Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number NIHR135450. The contractual start date was in November 2021. The final report began editorial review in November 2022 and was accepted for publication in February 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 de Bell et al. This work was produced by de Bell et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 de Bell et al.
Chapter 1 Background
The problem, condition or issue
Changing population demographics and rising rates of non-communicable diseases are placing new demands on the health and social care services. 1 It is estimated that one in seven people in the UK will be aged over 75 years by 2040. 1 Similar patterns are being seen worldwide; by 2050, the proportion of the population over the age of 60 years will double. 2 Owing both to the likelihood of developing chronic conditions with age and lifestyle factors (e.g. low rates of physical activity), there has been an increase in the number of people living with non-communicable diseases such as type 2 diabetes, chronic obstructive pulmonary disorder (COPD) and cardiovascular disease (CVD). 1,3 New models of care are needed to meet the challenges this situation creates for health and social care.
Technology offers opportunities for innovation in service provision that could be used to address some of these challenges. 1,4 This has been recognised in policy, with the World Health Organization (WHO) digital health strategy advocating the use of technology that ‘strengthens and scales up health promotion, disease prevention, diagnosis, management, rehabilitation and palliative care’. 5 Within the NHS England Long Term Plan, there are plans to invest in and increase the use of technology in the healthcare system. 6 This aim has been accelerated by the COVID-19 pandemic, which led to rapid adoption of technologies that enabled the remote provision of health services around the world, demonstrating the potential of technology. 7,8
Defining remote monitoring
Recent years have seen both the development of new devices and systems capable of delivering health services, and the implementation of technology within the healthcare system. The terms used to refer to this provision vary, as do their definitions. 4 eHealth is generally considered to encompass the use of digital health records (often accessed through patient portals, specific websites with secure access for individuals), as well as the delivery of health care via electronic means. 9 Within eHealth, telehealth, telemedicine, telecare and mHealth are all used to refer to the delivery of different types of health care or services via new technologies (e.g. smartphone apps) or older technologies (such as telephones) to aid self-management, diagnosis or treatment. 9,10 Remote monitoring is a further subset of eHealth that could be particularly beneficial for people with long-term conditions.
While multiple definitions of remote monitoring also exist,11 we define it as:
An intervention, involving the monitoring of a patient (using medical devices, applications, clinical investigation results, or other assessment tools), including self-monitoring, and which allows care professionals from a healthcare provider to assess and manage a patient’s condition remotely, without the need for the patient to be seen face to face.
A variety of remote monitoring technologies are available, including invasive (e.g. pacemakers)12 and non-invasive (e.g. blood pressure monitors),11 wearable sensors13 and home sensing technologies, which could be used to monitor falls or night-time disturbances. 14 Some take constant or automatic measurements, while others require the patient to take readings periodically. 15,16 The use of some is specific to certain conditions, such as the measurement of blood glucose by patients with diabetes. Others may provide an indication of health status (e.g. blood pressure, which is used in the monitoring of a range of conditions).
The application of remote monitoring technologies also differs between interventions. Variations include:
-
frequency of data upload and whether this is automatic or manual
-
the type of healthcare professional involved in the intervention and whether and how they provide feedback
-
frequency and mode of contact with healthcare professionals, whether in person or via telephone or mobile application
-
the content of feedback, which might include a referral to another healthcare professional or changes to medication.
How the intervention might work
For the individual
Remote monitoring can contribute to effective self-management, improving individuals’ knowledge of their condition and assisting them in managing their symptoms. 17 Additionally, it can help to bridge the gap between this self-management and professional health care. 18 By providing data on health status, monitoring can give patients the confidence to contact professionals when necessary and support health assessment and clinical decision-making, including timeliness of care through the identification of exacerbations. 11 It can also enhance communication between patient and provider, assisting in shared decision-making and enabling the delivery of personalised and person-centred care, an important component of quality of care. 4,18
For the healthcare system
Remote monitoring could have wider benefits for the healthcare system. In the UK, there is increasing financial pressure on the NHS and social care services,19 creating a need to reduce the costs of health care where possible. Remote monitoring offers opportunities to increase the efficiency of care delivery in a number of ways. 10 First, through more effective use of time, by contributing to enhanced communication, as detailed above, and as it means neither patient nor healthcare professional needs to travel to appointments. 20 It can also reduce health service use, both through the avoidance of unnecessary routine appointments and reducing acute admissions. 21
In addition to enabling health and social care services to respond to current challenges, remote monitoring and other technologies could help address wider, and urgent, societal problems such as the climate emergency. The NHS Sustainability Annual Report 2020–21 recognised the sustainability benefits of the implementation of digital technology during the COVID-19 pandemic and discusses how its future use could deliver further benefits. 22 By reducing the need to travel and the associated carbon emissions, these technologies could contribute to improving the sustainability of the healthcare system and the NHS England ambition to reach net zero, as set out in the Health and Care Act 2022. 23
Existing evidence
Background scoping searches of the literature found reviews on the effectiveness of remote monitoring, as well as factors that influence its acceptability for patients and providers and implementation by healthcare providers.
Effectiveness
Previous reviews of remote monitoring vary in their conclusions on its effectiveness. McBain et al. 21 focused on self-monitoring for three chronic conditions (heart failure, hypertension and COPD) in their review of reviews, finding significant reductions in both hospitalisation and re-admissions to hospital as a result of monitoring. However, a 2020 meta-analysis in which the majority of patients had either CVD or pulmonary disease, or were overweight or obese, did not find any statistically significant effects. 15 A range of clinical outcomes were assessed, including body mass index, weight, waist circumference, body fat percentage, systolic blood pressure and diastolic blood pressure. 15 In a narrative synthesis of studies on the impact of using eHealth tools on changes to medication use, there was little evidence of improvement to outcomes such as medication use or quality of life, but tools did lead to positive medication change and improved patient symptoms. 17 These reviews suggest that the effectiveness of remote monitoring may differ depending on the targeted health conditions and outcomes.
Acceptability and implementation
A number of reviews detail barriers and facilitators to the implementation of remote monitoring interventions. Thomas et al. 24 identified six theories of intervention success in their realist review of potential mechanisms reducing or leading to acute care use: (1) targeting populations at high risk; (2) accurately detecting a decline in health; (3) providing responsive and timely care; (4) personalising care; (5) enhancing self-management; and (6) ensuring collaborative and co-ordinated care.
Reviews on the positive and negative aspects of remote monitoring have focused on the views of clinicians,20 patients25 and both clinicians and patients. 26 Both groups consider potential benefits to include reduced travel and clinician workload, while raising concerns regarding lower quality of care and additional burden for providers. 20,25,27 Reviews concentrating on the technology itself also indicate the potential for negative impacts on healthcare providers, for example due to the need for increased data processing. 28 Additional barriers to adoption include connectivity28 and usability issues ranging from difficulties reading devices to the importance of instructions for users. 29
Why it is important to do this review?
During our initial scoping searches of the literature, we identified a large number of systematic reviews focusing on the effectiveness of remote monitoring, and the acceptability and implementation of these interventions. Remote monitoring is used for a range of health conditions, varying in everything from the aspect of health that is monitored to the application of the technology in the intervention. Understanding this evidence, recognising where evidence is concentrated and identifying where there are gaps is important to support evidence-informed policy, commissioning and provision. 30 Our conversations with relevant stakeholders’ contacts at NHS England’s NHS @home initiative indicated that knowledge of the breadth of evidence on remote monitoring would be most useful in supporting their work. Concentrations of evidence for certain health conditions or technologies could help inform the development of interventions and the delivery of existing programmes.
It is also important to understand the current evidence base to direct research. 30 Identifying topics which have been the focus of research prevents the duplication of effort, while knowledge of gaps – populations, interventions or outcomes where there are no systematic reviews – can prioritise areas for the future. Remote monitoring is an important topic for research, given ambitions for the use of technology in the health services and its potential to support adaptation to meet changing demands for health care. While COVID-19 has demonstrated how rapidly digital technology can be deployed, there are still many unknowns, with devices often developed by technology firms for the fitness market then adapted for other uses. 31
Chapter 2 Research question
We aimed to identify, classify, appraise and map recent systematic reviews of the effectiveness of remote monitoring and its acceptability and implementation in people living with long-term physical health conditions. Our research question was:
-
What is the volume, diversity and nature of recent systematic reviews about the use of remote monitoring interventions for adults living with long-term physical health conditions?
Our specific research objectives were to:
-
map recent systematic reviews of the effectiveness of remote monitoring interventions for adults living with long-term physical health conditions
-
map recent systematic reviews of the acceptability and implementation of remote monitoring interventions for adults living with long-term physical health conditions.
Chapter 3 Methods
Defining evidence and gap maps
Evidence and gap maps (EGMs) collate the research on a particular topic, providing an overview by summarising key characteristics of existing studies. 32 They are produced using similar methods to other forms of evidence synthesis, such as systematic reviews. However, unlike systematic reviews, they do not synthesise the findings of research; instead, they allow users to identify and access the research evidence most relevant to their patient groups and intervention focus, or to see where evidence gaps exist. 30,32 To produce an EGM, studies are categorised according to key dimensions (e.g. aims, methods, type of intervention, type of condition). A ‘map’ is then created by visually representing the number of studies in particular combinations of categories (usually in a two-dimensional grid). 33
Below, we describe the steps taken to produce this EGM on the effectiveness, acceptability and implementation of remote monitoring for long-term health conditions, as specified in our protocol. 34
Inclusion criteria
Inclusion criteria for reviews in the map are summarised below and in Table 1, with further details provided in Appendix 1, Table 5. Some systematic reviews included studies that did not meet our criteria; for example, they evaluated other eHealth interventions or were conducted in high- and low-income countries, in addition to relevant primary studies. As specified in our protocol,34 we considered reviews eligible for inclusion if 75% or more of the included studies met our inclusion criteria. We did not check individual primary studies; our decisions were based on information reported in the review.
| Include | Exclude | ||
|---|---|---|---|
| Acceptability | Implementation | ||
| Study design | Systematic reviews including comparative outcome evaluations | Systematic reviews including comparative outcome evaluations, other quantitative designs and/or qualitative studies | Any other study design |
| Population | Adult (≥ 18 years) | < 18 years | |
| Long-term physical health condition | No long-term condition | ||
| Participants | Patients as described above | Patients, carers and/or healthcare professionals | |
| Intervention | Any intervention where:
|
Interventions that are too poorly described to determine whether they meet this definition | |
| Multicomponent interventions | |||
| Outcomes | Any outcome related to effectiveness, including risk of adverse events and self-efficacy | Any outcome related to acceptability or implementation, including adherence | Cost effectiveness |
| Publication date | Systematic reviews published in 2018 or later | ||
Following title and abstract screening and after establishing the volume and nature of the available evidence, we decided to limit inclusion to reviews published since January 2018 for several reasons, as detailed below and further in the section Departures from the protocol:
-
To make the map more relevant to decision-makers. Remote monitoring technology is changing rapidly (e.g. use of smartphones) and older systematic reviews included studies evaluating technology that is out of date in terms of capability (e.g. unable to automatically transfer data), with associated implications for the generalisability of findings on acceptability and implementation.
-
To include reviews containing studies from both before and after the COVID-19 pandemic, which led to the rapid uptake of remote monitoring technology.
-
To reduce the number of papers that needed to be screened. Our title and abstract screening produced a large number of full-text articles (n = 829), the double-screening of which was beyond our capacity. We, therefore, decided to focus on the more recent and relevant portion of the identified papers.
Types of evidence
This map contains systematic reviews, defined as studies that have collected all the research on a given topic and synthesised it to answer a specific question, usually using prespecified methods to reduce bias. 35 To meet our definition of a systematic review, studies had to have defined a clear research question, used a reproducible search strategy, prespecified inclusion/exclusion criteria and screening methods, conducted quality assessment of included studies, and reported their method of data analysis. 36
We also considered the design of primary studies included within the systematic review. For reviews of effectiveness, we included those where at least 75% of studies were comparative outcome evaluations, whereas for reviews of acceptability or implementation we included all empirical research regardless of study design. When a review aimed to answer both effectiveness and acceptability or implementation questions and the primary studies addressing the effectiveness question did not meet our study design criteria, we included the review but extracted only data on the primary studies related to acceptability or implementation (see Types of outcome for detail on included acceptability and implementation outcome measures).
Type of population
This EGM focused on adult populations (18 years or over) with a long-term physical health condition. We considered long-term physical conditions to be any chronic disease of long duration that is unlikely to be cured completely. 37 These included conditions that typically develop early in life, for example asthma, as well as non-communicable diseases often associated with ageing such as CVD, and the long-term consequences of acute events/treatments, for example transplant patients, or cancer survivors. We excluded interventions that were preventative or focusing on the acute stages of treatment for what might be a long-term condition; for example, reviews of patients undergoing cancer treatment were excluded.
For reviews of effectiveness, we included only those where at least 75% of included primary studies focused on adults with a long-term physical health condition as participants. Additionally, for reviews of acceptability or implementation, we included those seeking the views of carers of adult patients and healthcare professionals using or providing remote monitoring. However, these reviews still had to focus on remote monitoring for adults with a long-term physical health condition as a population.
Types of intervention
Our intervention of focus was remote monitoring, defined as:
An intervention, involving the monitoring of a patient (using medical devices, applications, clinical investigation results, or assessment tools), including self-monitoring, and which allows care professionals from a healthcare provider to assess and manage a patient’s condition remotely – without the need for the patient to be seen face to face.
We included monitoring:
-
of objective or self-reported health status
-
occurring in the place where a person lives, either their home or a residential setting such as a care home
-
using a device or written output, as long as data are transferred to a care professional.
Reviews focusing on multicomponent interventions, such as those where participants attended education or counselling sessions as well as monitoring their health status, were excluded, unless the effects of remote monitoring alone could be distinguished due to the inclusion of an appropriate control or additional intervention group. This is because of the difficulty in determining the effectiveness of remote monitoring if combined with other components. 38 We considered interventions where some education was provided as part of feedback based on data submitted through monitoring, rather than in a separate session, as meeting our definition of remote monitoring. Reviews were only included if at least 75% of primary studies met our definition of remote monitoring.
Types of outcome
We were interested in all outcomes relating to effectiveness and acceptability or implementation. Outcomes of effectiveness included objective (e.g. heart rate, blood pressure) and subjective (e.g. quality of life, self-efficacy) measures as well as outcomes such as the occurrence of adverse events targeted by the intervention (e.g. risk of stroke) or caused by the intervention (e.g. inappropriate shocks from implantable cardioverter defibrillators when used to monitor patients with heart failure). Although we included reviews on use of the health service, those focusing solely on cost effectiveness were excluded, as consultation with stakeholders indicated a greater interest in health-related effectiveness outcomes.
We included reviews of quantitative and qualitative measures of acceptability or implementation, including patient adherence and patient satisfaction. Although acceptability is often considered an aspect of implementation, we decided to report it separately to make it more visible for map users, especially patients, carers and healthcare professionals, who might have a particular interest in this topic.
Types of location
This map contains systematic reviews in which at least 75% of the included primary studies were conducted in high-income countries, as defined by the World Bank (at 3 October 2022). 39 This is both because the funders of this map are working within a healthcare system in a high-income country and as a result of consultation with our stakeholders. While not all healthcare systems in high-income countries are comparable, this criterion ensured the included reviews contained primary studies that were most relevant to users in terms of healthcare system, patient population and social context.
Types of setting
Owing to the focus on remote monitoring, we included only reviews of interventions that took place in the participants’ homes, including care homes and other residential settings. Reviews containing primary studies in which initial training on how to use remote monitoring equipment occurred in a hospital or other medical facility were included.
Search methods and sources
Information specialists (NS and AB) developed the bibliographic database search strategies using MEDLINE (via Ovid) in consultation with the review team. The search strategy combined search terms for remote monitoring and evidence syntheses using both controlled vocabulary (e.g. MeSH in MEDLINE) and free-text search terms. Search terms were partly derived from the titles and abstracts of preidentified systematic reviews of remote monitoring and from initial scoping searches.
Search results were date limited to 2012. However, following title and abstract screening, a post hoc decision was made to further limit the inclusion to reviews published since January 2018 (see Inclusion criteria and Departures from the protocol for further details).
Electronic searches
We searched the following bibliographic databases in March 2022:
-
Cochrane Database of Systematic Reviews (via the Cochrane Library)
-
Cumulative Index to Nursing and Allied Health Literature Complete (EBSCOhost)
-
Embase (Ovid)
-
MEDLINE (Ovid)
-
Web of Science Core Collection (Clarivate)
-
Scopus (Elsevier)
-
PEDro
-
OTseeker
-
ProQuest Dissertations & Theses Global (via ProQuest).
Full search strategies for all bibliographic databases and other sources are included in Appendix 2.
All records from bibliographic database searches were imported into EndNote™ X9.3 (Clarivate, London, UK) and deduplicated using EndNote functionality and manual checks.
Searching other resources
Epistemonikos (www.epistemonikos.org) was searched on 30 March 2022 to identify relevant systematic reviews. Web searching was completed via Google Scholar using Publish or Perish (Harzing). Citation searching (forwards and backwards) was conducted on reviews that met our inclusion criteria using Scopus (Elsevier), Web of Science (Clarivate), Spidercite (available from SR-Accelerator: https://sr-accelerator.com) and Citation Chaser (available from: https://estech.shinyapps.io/citationchaser). Results from citation chasing were downloaded into EndNote and deduplicated against records retrieved from bibliographic database searches. To identify evidence syntheses from results of citation chasing, a search of All Fields in EndNote for review or meta or systematic or synthesis was applied.
Searches of the PROSPERO register (of systematic review protocols, available from: https://www.crd.york.ac.uk/prospero) were conducted on 23 March 2022 to identify continuing reviews. The publication status of each review was checked both in PROSPERO and through a search of title and author names in Google. Records for completed reviews (n = 106) identified from PROSPERO were added to the results from citation chasing and deduplicated against records identified from bibliographic database searches. Records for continuing reviews identified from published protocols or PROSPERO were screened separately as described in Stage 1: title and abstract below.
Screening and study selection
Stage 1: title and abstract
On completion of the searches, each member of the review team (SDB, ZZ, NS, AB, JTC, RA) independently applied the inclusion and exclusion criteria (Table 1 and Appendix 1, Table 5) to a random sample of citations (n = 100). This pilot screening exercise was intended to establish consistent interpretation of the inclusion criteria. Decisions were discussed in a group meeting, with some clarifications made to the criteria to ensure they were applied in the same way by different reviewers.
