Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 13/29/83. The contractual start date was in June 2016. The final report began editorial review in June 2020 and was accepted for publication in January 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 White et al. This work was produced by White et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 White et al.
Chapter 1 Introduction
Aneurysmal subarachnoid haemorrhage (aSAH) is one of the major causes of haemorrhagic stroke. In the UK and developed world its incidence is ≈ 80 per million population per year,1 and it often affects young, previously fit people, with peak occurrence in the 40–60 years age range. It often has a poor prognosis and so it carries a disproportionate socioeconomic burden. aSAH accounts for just 5% of strokes, but 20% of the quality-adjusted life-years lost to stroke in the UK and developed world, with much of that loss being concentrated in poor-grade aSAH patients. 1 The outcome of aSAH patients is often linked to the severity of the initial haemorrhage and the degree of neurological disability at the time of presentation. The total socioeconomic burden of stroke is approximately £26B per annum in the UK. 2
Existing research
To assess patients systematically on the basis of their initial neurological status, various grading systems have been introduced, with the World Federation of Neurosurgical Societies (WFNS) grading system being the most widely used. 3 Patients with WFNS grades 1–3 are considered ‘good grade’. These patients mostly make a reasonable physical recovery and are usually managed aggressively with early coiling or clipping of their aneurysms. Patients with WFNS grades 4 or 5 are considered ‘poor grade’ and generally have considerably worse outcomes than those with grades 1–3. Traditionally, neurosurgical clipping of aneurysms in these patients has been deferred until their neurological status improves. This is because surgery at an early stage in this group of patients is thought to be associated with an unacceptably high risk of stroke. 4
In more recent years, intracranial aneurysms have been treated primarily by endovascular coiling [85% coiling rate in the 2013 National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report]. 1 Packing the aneurysm with platinum coils through a minimally invasive endovascular route avoids the need for craniotomy and retraction/manipulation of an already oedematous brain. There is high-quality evidence favouring coiling in grade 1–3 patients. 5–7 However, there is no good-quality evidence to indicate whether coiling should be undertaken early or only after neurological improvement in grade 4–5 patients. Grade 4–5 aSAH patients are not usually considered for clipping unless they make substantial clinical improvement. The landmark, UK-led, international subarachnoid aneurysm trial (ISAT)6 compared coiling with clipping but included predominantly grade 1–3 patients. Just 5% of patients recruited into ISAT were grades 4 or 5 (most were grade 4). The small number of poor-grade patients enrolled, and probably the differential centre enrolment bias around grade, meant that no conclusions on poor-grade management could be drawn. Overall, 37% (46/123) of grade 4–5 patients enrolled in ISAT had a good outcome at 1 year (alive and independent), compared with 75% of grade 1–3 patients. 6 In the only other substantial trial of aneurysm coiling versus clipping of which we are aware, a more representative 19% (91/471) of patients were grades 4 or 5, but outcome data by individual clinical grade (on randomisation) were not presented; however, the odds ratio (OR) for poor outcome was 3.51 [95% confidence interval (CI) 2.21 to 5.68] for grade 3–5 patients combined, compared with grade 1–2 patients, on multivariable analysis. 7
The conventional management strategy for grade 4–5 patients [treatment on neurological improvement (TONI) (control) arm] incurs a risk of aneurysm rebleed. The patient outcome if a rebleed occurs prior to aneurysm treatment is dismal, as > 80% of patients have a poor outcome. 6,7 Grade 4–5 patients are also thought to have a higher aneurysm rebleed rate than grade 1–3 patients, and this risk is highest quite soon after the first bleed. 4
Therefore, reducing the chance of rebleeding by early aneurysm treatment may improve patient outcome. Based on this assumption, an early coiling strategy in grade 4–5 patients is being practised in many centres and some of the results are encouraging. 8–15 One larger (459 patients) heterogeneous population-based non-randomised prospective study found evidence that treatment of ruptured intracranial aneurysms within 24 hours of aSAH improves medium- and long-term clinical outcome. 16 The benefit of such ultra-early treatment was even more apparent for patients treated with endovascular coiling.
Review of prior literature on early coiling in poor-grade patients published from 2002 (when coiling became a proven aneurysm therapy) until 2015 identified eight relevant studies. 8–15 Unfortunately, none of these studies had a control group and most were small, retrospective studies carried out in a single centre, therefore suffering from inherent selection, review and recall bias. Overall, this was a very heterogeneous group of studies in terms of methodology, inclusion criteria, treatment timing and outcome measures used (and their timing).
Summary analysis of the eight studies identified a combined mortality rate of 36%, with good outcome in 52% (258/495). That represents an absolute 15% improvement over ISAT results, despite studies in the summary analysis including proportionately more WFNS grade 5 patients than were enrolled in ISAT. The primary and over-riding difference between ISAT and the subsequent acute aneurysm treatment literature is the timing of the aneurysm treatment.
A Chinese registry of poor-grade aSAH patients was published in early 2014. 17 This was an observational rather than a randomised study, examining outcomes rather than management strategy. A randomised poor-grade trial protocol for a single Chinese centre has also been published recently. 18 However, this proposed a trial of 99 patients examining timing of clipping in three groups of 33 (at < 3 days, 3–7 days and > 7 days), none of which is truly early aggressive aneurysm treatment. On both grounds (treatment modality and timelines), it is not comparable with the treatment of poor-grade subarachnoid haemorrhage trial 2 (TOPSAT2).
Newcastle feasibility study: TOPSAT119
The treatment of poor-grade subarachnoid haemorrhage trial 1 (TOPSAT1) was carried out in a single UK neuroscience centre (Newcastle upon Tyne Hospitals NHS Foundation Trust). Adult patients with WFNS grade 4–5 aSAHs were randomised within 24 hours of admission to neurocritical care to either the emergent treatment (ET) arm or the TONI arm with analysis on an intention-to-treat basis. If randomised to ET, the aneurysm was treated endovascularly (coiled) within 24 hours of randomisation. Feasibility of randomisation, recruitment rate, safety profile and functional outcome at the time of discharge and at 6 months were assessed. If the patient was initially admitted to a different hospital, confirmation of the Glasgow Coma Scale (GCS) (and thus derivation of WFNS grading) prior to intubation/ventilation was sought from the hospital transfer/referral letter.
The exclusion criteria were as follows:
-
age > 75 years
-
signs of brainstem death not promptly reversed by anti-cerebral oedema treatment
-
pure intra-ventricular haemorrhage
-
large intracerebral haematoma requiring immediate surgical clot evacuation
-
pregnancy
-
cardiorespiratory instability
-
lack of clinical equipoise.
An appropriate clinician [e.g. an intensive therapy unit (ITU) consultant/registrar, neurological consultant/registrar or interventional neuroradiology consultant/registrar] discussed the trial and provided written information to the next of kin. The clinician returned after a maximum of 4 hours to allow adequate time for reflection and obtained informed assent for the trial from the next of kin. If assent was not obtained from the next of kin, the reason for this was documented.
Fifty patients who were admitted to neurocritical care with grade 4–5 aSAHs were screened from August 2008 to January 2011. Fourteen patients were eligible for TOPSAT1 (28%). Eight out of fourteen patients were randomised (57%): four male and four female, with a mean age of 53 years. In six patients, relatives were not available to give assent (four cases) or assent was refused (two cases). Five patients were randomised to the ET arm and three patients were randomised to the TONI arm. Of the patients in the ET arm, three patients had a WFNS grade of 5 and two had a WFNS grade of 4. Of patients in the TONI arm, two patients had a WFNS grade of 5 and one had a WFNS grade of 4. There were no treatment-related adverse events (AEs) related to endovascular aneurysm treatment in either arm. No patients were lost to follow-up or crossed over arms in TOPSAT1.
Functional outcomes were assessed at the time of discharge and at 6 months following ictus using the standard modified Rankin Scale (mRS) questionnaire. There was no statistically significant difference between the arms (but the number of patients in this feasibility study was very small and it was not powered for formal analysis of efficacy).
It was demonstrated in TOPSAT1 that recruitment into a randomised controlled trial (RCT) of management policy for grade 4–5 aSAH patients was feasible. The recruitment rate among patients eligible for the study was encouraging, at 57%. However, TOPSAT1 did not have Stroke Research Network (SRN)/Comprehensive Local Research Network (CLRN) support, which limited recruitment to 5 days per week rather than 7 days; no specific trial funding was secured and TOPSAT1 had narrower eligibility criteria than were proposed for TOPSAT2. In addition, since TOPSAT1 ended, the use of an appropriate consultee to gain assent in urgent acute trials involving incapacitated patients has become widely accepted in the NHS. Therefore, applying all these improvements in resource/practice to the TOPSAT1 screening log, we estimated that at least 12 additional patients would have been eligible for TOPSAT2 (52% overall eligibility): 10% by eliminating delays to randomisation by SRN/CLRN support, 6% by including patients aged up to 80 years and 8% by utilisation of an appropriate consultee for assent. Therefore, it was felt that there was good evidence to support an appreciably higher participation rate being achieved from the aSAH population in TOPSAT2 than in TOPSAT1.
Manchester audit of high-grade subarachnoid haemorrhage
Additional data on grade 4–5 patients were sought from the earlier Manchester audit of high-grade subarachnoid haemorrhage (SAH) (Mr Hiren Patel, Consultant Neurosurgeon, Salford Royal NHS Foundation Trust, 20 November 2013, personal communication). Eighty patients were admitted to Salford neurocritical care over a 2-year period, 21 of whom improved in neurological status quickly (26%); 44 out of the 59 remaining ‘true grade 4–5’ patients had the ruptured aneurysm treated early, with 23 good outcomes (39%). Most of those 44 patients would have been eligible for TOPSAT2, so again an approximately 50% eligibility rate.
National Confidential Enquiry into Patient Outcome and Death report
The 2013 NCEPOD report1 revealed many grade 4–5 aSAH patients were simply not admitted to NSCs; 124 out of 404 (31%) SAH patients referred to NSCs were not transferred, with poor clinical grade being the overwhelming reason for this. In the absence of evidence for benefit with early grade 4–5 aneurysm treatment this can be medically justified, but the practice is undoubtedly associated with poor outcome in terms of death and disability.
The NCEPOD report1 also highlighted the heterogeneity of UK management of grade 4–5 aSAH patients in terms of both admission rates and subsequent management. Grade 4–5 patients accounted for between 8% and 50% of admissions and some units never admitted grade 5 patients. Overall, 22% of patients in the NCEPOD report1 were WFNS grades 4 or 5 on admission to a neurosciences centre (NSC), with approximately equal numbers in each grade. Twenty-eight per cent of grade 4 patients and 14% of grade 5 patients had a reasonable functional outcome on discharge from the NSC (a formal mRS assessment was not available) but no longer-term follow-up was obtained. This indicates that the ‘real-world’ current good outcome rate even in grade 4–5 aSAH patients admitted to NSCs in the NHS averages only around 20%, compared with case series literature indicating > 50% good outcomes with early aneurysm treatment. 1
Biomarkers of aneurysmal subarachnoid haemorrhage outcome
One of the challenges in managing patients with grade 4–5 aSAHs is that the only accepted tool in predicting a patient’s outcome is the admission clinical grading. However, all indications are that patients with high-grade aSAHs are not a homogeneous group, and some patients’ true clinical grading is unknown because they have been previously ventilated and intubated for brain imaging and/or transfer. There could be other more accurate early predictors of outcome that would help select patients for the most appropriate management strategies, including whether or not to transfer to neurocritical care and whether or not to treat the ruptured aneurysm early and aggressively.
Neurological damage following aSAH is a complex and evolving process. The initial phase starts with the ictus and is a response to the initial haemorrhage. A further variable phase is a consequence of vasospastic ischaemia. This is substantially absent for approximately 3 days following ictus and then evolves to a variable degree of neurological damage up to around 4 weeks post haemorrhage, with a peak typically at days 4–12. Another phase may occur related to hydrocephalus. This may arise at any point up to some months post ictus but is concentrated in the first 2–3 weeks. The proposed exploratory magnetic resonance imaging (MRI) mechanistic study had two rationales. The first was to establish whether or not MRI could be used to guide the decision on early versus deferred treatment. The second was to measure the risks posed to nervous tissue by aneurysm repair and to relate this to timing.
Imaging biomarkers
We identified a number of specific MRI-based biomarkers20–22 that may be hypothesised to have potential predictive power in this setting. These included changes in overall cerebral perfusion, the presence of increased intracerebral pressure with associated changes in cerebral tissue compliance, the presence of dysfunctional autoregulatory hydrodynamics and the presence of early inflammatory change. Diffusion-weighted imaging (DWI) is well established as an imaging marker of acute ischaemia and highly relevant to correlate risks posed by early treatment. It has been demonstrated that DWI on early MRI in SAH patients shows substantially more changes in grade 4–5 patients than in grade 1–2 patients. 20 Furthermore, studies suggest that the more extensive the changes on DWI in grade 4–5 aSAH patients, the worse the prognosis. 21 Another promising technique is diffusion tensor imaging (DTI), which provides information on the integrity of fibre tracts in the brain. Studies have shown DTI changes in the corticospinal tract in patients with SAH who have focal limb weakness. 22 Comparison of DTI studies in grade 4–5 aSAH patients can potentially give us insight into the mechanism of cerebral damage and help predict outcome. Dynamic contrast magnetic resonance has been demonstrated to reveal breakdown in the blood–brain barrier (quantification of contrast leakage in ischaemic and inflammatory diseases) and the integrity of this post SAH is hypothesised to be a biomarker for complications such as vasospasm, and possibly even as an independent predictor of outcome.
Rationale
There is genuine uncertainty about the optimal management strategy for grade 4–5 aSAH patients and a lack of high-quality research evidence in this area, confirmed by the NCEPOD report. 1
Although there is reasonable evidence that fewer rebleeds occur in patients with good clinical grades treated by coiling ultra-early, there are additional procedural risks in poor-grade patients. 23 Therefore, the management of poor-grade aSAH is based on individual or team experience, although there is a clear trend for these poor-grade patients being treated more aggressively, mostly with early coiling. This is mainly because, with the availability of coiling as a less invasive alternative to clipping, most clinicians are not comfortable with leaving a ruptured aneurysm unprotected at a stage when the risk of rebleed is greatest. However, this is not an evidence-based approach and potentially exposes health-care systems to the following considerable extra costs:
-
A substantial additional demand on already stretched neurocritical care bed and staff resources.
