Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as award number 14/23/09. The contractual start date was in February 2016. The draft manuscript began editorial review in August 2022 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Boughton et al. This work was produced by Boughton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Boughton et al.
Chapter 1 Introduction
Management of newly diagnosed type 1 diabetes in children and adolescents is challenging for patients, families, carers and healthcare professionals. Glucose is the dominant metabolic substrate for brain function1 and the glycaemic instability inherent in type 1 diabetes is known to affect brain structure and function in those with poorly controlled disease. 2 Severe hypoglycaemia, particularly nocturnal episodes, is more common in children3 and has a negative impact on the developing brain. 2,4 Fear of hypoglycaemia is common,5 impacts quality of life and psychological well-being of young people and their families6 and often leads to suboptimal glucose control. 6 Glycaemic control usually deteriorates during adolescence. The Diabetes Control and Complications Trial (DCCT) revealed both higher glycated haemoglobin A1c (HbA1c) levels and a 50% increase in the rate of severe hypoglycaemia in intensively treated adolescents compared to adults. 7 Teenagers with type 1 diabetes face the burden of diabetes management in addition to major physiological and psychological changes accompanying puberty. Only 22% of children and adolescents aged 19 years and younger in the UK reach the international target glycated haemoglobin (HbA1c) of < 53 mmol/mol (< 7.0%). 8
Preservation of C-peptide: evidence and approach
Type 1 diabetes is characterised by autoimmune destruction of pancreatic beta-cells. 9 Loss of beta-cells is gradual10 and at the clinical diagnosis of diabetes most people with type 1 diabetes have residual pancreatic beta-cells which can continue to secrete insulin for several additional years. Amelioration of hyperglycaemia after diagnosis allows partial recovery of beta-cell insulin secretory function, leading to a ‘honeymoon period’ with relatively low exogenous insulin requirements. 9 In the DCCT, 35% of participants with diabetes duration of 1–5 years had persistent islet cell function (meal-stimulated C-peptide levels of 0.2–0.5 pmol/ml). 7
Persistence of residual functioning beta-cells, measured by C-peptide secretion, is associated with improved glycaemic control, reduced risk of hypoglycaemia and lower incidence of microvascular complications. 11,12 In the DCCT, those who had ≥ 0.20 pmol/ml C-peptide initially or sustained over a year had markedly fewer complications – a 79% decrease in the relative risk of retinopathy. 12 Importantly, these benefits were seen in the face of less hypoglycaemia events. Individuals in the intensive treated group with ≥ 0.20 pmol/ml C-peptide had about the same frequency of severe hypoglycaemia as those in the standard care group; a 30% reduction as compared to those in intensive therapy without this level of C-peptide. A linear relationship between frequency of retinopathy progression and C-peptide as low as 0.03 pmol/ml has been reported. 11 Islet transplant studies have also shown that even small amounts of residual beta-cell function are clinically important. Vantyghem et al. showed that, while significant beta-cell function was required to improve mean glucose, lower glucose excursions, and result in insulin independence, participants who maintained minimal beta-cell function experienced almost no severe hypoglycaemic events. 13 Interventions which can preserve endogenous insulin secretion prior to and following clinical diagnosis of type 1 diabetes are therefore clinically important. 14,15
Assignment to the intensively managed group in the DCCT reduced the risk for loss of C-peptide by 57% over the mean 6.5 years of study. This suggests that metabolic control soon after the onset of type 1 diabetes may have a significant effect on preservation of residual islet cell function. However, intensification of insulin therapy inevitably hits the barrier of hypoglycaemia. 16
Previous studies have investigated whether an early period of islet cell rest achieved by intensive glycaemic control following diagnosis of type 1 diabetes can prevent the decline in endogenous insulin secretion with conflicting results. An early exploratory study in adolescents reported improved stimulated C-peptide levels (0.51 pmol/ml) at 12 months following a period of intensive insulin treatment in hospital for 2 weeks after diagnosis. 17 However, a more recent study applying a short period of hybrid closed-loop (CL) within 7 days of diagnosis, followed by sensor-augmented pump therapy, did not alter C-peptide secretion at 12 months compared with standard care, but there was no difference in glucose control between the two treatment groups over the 12-month study period. 18
It has yet to be determined whether sustained intensive glycaemic control following diagnosis can ameliorate the decline in endogenous insulin secretion in youth with type 1 diabetes.
Closed-loop technology
The emergence of new technologies including continuous glucose monitoring (CGM),19 sensor-augmented pump therapy (SAP)20 and threshold pump suspend21,22 provides new opportunities to improve outcomes for people with type 1 diabetes. The most promising approach is CL insulin therapy23 which combines real-time CGM with insulin pump therapy to achieve glucose responsive subcutaneous (s.c. ) insulin delivery. The vital component of such a system, also known as an artificial pancreas (AP), is a computer-based algorithm. The role of the control algorithm is to translate, in real time, the information it receives from the CGM and to compute the amount of insulin to be delivered by the pump. The other components include a real-time continuous glucose monitor and an infusion pump to titrate and deliver insulin.
Cambridge closed-loop research
Previous research conducted at the University of Cambridge focused on developing a CL system for overnight glucose initially and then day-and-night control in people with type 1 diabetes. Studies employed model predictive control (MPC) – this algorithm estimates user-specific parameters from CGM measurements taken every 1–15 minutes and makes predictions of glucose excursions, which are then used to direct insulin infusion between meals and overnight while a standard bolus calculator is used to deliver prandial insulin. 24 The MPC algorithm has been studied extensively using in silico testing, utilising a simulator developed by members of the study team. 25 The simulations suggested a reduced risk of nocturnal hypoglycaemia and hyperglycaemia with the use of the MPC algorithm. 26
Previous studies of CL in children and adolescents with type 1 diabetes in the clinical research facility showed that overnight CL therapy increased the time spent in target glucose range by 37% and reduced the risk of overnight hypoglycaemia eightfold, as compared to conventional insulin pump therapy in a randomised, crossover design. 27
Following demonstration of safety and efficacy of CL insulin delivery in the research facility, in a multicentre, crossover, randomised, controlled study, we compared 12-week use of an overnight CL insulin delivery system with SAP in children and adolescents aged 6–18 years. 28 The proportion of time with the night-time glucose level in the target range (3.9–8.0 mmol/L) was higher during the CL phase than during the control phase [by 24.7 percentage points; 95% confidence interval (CI) 20.6 to 28.7; p < 0.001], and the mean night-time glucose level was lower (difference, −1.6 mmol/l; 95% CI−2.2 to − 1.1; p < 0.001).
We completed a randomised, crossover design study in adolescents aged 10–18 years who underwent two 7-day home periods of sensor-augmented insulin pump therapy or CL insulin delivery without supervision or remote monitoring. 29 The proportion of time when the sensor glucose level was in the target range (3.9–10.0 mmol/l) was increased during CL insulin delivery compared with sensor-augmented pump therapy (72% vs. 53%, p < 0.001), the mean glucose concentration was lower (8.7 vs. 10.1 mmol/l, p = 0.028), and the time spent above the target level was reduced (p = 0.005) without changing the total daily insulin amount (p = 0.55). The time spent in the hypoglycaemic range was low and comparable between interventions.
The CL approach has been successfully evaluated in children and adolescents in controlled laboratory studies27,30,31 and in home settings. 28,29,32–37 The results demonstrate improved glucose control and reduced risk of hypoglycaemia events. Hybrid CL systems have also been shown to accommodate variability in exogenous insulin requirements. 38,39 Psychosocial assessments support acceptability and positive impact of this novel therapeutic approach among children/adolescents and carers,40 although the potential benefit in preserving cognitive function is, as yet, unknown. The CL approach promises to transform management of type 1 diabetes and may provide a tangible option to improve residual beta-cell function.
Rationale for the study
The purpose of the study is to test the impact of continued intensive metabolic control using CL insulin delivery after diagnosis on preservation of C-peptide residual secretion. The study enrols children aged 10 and older, as they are characterised by higher residual C-peptide secretion at diagnosis compared to younger children. The present study will also test the feasibility and acceptance of this therapy so that it could be considered as a standard treatment modality in the future.
External funding has been secured to continue with the treatment allocated by randomisation for 48 months to allow ongoing assessment of the impact of continued intensive metabolic control using CL insulin delivery on residual C-peptide and will test acceptability of this therapy over a longer duration.
Hypothesis
We hypothesised that a sustained period of intensive glucose control with hybrid CL for 12 months following diagnosis of type 1 diabetes in children and adolescents can preserve C-peptide secretion compared to standard insulin therapy.
Chapter 2 Objectives
Primary objective
The primary objective is to evaluate the effect of continued intensive metabolic control using CL insulin delivery after diagnosis on preservation of C-peptide residual secretion by comparing the area under the stimulated C-peptide curve (AUC) of a mixed-meal glucose tolerance test conducted at the 12-month visit in participants receiving CL insulin delivery with those receiving standard therapy – that is, multiple daily injections applying basal bolus regimen.
The objective of the internal pilot phase is to carry out preliminary evaluation of recruitment, randomisation, treatment and follow-up assessments at the five participating sites.
Secondary objectives
Glucose control
The objective is to examine the efficacy of day-and-night CL compared with standard basal bolus regimen as far as glucose control is concerned. We will compare between-group differences in HbA1c, and parameters based on subcutaneous CGM, such as the percentage of time spent within, below and above the target range from 3.9 to 10.0 mmol/l.
Safety
The objective is to evaluate the safety of day-and-night automated CL glucose control in terms of episodes of severe hypoglycaemia and other adverse events (AEs).
Utility
The objective is to determine the frequency and duration of use of the automated CL system.
Human factors
The objective is to assess emotional and behavioural characteristics of participating subjects and family members and their response to the CL system and clinical trial in order to aid interpretation of trial results and inform recommendations for future use of CL systems.
Interviews were conducted with healthcare professionals to explore their views and experiences of delivering the trial and supporting participants using the CL system.
Chapter 3 Trial design
The study adopted an open-label, multicentre, randomised, parallel design comparing hybrid CL insulin delivery and standard insulin therapy (control) over 12 months. The study protocol has been previously reported. 41
Internal pilot phase
The purpose of the internal pilot study was to estimate the rate of recruitment, and to pilot randomisation, treatment and follow-up assessments at the initial participating sites. During the pilot phase, we aimed to recruit at least 10 subjects – that is, 2 per site. All participants recruited during the pilot phase proceeded to the full study.
Full study
Following the internal pilot phase and consecutive re-evaluation of recruitment procedures and follow-up assessments, recruitment for the study resumed at full rate, and all randomised subjects were followed up until study completion. External funding was secured to allow participants to continue with the treatment allocated at randomisation for a further 12 months. Study processes are therefore referred to over the 24-month study period, but this report focuses on the 12-month results which includes the primary end point. The study flow chart is outlined in Figure 1.
FIGURE 1.
Study flow chart.

Extension phase
At 24 months, all participants were invited to participate in an optional extension phase to continue with their current treatment (automated CL glucose control or standard insulin therapy) for a further 24 months. Permission has also been sought from participants and their carers for ongoing submission of routine clinical data to the research team for a further 9 years to enable long-term outcomes to be reported.
Approval was received from Cambridge East Research Ethics Committees (16/EE/0286) and Medicines and Healthcare products Regulatory Agency. Safety aspects were overseen by an independent data safety monitoring board. The study is registered with clinicaltrials.gov (NCT02871089).
Study participants
Participants were recruited from seven paediatric diabetes clinics in the UK (Cambridge, Edinburgh, Leeds, Liverpool, Nottingham, Oxford, Southampton). Eligible participants were identified by clinical teams at each centre. Participants aged 16 years and parents/guardians of participants < 16 years gave written informed consent. Written assent was obtained from participants < 16 years.
Following the initial 24 months of the study, all participants opting to continue with the extension phase of the study were re-consented.
Inclusion criteria
-
Diagnosis of type 1 diabetes within previous 21 days. Day 1 will be defined as the day insulin was first administered. Type 1 diabetes will be defined according to World Health Organization (WHO) criteria using standard diagnostic practice. WHO definition: ‘The aetiological type named type 1 encompasses the majority of cases with are primarily due to beta-cell destruction, and are prone to ketoacidosis. Type 1 includes those cases attributable to an autoimmune process, as well as those with beta-cell destruction for which neither an aetiology nor a pathogenesis is known (idiopathic). It does not include those forms of beta-cell destruction or failure to which specific causes can be assigned (e.g. cystic fibrosis, mitochondrial defects, etc.).’
-
The subject is at least 10 years and not older than 16.9 years.
-
The subject/carer is willing to perform regular capillary blood glucose monitoring, with at least four blood glucose measurements taken every day.
-
The subject is literate in English.
-
The subject is willing to wear glucose sensor.
-
The subject is willing to wear CL system at home.
-
The subject is willing to follow study-specific instructions.
-
The subject is willing to upload pump and CGM data at regular intervals.
Exclusion criteria
-
Physical or psychological condition likely to interfere with the normal conduct of the study and interpretation of the study results as judged by the investigator.
-
Current treatment with drugs known to interfere with glucose metabolism – for example, systemic corticosteroids, non-selective beta-blockers and MAO inhibitors, etc.
-
Known or suspected allergy to insulin.
-
Regular use of acetaminophen.
-
Lack of reliable telephone facility for contact.
-
Pregnancy, planned pregnancy or breastfeeding.
-
Living alone.
-
Severe visual impairment.
-
Severe hearing impairment.
-
Medically documented allergy towards the adhesive (glue) of plasters or unable to tolerate tape adhesive in the area of sensor placement.
-
Serious skin diseases (e.g. psoriasis vulgaris, bacterial skin diseases) located at places of the body, which potentially are to be used for localisation of the glucose sensor.
-
Illicit drugs abuse.
-
Prescription drugs abuse.
-
Alcohol abuse.
-
Sickle cell disease, haemoglobinopathy, receiving red blood cell transfusion or erythropoietin within 3 months prior to time of screening.
-
Eating disorder such as anorexia or bulimia.
-
Milk protein allergy.
Randomisation
Eligible participants were randomised after baseline mixed-meal tolerance test (MMTT) using block randomisation (block sizes of two and four were used with equal probability) and central randomisation software to CL therapy or standard insulin therapy. Randomisation was stratified by site and age (10–13 years and 14–16 years); the randomisation ratio was 1 : 1 within each stratum.
Closed-loop system
The Cambridge MPC algorithm (version 0.3.71) was run in two hardware configurations, the initial FlorenceM configuration followed by the CamAPS FX (CamDiab, Cambridge, UK) configuration to improve usability and therapy adherence (Figure 2).
FIGURE 2.
Hybrid CL configurations used in the CL group. Panel A shows FlorenceM configuration and panel B shows CamAPS FX configuration.

FlorenceM
The FlorenceM configuration comprised a locked smartphone (Samsung Galaxy S4, South Korea) running an app with the Cambridge control algorithm (version 0.3.71), a Medtronic prototype phone enclosure with an embedded modified Carelink USB to allow the smartphone to wirelessly communicate with a modified Medtronic MiniMedTM 640G insulin pump (Medtronic, Northridge, CA, USA). This pump had low glucose suspend enabled and received glucose sensor data from the Medtronic MiniMedTM GuardianTM 3 sensor, which requires finger-stick calibrations.
CamAPS FX
The CamAPS FX configuration superseded FlorenceM in July 2019. The CamAPS FX system comprised an unlocked smartphone (Samsung Galaxy S8, South Korea) hosting the CamAPS FX app running the Cambridge control algorithm (version 0.3.71), which communicated wirelessly with both the Dana Diabecare RS insulin pump (Sooil, Seoul, South Korea) and Dexcom G6 transmitter (Dexcom, San Diego, CA, USA).
In both configurations, algorithm-driven insulin delivery was adjusted automatically every 8–12 minutes, with the app-based control algorithm communicating the insulin infusion rate to the insulin pump wirelessly. The control algorithm was initialised using total daily insulin dose and body weight, and incorporated adaptive learning with regard to total daily insulin requirements, diurnal variations, meal patterns, and duration of insulin action.
In both configurations, when auto mode was not operational, the insulin pump reverted to pre-programmed basal rates. The treat-to-target adaptive control algorithm had a nominal glucose target level of 5.8 mmol/l, which was adjustable in the CamAPS FX configuration between 4.0 mmol/l and 11.1 mmol/l across different times of day. The CamAPS FX app contained a bolus calculator to initiate bolus delivery from the phone, a user-selectable ‘Ease-off’ mode to reduce insulin delivery around activity/exercise, and a ‘Boost’ mode to intensify insulin delivery when insulin needs were elevated. The CamAPS FX app streamed data to Diasend data ecosystem (Glooko/Diasend, Sweden).
Procedures
Screening and baseline evaluation
The screening assessment included written informed consent/assent, checking inclusion and exclusion criteria, medical (diabetes) history, body weight and height measurement, calculation of body mass index (BMI), blood pressure measurement, record of current insulin therapy and urine pregnancy test (females of child-bearing potential). If not done at diagnosis, a blood sample was taken for assessment of full blood count, thyroid function (TSH, fT4), anti-transglutaminase antibodies and IgA (measured locally).
Education for both arms
At entry to the study, all participants completed a structured educational programme delivered to the participants and their families in accordance with the standards of the International Society for Pediatric and Adolescent Diabetes (ISPAD). 42 All participants were trained on the use of multiple daily injection therapy (MDI) regimen. Participants and their families were educated in:
-
type 1 diabetes
-
the use and administration of insulin
-
hyperglycaemia and correction doses
-
hypoglycaemia symptoms and treatment
-
exercise
-
sick-day rules
-
carbohydrate counting and dietetic education
-
the benefits of maintaining optimal glycaemic control for long-term health
-
blood glucose monitoring.
Baseline visit
Within 21 days of diagnosis, participants attended for the baseline MMTT. Blood samples were taken for HbA1c, lipid profile and immunological parameters. Computerised cognitive tests and validated questionnaires to assess quality of life and diabetes management were completed. A masked CGM device was applied to assess baseline glycaemic control.
Mixed-meal tolerance tests
Mixed-meal tolerance tests were conducted at baseline, 6, 12 and 24 months post diagnosis following an overnight fast. Participants were given a liquid meal (Boost, Nestlé, Switzerland) according to bodyweight [6 ml/kg (maximum 360 ml), 17 g carbohydrate, 4 g protein, 3 g fat per 100 ml] and venous blood samples for measurement of C-peptide and glucose were collected at –10, 0, 15, 30, 60, 90 and 120 minutes.
Questionnaires
At baseline, 12 and 24 months participants and caregivers completed the following questionnaires:
-
Paediatric Quality of Life Inventory (PedsQL) Diabetes;
-
Strengths and Difficulties Questionnaire (SDQ);38
-
Pittsburgh Sleep Quality Index (PSQI). 43
At baseline, 12 and 24 months participants and caregivers in the CL arm completed the INsulin Dosing Systems: Perceptions, Ideas, Reflections and Expectations (INSPIRE) questionnaire. At baseline, 12 and 24 months participants completed the problem areas in diabetes (PAID)-Teen questionnaire.
