Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 17/59/13. The contractual start date was in March 2019. The final report began editorial review in February 2022 and was accepted for publication in September 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Cella et al. This work was produced by Cella et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Cella et al.
Background
Negative symptoms (NS) are common in people with schizophrenia with at least one in three experiencing debilitating and long-lasting NS. 1 These include poor motivation, social withdrawal, difficulty in experiencing pleasure, blunted affect and reduced communication. 2 Extensive evidence points at the critical role NS play in negatively affecting patients’ recovery goals, quality of life and functioning levels. 3
Despite their importance to illness prognosis and functioning, the development of interventions for NS has received very limited attention. 4 Current treatment recommendation guidelines for both pharmacological and psychosocial interventions centre on efficacy evidence for positive symptoms reduction. 5 First-line interventions for people with schizophrenia such as antipsychotic medications and cognitive–behaviour therapy (CBT) for psychosis target prominent positive symptoms and have a small effect on NS. 6,7
Psychosocial interventions have shown some promise in targeting NS. 8,9 However, only a handful of approaches have been designed and tailored to tackle these symptoms. An issue that has hampered therapy development is the absence of a clear therapy target or mechanisms. Adapted CBT approaches for NS have focused on tackling clients’ defeatist belief, while cognitive remediation approaches have targeted the cognitive underpinning of NS. 10,11
Research has shown a consistent association between reward-processing abnormalities and NS in people with schizophrenia. 12,13 Reward learning is considered a key cognitive function in determining our behaviour; it pertains to estimating the pleasure and the value of everyday situations. We use this skill constantly to make decisions that define who we are and determine our role and function in society. Given its relevance, reward learning has been the focus of a substantial body of basic research. This process is mediated by the mesolimbic dopamine system, in particular by the ventral and dorsal striatum and ventral tegmental area and the nucleus accumbens. 14,15 Dopamine neurons in these areas have been found to respond to rewards but also, overtime, learn to ‘fire’ to predict reward. 16 The affinity between the brain areas involved in this process and those considered responsible for a range of psychiatric conditions prompted the investigation of reward learning in mental health conditions. The consistent finding that reward-learning abnormalities are found in many psychiatric disorders, including schizophrenia, has prompted the United States National Institute of Mental Health to highlight this as one of the core psychopathological processes of psychiatric conditions. 17–20
Studies have found that difficulties in motivation and pleasure experience were associated with reduced sensitivity to feedback and reward learning. 19 Previous studies have found that more feedback (either positive or negative) is necessary to modify behaviour,21 and that people with schizophrenia have impaired feedback sensitivity to learning from rewards, but learning from punishments is maintained, suggesting that this pattern could lead to motivational difficulties. 22 Overcoming motivational issues is a long-standing challenge for effectively delivering interventions in people with psychosis. 23
Studies have attempted to address difficulties in reward sensitivity by using contingency measures (e.g. financial incentives24), but these approaches circumvent reward-processing difficulties by disproportionally increasing reward and may not allow the reward-processing system to recalibrate. This may limit the potential for benefits to generalise to other areas of life. There is evidence suggesting that changes in reward learning following therapy are associated with NS reduction. 25 A therapy targeting reward-processing difficulties may be appropriate to reduce NS. However, NS are a key barrier to therapy engagement. 26 For example, motivational issues, flat affect and difficulties engaging with social contact may make attending therapy sessions difficult. Expressive and cognitive difficulties may also make it difficult for people with high levels of NS to engage with long sessions of talking therapy. While there is recognition that in vivo and behavioural work may be of help, these techniques have rarely been employed systematically in this filed.
Virtual reality (VR) is a computer-generated realistic scenario that can effectively mirror everyday life experiences. It is an experience allowing participants to interact with an environment and feel immersed in it. 27 This technology is increasingly used for health-care applications including in mental health. 28,29 A specific advantage of VR in mental health is the possibility to conduct exposure procedures in a controlled virtual environment. This is important as it may allow exposure intensity to increase at clients’ individual pace. There have been a number of applications of VR technology targeting the symptoms of psychosis with all of the therapeutic attempts to date targeting delusions or hallucinations. 30,31 This technology has potential in the treatment of the NS of psychosis. It may allow exposure to situations that are motivationally challenging and to appraise and evaluate patients’ enjoyment experience. With activity levels often severely reduced in people experiencing severe and debilitating NS, VR may also represent a form of behavioural activation. For these reasons, VR was incorporated as part of a novel intervention for NS.
The novelty of the intervention coupled with the limited access people with psychosis tend to have to this technology, due to cost and potential usage complexity, warrant initial investigations to focus on the acceptability and feasibility of the therapy. For this reason, the main objective of this study will be to assess the acceptability and feasibility of a novel VR-supported therapy for the NS of psychosis called V-NeST (Virtual Reality Therapy for the Negative SympToms of Psychosis).
Methods
Design
This is a two-arm single-blind randomised controlled trial comparing V-NeST plus treatment as usual (TAU) to TAU alone. Participants were assessed at baseline and at 12 weeks postrandomisation (i.e. end of therapy for those randomised to V-NeST). The primary outcome was participants’ progress on personal recovery goals measured by the Goal Attainment Scale (GAS) at week 12 postrandomisation. Secondary outcomes were NS and functioning. Apart from the participants, the therapists and the trial principal investigator, all other study staff including outcome assessors and the trial statistician were blind to trial arm allocation, until primary analysis completion.
The study protocol was pre-registered on ClinicalTrials.gov (identifier: NCT03995420). The study procedures were reviewed and approved by the London Camberwell and St. Giles NHS ethics committee (approval number 19/LO/0830).
Randomisation
Consented participants were randomised using a web-based randomisation service at the UKCRC registered King’s Clinical Trials Unit (KCTU). Randomisation used variable block size (i.e. 2, 4 and 6) with equal allocation. The randomisation sequence was generated by a KCTU statistician independent of the study statistician.
Participants
Participants were recruited from community mental health teams, which were part of the South London and Maudsley NHS Foundation Trust. Inclusion criteria were as follows: (1) currently under the care of a community psychosis services; (2) older than 18 years; (3) in a stable clinical condition; (4) with a documented episode of psychosis (e.g. first-episode psychosis) and/or a diagnosis of schizophrenia; (5) no current episode or history of epilepsy (as it is a contraindication for VR); (6) experiencing disabling NS as identified by care staff. Exclusion criteria were as follows: (1) having a comorbid organic condition affecting behaviour; (2) severe learning disability; (3) insufficient communication skills for consenting, undertaking the research assessment and therapy.
Protocol changes
The onset of the COVID-19 pandemic prompted the following protocol amendments approved by the Trial Steering Committee. All protocol changes were submitted and approved by the NIHR.
-
Participants were screened for health conditions or characteristics associated with COVID-19 complications (e.g. diabetes, respiratory problems, cancer). The study adopted a screening tool (see Appendix 1) to exclude participants at high risk of COVID-19 complications.
-
Research assessments were adapted and conducted remotely. All self-assessed measures were adapted to be completed using an online platform. All interviewer-administered measures were adapted to be completed over the phone or using a web-based video conferencing system. Computer tasks were adapted to be completed remotely on participants’ computers.
-
All therapy contact was conducted face-to-face but with COVID-19 risk mitigation measures in place. All equipment was sterilised with UV light before and after each session.