Following the initial calibration exercise, two reviewers (SDB and ZZ) independently applied the revised inclusion and exclusion criteria to the title and abstract of each identified citation. Disagreements were solved through discussion. Full-text papers of studies were obtained when both reviewers judged the study to meet the inclusion criteria and for those studies where it was not clear whether the criteria were met from the information in the title and abstract alone.
Two reviewers (SDB and ZZ) also independently screened the published protocols of all continuing systematic reviews identified in the searches. The information reported in the protocols was limited and, for many protocols, it was not possible to establish with certainty whether they meet our inclusion criteria. We therefore included all continuing reviews that were selected for inclusion by at least one of the reviewers and reported them separately in Appendix 3.
Stage 2: full text
The full text of each record was assessed independently by two reviewers (SDB and ZZ) to determine whether they met our inclusion criteria (as described above and in Appendix 1, Table 5). Decisions were made based on the information reported in the review and disagreements were settled through discussion with a third reviewer if necessary.
Data extraction and management
We imported records of the included reviews from the Endnote libraries used for screening into EPPI-Reviewer 4 (EPPI Centre, Social Science Research Unit, UCL Institute of Education, University of London, London, UK). A standardised data extraction coding form was then constructed in EPPI-Reviewer 4. The categories in this form are those from the framework detailed below in Developing the framework and can be found in Report Supplementary Material 1. The form was piloted by two reviewers (SDB and ZZ) on a sample of included reviews (n = 10) and discussed by the whole review team (SDB, ZZ, NS, AB, JTC, RA). Once revised to ensure that information provided in the reviews was being represented accurately by the categories in the form, data on each category were collected from all included full-text items. We defined items as a single review where they were based on the same searches; these could include multiple reports or publications. Data extraction was conducted by one reviewer (SDB or ZZ) and checked by a second reviewer (SDB or ZZ), with disagreements settled through discussion and, if necessary, the involvement of a third reviewer.
We did not check for duplication of primary studies between reviews. Besides being a difficult and time-consuming process, similar reviews often had a slightly different focus which means that even if most of the included studies overlapped, we still would have had to include the review to capture the breadth of evidence available.
Continuing reviews were grouped according to the patient population on which they focused. One reviewer (SDB) classified the continuing reviews and these classifications were then checked by a second reviewer (ZZ).
Developing the framework
The development of our framework was an iterative process. An initial framework was created using information from key literature (e.g. 9,15,21) identified during our initial scoping searches and by stakeholders at NHS @home. This was revised and refined following our first meeting with our patient and public involvement (PPI) group (as detailed in Public and patient involvement) and through discussion with stakeholders at NHS @home.
Categories were designed to describe the breadth of remote monitoring interventions and outcomes reported in the included reviews, as well as being accessible and easy to use in the interactive map. During data extraction, when information in the included reviews did not fit any categories in the framework, we renamed or adjusted the categories to ensure that all characteristics of the interventions and measured outcomes were included in the EGM. These adjustments were discussed and agreed upon in team meetings. Categories included in the framework are described briefly below, with details given in the data extraction form in Report Supplementary Material 1; full definitions are provided in the EGM glossary, which can be found in Report Supplementary Material 2.
Within the framework, we aimed to extract data on factors related to diversity and inclusion such as age and gender. A lack of consistent reporting meant that there was not enough information included in reviews on these factors to form categories in the framework.
Methods for mapping
The data on each review entered into EPPI-Reviewer 4 were visualised in an interactive map using EPPI-Mapper, version 1.2.5 (EPPI-Centre, UCL Social Research Institute, University College London, London, UK). Each record in the map contains one review and details the author, year of publication, title, journal and abstract, as well as giving the digital object identifier and a summary of basic information in the review; for example, the number of primary studies included in the review and the definition of remote monitoring used by the authors. Where we found publications that were based on the same searches, we treated these as a single review, providing the details of the additional publications at the end of the study abstract, together with a link to the relevant publication(s).
Characteristics of remote monitoring interventions
The included reviews contained a wide range of remote monitoring interventions. We detail important characteristics of the interventions in the EGM. These are: (1) what was monitored; (2) how it was monitored; (3) the method of passing on the data; (4) the healthcare professional involved; (5) the method of feedback; and (6) the content of feedback. Further information on the subcategories within these categories is provided in the data extraction forms in Report Supplementary Material 1 and the EGM glossary in Report Supplementary Material 2.
Categorisation of outcomes
We included any outcomes on effectiveness, acceptability or implementation in the EGM. We grouped effectiveness outcomes into four broad categories: (1) physical health; (2) mental health and well-being; (3) health behaviours and self-regulation; and (4) health service use. As most reviews of acceptability or implementation were qualitative, we grouped related outcomes within one broad category. Finally, we included one broad category in the map, adherence and compliance, which contained subcategories relating to both effectiveness and acceptability/implementation. Table 2 lists the subcategories within each of the broad categories, together with examples of measures used to assess them in included reviews.
| Outcome | Subcategory | Examples |
|---|---|---|
| Physical health | Mortality | All-cause mortality |
| Blood glucose/glycaemic control | Level of glycated haemoglobin; time in glycaemic range | |
| Blood pressure | Mean arterial pressure | |
| Other cardiovascular metrics | Peak oxygen consumption; left ventricular ejection fraction | |
| Detection rate | Detection rate of atrial arrhythmia | |
| Risk of adverse events | Incidence of stroke | |
| Weight/body mass index/waist circumference | ||
| General health | Six-minute walk distance test | |
| Other | Kidney related (e.g. serum creatinine); change in Epworth Sleepiness Scale | |
| Mental health/well-being | Anxiety/depression | Hospital Anxiety and Depression Scale; Goldberg anxiety or depression subscale scores |
| Quality of life | Short Form Survey SF-36; St George’s Respiratory Questionnaire | |
| Health behaviours/self-regulation | Self-management or self-care | Heart failure medication management; frequency of communicating with physicians |
| Knowledge, understanding | Diabetes knowledge | |
| Risk factors | Frequency of smoking; frequency of drinking | |
| Self-efficacy | Ability to monitor the conditions and having insights into living with the conditions | |
| Health care/service use | Hospitalisation | Admission or re-admission (e.g. heart failure-related admission), length of stay |
| Emergency room visits | ||
| Acceptability and implementation | Acceptability and satisfaction | Diabetes Treatment Satisfaction Questionnaire; qualitative themes (e.g. lack of trust, peace of mind) |
| Usability | Qualitative themes (e.g. functionality) | |
| Implementation-related | Qualitative themes (e.g. concern about additional burden, out-of-pocket costs for patients, accessibility, difficulties with physical installation of equipment such as finding space) | |
| Adherence/compliance | With treatment | Continuous positive airway pressure machine usage; adherence to lipid-modifying drugs |
| With intervention | Recording weight, pulse and blood pressure; adherence to blood glucose monitoring |
Filters for presentation
Evidence and gap maps are usually presented in two primary dimensions as a table, with different outcomes as columns and different intervention features as rows (as detailed above). We added additional filters to this EGM. Selecting a filter means the map will only display reviews containing evidence on the specified filter. This allows users to change the subset of reviews shown in the map to those most relevant to their needs (e.g. reviews that include at least one UK-based study). The filters are listed below, with detailed definitions available in Report Supplementary Material 2:
-
publication year
-
type of synthesis: meta-analysis, narrative, qualitative, other
-
included study designs: randomised controlled trial plus other study design, other quantitative (e.g. cohort studies, observational studies, other qualitative)
-
population: patients, carers, healthcare professionals
-
patient categories: CVD; neurological conditions; diabetes; respiratory conditions; cancer survivors; kidney disease; other; not clearly defined
-
study region (all regions where studies included in the review were conducted were selected): UK; Europe (not UK); North America; Australia or New Zealand; other; not clearly reported
-
duration of interventions: mean/median duration ≥ 12 months (as reported in the paper); at least one of the included studies had duration ≥ 12 months; not clearly defined
-
study quality (based on AMSTAR 2): high, moderate, low, critically low.
To accompany the map, we produced a brief narrative synthesis, which can be found in the Results section below, together with supporting tables and figures. 33 This synthesis details the distribution of reviews across the different intervention and outcome categories as well as the filters for the map. 32
Quality assessment
An adapted version of AMSTAR 2 was used to assess the quality of reviews included in the map. Quality appraisal was performed by one reviewer (SDB or ZZ) and checked by a second (SDB or ZZ), with disagreements settled by discussion and, if required, a third reviewer.
AMSTAR 2
AMSTAR 2 is a 16-item checklist which considers all aspects of the conduct of a systematic review, from prespecifying a protocol to the assessment and discussion of risk of bias within the review. 40 AMSTAR 2 is intended to critically appraise reviews of quantitative studies of healthcare interventions with randomised or non-randomised designs. This map includes reviews containing a broader range of study designs; accordingly, we adapted certain questions to allow us to appraise the quality of these reviews. These adaptations are based on Lam et al. 41 and can be found in Appendix 4, Table 7.
Items from the checklist are chosen as critical domains and used to determine the overall quality of the review. 40 There are four categories of overall quality: high, moderate, low and critically low. To be considered high-quality, a review can have no more than one non-critical weakness, while to be moderate-quality a review can have more than one non-critical weakness but no critical flaws. Low-quality reviews have a flaw in one critical domain and may have non-critical weaknesses; reviews of critically low quality have more than one critical flaw.
We reflected on the domains used by other researchers for similar topics41,42 and discussed the most important domains to accurately represent the quality of the included reviews for this area of research within the team. 40 To be considered high-quality, reviews had to have a prespecified protocol, comprehensive search strategy, have described included studies in adequate detail, assessed risk of bias in included primary studies appropriately, and investigated any heterogeneity in their results (for further detail see Appendix 4, Table 7).
External engagement
Engaging users in the process of evidence synthesis is important to ensure that that outputs produced meet their needs. 43
Stakeholder engagement
The core stakeholder group for this EGM were members of the NHS @home team within NHS England. A total of seven stakeholders, including the head of implementation, the evaluation lead and team members involved with specific NHS @home programmes (e.g. for heart failure @home and lung health @home), were consulted via e-mail and video meetings throughout the process of developing the EGM. These discussions determined the scope of our review question, the potential value of an EGM given the number of existing studies and systematic reviews, and the inclusion of key intervention and outcome categories in the framework for the EGM, as well as refining the interactive map. Table 3 details specific changes made to the map as a result of feedback from stakeholders.
| Type of change | Comment | Actions and response |
|---|---|---|
| Definitions and language | Stakeholders provided feedback on conditions and interventions included in the map and how they were grouped. Some (e.g. implantable cardiac monitors) were less relevant to NHS @home | We have clearly categorised health conditions and interventions in the map so that users can find reviews that are of most relevance to their needs |
| Stakeholders wanted to be able to distinguish between low- and high-quality reviews | Reviews are grouped and displayed in the map according to their quality; we have also added quality as a filter so that users can choose to look at only high- or low-quality reviews | |
| The PPI groups commented on barriers and facilitators to remote monitoring such as digital literacy | We considered these comments while constructing the data extraction form for the map. These factors were rarely reported so we were not able to collect data on them, but we have commented on them in the report | |
| The PPI group considered that receiving feedback on the data they were collecting was an important part of remote monitoring | We included method and content of feedback as two data extraction categories | |
| Map presentation | The PPI group thought that the colours representing study quality were not intuitive (darker colours representing lower quality) | We changed the colours representing study quality, so that darker colours indicated higher quality, and added an explanation of this beneath the title (together with other instructions for using the map) |
| Stakeholder and PPI groups wanted to know the number of UK studies included in reviews | We have included the number of UK studies in each review in the study summary and there is a filter that can be used to select UK-based studies only | |
| Useability | The PPI group found the size of the map overwhelming when first viewed and were worried about navigating away from the map to view instructions for use | We added basic instructions, including an explanation of how to reduce the size of the map, under the title, so they are easily seen when the map is first opened |
| The PPI group commented that an easy-to-read font would make the map more accessible | We changed the font used in the map to Verdana, which is a sans-serif font considered legible for online reading | |
| The PPI group commented that the white map background made the map harder to read, as did a grey background and pale text in the headers | We changed the header background to dark blue. EPPI-Mapper does not currently have functionality to change the colour of the map background, but we have passed this comment to their development team |
Public and patient involvement
We recruited a PPI group at the beginning of the project to gain feedback from people who use remote monitoring technology to manage their heath conditions. The group consisted of five people: one man and four women. Members of the group had a range of health conditions, including hypertension, COPD and sleep apnoea, and experiences of using different technologies (e.g. blood pressure monitors, heart rate monitors) as a patient, carer or both. We held three meetings with this group over the course of the project, arranging meetings to suit the project progress and participant availability. These meetings discussed:
-
– their experiences of using remote monitoring
-
– a draft version of the EGM
-
– the plain language summary and dissemination plans for the EGM.
Changes made to the map as a result of consultation with the PPI group can be found in Table 3.
Departures from the protocol
Title and abstract screening resulted in a large number of studies (n = 829) which needed to be checked at full text. As a result, we decided to restrict our inclusion criteria and limit full-text screening to articles published from 2018 onwards. This was a pragmatic decision, based on the need to reduce the number of studies to screen, but was made following discussion with NHS @home to ensure the relevance of the EGM to stakeholders. Remote monitoring technology is changing rapidly, so the results and conclusions of older systematic reviews are less reliable as they contain more studies on out-of-date technology and do not include more recent primary research studies. Older systematic reviews may also have been duplicated by more recent systematic reviews. Finally, even though the COVID-19 pandemic has accelerated the uptake and experience of remote monitoring in many patient groups, we wanted to capture reviews containing evidence from both before and after February 2020.
Chapter 4 Results
Results of the search and reviews included in the evidence and gap map
Figure 1 provides an overview of the search and screening process. Bibliographic database searches retrieved 12,124 records; 11,256 additional records were then identified through citation chasing or as completed reviews identified from PROSPERO searches. After deduplication, 7063 records from database searches and 1022 records from other sources were double-screened at title and abstract. This resulted in 986 reports which were eligible to be assessed at full text, 639 of which were published from 2018 onwards. These 639 were screened at full text, resulting in 72 systematic reviews (reported in 73 publications) being included in the EGM. The number of primary studies included in the reviews ranged from 3 to 118, median 16 (interquartile range 10–27; Figure 2). We found 86 continuing reviews (see Appendix 3).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.
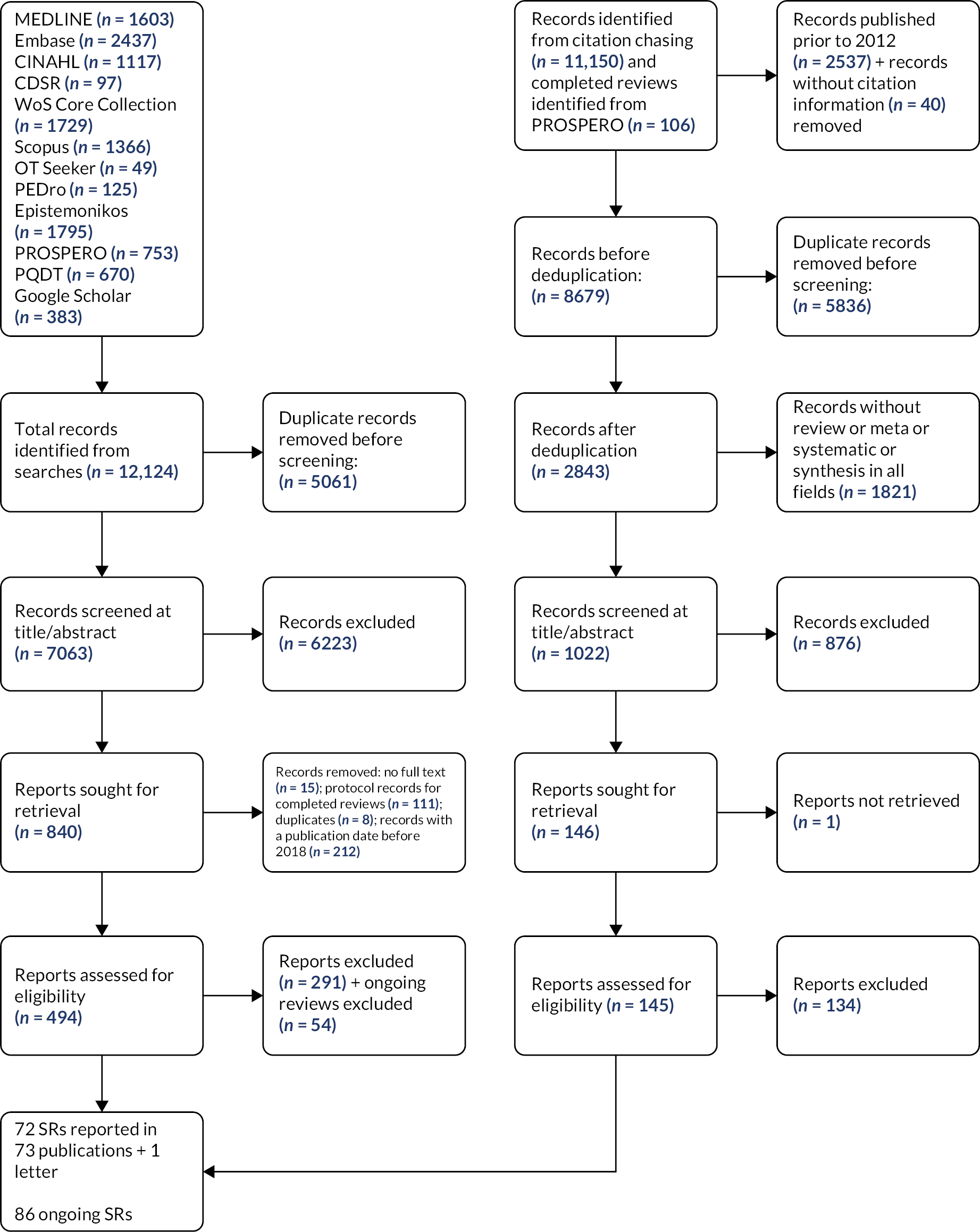
FIGURE 2.
Number of studies included in the reviews.

A list of studies excluded after screening at full text, along with reasons for exclusion, can be found in Report Supplementary Material 3. The primary reasons for exclusion were that the included interventions did not meet our definition of remote monitoring (n = 161) or that the study design did not fit our definition of a systematic review (n = 165).
Map of included reviews
The interactive EGM can be found at https://eppi.ioe.ac.uk/cms/Portals/35/Maps/ExeterNIHR/RemoteMonitoring.
