Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as award number 13/179/01. The contractual start date was in January 2015. The draft manuscript began editorial review in October 2022 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Quraishi et al. This work was produced by Quraishi et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Quraishi et al.
Chapter 1 Introduction
In inflammatory bowel diseases (IBDs), Crohn’s disease (CD) and ulcerative colitis (UC), there is imbalance or ‘dysbiosis’ of the gut microbiota compared with healthy bowel, with individuals having IBD showing reduced bacterial diversity compared with healthy individuals. 1,2 Indeed, the currently accepted hypothesis concerning the pathogenesis of IBD involves an aberrant immunological response to the intestinal microbiota in a genetically susceptible host. 3 Whether the observed dysbiosis represents ‘cause’ or ‘effect’ remains unanswered. Faecal microbiota transplant (FMT) is the infusion of a faecal suspension from a healthy donor into the gastrointestinal tract of a patient with disease, and there is interest in the potential of this technique for modifying the gut microbiome as a possible treatment for IBD. 4
Since the first descriptions of CD and UC at the beginning of the 20th century, it has been strongly suspected that the gut microbiota may have a defining role in the pathogenesis of IBD. 5 Early culture-based studies underestimated the complexity of the microbiota and were hampered by inherent challenges associated with growing fastidious gut bacteria using traditional culturing techniques. With the advent of cheap high-throughput genetic sequencing techniques allied with complex bioinformatics capability, there has been a revolution in our understanding of the composition and function of the colonic microbiome. As a result of studies on the microbiome both in patients with IBD and animal models, we know that patients with IBD (either CD or UC) have, at the phylum level, a reduction in Firmicutes and a relative increase in Proteobacteria. 2
Accumulating data suggest that alteration in the gut microbiome plays a central role in driving UC; data sets highlighting the importance of Roseburia hominis,6 Faecalibacterium prausnitzii7 and Akkermansia muciniphila8 mediating anti-inflammatory responses in UC have been published. Attempts to alter the microbiome with probiotics, while disappointing in CD,9 have shown some promise in UC. 10,11 Since the first exploratory use of FMT to treat UC in 1989,12 numerous case series have been published demonstrating encouraging efficacy signals,13 and this has led to investigators testing FMT as a treatment for UC. To date, there have been five randomised controlled trials (RCTs) of FMT for the treatment of UC. 14-18 The first was a trial of fresh and frozen enemas, which were delivered weekly for 6 weeks, with water being used as a placebo. 14 In this seminal study, of the 75 patients included approximately 20% were on immune-modulating treatment and 10% biologics. Similarly in the other three original FMT studies in UC, similar proportions of patients had used immune-suppressants or biologics; 31% and 20% in the study from Rossen et al. ,15 43% and 20% in the Paramsothy study from Australia16 and 40% and 10% in the second multidonor study from Australia. 17 This indicates that these original patient cohorts included patients with established IBD indicated by the inclusion of patients who had tried and failed second-line treatments. There was a significant increase in remission in patients receiving treatment compared to those randomised to placebo. In the second study, fresh FMT by naso-jejunal delivery was used and autologous stool was used as a placebo. 15 Although the outcome favoured active treatment, the difference between the FMT and placebo group was not statistically significant. Two RCTs were reported in 2017 and 201916,17 that both used frozen FMT from pooled donors and involved intensive treatment regimens. Both achieved statistically significant results in favour of active treatment, with remission rates of 32% versus 9%16 and 27% versus 8%17 for the active and placebo groups, respectively. The most recent (fifth) trial had three arms comparing FMT alone, FMT with dietary modification and dietary modification alone; there was no placebo arm. 18 This last study did not show a benefit for FMT. However, it was noted that the majority of adult patients in this study had failed biologics and that this was perhaps the most challenging adult UC cohort recruited to FMT trials to date.
As a result of this work, there is great interest in FMT as a possible treatment for UC, but the optimal route of delivery remains unknown. The purpose of this pilot study was to determine the optimum route of FMT delivery and assess the feasibility (recruitment, treatment adherence, retention) of undertaking a large-scale trial of FMT in UC.
Chapter 2 Methods of clinical study
Trial design
This was a prospective, multicentre, open-label, randomised pilot study to assess two possible routes of FMT delivery for the treatment of UC. Patients with UC were randomised to receive open-label FMT delivered either via a nasogastric (NG) tube for delivery to the stomach (foregut-NG) or by a combination of delivery through a colonoscope followed by 7 weekly enemas (hindgut-COLON). All patients underwent a treatment schedule using FMT derived, in each case, from a single donor. Details regarding the objectives, design and methods of the trial have been published previously. 19
The pilot study also included a qualitative assessment (see Chapter 4) and a nested-mechanistic study (see Chapter 5). The trial had favourable ethics opinion from the East Midlands Research Ethics Committee.
Aims and objectives
The aims of the pilot study were as follows:
-
to determine which FMT administration route (NG or COLON) should be investigated in a randomised double-blind, placebo-controlled trial
-
to determine whether a full-scale RCT was feasible.
In order to achieve these aims, the pilot study had the below clinical objectives to assess the following:
-
whether FMT by the NG route induces clinical response in patients with mild to moderately active UC (partial Mayo score of ≥4 and ≤8)
-
whether FMT by the COLON route induces clinical response in patients with mild to moderately active UC
-
tolerability and safety
-
which route of FMT delivery (if any) was suitable to investigate in a full-scale RCT.
The aims of the qualitative research were to assess the following:
-
patient and clinician acceptability of FMT (NG route)
-
patient and clinician acceptability of FMT (COLON route).
The aims of the nested-mechanistic substudy were to assess the following:
-
whether FMT by either route is associated with a change in faecal calprotectin as a surrogate marker of colonic inflammation
-
changes in the colonic microbiome and metabolome [short-chain fatty acids (SCFAs)] induced by FMT via each route
-
effect of diet (donors)
-
time from stool donation to treatment.
Further details of the qualitative and translational research are provided in Chapters 4 and 5, respectively.
Recruitment
Patients with UC were recruited from three hospitals in the UK (Queen Elizabeth Hospital, Birmingham; St Mark’s Hospital, London; and Glasgow Royal Infirmary). Potentially eligible patients who expressed an interest in participating in the trial were consented via a two-stage consent process, namely registration and randomisation. Participant information sheets were provided to facilitate the consent process. The first stage, registration, involved consent for trial-specific screening activities, and consent to collect stool and urine samples for the mechanistic substudies. Patients underwent basic physiological assessments (pulse, blood pressure, temperature, height and weight) and baseline blood tests. They were provided with a diary to record bowel symptoms (so that the partial Mayo score could be calculated at the randomisation visit), stool sample kits and a bowel preparation kit (MoviprepTM, Middlesex, NJ), which would need to be taken before the randomisation visit (if eligible) for a colonoscopy. They were asked to return the stool sample as soon as possible so that the stool could be tested for Clostridioides difficile. Following the screening visit, the qualitative researcher arranged for an interview with the patient to take place prior to their randomisation visit.
For the second stage of the recruitment process, the research team at the hospital contacted the patient to notify them of their stool result. If they tested negative for Clostridioides difficile, they were invited to attend the randomisation visit. Instructions were given on when to take the bowel preparation, and they were asked to collect a stool sample on the same day prior to taking the bowel preparation, which they brought with them to the randomisation visit. At the randomisation visit, the patient’s eligibility for the trial was confirmed and consent for randomisation taken. Basic physiological assessments were undertaken, blood test results were checked, a urine sample was taken for pregnancy testing in women and for urinary metabolomics, and the partial Mayo score was calculated from patient diaries. All patients were to have a colonoscopy to assess disease (following randomisation), so that a full Mayo score could be calculated, and to collect mucosal biopsies.
This study was approved by the East Midlands-Nottingham Research Ethics Committee (REC 17/EM/0274).
Eligibility criteria
Potential participants were assessed for eligibility by an appropriately trained doctor. The participants needed to meet the following criteria:
Inclusion criteria
-
Adult patients (aged 16–70 years) with clinically confirmed (clinical, endoscopic and histological proven) UC for at least 12 weeks prior to the screening visit.
-
Partial Mayo score of ≥4 and ≤8 despite stable disease maintenance treatment with 5-aminosalicylates (5ASA) with or without immunomodulators, or on no treatment.
-
Rectal bleeding subscore of ≥1 on the partial Mayo.
-
Able to give written, signed informed consent.
Exclusion criteria
-
Stool positive for C. difficile or infection by either polymerase chain reaction or enzyme-linked immunoassay (ELISA).
-
Sero-positive for hepatitis A/B/C and/or human immunodeficiency virus (HIV) infection.
-
Antibiotics in the preceding 12 weeks prior to date of the screening visit.
-
Systemic/topical steroids (prednisolone or beclomethasone) in the preceding 2 weeks prior to the date of the screening visit.
-
Biologics in the preceding 12 weeks prior to the date of the screening visit.
-
Commercial probiotics and prebiotics in the preceding 12 weeks prior to the date of the screening visit.
-
On oral nutritional supplements or enteral/parenteral nutrition in the preceding 4 weeks prior to the date of the screening visit.
-
Pregnant or lactating.
-
Not willing to take appropriate contraceptive measures to prevent pregnancy during trial participation.
Concomitant drugs
Participants were not able to take oral or systemic steroids or change their maintenance treatment for UC for the first 8 weeks of the pilot study. Maintenance medications for UC (e.g. oral 5ASA compounds, thiopurine or methotrexate but not biologics) were allowed if the dosage had been stable for 3 months prior to study entry.
Randomisation
Eligible patients were randomised into the STOP-COLITIS pilot study by the research staff at sites using a secure online randomisation service provided by the Birmingham Clinical Trials Unit (BCTU). Randomisation was at the level of the individual in a 1 : 1 ratio to either NG or colonic delivery of FMT. A minimisation algorithm, incorporating a random element, was used to avoid chance imbalances in important prognostic variables. The variables used in the minimisation algorithm were as follows:
-
partial Mayo score (4–5 or 6–8)
-
current smoking status [yes or no (not smoked for the past 12 months)].
Donors
Donor sample acquisition and processing
Donors were recruited following advertisement from healthy unrelated anonymous individuals living in Birmingham. We excluded healthcare workers due to their potential exposure to microbes affecting the microbiome. This was as a result of comments received following protocol review by the funder in which reservations were expressed regarding a possible dysbiosis of colonic microbiota in healthcare workers compared to the healthy population.
Donors were ≥18 and <50 years of age, had a normal morning bowel habit, normal body mass index (≥18.5 and ≤25 kg/m2), were non-smokers (not smoked for at least 12 months) and had no recent history of diarrhoea or rectal bleeding. The requirement for donors to usually open their bowels in the morning was to facilitate delivery of the donation to the laboratory in time for immediate processing during the working day. Potential donors underwent rigorous screening using a health-screening questionnaire (see Appendix 1).
Those who were eligible following this screening were consented for the donation process and had blood and faecal samples taken to test for transmissible pathogens in accordance with UK, American Gastroenterology Association (AGA) and European guidelines. 20,21 Individuals who passed the screening process were invited to donate morning faecal samples for 10 days and to deliver these for processing within 6 hours of defaecation. At first donation, they were also asked to complete the EPIC-Norfolk food frequency questionnaire (see Appendix 2).
In order to carry out this study, it was necessary to set up the first laboratory in the UK to produce FMT under strict good manufacturing practice (GMP) conditions. This was achieved by setting up a parallel laboratory to an existing cell therapy centre at the University of Birmingham and amending that existing licence. Donations were then processed under a Medicines and Healthcare products Regulatory Agency (MHRA) manufacturing licence at the University of Birmingham Microbiome Treatment Centre, in a dedicated GMP laboratory and the resulting FMT was stored in temperature-monitored −80 °C freezers. Stool collection and processing were performed under aerobic conditions. Donations were transferred into a Whirl-Pak filter bag and homogenised with 0.9% w/v saline and 10% glycerol in a 400-circulating stomacher (Seward, UK) for 2 minutes at 230 rpm. The liquid portion of the sample (FMT), containing 0.6 g/ml faeces was then transferred into labelled sterile sample pots in volumes of 50 ml, and stored frozen at −80 °C for up to 24 weeks. Samples were dispatched for use at each site as required. An aliquot from each donated sample was kept for analysis in the event of adverse events (AEs) occurring as a result of FMT and samples from each donation period were processed for genetic sequencing and metabolic analysis. At the end of each 10-day donation period, donors received compensation for expenses incurred and underwent an exit health questionnaire. Pooled samples during the donation period were screened for pathogens and were collected from the donated stool by staff at the Microbiome Treatment Centre before FMT manufacture.
Donor assessment of dietary intake
The habitual dietary pattern of the donors was assessed using the validated food frequency questionnaire as used in the EPIC-Norfolk study in the UK. 22 Data from the EPIC questionnaires were transferred to the University of Glasgow for analysis. Energy, macronutrient and fibre intake was estimated and expressed in nutrient ranks and quartiles and compared against Department of Health recommendations and UK National Diet and Nutrition Survey results.
Interventions
This pilot study assessed two possible routes of FMT delivery, NG and colonic. Before the initial FMT treatment, all participants received standard bowel preparation comprising 2 l of reconstituted Moviprep solution (Norgine Ltd) within the 24 hours prior to the procedure.
Participants randomised to the NG route of FMT delivery were pre-treated orally with a proton pump inhibitor (lansoprazole 30 mg) and a prokinetic agent (domperidone 10 mg) at least 30 minutes prior to each FMT infusion to reduce gastric acid secretion and prevent the risk of gastro-oesophageal regurgitation. NG tubes were positioned and checked for correct position as per local protocol (with the tip below the diaphragm as assessed by X-ray or evidence of stomach acid on aspiration and pH testing of a sample from the tube after placement). Following thawing at room temperature, 50 ml of thawed FMT containing 30 g faeces was to be infused. Following the first treatment, participants were to return for the next 3 days for further treatment following an overnight fast. It was the individual’s choice whether they wished to retain the NG tube in place for the 4-day treatment course or have the tube removed and repassed every day. On the last day of treatment, the NG tube was removed to be replaced prior to the start of the second course of treatment. At week 4, participants returned for a further four FMT infusions over 4 consecutive days following a fast from midnight. In summary, participants in this arm of the trial were to receive 30 g of FMT in 50 ml aliquots for NG administration each day for 4 days at the start of the trial (starting on the day of randomisation), and then again for 4 days in week 4 (total FMT dose 240 g).
Participants randomised to the colonic route of FMT delivery received a thawed 250 ml aliquot of FMT (normal saline and 10% glycerol containing 150 g of faeces) on the day of randomisation. This was delivered to the colon using a spray catheter, with 125 ml sprayed into the caecum to treat the right side of the bowel and the remaining 125 ml sprayed directly onto the rest of the colon (from proximal transverse colon to rectum). Care was taken to avoid any pools of prep liquid and gas insertion was carefully limited during the treatment to avoid the need for aspiration as this would risk extracting the FMT product. Participants then returned on a weekly basis to receive weekly enemas of 30 g of faeces (made up to 100 ml with normal saline in 10% glycerol) up to week 7. Participants were positioned in the recumbent left lateral position for treatment and asked to stay in position for an hour to promote retention of the FMT. In summary, participants in this arm of the trial were to receive 150 g of stool (in 250 ml aliquot) for colonic administration on the day of randomisation followed by 30 g of stool (in 100 ml aliquot) for administration by enema for 7 weeks (total FMT dose 360 g).
All participants underwent a treatment schedule using FMT derived from a single donor. In both the NG and colonic arms, following each FMT treatment, participants received a single dose of loperamide.
Scheduled trial appointments
Participants were followed up weekly up to week 8 and then again at week 12. At each visit, the partial Mayo score was calculated from the patient diaries, medication use and any AEs were recorded.
Stool samples were collected at randomisation and weeks 2, 4, 6, 8 and 12 for storage and assessment of faecal calprotectin, 16S rRNA sequencing, and faecal metabolomics profile. Blood samples were collected at weeks 4, 6 and 8 for C-reactive protein (CRP) assessment. At weeks 8 and 12, physiological data, a urine sample (for SCFA measurement) and the quality of life (QoL) questionnaires, the short-form 36 (SF-36) and inflammatory bowel disease questionnaire (IBDQ), were collected. At week 8, a flexible sigmoidoscopy was undertaken to calculate the full Mayo score. Biopsies were taken from the colon at baseline and week 8 for mucosal microbiome assessment. A schedule of assessments for the NG and colonic arms is provided in Table 1.
| Registration and screening | Randomisation and treatment start | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 12 | ETV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent | X | X | ||||||||||
| Physiological measurements | X | X | X | X | ||||||||
| Eligibility | X | X | ||||||||||
| IBD diaries | X | X | X | X | X | X | X | X | X | X | X | |
| IBDQ/SF-36 | X | X | X | |||||||||
| Qualitative interview | X | X | ||||||||||
| Partial Mayo | X | X | X | X | X | X | X | X | X | X | X | X |
| Stool (microbiome) collected | X | X | X | X | X | X | ||||||
| Stool (C. difficile) | X | |||||||||||
| FBC/BIO/CRP | X | X | X | X | ||||||||
| Urine test | X | |||||||||||
| Bowel preparation | X | |||||||||||
| Urine sample | X | X | X | |||||||||
| Domperidone/lansoprazole | Xa (4 days) | Xa (4 days) | ||||||||||
| Colonoscopy | X | |||||||||||
| Colonic biopsies | X | X | ||||||||||
| Full Mayo | X | X | ||||||||||
| Loperamide | Xb (4 daysa) | Xb | Xb | Xb | Xb (4 daysa) | Xb | Xb | Xb | Xb | |||
| AEs | X | X | X | X | X | X | X | X | X | X | ||
| Medication | X | X | X | X | X | X | X | X | X | X | ||
| Flexible sigmoidoscopy | X | |||||||||||
| FMT NG (X-ray if applicable) | Xa (4 days) | Xa (4 days) | ||||||||||
| FMT COLON | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb |
Adverse events
AEs are uncommonly encountered in people receiving FMT; however, this has been mostly in the setting of C. difficile treatment, with FMT having been rarely used for treating UC. All AEs experienced whether during or after treatment were recorded on STOP-COLITIS case report forms by research staff at the recruiting sites. The following AEs were expected in participants from a review of the existing literature:
-
bloating
-
transient change of bowel habit (constipation or diarrhoea)
-
nausea
-
vomiting
-
transient pyrexia
-
epistaxis (due to NG tube)
-
abdominal pain
-
AEs clearly associated with the use of standard immune-suppressive medications used as part of UC maintenance treatment.
There was a requirement for any serious adverse events (SAEs) to be recorded on the STOP-COLITIS SAE form and faxed to the STOP-COLITIS trial office immediately and no later than 24 hours of the site becoming aware of the SAE. For the purposes of STOP-COLITIS, the following hospitalisations were NOT considered as SAEs:
-
routine treatment or monitoring of the studied indication, not associated with any deterioration in UC or trial procedures
-
treatment which was elective or pre-planned, for a pre-existing condition that is unrelated to UC and did not worsen
-
admission to a hospital or other institution for general care, not associated with any deterioration in UC or trial procedures.
Participant withdrawal
Participants were made aware at study entry that they could freely withdraw from the trial or any aspect of it at any time and their subsequent care would not be affected. In any case of withdrawal, patients were offered standard care as per local hospital protocol for UC management. If a participant wished to cease to participate in a particular aspect of the trial, they were considered to have changed their status within the trial. The change of status was categorised into three groups:
-
No trial intervention: the participant no longer wished to receive FMT treatment but was willing to be followed up in accordance with the schedule of assessments up to week 12 and if applicable using any central UK NHS bodies for long-term outcomes. A post-12-week qualitative research interview was arranged with the participant.
-
No trial intervention and no further data collection: the participant no longer wished to receive FMT treatment and was not willing to be followed up in any way for the purposes of the trial and therefore no further data were to be collected (i.e. only data collected prior to the withdrawal could be used in the trial analysis). This was regarded as an Early Termination from the trial.
-
No qualitative interview: the participant wished to withdraw from the qualitative research interview at week 12.
Outcomes
The primary outcome was a composite assessment of both quantitative and qualitative data based on the efficacy, acceptability and safety of FMT via either the NG or COLON route, in order to assess the feasibility and acceptability of a full-scale RCT of FMT versus placebo in UC.
STOP/GO criteria for progression to a main randomised controlled trial
The STOP-COLITIS pilot study was designed to determine which FMT administration route (NG or colonic) should be used in the main trial and to assess if a full-scale RCT using either route was feasible. A two-stage STOP/GO process with pre-defined progression criteria was used to assess this. An Independent Oversight Committee (IOC) reviewed the STOP/GO criteria at the end of the pilot study in order to make recommendations to the Trial Management Group (TMG) regarding progression to the full RCT. The first stage of the STOP/GO was based on which route of FMT to use for the full trial. The following data were reviewed by the IOC to inform this decision:
-
The proportion of participants in each arm who achieved clinical response (as defined below) at week 8 following FMT.