Intervention period
Study visit schedules and flow chart are shown in Table 1, Table 2 and Figure 1. Following randomisation, participants randomised to the CL group were trained to use the study insulin pump and glucose sensor prior to starting CL insulin delivery within 6 weeks of diagnosis. Participants continued with CL therapy for 24 months with no remote monitoring or study-related restrictions. Participants randomised to standard insulin therapy received additional training to complement the core training and to match contact time with the CL group. Participants continued with standard insulin therapy for 24 months but could switch to insulin pump therapy and/or use flash/CGM or approved CL systems if clinically indicated, applying National Institute for Health and Care Excellence (NICE) criteria. 43 The recommended glycaemic target for both groups was HbA1c < 48 mmol/mol (6.5%) as per the NICE guidelines. 43
| Visit/contact | Description | Start relative to previous/next visit/activity | Duration | |
|---|---|---|---|---|
| Run-in period | Visit 1 | Recruitment and screening visit: Consent/assent; inclusion, exclusion; screening blood sample | Within 21 days of diagnosis | 2 hours |
| Visit 2 | Baseline visit: HbA1c, MMTT, blinded CGM, questionnaires, computerised cognitive testing, bloods for immunological analyses | 7–21 days after diagnosis | 3–4 hours | |
| Randomisation | ||||
| Insulin pump and CGM training | Visit 3 | Insulin pump training, initiation study pump | Within 1 week of Visit 2 | 3–4 hours |
| Visit 4 | CGM training, initiation of CGM | Within 0–7 days of Visit 3 (Visit 4 may coincide with Visit 3; Training visits can be repeated) | 2 hours | |
| Closed-loop insulin delivery (24 months) | Visit 5a | CL initiation at clinic/home | Within 6 weeks of diagnosis | 3–4 hours |
| Contact | Review use of study devices, study update | 1 week after Visit 5 (± 3 days) | < 0.5 hour | |
| Visit 6a | HbA1c, data download, blinded CGM | After 3 months of diagnosis (± 1 week) | < 1 hour | |
| Visit 7 | MMTT, HbA1c, bloods for immunological analyses, data download, blinded CGM, sleep quality assessment | After 6 months of diagnosis (± 2 weeks) | 3–4 hours | |
| Visit 8a | HbA1c, data download, blinded CGM | After 9 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 9 | MMTT, HbA1c, bloods for immunological analyses, data download, blinded CGM, questionnaires, computerised cognitive testing, interviews, sleep quality assessment | After 12 months of diagnosis (± 2 weeks) | 3–4 hours | |
| Visit 10a | HbA1c, data download, blinded CGM | After 15 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 11a | HbA1c, data download, blinded CGM | After 18 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 12a | HbA1c, data download, blinded CGM | After 21 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 13a | Blinded CGM, sleep quality assessment | Between Visit 12 and Visit 14 (Visit 13 may coincide with Visit 14) | < 0.5 hour | |
| Visit 14 | End of CL treatment: MMTT, HbA1c, data download, bloods for immunological analyses, questionnaires, computerised cognitive testing, focus groups |
After 24 months of diagnosis (± 2 weeks) | 4–5 hours |
| Visit/contact | Description | Start relative to previous/next visit/activity | Duration | |
|---|---|---|---|---|
| Run-in period | Visit 1 | Recruitment and screening visit: Consent/assent; inclusion, exclusion; screening blood sample | Within 21 days of diagnosis | 2 hours |
| Visit 2 | Baseline visit: HbA1c, MMTT, blinded CGM, questionnaires, computerised cognitive testing, bloods for immunological analyses | 7–21 days after diagnosis | 3–4 hours | |
| Randomisation | ||||
| Additional training | Visit 3 | Training on carbohydrate counting | Within 1 week of Visit 2 | 2 hours |
| Visit 4 | Training on insulin dose adjustment | Within 0–7 days of Visit 3 (Visit 4 may coincide with Visit 3; Training visits can be repeated) | 2 hours | |
| Standard insulin therapy (24 months) | Visit 5a | Control arm start visit | Within 6 weeks of diagnosis | < 1 hour |
| Contact | Study update | 1 week after Visit 5 (± 3 days) | < 0.5 hour | |
| Visit 6b | HbA1c, blinded CGM | After 3 months of diagnosis (± 1 week) | < 1 hour | |
| Visit 7 | MMTT, HbA1c, bloods for immunological analyses, blinded CGM, sleep quality assessment | After 6 months of diagnosis (± 2 weeks) | 3–4 hours | |
| Visit 8b | HbA1c, blinded CGM | After 9 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 9 | MMTT, HbA1c, bloods for immunological analyses, blinded CGM, questionnaires, computerised cognitive testing, sleep quality assessment |
After 12 months of diagnosis (± 2 weeks) | 3–4 hours | |
| Visit 10b | HbA1c, blinded CGM | After 15 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 11b | HbA1c, blinded CGM | After 18 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 12b | HbA1c, blinded CGM | After 21 months of diagnosis (± 2 weeks) | < 1 hour | |
| Visit 13b | Blinded CGM, sleep quality assessment | Between Visit 12 and Visit 14, (may coincide with visit 14) | < 1 hour | |
| Visit 14 | End of control treatment: MMTT, HbA1c, bloods for immunological analyses, questionnaires, computerised cognitive testing | After 24 months of diagnosis (± 2 weeks) | 4–5 hours |
Study contacts
Participants were followed up at 3-monthly intervals. At each follow-up visit, HbA1c was measured and participants wore a masked glucose sensor (LibrePro; Abbott Diabetes Care, Alameda, CA, USA) for 14 days. MMTTs were conducted at 6, 12 and 24 months post diagnosis following an overnight fast.
Participants/parents and/or the local diabetes clinical team were free to adjust insulin therapy, but no active treatment optimisation was undertaken by the research team. Participants were able to contact a 24-hour telephone helpline to the local research team.
Assays
C-peptide and glucose were measured centrally (Swansea University, Swansea, UK); C-peptide was measured using a sensitive, luminescence immunoassay (IV2-004, Invitron, UK) and glucose using a glucose oxidase method (YSI 2300 stat plus, YSI Life Sciences, USA). HbA1c was measured centrally (Swansea University) using an International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)-aligned method and following National Glycohemoglobin Standardisation Program (NGSP) standards; Tosoh GX (Tosoh Bioscience, UK). Lipid profile was measured locally.
Study end points
The primary end point was the between-group difference in mean stimulated C-peptide AUC of the 12-month MMTT. Key secondary end points included time in target glucose range 3.9–10.0 mmol/l, HbA1c, and time < 3.9 mmol/l at 12 months tested sequentially to control the type I error. Sensor glucose end points were based on data from a masked glucose sensor worn for 14 days.
Additional secondary end points included mean stimulated C-peptide at 6 and 24 months, plasma glucose and fasting C-peptide divided by fasting glucose at 6, 12 and 24 months during MMTT, HbA1c at 6 and 24 months, percentage of participants in each group with HbA1c < 7.5% (58 mmol/mol) at 12 and 24 months. Secondary end points based on masked sensor glucose data collected every 3 months included time in range, mean glucose, standard deviation (SD) and coefficient of variation of glucose, time spent in hyperglycaemia, time with glucose < 3.5 mmol/l, < 3.0 mmol/l and < 2.8 mmol/l and AUC of glucose < 3.9 mmol/l and < 3.5 mmol/l. Insulin metrics (total, basal and bolus insulin dose), BMI SD score, blood pressure and lipid profile were compared between groups at 12 and 24 months.
Safety evaluation comprised the frequency of severe hypoglycaemia and diabetic ketoacidosis (DKA), and other AEs and SAEs.
Power calculation
The primary analysis compares the difference between groups in the 2-hour AUC-mean using the ln(mean C-peptide + 1). The residual SD of the ln(x + 1) transformed C-peptide AUC analysis of covariance is referred to by TrialNet44 as the root mean squared error (RMSE). The back-transform, exp(y) – 1, of the mean of the transformed values is referred to by TrialNet as the geometric-like mean. In the DirecNet/TrialNet new-onset studies,44 the point estimate for RMSE was 0.18 (transformed scale) and was used in the power calculations. As in the TrialNet sample size calculations, a 50% improvement was assumed in the geometric-like mean C-peptide AUC. The sample size depends on the geometric-like mean value in the control group. The original TrialNet sample size calculations assumed a value of 0.37 pmol/ml for the control group based on the lower 90% confidence limit from previous data. 44 The present power calculation applied the same value. A 50% increase in the intervention group of the geometric-like mean C-peptide AUC gives 0.37*1.50 = 0.555 pmol/ml. After ln(x + 1) transformation, the mean values in the control and treatment groups are 0.315 and 0.441 (transformed scale), respectively, the treatment effect is 0.441–0.315 = 0.126. The treatment effect of 0.126 with a SD of 0.18 requires 44 subjects per group at 90% power for a two-sided test at the 0.05 level. Allowing for 10% loss to follow-up means we aimed to recruit a total of 96 randomised participants (48 per group).
Statistical analysis
Primary analyses were performed on an intention-to-treat basis with each participant analysed according to the treatment assigned by randomisation. All randomised participants were included in the intention-to-treat population and all enrolled participants were included in the safety cohort. Comparison of safety outcomes between the two treatment groups only included those events occurring on or after randomisation. All randomised participants with at least one CGM reading were included in the CGM analyses following a modified intention-to-treat approach. Treatment interventions were compared using a longitudinal mixed effects linear model adjusting for baseline value, gender, presence/absence of DKA at diagnosis and age as fixed effects, and clinical site as a random effect. A 95% CI was reported for the difference between the interventions based on the model. The log (C-peptide AUC + 1) values at 12 months post randomisation were compared for the primary analysis. For highly skewed residuals for secondary outcomes winsorisation was used. Mixed effects regression models addressed missing data by using maximum likelihood estimation incorporating data from all randomised participants, which assumes data were missing at random.
A per-protocol analysis restricted to participants in the CL group who used the system at least 60% of the time and those in the control group who did not start insulin pump therapy was conducted.
The primary and key end points comparing between-group differences at 12 months post diagnosis were tested sequentially to maintain a type 1 error rate of 5%. If C-peptide AUC was significant at α = 0.04, then key end points were tested at α = 0.05. Otherwise, they were tested at α = 0.01. If all three key end points were significant at α = 0.01, then this alpha was recycled to the primary outcome and C-peptide AUC was tested at α = 0.05. Secondary end points were adjusted for multiple comparisons to control the false discovery rate using the two-stage adaptive Benjamini–Hochberg method. 45 Analyses were conducted with SAS software version 9.4 (SAS Institute, Inc.).
Health economics analysis was not included in the NIHR Efficacy and Mechanism Evaluation contract and is therefore not included in this report.
Participant withdrawal criteria
The following pre-randomisation withdrawal criterion will apply:
-
Subject/Family is unable to demonstrate safe application of MDI during run-in period as judged by the investigator.
The following pre- and post-randomisation withdrawal criteria will apply:
-
Subject is unable to demonstrate safe use of MDI or study insulin pump and/or CGM during post-randomisation training period as judged by the investigator.
-
Subject fails to demonstrate compliance with MDI therapy or study insulin pump and/or CGM during post-randomisation training period.
-
Subjects may terminate participation in the study at any time without necessarily giving a reason and without any personal disadvantage.
-
Significant protocol violation or non-compliance.
-
Recurrent severe hypoglycaemia events not related to the use of the CL system.
-
Recurrent severe hyperglycaemia event/DKA unrelated to infusion site failure and related to the use of the CL system.
-
Decision by the investigator or the Sponsor that termination is in the subject’s best medical interest.
-
Allergic reaction to insulin.
-
Allergic reaction to adhesive surface of infusion set or glucose sensor.
-
If patient cannot be contacted in 12 weeks subject will be considered lost to follow-up.
Protocol and statistical analysis plan amendments can be found in Appendices 2 and 3.
Chapter 4 Results
Recruitment
Pilot study for recruitment
Recruitment for CLOuD commenced in February 2017. An internal pilot study randomising 10 participants tested the feasibility of recruitment to the study protocol. The first 10 participants were recruited within the first 4 months after recruitment commenced. Over 30% of subjects who were eligible and invited to participate in the study were successfully recruited and therefore the internal pilot study continued to the full study.
Full study recruitment
Table 3 shows the recruitment success rate overall and by individual site. We applied no exclusions at enrolment such as technology propensity or healthcare professional considerations about suitability, minimising selection bias. The study population is therefore representative of the general population of youth newly diagnosed with type 1 diabetes, improving generalisability of the findings.
| Site | Number of newly diagnosed T1D patients aged 10–16.9 years | Number of patients approached (given PIS) | Number of patients eligible for CLOuD | Number of participants recruited to CLOuD | Recruitment success rate (%) |
|---|---|---|---|---|---|
| 1 | 37 | 35 | 33 | 19 | 57.6 |
| 2 | 15 | 15 | 14 | 6 | 42.9 |
| 3 | 23 | 23 | 23 | 12 | 52.2 |
| 4 | 25 | 21 | 20 | 17 | 85.0 |
| 5 | 29 | 26 | 26 | 21 | 80.8 |
| 6 | 32 | 30 | 27 | 14 | 51.9 |
| 7 | 21 | 21 | 19 | 12 | 63.2 |
| Total | 182 | 171 | 162 | 101 | 62.4 |
The reasons potential participants declined to take part in CLOuD were recorded and are shown in Figure 3. The most common reason for declining to take part in the study was concerns using an insulin pump (21%) or being uninterested in research (16%). Recruitment completed in July 2019. The cumulative rate of recruitment is shown in Figure 4.
FIGURE 3.
Reasons given for declining to participate in the CLOuD study (%).
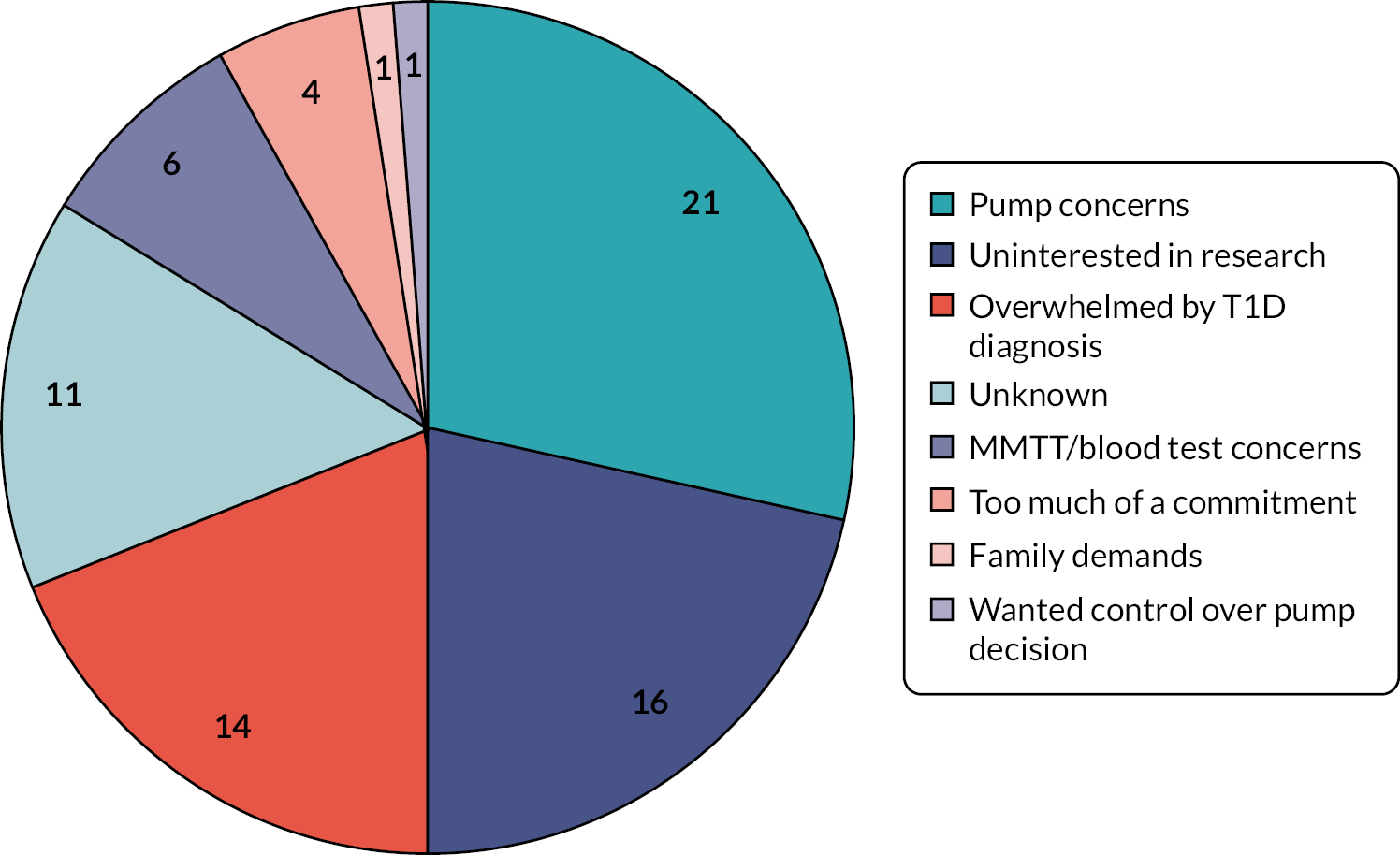
FIGURE 4.
Rate of recruitment.
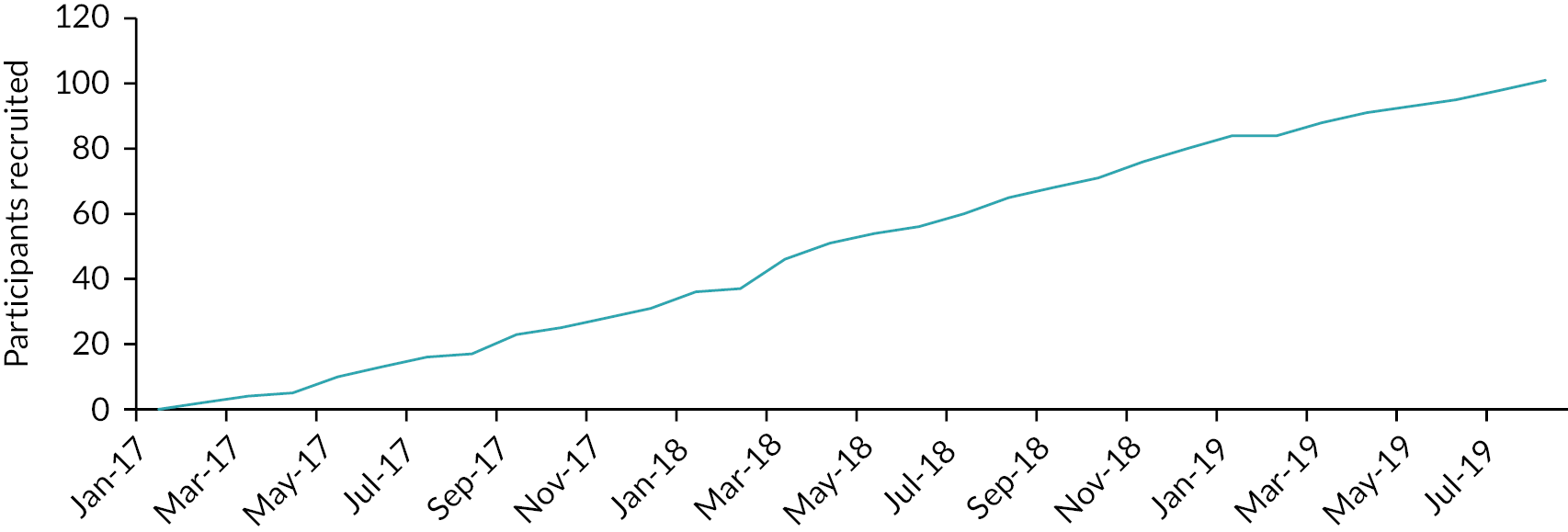
Between 6 February 2017 and 18 July 2019, 101 participants were enrolled. Four participants withdrew prior to randomisation; 97 participants were randomised [mean ± SD age 12 ± 2 years, 44% (n = 43) female, baseline HbA1c 93 ± 18 mmol/mol (10.6 ± 1.7%) and 29% (n = 28) presented in DKA at diagnosis], 51 to the CL group and 46 to control group (Table 4). Mean ± SD time to randomisation from diagnosis was 9.5 ± 6.2 days (Figure 5).
| Overall (N = 97) | Closed-loop (N = 51) | Control (N = 46) | |
|---|---|---|---|
| Age (years) | 12 ± 2 | 12 ± 2 | 12 ± 2 |
| Distribution, n (%) | |||
| 10–13 years | 79 (81) | 41 (80) | 38 (83) |
| 14–< 17 years | 18 (19) | 10 (20) | 8 (17) |
| Sex, n (%) | |||
| Female | 43 (44) | 25 (49) | 18 (39) |
| Male | 54 (56) | 26 (51) | 28 (61) |
| BMI percentile | 52 ± 31 | 53 ± 29 | 51 ± 34 |
| Ethnicity, n (%) | |||
| White | 79 (81) | 44 (86) | 35 (76) |
| Black | 3 (3) | 1 (2) | 2 (4) |
| Asian | 6 (6) | 2 (4) | 4 (9) |
| More than one race | 5 (5) | 4 (8) | 1 (2) |
| Unknown/not reported | 4 (4) | 0 (0) | 4 (9) |
| Baseline glycated haemoglobin (mmol/mol) | 93 ± 18 | 94 ± 20 | 91 ± 17 |
| Baseline glycated haemoglobin (%) | 10.6 ± 1.7 | 10.7 ± 1.8 | 10.5 ± 1.6 |
| Presence of DKA at diagnosis, n (%) | 28 (29) | 17 (33) | 11 (24) |
FIGURE 5.
