Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 14/23/36. The contractual start date was in July 2018. The final report began editorial review in April 2022 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Lambert et al. This work was produced by Lambert et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Lambert et al.
Chapter 1 Introduction
Background
There are several populations of babies requiring renal replacement therapy (RRT). Those included in this study were critically ill infants in paediatric intensive care units (PICUs), who mostly did not have intrinsic renal disease and therefore were likely to have had good potential for renal recovery. Many were postoperative, especially post cardiac surgery, whose major problem was an acute kidney insult, fluid overload and poor urine output, and others who are septic or have renal failure as part of multiorgan failure. Although mortality and morbidity in PICU vary and are related to the underlying diagnosis, survival of babies in PICU is worse in those with fluid overload or needing RRT, of whom up to 20–40% may die. 1–6 RRT is supportive until kidney recovery, and although most survivors are independent of RRT at discharge from PICU, data on chronic renal sequelae are lacking. Children requiring RRT in PICU have been reported to have longer lengths of stay and have required more days of ventilator support. 5 There are over 200 infants per year in the UK receiving treatment with continuous RRT in PICU. 7,8 Some babies were excluded – for example, those with an inborn error of metabolism, such as urea cycle defects, causing hyperammonaemia, as they require emergency, very rapid removal of toxic metabolites by higher-than-normal dialysis clearances, and babies with severe intrinsic renal disease, which is often congenital, who are usually treated with chronic peritoneal dialysis (PD) at home, unless they required urgent RRT because of failure of chronic dialysis. 9
Types of ‘dialysis’ treatment
The word ‘dialysis’ is frequently used as a lay term to encompass all of the processes involved in replacing the function of the kidneys, that is cleaning the blood of waste chemicals and removing fluid from the body in a controlled way. However, medically it has a specific and more limited meaning and describes waste products being removed from the blood into fluid by a process of diffusion down their concentration gradients, and this may lead to confusion. In this report, we only use the term in a specific sense, as defined below.
Peritoneal dialysis is the process by which fluid is instilled cyclically into the abdomen and allowed to dwell, and during this time waste chemicals move across the lining peritoneal membrane between the blood that supplies the abdominal organs and the fluid by diffusion, prior to drainage. In PD, fluid removal or ultrafiltration (UF) is generated by the osmotic gradient between the blood and the dialysis fluid which causes water to cross into the peritoneal space.
Haemotherapy; both haemodialysis (HD) and continuous veno-venous haemofiltration (CVVH) are types of treatment in which blood is drawn from the patient into a disposable plastic circuit on a machine, processed through a filter which has a membrane that separates the blood from treatment fluids, and then returns it to the patient. We will call both of these treatments haemotherapies, and they share the need for vascular access into a central vein, an extracorporeal blood circuit which has a tendency to clot and typically requires anticoagulation treatment to stop that, but the mechanisms by which they remove chemical wastes differ.
Continuous veno-venous haemofiltration removes waste chemicals by filtering large volumes of plasma water and replenishing it with a chemically balanced replacement fluid. It generates UF by replacing slightly less fluid than it filters. CVVH machines are the devices conventionally used to provide haemotherapy to critically ill babies in a PICU setting, of which the Prismaflex® (Baxter Healthcare, www.baxterhealthcare.co.uk) and Aquarius® (Nikkiso, www.Nikkosomedical.com) are the most commonly used in the UK.
Haemodialysis removes waste chemicals by dialysis as they diffuse across the filter membrane from the plasma into dialysate fluid being pumped across the other side. It generates UF by increasing the pressure gradient between the plasma and the dialysate fluid. Conventional HD devices are widely used to provide therapy to children with chronic renal failure. NIDUS® (named for the Newcastle Infant Dialysis and Ultrafiltration System) has been developed specifically to treat small infants, either in an acute PICU setting or for chronic use as required. Conventional HD devices generate the pressure gradient necessary for UF by a computer-controlled regulation of the blood and fluid pump speeds. The NIDUS generates the necessary UF pressures by volumetrically controlling the blood flow using syringes.
Haemotherapies can be provided to babies who are on extracorporeal membrane oxygenation (ECMO) support using a simple technique in which the ECMO blood circuit can be used to pump blood through a dialysis filter. Using ECMO plus haemodialysis (ECMO + HD), the flow of dialysis fluid is controlled by adapting intravenous infusion pumps, and UF is controlled by adjusting the difference between the speeds of the inflow and outflow pumps.
The problems
Providing RRT to young babies may be severely challenging because of their small size and immaturity, both for PD, and for haemotherapy solutions which require the use of devices that have disposable extracorporeal blood circuits. Publications indicate similar problems faced by clinicians worldwide who use adult devices because of a lack of alternatives, and the need for new solutions including improved device technology. 10,11
Acute peritoneal dialysis
Acute PD is the technically easiest method for providing RRT, and is carried out manually using simple circuits in small infants, with fresh dialysis fluid being run in and out of the patient’s abdomen through a catheter under gravity, as no suitable automated cycling devices exist. There is no lower size limit, and it is used frequently to support infants after open-heart surgery. 2,12 Complications are common in the smallest participants including leakage of dialysate from the access entry point, drainage difficulties and the risk of developing peritonitis or hyperglycaemia, and it cannot be used in babies who have had abdominal surgery such as for necrotising enterocolitis, which is common in small, unwell babies, or in infants with congenital abdominal wall defects. 1 UF is unpredictable, but can be monitored easily as the dialysate is drained and collected using calibrated burettes. The clearance of waste chemicals is relatively slow. Both the UF and biochemical clearances may fall or fail altogether, especially in unstable babies who develop splanchnic vasoconstriction which limits the supply of blood to the peritoneum.
Continuous veno-venous haemofiltration
Most small infants on RRT haemotherapy are treated with CVVH. These devices were initially designed and built to treat adults, then Gambro developed the Prisma® with smaller volume circuits which was approved for use in all sizes of children by the Food and Drug Administration (FDA) in the USA, and obtained Conformité Européenne (CE) marking in Europe. However, it was subsequently recognised that its control and reporting of UF was insufficiently precise to guarantee delivering safe treatment to smaller children, even in those being kept in neutral fluid balance. It should be remembered that the typical circulating blood volume of an infant is approximately 80 ml/kg of body weight. For this reason, the FDA withdrew its approval for using the Prisma in children of <20 kg, and Europe limited its CE mark to children <8 kg. 13 Gambro report that their latest iteration, the Prismaflex, has a fluid removal accuracy of ±30 ml/hour or 300 ml/day. 14 Unfortunately, poor fluid control in conventional haemotherapy is a consequence of its inherent technology which relies upon the device computing pressure gradient measurements rather than volumetric monitoring, and erratic and unrecognised variations in the UF have been shown in vitro in the two CVVH devices commonly used in the UK, the Prismaflex and the Aquarius. 15 These volume changes have the potential to cause dehydration or fluid overload in small babies, but such machines are used extensively worldwide outside practice recommendations due to a lack of more suitable devices.
Other problems with using CVVH in babies also result from their small size compared with the blood access, flow and volume requirements of conventional haemotherapy circuits. Specifically, existing CVVH devices require double-lumen central venous access lines with recommended minimum 7-French, size, and continuous 40 ml/minute blood flows, both of which may be difficult to achieve in the smallest babies. Also, they have extracorporeal circuit volumes of 50–70 ml, which exceeds the safe limit of removal of 10–15% of blood volume in babies under 5 kg. This necessitates priming their circuits with blood products, or by using saline and precipitating a sudden haemodilution as the therapy is started. Exposure to foreign blood carries a risk of causing tissue sensitisation, with potential consequences for children who may later require organ transplantation. The sudden exposure of a baby to a relatively large transfusion also has the inherent risk or causing abrupt pH and other aberrant chemical changes, which may be reduced by pre-dialysing the circuit. 16
Because of these device limitations, common clinical consequences of these limitations of CVVH devices include cardiovascular instability with hypotensive episodes on connecting and commencing therapy and at any time due to variations in UF control, difficulties providing vascular access and sufficient blood flows, which may result in the clotting of circuits and multiple blood transfusions. 3–6,17,18
NIDUS technology
The NIDUS began development in 1995 specifically to provide RRT to very small infants, and has a novel circuit that operates using different physical principles from conventional systems. 19 It uses syringes rather than peristaltic pumps to drive the blood flow, which provide precise volumetric control of UF, and uncouple the baby’s blood flow capacity from the requirements of the dialysis filter, allowing it to sample more slowly. Its minimum circuit volume of <10 ml does not require blood priming and only requires a relatively small single-lumen central venous access line. By 2005 an early automated version had been used to treat four babies of 0.8 to 3.4 kg, and this was subsequently re-engineered to produce the I-KID study intervention device, the NIDUS. 20 During development it was tested on piglets of 1 to 8 kg, and was used to dialyse 10 babies of 1.8 to 5.9 kg in a PICU and a paediatric nephrology setting, including 354 dialysis sessions totalling 2475 hours, where it was found to be safe. 21 Its UF precision and biochemical clearances were consistently superior to PD in both the animal and clinical studies.
Other novel infant RRT devices
There are two other haemotherapy devices being developed to provide RRT in babies as small as 2.5 kg, but neither was available for us to include in the I-KID study. A group in the USA have used the Aquadex® (Nuwellis, www.nuwellis.com) adult haemofiltration device in parallel with intravenous pump controllers to regulate the flow of replacement fluid and to generate UF. 22 An Italian group has produced and CE marked an infant CVVH device, the CARPEDIEM®, using a miniaturised conventional circuit. 23,24 Although this has been used in some European centres, and has recently gained FDA approval, it was not available in the UK to enable comparisons when the I-KID study started.
Rationale
The need for improved device technology for infant RRT has been widely stated. 10,11,25,26 Increasing success and breakthroughs in neonatal surgery including cardiac, will continue to produce a need for safe and effective postoperative management of fluid overload, acidaemia and biochemical disturbance in the smallest newborns.
The I-KID clinical investigation was designed to determine the clinical efficacy, outcomes and safety of a novel non-CE-marked infant HD machine, the NIDUS, compared with currently available RRT in the UK. NIDUS is specifically designed for use in babies between 0.8 kg and 8 kg. A pilot trial of NIDUS used in 10 babies in a single cardiothoracic PICU gave strong support for a study to provide evidence for the efficacy and safety of NIDUS in wider clinical use. 21 In vitro comparison of NIDUS with Prismaflex and Aquarius has lent support to possible improvement in control of UF. 15 For this reason, the I-KID clinical investigation was designed to determine the efficacy and safety of the NIDUS in PICUs across the UK, and compare it with conventional therapies, including PD, and Prismaflex and Aquarius CVVH machines in a randomised controlled trial (RCT). Safety monitoring, an important focus of the study, was enhanced by the fact that the NIDUS makes a continuous downloadable recording of all of its activity data for subsequent analysis and scrutiny, including volumes, flows, pressures, alarms and responses to alarms and events.
The NIDUS was developed by a team of clinicians, scientists and academics in Newcastle upon Tyne, with significant public involvement, and the devices used in the I-KID study were manufactured under licence by Allmed. The study was therefore designed such that the team that developed the device could provide training and support for the other centres.
Research questions, aims and objectives
Main study objectives
To compare the novel non-CE-marked infant HD machine, the NIDUS, to conventional standard RRT in children under 8 kg in PICU. The study aimed to evaluate the clinical efficacy of the NIDUS in improving accuracy of UF fluid removal and to monitor safety and patient outcomes using a cluster-randomised stepped wedge (SW) study design. The study also compared NIDUS separately with each of CVVH and PD.
In addition, the study was designed to look at the incidence and severity of the adverse effects of renal replacement, and to generate a safety profile in the application of NIDUS in the clinical environment.
Chapter 2 Methods
Chapter 2 contains material reproduced from Lambert HJ et al. 2021. 27 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Objectives
To compare the use of the NIDUS with conventional RRT in children and babies under 8 kg treated in PICU.
Study design
This multi-site clinical investigation used a randomised SW cluster design with four periods and three sequences. 28 Conventional therapy (PD or CVVH) was used in the control cells, with NIDUS used in the intervention cells. In all sequences, the treatment in the first period was conventional therapy, while in the last treatment period all sites used NIDUS. The sequences differed in the timing at which the change from conventional therapy to NIDUS occurred, as shown in Figure 1. The nature of the study meant there was no blinding.
FIGURE 1.
I-KID study design sequence.
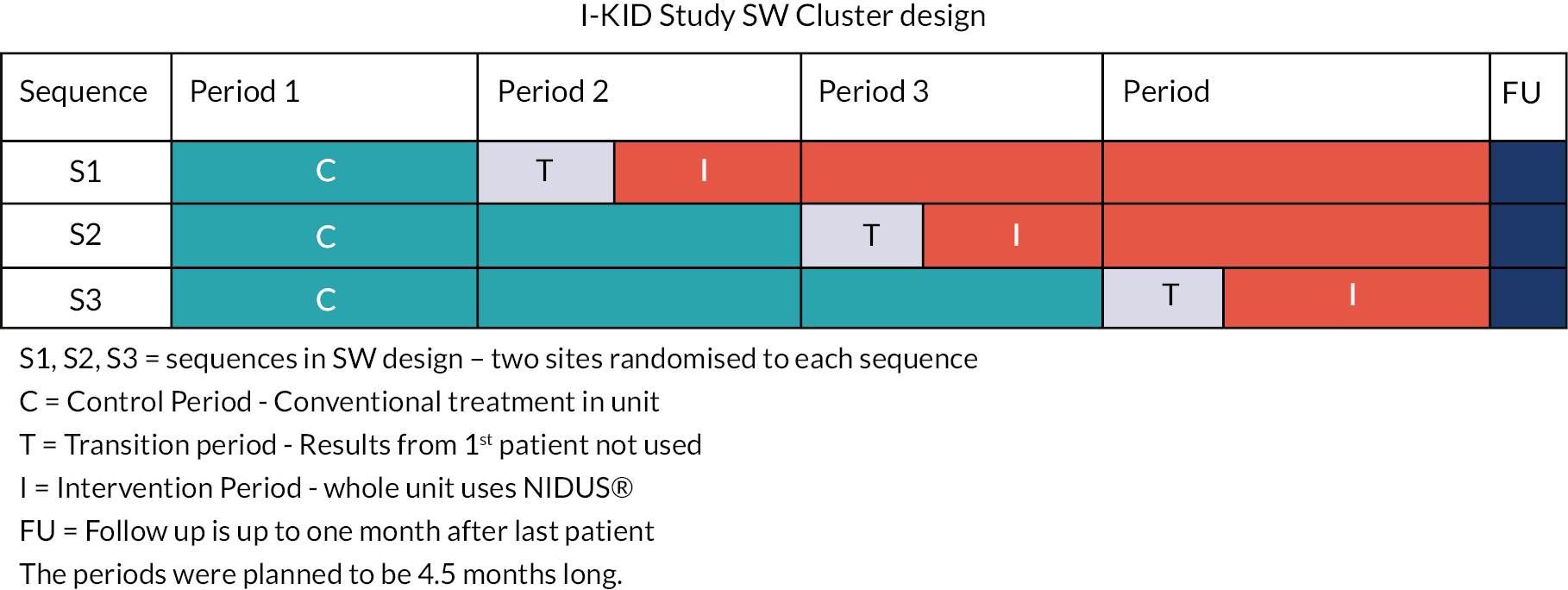
The six clusters in the SW design were the PICUs in six National Health Service Hospital Trusts with tertiary nephrology units in the UK where the study was conducted. Each site was randomly allocated to one of the sequences in the design, with two sites allocated to each sequence. See Randomisation of the SW design for further details.
Each site was trained in setting up and using the NIDUS before switching to the intervention period. The design meant that all participating sites had the chance to use both treatments during the study. Using the SW design permitted the phased training on the NIDUS and allowed within-site comparisons to contribute to the treatment effect estimate.
The SW design was chosen over a conventional RCT with individual patient randomisation for reasons of safety, ethics, acceptability and efficiency. This study took place in the PICU environment, necessitating a level of urgency to recruit, consent and initiate RRT without compromising the participants’ health further which raises ethical concerns. 29 Further discussion on the choice of the design is given in Chapter 4.
Data on UF accuracy and biochemical clearance from the first patient in each site treated after transition to NIDUS was not used in the analysis. This was because the lack of familiarity of staff using NIDUS for the first time under clinical conditions which could lead to unreliable observations. This was not applied in the Newcastle site, due to prior use/experience of the NIDUS device.
Participants
The study recruited participants in PICU who met the following eligibility criteria:
Inclusion criteria
-
Participants with a body weight of 0.8 kg–7.99 kg who require continuous RRT for acute renal insufficiency or fluid overload as part of their standard clinical care.
-
Person with legal parental responsibility (PR) for the patient able to provide written informed consent for the patient to take part in the study.
Exclusion criteria
-
Patient with known chronic renal failure already on established adequate RRT.
-
Patient already established on adequate RRT for whom entry into the study would require additional central venous access, if that access is not clinically indicated.
-
Patient with an underlying metabolic diagnosis, including hyperammonaemia.
-
A clinical decision is made that the patient should not receive RRT using NIDUS.
-
Unable to receive written informed consent for data collection from a person with legal PR for the patient.
Two main changes were made to the eligibility criteria during the course of the study as shown in Appendix 1, Table 33. These were the introduction of deferred consent for the study, reflecting common practice in emergency situations in PICU so as not to delay treatment, and the acceptance of use of estimated body weight.
Settings and locations
The study was conducted in PICUs in six NHS Hospital Trusts with tertiary nephrology units in the UK. The participating sites were Birmingham Children’s Hospital, University Hospitals Bristol, Evelina London Children’s Hospital, Great Ormond Street Hospital, Newcastle upon Tyne Hospitals (Royal Victoria Infirmary and Freeman Hospital) and University Hospital Southampton.
Identification and screening
Potential participants were identified as they presented on PICU by the doctor or nurse at the site with delegated responsibility. They were screened against the study inclusion and exclusion criteria using the patient medical notes.
As part of standard care, parents/guardians were told about the clinical need for the patient to receive dialysis treatment. Parents/guardians were also told that the PICU at their hospital was taking part in the I-KID study and the rationale for the study was introduced.
This initial approach was done sensitively by clinical staff, communicating carefully, and taking into consideration how the parents/guardians were feeling at that time and the individual situation of the patient.
A log was completed to document all participants who fulfilled the eligibility criteria for the study. This included those who were approached and were subsequently included or excluded, as well as those who were not approached and the reasons why.
Recruitment and consent
The decision to start the patient on RRT was always clinical and would commence at the discretion of the responsible clinician before consent was obtained if it was in the patient’s best interest to not delay treatment. As part of standard care, staff discussed with the parent/guardian the need for dialysis and the current methods of RRT being used within the PICU. This included the NIDUS during the intervention period.
Study Information Sheets, including Summary Information Sheets which were produced in collaboration with parent advisors, were provided to parents/guardians of all eligible participants. Tailored consent was obtained appropriate to the phase of the study (usual treatment/intervention).
A parent who was involved in the study development from the start was included as a co-applicant to ensure that methods used were acceptable and sensitive.
For all study periods in this emergency situation, the patient’s parents or legal guardians were approached for written consent, as soon as practicable after starting RRT, ideally within 48 hours (deferred consent).
Parents/guardians of a baby who was confirmed as eligible to take part in the study but who passed away were also be given the opportunity to take part in the study. Delayed consent from bereaved parents was in line with best practice recommendations from the CONNECT study. 30 Consent from bereaved parents was received either in hospital or by post and may have involved a telephone discussion where appropriate because of distance, using the bereaved parent/guardian information sheet and consent form.
Parents/guardians who provided consent were given further opportunities to discuss the study and ask questions. All parents/guardians had the right to withdraw the patient from the study at any time without having to give a reason.
If consent was not received, the method of dialysis used was decided by the clinician considering the best option for that patient and what methods were available in the PICU at that time. The patient was not entered/or continued in the I-KID study and no (further) study data were collected.
The parent/guardian specifically consented to the patient’s general practitioner (GP) being informed of their participation in the study.
Details of study interventions
Parents/guardians continued to receive full supportive care as required whether the patient received the control or intervention therapy. The initial requirement for the patient to have RRT was made by the lead clinician in PICU and was initiated according to the usual indications practiced by the attending clinical team. The control and intervention therapies were administered by the NHS clinical team ordinarily treating the patient with support from research nurses.
Control therapy (usual treatment)
Participants were treated with current RRT options available at the participating site, either PD or CVVH, when in the control phase of the design. Staff in PICU were already trained in the clinical use of these RRT methods. Additional training was given regarding the procedures needed to obtain measurements of UF and biochemical clearance.
All sites used PD. Each site was also able to perform CVVH either using the Gambro Prismaflex, or the Baxter Aquarius. In the absence of suitable and safe alternatives, these machines are used off licence during standard care. In one site, RRT was also provided for infants on ECMO by connecting a dialysis filter between the arterial and venous ends of the ECMO circuit and controlling the rate of dialysate flow using pumps designed to regulate intravenous infusion lines (ECMO + HD). The NIDUS machine was not available for use during the control period.
Control therapy was used in the control period according to usual clinical practice until changeover to NIDUS according to the SW design. Eligible participants who declined consent to the I-KID study received standard therapy.
Tests of RRT efficacy
Children recruited to this study had two types of tests of the efficacy of their RRT, measurement of the (UF precision) and measurement of the rate of clearance of chemicals from their blood. The details of these are given below, and separate detailed information and bedside data recording sheets for PD, the Prismaflex, the Aquarius, for ECMO + HD, and the NIDUS are available in the project documents.
Measurement of UF precision
The quantity of fluid removed by RRT was measured over an accurately timed period to calculate the actual UF rate that the therapy had achieved, and for each modality this was compared with the UF rate that the clinical team had documented they required. For the CVVH and NIDUS therapies, the measured UF was also compared with the volume of UF that the device displayed it had achieved.
For PD, the total volumes of dialysate fluid infused and drained were measured volumetrically using the calibrated burettes that are integral to the clinical circuits, as is undertaken for every complete dialysis cycle in normal clinical practice. To minimise errors that may occur due to variations in the completeness of emptying of the peritoneal space during some PD cycles, the collection periods were between 5 and 7 hours long, and were timed to ensure they only included completed cycles. The achieved hourly UF rates were calculated by subtracting the mean volume infused from the mean volume drained.
For CVVH and the NIDUS, the UF rates which were set by the operators and recorded by the devices were volumetric (ml/hour), and these were compared with the actual UF rates that were measured gravimetrically by assuming that all of the fluids had a density of 1 g/ml. This method was employed because both types of infant haemotherapy use closed fluid circuits, which means that the total combined weight of the fresh and waste fluid bags remains constant in the absence of any UF, and it can be assumed that any increase in their net weight will represent fluid added by removal from the baby’s circulation. In the case of CVVH machines, the saline infusion used to deliver heparin into the circuit also enters the device’s closed circuit and adds to the increase in net weight of the bags, so it was deducted from the measured weight gain, but this was not so for the NIDUS because in that circuit it is removed without entering the closed fluid system.
Thus, the weights of all of the dialysate and/or replacement fluid bags, plus the waste-fluid drainage bags were recorded at the start and end of each study period by suspending them over a stable weighing balance capable of weighing up to 16 kg, and sensitive to changes of 0.1 g, and by recording the volume of heparin solution infused by the CVVH devices. The calibration of the weigh-scales was checked to be accurate to within ±1 g using a 5 kg weight, and ±0.1 g using a 10 g weight before each study, and the fluid bags were suspended carefully in precisely the same manner each time, avoiding any stretching or contact of their connection lines with the device. For both types of device, the minimum collection study period was set at one hour. If the clinical requirement for UF changed during any of the study periods, the timing of the altered settings was recorded, and this was accounted for in the rate calculations.
For ECMO + HD, the change in the weight of the fresh dialysate fluid bag was recorded, and the volume of waste dialysate fluid was measured in a calibrated drainage container. The rates of the dialysate inflow and outflow infusion pumps were recorded, and the clinically set UF rate was taken as the difference between these rates.
Measurement of biochemical clearance
We calculated the clearance of creatinine, urea and phosphate by measuring the rate of accumulation of each chemical in the dialysate and/or replacement fluid and comparing that to its plasma concentration. This requires the measurement of the plasma and waste fluid concentrations of these three metabolites, and a knowledge of the waste fluid flow rate. The urea and phosphate were measured using standard methods in the clinical laboratories of the participating centres, and creatinine was measured by an enzymatic assay to avoid measuring non-creatinine chromogens in the plasma by the previously widely used alkaline picrate reaction. Clearances were expressed as ml of plasma totally cleared of that chemical per minute of therapy.
For PD, the biochemical clearance test was performed at the same time as the UF test, that is over a timed period of five to seven hours. The whole volume of waste fluid collected during that study was mixed, and a sample of this was used for the chemical assays.
For the CVVH and NIDUS the biochemical clearances were measured either just before or just after doing the UF test because each study would have interfered with the precision of the other one. When the standard extracorporeal circuit was mounted on the device, an extra extension tube and three-way tap were inserted into the waste fluid drain line close to the main drainage bag, with a small collection bag attached to the side connection. During the collection period of at least 20 minutes, the tap was turned to allow waste fluid to be collected as it was produced, and a sample of this was assayed for the metabolites. The rate of fluid drainage was calculated as the total of dialysis and/or replacement fluid flow rates set on the machine plus the volume of ultrafiltrate set.
For EMCO + HD, as for PD, the whole volume of waste fluid collected during the study period was mixed, and an aliquot was chemically assayed.
In all clearance studies, the test was performed within one hour of a blood sample being taken for creatinine, urea and phosphate measurement for clinical reasons. No extra blood samples were taken for the tests; instead, the timing of the biochemical clearance tests was adjusted to coincide with routine blood sampling, which is typically twice daily in babies on RRT in PICUs.
Details of the calculations used for each modality for both UF and clearances are available in Report Supplementary Material 1.
Intervention therapy (NIDUS)
The NIDUS was only available for use by trained staff during the intervention period for that site.
The main instrument is used in conjunction with a device-specific blood tubing set and a NeoFlux1® (Allmed, www.allmedgroup.com) HD filter, and withdraws small volumes of blood (5–10 ml) from the patient, passes it through the filter and then returns it to the patient, via a single lumen vascular access catheter. Blood movement is controlled using driven syringes and pinch valves. Its extracorporeal circuit volume with the syringes empty is 4.9 ml, which is small enough to prime safely with saline, without the need for blood products. UF is controlled by the differential movement of the two operating syringes, with the difference in volume between the two syringes is removed as UF. Dialysate is pumped around the outside of the filter via a peristaltic pump, allowing dialysis to occur by diffusion. The NIDUS makes a constant recording of all activity data, including volumes, flows, pressures, alarms and response to alarms, downloadable for safety purposes.
Allmed are the manufacturers of NIDUS device, circuit and filters. Devices were loaned, three to each site (except Newcastle that has two), in case of breakdown or multiple recruits.
Sites were supported in their use of the investigational device, both clinically and technically. A 24-hour helpline was available to contact on-call renal nurses from Newcastle upon Tyne Hospitals (NuTH), experienced in the use of NIDUS, in order to provide immediate support to study nurses at the bedside. Clinical telephone or videolink support was also available from Dr Heather Lambert (Chief Investigator) and Dr Malcolm Coulthard at all times as backup to the nursing advice. Allmed and NuTH Medical Physics provided continued support in response to immediate technical queries.
Support was both reactive and proactive. A monthly teleconference with representatives from each site and the I-KID Trial Management Group (TMG) enabled users from different sites, with different experiences, a platform to feedback to one another and Trial Steering Committee (TSC) members. Regular meetings between the clinical and technical teams were essential to manage and review the ongoing support offering and to respond to user requests regarding interventional aspects of the study.
During the intervention period, standard therapy continued to be used for those participants who did not meet the criteria for the NIDUS machine (see Inclusion criteria and Exclusion criteria in the Introduction).
Clinicians caring for participants patients under 8 kg who started on conventional dialysis methods, which had failed and where the patients did not meet the inclusion criteria for the study, had the option to use/switch to the NIDUS machine for compassionate use. These cases were initially discussed with the Chief Investigator. Local trust process and the process set out by the Medicines and Healthcare products Regulatory Agency (MHRA) processes for compassionate use were followed by sites when appropriate. No data from these patients were used for the study.
Site training and delivery of interventions
Study induction and control phase
During the control phase, sites followed routine clinical practice with the addition of conducting a number of research activities; and so, training involved the detailing and demonstration of these activities, namely, bag-weighing and fluid sampling methods. Initiation visits were conducted at each site in the months prior to the start of the study. The site PI and senior members of the clinical and/or research team received the training in person. These trained individuals then cascade-trained other team members at their own site. In addition to the introduction of research activities, information was disseminated with regard to the study rationale and protocol, the principles of the NIDUS (how it differs from conventional RRT) and the content and location of specific documentation required when carrying out research activities. This included an overview of safety reporting procedures and document version control.
Intervention phase
As per study design, intervention phase training followed a stepped approach, where sites were trained approximately two months before they were due to crossover to use the NIDUS. Each site had a minimum of four training days.
These sessions aimed to ensure that key members were competent to use the device and its components. This involved detailing and demonstrating how to set up and run the NIDUS, and how to troubleshoot potential issues that may occur in practice. The device’s operating principles, and key differences compared with conventional RRT devices were discussed. Largely, the emphasis of this face-to-face training was to allow hands-on time with the device. As with the control phase, members were also shown, and then asked to demonstrate, how to accurately perform device-related research activities, i.e. bag-weighing and fluid sampling methods.
Trainees were considered, and signed off as, competent to use the device if they showed that they could correctly follow guidance documentation to set up the device, perform procedures required when running the device, and if they showed a comprehensive understanding of the NIDUS and how they might troubleshoot issues via answering a set of trainer-led questions and discussion. A number of key individuals including senior nurses and the PI at each site were signed off as competent to both use the device and cascade train others. Sites were responsible for ensuring their skills were maintained during the study and this was supported by the I-KID training team, for example by conducting additional training sessions.
Parents/guardians were asked about their experiences and staff were asked about acceptability and usability of the device, using questionnaires.
Updates to guidance and training documents
User documentation, including documents used for training, device-use guidance documents and research activity guidance and recording documents (to be used at the bedside), were continuously reviewed and amended throughout the trial. On-going user feedback from sites largely facilitated this process. Aspects of accessibility and clarity of content were reviewed. Documentation, such as the training packages, began as paper copies that were also available online via study specific shared drives (Microsoft Teams and Google Drive); then, were adapted to become direct online material (accessible via a QR code or web link), in an app-like format, created using the Google Forms forum. This ‘app’, in its final form, provided a step-by-step, image-guided walkthrough of device set up and running procedures; it was used as a training tool and for guidance during clinical use of the NIDUS. Additional training was provided where appropriate, including prior to resumption of the study post the interruption caused by the COVID-19 pandemic, and included virtual video-linked training.
Outcomes
The principal aim of this study was to assess the precision of fluid removal compared with prescription – a measure that represents fluid removal precision of the dialysis system. Namely, does the dialysis methodology provide the hourly fluid removal that the clinical team wanted? The measurement of the required quantities is described in the section Measurement of UF precision and in Figure 2, below.
FIGURE 2.
Data collection timeline for Prismaflex, Aquarius and NIDUS (top); data collection timeline for PD (bottom).

