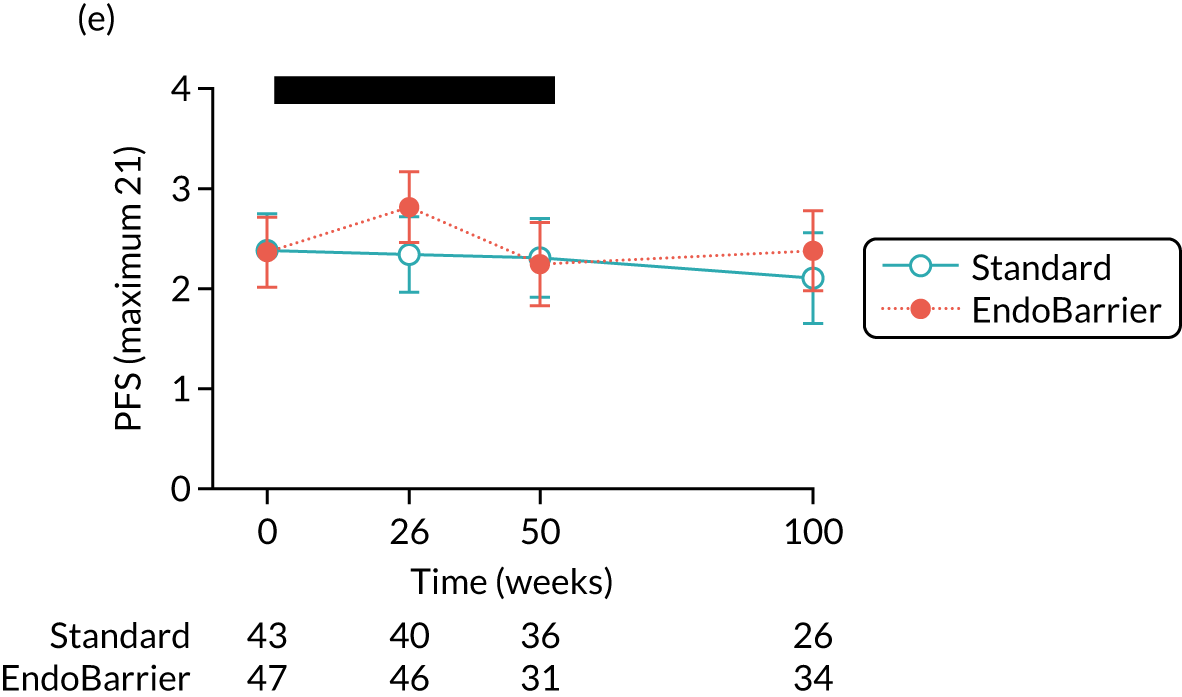


Figures 22a–d show the comparison of scores for TFEQ-hunger-related eating, TFEQ-disinhibited eating, DEBQ-emotional eating and alcohol misuse from the Alcohol Use Disorders Identification Test (AUDIT), respectively, between the standard treatment group (blue unfilled circles, solid line) and the EndoBarrier group (red filled circles, dotted line) over time (week 0 at baseline, and 26, 50 and 100 weeks after EndoBarrier insertion; black bar indicates period when EndoBarrier in situ).
FIGURE 22.
Questionnaires measuring hunger and emotional-related eating behaviours and alcohol misuse. (a) TFEQ-hunger; (b) TFEQ-disinhibition; (c) DBEQ-emotional; and (d) AUDIT. Data presented as mean ± SEM, numbers at each time point are given beneath graph. Fixed-effects mixed-model ANOVA with post hoc Fisher’s LSD test: p-value vs. week 0. For ANOVA results see Table 30.

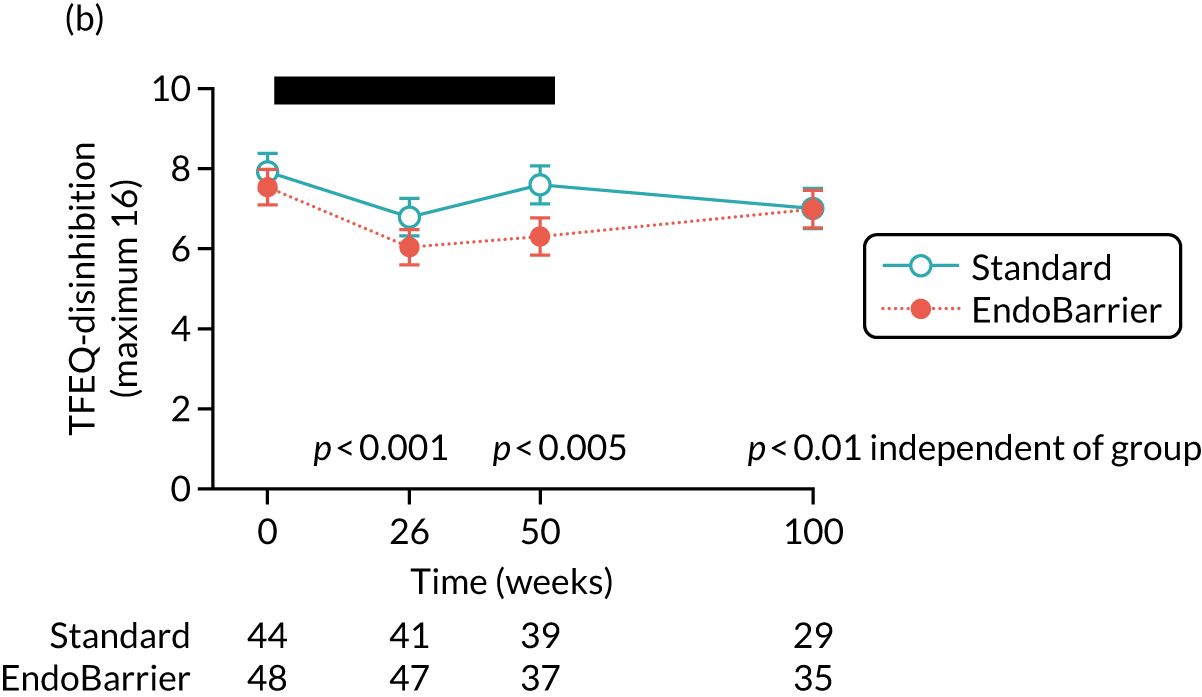
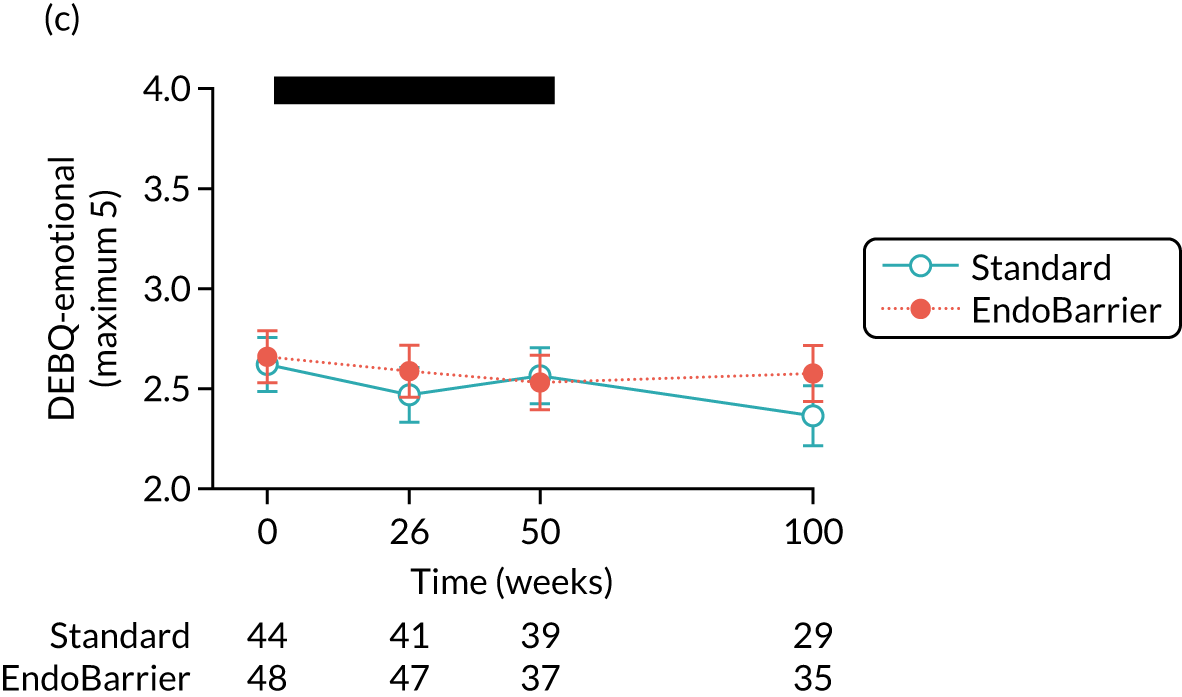
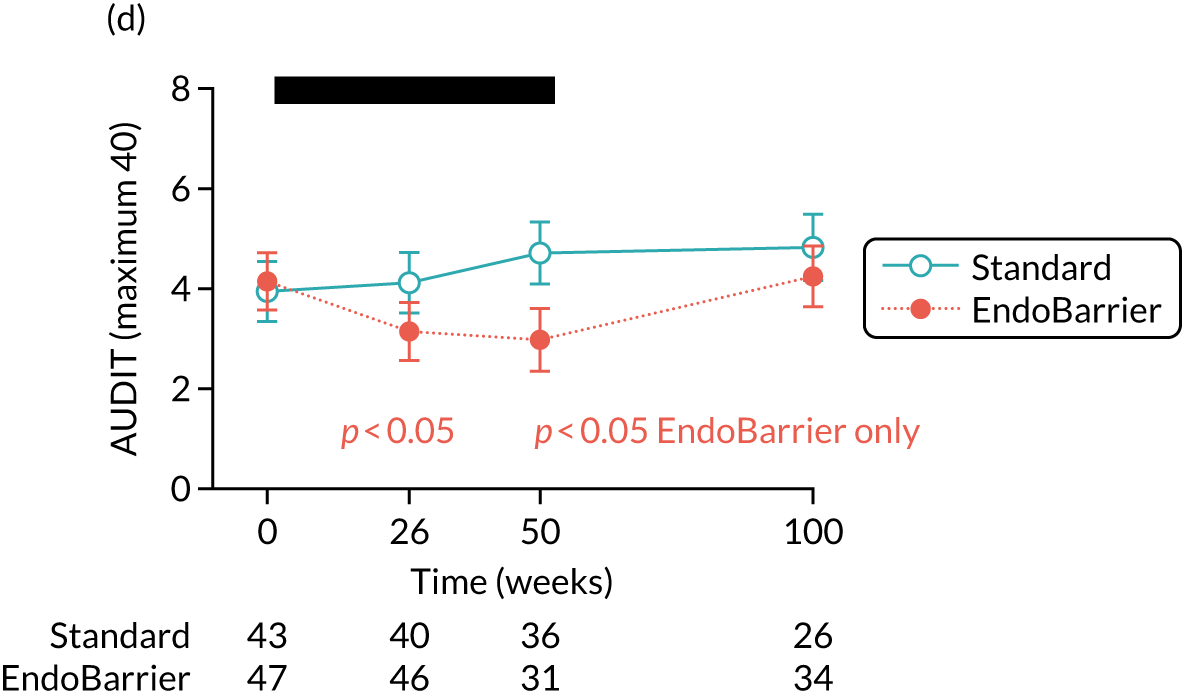
| Questionnaire | Group × time | Group | Time | |||
|---|---|---|---|---|---|---|
| F | p-value | F | p-value | F | p-value | |
| Eating behaviour | ||||||
| Dietary restraint | ||||||
| DEBQ | F(3,243.1) = 1.60 | 0.19 | F(1,93.1) = 0.62 | 0.44 | F(3,234.1) = 28.42 | < 0.001 |
| TFEQ | F(3,232.6) = 1.24 | 0.30 | F(1,91.5) = 0.03 | 0.87 | F(3,232.6) = 43.66 | < 0.001 |
| EDEQ | F(3,241.5) = 0.69 | 0.56 | F(1,92.5) = 2.29 | 0.13 | F(3,241.5) = 14.80 | < 0.001 |
| Food hedonics | ||||||
| DEBQ-external | F(3,236.2) = 1.16 | 0.33 | F(1,96.3) = 3.66 | 0.059 | F(3,236.3) = 15.23 | < 0.001 |
| PFS | F(3,185.7) = 0.32 | 0.81 | F(1,58.5) = 0.20 | 0.66 | F(3,185.7) = 0.43 | 0.74 |
| YFAS | F(3,226.7) = 0.66 | 0.58 | F(1,93.8) = 0.01 | 0.95 | F(3,226.7) = 9.04 | < 0.001 |
| BES | F(3,219.4) = 2.62 | 0.051 | F(1,93.1) = 0.04 | 0.84 | F(3,219.4) = 14.12 | < 0.001 |