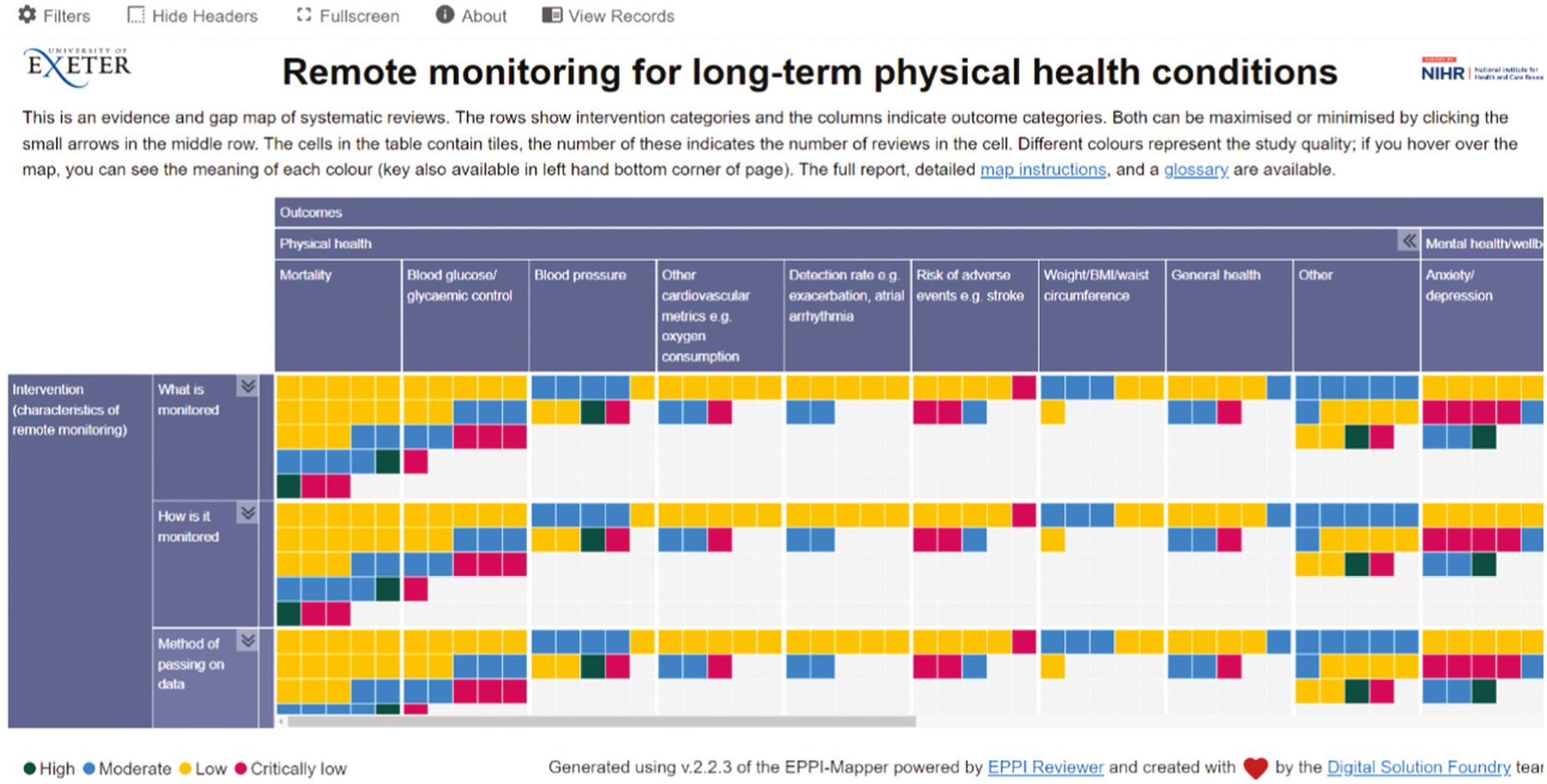
An example of the EGM is shown in Figure 3. Intervention categories are displayed as rows, outcome categories as columns, and the number of tiles indicate the number of reviews found in the cell. Colour represents study quality (as assessed by AMSTAR 2): dark green tiles indicate high-quality reviews, blue indicates moderate quality reviews, yellow low-quality reviews and pink critically low quality. The map has been prepared to be colour-blind friendly by using a colour palette with suitable shades and levels of contrast. 44
FIGURE 3.
Evidence and gap map of included reviews, showing intervention categories as rows and outcome categories as columns (subcategories can be accessed in the interactive map) and study quality (green indicates high quality, blue moderate quality, yellow low quality and pink critically low quality).
Individual reviews may be included in more than one category in the EGM, as they measure multiple outcomes, or report on several different types of intervention. Both in the narrative synthesis and in the figures and tables, the number of reviews reported is the total number of reviews found in that category. The sum of reviews for a figure or table, or in a descriptive summary, may therefore be greater than the number of unique reviews included within the category.
Below, we report areas of evidence synthesis concentration and ‘gaps’ in the EGM. ‘Gaps’ may show that remote monitoring has not been implemented for a certain combination of characteristics/outcomes (i.e. an ‘implementation gap’), that it has been implemented but not evaluated (i.e. an ‘evidence gap’) or that it has been implemented and evaluated through primary research, but not yet included in a systematic review (i.e. an ‘apparent evidence gap’).
Year of publication of included reviews
We included systematic reviews published from 2018 to March 2022. Between 2018 and 2020, the number of reviews published ranged from 11 to 14; a large increase was seen in 2021, with 29 reviews published in that year.
Populations and participants in included reviews
The included systematic reviews focused on patients, with all 72 reporting outcomes from patient populations. There were some reviews that also included data from carers (n = 3) and healthcare professionals (n = 5), but a gap was evident regarding reviews of these populations.
A range of health conditions were represented in the included reviews (Figure 4). There was a concentration of evidence synthesis concerning patients with CVD (n = 45), with diabetes (n = 25) and respiratory conditions (n = 23) being the next most studied populations. Reviews tended to concentrate on individual long-term conditions, with only three focusing on patients with multiple morbidities. ‘Gaps’ in secondary research were evident with respect to cancer survivors and patients with neurological conditions such as dementia (n = 3). Three reviews included primary studies on patient groups that were not clearly defined, referring to, for example, ‘general chronic conditions’, while seven reviews included studies on other conditions such as inflammatory bowel disease or thyroid disease.
FIGURE 4.
Number of included reviews reporting on each patient category. PAD, peripheral arterial disease.

We aimed to extract further data on patient populations (e.g. age, gender, health literacy and digital literacy), so that the map could represent the diversity of populations in which remote monitoring is implemented, as well as factors that might influence the effectiveness or acceptability of remote monitoring for specific populations. However, the inconsistent reporting of these characteristics within the included reviews meant that this was not possible and indicates an evidence ‘gap’.
Continuing reviews were classified according to their patient population of focus. Similar to reviews included in the EGM, CVD, diabetes and respiratory conditions are the most common patient populations (Table 4). However, a larger proportion of continuing reviews focus on neurological conditions than among the included reviews.
| Patients | Reviews (n) |
|---|---|
| Cardiovascular disease | 36 |
| Respiratory conditions | 13 |
| Not clearly defined/reported | 11 |
| Diabetes | 10 |
| Neurological conditions | 8 |
| Other | 5 |
| Kidney disease | 3 |
| Cancer survivors | 0 |
Type of remote monitoring in included reviews
Remote monitoring was used to measure a range of indicators of health status in the included systematic reviews, with several areas of evidence synthesis concentration evident regarding the format and delivery of interventions.
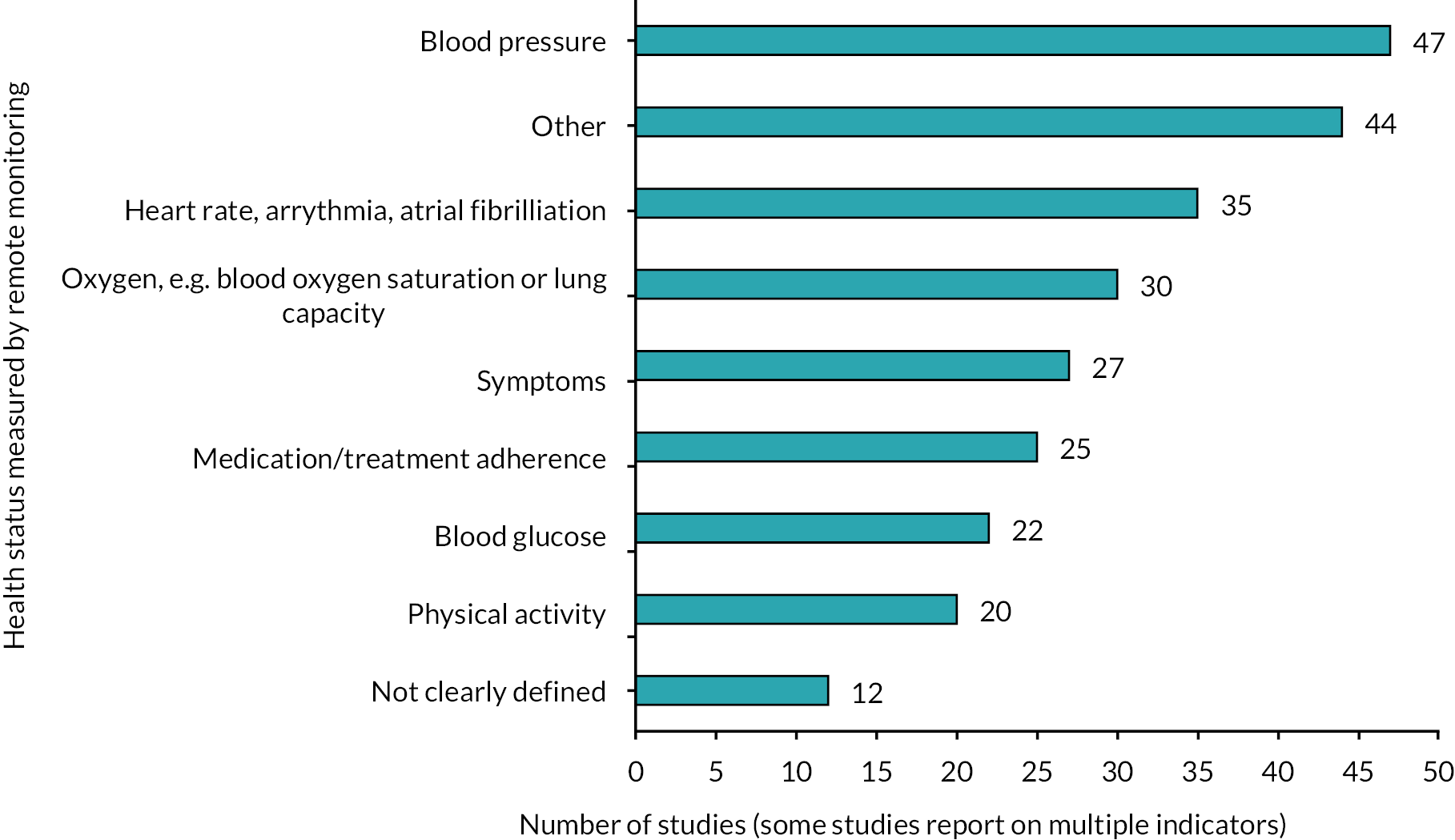
In terms of the indicator(s) of health status measured by remote monitoring, blood pressure (n = 47) was the most commonly used by primary studies in the included reviews. There were also concentrations of evidence synthesis relating to other cardiovascular measures (e.g. heart rate, arrythmia, atrial fibrillation; n = 35) and oxygen-related measures (e.g. blood oxygen saturation or lung capacity; n = 30). Medication/treatment adherence (n = 25), blood glucose (n = 22) and physical activity (n = 20) were the next most measured aspects of health (Figure 5). In 44 reviews, other indicators of health were monitored (e.g. weight). While the majority of measures were objective, 27 reviews included studies in which the symptoms were measured, often subjectively; for example, through questions on mood.
FIGURE 5.
Number of included reviews reporting on each category of health status measured by remote monitoring.
Health status was measured using implantable (n = 17) or wearable devices (n = 20) in some primary studies in the included reviews, but there was a concentration of evidence synthesis regarding the use of ‘other’ devices (n = 48; Figure 6). These included spirometers, weighing scales and blood pressure monitors. There were 29 reviews containing studies that used symptom tracking – this is more than the 27 reviews including studies on the monitoring of symptoms as this category also included the use of logbooks to record health indicators such as levels of physical activity. Data were passed from these devices to a healthcare professional via an app, website, e-mail or patient portal in primary studies included in 58 reviews, with 46 containing studies in which data were passed on automatically (Figure 7). There were fewer reviews containing studies where short messaging services (SMS; n = 10) or face-to-face meetings (n = 1) were used to pass on data, although, as noted in the Discussion, this does not necessarily indicate a ‘gap’.
FIGURE 6.
Number of included reviews reporting on how health status is measured in remote monitoring interventions.

FIGURE 7.
Number of included reviews reporting on each category for the (a) method of passing on the data; and (b) for the method of feedback to patients.
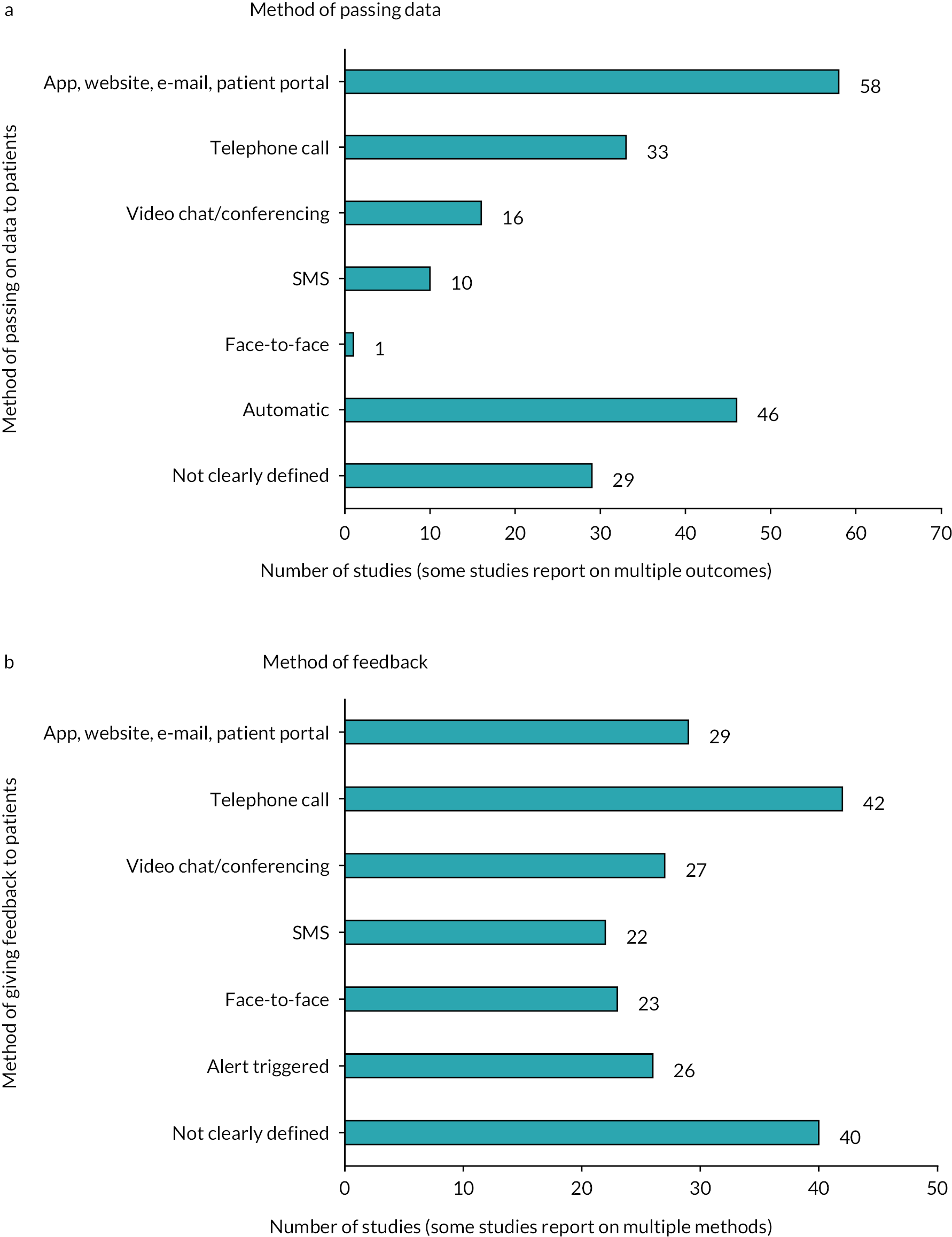
The majority of reviews included primary studies in which patients were provided with feedback as a result of remote monitoring. The type of healthcare professional with whom patients had contact was often not clearly defined (n = 43); where the role was defined, nurses were most frequently involved (n = 41), followed by doctors (n = 36) and other healthcare professionals (n = 24), for example physiotherapists. A concentration of evidence synthesis was present on the use of telephone calls (n = 42) by healthcare professionals to provide feedback to patients, with apps, websites, e-mails or patient portals (n = 29), and videoconferencing (n = 27) being the next most used methods of providing feedback (see Figure 7). No significant gaps were seen in terms of other methods of feedback, with 22 reviews reporting on feedback provided by SMS and 23 on face-to-face feedback. In 26 reviews, abnormal readings from monitoring resulted in an alert being triggered, prompting action by healthcare professionals.
The content of feedback found most often in the included reviews was motivation or education (n = 33) and changes to treatment/medication (n = 28). There were fewer reviews containing studies in which patients were referred, for example, to the emergency department (n = 12) as a result of monitoring. Most reviews also contained studies in which the content of feedback was not clearly defined (n = 46).
Outcomes reported in included reviews
The EGM includes 61 reviews that reported on the effectiveness of remote monitoring and 24 concerning its acceptability or implementation. Corresponding to the proportion of reviews that reported on effectiveness, the most common type of synthesis was meta-analysis (n = 48). Any outcome relating to effectiveness, acceptability or implementation was included in the map. By outcome, we mean what the remote monitoring intervention was intending to influence. For some interventions, the health indicator that was measured as part of the intervention was the same as the outcome that the intervention intended to influence (e.g. measuring and aiming to improve blood glucose levels in patients with diabetes), whereas in others the indicators measured were different (e.g. measuring heart rate in patients with CVD with the aim of reducing hospitalisations).
We grouped these outcomes into six broad categories, containing subcategories for specific outcomes (Figure 8). Four of these broad categories: (1) physical health, (2) mental health and well-being, (3) health behaviours and self-regulation and (4) health service use, contained outcomes associated with effectiveness. We used one broad category for acceptability and implementation, and one for adherence and compliance, which contained subcategories for both effectiveness and acceptability outcomes.