-
Long-term care costs if early coiling results in survival with major disability rather than improving the proportion of patients with a truly good outcome (alive and independent or with minor disability in the medium to long term at follow-up).
-
Costs of possibly unnecessary aneurysm coiling (staff, infrastructure and consumables).
-
Drive to deliver weekend coiling services locally rather than by potentially cheaper networking (networking between centres may be a good option for grade 1–3 aSAH patients but not so for grade 4–5 patients for whom extra transfer between centres may be risky, is very staff/equipment resource intensive, risks ITU overload at a centre and is costly).
Conversely, if an early-treatment strategy (primarily with coiling) of grade 4–5 aSAH patients was proven to be superior in a RCT, there is a compelling argument that it should be provided to all patients. Endovascular coiling services would need to be extended to cover 7 days, as it would not be logical to admit a critically ill patient to an intensive care bed from a peripheral hospital and then delay coiling treatment because of lack of endovascular service. In 2013, approximately one-quarter of UK NSCs offered a robust weekend coiling service [NCEPOD1 + UK Neurointerventional Group (UKNG) survey 2013, undertaken on behalf of the UKNG by Professor Phillip White (Newcastle University, 2013, personal communication)].
Crucially, there is a need for better understanding of the mechanisms involved in determining outcome in these patients. The current practice, which is reliant on crude clinical grading and to some extent the initial computerised tomography (CT) scan for risk stratification, needs to be refined. There is an urgent need for biomarkers for better understanding of the disease and, therefore, better selection of patients for aggressive management, as well as potentially stratifying long-term care and rehabilitation needs. This would help to ensure that appropriate individualised medicine is practised in an area where treatment/care costs for each patient are relatively high, but societal socioeconomic impact is also disproportionately high.
Patient and public involvement (PPI) was central to developing TOPSAT2. Feedback following presentations to the Newcastle SAH survivors’ group fed into the development of the process of obtaining assent from relatives and the way that follow-up would be conducted. The Trial Steering Committee (TSC) also included two PPI members, an aSAH survivor and a relative, who were involved at every stage of the study, including contributing to the Plain English summary and the final report.
For many neurosurgeons/interventional neuroradiologists in UK NSCs, surveys confirmed that there was genuine uncertainty (clinical equipoise) regarding whether or not to treat all grade 4–5 aSAH patients as soon as possible; this also has service provision implications. The main risk in TOPSAT2 was that more patients would undergo aneurysm treatment, mostly by coiling, with some attendant risks that would otherwise not be the case. Some of these patients would die after coiling but before neurological improvement. In relation to the early-treatment procedure, this was estimated to occur in no more than 10–15 out of 170 patients enrolled on the ET arm.
The risk of modern aneurysm coiling-related long-term morbidity/mortality is around 3–5%, although it may be slightly higher in grade 4–5 patients (unclear from existing RCT data). 13,24 However, we know that rebleeding from an aneurysm has an awful prognosis (82% poor outcome in ISAT across patients of all grades) and that the rebleed rate is also higher in grade 4–5 patients. 4,6
By contrast, the potential benefits of determining the optimum management strategy for aSAH were considerable. If early treatment was proved, service reconfiguration would be necessary but the outcome for grade 4–5 aSAH patients in the UK could be transformed. Studies on early treatment for grade 4–5 patients indicate good outcome rates around 50%, yet the NCEPOD report1 found that many grade 4–5 patients were not admitted to a NSC in the UK. Even when patients are admitted, very few NSCs provide treatment 7 days per week. Furthermore, delays to treatment are correlated with poor outcome. There are almost 1300 grade 4–5 aSAH patients per annum in the UK and NCEPOD data show that ≈ 20% currently have a good outcome, yet almost 50% might have a good outcome with consistent early aggressive (emergent) treatment (with substantial associated societal health benefit). Although an early intervention strategy for all grade 4–5 patients would be very expensive initially, it would carry substantial long-term care and social benefits savings. However, that expense provides a strong case for the care of poor-grade aSAH to be truly individualised, which the use of imaging biomarkers can potentially help to deliver.
If TONI was at least as good as ET, fewer patients would need NSC admission and some might avoid ITU admission; there would be savings on coils and other consumables that could be made immediately, and simpler options would be viable for coiling services out of hours.
Objectives
Primary objective
To establish the efficacy of a strategy of early aneurysm treatment (within 72 hours of ictus) in a population of WFNS grade 4–5 aSAH patients in comparison with the conventional strategy of treatment of aneurysm only after neurological improvement (defined for the trial as improvement to WFNS grades 1–3) by comparing functional outcome at 12 months between the two arms – by ordinal analysis of mRS outcomes.
Secondary objectives
-
Dichotomised mRS: cut-off points 0–3 versus 4–6; 0–2 versus 3–6.
-
Mortality rate (30 days and 12 months).
-
Rebleeding rate from randomisation.
-
Treatment-related complication rate and serious adverse event (SAE) report rates. SAEs followed until resolution.
-
Time in hospital to discharge (from NSC) and length of neurocritical care stay.
-
mRS at discharge (or 30 days).
-
Functional outcome at 6 months determined by ordinal analysis of mRS.
Magnetic resonance imaging substudy objective
To explore whether or not brain MRI markers in patients with poor-grade aSAH are related to outcome and whether or not they might be used to identify patients who would benefit from each treatment strategy, that is to stratify the management of grade 4–5 aSAH patients.
Chapter 2 Methods
Design
The TOPSAT2 trial was a pragmatic, randomised, open-blinded, end-point (PROBE), controlled, parallel-group study conducted in the UK and Eastern Europe. Extending recruitment beyond the UK was necessary to obtain the desired sample size within a reasonable time frame. Eastern Europe was chosen because of established links from surgical trials in intracerebral haemorrhage (STICHs). 25–27 It was planned to open 20 centres in the UK and 10 outside the UK, recruiting 246 UK and 100 non-UK participants. The proposed MRI substudy was to recruit a total of 100 consecutive participants from the UK only, in a subset of sites able to participate in it.
Participants were randomised to one of two groups in a 1 : 1 ratio to receive the standard local treatment for aneurysm either (a) as soon as possible within 72 hours of ictus (ET arm) or (b) after neurological improvement to WFNS grades 1–3 (TONI arm).
Eligibility
Eligible participants were adults aged 18–80 years and had been diagnosed with aSAH grades 4 or 5 on the WFNS scale. For trial eligibility purposes, the grade was that recorded at their first medical assessment following hospital attendance and confirmation of the diagnosis of SAH by CT (or MRI) and/or lumbar puncture. The protocol stressed that before including a participant in the trial it must also be confirmed that it would be possible to treat them within 72 hours of ictus, should they be randomised to the ET arm.
The exclusion criteria were as follows:
-
Age > 80 years (the prognosis of poor-grade aSAH patients in this subgroup is extremely poor and they are a very small percentage of NSC aSAH admissions).
-
WFNS grades 1–3, or uncertain WFNS grade. However, patients with aneurysms of uncertain grade on transfer to a NSC where a formal sedation hold was undertaken and the patient was subsequently established to be truly grades 4 or 5 were eligible for the trial. This also applied to patients with aneurysms of uncertain grade undergoing sedation hold after insertion of an external ventricular drain (EVD) or other early intervention for hydrocephalus.
-
Signs of coning or brain death on arrival at the NSC.
-
Pure aneurysmal intraventricular haemorrhage (no SAH).
-
Large intracerebral haematoma from an aneurysm requiring immediate surgical clot evacuation.
-
Significant aSAH-related haemodynamic instability.
-
Lack of clinical equipoise.
-
Lack of assent/consent.
-
Pregnancy.
-
Pre-SAH mRS > 2.
-
Pre-existing severe comorbidity such that clinical follow-up at 12 months was judged unlikely.
-
Non-saccular, mycotic, giant or other atypical aneurysm (as these are conditions for which early simple coiling/clipping would not be appropriate).
Recruitment
Locations
It was anticipated that up to 20 NSCs in the UK would participate. All UK NSCs (n = 26) with a functional interventional neuroradiology service were contacted with a feasibility form and asked for expressions of interest.
Feasibility was also initially carried out at seven non-UK centres in Czechia, Hungary, Latvia, Macedonia, Poland and two sites in Lithuania, using established contacts from the STICH. Two expressions of interest to participate were returned. Further forms were sent out to potential non-UK principal investigators (PIs) during the course of the study as more sites were sought (Romania and additional contacts in Czechia, Latvia, Poland and Hungary). Non-European centres (in Asia, Oceania and North America) did approach the TOPSAT2 team about joining but sponsorship arrangements were not in place to include non-European sites at the time suspension of trial recruitment was agreed by the TSC.
Overall, out of the 26 UK NSCs with functional interventional neuroradiology services, 25 were approached to participate [Edinburgh was not eligible as a site as the Data Monitoring Committee (DMC) chairperson was based in ITU there; the Efficacy and Mechanism Evaluation (EME) programme would not accept this unit as a trial site]. Fifteen centres were opened to recruitment between September 2016 and August 2018: 12 in the UK (Newcastle, Leeds, Sheffield, Middlesbrough, University College London, King’s College London, Oxford, Royal London Hospital, Birmingham, Stoke-on-Trent, Nottingham and Cambridge) and three not in the UK (Riga, Latvia; Łódź, Poland; and Timişoara, Romania). A further two centres (Liverpool Walton Centre, UK, and Prague, Czechia) signed contracts but, unfortunately, did not open to recruitment before recruitment was suspended.
Process
Once a poor-grade SAH patient was admitted to neurocritical care at a trial centre, the patient was stabilised from neurological and cardiorespiratory points of view as per local protocol. If the patient was initially admitted to a different hospital, confirmation of the WFNS grade prior to transfer was sought from the referral letter or by directly contacting the referring team. If the WFNS grade before transfer could not be established (e.g. the patient was immediately intubated/ventilated), the patient was considered to be of uncertain grade (WFNS U) and was ineligible for the trial unless subsequent formal sedation hold and reassessment confirmed poor grade, as described in Eligibility. However, there was no requirement at the trial centre to confirm the patient’s WFNS grade by reversing sedation in the ITU. Once aSAH was confirmed and the patient was stable, the admitting neurosurgical/anaesthetic team assessed the patient with regard to eligibility for the trial.
An appropriate clinician (e.g. an ITU consultant/registrar, neurological consultant/registrar or interventional neuroradiology consultant/registrar) with documented responsibility on the delegation log discussed the trial with the patient’s next of kin and provided them with the participant information sheet. A delegated individual then returned after an appropriate interval (maximum 4 hours) to allow adequate time for reflection, and obtained assent for the trial from the appropriate consultee. The PI was responsible for ensuring that informed assent for trial participation was given by each patient’s next of kin or relative, or by a nominated consultee, for patients fulfilling the TOPSAT2 eligibility criteria.
An important part of the design of the consent process was PPI. Through contact with the Newcastle SAH survivors’ group, former SAH patients and their relatives were able to advise on this and provided insight into how best to approach a potential participant’s next of kin, as the incapacitating nature of the condition precluded obtaining prospective informed consent from participants themselves. Wherever possible, an attempt was made to establish the views of the patient with regard to involvement in research from a personal consultee. If no personal consultee or next of kin could be identified, the researcher approached a person with no connection to the study who was pre-identified as willing to be consulted about the participation of a person lacking capacity, to act as a consultee. The nominated professional/personal consultee completed a consultee declaration form that was countersigned by the PI or delegated personnel. Professional consultees were (medical) consultants who could not be otherwise actively involved in the trial.
If a participant regained mental capacity, they were fully informed about the trial and their consent was sought to continue. If at that point they did not wish to remain in the trial, then they were withdrawn. This is in accordance with the Mental Capacity Act 200528 (England and Wales).
Interventions
Standard procedures at the centre for coiling (or clipping) the aneurysm were followed. If the patient had more than one aneurysm, the neurovascular team treated the aneurysm that, in their judgement, was most likely to have caused the SAH. Standard care for grade 4–5 aSAH patients was provided to all trial participants; no additional clinical intervention and no new treatment method was undertaken as part of the trial.
Outcomes
Primary end point/outcome
The primary end point was functional outcome at 12 months determined by ordinal analysis of the mRS. The mRS is a widely used outcome measure in stroke (including aSAH) and is based on the ability to carry out usual day-to-day activities.
The mRS at 12 months was established using a questionnaire for the participant (or their carer) to complete (see Appendix 1). After hospital discharge in the UK, a member of the research team at Newcastle University contacted the general practitioner to establish whether or not the participant was still alive and to obtain information about the participant’s clinical condition. If the participant was known to be alive, the study questionnaire was sent by post to the address given at recruitment, with a reply postage-paid envelope. Alternatively, patients could, if they wished, complete the questionnaire online through the trial website (www.topsat2.co.uk). For participants outside the UK, follow-up, including establishing the participant’s vital status, was to be carried out by staff at the recruiting centre. This difference was because of sponsorship arrangements that had to be changed during the trial (see Scientific summary for detailed discussion of this).
Secondary end points/outcomes
-
Dichotomised mRS: 0–3 versus 4–6; 0–2 versus 3–6.
-
Mortality rate (at 30 days and 12 months).
-
Rebleeding rate from randomisation.
-
Treatment-related complication rate and number of reported SAEs. SAEs followed until resolution.
-
Time in hospital to discharge (from NSC) and length of stay in neurocritical care.
-
mRS at discharge.
-
Functional outcome at 6 months determined by ordinal analysis of mRS – by questionnaire, as above.
Magnetic resonance imaging substudy outcomes
-
Lesion load on DWI.
-
Fractional anisotropy values on DTI.
-
Brain perfusion and cerebrospinal fluid (CSF) parameters.
-
Endothelial permeability.
-
Blood–brain barrier integrity on MRI.
