Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as award number 12/10/18. The contractual start date was in June 2014. The draft manuscript began editorial review in January 2022 and was accepted for publication in March 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Schoeler et al. This work was produced by Schoeler et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Schoeler et al.
Chapter 1 Introduction
Background
Epilepsy is a condition whereby individuals are prone to recurrent epileptic seizures, a change in behaviour or movement that is the direct result of a primary change in the electrical activity in the brain. It is not a single condition ‒ there are many different underlying causes and, more accurately, they should be referred to as the epilepsies. Up to 65% of individuals with epilepsy will have seizures controlled with antiseizure medicines (ASMs) or enter spontaneous remission in their lifetime; however, this leaves 35% who will continue with seizures despite treatment. Standard first-line management of an individual presenting with epilepsy is ASM, chosen on the basis of the type of epilepsy. Although guidelines exist on which drug to use (www.nice.org.uk/cg217), management is still based on a ‘trial and error’ approach. When the type of epilepsy is unclear, it can be difficult to optimise treatment at the outset. 1
The incidence of epilepsy is greatest in the first 2 years of life (56–88/100,000 children/year),2 a population who remain most at risk for neurodevelopmental compromise in the longer term. Early control of seizures is associated with better developmental outcome3 but, unfortunately, many of the epilepsies presenting in infancy are associated with a poor prognosis for seizure control. 4 Few data are available with regard to effective treatments and, even where seizure freedom is achieved, this is unlikely to be sustained long term;5 this group are the least likely to achieve longer-term remission. 6 Over 50% of infants presenting with seizures have infantile spasms, for which first-line treatment options (corticosteroids or vigabatrin) lead to seizure freedom in up to 70% of cases,7 but side effects limit their duration of use and relapse rates are not insignificant (40%). 8 This group of infants therefore place a large burden on NHS services, with a need for regular clinical review and ongoing medication, as well as clinical and therapy support. This is especially true for those who remain resistant to medication, this group being among the costliest for medical and care services long term. It is therefore imperative that all other treatment options are explored as early as possible. 1
Ketogenic diet therapies (KDTs), a group of high-fat, low-carbohydrate diets designed to mimic the effects of starvation on the body, are a non-pharmacological treatment option for individuals with drug-resistant epilepsy. The main energy intake is fat, which is utilised in the body to produce ketones. There are several forms of KDT used, but the classical form of the diet, based on a ratio of grams of fat to grams of protein and carbohydrate combined, is that most commonly used for infants. 9
Although KDT has been used in excess of 100 years in the treatment of epilepsy, our group previously published the first randomised control trial (RCT) of the KD, demonstrating effectiveness in children aged 2–16 years. 10 In this trial, 145 children aged 2–16 years, who had failed at least 2 ASMs and had at least 7 seizures weekly, were randomised to receive either a classical KD or medium-chain triglyceride (MCT) KD, either immediately or after a 3-month delay with no additional treatment changes (control group). After 3 months, the mean percentage of baseline seizures (on an intention-to-treat analysis) was significantly lower in the diet group (62%) than in the controls (137%, P < 0.0001). Twenty-eight (38%) of the diet group had >50% seizure reduction, compared to four (6%) controls (p < 0.0001). This study, together with three other RCTs since published looking at KDTs compared to usual care in children, was included in a recent Cochrane review;11 seizure freedom [relative risk (RR) 3.16, 95% CI 1.20 to 8.35; p = 0.02; 4 studies, 385 participants; very-low-certainty evidence] and seizure reduction (RR 5.80, 95% CI 3.48 to 9.65; p < 0.001; 4 studies, 385 participants; low-certainty evidence) favoured KDTs compared to usual care for children. The authors were ‘not confident that these estimated effects are accurate’. The most commonly reported adverse effects were vomiting, constipation and diarrhoea for both intervention and usual-care groups, but ‘the true effect could be substantially different (low-certainty evidence)’. The authors reported that there is a ‘lack of evidence for the use of KDs in infants with epilepsy, therefore, further research would be of benefit’.
A recent systematic review conducted by our group showed KD efficacy data to have been published for 534 infants across 33 studies: 2 were RCTs (one assessing use of classical KD versus adrenocorticotropic hormone in infants with infantile spasms, and one assessing classical KD versus modified Atkins diet in children, including 37 aged under 2 years) and the remainder were uncontrolled cohort studies. All studies were categorised as low quality. Meta-analyses of uncontrolled studies estimated that 59% (95% CI 53 to 65) of infants achieved ≥ 50% seizure reduction and 33% (95% CI 26 to 43) of infants achieved seizure freedom when following a KD. The need was identified for an adequately powered RCT assessing KD versus standard pharmacological treatment in infants (in particular aged under 2 years, as no RCT has focused on this age group) with epilepsy with a range of seizure types.
Despite its name, the efficacy of KDTs cannot be explained solely by brain ketone body accumulation. Various mechanisms with regard to its action have consequently been proposed. 12 Of particular interest to us is the potential role that medium-chain fatty acids play in the effect of KDTs. In experimental animals, a KD leads to mitochondrial biogenesis, alterations in brain energy metabolism and a consequent elevation of the seizure threshold. 13 However, neither the mechanism for this mitochondrial proliferation nor the metabolic changes associated with such changes are currently known. In addition to causing an elevation of ketones, KDTs increase the plasma concentration of medium-chain fatty acids. 14 Furthermore, such an increase in plasma concentration will lead to increased brain availability as medium-chain fatty acids are transported across the blood–brain barrier. 15,16 In the younger child, there is evidence that a switch to fatty-acid oxidation is undertaken more readily than in older children. 17 The increased availability of medium-chain fatty acids has, so far, received little attention. However, it is reported that fatty acids can influence mitochondrial function. 18 Recent work in our group (funded by Vitaflo International Ltd) has demonstrated that medium-chain fatty acids, particularly C10, may enhance neuronal mitochondrial function by stimulating mitochondrial proliferation. Medium-chain fatty acids, particularly C10, have also been shown more recently to have an antiepileptic effect. 19 These data raise the possibility that C10 alone has the ability to mimic aspects of KDs. Whether this has a role in a possible enhanced action of KDs in infancy should be determined and the biochemical basis for effectiveness identified.
We here report initial results of our RCT of infants comparing the efficacy of the classical KD to a further appropriate ASM when a child has failed two ASMs, with a component to examine the possible role of medium-chain fatty acids. The study is the first of its kind in infants, and will make a significant contribution to the research evidence-base for treatment of infants with epilepsy.
Chapter 2 Methods
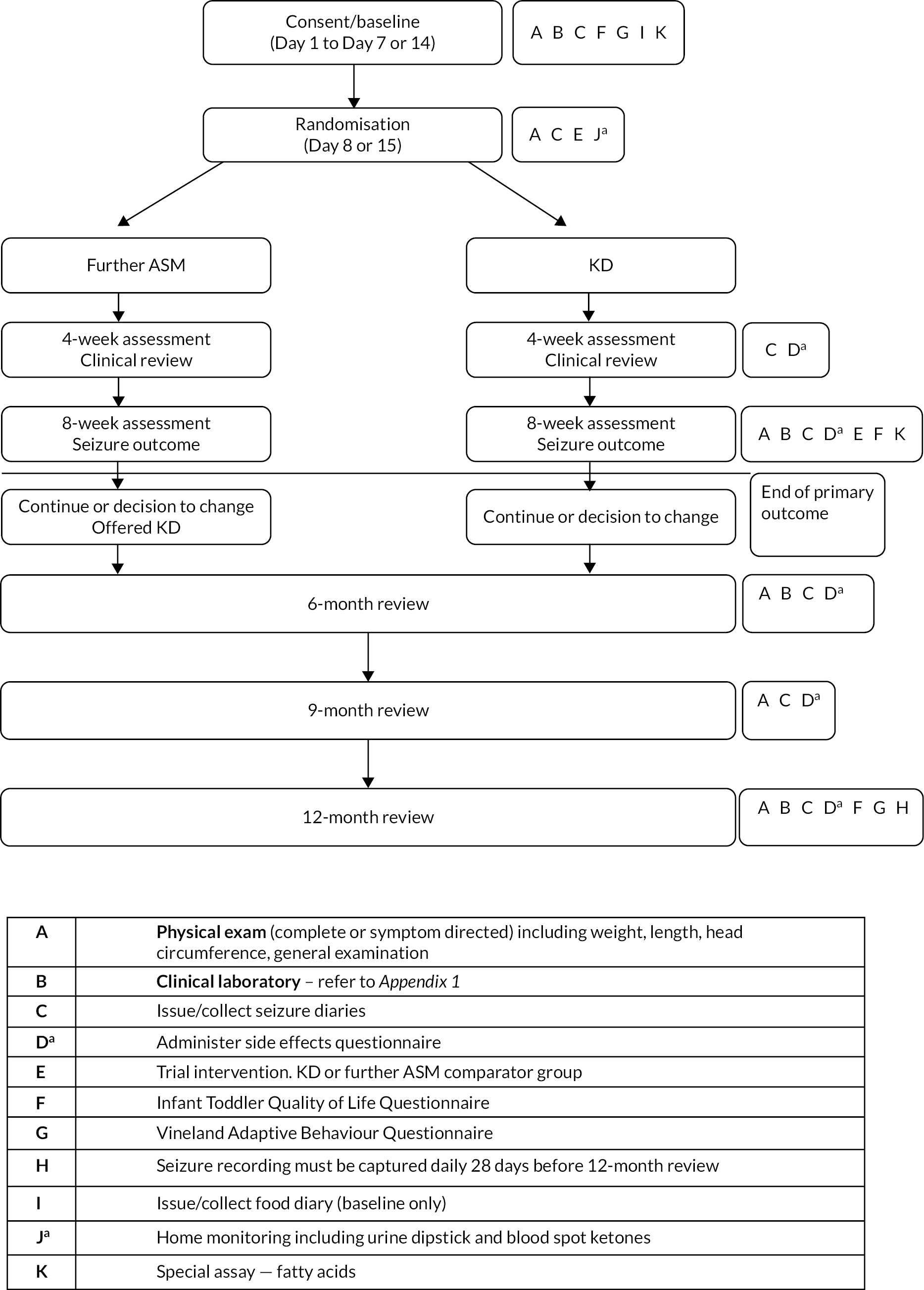
This was an open-label, randomised controlled multicentre clinical trial comparing KD to further ASM in infants with epilepsy, with an allocation ratio of 1 : 1.35 to account for the therapist effect in the KD group only. The protocol has been previously described. 1 Eligible infants were consented via their parents to undergo baseline assessment; they then started a 1–2 week observation period with documentation of seizure frequency; randomisation occurred on Day 8 or Day 15 for them to receive a KD or a further ASM. Assessments were repeated at 8 weeks after the start of treatment, and all infants were followed up for 12 months following randomisation for retention, seizure outcome and neurodevelopmental status (Figure 1).
FIGURE 1.
Schematic of trial design. a, KD arm only.
Patient selection
Inclusion criteria
-
Age between 1 month and 24 months (not beyond second birthday at baseline).
-
Diagnosis of epilepsy confirmed.
-
Seizure frequency ≥ 4 seizures/week on average in the baseline period.
-
Failed response to previous trial of two antiseizure medicines. In the case of infantile spasms this could include a trial of corticosteroids.
-
Infants with written informed consent from parent/carer.
Exclusion criteria
-
Age < 1 month or > 24 months.
-
No secure diagnosis of epilepsy.
-
On average < 4 seizures/week in the baseline period.
-
Trial of < 2 ASMs.
-
Continued on corticosteroids < 2 weeks prior to randomisation.
-
Metabolic disease contraindicating use of the KD, for example pyruvate carboxylase deficiency, medium-chain acyl-CoA dehydrogenase (MCAD) deficiency from previous medical investigation, and screening at baseline.
-
Progressive neurological disease.
-
Severe gastroesophageal reflux.
-
Previous treatment with the KD.
-
Concurrent participation in another clinical trial of an investigational medicinal product (IMP).
-
Infants who are prescribed investigational or unlicensed ASMs.
-
Infants who have a listed contraindication as per the SmPC to any of the ASMs listed in the trial IMPs.
Study sites
-
Great Ormond Street Hospital for Children.
-
Royal Aberdeen Children’s Hospital.
-
Bristol Royal Hospital for Children.
-
Birmingham Children’s Hospital.
-
Cambridge University Hospitals.
-
Tayside Children’s Hospital (Dundee).
-
Evelina London Children’s Hospital (recruited in pilot phase only).
-
Royal Hospital for Children (Glasgow).
-
Royal Hospital for Sick Children (Edinburgh).
-
Leeds Children’s Hospital.
-
Leicester Royal Infirmary.
-
Alder Hey Children’s Hospital.
-
Royal Manchester Children’s Hospital.
-
The Great North Children’s Hospital.
-
Nottingham Children’s Hospital.
-
Oxford University Hospitals.
-
Royal Preston Hospital.
-
Sheffield Children’s Hospital.
-
St Georges Hospital.
Informed consent procedure
Full ethical approval was sought and approved by the Research Ethics Committee (REC reference 14/LO/1230; IRAS project ID 142888) on 2 September 2014 prior to trial start.
Medicines and Healthcare Products Regulatory Agency (MHRA) approval was sought with annual review in view of the use of medicinal compounds as a comparator.
Parents/carers of potential participants were approached initially by a member of their direct healthcare team, who provided them with the REC-approved version of the patient information sheet. Written informed consent was obtained from each parent/carer prior to undergoing baseline assessment, following a face-to-face or telephone consultation with an adequate explanation of the aims, methods, anticipated benefits and potential hazards of the study. Consent was taken by the local site principal investigator (PI) (paediatric neurologist) or delegate.
Baseline assessments
Infants started an observation period of 2 weeks (or 1 week if the child was prone to particularly frequent seizures in excess of 2/day), during which there were no changes of regular ASMs, but emergency seizure treatments continued as required. The following data were recorded in a standardised diary: seizure types, seizure frequency, number of emergency seizure treatments required, and contacts with the NHS due to seizure exacerbation [hospital admissions – number of days, accident and emergency unit visits and/or general practitioner (GP) attendances]. The following investigations were performed in all children: administration of the Infant Toddler Quality of Life Questionnaire™ (ITQOL-97; © HealthActCHQ Inc. 2013, US Norms. Boston, MA) and Vineland Adaptive Behaviour Scales (Vineland™-II);20 clinical laboratory assessments (see Appendix 1), results of which had to received prior to randomisation (except for the special assay) to check for any contraindications to use of the KD. Food diaries required for diet calculation were returned from all enrolled infants a maximum of 1 week into the observation period.
