Notes
Article history
The contractual start date for this research was in August 2017. This article began editorial review in July 2023 and was accepted for publication in June 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Global Health Research editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Mills et al. This work was produced by Mills et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Mills et al.
Background
Low- and middle-income countries (LMICs) account for up to 98% of 2 million annual global stillbirths and 2.4 million neonatal deaths, with around 75% concentrated in sub-Saharan Africa and South Asia. 1 Beyond these appalling numbers, each death is a tragedy for parents who endure long-lasting grief, exacerbated by the lack of social validation of baby death compared to other bereavements. 2 Stigma, isolation and relationship breakdown are common. Negative social consequences often disproportionately impact women, particularly in contexts where childbearing is considered their predominant role. 3 In resource-limited settings, debilitating physical comorbidities associated with traumatic birth, including obstetric fistula, compound psychological morbidity. 4 Following perinatal death, an estimated 60–70% of women experience depressive symptoms and at least 30% develop intense grief reactions, increasing the risk of chronic anxiety, depression and post-traumatic stress disorder. 5 Perinatal mental ill health is a major risk factor for maternal suicide, a mounting contributor to maternal deaths globally. 6
Accumulating evidence demonstrates the importance of compassionate care in health facilities and social support in communities as key to prevention of adverse psychological outcomes following the death of a baby. 7 However, evidence, largely from high-income countries (HICs), suggests inequitable provision, training and resources, meaning that many parents still do not receive respectful bereavement care. 8 Our exploratory work in Kenya and Uganda confirmed and extended previous findings of lack of information and woman-centred care contributing to poor experiences. 9 Parents also described negative responses, isolation and stigma in their communities after discharge from hospital. Health workers were often poorly prepared, lacking essential knowledge and skills, with deficiencies in organisational support and environmental challenges also contributing. 10 Developments in perinatal bereavement support in many HICs have had beneficial impacts on parents’ experiences; however, robust evidence to support specific intervention components or packages is limited. 11,12
Interventions to improve bereavement support are urgently required for parents in LMIC settings including Kenya and Uganda, but they need to reflect the context of implementation. Broad consensus, including our exploratory data, supports common processes of ‘recognition’, ‘remembering’ and ‘rebuilding’ as key to adjustment,13 which require support through respectful communication, information provision, opportunities to create memories of the baby and ongoing psychosocial support in the days and weeks after baby death. Improving practice in facilities requires increased knowledge and skills, which are translated into changes in health worker behaviour. Evidence suggests training alone is unlikely to be sufficient, and leadership and support structures within facilities to promote evidence-based practice are needed. 14 Change ‘champions’ have been used to support successful implementation of interventions in a variety of healthcare contexts, using both individual and collective approaches. 15 There is no universal definition, but champions are normally internal to the organisation, interested in the topic area and committed to the change, with personal qualities including dynamism, energy and persistence. 16 However, their role and impact in perinatal bereavement care in LMIC contexts have not been widely explored.
Although better facility care after stillbirth or neonatal death would be anticipated to improve experiences, hospital stay is usually brief and follow-up by health workers in many LMIC settings is limited. Our data and others also suggest a need for enhanced community support after perinatal death. 17,18 In HICs, peer support networks, involving parents who have previously experienced the death of a baby providing support via groups, telephone contacts or online, have evolved in response to this gap. While robust evaluation is lacking, these services have tangible benefits and are integrated in the continuum of care. Although peer support is not designed to replace professional psychological therapy, it has potential benefit in assisting women and families to adjust to the death of the baby in Kenya and Uganda, where other sources are lacking. 19,20 Following exploratory work in the NIHR Global Health Group (16/137/53), co-production activities identified the potential of an intervention encompassing ‘change champions’ and peer support to improve care and support in the days and weeks following death of a baby, before, during or soon after birth.
Aim
To assess the feasibility of a full-scale evaluation of the effectiveness of a co-produced multicomponent intervention to improve bereavement care, after stillbirth or early neonatal death in Kenya and Uganda.
Objectives
The objectives for feasibility were as follows:
-
To assess recruitment and retention of women into the study; planned sample 120 women.
-
To explore the acceptability, implementation and uptake of the key components of the intervention.
-
To explore the impacts of the research on practice/services and delivery of the intervention.
-
To inform preparation of a full-scale evaluation.
Methods
Design and study setting
This work was part of the NIHR Global Health Group programme of improving care and support after stillbirth and neonatal death in sub-Saharan Africa (NIHR 16/137/53). A prospective, observational feasibility study was conducted in urban tertiary maternity facilities in Kenya (1) and Uganda (1) from November 2019 to December 2020, using a ‘pre’ and ‘post’ design. Research governance approvals were obtained from Kenyatta National Hospital/University of Nairobi (P828/09/2019), Makerere University/Uganda National Council for Science and Technology (UNCST; 2019-059) and University of Manchester (2019-7322-12550) and the study was prospectively registered (protocol available, ISRCTN68506895).
Community engagement and involvement
The NIHR Global Health Group community engagement and involvement (CEI) groups of parents with lived experience of the death of a baby, and stakeholder group of health workers, researchers and policy-makers in Kenya and Uganda contributed to all stages of the research, including planning, conduct and final interpretation of the findings.
Study interventions
The study intervention, informed by evidence synthesis and previous exploratory research conducted in both countries,9,10,17,21 was complemented with use of the behaviour change wheel, a structured approach to designing behaviour change interventions. 22 Two intervention components, addressing CEI group and stakeholder priorities of improving facility care and postnatal support for women after discharge identified, were co-produced with CEI members, health workers and service managers at a series of meetings.
Component one
Perinatal bereavement care ‘champions’ and champion group
Health workers (8–12), working across the included facility maternity (antenatal clinics/wards, labour ward, postnatal wards) and neonatal services (neonatal and paediatric wards) who were interested in improving bereavement support and willing to be involved in the research, were identified through advertising, word of mouth and liaison with managers. Volunteers were invited to a 2-day training workshop, facilitated by the research team, including the principles of respectful bereavement support, good communication and behaviour change techniques. At the end of the training, the champion group identified potential areas of focus, relevant to the local context and initial activities to improve support for bereaved women and families using SMART objectives. Champion group meetings, facilitated by the local research team were scheduled monthly during the intervention period, to enable progress updates and sharing of experiences.
Component two
Telephone peer support
Peer supporters were women who had personal lived experience of the death of a baby, at least 12 months prior to their involvement in the research, and who were identified via local networks including the NIHR Group CEI groups and following an information event. Those interested were invited to a 2-day training workshop and completed a peer supporter agreement outlining the role including the expectations/limitations of confidentiality. Peer support was offered following study recruitment, during phase 2 of the study, not sooner than 2 weeks after the birth or death of the baby and available up to 8 weeks after hospital discharge, the maximum duration for each woman was approximately 6 weeks. If women accepted, initial contact was initiated via the research assistant according to the woman’s preference (women or peer supporter to initiate contact via voice call or WhatsApp). Subsequent contacts were agreed between the woman and peer supporter. To minimise overburden, it was agreed that no more than four women were allocated to each peer supporter at any one time. Support and supervision for peer supporters were planned through regular contacts with the research team, both individually, at regular peer supporter meetings and through a peer supporter WhatsApp group.
Sample and recruitment
Informed by guidance for feasibility studies,23 the planned sample was 120 women (60 per country) ≥ 18 years who had experienced the death of a baby after 28 weeks’ gestation and before, during or 0–6 days after birth (neonatal death in the health facility) following the current pregnancy. This was considered sufficient to permit implementation of the study intervention in two sites and assess the feasibility of a large-scale trial. In the pre-intervention phase (phase 1; scheduled in month 1–6), recruitment of 60 women (30 per country) offered the usual care and support after the death of their baby was planned; in the post-intervention phase (phase 2; scheduled in month 7–12), a further 60 women would be recruited. Recruitment was planned to be completed in the first 3–4 months of each phase, to allow follow-up within 8 weeks after birth. Staff workshops were held in the included facilities, prior to commencement, to inform all staff about the study. Eligible women were identified and approached, prior to discharge from hospital, by health workers providing postnatal care or community health workers. Women were given a brief introduction to the research and those who were interested provided written consent to be contacted by the research team, not sooner than 2 weeks after the birth. At this contact, the research assistant (midwife or nurse) provided verbal and written information, gave an opportunity to ask further questions and allowed the potential participant time to consider. Informed consent was then confirmed in writing. During phase 2, the bereavement champion intervention was implemented on the facility level; therefore, individual consent was not sought for this component. Peer support was offered to all women participating in the study during phase 2; however, it was emphasised during recruitment that women could decline peer support and still participate in the research. Partners (or family members) of women participating in phase 2 of the study were also approached, via woman participants, to explore experiences of care and the research via qualitative interviews. A partner or family member’s unavailability or unwillingness to participate did not impact the woman’s eligibility. Health workers and others directly involved in the research as bereavement champions or peer supporters were also invited to participate in qualitative interviews at the end of phase 2.