Measures
The primary outcome of this study was GAS, which is a structured measure of personal recovery goals32,33 and has been used widely to evaluate intervention in mental health. 34–36 The following measures were secondary outcomes: The Clinical Assessment Interview for Negative Symptoms (CAINS), which is an interviewer-based assessment of NS. 37 The self-evaluation of negative symptoms (SNS), which provides an assessment of NS from the participant’s perspective. 38 The Work and Social Adjustment Scale (WSAS) was used to assess functioning. 39 The mechanistic elements of the intervention were assessed using the Effort Expenditure for Reward Task (EEfRT) and the Wisconsin Card Sorting Task (WCST). 40,41 The following measures were used to characterise the sample: The Psychotic Symptom Rating Scales (PSYRATS) to assess the positive symptoms of psychosis. 42 The Hospital Anxiety and Depression Scale (HADS) to assess anxiety and depression. 43 The Rosenberg Self-Esteem Scale (RSS) to assess self-esteem. 44 The Digit Span to assess working memory. 45 The Trail Making (A and B) to assess processing speed and executive function. 46
Participants randomised to V-NeST were invited to take part in a feedback semistructured interview assessing acceptability (adapted from Sedgwick et al. 47 and Reeder et al. 48). The interview asked questions in relation to the therapy and assessment procedures, use of VR and asked suggestions for therapy and research improvement. All interviews were recorded and transcribed.
Service user involvement
People experienced in using mental health services were consulted at different stages of this study, including the initial discussions on study procedures, to revise wording on the information sheet and consent forms and for feedback on VR development. Service users were part of the trial management group and supported the interpretation and dissemination of the results and are also authors of this report.
Sample size
Because of the feasibility and acceptability objectives of this study, power calculations to determine sample size are not appropriate. 49 On the basis of previous research and on Lancaster,50 we have considered a sample size of 30 participants to be adequate for obtaining reliable feasibility parameter estimates. And on the basis of previous similar studies conducted on our site, we have estimated for a dropout rate of 20% over the study period. 51 This will allow this study to have completed follow-up data for at least 24 participants.
Statistical analyses
Analyses were conducted using STATA® 17 (StataCorp LP, College Station, TX, USA). 52 The aim of the analyses was to provide estimates of key feasibility and acceptance parameters and to inform power calculations for a future definitive trial. Descriptive data are presented using means and standard deviations for continuous data and frequencies and proportions for categorical variables.
Feasibility evaluation
The feasibility of the trial procedures was examined using proportions and 95% exact Clopper Pearson’s 95% confidence intervals (CIs) for rates of recruitment, consent and availability for screening, eligibility, availability for baseline assessment and randomisation, treatment retention and follow-up assessments, and availability and consent to be approached by a research therapist.
Explorative treatment effect estimate
These analyses followed the intention-to-treat principle, with data from all participants who took part in the study included in the analysis irrespective of whether they attended the intervention or not. Clinical outcomes were analysed using a linear regression model. A linear regression with clinical outcome at follow-up and treatment arm (treatment or TAU) and baseline values of clinical outcomes as independent variables was used to estimate the likely range of intervention effects at follow-up. Baseline values of the outcome were included as a covariate to control for potential baseline differences (ANCOVA approach). Treatment differences with 95% CIs at follow-up are presented. In addition, standardised effect sizes (Cohen’s d calculated as the adjusted mean difference between treatment arms estimates divided by the within-groups pooled standard deviation) with 95% CIs will be presented. Pilot and feasibility studies are generally not powered to formally assess treatment effects and do not provide robust parameter estimates for assessing efficacy. 53 Emphasis was, therefore, placed on 95% CIs of effect size estimates, as opposed to hypothesis testing, allowing for exploration of imprecision around intervention effect sizes at follow-up.
Acceptability evaluation
Thematic analysis was used to analyse the post-therapy feedback interview transcripts. This explored participants’ experiences of receiving the therapy and taking part in this research study. Emergent themes were initially identified by one member of the team after reading and annotating the transcripts. This process was supported and the themes validated by a person with relevant lived experience. We focused on themes relating to the feasibility and acceptability of the therapy and trial and created a coding framework based on those themes. Themes and coding framework were then reviewed and discussed with the other four members of the research team, including the project lead and coinvestigator and additional two members who had relevant lived experience. The team reviewed the transcripts by using the coding framework. This provided a sense-check on the analysis process from both a clinical and a lived experience perspective.
Project timeline
Table 1 summarises the key project events and milestone dates.
| Study event | Date |
|---|---|
| Study start date | 1 March 2019 |
| Ethics and R&D approval in place | 6 August 2019 |
| VR therapy development completed | 16 September 2019 |
| Therapy manual completed | 16 September 2019 |
| First participant recruited in the trial | 22 November 2019 |
| Study paused (due to COVID-19) | 21 March 2020 |
| Protocol changed | 1 September 2020 |
| Study restarted | 14 September 2020 |
| Variation to contract approved (extension) | 12 March 2021 |
| Recruitment completed | 16 July 2021 |
| Last participant completed the study | 22 October 2021 |
| Study completion date | 31 October 2021 |
VR development work
The initial 6 months of the programme were dedicated to developing the VR software. We worked closely with our industrial partner Virtualware (Bilbao, Spain) to develop the final product. There were four iterations of software development, each including feedback from different stakeholders including three clinicians, two service users and two technology experts. The feedback provided was addressed over successive iterations.
The resulting VR software included five distinct VR environments: (1) resting area where participants can experience a low-stimulation environment (i.e. sitting on the sofa); (2) TV room where participants can select a video clip to watch from a number of options; (3) games room where participants can choose to engage in different games (e.g. darts and ball throwing and catching); (4) social space where participants can take part in conversations on a mundane topic with other two avatars; (5) factory where participants are asked to perform a task (e.g. sorting out objects). Illustrative screenshots of the environments are provided in Appendix 2.
Therapy procedures development
Alongside the VR development, the initial part of the programme was spent in developing the therapy procedures and the therapy manual. The manual drafts received feedback from team members and therapists ahead of use. Procedure and sessions layout were discussed with the members of the trial steering group, which involved service users, clinicians (i.e. clinical psychologist and psychiatrist), mental health researchers and technology experts. The resulting manual was used to guide therapy for all the participants randomised to V-NeST.
Intervention and virtual reality equipment
The resulting intervention (i.e. V-NeST) is a 12-session therapy using psychological intervention principles based on CBT and cognitive remediation. Each therapy session involves therapist-supported activities including psychoeducation, behavioural activation, developing insight on pleasure experience and emotions and learning to use feedback to improve goal-directed behaviour. These activities are supported by using a purposefully designed and built VR software taking advantage of five VR environments. The environments present unique and tailored motivational challenges that participants are encouraged to approach while reflecting (and rating) on their experiences. These are then discussed with the therapist. A typical session involves emotional check-in and check-out, psychoeducation, discussion on strategies to improve activity levels, goal monitoring and tracking, reflection on feedback and VR practice. For this study, we will consider participants completing six or more sessions as having received a suitable therapy dose (i.e. completers). Those receiving fewer than six sessions will be considered therapy dropouts.
The VR environments were designed by MC and LV in collaboration with people with lived experience and developed by Virtualware using Unity. The V-NeST software has a therapist back end allowing the therapist to create participants’ identity cards and individual therapy sessions. The software runs on a VR-ready laptop and Oculus Rift-S was used as the head-mounted display. Ear covering headphones were used for the sound. The experience was designed as a sitting experience.