Randomisation procedures
Randomisation occurred on Day 8 or Day 15, at the end of the baseline observation period, for infants to receive the KD or a further ASM. An independently generated web-based randomisation service provided by Sealed Envelope™ (Sealed Envelope Ltd, London, UK) was used. The randomisation schedule was generated by computer, using a simple randomisation method with no stratification. Allocations were released by e-mail to centres after the research nurse had entered participant information onto the randomisation website. This concealed the allocation to treatment from research nurses involved in patient care. While it was not possible to blind infants to their treatment allocation, efforts were made to minimise expectation bias by emphasising in the patient information sheet that evidence supporting KD for seizure control is currently limited. Assessment of harms [serious adverse events (SAEs)] were initially assessed by local investigators but were blinded from the Safety Monitoring Board for further review. Treatment procedures were started within 5 days of randomisation.
Treatment procedures
Trial arm 1: Classical ketogenic diet (KD arm)
Infants randomised to the intervention group had a KD individually calculated by a paediatric dietitian with consideration of daily calorie requirements, adequate protein intake for growth and vitamin and mineral supplementation. All diets were implemented according to a classical KD protocol, based on a ratio of fat to carbohydrate and protein, usually between 2 : 1 and 4 : 1, with non-fasting initiation. Further adjustments to the KD were determined by regular growth monitoring, seizure diaries and daily home measurement of urine or blood ketones.
Parents or carers of infants randomised to the KD group underwent a thorough teaching programme prior to diet commencement, including how to manage possible early side effects such as excess ketosis and hypoglycaemia. Infants under the age of 12 months were admitted for diet initiation.
A KD Intervention Manual (see project document https://www.fundingawards.nihr.ac.uk/award/12/10/18; accessed March 2024.) was created and provided to sites to ensure consistency of KD implementation across centres. All dietitians involved in ketogenic diet in infants with epilepsy (KIWE) were in regular contact with the dietetic assistant and meetings were organised to ensure continued cross-site consistency. Consistency of KD implementation was monitored after the 8-week and 12-month visits by the dietetic assistant.
Trial arm 2: Further antiseizure medicine (ASM arm)
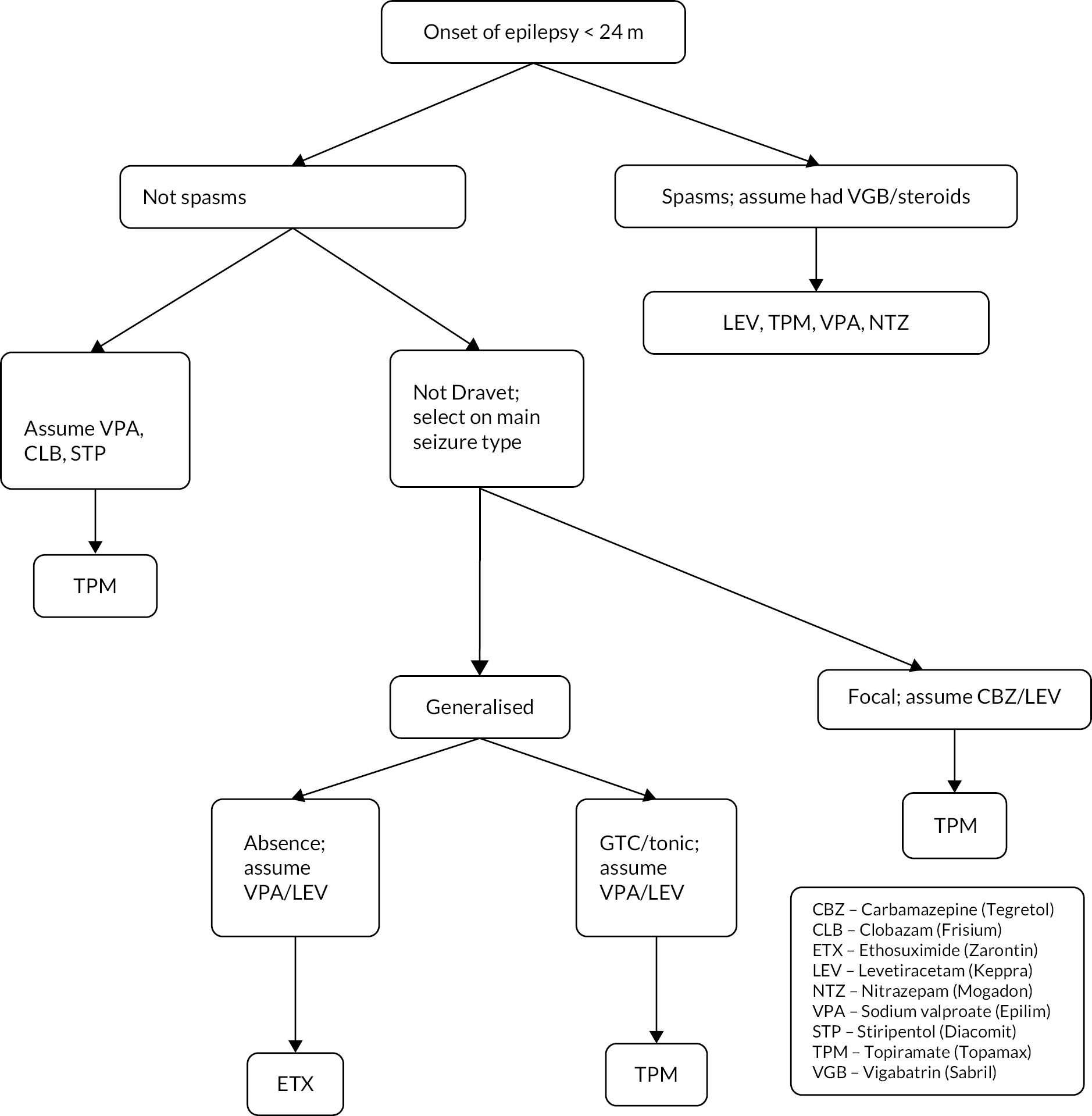
Infants randomised to the intervention group had the most appropriate further ASM chosen for them by the expert clinician responsible for management of the participant’s epilepsy, depending on their presenting seizures, syndrome and previous drugs used. Paediatric neurologists met at an initial workshop to discuss clinical practice, forming the basis of a consensus protocol to ensure the consistency of ASM treatments delivered (see Appendix 2). Cross-site consistency of IMP prescription according to the protocol was monitored by the dietetic assistant.
A general discussion about diet was undertaken with families of infants randomised to the ASM arm at the randomisation visit. If the infant was already under local dietetic support, it was ensured that this monitoring continued; if the infant did not have local dietetic support but it was deemed necessary by the ketogenic dietitian, an appropriate referral was made. A brief discussion about general infant or toddler nutrition was had, including details such as promotion of breastfeeding, age-appropriate texture progression for weaning, food groups and the important of iron-rich foods.
Subsequent assessments
Seizure diaries were kept daily by parents throughout the 8-week treatment periods for infants in both arms. Thereafter, parents were requested to reduce seizure recording to 1–2 days per week, as clinically indicated, until 28 days before the final 12-month follow-up visit, when daily seizure recording recommenced.
Four-week assessment: clinical review, including weight and documentation of seizure frequency from seizure diaries; completion of tolerability questionnaire; and review of adverse events and concomitant medication.
Eight-week assessment: clinical review, including a symptom-directed physical examination, weight, length and head circumference; documentation of seizure frequency from seizure diaries; completion of tolerability questionnaire and ITQOL-97; review of adverse events and concomitant medication; and clinical laboratory assessments (see Appendix 1).
After the 8-week assessment, according to the infant’s clinical response to treatment with regard to seizure outcome and tolerability, the KD or ASM was then continued or changed. Those in the ASM group who failed to achieve seizure control at the 8-week assessment were then offered the KD outside the context of the trial, depending on KD waiting lists at the study site, and they received usual clinical care under their KD service. Those on the KD who failed to achieve seizure improvement at the 8-week assessment continued with medical management under routine clinical care, as per clinician decision.
Six- and nine-month assessments: clinical review, including a symptom-directed physical examination, weight, length and head circumference; documentation of seizure frequency from seizure diaries; review of adverse events and concomitant medication; completion of tolerability questionnaire and clinical laboratory assessments (at month 6 only; Appendix 1).
Twelve-month (final) assessment: clinical evaluation of seizure frequency (seizure diaries maintained and seizure frequency taken as an average daily frequency over the 28 days prior to review), complete physical examination, review of adverse events and concomitant medication; completion of tolerability questionnaire, ITQOL-97 and Vineland-II questionnaires; and clinical laboratory assessments (see Appendix 1).
Infants who withdrew during the trial completed the 12-month follow-up assessment.
During the COVID-19 pandemic, visits were conducted remotely by telephone or secure videoconference facility if the parent/carer did not wish to travel and/or bring the infant into the hospital, or if there were concerns around safety. In all cases, this was up to the discretion of the treating consultant and the parent/carer was advised by the local KIWE team. Remote methods were also employed for the issuing and collection of seizure diary data or the completion of questionnaires. Blood tests could be carried out locally (such as at a GP surgery or local hospital), and existing laboratory samples could be used for screening if the blood was no older than 6 weeks.
A summary of protocol amendments can be found in Appendix 3.
Outcomes
The primary outcome was the number of seizures recorded for up to 14 days during weeks 6–8, accounting for the baseline rate and randomised group.
Secondary outcomes include:
(at 8 weeks)
-
number of infants seizure-free
-
responder rate, defined as the number showing more than a 50% in improvement in seizure frequency compared to baseline (taken as the mean daily seizure frequency over the 2-week period immediately preceding the 8-week review)
-
tolerance to KD as assessed by the questionnaire and blood results
-
relationship between medium-chain fatty acids and seizure control.
(at 12 months)
-
retention on treatment
-
quality of life (as measured by the Infant Toddler Quality of Life Questionnaire)
-
neurodevelopmental outcome (as measured by Vineland-II, including all four domains: Communication, Daily living, Socialisation and Motor skills).
Sample size calculation
For the primary outcome, based on data from our previous study,10 the mean percentage change in seizures from a baseline of 62% (SD 45) in the diet group was used, assuming a change to 90% of baseline seizure level in the comparison group (SD 50) (100 = no change in frequency of seizures from baseline) at 90% power and 5% significance, with a superiority study design. An inflation factor of 1.35 was used to account for therapist effect (dietitian), assuming nine centres with an average cluster size of eight and an intraclass correlation coefficient of 0.05. We also inflated for a 10% dropout or other methodological challenges. This gave a sample size of 68 in the ASM group and 92 in the KD group (total 160). Due to slow recruitment, the sample size was recalculated, assuming a 25% dropout allowing but keeping all other parameters the same as in the original sample size. With 75 in the KD group and 62 in the ASM group (137 total), this gave 80% power for the primary outcome. Type 1 error was two-tailed.
Statistical analysis plan
Analysis was on an intention-to-treat model. A full statistical analysis plan was created by the KIWE statisticians (see project document https://www.fundingawards.nihr.ac.uk/award/12/10/18).
The primary outcome was seizure count for up to 14 days in weeks 6–8 of the intervention period and in the baseline assessment period. Primary outcome data were analysed using a Poisson mixed model accounting for clustering by centre (synonymous with therapist), with randomised allocation and time point (baseline or 8 weeks) entered into the model as a fixed effect and centre as a random effect. Loge of number of days’ data included in the analysis from 6 to 8 weeks was included as an offset.
Secondary outcomes (those seizure-free and responders) were analysed using random effects logistic models, with centre as the random effect and randomised group as a fixed effect. The process outcome relating to tolerability, quality of life and neurodevelopment were analysed using random effects linear modelling.
Analysis was carried out to assess the potential impact of missing data on the primary outcome, assuming those who have missing data in the intervention group have the maximum total number of seizures for that randomised group and time point; the treatment-as-usual group was assigned the median total number of seizures (worst-case scenario). The primary analyses were complete case, and analyses looking at the impact of missing data were considered supportive.