Outcome measures
Recruitment and retention of women in the study were the primary feasibility outcomes. The criteria for a trial to be considered feasible was achievement of recruitment targets (n = 120 women) and ≥ 70% of women being retained until study completion. Other key outcomes included the acceptability of the intervention, uptake of peer support and experiences of the research. The feasibility and acceptability of data collection, characteristics of the proposed psychological and clinical outcome measures and process outcomes, including quality of implementation, were also assessed.
Data collection
A screening and recruitment log included data for women providing consent to contact, those recruited and participants leaving the study before completion. Women who declined to participate or left the study early were requested to provide reasons, if they were willing. Data were collected for each participant at two visits with the study research assistant, first at recruitment (around 2 weeks after the birth) and the second follow-up visit (approximately 6–8 weeks after birth) at study completion. Investigator-designed case report forms (demographic, pregnancy and birth, clinical data including healthcare and external support use) were completed at both visits and questionnaires (researcher-administered psychological measures) at follow up. Psychological measures included the perinatal grief scale (PGS) a 33-item measure assessing perinatal grief24 and the Edinburgh Postnatal Depression Scale (EPDS), a 10-item tool, originally designed to assess risk of postnatal depression but subsequently validated to assess anxiety and depressive symptoms in pregnancy. 25,26 Semistructured qualitative interviews with women (n = 37) and partners/family members (n = 13), interviewed separately, were conducted at or shortly after the follow-up visit (in phase 2 only) and with health workers champions (n = 16) and peer supporters (n = 10). Depending on COVID-19 restrictions in place locally, interviews were conducted at a place of the participant’s choice, for example, home or place of work following a risk assessment confirming physical distancing was possible, or by telephone. Interviews were audio-recorded and transcribed verbatim (Topic guides, Report Supplementary Materials S1–S5). To capture wider staff experiences of the intervention and research, a short investigator-designed questionnaire was distributed to health workers in maternity and neonatal wards in the facility at the end of phase 2 (see Report Supplementary Material S6). An intervention log, completed by the research assistants, captured training and meeting records for the bereavement champions and peer supporters. Peer supporters kept a log of the number and length of calls for each woman, space for brief comments was included, but it was not expected that the content of calls be documented.
Data analysis
Case report data, collected on paper, or directly inputted into a custom-designed REDCap database, was verified by a second researcher and exported to SPSS (SPSS Inc., Chicago, IL, USA) for analysis. Demographic, clinical and other outcome data were compared descriptively, reporting frequencies and percentages for categorical variables and descriptive statistics including means, standard deviations (SDs), medians and ranges for numerical variables. Scores for psychological tools were compared to determine whether characteristics were comparable across different measures, including a comparison between rates of missing data for different tools and their component items. For the psychological outcomes, adjusted (for country) 95% confidence intervals (CIs) for differences between the groups (estimated using analysis of covariance) and within-group SDs were calculated to inform the design of a subsequent evaluation.
Qualitative interview data were analysed using an inductive approach, following the five interlinked phases of framework analysis, moving from descriptive accounts to conceptualisation of meaning. 27 Initial analyses were conducted by two researchers and focused on experiences of research participation, recruitment processes, the acceptability and implementation of the intervention, impacts on care and burdens of data collection. The final interpretation of the findings was discussed and agreed by the wider research team.
Findings
Recruitment and retention
Recruitment log data were used to assess the willingness of bereaved women to participate and for those consenting, to continue in the research (Table 1). During phase 1 (usual care), 56 women were recruited: 30 women in Kenya and 26 in Uganda (93% of the planned sample of 60 women). Phase 2 (intervention) recruitment commenced as planned in late February (Kenya)/mid-March (Uganda) 2020. Due to the onset of the COVID-19 pandemic, an urgent review of activities was undertaken in late March 2020. In Kenya, a partial ‘lockdown’ was in place with restrictions to movement, access to and within healthcare facilities. Follow-up data collection for phase 1 was completed and 21 women had agreed to participate in phase 2 of the study. With ethics committee approval, recruitment was paused, and all data collection switched to remote means (via telephone), with research staff working remotely. In Uganda, similar restrictions on contact and movement were also in place. Only one woman had been recruited to phase 2 at this point. Further recruitment was paused and follow-up data (case report forms and questionnaires) for the remaining eight phase 1 participants were collected by phone. Participant recruitment was not restarted in Kenya, as no further funding for researcher time was available. In Uganda, following relaxation of restrictions, sponsor, local and UNCST review study activities were recommenced in late July 2020 and recruitment of a further 29 participants completed by end of September 2020. In phase 2, 51 participants (85% of the planned sample) were recruited, despite COVID 19. The total sample was 107 women (89% of total planned sample).
| Recruitment | Kenya | Uganda | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 2 | Total | 1 | 2 | Total | ||
| Consent to contact | 48 | 60 | 108 | 43 | 57 | 100 | 208 | |
| No contact (%) | 1 (0.5%) | 21 (35%) | 22 (24%) | 7 (16%) | 20 (35%) | 27 (27%) | 49 (24%) | |
| Not eligible (% approached) | 4 (9%) | 3 (7%) | 7 (8%) | 8 (22%) | 6 (16%) | 14 (19%) | 21 (11%) | |
| Reasons not eligible | < 18 years | 0 | 0 | 0 | 1 | 1 (2%) | 2 (2%) | 2 (1%) |
| < 28 weeks’ gestation | 1 (2%) | 0 | 1 (1%) | 0 | 0 | 0 | 1 (0.5%) | |
| Moving out of area | 3 (6%) | 2 (5%) | 5 (5%) | 6 (17%) | 4 (7%) | 10 (10%) | 15 (8%) | |
| Language barrier | 0 | 1 (2%) | 1 (1%) | 0 | 0 | 0 | 1 (0.5%) | |
| Neonatal death (NND) over 7 days | 0 | 0 | 0 | 1 (3%) | 1 (2%) | 2 (2%) | 2 (1%) | |
| Recruited (% eligible) | 30 (70%) | 21 (58%) | 51 (65%) | 26 (93%) | 30 (97%) | 56 (95%) | 107 (77%) | |
| Declined (% eligible) | 13 (30%) | 15 (26%) | 28 (35%) | 2 (7%) | 1 (3%) | 3 (10%) | 31 (22%) | |
| Reasons for declining | No reason given | 10 | 13 | 23 (82%) | 0 | 1 | 1 | 24 (77%) |
| Not interested in research | 1 | 0 | 1 (3.5%) | 2 | 0 | 2 | 3 (10%) | |
| Unwilling for questionnaires/interview | 2 | 1 | 3 (11%) | 0 | 0 | 0 | 3 (10%) | |
| Partner objection | 0 | 1 | 1 (3.5%) | 0 | 0 | 0 | 1 (3%) | |
| Retention | ||||||||
| Completed study (% recruited) | 30 (100%) | 14 (66%) | 44 (86%) | 22 (85%) | 25 (83%) | 47 (83%) | 91 (85%) | |
| Withdrew (% recruited) | 0 | 6 | 6 (12%) | 0 | 4 (13%) | 4 (7%) | 10 (4%) | |
| Reasons for withdrawal | Returned to work | N/A | 0 | 0 | N/A | 2 | 2 | 2 |
| Partner objection | N/A | 2 | 2 | N/A | 0 | 0 | 2 | |
| Moved away | N/A | 2 | 2 | N/A | 1 | 1 | 3 | |
| Other | N/A | 2 | 2 | N/A | 1 | 1 | 3 | |
| Lost to follow-up (no contact) | 0 | 1 (33%) | 1 (2%) | 4 (15%) | 1 (3%) | 5 (9%) | 6 (6%) | |
Recruitment rates
During the recruitment periods, 501 perinatal deaths were reported, 317 women experienced stillbirth and 174 neonatal deaths at the included facilities (see Appendix 1, Table 8). Of those approached, 208 women expressed interest in study participation by completing consent to contact forms. Contact was initiated with 159 women; of these, 21 (11%) were found to be ineligible. The main reasons were planned relocation away from the study area [n = 15 (8%)], age < 18 years [n = 2 (1%)] or late neonatal death [baby over 7 days old: n = 2 (1%)]. Failure to contact interested participants was more frequent in both countries in phase 2, after the onset of COVID-19 (n = 41 vs. n = 8 women in phase 1). Of 138 women identified as eligible, 107 agreed to participate (78%). Of 31 declining, only 7 supplied specific reasons, 3 women were not interested in research, 3 were unwilling to complete questionnaires or interviews and 1 cited partner objection.
