Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 15/136/45. The contractual start date was in June 2017. The final report began editorial review in June 2021 and was accepted for publication in March 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Ramnarayan et al. This work was produced by Ramnarayan et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Ramnarayan et al.
Chapter 1 Background
Over recent decades, evidence linking higher volume to better outcomes has led to the centralisation of specialist services, such as cancer surgery, perinatal care and trauma. 1–3 Prior to 1997, the care of critically ill or injured children (aged < 16 years) in the UK was quite fragmented. 4 In 1997, based on expert opinion and scientific evidence, the UK Department of Health and Social Care recommended that paediatric intensive care (PIC) services be centralised. 4 Dedicated regional paediatric intensive care units (PICUs) were established, and specialist paediatric critical care transport teams (PCCTs) were set up to transport critically ill children from general hospitals to PICUs. PCCTs act as ‘mobile intensive care’ teams. PCCTs travel to general hospitals and start PIC, ensuring that specialist expertise is not delayed until the patient reaches the PICU. 5
Currently, only 25 (12%) of the 215 acute hospitals in the UK where children may first present when they are critically ill have a PICU. Consequently, children presenting to hospitals without a PICU require transfer to a hospital with a PICU, which can be located at a median distance of 32 km away [interquartile range (IQR) 14–57 km]. 6 The use of PCCTs (rather than non-specialist teams) for the interhospital transport of critically ill children improves the odds of their survival by 42%. The majority (≈ 85%) of interhospital transports of critically ill children in the UK are currently performed by PCCTs. 7
Variations in current service provision
Each year in England and Wales, nearly 5000 critically ill children are transported by PCCTs to receive care in an appropriate location, such as a PICU. National audit data from the Paediatric Intensive Care Audit Network (PICANet) reveal wide variations in the timeliness of access to PIC in these patients. 7 First, the median time taken for PCCTs to reach critically ill children at general hospitals ranges from 1 hour to 4 hours, reflecting considerable differences in how soon a critically ill child can expect to start receiving PIC. During the winter surge period, some children may wait up to 12–24 hours for the PCCT to arrive. Second, there is variation in the time taken by PCCTs to transport children into the admitting PICU, reflecting differences in how soon a critically ill child can start receiving definitive care (e.g. surgery) available in a specialist centre only. Similarly, PICANet data indicate that there is considerable variation in the care provided to critically ill children by PCCTs prior to PICU admission, in terms of the seniority of the transport team leader [e.g. consultant, junior doctor or advanced nurse practitioner (ANP)], critical care interventions performed by the transport team (e.g. intubation or central venous catheterisation and delivery of vasoactive drug infusions) and the frequency of critical incidents (e.g. accidental extubation) occurring during transport.
Common reasons for delays in timeliness of access to emergency PIC and variations in care provided by PCCTs are graphically shown in Figure 1.
FIGURE 1.
Reasons for variation in timeliness of access to emergency PIC and care provided by PCCTs. CYP, children and young people; PICRT, paediatric intensive care retrieval team.
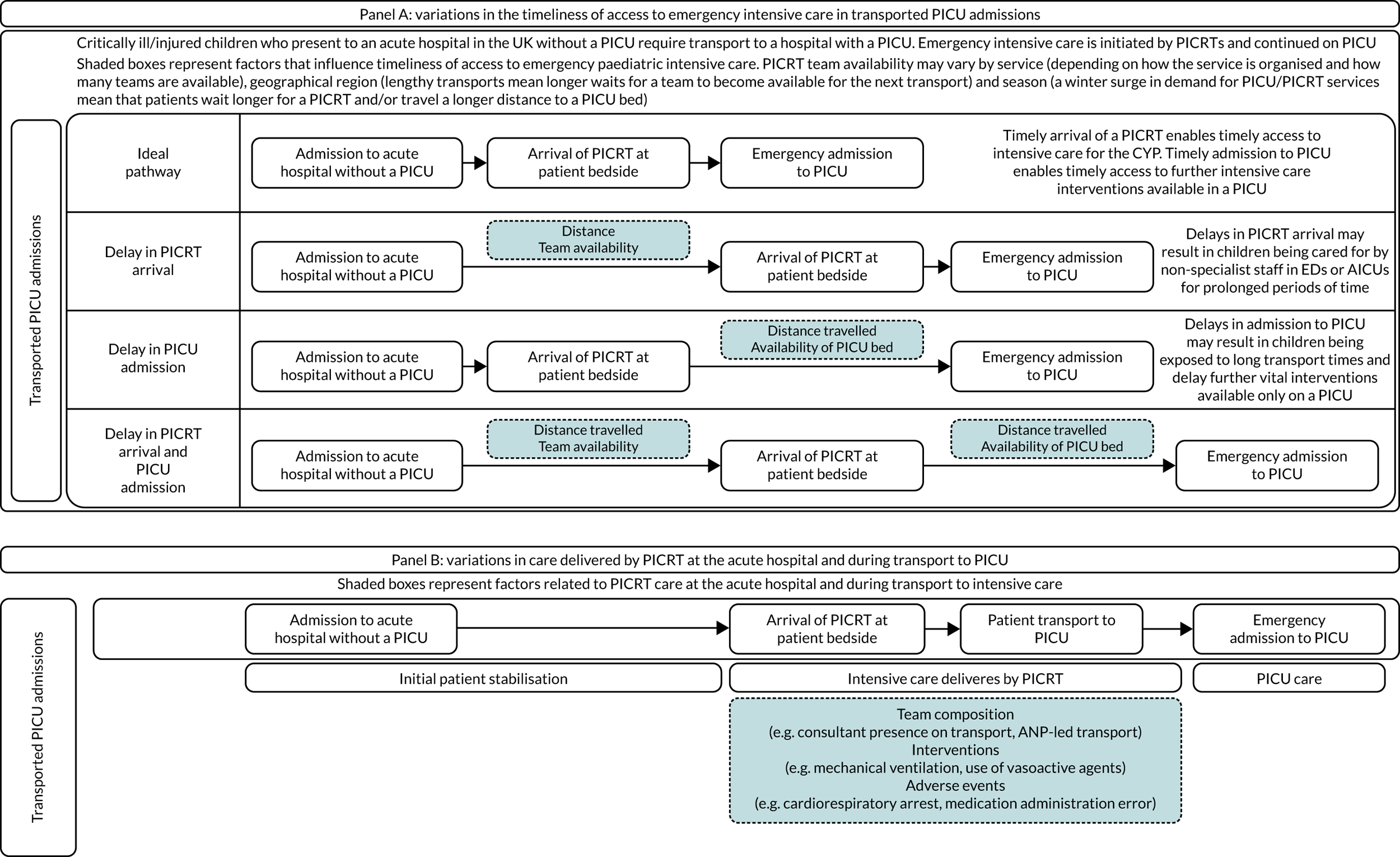
We do not know if these differences in timeliness of access to PIC and care delivered during stabilisation and transport by PCCTs matter in terms of clinical outcomes and patient experience, and it is also unclear what their impact on health-care costs is. This lack of scientific evidence has led over the years to the evolution of different models of PCCT provision, the development of national standards based on expert opinion rather than scientific evidence and has contributed to the lack of progress in improving care at the crucial interface between secondary and tertiary paediatric care.
Impact on patient outcomes and experience
Transported children represent one-third of all PICU admissions (and half of all emergency admissions). Yet, compared with the two other main patient groups (i.e. planned admissions and emergency admissions from within the same hospital where the PICU is located), transported children have the poorest clinical outcomes. Transported children’s PICU mortality is nearly double that of planned PICU admissions (8% vs. 4%) and higher than for internal emergency admissions (6%), and they have a significant risk of long-term complications and considerable associated health and social care costs. 6 It remains unclear whether or not this is solely because transported children are much sicker than other groups of critically ill children, or whether or not the timeliness of access to PICU expertise and the quality of care delivered by PCCTs prior to PICU admission may have an additional influence on clinical outcomes. From a family perspective, parents of sick children have described the process of PICU retrieval as the ‘the worst journey of their lives’ and demonstrate evidence of psychological trauma long afterwards. 8–10
Rationale for the study
Evidence is urgently required to enable understanding of if and how delays in access to PIC and variations in the quality of care provided during acute stabilisation and transport affect clinical outcomes and patient experience. National audit data relating to the referral and transport of critically ill children have been collected by PICANet since 2012. These data clearly show national differences in the timeliness of access to PIC (i.e. time taken by PCCT to reach the patient bedside) and in the care delivered by PCCT during transport (i.e. team composition, interventions performed and frequency of critical incidents). As the primary means through which critically ill children at general hospitals access PIC in an emergency, it is plausible that variations in PCCT provision compromise equity of access and may adversely affect their clinical outcomes and patient experience. Over the past decade, in the absence of scientific evidence, the development of PCCT services and national quality standards have been guided by expert opinion.
Provision of early high-quality acute care has been shown to improve clinical outcomes in specific diseases, such as paediatric sepsis and head trauma. 11,12 It is unclear how these findings apply to the vast majority of critically ill children who require stabilisation and transport to a PICU by PCCTs. From a wider NHS perspective, centralisation of specialist acute care has occurred in several NHS services, such as stroke, trauma and specialist paediatrics. 13,14 The findings from our research can provide evidence that can be generalised to evaluate other such centralisations, and this is especially relevant to questions relating to the trade-off between timeliness of access to acute care and provision of high-quality cost-effective specialist care.
Study aims
-
To understand if and how clinical outcomes and experiences of critically ill children transported to PICU are affected by national variations in timeliness of access to PIC and care provided by PCCTs prior to PICU admission.
-
To study the relative cost-effectiveness of current PCCT services and to use mathematical modelling to evaluate whether or not alternative models of PICU/PCCT service delivery can improve clinical and cost-effectiveness.
-
To generate evidence for the development of future clinical standards.
Study objectives
-
To perform a quantitative analysis using linked routinely collected data to study the association between timeliness of access to PIC and clinical outcomes in a national cohort of critically ill children transported to PICU.
-
To perform a quantitative analysis using linked routinely collected data to study the association between care delivered by PCCTs and clinical outcomes in a national cohort of critically ill children transported to PICU.
-
To explore, using qualitative methods (i.e. individual interviews and workshops) and questionnaires, the experiences and perspectives of a purposively sampled national cohort of parents of transported critically ill children and young people (CYP), and, if and where feasible, to use innovative methods to explore the experiences of transported critically ill CYP.
-
To explore, using qualitative methods (i.e. individual interviews and workshops), the experiences and perspectives of a purposively sampled national cohort of clinicians from a range of settings (e.g. acute general hospitals, PCCTs and PICUs) and service managers/NHS commissioners.
-
To perform cost-effectiveness analyses of PCCT provision for critically ill children, comparing different service models currently in use.
-
To use mathematical modelling and location–allocation optimisation methods to explore whether or not alternative models of service delivery for PICU/PCCT services can improve clinical outcomes while remaining cost-effective.
-
To synthesise study findings to inform the development of evidence-based national standards of care and information resources for families and clinicians.
Chapter 2 Overview of methods
Study design
The study design was a multiworkstream mixed-methods design. There were four linked workstreams and a final workstream that involved stakeholder workshops to draw together findings from the other workstreams.
Workstream A
-
A quantitative analysis of national PICANet data linked to routine administrative data [Hospital Episode Statistics (HES)], death registrations [Office for National Statistics (ONS)] and adult critical care data [case mix programme (CMP)].
Workstream B
-
A qualitative and questionnaire study involving interviews of parents of critically ill children transported to PICU (and children themselves, where feasible), interviews with clinicians working in PICUs, PCCTs and acute general hospitals, as well as service managers/commissioners, and questionnaires to collect feedback from parents of transported children.
Workstream C
-
A health economic evaluation of the costs and value for money of different models of PCCTs to identify cost-effective models of service delivery.
Workstream D
-
Mathematical modelling, including the use of location–allocation optimisation, to explore the potential clinical and cost impact of alternative models of service and geographical locations where PCCTs could be based.
Workstream E
-
Workshops involving key stakeholders (i.e. CYP, parents, clinicians and service managers/commissioners).
Integration of workstreams
We adopted a mixed-methods approach with a convergent triangulation study design whereby quantitative and qualitative data were collected concurrently, with equal weight being given to both workstreams. At the interpretation stage, we integrated findings from the qualitative and questionnaire study (of how national variations in the timeliness of access to emergency intensive care and care delivered by PCCTs affect patient/family experience) with findings from the quantitative study (of how clinical outcomes are affected by national variations) to generate complementary views of paediatric retrieval. Uniquely, the qualitative study gathered rich narrative detail from patient experiences and clinician perspectives that cannot be obtained from quantitative analysis of routine data alone. Integration was facilitated by regular discussions between the study team members at monthly study meetings. Further integration of study findings from the health economic evaluation and mathematical modelling were attempted at the workshop stage, although this was not completed as proposed because of changes in study conduct during the COVID-19 pandemic (see Salient issues faced). The dependencies between the workstreams and what was achieved during the study are shown in Appendix 1.
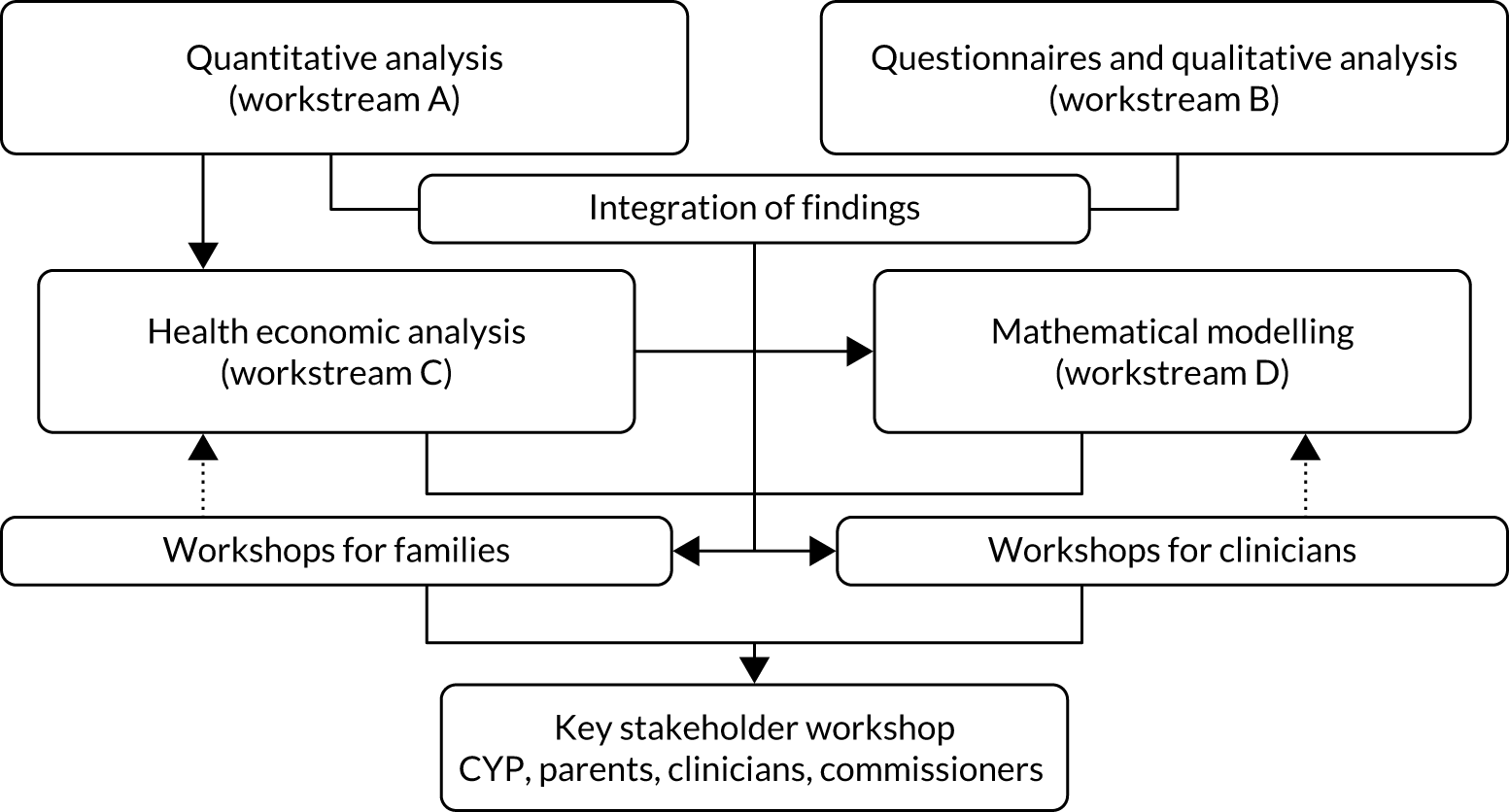
The overall study design is displayed in Figure 2, including points at which where findings are integrated.
FIGURE 2.
Study design and relationship between workstreams.
Ethics approval
Ethics approval for the DEPICT (Differences in access to Emergency Paediatric Intensive Care and care during Transport) study was provided by the Health Research Authority and the National Research Ethics Service – London Riverside Committee (reference 17/LO/1267). Approval for the use of patient-identifiable information without patient consent was provided by the Health Research Authority Confidentiality Advisory Group (reference CAG 0129). Relevant approvals were also provided by NHS Digital for data linkage and access to HES and ONS data.
Patient and public involvement
Two members of the public (ME and AP) were co-applicants on the proposal for the DEPICT study and were active members of the Study Management Group, contributing to the study design, operationalisation of the protocol and interpreting emerging findings. Matthew Entwistle and Anna Pearce critically reviewed patient information sheets (PISs) and consent forms and helped design the study questionnaires and interview topic guides. Anna Pearce was also part of the interview panel to recruit the qualitative researchers for the study. In addition, an independent lay member (Dermot Shortt) was part of the Study Steering Committee and provided input into the study conduct as it progressed and helped with the interpretation of emerging findings.
Salient issues faced
The COVID-19 pandemic had an adverse impact on the DEPICT study and necessitated the following changes to study procedures:
-
The DEPICT study data set, including the HES data, was held on secure servers at the University of Leicester (Leicester, UK). With the advent of the pandemic, all workstream A analyses had to be performed remotely using virtual private network (VPN) access. However, the HES data set was too large to manipulate effectively over VPN and, therefore, two specific secondary outcome measures [i.e. total length of stay (LOS) in hospital and hospital re-admission after PICU discharge] were unable to be studied. However, we feel that the absence of these outcomes does not detract from the importance of our findings.
-
The health economic analysis relied on the Cambridge team accessing workstream A data at Leicester. However, restrictions on physical access to the university since lockdown in March 2020, and inability to get remote access for honorary researchers, resulted in significant challenges. The full impact of COVID-19 on workstream C is detailed in Chapter 5.
-
The workshops for parents and clinicians were unable to be held as planned. One workshop for clinicians and parents was held in March 2020 just prior to lockdown, but was poorly attended. The methods for this workshop were planned beforehand; however, owing to the poor attendance, we were unable to draw meaningful conclusions. The final stakeholder workshop was changed to a virtual dissemination event in November 2020, making it difficult to collect systematic feedback to refine study conclusions.
Chapter 3 Workstream A: quantitative analysis
Parts of this chapter have been reproduced from Seaton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
The centralisation of PIC in the 1990s led to a reduced number of PICUs across the UK. In turn, this meant that critically ill children often presented at hospitals without the facilities to care for them, meaning that they needed to be transported to a hospital with a PICU. In more recent years, there has been a centralisation of the PCCTs rather than transports undertaken by ad hoc created teams from locally sourced staff. Therefore, children may now have to wait longer to be transported to PICU to receive care, but the trade-off is that this care will be provided by specialist teams.
Targets have been set surrounding access to care, for example the Paediatric Critical Care Society (London, UK) recommends that PCCTs should aim to arrive at the bedside of the child within 3 hours of the request to transport them to a PICU. However, these targets have been selected using expert consensus, rather than evidence-based research. To the best of our knowledge, no one has assessed whether or not these targets impact on outcome for children or whether or not they should be changed. Similarly, there is no guidance surrounding the way in which care is provided by the PCCT, for example what the level of the seniority of the staff leading the transport should be.
In this chapter, we will investigate the impact of the time taken to reach a critically ill child and whether or not the care and the PCCT impact on the outcome.
Aims and objectives
Workstream A has two broad questions to investigate:
-
Does timeliness of access to PIC impact on the outcomes of critically ill children? (For brevity, we refer to this as the ‘time-to-bedside’ work.)
-
Does care delivered by the PCCT, including the team composition, the stabilisation approaches and the occurrence of critical incidents, impact on the outcomes of critically ill children? (For brevity, we refer to this as the ‘models of care’ work.)
Methods
Children aged < 16 years who were admitted for PIC in England and Wales were included in this workstream. More detail about the cohort will be provided in Chapter 3, DEPICT study cohort. No data collection or study recruitment was undertaken as part of workstream A, as we made use of routinely available data that are collected by organisations that have permission to collect information about care received in health-care settings without explicit patient consent. We applied for Research Ethics Committee (reference 17/LO/1267) and Confidentiality Advisory Group (reference CAG0129) approvals to allow us to link together these data sources. In addition, we obtained permission from the appropriate organisations for use of their data [e.g. Healthcare Quality Improvement Partnership (HQIP) for PICANet and NHS Digital for HES/ONS].
The data set for workstream A is formed from linking the following data sets.
PICANet collects information about the referral, transport and admission to PICU of all critically ill children in the UK and Ireland (note that we only used data from England and Wales). Data relating to the child’s demographics (e.g. age, sex), their clinical condition [e.g. Paediatric Index Mortality 2 (PIM2) score, diagnoses], events that occur during their transport to PICU (e.g. vehicle accidents) and the clinical interventions and support they receive (e.g. ventilator support) are captured. Data were required to be entered into the PICANet data system within 3 months of the event occurring. Information about the data extracted from PICANet can be found in Appendix 3.
The Intensive Care National Audit & Research Centre (ICNARC) collects information about all admissions to general adult intensive care units. Similar to PICANet, information is collected about patients’ demographics and the care they receive. We extracted information about children (aged < 16 years) receiving care in general adult intensive care units who were subsequently transported to a PICU.
The ONS collects information from death certificates, including date and cause of death.
The Patient Episode Database for Wales (PEDW) and HES collects information about care, treatment and interventions received in hospital settings [i.e. wards and emergency departments (EDs)].
Data linkage
There were two separate data linkages in workstream A. First, linking of the PICANet referral, transport and admissions data sets. This linkage was undertaken by Sarah Seaton, with support from the PICANet team at the University of Leeds (Leeds, UK). Second, linking of the PICANet, ICNARC, HES and ONS mortality data. The linkage of all data sets was undertaken by NHS Digital under direction from Sarah Seaton and the DEPICT team.
PICANet linkage
The data related to a child’s referral, transport and subsequent admission to PIC are stored in three separate databases:
-
Referral data contains limited information relating to the hospital and clinical team requesting the transport. Referral data include information about whether or not the child was being ventilated at the time of referral.
-
Transport data contains information on the child’s transport, including demographic information about the child, all times associated with the transport, medical interventions received by the child and critical incidents that occurred during the transport.
-
Admission data contains information on the child’s admission to PICU, including demographic information, diagnoses and care they received during their time in PICU.
Copies of data collection forms used by PICANet can be found at www.picanet.org.uk/data-collection/ (accessed 21 June 2022).
All data sets contain personally identifiable data (e.g. name, date of birth, NHS number), but there is no in-built linkage between the data sets. Personally identifiable data can vary between data sources even when related to the same child. This variance can be accidental (e.g. typographical errors, such as spelling a name multiple ways) or can be due to updating of data (e.g. a transport of a child named ‘baby girl’ who on admission to PICU had been given a name by the family).
The PICANet system uses an algorithm to allocate individual children a unique pseudo-anonymised patient identifier (ID). The system identifies children who have been admitted previously (e.g. by looking for the same NHS number and other identifiable characteristics) and then allocates the same patient ID. Some children have multiple transport and admission events over a period of time.
The patient ID algorithm uses a probabilistic matching approach to consider several unique identifiers (e.g. NHS number, date of birth) for each child, before assigning the patient ID. Therefore, theoretically, the same child should be allocated the same patient ID for all their transport and admission events, although it is possible for one child to be allocated two different patient IDs if differing identifiable data are available for each record. In our linkage we, therefore, used a combination of both the patient ID and the original personally identifiable data.
Linkage was undertaken by the PICANet team at the University of Leeds, which has permission to hold the personally identifiable data. To facilitate the linkage, Sarah Seaton was provided with an honorary research fellow position at the University of Leeds.
Attempts to link between the transport and admissions data sets followed a hierarchy (if matched via any approach, then no further match attempts were made). There are four initial ‘rounds’ or ‘steps’:
-
The same patient ID in both data sets (i.e. transport and admissions data sets). The admissions data set to indicate that the child was a retrieval/transfer. The destination/admission PICU to match (i.e. transport and admissions data sets). The date and time of arrival at unit (i.e. transport data set) and admission (i.e. admissions data set) to match within ± 1 hour.
-
The same patient ID in both data sets (i.e. transport and admissions data sets). No indication from the admissions data set that the child was a retrieval/transfer. The destination/admission PICU to match (i.e. transport and admissions data sets). The date and time of arrival at unit (i.e. transport data set) and admission (i.e. admissions data set) to match within ± 1 hour.
-
The same NHS number, family name (and alternative family name) and date of birth in both data sets (.e. transport and admissions data sets). The admissions data set to indicate that the child was a retrieval/transfer. The destination/admission PICU to match (i.e. transport and admissions data sets). The date and time of arrival at unit (i.e. transport data set) and admission (i.e. admissions data set) to match within ± 1 hour.
-
The same NHS number, family name and date of birth in both data sets (i.e. transport and admissions data sets). No indication from the admissions data set that the child was a retrieval/transfer. The destination/admission PICU to match (i.e. transport and admissions data sets). The date and time of arrival at unit (i.e. transport data set) and admission (i.e. admissions data set) to match within ± 1 hour.
Following these initial four rounds, further rounds were repeated with increasing levels of flexibility in the matching of the difference in time between the date and time of arrival (i.e. transport data set) and admission (i.e. admissions data set):
-
In rounds 5–8 repeat steps 1–4, but relax the time difference to ± 2 hours.
-
In rounds 9–12 repeat steps 1–4, but relax the time difference to ± 6 hours.
-
In rounds 13–16 repeat steps 1–4, but relax the time difference to ± 12 hours (this is to account for errors in use of the 24-hour clock).
-
In rounds 17–20 repeat steps 1–4, but relax the time difference to ± 24 hours (this is to account for errors in the recording of the date).
We also undertook a manual review of the remaining unlinked data to ‘force’ a match if it was apparent that there was another reason that the records had not linked. In reality, ≈ 92% of all successful matches were obtained in step 1. Subsequent steps (i.e. steps 2–4) added minimal records, but provided assurance we had been as thorough as possible.
We tried and abandoned two additional approaches, which we document here for future researchers:
-
We attempted a match with no time restriction placed on it (i.e. any admission event at any date or time could match with a transport event of the same child from any date or time). Theoretically, we could link events that were several years apart in time. However, this provided only a small number of additional matches and hugely compromised data quality.
-
To ensure that we captured instances of the same child where the patient ID may have failed to allocate the same patient ID, we also investigated a match with same family name, postcode and date of birth. Although this identified a small number of additional matches (which we reviewed manually), this more often falsely identified sets of twins as if they were the same child. Care should be taken when using this approach.
Beginning with the transport data (as all children needed to have a transport record to be in our study), our match rate success between transport and admission data sets was ≈ 97%. We followed a similar process for matching the transport data with the referrals data set and achieved a match rate success of ≈ 96%.
The entire PICANet linkage took approximately 3 months of work to develop, check and implement. We have provided the detail here in the hope that this helps future researchers working to link this and other similar data sets.
NHS Digital linkage
NHS Digital was sent personally identifiable information about the unplanned (emergency) admissions of children aged < 16 years from PICANet and ICNARC. NHS Digital compared the data sets and informed us of any matches (using NHS number and other personally identifiable data) between the data sources and added in information related to hospital care (HES), ED attendances (HES) and mortality (ONS). The data flow for NHS Digital can be found in Appendix 2. We followed a similar approach with the data from NHS Wales (PEDW).
We faced significant delays with the NHS Digital data, beginning the process in spring 2018, submitting for review with NHS Digital in summer 2018, receiving approval in October 2018 and receiving the first download of data in late 2018. These data were re-supplied in February 2019 because of issues during the ICNARC data upload process. We were required to design and undertake a data destruction process approved by both NHS Digital and the University of Leicester.
However, on receipt of the revised data, it was clear that there were issues with data completeness and after detailed investigation during summer 2019 it became clear that we had not received the hospital data of children aged < 1 year (a large portion of our cohort) due to an oversight by NHS Digital. We contacted NHS Digital and the issue was confirmed. We were in the process of amending and updating our data-sharing agreement and so the corrected data were supplied in November 2019.
Despite these delays, we continued the aspects of workstream A that relied on the PICANet data and linked mortality data (unaffected by the outlined issues), and we have been able to produce two papers with only one outcome omitted,15,16 which we would have ideally included (i.e. re-admission to hospital within 1 year of discharge from PICU). In mid-2019, we had begun analysis of the HES data in collaboration with our colleagues from workstream C (health economics), which led to the identification of the issue with the unsupplied data. However, in November 2019, on receipt of the revised data, we had only limited time to begin analysis before the nationwide lockdown in early 2020 prevented continued work. A VPN at the University of Leicester allows substantively employed staff with the correct equipment to continue work on research projects while maintaining required data security, and we were able to continue the main analysis via this route. However, the HES data set was too large to access in this way because of the speed of personal home WiFi and so work related to this will be completed when we can physically access our offices again (after September 2021). Workstream C colleagues were unable to access the University of Leicester VPN, but support was provided from their colleagues working on workstream A.
DEPICT study cohort
Children were eligible for inclusion in the DEPICT study if they were aged < 16 years and transported as an emergency (non-elective) to an NHS PICU between 1 January 2014 and 31 December 2016. Children were included if their PICANet transport record linked to a corresponding PICU admission. Children with missing referral data were excluded. If a child was transported multiple times during the DEPICT study, then we included their final transport only. Specific exclusions were applied for missing data for each specific aspect of the research.
Time-to-bedside methods
Inclusion and exclusion
Children were included as outlined in DEPICT study cohort, that is, they were transported to an NHS PICU in England and Wales in 2014–16 as a result of an emergency (non-elective) situation. Children were excluded if there was missing information about ventilation status at referral or if it was not possible to calculate time to bedside. Time to bedside is the difference between the time it was agreed that the child required transport to PICU and the time when the team arrived at the child’s bedside. For the secondary outcomes of LOS and length of invasive ventilation (LOV), additional exclusions were made if there were missing data.
Time to bedside statistical methods
Summary statistics were reported as counts/percentages for categorical variables or median/range or mean/standard deviation (SD) for continuous variables. To investigate the impact of time to bedside on 30-day mortality, logistic regression models with clustered standard errors for the PCCT were fitted. Key confounders were selected a priori via discussion with the clinical members of the Study Management Group and were age of the child, PIM2 score,17 clinical diagnosis (based on PICANet diagnostic groups), ventilation status at referral (yes/no not indicated/no advised to intubate), number of transport requests from the collection hospital during the study (categorised as < 50 requests, 50–99 requests or ≥ 100 requests) and whether or not the child was receiving critical care around the time of the transport request (collected from intensive care or receiving care in a general intensive care unit in the 2 days preceding transport, yes/no).
Variables were included regardless of any statistically significant association with mortality (as the DEPICT team had made the decision not to focus on statistical significance in workstream A). Time to bedside was categorised as ≤ 60 minutes, 61–90 minutes, 91–120 minutes, 121–180 minutes and ≥ 181 minutes. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated alongside the (adjusted) probability of mortality by time to bedside. Logistic models were also fitted with secondary mortality end points of death on PICU, death within 2 days, death within 90 days and death within 1 year following PICU admission.
Clinical subgroups of children were selected a priori for investigation with regard to mortality, including children admitted with cardiac/neurological conditions, children with a low/high PIM2 score (low: PIM2 ≤ 0.10; high: PIM2 > 0.10) and children transported to PICU in summer/winter (summer: June/July/August; winter: December/January/February).
The outcomes of LOS and LOV were highly skewed (most children had a short LOS/LOV) and, therefore, negative binomial models were used with the same adjustments as the primary analysis. The expected (adjusted) LOS and LOV were estimated and presented graphically by time to bedside.
Sensitivity analyses
Sensitivity analyses were performed on our primary analysis investigating the outcome of mortality analyses. First, we assessed the impact of using categorical variables to model age and the PIM2 score by re-fitting our analyses using fractional polynomials. Fractional polynomials allow more flexible parameterisations of continuous variables, compared with including them as linear terms,21 and are sometimes favoured over categorisation like that used here, which, although simpler to interpret, can lead to a loss of data/power. 22
Second, we investigated the impact of using the final transport for children transported multiple times by repeating the analysis using their first transport in the DEPICT study time window. Finally, the impact of missing data was investigated by re-fitting the model with different scenarios for these.
Models of care methods
Inclusion and exclusion
Children were excluded if there was missing information about ventilation status at referral or if they had missing or implausible time data (defined as > 24 hours) for the time to bedside, time spent at the bedside or the total time taken to reach the PICU. In the analysis of team composition, children were excluded if they had missing data about the team leader of the transport. In the analysis of secondary health-care outcomes, children were excluded if they had missing data for LOS or LOV.
Models of care statistical methods
Summary statistics were reported as counts/percentage for categorical variables and median/range for continuous variables. Adjustments were selected a priori and included time to reach the bedside, age of the child, PIM2 score, clinical diagnosis, ventilation status at the time of referral and whether or not the child was receiving critical care around the time of the transport request.
Team leader
Transports are led by the most senior member of staff, who can be:
-
a junior doctor
-
an ANP
-
a consultant.
We undertook the following three comparisons regarding the team leader using logistic regression models with mortality as the outcome: (1) consultant compared with not a consultant, (2) junior doctor compared with ANP and (3) comparison of all three options. We also considered LOS and LOV using negative binomial models.
Prolonged stabilisation by the paediatric critical care transport team compared with short stabilisation
We investigated key clinical interventions provided to the child, including the provision of intubation and re-intubation (i.e. airway procedures); central venous access, arterial access and intraosseous access (i.e. vascular access procedures); and initiation of vasoactive infusions. Clinical interventions were provided either by the referring hospital prior to the arrival of the PCCT or when the PCCT was in attendance.
We compared the scenario where the PCCT spent substantial time preparing the child for transport (i.e. prolonged stabilisation, defined as two or more interventions provided while the PCCT were in attendance) compared with short stabilisation (i.e. two or fewer interventions provided by the PCCT). For outcomes relating to mortality, we used logistic regression models. For LOS and LOV, we used negative binomial models.
Number and types of interventions
We investigated the impact of the total number of interventions received by the child (from the referring hospital and while the PCCT were in attendance) and the percentage of interventions that were provided while the PCCT was present. We investigated mortality using logistic regression and considered the impact of interventions on our secondary outcomes of LOS and LOV via use of negative binomial models.
Critical incidents
We investigated instances of critical incidents involving the child, vehicle or an equipment failure that occurred during the transport and affected the child’s care, including whether or not these incidents affected the adjusted odds of mortality. Critical incidents involving the child were accidental extubation, required intubation in transit, complete ventilator failure, loss of medical gas supply, loss of all intravenous access, cardiac arrest and medication administration error. Vehicle incidents included accidents and breakdown. We fitted logistic models to adjust for characteristics of the child for each type of incident and investigated the odds of mortality within 30 days of admission to PICU.
Model fit
Model performance for our mortality analyses was assessed using the AUC, Hosmer–Lemeshow test and Brier score.
Reporting conventions
Emphasis is on the clinical importance of the observed trends, associations or differences. p-values and statistical significance are not reported in line with the DEPICT study protocol. 23 CIs are reported throughout.
Findings
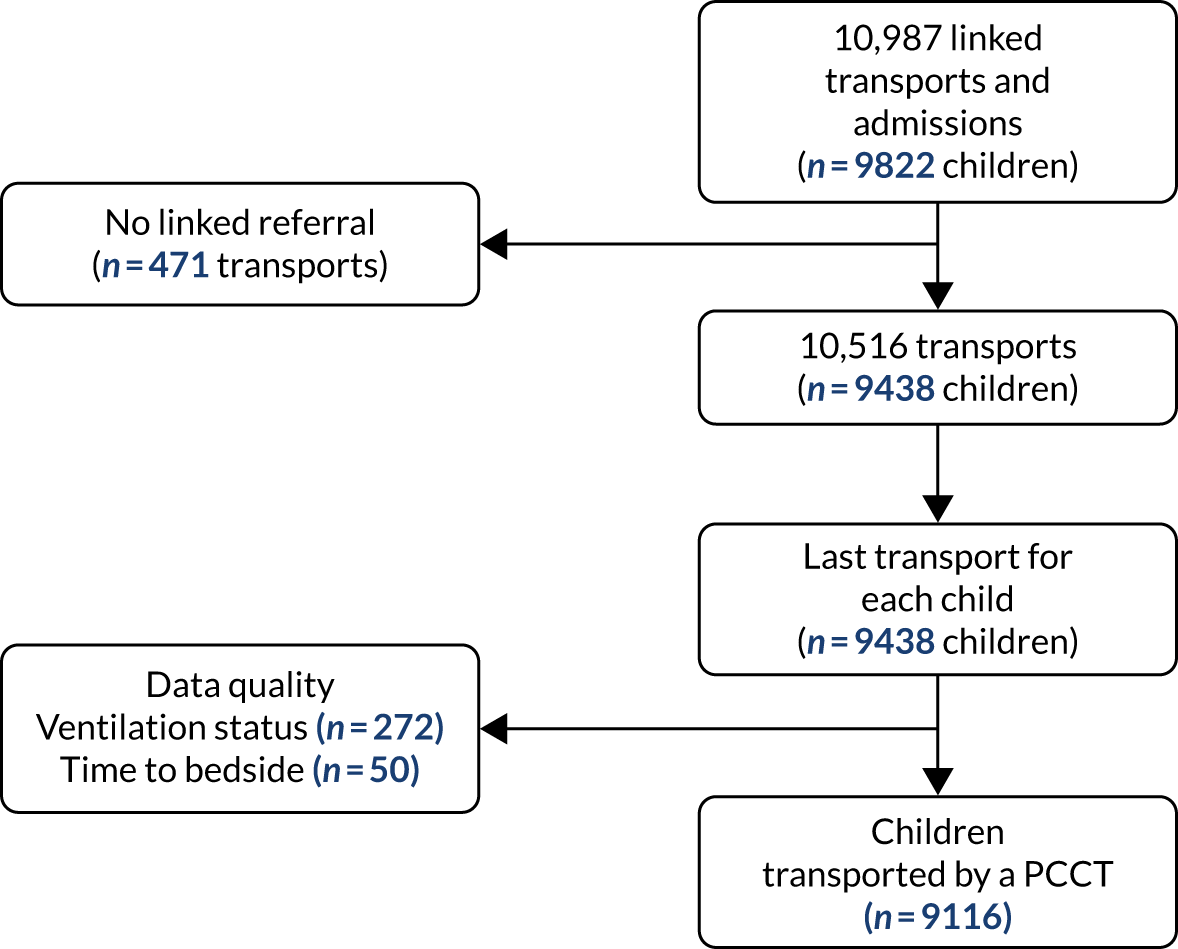
DEPICT study cohort
There were 10,987 emergency transports by a PCCT of children aged < 16 years with a linked admission record to a PICU during the study. Transports not linked with a corresponding referral event were excluded (n = 471, 4.3%), leaving 10,516 transports (Figure 3). For children with multiple transports, we used the latest transport, providing 9438 transported children. Additional exclusions were made for missing data in each section of our work, as outlined in the flow chart (see Figure 3).
FIGURE 3.
Time to bedside flow chart. Reproduced from Seaton et al. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Time-to-bedside results
Inclusion and exclusion
Additional exclusions were made for children whose ventilation status at the time of referral was missing (n = 272) and children with missing or implausible data (defined as > 24 hours) for the time to bedside (n = 50), leaving 9116 children in the primary analysis (see Figure 3). Summary statistics for the included children can be found in Table 1. Approximately half of the children were aged < 1 year at the time of transport, and the most common reason for admission was respiratory problems. The median LOS in the PICU was 5 (mean 7.5) days.
| Characteristic | Total (N = 9116) | Arrived at the bedside in ≤ 60 minutes (N = 2654) | Arrived at the bedside in 61–180 minutes (N = 5271) | Arrived at the bedside in ≥ 181 minutes (N = 1191) |
|---|---|---|---|---|
| Age (years), n (%) | ||||
| < 1 | 4669 (51.2) | 1371 (51.7) | 2685 (50.9) | 613 (51.5) |
| 1 to < 5 | 2438 (26.7) | 682 (25.7) | 1437 (27.3) | 319 (26.8) |
| 5 to < 11 | 1174 (12.9) | 344 (13.0) | 679 (12.9) | 151 (12.7) |
| 11 to < 16 | 835 (9.2) | 257 (9.7) | 470 (8.9) | 108 (9.1) |
| Sex of child, n (%) | ||||
| Male | 5183 (56.9) | 1552 (58.5) | 2962 (56.2) | 669 (56.2) |
| Female | 3932 (43.1) | 1102 (41.5) | 2308 (43.8) | 522 (43.8) |
| Unknown | 1 (< 0.5) | 0 | 1 (< 0.1) | 0 |
| PIM2 score (%), n (%) | ||||
| < 1 | 1039 (11.4) | 314 (11.8) | 625 (11.9) | 100 (8.4) |
| 1 to < 5 | 4089 (44.9) | 1104 (41.6) | 2409 (45.7) | 576 (48.4) |
| 5 to < 15 | 2985 (32.7) | 845 (31.8) | 1710 (32.4) | 430 (36.1) |
| 15 to < 30 | 579 (6.4) | 220 (8.3) | 305 (5.8) | 54 (4.5) |
| ≥ 30 | 424 (4.7) | 171 (6.4) | 222 (4.2) | 31 (2.6) |
| LOS in PICU (days), median (10th, 90th) | 5 (2, 14) | 5 (2, 15) | 5 (2, 14) | 5 (2, 15) |
| LOS in PICU (days), mean (SD) | 7.5 (13.2) | 7.5 (15.1) | 7.4 (11.6) | 8.2 (15.2) |
| Child received multiple transports during the time window of DEPICT, n (%) | 775 (8.5) | 206 (7.8) | 459 (8.7) | 110 (9.2) |
| Parent accompanied the child in the ambulance, n (%) | ||||
| Yes | 6974 (76.5) | 2188 (82.4) | 3966 (75.2) | 820 (68.9) |
| No, parent not present | 432 (4.7) | 135 (5.1) | 237 (4.5) | 60 (5.0) |
| No, parent declined to accompany | 1150 (12.6) | 233 (8.8) | 713 (13.5) | 204 (17.1) |
| No, parent not permitted to accompany | 385 (4.2) | 41 (1.5) | 259 (4.9) | 85 (7.1) |
| Unknown | 175 (1.9) | 57 (2.2) | 96 (1.8) | 22 (1.9) |
| Collection area, n (%) | ||||
| PICU | 259 (2.8) | 88 (3.3) | 127 (2.4) | 44 (3.7) |
| GICU | 731 (8.0) | 45 (1.7) | 430 (8.2) | 256 (21.5) |
| NICU | 822 (9.0) | 332 (12.5) | 374 (7.1) | 116 (9.7) |
| Theatre/recovery and theatre | 1978 (21.7) | 392 (14.8) | 1328 (25.2) | 258 (21.7) |
| X-ray/CT/endoscopy/A&E | 2773 (30.4) | 1084 (40.8) | 1464 (27.8) | 225 (18.9) |
| Ward/HDU/other intermediate area | 2536 (27.8) | 710 (26.8) | 1539 (29.2) | 287 (24.1) |
| Other/unknown | 17 (0.2) | 3 (0.1) | 9 (0.2) | 5 (0.4) |
| Diagnostic group, n (%) | ||||
| Respiratory | 4355 (47.8) | 1102 (41.5) | 2591 (49.2) | 662 (55.6) |
| Cardiovascular | 1310 (14.4) | 477 (18.0) | 681 (12.9) | 152 (12.8) |
| Endocrine | 219 (2.4) | 65 (2.5) | 133 (2.5) | 21 (1.8) |
| Haematology/oncology | 153 (1.7) | 56 (2.1) | 78 (1.5) | 19 (1.6) |
| Infection | 820 (9.0) | 261 (9.8) | 484 (9.2) | 75 (6.3) |
| Neurological | 1505 (16.5) | 403 (15.2) | 907 (17.2) | 195 (16.4) |
| Trauma and accidents | 338 (3.7) | 121 (4.6) | 184 (3.5) | 33 (2.8) |
| Other | 416 (4.6) | 169 (6.4) | 213 (4.0) | 34 (2.9) |
| Ventilated at time of referral call, n (%) | ||||
| Yes | 3814 (41.8) | 1129 (42.5) | 2109 (40.0) | 576 (48.6) |
| No (not indicated) | 2886 (31.7) | 911 (34.3) | 1652 (31.1) | 323 (27.1) |
| No (advised to intubate) | 2416 (26.5) | 614 (23.1) | 1510 (28.7) | 292 (24.5) |
| Size of acute hospital (based on transport requests in the DEPICT study time window), n (%) | ||||
| Small (< 50 requests) | 2274 (25.0) | 635 (23.9) | 1308 (24.8) | 331 (27.8) |
| Medium (50 to < 100 requests) | 3802 (41.7) | 1118 (42.1) | 2129 (40.1) | 555 (46.6) |
| Large (≥ 100 requests) | 3040 (33.4) | 901 (34.0) | 1834 (34.8) | 305 (25.6) |
| Receiving care in a critical care setting at collection organisation, n (%) | ||||
| Yes | 1951 (21.4) | 479 (18.1) | 1022 (19.4) | 450 (37.8) |
| No | 7165 (78.6) | 2175 (82.0) | 4249 (80.6) | 741 (62.2) |
| Mortality, n (%) | ||||
| Died within 2 days of admission | 278 (3.1) | 105 (4.0) | 153 (2.9) | 20 (1.7) |
| Died in PICU | 571 (6.3) | 200 (7.5) | 316 (6.0) | 55 (4.6) |
| Died within 30 days of admission | 645 (7.1) | 226 (8.5) | 357 (6.8) | 62 (5.2) |
| Died within 1 year of admission | 949 (10.4) | 331 (12.5) | 520 (9.9) | 98 (8.2) |
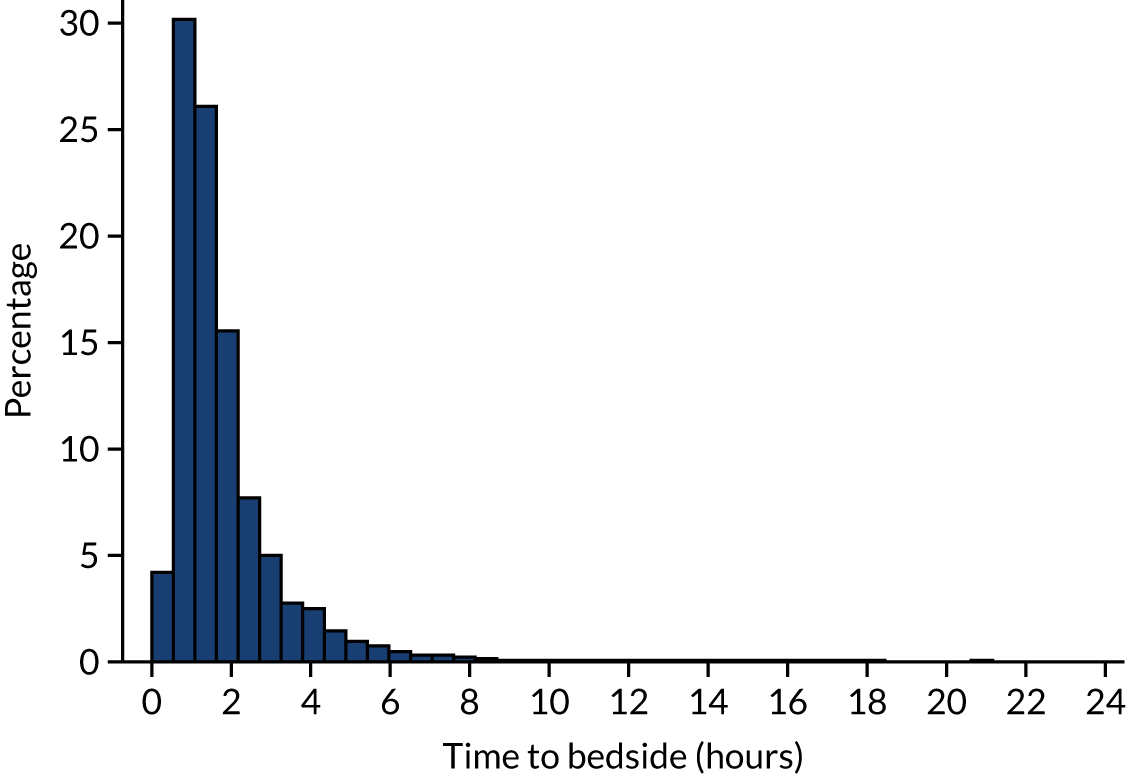
The target set by the Paediatric Intensive Care Society is to reach the bedside of the child within 3 hours of it being agreed that the child requires emergency transport to a PICU. This target can be relaxed to 4 hours when the child is being referred from a more remote location. However, further issues may prevent teams from meeting this target, for example the team being busy on other transports, poor weather and a long distance to travel to the referring hospital. Despite this, most (> 85%) of the children were met by the PCCT in < 3 hours (Figure 4) and very few children were left waiting for > 4 hours. We restricted our analysis to children whose time to bedside was < 24 hours; however, in reality, no child waited longer than ≈ 21 hours for a PCCT to arrive at their bedside.
FIGURE 4.