Sample size
The outcome measure in TOPSAT2 was based on the mRS, a 7-point scale, with values 0–5 representing increasing disability and a value of 6 representing death. In the past it was common to dichotomise such a scale for outcome analysis, resulting in a loss of information as not all patients contribute to the detection of a treatment effect. The Optimising Analysis of Stroke Trials Collaboration and other authors have shown the benefit of ordinal analysis in the field of stroke. 29 Using an ordinal analysis achieves substantially greater statistical power to detect a treatment effect with equal sample size. The sample size calculation was therefore based on a proportional odds regression ordinal analysis of the mRS.
The best available literature was used to provide the expected distribution of mRS after grade 4–5 aSAH patients, although it was a non-randomised study. 30 Detecting a difference in clinical outcome (i.e. favouring one treatment strategy over the other) by an OR of > 1.5 would be compelling evidence to rapidly change to a uniform practice, as would a number needed to treat (NNT) of < 10. Expected mRS distribution data (0, no symptoms 17%; 1, minor symptoms 10%; 2, some restriction in lifestyle 6%; 3, significant restriction in lifestyle 19%; 4, partially dependent 11%; 5, fully dependent 10%; and 6, dead 27%)30 was entered into the ‘sample size for ordered categories’ routine of the Compare2 program in WinPepi version 11.43 during July 2014 and using two-sided significance of 5%, power of 80%, and 1 : 1 ratio of sample size, the sample sizes needed for different ORs (where the OR is assumed to be the same at all cutting points) were examined.
With 167 participants in each arm, a proportional odds model ordinal analysis of mRS gave a cumulative OR in favour of better mRS in one treatment arm of 1.7 at a 5% significance level and 80% power. A margin was built in to allow for losses to follow-up and crossovers, giving a total sample size of 346 (173 per arm). Loss to follow-up of < 2% was based on data from multiple UK-based aneurysm coiling trials. 6,31,32
Using the expected distribution from the best available evidence30 as the control event rate and calculating the expected number of patients to fall in each outcome for the treatment group given an OR of 1.7, the NNT was calculated as the inverse of the proportion of pairwise comparisons, with a better result in the treatment group minus the proportion with a worse result. This gave a NNT of 5.7.
A sample size of 346 was sufficient that some secondary outcome analyses, including a dichotomised mRS, could also reach statistical significance. For instance, for a mRS 0–2 (alive and independent) versus 3–6 (dead or dependent) comparison, a 15% absolute difference in treatment effect would be statistically detectable.
If equal numbers of grades 4 and 5 patients had been recruited (and they are approximately equal in proportion of aSAH patients), with 346 patients overall, the trial was powered to detect a statistically significant OR of 2.2 for improved clinical outcome, favouring one treatment strategy over the other by individual grade, particularly for grade 5. This is clinically relevant given the appreciable differences between grades 4 and 5 patients; the optimum management strategy may differ between grades.
Randomisation
Randomisation was carried out by the research team at sites through the use of an online system accessed through the trial website (www.topsat2.co.uk), and hosted by the Centre for Healthcare Randomised Trials, University of Aberdeen. Randomisation utilised a minimisation algorithm with an 80% chance of being allocated to the minimisation group to reduce differences in the two arms with respect to the following:
-
WFNS grades 4 or 5 (so distributed equally between the two arms)
-
participant age at the time of randomisation (age bands 18–50 years, 51–65 years and 66–80 years)
-
presence of clinically significant hydrocephalus requiring CSF drainage procedure (yes/no)
-
UK/non-UK site.
The remaining 20% followed a totally random allocation.
If the participant was randomised to the ET arm, the result of randomisation was communicated to the neurovascular team treating the aneurysm. They decided on the most appropriate treatment strategy but, as per the RCT evidence base, if the aneurysm was technically amenable to coiling, coiling was the initial therapeutic option. Not all Eastern European centres provided an endovascular service, so only neurosurgical treatment (clipping) was available at those centres. The usual institutional surgical consent form for the procedure was completed, and the aneurysm was treated within 24 hours of randomisation and 72 hours of the ictal bleed that led to hospital admission.
If the participant was randomised to the TONI arm, the result was communicated to the ITU and neurovascular team, who continued to manage the patient as per the established local protocol. Once the patient’s neurological status improved to WFNS grade 3 or better, the aneurysm was treated expeditiously. There was no specific time delay criterion for aneurysm treatment in this arm, but it was anticipated that treatment beyond 1 month post randomisation would be exceptional owing to both the lower risk of rebleeding after that time and the very guarded prognosis if a participant had not recovered sufficiently neurologically for treatment within 1 month.
Statistical methods
Analysis of the primary outcome measure
Trial analysis was on a modified intention-to-treat basis. Where patients were lost to follow-up, they were removed from the primary outcome analysis; however, a sensitivity analysis was performed to assess the effect of missing data. The primary outcome is a comparison of the mRS, treated as an ordinal scale, at 12 months (including death coded as 6) under the two treatment strategy arms using a Wilcoxon rank-sum test. Various planned analyses were not undertaken because of the small sample size. These include a proportional odds model of the primary outcome, a sensitivity analysis of the proportional odds model (of primary outcome), adjusting for the minimisation criteria (WFNS grade, age band, hydrocephalus requiring drainage, and whether or not the patient was randomised within the UK) and a per-protocol analysis.
Analysis of secondary outcome measures
Secondary outcomes of dichotomised mRS (0–3 vs. 4–6; 0–2 vs. 3–6) at discharge, 6 and 12 months, mortality rate at 30 days, 6 and 12 months, rebleeding rate and treatment-related complication rate between the arms were to be compared using a chi-squared test or a Fisher’s exact test as appropriate. mRS at discharge and 6 months were to be compared using the Wilcoxon rank-sum test. Survival time, time to discharge and length of ITU/high dependency unit stay were to be compared between arms using survival plots, and the log-rank test has been reported. Owing to early termination of the trial and the small sample size, the ordered outcomes were not compared using the proportional odds models.
Subgroup analyses
Analyses were planned for the following subgroups: WFNS grade at randomisation (4–5), age band (18–50 years, 51–65 years, 66–80 years), if there was clinically significant hydrocephalus requiring CSF drainage (yes/no), location of the site (UK/non-UK) and treatment actually received (coil vs. clip). Had the trial not been terminated early, ORs and CIs would have been reported and interaction tests undertaken.
Safety
Monitoring for safety was undertaken in accordance with good clinical practice. Full details and definitions were provided in the trial protocol. The local investigator responsible for the care of the participant was asked to assign causality and expectedness of SAEs, in accordance with the trial protocol.
It was anticipated that most AEs that would occur during the trial, whether serious or not, would be expected because of the clinical indication and the standard treatment that the patient was receiving. A full list of expected SAEs related to acute SAH and aneurysm treatment is provided in Appendix 2.
All AEs related to the trial intervention (randomisation to either the ET arm or the TONI arm) were recorded in the case report form/electronic case report form. Those classified by the trial centre as SAEs were reported to the chief investigator, Clinical Trials Unit and sponsor on a SAE form, which was sent using either secure fax-to-e-mail transmission or secure e-mail. Details of the events were entered into the trial database. SAEs were followed up until resolution.
Chapter 3 Results
Participant flow
Figure 1 shows the participant flow through the study from screening to 12-month follow-up.
FIGURE 1.
The TOPSAT2 CONSORT flow diagram.
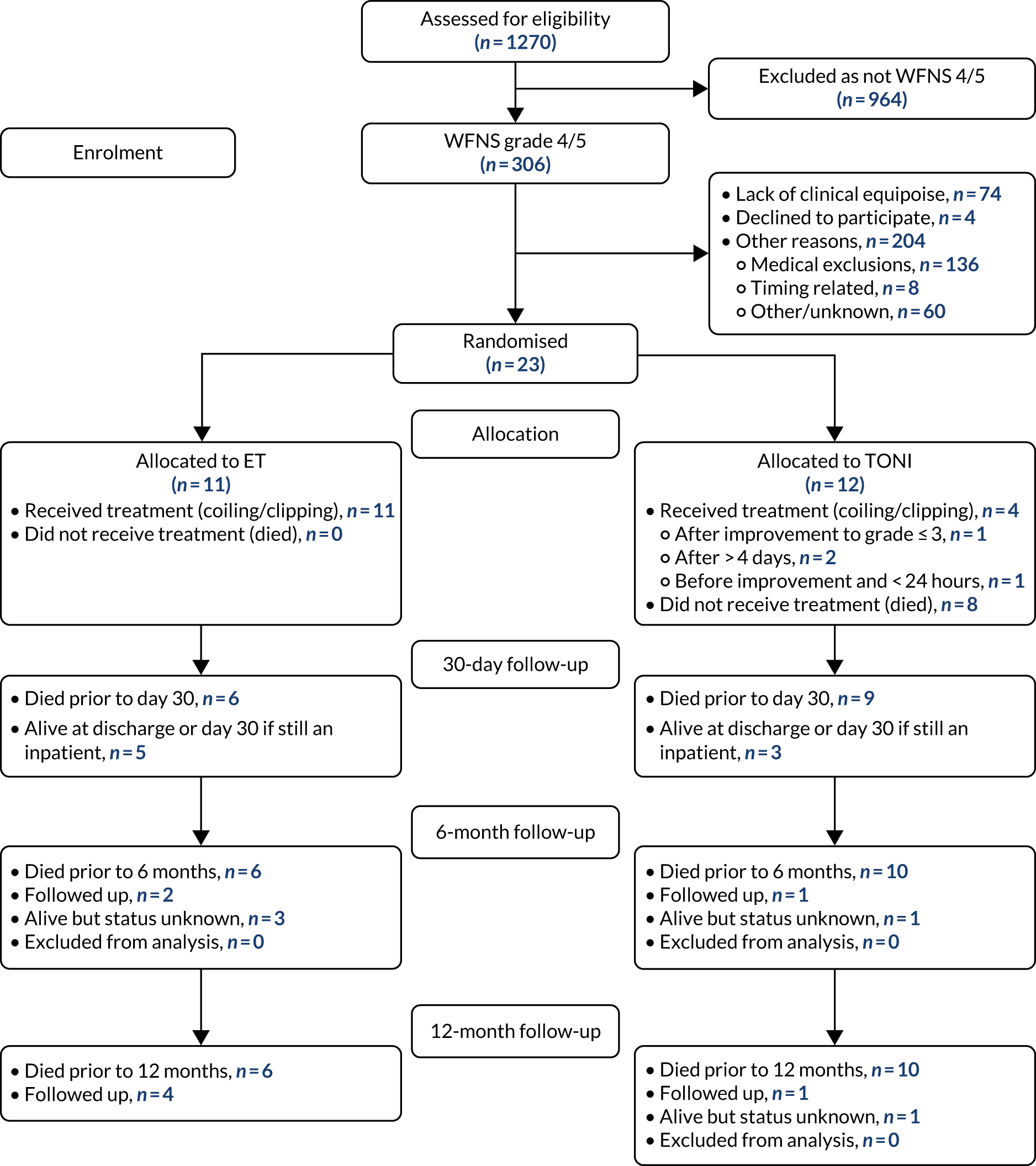
Screening
A total of 1269 grade 1–5 aSAH patients were screened (1189 from UK sites, 80 from non-UK sites), of whom 964 (936 UK, 28 non-UK) were immediately excluded because their aneurysm was not classified as poor grade (WFNS grades 4 or 5). Of the 305 poor-grade patients screened (286 UK, 19 non-UK), 23 were randomised; 16 were from UK sites and seven were from a single, non-UK site. Table 1 shows the number of patients screened at each site with the proportion of those recruited. See Tables 2 and 3 for more details of screened patients.