Participants
Demographic, pregnancy and birth data for participants are presented in Table 2.
| Baseline demographic characteristics | Phase 1 (pre intervention) | Phase 2 (intervention) | ||||
|---|---|---|---|---|---|---|
| Kenya | Uganda | Total | Kenya | Uganda | Total | |
| Participants (n) | 30 | 26 | 56 | 21 | 30 | 51 |
| Age [years; mean (SD)] | 31.0 (6.0) | 25.8 (5.4) | 28.6 (6.2) | 27.9 (5.8) | 26.7 (5.1) | 27.2 (5.4) |
| Country of birth | ||||||
| Kenya | 29 (97%) | 0 | 29 (52%) | 21 (100%) | 0 | 21 (41%) |
| Uganda | 1 (3%) | 26 (100%) | 27 (48%) | 0 | 30 (100%) | 30 (59%) |
| Location | ||||||
| Urban dwelling | 22 (73%) | 24 (92%) | 46 (82%) | 18 (85.7%) | 30 (100%) | 48 (94%) |
| Religion | ||||||
| Christian | 28 (93%) | 23 (88%) | 51 (91%) | 19 (91%) | 26 (87%) | 45 (88%) |
| Muslim | 2 (7%) | 3 (12%) | 5 (9%) | 1 (5%) | 4 (13%) | 5 (10%) |
| Relationship status | ||||||
| Married/partner | 25 (83%) | 20 (77%) | 45 (80%) | 18 (86%) | 27 (90%) | 45 (88%) |
| Separated/divorced | 2 (7%) | 0 | 2 (4%) | 0 | 0 | 0 |
| Single | 3 (10%) | 5 (19%) | 8 (14%) | 3 (14%) | 3 (10%) | 6 (12%) |
| Widowed | 0 | 1 (4%) | 1 (2%) | 0 | 0 | 0 |
| Education | ||||||
| Received secondary education | 26 (87%) | 20 (77%) | 46 (85%) | 15 (72%) | 18 (60%) | 33 (65%) |
| Employment | ||||||
| Full time | 10 (33%) | 12 (46%) | 22 (39%) | 6 (29%) | 18 (60%) | 24 (47%) |
| Part time | 2 (7%) | 3 (11%) | 5 (9%) | 7 (33%) | 1 (3%) | 8 (16%) |
| Unemployed | 5 (17%) | 4 (15%) | 9 (16%) | 0 | 1 (3%) | 1 (2%) |
| Homemaker | 13 (43%) | 7 (27%) | 20 (35%) | 8 (38%) | 10 (33%) | 18 (35%) |
| Pregnancy history | ||||||
| First pregnancy | 5 (17%) | 7 (27%) | 12 (21%) | 7 (33%) | 8 (26%) | 15 (29%) |
| No living children | 2 (6%) | 6 (23%) | 8 (14%) | 5 (24%) | 2 (7%) | 7 (14%) |
| Previous stillbirth | 2 (6%) | 2 (8%) | 4 (7%) | 2 (10%) | 2 (7%) | 4 (8%) |
| Previous neonatal death | 1 (3%) | 2 (8%) | 3 (5%) | 1 (5%) | 2 (7%) | 3 (6%) |
The mean age of women was 28 years (SD 5.8), similar across study sites and phases, all were born in Kenya or Uganda and most resided in urban locations (88%). A majority were married or living with a partner (83%), 90% described themselves as ‘Christian’, with 9% as ‘Muslim’. Secondary education was received by 75% of the sample. Over half women were employed, either full time or part time (55%), more women in Uganda were employed (61% vs. 47% Kenya). The proportion of women in their first pregnancy was similar across phases (phase 1, 21% vs. phase 2, 29%). Eight women reported having a previous stillbirth (four women in each phase) and six had experienced a previous neonatal death (three in each phase). Index pregnancy, birth and postnatal data are presented in Table 3.
| Pregnancy and birth data | Phase 1 (pre intervention) | Phase 2 (intervention) | ||||
|---|---|---|---|---|---|---|
| Kenya | Uganda | Total | Kenya | Uganda | Total | |
| Births (n) | 30 | 26a | 56 | 21 | 30 | 51 |
| Type of death | ||||||
| Stillbirth (SB) | 20 (67%) | 13 (48%) | 33 (58%) | 14 (67%) | 17 (57%) | 31 (61%) |
| Intrapartum SB (no/% all SB) | 3 (15%) | 7 (53%) | 10 (30%) | 7 (50%) | 8 (47%) | 15 (48%) |
| Neonatal death | 10 (33%) | 14 (52%)a | 24 (42%) | 7 (33%) | 13 (43%) | 20 (39%) |
| Sex of baby | ||||||
| Male | 13 (43%) | 17 (66%) | 30 (54%) | 13 (62%) | 22 (73%) | 35 (69%) |
| Female | 16 (53%) | 10 (38%) | 26 (46%) | 8 (38%) | 8 (27%) | 16 (31%) |
| Unknown | 1 (3%) | 0 | 1 (2%) | 0 | 0 | 0 |
| Gestation at birth [weeks; median (IQR)] | 32.5 (28.0–38.0) | 32.0 (28.0–38.0) | 32.0 (28.0–38.0) | 36 (29.2–39.8) | 37.5 (30.0–40.0) | 36 (29.00–40.00) |
| Neonatal survival [live birth; days median (IQR)] | 1 (1–3) | 1 (1–3) | 1 (1–3) | 1 (1–3) | 1 (1–3) | 1 (1–3) |
| Booked for antenatal care | 28 (93%) | 25 (96%) | 53 (95%) | 18 (86%) | 29 (97%) | 47 (92%) |
| Mode of birth | ||||||
| Spontaneous | 11 (37%) | 18 (67%) | 29 (51%) | 10 (48%) | 18 (60%) | 28 (55%) |
| Vacuum/forceps | 2 (6%) | 0 | 2 (3%) | 1 (5%) | 0 | 1 (2%) |
| Caesarean section | 17 (57%) | 9 (3%) | 26 (46%) | 9 (43%) | 12 (40%) | 21 (41%) |
| Birth weight [g; mean (SD)] | 2048 (1158.0) | 2144 (967.3) | 2086 (1078.6) | 2191 (1080.0) | 2736 (1000.9) | 2494 (1065.2) |
| Main cause of death | ||||||
| Obstructed labour | 0 | 3 (11%) | 3 (5%) | 1 (5%) | 1 (3%) | 2 (4%) |
| Haemorrhageb | 4 (13%) | 1 (4%) | 5 (9%) | 1 (5%) | 4 (13%) | 5 (10%) |
| Preterm birth | 2 (7%) | 6 (22%) | 8 (14%) | 3 (14%) | 5 (17%) | 8 (16%) |
| Cord accident | 1 (3%) | 1 (4%) | 2 (3%) | 2 (10%) | 3 (10%) | 5 (10%) |
| Maternal hypertension | 13 (43%) | 0 | 13 (23%) | 6 (29%) | 4 (13%) | 10 (20%) |
| Fetal compromise | 4 (13%) | 3 (11%) | 7 (12%) | 1 (5%) | 5 (17%) | 6 (12%) |
| Other | 0 | 1 (4%) | 1 (2%) | 3 (14%) | 2 (7%) | 5 (10%) |
| Unknown | 6 (20%) | 11 (41%) | 17 (30%) | 4 (19%) | 6 (20%) | 10 (20%) |
| Post-mortem performed | 1 | 0 | 1 (2%) | 0 | 0 | 0 |
| Postnatal hospital stay [mother; hours median (IQR)] | 96 (60–144) | 48 (19–72) | 72 (26–120) | 84 (48–168) | 48 (18–72) | 72 (24–96) |
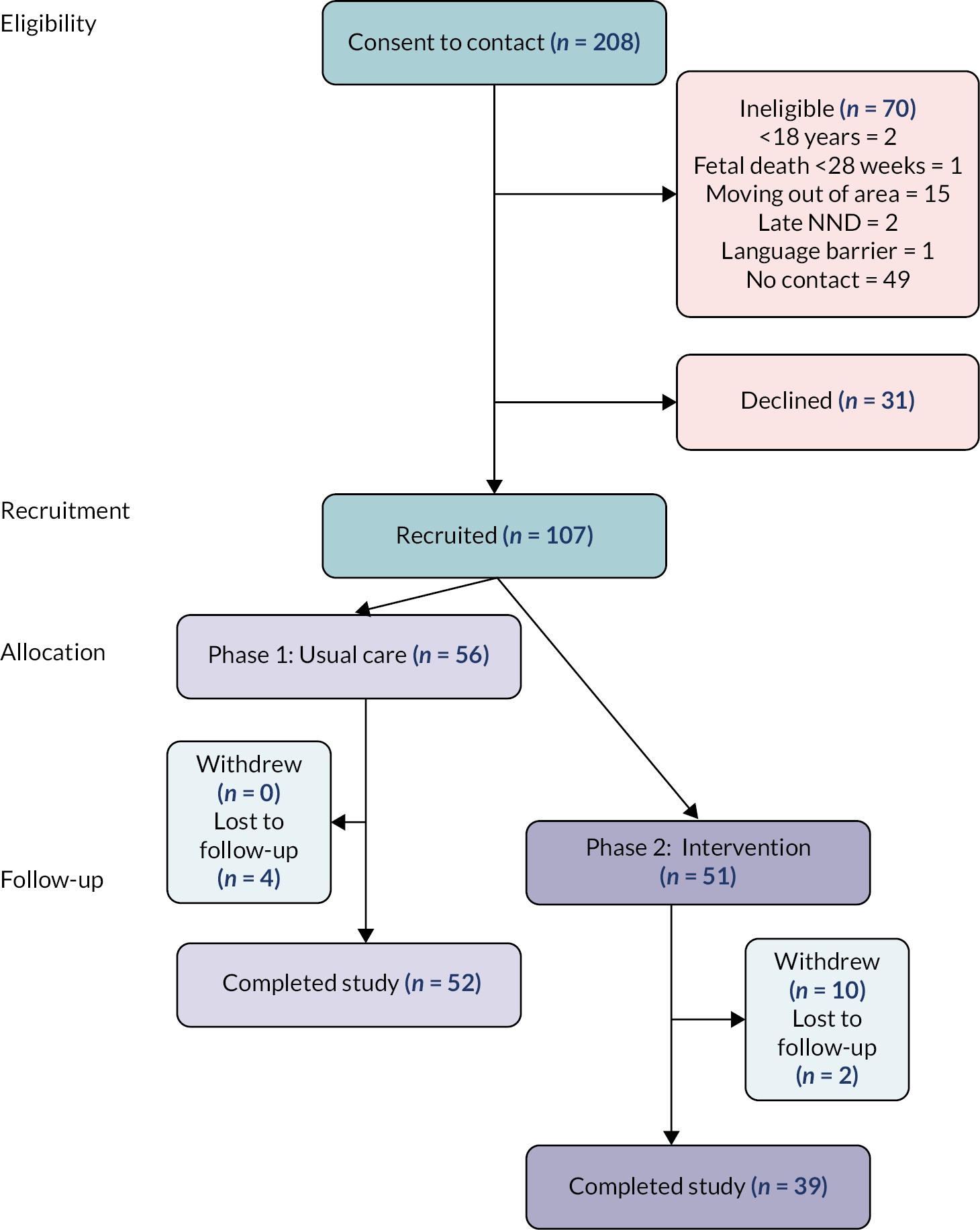
Most women were booked for antenatal care (93%); 64 babies were stillborn, 21 were intrapartum stillbirths, 44 babies died in the early neonatal period (including one set of twins), surviving for median 1 day. Gestation and birthweight were lower in phase 1 (32.0 weeks vs. 36.5 weeks; 2086 g vs. 2494 g, phase 2). Among reasons given by women for the baby’s death, fetal compromise (13%), preterm birth (16%) and maternal hypertension (19%) were the most common; however, 27 (25%) stated they did not know the cause. Only one baby was reported to have had a post-mortem examination. Participant flow is presented in Figure 1; of 107 women recruited, 91 (85%) completed all data collection. In Kenya, 86% of participants completed all data collection, during phase 2 recruitment was 70% of target (vs. 100% phase 1), six women withdrew from the study, and one was lost to follow-up (vs. 0 phase 1). In Uganda, 86% of participants completed all data collection, four women withdrew from the study before the follow-up visit (all during phase 2). Additionally, five women (four during phase 1, and one in phase 2) were not contactable for visit two and lost to follow-up. Among reasons provided for withdrawal, three women moved away from the research site, two returned to full-time work and two cited partner objections. For comparison, data for all stillbirths and neonatal deaths occurring at the included facilities during the recruitment period, was collated from the hospital birth registers and is presented in Appendix 1, Table 8.