Histogram of time taken to reach the bedside of the child by the PCCT. Reproduced from Seaton et al. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Time to bedside: mortality
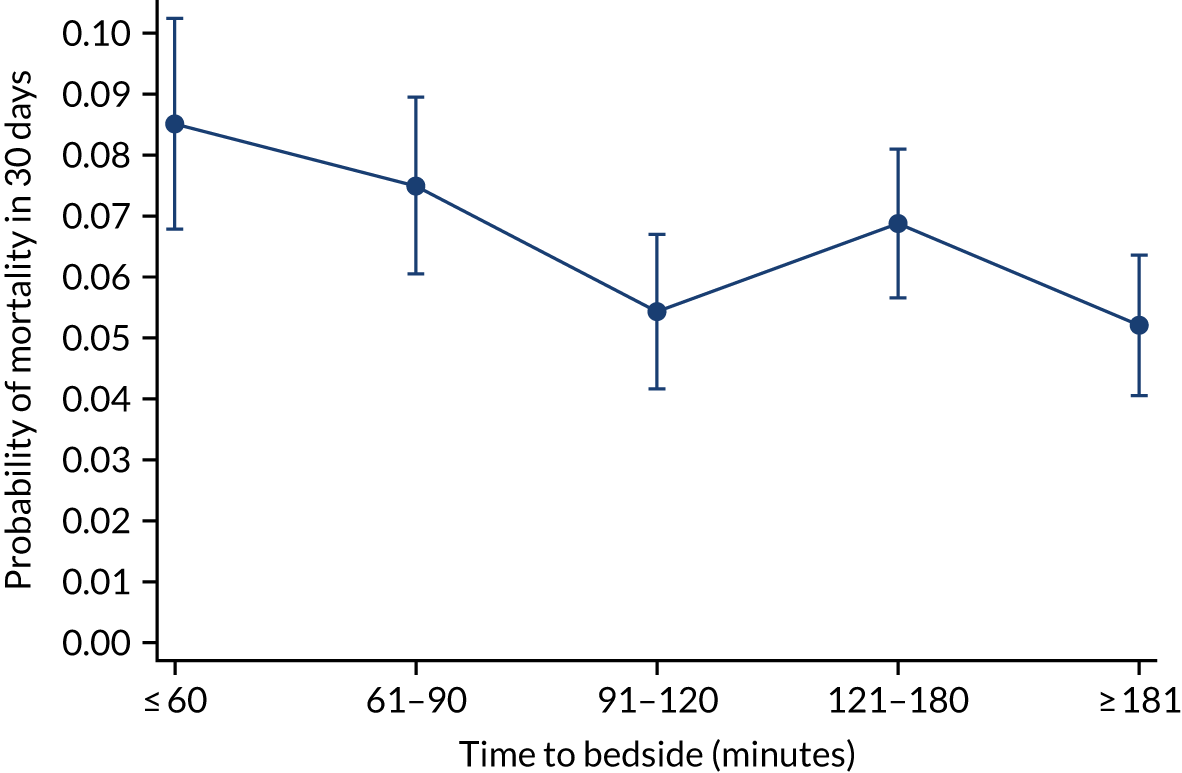
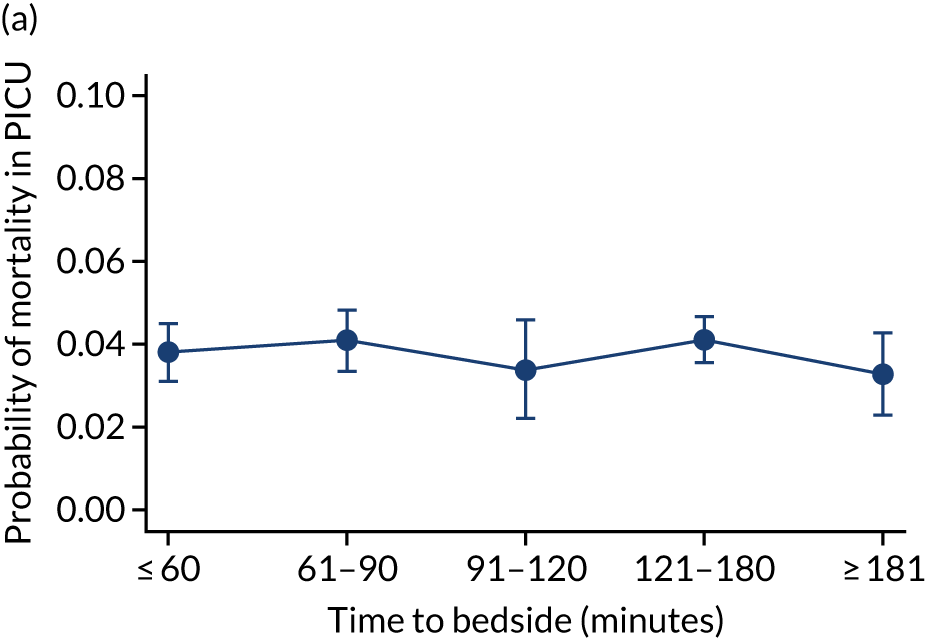
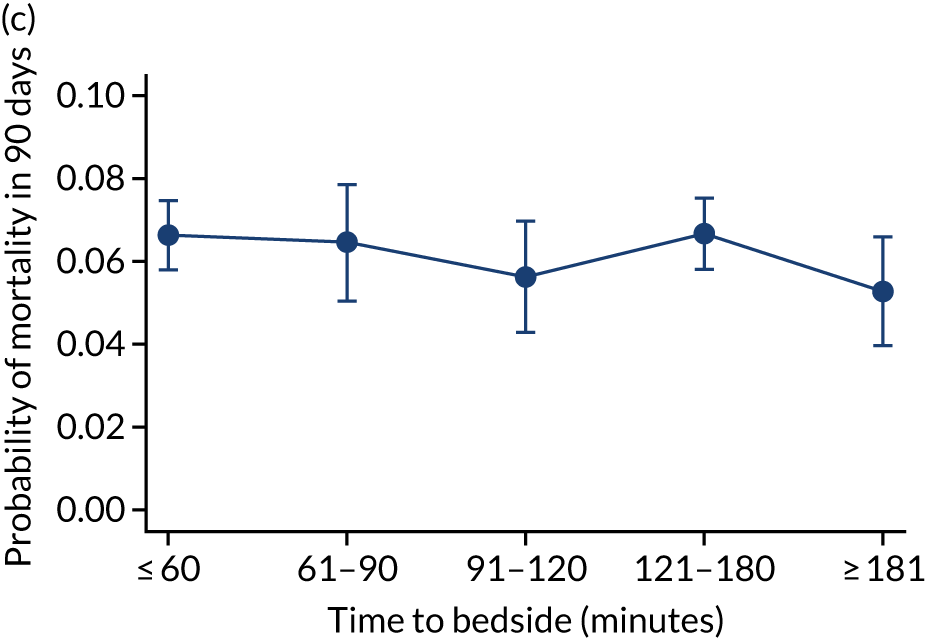
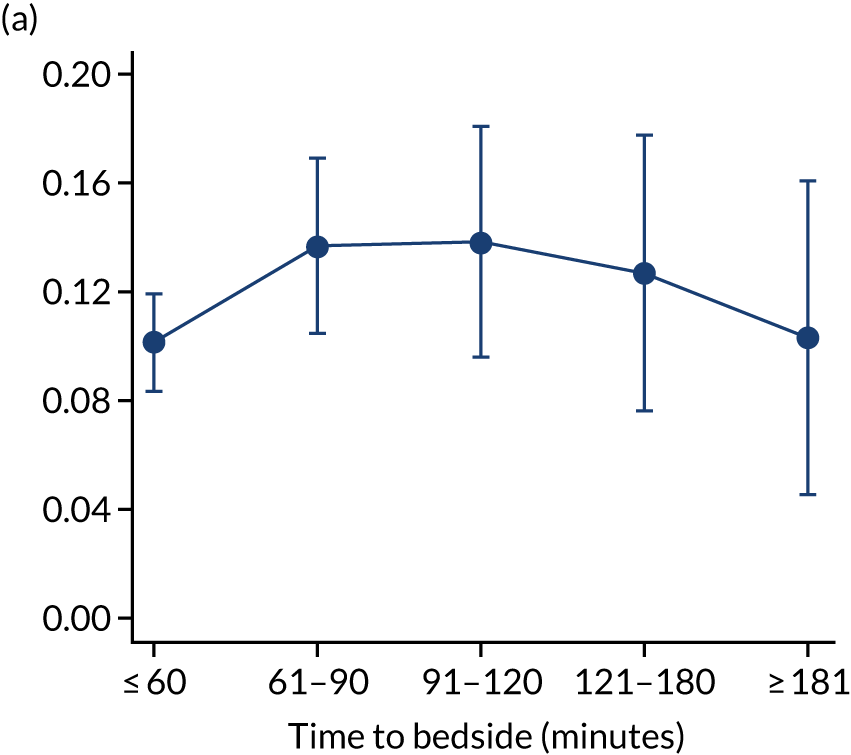
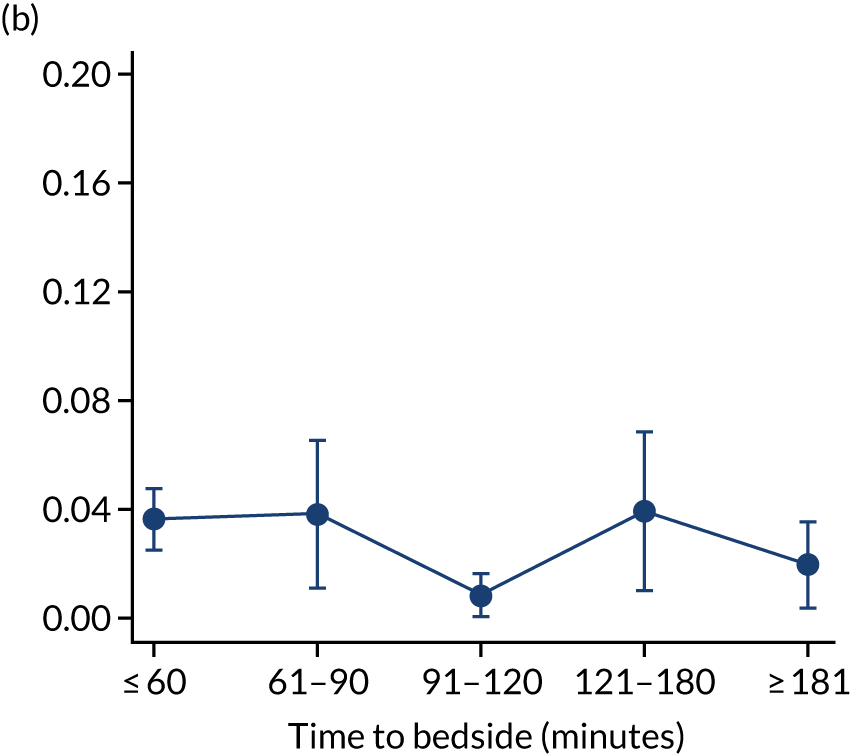
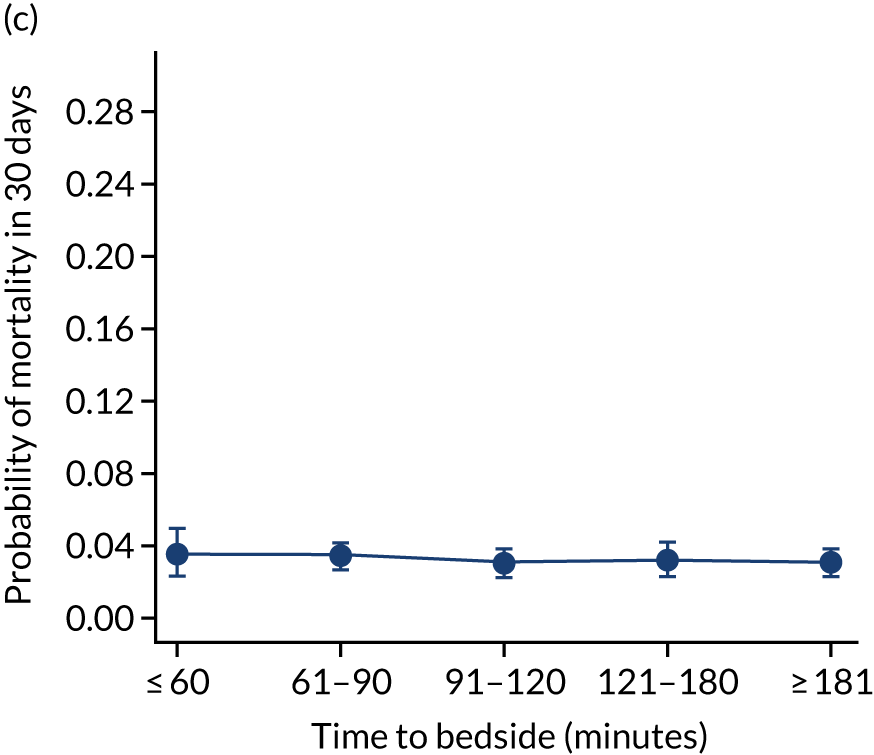
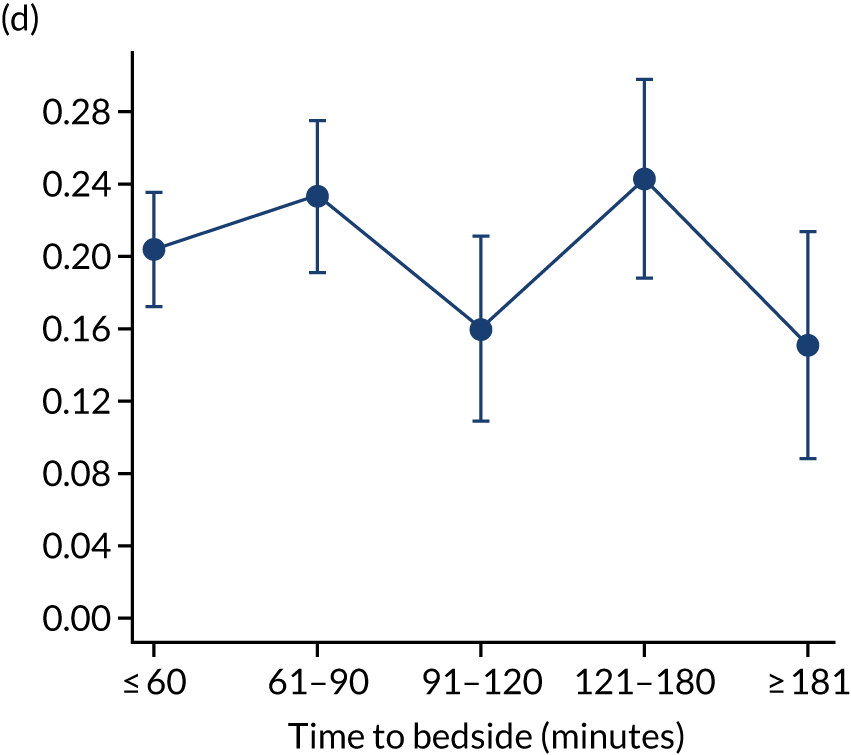
We investigated the impact of time to bedside on our primary outcome of mortality within 30 days of admission to PICU. Before adjustment, as time to bedside increased the probability of mortality 30 days after admission to PICU decreased (Figure 5) and this is likely to reflect that the children who are most critically ill, and have the highest probability of mortality, are not left waiting long periods of time for a PCCT to arrive. We fitted a logistic regression model that adjusted for age of the child, PIM2 score, diagnosis, receiving critical care, size of collection unit and whether or not the child was being ventilated at the time of the referral for PICU. We used clustered standard errors for the transport teams. After adjustment, there was no association between time to bedside and mortality within 30 days of admission to PICU (Table 2 and Figure 6). Similar relationships were seen for our alternative mortality end points of died during admission to the PICU, died within 2 days of admission, died within 90 days of admission and died within 1 year of admission (Figure 7).
FIGURE 5.
Unadjusted probability of mortality within 30 days of admission to PICU by time to bedside.
| Characteristic | Mortality in 30 days | |
|---|---|---|
| OR | 95% CI | |
| Time (minutes) to arrive at bedside | ||
| ≤ 60 | Baseline | Baseline |
| 61–90 | 1.06 | 0.87 to 1.31 |
| 91–120 | 0.84 | 0.66 to 1.08 |
| 121–180 | 1.07 | 0.91 to 1.26 |
| ≥ 181 | 0.82 | 0.66 to 1.02 |
| Age (years) | ||
| < 1 | Baseline | Baseline |
| 1 to < 5 | 0.96 | 0.79 to 1.16 |
| 5 to < 11 | 1.40 | 1.11 to 1.77 |
| 11 to < 16 | 1.24 | 0.94 to 1.64 |
| PIM2 (%) | ||
| < 1 | Baseline | Baseline |
| 1 to < 5 | 2.22 | 1.17 to 4.23 |
| 5 to < 15 | 3.61 | 1.98 to 6.60 |
| 15 to < 30 | 11.31 | 5.77 to 22.19 |
| ≥ 30 | 34.47 | 18.22 to 65.20 |
| Diagnosis | ||
| Respiratory | Baseline | Baseline |
| Cardiovascular | 2.41 | 1.62 to 3.57 |
| Endocrine | 2.73 | 1.85 to 4.05 |
| Haematology/oncology | 2.59 | 1.26 to 5.33 |
| Infection | 1.73 | 1.22 to 2.47 |
| Neurological | 1.28 | 0.76 to 2.16 |
| Trauma and accidents | 1.31 | 0.94 to 1.83 |
| Other | 1.81 | 0.96 to 3.44 |
| Ventilated at referral | ||
| No (not indicated) | Baseline | Baseline |
| Yes | 1.37 | 1.19 to 1.57 |
| No (advised to intubate) | 0.94 | 0.79 to 1.12 |
| Collection unit size | ||
| Small | Baseline | Baseline |
| Medium | 1.12 | 1.01 to 1.24 |
| Large | 1.04 | 0.88 to 1.23 |
| Receiving critical care | ||
| No | Baseline | Baseline |
| Yes | 1.06 | 0.90 to 1.25 |
FIGURE 6.
Adjusted probability of mortality within 30 days of PICU admission by time taken to reach the bedside while holding other variables in the model at the mean value. Reproduced from Seaton et al. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
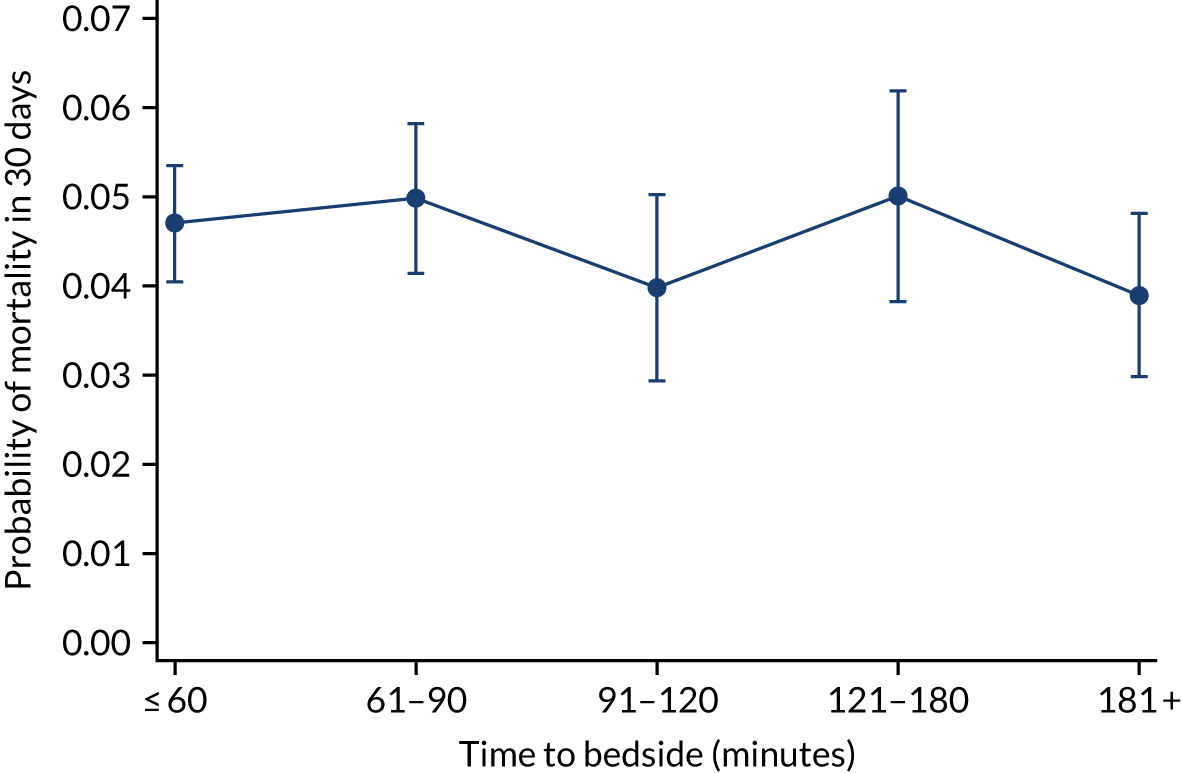
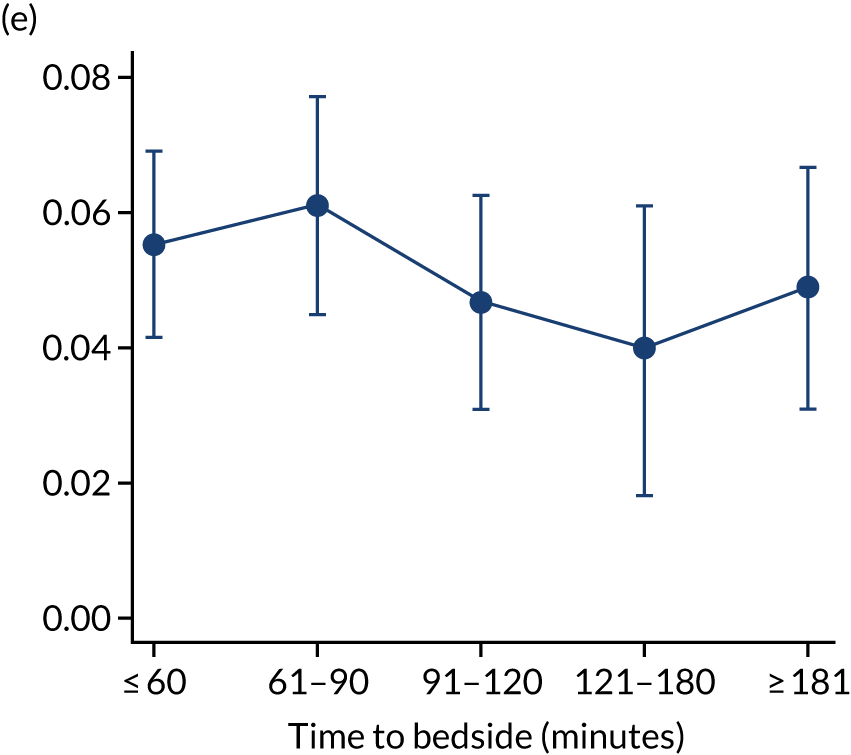
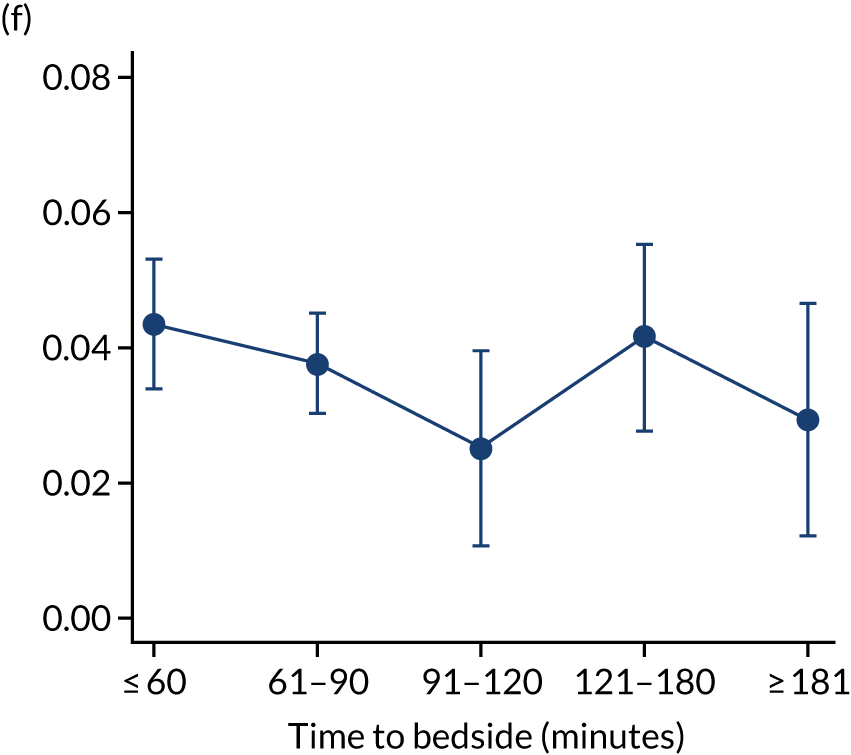
FIGURE 7.
Adjusted probability of mortality (a) in the PICU; (b) in 2 days; (c) in 90 days; and (d) within 1 year of admission against time taken to reach the bedside while holding other variables in the model at the mean value. Reproduced from Seaton et al. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.


Subgroup analyses
We made a priori decisions about clinically important subgroups to investigate further to see if the impact of time to bedside differed within them. The clinical subgroups selected were children admitted with cardiac/neurological conditions, children with a low/high PIM2 score (low: PIM2 ≤ 0.10; high: PIM2 > 0.10) and children transported to PICU in summer/winter (summer: June/July/August; winter: December/January/February). The analysis was fitted to each of these subgroups rather than via use of statistical interactions, and, therefore, our sample size was much reduced in our subgroup analyses, evidenced by the wider CIs and indicated in Figure 8. However, in each of our subgroups, we saw a similar lack of association between time to bedside and mortality within 30 days of admission to PICU (see Figure 8) and this suggests that the time taken by the PCCT to arrive at the bedside of the child is not associated with mortality for any of our subgroups.
FIGURE 8.
Clinically important subgroups and time taken to reach the bedside on mortality within 30 days of admission to PICU. Other adjustments are as in the primary analysis and those variables are held at the average value. (a) Children admitted with cardiac conditions (n = 1310); (b) children admitted with neurological conditions (n = 1505); (c) children with a low PIM2 score (n = 7511); (d) children with a high PIM2 score (n = 1605); (e) children transported to PICU in summer (n = 1777); and (f) children transported to PICU in winter (n = 2814). Reproduced from Seaton et al. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Length of stay/length of invasive ventilation
In addition to investigating the impact of time to bedside on mortality, we considered other important health-care resource outcomes, including LOS and LOV on PICU. For this analysis, we excluded children with missing data relating to LOS (n = 0) or LOV (n = 1).
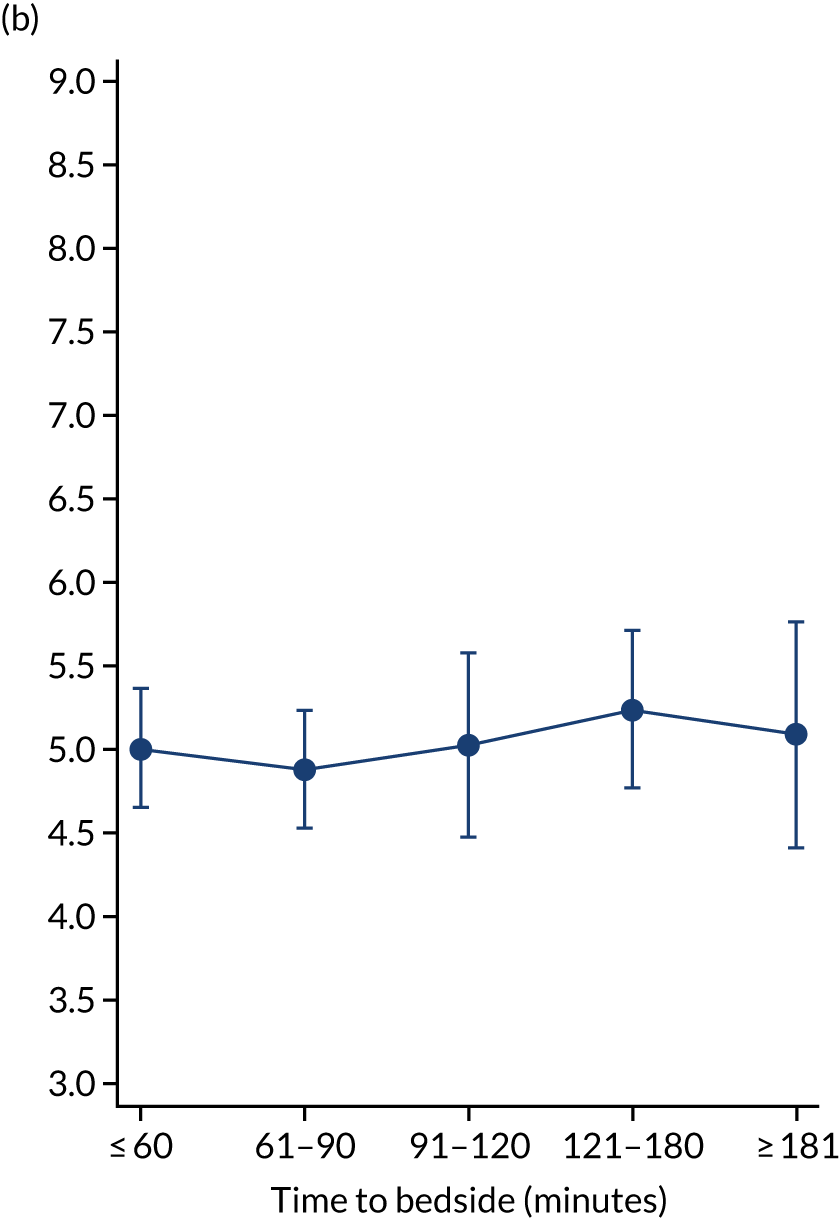
We fitted negative binomial models for each outcome. After adjustment, as time to bedside increased the LOS increased slightly from 7.17 days to 7.58 days (Figure 9) and this suggested a small association between time to bedside and the child’s LOS in PICU. However, the LOV remained static as time to bedside increased (change from 5.01 days to 5.09 days).
FIGURE 9.
Time to bedside and (1) expected LOS; and (b) expected LOV estimated while holding other variables at their average values. Reproduced from Seaton et al. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Model fit
The model fit of our mortality analyses was assessed using the AUC,18 Hosmer–Lemeshow test19 and Brier score. 20 All models had good fit (Table 3), with high values for the AUC, non-significant results for the Hosmer–Lemeshow test and low values for the Brier score. Similarly, despite smaller sample sizes, we noted good model fit for our subgroup analyses.
| Model | AUC | Hosmer–Lemeshow test | Brier score |
|---|---|---|---|
| Mortality analyses | |||
| Mortality in 30 days of admission to PICU | 0.79 | 0.86 | 0.056 |
| Mortality in PICU | 0.80 | 0.57 | 0.050 |
| Mortality in 2 days of admission to PICU | 0.84 | 0.65 | 0.026 |
| Mortality in 90 days of admission to PICU | 0.76 | 0.96 | 0.069 |
| Mortality in 1 year of admission to PICU | 0.75 | 0.51 | 0.082 |
| Subgroup analyses (all mortality in 30 days) | |||
| Cardiac diagnoses | 0.77 | 0.36 | 0.072 |
| Neurological diagnoses | 0.85 | 0.34 | 0.065 |
| Low PIM2 score | 0.66 | 0.59 | 0.065 |
| High PIM2 score | 0.69 | 0.62 | 0.081 |
| Winter | 0.80 | 0.33 | 0.058 |
| Summer | 0.77 | 0.81 | 0.058 |
Sensitivity analyses
Modelling covariates as fractional polynomials
We chose to categorise time taken to arrive at the bedside into meaningful groups according to the current target and because few children waited longer than 4 hours for the PCCT to arrive. In our primary analyses, we also categorised age and PIM2 score and, to investigate the impact of this, we re-fitted our models using fractional polynomials for age and PIM2 score. We explored the conclusions of our primary analysis (i.e. mortality in 30 days) and the results remained consistent (see Appendix 4), with no increasing or decreasing trend observed as time to bedside increased. Our conclusions for other mortality end points and unrepresented outcomes also remained unchanged, and this indicates that our results are robust despite any decisions that we made about how to model certain covariates.
Using the first transport
Approximately 9% of children (see Table 1) in the final DEPICT study cohort had more than one transport included during the time window of the study. Those children with only one transport may have had other transports prior to 2014 or after 2016, which we did not include. We decided to undertake our analysis using the final transport of the child during the time window of the study. We investigated the impact of this decision by repeating our analyses using the first observed transport (see Figure 8). The adjusted probability of mortality was reduced, which was as expected, as children who are transported more than once during the DEPICT study time window have to survive the first transport to receive subsequent transports, and their outcome is attributed to the final transport. However, the lack of association between time to bedside and mortality 30 days after admission to PICU remained consistent (i.e. we saw no increasing or decreasing probability of mortality as time to bedside increased). Our results remained unchanged when analysing the first, instead of the final, transport.
Missing data
To investigate the impact of missing data, we examined the children with missing information concerning their ventilation status at the time of referral, as we believed this to be a key confounder between time to bedside and mortality. In addition, ventilation status at the time of referral was the only variable included in our adjustment with substantial missing data. We re-fitted the analysis three times, assuming that (1) all missing data belonged to children who were ventilated at the time of referral call, (2) all children were not ventilated and (3) all missing data were for children where advice was given to ventilate the child. In all scenarios, the lack of relationship between time to bedside and mortality remained consistent (see Appendix 5).
Time to bedside: conclusions
We saw no evidence to suggest any association between time to bedside and mortality at our primary end point (i.e. died within 30 days of admission to PICU) or any other time point. Our models had good fit and a robust sensitivity analysis indicates that our results are not affected by missing data or modelling decisions. We observed limited evidence of an increasing LOS as time to bedside increased, but no evidence of a similar relationship with LOV. A subset of the results in this section have been published in BMC Pediatrics15 and Intensive Care Medicine. 24
Models of care: results
To investigate the models of care provided to critically ill children around the time of transport, we considered the following three areas: (1) team composition (specifically the choice of team leader), (2) approaches to stabilisation and (3) the occurrence of critical incidents.
Inclusion and exclusion
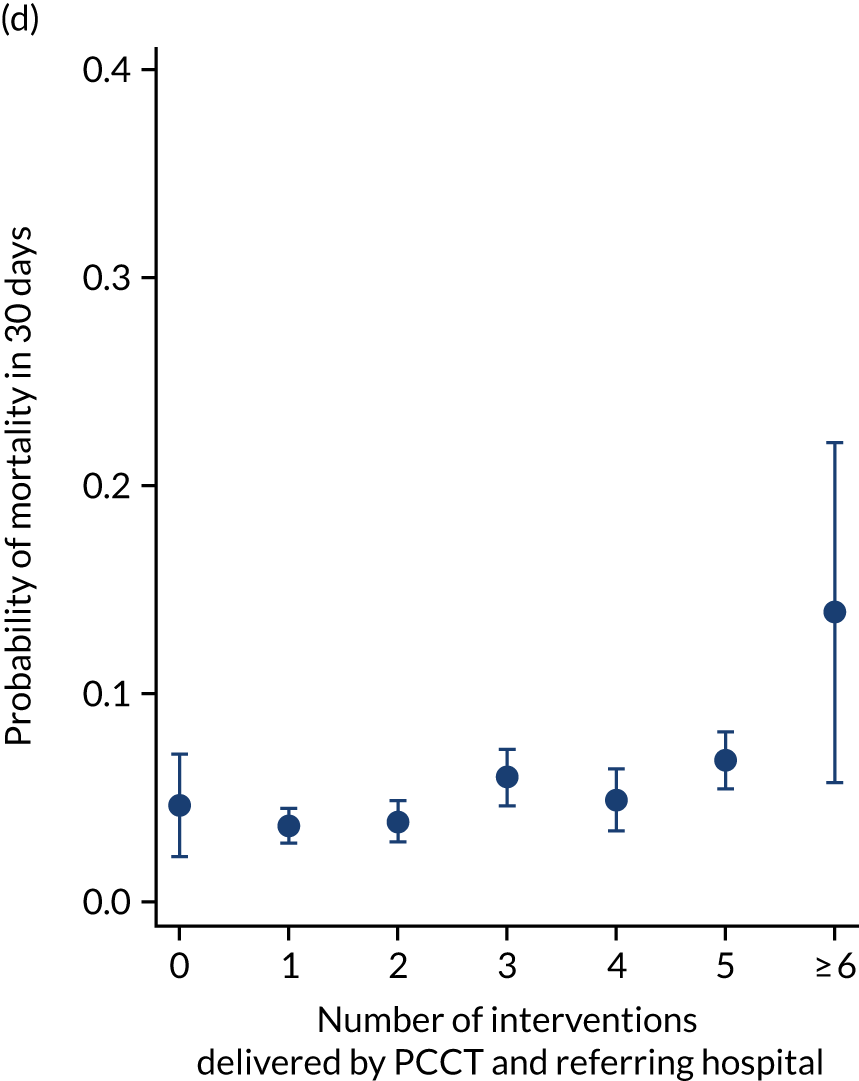
Exclusions were made for children whose ventilation status at the time of referral was missing (n = 272), for children with missing or implausible data (defined as > 24 hours) for the time to bedside (n = 50) or time to PICU (n = 2) and for children with a missing stabilisation time (n = 2). A total of 9112 children were included in the primary analysis (see Appendix 6). Summary statistics are provided in Table 4. The median time spent at the bedside of the child in the referring hospital preparing them for transport (i.e. stabilisation time) was 105 (IQR 56 to 191) minutes, and as the number of interventions provided by the PCCT increased so did the median stabilisation time (hence the phrase ‘prolonged stabilisation’).
| Characteristic | Total (N = 9112) |
|---|---|
| Age (years) of the child, n (%) | |
| < 1 | 4668 (51.2) |
| 1 to < 5 | 2436 (26.7) |
| 5 to < 11 | 1174 (12.9) |
| 11 to < 16 | 834 (9.2) |
| Sex of child, n (%) | |
| Male | 5181 (56.7) |
| Female | 3930 (43.1) |
| Unknown | 1 (< 0.1) |
| PIM2 group (%), n (%) | |
| < 1 | 1039 (11.4) |
| 1 to < 5 | 4087 (44.9) |
| 5 to < 15 | 2983 (32.7) |
| 15 to < 30 | 579 (6.4) |
| ≥ 30 | 424 (4.7) |
| Diagnosis of the child, n (%) | |
| Cardiovascular | 1309 (14.4) |
| Endocrine | 219 (2.4) |
| Haematology/oncology | 153 (1.7) |
| Infection | 820 (9.0) |
| Neurological | 1504 (16.5) |
| Trauma and accidents | 338 (3.7) |
| Respiratory | 4353 (47.8) |
| Other | 416 (4.6) |
| Time to bedside (minutes), median (10th, 90th centile) | 83 (42, 208) |
| Time at bedside (minutes), median (10th, 90th centile) | 105 (56, 191) |
| Journey time to PICU (minutes), median (10th, 90th centile) | 50 (25, 100) |
| Stabilisation time by number of interventions delivered by PCCT, median (10th, 90th centile) | |
| None | 90 (50, 150) |
| One | 125 (80, 200) |
| Two | 157 (100, 241) |
| Three | 180 (110, 279) |
| Four or more | 207 (135, 315) |
| Grade of team leader, n (%) | |
| Consultant | 3028 (33.2) |
| Junior doctor | 4726 (51.9) |
| ANP | 1342 (14.7) |
| Unknown | 16 (0.2) |
| Critical incidents, n (%) | |
| Child incident | 121 (1.3) |
| Vehicle incident | 55 (0.6) |
| Equipment failure | 333 (3.7) |
| Any incident | 496 (5.4) |
| Died in 2 days of admission to PICU, n (%) | 278 (3.1) |
| Died in PICU, n (%) | 571 (6.3) |
| Died in 30 days of admission to PICU, n (%) | 645 (7.1) |
| Died in 1 year of admission to PICU, n (%) | 949 (10.4) |
Models of care: team leader
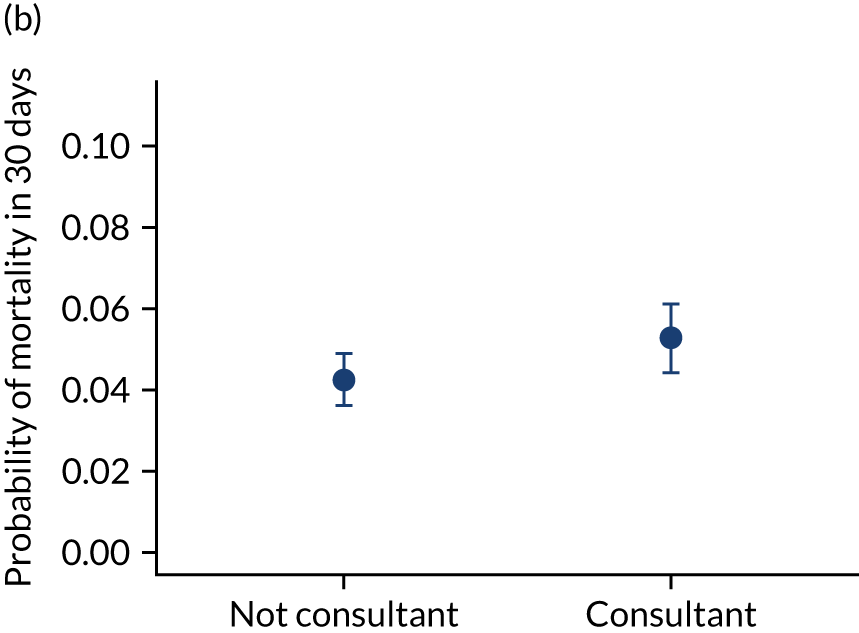
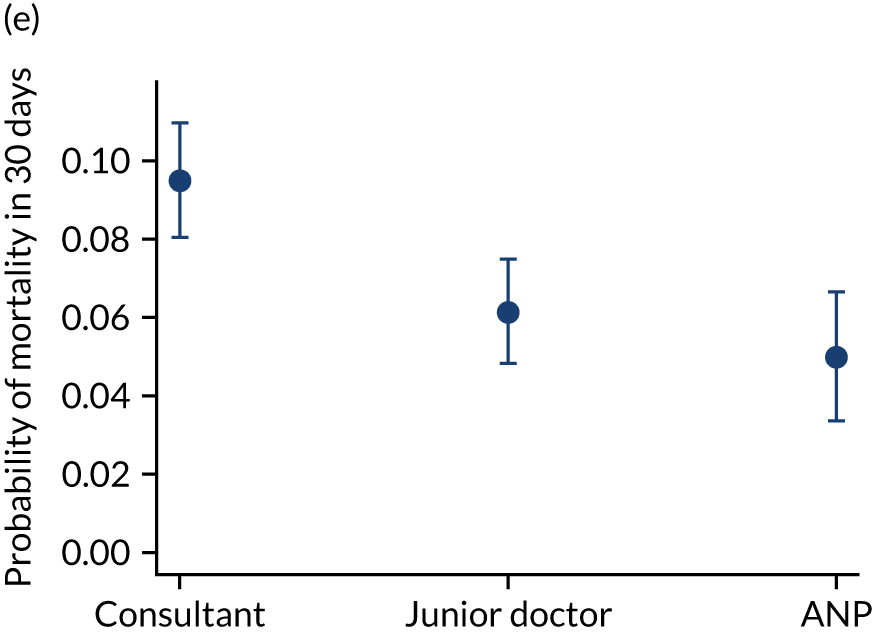
Additional exclusions were made in this section of the analysis for children with missing information about the team leader of the transport (n = 16) (see Table 4). Before adjustment, consultant-led transports had the highest probability of mortality (consultant 0.095 vs. not a consultant 0.059) (Figure 10). After adjustment, consultant-led transports still had the highest mortality, although the difference was substantially diminished (consultant 0.053 vs. not a consultant 0.043) (see Figure 10).
FIGURE 10.
Team leader and adjusted mortality 30 days after admission to PICU (while holding other variables at their average values). (a) Consultant vs. not a consultant (n = 9096) unadjusted; (b) consultant vs. not a consultant (n = 9096) adjusted; (c) ANP vs. junior doctor (n = 6068) unadjusted; (d) ANP vs. junior doctor (n = 6068) adjusted; (e) all team leaders (n = 9096) unadjusted; and (f) all team leaders (n = 9096) adjusted. Reproduced from Seaton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
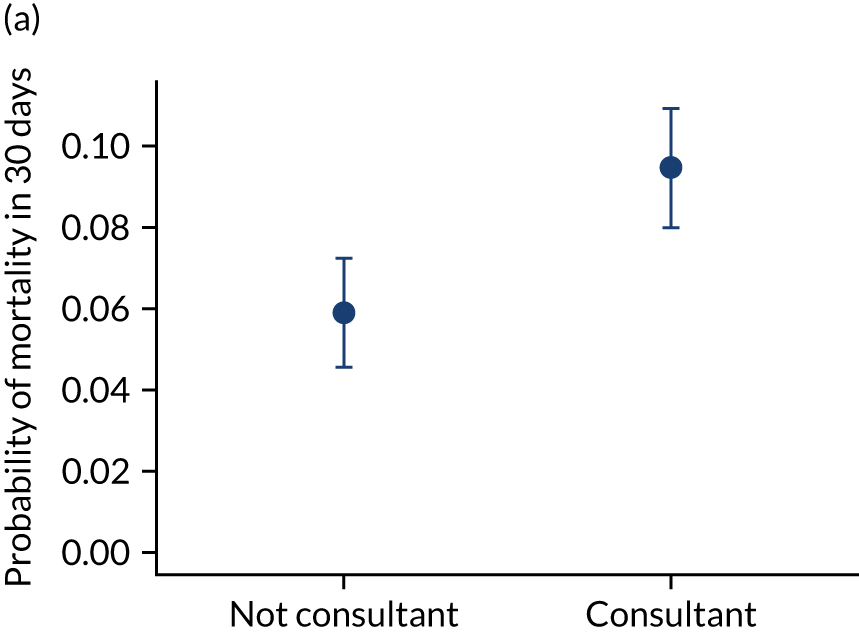
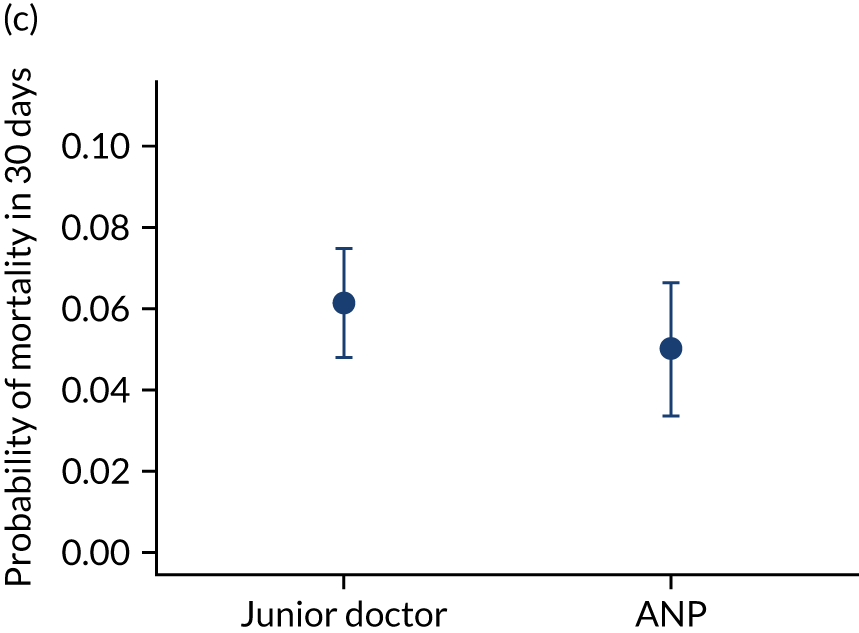
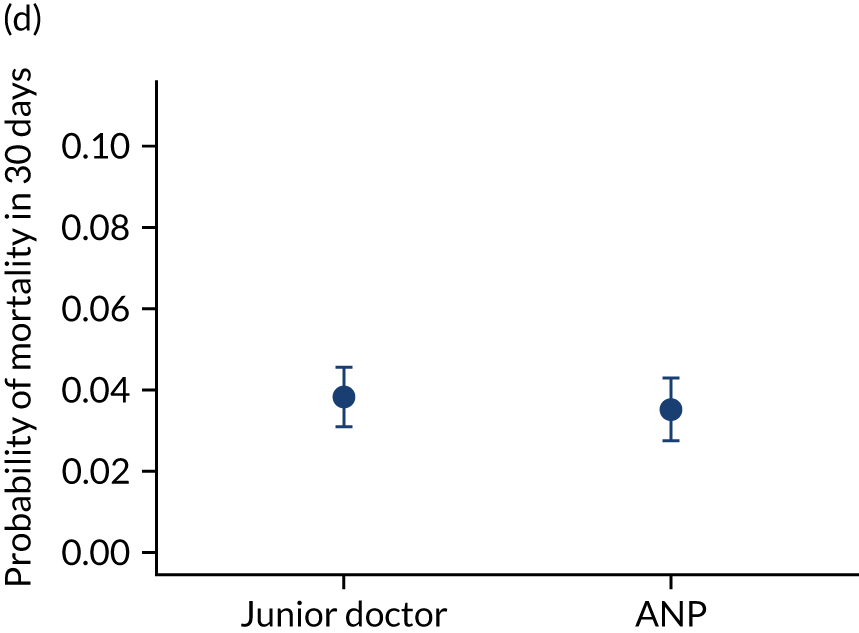
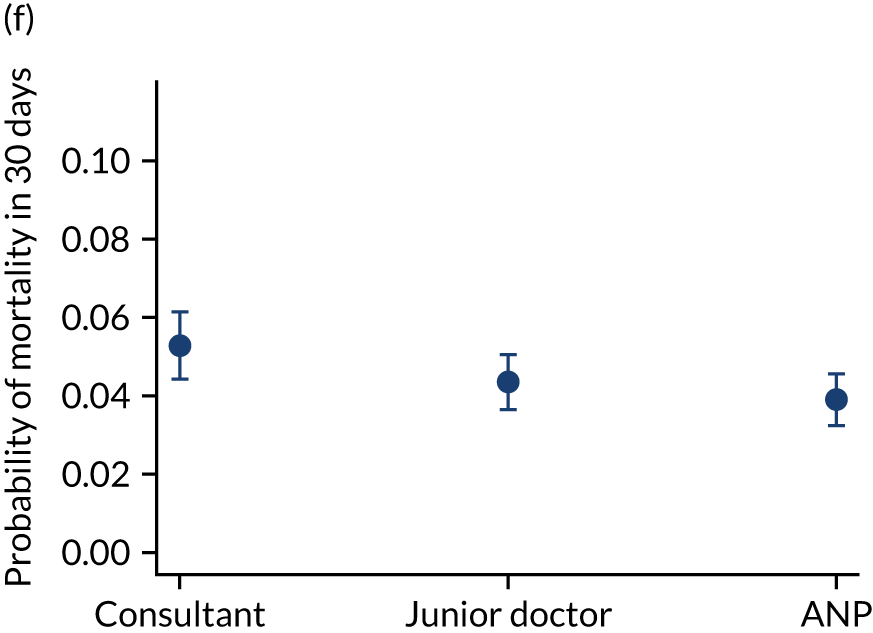
There were no differences between transports led by ANPs and junior doctors when considering adjusted mortality 30 days after admission to PICU (ANP 0.035 vs. junior doctor 0.038) (see Figure 10). Similar results were seen for our secondary mortality outcomes of died in PICU and died in 90 days of admission. All models investigating mortality had good model fit (Table 5).
| Model | AUC | Hosmer–Lemeshow test | Briers score |
|---|---|---|---|
| Team leader: mortality in 30 days | |||
| Consultant vs. not a consultant | 0.75 | 0.75 | 0.056 |
| ANP vs. junior doctor | 0.79 | 0.49 | 0.048 |
| All three team leaders | 0.79 | 0.77 | 0.056 |
| Team leader: mortality in PICU | |||
| Consultant vs. not a consultant | 0.80 | 0.17 | 0.05 |
| ANP vs. junior doctor | 0.79 | 0.82 | 0.04 |
| All three team leaders | 0.80 | 0.31 | 0.05 |
| Team leader: mortality in 90 days | |||
| Consultant vs. not a consultant | 0.76 | 0.18 | 0.07 |
| ANP vs. junior doctor | 0.76 | 0.73 | 0.06 |
| All three team leaders | 0.77 | 0.84 | 0.07 |
When investigating our secondary outcomes of LOS or LOV, children were excluded if they had missing LOS or LOV data, leading to the exclusion of one additional child from the LOV analysis. There were no substantial differences in the adjusted expected LOS by the seniority of the team leader. In the analysis where we directly compared all three types of team leader, the adjusted expected LOS was 7.55 days, 7.05 days and 7.22 days for consultants, junior doctors and ANPs, respectively (see Appendix 7). We did observe small differences in LOV in our three-way comparison, that is, consultant-led transports had the highest expected LOV (5.45 days) and junior doctors had the lowest expected LOV (4.77 days) (see Appendix 8).
Sensitivity analyses and model fit
All models in our team leader analysis had good model fit (see Table 5). To investigate the impact of any modelling decisions, as before, we re-fitted our primary analysis (i.e. mortality in 30 days of admission to PICU) allowing fractional polynomials to model age and PIM2 score. We compared the results from this sensitivity analyses with the results of our original adjusted analysis (see Appendix 9) and observed no noticeable changes to our conclusions.
To investigate the impact of missing data, we inputted values for the only variable in our analysis that had substantial missing data, namely whether or not the child was ventilated at the time of referral call. We assumed, in turn, that missing data represented children who were ventilated, children where it was indicated they should be ventilated and children who were not advised to be ventilated. We re-fitted the three models that investigated the impact of team leader on mortality within 30 days (i.e. the primary analysis), mortality while in the PICU and mortality in 90 days and our conclusions remained unchanged for each of these assumptions.
Models of care: stabilisation approaches
For the primary outcome, there was a marked difference in the unadjusted mortality between children who had received prolonged stabilisation and those who had not (0.137 vs. 0.060) (Table 6). Previously (see Table 4), we demonstrated that as the number of interventions delivered by the PCCT increased, so did the median stabilisation time, therefore, we refer to this as ‘prolonged’ compared with ‘short’ stabilisation. After adjustment, the difference was reduced substantially (0.059 vs. 0.044) (see Table 6), although differences did remain. Differences were seen in mortality at other time points between the children who had received prolonged stabilisation and those who had not, but differences were reduced markedly following adjustment (see Table 6).
| Stabilisation | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Probability | 95% CI | Probability | 95% CI | |
| Mortality in 30 days | ||||
| Short stabilisation | 0.060 | 0.051 to 0.069 | 0.044 | 0.039 to 0.048 |
| Prolonged stabilisation | 0.137 | 0.122 to 0.151 | 0.059 | 0.040 to 0.079 |
| Mortality in PICU | ||||
| Short stabilisation | 0.051 | 0.041 to 0.061 | 0.035 | 0.030 to 0.039 |
| Prolonged stabilisation | 0.135 | 0.123 to 0.147 | 0.056 | 0.036 to 0.076 |
| Mortality in 90 days | ||||
| Short stabilisation | 0.075 | 0.063 to 0.087 | 0.059 | 0.052 to 0.066 |
| Prolonged stabilisation | 0.158 | 0.143 to 0.174 | 0.079 | 0.055 to 0.103 |
| Expected number of days | Expected number of days | |||
| LOS | ||||
| Short stabilisation | 7.28 | 6.63 to 7.93 | 7.04 | 6.65 to 7.42 |
| Prolonged stabilisation | 9.15 | 8.12 to 10.19 | 8.47 | 7.56 to 9.39 |
| LOV | ||||
| Short stabilisation | 5.09 | 4.68 to 5.50 | 4.84 | 4.53 to 5.15 |
| Prolonged stabilisation | 6.74 | 5.88 to 7.59 | 6.18 | 5.33 to 7.02 |
Differences were apparent for LOS in PICU, for which adjustment differences of more than 1 day were noted between the two groups of children (short stabilisation 7.04 days vs. prolonged stabilisation 8.47 days) (see Table 6). Similarly, differences remained after adjustment for the expected LOV between the two groups (short stabilisation 4.84 days vs. prolonged stabilisation 6.18 days) (see Table 6).
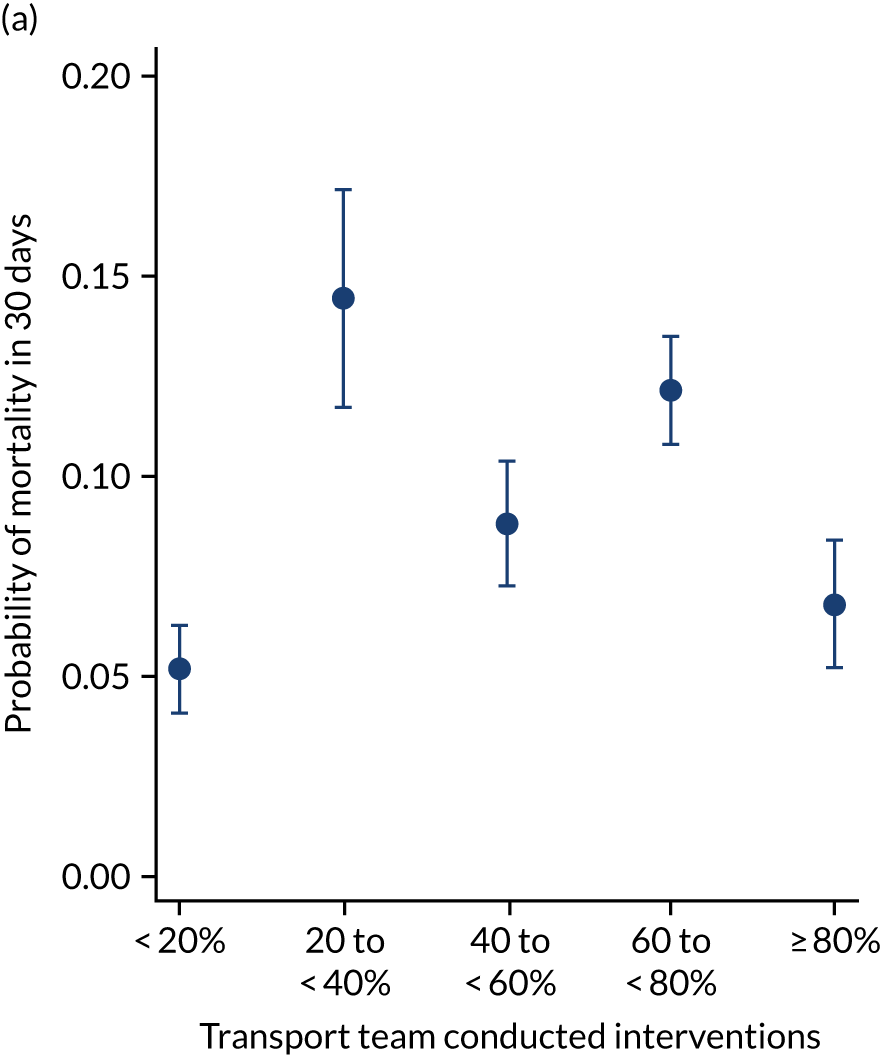
As an alternative way of considering the impact of stabilisation we considered the percentage of interventions conducted by the PCCT and the total number of interventions received by the child (Figure 11). There appeared to be no relationship in the unadjusted or adjusted analysis between the percentage of interventions that were delivered by the PCCT and mortality. When considering the total number of interventions provided to the child both by the referring hospital and the PCCT, the unadjusted probability of mortality at 30 days increased markedly as the number of interventions increased (see Figure 11). After adjustment, the trend was markedly diminished, although there was still an increase in mortality in the children receiving the most interventions, again, potentially indicating that the number of interventions was another proxy of the sickness of the child that was not entirely captured by the other variables in our adjustment.
FIGURE 11.
Total number of interventions delivered by PCCT and referring hospital and the percentage that were delivered by the PCCT. (a) Percentage of interventions delivered by the PCCT unadjusted; (b) percentage of interventions delivered by the PCCT adjusted; (c) total number of interventions delivered by PCCT and referring hospital unadjusted; and (d) total number of interventions delivered by PCCT and referring hospital adjusted. Reproduced from Seaton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.