| Site name | Number screened | Number grade 4/5 screened | Number medically eligible | % grade 4/5 medically eligiblea | % eligible of those screened | Number recruited | % recruited of those screened |
|---|---|---|---|---|---|---|---|
| Newcastle | 134 | 25 | 0 | 0 | 19 | 0 | 0 |
| Sheffield | 166 | 25 | 17 | 68 | 15 | 0 | 0 |
| Stoke-on-Trent | 124 | 42 | 21 | 50 | 30 | 4 | 3 |
| Leeds | 182 | 48 | 11 | 23 | 26 | 2 | 1 |
| King’s College London | 146 | 34 | 33 | 97 | 23 | 1 | 1 |
| Oxford | 118 | 34 | 23 | 68 | 29 | 3 | 3 |
| Nottingham | 150 | 34 | 9 | 26 | 23 | 0 | 0 |
| University College London | 77 | 12 | 4 | 36 | 16 | 2 | 3 |
| Middlesbroughb | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Łódź | 34 | 6 | 0 | 0 | 18 | 0 | 0 |
| Royal London | 16 | 2 | 2 | 100 | 13 | 2 | 13 |
| Birmingham | 59 | 25 | 10 | 40 | 42 | 1 | 2 |
| Cambridge | 17 | 6 | 1 | 17 | 35 | 1 | 6 |
| Riga | 8 | 3 | 0 | 0 | 38 | 0 | 0 |
| Timişoara | 38 | 10 | 8 | 80 | 26 | 7 | 18 |
| Overall | 1269 | 306 (24%) | 139 | 48 | 24 | 23 | 2 |
| Reason not recruited | Number of patients |
|---|---|
| Not aged 18–80 years | 6 |
| Lack of clinical equipoise | 74 |
| Lack of assent | 4 |
| Patient died/fixed GCS score of 3/deteriorated (before enrolment feasible) | 16 |
| WFNS grades 1–3, or uncertain WFNS grade (where patient recovers quickly and proves not to be of true poor grade) | 18 |
| Signs of coning or brain death not promptly reversed by anti-cerebral oedema treatment | 28 |
| Pure intraventricular haemorrhage (no SAH) | 3 |
| Large intracerebral haematoma that requires immediate clot evacuation | 24 |
| Significant aSAH-related haemodynamic instability | 6 |
| Pre-SAH mRS score > 2 | 11 |
| Pre-existing severe comorbidity such that clinical follow-up at 12 months is judged unlikely | 21 |
| Non-saccular, mycotic, giant or other atypical aneurysm | 3 |
| Cannot be treated within 72 hours of ictus if allocated to ET arm | 8 |
| Other | 18 |
| Unknown reason | 42 |
| Reason not recruited | Number of patients |
|---|---|
| Combination of reasons | 3 |
| Not appropriate for study | 5 |
| Withdrawal of treatment | 1 |
| Administrative issues | 1 |
| Out of time (to meet protocol requirement on random and/or Rx) | 3 |
| Elected for early coiling | 5 |
| Total | 18 |
Recruitment
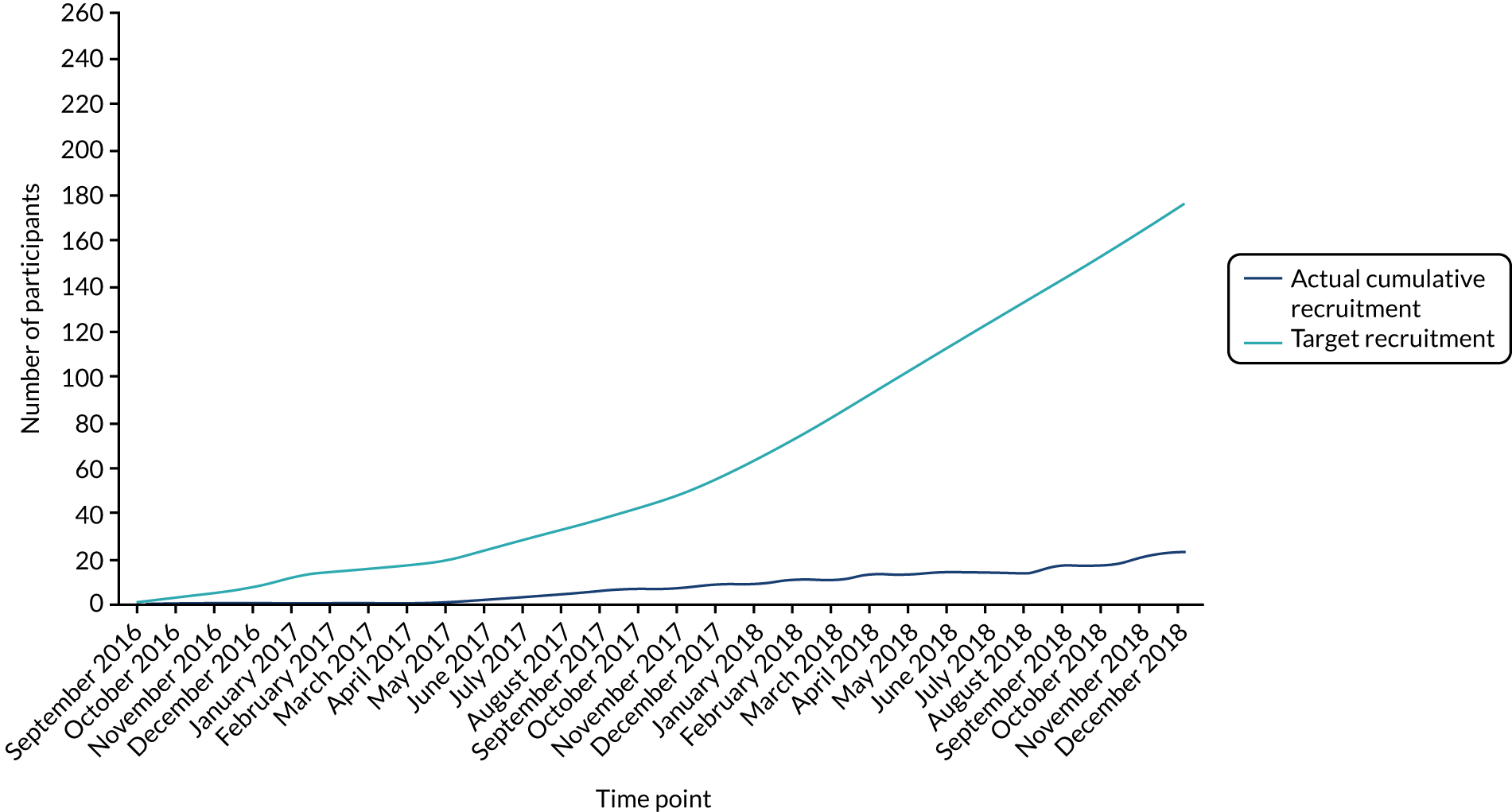
Recruitment opened on 20 September 2016 in Newcastle upon Tyne and a further 14 sites were opened over the subsequent 22 months, including three non-UK sites (Łódź, Poland; Riga, Latvia; and Timişoara, Romania).
The first participant was randomised on 2 March 2017, 6 months after screening started. A chart of actual cumulative recruitment by month (up until recruitment was paused in December 2018) against target recruitment over the same period is shown in Figure 2.
FIGURE 2.
Planned and actual recruitment for TOPSAT2.
Baseline characteristics
Twenty-three participants were recruited to TOPSAT2. Eleven were randomised to the ET arm and 12 to the TONI arm. The baseline characteristics of each of the two randomisation arms are shown in Table 4. Participants ranged in age from 44 to 75 years with a median age of 63 years. They were more likely to be female (65%) than male, and more were recruited in the UK (70%) than outside the UK. Most participants had an aneurysm of WFNS grade of 5 (74%) and Fisher grade 4 (87%), confirming their poor-grade status, although nearly all participants had a pre-stroke mRS of 0 (70%) or 1 (26%). The average time from ictus to randomisation was 20.9 hours. Randomisation resulted in two well-matched treatment arms.
| Randomised | ET (N = 11) | TONI (N = 12) | Total (N = 23) |
|---|---|---|---|
| Age (years) | |||
| Mean | 62.2 | 60.6 | 61.3 |
| SD | 11.4 | 9.8 | 10.1 |
| Median | 65 | 59 | 63 |
| Q1, Q3 | 52, 73 | 52, 70 | 52, 70 |
| Minimum, maximum | 44, 75 | 47, 75 | 44, 75 |
| Age band (years), n (%) | |||
| 18–50 | 2 (18) | 2 (17) | 4 (17) |
| 51–65 | 4 (36) | 5 (42) | 9 (39) |
| 66–80 | 5 (45) | 5 (42) | 10 (43) |
| Sex, n (%) | |||
| Male | 4 (36) | 4 (33) | 8 (35) |
| Female | 7 (64) | 8 (67) | 15 (65) |
| Site location, n (%) | |||
| UK | 8 (73) | 8 (67) | 16 (70) |
| Non-UK | 3 (27) | 4 (33) | 7 (30) |
| Pre-stroke residence, n (%) | |||
| At home alone | 3 (27) | 3 (25) | 6 (26) |
| Home with family/friends | 7 (64) | 9 (75) | 16 (70) |
| Sheltered housing | 0 | 0 | 0 |
| Other | 1 (9) | 0 | 1 (4) |
| Previous SAH, n (%) | |||
| No | 11 (100) | 12 (100) | 23 (100) |
| Yes | 0 | 0 | 0 |
| Previous medical history, n (%) | |||
| High blood pressurea | 5 (45) | 5 (42) | 10 (43) |
| Known diabetic | 0 | 1 (8) | 1 (4) |
| Severe dementia | 0 | 0 | 0 |
| Severe renal failure | 0 | 1 (8) | 1 (4) |
| Severe cardiac failure | 0 | 0 | 0 |
| Metastatic cancer (not controlled) | 0 | 0 | 0 |
| Smoker | 4 (36) | 5 (42) | 9 (39) |
| GCS – eye prior to intubation, n (%) | |||
| E1 | 7 (64) | 9 (75) | 16 (70) |
| E2 | 0 | 1 (8) | 1 (4) |
| E3 | 1 (9) | 1 (8) | 2 (9) |
| E4 | 2 (18) | 1 (8) | 3 (13) |
| Not recorded | 1 (9) | 0 | 1 (4) |
| GCS – motor prior to intubation, n (%) | |||
| M1 | 3 (27) | 3 (25) | 6 (26) |
| M2 | 1 (9) | 1 (8) | 2 (9) |
| M3 | 3 (27) | 2 (17) | 5 (22) |
| M4 | 0 | 3 (25) | 4 (17) |
| M5 | 3 (27) | 2 (17) | 5 (22) |
| M6 | 0 | 1 (8) | 1 (4) |
| Not recorded | 1 (9) | 0 | 1 (4) |
| GCS – verbal prior to intubation, n (%) | |||
| V1 | 6 (55) | 9 (75) | 15 (65) |
| V2 | 2 (18) | 3 (25) | 5 (22) |
| V3 | 0 | 0 | 0 |
| V4 | 1 (9) | 0 | 1 (4) |
| V5 | 0 | 0 | 0 |
| Not recorded | 2 (18) | 0 | 2 (9) |
| WFNS, n (%) | |||
| Grade 4 | 2 (18) | 4 (33) | 6 (26) |
| Grade 5 | 9 (82) | 8 (67) | 17 (74) |
| Fisher grade, n (%) | |||
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 3 (27) | 0 | 3 (13) |
| 4 | 8 (73) | 12 (100) | 20 (87) |
| Hydrocephalus requiring drainage, n (%) | |||
| No | 3 (27) | 3 (25) | 6 (26) |
| Done or planned | 8 (73) | 9 (75) | 17 (74) |
| Pre-stroke mRS, n (%) | |||
| 0 | 6 (55) | 10 (83) | 16 (70) |
| 1 | 4 (36) | 2 (17) | 6 (26) |
| 2 | 1 (9) | 0 | 1 (4) |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 |
| Time from ictus to randomisation | |||
| Median (hours) | 17.9 | 21.3 | 20.9 |
| Minimum, maximum | 2.1, 47.8 | 2.8, 47.4 | 2.1, 47.8 |
| Q1, Q3 | 8.1, 39.6 | 14.5, 35.7 | 8.8, 39.2 |
| Abnormal blood readings (clinically significant), n (%) | |||
| APTT | 0 | 0 | 0 |
| Prothrombin time | 0 | 0 | 0 |
| International normalised ratio if on warfarin | 0 | 0 | 0 |
| Platelets | 0 | 0 | 0 |
| Haemoglobin | 2 (18) | 1 (8) | 3 (13) |
| eGFR | 0 | 0 | |
| Glucose | 0 | 1 (8) | 1 (4) |
| Sodium | 0 | 1 (8) | 1 (4) |
| Potassium | 1 (9) | 0 | 1 (4) |
| Urea | 0 | 0 | 0 |
| Creatinine | 1 (9) | 0 | 1 (4) |
| Number of aneurysms, n (%) | |||
| 1 | 8 (73) | 9 (75) | 17 (74) |
| 2 | 2 (18) | 2 (17) | 4 (17) |
| 3 | 1 (9) | 0 | 1 (4) |
| 4 | 0 | 0 | 0 |
| 5 | 0 | 1 (8) | 1 (4) |
A table of baseline characteristics by WFNS grade is provided in Appendix 3, and a table of baseline characteristics by location of site (UK vs. non-UK) is provided in Appendix 4.
Aneurysm treatment details
The aneurysm characteristics are shown in Table 5. The majority of aneurysms were located in the anterior communicating artery (43%) or posterior communicating artery (30%). The planned treatment of the aneurysm was endovascular (coiling) in 30% of patients and neurosurgical (clipping) in 52% of patients but was not stated for 17% of patients who were in the TONI arm. Although there did appear to be a difference in planned treatment between the two treatment arms, with 55% planned coiling in the ET arm and 8% in the TONI arm, it is likely that coiling would have been the eventual preferred treatment in the ‘not yet planned’ participants, as these were all UK patients where coiling was more prevalent. The time from ictus to securing the aneurysm in the ET arm varied between 3 and 71 hours, with a median of 26 hours, whereas for the TONI arm it varied between 15 and 434 hours, with a median of 163 hours (over 6 days) for the four patients treated.
| Randomised | ET (N = 11) | TONI (N = 12) | Randomised (N = 23) |
|---|---|---|---|
| Location of aneurysm (primary outcome), n (%) | |||
| Anterior cerebral artery | |||
| Anterior communicating | 5 (45) | 5 (42) | 10 (43) |
| Proximal to anterior | 0 | 0 | 0 |
| Communicating | 1 (9) | 0 | 1 (4) |
| Pericallosal | 0 | 0 | 0 |
| Internal carotid artery | |||
| Proximal or ophthalmic region | 0 | 0 | 0 |
| Posterior communicating | 3 (27) | 4 (33) | 7 (30) |
| Bifurcation | 0 | 1 (8) | 1 (4) |
| Other internal carotid artery | 1 (9) | 0 | 1 (4) |
| Middle cerebral artery | |||
| Proximal to bifurcation | 0 | 0 | 0 |
| Bifurcation | 1 (9) | 1 (8) | 2 (9) |
| Distal to bifurcation | 0 | 0 | 0 |
| Posterior circulation | |||
| Basilar bifurcation | 0 | 0 | 0 |
| Basilar trunk | 0 | 0 | 0 |
| Superior cerebellar | 0 | 0 | 0 |
| Posterior cerebral | 0 | 1 (8) | 1 (4) |
| Posterior inferior cerebellar | 0 | 0 | 0 |
| Planned treatment, n (%) | |||
| Endovascular | 6 (55) | 1 (8) | 7 (30) |
| Neurosurgical | 5 (45) | 7 (58) | 12 (52) |
| Other (not yet planned) | 4 (33) | 4 (17) | |
| Time from ictus to treatment for those treated, n (%) | |||
| Median (hours) | 26 | 163 | 27 |
| Minimum, maximum | 3, 71 | 15, 434 | 3, 434 |
| Q1, Q3 | 20, 45 | 39, 270 | 20, 71 |
| Not treated | 0 | 8 | 8 |
Emergent treatment arm
All 11 patients in this arm each had only one aneurysm treated because it was either the only aneurysm or considered to be the cause of the bleed. At the time of treatment the WFNS grade was the same as at randomisation for all patients, confirming that the aneurysm was secured prior to neurological improvement of the participant. Seven patients had coiling and four had clipping.
Treatment on neurological improvement arm
A total of 4 out of 12 patients randomised to this arm went on to receive treatment: three patients had one aneurysm treated and one patient had two aneurysms treated. At the time of treatment, two of these aneurysms were grade 5, one was grade 4 and one was grade 2, demonstrating that only one TONI participant had actually shown the required degree of neurological improvement prior to the aneurysm being secured. One grade 5 patient had treatment within 24 hours and was therefore a crossover. The other two did not have their aneurysm secured for > 4 days. One patient had coiling and three had clipping.