FIGURE 1.
Participant flow.
Intervention implementation
The study intervention was implemented, mostly as planned in both countries. To minimise potential contamination between the study phases, bereavement champions and peer supporters were recruited and trained shortly before the start of phase 2. Champions were recruited from clinical staff [n = 19; (n = 7 Kenya, n = 12 Uganda)], following information workshops, and with the agreement of line managers. Midwives and nurses from maternity (antenatal, labour and postnatal wards), neonatal and paediatric departments were included in both countries; in Uganda a hospital social worker was also involved. Peer supporters (n = 12, all bereaved mothers, n = 5 Kenya, n = 7 Uganda) were identified via following an information session in December 2019. Three-day interactive training workshops were held in both sites, facilitated by the UK and country research teams, including a mental health specialist, in January 2020. Both bereavement champions and peer supporters attended an introductory day; this included the experiences and needs of bereaved parents, an introduction to the study intervention and opportunities for both to understand their complementary roles and develop supportive networks. Bereavement champions, alone, attended Day 2, which included strategies for set up and sustaining groups, and workshop on communication skills, including an introduction to behaviour change. Day 3 included peer supporters alone and focused on listening skills, managing relationships and resolving difficult situations, boundaries, and self-care. Copies of all training materials were provided to all participants. Peer supporters also received a study mobile phone, pre-paid airtime and signed a ‘peer supporter’ agreement outlining expectations, limitations of confidentiality and support available during delivery of the intervention. WhatsApp groups were set up in each country for peer supporters and research teams to enable communication of any issues.
Bereavement champions
In-person champion group meetings were initiated in February/March 2020, but paused after the first meeting in both countries due to COVID-19 restrictions. It was not possible to resume group meetings in Kenya due to restrictions on facility gathering, staffing pressures, redeployment and lack of devices to facilitate remote meetings. However, the research assistants met individual champions one-to-one, providing support and feedback in developing planned actions. In Uganda, champion meetings restarted in late July and continued monthly until the end of the intervention period in December 2020 (5 meetings), attended by 8–12 champions and research team facilitators. Potential activities to develop care for bereaved parents were discussed during the initial training workshop and activities were agreed at the first meetings. In Kenya, the priority was to improve the environment for bereaved women through identifying private spaces for postnatal care and facilitating mothers and families contact with the baby after birth if they wished. In Uganda, the group targeted improved communication between staff and bereaved women, including introductions, information giving and providing opportunities to discuss experiences and emotions before discharge home.
Peer support
Peer support was offered to all women accepting participation in phase 2 at recruitment. All participants in Kenya, and 27 of 30 (90%) in Uganda agreed to be linked with a peer supporter. Following this, verbal and written information to explain the service was provided. Some women chose to call the peer at a pre-arranged time, while others preferred to use WhatsApp message. In Uganda, women usually initiated the first contact with call or text, while in Kenya some women preferred that the peer supporter called or messaged them. One peer supporter in Uganda declined to continue involvement after the first woman was matched, but before contact was initiated; therefore, the woman was reallocated to another peer. Peers could not establish contact with one woman in Kenya and six women in Uganda. During the first call, the peer supporter explained the role, her volunteer status and provided availability for next call. According to the preferences of the woman receiving support, further contacts, either by phone or WhatsApp were arranged. All peer supporters returned the logs at the end of the invention period. Table 4 summarises peer support contacts, peers provided support for 1–6 women (median 4) during the intervention period. Women received median of 3 contacts (range 1–12), mostly voice calls. Five women in Kenya and three women in Uganda received only one contact, with no response to subsequent call or messages from the peer. Some women and peers exchanged messages by WhatsApp, mostly once regular contact was established. The length of calls varied considerably; the median call duration was 6 minutes (range 1–80 minutes), call duration was longer in Uganda, at 9 minutes (range 1–80 minutes), than in Kenya 3.5 minutes (range 1–27 minutes). However, a similar pattern of longer initial calls, with shorter follow-up contacts was observed in both. Research assistants, who were experienced midwives, provided individual and group support to peers throughout intervention delivery, including facilitating a peer WhatsApp group. One woman requested a change of peer for personal preference, but no other significant issues were reported.
| Kenya | Uganda | Total | |
|---|---|---|---|
| Peer supporters trained | 5 | 7 | 12 |
| Provided peer support | 5 | 6 | 11 |
| No. of women linked to peer supporters | 21 (100%) | 27 (90%) | 48 (94%) |
| No. of established contact with peer supporter | 20 (95%) | 21 (78%) | 41 (80%) |
| No. linked to each peer supporter: median (range) | 4 (3–5) | 4 (2–6) | 4 (2–6) |
| No. of women supported per peer supporter: median (range) | 3.5 (3–5) | 3 (1–5) | 3.5 (1–5) |
| No. of contacts per participant (phone and text): median (range) | 2 (1–12) | 4 (1–11) | 3 (1–12) |
| Length of contacts (minutes): median (range) | 3.5 (1–27) | 9 (1–80) | 6 (1–80) |
Key resources
Data for attendance at training for the bereavement champions and peer supporter were collected following the events and reported above. In the intervention phase of the study, the restrictions associated with COVID-19 interrupted access to facilities and participants, intermittently. As a consequence, research assistant workload increased and availability was reduced. These constraints prevented other resource data being collected as planned.