Participant flow CONSORT diagram.

Retention
There were 10 post-randomisation withdrawals by 12 months, 4 in the CL group and 6 in the control group. Two participants, one in each treatment group, were withdrawn by the clinic due to safety concerns and the other eight participant withdrawals were voluntary (Table 5).
| Treatment | Last visit | Reason for withdrawal |
|---|---|---|
| Closed-loop | Randomisation | Participant decided they did not want to use the study pump. |
| Closed-loop | Randomisation | Participant struggled with numerous device issues and did not wish to continue to be involved in the research. |
| Control | Randomisation | Participant did not wish to be cannulated during MMTTs and also wanted to be randomised to CL treatment. |
| Control | Randomisation | Participant was unhappy that they were not randomised to CL. |
| Closed-loop | 3 months | Parent asked that participant be dropped from the study. |
| Control | 3 months | Participant was unhappy they were not randomised to CL. |
| Control | 3 months | Clinicians felt the participant was more likely to have type 2 diabetes. Participant was withdrawn by the site. |
| Closed-loop | 6 months | Decision by ethics board following intentional over bolusing and concerns regarding impact of study on mental health. |
| Control | 9 months | Participant indicated they did not want to continue with study-related activities. |
| Control | 9 months | Participant withdrew as they had a funny turn when getting bloods taken at their previous visit. |
Primary and key end points
Primary and key end points for all randomised participants at 12 months are shown in Table 6. There was no difference in C-peptide AUC between treatment groups at 12 months {primary end point: geometric mean CL 0.35 pmol/ml [interquartile range (IQR) 0.16–0.49] compared with control 0.46 pmol/ml (IQR 0.22–0.69); mean adjusted difference –0.06 pmol/ml (95% CI –0.14 to 0.03)}.
| Baseline | 12 months | Mean adjusted difference (95% CI)a | |||
|---|---|---|---|---|---|
| Closed-loop | Control | Closed-loop | Control | Closed-loop minus control at 12 months | |
| Primary end point (at 12 months) | |||||
| C-peptide AUC (pmol/ml)b | (N = 49) | (N = 45) | (N = 46) | (N = 37) | |
| 0.56 (0.41, 0.74) |
0.64 (0.43, 0.81) |
0.35 (0.16, 0.49) |
0.46 (0.22, 0.69) |
–0.06 (–0.14 to 0.03) |
|
| Key end points (at 12 months) | |||||
| Time spent at glucose level (%)c | (N = 50) | (N = 43) | (N = 44) | (N = 33) | |
| 3.9–10.0 mmol/l | 74 ± 14 | 72 ± 13 | 64 ± 14 | 54 ± 23 | 10 (2 to 17) |
| (N = 51) | (N = 46) | (N = 46) | (N = 39) | ||
| HbA1c (mmol/mol) | 94 ± 20 | 91 ± 17 | 52 ± 8 | 56 ± 12 | –4 (–8 to 0) |
| HbA1c (%) | 10.7 ± 1.8 | 10.5 ± 1.6 | 6.9 ± 0.7 | 7.3 ± 1.1 | –0.4 (–0.7 to 0.0) |
| Time < 3.9 mmol/l (%)c,d | (N = 50) | (N = 43) | (N = 44) | (N = 33) | |
| 9.1 ± 6.3 | 10.7 ± 7.1 | 6.2 ± 3.8 | 5.4 ± 4.7 | 0.9 (–1.0 to 2.8) | |
| Secondary end points | |||||
| Sensor glucose end points c | (N = 50) | (N = 43) | (N = 44) | (N = 33) | |
| Mean glucose (mmol/l) | 7.2 ± 1.6 | 7.0 ± 1.6 | 8.5 ± 1.6 | 9.8 ± 3.3 | –1.5 (–2.6 to –0.5) |
| Standard deviation (mmol/l) | 2.7 ± 0.7 | 2.7 ± 0.8 | 3.6 ± 0.9 | 3.7 ± 1.4 | –0.2 (–0.6 to 0.2) |
| CV of glucose (%) | 38 ± 7 | 39 ± 7 | 42 ± 7 | 39 ± 8 | 4 (1 to 8) |
| Time spent at glucose level (%)d | |||||
| < 3.5 mmol/l | 5.2 ± 4.8 | 6.6 ± 5.2 | 3.6 ± 2.6 | 3.3 ± 3.1 | 0.3 (–1.0 to 1.6) |
| < 3.0 mmol/l | 2.0 ± 2.5 | 2.8 ± 2.8 | 1.4 ± 1.2 | 1.5 ± 1.6 | –0.2 (–0.8 to 0.5) |
| < 2.8 mmol/l | 1.3 ± 1.8 | 1.9 ± 2.2 | 0.9 ± 0.9 | 1.1 ± 1.4 | –0.3 (–0.8 to 0.2) |
| > 10.0 mmol/l | 15 ± 9 | 14 ± 10 | 29 ± 14 | 40 ± 25 | –11 (–19 to –3) |
| > 16.7 mmol/l | 1.0 ± 1.6 | 1.0 ± 1.5 | 4.2 ± 3.8 | 10.0 ± 12.4 | –5.9 (–9.7 to –2.1) |
| Area above curve 3.9 mmol/l | 1.0 ± 0.9 | 1.3 ± 1.1 | 0.7 ± 0.5 | 0.6 ± 0.6 | 0.0 (–0.2 to 0.3) |
| Area above curve 3.5 mmol/l | 0.5 ± 0.5 | 0.6 ± 0.6 | 0.3 ± 0.3 | 0.3 ± 0.4 | 0.0 (–0.2 to 0.1) |
| HbA1c < 7.5%, n (%) | (N = 51) | (N = 46) | (N = 46) | (N = 39) | |
| 0 (0) | 1 (2) | 36 (78) | 22 (56) | 21 (–1 to 42) | |
| Insulin end points (U/kg/day) | (N = 47) | (N = 44) | (N = 46) | (N = 39) | |
| Total daily insulin | 0.87 ± 0.33 | 0.82 ± 0.38 | 0.96 ± 0.45 | 0.84 ± 0.39 | 0.10 (–0.11 to 0.30) |
| Total daily basal insulin | 0.33 ± 0.12 | 0.36 ± 0.21 | 0.52 ± 0.31 | 0.37 ± 0.26 | 0.14 (–0.01 to 0.29) |
| Total daily bolus insulin | 0.54 ± 0.24 | 0.46 ± 0.28 | 0.44 ± 0.22 | 0.46 ± 0.23 | –0.06 (–0.17 to 0.05) |
| Fasting C-peptide divided by fasting glucose (pmol/ml per mmol/l)e | (N = 49) | (N = 45) | (N = 44) | (N = 36) | |
| 0.04 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.02 | 0.03 ± 0.02 | –0.01 (–0.02 to 0.00) | |
| Plasma glucose AUC (mmol/l)e | 12.6 ± 2.6 | 12.3 ± 2.1 | 14.2 ± 2.3 | 14.4 ± 3.0 | –0.4 (–1.9 to 1.0) |
| BMI percentile (%) | (N = 51) | (N = 46) | (N = 43) | (N = 37) | |
| 53 ± 29 | 51 ± 34 | 70 ± 26 | 68 ± 29 | 0.0 (–0.1 to 0.1) | |
| Blood pressure (mmHg)d | (N = 51) | (N = 46) | (N = 44) | (N = 37) | |
| Systolic | 110 ± 9 | 108 ± 8 | 113 ± 8 | 111 ± 9 | 2 (–2 to 6) |
| Diastolic | 65 ± 6 | 66 ± 8 | 65 ± 7 | 64 ± 8 | 1 (–2 to 4) |
| Lipid profile (mmol/l) d | (N = 46) | (N = 44) | (N = 42) | (N = 38) | |
| Total cholesterol | 4.5 ± 0.7 | 4.4 ± 0.7 | 3.9 ± 0.5 | 4.0 ± 0.7 | –0.1 (–0.3 to 0.1) |
| Triglycerides | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.3 | –0.1 (–0.2 to 0.1) |
| HDL cholesterol | 1.6 ± 0.3 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.3 | –0.1 (–0.2 to 0.1) |
| LDL cholesterol | (N = 46) | (N = 44) | (N = 41) | (N = 36) | |
| 2.5 ± 0.6 | 2.5 ± 0.7 | 2.1 ± 0.3 | 2.1 ± 0.6 | 0.0 (–0.2 to 0.2) | |
The proportion of time in target range 3.9–10.0 mmol/l based on masked LibrePro sensor glucose data at 12 months was 10 percentage points (95% CI 2 to 17) higher in the CL group (mean ± SD 64 ± 14%) compared to control group (mean ± SD 54 ± 23%) (Table 6). As this end point did not reach the threshold of 0.01 in the analysis, other key secondary end points were not tested for statistical significance. HbA1c was lower in the CL group by 4 mmol/mol (0.4%) [95% CI 0 to 8 mmol/mol (0.0% to 0.7%)] at 12 months. The mean difference in time spent < 3.9 mmol/l between groups was 0.9 percentage points (95% CI –1.0 to 2.8).
Secondary end points
Secondary end points for all randomised participants at 12 months are shown in Table 6.
C-peptide end points
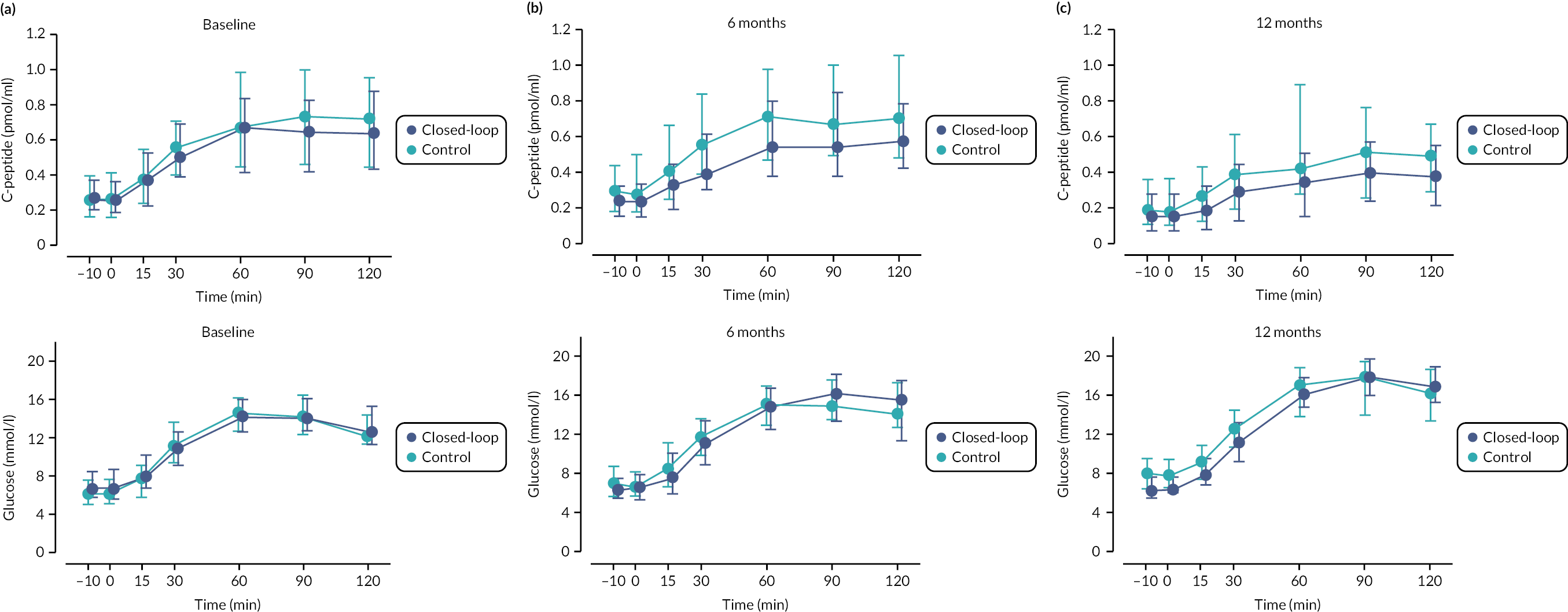
C-peptide AUC declined following diagnosis in both treatment groups (see Table 6, Figures 6 and 7). Plasma glucose AUC was similar between groups at 12 months. There was no difference in fasting C-peptide divided by fasting glucose between treatment groups at 12 months. The proportion of participants with negative C-peptide stimulation in response to mixed-meal test (defined as non-fasted C-peptide < 0.2 pmol/ml), increased in both groups from baseline to 12 months but was similar between treatment groups and is provided in Table 7.
FIGURE 6.
The area under the curve for plasma C-peptide in response to a mixed-meal tolerance test at baseline, 6 and 12 months post diagnosis of type 1 diabetes. Panel A; geometric mean [IQR] and the glycated haemoglobin from baseline to 12 months. Panel B; median [IQR]. C-peptide AUC at baseline (n = 49 CL, n = 45 control), 6 months (n = 45 CL, n = 38 control), 12 months (n = 46 CL, n = 37 control). HbA1c at baseline (n = 51 CL, n = 46 control), 3 months (n = 48 CL, n = 44 control), 6 months (n = 46 CL, n = 42 control), 9 months (n = 46 CL, n = 38 control), 12 months (n = 46 CL, n = 39 control).

FIGURE 7.
Stimulated C-peptide at MMTT at baseline (panel A), 6 (panel B) and 12 (panel C) months. Circles indicate the median, and the bars represent the 25th and 75th percentiles. Baseline (n = 49 CL, n = 45 control), 6 months (n = 45 CL, n = 38 control), 12 months (n = 46 CL, n = 37 control).
| Closed-loop | Control | |
|---|---|---|
| Baseline | (n = 51) | (n = 46) |
| 1 (2) | 1 (2) | |
| 6 months | (n = 45) | (n = 41) |
| 4 (9) | 3 (7) | |
| 12 months | (n = 46) | (n = 38) |
| 10 (22) | 5 (13) |
Glycaemic end points
End points by treatment group at 6 months is shown in Table 8. C-peptide AUC was lower in the CL group compared to the control group (0.51 vs. 0.70 pmol/ml). Time in target glucose range was higher (70 vs. 65%) and mean glucose lower (8.0 vs. 8.9 mmol/l) in the CL group compared to control group at 6 months but HbA1c was similar. Time in hypoglycaemia (< 3.9 mmol/l) was higher (6.1 vs. 4.2%) in the CL group compared to control group at 6 months.
| Closed-loop | Control | |
|---|---|---|
| C-peptide AUC (pmol/ml)a,b | (N = 45) | (N = 38) |
| 0.51 (0.36–0.71) | 0.70 (0.44–0.89) | |
| Time spent at glucose level 3.9–10.0 mmol/l (%) | (N = 44) | (N = 37) |
| 70 ± 15 | 65 ± 22 | |
| (N = 46) | (N = 42) | |
| HbA1c (%) | 6.7 ± 0.9 | 6.7 ± 0.9 |
| HbA1c (mmol/mol) | 49 ± 9 | 50 ± 9 |
| Time spent at glucose level < 3.9 mmol/l (%)c | (N = 44) | (N = 37) |
| 6.1 ± 5.7 | 4.2 ± 3.9 | |
| CGM end pointsd | (N = 44) | (N = 37) |
| Mean glucose (mmol/l) | 8.0 ± 1.9 | 8.9 ± 2.7 |
| Standard deviation (mmol/l) | 3.1 ± 0.9 | 3.3 ± 1.3 |
| CV of glucose (%) | 38 ± 6 | 36 ± 7 |
| Time spent at glucose level (%) | ||
| < 3.5 mmol/lc | 3.4 ± 3.4 | 2.3 ± 2.4 |
| < 3.0 mmol/lc | 1.4 ± 1.7 | 0.8 ± 0.9 |
| < 2.8 mmol/lc | 1.0 ± 1.3 | 0.5 ± 0.6 |
| > 10.0 mmol/lc | 23 ± 16 | 30 ± 21 |
| > 16.7 mmol/lc | 2.5 ± 3.6 | 4.8 ± 6.4 |
| Area above curve 3.9 mmol/l (mmol/l) | 0.7 ± 0.7 | 0.4 ± 0.4 |
| Area above curve 3.5 mmol/l (mmol/l) | 0.3 ± 0.4 | 0.2 ± 0.2 |
| HbA1c < 7.5%, n (%) | (N = 46) | (N = 42) |
| 38 (83) | 33 (79) | |
| Insulin end points (U/kg/day) | (N = 45) | (N = 40) |
| Total daily insulin | 0.73 ± 0.33 | 0.69 ± 0.41 |
| Total daily basal insulin | 0.33 ± 0.16 | 0.29 ± 0.20 |
| Total daily bolus insulin | 0.40 ± 0.23 | 0.40 ± 0.24 |
| Fasting C-peptide divided by fasting glucose (pmol/ml per mmol/l)b | (N = 44) | (N = 41) |
| 0.04 ± 0.02 | 0.05 ± 0.04 | |
| Plasma glucose AUC (mmol/l)b | 13.0 ± 2.7 | 13.3 ± 2.9 |
| (N = 42) | (N = 40) | |
| BMI percentile (%) | 66 ± 26 | 60 ± 33 |
| Blood pressure (mmHg) | (N = 46) | (N = 41) |
| Systolicc | 111 ± 7 | 109 ± 10 |
| Diastolicc | 64 ± 8 | 66 ± 8 |
Day and night glucose control is shown in Table 9. Daytime glucose control deteriorated in both groups from baseline to 12 months. Daytime glucose control measured by time in target glucose range and mean glucose was better in the CL group than the control group at 12 months. Night-time glucose control deteriorated in the control group from baseline to 12 months (72% at baseline and 57% at 12 months) but was maintained in the CL group (75% at baseline and 71% at 12 months).