| Other | ||||||
| TFEQ-hunger | F(3,234.1) = 2.77 | 0.042 | F(1,94.6) = 0.05 | 0.83 | F(3,234.1) = 15.98 | < 0.001 |
| TFEQ-disinhibition | F(3,232.6) = 2.00 | 0.12 | F(1,94.3) = 1.13 | 0.29 | F(3,232.6) = 10.00 | < 0.001 |
| DEBQ-emotional | F(3,232.5) = 1.55 | 0.20 | F(1,93.9) = 0.25 | 0.61 | F(3,232.5) = 1.55 | 0.20 |
| AUDIT | F(3,216.6) = 3.19 | 0.025 | F(1,91.5) = 1.02 | 0.31 | F(3,216.6) = 2.52 | 0.059 |
| Dumping syndrome | ||||||
| Sigstad score | F(3,212.0) = 0.07 | 0.97 | F(1,88.9) = 1.48 | 0.23 | F(3,212.0) = 9.45 | < 0.001 |
| Prevalence of Sigstad score > 6 | F(3,217.8) = 0.72 | 0.54 | F(1,91.3) = 0.59 | 0.45 | F(3,217.8) = 4.06 | 0.008 |
| Arts (modified) | F(3,212.3) = 1.66 | 0.18 | F(1,89.2) = 0.49 | 0.49 | F(3,212.3) = 8.33 | < 0.001 |
Figures 23a–d show the comparison of symptoms of dumping syndrome using absolute Sigstad questionnaire score, prevalence of Sigstad score of > + 6, modified Arts questionnaire score and VAS rating for nausea after an overnight fast, between the standard treatment group (blue unfilled circles, solid line) and the EndoBarrier group (red filled circles, dotted line), over time (week 0 at baseline, and 26, 50 and 100 weeks after EndoBarrier insertion; black bar indicates period when EndoBarrier in situ).
FIGURE 23.
Questionnaires assessing aversive symptoms of dumping syndrome and nausea. (a) Absolute Sigstad questionnaire score; (b) prevalence of Sigstad score; (c) modified Arts questionnaire score; and (d) VAS rating for nausea. Data presented as mean ± SEM, numbers at each time point are given beneath graph. Fixed-effects mixed-model ANOVA with post hoc Fisher’s exact LSD test: p-value vs. week 0 for EndoBarrier group only (orange), independent of group (black) or EndoBarrier vs. standard therapy [light blue in part (d)]. For ANOVA results see Table 30.