To explore the impact of stabilisation further, we estimated the adjusted change in time spent at the bedside of the child in the referring hospital (i.e. preparing the child for transport), according to interventions that the PCCT delivered (Table 7). For example, after adjustment for characteristics of the child, children who required intubation from the PCCT took on average 35.9 minutes longer to stabilise (95% CI 32.7 to 39.1 minutes) than those who did not require the PCCT to intubate them (see Table 7).
| Characteristic | Minutes change in stabilisation time (minutes) | 95% CI |
|---|---|---|
| Intervention conducted while PCCT in attendance | ||
| Specified intervention not provided | Baseline | Baseline |
| Intubation | 35.9 | 32.7 to 39.1 |
| Central venous access | 41.4 | 37.8 to 44.9 |
| Arterial access | 26.2 | 23.2 to 29.2 |
| Intraosseous | 41.8 | 34.3 to 49.2 |
| Vasoactive infusion | 22.2 | 19.1 to 25.4 |
| Age group (years) | ||
| < 1 | Baseline | Baseline |
| 1 to < 5 | –1.2 | –3.8 to 1.3 |
| 5 to < 11 | 3.0 | –0.3 to 6.2 |
| 11 to < 16 | 6.5 | 2.7 to 10.2 |
| PIM2 group (%) | ||
| < 1 | Baseline | Baseline |
| 1 to < 5 | 11.5 | 8.1 to 15.0 |
| 5 to < 15 | 18.8 | 15.1 to 22.5 |
| 15 to < 30 | 29.4 | 24.1 to 34.7 |
| ≥ 30 | 29.1 | 23.1 to 35.1 |
| DEPICT diagnosis | ||
| Respiratory | Baseline | Baseline |
| Cardiovascular | –15.5 | –18.8 to –12.2 |
| Endocrine | –3.0 | –9.8 to 3.7 |
| Haematology/oncology | –10.5 | –18.5 to –2.5 |
| Infection | –5.8 | –9.6 to –1.9 |
| Neurological | –11.4 | –14.5 to –8.4 |
| Trauma and accidents | –9.2 | –14.8 to –3.7 |
| Other | –15.0 | –20.0 to –10.0 |
| Ventilated at referral | ||
| No (not indicated) | Baseline | Baseline |
| Yes | 7.1 | 4.5 to 9.6 |
| No (advised) | 1.2 | –1.6 to 3.9 |
| Receiving critical care | ||
| No | Baseline | Baseline |
| Yes | 7.9 | 5.3 to 10.6 |
| Constant | 84.5 | 81.0 to 88.0 |
All models investigating our primary outcome of mortality had good model fit (see Appendix 10).
There were more apparent relationships when considering our secondary outcomes of LOS and LOV. The adjusted LOS increased as the percentage of interventions delivered by the PCCT increased (from 6.9 to 8.3 days) (see Appendix 11). Similarly, the adjusted LOS increased as the total number of interventions increased (from 6.5 to 8.6 days) (see Appendix 11). The adjusted LOV increased as the percentage of interventions delivered by the PCCT increased (from 4.7 to 6.4 days) (see Appendix 12).
Sensitivity analyses
To investigate the sensitivity of our results we re-fitted our primary analysis (see Table 6) using fractional polynomials for age and PIM2 score (see Appendix 13). We observed minimal differences and, therefore, concluded that our results were robust to the decisions made about the modelling of confounders. We also reproduced the adjusted analysis, using fractional polynomials, of the impact on mortality of the total number of interventions and the percentage delivered by the PCCT. Our conclusions remained unchanged.
Models of care: critical incidents
We considered the following three forms of critical incidents: (1) critical incidents involving the child, (2) critical incidents involving the vehicle and (3) critical incidents involving the equipment. Critical incidents due to equipment failures were the most common (n = 333, 3.7%) reason for critical incidents and those involving the vehicle were least common (n = 55, 0.6%) (see Table 4).
Before adjustment, there were elevated odds of mortality for all types of incidents except those involving the vehicle, although these incidents were the least common. After adjustment, the odds of mortality were reduced, but still remained high, most noticeably for incidents involving the child (OR 3.07) compared with those who did not experience a child incident (Table 8).
| Category of critical incident | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Any critical incident (baseline: no incident) | 1.96 | 1.46 to 2.65 | 1.60 | 1.05 to 2.45 |
| Incident involving the child (baseline: no child incident) | 4.29 | 2.68 to 6.85 | 3.07 | 1.48 to 6.35 |
| Incident involving equipment (baseline: no equipment incident) | 1.37 | 0.92 to 2.02 | 1.15 | 0.75 to 1.74 |
| Incident involving the vehicle (baseline: no vehicle incident) | 0.76 | 0.28 to 2.06 | 0.47 | 0.23 to 0.96 |
Discussion
Centralisation of paediatric intensive care unit care
The centralisation of PIC, which began with PICUs in the 1990s,4,25–27 has now also been introduced within the PCCTs that operate throughout England and Wales. In England and Wales, there are now 25 NHS PICUs that are served by nine PCCTs based at 11 geographical sites. Centralisation allows for a focusing of expertise and the development of specialist skills in a small number of sites. However, the consequence of this is that children may have to travel further, or wait longer, to receive access to the specialist care that they require. In other clinical areas, provision of early high-quality care is known to improve outcomes. 11,28
Internationally, a single-centre study from Canada29 identified that children who were transported long distances (> 350 km) by non-specialist teams had longer LOS in PICU and hospital. However, distance from the hospital did not affect outcomes in studies where a specialist team was used6,30 and this suggests that centralisation may not have led to detrimental outcomes for children who have to, potentially, wait longer for their care. However, no one, to the best of our knowledge, has investigated the impact of the centralisation of PIC on the outcomes of critically ill children in the UK in detail and on a larger scale. Despite this lack of evaluation, standards have been created in the UK for PCCTs to achieve, using expert consensus as there is a paucity of evidence-based research. The Paediatric Critical Care Society31 states that PCCTs should aim to reach the bedside of a critically ill child within 3 hours of the request for them to be transported to PICU and this can be relaxed to 4 hours when the child is being collected from a remotely located hospital. The NHS England Quality Dashboard also reports how frequently teams depart their base within 30 minutes of accepting a child for transport. 32
As well as the lack of evidence surrounding centralisation of care, currently no national guidance exists about the selection of a transport team leader for PCCTs, or whether or not transports should be triaged to different team leaders depending on the sickness of the child. Therefore, over the years, PCCTs have evolved dynamically in response to the resources available, with some PCCTs having the majority of transports led by consultants, whereas in other PCCTs consultants are triaged for sicker children only. Any triaging of team leaders in the UK is carried out in an informal manner, rather than via use of transport risk scores (as in other countries). 33
There is variation in the composition of teams used across the country, most notably some areas make use of ANPs, whereas others do not,7 but the impact of this has been unclear. Within neonatal transports, research suggests that nurse-led transports had similar outcomes to those led by junior doctors. 34,35
As well as the importance of selecting the appropriate team composition for the transport, there is no guidance surrounding different approaches to stabilising the child, with two broad approaches being commonplace: (1) taking time to stabilise the child before transport or (2) ‘scoop and run’. Previous research from a single London site has suggested that there is no association between the time spent stabilising the child before transport and short-term mortality in 24 hours. 36 We also considered the impact of critical incidents while the child was being transported to the PICU.
In this chapter, we assessed the two key areas of (1) the impact of time to bedside and (2) the different models of care used by PCCTs in England and Wales.
Time to bedside
In our work, the majority (> 85%) of children were collected within the 3-hour target window currently recommended by the Paediatric Critical Care Society. Therefore, it is difficult to draw conclusions about relaxing the target beyond 3 hours. However, we found no association between the time taken to reach the bedside of the child and their adjusted probability of 30-day mortality. We considered this finding for clinically important previously selected subgroups of children, including children with cardiac conditions, neurological conditions, high PIM2 score and low PIM2 score and children transported in summer/winter. Across all subgroups, our conclusions remained consistent, that is, we saw no relationship between time to bedside and 30-day mortality. Similarly, across all our sensitivity analyses, our results remained consistent. Therefore, we are confident in concluding that, after adjustment, there is no association between time to bedside and mortality, and this suggests that there is no apparent benefit in terms of survival from reducing the target lower than 3 hours, as to do this would require significant service re-configuration with little apparent benefit.
The reason for the lack of an association between time to bedside and mortality may be because UK hospitals perform a number of the critical care interventions before the arrival of the PCCT and, in the short term, hospitals are capable of caring for these children while being supported by the PCCT from a distance. However, this may be at the risk of reduced capacity to care for other children or patients adequately if resources are being re-directed. This provides an additional pragmatic argument to not relax the 3-hour target further, but to replace it with a 4- or 5-hour target.
Finally, although we observed no association with mortality, we did note that PICU LOS increased as time to bedside increased. Therefore, it is possible that the main impact of the early arrival of a PCCT is the physiological stability achieved before admission by the arrival of specialist care in the form of the PCCT at an earlier stage. At the other end of the child’s time in PICU, it could be that these children are kept in the PICU longer, as the hospital nearest their home is a longer distance away, and this may create concerns over the ability to discharge children because of logistics (e.g. getting an ambulance to transfer children) or safety (e.g. keeping children in PICU until certain of their recovery). However, the observed reduction in half a day of the expected PICU LOS should be considered alongside the fact the average PICU stay is only 7.5 days.
Models of care
More than half of transports in England and Wales were led by junior doctors. We found that there were no differences in mortality between ANP- and junior doctor-led paediatric transports after adjustment. Consultant-led transports had the highest mortality, potentially indicating that consultants were being triaged for sicker patients, and this persisted even after adjustment. The continued slightly elevated mortality may indicate that our adjustment did not fully account for the severity of illness of the patient when a consultant led the transport. However, it may also reflect that consultant-led transports are associated with higher mortality. We were unable to assess if consultants were supporting the training of new doctors or nurses or if they were informally triaged for more complex cases.
Similarly, when the transport was led by a consultant there was an elevated adjusted LOS and LOV, although we believe that this represents the same issues with residual confounding. The LOS and LOV were similar when compared transports led by ANPs with those led by junior doctors.
In terms of stabilising the child before transport, our work supported the findings of a smaller London-based study,36 that is, after adjustment the differences in 30-day mortality between children with prolonged stabilisation and other children were substantially reduced. However, we did still observe a persistent difference, although we hypothesise that this may be the result of residual confounding rather than a true difference.
Alongside the different approaches to stabilisation, we also considered the total interventions provided to the child by the referring hospital and the PCCT, which were likely to be a proxy for the sickness of the child, as seen by the marked increase in mortality as the number of interventions increased before our case mix adjustment. When considering the percentage of those interventions that were delivered by the PCCT, there was no association with mortality as the PCCT delivered a higher percentage of the total interventions, and this further supports our tentative conclusion that it is safe for PCCTs to take the necessary time to provide the child with the interventions they require before transport. This is likely to be because the referring hospital and the PCCT are working together, from around the time of the request to transport the child to PICU, to provide the child with the most important intensive care interventions that they will ultimately require on the PICU.
Larger differences did persist for our secondary outcomes, with children requiring prolonged stabilisation having longer LOS and LOV. These differences could represent residual confounding, as we have previously theorised, or they could represent a true difference. In addition , these differences may indicate that keeping children away from PICU for longer, by taking longer to prepare them for transport, means that they require longer to achieve clinical stability within PICU. Similarly, it may be that children transported further from their home may be kept in PICU until clinical stability is assured before transporting them back to a hospital nearer their home for ongoing recovery, therefore, increasing their PICU LOS. However, although we believe that there may be a true difference here, the size of these differences may still be exaggerated by confounding, as the PIM2 score used in our risk adjustment was created to account for mortality rather than other outcomes.
In our research, the impact of critical incidents on the odds of mortality for the child was notable. Incidents involving the child appeared to have the largest impact and, although these incidents were uncommon, occurring in ≈ 1% of transports, they led to the highest odds of mortality, which remained very high even after adjustment. This could also represent residual confounding, although we believe the differences are so pronounced that they are unlikely to completely disappear even after further adjustment. Child-related incidents included accidental extubation, required intubation in transit, complete ventilator failure, loss of medical gas supply, loss of all intravenous access, cardiac arrest and medication administration error. Although some of these incidents may be unavoidable, we recommend that PCCTs evaluate and examine every occurrence of any of these incidents to ensure that lessons are learnt to minimise a repeat event. This review could be formally via local mortality and morbidity meetings or case note review, or informally via team debriefs at the end of a shift.
Additional work delayed because of COVID-19
Most of the analysis for workstream A was able to be completed in a timely fashion, despite the COVID-19 pandemic. However, we were unable to fully analyse the data relating to hospital care, as the data set was too large to effectively manipulate over the secure VPN while working remotely. The impact of this was that we were unable to investigate the following two specific end points of interest: (1) total LOS in hospital and (2) re-admission to hospital after discharge from PICU. However, we feel that the absence of these outcomes does not detract from the importance of our findings.
Publication of work from workstream A
The time to bedside and models of care analysis presented in this chapter have been published in peer-reviewed journals as follows:
-
Seaton SE, Ramnarayan P, Davies P, Hudson E, Morris S, Pagel C, et al. Does time taken by paediatric critical care transport teams to reach the bedside of critically ill children affect survival? A retrospective cohort study from England and Wales. BMC Pediatr 2020;20:301.
-
Seaton SE, Draper ES, Pagel C, Rajah F, Wray J, Ramnarayan P, DEPICT Study Team. The effect of care provided by paediatric critical care transport teams on mortality of children transported to paediatric intensive care units in England and Wales: a retrospective cohort study. BMC Pediatr 2021;21:217.
Strengths and limitations
The UK is unique across the world in having a national data collection system for all PICU transports and admissions. Information relating to all aspects of a child’s care is required to be submitted to PICANet within 3 months of transport/discharge and case ascertainment is known to be very high (> 99%). Therefore, our study is, to the best of our knowledge, the first large national study to be able to investigate these important questions using a population-based cohort.
Although coverage of the data is more or less complete, different data sources were required for this work and we had to develop a robust, transparent approach to data linkage both within PICANet and to external data sources. Our PICANet linkage, which we detailed here to allow other researchers to re-produce in their own research, was successful, leading to minimal loss of data due to failed linkage.
Data completeness was high, as evidenced by the minimal exclusions for missing data for variables required in our analysis. The only variable with substantial missing data was whether or not the child was ventilated at the time of the referral call and we undertook a robust sensitivity analysis to ensure that the impact of excluding children with missing data was minimal.
Of course, data being of a high level of completeness does not necessarily equate with data being good quality. For example, over time, the accurate recording of the time when the child was accepted for transport to PICU is likely to have improved. However, we believe that these issues are likely to be minimal, as the variables we used in this analysis had been collected by the PCCTs for a number of years, allowing for familiarity and improved data collection. The PCCTs are regularly contacted to participate in data cleaning as part of the PICANet annual reporting process, and the PCCTs had an additional opportunity to clean data used in the DEPICT study as part of this review. The statistician for the referral and transport sections of the PICANet annual report is the same as the statistician who undertook this analysis (SS). Since the conclusion of the DEPICT study time window, the data collected relating to transports have been cleaned regularly and we believe that it will have continued to improve and that this represents an excellent resource for future research.
With regard to our analysis, we selected confounders via clinical discussions, with no awareness of statistical significance. We felt that this was most appropriate to develop a clinically meaningful analysis that focused on trends and associations. We made the decision not to present p-values in this section of our work and, to date, we have published from workstream A with this ethos in mind. Our analysis was determined in advance of any work being conducted and was made publicly available on our website [URL: www.depict-study.org.uk (accessed 22 June 2022)]. We chose clinically meaningful groupings for some of our variables and to ensure that this did not affect our results we undertook a robust series of sensitivity analyses that investigated the impact of our modelling choices. In addition, we considered the potential impact of missing data, and our conclusions remained unchanged throughout.
However, despite careful selection of our variables for adjustment, we have indicated instances when we believe that there may still be the potential for residual confounding, particularly in the models of care work. We have presented unadjusted and adjusted results to demonstrate the impact of adjustment. However, we do not believe that the PIM2 score is entirely adequate for risk adjustment in research such as this, as this score was developed for mortality alone without consideration of morbidity outcomes and, therefore, we recommend that future research investigate methods and approaches for risk adjustment in paediatric critical care research.
Finally, we selected the primary outcome of mortality within 30 days of admission to PICU to allow capturing of deaths in locations other than the PICU (e.g. hospices). However, it is unlikely that deaths occurring some days after the transport will be due to care received during transport, rather than care and interventions received on the PICU. Therefore, we presented other shorter-term outcomes, including death on the PICU.
Chapter 4 Workstream B: questionnaires and interviews
Sections of this chapter have been reproduced from Ramnarayan et al. 23 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
Reviews and reform of the UK NHS provision in recent years have seen an increasing recognition of, and emphasis on, the importance of patient experience, with good patient and family experience identified as a core outcome together with clinical effectiveness and safety. 37,38 Traditionally, feedback has been collected from patients about events that have happened – transactions between patients/families and staff – but there is a growing understanding that what also matters to users is the ‘relational’ experience (i.e. how staff made patients and their families feel). 37 Within paediatric services, parents are often asked to provide feedback about their experiences of the care their child has received, frequently because children are unable to provide feedback themselves because of their age, cognitive ability and/or health condition, although the importance of asking CYP to provide feedback on their experience is recognised. 39 Ideally, both parents and CYP should be asked to provide feedback, as they can experience the same situation differently. 40
One of the principles of family-centred care is the involvement of parents as essential partners in their children’s care. There is now a wealth of evidence supporting the opportunity for parental involvement at every stage of their child’s interaction with health services, including the critical care arena. For example, parents want the choice of being able to stay with their children during invasive procedures, such as resuscitation, even if they choose not to remain. 41,42 A child’s transfer to PICU is one element of families’ critical care experience and understanding this from the perspective of both parents and staff is important for a number of reasons. First, there is evidence that parents’ emotional well-being – in the short and longer term – is affected by their child’s PICU admission. 43 Second, longer-term parent mental health is related to distress on arrival to PICU. 44 Finally, there is an association between parent emotional well-being and the longer-term recovery trajectory of their child after discharge from PICU. 43,45
Although the stresses associated with a PICU admission are well documented, patients’ and families’ experiences associated with retrieval to PICU are less well understood, but parents have described the journey to PICU as the ‘worst journey’ of their lives. 8 Parents’ transport experience is likely to contribute to how they feel at the time of the PICU admission and this may, in turn, influence the way in which they interact with PICU staff and how they participate in decision-making, as well as their ability to effectively engage with their child and their care. Since the publication of early work detailing parents’ experiences of their critically ill child’s transfer to PICU,7 approaches to parental presence have changed in the UK PCCT community and, where possible, parents are now offered the opportunity for at least one parent to travel in the ambulance. However, the experiences and perceptions of parents, CYP and staff in relation to this and other elements of the transfer, from arrival of the PCCT at the referring hospital to handover of the child at PICU, have, to the best of our knowledge, not been comprehensively evaluated.
Aims and objectives
This workstream addressed the following two objectives of the DEPICT study:
-
to explore parents’ and, where feasible, children’s experiences of emergency transport to PICU
-
to explore clinicians’ and service managers’/commissioners’ perspectives of PICU transport and its impact on care provided to critically ill CYP, as well as the wider impact on other patients and services.
Methods
Recruitment of transported children and their parents
Parents/guardians of critically ill CYP admitted to 1 of 24 participating PICUs (located in 21 NHS trusts) in England and Wales from January 2018 to January 2019 were recruited. All emergency admissions to participating PICUs over this period were screened for eligibility and CYP arriving to PICU via interhospital transport (i.e. using PCCTs or other transport teams) were eligible. Parents/guardians of eligible patients were approached by clinical/research staff while their child was on PICU to discuss the study and to obtain written informed consent for (1) completion of a questionnaire relating to the experience of PIC transport, (2) potential contact by a researcher for participation in an interview 2–8 months after PICU admission, (3) contact by researchers for completion of a follow-up questionnaire 12 months after their child’s PICU admission and (4) use of patient identifiers to extract PICANet data relating to the child’s transport episode. The consent approach protocol recommended speaking to families within the first 24–48 hours, taking into account the short LOS for many children on the unit. However, recognising that families of critically ill children are likely to be highly stressed during transport and their child’s admission, approach was deferred to a suitable time after PICU admission, as judged by the clinical/research nurse team. Participating PICU teams were asked to log all eligible participants and the outcome of decisions to approach and consent, as well as details of which service transferred children to PICU, on a standardised screening log prepared by the core research team. The sites sent a password-protected copy of the screening log to the core research team once a week initially and then monthly as the study progressed via NHS e-mail so that recruitment across the sites could be monitored.
Strategies to improve participation
Study materials were available in English and in five other languages (i.e. Bengali, Gujarati, Punjabi, Urdu and Polish) to support the process of informed consent. Languages were chosen based on the most frequently spoken languages in England and Wales, based on 2011 census data, data identifying languages where self-reported English proficiency is lower,46 data indicating that PICU admissions are more likely if a child is of South Asian ethnicity and data suggesting that regions that have seen the greatest increases in admissions are those with the largest number of live births to mothers of East European origin, especially Polish. 47 A language translation company developed the alternative language versions and proofread all documents to check for accuracy. The non-English versions were also circulated to independently recruited volunteers (i.e. one or two volunteers per language version) who sense-checked the language versions and feedback was sent to the translation company to action any changes needed. The alternative language study materials were e-mailed to the sites as a PDF for them to print as required.
Parents were eligible to participate in interviews (see Parent interviews) if they returned a questionnaire and consented to be contacted about participation. If non-English-speaking parents consented to be contacted, then our intention was to use interpreting services to contact them about participation and, if parents agreed, to enable the interview to be conducted in parents’ preferred language.
Separate procedures were devised to recruit families whose child had died on PICU prior to approach and this involved a personalised postal letter sent from the site principal investigator (PI), approximately 6–8 weeks after the child’s death. This was managed in accordance with each PICU’s local bereavement policy. Documents enclosed with the personalised letter included adapted participant information sheets, two consent forms, two copies of the transport questionnaires and a pre-paid return envelope to return the completed questionnaire and consent forms. Bereaved families who wanted to participate were asked for consent for completion of the transport questionnaire and for being contacted for participation in an interview, but not for the 12-month follow-up. Sites were provided with bereavement materials on request and these were printed on yellow paper so that they could be easily distinguishable from the other questionnaires.
Questionnaire study
Based on the number of children transported to PICU and data about response rates in questionnaire-based research,48 we estimated receiving 800–1000 completed questionnaires.
Families who were approached and expressed an interest in participating in the DEPICT study were given a pack of information that included all that they would need to give feedback on their transport experience, including a DEPICT study pen, a free-post reply envelope, an information leaflet about the study, a consent form and a paper copy of the questionnaire, which also detailed how to access a REDCap (Vanderbilt, Nashville, TN< UA) electronic version via an online link or quick response code (which was smartphone enabled and had a speak-aloud functionality option to facilitate completion for participants with low literacy levels or impaired visual abilities). Where appropriate, two copies of the questionnaire were offered to families, recognising that parents may have different perspectives and capturing both would enrich the description of family experiences. If a second questionnaire was issued, then the study number was slightly modified to indicate that two questionnaires had been completed for the one transfer. Each parent was asked to complete a consent form; however, for calculating response rates, only one parent per family was included, as both questionnaires related to the same transfer. We primarily intended to recruit parents of transported CYP, but if other family members were present during the transfer and wanted to participate then they could consent to take part. Any CYP aged > 16 years who was conscious during transfer and interested at the time of approach was offered the opportunity to participate.
Particular attention was given to the ‘look and feel’ of the questionnaire to increase parents’ perceptions of the integrity of the study. Each pack was printed in colour on high-quality paper, pre-labelled with a unique study number and localised to the 21 NHS trust sites (with logos and PI information). Each site was sent, via recorded delivery, an initial batch of pre-packed envelopes based on supplying 50% of the total estimated consent rates, and further batches were sent as needed. We chose to send pre-packed envelopes rather than electronic (PDF) versions for each site to print to reduce local researcher burden and to ensure consistency in the presentation of the study materials.
The eight-page questionnaire was developed specifically for this study based on (1) existing user feedback questionnaires used prior to the study, within the retrieval teams, (2) review of relevant literature and evaluation measures, including the Friends and Family Test, and (3) the experience of the Study Team, Steering Group and parent representatives to further inform the format and content. The questionnaire was piloted with six families at two PICUs to check coherence, clarity and acceptability. The questionnaire included a mixture of rating scales, tick boxes and free-text boxes to collect parental responses to specific questions regarding their experience before, during and after their child’s transport to PICU. Examples included perceptions of the wait for the PCCT to arrive at the referring hospital, whether verbal or written information about the transfer was provided, whether or not parents travelled with their child in the ambulance and details about the arrival and handover at the PICU. In addition, we developed a 17-item transport experience questionnaire as part of the larger questionnaire, details of which are provided in Appendix 14. 49 Finally, we included some measures of overall quality and satisfaction with the service provided by the PCCT and the mandatory Friends and Family Test items (see Appendix 14). The questions were developed to ensure that they were relevant to the families’ experiences. Demographic questions included child’s age at the time of transfer, reasons for PICU admission, prior experience of transfer to PICU and whether or not their child had any existing medical problems. At the end of the questionnaire, parents were asked about their employment status and occupation, details of who lives at home with their child and languages spoken at home. No identifiable information was recorded.
Families had the option of posting their completed questionnaires back to the research team via the Royal Mail (Royal Mail Group plc, London, UK) (using the free-post envelope) or they could post the questionnaires in a bespoke post box (designed to represent an ambulance) supplied to each PICU, which, together with the posters, study pens and mugs, acted as an additional cue and signposting about the study.
PICANet data
The PICANet database collects national audit data for all children admitted to PICUs in the UK and includes details of each transfer of a critically ill child to a PICU by each retrieval team [URL: www.picanet.org.uk (accessed 22 June 2022)]. PCCTs supplied audit transport data from the PICANet database where families consented to this so that data from the questionnaires could be linked with PICANet audit data to provide additional information regarding the transport for the analysis.
Parent interviews
Based on previous studies with parents in PICU,50,51 recommendations regarding sample sizes in qualitative research52 and our aim to recruit parents of children transported by each of the PCCTs, we planned to recruit approximately 50 parents, which we considered to be a sufficient number to reach data saturation and to capture the range of parents’ experiences across the different models of care delivery.
A purposive sample of families of critically ill CYP transported to PICU were invited to participate in an interview if they had completed the initial transport questionnaire and consented to being contacted about participation. A member of the research team sent a personalised e-mail invitation to selected families or telephoned them (depending on parents’ expressed preference for contact on the consent form). A second contact (e-mail or telephone) was made to the families who had not responded to the initial contact attempt. Information sheets and consent forms were sent via e-mail or post before the interview and a convenient time and place for the interview was arranged. One of two researchers undertook open-ended semistructured interviews using an interview guide (see Appendix 15) and this format was chosen to ensure that core questions were asked of all participants while providing scope for participants to explore relevant but unanticipated domains of experience and reflection that were important to them. Experts in critical care and qualitative research, as well as parent representatives, developed the interview guides. Families were encouraged to talk about their experiences at different stages of the transport journey, with a specific focus on talking about what went well, what worked less well and what an optimum service would look like. All interviews started with open-ended questions to build rapport between the interviewer and participant and to allow insight into future lines of questioning. In accordance with good practice in qualitative research, the interview guide was reviewed throughout the interview phase by the researchers and modified as understanding of the salient issues developed. It was made clear to participants at the outset that the interview could be stopped at any time should they wish. Recognising that it can sometimes be difficult for participants to ask for an interview to be stopped, a stop signal was agreed at the start so that the participant could express this clearly to the researcher. This technique has been successfully employed in previous studies53 and participants have reported that knowing that they have a mechanism of stopping the interview has been reassuring and empowering should they want to do so.
Purposive sampling was used to ensure diversity in terms of child’s age, diagnosis and previous use of PCCTs and whether or not parents travelled with the child in the ambulance. Family interviews were completed face to face at participants’ place of work, at their home or at a local café, or, if requested, over the telephone. Interviews were expected to last between 30 and 60 minutes and were audio-recorded and transcribed verbatim.
Children’s and young people’s perspectives
The vast majority of children were expected to be sedated and ventilated and, therefore, unable to remember their illness or transport experience. We planned to interview 20–30 CYP aged 5–16 years who could remember some of their transport journey and were able to provide age-appropriate assent for their participation. We identified a small number of CYP who met our recruitment criteria, discussed the CYP interview in a telephone conversation with their parents and provided age-appropriate information sheets. Described details about the interview included duration (i.e. 30–60 minutes), the types of methods we would use to encourage conversation {i.e. draw and write, talking mats, Playmobil® [Playmobil (UK) Ltd, Essex, UK]} and confirmation that parents could be present.
Clinician and service manager perspectives
Using purposive sampling, we aimed to identify and recruit between 35 and 40 health professionals from PCCTs, PICUs and referring hospitals. We aimed to create a diverse sample in terms of professional background, including experience (e.g. time in role), place of work (e.g. referring hospital, PICU, PCCT, PICU-based transport team) and proximity to referring hospital (e.g. < 60 minutes, > 60 minutes).
A study poster that included a brief summary of the study, participation details and researcher contact information was sent to PIs at each participating site. PIs were asked to circulate the study poster to all eligible staff within their hospital/PCCT service. Interested staff were encouraged to contact the study researchers via e-mail or telephone to be sent an information sheet and consent form to complete before participation, answer any questions they had about the study and arrange a mutually convenient time for the interview to take place. Clinicians were offered the opportunity to complete the interview either by telephone or face to face, depending on their preference. We did not want to assume that staff would want to complete the study in their own time and recognised that if they participated while on a clinical shift then they might need to cancel or change the interview time at short notice and, therefore, the interview method was flexible to reflect this. The semistructured interviews were designed to elicit staff experiences and perceptions of the transport of critically ill or injured CYP, the impact of the service on the care provided to the CYP themselves, any wider impact on other patients and services and to describe what they felt an optimal service would look like. A topic guide (see Appendix 16) was developed based on relevant literature and the experience of the Study Team and Steering Group, and was piloted with four clinicians. Interviews were expected to last between 30 and 90 minutes and were audio-recorded and transcribed verbatim.
Post-study evaluation survey by paediatric intensive care unit staff
Paediatric intensive care unit staff from the 21 NHS trusts involved in the DEPICT study were invited to provide feedback on the study set-up, recruitment and support from the central research team via a brief, anonymous questionnaire. The short survey included a mixture of rating scales, tick boxes and free-text boxes to collect staff responses to specific questions regarding their experiences of the DEPICT, including factors that worked well and less well, in addition to asking them to provide suggestions of how to improve any future collection of parent feedback about the retrieval service. The PI from each participating site was sent a link to the online survey and was asked to distribute it to the staff members who were involved in the study.
Data analysis
Quantitative analysis
Questionnaires
Questionnaire data were entered into IBM SPSS Statistics V.21 (IBM Corporation, Armonk, NY, USA) and were analysed using non-parametric statistics (e.g. frequencies, medians, IQRs, Kendall’s tau correlations, Mann–Whitney, chi-squared and Kruskal–Wallis, as appropriate) to describe and examine associations between key variables. Non-parametric statistics were used as the key outcome variables were positively skewed (i.e. the majority of respondents answered very positively about their experiences) and, therefore, did not meet the normality distribution assumptions necessary to reliably use parametric statistics.
PICANet data and data linkage with the questionnaire
If consent was provided, then questionnaire responses were linked with PICANet data on that specific patient transport to provide additional information for analysis (e.g. exact time of arrival at patient bedside). The PICANet data fields are provided in Appendix 17. Objective characteristics of the transfer (e.g. retrieval times and team composition) were included as variables in the quantitative analysis of the parent questionnaire data.
Post-evaluation survey by paediatric intensive care unit staff
Descriptive statistics were used to characterise the responses. For the questions with a Likert response scale, a visual individualised Likert data chart was used to display the data, which enabled responses to be colour coded for individual respondents for groups of questions, thereby providing an easily assimilated overview of the data. 54
Qualitative analysis
Baseline and follow-up questionnaire free-text comments analysis
Content analysis55 was used to convert free-text comments in the questionnaire responses into numerical data that could be summarised alongside the quantitative responses. A coding framework was developed for the content analysis to describe free-text responses to capture the range and numerical frequency with which they occurred. The framework was developed by one researcher using a thematic approach and reviewed by the remaining authors. The free-text comments were coded separately by two authors and an inter-rater reliability was calculated (Cohen’s kappa).
Family and clinician interviews
Interview audio-recordings were transcribed verbatim by a third-party transcription company TakeNote™ (London, UK) that are the approved providers of transcription services for Great Ormond Street Hospital (London, UK). Transcripts were individually checked for accuracy and appropriate redaction of identifying details by the researchers, and were then entered into NVivo version 10 (QSR International, Warrington, UK), a qualitative data analysis program. The framework approach was used to enable an inductive thematic analysis of described experiences. Framework is particularly suitable for analysis of large data sets by a team of researchers56 and it facilitates rigorous and transparent data management, involving the six distinct stages of familiarisation, identifying a thematic framework, indexing, charting, mapping and interpretation. 52 The same team members who conducted the interviews completed the analysis. Once familiar with the data (i.e. transcripts read and analytical notes, thought and impressions recorded in the margins), researchers completed line-by-line coding independently on the first few transcripts. Coding aims to classify all of the data so that they can be compared systematically with other parts of the data set. The two researchers then met to compare codes that they had applied and, with a third member of the core research team, agreed a set of codes to apply to the subsequent transcripts. Codes were grouped together into clearly defined categories to form an analytical framework to then apply to all the transcripts. At the end of this indexing stage, the analysis team met to review insights gained from the thorough review of all the transcripts, including emerging themes that related to parents’ transfer experiences. A thematic matrix sheet was created for each of these themes and subthemes were created using combinations of index codes developed earlier. These subthemes were used as column headings in the matrix sheet, with participants being represented as rows in the matrix. Interview data were then ‘charted’, which involved paraphrasing relevant sections of the interview transcript, retaining the language of the participants, into the matrix at the relevant cell. Styles of summarising were compared and contrasted in the early stages of the analysis process to ensure consistency within the team.
Post-evaluation survey by paediatric intensive care unit staff
Free-text comments were categorised into themes by one researcher and the themes were independently verified by the second qualitative researcher.
The researchers
Both researchers who conducted the interviews were female post-doctoral psychologists who were experienced in qualitative methods, including in-depth interviewing and framework analysis. The third psychologist in the team was a senior researcher in paediatric health psychology with experience and expertise in mixed-methods research.
Ethics issues relating to informed consent and the collection of questionnaire and interview data
Specific ethics considerations relating to the questionnaires and interviews are identified in Table 9.
| Ethics consideration | Mitigating action(s) |
|---|---|
| Questionnaires | |
| Families of critically ill children are likely to be highly stressed during transport and child’s admission to PICU |
|
| Proportion of non-English-speaking families transported to PICU |
|
| Changing situations after consenting |
|
| Inclusion of families whose child died before approach |
|
| Interviews | |
| Potential for recollecting a difficult experience in their child’s life to be upsetting for parents |
|
| Stopping if a participant becomes distressed |
|
| Difficulty for participant to ask for an interview to be stopped |
|
| Participant need for further support/referral |
|
| Safety of interviewer |
|
Findings
Description of sample
Parent sample: consent to participate in the DEPICT study
During the period January 2018 to January 2019, 4558 emergency transfers into 24 participating NHS PICUs in England and Wales that were screened for eligibility. Families of the child transferred in 3439 retrievals were approached for consent (75.5% of eligible), with consent to participate received for 2838 transfers (62.2% of eligible). Figure 12 provides details of reasons for non-approach and non-consent of families.
FIGURE 12.
Flow diagram of participants recruited into the DEPICT study. GDPR, General Data Protection Regulation; NAI, non-accidental injury; PR, parental responsibility.
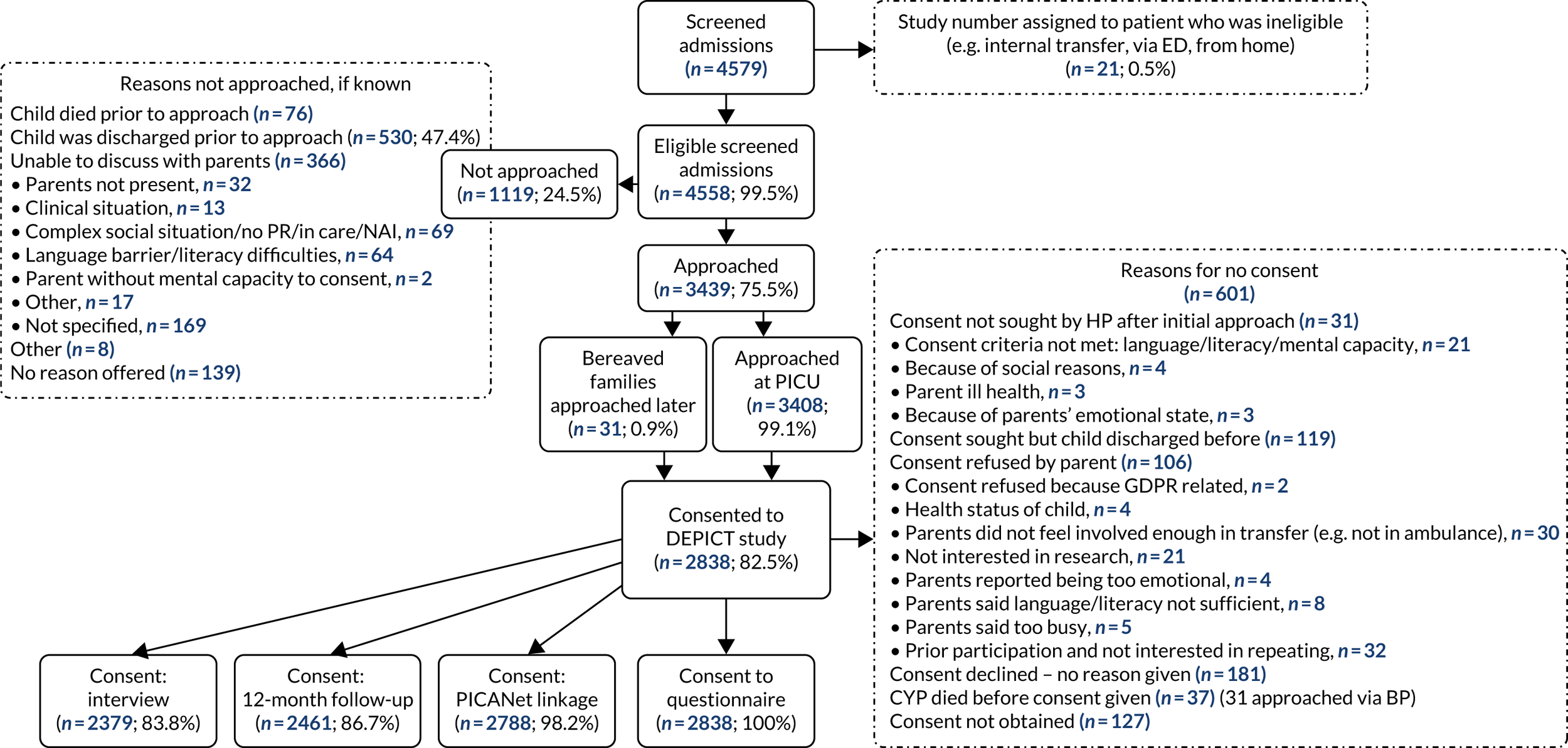
Most parents who consented to participate in the DEPICT study gave consent for the additional activities, including the interview (n = 2379, 83.8% of consented families), PICANet linkage (n = 2788, 98.2% of consented families) and 12-month follow-up (n = 2468, 86.8% of consented families).
Thirty-one families where the child died while being cared for on PICU, but before approach for participation in the DEPICT study, were approached at a later date as per the bereaved protocol, and four of these families (12% of those approached) consented to participate in the study.
Seventeen families were approached and offered participation using the translated DEPICT study consent materials, six of whom consented (three Bengali speaking, two Polish speaking and one Urdu speaking). Although five of these six families also consented to be contacted about participating in an interview, we were not able to contact any of the families.
Children can be transferred multiple times into PICU in any given year and each journey identified in the DEPICT study data collection period was considered to be a unique event. Parents’ experiences are likely to be different on every transfer, for example the staff involved in transferring the child are likely to be different in each case, reasons for transfer may be different and families may travel at a different time of day or to a different PICU.
Questionnaire respondents
We received 2143 returned DEPICT study questionnaires, 10 of which were excluded because they were returned blank. Most parents opted to complete the paper version of the questionnaire, with just eight (0.4%) families completing the REDCap online version. The 2133 at least partially completed questionnaires received related to 2084 unique transfers (i.e. there were 49 questionnaires where two family members reported on the same journey). Taking into account the children who travelled more than once, the 2084 unique transfer questionnaires involved 1998 children. Parents of children who travelled multiple times returned questionnaires relating to between one and three of these journeys (Figure 13).
FIGURE 13.
Flow of participants through the DEPICT study: questionnaire and PICANet linkage.
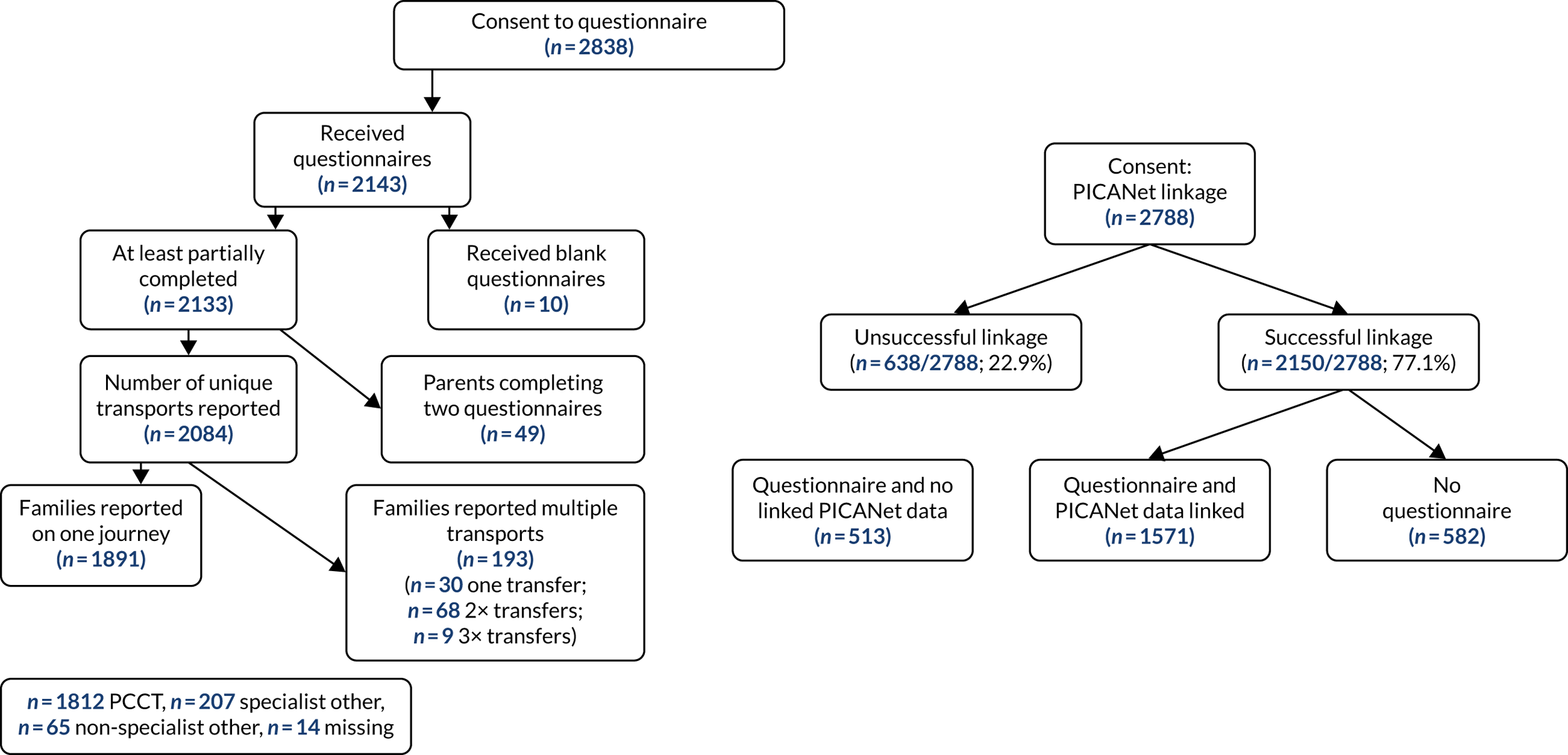
Questionnaires included in the main analyses (i.e. ‘parent 1’ questionnaires) were more frequently completed by mothers (n = 1408, 67.6%). The ‘parent 2’ questionnaires were more frequently completed by fathers (n = 38, 77.6%). Reporting on the comparison of the 49 journeys where responses were collected from two different family members is beyond the scope of this report. As there is a significant proportion of fathers included as respondents in the main analyses, it is not anticipated that there will be a bias from excluding the ‘second parent’ sample (Table 10).
| Participant | Parent 1 (N = 2084), n (%) | Parent 2 (N = 49), n (%) |
|---|---|---|
| Mother | 1408 (68) | 8 (16) |
| Father | 403 (19) | 38 (77.6) |
| Two parents | 119 (6) | |
| Parent (not specified) | 74 (4) | 1 (2) |
| Legal guardian (not parent) | 7 (0) | |
| Other family | 4 (0) | |
| Child participant | 1 (0) | 1 (2) |
| Missing | 68 (3) |
Questionnaires were completed between 0 and 194 days after their child’s admission to PICU, with a median interval of 1 day between admission and questionnaire completion.
Respondents were asked what languages were spoken at home and the majority (n = 1394, 70%) of respondents listed English as the only language or alongside an additional language (n = 298, 15%). In total, ≈ 80 languages other than English were listed. Approximately 40% of families said that they spoke one of the languages in which translated materials were available; Romanian was the most common language reported for which there were no translated materials [6.7% (n = 26) of families reporting speaking a language at home other than English] (see Appendices 18 and 19).
About the child transported
Approximately half (n = 1031, 49.5%) the children transferred in our sample were aged ≤ 6 months, with respiratory and infection being the most frequently occurring reasons for admission to PICU [n = 630 (30.2%) and n = 453 (21.7%), respectively] (Table 11). For the majority of families (n = 1687, 81.0%) this was their child’s first transfer to a PICU and nearly 90% (n = 1802, 87%) of the transfers were completed by PCCTs.
| Variable | Number (%) |
|---|---|
| Age of the child | |
| 0–6 months | 1031 (50) |
| 7–12 months | 196 (9) |
| 13–23 months | 191 (9) |
| 2–5 years | 300 (14) |
| 6–10 years | 181 (9) |
| 11–15 years | 149 (7) |
| ≥ 16 years | 12 (1) |
| Missing | 24 (1) |
| Reason for admission | |
| Respiratory | 630 (30) |
| Cardiac condition | 293 (14) |
| Neurological | 188 (9) |
| Surgery | 116 (6) |
| Diabetes | 7 (0) |
| Trauma | 43 (2) |
| Infection | 453 (22) |
| Other | 182 (9) |
| Too complex/unable to identify primary reason | 151 (7) |
| Missing | 21 (1) |
| Admission to PICU | |
| Previous admission to PICU | 296 (15) |
| No previous admission to PICU | 1681 (84) |
| Missing | 21 (1) |
| Transport team | |
| T001 | 494 (24) |
| T002 | 110 (5) |
| T003 | 152 (7) |
| T004 | 335 (16) |
| T005 | 174 (8) |
| T008 | 205 (10) |
| T024 | 148 (7) |
| T026 | 84 (4) |
| T027 | 100 (5) |
| Specialist other | 204 (10) |
| Non-specialist other | 64 (3) |
| Missing | 14 (1) |
Matching with PICANet data
Of the 2788 journeys for which there was permission to access the PICANet audit data, it was possible to retrieve information relating to 2150 (77.1%) journeys and to link these data to 1571 questionnaires (i.e. 75.4% of all completed questionnaires received) (see Appendix 20). Approximately 20% of parent questionnaires could not be linked with PICANet data and the PICANet data provided information on an additional 582 journeys for which parents had not submitted a self-report questionnaire (i.e. 21% of consented families) (see Figure 13 and Appendix 21).
We compared the age of the child, presenting illness and prior experience of transfer to PICU, between questionnaire respondents with PICANet data and questionnaire respondents without PICANet data. There were significant differences in the primary reason for admission {χ2 [degrees of freedom (df) 8] = 206.18; p < 0.0001} and age of the child [χ2 (df 6) = 15.41; p = 0.017], but no differences in the proportion of respondents reporting prior experience of transfer to PICU [χ2 (df 1) = 0.085; p = 0.770] (see Appendix 21). In addition, we compared the age and sex of the child patient in respondents for whom there were PICANet data with and without linked questionnaire data, and there were no significant differences [z = –1.173, p = 0.241; χ2 (df 1) = 2.340, p = 0.126].
Interview participants
Parents of seven children who were contacted about participating in an interview declined because they were too busy, their child was unwell or they were unwell themselves. Parents of 30 children were interviewed. Nine interviews were with both parents and 21 interviews were with either the mother or father. Interviews were completed between 2 and 8 months from the admission date. The duration of the interviews ranged from 33 minutes to 65 minutes, with a mean of 49.7 minutes. Interviews were largely face to face (n = 27) in the family home, with a small number over the telephone (n = 3). Characteristics of interviewed parents are shown in Table 12.
| Characteristic | Number (%) |
|---|---|
| Parents | |
| Mother | 18 (60) |
| Father | 3 (10) |
| Two parents | 9 (30) |
| Child’s presenting illness | |
| Respiratory | 18 (59.9) |
| Cardiac condition | 3 (10) |
| Severe infection (e.g. sepsis) | 6 (20) |
| Neurological | 2 (6.6) |
| Accident/injury | 1 (3.3) |
| Prior experience of retrieval | |
| Yes | 5 (16.6) |
| No | 25 (83.3) |
| Patient’s age (years) | |
| < 1 | 20 (66.6) |
| 1–4 | 3 (10) |
| 5–12 | 7 (23.3) |
| Siblings | |
| Yes | 23 (77) |
| No | 7 (33) |
| Transport team | |
| Paediatric intensive care retrieval team | 28 (93) |
| Non specialist/other specialist | 2 (7) |
| Mode of transport | |
| Road ambulance | 29 (97) |
| Helicopter | 1 (3) |
| Who travelled in ambulance | |
| One parent | 21 (70) |
| Two parents | 2 (7) |
| No parents | 7 (23) |
Staff were recruited from nine PCCTs, 24 PICUs and seven referring hospitals across England and Wales between July 2018 and July 2019. The duration of the interviews ranged from 27 minutes to 104 minutes, with a mean of 50.2 minutes. Interviews were largely over the telephone (n = 46), with a small number of face-to-face interviews at the person’s place of work (n = 2). Staff characteristics are shown in Table 13.
| Staff characteristic | Detail | Number (%) |
|---|---|---|
| Staff role | ||
| Consultant | Paediatrician | 5 (10) |
| Intensivist | 3 (6) | |
| Anaesthetist | 3 (6) | |
| Specialty unknown | 4 (8) | |
| Registrar | 5 (10) | |
| Nurse (bands 5–7) | 20 (42) | |
| ANP | 3 (6) | |
| Ambulance driver/technician | 5 (10) | |
| Location of role | ||
| Referring hospital | > 60 minutes travel time from a PCCT | 9 (56) |
| < 60 minutes travel time from a PCCT | 7 (15) | |
| PCCT | 11 (23) | |
| PICU and PCCT | 6 (13) | |
| PICU | 15 (31) | |
| Experience/time in role (years) | ||
| < 1 | 5 (10) | |
| 1–5 | 17 (35) | |
| > 5 | 26 (54) | |
Post-study evaluation survey by paediatric intensive care unit staff
Twenty-seven surveys were received from 16 of the 21 participating trusts and results about specific measures put in place to support staff are summarised in Appendix 22. Study set-up, study materials and support received from the research team were positively evaluated by most staff. Staff highlighted the difficulty of approaching families when there was a language barrier and suggested that having finances available to use translators would have been useful to help encourage non-English-speaking families to participate. Suggestions for future collection of parent feedback included using a shorter anonymous questionnaire with different options for completion, including provision of tablets with preloaded surveys.
Experience outcomes
Parents’ experiences were measured in different ways. Quantitative assessment was via responses in the feedback questionnaire, and this was supplemented with described experiences in the qualitative interviews. Associations between parents’ experiences and characteristics of the transports were explored using both kinds of data.
Qualitative description of parental experience of the transfer
Parents’ experiences of their child’s transfer to PICU were explored qualitatively through interviews and supplemented with descriptions of how staff perceived parents experienced the transfer, including reference to staff-related factors that staff felt could be influential in how parents experienced the retrieval service. Three main themes (with subthemes), which map chronologically to the transfer journey, were described, starting at the referring hospital prior to the retrieval team’s arrival, then time spent with the retrieval team both at the referring hospital and during transit, through to arrival at the PICU (Figure 14).
FIGURE 14.
Map of themes relating to the journey to PICU, starting at the district general hospital. DGH, district general hospital.

The context: experiences and emotions prior to transfer
Prior to travel, parents described being distressed, sleep deprived, feeling helpless, having difficulties processing information and wanting reassurance. Parents looked to the referring hospital staff to try and make sense of their situation, and perceptions of its seriousness were influenced by observations of staff behaviour (e.g. increased numbers of staff and activity at the bedside). The decision to transfer was perceived by parents as a sign of the seriousness of their child’s medical condition and some parents expressed relief, believing the PICU to be where their child would receive specialist care. Anxieties were described about the safety of the transfer journey and relocating to an unfamiliar hospital, and some parents reported being upset at how their child looked when prepared for travel. Waiting for the retrieval team was stressful, especially if there was uncertainty, perceived delay and if parents felt that their child’s condition was deteriorating. Referring hospital staff described how caring for critically ill patients could be stressful, presenting a challenge both technically and in terms of resources. For example, there is often a need to implement infrequently used care procedures and drugs, sometimes at fast pace, and with senior staff, who usually work across clinical areas, exclusively looking after the one child, which affected workflow in other parts of the hospital.
With the retrieval team
Paediatric critical care transport team staff described how they aim to talk with parents, including introducing themselves and learning more about the patient, when they arrive at the referring hospital prior to, or just after, a clinical handover. How much time clinical staff can spend with parents at that time appears to vary depending on the medical acuity of the child, and ambulance drivers/technicians can take on the role of offering support to families:
Literally, when we first started the contract all they wanted the ambulance tech[nician] to do was drive the ambulance to the hospital . . . the role has actually evolved now. We spend a lot of time reassuring and liaising with the parents, while the clinical team are very much focussed on looking after the child . . . spend a lot of time really calming down at times the parents and looking after them . . . it’s such a difficult time for them, often they can get forgotten if the child’s really poorly.