Primary outcome
The first observation of precision of fluid removal (UF) from an episode lasting at least an hour within 48 hours of the start of RRT.
Secondary outcomes: related to the primary outcome
-
average of all precision values observed on the patient
-
biochemical clearance rates for creatinine, urea and phosphate
-
precision of observed versus reported fluid removal.
Secondary outcomes: mortality data
-
death within 30 days of the start of RRT (collected by the I-KID team)
-
death before discharge from PICU [collected via PICANet (Paediatric Intensive Care Audit Network) – see below].
Secondary outcomes: collected through PICANet
Paediatric Intensive Care Audit Network is an audit database recording details of the treatment of all critically ill children in PICU. Data are routinely collected on admission to PICU and thereafter daily returns are made, with detailed renal data. This custom renal data set uses precise definitions and terms for the information collected. PICANet publishes an annual report and makes regular download of data to the site of origin for audit and quality improvement purposes. Some of the descriptive and secondary outcome data in I-KID was collected via PICANet, and with consent from parents/guardians as part of the I-KID study-specific data were shared with the I-KID study by the sites. The reason for using this process for some data collection was to try to ensure as near as possible complete data collection as these data were being collected routinely by sites who were used to doing this in a regular way. The I-KID study also aimed to not cause additional workload by duplicating similar data sets.
-
haemodynamic status (drop in blood pressure after connection to a RRT device, requiring intervention of fluid bolus or administration of inotropes)
-
completion of intended RRT course
-
need for additional vascular or dialysis access
-
unplanned change in circuits
-
exposure to blood transfusion
-
bleeding events
-
anticoagulant use
-
number of ventilator-free days (calculated as number of days on RRT minus number of days on RRT and on ventilator).
Secondary outcomes: questionnaire results
-
parent/guardian experience measured using questionnaires
-
staff acceptability and usability of device measured using questionnaires.
Changes to primary outcome variable
The protocol initially specified that the primary outcome variable should be measured during data phase 1 (0–7 hours), provided the data collection episode exceeded one hour. However, because of the time constraints for clinical teams managing critically ill babies and the complexity of the study testing, the protocol was formally updated so that; if the site team were unable to collect the primary outcome during the first phase of data collection, then the next available collection period was used to measure the primary outcome, provided this was within the first 48 hours of RRT.
Sample size
The sample size calculation followed the model of Hussey and Hughes, adapted to accommodate unequal cluster sizes. 28 No interim analyses were planned and the study was monitored by a DMEC.
If the observed fluid removal rate is X and the rate prescribed by the treating physician is A, then the aim of the primary analysis is to compare the different treatments with respect to how closely X conforms to A. To this end the primary outcome is defined as Y = log|X-A|, where log denotes the natural logarithm. If X is distributed as a Normal variable with mean A and standard deviation (SD) σ, then Y will be distributed, log σ + log |Z|, where Z has a standard Normal distribution. This variable is approximately Normally distributed with variance ⅛π2 and mean difference between treatment groups of log(σcontrol/σNIDUS): the ratio of SDs is the parameter of principal interest.
The study uses a SW cluster design and historical data from PICANet indicated that the annual numbers of participants for the six sites could be taken to be N = 14, 14, 14 (Evelina, GOSH, Southampton) 9, 9 (Birmingham, Bristol) and 3 (Newcastle). The aim of the power calculation was therefore to determine the length, L, of periods in the design to achieve the desired power.
The model initially assumed for the primary outcome is the linear mixed model applied to SW designs by Hussey and Hughes, namely the kth response in cluster i in period j is
where πj is the period effect, Xij is 0 or 1, being 1 only if NIDUS is allocated to cluster i in period j: ξi is a random term accounting for the extra variation in y due to site i, and the εijk is the individual-level residual with variance assumed to be ⅛π2. 28 The treatment effect of NIDUS relative to control is θ and the variance of ξi is such as to yield the postulated intraclass correlation of ICC.
The formula for the standard error of the estimate of θ can be obtained using standard methods, such as those in Matthews and Forbes but amended to allow for different numbers of participants being allocated to each site. 31 On the grounds of simplicity, and because there is no indication to the contrary, it was assumed that there would be no period-to-period variation in recruitment within a site.
If the length of each period is L months, then it was assumed that the number recruited in each period and each site would be NL/12. A clinically important change in the precision of the UF rate was judged to be a ratio of 3 between the SDs, so the aim was to detect a change of log 3, that is, 1.098. The power will be 80% using a two-sided significance level of 5%. The sample size calculation will determine a value of L to achieve this.
The power of the trial will vary according to which sequences were allocated sites of the different sizes. The randomisation was restricted as described below, which reduced the size of this effect. A purpose-written R program showed that the power when L = 4.5 months was 0.80, if the smallest site was allocated to sequence 2 and 0.79 otherwise. This was for an ICC = 0.1. For ICC = 0.05, the values were 0.84 and 0.82, respectively and for ICC = 0.2, 0.77 and 0.75. With such a novel outcome variable there was no specific prior knowledge of the ICC. In cluster randomised studies values of ICC below 0.1 are common. The sample size was based on this value: smaller values would not compromise the power of the study; the larger values are judged to be unlikely but, if true, would lead to only small loss of power.
From analysis of PICANet audit data, in 2011–13, approximately 200 children under one year old received were provided with renal support (CVVH, PD or both) annually in the participating PICUs. Of these about 50% were under one month old. PICANet data did not include weight but it was all those under one month would weigh under 8 kg, and around 70–80% of the older group would weigh less than 8 kg. Overall, it was anticipated that 35–40% of these children would receive CVVH or CVVH + PD. Taking account of these figures and making conservative allowance for those refusing consent or dropping out for clinical reasons, it was anticipated that I-KID would be able to recruit about 63 babies a year from the combined units, with a target of 95 participants recruited over 20 months. In reality, I-KID recruited 97 participants over 24 recruiting months.
Randomisation of the SW design
The design for I-KID was a three-sequence SWD and six sites, allocating two sites to each sequence. As indicated previously, three sites (Evelina, GOSH and Southampton) were expected to recruit at rates about 50% higher than Bristol and Birmingham and much higher than Newcastle. It was convenient to designate the first three sites as large sites and the others as small sites. If two large sites were allocated to the same sequence, then two small sites would be allocated to another, and the design would have become rather unbalanced – for example if Newcastle and another small site were on the same sequence then the probability of periods with no recruits in that sequence might have been too high. Moreover, if the allocation process had allowed very different numbers of participants to be allocated to the different sequences, the variation in power between allocations could have been noticeable.
On the basis of these considerations, it was decided to restrict the randomisation so that one large site and one small site were allocated to each sequence. This will mitigate the risk of a sequence with very low recruitment and control the variation in power between different allocations.
The procedure for randomisation was as follows. The Senior Trial Manager produced a list in which each of the symbols A, B and C was associated with one of the large sites in an arbitrary order. A second list associated the symbols a, b and c with the small sites. These lists were not revealed to any other member of the TMG. The Senior Trial Statistician used the base function sample in R to produce a random permutation of the symbols A, B and C, with the first element of the permuted list being allocated to sequence 1, the second to sequence 2 and the last to sequence 3. This was repeated for the symbols a, b and c. This allocation, with one upper case and one lower case letter allocated to each sequence was passed to the Senior Trial Manager, who was able to form the random allocation of sites to sequences by substituting the actual site names for the symbols.
The allocations were revealed to the sites in sequence 1 only as far ahead of the end of period 1 as was necessary for training and for the sites to make appropriate practical arrangements. At this stage the allocations for the remaining sites were not revealed. The same procedure was adopted for sites changing over after period 2, although in this case concealment of the allocation of the sites to sequence 3 was unnecessary.
All parents/guardians were fully aware and informed of the treatment that the patient received.
Statistical analyses
The analyses undertaken at the end of the study are outlined below. No interim analyses of efficacy variables were planned or undertaken.
Primary analysis
The primary analysis of the primary outcome used a linear model with a categorical period effect, to allow for time effect during the study, and a binary treatment effect comparing NIDUS and controls. Differences between sites were accommodated by a fixed categorical effect. The log of the duration over which X and A were observed was included as a covariate. The fit of the model was assessed using standard diagnostic methods. The treatment effect estimated log σcontrol/σNIDUS and results are presented in terms of the estimated ratio and 95% confidence interval (CI). It should be noted that the outcome measure is based on the assumption that X-A has zero mean: if this assumption was not borne out by the data, alternative methods would be used.
Sensitivity analyses
-
The choice of a fixed effect for sites was unusual but obviated the need for the form of the dispersion structure of the responses to be specified. This approach is one of the methods outlined in Matthews and Forbes and was adopted because of the very uneven and fragmented intervals between recruitment periods consequent on recruitment suspensions, largely due to the COVID-19 pandemic. 31 A sensitivity analysis uses the more usual approach of a generalised estimating equation (GEE), adjusted for the small number of clusters using the method of Morel-Bokassa-Neerchal. 32 This correction differs from that specified in the original Statistical Analyses Plan (SAP) and is preferred because it has recently been found to have good properties with SW designs and can be implemented in Stata. 33
-
The definition of the primary outcome in the original protocol required that the values of X and A be observed within six hours of the inception of RRT. This was amended when it became clear that initial attempts at RRT were often interrupted because of the extreme clinical condition of the participants, which meant that other clinical procedures supervened (see Changes to primary outcome variable in Outcomes section). However, a sensitivity analysis was performed with the outcome following the original definition.
-
Recruitment had to be paused on several occasions, for varying durations and patterns. Pauses due to COVID-19 affected the whole study, whereas technical problems with NIDUS only affected those sites using NIDUS at the time. The intention to treat (ITT) principle was followed, with all available data analysed. However, for pauses in periods 2 and 3 when both control and NIDUS were in use, pauses only to NIDUS sites for some of those periods could lead to difficulty in the interpretation of the associated period effect. A sensitivity analysis omitted all participants recruited to control groups during the intervals when recruitment to NIDUS was paused.
Secondary analyses
Variables related to primary outcome
The values of X and A were observed in several episodes during the treatment of a patient. While the first computable value of log|X-A| was used as the primary outcome, the mean of all valid values of log|X-A| on a patient was used as a secondary outcome. To be valid the value had to be based on a collection period that started within 48 hours of the inception of RRT and lasted for at least an hour. The linear model used had the same form as for the primary analysis.
Biochemical clearance values: first recorded value
The rate of clearance of each of creatinine, urea and phosphate (PO4) was computed during each episode of RRT. The first such observation from each patient was analysed. A linear model with categorical covariates for centre, period and a binary treatment indicator with constant residual variance was proposed in the SAP. However, as will be shown in the results section, for these variables it was subsequently realised that it would be misleading to combine participants who received PD with those who received CVVH. It was also found that assuming a common residual variance was inappropriate, so the analysis used generalised least squares to compare the three treatment groups, assuming separate residual variances for each group.
Biochemical clearance values: average of recorded values
The analysis for the first recorded value was repeated, with outcome now being the mean of each of the clearances computed on each patient. Only observations from treatment episodes starting within 48 hours of the start of RRT are used.
Analysis of mortality
Descriptive summaries of mortality at 30 days after the start of RRT and on discharge from PICU were presented, and standard methods for binary variables were used to compare between NIDUS and control groups.
Analysis of PICANet outcomes
Information on variables collected from PICANET were analysed using descriptive summaries and, where indicated in the SAP, standard methods for comparing binary variables between NIDUS and Control were employed. Numbers of observations in each site by period group were sufficiently small that more sophisticated models were avoided. Period effects were seldom observed in analyses of other variables, which gives some support to the use of these simpler methods.
Subgroup analyses (all pre-specified)
Primary outcome by NIDUS, CVVH and PD
This analysis presented comparisons of the primary outcome between treatment groups of NIDUS, PD and CVVH, that is the primary analysis but with the control group split into CVVH and PD. The mean log|X-A| was also compared between these groups.
Mortality data by NIDUS, CVVH and PD
Descriptive statistics comparing mortality 30-day post RTT and on discharge from PICU were presented by NIDUS, CVVH and PD.
PICANet Variables by NIDUS, CVVH and PD
Descriptive statistics comparing the variables collected via PICANet were presented by NIDUS, CVVH and PD.
Actual versus reported fluid removal rates
The Aquarius, Prismaflex and NIDUS devices report the amount of fluid that they claim to have removed (A2). For these devices it was therefore possible to compare the amount actually removed, X, with that reported to have been removed, A2. This is of clinical importance, as the management of a patient could be seriously compromised if these two quantities are discrepant. The SAP anticipated analysing log|X-A2| provided that X-A2 appeared to have mean zero. This was not the case, so generalised least squares were used, with categorical covariates for centre and period and a binary indicator to distinguish NIDUS and CVVH. Different residual variances were allowed in the two treatment arms.
Study oversight and management
Trial management
The TMG was responsible for overseeing management of the study. The TMG met approximately every four to eight weeks during the course of the study. TMG meetings involved the Chief Investigator, trial statisticians, local co-applicants, members from the Northern Medical Physics and Clinical Engineering Directorate for technical support and development of the NIDUS device, a sponsor representative and trial management team members from Newcastle Clinical Trials Unit (NCTU).
Data management
A study-specific MACRO database was designed and built by the database manager with input from the TMG. Data for participants were entered into the electronic case report forms by local site staff.
Sites were asked to send all serious adverse device event (SADE) reporting forms, serious adverse event (SAE) logs and device deficiency (DD) logs were sent from site to the central study team in Newcastle using secure email.
The occurrence of events such as blood transfusions and access line changes were recorded by local site staff via the PICANet enhanced renal audit reporting system. Staff at site downloaded the PICANet data for their participants who had been consented to the study. The downloaded dataset was sent to the Database Manager at NCTU by secure email.
Data were handled, digitalised and stored in accordance with the Data Protection Act 2018.
Study oversight
Study oversight was provided by the study sponsor (the NuTH NHS Foundation Trust), the TSC and the Independent Data Monitoring Committee. A Clinical Safety Sub-Group consisting of clinicians from the TMG, representatives from Medical Physics and a sponsor representative reviewed all SADE and SAE safety reports. Device deficiencies that led to a SADE or had the potential to become a SADE underwent expedited reporting by Sponsor to the MHRA, in line with the requirements for a clinical investigation (see Appendix 7, Figure 28). SAEs that were not consistent with the usual clinical pattern for participants requiring RRT in PICU were recorded by site on the study SAE log. SAEs which were excluded from reporting in the protocol were recorded in the study database. All device deficiencies in the intervention arm were recorded by site on a DD log.
Patient and public involvement
Patient, Care and Public Involvement (PCPI) heavily shaped the study design. Feedback was sought from a group of parents with children on dialysis in Newcastle upon Tyne where considerable support was given to the study and the SW design. It was felt that obtaining consent for the type of dialysis method to be used would add to families’ stress and anxiety. Also, that parents were likely to default to the position of the medical team.
The choice of a SW design was strongly influenced by advice given through PCPI. The method of randomising the site, rather than the patient, and delayed consent to collect and record information for the study was supported by a Newcastle University Research Consumer Group, parents who were consulted and health professionals. This study took place in the Paediatric Intensive Care environment, necessitating a level of urgency to recruit, consent and initiate RRT without compromising the participants’ health further which raised ethical concerns. 29
One of the study’s co-applicant is a parent who has experience of the NIDUS in use and has been involved in the study development from the start to ensure that methods were acceptable, inclusive and sensitive. They were also involved in presenting at the study launch and the PICANet study day. The Study Information Sheets were produced in collaboration with several parents and advice from parents has been sought on how best to disseminate the study results.
Initial development of NIDUS infant dialysis device was in response to concerns raised by parents of babies in whom other dialysis had failed and for whom there were no alternatives. One parent, CB, has been involved in study development from start and brings an important perspective, ensuring inclusion of compassionate use and that methods are acceptable and sensitive. Feasibility and ethical concerns of three families about individualised randomisation and consent in a life-threatening situation, which then have shaped the study design. Discussion with Newcastle Research Consumer Group provided invaluable feedback about how very important they considered this study to be; they discussed the problems of consent and individual randomisation and had favourable views of the cluster wedge step design proposed as units are randomised to intervention not individuals; they supported inclusion of compassionate use in the study. Parents in charity parent group Children’s Heart Unit Fund were asked to comment on drafts of the Plain language summary.
Chapter 3 Results
Patient flow
The CONSORT flow diagram has been prepared in accordance with the CONSORT extension for SW designs and is available as Figure 3. 34 Six sites were allocated into one of three sequences with two sites per sequence. There were five recruitment pauses that occurred throughout the study; most of which were specific to NIDUS recruitment with the exception of the period when COVID-19 pandemic restrictions were in place.
FIGURE 3.
CONSORT flow diagram. 34 Shaded boxes indicate that the sites were under intervention conditions and white boxes indicate that sites were under control conditions. Screening of participants continued during recruitment pauses. Only the numbers of participants not recruited due to recruitment pauses and high screening weight are reported in the boxes; details of other non-recruited cases are in Table 1. Newcastle switched to NIDUS later than GOSH as Newcastle still needed to complete the function testing of the device. * The site was recruiting into control during this period but had confused the pause to recruitment to NIDUS with the end of the study. The reason documented was ‘Study finished early’.

| Sequence | 1 | 2 | 3 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Patient eligible but not included | |||||||||||||
| No legal guardian/parent present | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Missed by staff | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 3 | 6 | 1 | 16 |
| Other | 2 | 3 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 16 |
| Patient died before consent obtained | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 4 |
| Not interested in participating | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 6 |
| Not appropriate – child deteriorated | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Research staff availability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Study pauses (MHRA withdrawal of notice of no objection, safety and COVID-19 pauses) | 0 | 1 | 6 | 0 | 0 | 1a | 2 | 3 | 0 | 0 | 28 | 16 | 57 |
| Patient not eligible | |||||||||||||
| Weight >7.99 kg | 8 | 15 | 18 | 22 | 0 | 0 | 0 | 0 | 2 | 3 | 3 | 7 | 78 |
| Weight <0.8 kg | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Known chronic renal failure | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 6 | 12 |
| Already on established RRT (for whom entry into the study would require additional central venous access) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 5 |
| Underlying metabolic diagnosis | 0 | 0 | 3 | 1 | 1 | 2 | 3 | 4 | 2 | 2 | 3 | 1 | 22 |
| Clinical decision – not to receive RRT using NIDUS | 0 | 1 | 1 | 17 | 0 | 0 | 6 | 14 | 0 | 0 | 1b | 6 | 46 |
| Other | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 3 | 13 |
Screening
Recruitment began on 5 December 2018 and ended on 31 August 2021. A total of 376 participants were screened of which 102 were deemed eligible but not recruited, and 97 were recruited into I-KID. Reasons for the 279 participants not included into the study are summarised in Table 1. Details of those labelled as ‘Other’ are presented in Report Supplementary Material 1 Table SCR1 and Table SCR2. A weight exceeding 7.99 kg (n = 78) and the clinical decision by the attending clinician not to use NIDUS were the most common reasons for ineligibility of screened participants. For most of the participants who were eligible but not recruited, this was because of the various recruitment pauses.
Of the 102 eligible participants who were not included, 59 were from the control phases and 43 from the intervention phases. Of the 177 ineligible participants, 42 were from the control phases and 135 from the intervention phases.
Recruitment
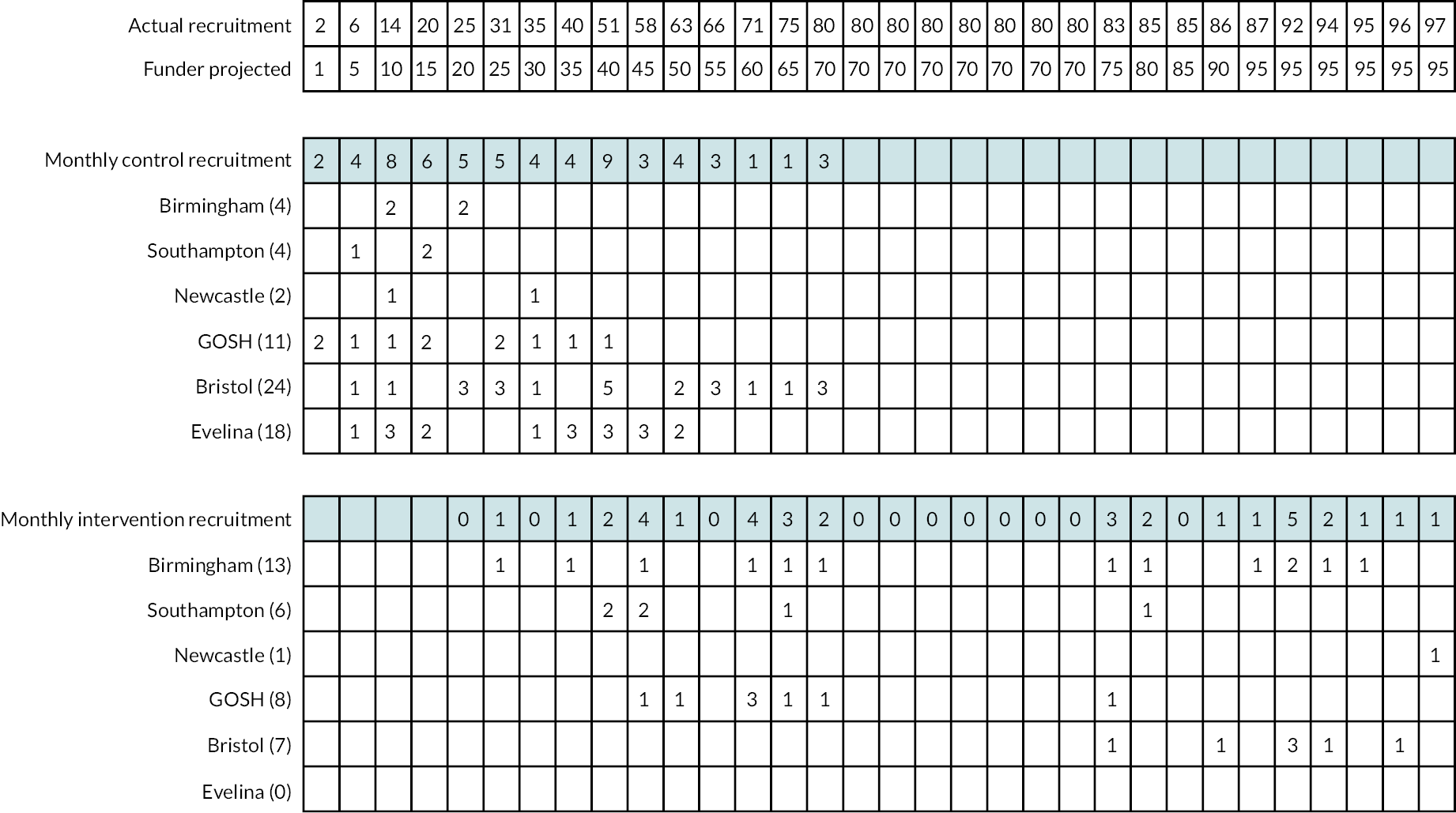
Study recruitment, together with the periods in which this was paused, along with the associated reasons is shown in Figure 4. A diagram of participant recruitment over time is presented in Figure 5. Line graph of actual versus projected recruitment with observed recruitments numbers by site and arm in Figure 6.
FIGURE 4.
Cumulative actual and projected recruitment by intervention arm by month.

FIGURE 5.
Line graph of actual vs. projected recruitment. Shaded areas describe the various recruitment pauses: (a) recruitment pause to NIDUS [20/05/2019–26/06/2019]; (b) recruitment pause to NIDUS [23/09/2019–18/10/2019]; (c) COVID-19-related pause [02/03/2020–05/10/2020]; (d) recruitment pause to study [04/11/2020–18/12/2020]. One patient was recruited into the intervention arm with MHRA approval; (e) Evelina recruitment pause [04/11/2020–06]. The black vertical line indicates the date the study restarted post COVID-19 [05/10/2020].
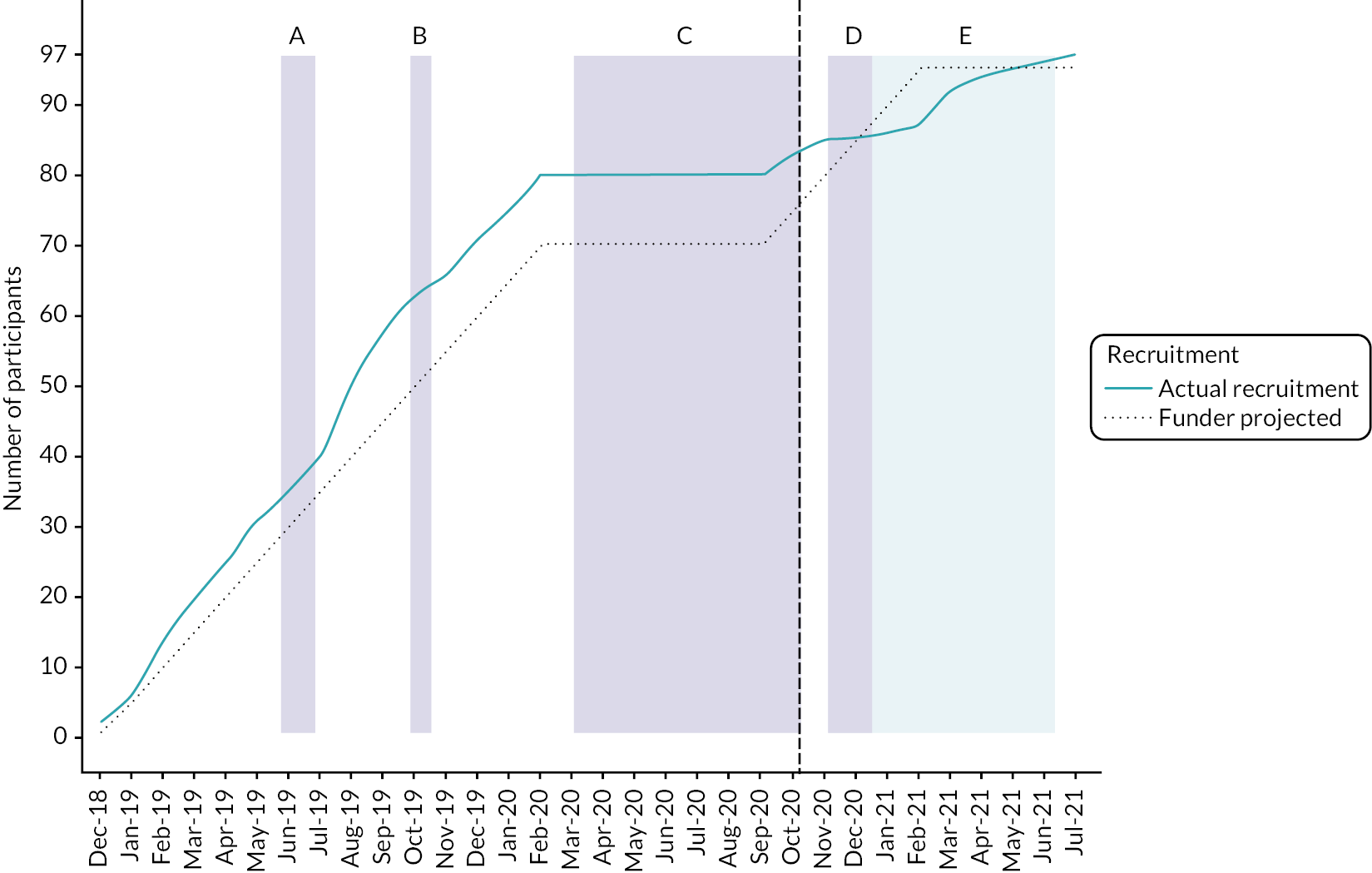
FIGURE 6.
Patient recruitment over time. Top table: The cumulative actual and projected recruitment numbers. Middle table: The monthly recruitment figures into the control arm by site. Figures in parentheses next to the site are the total numbers recruited into the control arm for that site. Bottom table: The monthly recruitment figures into the intervention arm by site. Figures in parentheses next to the site are the total numbers recruited into the intervention arm for that site.
Protocol deviations, study losses and compliance
Protocol deviations and violations
In total there were 23 deviations and violations. Full details are in Appendix 2, Table 34.
Losses to follow up, withdrawals and death
There were no withdrawals and no losses to follow up. Twenty-two participants died by the 30-day follow-up or by the time they were discharged from PICU, whichever came earlier (10 control, 12 NIDUS). Full details are in Appendix 2, Table 35 and see also Key findings in Chapter 4.
Treatment compliance
Details of treatment compliance can be found in the section on treatment compliance in Appendix 2.
Baseline characteristics
The baseline characteristics of the 97 participants (62 control, 35 intervention) recruited to the study are described in Table 2. Descriptive statistics of laboratory measures before the initiation of RRT are presented in Table 3. The treatment groups appeared balanced with respect to baseline characteristics and pre-RRT laboratory measurements, though with a slightly higher proportion of males and higher creatinine and urea values in the intervention arm. Tables giving baseline characteristics by the modality of RRT are in Appendix 3, Tables 38 and 39. Further descriptive statistics of age (days) at screening, weight (kg) at RRT initiation, and Paediatric Index of Mortality 3 (PIM3) scores by period and sequence are presented in Report Supplementary Material 1 Section SBL1.
| Control (n = 62) | Intervention (n = 35) | Total (n = 97) | |
|---|---|---|---|
| Age at screening, days | |||
| Mean (SD) | 52.10 (100.80) | 91.66 (136.85) | 66.37 (115.99) |
| Median (IQR) | 10.50 (7.00–38.00) | 11.00 (7.00–124.00) | 11.00 (7.00–61.00) |
| Range | 1.00–477.00 | 1.00–443.00 | 1.00–477.00 |
| Available, n | 62 | 35 | 97 |
| Sex, n (%) | |||
| Female | 27 (43.55) | 8 (22.86) | 35 (36.08) |
| Male | 35 (56.45) | 27 (77.14) | 62 (63.92) |
| Weight at RRT initiation, kga | |||
| Mean (SD) | 3.76 (1.59) | 4.33 (1.72) | 3.97 (1.65) |
| Median (IQR) | 3.20 (2.90–3.90) | 3.70 (3.10–5.60) | 3.50 (3.00–4.60) |
| Range | 1.80–10.10 | 1.00–7.80 | 1.00–10.10 |
| Available, n | 62 | 35 | 97 |
| Type of weight measurement, n (%) | |||
| Actual weight | 51 (82.26) | 34 (97.14) | 85 (87.63) |
| Estimated weight | 11 (17.74) | 1 (2.86) | 12 (12.37) |
| Gestational age at delivery (completed weeks) | |||
| Mean (SD) | 37.93 (2.25) | 36.49 (3.74) | 37.41 (2.95) |
| Median (IQR) | 38.00 (37.00–39.00) | 38.00 (35.00–39.00) | 38.00 (37.00–39.00) |
| Range | 28.00–41.00 | 26.00–41.00 | 26.00–41.00 |
| Available, n | 61 | 35 | 96 |
| Type of admission to unit, n (%) | |||
| Planned – following surgery | 31 (50.00) | 13 (37.14) | 44 (45.36) |
| Unplanned – following surgery | 1 (1.61) | 1 (2.86) | 2 (2.06) |
| Planned – other | 2 (3.23) | 4 (11.43) | 6 (6.19) |
| Unplanned | 28 (45.16) | 17 (48.57) | 45 (46.39) |
| Previous ICU admission, n (%) | |||
| ICU | 0 | 1 (2.86) | 1 (1.03) |
| PICU | 4 (6.45) | 4 (11.43) | 8 (8.25) |
| Neonatal ICU | 29 (46.77) | 12 (34.29) | 41 (42.27) |
| None | 26 (41.94) | 18 (51.43) | 44 (45.36) |
| Unknown | 3 (4.84) | 0 | 3 (3.09) |
| Source of admission, n (%) | |||
| Same hospital | 40 (64.52) | 23 (65.71) | 63 (64.95) |
| Other hospital | 22 (35.48) | 12 (34.29) | 34 (35.05) |
| Elective admission, n (%) | |||
| No | 29 (46.77) | 18 (51.43) | 47 (48.45) |
| Yes | 33 (53.23) | 17 (48.57) | 50 (51.55) |
| Main reason for PICU admission, n (%) | |||
| Other | 30 (48.39) | 20 (57.14) | 50 (51.55) |
| Bronchiolitis | 1 (1.61) | 2 (5.71) | 3 (3.09) |
| Recovery from surgery | 31 (50.00) | 12 (34.29) | 43 (44.33) |
| Seizure disorder | 0 | 1 (2.86) | 1 (1.03) |
| If admission was recovery from surgery, what was procedure, n (%) | |||
| Bypass cardiac procedure | 30 (96.77) | 10 (83.33) | 40 (93.02) |
| Non–bypass cardiac procedure | 0 | 1 (8.33) | 1 (2.33) |
| Other procedure | 1 (3.23) | 1 (8.33) | 2 (4.65) |
| Is evidence available to assess past medical history? n (%) | |||
| Yes | 55 (88.71) | 35 (100.00) | 90 (92.78) |
| No | 7 (11.29) | 0 | 7 (7.22) |
| Systolic blood pressure, mmHg | |||
| Mean (SD) | 72.20 (19.45) | 74.86 (22.33) | 73.19 (20.49) |
| Median (IQR) | 68.00 (59.00–78.00) | 68.00 (60.00–86.00) | 68.00 (60.00–82.00) |
| Range | 40.00–137.00 | 36.00–134.00 | 36.00–137.00 |
| Available, n | 59 | 35 | 94 |
| Base excess source, n (%) | |||
| Arterial | 44 (70.97) | 23 (65.71) | 67 (69.07) |
| Capillary | 6 (9.68) | 6 (17.14) | 12 (12.37) |
| Venous | 4 (6.45) | 3 (8.57) | 7 (7.22) |
| Available, n | 54 | 32 | 86 |
| Lactate source, n (%) | |||
| Arterial | 44 (70.97) | 23 (65.71) | 67 (69.07) |
| Capillary | 7 (11.29) | 7 (20.00) | 14 (14.43) |
| Venous | 4 (6.45) | 3 (8.57) | 7 (7.22) |
| Available, n | 55 | 33 | 88 |
| Mechanical ventilation, n (%) | |||
| Yes | 50 (80.65) | 30 (85.71) | 80 (82.47) |
| No | 12 (19.35) | 5 (14.29) | 17 (17.53) |
| Received continuous positive airway pressure within first hour, n (%) | |||
| Yes | 5 (8.06) | 2 (5.71) | 7 (7.22) |
| No | 57 (91.94) | 33 (94.29) | 90 (92.78) |
| Pupil reaction, n (%) | |||
| Both fixed and dilate | 0 | 1 (2.86) | 1 (1.03) |
| Other reaction | 56 (90.32) | 28 (80.00) | 84 (86.60) |
| Unknown | 6 (9.68) | 6 (17.14) | 12 (12.37) |
| PIM3 score | |||
| Mean (SD) | 0.070 (0.100) | 0.095 (0.172) | 0.079 (0.130) |
| Median (IQR) | 0.023 (0.013–0.065) | 0.027 (0.014–0.131) | 0.025 (0.014–0.093) |
| Range | 0.005–0.445 | 0.006–0.972 | 0.005–0.972 |
| Available, n | 62 | 35 | 97 |
| Logit of PIM3 score | |||
| Mean (SD) | −3.36 (1.29) | −3.05 (1.67) | −3.24 (1.44) |
| Median (IQR) | −3.74 (−4.30 to −2.67) | −3.58 (–4.24 to −1.90) | –3.68 (−4.28 to −2.28) |
| Range | –5.29 to −0.22 | −5.12 to 3.54 | −5.29 to 3.54 |
| Available, n | 62 | 35 | 97 |
| Control (n = 65) | Intervention (n = 32) | Total (n = 97) | |
|---|---|---|---|
| Sodium, mmol/l | |||
| Mean (SD) | 145.42 (6.33) | 141.57 (6.52) | 144.03 (6.63) |
| Median (IQR) | 146.00 (141.00–149.00) | 143.00 (136.00–146.00) | 144.00 (140.00–148.00) |
| Range | 130.00–157.00 | 128.00–156.00 | 128.00–157.00 |
| Available, n | 62 | 35 | 97 |
| Potassium, mmol/l | |||
| Mean (SD) | 4.67 (0.81) | 4.88 (1.00) | 4.75 (0.89) |
| Median (IQR) | 4.45 (4.00–5.20) | 4.80 (4.20–5.50) | 4.60 (4.10–5.20) |
| Range | 3.50–6.70 | 3.30–8.30 | 3.30–8.30 |
| Available, n | 60 | 35 | 95 |
| Creatinine, mmol/l | |||
| Mean (SD) | 77.24 (98.21) | 106.00 (113.38) | 87.62 (104.28) |
| Median (IQR) | 51.50 (40.00–68.00) | 74.00 (56.00–110.00) | 60.00 (42.00–87.00) |
| Range | 12.00–623.00 | 9.00–678.00 | 9.00–678.00 |
| Available, n | 62 | 35 | 97 |
| Urea, mmol/l | |||
| Mean (SD) | 8.33 (7.96) | 12.54 (9.81) | 9.85 (8.86) |
| Median (IQR) | 6.15 (3.50–10.60) | 9.50 (4.70–17.20) | 7.20 (3.70–11.70) |
| Range | 1.70–45.40 | 2.20–36.80 | 1.70–45.40 |
| Available, n | 62 | 35 | 97 |
| Phosphate, mmol/l | |||
| Mean (SD) | 2.21 (0.64) | 2.43 (0.68) | 2.29 (0.66) |
| Median (IQR) | 2.20 (1.75–2.70) | 2.43 (2.13–2.93) | 2.36 (1.79–2.75) |
| Range | 0.61–3.58 | 0.65–3.53 | 0.61–3.58 |
| Available, n | 62 | 35 | 97 |
| Actual bicarbonate, mmol/l | |||
| Mean (SD) | 21.74 (4.32) | 20.53 (4.79) | 21.31 (4.50) |
| Median (IQR) | 21.50 (18.40–25.40) | 20.20 (17.50–24.00) | 21.40 (18.05–24.15) |
| Range | 12.70–33.00 | 6.50–31.60 | 6.50–33.00 |
| Available, n | 62 | 34 | 96 |
| Base excess, mmol/l | |||
| Mean (SD) | −3.44 (6.01) | −4.95 (6.73) | −3.97 (6.27) |
| Median (IQR) | −4.25 (−7.75 to 0.85) | −4.55 (−8.00 to −1.45) | −4.25 (−7.75 to 0.20) |
| Range | −18.00 to 10.30 | −26.70 to 9.10 | −26.70 to 10.30 |
| Available, n | 60 | 32 | 92 |
| pH | |||
| Mean (SD) | 7.33 (0.12) | 7.28 (0.13) | 7.31 (0.12) |
| Median (IQR) | 7.33 (7.24–7.43) | 7.29 (7.21–7.36) | 7.32 (7.24−7.40) |
| Range | 7.02−7.52 | 6.86−7.54 | 6.86−7.54 |
| Available, n | 62 | 35 | 97 |
| Haemoglobin, g/l | |||
| Mean (SD) | 125.89 (28. 99) | 114.46 (26.86) | 121.76 (28.63) |
| Median (IQR) | 125.50 (111.00–148.00) | 120.00 (90.00–129.00) | 123.00 (101.00–140.00) |
| Range | 56.00–194.00 | 65.00–184.00 | 56.00–194.00 |
| Available, n | 62 | 35 | 97 |
| Platelets, ×109/l | |||
| Mean (SD) | 201.18 (112.39) | 146.53 (82.21) | 181.82 (105.59) |
| Median (IQR) | 207.50 (113.00–257.00) | 124.00 (95.00–209.00) | 163.50 (101.50–241.50) |
| Range | 34.00–582.00 | 20.00–337.00 | 20.00–582.00 |
| Available, n | 62 | 34 | 96 |
| Primary indication for starting RRT, n (%) | |||
| Fluid volume control | 32 (51.61) | 17 (48.57) | 49 (50.52) |
| Biochemical control | 8 (12.90) | 9 (25.71) | 17 (17.53) |
| Fluid and chemical equally | 22 (35.48) | 9 (25.71) | 31 (31.96) |
Primary outcome measure
Availability of primary outcome
The actual fluid removal rate, X, and the prescribed removal rate, A, were observed during several episodes throughout the period that a patient was on RRT. The primary outcome variable was defined as log|X-A| from the first episode at which X and A were available, provided that the episode lasted at least an hour and started within 48 hours of the inception of RRT.
For the 62 participants who received the control treatment, the primary outcome was available for 61 participants. For one patient, who was receiving PD, the first episode to provide X-A started 67 hours after the inception of RRT. However, as the analysis of the primary outcome was by ITT, this observation was included in the analysis of the primary outcome.
For the 35 participants who received NIDUS, a primary outcome was available for 21 participants. One of these 21 participants was a transition baby and was excluded from the primary analysis. For 14 participants receiving NIDUS no value of X-A was available. The reasons for the 14 missing values are summarised in Table 4, with more detail provided in Appendix 2, Table 37. In most cases the reason why no value of the primary outcome was available was that technical difficulties were experienced in establishing or sustaining RRT using NIDUS. In some of these 14 cases, the patient spontaneously started to pass urine so RRT was no longer needed; others were so sick that they died very quickly. In five cases it was recorded that another method of RRT was attempted but in none of these cases was a value of X-A available, which probably reflects the clinical circumstances at the time. Three of the 14 participants without a primary outcome were transition babies, so would not have been included in the primary analysis even if they had had outcomes recorded.
| Centre | No. allocated to NIDUS | No. allocated to NIDUS without primary outcomea | Reasons for missing outcome | Outcome for participants without primary outcome | No. without primary outcome with a reported DD |
|---|---|---|---|---|---|
| Birmingham | 13 | 4a | Multiple filter changes and access issues (n = 1) Problems with circuit and ACT (n = 1)b Multiple filter clots (n = 1) Air bubbles in syringe withdraw pack and filter clot (n = 1) |
Changed to Aquarius (n = 2) Not known (n = 2)b |
0 |
| Southampton | 6 | 4a | Blood leaking into waste bag (n = 1) Problems with blood in circuit and filter clots before patient passed urine (n = 1)b Patient needed ECMO after a few hours (n = 1) Multiple filter clots (n = 1) |
Changed to Prismaflex (n = 2) Decision to insert a PD catheter instead for RRT (n = 1) Started passing urine (n = 1)b |
2 |
| Newcastle | 1 | 0 | NA | ||
| GOSH | 8 | 5a | Machine malfunction (n = 1)b Filter clot 20 minutes after start. Was not connected to new filter as patient passed urine (n = 1) Filter clot after cryoprecipitate and platelets administered; futility of treatment agreed between medical team and family (n = 1) Filter clot despite a good line (n = 1) Filter clot due to non-compliance with NIDUS-specific heparin guidelines (n = 1) |
Started passing urine (n = 1) Died on day of RRT initiation (n = 1) Not known (n = 3)b |
5 |
| Bristol | 7 | 1 | Air bubbles and filter clot (n = 1) | Not known (n = 1) | 1 |
| Evelina | 0 | 0 | NA | ||
| Total | 35 | 14 |
Description of primary outcome
Summaries of the baseline characteristics of the 20 participants receiving NIDUS who were included in the primary analysis, are given in Appendix 3, Tables 40 and 41. Also, in these tables are the summaries of the 11 participants allocated to NIDUS that would have been included in the primary analysis had values for X-A been available. Summaries of the same variables comparing the 62 control participants and the 20 Intervention participants included in the primary analysis are in Appendix 3, Tables 42 and 43.
Descriptive statistics for the data used for the primary analysis are in Table 5. The use of log|X-A| for the primary outcome was based on the assumption that the expectation of X-A is zero. The means and SDs for X-A shown in Table 5 lend support to this assumption. A histogram and boxplot of X-A are shown in Figures 5 and 6 to illustrate that this variable appears to be symmetrically distributed about zero: it should also be noted that the spread is considerably less in the intervention than the control group. Further descriptive statistics of X-A by period and sequence are presented in Report Supplementary Material 1 Table SPO1.
FIGURE 7.
Boxplot and histogram of first computable precision (X-A) by arm. Top: Boxplot of precision by arm for n = 83 participants with a computable precision measurement. Mid-blue points denote the participants in the control arm and the Orange points denote participants in the intervention arm. Point in dark blue indicates the NIDUS participant recruited during a transition period (precision = −13.12). Bottom: Histogram of precision by arm. Colour scheme is in accordance with the boxplot.
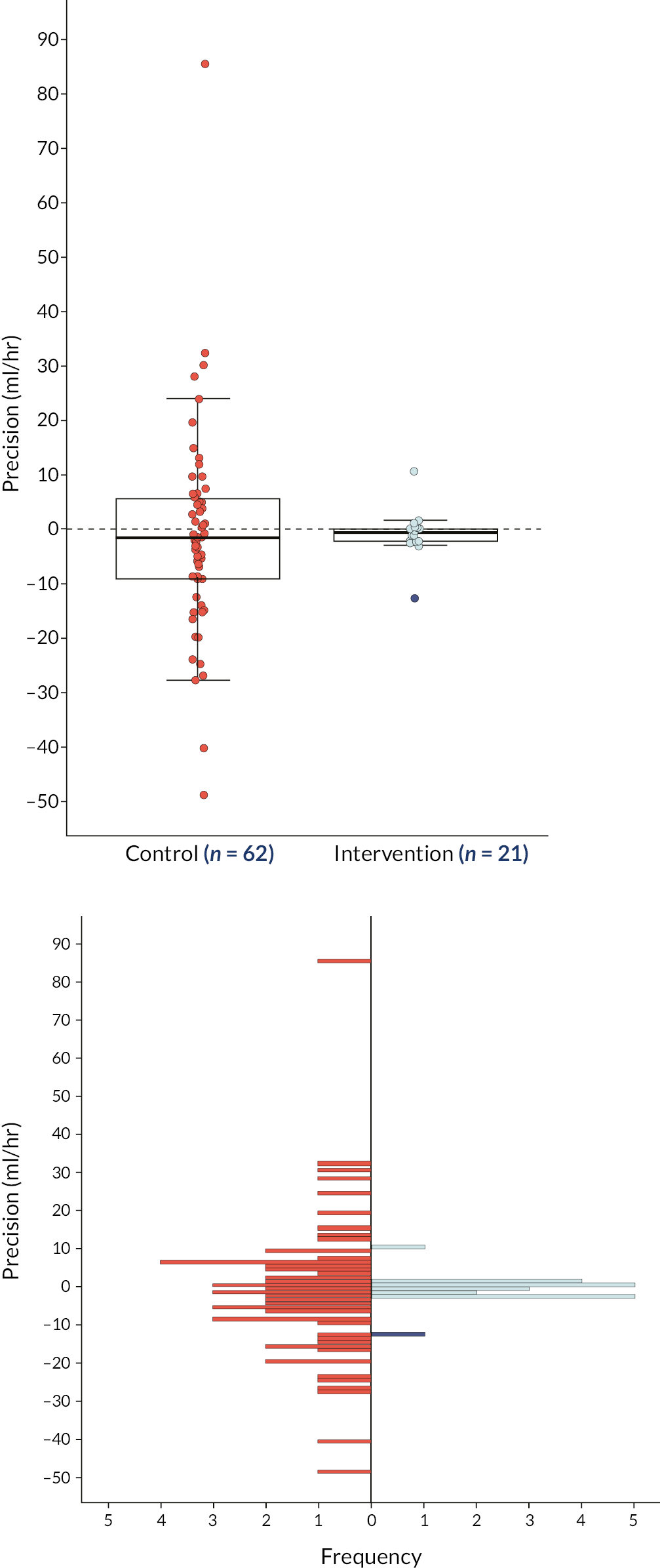
| Control (n = 62) | Intervention (n = 20) | |
|---|---|---|
| X, ml/hour | ||
| Mean (SD) | 14.42 (21.93) | 14.88 (9.37) |
| Median (IQR) | 9.67 (4.00–16.83) | 12.88 (9.08–19.57) |
| Range | −5.17 to 143.32 | 0.54 to 36.67 |
| A, ml/hour | ||
| Mean (SD) | 15.87 (15.14) | 15.36 (10.14) |
| Median (IQR) | 10.00 (5.00–28.48) | 12.26 (8.58–21.15) |
| Range | 0.00–60.00 | 0.00–40.00 |
| Precision (X-A), ml/hour | ||
| Mean (SD) | −1.45 (18.75) | −0.48 (2.95) |
| Median (IQR) | −1.67 (−9.36 to 5.58) | −0.55 (−2.48 to 0.26) |
| Range | −49.17 to 85.32 | −3.33 to 10.32 |
| log|X-A| | ||
| Mean (SD) | 1.85 (1.40) | −0.02 (1.41) |
| Median (IQR) | 1.93 (1.26–2.75) | 0.31 (−0.72 to 0.94) |
| Range | −3.03 to 4.45 | −3.91 to 2.33 |
A linear model with categorical covariates for centre, period and treatment arm was fitted to X-A to investigate further. Only the effect for period 2 was significant out of the 11 fitted parameters and this seemed to be due to a marked outlier in period 2 and in the control group (see Appendix 4, Figure 14). There was no compelling evidence against the assumption that X-A has zero mean, so the primary analysis was based on log|X-A|.
Analysis of log|X-A|
The linear model described in Primary analysis under the Statistical analyses section was fitted. The difference in means Control – Intervention is −2.00, which corresponds to a ratio of the SDs of X-A of exp(−2.00) = 0.13, that is the SD of the true fluid removal rate around the prescribed rate for NIDUS is 13% of that under control, with 95% CI from 3 to 70%. This is shown in the first row of Table 6. Note that this figure is consistent with the ratio of the sample SDs in Table 5, namely 2.75/18.75 = 0.16. The fit of the model was assessed using standard methods and found to be satisfactory: full details of the fitted model are in Appendix 4, Tables 44 and 45.
| Estimated treatment effect | ||
|---|---|---|
| Mean difference (95% CI); p-value | Ratio of SNIDUS/Scontrol (95% CI) | |
| Primary outcome (full analysis population, ITT) | −2.00 (−3.64 to −0.35); p = 0.018 | 0.13 (0.03 to 0.71) |
| n | 82 | |
| Sensitivity analysis: data from 0 to 6 hours only | −1.97 (−3.95 to 0.02); p = 0.052 | 0.14 (0.02 to 1.02) |
| n | 63 | |
| Sensitivity analysis accounting for recruitment interruptionsa | −2.13 (−3.83 to −0.43); p = 0.015 | 0.12 (0.02 to 0.65) |
| n | 75 | |
| Sensitivity analysis: GEE applied to full analysis population (ITT) | ||
| GEE with (Liang and Zeger) robust sandwich standard errors | −2.29 (−3.50 to −1.08); p < 0.001 | 0.10 (0.03 to 0.34) |
| GEE with Morel-Bokossa-Neerchal bias-corrected standard errors | −2.29 (−3.41 to −1.17); p < 0.001 | 0.10 (0.03 to 0.31) |
Three forms of sensitivity analysis are also described in the Statistical analyses section. The first two used the same statistical model, applied to outcomes (1) collected in the first six hours of RRT (as per original protocol) and (2) outcomes adjusted for periods when recruitment to NIDUS was interrupted. In these two cases P = 0.017, and 0.015, with the ratio of SDs estimated at 10% and 12%, respectively. The final form uses a GEE to allow for between-centre variation, with fixed effects for treatment and period effects. Two versions were used: the first computed standard errors from the robust sandwich estimator (Liang and Zeger, 1986) and the second adjusted the standard errors for the small number of centres using the method of Morel, Bokossa and Neerchal. 32,35 Both analyses gave P < 0.001 and estimated the SD ratio as 10%, and with slightly narrower CIs than for the models with fixed centre effects.
The estimate of the ratio of the SDs of X-A remains stable in the region 10% to 14% across all analyses: the upper end of the CI remains less than one except for the 0–6 hours sensitivity analysis, which is based on the smallest dataset used here.
Secondary outcome measures
The secondary outcomes fall into four categories: (1) the same variable used for the primary outcome but averaged over all available episodes; (2) outcomes related to biochemical clearances; (3) measures of mortality and (4) variables collected from PICANet. The data from transition babies were excluded from analyses (1) and (2) but not from (3) and (4) as these data could have been unaffected by a lack of familiarity with NIDUS.
Average value of log|X-A|
The average value of all the available log|X-A| collected within 48 hours of inception of RRT was computed (see Table 7). This analysis excluded the patient on PD whose only value of log|X-A| was collected more than 48 hours from the start of RRT. There were up to four observations on each patient: in the control group the number of participants providing 1, 2, 3 or 4 observations was 6, 18, 13, 24, respectively, whereas in the NIDUS group the corresponding numbers were 9, 3, 5, 4.
| Control (n = 61) | Intervention (n = 20) | Total (n = 81) | |
|---|---|---|---|
| Average log|X-A| | |||
| Mean (SD) | 1.88 (1.12) | 0.15 (0.97) | 1.45 (1.31) |
| Median (IQR) | 1.88 (1.26–2.48) | 0.29 (−0.38–0.69) | 1.55 (0.52–2.24) |
| Range | −0.69 to 4.11 | −1.96 to 2.33 | −1.96 to 2.33 |
The same linear model used for the primary outcome was fitted. The estimated treatment difference (NIDUS – Control) (95% CI) was −2.05 (−3.22 to −0.88), with P = 0.001. This corresponds to a ratio of SDs NIDUS: control of 0.13 (0.04 to 0.41), which is in line with the primary analysis but with a narrower CI. Full details of the fitting of the model are in Appendix 5.
Rates of biochemical clearance
The rates of clearance of creatinine, urea and phosphate (in ml/min) were computed from all episodes of RRT where the necessary data were available. Data from transition babies were excluded (three for each metabolite). Two analyses were performed for each metabolite, one on the first observed clearance and the second on the mean of all clearances available on a patient.
The SAP prescribed comparing two groups, namely NIDUS and non-NIDUS groups. The results of this analysis are shown in Tables 8 and 9 where we see that for each metabolite there is a difference between NIDUS and control for the residual SD but no evidence of any difference in the mean clearances.
| Control | NIDUS | |
|---|---|---|
| Creatinine clearance, ml/min/kg | ||
| Mean (SD) | 0.33 (0.56) | 0.46 (0.30) |
| Median (IQR) | 0.10 (0.07–0.14) | 0.39 (0.31–0.47) |
| Range | 0.02–2.91 | 0.06–1.50 |
| Available, n | 61 | 25 |
| Urea clearance, ml/min/kg | ||
| Mean (SD) | 0.35 (0.53) | 0.48 (0.30) |
| Median (IQR) | 0.13 (0.09–0.30) | 0.43 (0.33–0.57) |
| Range | 0.03–2.69 | 0.01–1.60 |
| Available, n | 60 | 27 |
| Phosphate clearance, ml/min/kg | ||
| Mean (SD) | 0.31 (0.54) | 0.44 (0.27) |
| Median (IQR) | 0.08 (0.06–0.14) | 0.35 (0.27–0.50) |
| Range | 0.03–2.58 | 0.11–1.41 |
| Available, n | 58 | 26 |
| Mean difference (95% CI); p-value NIDUS – control |
SD SNIDUS |
SD control and ratio SDs SNIDUS/Scontrol (95% CI) |
|
|---|---|---|---|
| First computable creatinine clearance (ml/min/kg) | 0.0 (−0.36 to 0.35); p = 0.99 | 0.29 | 0.55 0.52 (0.37 to 0.77) |
| First computable urea clearance (ml/min/kg) | 0.01 (−0.32 to 0.34); p = 0.95 | 0.28 | 0.52 0.54 (0.39 to 0.79) |
| First computable phosphate clearance (ml/min/kg) | 0.0 (−0.32 to 0.33); p = 0.98 | 0.26 | 0.52 0.49 (0.35 to 0.71) |
However, this analysis is misleading because, for each metabolite, the mean clearance rate was generally larger for CVVH than for NIDUS and for NIDUS was larger than PD (see Figures 8–10). To combine these two groups, one with generally larger values and one with smaller values than for NIDUS would seriously misrepresent the data. The analysis comparing three interventions, CVVH, PD and NIDUS (with the single Manual HD and ECMO excluded) was specified as a subgroup analysis in the SAP, and this is presented in Tables 10 and 11. It is also clear from Figures 8–10 that the residual SD differs between PD, NIDUS and CVVH, so generalised least squares was used to fit a model with categorical covariates for centre, period, treatment at three levels and separate residual variances in each treatment group.
FIGURE 8.
Boxplot of first computable biochemical clearance by mode of RRT – creatinine.