Ethical approval
The study procedures were reviewed and approved by the London Camberwell and St. Giles NHS ethics committee (approval number 19/LO/0830).
Study registration
The study protocol was pre-registered on ClinicalTrials.gov (identifier: NCT03995420).
Randomisation
Consented participants were randomised using web-based randomisation service provided by the UKCRC registered KCTU. Randomisation used variable block size with a 1 : 1 ratio between the two conditions.
Participant recruitment
The study recruited participants from a single large NHS trust: the South London and Maudsley NHS Foundation Trust. We recruited participants from the trust community teams providing care for people with psychosis, including early intervention teams, recovery teams, assertive outreach, and home treatment teams. Eligible participants were first identified by a member of the care team and referred to a study researcher for further consideration if interested to take part. We were also able to use our local NHS trust register called Consent for Contact. This register includes trust service users who have given consent to be contacted directly for invitation to take part in research projects.
DMEC and TSC
The trial had a DMEC and a TSC. The DMEC membership included a senior statistician (also functioning as the DMEC chair), a research academic and a clinical psychologist. The study principal investigator and statistician were present at all DMEC meetings. The DMEC met four times approximately every 6 months throughout the duration of the trial. The DMEC monitored the trial procedures and AEs.
The TSC membership included a clinical academic chair, two service users, a clinician and a statistician; it met three times and discussed trial management issues.
Results
Recruitment and retention
A total of 190 people were assessed for eligibility of which 160 (84.2%, 95% CI 78.2% to 89.1%) were excluded: 39 people (20.5%, 95% CI 15.0% to 27.0%) declined to participate and reasons for declining participations included time commitment (17), travelling time (12) and not wanting to receive therapy using VR (5) or a talking therapy (5); 44 people (23.2%, 95% CI 17.4% to 29.8%) were not contactable and this included individuals not answering phone calls, messages or returning phone calls; and the remaining 77 people [40.1% (95% CI 33.5% to 47.9%)] did not meet the inclusion criteria. Thus a total of 30 (15.8%, 95% CI 10.9% to 21.8%) were assessed at baseline and randomised into V-NeST plus TAU (N = 15; 50%) or TAU alone (N = 15; 50%). Out of these 30 participants, 29 (96.7%, CI 82.8% to 99.9%) received the allocated treatment [V-NeST plus TAU: N = 14 (93.3%, 95% CI 68.1% to 99.8%) and TAU alone: N = 15 (100%, 95% CI 78.2% to 99.99%)]. The participant not receiving the intervention moved out of the area. Four participants (16.6%, 95% CI 5.6% to 34.7%) did not provide data at follow-up. They were either ‘lost at follow-up’ [V-NeST plus TAU: N = 1 (3.1%, 95% CI 0.07% to 16.2%) and TAU: N = 2 (6.3%, 95% CI 0.8% to 20.8%)] or discontinued the study [V-NeST plus TAU: N = 1 (3.1%, 95% CI 0.07% to 16.2%)]. All 30 participants were considered for the primary analyses. Figure 1 illustrates the recruitment flow for the study.
FIGURE 1.
CONSORT diagram showing the study recruitment flow.

In the treatment arm, 14 out of 15 participants attended at least one therapy session with an average of 9.7 [standard deviation (SD) = 3.77, range 1–12] sessions. Two participants did not receive the minimum therapy dose of six sessions and completed only one and four therapy sessions, respectively.
Assessment completion
Baseline data collection
All clinical outcome measures (primary and secondary) were completed by all participants (100%, 95% CI 88.4% to 100%). Twenty-three participants (76.7%, 95% CI 57.7% to 90.0%) completed the Trail Making Test (TMT) and 25 participants (83.3%, 95% CI 65.3% to 94.4%) completed the EEfRT and the WCST.
Follow-up data collection
Twenty-six participants (86.7% 95% CI 69.3% to 92.2%) completed primary clinical outcome (i.e. the GAS) and the secondary outcomes, including the CAINS, the SNS and the WSAS. There were no differences between the two arms in data completion.
Only eight participants (26.7%, 95.CI 12.3% to 45.9%) provided data for TMT, seven (23.3%, 95% CI 9.9% to 42.3%) for EEfRT and 14 (46.7%, 95% CI 28.3% to 65.7%) for the digit span. There were no differences between treatment and control groups in TMT and digit span, but participants of control arm of the trial (i.e. TAU alone) did fewer effort tasks (TAU = 2/15 vs. V-NeST = 5/15).
The impact of COVID-19 on data collection varied for different study outcomes. Only two participants were not affected by the COVID-19 outbreak and the corresponding restrictions because they completed the trial before the pandemic outbreak in the United Kingdom (UK). Fourteen participants were partially affected (COVID-19 restrictions were imposed during the study period) and 14 provided consent during COVID-19 restriction periods. COVID-19 restrictions affected both arms similarly (TAU: 1 not affected, 8 partially affected and 6 affected for the whole duration of the study; V-NeST: 1 not affected, 6 partially affected and 8 affected for the whole duration of the study).
COVID-19 restrictions had little influence on collecting the primary outcome. Out of the four participants who did not complete the study primary outcome, one was not affected by the COVID-19 restrictions, one was partially affected and two were recruited and completed the study under COVID-19 restrictions.
Adverse events
There were two serious adverse events (SAE) (from two individuals) recorded in this study: one was the deterioration of mental state requiring urgent assessment and the other was of a miscarriage. There were no events of deterioration of physical health requiring an urgent appointment or hospital admission and no deaths (see Tables 2 and 3). No AE was considered related to study participation.
| N | SAE category | Group | Detail |
|---|---|---|---|
| 1 | Other | TAU | Miscarriage at week 8 (identified via clinical record review) |
| 2 | Mental health | TAU | Stopped taking antipsychotic medication |
| SAE | SAE % | Participants experiencing SAE | Participants experiencing SAE % | |
|---|---|---|---|---|
| TAU (N = 15) | 2 | 13 | 2 | 13 |
| V-NeST (N = 14) | 0 | 0 | 0 | 0 |
There were 11 AEs recorded from 8 participants. Seven were recorded from participants randomised to V-NeST and four were from participants randomised to TAU (see Tables 4 and 5). No AE was considered related to study participation.
| N | AE category | Group | Detail |
|---|---|---|---|
| 1 | Mental health | V-NeST | Suicidal thoughts (no plan, no intention) |
| 2 | Physical health | V-NeST | Vomiting (after medication intake)a |
| 3 | Physical health | V-NeST | Vomiting (after medication intake)a |
| 4 | Physical health | V-NeST | Vomiting (after medication intake)a |
| 5 | Mental health | V-NeST | Feeling anxious |
| 6 | Physical health | V-NeST | Feeling lightheaded (low blood sugar) |
| 7 | Physical health | V-NeST | Viral gastroenteritis (diarrhoea)a |
| 8 | Mental health | TAU | Distressing thoughts |
| 9 | Mental health | TAU | Frequent, intrusive and distractive religious thoughts |
| 10 | Physical health | TAU | Neck stiffness |
| 11 | Mental health | TAU | Distressing thoughts |
| AE | AE % | Participants experiencing AE | Participants experiencing AE % | |
|---|---|---|---|---|
| TAU (N = 15) | 4 | 26 | 4 | 26 |
| V-NeST (N = 14) | 7 | 5 | 4 | 28 |
Treatment effect estimate
Table 6 presents characteristics of participants who were randomised.