-
Tolerability of each route assessed by the proportion of participants who were considered adherent in each arm. Adherence was defined as participants who received FMT by the route to which they were randomly allocated and received ≥70% of the intended dose.
-
Safety assessed by the proportion of participants who experienced an AE or SAE, and number of events in each arm.
-
Patient and clinician acceptability of FMT (in each arm) described via qualitative interview data (including advantages and disadvantages of each route).
If the IOC were able to recommend a route of FMT delivery, the second stage of the STOP/GO was to determine whether a full-scale RCT was feasible. The following criteria were reviewed by the IOC to inform this decision:
-
Recruitment of 30 participants in the pilot averaged 0.7 participants per week in each open site, including assessment of the potential barriers and facilitators to patient participation in the study through the qualitative interviews.
-
That 10 of the 15 participants in the route cohort selected for the main study were considered adherent.
-
That the IOC had not identified any safety concerns.
Clinical outcome measures
-
Clinical response (primary measure of efficacy) defined as ≥3 point reduction in the full Mayo score (see Appendix 3) from randomisation to week 8, and 30% reduction from randomisation and at least one point reduction of rectal bleeding subscore or an absolute rectal bleeding subscore of 0 or 1.
-
Time to clinical response (where clinical response was defined as ≥2 point reduction in partial Mayo).
-
Clinical remission at week 8 (full Mayo score of ≤2, with no subscore >1).
-
Participant’s weight at weeks 8 and 12.
-
QoL using the SF-36 and the IBDQ at weeks 8 and 12.
-
CRP at weeks 4, 6 and 8.
-
Adherence to FMT.
-
AEs and SAEs.
Qualitative research
-
Patient and clinician acceptability of FMT and preference of treatment route was assessed through semi-structured qualitative research interviews (see Appendix 4).
Translational and exploratory outcome measures
-
Faecal calprotectin.
-
Measures of patient microbiome (faecal and mucosal).
-
Mucosal healing.
-
Urinary metabolome (SCFA).
-
Donor faecal microbiome.
-
Association between the donor’s dietary profile and microbiome.
-
Time (days) from stool donation processing to treatment of the patient.
Further details of the qualitative and translational research outcomes can be found in Chapters 4 and 5, respectively.
Statistical considerations
Sample size
For this pilot study, no formal sample size calculation was undertaken as the study was not designed or powered to detect a statistically significant difference in efficacy between the two FMT methods of delivery. The recruitment target for this study was 30 patients.
Statistical analysis
Baseline characteristics were summarised with numbers and percentages for categorical variables, means and standard deviations (SDs) for normally distributed continuous variables, or medians and interquartile ranges (IQR) for non‐normal continuous variables.
For the STOP/GO criteria, clinical response was summarised using the number of responses and percentages. The binomial normal approximation was used to generate 95% confidence intervals (CIs) around the proportion of participants who achieved a clinical response in the NG arm and the colonic arm separately. Treatment adherence was analysed in the same manner as clinical response. AEs and SAEs were summarised descriptively only using the number of responses and percentages, alongside the number of events.
For clinical response (primary measure of efficacy for STOP/GO), a log-binomial model was also fitted to generate a risk ratio (RR) (and 95% CI) adjusting for the minimisation variable smoking status and baseline full Mayo score. Clinical remission was reported using the number of responses and percentages. A log-binomial model was fitted to generate a RR (and 95% CI) adjusting for the minimisation variables. Continuous clinical outcome measures (weight and QoL measures) were summarised using means and SDs, and a linear model was fitted to generate mean differences between arms (and 95% CI) adjusting for the minimisation variables and baseline value. Time to clinical response (using the partial Mayo score) was summarised using the number of events. A Cox regression model was used to generate a hazard ratio (HR) (and 95% CI) adjusting for the minimisation variables. A Kaplan–Meier plot was also produced by treatment arm. All analyses were based on the intention‐to‐treat (ITT) principle using complete case data. No formal hypothesis testing was undertaken and no p-values are presented. No subgroup analyses were performed and sensitivity analyses were limited to clinical response only. These included a per-protocol analysis and an assessment of missing primary outcome data by means of a worse-case analysis under two scenarios. For scenario A, any participants with missing outcome data in the NG arm were treated as a treatment failure (participant did not achieve clinical response) and missing outcome data in the colonic arm were treated as a treatment success (participant did achieve clinical response). For scenario B, any participants with missing outcome data in the NG arm were treated as a treatment success (participant did achieve clinical response) and missing outcome data in the colonic arm were treated as a treatment failure (participant did not achieve clinical response). All analyses were performed using SAS version 9.4 and Stata version 16 or higher.
Patient and public involvement
Our protocol was developed in consultation with a patient and public involvement (PPI) group referred to as the Clinical Research Ambassador Group, based at University Hospitals Birmingham NHS Foundation Trust. One of the co-applicants was a patient with IBD and also Chairperson for the West Midlands Group of Crohn’s and Colitis UK (CCUK), and they helped develop the proposal. The PPI group has strong links with CCUK, which will aid dissemination of findings nationally to patients, relatives and health professionals.
As we were aware that the acceptability of FMT in UC had not been extensively studied, we designed the pilot study to investigate patient acceptability of the therapy by regular questionnaires, individual interviews and group discussions. We conducted a survey of our own IBD patients from the outpatient department at University Hospital Birmingham. Of the 74 patients surveyed, the vast majority of patients would accept FMT as a treatment for IBD and over 80% would consider involvement in a trial of FMT in IBD. Qualitative interviews with patients would be conducted at baseline and at the end of the study and these findings would feed into the discussions regarding progression to a full-scale RCT (see Chapter 4).
Trial oversight
Study oversight was provided by an IOC that was chaired by Dr Robert Logan (Consultant Gastroenterologist at King’s College NHS Foundation Trust). The IOC provided independent oversight for the trial, and provided advice to the chief investigator and coinvestigators on progression to a full RCT.
Chapter 3 Results of clinical study
This chapter reports the results from the quantitative data of the pilot study.
Recruitment
Between March 2018 and April 2019, 76 patients with active UC were assessed for eligibility. Of these, 37 were registered and 30 were subsequently randomised into the pilot study; 16 to the NG arm and 14 to the colonic arm. Follow-up was completed in July 2019.
Reasons for non-participation are provided in the Consolidated Standards of Reporting Trials flow diagram (Figure 1). The recruitment rate was slower than anticipated with the average number of participants recruited per week being 0.53. Seven participants in the NG arm and two participants in the colonic arm withdrew from the trial.
FIGURE 1.
Consolidated Standards of Reporting Trials flow chart.
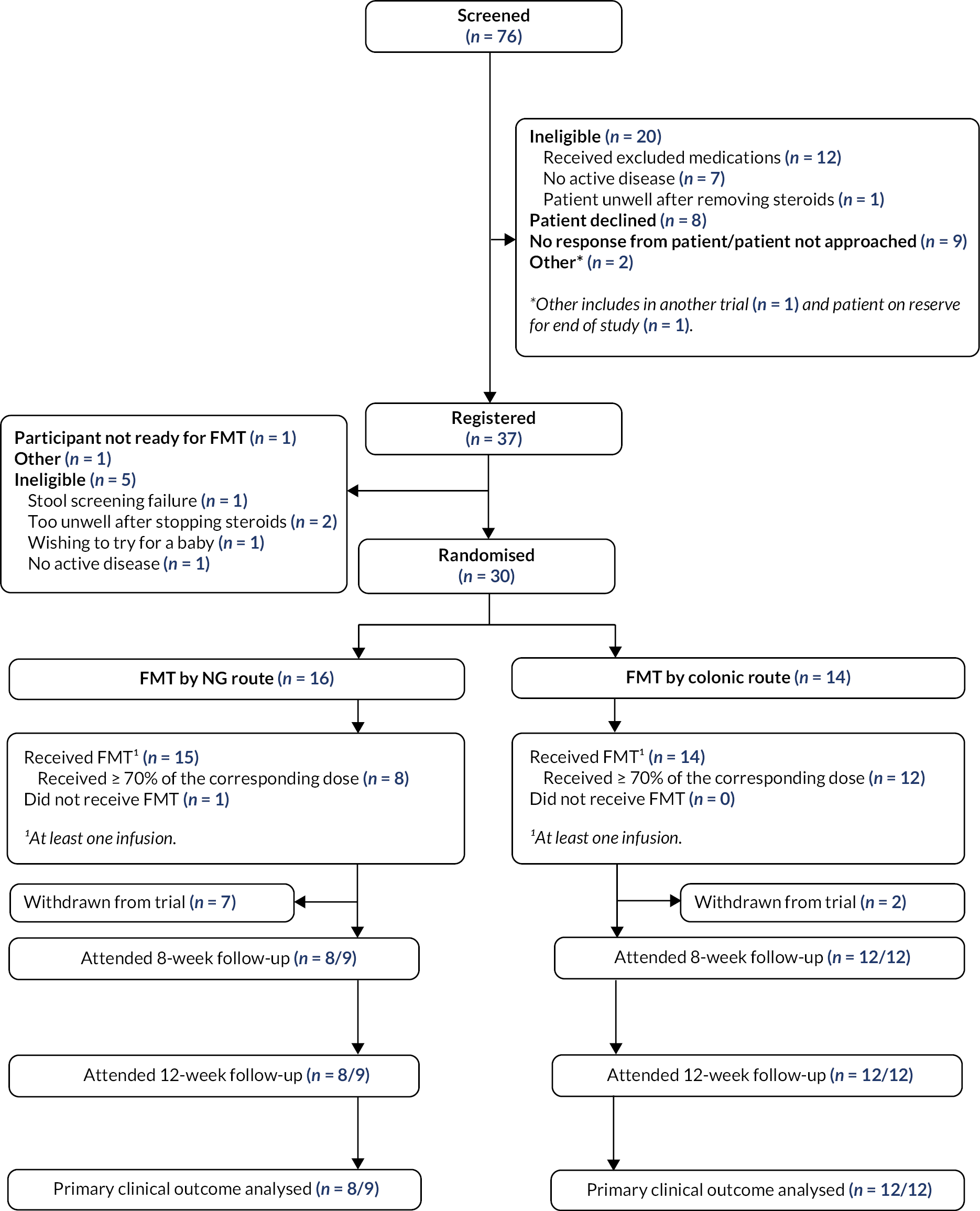
Participant characteristics
Baseline participant demographics, disease activity and inflammation indices appeared generally well balanced between the two treatment arms. Participants were younger in the NG arm than the colonic arm (mean age 37.3 vs. 46.1 years) and appeared to have a more recent diagnosis of UC than those participants in the colonic arm (time since diagnosis of UC 4.4 vs. 8.5 years) (Table 2). Mean faecal calprotectin was lower in the NG arm compared with the colonic arm at baseline [670.8 vs. 750.7 (mg/kg)2].
| FMT NG route (N = 16) | FMT colonic route (N = 14) | |
|---|---|---|
| Recruiting centre – n (%) | ||
| Queen Elizabeth Hospital, Birmingham | 9 (56) | 8 (57) |
| St Mark’s Hospital, London | 2 (13) | 3 (21) |
| Glasgow Royal Infirmary | 5 (31) | 3 (21) |
| Partial Mayo scorea,b – n (%) | ||
| 4–5 | 5 (31) | 5 (36) |
| 6–8 | 11 (69) | 9 (64) |
| Full Mayo scorec – mean (SD, N) | 7.8 (1.3, 16) | 8.1 (1.5, 14) |
| Current smokerb,d – n (%) | 2 (13) | 2 (14) |
| Duration of diagnosis of UC (years)e – median (IQR, N) | 4.4 (1.5–6.4, 16) | 8.5 (3.0–12.0, 14) |
| Disease extent – n (%) | ||
| Left-sided disease | 9 (56) | 9 (64) |
| Pancolitis | 7 (44) | 3 (22) |
| Proctitis | 0 (–) | 2 (14) |
| Gender – n (%) | ||
| Male | 9 (56) | 5 (36) |
| Female | 7 (44) | 9 (64) |
| Age at randomisation (years) – mean (SD, N) | 37.3 (11.0, 16) | 46.1 (11.7, 14) |
| Weight at registrationf (kg) – mean (SD, N) | 77.0 (24.9, 16) | 73.9 (14.1, 14) |
| BMI at randomisation (kg/m²) – mean (SD, N) | 25.8 (7.2, 16) | 24.9 (5.1, 12) |
| Previous biologics used – n (%) | ||
| Infliximab | 3 (19) | 3 (21) |
| Vedolizumab | 4 (25) | 2 (14) |
| Adalimumab | 1 (6) | 0 (–) |
| Golimumab | 1 (6) | 1 (7) |
| Taking maintenance therapy for UCg – n (%) | 13 (81) | 14 (100) |
| Oral 5ASA compound | 10 | 12 |
| Immunosuppressantsh | 5 | 3 |
| Other concomitant medications | 5i | 7j |
| Haemoglobin (g/l) – mean (SD, N) | 126.0 (18.9, 16) | 129.6 (17.1, 14) |
| Albumin (g/l) – mean (SD, N) | 41.9 (5.3, 15) | 43.5 (3.9, 13) |
| CRP (mg/l) – mean (SD, N) | 11.3 (13.0, 16) | 4.2 (3.4, 14) |
| Faecal calprotectin [(mg/kg)2] – mean (SD, N) | 670.8 (582.6, 11) | 750.7 (343.7, 11) |
STOP/GO criteria: stage 1
The IOC met in July 2019 to discuss the trial progress and to review the data against the pre-agreed STOP/GO criteria in order to make a recommendation as to continuation to a full RCT. The STOP/GO was a two-stage process, with the first stage assessing which route of FMT delivery to use for the full trial. The quantitative assessment was based on clinical response, tolerability and safety of FMT via each route of delivery.
Clinical response
Clinical response (the primary measure of efficacy) was achieved in 2 of 8 participants (25%, 95% CI 0% to 55%) in the NG arm compared with 9 of 12 participants (75%, 95% CI 51% to 100%) in the colonic arm (RR 2.94, 95% CI 0.84 to 10.30). Results from pre-specified sensitivity analyses drew similar conclusions with the primary analysis (Table 3).
| FMT NG route (N = 16) | FMT colonic route (N = 14) | Adjusted RRa (95% CI) | |
|---|---|---|---|
| Clinical response-ITT analysis | |||
| Yes | 2 (25) | 9 (75) | 2.94 (0.84 to 10.30) |
| No | 6 (75) | 3 (25) | |
| Missingb | 8 | 2 | – |
| Clinical response-per-protocol analysisc | |||
| Yes | 2 (25) | 9 (75) | 2.94 (0.84 to 10.30) |
| No | 6 (75) | 3 (25) | |
| Clinical response-sensitivity analysis for missing data (scenario A) | |||
| Yes | 2 (13) | 11 (79) | 6.30 (1.68 to 23.58) |
| No | 14 (87) | 3 (21) | |
| Clinical response-sensitivity analysis for missing data (scenario B) | |||
| Yes | 10 (63) | 9 (64) | 0.98 (0.57 to 1.68) |
| No | 6 (37) | 5 (36) | |
Tolerability and safety
The number and proportion of participants who were deemed adherent was 8/16 (50%, 95% CI 26% to 75%) in the NG arm compared with 12/14 (86%, 95% CI 67% to 100%) in the colonic arm. Table 4 provides further details regarding adherence to FMT in each arm.
| FMT NG route | FMT colonic route | |
|---|---|---|
| Adherenta – n/N (%) | 8/16 (50) | 12/14 (86) |
| Total FMT dose received (mg) | ||
| Median (IQR, N) | 195 (75–240, 16) | 360 (360–360, 14) |
| Minimum–maximum | 0–240 | 210–360 |
| Number of FMT infusions received – n/N (%) | ||
| 0 | 1/16 (6) | 0/14 (–) |
| 1 | 3/16 (19) | 0/14 (–) |
| 2 | 0/16 (–) | 0/14 (–) |
| 3 | 0/16 (–) | 1/14 (7) |
| 4 | 3/16 (19) | 1/14 (7) |
| 5 | 1/16 (6) | 0/14 (–) |
| 6 | 0/16 (–) | 0/14 (–) |
| 7 | 0/16 (–) | 1/14 (7) |
| 8 | 8/16 (50) | 11/14 (79) |
The number of participants who experienced an AE in the NG arm was 11/16 (69%), and 30 AEs were reported; the most common AE being abdominal pain. The number of participants who experienced an AE in the colonic arm was 11/14 (79%), and 26 AEs were reported; the most common AE being diarrhoea (Table 5). Two participants in the NG arm experienced three SAEs. One participant experienced two separate episodes of abdominal pain, nausea, vomiting and constipation/diarrhoea. One participant with moderate to severe UC was admitted to hospital after experiencing fever (38.9 °C), tachycardia and abdominal pain immediately after administration of their first FMT infusion. Both participants who experienced a SAE ultimately withdrew from subsequent follow-up. No SAEs were reported in the colonic arm.
| FMT NG route | FMT colonic route | |
|---|---|---|
| Participants experiencing an AE – n/N (%) | 11/16 (69) | 11/14 (79) |
| Number of AEs reported – N | 30 | 26 |
| Diarrhoea | 4 | 10 |
| Nausea | 5 | 1 |
| Vomiting | 3 | 1 |
| Abdominal pain | 7 | 4 |
| Hypotensiona | 0 | 0 |
| Pyrexia (≥38ºC) | 1 | 0 |
| Tachycardiab | 1 | 0 |
| Perforation due to colonoscopy | 0 | 0 |
| Haemorrhage due to colonoscopy | 0 | 0 |
| Other | 9 | 10 |
| Participants experiencing a SAE – n/N (%) | 2/16 (13) | 0/14 (–) |
| Number of SAE’s reported – N | 3c | – |
Based on the quantitative data reported above and the qualitative data reported in Chapter 4, the IOC recommended that the colonic route could be taken forward to a full trial of FMT versus placebo.
STOP/GO criteria: stage 2
The second stage of the STOP/GO was to determine whether a full-scale RCT was feasible. This decision was based on recruitment, tolerability and safety.
The recruitment target was 30 participants, with an average recruitment rate of 0.7 participants per week in each open centre. The recruitment rate was 0.30 participants per week at Birmingham, 0.18 participants per week at Glasgow and 0.17 participants per week at London.
The tolerability criteria in stage 2 were based on 10 of the 15 participants in the route selected for the main study received at least 70% of their intended FMT dose. This was achieved in the colonic arm, with 12 of 14 participants receiving at least 70% of their intended dose. The IOC also had no safety concerns.
The IOC recognised that although the pilot study did not satisfy the STOP/GO in relation to recruitment rates, the pilot did recruit the planned 30 participants. The IOC felt that it could still recommend moving forward to a full trial, but recommended that an internal pilot study was included in the design of the main trial, with clear progression rules set in relation to recruitment targets.