Coiling
Of the eight patients who had coiling, a balloon was used for four of the ET patients and for the single TONI patient; a stent was used for one ET patient. The rest had unassisted coiling (no balloon). No patients had a flow diverter, web or other permanently implanted neck bridge device.
All patients had intravenous heparin, which was administered during the procedure in all patients except one ET patient, in whom it was administered post procedure. Two ET patients received aspirin, one of whom also received oral aspirin. Two other ET patients and the TONI patient received oral aspirin alone. No patients received clopidogrel or equivalent.
No patients suffered from aneurysm rupture or parent artery occlusion, but one ET patient had a coil migration or significant protrusion, and two ET patients had a dissection or vessel perforation. No patients had a groin haemorrhage, thromboembolic complication or required an additional procedure.
Clipping
The procedure was completed for all seven patients across both arms with no aneurysm rupture, parent artery occlusion or vasospasm.
Complications
Only one treated participant reported delayed ischaemic deficit (a TONI patient who had clipping). This was the only participant for whom a haematoma was reported and who also suffered a rebleed at this time point. The rebleed occurred after treatment and was confirmed by CT but did not require treatment.
Other neurosurgical procedures
Of the ET patients, two coiled patients had an EVD and one clipped patient had a ventriculoperitoneal shunt.
Of the TONI patients, two untreated participants had an EVD and one clipped participant had a ventriculoperitoneal shunt.
Four ET patients (one coiled, three clipped) had triple-H therapy and one untreated patient also had triple-H therapy. No balloon angioplasty procedures were reported.
There were no responses provided to these questions for four untreated participants.
Outcomes
The primary objective for TOPSAT2 was functional outcome at 12 months using the mRS. Outcomes for each arm at 6 and 12 months after randomisation are shown in Table 6.
| Randomised | ET (N = 11) | TONI (N = 12) | Randomised (N = 23) |
|---|---|---|---|
| mRS at 12 months (primary outcome), n | |||
| 0 | 1 | 0 | 1 |
| 1 | 0 | 0 | 0 |
| 2 | 1 | 0 | 1 |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 1 | 1 |
| 5 | 2 | 0 | 2 |
| 6 | 6 | 10 | 16 |
| Lost to follow-up | 1 (at home) | 1 (at home) | 2 |
| mRS at 6 months, n | |||
| 0 | 1 | 0 | 1 |
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 1 | 1 | 2 |
| 6 | 6 | 10 | 16 |
| Lost to follow-up | 3 | 1 | 4 |
| Place of residence at 6 months, n | |||
| Home | 1 | 0 | 1 |
| Residential home | 0 | 0 | 2 |
| Nursing home | 0 | 0 | 16 |
| Hospital | 1 | 1 | 4 |
| Rehabilitation unit | 0 | 0 | |
| Other | 0 | 0 | |
| Not applicable – deceased | 6 | 10 | |
| Lost to follow-up | 3 | 1 | |
| Place of residence at 12 months, n | |||
| Home | 2 | 1 | 3 |
| Residential home | 0 | 0 | 0 |
| Nursing home | 1 | 1 | 2 |
| Hospital | 1 | 0 | 1 |
| Rehabilitation unit | 1 | 0 | 1 |
| Other | 0 | 0 | 0 |
| Not applicable – deceased | 6 | 10 | 16 |
Primary outcome
Using a Wilcoxon rank-sum test (Mann–Whitney U-test) there was no evidence of a difference in 12-month mRS between patients receiving ET and patients receiving TONI (p = 0.11). However, there were two patients who did not respond but who were known (from general practitioner or hospital records) to be living at home. Patients living at home are most likely to have a mRS of between 0 and 3. To carry out a sensitivity analysis to include the patients with missing data, two possible scenarios were examined: first, assuming the missing case in the ET arm has the best outcome (mRS = 0) and in the TONI arm has the worst outcome (mRS = 3), and, second, vice versa. These two analyses give rank-sum test results of p = 0.13 and p = 0.19, respectively. The two TONI patients alive at 12 months had their aneurysms secured after more than 4 days.
Secondary outcomes
At 6 months, the Wilcoxon rank-sum test gives a value of p = 0.33 for the patients with known mRS. However, at 6 months there were four patients alive but for whom mRS was unknown (three ET patients and one TONI patient), and at this time their place of residence was also not known. Their best possible mRS was 0 and their worst possible mRS was 5. Assuming that the patients in the ET arm whose mRS were unknown had a value of 0 and the patient in the TONI arm whose mRS was unknown had a value of 5, and then assuming the opposite scores for those in each arm, the Wilcoxon rank-sum test produced p-values of 0.08 and 0.18, respectively.
Table 7 shows the outcomes for mortality and for dichotomised mRS, with the break between mRS 3 and mRS 4 and between mRS 2 and mRS 3, respectively. There is no evidence of a difference between the two treatment arms. Only one patient died after 30 days. Figure 3 shows the results of the survival analysis (Log-rank p = 0.32).
| Secondary outcomes | ET (N = 11), n (%) | TONI (N = 12), n (%) | Fisher’s exact test |
|---|---|---|---|
| Dead at 30 days | 6 (55) | 9 (75) | p = 0.40 |
| Dead at 6 or 12 months | 6 (55) | 10 (83) | p = 0.19 |
| 12-month mRS 0–3 vs. 4–6a | 3 (27) | 1 (8) | p = 0.32 |
| 12-month mRS 0–2 vs. 3–6a | 2 (18) | 0 | p = 0.22 |
FIGURE 3.
Kaplan–Meier plot.

Table 8 shows cause of death for all patients who died in the trial and demonstrates that the main cause of death in both arms for these poor-grade patients is subarachnoid haemorrhage.
| Cause | ET (N = 6), n (%) | TONI (N = 10), n (%) | Total (N = 16), n (%) |
|---|---|---|---|
| Subarachnoid haemorrhage | 3 (50) | 6 (60) | 9 (56) |
| Cardiac arrest | 1 (17) | 2 (20) | 3 (19) |
| Pneumonia | 1 (17) | 1 (6) | |
| Cerebral infarction | 1 (17) | 2 (20) | 3 (19) |
Table 9 shows outcomes at 30 days. By day 30, six ET patients and 10 TONI patients had died. Of the survivors, two ET patients had been discharged: one to be home alone (mRS = 2) and the other into rehabilitation (mRS = 3). The remaining three ET patients (all mRS 5) and two TONI patients (mRS 4 and 5) were still in hospital. There was no evidence of a difference in mRS at 30 days (Wilcoxon rank-sum test p = 0.33). Length of time in ITU by day 30 varied between 3 and 30 days with a median in the ET group of 13 days [mean 11.8 days; standard deviation (SD) 7.7 days] and a median in the TONI group of 10 days (mean 14.8 days; SD 9.2 days) (Wilcoxon rank-sum test p = 0.68).
| Randomised | ET (N = 11) | TONI (N = 12) | Randomised (N = 23) |
|---|---|---|---|
| Status by day 30, n (%) | |||
| Died before discharge | 6 (55) | 9 (75) | 15 (65) |
| Discharged | 2 (18) | 0 | 2 (9) |
| Still in hospital | 3 (27) | 3 (25) | 6 (26) |
| Discharged to, n (%) | |||
| Home alone | 1 | 0 | 1 (50) |
| Home not alone | 0 | 0 | 1 (50) |
| Residential home | 0 | 0 | 21 |
| Nursing home | 0 | 0 | |
| Referring hospital | 0 | 0 | |
| Rehabilitation unit | 1 | 0 | |
| Other | 0 | 0 | |
| Not applicable | 9 | 12 | |
| Number of days in ITU by 30 days | |||
| Mean | 11.8 | 14.8 | 13.4 |
| SD | 7.7 | 9.2 | 8.3 |
| Median | 13 | 10 | 10 |
| Q1, Q3 | 5, 15 | 9.2, 24.8 | 8, 15 |
| Minimum, maximum | 3, 30 | 4, 30 | 3, 30 |
| mRS score at 30 days, n (%) | |||
| 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 |
| 2 | 1 (9) | 0 | 1 (4) |
| 3 | 1 (9) | 0 | 1 (4) |
| 4 | 0 | 1 (8) | 1 (4) |
| 5 | 3 (27) | 1 (8) | 4 (17) |
| 6 | 6 (55) | 10 (83) | 16 (70) |
Adverse events
There were 41 AEs recorded: 19 in the ET arm (mean 1.7 per participant) and 22 in the TONI arm (mean 1.8 per participant). The number of AEs for an individual participant ranged from 0 to 5, as shown in Figure 4.
FIGURE 4.
Number of AEs per participant.
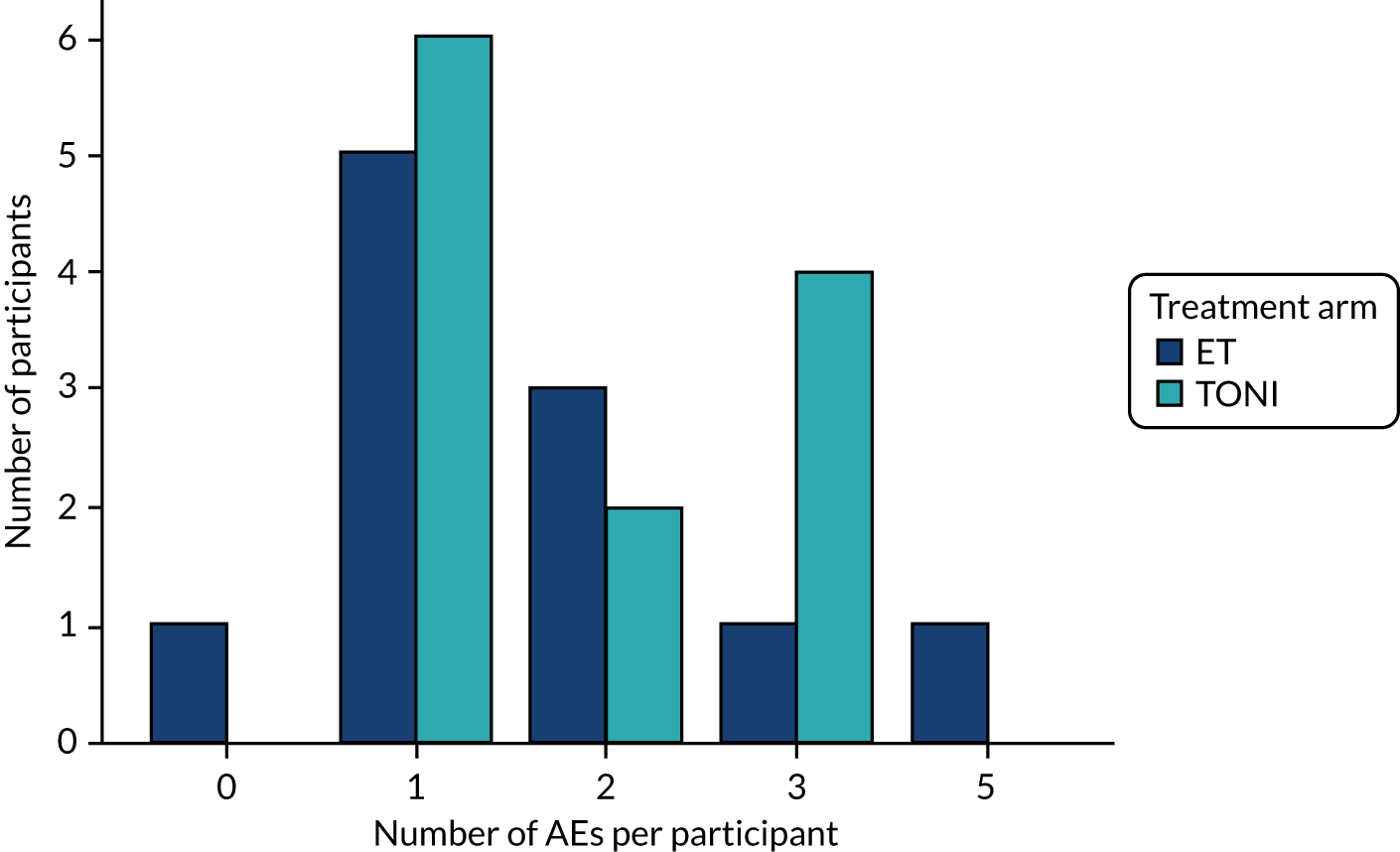
The nature of AEs is shown in Table 10. There are no statistically significant differences between the arms, although infections were more likely to be reported in patients allocated to the ET arm (Fisher’s exact p = 0.08), whereas brain oedema (p = 0.35) and cardiorespiratory (p = 0.17) events were more likely to be reported in patients allocated to the TONI arm. Most of the ‘other’ events (four out of the five) were death (in the fifth a new infarction was reported).
| Type of AE | ET (n = 11) | TONI (n = 12) | Total |
|---|---|---|---|
| Neurological | |||
| Impaired CSF drainage | 3 | 3 | 6 |
| Vasospasm related | 2 | 0 | 2 |
| Rebleed | 0 | 2 | 2 |
| Brain swelling/oedema | 1 | 4 | 5 |
| Stroke | 1 | 0 | 1 |
| Systemic | |||
| Cardiorespiratory | 3 | 8 | 11 |
| Renal | 2 | 1 | 3 |
| Infections | 5 | 1 | 6 |
| Other | 2 | 3 | 5 |
| Total | 19 | 22 | 41 |
Serious adverse events
Seventeen SAEs were reported, all resulting in the death of the patients: six in the ET arm and 10 in the TONI arm (one TONI patient was reported as having two separate SAEs: a rebleed followed by a stroke 6 days later). All SAEs in the ET arm resulted in the death of the patients but were judged to be unrelated to the intervention by both site and chief investigator. Eight of the SAEs in the TONI arm were unrelated; of the others, one was probably related (a rebleed) and two were judged probably unrelated (a myocardial infarction and a new cerebral infarction). All of these were expected outcomes of the disease.