Outcomes and acceptability
Postnatal health care and use of support
Table 5 presents the data for routine postnatal follow-up, unplanned healthcare contacts and postnatal support used. In Kenya, all data were collected in person, whereas in Uganda five participants (four, phase 1 and one, phase 2) were followed up by phone. Fewer women received routine postnatal follow-up in Uganda (30% vs. 100% in Kenya). Most women (75%) reported accessing postnatal psychological support, often in multiple forms; family and friends were the most common source (56%) with religious organisations also mentioned. In phase 2, individual peer support was cited by 76% versus 0% in phase 1. Few women reported accessing support groups, professional counselling, or websites.
| Kenya | Uganda | |||
|---|---|---|---|---|
| Phase 1 (n = 30) | Phase 2 (n = 14) | Phase 1 (n = 22) | Phase 1 (n = 25) | |
| Healthcare follow-up | ||||
| Routine postnatal follow-up | 30 (100%) | 14 (100%) | 7 (32%) | 7 (28%) |
| No. of visits: median (range) | 2 (2–3) | 2 (1–3) | 1 (1–4) | 1 (1–4) |
| Hospital follow-up | 71 (100%) 1 with counsellor |
18/33 (55%) 1 with counsellor |
11 (100%) | 7/11 (64%) |
| Postnatal health complications | 6 (20%) | 6 (43%) | 7 (32%) | 9 (36%) |
| Hypertension = 4 Other = 2 |
Anaemia = 1 Hypertension = 3 Other infection = 2 |
Malaria = 1 Other infection = 5 Post partum haemorrhage = 1 |
Malaria = 2 Hypertension = 1 Other infection = 1 Other = 5 |
|
| Unplanned postnatal health contacts | 2 (7%) | 6 (43%) | 6 (27%) | 9 (36%) |
| Postnatal hospital admission | 1 (Hypertension) | 0 | 0 | 1 (Malaria) |
| Postnatal support | ||||
| Used support | 10 (33%) | 14 (100%) | 20 (90%) | 24 (96%) |
| Friends/familya | 3 (30%) | 12 (86%) | 16 (80%) | 20 (83%) |
| Religious groupsa | 8 (80%) | 1 (7%) | 0 | 0 |
| Individual peer supporta | 0 | 14 (100%) | 0 | 15 (63%) |
| Support groupsa | 0 | 0 | 1 (5%) | 1 (4%) |
| Social mediaa | 0 | 0 | 0 | 0 |
| Professionala support | 1 (1%) | 1 (7%) | 1 (5%) | 0 |
| Websitesa | 0 | 2 | 0 | 0 |
| Employment status at follow-up | ||||
| Resumed employment at follow-up visit | 9 (30%) | 7 (50%) | 9 (41%) | 9 (36%) |
Psychological outcome measures
Psychological outcome data for the EPDS and the PGS are presented in Table 6.
| Phase 1 (n = 52) | Phase 2 (n = 39) | Phase 1 – Phase 2 | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Adjusteda difference (95% CI) | |
| EPDS | 14.0 (5.1) | 9.1 (7.0) | 5.0 (2.0 to 7.2) |
| PGS | 106.2 (18.2) | 84.7 (25.4) | 22.6 (13.3 to 21.9) |
Questionnaires were completed by all women remaining in the study at follow-up and no required question on either scale was repeatedly omitted. During interviews with participants, no issues were raised with wording or content of questions, or burden or completion for either instrument. These findings support acceptability for use in a subsequent evaluation. The mean EPDS score in phase 1 was 14 and scores were higher in phase 1 than phase 2 (14 vs. 9.1; adjusted difference 4.6, 95% CI 2.0 to 7.2). The mean PGS score in phase 1 was higher than phase 2 (106.2 vs. 84.7; adjusted difference 22.6, 95% CI 13.3 to 31.9). Where EDPS scores were above 10 or above 1 on item 10 (thoughts of self-harm) at follow-up, further assessment, referral to secondary care or psychological counselling services was offered. In Kenya, 5 women recruited in phase 1 scored > 1 on question 10 indicating potential thoughts of self-harm (all in phase 1), in Uganda 2 women scored > 1 (1 in phase 1, 1 in phase 2), all were offered immediate support and referral to secondary/psychological services. These, along with unexpected adverse events affecting participants, were reviewed by principal investigator and presented to the NIHR Stillbirth Group Advisory Board, and none were considered related to the research or the study intervention.
Experiences of the research and intervention
Interviews were conducted with 35 women and 13 partners or family members, to explore experiences of the research and study intervention. Two main themes were identified, common to both countries; ‘she made me feel like I am not alone’ described a largely positive perception of peer support, women and families also valued taking part in the research, as one father remarked it ‘gave us a voice’. The overwhelming majority of women who received peer support felt that it was helpful. The opportunity to talk to another woman with lived experience of the death of a baby was of key importance:
There are some things you cannot know unless you go through it yourself and it if happens to you then you understand. But, if it hasn’t happened to you, you can hear from hearsay and you can sympathize and empathize but until it really happens to you and you go through the journey, the pain, the sadness the denial, the grief, the loss, the pain, you cannot really understand.
Mary, mother
Bereaved mothers remarked that they gained a sense of hope from these conversations, hearing about the peer supporter’s experiences, what they had found useful, and less useful and how they had adapted to life after the baby’s death. Contact with the peer was especially appreciated by those who struggled to express their feelings to partners and family.
Yes, she did me good because sometimes I tried to talk to my husband, but I realised he wasn’t telling me words that I needed to hear. So, I just said – no, let me talk to the peer supporter, even when I had some challenges with people here at home, I would be like, ‘no, let me talk my peer supporter’.
Florence, mother
For the few who did not establish contact, access to a phone, sometimes shared with a partner or family member, appeared to be the most significant barrier. Other women mentioned lack of airtime, although peer supporters called them back once contact had been made. One woman chose not to make contact, because she did not feel ready to talk about her feelings. Regarding the timing and frequency of contacts, most women appeared satisfied with the service offered and appreciated the peer’s flexibility. Views surrounding the mode of delivery were mixed. Although many women appreciated the convenience and relative anonymity of telephone support, some would have preferred to meet the peer supporter in person, perceiving personal contact as essential to develop a ‘connection’.
I don’t believe in calls. I would want to meet someone, sit down and you see my tears. But on phone, it’s audio, you are not seeing my facial expression. But when am seated down, you see my tears.
Joy, mother
Parents and families were also very positive about taking part in research. Both mothers and fathers related the key importance of respectful care in facilities, sharing both good and challenging experiences. Fathers particularly described limited opportunities to talk about their baby and express their grief. They wanted to be more actively involved and needed better support themselves, research was therefore important to identify issues:
When you release what you have at your heart, those responsible can read it and maybe change. Maybe they might change a little bit.
Godfrey, father
Ten peer supporters and 16 bereavement champions were also interviewed. Peers spoke of their motivation for involvement, often rooted in their own positive or negative experiences. Both champions and peer supporters felt they were ‘well prepared and supported’ and had clear understanding of their roles. The training workshops were evaluated positively, and activities helped develop skills for both groups, such as, empathetic communication with women:
We were trained on how to approach these women and then there are things we didn’t know about how to talk to them because at times we start by saying it was God’s plan or God knows and yet we don’t know if they are Christians. And so, we were given additional skills on how to approach these mothers and counsel them.
Ivy, peer supporter
Some champions and peers would have welcomed extended training, specifically more time for role play and practising skills. Contact between the groups, during training, was also felt to be helpful, creating mutual understanding of complementary roles. To increase reach and sustain change, some champions felt more support from service managers was needed, and they gave examples of encountering resistance to environmental changes. They also felt increasing access to training for all staff members who care for bereaved women should be prioritised:
Yeah, the only thing I would think of was involving more people because now in this kind of this training, it is we really had to involve maybe more health workers if funds allow because at one moment, I may not be there or the people who were trained may not be there. But if everybody was trained, so it would make it, would create more big impact with or without any other person whoever is on at station on duty could handle any case.
Georgina, bereavement champion
Many of the peer supporters described a simultaneously ‘emotional and emotionally rewarding experience’. First contacts were often perceived as ‘difficult’, women were sometimes distressed or uncommunicative and listening to their experiences sometimes triggered painful memories for peers of their own loss. However, witnessing the progress made when relationships were formed, and women gained confidence to ‘open up’ was very satisfying:
You know the first time is not easy, you talk and they [women] are like listening. Yeah, but as you go along, you feel the person is a little bit better. You feel [she] come[s] out of the situation. Sometimes [she] tell[s] you that ‘yeah I am okay, I am doing this and that, and I am working’. Maybe the person thanks you so much for what you have done. So [she] is happy, they are happy actually. Yeah.