Other clinical measures
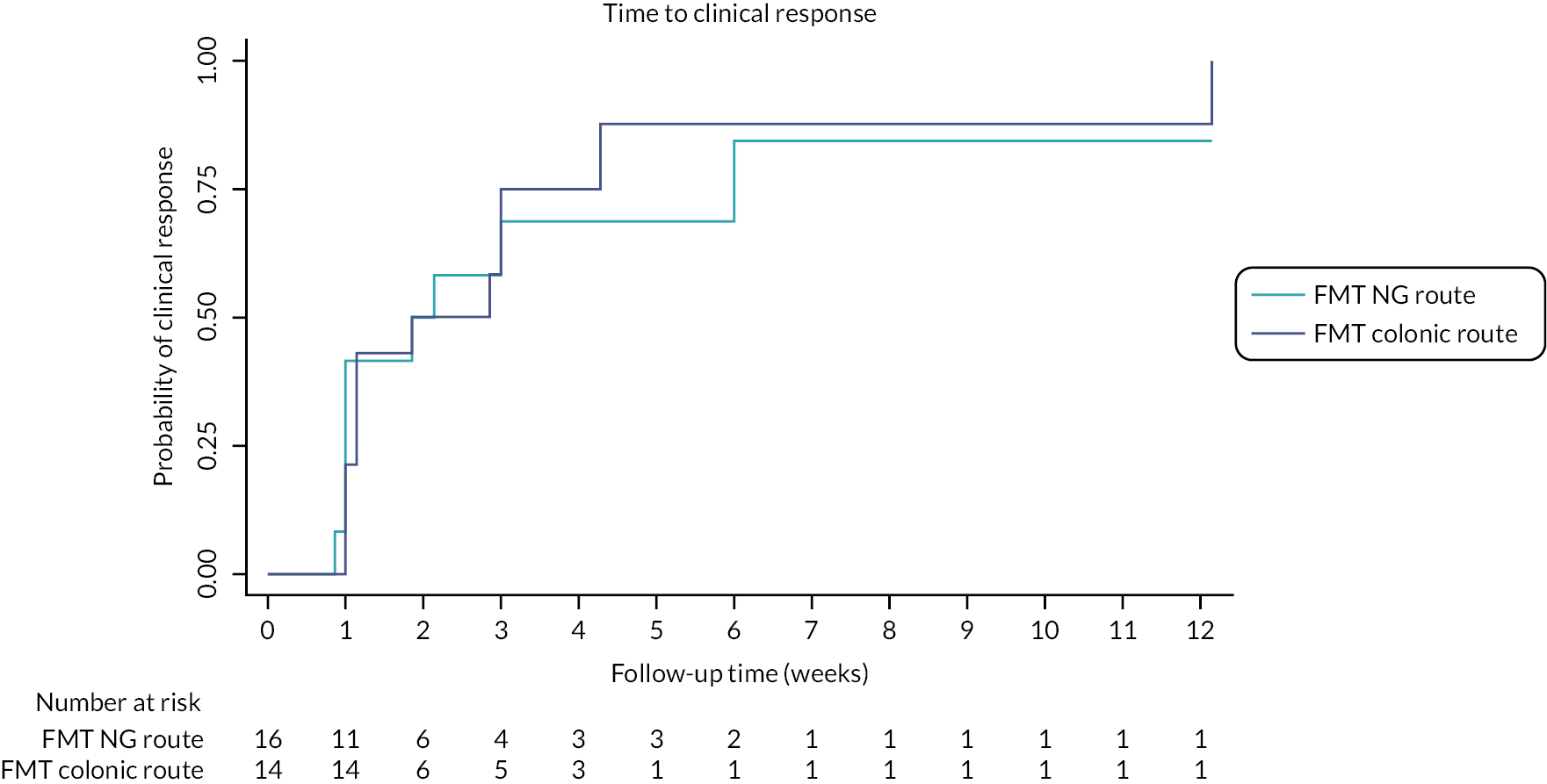
More participants in the colonic arm (6/12; 50%) achieved clinical remission compared with the NG arm (2/8; 25%) (RR 1.89, 95% CI 0.51 to 6.99) (see Table 6). Clinical response based on the partial Mayo (defined as ≥2 point reduction in partial Mayo from randomisation) was observed in 9/16 (56%) in the NG arm and 12/14 (86%) participants in the colonic arm (HR 1.16, 95% CI 0.48 to 2.80) (see Table 6 and Figure 2). Mucosal healing, defined as an endoscopic subscore of zero on the Mayo at week 8, occurred in 2/8 (25%) in the NG arm and 3/12 (25%) in the colonic arm.
| Baselinea | Week 8 | Week 12 | ||||||
|---|---|---|---|---|---|---|---|---|
| FMT NG route | FMT colonic route | FMT NG route | FMT colonic route | Estimate (95% CI) | FMT NG route | FMT colonic route | Estimate (95% CI) | |
| Clinical remission – n/N (%) | – | – | 2/8 (25) | 6/12 (50) | 1.89b (0.51 to 6.99) | – | – | – |
| Time to clinical responsec – n/N (%) | – | – | – | – | – | 9/16 (56) | 12/14 (86) | 1.16d (0.48 to 2.80) |
| Weight (kg) – mean (SD, N) | 77.0 (24.9, 16) | 73.9 (14.1, 14) | 72.5 (17.4, 7) | 75.1 (14.0, 12) | −0.24e (−2.17 to 1.69) | 74.7 (16.8, 8) | 73.7 (12.2, 10) | 0.19e (−3.21 to 3.59) |
| Faecal calprotectin (mg/kg) – mean (SD, N) | 670.8 (582.6, 11) | 750.7 (343.7, 11) | 640.3 (456.6, 6) | 770.4 (445.3, 10) | 33.4f (−480.1 to 547.0) | 742.3 (494.8, 6) | 504.0 (585.8, 9) | −111.7f (−701.0 to 477.7) |
FIGURE 2.
Kaplan–Meier plot of time to clinical response.
At week 8, the mean CRP was 10.2 mg/l (SD 13.3 mg/l) in the NG arm and 4.2 mg/l (SD 4.1 mg/l) in the colonic arm (mean difference −0.02, 95% CI −5.8 to 5.8) (see Table 7). CRP values at weeks 4, 6 and 8 were all lower in the colonic arm compared with the NG arm, although the CRP values at baseline were also lower in the colonic arm. The mean faecal calprotectin was 640.3 mg/kg (SD 456.6 mg/kg) in the NG arm and 770.4 mg/kg (SD 445.3 mg/kg) in the colonic arm at 8 weeks (mean difference 33.4, 95% CI −480.1 to 547.0), and 742.3 mg/kg (SD 494.8 mg/kg) in the NG arm and 504.0 mg/kg (SD 585.8 mg/kg) in the colonic arm at 12 weeks (mean difference −111.7, 95% CI −701.0 to 477.7). Participant weight at 8 and 12 weeks were similar between arms (Table 6).
| Baseline | Week 4 | Week 6 | Week 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FMT NG route | FMT colonic route | FMT NG route | FMT colonic route | Estimatea (95% CI) | FMT NG route | FMT colonic route | Estimatea (95% CI) | FMT NG route | FMT colonic route | Estimatea (95% CI) | |
| CRP (mg/l) – mean (SD, N) | 11.3 (13.0, 16) | 4.2 (3.4, 14) | 14.1 (28.9, 11) | 5.9 (6.3, 13) | −9.0 (−27.7 to 9.7) | 13.0 (13.2, 13) | 6.9 (10.2, 10) | −2.6 (−12.7 to 7.4) | 10.2 (13.3, 10) | 4.2 (4.1, 11) | −0.02 (−5.8 to 5.8) |
Quality-of-life measures
At 8 and 12 weeks, participant QoL scores using the IBDQ and SF-36 were similar between arms. Summary scores and point estimates are provided in Table 8.
| Baseline | Week 8 | Week 12 | ||||||
|---|---|---|---|---|---|---|---|---|
| FMT NG route | FMT colonic route | FMT NG route | FMT colonic route | Mean differencea (95% CI) | FMT NG route | FMT colonic route | Mean differencea (95% CI) | |
| Inflammatory bowel disease questionnaireb – mean (SD, N) | ||||||||
| Total | 119.1 (31.4, 15) | 129.3 (19.9, 14) | 140.7 (51.6, 8) | 171.7 (35.8, 11) | 24.95 (−25.85 to 75.75) | 140.8 (42.6, 8) | 179.3 (30.2, 10) | 20.03 (−21.91 to 61.98) |
| Bowel systems | 3.8 (1.2, 15) | 4.0 (0.6, 14) | 4.9 (1.3, 8) | 5.5 (1.1, 11) | 0.74 (−0.60 to 2.09) | 4.8 (1.0, 8) | 5.7 (1.1, 10) | 0.78 (−0.37 to 1.93) |
| Systemic systems | 3.3 (1.0, 15) | 4.0 (0.9, 14) | 3.9 (1.5, 8) | 5.2 (1.1, 12) | 0.91 (−0.47 to 2.29) | 3.9 (1.3, 8) | 5.5 (0.8, 10) | 1.10 (−0.25 to 2.46) |
| Emotional health | 3.6 (1.2, 15) | 4.0 (0.8, 14) | 4.1 (1.7, 8) | 5.1 (1.3, 11) | 0.56 (−1.31 to 2.43) | 4.2 (1.4, 8) | 5.4 (1.0, 10) | 0.51 (−1.00 to 2.02) |
| Social function | 4.3 (1.6, 15) | 4.4 (1.1, 14) | 4.5 (2.5, 8) | 5.7 (1.0, 12) | 1.37 (−0.51 to 3.24) | 4.5 (2.3, 8) | 6.0 (1.2, 10) | 1.39 (−0.81 to 3.59) |
| Short-form 36 questionnairec – mean (SD, N) | ||||||||
| Physical functioning | 76.1 (20.7, 14) | 77.1 (22.6, 14) | 75.0 (24.8, 8) | 80.0 (27.1, 12) | 6.76 (−18.48 to 31.99) | 81.9 (17.3, 8) | 80.0 (27.6, 10) | −2.16 (−28.98 to 24.66) |
| Role limitations due to physical problems | 75.0 (35.4, 14) | 75.0 (29.4, 14) | 46.9 (47.1, 8) | 43.8 (44.1, 12) | 8.45 (−45.81 to 62.71) | 59.4 (49.9, 8) | 27.3 (46.7, 11) | −34.05 (−99.80 to 31.70) |
| Role limitations due to emotional problems | 64.4 (40.8, 15) | 54.8 (46.4, 14) | 45.8 (50.2, 8) | 27.8 (39.8, 12) | −30.04 (−77.79 to 17.71) | 62.5 (41.5, 8) | 18.2 (40.5, 11) | −40.98 (−84.70 to 2.73) |
| Energy/fatigue | 25.5 (16.3, 11) | 31.2 (19.4, 13) | 34.3 (23.7, 7) | 56.3 (25.7, 12) | 15.16 (−17.12 to 47.45) | 35.0 (27.9, 6) | 53.0 (27.3, 10) | 18.96 (−15.13 to 53.06) |
| Emotional well-being | 53.6 (15.1, 15) | 63.7 (19.4, 14) | 48.0 (19.1, 8) | 64.7 (25.3, 12) | 13.00 (−13.08 to 39.07) | 53.5 (20.9, 8) | 69.5 (22.6, 11) | 9.95 (−14.36 to 34.26) |
| Social functioning | 41.3 (27.2, 13) | 53.8 (15.6, 13) | 50.0 (28.0, 7) | 77.5 (21.9, 10) | 18.84 (−31.30 to 68.98) | 50.0 (12.5, 7) | 77.8 (25.6, 9) | 34.08 (−13.78 to 81.94) |
| Pain | 55.2 (25.0, 15) | 59.1 (24.7, 14) | 56.9 (32.4, 8) | 73.1 (27.9, 12) | 5.57 (−23.09 to 34.24) | 60.0 (26.6, 8) | 81.4 (19.2, 11) | 24.57 (−4.92 to 54.05) |
| General health | 32.7 (15.0, 15) | 39.6 (18.1, 14) | 30.6 (17.0, 8) | 45.4 (21.8, 12) | 4.01 (−16.88 to 24.91) | 30.0 (15.8, 8) | 53.5 (20.0, 10) | 10.09 (−9.04 to 29.23) |
Conclusion
In this small pilot study comparing two different routes of FMT administration for the treatment of UC, we observed a difference in adherence to treatment between the NG and colonic routes. Adherence was defined as receiving at least 70% of the prescribed treatment. Only 8 out of 16 participants randomised to the NG arm were adherent compared to 12 out of 14 randomised to the colonic arm. Of those who were adherent to the NG route, 2 out of 8 responded compared to 9 of 12 participants treated via the colonic route. A high proportion of participants in both treatment arms experienced an AE (>65% of participants). These were generally transient and typical of the mild and transient symptoms usually seen in the first 24 hours after FMT in routine clinical practice in the management of Clostridioides difficile infection (CDI); self-limiting diarrhoea and mild abdominal discomfort. Three SAEs were seen in two participants treated by the NG route both of whom withdrew.
At the end of the study, the IOC were able to recommend that the pre-specified STOP/GO criteria had been met in the colonic arm and this route of FMT delivery could be taken forward to be evaluated in the proposed efficacy RCT.
Chapter 4 Qualitative research
Qualitative objectives
The qualitative study undertaken as part of this study aimed to assess the following:
-
patient and clinician acceptability of FMT (NG route)
-
patient and clinician acceptability of FMT (COLON route).
Methods
Patient and clinician acceptability of FMT and preference of treatment route was assessed through semistructured qualitative research interviews (see Appendix 4 for interview schedules). Patients were interviewed face to face or by telephone at two time points; first following the screening visit and prior to randomisation, and second after the 12-week follow-up period. During these interviews, participants from both groups were asked about their perspectives and experience of FMT during the pilot trial, including receiving the intervention, recovering from the intervention, their symptoms and impact on QoL. Further examples of interview questions are available as part of the published trial protocol. 19 Additional interviews were conducted with patients who withdrew from FMT treatment early and with a small sample who declined to take part in the study. Interviews were also conducted with clinical staff to describe their views on the acceptability of the intervention and their experience of trial processes. Qualitative data were analysed using thematic approaches informed by the framework analytic approach. 23
Results: patient interviews
Interview sample
One patient who withdrew from the pilot trial prior to treatment allocation could not be contacted for interview. In total, we conducted interviews with 31 patients. Two patients who withdrew prior to treatment allocation took part in a one-off interview. Of the remaining patients; baseline and follow-up interviews were conducted with 19 patients who completed the pilot, and of those who withdrew after allocation 3 participated in a baseline and a withdrawal interview, 2 in a withdrawal interview only, and 5 did not want to participate in a withdrawal interview having already completed a baseline interview.
Patient interviewees were recruited from across all three pilot sites: Birmingham (n = 18), Glasgow (n = 7) and London (n = 6). The sample was made up of 15 men and 16 women, and included 20 patients from a white background, 10 from an Asian/Asian British background and 1 from a mixed/multiple ethnic background. The age of the patients ranged between 21 and 63 years. Patients had been diagnosed with UC between 4 months and 14 years before the baseline interviews.
We present the findings under two headings: (1) acceptability of the trial and FMT in principle and (2) acceptability of the trial and FMT in practice. Findings described as part of (1) relate to the baseline interviews undertaken prior to both randomisation and treatment where patients were talking hypothetically about their views regarding FMT. Findings described as part of (2) relate to follow-up interviews where interviewees have actual experience of FMT treatment and of the STOP-COLITIS pilot trial processes.
1. Acceptability of the STOP-COLITIS pilot and of FMT in principle (baseline interviews).
During the baseline interviews, patients discussed their
-
views and expectations related to the trial and FMT
-
knowledge and awareness of FMT
-
views on the acceptability of FMT as a treatment for UC
-
general concerns and considerations related to the trial and FMT
-
FMT administration route preferences (NG vs. colonic).
Views and expectations related to the STOP-COLITIS pilot and faecal microbiota transplant
In general, patients were enthusiastic about the STOP-COLITIS pilot and stated that the trial was a good idea. Patients also discussed what they might hope for and expect from FMT, including a reduction in symptoms, a hope to get back to ‘normal’, and, however unlikely, the hope of being cured. Sometimes the latter was because of a fear of developing colon cancer.
Patients also talked about a desire to take part in the STOP-COLITIS pilot due to the lack of alternative treatment options that were effective for them. Several patients felt that their medication had not been working and that the trial was a good opportunity to try something different:
Being at the end of the line with other medications, my consultants and nurse, my IBD nurse has advised me that there wasn’t any other options medication-wise aside from surgery, and then they said it’s worth considering.
Interviewee 14
Knowledge and awareness of faecal microbiota transplant prior to STOP-COLITIS
Before consenting to take part in STOP-COLITIS, most patients that we spoke to expressed at least some prior knowledge and awareness of FMT. Several stated that they were aware of FMT through personal interest and research:
It’s something I’ve done a lot of reading about and I’ve read a lot of success stories.
Interviewee 5
Several patients were aware that FMT was a treatment option for CDI. Other patients had only become aware of FMT in the run-up to the commencement of STOP-COLITIS via conversations with their consultant about the trial. Another interviewee who had not had earlier conversations about STOP-COLITIS talked about their initial surprise about the nature of FMT when it was mentioned to them at first by their consultant during recruitment:
I never heard of it, it sounds really weird when they mentioned it.
Interviewee 2
On the whole patients seemed to have a good understanding of FMT. When asked to give a definition of FMT, it was often described as a ‘donation of good bacteria’.
Views on the acceptability of faecal microbiota transplant as a treatment for ulcerative colitis
In principle, patients found the idea of FMT acceptable. Several likened the FMT process to a blood transplant, implying that if they did not mind receiving someone else’s blood, they would not mind receiving someone else’s stool:
Because I understand the concept of it [FMT] and that I could receive somebody else’s blood, and I could receive somebody else’s … other things.
Interviewee 4
Several interviewees stated that they considered it a more natural treatment than the medication that they had been taking. This was an important feature of FMT, and it was felt that it played a significant role in their decision to take part in the trial:
It’s natural, it’s not a pharmaceutical product, it’s not a chemical product, it’s from the donors, so it’s a natural resource if you like. So that to me is compelling.
Interviewee 20
Perhaps related to this, some patients seemed to consider FMT to be simple and uncomplicated:
I suppose it’s just it’s a really simple idea isn’t it? … by the sounds of it I could drink it couldn’t I? Let’s stop there, but it’s that simple realistically.
Interviewee 4
As well as finding FMT natural and simple, unlike their medication, some patients felt very at ease about the nature of FMT and showed no embarrassment when talking about it during interviews. This was reflected in how they referred to FMT. For instance, some interviewees appeared very comfortable using words such as ‘poo’ and ‘stool’:
Poo has been a big part of my life for ten years and I don’t think of it as a dirty word no more, so to speak.
Interviewee 4
Other patients, however, were not as open during their interviews and seemed to avoid using words such as ‘poo’ or ‘stool’, and even ‘colon’. Instead, they found other ways to refer to these. For example, when talking about their FMT route preference, some made a gesture to refer to the colon or called it ‘the other way’. In addition, several patients could not help laughing nervously when talking about FMT during the interviews, highlighting some awkwardness felt when talking about the nature of the treatment. As a result of this, a couple of patients told the interviewer that they did not tell their partners and work colleagues about the details of the FMT:
It’s just embarrassment really […] I haven’t told people at work, it’s one of those if people want to know about it, I just don’t want to talk about bowel problems unless people say or ask me. I am happy to talk about it, but I would rather they say what’s happening than me going this is what’s happening.
Interviewee 27
When discussing donors there wasn’t any consensus of views. Some expressed a preference for an anonymous donor, some that they would feel more comfortable with the donor being a friend or relative, and others that they had no preference.
General concerns and considerations related to the trial and faecal microbiota transplant
Although patients understood what the FMT intervention consisted of, several seemed slightly unclear about the process that the donation went through before being transplanted. Interviewees expressed a desire to know more about this process and suggested they could have been told more about it.
Other patients suggested that another concern was the potential for side effects and what these could be:
The only questions I would have had is 'are there any side effects?'. But who knows until you do it?
Interviewee 2
Other practical concerns regarding the FMT intervention were mentioned by several patients and they included issues such as getting to the hospital on time for the treatment, the cost of attending and being reimbursed for expenses incurred and being able to take time off work to attend hospital.
Faecal microbiota transplant administration route preferences
At baseline interview, all but one patient stated a preference to be allocated to the colonic route. Although most of the patients stated that they would still be happy to go ahead if they were allocated to the NG group, a minority of them admitted not being sure whether they would do so.
The main reason stated for the preference for the colonic route was patients’ familiarity with colonoscopies, therefore knowing what to expect:
I am familiar with the colonoscopy and enemas and things like that, and the fact that’s probably only going to be once a week for eight weeks. I probably if I had a choice would feel more comfortable with that route but only because of familiarity.
Interviewee 14
Other reasons given included the perception that the colonic route would be more effective and less painful than the NG route:
But also from a scientific point of view putting the bacteria into the base of the stomach which has got that … the low acidic pH and then having to work its way through the small intestine before it gets to the colon where it’s got to act, where if you are putting it straight into the colon that makes me think it would be more effective.
Interviewee 5
Because I’ve had the colonoscopy loads of times and I feel like it’s less painful and invasive.
Interviewee 28
The only slight concern regarding the colon route mentioned by one patient was whether or not FMT would be able to reach all of the colon:
It’s if it reaches far enough or will it get to where it needs to be?
Interviewee 2
Patients who preferred not to be randomised to the NG group did so because they feared the tube being inserted in the nose. As opposed to the colon route, patients were not familiar with the procedure and some said how they felt quite nervous and anxious about the prospect and any potential discomfort from the tube:
I don’t like anything in my nose or mouth, I’ve got an extremely bad gag reflex, extremely bad, to a point where I can be sick while brushing my teeth, and it’s always been that way since a child. Anything that’s anywhere near my nose or mouth I have always hated from as far back as I can remember.
Interviewee 8
Other concerns included the need to keep the tube in situ for 4 days while going out or going to work. Patients were concerned about how other people might look at them. They were also concerned about disruptions to work if needing to come to the hospital 4 days in a row, which for some, was difficult due to work commitments.
Although patients understood what the intervention entailed, several were confused about the way the donors’ samples would be transplanted, especially with the NG route and expressed some concerns around the acidity in the stomach preventing the stool sample from being effective:
I just didn’t understand how it can go into the stomach and you get all the stomach acid, and then it would have to get into the digestive system. I thought it would all be killed in the stomach basically.
Interviewee 3
2. Acceptability of the trial and FMT in practice (follow-up interviews).
During the follow-up interviews, patients discussed
-
their experience of receiving the FMT procedure
-
their experience of the trial and trial visits
-
impact on their symptoms and QoL
-
general concerns and considerations related to the trial and FMT
-
barriers to patient participation in trial
-
reasons for withdrawing from the pilot trial.