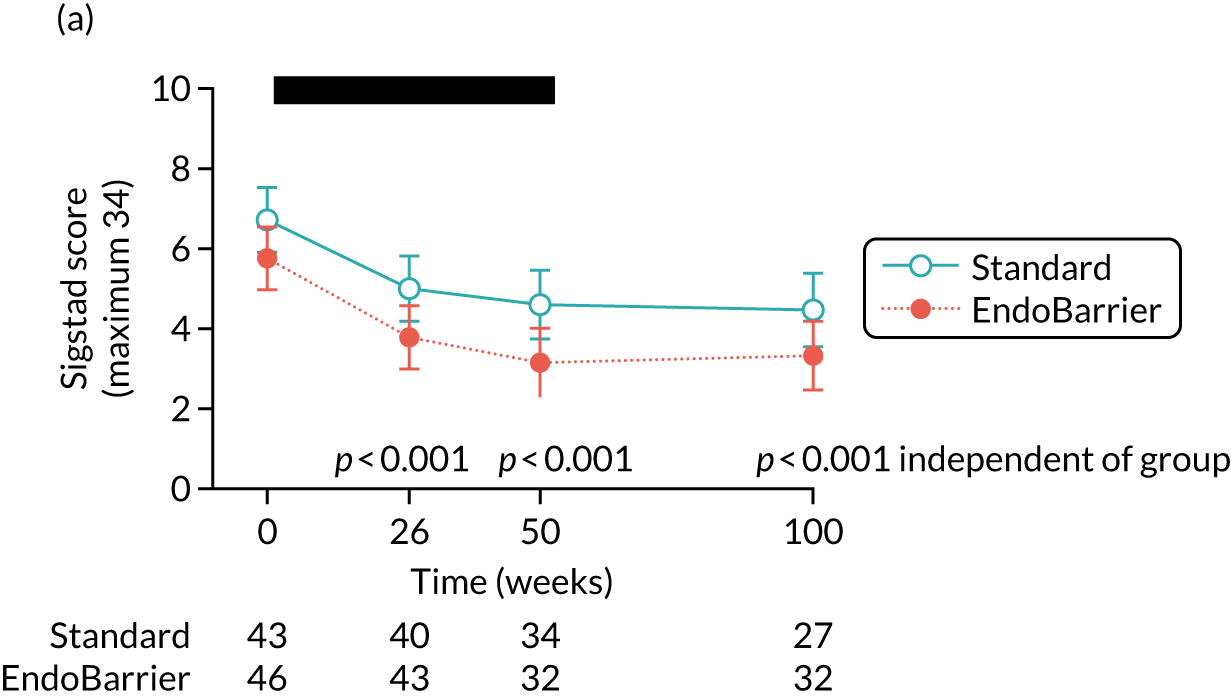



| Baseline characteristic | EndoBarrier (N = 15), mean ± SD | Standard therapy (N = 17), mean ± SD |
|---|---|---|
| Age (years) | 52 ± 6 | 53 ± 9 |
| Gender, n (%) | ||
| Female | 7 (47) | 4 (24) |
| Weight (kg) | 107.6 ± 17.4 | 104.4 ± 14.6 |
| BMI (kg/m2) | 36.8 ± 5.0 | 33.9 ± 3.3 |
| Fasting glucose (mmol/l) | 11.0 ± 2.9 | 10.4 ± 2.7 |
| Fasting insulin (mU/l) | 11.5 ± 4.2 | 11.7 ± 5.6 |
| HbA1c (mmol/mol) | 71.9 ± 9.0 | 71.0 ± 7.3 |
| Anthropometric measure | EndoBarrier | Standard therapy | Mixed-model analysis | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | Effect | p-value | |
| Weight (kg) | ||||||
| Baseline | 15 | 107.6 ± 17.4 | 17 | 104.4 ± 14.6 | Group | 0.911 |
| 10 days | 14 | 98.7 ± 14.8a | 17 | 100.0 ± 13.5a | Visit | < 0.001 |
| 6 months | 13 | 91.9 ± 13.5a,b | 15 | 100.2 ± 13.2a | Group × visit | < 0.001 |
| BMI (kg/m2) | ||||||
| Baseline | 15 | 36.8 ± 5.0 | 17 | 33.9 ± 3.3 | Group | 0.210 |
| 10 days | 14 | 33.7 ± 3.8a | 17 | 32.4 ± 3.0a | Visit | < 0.001 |
| 6 months | 13 | 32.3 ± 4.2a,b | 15 | 32.5 ± 3.1a | Group × visit | < 0.001 |
| Absolute weight loss (kg) | ||||||
| Baseline | 15 | – | 17 | – | Group | < 0.001 |
| 10 days | 14 | 7.2 ± 3.3 | 17 | 4.4 ± 2.1 | Visit | < 0.001 |
| 6 months | 13 | 13.3 ± 6.3b,c | 15 | 5.7 ± 5.0c | Group × visit | 0.003 |
| Total weight loss (%) | ||||||
| Baseline | 15 | – | 17 | – | Group | < 0.001 |
| 10 days | 14 | 6.6 ± 2.4 | 17 | 4.2 ± 1.7 | Visit | < 0.001 |
| 6 months | 13 | 12.3 ± 4.8b,c | 15 | 5.2 ± 4.3c | Group × visit | 0.002 |
| Glycaemic measure | EndoBarrier | Standard therapy | Mixed-model analysis | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | Effect | p-value | |
| Fasting glucose (mmol/l) | ||||||
| Baseline | 15 | 11.0 ± 2.9 | 17 | 10.4 ± 2.7 | Group | 0.807 |
| 10 days | 14 | 8.5 ± 3.0a | 17 | 7.6 ± 1.8a | Visit | < 0.001 |
| 6 months | 13 | 8.7 ± 2.4a | 15 | 9.2 ± 2.6b,c | Group × visit | 0.053 |
| Fasting insulin (mU/l) | ||||||
| Baseline | 14 | 11.5 ± 4.2 | 17 | 11.7 ± 5.6 | Group | 0.643 |
| 10 days | 14 | 9.8 ± 4.3 | 16 | 8.7 ± 5.1d | Visit | 0.002 |
| 6 months | 13 | 8.5 ± 2.8a | 15 | 11.4 ± 5.5c | Group × visit | 0.020 |
| HbA1c (mmol/mol) | ||||||
| Baseline | 15 | 71.9 ± 9.0 | 17 | 71.0 ± 7.3 | Group | 0.784 |
| 10 days | 14 | 62.9 ± 17.2d | 17 | 58.1 ± 8.2a | Visit | < 0.001 |
| 6 months | 13 | 54.6 ± 16.3a,c | 15 | 57.1 ± 15.4a | Group × visit | 0.266 |
| Clamp outcome | EndoBarrier | Standard therapy | Mixed-model analysis | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | Effect | p-value | |
| Low-dose Ra (µmol/kg/minute) | ||||||
| Baseline | 15 | 4.7 ± 1.6 | 17 | 4.6 ± 2.4 | Group | 0.986 |
| 10 days | 14 | 4.0 ± 1.6a | 17 | 3.7 ± 1.4a | Visit | 0.009 |
| 6 months | 13 | 4.0 ± 1.2 | 15 | 4.4 ± 2.6 | Group × visit | 0.440 |
| Corrected low-dose Ra [µmol/kg/minute/(mU/l)] | ||||||
| Baseline | 14 | 170.0 ± 79.0 | 17 | 155.6 ± 77.3 | Group | 0.690 |
| 10 days | 13 | 122.9 ± 43.3a | 17 | 118.0 ± 42.1a | Visit | 0.004 |
| 6 months | 12 | 107.9 ± 36.6b | 15 | 151.3 ± 79.2 | Group × visit | 0.110 |
| High-dose Rd (µmol/kg/minute) | ||||||
| Baseline | 14 | 22.2 ± 10.1 | 15 | 21.3 ± 8.51 | Group | 0.313 |
| 10 days | 14 | 28.5 ± 10.6a | 15 | 31.9 ± 8.42c | Visit | < 0.001 |
| 6 months | 13 | 39.5 ± 15.8c,d,e | 14 | 26.4 ± 11.1a,e,f | Group × visit | < 0.001 |
| Corrected high-dose Rd [µmol/kg/minute/(mU/l)] | ||||||
| Baseline | 14 | 0.186 ± 0.105 | 15 | 0.169 ± 0.061 | Group | 0.124 |
| 10 days | 14 | 0.257 ± 0.116a | 15 | 0.280 ± 0.096c | Visit | < 0.001 |
| 6 months | 13 | 0.422 ± 0.231c,d,g | 14 | 0.229 ± 0.087a,g | Group × visit | < 0.001 |
| Anthropometric variable | Group | Mixed-model analysis | ||||
|---|---|---|---|---|---|---|
| EndoBarrier | Standard therapy | Effect | p-value | |||
| n | Mean ± SD | n | Mean ± SD | |||
| Weight (kg) | ||||||
| Baseline | 27 | 109.4 ± 18.9 | 20 | 101.3 ± 14.4 | ||
| 10 days | 26 | 103.7 ± 18.9a | 19 | 96.3 ± 13.9a | Group | 0.1 |
| 6 months | 22 | 97.7 ± 19.3a | 16 | 91.3 ± 12.8a | Time | < 0.001 |
| 12 months | 16 | 98.0 ± 17.0a | 16 | 90.9 ± 13.2a | Group × time | 0.02 |
| 24 months | 15 | 103.3 ± 18.9a | 15 | 91.5 ± 13.1a,b | ||
| % weight loss | ||||||
| 10 days | 27 | –5 ± 3 | 19 | –4 ± 1 | Group | 0.6 |
| 6 months | 22 | –10 ± 4a | 16 | –8 ± 6c | Time | < 0.001 |
| 12 months | 16 | –11 ± 5a | 16 | –8 ± 8c | Group × time | 0.02 |
| 24 months | 15 | –4 ± 5 | 14 | –7 ± 7d | ||
| % of body fat (female) | ||||||
| Baseline | 9 | 46 ± 3 | 12 | 47 ± 4 | ||
| 10 days | 8 | 45 ± 4 | 11 | 46 ± 5 | Group | 0.9 |
| 6 months | 7 | 43 ± 6d | 9 | 41 ± 5a | Time | < 0.001 |
| 12 months | 5 | 43 ± 2d | 9 | 40 ± 7a | Group × time | 0.05 |
| 24 months | 5 | 42 ± 3d | 9 | 40 ± 7a | ||
| % of body fat (male)e | ||||||
| Baseline | 18 | 35 ± 5 | 8 | 33 ± 3 | ||
| 10 days | 17 | 35 ± 6 | 8 | 32 ± 3 | Group | 0.9 |
| 6 months | 14 | 31 ± 5a | 7 | 29 ± 3c | Time | 0.5 |
| 12 months | 11 | 29 ± 6a | 7 | 29 ± 4c | Group × time | 0.6 |