| Daytime (8:00–23:59) | Night-time (00:00–07:59) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | |||||
| Closed-loop | Control | Closed-loop | Control | Closed-loop | Control | Closed-loop | Control | |
| (N = 50) | (N = 43) | (N = 44) | (N = 33) | (N = 50) | (N = 43) | (N = 44) | (N = 33) | |
| Time in range 3.9–10.0 mmol/l (%) | 73 ± 14 | 72 ± 13 | 60 ± 15 | 52 ± 23 | 75 ± 16 | 72 ± 17 | 71 ± 15 | 57 ± 26 |
| Mean glucose (mmol/l) | 7.3 ± 1.7 | 7.2 ± 1.7 | 8.9 ± 1.7 | 10.1 ± 3.4 | 7.0 ± 1.6 | 6.5 ± 1.9 | 7.5 ± 1.6 | 9.1 ± 3.1 |
| Glucose SD (mmol/l) | 2.8 ± 0.7 | 2.7 ± 0.8 | 3.7 ± 1.0 | 4.0 ± 1.5 | 2.4 ± 0.8 | 2.3 ± 0.9 | 2.9 ± 0.9 | 3.0 ± 1.2 |
| Time < 3.0 mmol/l (%)a | 1.5 ± 1.6 | 1.9 ± 1.9 | 0.9 ± 0.9 | 1.5 ± 1.8 | 2.6 ± 4.1 | 4.0 ± 4.8 | 2.2 ± 2.4 | 1.7 ± 2.5 |
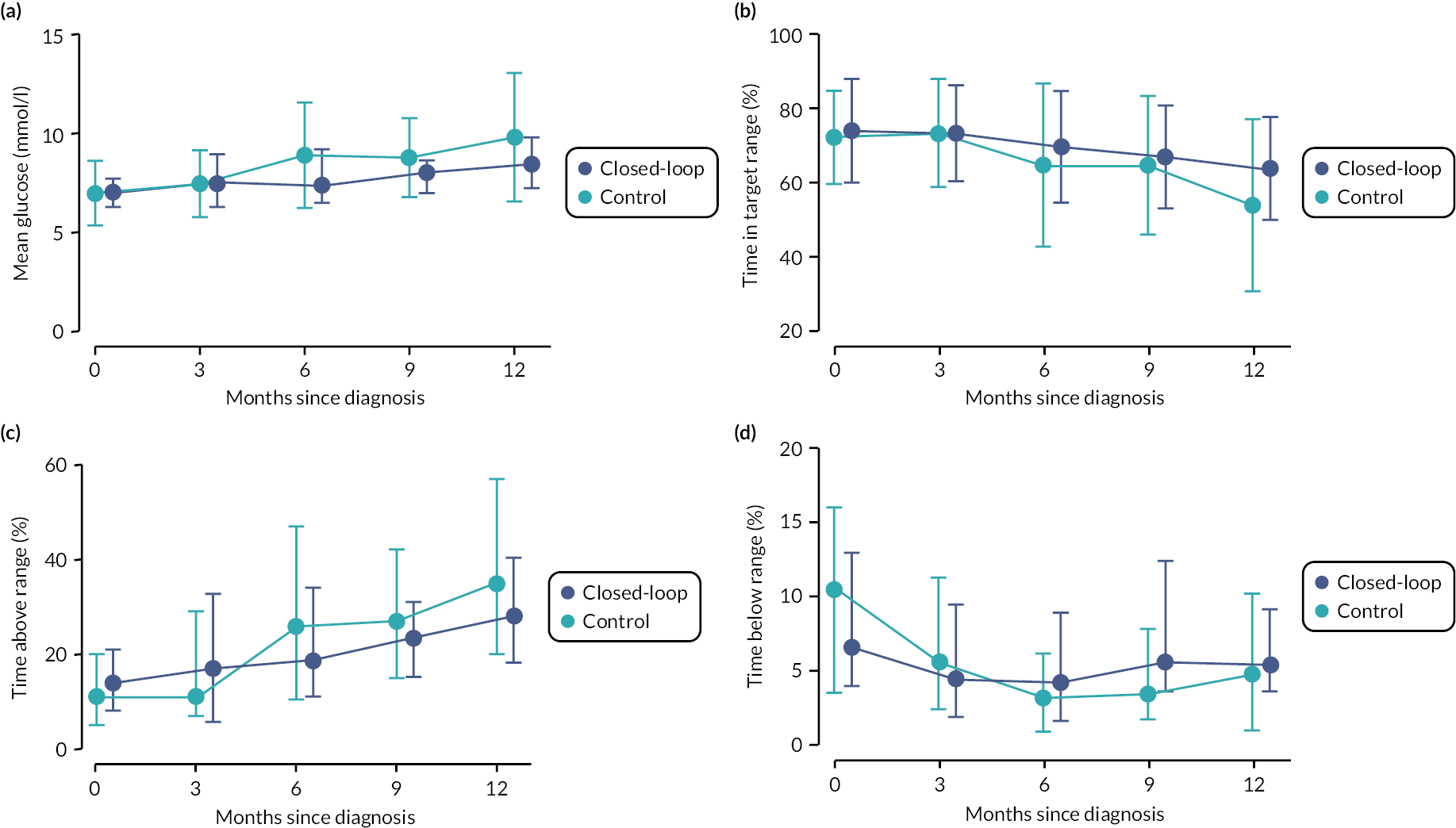
Longitudinal sensor glucose end points are shown in Table 10 and Figure 8. Although glucose control as measured by sensor glucose metrics time in range, time above range and mean glucose deteriorated in both groups from baseline to 12 months, this was more pronounced in the control group. There was a trend towards a reduction in the proportion of time spent in hypoglycaemia in both groups over time.
| Baseline | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|
| Closed-loop (N = 50), control (N = 43) | Closed-loop (N = 46), control (N = 41) | Closed-loop (N = 44), control (N = 37) | Closed-loop (N = 42), control (N = 35) | Closed-loop (N = 44), control (N = 33) | |
| Time in range 3.9–10.0 mmol/l (%) | |||||
| Closed-loop | 74 ± 14 | 73 ± 13 | 70 ± 15 | 67 ± 14 | 64 ± 14 |
| Control | 72 ± 13 | 73 ± 15 | 65 ± 22 | 65 ± 19 | 54 ± 23 |
| Mean glucose (mmol/l) | |||||
| Closed-loop | 7.2 ± 1.6 | 7.6 ± 1.6 | 8.0 ± 1.9 | 8.0 ± 1.6 | 8.5 ± 1.6 |
| Control | 7.0 ± 1.6 | 7.4 ± 1.7 | 8.9 ± 2.7 | 8.8 ± 2.0 | 9.8 ± 3.3 |
| Glucose SD (mmol/l) | |||||
| Closed-loop | 2.7 ± 0.7 | 2.8 ± 0.9 | 3.1 ± 0.9 | 3.3 ± 1.0 | 3.6 ± 0.9 |
| Control | 2.7 ± 0.8 | 2.7 ± 0.9 | 3.3 ± 1.3 | 3.4 ± 1.1 | 3.7 ± 1.4 |
| Glucose CV (%) | |||||
| Closed-loop | 38 ± 7 | 37 ± 7 | 38 ± 6 | 41 ± 8 | 42 ± 7 |
| Control | 39 ± 7 | 37 ± 6 | 36 ± 7 | 38 ± 7 | 39 ± 8 |
| Time < 3.9 mmol/l (%)a | |||||
| Closed-loop | 9.1 ± 6.3 | 6.3 ± 5.3 | 6.1 ± 5.7 | 7.5 ± 5.2 | 6.2 ± 3.8 |
| Control | 10.7 ± 7.1 | 7.0 ± 5.4 | 4.2 ± 3.9 | 4.4 ± 3.2 | 5.4 ± 4.7 |
| Time < 3.5 mmol/l (%)a | |||||
| Closed-loop | 5.2 ± 4.8 | 3.7 ± 3.8 | 3.4 ± 3.4 | 4.6 ± 4.0 | 3.6 ± 2.6 |
| Control | 6.6 ± 5.2 | 3.8 ± 3.7 | 2.3 ± 2.4 | 2.4 ± 2.1 | 3.3 ± 3.1 |
| Time < 3.0 mmol/l (%)a | |||||
| Closed-loop | 2.0 ± 2.5 | 1.6 ± 1.9 | 1.4 ± 1.7 | 2.2 ± 2.6 | 1.4 ± 1.2 |
| Control | 2.8 ± 2.8 | 1.3 ± 1.8 | 0.8 ± 0.9 | 1.1 ± 1.3 | 1.5 ± 1.6 |
| Time < 2.8 mmol/l (%)a | |||||
| Closed-loop | 1.3 ± 1.8 | 1.1 ± 1.4 | 1.0 ± 1.3 | 1.6 ± 2.1 | 0.9 ± 0.9 |
| Control | 1.9 ± 2.2 | 0.8 ± 1.1 | 0.5 ± 0.6 | 0.7 ± 0.9 | 1.1 ± 1.4 |
| Area over curve < 3.9 mmol/l (mmol/l)a | |||||
| Closed-loop | 1.0 ± 0.9 | 0.7 ± 0.8 | 0.7 ± 0.7 | 0.9 ± 0.8 | 0.7 ± 0.5 |
| Control | 1.3 ± 1.1 | 0.7 ± 0.7 | 0.4 ± 0.4 | 0.5 ± 0.4 | 0.6 ± 0.6 |
| Area over curve < 3.5 mmol/l (mmol/l)a | |||||
| Closed-loop | 0.5 ± 0.5 | 0.4 ± 0.5 | 0.3 ± 0.4 | 0.5 ± 0.5 | 0.3 ± 0.3 |
| Control | 0.6 ± 0.6 | 0.3 ± 0.4 | 0.2 ± 0.2 | 0.2 ± 0.3 | 0.3 ± 0.4 |
| Time > 10.0 mmol/l (%)a | |||||
| Closed-loop | 15 ± 9 | 19 ± 14 | 23 ± 16 | 24 ± 11 | 29 ± 14 |
| Control | 14 ± 10 | 18 ± 13 | 30 ± 21 | 30 ± 16 | 40 ± 25 |
| Time > 16.7 mmol/l (%)a | |||||
| Closed-loop | 1.0 ± 1.6 | 1.9 ± 2.7 | 2.5 ± 3.6 | 2.5 ± 2.7 | 4.2 ± 3.8 |
| Control | 1.0 ± 1.5 | 1.4 ± 2.4 | 4.8 ± 6.4 | 3.1 ± 3.1 | 10.0 ± 12.4 |
FIGURE 8.
Longitudinal glycaemic control from baseline to 12 months based on masked sensor glucose data collected by Freestyle LibrePro for up to 14 days. Panel A shows mean glucose levels. Panel B shows time in target glucose range 3.9–10.0 mmol/l. Panel C shows time with glucose above 10.0 mmol/l. Panel D shows time with glucose below 3.9 mmol/l. Full circles indicate the median, and the bars represent the 25th and 75th percentiles. Baseline (n = 50 CL, n = 43 control), 3 months (n = 46 CL, n = 41 control), 6 months (n = 44 CL, n = 37 control), 9 months (n = 42 CL, n = 35 control), 12 months (n = 44 CL, n = 33 control).
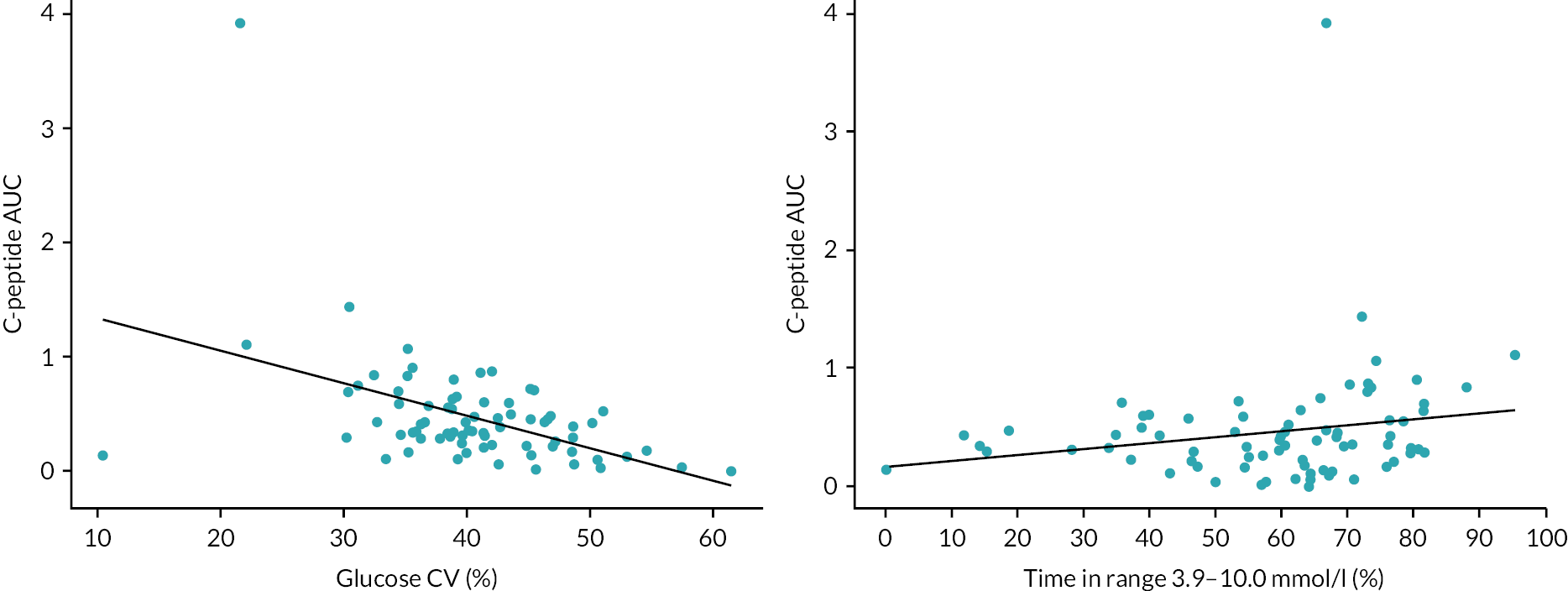
C-peptide AUC by HbA1c, coefficient of variation of glucose, and time in target range 3.9–10.0 mmol/l are shown in Table 11 and Figure 9. Higher C-peptide AUC was associated with lower glucose variability as measured by coefficient of variation of glucose, and higher time in target glucose range (see Figure 9). There was no clear relationship between C-peptide AUC quartiles and HbA1c quartiles (see Table 11).
| HbA1c (mmol/mol) quartiles | C-peptide AUC (pmol/ml) quartiles | |||
|---|---|---|---|---|
| < 0.16 | 0.16–< 0.32 | 0.32–< 0.49 | ≥ 0.49 | |
| < 46 | 1 (9) | 5 (42) | 2 (17) | 4 (36) |
| 46–< 50.5 | 6 (55) | 1 (8) | 1 (8) | 3 (27) |
| 50.5–< 57 | 3 (27) | 2 (17) | 4 (33) | 2 (18) |
| ≥ 57 | 1 (9) | 4 (33) | 5 (42) | 2 (18) |
FIGURE 9.
Relationship between glucose CV, time in range and C-peptide AUC at 12 months (CL and control participants combined). The black line represents the least squares linear regression line. Baseline (n = 48 CL, n = 42 control), 12 months (n = 44 CL, n = 32 control).
Insulin end points
Total, basal and bolus insulin dose were similar between treatment groups at 12 months (see Table 6). The ratio of basal to bolus insulin was higher in the CL group compared to control group at 12 months (1.2 vs. 0.8).
Clinical end points
Blood pressure, lipid profile and BMI percentile were similar between treatment groups at 12 months (see Table 6). Total and LDL cholesterol decreased and BMI increased in both groups from baseline to 12 months. There were no clinically relevant between-group differences in blood pressure, BMI percentile or components of lipid profile at 12 months.
Per-protocol analysis
The primary end point was similar in a per-protocol analysis using data from randomised participants in the CL group with at least 60% CL use and those in the control group who did not start insulin pump therapy (Table 12). The improved glycaemic control in the CL group compared to the control group was slightly more pronounced in the per-protocol analysis of key secondary end points.
| Baseline | 12 months | |||
|---|---|---|---|---|
| Closed-loop | Control | Closed-loop | Control | |
| Primary end point | ||||
| C-peptide AUC (pmol/ml)a | (N = 31) | (N = 37) | (N = 32) | (N = 32) |
| 0.54 (0.34, 0.70) | 0.65 (0.43, 0.81) | 0.33 (0.13, 0.43) | 0.47 (0.22, 0.70) | |
| Key end points | ||||
| Time in range 3.9–10.0 mmol/l (%)b | (N = 33) | (N = 37) | (N = 32) | (N = 29) |
| 76 ± 10 | 74 ± 11 | 68 ± 10 | 53 ± 24 | |
| (N = 33) | (N = 38) | (N = 32) | (N = 34) | |
| HbA1c (%) | 10.5 ± 1.6 | 10.5 ± 1.6 | 6.8 ± 0.7 | 7.3 ± 1.1 |
| HbA1c (mmol/mol) | 92 ± 17 | 91 ± 18 | 50 ± 7 | 57 ± 12 |
| Time with glucose < 3.9 mmol/l (%)b | (N = 33) | (N = 37) | (N = 32) | (N = 29) |
| 8.0 ± 5.9 | 11.2 ± 6.9 | 6.8 ± 4.0 | 5.3 ± 4.7 | |
Adverse events
Safety-related events are summarised in Table 13. Three severe hypoglycaemia events occurred in two participants randomised to the CL group and one severe hypoglycaemia event occurred in one participant randomised to control group. There was one DKA in the CL group and none in control group. Details of the events are in Table 14. Two non-treatment related SAEs occurred in the CL group and four in control group. A total of 71 other AEs (34 in the CL group, 37 in control group) were reported (Table 15).
| Closed-loop (N = 51) | Control (N = 46) | p-valuea | |
|---|---|---|---|
| Severe hypoglycaemic events, n | 3 | 1 | |
| Number of events per subject, mean ± SD | 0.06 ± 0.31 | 0.02 ± 0.15 | 0.39 |
| Incidence rate per 100 person-years | 3 | 1 | 0.41 |
| Number of subjects with ≥ 1 event, n (%) | 2 (4) | 1 (2) | > 0.99 |
| Diabetic ketoacidosis events, n | 1 | 0 | |
| Number of events per subject, mean ± SD | 0.02 ± 0.14 | 0.00 ± 0.00 | 0.97 |
| Incidence rate per 100 person-years | 1 | 0.0 | 0.97 |
| Number of subjects with ≥ 1 event, n (%) | 1 (2) | 0 (0) | 0.50 |
| Serious adverse events, n | 2 | 4 | |
| Number of events per subject, mean ± SD | 0.04 ± 0.20 | 0.09 ± 0.28 | 0.53 |
| Other AEs, n | 34 | 37 | |
| Number of events per subject, mean ± SD | 0.67 ± 1.18 | 0.80 ± 1.31 | 0.43 |
| Event | Treatment group | Details |
|---|---|---|
| Severe hypoglycaemia | Closed-loop | Participant administered meal-time bolus but did not finish the meal. Participant felt dizzy, collapsed and had seizures. |
| Severe hypoglycaemia | Closed-loop | Participant had a large carbohydrate meal and then walked up hill to school. Despite oral hypoglycaemia treatment the participant collapsed, had a hypoglycaemic seizure and required paramedic treatment. |
| Severe hypoglycaemia | Control | Participant administered pre-breakfast bolus but breakfast was delayed. The participant felt dizzy and shaky and required the parent to give dextrose tablets. |
| Severe hypoglycaemia | Closed-loop | Participant had hypoglycaemic seizure after participating in rugby and gymnastics without adjustment of meal-time bolus and inadequate treatment of hypoglycaemia when sensor alerted. |
| Diabetic ketoacidosis | Closed-loop | Participant developed abdominal pain and vomiting while fasting during Ramadan. Admitted to hospital in DKA with severe dehydration, contributed to by fasting and gastroenteritis. |
| Closed-loop (N = 51) | Control (N = 46) | |
|---|---|---|
| Skin or soft tissue | 7 | 12 |
| Upper respiratory tract symptoms | 7 | 9 |
| Headache or visual symptoms | 9 | 4 |
| Abdominal symptoms | 4 | 6 |
| Injury | 4 | 2 |
| Hypoglycaemia | 2 | 1 |
| Other | 1 | 3 |
Technology usage
In the CL group, CL use was 66% (IQR 44–80) over the 12-month period over the two CL platforms (Table 16). At 12 months, 10% of participants in the control group (n = 4) were using insulin pump therapy and 57% (n = 21) were using a flash or real-time continuous glucose sensor (Table 17).
| Baseline to 12 months (N = 50) | |
|---|---|
| Time using the CL system, n (%)a | |
| 0–< 20% | 3 (6) |
| 20–< 40% | 6 (12) |
| 40–< 60% | 8 (16) |
| 60–< 80% | 21 (42) |
| ≥ 80% | 12 (24) |
| Median (IQR) use (%) | 66 (44, 80) |
| 6 months | 12 months | |
|---|---|---|
| (N = 42) | (N = 39) | |
| Use of an insulin pump, n (%) | 4 (10) | 4 (10) |
| (N = 32) | (N = 37) | |
| Use of a glucose sensor, n (%)a | 17 (53) | 21 (57) |
Chapter 5 Discussion
The present study shows that CL glucose control over a period of up to 12 months does not slow the decline in C-peptide secretion in children and adolescents with new-onset type 1 diabetes.