FIGURE 9.
Boxplot of first computable biochemical clearance by mode of RRT – urea.

FIGURE 10.
Boxplot of first computable biochemical clearance by mode of RRT – phosphate.

| PD | CVVH | NIDUS | |
|---|---|---|---|
| Creatinine clearance, ml/min/kg | |||
| Mean (SD) | 0.08 (0.03) | 1.20 (0.72) | 0.46 (0.30) |
| Median (IQR) | 0.08 (0.06–0.10) | 0.93 (0.75–1.47) | 0.39 (0.31–0.47) |
| Range | 0.02–0.15 | 0.47–2.91 | 0.06–1.50 |
| Available, n | 47 | 13 | 25 |
| Urea clearance, ml/min/kg | |||
| Mean (SD) | 0.12 (0.06) | 1.15 (0.67) | 0.48 (0.30) |
| Median (IQR) | 0.11 (0.09–0.14) | 0.91 (0.83–1.24) | 0.43 (0.33–0.57) |
| Range | 0.03–0.30 | 0.42–2.69 | 0.01–1.60 |
| Available, n | 46 | 13 | 27 |
| Phosphate clearance, ml/min/kg | |||
| Mean (SD) | 0.07 (0.04) | 1.16 (0.71) | 0.44 (0.27) |
| Median (IQR) | 0.06 (0.05–0.08) | 0.99 (0.62–1.39) | 0.35 (0.27–0.50) |
| Range | 0.03–0.23 | 0.34–2.58 | 0.11–1.41 |
| Available, n | 45 | 12 | 26 |
| Mean difference (95% CI); p-value NIDUS – PD |
Mean difference (95% CI); p-value NIDUS – CVVH |
SD SNIDUS |
SD PD and ratio SDs SNIDUS/SPD (95% CI) |
SD CVVH and ratio SDs SNIDUS/SCVVH (95% CI) |
|
|---|---|---|---|---|---|
| First computable creatinine clearance (ml/min/kg) | 0.32 (0.13 to 0.5); p = 0.001 | −0.80 (−1.23 to −0.37); p < 0.001 | 0.30 | 0.03 10.92 (7.90 to 15.16) |
0.72 0.42 (0.26 to 0.63) |
| First computable urea clearance (ml/min/kg) | 0.31 (0.13 to 0.50); p = 0.001 | −0.72 (−1.12 to −0.31); p < 0.001 | 0.30 | 0.06 5.09 (3.66 to 7.07) |
0.67 0.45 (0.27 to 0.67) |
| First computable phosphate clearance (ml/min/kg) | 0.31 (0.15 to 0.47); p < 0.001 | −0.78 (−1.21 to −0.34); p < 0.001 | 0.27 | 0.04 7.42 (5.29 to 10.38) |
0.71 0.38 (0.23 to 0.58) |
Descriptive statistics of the first computable clearances are given in Table 10, with the results from fitting the model given in Table 11.
Further details of the fitting of the model and its assessment are in Appendix 5 and Appendix 6 (Tables 46 and 47).
Table 11 shows that the biochemical clearance rate for PD was smaller than that for NIDUS, which in turn was smaller than that for CVVH for each metabolite. The SD of the clearance rate was also smaller for PD than NIDUS, and smaller for NIDUS than CVVH, again for each metabolite.
The results for the mean biochemical clearances were very similar to those shown here for the first clearance (details not shown).
Analysis of mortality and of variables collected through PICANet
Descriptive statistics for mortality, using measures collected through PICANet and by the NIDUS team, are shown in Table 12. Other variables collected through the routine returns made to PICANet are presented in Table 13.
| Control (n = 62) | Intervention (n = 35) | Total (n = 97) | χ2, df, p | Difference in percentage (95% CI) | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Survival until PICU dischargea, n (%) | ||||||
| Alive | 52 (83.87) | 23 (65.71) | 75 (77.32) | χ2(1) = 4.21, p = 0.040 | 18.2 (0.0 to 36.4) | 2.71 (1.03 to 7.27) |
| Dead | 10 (16.13) | 12 (34.29) | 22 (22.68) | |||
| Survival until 30 days post RRT, n (%) | ||||||
| Alive | 54 (87.10) | 25 (71.43) | 79 (81.44) | χ2(1) = 3.63, p = 0.057 | 15.7 (−1.5 to 32.8) | 2.70 (0.95 to 7.67) |
| Dead | 8 (12.90) | 10 (28.57) | 18 (18.56) | |||
| Control (n = 62) | Intervention (n = 35) | Total (n = 97) | χ2, df, p | Difference in percentage (95% CI) | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Completion of planned RRT, n (%) | ||||||
| No | 4 (8.16) | 11 (52.38) | 15 (21.43) | χ2(1) = 17.07, p < 0.001 | −44.2 (−66.9 to −21.5) | 0.08 (0.02 to 0.31) |
| Yes | 45 (91.84) | 10 (47.62) | 55 (78.57) | |||
| Missing | 13 | 14 | 27 | |||
| Survival until PICU discharge, n (%) | ||||||
| Alive | 52 (83.87) | 23 (65.71) | 75 (77.32) | χ2(1) = 4.21, p = 0.040 | 18.2 (0.0 to 36.4) | 2.71 (1.03 to 7.27) |
| Dead | 10 (16.13) | 12 (34.29) | 22 (22.68) | |||
| Survival until 30 days follow-up, n (%) | ||||||
| Alive | 54 (87.10) | 25 (71.43) | 79 (81.44) | χ2(1) = 3.63, p = 0.057 | 15.7 (−1.5 to 32.8) | 2.70 (0.95 to 7.67) |
| Dead | 8 (12.90) | 10 (28.57) | 18 (18.56) | |||
| Need for additional vascular or dialysis access on RRT while in PICU, n (%) | ||||||
| Yes | 62 (100.00) | 35 (100.00) | 97 (100.00) | |||
| Haemodynamic status (drop in blood pressure after connection requiring intervention), n (%) | ||||||
| No | 3 (4.84) | 4 (11.43) | 7 (7.22) | χ2(1) = 1.41, p = 0.228 | −6.6 (−18.4 to 5.2) | 0.39 (0.08 to 1.87) |
| Yes | 59 (95.16) | 31 (88.57) | 90 (92.78) | |||
| Fluid bolus given a | ||||||
| No | 57 (91.94) | 33 (94.29) | 90 (92.78) | |||
| Yes | 5 (8.06) | 2 (5.71) | 7 (7.22) | |||
| Summaries (see footnote) | 0.0, 0.0, 0.0, 0.0, 1.0 | 0.0, 0.0, 0.0, 0.0, 1.0 | 0.0, 0.0, 0.0, 0.0, 1.0 | |||
| Inotropes administered a | ||||||
| No | 4 (6.45) | 4 (11.43) | 8 (8.25) | |||
| Yes | 58 (93.55) | 31 (88.57) | 89 (91.75) | |||
| Summaries (see footnote) | 0.0, 1.0, 1.0, 1.0, 1.0 | 0.0, 0.75,1.0, 1.0, 1.0 | 0.0, 0.9, 1.0, 1.0, 1.0 | |||
| Unplanned filter change on RRT while in PICU, n (%) | ||||||
| No | 55 (88.71) | 14 (40.00) | 69 (71.13) | χ2(1) = 25.85, p < 0.001 | 48.7 (30.7 to 66.8) | 11.79 (4.18 to 33.25) |
| Yes | 7 (11.29) | 21 (60.00) | 28 (28.87) | |||
| Exposure to blood transfusion on RRT while in PICU, n (%) | ||||||
| No | 42 (67.74) | 8 (22.86) | 50 (51.55) | χ2(1) = 18.05, p < 0.001 | 44.9 (26.8 to 63.0) | 7.09 (2.74 to 18.36) |
| Yes | 20 (32.26) | 27 (77.14) | 47 (48.45) | |||
| Heparin use on RRT while in PICU, n (%) | ||||||
| No | 45 (72.58) | 2 (5.71) | 47 (48.45) | χ2(1) = 40.05, p < 0.001 | 66.9 (53.4 to 80.4) | 43.68 (9.43 to 202.20) |
| Yes | 17 (27.42) | 33 (94.29) | 50 (51.55) | |||
| Citrate use on RRT while in PICU, n (%) | ||||||
| No | 59 (95.16) | 34 (97.14) | 93 (95.88) | χ2(1) = 0.22, p = 0.637 | −2.0 (−9.7 to 5.7) | 0.58 (0.06 to 5.78) |
| Yes | 3 (4.84) | 1 (2.86) | 4 (4.12) | |||
| Prostacyclin use on RRT while in PICU, n (%) | ||||||
| No | 62 (100.00) | 35 (100.00) | 97 (100.00) | |||
| Other anticoagulant use on RRT while in PICU, n (%) | ||||||
| No | 60 (96.77) | 35 (100.00) | 95 (97.94) | χ2(1) = 1.15, p = 0.283 | −3.2 (−7.6 to 1.2) | 0 (0 to 9.46) |
| Yes | 2 (3.23) | 0 | 2 (2.06) | |||
| Any anticoagulant use on RRT while in PICU, n (%) | ||||||
| No | 44 (70.97) | 2 (5.71) | 46 (47.42) | χ2(1) = 38.20, p < 0.001 | 65.3 (51.6 to 78.9) | 40.33 (8.74 to 186.08) |
| Yes | 18 (29.03) | 33 (94.29) | 51 (52.58) | |||
| PD | 5 | 0 | 5 | |||
| Prismaflex | 4 | 0 | 4 | |||
| Aquarius | 8 | 0 | 8 | |||
| Manual HD and ECMO | 1 | 0 | 1 | |||
| NIDUS | 0 | 33 | 33 | |||
| Ventilation-free days on RRT while in PICU | ||||||
| Mean (SD) | 0.37 (1.62) | 1.66 (8.32) | 0.84 (5.16) | |||
| Median (IQR) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | |||
| Range | 0.00–12.00 | 0.00–49.00 | 0.00–49.00 | |||
| Available, n | 62 | 35 | 97 | |||
The mortality outcomes are binary as are the other PICANet variables apart from ventilator-free days. The intervention and control groups were compared for binary outcomes using standard χ2 tests, with differences described by both absolute differences and odds ratios, each with associated 95% CIs.
Comparisons were based on the full data, including transition babies, as these variables should not be affected by unfamiliarity with a new device and most are routinely collected through PICANet.
Subgroup analyses
Pre-specified subgroup analyses
The SAP specified the following subgroup analyses:
-
Repeat the primary analysis but with the control group split into CVVH and PD, so that NIDUS is compared with CVVH and PD separately.
-
Descriptive statistics of mortality and of the variables collected through PICANet presented separately for those allocated to PD, CVVH and NIDUS.
-
Comparison of the actual filtration rate (X) and the filtration rate reported by the dialysis device (A2). This quantity is only relevant to participants allocated to NIDUS or CVVH.
The patient receiving Manual HD with ECMO was omitted from these analyses.
Primary outcome compared between NIDUS, CVVH and PD
Descriptive statistics of the primary outcome between NIDUS, PD and CVVH are presented in Table 14, with the results of analysis in Table 15. The analysis used the same model used for the primary analysis, but with the treatment factor now having three levels: full details of the fitted model are in Appendix 6. The results were similar to the results when CVVH and PD are combined, with the SD of log|X-A| on NIDUS being 14% of that for PD and 11% of that on CVVH.
| Variable | PD (n = 48) | CVVH (n = 13) | NIDUS (n = 20) |
|---|---|---|---|
| X, ml/hour | |||
| Mean (SD) | 8.13 (7.92) | 38.74 (37.19) | 14.88 (9.37) |
| Median (IQR) | 6.67 (2.75–12.42) | 31.85 (20.64–40.00) | 12.88 (9.08–19.57) |
| Range | −5.17 to 27.83 | 0.47 to 143.32 | 0.54 to 36.67 |
| Available, n | 48 | 13 | 20 |
| A, ml/hour | |||
| Mean (SD) | 11.79 (12.57) | 30.56 (15.60) | 15.36 (10.14) |
| Median (IQR) | 10.00 (4.00–13.50) | 30.22 (20.00–39.03) | 12.26 (8.58–21.15) |
| Range | 0.00–60.00 | 6.23–58.00 | 0.00–40.00 |
| Available, n | 48 | 13 | 20 |
| Precision (X-A), ml/hour | |||
| Mean (SD) | −3.67 (15.10) | 8.19 (27.29) | −0.48 (2.95) |
| Median (IQR) | −1.40 (−10.92 to 5.12) | −1.67 (−6.13 to 7.24) | −0.55 (−2.48 to 0.26) |
| Range | −49.17 to 27.83 | −15.65 to 85.32 | −3.33 to 10.32 |
| Available, n | 48 | 13 | 20 |
| log|X-A| | |||
| Mean (SD) | 1.82 (1.30) | 1.90 (1.80) | −0.02 (1.41) |
| Median (IQR) | 1.88 (1.15–2.73) | 1.98 (1.59–2.75) | 0.31 (−0.72 to 0.94) |
| Range | −3.00 to 3.90 | −3.03 to 4.45 | −3.91 to 2.33 |
| Available, n | 48 | 13 | 20 |
| NIDUS – PD | NIDUS – CVVH | |
|---|---|---|
| Mean difference (95% CI); p-value | −2.00 (−3.65 to −0.35); p = 0.019 | −2.19 (−3.94 to −0.44); p = 0.015 |
| Ratio of sNIDUS/scontrol (95% CI) | 0.14 (0.03 to 0.70) | 0.11 (0.02 to 0.64) |
Descriptive statistics of mortality by NIDUS, CVVH and PD
Mortality statistics by PD, CVVH and NIDUS are shown in Table 16.
| PD (n = 48) | CVVH (n = 13) | NIDUS (n = 35) | |
|---|---|---|---|
| Survival until PICU discharge, n (%) | |||
| Alive | 46 (95.83) | 6 (46.15) | 23 (65.71) |
| Dead | 2 (4.17) | 7 (53.85) | 12 (34.29) |
| Survival until 30 days post RRT, n (%) | |||
| Alive | 47 (97.92) | 7 (53.85) | 25 (71.43) |
| Dead | 1 (2.08) | 6 (46.15) | 10 (28.57) |
Descriptive statistics of variables collected through PICANet, by PD, CVVH and NIDUS
Descriptive statistics of these variables are in Table 17.
| PD (n = 48) | CVVH (n = 13) | NIDUS (n = 35) | |
|---|---|---|---|
| Completion of planned RRT, n (%) | |||
| No | 3 (6.25) | 1 (7.69) | 11 (31.43) |
| Yes | 41 (85.42) | 4 (30.77) | 10 (28.57) |
| Missing | 4 (8.33) | 8 (61.54) | 14 (40.00) |
| Survival until PICU discharge, n (%) | |||
| Alive | 46 (95.83) | 6 (46.15) | 23 (65.71) |
| Dead | 2 (4.17) | 7 (53.85) | 12 (34.29) |
| Survival until 30 days follow-up, n (%) | |||
| Alive | 47 (97.92) | 7 (53.85) | 25 (71.43) |
| Dead | 1 (2.08) | 6 (46.15) | 10 (28.57) |
| Need for additional vascular or dialysis access on RRT while in PICU, n (%) | |||
| Yes | 48 (100.00) | 13 (100.00) | 35 (100.00) |
| Haemodynamic status (drop in blood pressure after connection requiring intervention), n (%) | |||
| No | 0 | 3 (23.08) | 4 (11.43) |
| Yes | 48 (100.00) | 10 (76.92) | 31 (88.57) |
| Fluid bolus givena | |||
| No | 48 (100.00) | 9 (69.23) | 33 (94.29) |
| Yes | 0 | 4 (30.77) | 2 (5.71) |
| Summaries (see footnote) | 0.0, 0.0, 0.0, 0.0, 0.0 | 0.0, 0.0, 0.0, 0.1, 1.0 | 0.0, 0.0, 0.0, 0.0, 1.0 |
| Inotropes administereda | |||
| No | 0 | 4 (30.77) | 4 (11.43) |
| Yes | 48 (100.00) | 9 (69.23) | 31 (88.57) |
| Summaries (see footnote) | 0.4, 1.0, 1.0, 1.0, 1.0 | 0.0, 0.0, 0.7, 1.0, 1.0 | 0.0, 0.7, 1.0, 1.0, 1.0 |
| Unplanned filter change on RRT while in PICU, n (%) | |||
| No | 48 (100.00) | 7 (53.85) | 14 (40.00) |
| Yes | 0 | 6 (46.15) | 21 (60.00) |
| Exposure to blood transfusion on RRT while in PICU, n (%) | |||
| No | 41 (85.42) | 1 (7.69) | 8 (22.86) |
| Yes | 7 (14.58) | 12 (92.31) | 27 (77.14) |
| Heparin use on RRT while in PICU, n (%) | |||
| No | 43 (89.58) | 2 (15.38) | 2 (5.71) |
| Yes | 5 (10.42) | 11 (84.62) | 33 (94.29) |
| Citrate use on RRT while in PICU, n (%) | |||
| No | 48 (100.00) | 10 (76.92) | 34 (97.14) |
| Yes | 0 | 3 (23.08) | 1 (2.86) |
| Prostacyclin use on RRT while in PICU, n (%) | |||
| No | 48 (100.00) | 13 (100.00) | 35 (100.00) |
| Other anticoagulant use on RRT while in PICU, n (%) | |||
| No | 48 (100.00) | 11 (84.62) | 35 (100.00) |
| Yes | 0 | 2 (15.38) | 0 |
| Any anticoagulant use on RRT while in PICU, n (%) | |||
| No | 43 (89.58) | 1 (7.69) | 2 (5.71) |
| Yes | 5 (10.42) | 12 (92.31) | 33 (94.29) |
| Ventilation-free days on RRT while in PICU | |||
| Mean (SD) | 0.06 (0.24) | 1.54 (3.36) | 1.66 (8.32) |
| Median (IQR) | 0 (0–0) | 0 (0–2) | 0 (0–0) |
| Range | 0–1 | 0–12 | 0–49 |
Difference between actual fluid removal and value reported by the procedure (X-A2)
For NIDUS and CVVH, an important measure was to compare how close the actual fluid removal (X) was to that reported by the device (A2). As this was not relevant for PD nor for HD and ECMO, the analysis used data from the 48 NIDUS and CVVH participants in the study. At least one measurement of X-A2 was available for 34 participants (13/13 CVVH and 21/35 NIDUS). Descriptive statistics of X-A2 are presented in Table 18.
| CVVH (n = 13) | NIDUS (n = 20) | Total (n = 33) | |
|---|---|---|---|
| X, ml/hour | |||
| Mean (SD) | 38.74 (37.19) | 14.88 (9.37) | 24.28 (26.67) |
| Median (IQR) | 31.85 (20.64–40.00) | 12.88 (9.08–19.57) | 19.35 (9.98–30.96) |
| Range | 0.47–143.32 | 0.54–36.67 | 0.47–143.32 |
| A2, ml/hour | |||
| Mean (SD) | 27.10 (21.49) | 15.32 (10.56) | 19.96 (16.54) |
| Median (IQR) | 26.40 (16.15–36.77) | 12.30 (7.37–22.50) | 17.39 (8.65–28.89) |
| Range | −16.67 to 61.60 | 0.00–39.24 | −16.67 to 61.60 |
| X-A2, ml/hour | |||
| Mean (SD) | 11.64 (28.43) | −0.44 (3.18) | 4.32 (18.58) |
| Median (IQR) | −0.46 (−4.52 to 29.39) | −1.01 (−1.75 to −0.12) | −0.84 (−2.39 to 0.37) |
| Range | −22.10 to 81.72 | −3.65 to 12.20 | −22.10 to 81.72 |
| Duration, hours | |||
| Mean (SD) | 5.81 (0.73) | 5.31 (1.34) | 5.51 (1.15) |
| Median (IQR) | 6.00 (5.20–6.00) | 5.77 (4.44–6.00) | 6.00 (5.00–6.00) |
| Range | 4.58–7.20 | 2.60–7.50 | 2.60–7.50 |
The analysis anticipated in the SAP assumed that X-A2 would have zero mean but stipulated that this should be confirmed before proceeding to the proposed analysis. A general linear model fitted to the first computable X-A2 on treatment arm, site, period and duration was used to assess this assumption (see Appendix 6, Table 48). This suggested that X-A2 does not have zero mean and also that the residual SDs varied between arms. Consequently, the analysis fitted the usual model of centre, period, treatment and duration to the difference in X-A2 using generalised least squares, with different residual variances in the two treatment groups. Results are in Table 19. Further details on the fitting and assessment of the model are presented in Appendix 6, Table 49.
| Mean difference (95% CI); p-value NIDUS – CVVH |
Ratio of SDs sNIDUS/sCVVH (95% CI); p-value |
Estimate of SD sNIDUS |
Estimate of SD sCVVH |
|
|---|---|---|---|---|
| First computable X-A2 | −28.18 (−49.53 to −6.84); p = 0.017 | 0.17 (0.09 to 0.31); p < 0.001 | 3.68 | 22.19 |
| n | 33 |
Table 19 shows that X-A2 is substantially smaller on NIDUS than on CVVH, with a SD on NIDUS of about 17% that on CVVH and the adjusted mean of X-A2 is 28.2 ml/hour (95% CI 6.8 to 49.5) smaller on NIDUS than CVVH. However, some caution should be exercised in interpreting this analysis, which considers only 33 participants. The adjusted mean difference is substantially larger than the difference in unadjusted means (mean 11.6 ml/hour on CVVH vs. −0.4 on NIDUS), so the conclusions could be highly dependent on the effect of a small number of influential observations on the fitted model.
The adjusted difference in means is very different to the difference in unadjusted means. The results should be treated with caution as this analysis is based on small samples and the adjusted estimates could be affected by a few points that have a marked effect on the fitted model.
Safety
Adverse device effects (ADE), SADE, non-SAE which were not consistent with the usual clinical pattern for participants requiring RRT in PICU, and SAE, regardless of consistency with usual clinical pattern, were recorded. ADEs and SADEs were specific to participants in the intervention arm as the event had to be judged to be possibly, probably or definitely caused by the NIDUS device/tubing set.
A total of 35 events were initially recorded for 29 participants. Two participants on NIDUS, from the same site, were reported to have had small intraventricular haemorrhage (IVH) detected via ultrasound. It was noted from the site team that at the time of the two events, they had only recently introduced routine head ultrasound scans for participants that were being treated on NIDUS. The reasons for the scans were to look for intracranial bleeds but it is not known whether such bleeds were related to the disease severity of the patient or if they were related to the use of heparin. The scans had not been carried out for participants who had previously been treated on the NIDUS or had been dialysed using other modalities that required anticoagulation. It was clarified that ultrasound scans of the head were performed routinely in neonatal units as preterm babies are at risk of developing an intracranial haemorrhage. However, the scans were not routinely carried out in most PICUs. Thus, these two events are reported in a line listing (see Report Supplementary Material 1 Table SAF1) and separate from tables where the frequency (%) of adverse events (AEs) are tabulated. Additionally, after discussion with the CI, it was decided that six recorded safety events concerning filter clots were to be recorded as device deficiencies instead.
Thus, a remainder of 27 events across 23 participants were tabulated (15 control, 8 intervention) (see Table 20). The number of participants affected by each adverse event is presented in Table 21 and the number of occurrences of each adverse event is presented in Table 22.
| Control (n = 65) | Intervention (n = 32) | |
|---|---|---|
| No event | 50 | 24 |
| One event | 13 | 6 |
| Two events | 2 | 2 |
| Control (n = 15) | Intervention (n = 8) | Overall (n = 23) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | N | % | |
| Blood pressure decreased | 0 | 0 | 1 | 12.50 | 1 | 4.35 |
| Mild | 0 | 0 | 1 | 1 | ||
| Bradycardia | 0 | 0 | 1 | 12.50 | 1 | 4.35 |
| Severe | 0 | 1 | 1 | |||
| Cardiac arrest | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Severe | 1 | 0 | 1 | |||
| Chylothorax | 2 | 13.33 | 0 | 0 | 2 | 8.70 |
| Moderate | 2 | 0 | 2 | |||
| Death | 4 | 26.67 | 7 | 87.50 | 11 | 47.83 |
| Severe | 2 | 4 | 6 | |||
| Missing severity | 2 | 3 | 5 | |||
| Debridement | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Missing severity | 1 | 0 | 1 | |||
| Dyskinesia | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Mild | 1 | 0 | 1 | |||
| Manufacturing equipment issue | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Moderate | 1 | 0 | 1 | |||
| Mediastinitis | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Moderate | 1 | 0 | 1 | |||
| Necrotising enterocolitis neonatal | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Moderate | 1 | 0 | 1 | |||
| PD complication | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Mild | 1 | 0 | 1 | |||
| Pneumothorax | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Missing severity | 1 | 0 | 1 | |||
| Postoperative wound infection | 2 | 13.33 | 0 | 0 | 2 | 8.70 |
| Moderate | 2 | 0 | 2 | |||
| Pulmonary haemorrhage | 0 | 0 | 1 | 12.50 | 1 | 4.35 |
| Severe | 0 | 1 | 1 | |||
| Vocal cord paralysis | 1 | 6.67 | 0 | 0 | 1 | 4.35 |
| Mild | 1 | 0 | 1 | |||
| Number of AEs in Control Arm (n = 17) | Number of AEs in Intervention Arm (n = 10) | Overall Number of Events (n = 27) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | N | % | |
| Blood pressure decreased | 0 | 0 | 1 | 10.00 | 1 | 3.70 |
| Mild | 0 | 1 | 1 | |||
| Bradycardia | 0 | 0 | 1 | 10.00 | 1 | 3.70 |
| Severe | 0 | 1 | 1 | |||
| Cardiac arrest | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Severe | 1 | 0 | 1 | |||
| Chylothorax | 2 | 11.76 | 0 | 0 | 2 | 6.30 |
| Moderate | 2 | 0 | 2 | |||
| Death | 4 | 23.53 | 7 | 70.00 | 11 | 40.74 |
| Severe | 2 | 4 | 6 | |||
| Missing severity | 2 | 3 | 5 | |||
| Debridement | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Missing severity | 1 | 0 | 1 | |||
| Dyskinesia | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Mild | 1 | 0 | 1 | |||
| Manufacturing equipment issue | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Moderate | 1 | 0 | 1 | |||
| Mediastinitis | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Moderate | 1 | 0 | 1 | |||
| Necrotising enterocolitis neonatal | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Moderate | 1 | 0 | 1 | |||
| PD complication | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Mild | 1 | 0 | 1 | |||
| Pneumothorax | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Missing severity | 1 | 0 | 1 | |||
| Postoperative wound infection | 2 | 11.76 | 0 | 0 | 2 | 6.40 |
| Moderate | 2 | 0 | 2 | |||
| Pulmonary haemorrhage | 0 | 0 | 1 | 10.00 | 1 | 3.70 |
| Severe | 0 | 1 | 1 | |||
| Vocal cord paralysis | 1 | 5.88 | 0 | 0 | 1 | 3.70 |
| Mild | 1 | 0 | 1 | |||
A total of eight non-SAEs which were not consistent with the usual clinical pattern for participants requiring RRT in PICU occurred across seven participants (6 PD, 1 Prismaflex) (see Table 23). There was one ADE which was possibly related to the NIDUS device/tubing set (see Table 24). There were 17 SAEs across 15 participants (8 control, 7 intervention) (see Table 25). One SADE was reported during the study (see Table 26).
| Index | Device | Days from RRT initiation to AE Start | Days from RRT initiation to AE Resolution | AE | Description | Causality | Severity | Action(s) taken | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PD | 2 | 3 | PD complication | PD stopped working so removed | Definitely | Mild | Treatment adjusted/interrupted | Resolved |
| 2 | PD | 3 | 14 | Necrotising enterocolitis neonatal | Necrotising enterocolitis | Moderate | Concomitant medication | Resolved | |
| 3 | PD | 7 | 55 | Chylothorax | Chylothorax | Unrelated | Moderate | Non-drug therapy given | Resolved |
| 4 | PD | 3 | 4 | Dyskinesia | Abnormal movements – repetitive gasping associated with bilateral symmetrical upper limb extensor movement and abdominal flexion. EEG not suggestive of seizures. MRI structurally normal. | Unrelated | Mild | Concomitant medication | Resolved |
| 11 | Vocal cord paralysis | Left vocal cord palsy | Unrelated | Mild | None | Ongoing | |||
| 5 | PD | 19 | Postoperative wound infection | Surgical site sternal wound infection | Unrelated | Moderate | Concomitant medication | Ongoing | |
| 6 | PD | 18 | 49 | Chylothorax | Chylothorax | Unrelated | Moderate | Concomitant medication | Resolved |
| 7 | Prismaflex | 2 | 5 | Manufacturing equipment issue | Filter not working | Possible | Moderate | Treatment adjusted/interrupted | Resolved |
| Index | Device | Days from RRT initiation to AE start | Days from RRT initiation to AE resolution | AE | Description | Causality | Severity | Action(s) taken | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NIDUS | 6 | Blood pressure decreased | NIDUS restarted post a failed trial of a furosemide infusion on 16/01/2021 at 13:30. Patient dropped blood pressure from a MAP of 45 to a MAP of 40. Adrenaline infusion increased to support BP. Able to wean this rate back down to pre NIDUS rate | Possible | Mild | None | Resolved |
| Index | Device | Days from RRT initiation to AE start | Days from RRT initiation to AE resolution | AE | Description | Causality | Severity | Action(s) taken | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NIDUS | 6 | 6 | Death | Patient death – patient became progressively bradycardic and hypotensive over several hours, was not responding to any treatment, eventually arrested. Patient was not an appropriate candidate for ECPR and unfortunately passed away. Patient was not on the NIDUS at the time of deterioration | Unrelated | None | Fatal | |
| 2 | NIDUS | 2 | 2 | Death | Patient passed away | Unrelated | None | Fatal | |
| 3 | NIDUS | 19 | 19 | Death | Death | Unrelated | Severe | None | Fatal |
| 4 | NIDUS | 3 | 3 | Death | Death | Unrelated | Severe | None | Fatal |
| 5 | NIDUS | 11 | 11 | Death | Death | Unrelated | Severe | None | Fatal |
| 6 | NIDUS | 22 | 22 | Death | Patient death unrelated to NIDUS | Unrelated | None | Resolved | |
| 7 | NIDUS | 2 | 2 | Bradycardia | Bradycardia requiring chest compressions | Unrelated | Severe | Concomitant medication | Resolved |
| 18 | 18 | Death | Death during follow-up period, following redirection of care | Unrelated | Severe | None | Fatal | ||
| 8 | Aquarius | 4 | 4 | Death | Patient passed away | Unrelated | None | Fatal | |
| 9 | Aquarius | 20 | 20 | Death | Patient death – patient progressively deteriorated, his bowel was no longer viable and unfortunately care was withdrawn and the patient passed away | Unrelated | None | Fatal | |
| 10 | Aquarius | 1 | 4 | Death | Death – Group B streptococcal sepsis, persistent pulmonary hypertension of the newborn, catastrophic neurological injury. | Unrelated | Severe | Treatment discontinued | Fatal |
| 11 | Aquarius | 19 | 19 | Death | Death – palliation | Unrelated | Severe | None | Fatal |
| 12 | PD | 13 | 22 | Mediastinitis | Mediastinitis | Unrelated | Moderate | Hospitalisation | Resolved |
| 13 | PD | 5 | 12 | Debridement | Surgical wound debridement | Unrelated | Concomitant medication | Resolved | |
| 1 | 1 | Pneumothorax | Right pneumothorax, left-sided white out. ETT pulled back. Cardiothoracic fellow manipulated right pleural and mediastinal drain to unblock. Subsequently there was partial re-expansion of pneumothorax and re-expansion of left lung | Non-drug therapy given | Resolved | ||||
| 14 | PD | 2 | 2 | Cardiac arrest | Cardiac arrest | Unrelated | Severe | Concomitant medication | Resolved |
| 15 | PD | 7 | 7 | Postoperative wound infection | Chest re-exploration for sternotomy wound site infection | Unrelated | Moderate | Resolved |
| Index | Device | Days from RRT initiation to AE Start | Days from RRT initiation to AE Resolution | AE | Description | Causality | Severity | Action(s) taken | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NIDUS | 2 | 2 | Pulmonary haemorrhage | Pulmonary haemorrhage in context of multiorgan failure and pulmonary hypertension, death | Possible | Severe | Fatal |
Parent questionnaire
All parents/guardians of the patient were given the opportunity to answer a questionnaire about their experiences of having a baby on dialysis and their experiences taking part in the study. A total of 106 observations for the parent questionnaire were recorded in MACRO. It should be noted that this figure is purely the number of observations in the parent questionnaire data. The study team did not record whether a questionnaire was actually handed out to parents and so it cannot be determined if the observations with missing data are actually non-responders or perhaps empty entries in the database. Of the 106 parent questionnaires recorded in MACRO, only 34 contained non-null responses.
The 34 observations corresponded to the experiences of carers of 34/97 participants in the study [4 CCVH (2 Prismaflex, 2 Aquarius), 15 PD, and 15 NIDUS]. Descriptive statistics of close-ended questions are presented in Table 27 while a listing of responses to open-ended questions is presented in Table 28.
| Carers of participants on CVVH (n = 4) | Carers of participants on PD (n = 15) | Carers of participants on NIDUS (n = 15) | Total carer responses (n = 34) | |
|---|---|---|---|---|
| Questionnaire completed by, n (%) | ||||
| Mother | 0 | 6 (40.00) | 5 (33.33) | 11 (32.35) |
| Father | 1 (25.00) | 2 (13.33) | 2 (13.33) | 5 (14.71) |
| Both parents | 1 (25.00) | 2 (13.33) | 2 (13.33) | 5 (14.71) |
| Missing | 2 (50.00) | 5 (33.33) | 6 (40.00) | 13 (38.24) |
| How helpful was the information provided in increasing your understanding about the dialysis used for your baby?, n (%) | ||||
| Very unhelpful | 0 | 0 | 0 | 0 |
| Unhelpful | 0 | 0 | 0 | 0 |
| Made no difference | 0 | 1 (6.67) | 0 | 1 (2.94) |
| Helpful | 0 | 7 (46.67) | 4 (26.67) | 11 (32.35) |
| Very helpful | 2 (50.00) | 2 (13.33) | 5 (33.33) | 9 (26.47) |
| Missing | 2 (50.00) | 5 (33.33) | 6 (40.00) | 13 (38.24) |
| How would you rate your baby’s comfort level while being treated with this dialysis?, n (%) | ||||
| Very uncomfortable | 0 | 0 | 1 (6.67) | 1 (2.94) |
| Uncomfortable | 0 | 0 | 0 | 0 |
| Neutral | 0 | 1 (6.67) | 1 (6.67) | 2 (5.88) |
| Comfortable | 1 (25.00) | 4 (26.67) | 3 (20.00) | 8 (23.53) |
| Very comfortable | 1 (25.00) | 5 (33.33) | 4 (26.67) | 10 (29.41) |
| Missing | 2 (50.00) | 5 (33.33) | 6 (40.00) | 13 (38.24) |
| Given your baby’s medical condition, overall how acceptable did you find this dialysis treatment for your baby?, n (%) | ||||
| Very unacceptable | 0 | 1 (6.67) | 1 (6.67) | 2 (5.88) |
| Unacceptable | 0 | 0 | 0 | 0 |
| Neutral | 0 | 0 | 1 (6.67) | 1 (2.94) |
| Acceptable | 1 (25.00) | 6 (40.00) | 1 (6.67) | 8 (23.53) |
| Very acceptable | 1 (25.00) | 3 (20.00) | 6 (40.00) | 10 (29.41) |
| Missing | 2 (50.00) | 5 (33.33) | 6 (40.00) | 13 (38.24) |
| Given that your baby was unwell, in your opinion, how acceptable was it to be asked to take part in a research study about baby dialysis?, n (%) | ||||
| Very unacceptable | 0 | 0 | 0 | 0 |
| Unacceptable | 0 | 0 | 0 | 0 |
| Neutral | 0 | 1 (6.67) | 0 | 1 (2.94) |
| Acceptable | 1 (25.00) | 5 (33.33) | 2 (13.33) | 8 (23.53) |
| Very acceptable | 1 (25.00) | 4 (26.67) | 7 (46.67) | 12 (35.29) |
| Missing | 2 (50.00) | 5 (33.33) | 6 (40.00) | 13 (38.24) |
| How likely would you be to recommend to other parents with a baby with similar medical needs, that they take part in a research study like this one?, n (%) | ||||
| Very unlikely | 1 (25.00) | 0 | 0 | 1 (2.94) |
| Unlikely | 0 | 0 | 0 | 0 |
| Neither likely nor unlikely | 0 | 0 | 0 | 0 |
| Likely | 0 | 3 (20.00) | 2 (13.33) | 5 (14.71) |
| Very likely | 1 (25.00) | 7 (46.67) | 7 (46.67) | 15 (44.12) |
| Missing | 2 (50.00) | 5 (33.33) | 6 (40.00) | 13 (38.24) |
| CVVH | PD | NIDUS | |
|---|---|---|---|
| In your opinion, what were the benefits for your baby of this dialysis treatment? |
|
|
|
|
|
||
|
|
||
|
|||
| In your opinion, what were the difficulties for your baby with this dialysis treatment? |
|
|
|
|
|
||
|
|
||
|
Across all dialysis types, parents gave positive feedback about information provided to them, their baby’s comfort during dialysis, and the acceptability of the different dialysis treatments. Parents also indicated that, despite the inevitably high levels of stress for them at the time of their baby being unwell, they found it acceptable to be asked to participate in research, and would recommend this to other parents. Many parents commented on the importance of research in developing new treatments, and their wish to help other babies and families in similar situations.
Staff questionnaire
Staff using the CVVH, PD and/or NIDUS machine were asked to complete a questionnaire about the acceptability and usability of the RRT device. A total of 140 observations for the staff questionnaire were recorded in MACRO. It should be noted that this figure is purely the number of observations in the staff questionnaire data. The study team did not record whether a questionnaire was actually handed out to staff and so it cannot be determined if the observations with missing data are actually non-responders or perhaps empty entries in the database.