Maureen, peer supporter
Several peers described a pattern of reduced contacts over the weeks, with brief calls to check-in or text messages. Peer supporters were generally complementary about the support they received from the local research team, individual and monthly group meetings were reported, mutual support was also received from the study WhatsApp group. Findings of the staff survey, completed by 31 health workers, are presented in Table 7. Most participants were aware of the bereavement champion group, around half could name their ward or area champion. Over three-quarters were able to provide examples of changes made to improve bereavement care for women in their facility during the previous 6 months. Visibility of the bereavement champion group and activities appeared higher in Uganda than Kenya.
| Kenya (n = 15) | Uganda (n = 16) | Total (n = 31) | |
|---|---|---|---|
| Role | |||
| Nurse/midwife | 14 (93%) | 13 (2 managers; 81%) | 27 (87%) |
| Doctor | 0 | 2 (13%) | 2 (6.5%) |
| Other | 1 (7%) | 1 (6%) | 2 (6.5%) |
| Main area of work | |||
| Antenatal (AN)/Postnatal (PN) | 2 (13%) | 7 | 9 |
| Labour ward | 11 (73%) | 3 | 14 |
| Neonatal unit (NNU) | 0 | 3 | 3 |
| Rotational/other | 1 (7%) | 3 | 4 |
| Length of experience (years) | 6 (1–25) | 12.5 (2–30) | 1–30 |
| Aware of champion group | 6 (40%) | 13 (81%) | 19 (61%) |
| Could identify ward/department champion | 4 (27%) | 12 (80%) | 16 (52%) |
| Aware of recent changes in care for bereaved women | 10 (67%) | 14 (87.5%) | 24 (77%) |
Discussion
This study was the first to explore the feasibility of implementation and large-scale evaluation of a new intervention to improve care and support for parents after stillbirth or neonatal death in Kenya and Uganda. Although understanding of parents’ experiences after perinatal death in LMICs is developing,28 no study that we are aware of has evaluated the effectiveness of perinatal bereavement support interventions in sub-Saharan Africa. This reflects a dearth of global evidence to underpin optimal care and support for parents after stillbirth and neonatal death; a recent review identified only six trials, all conducted in HIC, and assessed as having significant methodological or reporting limitations. 11 Despite the COVID-19 pandemic limiting some activities, the study intervention, addressing local community and stakeholder priorities of improving the quality of bereavement care in facilities and community support, was implemented, largely as planned in both Kenya and Uganda. Recruitment was 89% of target and notwithstanding COVID interruption was completed within overall planned timescales, with retention ≥ 75% which demonstrated a willingness among bereaved women in the study settings to participate in research to improve care. Women, families and peers affirmed the potential of peer support to improve postnatal experiences after bereavement. Health workers appreciated new knowledge and skills gained through their role as champions and opportunities to lead improvement in their facilities. Research processes and data collection methods were generally considered acceptable and, confirming previous experiences, participants valued the opportunity to contribute to developing future care and services.
Lessons learned
Alongside prevention of stillbirth and neonatal death, the World Health Organization/UNICEF ‘Renewed Call for Collective Action’ (2020) stressed the urgency of provision of appropriate bereavement support for all women and families. To date, few studies have addressed improving facility care or community support in LMICs including Kenya and Uganda. While this study did not evaluate the impact of the intervention on outcomes, EPDS scores were above suggested cut offs for depressive symptoms in this context29,30 and PGS scores suggested intense grief. 31 This reflects qualitative evidence suggesting commonality of psychological responses across settings and supports assessment of grief and depressive symptoms as outcome measures for studies exploring experiences and improvement of care. The intervention components were designed to address prominent factors identified in negative experiences in Kenya and Uganda including poor communication, lack of supportive care after birth and social support at home.
Introducing trained ‘bereavement champions’ in wards and departments where bereaved women and families were cared for in included facilities was designed to address key ‘opportunity’(physical and social resources) and ‘motivation’ (conscious intention and subconscious experience) barriers, in addition to traditional strategies targeting knowledge and skills. 32 Activities revolved mainly around promoting good practice and role modelling, particularly in communication, although environmental changes including privacy and separation from other mothers and babies for bereaved women were also achieved. Group activities were curtailed in Kenya due to COVID-19 restrictions within facilities, but regular meetings were facilitated in Uganda via video conference. Facility surveys confirmed that champions were visible to other maternity staff, who also recognised increased attention to bereavement care and changes made during the study period. However, this was less evident in Kenya, also likely due to COVID-19 restrictions. The champions appeared to possess many characteristics, identified by Bonawitz et al. (2020), as key in leveraging change including presence, influence, investment in improvement and tenacity. 33 However, while the group included senior ward-based staff (‘in-charges’), service managers were not directly involved. Including managers as bereavement champions could increase success in overcoming organisational barriers encountered, through strategic knowledge and relationships within the institution. Another issue raised by champions was the lack of knowledge of bereavement care among maternity and neonatal staff in facilities. Additional formal training was perceived as important and could also lead to further champions ‘emerging’ through self-selection. This has been identified as a mechanism of sustaining effective champion networks in other health systems with benefits over reliance on nominations alone. 34
Peer support for bereaved parents evolved in HIC, as a response to unmet needs for psychological support and is often provided by third-sector organisations. In this study, telephone peer support was offered. Peer supporters were past or present members of the CEI groups supporting the research programme in Kenya and Uganda, and highly committed to improving care and support for women. Most women offered peer support accepted it, and established contact with the peer. In Uganda, the requirement for the woman to initiate the first call was identified as a potential issue and several peer supporters reflected that giving women a choice to be contacted, as was done in Kenya, might have increased reach. Considerable variation in contacts was observed; it was notable the Uganda participants had greater frequency and length of contacts, which could be related to less routine postnatal follow-up with health workers than observed in Kenya. Overall, women were positive, citing shared experiences, hope and validation of loss, as distinctive benefits of peer support in perinatal bereavement similar to those reported in HIC. 35,36 A review of the peer support used across multiple maternal and newborn health contexts in sub-Saharan Africa also demonstrated a range of positive impacts including empowerment through knowledge and agency and reduced perception of stigma. 37
Peer supporters generally understood their role, felt prepared and supported during the research and gained considerable satisfaction, reflecting experiences reported in HIC. 21 Peer support was a significant commitment of time, in addition to emotional resources. Co-developed guidance shared with participating women, provision of study specific phones, limiting the number of women allocated to each peer and the fixed duration of support offered in the study, helped most peer supporters balance their commitments. Several peers expressed a desire for further or extended training to build confidence, and provision of ongoing development activities have also been highlighted as important for the long-term sustainability of similar services in Australia and Finland. 21,38 Providing refresher training sessions at regular intervals or during meetings with the research team would potentially increase peer supporters’ confidence in the quality of their interactions with women.
Strengths and limitations
This feasibility study was conducted in two urban public maternity facilities in Kenya and Uganda. These are lower middle- and low-income settings with stillbirth and neonatal death rates significantly above international targets. The study was impacted by the COVID-19 pandemic, during phase 2, restrictions on contact and access to health facilities impacted recruitment and some aspects of intervention implementation and data collection, in both sites. In both countries, participant retention was also decreased in phase 2, which might also be related to impacts of COVID-19. The focus on urban sites might also reduce the transferability of findings to rural communities in sub-Saharan Africa, where birth outside facilities, geographical distance and sociocultural differences might impact.
Conclusion
Guided by the NIHR/Medical Research Council framework for developing and evaluating complex interventions in health care,39 this study assessed the feasibility of an effectiveness evaluation of an intervention to improve perinatal bereavement support for women and families after stillbirth or neonatal death in Kenya and Uganda. Successful recruitment and retention of participants demonstrated the feasibility and acceptability of the recruitment processes used in this study and confirm womens’ and families’ willingness to participate in bereavement care research. Data collection tools, including the perinatal grief and depression measures used, were also acceptable, and could be considered to assess the impact of interventions on outcomes for a wider evaluation. The intervention was largely successfully implemented and acceptable to women, health workers and peer supporters. Learning gained from this study, for example inclusion of clinical leaders and incorporation of an element of bereavement care training for all staff caring for bereaved parents, will be used to refine the package, implementation and research processes. A large-scale evaluation, potentially a stepped-wedge randomised controlled trial, is required to determine the effectiveness of the intervention in improving outcomes for women and families and explore future scale-up.
Additional information
CRediT contribution statement
Tracey A Mills (https://orcid.org/0000-0002-2183-7999): Conceptualisation (equal), Data curation (lead), Formal analysis (equal), Funding acquisition (supporting), Methodology (lead), Project administration (lead), Supervision (equal), Visualisation, Writing – original draft (lead), Writing – reviewing and editing (lead).
Valentina Actis Danna (https://orcid.org/0000-0003-2476-1659): Data curation (supporting), Formal analysis (supporting), Writing – reviewing and editing (supporting).
Elizabeth Ayebare (https://orcid.org/0000-0002-5636-5258): Funding acquisition (supporting), Project administration (supporting), Investigation (lead) Writing – reviewing and editing (supporting).
Carol Bedwell (https://orcid.org/0000-0001-8031-7793): Funding acquisition (supporting), Writing – reviewing and editing (supporting).
Lucie Byrne Davis (https://orcid.org/0000-0002-9658-5394): Methodology (supporting), Writing – reviewing and editing (supporting).
Karina Lovell (https://orcid.org/0000-0001-8821-895X): Methodology (supporting), Writing – reviewing and editing (supporting).
Raheli Mukwhana (https://orcid.org/0000-0002-0345-6316): Investigation (supporting), Writing – reviewing and editing (supporting).
Allen Nabisere (https://orcid.org/0009-0003-9397-6351): Formal analysis (supporting), Investigation (supporting), Writing – reviewing and editing (supporting).
Marion Okello (https://orcid.org/0009-0008-8166-6811): Writing – reviewing and editing (supporting).