| N | V-NeST | SD | N | TAU | SD | N | Total | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % or mean | % or mean | % or mean | ||||||||
| Gender | Male | 10 | 33% | – | 11 | 27% | – | 21 | 70% | – |
| Female | 5 | 67% | – | 4 | 73% | – | 9 | 30% | – | |
| Age | Years | 15 | 35.7 | 9.1 | 15 | 38.2 | 12.5 | 30 | 37.1 | 10.8 |
| Time since first episode | Months | 15 | 122.2 | 117.1 | 15 | 138.8 | 168.7 | 30 | 120 | 142.9 |
| WSAS | Functioning | 15 | 29.2 | 5.2 | 15 | 27.4 | 9.4 | 30 | 28.33 | 7.54 |
| CAINS | NS | 15 | 32.1 | 6.8 | 15 | 31.3 | 9.1 | 30 | 31.63 | 7.94 |
| SNS | NS | 15 | 20.6 | 7.5 | 15 | 20.1 | 11.5 | 30 | 20.37 | 9.55 |
| PSYRATS_H | Hallucination | 15 | 10.7 | 13.9 | 15 | 7.7 | 13.4 | 30 | 9.23 | 13.55 |
| PSYRATS_D | Delusion | 15 | 6.8 | 8 | 15 | 5.1 | 8.1 | 30 | 6.13 | 7.99 |
| HADS-A | Anxiety | 15 | 9.9 | 4.4 | 15 | 10.9 | 6.6 | 30 | 10.43 | 5.56 |
| HADS-D | Depression | 15 | 10.4 | 4.7 | 15 | 10.6 | 5.5 | 30 | 10.53 | 5.06 |
| RSS | Self-esteem | 15 | 28.7 | 3.3 | 15 | 25.8 | 2.2 | 30 | 27.27 | 3.16 |
| TMT-a | Processing speed (time in seconds) | 11 | 47.8 | 15.1 | 12 | 37.3 | 10.7 | 23 | 42.32 | 13.83 |
| TMT-b | Executive function (time in seconds) | 11 | 114.1 | 41.9 | 12 | 108.7 | 49.3 | 23 | 111.31 | 45.35 |
| Digit span | Working memory | 15 | 15.3 | 3.35 | 15 | 14.2 | 2 | 30 | 14.87 | 2.80 |
| EffRT-h | N hard trials completed | 12 | 11.6 | 11.4 | 13 | 11.5 | 11.2 | 11.5 | 11.2 | |
| EffRT-e | N easy trial completed | 12 | 52.6 | 28.4 | 13 | 40.7 | 26.3 | 25 | 46.48 | 27.43 |
| WCST-c | Correct | 12 | 64.9 | 16.4 | 13 | 66 | 12.3 | 25 | 65.48 | 14.13 |
| WCST-e | Error | 12 | 55.1 | 15.7 | 13 | 52.8 | 22.1 | 25 | 53.96 | 18.95 |
| WCST-p | Perseverative errors | 12 | 8.8 | 4.7 | 13 | 6.9 | 8.1 | 25 | 7.84 | 6.69 |
Table 7 shows the results of linear regression model analyses with the clinical outcome as the dependent variable and group as categorical independent variable. The primary clinical outcome (i.e. the GAS) had a score of 0 for all participants and baseline values were not included in the model. Effect sizes (expressed in Cohen’s d) and 95% CIs are presented. Because of the small sample size, statistical techniques for handling missing data were not applied. Change in outcome scores and variance in the two study groups is shown in Figure 2.
| Variable | N | Favouring arm | b | 95% CI | Cohen’s d | 95% CI |
|---|---|---|---|---|---|---|
| GAS | 26 | V-NeST | 2.77 | 1.22 to 4.32 | 1.48 | 0.61 to 2.35 |
| CAINS | 30 | V-NeST | −4 | −10.15 to 2.15 | −0.46 | −1.24 to 0.32 |
| SNS | 30 | V-NeST | −3.84 | −9.8 to 2.13 | −0.34 | −1.11 to 0.43 |
| WSAS | 30 | V-NeST | −2.85 | −7.77 to 2.07 | −0.28 | −1.05 to 0.5 |
FIGURE 2.
Boxplots of (a) primary clinical outcome GAS at post-treatment and main secondary outcomes, (b) CAINS, (c) NS and (d) WSAS at baseline and post-treatment for each arm.
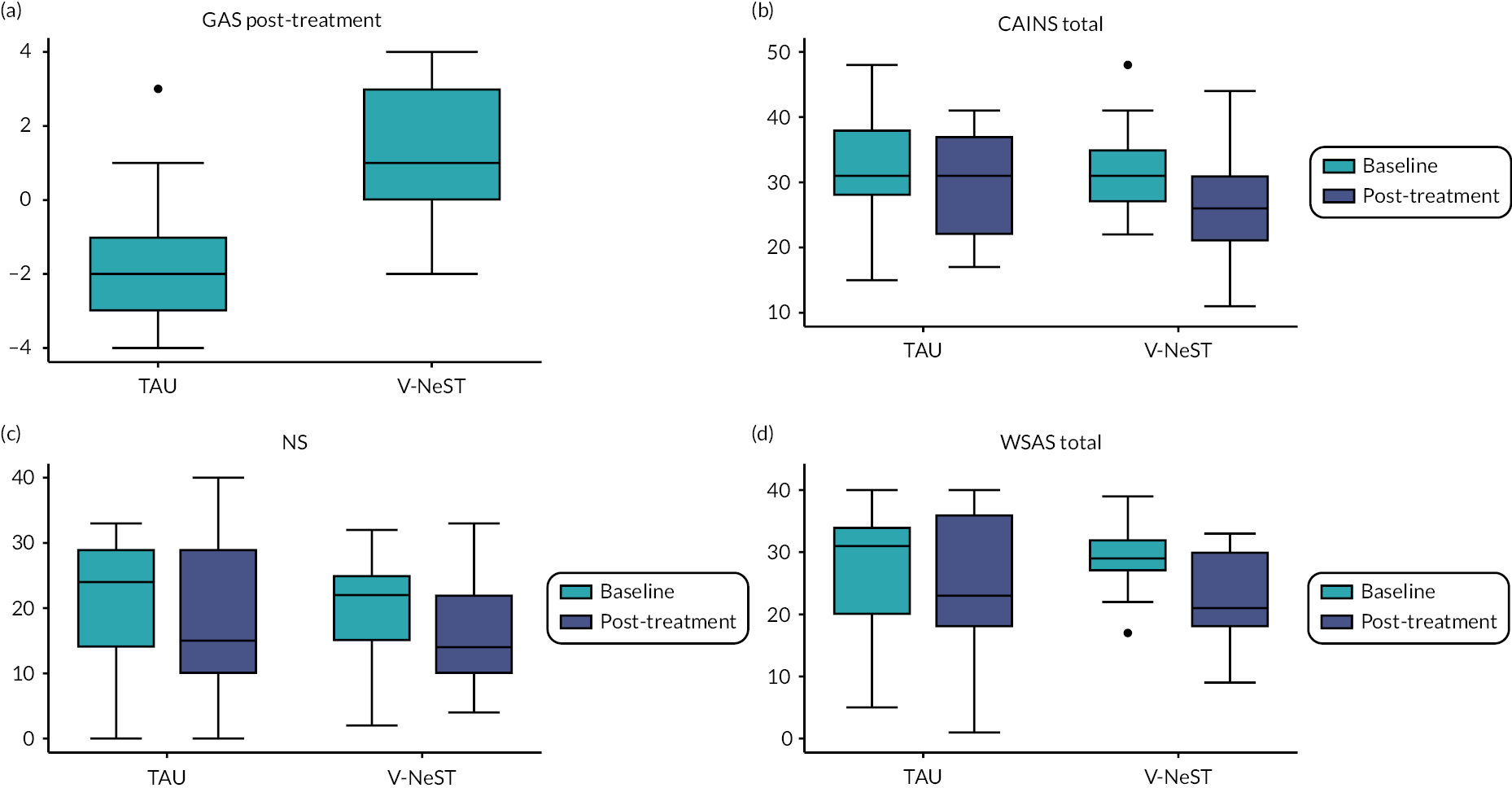
A large treatment effect was observed for the GAS and assessment of the 95% CIs suggests that the treatment effect is at least d = 0.61. Treatment effects of main secondary outcomes (CAINS, NS, WSAS and digit span) are smaller and the 95% CIs are too large to derive any conclusion.