| 24 months | 10 | 32 ± 7c | 6 | 28 ± 2c | ||
| Time point | Group | Mixed-model analysis | ||||
|---|---|---|---|---|---|---|
| EndoBarrier | Standard therapy | Effect | p-value | |||
| n | Mean ± SD | n | Mean ± SD | |||
| Calories (kcal) from the 3-day diary | ||||||
| Baseline | 24 | 1911 ± 506 | 17 | 1740 ± 285 | ||
| 10 days | 22 | 1097 ± 407a | 17 | 1194 ± 203a | Group | 0.5 |
| 6 months | 16 | 1575 ± 410b | 14 | 1443 ± 321c | Time | < 0.001 |
| 12 months | 13 | 1423 ± 647a | 13 | 1504 ± 470c | Group × time | 0.3 |
| 24 months | 12 | 1788 ± 761 | 14 | 1525 ± 494 | ||
| Calories (kcal) from 24-hour recall | ||||||
| Baseline | 23 | 2049 ± 851 | 18 | 1939 ± 758 | ||
| 10 days | 21 | 1122 ± 436a | 17 | 1145 ± 386a | Group | 0.4 |
| 6 months | 20 | 1655 ± 421c | 14 | 1463 ± 641c | Time | < 0.001 |
| 12 months | 15 | 1382 ± 716a | 15 | 1441 ± 495b | Group × time | 0.6 |
| 24 months | 12 | 1683 ± 660 | 14 | 1337 ± 454b | ||
| Time point | Group | Mixed-model analysis | ||||
|---|---|---|---|---|---|---|
| EndoBarrier | Standard therapy | Effect | p-value | |||
| n | Mean ± SD | n | Mean ± SD | |||
| Carbohydrates (% of total calories) | ||||||
| Baseline | 24 | 40 ± 7 | 17 | 40 ± 8 | ||
| 10 days | 22 | 46 ± 6a | 17 | 47 ± 2a | Group | 0.8 |
| 6 months | 16 | 41 ± 7 | 14 | 39 ± 9 | Time | < 0.001 |
| 12 months | 13 | 37 ± 8 | 13 | 41 ± 9 | Group × time | 0.5 |
| 24 months | 12 | 42 ± 7 | 14 | 40 ± 7 | ||
| Protein (% of total calories) | ||||||
| Baseline | 24 | 19 ± 5 | 17 | 19 ± 4 | ||
| 10 days | 22 | 19 ± 6 | 17 | 16 ± 1b | Group | 0.9 |
| 6 months | 16 | 21 ± 6 | 14 | 24 ± 5a | Time | < 0.001 |
| 12 months | 13 | 22 ± 7b | 13 | 21 ± 4 | Group × time | 0.05 |
| 24 months | 12 | 19 ± 5 | 14 | 22 ± 7 | ||
| Fat (% of total calories) | ||||||
| Baseline | 24 | 38 ± 6 | 17 | 38 ± 7 | ||
| 10 days | 22 | 35 ± 4b | 17 | 37 ± 2 | Group | 0.9 |
| 6 months | 16 | 36 ± 7 | 14 | 36 ± 10 | Time | 0.5 |
| 12 months | 13 | 38 ± 7 | 13 | 36 ± 9 | Group × time | 0.6 |
| 24 months | 12 | 36 ± 7 | 14 | 37 ± 8 | ||
| Time point | Group | Mixed-model analysis | ||||
|---|---|---|---|---|---|---|
| EndoBarrier | Standard therapy | Effect | p-value | |||
| n | Mean ± SD | n | Mean ± SD | |||
| Carbohydrates (% of total calories) | ||||||
| Baseline | 23 | 44 ± 7 | 18 | 40 ± 11 | ||
| 10 days | 21 | 47 ± 8 | 17 | 45 ± 7 | Group | 0.3 |
| 6 months | 20 | 40 ± 10 | 14 | 33 ± 9a | Time | 0.001 |
| 12 months | 15 | 37 ± 10a | 15 | 41 ± 10 | Group × time | 0.2 |
| 24 months | 12 | 41 ± 10 | 14 | 42 ± 11 | ||
| Protein (% of total calories) | ||||||
| Baseline | 23 | 17 ± 5 | 18 | 19 ± 5 | ||
| 10 days | 21 | 19 ± 4 | 17 | 17 ± 4 | Group | 0.2 |
| 6 months | 20 | 21 ± 7a | 14 | 26 ± 11b | Time | < 0.001 |
| 12 months | 15 | 20 ± 7 | 15 | 21 ± 6 | Group × time | 0.09 |
| 24 months | 12 | 20 ± 5 | 14 | 20 ± 6 | ||
| Fat (% of total calories) | ||||||
| Baseline | 23 | 37 ± 8 | 18 | 38 ± 8 | ||
| 10 days | 21 | 34 ± 8 | 17 | 37 ± 5 | Group | 0.9 |
| 6 months | 20 | 39 ± 10 | 14 | 40 ± 11 | Time | 0.4 |
| 12 months | 15 | 40 ± 8 | 15 | 36 ± 13 | Group × time | 0.4 |
| 24 months | 12 | 38 ± 9 | 14 | 35 ± 9 | ||
Figure 24 shows the curves of the mean corrected hit rate over time for the control group and the EndoBarrier group as a function of sucrose concentration. The EC50 (half maximal effective concentration) was derived from the C parameter in the curve fit and represented the concentration at which the corrected hit rate reaches 50% of the maximum asymptote.
FIGURE 24.
Sucrose concentration: curves of the mean corrected hit rate over time for (a) the control group and (b) the EndoBarrier group. 6M, 6 months; 10D, 10 days; BL, β-lactam; EC50, half maximal effective concentration.


Figure 25 shows the intensity ratings functions of the five concentrations of sweet taste over time for the control group (n = 19) and the EndoBarrier group (n = 26). Results are shown as a mean rating of each concentration.
FIGURE 25.
Intensity ratings of concentrations for (a) the control group and (b) the EndoBarrier group.
Figure 26 shows the consummatory reward value of sweet taste assessed by the Just About Right scale over time for the control group (n = 19) and the EndoBarrier group (n = 26), with 0 value in the middle corresponding to ‘just right sweetness’, 1 to 100 corresponding to above the preferred level of sweetness, and –1 to –100 corresponding to below the preferred level of sweetness. Data are presented as the mean of each concentration.
FIGURE 26.
Consummatory reward value (Just About Right scale) for (a) the control group and (b) the EndoBarrier group.
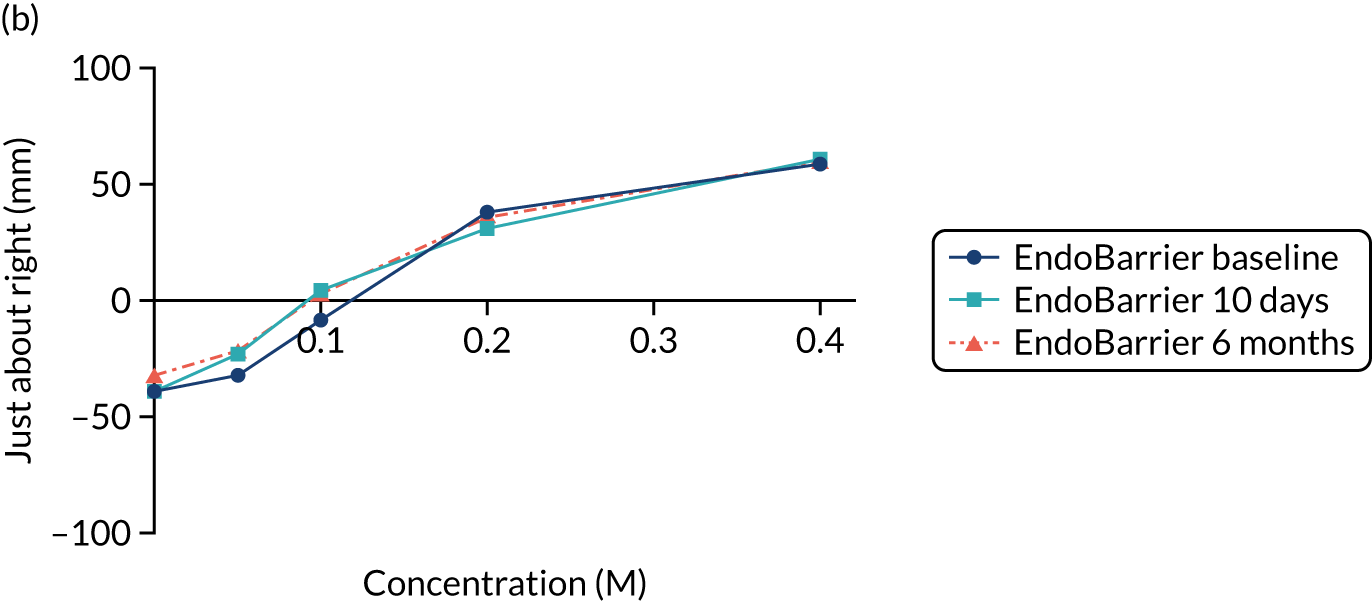
Figure 27 shows the consummatory reward value of sweet taste assessed by the VAS of liking over time for the control group (n = 19) and the EndoBarrier group (n = 26), with 0 value in the middle corresponding to ‘neutral’, 1 to 100 representing levels of liking, and –1 to –100 representing levels of disliking. Data are presented as the mean of each concentration.