S17, PCCT, ambulance technician
Parents’ perceptions of staff as confident and competent through observations of caring for their child, as well as positive communication experiences, seems related to trust in the team and reports of ‘relief from distress’, as well as descriptions of ‘parental support’. Parents who do not travel in the ambulance have fewer opportunities to observe and interact with retrieval staff than parents who do. Policy about where parents sit in the ambulance varied between different services, and this influenced which staff member parents interacted with (driver/technician or clinical staff), as well as the parent’s proximity to their child (some services allowed parents to sit only in the front of the ambulance, whereas some services explicitly excluded this because of the risk of the driver being distracted).
Relief from distress
Parents described relief from distress when distracted by talking with staff about what their child was usually like or, conversely, unrelated ‘small talk’. Reassurance was gained directly from the things staff said about what was happening in the moment and what to expect at the PICU, as well as through observation of the confident, competent staff, and calm was ‘borrowed’ from the emotional demeanour of the retrieval team:
He just made me feel so calm . . . he obviously knew what he was doing, he wasn’t panicky like I was . . . In [local hospital] because they were a bit unsure, it made us more unsure, but because they [PCCT] were more confident in what they were doing it made me feel confident.
P6, mother, PCCT-7
Parental support
Feeling cared for by the retrieval team appeared to be derived through empathetic, sometimes light-hearted, general conversation, in addition to receiving care packs (containing food and toiletries) and self-care advice (e.g. to eat or rest during the journey). Parents were impressed by the capacity of staff to offer them support while caring for their critically ill child:
. . . they did an excellent job of looking after me and obviously they did a brilliant job of looking after [patient].
P24, father, PCCT-4
. . . they would be watching her, but talking about other things and involving me as well . . . I’m not sure whether it’s partly to calm me down or whether it’s just what they would have done anyway, but it had that effect . . . That was one of the least stressful bits of the day.
P17, mother, PCCT-6
Not every parent felt supported. Some parents felt alone during the transfer, and self-care advice around eating was interpreted by one parent as being ‘guilt-tripped’:
. . . they made me eat something . . . they did the whole guilt-trip, ‘What are you going to do if you get there and you pass out because you’ve not eaten, and then you won’t be there’, and it’s like, ‘Shut up’.
P9, mother, PCCT-2
I know they’ve got work to do, but just a little reassurance, you’re not on your own . . . just talking it through . . .
P18, mother, PCCT-1
Some parents discussed the lack of support for themselves alongside recognising that, despite this, their priority (along with the PCCT) was the care for their child:
. . . all you want is for your child to be OK and their job is to look after them, so they are doing the exact right thing . . . It was quite a scary thing, and not having that reassurance – , I have no doubt about their technical capabilities . . . there were no complaints there at all. It’s not a complaint, it was just simply that it wasn’t focused on the parents at all . . . it shouldn’t be.
P4, mother, PCCT-1
Parents varied in their preference for communication and negative appraisal may have been related to a mismatch between preferences/expectation and what happened. For example, jovial small talk perceived to be superficial or inappropriate or conversation perceived as lacking empathy or simply not enough conversation:
. . . the hardest bit for me, was that driver making that stupid comment [joke about a missed opportunity to watch football], because it just upset everybody and it just made the whole situation really scary and worse.
P18, mother, PCCT-1
Staff attitudes towards parents travelling in the ambulance were generally supportive for the following reasons. The time with parents in the ambulance was perceived as beneficial to staff to learn more about the patient (although there were different opinions if the child had the potential to arrest during transfer). In addition, there was recognition that conversations could support families emotionally, as well as ‘boost’ and ‘prep’ parents for the next stage at the PICU. Staff described how parents’ responses to PICU transfer varied and how this response can be influenced by prior retrieval experiences or communication preferences. Communication efficacy was suggested to vary between individuals and professional roles, as well as situational factors:
. . . certainly some of them [medics on PCCT] have that ability just to, get on at the parent’s level and acknowledge that it’s a horrendous situation they’re in . . . some of the medics definitely would and certainly all of our consultants do . . . if you go out with the more junior registrars, as a nurse I think sometimes we take the lead on doing . . . the nicety-nice just to free them up to do the . . . more technical tasks.
S11, PICU/PCCT, nurse
I didn’t have time to really speak to the mother . . . because it was a bit of a hairy transfer.
S3, PICU, nurse
On arrival to the PICU, some parents described being encouraged to wait in the ‘family room’ either straight away or after being shown the bed space where their child would be cared for. There was variation in how long parents waited before contact with PICU staff, ranging from 5 minutes to a very long time:
I was only left alone for about 5 minutes. I was shuffled through quite fast.
P12, mother, PCCT-7
. . . somebody showed me where the parents’ room was and left me there. This is another one of the bad bits of the day, because I was waiting there a very long time for news.
P17, mother, PCCT-6
Despite travelling with their child in the ambulance, separation at this point was still difficult for some families and a variety of reasons were given for this, including not having the opportunity to observe and, potentially, contribute to the handover, a longer than expected wait leading to worries that their child had deteriorated and not being able to soothe their baby who had become increasingly distressed on the journey:
She’d gotten worked up in the ambulance and then to be taken away from her and not being able to reassure her, and you just think she’s going into this room with all these people who are going to poke and prod her, she’s going to be really upset.
P4, mother, PCCT-1
Some parents described being prepared for this stage by staff in the ambulance, which helped with the wait, whereas others reported that they had wished that this had happened:
. . . it would only take 2 minutes to say, ‘Right, this is where she’s going to be. I’m going to walk you to the parents’ room, we’re going to have a conversation, we’re going to come in and we’re going to say goodbye, and someone else will come in and tell you –,’ it doesn’t take very much at all just to do those various things.
P4, mother, PCCT-1
Staff recognised that parents found it difficult to wait to see their child:
. . . that’s the one thing that generally they’ve fed back to us is that there’s a lot of waiting . . . all they want to do is see their child . . . sometimes, that’s out of our control and we’ve said to them, unfortunately, there are things we need to do before they can come in . . .
S30, PICU, nurse
Before travelling, retrieval teams confirmed whether or not parents could accompany their child in the ambulance. The parents who we interviewed described being upset when no opportunity to travel was offered, especially if this was contrary to expectation. Some parents chose not to travel and this could be for practical reasons (e.g. picking up clothes from home) or emotional reasons. Some staff suggested that parents were ‘relieved’ when they could not travel and benefited from time away to process information.
Independent of whether or not they travelled with their child, parents expressed that they would have liked to have had, or described benefits of having, a parent on board, and for some parents this was contingent on travelling as a couple. Acceptance of travelling separately was associated with parent trust in the capabilities of the medical team and parents’ evaluation of their ability to cope emotionally in the ambulance and or ability to help their child, as well as the state of consciousness of the child:
I didn’t want to leave her . . . because you don’t want your baby to go out of your hands, but she [doctor] honestly made me feel like she had this and she was going to do her best by her . . . if she’d have been awake, then I would absolutely have not left her.
P11, mother, non-specialist
Staff recognised the importance of trust gained at the referring hospital to facilitate parents’ independent travel and described how they worked to achieve this through introductions on arrival, making clinical decisions confidently in front of parents and sometimes involving parents in preparation and planning for transfer.
Parents’ descriptions of their journeys to PICU included challenges, such as making the journey alone, sleep deprivation, distance, unfamiliarity of the PICU location, time of day, traffic and weather conditions, along with emotional distress about their child’s condition, being separated from their child and not knowing what was happening in the ambulance. Finding the PICU and gaining access was not always easy, especially if the journey was made ‘out of hours’, and finding out the health status of the child on arrival was emotionally difficult:
. . . you’re searching for people’s eye contact and conversation . . . I wish we’d had a chattier nurse and doctor when we arrived . . . your emotions are everywhere . . . you’re a bit angry, you’re a bit scared, you’re a bit anxious, you’re everything really, and for a parent, we just want to make sure she’s all right really, ‘Is she OK?’
P16, mother, PCCT-9
Parents who travelled together were able to share the burden of navigation and one parent, who was initially disappointed not to be able to travel with their child, reported that, in hindsight, travelling together with their partner was a valued opportunity to express emotion and process together what had happened up to that point.
Referring hospital and PCCT staff described actions to mitigate the burden of travelling independently by focusing on the benefits of travelling separately to distract parents from the disappointment of no seat offered, such as loan of a ‘Sat Nav’, leaflets with travel directions, maps and contact telephone numbers of PCCT and PICU staff. One parent described how referring hospital staff encouraged him to eat and rest prior to setting off to PICU and another parent explained how their ambulance driver (once he had delivered the parent’s child) was able to give additional support on the telephone to help navigate road closures. Although all the parents interviewed travelled in their own cars, referring hospital staff talked about organising transport (e.g. taxis and police escorts) for parents.
Some retrieval teams take a parent’s telephone number and/or give out a telephone number to parents with the agreement that calls will happen if the child deteriorates during transfer. Parents and staff reported that this offered reassurance; however, it also offered false reassurance for one parent interviewed:
. . . they got there a bit before us because they blue-lighted . . . whereas we got stuck in all the 40 mph [miles per hour] roadworks . . . by the time we’d got there he’d already got a lot worse . . . a lot had happened within that time that obviously we were completely unaware of driving up . . . they did say that if there were any problems, that anything massively changed, then they’d give us a call and I think, because we didn’t hear anything, we went up thinking, ‘It must just be an infection then, and it’ll be fine when we get there’ . . .
P29, mother, PCCT-2
Quantitative measures of experience
Parent transfer experience measure
The ‘parent transfer experience’ measure encompasses ratings relating to parents’ perceptions of health professionals’ behaviour and interactions, as well as parents’ comprehension of the events involved in retrieval. The scale ranges from 1 to 5, with a composite mean score of 1 indicating least positive experience and a score of 5 representing the most positive transfer experience. Table 14 shows the proportion of parents who rated each category for the nine individual items that contributed to the composite score. The experience composite score could be calculated for 1759 (85%) of the journeys with a median score of 5 (first quartile = 5 and third quartile = 5). A composite score was not calculated for participants who had one or more missing items in the nine individual responses.
| Item | Response category, n (%) | ||||
|---|---|---|---|---|---|
| Disagree a lot | Disagree a bit | Neither | Agree a bit | Agree a lot | |
| I felt that my child was safe during the transfer | 32 (2) | 19 (1) | 41 (2) | 191 (9) | 1741 (86) |
| When I asked questions I received answers that I understood | 9 (0) | 9 (0) | 35 (2) | 87 (4) | 1745 (93) |
| Transport team listened to me | 19 (1) | 10 (1) | 84 (4) | 173 (9) | 1662 (85) |
| I trusted the transport team | 9 (0) | 8 (0) | 25 (1) | 134 (7) | 1868 (91) |
| My child’s transfer went well | 10 (0) | 5 (0) | 13 (1) | 74 (4) | 1937 (95) |
| The transport team were caring and understanding | 6 (0) | 3 (0) | 29 (1) | 98 (5) | 1891 (93) |
| I was satisfied with the care received | 7 (0) | 4 (0) | 13 (1) | 70 (3) | 1941 (95) |
| Transport team treated me and my family with respect | 8 (0) | 6 (0) | 23 (1) | 65 (3) | 1916 (95) |
| I felt reassured by the transport team | 13 (1) | 7 (0) | 36 (2) | 117 (6) | 1826 (91) |
Other quantitative measures of experience and satisfaction (categorical)
Families also provided a categorical rating of their experience and satisfaction with the retrieval team and completed the NHS Friends and Family Test, which was current at the time. Table 15 indicates that measures of satisfaction were very high.
| Measure of satisfaction | Rating, n (%) | Missing, n (%) | ||||
|---|---|---|---|---|---|---|
| Extremely poor | Poor | Fair | Good | Excellent | ||
| Overall experience of transport service | 1 (0) | 9 (0) | 25 (1) | 193 (9) | 1822 (87) | 34 (2) |
| Overall satisfaction with transport service | 1 (0) | 6 (0) | 21 (1) | 180 (9) | 1836 (88) | 40 (2) |
| NHS Friends and Family Test: if child in this situation would like them to be transported by this team | 5 (0) | 8 (0) | 34 (2) | 86 (4) | 1921 (92) | 32 (1) |
| NHS Friends and Family Test: would recommend transport team to anybody whose child needed to be transported to PICU | 7 (0) | 9 (0) | 32 (2) | 71 (3) | 1933 (93) | 35 (2) |
Timeliness
A primary objective of the DEPICT study was to assess the impact of timeliness of access to PIC on parents’ experience of retrieval and perceived satisfaction with this experience. Timeliness was defined as the time in which it took for the retrieval team to reach the bedside of the patient at the referring hospital, as well as other stages of the retrieval journey, such as stabilisation at the referring hospital (i.e. the time the retrieval team spent at the referring hospital) and time to get to the PICU. Both quantitative data (i.e. parent questionnaire responses and PICANet transport audit) and qualitative data (i.e. staff and parent interviews) were used to describe the impact of timeliness on retrieval experience (including satisfaction), as well as to identify the factors that may influence timeliness.
Parent perceptions of timeliness
Families were asked to indicate whether they perceived the retrieval team to arrive at the referring hospital ‘earlier than expected’, ‘on time’ or ‘later than expected’, or to indicate that they could not remember or were not told a time frame. For the majority of journeys (n = 1375, 66.0%), parents perceived the transport team to arrive either early or on time (see Appendix 23).
Association between parent perceptions of timeliness and experience/satisfaction with the transfer to the paediatric intensive care unit
Being aware of an arrival time and satisfaction/family transfer experience
Being told a time, compared with not being told a time, regardless of when the team actually arrived (i.e. earlier, on time or later), was associated with significantly higher ratings of satisfaction and positive experiences with the retrieval (Tables 16 and 17).
| Satisfaction measure | Aware of a time, n (%) | Not aware of a time, n (%) | Group difference (Mann–Whitney) |
|---|---|---|---|
| If my child was ever in this situation again then I would like them to be transported by this team again | |||
| Disagree at lot | 2 (0) | 1 (0) | U = 254,933.5; z = –2.721; p = 0.007 |
| Disagree a bit | 8 (1) | 0 (0.0) | |
| Neither agree nor disagree | 18 (1) | 14 (4) | |
| Agree a bit | 65 (4) | 17 (5) | |
| Agree a lot | 1522 (94) | 297 (90) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | |||
| Disagree at lot | 4 (0) | 1 (0) | U = 249,818.0; z = –3.784; p < 0.0001 |
| Disagree a bit | 8 (1) | 1 (0) | |
| Neither agree nor disagree | 14 (1) | 16 (5) | |
| Agree a bit | 52 (3) | 15 (5) | |
| Agree a lot | 1536 (95) | 294 (90) | |
| Overall experience of the transport service | |||
| Extremely poor | 0 (0) | 1 (0) | U = 247,174.0; z = –3.316; p = 0.001 |
| Poor | 8 (1) | 1 (0) | |
| Fair | 20 (1) | 5 (2) | |
| Good | 127 (8) | 45 (14) | |
| Excellent | 1457 (90) | 275 (84) | |
| Overall satisfaction with the transport service | |||
| Extremely poor | 0 (0) | 1 (0) | U = 249,413.5; z = –2.814; p = 0.005 |
| Poor | 5 (0) | 1 (0) | |
| Fair | 19 (1) | 2 (1) | |
| Good | 119 (8) | 42 (13) | |
| Excellent | 1464 (91) | 281 (86) | |
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group difference (Mann–Whitney) | |
|---|---|---|---|
| Median | IQR | ||
| Aware of a time (n = 1404) | 5 | 5–5 | U = 175,783.5; z = –2.314; p = 0.021 |
| Not aware of a time (n = 268) | 5 | 4.89–5 | |
Association between perceived earlier/on-time arrival time and satisfaction/experience
More parents endorsed higher satisfaction categories and had higher positive experience scores if the transfer team’s arrival to the referring hospital was perceived to be earlier or on time (Table 18 and see Appendix 24).
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group difference (Mann–Whitney) | |
|---|---|---|---|
| Median | IQR | ||
| Early/on time (n = 1185) | 5 | 5–5 | U = 105,579; z = –6.053; p < 0.0001 |
| Later (n = 219) | 5 | 4.78–5 | |
PICANet data of time to bedside, stabilisation time, journey time (i.e. time in ambulance) and total time to the paediatric intensive care unit
The PICANet audit database records the date and time of arrivals and departures of transfer teams at various different locations that make up the transfer journey. These data relate to PCCTs only, and no equivalent data are collected for the other teams that might complete the retrieval. From these times, it was possible to calculate the durations (in minutes) at different stages of the journey to the PICU, including time to bedside (i.e. the interval time from departure time from PCCT base and arrival to the referring hospital), stabilisation time (i.e. the interval between time of PCCT arrival and departure at the referring hospital), journey time (i.e. the interval between PCCT departure at the referring hospital and arrival at the PICU) and, finally, total time to PICU (i.e. the sum of the previous three intervals). The median times for each of these stages for all participants with PICANet data are reported in Appendix 25. There were no significant differences in the median times for journeys with and without linked parent questionnaire data (Mann–Whitney z-scores ranged from–0.085 to 0.81 and p-values ranged from 0.419 to 0.933).
Association between PICANet transfer time intervals and parent experience
Time intervals were correlated with the family transfer experience scale. Appendix 26 shows that there is a small but significant negative correlation for the time periods ‘stabilisation’ and ‘patient journey time’ and experience score, suggesting that the longer these periods were the less positively parents’ reported their transfer experience. There was no significant relationship between time to bedside and parent experience.
Qualitative description of parent and staff perceptions of timeliness
Timeliness factors influencing arrival of paediatric critical care transport teams and access to paediatric intensive care: parent and staff perspectives
Seven main factors influencing timeliness of access to PIC at each stage of the transfer process were identified (Figure 15), starting at the referring hospital prior to the retrieval team’s arrival through to the arrival at the PICU (Figure 16). A number of barriers and facilitators that influenced the likelihood of the factor affecting the retrieval process were also identified by staff and families, and these are summarised in Figure 17 and described in more detail below, with illustrative quotes.
FIGURE 15.
Model illustrating the factors that influence timeliness of access to the PICU from parent and staff perspectives.
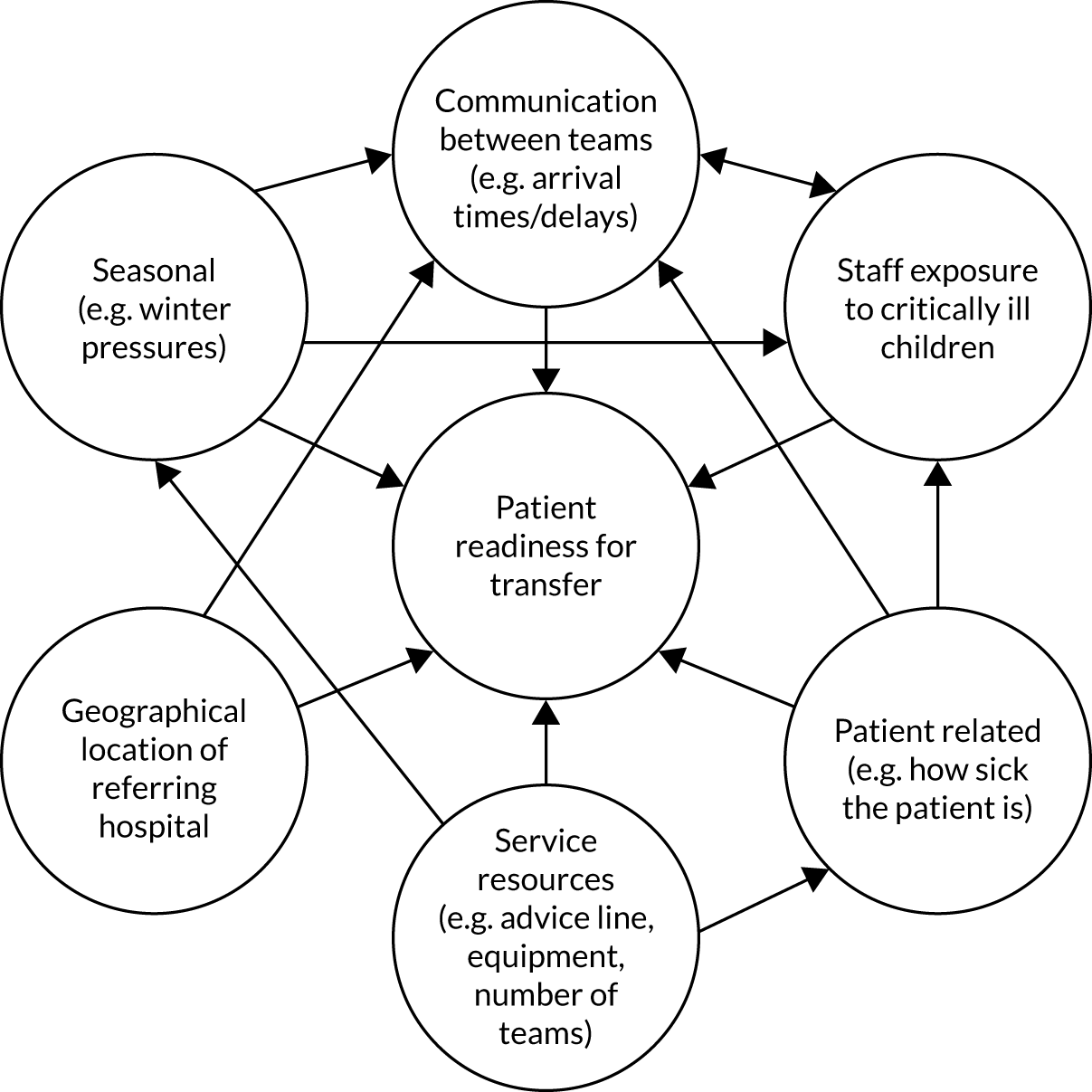
FIGURE 16.
Map of factors influencing timeliness of access to the PICU, starting at the referring hospital prior to the retrieval team’s arrival through to the arrival at the PICU. ICU, intensive care unit; RH, referring hospital.
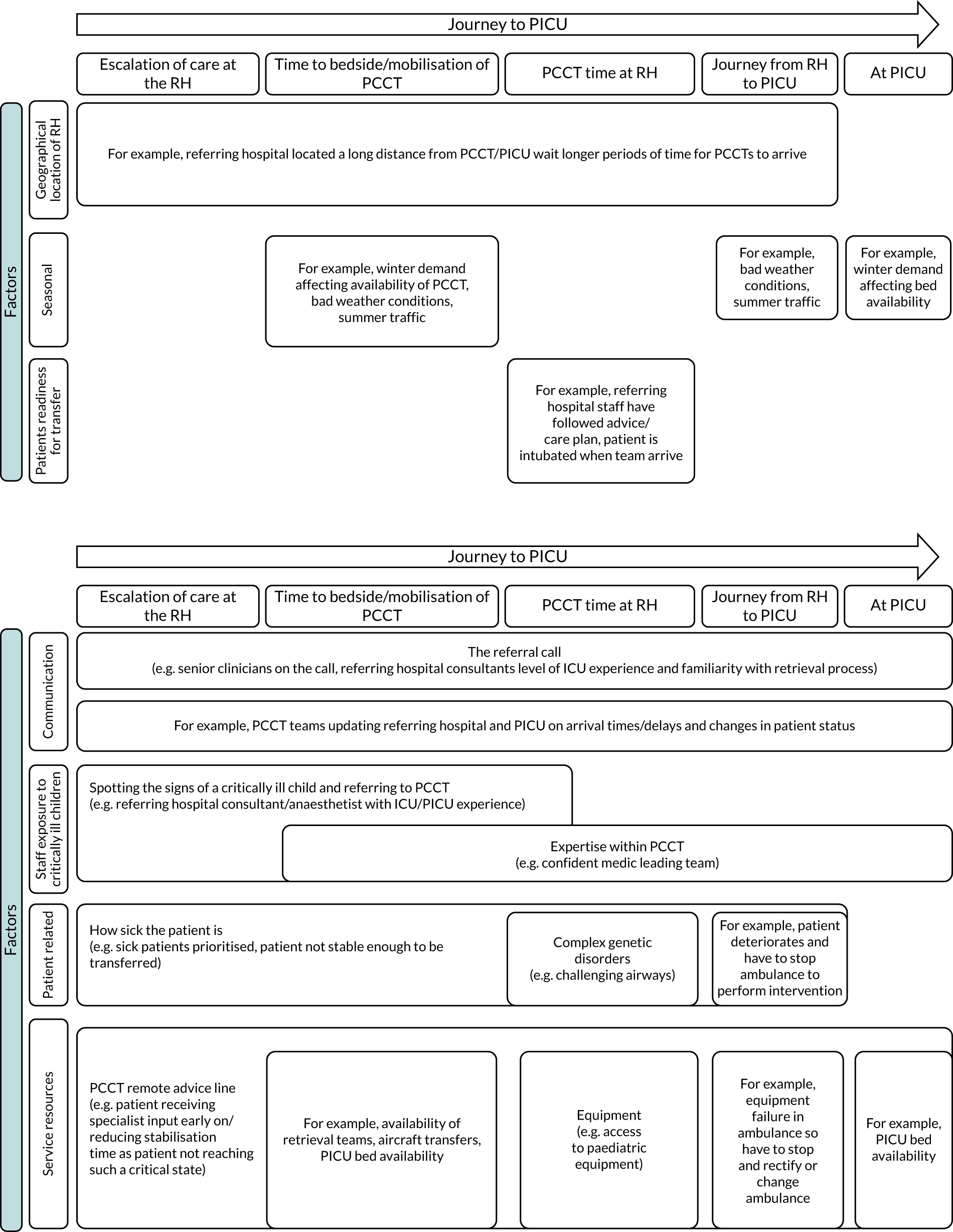
FIGURE 17.
A summary of staff- and family-identified barriers and facilitators that influenced the likelihood of the factor affecting the retrieval process. AICU, acute intensive care unit; HDU, high-dependency unit; ICU, intensive care unit.
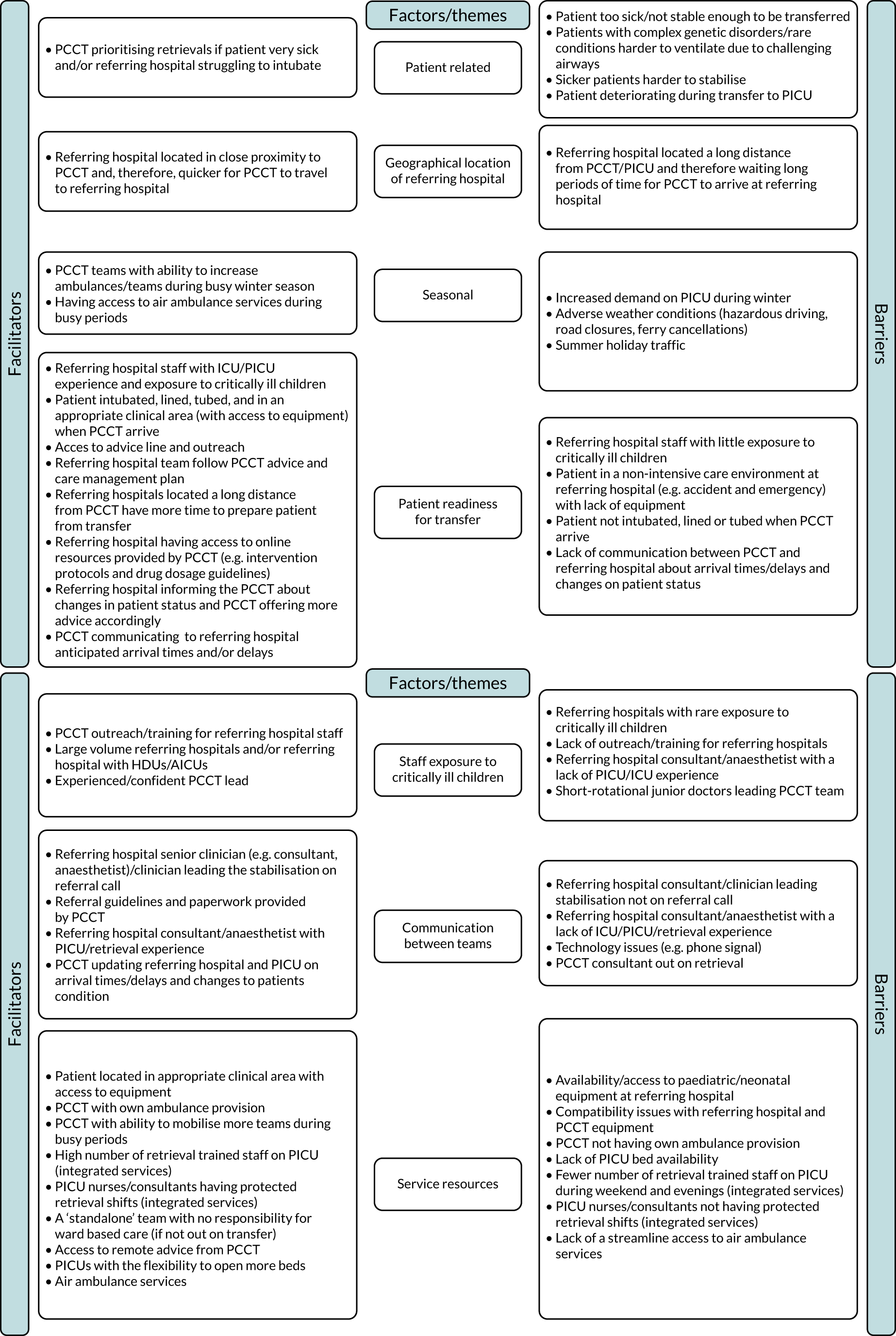
Both families and staff highlighted the importance of efficient escalation of care at the referring hospital to allow specialist input as soon as possible and to prevent delay of receiving PIC (i.e. at the referring hospital) and definitive care (i.e. at the PICU).
The level of exposure to critically ill patients varied across the referring hospitals. Some staff reported that they look after this type of patient only two or three times a year and referred to this affecting their ability to spot the signs of a critically ill child, whereas staff in ‘higher-volume’ sites [e.g. those with high-dependency units (HDUs)] reported greater confidence in identifying children needing escalation because of their greater exposure to the ‘sicker’ patients:
. . . making the decision that they’re critically unwell and that they might need PICU is a tricky thing . . . what makes it tricky for us is, we see a small volume, so we don’t make those decisions as often as big places do.
S13 referring hospital consultant
In addition, characteristics of referring hospital staff, particularly consultants and anaesthetists with intensive care experience, and staff who receive education and training (outreach) from PCCTs, as well as the patient being known to the team can facilitate escalation of care:
They [referring hospital] have specific training, outreach, support . . . They recognise a sick child much quicker than they did 15 years ago, so therefore they resuscitate that child properly and they’ve all got a plan.
S45 PICU nurse
. . . one of the doctors that knew him very well from [PICU] was on a rotation . . . so could see that he needed acting on.
P2 father
Families’ experiences of the speed of their child’s escalation of care varied. Some families described the decision to transfer as ‘slow’, with lots of discussion between staff with regard to a diagnosis and a treatment plan, trialling of interventions and waiting for senior clinicians to assess the child:
There was so much umming and ahing for hours in A&E [accident and emergency] whether he was going to go up to the ward or be transferred.
P5 mother
However, in contrast one mother stated:
. . . the team at [referring hospital] reacted really quickly. . . . they were really quick to recognise that he just was getting worse and worse.
P21 mother
The impact of a delay in a child’s escalation of care at the referring hospital was described by families as distressing and confusing, particularly if there was a lack of communication from the referring hospital staff about the child’s condition:
So, there was lots of to-ing and fro-ing and lots of people not really knowing what was going on . . . lots of different specialists coming to see him and lots of different doctors and nurses coming in and out and no-one really talking to us that much . . . at that point, both myself and my husband were quite confused and upset, because just nobody seemed to really know what was going on.
P12 mother
In contrast, some families praised the quick decision-making and competence of the referring hospital team, with one mother having ‘no doubt, that had they not reacted as quickly, well, who knows what would have happened?’ (P21 mother).
Staff working in referring hospitals located far from a PICU/PCCT reported having to wait longer for PCCTs to arrive and suggested that this made them quicker to trigger a referral:
So, timing and just sitting, watching and waiting and seeing over a couple of hours is not a luxury we have often, whereas I think if you’re in a [location closer to PICU], you can go, ‘OK, we’ll try this. We’ll give drug A or we’ll give fluid A’.
S13 referring hospital consultant
PCCT staff described the referral call as ‘the starting block’ of the retrieval process and, as PCCTs do not have ‘eyes on the patient’ until they arrive at the referring hospital, gaining accurate and detailed information about the patient is paramount to a smooth and timely transfer:
. . . if your referring hospital hasn’t given you all the information, or has misinterpreted some information, the starting block can be incorrect, and can, obviously, have an influence on what the retrieval team has to do.
S48 PICU nurse
Guidelines and paperwork provided by PCCTs were reported to facilitate a ‘structure’ for the staff to follow during the referral, which made for a more efficient referral:
. . . to try and make the structure of the phone call easier, I think most of the retrieval services have pre-filled forms . . . it prompts you over all the information that you need . . . It’s all clear, concise, you get all the correct information, and agree a plan.
S14 consultant PCCT/PICU
Having the senior referring hospital clinician – who is leading the stabilisation – on the referral call rather than relaying information through another colleague was reported by PCCT staff to facilitate communication and to ‘minimise miscommunication’.
Referring hospital staff described a ‘good response time in terms of when the phone is picked up, and how the information is relayed’ (S5 referring hospital registrar) as important when contacting the specialist team, although one example of a factor that can cause delay was difficulty in accessing specialist input from a PCCT consultant because they were out on retrieval and lost phone signal:
There isn’t necessarily someone specifically available for advice calls . . . it might be people in the back of an ambulance. You ring through, it’s a dodgy mobile line, they’re in the back of an ambulance.
S26 referring hospital consultant
The number of teams and ambulances varied across PCCTs, ranging from one to four teams at any one time, with some PCCTs having resources to increase teams during busy periods (e.g. winter). In some PCCTs, a rapid response vehicle was available to get a consultant to the referring hospital quickly if their skills were required or to release the team if they were at the end of a shift. At the time of the DEPICT study, one team did not have its own dedicated ambulance, relying, instead, on local NHS ambulance service provision, an arrangement that was described as causing additional work and delays relating to contacting and organising an ambulance, conveying specialist equipment to the ambulance and lack of availability in busy periods.
The speed of mobilising a team for integrated PCCT services (where staff work on PICU until needed) was reported to be influenced by a number of factors, including PICU demand at the time, time of day (with less staff on shift at night and at weekends), the number of retrieval-trained staff on the PICU at the time, whether or not the retrieval nurses and consultants had protected time for retrievals (i.e. supernumerary to the patient care roster) and whether or not the consultant was on call and had to be called in from home. Mobilisation was suggested to be longer when staff needed to handover patient care because they were not supernumerary. A ‘stand-alone’ team with no responsibility for ward-based care was suggested to be quicker to mobilise (if not out on transfer).
When PCCTs travel long distances to retrieve a patient, PCCT staff reported higher levels of fatigue and described a feeling similar to ‘jet lag’, which can impact the efficiency of subsequent transfers. Long-distance transfers (as well as spending long periods of time at the referring hospital) were reported to impact the number of transfers possible in a shift and could cause shifts to overrun, potentially meaning that staffing levels the next day were too low to allow sufficient rest breaks:
. . . we ensure, that our staff have a minimum of eleven hours off between shifts, because of the driving regulations and working time. So, for example, they finish at 2 o’clock in the afternoon, they won’t come in until 2.00 a.m. the following morning, but then it has a knock on impact on the service, because you are short of a driver for that period of time to allow them to have the sufficient rest breaks.
S17 ambulance technician
On occasion, PCCTs reported using air ambulance services to reduce transfer time when covering large distances to reach the PICU and some PCCTs suggested that increasing the use of air transfer was beneficial to the speed of access to PIC for patients and to increasing efficiency for PCCTs:
Why is it always on the roads? [Air travel] that would be my optimal, that they would get here quicker and children would get transferred quicker. You know, I just think it would be safer to get them there quicker. On the unit . . . they would be down quicker. I think they could do more transfers if that happened.
S35 referring hospital nurse
I was there and back in 5 hours. So, dramatically different impact on the team, you know. The team are not out of region for long. It’s much better. You don’t have as quite a numb bum, basically, from sitting down for hours in the back of an ambulance.
S24 PCCT nurse
However, this view was challenged by some PCCT staff who described the additional practical (e.g. different equipment needed, transfer to safe landing site) and administrative processes involved in air transfer:
Sometimes, taking a helicopter or a flight, it’s supposed to save time and it doesn’t. Sometimes, it’s quicker to get there by road because you’ve got the time delay of getting the flight . . . Then, you’ve got to transfer all the equipment onto the aircraft that you’re taking. So, all that time does add up and the time you’ve waited for the vehicle to come.
S1 PCCT ambulance technician
Locating a PICU bed was cited by both staff and families as a challenge that can cause delays in the PCCT arriving. Some teams described not mobilising until a bed was located and PCCT staff reported going to the referring hospital without a confirmed bed to support patient care if they were ‘struggling’; however, this was suggested to be rare and ‘risky’, as the time could be used to transfer another patient:
Although it all escalated quite quickly, the actual transfer itself did take a fair while. I think she must have been in the one in [referring hospital] for 3 hours . . . trying to get a bed and then when they had to get a bed, they had to wait for the transport team to come, for an ambulance to be booked.
P20 father
During winter (i.e. between October and March), staff reported higher volumes of ‘sicker children’ needing a PICU, which was linked to higher bed occupancy and, therefore, finding a bed was more time-consuming. Some PICUs reported continually running at capacity, with no ‘summer lull’. PICUs’ capacity to open more beds was suggested to vary. Some units had the capacity to open more beds by shifting staff from other parts of the hospital (e.g. HDUs), whereas other units did not have this flexibility. Finding a bed could be time-consuming:
. . . our doctors will spend hours on the phone to various different hospitals.
S5 referring hospital nurse
A lack of a nearby PICU bed could lead to out-of-region transfers, which increased travel time both to and from the referring hospital and then back to base, causing delays to subsequent transfers:
The winter pressure makes it extra difficult because your transport teams are busier, but also the PICU beds are at capacity. So, you know, they can go out and get a patient, but they might have to take a patient a long way to find a PICU bed.
S19 PICU consultant
I’ve done a long-distance transfer where the decision to move the child happened later on in the day . . . I said to them, ‘If I do it, I’m not coming in tomorrow’ . . . if you are undertaking a long transfer, you will be unsafe to be back on duty the next day.
S24 PCCT nurse
The weather (e.g. fog, high winds, snow) in winter could also affect travel time; however, summer also affected PCCT journey times when retrieving from tourist areas.
Parents reported increased anxiety and frustration if there were delays in the retrieval team arriving, particularly if parents perceived the referring hospital team to be struggling and perceived that their child’s condition was deteriorating or could deteriorate if they waited any longer. Some parents attributed the delay in the retrieval team arriving to further deterioration and to subsequent challenges with intubation:
. . . when the severity of his illness was more and more apparent, and his deterioration was more apparent, then it [timing of PCCT arrival] became a real worry and then, actually, when he did come to get moved, it was quite an ordeal to put him under . . . and it just made everything worse.
P2 father
Fears around not being retrieved were also present among some families:
Obviously when they said there were only two ambulances covering the whole of the [region], that worried me, because I thought, ‘It could be hours until he’s transferred’. I was very worried that he would deteriorate and be stuck at [referring hospital] and there would be no one available to move him.
P5 mother
One parent described her distress while waiting all day for the PCCT to arrive and eventually being transferred by the referring hospital team because there was no transport team available. The parent’s trust in the referring hospital safely transferring her child was challenged because she was initially told that the specialist team was required to safely transfer her child:
So, it was like, ‘We absolutely have to have the transfer team because she will not survive in a normal ambulance’ . . . Then the next minute . . . They were saying that the specialist transfer team were not available, so they would have to prepare an ordinary ambulance and make sure that they had everything to go with her. It just took all day . . . I just don’t understand why a transfer wasn’t sooner.
P11 mother
The need for the referring hospital to mobilise a team because of a lack of available PCCTs was described by staff as a rare event and dependent on the expertise and availability of the team at the time:
There has been occasions where the [PCCT], they’re too busy, they don’t have a retrieval team, where we’ve said, ‘OK, we’ll put into motion’ . . . But that’ll be done, again, with a shared decision-making process, and luckily, we haven’t had to do that for a while.
S27 referring hospital consultant
Clear communication about the process of a transfer and the arrival time of PCCTs was especially important. However, some families felt that they were not updated sufficiently about the expected arrival times or delays, which caused further distress and confusion:
I think from the point on Thursday at which we were diagnosed, there was the potential that we were going to be moved every hour . . . We were just, kind of, waiting. So, you know, every time someone comes around, you’re, kind of, asking, ‘What’s happening next?’ and nobody can tell you.
P7 mother
One family reported that they were not informed that the PCCT had arrived and learnt that they had arrived and were in theatre with their child through asking a referring hospital nurse for an update:
Every time we saw a blue light, we thought, ‘Oh, I wonder if that’s the ambulance’, and then when they did arrive, we weren’t told . . . because it had been that long . . . we tried to find someone to ask had they heard, and then they had to ring the theatre and say that the team were there and working on him.
P21 mother
For referring hospital staff, communication from PCCTs about arrival times and delays was important so that staff could prepare for a potentially long wait, staff could be allocated efficiently and the child could be moved to a safer environment within the hospital [e.g. acute intensive care unit (AICU), HDU]:
They keep phoning us and they keep us very well informed. So, we’ve never been in a situation where we’re just waiting, they give you the exact situation . . . Which helps greatly, so you are not left not knowing, you are left knowing, but you know that it is going to be a long night.
S32 referring hospital consultant
Delays to PCCTs arriving at the referring hospital were described as putting ‘a huge amount of stress on the internal workings of the hospital’ (S27 referring hospital consultant) due to the need to pull senior staff from the wider hospital to help with the care of the patient until the patient is retrieved, causing delay to patient flow across other parts of the hospital (e.g. increasing accident and emergency waiting times, delays in triage) and the need to cancel or reschedule operation lists:
. . . once I have a sick child, if I’m on call and I’m the only consultant after-hours, I’d stay with the child, I can’t leave the child. In the system, we may have an elderly patient who needs an urgent operation and they will be on hold because probably, my registrar team doesn’t have the capacity to handle the very sick patient, so this leads to delay in the care for another patient.
S6 referring hospital consultant
The impact was described as greater during the weekend and at night because of the smaller number of staff across the hospital. If care of a critically ill child was needed throughout the night or delays caused shifts to overrun, then it could impact the service the following day:
. . . the quicker the process can be, the less impact it’s going to have . . .during the weekday, generally, there’s enough, senior people, extra numbers around that can come in and help out as needed. Certainly, nights and weekends, a child being retrieved has a huge impact on the service.
S4 referring hospital nurse
There have been situations where the service next day has suffered because the consultant who’s been through the night frankly says, ‘I really cannot carry on. I need to go home’, which is absolutely fine, but that makes the workload for the people on the floor the next day a lot more busier.
S32 referring hospital consultant
The impact of delays to PCCTs arriving at the referring hospital is lessened among referring hospitals that are better equipped to look after critically ill children, such as hospitals with a higher demand and a higher level of exposure (i.e. the bigger hospitals), hospitals with a capacity to use HDUs and AICUs resources within the hospital and hospitals with higher staffing levels and a higher level of training for staff:
I think we look after probably between 50 and 60 children a year here of that level of acuity. So, we have quite frequent interaction I suppose with [PCCT] . . . we have to look after them for longer because it takes them longer to get to us . . . and therefore looking after those children for longer means that our medical and nursing are probably more comfortable to look after a child for another 8 or 10 hours if that’s all it might take.
S34 referring hospital consultant
Where the child was looked after until the PCCT arrived, and when there was also a lack of access to AICUs and HDUs, was also described to affect wider hospital function (e.g. the impact on surgery lists if child was looked after in an operating theatre):
. . . you are taken away for a few hours to look after that sick child in the theatre recovery area, or the theatre annexe, because we don’t have the, sort of, access to the other intensive care bed . . . Normally that’s why we have a huge backlog.
S32 referring hospital consultant
Preparation and planning at the referring hospital were seen as integral parts of the retrieval process by the PCCT staff, as they directly affected the amount of time the team spent at the referring hospital. The PCCT staff described the ‘ideal transfer’ to be when the referring hospital team had followed the advice/care management plan and, therefore, when the PCCT staff arrived at the referring hospital the patient was ready for transfer ‘perfectly packaged’ [i.e. intubated, lined and tubed, in an appropriate area (e.g. intensive care environment with access to equipment) and paperwork completed] and, therefore, minimal clinical input was required by the PCCT. There was some variability across PCCTs in terms of expectations of the referring team intubating the patient before the team arrived. Some PCCTs generally did not mobilise until the patient was intubated:
There might be times when you arrive, and the patient hasn’t been intubated, or they don’t have lines in, or they haven’t had certain medicine started . . . the ideal really is that the referring hospital does the stabilisation bit, and you as the retrieval service, sort of, arrive and move the patient, so that then you’re free to go and move the next patient . . . It just takes longer.
S14 PICU/PCCT consultant
Whether or not a patient was prepared for transfer was reported to be influenced by a number of factors, including the level of intensive care unit experience of referring hospital staff and their exposure to critically ill patients; the geographical location of the referring hospital (e.g. referring hospitals located a long distance away from PCCT/PICU typically waited longer for the PCCT to arrive and, therefore, had more time to prepare the patients for transfer); access to online resources (e.g. drug dosage guidelines) and protocols for interventions provided by the PCCT; communication between the teams (e.g. referring hospital informing the PCCT about a change in patient status and PCCT offering more advice accordingly and the PCCT ensuring that the referring hospital were aware of anticipated arrival times and/or any delays); and access to outreach/training provided by the PCCT:
. . . one of the major benefits that both parties get out of it [referring to outreach] is the fact, they learn more of how to use the service, but also to know what to prepare for us for our arrival. Which, obviously then has an impact on our resting time at the referring hospital.
S48 PICU nurse
When PCCTs arrived at the referring hospital and the patient was prepared for the transfer (with minimal interventions to perform) there was more time for PCCTs to (1) establish relationships and build rapport with families, (2) gather more information about the child from the families and referring hospital staff and (3) to talk families through the retrieval process, which was perceived by staff to help instil trust and reduce anxiety among families:
. . . the patient was really perfectly packaged by the time we got there, we could just take over care and not have to jump in and do lots of things, and make that a really smooth transition for the family, making sure that they were fully up to date . . . we could actually make sure that there was time to explain exactly what we were doing and how the process was gonna run.
S11 PCCT/PICU nurse
There was consensus across staff that a child receiving specialist input at the referring hospital and definitive care at the PICU as quickly as possible were important for patient outcome. Although ensuring the safety of the patient prior to retrieval took priority, PCCTs had divergent perceptions about how much time should be spent at the referring hospital administering care:
. . . there is no doubt that the earlier the paediatric intensive care team are presented by the team that are involved, the better the outcome and the better the management of the child. There is no doubt about it because they are more skilled than the local team.
S32 referring hospital consultant
. . . transport is all about transporting them from A to B. It’s not about providing mobile intensive care a lot of the time, it’s about getting them to the best place as soon as you can. Sometimes it is about doing that, sort of, mobile ICU [intensive care unit] thing, I suppose, but I think mainly it’s just trying to make them as safe as possible and balancing those risks.
S42 PICU/PCCT registrar
One staff member challenged the emphasis that their team had on the time spent at the referring hospital:
We wouldn’t move anyone who wasn’t safe to move but we just seem very conscious of time and I don’t always feel that, as an example, being in and out of referring hospital within an hour is a marker necessarily of success. I think, if you have a sick patient, getting them to definitive care is important but they always have to be safe to transfer.
S10 PICU/PCCT registrar
The team structure of some PCCTs was that medical fellows led the team on retrievals after an induction period. Medical fellows could be transient, typically working in the role for 3–6 months, and they varied in their prior clinical training specialties (e.g. paediatrics, anaesthesia) and prior exposure to acute paediatric medical situations. Some referring hospital and PCCT staff described how if a new medic or rotating fellow was leading the team, then more time could be spent at the referring hospital due to interventions taking longer (particularly stabilisation) and other staff members having to support and teach the medic:
There are times where it didn’t really go very well because the [PCCT] fellow, sometimes the [PCCT] fellows are quite inexperienced. And if they’re inexperienced then stabilisation, getting the child out could be very complicated. And sometimes, they spend about three or four hours here.
S44 referring hospital consultant
Both families and staff reported that availability of particular neonatal/paediatric equipment (e.g. paediatric intravenous therapy lines and ventilators) at the referring hospital could limit the capacity of referring hospital staff to prepare and stabilise the patient and, therefore, increased what the PCCT had to do when they arrived. Access to equipment was reported to be influenced by where the patient was located in the referring hospital (e.g. ward, theatre, ED, paediatric department) when the PCCT arrived and whether or not the referring hospital staff stayed and helped locate the required equipment:
It works well when you arrive in hospitals and there are people available that know where to find things that you ask for. There’s a very different experience to some hospitals where you’re looking around for things, you can’t find what you need.
S15 PICU/PCCT consultant
Some families described waiting for particular equipment to become available across the wider hospital or waiting for equipment to be delivered from other referring hospitals. Compatibility issues between the referring hospital and PCCT equipment (e.g. ventilation leads/masks) was also reported by families and staff to cause delays:
. . . the only other thing, that just frustrated us, is equipment. They would often turn up with – , might not necessarily be the right stuff for [patient’s name]. So, because he was on the CPAP [continuous positive airway pressure], like, the wrong-size mask . . . One time they were expecting him to have i.v. [intravenous] access. He didn’t. So, again, that just added to the time getting him into the ambulance.
P1 mother
Paediatric critical care transport team and referring hospital staff reported that the remote advice service offered to the referring hospitals had reduced stabilisation time and the number of transfers required, as the staff were receiving specialist input early on and, therefore, the patients were not reaching such a critical state:
I think we transfer far less patients now than we ever did, and our stabilisation time is much, much quicker because they know what they’re doing, they know what the plan is, they know they can, and they’ve been liaising with us.
S9 PICU/PCCT nurse
Patients with complex genetic disorders and rare conditions were reported by both PCCT and referring hospital staff as being harder to ventilate because of challenging airways, which could increase the length of time the PCCTs spent at the referring hospital. ‘Sicker’ children were also sometimes reported to take longer to stabilise:
. . . children who may have syndromes, genetic diseases that are really difficult to manage clinically for anybody . . . These are challenges to everybody, but then, we have syndromes who may have, for example, difficult ventilation issues, [congenital or structural abnormalities] that will make it really hard to manage.
S6 referring hospital consultant anaesthetist
It’s only really the children that are very sick now that you have to, sort of, stay and do a lot more stuff with the team with.
S45 PICU/PCCT nurse
Paediatric critical care transport team staff described rare occasions of having to stop the ambulance on route to PICU if the child’s condition suddenly deteriorated and they needed intervention or if there were issues with equipment, which caused delays in reaching PICU:
Those are the ones that don’t go so well when you have to then say, ‘Please stop somewhere safe as possible and as soon as possible’, . . . and that’s either because a cannula has fallen out or a child has suddenly deteriorated a little bit.
S45 PICU/PCCT nurse
Having to stop on route to the PICU because of issues with equipment or to perform an intervention on the patient was stressful for the team, as they had to wait until they could stop to administer critical care, often in potentially dangerous environments (e.g. side of the road):
. . . If something unexpected goes on, you can’t get to the stuff without stopping and opening a door . . . But you want it now because the child is doing something funny, but it’s going to take 30 seconds at least to get. So, that time lag is always a bit uncomfy and that time lag can actually have quite a significant impact . . . you end up in a bit of a farm gateway or on a hard shoulder or in a lay-by and you have to do some fiddling, and that is always much more stressful . . . being on the side of the road is a vulnerable thing.
S45 PICU/PCCT nurse
The time it took to travel to the PICU was significant for families, particularly if they did not travel in the ambulance with their child because of fears of not being present if anything happened during the journey and not finding out until they arrived at the PICU, as well as the perceived risks to their child associated with travelling long distances:
So I was slightly concerned because I know there’s risks when they’re being transported and it was so far away. I obviously would have much preferred [specific PICU] because it’s only about 35 minutes away as opposed to over an hour . . . I was just really worried that something was going to happen to him whilst he was being transported.
P21 mother
Parents’ perceptions about the length of a journey varied. Some parents reported a journey being quick, whereas other parents reported a similar length journey as being long.
How prepared PICU staff were for the arrival of the patient relied on the PCCT updating PICU staff on arrival times, delays and any changes in the patient’s condition:
Good communication channels between both ends of the service . . . I think that communication always makes a difference to how ready we are.
S36 PICU nurse
Paediatric critical care transport teams updating PICU staff on arrival times and delays (as well as any changes in the patient’s condition) was reported to affect how prepared PICU staff were for the arrival of the patient. When the PCCTs arrived at the PICU with little or no warning, then the process could become rushed, as a bed space and staff had not been allocated and drugs and equipment had not been prepared. Consequently, this could slow down treatment and affect the wider unit, and staff perceived that this might affect how parents perceived the PICU staff and the care that their child was going to receive:
I think that communication always makes a difference to how ready we are . . . if you’re all ready, and make it look like a competent service, they’re confident that their children are in safe hands because, you know, we’re fully prepared.
S36 PICU nurse
The quality and efficiency of handovers at the PICU were influenced by the experience level of the PCCT lead. When a junior doctor led the team the handovers were described as sometimes being ‘muddled’, lacking detail and taking longer:
If it’s all over the place, it just takes longer, and it’s a bit confusing, but when it’s smooth, it works really well, and you can see people who’ve had experience in retrieval so much, from people who haven’t had experience. Especially when they have new retrieval leads, who are quite new into the service, they require quite a lot of support and prompting about what they should hand over, what they shouldn’t hand over.
S22 PICU nurse
Paediatric critical care transport teams usually delivered children to PICUs in their region and were familiar with the physical location of these PICUs and their working practices; however, there were occasions when PCCTs travelled to PICUs that were out of their region and these PICUs were, therefore, less familiar. Familiarity could influence the cohesion and flow of the transfer when the PCCT was familiar with the systems, protocols and paperwork and, therefore, knew how to prepare a patient for that particular PICU.
Aspects of care
A second key objective of the DEPICT study was to explore how variations in how care was organised influenced experiences. In particular, there was a focus on team composition; working relationships within teams, as well as the wider care network (referring hospitals and PICUs); the influence of critical incidents (i.e. events where there were errors or issues with care delivery, e.g. equipment failures or cardiac arrest); and, finally, the involvement of parents in the transfers, including whether or not parents were able to travel with their child in the ambulance to the PICU. Parental questionnaire responses, PICANet audit data and qualitative interviews were used to describe experiences and explore associations between these aspects of care and satisfaction.
Team composition
Perceived team lead
Families were asked about who led the retrieval team and were offered five response options and an accompanying free-text option to give additional information. The majority (46%) of team leads were identified as a doctor, with just under 20% of team leads perceived to be led by a nurse. Some of the respondents who indicated ‘other’ wrote in the free-text comments that there was no leader, as they were either told, or perceived, that the clinicians work as a team, for example ‘they told me they work as a team and there isn’t really a leader’ (P44, mother) and ‘didn’t appear to be a leader. They worked as a team!’ (P45, mother) (see Appendix 27).