A total of 65 observations of the staff questionnaire had non-null records in MACRO, with a response to at least one item on the questionnaire. It was possible for responses to several questionnaires to be based on the experience of delivering RRT to one study participant. These 65 observations corresponded to the experience of delivering RRT to 43/97 participants in the trial [18 PD, 5 CVVH (Aquarius) and 20 NIDUS] – with a range of one to five staff questionnaires per patient. Descriptive statistics of close-ended questions are presented in Table 29. Responses to the open-ended questions are not presented in this report.
| Staff delivering care to participants on CVVH (n = 9) | Staff delivering care to participants on PD (n = 24) | Staff delivering care to participants on NIDUS (n = 32) | Total staff responses (n = 65) | |
|---|---|---|---|---|
| Role, n (%) | ||||
| PICU nurse | 9 (100.00) | 22 (91.67) | 31 (96.88) | 62 (95.38) |
| Missing | 0 | 2 (8.33) | 1 (3.13) | 3 (4.62) |
| Grade, n (%) | ||||
| Band 5 | 1 (11.11) | 11 (45.83) | 8 (25.00) | 20 (30.77) |
| Band 6 | 8 (88.89) | 11 (45.83) | 17 (53.13) | 36 (55.38) |
| Band 7 | 0 | 0 | 6 (18.75) | 6 (9.23) |
| Band 8 | 0 | 0 | 1 (3.13) | 1 (1.54) |
| Missing | 0 | 2 (8.33) | 0 | 2 (3.08) |
| CVVH trained, n (%) | ||||
| No | 0 | 7 (29.17) | 4 (12.50) | 11 (16.92) |
| Yes | 9 (100.00) | 13 (54.17) | 27 (84.38) | 49 (75.38) |
| Missing | 0 | 4 (16.67) | 1 (3.13) | 5 (7.69) |
| If yes, years of experience with CVVH | ||||
| Mean (SD) | 5.56 (3.64) | 4.12 (2.41) | 5.68 (4.64) | 5.23 (3.94) |
| Median (IQR) | 6.00 (2.00–9.00) | 4.00 (2.00–5.00) | 4.00 (2.00–7.00) | 4.00 (2.00–7.00) |
| Range | 1.00–10.00 | 1.00–10.00 | 0.50–20.00 | 0.50–20.00 |
| Available, n | 9 | 13 | 25 | 47 |
| PD trained, n (%) | ||||
| No | 2 (22.22) | 3 (12.50) | 8 (25.00) | 13 (20.00) |
| Yes | 6 (66.67) | 19 (79.17) | 21 (65.63) | 46 (70.77) |
| Missing | 1 (11.11) | 2 (8.33) | 3 (9.38) | 6 (9.23) |
| If yes, years of experience with PD | ||||
| Mean (SD) | 7.00 (4.29) | 5.09 (4.46) | 7.67 (4.41) | 6.49 (4.49) |
| Median (IQR) | 7.00 (4.00–10.00) | 4.75 (2.00–7.00) | 9.00 (4.00–10.00) | 6.00 (3.00–10.00) |
| Range | 1.00–13.00 | 0.00–18.00 | 2.50–20.00 | 0.00–20.00 |
| Available, n | 6 | 18 | 19 | 43 |
| NIDUS trained, n (%) | ||||
| No | 5 (55.56) | 18 (75.00) | 4 (12.50) | 27 (41.54) |
| Yes | 3 (33.33) | 1 (4.17) | 27 (84.38) | 31 (47.69) |
| Missing | 1 (11.11) | 5 (20.83) | 1 (3.13) | 7 (10.77) |
| If yes, years of experience with NIDUS | ||||
| Mean (SD) | 0.08 (0.14) | 1.00 (·) | 0.41 (0.48) | 0.40 (0.47) |
| Median (IQR) | 0.00 (0.00–0.25) | 1.00 (1.00–1.00) | 0.33 (0.08–0.50) | 0.33 (0.00–0.50) |
| Range | 0.00–0.25 | 1.00–1.00 | 0.00–2.00 | 0.00–2.00 |
| Available, n | 3 | 1 | 22 | 26 |
| I have received adequate training in how to use this type of dialysis, n (%) | ||||
| Strongly disagree | 2 (22.22) | 2 (8.33) | 2 (6.25) | 6 (9.23) |
| Disagree | 1 (11.11) | 3 (12.50) | 4 (12.50) | 8 (12.31) |
| Neither agree nor disagree | 0 | 3 (12.50) | 4 (12.50) | 7 (10.77) |
| Agree | 1 (11.11) | 11 (45.83) | 17 (53.13) | 29 (44.62) |
| Strongly agree | 5 (55.56) | 3 (12.50) | 5 (15.63) | 13 (20.00) |
| Missing | 0 | 2 (8.33) | 0 | 2 (3.08) |
| Compared to using other types of dialysis, I found this type of dialysis, n (%) | ||||
| Much harder to learn to use | 0 | 0 | 0 | 0 |
| Slightly harder to learn to use | 2 (22.22) | 0 | 1 (3.13) | 3 (4.62) |
| As easy as other types to learn to use | 4 (44.44) | 5 (20.83) | 16 (50.00) | 25 (38.46) |
| Slightly easier to learn to use | 1 (11.11) | 3 (12.50) | 10 (31.25) | 14 (21.54) |
| Much easier to learn to use | 2 (22.22) | 9 (37.50) | 5 (15.63) | 16 (24.62) |
| Missing | 0 | 7 (29.17) | 0 | 7 (10.77) |
| After training, I felt confident I could use this type of dialysis safely, n (%) | ||||
| Strongly disagree | 2 (22.22) | 1 (4.17) | 1 (3.13) | 4 (6.15) |
| Disagree | 1 (11.11) | 1 (4.17) | 2 (6.25) | 4 (6.15) |
| Neither agree nor disagree | 1 (11.11) | 3 (12.50) | 6 (18.75) | 10 (15.38) |
| Agree | 3 (33.33) | 13 (54.17) | 22 (68.75) | 38 (58.46) |
| Strongly agree | 2 (22.22) | 4 (16.67) | 1 (3.13) | 7 (10.77) |
| Missing | 0 | 2 (8.33) | 0 | 2 (3.08) |
| This type of dialysis machine is user-friendly, n (%) | ||||
| Strongly disagree | 0 | 1 (4.17) | 0 | 1 (1.54) |
| Disagree | 0 | 0 | 0 | 0 |
| Neither agree nor disagree | 2 (22.22) | 3 (12.50) | 4 (12.50) | 9 (13.85) |
| Agree | 6 (66.67) | 13 (54.17) | 22 (68.75) | 41 (63.08) |
| Strongly agree | 1 (11.11) | 13 (54.17) | 6 (18.75) | 38 (58.46) |
| Missing | 0 | 2 (8.33) | 0 | 2 (3.08) |
Most staff reported that they had received sufficient training in each type of dialysis and felt confident in using, however, some did express some concerns about this, which would need addressing in the future.
Chapter 4 Discussion
The research question
The I-KID study was undertaken because of an unmet clinical need for improved technology to perform RRT in babies in PICU. Current techniques of PD and CVVH were compared with a novel device, NIDUS, specifically designed for babies between 0.8 kg and 8.0 kg. While PD sets used are licensed there was no licensed HD or CVVH device available in the UK at present, for this size of baby. Clinically it was well recognised that RRT methods were challenging in babies. 10
Having shown promising results in pilot studies, the main research question was how does the novel device NIDUS perform in a normal clinical PICU environment?21 The primary outcome was the precision of UF delivered compared with clinician prescription, with related secondary outcomes of precision of UF compared with information from the device, and the clearances achieved, with a range of other secondary outcomes largely provided via PICANet data. It was important to establish a safety profile of the new device.
Summary of key findings
From the primary outcome in this randomised cluster SW study comparing dialysis methods in babies in PICU, we found that the intervention device NIDUS delivered greater precision of UF than the control methods. The comparison of how close actual fluid removal was to that reported by the device showed that the difference between observed and machine reported was substantially smaller for NIDUS compared with CVVH, but it should be noted that this analysis was on a small sample of 33 cases and was influenced by some outlying values. The clearances of creatinine, urea and phosphate were lowest using PD, better using NIDUS – which provides creatinine clearance of a similar magnitude to a newborn baby with normal renal function – and highest using the CVVH devices. Overall, 77% of babies survived to PICU discharge, 96% of those receiving PD, 46% of those receiving CVVH and 66% of those receiving NIDUS. These figures reflect the high mortality associated with the underlying clinical diagnoses. The study faced a number of challenges to delivery, including a delay in the start of the study due to regulatory issues and the wider effects of the COVID-19 pandemic.
The study was found acceptable to parents who provided feedback.
Recruitment
Recruitment to the I-KID study from opening in December 2018 through to March 2020 (periods 1, 2 and 3) was much as expected. The majority of the reasons for ineligibility were due to weight exceeding 7.99 kg and these were evenly distributed across time periods but primarily from sequence 1 (63 of 78 cases), which suggests screening was interpreted differently by different sites. Another group of babies excluded were those with an underlying metabolic diagnosis (22) who were evenly distributed across time and sites.
There were 46 patients in the NIDUS phase of the study who were excluded because the attending clinician did not wish to use NIDUS, and this is potentially a notable source of bias. However, two items should be noted. First, the exclusion criterion ‘clinical decision by the attending clinician to not use NIDUS’ was in the study protocol from the early development phase and was included at the insistence of PICU consultants who were clear that in the very difficult and complex clinical situations in which they worked, this had to be a clinical management option. Without this exclusion the I-KID study would not have gained clinical support necessary for the trial to happen. Second, it should be borne in mind that this is a device trial: the primary outcome assesses how well the method of RRT delivers the prescribed UF rate. It is believed that the clinical condition of the patient will affect such an outcome much less than it would some measure of efficacy.
While there will be multiple reasons for the exclusion under this heading, it should be noted that 37 of the 46 exclusions were in the 4th time period. The outcomes in I-KID that were measured at the bedside were time-consuming and onerous. It is therefore possible that when making a decision which is finely balanced, clinicians might have been influenced by knowing the workload recruitment would impose on staff who, at that time, had been facing the challenges of the COVID-19 pandemic for many months.
Pauses to recruitment
The longest pause to recruitment was from March to October 2020 for five sites (March 2020 to June 2021 for one site) and was largely as a result of COVID-19 and the withdrawal of non-COVID-19 research activity in UK hospitals from March 2020. The beginning of this period was a little confusing. As a result of a protocol amendment submitted to request to do some additional anticoagulation studies (to answer clinical questions raised by site clinicians), the MHRA requested further clarification and information and withdrew their notice of no objection until they had reviewed that additional information which was submitted on 6 March 2020. The notice of no objection was reinstated by MHRA based on the submission of information and clarification and without any further changes.
There were three pauses to recruitment for technical issues related to problems with consumables used on the NIDUS device (see Table 30). All were instigated as a result of a cautious approach by the Chief Investigator on receipt of the first information from sites about any issues that could pose a risk to the safety of participants.
| Reason | Solution | Comments | |
|---|---|---|---|
| 20/05/2019–26/06/2019 | Fine tubing on sets at one site peeled away from connector if too much tension applied | More robust tubing used and glued to connector Additional training to sites rehandling of tubing |
Tubing sets for I-KID study were assembled by hand by Allmed. Sites were used to using more robust adult-sized tubing sets |
| 23/09/2019–18/10/2019 | Blood leak noted in filter | Faulty batch of filters identified and withdrawn; new filters supplied to sites | Allmed identified a change in manufacturing process which resulted in glue-weakening filter fibres. Process amended and quality control tightened |
| 02/03/2020–05/10/2020 | MHRA withdrawal of no objection; coincided with site shutdown of recruitment to all non-COVID studies from March 2020 | MHRA awaiting clarification following protocol amendment. Sites still closed to all non-COVID study recruitment | MHRA reinstated on 26/06/2020 following our withdrawal of protocol amendment relating to additional coagulation studies and submission of additional documentation for clarification (06/03/2022) Communication with MHRA to receive more urgent response was not pursued as MHRA engaged in COVID-related work and sites unable to recruit to non-COVID studies |
| 04/11/2020–18/12/2020 | Blood leak from disconnect of three-way tap above bubble detector | Safety warning issued and additional training provided to remind users to ensure air bubble detectors remain clean and dry | Air bubble detectors are standard and similar to those used on other devices; user handbooks instruct users to ensure clean and dry. To prevent/reduce risk of this happening in future the air bubble detectors will be repositioned in the next version of NIDUS device |
All technical problems were fully investigated and assessed by the Clinical Safety Subgroup and by MHRA before restarting. Liaison with the manufacturer Allmed was critical and they were supportive in addressing manufacturing issues.
Baseline characteristics of the 97 participants
Composition of control group
Of the 62 babies in the control group 48 received PD, 13 CVVH and one baby on ECMO had HD via a filter in the blood circuit. From baseline PICANet data from 2011 to 2013 when this study was first proposed, we had expected in I-KID that the modality of RRT in the control group to be more evenly split between PD and CVVH. In the study development we had sought data on dialysis modality by weight but as PICANet collected age and not weight data at that time, we therefore made some assumptions using weight for age centiles to estimate numbers of potential recruits.
There are no clinical guidelines for choice of dialysis modality in babies under 8 kg and the modality used is influenced by individual clinicians own experience. Moreover, it is well recognised that simply performing a study in a clinical environment can subtly change clinical practice and fine details of routine data collection. 36 Although it was not intended to influence current practice for the control group it is possible that starting I-KID actually influenced choice of RRT modality. At study launch, site initiation and study training visits the issues of reported problems and the current regulatory status of CVVH devices being used off-licence and against manufacturers advice was highlighted. In addition, on reflection, the study tasks for collection of data for the primary outcome and related secondary outcomes (filtration and clearances) were probably easier to perform for the babies on PD than babies on CVVH or NIDUS because of wider nursing familiarity with PD and how these tasks fitted with normal clinical practice. For the volumetric study for babies on PD the main bedside study tasks were accurate timing and collecting and sending of a fluid sample, whereas for the CVVH devices and intervention NIDUS device there were additional bag-weighing tasks and the collection of fluid for clearance calculation was more complex for bedside staff.
Age and weight of participants
There was a wide age range of participants: the median [interquartile range (IQR)] age in controls 10.5 (7–38) days was similar to that in the intervention group 11 (7–61) days; the range of age of participants was between 1 and 477 days (c. 16 months). It is recognised from previous PICANet reports that use of RRT is skewed towards the younger end of the age range of babies expected to fall in the under 8 kg weight range. 7,8 The 50th centile for weight of a 9-month-old baby is around 8 kg, whereas, at the upper end of the age range of 15 months, 8 kg is on the 2nd centile for weight. However, it should be noted that weight and age do not closely correlate in sick babies. 37
Admission to PICU and use of RRT commonly occur for babies following surgery for congenital abnormalities especially cardiac, which tends to be required in the early postnatal period. This is also reflected in the median (IQR) weights 3.2 (2.9–3.9) kg and 3.7 (3.1–5.6) kg which were similar between control and intervention and not dissimilar to the average birth weight of a term baby (37–40 weeks gestation) in the UK which is around 2.5–4 kg.
One participant was 10 kg with estimated dry weight of 7.7 kg; estimated dry weight was permitted in the protocol, as some babies can become severely oedematous by several litres of fluid. The median gestational age was 38 weeks in both the control and intervention groups with a range of 26–41 weeks which is not unexpected.
Descriptive data were similar in both control and intervention groups; around half the participants had unplanned admissions to PICU and approximately a third were transferred from outside hospitals. RRT was required post surgery in 52% of control and 40% of intervention participants. For those requiring RRT post surgery this involved cardiac bypass surgery in 97% of controls and 84% of intervention participants. Systolic blood pressure median (IQR) was 68 (59–78) mmHg for control, and 68 (60–86) mmHg for intervention, and the need for mechanical ventilation (>80%) was similar in the two groups.
PIM3 score
The Paediatric Index of Mortality 3 (PIM3) is a score to quantify a patient’s probability of death was developed as mortality risk prediction model (range 0–1) for all admissions to PICUs, a heterogeneous patient population of which those receiving RRT from a very small subset. It is based upon information from the first hour of admission. The median (IQR) PIM score was similar in control 0.023 (0.013–0.065) and in intervention 0.027 (0.014–0.131) groups, although the corresponding values for PD of 0.02 (0.01–0.05) and CVVH 0.06 (0.01–0.20) suggest a lack of homogeneity in the control group.
Laboratory data pre-initiation of RRT
Laboratory data collected pre-initiation of RRT were similar for control and intervention groups for plasma sodium, median (IQR) control 146 (141–149) mmol/l, intervention 143 (136–146) mmol/l, and for plasma potassium control 4.45 (4.0–5.2) mmol/l, intervention 4.8 (4.2–5.5) mmol/l. Plasma creatinine and urea appeared slightly higher in the intervention group than in the control group: creatinine 74 (56–110) µmol/l versus 51.5 (40–68) µmol/l and urea 6.15 (3.5–10.6) µmol/l versus intervention 9.5 (4.7–17.2) µmol/l, respectively. Within the control group plasma creatinine was higher in the CVVH group 94 (42–218) µmol/l than the PD group 51 (39.5–62) µmol/l. This raises the possibility that the severity of renal failure was different in these subgroups or that the threshold for starting this modality of RRT may have been different.
The median platelet count was lower in the intervention, 124 (95–209), than control 207 (113–257) groups.
Indication for starting RRT
The clinical decision that the participant required RRT was made by the attending clinical team irrespective of the I-KID study. Decisions about clearances and UF aims and prescriptions were made based on individual patient clinical parameters by the clinical team. For the NIDUS device, advice was given in training sessions and as written information about the need for anticoagulation with heparin, and how to monitor this. The need for very frequent (hourly) monitoring of activated clotting time (ACT) assays was reinforced after we received early reports of filter clotting.
The primary indication for starting RRT in the control and intervention groups, was similar in that in about half of the cases it was for fluid volume control alone, the rest having RRT started for biochemical control or both.
Key findings
Ultrafiltration (fluid removal)
The results show that the UF obtained with NIDUS was closer to that prescribed than with control. If the precision denotes the UF obtained minus the UF prescribed, X-A, then when calculated for the primary outcome, that is the first observation on X-A from each patient, Figure 7 shows this is much less dispersed about 0 with NIDUS than with control. Results in Tables 5 and 6 indicate that SD of X-A using NIDUS is about 13% of that using control 95% CI (3 to 71). If the information from all determinations of X-A in the first 48 hours post RRT is considered, then the SD using NIDUS is still estimated to be 13% of that using control, but with a narrower 95% CI (4 to 41).
When the results for the primary outcome in participants receiving CVVH and PD are each compared with NIDUS the picture is very similar. The SD for NIDUS is 14% of that for PD, 95% CI (3 to 70) and 11% of that for CVVH 95% CI (2 to 64).
A concern is that while UF values were obtained for all participants allocated to control, values were obtained from only 21 of the 35 participants receiving NIDUS. Of the 14 participants with no primary outcome, three were transition babies in the transition period so would have been excluded from the primary analysis. The reasons for this are given in Table 4 with more details in Appendix 2, Table 37 and are almost all related to technical issues with NIDUS, in particular its filter. Nevertheless, the failure to obtain the primary outcome in such a high proportion of one treatment group in a conventional therapeutic study would raise serious concerns about bias. While these missing data are a definite weakness, it should be borne in mind that in this study the comparison is between properties of methods of RRT – we are concerned with how closely the method of RRT delivers the prescribed UF – and this is likely to be less affected by patient characteristics.
We note that prescribed UF rates were substantially lower for NIDUS median 12 (8.6–21) range 0–40 ml/hour, and for PD median 10 (4–13.5) range 0–60 ml/hour than for CVVH median 30 (20–39) range 6–58 ml/hour. The difference in settings may be related to the understanding that CVVH achieves biochemical clearance by convection (and is increased by increasing UF and replacement/dilution fluid). Whereas PD and NIDUS primarily achieve biochemical clearance by diffusion.
Precision of RRT device
For NIDUS and CVVH devices, an important measure is to compare the difference between the actual fluid removal and that reported by the device: The results in Table 18 show this to have a mean closer to zero for NIDUS than CVVH (means −0.44 vs. 11.6 ml/hour, respectively), with less variation in NIDUS than CVVH (SDs 3.2 vs. 28.4 ml/hour). The formal analysis reported in Table 19 shows a similar ratio of SDs (17%, 95% CI 9% to 31%), but with a markedly different adjusted mean difference. This, and the difference in SDs, may be heavily influenced by large discrepancies on CVVH in period 2.
It is clinically very important to be able to rely on the information given by the device is accurate; then the clinician is able to make adjustments to the participants overall fluid input to counter discrepancies. Conversely if the device gives inaccurate information to the clinical team it contributes to uncertainty and difficulty in overall fluid management. Manufacturers are aware of the inherent imprecision of their devices and give warning in their technical documentation (e.g. Prismaflex ±30 ml/h, ±300 ml per 24 hours). In vitro studies of the fluid removal precision of RRT devices when set to ‘treat’ bags of saline using infant settings showed that the variances between the displayed and actual UF volumes for Prismaflex and Aquarius were wide at 14.0 and 30.3 ml by 15 minutes, respectively, while that for NIDUS was close to zero at 0.17 ml by 15 minutes. 15 These variances were seen whether the devices were set at relatively high UF rates (40 ml/hour) or to maintain neutral fluid balance.
Clearances
Biochemical clearance rates for creatinine, urea and phosphate
There is clear evidence that the mean clearance for each metabolite was lower on PD than NIDUS, and was lower on NIDUS than CVVH (see Table 11). It was also shown that the variation in the clearance for each metabolite was lowest for PD, highest for CVVH, with NIDUS having intermediate variation in clearance rates.
For PD the median (IQR) creatinine clearance was 0.08 (0.06–0.10) ml/min/kg, for CVVH 0.93 (0.75–1.47) ml/min/kg, and for NIDUS 0.39 (0.31–0.47) ml/min/kg. The results for biochemical clearance of creatinine, urea and phosphate are similar in that the biochemical clearance delivered by PD is less than that delivered by NIDUS which is less than that delivered by CVVH.
The clearance comparison between PD and NIDUS reflects that found in previous study whereas this is the first comparison between CVVH (Prismaflex and Aquarius) and NIDUS devices. 21 Given the greater blood flow and larger filter surface area of the CVVH devices these results are as anticipated. Clinically, the NIDUS would provide adequate biochemical clearance for controlling biochemical disturbance in babies with acute renal failure.
Secondary outcomes (mortality)
Of the 62 participants receiving control 54 survived to 30 days (87%) and 52 (84%) survived until discharge. For the 35 participants in the NIDUS group, 25 survived to 30 days (71%) and 23 (66%) survived to discharge. These comparisons gave p = 0.057 and 0.040, respectively (see Table 12).
When the groups receiving PD and CVVH are compared with NIDUS there is a suggestion that survival was highest in PD: out of 48 participants 47 survived to 30 days (98%) and 46 (96%) to discharge, whereas for the 13 participants on CVVH the corresponding values were 7 (54%) and 6 (46%) (see Table 16).
No formal statistical modelling of the effect of variables on death rates in the groups has been carried out. However, it is noticeable that the means of a variables measuring the risk of death (PIM3) and extent of renal disease (baseline creatinine) are in the same order as the death rates in the treatment groups: in the order PD, NIDUS, CVVH the means of PIM3 are 0.02, 0.027, 0.06 and for creatinine before RRT 52, 106 and 172 mmol/l.
While I-KID has provided evidence that NIDUS delivers more precise UF, there is no indication that this has translated into lower mortality. I-KID was not designed to detect differences in mortality. While it is to be hoped that improved methods for RRT will lead to better patient outcomes, mortality will be principally dependent on the diagnosis, so it is likely that the effect of the performance of the method of RRT on mortality will only be detected in larger studies.
Causes of death are presented in Table 31. Most deaths were assessed to be unrelated to the RRT device with the exception of two which were possibly related to the ECMO + HD and NIDUS devices, respectively.
| Index | Device | Status at 1 month | On RRT at death | PI notes/AE TERM on MACRO | PI view of causality being due to RRT therapy |
|---|---|---|---|---|---|
| 1 | NIDUS | Alive | No | Hemophagocytic lymphohistiocytosis (HLH) type 2, leading to multiorgan failure | No |
| 2 | NIDUS | Alive | On chronic PD | Complex unexplained illness including sepsis and hypogammaglobulinaemia, leading to multiorgan failure | No |
| 3 | NIDUS | Dead | No | Group A streptococcal septicaemia, leading to multiorgan failure | No |
| 4 | NIDUS | Dead | No | Hemophagocytic lymphohistiocytosis (HLH), veno-occlusive disease, disseminated adenovirus infection, and stem cell transplant, leading to multiorgan failure | No |
| 5 | Prismaflex | Alive | No | Complex congenital heart disease requiring cardiac surgery Cardiac arrest |
No |
| 6 | Prismaflex | Dead | No | Complex congenital heart disease requiring cardiac surgery, leading to multiorgan failure | No |
| 7 | Prismaflex | Dead | No | Medulloblastoma treatment leading to veno-occlusive disease (VOD), leading to multiorgan failure | No |
| 8 | PD | Alive | No | Complex congenital heart disease requiring cardiac surgery, and tracheo-broncho-malacia, leading to multiorgan failure | No |
| 9 | PD | Dead | No | Complex congenital heart disease requiring cardiac surgery, and prematurity | No |
| 10 | ECMO + HD | Dead | No | Complex congenital heart disease requiring ECMO, and leading to multiorgan failure | Possibly related to ECMO complications |
| 11 | NIDUS | Dead | No | Diabetic fetopathy (infant of diabetic mother), hypertrophic cardiomyopathy, respiratory distress syndrome at 35 weeks gestation Congenital nephrotic syndrome |
No |
| 12 | NIDUS | Dead | No | Prematurity (32 weeks gestation), patent ductus arteriosus, necrotising enterocolitis totalis with perforation (operated), gastric necrosis and perforation (partial gastrectomy), Escherichia coli sepsis | No |
| 13 | NIDUS | Dead | No | Complex congenital heart disease requiring cardiac surgery and ECMO. E. coli (ESBL) sepsis, necrotising enterocolitis, necrotising pneumonia, pneumothoraces | No |
| 14 | NIDUS | Dead | No | Complex congenital heart disease requiring cardiac surgery, leading to multi-organ failure | No |
| 15 | NIDUS | Dead | No | Complex congenital heart disease requiring cardiac surgery and ECMO, and leading to multiorgan failure | No |
| 16 | NIDUS | Dead | No | Complex congenital heart disease requiring cardiac surgery and ECMO. Cardiac arrest | No |
| 17 | NIDUS | Dead | No | Chronic lung disease causing pneumonitis and prolonged hypoxia, developed multiorgan failure and pulmonary haemorrhage | SADE reported Possibly related to NIDUS therapy |
| 18 | NIDUS | Dead | No | Prematurity plus massive cystic hygroma, hypotension and cerebral haemorrhage. Sepsis with systemic inflammatory response syndrome | No |
| 19 | Aquarius | Dead | No | Congenital lung hypoplasia, plus congenital myopathy. (Was also on ECMO treatment) | No |
| 20 | Aquarius | Dead | No | Congenital left diaphragmatic hernia (operated), hypoplastic lungs requiring ECMO therapy, pulmonary embolism, pulmonary hypertension, bilateral chylothoraxes | No |
| 21 | Aquarius | Dead | No | Group B streptococcal sepsis, persistent pulmonary hypertension, neurological injury | No |
| 22 | Aquarius | Dead | No | Congenital alveolar capillary dysplasia, treated with ECMO | No |
Secondary outcomes (collected through PICANet)
Completion of planned RRT
While 41 (85%) of the 48 babies on PD completed the planned RRT, there is a suggestion that fewer babies on CVVH and NIDUS completed planned RRT but the data were missing for 60% and 40% of these two groups.
Need for additional vascular or dialysis access
All the babies requiring RRT needed additional vascular or peritoneal access to facilitate this.
Haemodynamic status (drop in blood pressure after connection, requiring intervention)
We collected data on haemodynamic instability requiring intervention related to commencement of RRT. We requested data on additional fluid bolus (PICANet data collection defines this as >80 ml/kg) and/or inotropes used in the first day and second day after RRT started. Hundred per cent of participants on PD required intervention, 77% of those on CVVH and 89% of those on NIDUS. These data may simply reflect that nearly all babies were haemodynamically unstable or that babies, particularly post cardiac surgery, are on inotropes almost routinely.
Unplanned filter change
Unplanned filter changes cause additional work for bedside nursing staff, interfere with continuity of delivery of RRT (UF and clearance) and blood may be lost from the baby in the filter – the amount being related to whether the loss of filter was due to high pressures (‘sludging’) or blood clot and whether any ‘washing back’ of the circuit and filter is achieved and its priming volume (Prismaflex HF20 circuit = 60 ml; Aquarius HF03 circuit = 96 ml; NIDUS Neoflux1 circuit = 14.8 ml). Forty-six per cent of babies on CVVH and 60% on NIDUS required an unplanned filter change. The high rate of filter loss on the NIDUS due to high-pressure alarms and in some cases clotting of the filter have reinforced the case for requiring a new design of filter geometry specifically for this device. The NeoFlux1 filter used on the NIDUS was chosen because it was the only one of appropriate size (surface area and priming volume) that was CE marked and manufactured.
Exposure to blood transfusion while on RRT
Babies who are unwell and particularly post surgery may require blood transfusion for a number of reasons. Median (IQR) haemoglobin concentrations prior to starting RRT were similar. However, only 7 (15%) participants on PD required a blood transfusion, whereas 12 (92%) on CVVH required blood transfusion and 27 (77%) of those on NIDUS. This suggests that the process of haemotherapy RRT increases the need for blood transfusion, which fits with clinical experience of CVVH and HD. Three of the 13 cases of CVVH obtained their venous access from the baby’s ECMO circuit, and of the remaining ten, five had their CVVH circuits primed with a combination of packed red blood cells and either crystalloid or plasma products, and five were primed with saline. All of the NIDUS circuits were primed with saline. In retrospect, it was an oversight of the study that the volume of transfusion given was not recorded.
Anticoagulation