Stimulated C-peptide declined in both treatment groups by 12 months. The proportion of participants with negative C-peptide stimulation in response to mixed-meal test also increased over time and was similar between treatment groups. The stimulated C-peptide at 12 months in the present study (0.35 pmol/ml in the CL group and 0.46 pmol/ml in the control group) are in keeping with those reported by Buckingham et al. where there was also no difference between a 3-day period of early intensive CL glucose control, initiated within the first 7 days following diagnosis and usual care (0.43 pmol/ml in the intensive group and 0.52 pmol/ml in the control group). 18 However, as glycaemic control in the Buckingham et al. study was similar between groups following the initial intensive period, the study was not able to determine the impact of a sustained period of optimised glucose control on C-peptide secretion. 18
Total daily exogenous insulin requirements, a surrogate marker of residual insulin secretion, were similar between groups at all time points after diagnosis. However, this comparison may be hampered by any between-group differences in glycaemic control.
Mean time in range was 10 percentage points higher and mean HbA1c was 0.4% (4 mmol/mol) lower in the CL group compared with the control group at 12 months, but these end points did not reach the pre-specified significance thresholds and it is possible that a greater improvement in glucose control with attainment of normoglycaemia could prevent the decline in C-peptide secretion. 46 Further work is needed to definitively rule out a role of glycaemic burden in the decline of C-peptide secretion. Additionally, the greater mean time below range and mean glycaemic coefficient of variation observed in the CL group may have reduced beta-cell viability. 47
It is likely that there are factors other than glycaemic control, such as autoimmune response that determine the rate of C-peptide decline following diagnosis of type 1 diabetes, and CL glucose control for 12 months following diagnosis is unable to preserve endogenous insulin secretion. It is possible that other factors act in concert with dysglycaemia on C-peptide secretion. Future research may utilise CL therapy to optimise glucose control as an adjunctive tool to evaluate the impact of immunotherapies on preserving residual C-peptide.
The present study demonstrates that hybrid CL therapy is effective in new-onset type 1 diabetes in youth and can safely accommodate the variability in exogenous insulin requirements which occur with beta-cell recovery post diagnosis. Glycaemic control was sustained over 1 year in the CL group, whereas glycaemic control started to deteriorate in the control group 6 to 9 months after diagnosis (see Figure 6). At 12 months post diagnosis, only 56% of youth in the control group (78% in the CL group) were able to achieve a HbA1c of < 58 mmol/mol (< 7.5%) which is above the current national and international glycaemic targets43,48 despite high uptake of diabetes technology in the control group. Analysis of data from the Epidemiology of Diabetes Interventions and Complications study suggests reduced risk of renal and cardiovascular complications with earlier implementation of intensive therapy compared to later implementation, despite similar overall glycaemic control. 49 This highlights the need for improved therapies to allow youth to achieve recommended glycaemic targets from onset of type 1 diabetes irrespective of the lack of effect on residual C-peptide secretion.
Strengths of this study include the multicentre, randomised parallel design and the 1-year study duration. We applied no exclusions at enrolment such as technology propensity or healthcare professional considerations about suitability, minimising selection bias. The study population is representative of the general population of youth newly diagnosed with type 1 diabetes. There were no limitations to diabetes therapies used in the control group supporting generalisability of the findings. The study had limitations. There was no central measurement of auto-antibodies at diagnosis. There was imbalance in the rate of DKA at diagnosis which is associated with a more rapid decline in C-peptide secretion. 50 The rate was higher in the CL group (33%) than in the control group (24%) but this was adjusted for in the analyses. Retention of participants was lower in the control group reflecting a lower level of motivation compared to the CL group, but this was within the anticipated withdrawal rate for the analysis of the primary end point. We did not undertake separate analysis of glycaemic control by CL system as participants switched systems at different time points which would limit any comparisons given the different time after diagnosis that this occurred. We recorded a higher number of unscheduled contacts in the CL group. Recording of these contacts was inconsistent longitudinally within and between clinical sites preventing coherent interpretation.
In conclusion, a sustained period of hybrid CL glucose control following diagnosis of type 1 diabetes in children and adolescents does not appear to prevent the decline in residual C-peptide secretion.
Chapter 6 Human factors assessments
We broadly refer to human factors as the emotional and behavioural characteristics of the participants in the study. The human factors assessment battery is grounded in two principles: (1) it is critical to use evidence-based methods that are reliable, valid, and have parallel forms for youth and their caregivers and (2) both quantitative (i.e. surveys) and qualitative (i.e. interviews) data need to be gathered to provide the richest, most comprehensive characterisation of the sample and their response to the CL system and clinical trial.
Questionnaires
Surveys and tests used in this trial are listed in Table 18. Participants/guardians completed the questionnaires at time points as indicated in the table. Additionally, feedback questionnaires on CL specific experience were distributed to participants/guardians randomised to the CL intervention arm.
| Measure | Respondent | Construct measured/relevant points | Time point |
|---|---|---|---|
| Paediatric Quality of Life Inventory (PedsQL) Diabetes51 | All youth and all parents | All youth ages 10–18 completed age-appropriate PedsQL Diabetes module. Parents also completed a proxy version. | Baseline, 12 months |
| Strengths and Difficulties Questionnaire (SDQ)52 | All youth and all parents | Self-report inventory behavioural screening questionnaire for children and adolescents. The same items are included in questionnaires for completion by the parents. | Baseline, 12 months |
| Hypoglycaemia Fear Survey (HFS)53–55 | All youth and all parents | Validated questionnaires (HFS child version, HFS parent version) to measure several dimensions of fear of hypoglycaemia. They consist of a ‘Behaviour subscale’ that measures behaviours involved in avoidance and over-treatment of hypoglycaemia and a ‘Worry subscale’ that measures anxiety and fear surrounding hypoglycaemia. | 12 months |
| INSPIRE | Youth and parents in CL arm | Measures the psychological side of automated insulin delivery. Child (6–12), adolescent versions (13–18) and parent versions. | 12 months |
| PAID-Teen | All youth | Measures related to the daily hassles of managing type 1 diabetes, and the degree of diabetes distress that arises from diabetes management. | 12 months |
Questionnaire results
Responses to the HFS (Table 19), PedsQL (Table 20), PAID (Table 21) and SDQs (Table 22) were similar in both children and parents between treatment groups at 12 months. Scores for the INSPIRE questionnaire were high in children, teenagers and parents suggesting positive expectancies regarding automated insulin delivery in this population (Table 23).
| 12 months | p-valueb | ||
|---|---|---|---|
| Closed-loop (N = 43) | Control (N = 37) | ||
| Child Questionnaire Total Score (range 0–92) mean (SD) | 28 (15) | 27 (12) | 0.95 |
| Behaviour Subscale Total Score (range 0–40) mean (SD) | 15 (7) | 16 (7) | 0.87 |
| Worry Subscale (range 0–52) Total Score mean (SD) | 13 (10) | 11 (7) | 0.64 |
| Parent Questionnaire Total Score (range 0–92) mean (SD) | 40 (14) | 41 (15) | 0.99 |
| Behaviour Subscale Total Score (range 0–40) mean (SD) | 18 (7) | 18 (7) | 0.99 |
| Worry Subscale Total Score (range 0–52) mean (SD) | 22 (9) | 22 (10) | 0.99 |
| Baseline | 12 months | p-valuea | |||
|---|---|---|---|---|---|
| Closed-loop (N = 51) | Control (N = 46) | Closed-loop (N = 44) | Control (N = 38) | ||
| Child Questionnaire Total Score (range 0–100) mean (SD) | 72 (13) | 68 (13) | 74 (13) | 71 (12) | 0.99 |
| Diabetes Subscale Total Score (range 0–100) mean (SD) | 62 (13) | 57 (15) | 65 (13) | 62 (13) | 0.99 |
| Treatment I Subscale Total Score (range 0–100) mean (SD) | 78 (13) | 77 (12) | 82 (14) | 77 (15) | 0.35 |
| Treatment II Subscale Total Score (range 0–100) mean (SD) | 85 (13) | 84 (12) | 89 (11) | 85 (12) | 0.45 |
| Worry Subscale Total Score (range 0–100) mean (SD) | 77 (19) | 69 (20) | 74 (22) | 71 (22) | 0.99 |
| Communication Subscale Total Score (range 0–100) mean (SD) | 82 (15) | 82 (16) | 79 (19) | 83 (17) | 0.64 |
| Parent Questionnaire Total Score (range 0–100) mean (SD) | 64 (15) | 65 (12) | 70 (12) | 65 (14) | 0.35 |
| Diabetes Subscale Total Score (range 0–100) mean (SD) | 58 (16) | 58 (15) | 64 (13) | 61 (16) | 0.95 |
| Treatment I Subscale Total Score (range 0–100) mean (SD) | 66 (16) | 69 (15) | 70 (12) | 63 (15) | 0.11 |
| Treatment II Subscale Total Score (range 0–100) mean (SD) | 75 (15) | 77 (14) | 84 (13) | 80 (15) | 0.37 |
| Worry Subscale Total Score (range 0–100) mean (SD) | 62 (19) | 58 (18) | 73 (17) | 65 (20) | 0.37 |
| Communication Subscale Total Score (range 0–100) mean (SD) | 72 (22) | 76 (21) | 68 (23) | 66 (24) | 0.73 |
| 12 months | p-valueb | ||
|---|---|---|---|
| CL (N = 26) | MDI (N = 20) | ||
| PAID-Teen Questionnaire Total Score (range 0–130) mean (SD) | 25 (19) | 23 (17) | 0.99 |
| Baseline | 12 months | p-valueb | |||
|---|---|---|---|---|---|
| Closed-loop (N = 51)a | Control (N = 46)a | Closed-loop (N = 46)a | Control (N = 38)a | ||
| Child Questionnaire Results mean (SD) | |||||
| Emotional Symptoms Subscale Total Score (range 0–10) | 2.4 (1.9) | 2.8 (2.0) | 2.4 (2.3) | 2.8 (2.1) | 0.69 |
| Conduct Problems Subscale Total Score (range 0–10) | 1.3 (1.2) | 1.8 (1.4) | 1.6 (1.6) | 1.8 (1.3) | 0.87 |
| Hyperactivity/Inattention Subscale Total Score (range 0–10) | 3.5 (2.6) | 4.1 (2.2) | 3.8 (2.5) | 4.2 (2.6) | 0.99 |
| Peer Relationship Problems Subscale Total Score (range 0–10) | 1.3 (1.4) | 1.7 (1.6) | 1.6 (1.2) | 1.9 (1.8) | 0.99 |
| Total Difficulties Score (range 0–40) | 8.8 (5.7) | 10.8 (5.4) | 9.5 (6.3) | 11.1 (6.1) | 0.99 |
| Prosocial Behaviour Subscale Total Score (range 0–10) | 8.2 (1.4) | 8.1 (1.4) | 7.9 (1.8) | 7.9 (1.5) | 0.99 |
| Internalising Problems Subscale Total Score (range 0–20) | 4.0 (3.4) | 4.9 (3.3) | 4.1 (3.6) | 4.9 (3.4) | 0.95 |
| Externalising Problems Subscale Total Score (range 0–20) | 4.8 (3.4) | 5.9 (3.3) | 5.4 (3.8) | 6.2 (3.8) | 0.99 |
| Impact Supplement Total Score (range 0–10) | 0.9 (1.1) | 1.0 (1.0) | 0.3 (0.5) | 0.8 (0.8) | 0.37 |
| Parent Questionnaire Results mean (SD) | |||||
| Emotional Symptoms Subscale Total Score (range 0–10) | 3.0 (2.3) | 3.0 (2.3) | 2.3 (2.2) | 2.7 (2.3) | 0.64 |
| Conduct Problems Subscale Total Score (range 0–10) | 1.3 (1.2) | 1.5 (1.2) | 1.5 (1.1) | 1.8 (1.5) | 0.99 |
| Hyperactivity/Inattention Subscale Total Score (range 0–10) | 3.5 (2.5) | 3.2 (2.7) | 2.7 (2.0) | 3.5 (2.6) | 0.35 |
| Peer Relationship Problems Subscale Total Score (range 0–10) | 1.7 (1.8) | 1.6 (1.8) | 1.8 (2.0) | 1.9 (1.9) | 0.95 |
| Total Difficulties Score (range 0–40) | 9.9 (6.2) | 9.6 (6.2) | 8.4 (5.5) | 10.2 (6.4) | 0.37 |
| Prosocial Behaviour Subscale Total Score (range 0–10) | 8.8 (1.4) | 8.3 (1.4) | 8.3 (1.7) | 8.2 (1.4) | 0.68 |
| Internalising Problems Subscale Total Score (range 0–20) | 4.9 (3.9) | 4.7 (3.8) | 4.2 (3.6) | 4.8 (3.5) | 0.60 |
| Externalising Problems Subscale Total Score (range 0–20) | 5.0 (3.3) | 4.8 (3.5) | 4.3 (3.0) | 5.4 (3.7) | 0.45 |
| Impact Supplement Total Score (range 0–10) | 1.6 (1.5) | 1.2 (1.3) | 1.0 (1.3) | 1.2 (1.5) | 0.81 |
| 12 months | |
|---|---|
| Child Questionnaire Mean Score (range 0–100) mean (SD) | 81 (12) |
| Teen Questionnaire Mean Score (range 0–100) mean (SD) | 83 (16) |
| Parent Questionnaire Mean Score (range 0–100) mean (SD) | 90 (11) |
Closed Loop from Onset in Type 1 Diabetes: qualitative substudy
Introduction
This report details work undertaken by Edinburgh University on two qualitative substudies conducted as part of the NIHR-funded Closed Loop from Onset in Type 1 Diabetes (CLOuD) trial and an interim analysis of data to provide feedback to the trial team while the qualitative work was ongoing.
In the original trial protocol, the Edinburgh team had proposed to carry out one study to compare the experiences of youths in the intervention (CL) arm, and their parents’ views, with those of youths and their parents taking part in the control [multiple daily injections (MDIs)] arm. Early consultation with the trial team highlighted the pressing importance of seeking staff perspectives on the implications for roll-out of CL technology in routine clinical care. In addition, a review of an extensive body of literature, which has explored this age group and their parents’ experiences of using MDI, indicated that no further primary research involving these groups was required. Following the consultation and literature review, a whole-team decision was taken to revise the aims and scope of the original substudy to no longer interview those in the MDI arm in order to focus on exploring the views and experiences of participants using closed loops and their parents’ views, and to compare these with youth MDI users’ and their parents’ accounts in the existing literature. In so doing, this enabled us to free up capacity to develop and undertake a second substudy to look at the experiences and views of staff members delivering the CLOuD trial and supporting participants in the CL arm.
In light of these changes and having sought requisite ethics approvals, the two qualitative substudies included: (1) a substudy which explored youth participants’ and their parents’ experiences of using the FlorenceM CL in everyday life; (2) a substudy which explored the views and experiences of health professionals who delivered the trial and provided support to study participants in the CL arm. The remainder of this document reports the aims, methods, key findings and recommendations which resulted from these two substudies. We also report interim feedback about CL users’ and parents’ experiences of recruitment and views about the training they received, which was given to the trial team while data collection was ongoing.
Study 1: participants’ and their parents’ experiences of using the FlorenceM CL system
Aims
Original aims: In the original protocol, it was proposed that the qualitative study would explore parents’ and youths’ views about using the FlorenceM CL system and how these compare to accounts of those using MDI to:
-
understand the impact of using CL systems (as compared to MDI) on diabetes management practices and everyday family life;
-
identify parents’ and youths’ information and support needs when using CL systems.
Revised aims: In line with the revisions to the protocol reported above, the qualitative study explored parents’ and youths’ views about using the FlorenceM CL system to:
-
understand the impact of using CL systems on diabetes management practices and everyday family life [as compared to accounts from users of conventional regimens (MDI and insulin pumps) and parents reported in existing literature];
-
Identify parents’ and youths’ information and support needs when using CL systems.
Methods
Recruitment, data collection and data analysis were successfully executed as detailed below – and in line with the study protocol. The qualitative work involved:
-
Telephone interviews with youths and their parents which took place 12 months after participants had commenced use of the FlorenceM CL to allow time for the technology to be embedded in everyday (family) life.
Participants
The proposed sample was achieved in full and comprised of 18 youths (aged 11–17 years) and 21 parents recruited from 6 of the 7 centres delivering the trial. These were: Addenbrooke’s Hospital, Cambridge; Alder Hey Children’s Hospital, Liverpool; Nottingham Hospital, Nottingham; Oxford Children’s Hospital, Oxford; Southampton Children’s Hospital, Southampton; and Royal Hospital for Sick Children, Edinburgh. No participants were recruited to the qualitative study from Leeds Teaching Hospital as none had gained 12 months’ experience using CL when the qualitative research was undertaken. Four youths declined to take part. This included two individuals who had withdrawn from the trial, and two who cited anxiety about speaking to the researcher. Purposive sampling was used to ensure diversity in terms of (1) youths’ age and gender, (2) parents’ occupation/education and (3) family forms. Demographic data are presented in Table 24.
| Characteristic | N (%) | Mean ± SD and range |
|---|---|---|
| Youths (n = 18) | ||
| Female | 9 (50) | |
| Age at time of interview – all children (years) | 13.9 ± 1.7 range 11–17 |
|
| Parents of adolescents (n = 21) | ||
| Female | 15 (72) | |
| Age at time of interview – all parents (years) | 46.6 ± 5.3 range 36–56 |
|
| Occupationa | ||
| Professional | 12 (57) | |
| Semi-skilled | 7 (33) | |
| Unemployed/Full-time carer | 2 (10) | |
Data collection
Interviews were conducted by DR, an experienced non-clinical qualitative researcher. Youths and parents were interviewed separately at a time of their choosing. Interviews were informed by topic guides which were developed to take into account the study aims, literature reviews, consultations with members of the trial team, and revised in light of emerging findings. Interviews took place between February 2018 and July 2019, typically lasted between 45 and 120 minutes, were digitally recorded, and transcribed in full.
Data analysis
Data were analysed by JL and DR using a thematic approach informed by the method of constant comparison. 56 Each researcher performed an independent analysis by repeatedly reading and cross-comparing all interviews to identify recurrent themes. Meetings were held to compare and reach agreement on key themes in order to develop a coding framework which captured these themes and contextual data needed to aid data interpretation. Data were coded using NVivo11, a qualitative software package (QSR International, Doncaster, Australia). Data sets were subject to further analyses to develop more nuanced interpretations and findings compared with existing literature which explored individuals’ experiences of using MDI regimens.
Findings
Findings are reported under each of the aims for the youth and parent substudy.
To understand the impact of using CL systems on diabetes management practices and everyday family life
This aim is addressed in material presented in the published paper57 and a summary given below. Throughout our reporting, we compare participants’ accounts with those reported by parents and youths who used standard insulin regimens (MDI or insulin pump) in existing published literature.
Most parents described how their child had taken on much of the responsibility for using the CL system to manage diabetes on a daily basis. This included tasks such as checking their glucose levels using the CGM reading, performing finger-pricks to monitor blood glucose levels and calibrate the CGM, counting carbohydrates and entering these data into the pump, administering bolus insulin doses and treating hypoglycaemia. Parents described having different types of input and involvement depending on their child’s needs and desire for independence. Many parents discussed providing help to count carbohydrates and maintain medical supplies and practical assistance to insert cannulas and sensors.