Association between team lead and satisfaction/experience
There was no difference in satisfaction between families who reported that the transfer team was led by a nurse or a doctor; however, the proportion of families rating the highest levels of satisfaction was lowest when families reported that they were not told or if it was not clear to them who the team lead was.
Similarly, there were significant differences in the parent experience scale score. Although the median score was 5 for all groups, there was a significantly different IQR score of 4.56–5 for the group that reported not being told who led the team (Tables 19 and 20).
| Measure | Who led the team?, n (%) | Group difference (Kruskal–Wallis) | ||
|---|---|---|---|---|
| Nurse | Doctor | Was not told | ||
| If my child was ever in this situation again then I would like them to be transported by this team again | ||||
| Disagree at lot | 0 (0) | 3 (0) | 1 (1) | χ2 (df 2) = 69.70; p < 0.0001 |
| Disagree a bit | 1 (0) | 4 (00) | 1 (1) | |
| Neither agree nor disagree | 3 (1) | 7 (1) | 13 (6) | |
| Agree a bit | 21 (5) | 23 (2) | 26 (13) | |
| Agree a lot | 362 (94) | 913 (96) | 165 (80) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | ||||
| Disagree at lot | 1 (0) | 3 (0) | 0 (0) | χ2 (df 2) = 71.91; p < 0.0001 |
| Disagree a bit | 0 (0) | 4 (0) | 4 (2) | |
| Neither agree nor disagree | 4 (1) | 5 (1) | 14 (7) | |
| Agree a bit | 14 (4) | 20 (2) | 20 (10) | |
| Agree a lot | 369 (95) | 914 (97) | 168 (82) | |
| Overall experience of the transport service | ||||
| Extremely poor | 0 (0) | 0 (0) | 1 (0) | χ2 (df 2) = 72.82; p < 0.0001 |
| Poor | 2 (1) | 5 (1) | 1 (0) | |
| Fair | 2 (1) | 9 (1) | 9 (4) | |
| Good | 35 (9) | 59 (6) | 47 (23) | |
| Excellent | 348 (90) | 876 (92) | 148 (72) | |
| Overall satisfaction with the transport service | ||||
| Extremely poor | 0 (0) | 0 (0) | 1 (1) | χ2 (df 2) = 83.784; p < 0.0001 |
| Poor | 0 (0) | 3 (0) | 2 (1) | |
| Fair | 4 (1) | 7 (1) | 6 (3) | |
| Good | 35 (9) | 51 (5) | 48 (23) | |
| Excellent | 346 (90) | 885 (94) | 148 (72) | |
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group statistic (Kruskal–Wallis) | |
|---|---|---|---|
| Median | IQR | ||
| Nurse (n = 346) | 5 | 5–5 | χ2 (df 2) = 45.23; p < 0.0001 |
| Doctor (n = 840) | 5 | 5–5 | |
| Was not told (n = 145) | 5 | 4.56–5 | |
PICANet leader grade
The PICANet audit database collects data on who led the team, classifying staff as ANP or medical staff [i.e. consultant grade (associate specialist/staff grade) or grades ST1–3 and ST4–8]. The categories of ST1–3 and ST4–8 were merged to form ‘junior doctor’, as there were only five instances of grade ST1–3 staff leading the team (see Appendix 28). The proportions of family-reported medical and nurse leads were broadly similar to the PICANet data. There were no significant differences in the proportions of the three staff groups (i.e. consultant, junior doctor, ANP) for journeys with and without linked parent questionnaire data [χ2 (df 2) = 0.539; p = 0.764].
Association between PICANet leader grade and family transfer satisfaction/experience
More parents rated the highest satisfaction categories when an ANP led the transfer compared with the two medic groups (i.e. consultant and junior doctor), but the differences in proportions rating each satisfaction category for each staff group was not significant. Although the median experience score for each of the staff groups was 5, there was a significant difference in the distribution of scores with an IQR of 4.89–5 for the consultant group compared with an IQR of 5–5 for the other two groups, indicating a greater variability of parent experience score for the consultant group (see Appendices 29 and 30).
Qualitative description of parent and staff perceptions of aspects of care
Perceptions of team composition
Staff described the importance of familiarity (as well as level of experience) between staff within PCCTs, as it facilitated ‘good teamwork’, which made for a smoother transfer. Effective team working was described as happening when staff had trust in their peers’ skills and each team member knew their individual role and what was expected of them:
. . . you know what each other are thinking so we just get on with our jobs and it just, like, flows really easily.
S1 PCCT ambulance technician
For me, it’s knowing your team members and knowing what their skills and their capabilities are, and being able to communicate with them effectively. The retrievals that generally go most smoothly and most quickly are the ones where I’m working with a nurse and a technician who have worked on the service for a long time. That’s because we all just kind of slip into – it’s almost like autopilot because we’ve done it so many times, it’s second nature. Everything just happens. The ones where it’s more challenging, not necessarily bad teamwork, but it’s not as smooth, and you feel it’s not as smooth when you get new team members in and they’re still learning.
S18 PCCT ANP
Competent medics were identified as part of a good retrieval:
I was out with a very competent medic, which always makes a big difference . . . Clinically there was a lot to be done, but we were in our comfort zones with the procedures that we were doing.
S38 PCCT nurse
When PCCTs leads were less experienced (e.g. junior doctors on short rotation) PCCT staff described the need for other team members to support the lead during the transfer, with more experienced staff, such as ANPs and nurses, playing a key role in this. Some nursing staff preferred ANP-led retrieval because it was more consistent with the role and their skill set:
. . . when they have new retrieval leads . . . they require quite a lot of support and prompting, when they’ve had a new medic on the retrieval service, and a nurse practitioner is the one leading, they often have a better transition about how to retrieve than they do when they have it medic to medic.
S22 PICU nurse
Referring hospital staff reported how a perceived lack of confidence in the PCCT lead can affect how ‘smoothly’ a transfer goes and can affect how much involvement the PCCT lead requires from senior referring hospital staff (e.g. with managing ventilation):
I think the only reason why I would say it went less well, I think maybe the [PCCT] fellow . . . I’m not sure if it was maybe one of the first transfers that he was doing. I think things were just . . . not as smooth as you would expect. The child was not compromised in any way. The child was managed very safely . . . I think it was just the confidence in terms of managing the ventilation to the child which actually, the anaesthetic registrar who had intubated the child thankfully stayed for quite a while.
S43 referring hospital consultant
Staff also described that an additional challenge for a new medic was not just getting to know the retrieval system/service, but also getting to know how a particular team worked together, particularly the need to communicate to work as a team rather than autonomously:
. . . the doctors mainly, they work from 2 months, 3 months, 6 months so sometimes, when they come in first of all, it’s them getting used to how the service is running and what their role is expected to be. I’m sure that they know what they’ve got to do but within the system that we run, it’s getting to know how we work as a team as well as them doing their job.
S1 ambulance technician
. . . when new medics come into a retrieval team . . . they have been taught to be autonomous, and treating to cure. Whereas, when it comes to retrieval, it is, you are part of a team, you are treating to transport, but, you must communicate your thoughts, because the thing is, if I don’t know what my medic’s thoughts are, I can’t support them.
S48 PICU nurse
Handovers at PICU could also be more ‘muddled’ when a less-experienced medic was leading the team, as more support was needed about what they should handover:
. . . you can pick up on levels of experience of the staff handing over and some of the more junior medical staff, when they do their patient handover, sometimes they can be quite muddled. On the whole, everyone follows a fairly, kind of, systematic, body-systems approach. . . . but I have had handovers where it’s flipped from one to another, back to the other and you get the impression that, ‘Do you actually know the proper course of events and exactly what’s going on?’
S36 PICU nurse
Parents described good teamwork within PCCTs teams as the team ‘constantly’ communicating with each other and anticipating what might be required of them individually:
. . . one was doing something, and the other’s doing the other thing, and they’re talking to each other constantly.
Yes, it was communication, wasn’t it? I’m sure there was one stage where the doctor asked the technician for something, he’d already prepped it, it was already ready . . . They just worked really well with each other, didn’t they? They communicated. Talking, and also handling us, as well.
Some parents also picked up on less confident PCCT leads, and one mother described them as appearing ‘panicked, faffing around . . . and the aura that he had, he didn’t seem confident at all’ (P18 mother). However, other members of the team who appeared confident and competent reduced any parental concerns about the safety of their child:
I had . . . no trust in the doctor that he was going to keep my son safe, but I had absolutely 100% trust in the other lady. I felt comfortable, but then again, you feel like, that doctor doesn’t know what he’s doing, she’s trying to do everything.
P18 mother
The ANP role is relatively new to retrieval and some staff talked about the challenges of the role not being recognised by some referring hospital staff, although this seems to have improved over time:
. . . in the early days of me going out as a nurse practitioner, leading the team, there often would be a little bit of a resistance and a lack of understanding of my role, and that there was no doctor on our team. Occasionally, we still get that now, but the fact that I do it full-time means that my face is known, and so I know a lot of the consultants in the region . . . So, that helps with the relationship, in that when I turn up they know me, they know what my capabilities are, and we’ve already got a degree of a relationship straight away.
S18 ANP PCCT
Paediatric critical care transport team staff described the valuable role that ambulance drivers/technicians play in supporting the clinical team and in offering support and reassurance to the families while the team were busy with patients:
He [ambulance technician] knows I want her put on the monitor, so as soon as we come in, he’s putting her on our monitor . . . Our ambulance techs do quite a lot of things . . . just things that we need to have for the transfer, so it goes smoothly.
S8 PICU/PCCT ANP
. . . Sometimes when they really come into their own is the support of the parents. If we are really, really busy with the child sometimes they’ll just sit with the parents, because they have enough knowledge to talk them through little bits without going into too much detail, but just there to support them. That can be a big help, I think, to the team, because one of my worries is who’s supporting the parents while all this is going on.
S38 PCCT nurse
However, there was variation across the PCCTs in how involved the driver/technicians were within the team, with some driver/technicians only driving the ambulance. Staff described the challenges associated with drivers not directly being employed by the PCCT and, therefore, not having control over their training and involvement within the team.
Some parents echoed the role that ambulance drivers had on their experience, acting as a liaison with parents, finding out information and relaying it to families when the clinical team were busy, and offering emotional support to the families:
The ambulance driver sat with us, he’d be, kind of, like our go-between, for a general update, getting us cups of tea . . . keep us, kind of, sane. He was brilliant.
P8 father
Working relationships
Paediatric critical care transport team staff described having good working relationships and feeling supported by the team’s consultant back at base (if not out on a retrieval) throughout the retrieval:
. . . never feeling like you’re left doing difficult decision-making on your own.
S2 PCCT registrar
We have a very good working relationship with the consultants and they’re incredibly supportive . . . and you don’t have to justify it, if we ring up and say we’re not happy, that’s good enough for them. You never felt like you’re failing or you should be doing better or it’s not good enough, and they will either just give telephone advice, which very often is enough to get us through, or they’ll come out, it’s as simple as that . . . they will come out if we are struggling.
S38 PCCT nurse
Effective working relationships between the referring hospital and PCCT teams were described by staff. Examples of effective working relationships included when teams communicated clearly with each other throughout the transfer, when the PCCT arrived at the referring hospital and their role was clearly defined and when the PCCT and referring hospital staff worked together:
The fact that the two teams worked well together . . . It worked well, because each body was doing their role, and was then, obviously, it was clear communication, there was nobody talking over anybody. If one person was talking, nobody else was. So, it was good from the point of, people took on an individual role, there was only one leader, and there was clear instruction, and there was closed communication.
S48 PICU nurse
When the teams worked well together it encouraged the referring hospital staff to stay and support the PCCT when they arrived, which facilitated access to medication and equipment:
We have to have a good working relationship with them . . . if we haven’t got a relationship with them and they all disappear, it’s very hard for us to work as a small team of three in a hospital that’s not our own hospital. So, we need them to work with us as well.
S18 ANP PCCT
Both PCCT and referral hospital staff recognised the importance of the PCCTs teams not just arriving and ‘taking over’, but validating and respecting the work the referring hospital staff had carried out prior to arrival:
Instead of being dismissive, and saying, ‘Thanks very much, we’ll just take over now’ and almost ignoring the fact that what we’ve done for the previous X amount of hours, they were, sort of, very engaging.
S33 referring hospital nurse
Staff recognised the importance of the critical care network (e.g. outreach and training/simulation days provided by PCCTs), as it provided an opportunity for learning and knowledge exchange, as well as facilitating good working relationships between the teams. PCCT staff reported that, historically, staff at the referring hospital would not always stay around to support the PCCT when they arrived, but outreach and relationship-building outside a referral appeared to have encouraged a change, with more staff wanting to be involved and, therefore, providing opportunity for knowledge exchange:
Outreach is amazing. So, the face-to-face contact that we provide, it went into the contracting, that we would supply outreach to each hospital annually. Also, we do teaching at base as well, so, they come into us for teaching as well. That face-to-face contact makes a massive difference when you’re actually then engaging over, you know, they’re faced with a sick child and they want advice. Actually having that personal relationship first does really help.
S24 PCCT nurse
I think the training that we do is a huge part because when we get there they say, ‘Oh, hello. Are you all right?’ and they often say, ‘Can we do the takes for you today?’ because you know we’ve done training maybe a couple of months ago and they say, ‘Oh, would you mind letting us do it?’ . . . So actually, we try and involve them in the retrieval . . . what previously happened was everybody would step back and leave us to it. Like I say, that’s not what we want. We want them to be working with us so that then they feel confident.
S9 PCCT nurse
Variability of access to outreach across the referring hospitals was reported, with some staff highlighting a need for more contact (e.g. more than once a year) with the PCCTs outside the transfer and, in particular, a need for regular, bespoke training for referring hospital anaesthetists:
. . . the most important thing . . . is if people are not exposed enough or not trained enough, although they’re doing the job, they do what is necessary but the stress they are getting . . . they will just have difficulties because the stress . . . if you’re working uncomfortably you are, you may make mistakes, you may not do as you would do if you’re comfortable . . . With the help from the retrieval team, with the help from the Paediatric Intensive Care Society is to do, like, regularly refreshing training, specified for us as anaesthetists to manage these cases.
S6 referring hospital consultant
Some PCCT staff highlighted the challenges of providing outreach due to staff availability and the need to pull staff from clinical duties to run the sessions:
I think the outreach education is somewhat hit and miss at the moment. Twofold really, one is it relies on the referring hospital’s requesting dates, requesting outreach sessions, and also the availability of team members to go out because we don’t have anybody in post who, you know, their role is dedicated to education. So, they have to be released from clinical duties . . . so to release team members for that is a challenge every time.
S18 PCCT ANP
Some parents picked up on good working relationships between the referring hospital and PCCTs, and observing team cohesion and rapport reduced parental anxiety and increased trust in the teams looking after their child:
No, I mean like, you’d think they’d of known each other before, because then they were on about the doctor was helping was coughing and they was like, I think you need to be ventilated, and I know like, it sounds like, horrible saying it but it was sort of like, a bit of a laugh sort of. It sort of like, calmed us down a bit, sort of thing.
P23
One parent described observing a ‘tension’ between the teams and, although it did not concern the mother in terms of the care her child was receiving, it increased the mother’s anxiety about an unfamiliar team caring for her child:
I definitely got the sense that the local hospital and the PCCT team, there’s a little bit of tension between them. The [PCCT] team come in and do their own assessment and they don’t listen to anything that the local hospital is saying to them because they’re doing their own thing and making their own decisions based on all of that information. You could see it when they arrived, . . . It didn’t concern me in her care but I think it added to that, ‘Who are these people, are they going to take care of her?’
P4 mother
Critical incidents
The PICANet audit data base collects information on critical incidents that occurred during transports. A critical incident could be related to such things as mechanical/equipment failure or directly related to the child’s clinical condition (e.g. cardiac arrest while in transit). The PICANet data collection form has nine separate categories (see Appendix 17); however as these incidences were low (affecting 6% of journeys), a binary category was created to summarise these data (see Appendix 31). We then assessed whether or not any reported critical incident was related to parents’ reported experience or satisfaction. There was no significant difference in the proportion of journeys with a reported critical incident between journeys with and without a linked parent questionnaire [χ2 (df 1) = 2.037; p = 0.154].
Association between critical incident and parent experience/satisfaction
There was no significant association between a critical incident on a journey and parent satisfaction/experience scores (see Appendices 32 and 33).
Parental involvement in the transfer
Parental presence in the ambulance
Across all services, at least one parent accompanied their child in the ambulance to the PICU in three-quarters (n = 1563, 77%) of transfers, but there was significant variation (25–94%) in parental presence in the ambulance between different PCCT services [Kruskal–Wallis χ2 (df 8) = 377.06; p < 0.0001]. The transfer team could not be identified for 13 journeys for which parents had reported whether or not they travelled in the ambulance. The proportion of retrievals without a parent present in the ambulance was generally higher when non-PCCT services carried out the transfer (Table 21).
| Transport team | Did you (and other family members) travel with your child (in the ambulance) to this PICU? (%) | ||
|---|---|---|---|
| Yes, two of us | Yes, just me | None of us | |
| T001 | 39 | 51 | 10 |
| T002 | 1 | 82 | 17 |
| T003 | 6 | 70 | 25 |
| T004 | 28 | 66 | 6 |
| T005 | 25 | 58 | 17 |
| T008 | 4 | 68 | 29 |
| T024 | 6 | 58 | 36 |
| T026 | 6 | 76 | 18 |
| T027a | 0 | 25 | 75 |
| Specialist (other) | 5 | 44 | 52 |
| Non-specialist | 13 | 54 | 33 |
| Total | 18 | 58 | 23 |
Parents who did not accompany their child in the ambulance (n = 478 journeys, 23%) were invited to offer an explanation about why they did not travel, and most (n = 442, 93%) provided an explanation. Categorised reasons are summarised in Table 22 and are subdivided into reasons parents attributed to transfer team decision-making and reasons relating to parents’ own decisions/perceptions of the situation. Responses were further split by the transfer team, that is, either PCCT or non-PCCT. For three journeys, where parents gave reasons, the transport team was not reported (in two of these journeys the reason reported was that parents were not permitted to travel and in the third journey it was not clear). Parents were not limited in how many reasons they offered in their explanation.
| Reason | Transfer team, n (%)a | |
|---|---|---|
| PCCT | Non-PCCT | |
| Not permitted to travel: only reason offered (e.g. space limited because of equipment, staff numbers or staff want extra space to work, insurance issues or family member not permitted, e.g. pregnant/grandparent) | 77 (24) | 55 (48) |
| Not permitted to travel: reason offered (as above) alongside other reasons | 7 (2) | 6 (5) |
| Parents wanted to travel together (e.g. for emotional or practical reasons and it was not possible to do so in the ambulance, the seat was in the front of ambulance and not with child and, therefore, there was greater benefit to be with partner) | 73 (23) | 5 (4) |
| Practical considerations (e.g. parents needed to go elsewhere prior to PICU, such as home to sort siblings, other family, pets or to pick up clothes, their luggage did not fit or parents need to transfer their car to a new hospital) | 100 (31) | 16 (14) |
| Parent health or emotional status (e.g. postnatal and not discharged, ill, motion sickness, concerned about how they would react in the ambulance if things went wrong) | 49 (15) | 23 (20) |
| Perceptions of retrieval team (e.g. trusted, parents felt that they might be in the way) | 43 (13) | 5 (4) |
| Child health status perceptions by parent (e.g. no need for parent to travel because child was sedated/stable, the child was happy to travel alone or the parent did not want to travel because child too ill) | 28 (9) | 9 (8) |
| Other/not clear | 38 (12) | 13 (11) |
When parents chose to travel separately reasons for this included parents’ emotional state, for example ‘Needed headspace from the stress, didn’t want to be stuck in a small space if they had to make an emergency stop; (P31, mother and father PCCT-4]). Some families travelled separately for practical reasons, for example ‘Collecting essentials for overnight stay’ (P32, mother, PCCT-4). Other families offered reasons relating to their child, such as ‘he was sedated he would not know we were there’ (P33, mother, PCCT-9), ‘our daughter was stable and being well cared for’ (P34, mother, PCCT-9) and, in contrast, ‘I felt that my child was too poorly’ (P35, mother, PCCT-6).
For some families, it was a combination of factors that influenced their decision, for example where one seat was offered this was sometimes declined because one parent wanted the emotional support of a partner, rather than to travel separately:
Because we were both in a state we wanted to go together as a team. Rather than one person travel to [city] alone.
P36, mother, PCCT-8
Some families chose to travel together because they were being transferred to an unfamiliar hospital, potentially in an unfamiliar city, and needed to work together to navigate the way:
We didn’t know what hospital he was going to be taken to. My husband hadn’t slept for 2 nights before. I was worried about him having an accident driving 3+ hours with the worry of getting to the hospital quickly.
P37, mother, PCCT-9
A seat offered in the front cab rather than in the body of the ambulance (in proximity to their child) was cited as a reason to choose to travel with a partner:
As we weren’t able to sit in the back with him. We decided to go up together.
P38, father, other specialist
Some parents reported that trust and perceived efficacy of the retrieval team enabled them to travel separately from their child:
. . . my husband and I felt our child was in great care. She was safe and settled and didn’t feel we would be able to drive 2.5 hours independently and needed to share the journey.
P39, mother, PCCT-9
A proportion of parents did not travel because the mother was receiving medical care (often postnatal) and the father chose to remain with the mother for emotional support:
I was looking after my wife who had just given birth.
P40, father, PCCT-7
In around one-third (145/477, 30%) of cases where parents did not travel in the ambulance, this was because the retrieval team did not permit it, frequently because of space limitations. We did not specifically ask parents to report the emotional impact of being unable to travel with their child, but some parents offered their responses and there was variation. For example, some parents reported distress and wanting change:
. . . not enough room in the ambulance. This is a point that can be improved.
P41, mother, PCCT-2
No room in the ambulance. This distressed me a lot.
P42, mother and father, PCCT-2
Other parents reported a positive acceptance:
They asked if we could follow and didn’t want one of us in the ambulance which was fine with us.
P43, parent, non-specialist service
Taking the sample as a whole (i.e. PCCT-transported families, non-PCCT-transported families and where no transport team was identified), parental presence in the ambulance was associated with greater satisfaction. More journeys were in the ‘excellent’ category when both parents travelled in the ambulance and the smallest number rated ‘excellent’ when no parents were present. More families where two parents travelled, compared with no parents travelled, agreed strongly that they would recommend their transfer team to others and would consider using them again if needed (Tables 23 and 24).
| Measure | Who travelled in the ambulance?, n (%) | Group difference (Kruskal–Wallis) | ||
|---|---|---|---|---|
| Two of us | One of us | None of us | ||
| If my child was ever in this situation again then I would like them to be transported by this team again | ||||
| Disagree at lot | 2 (1) | 2 (0) | 1 (0) | χ2 (df 2) = 16.98; p < 0.0001 |
| Disagree a bit | 1 (0) | 4 (0) | 3 (1) | |
| Neither agree nor disagree | 1 (0) | 17 (1) | 15 (3) | |
| Agree a bit | 14 (4) | 40 (3) | 30 (6) | |
| Agree a lot | 354 (95) | 1123 (95) | 418 (90) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | ||||
| Disagree at lot | 2 (1) | 3 (0) | 2 (0) | χ2 (df 2) = 18.37; p < 0.0001 |
| Disagree a bit | 1 (0) | 6 (1) | 2 (0) | |
| Neither agree nor disagree | 1 (0) | 14 (1) | 16 (3) | |
| Agree a bit | 10 (3) | 33 (3) | 25 (5) | |
| Agree a lot | 359 (96) | 1128 (95) | 421 (90) | |
| Overall experience of the transport service | ||||
| Extremely poor | 0 (0) | 0 (0) | 1 (0) | χ2 (df 2) = 31.80; p < 0.0001 |
| Poor | 1 (0) | 1 (0) | 6 (1) | |
| Fair | 1 (0) | 17 (1) | 7 (2) | |
| Good | 23 (6) | 95 (8) | 68 (15) | |
| Excellent | 348 (93) | 1073 (91) | 380 (82) | |
| Overall satisfaction with the transport service | ||||
| Extremely poor | 0 (0) | 0 (0) | 1 (0) | χ2 (df 2) = 37.17; p < 0.0001 |
| Poor | 1 (0) | 2 (0) | 2 (0) | |
| Fair | 1 (0) | 11 (1) | 9 (2) | |
| Good | 22 (6) | 84 (7) | 68 (15) | |
| Excellent | 347 (94) | 1085 (92) | 382 (83) | |
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group statistic (Kruskal–Wallis) | |
|---|---|---|---|
| Median | IQR | ||
| Two of us (n = 342) | 5 | 5–5 | χ2 (df 2) = 12.281; p = 0.002 |
| One of us (n = 1082) | 5 | 5–5 | |
| None of us (n = 316) | 5 | 4.89–5 | |
When comparing parents who chose not to travel with parents who were not permitted to travel, a similar pattern of significant differences was found, with a higher proportion of parents who chose not to travel endorsing the higher satisfaction categories and rating their experience more positively (Table 25 and see Appendix 34).
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group difference (Mann–Whitney) | |
|---|---|---|---|
| Median | IQR | ||
| Chose not to travel (n = 230) | 5 | 4.89–5 | U = 8575; z = –2.252; p = 0.024 |
| Not permitted to travel (n = 86) | 5 | 4.78–5 | |
Paediatric critical care transport team services operate under the same service standards57 and so, although there was variation between the teams in terms of parental presence, it could be argued that these teams may be more similar in ways that could potentially affect satisfaction, compared with the other teams (e.g. ‘specialist other’ and ‘non-specialist’ groups) that also transferred patients into the PICU. With this in mind, the analyses were re-run, looking at associations between satisfaction and parental presence in the ambulance for just the PCCT journeys. Differences in the categorical satisfaction measures were still significant; however, differences in the experience scale were no longer significant [Kruskal–Wallis χ2 (df 2) = 4.28] and differences between those who chose not to travel and those who were not permitted to travel in the ambulance were no longer significant for all measures of satisfaction and experience (Mann–Whitney z-scores ranged from –1.258 to–0.858 and p-values ranged from 0.559 to p = 0.94).
Discussion
In this chapter, we have described our mixed-methods approach to exploring (1) parents’ experiences of emergency transport to the PICU and (2) clinicians’ perspectives of PICU transport and its impact on care provided to critically ill CYP. The qualitative element enabled us to gather a richer narrative from parent experiences and clinician perspectives than could be obtained from quantitative analyses of either routine data or experience-related data.
Sample
Our strategy for recruitment to the questionnaire study enabled us to recruit a large sample of parents from all PICUs in England and Wales, representing all of the transport teams, which exceeded our forecasted recruitment targets. However, this success was tempered by the challenges of recruiting CYP. Although the DEPICT study was an assessment of the specialist PCCTs, we also included parents whose children were transported by non-specialist PCCT services, in part, because it made recruitment easier in the busy PICU setting. We could have excluded families transported by non-specialist teams because these teams are not governed by the same protocols and standards, and it is not as possible for us to influence that experience; however, it was important to include these families from a parent/patient perspective. Regardless of which way a family is transported to PICU, as soon as they arrive they become part of the same group (i.e. PICU families who will need support as their journey progresses) and so it is important to know what their transfer experience is, as it may have an influence on their recovery/PICU experiences going forward.
Experience outcomes
Levels of satisfaction with the transport services were very high and experiences were positive, as measured by the questionnaire. Although there were some significant differences these were really very small variances in the sample and may have been found because of the large sample size. We received more nuanced and revealing descriptions of experiences when families were able to talk about their experiences as they wanted to, with time to add more detail and provide a richer narrative of their journey with their child.
Measuring experience
As part of this study we also developed a brief measure of parents’ experience of critical care transport, providing a standardised measure that can be used across all PCCTs. This measure will enable national benchmarking of services, and offers the potential to increase the collection and use of parent experience data to improve services.
Timeliness
Timeliness matters to parents; however, sometimes it is the perceived, rather than the objective, time that has the greater influence on perceived satisfaction (e.g. perceptions of the time it took for the transport team to reach their child’s bedside). Communication of timeliness was identified as particularly important and parents were less satisfied when they were not told a time frame for the arrival of the team, for example. Our results highlight the importance of managing parent expectations around timeliness, providing clear communication and continuous updates on a fluid and changing situation.
Aspects of care
Leadership
Team composition appeared to have a greater impact on staff, although there was evidence that some parents picked up on staff’s confidence (or lack of). Transport teams often have some team members who are transient, with varying prior experience, and this was identified by staff in all locations, and by parents, as presenting some challenges. How best to prepare such staff to enable them to feel confident working in the retrieval environment is something that needs to be addressed in future.
Parental presence in the ambulance
There was mixed evidence about parental presence in the ambulance, but the findings suggest greater satisfaction if parents can travel, with satisfaction levels being highest if both parents are given the opportunity to travel. Some families chose to travel separately because only one seat was offered and findings from the interviews provided some insight into why that might be. What was very clear was that offering parents choice, rather than the choice that they made, was the salient issue.
Limitations of the experience work
There were some limitations to this work, including the lack of PICANet linked data for all questionnaire respondents; differences in demographic characteristics (e.g. primary reason for transfer to PICU and age of child) between those with and without PICANet linked data, which may limit generalisability of our findings; and our failure to recruit any CYP to be interviewed. This latter point, while disappointing, was not wholly unexpected, given the young age of the majority of transported patients and the high proportion of patients who were ventilated (and sedated) and, therefore, unable to recall their transport journey. In those very few instances where a child was old enough and awake during the journey, parents were reluctant to allow them to participate in an interview. The parents had very reasonable concerns that talking about their experience might upset children or force them to revisit a situation from which they had moved on. In addition, there were concerns about children’s ability to accurately recall what happened. Future research should, therefore, focus on capturing children’s and young people’s experiences in a more timely fashion, closer to the time of the journey and possibly using quantitative methods that may be easier and less onerous.
Summary of influencing factors on parents’ experience of the journey to paediatric intensive care unit
Our findings indicate that the factors that influence parents’ experience of transfer to a PICU are multiple and varied, and the model in Figure 18 represents a summary of those factors identified in this study, with the additional aim of identifying factors that may be modifiable through future interventions.
FIGURE 18.
Influencing factors on parents’ experience of their child’s transfer to a PICU.
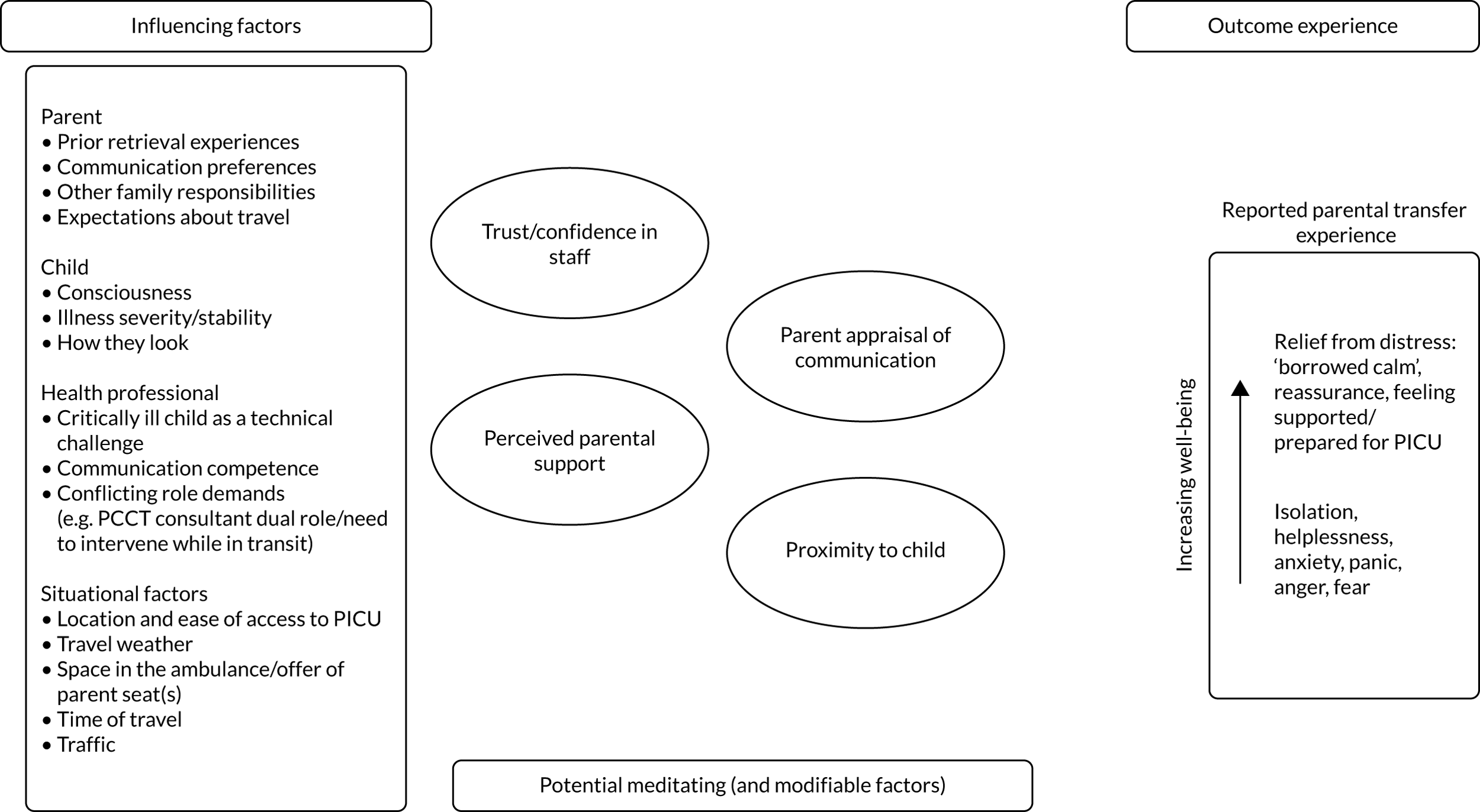
Final comments about workstream B
Effective communication is clearly central to parent and staff experience and is an indicator of effective team working. Effective communication also helps with making sense of, and coping with, stressful experiences. What we have not been able to explore and test in this study is what inhibits and what promotes effective communication and whether or not there are points in the process where effective communication is more important.
Chapter 5 Workstream C: health economic evaluation
Introduction
The centralisation of paediatric critical care across a small number of specialist centres in the UK means that a transport service must be commissioned to retrieve and transfer children who require a level of care that is not available in hospitals without PICUs. On average, around 5000 children are transported as emergency referrals each year by PCCTs. Providing a round-the-clock emergency transport service for critically ill children is likely to be expensive for providers, but, to our knowledge, no data have been published that investigate and evaluate the costs and cost-effectiveness of such transport by PCCTs.
The DEPICT study has shown that there is variation in the ways in which PCCTs across England and Wales deliver their service. Although there are uniform service standards for all PCCTs,58 how teams choose to operationalise their service is not mandated and each PCCT has a different configuration. The variations between services across the country exist in terms of the numbers and types of staff on teams, as well as the numbers of teams made available on weekends, at night and by season. Teams also vary by the proximity of the service base from the hospitals they serve. These variations in service provision will affect the costs of the service to the NHS (e.g. the costs associated with greater resource requirements), as well as the costs of the service to families, who may need to travel further to a hospital that their child has been transferred to. The aim of this economic evaluation was to determine the most cost-effective PCCT configuration.
Methods
Overview of approach
We utilised the quantitative data sources used in workstreams A (see Chapter 3) and B (see Chapter 4). We calculated the costs of different ways of organising PCCTs, based on staffing associated with different team compositions, as a potentially modifiable factor. We evaluated costs that were associated with the different ways of organising PCCTs in terms of interventions performed and critical incidents during transport, hospital costs, primary care costs and costs borne by families. Outcomes were measured in terms of mortality and health-related quality of life. We evaluated the impact of team composition on different components of NHS hospital costs and primary care costs, costs borne by families and outcomes. All costs were calculated in 2019/20 GBP.
Patients
We used two samples for this analysis. The first sample was from workstream A (see Chapter 3), that is, 9112 children transported by a PCCT in the ‘models of care cohort’. The second sample was from workstream B (see Chapter 4), that is, 395 children who were included in the questionnaire study and who were linked with PICANet transport data.
Measuring costs
Costs of different ways of organising paediatric critical care transport teams
We calculated the costs of different ways of organising PCCTs based on the staff costs associated with different team compositions. In our protocol, we originally planned to measure the costs of each identified PCCT model in terms of team composition, interventions performed and critical incidents. We identified that the number and type of interventions performed and the number and type of critical incidents were potential consequences of the child’s health and the transport, rather than costs associated with the ways of organising PCCTs, and, therefore, we analysed these factors independently to determine any association with team composition. We did not include driver costs or non-staff costs, such as overheads and petrol, as these fixed costs would be incurred irrespective of how the PCCT was organised.
Although PICANet transport-level data are available on the grade of the clinical team leader and the grade of the most senior nurse, no further data are available on the composition of the whole team. Therefore, to calculate the staff costs per transport, we obtained data on typical team composition used by individual transport teams. Each PCCT was asked to record the staff resource allocated to providing the service. All PCCTs report different configurations of service in terms of the roles and seniority (staff grade) of staff, the number of ambulances and teams that were resourced, and the variations of provision on weekends, at nights and by season.
We calculated the cost of each of the configurations using published unit costs. 59 When a ‘consultant’ was recorded, then we allocated the unit cost per unit hour for a hospital-based medical consultant. If the team reported a ‘medic’ or a ‘doctor’, then we allocated the unit cost per hour of a hospital registrar. When a nurse was present on the team, then we determined the weighted average of the unit cost using the percentage of each band (5–8) as they were reported in the workstream A data set. ANPs were allocated the unit cost per hour at band 8a. Several teams recorded ‘ANP/registrar’, as these roles were interchangeable, and for this we used the mean cost of the registrar and the ANP. The total hourly rate of each team was calculated and then divided by 60 to give a cost per minute. This value was multiplied by the average time per trip calculated across all teams to demonstrate the variation in costs of the different staffing models.
Hospital costs
We considered hospital costs in the following three components: (1) length of index stay in the PICU, (2) total index LOS in the hospital and (3) outpatient and ED visits in the 12 months following the transport. Length of index stay in the PICU was derived from the linked PICANet admissions data set described in Chapter 2. For each day that the child was in the PICU during the index stay, data were recorded on the level of care (e.g. intensive care extracorporeal membrane oxygenation/extracorporeal life support, intensive care advanced enhanced, intensive care advanced, intensive care basic enhanced, intensive care basic, high dependency advanced, high dependency, enhanced care) that they received. Unit costs were obtained, which were daily costs for each level of care,60 and these were applied to the number of days spent receiving that level of care. LOS of the index stay was calculated, as described in Chapter 2. Outpatient and ED costs were derived from a different cohort included in the questionnaire study, described in Chapter 3. Parents of the transported children were asked how many times they had visited the ED or had attended an outpatient appointment during the last 12 months (i.e. since their child’s initial PICU stay) for their child as a result of the reason for their PICU stay or because another family member needed to because of their child’s admission to the PICU. Unit costs for outpatient visits were based on national average costs per attendance for paediatrics outpatient visits (£135). 61 In the regression analysis, we evaluated the combined outpatient and ED costs.
Transport-related costs
We included the interventions performed by the transport team and critical incidents relating to the child during transport, as derived in Chapter 2. The number of critical incidents relating to the child were small and so this was included as a binary variable, measuring if there was a critical incident (i.e. yes or no). The number of interventions performed was measured as a count of potential interventions (i.e. any form of intubation, central venous access, arterial access or intraosseous access, or use of inotropes by the transport team).
Primary care costs
Primary care costs were derived from the questionnaire study described in Chapter 3. Parents of the transported children were asked how many times they had contacted the general practitioner (GP) or a nurse during the 12 months since their child’s initial PICU stay for their child as a result of (1) the reason for their PICU stay or (2) because another family member needed to because of their child’s admission to the PICU. We assumed that all GP contacts were face-to-face visits and that all contacts with the nurse were with the practice nurse. Unit costs per contact were based on previously published figures (£39 and £14, respectively). 62 In the regression analysis, we evaluated the combined GP and nurse costs.
Costs borne by families
In the questionnaire study, families were asked about the costs that they had incurred following their child’s transfer to the PICU. Parents were asked to report separately how much they had spent on accommodation, travel, food and drink, as well as other out-of-pocket expenses. For each item, respondents were presented with options (i.e. £0, £1–100, £101–200) and were asked to select the one that applied to their situation. For each child, we used point estimates based on £0 and the mid-point of each range. In the regression analysis, we evaluated the combined family costs.
Measuring outcomes
Parts of this section have been reproduced from Brown et al. 63 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
We measured mortality within 30 days of admission and within 1 year of admission using the methods described in Chapter 2.
For surviving infants included in the questionnaire study, generic health-related quality of life was described using the Health Utilities Index Mark 2 (HUI2)64 descriptive system and was measured 12 months after their child’s initial PICU stay. The HUI2 is a preference-based multiattribute health-related quality-of-life instrument that was specifically developed for use with children. HUI2 consists of seven dimensions (i.e. sensation, mobility, emotion, cognition, self-care, pain and fertility), each of which has between three and five levels, ranging from ‘normal functioning for age’ to ‘extreme disability’. The use of the fertility dimension is discretionary and was not used in the present study. The questionnaire was completed by parent proxy for children in the questionnaire study. The HUI2 health states were converted into utility values using a formula that attaches weights to each level in each dimension, based on valuations by a UK general population sample. 65 Utility values of 1 represent full health, values of zero are equivalent to death and negative values represent states worse than death. 63 Patients who died were not included in the questionnaire study. Given the high proportion of responses equal to 1, we also created a binary version of this variable, taking the value of 1 if the HUI2 scores was equal to 1 and zero otherwise.
Analysis
We used regression analysis to evaluate the association between team composition and the cost and outcome measures described above. Team composition was delineated by leader grade and grade of the most senior nurse (see Chapter 2). The regressions we ran are summarised in Table 26. The dependent variables and data sets are as described above. The regression models were based on the nature of the dependent variables. Where we used the generalised linear model with gamma family and log-link to account for the skewness of the dependent variable, we also experimented with using the Gaussian family and log-link models; however, the gamma model was preferred because of the Akaike information criterion. For models using the workstream B data, we had a relatively small number of observations (maximum n = 395) (Table 27 gives descriptive statistics for this data set). We originally ran the models using the same covariates as for the analyses using the workstream A data. All of the team composition variables were non-significant; however, given the low power, we re-ran the models without covariates, reported here. For every regression model we present the results in terms of predictive margins, that is, the adjusted mean value of the dependent variable for each category.
| Dependent variable | Data set | Regression model | Covariate(s) |
|---|---|---|---|
| Mortality within 30 days of admission | ‘Models of care’ data set in (workstream A) | Logistic regression | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time |
| Mortality within 1 year of admission | ‘Models of care’ data set in (workstream A) | Logistic regression | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time |
| HUI2 score at 1 year | Questionnaire study data set (workstream B) | Ordinary least squares regression | |
| HUI2 score at 1 year = 1 | Questionnaire study data set (workstream B) | Ordinary least squares regression | |
| Total length of index hospital stay | ‘Models of care’ data set in (workstream A) | Generalised linear model with gamma family and log-link | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time |
| Cost of length of index stay in the PICU | ‘Models of care’ data set in (workstream A) | Generalised linear model with gamma family and log-link | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time |
| Critical incidents relating to the child | ‘Models of care’ data set in (workstream A) | Logistic regression | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time |
| Total interventions by the transport team | ‘Models of care’ data set in (workstream A) | Negative binomial regression | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time |
| Primary care costs up to 12 months | Questionnaire study data set (workstream B) | Generalised linear model with gamma family and log-link | |
| Outpatient and ED costs up to 12 months | Questionnaire study data set (workstream B) | Generalised linear model with gamma family and log-link | |
| Family costs following child’s transfer to the PICU | Questionnaire study data set (workstream B) | Generalised linear model with gamma family and log-link |
| Variable | Observation | Mean | SD |
|---|---|---|---|
| Health-related quality of life | |||
| HUI2 score at 1 year | 353 | 0.83 | 0.24 |
| HUI2 score at 1 year = 1 | 353 | 0.48 | 0.50 |
| Resource use and costs over 12 months | |||
| GP visits (n) | 298 | 12 | 17 |
| Nurse visits (n) | 290 | 10 | 21 |
| ED visits (n) | 289 | 5 | 14 |
| Outpatient visits (n) | 293 | 12 | 19 |
| Primary care costs (£) | 288 | 579 | 820 |
| Outpatient costs (£) | 293 | 1607 | 2620 |
| Family costs (£) | |||
| Accommodation costs | 335 | 78 | 264 |
| Travel costs | 338 | 217 | 410 |
| Food and drink costs | 334 | 191 | 200 |
| Other family costs | 281 | 144 | 282 |
| Total family costs | 276 | 603 | 858 |
| Leader grade, n (%) | |||
| Consultant | 117 (30) | ||
| Junior doctor | 190 (48) | ||
| Nurse practitioner | 70 (18) | ||
| Missing | 18 (5) | ||
| Total | 395 (100) | ||
| Nurse band, n (%) | |||
| Not present/missing | 22 (6) | ||
| Grade 5 | 53 (13) | ||
| Grade 6 | 250 (63) | ||
| Grade 7 | 51 (13) | ||
| Grade 8 | 19 (5) | ||
| Total | 395 (100) | ||
Changes from the original protocol
The analysis presented in this chapter is different from the analysis proposed in the study protocol66 for three reasons. First, workstream A data access was restricted to a single data guardian and held on secure servers at the University of Leicester. To analyse workstream A data, it was necessary for the workstream C researcher to hold an honorary contract with the University of Leicester and to be on-site when accessing the data set. Unfortunately, access to this large data set could not be managed remotely and, therefore, the researcher lost access to the workstream A data set at the beginning of the first UK lockdown in March 2020. It was not possible to obtain permission to return to the University of Leicester for the remaining duration of the DEPICT study. Restricted access to data meant that it was, therefore, not possible to evaluate the cost (as opposed to the length) of the index hospital stay, the cost (as opposed to number) of critical incidents relating to the child and interventions provided by the transport team, or the number and cost of inpatient and day case re-admissions up to 1 year.
Second, we originally planned to undertake the primary analysis of team composition on QALYs and all costs using the workstream A data set. This data set did not have utility measures suitable for estimating QALYs, primary care costs or family costs; however, all patients in both the workstream A and workstream B data sets met the study inclusion criteria and both data sets include PICANet data. Our original plan was to link the data in two stages. First, using regression analysis on the workstream B data set to relate the collected data on utility scores, primary care costs, outpatient and ED costs and family costs to transport and patient characteristics available in the PICANet transport data set. Second, using the coefficients from these regression models to predict utility scores and costs on to patients in the workstream A data set, producing patient-level predicted values, allowing us to evaluate QALYs and the full range of costs using workstream A data. As shown below, from the regression analyses at stage 1, utility scores and costs during the 12 months’ follow-up were not related to team composition and so the predictions at stage 2 were unnecessary.
Third, in the protocol we had planned to estimate long-term cost-effectiveness beyond 1 year by predicting long-run costs and outcomes using data from published studies; however, as shown below, our regression analyses showed that team composition was largely non-significant in terms of predicting costs and outcomes at time points up to 1 year and so the analysis of long-term effects was unnecessary.
Results
Association between team characteristics and costs
Conditional on the covariates, critical incidents relating to the child (Table 28) were more likely if the clinical team leader was a consultant (as opposed to a more junior doctor or nurse practitioner), although the probability of such an incident was small (< 2%). Critical incidents were not associated with the grade of the most senior nurse. The total number of interventions by the transport team was not associated with the grade of the team leader.
| Team characteristic | Critical incidents relating to the child | Total interventions by the transport team | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Predictive margina | Coefficient | 95% CI | Predictive marginb | |
| Leader grade | ||||||
| Consultant | Baseline | 0.018 | Baseline | 0.60 | ||
| Junior doctor | 0.58 | 0.38 to 0.89 | 0.011 | –0.06 | –0.26 to 0.14 | 0.56 |
| Nurse practitioner | 0.62 | 0.45 to 0.86 | 0.012 | –0.08 | –0.35 to 0.19 | 0.55 |
| Nurse band | ||||||
| Not present/missing | Baseline | 0.014 | Baseline | 0.40 | ||
| Grade 5 | 1.18 | 0.51 to 2.71 | 0.016 | 0.48 | 0.35 to 0.61 | 0.64 |
| Grade 6 | 1.01 | 0.88 to 1.17 | 0.014 | 0.41 | 0.20 to 0.62 | 0.60 |
| Grade 7 | 0.97 | 0.56 to 1.68 | 0.013 | 0.41 | 0.18 to 0.63 | 0.60 |
| Grade 8 | 0.35 | 0.06 to 2.25 | 0.005 | 0.56 | 0.41 to 0.72 | 0.70 |
| Regression model | Logistic regression | Negative binomial regression | ||||
| Observations, n | 8794 | 9012 | ||||
| Pseudo-R2 | 0.0729 | 0.1578 | ||||
| Covariates | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time | ||||
In terms of hospital-admitted patient costs (Table 29), total length of index hospital stay was longer and cost of length of index stay in the PICU was higher if the PCCT leader was a consultant. The same effect was seen in terms of nurse grade, where the more senior nurse (i.e. grade 8) was associated with a longer LOS in the PICU and an increased cost.
| Team characteristic | Total length of index hospital stay | Cost (£) of length of index stay in the PICU | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Predictive margina | Coefficient | 95% CI | Predictive marginb | |
| Leader grade | ||||||
| Consultant | Baseline | 7.8 | Baseline | 14,752 | ||
| Junior doctor | –0.067 | –0.116 to –0.018 | 7.4 | –0.113 | –0.160 to –0.067 | 13,172 |
| Nurse practitioner | –0.050 | –0.126 to 0.24 | 7.5 | –0.096 | –0.191 to 0.0001 | 13,409 |
| Nurse band | ||||||
| Not present/missing | Baseline | 7.4 | Baseline | 13,532 | ||
| Grade 5 | –0.094 | –0.230 to 0.040 | 6.7 | –0.137 | –0.270 to –0.004 | 11,800 |
| Grade 6 | 0.021 | –0.054 to 0.095 | 7.6 | 0.016 | –0.067 to 0.099 | 13,755 |
| Grade 7 | 0.097 | –0.014 to 0.208 | 8.2 | 0.110 | –0.006 to 0.226 | 15,106 |
| Grade 8 | 0.211 | 0.054 to 0.368 | 9.1 | 0.247 | 0.028 to 0.461 | 17,286 |
| Regression model | Generalised linear model with gamma family and log-link | Generalised linear model with gamma family and log-link | ||||
| Observations, n | 9012 | 8437 | ||||
| Covariates | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time | ||||
Conditional on the covariates, critical incidents relating to the child (Table 30) were more likely if the clinical team leader was a consultant (as opposed to a more junior doctor or nurse practitioner), although the probability of such an incident was small (< 2%). Critical incidents were not associated with the grade of the most senior nurse. The total number of interventions by the transport team was not associated with the grade of the team leader.
| Team characteristic | Primary care costs (£) up to 12 months | Outpatient and ED costs (£) up to 12 months | Family costs (£) following child’s transfer to the PICU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Predictive margin | Coefficient | 95% CI | Predictive margin | Coefficient | 95% CI | Predictive margin | |
| Leader grade | |||||||||
| Consultant | Baseline | 709 | Baseline | 1815 | Baseline | 704 | |||
| Junior doctor | –0.233 | –0.568 to 0.103 | 562 | –0.23 | –0.60 to 0.15 | 1446 | –0.15 | –0.55 to 0.25 | 605 |
| Nurse practitioner | –0.482 | –0.903 to –0.060 | 438 | –0.30 | –0.78 to 0.18 | 1348 | –0.16 | –0.67 to 0.35 | 600 |
| Nurse band | |||||||||
| Not present/missing | –0.560 | –1.243 to 0.124 | 525 | –0.13 | –0.87 to 0.61 | 1760 | 0.25 | –0.62 to 1.11 | 701 |
| Grade 5 | Baseline | 919 | Baseline | 2008 | Baseline | 547 | |||
| Grade 6 | –0.724 | –1.196 to –0.251 | 446 | –0.42 | –0.92 to 0.09 | 1324 | 0.15 | –0.40 to 0.69 | 633 |
| Grade 7 | –0.286 | –0.919 to 0.347 | 691 | –0.03 | –0.71 to 0.65 | 1955 | 0.15 | –0.56 to 0.87 | 638 |
| Grade 8 | 0.315 | –0.446 to 1.076 | 1260 | 0.54 | –0.33 to 1.40 | 3429 | 0.01 | –0.99 to 1.00 | 552 |
| Regression model | Generalised linear model with gamma family and log-link | Generalised linear model with gamma family and log-link | Generalised linear model with gamma family and log-link | ||||||
| Observations, n | 275 | 280 | 262 | ||||||
In terms of hospital-admitted patient costs (Table 31), total length of index hospital stay was longer and cost of length of index stay in the PICU was higher if the PCCT leader was a consultant. The same effect was seen in terms of nurse grade, where the more senior nurse (i.e. grade 8) was associated with a longer LOS in the PICU and an increased cost.
| Team characteristic | Mortality within 1 year of admission | Mortality within 30 days of admission | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Predictive margin | OR | 95% CI | Predictive margin | |
| Leader grade | ||||||
| Consultant | Baseline | 0.12 | Baseline | 0.08 | ||
| Junior doctor | 0.79 | 0.71 to 0.89 | 0.10 | 0.77 | 0.65 to 0.91 | 0.07 |
| Nurse practitioner | 0.79 | 0.62 to 1.00 | 0.10 | 0.63 | 0.51 to 0.78 | 0.06 |
| Nurse band | ||||||
| Not present/missing | Baseline | 0.11 | Baseline | 0.08 | ||
| Grade 5 | 0.69 | 0.53 to 0.92 | 0.08 | 0.56 | 0.45 to 0.71 | 0.05 |
| Grade 6 | 1.03 | 0.89 to 1.20 | 0.11 | 0.86 | 0.78 to 0.94 | 0.07 |
| Grade 7 | 0.99 | 0.76 to 1.29 | 0.11 | 0.77 | 0.57 to 1.05 | 0.07 |
| Grade 8 | 0.99 | 0.77 to 1.28 | 0.11 | 1.23 | 0.93 to 1.62 | 0.09 |
| Regression model | Logistic regression | Logistic regression | ||||
| Observations, n | 9012 | 9012 | ||||
| Pseudo-R2 | 0.143 | 0.187 | ||||
| Covariates | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time | Age, PIM2 score, diagnosis, ventilated at referral, collection hospital size, critical care, time to bedside, stabilisation time, patient journey time | ||||
The distribution of costs from the questionnaire study in workstream B are shown in Figures 19–21. In all cases, the costs are positively skewed. Mean (SD) and median (IQR) primary care costs were £579 (SD £820) and £312 (IQR £92–717), respectively, per child. For combined outpatient and ED costs, the figures were £1607 (SD £2620) and £810 (IQR £270–1755), respectively. For family costs, the figures were £603 (SD £858) and £300 (IQR £150–625). There was no association between the team composition and any of the out-of-hospital cost measures (see Table 30); however, the single exception was that primary care costs were lower if a grade 6 nurse was the most senior nurse on the team.