FIGURE 27.
Consummatory reward value (VAS) for (a) the control group and (b) the EndoBarrier group.
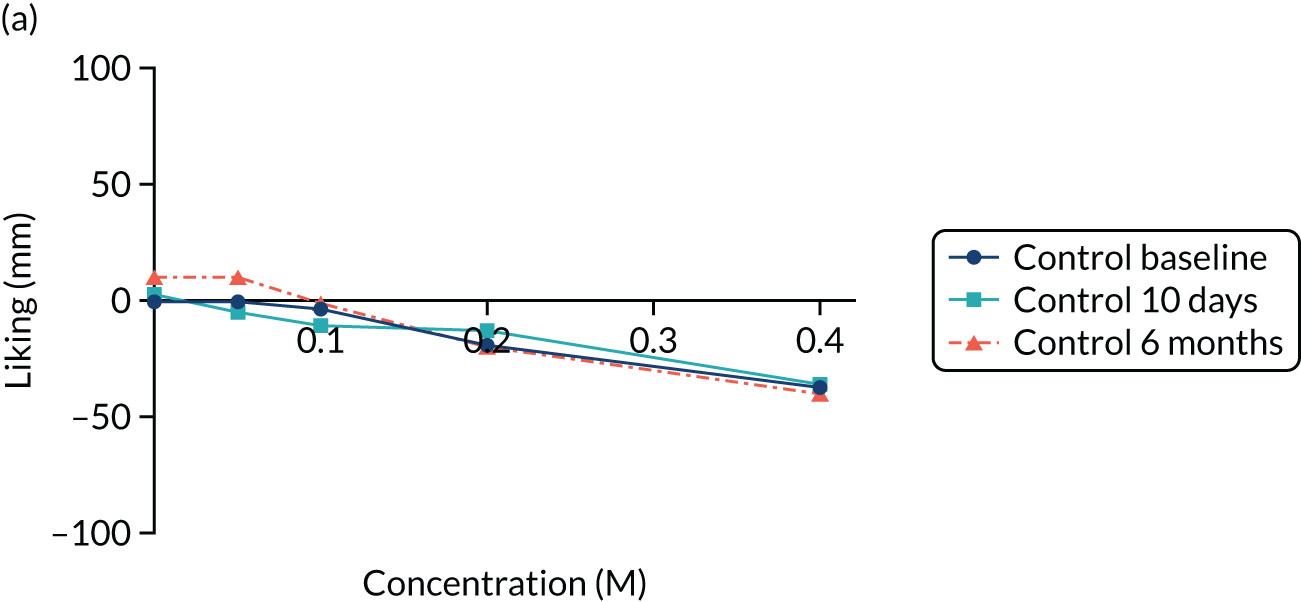

Figure 28 shows the ratings of hunger, fullness, pleasantness to eat and prospective food intake using VASs during the mixed-meal tolerance test.
FIGURE 28.
Mixed-meal tolerance test (VAS). (a) Hunger (control); (b) hunger (EndoBarrier); (c) fullness (control); (d) fullness (EndoBarrier); (e) pleasantness (control); (f) pleasantness (EndoBarrier); (g) amount to eat (control); and (h) amount to eat (EndoBarrier).


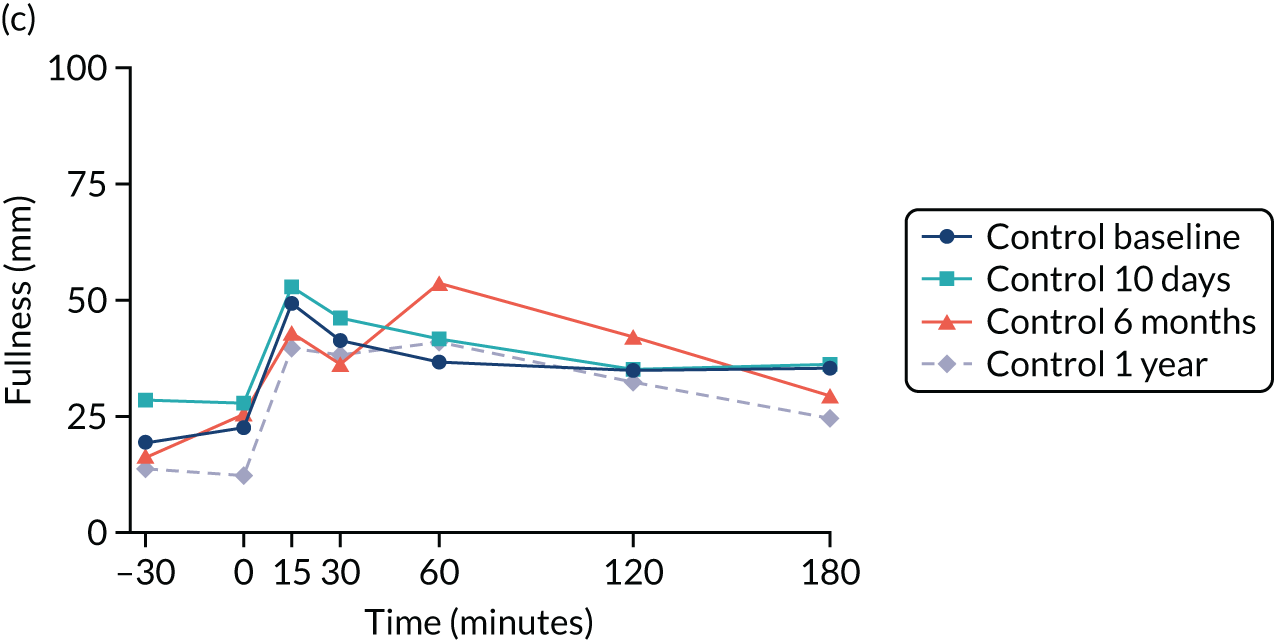

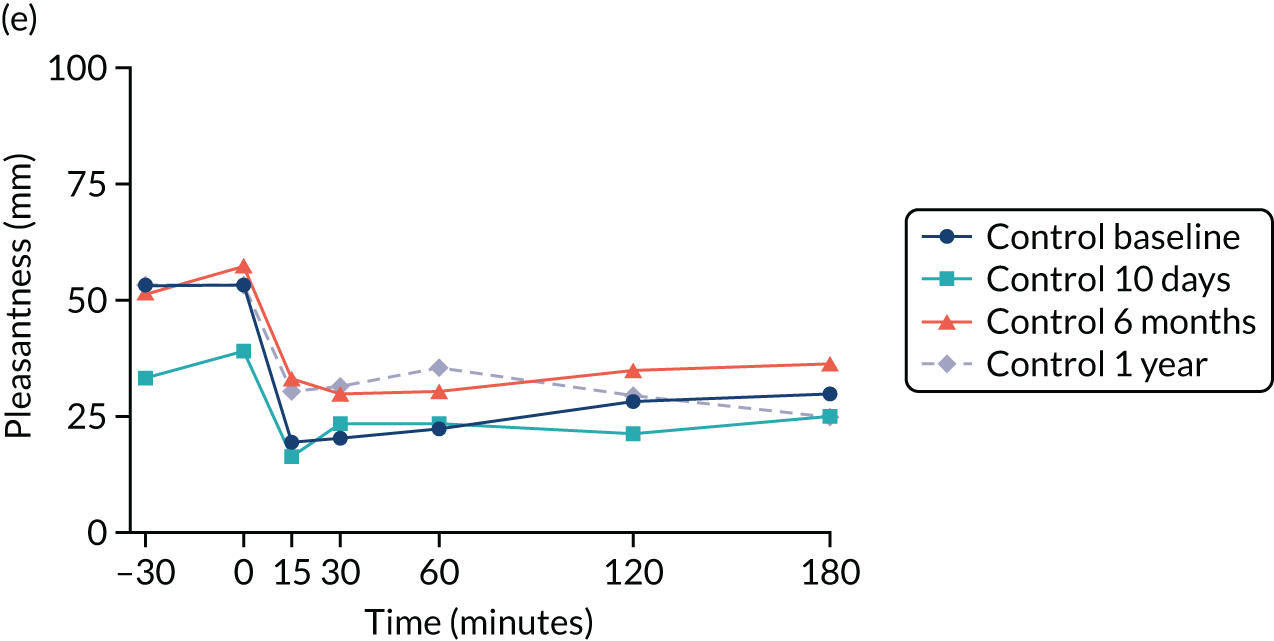
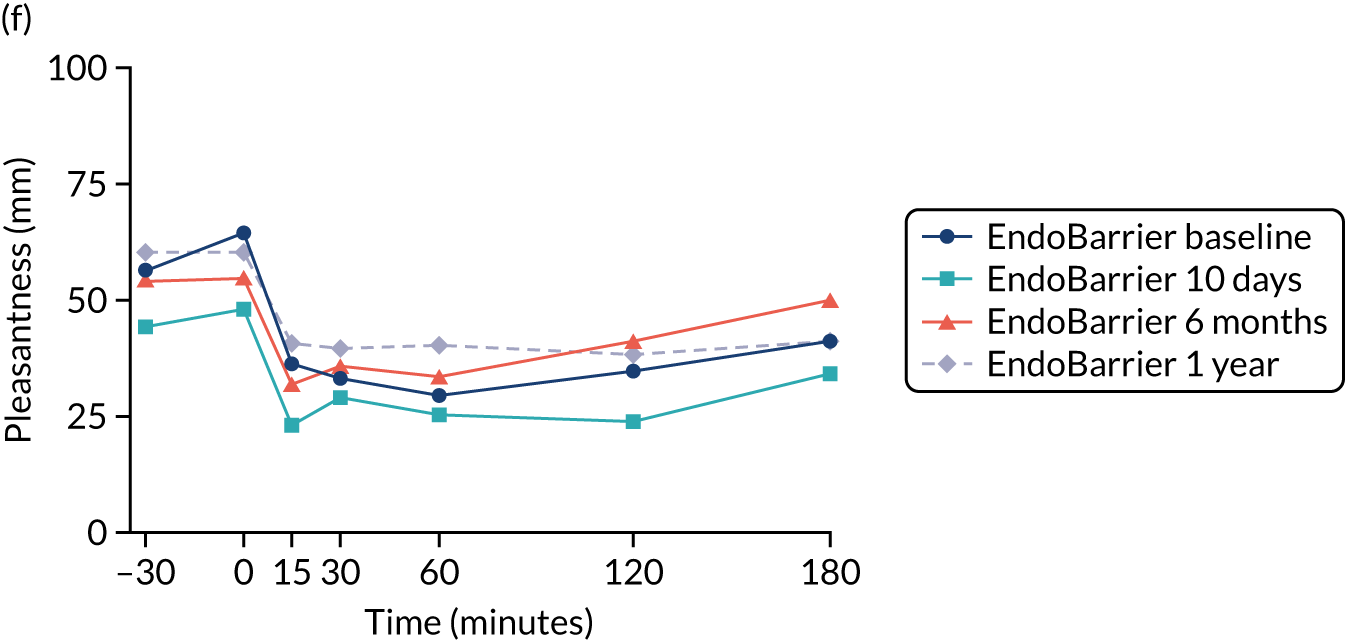


Metabonomic figures
FIGURE 29.
Typical 1D standard 1H-NMR spectra of plasma from (a) the control group and (b) the EndoBarrier group at 6 months: chemical shift (p.p.m.).