Only six participants were able to complete the trial mechanistic measures (i.e. EEfRT task and WCST) at follow-up because of the pandemic-imposed restrictions on face-to-face research assessment procedures. Statistical analyses were not conducted on these outcomes.
Acceptability
Nine out of fifteen participants (60%; 95% CI 32.3% to 83.7%) in the intervention arm of the trial were interviewed. Two participants did not want to be recorded and declined to be interviewed and two did not want to participate in this additional part of the study (one declined due to time demand and the other participant did not want to provide a reason). Two participants did not complete the intervention (one relocated and one was not contactable any longer). Two-thirds of the participants interviewed (six out of nine) were men.
The following themes emerged from the interview:
-
Therapy goals: Positive experiences of therapy often centred around the goals and activities set. Participants described specific goals from their sessions and how the therapy had contributed to goals progress (see Table 8 for examples of quotes).
-
Impact of the pandemic: In some cases, the pandemic infringed on participants’ abilities to practice what they had learnt in therapy in real life. This meant that certain goals could not be achieved because the places where participants could practice or see friends were closed.
-
Issues experienced with symptoms. Participants described how difficulties with motivation and ‘feeling low’ made it difficult to attend therapy sessions. In some cases, the therapy was seen as making a positive contribution.
-
Using VR: Only one had tried VR before and most described it as their first experience. Most were curious about the VR and valued the chance to do something different. Responses were positive, and most participants said they would recommend it to a friend (n = 7, none said that they would not). The combination of VR use and therapy allowed participants to ‘relax’, particularly at the end of the session.
-
VR relevance: Participants’ opinions varied in how important they saw the VR. Some saw it as integral to the therapy. The therapist provided structure and guidance; the VR added a chance to practice aspects of the therapy. These participants connected the practical aspects of the therapy with the chance to practice in VR. However, participants were not always able to say why the intervention was helpful (e.g. participant #18, see Table 4). The benefits of therapy could be more diffuse, and some found it difficult to understand how the VR fitted with the wider therapy.
-
Therapy procedures: There were different opinions about the optimal session length and number of sessions, likely reflecting participants’ preferences. One participant mentioned that group sessions might be useful.
-
Suggestions for improvement: One of the challenges for people experiencing NS was leaving the house to attend the therapy. Some welcomed the idea of doing the therapy remotely. Some practical difficulties were described with the VR aspects of the sessions. Some participants experienced mild discomfort when wearing the headset. There were occasional criticisms of both the hardware (e.g. hand controllers) and the software (e.g. avatar and environment appearance) while others struggled with the mechanical aspects of controlling the VR handset.
-
Research procedures: Most participants found that the length of the assessment was appropriate and that they understood the process they were consenting to. There were comments from some participants about the length and repetitive nature of some of the assessments.
| Theme | Example quotes |
|---|---|
| Therapy goals | ‘I wanted to restart playing music and I sort of did … I still haven’t found a band, but I now do practice a little at home.’ (#3) ‘It helped me [the therapy] with my weight, losing weight … for example, my homework was to do some exercise at home, or cook few meals a week’ (#4) ‘just going to the sessions would make me passively reflect on what I experience and what could change my experience in life [ … ] I would reflect on the sessions it would push and drive me more to like to achieve and get things done, push my life forward. Push my life in the direction envisaged it to be.’ (#23) |
| Impact of the pandemic | ‘the lockdown obviously is preventing a lot of things … A lot of things I would like to get done means being out of my house.’ (#18) ‘I wanted to do more African dancing. But I couldn’t, the centre was closed for COVID and when they open again, they stopped all group classes and activities.’ (#31) |
| Issues experienced with symptoms | ‘My mood can be very low, and I don’t end up leaving the house for days. This [therapy] helped me. It helped with the mood, and I left the house to attend sessions.’ (#31) ‘It supposed to be action then gives motivation and that’s how you change the cycle.’ (#17) ‘My motivation went up … only a little but it did go up’ (#18) ‘After a few sessions I thought this is boring, I didn’t want to go back. But this was what the therapy was about.’ (#31) |
| Using VR | ‘I think the therapy in combination with the VR, was good. Sometimes like, when I have therapy, I don’t really like the constant back and forth talking. It becomes a bit heavy after a while … So taking the VR time with it helped to break out the heaviness or tension. [ … ] everything was really well done. I felt like it was a good idea. Like, having a therapy session with a VR session where you can listen to music and have choice.’ (#23) ‘it relaxes you and it is helpful …’ (#31) ‘Having the sessions going into details about like, intense things. To have like, almost like a cool off at the end was really nice. [ … ] Like in the living room or tv room that was just like really relaxing, quite sombre, kind of zen.’ (#25) |
| VR relevance | ‘without VR … I think it would be worse. [ … ] without the therapist you’d be lost … the therapist is telling me what to do.’ (#4) ‘How would you pull it off without VR? I think it will be less effective … It just adds the practical part to it.’ (#17) ‘It was like a video game, it was easy and also it took you somewhere else [ … ] you feel like being somewhere else, but you are not and you don’t have to travel or do other things that can be difficult’ (#3) |
| ‘I visited the pub and the factory. The factory I treated like a game. The pub was very useful. Very useful, because I was scared to go in the pub but after going into the pub, I was less scared. [ … ] I don’t know if I have the vocabulary to describe how he was helpful, but he was helpful but even if he wasn’t just as a sounding board as someone to talk to with my problems’ (#18) ‘In day-to-day life, we have so much going on that we generally don’t realise, like really sit back and think am I ok?’ (#25) |
|
| Therapy procedures | ‘The first one [session] was erm, kind of long.’ (#17) ‘Twelve sessions was good but it definitely could be longer because you get to a point where you are finally getting that momentum. It takes a while if you are having negative symptoms. It takes a while to even start the ball rolling. [ … ] I think it could be maybe like a little bit longer, maybe by like I don’t know … 10, 15 minutes … So maybe have like a full-length session for maybe like an hour. And then a separate 15 minutes for the VR. Rather than putting it all into one.’ (#25) ‘rather than just continuously doing it 1-1. I feel like having one group session wouldn’t hurt … Just to hear other people’s perspectives.’ (#23) |
| Suggestions for improvement | ‘maybe if it could be done a bit more remotely. Because like, for me, one of the hardest things I think was physically getting there some days. Erm, because, once the anxiety or the depressive thought set in, it is very hard to physically get out of bed to leave the house, to physically go to that location.’ (#25) ‘I needed more therapy, for depression rather than motivation. I know the two are linked but I think if I wasn’t depressed the therapy for the motivation would have been more helpful.’ (#18) ‘It wasn’t always comfortable to have on and the goggles view sometimes blurred’ (#3) ‘one thing I found a bit of a hindrance was the physical shape of the headset. Like I know, I think that was like the first gen of that. But this is more of a design thing. Like, my hair, is very voluminous … They have obviously designed it for people with flatter hair …’ (#25) ‘I was little bit reluctant to use my right hand, doing the job [referring to factory environment]. It wasn’t great, with my right hand but after a while it was ok.’ (#33) |
Interviewing participants with high levels of NS presents challenges. Maintaining good engagement levels and conversation flow was not always easy. As a result, some of the answers to the interview questions were short and provided limited information.