FIGURE 8.
Number of included reviews reporting on each broad outcome category.
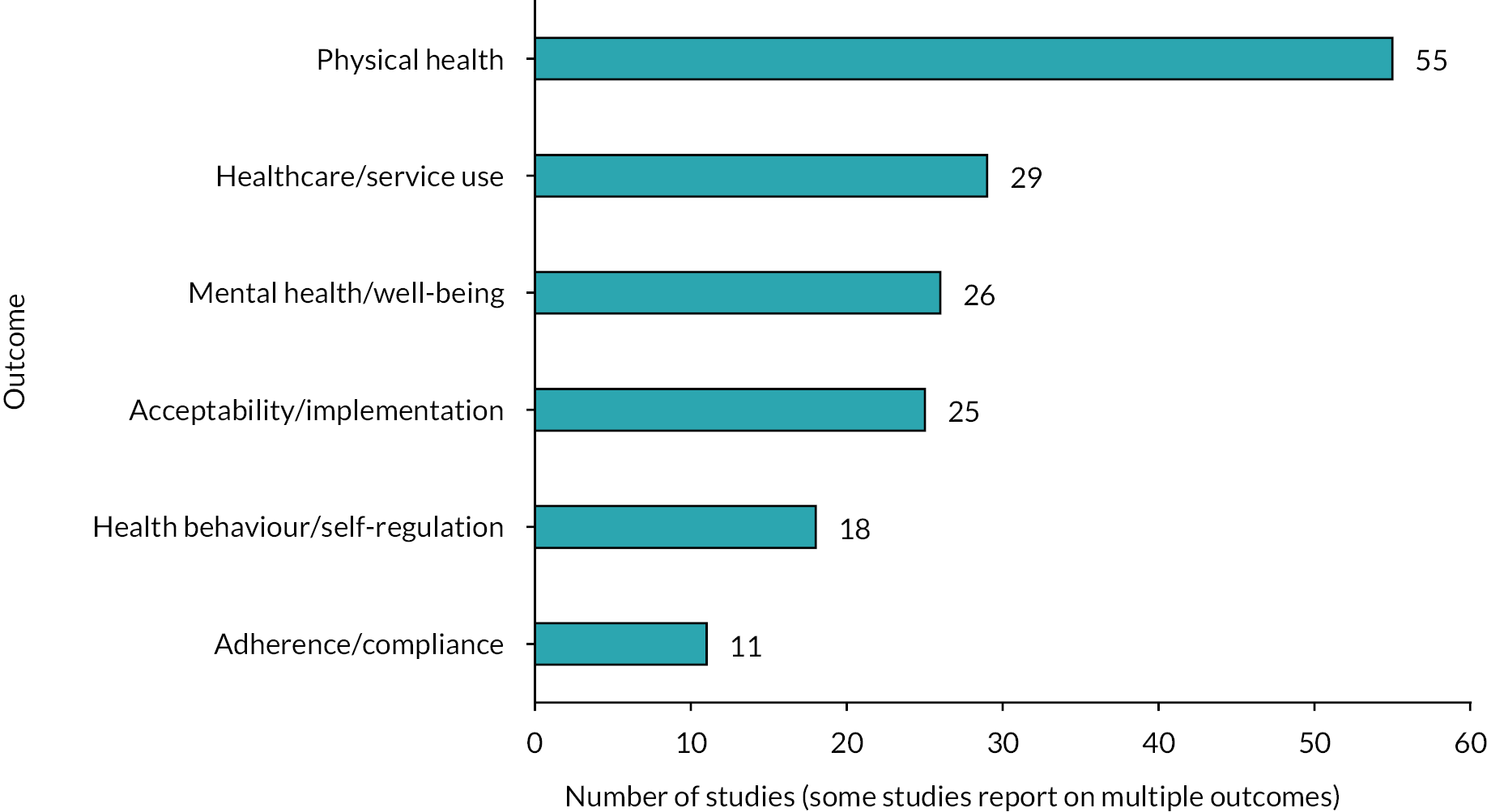
There was a concentration of evidence synthesis on physical health outcomes (n = 55). Mortality (n = 23) and glycaemic control (n = 16) were the most frequently described in the reviews. Similar numbers of systematic reviews reported outcomes such as blood pressure (n = 9), oxygen consumption (n = 8) and risk of adverse events such as stroke (n = 8). Fourteen reviews included ‘other’ physical health outcomes such as cholesterol levels or fatigue. While the focus of most remote monitoring interventions in the reviews was on measuring physical aspects of health, some reported the benefits of these interventions for mental health and well-being (n = 26). Outcomes related to anxiety/depression were reported in 13 reviews, while there was a concentration of evidence synthesis on quality-of-life outcomes, with 24 reviews reporting these outcomes.
Self-management or self-care (n = 14) was the main outcome reported for the broad category health behaviours and self-regulation (n = 18). There were few reviews that included studies on risk factors, for example low physical activity (n = 4) or self-efficacy (n = 5). Reviews containing information on the impact of remote monitoring on health service use (n = 29) tended to focus on hospitalisation (n = 29), with fewer focusing on emergency room visits (n = 16). There were several aspects of health service use for which we found no evidence of secondary research, such as primary care visits and staff time.
Regarding the acceptability and implementation of remote monitoring (n = 25), there was a concentration of evidence synthesis related to the acceptability and satisfaction (n = 24) of remote monitoring interventions. There was less secondary research reporting on usability (n = 7) and other implementation-related factors (n = 9). There were 11 reviews that included studies reporting on adherence and compliance with the intervention.
Certain outcomes had evidence synthesis concentrations for specific health conditions. For CVD, the most common condition in the EGM, 23 reviews reported on hospitalisation, 18 on mortality and 13 on quality of life, whereas only 2 reviews reported on self-efficacy. Blood glucose (n = 15) was reported as an outcome for the majority of reviews focusing on diabetes. Few reviews reported on other physical health-related indicators for patients with diabetes; further outcomes with greater evidence synthesis included acceptability and satisfaction (n = 11), self-management or self-care (n = 7) and quality of life (n = 6). Respiratory conditions had evidence synthesis concentrations for acceptability and satisfaction (n = 13), hospitalisation (n = 12) and quality of life (n = 10), with fewer reviews reporting on health behaviours and self-regulation.
Location of studies in the included reviews
Primary studies included in the reviews were global in origin. There was a concentration of evidence from North America and Europe (excluding the UK), with the majority of reviews containing primary studies from these locations (n = 52 and 50, respectively). No significant gaps were seen regarding geographic location, with 37 reviews including studies from the UK, 32 from other locations such as Argentina, Japan and Singapore, and 28 from Australia or New Zealand.
Quality of included reviews
The critical appraisal tool for systematic reviews AMSTAR 2 was used to assess the quality of included reviews. The majority of reviews in the map were of low quality (n = 33). While few were rated as high quality (n = 5), 22 were found to be of moderate quality and 12 were of critically low quality (Figure 9). In 56% of included reviews, the reason they were rated of low quality was the lack of a protocol. The majority of reviews described reasons for heterogeneity (92%) and adequately assessed the risk of bias in quantitative comparative evaluations (86%). However, it was often unclear whether the risk of bias in other quantitative study designs or qualitative studies had been assessed adequately (70%). For many reviews, while their searches were adequate as they searched at least two databases and provided keywords/a search strategy, their search strategies were not rated as fully comprehensive (68%), as they did not search as extensively as possible, for example in the grey literature or the reference lists of included studies. In terms of non-critical domains, few reviews described the funding sources of studies (82%) or gave full details of excluded studies (71%) but most provided details on the population, intervention, comparator and outcome(s) of focus (96%) and used appropriate methods of synthesis (97%). Additional details can be found in Appendix 4, Table 8.
FIGURE 9.
Overview of quality of included reviews, by AMSTAR 2 item; **indicates critical domains used to determine overall quality. PICO, population, interventions, comparison type and outcomes.
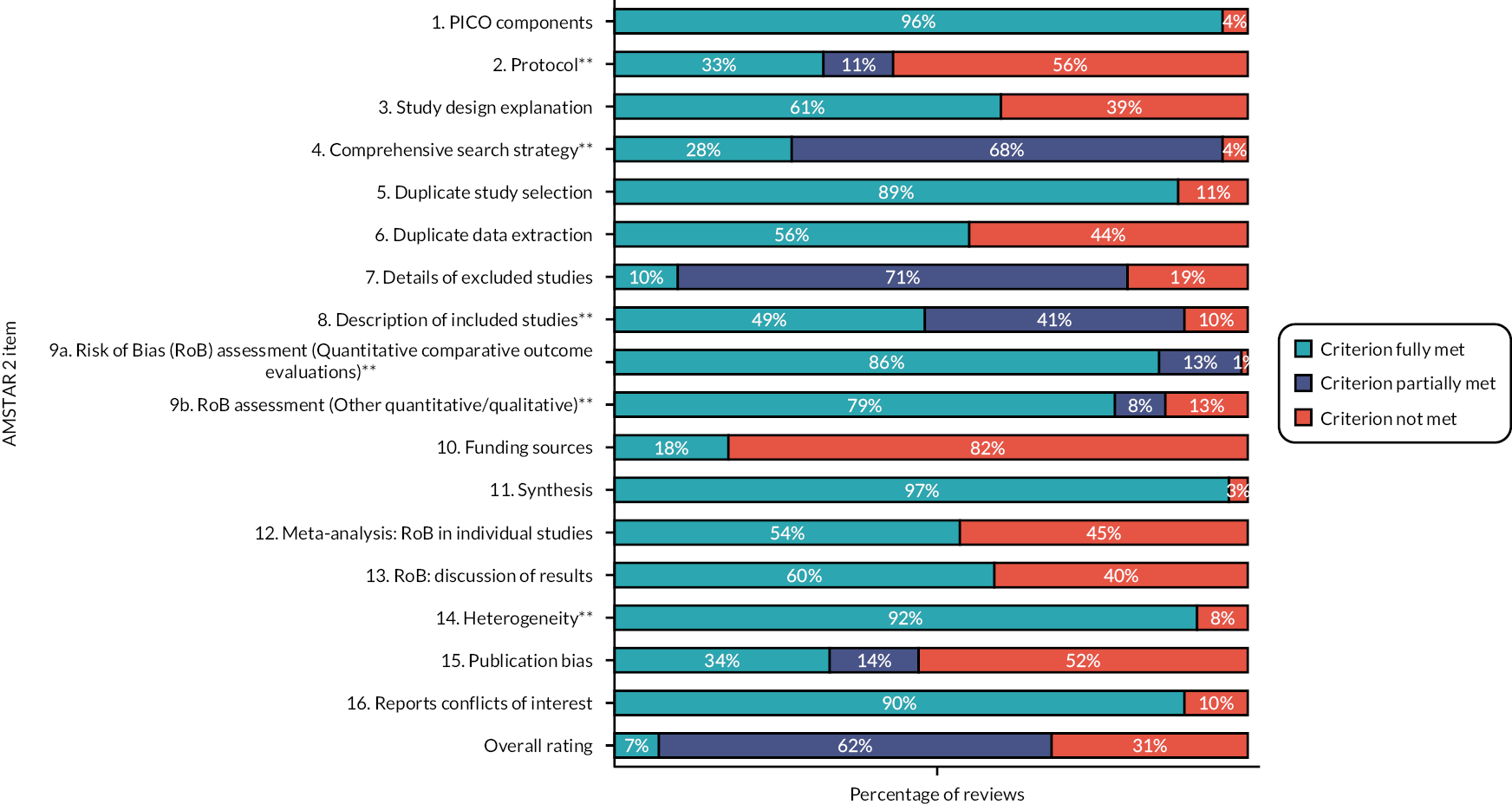
Patterns of evidence synthesis concentration and gaps regarding outcome and intervention categories were similar to those reported above across low- and moderate-quality studies. Of the five high-quality reviews, two reported mortality and three acceptability and satisfaction. Of those rated of critically low quality, four reviews included blood glucose as an outcome and three contained acceptability or implementation outcomes. A greater proportion of reviews assessed as critically low reported on patients with diabetes (7 of 25 reviews) than any other patient population.
Chapter 5 Discussion
Summary of main results
This EGM contains systematic reviews of primary studies reporting the effectiveness, acceptability or implementation of remote monitoring interventions. Owing to our stakeholders’ priorities, we did not explicitly seek or summarise systematic reviews of relevant economic or cost-effectiveness evidence. We found a considerable volume of research, particularly relating to the effectiveness of remote monitoring. There were some clear areas of evidence synthesis concentration and apparent gaps in the evidence; these are discussed below.
Areas of evidence concentration
Evidence synthesis concentrations in the map indicate that reviews of remote monitoring interventions have focused on certain health conditions, particularly CVD, diabetes and respiratory conditions. Accordingly, certain types of remote monitoring interventions are more represented in the map. For example, those measuring aspects of health related to CVD, such as blood pressure and heart rate, or respiration-related indicators, such as blood oxygen. Understandably, reported outcomes also varied depending on the condition, with concentrations of evidence synthesis for blood glucose for diabetes, mortality for CVD and hospitalisations for both CVD and respiratory conditions. There were also evidence syntheses regarding quality of life as an outcome of remote monitoring for all three of the most common conditions in the EGM.
The map contains a considerable number of reviews on ‘other’ devices (e.g. blood pressure or blood glucose monitors), reflecting the variety of health indicators that were measured by remote monitoring interventions and the range of technologies available. There was a greater volume of synthesised research on interventions where data were passed on via app, website, e-mail or patient portal, or automatically, than methods such as telephone calls or SMS. This perhaps reflects the fact that remote monitoring is often a form of digital innovation, and that a key aim of these interventions is to improve the efficiency of health care,9,45 for example through reducing re-admissions. 21 There were further concentrations of evidence synthesis relating to feedback, with feedback being most likely to be provided via a telephone call, and from a nurse, if the healthcare professional involved was reported.
Areas of major gaps in the evidence map and confidence considerations
Fewer reviews were found on the acceptability and implementation of remote monitoring than its effectiveness. This is not necessarily a gap, as separate effectiveness reviews are often conducted for different outcomes, meaning that they are likely to outnumber reviews on implementation-related factors, which typically summarise a wider range of measures within a single review. However, there was a clear gap in reviews reporting on the acceptability of remote monitoring to carers and healthcare professionals, and on factors affecting implementation in specific health conditions.
Actual or apparent gaps in secondary research on outcomes related to the potential benefits of remote monitoring should be highlighted. Some of the benefits of remote monitoring to patients are thought to be as a result of improved knowledge and self-management of their condition;18 we found a relative lack of reviews focusing on these outcomes. It has been suggested that remote monitoring could improve efficiency in the healthcare system,10 but reviews of health service use tended to focus on hospitalisations. We did not find any reviews looking at the effectiveness of remote monitoring for outcomes such as reducing staff workload. A small number of reviews reported risk of adverse events targeted by the intervention (e.g. adverse cardiovascular events) or caused by the intervention (e.g. inappropriate shocks from implantable cardioverter defibrillators when used for monitoring patients with heart failure), but there were no reviews for other adverse events such as communication errors.
The reviews reported a wide range of outcomes, which reflect the diverse impact that remote monitoring can have on patients’ physical and mental health, and the healthcare system as a whole (e.g. resource use). Twenty-three reviews reported mortality and eight reported risk of adverse events (e.g. stroke or cardiovascular events). Many of the reported surrogate outcomes (e.g. blood pressure, cholesterol and HbA1c) are well-established predictors of ‘harder’ outcomes (e.g. mortality, stroke and myocardial infarction) and could be more feasible to use than ‘harder’ outcomes (e.g. in younger patients with diabetes). Also, the studies reported a wide range of outcomes, including the impact on patients’ mental health, well-being and self-efficacy, which are also important to patients. The map could be used to explore to what extent outcomes important to patients are reported in a specific area, but this question as a whole requires further investigation and is beyond the scope of the current project.
Certain patient populations were also under-represented in the map: there was a lack of evidence synthesis on cancer survivors, those with neurological conditions and for other conditions such as inflammatory bowel disease. It should be noted that these were identified as ‘gaps’ as we found some evidence synthesis for these conditions. As discussed below in Implications for research, there are chronic conditions for which we found no secondary research, which are therefore not represented in the map.
There were few reviews that included interventions where SMS or face-to-face contact was used as a method of passing on data resulting from remote monitoring. However, as discussed in Areas of evidence concentration, this does not necessarily indicate a gap in the evidence. There were also few reviews that included studies where patients were referred for further medical intervention as a result of remote monitoring. This may be a gap in primary or secondary research, as one purpose of remote monitoring is to identify and react to exacerbations in a patient’s condition. 46,47 However, few interventions aimed to identify and react to exacerbations so this may indicate an implementation gap.
We aimed to extract demographic data and factors such as health and digital literacy, which might influence the effectiveness of remote monitoring from included reviews. There was a gap regarding these factors, with a lack of consistent reporting in the reviews, and there is, therefore, an evidence synthesis gap relating to diversity and inclusion in remote monitoring interventions and their impact on health equity.
In general, there was a lack of high-quality reviews in the map. In terms of critical flaws, less than half of the reviews had a published protocol and were rated as having an adequate but not comprehensive search strategy or description of the included interventions. Most of the reviews used appropriate methods for quality appraisal, data synthesis and investigation of heterogeneity. This means that the results from the majority of the included reviews might be biased and should be interpreted with caution, even when the included primary studies are of high quality.
Implications for research
Funders of systematic reviews and review authors should try to address the following issues:
-
lack of systematic reviews on remote monitoring in specific health conditions
-
failure to adhere to best practice guidelines for conducting systematic reviews and meta-analyses
-
failure to report (by review authors and/or authors of studies included in the reviews) essential information related to the intervention, participant characteristics or other aspects of study design.
Cardiovascular disease, diabetes and respiratory conditions such as COPD are among the most prevalent long-term conditions in the UK and worldwide,48,49 so the focus of research on these diseases is important. However, remote monitoring offers the potential to manage a range of health conditions and, while these conditions may affect smaller numbers of people, remote monitoring could offer them significant benefits. We found few systematic reviews on monitoring for neurological conditions, such as dementia, although there are several continuing reviews in this area and reviews that did not meet our inclusion criteria. As the number of older people living with dementia in the UK is predicted to increase by 80% from 2019 to 2040, and the cost of care is expected to be £94.1 billion by 2040,50 there is a particular need for evidence synthesis of research on remote monitoring in this patient population. Similarly, systematic reviews are needed on conditions where remote monitoring could increase quality of life, such as inflammatory bowel disease,51 epilepsy and allergies;48 these are either potential areas for further research or, if primary research exists, for evidence synthesis.