Other analysis
Appendix 4 reports the baseline characteristics of UK and non-UK participants. Non-UK participants tended to be older and were more likely to be male and to have high blood pressure. The planned treatment to secure all aneurysms for non-UK participants was neurosurgical clipping. The mortality rate differed between UK (56%) and non-UK (100%) participants (Fisher’s exact p = 0.06).
Appendix 3 reports the baseline characteristics by WFNS grade. Participants with WFNS grade 4 tended to be older, but the other baseline variables showed little difference, and there was no evidence of a difference in mortality rate, with rates of 83% in grade 4 patients and of 65% in grade 5 (Fisher’s exact p = 0.62).
Summary of results
Of the 1269 aSAH patients screened, 305 were identified as poor grade. From this group, 23 patients were recruited and randomised to the ET group (11 patients) or TONI group (12 patients). Patients had a median age of 63 years, 65% were female and 74% were WFNS grade 5. All patients randomised to the ET group had their aneurysm clipped or coiled within 3 days and prior to any change in their WFNS grade. Only four patients randomised to the TONI group went on to have their aneurysm secured: three before neurological improvement and one after improvement to grade 2. As expected, the mortality rate was high among poor-grade patients: 55% for the ET group and 83% for the TONI group. There was no evidence of a statistically significant difference in the mRS between the two groups at 12 months, as per the Wilcoxon rank-sum test (p = 0.11).
Chapter 4 Discussion
Overview of recruitment and comparison of clinical results with other key studies
Recruitment to TOPSAT2 proved to be very challenging in both the UK and Eastern Europe, and the reasons are explored in detail in Reasons for early termination.
Comparison with previous studies
Perhaps the most relevant comparison is with the NCEPOD report1 into SAH in the UK (excluding Scotland) in November 2013. The median age in the NCEPOD sample overall was 57 years compared with 63 years in TOPSAT2, but no breakdown of age by grade was performed on the NCEPOD sample. However, as approximately 19% of patients in the NCEPOD sample were managed conservatively and not admitted to a NSC, with poor grade as a reason for conservative management in many cases, it is reasonable to suppose that the more elderly and comorbid patients were actually under-represented in the NCEPOD NSC admissions. Therefore, it is likely that the underlying age differential between TOPSAT2 and NCEPOD poor-grade patients would probably have been somewhat greater than 6 years. It is also important to note that the clinical outcome from aSAH is strongly linked to age with a fairly linear effect; for instance, in the ISAT trial those patients < 60 years had a poor outcome rate of 24%, whereas for those 60 years and over it was 37%, a 54% relative risk increase. 6
The sex profiles of the NCEPOD sample and TOPSAT2 RCT are similar at nearly 70% and 65% female, respectively. The high rates of smoking (39%) and hypertension (43%) seen in the TOPSAT2 trial are typical of the aSAH population; 70% of the TOPSAT2 population was recruited in the UK where in 2017–18 overall smoking prevalence was < 15% and overall hypertension prevalence was < 30% for adults. No participants had a prior history of SAH, but this may well be a small-sample phenomenon. Interestingly, hypertension rates were much higher but smoking rates were lower in non-UK participants than in UK participants, although these might be confounded by the higher average age and 57% male profile of non-UK participants (smoking generally being more common in men). The sex mix in non-UK recruitment is most likely to be another small-numbers effect, as across Europe aSAH is universally more common in females than in males.
The time from ictus to randomisation in the TOPSAT2 trial was a median of just 21 hours and, as randomisation could be performed only after admission to a NSC, this indicates a fairly rapid care pathway. That is both reassuring and indicates a real improvement since the ISAT trial,33 in which time from ictus to randomisation was a median of 2 days. There was also an acknowledged appreciably lower time to randomisation in ISAT for the 22% of international participants than for UK participants. 33,34 That differential national timeline (ictus to randomisation) effect was seen to a much lesser extent in TOPSAT2 with only a 4-hour lower median time in non-UK compared with UK centres. With regard to aneurysm treatment undertaken/planned, the Eastern European recruiting centre did not provide an endovascular service, so only neurosurgical treatment was available and that does have an impact on the overall clipping versus coiling rates in the trial.
Predominantly good-grade patients were recruited by ISAT, with < 5% being WFNS grade 4–5 patients and with no detailed results being presented for poor-grade patients, therefore it tells us relatively little about poor-grade aSAH. In NCEPOD, which was a population-based sample, ≈ 22% of the patients admitted with aSAH to NSCs were WFNS grades 4 or 5, with only slightly more grade 5 than grade 4 patients. In contrast, recruitment into the TOPSAT2 trial did not reflect those earlier population-based data; again, only NSC-admitted patients could be included in TOPSAT2, but 74% were grade 5 and not the 50–55% range expected if randomisation reflected admissions in the NCEPOD national sample. Therefore, there was a clear selection bias in TOPSAT2 towards enrolling grade 5 and older poor-grade patients, and this probably reflects equipoise towards grade 5 patients but not grade 4 patients (see below for detailed discussion of clinical equipoise in relation to TOPSAT2). The balance (%) of grade (4 vs. 5) recruitment in the non-UK centre was more as expected from the UK-based NCEPOD sample, indicating that in actively recruiting centres, the (lack of) clinical equipoise issue seemed to be a UK phenomenon, with predominantly grade 5 and older grade 4 patients recruited in the UK. Two-thirds of grade 4 patients were in the 66–80 years age band compared with only 35% of grade 5 patients.
In the NCEPOD report,1 62% of aSAH patients admitted to NSCs required some intervention for hydrocephalus, but this includes patients with aneurysms of all grades, and the rate of hydrocephalus is correlated with the extent of blood load on CT (the Fisher grade). That, in turn, correlates with the clinical (WFNS) grade; simply put, the more blood on the CT scan the greater the chance of being in a poorer clinical grade. Given that all TOPSAT2 patients were poor grade, it is unsurprising that the actual or planned intervention rate for hydrocephalus was rather greater in TOPSAT2 than in the NCEOPD report1 at 74%.
Regarding clinical outcomes, in NCEPOD at discharge, 16% of grade 5 patients were mRS 0–2 and 65% died prior to discharge, whereas 40% of grade 4 patients attained mRS of 0–2 and only 24% died. The timeline for NSC discharge used in NCEPOD roughly equates to the 30-day clinical outcomes in TOPSAT2, especially as few poor-grade patients are discharged before 30 days. In TOPSAT2 overall, 70% died, reflecting both the preponderance of grade 5 patients and the higher average age of the TOPSAT2 cohort. Again, in TOPSAT2 data there are clear differences in outcomes between WFNS grade 4 and grade 5 patients, albeit very few WFNS grade 4 patients were enrolled in TOPSAT2 (only six), so that formal statistical testing for differences is not appropriate. Given that there have been no major management changes in the 3–5 years since the NCEPOD report1 and the period of TOPSAT2 recruitment (December 2016–December 2018), except a drive in the UK to admit and treat aSAH within 48 hours, this similarity in outcomes is as expected.
The outcomes for those patients who experience rebleeding are universally acknowledged as being very poor: 82% bad outcome in ISAT and 75% in NCEPOD. In TOPSAT2, as the trial numbers are very small and the frequency of rebleeding is fairly low, these events were rare, with only two cases of rebleeding reported.
In the limited data provided in a trial with 23 patients enrolled, TOPSAT2 does not support the concept that a TONI approach is inevitably associated with a much higher rate of rebleeding events than treating all emergently regardless of neurological status. The Kaplan–Meier survival analysis (see Figure 3) does not indicate any statistically significant difference in survival between the ET and TONI arms (p = 0.32). Comparison of mortality rates at 30, 180 and 365 days found no statistically significant difference between groups at any time point.
In terms of overall clinical outcome, not only mortality, there was again no statistically significant difference identified between the arms within the limitations of such a modest sample size. Sensitivity analysis was performed to allow for missing Rankin Scale outcome data, but this also did not indicate any outcome differences.
The mortality rate differed between UK (56%) and non-UK (100%) centres but did not quite reach statistically significant difference. This trend could reflect a number of factors, including that the Romanian centre did not have access to coiling as a treatment option; it recruited older patients with more comorbidities and had different critical care provision (to UK). Of course, it could also be a small-numbers effect.
All SAEs were expected events related to poor-grade aSAH, with no statistically significant difference between the arms. Only one event in the TONI arm, a rebleed before aneurysm treatment, could be adjudicated as probably related to the intervention. Likewise, regarding AEs, there were no statistically significant differences between the arms. As expected, the occurrence of any AE was very common in the enrolled population, predominantly grade 5 and of older average age than in most aSAH trials.
Reasons for early termination
The trial recruited only 23 patients over 25 months; therefore, feasibility could not be confirmed and recruitment had to be terminated early. Furthermore, although by then centres had been opened in four European countries, participants were actually recruited only in the UK and Romania, and only 9 out of 15 open centres recruited participants. It was clear that this patchy enrolment pattern applied across both UK and Eastern European centres so that opening additional European centres did not seem likely to overcome the very slow recruitment. Various measures were taken to attempt to stimulate recruitment: we conducted local training with ITUs/neurosurgery departments and we offered feedback calls for units, as required, following on from site initiation visits/issues arising. We presented TOPSAT2 at multiple relevant conferences including the UK Neurointerventional Group, the European Association of Neurological Societies, the European Society of Minimally Invasive Neurological Therapy, the Society of British Neurological Surgeons, the British Neurovascular Group and the British Society of Neuroradiologists. We ran stands at multiple national/international conferences to raise awareness/profile. We held trial webinars for after ‘conference season’ as an ongoing awareness raiser and to specifically address/discuss equipoise issues as well as to assist with site/PI training. We addressed any issues arising and focused on recruitment in the trial monthly newsletter.
In addition, through one of the co-applicants, Mr Paul Brennan, we engaged further with the British Neurosurgical Trainees Research Collaborative to assist with TOPSAT2 screening and enrolment. We developed training materials/a slide-set for ITU staff and neurosurgeons. We also agreed on a policy of preferential enrolment with the EME-funded Subcutaneous Interleukin-1 Receptor Antagonist (SCIL) trial run from Manchester (this recruited patients of all SAH grades), so that neither trial interfered with recruitment of the other. Both trials actively encouraged their centres to recruit any SAH case eligible into either TOPSAT2 or SCIL, as appropriate (see Appendix 5).
A total of 1269 patients were assessed by centre using screening logs. These findings are summarised in Table 2.
Data from screening logs indicate that the proportion of poor-grade SAH patients was within the anticipated range (expected 22% and actual 24%) and that the medically eligible proportion of poor-grade patients was also very close to expected, at 48% (50% expected). The reasons for screened poor-grade SAH patients not being eligible were summarised in Tables 2 and 3.
The reason for non-eligibility was known for 240 out of 305 patients. Definite (known) contraindications (to trial enrolment) account for a majority of reasons for non-eligibility (147/240). Of non-medical reasons for non-eligibility, the lack of clinical equipoise (74/240) is by far the largest factor and accounts for 28% of all poor-grade patients overall (where the reason for non-eligibility was recorded). Of the 42 grade 4–5 patients screened as non-eligible but with no reason given for non-eligibility, it is possible, indeed probable, that a fair proportion of these were also medically eligible but lack of equipoise was not declared and recorded. It was the unexpectedly high ‘lack of clinical equipoise’ rate in otherwise medically eligible poor-grade patients that undermined recruitment, not timelines (15 patients) or lack of assent (four patients). Centres had to confirm such clinical equipoise regarding the question of treat all early regardless of improvement versus TONI (irrespective of whether that improvement was early or late) to participate in the trial and, indeed, several large UK centres that declared they lacked equipoise declined to participate on those grounds. ‘Other’ (18/240) comprises a large number of reasons but can be aggregated into three main groupings (more than one reason applying in some cases): timeline related (delayed presentation or admission to NSC, etc.) for 15 patients, query over grade for five patients, and a combination of reasons for non-eligibility for three patients.
There was a good conversion rate, of 23 out of 45 for patients screened as eligible, in TOPSAT2 (51% of eligible enrolled vs. 20% expected). Therefore, had the lack of clinical equipoise rate been remotely similar to that seen in the TOPSAT1 pilot (4%), recruitment would have been increased by ≈ 35 patients. In other words, there would have been a < 150% increase in recruitment during the trial recruitment period, rendering it feasible to have completed the trial with more centres opened.
It is uncertain what part the UK national drive towards treating aSAH within 48 hours may have played in that equipoise shift. 1,35 Although the National Clinical Guideline for Stroke35 is clear that treatment within 48 hours refers to good-grade patients (as early coiling was proven in good-grade patients in the ISAT trial), it may well have influenced perceptions quite widely within NSCs, such that units may have been concerned that TOPSAT2 enrolment might make it appear that they were missing the ‘48 hours to secure aneurysm’ target repeatedly. That was one of the contributory factors stated for not joining TOPSAT2 by some of the centres that declined to participate in the trial. The equipoise change is probably also due to a number of other reasons; all consultants and senior nursing staff managing a case had to be content with randomisation for this to be feasible. It transpired that a team consensus to clinical equipoise in participating centres was not the prevailing situation, whereas it might have tended to be so in the past if agreement to participate in a trial had been given by all relevant consultant groups. Indeed, even the understanding of the concept of clinical equipoise as it applies to an individual patient may have worsened with time, and/or a mindset of defensive medicine may affect clinical care trials such as this. Anything potentially perceived (even erroneously) as ‘conservative management’ is perceived as risky, although, of course, randomisation into a clinical care trial like TOPSAT2 is actually a very positive decision. 36,37
Limitations
The TOPSAT2 trial aimed to compare a policy of securing a ruptured aneurysm in poor-grade patients early in all cases, with the aneurysm being treated expeditiously on neurological improvement (whether that was early or late was irrelevant). TONI had been the standard approach to management when aneurysm clipping was the main/only proven treatment (up until at least 2002). The trial PROBE design used in TOPSAT2 is a very well-validated and robust one that has proven successful in the neurointerventional field before. However, such an approach has been less successful in prior trials where neurointerventional treatment has been compared with ‘conservative’ management. 33,34
Although TOPSAT2 was not a trial of conservative management, it was clear that the actual policy applied in most trial centres was ET in all cases, even though they had stated equipoise regarding the approach in response to pre-trial enquiries and in centre-declared expressions of interest. In other words, it seems that many clinicians in participating centres viewed TOPSAT2 as a de facto conservative management trial, even though it was not, and, therefore, they felt that they (or at least one of the team) were not in personal clinical equipoise to randomise.