Most babies on haemotherapy RRT require anticoagulation to prevent clotting of the dialysis/CVVH circuit. However, in some babies who have a clinical coagulopathy there is an attempt to use devices without additional anticoagulation to reduce the risk of bleeding. Usually the anticoagulation used was heparin – used in 85% of the babies on CVVH. Regional citrate anticoagulation is being increasingly used for children on CVVH to prevent the need for systemic anticoagulation but in the I-KID study was only used in two of the 13 babies. The NIDUS device (because of the single vascular access line technology) cannot be used with citrate and thus heparin anticoagulation is required. It was reported as being used in 33 of the 35 babies on NIDUS, with two babies reported as having no anticoagulation used.
Safety profile
It was obligatory to report safety to the MHRA (device deficiencies, SADEs, SAEs) on the NIDUS as this was the device under investigation. Although CVVH in children weighing <8 kg is currently undertaken without regulatory approval (off-licence) and against the advice of the device manufacturers, it is not common practice to make regular safety reports on clinical use outside the I-KID study. Similarly, few clinicians report complications encountered when undertaking manual PD in infants during normal clinical practice. However, the participating sites were encouraged to make regular safety reports about problems they encountered during the control period of the I-KID study in a manner similar to that which they would be doing for NIDUS during the intervention period.
The details of the safety profile are tabulated (see Tables 20–26, 31 and 34). ADEs, SADEs, AEs which were not consistent with the usual clinical pattern for participants requiring RRT in PICU, and, SAEs, regardless of whether they were consistent with usual clinical patterns, were recorded.
ADEs and SADEs were specific to participants in the intervention arm only as the event had to be judged to be possibly, probably or definitely caused by the NIDUS device/tubing set.
A total of 35 AEs were initially recorded spanning 29 participants, but six of these were due to the loss of filter/filter clot which is an expected occurrence in this type of therapy. In addition, two participants in the intervention group were reported to have small intraventricular haemorrhage (IVH) detected via routine head ultrasound, but they were not reported as symptomatic. This investigation which was introduced by one site for NIDUS participants only and was not available for control babies at that site. Thus, there were 27 AEs across 23 participants (15 control, 8 intervention).
There was one SADE reported during the study and one ADE which was possibly related to the NIDUS device/tubing set. There were 17 SAEs across 15 participants (8 control, 7 intervention).
Study design
The study design chosen was a result of various factors. We knew from PICANet data that most RRT in small babies in the UK is concentrated at 11 sites (this is largely related to case mix; those sites with a paediatric cardiothoracic surgery unit doing more RRT. 7 As we only had 18 NIDUS devices the maximum number of sites, we could use was six to enable sites to have three intervention devices each – potentially one in use, one for a second baby recruited and one as backup in case of device problem. By the time the study started there were only 17 devices available, one having been lost to regulatory testing, thus it was decided that Newcastle as the original development site with small recruitment potential and with onsite engineers and scientists should have two devices.
In most circumstances the best design for a clinical study comparing two interventions is to randomise individual participants to one or other treatment. However, in I-KID this would open the possibility of two participants on the same PICU being treated differently. Discussion with parents and public revealed a strong feeling that asking parents to consent to individualised randomisation to dialysis type was not possible or desirable when most parents would have no prior knowledge of what dialysis was. Moreover, they would already be overwhelmed by the sheer amount of medical information they were being given at the time and thus most parents in that situation would defer to the clinician’s decision. It was also felt that having babies in the same unit on different devices at the same time could add to staff confusion and cause additional distress to parents to see an adjacent baby had the ‘new’ device when theirs had not been offered it – or vice versa.
Consequently, we opted for a form of cluster-randomised design, in which random allocation was applied to PICUs, not participants. As pointed out, I-KID was designed to study RTT in participants under 8 kg, where only a limited number of sites in the UK could participate and where resources limited this further to just six sites. With six participating sites it was always going to be difficult, notwithstanding random allocation, to ensure that the intervention groups in a conventional parallel-group cluster-randomised trial were comparable. This difficulty was heightened because the tertiary centres which participated in I-KID will inevitably have developed slightly different specialisms – for example some are co-located with quaternary cardiothoracic units.
This led to consideration of forms of cluster-randomised trials which can yield within-cluster information on intervention effects. A cluster-randomised crossover design would achieve this, in which three sites received the control intervention at the outset, and the other three NIDUS. Halfway through the study the sites would change to offering the other intervention. This form of design was not adopted because (1) there could be carryover effects, which in this case would largely be to do with the way staff treat participants needing RRT and, more importantly, (2) because there was a strong indication from staff in Newcastle who had used NIDUS during its development and compassionate use, of a reluctance to change from NIDUS to conventional therapy for these very sick children. Taken together, these considerations, where cluster allocation, availability of within-cluster information on intervention effects and reluctance to change back from NIDUS once used, led to the choice of SW design.
We anticipated that training sites to use new technology in a busy clinical environment and supporting them in the early stages of use would be challenging and time consuming. A further advantage of the SW design was that not all sites crossed over to the new device at the same time.
The SW design is a form of crossover designs, one in which there is substantial confounding of the treatment effect with time. Consequently, a valid analysis will almost certainly have to allow for the effect of time through the study, as well as any effect of treatment. In addition, the model needs to accommodate the fact that observations from each cluster, that is site, may be correlated. This is done by allowing a separate term in the model for each site, and usually this term is assumed to be random with a dispersion matrix taking a form specified up to the value(s) of the dispersion parameters in the model.
The model originally posited for I-KID assumed a general period effect, modelled by a categorical variable, and assumed that different observations within a site would have a given correlation, with those from different sites being uncorrelated – an equi-correlation structure. While there were some relatively short interruptions to the study when recruitment to NIDUS alone was paused, there was a much more extended pause due to COVID-19 from March 2020 for eight months for five sites and longer for a sixth site. This interruption was from the start of period 4 in the design and the general form of the period effect meant that this did not need modification. However, the much greater interval between observations taken in the fourth period compared with the intervals in earlier periods, called into question the form of the dispersion assumptions that had been made. Moreover, for a study with fewer than 100 participants, modelling a more elaborate dispersion structure would be inadvisable. A compromise was to assume a fixed site effect, so no dispersion structure needed to be specified. A disadvantage is that this model is potentially less efficient (Matthews and Forbes) but will be less vulnerable to the effect of mis-specification of the dispersion structure.
There were technical problems in establishing RRT in some participants using NIDUS and, when this occurred in the earlier parts of the intervention phase at a site (period 2 in sequence one and period 3 in sequence two), the rate of recruitment to NIDUS seemed slow. In parallel group studies, there is always an option to extend the study, however, with a SW design of three sequences and four periods, by the end of period 3 the only option would be to extend period 4. However, the information on the treatment effect contained in period 4 is limited – in the extreme case where the intra-class correlation is zero, then there is no information on the treatment effect in period 4. While it remains the case that there less information on the treatment effect in the final period than in periods 2 and 3, the reduction in information is lessened if fixed centre effects are fitted, which was a useful consequence of the decision to use fixed centre effects. For a related discussion of the information content of cells in a SWD, see Kasza and Forbes. 38
Questionnaires
i) Parents/carers questionnaires
We were very keen to learn from the experiences of parents/carers of participants in the I-KID study. We received 34 responses, 17 from control participants and 15 from intervention, of which approximately half were filled in by fathers or both parents, and half by mothers alone. Given all the other demands on parent and carers when their child is in PICU this is perhaps as expected, and the I-KID team are very grateful to the families for taking the time to give important feedback. The responses were generally very positive about the provision of information in increasing understanding about the dialysis used for their baby. Most respondents found it acceptable to be asked to take part in a research study about baby dialysis given that their child was so unwell, and importantly most would be likely to recommend other parents to take part in a similar research study if they had a baby with similar medical needs. We credit these positive findings to the input that the I-KID team had from parents in the design of the study, and in the production of parent-facing information.
ii) Staff questionnaires
One of the fundamental reasons for doing the I-KID study was to learn about staff experience of using the novel NIDUS device in a normal PICU clinical environment. We thus attempted to collect data on use of NIDUS and other methods of RRT, and received 65 responses relating to the experience of delivering therapies to 43 participants.
Staff views on training were similar for the three different modalities of RRT, with most respondents reporting that learning each technique was of similar difficulty, and feeling confident to use them all safely once they had received adequate training. It is notable that for each type of RRT, there were some respondents who did not feel confident to use it.
Strengths of study
The I-KID study had high input from public and parents at all stages from the early development phase onwards and this has been crucial in ensuring acceptability to participant parents. As the first comparison of these three different dialysis modalities in infants in PICU, I-KID provides important new information about RRT in babies on PICU. The study achieved a high degree of enthusiasm and support from clinicians and nursing staff, who worked very hard to make it work in sometimes challenging circumstances.
Largely the results were in concordance with clinical experience of RRT in babies and with previous NIDUS animal and compassionate use reports. 21 The results for UF and clearances support the view that NIDUS is worth pursuing through regulatory procedures as it has a potential place alongside established RRT modalities, particularly PD in clinical therapy.
An important safety profile has been created and I-KID has provided vital information on improvements required to the NIDUS device to improve usability.
Limitations of study
Recruitment was high in the first part of the study in the control phase but was less good as the study progressed through the intervention phase. There were pauses to recruitment for technical (consumables) reasons, but the largest interruption was due to the COVID-19 pandemic. This impacted directly on the ability to recruit to the study with all sites unable to recruit from March to October 2020. For one site (which recruited large numbers to control), ongoing COVID issues affected recruitment to intervention through June 2021. More indirectly, and difficult to quantify was the effect of the ongoing aftermath, reopening the study when PICU staff were described as tired and facing staffing problems due to COVID-19 absences. In addition, we underestimated the effect on nursing staff of having to learn a new technology and do additional study tasks – bag-weighing and timed sample collections - at the bedside. It is of note that despite provision of additional training sessions and updates, 37 of the 46 cases where the clinician chose not to use NIDUS were in the 4th time period that is post-COVID recruitment pause. We noted that study financial provision of additional nursing support does not always translate to availability when needed, which was unpredictable.
There were more missing data than was ideal, especially for the primary outcome in the intervention group. The number of patients not recruited to NIDUS due to consultants exercising their clinical judgement not to use the device on some patients was also larger than anticipated. The number of control cases on PD (vs. CVVH) was higher than we had estimated and expected. It may be that these factors combined to leave a slight imbalance in creatinine, urea and PIM3 between intervention and control, with potentially higher morbidity in the HD groups. However, this would have limited effect on device performance, the principal focus of I-KID.
Because of the unanticipated need for additional circuits and filter changes, study sites faced some additional costs for intervention cases.
Public and patient involvement
We had high engagement from parents and patient groups as summarised in Table 32 below.
| Section | PPI | Page |
|---|---|---|
| 1. Aims | The aims of PPI for the I-KID study were:
|
|
| 2. Methods | A presentation and discussion with the Newcastle University Research Consumer Group. View of parents with children who had experienced dialysis, including parents of participants who had been involved in previous pilot studies and compassionate use were approached. | |
| 3. Study results | PPI was a very positive contribution to the initial pre-grant application development and discussion. A parent was invited to all the early group telephone discussions between clinicians and this led to considerable clarity and respectfulness of communication. A parent contributed to telephone discussions with NIHR in the grant awarding process and jointly presented the study to the REC, and spoke at the study launch event for site clinicians. Parents have been involved in discussing the study results and guiding our approaches to planning results dissemination to participant families in an appropriate and sensitive way. | |
| 4. Discussion and conclusions | We think the high acceptability of the study to parents approached was a reflection of the excellent contribution to design and parent-facing information by PPI. | |
| 5. Reflections/critical perspective | It was important to have involved PPI at the earliest opportunity as their views helped formulate the study design and protocol. Their involvement improved the quality of the clinical research planning discussions and discussions with ethics committee and grant awarding body (NIHR). Their advice on sensitivity of approach to participant families for dissemination of results is invaluable. |
Participant representation
Patients became eligible for screening and subsequent inclusion in the study based purely on their clinical condition and need for RRT. Eligibility criteria (see Chapter 2) were deliberately broad to allow inclusion of a wide range of infants, with no exclusions on the basis of sex, biological age, race, ethnicity or other protected characteristics.
The sites, in particular those in London and Birmingham, have catchment areas with significant ethnic diversity.
For ethical reasons, it was essential that there was a person with legal PR for the patient who was able and willing to provide written informed consent for the patient to take part in the study. Only one infant had to be excluded from the study because of lack of availability of parent/guardian.
The I-KID TMG and PIs considered and discussed the possibility of potential language and communication barriers (both in respect of non-English speakers and deafness) to the provision of informed consent. Because of the severity of the participants’ illness and to minimise burden on the parents of eligible infants, we asked clinical teams to use the same routine translation services they were using for clinical discussions to explain the study and discuss the content of the participant facing information, either in person or via phone (e.g. big word type services in common use in the NHS). Our rationale was that seeking consent for a study such as I-KID, in such a complex high-risk clinical PICU situation, required a conversation between clinician and parent/guardian, not simply a translation of information sheets into a range of community languages, which therefore was not undertaken. Parental input to the design of the participant information materials was sought, to ensure comprehensibility and accessibility of these documents (see Public and patient involvement).
To minimise the burden of data collection on participating sites, we did not seek to record ethnicity or socioeconomic status of participants or their families; nor are these variables routinely captured by PICANet. Due to the young age of study participants, we recorded their sex assigned at birth, rather than gender. Of the 97 infants recruited to the study, 35 (36%) were female and 62 (64%) were male. Median age at screening was 11 days (IQR 7–61 days). PICANet data, across all PICUs participating in PICANet, for 2018–20 all-cause admissions (https://www.picanet.org.uk/annual-reporting-and-publications/) for infants aged under two months shows a 60% male, 40% female split, roughly in line with the pattern observed in I-KID, suggesting that there was no participation bias with respect to sex of infant.
Research team and wider involvement
I-KID research team composition reflected the range of professional disciplines, skills, expertise and experience required to deliver the study: paediatric intensive care medicine and nursing; medical physics; biostatistics; clinical trial design and management; data management (including representation from the central PICANet team at Leeds University); project management. The I-KID study co-applicants were all experienced in their respective disciplines, and generally at a relatively senior point in their careers. Within Newcastle University and the NuTH NHS Foundation Trust (NuTH), allocation of staff (e.g. trial and data management staff from the NCTU, project management staff from NUTH) was from within the existing complement of staff (i.e. no new staff were recruited specifically to work on I-KID), and was determined by capacity of the available staff members. NCTU staff allocation followed the unit’s normal model of a senior trials manager, trial manager, clinical trials assistant and database manager, and therefore included members of staff with various levels of seniority and experience. As might be expected in a study carried out over a number of years, there was some staff turnover (especially in the medical physics, NCTU and NUTH project management teams) over the course of the study. Replacement of members of staff who left was again determined by experience and spare capacity within the relevant organisation.
At site level, those delivering the clinical aspects of the study, and collecting and entering study data, were also drawn from the existing staff of the participating PICUs. In keeping with the responsibilities of the role, the Principal Investigators at all six sites were experienced paediatric intensive care consultants. As indicated in Chapter 2, all site staff participated in training for the roles in the I-KID study.
The I-KID study was closely aligned to and fully supportive of the equality, diversity and inclusion policies, including in respect of staff recruitment and training, of the university and NHS organisations employing the research team and site staff. Nonetheless, we note and are critical of these organisations still having a gender pay gap.
We did not collect data on the age, sex, gender, race, ethnicity or other protected characteristics of research team members or of site staff.
Collaboration with manufacturer (Allmed)
The I-KID study was designed, managed and analysed completely independently from Allmed, the device manufacturer, and no funding has been received from them.
However, this study could not have taken place without their support; the 17 NIDUS devices used were loaned by Allmed free of charge to study sites, and delivery and movement of devices was undertaken by them. The consumables used (filters and tubing) were purchased from Allmed by study sites for the NIDUS device and for the alternative forms of RRT (Prismaflex, Aquarius) and PD through the usual NHS purchasing routes. NUTH medical physics/engineering provided practical support in troubleshooting, training, maintenance and minor NIDUS device repair as the on-site NHS dialysis technicians would not have had sufficient knowledge and expertise to provide this specialist support of a new device.
Chapter 5 Conclusions
Implications for health care
RRT in babies under 8 kg poses considerable challenges related to the size of the patient and the technology available. The data from the I-KID study add to the knowledge base of RRT methods used in babies of this size in PICU, and the study was only possible because of close co-operation, working and clinical discussion between medical, nursing and allied staff. We have been able to collect data on ‘standard’ forms of RRT in addition to the new device under investigation. Furthermore, the I-KID study has raised important discussion and dialogue about clinical issues relating to RRT like difficulties of establishing adequate vascular access, lack of availability of appropriate vascular access catheters, anticoagulant use, assessment of fluid status and distribution, investigation and monitoring of babies undergoing RRT and the need to measure outcomes. The study faced significant challenges and the extent of missing data underlines the difficulties of doing research studies requiring additional bedside tasks in a PICU environment. The generous contribution of families in the study planning and oversight and as participants has been exceptional. Parental support for the concept of performing research despite their child being very unwell, and in difficult and emotional circumstances for them is very important and encouraging to healthcare researchers.
Many babies requiring RRT in PICU are very unwell as reflected by the vast majority in I-KID participants having multi-organ failure; most were on positive pressure ventilatory support and there was a very high use of inotropes to support blood pressure. Many babies required blood transfusion and the overall mortality was around 20%.
The intervention device NIDUS was shown to work effectively and delivers appropriate blood clearances and accurate, controllable fluid removal (UF), indicating that it has an important place alongside other dialysis modalities in the management of babies with renal failure.
There were AEs reported in both control subgroups and in intervention cases. NIDUS was shown to have an acceptable safety profile compared with other modalities used in this very unwell population.
PD is likely to remain a commonly used method for babies with less severe renal failure who require less prolonged/intensive dialysis. Many postoperative (especially those undergoing cardiac surgery) babies have a PD catheter inserted before emerging from theatre which is sometimes just used for draining ascitic fluid and can be easily used for dialysis as required. However, insertion of a PD catheter is not without its risks and there is room for future studies questioning the best immediate postoperative renal support modality. Where PD is not possible or fails it is clear that NIDUS provides a good therapeutic option to be considered.
Feedback from users through the I-KID study has been invaluable in identifying that the NIDUS device requires certain improvements regarding ease of setup, usability, and training, guiding towards appropriate solutions. There was substantial inter-case and inter-centre variation in the ease of use of the NIDUS device. There were similar problems with filter loss and the requirement for filter and circuit changes. Most HD devices have consumables which include a choice of filters that can be used in different circumstances. Experience of users through I-KID strongly supports the view that a new filter needs to be made available with improved geometry as the need to change filters and circuits was a recurrent problem for some babies, which sometimes interfered with continuity of renal replacement.
Recommendations for the future
Regulatory
The NIDUS device requires completed approvals by appropriate regulatory authorities (CE, UKCA, FDA, etc.) in order to be introduced more widely, and these should be pursued without delay.
Research
Short-term outcome of babies by RRT
Data from PICANet on RRT and short-term outcomes and survival to 30 days and discharge from PICU should continue to be collected and published.
Long-term outcome of babies who received RRT
Babies who receive RRT in PICU are among the sickest and have considerable morbidity and mortality. Long-term follow-up of overall outcomes for the child and for their renal function is required. Such babies are born with renal function which is less well developed than in later childhood and there is a period of renal maturation that happens over the first 12–18 months. Thus, a period of acute renal failure/renal insult occurring early in life has the possibility of affecting renal maturation. While babies who remain dependent on RRT are for obvious reasons closely followed up by paediatric nephrology units, those who become independent of RRT are sometimes followed up but not in a comprehensive and systematic way. Renal function is not a binary function, but a spectrum and independence from dialysis does not equate to normal renal function. Currently The Renal Registry collects data on RRT outside of PICU, but we recommend approaches be made to The Renal Registry to propose ongoing collection of data from all children (with priority to introduce data collection for all those under two years) who receive RRT in PICU.
Comparison of PD vs. NIDUS immediately post cardiac surgery
Insertion of a PD catheter after complex neonatal cardiac surgery has become almost routine. But PD catheter insertion is not without side effects and a study posing the question of what the most appropriate postsurgical method of fluid removal is and ensuring biochemical stability would be an appropriate research development.
Acknowledgements
We thank the parents and families of babies recruited to the study for their important contribution, particularly at a such stressful and anxious time when their baby is unwell.
Parents and families who advised at various stages in the study are thanked for their invaluable contribution.
We acknowledge the work, time and enthusiasm of the site research and clinical staff in making this study happen, their continuous support and for enabling restart to recruitment following interruption due to the COVID pandemic. In particular:
-
The Birmingham Women’s & Children’s research team Samantha Owen, Ceri Robbins, Margaret Farley & Helen Winmill
-
Dr Michael Griksaitis, Consultant paediatric Intensivist at Southampton Children’s Hospital
-
Lauran O’Neill, Ana Luisa Tomas, Holly Belfield, Helen Vander-Johnson at Great Ormond Street Hospital for Children
-
Helen Marley and Becca Lean at Bristol Royal Hospital for Children
-
Additionally, we acknowledge the CVVH nurse teams in all of the participating hospitals.
We acknowledge the commitment, work and dedication of the TSC, TMG and Independent Data Monitoring Committee as well as the important input from the Sponsor Team at NuTH in particular Chris Price (Regulatory Compliance Specialist).
We acknowledge the long standing and considerable contribution of members of the Northern Medical Physics and Clinical Engineering Department to the NIDUS project.
Contributions of authors
Heather Lambert (https://orcid..org/0000-0003-4389-4078) (Consultant Paediatric Nephrologist) conceived the original idea for the study, and supported by a team, led all stages of the NIHR grant application, was Chief Investigator throughout the study, was on call for clinical/device advice and queries throughout the recruitment period of the study. Responsible for interpretation of the results together with clinical and statistical colleagues, authored the introductory, discussion and conclusion chapters of the final report, and had substantial input into review of all other sections of the final report.
Shaun Hiu (https://orcid..org/0000-0003-1699-4348) (Trial Statistician) conducted the main trial analysis, co-wrote the results chapter and had substantial input into review of all other sections of the final report.
Malcolm Coulthard (https://orcid..org/0000-0001-8316-8957) (retired Paediatric Nephrologist) was responsible for the original design and concept of the NIDUS device, provided technical support to the trial sites, contributed to the trial design, protocol and SAP; co-applicant for funding; interpreted trial data; and had substantial input into review of all other sections of the final report.
John N S Matthews (https://orcid..org/0000-0003-2559-037X) (Professor of Medical Statistics) helped to design the study and co-wrote the SAP, conducted the main trial analysis, wrote the results chapter and had substantial input into review of all other sections of the final report.
Ruth Wood (https://orcid..org/0000-0002-8296-1774) (Database Manager) was involved in the data management aspects of the study, including setting up the database and making a substantial contribution to the design of the study database as well as liaising with the PICANet Audit to obtain specific data related to the Study and data cleaning. Provided input into the report and has critically reviewed it, with particular reference to sections related to the data collection.
Jean Crosier (https://orcid..org/0000-0001-8498-8412) (Senior Children’s Kidney Nurse) contributed to the design of the protocol, was training lead, provided clinical/device advice and queries throughout the recruitment period of the study, provided technical support to the trial sites, contributed to the design of the trial protocol contributed to trial recruitment and reviewed and contributed to the final report.
Rachel Agbeko (https://orcid..org/0000-0001-8931-6869) (Consultant Paediatric Intensivist, Site PI) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
Thomas Brick (https://orcid..org/0000-0002-2806-0000) (Consultant Paediatric Intensivist, Site PI) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
Heather Duncan (https://orcid..org/0000-0003-1771-8644) (Consultant in Paediatric Intensive Care, Site PI) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