The fact that 33 (46%) of the 72 reviews included in the map were judged to be of low quality is of particular concern and casts doubt on the usability of the review results for decision-making. Researchers should consult guidance documents such as those produced by the Centre for Reviews and Dissemination52 and the Cochrane Collaboration53 when conducting further reviews, as well as referring to PRISMA when reporting reviews. 54 There was a lack of high-quality reviews found, with the absence of a prespecified review protocol being the most common reason for reviews being judged as low quality. Registering a protocol on a recognised database such as PROSPERO (Centre for Reviews and Dissemination, University of York) is an important step in the conduct of a review, avoiding duplication of reviews, providing an understanding of the methods applied and reducing the risk of bias in the review. 52
Fifty-one per cent of the included reviews failed to report essential information about the intervention, the participants or some other aspect of study design that could affect the interpretation of results. The effectiveness and acceptability of remote monitoring interventions could be affected by a wide range of participant characteristics, such as age, professional role, educational status, health and digital literacy. 55 Future reviews should report such information as fully as possible, and should signal gaps in the reporting of primary studies, to improve the existing evidence base and help determine the impact of remote monitoring on equity of access to services.
Given the complexity of remote monitoring, detailed description of the included interventions and their variation is essential for readers to make informed decisions about the applicability and reliability of results. Researchers may find the TIDier (template for intervention description and replication) checklist useful,56 which is specifically designed to improve the reporting of healthcare interventions and could be used in conjunction with other CONSORT tools.
Eighty-two per cent of the reviews failed to report information on the funding of the included studies. Reporting such information is important, as this is an area where technologies may be, and often are, commercially produced. In other areas where this is the case, such as drug trials, sources of funding are routinely reported.
Implications for practice and/or policy
The EGM contains concentrations of evidence synthesis on the effectiveness of remote monitoring that could be used to support the commissioning of remote monitoring interventions by healthcare providers. The COVID-19 pandemic resulted in a rapid shift to the use of remote monitoring and other technologies. 7,8 Although there has been a return to face-to-face provision for many services, the pandemic demonstrated both the potential of such technology and its wider acceptability. The NHS plans to increase the use of remote monitoring in the future,6 through initiatives such as NHS @home, which is developing home monitoring programmes for various conditions such as heart and lung disease. As can be seen in the increasing number of reviews per year in the map, and the continuing reviews noted in Appendix 3, further evidence synthesis is likely to be available to support the design and delivery of remote monitoring. That said, it is conceivable that evidence from studies conducted pre-COVID-19 might now be less applicable given the recent scale of uptake and levels of acceptability in some contexts. With the pace of developments in remote monitoring technologies and the post-pandemic shifts in the context of their use, there may be a case for conducting reviews exclusively of more recent studies. This evidence could assist in achieving goals regarding the use of digital technologies, such as those set out in the NHS Long Term Plan6 and the WHO Global Health Strategy. 5
Diabetes, cardiovascular and respiratory conditions are some of the most common long-term conditions in the UK. 48 As the greatest quantity of evidence syntheses in the EGM relates to these conditions, the map could be particularly beneficial in supporting the commissioning or delivery of remote monitoring for people with these conditions. The map also contains evidence syntheses on the measurement of different health indicators and the use of different types of device, with many then passing on that data using apps, websites or patient portals. Information on the effectiveness of these different intervention features could be used by those delivering remote monitoring to design interventions with the most suitable features for their target populations.
While the map focuses on patients with long-term physical health conditions, evidence in the included reviews could aid healthcare professionals in supporting multiple aspects of patient’s health. Having a long-term physical health condition can have implications for mental health,57 and there is evidence synthesis in the EGM on the impact of remote monitoring on the mental health of those with physical health conditions, particularly quality of life.
The apparent lack of secondary research on families and carers is a problem for the successful implementation of remote monitoring interventions, as these groups often have the main responsibility for monitoring. 55 While there were fewer reviews reporting on the acceptability or implementation of remote monitoring interventions than on their effectiveness, a number were found, including a realist review,24 which could be used by healthcare professionals to identify key factors to ensure the successful delivery of these interventions.
Limitations
This is a map of systematic reviews, not trials, which is a strength, as high-quality systematic reviews are usually regarded as better for aiding decision-making. However, only including reviews is also a limitation because we were only able to include evidence for remote monitoring interventions that have been included in a systematic review. While some gaps in the map may be implementation gaps or may indicate a lack of primary research, for others, evidence may be available that has not yet been reviewed. As we did not check for duplication between reviews, the EGM may also misrepresent the true volume of evidence within some categories in the map.
As an umbrella term, eHealth, and terms related to the delivery of health care using technology which fall under it, such as telemedicine, are not used consistently in the literature. 4 Whether they encompassed remote monitoring was dependent on how they were defined by the authors in individual reviews. This meant that, in order not to miss any relevant reviews, we had to search for all relevant terms, with the fact that our database searches found only around half of the potentially relevant studies perhaps reflecting the challenges created by these differences in definitions. As definitions were rarely evident in the abstract, this also resulted in a large volume of literature to screen at full text.
The volume of literature meant that we applied strict inclusion and exclusion criteria; as a result, some relevant evidence may have been excluded. Included reviews were published after 2018, so earlier reviews with relevant information, particularly regarding the implementation of remote monitoring, will have been excluded. However, there have been considerable advances in technology in recent years, including capabilities that aid the implementation of interventions such as passing data automatically from the device to a healthcare provider. These advances make the findings of older reviews less applicable; for example, older technology might need specialist installation and maintenance, whereas new devices can be used immediately, so the impact of these exclusions is not likely to be significant. We also made the decision to include reviews only when 75% or more of included studies met our inclusion criteria, to ensure most evidence in the EGM is of relevance to users. However, a different cut-off point would change the evidence contained in the map. Despite this comprehensive search, authors often failed to clearly report either their interpretation of remote monitoring or the details of the interventions included in the review. When little information was given, we were inclusive, meaning that some information in the map may relate to interventions that do not fit our definition of remote monitoring.
Last, owing to the priorities expressed by our stakeholders (effectiveness, acceptability and implementation), we did not seek to include reviews of economic or cost-effectiveness evidence relating to remote monitoring interventions in the EGM. Some would regard this as a significant limitation of the evidence that we have summarised, given the cost-saving intentions of some types of remote monitoring, and as systematic reviews of economic evaluations or cost-effectiveness studies have been conducted in conjunction with some of the reviews of effectiveness included in our EGM. This could be addressed if an update of this EGM is conducted in the future.
Equality, diversity and inclusion
As stated in the protocol for this EGM,34 we aimed to extract data on factors such as age and gender, which might relate to the effectiveness of remote monitoring, from the included systematic reviews. However, inconsistencies in the reporting of these variables between reviews meant that this was not possible. While many meta-analyses included in the EGM conducted subgroup analyses, these focused on condition or length/type of intervention rather than demographic factors. It has been noted that remote monitoring tends to focus on narrow patient populations, rather than considering how factors such as age, gender, ethnicity, income and the intersection of these identities might impact its success. 55 Additionally, health and digital literacy were noted as important by a number of reviews (e.g., refs. 55,58,59) and need further consideration in research.
Our team was small, making it difficult to ensure diversity across a range of groups; we also did not feel comfortable asking team members to disclose information on diversity unless they wished to share this information. However, we did recruit a PPI group with a range of conditions to inform the review, representing the experience of the varied application of remote monitoring. All team members had experience of producing evidence syntheses, including EGMs, and working with stakeholders and PPI groups to achieve this. The review offered opportunities for the development of skills, through sharing knowledge on the conduct of EGMs for team members who had less experience of producing this form of evidence synthesis and mentoring of junior members by team leads in project management.
Public and patient involvement and engagement
While the topic and focus of the EGM were determined by the policy customers at the start of the project, discussions with the PPI group provided context and developed our understanding of the topic as well as confirming its importance. The input of the group informed the categories included in the data extraction form and the design of the EGM, particularly in improving clarity for non-expert users. Overall, PPI was valuable in improving the EGM; the main difficulty we encountered was with the programme (EPPI-Mapper 4) used to develop the EGM, which meant it was not easy to share in its draft stages.
Chapter 6 Conclusions
This EGM is an accessible and interactive tool that provides a comprehensive overview of recent systematic reviews on the effectiveness, acceptability and implementation of remote monitoring interventions for adults with long-term physical health conditions. It could be used by a wide range of stakeholders (e.g. policy-makers, commissioners, patients, clinicians and researchers) to interrogate the available secondary research evidence and access systematic reviews on specific topics (e.g. remote monitoring using implantable devices). This could support the commissioning and delivery of interventions, while identifying apparent gaps in evidence synthesis, which could inform future research and technology development.
The majority of the included reviews investigate the effectiveness of remote monitoring in patients with CVD, diabetes and chronic respiratory conditions, while the number of reviews on other chronic conditions is limited. Reviews on acceptability and implementation focus almost entirely on the patients’ perspective, with only a small number on the perceptions and experiences of carers and healthcare professionals.
More than half of the included reviews have critical methodological flaws so their results should be interpreted cautiously, even when the included primary studies are reported to be of high quality. Many of them provide very scant descriptions of the included interventions, which makes the interpretation of results difficult. Additionally, a lack of consistent reporting on patient characteristics such as age, gender and digital literacy means that it is difficult to assess the impact of remote monitoring on equity of access to services. This may reflect either a lack of application of remote monitoring or its evaluation in specific groups.
Future reviews should:
-
adhere more closely to the recommended systematic review methods
-
report their methods and findings as fully as possible
-
provide detailed description of the included interventions ideally using intervention characteristics such as those listed in the map as a template
-
report the effectiveness, acceptability and implementation of remote monitoring in all relevant patient groups or highlight the lack of such evidence
-
investigate the application of remote monitoring in chronic conditions for which evidence is missing
-
explore acceptability and implementation from a wider range of perspectives.
Acknowledgements
The Exeter HS&DR Evidence Synthesis Centre team would like to acknowledge and thank the NHS @home team at NHS England for their support in developing this evidence and gap map and the PPI group for their valuable feedback. We would also like to thank Sue Whiffin for administrative support throughout this review.
Contributions of authors
Siân de Bell (https://orcid.org/0000-0001-7356-3849) (Research Fellow) contributed to the development of the protocol, carried out screening, data extraction and quality appraisal, developed the interactive EGM, led PPI engagement, and led drafting of the final report.
Zhivko Zhelev (https://orcid.org/0000-0002-0106-2401) (Research Fellow) carried out screening, data extraction and quality appraisal, developed the interactive EGM, supported PPI engagement, drafted sections of the report and read, provided feedback on, edited and approved the final version of the report.
Naomi Shaw (https://orcid.org/0000-0001-7387-1809) (Information Specialist) contributed to the development of the protocol, designed and ran the search strategies, carried out citation chasing and managed the bibliographic libraries, drafted sections of the report and read, provided feedback on, edited and approved the final version of the report.
Alison Bethel (https://orcid.org/0000-0002-0963-9201) (Information Specialist) contributed to the development of the protocol, designed and ran the search strategies, carried out citation chasing and managed the bibliographic libraries, drafted sections of the report and read, provided feedback on, edited and approved the final version of the report.
Rob Anderson (https://orcid.org/0000-0002-3523-8559) (Professor of Health Services and Implementation Research) provided overall project management and contributed to the scoping process, refining of research questions and protocol, development of interactive EGM, and read, provided feedback on, edited and approved the final version of the report.
Jo Thompson Coon (https://orcid.org/0000-0002-5161-0234) (Professor of Evidence Synthesis and Health Policy) contributed to the scoping process, refining of research questions and protocol, development of interactive EGM, and read, provided feedback on, edited and approved the final version of the report.
Publications
The evidence and gap map is available at: https://eppi.ioe.ac.uk/cms/Portals/35/Maps/ExeterNIHR/RemoteMonitoring.
Price A, de Bell S, Shaw N, Bethel A, Anderson R, Coon JT. What is the volume, diversity and nature of recent, robust evidence for the use of peer support in health and social care? An evidence and gap map. Campbell System Rev 2022;18:e1264. https://doi.org/10.1002/cl2.1264
Ethics statement
This was an evidence review, based on published systematic reviews, so did not require ethical approval.
Data-sharing statement
This is an evidence synthesis study based on published systematic reviews. It did not generate new data. All data extracted from the reviews, together with links to each publication, can be found in the evidence and gap map, available at: https://eppi.ioe.ac.uk/cms/Portals/35/Maps/ExeterNIHR/RemoteMonitoring. Further information can be obtained from the corresponding author.
Information governance statement
This study did not involve primary research or therefore the handling of any personal information.
Department of Health and Social Care disclaimer
This report presents independent research commissioned by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, Medical Research Council, Central Commissioning Facility, NIHR Evaluation, Trials and Studies Coordinating Centre, the HSDR programme or the Department of Health and Social Care.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Government Office for Science, Foresight . Future of an Ageing Population 2016. www.gov.uk/government/publications/future-of-an-ageing-population (accessed 27 June 2023).
- World Health Organization . Ageing and Health 2022. www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed 8 March 2022).
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. https://doi.org/10.1016/S0140-6736(20)30925-9.
- Harst L, Lantzsch H, Scheibe M. Theories predicting end-user acceptance of telemedicine use: systematic review. J Med Internet Res 2019;21. https://doi.org/10.2196/13117.
- World Health Organization . Global Strategy on Digital Health 2020–2025 2021. https://apps.who.int/iris/handle/10665/344249 (accessed 27 June 2023).
- NHS England . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk/publication/nhs-long-term-plan (accessed 27 June 2023).
- Vindrola-Padros C, Singh KE, Sidhu MS, Georghiou T, Sherlaw-Johnson C, Tomini SM, et al. Remote home monitoring (virtual wards) for confirmed or suspected COVID-19 patients: a rapid systematic review. EClinicalMedicine 2021;37. https://doi.org/10.1016/j.eclinm.2021.100965.
- Horton T, Hardie T, Mahadeva S, Warburton W. Securing a Positive Health Care Technology Legacy from COVID-19. London: Health Foundation; 2021.
- Barbabella F, Melchiorre MG, Quattrini S, Papa R, Lamura G. How Can EHealth Improve Care for People With Multimorbidity in Europe? 2016. www.ncbi.nlm.nih.gov/books/NBK464571 (accessed 26 October 2022).
- Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood) 2014;33:194-9. https://doi.org/10.1377/hlthaff.2013.0992.
- Iqbal FM, Lam K, Joshi M, Khan S, Ashrafian H, Darzi A. Clinical outcomes of digital sensor alerting systems in remote monitoring: a systematic review and meta-analysis. NPJ Digit Med 2021;4. https://doi.org/10.1038/s41746-020-00378-0.
- Veenis JF, Radhoe SP, Hooijmans P, Brugts JJ. Remote monitoring in chronic heart failure patients: is non-invasive remote monitoring the way to go?. Sensors 2021;21. https://doi.org/10.3390/s21030887.
- Lunde P, Nilsson BB, Bergland A, Kværner KJ, Bye A. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res 2018;20. https://doi.org/10.2196/jmir.9751.
- Husebo BS, Heintz HL, Berge LI, Owoyemi P, Rahman AT, Vahia IV. Sensing technology to monitor behavioral and psychological symptoms and to assess treatment response in people with dementia. A systematic review. Front Pharmacol 2019;10. https://doi.org/10.3389/fphar.2019.01699.
- Noah B, Keller MS, Mosadeghi S, Stein L, Johl S, Delshad S, et al. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med 2018;1. https://doi.org/10.1038/s41746-017-0002-4.
- Choi WS, Shin I-S, Yang J-S. Understanding moderators of home blood pressure telemonitoring systems in urban hypertensive patients: a systematic review and meta-analysis. Telemed E-Health 2020;26:1016-34. https://doi.org/10.1089/tmj.2019.0205.
- Lancaster K, Abuzour A, Khaira M, Mathers A, Chan A, Bui V, et al. The use and effects of Electronic Health Tools for patient self-monitoring and reporting of outcomes following medication use: systematic review. J Med Internet Res 2018;20. https://doi.org/10.2196/jmir.9284.
- Zhang W, Cheng B, Zhu W, Huang X, Shen C. Effect of telemedicine on quality of care in patients with coexisting hypertension and diabetes: a systematic review and meta-analysis. Telemed J E Health 2021;27:603-14. https://doi.org/10.1089/tmj.2020.0122.
- Health Foundation, Kings Fund, Nuffield Trust . Budget 2018: What It Means for Health and Social Care 2018. www.kingsfund.org.uk/sites/default/files/2018-11/budget-2018-what-it-means-for-health-and-social-care_0.pdf (accessed 26 October 2022).
- Davis MM, Freeman M, Kaye J, Vuckovic N, Buckley DI. A systematic review of clinician and staff views on the acceptability of incorporating remote monitoring technology into primary care. Telemed E-Health 2014;20:428-38. https://doi.org/10.1089/tmj.2013.0166.
- McBain H, Shipley M, Newman S. The impact of self-monitoring in chronic illness on healthcare utilisation: a systematic review of reviews. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-1221-5.
- NHS Digital . Sustainability Annual Report 2020–2021 n.d. https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/sustainability/sustainability-reports/sustainability-annual-report-2020-21 (accessed 26 October 2022).
- NHS England . Delivering a ‘Net Zero’ National Health Service 2022. www.england.nhs.uk/greenernhs/a-net-zero-nhs (accessed 27 June 2023).
- Thomas EE, Taylor ML, Banbury A, Snoswell CL, Haydon HM, Gallegos Rejas VM, et al. Factors influencing the effectiveness of remote patient monitoring interventions: a realist review. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2021-051844.
- Walker RC, Tong A, Howard K, Palmer SC. Patient expectations and experiences of remote monitoring for chronic diseases: systematic review and thematic synthesis of qualitative studies. Int J Med Inf 2019;124:78-85. https://doi.org/10.1016/j.ijmedinf.2019.01.013.
- Ferguson C, Inglis S, Hickman L, Byiers V, Macdonald P, Breen P, et al. 766 Acceptance and uptake of wearable cardiac technologies in older adults: a systematic review and meta-synthesis. Heart Lung Circ 2020;29. https://doi.org/10.1016/j.hlc.2020.09.773.
- Ferguson C, Hickman LD, Turkmani S, Breen P, Gargiulo G, Inglis SC. ‘Wearables only work on patients that wear them’: barriers and facilitators to the adoption of wearable cardiac monitoring technologies. Cardiovasc Digit Health J 2021;2:137-47. https://doi.org/10.1016/j.cvdhj.2021.02.001.