Discussion
Recent years have seen a renewed interest in the assessment and consideration of the NS of psychosis. 4 Unfortunately, this has not yet led to novel, effective and widespread interventions. Digital technology tools may be a way to enhance the usefulness of interventions by improving adherence and by reducing motivation difficulties and barriers to accessing therapy. In this study we developed and evaluated a novel therapy for NS using VR (called V-NeST). The primary aim of this study was to evaluate the acceptability and feasibility of V-NeST.
V-NeST demonstrated good acceptability and feasibility parameters particularly considering that this study involved participants with severe and disabling levels of NS. The therapy procedures were considered acceptable, and the VR aspects were well tolerated and found to be engaging. Several therapy features were suggested for revision, including ways of interacting with the virtual environment (e.g. hands movements) and some therapy components (e.g. making psychoeducation more engaging). Only one of the participants had tried VR before and most had limited digital technology skills. This was not a barrier to therapy use and the VR proved intuitive and easy to learn. One of the suggested improvements was the possibility to complete V-NeST sessions remotely. This would be possible if participants can be provided with an adequate headset in their homes and have an appropriate internet connectivity. However, the independent use of the VR software and equipment may be complex for this group of participants and appropriate resource to support this (e.g. training videos) will have to be developed. The feedback interviews also recommended improvements for the virtual hand controllers. This comment is likely related to the limited familiarity some participants had with controlling technology with their hands. Developing or using a tutorial for familiarising with the hand controller device may minimise this issue in future trial and clinical applications. The feasibility of the research procedure was also good with most research procedures being well tolerated by all participates. The study recruited to target (30 participants) but considered 190 referrals to meet its target. This means approximately one in six of the people referred was able to take part in the trial. While this study had comprehensive inclusion criteria, there is consensus that recruiting people with NS in research studies may be complex. 54 However, we proved that it is possible and that once participants entered the trial, we were able to retain more than 80% of those randomised to treatment. This is in line with other randomised controlled trials in people with psychosis. 51,55,56
The explorative analysis on the V-NeST prespecified primary outcome suggested that the intervention may be helpful in supporting people’s recovery goals. We chose this outcome as this was what service users suggested to be the most valuable. This result is encouraging and taken together with the acceptability and feasibility findings supports further development and evaluation of this therapy.
Given the small sample size and the objectives of this study around feasibility and acceptability, the current results have limited internal and external validity. Results cannot be generalised and should not be used to inform efficacy or effectiveness. One important caveat in considering the effect size is how pandemic-related restrictions may have impacted therapy goals. It is likely that, as some participants expressed, restriction may have limited the ability of participants to achieve goals (e.g. limitation in social contact or gym being closed). However, this would have impacted both the groups equally. With studies suggesting that pandemic restrictions and COVID-19 have a negative effect accessing therapy for mental health, it is likely to have been a positive experience for those who received it. 57
This study has its limitations. We were not able to evaluate the mechanistic element of the therapy, that is, feedback sensitivity, as planned. This was consequent to limitations imposed on research procedures during different periods of social contact restrictions in the UK. While we were able to continue to deliver therapy sessions and assessment remotely, many participants did not have a computer or were unable to complete computer tasks at home. The therapy developed has different potential active mechanisms including the novelty and engaging nature of a VR intervention and the role and input of the therapist. While the current study was not set out to formally evaluate the relative contribution of these therapy elements, it may be important for a future investigation to consider the contribution of different therapy aspects to the therapy outcome. A further limitation of this study is the lack of a follow-up assessment to consider assessment completion level and to evaluate exploratively how treatment effect estimates may be retained over time. Data were also collected from a single large NHS trust located in an urban environment.
The development and evolution of digital therapies has enormous potential to reduce the impact of NS on the recovery prospect of people with schizophrenia. There is the promise of better and more engaging therapies coupled with the prospect of these being easier to deliver for staff and services.
Future steps
Further developments of V-NeST will include VR software modifications and the adaptation of therapy procedures in line with the feedback received from participants. A formal evaluation of efficacy will require an appropriately powered trial.
Variance estimates for future sample size calculations are provided in Table 9. The use of the upper 80% CI of the standard deviation is recommended by Browne49 for robust estimates of the standard deviations. Using upper 80% CIs as an estimate for future power and sample size calculations will result in 15–21% larger values (< 10%) compared with the observed standard deviations.
| Outcome | Observed SD | Lower 95% | Upper 95% | Lower 80% CI | Upper 80% CI |
|---|---|---|---|---|---|
| GAS post-therapy (TAU alone) | 1.74 | 1.17 | 2.31 | 1.37 | 2.11 |
| GAS post-therapy (TAU + V-NeST) | 2.07 | 1.26 | 2.88 | 1.54 | 2.60 |
| CAINS | 7.94 | 6.03 | 9.84 | 6.69 | 9.18 |
| SNS | 9.55 | 7.28 | 11.82 | 8.07 | 11.04 |
| WSAS | 7.54 | 5.08 | 10.00 | 5.93 | 9.15 |
| Digit span | 2.80 | 2.11 | 3.50 | 2.35 | 3.26 |
Sample size calculations
We estimated the sample size for a minimally clinically important difference defined as the smallest difference on the primary outcome, which would be of benefit to a service user (i.e. 1 GAS point). The variance of the GAS had to be estimated from the follow-up data because the GAS scores were all 0 at baseline. We used the upper 80th percentile of the standard deviations estimated from our data to account for the small sample size and lack of evidence of generalisability to other settings. For future sample size estimates, we used the mean-variance of the two groups.