FIGURE 19.
Distribution of primary care costs over 12 months. Note that the mean (SD) value was £579 (SD £820) and the median (IQR) was £312 (IQR £92–717).
FIGURE 20.
Distribution of outpatient and ED costs over 12 months. Note that the mean (SD) value was £1607 (£2620) and the median (IQR) was £810 (£270–1755).

FIGURE 21.
Distribution of family costs following child’s transfer to the PICU. Note that the mean (SD) value was £603 (£858) and the median (IQR) was £300 (£150–625).
Association between team characteristics and outcomes
We observed a higher mortality probability at 30 days and at 12 months when the team leader was a consultant, compared with where a junior doctor or ANP lead the team, although the absolute impacts on mortality were small (see Table 31). Mortality was lowest when the most senior nurse was band 5.
Health-related quality-of-life scores at 1 year were negatively skewed (Figure 22), with around 50% of respondents reported as being in full health. The mean (SD) and median (IQR) values were 0.83 (SD 0.24) 0.95 (IQR 0.73–1.00), respectively. Neither leader grade nor grade of the most senior nurse was associated with utility scores at 1 year.
FIGURE 22.
Distribution of HUI2 scores at 1 year. Note that the mean (SD) value was 0.83 (0.24) and the median (IQR) was 0.95 (0.73–1.00). One hundred and sixty-eight (48%) respondents reported a HUI2 score equal to 1.
Discussion
Key findings
In this chapter, we aimed to evaluate the costs and value for money of different models of PCCTs to compare the cost-effectiveness of different models of service delivery. The PCCT models varied by factors that affected their costs. We investigated the impact of team composition on various cost and outcome measures and the findings suggest that either the team leader was not associated with costs or outcomes (i.e. total interventions by the transport team, primary care costs, outpatient and ED costs, family costs, health-related quality of life), or that having a more senior team leader (i.e. consultant) was associated with higher resource use/costs and worse outcomes (i.e. total length of index hospital stay, cost of length of index stay in the PICU, mortality) compared with a less senior team leader (i.e. junior doctor, nurse practitioner). With few exceptions, the grade of the most senior nurse was not associated with higher or lower costs or better or worse outcomes. Although we control for a range of factors likely to affect costs and outcomes, including PIM2 score and diagnosis, the finding that some costs are higher and outcomes worse when the team leader is a consultant would suggest that there is a confounding factor relating to the child’s severity of illness, which our measures did not detect, that has introduced a bias into our regression analysis (e.g. severity that is positively correlated with the seniority of the team leader).
Implications
The findings suggest that team composition has little impact on health-care costs and outcomes. There are limitations to our study, which mean that any implications need to be treated with caution. However, when taken at face value, the findings suggest that cost-effectiveness considerations should not affect how transport services are organised.
Limitations and further research
There were a number of limitations to our study, some of which were due to the restricted access to the data set that we had originally planned to use for our analysis, and others resulted from the lack of sensitivity in the available data. For example, although the allocation of a consultant as team leader and the highest grade of the team nurse tended to be associated with higher costs and worse outcomes, it is highly likely that this would have been due to the greater severity of illness among children being transported by senior teams, as the determination of who should attend the transfer is based on clinical decision-making prior to transport. The impact of seniority among the team composition needs further investigation, as the benefit of clinical experience and seniority should be analysed with robust methods that are sufficiently sensitive to measure severity of illness and facilitate an analysis that can adequately control for this. Lack of data access meant that we were limited in the range of cost measures we were able to include, particularly hospital readmissions data. A more comprehensive comparison of the cost-effectiveness of the models of service delivery would require access to the patient-level data, and this is something that should be carried out in the future to provide PCCTs with information that could enable them to rationalise their resource usage and to optimise services. The measures of team composition did not appear to affect outcome and, therefore, it is possible that service changes could be considered in terms of optimising resource efficiency. However, our data were not sufficiently detailed to evaluate this, for example analysis of the team composition was limited to the grade of the team leader and the grade of the most senior nurse. Greater accuracy could be gained from using a true time-and-motion study to inform future decision-making on how best to resource this service. In particular, further research would be beneficial to understand the rationale for different team compositions selected by PCCTs.
Summary
In summary, when using the available data, it is difficult to draw firm conclusions around the most cost-effective organisation of services; however, based on the limited evidence presented here, the findings suggest that cost-effectiveness considerations should not affect how transport services are organised, although further investigation as to how best to improve the cost-effectiveness of the service is warranted.
Chapter 6 Workstream D: mathematical modelling
Sections of this chapter have been reproduced from Ramnarayan et al. 23 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
When PIC services were centralised in the UK, and when specialist PCCTs started being used to transport critically ill children from district general hospitals (DGHs) to PICUs, there was a necessary trade-off in longer durations between critically ill children arriving at local hospitals and arriving a specialist PICU. In recognition of this, timely arrival at the patient’s bedside has become a key performance indicator for PCCTs and this has formed the basis of a current national quality standard57 that specifies that PCCTs should reach the patient bedside within 3 hours of accepting a referral. Although workstream A concentrated on investigating how much impact time to bedside makes on clinical outcomes, in this workstream we use mathematical modelling and location–allocation optimisation methods to explore if different configuration of PCCT services can improve times to bedside and team availability.
We note that location–allocation methods are not statistical modelling. Location allocation methods are, instead, a form of optimisation modelling where the aim is to optimise a given decision-dependent goal (e.g. making journey times as short as possible by changing the location of retrieval teams) subject to various constraints (e.g. maximum number of retrieval team locations, minimum level of demand per location). In simple optimisation models there are exact solutions; however, heuristic algorithms are used to obtain a solution for more complex problems that are computationally complex.
We used location–allocation optimisation to investigate, for a given number of transport services and teams and a set of possible locations, where PCCTs should be based to minimise travel time to the local hospitals they serve and to the receiving PICUs. The constraints on the numbers of services, numbers of teams and possible locations were defined through conversations with the PCCT services and commissioners. In particular, we explored the following different and possible models of service on the potential impact on outcome:
-
more transport services to reduce distance (either with same number of teams or more teams)
-
the same number of transport services with more teams to increase team availability
-
seasonal allocations of teams to plan for the winter surge.
Proposed service models were further re-examined in the light of the feedback from stakeholders (i.e. parents and families, PICUs, PCCTs and local hospital clinicians).
Current paediatric critical care transport team services
In the current service, there are 212 DGHs, 24 PICUs and 11 operating PCCT stations. We allowed any PICU to be a potential location for a PCCT station, giving us 28 possible PCCT locations (i.e. 24 PICUs plus four further locations that currently host PCCTs). The 212 DGHs are hospitals, including some hospitals with PICUs, that have used the PCCT service at least once from 2014 to 2018. The configuration of current retrieval services is shown in Table 32. The current configuration was compiled by the clinical co-applicant team and this was then simplified to give a baseline configuration for the purposes of the mathematical modelling of team allocation (see Table 32, right-hand column).
| PCCT | Current team allocation | Assumed current team allocation for the purposes of workstream D |
|---|---|---|
| Birmingham KIDS | One day and night team, summer. Can add an extra nurse/medic for 8 weeks in winter | 1 |
| Bristol royal WATCH | Two day teams and one night team all year | 2 |
| Leicester COMET | One day team and one night team all year (some flexibility to go to two teams if required) | 1 |
| Nottingham COMET | One day team and one night team all year | 1 |
| North West and North Wales (NWTS) | One day team and one night team all year | 1 |
| Newcastle NECTAR | Two day teams and one night all year | 2 |
| Oxford SORT | One day team and no night team all year | 1 |
| Southampton SORT | One day team and one night team all year | 1 |
| Sheffield EMBRACE | Four day teams all year, one night team in summer and one night team in winter | 2 |
| St Thomas’ London STRS | Two day teams and two night teams all year | 2 |
| Great Ormond Street London CATS | Two day teams and two night teams all year | 2 |
Workstream A provided a summary of historic demand for each PCCT from each of the 212 hospitals over 1 year. Demand for services varies by time of day (lower at night), day of week (lower at weekends) and season (highest in winter). 67 The overall aim of this workstream was to explore configurations of services for the busiest times and so we concentrated on daytime allocation for the baseline configuration of teams. We did, however, explore winter and non-winter configurations as separate scenarios, as the clinical partners indicated that understanding better how to flexibly staff for the annual winter surge was an important research question.
Anatomy of a single paediatric critical care transport team retrieval
A PCCT retrieval begins with a request from a DGH. Once that request is accepted, time starts ticking for the 3-hour time-to-bedside target. First, a team needs to be available to transport the child (which is not always the case). Then, the team needs to mobilise (which takes some time) and travel to the DGH. Travel time can depend on time of day, day of week, season and traffic. Once at the DGH, the team needs to find the patient (which can take some further time). Finally, at the bedside, the ‘time-to-bedside’ clock ends. However, the team then typically takes 1–2 hours to stabilise the patient before transport and then there is further time while the team takes the child to the receiving PICU (which is not necessarily the closest one). Once handover is completed, then the team returns to the PCCT base and it is only then that the team is available for another retrieval. Roundtrips can easily take ≥ 5 hours. The different steps of a single PCCT retrieval are illustrated in Figure 23.
FIGURE 23.
Anatomy of a single PCCT retrieval.
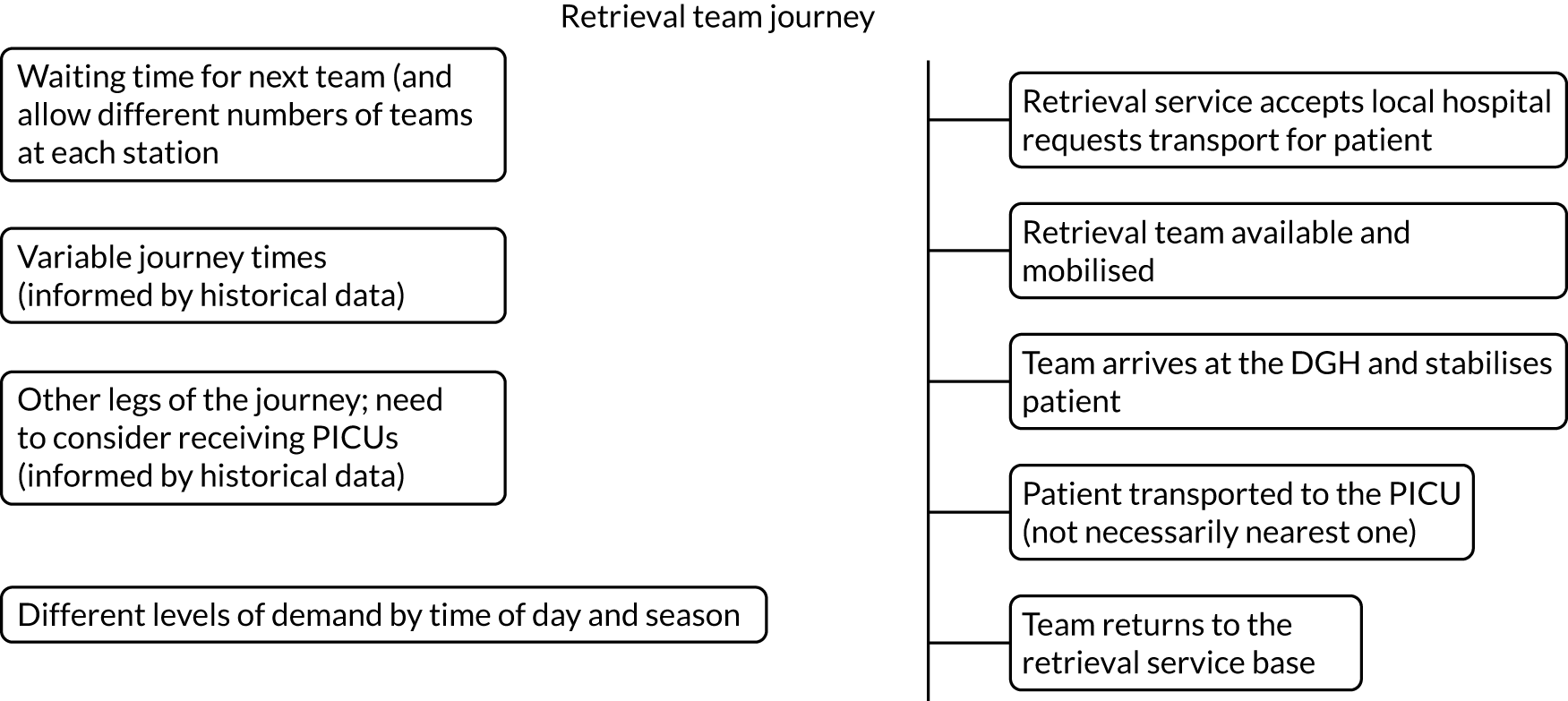
Methods
Overview of approach
We started with a number of simplifying assumptions to gain initial important insights into the current configuration and potential impact of changes to either the configuration of services or to the national standards for time to bedside. We then worked with clinical partners to define scenarios of interest to explore with more sophisticated modelling, relaxing key assumptions about team availability, travel times and demand. Note that the methods described in this chapter have been published in peer-reviewed journals by King et al. 68 and Kung et al. 69
Brief overview of location–allocation modelling
The question of where to locate ‘resource hubs’ serving a number of ‘demand points’ is perfectly suited to a branch of mathematics called ‘location–allocation optimisation’. 70–72 In the context of this project, the resource hubs are the PCCTs and the demand points are DGHs with acute paediatric services and also PICUs (in case of interPICU transfer).
Toregas et al. 73 and Li et al. 74 provide an overview of the basics of location–allocation analysis and its historical development. A general objective in location–allocation research is to consider facilities as a network and then try to determine the ‘effectiveness’ of that network. Popular metrics for effectiveness could be to reduce the total travel distance or time between facilities, such as those used for p-median problems. 73,74 Another metric could be to maximise the coverage of the population with a minimal number of locations, which are called location set and maximal coverage location problems. Problem-specific metrics can be used as well, depending on the context. For example, ambulance location problems can use expected patient survival as a measurement of the quality of facility locations, such as the Maximal Survival Location Problems in Erkut et al. 75 and McCormack and Coates. 76 Most location–allocation problems are solved numerically using either optimisation packages or simulation methods.
A variety of extensions have been developed for location–allocation analysis. Stochastic elements (allowing for some randomness) have been introduced in different ways, including the use of queuing theory and probability. The work of Mirchandani and Odoni77 considered the fluctuations of travel time due to traffic conditions. Daskin78 developed a covering model to account for the possible unavailability of facilities. The hypercube queueing model by Larson79 was employed to model the state of service availability of facilities as a more convenient way to search for optimal locations. Church and ReVelle80 introduced stochasticity into the covering model by guaranteeing coverage to those demanding service with a likelihood above a certain threshold probability, which was later extended81 to consider service availability also.
Location analysis has been applied to the realm of police, ambulance and other emergency services. Larson79 used the hypercube queuing model to divide a district into police patrol beats so as to equalise the workload of each police patrol while minimising response time. The ambulance network was similarly studied,75,82 with the aim to minimise the time of arrival to incident by extending parameters such as capacity requirements and ambulance availability. 82 Queuing models, such as the priority queuing covering location problem,83 have been applied to emergency services as a covering model that allows prioritisation of calls for service. Further analyses have been undertaken on emergency service planning, such as the trade-off between equity and efficiency in the distribution of service to urban and rural areas. 84,85
Initial modelling
We explored scenarios where all PICUs could act as potential hosts for PCCTs and, therefore, as potential resource hubs. Such models have been used to explore the location of emergency medical facilities73,78,86 and general ambulances services. 87 Location–allocation modelling can address questions such as ‘What is the minimum number of PCCTs needed to reach all demand points within a specified period of time?’ and ‘For a given number of PCCTs, where should they be located to minimise the journey time across all demand points and to which PCCT should each demand point be allocated?’. The latter formulation is most relevant to the current situation in England and Wales, as there are 11 current PCCTs, several hundred demand points and the Paediatric Intensive Care Society’s standard is 3 hours from referral acceptance to bedside. We can weight models by volume of demand from DGHs so that hospitals that require PCCT services more are given greater priority in terms of minimising journey time to them from their allocated PCCT.
We assumed that a PCCT was always available to meet a referral and, therefore, time to bedside depended on only mobilisation time, journey time and time between the ambulance parking bay and the patient’s bedside at the DGH. We assumed a constant mobilisation time of 30 minutes (i.e. the Paediatric Intensive Care Society’s standard57) and a constant time of 10 minutes from arriving at the demand hospital to the child’s bedside. Journey times were, therefore, constrained to be 40 minutes less than the required time to bedside. We considered transports performed by ground ambulances only (i.e. not transports performed by air, which comprise < 2% of transports). Another key assumption was that journey times were constant between any two locations, and we weighted our models by historic demand from each DGH.
The initial questions we explored were:
-
What proportion of overall demand can be covered from existing PCCTs for the time-to-bedside standard of 3 hours and if standards were to be reduced following evidence from the DEPICT study? We considered times to bedside of 1 hour (20 minutes travel time), 75 minutes (35 minutes travel time), 90 minutes (50 minutes travel time), 2 hours (80 minutes travel time) and 3 hours (140 minutes travel time).
-
What is the impact of reducing or increasing the number of PCCT locations on meeting different time-to-bedside standards assuming (1) the current locations of PCCTs and (2) PCCTs could be located at any existing PICU or PCCT location?
‘Current locations’ indicated that the mathematical model would be constrained to select PCCT locations from the pool of existing PCCTs if the number of PCCT locations is 11 or fewer. For more than 11 PCCTs, the model used the existing 11 PCCT locations first and could then choose additional PCCTs from any PICUs that are not already PCCTs. The ‘best locations’ formulation allowed the model to choose PCCT locations from any existing PICU or PCCT location, regardless of whether or not a PCCT is currently based there.
Travel time determination
For this initial work, distance and travel time between each PCCT, PICU and DGH was calculated using postcodes within the Google Maps Distance Matrix Application Programming Interface (API) (Google Inc., Mountain View, CA, USA)88 and the R package gmapsdistance (The R Foundation for Statistical Computing, Vienna, Austria). 89 Google’s ‘best guess’ traffic model was used to estimate the travel time in minutes. We performed a sensitivity analysis of the estimated travel times by comparison with the ‘optimistic’ and ‘pessimistic’ traffic models, and this did not change our results.
Software
The model was coded in Python (Python Software Foundation, Wilmington, DE, USA) using the library IBM Decision Optimization CPLEX modelling for Python. 90 Results were mapped using the Google Maps Geocoding API88 and the Python module gmplot. 91
Mathematical formulation
We formulate the problem as a p-median location–allocation optimisation model.
The model is constrained by the number of facilities available to locate. 74,92 In our model, current PCCTs and PICUs represent potential facility locations and DGHs represent demand points. The model’s objective is to minimise travel time between a DGH and its assigned PCCT. Every DGH within the time threshold must be assigned to a PCCT. In some scenarios, an additional constraint was added to select PCCTs from the current PCCTs before adding new locations at PICUs.
The model has the following notation:
-
Inputs:
-
I – demand nodes, indexed by i
-
J – candidate sites, indexed by j
-
K – existing candidate sites, indexed by k
-
dij – travel time between demand node i ϵ I and candidate site j ϵ J
-
p – number of PCCTs to locate
-
r – time threshold for a demand node to be considered covered by a facility
-
hi – population size of demand node i ϵ I, assumed to be 1 ∀ i ϵ I.
-
-
Decision variables:
-
Xj – 1 if we locate at candidate site j ϵ J, 0 otherwise.
-
Yij – 1 if demand at node i ϵ I is allocated to facility at node j ϵ J, 0 otherwise.
-
-
Minimise:
-
Σiϵ1ΣjϵjhidijYij.
-
Subject to:
-
ΣjϵJYij=1∀iϵI (each demand node allocated to one and only one PCCT)
-
ΣjϵJXj=p (exactly p PCCTs located)
-
Yij−Xj≤0∀iϵI;jϵJ (cannot assign a demand node to a PCCT that does not exist)
-
ΣkϵKXk=p,p≤11 (locate existing PCCTs before choosing locations at PICUs)
-
Xj{ϵ{0,1}}∀jϵJ
-
Yij{ϵ{0,1}}∀iϵI;jϵJ.
Extending the model by allowing stochastic journey times and considering the whole journey
We then significantly extended the previous formulation by developing an optimisation framework that generalises the model by dropping some of the more unrealistic assumptions, in particular the assumption of constant travel times and that only time to bedside matters. We now allow the other journey elements to contribute to the objective function, with their contribution weighted according to their importance, as determined by the service user. Setting the weight of time to bedside to 1 and the other weights to zero recovers the initial objective of minimising only time to bedside.
As above, journey times between hospitals are scaled by demand, where demand is the number of requests for PCCT services from each DGH, so that journeys taken more frequently are given more weight. Although demand can vary throughout the year, or even time of day, demand is assumed constant within our optimisation framework. The impact of different demand levels is, instead, examined by exploring different realisations of the optimisation model under different scenarios of demand (e.g. winter vs. non-winter). Note that the methods described in the rest of this section have been published in Kung et al. 69
Notation
The list of notation used in the extended model is given in Table 33.
| Notation | Meaning |
|---|---|
| X j | 1 if station j is operational, 0 otherwise |
| Y ji | 1 if hospital i is served by station j, 0 otherwise |
| I, I | Set of DGHs, number of DGHs |
| J, J | Set of potential PCCT stations, number of potential PCCT stations |
| R, R | Set of PICUs, number of PICUs |
| N | Number of operational PCCT stations |
| d i | Demand for PCCT services over a year for hospital i |
| w k | Weight of the k-th journey leg for k = 1, 2, 3 |
| r(i) | The closest PICU to hospital i |
| tji1 | Journey time from PCCT station j to hospital i |
| tir(i)2 | Journey time from hospital i to PICU r(i) |
| tr(i)j3 | Journey time from PICU r(i) to station j |
| T m | Mobilisation time (30 minutes is assumed) |
| T a | Time from arrival to hospital to patient bedside (10 minutes is assumed) |
| T p | Stabilisation time before transport to PICU (2 hours is assumed) |
| T ji | Combined travel time Tm+tji1+Ta+Tp+tir(i)2+tr(i)j3 |
| Z i | Zi = j if hospital i is served by station j Yji = 1 |
| λ j | Request for transport per minute for PCCT station j (demand) |
| μ j | Service rate: number of patients served per minute for station j (reciprocal of overall time away from base) |
| c j | Number of retrieval teams working at station j |
| ρ j | Utilisation rate: λjcjµj |
Let I, J and R denote the set of DGHs, PCCT stations and PICUs, respectively. The size of these sets are correspondingly denoted by I, J, and R. The set J comprises both the PICUs in the set R and other existing PCCT station locations. Of the J PCCT stations, we limit the number of operating stations to be N. For example, in the current situation N = 11.
Decision variables that identify the operational stations and the hospital allocations are given by:
The objective function is a weighted sum of the three journey times in the PCCT’s round trip. For the trip between the DGH and PICU, we calculate the expected travel time averaged proportionately over all possible PICU destinations from each DGH. The travel times of a team’s journey are labelled tji1 (station j to DGH i), tir(i)2 [DGH i to PICU r(i)] and tr(i)j3 [PICU r(i) to station j]. These times are scaled by the demand of each hospital, denoted by di, and also by weighting the different parts of the journey by a parameter wk, where k = 1, 2, 3. We also extend the formulation by allowing the decision-maker to put different importance on the three main legs of the journey (i.e. time to bedside, time to receiving PICU and time back to base), using weights.
The result is a linear integer optimisation problem:
which is subject to the following constraints:
-
(C1) ΣjYji = 1 for i = 1, 2, . . . , I (each hospital is served by one and only one PCCT station)
-
(C2) Yji – Xj ≤ 0 for i = 1, . . . I and j = 1, . . . J (hospitals must be served by an operational station)
-
(C3) ΣjXj ≤ N (the number of operational stations are at most a fixed number N)
-
(C4) Xj, Xji ϵ {0,1} (variables are binary).
The problem can be solved by standard integer optimisation packages, using mean travel times between pairs of hospitals (either estimated from historical data or using online software such as Google Maps).
Extending the model further by allowing there to be no team available for transport
Another key reason for including all legs of the journey is that total time away from base determines the availability of a team for a new referral and so we are now in a position to be able to allow for the possibility that a team might not be available for a referral, and we do this by turning to a branch of mathematics called queuing theory. 93
Each team at a PCCT station can be considered as a server and the patients as forming a queue, waiting for the server to be free. The queue can be arbitrarily long, as patients are not in a physical queue but are waiting in local hospitals. We also assume that the service is first come, first served. As is standard in modelling demand for emergency services, arrivals are taken to be random, following a Poisson distribution. We cannot, however, assume a simple probability distribution for the service time (i.e. the time that a retrieval team is away from base) and so we assign it a general probability distribution. Therefore, for PCCT station j, we have what is called an M|G|cj|∞ queue. The parameter cj refers to the number of teams working in PCCT station j.
A result from queueing theory allows us to approximate the average waiting time for an available team experienced by a new referral during what is called ‘steady state’ for a simpler M|M|cj|∞ queue, where the waiting time can be explicitly stated in the following way:
where λj = ΣidiYji is the total arrival rate, µj=λjΣidiYjiE[Tji] is the service rate, Tji is the random variable of the round-trip travel time of a team from leaving the station to pick up a patient and returning to the station and ρj=λjcjµj is the utilisation rate or traffic intensity.
We then use Kingman’s formula,94 stated in Equation 4,68 which provides an approximation for the waiting time, Wi, for a more general queue:
where mj is the mean service time and σj is the SD of the service time. The mean service time and the SD of the service time can be estimated from historical data of the journey times between different hospitals. Our final objective function is then given by:
and is subject to the constraints in Equations 1, 2, 4 and 5. This objective function is no longer linear and so requires a different approach to solving it within a reasonable computational time frame.
Solving the non-linear objective problem using a genetic algorithm
We applied a genetic algorithm to approach the non-linear optimisation problem. Although there are various possible heuristic approaches to solve this optimisation, we chose to use a genetic algorithm because of its simplicity in sorting through a vast pool of potential solutions and its ability to combine fragments of optimal features from the population of solutions. Another advantage of a genetic algorithm is that it can be implemented simply and is flexible enough to handle different objective functions for future use (for a detailed introduction to genetic algorithms, see Mitchell). 95 The main challenges in the application of a genetic algorithm are the number of variables and the inclusion of constraints, and we have overcome both obstacles by restructuring our optimisation problem.
Instead of solving both the optimal locations for the PCCT stations and the allocation of DGHs together, the optimisation is split into two stages, where (1) we assume the number and location of PCCT stations are given and solve for them the optimal allocation of DGHs, and then afterwards (2) optimise the configuration of PCCT stations. This process allows us to deconstruct a large problem into several manageable parts, and both problems can be solved by applying a genetic algorithm.
Part 1: paediatric critical care transport team station locations and their number
Given a set of operational PCCT stations, we can reduce the number of variables, Yji, as there are now only N number of possible values of j. The objective function in Equation 3 remains the same, but only the terms where station j is an operational PCCT station remain. The constraints in Equations 1, 2 and 5 will be imposed, whereas the constraints in Equation 4 do not matter at this stage because the operational PCCT stations are already known.
There is another constraint that can be imposed on the variables based on the idea that the practical solution must be one for which each station is able to satisfy the demand of the DGHs allocated to it and not be overloaded and that is rate of new requests should not exceed the rate at which they can be served. Mathematically, the requirement is expressed as:
In summary, when the set of operational PCCT stations are given, we solve the problem:
subject to Equations 1, 2 and 6.
It is possible that this has no feasible points, in which case we arbitrarily assign the answer to be an extremely high number, say 1010.
Part 2: optimising paediatric critical care transport team station locations
We now have a map from the variables ’s to the minimum value obtained by solving Equation 5 (i.e. the allocation of DGHs to PCCT locations j), which we write as F(Xj). The resulting minimisation problem is:
subject to Equation 3.
These two minimisation problems working together yield the optimal location of PCCT stations and allocation of DGHs.
Distribution of teams among selected paediatric critical care transport team locations
The final important problem is to assign the optimal number of teams to each operational station given an overall number of teams. A natural solution would be to treat the number of teams at location j, cj, as a decision variable instead of a parameter, along with the optimal operating stations and hospital allocations, and then apply the genetic algorithm.
However, owing to the non-linearity of the objective function and the size of our problem, we used a simpler method to arrive at team distributions to ensure sensible computational solution times. Our strategy is to overprescribe teams to each station and to use this overprescribed team profile to solve the objective function given in Equation 5. Then, using the obtained retrieval team locations and hospital allocations, teams are sequentially removed from each station using a greedy algorithm, that is, a team is removed if its removal leads to the smallest increase in the optimised objective function value.
Let C denote the number of teams to be distributed and let c′ be the number of teams prescribed to each station. c′ is chosen such that c′ N ≥ C, where N is the number of operational stations. After obtaining the operational station locations and the hospital allocations, the greedy algorithm is employed. The choice of c′, however, can affect the result of its corresponding stations and hospital allocations obtained from Equation 5. For example, the higher value of c′, then the closer the solution would be to the linear optimal solution (without wait time). To mitigate this limitation, we perform the optimisation for several values of c′, apply the greedy algorithm and choose the resulting solution that yields the lowest value of the objective function.
Software
The model was coded in MATLAB® (The MathWorks, Inc., Natick, MA, USA) and solved using intlinprog for integer linear programs and ga for the integer genetic algorithm. The Google Maps Distance Matrix API88 is used to obtain the mean travel times not obtainable from historical PCCT transport data. The Google Maps Geocoding API88 is used to plot the allocations through the gmplot module in Python.
Findings
Initial results
We first solved the simple model to gain insight into the current allocation of PCCT locations across England and Wales. The 24 PICUs and 11 PCCT locations in England and Wales are shown in Figure 24. 68 For this initial work, we used a list of 212 DGHs with acute paediatric services and PICUs that generated demand for PCCT services at least once between 1 January and 31 December 2017 (i.e. about 5000 transports). The list of DGHs with acute paediatric services was obtained from the Royal College of Paediatrics and Child Health and the annual demand for 2017 from each was provided by PICANet. The demand and location from each demand hospital is shown in Figure 25. 68
FIGURE 24.
The 24 PICUs (red) and 11 current PCCT locations (blue) in England and Wales. Map data © 2018 Google. Reproduced with permission from King et al. 68
FIGURE 25.
Map of demand at DGHs, by quintile of demand for PCCT transports during 2017. Map data © 2018 Google. Reproduced with permission from King et al. 68

Question 1: what would the impact of different time-to-bedside thresholds using the 11 existing paediatric critical care transport team locations be?
Figure 2668 shows demand hospitals colour coded by time to patient bedside for the current locations of 11 PCCTs after optimal allocation of demand hospitals to PCCTs (to minimise journey time). There are five hospitals (highlighted in black in Figure 26) not reachable within 3 hours (accounting for 1.3% of total demand), and one is in Cornwall, two are in Wales and two are in Norfolk.
FIGURE 26.
Demand hospitals colour coded by time to bedside with the current configuration of PCCTs (light blue). Demand hospitals reachable within 1 hour, 75 minutes, 90 minutes, 2 hours and 3 hours of their assigned PCCT are coloured blue, green, yellow, orange and red, respectively. Black markers indicate hospitals not reachable within 3 hours. Map data © 2018 Google. Reproduced with permission from King et al. 68
For the current PCCT locations, the vast majority (98%) of demand is reachable within 3 hours (i.e. all but the black-marked hospitals in Figure 26). Significant loss of coverage occurs if the standard is reduced to 2 hours (orange-marked hospitals in Figure 26), 90 minutes (yellow-marked hospitals in Figure 26) or 75 minutes (green-marked hospitals in Figure 26), with 86%, 59% and 33% of demand hospitals reachable, respectively. Less than 20% of the demand can be reached within 1 hour (20 minutes’ travel time once mobilisation time and time after arrival at local hospital are accounted for).
The question then becomes whether or not different PCCT locations can improve the coverage of demand for PCCT services at the different possible time to bed side thresholds.
Question 2: what is the impact of reducing or increasing the number of paediatric critical care transport team locations on meeting different time-to-bedside standards?
We examined the trade-off between the number of PCCT locations and the proportion of demand hospitals reachable within the five different ‘time-to-bedside’ time thresholds, using (1) the current 11 PCCT locations as a starting point and (2) any PICU as a potential PCCT location (Figure 27). 68
FIGURE 27.
Per cent of demand covered with a time to bedside of 1–3 hours for current and optimal PCCT locations (3–27) and optimal demand hospital allocation. The black vertical line indicates the coverage for 11 PCCT locations. Solid and dashed lines represent the current and best locations, respectively.
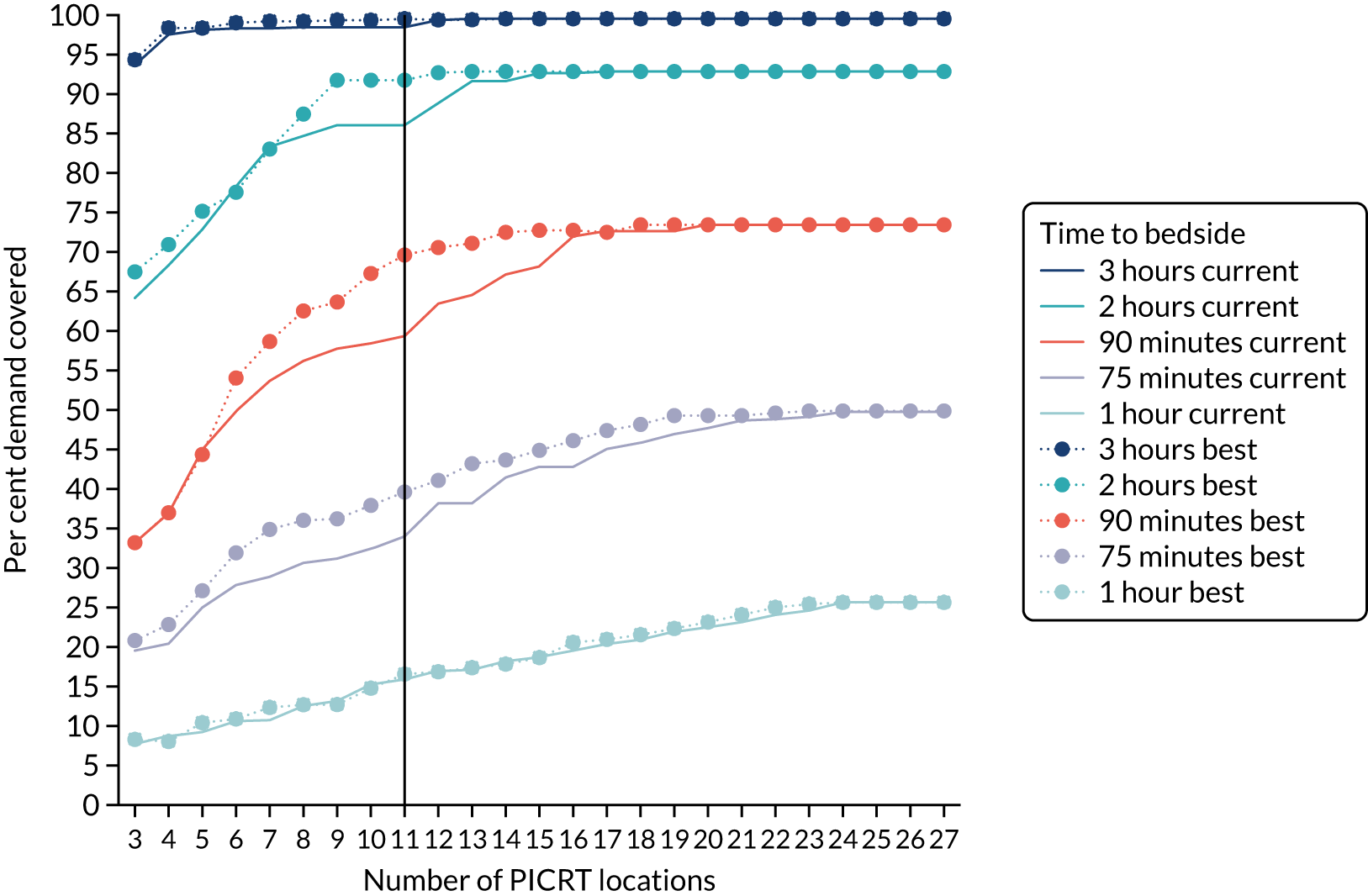
For a 3-hour threshold, there are only marginal gains in coverage for more than eight PCCT locations (see Figure 27). If the time standard is reduced to 2 hours, however, then at least 13 PCCTs are needed to achieve a coverage of around 91% of demand (see solid mid-blue line in Figure 27). This requirement increases to 16, 21 and 24 PCCT locations for 90 minutes, 75 minutes and 1 hour, respectively, and with low achievable coverage (see Figure 27).
When we consider exactly 11 PCCT locations (i.e. the current number of locations), could we improve on time to bedside by allowing them to be located at any of the currently unused PICUs? There is no meaningful difference for a 3- or 1-hour time-to-bedside threshold; however, there are potentially substantial improvements for the other three thresholds, that is, for 11 optimally located PCCTs, 91% of demand is reachable within 2 hours (compared with 86% currently), 69% of demand is reachable with 90 minutes (compared with 59% currently) and 39% of demand is reachable within 75 minutes (compared with 33% currently).
Our initial assumptions are quite basic and unrealistic, but by removing variability they will tend to underestimate time to bedside and so provide an overly optimistic picture. We found that 98% of retrieval demand can be met within the 3-hour standard, with five DGHs not reachable by road within that time, making them candidates for the use of air transport. If the time-to-bedside standard was made more stringent, then a smaller number of DGHs would be accessible within the standard. Recent analysis showed that the median PCCT mobilisation time was 29 minutes (IQR 17–65 minutes). 96 Reducing the PCCT mobilisation time (even by 15 minutes) could have a significant impact, but would, nonetheless, be insufficient to meet most demand for any thresholds below 1.5 hours. Currently, less than 2% of UK PCCT transports involve the use of rotary or fixed wing aircraft, mainly because of the limited availability of aircraft for emergency interhospital transports. More stringent targets could start a national conversation about greater use of air transport or about adding more PCCT locations to reduce road journey times. Note that these results have been previously published in King et al. 68
However, workstream A showed that time to bedside was not significantly associated with worse outcomes and so there was no longer the need to consider service configurations that could significantly shorten time to bedside. Instead, our focus moved to considering if and what changes to services could support the annual winter surge in demand for services. 67,97
First, we expanded the methodology significantly to (1) account for stochastic journey times, (2) use distributions of historical journey times to reflect the availability of ‘blue light’ travel, (3) incorporate queuing theory to take account of the likelihood of a transport team being available at referral (affecting mobilisation times), (4) incorporate journey times for the rest of the transfer, that is, time between local hospital and the receiving PICU and then time from the receiving PICU back to the PCCT base (which affects availability of teams for subsequent transports) and (5) incorporate seasonal effects to capture the winter.
Applying the full methodology to explore service configuration for winter
Data processing
We received pseudonymised data from PICANet for all journeys by PCCT services between 2014 and 2018. These data comprised over 15,000 transports that were used to estimate demand for service from each DGH by time of day and month of year, mean and variance of journey times between hospitals, and the proportion of each hospital’s demand that went to each receiving PICU. Where no journeys between a pair of hospitals was recorded (e.g. a hospital in the South would not be served by a PCCT in the North and so we would not expect a journey between the two), we estimated the mean travel times using Google Maps (see Initial results). The PCCTs, PICUs, DGHs and possible PCCTs remain the same as before. We used weights of w1 = 2, w2 = 2 and w3 = 1 in Equation 5 (i.e. prioritising time to bedside and time to PICU over return to base); however, we note that results were quite insensitive to weights.
A survey of teams carried out by clinical co-applicants showed that there were 16 daytime teams spread across the 11 current PCCT locations (see Table 32).
Specific scenarios considered
Demand varies by time of day and season. 67 As we are considering service configuration to minimise times to bed site and maximise team availability, it makes sense to concentrate on the busiest periods because (1) if this demand can be met, then services will certainly be able to meet demand in less-busy periods and (2) if we use demand averaged out over busy and less-busy periods, then we will overestimate team availability during busy periods.
Therefore, we concentrated on daytime periods, which were as defined as 8 a.m. to 8 p.m. Given the large annual winter surge, we further considered just the winter months (i.e. November–January) and then ‘non-winter’ months (i.e. February–October). The winter period is a month earlier than what is often considered winter because the surge for PIC normally starts in mid-November and ends in mid-January. We considered how best to meet demand during ‘non-winter’ daytimes and then explored the impact of increased demand in winter and if and how that could be mitigated through additional teams allocated to specific PCCT locations.
In consultation with clinical, parent and commissioning partners, we decided to tackle these four questions in turn:
-
Can we better redistribute the 16 teams among current 11 locations?
-
Would deploying 16 teams across a reduced number of the current locations bring benefit?
-
Would adding more teams to locations provide benefit, particularly in winter?
-
Would changing the locations of the current PCCT services provide benefit?
In the results below, we do not show results for fewer than eight locations or for more than 22 overall teams because metrics were consistently much worse for fewer than eight locations on account of journey times being much longer for many DGHs, and waiting times were reduced to near zero and so adding more teams would make no difference to the outcome metrics when there were more than 22 teams.
Question 1: can we better redistribute the 16 teams among current 11 locations?
First, we looked at whether or not we could better redistribute current teams. The optimal allocation of DGHs to the current 11 PCCTs and their current 16 team allocation (see Table 3) resulted in a mean modelled time to bedside of 108 minutes during non-winter daytime. Redistributing those 16 teams across the 11 locations reduced this mean time to bedside to 100 minutes. The improvement in winter is a bit greater, with the average time to bedside reduced from 124 minutes to 110 minutes (the average times to bedside are higher because teams are less likely to be available for immediate deployment because of higher demand). This reduction in time to bedside carries over to overall time to receiving PICU. Perhaps most importantly, the proportion of children reached within 3 hours increases from 87% to 97% of children. In addition, the switch in team allocation to achieve this benefit is minimal, as it moves one of the teams currently allocated to Newcastle Nectar to North West and North Wales, whereas all other allocations stay the same.
Question 2: would deploying 16 teams across a reduced number of the current locations bring benefit?
We now considered if allocating different numbers of teams (i.e. 14–19 teams) across 8, 9 or 10 of the existing 11 PCCT locations provided further benefit, and the results are shown in Appendix 35. In Appendix 35, we have shown the average time to bedside from the current baseline allocation of 16 teams across the 11 locations and the optimal allocation of 16 teams across the 11 locations for ease of comparison.
For 16 available teams, we see that the optimal number of locations is nine, which gives just over 96 minutes average time to bedside; however, the times to bedside are very similar for configurations of 16–19 teams across 8–11 locations, with all possible configurations reaching between 98% and 99% of demand in this scenario.
If we now consider the situation in winter daytime, then we see more variation in times to bedside by number of locations and teams (Figure 28).
FIGURE 28.
Comparing average time to bedside for different numbers of teams across different numbers of PCCT locations (chosen from the current 11 locations) for winter daytime.
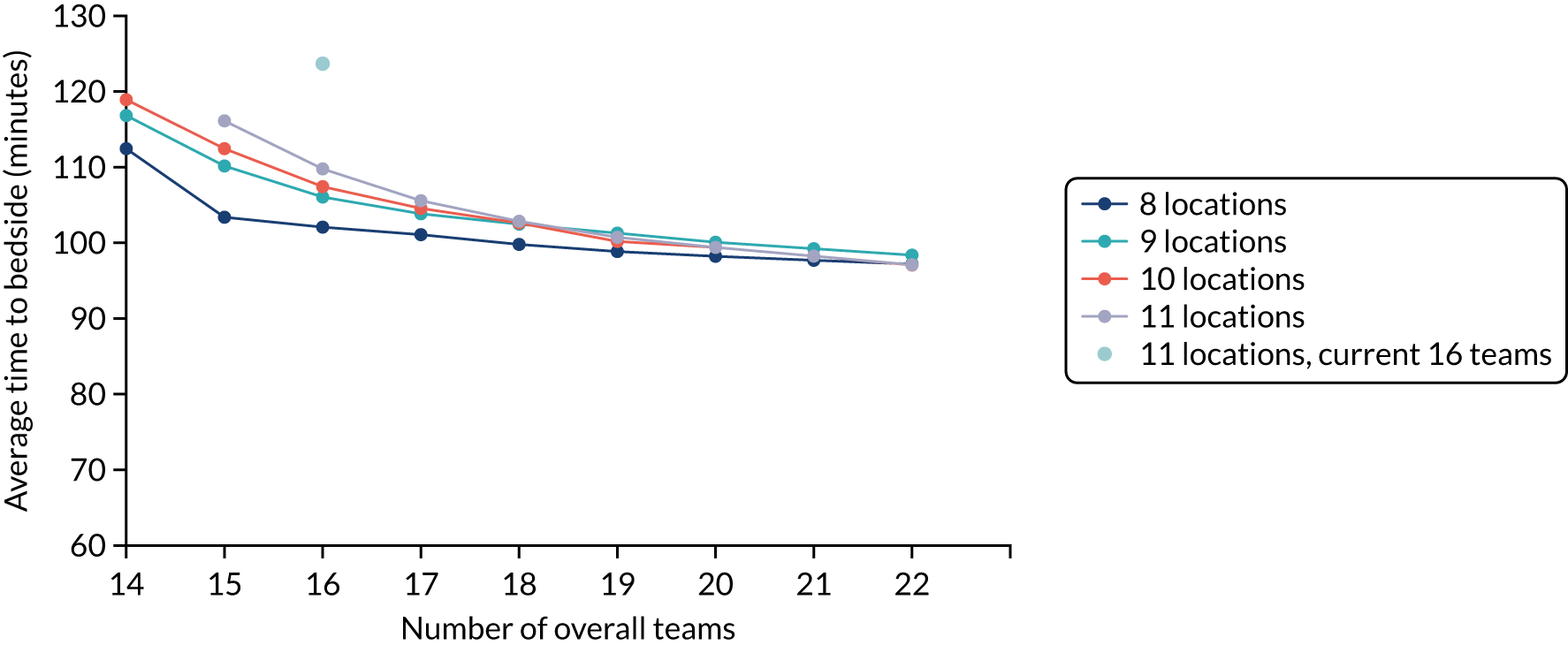
We now see a much clearer beneficial effect in reducing the number of PCCT locations, particularly where the number of teams is less than 19. For the current number of 16 teams, eight locations is preferred, reducing time to bedside by over 20 minutes (i.e. from 124 minutes to 102 minutes) from current team allocation. Changing the optimal 16 team allocation across 11 locations to the optimal 16 team allocation for eight locations reduces time to bedside by almost 10 minutes (i.e. from 110 minutes to 102 minutes). The big impact in moving to fewer locations is that it allows for more teams at each location. A well-known insight from queuing theory is that having more servers (in this case teams) per queue (in this case each PCCT location) results in shorter waiting times, and this effect is more pronounced for higher demand (because you are more like to be in a queue on arrival – in this case, a team is more likely to be unavailable for a new referral). If we look at average waiting time during winter daytime for each configuration of number of PCCT locations and teams, we can see very clearly that fewer locations for any given number of teams results in lower waiting times (see Appendix 36).
The reason that the scenarios are closer together when considering time to bedside (see Figure 28) is that the gains in reduced waiting time for fewer locations is offset, to some extent, by needing to travel further to some DGHs if there are fewer locations.
As well as the reduction of almost 10 minutes in time to bedside when moving from 16 optimally allocated teams across 11 locations to 16 teams across eight locations, there is a reduction of about 7 minutes in time to receiving PICU. However, the proportion of children reached within 3 hours improves by only 0.5 percentage points to 97.4%. When the 16 teams are optimally placed among the 11 locations, then the chances of breaching the 3-hour threshold are greatly reduced.
The map of what eight PCCT locations would look like in terms of geographical coverage of DGHs is shown in Appendix 37. Locations with large patch areas (such as London and the South East) tend to have more teams allocated (however, it also depends on demand from each DGH).
Question 3: would adding more teams to locations provide benefit, particularly in winter?
The second important insight from considering time to bedside for different configurations in Figure 28 is that the average time to bedside of 100 minutes achieved for 11 locations and 16 (optimally allocated) teams can be achieved during winter daytime for 11 locations and 19 teams. This insight suggests that adding three additional teams in winter could go some way to alleviating the winter surge experienced by services. However, there is very little additional benefit to adding more teams beyond that (i.e. time to bedside does not change appreciably after 19 teams in Figure 28).
The other insight from Figure 28 is that when there are 19 teams, then there is little incentive to move from 11 locations to eight locations.
The distribution of 19 teams across the 11 current PCCT locations suggested by the optimisation model in winter daytime are given in Table 34. In the third column of Table 34 we have shown the optimal allocation of 16 teams across the 11 locations. In winter, the optimisation framework suggests allocating an extra team each to Birmingham, London STRS and London CATS.
| PCCT location | Average number of currently allocated daytime teams | 16 optimally allocated PCCTs | 19 optimally allocated PCCTs (winter daytime) |
|---|---|---|---|
| Birmingham KIDS | 1 | 1 | 2 |
| Bristol Royal Infirmary WATCH | 2 | 2 | 2 |
| Leicester COMET | 1 | 1 | 1 |
| Nottingham COMET | 1 | 1 | 1 |
| NWTS (Manchester/Liverpool) | 1 | 2 | 2 |
| Newcastle NECTAR | 2 | 1 | 1 |
| Oxford SORT | 1 | 1 | 1 |
| Southampton SORT | 1 | 1 | 1 |
| EMBRACE (Sheffield) | 2 | 2 | 2 |
| St Thomas’ Hospital STRS (London) | 2 | 2 | 3 |
| Great Ormond Street CATS (London) | 2 | 2 | 3 |
Question 4: would changing the locations of the current paediatric critical care transport team services provide benefit?
Moving existing PCCT locations is the biggest disruption to current service configuration and would carry a cost of reorganisation and, potentially, team morale. Compared with the time to bedside for the optimal 19 teams across eight locations (i.e. 99 minutes), allowing any locations results in an optimal time to bedside that is 4 minutes quicker (i.e. 95 minutes) and an increase in the percentage of children reached within 3 hours from 97.9% to 98.6%. Although it is inevitable that allowing more options will results in improvement, the improvements are not that large. Given the costs associated with such a major configuration, it is unlikely that the marginal benefit would be worth it, especially given workstream A results showing that time to bedside was not significantly associated with outcome.
An overview of the incremental improvements achieved with each configuration change compared with the baseline current configuration is provided in Figure 29 for the highest-demand winter daytime context. When considering the percentage of children reached within 3 hours, there is only marginal benefit in moving from the 11 current locations with 19 teams to the optimal PCCT locations with 19 teams.
FIGURE 29.
Improvements in various metrics for different configurations compared with the baseline scenario of the current 11 locations and 16 allocated teams.

Discussion
Key findings
In this chapter, we developed a mathematical optimisation framework that could be used to explore the impact of different service configurations on key metrics, such as time to bedside, time to PICU and percentage of referred children reached within 3 hours. After starting with a simple framework, we then significantly expanded it to allow for variable journey times and variable team availability, and used detailed historical data on journey times and demand for PCCT services as inputs into the framework.
The simple formulation highlighted that almost all children could be reached within 3 hours of referral and the exceptions were five local hospitals (DGHs) that were very far from the nearest PICU, in Cornwall, Norfolk and Wales. The same analysis also showed that the system was quite finely balanced. For example, if the threshold for time to bedside was to be reduced to ≤ 2 hours, then more locations would be needed to meet demand and, likewise, if the threshold was reduced to < 1 hour, then it would be extremely difficult to meet with centralised PCCT services.
The emerging evidence from workstream A that time to bedside was not significantly associated with outcome prompted us to shift our question to whether or not different team allocations across the current 11 PCCT locations (or a subset of these 11 locations) could improve the proportion of children reached within 3 hours, particularly in winter when services are often severely stretched.
We showed that the current allocation of 16 teams across the 11 PCCT locations could be markedly improved by allocating two teams to NWTS (instead of one team) and one team to Newcastle NECTAR (instead of two teams). During winter, the additional burden of the winter surge could be mitigated by adding three teams to Birmingham KIDS, London CATS and London STRS (one each) for a total of 19 teams across 11 locations.
In general, the modelling showed that in non-winter periods a range of different configurations (in terms of numbers of teams/locations) have very similar performance. At times of higher demand in winter, for a given number of overall teams, fewer locations (e.g. eight locations) tended to give better performance, which is consistent with standard insights from queuing theory. When there were fewer than eight locations, then the improvements in team availability were offset by longer journey times, as locations are inevitably further away from many DGHs.
We note that because workstream A showed no difference in outcomes for different models of care delivery or team composition, we did not incorporate such factors in our optimisation framework.
Implications
The findings suggest that there is, at best, marginal benefit to changing the current PCCT locations. However, significant benefit could be achieved by tweaking the current team allocation (or at least adding a team to NWTS). It is also worth exploring funding additional resource in winter to a total of 19 teams across the 11 locations. Of course, the benefit of having a retrieval team available to transport a child is of less use if PICU beds are scarce in winter, which can equally cause delays in time to PICU.
More broadly, this sort of location–allocation modelling can be a useful tool for exploring different configurations of services, particularly for situations where there is flexibility in the degree of centralisation.
Limitations and further research
Although our model has generated several useful insights, there are some limitations.
We currently assume that the mobilisation time, the time for the team to get to the bedside from ambulance arrival at the DGH and the treatment time at the bedside before transport to the PICU are constant and the same for all PCCT and DGH locations. The mobilisation time and time to reach the child’s bedside are small compared with journey time, but the time at bedside can be long (several hours) and quite variable, depending on the condition of the child. However, for informing decisions about location of services and number of teams at each service, which will depend most on demand and journey times, treating these variables as constant (using observed mean values) seems reasonable.
The Kingman’s formula approximation for waiting time of a general queue presents the main limitation to our modelling framework. One problem posed by the approximation is the assumption that the system is in steady state. Depending on how fast a given scenario converges to the steady state and the volatility of service demand, it may be reasonable to make this assumption and one could argue that assuming steady state is sensible for decisions on long-term service provision. However, if the system does not converge quickly, then this assumption may not hold. In that case, the waiting time could be obtained using alternative methods or the framework must adapt to the change in demand throughout the year. Furthermore, the heuristic approach to solve the non-linearity introduced by the formula cannot guarantee the solution to be globally optimal.
Another limitation is the assumption of a first come, first served protocol for attending new referrals. Although this is normally the case, where two referrals come in at once and only one team is available, then the sicker child is prioritised. However, we have not incorporated such complexity into our framework.
Finally, we have set up a simplified way to determine the number of teams to be allocated to each PCCT location. Having the distribution of teams included as a variable to the optimisation greatly increases the size of the problem and the computational times to solve it are not practical (on the order of days, as opposed to minutes). However, future work could include designing a more efficient algorithm that incorporates the distribution of teams as a variable along with the other variables.