Participants did not report experiencing the types of diet-related family conflict reported by individuals using MDI regimens. 58–61 As youths indicated, having the CGM data made them aware of the impact of carbohydrate on glucose levels, which resulted in many reducing their intake of sugary foods and, as a consequence, making their own dietary choices without parents feeling they needed to interfere to encourage more ‘healthy’ options. Parents also reported feeling confident that the closed loop would administer basal insulin to correct rises in glucose levels or suspend insulin delivery to address low blood glucose if they or their child were to miscalculate carbohydrate in food.
Compared to studies involving MDI users,59,62–64 very few parents or youths reported having disagreements about the requirement to perform finger-pricks to monitor blood glucose. Youths reported benefiting from using the CGM as this reduced their need to perform painful finger-pricks. Parents described feeling relieved that their child no longer needed to perform finger-pricks and because they did not need to prompt or nag them to do these checks. Parents also described feeling reassured by the accuracy of the sensor if their child omitted doing finger-prick checks, for example before going to bed or when doing physical activity.
While family conflict was lessened as a result of using the closed loop, it was not ameliorated altogether. To ensure the closed loop operated effectively, parents reported often having to remind and/or prompt their child to carry out practical tasks, including: checking that the study handset was connected to the pump and kept within range; charging the phone; performing calibrations; responding to system alarms; and changing cannulas and sensors at appropriate intervals.
Youths discussed how using the closed loop had helped them to live a normal life. Some of this benefit was due to the component parts of the system as the CGM lessened youths’ need to check glucose levels in public and because the pump enabled insulin to be administered quickly and discretely. In addition, youths reported that the closed loop enabled them to better fit in with their friends, particularly when eating meals away from home, because the system would adjust the amount of insulin delivered in response to high/low glucose levels resulting from errors in carbohydrate counting. Similarly, parents described feeling confident and reassured that the closed loop would regulate their child’s glucose levels when they were socialising with friends.
Several youths reported feeling self-conscious when using the equipment in public or disliked having to wear and carry devices on their person. As parents noted, this could result in their child stopping doing some activities (e.g. swimming), choosing to wear clothes to cover devices in public settings or, in some public settings, using open-loop settings to reduce the likelihood of alarms going off (e.g. at school).
Unlike the accounts of individuals who use MDI regimens,62,64–66 youths rarely reported elevating their glucose levels due to fears about developing hypoglycaemia (e.g. before bed or doing physical activity). As youths indicated, the closed loop helped to limit the occurrence of hypoglycaemia in the daytime by reducing the rate of insulin delivery in response to falling glucose levels or aided their recovery from hypoglycaemia by adjusting the rate of insulin delivery to counter rises in glucose levels after treatment had been administered. All participants reported that the closed loop maintained glucose levels in range at night and would suspend insulin delivery if the young person developed hypoglycaemia. Parents reported benefiting from being able to check the graph on the handset to monitor their child’s readings without needing to disturb them when they were asleep. Parents also noted that they had slept better because they could use the graph to see that their child’s glucose levels were stable overnight which meant they did not feel it was necessary to wake them up in order to carry out further checks.
As previous studies have shown, youths exhibit two main styles of adaptation when diagnosed with type 1 diabetes. 63,67–69 Participants indicated how using the closed loop benefited both individuals who wanted to be in control of managing their diabetes and those who preferred to distance themselves from and sometimes neglected to perform self-management tasks. Parents of children in both groups noted that their child was adept at using smartphone and other digital technologies and had been quick to explore and become comfortable navigating the handset and pump screen interfaces. Similarly, youth in both groups described finding the CL technology straightforward to operate. Youths in the former category described feeling motivated by seeing how the closed loop was regulating their glucose levels on the handset graph. By reviewing their data, youths reported making informed decisions to administer correction doses to bring levels into range more quickly. Participants also reported using the data on the handset to inform decisions to increase or decrease insulin-to-carbohydrate ratio settings on the pump, usually by seeking health professionals’ support or, in a few cases, by making changes independently. In the latter category, participants described how the components and automated features of the closed loop helped to create distance and lessen the feeling of being controlled by having to manage type 1 diabetes. This was because users could minimise their involvement and rely on the system administering and suspending insulin delivery to address high and low glucose readings.
To identify parents’ and youths’ information and support needs when using closed-loop systems
Training: As indicated in the interim feedback provided to the Cambridge team (see below), participants reported receiving comprehensive training before transitioning to the closed loop. However, some parents reported feeling overloaded soon after diagnosis, being sleep-deprived, and struggling to assimilate information about the closed loop. Participants suggested that staff deliver training on the CGM, pump and CL algorithm on separate days to allow time for them to become familiar with each of the component parts.
Support needs: Participants reported receiving comprehensive and age-appropriate support from appropriately trained staff at their local site during both clinic and trial visits. Participants who discussed having contacted staff using the 24-hour telephone number described receiving prompt feedback to address issues which included: responding to system alarms, adjusting insulin-to-carbohydrate ratios, changing settings on the pump, support to use the closed loop in schools, and addressing system failures including replacement of failed components. Parents indicated that staff were very responsive to their requests for support and would, if necessary, contact the main trial team to seek information on their behalf. Parents suggested that future users would benefit from having access to a 24-hour helpline to resolve problems and, if required, seek replacement components. Several youths and parents suggested that potential future users might benefit from speaking with individuals who had experience of using a closed loop to gain an ‘insider’ perspective to inform decisions about using the technology.
Future development of CL systems: In keeping with findings from other studies,40,70 participants suggested that future CL systems could be developed which used integrated, lighter and more portable devices (e.g. by integrating the CL algorithm within the pump). Youths and parents also suggested that future systems include an app to run the control algorithm which users could host on their own smartphone or smartwatch. Several participants felt that future systems could be developed to allow users to more easily adjust the number of alarms/alerts issued by the closed loop.
Study 1: interim analysis
At the request of the trial team, an interim analysis of parent and youth interviews was conducted while data collection was being undertaken. This was to inform ongoing recruitment to the trial and identify ways to improve the training and support given to newly recruited participants and their parents. As reported below, most youths and parents raised concerns about how their lives might be affected after handing back the closed loop at the end of the trial. We decided to report this important finding to the trial team so, if necessary, they could prepare to manage participants’ distress at close-out and develop strategies to mitigate this. It subsequently transpired that this feedback was unnecessary because, at the CLOuD Annual Meeting in 2018, the trial team advised that participants would have the opportunity to continue using a closed loop for a further 2 years if they opted to take part in an extension phase of research. In addition to this finding, the interim analysis identified the following issues on trial conduct:
-
Making approaches to parents/participants post diagnosis. Some parents speculated that information about the trial and timelines for deciding whether to take part might get ‘lost’ when people are confronted by the shock of diagnosis, medical response and care needed/received by the young person, and the amount of training provided to enable people to be discharged and self-manage thereafter. Keen to ensure that others benefited from the opportunity to participate in the trial, parents suggested that members of the trial team follow-up more often after making initial approaches to prompt people who might have forgotten they had been invited to consider whether they/their child would like to take part.
-
Training was very comprehensive but could, at times, be overwhelming. It was suggested that staff take care to avoid over-burdening parents and participants who might be feeling very emotional and sleep-deprived, and who have difficulty assimilating new information. It was also suggested that trial staff deliver pump and CGM training on separate days to avoid overloading participants.
-
Use of the exercise function (temporary basal rate). Approximately half the participants were aware of and/or had used the exercise function. Those who were unaware of this function indicated that they would raise this with members of the trial team when they next attended clinic.
-
Concerns about the time taken to despatch/receive new equipment when a component of the CL breaks or malfunctions. Parents indicated that they had sometimes waited over a week for new equipment to be ordered/despatched and set up for their child. Others highlighted delays in the time taken to receive advice if staff at local sites were unable to answer questions. Parents noted that it could take a significant amount of time for local staff to relay an issue/problem to Cambridge and receive a response before information was relayed back to them.
-
Concerns for the future. Most participants raised issues about what would happen at the end of the trial when CL equipment was to be handed back. Some parents highlighted concerns about children sitting exams close to the trial end date and disruption which might ensue were they to switch to a new regimen. Most parents reported that their child would be considered for a pump; however, none indicated that they had any firm commitments in place. A few indicated that they were considering self-funding a CGM when the trial ends. Others reported that they had heard that the trial might continue for longer than originally planned and expressed a hope that their child might be able to continue using the closed loop. Some raised concerns that it might be unethical to remove the closed loop from their child.
At the request of the Cambridge team, the Edinburgh team undertook three dissemination activities to enable these interim findings to be cascaded to staff and other individuals and fed back into the conduct and delivery of the trial. These were as follows:
-
Summary document of interim analysis (July 2018). This was circulated to staff at each of the seven sites delivering the trial.
-
Briefing document sent to Cambridge (July 2018). This document detailed participants’ anxieties and concerns about returning the closed loop at the end of the trial.
-
Presentation delivered at the CLOuD Annual Meeting (November 2018).
Recommendations: study 1
Findings from study 1 were discussed at the annual CLOuD meeting in November 2019 and the following recommendations generated:
-
Diabetes teams should ensure that sufficient time and resources are made available to provide training and support on CL technology to individuals newly diagnosed with type 1 diabetes. It is recommended that training on each component (CGM, insulin pump and closed loop) be provided in different sessions at a pace appropriate to the individual.
-
Staff should encourage family members (parents) to engage with the data on the handset to ensure that youths keep component devices in range of each other, are regularly bolusing for meals, and to provide support and encouragement.
-
Staff should discuss with young people and their parents situations in which family conflict may arise, for example when prompts are required to carry out practical and diabetes-related tasks and highlight their importance to ensure the closed loop operates effectively.
Study 2: staff members’ experiences of delivering the CLOuD trial and providing support to trial participants using the closed loop.
Aims
The aims for the study involving staff members delivering the trial were to:
-
Understand and explore the benefits, issues and challenges arising from introducing and using CL systems to support diabetes self-management among youth with type 1 diabetes and parents/carers who assist their child with diabetes management.
-
Establish what information, training and resources health professionals may need to help individuals using a CL system achieve optimal glycaemic control in routine clinical care.
-
Explore health professionals’ views about which individuals gain greatest clinical benefit from using a CL system and who should be prioritised for access to the technology in routine clinical care.
Methods
Recruitment, data collection and data analysis were successfully executed as detailed below. The qualitative work involved:
-
Telephone interviews with health professionals which took place after staff had gained at least 6 months experience of delivering CLOuD in their site.
Participants
The proposed sample was achieved in full and comprised 22 health professionals (7 doctors, 9 diabetes specialist nurses and 6 research nurses) actively involved in delivery of training and care to participants (and parents) in the CL arm of the trial. Participants were recruited from all seven participating centres. Participants’ characteristics are presented in Table 25.
| CLOuD sites | 7 |
| Total number of interviewees | 22 |
| Interviewees per site – range (mode) | 1–5 (4) |
| Role, n (%) | |
| Diabetes consultants | 7 (32) |
| Diabetes nurses | 9 (41) |
| Research nurses | 6 (27) |
| Number of staff with previous CL experience | 5 (23) |
Data collection
Interviews were undertaken by BK, an experienced non-clinical qualitative researcher. Interviews were informed by a topic guide which was developed in light of literature reviews, inputs from clinical coinvestigators and revised in response to emerging findings. Interviews took place between August 2018 and June 2019, averaged 70 minutes, were digitally recorded and transcribed in full.
Data analysis
Data were analysed thematically using the method of constant comparison56 by JL and BK. Individual interviews were read through repeatedly before being cross-compared to identify issues which cut across different accounts. Each researcher undertook separate analyses and wrote separate reports before meeting to discuss and reach agreement on key themes and develop a coding frame that captured these themes. The qualitative analysis software package NVivo10 (QSR International, Doncaster, Australia) was used to facilitate data coding and retrieval. Coded data sets were subjected to further analysis to allow more nuanced interpretations of the data and identify illustrative quotations.
Findings
Findings are reported under each of the aims for the staff sub study. Aims 1 and 2 are reported jointly and addressed in material presented in the attached paper,71 while Aim 3 is addressed in a further paper. 72 A summary for each of these aims is given below:
-
Understand and explore the benefits, issues and challenges arising from introducing and using CL systems to support diabetes self-management among youth with type 1 diabetes and parents/carers who assist their child with diabetes management.
-
Establish what information, training and resources health professionals may need to help individuals using a CL system achieve optimal glycaemic control in routine clinical care.
Staff reported finding it straightforward to teach families how to use the closed loop because many of the people they encountered were familiar with, and felt confident about, using technology in everyday life. However, staff noted that more time was required to teach families with a new diagnosis of diabetes how to use each of the CL component devices because they only had a basic understanding of diabetes management compared to individuals with longer durations of diabetes who they would usually support at clinic. After experimenting with different educational approaches, staff concluded that training was best delivered by introducing the system’s component parts one-by-one at a pace appropriate to the individual.
After being set up on the system, staff noted that people initially required more support to become confident in using the CL devices than individuals using other regimens. However, staff explained that after an initial period of adjustment, users subsequently sought less clinical input than was typically needed by people using MDI or Continuous Subcutaneous Insulin Infusion (CSII) because using the closed loop meant there was less need to regularly review data to adjust insulin titration. Hence, the increased support required during set-up was offset by less need for clinical support thereafter. However, staff observed that reduced levels of contact could have unanticipated consequences because they had fewer opportunities to proactively educate users and parents how to interpret patterns in glucose data to inform adjustments to basal rates and insulin-to-carbohydrate ratios. Staff also described how having less contact impacted on their ability to identify whether youths also required psychological support. Some staff suggested that using the closed loop might have delayed the time taken by some participants to emotionally adjust to having diabetes because it masked much of the work involved in diabetes self-care using conventional regimens.
Staff noted that the closed loop could heighten some parents’ existing anxieties because they had access to near real-time glucose data. Staff described how this could result in parents micromanaging by over-riding settings and, hence, interfering with the system’s ability to adapt to a user’s individual insulin requirements. Worried parents were also reported to have placed excessive demands on staff by contacting them to discuss what they erroneously perceived as dangerous glucose levels. As a result, staff described the importance of educating parents about glucose fluctuations and managing people’s expectations about glucose stability when using a CL system.
Staff considered themselves proficient with the pump and sensor technology and described finding it straightforward to learn how to operate the handset containing the CL algorithm. Many suggested that all team members involved in supporting patients using a closed loop in routine clinical care be provided at least basic training which could be delivered via webinars, recorded TED talks, in-depth training from device manufacturers, an accredited training scheme and competency assessments. All staff emphasised the importance of being given time to become familiar with and feel confident using the technology prior to using it in clinical practice. They suggested future staff would benefit from having access to simulation equipment and a demonstration system to support families, alongside a manual for ongoing reference. Staff also suggested that structured guidance on how best to advise CL users be issued, including on atypical cases (e.g. active individuals or non-routine events such as travel across time zones).
To support CL users in routine clinical care, staff emphasised that health professionals must be proficient in advising patients how to use insulin pumps and sensors. Some raised concerns that this expertise was currently not always available in all diabetes centres and suggested CL care be delivered by specialist centres. Conversely, others indicated that local diabetes teams should be conversant in CL technology to ensure users receive appropriate care and support during emergencies. However, staff felt that a reliable throughput of patients using the closed loop would be necessary to help develop and maintain an appropriate skills base. Some suggested having national standards in place to guide resource requirements for teams providing support to CL users. Staff differed in their views about the provision of out-of-hours support. While some indicated that a 24-hour national helpline could be developed, others felt this could result in problems if health professionals were unfamiliar with individual patients.
Staff suggested that the roll-out of CL systems in routine clinical care might affect current levels of resource because longer clinic appointments would be required to download and review data. Relatedly, staff noted that centres would require robust IT systems to facilitate working with large downloads of data. Staff anticipated that the introduction of closed loops to routine clinical care would present similar challenges in terms of training needs and managing patient expectations to those encountered when other new regimens were introduced.
-
Explore health professionals’ views about which individuals gain greatest clinical benefit from using a CL system and who should be prioritised for access to the technology in routine clinical care.
Staff perceived CL systems as offering better and more fine-tuned glycaemic control than is possible using other regimens. However, for the glycaemic benefits to be realised, staff noted that essential tasks (e.g. changing cannulas, calibrations) required time and effort to be done correctly to enable youths to optimise their use of the technology. Staff also highlighted the importance of family involvement by having parents engage with the data on the handset to ensure that youths kept component devices in range of each other, were regularly bolusing for meals, and to provide ongoing support and encouragement.
Staff described having held strong and seemingly stereotypical views about the kinds of people who would use the technology most effectively in advance of the trial. However, staff reported that these views were challenged by observing individuals who engaged with the closed loop in ways they had not expected. For example, staff described having assumed that individuals who are well-educated and familiar with technology might be better candidates for CL technology. While this was sometimes found to be the case, staff reported examples of well-educated individuals over-interacting with the system resulting in a detrimental impact on glucose control. Others noted how families which might have been considered less able candidates were willing to follow guidelines without interfering and experienced greater glycaemic benefits from using the technology. Staff also reported finding it a challenge to gauge family dynamics to determine whether the young person would receive the practical and emotional support needed to use the system effectively. Furthermore, some noted how using the closed loop could act as a tipping point and lead to increased levels of engagement in diabetes self-management.
Staff concluded that individual, family, and psychological attributes are inappropriate pre-selection criteria and, ideally, all individuals should be given the chance to try the technology. Based on their experiences, staff recommended individuals be given a probationary period to ascertain whether the closed loop is being used effectively to achieve better glycaemic control than was possible using less-costly regimens (e.g. MDI). They also suggested the need for a longer-term review to ensure the system continued to be used safely and effectively, and to ensure that key self-management behaviours (e.g. bolusing for meals) were not being relaxed over time.
Staff indicated that clinical guidelines will be needed to inform difficult decisions about who should be prioritised and given access to closed loops in routine clinical care. Staff discussed how teenagers would benefit due to the physiological and social changes experienced by this age group. However, staff suggested a more pressing case could be made for access to be given to young children and infants due to unpredictable eating habits, requirement for tiny and variable doses of insulin and because this group would have diabetes for the longest and would benefit most from the improved glycaemic control afforded by the closed loop. To facilitate roll-out for this younger age group, staff suggested that an adapted system would be necessary with the algorithm integrated into the pump or another easily transportable device. When considering adults, some staff indicated that those already meeting clinical (e.g. NICE) criteria for insulin pump therapy should be prioritised for access. This included pregnant women and those with problematic hypoglycaemia. However, others contended that no-one should be excluded from being given access to a closed loop because of its potential to offer better glycaemic control and improved quality of life for all groups.
Recommendations: study 2
The following recommendations include those reported in the two papers detailing staff experiences. These were discussed with the investigator team at the annual CLOuD meeting in November 2019 and their feedback was incorporated into the final set of recommendations:
-
Each service delivery hub should have at least one health professional with formal training in CL technology. Health professionals wishing to provide care to users of CL systems should undergo accredited training and evidence relevant continued professional development.
-
Develop formal standards, which set out the core competencies expected of health professionals delivering CL system education and care.
-
Health professionals should be encouraged and enabled to access existing technology training to help them acquire the necessary skills and confidence to support CL technology upon its wider roll-out. To support the roll-out of CL technology, this might include: organisational and managerial support through funding and study time, placements, mentorship schemes, peer support and professional development using video-conferencing facilities.
-
The roll-out of CL technology into routine clinical practice should include the following training and support:
-
Device manufacturers could provide accredited training options, including online resources that enable flexible access for busy professionals.
-
Manufacturers could look to develop simulation CL systems to facilitate individual learning and understanding, and to promote awareness and adoption of this technology among the wider community of diabetes professionals.
-
-
In addition to training staff in specialist centres, consideration should be given to providing training to health professionals in local diabetes teams who support CL users, as they are typically the first port-of-call for diabetes-related emergencies:
-
Local diabetes teams should be empowered to deliver CL system care, which will require access to comprehensive technology training, standardised clinical guidance and appropriate resourcing.