Stata 17 (Stata Statistical Software: Release 17: StataCorp LLC, College Station: TX; 2021) was used for all analyses except for SAS 9.4, which was also used for the primary outcome. 21
Chapter 3 Results
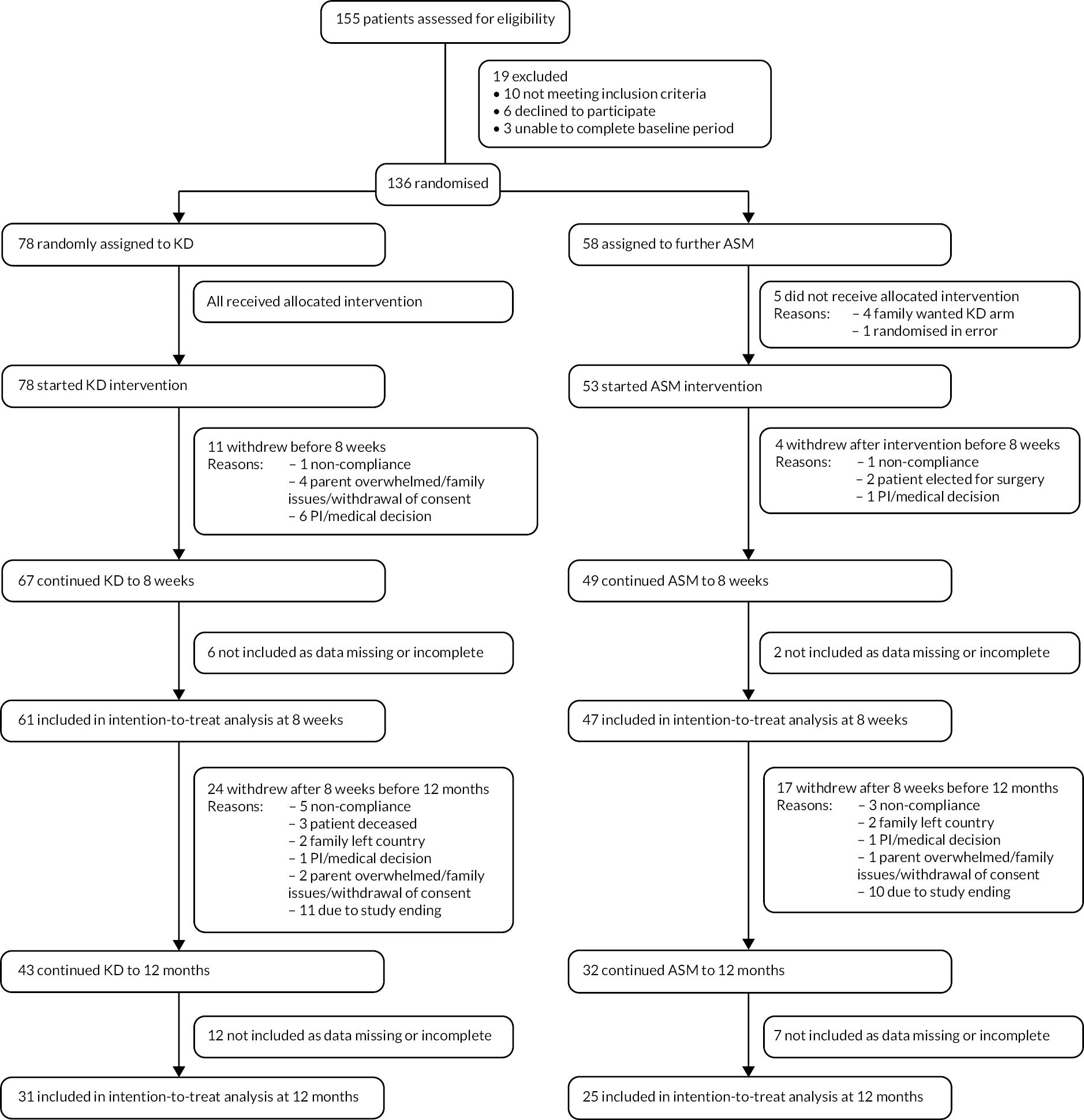
Between 1 January 2015 and 30 September 2021, of 155 infants assessed for eligibility, 136 met criteria and were randomised. Seventy-eight infants were assigned to the KD and 58 to the further ASM group (Figure 2). Of 78 infants who started KD, 67 (86%) continued to 8 weeks, of which 61 (78%) had data available; 53 (91% of those randomised to ASM group) started a further ASM, 49 (84%) continued to 8 weeks and 47 (81%) had data available. Of those who did not complete the intervention period, 15 withdrew from treatment before 8 weeks (7 the result of a decision by the clinical care team, 4 due to parents feeling overwhelmed, 2 because of non-compliance, and 2 proceeding surgery), 8 did not provide adequate data and 5 did not receive their intervention (all in ASM arm).
FIGURE 2.
CONSORT diagram.
Baseline clinical and demographic characteristics, including quality of life (ITQOL-97) and neurodevelopment (Vineland-II), were similar in individuals randomised to KD or further ASM (Table 1).
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Age at randomisation, years | 1.10 | (0.48) | 1.23 | (0.54) |
| Male | 36/58 | 62 | 39/78 | 50 |
| White | 40/55 | 73 | 59/75 | 79 |
| EEG abnormal | 45/51 | 88 | 59/68 | 87 |
| Epilepsy syndrome diagnosis | 29/43 | 67 | 45/67 | 67 |
| Epilepsy syndrome/type | ||||
| Early myoclonic encephalopathy | 0/38 | 0 | 1/46 | 2 |
| Early infantile epileptic encephalopathy | 11/38 | 29 | 13/46 | 28 |
| Migrating focal seizures of infancy | 1/38 | 3 | 0/46 | 0 |
| Infantile epileptic spasms syndrome | 19/38 | 50 | 23/46 | 50 |
| Dravet syndrome | 1/38 | 3 | 2/46 | 4 |
| Epilepsy with myoclonic atonic seizures (Doose syndrome) | 1/38 | 3 | 0/46 | 0 |
| Lesional focal epilepsy | 5/38 | 13 | 7/46 | 15 |
| Genetic diagnosis | 14/54 | 26 | 18/68 | 26 |
| Other neurological diagnosis | 19/54 | 35 | 26/67 | 39 |
| Developmental delay | 49/55 | 89 | 65/73 | 89 |
| Hemiplegia | 3/55 | 5 | 8/72 | 11 |
| Seizure type | ||||
| Focal | 22/52 | 42 | 30/69 | 43 |
| Spasms | 30/52 | 58 | 41/69 | 59 |
| Absence | 6/52 | 12 | 4/69 | 6 |
| Myoclonic | 10/52 | 19 | 9/70 | 13 |
| Clonic | 3/52 | 6 | 4/69 | 6 |
| Tonic | 10/52 | 19 | 18/69 | 26 |
| Tonic–clonic | 10/52 | 19 | 7/69 | 10 |
| Atonic | 4/52 | 8 | 3/69 | 4 |
| Seizures per day median (IQR) | 9 | (3, 19) | 7 | (4, 21) |
| Systolic blood pressure (mmHg) | 94 | (13) | 98 | (16) |
| Diastolic blood pressure (mmHg) | 56 | (13) | 62 | (16) |
| Pulse beats/minute | 126 | (17) | 126 | (22) |
| Temperature (°C) | 36.8 | (0.3) | 36.6 | (0.4) |
| Weight (kg) | 9.9 | (2.9) | 9.6 | (2.7) |
| Weight SDS | −0.02 | (1.76) | −0.09 | (1.52) |
| Length (m) | 0.75 | (0.11) | 0.76 | (0.09) |
| Length SDS | −0.45 | (1.90) | −0.27 | (1.79) |
| Head circumference (cm) | 44.1 | (4.1) | 44.1 | (3.5) |
| Head circumference SDS | −1.07 | (2.58) | 1.08 | (2.28) |
| ITQOL-97 | ||||
| Infant’s overall health median (IQR) | 60 | (30, 60) | 60 | (30, 60) |
| Infant’s physical abilities median (IQR) | 25 | (10, 56) | 23 | (10, 62) |
| Satisfaction with infant’s overall growth and development median (IQR) | 45 | (33, 58) | 51 | (39, 70) |
| Infant’s pain median (IQR) | 58 | (42, 75) | 58 | (42, 75) |
| Infant’s temperament and mood median (IQR) | 56 | (45, 66) | 61 | (47, 72) |
| Infant’s behaviour overall median (IQR) | 65 | (58, 79) | 69 | (56, 81) |
| Infant’s global behaviour median (IQR) | 85 | (60, 85) | 73 | (60, 100) |
| Infant getting along with others median (IQR) | 60 | (50, 66) | 55 | (48, 70) |
| General health perceptions median (IQR) | 36 | (25, 54) | 41 | (27, 50) |
| Change in infant’s health | ||||
| Much worse than a year ago | 8/29 | 28 | 7/43 | 16 |
| Somewhat worse than a year ago | 3/29 | 10 | 7/43 | 16 |
| About the same now as a year ago | 11/29 | 38 | 10/43 | 23 |
| Somewhat better than a year ago | 3/29 | 10 | 11/43 | 26 |
| Much better than a year ago | 4/29 | 14 | 8/43 | 19 |
| Parental impact emotional median (IQR) | 39 | (21, 54) | 50 | (29, 64) |
| Parental impact time median (IQR) | 52 | (33, 71) | 62 | (33, 76) |
| Family cohesion median (IQR) | 85 | (85, 100) | 85 | (60, 100) |
| Vineland-II | ||||
| Communication receptive v-scale score median (IQR) | 7 | (5, 10) | 8 | (6, 10) |
| Communication expressive v-scale score median (IQR) | 6 | (4, 11) | 8 | (6, 11) |
| Communication sum of v-scale scores median (IQR) | 12 | (9, 19) | 16 | (11, 20) |
| Communication domain standard score median (IQR) | 44 | (37, 62) | 60 | (46, 66) |
| Daily living personal v-scale score median (IQR) | 9 | (8, 11) | 9 | (8, 12) |
| Daily living domestic v-scale score median (IQR) | 12 | (6, 13) | 11 | (0, 12) |
| Daily living community v-scale score median (IQR) | 10 | (0, 10) | 10 | (0, 10) |
| Daily living sum of v-scale scores median (IQR) | 29 | (10, 32) | 26 | (10, 32) |
| Daily living domain standard score median (IQR) | 66 | (57, 72) | 68 | (61, 75) |
| Socialisation interpersonal relationships v-scale score median (IQR) | 7 | (5, 9) | 8 | (5, 10) |
| Socialisation play v-scale score median (IQR) | 8 | (7, 10) | 9 | (7, 10) |
| Socialisation coping v-scale score median (IQR) | 0 | (0, 9) | 0 | (0, 9) |
| Socialisation sum of v-scale scores median (IQR) | 21 | (18, 26) | 22 | (19, 26) |
| Socialisation domain standard score median (IQR) | 59 | (53, 65) | 65 | (54, 73) |
| Motor gross v-scale score median (IQR) | 6 | (5, 8) | 6 | (6, 8) |
| Motor fine v-scale score median (IQR) | 6 | (6, 8) | 7 | (6, 9) |
| Motor sum of v-scale scores median (IQR) | 12 | (10, 15) | 14 | (12, 19) |
| Motor skills domain standard score median (IQR) | 50 | (49, 55) | 55 | (50, 61) |
| Sum of domain standard scores/adaptive behaviour composite median (IQR) | 228 | (199, 244) | 236 | (208, 276) |
| Standardised score median (IQR) | 54 | (48, 58) | 56 | (50, 66) |
Baseline clinical laboratory parameters were also similar between the two groups at baseline (see Appendix 4).
Efficacy
At 8 weeks, the median number of seizures per day compared to baseline was not significantly different in both groups [KD 5 (1, 16); ASM 3 (2, 11), incidence rate ratio (IRR) 1.33, 95% CI 0.84 to 2.11; p = 0.22]. Of 63 infants in the KD group, 28 (44%) had > 50% seizure reduction compared with 19/47 (40%) in the ASM group OR 1.21 (95% CI 0.55 to 2.65); 7/63 infants (11%) in the KD group were seizure-free, compared with 6/48 (13%) in the ASM group OR 0.88 (95% CI 0.27 to 2.80).
A higher proportion of infants in the ASM group [24/48 (50%)] had changes to the number or dose of concurrent ASMs during the intervention period compared to the KD group [9/66 (14%)]. This was advised against in the protocol, although allowed if required due to clinical need. This included dose increases of concurrent ASMs or short courses of new ASMs due to seizure escalation, or prophylaxis for planned admission, with the exception of 1/66 (2%) infants in the KD group and 2/48 (4%) infants in the ASM group for whom the dose of a concurrent ASM was decreased during the intervention period. A similar proportion of infants in both groups had changes to concomitant (non-ASM) medications (excluding concurrent ASMs) during the intervention period [25/47 (53%) in ASM group; 33/67 (49%) in KD group].
Tolerability
The side-effect score was similar in the KD (median 40 IQR 38–42) and ASM groups (median 41 IQR 39–44) at 4 weeks (ASM median 44 IQR 41–44; KD median 40 IQR 36–42), 8 weeks (ASM median 41 IQR 39–44; KD median 40 IQR 38–42), 6 months (ASM mean 40 SD 4; KD mean 39 SD 4), 9 months (ASM mean 41 SD 3; KD mean 41 SD 3) and 12 months (ASM median 41 IQR 39–43; KD median 40 IQR 36–42) – a lower score refers to more and/or more severe symptoms.
Mean measurements for laboratory parameters, blood pressure, pulse and body temperature were similar in both groups at 8 weeks, with the exception of differences in beta-hydroxybutyrate, glucose, bicarbonate, urate, creatinine, free carnitine, urine organic acids, urine creatinine ratio, lipids and acylcarnitine profiles, which were as expected for individuals following a KD (see Appendix 5). No out-of-range laboratory parameters were considered clinically significant in either group.
Mean measurements for laboratory parameters, anthropometry standard deviation scores, blood pressure, pulse and body temperature were similar in both groups at 12 months (see Appendix 6).
Quality of life
At 8 weeks, median scores were numerically higher (indicating better health) in the KD group for the following scales within the ITQOL-97: infant’s overall health, infant’s physical abilities, satisfaction with infant’s overall growth and development, infant’s temperament and mood, infant’s overall behaviour, infant getting along with others and impact on parental emotion (Table 2).
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Infant’s overall health median (IQR) | 30 | (30, 60) | 60 | (30, 60) |
| Infant’s physical abilities median (IQR) | 17 | (7, 52) | 35 | (17, 62) |
| Satisfaction with infant’s overall growth and development median (IQR) | 43 | (28, 58) | 48 | (38, 63) |
| Infant’s pain median (IQR) | 67 | (33, 75) | 67 | (42, 75) |
| Infant’s temperament and mood median (IQR) | 58 | (46, 68) | 63 | (56, 71) |
| Infant’s behaviour overall median (IQR) | 60 | (52, 71) | 69 | (58, 83) |
| Infant’s global behaviour median (IQR) | 60 | (30, 85) | 60 | (30, 85) |
| Infant getting along with others median (IQR) | 50 | (48, 59) | 58 | (50, 68) |
| General health perceptions median (IQR) | 39 | (27, 52) | 36 | (24, 47) |
| Change in infant’s health | ||||
| Much worse than a year ago | 8/32 | 25 | 2/40 | 5 |
| Somewhat worse than a year ago | 6/32 | 19 | 10/40 | 25 |
| About the same now as a year ago | 7/32 | 22 | 12/40 | 30 |
| Somewhat better than a year ago | 8/32 | 25 | 6/40 | 15 |
| Much better than a year ago | 3/32 | 9 | 10/40 | 25 |
| Parental impact emotional median (IQR) | 43 | (21, 61) | 46 | (32, 64) |
| Parental impact time median (IQR) | 60 | (29, 81) | 60 | (43, 81) |
| Family cohesion median (IQR) | 85 | (60, 100) | 85 | (60, 100) |
The infant’s pain, infant’s global behaviour, impact on parental time and family cohesion were equal between the two groups, although general perceptions of the infant’s health were numerically higher in the ASM arm (see Table 2).