Table 10 shows the required sample sizes for the primary and the secondary outcomes for these scenarios, including scenarios assuming 20% loss at follow-up.
| Outcome | Minimum clinical difference | SD based on upper 80% CI | Cohen’s d based on 80% CI | Sample size per group | Sample size adjusted for 20% dropout |
|---|---|---|---|---|---|
| GAS baseline mean | 1 | 2.37 | 0.423 | 119 | 149 |
| CAINS | 3 | 9.18 | 0.327 | 198 | 248 |
| SNS | 3 | 11.04 | 0.272 | 286 | 356 |
| WSAS | 3 | 9.15 | 0.328 | 197 | 247 |
| Digit span | 1 | 3.26 | 0.307 | 224 | 280 |
Future trial considerations
The estimate gathered in this study in addition to the information on minimal clinical usefulness on the primary outcomes suggests that a future trial (randomising to two conditions) aiming to assess the efficacy of V-NeST will need to recruit between 298 (using our primary outcome) and 712 participants, including a conservative 20% loss of follow-up. This future evaluation may also consider the cost-efficacy of V-NeST and how it may be implemented in clinical settings (e.g. therapist training or access to VR). The large observed effect size for our primary outcome may suggest considering an adaptive trial that allows for early stopping or sample size reassessment without undermining its validity and integrity. 58
Our feasibility study is a small single-centre trial, and our range of possible effect sizes and estimates of standard deviations needs to be treated with caution because our results may not generalise to different and larger populations. This may lead to the need for larger sample sizes, especially if long-term improvements are assessed. 53
Acknowledgements
We are grateful for the input of the King’s College London Biomedical Research Centre Patient and Public Involvement theme for providing feedback on the study design, procedures and material development. We also thank Jerome DiPietro for his technical support.
Contributions of authors
Matteo Cella (https://orcid.org/0000-0002-5701-0336) (Clinical Psychologist and Senior Lecturer) prepared the result for publication and drafted the manuscript.
Paul Tomlin (Research Worker) prepared data for analyses.
Daniel Robotham (https://orcid.org/0000-0003-2968-2415) (Head of Research at the McPin Foundation) conducted the qualitative analyses.
Patrick Green (Service User) supported analyses and manuscript drafting.
Helena Griffiths (Service User) supported the qualitative analyses.
Daniel Stahl (https://orcid.org/0000-0001-7987-6619) (Biostatistician and Professor) conducted the quantitative data analyses.
Lucia Valmaggia (https://orcid.org/0000-0001-6099-8464) (Clinical Psychologist and Reader) commented on the manuscript.
All authors contributed and approved the final version of the manuscript.
Ethics statement
The study protocol was pre-registered on ClinicalTrials.gov (identifier: NCT03995420). The study procedures were reviewed and approved by the London Camberwell and St. Giles NHS ethics committee (approval number 19/LO/0830) and received R&D approval on 9 August 2019.
Data-sharing statement
This is a primary research study. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. Data collected as part of this study are stored, kept safe and secure, to protect everyone’s privacy.
Funding
This project was funded by the National Institute for Health and Care Research (NIHR) Efficacy and Mechanism Evaluation (EME) programme, an MRC and NIHR partnership (NIHR-EME: 17/59/13). This will be published in full in Efficacy and Mechanism Evaluation. See the NIHR Journals Library website for further project information.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Bobes J, Arango C, Garcia-Garcia M, Rejas J, Group CSC. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry 2010;71:280-6.
- Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017;16:14-2.
- Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 2018;5:664-77.
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, et al. Treatment of negative symptoms: Where do we stand, and where do we go?. Schizophr Res 2017;186:55-62.
- Psychosis and Schizophrenia in Adults: Prevention and Management. London: National Institute for Health and Care Excellence: Guidelines; 2014.
- Krause M, Zhu Y, Huhn M, Schneider-Thoma J, Bighelli I, Nikolakopoulou A, et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2018;268:625-39.
- Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014;204:20-9.
- Cella M, Preti A, Edwards C, Dow T, Wykes T. Cognitive remediation for negative symptoms of schizophrenia: a network meta-analysis. Clin Psychol Rev 2017;52:43-51.
- Turner DT, McGlanaghy E, Cuijpers P, van der Gaag M, Karyotaki E, MacBeth A. A meta-analysis of social skills training and related interventions for psychosis. Schizophr Bull 2018;44:475-91.
- Ventura J, Subotnik KL, Gretchen-Doorly D, Casaus L, Boucher M, Medalia A, et al. Cognitive remediation can improve negative symptoms and social functioning in first-episode schizophrenia: a randomized controlled trial. Schizophr Res 2019;203:24-31.
- Cella M, Stahl D, Morris S, Keefe RSE, Bell MD, Wykes T. Effects of cognitive remediation on negative symptoms dimensions: exploring the role of working memory. Psychol Med 2017;47:2593-601. https://doi.org./10.1017/S0033291717000757.
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull 2008;34:835-47.
- Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull 2014;40:S107-16.
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013;76:412-27.
- Diekhof EK, Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci 2010;30:1488-93.
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001;21.
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009;166:702-10.
- Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology 2015;40:2258-68.
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 2015;28:7-12.
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013;11.
- Vogel SJ, Strauss GP, Allen DN. Using negative feedback to guide behavior: impairments on the first 4 cards of the Wisconsin Card Sorting Test predict negative symptoms of schizophrenia. Schizophr Res 2013;151:97-101.
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry 2011;69:424-31.
- Tobe M, Nemoto T, Tsujino N, Yamaguchi T, Katagiri N, Fujii C, et al. Characteristics of motivation and their impacts on the functional outcomes in patients with schizophrenia. Compr Psychiatry 2016;65:103-9.
- Noordraven EL, Wierdsma AI, Blanken P, Bloemendaal AF, Staring AB, Mulder CL. Financial incentives for improving adherence to maintenance treatment in patients with psychotic disorders (Money for Medication): a multicentre, open-label, randomised controlled trial. Lancet Psychiatry 2017;4:199-207.
- Cella M, Bishara AJ, Medin E, Swan S, Reeder C, Wykes T. Identifying cognitive remediation change through computational modelling—effects on reinforcement learning in schizophrenia. Schizophr Bull 2014;40:1422-32.
- Browne J, Wright AC, Berry K, Mueser KT, Cather C, Penn DL, et al. The alliance-outcome relationship in individual psychosocial treatment for schizophrenia and early psychosis: A meta-analysis. Schizophr Res 2021;231:154-63.
- Slater M. Presence and emotions. Cyberpsychol Behav 2004;7.
- Dermody G, Whitehead L, Wilson G, Glass C. The role of virtual reality in improving health outcomes for community-dwelling older adults: systematic review. J Med Internet Res 2020;22.
- de Rooij IJM, van de Port IGL, Punt M, Abbink-van Moorsel PJM, Kortsmit M, van Eijk RPA, et al. Effect of virtual reality gait training on participation in survivors of subacute stroke: a randomized controlled trial. Phys Ther 2021;101. https://doi.org./10.1093/ptj/pzab051.
- Rus-Calafell M, Garety P, Sason E, Craig TJK, Valmaggia LR. Virtual reality in the assessment and treatment of psychosis: a systematic review of its utility, acceptability and effectiveness. Psychol Med 2018;48:362-91.
- Torous J, Bucci S, Bell IH, Kessing LV, Faurholt-Jepsen M, Whelan P, et al. The growing field of digital psychiatry: current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry 2021;20:318-35.
- Kiresuk TJ, Sherman RE. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment Health J 1968;4:443-53.
- Turner-Stokes L. Goal Attainment Scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil 2009;23:362-70.
- Hurn J, Kneebone I, Cropley M. Goal setting as an outcome measure: a systematic review. Clin Rehabil 2006;20:756-72.
- Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Goal-oriented cognitive rehabilitation for early-stage Alzheimer’s and related dementias: the GREAT RCT. Health Technol Assess 2019;23:1-242.
- Lee CE, Shogren KA, Segal J, Pezzimenti F, Aleman-Tovar J, Taylor JL. Goal attainment scaling-community-based: A method to incorporate personalized outcomes into intervention research with youth and adults on the autism spectrum. Autism 2021;26:178-87. https://doi.org./10.1177/13623613211024492.