These observations are based on 24 interviews: 11 with patients allocated to the colonic route; 8 to the NG route; and 5 who withdrew from the pilot following allocation to the NG route and who agreed to take part in an interview. In this section, we present findings separately for the colonic and NG groups, starting with the colonic group.
Colonic group
Experience of the trial, trial visits and the faecal microbiota transplant procedure
At follow-up interview, patients allocated to the colonic route were on the whole very positive about the STOP-COLITIS pilot. They all reported to have been happy and relieved to have been allocated to the colonic group. Similar to baseline interview, several patients suggested that they would not have completed the pilot trial had they been allocated to the NG group:
I don’t think I would have done, no, because that’s my main worry, that I wanted to go on the trial, and my main worry I was getting so tearful about the run up to it thinking … I don’t think I could have gone that way. So when … and the … when they came back and they randomised it and they came back into the hospital ward the two nurses was like oh [name] guess what? And I was like oh God what, and then the doctor come in and they went you’ve got the colonic and I was like yeah, and I was jumping up and down, I really was ecstatic.
Interviewee 9
Attending the clinical research facility once a week for 8 weeks was not seen as an issue for patients. They believed that it was easier for them to attend 1 day during 8 weeks rather than 4 days in a row twice during the trial.
Patients found the administration of the FMT via the colonic route to be as comfortable as expected. Although the colonoscopy was slightly uncomfortable for some, no major issues were reported, highlighting, as per baseline interview that participants were already used to the procedure.
No interviewees reported being able to smell or see the colonic FMT during the procedure. Patients from the colonic group also found the recovery period to be very straightforward.
Perceptions of treatment effect
During the follow-up interview with those allocated to colonic FMT, the majority of patients reported noticing improvements in their symptoms during the pilot trial. These included less urgency, fewer or no stomach pains, less frequent bowel movements, less or no blood or mucus in stools, and better formed stools. The following quote illustrates how one patient described not feeling debilitating urgency anymore leading to a feeling of regaining control over the condition:
Well it’s all improved, I’ve got more control, I had to go to the toilet before, I would get the sudden urge, and if I wasn’t, I don’t know, five minutes, four minutes from the toilet I might have an accident. Now I feel like I’ve got to go I know I’ve got at least half an hour or so to plan when I’m going, so I can hold on where I used to not be able to do ... But that’s the big improvement for me, I do still get the … I still occasionally get that I feel a bit bloated, but that might have been how it used to be before I got the condition, I can’t really remember what’s normal if you know what I mean. But apart from that it’s all been good.
Interviewee 19
Although the perceived and reported level of improvements varied between participants, the majority discussed considerable improvements in their symptoms. A small number reported that they were now ‘symptom-free’:
There’s actually a 100% improvement now. I’ve got no symptoms at all.
Interviewee 14
The timing of improvements in symptoms varied between participants. While some reported noticing improvements following the first trial treatment, others reported seeing improvements from weeks 2 or 3:
The first day of having the treatment, straight away I noticed an improvement.
Interviewee 14
During the trial actually after about two or three weeks after starting FMT there was improvement.
Interviewee 10
A few interviewees in the colonic group reported decreasing their medication as a result of the intervention:
I was on Salofalk foam and suppositories, so stopped the foam but I just do the suppository now one a night, and then the other tablets I’m on, the oh gosh, I can’t remember the name now, balsalazide, it’s half dose.
Interviewee 2
Patients who discussed improvements in symptoms reported feeling happier, being less anxious, less fatigued, sleeping better and having more energy. They also mentioned being able to engage in activities that they had not been able to before the pilot trial, such as going to the gym, socialising with friends or planning a holiday:
One thing I have done is booked holidays. I’ve booked a couple. I’m going away with the confidence that I am not going to be a problem on the airplane or anything like that.
Interviewee 4
While patients on the whole reported improvements in their symptoms during the pilot, some said that these improvements did not last right up until the 12-week follow-up interview, at least not as first experienced. A few patients said that these improvements only lasted a few weeks and that some of their symptoms came back, for some during treatment:
Right after … two weeks after we stopped FMT my symptoms they came back.
Interviewee 23
Well it’s been a bit of a rollercoaster if I’m honest. I saw some really good improvements for a couple of weeks during the trial, and I can honestly say that was the best I felt… I felt normal for two weeks, and it made me realise just how rubbish I feel most of the time if I’m honest. It wasn’t perfect for me unfortunately, I still had a couple of what I call little flares if you like, I did see blood and mucus once.
Interviewee 10
Some patients who were still experiencing improvements in their symptoms at the follow-up interview expressed concern regarding the longevity of these improvements. They thought that this would only be a temporary situation and that their pre-FMT symptoms would eventually return:
I just wonder how long it [the improvements] will last … if it does go back into remission or when it goes back then at least I know something could be done for it.
Interviewee 4
However, in general, when patients reported a lack of sustained improvement they stated that when symptoms returned they were less severe than prior to the pilot trial:
To be honest no, so the symptoms were not as big, as many of them … it was milder than before.
Interviewee 23
Without longer follow-up, it is not possible to say anything about interviewees’ experience of symptom remission or relapse beyond the 12-week follow-up interview.
Nasogastric group
Experience of the trial, trial visits and the faecal microbiota transplant procedure
Overall, patients reported having mixed feelings about their participation in the NG group, with the majority of them being disappointed at having been allocated to this group. Attending the clinical research facility for 4 days in a row was not always practical, especially for participants that lived far from the hospital. Of the patients allocated to the NG group that took part in an interview after the procedure just over half reported that their experience of tube insertion was negative, being uncomfortable, painful and even distressing:
It was pretty horrible to be honest. The tube was just horrible, the putting it in, the keeping it in, the … it was just invasive is the only way to describe it.
Interviewee 5
Other patients stated that the insertion of the tube had been relatively comfortable:
I found that okay, so I know there were other patients that struggled with it, but if you’re talking about so I had the NG tube, and I found it was a couple of seconds of discomfort as they inserted it … then just a couple of seconds drawing it out where it’s a bit uncomfortable. But the way I looked at it was in total about a minute’s worth of discomfort in your day is not really a huge deal.
Interviewee 7
Interviewees also reported irritation of the nose and experiencing the gag reflex. Most of the patients who withdrew from the pilot trial whom we interviewed did so because they could not tolerate the tube:
Yeah it did go in, I had it in for a day, and then after a day I took it out, because I just couldn’t tolerate it, and they tried to put it back in but because one my nose had already had a blood clot and everything from it.
Interviewee 6
Some of the patients allocated to the NG group that we spoke to chose to keep the tube in situ for the 4 days of intervention delivery. They discussed the difficulties they encountered during these 4 days, including difficulty in eating, drinking, swallowing and sleeping:
It was unpleasant … the first time was such a shock, and then by the second time I was out in the supermarket and doing other things and trying not to let it get in the way too much […] It wasn’t pleasant, eat and drink were less solid, I just tried to have things that would slip down easily, because it pushed it back up if I swallowed in a way, it pushed in an unpleasant way.
Interviewee 29
Things aren’t meant to go up like that up your nose, and just keeping it in and trying to swallow around it, because it’s designed for when people can’t swallow, so when you can swallow and you are trying to eat or drink anything, I couldn’t eat or drink properly when it was in, and sleeping was more of a nightmare than normal, just because it’s always there and it’s down the back of your throat, and if it moves it triggers your gag reflex, and the actual process of putting it in.
Interviewee 5
More patients preferred to take the tube out after each day because of the discomfort and the pain they thought the tube might cause them and the way they believed other people would look at them:
It literally takes seconds to whip it out and put it back in again, and actually when it’s in there is discomfort with it being in because every time it moves ever so slightly it hits against your nose which is very sensitive, so your eyes water and it’s just uncomfortable. So you have to worry about it and wander around with it sticking out your nose all day, it made much more sense to just whip it out and put it back in again.
Interviewee 7
Patients praised the clinical staff who delivered the intervention through the NG route and acknowledged the way they tried to make the process of inserting the tube as painless as possible:
The nurses were quite good at running a bit of distraction whilst they were putting the … so they flush it through first with something like non-FMT, and then they add the FMT and then they flush it through, and the only way I could tell when the FMT started it was slightly colder because it had been defrosted, and so the nurses were very good at basically hiding it.
Interviewee 7
Nevertheless, a couple of patients highlighted a perceived lack of procedural experience of some of the nurses, which they believed, resulted in the procedure being more uncomfortable for them. In addition, they mentioned having different nurses inserting the tube every day and a preference for continuity.
As well as patients describing how cold the FMT felt as it went down the NG tube, a small number reported smelling the FMT during the procedure:
I could smell when they are taking the tube out you can right at the end every time I went in you could smell it slightly just for about a second or so, but that was about it.
Interviewee 13
It did have a certain smell … as they were putting it in tried to hold my breath and not to smell or look at it at all.
Interviewee 30
Perceptions of treatment effect
Similar to the colonic group, the majority of patients whom we interviewed at follow-up reported some improvement in symptoms, including less urgency, fewer bowel movements, less stomach pain, better quality of stools and less blood or mucus in stool. Unlike the colonic group, those reporting improvements did not mention that these improvements tailed off after finishing treatment. A few patients reported substantial improvements in their symptoms:
I don’t see no blood, nothing really, I don’t see no blood and my stool has before it used to be all watery but now it’s formed, and before I used to get pain in my stomach, cramps and pain, and now the pain it’s gone. I just get a pain when I have the urgency to go to the bathroom, but apart from that it’s gone, so I am very happy.
Interviewee 21
Again similar to the colonic group, a small number of patients saw little change in their symptoms:
To be honest I am no different.
Interviewee 5
It should be noted that four patients who withdrew from the pilot trial in the NG group did not want to participate in a follow-up interview. Patients who reported improvements in their symptoms also reported feeling happier and having a better QoL.
Reasons for withdrawing from pilot trial
Patients who withdrew from the pilot trial did so before and after random allocation. Two patients were withdrawn prior to allocation as their condition worsened, requiring further treatment. A third patient who withdrew before allocation could not be contacted for interview. Four of the five patients who withdrew after randomisation and who agreed to speak to the qualitative researcher were allocated to the NG group. They chose to withdraw for two reasons: most found the NG tube intolerable and one patient reported seeing no improvement in their condition. The fifth patient, allocated to the colonic group, experienced deterioration in their condition, requiring further treatment.
Barriers to participation in main STOP-COLITIS randomised controlled trial
When asked what could prevent patients taking part in a full-scale STOP-COLITIS RCT, the main reason mentioned by patients who took part in the pilot trial was the NG group and the NG tube in particular. The idea of a placebo-controlled trial was also another potential barrier for participation in the main RCT hypothesised by patients.
Results: staff interviews
Semistructured interviews were conducted with 11 members of staff including site Principal Investigators, research nurses, clinical research fellows and staff at the microbiome treatment centre. During the interviews staff discussed
-
views on the acceptability of FMT as a treatment for UC
-
their experience of delivering the FMT procedure
-
their experience of the trial and trial visits
-
general concerns and considerations related to the trial and FMT
-
barriers to patient participation in the trial.
1. Views of STOP-COLITIS and FMT.
Overall, during interviews, staff involved in the STOP-COLITIS pilot were very enthusiastic about the study. They believed it could provide a new way to manage IBD and highlighted the importance of focusing on the microbiome. Staff interviewees thought the study important and were very motivated in delivering it:
I think it’s been a very important trial to get involved in. We’re very motivated to deliver it.
Interviewee S2
Staff interviewed were also very positive about using FMT in order to try to treat UC. This was based mainly on evidence relating to use in CDI:
FMT is very promising and exciting.
Interviewee S8
There is a significant literature base now not just from ulcerative colitis but from C. diff, the patients are very up for this really […] There is good evidence there, some evidence that it works.
Interviewee S11
2. FMT delivery routes.
Colonic route
All the staff involved in the administration of FMT via the colonic route were very positive about their experience and that of the patients. They found that it was an easy and straightforward way of delivering FMT. As per patient interviews, staff reported that patients had no issues with the colonic route, preferring it to NG administration. During the administration of FMT through the colon, some staff made a conscious effort to cover the colonic bottle to make sure patients could not see or smell the content:
I think just the look of it, 50 mls poo in a syringe the patient might think oh God, or even drawn up in the colonic bottle that we did, keep it all covered from the patients so they didn’t see it really, or smell it.
Interviewee S1
The only concerns mentioned by a few of the staff related to time and staffing resources needed to administer FMT. Some reported that the FMT can take some time to administer via the catheter:
Can be quite difficult depending on the size of the syringe used but it can be difficult to push it through the catheter, takes quite a long time.
Interviewee 6
Others reflected on the staffing resources and related capacity for trial patients:
It involves the scope rooms, that always takes three nurses out, so it’s not like you can suddenly fit ten in […] you would need two in the room because it’s looking after the patient, assisting the doctor with the scope. I would always have two nurses in there, sometimes three if it was the randomisation day. Someone has got to then draw up the product and two could potentially do it but I try and get three in there if I could.
Interviewee S1
Nasogastric route
Some of the research nurses/clinical research fellows who delivered the intervention reported having initial concerns about the NG route:
Well at first I had apprehensions about the nasogastric route. We didn’t know whether patients were going to be sick, vomit, just the thought of it.
Interviewee S1
Although some research nurses/fellows stated that they had no issues with tube insertion, some reported not liking administering FMT through the NG route as they found it was distressful for the patients and made them retch:
Definitely the NG path put patients off big time […] I think they struggled the fact that they were getting NG, and wasn’t accepted by everyone. People were just saying on the randomisation they wish it was colonic.
Interviewee S10
I don’t actually like doing them very much, no. I think it’s because it’s so distressful for the patient, they’re all retching and their eyes are watering.
Interview S3
Nurses also highlighted the need for training in NG tube insertion.
One of the reasons why staff believed patients did not like the NG route was because they were not used to it and did not know what to expect:
The vast majority of patients have never had an NG tube, and they don’t actually know what that is. So they are saying yes to it but they don’t know as much about it as they do about having an enema.
Interviewee S6
As reported by patients, staff tried to find ways to make the process more comfortable for patients, including keeping the tube out of sight, explaining the procedure step by step, or involving the patients in conversation:
I tend to take the tube from behind their shoulder now, just things like that I’ve picked up whilst doing it, so that they are not seeing it before you are putting it down the tube.
Interviewee S3
I just explained why I’ve done that and they know what’s coming.
Interviewee S6
We do a tactic where another nurse would be chatting while the NG is here standing behind administering it slowly, but someone is taking their mind off it while they are chatting.
Interviewee S1
Some of the nurses involved in the preparation of the FMT reported that although there were no issues drawing up and preparing the FMT before administering to the patients, they could smell faeces during this preparation phase, which could be an issue for patients.
Reasons for withdrawals
The main reason for patients withdrawing from the study mentioned by staff during interviews was the ability of patients to tolerate the NG tube:
The other patient who pulled out it was because he took the NG out himself, and then just didn’t want it again so he couldn’t tolerate it.
Interviewee S1
Another reason mentioned by staff was that some patients in the NG group had reported being able to burp and taste the FMT after delivery:
The first one we gave the NG route to he took the first dose, was alright with it, but he came back the next day and said he just couldn’t do it because when he burped that was all he could taste, and he was in a panic about it. He was out in a cold sweat just thinking about it, and that was he just couldn’t handle it, it was just too much for him to process.
Interviewee S3
This was not apparent in our interviews with patients who withdrew from the NG group, although several patients who withdrew did not want to take part in a further interview. Staff also reported that some patients who perceived no benefit wanted to withdraw:
One of the ladies as well [nasogastric group], because her symptoms were no better she only had the first lot, she said to me, 'I’m not coming back for the next lot, symptoms are no better, I would rather go and have other treatment'.
Interviewee S1
Conclusion
Qualitative research found a high level of enthusiasm for the trial among both patients and staff. Patients were hopeful that FMT would provide a viable and effective adjunct or alternative to medical options, and staff expressed similar hopes and expectations. Patients were more positive about the colonic treatment route than the NG route, both in advance of treatment allocation, but also at follow-up interview where the colonic route was described as familiar and comfortable on the whole. The NG route, on the other hand, was unfamiliar, thought to be a potential barrier to trial participation, and described negatively by several of the patients allocated to this route at their second interview. Staff interviewees expressed very similar experiences of administering FMT via the two routes, corroborating the accounts of the patients that we spoke with. The qualitative data gave a strong indication that the colonic route was more acceptable to both patients and staff.
Chapter 5 Translational research
Translational research objectives
As part of the nested-mechanistic substudy, donor and participant samples were collected for translational research to assess the following:
-
whether FMT by either route is associated with a change in faecal calprotectin as a surrogate marker of colonic inflammation
-
changes in the colonic microbiome and metabolome (SCFAs) induced by FMT via each route
-
effect of diet (donors)
-
time from stool donation to treatment.
Translational and exploratory outcome measures
-
Changes in faecal SCFAs and faecal calprotectin in recipients induced by FMT via each route.
-
Assessment of microbiome and metabolites in donor and recipient stool.
-
Analysis of microbiome (faecal and mucosal) in recipient.
-
Association between the donor’s dietary profile and microbiome.
-
Time from stool donation to treatment, that is the number of days from donor stool processing to treatment of the patient (recipient) at site for association between efficacy and freezer life of FMT (effect of ‘freezer time’ on efficacy of FMT).
As a decision was made not to proceed to the full RCT, urinary metabolomics were not performed as specified in the trial protocol.
Methods
Sample processing
Donor faecal microbiota transplant
FMT was produced through the University of Birmingham Microbiome Treatment Centre. Briefly, healthy unrelated anonymous individuals were recruited as potential donors following advertisement from the University of Birmingham. We excluded healthcare workers due to the higher risk of exposure to antimicrobial-resistant genes in clinical environments. Donors were non-smokers aged ≥18 and <50 years with a body mass index ≥18.5 and ≤25 kg/m2 with no known comorbidities or gastrointestinal symptoms. Potential donors underwent rigorous screening using a health-screening questionnaire along with blood and faecal samples to test for transmissible pathogens in accordance with UK, AGA and European guidelines. 20,21 Individuals who passed the screening process were then invited to donate stool samples for 10 consecutive weekdays. At the end of the 10-day donation period, donors received financial compensation and underwent an exit health questionnaire. Aliquots from the first 5 days and second 5 days of donated stool underwent repeat testing for pathogens. Donations were processed within 6 hours of defaecation and FMT was stored under an MHRA manufacturing licence at the University of Birmingham Microbiome Treatment Centre. Stool donations for this study completed in 2018 and pre-dated the COVID-19 pandemic.
Donated stool was checked for colour, presence of blood and mucus and was checked against the Bristol stool chart, prior to processing in the Microbiome Treatment Centre production laboratory at ambient temperature under aerobic conditions. Stool was weighed into one side of a sterile Nasco Whirl-Pak filter bag (SLS) and 0.9% w/v saline and 10% glycerol v/v added to a final concentration 0.6 g/ml. The Nasco filter bag and content were transferred into a 400 circulator stomacher (Seward) and homogenised for 2 minutes at 230 rpm. The solid particles from the homogenised sample were retained on one side of the filter, while the resulting liquid portion (FMT) was poured into sterile 60 ml labelled Biotite pots (Alpha Laboratories Ltd, Eastleigh, UK) from the other side of the filter. An aliquot (0.5 ml) of FMT from each pot was retained for analysis in the event of any AEs occurring as a result of FMT. All pots of FMT were sealed with Parafilm M (Fisher Scientific, Loughborough, UK) and stored at −80 °C for up to 24 weeks. Each participant recruited into the trial was matched with a donor to receive FMT from a single treatment set. FMT was removed from −80 °C storage on the morning of treatment and allowed to defrost completely prior to use within 6 hours of removal from storage.
Throughout the trial, FMT prepared donor stool was stored for variable amounts of time prior to treatment as dictated by the practicalities of recruitment and protocol stipulated procedures. The time from donation to treatment was assessed both in terms of the diversity of the samples at the time of treatment administration and also with respect to any difference in clinical response in relation to storage time of donated FMT.
Samples for mechanistic substudies
Bio-samples (mucosal biopsies, stool samples and urine) were collected from participants throughout the study at the three sites (Table 9) and stored at –80 °C in dedicated freezers at each site prior to batch dispatch to either the University of Glasgow for calprotectin and SCFA analysis or the University of Birmingham for genetic sequencing. Sequence data files were subsequently transferred to the Earlham Institute, Norwich Research Park for bioinformatics analysis.