- Baig MM, Gholam Hosseini H, Moqeem AA, Mirza F, Linden M. A systematic review of wearable patient monitoring systems – current challenges and opportunities for clinical adoption. J Med Syst 2017;41. https://doi.org/10.1007/s10916-017-0760-1.
- Saeed N, Manzoor M, Khosravi P. An exploration of usability issues in telecare monitoring systems and possible solutions: a systematic literature review. Disabil Rehabil Assist Technol 2020;15:271-81. https://doi.org/10.1080/17483107.2019.1578998.
- Snilstveit B, Vojtkova M, Bhavsar A, Gaarder M. Evidence Gap Maps: A Tool for Promoting Evidence-Informed Policy and Prioritizing Future Research 2013. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/212651468163487838/evidence-gap-maps-a-tool-for-promoting-evidence-informed-policy-and-prioritizing-future-research (accessed 27 June 2023).
- Davis K, Hobbs A. Innovation in adult social care. UK Parliament POST 2022:1-20. https://researchbriefings.files.parliament.uk/documents/POST-PN-0670/POST-PN-0670.pdf (accessed 26 October 2022).
- White H, Albers B, Gaarder M, Kornør H, Littell J, Marshall Z, et al. Guidance for producing a Campbell evidence and gap map. Campbell Syst Rev 2020;16. https://doi.org/10.1002/cl2.1125.
- Saran A, White H. Evidence and gap maps: a comparison of different approaches. Campbell Syst Rev 2018;14:1-38. https://doi.org/10.4073/cmdp.2018.2.
- de Bell S, Shaw N, Bethel A, Anderson R, Thompson-Coon J. Remote monitoring for long-term physical health conditions: protocol for an evidence and gap map. OSF Registries 2022. https://doi.org/10.17605/OSF.IO/6Q7P4.
- Chandler J, Cumpston M, Thomas J, Higgins J, Deeks J, Clarke M. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated August 2022). London: Cochrane; 2022.
- Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol 2019;19. https://doi.org/10.1186/s12874-019-0855-0.
- Australian Institute of Health and Welfare . Chronic Diseases and Associated Risk Factors in Australia 2001 2002. www.aihw.gov.au/reports/chronic-disease/chronic-diseases-risk-factors-australia-2006/report-editions (accessed 26 October 2022).
- Lundell S, Holmner A, Rehn B, Nyberg A, Wadell K. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med 2015;109:11-26. https://doi.org/10.1016/j.rmed.2014.10.008.
- World Bank . Countries and Economies 2021. https://data.worldbank.org/country (accessed 26 October 2022).
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358. https://doi.org/10.1136/bmj.j4008.
- Lam AA, Lepe A, Wild SH, Jackson C. Diabetes comorbidities in low- and middle-income countries: an umbrella review. J Glob Health 2021;11. https://doi.org/10.7189/jogh.11.04040.
- Finucane AM, O’Donnell H, Lugton J, Gibson-Watt T, Swenson C, Pagliari C. Digital health interventions in palliative care: a systematic meta-review. NPJ Digit Med 2021;4. https://doi.org/10.1038/s41746-021-00430-7.
- Konnerup M, Sowden A. User Involvement in the Systematic Review Process, a Campbell Policy Brief. Philadelphia, PA: Campbell Collaboration; 2008.
- Nichols D. Coloring for Colorblindness n.d. https://davidmathlogic.com/colorblind/#%23D81B60-%231E88E5-%23FFC107-%23004D40 (accessed 27 June 2023).
- Clark A, Sousa B, Smith A. CADTH health technology review: remote monitoring programs for cardiac conditions. Can J Health Technol 2021;1. https://www.cadth.ca/remote-monitoring-programs-cardiac-conditions (accessed 17 January 2023).
- Lu JW, Wang Y, Sun Y, Zhang Q, Yan LM, Wang YX, et al. Effectiveness of telemonitoring for reducing exacerbation occurrence in COPD patients with past exacerbation history: a systematic review and meta-analysis. Front Med 2021;8. https://doi.org/10.3389/fmed.2021.720019.
- Althobiani MA, Evans RA, Alqahtani JS, Aldhahir AM, Russell A-M, Hurst JR, et al. Home monitoring of physiology and symptoms to detect interstitial lung disease exacerbations and progression: a systematic review. ERJ Open Res 2021;7:00441-2021. https://doi.org/10.1183/23120541.00441-2021.
- Office for National Statistics . People With Long-Term Health Conditions, UK: January to December 2019 2020. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/adhocs/11478peoplewithlongtermhealthconditionsukjanuarytodecember2019 (accessed 26 October 2022).
- World Health Organization 2022. www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=Cardiovascular%20diseases%20account%20for%20most,disease%20deaths%20caused%20by%20diabetes (accessed 26 October 2022).
- Wittenberg R, Hu B, Barraza-Araiza L, Rehill A. Projections of Older People with Dementia and Costs of Dementia Care in the United Kingdom, 2019–2040. London, UK: London School of Economics and Political Science; 2019.
- Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life 2019;12:113-22. https://doi.org/10.25122/jml-2018-0075.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed 26 October 2022).
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated August 2022). London: Cochrane; 2022.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10. https://doi.org/10.1186/s13643-021-01626-4.
- Smith A, Harrington E, DeJean D, MacDougall D. Remote monitoring programs for cardiac conditions. CADH Health Technology Review. Can J Health Technol 2021;1. www.cadth.ca/sites/default/files/attachments/2021-09/OP0549-RM%20for%20Cardiac%20Conditions%20Final.pdf (accessed 26 October 2022).
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDier) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC, et al. Depression–anxiety relationships with chronic physical conditions: results from the World Mental Health surveys. J Affect Disord 2007;103:113-20. https://doi.org/10.1016/j.jad.2007.01.015.
- Barken TL, Söderhamn U, Thygesen E. A sense of belonging: a meta-ethnography of the experience of patients with chronic obstructive pulmonary disease receiving care through telemedicine. J Adv Nurs 2019;75:3219-30. https://doi.org/10.1111/jan.14117.
- Wiegel J, Seppen B, van der Leeden M, van der Esch M, de Vries R, Bos W. Adherence to telemonitoring by electronic patient-reported outcome measures in patients with chronic diseases: a systematic review. Int J Environ Res Public Health 2021;18. https://doi.org/10.3390/ijerph181910161.
- Alotaibi S, Hernandez-Montfort J, Ali OE, El-Chilali K, Perez BA. Remote monitoring of implantable cardiac devices in heart failure patients: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev 2020;25:469-79. https://doi.org/10.1007/s10741-020-09923-1.
- Aronow WS, Shamliyan TA. Comparative effectiveness of disease management with information communication technology for preventing hospitalization and readmission in adults with chronic congestive heart failure. J Am Med Dir Assoc 2018;19:472-9. https://doi.org/10.1016/j.jamda.2018.03.012.
- Auener SL, Remers TEP, van Dulmen SA, Westert GP, Kool RB, Jeurissen PPT. The effect of noninvasive telemonitoring for chronic heart failure on health care utilization: systematic review. J Med Internet Res 2021;23. https://doi.org/10.2196/26744.
- Batalik L, Filakova K, Batalikova K, Dosbaba F. Remotely monitored telerehabilitation for cardiac patients: a review of the current situation. World J Clin Cases 2020;8:1818-31. https://doi.org/10.12998/wjcc.v8.i10.1818.
- Bauce K, Fahs DB, Batten J, Whittemore R. Videoconferencing for management of heart failure: an integrative review. J Gerontol Nurs 2018;44:45-52. https://doi.org/10.3928/00989134-20180207-01.
- Blok S, Linden ELV, Somsen GA, Tulevski II, Winter MM, Born B. Success factors in high-effect, low-cost eHealth programs for patients with hypertension: a systematic review and meta-analysis. Eur J Prev Cardiol 2021;28. https://doi.org/10.1177/2047487320957170.
- Cano Portillo CE. Effect of Telemonitoring on Continuous Airway Pressure Treatment Compliance in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Ann Arbor, MI: Rush University; 2018.
- Castelyn G, Laranjo L, Schreier G, Gallego B. Predictive performance and impact of algorithms in remote monitoring of chronic conditions: a systematic review and meta-analysis. Int J Med Inform 2021;156. https://doi.org/10.1016/j.ijmedinf.2021.104620.
- Chan C, Sounderajah V, Normahani P, Acharya A, Markar SR, Darzi A, et al. Wearable activity monitors in home based exercise therapy for patients with intermittent claudication: a systematic review. Eur J Vasc Endovasc Surg 2021;61:676-87. https://doi.org/10.1016/j.ejvs.2020.11.044.
- Chan CB, Popeski N, Hassanabad MF, Sigal RJ, O’Connell P, Sargious P. Use of virtual care for glycemic management in people with types 1 and 2 diabetes and diabetes in pregnancy: a rapid review. Can J Diabetes 2021;45:677-688.e2. https://doi.org/10.1016/j.jcjd.2021.02.007.
- Chen C, Wang J, Pang L, Wang Y, Ma G, Liao W. Telemonitor care helps CPAP compliance in patients with obstructive sleep apnea: a systemic review and meta-analysis of randomized controlled trials. Ther Adv Chronic Dis 2020;11. https://doi.org/10.1177/2040622320901625.
- Choi WS, Choi JH, Oh J, Shin IS, Yang JS. Effects of remote monitoring of blood pressure in management of urban hypertensive patients: a systematic review and meta-analysis. Telemed J E Health 2020;26:744-59. https://doi.org/10.1089/tmj.2019.0028.
- Choi WS, Kim NS, Kim AY, Woo HS. Nurse-coordinated blood pressure telemonitoring for urban hypertensive patients: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18. https://doi.org/10.3390/ijerph18136892.
- Cowart K, Updike W, Bullers K. Systematic review of randomized controlled trials evaluating glycemic efficacy and patient satisfaction of intermittent-scanned continuous glucose monitoring in patients with diabetes. Diabetes Technol Ther 2020;22:337-45. https://doi.org/10.1089/dia.2019.0345.
- De Ridder F, den Brinker M, De Block C. The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials. Ther Adv Endocrinol Metab 2019;10. https://doi.org/10.1177/2042018819871903.
- Drews TEI, Laukkanen J, Nieminen T. Non-invasive home telemonitoring in patients with decompensated heart failure: a systematic review and meta-analysis. ESC Heart Fail 2021;8:3696-708. https://doi.org/10.1002/ehf2.13475.
- Foong HF, Kyaw BM, Upton Z, Car LT. Facilitators and barriers of using digital technology for the management of diabetic foot ulcers: a qualitative systematic review. Int Wound J 2020;17:1266-81. https://doi.org/10.1111/iwj.13396.
- Gao W, Lv X, Xu X, Zhang Z, Yan J, Mao G, et al. Telemedicine interventions to reduce blood pressure in a chronic disease population: a meta-analysis. J Telemed Telecare 2022;28:621-31. https://doi.org/10.1177/1357633X20959581.
- Golledge J, Fernando ME, Alahakoon C, Lazzarini PA, Aan de Stegge WB, van Netten JJ, et al. Efficacy of at home monitoring of foot temperature for risk reduction of diabetes-related foot ulcer: a meta-analysis. Diabetes Metab Res Rev 2022;38. https://doi.org/10.1002/dmrr.3549.
- Hajduczok AG, Muallem SN, Nudy MS, DeWaters AL, Boehmer JP. Remote monitoring for heart failure using implantable devices: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Heart Fail Rev 2021;27:1281-300. https://doi.org/10.1007/s10741-021-10150-5.
- Halawa A, Enezate T, Flaker G. Device monitoring in heart failure management: outcomes based on a systematic review and meta-analysis. Cardiovasc Diagn Ther 2019;9:386-93. https://doi.org/10.21037/cdt.2019.01.02.
- Hong Y, Lee SH. Effectiveness of tele-monitoring by patient severity and intervention type in chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. Int J Nurs Stud 2019;92:1-15. https://doi.org/10.1016/j.ijnurstu.2018.12.006.
- Hu Y, Wen X, Wang F, Yang D, Liu S, Li P, et al. Effect of telemedicine intervention on hypoglycaemia in diabetes patients: a systematic review and meta-analysis of randomised controlled trials. J Telemed Telecare 2019;25:1357633X-8776823. https://doi.org/10.1177/1357633X18776823.
- Hu Y, Su Y, Hu S, Ma J, Zhang Z, Fang F, et al. Effects of telemedicine interventions in improving continuous positive airway pressure adherence in patients with obstructive sleep apnoea: a meta-analysis of randomised controlled trials. Sleep Breath 2021;25:1761-71. https://doi.org/10.1007/s11325-021-02292-5.
- Ikpeama B. Telehealth and Type 2 Diabetes Management. Minneapolis, MN: Walden University; 2019.
- Jang JP, Lin HT, Chen YJ, Hsieh MH, Huang YC. Role of remote monitoring in detection of atrial arrhythmia, stroke reduction, and use of anticoagulation therapy – a systematic review and meta-analysis. Circ J 2020;84:1922-30. https://doi.org/10.1253/circj.CJ-20-0633.
- Jang S, Kim Y, Cho WK. A systematic review and meta-analysis of telemonitoring interventions on severe COPD exacerbations. Int J Environ Res Public Health 2021;18. https://doi.org/10.3390/ijerph18136757.
- Janjua S, Carter D, Threapleton CJD, Prigmore S, Disler RT. Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2021. https://doi.org/10.1002/14651858.CD013196.pub2.
- Kaihara T, Intan-Goey V, Scherrenberg M, Falter M, Frederix I, Dendale P. Impact of activity trackers on secondary prevention in patients with coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol 2021;29:1047-56. https://doi.org/10.1093/eurjpc/zwab146.
- Kirakalaprathapan A, Oremus M. Efficacy of telehealth in integrated chronic disease management for older, multimorbid adults with heart failure: a systematic review. Int J Med Inform 2022;162. https://doi.org/10.1016/j.ijmedinf.2022.104756.
- Kitsiou S, Vatani H, Pare G, Gerber BS, Buchholz SW, Kansal MM, et al. Effectiveness of mobile health technology interventions for patients with heart failure: systematic review and meta-analysis. Can J Cardiol 2021;37:1248-59. https://doi.org/10.1016/j.cjca.2021.02.015.
- Kłak A, Mańczak M, Owoc J, Olszewski R. Impact of continuous glucose monitoring on improving emotional well-being among adults with type 1 diabetes mellitus: a systematic review and meta-analysis. Pol Arch Intern Med 2021;131:808-18. https://doi.org/10.20452/pamw.16047.
- Lee JA, Choi M, Lee SA, Jiang N. Effective behavioral intervention strategies using mobile health applications for chronic disease management: a systematic review. BMC Med Inform Decis Mak 2018;18. https://doi.org/10.1186/s12911-018-0591-0.
- Lelli D, Antonelli Incalzi R, Adiletta V, Pedone C. Is telemonitoring effective in older adults affected by heart failure? A meta-analysis focused on this population. J Gerontol Geriatr 2019;2019:87-95.
- Leo D, Buckley B, Chowdhury M, Harrison S, Isanejad M, Lip G, et al. Interactive remote patient monitoring devices for managing chronic health conditions: systematic review and meta-analysis. J Med Internet Res 2021;24. https://doi.org.10.2196/35508.
- Li W, Liu W, Liu S, Li J, Wang W, Li K. Perceptions of patients with chronic obstructive pulmonary disease towards telemedicine: a qualitative systematic review. Heart Lung 2021;50:675-84. https://doi.org/10.1016/j.hrtlng.2021.03.081.
- Liu K, Xie Z, Or CK. Effectiveness of mobile app-assisted self-care interventions in improving patient outcomes in type 2 diabetes and/or hypertension: a systematic review and meta-analysis of randomized controlled trials. JMIR MHealth UHealth 2020;8.
- Luo L, Ye M, Tan J, Huang Q, Qin X, Peng S, et al. Telehealth for the management of blood pressure in patients with chronic kidney disease: a systematic review. J Telemed Telecare 2019;25:1357633X-7743276. https://doi.org/10.1177/1357633X17743276.
- Ma X, Li J, Ren X. The efficacy of telemedical care for heart failure: a meta-analysis of randomized controlled trials. Am J Emerg Med 2021;47:1-5. https://doi.org/10.1016/j.ajem.2021.01.032.
- Maiorino M, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020;43:1146-56. https://doi.org/10.2337/dc19-1459.
- McFarland S, Coufopolous A, Lycett D. The effect of telehealth versus usual care for home-care patients with long-term conditions: a systematic review, meta-analysis and qualitative synthesis. J Telemed Telecare 2019;27:69-87. https://doi.org/10.1177/1357633X19862956.
- Mhanna M, Beran A, Nazir S, Al-Abdouh A, Barbarawi M, Sajdeya O, et al. Efficacy of remote physiological monitoring-guided care for chronic heart failure: an updated meta-analysis. Heart Fail Rev 2021;27:1627-37. https://doi.org/10.1007/s10741-021-10176-9.
- Morken IM, Storm M, Soreide JA, Urstad KH, Karlsen B, Lunde Husebo AM. Posthospitalization follow-up of patients with heart failure using ehealth solutions: restricted systematic review. J Med Internet Res 2022;24. https://doi.org/10.2196/32946.
- Murphie P, Little S, McKinstry B, Pinnock H. Remote consulting with telemonitoring of continuous positive airway pressure usage data for the routine review of people with obstructive sleep apnoea hypopnoea syndrome: a systematic review. J Telemed Telecare 2019;25:17-25. https://doi.org/10.1177/1357633X17735618.
- Nick JM, Roberts LR, Petersen AB. Effectiveness of telemonitoring on self-care behaviors among community-dwelling adults with heart failure: a quantitative systematic review. JBI Evid Synth 2021;19:2659-94. https://doi.org/10.11124/JBIES-20-00329.
- Health Quality Ontario . Remote monitoring of implantable cardioverter-defibrillators, cardiac resynchronization therapy and permanent pacemakers: a health technology assessment. Ont Health Technol Assess Ser 2018;18:1-199.
- Park SH, Shin JH, Park J, Choi WS. An updated meta-analysis of remote blood pressure monitoring in urban-dwelling patients with hypertension. Int J Environ Res Public Health 2021;18. https://doi.org/10.3390/ijerph182010583.
- Pekmezaris R, Tortez L, Williams M, Patel V, Makaryus A, Zeltser R, et al. Home telemonitoring in heart failure: a systematic review and meta-analysis. Health Aff (Millwood) 2018;37:1983-9. https://doi.org/10.1377/hlthaff.2018.05087.