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry 2013;170:165-72.
- Dollfus S, Mach C, Morello R. Self-evaluation of negative symptoms: A novel tool to assess negative symptoms. Schizophr Bull 2016;42:571-8.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4.
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLOS ONE 2009;4.
- Tien AY, Spevack TV, Jones DW, Pearlson GD, Schlaepfer TE, Strauss ME. Computerized Wisconsin Card Sorting Test: comparison with manual administration. Kaohsiung J Med Sci 1996;12:479-85.
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychol Med 1999;29:879-89.
- Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes 2003;1.
- Rosenberg M. Society and the Adolescent Self-image. Middletown, Conn: Wesleyan University Press; 1989.
- Wechsler D. WASI-II: Wechsler Abbreviated Scale of Intelligence: PsychCorp. Bloomington, MN: Pearson; 2011.
- Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004.
- Sedgwick O, Hardy A, Greer B, Newbery K, Cella M. ‘I wanted to do more of the homework!’ – feasibility and acceptability of blending app-based homework with group therapy for social cognition in psychosis. J Clin Psychol 2021;77:2701-24. https://doi.org./10.1002/jclp.23193.
- Reeder C, Pile V, Crawford P, Cella M, Rose D, Wykes T, et al. The feasibility and acceptability to service users of CIRCuiTS, a Computerized Cognitive Remediation Therapy Programme for schizophrenia. Behav Cogn Psychother 2016;44:288-305.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12.
- Reeder C, Huddy V, Cella M, Taylor R, Greenwood K, Landau S, et al. A new generation computerised metacognitive cognitive remediation programme for schizophrenia (CIRCuiTS): a randomised controlled trial. Psychol Med 2017;1.
- StataCorp LP. Stata Data Analysis and Statistical Software. Special Edition Release 2007;10.
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res 2011;45:626-9.
- Marder SR, Alphs L, Anghelescu I-G, Arango C, Barnes TR, Caers I, et al. Issues and perspectives in designing clinical trials for negative symptoms in schizophrenia. Schizophr Res 2013;150:328-33.
- Craig TK, Rus-Calafell M, Ward T, Leff JP, Huckvale M, Howarth E, et al. AVATAR therapy for auditory verbal hallucinations in people with psychosis: a single-blind, randomised controlled trial. The Lancet Psychiatry 2018;5:31-40.
- Morrison AP, Pyle M, Gumley A, Schwannauer M, Turkington D, MacLennan G, et al. FOCUS trial group . Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): an assessor-blinded, randomised controlled trial. The Lancet Psychiatry 2018;5:633-43.
- Hossain MM, Tasnim S, Sultana A, Faizah F, Mazumder H, Zou L, et al. Epidemiology of mental health problems in COVID-19: a review. F1000Research 2020;9.
- Thorlund K, Haggstrom J, Park JJ, Mills EJ. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ 2018;360.
Appendix 1 V-NeST Study – Research Participant Coronavirus Risk Assessment
This document
This COVID-19 Risk Matrix is to be used to assess suitability of participants to take part in the V-NeST trial. This matrix is to be used only for the assessments of potential participants’ health factors and not for household members. This risk matrix should be completed on the phone ahead of any face-to-face meeting. This risk matrix scoring is based on NHS risk assessment criteria for NHS for staff returning to work. It has been modified by the V-NeST trail management group and updated to be used for this study. Any query about this document or scoring should be addressed to the study Principal Investigator:
Dr. Matteo Cella
Department of Psychology,
Institute of Psychiatry, Psychology & Neuroscience
King’s College London,
PI
Telephone: (+44) 020 7848 5001
Email: matteo.cella@kcl.ac.uk
Inclusion in the V-NeST study
The V-NeST protocol exclusion criteria were amended on the 30 of September 2020 to include that people scoring in the high-risk range on this assessment will not be considered for participation in this study.
Participant initials or study ID: …… ……… ……… …… …… …… …… …… …… …… …… …… …… …… …… …… …… ……
Date of assessment: …… …… …… …… …… …… …… Assessor: …… …… …… …… …… …… …… …… …… …… …… ……
| Risk factor | Indicator | Adjustment | Comment | Score |
|---|---|---|---|---|
| Personal characteristics | ||||
| Age | < 50 | 0 | ||
| > 50 | 1 | |||
| > 60 | 3 | |||
| > 70 | > 6 | |||
| Sex at birth | Female | 0 | ||
| Male | 1 | |||
| Ethnicity | Caucasian | 0 | ||
| Black African descent | 1 | |||
| Indian Asian descent | 1 | |||
| Bangladeshi | 1 | |||
| Other (including mixed race) | 1 | |||
| Age, sex, ethnicity subtotal | ||||
| Health factors | ||||
| Pregnancy | > 28 weeks | > 6 (high risk) | ||
| < 28 weeks | > 6 (high risk) | |||
| Obesity | BMI ≥ 35 kg/m2 | 1 | ||
| ≥ 40 kg/m2 | 2 | |||
| Cardiology | Angina, previous MI stroke or | 1 | ||
| Heart failure | 2 | |||
| Diabetes | Type 1, type 2 diabetes | 1 | ||
| 2 | ||||
| Renal | Chronic renal disease (GFR < 60) | 2 | ||
| Respiratory | Asthma (not severe) | 0 | ||
| Asthma (taking oral steroids in the past year) | 1 | |||
| Other chronic pulmonary condition | 2 | |||
| Cancer | Active malignancy | 3 | ||
| Malignancy in remission 1–5 years ago |
1 | |||
| > 5 years | 0 | |||
| Blood cancer | < 1 year ago | 4 | ||
| 1–5 years | 3 | |||
| > 5 years | 1 | |||
| Liver disease | Any | 1 | ||
| Neurological disease | Any | 2 | ||
| Rheumatological | Active treated conditions | 1 | ||
| Immunosuppression | Any indication | 2 | ||
| Advised to shield by government or health provider | 6 | |||
| Health factors subtotal | ||||
| Total of personal characteristics and health factors | ||||
Interpretation
| Score | Inclusion in study | |
|---|---|---|
| Low risk | < 3 | Yes |
| Medium risk | 3-5 | Yes |
| High risk | ≥ 6 | No |
Appendix 2 Screenshots of the V-NeST VR environments: (1) factory; (2) social space; (3) TV room; (4) music room; and (5) games room

List of abbreviations
- AE
- adverse event
- CAINS
- The Clinical Assessment Interview for Negative Symptoms
- CBT
- cognitive–behavioural therapy
- CIs
- confidence intervals
- EEfRT
- Effort Expenditure for Reward Task
- GAS
- Goal Attainment Scale
- HADS
- The Hospital Anxiety and Depression Scale
- KCTU
- King’s Clinical Trials Unit
- NS
- negative symptoms
- PSYRATS
- The Psychotic Symptom Rating Scales
- RSS
- The Rosenberg Self-Esteem Scale
- SAE
- serious adverse event
- SD
- standard deviation
- SNS
- self-evaluation of negative symptoms
- TAU
- treatment as usual
- TMT
- Trail Making Test
- UK
- United Kingdom
- V-NeST
- Virtual reality therapy for the Negative SympToms of psychosis
- VR
- virtual reality
- WCST
- Wisconsin Card Sorting Task
- WSAS
- Work and Social Adjustment Scale