Summary
Location–allocation modelling can be a powerful tool to support service configuration. We showed that for periods of moderate or low demand (outside winter), many different configurations had similar outcomes, providing a lot of flexibility in designing future changes. During winter, we showed that, in general, more teams in fewer locations provided better performance for at least eight locations and fewer than 20 overall teams (too few locations means that DGHs are too far away and many teams means that a team is nearly always available). The current PCCT service could be improved with minor tweaks to current allocation and the addition of three teams overall during winter.
Chapter 7 Discussion
Key findings and interpretation
In this national mixed-methods study of paediatric critical care transport services, we found that the time taken by the transport team to reach the patient’s bedside at the referring hospital (i.e. a national quality standard, with a target of 3 hours) did not affect mortality and length of ventilation, although intensive care LOS was slightly longer when the time to bedside increased. Parents of sick children valued the timely arrival of a transport team, although this was relative to what they expected based on what the local clinicians communicated to them, rather than actual times. Staff interviews revealed that delays in the arrival of a transport team had wider implications for the care of other patients in the hospital. Taken together, these findings suggest that, in the current centralised model of care, the vast majority of referring hospitals do initiate and maintain high-quality care for sick children while waiting for the transport team, but this involves mobilising considerable local resources. Better communication between transport teams and local teams, and with the parents of sick children, regarding expected arrival times and reasons for delay is an important area for quality improvement. The multidisciplinary research team allowed us to draw these conclusions by interpreting the study findings through the lens of clinical experience.
Although there was variation in the way in which different transport teams provided transport care, these care models, for example those relating to team composition and stabilisation approach, did not affect patient outcome. Our finding that a consultant team leader was associated with higher mortality even after adjustment probably reflects the lack of suitable risk-adjustment scores specific for the transport setting. Effective working relationships between the PCCTs and referring hospitals, by close communication and through outreach education, were emphasised as a key factor in ensuring a safe continuum of care from the point the child arrives at the local hospital through to transport and admission to the intensive care unit. The ability of parents to travel with their child in the specialist ambulance (being offered the choice rather than whether or not this choice was used) was highlighted during interviews as an important means to improve patient/family experience.
The health economic evaluation was limited by the availability of complete data relating to team composition; however, team composition did not appear to affect health-care costs and outcomes. This indicates that cost-effectiveness considerations alone should not be used to inform service improvements in paediatric critical care transport. The findings from our mathematical modelling workstream have the potential to inform service commissioners regarding the most optimal locations to base transport teams and how many teams are required nationally. A range of different configurations (in terms of numbers of teams/locations) had similar performance in terms of reaching the child within 3 hours in non-winter (normal-demand) periods; however, during the winter (high-demand) period, adding three teams across the current 11 PCCT locations (up to a total of 19 teams nationally) had the most impact on performance. Rather than planning for paediatric critical care transport on a regional basis, our modelling shows that planning for paediatric transport as a national resource allows cost-effective delivery of high-quality care.
Implications for health care
-
National quality standards from the Paediatric Critical Care Society and quality metrics reported on the NHS England specialised services dashboard should take into account the evidence generated from this study, particularly for the future development of time-to-bedside targets for transport teams, as we did not find that reduction in the time taken to reach the bedside reduced mortality at 30 days.
-
Future national standards, as appropriate, should be developed using both evidence-based research and expert consensus from families, young people and health-care professionals. Regular monitoring should assess the ability of teams within paediatric critical care to meet those standards.
-
Routine measurement of patient/family experience using a standardised questionnaire, based on processes illustrated in this study and associated with a high return rate, should be prioritised by transport teams for national benchmarking.
-
The ability for (both) parents to travel in the ambulance with their child should be incorporated within the service specifications of paediatric critical care transport services.
-
Transport teams should have systems in place to regularly evaluate patient-related critical incidents, either through monthly multidisciplinary mortality/morbidity meetings or case note review, to ensure that relevant lessons are learnt to minimise the chances of a repeat event.
-
Improving clinical communication between the transport team and the referring hospital team should be an area for quality improvement so that parents can be kept fully informed of the need for their child’s transport, the time a transport team is expected and reasons for any delay.
-
Transport teams should focus on initiatives to build confidence among staff in managing the sick child at the referring hospital and to improve senior supervision of more junior members of the team, and staff training should be given on how best to communicate with parents/families at a particularly vulnerable time in their lives.
-
There should be more formal and appropriately resourced outreach education delivered by transport teams to build and maintain effective relationships between the transport team, referring hospitals and intensive care units in the network.
-
Winter surge planning relating to transport should be done at supraregional level, as well as at a regional level, to improve the efficiency of use of limited transport resources.
Recommendations for research
-
The PICANet national audit database is a unique resource, being the only such national data set of paediatric critical care transport and admission to PICU. Future high-quality research in this area is crucially dependent on PICANet data. Improvements to the referrals and transport data sets in terms of collecting more details on the exact staffing of the transport team and greater clinical detail on patient condition at initial referral would be valuable. Similarly, the development of an automated regular linkage between referral, transport and admission events in the PICANet database would allow ready availability of rich clinical data across the entire critically ill child pathway.
-
A key recommendation for data controllers of national data sets, such as HES, ONS, PICANet and PEDW, would be to adopt a much more joined-up and streamlined approach to data access for researchers to allow quicker and easier analysis and, therefore, enable a greater range of research questions to be answered in a timely fashion.
-
Robust risk-adjustment methods that extend beyond mortality outcomes alone in the paediatric critical care research setting are required, as these are essential to minimise the impact of unmeasured confounding, as seen in this study.
-
Critical incidents are a key area for further research, in particular whether or not critical incidents other than those studied are associated with poor outcomes and how to reduce the occurrence and impact of critical incidents. A detailed taxonomy of critical incidents in the transport setting needs to be developed and validated, as this will allow collection of standardised data internationally and benchmarking.
-
Further research is needed to validate the short questionnaire developed in this study to collect patient/family experience in the paediatric transport setting.
-
Better understanding of the factors that help and limit effective communication between transport teams, referring hospitals and patients/parents is needed. In addition, there is a need to identify specific points in the transport process where interventions to improve communication might be beneficial.
-
Future research should explore how best to maximise the involvement of critically ill children in future critical care transport research, recognising that the majority of children will not be able to provide feedback because they are usually sedated on a ventilator during transport, and that over half of the patients are infants aged < 1 year. However, some children will be able to contribute their views and it is important to maximise the opportunities to collect feedback from children who can provide it, including a flexible approach to individualising the way in which such information is collected.
Acknowledgements
We thank the National Institute for Health and Care Research Health and Social Care Delivery Research programme for funding this study. We are very grateful to all of the parents and staff members who took part in the DEPICT study.
We would like to thank Lee Norman (PICANet) and Izabella Orzechowska (ICNARC) for their assistance with the data extraction for this study. We would like to acknowledge Rachel Lundy for her assistance in interpretation of the study findings. We would like to thank PICANet and ICNARC for providing data for this study, and particularly acknowledge the support of the transport teams and PICUs that have submitted data used in this study. PICANet is commissioned by the HQIP as part of the National Clinical Audit and Patient Outcomes Programme. HQIP is led by a consortium of the Academy of Medical Royal Colleges, the Royal College of Nursing and National Voices. The aim of HQIP is to promote quality improvement in patient outcomes and, in particular, to increase the impact that clinical audit, outcome review programmes and registries have on health-care quality in England and Wales. HQIP holds the contract to commission, manage and develop the National Clinical Audit and Patient Outcomes Programme, comprising around 40 projects covering care provided to people with a wide range of medical, surgical and mental health conditions. The programme is funded by NHS England, the Welsh Government and, with some individual projects, other devolved administrations and crown dependencies [URL: www.hqip.org.uk/national-programmes (accessed 28 June 2022)].
Participating sites (principal investigators)
We acknowledge that there have been many other individuals who made a contribution within the participating sites.
Paediatric intensive care unit sites
-
Great Ormond Street Hospital NHS Foundation Trust: Padmanabhan Ramnarayan and Samiran Ray.
-
Birmingham Children’s Hospital NHS Foundation Trust: Sanjay Revanna.
-
Guy’s and St Thomas’ NHS Foundation Trust: Benedict Griffiths.
-
Central Manchester University Hospitals NHS Foundation Trust: Dorthe Grainger.
-
Sheffield Children’s NHS Foundation Trust: Fatemah Rajah.
-
University Hospitals of Bristol NHS Foundation Trust: Peter Davis.
-
The Newcastle upon Tyne Hospitals NHS Foundation Trust: Aravind Kashyap.
-
Nottingham University Hospitals NHS Trust: Patrick Davies.
-
University Hospitals of Leicester NHS Trust: Peter Barry.
-
University Hospital Southampton NHS Foundation Trust: Gareth Jones.
-
Imperial College Healthcare NHS Trust: David Inwald.
-
King’s College Hospital NHS Foundation Trust: Akash Deep.
-
Addenbrookes Hospital NHS Trust: Nazima Pathan.
-
Royal Brompton and Harefield NHS Foundation Trust: Angela Aramburo.
-
Alder Hey Children’s NHS Foundation Trust: Nayan Shetty.
-
University Hospitals of North Midlands NHS Trust: John Alexander.
-
The Leeds Teaching Hospitals NHS Trust: Joanne Lumsden.
-
St George’s University Hospital NHS Foundation Trust: Nick Prince.
-
Barts Health NHS Trust: Naomi Edmonds.
-
Oxford University Hospitals NHS Foundation Trust: Avishay Sarfatti.
-
Noah’s Ark Children’s Hospital for Wales: Siva Oruganti.
District general hospital sites
-
St Mary’s Hospital, Isle of Wight: Mariam Rice.
-
Countess of Chester Hospital NHS Foundation Trust: Susie Holt.
-
United Lincolnshire Hospitals NHS Trust: David Broodbank.
-
Aneurin Bevan University Health Board, Royal Gwent Hospital: Youssef Abourahma.
-
Royal Cornwall Hospitals NHS Trust: Julian Berry.
-
Basildon University Hospital: Sanjay Rawal.
Chief investigator and co-investigators
Dr Padmanabhan Ramnarayan, Professor Elizabeth Draper, Dr Jo Wray, Professor Steve Morris, Dr Christina Pagel, Dr Will Marriage, Dr Fatemah Rajah, Dr Patrick Davies, Eithne Polke, Paul Mouncey, Rachel Lundy, Matthew Entwistle and Anna Pearce.
Study Management Group
Victoria Barber, Robert Darnell, Patrick Davies, Matthew Entwistle, Ruth Evans, Emma Hudson, Enoch Kung, Will Marriage, Stephen Morris, Paul Mouncey, Anna Pearce, Eithne Polke, Elizabeth S Draper, Christina Pagel, Fatemah Rajah, Padmanabhan Ramnarayan, Sarah E Seaton and Jo Wray (previous members: Laura Drikite and Nicholas Hudson).
Study Steering Committee
Dr Mark Terris (chairperson), Mr Dermot Shortt, Dr Padmanabhan Ramnarayan, Ms Shelley Marsh, Dr Richard Feltbower, Dr Jo Wray, Professor Elizabeth Draper, Dr Mary Cardwell, Dr Anne Davies, Mr Gezz Zwanenberg, Dr Tracy Long-Sutehall and Ms Lynsey Freeburn.
Independent Data Monitoring and Ethics Committee
Dr Jillian MacFadzean (chairperson), Professor Stavros Petrou, Dr Lyvonne Tume, Ms Melpo Kapetanstrataki and Ms Madeleine Wang.
Contributions of authors
Padmanabhan Ramnarayan (https://orcid.org/0000-0003-0784-8154) (Consultant in PIC and Retrieval) conceived the study, led the grant application and wrote the study protocol; was responsible for ethics and governance, budget management and development of patient information; contributed to acquisition, analysis and interpretation of the data; and drafted and critically reviewed the manuscript.
Sarah Seaton (https://orcid.org/0000-0001-8711-4817) (Study Statistician) contributed to the conduct of the study; led the acquisition, analysis and interpretation of the data in workstream A; and drafted and critically reviewed the manuscript.
Ruth Evans (https://orcid.org/0000-0002-4168-3544) (Qualitative Researcher) contributed to the conduct of the study; developed patient information resources; led the acquisition, analysis and interpretation of the data in workstream B; and drafted and critically reviewed the manuscript.
Victoria Barber (https://orcid.org/0000-0003-2054-4163) (Qualitative Researcher) contributed to the conduct of the study; led the acquisition, analysis and interpretation of the data in workstream B; and drafted and critically reviewed the manuscript.
Emma Hudson (https://orcid.org/0000-0002-0505-5049) (Health Economist) contributed to the conduct of the study; led the acquisition, analysis and interpretation of the data in workstream C; and drafted and critically reviewed the manuscript.
Enoch Kung (https://orcid.org/0000-0001-7174-8057) (Operational Researcher) contributed to the conduct of the study; led the acquisition, analysis and interpretation of the data in workstream B; and drafted and critically reviewed the manuscript.
Matthew Entwistle (https://orcid.org/0000-0001-7964-1588) (Patient Representative) reviewed patient information; contributed to the design of the trial; contributed to the interpretation of the data; and critically reviewed the manuscript.
Anna Pearce (Patient Representative) reviewed patient information; contributed to the design of the trial; contributed to the interpretation of the data; and critically reviewed the manuscript.
Patrick Davies (https://orcid.org/0000-0001-6540-6368) (Clinical Representative) contributed to the design of the study; contributed to the interpretation of the data; and critically reviewed the manuscript.
Will Marriage (https://orcid.org/0000-0001-7081-4913) (Clinical Representative) contributed to the design of the study; contributed to the interpretation of the data; and critically reviewed the manuscript.
Paul Mouncey (https://orcid.org/0000-0002-8510-8517) (Clinical Trials Unit Head of Research) contributed to the design of the study; contributed to the acquisition, analysis and interpretation of the data; and critically reviewed the manuscript.
Eithne Polke (Nurse Representative) contributed to the design of the study; contributed to the interpretation of the data; and critically reviewed the manuscript.
Fatemah Rajah (https://orcid.org/0000-0003-2053-6702) (Clinical Representative) contributed to the design of the study; contributed to the interpretation of the data; and critically reviewed the manuscript.
Nicholas Hudson (https://orcid.org/0000-0002-0127-421X) (Research Assistant) supported management of the study; contributed to the acquisition and interpretation of the data; and critically reviewed the manuscript.
Robert Darnell (https://orcid.org/0000-0003-4490-1962) (Study Administrator) supported management of the study; contributed to the acquisition and interpretation of the data; and drafted and critically reviewed the manuscript.
Elizabeth Draper (https://orcid.org/0000-0001-9340-8176) (Professor of Perinatal and Paediatric Epidemiology) conceived the study; contributed to the grant application and wrote the study protocol; led workstream A (data linkage and analysis); contributed to acquisition, analysis and interpretation of the data; and drafted and critically reviewed the manuscript.
Jo Wray (https://orcid.org/0000-0002-4769-1211) (Senior Research Fellow, Health Psychology) conceived the study; contributed to the grant application and wrote the study protocol; led workstream B (questionnaires and qualitative study); contributed to acquisition, analysis and interpretation of the data; and drafted and critically reviewed the manuscript.
Stephen Morris (https://orcid.org/0000-0002-5828-3563) (Professor of Health Services Research) contributed to the grant application and wrote the study protocol; led workstream C (health economic evaluation); contributed to analysis and interpretation of the data; and drafted and critically reviewed the manuscript.
Christina Pagel (https://orcid.org/0000-0002-2857-1628) (Professor of Clinical Operational Research) conceived the study; contributed to the grant application and wrote the study protocol; led workstream D (mathematical modelling); contributed to acquisition, analysis and interpretation of the data; and drafted and critically reviewed the manuscript.
Publications
King M, Ramnarayan P, Seaton SE, Pagel C, DEPICT Study Group. Modelling the allocation of paediatric intensive care retrieval teams in England and Wales. Arch Dis Child 2019;104:962–6.
Ramnarayan P, Evans R, Draper ES, Seaton SE, Wray J, Morris S, Pagel C, DEPICT Study Investigators. Differences in access to Emergency Paediatric Intensive Care and care during Transport (DEPICT): study protocol for a mixed methods study. BMJ Open 2019;9:e028000.
Seaton SE, Ramnarayan P, Davies P, Hudson E, Morris S, Pagel C, et al. Does time taken by paediatric critical care transport teams to reach the bedside of critically ill children affect survival? A retrospective cohort study from England and Wales. BMC Pediatr 2020;20:301.
Seaton SE, Draper ES, Pagel C, Rajah F, Wray J, Ramnarayan P, DEPICT Study Team. The effect of care provided by paediatric critical care transport teams on mortality of children transported to paediatric intensive care units in England and Wales: a retrospective cohort study. BMC Pediatr 2021;21:217.
Evans REC, Barber V, Seaton S, Draper ES, Rajah F, Pagel C, et al. Development of a parent experience measure for paediatric critical care transport teams. Nurs Crit Care 2022;27:367–74.
Data-sharing statement
Data can be obtained from the corresponding author, following review and approval.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Metcalfe D, Bouamra O, Parsons NR, Aletrari MO, Lecky FE, Costa ML. Effect of regional trauma centralization on volume, injury severity and outcomes of injured patients admitted to trauma centres. Br J Surg 2014;101:959-64. https://doi.org/10.1002/bjs.9498.
- Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. https://doi.org/10.1001/jama.280.20.1747.
- Field D, Hodges S, Mason E, Burton P. Survival and place of treatment after premature delivery. Arch Dis Child 1991;66:408-11. https://doi.org/10.1136/adc.66.4_Spec_No.408.
- Department of Health and Social Care, Health Service Directorate . Paediatric Intensive Care: ‘A Framework for the Future’ – Report from the National Coordinating Group on Paediatric Intensive Care to the Chief Executive of the NHS Executive 1997.
- Ramnarayan P, Polke E. The state of paediatric intensive care retrieval in Britain. Arch Dis Child 2012;97:145-9. https://doi.org/10.1136/adc.2010.204503.
- Ramnarayan P, Thiru K, Parslow RC, Harrison DA, Draper ES, Rowan KM. Effect of specialist retrieval teams on outcomes in children admitted to paediatric intensive care units in England and Wales: a retrospective cohort study. Lancet 2010;376:698-704. https://doi.org/10.1016/S0140-6736(10)61113-0.
- Paediatric Intensive Care Audit Network . Paediatric Intensive Care Audit Network: Annual Report 2015 2015.
- Colville G, Orr F, Gracey D. ‘The worst journey of our lives’: parents’ experiences of a specialised paediatric retrieval service. Intensive Crit Care Nurs 2003;19:103-8. https://doi.org/10.1016/S0964-3397(03)00022-3.
- Colville G, Darkins J, Hesketh J, Bennett V, Alcock J, Noyes J. The impact on parents of a child’s admission to intensive care: integration of qualitative findings from a cross-sectional study. Intensive Crit Care Nurs 2009;25:72-9. https://doi.org/10.1016/j.iccn.2008.10.002.
- Colville GA, Gracey D. Mothers’ recollections of the paediatric intensive care unit: associations with psychopathology and views on follow up. Intensive Crit Care Nurs 2006;22:49-55. https://doi.org/10.1016/j.iccn.2005.04.002.
- Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the Brain Trauma Foundation guidelines, executive summary. Neurosurgery 2019;84:1169-78. https://doi.org/10.1093/neuros/nyz051.
- Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 2020;21:e52-106. https://doi.org/10.1097/PCC.0000000000002198.
- Ramsay AI, Morris S, Hoffman A, Hunter RM, Boaden R, McKevitt C, et al. Effects of centralizing acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46:2244-51. https://doi.org/10.1161/STROKEAHA.115.009723.
- Moran CG, Lecky F, Bouamra O, Lawrence T, Edwards A, Woodford M, et al. Changing the system: major trauma patients and their outcomes in the NHS (England) 2008–17. E Clinical Medicine 2018;2–3:13-21. https://doi.org/10.1016/j.eclinm.2018.07.001.
- Seaton SE, Draper ES, Pagel C, Rajah F, Wray J, Ramnarayan P. DEPICT Study Team . The effect of care provided by paediatric critical care transport teams on mortality of children transported to paediatric intensive care units in England and Wales: a retrospective cohort study. BMC Pediatr 2021;21. https://doi.org/10.1186/s12887-021-02689-x.
- Seaton SE, Ramnarayan P, Davies P, Hudson E, Morris S, Pagel C, et al. Does time taken by paediatric critical care transport teams to reach the bedside of critically ill children affect survival? A retrospective cohort study from England and Wales. BMC Pediatr 2020;20. https://doi.org/10.1186/s12887-020-02195-6.
- Slater A, Shann F, Pearson G. Paediatric Index of Mortality (PIM) Study Group. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003;29:278-85. https://doi.org/10.1007/s00134-002-1601-2.
- Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007;115:654-7. https://doi.org/10.1161/CIRCULATIONAHA.105.594929.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: Wiley; 2000.
- Brier GW. Verification of forecasts expressed in terms of probability. Mon Weather Rev 1950;78:1-3. https://doi.org/10.1175/1520-0493(1950)078<0001:VOFEIT>2.0.CO;2.
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999;28:964-74. https://doi.org/10.1093/ije/28.5.964.
- Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332. https://doi.org/10.1136/bmj.332.7549.1080.
- Ramnarayan P, Evans R, Draper ES, Seaton SE, Wray J, Morris S, et al. DEPICT Study Investigators . Differences in access to Emergency Paediatric Intensive Care and care during Transport (DEPICT): study protocol for a mixed methods study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028000.
- Seaton SE, Ramnarayan P, Pagel C, Davies P, Draper ES. DEPICT Study Team . Impact on 30-day survival of time taken by a critical care transport team to reach the bedside of critically ill children. Intensive Care Med 2020;46:1953-5. https://doi.org/10.1007/s00134-020-06149-5.
- Pearson G, Shann F, Barry P, Vyas J, Thomas D, Powell C, et al. Should paediatric intensive care be centralised? Trent versus Victoria. Lancet 1997;349:1213-17. https://doi.org/10.1016/S0140-6736(96)12396-5.
- Gemke RJ. Centralisation of paediatric intensive care to improve outcome. Lancet 1997;349:1187-8. https://doi.org/10.1016/S0140-6736(05)62407-5.
- Pollack MM, Alexander SR, Clarke N, Ruttimann UE, Tesselaar HM, Bachulis AC. Improved outcomes from tertiary center pediatric intensive care: a statewide comparison of tertiary and nontertiary care facilities. Crit Care Med 1991;19:150-9. https://doi.org/10.1097/00003246-199102000-00007.
- Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017;45:1061-93. https://doi.org/10.1097/CCM.0000000000002425.
- Vos GD, Nissen AC H M, Nieman F, Meurs MMB, van Waardenburg DA, Ramsay G, et al. Comparison of interhospital pediatric intensive care transport accompanied by a referring specialist or a specialist retrieval team. Intensive Care Med 2004;30:302-8. https://doi.org/10.1007/s00134-003-2066-7.
- Moynihan K, McSharry B, Reed P, Buckley D. Impact of retrieval, distance traveled, and referral center on outcomes in unplanned admissions to a national PICU. Pediatr Crit Care Med 2016;17:e34-42. https://doi.org/10.1097/PCC.0000000000000586.
- Paediatric Intensive Care Society . Quality Standards for the Care of Critically Ill Children 2015.
- NHS England . PICU Quality Dashboard 2018 19 n.d. www.england.nhs.uk/wp-content/uploads/2018/03/picu-metric-definitions-2018-19.pdf (accessed 29 March 2021).
- Kandil SB, Sanford HA, Northrup V, Bigham MT, Giuliano JS. Transport disposition using the Transport Risk Assessment in Pediatrics (TRAP) score. Prehosp Emerg Care 2012;16:366-73. https://doi.org/10.3109/10903127.2012.664246.
- Leslie A, Stephenson T. Neonatal transfers by advanced neonatal nurse practitioners and paediatric registrars. Arch Dis Child Fetal Neonatal Ed 2003;88:F509-12. https://doi.org/10.1136/fn.88.6.F509.
- Fenton AC, Leslie A. Who should staff neonatal transport teams?. Early Hum Dev 2009;85:487-90. https://doi.org/10.1016/j.earlhumdev.2009.05.006.
- Borrows EL, Lutman DH, Montgomery MA, Petros AJ, Ramnarayan P. Effect of patient- and team-related factors on stabilization time during pediatric intensive care transport. Pediatr Crit Care Med 2010;11:451-6. https://doi.org/10.1097/PCC.0b013e3181e30ce7.
- National Institute for Health and Care Research (NIHR) . Themed Review: Improving Care by Using Patient Feedback 2019.
- de Silva D. Measuring Patient Experience. London: The Health Foundation; 2013.
- Wray J, Hobden S, Knibbs S, Oldham G. Hearing the voices of children and young people to develop and test a patient-reported experience measure in a specialist paediatric setting. Arch Dis Child 2018;103:272-9. https://doi.org/10.1136/archdischild-2017-313032.
- Lindeke L, Fulkerson J, Chesney M, Johnson L, Savik K. Children’s perceptions of healthcare survey. Nurs Adm Q 2009;33:26-31. https://doi.org/10.1097/01.NAQ.0000343345.70666.6d.
- Mangurten J, Scott SH, Guzzetta CE, Clark AP, Vinson L, Sperry J, et al. Effects of family presence during resuscitation and invasive procedures in a pediatric emergency department. J Emerg Nurs 2006;32:225-33. https://doi.org/10.1016/j.jen.2006.02.012.
- Curley MA, Meyer EC, Scoppettuolo LA, McGann EA, Trainor BP, Rachwal CM, et al. Parent presence during invasive procedures and resuscitation: evaluating a clinical practice change. Am J Respir Crit Care Med 2012;186:1133-9. https://doi.org/10.1164/rccm.201205-0915OC.
- Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, et al. Life after critical illness in children: toward an understanding of pediatric post-intensive care syndrome. J Pediatr 2018;198:16-24. https://doi.org/10.1016/j.jpeds.2017.12.084.
- Bronner MB, Peek N, Knoester H, Bos AP, Last BF, Grootenhuis MA. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol 2010;35:966-74. https://doi.org/10.1093/jpepsy/jsq004.
- Marsac ML, Kassam-Adams N, Delahanty DL, Widaman KF, Barakat LP. Posttraumatic stress following acute medical trauma in children: a proposed model of bio-psycho-social processes during the peri-trauma period. Clin Child Fam Psychol Rev 2014;17:399-411. https://doi.org/10.1007/s10567-014-0174-2.
- Office for National Statistics . 2011 Census n.d. www.ons.gov.uk/census/2011census (accessed 4 May 2021).
- Davis P, Stutchfield C, Evans TA, Draper E. Increasing admissions to paediatric intensive care units in England and Wales: more than just rising a birth rate. Arch Dis Child 2018;103:341-5. https://doi.org/10.1136/archdischild-2017-313915.
- Toomey SL, Elliott MN, Zaslavsky AM, Quinn J, Klein DJ, Wagner S, et al. Improving response rates and representation of hard-to-reach groups in family experience surveys. Acad Pediatr 2019;19:446-53. https://doi.org/10.1016/j.acap.2018.07.007.
- Evans REC, Barber V, Seaton S, Draper ES, Rajah F, Pagel C, et al. Development of a parent experience measure for paediatric critical care transport teams [published online ahead of print May 24 2021]. Nurs Crit Care 2021. https://doi.org/10.1111/nicc.12648.
- Geoghegan S, Oulton K, Bull C, Brierley J, Peters M, Wray J. The experience of long-stay parents in the ICU: a qualitative study of parent and staff perspectives. Pediatr Crit Care Med 2016;17:e496-501. https://doi.org/10.1097/PCC.0000000000000949.
- Tinsley C, Hill JB, Shah J, Zimmerman G, Wilson M, Freier K, et al. Experience of families during cardiopulmonary resuscitation in a pediatric intensive care unit. Pediatrics 2008;122:e799-804. https://doi.org/10.1542/peds.2007-3650.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Helseth S, Slettebø A. Research involving children: some ethical issues. Nurs Ethics 2004;11:298-30. https://doi.org/10.1191/0969733004ne697oa.
- Wray J, Oldham G. Using parent-reported experience measures as quality improvement tools in paediatric cardiothoracic services: making it happen. Int J Qual Health Care 2020;32:140-8. https://doi.org/10.1093/intqhc/mzaa001.
- Neuendorf KA. The Content Analysis Guidebook. Thousand Oaks, CA: SAGE Publications Ltd; 2002.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Paediatric Intensive Care Society . Standards for the Care of Critically Ill Children 2010. www.picsociety.uk/wp-content/uploads/2015/10/PICS_standards_2010.pdf (accessed 14 July 2018).
- NHS England . Schedule 2: The Services – Paediatric Critical Care Transport 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- NHS England and NHS Improvement . National Cost Collection 2019 2020. www.england.nhs.uk/wp-content/uploads/2020/08/1_-_NCC_Report_FINAL_002.pdf (accessed 26 May 2021).
- NHS England . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection (accessed 25 May 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: PSSRU, University of Kent; 2020.
- Brown KL, Pagel C, Ridout D, Wray J, Tsang VT, Anderson D, et al. Early morbidities following paediatric cardiac surgery: a mixed-methods study. Health Serv Deliv Res 2020;8. https://doi.org/10.3310/hsdr08300.
- University of Sheffield . Measuring and Valuing Health: Health Utilities Index (HUI2) n.d. www.sheffield.ac.uk/scharr/sections/heds/mvh/hui2 (accessed 17 February 2021).
- McCabe C, Stevens K, Roberts J, Brazier J. Health state values for the HUI 2 descriptive system: results from a UK survey. Health Econ 2005;14:231-44. https://doi.org/10.1002/hec.925.
- National Institute for Health and Care Research . Critically Ill Children and Young People: Do National Differences in Access to Emergency Paediatric Intensive Care and Care During Transport Affect Clinical Outcomes and Patient Experience? The DEPICT Study n.d. www.fundingawards.nihr.ac.uk/award/15/136/45 (accessed 26 May 2021).
- Pagel C, Lutman D, Polke E, Ray S, Ramnarayan P. Managing the winter surge in demand for resources. Br J Health Care Manag 2016;22:370-9. https://doi.org/10.12968/bjhc.2016.22.7.370.
- King M, Ramnarayan P, Seaton SE, Pagel C. DEPICT Study Group . Modelling the allocation of paediatric intensive care retrieval teams in England and Wales. Arch Dis Child 2019;104:962-6. https://doi.org/10.1136/archdischild-2018-316056.
- Kung E, Seaton SE, Ramnarayan P, Pagel C. Using a genetic algorithm to solve a non-linear location allocation problem for specialised children’s ambulances in England and Wales. Health Syst 2021:1-11. https://doi.org/10.1080/20476965.2021.1908176.
- Smith HK, Laporte G, Harper PR. Locational analysis: highlights of growth to maturity. J Oper Res Soc 2009;60:S140-S8. https://doi.org/10.1057/jors.2008.172.
- Batta R, Lejeune M, Prasad S. Public facility location using dispersion, population, and equity criteria. Eur J Oper Res 2014;234:819-29. https://doi.org/10.1016/j.ejor.2013.10.032.
- Hakimi SL. Optimum distribution of switching centers in a communication network and some related graph theoretic problems. Oper Res 1965;13:462-75. https://doi.org/10.1287/opre.13.3.462.
- Toregas C, Swain R, ReVelle C, Bergman L. The location of emergency service facilities. Oper Res 1971;19:1363-73. https://doi.org/10.1287/opre.19.6.1363.
- Li X, Zhao Z, Zhu X, Wyatt T. Covering models and optimization techniques for emergency response facility location and planning: a review. Math Methods Oper Res 2011;74:281-310. https://doi.org/10.1007/s00186-011-0363-4.
- Erkut E, Ingolfsson A, Sim T, Erdoğan G. Computational comparison of five maximal covering models for locating ambulances. Geogr Anal 2009;41:43-65. https://doi.org/10.1111/j.1538-4632.2009.00747.x.
- McCormack RJ, Coates G. A simulation model to enable the optimization of ambulance fleet allocation and base station location for increased patient survival. Eur J Oper Res 2015;247:294-309. https://doi.org/10.1016/j.ejor.2015.05.040.
- Mirchandani PB, Odoni AR. Locations of medians on stochastic networks. Transp Sci 1979;13:85-97. https://doi.org/10.1287/trsc.13.2.85.
- Daskin MS. Application of an expected covering model to emergency medical service system design. Decis Sci 1982;13:416-39. https://doi.org/10.1111/j.1540-5915.1982.tb00159.x.
- Larson RC. A hypercube queuing model for facility location and redistricting in urban emergency services. Comput Oper Res 1974;1:67-95. https://doi.org/10.1016/0305-0548(74)90076-8.
- Church R, ReVelle C. The maximal covering location problem. Pap Reg Sci Assoc 1974;32:101-18. https://doi.org/10.1007/BF01942293.
- ReVelle C, Hogan K. The maximum availability location problem. Transp Sci 1989;23:192-200. https://doi.org/10.1287/trsc.23.3.192.
- Shiah D-M, Chen S-W. Ambulance Allocation Capacity Model n.d. https://doi.org/10.1109/HEALTH.2007.381600.
- Silva F, Serra D. Locating emergency services with different priorities: the priority queuing covering location problem. J Oper Res Soc 2008;59:1229-38. https://doi.org/10.1057/palgrave.jors.2602473.
- Burkey ML, Bhadury J, Eiselt HA. A location-based comparison of health care services in four U.S. states with efficiency and equity. Socioecon Plann Sci 2012;46:157-63. https://doi.org/10.1016/j.seps.2012.01.002.
- Achabal DD, Schoeman ME. An examination of alternative emergency ambulance systems: contributions from an economic geography perspective. Soc Sci Med Med Geogr 1979;13:81-6. https://doi.org/10.1016/0160-8002(79)90054-6.
- Coskun N, Erol R. An optimization model for locating and sizing emergency medical service stations. J Med Syst 2010;34:43-9. https://doi.org/10.1007/s10916-008-9214-0.
- Brotcorne L, Laporte G, Semet F. Ambulance location and relocation models. Eur J Oper Res 2003;147:451-63. https://doi.org/10.1016/S0377-2217(02)00364-8.
- Google Developers . Distance Matrix API Overview n.d. https://developers.google.com/maps/documentation/distance-matrix/intro (accessed 23 July 2013).
- Melo RA, Zarruck D. Gmapsdistance: Distance and Travel Time Between Two Points from Google Maps n.d. https://CRAN.R-project.org/package=gmapsdistance (accessed 10 April 2021).
- IBM . IBM® Decision Optimization CPLEX® Modeling for Python n.d. http://ibmdecisionoptimization.github.io/docplex-doc/index.html (accessed 10 April 2021).
- Gmplot n.d. www.github.com/vgm64/gmplot (accessed 10 April 2021).
- Daskin MS. Network and Discrete Location Models, Algorithms, and Applications. Hoboken, NJ: John Wiley & Sons, Inc.; 2013.
- Bunday BD. An Introduction to Queueing Theory. London: Arnold; 1996.
- Kingman JFC. The single server queue in heavy traffic. Math Proc Camb Philos Soc 1961;57:902-4. https://doi.org/10.1017/S0305004100036094.
- Mitchell M. An Introduction to Genetic Algorithms. Cambridge, MA: MIT Press; 1998.
- Ramnarayan P, Dimitriades K, Freeburn L, Kashyap A, Dixon M, Barry PW, et al. Interhospital Transport of Critically Ill Children to PICUs in the United Kingdom and Republic of Ireland: Analysis of an International Dataset. Pediatr Crit Care Med 2018;19:e300-1. https://doi.org/10.1097/PCC.0000000000001506.
- Pagel C, Ramnarayan P, Ray S, Peters MJ. A Novel method to identify the start and end of the winter surge in demand for pediatric intensive care in real time. Pediatr Crit Care Med 2015;16:821-7. https://doi.org/10.1097/PCC.0000000000000540.
Appendix 1 Interdependencies between the workstreams and how findings were integrated from the workstreams
| Workstream | Takes inputs from | Expected outputs | Outputs used by | Comments |
|---|---|---|---|---|
| Quantitative analysis | n/a |
|
|
This was completed. As workstream A findings did not support any association between timeliness and outcome, there was no specific impact on workstreams C and D |
| Parental questionnaires | n/a |
|
|
This was completed. As workstream B did not suggest that parent experience was influenced by timeliness, there was no impact on the mathematical modelling |
| Interviews of children and parents | n/a |
|
|
This was completed. As the interviews did not suggest that changes to the current transport team locations were required, the modelling did not explore the option of placing teams at other non-PICU-based locations |
| Interviews of clinical staff and managers | n/a |
|
|
This was completed. As the interviews of staff did suggest that there was a wider impact of waiting for the transport team on the care of other patients, the constraints applied to the mathematical modelling prioritised the overall efficiency of the transport system as the key goal |
| Health economic evaluation |
|
|
|
This was completed. There was no difference in the costs and benefits of the different transport team models |
Appendix 2 Data flow
FIGURE 30.
Flow of data in the data linkage part of the DEPICT study.

Appendix 3 Extracted data items from PICANet transport and admission data sets
| Data field/variable | Description of variable and manipulation applied |
|---|---|
| NHS number | Required for NHS Digital to link PICANet data with HES, ONS and CMP data, but not provided directly to the study team |
| Sex | Recorded as male, female, ambiguous and unknown. Provided to NHS Digital to link PICANet data with HES, ONS and CMP data |
| Postcode | Required for NHS Digital to link PICANet data with HES, ONS and CMP data, but not provided directly to the study team |
| Date of birth | Required for NHS Digital to link PICANet data with HES, ONS and CMP data, but not provided directly to the study team. Used by the study team to calculate the child’s age at the time of admission |
| Unique DEPICT study number | A unique study identifier for each individual PICU admission episode |
| Date of PICU admission | Provided as a date and used to confirm link with transport record, to check study eligibility and to calculate age of child on admission to PICU |
| Type of admission | `Recorded by PICANet as planned (following surgery), unplanned (following surgery), planned (other) or unplanned (other). The DEPICT study received information about unplanned (emergency) admissions only |
| Source of admission | Recorded as same hospital, clinic, another hospital or home |
| Care area admitted from | Recorded as X-ray/scanner/CT scanner; ICU/PICU/NICU; recovery; ward; HDU; theatre and recovery; immediate care area and A&E. This was used to identify children collected from an intensive care environment |
| Retrieval/transfer? | Recorded whether or not the child was transported to the PICU and was used to confirm the link between admission and transport record |
| Type of transport team | Recorded who transported the child to PICU. Used to confirm the link between admission and transport record |
| PIM2 score | A score calculated by the PICANet team from appropriate medical variables, as described previously. The PIM2 score is collected within the first hour of interaction with intensivist, which could be during transport or on admission to PICU |
| PIM2 score medical history variables | Individual components used in the calculation of the PIM2 score |
| PIM2 score physiology variables | Individual components used in the calculation of the PIM2 score |
| Primary diagnosis | Main reason for the admission provided as a READ Code with text description. With support from our clinical team, we adapted agreed PICANet clinical groupings to form groups of: respiratory, cardiovascular, endocrine, haematology/oncology, infection, neurological, trauma and accidents and other |
| Comorbidities | Recorded as a READ Code |
| Daily activity data in PICU | Information relating to the care received in PICU. We focused on the number of days spent receiving invasive ventilation |
| Vital status at PICU discharge | Whether or not the child was alive at the end of PICU stay. Confirmed with mortality information received from NHS Digital |
| Date of discharge (or death) | Date of discharge from PICU (not hospital) used to calculate PICU LOS |
| Destination following discharge | Normal residence, hospice, same hospital or another hospital. Used to confirm data collected within HES |
| Referring unit | Information about the hospital that requested the child be transported to PICU. This was amalgamated into how many requests each referring hospital made during the DEPICT study to give a proxy of the size/experience of the referring hospital |
| Invasively ventilated at referral? | Recorded as yes, no (not indicated), no (advised to intubate) and unknown |
| Identifier of transport team | Name of the transport team to ensure that we included eligible teams. We used this as a cluster term in certain analyses; however, we do not present any work where we name or compare the PCCTs in this chapter |
| Time intervals for transport | Using dates/times recorded by PICANet, we calculated time to bedside, stabilisation time and time taken to travel from the referring hospital to PICU |
| Grade of team leader | Recorded by PICANet as consultant, ST1–3, ST4–8 or nurse practitioner. We combined ST1–3 and ST4–8 into ‘junior doctor’ |
| Grade of senior nurse | If a nurse was present, then this indicates their pay band as being band 5, 6, 7 or 8 |
| Parent accompany patient? | Recorded by PICANet as yes, no (parent not present), no (parent declined) or no (parent not permitted) |
| Interventions performed by the referring team | Information about the care provided by the referring hospital before the arrival of the PCCT, which the DEPICT team grouped into intubation, inotropes, central venous access and intraosseous access |
| Interventions performed by the transport team | Information about the care provided while the PCCT was in attendance before the arrival of the PCCT, which the DEPICT team grouped into intubation, arterial access, vasoactive infusion, central venous access and intraosseous access |
| Critical incidents during transport | Information about critical incidents in the transport, which the DEPICT team grouped into intubation, arterial access, vasoactive infusion, central venous access and intraosseous access |
| PIM2 score (transport) | A score calculated by the PICANet team from appropriate medical variables, as described previously. The PIM2 score is collected within the first hour of interaction with intensivist, which could be during transport or on admission to the PICU |
| PIM2 score medical history variables (transport) | Individual components used in the calculation of the PIM2 score |
| PIM2 score physiology variables (transport) | Individual components used in the calculation of the PIM2 score |
| Weight | Weight of the child in kg |
| Height/length | Height/length of the child in cm |
| Ethnicity | Ethnicity of the child, as recorded by PICANet |
Appendix 4 Adjusted probability of mortality within 30 days of PICU admission by time taken to reach the bedside while holding other variables in the model at the mean value
FIGURE 31.
Adjusted probability of mortality within 30 days of PICU admission by time taken to reach the bedside while holding other variables in the model at the mean value (sensitivity analysis allowing PIM2 and age to be modelled using fractional polynomials).

Appendix 5 Sensitivity analyses of the impact of missing data for whether the child was ventilated at the time of the referral call
FIGURE 32.
Sensitivity analyses of the impact of missing data for whether the child was ventilated at the time of the referral call. (a) Ventilated; (b) not ventilated; and (c) advised to ventilate.
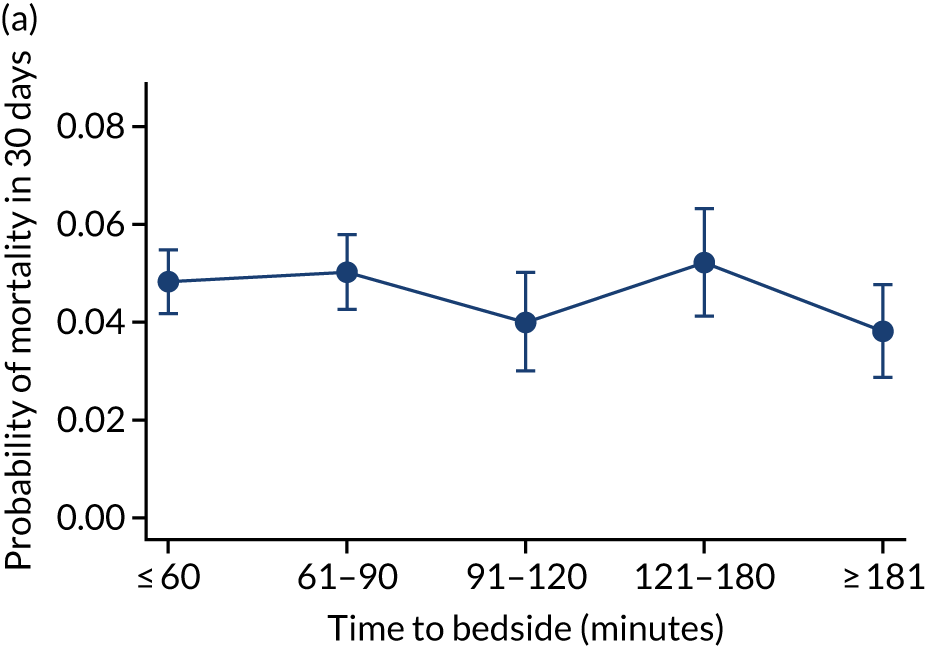
Appendix 6 Models of care flow chart
FIGURE 33.
Models of care flow chart. Reproduced from Seaton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
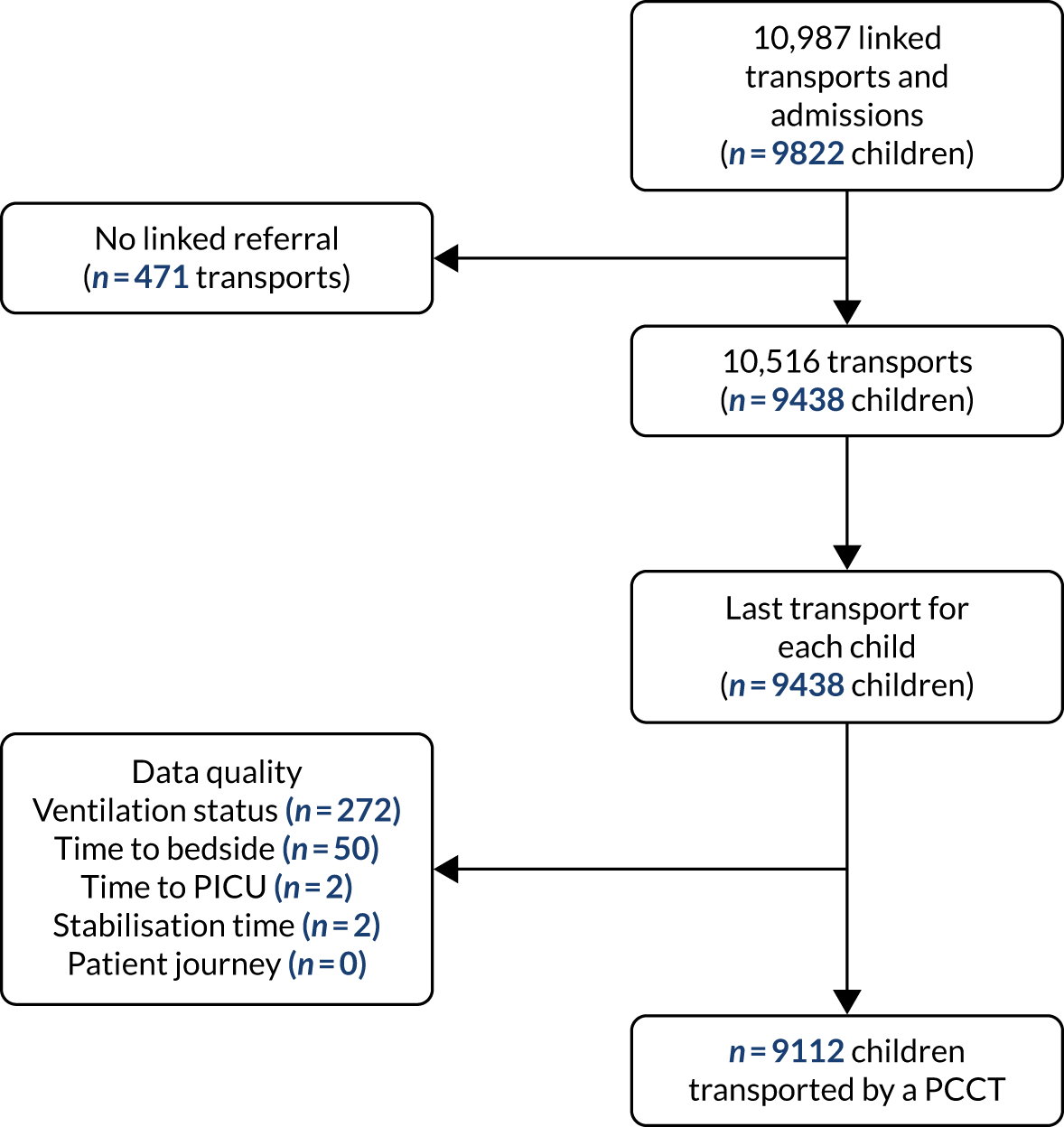
Appendix 7 Expected length of stay by team leader
FIGURE 34.
Expected LOS by team leader: (a) consultant vs. not a consultant (n = 9096) unadjusted; (b) consultant vs. not a consultant (n = 9096) adjusted; (c) ANP vs. junior doctor (n = 6068) unadjusted; (d) ANP vs. junior doctor (n = 6068) adjusted; (e) all team leaders (n = 9096) unadjusted; and (f) all team leaders (n = 9096) adjusted. Adjustment calculated while holding other variables at their average values.
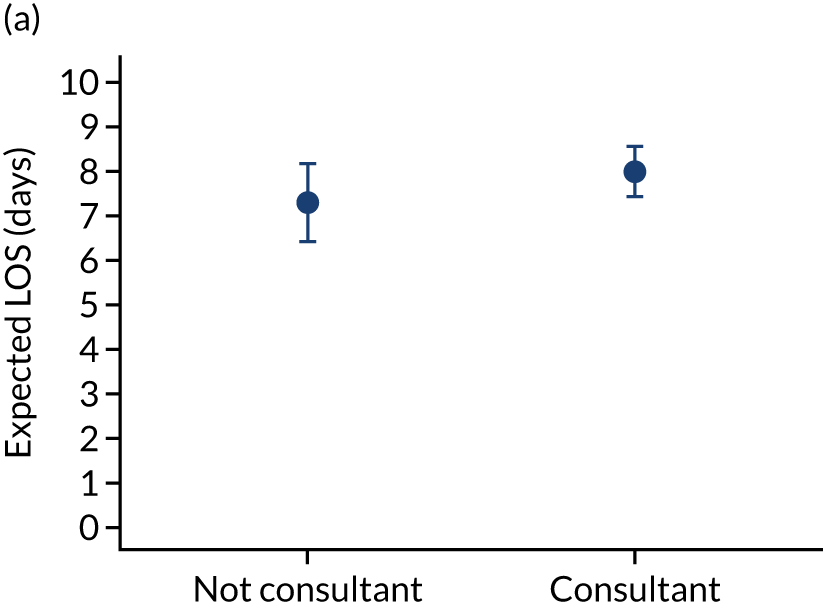
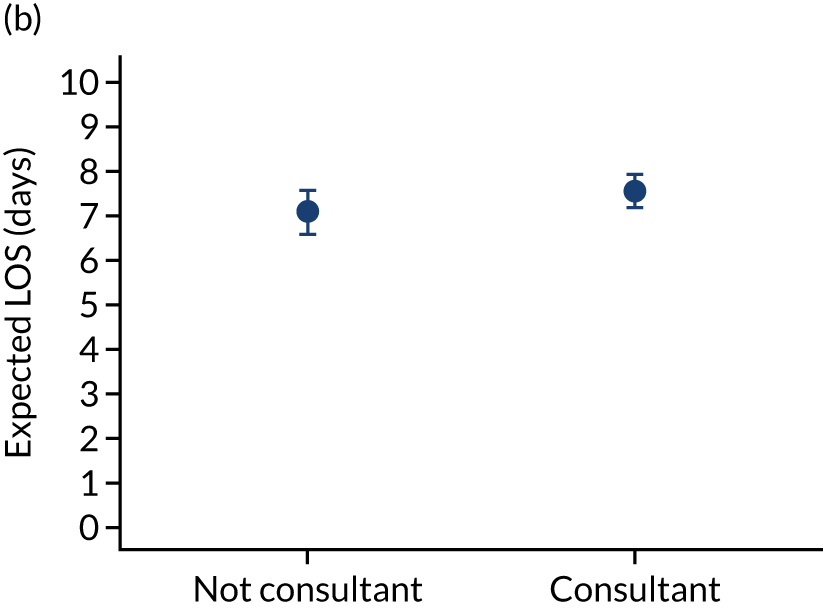
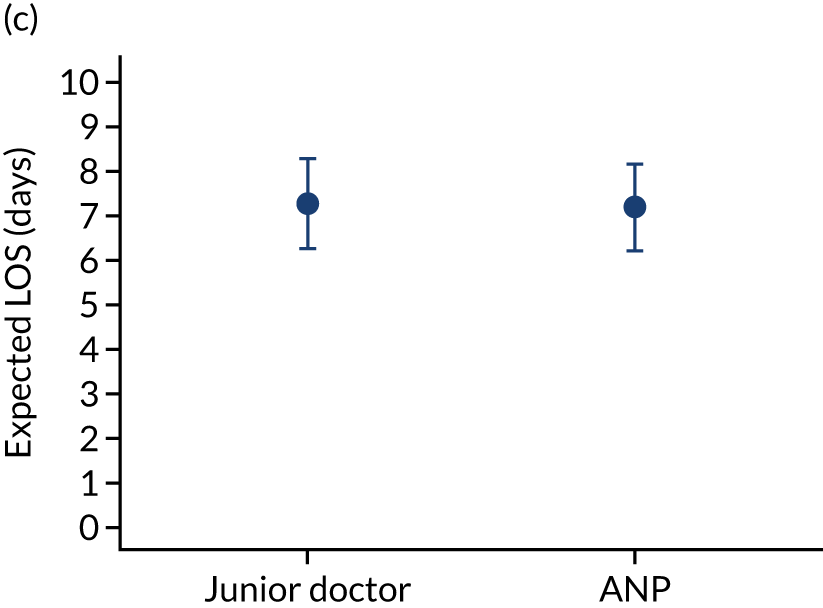
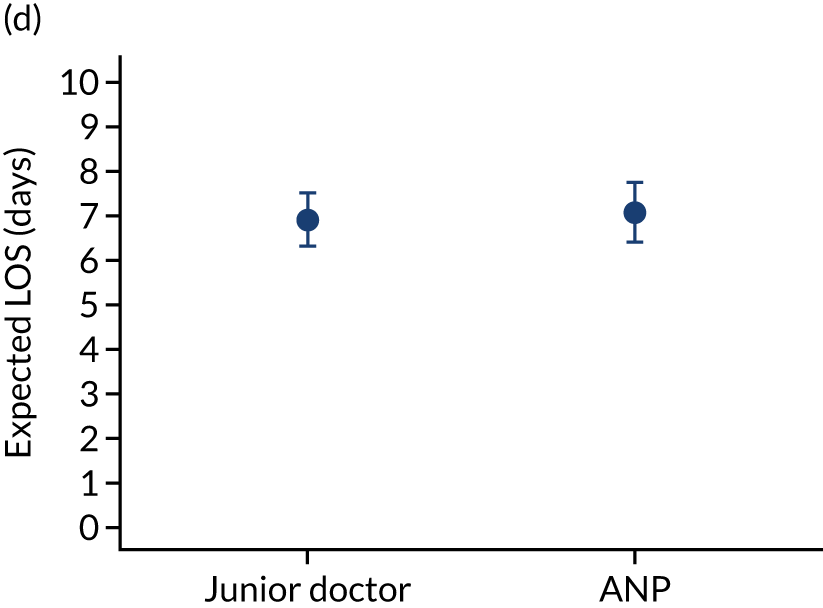
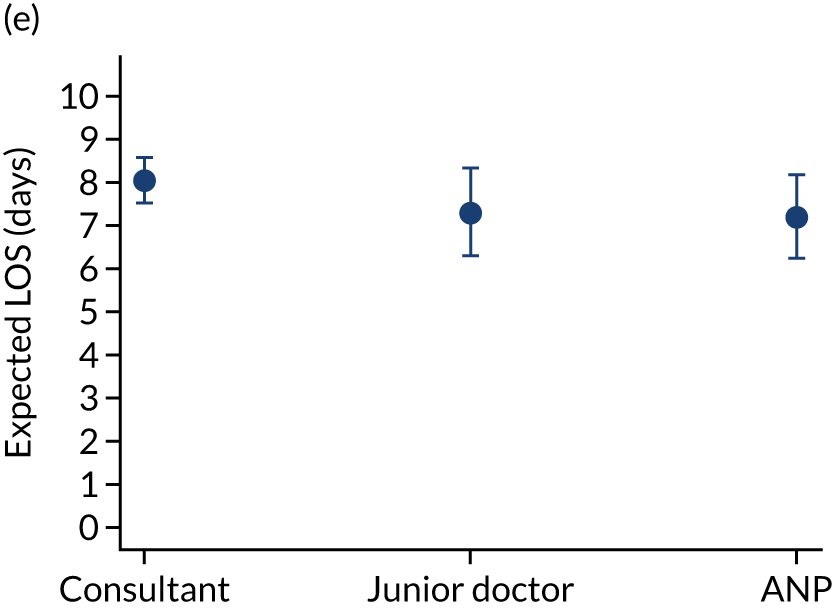
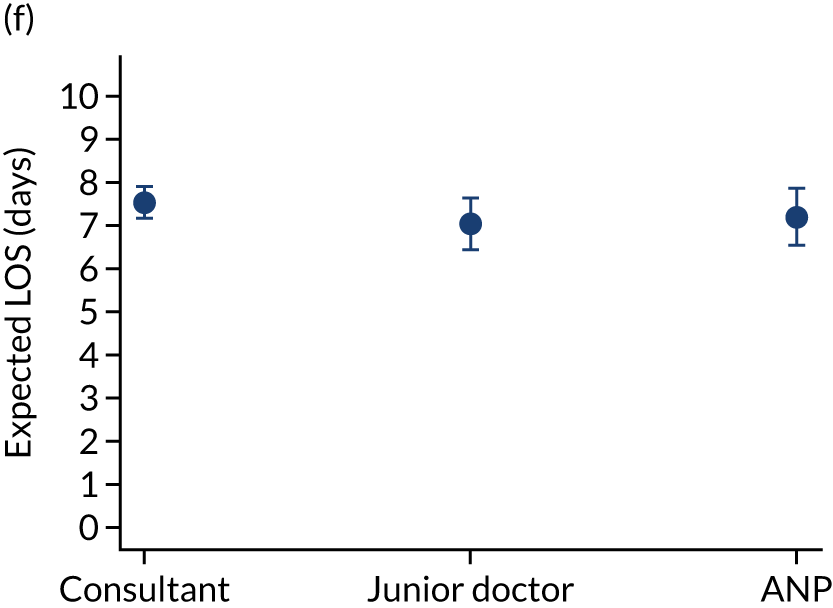
Appendix 8 Expected length of invasive ventilation by team leader
FIGURE 35.