Grace Omoni (https://orcid.org/0000-0002-0345-6316): Funding acquisition (supporting), Project administration (supporting), Investigation (lead), Writing – reviewing and editing (supporting).
Chris J Sutton (https://orcid.org/0000-0002-6406-1318): Methodology (supporting), Formal analysis (supporting), Writing – reviewing and editing (supporting).
Vicky P Taxiarchi (https://orcid.org/0000-0003-0737-098X): Formal analysis (supporting), Writing – reviewing and editing (supporting).
Sabina Wakasiaka (https://orcid.org/0000-0002-7925-506X): Project administration (supporting), Investigation (lead), Writing – reviewing and editing (supporting).
Tina Lavender (https://orcid.org/0000-0003-1473-4956): Conceptualisation (equal), Funding acquisition (lead), Methodology (supporting), Supervision (equal), Writing – original draft(supporting), Writing – reviewing and editing (supporting).
Other contributions
Dr Carol Bedwell co-ordinated community engagement and involvement input for this study, Ms Marion Okello provided input and critical review for the writing up of the paper on behalf of the CEI groups in Kenya and Uganda. Mr Jonan Mweteise, Ms Nerea Ojanga and the late Ms Anne Nendela also supported data collection. Special thanks to the CEI members in both countries for their input into the research at all stages of the design, conduct and interpretation. We would also like to thank Ms Claire Storey (UK) for her advice for peer support training, and the health workers and peer supporters in Kenya and Uganda involved in delivering the study intervention.
Data-sharing statement
The data generated for this study are not publicly available due to arrangements specified in the ethics approvals, but requests for anonymised data will be considered on application to the corresponding author.
Ethics statement
The study was approved by the Kenyatta National Hospital/University of Nairobi (P828/09/2019) 27 November 2019, Makerere University/Uganda National Council for Science and Technology (UNCST; 2019-059) 29 October 2019 and The University of Manchester (2019-7322-12550) 31 July 2019. Written informed consent was obtained from all participants, including for use of anonymised verbatim quotes. All study processes including recruitment, data collection and data processing were carried out in accordance with relevant guidance and regulations.
Information governance statement
The University of Manchester is committed to handling all personal information in line with the UK Data Protection Act, and the General Data Protection Regulations (EU GDPR) 2016/679. Under the Data Protection legislation The University of Manchester is the Data Controller and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer by contacting: dataprotection@manchester.ac.uk
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/JNWA6983.
Primary conflicts of interest: Dr Tracey A Mills is a member of the NIHR Academy Pre-doctoral Clinical Academic Fellowship (PCAF) Selection Panel and Co-I on NIHR Global Health Group (16/137/53) and Unit (132027). Professor Karina Lovell is a member of the NIHR Advanced Fellowship and Senior Investigator Selection Panels. Dr Chris J Sutton is a member of the NIHR Health Technology Assessment Commissioning Funding Committee and Co-I on a number of NIHR funded grants/projects, including HTA (133518, 133418, 131483) Global Health Groups (16/137/53). Other NIHR funded work EME 132622, RFPB 203507, 201093, 203475, 203468, Programme development grant 202044 HSDR 130581. Professor Dame Tina Lavender was chair of NIHR Global Health Groups Round 4 Selection Panel and is a member of NIHR Global Health Groups Round 5 selection panel, was a member of the HTA Obesity Themed Call Board and Independent Steering Group Chair for NIHR CRIBBS Sierra Leone. She is also principal investigator on NIHR Global Health Group (16/137/53) and Unit (132027). The other authors declare they have no competing interests.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the Global Health Research programme or the Department of Health and Social Care.
This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Study registration
This study is registered as ISRCTN68506895.
Funding
This research was funded by the National Institute for Health and Care Research (NIHR) Global Health Research programme (NIHR award ref: 16/137/53) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK government.
This article reports on one component of the research award NIHR Global Health Research Group on Stillbirth Prevention and Management in Sub-Saharan Africa, Liverpool School of Tropical Medicine. For more information about this research please view the award page (https://www.fundingawards.nihr.ac.uk/award/16/137/53)
About this article
The contractual start date for this research was in August 2017. This article began editorial review in July 2023 and was accepted for publication in June 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Global Health Research editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Copyright
Copyright © 2024 Mills et al. This work was produced by Mills et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
Notes
-
Interview topic guide healthworkers delivering the intervention
-
Interview topic guide peer supporters delivering the intervention
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/JNWA6983).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
List of abbreviations
- CEI
- community engagement and involvement
- Ci
- confidence interval
- EPDS
- Edinburgh Postnatal Depression Scale
- HICs
- high-income countries
- LMICs
- low- and middle-income countries
- PGS
- Perinatal Grief Scale
- UNCST
- Uganda National Council for Science and Technology
References
- UNICEF, WHO, World Bank, UN . Levels and Trends in Child Mortality Report 2018: Estimates Developed by the United Nations Inter-Agency Group for Child Mortality Estimation 2019.
- Cacciatore J. Psychological effects of stillbirth. Semin Fetal Neonatal Med 2013;18:76-82. https://doi.org/10.1016/j.siny.2012.09.001.
- Kiguli J, Munabi IG, Ssegujja E, Nabaliisa J, Kabonesa C, Kiguli S, et al. Stillbirths in sub-Saharan Africa: unspoken grief. Lancet 2016;387:e16-8. https://doi.org/10.1016/S0140-6736(15)01171-X.
- Tebeu PM, de Bernis L, Doh AS, Rochat CH, Delvaux T. Risk factors for obstetric fistula in the Far North Province of Cameroon. Int J Gynaecol Obstet 2009;107:12-5. https://doi.org/10.1016/j.ijgo.2009.05.019.
- Heazell AEP, Siassakos D, Blencowe H, Burden C, Bhutta ZA, Cacciatore J, et al. Stillbirths: economic and psychosocial consequences. Lancet 2016;387:604-16. https://doi.org/10.1016/S0140-6736(15)00836-3.
- Chin K, Wendt A, Bennett IM, Bhat A. Suicide and maternal mortality. Curr Psychiatry Rep 2022;24:239-75. https://doi.org/10.1007/s11920-022-01334-3.
- Flenady V, Boyle F, Koopmans L, Wilson T, Stones W, Cacciatore J. Meeting the needs of parents after a stillbirth or neonatal death. BJOG 2014;121:137-40. https://doi.org/10.1111/1471-0528.13009.
- Flenady V, Wojcieszek AM, Middleton P, Ellwood D, Erwich JJ, Coory M, et al. Lancet Ending Preventable Stillbirths study group . Stillbirths: recall to action in high-income countries. Lancet 2016;387:691-702. https://doi.org/10.1016/S0140-6736(15)01020-X.
- Mills TA, Ayebare E, Mukhwana R, Mweteise J, Nabisere A, Nendela A, et al. Parents’ experiences of care and support after stillbirth in rural and urban maternity facilities: a qualitative study in Kenya and Uganda. BJOG 2020;128:101-9. https://doi.org/10.1111/1471-0528.16413.
- Mills TA, Ayebare E, Mweteise J, Nabisere A, Mukhwana R, Nendela A, et al. ‘There is trauma all round’: a qualitative study of health workers’ experiences of caring for parents after stillbirth in Kenya and Uganda. Women Birth 2022;36:56-62. https://doi.org/10.1016/j.wombi.2022.02.012.
- Ainscough T, Fraser L, Taylor J, Beresford B, Booth A. Bereavement support effectiveness for parents of infants and children: a systematic review. BMJ Support Palliat Care 2022;12:e623-31. https://doi.org/10.1136/bmjspcare-2019-001823.
- Koopmans L, Wilson T, Cacciatore J, Flenady V. Support for mothers, fathers and families after perinatal death. Cochrane Database Syst Rev 2013;2013. https://doi.org/10.1002/14651858.CD000452.pub3.
- Rando T. The increasing prevalence of complicated mourning: the onslaught is just beginning. OMEGA J Death Dying 1993;26:420-36. https://doi.org/10.2190/7MDL-RJTF-NA2D-NPQF.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Miech EJ, Rattray NA, Flanagan ME, Damschroder L, Schmid AA, Damush TM. Inside help: an integrative review of champions in healthcare-related implementation. SAGE Open Med 2018;6. https://doi.org/10.1177/2050312118773261.
- Shaw EK, Howard J, West DR, Crabtree BF, Nease DE, Tutt B, et al. The role of the champion in primary care change efforts: from the State Networks of Colorado Ambulatory Practices and Partners (SNOCAP). J Am Board Fam Med 2012;25:676-85. https://doi.org/10.3122/jabfm.2012.05.110281.
- Ayebare E, Lavender T, Mweteise J, Nabisere A, Nendela A, Mukhwana R, et al. The impact of cultural beliefs and practices on parents’ experiences of bereavement following stillbirth: a qualitative study in Uganda and Kenya. BMC Pregnancy Childbirth 2021;21. https://doi.org/10.1186/s12884-021-03912-4.