David Grant (https://orcid..org/0000-0003-4584-1498) (Consultant Paediatric Intensivist, Site PI) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
Quen Mok (https://orcid..org/0000-0003-4807-9275) (Consultant Paediatric Intensivist Site PI) contributed to protocol development and funding submission, trial recruitment, aided in the critical analysis of the results and reviewed and contributed to the final report.
Andrew Gustaf Nyman (https://orcid..org/0000-0001-7363-6120) (Consultant Paediatric Intensivist, Site PI) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
John Pappachan (https://orcid..org/0000-0002-3559-0595) (Consultant Paediatric Intensivist, Site PI) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
Paul Wellman (https://orcid..org/0000-0001-6404-1758) (Paediatric Intensive Care Research Charge Nurse) contributed to the protocol design and amendments. Contributed to national patient recruitment, data acquisition and entry, study coordination and critical review of the final report.
Chris Boucher (https://orcid..org/0000-0003-1683-2584) (Lay Person Advisor) played a large role in the development of the protocol and associated documents from a patient-representative perspective. Contributed to the TOC and was a part of the ongoing development of the trial processes, reviewed and advised on the content of the final report.
Joe Bulmer (https://orcid..org/0000-0001-7778-0840) (Research Scientist) involved in the creation and dissemination of training materials and guidance for those collecting data (via the investigational device) and in aspects of troubleshooting during the trial (e.g. with the investigational device), aiding in the acquisition of adequate, accurate data. Involvement in TMG meetings which included discussions with regards to layout and content of the work. Write up/first draft of the methods and discussion sections. Revision/comments on the entire document and associated works.
Denise Chisholm (https://orcid..org/0000-0002-8073-0980) (Specialist Renal Nurse) contributed substantially to the concept and development of the study protocol, provided technical support to the trial sites, contributed to the design of the trial protocol on call for clinical/device advice and queries throughout the recruitment period of the study.
Kirsten Cromie (https://orcid..org/0000-0002-2859-5291) (PICANet Research Statistician) involved in the statistical analyses of PICANet data for the study (production of summary statistics). Provided continued statistical input and advice which contributed to the maintenance of the database and interpretation of study data for research purposes.
Victoria Emmet (https://orcid..org/0000-0002-5211-9455) (Specialist Renal Nurse) contributed substantially to the concept and development of the study protocol, provided technical support to the trial sites, contributed to the design of the trial protocol on call for clinical/device advice and queries throughout the recruitment period of the study.
Richard Feltbower (https://orcid..org/0000-0002-1728-9408) (Professor of Epidemiology) oversaw the PICANet data collection and reporting, contributed to revising the draft final report and approving the final version.
Michael Grayling (https://orcid..org/0000-0002-0680-6668) (Trial Statistician) co-wrote the SAP, conducted the main trial analysis, wrote the results chapter and reviewed and contributed to the final report.
Rebecca Harrison (https://orcid..org/0000-0001-8355-2406) (Research Scientist) Device training on-site, troubleshooting and machine maintenance. Device troubleshooting data analysis and report write-up, training materials authorship and quick-guide design.
Eva-Maria Holstein (https://orcid..org/0000-0002-3027-9052) (Clinical Trials Administrator) provided day-to-day administration of trial conduct and adherence to GCP, edited and contributed to the final report.
Ciara A Kennedy (https://orcid..org/0000-0003-2987-0977) (Clinical Trials Manager) was Trial Manager for the study, provided day-to-day management of trial conduct, edited, reviewed and contributed to the final report.
Elaine McColl (https://orcid..org/0000-0001-8300-3204) (Professor of Health Services Research) co-applicant for funding; contributing to the conception and design of the protocol and a member of Trial Management Group; contributed to discussions of data acquisition, analysis and interpretation. Wrote the equality, diversity and inclusion section and contributed to the critical review of the final report.
Kevin Morris (https://orcid..org/0000-0001-8911-6306) (Consultant in Paediatric Intensive Care, Co-applicant) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
Lee Norman (https://orcid..org/0000-0002-8684-8787) (PICANet Database Manager) built, designed and managed the PICANet software and database including the study data collection system.
Julie Office (https://orcid..org/0000-0002-7922-9830) (Specialist Renal Nurse) contributed substantially to the concept and development of the study protocol, provided technical support to the trial sites, contributed to the design of the trial protocol on call for clinical/device advice and queries throughout the recruitment period of the study.
Roger Parslow (https://orcid..org/0000-0002-3945-5294) (Visiting Senior Lecturer in Epidemiology) designed and provided the data collection instrument, advised on data quality and provided analysis of preliminary data for power calculations; contributed to the critical review of the final report.
Christine Pattinson (https://orcid..org/0000-0002-2256-1541) (Specialist Renal Nurse) contributed substantially to the concept and development of the study protocol, provided technical support to the trial sites, and contributed to the design of the trial protocol on call for clinical/device advice and queries throughout the recruitment period of the study.
Shriya Sharma (https://orcid..org/0000-0001-5237-757X) (Clinical Trials Manager) was Trial Manager for the study, provided day-to-day management of trial conduct, performed monitoring to ensure the trial was conducted to GCP requirements, edited and co-wrote the final report.
Jonathan Smith (https://orcid..org/0000-0001-8300-4426) (Consultant in Paediatric Cardiothoracic Anaesthesia and Intensive Care) was involved in the initial setting up and ideas behind the study, and contributed to critical review of the final report.
Alison Steel (https://orcid..org/0000-0003-1279-1455) (Senior Trial Manager) contributed to the significant changes required to bring the study in line with the regulatory requirements for a clinical investigation of a non-CE marked device; member of Trial Management Group, contributing to discussions about data acquisition, analysis and interpretation drafted Methods chapter and critically reviewed and commented on other chapters.
Rachel Steel (https://orcid..org/0000-0001-7295-5744) (Specialist Renal Nurse) contributed substantially to the concept and development of the study protocol, provided technical support to the trial sites, and contributed to the design of the trial protocol on call for clinical/device advice and queries throughout the recruitment period of the study.
Jayne Straker (https://orcid..org/0000-0003-3524-0744) (Specialist Renal Nurse) contributed substantially to the concept and development of the study protocol, provided technical support to the trial sites, and contributed to the design of the trial protocol on call for clinical/device advice and queries throughout the recruitment period of the study.
Lamprini Vrana (https://orcid..org/0000-0003-2454-9842) (Consultant Paediatric Intensivist) contributed to trial recruitment, aided in the analysis of the results and reviewed and contributed to the final report.
Jenn Walker (https://orcid..org/0000-0002-7442-3060) (Senior Trial Manager) led NCTU’s involvement in the trial, giving guidance on all aspects of governance and trial delivery, and reviewing and editing the final report for important intellectual content.
Mike Whitaker (https://orcid..org/0000-0002-2390-3056) (Research Scientist) contributed to the draft and submission of the study application. Developed and delivered training and site setup. Facilitated introduction of NIDUS instruments into study sites. Produced worksheets/timelines guiding data-collection and adapted to differing practices/instruments in the various sites, worked with I-KID team on necessary protocol updates through the course of the study.
Jim Wightman (https://orcid..org/0000-0002-9158-8684) (Research Scientist) contributed to the device design work, technical support, testing and maintenance of NIDUS. Data analysis and report write-ups. Draft revisions, discussions and advice on interim and final reports.
Nina Wilson (https://orcid..org/0000-0001-5908-1720) (Trial Statistician) wrote the SAP, contributed to interim trial analysis.
Lucy Wirz (https://orcid..org/0000-0003-2212-5675) (Consultant Clinical Psychologist) designed and piloted the study-specific questionnaires used to gather parent/guardian views (experience and acceptability of infant dialysis and study participation) and staff views (experience, acceptability and usability of the NIDUS). Advised on reporting and analysis of quantitative and qualitative data from parent/guardian and staff questionnaires. Advice and feedback on patient-facing information provided to the parents/guardians of participants about the study. Was named support availability for parent representatives on the study.
Publication
Lambert HJ, Sharma S, Matthews JNS. I-KID study protocol: evaluation of efficacy, outcomes and safety of a new infant haemodialysis and ultrafiltration machine in clinical use: a randomised clinical investigation using a cluster stepped-wedge design. BMJ Paediatrics Open 2021;5:e001224. https://doi.org./10.1136/bmjpo-2021-001224
Ethics statement and regulatory approvals
The study was awarded NIHR Efficacy and Mechanism Evaluation funding in 2015 as a UK multicentre study of a CE (Conformité Européenne) marked medical device and received favourable ethical opinion from the North East – Tyne & Wear South Research Ethics Committee (REC) (Reference: 16/NE0008) on 8 February 2016. One of the conditions the REC favourable opinion was subject to the NIDUS device receiving CE marking. Due to unexpected delays in the CE marking process, the study was submitted to the MHRA as a clinical investigation of a non-CE marked medical device. The study received Clinical Trial Authorisation from the MHRA on 9 March 2018. Favourable ethical opinion for the clinical investigation was received from the North East – Tyne & Wear South Research Ethics Committee on 21 February 2018, followed by Health Research Authority approval on 28 February 2018. The Research and Development departments at each participating site granted approvals for the study and subsequent amendments.
The study was conducted in accordance with Good Clinical Practice and the principles of the Declaration of Helsinki.
Data-sharing statement
Anonymised data from this study may be available to the scientific community subject to regulatory and ethical approval. Request for data should be directed to the corresponding author.
Investigators and administrative structure of clinical investigation
The NIDUS was supplied by the Allmed Medical Care Holdings Limited
Company No. 07966208
VAT No. GB 177361487
Address: Allmed Group, Building 7, Chiswick Park, 566 Chiswick High Road London W4 5YG
Website: www.allmedgroup.com
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Sutherland SM, Alexander SR. Continuous renal replacement therapy in children. Pediatr Nephrol 2012;27:2007-16. https://doi.org/10.1007/s00467-011-2080-x.
- Westrope CA, Fleming S, Kapetanstrataki M, Parslow RC, Morris KP. Renal replacement therapy in the critically ill child. Pediatr Crit Care Med 2018;19:210-7. https://doi.org/10.1097/PCC.0000000000001431.
- Santiago MJ, López-Herce J, Urbano J, Solana MJ, del Castillo J, Ballestero Y, et al. Clinical course and mortality risk factors in critically ill children requiring continuous renal replacement therapy. Intensive Care Med 2010;36:843-9. https://doi.org/10.1007/s00134-010-1858-9.
- Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, et al. Continuous renal replacement therapy for children ≤10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr 2013;162:587-92.e3. https://doi.org/10.1016/j.jpeds.2012.08.044.
- Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 2017;1:184-94. https://doi.org/10.1016/S2352-4642(17)30069-X.
- Menon S, Broderick J, Munshi R, Dill L, DePaoli B, Fathallah-Shaykh S, et al. Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol 2019;14:1432-40. https://doi.org/10.2215/CJN.03240319.
- Paediatric Intensive Care Audit Network, Universities of Leeds and Leicester . Annual Report 2010 - 2012, 2013 n.d. http://www.picanet.org.uk/Audit/Annual-Reporting/PICANetAnnualReport2013Summary.pdf.
- Paediatric Intensive Care Audit Network, Universities of Leeds and Leicester . Annual Report 2020, 2021 n.d. https://www.picanet.org.uk/wp-content/uploads/sites/25/2021/02/PICANet2020_AnnualReportSummary_v1.0.pdf.
- Westrope C, Morris K, Burford D, Morrison G. Continuous hemofiltration in the control of neonatal hyperammonemia: a 10-year experience. Pediatr Nephrol 2010;25:1725-30. https://doi.org/10.1007/s00467-010-1549-3.
- Tal L, Angelo JR, Akcan-Arikan A. Neonatal extracorporeal renal replacement therapy-a routine renal support modality?. Pediatr Nephrol 2016;31:2013-5. https://doi.org/10.1007/s00467-016-3423-4.
- Lee ST, Cho H. Fluid overload and outcomes in neonates receiving continuous renal replacement therapy. Pediatr Nephrol 2016;31:2145-52. https://doi.org/10.1007/s00467-016-3363-z.
- Bojan M, Gioanni S, Vouhé PR, Journois D, Pouard P. Early initiation of peritoneal dialysis in neonates and infants with acute kidney injury following cardiac surgery is associated with a significant decrease in mortality. Kidney Int 2012;82:474-81. https://doi.org/10.1038/ki.2012.172.
- FDA Updated public health notification: Gambro Prisma® continuous renal replacement system n.d. https://www.fda.gov/cdrh/safety/022706-gambro.html.
- Diaa M. Prismaflex User’s Manual n.d. http://www.scribd.com/doc/141000459/Prismaflex-user-manual.
- Crosier J, Whitaker M, Lambert HJ, Wellman P, Nyman A, Coulthard MG. In vitro measurements of ultrafiltration precision in hemofiltration and hemodialysis devices used in infants. Pediatr Nephrol 2022;37:3189-94. https://doi.org/10.1007/s00467-022-05439-y.
- Hackbarth RM, Eding D, Gianoli Smith C, Koch A, Sanfilippo DJ, Bunchman TE. Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol 2005;20:1328-33. https://doi.org/10.1007/s00467-005-1970-1.
- Hothi DK, St George-Hyslop C, Geary D, Bohn D, Harvey E. Continuous renal replacement therapy (CRRT) in children using the AQUARIUS. Nephrol Dial Transplant 2006;21:2296-300. https://doi.org/10.1093/ndt/gfl265.
- Sohn YB, Paik KH, Cho HY, Kim SJ, Park SW, Kim ES, et al. Continuous renal replacement therapy in neonates weighing less than 3 kg. Korean J Pediatr 2012;55:286-92. https://doi.org/10.3345/kjp.2012.55.8.286.
- Coulthard MG, Sharp J. Haemodialysis and ultrafiltration in babies weighing under 1000 g. Arch Dis Child Fetal Neonatal Ed 1995;73:F162-5. https://doi.org/10.1136/fn.73.3.F162.
- Everdell NL, Coulthard MG, Crosier J, Keir MJ. A machine for haemodialysing very small infants. Pediatr Nephrol 2005;20:636-43. https://doi.org/10.1007/s00467-004-1785-5.
- Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, et al. Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (NIDUS): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 2014;29:1873-81. https://doi.org/10.1007/s00467-014-2923-3.
- Askenazi D, Ingram D, White S, Cramer M, Borasino S, Coghill C. Smaller circuits for smaller participants: improving renal support therapy with Aquadex™. Pediatr Nephrol 2016;31:853-60. https://doi.org/10.1007/s00467-015-3259-3.
- Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, et al. Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 2014;383:1807-13. https://doi.org/10.1016/S0140-6736(14)60799-6.
- Lorenzin A, Garzotto F, Alghisi A, Neri M, Galeano D, Aresu S, et al. CVVHD treatment with CARPEDIEM: small solute clearance at different blood and dialysate flows with three different surface area filter configurations. Pediatr Nephrol 2016;31:1659-65. https://doi.org/10.1007/s00467-016-3397-2.
- Hothi DK. Designing technology to meet the therapeutic demands of acute renal injury in neonates and small infants. Pediatr Nephrol 2014;29:1869-71. https://doi.org/10.1007/s00467-014-2910-8.
- Laskin BL, Foster BJ. Extracorporeal therapy for the smallest children. Lancet 2014;383:1785-6. https://doi.org/10.1016/S0140-6736(14)60650-4.
- Lambert HJ, Sharma S, Matthews JNS. I-KID study protocol: evaluation of efficacy, outcomes and safety of a new infant haemodialysis and ultrafiltration machine in clinical use: a randomised clinical investigation using a cluster stepped-wedge design. BMJ Paediatr Open 2021;5.
- Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 2007;28:182-91. https://doi.org/10.1016/j.cct.2006.05.007.
- Brierley J, Larcher V. Compassionate and innovative treatments in children: a proposal for an ethical framework. Arch Dis Child 2009;94:651-4. https://doi.org/10.1136/adc.2008.155317.
- University of Liverpool . Research Without Prior Consent (Deferred Consent) in Trials Investigating the Emergency Treatment of Critically Ill Children: CONNECT Study Guidance 2015. https://www.liverpool.ac.uk/media/livacuk/iphs/Research, without,prior,consent,in, trials,investigating,the, emergency,treatment,of, critically,ill,children, CONNECT, guidance,July,2015.pdf.
- Matthews JNS, Forbes AB. Stepped wedge designs: insights from a design of experiments perspective. Stat Med 2017;36:3772-90. https://doi.org/10.1002/sim.7403.
- Morel JG, Bokossa MC, Neerchal NK. Small sample correction for the variance of GEE estimators. Biom J 2003;45:395-409.
- Gallis JA, Li F, Turner EL. xtgeebcv: A command for bias-corrected sandwich variance estimation for GEE analyses of cluster randomized trials. Stata J 2020;20:363-81.
- Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ 2018;363.
- Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika 1986;73:13-22.
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;67:267-77.
- Pollack MM, Wiley JS, Kanter R, Holbrook PR. Malnutrition in critically ill infants and children. JPEN J Parenter Enteral Nutr 1982;6:20-4.
- Kasza J, Forbes AB. Information content of cluster-period cells in stepped wedge trials. Biometrics 2019;75:144-52. https://doi.org/10.1111/biom.12959.
Appendix 1 Changes to the eligibility criteria during the course of the study
| Initial wording of eligibility criterion | Amended wording of eligibility criterion | Date word change approved |
|---|---|---|
| Participants in PICU with a body weight of 0.8 kg–7.99 kg who require continuous RRT for acute renal insufficiency or fluid overload as part of their standard clinical care | Participants in PICU with a body weight of 0.8 kg–7.99 kg (note: includes estimated body weight in emergency situation) who require continuous RRT for acute renal insufficiency or fluid overload as part of their standard clinical care | 31 October 2018 |
| Person with legal parental responsibility (PR) for the patient has provided written informed consent for the patient to take part in the study | * Person with legal parental responsibility (PR) for the patient provides written informed consent for the patient to take part in the study * This may be after the patient has started dialysis in an emergency situation so as not to delay treatment |
31 October 2018 |
| Unable to receive written informed consent for data collection from a person with legal PR for the patient | Eligibility criteria deleted | 31 October 2018 |
| Patient with known chronic renal failure already on established adequate RRT’ | Patient with known chronic renal failure already on established adequate RRT (This exclusion should not apply when chronic RRT has failed and patient requires acute RRT during the PICU admission) | 28 August 2020 |
| Patient already established on adequate RRT for whom entry into the study would require additional central venous access, if that access is not clinically indicated | Patient already established on adequate RRT for whom entry into the study would require additional central venous access, if that access is not required in the view of the clinical team | 28 August 2020 |
| Patient has an underlying metabolic diagnosis, including hyperammonaemia | Patient has an underlying (or clinically suspected) diagnosis of a metabolic disease, including hyperammonaemia and no other indication for RRT | 28 August 2020 |
Appendix 2 Further material on protocol deviations and violations, and losses from the study
Protocol deviations and violations
Protocol deviations and violations are presented in Appendix 2, Table 34. In total there were 23 deviations and 2 violations. Seven were related to consent procedures, two were unclassified, four related to study procedures, three related to eligibility criteria, one related to laboratory assessments and six were due to confidentiality breaches.
| Index | Site | Treatment modea | Deviation type | Details |
|---|---|---|---|---|
| 1 | Site 1 | NIDUS | A. Consent Procedure | Participant passed away before consent could be received from parents. The parents have given ‘verbal’ telephone consent. The issue was discussed with the Birmingham team by the Chief Investigator, as the parents had initially been approached about the study. Following correspondence between site teams and PI, it was agreed by the Chief Investigator, Sponsor and CTU to ask REC for permission to use the data already collected. (since this deviation, the site team has managed to receive written consent during the parents’ visit to the hospital) |
| 2 | Site 1 | Control | Unclassified by site | Patient put on study 22 hours after RRT had started. Due to the participant being unstable and on extracorporeal life support (ECMO) with the team at site being unable to initiate study procedures. Site team had contacted the Chief Investigator, who had confirmed it was fine for the patient to start the study from period 1 |
| 3 | Site 1 | A. Consent Procedure | Consent taken by nurse not on the delegation log | |
| 4 | Site 1 | A. Consent Procedure | Patient put on NIDUS for 30 minutes. Taken off. Died later that day (unrelated to the NIDUS). TM confirmed with Sponsor that a deviation needs to be documented centrally | |
| 5 | Site 1 | E. Study Procedures | De-identified, password-protected PICANet data sent to NCTU prior to gaining consent | |
| 6 | Site 2 | Control | B. Eligibility Criteria | Research nurse signed eligibility proforma |
| 7 | Site 2 | Control | B. Eligibility Criteria | Date discrepancy on eligibility proforma |
| 8 | Site 2 | Control | F. Laboratory Assessments/Procedure | No PD samples taken for this participant or the samples have not been processed by labs |
| 9 | Site 2 | Control | H. Other (confidentiality breach) | PICANet data for participants who were not in the study were sent to the Data Manager due to error at site |
| 10 | Site 2 | Control | A. Consent Procedure | Parent placed participants date of birth instead of the date of consent within the consent form |
| 11 | Site 2 | Control | A. Consent Procedure | Parent had not signed box stating ‘I agree for my baby to take part in the I-KID study’ |
| 12 | Site 2 | NIDUS | A. Consent Procedure | Consent collected via email due to participant in social care and covid visiting restrictions meant they could not attend unit |
| Site 3 | NK | No reported deviations from Evelina | ||
| All participants | Site 4 | E. Study Procedures | Deviation found during monitoring visit by TM. Eligibility proformas, consent forms and GP letters not scanned consistently for recruited participants into the electronic records | |
| 13 | Site 4 | Control | A. Consent Procedure | Deviation found during monitoring. Superseded summary PIS used for consent. Version 2.0 was used, despite being superseded by version 3.0 in November 2018 |
| All participants | Site 4 | H. Other (confidentiality breach) | DD logs, SAE log, eligibility and consent proformas, SADE forms sent to non-confidential addresses. There were no patient information in these forms | |
| All participants | Site 4 | H. Other (confidentiality breach) | PICANet data was mistakenly sent without password encryption and from a non-NHS.net email address | |
| All participants | Site 5 | E. Study Procedures | Two IDs were assigned by Site 5 staff in the I-KID main database, with screening information for ineligible participants. When the Freeman Hospital downloaded their PICANet data to send to the NCTU, it included the data for the two ineligible participants (who have not consented to be part of the study). All the data for the ineligible participants from the NCTU systems. The deviation log from site is pending | |
| Site 5 | E. Study Procedures | PID sent to NCTU DM by site staff | ||
| One non-recruited patient only | Site 5 | B. Eligibility Criteria | The previous/superseded version of the PIS (v3.0 28/09/2018) and consent form had been used/given to the parents instead of the current versions | |
| 14 | Site 6 | H. Other | Data entered into Macro before informed consent obtained | |
| 15 | Site 6 | Unclassified by site | Eligibility not confirmed in writing before starting I-KID procedures | |
| 16 | Site 6 | H. Other | PICANet data sent for participants not in study | |
| All participants | Site 6 | H. Other | PICANet data sent for participants not in study – again |
Lost to follow up, withdrawals and death
There were no withdrawals. None of the participants were lost to follow up as their mortality status at the 30-day follow-up was known. Twenty-two participants died by the 30-day follow-up or by the time they were discharged from PICU, whichever came earliest (10 control, 12 NIDUS). Of the 22 participants, 18 died by their 30-day follow-up while 4 were alive and in PICU at their 30-day follow-up visit but subsequently died while in PICU (see Appendix 5, Table 35).
| Index | Device | Description | Days from RRT initiation to death | AE? |
|---|---|---|---|---|
| 1 | PD | Patient alive and in PICU by their 30-day follow-up date and later died in PICU | 110 | N |
| 2 | PD | 23 | N | |
| 3 | Prismaflex | Patient alive and in PICU by their 30-day follow-up date and later died in PICU | 40 | N |
| 4 | Prismaflex | 11 | N | |
| 5 | Prismaflex | 16 | N | |
| 6 | Aquarius | Patient passed away | 4 | Y |
| 7 | Aquarius | Patient passed away | 20 | Y |
| 8 | Aquarius | Death – Group B streptococcal sepsis, persistent pulmonary hypertension of the newborn, catastrophic neurological injury | 4 | Y |
| 9 | Aquarius | Death – Palliation | 19 | Y |
| 10 | NIDUS | Patient passed away | 6 | Y |
| 11 | NIDUS | Patient passed away | 2 | Y |
| 12 | NIDUS | Death | 19 | Y |
| 13 | NIDUS | Patient alive and in PICU by their 30-day follow-up date and later died in PICU. Follow-up was done early as patient was unwell and there were concerns he would pass away before his 1 month follow up which the protocol states is allowed | 31 | N |
| 14 | NIDUS | Death | 3 | Y |
| 15 | NIDUS | Death | 11 | Y |
| 16 | NIDUS | Patient death unrelated to NIDUS | 22 | Y |
| 17 | NIDUS | Patient alive and in PICU by their 30-day follow-up date and later died in PICU | 81 | N |
| 18 | NIDUS | 1 | N | |
| 19 | NIDUS | 2 | N | |
| 20 | NIDUS | Death during follow-up period, following redirection of care | 18 | Y |
| 21 | NIDUS | 8 | N | |
| 22 | ECMO + HD | 26 | N |
Treatment compliance
To investigate the compliance with the data collection procedure during the collection phases [phase 1 (one collection) and phase 2 (three collections)], we computed availability of the precision variable and biochemical clearance measurements and the time from RRT initiation to a patient’s last observed start of a collection. The availability of the precision variable and biochemical clearance measurements was generally higher in the control arm compared with the intervention arm (see Appendix 2, Table 36). A total 93 participants had a last observable start date and time to a collection phase (see Appendix 2, Table 36). Compliance with the 48-hour data collection window post-RRT initiation was met for most participants. For a minority of participants (8/93; 8.60%), their last observed collection start dates, and times were beyond 48 hours from the start of their RRT (6 control, 2 intervention). For one control participant, this was because their phase 1 collection began after 48 hours post-RRT initiation (see Table 36). A diagram of the distribution of duration from the start of RRT to their last known start of a collection is presented in Appendix 2, Figure 11.
| Control (n = 62) | Intervention (n = 35) | Total (n = 97) | |
|---|---|---|---|
| Treatment allocated, n (%) | |||
| PD | 48 (77.42) | 0 | 48 (49.48) |
| Prismaflex | 5 (8.06) | 0 | 5 (5.15) |
| Aquarius | 8 (12.90) | 0 | 8 (8.25) |
| Manual HD and ECMO | 1 (1.61) | 0 | 35 (36.08) |
| NIDUS | 0 | 35 (100) | 35 (36.08) |
| Phase 1: Collected treatment data 0–6 hours a | |||
| Collection duration, hours | |||
| Mean (SD) | 5.96 (0.49) | 4.77 (2.08) | 5.62 (1.29) |
| Median (IQR) | 6 (6–6) | 5.7 (3.25–6) | 6 (5.58–6) |
| Range | 4.58–7.2 | 0.15–9 | 0.15–9 |
| Available, n | 62 | 25 | 87 |
| Computable precision X-A, n (%) | 60 (96.77) | 15 (42.86) | 75 (77.32) |
| Computable biochemical clearance, n (%) | |||
| Creatinine | 57 (91.94) | 27 (77.14) | 84 (86.60) |
| Urea | 56 (90.32) | 30 (85.71) | 86 (88.66) |
| Phosphate | 51 (82.26) | 27 (77.14) | 78 (80.41) |
| Phase 2: Collected treatment data 6–48 hours b | |||
| Computable precision X-A, n (%) | |||
| None computable | 6 (9.68) | 16 (45.71) | 22 (22.68) |
| 1 computable | 15 (24.19) | 7 (20.00) | 22 (22.68) |
| 2 computable | 14 (22.58) | 7 (20.00) | 21 (21.65) |
| 3 computable | 27 (43.55) | 5 (14.29) | 32 (32.99) |
| Computable biochemical clearance | |||
| Creatinine, n (%) | |||
| None computable | 14 (22.58) | 15 (42.86) | 29 (29.90) |
| 1 computable | 11 (17.74) | 6 (17.14) | 17 (17.53) |
| 2 computable | 15 (24.19) | 5 (14.29) | 20 (20.62) |
| 3 computable | 22 (35.48) | 9 (25.71) | 31 (31.96) |
| Urea, n (%) | |||
| None computable | 15 (24.19) | 15 (42.86) | 30 (30.93) |
| 1 computable | 12 (19.35) | 7 (20.00) | 19 (19.59) |
| 2 computable | 14 (22.58) | 5 (14.29) | 19 (19.59) |
| 3 computable | 21 (33.87) | 8 (22.86) | 29 (29.90) |
| Phosphate, n (%) | |||
| None computable | 13 (20.97) | 15 (42.86) | 28 (28.87) |
| 1 computable | 12 (19.35) | 8 (22.86) | 20 (20.62) |
| 2 computable | 16 (25.81) | 3 (8.57) | 19 (19.59) |
| 3 computable | 21 (33.87) | 9 (25.71) | 30 (30.93) |
| All phases: Collected treatment data 0–48 hours a , b | |||
| Computable precision X-A, n (%) | |||
| None computable | 0 | 14 (40.00) | 14 (14.43) |
| 1 computable | 6 (9.68) | 5 (14.29) | 11 (11.34) |
| 2 computable | 16 (25.81) | 6 (17.14) | 22 (22.68) |
| 3 computable | 14 (22.58) | 6 (17.14) | 20 (20.62) |
| 4 computable | 26 (41.94) | 4 (11.43) | 30 (30.93) |
| Computable biochemical clearance | |||
| Creatinine, n (%) | |||
| None computable | 1 (1.61) | 7 (20.00) | 8 (8.25) |
| 1 computable | 14 (22.58) | 8 (22.86) | 22 (22.68) |
| 2 computable | 11 (17.74) | 6 (17.14) | 17 (17.53) |
| 3 computable | 16 (25.81) | 6 (17.14) | 22 (22.68) |
| 4 computable | 20 (32.26) | 8 (22.86) | 28 (28.87) |
| Urea, n (%) | |||
| None computable | 2 (3.23) | 5 (14.29) | 7 (7.22) |
| 1 computable | 15 (24.19) | 10 (28.57) | 25 (25.77) |
| 2 computable | 10 (16.13) | 7 (20.00) | 17 (17.53) |
| 3 computable | 16 (25.81) | 5 (14.29) | 21 (21.65) |
| 4 computable | 19 (30.65) | 8 (22.86) | 27 (27.84) |
| Phosphate, n (%) | |||
| None computable | 4 (6.45) | 6 (17.14) | 10 (10.31) |
| 1 computable | 11 (17.74) | 9 (25.71) | 20 (20.62) |
| 2 computable | 11 (17.74) | 9 (25.71) | 20 (20.62) |
| 3 computable | 19 (30.65) | 3 (8.57) | 22 (22.68) |