FIGURE 30.
Typical 600-MHz 1H-NMR spectra of urine from (a) the control group and (b) the EndoBarrier group at 6 months. TMAO, trimethylamine N-oxide.


The spectra in Figure 29 are from the chemical shift regions of 0.5–5.5 p.p.m. and the spectra in Figure 30 are from the chemical shift regions of 7–8.5 p.p.m.
FIGURE 31.
Representative partial 1H-NMR spectra of urinary samples from (a) the control group and (b) the EndoBarrier group.

Chemical shift
FIGURE 32.
Typical 600-MHz 1H-NMR spectra of faeces. (a) Control group at 6 months; (b) EndoBarrier group at 6 months; (c) control group at 12 months; and (d) EndoBarrier group at 12 months.
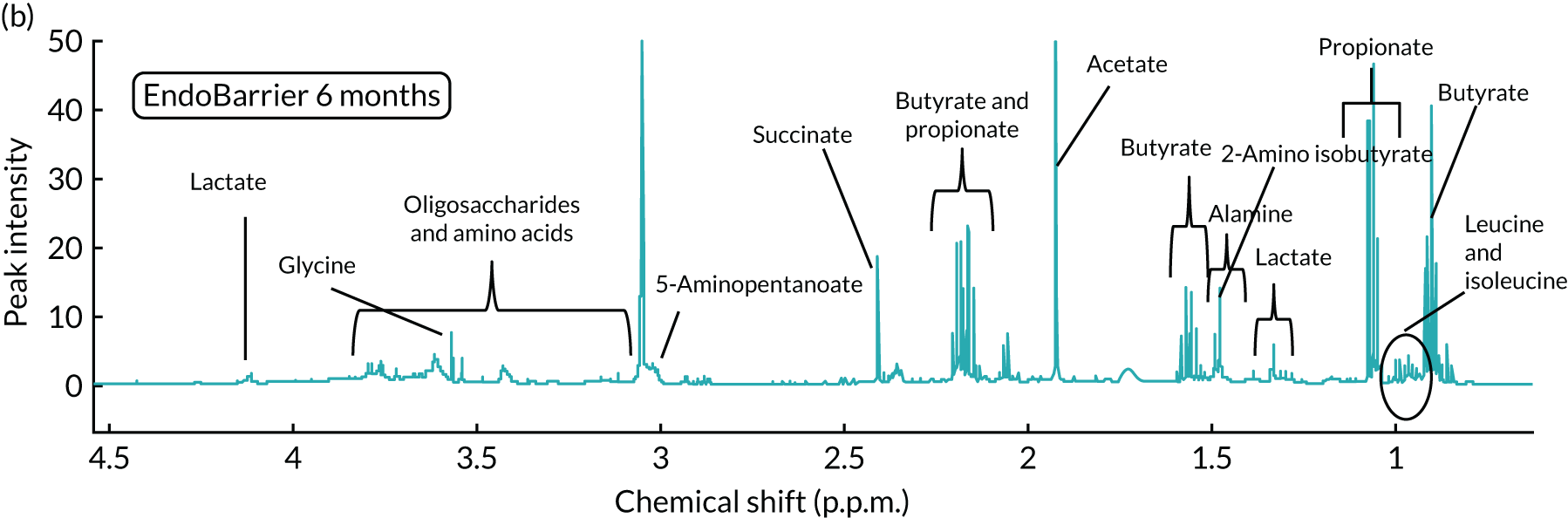
FIGURE 33.
Urine OPLS-DA: EndoBarrier vs. control at 1 year.

FIGURE 34.
Plasma Carr-Purcell-Meiboom-Gill OPLS-DA: EndoBarrier vs. control at 1 year.