This lack of equipoise was not seen in the earlier feasibility TOPSAT1 trial. 19 This change is probably for a number of reasons discussed above.
Relatives’ assent was required for all TOPSAT2-screened eligible patients, and the relatives’ approach to this may also have shifted towards ET. This is unsurprising given that they were often told that their relative was being transferred to a regional NSC for the aneurysm to be coiled, raising expectations of definite and early treatment. These factors combined led to an unfeasibly low recruitment rate (relative to time and target necessary), even though the conversion rate of screened, eligible patients was somewhat better than predicted.
The very modest sample size recruited is the major limitation of TOPSAT2. The preponderance of WFNS grade 5 patients would have been only a relatively minor limitation of the trial (as grade was a minimisation criterion) if recruitment targets were able to have been met. The PROBE design and the multicentre/multinational recruitment with blinded outcome assessment are indeed strengths, but cannot negate the small sample size achieved.
Magnetic resonance imaging substudy
This was an exploratory and mechanistic component to the study and was intended to be run in the UK centres only. However, none of the UK centres that recruited patients was able to sign up to the substudy from the start of their participation. This was due to the logistical difficulties of performing detailed advanced MRI in this critically ill and unstable patient population (population as enrolled) on top of the effort required by centres to screen and enrol patients. This is despite all NSCs regularly performing MRI under general anaesthesia in children and some adults. However, general anaesthesia plus critically ill patient monitoring within the MRI environment is both technically challenging and very time-consuming of sparse human resources, such as critical care anaesthetists and nurses. Two centres did agree to the MRI substudy from the start but failed to enrol any patients into TOPSAT2, Newcastle being one of them. Several centres intended to enrol into the substudy once they had recruited 5–10 patients and they and their ITU had become familiar with the trial processes and protocol, etc. However, the trial closing very early precluded that extension.
Research MRI units exist in or near most UK university hospitals but they rarely have the facilities for general anaesthesia let alone the monitoring and care of critically ill patients, and they are most often not located within the main hospital building. Therefore, transport would be required as well, which would occupy even more extensively the time of a whole team of critical care staff to undertake MRI in research MRI facilities. Ultimately, no patients were enrolled in the MRI substudy. That of itself indicates that, at present, carrying out MRI in WFNS grade 5 aSAH patients in the UK is not feasible on a regular basis, and, thus, MRI biomarker studies in poor-grade SAH do not seem plausible currently within the UK NHS health-care environment.
Generalisability of trial findings
The low recruitment inevitably limits drawing any substantial conclusions about the management of poor-grade aSAH. However, the demographics in terms of sex and key comorbidities were as expected, although the population was, on average, older than is typical of aSAH patients. There were also some trends emerging within the population actually recruited into TOPSAT2 indicating that it may not reflect the generality of poor-grade aSAH. For instance, younger patients and those in WFNS grade 4 are under-represented in the TOPSAT2 RCT compared with the NCEPOD national sample.
The TOPSAT2 trial gives an indication of outcomes really only for WFNS grade 5 SAH patients and grade 4 patients aged > 60 years, neither group being well represented in either ISAT or the NCEPOD sample (regarding NSC admissions). Given that the clinical outcomes statistically are the same between arms, the results are likely to reflect the true clinical outcome for the sample population (WFNS grade 5 and older grade 4 patients).
Interpretation
Unfortunately, the low recruitment and early termination of TOPSAT2 preclude making many clear statements or recommendations. The subgroup of poor-grade SAH patients proved to be a difficult population in which to undertake a randomised trial, at least in the neurovascular field. The trial data do suggest that the care pathway for aSAH has improved in the 15 years since the ISAT trial, at least in part driven by a 48-hour target to treat aneurysms in good-grade patients. However, as a by-product, that 48-hour aspiration may have contributed to undermining equipoise to randomise patients into the trial. Younger patients, especially those in WFNS grade 4, were under-represented; this may well be because the team/unit preference to treat immediately may have been more compelling given that, on average, both worse grade and older age are independent poor prognostic factors following aSAH.
However, if there are differences between the ET and TONI approaches they would appear to be modest at best, as no strong trends let alone statistically significant differences between the two management approaches were detected for mortality or functional clinical outcome in TOPSAT2 (or indeed in the preceding small TOPSAT1 feasibility study).
Although any interpretation of TOPSAT2 data must necessarily be limited, it is reassuring that no evidence was demonstrated that supported the hypothesis that ET would reduce mortality but lead to a large proportion of highly dependent survivors. The combined mRS 4–5 rate at 12 months was 2/11 for the ET arm compared with 1/12 for the TONI arm, and mortality was 6 versus 10 and not statistically different.
Chapter 5 Conclusions
Implications for health care
With regard to poor-grade aSAH management, at present, some units employ ET in nearly all patients routinely, some apply both ET and TONI on an ad hoc individual basis and others are (variably) selective in admissions regarding poor-grade SAH and some restrict admission to grade 4. 1 The TOPSAT2 data indicate no difference in outcome for the strategies of ET and TONI, albeit with the caveats discussed above (see Chapter 4). Therefore, the evidence suggests that TOPSAT2 is not able to provide data to indicate that the current heterogeneous approach to poor-grade management should change in one or another direction and, thus, that no service reconfiguration is necessary.
The use of MRI remains very challenging in aSAH patients, particularly in poor-grade patients. Therefore, caution should be employed when assessing any data on MRI in SAH – especially if generated on small sample sizes and/or extrapolating results from predominantly good-grade patients.
Future research implications
The RCT approach to poor-grade SAH management of TOPSAT2 proved unfeasible, and funding for a prospective observational study specific to this group was rejected by the National Institute for Health Research (NIHR) EME programme. It is also not likely to appeal to any other UK funder currently supporting studies in this clinical field given the scale of resources and study duration required, especially given the impact of the COVID-19 pandemic on charitable incomes. This leaves clinical audit approaches. A systematic comprehensive SAH patient audit has been proposed in the UK but does not yet exist. Anything less than a universal (all NSC units) and comprehensive (near 100% case ascertainment and inclusion) audit is inherently afflicted by many biases. These include admission and inclusion selection biases, data collection bias, transfer bias (losses to follow-up not random), interviewer bias, recall bias, outcome misclassification bias, confounding bias and performance bias. There is an ongoing voluntary UK and Ireland aSAH audit but it involves < 70% of NSCs; no measures exist to ensure 100% case ascertainment and all of the follow-up is undertaken and adjudicated by the clinical team looking after the patient, which is inherently open to major bias. Extension of the Stroke Sentinel National Audit Programme (SSNAP) to include SAH would probably be a better way to achieve that universal audit. However, no funding exists for such a development at present. Although it would not eliminate all the biases mentioned above, it could deal with some and ameliorate others. The linkage of SSNAP data to quality improvement markers, and thus NHS funding, has proven successful in the ischaemic stroke care setting.
Randomised controlled trials of proven design overcome most biases. However, RCTs of management pathways or so called ‘clinical care trials’, although often called for, have proven difficult, certainly in the neurovascular field. 38 If we are to provide patients with optimal, prudent care in the context of uncertainty and perform such trials then it does seem to require a major change in the mentalities of clinicians, other health-care staff and patients/relatives alike. Recognition that the answer to a care question was valuable and needed (with supporting survey and indeed feasibility study evidence) no longer seems to be enough. Innovative trial methodologies, such as step-wedge or cluster RCTs, may not map well onto the sort of specialised management care pathways that TOPSAT2, TEAM36 and ARUBA39 have attempted to investigate – where units that perform the treatment are relatively few and where key team leaders will frequently interact regionally, nationally and internationally. These trials’ collective experience does raise the question of whether or not it is appropriate that trials aimed at evaluating clinical care have a fixed, limited time period to provide an answer (recruitment ‘feasibility’) while potentially unjustified clinical care continues ‘unquestioned’ over an unlimited time period. In such clinical care questions, it is more likely than not that one approach is indeed somewhat better than the other, but if both approaches continue in the long term it is clearly likely to be to the detriment of some of those receiving the clinical care in question.
The use of MRI biomarkers looks promising in aSAH but the TOPSAT2 experience indicates that, at least in the subgroup of poor-grade patients, routine use of such MRI in the UK remains some way off and is associated with major logistical barriers in any health-care system. More traction might exist for the investigation and validation of MRI biomarkers in good-grade patients (not requiring a critical care support team for scanning), but studies would need to be correspondingly much larger, given that the number of (poor) outcome events is much lower in good-grade patients, amplifying the costs of such studies, which per patient are already high.
Acknowledgements
Contributions of others
Professor Peter Andrews (Consultant and Honorary Professor) was chairperson of the DMC and reviewed the final report.
Professor Jens Fiehler (Director of Diagnostic and Interventional Radiology) was a member of the TSC and reviewed the final report.
Professor Alex McConnachie (Professor of Clinical Trial Biostatistics) was an independent statistician on the DMC and reviewed the final report.
Dr Andrew Molyneux (Consultant Neuroradiologist) was a member of the DMC and reviewed the final report.
Contributions of authors
Philip White (https://orcid.org/0000-0001-6007-6013) (Translational and Clinical Research Institute, Newcastle University) was chief investigator for TOPSAT2, helped conceive the multicentre trial and develop the trial protocol, chaired the Trial Management Group (TMG), helped draft the report and edited it for important intellectual content.
Barbara Gregson (https://orcid.org/0000-0003-4868-9137) (Principal Research Associate, Neurosurgical Trials Group) was co-investigator and statistician for TOPSAT2, helped conceive the multicentre trial and develop the trial protocol, analysed the data, helped draft the report and edited it for important intellectual content.
Elaine McColl (https://orcid.org/0000-0001-8300-3204) (Professor of Health Services Research, Newcastle University) helped finalise the trial protocol, was a TMG member and edited the report for important intellectual content.
Paul Brennan (https://orcid.org/0000-0002-7347-830X) (Senior Clinical Lecturer, Neurosurgery, University of Edinburgh) contributed to neurosurgical engagement and recruitment.
Alison Steel (https://orcid.org/0000-0003-1279-1455) (Senior Trial Manager, Newcastle Clinical Trials Unit) helped finalise the trial protocol, was a TMG member and edited the report for important intellectual content.
Philippa Watts (https://orcid.org/0000-0003-3523-5101) (Trial Manager, Newcastle Clinical Trials Unit) set up and worked with study sites, conducted trial management and contributed to drafting and editing the final report for important intellectual content.
Ruth Wood (https://orcid.org/0000-0002-8296-1774) (Data Manager, Newcastle Clinical Trials Unit) designed and set up the trial-specific database, managed central data processes, reviewed study results and edited the final report.
Clare Bowes (https://orcid.org/0000-0002-9212-5315) (Professional and Administrative Services Officer, Faculty of Medical Sciences, Newcastle University) carried out data collection, data entry and revision of the report manuscript for important intellectual content.
Mohsen Javadpour (https://orcid.org/0000-0001-9836-1741) (Consultant Neurosurgeon, National Neurosurgical Centre, Beaumont Hospital) was chairperson of the TSC and had input in and final approval of the version of the report.
Amanda Weston (https://orcid.org/0000-0002-8119-1050) (independent layperson) contributed to public and patient involvement and reviewed and edited the final report.
Dipayan Mitra (https://orcid.org/0000-0002-1891-5415) (Consultant Neuroradiologist, Department of Neuroradiology, Royal Victoria Infirmary) contributed to the development of the study hypothesis, collection of pilot data, grant application, trial management, initiation of sites and writing of the manuscript.
Data-sharing statement
Fully anonymised data from this trial will be available to the scientific community subject to appropriate ethics approval and TSC agreement. Requests for anonymised data should be directed to the corresponding author.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- National Confidential Enquiry into Patient Outcome and Death (NCEPOD) . Managing the Flow? A Review of the Care Received by Patients Who Were Diagnosed With an Aneurysmal Subarachnoid Haemorrhage 2013.
- Patel A BV, King D, Quayyum Z, Wittenberg R, Knapp M. Executive Summary Part 2: Burden of Stroke in the Next 20 Years and Potential Returns from Increased Spending on Research. London: Stroke Association; 2017.
- Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988;51. https://doi.org/10.1136/jnnp.51.11.1457.
- Whitfield PC, Kirkpatrick PJ. Timing of surgery for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2001;2. https://doi.org/10.1002/14651858.CD001697.
- Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, et al. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke 2013;44:29-37. https://doi.org/10.1161/STROKEAHA.112.663559.
- Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. international subarachnoid aneurysm trial (ISAT) collaborative group . International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809-17. https://doi.org/10.1016/S0140-6736(05)67214-5.
- McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The barrow ruptured aneurysm trial. J Neurosurg 2012;116:135-44. https://doi.org/10.3171/2011.8.JNS101767.
- Bergui M, Bradac GB. Acute endovascular treatment of ruptured aneurysms in poor-grade patients. Neuroradiology 2004;46:161-4. https://doi.org/10.1007/s00234-003-1143-5.
- Bracard S, Lebedinsky A, Anxionnat R, Neto JM, Audibert G, Long Y, et al. Endovascular treatment of Hunt and Hess grade IV and V aneuryms. AJNR Am J Neuroradiol 2002;23:953-7.
- Suzuki S, Jahan R, Duckwiler GR, Frazee J, Martin N, Viñuela F. Contribution of endovascular therapy to the management of poor-grade aneurysmal subarachnoid hemorrhage: clinical and angiographic outcomes. J Neurosurg 2006;105:664-70. https://doi.org/10.3171/jns.2006.105.5.664.
- Taylor CJ, Robertson F, Brealey D, O’shea F, Stephen T, Brew S, et al. Outcome in poor grade subarachnoid hemorrhage patients treated with acute endovascular coiling of aneurysms and aggressive intensive care. Neurocrit Care 2011;14:341-7. https://doi.org/10.1007/s12028-010-9377-7.
- van Loon J, Waerzeggers Y, Wilms G, Van Calenbergh F, Goffin J, Plets C. Early endovascular treatment of ruptured cerebral aneurysms in patients in very poor neurological condition. Neurosurgery 2002;50:457-64. https://doi.org/10.1097/00006123-200203000-00005.