Table 9 shows the protocol schedule for bio-sample collection, storage and transfer during the study.
| Sample to be dispatched | Quantities | Timelines | Recipient |
|---|---|---|---|
| Donor urine | 10–20 ml | Day 1 and 10 | University of Glasgow |
| Donor stool (fresh and frozen) | Approx. 10–20 g | Day 1 and 10 | University of Glasgow |
| Donor stool (fresh and frozen) | Approx. 10–20 g | Day 1 and 10 | University of Birmingham |
| Participant stool for calprotectin | Approx. 10–20 g | Randomisation visit, weeks 2, 4, 6, 8, 12, ETV | University of Glasgow |
| Participant urine for SCFA | 10–20 ml | Randomisation visit, weeks 8, 12, ETV | University of Glasgow |
| Participant stool for SCFA | Approx. 10–20 g | Randomisation visit, weeks 2, 4, 6, 8, 12, ETV | University of Glasgow |
| Participant mucosal biopsies | 6 biopsies | Randomisation visit, week 8 | University of Birmingham |
| Participant stool for 16S rRNA | Approx. 10–20 g | Randomisation visit, weeks 8, 12, ETV | University of Birmingham |
Faecal calprotectin analysis
Measurements of faecal calprotectin were performed using a standard protocol with commercially available ELISA kits and according to the specifications of the manufacturer. Briefly, 100 mg of thawed stool sample was extracted with a proprietary buffer, then diluted and loaded on a 96-well plate coated with calprotectin antibodies. The concentration of faecal calprotectin was calculated in mg/kg of wet and dry faecal matter. Faecal extracts were assayed in duplicates.
Absolute quantification was performed against calibration curves created using the manufacturer’s standards and assay validity was ascertained against quality-control samples provided in the kit. If the calculated concentrations of the quality-control samples were outside the expected range of values, the data were discarded and the experiment was repeated. As measurements vary according to the laboratory and kits used, all measurements were performed in one central laboratory at the University of Glasgow. All serial samples from the same participant were assayed on the same kit to minimise interassay variation.
As there is no standard value regarding an accepted lower limit for faecal calprotectin in UC, no threshold was set at baseline; rather, the change from baseline was explored in this interventional study.
Microbiome-related assays: 16S ribosomal DNA sequencing and metagenomics
In order to profile the microbial community of both donors and participants, 16S rRNA gene sequencing of faecal and biopsy samples was performed. This method of sequencing a universal marker gene provides a cost-effective means to determine the microbes present in a community. It is a well-established tool in microbiome research and has been shown, despite noise and biases, to enable broad-scale changes in microbiome structure to be detected. It does, however, have drawbacks, it has limited taxonomic resolution, we performed analysis to the genus level, and it cannot determine potential function, both of these can be addressed by using shotgun metagenomics instead,24 but that was outside the scope of this pilot study.
16S rRNA marker gene sequencing was performed on all samples collected during the study: donor faeces (fresh and frozen from day 1 to day 10 donations), faeces from the recipient at baseline, weeks 2, 4, 6, 8 and 12 and mucosal biopsies taken from the right colon, left colon and rectum at baseline and from left colon and rectum at week 8. DNA was obtained from these samples using the QIAamp PowerFecal Pro DNA Kit (Qiagen, Crawley, UK) operated according to the manufacturer’s protocol. The V4 region of the 16S rRNA gene was amplified from these samples using standard 515F forward and 806R degenerate primers with Golay barcodes and 30 rounds of amplification. Following clean-up with Ampure reads, the barcoded libraries were sequenced to 2X250 bp on the Illumina MiSeq platform. Samples were run together with negative (extraction blank) and positive (Zymo mock community) controls.
Microbiome-related assays: major bacterial metabolites and metabolomics on faecal samples
Major bacterial metabolites including faecal short- and medium-chain fatty acids previously implicated in the aetiology and mucosal inflammation of IBD were measured using gas chromatography and other assays as described previously. 25-28
The SCFAs (acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, heptanoic acid and caprylic acid) and branched SCFAs (iso-butyric acid and iso-valeric acid) were extracted from acidified slurries three times in total using diethyl ether. Extracts were analysed using gas chromatography (Agilent 7890A) with flame ionisation detector, as described previously. 25 Each of the SCFAs was quantified against calibration curves plotted using authentic external standards [acetic acid (185.8 mM), propionic acid (144.5 mM), butyric acid (114.2 mM), valeric acid (83.4 mM), caproic acid (52.6 mM), heptanoic acid (65.8 mM), caprylic acid (53.2 mM), isobutyric acid (97.3 mM) and isovaleric acid (87.0 mM)] and using 2-ethylbutyric acid (74.0 mM) as internal standard. All samples from the same participant were analysed in the same run to minimise interassay variation. Each sample was measured twice, and in all cases the average concentration was calculated unless the percentage coefficient of variation was >10% in which case a third replicate was analysed. Concentration of SCFAs (μmol) is reported per wet and dry faecal matter.
Statistical analysis
Bioinformatics for 16S rRNA analysis on stool and mucosa
Amplicon sequence variants (ASVs) were generated from the raw data using the dada2 pipeline, version 1.16 https://benjjneb.github.io/dada2/tutorial.html (accessed 1 June 2023). Quality filtering was performed with a maximum expected error value for merged sequences of two, and sequences were truncated at the first instance of a quality score <2. The first 18 nucleotides in forward reads and the first two in reverse reads were trimmed to account for non-templated nucleotides, which were present in the data. The core dada2 algorithm was applied to remove sequencing noise, forward and reverse sequences were merged, and an ASV table was generated. Chimeras were removed using the dada2 de novo method and sequences longer than 240 bp and shorter than 225 bp were filtered out. The resulting ASVs were clustered into operational taxonomic units (OTUs) at the 97% similarity level using VSEARCH. A phylogenetic tree was generated by forming alignments of ASVs using mafft followed by FastTree. This procedure was repeated to generate another phylogenetic tree for OTUs. ASVs and OTUs were taxonomically classified to genus level against the SILVA 16S reference data set, release 138, using IDTAXA29 with species level added for those with a 100% match to the SILVA data set using the addSpecies function, as per the dada2 pipeline.
Short-chain fatty acids
For the faecal SCFA analysis, concentration per wet faecal matter for C2, C3, C4, IC4 and IC5 were considered the primary metabolites of interest. These concentrations were compared between baseline and the start of FMT (week 2) and the end of FMT (week 8) using paired t-tests on the log-transformed values. Patients were also divided into responders and non-responders and the analysis repeated.
This analysis was augmented by a repeated-measures model. All assessment times were included in the model (weeks 2, 4, 6, 8 and 12), with baseline value included as a covariate in the model. Assessment time was considered a continuous variable in the model. The model was further adjusted for smoking status, partial Mayo score, allocated treatment and clinical response (as measured by the primary measure of efficacy for the STOP/GO). A general ‘unstructured’ covariance structure was assumed. For allocated treatment and clinical response covariates, time-by-covariate effects were explored by including the corresponding parameter in the model; if significant (p ≤ 0.05), a constant covariate effect was not assumed and estimates of effect size (and 95% CI) were generated at each time point (weeks 2, 4, 6, 8, 12).
Time from stool donation to treatment
The number of days between stool donation and treatment was calculated as the number of days between the date of FMT manufacture and the date the FMT was removed from the freezer for thawing for first FMT treatment. Time from stool donation to treatment was summarised using means and SDs by clinical response (primary measure of efficacy). To assess if time from stool donation to treatment was a predictor for clinical response, an additional analysis was conducted where time from stool donation to treatment was included as a continuous covariate to the ITT analysis model of clinical response.
Association between the donor’s dietary profile and microbiome
For association between the donor’s dietary profile and microbiome, donor microbiome indices (rarefied Shannon diversity and rarefied species) and dietary profiles were averaged across sampling periods. Pearson’s correlation coefficients were produced to assess the association between each dietary component and rarefied Shannon diversity and rarefied species.
Results
Changes in faecal calprotectin and short-chain fatty acids in recipients’ faecal samples induced by faecal microbiota transplant via each route
Individual calprotectin trajectories were noisy, in the sense that the levels in an individual subject fluctuated substantially from week to week. To address this, we averaged values for each subject over the last half of the treatment course (weeks 4, 6 and 8). Using these averaged values, we observed significantly lower calprotectin levels (t-test p = 0.03), in the responders (N = 11, mean 508.6 mg/kg) versus the non-responders (N = 9, mean 853.6 mg/kg) (Figure 3).
FIGURE 3.
Average faecal calprotectin levels against response.
Short-chain fatty acids
For the primary SCFA metabolites of interest, the mean concentration was similar in participants who achieved clinical response compared with those who did not achieve clinical response at each time point (Table 10). For allocated treatment and clinical response in the repeated measures models, a constant variable effect across time was demonstrated in all metabolites.
| Time point | Clinical response | Mean differencea (95% CI) | |
|---|---|---|---|
| Yes | No | ||
| C2 (acetate) concentration per wet faecal matter (mol/g) | |||
| Baseline | 83.2 (40.3, 9) | 64.9 (37.8, 7) | −6.2 (−26.0 to 13.6) |
| Week 2 | 90.3 (25.6, 9) | 92.9 (36.8, 7) | |
| Week 4 | 95.9 (57.1, 9) | 111.2 (45.8, 7) | |
| Week 6 | 89.1 (33.0, 9) | 70.9 (22.9, 6) | |
| Week 8 | 90.4 (29.5, 9) | 91.1 (35.5, 7) | |
| Week 12 | 87.2 (25.3, 9) | 102.6 (46.8, 6) | |
| C3 (propionate) concentration per wet faecal matter (mol/g) | |||
| Baseline | 25.6 (26.1, 9) | 12.3 (14.3, 7) | −3.5 (−7.9 to 0.9) |
| Week 2 | 21.3 (16.5, 9) | 14.9 (16.9, 7) | |
| Week 4 | 30.7 (30.7, 9) | 16.6 (17.7, 7) | |
| Week 6 | 21.7 (17.0, 9) | 12.1 (9.9, 6) | |
| Week 8 | 17.1 (8.5, 9) | 17.1 (14.7, 7) | |
| Week 12 | 23.9 (12.4, 9) | 20.2 (17.5, 6) | |
| C4 (butyrate) concentration per wet faecal matter (mol/g) | |||
| Baseline | 12.4 (9.1, 9) | 13.9 (13.1, 7) | −5.9 (−14.8 to 3.1) |
| Week 2 | 21.3 (15.5, 9) | 21.1 (16.5, 7) | |
| Week 4 | 19.3 (12.6, 9) | 26.8 (19.0, 7) | |
| Week 6 | 19.1 (16.2, 9) | 19.8 (11.7, 6) | |
| Week 8 | 14.8 (10.2, 9) | 22.6 (15.0, 7) | |
| Week 12 | 20.7 (11.7, 9) | 34.9 (28.5, 6) | |
| IC4 (valerate) concentration per wet faecal matter (mol/g) | |||
| Baseline | 1.4 (1.1, 9) | 1.0 (1.2, 7) | 0.3 (−0.1 to 0.7) |
| Week 2 | 1.8 (1.6, 9) | 1.1 (2.1, 7) | |
| Week 4 | 1.6 (1.3, 9) | 1.3 (2.5, 7) | |
| Week 6 | 1.3 (0.5, 9) | 1.1 (1.2, 6) | |
| Week 8 | 1.7 (1.0, 9) | 1.9 (2.1, 7) | |
| Week 12 | 1.7 (1.0, 9) | 0.9 (1.0, 6) | |
| IC5 concentration per wet faecal matter (mol/g) | |||
| Baseline | 1.7 (1.4, 9) | 1.3 (1.6, 7) | 0.1 (−0.3 to 0.5) |
| Week 2 | 2.0 (1.7, 9) | 1.4 (2.9, 7) | |
| Week 4 | 2.0 (1.9, 9) | 1.7 (3.8, 7) | |
| Week 6 | 1.5 (0.7, 9) | 1.3 (1.6, 6) | |
| Week 8 | 2.1 (1.2, 9) | 2.2 (2.7, 7) | |
| Week 12 | 1.8 (1.1, 9) | 1.1 (1.3, 6) | |
There were significant and large increases observed in the concentration of acetate (45% increase, p = 0.05) and butyrate (57% increase, p = 0.03) across all participants (N = 19) from the baseline to 2 weeks during the course of FMT. This effect was also observed over the course of the entire FMT from baseline to 8 weeks (N = 16, acetate 21% increase, p = 0.02; butyrate 39% increase, p = 0.08). Significant changes were not observed for the other SCFAs measured, notably propionate (2 weeks p = 0.46, 8 weeks p = 0.36). The observed increase in acetate and butyrate was not associated with clinical response, and in fact when restricted to the responder group (N = 9) changes at both 2 weeks and 8 weeks were no longer significant, although increases were still observed.
Assessment of microbiome in donor and recipients
In total, 166 faecal microbiome samples were sequenced. We obtained a median of 50,726 reads per sample. Of these 166 samples, 14 had <10,000 reads and these were not used for further analysis. We observed 1000 3% OTUs in these 152 samples and unless otherwise noted results below are reported at this 3% OTU level. In order to account for differing sample sizes, we rarefied or subsampled OTU profiles to the smallest remaining sample size of 21,433 reads prior to diversity calculations. Alpha diversity was quantified using the Shannon entropy of the community profile, after normalising by total read count to get species proportions. The Shannon entropy effectively combines both evenness and overall species number, that is a more even distribution will have a higher Shannon diversity as will a community with more species. 30
We also obtained 16S rRNA reads from 124 mucosal biopsy samples, these had a median of 47,430 reads per sample. Of these samples, 13 had <10,000 reads and were filtered to avoid noise from small read number. In these 111 samples, we observed 753 3% OTUs, and as for the faecal samples, we based the diversity analyses on these 3% OTUs following rarefaction to the smallest sample size of 10,935.
Microbiome in donor stool
Microbiome diversity, as measured by rarefied 16S rRNA gene Shannon diversity, varied substantially between donors (Figure 4). There was a non-significant positive association between clinical response and donor diversity (p = 0.19) with treatment arm taken into account (logistic regression response against treatment and donor rarefied Shannon diversity).
FIGURE 4.
Donor microbiome diversity as measured by 16S rRNA rarefied Shannon diversity.
Taking into account the small number of donors in this study, no differences in time to donation and microbiome diversity were seen.
Analysis of microbiome (faecal and mucosal) in recipient
Across all patients that completed FMT to day 56 (N = 13 for participants with 16S rRNA data at both day 1 and day 56), there was no significant change in faecal microbiome alpha diversity, as measured by a rarefied Shannon diversity of 3% OTUs (paired t-test = −0.81907, p = 0.43). However, when the analysis was restricted to the colonic arm (N = 8 for participants with 16S rRNA data at both day 1 and day 56), there was a significant increase in alpha diversity (p = 0.01) after FMT. Consistently, for all those participants that responded to FMT (N = 9 for participants with 16S rRNA data at both day 1 and day 56), there was a significant increase in alpha diversity (rarefied Shannon diversity, p < 0.01) (Figure 5). This was also observed for rarefied species number although with less significance (p = 0.05).
FIGURE 5.
Rarefied Shannon diversity faecal microbiome (3% OTUs) at start (day 0) and end of FMT (day 56) for patients (N = 9) that responded to FMT.
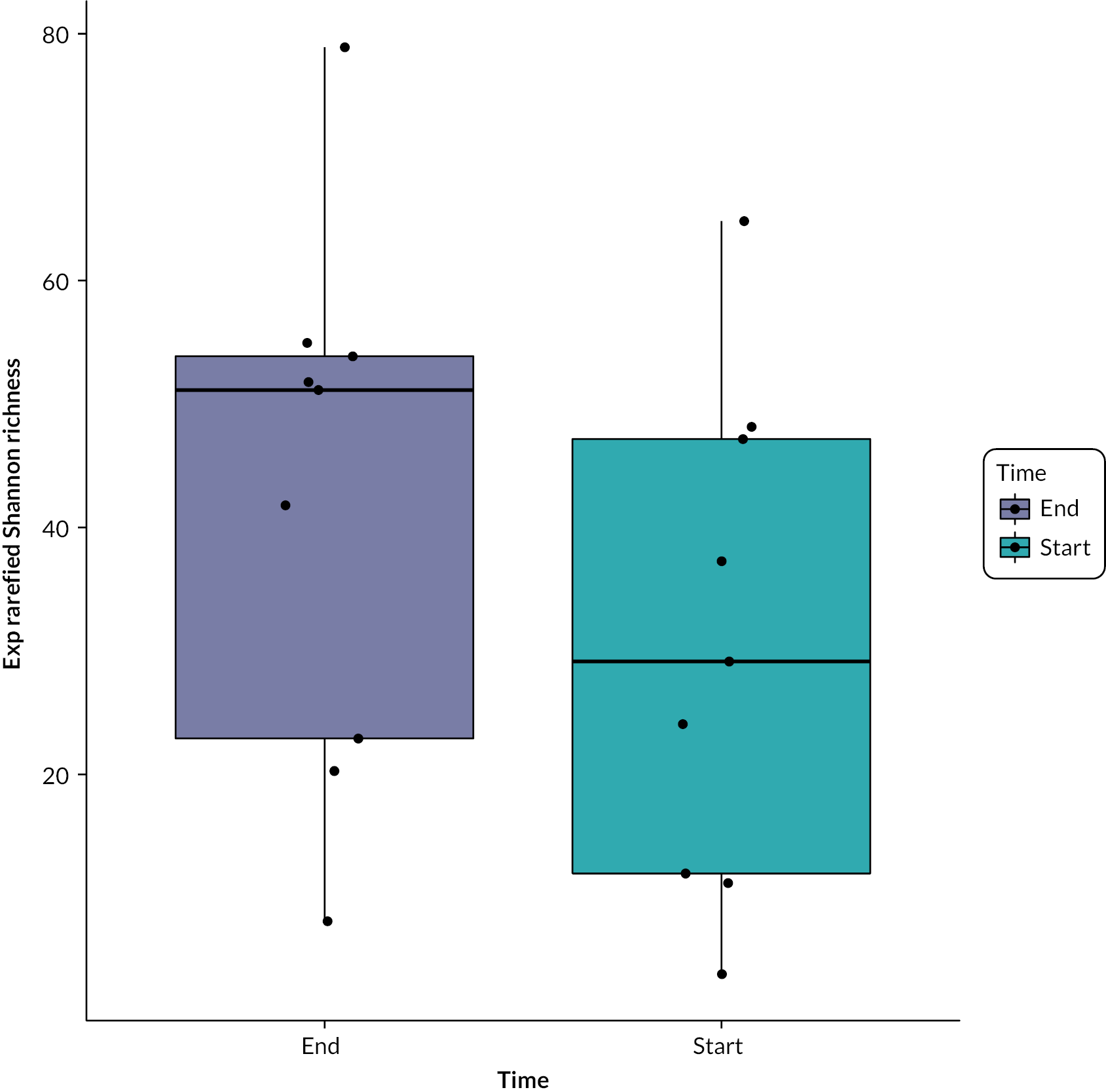
We did not observe similar changes in mucosal microbiome alpha diversity. We observed no significant difference across tissue types (N = 85 for participants with mucosal biopsies both pre and post treatment) for rarefied Shannon diversity (p = 0.37); therefore we considered all tissue types when comparing between pre and post treatment. Across all subjects as for the faecal microbiome, we observed no significant difference in alpha diversity between pre and post treatment (two-way analysis of variance of rarefied Shannon diversity against patient ID and pre and post treatment, N = 85, p = 0.86). However, this was also true when restricted to responders (N = 45, p = 0.84) or the colonic arm (N = 51, p = 0.31). In contrast, in all these tests, patient ID was significantly associated with mucosal microbiome diversity.
Of interest, there was a negative association between faecal microbiome alpha diversity (rarefied total OTU richness) and calprotectin levels (Kendall’s tau z = −2.8231, p-value = 0.004757) (Figure 6).
FIGURE 6.
Correlation between faecal calprotectin and rarefied 3% OTU diversity across all time points.