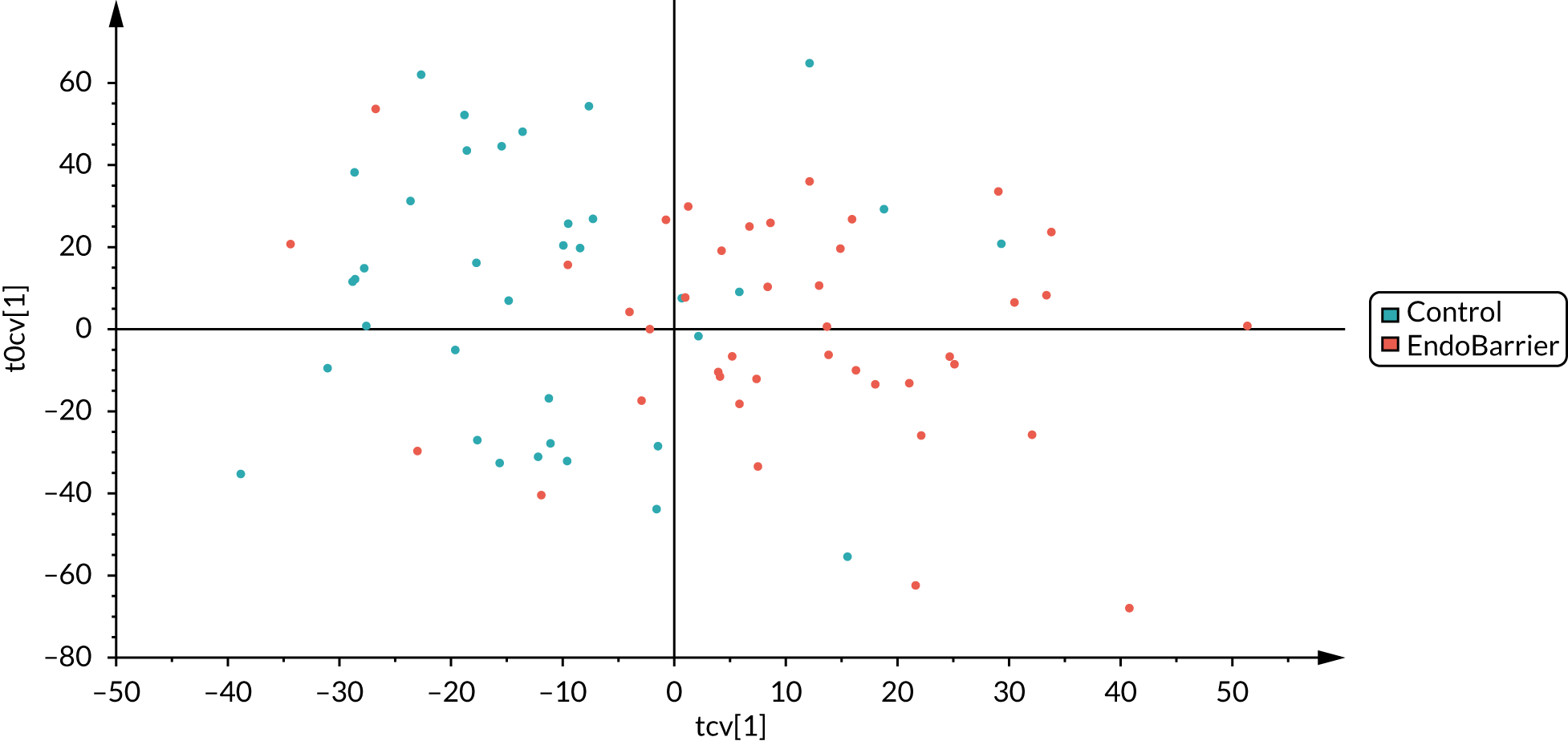
FIGURE 35.
Faeces OPLS-DA: EndoBarrier vs. control at 1 year.

Quality-adjusted life-years
| Visit | Day | EndoBarrier (N = 85) | Standard therapy (N = 85) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Lower 95% CI | Upper 95% CI | n | Mean | Lower 95% CI | Upper 95% CI | ||
| 3 | 0 | 80 | 0.878 | 0.840 | 0.915 | 73 | 0.877 | 0.835 | 0.919 |
| 5 | 10 | 72 | 0.831 | 0.777 | 0.885 | 68 | 0.898 | 0.863 | 0.932 |
| 6 | 30 | 69 | 0.899 | 0.864 | 0.934 | 66 | 0.894 | 0.838 | 0.949 |
| 7 | 91 | 69 | 0.878 | 0.829 | 0.927 | 64 | 0.872 | 0.817 | 0.928 |
| 8 | 183 | 63 | 0.860 | 0.804 | 0.916 | 61 | 0.840 | 0.796 | 0.884 |
| 10 | 365 | 55 | 0.855 | 0.811 | 0.899 | 58 | 0.834 | 0.790 | 0.878 |
| 14 | 730 | 48 | 0.847 | 0.784 | 0.910 | 48 | 0.803 | 0.745 | 0.862 |
| QALYs | 46 | 1.685 | 1.601 | 1.770 | 44 | 1.642 | 1.564 | 1.720 | |
| Visit | Day | EndoBarrier (N = 85) | Standard therapy (N = 85) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Lower 95% CI | Upper 95% CI | n | Mean | Lower 95% CI | Upper 95% CI | ||
| 3 | 0 | 85 | 0.859 | 0.828 | 0.889 | 85 | 0.863 | 0.827 | 0.899 |
| 5 | 10 | 85 | 0.825 | 0.786 | 0.863 | 85 | 0.881 | 0.849 | 0.914 |
| 6 | 30 | 85 | 0.876 | 0.846 | 0.905 | 85 | 0.883 | 0.844 | 0.921 |
| 7 | 91 | 85 | 0.867 | 0.830 | 0.904 | 85 | 0.865 | 0.825 | 0.906 |
| 8 | 183 | 85 | 0.850 | 0.808 | 0.891 | 85 | 0.845 | 0.809 | 0.882 |
| 10 | 365 | 85 | 0.841 | 0.804 | 0.879 | 85 | 0.831 | 0.791 | 0.871 |
| 14 | 730 | 85 | 0.835 | 0.780 | 0.889 | 85 | 0.813 | 0.765 | 0.861 |
| QALYs | 85 | 1.660 | 1.596 | 1.723 | 85 | 1.643 | 1.581 | 1.705 | |
FIGURE 36.
Mean utility (EQ-5D-5L score) over 2 years, without imputation.
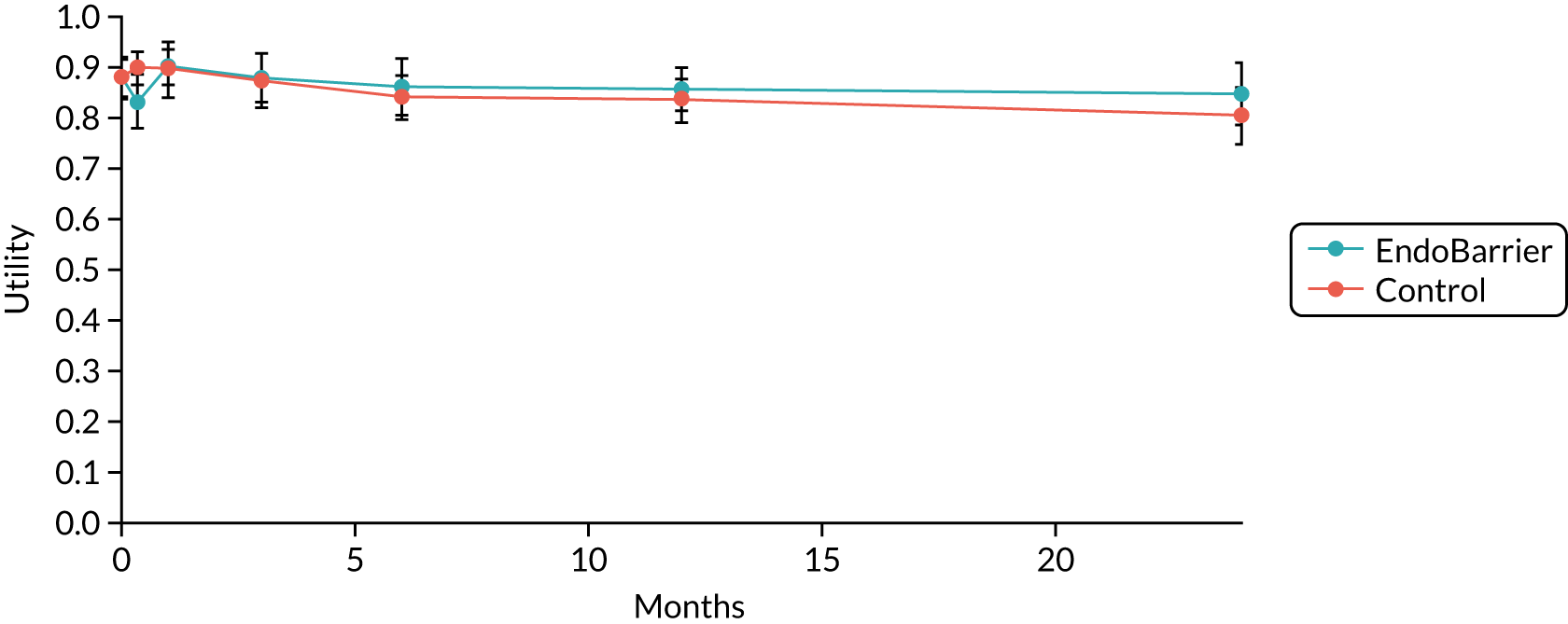
FIGURE 37.