-
-
Develop a structured, stepwise education package for CL system users and clinical guidance to support CL system consultations.
-
To ensure fair and equitable access to CL systems in routine clinical care, health professionals should be encouraged to explore any prejudicial and stereotypical assumptions they may have and given support to overcome these:
-
To help inform staff decision-making, case studies of individuals benefiting from the technology who do not conform to health professionals’ preconceptions of candidacy could be used as part of their training.
-
-
To help individuals adopt and use CL technology appropriately, staff should explore their initial understandings of the CL system in order to clarify its capabilities and limitations.
-
When individuals begin to use a closed loop after diagnosis, staff should be aware of the following:
-
Individuals using closed loops may require less clinical input than is typically required by those using CSII and MDI. Hence, diabetes teams should ensure that, like other individuals newly diagnosed with type 1 diabetes, people who use a closed loop soon after diagnosis are given access to high-quality, structured education programmes.
-
Reduced contact with users, coupled with the CL system’s ability to rectify (and therefore mask) suboptimal self-management practices (e.g. meal boluses being miscalculated or omitted), might compromise timely detection of issues including: emotional adjustment or psychosocial problems. Staff should ensure that new users of CL systems, particularly adolescents, receive ongoing psychological assessment and referral to psychological services if required.
-
-
Staff should provide individuals with a regular and ongoing review to ensure that CL technology is being used optimally. Staff should be aware that the system’s ability to compensate for behaviour lapses, such as missed boluses, might lead to self-management tasks being neglected over time. Hence, regular, ongoing review of individuals using a CL system will be essential, in line with recommendations for those using other diabetes technologies:
-
Staff should provide an ongoing review that is responsive to, and supportive of, an individual’s personal needs as users are more likely to be receptive to receiving tailored advice delivered using language which is not stigmatising.
-
-
Staff should be given guidance about removing CL systems from individuals who do not use them in clinically and cost-effective ways over time.
-
Healthcare providers should consider whether service reconfiguration, reallocation of staffing and resources, or improvements to IT infrastructure may be required to ensure that local teams can deliver care for CL users:
-
Local diabetes teams should ensure that robust IT infrastructure is in place to facilitate the large data downloads that inform the clinical input and advice given to system users.
-
Chapter 7 Final conclusions
The CLOuD study demonstrates, applying a multicentre, randomised parallel design, that a sustained period of hybrid CL glucose control over a period of 12 months does not slow the decline in C-peptide secretion in children and adolescents with new-onset type 1 diabetes. It is possible that a greater improvement in glucose control with attainment of normoglycaemia could prevent the decline in C-peptide secretion. However, it is likely that factors other than glycaemic control, such as autoimmune response, determine the rate of C-peptide decline following diagnosis of type 1 diabetes.
We have shown that hybrid CL therapy is effective in new-onset type 1 diabetes in youth and can safely accommodate the variability in exogenous insulin requirements which occur with beta-cell recovery post diagnosis.
Glycaemic control was sustained over 12 months in the CL group, whereas glycaemic control started to deteriorate in the control group 6–9 months after diagnosis, highlighting the need for improved therapies to allow youth to achieve recommended glycaemic targets from onset of type 1 diabetes irrespective of the lack of effect on residual C-peptide secretion.
Qualitative assessments demonstrate that CL has life-enhancing consequences for both adolescents and parents when initiated from onset of type 1 diabetes and helps to reduce the biographical disruption of type 1 diabetes in this age group.
Chapter 8 Equality, diversity and inclusion
Participant representation
We applied no exclusions at enrolment such as technology propensity or healthcare professional considerations about suitability, minimising selection bias. We included clinical sites and Participant Identification Centres in areas where children and young people from diverse backgrounds attend clinic. The participants in the present trial demonstrated the expected ratio of male to female children and young people. The proportion of participants from ethnic minorities enrolled in the trial was 14%; approximately 15–20% of children and young people with type 1 diabetes in the UK are from ethnic minority backgrounds. The presence of DKA at diagnosis in the present trial was 28%, which is similar to that expected of a UK population. The study population are representative of the general population of youth newly diagnosed with type 1 diabetes.
The research team
There was a wide range of experience and expertise across the research team and opportunities were provided for more junior members of the team. A parent of a young person with type 1 diabetes was a member of the Trial Steering Committee (TSC) and provided valuable insight throughout the duration of the trial.
Chapter 9 Patient and public involvement
Throughout the duration of the CLOuD project to date, numerous activities involving people living with diabetes and the public have been undertaken.
Patient and public engagement activities:
-
CLOuD was publicised in the Nottingham Children’s Hospital Newsletter (Spring 2017).
-
Twitter hashtag #cloudT1D created for publicising news about CLOuD recruitment and study progress.
-
The details of the CLOuD study were mentioned by Professor Roman Hovorka in an interview published in a Diabetes special supplement of The Telegraph newspaper in June 2017.
-
Professor Hovorka and a study participant were interviewed for the BBC television programme Trust me, I’m a Doctor, aired in January 2018.
-
A public study website has been developed.
-
Professor Hovorka was nominated for The Sun’s Who Cares Wins award on 8 October 2018 for his work on the artificial pancreas.
-
Dr Charlotte Boughton and Ms Janet Allen (Professor Hovorka’s Group) were invited on to the Today programme on BBC Radio 4 to talk about the artificial pancreas in type 1 diabetes on 4 October 2018.
-
Dr Martin Tauschmann (Former Clinical Research Associate in Professor Hovorka’s Group) was awarded ‘Young Investigators Award’ at the International Society for Pediatric and Adolescent Diabetes (ISPAD) Annual Conference held in India on 14 October 2018. CLOuD was not mentioned specifically, but it is excellent publicity for artificial pancreas and type 1 diabetes research.
-
Dr Rachel Besser (PI at Oxford) was interviewed by BBC Oxford on 14 November 2018 for World Diabetes Day to speak about the CLOuD study.
-
Article published in the Oxford Mail, Banbury Guardian and The Mail on Sunday on 25 November 2018 about the artificial pancreas and participants’ experience of being in the CLOuD study.
-
Her Royal Highness the Duchess of Cornwall visited Cambridge University Hospital on 27 November 2018 to see the world-leading type 1 diabetes technology being developed at the Cambridge Clinical Research Centre by Professor Roman Hovorka.
-
Professor Roman Hovorka was awarded a British Citizen Award for his work on the artificial pancreas on 24 January 2019. The event was hosted at the Palace of Westminster.
-
Type I Diabetes: The Rise of the Machines Conference, 2 February 2019 – Professor Roman Hovorka engaged in a presentation about CL research in London.
-
Press coverage for CLOuD with an interview of the first recruited participant at the Oxford site by ITV Meridian South News broadcast on 7 February 2019.
-
The father of one of the Nottingham CLOuD participants took part in the London Marathon on Sunday 28 April 2019 for JDRF as an appreciation of the benefits received for his child from the JDRF work.
-
One of the Nottingham CLOuD participants visited 10 Downing Street to attend an event hosted by the then prime minister Theresa May on 24 June 2019 to celebrate the work of NHS staff and diabetes charities.
-
The parents of two siblings who have been diagnosed with type 1 diabetes (one child is enrolled in the study and randomised to CL, and the other child is not in the study but is using standard insulin therapy) reported the excellent performance of the CL system in comparison to multiple daily insulin injections while they were doing Hadrian’s Wall Walk. They raised £3500 for JDRF.
-
The mother of a child with type 1 diabetes is a member of the TSC and actively participates in the 6-monthly TSC meetings, providing valuable insights from a patient perspective as to the conduct of the study.
-
During the design of the new CamAPS FX CL app, the study team held focus groups with people with type 1 diabetes (children, teens and adults) and caregivers of children with type 1 to help inform the developers on the design and functionality of the new app.
Chapter 10 Impact, outputs and dissemination
An extensive multistranded dissemination strategy was undertaken to ensure that outputs from the study reach wide-spread audiences and relevant stakeholders. Manuscripts from the study have been published in high-impact open access peer-reviewed journals.
Publications
The primary manuscript has been accepted for publication in the New England Journal of Medicine (July 2022). The protocol paper was published in BMJ Open. The qualitative evaluation has led to five publications in high impact peer reviewed journals. Data from CLOuD study participants have also been included in several secondary analyses with several more underway.
-
Boughton CK, Allen JM, Ware J, Wilinska ME, Hartnell S, Thankamony A, et al. ; on behalf of the CLOuD Consortium. Closed-loop and preservation of C-peptide secretion in type 1 diabetes. Accepted in NEJM.
-
Boughton C, Allen JM, Tauschmann M, Hartnell S, Wilinska ME, Musolino G, et al. ; CLOuD Consortium. Assessing the effect of closed-loop insulin delivery from onset of type 1 diabetes in youth on residual beta-cell function compared to standard insulin therapy (CLOuD study): a randomised parallel study protocol. BMJ Open 2020;10(3):e033500.
-
Rankin D, Kimbell B, Hovorka R, Lawton J; on behalf of the CLOuD Consortium. Adolescents’ and their parents’ experiences of using a closed-loop system to manage type 1 diabetes in everyday life: qualitative study. Chronic Illn 2021:1742395320985924.
-
Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, Boughton CK, et al.; on behalf of CLOuD Consortium. What training, support and resourcing do health professionals need to support people using a closed-loop system? A qualitative interview study with health professionals involved in the Closed Loop from Onset in Type 1 Diabetes (CLOuD) trial. Diabetes Technol Ther 2020;22(6):468–75.
-
Lawton J, Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, et al.; on behalf of the CLOuD Consortium. Health professionals’ views about who would benefit from using a closed-loop system: qualitative study. Diabet Med 2020;37(6):1030–7.
-
Rankin D, Kimbell B, Allen JM, Besser REJ, Boughton CK, Campbell F, et al. ; on behalf of the CLOuD Consortium. Adolescents’ experiences of using a smartphone application hosting a closed-loop algorithm to manage type 1 diabetes in everyday life: qualitative study. J Diabetes Sci Technol 2021;15(5):1042–51.
-
Lawton J, Hart RI, Kimbell B, Allen JM, Besser REJ, Boughton CK, et al.; on behalf of the CLOuD Consortium. Data sharing while using a closed-loop system: qualitative study of adolescents’ and parents’ experiences and views. Diabetes Technol Ther 2021;23(7):500–7.
-
Chen NS, Boughton CK, Hartnell S, Fuchs J, Allen JM, Willinska ME, et al. ; AiDAPT, AP@home04, CLOuD, DAN05, DAN06, and KidsAP consortia. User engagement with the CamAPS FX hybrid closed-loop app according to age and user characteristics. Diabet Care 2021;44(7):e148–50.
Presentations
The primary results were presented as an oral presentation at the American Diabetes Association in New Orleans in June 2022. Results from the qualitative study were presented at the Diabetes Professional Care Conference in London in November 2019. The Edinburgh team have given a series of presentations at the CLOuD Annual Meetings. We extended the invitation to the CLOuD Annual Meeting in 2020 and 2021 to include a wider audience of relevant stakeholders and other interested parties:
-
Boughton CK, Allen JM, Ware J, Wilinska ME, Hartnell S, Thankamony A, et al. ; on behalf of the CLOuD Consortium. Effect of 24 Months of Optimised Glucose Control on Residual C-peptide Secretion in Youth with New-onset Type 1 Diabetes. American Diabetes Association, New Orleans, June 2022.
-
Kimbell B. Closed-loop Technology: Who Should Be Given Priority and What Training, Resourcing and Support Will Healthcare Professionals Need? Diabetes Professional Care Conference, London, November 2019.
-
Rankin D, Kimbell B, Lawton J. Adolescents’ Experiences of Using the CamAPS FX Closed-loop. Closed-loop from Onset of type 1 Diabetes – Annual Meeting, Cambridge, November 2020.
-
Kimbell B, Rankin D. Qualitative Interviews – Staff Study. Closed-loop from Onset of type 1 Diabetes – Annual Meeting, Cambridge, November 2019.
-
Rankin D, Lawton J. Initial Findings from Qualitative Interviews with CLOuD Participants and Their Parents. Closed-loop from Onset of type 1 Diabetes – Annual Meeting, Cambridge, November 2018.
Webinars
Eleven webinars were delivered on the hybrid CL system for healthcare professionals, people living with diabetes and teachers/education support staff. These are hosted by the Cambridge Diabetes Education Platform (CDEP) and are publicly available on YouTube and have been watched over 10,000 times. CLOuD participants have featured in these webinars sharing their experience of the impact CL technology has had on their lives including managing sporting activities and mental health. David Rankin (Edinburgh) also presented findings from the CLOuD participants experience in a webinar focussing on CL insulin therapy and quality of life.
Social media
-
The CLOuD website (http://cloud.mrl.ims.cam.ac.uk/) has been kept updated with recruitment data.
-
A CLOuD participant from Nottingham took to Twitter to share their experience of the MMTT.
-
A CLOuD participant from Oxford features in an NIHR case study https://local.nihr.ac.uk/case-studies/artificial-pancreas-helps-jack-manage-his-diabetes/23305.
Newsletters
Two newsletters have been sent out to participants to date to share updates on the trial progress, recent trial related publications and news from other trial participants.
Additional information
Contributions of authors
Charlotte K Boughton (https://orcid.org/0000-0003-3272-9544) (Clinical Lecturer) co-designed the study, recruited and provided support for trial participants, carried out or supported data analysis, including the statistical analyses, prepared the results for publication and wrote the final report.
Janet M Allen (Research Nurse) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
Julia Ware (https://orcid.org/0000-0002-4497-0979) (Clinical Research Fellow) screened and enrolled participants, provided patient care and took study samples.
Malgorzata E Wilinska (https://orcid/0000-0002-1564-8083) (Clinical Research Associate) screened and enrolled participants, provided patient care, took study samples, carried out or supported data analysis, including the statistical analyses.
Sara Hartnell (Diabetes Educator) screened and enrolled participants, provided patient care and took study samples.
Ajay Thankamony (https://orcid.org/0000-0001-6290-3553) (Principal Investigator, Cambridge) screened and enrolled participants, provided patient care and took study samples.
Tabitha Randell (https://orcid.org/0000-0002-1703-1589) (Principal Investigator, Nottingham) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
Atrayee Ghatak (Principal Investigator, Liverpool) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
Rachel EJ Besser (https://orcid.org/0000-0002-4645-6324) (Principal Investigator, Oxford) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
Daniela Elleri (Principal Investigator, Edinburgh) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
Nicola Trevelyan (https://orcid.org/0000-0002-5991-2691) (Principal Investigator, Southampton) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
Fiona M Campbell (https://orcid.org/0000-0002-7618-6759) (Principal Investigator, Leeds) co-designed the study, screened and enrolled participants, provided patient care and took study samples.
David Rankin (https://orcid.org/0000-0002-5835-3402) (Research Fellow) undertook and supported analysis of the qualitative interviews.
Barbara Kimbell (https://orcid.org/0000-0003-4510-9862) (Research Fellow) undertook and supported analysis of the qualitative interviews.
Julia Lawton (https://orcid.org/0000-0002-8016-7374) (Professor of Health and Social Science) undertook and supported analysis of the qualitative interviews.
Judy Sibayan (https://orcid.org/0009-0005-9298-133X) (Study coordinator) supported study set-up and randomisation.
Peter Calhoun (https://orcid.org/0000-0002-5325-7200) (Statistician) carried out or supported data analysis, including the statistical analyses.
Ryan Bailey (Statistician) carried out or supported data analysis, including the statistical analyses.
Gareth Dunseath (https://orcid.org/0000-0001-6022-862X) (Senior Research Officer) undertook sample analysis.
Roman Hovorka (https://orcid.org/0000-0003-2901-461X) (Chief Investigator) co-designed the study, designed and implemented the glucose controller, carried out or supported data analysis, including the statistical analyses and wrote the report.
All authors critically reviewed the report and contributed to the interpretation of the results. Charlotte K Boughton, Peter Calhoun, Malgorzata E Wilinska and Roman Hovorka are the guarantors of this work and, as such, had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the paper prior to publication.
We are grateful to study volunteers for their participation. We acknowledge support by the staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility. We thank the members of the TSC (see Appendix 4) and Data Safety Monitoring Board (see Appendix 5) for their oversight of the trial. The study was co-coordinated by Cambridge Clinical Trial Unit.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/KTFR5698.
Primary conflicts of interest: Charlotte K Boughton has received consulting fees from CamDiab and speaker honoraria from Ypsomed. Julia Ware reports receiving speaker honoraria from Ypsomed. Malgorzata E Wilinska reports patents related to closed-loop and being a consultant at CamDiab. Sara Hartnell serves as a member of Medtronic advisory board, is a director of Ask Diabetes Ltd providing training and research support in healthcare settings, and reports having received training honoraria from Medtronic and Sanofi and consulting fees for CamDiab. Tabitha Randell receives consultancy fees from Abbott Diabetes Care and has received honoraria from NovoNordisk for delivering educational meetings. Rachel EJ Besser reports receiving speaker honoraria from Eli Lilly and Springer Healthcare, and reports sitting on the NovoNordisk UK Foundation Research Selection Committee on a voluntary basis. Julia Lawton was a member of HTA General Committee 2018–9. Roman Hovorka reports receiving speaker honoraria from Eli Lilly, Dexcom and Novo Nordisk, receiving licence and/or consultancy fees from B Braun and Abbott Diabetes Care; patents related to closed-loop, and being director at CamDiab. Janet M Allen, Ajay Thankamony, Atrayee Ghatak, Daniela Elleri, Nicola Trevelyan, Fiona M Campbell, David Rankin, Barbara Kimbell, Judy Sibayan, Peter Calhoun, Ryan Bailey and Gareth Dunseath declare no competing financial interests exist.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
De‐identified data sets will be made available on a case-by-case basis on reasonable request for research purposes. All requests should be addressed to the corresponding author.
Ethics statement
Approval was received from Cambridge East Research Ethics Committee on the 5 August 2016 (16/EE/0286) and Medicines and Healthcare products Regulatory Agency.
Information governance statement
The University of Cambridge is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, the University of Cambridge is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.research-integrity.admin.cam.ac.uk/research-governance.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the EME programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publications
Boughton C, Allen JM, Tauschmann M, Hartnell S, Wilinska ME, Musolino G, et al. Assessing the effect of closed-loop insulin delivery from onset of type 1 diabetes in youth on residual beta-cell function compared to standard insulin therapy (CLOuD study): a randomised parallel study protocol. BMJ Open 2020;10(3):e033500.
Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, Boughton CK, et al.; on behalf of CLOuD Consortium. What training, support and resourcing do health professionals need to support people using a closed-loop system? A qualitative interview study with health professionals involved in the Closed Loop from Onset in Type 1 Diabetes (CLOuD) trial. Diabetes Technol Ther 2020;22(6):468–75.
Lawton J, Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, et al.; on behalf of the CLOuD Consortium. Health professionals’ views about who would benefit from using a closed-loop system: qualitative study. Diabet Med 2020;37(6):1030–7.
Chen NS, Boughton CK, Hartnell S, Fuchs J, Allen JM, Willinska ME, et al. User engagement with the CamAPS FX hybrid closed-loop app according to age and user characteristics. Diabetes Care 2021;44(7):e148–50.
Lawton J, Hart RI, Kimbell B, Allen JM, Besser REJ, Boughton CK, et al. ; on behalf of the CLOuD Consortium. Data sharing while using a closed-loop system: qualitative study of adolescents’ and parents’ experiences and views. Diabetes Technol Ther 2021;23(7):500–7.
Rankin D, Kimbell B, Allen JM, Besser REJ, Boughton CK, Campbell F, et al. Adolescents’ experiences of using a smartphone application hosting a closed-loop algorithm to manage type 1 diabetes in everyday life: qualitative study. J Diabetes Sci Technol 2021;15(5):1042–51.