Association between the donor’s dietary profile and microbiome
Correlation coefficients between donor’s dietary profile and rarefied Shannon diversity and rarefied species are provided in Table 11. The Pearson correlation coefficient for milk and milk products (g)/1000 kcal, and rarefied species was 0.67 indicating moderate positive correlation; however numbers are small.
| Pearson’s correlation coefficients, N | Number of donors | Rarefied Shannon diversity | Rarefied species |
|---|---|---|---|
| Energy kcal | 9 | 0.12 | −0.14 |
| Energy/BMR | 7 | 0.42 | −0.16 |
| %CHO | 9 | 0.24 | −0.01 |
| %PRO | 9 | 0.17 | 0.25, |
| %FAT | 9 | −0.43 | −0.14 |
| %MUFA | 9 | −0.22 | −0.16 |
| %PUFA | 9 | −0.04 | −0.33 |
| %SFA | 9 | −0.52 | 0.04 |
| Non-starch polysaccharides (NSP) (g)/1000 kcal | 9 | 0.64 | −0.01 |
| Englyst fibre – NSP (g) | 9 | 0.37 | −0.18 |
| Alcoholic beverages (g)/1000 kcal | 9 | 0.33 | 0.05 |
| Cereals and cereal products (g)/1000 kcal | 9 | 0.02 | −0.06 |
| Eggs and egg dishes (g)/1000 kcal | 9 | 0.54 | 0.49 |
| Fats and oils (g)/1000 kcal | 9 | −0.22 | −0.20 |
| Fish and fish products (g)/1000 kcal | 9 | 0.18 | 0.02 |
| Fruit (g)/1000 kcal | 9 | 0.49 | −0.11 |
| Meat and meat products (g)/1000 kcal | 9 | −0.21 | 0.05 |
| Milk and milk products (g)/1000 kcal | 9 | 0.04 | 0.67 |
| Non-alcoholic beverages (g)/1000 kcal | 9 | 0.30 | 0.44 |
| Nuts and seeds (g)/1000 kcal | 9 | −0.44 | −0.69 |
| Potatoes (g)/1000 kcal | 9 | −0.10 | 0.05 |
| Soups and sauces (g)/1000 kcal | 9 | 0.59 | 0.31 |
| Sugars; preserves and snacks (g)/1000 kcal | 9 | −0.02 | −0.07 |
| Vegetables (g)/1000 kcal | 9 | 0.55 | 0.13 |
Time from stool donation to treatment
The mean number of days between time of stool donation to treatment was similar in participants who achieved a clinical response compared with those who did not achieve a clinical response (Table 12). There was no association between time from stool donation and treatment and clinical response when added to the primary ITT analysis model (RR 1.00, 95% CI 0.96 to 1.04).
| Clinical response | ||
|---|---|---|
| Yes | No | |
| Time from stool donation to treatment (days) | ||
| Mean (SD, N) | 70.6 (20.0, 11) | 74.0 (26.7, 9) |
| Minimum–maximum | 28.0–96.0 | 40.0–126.0 |
Conclusion
Taking into account the fact that this was a small feasibility study designed to pave the way for a subsequent fully powered efficacy trial some important mechanistic signals were seen in the trial.
Faecal calprotectin is the main non-invasive biomarker used in day-to-day clinical practice to assess inflammation in UC. Faecal calprotectin levels depend on the extent as well as the severity of inflammation. 31 There was considerable variation in baseline calprotectin levels in the participant cohort as a whole, and this probably reflects the variation in phenotype of recruits with respect to extent of disease (Table 2). When comparing calprotectin results in those who showed a clinical response to FMT compared to those who did not, despite the small numbers, there was a significant reduction in calprotectin, representing a ‘hard indication’ of an improvement in colonic inflammation. Higher microbiome diversity is a recognised ‘high-level’ marker of gut microbiome health. In this study, assessing all available stool samples taken during the study, we have seen for the first time a negative correlation between faecal calprotectin and microbiome diversity. While this has no direct message regarding the efficacy of FMT for UC, it shows a useful link between these two markers of colonic health.
Short-chain fatty acids are produced by the action of ‘beneficial’ obligate anaerobes on microbially available carbohydrates in the colon and, as such, are commonly used as a surrogate marker of the ‘general health’ of the gut microbiota community. 32 In this study, we observed an increase in acetate and butyrate levels in faecal samples after FMT compared to baseline, although there were no signals correlating SCFA levels with efficacy. Rather SCFA levels seem to indicate ‘successful FMT’ rather than clinical efficacy in this investigation.
Considering changes in microbiome diversity in this study, in the whole treated cohort, no significant difference was seen between baseline and post FMT. However, taking the most successful treatment group both in terms of adherence and clinical response, that is the participants treated with colonic FMT, we did see a significant increase in diversity in those who responded to FMT. No clear donor effects were seen in this study, which employed only nine donors. There was considerable baseline variability in diversity among the donors and, although not reaching clinical significance in this study, a trend was seen for a better treatment outcome from donors who had higher baseline diversity. It was not possible to draw conclusions regarding engraftment of individual components of the microbiome from donors to participants associated with clinical response as the pilot study was not funded for shotgun metagenomic sequencing. We observed no effects of donor diet nor time from donation to treatment, neither in terms of baseline donor diversity nor clinical response.
In summary, despite the limitations of this small feasibility study, important mechanistic signals have been observed linking inflammation with the gut microbiota and suggesting mechanistic efficacy of FMT, which should be further explored.
Chapter 6 Discussion
Previously published trials of FMT in the treatment of UC have been undertaken with variable methodology,14 18 in particular with regard to the two possible routes of delivery, that is via the foregut or hindgut. Furthermore, the most recent trials have employed a mixture of donor samples with a view of maximising microbiome diversity in the delivered FMT. 16,17 However, this approach limits the possibility of linking microbial engraftment from donor to recipient. None of the previous trials examined the effect of the FMT treatment on faecal calprotectin, the most common non-invasive biomarker used in clinical practice.
This prospective, three-centre, open-label, randomised pilot trial aimed to identify the optimal route of administration of FMT comparing delivery to the foregut with hindgut to further explore in a full-scale RCT. In the study, each treatment course for a participant was undertaken using donated stool from a single donor. Extensive qualitative data were collected from trial participants and healthcare staff involved in the trial to assess the acceptability and tolerability of FMT treatment, and to determine if there was a preference for either route of delivery. Serum and stool samples were taken frequently during the trial to examine biological mechanisms underlying the effect of FMT.
Thirty adults with UC were recruited. FMT delivered by the colonic route was much better adhered to than NG delivery. Eleven of the 14 participants (79%) randomised to colonic delivery completed the full FMT treatment course compared to 8 of 16 (50%) participants randomised to FMT via the NG route. Interestingly, although participants were enthusiastic about the idea of the trial when approached, all but one expressed a preference for the colonic route prior to randomisation. Prior to enrolment, patients were worried about the tolerability of NG treatment. Findings from this pilot study showed that lack of tolerability of the NG route was as a result of problems tolerating the NG tube, rather than the FMT. All members of the nursing team administering FMT were positive about their experience of taking part in the study.
This pilot study was not powered to assess efficacy and interpretation of the findings is limited by the small sample size, which corresponds to high levels of uncertainty. Clinical response, the primary measure of efficacy, was achieved in 9/12 (75%) colonic-treated participants compared to 2/8 (25%) NG-treated participants. However, clinical response data were unavailable for 10 of the 30 randomised participants, 9 of which were due to participant withdrawal. Clinical remission was also higher in the colonic arm (6/12) compared to the NG arm (2/8). Five participants (two NG and three colonic) achieved mucosal healing during the course of this study. CRP levels were similar between the arms during follow-up. This is perhaps not surprising as in UC, as opposed to CD, inflammation is confined to the mucosa and is not systemic; CRP is rarely elevated significantly. 33
With regard to mechanistic signals, in this small pilot study, it was demonstrated, for the first time in a FMT trial in UC that faecal calprotectin levels were lower in responders compared to non-responders (treatment routes combined). There was also an increase in microbiome alpha diversity in responders compared to non-responders. Interestingly, faecal calprotectin was negatively correlated with microbiome diversity, adding further support for a positive biological response to FMT. No differences in diversity of mucosa-associated bacteria were seen when comparing participant samples before and after FMT.
A significant increase was seen in the SCFAs acetate and butyrate within 2 weeks of FMT although, in this small cohort, these changes did not correlate with clinical response, rather representing ‘successful’ FMT as has been shown in other studies. 34
As the investigative medicinal product in this study (donated stool) is highly dependent on the diet taken by the donor, we also looked for any associations between habitual donor diet as reported by self-completed diet questionnaires and microbiota diversity, which is well recognised to be an important indicator of the ‘healthiness’ of the intestinal ecological niche. 35 Not surprisingly, given the small number of donors (N = 9) used in this pilot study, no significant associations were seen, although there was a trend between milk and milk product intake and diversity.
Bacterial diversity has been shown to diminish with storage of frozen FMT,36 and we therefore sought to look for any associations between time of storage and efficacy. No signal was seen in this small study. It is to be noted that the mean time between donation and use was between 70 and 75 days. This is quite rapid given evidence that frozen FMT maintains bacterial viability for many months and is stored for clinical use for up to 12 months. 21
The small number of patients recruited to this clinical trial and the high number of ‘drop-outs’ particularly in the NG-treated patients may be considered a ‘limitation’. However, the pilot study did achieve its objective to both show a difference in feasibility of using one of the comparative routes in a larger RCT to treat patients with UC and to demonstrate the general feasibility of such a study. Overall, the FMT treatment was well tolerated and side effects, although not uncommon were transient. Participants (both patients and clinicians) who took part in this study were enthusiastic at the start and remained so at the end.
The limited numbers in this study make extrapolations regarding mechanisms difficult, in particular with respect to identifying engrafting components of the microbiota from donor to recipient and metabolites associated with effect. Nevertheless, it is remarkable, given the size of the study, that such clear mechanistic signals regarding efficacy emerged, highlighting just how powerful this crude manipulation of the microbiome is in effect.
The signals observed within the STOP-COLITIS pilot need further study and much more attention needs to be directed towards donor selection. More extensive work on diet and engraftment of specific microbiota from donors to recipients and the longevity of these affects also requires further study. Future research should also focus on carefully considered mechanistic insights regarding the metabolic consequences of FMT beyond just looking at SCFAs.
This study was not without its challenges. The initiation of the trial was greatly delayed by a change in regulations around FMT that coincided with the grant starting. Having previously been regarded as a ‘tissue’ and manufactured under Health Technology Assessment regulation in a Public Health England laboratory in Birmingham, FMT was subsequently classified as a medicine, requiring us to set up a new GMP production facility at the University of Birmingham prior to starting the trial. This was achieved in 48 months and the University of Birmingham remains the only institution in the UK public sector to hold a ‘specials licence’ for FMT.
This pilot study adds to the growing evidence base for the benefit of microbiome manipulation using FMT as an effective treatment option for UC. This is supported not only by tolerability of the approach by participant and treating nurses, but also by efficacy signals which correlate with positive mechanistic outcomes.
Patient and public involvement
Our protocol was developed in consultation with a PPI group. One of the co-applicants was a patient with IBD and also Chairperson for the West Midlands Group of CCUK. They provided invaluable help with the development of the project, and then ongoing support as an active member of the TMG ensuring the patient voice was central to the research. They also contributed and are a coauthor of this report. The PPI group has strong links with CCUK, and this will significantly aid dissemination of findings nationally to patients, relatives and health professionals.
In the development of this project, we conducted a survey of our own IBD patients from the outpatient department at University Hospital Birmingham. Of the 74 patients surveyed, the vast majority of patients said they would accept FMT as a treatment for IBD and over 80% would consider involvement in a trial of FMT in IBD. However, since we were aware that the acceptability of FMT in UC had not been extensively studied, we designed the pilot study to investigate extensive assessment of patient acceptability of the therapy by regular questionnaires, individual interviews and group discussions.
Equality, diversion and inclusion
Research in the UK has highlighted that people of South Asian and African-Caribbean heritage have an equivalent, if not higher prevalence of UC compared to white people. 37 Our 74-patient survey of attitudes to the proposed study carried out in Birmingham included patients of South Asian background (predominantly Punjabi, Bangladeshi and Mirpuri). Patients of African-Caribbean origin were also included in the survey. The Chief Investigator and several members of the clinical research team are bilingual in English and at least one South Asian language, and they were able to explain the survey to patients in languages other than English where required. All recruitment sites for the pilot were located in areas with a multiethnic population. These factors would account for the fact that a third of participants recruited to this study were of non-white background.
Chapter 7 Conclusion
The main aim of this small pilot study was to determine the optimal route by which to administer FMT to patients with UC. In the clinical study, we observed a difference in adherence to treatment between the NG and colonic routes of FMT delivery. Only 8 out of 16 (50%) participants randomised to the NG arm were considered adherent compared to 12 out of 14 (86%) randomised to the colonic arm. Of those who were adherent to the NG route, 2 out of 8 (25%) responded compared to 9 of 12 (75%) participants treated via the colonic route. As is common after FMT treatment, many participants (>65%) developed mild and transient gastrointestinal symptoms in the first 24 hours after treatment. However, only two participants, both in the NG arm, developed SAEs; one with troublesome gastrointestinal symptoms requiring admission and the other with a severe exacerbation of UC – both withdrew from the trial. Unfortunately, given the protocol requirement for a complete wean off steroids prior to recruitment, it is not possible to distinguish whether the disease flare was due to the withdrawal of steroid treatment or an effect of FMT.
Our qualitative research found a high level of enthusiasm for the trial among both patients and staff. Patients were hopeful that FMT would provide a viable and effective adjunct or alternative to medical options, and staff expressed similar hopes and expectations. Patients were more positive about the colonic treatment route than the NG route, both in advance of treatment allocation, but also at follow-up interview, with the colonic route described as familiar and comfortable on the whole. The NG route, on the other hand, was unfamiliar, thought to be a potential barrier to trial participation, and described negatively by several of the patients allocated to this route at their second interview. Staff interviewees expressed very similar experiences of administering FMT via the two routes, corroborating the accounts of the patients that we spoke with. The qualitative data gave a strong indication that the colonic route was more acceptable to both patients and staff.
This small pilot study was not powered to determine mechanisms; nevertheless, some important mechanistic associations were observed during the course of the trial. Faecal calprotectin is the main non-invasive biomarker used in day-to-day clinical practice to assess inflammation in UC. Faecal calprotectin levels depend on the extent, as well as the severity of inflammation. 31 Those who responded to FMT showed a significant reduction in calprotectin, and for the whole cohort there was a negative correlation between calprotectin and microbiome diversity implying lower diversity associating with colonic inflammation.
Short-chain fatty acids are produced by the action of ‘beneficial’ obligate anaerobes on microbially available carbohydrates in the colon and, as such, are commonly used as a surrogate marker of the ‘general health’ of the gut microbiota community. 32 The data in this study point to SCFAs representing ‘general health’ of the microbiota community as a whole rather than associating with clinical efficacy as we saw an increase in both butyrate and acetate after FMT.
With regard to microbiome changes, there was no significant change in the whole cohort after FMT. However, considering the participants in the colonic group, there was an increase in diversity in those who responded clinically. In this small study, in which only nine donors were used, no clear ‘donor effect’ was observed. It was not possible to draw conclusions regarding engraftment of individual components of the microbiome from donors to patients associated with clinical response as the pilot study was not funded for shotgun metagenomic sequencing. We saw no effects of donor diet nor time from donation to treatment, neither in terms of baseline donor diversity nor clinical response.
In summary, data from this small pilot study suggest that FMT for UC appears safe and was well-tolerated, at least when administered to the hindgut in patients with UC. Mechanistic signals, while not definitive, point to a beneficial effect of FMT for UC. At the end of the study, the IOC were able to recommend that the pre-specified STOP/GO criteria had been met in the colonic arm and this route of FMT delivery could be taken forward to be evaluated in the proposed efficacy RCT.
The positive signals from the current study and the obvious enthusiasm of patients for this modality of treatment suggest that future studies using FMT to treat UC should be undertaken. FMT should be developed to treat patients, understand underlying mechanisms and to develop the place of microbiome manipulation in the current treatment algorithm of IBD. Another important question remains the effectiveness of novel lyophilised capsules, which have been developed for treatment of C. difficile, but not fully tested in IBD. 37 We believe that FMT remains a useful tool for further human interventional ‘discovery science’ on the pathway to develop rational novel biotherapeutics to treat chronic inflammation.
Recommendations for future research
This pilot study has demonstrated that FMT is a safe and acceptable treatment for UC and the colonic delivery route has been clearly identified as the better route of delivery. Randomised studies should be undertaken to further investigate the mechanisms (microbial and metabolic) to better understand the observed effects, and to both establish a role for microbiome manipulation in the management of UC and to develop novel therapies based on improved understanding of underlying mechanisms.
Additional information
Contributions of authors
Mohammed Quraishi (https://orcid.org/0000-0003-2338-8397) was the research fellow in Birmingham involved in developing the protocol, recruiting patients, undertaking clinical interventions and report writing.
Catherine A Moakes (https://orcid.org/0000-0002-0473-0532) (Senior Medical Statistician) conducted the statistical analyses and contributed to interpretation of the results and to the writing and editing of this report.
Mehmet Yalchin (https://orcid.org/0000-0001-5445-6458) (Research Fellow at St Marks Hospital) was involved in screening and recruiting patients to the trial and undertaking clinical procedures.
Jonathan Segal (https://orcid.org/0000-0002-9668-0316) (Research Fellow at St Marks Hospital) was involved in screening and recruiting patients to the trial and undertaking clinical procedures.
Natalie J Ives (https://orcid.org/0000-0002-1664-7541) (Reader in Clinical Trials, Senior Statistician) contributed to the conception and design of the study, supervised the statistical analyses and contributed to the interpretation of the results and to the writing and editing of the report.
Laura Magill (https://orcid.org/0000-0003-2498-8407) (Senior Lecturer in Clinical Trials) contributed to the conception and design of the project and oversaw the conduct of the study.
Susan Manzoor (https://orcid.org/0000-0003-3411-0315) (MTC Production Manager) led the production of FMT from stool donations and wrote many of the SOPs required for our MHRA licence.
Konstantinos Gerasimidis (https://orcid.org/0000-0001-9432-2200) (Professor of Clinical Nutrition) was scientific lead for metabolomics and analysis of donor diets.
Shrushma Loi (https://orcid.org/0000-0002-3815-2320) (Senior Trial Manager) contributed to the conduct and co-ordination of the study, collation of the data and interpretation of the results.
Christel McMullan (https://orcid.org/0000-0002-0878-1513) (Qualitative Researcher) was responsible for the qualitative data collection and analysis of this data, and wrote the qualitative chapter of this report.
Jonathan Mathers (https://orcid.org/0000-0001-6651-6286) (Associate Professor in Qualitative and Mixed Methods Applied Research, Qualitative Researcher) contributed to the conception and design of the study, supervised the delivery of the qualitative study and contributed to the writing and editing of the report.
Christopher Quince (https://orcid.org/0000-0003-1884-8440) (Associate Professor) undertook the majority of the bioinformatics work on microbiome sequencing.
Manjinder Kaur (https://orcid.org/0000-0002-3412-0276) (Trials Management Team Leader) contributed to the conduct of the study, collation of the data and the interpretation of the results, and the writing and editing of the report.
Nicholas J Loman (https://orcid.org/0000-0002-9843-8988) (Professor of Microbial Genomics and Bioinformatics) managed the 16S microbiome sequencing work.
Naveen Sharma (https://orcid.org/0000-0002-2298-654X) (Consultant Gastroenterologist), as associate PI in Birmingham, was involved in patient recruitment and all clinical procedures associated with this study.
Peter Hawkey (https://orcid.org/0000-0001-8698-7085) (Professor of Clinical and Public Health Bacteriology) was microbiology lead and was key to the process required to attain an MHRA licence.
Victoria McCune (https://orcid.org/0009-0000-0807-4512) (Microbiologist) worked closely with Professor Hawkey on microbiological aspects of this project and with Dr Manzoor in the development of Standard Operating Procedures for the laboratory.
Ben Nichols (https://orcid.org/0009-0006-2747-0805) (Research Fellow in Glasgow) processed the microbiome sequencing data and QC.
Vaios Svolos (https://orcid.org/0000-0002-7785-4245) (Research Fellow in Glasgow) performed the measurements of SCFA and FCAL and dietary analysis.
Caroline Kerbiriou (https://orcid.org/0000-0002-7927-2740) (Postdoctoral Research Assistant, Human Nutrition) performed the measurements of SCFAs and FCAL.
Claire McMurray (https://orcid.org/0009-0008-7550-9887) (Postdoctoral Research Fellow) developed and validated the 16S extraction and sequencing protocol and performed 16S stool sequencing.
Andrew Beggs (https://orcid.org/0000-0003-0784-2967) (Professor of Cancer Genetics and Surgery) provided sequencing capacity in the genomics laboratory at the University of Birmingham.
Richard Hansen (https://orcid.org/0000-0002-3944-6646) (Consultant Paediatric Gastroenterologist) was a member of the TMG and cowrote the protocol.
Ailsa L Hart (https://orcid.org/0000-0002-7141-6076) (Consultant Gastroenterologist) was PI at St Marks Hospital and involved in all intellectual aspects of the project, that is designing the protocol, attending TMG, recruiting patients and coauthoring the final report.
Daniel R Gaya (https://orcid.org/0000-0003-1942-7568) (Consultant Gastroenterologist) was PI in Glasgow and involved in all intellectual aspects of the project, that is designing the protocol, attending TMG, recruiting patients and coauthoring the final report.
Tariq H Iqbal (https://orcid.org/0000-0002-6681-9882) (Consultant Gastroenterologist) led the conception and design of the project, the conduct and oversight of the study, recruitment, data acquisition, the interpretation of the results and the writing and editing of this report.
Acknowledgements
Nicola Crees (Public and Patient Representative, Crohn’s and Colitis UK) was involved in the inception of the project, throughout as a member of the TMG and reviewed the draft report. Sam Nicholls developed the lab informatics infrastructure. Josh Quick provided guidance on the lab work.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they arestored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
STOP-COLITIS adopts a controlled access data-sharing policy. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review by the BCTU data-sharing committee.