A larger proportion of parents of infants in the KD group perceived their child’s health to be ‘much better than a year ago’ (10/40, 25%) compared to those in the ASM group (3/32, 9%); more parents of infants in the ASM group perceived their child’s health to be ‘much worse than a year ago’ (8/32, 25%) compared to those in the KD group (2/40, 5%) (see Table 2).
There were no significant differences between groups for any scale within the ITQOL-97 at 12 months, except for infant’s temperament and mood (coefficient −6.09, 95% CI −11.63 to −0.54) and infant getting along with others (coefficient −6.79, 95% CI −12.97 to −0.60), which favoured the ASM group (Table 3).
| Outcome | ASM group | KD group | Estimate | 95% CI |
|---|---|---|---|---|
| n/N (%) | n/N (%) | |||
| Median (IQR) | Median (IQR) | |||
| ITQOL-97 | ||||
| Infant’s overall health n = 52 ASM n = 24, KD n = 28 | 30 (30, 85) | 60 (30, 60) | 1.23 | −12.70 to 15.17 |
| Infant’s physical abilities n = 42 ASM n = 20, KD n = 22 | 47 (7, 70) | 27 (13, 58) | −0.59 | −14.58 to 13.40 |
| Satisfaction with child’s overall growth and development n = 54 ASM n = 24, KD n = 30 | 58 (38, 78) | 45 (38, 70) | −4.14 | −14.22 to 5.94 |
| Infant’s pain n = 54 ASM n = 24, KD n = 30 | 75 (50, 83) | 67 (33, 83) | −11.14 | −24.65 to 2.36 |
| Infant’s temperament and mood n = 53 ASM n = 23, KD n = 30 | 68 (60, 79) | 65 (56, 71) | −6.09 | −11.63 to −0.54 |
| Infant’s behaviour overall n = 28 ASM n = 14, KD n = 14 | 67 (60, 83) | 65 (56, 77) | −7.23 | −15.96 to 1.50 |
| Infant’s global behaviour n = 28 ASM n = 14, KD n = 14 | 85 (60, 100) | 85 (60, 100) | 12.72 | −1.56 to 27.00 |
| Infant getting along with others n = 28 ASM n = 12, KD n = 16 | 65 (52, 72) | 58 (50, 66) | −6.79 | −12.97 to −0.60 |
| General health perceptions n = 54 ASM n = 24, KD n = 30 | 41 (30, 52) | 30 (16, 52) | −6.37 | −14.29 to 1.56 |
| Parental impact emotional n = 54 ASM n = 24, KD n = 30 | 57 (36, 79) | 54 (39, 68) | −5.00 | −15.52 to 5.53 |
| Parental impact time n = 54 ASM n = 24, KD n = 30 | 62 (33, 90) | 57 (43, 76) | −3.11 | −16.80 to 10.58 |
| Family cohesion n = 54 ASM n = 24, KD n = 30 | 85 (60, 85) | 85 (60, 100) | −1.52 | −9.48 to 6.45 |
| Vineland-II | ||||
| Communication receptive v-scale score n = 17 ASM n = 9, KD n = 8 | 7 (7, 8) | 7 (5, 7) | 0.09 | −1.22 to 1.39 |
| Communication expressive v-scale score n = 23 ASM n = 9, KD n = 14 | 5 (3, 7) | 5 (3, 8) | 0.68 | −1.49 to 2.85 |
| Communication sum of v-scale scores n = 10 ASM n = 5, KD n = 5 | 10 (8, 14) | 11 (9, 13) | 1.17 | −3.42 to 5.77 |
| Communication domain standard score n = 10 ASM n = 5, KD n = 5 | 48 (43, 59) | 49 (44, 55) | 2.79 | −8.14 to 13.72 |
| Daily living personal v-scale score n = 20 ASM n = 11, KD n = 9 | 6 (5, 7) | 5 (4, 7) | −1.53 | −3.38 to 0.32 |
| Daily living domestic v-scale score n = 41 ASM n = 21, KD n = 20 | 10 (9, 11) | 11 (9, 11) | 0.01 | −0.38 to 0.41 |
| Daily living community v-scale score n = 39 ASM n = 17, KD n = 22 | 10 (9, 10) | 10 (9, 10) | 0.22 | −0.22 to 0.67 |
| Daily living sum of v-scale scores n = 18 ASM n = 10, KD n = 8 | 16 (15, 17) | 15 (13, 16) | −2.23 | −4.22 to −0.25 |
| Daily living domain standard score n = 18 ASM n = 10, KD n = 8 | 25 (21, 34) | 25 (21, 28) | −0.69 | −7.68 to 6.31 |
| Socialisation interpersonal relationships v-scale score n = 24 ASM n = 12, KD n = 12 | 6 (5, 9) | 6 (3, 7) | −1.30 | −3.17 to 0.57 |
| Socialisation play v-scale score n = 25 ASM n = 13, KD n = 12 | 8 (7, 9) | 8 (7, 9) | 0.77 | −1.12 to 2.66 |
| Socialisation coping v-scale score n = 27 ASM n = 11, KD n = 16 | 9 (8, 9) | 9 (8, 9) | −0.07 | −0.99 to 0.84 |
| Socialisation sum of v-scale scores n = 10 ASM n = 5, KD n = 5 | 21 (18, 23) | 22 (19, 24) | 1.55 | −4.04 to 7.14 |
| Socialisation domain standard score n = 10 ASM n = 5, KD n = 5 | 56 (47, 59) | 54 (53, 58) | 1.12 | −17.13 to 19.36 |
| Motor gross v-scale score n = 37 ASM n = 18, KD n = 19 | 5 (4,7) | 5 (4, 6) | −0.53 | −1.54 to 0.48 |
| Motor fine v-scale score n = 33 ASM n = 15, KD n = 18 | 5 (4, 7) | 5 (3, 6) | −0.33 | −1.85 to 1.19 |
| Motor sum of v-scale scores n = 29 ASM n = 14, KD n = 15 | 9 (9, 12) | 9 (8, 10) | −0.46 | −1.95 to 1.03 |
| Motor skills domain standard score n = 29 ASM n = 14, KD n = 15 | 48 (45, 54) | 43 (45, 50) | −1.53 | −5.94 to 2.88 |
| Sum of domain standard scores/adaptive behaviour composite n = 6 ASM n = 4, KD n = 2 | 165 (160, 171) | 168 (162, 176) | 0.96 | −18.12 to 20.03 |
| Standardised score n = 6 ASM n = 4, KD n = 2 | 40 (39, 41) | 41 (39, 43) | 0.16 | −5.34 to 5.67 |
A similar proportion of parents of infants in both groups perceived their child’s health to be ‘much better than a year ago’ (12/24 50% ASM; 11/30 37% KD) or ‘much worse than a year ago’ (0/24, 0% ASM; 1/30, 3% KD) (see Appendix 7).
Neurodevelopment
At 12 months, median standardised scores for Communication, Daily living and Socialisation domains, and the adaptive behaviour composite standardised score were ‘low’ in both groups (see Appendix 7), equivalent to approximately 3–4 SD below the mean. All subdomain scores (too few data points for Motor skills gross and fine) were also ‘low’ or ‘moderately low’.
There were neither significant differences between the two groups in the overall standardised score nor domain standard scores at 12 months (see Table 3). Domain standard scores for communication (2.79, 95% CI −8.14 to 13.72) and socialisation (1.12, 95% CI −17.13 to 19.36) numerically improved in the KD group compared to ASM. The Daily living domain sum of v-scale scores was nominally improved in the ASM group (coefficient 2.23, 95% CI −4.22 to −0.25).
Safety
A total of 73 SAEs were reported in the ASM group and 161 in the KD group. The proportion of SAEs classified into each MedDRA system organ was similar in each group (Table 4).
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| At least one SAE at any time | 24/56 | 43 | 40/78 | 51 |
| Number of SAE | 73 | 161 | ||
| MedDRA system organ class | ||||
| Cardiac disorders | 0/73 | 0 | 1/161 | 1 |
| Gastrointestinal disorders | 7/73 | 10 | 8/161 | 5 |
| General disorders and administration site conditions | 3/73 | 4 | 2/161 | 1 |
| General system disorders not elsewhere classified | 1/73 | 1 | 0/161 | 0 |
| Immune system disorders | 1/73 | 1 | 0/161 | 0 |
| Infections and infestations | 11/73 | 15 | 64/161 | 40 |
| Injury, poisoning and procedural complications | 1/73 | 1 | 0/161 | 0 |
| Investigations | 1/73 | 1 | 2/161 | 1 |
| Metabolism and nutrition disorders | 1/73 | 1 | 9/161 | 6 |
| Nervous system disorders | 34/73 | 47 | 56/161 | 35 |
| Respiratory, thoracic and mediastinal disorders | 10/73 | 14 | 23/161 | 14 |
| Surgical and medical procedures | 5/73 | 7 | 2/161 | 1 |
| Vascular disorders | 0/73 | 0 | 1/161 | 1 |
Three infants died during the course of the trial, all of whom were randomised to the KD arm but deaths were considered to be unrelated to treatment. One infant was found not breathing at home; cardiopulmonary resuscitation was attempted without success in the emergency department. One infant suffered sudden unexpected death at home; another became bradycardic and went into cardiac arrest during routine surgery under anaesthetic.
Medium-chain fatty acids
Seventy-one samples were sent for medium-chain fatty acid analysis. However, sample stability was compromised in 39 samples in storage, so medium-chain fatty acid data were available on only 17 samples at baseline and 15 at 8 weeks, from infants receiving the KD. There was a wide range of baseline plasma medium-chain fatty acid levels in those receiving a KD (n = 17) (Table 5). There was an increase in octanoic acid (C8) and decanoic acid (C10) in samples taken 8 weeks after diet initiation (n = 15). Dodecanoic acid levels were very similar in baseline and post-intervention samples. In view of the small number of samples available for analysis, no attempt was made to perform any statistical comparison, or regression analysis to determine whether there was an association between seizures and fatty acid levels.
| Baseline (n = 17) median (IQR) |
8 weeks (n = 15) median (IQR) |
|
|---|---|---|
| Octanoic acid (μmol/L) | 6.7 (2.4–9.1) | 10.1 (4.2–14.8) |
| Decanoic acid (μmol/L) | 4.3 (3.2–6.6) | 10.2 (4.2–18.1) |
| Dodecanoic acid (μmol/L) | 11.6 (8.1–21.9) | 12.9 (9.2–18.5) |
Retention
At 8 weeks, 33/58 (57%) infants randomised to the ASM group commenced the KD, and 12/78 (15%) infants randomised to the KD group discontinued the diet and started a further ASM.
The trial was terminated before all participants had a chance to achieve 12 months’ follow-up, as the revised sample size was reached. Of 66 infants randomised to KD > 12 months before the study end date, 31 (47%) continued the diet to 12 months; of 47 randomised to further ASM > 12 months before the study end date, 21 (45%) continued the ASM to 12 months. Of the 78 infants randomised to KD, 31 (40%) were included in the intention-to-treat analysis at 12 months; of the 58 infants randomised to ASM, 25 (43%) were included in the intention-to-treat analysis at 12 months (see Figure 2).
Chapter 4 Discussion
We here present the findings of the first RCT assessing the effectiveness of the KD in infants with drug-resistant epilepsy, compared to standard ASM treatment. Designed as a superiority study, there was no evidence that KD was better than further ASM in achieving seizure control in infants aged 1–24 months, and the two treatments were similarly tolerated. KD may be considered as a treatment option alongside standard ASMs for infants who continue to have seizures after having tried two ASMs.
Seizure frequency
Our responder rate (> 50% seizure reduction) of approximately 40% is consistent with other KD studies: RCTs comparing KD to usual care in children report responder rates between 34% and 50% after 3–4 months;11 a meta-analysis conducted by our group of uncontrolled studies of KD use in infants with epilepsy estimated a responder rate of 59%. 22 Our seizure freedom rates are on the higher end of the range reported in previous RCTs of older children, between 1% and 10%, yet lower than the 33% from uncontrolled studies in infants. One further RCT has examined efficacy of KD compared to adrenocorticotropic hormone (ACTH) in infantile spasms alone (a standard treatment for this seizure type): 10/16 (62%) of those on KD achieved electroclinical remission at 28 days compared to 11/16 (69%) on ACTH, and relapse rates were similar (40% vs. 36%). 18 This study, however, was underpowered with very small numbers, and in a single seizure type; therefore results were reported to be considered with caution. It also has to be acknowledged that there is the potential for false-negative results if seizures were not seen and/or recorded within the intervention period, but this would apply to individuals in both arms, as well as throughout the study (baseline and intervention periods), as with all clinical trials.
Despite the similar changes in seizure frequency in the KD and ASM groups in this study, a higher proportion of infants randomised to ASM had changed in other ASMs during the intervention period compared to those randomised to KD, suggesting that those on KD may have been more clinically stable. We acknowledge that, although the revised study sample size gave 80% power for the primary outcome, the confidence intervals (CIs) are wide and the upper CI is > 2 for the IRR; therefore, a small (but important) effect may have been missed. Furthermore, there is the potential for false-negative results if seizures were not seen and/or recorded within the intervention period, but this would apply to individuals in both arms, as well as throughout the study (baseline and intervention periods), as with all clinical trials.
In this study, we used 8 weeks as the primary outcome period rather than 3 months as, in infants, many epilepsy syndromes are characterised by high seizure frequency and it was felt this was the longest tolerable period for assessment. In our clinical experience, seizure response to the KD or an ASM is generally determined in 4 weeks in this population, which leaves a 2–4 week period for seizure assessment following the initial titration period. Previous ASM studies in this age group have used titration periods of between 1 day and 4 weeks, with 4-day to 4-week stabilisation periods. 23–25
Quality of life and neurodevelopment
Most sections of the quality-of-life measure at 8 weeks were in favour (although only numerically) of the KD group, and a larger proportion of parents perceived their child’s health to be ‘much better than a year ago’ compared to those in the ASM group. The feeling on the part of the parents’ ‘doing something worthwhile’ when administering a KD (despite the common perception that dietary treatment is an imposition on parents) and their perception of its benefit for their child should not be diminished, independent of its efficacy in terms of seizure reduction. Perhaps being supported by a KD team is therapeutic in itself?