Mean utility (EQ-5D-5L score) over 2 years, with imputation for missing data.

| Cost type | EndoBarrier (N = 85) | Standard therapy (N = 85) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | Lower 95% CI | Upper 95% CI | n | Mean | Lower 95% CI | Upper 95% CI | |
| Intervention | 85 | £2681 | £2647 | £2714 | 85 | £0 | – | – |
| Medications | 80 | £1759 | £1534 | £1985 | 74 | £1105 | £869 | £1342 |
| Other | 46 | £1277 | £610 | £1943 | 47 | £1104 | £772 | £1436 |
| Total | 46 | £5717 | £5020 | £6414 | 47 | £2209 | £1768 | £2651 |
| Cost type | EndoBarrier (N = 85) | Standard therapy (N = 85) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | Lower 95% CI | Upper 95% CI | n | Mean | Lower 95% CI | Upper 95% CI | |
| Intervention | 85 | £2489 | £2257 | £2721 | 85 | £0 | – | – |
| Medications | 85 | £1802 | £1621 | £1984 | 85 | £1089 | £900 | £1279 |
| Other | 85 | £1153 | £741 | £1566 | 85 | £1135 | £840 | £1431 |
| Total | 85 | £5445 | £4921 | £5968 | 85 | £2225 | £1853 | £2596 |
| Medication category | EndoBarrier | Standard therapy | ||
|---|---|---|---|---|
| n | Mean costs | n | Mean costs | |
| Diabetes | 80 | £798 | 74 | £704 |
| GI | 80 | £772 | 74 | £32 |
| Cardiovascular | 80 | £103 | 74 | £93 |
| Musculoskeletal | 80 | £22 | 74 | £59 |
| Nutritional | 80 | £37 | 74 | £44 |
| Other | 80 | £120 | 74 | £119 |
| Time period | EndoBarrier (N = 85) | Standard therapy (N = 85) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | Lower 95% CI | Upper 95% CI | n | Mean | Lower 95% CI | Upper 95% CI | |
| P1 (0–10 days) | 72 | £337 | £118 | £557 | 70 | £35 | £11 | £60 |
| P2 (10–30 days) | 69 | £1875 | £1603 | £2147 | 68 | £83 | £14 | £153 |
| P3 (1–3 months) | 69 | £576 | £349 | £804 | 64 | £231 | £120 | £342 |
| P4 (3–6 months) | 63 | £566 | £399 | £732 | 61 | £260 | £176 | £344 |
| P5 (6–12 months) | 55 | £871 | £537 | £1206 | 58 | £543 | £367 | £720 |
| P6 (12–24 months) | 48 | £1491 | £1248 | £1735 | 48 | £1057 | £811 | £1303 |
| Time period | EndoBarrier (N = 85) | Standard therapy (N = 85) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | Lower 95% CI | Upper 95% CI | n | Mean | Lower 95% CI | Upper 95% CI | |
| P1 (0–10 days) | 85 | £278 | £131 | £425 | 85 | £40 | £18 | £61 |
| P2 (10–30 days) | 85 | £1768 | £1539 | £1996 | 85 | £131 | £37 | £225 |
| P3 (1–3 months) | 85 | £530 | £364 | £695 | 85 | £220 | £143 | £297 |
| P4 (3–6 months) | 85 | £538 | £422 | £654 | 85 | £305 | £207 | £403 |
| P5 (6–12 months) | 85 | £861 | £652 | £1070 | 85 | £533 | £400 | £665 |
| P6 (12–24 months) | 85 | £1471 | £1254 | £1687 | 85 | £996 | £789 | £1203 |
| Scenario | Incremental costs (£) | Incremental QALYs | ICERa (£/QALY) | |
|---|---|---|---|---|
| Base case | £3507 | 0.042 | £83,775 | |
| Incremental cost | Lower limit | £2697 | 0.042 | £64,427 |
| Upper limit | £4318 | 0.042 | £103,122 | |
| Incremental QALY | Lower limit | £3507 | –0.047 | Dominated |
| Upper limit | £3507 | 0.130 | £26,930 | |
| Scenario | Incremental costs (£) | Incremental QALYs | ICERa (£/QALY) | |
|---|---|---|---|---|
| Base case | £3220 | 0.022 | £147,408 | |
| Incremental cost | Lower limit | £2585 | 0.022 | £118,318 |
| Upper limit | £3856 | 0.022 | £176,497 | |
| Incremental QALY | Lower limit | £3220 | –0.047 | Dominated |
| Upper limit | £3220 | 0.090 | £35,700 | |
| Cost of DJBL device and consumables | Incremental costs (£) | Incremental QALYs | ICERa (£/QALY) |
|---|---|---|---|
| £5000 | £7037 | 0.042 | £168,073 |
| £2500 | £4831 | 0.042 | £115,387 |
| Base case: £1000 | £3507 | 0.042 | £83,775 |
| £500 | £3066 | 0.042 | £73,237 |
| £0 | £2625 | 0.042 | £62,700 |
| Cost of DJB device and consumables | Incremental costs (£) | Incremental QALYs | ICERa (£/QALY) |
|---|---|---|---|
| £5000 | £6750 | 0.022 | £308,957 |
| £2500 | £4544 | 0.022 | £207,989 |
| Base case: £1000 | £3220 | 0.022 | £147,408 |
| £500 | £2779 | 0.022 | £127,214 |
| £0 | £2338 | 0.022 | £107,020 |
List of abbreviations
- ADA
- American Diabetes Association
- ADE
- adverse device effect
- AE
- adverse event
- ALT
- alanine aminotransferase
- ANOVA
- analysis of variance
- AST
- aspartate aminotransferase
- AUDIT
- Alcohol Use Disorders Identification Test
- BES
- Binge Eating Scale
- BMI
- body mass index
- BNF
- British National Formulary
- BOLD
- blood oxygen level dependent
- CI
- confidence interval
- CRF
- case report form
- CVD
- cardiovascular disease
- DEBQ
- Dutch Eating Behaviour Questionnaire
- DJBL
- duodenal–jejunal bypass liner
- DMEC
- Data Monitoring and Ethics Committee
- DNA
- deoxyribonucleic acid
- eCRF
- electronic case report form
- EDEQ
- Eating Disorder Examination Questionnaire
- ELISA
- enzyme-linked immunosorbent assay
- EME
- Efficacy and Mechanism Evaluation
- EPI
- echoplanar imaging
- EPIC
- European Prospective Investigation into Cancer and Nutrition
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FDA
- Food and Drug Administration
- FGF-19
- fibroblast growth factor-19
- fMRI
- functional magnetic resonance imaging
- FPG
- fasting plasma glucose
- fROI
- functional region of interest
- GI
- gastrointestinal
- GIP
- gastric inhibitory polypeptide
- GLM
- general linear model
- GLP-1
- glucagon-like peptide 1
- GM
- gut microbiota
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HE
- high energy
- HF
- high fat
- HGP
- hepatic glucose production
- HOMA-IR
- homeostatic model assessment of insulin resistance
- ICER
- incremental cost-effectiveness ratio
- ICTU
- Imperial Clinical Trials Unit
- IDF
- International Diabetes Federation
- ITT
- intention to treat
- LE
- low energy
- LF
- low fat
- LFPQ
- Leeds Food Preference Questionnaire
- LSD
- least significance difference
- MAR
- missing at random
- MI
- multiple imputation
- MICE
- multiple imputation by chained equations
- MRI
- magnetic resonance imaging
- MS
- mass spectrometry
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMR
- nuclear magnetic resonance
- OPLS-DA
- orthogonal partial least squares discriminant analysis
- OR
- odds ratio
- PFS
- Power of Food Scale
- PIS
- patient information sheet
- PMM
- predictive mean matching
- PPI
- proton pump inhibitor
- PRT
- progressive ratio task
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- PYY
- peptide tyrosine tyrosine
- QALY
- quality-adjusted life-year
- Ra
- endogenous glucose appearance
- RCT
- randomised controlled trial
- Rd
- glucose disappearance
- REC
- Research Ethics Committee
- RNA
- ribonucleic acid
- RYGB
- Roux-en-Y gastric bypass
- SAE
- serious adverse event
- SD
- standard deviation
- SEM
- standard error of the mean
- SOP
- standard operating procedure
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- TFEQ
- Three Factor Eating Questionnaire
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- VSG
- vertical sleeve gastrectomy
- WHO
- World Health Organization
- YFAS
- Yale Food Addiction Scale