Ethics statement
This study was approved by the UK National Ethics Committee East Midlands-Nottingham 2 Research Ethics Committee (REC 17/EM/0274) and by the relevant regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA) (CTA#21761/0337/001-0001 and EudraCT# 2015-005753-12) November 2017. Subsequent amendments were also reviewed and approved by the UK National Ethics Committee East Midlands-Nottingham 2 Research Ethics Committee.
Information governance statement
The University of Birmingham is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation the University of Birmingham is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: The Data Protection Office, Legal Services, the University of Birmingham, Edgbaston, Birmingham B15 2TT.
E-mail: dataprotection@contacts.bham.ac.uk Telephone: 0121 414 3916
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YCJD4579.
Primary conflicts of interest: Ailsa Hart has received funding from industry for consultancy work, support for attending meetings and honoraria for lectures. Ailsa Hart also reports being a Governing Board member (unpaid) for European Crohn’s and Colitis Organisation and committee member (unpaid) for British Society of Gastroenterology CRG and GMfH. Christel McMullan reports receiving support from NIHR EME, NIHR UKRI, NIHR BTRU, NIHR SRMRC and Innovate UK paid directly to the Institution. Christel McMullan reports receiving consulting fees from Aparito Ltd. Christopher Quince reports receiving consulting fees from Takeda pharmaceuticals. Daniel R Gaya has undertaken paid lectures for the following pharmaceutical companies; Abbvie, Janssen, Pfizer, Galapagos & Takeda and also reports providing opinions as an expert witness in numerous court cases. Jonathan Segal reports receiving consulting fees and support for attending meetings from BMS Medical and payments for lectures from Pfizer. Konstantinos Gerasimidis reports receiving consulting fees from Nestle Health Science, Nutricia-Danone, Baxter and payments or honoraria for lectures and presentations from Nestle Health Science, Nutricia-Danone, Baxter Abbott, Servier, Abbvie, Janssen, DrFalk. Mohammed Quraishi reports receiving consulting fees from Parapharm and Vifor and payments or honoraria for lectures and presentations from BMS, Takeda, Janssen and support for attending meetings from Tillotts. Naveen Sharma reports receiving consulting and DSMB advisory board fees from Pharmacosmos. Richard Hanson reports being Honorary Medical Director Crohn’s in Childhood Research Association 2017–20, Vice Chair Gut Microbiota for Health Expert Panel of British Society of Gastroenterology 2021–3; Chair 2023–5, Member British Society of Gastroenterology IBD Guidelines Committee 2016–9; 2021–present and Paediatric committee member European Crohn’s and Colitis Organisation 2022–5. Tariq Iqbal reports holding an Educational Grant from Hofmann Roche, consulting fees from Pharmacosmos and Janssen. Tariq Iqbal reports being a member of the NICE committee for FMT 2022 and Gut Microbiome for Health Expert panel (both unpaid). Vaios Svolos reports receiving support to attend the ECCO congress meeting as ECCO Officer and reports being an Elected member of the D-ECCO Working Group (Dietitian Working Group of European Crohn’s Colitis Organisation).
Disclaimers
This article presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59-65.
- Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm Bowel Dis 2014;20:861-71.
- de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016;13:13-27.
- Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther 2012;36:503-16.
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146:1489-99.
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2013;63:1275-83.
- Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, et al. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther 2013;38:151-61.
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012;9:599-608.
- Limketkai BN, Akobeng AK, Gordon M, Adepoju AA. Probiotics for induction of remission in Crohn’s Disease. Cochrane Database Syst Rev 2020;7. https://doi.org/10.1002/14651858.CD006634.pub3.
- Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2009;7:1202-1209.e1.
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 2010;105:2218-27.
- Bennet J, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989;333.
- Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2014;8:1569-81.
- Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102-109.e6.
- Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JHA, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110-118.e4.
- Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218-28.
- Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-Week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019;321:156-64.
- Shabat CS, Scaldiferri F, Zittan E, Hirsch A, Mentella MC, Musca T, et al. Use of faecal transplantation with a novel diet for mild to moderate active ulcerative colitis: The CRAFT UC randomised controlled trial. J Crohns Colitis 2022;16:369-78.
- Quraishi M, Yalchin M, Blackwell C, Segal J, Sharma N, Hawkey P, et al. STOP-Colitis pilot trial protocol: a prospective, open-label, randomised pilot study to assess two possible routes of faecal microbiota transplant delivery in patients with ulcerative colitis. BMJ Open 2019;9:e030659-9.
- Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 2015;149:223-37.
- Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018;67:1920-41.
- FFQ n.d. epic-norfolk.org.uk (accessed 1 June 2023).
- Ritchie J, Spencer L, O’Connor W. Qualitative Research Practice. London: SAGE Publications Ltd; 2003.
- Quince C, Walker A, Simpson J, Loman NJ, Segata N. Shotgun metagenomics from sampling to analysis. Nat Biotechnol 2017;35:833-44.
- Kamleh A, Barrett MP, Wildridge D, Burchmore RJS, Scheltema RA, Watson DG. Metabolomic profiling using Orbitrap Fourier transform mass spectrometry with hydrophilic interaction chromatography: a method with wide applicability to analysis of biomolecules. Rapid Commun Mass Spectrom 2008;22:1912-8.
- Creek DJ, Jankevics A, Burgess KEV, Breitling R, Barrett MP. IDEOM: an Excel interface for analysis of LC-MS-based metabolomics data. Bioinformatics 2012;28:1048-9.
- t’Kindt R, Jankevics A, Scheltema RA, Zheng L, Watson DI, Dujardin JC, et al. Towards an unbiased metabolic profiling of protozoan parasites: optimisation of a Leishmania sampling protocol for HILIC-orbitrap analysis. Anal Bioanal Chem 2010;398:2059-69.
- Creek DJ, Jankevics A, Breitling R, Watson DG, Barrett MP, Burgess KEV. Toward global metabolomics analysis with hydrophilic interaction liquid chromatography-mass spectrometry: improved metabolite identification by retention time prediction. Anal Chem 2011;83:8703-10.
- Murali A, Bhargava A, Wright ES. IDTAXA: a novel approach for accurate taxonomic classification of microbiome sequences. Microbiome 2018;6.
- Reese AT, Dunn RR. Drivers of microbiome biodiversity: a review of general rules, feces, and ignorance. MBio 2018;9.
- Kawashima K, Ishihara S, Yuki T, Fukuba N, Oshima N, Kazumori H, et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol 2016;16.
- Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014;20:779-86.
- Fagan EA, Dyck RF, Maton PN, Hodgson HJ, Chadwick VS, Petrie A, et al. Serum levels of c-reactive protien in Crohn’s disease and ulcerative colitis. Eur J Clin Invest 1982;12:351-9.
- El-Salhy M, Valeur J, Hausken T, Gunnar Hatlebakk J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil 2021;33.
- Moeller AH. The shrinking human gut microbiome. Curr Opin Microbiol 2017;38:30-5.
- Burz SD, Abraham AL, Fonseca F, David O, Chapron A, Béguet-Crespel F, et al. A guide for ex-vivo handling and storage of stool samples intended for fecal microbiota transplantation. Sci Rep 2019;9.
- Carr I, Mayberry JF. The effects of migration on ulcerative colitis: a three-year prospective study among Europeans and first- and second-generation South Asians in Leicester (1991–1994). Am J Gastroenterol 1999;94:2918-22.
Appendix 1 Participant donor health screening questionnaire



Appendix 2 EPIC questionnaire

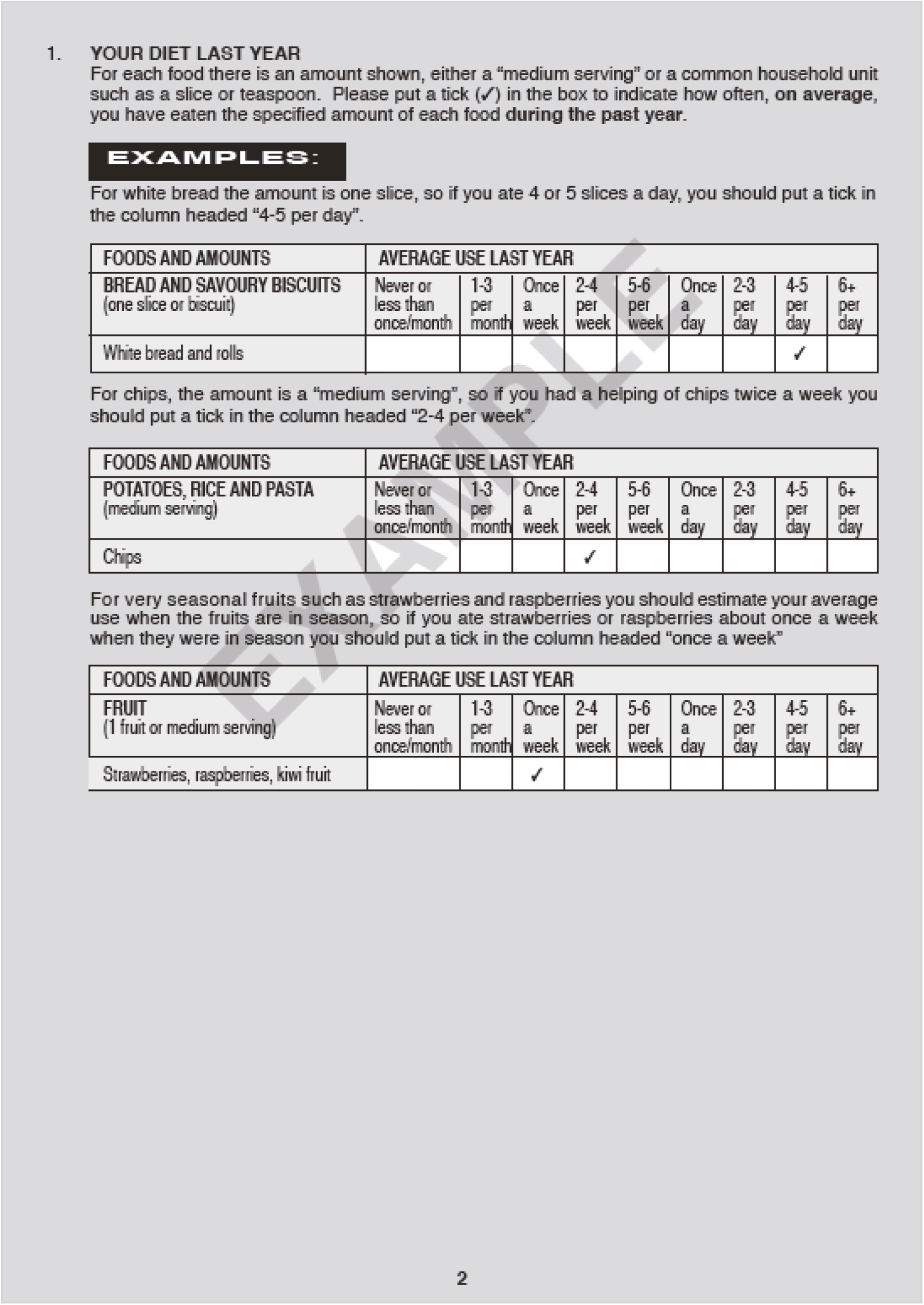
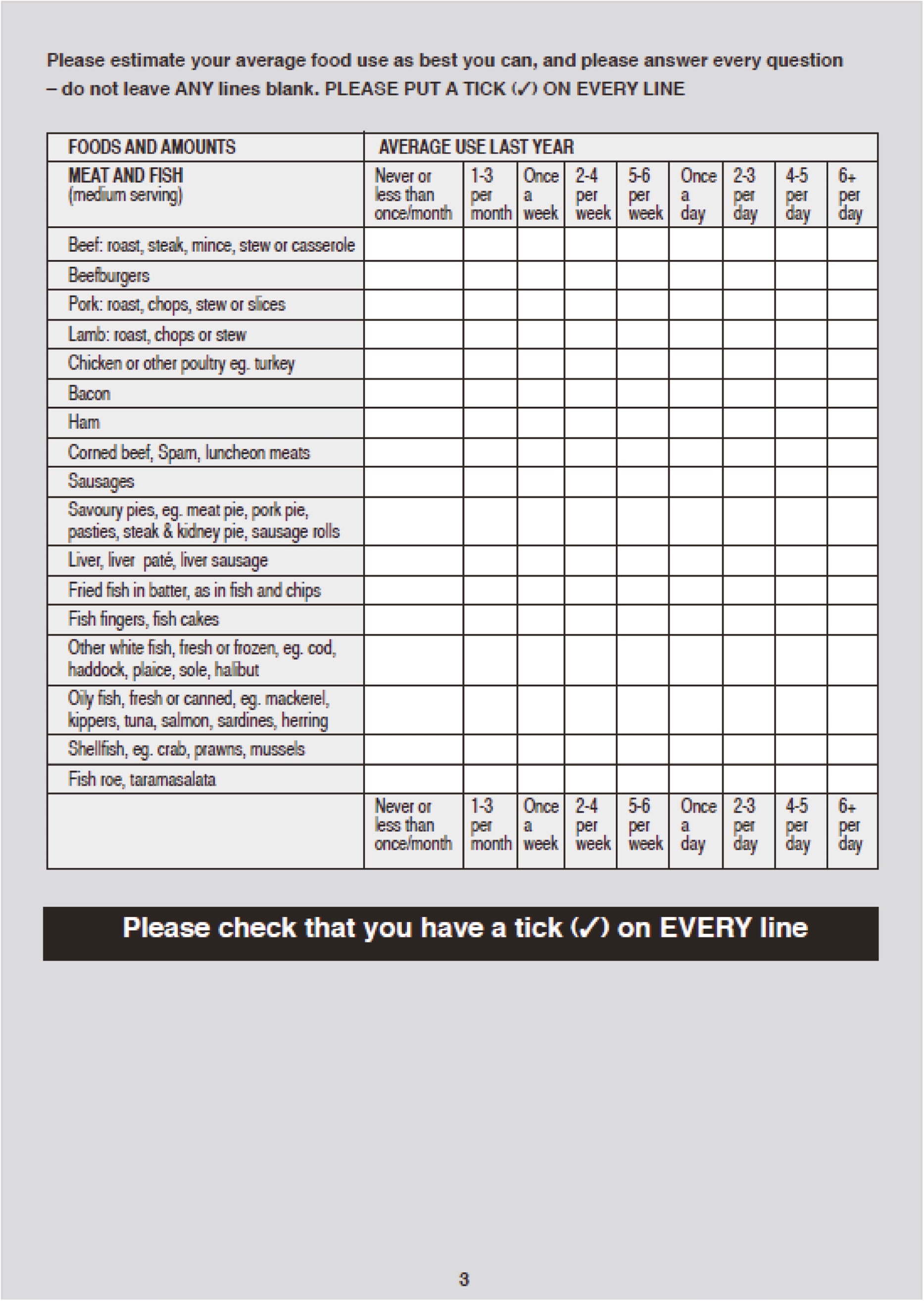
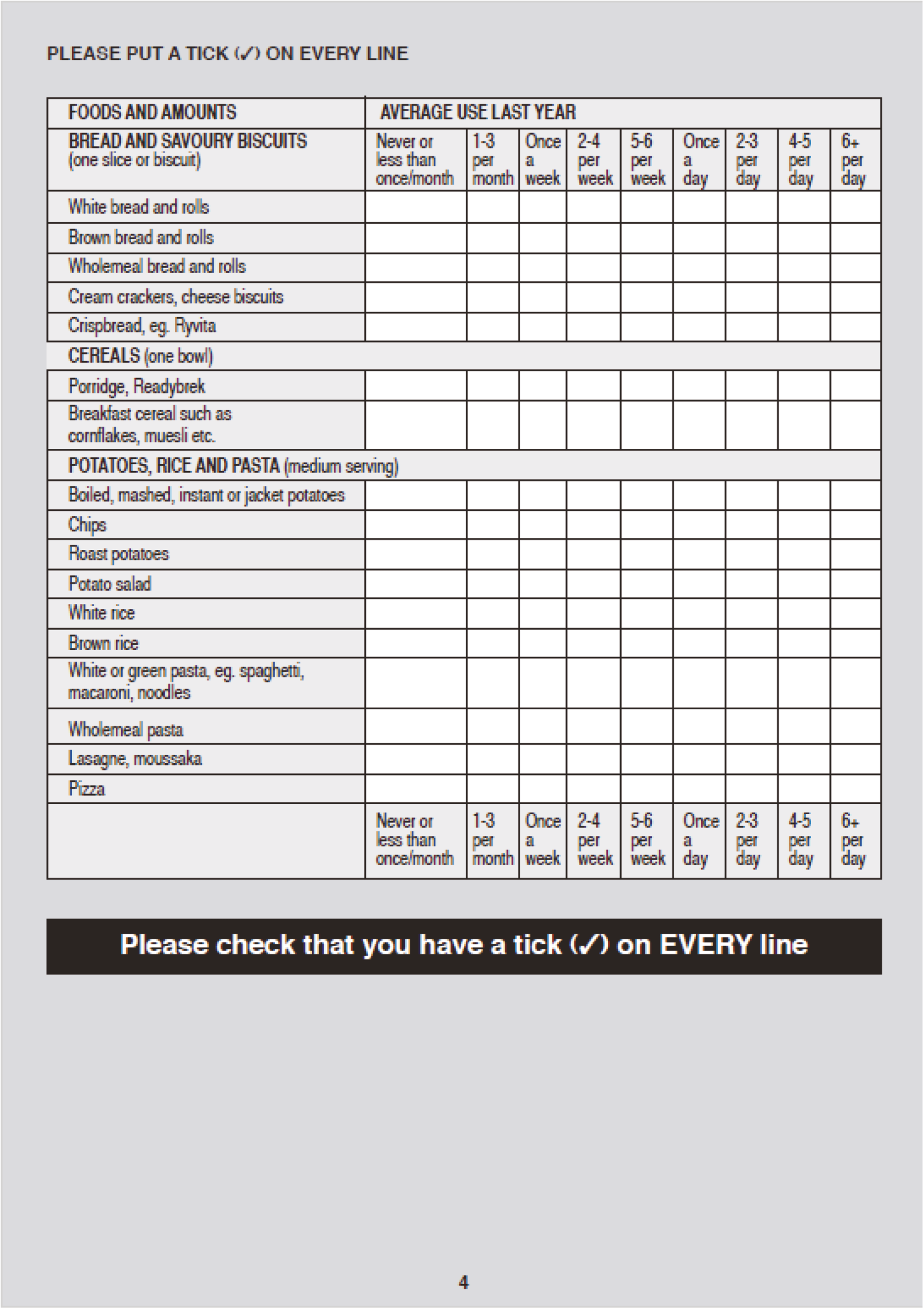
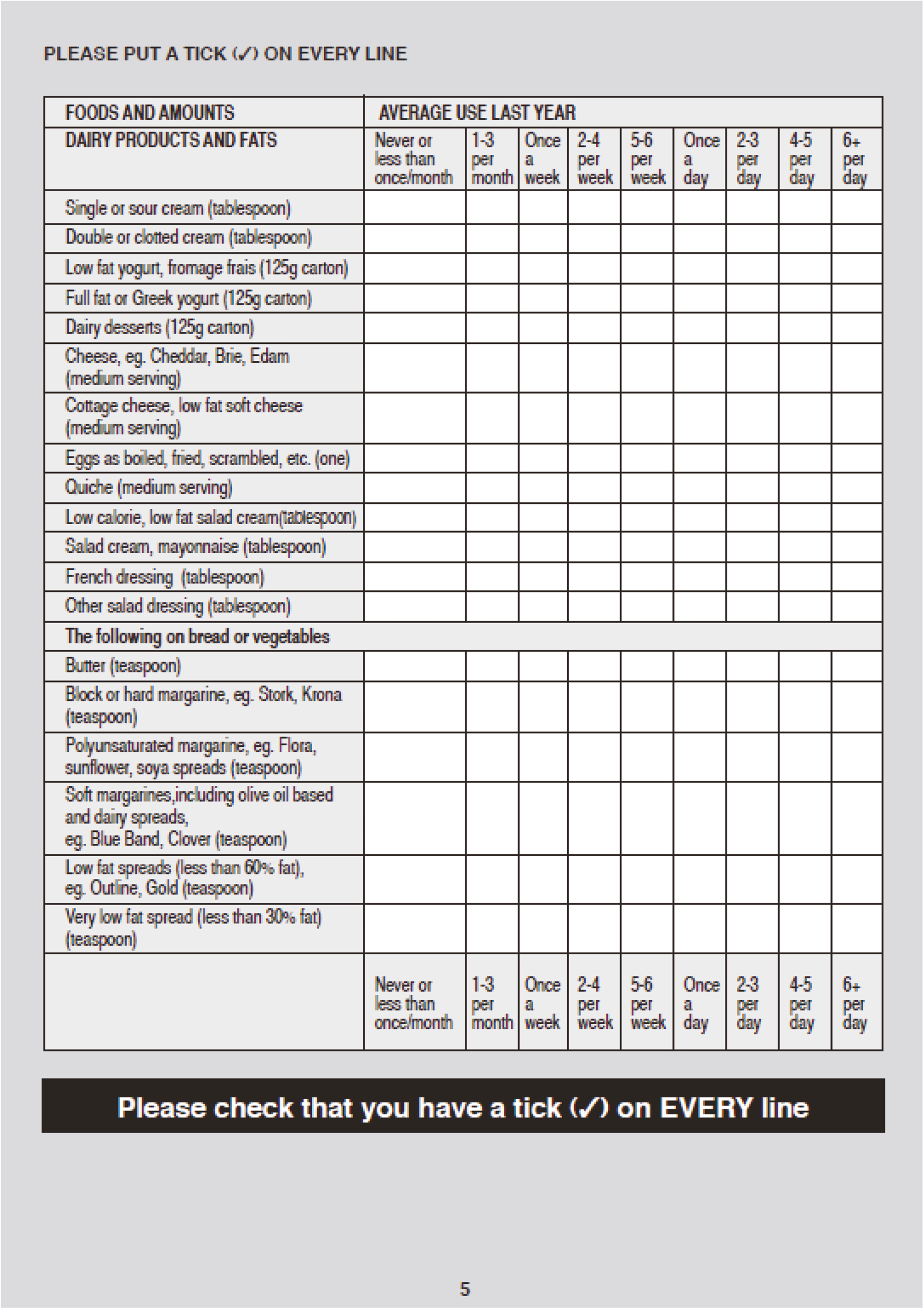
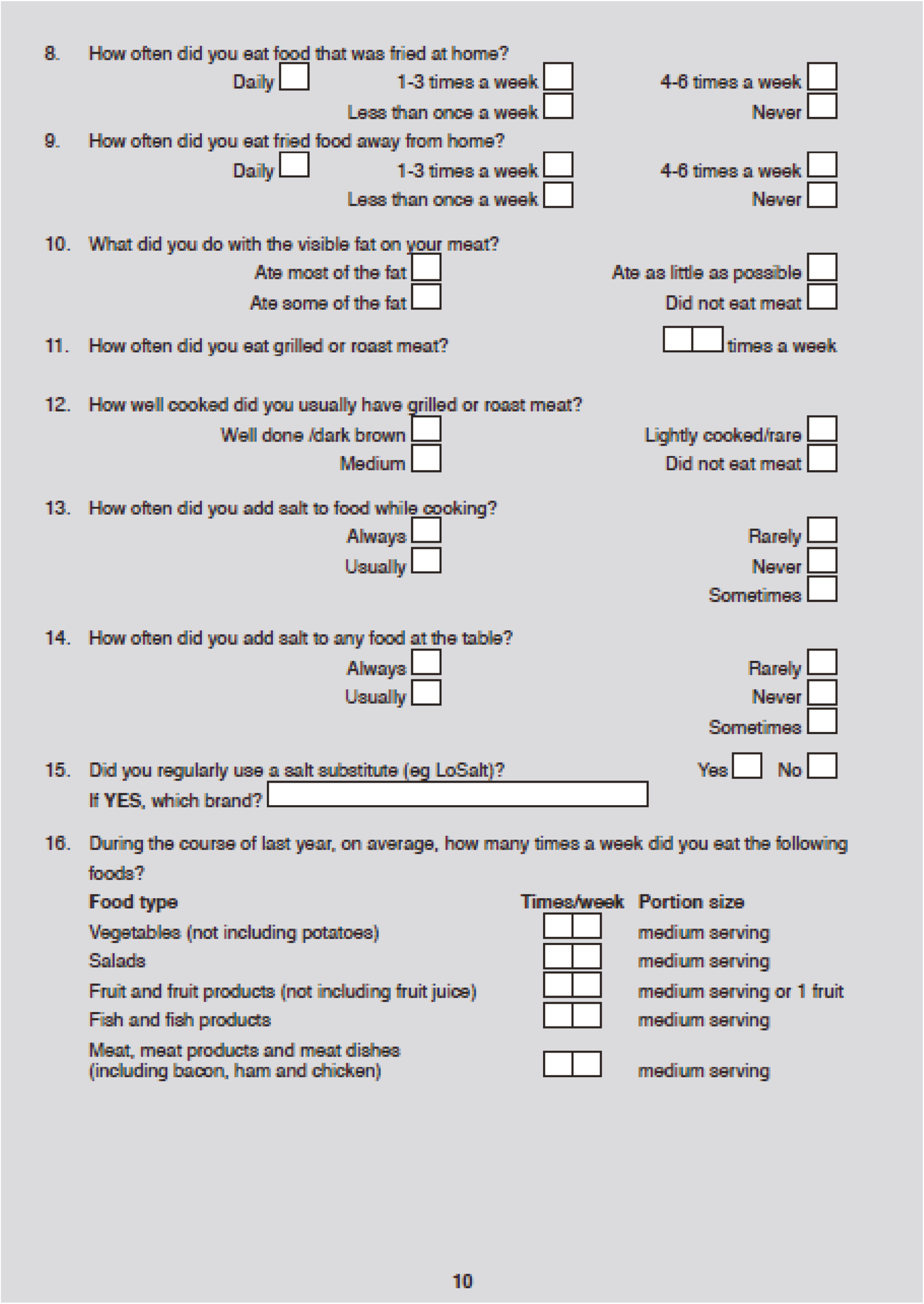
Appendix 3 MAYO scoring

Appendix 4 Qualitative research interviews, questions and schedules
INTERVIEW SCHEDULE STOP-COLITIS T1 (Baseline) – PATIENTS
INTRODUCTION
Thank the participant for agreeing to take part.