- Pereira AR, Sanchez-Peña P, Biondi A, Sourour N, Boch AL, Colonne C, et al. Predictors of 1-year outcome after coiling for poor-grade subarachnoid aneurysmal hemorrhage. Neurocrit Care 2007;7:18-26. https://doi.org/10.1007/s12028-007-0053-5.
- Luo YC, Shen CS, Mao JL, Liang CY, Zhang Q, He ZJ. Ultra-early versus delayed coil treatment for ruptured poor-grade aneurysm. Neuroradiology 2015;57:205-10. https://doi.org/10.1007/s00234-014-1454-8.
- Weir RU, Marcellus ML, Do HM, Steinberg GK, Marks MP. Aneurysmal subarachnoid hemorrhage in patients with Hunt and Hess grade 4 or 5: treatment using the Guglielmi detachable coil system. AJNR Am J Neuroradiol 2003;24:585-90.
- Phillips TJ, Dowling RJ, Yan B, Laidlaw JD, Mitchell PJ. Does treatment of ruptured intracranial aneurysms within 24 hours improve clinical outcome?. Stroke 2011;42:1936-45. https://doi.org/10.1161/STROKEAHA.110.602888.
- Zhao B, Tan X, Yang H, Zheng K, Li Z, Xiong Y, et al. AMPAS investigators . A multicenter prospective study of poor-grade aneurysmal subarachnoid hemorrhage (AMPAS): observational registry study. BMC Neurol 2014;14. https://doi.org/10.1186/1471-2377-14-86.
- Zhang Q, Ma L, Liu Y, He M, Sun H, Wang X, et al. Timing of operation for poor-grade aneurysmal subarachnoid hemorrhage: study protocol for a randomized controlled trial. BMC Neurol 2013;13. https://doi.org/10.1186/1471-2377-13-108.
- Mitra D, Gregson B, Jayakrishnan V, Gholkar A, Vincent A, White P, et al. Treatment of poor-grade subarachnoid hemorrhage trial. AJNR Am J Neuroradiol 2015;36:116-20. https://doi.org/10.3174/ajnr.A4061.
- Hadeishi H, Suzuki A, Yasui N, Hatazawa J, Shimosegawa E. Diffusion-weighted magnetic resonance imaging in patients with subarachnoid hemorrhage. Neurosurgery 2002;50:741-7. https://doi.org/10.1097/00006123-200204000-00010.
- Sato K, Shimizu H, Fujimura M, Inoue T, Matsumoto Y, Tominaga T. Acute-stage diffusion-weighted magnetic resonance imaging for predicting outcome of poor-grade aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2010;30:1110-20. https://doi.org/10.1038/jcbfm.2009.264.
- Yeo SS, Choi BY, Chang CH, Kim SH, Jung YJ, Jang SH. Evidence of corticospinal tract injury at midbrain in patients with subarachnoid hemorrhage. Stroke 2012;43:2239-41. https://doi.org/10.1161/STROKEAHA.112.661116.
- Rosenørn J, Eskesen V, Schmidt K, Rønde F. The risk of rebleeding from ruptured intracranial aneurysms. J Neurosurg 1987;67:329-32. https://doi.org/10.3171/jns.1987.67.3.0329.
- Naggara ON, White PM, Guilbert F, Roy D, Weill A, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology 2010;256:887-97. https://doi.org/10.1148/radiol.10091982.
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): a randomised trial. Lancet 2005;365:387-97. https://doi.org/10.1016/S0140-6736(05)70233-6.
- Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P, et al. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage [STITCH(trauma)]: the first randomized trial. J Neurotrauma 2015;32:1312-23. https://doi.org/10.1089/neu.2014.3644.
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013;382:397-408. https://doi.org/10.1016/S0140-6736(13)60986-1.
- Great Britain . Mental Capacity Act 2005 2005. www.legislation.gov.uk/ukpga/2005/9/contents (accessed 25 May 2021).
- Bath PM, Gray LJ, Collier T, Pocock S, Carpenter J. Optimising Analysis of Stroke Trials (OAST) collaboration. Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke 2007;38:1911-15. https://doi.org/10.1161/STROKEAHA.106.474080.
- Sarrafzadeh A, Haux D, Küchler I, Lanksch WR, Unterberg AW. Poor-grade aneurysmal subarachnoid hemorrhage: relationship of cerebral metabolism to outcome. J Neurosurg 2004;100:400-6. https://doi.org/10.3171/jns.2004.100.3.0400.
- White PM, Lewis SC, Gholkar A, Sellar RJ, Nahser H, Cognard C, et al. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): a randomised controlled trial. Lancet 2011;377:1655-62. https://doi.org/10.1016/S0140-6736(11)60408-X.
- Coley S, Sneade M, Clarke A, Mehta Z, Kallmes D, Cekirge S, et al. Cerecyte coil trial: procedural safety and clinical outcomes in patients with ruptured and unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2012;33:474-80. https://doi.org/10.3174/ajnr.A2836.
- Reeves BC, Langham J, Lindsay KW, Molyneux AJ, Browne JP, Copley L, et al. Findings of the international subarachnoid aneurysm trial and the national study of subarachnoid haemorrhage in context. Br J Neurosurg 2007;21:318-23. https://doi.org/10.1080/02688690701534748.
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. international subarachnoid aneurysm trial (ISAT) collaborative group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267-74. https://doi.org/10.1016/S0140-6736(02)11314-6.
- Royal College of Physicians (RCP) Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 2016.
- Raymond J. Reflections on the TEAM trial: why clinical care and research should be reconciled. Can J Neurol Sci 2011;38:198-202. https://doi.org/10.1017/S0317167100011343.
- Gifford F. So-called ‘clinical equipoise’ and the argument from design. J Med Philos 2007;32:135-50. https://doi.org/10.1080/03605310701255743.
- Raymond J, Darsaut TE, Molyneux AJ. TEAM collaborative group . A trial on unruptured intracranial aneurysms (the TEAM trial): results, lessons from a failure and the necessity for clinical care trials. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-64.
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicenter, non-blinded, randomized trial. Lancet 2014;383:614-21. https://doi.org/10.1016/S0140-6736(13)62302-8.
Appendix 1 Twelve-month follow-up questionnaire
Appendix 2 Expected adverse reactions
Frequencies are defined as common (≥ 1/100 to < 1/10); uncommon (≥ 1/1000 to < 1/100); rare (≥ 1/10,000 to < 1/1000); very rare (< 1/10,000); or not known (cannot be estimated from the available data).
Adverse events related to acute subarachnoid haemorrhage (neurological or systemic)
| Condition | Common | Uncommon | Very rare |
|---|---|---|---|
| Neurological | Death, impaired CSF drainage related, vasospasm related, rebleed, brain swelling/oedema, neuropsychological and cognitive, stroke, epilepsy (and sequelae); depression | Additional aneurysm related, visual, cranial nerve palsy | Visual loss |
| Systemic | Cardiorespiratory; renal; infections (including pneumonia, urinary tract infection, cellulitis, ventriculitis and hospital-acquired infections); complications of prolonged immobility including falls (and sequelae), deep-vein thrombosis, pulmonary embolism, pressure sores, spasticity, pain; fluid balance and electrolytic disturbances including syndrome of inappropriate antidiuretic hormone secretion and cerebral salt wasting; frailty | Myocardial infarction |
Adverse events related to aneurysm treatment (coiling or clipping)
| Procedure | Common | Uncommon | Very rare |
|---|---|---|---|
| Coiling | Rupture, dissection/vessel perforation, anaesthetic related, arterial puncture site related, adjunctive drug related (such as heparin, aspirin, nimodipine), stroke, coil prolapse/parent vessel occlusion, vasospasm | Epilepsy, myocardial infarction, contrast media related, radiation related, death | Visual loss |
| Clipping | Stroke, infection, anaesthetic, drain insertion related, epilepsy, vasospasm | Brain retraction related, rebleed death, myocardial infarction |
Appendix 3 Baseline characteristics of participants by World Federation of Neurosurgical Societies grade
| Randomised | WFNS 4 (N = 6) | WFNS 5 (N = 17) | Total (N = 23) |
|---|---|---|---|
| Age band (years), n (%) | |||
| 18–50 | 0 | 4 (24) | 4 (17) |
| 51–65 | 2 (33) | 7 (41) | 9 (39) |
| 66–80 | 4 (67) | 6 (35) | 10 (43) |
| Sex, n (%) | |||
| Male | 2 (33) | 6 (35) | 8 (35) |
| Female | 4 (67) | 11 (65) | 15 (65) |
| Site location, n (%) | |||
| UK | 3 (50) | 13 (76) | 16 (70) |
| Non-UK | 3 (50) | 4 (24) | 7 (30) |
| Pre-stroke residence, n (%) | |||
| At home alone | 1 (17) | 5 (65) | 6 (26) |
| Home with family/friends | 5 (83) | 11 (29) | 16 (70) |
| Sheltered housing | 0 | 0 | 0 |
| Other | 0 | 1 (6) | 1 (4) |
| Previous medical history, n (%) | |||
| High blood pressure | 4 (67) | 6 (35) | 10 (43) |
| Smoker | 1 (17) | 8 (47) | 9 (39) |
| Fisher grade, n (%) | |||
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 1 (17) | 2 (12) | 3 (13) |
| 4 | 5 (83) | 15 (88) | 20 (87) |
| Pre-stroke mRS, n (%) | |||
| 0 | 5 (83) | 11 (65) | 16 (70) |
| 1 | 1 (17) | 5 (29) | 6 (26) |
| 2 | 0 | 1 (6) | 1 (4) |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 |
| Time from ictus to randomisation | |||
| Median (hours) | 23.4 | 17.9 | 20.9 |
| Minimum, maximum | 13.8, 47.8 | 2.1, 47.5 | 2.1, 47.8 |
| Q1, Q3 | 19.1, 45.5 | 8.1, 31.2 | 8.8, 39.2 |
| Planned treatment, n (%) | |||
| Endovascular | 1 (17) | 6 (35) | 7 (30) |
| Neurosurgical | 3 (50) | 9 (53) | 12 (52) |
| Other (not yet planned) | 2 (33) | 2 (12) | 4 (17) |
Appendix 4 Baseline characteristics of participants by location of centre (UK/non-UK)
| Randomised | UK (N = 16) | Non-UK (N = 7) | Total (N = 23) |
|---|---|---|---|
| Age band (years), n (%) | |||
| 18–50 | 3 (19) | 1 (14) | 4 (17) |
| 51–65 | 7 (44) | 2 (29) | 9 (39) |
| 66–80 | 6 (38) | 4 (57) | 10 (43) |
| Sex, n (%) | |||
| Male | 4 (25) | 4 (57) | 8 (35) |
| Female | 12 (75) | 3 (43) | 15 (65) |
| Pre-stroke residence, n (%) | |||
| At home alone | 3 (19) | 3 (43) | 6 (26) |
| Home with family/friends | 12 (75) | 4 (57) | 16 (70) |
| Sheltered housing | 0 | 0 | 0 |
| Other | 1 (6) | 0 | 1 (4) |
| Previous medical history, n (%) | |||
| High blood pressure | 4 (25) | 6 (86) | 10 (43) |
| Smoker | 7 (44) | 2 (29) | 9 (39) |
| WFNS, n (%) | |||
| Grade 4 | 3 (19) | 3 (43) | 6 (26) |
| Grade 5 | 13 (81) | 4 (57) | 17 (74) |
| Fisher grade, n (%) | |||
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 2 (13) | 1 (14) | 3 (13) |
| 4 | 14 (88) | 6 (86) | 20 (87) |
| Pre-stroke Rankin, n (%) | |||
| 0 | 9 (56) | 7 (100) | 16 (70) |
| 1 | 6 (38) | 0 | 6 (26) |
| 2 | 1 (6) | 0 | 1 (4) |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 |
| Time from ictus to randomisation | |||
| Median (hours) | 21.9 | 17.9 | 20.9 |
| Minimum, maximum | 2.1, 47.8 | 4.4, 44.7 | 2.1, 47.8 |
| Q1, Q3 | 8.5, 39.5 | 8.8, 25.1 | 8.8, 39.2 |
| Planned treatment, n (%) | |||
| Endovascular | 7 (44) | 0 | 7 (30) |
| Neurosurgical | 5 (31) | 7 (100) | 12 (52) |
| Other (not yet planned) | 4 (25) | 0 | 4 (17) |
Appendix 5 The SCIL/TOPSAT2 recruitment flow chart
List of abbreviations
- AE
- adverse event
- aSAH
- aneurysmal subarachnoid haemorrhage
- CI
- confidence interval
- CLRN
- Comprehensive Local Research Network
- CSF
- cerebrospinal fluid
- CT
- computerised tomography
- DMC
- Data Monitoring Committee
- DTI
- diffusion tensor imaging
- DWI
- diffusion-weighted imaging
- EME
- Efficacy and Mechanism Evaluation
- ET
- emergent treatment
- EVD
- external ventricular drain
- GCS
- Glasgow Coma Scale
- ISAT
- international subarachnoid aneurysm trial
- ITU
- intensive therapy unit
- MRI
- magnetic resonance imaging
- mRS
- modified Rankin Scale
- NCEPOD
- National Confidential Enquiry into Patient Outcome and Death
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- NSC
- neurosciences centre
- OR
- odds ratio
- PI
- principal investigator
- PPI
- patient and public involvement
- PROBE
- pragmatic randomised open-blinded end point
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAH
- subarachnoid haemorrhage
- SCIL
- Subcutaneous Interleukin-1 Receptor Antagonist
- SD
- standard deviation
- SRN
- Stroke Research Network
- SSNAP
- Sentinel Stroke National Audit Programme
- STICH
- surgical trial in intracerebral haemorrhage
- TMG
- Trial Management Group
- TONI
- treatment on neurological improvement
- TOPSAT1
- treatment of poor-grade subarachnoid haemorrhage trial 1
- TOPSAT2
- treatment of poor-grade subarachnoid haemorrhage trial 2
- TSC
- Trial Steering Committee
- WFNS
- World Federation of Neurosurgical Societies