- Salehi S, Olyaeemanesh A, Mobinizadeh M, Nasli-Esfahani E, Riazi H. Assessment of remote patient monitoring (RPM) systems for patients with type 2 diabetes: a systematic review and meta-analysis. J Diabetes Metab Disord 2020;19:115-27. https://doi.org/10.1007/s40200-019-00482-3.
- Shaw G, Whelan ME, Armitage LC, Roberts N, Farmer AJ. Are COPD self-management mobile applications effective? A systematic review and meta-analysis. NPJ Prim Care Respir Med 2020;30. https://doi.org/10.1038/s41533-020-0167-1.
- So CF, Chung J. Telehealth for diabetes self-management in primary healthcare – a systematic review and meta-analysis. J Telemed Telecare 2018;24:1357633X-7700552. https://doi.org/10.1177/1357633X17700552.
- Sul AR, Lyu DH, Park DA. Effectiveness of telemonitoring versus usual care for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2020;26:189-99. https://doi.org/10.1177/1357633X18811757.
- Tan X, Wu X, Zhang J, Song Z, Qiu Y, Chen Z, et al. The efficacy and safety of insertable cardiac monitor on atrial fibrillation detection in patients with ischemic stroke: a systematic review and meta-analysis. J Neurol 2021;269:2338-45. https://doi.org/10.1007/s00415-021-10903-0.
- Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health 2019;25:569-83. https://doi.org/10.1089/tmj.2018.0128.
- Tse G, Chan C, Gong M, Meng L, Zhang J, Su XL, et al. International Health Informatics Study (IHIS) Network . Telemonitoring and hemodynamic monitoring to reduce hospitalization rates in heart failure: a systematic review and meta-analysis of randomized controlled trials and real-world studies. J Geriatr Cardiol 2018;15:298-309. https://doi.org/10.11909/j.issn.1671-5411.2018.04.008.
- Tse G, Gong MQ, Meng L, Ng EMC, Tsang NS, Ali-Hasan-Al-Saegh S, et al. Effects of telemonitoring and hemodynamic monitoring on mortality in heart failure: a systematic review and meta-analysis. Curr Emerg Hosp Med Rep 2019;7:36-47. https://doi.org/10.1007/s40138-019-00181-6.
- Udsen FW, Hangaard S, Bender C, Andersen J, Kronborg T, Vestergaard P, et al. The effectiveness of telemedicine solutions in type 1 diabetes management: a systematic review and meta-analysis. J Diabetes Sci Technol 2022;17:782-93. https://doi.org/10.1177/19322968221076874.
- Van Opstal J, Zhao AT, Kaplan SJ, Sung AD, Schoemans H. Ehealth-generated patient data in an outpatient setting after hematopoietic stem cell transplantation: a scoping review. Transplant Cell Ther 2022;28:463-71. https://doi.org/10.1016/j.jtct.2022.05.016.
- Woo K, Dowding D. Factors affecting the acceptance of telehealth services by heart failure patients: an integrative review. Telemed J E Health 2018;24:292-300. https://doi.org/10.1089/tmj.2017.0080.
- Yun JE, Park JE, Park HY, Lee HY, Park DA. Comparative effectiveness of telemonitoring versus usual care for heart failure: a systematic review and meta-analysis. J Card Fail 2018;24:19-28. https://doi.org/10.1016/j.cardfail.2017.09.006.
- Zhu Y, Gu X, Xu C. Effectiveness of telemedicine systems for adults with heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev 2020;25:231-43. https://doi.org/10.1007/s10741-019-09801-5.
Appendix 1 Inclusion and exclusion criteria
| Category | Include | Exclude | |
|---|---|---|---|
| Effectiveness | Acceptability/implementation | ||
| Population | Adults (≥ 18 years) with a long-term physical health condition, defined as ‘a chronic disease, defined as a physical illness that is prolonged in duration, does not often resolve spontaneously, and is rarely cured completely’.37 This definition included:
|
|
|
| Study participants | Adults (≥ 18 years) with a long-term physical health condition |
|
|
| Interventions | Interventions must involve delivery of remote monitoring, defined as: ‘An intervention, involving the monitoring of a patient (using medical devices, applications, clinical investigation results, or assessment tools), including self-monitoring, and which allows care professionals from a healthcare provider to assess and manage a patient’s condition remotely, without the need for the patient to be seen face-to-face’ | Interventions not meeting the definition or described poorly enough to preclude assessment of intervention type | |
Include monitoring:
Include tele-rehabilitation unless it is obvious there is not a remote monitoring element (e.g. the intervention focuses solely on physical exercises) or the intervention is time limited (e.g. following angioplasty) Include if another component is communication with a healthcare provider (e.g. measuring blood pressure and having regular remote consultations) |
Exclude studies focusing on:
|
||
| Comparator(s)/control | Any comparator eligible for inclusion. Examples may include wait-list control or treatment as usual, but there has to be either no remote monitoring or a different level or type of remote monitoring | No exclusion | |
| Outcomes | All reported outcomes on effectiveness are of interest, including:
|
All reported outcomes on acceptability or implementation are of interest, including:
|
Exclude measurement of technical efficacy/aspects of remote monitoring devices, for example diagnostic accuracy of gait analysis using a device compared to a lab-based assessment, as these are not related to effectiveness or acceptability/implementation. Exclude monitoring of outcomes related to acute events (e.g. surgical outcomes) Exclude if only outcomes are economic/cost effectiveness |
| Literature type | Published journal articles; theses; continuing systematic review protocols | Conference abstracts or posters without full details; commentary or conceptual papers; editorials; case studies | |
| Study design | Systematic reviews that aim to evaluate the effectiveness, acceptability and/or implementation of remote monitoring interventions, and which:
Reviews that contained separate analyses or meta-analyses, where not all were relevant, were included |
|
|
| Systematic reviews including comparative outcome evaluations (randomised and non-randomised controlled trials and other study designs, e.g. controlled before-and-after trials, interrupted time series designs) | Systematic reviews including comparative outcome evaluations, other quantitative designs (e.g. single-arm trials, cohort studies, surveys) and/or qualitative studies | ||
| Date | Only systematic reviews published in 2018 or later were included in the evidence map. The publication date specified in our protocol was systematic reviews with searches conducted in 2012 or later. However, prior to screening we decided to change this to systematic reviews published in 2018 or later, to focus the map on more recent and relevant evidence and avoid unnecessary screening of a large number of records | Systematic reviews with searches conducted prior to 2012 | |
| Context | Reviews reported in English (primary studies contained in the reviews may have been reported in other languages) | Reviews not reported in English, due to study team expertise and time, and resources constraints | |
| Conducted within any high-income countries as defined by the World Bank list as published in 2022 If review includes studies from high-income and low- or middle-income countries, include if majority (75%) is high income |
Studies conducted in low- or middle- income countries | ||
| Duplicate | If the same study (using the same sample) but different publication (e.g. focus on moderating factors) include both (this is counted as one study with multiple reports) | If it is the same publication published in two sources | |
Appendix 2 Search strategy and databases
MEDLINE (Ovid; search date 24 March 2022)
Ovid MEDLINE ALL <1946 to March 22, 2022>
-
Remote Sensing Technology/ 3617
-
Telemetry/ 10077
-
Telemedicine/ 32700
-
monitor*.ti,ab. 900789
-
3 and 4 [combined with monitor* as telemedicine/ concept much broader to include remote consultations etc] 4977
-
Monitoring, ambulatory/ 8593
-
Wearable electronic devices/ 5748
-
Fitness trackers/ 986
-
((remote* or home* or digital or virtual* or telephon* or smartphone* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) adj5 monitor*).ti. 10564
-
((remote* or home* or digital or virtual* or telephon* or smartphone* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) adj2 monitor*).ab. 21761
-
((remote* or digital or home*) adj2 (sensor* or sensing or tracker or tracking)).ti,ab. 11072
-
(remote* adj2 (measurement* or supervision or surveillance)).ti,ab. 911
-
“distant patient monitoring”. ti,ab. 1
-
(biosensor* or biosensing).ti. 18621
-
((body or motion or inertia* or wearable* or worn or activity or ingestible* or implant* or insertable or patch* or location* or GPS or global positioning or acceleromet* or gyroscop* or wireless or fitness) adj2 (sensor* or sensing or tracker* or tracking)).ti,ab. 23838
-
((wearable* or sensing) adj2 (device* or system* or technolog*)).ti,ab. 18640
-
(virtual adj2 (ward* or healthcare or “health care” or hospital* or monitor*)).ti,ab. 474
-
telemonitoring.ti,ab. 1805
-
((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or electronic health*) adj8 monitor*).ti,ab. 3017
-
(assistive technolog* adj5 monitor*).ti,ab. 17
-
(smart home* adj5 monitor*).ti,ab. 74
-
(smart house* adj5 monitor*).ti,ab. 2
-
(home automation adj5 monitor*).ti,ab. 9
-
(“Internet of things” adj5 monitor*).ti,ab. 155
-
(gerontechnolog* adj5 monitor*).ti,ab. 1
-
“electronic patient reported outcome”.ti,ab. 173
-
(ePROM or ePROMs or ePRO or ePROs).ti,ab. 274
-
1 or 2 13626
-
or/5-27 108332
-
28 or 29 117401
-
(metaanalysis or meta-analysis or metasynthesis or meta-synthesis).ti,ab. 198936
-
(systematic adj (review or overview or search*)).ti,ab. 228569
-
(systematically adj (review* or search*)).ab. 30524
-
evidence synthesis.ti,ab. 5678
-
thematic synthesis.ti,ab. 1109
-
(evidence adj2 map*).ti,ab. 1170
-
((scoping or rapid or realist or mapping or umbrella) adj2 review).ti,ab. 16692
-
(qualitative adj2 synthesis).ti,ab. 3925
-
((mixed-stud* or (mixed adj stud*) or (mixed adj method*) or mixed-method*) adj2 review).ti,ab. 836
-
cochrane.jw. 15903
-
systematic reviews.jn. 2245
-
systematic review/ 189020
-
“review of reviews”.ti,ab. 711
-
or/31-43 374135
-
30 and 44 1768
-
limit 45 to yr=“2012 -Current” 1603
Embase (Ovid; search date: 24 March 2022)
Embase <1974 to 2022 March 23>
-
Remote Sensing/ [not exploded as satellite imagery is narrower term] 11917
-
Telemetry/ 19178
-
telephone telemetry/ 474
-
exp biotelemetry device/ [includes telemetric capsule, implant, electrocardiogam] 2001
-
telemonitoring/ 4378
-
exp telehealth/ 68732
-
monitor*.ti,ab. 1237541
-
6 and 7 [combined with monitor* as telehealth/ concept much broader to include remote etc] 9619
-
ambulatory monitoring/ 12001
-
exp wearable computer/ [narrower terms include smartwatch and activity tracker] 6151
-
wearable sensor/ 1070
-
((remote* or home or digital or virtual* or telephon* or smartphone* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) adj5 monitor*).ti. 14606
-
((remote* or home or digital or virtual* or telephon* or smartphone* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) adj2 monitor*).ab. 32169
-
(home* adj5 monitor*).ti. 2974
-
(home* adj2 monitor*).ab. 5612
-
((remote* or digital or home*) adj2 (sensor* or sensing or tracker* or tracking)).ti,ab. 10448
-
(remote* adj2 (measurement or supervision or surveillance)).ti,ab. 698
-
“distant patient monitoring”.ti,ab. 1
-
(biosensor* or biosensing).ti. 19868
-
((body or motion or inertia* or wearable* or worn or activity or ingestible* or implant* or insertable or patch* or location* or GPS or global positioning or acceleromet* or gyroscop* or wireless or fitness) adj2 (sensor* or sensing or tracker* or tracking)).ti,ab. 27879
-
(virtual adj2 (ward* or healthcare or “health care” or hospital* or monitor*)).ti,ab. 670
-
telemonitoring.ti,ab. 2720
-
((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or electronic health*) adj8 monitor*).ti,ab. 4680
-
(assistive technolog* adj5 monitor*).ti,ab. 22
-
(smart home* adj5 monitor*).ti,ab. 74
-
(smart house* adj5 monitor*).ti,ab. 2
-
(home automation adj5 monitor*).ti,ab. 7
-
(“Internet of things” adj5 monitor*).ti,ab. 152
-
(gerontechnolog* adj5 monitor*).ti,ab. 1
-
“electronic patient reported outcome”.ti,ab. 305
-
(ePROM or ePROMs or ePRO or ePROs).ti,ab. 720
-
or/1-5 37406
-
or/8-31 120065
-
32 or 33 142626
-
(metaanalysis or meta-analysis or metasynthesis or meta-synthesis).ti,ab. 256658
-
(systematic adj (review or overview or search*)).ti,ab. 278501
-
(systematically adj (review* or search*)).ab. 37779
-
evidence synthesis.ti,ab. 6270
-
thematic synthesis.ti,ab. 1252
-
(evidence adj2 map*).ti,ab. 1277
-
((scoping or rapid or realist or mapping or umbrella) adj2 review).ti,ab. 17807
-
(qualitative adj2 synthesis).ti,ab. 4418
-
((mixed-stud* or (mixed adj stud*) or (mixed adj method*) or mixed-method*) adj2 review).ti,ab. 890
-
cochrane.jw. 23690
-
systematic reviews.jn. 2268
-
“systematic review”/ 337681
-
exp meta-analysis/ 241798
-
“review of reviews”.ti,ab. 818
-
or/35-48 578110
-
34 and 49 2743
CINAHL Complete (EBSCO; search date 24 March 2022)
-
S45 S29 AND S43 Limiters – Published Date: from 20120101 onwards (1117)
-
S44 S29 AND S43 (1281)
-
S43 S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 (220,592)
-
S42 TI “review of reviews” OR AB “review of reviews” (343)
-
S41 (MH “Meta Analysis”) (61,283)
-
S40 (MH “Systematic Review”) OR (MH “Scoping Review”) (111,189)
-
S39 JN systematic reviews (220)
-
S38 AB (((mixed-stud*) or (mixed N0 stud*) or (mixed N0 method*) or (mixed-method*)) N2 review) OR TI (((mixed-stud*) or (mixed N0 stud*) or (mixed N0 method*) or (mixed-method*)) N2 review) (666)
-
S37 AB qualitative N2 synthesis OR TI qualitative N2 synthesis (2438)
-
S36 AB ((scoping or rapid or realist or mapping or umbrella) N2 review) OR TI ((scoping or rapid or realist or mapping or umbrella) N2 review) (9786)
-
S35 AB evidence N2 map* OR TI evidence N2 map* (590)
-
S34 AB thematic synthesis OR TI thematic synthesis (926)
-
S33 AB evidence synthesis OR TI evidence synthesis (3539)
-
S32 AB systematically N1 (review* or search*) (13,064)
-
S31 TI (systematic N1 (review or overview or search*)) OR AB (systematic N1 (review or overview or search*)) (132,543)
-
S30 TI (metaanalysis or meta-analysis or metasynthesis or meta-synthesis) OR AB (metaanalysis or meta-analysis or metasynthesis or meta-synthesis) (92,251)
-
S29 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 (35,725)
-
S28 AB (ePROM or ePROMS or ePRO or ePROs) OR TI (ePROM or ePROMS or ePRO or ePROs) (123)
-
S27 AB electronic patient reported outcome* OR TI electronic patient reported outcome* (258)
-
S26 AB (gerontechnolog*) N5 monitor* OR TI (gerontechnolog*) N5 monitor* (3)
-
S25 AB (“internet of things”) N5 monitor* OR TI (“internet of things”) N5 monitor* (32)
-
S24 AB (home automation) N5 monitor* OR TI (home automation) N5 monitor* (2)
-
S23 AB (smart house*) N5 monitor* OR TI (smart house*) N5 monitor* (1163)
-
S22 AB (smart home*) N5 monitor* OR TI (smart home*) N5 monitor* (21)
-
S21 AB (assistive technolog*) N5 monitor* OR TI (assistive technolog*) N5 monitor* (21)
-
S20 AB ((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or electronic health*) N8 monitor*) OR TI ((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or electronic health) N8 monitor*) (1355)
-
S19 TI telemonitoring OR AB telemonitoring (916)
-
S18 TI (virtual N3 (ward* or healthcare or “health care” or hospital* or monitor*)) OR AB (virtual N3 (ward* or healthcare or “health care” or hospital* or monitor*)) (690)
-
S17 TI ((wearable* or sensing) N3 (device* or system* or technolog*)) OR AB((wearable* or sensing) N3 (device* or system* or technolog*)) (2702)
-
S16 TI ((body or motion or inertia* or wearable* or worn or activity or ingestible* or insertable or implant* or patch* or location* or GPS or global positioning or acceleromet* or gyroscop* or wireless or fitness) N3 (sensor* or tracker*)) OR AB ((body or motion or inertia or wearable* or worn or activity or ingestible* or insertable* or implant* or patch* or location* or GPS or global positioning or acceleromet* or gyroscop* or wireless or fitness) N3 (sensor* or sensing or tracker* or tracking) (5528)
-
S15 TI (biosensor* or biosensing) OR AB (biosensor* or biosensing) (635)
-
S14 TI “distant patient monitoring” OR AB “distant patient monitoring” (670)
-
S13 TI (remote* N3 (measurement* or supervision or surveillance)) OR AB (remote* N3 (measurement* or supervision or surveillance)) (224)
-
S12 TI ((remote* or digital or home*) N3 (sensor* or sensing or tracker* or tracking)) OR AB ((remote* or digital or home*) N3 (sensor* or sensing or tracker* or tracking)) (898)
-
S11 AB ((remote* or home* or digital or virtual or telephon* or smartphon* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) N3 monitor*) (9984)
-
S10 TI ((remote* or home* or digital or virtual or telephon* or smartphon* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) N3 monitor*) (3860)
-
S9 S1 OR S4 OR S5 OR S6 OR S7 OR S8 (18,509)
-
S8 S2 AND S3 (2957)
-
S7 (MH “Fitness Trackers”) (284)
-
S6 (MH “Wearable Sensors+”) (6386)
-
S5 (MH “Blood Pressure Monitoring, Ambulatory”) (4018)
-
S4 (MH “Electrocardiography, Ambulatory”) (3312)
-
S3 TI monitor* OR AB monitor* (172,095)
-
S2 (MH “Telehealth+”) (31,245)
-
S1 (MH “Telemetry”) (2178)
Web of Science Core Collection (Clarivate; search date 24 March 2022)
The web of science core collection includes the following databases: Science Citation Index Expanded; Social Sciences Citation Index; Arts and Humanities Citation Index; Conference Index – Science; Conference Proceedings Citation Index – Social Science and Humanities; and the Emerging Sources Citation Index.