Quality-of-life and neurodevelopmental measures at 12 months are to be interpreted with caution due to low numbers (predominantly due to missing data) and wide CIs. Furthermore, as an intent-to-treat analysis was used, many randomised to the ASM arm then started the KD after 8 weeks, but individuals remain in their randomised group for analysis. Communication and socialisation being numerically (not statistically significant) in favour of the KD group at 12 months are consistent with the only RCT assessing quality of life, cognitive and behavioural functioning on KD compared to usual care at 4 and 16 months: a trend was reported towards improved activation, increased productivity and less anxious and mood-disturbed behaviour,26 although no difference was found between quality-adjusted life-years when comparing KD to usual care. 27
Safety
Consistent with uncontrolled studies on use of KD in infants,22 there were no clinically significant differences in clinical or laboratory parameters between groups, except those as expected when following a KD. The proportion of infants with results out of normal range differed between groups only for specific clinical or laboratory parameters at varying time points, for example a higher proportion of infants in the KD group had vitamin D out-of-normal range at baseline compared to the ASM group. This is likely a reflection of the clinical complexity of the population. SAEs were as expected in both groups, most commonly an increase in seizures, followed by infections.
Retention
Retention rates of approximately 50% at 12 months in this study are similar to those reported in uncontrolled studies of infants on KD (aggregated rate of 43% at 12 months). 22 RCTs of KD versus usual care in children with epilepsy also found similar retention rates between groups (RR 1.08, 95% CI 0.74 to 1.57; p = 0.71). 11 Studies evaluating efficacy of ASMs in infancy are limited; those reviewing first-line treatment in spasms utilised protocols only requiring limited time for intervention and therefore retention rates longer term are not relevant. There are limited studies of second-line treatments, reviewing efficacy only over a relatively short time. These are therefore the first available data providing such information. It should be acknowledged that such long-term retention endpoints are difficult to relate to real-life clinical practice, due to the intention-to-treat principle of analysing individuals according to their randomised group irrespective of whether they continue on that treatment or not.
Interestingly, there is a greater proportion of infants in the ASM group who switched to KD, than those in the KD group who switched to ASM. Further, within the ASM group, despite changes to ASMs within the intervention period not recommended within the protocol, there was a greater number of changes to ASM or dose than within the KD arm. Although group efficacy was not determined to be different, this implies a general greater degree of satisfaction with the KD than ASM.
Equality, diversity and inclusion
Although we started the study with 12 sites, we ultimately invited all UK KDT centres to participate and eventually included 19 sites nationwide, encompassing secondary and tertiary centres, from the South of England to North of Scotland, with variation in the size of KD service. This ensured that a range of sociodemographic and clinical diversity typically encountered in this population was reflected in our recruitment pool. As expected, infantile epileptic spasms syndrome was the most common presenting epilepsy syndrome, as is seen in epidemiological studies in this age group1 and there were no obvious differences in characteristics between intervention groups. Study visits and assessments were conducted online during the COVID-19 pandemic, where possible, and interpreters were available as required.
Patient and public involvement
Throughout the study, Matthews Friends, a charitable organisation providing support and education for families utilising the KD, has been involved in reviewing progress. There were both parent and epilepsy charity representatives on the Trial Steering Committee (TSC). In 2020, a mother of a child who participated in KIWE spoke to parents of other participants and provided a document of concerns and suggestions, which was discussed during the TSC. As a result of this, it was then communicated to the Trial Management Group to ensure that patients/families were made aware of all support groups and resources available at that time.
Difficulties and limitations
We recognise there are limitations to this study. Recruitment was slower than anticipated, likely due to a number of factors. Infants presenting with epilepsy often have a high burden of seizures; there is a perceived urgency for the need to treat and change treatment, rather than wait for a period to document the required baseline. We also found the study tended to be introduced at a relatively late stage in the treatment timeline – not necessarily at a time when only two ASMs had been trialled, but after 4–5, at a time when there was limited equipoise as to the next treatment to try, that is a reluctance for randomisation to 1 of 2 arms as there was a predetermined view the KD should be trialled next. To mitigate some of these problems, we reduced the baseline period to 1 week for infants with frequent seizures, and we also increased the number of sites involved to aid recruitment rates. There has been much discussion as to a need to seek an alternative trial design in infants considering the difficulty recruiting to such studies and the limited information with regard to meaningful outcomes. A new trial design has recently been proposed where the baseline duration would be adjusted based on individual seizure burden, and treatment duration linked to seizure response according to timing of seizure occurrence compared to baseline using ‘time to Nth seizure’ as the primary outcome. 28 This should be considered with regard to trial design in this age group in the future. We acknowledge that some participants were withdrawn from the trial when they stopped their randomised treatment due to confusion over the protocol, leading to a risk of attrition bias. However, all participants with primary outcome data available were included in the results. We also acknowledge that, despite discussing with families in detail what following a KD would entail should the participant be randomised to start the diet and, as part of the trial, families measured blood ketone levels as a rough indicator of dietary adherence, we did not specifically record families’ competence or ability to afford following a KD, which may have impacted adherence.
Chapter 5 Conclusion
In this first RCT assessing the use of KD in infants with drug-resistant epilepsy, we did not find, with sufficient precision, that KD was more effective than further ASM. The diet appeared safe to use in this age group, although the limited sample size should be acknowledged. The KD may also improve some aspects of quality of life and neurodevelopment, but further trials are needed to assess these non-seizure-related outcomes with larger cohorts at 12 months’ follow-up and beyond, perhaps with alternative study design to aid recruitment. Acknowledging the difficulties we had with recruitment, many centres would need to be involved in any future trials to obtain sufficient participants in a timely manner. The KD should be considered a treatment option in infants who continue to have seizures despite having tried two ASMs.
Additional information
Contributions of authors
Natasha E Schoeler (https://orcid.org/0000-0001-6202-1497) (Senior Research Fellow, UCL Great Ormond Street Institute of Child Health) was the trial Dietetic Assistant, wrote the first draft of the report, and had final responsibility for the decision to submit for publication.
Louise Marston (https://orcid.org/0000-0002-9973-1131) (Professor of Clinical Trials Statistics, UCL) did the statistical analyses, directly accessed and verified the underlying data reported here and had final responsibility for the decision to submit for publication.
Laura Lyons (https://orcid.org/0009-0008-2069-5651) (Trial Manager, UCL Great Ormond Street Institute of Child Health) was part of the trial management team.
Sally Halsall (https://orcid.org/0000-0002-5798-5440) (ex-Trial Manager, UCL Great Ormond Street Institute of Child Health) was part of the trial management team.
Ruchika Jain (ex-Trial Manager, UCL Great Ormond Street Institute of Child Health) was part of the trial management team.
Siobhan Titre-Johnson (https://orcid.org/0000-0003-4985-8131) (ex-Trial Manager, UCL Great Ormond Street Institute of Child Health) was part of the trial management team.
Maryam Balogun (https://orcid.org/0009-0006-8006-0522) (Research Assistant, UCL Great Ormond Street Institute of Child Health) was part of the trial management team.
Simon J R Heales (https://orcid.org/0000-0002-9906-0200) (Consultant Clinical Scientist, Great Ormond Street Hospital for Children) contributed to protocol development and had the idea for the medium-chain fatty acid component of the study.
Simon Eaton (https://orcid.org/0000-0003-0892-9204) (Associate Professor, UCL Great Ormond Street Institute of Child Health) had the idea for the medium-chain fatty acid component of the study.
Michael Orford (https://orcid.org/0000-0002-6295-6804) (Postdoctoral Research Associate, UCL) analysed the medium-chain fatty acid samples.
Elizabeth Neal (https://orcid.org/0009-0003-3596-8118) (Research Dietitian, UCL Great Ormond Street Institute of Child Health) contributed to protocol development.
Christin Eltze (https://orcid.org/0000-0003-4411-2939) (Consultant Paediatric Neurologist, Great Ormond Street Hospital for Children) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Elma Stephen (https://orcid.org/0000-0001-9501-6010) (Consultant Paediatric Neurologist, Royal Aberdeen Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Andrew A Mallick (https://orcid.org/0000-0002-5882-8076) (Consultant Paediatric Neurologist, Bristol Royal Hospital for Children) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Finbar O’Callaghan (https://orcid.org/0000-0002-7499-6029) (Professor of Paediatric Neuroscience, UCL Great Ormond Street Institute of Child Health) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Shaki Agrawal (https://orcid.org/0000-0003-1298-3369) (Consultant Paediatric Neurologist, Birmingham Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Alasdair Parker (https://orcid.org/0000-0002-8768-2686) (Consultant Paediatric Neurologist, Cambridge University Hospitals) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Martin Kirkpatrick (https://orcid.org/0000-0002-8156-3010) (Professor of Child Health in the School of Medicine at the University of Dundee) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Andreas Brunklaus (https://orcid.org/0000-0002-7728-6903) (Consultant Paediatric Neurologist, Royal Hospital for Children) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Ailsa McLellan (Consultant Paediatric Neurologist, Royal Hospital for Sick Children) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Helen McCullagh (https://orcid.org/0000-0003-4896-5111) (Consultant Paediatric Neurologist, Leeds Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Rajib Samanta (Consultant Paediatric Neurologist, University Hospital of Leicester) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Rachel Kneen (https://orcid.org/0000-0002-8729-4122) (Consultant Paediatric Neurologist, Alder Hey Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Hui Jeen Tan (https://orcid.org/0000-0003-4963-5835) (Consultant Paediatric Neurologist, Royal Manchester Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Anita Devlin (https://orcid.org/0000-0002-3814-4777) (Consultant Paediatric Neurologist, Great North Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Manish Prasad (Consultant Paediatric Neurologist, Queens Medical Centre) oversaw site recruitment, clinical management of participants and data collection for his respective site.
Rohini Rattihalli (Consultant Paediatric Neurologist, Oxford University Hospitals) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Helen Basu (https://orcid.org/0009-0006-6350-9420) (Consultant Paediatric Neurologist, Royal Preston Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Archana Desurkar (Consultant Paediatric Neurologist, Sheffield Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Ruth Williams (Consultant Paediatric Neurologist, Evelina London Children’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Penny Fallon (Consultant Paediatric Neurologist, St George’s Hospital) oversaw site recruitment, clinical management of participants and data collection for her respective site.
Irwin Nazareth (https://orcid.org/0000-0003-2146-9628) (Professor of Primary Care and Population Science) gave input into the design of the study, and oversaw the study conduct.
Nicholas Freemantle (https://orcid.org/0000-0001-5807-5740) (Director, UCL Comprehensive Clinical Trials Unit) gave input into the design of the study, did the statistical analyses, directly accessed and verified the underlying data reported here, and had final responsibility for the decision to submit for publication.
J Helen Cross (https://orcid.org/0000-0001-7345-4829) (Director, UCL Great Ormond Street Institute of Child Health, The Prince of Wales’s Chair of Childhood Epilepsy) had the idea for the study, designed the study and had final responsibility for the decision to submit for publication.
All authors had full access to all study data.
Acknowledgements
The authors thank all participating patients and their families; the Trial Managers, Dr Laura Lyons, Ruchika Jain, Dr Sally Halsall and Siobhan Titre-Johnson; Maryam Balogun as Research Administrator; the PIs at each centre: Dr Christin Eltze (GOSH); Dr Elma Stephen (Aberdeen); Dr Andrew Mallick (Bristol); Dr Shakti Agrawal (Birmingham); Dr Alasdair Parker (Cambridge); Professor Martin Kirkpatrick (Dundee); Dr Ruth Williams (Evelina, recruited in pilot phase only); Professor Andreas Brunklaus (Glasgow); Dr Ailsa McLellan (Edinburgh); Dr Helen McCullagh (Leeds); Dr Rajib Samanta (Leicester); Dr Rachel Kneen (Liverpool); Dr Jeen Tan (Manchester); Dr Anita Devlin (Newcastle); Dr Manish Prasad (Nottingham); Dr Rohini Rattihalli (Oxford); Dr Helen Basu (Preston); Dr Archana Desurkar (Sheffield); Dr Penny Fallon(St Georges); all co-investigators, dietitians and research nurses in participating centres.
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Ethics statement
Full ethical approval was sought and approved by the Research Ethics Committee [REC reference 14/LO/1230] on 2 September 2014 prior to trial start. Approval from the Medicines and Healthcare products Regulatory Agency was gained with annual review as medicinal compounds without marketing authorisations in the target population were used as the comparator.
Information governance statement
UCL is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under Data Protection legislation UCL is the Data Processor, delegated to the CTU with a third part MOU with Sealed Envelope who hosted the database; UCL is the Data Controller, and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for UCL’s Data Protection Officer here: https://ucl.ac.uk/data-protection/data-protection
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YJTR9895.
Primary conflicts of interest: Natasha Schoeler was previously supported for a research post by Vitaflo (International) Ltd and she has received grants from Nutricia Advanced Medical Nutrition, Vitaflo (International) Ltd and Matthew’s Friends Charity, and honoraria from Nutricia Advanced Medical Nutrition, Vitaflo (International) Ltd. and Schaer. Simon Eaton, Simon Heales and J Helen Cross report grants from Vitaflo (International) Ltd. J Helen Cross also reports honoraria from Nutricia, grants from GW Pharma, Zogenix, Marinius and Ovid outside the submitted work. Simon Eaton, Simon Heales and J Helen Cross have a patent on Nutritional product (WO2013186570) and a patent on Anticonvulsant compound (WO2016038379A1) issued. Simon Heales reports consultancy fees and PhD studentship funding from Vitaflo (International) Ltd. Christin Eltze reports honorarium from GW JAZZ Pharma. Hui Jeen Tan reports honoraria from UCB Pharma, Nutricia and GW Pharma. Shakti Agrawal reports honorarium from Nutricia. Alasdair Parker reports honorarium from Biomarin. Elizabeth Neal reports honorarium from Vitaflo (International) Ltd. Anita Devlin reports consultancy fees from Nutricia, and honoraria from Nutricia, GW Pharma and Zogenix. Andrew Mallick reports honoraria from LivaNova PLC and Danone S.A. Nick Freemantle reports grants from NIHR, MRC, Cure Parkinson’s Trust and EU; he also reports consultancy fees from ALK, Sanofi Aventis, Gedeon Richter, Abbott, Galderma, AstraZeneca, Ipsen, Vertex, Thea, Novo Nordisk, Aimmune and Ipsen, and honorarium from Abbott Singapore. Irwin Nazareth has previously formed part of the Disease Prevention Panel, HTA Commissioning Sub-Board, HTA Primary Care Themed Call board and HTA Commissioning Committee, and CTUs funded by NIHR member. All other authors declare no competing interests.