Rankin D, Kimbell B, Hovorka R, Lawton J; on behalf of the CLOuD Consortium. Adolescents’ and their parents’ experiences of using a closed-loop system to manage type 1 diabetes in everyday life: qualitative study. Chronic Illn 2021:1742395320985924.
Boughton CK, Allen JM, Ware J, Wilinska ME, Hartnell S, Thankamony A, et al. Closed-loop and preservation of C-peptide secretion in type 1 diabetes. Accepted in NEJM.
Disclaimers
This article presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci 2008;9:36-45.
- Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes 2013;14:541-53.
- Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycaemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 1997;20:22-5.
- Blasetti A, Chiuri RM, Tocco AM, Di Giulio C, Mattei PA, Ballone E, et al. The effect of recurrent severe hypoglycaemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol 2011;26:1383-91.
- Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr 2010;10.
- Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabet Med 2013;30:1126-31.
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- Royal College of Paediatrics and Child Health; National Paediatric Diabetes Audit . Annual Report 2018–2019: Care Processes and Outcomes 2020.
- DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449-62.
- Greenbaum CJ, Anderson AM, Dolan LM, Mayer-Davis EJ, Dabelea D, Imperatore G, et al. SEARCH Study Group . Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care 2009;32:1839-44.
- Lachin JM, McGee P, Palmer JP. DCCT/EDIC Research Group . Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014;63:739-48.
- Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care 2003;26:832-6.
- Vantyghem MC, Raverdy V, Balavoine AS, Defrance F, Caiazzo R, Arnalsteen L, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) is required to abrogate hyperglycaemia, whereas a minimal function is necessary to suppress severe hypoglycaemia (β-score greater than 3). J Clin Endocrinol Metab 2012;97:E2078-83.
- Bluestone JA, Buckner JH, Herold KC. Immunotherapy: building a bridge to a cure for type 1 diabetes. Science 2021;373:510-6.
- Brusko TM, Russ HA, Stabler CL. Strategies for durable beta-cell replacement in type 1 diabetes. Science 2021;373:516-22.
- Cryer PE. The barrier of hypoglycaemia in diabetes. Diabetes 2008;57:3169-76.
- Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med 1989;320:550-4.
- Buckingham B, Beck RW, Ruedy KJ, Cheng P, Kollman C, Weinzimer SA, et al. Diabetes Research in Children Network (DirecNet) Study Group . Effectiveness of early intensive therapy on β-cell preservation in type 1 diabetes. Diabetes Care 2013;36:4030-5.
- Phillip M, Danne T, Shalitin S, Buckingham B, Laffel L, Tamborlane W, et al. Consensus Forum Participants . Use of continuous glucose monitoring in children and adolescents. Pediatr Diabetes 2012;13:215-28.
- Kordonouri O, Hartmann R, Pankowska E, Rami B, Kapellen T, Coutant R, et al. Sensor augmented pump therapy from onset of type 1 diabetes: late follow-up results of the Pediatric Onset Study. Pediatr Diabetes 2012;13:515-8.
- Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. ASPIRE In-Home Study Group . Threshold-based insulin-pump interruption for reduction of hypoglycaemia. N Engl J Med 2013;369:224-32.
- Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycaemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240-7.
- Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol 2011;7:385-95.
- Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 2004;25:905-20.
- Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R. Simulation environment to evaluate closed-loop insulin delivery systems in type 1 diabetes. J Diabetes Sci Technol 2010;4:132-44.
- Wilinska ME, Budiman ES, Taub MB, Elleri D, Allen JM, Acerini CL, et al. Overnight closed-loop insulin delivery with model predictive control: assessment of hypoglycaemia and hyperglycaemia risk using simulation studies. J Diabetes Sci Technol 2009;3:1109-20.
- Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743-51.
- Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129-40.
- Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168-74.
- Elleri D, Allen JM, Kumareswaran K, Leelarathna L, Nodale M, Caldwell K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838-44.
- Nimri R, Danne T, Kordonouri O, Atlas E, Bratina N, Biester T, et al. The ‘Glucositter’ overnight automated closed loop system for type 1 diabetes: a randomized crossover trial. Pediatr Diabetes 2013;14:159-67.
- Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, et al. Overnight closed loop insulin delivery in young people with type 1 diabetes: a free-living randomised clinical trial. Diabetes Care 2014;37:1204-11.
- Nimri R, Muller I, Atlas E, Miller S, Kordonouri O, Bratina N, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes 2014;15:91-9.
- Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. FLAIR Study Group . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021;397:208-19.
- Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836-45.
- Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707-17.
- Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321-9.
- Dovc K, Boughton C, Tauschmann M, Thabit H, Bally L, Allen JM, et al. APCam11, AP@Home, and KidsAP Consortia . young children have higher variability of insulin requirements: observations during hybrid closed-loop insulin delivery. Diabetes Care 2019;42:1344-7.
- Ruan Y, Thabit H, Leelarathna L, Hartnell S, Willinska ME, Dellweg S, et al. AP@home Consortium . Variability of insulin requirements over 12 weeks of closed-loop insulin delivery in adults with type 1 diabetes. Diabetes Care 2016;39:830-2.
- Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabet Res Care 2014;2.
- Boughton C, Allen JM, Tauschmann M, Hartnell S, Wilinska ME, Musolino G, et al. CLOuD Consortium . Assessing the effect of closed-loop insulin delivery from onset of type 1 diabetes in youth on residual beta-cell function compared to standard insulin therapy (CLOuD study): a randomised parallel study protocol. BMJ Open 2020;10.
- Swift PG. International Society for Pediatric and Adolescent Diabetes . International Society for Pediatric and Adolescent Diabetes ISPAD clinical practice consensus guidelines 2006–2007 Diabetes education. Pediatr Diabetes 2007;8:103-9.
- National Institute for Health and Care Excellence . Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management 2020.
- Lachin JM, McGee PL, Greenbaum CJ, Palmer J, Pescovitz MD, Gottlieb P, et al. Type 1 Diabetes Trial Network . Sample size requirements for studies of treatment effects on beta-cell function in newly diagnosed type 1 diabetes. PLOS ONE 2011;6.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 1995;57:289-300.
- The Diabetes Control and Complications Trial Research Group . Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. Ann Intern Med 1998;128:517-23.
- Kohnert KD, Freyse EJ, Salzsieder E. Glycaemic variability and pancreatic β-cell dysfunction. Curr Diabetes Rev 2012;8:345-54.
- DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19:105-14.
- Lachin JM, Bebu I, Nathan DM. DCCT/EDIC Research Group . The beneficial effects of earlier versus later implementation of intensive therapy in type 1 diabetes. Diabetes Care 2021;44:2225-30.
- Mortensen HB, Swift PG, Holl RW, Hougaard P, Hansen L, Bjoerndalen H, et al. Hvidoere Study Group on Childhood Diabetes . Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes 2010;11:218-26.
- Varni JW, Curtis BH, Abetz LN, Lasch KE, Piault EC, Zeytoonjian AA. Content validity of the PedsQL™ 32 diabetes module in newly diagnosed patients with Type 1 diabetes mellitus ages 8–45. Qual Life Res 2013;22:2169-81.
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 1997;38:581-6.
- Green LB, Wysocki T, Reineck BM. Fear of hypoglycaemia in children and adolescents with diabetes. J Pediatr Psychol 1990;15:633-41.
- Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycaemia: quantification, validation, and utilization. Diabetes Care 1987;10:617-21.
- Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, et al. Psychometric properties of the hypoglycaemia fear survey-ii for adults with type 1 diabetes. Diabetes Care 2011;34:801-6.
- Corbin J, Strauss A. Basics of Qualitative Research. Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: SAGE Publications Ltd; 2014.
- Rankin D, Kimbell B, Hovorka R, Lawton J. Adolescents’ and their parents’ experiences of using a closed-loop system to manage type 1 diabetes in everyday life: qualitative study. Chronic Illn 2021;18:742-56.
- Davidson M, Penney ED, Muller B, Grey M. Stressors and self-care challenges faced by adolescents living with type 1 diabetes. Appl Nurs Res 2004;17:72-80.
- Schilling LS, Knafl KA, Grey M. Changing patterns of self-management in youth with type I diabetes. J Pediatr Nurs 2006;21:412-24.
- Spencer JE, Cooper HC, Milton B. The lived experiences of young people (13–16 years) with Type 1 diabetes mellitus and their parents: a qualitative phenomenological study. Diabet Med 2013;30:e17-24.
- Walker A, Schatz D, Johnson C, Silverstein J, Lyles S, Rohrs H. Type 1 diabetes through two lenses: comparing adolescent and parental perspectives with photovoice. Int J Pediatr Endocrinol 2016;2016.
- Castensøe-Seidenfaden P, Teilmann G, Kensing F, Hommel E, Olsen BS, Husted GR. Isolated thoughts and feelings and unsolved concerns: adolescents’ and parents’ perspectives on living with type 1 diabetes: a qualitative study using visual storytelling. J Clin Nurs 2017;26:3018-30.
- Chilton R, Pires-Yfantouda R. Understanding adolescent type 1 diabetes self-management as an adaptive process: a grounded theory approach. Psychol Health 2015;30:1486-504.
- Ersig AL, Tsalikian E, Coffey J, Williams JK. Stressors in teens with type 1 diabetes and their parents: immediate and long-term implications for transition to self-management. J Pediatr Nurs 2016;31:390-6.
- Rising Holmström M, Häggström M, Audulv A, Junehag L, Coyne I, Söderberg S. To integrate and manage diabetes in school: Youth’s experiences of living with Type 1 diabetes in relation to school – a qualitative study. International Diabetes Nursing 2017;14:46-51.
- King KM, King PJ, Nayar R, Wilkes S. Perceptions of adolescent patients of the ‘lived experience’ of type 1 diabetes. Diabetes Spectr 2017;30:23-35.
- Commissariat PV, Kenowitz JR, Trast J, Heptulla RA, Gonzalez JS. developing a personal and social identity with type 1 diabetes during adolescence: a hypothesis generative study. Qual Health Res 2016;26:672-84.
- Dickinson JK, O’Reilly MM. The lived experience of adolescent females with type 1 diabetes. Diabetes Educ 2004;30:99-107.
- Kyngäs H, Barlow JD. an adolescent’s perspective. J Adv Nurs 1995;22:941-7.
- Barnard KD, Wysocki T, Ully V, Mader JK, Pieber TR, Thabit H, et al. Closing the loop in adults, children and adolescents with suboptimally controlled type 1 diabetes under free living conditions: a psychosocial substudy. J Diabetes Sci Technol 2017;11:1080-8.
- Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, Boughton CK, et al. What training, support, and resourcing do health professionals need to support people using a closed-loop system? A qualitative interview study with health professionals involved in the Closed Loop from Onset in Type 1 Diabetes (CLOuD) trial. Diabetes Technol Ther 2020;22:468-75.
- Lawton J, Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, et al. CLOuD Consortium . Health professionals’ views about who would benefit from using a closed-loop system: a qualitative study. Diabet Med: J Br Diabet Assoc 2020;37:1030-7.
Appendix 1 Members of the CLOuD Consortium
| Roman Hovorka | Chief Investigator | Department of Paediatrics, University of Cambridge, UK |
|---|---|---|
| Ajay Thankmonay | Principal Investigator | Department of Paediatrics, University of Cambridge, UK |
| Carlo Acerini | Principal Investigator | Department of Paediatrics, University of Cambridge, UK |
| David Dunger | Investigator | Department of Paediatrics, University of Cambridge, UK |
| Charlotte Boughton | Investigator | Department of Clinical Biochemistry, University of Cambridge, UK |
| Julia Ware | Investigator | Department of Paediatrics, University of Cambridge, UK |
| Martin Tauschmann | Investigator | Department of Paediatrics, University of Cambridge, UK |
| Klemen Dovc | Investigator | Department of Paediatrics, University of Cambridge, UK |
| Malgorzata Wilinska | Investigator | Department of Paediatrics, University of Cambridge, UK |
| Janet allen | Study nurse | Department of Paediatrics, University of Cambridge, UK |
| Sara Hartnell | Pump educator | Cambridge University Hospitals NHS Foundation Trust, UK |
| Alina Cezar | Study coordinator | Department of Paediatrics, University of Cambridge, UK |
| Nicole Ashcroft | Study coordinator | Department of Paediatrics, University of Cambridge, UK |
| Daniela Elleri | Principal Investigator | Royal Hospital for Sick Children, Edinburgh, UK |
| Morag McDonald | Study nurse | Royal Hospital for Sick Children, Edinburgh, UK |
| Fiona Campbell | Principal Investigator | Leeds Children’s Hospital, Leeds, UK |
| James Yong | Investigator | Leeds Children’s Hospital, Leeds, UK |
| Emily Metcalfe | Study nurse | Leeds Children’s Hospital, Leeds, UK |
| Atrayee Ghatak | Principal Investigator | Alder Hey Children’s Hospital, Liverpool, UK |
| Keith Thornborough | Study nurse | Alder Hey Children’s Hospital, Liverpool, UK |
| Jonathon Mimnagh | Study nurse | Alder Hey Children’s Hospital, Liverpool, UK |
| Joanne Shakeshaft | Administrative manager | Alder Hey Children’s Hospital, Liverpool, UK |
| Karen Phelan | Research assistants | Alder Hey Children’s Hospital, Liverpool, UK |
| Tabitha Randell | Principal Investigator | Nottingham Children’s Hospital, Nottingham, UK |
| Vreni Verhoeven | Study nurse | Nottingham Children’s Hospital, Nottingham, UK |
| Rachel Besser | Principal Investigator | University of Oxford, Oxford, UK |
| Rebecca Law | Study nurse | University of Oxford, Oxford, UK |
| Loraine Bunton | Study nurse | University of Oxford, Oxford, UK |
| Clare Megson | Study nurse | University of Oxford, Oxford, UK |
| Imogen Stamford | Study nurse | University of Oxford, Oxford, UK |
| Jane Haest | Pump educator | University of Oxford, Oxford, UK |
| Nicola Trevelyan | Principal Investigator | Southampton Children’s Hospital, Southampton, UK |
| Helen Dewar | Study nurse | Southampton Children’s Hospital, Southampton, UK |
| Rachel Brampton | Study nurse | Southampton Children’s Hospital, Southampton, UK |
| Gabrielle Price | Study nurse | Southampton Children’s Hospital, Southampton, UK |
| Gillian Crouch | Study nurse | Southampton Children’s Hospital, Southampton, UK |
| Julia Lawton | Investigator | University of Edinburgh Usher Institute, Edinburgh, UK |
| David Rankin | Investigator | University of Edinburgh Usher Institute, Edinburgh, UK |
| Barbara Kimbell | Investigator | University of Edinburgh Usher Institute, Edinburgh, UK |
| Judy Sibayan | Study coordinator | Jaeb Center for Health Research, Tampa, Florida, USA |
| Peter Calhoun | Statistician | Jaeb Center for Health Research, Tampa, Florida, USA |
| Ryan Bailey | Statistician | Jaeb Center for Health Research, Tampa, Florida, USA |
| Nate Cohen | Statistician | Jaeb Center for Health Research, Tampa, Florida, USA |
| Gareth Dunseath | Investigator | Swansea University, Swansea, UK |
| Stephen Luzio | Investigator | Swansea University, Swansea, UK |
| Elisabeth Northam | Investigator | Murdoch Children’s Research Institute, Parkville, Victoria, Australia |
| John Todd | Investigator | Wellcome Trust Centre for Human Genetics, Oxford, UK |
| Stéphane Roze | Investigator | Vyoo Agency, Lyon, France |
Appendix 2 Summary of protocol amendments
| Version number | Date | Amendment |
|---|---|---|
| 1.1 | 29 July 2016 |
|
| 2.0 | 6 March 2017 |
|
| 3.0 | 16 June 2017 |
|
| 4.0 | 23 February 2018 |
|
| 4.1 | 15 March 2018 |
|
| 5.0 | 29 November 2018 |
|
| 6.0 | 22 January 2019 |
|
| 7.0 | 30 April 2019 |
|
| 8.0 | 10 September 2019 |
|
| 9.0 | 8 March 2023 |
|
Appendix 3 Summary of statistical analysis plan amendments
| Version number | Author | Approver | Effective date | Study stage |
|---|---|---|---|---|
| 1.0 | Nathan Cohen | Craig Kollman | 9 February 2018 | Enrolment started |
| 1.1 | Nathan Cohen | Craig Kollman | 7 May 2018 | Enrolment started |
| 2.0 | Nathan Cohen | Peter Calhoun | 4 April 2019 | Enrolment started |
| 2.1 | Nathan Cohen | Peter Calhoun | 19 November 2019 | Follow-up |
| 2.2 | Nathan Cohen | Peter Calhoun | 14 May 2020 | Follow-up |
| 2.3 | Nathan Cohen | Peter Calhoun | 17 July 2020 | Follow-up |
| 2.4 | Nathan Cohen | Peter Calhoun | 23 October 2020 | Follow-up, unmasked year 1 results |
| 3.0 | Ryan Bailey | Peter Calhoun | 7 September 2021 | Follow-up, unmasked year 1 results |
| 4.0 | Ryan Bailey | Peter Calhoun | 5 February 2023 | Follow-up, unmasked year 2 results |
| Version number | Revision description |
| 1.1 | Added the INSPIRE and PAID-Teen questionnaires. Also added clarifications on how we will handle venous and capillary HbA1c. Reorganised calculation of secondary outcomes. |
| 2.0 | Added the Cogstate questionnaire and analyses for the extension phase. |
| 2.1 | Clarified that C-peptide AUC can be tested at α = 0.05 if all three key end points are significant at α = 0.01. Added a diagram to the multiple comparisons section for clarification. |
| 2.2 | Added language to explain how to handle out of window visits resulting from the COVID-19 pandemic. |
| 2.3 | Added analyses involving the plasma glucose data. |
| 2.4 | Added sensitivity analyses for the primary outcome. Clarified diagram of the multiple comparison section. Clarified calculation of CGM metrics. |
| 3.0 | Added CL use and CGM use as outcomes in the adherence analysis. Created separate FDR category for C-peptide AUC, fasting C-peptide and plasma glucose. Deleted section on focus groups. |
| 4.0 | Clarified separate FDR category for outcome comparisons at 36 months and 48 months. |
Appendix 4 Trial Steering Committee members
Professor John Gregory (Chair), Cardiff University
Professor Stephen Greene, University of Dundee
Professor Jo Blair, Alder Hey Children’s NHS Foundation Trust
Mrs Alexia Passmore (Public and Patient Involvement representative)
Appendix 5 Data Monitoring and Ethics Committee members
Professor Timothy Jones (Chair), Perth Children’s Hospital, Australia
Professor Chris Patterson, Queen’s University Belfast
Dr Peter Adolfsson, University of Gothenburg, Sweden
List of abbreviations
- AE
- adverse event
- AP
- artificial pancreas
- AUC
- area under the curve
- BMI
- body mass index
- CGM
- continuous glucose monitoring
- CL
- closed-loop
- CLOuD
- Closed Loop from Onset in Type 1 Diabetes
- CSII
- Continuous Subcutaneous Insulin Infusion
- DCCT
- Diabetes Control and Complications Trial
- DKA
- diabetic ketoacidosis
- HbA1c
- glycated haemoglobin A1c
- HFS
- hypoglycaemia fear survey
- INSPIRE
- INsulin Dosing Systems: Perceptions, Ideas, Reflections and Expectations
- ISPAD
- International Society for Pediatric and Adolescent Diabetes
- MDI
- multiple daily injection therapy
- MMTT
- mixed-meal tolerance test
- MPC
- model predictive control
- NICE
- National Institute for Health and Care Excellence
- PAID
- problem areas in diabetes
- PedsQL
- Pediatric Quality of Life Inventory
- RMSE
- root mean squared error
- s.c.
- subcutaneous
- SAE
- serious adverse event
- SAP
- sensor-augmented pump therapy
- SDQ
- Strengths and Difficulties Questionnaire
- T1D
- type 1 diabetes mellitus
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