| 4 computable | 17 (27.42) | 8 (22.86) | 25 (25.77) |
| Total duration from start of RRT to last observed start of collection, hoursc | |||
| Mean (SD) | 27.77 (17.62) | 20.94 (21.06) | 25.50 (19.00) |
| Median (IQR) | 28.17 (13–38.15) | 19.67 (0.83–31.08) | 24.40 (11.10–37.50) |
| Range | 0–82 | 0–93 | 0–93 |
| Available, n | 62 | 31 | 93 |
FIGURE 11.
Histogram of duration from start of RRT to last start of collection (hours) stratified by arm. Red reference line at 48 hours. Sample sizes based on n = 93 participants with available information on the duration from start of RRT to their last observed start of collection.
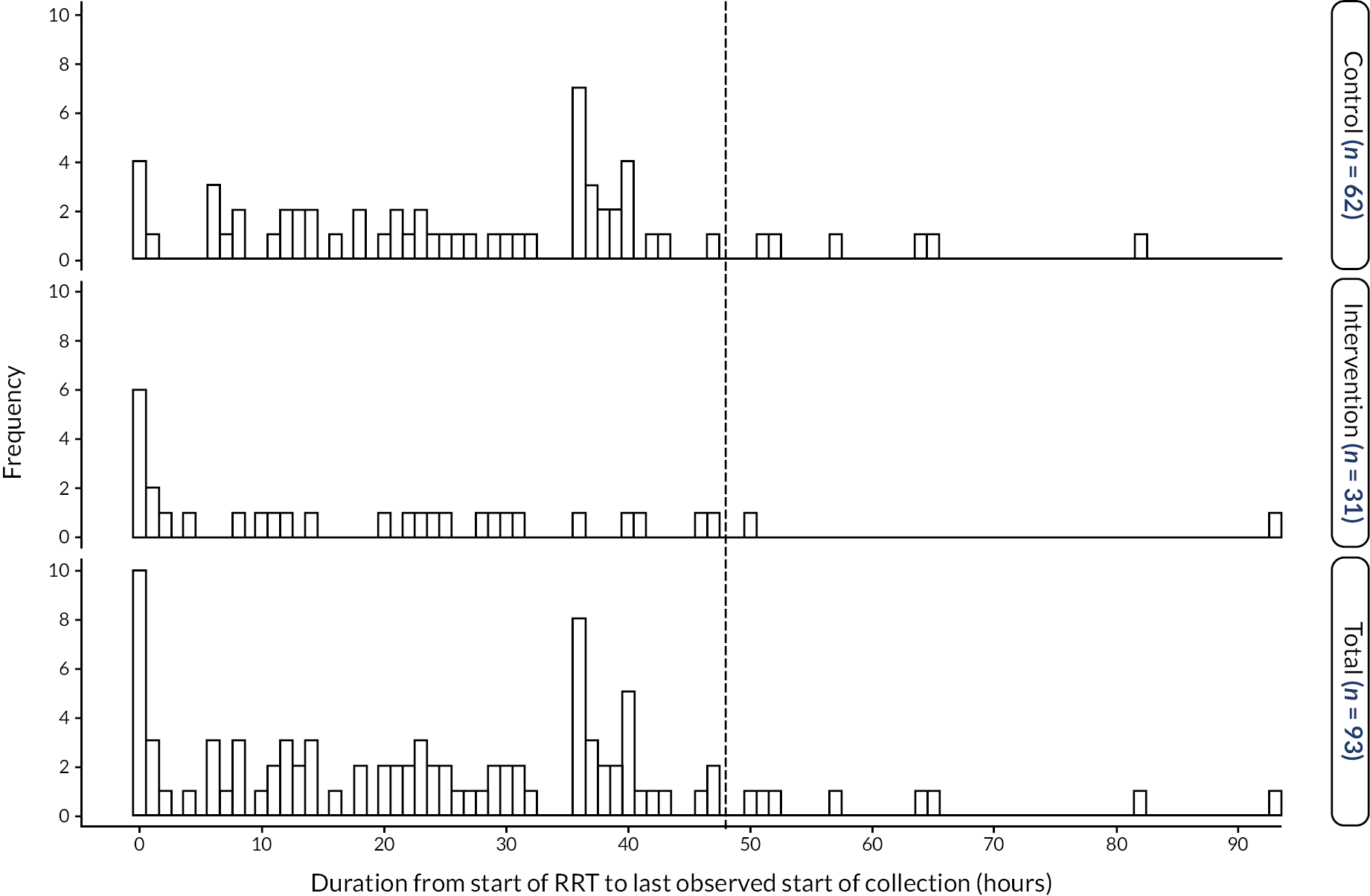
Missing primary outcome from those allocated to NIDUS group: further details
The details given by sites for the 14 participants allocated to NIDUS who did not provide a primary outcome.
| Index | Per | Seq | Number of UF collections | Missing variables | Description from summary of dialysis CRF | Notes |
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 |
|
Filter clotted and we were unable to establish in the beginning due to air alarms during the initial attempt. Problems with the circuit and ACTs as documented in the DD log. | First baby at transition to NIDUS |
| 2 | 3 | 1 | 0 | No data collected | 06:00 – NIDUS initiated, heparin bolus given, 07:30 – ACT reported as 197, issues with air bubbles ++, discontinued. 09:15 – circuit replaced and washed at least six times, withdrawn syringe from pack noted to have tiny air bubbles and leaking wash fluid (not connected to patient), reported as DD, syringe replaced with LOT no. 16H01C8, 09:43 – started dialysis, very negative withdraw pressure -ve 300 to -ve 400 due to line, improved when tension applied to line, set 5 ml/hour UF rate – reduced to 0 to see if improves, 10:20 – clearance test started, high return pressure and very low withdraw pressure, attempted to aspirate line – unsuccessful, unable to flush, aspirated at three-way tap further up line and aspirated clots ++, line flushed and dialysis restarted (×2), 10:34 – stroke vol reduced to 10 ml/hour, 10:41 – clearance test stopped, discontinued – wash cycle, air +++ coming through the filter to the left syringe – found to be clotted filter. Unable to start UF test. | |
| 3 | 4 | 1 | 0 | No data collected | Difficult process with multiple isolated filter changes and access issues. Decision after 24 hours to use the Aquarius machine to filter patient. | |
| 4 | 4 | 1 | 0 | No data collected | Patient on extracorporeal life support, anuric required dialysis. Unfortunately, multiple filters clotted despite high ACTs and no issues with access on the circuit. Two periods of a few hours on NIDUS with multiple clotting filters, decision for Aquarius made. | |
| 5 | 4 | 3 | 0 | No data collected | Lots of bubbles at the start of the run-filter clotted. Filter changed and washed through, dialysis rate at 0 ml/hour? Caused the next filter to clot. Overall, the patient on for 2.5 hours with no filtration or fluid removal achieved. | |
| 6 | 3 | 2 | 1 |
|
SADE for completed due to filter blood leak into effluent line. Malfunction detected about 2–3 minutes after treatment start and Newcastle on-call team contacted. Machine stopped. Malfunction explained to parents who were accepting and willing to try with different batch of filters but none available on site. Treatment stopped and child started PU with furosemide infusion, so no other RRT started. No sampling done post start. | First baby at transition to NIDUS; UF phase collection period <1 hour |
| 7 | 3 | 2 | 1 |
|
9 a.m. commenced setting up the NIDUS. Wash circuit 5–6 times but still air in. 12:15 called Site 5 liaison, it was realised we had thread the tubing through the pump on the left side back to front, so washed circuit again minimum three times. 13:00 first attempt to go on right femoral 18 g lumen, high pressures aspirating from line –400, participants position adjusted, started working, then high pressures again on returning blood, pressures of +350, tried multiple times to fix problem, when it was decided to stop cycle. Tried aspirating the line, really difficult to aspirate and clot found at tip of lumen. During procedure patient was stable and no adverse effects of going on to the NIDUS. I called Site 5 liaison and she said the patient needs new line, femoral lines difficult and if not aspirating and flushing well NIDUS is not going to work. I did a few wash cycles on circuit to save circuit and worked fine, did up to five wash cycles. Medical team inserted a new line. 15:15 attempted to connect to new line on LIJ 16 g, aspirating and flushing beautifully. Connected, pressures all low, about 10 cycles completed through filter then alarms began commencing high pressures. It was when the right syringe was pushing through the filter to the left syringe. I called the Site 5 liaison at 15:30 and she said there must have been a clot in the filter and it’s not salvageable. 16:00 new circuit was washed multiple times. Awaiting medical decision on whether to attempt again. Handed over primed circuit to night team. | |
| 8 | 3 | 2 | 1 |
|
As per bedside nurses: At 15:00 I arrived at the bed space and the machine being set up by Research team and SSN. Then we did many wash cycles, however not sure how many. Once the Site 5 team arrived, the discussion about anticoagulation happened and it took a long time before a decision was made. They finally agreed on following the CICU anticoagulation guidelines for NIDUS. I assessed the line at this point (nobody did it before me): the white lumen of the Cook 3 lumen CVL was flushing very well, a bit positional for withdrawing blood. All team aware about it. Decision to start NIDUS. We connected the machine to the patient and decided to do few cycles as neutral, just to see how the patient would have tolerated it. We soon saw high pressure while withdrawing and pushing back blood. I, with the help of the Newcastle technician, troubleshoot for about an hour on the CVL to try to make it work. After 30 minutes, as per I-KID study protocol we managed to send bloods and fluid to the lab. However, I was not able to take blood form the machine in order to run an ACT as it felt obstructed. At this point we had many alarms of high pressures, not being able to complete many cycles. After more troubleshooting, it was discussed with CICU consultant and decision to disconnect the patient and restart medical management of anuria with medications IV. We then found small clots in both access site on the NIDUS machine (photo of it take by Site 5 technician) and in the CVL connector. CICU Consultant decided to insert new line in the R femoral vein, planning to restart the NIDUS as soon as possible. New machine set up by Newcastle team’. 21:10. Patient commenced on NIDUS UF0. Tolerated well. 23:00. Patient ACT 140, heparin 2 ml/hour, no change. Patient UF increased to 5 ml/hour but not achieving. UF set back to 0 ml/hour (continued in comments). | |
| 9 | 3 | 2 | 1 |
|
Connected to the NIDUS on 13/11/2019 @ approx 7 a.m. due to renal failure and low U/O. Filter clotted approximately 20 minutes post start. New machine primed and support from on-call nurse obtained. Was not reconnected to new filter since started passing urine. | |
| 10 | 3 | 2 | 1 |
|
Decision made to start RRT via NIDUS at approximately 16:30. Machine set up and primed by research nurse and clinical B7, while additional access was placed (required for drugs as well). Access secured at approx. 18:00. Brief information about therapy and indication provided by fellow. Brief explanation about role and connection process provided by research nurse/clinical B6. Patient attached to NIDUS at 18:20. ACT at connection un-recordable (>350), heparin weaned twice in approximately 30-minute intervals to minimum 25 units/hour due to repeated un-recordable ACTs. Very deranged patient clotting, Cryoprecipitate and platelets administered. Filter clotted shortly after platelets administration - approximately 20:20. Futility of treatment agreed between medical team and family. RRT was not restarted. | |
| 11 | 2 | 1 | 1 |
|
Patient went onto the NIDUS. The machine was running for a couple of hours and then it was noticed that there was blood leaking into the waste bag. Appears to be a problem with the filter. Contacted Site 5 it was decided that we stop and await new filters from Allmed. Patient needed to go onto the Prismaflex. | |
| 12 | 2 | 1 | 1 |
|
Patient was put onto the NIDUS. We initially had problems with access, which was resolved. However, we kept the NIDUS washing. However, it still had blood in the circuit, and we noticed the filter had clotted with blood. We contacted the on-call team who suggested that changing the filter only should be enough to get the machine up and running again. The NIDUS was still alarming clot. We took the patient off as the patient had started to pass urine and did not require RRT. We spoke to the Site 5 team later on and we should have changed the whole set not just the filter. | First baby at transition to NIDUS |
| 13 | 2 | 1 | 1 |
|
Patient went onto the NIDUS for removal of fluid. Patient went on via vascath size 6.0. NIDUS working well, however after a few hours this baby needed ECMO, so the NIDUS was discontinued and Prismaflex put up via ECMO circuit. The baby was on ECMO for several days then was going to go back onto the NIDUS on the 28/8/19 eligibility was signed. We attached to size 6.0 however the machine did not work as the NIDUS was set up incorrectly. We did not take any fluid samples from the patient. Routine bloods were taken. | |
| 14 | 3 | 1 | 1 |
|
Baby started on the NIDUS. Good access obtained with a green cannula in the left femoral. First machine was set up as per setup app. Machine working well alarm pressures were minimal. However, after 45 minutes filter had clotted. Heparin was running at 20/u/kg/hour; no heparin bolus was given when patient first went on. So, machine taken down and new machine set up and connected. On new machine that was set up heparin bolus of 20/u/kg was given to patient and heparin increased to 25/u/kg/hour however after 30–45 minutes filter clotted again. An ACT was taken by nurse however was not recorded onto metavision. After the second filter clotted it was decided not to put another machine up and to insert a PD catheter instead for RRT. First NIDUS machine no – 16, second NIDUS machine no – 12 |
Appendix 3 Baseline values
Baseline values by machine type
| PD (n = 48) | CVVH (n = 13) | Manual HD (n = 1) | NIDUS (n = 35) | Total (n = 97) | |
|---|---|---|---|---|---|
| Age at screening, days | |||||
| Mean (SD) | 41.98 (85.91) | 92.62 (142.90) | 11.00 (.) | 91.66 (136.85) | 66.37 (115.99) |
| Median (IQR) | 9.00 (6.50–17.50) | 17.00 (9.00–81.00) | 11.00 (7.00–124.00) | 11.00 (7.00–61.00) | |
| Range | 1.00, 477.00 | 1.00, 466.00 | 1.00, 443.00 | 1.00, 477.00 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Sex, n (%) | |||||
| Female | 20 (41.67) | 6 (46.15) | 1 (100.00) | 8 (22.86) | 35 (36.08) |
| Male | 28 (58.33) | 7 (53.85) | 0 | 27 (77.14) | 62 (63.92) |
| Weight at RRT initiation, kg* | |||||
| Mean (SD) | 3.65 (1.29) | 4.25 (2.43) | 2.70 (.) | 4.33 (1.72) | 3.97 (1.65) |
| Median (IQR) | 3.25 (2.90–3.90) | 3.00 (2.75–4.00) | 3.70 (3.10–5.60) | 3.50 (3.00–4.60) | |
| Range | 1.80–7.40 | 2.60–10.10 | 1.00–7.80 | 1.00–10.10 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Type of weight measurement, n (%) | |||||
| Actual weight | 40 (80.33) | 10 (76.92) | 1 (100.00) | 34 (97.14) | 85 (87.63) |
| Estimated weight | 8 (16.67) | 3 (23.08) | 0 | 1 (2.86) | 12 (12.37) |
| Gestational age at delivery (completed weeks) | |||||
| Mean (SD) | 38.10 (1.93) | 37.25 (3.33) | 38.00 (.) | 36.49 (3.74) | 37.41 (2.95) |
| Median (IQR) | 38.00 (38.00–39.00) | 37.50 (36.50–39.50) | 38.00 (35.00–39.00) | 38.00 (37.00–39.00) | |
| Range | 31.00–41.00 | 28.00–41.00 | 26.00–41.00 | 26.00–41.00 | |
| Available, n | 48 | 12 | 1 | 35 | 96 |
| Type of admission to unit, n (%) | |||||
| Planned – Following surgery | 30 (62.50) | 1 (7.69) | 0 | 13 (37.14) | 44 (45.36) |
| Unplanned – Following surgery | 1 (2.08) | 0 | 0 | 1 (2.86) | 2 (2.06) |
| Planned – Other | 1 (2.08) | 1 (7.69) | 0 | 4 (11.43) | 6 (6.19) |
| Unplanned | 16 (33.33) | 11 (84.62) | 1 (100.00) | 17 (48.57) | 45 (46.39) |
| Previous ICU admission, n (%) | |||||
| ICU | 0 | 0 | 0 | 1 (2.86) | 1 (1.03) |
| PICU | 3 (6.25) | 1 (7.69) | 0 | 4 (11.43) | 8 (8.25) |
| NICU | 22 (45.83) | 7 (53.85) | 0 | 12 (34.29) | 41 (42.27) |
| None | 21 (43.75) | 4 (30.77) | 1 (100.00) | 18 (51.43) | 44 (45.36) |
| Unknown | 2 (4.17) | 1 (7.69) | 0 | 0 | 3 (3.09) |
| Source of admission, n (%) | |||||
| Same hospital | 33 (68.75) | 6 (46.15) | 1 (100.00) | 23 (65.71) | 63 (64.95) |
| Other hospital | 15 (31.25) | 7 (53.85) | 0 | 12 (34.29) | 34 (35.05) |
| Elective admission, n (%) | |||||
| No | 17 (35.42) | 11 (84.62) | 1 (100.00) | 18 (51.43) | 47 (48.45) |
| Yes | 31 (64.58) | 2 (15.38) | 0 | 17 (48.57) | 50 (51.55) |
| Main reason for PICU admission, n (%) | |||||
| Other | 18 (37.50) | 11 (84.62) | 1 (100.00) | 20 (57.14) | 50 (51.55) |
| Bronchiolitis | 0 | 1 (7.69) | 0 | 2 (5.71) | 3 (3.09) |
| Recovery from surgery | 30 (62.50) | 1 (7.69) | 0 | 12 (34.29) | 43 (44.33) |
| Seizure disorder | 0 | 0 | 0 | 1 (2.86) | 1 (1.03) |
| If admission was recovery from surgery, what was procedure, n (%) | |||||
| Bypass cardiac procedure | 30 (100.00) | 0 | 0 | 10 (83.33) | 40 (93.02) |
| Non–bypass cardiac procedure | 0 | 1 (100.00) | 0 | 1 (8.33) | 1 (2.33) |
| Other procedure | 0 | 1 (8.33) | 2 (4.65) | ||
| Is evidence available to assess past medical history, n (%) | |||||
| Yes | 42 (87.50) | 12 (92.31) | 1 (100.00) | 35 (100.00) | 90 (92.78) |
| No | 6 (12.50) | 1 (7.69) | 0 | 0 | 7 (7.22) |
| Systolic blood pressure, mmHg | |||||
| Mean (SD) | 68.44 (15.19) | 83.08 (27.16) | 100.00 (.) | 74.86 (22.33) | 73.19 (20.49) |
| Median (IQR) | 66.00 (57.00–74.00) | 77.00 (65.0092.00) | 68.00 (60.00–86.00) | 68.00 (60.00–82.00) | |
| Range | 40.00–118.00 | 44.00–137.00 | 36.00–134.00 | 36.00–137.00 | |
| Available, n | 45 | 13 | 1 | 35 | 94 |
| Base excess source, n (%) | |||||
| Arterial | 36 (75.00) | 7 (53.85) | 1 (100.00) | 23 (65.71) | 67 (69.07) |
| Capillary | 4 (8.33) | 2 (15.38) | 0 | 6 (17.14) | 12 (12.37) |
| Venous | 3 (6.25) | 1 (7.69) | 0 | 3 (8.57) | 7 (7.22) |
| Available, n | 43 | 10 | 1 | 32 | 86 |
| Lactate source, n (%) | |||||
| Arterial | 37 (77.08) | 6 (46.15) | 1 (100.00) | 23 (65.71) | 67 (69.07) |
| Capillary | 4 (8.33) | 3 (23.08) | 0 | 7 (20.00) | 14 (14.43) |
| Venous | 3 (6.25) | 1 (7.69) | 0 | 3 (8.57) | 7 (7.22) |
| Available, n | 44 | 10 | 1 | 33 | 88 |
| Mechanical ventilation, n (%) | |||||
| Yes | 41 (85.42) | 9 (69.23) | 0 | 30 (85.71) | 80 (82.47) |
| No | 7 (14.58) | 4 (30.77) | 1 (100.00) | 5 (14.29) | 17 (17.53) |
| Received CPAP within first hour, n (%) | |||||
| Yes | 4 (8.33) | 1 (7.69) | 0 | 2 (5.71) | 7 (7.22) |
| No | 44 (91.67) | 12 (92.31) | 1 (100.00) | 33 (94.29) | 90 (92.78) |
| Pupil reaction, n (%) | |||||
| Both fixed and dilate | 0 | 0 | 0 | 1 (2.86) | 1 (1.03) |
| Other reaction | 42 (87.50) | 13 (100.00) | 1 (100.00) | 28 (80.00) | 84 (86.60) |
| Unknown | 6 (12.50) | 0 | 0 | 6 (17.14) | 12 (12.37) |
| PIM3 score | |||||
| Mean (SD) | 0.06 (0.09) | 0.12 (0.13) | 0.02 (.) | 0.095 (0.172) | 0.079 (0.130) |
| Median (IQR) | 0.02 (0.01–0.05) | 0.06 (0.01–0.20) | 0.027 (0.014–0.131) | 0.025 (0.014–0.093) | |
| Range | 0.00–0.45 | 0.01–0.38 | 0.006–0.972 | 0.005–0.972 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Logit of PIM3 score | |||||
| Mean (SD) | −3.52 (1.17) | −2.68 (1.56) | −4.14 (.) | −3.05 (1.67) | −3.24 (1.44) |
| Median (IQR) | −4.01 (−4.35 to −2.97) | −2.74 (−4.18 to −1.40) | −3.58 (−4.24 to −1.90) | −3.68 (−4.28 to −2.28) | |
| Range | −5.29 to −0.22 | −5.14 to −0.51 | −5.12 to 3.54 | −5.29 to 3.54 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| PD (n = 48) | CVVH (n = 13) | Manual HD (n = 1) | NIDUS (n = 35) | Total (n = 97) | |
|---|---|---|---|---|---|
| Sodium, mmol/l | |||||
| Mean (SD) | 145.83 (5.46) | 143.08 (8.41) | 156.00 (.) | 141.57 (6.52) | 144.03 (6.63) |
| Median (IQR) | 146.00 (142.00–149.00) | 146.00 (139.00–149.00) | 143.00 (136.00–146.00) | 144.00 (140.00–148.00) | |
| Range | 130.00–157.00 | 130.00–154.00 | 128.00–156.00 | 128.00–157.00 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Potassium, mmol/l | |||||
| Mean (SD) | 4.74 (0.80) | 4.51 (0.83) | 3.70 (.) | 4.88 (1.00) | 4.75 (0.89) |
| Median (IQR) | 4.60 (4.10–5.40) | 4.30 (3.80–5.00) | 4.80 (4.20–5.50) | 4.60 (4.10–5.20) | |
| Range | 3.50–6.70 | 3.60–6.00 | 3.30–8.30 | 3.30–8.30 | |
| Available, n | 47 | 12 | 1 | 35 | 95 |
| Creatinine, mmol/l | |||||
| Mean (SD) | 52.27 (21.01) | 171.54 (187.37) | 50.00 (.) | 106.00 (113.38) | 87.62 (104.28) |
| Median (IQR) | 51.00 (39.50–62.00) | 94.00 (42.00–218.00) | 74.00 (56.00–110.00) | 60.00 (42.00–87.00) | |
| Range | 12.00–105.00 | 17.00–623.00 | 9.00–678.00 | 9.00–678.00 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Urea, mmol/l | |||||
| Mean (SD) | 6.97 (6.14) | 13.75 (11.54) | 3.20 (.) | 12.54 (9.81) | 9.85 (8.86) |
| Median (IQR) | 5.70 (3.40–8.45) | 14.40 (5.40–18.60) | 9.50 (4.70–17.20) | 7.20 (3.70–11.70) | |
| Range | 1.70–41.00 | 1.70–45.40 | 2.20–36.80 | 1.70–45.40 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Phosphate, mmol/l | |||||
| Mean (SD) | 2.29 (0.64) | 1.95 (0.63) | 2.00 (.) | 2.43 (0.68) | 2.29 (0.66) |
| Median (IQR) | 2.30 (1.78–2.73) | 1.80 (1.60–2.27) | 2.43 (2.13–2.93) | 2.36 (1.79–2.75) | |
| Range | 0.61–3.58 | 0.80–3.00 | 0.65–3.53 | 0.61–3.58 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Actual bicarbonate, mmol/l | |||||
| Mean (SD) | 21.88 (4.04) | 21.37 (5.53) | 19.80 (.) | 20.53 (4.79) | 21.31 (4.50) |
| Median (IQR) | 22.15 (18.25–24.90) | 20.40 (18.60–25.60) | 20.20 (17.50–24.00) | 21.40 (18.05–24.15) | |
| Range | 13.20–30.20 | 12.70–33.00 | 6.50–31.60 | 6.50–33.00 | |
| Available, n | 48 | 13 | 1 | 34 | 96 |
| Base excess, mmol/l | |||||
| Mean (SD) | −3.17 (5.71) | −4.20 (7.31) | −6.20 (.) | −4.95 (6.73) | −3.97 (6.27) |
| Median (IQR) | −3.50 (−8.10 to 0.90) | −5.30 (−7.40 to 0.80) | −4.55 (−8.00 to −1.45) | −4.25 (−7.75 to 0.20) | |
| Range | −15.00 to 7.20 | −18.00 to 10.30 | −26.70 to 9.10 | −26.70 to 10.30 | |
| Available, n | 46 | 13 | 1 | 32 | 92 |
| pH | |||||
| Mean (SD) | 7.33 (0.10) | 7.31 (0.16) | 7.23 (.) | 7.28 (0.13) | 7.31 (0.12) |
| Median (IQR) | 7.33 (7.24–7.43) | 7.34 (7.24–7.41) | 7.29 (7.21–7.36) | 7.32 (7.24–7.40) | |
| Range | 7.06–7.51 | 7.02–7.52 | 6.86–7.54 | 6.86–7.54 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Haemoglobin, g/l | |||||
| Mean (SD) | 133.94 (24.27) | 95.15 (25.83) | 139.00 (.) | 114.46 (26.86) | 121.76 (28.63) |
| Median (IQR) | 133.00 (116.50–153.00) | 93.00 (82.00–111.00) | 120.00 (90.00–129.00) | 123.00 (101.00–140.00) | |
| Range | 78.00–194.00 | 56.00–140.00 | 65.00–184.00 | 56.00–194.00 | |
| Available, n | 48 | 13 | 1 | 35 | 97 |
| Platelets, ×109/l | |||||
| Mean (SD) | 216.79 (97.92) | 146.38 (149.38) | 164.00 (.) | 146.53 (82.21) | 181.82 (105.59) |
| Median (IQR) | 221.50 (132.50–296.00) | 82.00 (61.00–203.00) | 124.00 (95.00–209.00) | 163.50 (101.50–241.50) | |
| Range | 50.00–422.00 | 34.00–582.00 | 20.00–337.00 | 20.00–582.00 | |
| Available, n | 48 | 13 | 1 | 34 | 96 |
| Primary indication for starting RRT, n (%) | |||||
| Fluid volume control | 25 (52.08) | 6 (46.15) | 1 (100.00) | 17 (48.57) | 49 (50.52) |
| Biochemical control | 6 (12.50) | 2 (15.38) | 0 | 9 (25.71) | 17 (17.53) |
| Fluid and biochemical equally | 17 (35.42) | 5 (38.46) | 0 | 9 (25.71) | 31 (31.96) |
Baseline summaries of participants allocated to NIDUS, intended for inclusion in primary analysis
The primary outcome was obtained for only 21 of the 35 participants allocated to NIDUS. One of the 21 participants with an outcome was a transition baby, so was not included in the primary analysis, and three of the participants without a primary outcome were also transition babies and would not have been included in the primary analysis. The baseline summaries and pre-RRT summaries of the babies allocated to NIDUS that were included in the primary analysis (n = 20) and that would have been included had values of X-A been obtained (n = 11) are shown in Tables 40 and 41.
| Missing primary outcome (n = 11) | With primary outcome (n = 20) | |
|---|---|---|
| Age at screening, days | ||
| Mean (SD) | 132.73 (161.92) | 85.85 (131.22) |
| Median (IQR) | 77.00 (7.00–273.00) | 12.50 (6.50–129.50) |
| Range | 1.00–443.00 | 1.00–367.00 |
| Available, n | 11 | 20 |
| Sex, n (%) | ||
| Female | 2 (18.18) | 6 (30.00) |
| Male | 9 (81.82) | 14 (70.00) |
| Weight at RRT initiation, kg* | ||
| Mean (SD) | 5.01 (1.65) | 4.07 (1.85) |
| Median (IQR) | 5.00 (3.50–6.29) | 3.60 (3.00–5.35) |
| Range | 3.00–7.80 | 1.00–7.40 |
| Available, n | 11 | 20 |
| Type of weight measurement, n (%) | ||
| Actual weight | 11 (100.00) | 20 (100.00) |
| Estimated weight | 0 | 0 |
| Gestational age at delivery (completed weeks) | ||
| Mean (SD) | 36.73 (3.58) | 35.75 (3.95) |
| Median (IQR) | 38.00 (36.00–38.00) | 36.50 (32.50–39.00) |
| Range | 27.00–41.00 | 26.00–40.00 |
| Available, n | 11 | 20 |
| Type of admission to unit, n (%) | ||
| Planned – following surgery | 6 (54.55) | 5 (25.00) |
| Unplanned – following surgery | 0 | 1 (5.00) |
| Planned – other | 0 | 3 (15.00) |
| Unplanned | 5 (45.45) | 11 (55.00) |
| Type of admission to unit, n (%) | ||
| ICU | 0 | 1 (5.00) |
| PICU | 2 (18.18) | 1 (5.00) |
| NICU | 2 (18.18) | 8 (40.00) |
| None | 7 (63.64) | 10 (50.00) |
| Unknown | 0 | 0 |
| Source of admission, n (%) | ||
| Same hospital | 7 (63.64) | 12 (60.00) |
| Other hospital | 4 (36.36) | 8 (40.00) |
| Elective admission, n (%) | ||
| No | 5 (45.45) | 12 (60.00) |
| Yes | 6 (54.55) | 8 (40.00) |
| Main reason for PICU admission, n (%) | ||
| Other | 5 (45.45) | 13 (65.00) |
| Bronchiolitis | 0 | 2 (10.00) |
| Recovery from surgery | 5 (45.45) | 5 (25.00) |
| Seizure disorder | 1 (9.09) | 0 |
| If admission was recovery from surgery, what was procedure?, n (%) | ||
| Bypass cardiac procedure | 4 (80.00) | 4 (80.00) |
| Non-bypass cardiac procedure | 1 (20.00) | 0 |
| Other procedure | 0 | 1 (20.00) |
| Is evidence available to assess past medical history?, n (%) | ||
| Yes | 11 (100.00) | 20 (100.00) |
| No | 0 | 0 |
| Systolic blood pressure, mmHg | ||
| Mean (SD) | 81.82 (21.61) | 73.15 (22.91) |
| Median (IQR) | 73.00 (66.00–88.00) | 64.50 (59.50–80.50) |
| Range | 60.00–134.00 | 36.00–129.00 |
| Available, n | 11 | 20 |
| Base excess source, n (%) | ||
| Arterial | 10 (90.91) | 10 (50.00) |
| Capillary | 1 (9.09) | 5 (25.00) |
| Venous | 0 | 3 (15.00) |
| Available, n | 11 | 18 |
| Lactate source, n (%) | ||
| Arterial | 10 (90.91) | 10 (50.00) |
| Capillary | 1 (9.09) | 6 (30.00) |
| Venous | 0 | 3 (15.00) |
| Available, n | 11 | 19 |
| Mechanical ventilation, n (%) | ||
| Yes | 10 (90.91) | 18 (90.00) |
| No | 1 (9.09) | 2 (10.00) |
| Received CPAP within first hour, n (%) | ||
| Yes | 1 (9.09) | 1 (5.00) |
| No | 10 (90.91) | 19 (95.00) |
| Pupil reaction, n (%) | ||
| Both fixed and dilate | 1 (9.09) | 0 |
| Other reaction | 9 (81.82) | 18 (90.00) |
| Unknown | 1 (9.09) | 2 (10.00) |
| PIM3 score | ||
| Mean (SD) | 0.15 (0.28) | 0.07 (0.08) |
| Median (IQR) | 0.02 (0.01–0.13) | 0.03 (0.02–0.08) |
| Range | 0.01–0.97 | 0.01–0.27 |
| Available, n | 11 | 20 |
| Logit of PIM3 score | ||
| Mean (SD) | −2.68 (2.40) | −3.21 (1.19) |
| Median (IQR) | −3.81 (−4.26 to −1.88) | −3.41 (−4.08 to −2.43) |
| Range | −4.69 to 3.54 | −5.12 to −0.98 |
| Available, n | 11 | 20 |
| Missing primary outcome (n = 11) | With primary outcome (n = 20) | |
|---|---|---|
| Sodium, mmol/l | ||
| Mean (SD) | 143.64 (4.18) | 140.30 (7.70) |
| Median (IQR) | 144.00 (142.00–148.00) | 138.50 (135.00–146.00) |
| Range | 134.00–148.00 | 128.00–156.00 |
| Available, n | 11 | 20 |
| Potassium, mmol/l | ||
| Mean (SD) | 4.84 (0.86) | 4.94 (1.16) |
| Median (IQR) | 4.60 (4.30–4.90) | 4.90 (4.00–5.65) |
| Range | 4.10–7.00 | 3.30–8.30 |
| Available, n | 11 | 20 |
| Creatinine, mmol/l | ||
| Mean (SD) | 69.27 (21.39) | 137.75 (142.14) |
| Median (IQR) | 65.00 (52.00–89.00) | 87.50 (72.00–163.50) |
| Range | 44.00–110.00 | 9.00–678.00 |
| Available, n | 11 | 20 |
| Urea, mmol/l | ||
| Mean (SD) | 9.32 (5.75) | 15.78 (11.22) |
| Median (IQR) | 10.00 (4.30–12.80) | 13.25 (6.80–22.90) |
| Range | 2.20–18.50 | 3.60–36.80 |
| Available, n | 11 | 20 |
| Phosphate, mmol/l | ||
| Mean (SD) | 2.62 (0.75) | 2.38 (0.57) |
| Median (IQR) | 2.74 (2.40–3.21) | 2.36 (2.10–2.88) |
| Range | 1.26–3.53 | 1.26–3.47 |
| Available, n | 11 | 20 |
| Actual Bicarbonate, mmol/l | ||
| Mean (SD) | 18.99 (5.10) | 20.48 (4.11) |
| Median (IQR) | 19.30 (16.90–23.50) | 20.20 (17.65–23.65) |
| Range | 6.50–24.90 | 11.80–29.10 |
| Available, n | 11 | 20 |
| Base excess, mmol/l | ||
| Mean (SD) | −7.84 (8.04) | −4.61 (5.33) |
| Median (IQR) | −6.65 (−12.00 to −3.70) | −4.55 (−8.50 to −1.30) |
| Range | −26.70 to 2.80 | −17.60 to 5.70 |
| Available, n | 10 | 18 |
| pH | ||
| Mean (SD) | 7.25 (0.16) | 7.27 (0.10) |
| Median (IQR) | 7.28 (7.15–7.36) | 7.29 (7.20–7.34) |
| Range | 6.86–7.43 | 7.00–7.40 |
| Available, n | 11 | 20 |
| Haemoglobin, g/l | ||
| Mean (SD) | 123.45 (27.05) | 104.15 (22.53) |
| Median (IQR) | 125.00 (102.00–130.00) | 105.00 (83.50–121.00) |
| Range | 87.00–184.00 | 65.00–148.00 |
| Available, n | 11 | 20 |
| Platelets, ×109/l | ||
| Mean (SD) | 124.30 (59.00) | 143.25 (83.16) |
| Median (IQR) | 117.50 (109.00–128.00) | 126.50 (86.50–203.00) |
| Range | 20.00–227.00 | 25.00–296.00 |
| Available, n | 10 | 20 |
| Primary indication for starting RRT, n (%) | ||
| Fluid volume control | 5 (45.45) | 9 (45.00) |
| Biochemical control | 4 (36.36) | 5 (25.00) |
| Fluid and biochemical equally | 2 (18.18) | 6 (30.00) |
The following tables show the baseline and pre-RRT values for those participants included in the primary analysis.
| Control (n = 62) | Intervention, included in primary analysis (n = 20) | |
|---|---|---|
| Age at screening, days | ||
| Mean (SD) | 52.10 (100.80) | 85.85 (131.22) |
| Median (IQR) | 10.50 (7.00–38.00) | 12.50 (6.50–129.50) |
| Range | 1.00–477.00 | 1.00–367.00 |
| Available, n | 62 | 20 |
| Sex, n (%) | ||
| Female | 27 (43.55) | 6 (30.00) |
| Male | 35 (56.45) | 14 (70.00) |
| Weight at RRT initiation, kg* | ||
| Mean (SD) | 3.76 (1.59) | 4.07 (1.85) |
| Median (IQR) | 3.20 (2.90–3.90) | 3.60 (3.00–5.35) |
| Range | 1.80–10.10 | 1.00–7.40 |
| Available, n | 62 | 20 |
| Type of weight measurement, n (%) | ||
| Actual weight | 51 (82.26) | 20 (100.00) |
| Estimated weight | 11 (17.74) | 0 |
| Gestational age at delivery (completed weeks) | ||
| Mean (SD) | 37.93 (2.25) | 35.75 (3.95) |
| Median (IQR) | 38.00 (37.00–39.00) | 36.50 (32.50–39.00) |
| Range | 28.00–41.00 | 26.00–40.00 |
| Available, n | 61 | 20 |
| Type of admission to unit, n (%) | ||
| Planned – following surgery | 31 (50.00) | 5 (25.00) |
| Unplanned – following surgery | 1 (1.61) | 1 (5.00) |
| Planned – other | 2 (3.23) | 3 (15.00) |
| Unplanned | 28 (45.16) | 11 (55.00) |
| Previous ICU admission, n (%) | ||
| ICU | 0 | 1 (5.00) |
| PICU | 4 (6.45) | 1 (5.00) |
| NICU | 29 (46.77) | 8 (40.00) |
| None | 26 (41.94) | 10 (50.00) |
| Unknown | 3 (4.84) | 0 |
| Source of admission, n (%) | ||
| Same hospital | 40 (64.52) | 12 (60.00) |
| Other hospital | 22 (35.48) | 8 (40.00) |
| Elective admission, n (%) | ||
| No | 29 (46.77) | 12 (60.00) |
| Yes | 33 (53.23) | 8 (40.00) |
| Main reason for PICU admission, n (%) | ||
| Bronchiolitis | 1 (1.61) | 13 (65.00) |
| Recovery from surgery | 31 (50.00) | 2 (10.00) |
| Seizure disorder | 0 | 5 (25.00) |
| Other | 30 (48.39) | 0 |
| If admission was recovery from surgery, what was procedure, n (%) | ||
| Bypass cardiac procedure | 30 (96.77) | 4 (80.00) |
| Non-bypass cardiac procedure | 0 | 0 |
| Other procedure | 1 (3.23) | 1 (20.00) |
| Is evidence available to assess past medical history, n (%) | ||
| Yes | 55 (88.71) | 20 (100.00) |
| No | 7 (11.29) | 0 |
| Systolic blood pressure, mmHg | ||
| Mean (SD) | 72.20 (19.45) | 73.15 (22.91) |
| Median (IQR) | 68.00 (59.00–78.00) | 64.50 (59.50–80.50) |
| Range | 40.00–137.00 | 36.00–129.00 |
| Available, n | 59 | 20 |
| Base excess source, n (%) | ||
| Arterial | 44 (70.97) | 10 (50.00) |
| Capillary | 6 (9.68) | 5 (25.00) |
| Venous | 4 (6.45) | 3 (15.00) |
| Available, n | 54 | 18 |
| Lactate source, n (%) | ||
| Arterial | 44 (70.97) | 10 (50.00) |
| Capillary | 7 (11.29) | 6 (30.00) |
| Venous | 4 (6.45) | 3 (15.00) |
| Available, n | 55 | 19 |
| Mechanical ventilation, n (%) | ||
| Yes | 50 (80.65) | 18 (90.00) |
| No | 12 (19.35) | 2 (10.00) |
| Received CPAP within first hour, n (%) | ||
| Yes | 5 (8.06) | 1 (5.00) |
| No | 57 (91.94) | 19 (95.00) |
| Pupil reaction, n (%) | ||
| Both fixed and dilate | 0 | 0 |
| Other reaction | 56 (90.32) | 18 (90.00) |
| Unknown | 6 (9.68) | 2 (10.00) |
| PIM3 score | ||
| Mean (SD) | 0.070 (0.100) | 0.07 (0.08) |
| Median (IQR) | 0.023 (0.013–0.065) | 0.03 (0.02–0.08) |
| Range | 0.005–0.445 | 0.01–0.27 |
| Available, n | 62 | 20 |
| Logit of PIM3 score | ||
| Mean (SD) | −3.36 (1.29) | −3.21 (1.19) |
| Median (IQR) | −3.74 (−4.30 to −2.67) | −3.41 (−4.08 to −2.43) |
| Range | −5.29 to −0.22 | −5.12 to −0.98 |
| Available, n | 62 | 20 |
| Control (n = 62) | Intervention, included in primary analysis (n = 20) | |
|---|---|---|
| Sodium, mmol/l | ||
| Mean (SD) | 145.42 (6.33) | 140.30 (7.70) |
| Median (IQR) | 146.00 (141.00–149.00) | 138.50 (135.00–146.00) |
| Range | 130.00–157.00 | 128.00–156.00 |
| Available, n | 62 | 20 |
| Potassium, mmol/l | ||
| Mean (SD) | 4.67 (0.81) | 4.94 (1.16) |
| Median (IQR) | 4.45 (4.00–5.20) | 4.90 (4.00–5.65) |
| Range | 3.50–6.70 | 3.30–8.30 |
| Available, n | 60 | 20 |
| Creatinine, mmol/l | ||
| Mean (SD) | 77.24 (98.21) | 137.75 (142.14) |
| Median (IQR) | 51.50 (40.00–68.00) | 87.50 (72.00–163.50) |
| Range | 12.00–623.00 | 9.00–678.00 |
| Available, n | 62 | 20 |
| Urea, mmol/l | ||
| Mean (SD) | 8.33 (7.96) | 15.78 (11.22) |
| Median (IQR) | 6.15 (3.50–10.60) | 13.25 (6.80–22.90) |
| Range | 1.70–45.40 | 3.60–36.80 |
| Available, n | 62 | 20 |
| Phosphate, mmol/l | ||
| Mean (SD) | 2.21 (0.64) | 2.38 (0.57) |
| Median (IQR) | 2.20 (1.75–2.70) | 2.36 (2.10–2.88) |
| Range | 0.61–3.58 | 1.26–3.47 |
| Available, n | 62 | 20 |
| Actual bicarbonate, mmol/l | ||
| Mean (SD) | 21.74 (4.32) | 20.48 (4.11) |
| Median (IQR) | 21.50 (18.40–25.40) | 20.20 (17.65–23.65) |
| Range | 12.70–33.00 | 11.80–29.10 |
| Available, n | 62 | 20 |
| Base excess, mmol/l | ||
| Mean (SD) | −3.44 (6.01) | −4.61 (5.33) |
| Median (IQR) | −4.25 (−7.75 to 0.85) | −4.55 (−8.50 to −1.30) |
| Range | −18.00 to 10.30 | −17.60 to 5.70 |
| Available, n | 60 | 18 |
| pH | ||
| Mean (SD) | 7.33 (0.12) | 7.27 (0.10) |
| Median (IQR) | 7.33 (7.24–7.43) | 7.29 (7.20–7.34) |
| Range | 7.02–7.52 | 7.00–7.40 |
| Available, n | 62 | 20 |
| Haemoglobin, g/l | ||
| Mean (SD) | 124.08 (32.34) | 104.15 (22.53) |
| Median (IQR) | 124.50 (110.00–148.00) | 105.00 (83.50–121.00) |
| Range | 13.00–194.00 | 65.00–148.00 |
| Available, n | 62 | 20 |
| Platelets, ×109/l | ||
| Mean (SD) | 201.18 (112.39) | 143.25 (83.16) |
| Median (IQR) | 207.50 (113.00–257.00) | 126.50 (86.50–203.00) |
| Range | 34.00–582.00 | 25.00–296.00 |
| Available, n | 62 | 20 |
| Primary indication for starting RRT, n (%) | ||
| Fluid volume control | 32 (51.61) | 9 (45.00) |
| Biochemical control | 8 (12.90) | 5 (25.00) |
| Fluid and biochemical equally | 22 (35.48) | 6 (30.00) |
Appendix 4 Statistical details: analysis of the primary outcome
Supplementary statistical details for the analysis of the primary outcome
Preliminary analysis of primary outcome
The primary analysis assumes that X-A has zero mean, and the following shows the fit of a linear model to X-A, with terms for centre, period treatment and duration.
FIGURE 12.
Residual vs. fitted plot for linear model of X-A.