Disclaimers
This article presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Titre-Johnson S, Schoeler N, Eltze C, Williams R, Vezyroglou K, McCullagh H, et al. Ketogenic diet in the treatment of epilepsy in children under the age of 2 years: study protocol for a randomised controlled trial. Trials 2017;18.
- Eltze CM, Chong WK, Cox T, Whitney A, Cortina-Borja M, Chin RF, et al. A population-based study of newly diagnosed epilepsy in infants. Epilepsia 2013;54:437-45.
- Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia 2005;46:561-7.
- Chevrie JJ, Aicardi J. Convulsive disorders in the first year of life: neurological and mental outcome and mortality. Epilepsia 1978;19:67-74.
- Altunbasak S, Incecik F, Herguner O, Refik BH. Prognosis of patients with seizures occurring in the first 2 years. J Child Neurol 2007;22:307-13.
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, et al. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia 2001;42:1553-62.
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet 2004;364:1773-8.
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. United Kingdom Infantile Spasms Study . The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol 2005;4:712-7.
- van der Louw E, van den Hurk D, Neal E, Leiendecker B, Fitzsimmon G, Dority L, et al. Ketogenic diet guidelines for infants with refractory epilepsy. Eur J Paediatr Neurol 2016;20:798-809.
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 2008;7:500-6.
- Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev 2020;6.
- Masino S, Rho J. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda, MD: National Center for Biotechnology Information (US); 2012.
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 2006;60:223-35.
- Heales SJ, Woolf DA, Robinson P, Leonard JV. Rapid diagnosis of medium-chain acyl CoA dehydrogenase deficiency by measurement of cis-4-decenoic acid in plasma. J Inherit Metab Dis 1991;14:661-7.
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 2003;23:5928-35.
- Spector R. Fatty acid transport through the blood-brain barrier. J Neurochem 1988;50:639-43.
- Heales SJ, Thompson GN, Massoud AF, Rahman S, Halliday D, Leonard JV. Production and disposal of medium-chain fatty acids in children with medium-chain acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis 1994;17:74-80.
- Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta 1993;1183:41-57.
- Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013;69:105-14.
- Sparrow SS, Cicchetti D, Balla DA. Vineland Adaptive Behavior Scales. (Vineland-II) PsycTests A; 2005.
- SAS Statistical Analysis Software v9.4 n.d.
- Lyons L, Schoeler NE, Langan D, Cross JH. Use of ketogenic diet therapy in infants with epilepsy: a systematic review and meta-analysis. Epilepsia 2020;61:1261-81.
- Pina-Garza JE, Espinoza R, Nordli D, Bennett DA, Spirito S, Stites TE, et al. Oxcarbazepine adjunctive therapy in infants and young children with partial seizures. Neurology 2005;65:1370-5.
- Pina-Garza JE, Levisohn P, Gucuyener K, Mikati MA, Warnock CR, Conklin HS, et al. Adjunctive lamotrigine for partial seizures in patients aged 1 to 24 months. Neurology 2008;70:2099-108.
- Pina-Garza JE, Nordli DR, Rating D, Yang H, Schiemann-Delgado J, Duncan B, et al. Adjunctive levetiracetam in infants and young children with refractory partial-onset seizures. Epilepsia 2009;50:1141-9.
- IJff D, Postulart D, Lambrechts D, Majoie M, de Kinderen RJA, Hendriksen JGM, et al. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: a randomized controlled trial. Epilepsy Behav 2016;60:153-7.
- Lambrechts D, de Kinderen RJA, Vles JSH, de Louw AJ, Aldenkamp AP, Majoie HJM. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol Scand 2017;135:231-39.
- Auvin S, French J, Dlugos D, Knupp KG, Perucca E, Arzimanoglou A, et al. Novel study design to assess the efficacy and tolerability of antiseizure medications for focal-onset seizures in infants and young children: a consensus document from the regulatory task force and the pediatric commission of the International League against Epilepsy (ILAE), in collaboration with the Pediatric Epilepsy Research Consortium (PERC). Epilepsia Open 2019;4:537-43.
Appendix 1 Laboratory procedures
Clinical laboratory investigations taken at baseline, week 8, month 6 and month 12 were:
-
FBC (white blood cell, platelets, haemoglobin).
-
U&Es (urea, sodium, potassium, chloride, bicarbonate, creatinine).
-
LFTs (total protein, bilirubin, albumin, alkaline phosphatase, alanine transaminase, aspartate transaminase).
-
Glucose.
-
Calcium.
-
Phosphate.
-
Vitamin D.
-
Selenium.
-
Zinc.
-
Magnesium.
-
Cholesterol.
-
Triglycerides.
-
Acylcarnitine profile.
-
Non-esterified fatty acids (NEFA).
-
Beta-hydroxybutyrate (BHB – to be taken at baseline and week 8 only).
-
Urine calcium/creatinine ratio.
-
Urine organic acids.
-
The special assay was taken at baseline and 8 weeks only.
The blood and urine samples for the secondary outcome were processed at local labs. The special assay blood samples to evaluate the plasma profiles of medium-chain fatty acids and for the assessment of mitochondrial function (respiratory chain enzymes) and enrichment (citrate synthase) were processed at the Chemical Pathology Laboratory at Great Ormond Street Hospital.. A sample management standard operating procedure was created to outline the details of sample collection and shipment to the central laboratory from local sites.
GC-MS analysis of medium-chain fatty acids
100 µl plasma, 30 µl internal standard mix (0.33 µM each of d15-octanoic acid, d5-decanoic acid and d23-dodecanoic acid) and 400 µl water was acidified with 125 µl 6N HCL. 3 ml ethyl acetate was added, and the upper organic layer was dried under N2 and derivatised at room temperature for 15 minutes using 50 µl 10% (v/v) 2,3,4,5,6-pentafluorobenzyl bromide in acetonitrile, plus 10 µl of triethylamine. Samples were dried under N2, redissolved in 100 µl of ethyl acetate and analysed by GC/MS (Thermo DSQ II) with Trace GC, RXI®-5Sil column (30 m × 0.25 mm I.D, 0.25 M film thickness), inlet temperature 280 °C, helium flow rate 0.8 ml/minute, 2 µl injection and 1 : 100 split ratio. The oven temperature gradient was 110 °C to 220 °C at 10 °C/minute, then to 300 °C at 30 °C/minute. Compounds were analysed by negative chemical ionisation (methane flow 2 ml/minute). The following fragment ions were detected in selected ion monitoring mode: m/z 143 (C8), 158 (d15-C8), 171 (C10), 176 (d5-C10), 199 (C12), 222 (d23-C12).
Appendix 2 ASM consensus flowchart
Appendix 3 Summary of protocol amendments
| Amendment name/code | Purpose of amendment | Classification (substantial/non-substantial) | Version/date of amended documents | Date submitted (if applicable) | Date approved for substantial amendments only | ||
|---|---|---|---|---|---|---|---|
| New | Old | REC | MHRA | ||||
| 1 (SA#1) | Response to grounds of non-acceptance from the MHRA. Changes to protocol – exclusion criteria | Substantial | V2.0 26 August 2014 | v1.0 17 June 2014 | 14 October 2014 | 24 October 2014 | (Response to GNA letter approved on 1 September 2014) |
| Notification only – MHRA | Notification of change of sponsor name | N/A | N/A | N/A | 27 October 2014 | N/A | N/A |
| 2 (NSA#1) | Change of lab name. Change of sponsor contact | Non-substantial | Protocol v2.1, 11 December 2014 | v2.0 26 August 2014 | 11 December 2014 | Site team notified REC on 11 December 2014 – letter from REC dated 12 December 2014 | N/A |
| 3 (NSA#2) | Change of lab name. Change of sponsor contact | Non-substantial | KIWE Food diary v1.1 dated 12 January 2015 KIWE Seizure diary v1.1 dated 12 January 2015 KIWE Treatment side effects v1.1 dated 12 January 2015 |
KIWE Food diary v1.0 dated 17 June 2014 KIWE Seizure diary v1.0 dated 17 June 2014 KIWE Treatment side effects v1.0 dated 17 June 2014 |
15 January 2014 12 January 2015 |
N/A | N/A |
| 4 (SA#2) | Ketones to be recorded for both groups of infants; KIWE side effects, seizure diary and food diary updated | Substantial | Protocol v3.0 1 May 2015 | Protocol v2.1, 11 December 2014 | 3 June 2015 | N/A | |
| 5 (NSA#3) | Amendment to seizure recording time points and update of KD Intervention Manual | Non-substantial | Protocol v3.1 9 June 2015 Seizure diary V1.2, 9 June 2015 |
Protocol v3.0 1 May 2015 Seizure diary V1.1, 1 May 2015 |
N/A | N/A | |
| 6 (SA#3) | Addition of PIC sites; schematic trial design flowchart to include seizure recording frequency; clarification of procedures within the main text and flowchart of assessments within the protocol | Substantial | Protocol v4.0; PIS v2.0 | Protocol v3.1; PIS v1.2 | 7 September 2015 | 30 September 2015 | N/A |
| 7 (SA#4) | Protocol – reducing seizure frequency to ≥ 4 seizures/week; If the child is prone to seizures in excess of 2/day, then minimum 1-week baseline instead of 2 weeks; +/5 days deviation window allowed at randomisation, 4 weeks and 8 weeks. Removal of ‘β hydroxybutyrate and acetoacetate’ as not required as part of haematology. Minor changes to the schematic of trial design, study procedures and schedule of assessments and flowchart of study assessments. MHRA amendment – updated reference safety information for certain SPCs |
Substantial | Protocol v5.0 | Protocol v4.0 | 12 November 2015 | 7 December 2015 | 18 December 2015 |
| 8 (SA#5) |
|
Substantial | Protocol v6; PIS V3.0; Invite letter V2.0; Seizure diary V3.0 | Protocol v5.0; Invite letter v1.0; Seizure diary V2.0;PIS V2.0 | 23 December 2016 | 10 January 2017 | |
|
|||||||
| 9 (NSA#4) | An administrative error was noted in protocol v6.0 where the asterisk was removed from the schedule of events. This was corrected in v6.1 to clarify that bloods should be taken for only the KD patients at months 6 and 12 | Non-substantial | Protocol V6.1 | Protocol V6.0 | N/A | N/A | |
| 10 (SA#6) | Change of PI at Leicester and Bristol hospitals; removal of co-investigators in protocol; removal of Matthew’s Friends as a recruiting centre; clarification of wording; notification of previous change in food diary | Substantial | Protocol V7.0, Food diary V2.0 dated 16 July 2015 | Protocol V6.1 | 22 June 2017 | N/A | |
| 11 (SA#7) | Updates to the reference safety information for certain SPCs. A no-cost extension to recruitment to end on 31 October 2018 |
Substantial | N/A | N/A | 2 January 2018 | 23 January 2018 | 6 February 2018 |
| 12 (NSA#5) | Update to GDPR information to be handed out to new patients and patients who participated in the trial on or after 25 May 2018 | Non-substantial, non-notifiable | Transparency Notice for Research Participants, v1 24 July 2018, GDPR Transparency Notice Distribution Log, v1 25 July 2018 | N/A | N/A | N/A (non-notifiable) | N/A |
| 13 (NSA#6) | Recruitment end date on 31 November 2019 | Non-substantial | N/A | N/A | 29 October 2018 | N/A | N/A |
| 14 (SA#8) | Recruitment end date on 30 April 2021. Manchester PI updated from Dr Timothy Martland to Dr Jeen Tan Protocol : amended wording of exclusion criterion so that patients who have been prescribed phenobarbital are not excluded. Trial manager details updated. Maryam Balogun added as Research Administrator. Sponsor details updated |