Ensure that the consent form has been signed. Keep a copy for the site file and give a copy back to the participant.
Statement on confidentiality, right to withdraw consent, recording of the interview.
Review the purpose of the study in general.
Emphasise the value of their views and opinions – there are no right or wrong answers.
Ask if the participant has any questions before starting the interview.
ICEBREAKER
Background, interviewee details and icebreaker, for example explore interviewee background, whether they have family, and why they were interested in taking part in this particular interview/research etc.
BACKGROUND INFORMATION (provides context for remainder of data) – INTERVIEWEES’ EXPERIENCE TO DATE WITH UC (symptoms, diagnosis, treatment, impact)
Brief discussion of period leading to diagnosis (e.g. when, symptoms, impact, presentation, initial treatment, knowledge of UC, reaction to diagnosis).
Brief discussion of experience postdiagnosis (e.g. experience of flare and remission, treatment, impact, adaptations).
PERSPECTIVES ON TRIAL RECRUITMENT, PARTICIPATION AND PROCEDURES
-
What are your reasons/motivations for taking part in STOP-COLITIS?
-
How long did it take you to decide to take part?
-
Did you make the decision on your own or did you ask other people’s opinion to help you decide? If so, who? And why?
-
-
Patients’ understanding of STOP-COLITIS.
-
In your own words, what do you think the aims of STOP-COLITIS are?
-
What were you told about it?
-
-
Patients’ understanding/expectations of FMT.
-
Were you aware of FMT before being introduced to STOP-COLITIS?
-
What is your understanding of FMT? NG? Colon?
-
What were you told about FMT before consenting to the trial?
-
How do you feel about receiving someone else’s stool sample?
-
How do you think it compares with other treatments (medical treatment)?
-
Do you know what the two groups (NG/colon) entail?
-
How would you react to being randomised the NG group? The Colonoscopy group?
-
Do you have any preferences as to which group you would like to be randomised to?
-
Have you told your partner/close family about what FMT is?
-
What do your close family and partner/parents think about FMT?
-
-
Expectations for trial participation.
-
What do you expect from taking part in STOP-COLITIS?
-
-
Experience of information and consent received from medical team.
-
Type of information received.
-
Who provided the information?
-
Was it useful?
-
Enough time to digest it? To think about the study?
-
What other information would patients have liked (if any)?
-
-
Potential barriers/facilitators to patients’ participation in STOP-COLITIS.
-
What might influence patients to participate?
-
Why might patients decide not to take part in STOP-COLITIS?
-
What could be done to encourage them taking part in STOP-COLITIS?
-
-
Experience of different stages of trial.
-
How would you describe your experience of consenting to STOP-COLITIS?
-
Who took consent?
-
Did you have enough time to think about it before consenting?
-
Do you know what ‘random allocation’ is in STOP-COLITIS and what it involves? Who did the randomisation?
-
Do you know what the follow-up process entails in STOP-COLITIS? Questionnaires/blood tests/stool samples etc…?
-
-
Outcomes.
-
What should we be comparing/measuring in these questionnaires etc…?
-
What is important to you (in terms of symptoms, treatment, QoL, etc…).
-
-
The main trial, after this pilot trial, will include a placebo group and an intervention group (NG or colon?), hypothetically, how would you feel about being in a placebo group?
-
I’ve come to the end of my questions, do you have any other issues you wish to talk about?
CLOSING
Tell the participants that they have reached the end of the interview.
Check understanding of any outstanding points and give the participant the opportunity to ask any further questions.
Check arrangements for scheduling the follow-up interview.
Remind them about confidentiality and thank them for their time.
INTERVIEW SCHEDULE STOP-COLITIS T2 (3 months) – PATIENTS
INTRODUCTION
Thank the participant for agreeing to take part.
Ensure that the consent form has been signed. Keep a copy for the site file and give a copy back to the participant.
Statement on confidentiality, right to withdraw consent, recording of the interview.
Review the purpose of the study in general.
Emphasise the value of their views and opinions – there are no right or wrong answers.
Ask if the participant has any questions before starting the interview.
HOW PATIENTS FEEL AFTER 3 MONTHS
-
How have you been in general since the last time I saw you?
-
What symptoms have you experienced over the last 3 months? (Any changes since initial interview?)
-
Have you had any flare-ups since initial interview? Types/severity/frequency of symptoms? Treatment?
-
Has anything changed since the last time I saw you?
PILOT TRIAL PROCESSES AND PROCEDURES
-
How did you feel about being randomised to NG/colon?
-
Were you hoping to be randomised to NG/colon?
-
-
What was your experience of FMT (NG)?
-
Colonoscopy?
-
Medication prior to/after FMT infusion?
-
Intubation?
-
Attending the Clinical Research Facility (CRF) daily?
-
Did it match your expectations?
-
Do you wish you had been randomised to the other group? Why? Why not?
-
-
What was your experience of FMT (colon)?
-
Colonoscopy?
-
Medication prior to/after FMT dose?
-
Endoscopy?
-
Enema?
-
Attending the CRF weekly?
-
-
What was your experience of the clinical reviews/assessments after the intervention?
-
What did you think of the QoL questionnaires you had to complete?
-
What did you think of IBD diaries? Did you complete them? How did you find it?
-
What did you think of the stool kit? Was it easy to use?
-
Is there anything that could have been done differently as far as these follow-up assessments are concerned?
-
-
Overall, have your initial expectations of taking part in the trial been met? Have these expectations changed?
-
What do you think are the barriers and facilitators to patients’ participation in STOP-COLITIS?
-
Is there anything that you think we should do differently?
CLOSING
Tell the participants that they have reached the end of the interview.
Check understanding of any outstanding points and give the participant the opportunity to ask any further questions.
Remind them about confidentiality and thank them for their time.
INTERVIEW SCHEDULE STOP-COLITIS INTERVIEW – CLINICIANS
INTRODUCTION
Thank the participant for agreeing to take part.
Ensure that the consent form has been signed. Keep a copy for the site file and give a copy back to the participant.
Statement on confidentiality, right to withdraw consent, recording of the interview.
Review the purpose of the study in general.
Emphasise the value of their views and opinions – there are no right or wrong answers.
Ask if the participant have any questions before starting the interview.
BACKGROUND/ICEBREAKER
-
What is your role and position?
-
What is your involvement in STOP-COLITIS?
RECRUITMENT (if applicable)
-
What has been your involvement in patient recruitment?
-
How many patients have been recruited to STOP-COLITIS?
-
Are you on track to reach your target?
-
If not, what steps will you take to reach your target?
-
-
Can you describe the recruitment pathway?
-
How have patients been approached?
-
How have patients been introduced to STOP-COLITIS?
-
What information has been given to them? By whom?
-
How have patients reacted to this information?
-
-
Has it worked well? If so, what has worked well?
-
If not, what would you change? What do you think we could do differently?
-
Have any patients declined to take part in STOP-COLITIS? Do you know why they have declined?
-
What do you think are the barriers to patient recruitment in STOP-COLITIS?
-
-
Have any patients withdrawn from STOP-COLITIS? Do you know why they have withdrawn?
TAKING CONSENT (if applicable)
-
What has been your involvement in taking consent?
-
Can you describe your experience of the consenting process?
-
How much time were patients given before consenting?
-
Who has been taking patient consent?
-
Has there been any issues with consenting patients?
-
How have patients reacted?
-
-
Has it worked well? If so, what has worked well?
-
If not, what has not worked well? What would you change? What do you think we should we do differently?
RANDOMISATION/ALLOCATION (if applicable)
-
What has been your involvement in the randomisation?
-
Can you describe your experience of the randomisation process?
-
Has there been any issues with the randomisation process?
-
How have patients reacted to allocation?
-
-
Has it worked well? If so, what has worked well?
-
If not, what has not worked well? What would you change? What do you think we should we do differently?
INTERVENTION (if applicable)
-
What has been your involvement in the delivering the intervention?
-
What are your views of FMT? NG? Colon?
-
Can you describe your experience with the process of FMT supply, collection, preparation and storage within STOP-COLITIS? (If applicable).
-
What have the main issues been so far?
-
Has it worked well? If so, what has worked well?
-
If not, what has not worked well? What would you change? What do you think we should do differently?
-
-
Can you describe your experience of delivering FMT?
-
NG.
-
COLON.
-
How have patients reacted to both?
-
Has it worked well? If so, what has worked well with NG/colon?
-
If not, what has not worked well with NG/colon? What would you change? What do you think we should do differently?
-
FOLLOW-UP VISITS (if applicable)
-
What has your involvement been in the follow-up assessments (clinical reviews/QoL questionnaires, colonoscopy/sig, stool samples, IBD diaries)?
-
What has your experience been of the follow-up assessments?
-
Has it worked well? If so, what has worked well with the follow-up assessments?
-
If not, what has not worked well with follow-up assessments? What would you change? What do you think we should do differently?
-
How patients have reacted with the follow-up assessments?
-
Have patients been complying with the visits/treatment?
-
-
What other outcomes/outcome measures should have been included?
OVERALL EXPERIENCE
-
Overall, what was your experience of STOP-COLITIS?
-
How is STOP COLITIS fitting with your clinical work?
-
What is working well within in STOP-COLITIS?
-
What are the main barriers/facilitators to the trial?
-
How could STOP-COLITIS be improved?
-
Close
Thank participant and answer any further questions.
INTERVIEW SCHEDULE STOP-COLITIS – PATIENT DECLINERS
INTRODUCTION
Thank the participant for agreeing to take part.
Ensure that the consent form has been signed. Keep a copy for the site file and give a copy back to the participant.
Statement on confidentiality, right to withdraw consent, recording of the interview.
Review the purpose of the study in general.
Emphasise the value of their views and opinions – there are no right or wrong answers.
Ask if the participant have any questions before starting the interview.
BACKGROUND/ICEBREAKER
Background, interviewee details and icebreaker, for example explore interviewee background, whether they have family, etc….
BACKGROUND INFORMATION (provides context for remainder of data) – INTERVIEWEES’ EXPERIENCE TO DATE WITH UC (symptoms, diagnosis, treatment, impact)
Brief discussion of period leading to diagnosis (e.g. when, symptoms, impact, presentation, initial treatment, knowledge of UC, reaction to diagnosis).
Brief discussion of experience postdiagnosis (e.g. experience of flare and remission, treatment, impact, adaptations).
PERSPECTIVES ON TRIAL RECRUITMENT AND PARTICIPATION
-
11 Patients’ understanding of STOP-COLITIS.
-
In your own words, what do you think the aims of STOP-COLITIS are?
-
Do you know what ‘random allocation’ is in STOP-COLITIS and what it involves?
-
-
12 Patients’ understanding/expectations of FMT.
-
Were you aware of FMT before being introduced to STOP-COLITIS?
-
What is your understanding of FMT? NG? Colon?
-
Do you know what the two groups (NG/colon) entail?
-
-
13 Experience of information received from medical team.
-
Type of information received
-
Who provided the information?
-
Was it useful?
-
Would patients have liked other information? If so, what information?
-
REASONS FOR DECLINING TO PARTICIPATE
-
14. What are your reasons/motivations for not taking part in STOP-COLITIS?
-
How long did it take you to decide not to take part?
-
Did you make the decision on your own or did you ask other people’s opinion to help you decide? If so, who? And why?
-
-
15. Potential barriers/facilitators to STOP-COLITIS.
-
What might influence patients to participate?
-
What else might make patients decide not to take part in STOP-COLITIS?
-
What could be done to encourage them taking part in STOP-COLITIS?
-
-
16. What do you think we could do differently overall?
-
17. Do you have any other issues you wish to talk about?
CLOSING
Tell the participants that they have reached the end of the interview.
Check understanding of any outstanding points and give the participant the opportunity to ask any further questions.
Remind them about confidentiality and thank them for their time.
INTERVIEW SCHEDULE STOP-COLITIS – WITHDRAWAL PATIENTS
INTRODUCTION
Thank the participant for agreeing to take part.
Ensure that the consent form has been signed. Keep a copy for the site file and give a copy back to the participant.
Statement on confidentiality, right to withdraw consent, recording of the interview.
Review the purpose of the study in general.
Emphasise the value of their views and opinions – there are no right or wrong answers.
Ask if the participant have any questions before starting the interview.
BACKGROUND/ICE BREAKER
What are your main reasons for agreeing to talk to me (qualitative researcher)?
HOW PATIENTS FEEL AFTER 3 MONTHS
-
How have you been in general since the last time I saw you?
-
What symptoms have you experienced over the last 3 months? (Any changes since initial interview?)
-
Have you had any flare-ups since initial interview? Types/severity/frequency of symptoms? Treatment?
-
Has anything changed since the last time I saw you?
PILOT TRIAL PARTICIPATION AND PROCEDURES
-
8 How did you feel about being randomised to NG/colon?
-
Were you hoping to be randomised to NG/colon?
-
-
9 What was your experience of FMT (NG)?
-
Colonoscopy?
-
Medication prior to/after FMT infusion?
-
Intubation?
-
Attending the CRF daily?
-
Did it match your expectations?
-
Do you wish you had been randomised to the other group? Why? Why not?
-
-
10 What was your experience of FMT (colon)?
-
Colonoscopy?
-
Medication prior to/after FMT dose?
-
Endoscopy?
-
Enema?
-
Attending the CRF weekly?
-
-
11 What was your experience of the clinical reviews/assessments after the intervention? (If applicable)
-
What did you think of the QoL questionnaires you had to complete?
-
What did you think of IBD diaries? Did you complete them? How did you find it?
-
What did you think of the stool kit? Was it easy to use?
-
What do you we should have done differently as far as these follow-up assessments are concerned?
-
-
12 Overall, have your initial expectations of taking part in the trial been met? Have these expectations changed?
REASONS FOR WITHDRAWING FROM STOP-COLITIS
-
13. What were your main reasons for withdrawing from the trial?
-
Did you take the decision on your own? Did you ask for other people’s advice? If so, who?
-
-
14. What else would make patients decide to withdraw from the study?
-
15. What could be done to prevent patients from withdrawing from the study?
-
16. What do you think we should do differently overall?
CLOSING
Tell the participants that they have reached the end of the interview.
Check understanding of any outstanding points and give the participant the opportunity to ask any further questions.
Remind them about confidentiality and thank them for their time.
List of abbreviations
- AE
- adverse event
- AGA
- American Gastroenterology Association
- ASV
- amplicon sequence variant
- BCTU
- Birmingham Clinical Trials Unit
- CCUK
- Crohn’s and Colitis UK
- CD
- Crohn’s disease
- CDI
- Clostridioides difficile infection
- CRP
- C-reactive protein
- ELISA
- enzyme-linked immunoassay
- FMT
- faecal microbiota transplant
- GMP
- good manufacturing practice
- IBD
- inflammatory bowel disease
- IBDQ
- inflammatory bowel disease questionnaire
- IOC
- Independent Oversight Committee
- ITT
- intention to treat
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NG
- nasogastric
- OTU
- Operational Taxonomic Unit
- PPI
- patient and public involvement
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SCFA
- short-chain fatty acids
- SF-36
- short-form 36
- TMG
- trial management group
- UC
- ulcerative colitis