FIGURE 13.
Normal plot of residuals for linear model of X-A.

FIGURE 14.
X-A by study period for n = 82 in primary analysis.
The plots of the residuals and other diagnostics above indicate that the models fit well and only the term for period 2 is significant. The boxplots show a noticeable outlier in period 2. On the basis of this analysis and observation in the main part of the report, it was decided to proceed with the primary analysis as prescribed in the SAP.
Primary analysis
The following output shows the results of fitting the prescribed model to log|X-A|, with the following diagnostic plots showing that the fit is satisfactory.
FIGURE 15.
Normal plot of residuals for primary analysis.
FIGURE 16.
Standardised residuals with kernel density curve by treatment arm for the primary analysis.

FIGURE 17.
Boxplot of standardised residuals by treatment arm for primary analysis.

FIGURE 18.
Residuals vs. fitted values plot for primary analysis.
Appendix 5 Statistical details: other analyses
Supplementary material on the analysis of the average log|X-A|
The full fit of the model is given below.
The diagnostic plots below indicate that the model provides a satisfactory fit.
FIGURE 19.
Normal plot of standardised residuals for analysis of average log|X-A|.
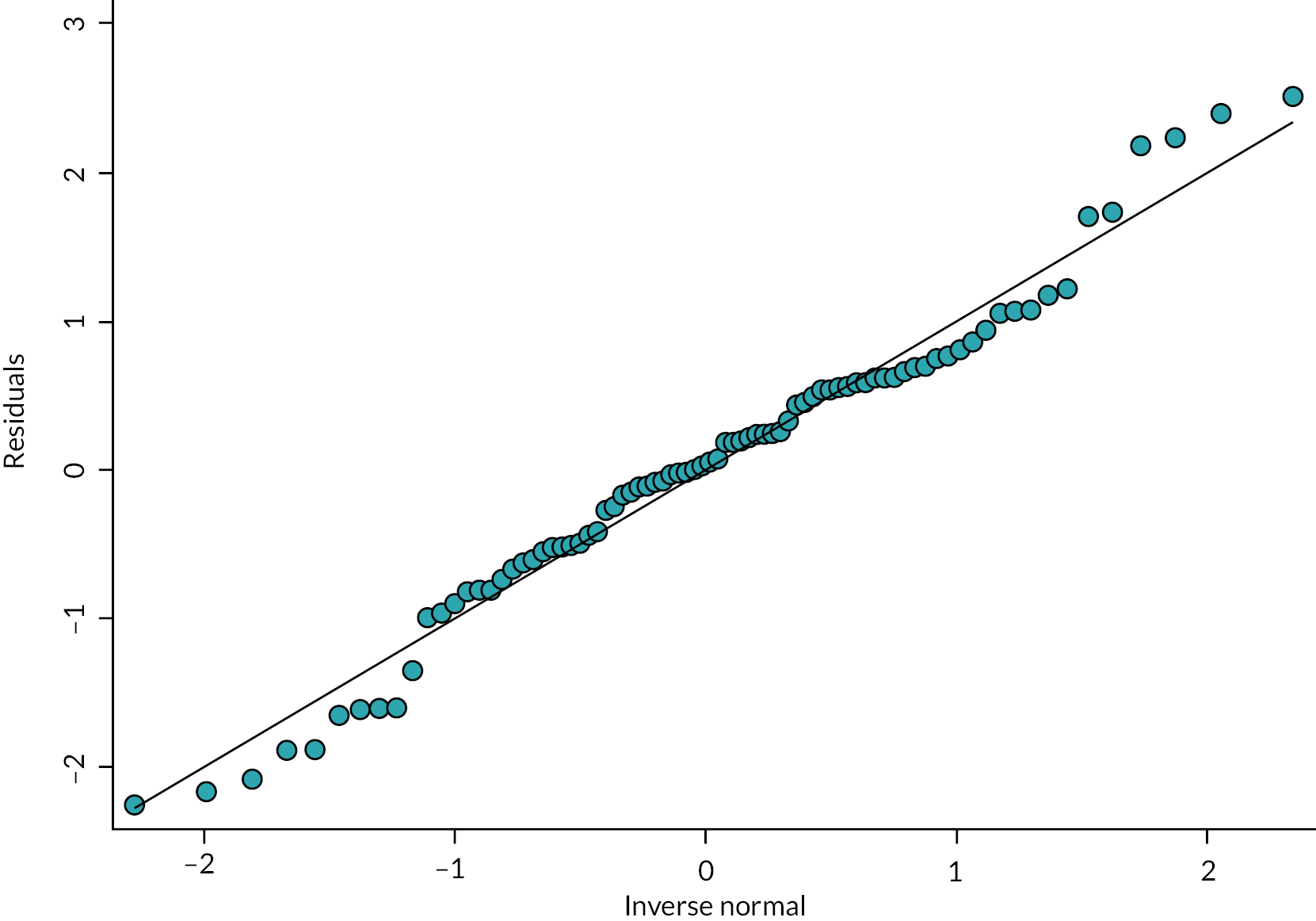
FIGURE 20.
Standardised residuals with kernel density curve by treatment arm for analysis of average log|X-A|.
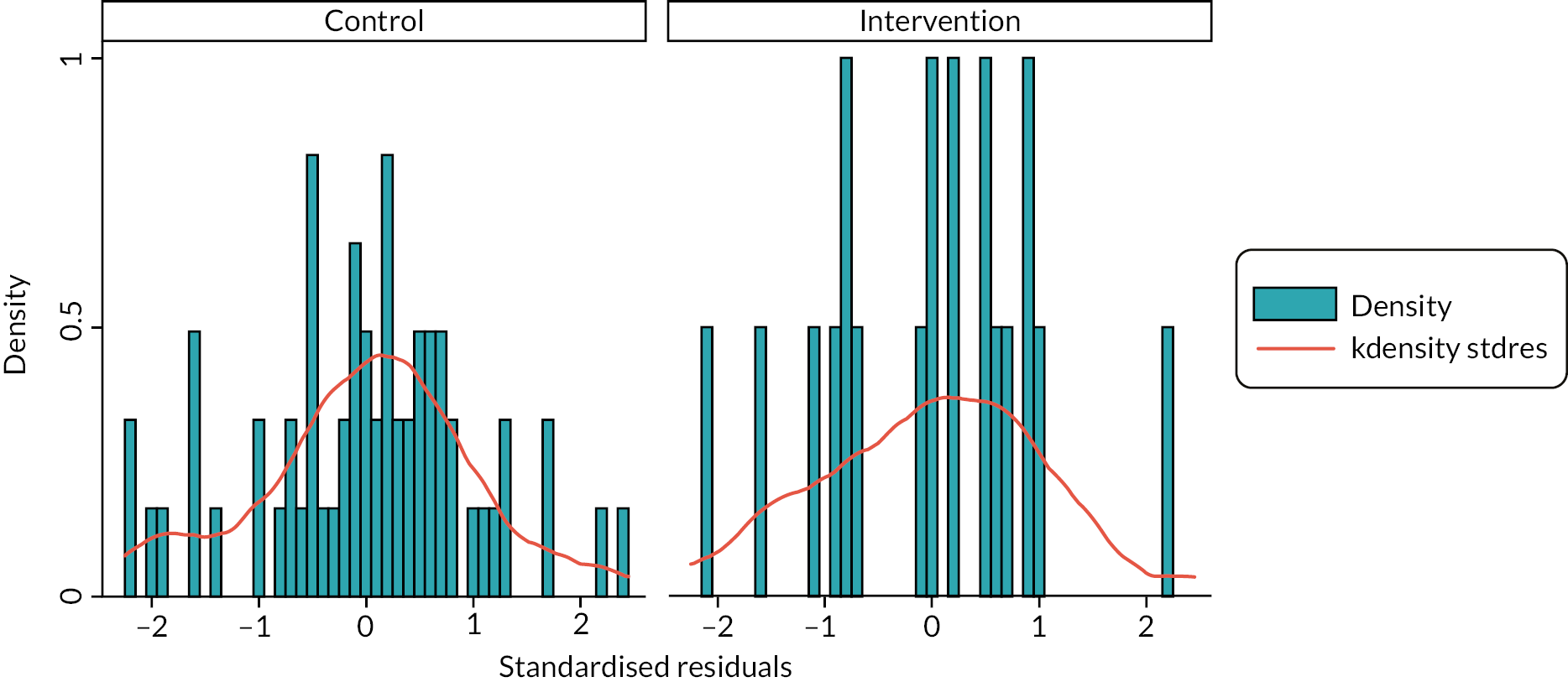
FIGURE 21.
Boxplot of standardised residuals by treatment arm for analysis of average log|X-A|.
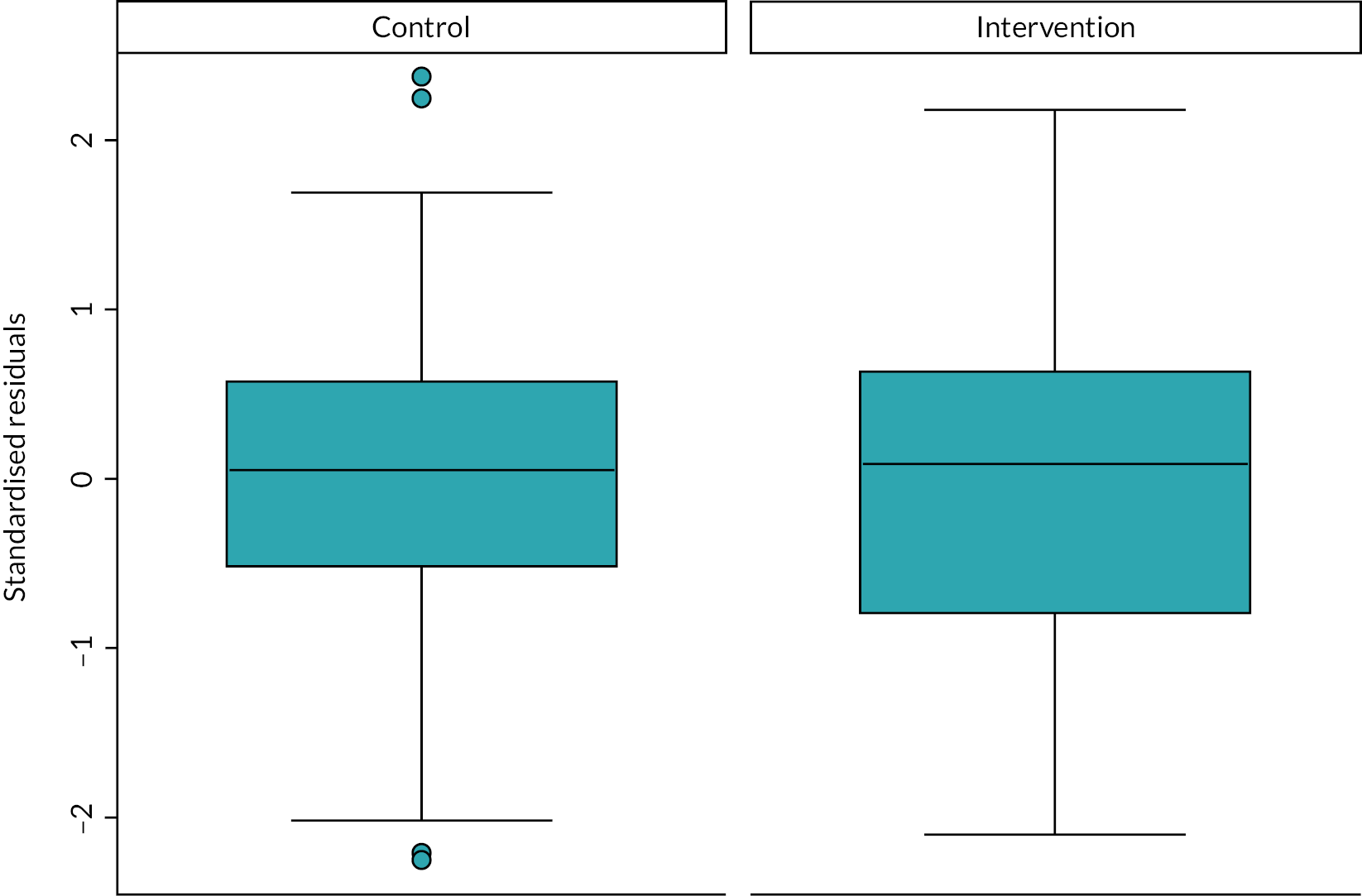
FIGURE 22.
Standardised residuals vs. fitted values plot for analysis of average log|X-A|.

Supplementary material on the analysis of biochemical clearances
Creatinine
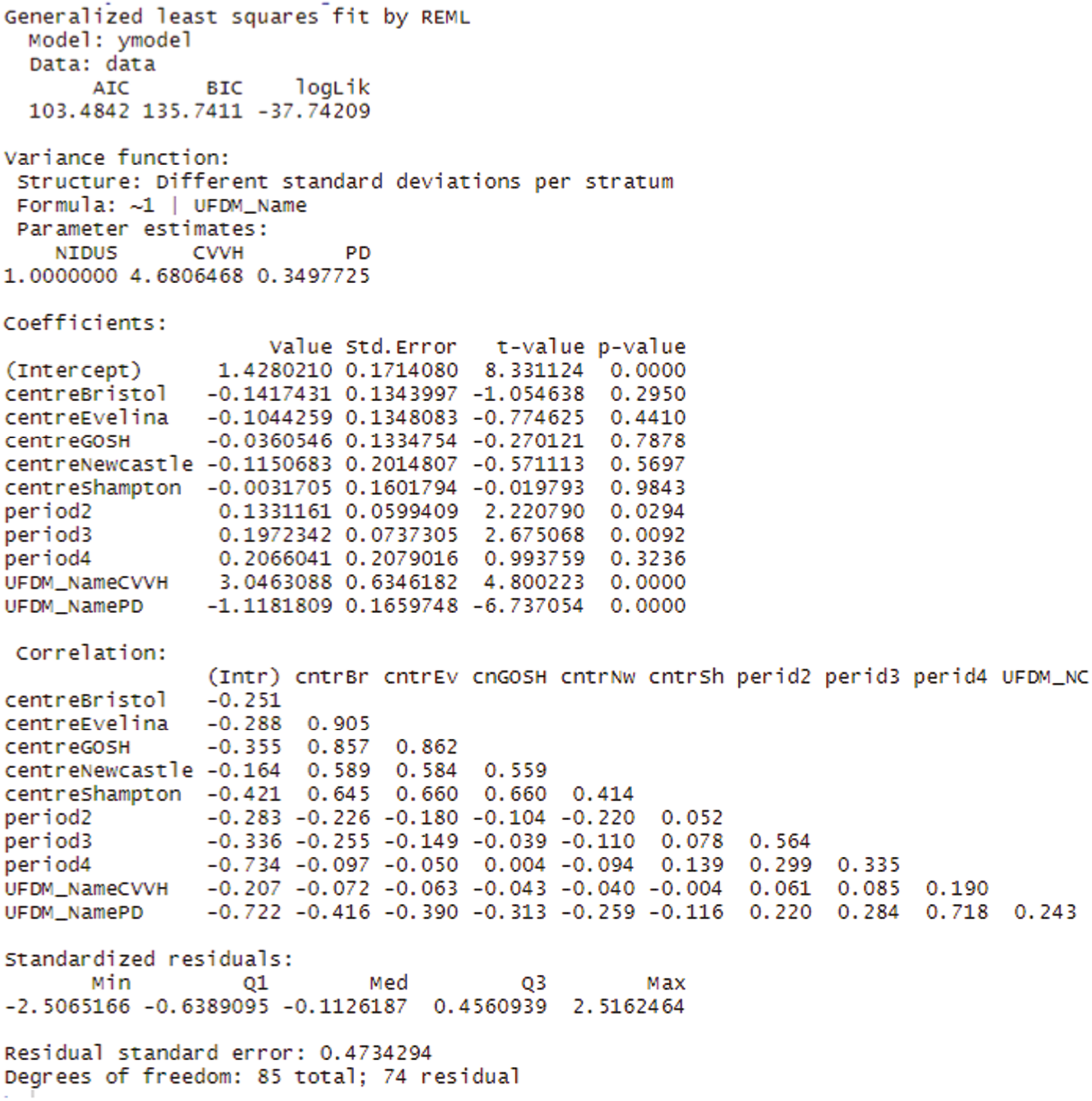
R output for GLS model of analysis of the first computable creatinine.
FIGURE 23.
Normal plot of standardised residuals for GLS model of first creatinine clearance.

Urea
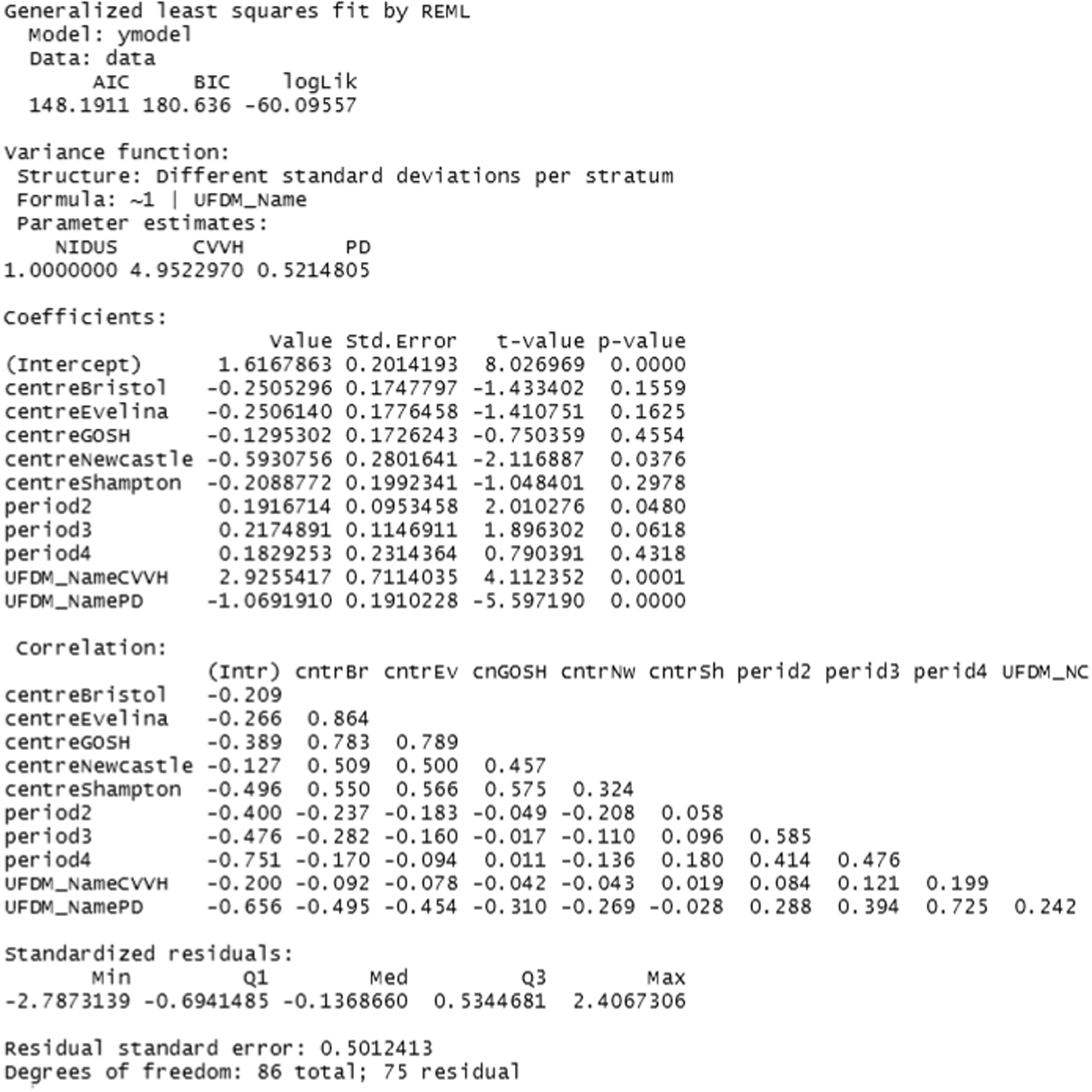
R output for GLS model of analysis of the first computable urea.
FIGURE 24.
Normal plot of standardised residuals for GLS model of first urea clearance.

Phosphate
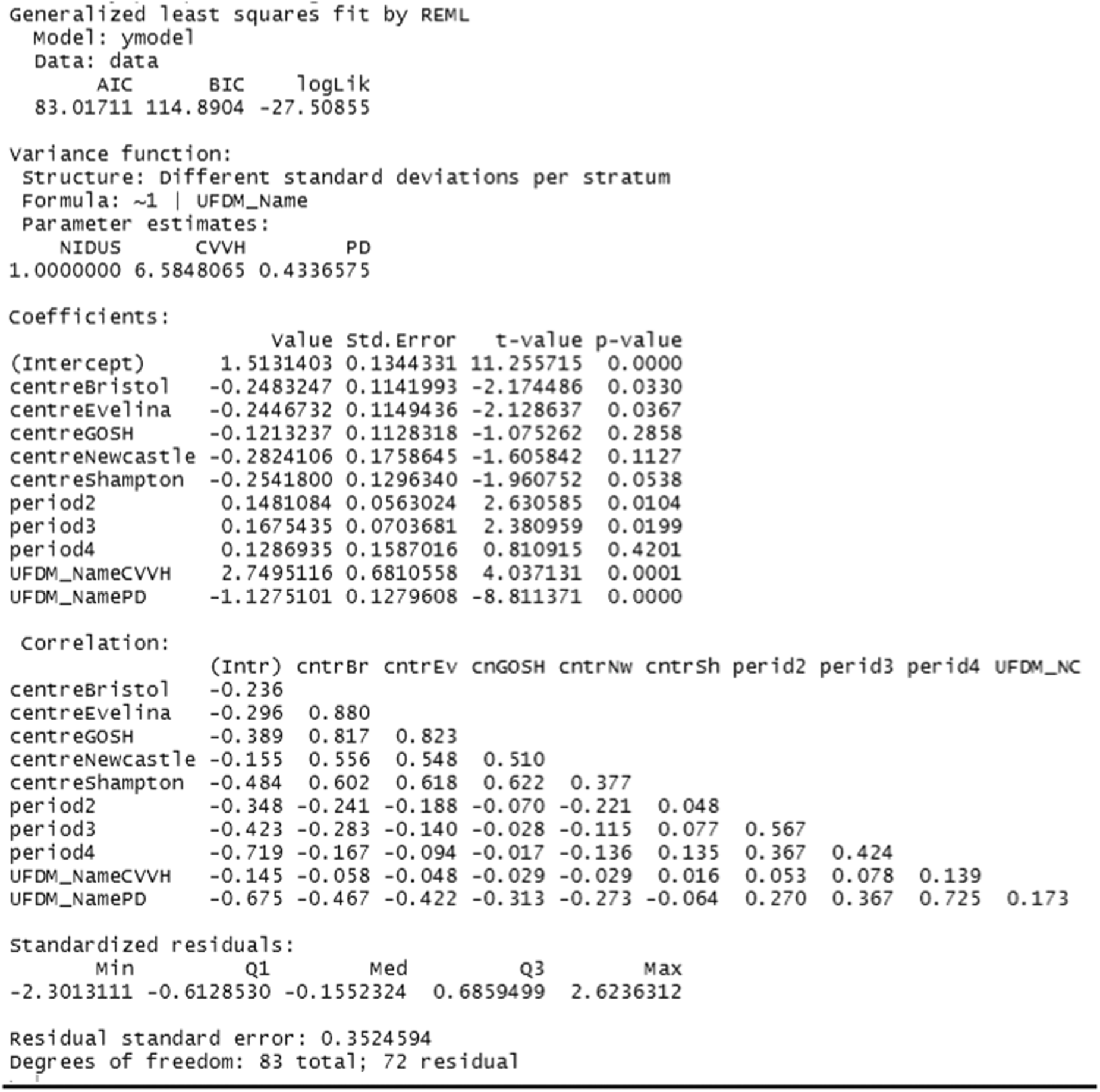
R output for GLS model of analysis of the first computable phosphate.
FIGURE 25.
Normal plot of standardised residuals for GLS model of first phosphate clearance.

Appendix 6 Statistical details: subgroup analyses
Further details of the model fitted to the primary outcome separating treatment into NIDUS, CVVH and PD.
Further details of the analysis comparing X-A2 between NIDUS and CVVH.
FIGURE 26.
Normal plot of residuals for general linear model of X-A2.

FIGURE 27.
Standardised residuals vs. fitted plot by treatment arm for general linear model of X-A2.
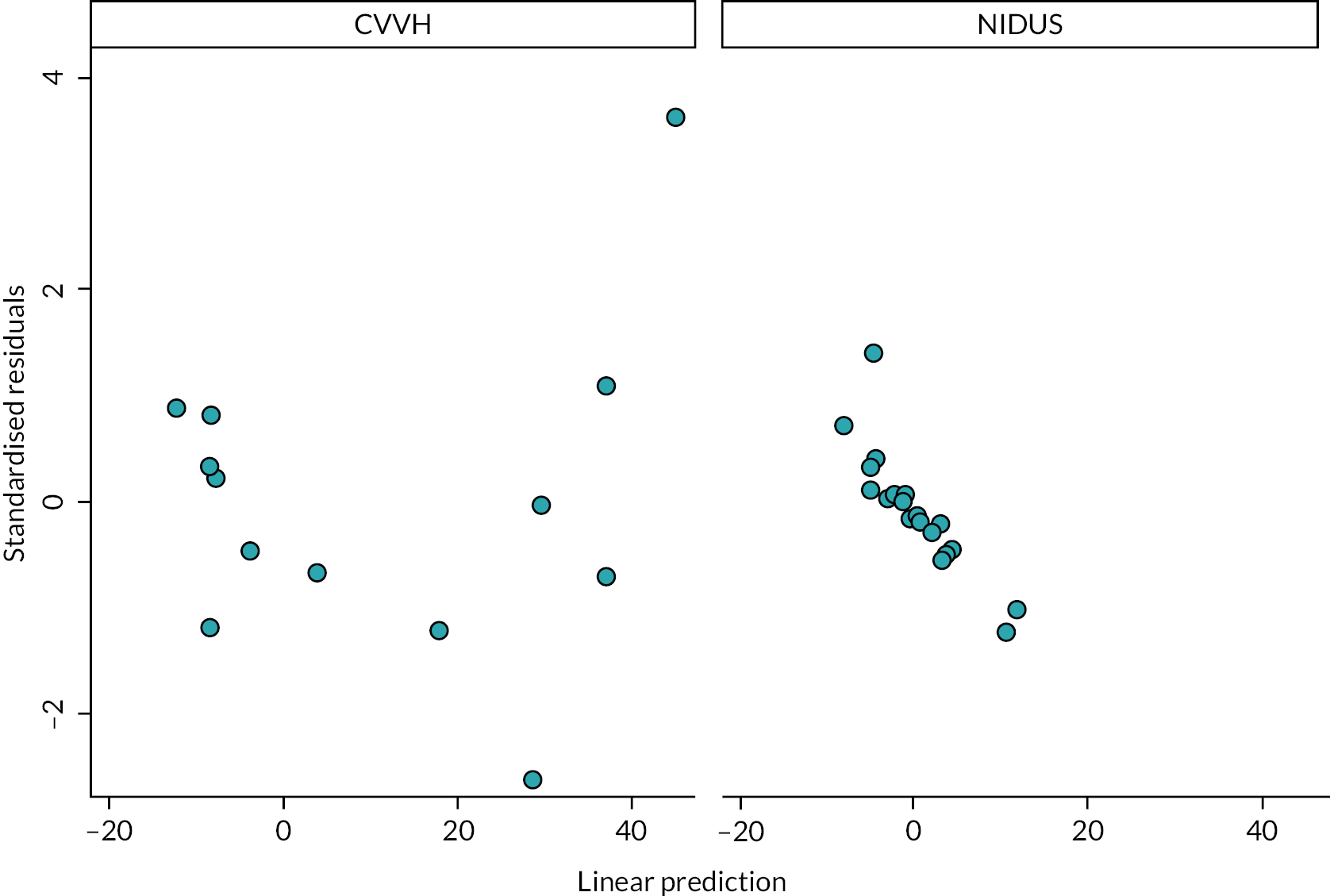
The above model, with strong effects for the period and treatment effects suggested that it would be unwise to analyse X-A2 on the assumption that it had zero mean. Consequently, the final analysis fitted a model to X-A2 using the same model for the means just presented using generalised least squares and assuming separate residual variances in for NIDUS and CVVH. The result of doing this using gls from the nlme library in R is below.
Appendix 7 Assessment of adverse events
FIGURE 28.
Assessment of AEs flow diagram: assessment of AEs.
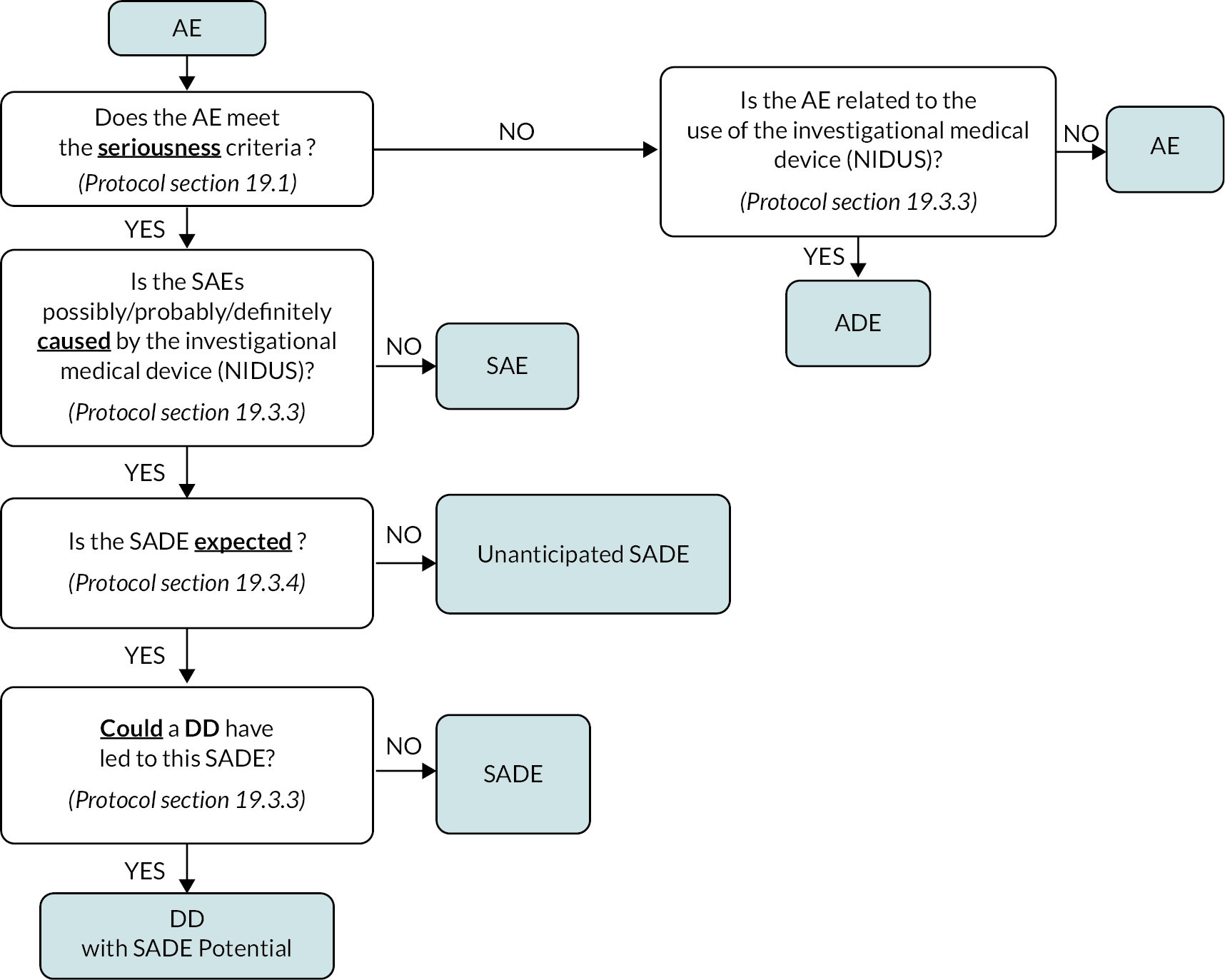
List of abbreviations
- ACT
- activated clotting time
- ADE
- adverse device effect
- AE
- adverse event
- CARPEDIEM
- The Cardio-Renal Pediatric Dialysis Emergency Machine
- CI
- confidence interval
- CE
- Conformité Européenne
- CVVH
- continuous veno-venous haemofiltration (hemofiltration)
- DD
- device deficiency
- ECMO
- extracorporeal membrane oxygenation
- ECMO + HD
- ECMO plus haemodialysis
- FDA
- Food and Drug Administration (US)
- GEE
- generalised estimating equations
- GP
- general practitioner
- HD
- haemodialysis
- ICC
- intraclass correlation coefficient
- ICU
- intensive care unit
- IQR
- interquartile range
- ITT
- intention to treat
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NCTU
- Newcastle Clinical Trials Unit
- NIDUS
- Newcastle Infant Dialysis and Ultrafiltration System
- NIHR
- National Institute for Health and Care Research
- NuTH
- Newcastle upon Tyne Hospitals
- PCPI
- Patient, Care and Public Involvement
- PD
- peritoneal dialysis
- PICANet
- Paediatric Intensive Care Audit Network
- PICU
- paediatric intensive care unit
- PR
- parental responsibility
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RRT
- renal replacement therapy
- SADE
- serious adverse device event
- SAE
- serious adverse event
- SAP
- statistical analyses plan
- SD
- standard deviation
- SW
- stepped wedge
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UF
- ultrafiltration
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/VGJT3714).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