Substantial | Protocol v8.0, 12 March 2019 | Protocol V7.0, 16 July 2015 | 25 March 2019 | 29 April 2019 | N/A |
| 15 (SA#9) | Addition of two research sites in England: Nottingham University Hospitals NHS Trust. Oxford University Hospitals NHS Foundation Trust. Addition of four research sites in Scotland: Royal Aberdeen Children’s Hospital – NHS Grampian Royal Hospital for Children – NHS Greater Glasgow and Clyde, Tayside Children’s Hospital – NHS Tayside (Dundee), Royal Hospital for Sick Children – NHS Lothian (Edinburgh) |
Substantial | N/A | N/A | 14 June 2019 | 18 June 2019 | N/A |
| 16 (SA 10) | SmPC updates as follows (see current approved RSI version and updated RSI version) | Substantial | Updated SmPCs – see ROT dates in column C | SmPCs | 21 April 2020 | 3 June 2020 | 28 May 2020 |
| 17 (SA11) | Consent can be taken over the phone with confirmation of consent via e-mail or post and signature gained at the next face-to-face visit. Existing bloods which are < 6 weeks old can be used for screening. Visits may be carried out remotely and data such as diaries and questionnaires can be collected via remote methods. Flexibility window for visits has been increased. Bloods may be taken locally. A contingency plan has been added if the central lab is unable to accept new samples. The sites will store the samples until the lab is ready to reopen |
Substantial | Protocol v9.0, 28 June 2020, PIS v4.0, 28 June 2020 | Protocol v8.0, 12 March 2019, PIS v3.0 | 6 July 2020 | E-mail on 14 July 2020 confirming non-substantial category C | 25 August 2020 |
| 18 (SA12) | Protocol update regarding informed consent procedure obtained during a telephone consultation. Update of the RSI of certain SmPCs. An extension to the recruitment period to end on 31 September 2021 and trial end date to 28 February 2022. Edit of trial manager e-mail address on protocol |
Substantial | Protocol v10.0 31 March 2021; Updated SmPCs | Protocol v9.0, 28 June 2020; SmPCs | 31 March 2021 | E-mail on 7 April 2021 confirming REC/HRA approval not required | 18 May 2021 |
Appendix 4 Laboratory measurements at baseline by randomised group
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| White blood cell count × 109/l | 9.45 | (3.85) | 9.67 | (3.84) |
| White blood cell out of normal range | 8/47 | 17 | 11/67 | 16 |
| Platelets × 109/l | 370 | (126) | 362 | (136) |
| Platelets out of normal range | 9/45 | 20 | 20/65 | 31 |
| Haemoglobin g/dl median (IQR) | 12.0 | (11.3, 13.3) | 12.3 | (11.3, 13.2) |
| Haemoglobin out of normal range | 8/47 | 17 | 8/66 | 12 |
| Sodium (mmol/l) | 140 | (3) | 140 | (2) |
| Sodium out of normal range | 4/51 | 8 | 2/71 | 3 |
| Potassium (mmol/l) | 4.5 | (0.5) | 4.6 | (0.6) |
| Potassium out of normal range | 4/50 | 8 | 5/71 | 7 |
| Chloride (mmol/l) | 104 | (4) | 105 | (3) |
| Chloride out of normal range | 3/37 | 8 | 7/61 | 11 |
| Bicarbonate (mmol/l) median (IQR) | 23 | (20, 25) | 23 | (20, 25) |
| Bicarbonate out of normal range | 4/34 | 12 | 13/54 | 24 |
| Urate (umol/l) median (IQR) | 6.2 | (2.8, 203.0) | 21.1 | (3.0, 172.0) |
| Urate out of normal range | 5/32 | 16 | 7/36 | 19 |
| Creatinine (µmol/l) | 20.9 | (6.5) | 21.2 | (5.0) |
| Creatinine out of normal range | 11/50 | 22 | 17/70 | 24 |
| Total protein (g/l) | 64 | (6) | 65 | (6) |
| Total protein out of normal range | 9/34 | 26 | 9/52 | 17 |
| Total bilirubin (µmol/l) median (IQR) | 4 | (3, 5) | 4 | (3, 5) |
| Total bilirubin out of normal range | 2/45 | 4 | 2/62 | 3 |
| Albumin (g/l) | 39 | (5) | 40 | (4) |
| Albumin out of normal range | 9/50 | 18 | 11/69 | 16 |
| Alkaline phosphatase (IU/l) median (IQR) | 215 | (154, 272) | 226 | (189, 309) |
| Alkaline phosphatase out of normal range | 7/49 | 14 | 9/67 | 13 |
| Alanine transaminase (ALT) (IU/l) median (IQR) | 16 | (9, 22) | 17 | (8, 29) |
| Alanine transaminase (ALT) out of normal range | 4/50 | 8 | 12/68 | 18 |
| Aspartate transaminase (AST) (IU/l) median (IQR) | 36 | (24, 53) | 39 | (29, 55) |
| Aspartate transaminase (AST) out of normal range | 6/30 | 20 | 8/44 | 18 |
| Calcium mmol/l median (IQR) | 2.54 | (2.46, 2.61) | 2.53 | (2.43, 2.60) |
| Calcium out of normal range | 13/49 | 27 | 18/66 | 27 |
| Glucose (mmol/l) | 4.6 | (4.2, 4.9) | 4.6 | (4.1, 5.1) |
| Glucose out of normal range | 5/43 | 12 | 1/57 | 2 |
| Beta-hydroxybutyrate (mmol/l) median (IQR) | 0.10 | (0.07, 0.30) | 0.10 | (0.09, 0.49) |
| Beta-hydroxybutyrate out of normal range | 1/24 | 4 | 1/33 | 3 |
| Acetoacetate (mmol/l) | a | a | ||
| Acetoacetate out of normal range | a | a | ||
| Vitamin D (nmol/l) | 93 | (44) | 93 | (35) |
| Vitamin D out of normal range | 3/35 | 9 | 9/41 | 22 |
| Selenium (µmol/l) | 0.79 | (0.20) | 0.82 | (0.20) |
| Selenium out of normal range | 9/37 | 24 | 13/51 | 25 |
| Zinc (µmol/l) median (IQR) | 11.9 | (10.9, 13.0) | 11.0 | (9.5, 12.6) |
| Zinc out of normal range | 5/36 | 14 | 12/54 | 22 |
| Magnesium (mmol/l) median (IQR) | 0.92 | (0.85, 0.97) | 0.95 | (0.88, 0.97) |
| Magnesium out of normal range | 6/44 | 14 | 7/65 | 11 |
| Cholesterol (mmol/l) | 4.1 | (0.8) | 4.2 | (1.2) |
| Cholesterol out of normal range | 7/46 | 15 | 9/67 | 13 |
| Triglycerides (mmol/l) median (IQR) | 1.60 | (1.10, 1.90) | 1.40 | (0.94, 1.93) |
| Triglycerides out of normal range | 14/45 | 31 | 13/67 | 19 |
| Non-esterified fatty acids (NEFA) (mmol/l) median (IQR) | 0.40 | (0.30, 0.52) | 0.45 | (0.24, 0.93) |
| Non-esterified fatty acids (NEFA) out of normal range | 2/18 | 11 | 1/25 | 4 |
| Phosphate (mmol/l) | 1.75 | (0.30) | 1.83 | (0.20) |
| Phosphate out of normal range | 5/28 | 18 | 6/52 | 12 |
| Free carnitine (µmol/l) | 36 | (15) | 33 | (11) |
| Free carnitine out of normal range | 3/35 | 9 | 3/50 | 6 |
| Acetyl carnitine (µmol/l) | 21.5 | (10.6) | 20.6 | (8.0) |
| Acetyl carnitine out of normal range | 8/31 | 26 | 2/35 | 6 |
| Propionyl carnitine (µmol/l) median (IQR) | 1.08 | (0.80, 1.76) | 1.00 | (0.67, 1.51) |
| Propionyl carnitine out of normal range | 4/27 | 15 | 2/29 | 7 |
| Butyryl carnitine (µmol/l) median (IQR) | 0.20 | (0.18, 0.27) | 0.22 | (0.16, 0.37) |
| Butyryl carnitine out of normal range | 4/26 | 15 | 4/28 | 14 |
| Isovaleryl carnitine (µmol/l) median (IQR) | 0.20 | (0.10, 0.29) | 0.13 | (0.10, 0.30) |
| Isovaleryl carnitine out of normal range | 6/26 | 23 | 3/27 | 11 |
| Hexanoyl carnitine (µmol/l) median (IQR) | 0.09 | (0.05, 0.10) | 0.08 | (0.05, 0.10) |
| Hexanoyl carnitine out of normal range | 6/25 | 24 | 2/27 | 7 |
| Octanoyl carnitine (µmol/l) | 0.11 | (0.06) | 0.11 | (0.06) |
| Octanoyl carnitine out of normal range | 5/26 | 19 | 1/27 | 4 |
| Tetradecenyl carnitine (µmol/l) | 0.09 | (0.04) | 0.09 | (0.05) |
| Tetradecenyl carnitine out of normal range | 3/25 | 12 | 4/24 | 17 |
| Palmitoyl carnitine (µmol/l) median (IQR) | 1.05 | (0.70, 1.20) | 0.80 | (0.70, 1.20) |
| Palmitoyl carnitine out of normal range | 3/23 | 13 | 0/25 | 0 |
| Urine calcium (mmol/l) median (IQR) | 2.23 | (0.85, 3.91) | 1.30 | (0.50, 2.45) |
| Urine calcium out of normal range | 4/39 | 10 | 2/46 | 4 |
| Creatinine ratio median (IQR) | 1.24 | (0.47, 2.18) | 1.00 | (0.57, 1.60) |
| Creatinine ratio out of normal range | 11/36 | 31 | 9/44 | 20 |
| Urine organic acids abnormal | 7/29 | 24 | 9/40 | 23 |
Appendix 5 Clinical and laboratory measurements at 8 weeks by randomised group
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Systolic blood pressure (mmHg) | 93 | (17) | 99 | (16) |
| Diastolic blood pressure (mmHg) | 57 | (10) | 61 | (13) |
| Pulse beats/minutes | 113 | (24) | 122 | (21) |
| Temperature (°C) | 36.7 | (0.4) | 36.7 | (0.3) |
| White blood cell count × 109/l | 11.81 | (9.36) | 10.95 | (4.25) |
| White blood cell count out of normal range | 2/28 | 7 | 5/50 | 10 |
| Platelets × 109/l | 356 | (179) | 387 | (344) |
| Platelets out of normal range | 7/28 | 25 | 6/49 | 12 |
| Haemoglobin (g/dl) median (IQR) | 12.3 | (11.6–14.2) | 13.1 | (12.0–14.4) |
| Haemoglobin out of normal range | 3/28 | 11 | 16/50 | 32 |
| Sodium (mmol/l) | 140 | (3) | 140 | (3) |
| Sodium out of normal range | 0/31 | 0 | 1/57 | 2 |
| Potassium (mmol/l) | 5.6 | (6.6) | 4.5 | (0.5) |
| Potassium out of normal range | 3/31 | 10 | 4/57 | 7 |
| Chloride (mmol/l) | 103 | (4) | 103 | (4) |
| Chloride out of normal range | 0/20 | 0 | 4/45 | 9 |
| Bicarbonate (mmol/l) median (IQR) | 23 | (19–24) | 20 | (19–22) |
| Bicarbonate out of normal range | 3/23 | 13 | 8/41 | 20 |
| Urate (µmol/l) median (IQR) | 119 | (5–198) | 84 | (2–271) |
| Urate out of normal range | 2/20 | 10 | 9/36 | 25 |
| Creatinine (µmol/l) | 22 | (7) | 19 | (5) |
| Creatinine out of normal range | 4/31 | 13 | 12/52 | 23 |
| Total protein (g/l) | 67 | (10) | 65 | (10) |
| Total protein out of normal range | 3/16 | 19 | 9/38 | 24 |
| Total bilirubin (umol/L) median (IQR) | 3 | (3–5) | 4 | (3–6) |
| Total bilirubin out of normal range | 0/29 | 0 | 3/48 | 6 |
| Albumin (g/l) | 39 | (5) | 41 | (5) |
| Albumin out of normal range | 4/32 | 13 | 11/55 | 20 |
| Alkaline phosphatase (IU/l) median (IQR) | 216 | (170–296) | 223 | (172–257) |
| Alkaline phosphatase out of normal range | 2/29 | 7 | 8/55 | 15 |
| Alanine transaminase (IU/l) median (IQR) | 13 | (7–21) | 18 | (9–24) |
| Alanine transaminase out of normal range | 3/31 | 10 | 5/54 | 9 |
| Aspartate transaminase (IU/l) median (IQR) | 36 | (26–53) | 43 | (31–62) |
| Aspartate transaminase out of normal range | 3/18 | 17 | 9/34 | 26 |
| Calcium (mmol/l) median (IQR) | 2.45 | (2.41–2.56) | 2.46 | (2.38–2.57) |
| Calcium out of normal range | 4/28 | 14 | 12/52 | 23 |
| Glucose (mmol/l) | 4.4 | (0.7) | 4.0 | (0.6) |
| Glucose out of normal range | 0/28 | 0 | 4/44 | 9 |
| Beta-hydroxybutyrate (mmol/l) median (IQR) | 0.20 | (0.07–3.94) | 2.98 | (1.40–5.25) |
| Beta-hydroxybutyrate out of normal range | 0/19 | 0 | 5/23 | 22 |
| Acetoacetate (mmol/l) | a | a | ||
| Acetoacetate out of normal range | a | a | ||
| Vitamin D (nmol/l) | 104 | (48) | 115 | (34) |
| Vitamin D out of normal range | 1/13 | 8 | 3/32 | 9 |
| Selenium (umol/l) | 0.92 | (0.25) | 1.19 | (1.56) |
| Selenium out of normal range | 6/22 | 27 | 10/39 | 26 |
| Zinc (µmol/l) median (IQR) | 12.2 | (11.3–14.0) | 12.3 | (10.0–14.0) |
| Zinc out of normal range | 1/23 | 4 | 7/39 | 18 |
| Magnesium (mmol/l) median (IQR) | 0.93 | (0.88–0.96) | 0.91 | (0.84–0.97) |
| Magnesium out of normal range | 2/29 | 7 | 7/48 | 15 |
| Cholesterol (mmol/l) | 4.43 | (0.86) | 4.34 | (1.39) |
| Cholesterol out of normal range | 5/27 | 19 | 6/51 | 12 |
| Triglycerides (mmol/l) median (IQR) | 1.51 | (1.11–2.38) | 1.81 | (1.31–3.10) |
| Triglycerides out of normal range | 8/24 | 33 | 23/53 | 43 |
| Non-esterified fatty acids (mmol/l) median (IQR) | 0.6 | (0.4–1.7) | 1.1 | (0.8–1.8) |
| Non-esterified fatty acids out of normal range | 0/18 | 0 | 1/21 | 5 |
| Phosphate (mmol/l) | 2.68 | (4.19) | 1.79 | (0.38) |
| Phosphate out of normal range | 2/26 | 8 | 4/44 | 9 |