Expected LOV by team leader: (a) consultant vs. not a consultant (n = 9095) unadjusted; (b) consultant vs. not a consultant (n = 9095) adjusted; (c) ANP vs. junior doctor (n = 6068) unadjusted; (d) ANP vs. junior doctor (n = 6068) adjusted; (e) all team leaders (n = 9095) unadjusted; and (f) all team leaders (n = 9095) adjusted. Adjustment calculated while holding other variables at their average values.
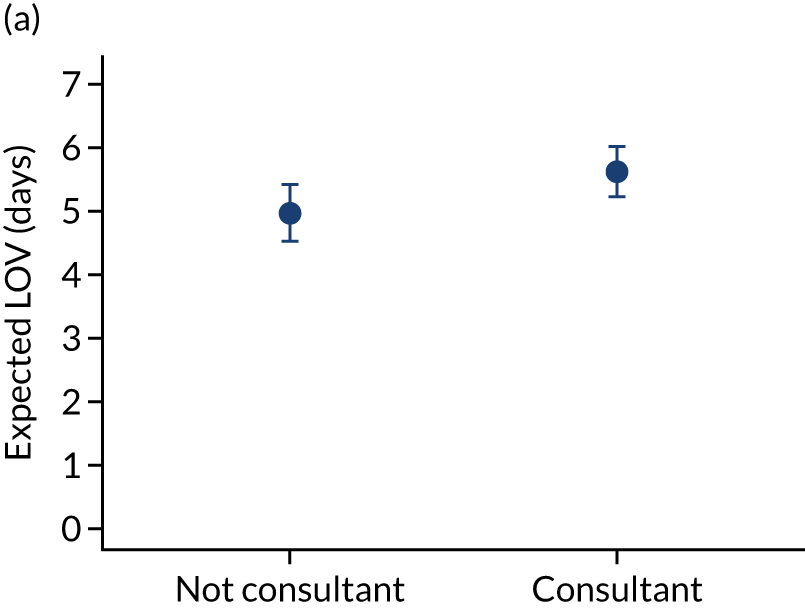
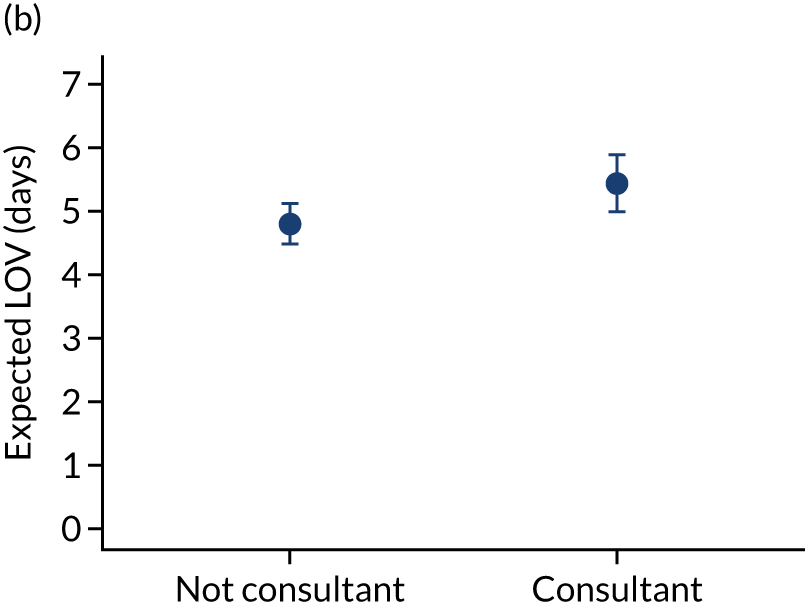
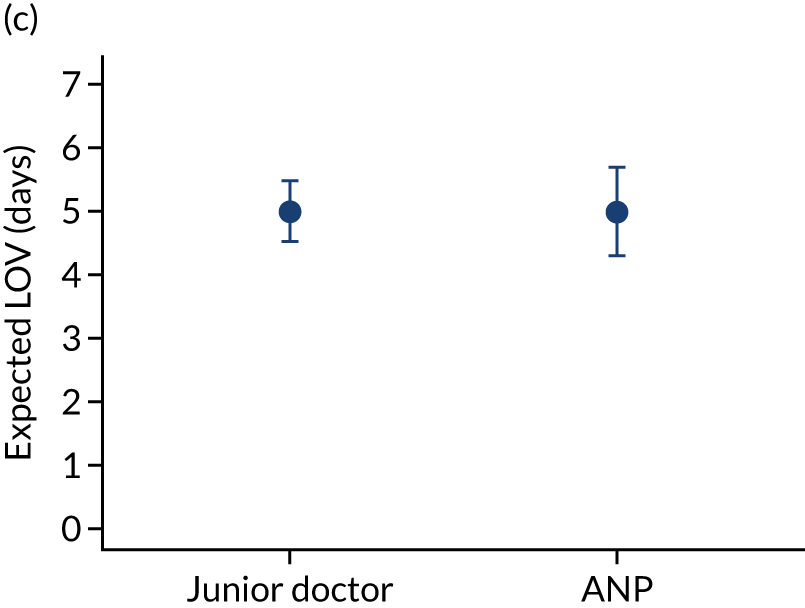
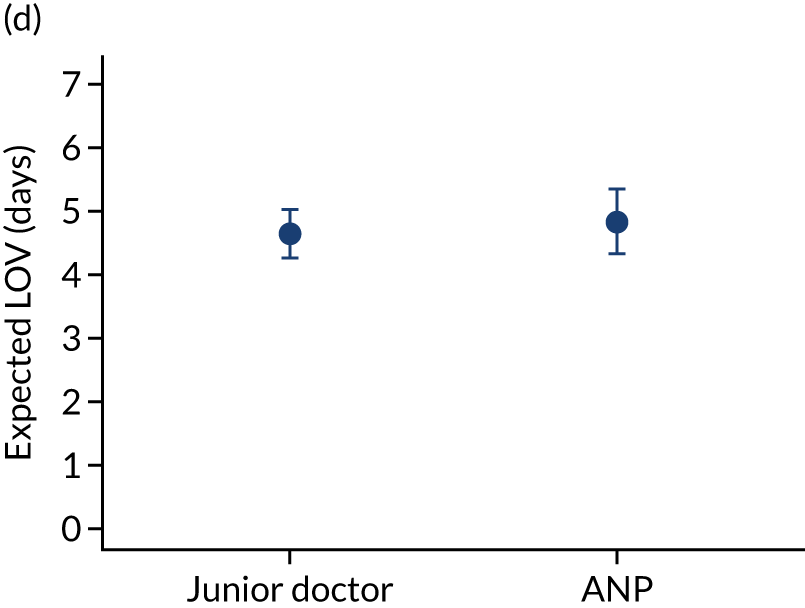
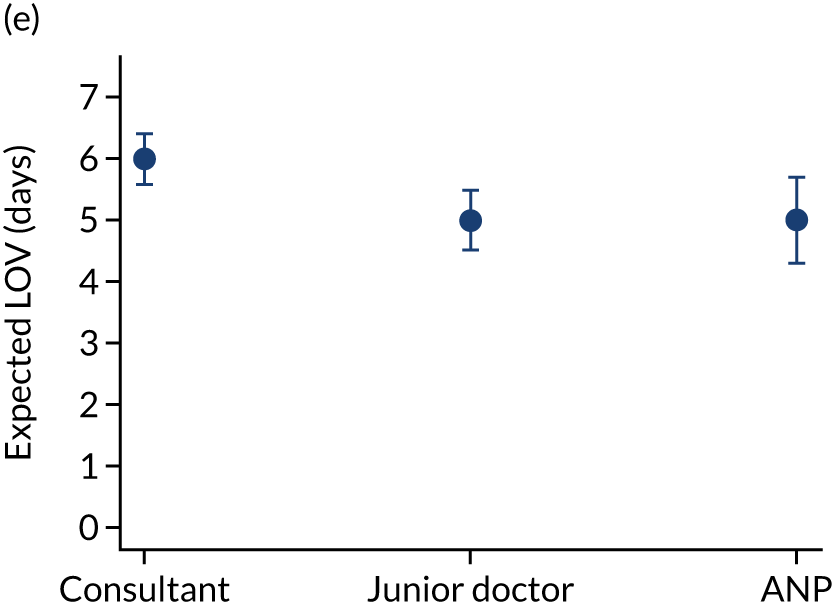

Appendix 9 Sensitivity analyses for team leader using fractional polynomials
FIGURE 36.
Sensitivity analyses (adjusted) for team leader using fractional polynomials: (a) consultant vs. not a consultant; (b) ANP vs. junior doctor; and (c) all team leaders.
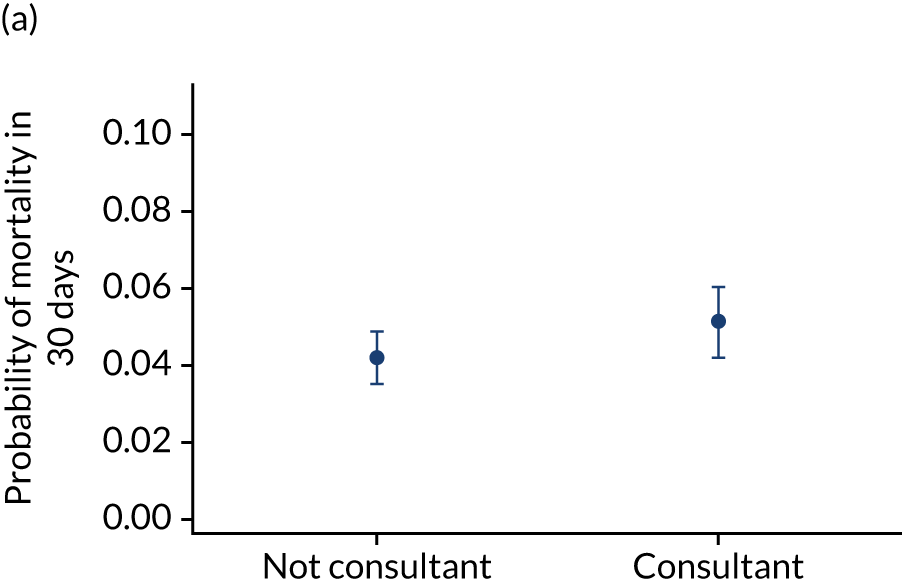
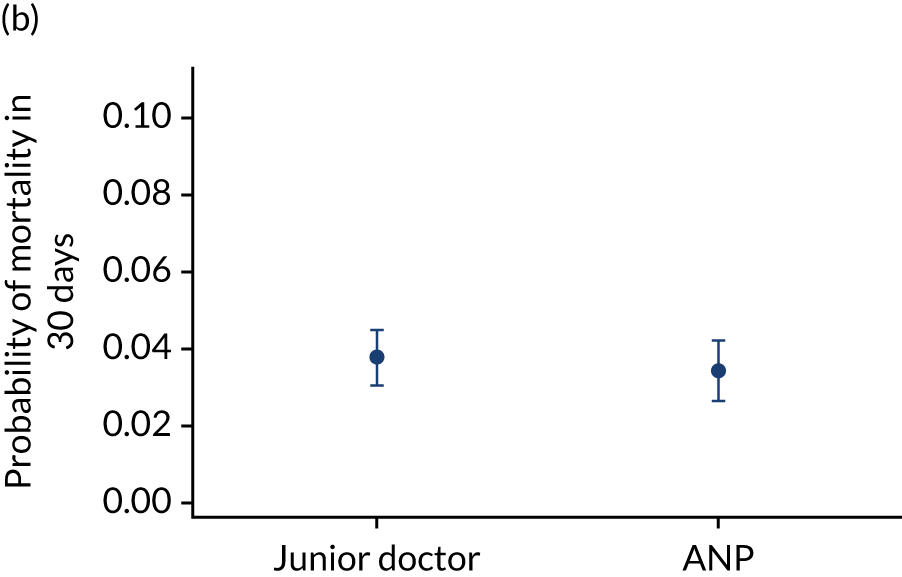
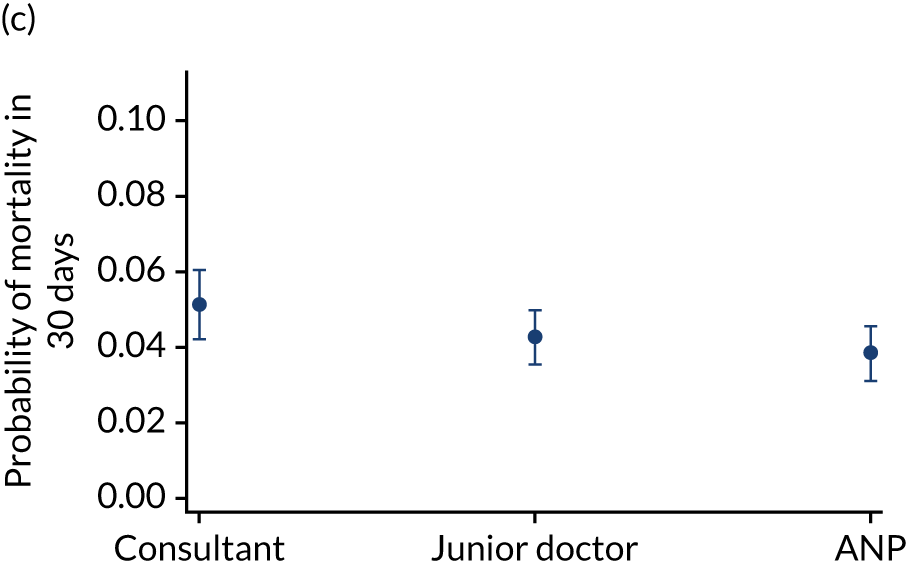
Appendix 10 Model fit for the stabilisation and intervention mortality models
| Model | AUC | Hosmer–Lemeshow test | Brier’s score |
|---|---|---|---|
| Stabilisation: mortality in 30 days | 0.79 | 0.51 | 0.06 |
| Stabilisation: mortality in PICU | 0.80 | 0.50 | 0.05 |
| Stabilisation: mortality in 90 days | 0.77 | 0.62 | 0.07 |
| Total interventions: mortality in 30 days | 0.79 | 0.95 | 0.06 |
| Percentage of interventions delivered by PCCT: mortality in 30 days | 0.79 | 0.60 | 0.06 |
Appendix 11 Percentage of interventions delivered by paediatric critical care transport teams and total number of interventions against length of stay
FIGURE 37.
Total number of interventions delivered by the PCCT and referring hospital and the percentage that were delivered by the PCCT against LOS. (a) Percentage of interventions delivered by the PCCT unadjusted; (b) percentage of interventions delivered by the PCCT adjusted; (c) total number of interventions delivered by PCCT and referring hospital unadjusted; and (d) total number of interventions delivered by PCCT and referring hospital adjusted.


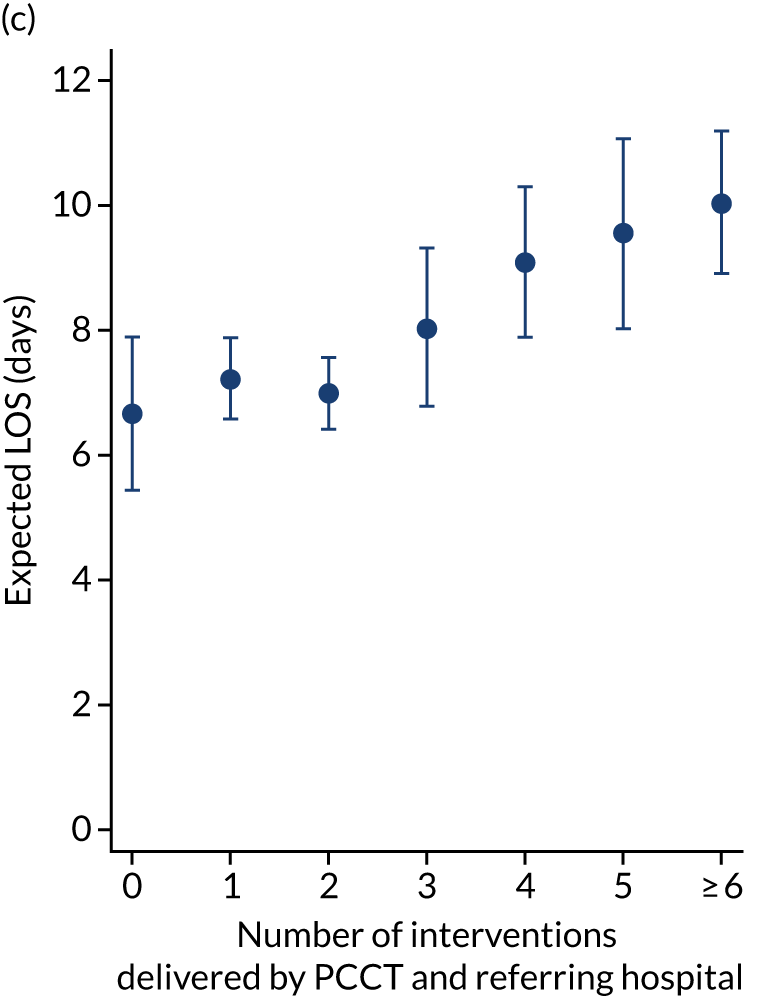
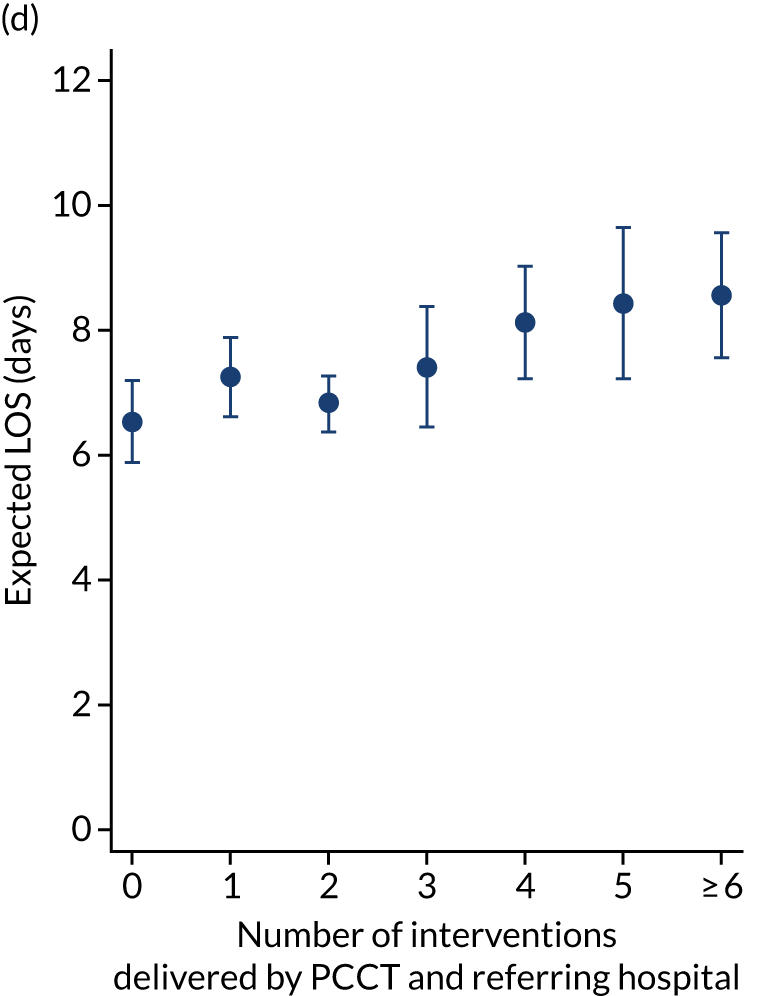
Appendix 12 Percentage of interventions delivered by paediatric critical care transport teams and total number of interventions against length of invasive ventilation
FIGURE 38.
Total number of interventions delivered by the PCCT and referring hospital and the percentage that were delivered by the PCCT against LOV. (a) Percentage of interventions delivered by the PCCT unadjusted; (b) percentage of interventions delivered by the PCCT adjusted; (c) total number of interventions delivered by PCCT and referring hospital unadjusted; and (d) total number of interventions delivered by PCCT and referring hospital adjusted.
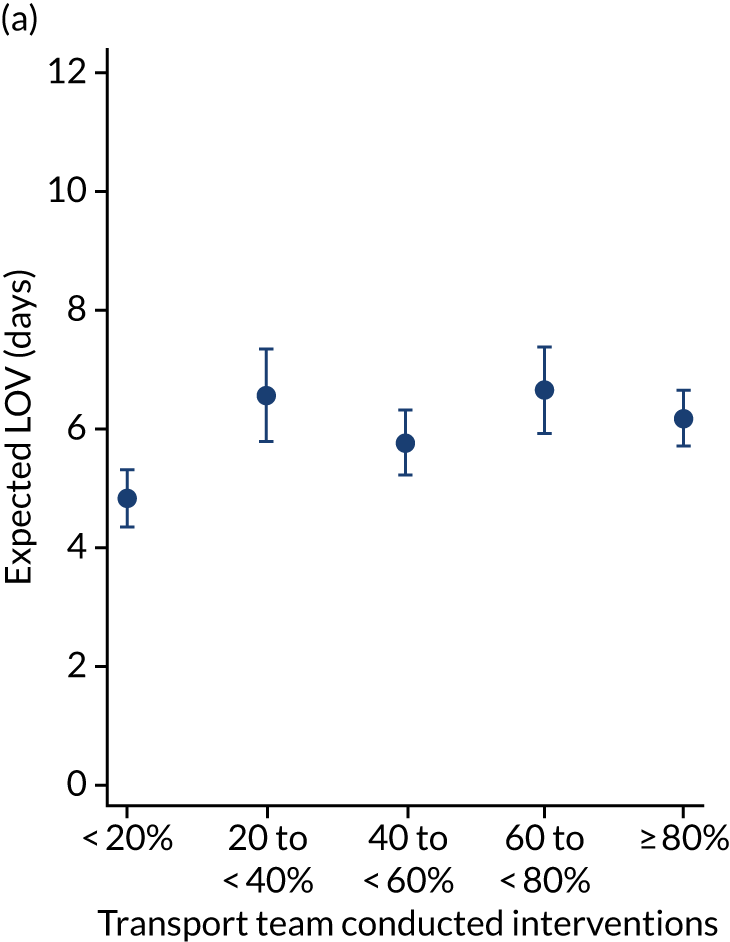
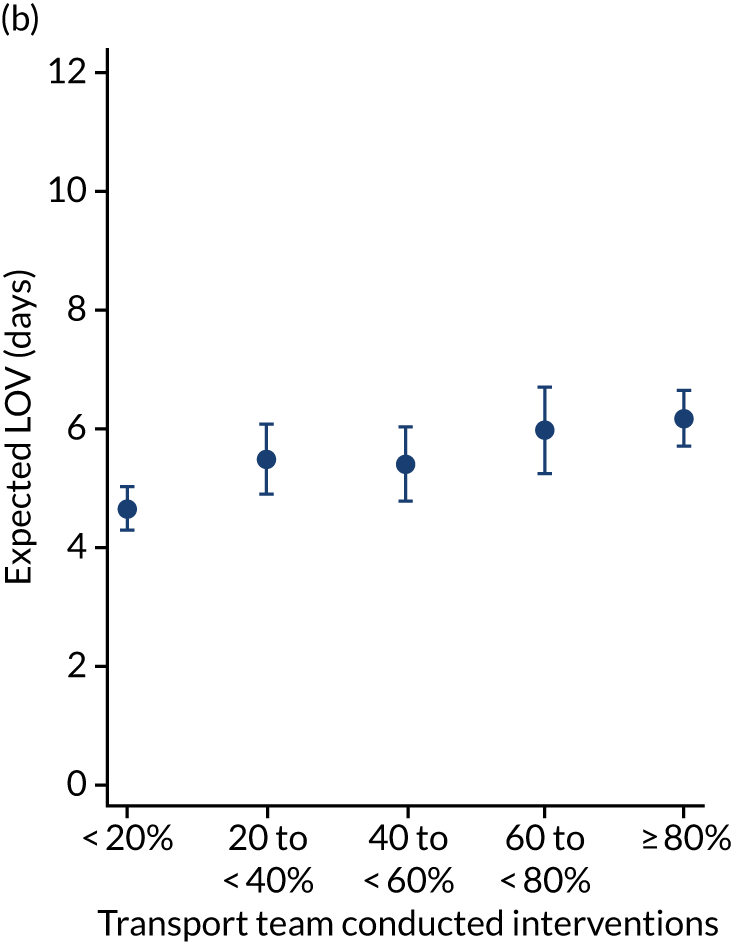
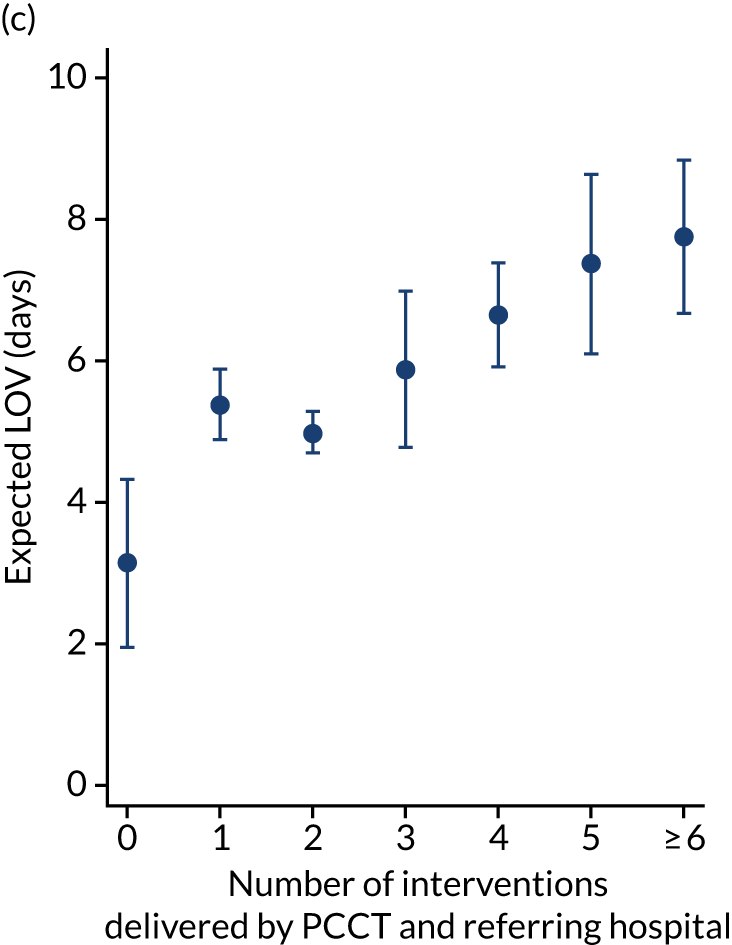
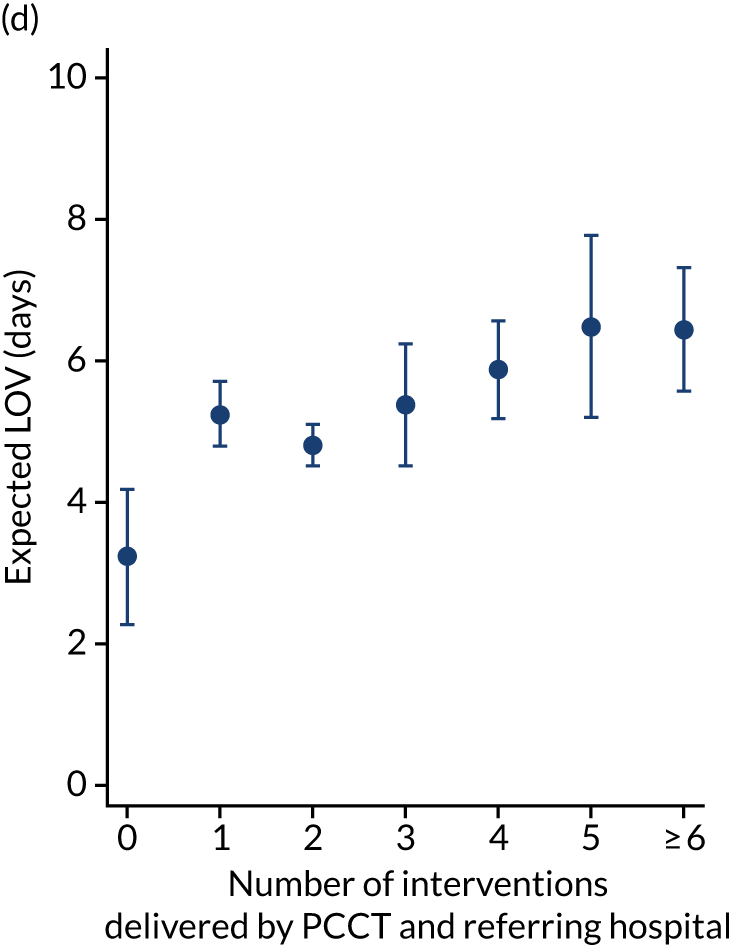
Appendix 13 Sensitivity analyses for adjusted mortality and length of stay/length of invasive ventilation
| Analysis | Adjusteda | 95% CI |
|---|---|---|
| Probability | ||
| Mortality in 30 days | ||
| Short stabilisation | 0.043 | 0.038 to 0.048 |
| Prolonged stabilisation | 0.057 | 0.037 to 0.076 |
| Mortality in PICU | ||
| Short stabilisation | 0.034 | 0.029 to 0.039 |
| Prolonged stabilisation | 0.054 | 0.035 to 0.072 |
| Mortality in 90 days | ||
| Short stabilisation | 0.057 | 0.049 to 0.065 |
| Prolonged stabilisation | 0.074 | 0.053 to 0.096 |
| Expected number of days | ||
| LOS | ||
| Short stabilisation | 7.06 | 6.65 to 7.46 |
| Prolonged stabilisation | 8.33 | 7.41 to 9.26 |
| LOV | ||
| Short stabilisation | 4.85 | 4.53 to 5.17 |
| Prolonged stabilisation | 6.05 | 5.20 to 6.90 |
Appendix 14 Initial 17-item parent experience measure and Friends and Family Test items included in the questionnaire
| Item number | Item |
|---|---|
| 1 | I was confident in the transport team |
| 2 | I understood what was going on overall |
| 3 | My child being transported to this PICU was difficult to cope with emotionally |
| 4 | I felt involved in my child’s care |
| 5 | The time it took to get to this PICU was bearable |
| 6 | I felt calm during the time my child was looked after by the transport team |
| 7 | I was confused about what was happening while my child was being transported to this PICU |
| 8 | I felt my child was safe during the transfer |
| 9 | The transfer to this PICU was chaotic |
| 10 | The transport team listened to me |
| 11 | I trusted the transport team |
| 12 | My child’s transfer to this PICU went well |
| 13 | The transport team were caring and understanding |
| 14 | I was satisfied with the care my child received from the transport team |
| 15 | The transport team treated my family and me with respect |
| 16 | When I asked questions, during the transfer, I received answers I understood |
| 17 | I felt reassured by the transport team |
How would you rate the quality of care of the whole team?
How would you rate the quality of care of the nurse?
How would you rate the quality of care of the nurse practitioner?
How would you rate the quality of care of the doctor?
How would you rate the quality of care of the ambulance driver?
Overall satisfaction with the transport service.
Appendix 15 Interview schedule/topic guide for parents/guardians
IRAS 218569, DEPICT parent/guardian interview topic guide: v3.0 – 20 November 2017
Interview schedule/ topic guide for parents/guardians
Critically ill children and young people: do national Differences in access to Emergency Paediatric Intensive Care and care during Transport affect clinical outcomes and patient experience?
The DEPICT study
Objectives
-
Describe carer experience of child being transported from their ‘local hospital’ to intensive care.
-
Encourage discussion of three sequential stages of the transport: local hospital, time with the transport team (including the physical journey) and the arrival at PICU.
-
Encourage description of the communication experience from a carer perspective: what they were told.
-
Encourage exploration of expectations at each different stage – what were the expectations and were they met? What went well at each stage of the transfer, what could be better?
-
Encourage discussion of emotional impact.
Introduction
-
Introduce self, DEPICT team at GOSH (Great Ormond Street Hospital), confidentiality, timing, OK to record, able to withdraw at any time. Introduce ‘stop’ signal.
-
Interview objectives – recap PIS: national study, nine retrieval services across country, all organised slightly differently, want to understand parent/carer experience with the aim of looking at what worked well for you and what could be improved? Hope to produce recommendations for service change, if needed.
The following questions and prompts will act as a guide of issues for the researchers to cover, rather as a list of questions to be asked in sequential order.
Background
I wonder if we can begin with you telling me what you remember about your child’s transport to PICU, starting with when your child was at your local hospital and how they got to be in the hospital.
Prompts:
-
What happened at your local hospital?
-
How long was your child there?
-
Why were they there?
-
Can you tell me a bit about your local hospital (location, type of ward child was on)?
-
Can you tell me a little bit about the care that your child received at the local hospital?
Was there anything you would have liked to have been different (about the care, about how things were explained)?
Decision to go to a paediatric intensive care unit
Can you tell me about when you were first told that your child was going to be transported to a PICU?
Prompts:
-
Who told you?
-
What did they tell you?
What was happening to your child at that time?
Can you tell me a little bit about the time after the decision was made to transfer your child?
Once you were told that your child needed to be transferred to PICU, did things happen in the way that you expected them to?
Prompts:
-
What happened while you were waiting for the transport to arrive?
-
Who was with your child?
-
Where was your child waiting?
-
Were they awake or asleep?
-
What support did you have?
How long was it before a place was found for your child at the PICU?
Did plans change?
Prompts:
-
What were you told when things changed, how did you feel at that time?
-
What words describe that time before the transport team arrived?
-
How did you feel during that time?
Is there anything, that you haven’t already mentioned, that you would have liked to have been different during this time?
The transport team
Thinking now about when the transport team arrived at your local hospital, can you tell me a bit about what happened then?
Prompts:
-
Can you remember who arrived as part of the transport team?
-
How did you feel when you met them?
-
What did they tell you?
-
Did you understand the things that they told you?
-
Were you able to ask questions?
-
If so, what did you think of the answer?
-
What did the transport team do?
-
How long did you have to wait for the team to arrive?
Was this different to what you had been expecting?
-
How long were the team there before your child went in the transport?
-
How did you feel during this time?
-
Is there anything, that you haven’t already mentioned, that you would have liked to have been different during this time?
Moving on the transport itself, can you tell me about that?
Prompts:
-
If parent was not in the transport, can you tell me a little bit more about the decision of who travelled with your child in the ambulance?
-
If parent was in the transport, what happened during the journey?
-
How long did the journey feel?
-
Can you remember how long it actually took?
-
Who was with your child?
-
How was your child?
-
How were the staff with you and your child?
-
What kinds of things did you talk about in the ambulance?
-
Who did you talk to?
How did it feel during this time?
Is there anything, that you haven’t already mentioned, that you would have liked to have been different during this time?
Arriving at the paediatric intensive care unit
What about when you arrived at the PICU, what happened then?
Prompts:
-
Can you remember who met you at the PICU?
-
Where did you wait?
-
For how long?
-
When did you get to see your child?
-
What were you told? By whom?
Concluding questions
What worked less well for you? What could have been done to improve your experience?
Thinking about the whole experience, what specific things about the transport worked well? Is there anything that comes to mind that you feel was particularly good?
Finally, as we are trying to understand how to provide the best service that we can, what do you think an ‘ideal’ transport service would look like when a child needs to be transferred to a PICU?
Appendix 16 Interview schedule for staff
IRAS 218569, DEPICT staff/clinician interview topic guide: version 3.0 – 20 November 2017
Interview schedule for staff
Critically ill children and young people: do national Differences in access to Emergency Paediatric Intensive Care and care during Transport affect clinical outcomes and patient experience?
The DEPICT study
Objectives
-
Description of how transport service works and participant’s role within this service.
-
Perception of impact of service on stakeholders: children, their families, staff and other services.
-
Evaluation of current service: identifying what works well and could be better.
Introduction
-
Introduce self (psychologist, employed to work on this project), DEPICT team at GOSH (Great Ormond Street Hospital) [independent of CATS (Children’s Acute Transport Service)/PICU], confidentiality (standard to say about disclosing anything that concerns me about your welfare or welfare of others), timing, OK to record, able to withdraw at any time without giving reason, consent form.
-
Explain interview objectives, recap PIS, national study, nine retrieval services across country, all organised slightly differently, want to understand staff perspective experience with the aim of looking at what worked well in your team, in your area, what could be improved?
-
Aim to evaluate need for service change and produce recommendations if required.
-
Going to ask you questions about PIC retrieval services but recognise that there are other services specialist and other that deliver children into PICU and may ask you to clarify which ones we are talking about.
-
I ask questions and encourage you to do the talking and so it might be a bit one sided, if you have questions for me I am more than happy to answer them at the end.
The following prompts will act as a guide of issues for the researchers to cover, rather than a list of questions to be asked in sequential order.
Could you tell me a bit about your role in the paediatric intensive care retrieval team/PICU/hospital?
Prompts:
-
How is the role linked to the transport service?
-
How long have you been working in this role?
Can you give me an example of a transfer that went well from your perspective and why it went well?
Prompts:
-
Why was it good?
-
Could it have been improved?
And an example of a transfer that went less well?
Prompts:
-
What were the reasons it went less well?
-
What would have made it better?
Are there any wider impacts of transport services?
Prompts:
-
On other patients?
-
Other families?
-
Any procedural considerations?
-
Other services?
-
Staff?
If you had to design the optimum transport service what would that look like? Why?
Prompts:
-
Characteristics of a good service?
-
Considerations in designing a service?
-
Who are the important stakeholders?
-
What would the challenges be?
-
What are the barriers to and facilitators of delivering such a service?
Thank you – anything else that you want to talk about that we have not covered in the questions that I have asked you today?
Appendix 17 Paediatric Intensive Care Audit Network data collection form
Appendix 18 Questionnaire respondents: language spoken at home
| Language | Number (%) |
|---|---|
| English | 1394 (70) |
| English and another | 298 (15) |
| Another language (other than English) | 90 (5) |
| Missing | 216 (11) |
Appendix 19 Frequency of languages selected for translated materials provision
| Language | Number (%) |
|---|---|
| Urdu | 41 (2) |
| Bengali | 17 (1) |
| Punjabi | 21 (1) |
| Polish | 23 (1) |
| Gujarati | 15 (1) |
| Welsh | 20 (1) |
| Urdu and Punjabi | 16 (1) |
| Urdu, Bengali/Punjabi | 1 (0) |
| Romanian | 26 (1) |
| Other | 208 (10) |
| Missing (English speakers) | 1610 (81) |
Appendix 20 PICANet and questionnaire data indicating proportions of matching
| Data | Number (%) |
|---|---|
| Questionnaire data onlya | 513 (19) |
| Questionnaire and PICANet data | 1571 (59) |
| PICANet data only | 582 (22) |
Appendix 21 Matching with PICANet data
| Variable | Questionnaire only, n (%) | Questionnaire plus PICANet, n (%) |
|---|---|---|
| Reason for PICU admission | ||
| Respiratory | 94 (19) | 536 (35) |
| Cardiac condition | 112 (22) | 181 (12) |
| Neurological | 37 (7) | 151 (10) |
| Surgery | 67 (13) | 49 (3) |
| Diabetes | 1 (0) | 6 (0) |
| Infection | 71 (14) | 382 (25) |
| Trauma | 25 (5) | 18 (1) |
| Other | 74 (15) | 108 (7) |
| Too complex/unable to identify primary reason | 28 (6) | 123 (8) |
| Age | ||
| 0–6 months | 290 (57) | 741 (48) |
| 7–12 months | 41 (8) | 155 (10) |
| 13–23 months | 37 (7) | 154 (10) |
| 2–5 years | 60 (12) | 240 (16) |
| 6–10 years | 41 (8) | 140 (9) |
| 11–15 years | 36 (7) | 113 (7) |
| ≥ 16 years | 4 (1) | 8 (1) |
| Child’s first transport to PICU | ||
| Yes | 426 (83) | 1307 (82) |
| No | 90 (17) | 288 (18) |
Appendix 22 Visual individualised Likert data chart showing perceptions of paediatric intensive care unit staff about measures put in place to support them
Appendix 23 Parents’ report of when the team arrived
| Perception | Number (%) |
|---|---|
| Earlier than expected | 363 (17) |
| On time | 1012 (49) |
| Later than expected | 261 (13) |
| I cannot remember | 95 (5) |
| I was not told a time frame | 333 (16) |
| Missing | 20 (1) |
Appendix 24 Satisfaction measures of transfer by group (earlier/on time vs. later)
| Measure | Earlier/on time, n (%) | Later, n (%) | Group difference (Mann–Whitney) |
|---|---|---|---|
| If my child was ever in this situation again then I would like them to be transported by this team again | |||
| Disagree at lot | 2 (0) | 0 (0) | U = 157,728.5; z = –5.681; p < 0.0001 |
| Disagree a bit | 4 (0) | 4 (2) | |
| Neither agree nor disagree | 10 (1) | 8 (3) | |
| Agree a bit | 43 (3) | 22 (9) | |
| Agree a lot | 1301 (96) | 221 (87) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | |||
| Disagree at lot | 3 (0) | 1 (0) | U = 158,338.0; z = –5.677; p < 0.0001 |
| Disagree a bit | 3 (0) | 5 (2) | |
| Neither agree nor disagree | 8 (1) | 6 (2) | |
| Agree a bit | 34 (3) | 18 (7) | |
| Agree a lot | 1312 (97) | 224 (14) | |
| Overall experience of the transport service | |||
| Extremely poor | 0 (0) | 0 (0) | U = 175,783.5; z = –2.314; p < 0.021 |
| Poor | 2 (0) | 6 (2) | |
| Fair | 12 (1) | 8 (3) | |
| Good | 85 (6) | 42 (17) | |
| Excellent | 1258 (93) | 199 (78) | |
| Overall satisfaction with the transport service | |||
| Extremely poor | 0 (0) | 0 (0) | U = 145,355.5; z = –8.057; p < 0.0001 |
| Poor | 2 (0) | 3 (1) | |
| Fair | 9 (1) | 10 (4) | |
| Good | 76 (6) | 43 (17) | |
| Excellent | 1265 (94) | 199 (78) | |
Appendix 25 Median time intervals for different stages of the transfer to paediatric intensive care unit
| Stage | Number of respondents | Median (minutes) | IQR (minutes) |
|---|---|---|---|
| Time to bedside | 2120 | 84.50 | 56–130 |
| Stabilisation time | 2131 | 100 | 75–140 |
| Time in ambulance to PICU | 2077 | 50 | 35–75 |
| Total time to PICU | 2153 | 255 | 200–230 |
Appendix 26 Association between PICANet time interval and parent experience
| Time interval (number of respondents) | Parent experience scale (τB ;p-value) |
|---|---|
| Time to bedside (n = 1331) | τB = –0.001; p = 0.954 |
| Stabilisation time (n = 1337) | τB = –0.075; p = < 0.001 |
| Time in ambulance to PICU (n = 1301) | τB = –0.020; p = 0.372 |
| Total time to PICU (n = 1349) | τB = –0.055; p = 0.010 |
Appendix 27 Family-perceived transport team leader
| Team lead | Number (%) |
|---|---|
| Nurse | 389 (19) |
| Doctor | 959 (46) |
| I do not remember | 408 (20) |
| I was not told/it was not clear | 209 (10) |
| Other | 74 (4) |
| Missing | 45 (2) |
Appendix 28 PICANet leader grade
| Staff leader grade | Number (%) |
|---|---|
| Consultant/associate specialist/staff grade | 617 (23) |
| ST4–8 | 996 (37) |
| ST1–3 | 5 (0) |
| Nurse practitioner | 388 (15) |
| Unknown | 147 (6) |
| Missing | 660 (25) |
Appendix 29 Satisfaction scores and team leader
| Measure | Who led the team? n (%) | Group difference (Kruskal–Wallis) | ||
|---|---|---|---|---|
| Consultant | Junior doctor | ANP | ||
| If my child was ever in this situation again then I would like them to be transported by this team again | ||||
| Disagree at lot | 1 (0) | 1 (0) | 0 (0) | χ2 (df 2) = 4.84; p < 0.089 |
| Disagree a bit | 0 (0) | 2 (0) | 0 (0) | |
| Neither agree nor disagree | 4 (1) | 11 (2) | 0 (0) | |
| Agree a bit | 13 (3) | 23 (3) | 6 (2) | |
| Agree a lot | 427 (96) | 677 (95) | 276 (98) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | ||||
| Disagree at lot | 0 (0) | 1 (0) | 0 (0) | χ2 (df 2) = 4.25; p < 0.120 |
| Disagree a bit | 1 (0) | 3 (0) | 0 (0) | |
| Neither agree nor disagree | 4 (1) | 11 (2) | 1 (0) | |
| Agree a bit | 11 (3) | 17 (2) | 4 (1) | |
| Agree a lot | 428 (97) | 682 (96) | 276 (98) | |
| Overall experience of the transport service | ||||
| Extremely poor | 0 (0) | 1 (0) | 0 (0) | χ2 (df 2) = 4.83; p < 0.089 |
| Poor | 0 (0) | 2 (0) | 0 (0) | |
| Fair | 5 (1) | 6 (1) | 0 (0) | |
| Good | 32 (7) | 56 (18) | 14 (5) | |
| Excellent | 405 (92) | 649 (91) | 267 (95) | |
| Overall satisfaction with the transport service | ||||
| Extremely poor | 0 (0) | 1 (0) | 0 (0) | χ2 (df 2) = 2.64; p = 0.268 |
| Poor | 1 (0) | 2 (0) | 0 (0) | |
| Fair | 2 (1) | 5 (1) | 0 (0) | |
| Good | 28 (6) | 48 (7) | 14 (5) | |
| Excellent | 412 (93) | 653 (92) | 265 (95) | |
Appendix 30 PICANet leadership and parent transfer experience
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group statistic (Kruskal–Wallis) | |
|---|---|---|---|
| Median | IQR | ||
| Consultant (n = 376) | 5 | 4.89–5 | χ2 (df 2) = 6.95; p = 0.031 |
| Junior doctor (n = 626) | 5 | 5–5 | |
| ANP (n = 253) | 5 | 5–5 | |
Appendix 31 Summary of any reported critical incident
| Critical incident occurred | Number (%) |
|---|---|
| No | 1995 (93) |
| Yes | 130 (6) |
| Missing | 28 (1) |
Appendix 32 Scores of satisfaction measures by critical incident group
| Measure | No critical incident, n (%) | Critical incident, n (%) | Group difference (Mann–Whitney) |
|---|---|---|---|
| If my child was ever in this situation again then I would like them to be transported by this team | |||
| Disagree at lot | 2 (0) | 0 (0) | U = 70,572.5; z = –0.792; p = 0.428 |
| Disagree a bit | 2 (0) | 0 (0) | |
| Neither agree nor disagree | 17 (1) | 0 (0) | |
| Agree a bit | 46 (3) | 3 (3) | |
| Agree a lot | 1369 (95) | 97 (97) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | |||
| Disagree at lot | 1 (0) | 0 (0) | U = 70,966.5; z = –0.510; p = 0.610 |
| Disagree a bit | 4 (0) | 0 (0) | |
| Neither agree nor disagree | 18 (1) | 0 (0) | |
| Agree a bit | 34 (2) | 3 (3) | |
| Agree a lot | 1377 (96) | 97 (97) | |
| Overall experience of the transport service | |||
| Extremely poor | 1 (0) | 0 (0) | U = 69,060; z = –1.276; p = 0.220 |
| Poor | 3 (0) | 0 (0) | |
| Fair | 13 (1) | 0 (0) | |
| Good | 102 (7) | 12 (12) | |
| Excellent | 1313 (92) | 88 (88) | |
| Overall satisfaction with the transport service | |||
| Extremely poor | 1 (0) | 0 (0) | U = 69,590; z = –0.871; p = 0.384 |
| Poor | 3 (0) | 0 (0) | |
| Fair | 10 (1) | 0 (0) | |
| Good | 93 (6) | 10 (10) | |
| Excellent | 1319 (93) | 90 (90) | |
Appendix 33 Parent experience of the transfer when there was or was not a critical incident during the transfer
| Category | Experience score (1 = extremely poor, 5 = excellent) | Group difference (Mann–Whitney) | |
|---|---|---|---|
| Median | IQR | ||
| No incident (n = 1247) | 5 | 5–5 | U = 51,051; z = –61.265; p = 0.206 |
| Critical incident (n = 87) | 5 | 4.89–5 | |
Appendix 34 Parents who did not travel and experience of, and satisfaction with, the retrieval
| Measure | Did not travel, n (%) | Group difference (Mann–Whitney) | |
|---|---|---|---|
| Chose not to | Not permitted to | ||
| If my child was ever in this situation again then I would like them to be transported by this team again | |||
| Disagree at lot | 0 (0) | 1 (1) | U = 19,623.5; z = –3.423; p = 0.001 |
| Disagree a bit | 0 (0) | 3 (2) | |
| Neither agree nor disagree | 11 (3) | 4 (3) | |
| Agree a bit | 14 (4) | 16 (12) | |
| Agree a lot | 311 (93) | 107 (82) | |
| I would recommend this transport team to anybody whose child needed to be transported to a PICU | |||
| Disagree at lot | 0 (0) | 2 (2) | U = 19,391; z = –3.667; p < 0.0001 |
| Disagree a bit | 0 (0) | 2 (2) | |
| Neither agree nor disagree | 10 (3) | 6 (5) | |
| Agree a bit | 12 (4) | 13 (10) | |
| Agree a lot | 314 (94) | 107 (82) | |
| Overall experience of the transport service | |||
| Extremely poor | 1 (0) | 0 (0) | U = 19,403; z = –2.542; p = 0.011 |
| Poor | 0 (0) | 6 (5) | |
| Fair | 5 (2) | 2 (2) | |
| Good | 44 (13) | 24 (19) | |
| Excellent | 282 (85) | 98 (75) | |
| Overall satisfaction with the transport service | |||
| Extremely poor | 1 (0) | 0 (0) | U = 19,299.5; z = –2.690; p = 0.007 |
| Poor | 0 (0) | 2 (2) | |
| Fair | 4 (1) | 5 (4) | |
| Good | 43 (13) | 25 (19) | |
| Excellent | 284 (86) | 98 (75) | |
Appendix 35 Comparing average time to bedside for different numbers of teams across different numbers of paediatric critical care transport team locations for non-winter daytime
FIGURE 39.
Comparing average time to bedside for different numbers of teams across different numbers of PCCT locations (chosen from the current 11 locations) for non-winter daytime (8 a.m. to 8 p.m.).
Appendix 36 Average wait time for an available team during winter daytime for different configurations of number of locations and teams
FIGURE 40.
Average wait time for an available team during winter daytime (8 a.m. to 8 p.m.) for different configurations of number of locations and teams.
Appendix 37 Map of the district general hospitals covered by paediatric critical care transport team locations when there are only eight locations (chosen out of the current 11)
FIGURE 41.
Map of the district general hospitals covered by paediatric critical care transport team locations when there are only eight locations (chosen out of the current 11). Map data © 2021 Google.
List of abbreviations
- AICU
- acute intensive care unit
- ANP
- advanced nurse practitioner
- API
- application programming interface
- AUC
- area under the curve
- CI
- confidence interval
- CMP
- case mix programme
- CYP
- children and young people
- DEPICT
- Differences in access to Emergency Paediatric Intensive Care and care during Transport
- df
- degrees of freedom
- DGH
- district general hospital
- ED
- emergency department
- GP
- general practitioner
- HDU
- high-dependency unit
- HES
- Hospital Episode Statistics
- HQIP
- Healthcare Quality Improvement Partnership
- HUI2
- Health Utilities Index Mark 2
- ICNARC
- Intensive Care National Audit & Research Centre
- ID
- identifier
- IQR
- interquartile range
- LOS
- length of stay
- LOV
- length of invasive ventilation
- NWTS
- North West and North Wales
- ONS
- Office for National Statistics
- OR
- odds ratio
- PCCT
- paediatric critical care transport team
- PEDW
- Patient Episode Database for Wales
- PI
- principal investigator
- PIC
- paediatric intensive care
- PICANet
- Paediatric Intensive Care Audit Network
- PICU
- paediatric intensive care unit
- PIM2
- Paediatric Index Mortality 2
- PIS
- patient information sheet
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- VPN
- virtual private network