- Kiguli J, Namusoko S, Kerber K, Peterson S, Waiswa P. Weeping in silence: community experiences of stillbirths in rural eastern Uganda. Glob Health Action 2015;8. https://doi.org/10.3402/gha.v8.24011.
- Dennis CL, Hodnett E, Kenton L, Weston J, Zupancic J, Stewart DE, et al. Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. BMJ 2009;338. https://doi.org/10.1136/bmj.a3064.
- Jones CC, Jomeen J, Hayter M. The impact of peer support in the context of perinatal mental illness: a meta-ethnography. Midwifery 2014;30:491-8. https://doi.org/10.1016/j.midw.2013.08.003.
- Boyle FM, Mutch AJ, Barber EA, Carroll C, Dean JH. Supporting parents following pregnancy loss: a cross-sectional study of telephone peer supporters. BMC Pregnancy Childbirth 2015;15. https://doi.org/10.1186/s12884-015-0713-y.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Developing Interventions. London: Silverback Publishing; 2014.
- Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016;25:1057-73. https://doi.org/10.1177/0962280215588241.
- Toedter LJ, Lasker JN, Alhadeff JM. The Perinatal Grief Scale: development and initial validation. Am J Orthopsychiatry 1988;58:435-49. https://doi.org/10.1111/j.1939-0025.1988.tb01604.x.
- Cox JL, Chapman G, Murray D, Jones P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J Affect Disord 1996;39:185-9. https://doi.org/10.1016/0165-0327(96)00008-0.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6. https://doi.org/10.1192/bjp.150.6.782.
- Richie J, Spencer L. Analyzing Qualitative Data. New York: Routledge; 1994.
- Kuforiji O, Mills TA, Lovell K. Women’s experiences of care and support following perinatal death in high burden countries: a metasynthesis. Women Birth 2023;36:e195-202. https://doi.org/10.1016/j.wombi.2022.07.170.
- Green EP, Tuli H, Kwobah E, Menya D, Chesire I, Schmidt C. Developing and validating a perinatal depression screening tool in Kenya blending Western criteria with local idioms: a mixed methods study. J Affect Disord 2018;228:49-5. https://doi.org/10.1016/j.jad.2017.11.027.
- Lawrie TA, Hofmeyr GJ, de Jager M, Berk M. Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. S Afr Med J 1998;88:1340-4.
- Toedter LJ, Lasker JN, Janssen HJ. International comparison of studies using the perinatal grief scale: a decade of research on pregnancy loss. Death Stud 2001;25:205-28. https://doi.org/10.1080/07481180125971.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Bonawitz K, Wetmore M, Heisler M, Dalton VK, Damschroder LJ, Forman J, et al. Champions in context: which attributes matter for change efforts in healthcare?. Implement Sci 2020;15. https://doi.org/10.1186/s13012-020-01024-9.
- George ER, Sabin LL, Elliott PA, Wolff JA, Osani MC, McSwiggan Hong J, et al. Examining health care champions: a mixed-methods study exploring self and peer perspectives of champions. Implement Res Pract 2022;3. https://doi.org/10.1177/26334895221077880.
- McCreight BS. Narratives of pregnancy loss: the role of self-help groups in supporting parents. Med Sociol Online 2007;2:3-16.
- Umphrey LR, Cacciatore J. Coping with the ultimate deprivation: narrative themes in a parental bereavement support group. OMEGA J Death Dying 2011;63:141-60.
- Wishlade T, Lavender T. Can peer support interventions assist women following fistula surgery?. Afr J Midwifery Womens Health 2016;10:181-7. https://doi.org/10.12968/ajmw.2016.10.4.181.
- Aho AL, Åstedt-Kurki P, Kaunonen M. Peer supporters’ experiences of a bereavement follow-up intervention for grieving parents. Omega (Westport) 2014;68:347-66.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374. https://doi.org/10.1136/bmj.n2061.
Appendix 1 Supplementary data
| Phase 1 | Phase 2 | Total | |
|---|---|---|---|
| (n births) | 172 | 329 | 501 |
| Age [years; mean (SD)] | 27.7 (5.9) | 28 (6.3) | 27.9 (6.2) |
| Country of birth/location (n) | n = 172 | n = 316 | n = 488 |
| Kenya | 86 (50%) | 147 (44.7%) | 233 (46.5%) |
| Uganda | 83 (48%) | 169 (51.4%) | 252 (94.7%) |
| Congo | 2 (1%) | 0 | 2 (0.4%) |
| Somalia | 1 | 0 | 1 (0.2%) |
| Sudan | 0 | 1 | 1 (0.2%) |
| Dwelling (n) | n = 154 | n = 267 | n = 421 |
| Urban | 131 (85.1) | 175 (65.5) | 306 (72.7%) |
| Religion (n) | n = 100 | n = 166 | n = 266 |
| Christian | 94 (94%) | 158 (95%) | 252 (94.7%) |
| Muslim | 5 (5%) | 8 (5%) | 13 (4.9%) |
| Other | 1 (1%) | 0 | 1 (0.4%) |
| Marital status (n) | n = 109 | n = 192 | n = 301 |
| Married/partner | 102 (94%) | 169 (88%) | 271 (90%) |
| Separated/divorced | 0 | 0 | 0 |
| Single | 7 (6%) | 21 (11%) | 28 (9.3%) |
| Widowed | 0 | 2 (1%) | 2 (0.7%) |
| Education (n) | n = 63 | n = 145 | n = 178 |
| Received secondary education | 42 (67%) | 97 (67%) | 139 (67%) |
| Employment (n) | n = 66 | n = 163 | n = 229 |
| Full time | 12 (18.2%) | 45 (27.6%) | 57 (24.9%) |
| Part time | 5 (7.6%) | 1 (1%) | 6 (2.6%) |
| Unemployed | 38 (57.6%) | 76 (46.6%) | 114 (49.8%) |
| Homemaker | 11 (16.7%) | 41 (25.2%) | 52 (22.7%) |
| Previous pregnancies (n) | n = 206 | n = 138 | n = 344 |
| First pregnancy | 35 (%) | 77 (37.4%) | 112 (32.6%) |
| Living children (n) | n = 91 | n = 124 | 215 |
| None | 6 (6.6%) | 12 (9.7%) | 18 (8.4%) |
| Previous perinatal death (n) | n = 80 | n = 117 | n = 197 |
| Previous stillbirth | 5 (6.3%) | 13 (11.1%) | 18 (9.1%) |
| Previous neonatal death | 3 (3.8%) | 5 (4.3%) | 8 (4.1%) |
| Type of death (n)a | n = 170 | n = 321 | n = 491 |
| Stillbirth (SB) | 111 (64.5%) | 206 (62.6%) | 317 (63.3%) |
| Intrapartum SB (no/% all SB) | 26 (23.4%) | 61 (28.8%) | 87 (26.9%) |
| Neonatal death | 59 (34.3%) | 115 (35%) | 174 (34.7%) |
| Sex of baby (n)a | n = 171 | n = 309 | n = 480 |
| Male | 96 (56.1%) | 156 (50.5%) | 252 (52.5%) |
| Female | 66 (38.6%) | 148 (47.9%) | 214 (44.6%) |
| Unknown | 9 (5.3%) | 5 (1.6%) | 14 (2.9%) |
| Gestation at birth [weeks; median (IQR)] | 34 (30–38) | 36 (30–38) | 35 (30–38) |
| Neonatal survival [days; median (IQR)] | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| AN booking (n) | n = 101 | n = 192 | n = 293 |
| Yes | 99 (98%) | 185 (96.4%) | 284 (96.9%) |
| Mode of birth (n) | n = 150 | n = 221 | n = 371 |
| Spontaneous | 84 (56.0%) | 140 (63.3%) | 224 (60.3%) |
| Vacuum/forceps | 3 (1.9%) | 0 | 3 (0.8%) |
| Caesarean section | 63 (40.4%) | 81 (35.7%) | 144 (38.8%) |
| Birthweight | 2170 (1010.2) | 2271 (1040.1) | 2235 (1029.8) |
| Cause of death (n)a | n = 174 | n = 337 | n = 511 |
| Obstructed labour | 15 (8.6%) | 28 (8.3%) | 43 (8.4%) |
| Haemorrhage | 14 (8%) | 35 (10.4%) | 49 (9.6%) |
| Preterm birth | 25 (14.3%) | 30 (8.9%) | 55 (10.7%) |
| Cord accident | 2 (1.1%) | 7 (2.1%) | 9 (2.1%) |
| Maternal hypertension | 23 (13.2) | 43 (12.8) | 66 (12.9) |
| Fetal compromise | 20 (11.5%) | 22 (6.5%) | 42 (8.2%) |
| Other | 23 (13.4%) | 59 (17.9%) | 82 (16.4%) |
| Unknown | 52 (29.9%) | 113 (33.5%) | 165 (32.3%) |
| Post-mortem performed | 2 (1.1%) | 5 (1.5%) | 7 (1.4%) |
| Length of postnatal stay (hours) | 72 (25–120) | 48 (24–72) | 48 (24–96) |