| Free carnitine (µmol/l) | 31 | (12) | 33 | (13) |
| Free carnitine out of normal range | 0/20 | 0 | 5/42 | 12 |
| Acetyl carnitine (µmol/l) | 24.8 | (16.6) | 35.7 | (13.4) |
| Acetyl carnitine out of normal range | 3/14 | 21 | 18/34 | 53 |
| Propionyl carnitine (µmol/l) median (IQR) | 1.02 | (0.70–1.55) | 0.60 | (0.45–1.11) |
| Propionyl carnitine out of normal range | 0/12 | 0 | 2/25 | 8 |
| Butyryl carnitine (µmol/l) median (IQR) | 0.28 | (0.26–0.30) | 0.33 | (0.21–0.54) |
| Butyryl carnitine out of normal range | 1/12 | 8 | 10/30 | 33 |
| Isovaleryl carnitine (µmol/l) median (IQR) | 0.20 | (0.16–0.27) | 0.20 | (0.10–0.29) |
| Isovaleryl carnitine out of normal range | 1/12 | 8 | 4/24 | 17 |
| Hexanoyl carnitine (µmol/l) median (IQR) | 0.08 | (0.07–0.15) | 0.10 | (0.07–0.11) |
| Hexanoyl carnitine out of normal range | 3/12 | 25 | 4/26 | 15 |
| Octanoyl carnitine (µmol/l) | 0.15 | (0.05) | 0.18 | (0.21) |
| Octanoyl carnitine out of normal range | 3/11 | 27 | 4/26 | 15 |
| Tetradecenyl carnitine (µmol/l) | 0.09 | (0.04) | 0.14 | (0.06) |
| Tetradecenyl carnitine out of normal range | 3/12 | 25 | 0/23 | 0 |
| Palmitoyl carnitine (µmol/l) median (IQR) | 0.9 | (0.6–1.2) | 1.2 | (0.6–1.5) |
| Palmitoyl carnitine out of normal range | 2/11 | 18 | 7/26 | 27 |
| Urine calcium (mmol/l) median (IQR) | 2.02 | (1.25–2.91) | 3.00 | (1.62–5.14) |
| Urine calcium out of normal range | 1/17 | 6 | 3/38 | 8 |
| Creatinine ratio median (IQR) | 1.1 | (0.5–2.2) | 1.7 | (1.1–4.3) |
| Creatinine ratio out of normal range | 5/19 | 26 | 18/37 | 49 |
| Urine organic acids abnormal | 0/10 | 0 | 12/23 | 52 |
Appendix 6 Clinical and laboratory measurements at 12 months by randomised group
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Changes in concomitant medications | 14/30 | 47 | 16/37 | 43 |
| Changes in ASM | 14/27 | 52 | 24/36 | 67 |
| Developmental delay | 22/23 | 96 | 33/34 | 97 |
| Hemiplegia | 3/23 | 13 | 7/34 | 21 |
| Weight (kg) | 16.7 | (19.8) | 12.7 | (3.0) |
| Length (m) | 0.84 | (0.06) | 0.87 | (0.09) |
| Head circumference (cm) | 45.0 | (3.5) | 45.4 | (5.4) |
| Weight SDS | 0.35 | (1.27) | 0.16 | (1.51) |
| Length SDS | −0.81 | (1.60) | −0.22 | (1.68) |
| Head circumference SDS | −1.89 | (2.29) | −1.06 | (2.30) |
| Systolic blood pressure (mmHg) | 91 | (11) | 106 | (16) |
| Diastolic blood pressure (mmHg) | 58 | (13) | 76 | (14) |
| Pulse beats/minute | 115 | (17) | 125 | (19) |
| Temperature (°C) | 36.6 | (0.2) | 36.6 | (0.4) |
| White blood cell count × 109/l | 9.71 | (3.36) | 9.20 | (2.97) |
| White blood cell out of normal range | 3/11 | 27 | 3/17 | 18 |
| Platelets × 109/l | 304 | (125) | 365 | (146) |
| Platelets out of normal range | 4/11 | 36 | 8/17 | 47 |
| Haemoglobin (g/dl) median (IQR) | 15 | (12–122) | 13 | (12–99) |
| Haemoglobin out of normal range | 3/11 | 27 | 3/17 | 18 |
| Sodium (mmol/l) | 140 | (3) | 139 | (2) |
| Sodium out of normal range | 1/11 | 9 | 1/20 | 5 |
| Potassium (mmol/l) | 4.5 | (0.9) | 4.3 | (0.5) |
| Potassium out of normal range | 2/11 | 18 | 0/20 | 0 |
| Chloride (mmol/l) | 102 | (3) | 101 | (3) |
| Chloride out of normal range | 2/10 | 20 | 2/13 | 15 |
| Bicarbonate (mmol/l) median (IQR) | 20 | (17–21) | 21 | (20–23) |
| Bicarbonate out of normal range | 3/11 | 27 | 2/13 | 15 |
| Urate (umol/l) median (IQR) | 11 | (3–195) | 2 | (1–246) |
| Urate out of normal range | 1/7 | 14 | 7/11 | 64 |
| Creatinine (µmol/l) | 22 | (4) | 19 | (4) |
| Creatinine out of normal range | 0/11 | 0 | 5/20 | 25 |
| Total protein (g/l) | 68 | (13) | 64 | (7) |
| Total protein out of normal range | 5/9 | 56 | 2/13 | 15 |
| Total bilirubin (µmol/l) median (IQR) | 10 | (5–18) | 3 | (2–5) |
| Total bilirubin out of normal range | 2/9 | 22 | 2/15 | 13 |
| Albumin (g/l) | 41 | (7) | 39 | (5) |
| Albumin out of normal range | 3/11 | 27 | 4/19 | 21 |
| Alkaline phosphatase (IU/l) median (IQR) | 187 | (123–248) | 226 | (164–258) |
| Alkaline phosphatase out of normal range | 1/10 | 10 | 5/20 | 25 |
| Alanine transaminase (IU/l) median (IQR) | 21 | (13–26) | 16 | (11–24) |
| Alanine transaminase out of normal range | 1/11 | 9 | 4/20 | 20 |
| Aspartate transaminase (IU/l) median (IQR) | 44 | (37–55) | 51 | (23–60) |
| Aspartate transaminase out of normal range | 1/8 | 13 | 3/11 | 27 |
| Calcium (mmol/l) median (IQR) | 2.45 | (2.27–2.49) | 2.43 | (2.36–2.51) |
| Calcium out of normal range | 2/11 | 18 | 4/17 | 24 |
| Glucose (mmol/l) | 3.9 | (0.7) | 3.8 | (0.6) |
| Glucose out of normal range | 2/11 | 18 | 3/11 | 27 |
| Beta-hydroxybutyrate (mmol/l) median (IQR) | 2.27 | (1.19–4.30) | 2.10 | (0.49–4.27) |
| Beta-hydroxybutyrate out of normal range | 0/3 | 0 | 0/6 | 0 |
| Acetoacetate (mmol/l) | a | a | ||
| Acetoacetate out of normal range | a | a | ||
| Vitamin D (nmol/l) | 116 | (39) | 110 | (68) |
| Vitamin D out of normal range | 2/7 | 29 | 2/13 | 15 |
| Selenium (umol/l) | 0.92 | (0.33) | 0.89 | (0.22) |
| Selenium out of normal range | 2/7 | 29 | 4/14 | 29 |
| Zinc (µmol/l) median (IQR) | 12 | (10–14) | 10 | (9–17) |
| Zinc out of normal range | 1/9 | 11 | 6/14 | 43 |
| Magnesium (mmol/l) median (IQR) | 0.96 | (0.90–1.01) | 0.88 | (0.82–0.94) |
| Magnesium out of normal range | 3/11 | 27 | 2/16 | 13 |
| Cholesterol (mmol/l) | 4.5 | (1.4) | 4.9 | (1.8) |
| Cholesterol out of normal range | 2/10 | 20 | 3/18 | 17 |
| Triglycerides (mmol/l) median (IQR) | 2.3 | (1.3–3.9) | 2.1 | (1.8–3.0) |
| Triglycerides out of normal range | 5/10 | 50 | 7/17 | 41 |
| Non-esterified fatty acids (mmol/l) median (IQR) | 0.82 | (0.62–1.09) | 1.15 | (0.50–1.77) |
| Non-esterified fatty acids out of normal range | 1/4 | 25 | 0/4 | 0 |
| Phosphate (mmol/l) | 1.59 | (0.23) | 1.63 | (0.23) |
| Phosphate out of normal range | 0/6 | 0 | 1/13 | 8 |
| Free carnitine (µmol/l) | 29 | (16) | 33 | (21) |
| Free carnitine out of normal range | 1/12 | 8 | 3/15 | 20 |
| Acetyl carnitine (µmol/l) | a | a | ||
| Acetyl carnitine out of normal range | 5/11 | 45 | 6/12 | 50 |
| Propionyl carnitine (µmol/l) median (IQR) | 0.62 | (0.49–1.00) | 0.51 | (0.45–0.97) |
| Propionyl carnitine out of normal range | 1/9 | 11 | 1/11 | 9 |
| Butyryl carnitine (µmol/l) median (IQR) | 0.31 | (0.20–0.52) | 0.32 | (0.18–0.59) |
| Butyryl carnitine out of normal range | 5/10 | 50 | 4/12 | 33 |
| Isovaleryl carnitine (µmol/l) median (IQR) | 0.20 | (0.15–0.28) | 0.10 | (0.08–0.29) |
| Isovaleryl carnitine out of normal range | 3/9 | 33 | 1/11 | 9 |
| Hexanoyl carnitine (µmol/l) median (IQR) | 0.08 | (0.07–0.12) | 0.09 | (0.07–0.10) |
| Hexanoyl carnitine out of normal range | 2/8 | 25 | 2/10 | 20 |
| Octanoyl carnitine (µmol/l) | 0.14 | (0.07) | 0.12 | (0.05) |
| Octanoyl carnitine out of normal range | 2/9 | 22 | 1/10 | 10 |
| Tetradecenyl carnitine (µmol/l) | 0.12 | (0.07) | 0.13 | (0.07) |
| Tetradecenyl carnitine out of normal range | 1/8 | 13 | 2/10 | 20 |
| Palmitoyl carnitine (µmol/l) median (IQR) | 1.05 | (0.75–1.70) | 0.95 | (0.40–1.40) |
| Palmitoyl carnitine out of normal range | 2/7 | 29 | 2/11 | 18 |
| Urine calcium (mmol/l) median (IQR) | 1.40 | (1.28–7.15) | 1.75 | (0.58–2.32) |
| Urine calcium out of normal range | 0/3 | 0 | 1/10 | 10 |
| Creatinine ratio median (IQR) | 1.8 | (1.1–2.4) | 1.0 | (0.5–2.6) |
| Creatinine ratio out of normal range | 0/3 | 0 | 4/10 | 40 |
| Urine organic acids abnormal | 0/2 | 0 | 2/6 | 33 |
Appendix 7 Modelling in terms of KD group at 12 months for Infant Toddler Quality of Life Questionnaire and Vineland Adaptive Behaviour Scales at 12 months by randomised group
| Characteristic | ASM group | KD group | ||
|---|---|---|---|---|
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| ITQOL-97 | ||||
| Infant’s overall health median (IQR) | 60 | (30–85) | 60 | (30–60) |
| Infant’s physical abilities median (IQR) | 47 | (7–70) | 27 | (13–58) |
| Satisfaction with infant’s overall growth and development median (IQR) | 58 | (38–78) | 45 | (38–70) |
| Infant’s pain median (IQR) | 75 | (50–83) | 67 | (33–83) |
| Infant’s temperament and mood median (IQR) | 68 | (60–79) | 65 | (56–71) |
| Infant’s behaviour overall median (IQR) | 67 | (60–83) | 65 | (56–77) |
| Infant’s global behaviour median (IQR) | 85 | (60–100) | 85 | (60–100) |
| Infant getting along with others median (IQR) | 65 | (52–72) | 58 | (50–66) |
| General health perceptions median (IQR) | 41 | (30–52) | 30 | (16–52) |
| Change in infant’s health | ||||
| Much worse than a year ago | 0/24 | 0 | 1/30 | 3 |
| Somewhat worse than a year ago | 1/24 | 4 | 1/30 | 3 |
| About the same now as a year ago | 5/24 | 21 | 7/30 | 23 |
| Somewhat better than a year ago | 6/24 | 25 | 10/30 | 33 |
| Much better than a year ago | 12/24 | 50 | 11/30 | 37 |
| Parental impact emotional median (IQR) | 57 | (36–79) | 54 | (39–68) |
| Parental impact time median (IQR) | 62 | (33–90) | 57 | (43–76) |
| Family cohesion median (IQR) | 85 | (60–85) | 85 | (60–100) |
| Vineland-II | ||||
| Communication receptive v-scale score median (IQR) | 7 | (7–8) | 7 | (5–7) |
| Communication expressive v-scale score median (IQR) | 5 | (3–7) | 5 | (3–8) |
| Communication sum of v-scale scores median (IQR) | 10 | (8–14) | 11 | (9–13) |
| Communication domain standard score median (IQR) | 48 | (43–59) | 49 | (44–55) |
| Daily living personal v-scale score median (IQR) | 6 | (5–7) | 5 | (4–7) |
| Daily living domestic v-scale score median (IQR) | 10 | (9–11) | 11 | (9–11) |
| Daily living community v-scale score median (IQR) | 10 | (9–10) | 10 | (9–10) |
| Daily living sum of v-scale scores median (IQR) | 16 | (15–17) | 15 | (13–16) |
| Daily living domain standard score median (IQR) | 25 | (21–34) | 25 | (21–28) |
| Socialisation interpersonal relationships v-scale score median (IQR) | 6 | (5–9) | 6 | (3–7) |
| Socialisation play v-scale score median (IQR) | 8 | (7–9) | 8 | (7–9) |
| Socialisation coping v-scale score median (IQR) | 9 | (8–9) | 9 | (8–9) |
| Socialisation sum of v-scale scores median (IQR) | 21 | (18–23) | 22 | (19–24) |
| Socialisation domain standard score median (IQR) | 56 | (47–59) | 54 | (53–58) |
| Motor gross v-scale score median (IQR) | a | a | ||
| Motor fine v-scale score median (IQR) | a | a | ||
| Motor sum of v-scale scores median (IQR) | a | a | ||
| Motor skills domain standard score median (IQR) | a | a | ||
| Sum of domain standard scores/adaptive behaviour composite median (IQR) | 165 | (160–171) | 168 | (162–176) |
| Standardised score median (IQR) | 40 | (39–41) | 41 | (39–43) |
List of abbreviations
- ASM
- antiseizure medicine
- CI
- confidence interval
- EudraCT
- European Clinical Trials Database
- IMP
- investigational medicinal product
- IQR
- interquartile range
- IRR
- incidence rate ratio
- KD
- ketogenic diet
- KDT
- ketogenic diet therapies
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- OR
- odds ratio
- PI
- principal investigator
- RCT
- randomised control trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SAE
- serious adverse event
- SDS
- standard deviation score
- SmPC
- summary of product characteristics
- TSC
- Trial Steering Committee