34 #10 AND #30 and 2022 or 2021 or 2020 or 2019 or 2018 or 2017 or 2016 or 2015 or 2014 or 2013 or 2012 (Publication Years) and Chemistry Analytical or Geosciences Multidisciplinary or Physics Applied or Ecology or Biodiversity Conservation or Water Resources or Environmental Studies or Meteorology Atmospheric Sciences or Engineering Civil or Forestry or Green Sustainable Science Technology or Geochemistry Geophysics or Electrochemistry or Construction Building Technology or Energy Fuels or Plant Sciences or Engineering Industrial or Food Science Technology or Geography or Marine Freshwater Biology or Zoology or Agronomy or Veterinary Sciences or Agriculture Dairy Animal Science or Agriculture Multidisciplinary or Polymer Science or Physics Condensed Matter or Chemistry Inorganic Nuclear or Engineering Manufacturing or Mathematics Applied or Oceanography or Regional Urban Planning or Urban Studies or Engineering Chemical or Physics Multidisciplinary or Soil Science or Astronomy Astrophysics or Chemistry Applied or Engineering Mechanical or Geology or Limnology or Materials Science Ceramics or Metallurgy Metallurgical Engineering or Mathematics or Physics Atomic Molecular Chemical or Agricultural Economics Policy or Archaeology or Architecture or Crystallography or Engineering Geological or Entomology or Fisheries or Folklore or Industrial Relations Labor or Ornithology or Paleontology or Mining Mineral Processing or Physics Fluids Plasmas or Physics Mathematical or Transportation or Transportation Science Technology (Exclude – Web of Science Categories) and Environmental Sciences (Exclude – Web of Science Categories) and Geography Physical or Materials Science Multidisciplinary or Mathematical Computational Biology (Exclude – Web of Science Categories) 1729
-
33 #10 AND #30 and 2022 or 2021 or 2020 or 2019 or 2018 or 2017 or 2016 or 2015 or 2014 or 2013 or 2012 (Publication Years) 2350
-
32 #10 AND #30 2638
-
31 #10 AND #30 2638
-
30 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 481,603
-
29 ((remote* or home* or digital or virtual* or telephon* or smartphone* or phone* or smartwatch* or “smart watch” or ambulatory or app or apps or mobile* or device* or location* or GPS or “global positioning” or acceleromet* or gyroscop* or wearable*) NEAR/5 monitor*) (Title) 26,217
-
28 ((remote* or home* or digital or virtual* or telephon* or smartphone* or phone* or smartwatch* or “smart watch” or ambulatory or app or apps or mobile* or device* or location* or GPS or “global positioning” or acceleromet* or gyroscop* or wearable*) NEAR/2 monitor*) (Abstract) 54,920
-
27 ((remote* or digital or home*) NEAR/2 (sensor* or sensing or tracker or tracking)) (Title) or ((remote* or digital or home*) NEAR/2 (sensor* or sensing or tracker or tracking)) (Abstract) 143,327
-
26 (remote* NEAR/2 (measurement* or supervision or surveillance)) (Title) or (remote* NEAR/2 (measurement* or supervision or surveillance)) (Abstract) 7137
-
25 “distant patient monitoring” (Title) or “distant patient monitoring” (Abstract) 5
-
24 biosensor* or biosensing (Title) 38,864
-
23 ((body or motion or inertia* or wearable* or worn or activity or ingestible* or implant* or insertable or patch* or location* or GPS or “global positioning” or acceleromet* or gyroscop* or wireless or fitness) NEAR/2 (sensor* or sensing or tracker* or tracking)) (Title) or ((body or motion or inertia* or wearable* or worn or activity or ingestible* or implant* or insertable or patch* or location* or GPS or “global positioning” or acceleromet* or gyroscop* or wireless or fitness) NEAR/2 (sensor* or sensing or tracker* or tracking)) (Abstract) 172,971
-
22 ((wearable* or sensing) NEAR/2 (device* or system* or technolog*)) (Title) or ((wearable* or sensing) NEAR/2 (device* or system* or technolog*)) (Abstract) 82,883
-
21 (virtual NEAR/2 (ward* or healthcare or “health care” or hospital* or monitor*)) (Title) or (virtual NEAR/2 (ward* or healthcare or “health care” or hospital* or monitor*)) (Abstract) 1,848
-
20 telemonitoring (Title) or telemonitoring (Abstract) 2478
-
19 ((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or “electronic health” or “electronic healthcare”) NEAR/8 monitor*) (Title) or ((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or “electronic health” or “electronic healthcare”) NEAR/8 monitor*) (Abstract) 5071
-
18 (assistive technolog* NEAR/5 monitor*) (Title) or (assistive technolog* NEAR/5 monitor*) (Abstract) 48
-
17 (smart home* NEAR/5 monitor*) (Title) or (smart home* NEAR/5 monitor*) (Abstract) 654
-
16 (smart house* NEAR/5 monitor*) (Title) or (smart house* NEAR/5 monitor*) (Abstract) 32
-
15 (home automation NEAR/5 monitor*) (Title) or (home automation NEAR/5 monitor*) (Abstract) 200
-
14 (“Internet of things” NEAR/5 monitor*) (Title) or (“Internet of things” NEAR/5 monitor*) (Abstract) 1880
-
13 (gerontechnolog* NEAR/5 monitor*) (Title) or (gerontechnolog* NEAR/5 monitor*) (Abstract) 1
-
12 “electronic patient reported outcome” (Title) or “electronic patient reported outcome” (Abstract) 217
-
11 ePROM or ePROMs or ePRO or ePROs (Title) or ePROM or ePROMs or ePRO or ePROs (Abstract) 958
-
10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 524,827
-
9 metaanalysis or meta-analysis or metasynthesis or meta-synthesis (Title) or metaanalysis or meta-analysis or metasynthesis or meta-synthesis (Abstract) 260,900
-
8 systematic NEAR/1 (review or overview or search*) (Title) or systematic NEAR/1 (review or overview or search*) (Abstract) 303,005
-
7 systematically NEAR/1 (review* or search*) (Abstract) 36,013
-
6 TI = (evidence synthesis or “review of reviews”) OR AB = (evidence synthesis or “review of reviews”) 70,987
-
5 thematic synthesis (Title) or thematic synthesis (Abstract) 2610
-
4 evidence NEAR/2 map* (Title) or evidence NEAR/2 map* (Abstract) 2847
-
3 TI=(((scoping or rapid or realist or mapping or umbrella) NEAR/2 review)) OR AB = (((scoping or rapid or realist or mapping or umbrella) NEAR/2 review)) 24,788
-
2 (qualitative NEAR/2 synthesis) (Title) or (qualitative NEAR/2 synthesis) (Abstract) 4813
-
1 ((mixed-stud* or mixed-method*) NEAR/2 review) (Title) or ((mixed-stud* or mixed-method*) NEAR/2 review) (Abstract) 1183
Scopus (Elsevier; search date 30 March 2022)
Restricted to: medicine, computer science, engineering, biochem, health professions, social sciences, psychology, pharmacy, immunology, dentistry
(((TITLE-ABS((telecare OR telemedicine OR telemetry OR telehealth* OR m-health* OR mhealth* OR e-health* OR ehealth* OR “electronic health*”) W/8 monitor*))) and (((TITLE-ABS({review of reviews}))) or ((TITLE-ABS((mixed-stud* OR (mixed W/1 stud*) OR (mixed W/1 method*) OR mixed-method*) W/2 review))) or ((TITLE-ABS(qualitative W/2 synthesis))) or ((TITLE-ABS(evidence W/2 map*))) or ((TITLE-ABS({thematic synthesis}))) or ((TITLE-ABS({evidence synthesis}))) or ((ABS(systematically W/1 (review* OR search*)))) or ((TITLE-ABS(systematic W/1 (review OR overview OR search*)))) or ((TITLE({metaanalysis} OR {meta-analysis} OR {metasynthesis} OR {meta-synthesis}))))) or ((((TITLE(virtual W/2 (monitor*)))) or ((TITLE-ABS(virtual W/2 (“health care”)))) or ((TITLE-ABS(virtual W/2 (healthcare)))) or ((TITLE-ABS(virtual W/2 (ward*)))) or ((TITLE-ABS(virtual W/2 (ward* OR healthcare OR “health care” OR hospital* OR monitor*)))) or ((TITLE-ABS({telemonitoring}))) or ((TITLE-ABS(“assistive technolog*” W/5 monitor*))) or ((TITLE-ABS(“smart home*” W/5 monitor*))) or ((TITLE-ABS(“smart house*” W/5 monitor*))) or ((TITLE-ABS(“home automation” W/5 monitor*))) or ((TITLE-ABS(“Internet of things” W/5 monitor*))) or ((TITLE-ABS(gerontechnolog* W/5 monitor*))) or ((TITLE-ABS({electronic patient reported outcome}))) or ((TITLE-ABS({ePROM} OR {ePROMs} OR {ePRO} OR {ePROs})))) and (((TITLE-ABS({review of reviews}))) or ((TITLE-ABS((mixed-stud* OR (mixed W/1 stud*) OR (mixed W/1 method*) OR mixed-method*) W/2 review))) or ((TITLE-ABS(qualitative W/2 synthesis))) or ((TITLE-ABS(evidence W/2 map*))) or ((TITLE-ABS({thematic synthesis}))) or ((TITLE-ABS({evidence synthesis}))) or ((ABS(systematically W/1 (review* OR search*)))) or ((TITLE-ABS(systematic W/1 (review OR overview OR search*)))) or ((TITLE({metaanalysis} OR {meta-analysis} OR {metasynthesis} OR {meta-synthesis}))))) or ((((TITLE-ABS({review of reviews}))) or ((TITLE-ABS((mixed-stud* OR (mixed W/1 stud*) OR (mixed W/1 method*) OR mixed-method*) W/2 review))) or ((TITLE-ABS(qualitative W/2 synthesis))) or ((TITLE-ABS(evidence W/2 map*))) or ((TITLE-ABS({thematic synthesis}))) or ((TITLE-ABS({evidence synthesis}))) or ((ABS(systematically W/1 (review* OR search*)))) or ((TITLE-ABS(systematic W/1 (review OR overview OR search*)))) or ((TITLE({metaanalysis} OR {meta-analysis} OR {metasynthesis} OR {meta-synthesis})))) and ((TITLE((remote* OR home* OR digital OR virtual* OR telephon* OR smartphone* OR phone* OR smartwatch* OR “smart watch*” OR ambulatory OR app OR apps OR mobile* OR device* OR location* OR GPS OR “global positioning” OR acceleromet* OR gyroscop* OR wearable*) W/5 (monitor*))))) or ((((TITLE-ABS({review of reviews}))) or ((TITLE-ABS((mixed-stud* OR (mixed W/1 stud*) OR (mixed W/1 method*) OR mixed-method*) W/2 review))) or ((TITLE-ABS(qualitative W/2 synthesis))) or ((TITLE-ABS(evidence W/2 map*))) or ((TITLE-ABS({thematic synthesis}))) or ((TITLE-ABS({evidence synthesis}))) or ((ABS(systematically W/1 (review* OR search*)))) or ((TITLE-ABS(systematic W/1 (review OR overview OR search*)))) or ((TITLE({metaanalysis} OR {meta-analysis} OR {metasynthesis} OR {meta-synthesis})))) and ((ABS((home* OR digital OR virtual* OR telephon* OR smartphone* OR phone* OR smartwatch* OR “smart watch*” OR ambulatory OR app OR apps OR mobile* OR location* OR GPS OR “global positioning” OR acceleromet* OR gyroscop* OR wearable*) W/2 (monitor*))))) or ((((TITLE-ABS({review of reviews}))) or ((TITLE-ABS((mixed-stud* OR (mixed W/1 stud*) OR (mixed W/1 method*) OR mixed-method*) W/2 review))) or ((TITLE-ABS(qualitative W/2 synthesis))) or ((TITLE-ABS(evidence W/2 map*))) or ((TITLE-ABS({thematic synthesis}))) or ((TITLE-ABS({evidence synthesis}))) or ((ABS(systematically W/1 (review* OR search*)))) or ((TITLE-ABS(systematic W/1 (review OR overview OR search*)))) or ((TITLE({metaanalysis} OR {meta-analysis} OR {metasynthesis} OR {meta-synthesis})))) and ((TITLE-ABS((remote* OR digital OR home*) W/2 (sensor* OR sensing OR tracker OR tracking))))) or ((((TITLE-ABS({review of reviews}))) or ((TITLE-ABS((mixed-stud* OR (mixed W/1 stud*) OR (mixed W/1 method*) OR mixed-method*) W/2 review))) or ((TITLE-ABS(qualitative W/2 synthesis))) or ((TITLE-ABS(evidence W/2 map*))) or ((TITLE-ABS({thematic synthesis}))) or ((TITLE-ABS({evidence synthesis}))) or ((ABS(systematically W/1 (review* OR search*)))) or ((TITLE-ABS(systematic W/1 (review OR overview OR search*)))) or ((TITLE({metaanalysis} OR {meta-analysis} OR {metasynthesis} OR {meta-synthesis})))) and ((TITLE-ABS((body OR motion OR inertia* OR wearable* OR worn OR activity OR ingestible* OR implant* OR insertable OR patch* OR location* OR GPS OR “global positioning” OR acceleromet* OR gyroscop* OR wireless OR fitness) W/2 (sensor* OR sensing OR tracker* OR tracking))))) AND (LIMIT-TO (PUBYEAR,2022) OR LIMIT-TO (PUBYEAR,2021) OR LIMIT-TO (PUBYEAR,2020) OR LIMIT-TO (PUBYEAR,2019) OR LIMIT-TO (PUBYEAR,2018) OR LIMIT-TO (PUBYEAR,2017) OR LIMIT-TO (PUBYEAR,2016) OR LIMIT-TO (PUBYEAR,2015) OR LIMIT-TO (PUBYEAR,2014) OR LIMIT-TO (PUBYEAR,2013) OR LIMIT-TO (PUBYEAR,2012)) AND (LIMIT-TO (SUBJAREA,”MEDI”) OR LIMIT-TO (SUBJAREA,”COMP”) OR LIMIT-TO (SUBJAREA,”ENGI”) OR LIMIT-TO (SUBJAREA,”BIOC”) OR LIMIT-TO (SUBJAREA,”HEAL”) OR LIMIT-TO (SUBJAREA,”SOCI”) OR LIMIT-TO (SUBJAREA,”PSYC”) OR LIMIT-TO (SUBJAREA,”PHAR”) OR LIMIT-TO (SUBJAREA,”IMMU”) OR LIMIT-TO (SUBJAREA,”DENT”))
Cochrane Database of Systematic Reviews (Wiley; search date 28 March 2022)
Custom date range: from 01/01/2012 onwards
-
#1 MeSH descriptor: [Remote Sensing Technology] explode all trees
-
#2 MeSH descriptor: [Telemetry] explode all trees
-
#3 MeSH descriptor: [Telemedicine] explode all trees
-
#4 (monitor*):ti,ab,kw
-
#5 #3 AND #4
-
#6 MeSH descriptor: [Monitoring, Ambulatory] explode all trees
-
#7 MeSH descriptor: [Wearable Electronic Devices] explode all trees
-
#8 MeSH descriptor: [Fitness Trackers] explode all trees
-
#9 ((remote* or home* or digital or virtual or telephon* or smartphon* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) near/2 monitor*):ti,ab,kw
-
#10 ((remote or digital or home*) near/2 (sensor* or sensing or tracker* or tracking)):ti,ab,kw
-
#11 (remote* near/2 (measurement* or supervision or surveillance)):ti,ab,kw
-
#12 (“distant patient monitoring”):ti,ab,kw
-
#13 (biosensor* or biosensing):ti
-
#14 ((body or motion or inertia* or wearable* or worn or activity or ingestible* or insertable or implant* or patch* or location* or GPS or global positioning or gyroscop* or wireless or fitness) near/2 (sensor* or sensing or tracker* or tracking)):ti,ab,kw
-
#15 ((wearable* or sensing) near/2 (device* or system* or technolog*)):ti,ab,kw
-
#16 (virtual near/2 (ward* or healthcare or “health care” or hospital* or monitor*)):ti,ab
-
#17 telemonitoring:ti,ab,kw
-
#18 ((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or electronic health*) near/8 monitor*):ti,ab,kw
-
#19 ((assistive technolog*) near/5 monitor*):ti,ab,kw
-
#20 ((smart home*) near/5 monitor*):ti,ab,kw
-
#21 ((smart house*) near/5 monitor*):ti,ab,kw
-
#22 ((“home automation”) near/5 monitor*):ti,ab,kw
-
#23 ((“internet of things”) near/5 monitor*):ti,ab,kw
-
#24 (gerontechnolog* near/5 monitor*):ti,ab,kw
-
#25 “electronic patient reported outcome”:ti,ab,kw
-
#26 (ePROM or ePROMs or ePRO or ePROs):ti,ab,kw
-
#27 #1 or #2 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 (113, limited to 1/1/2012: 97)
PROSPERO (Centre for Reviews and Dissemination, University of York; search date 28 March 2022)
-
#1 MeSH DESCRIPTOR Remote Sensing Technology EXPLODE ALL TREES 1
-
#2 MeSH DESCRIPTOR Telemetry EXPLODE ALL TREES 4
-
#3 MeSH DESCRIPTOR Telemedicine EXPLODE ALL TREES 724
-
#4 monitor* 7031
-
#5 #3 AND #4 199
-
#6 MeSH DESCRIPTOR Monitoring, Ambulatory EXPLODE ALL TREES 59
-
#7 MeSH DESCRIPTOR Wearable Electronic Devices EXPLODE ALL TREES 105
-
#8 MeSH DESCRIPTOR Fitness Trackers EXPLODE ALL TREES 28
-
#9 (((remote* or home* or digital or virtual* or telephon* or smartphon* or phone* or smartwatch* or smart watch* or ambulatory or app or apps or mobile* or device* or location* or GPS or global positioning or acceleromet* or gyroscop* or wearable*) AND monitor*)):TI 132
-
#10 (((remote* or digital or home*) AND (sensor* or sensing or tracker* or tracking))):TI 11
-
#11 (((remote*) AND (measurement* or supervision or surveillance))):TI 7
-
#12 (“distant patient monitoring”):TI 0
-
#13 “distant patient monitoring” 0
-
#14 (biosensor or biosensing):TI 2
-
#15 (((body or motion or inertia* or wearable* or worn or activity or ingestible* or implant* or insertable or patch* or location* or GPS or global positioning or acceleromet* or gyroscop* or wireless or fitness) AND (sensor* or sensing or tracker* or tracking))):TI 84
-
#16 (((wearable* or sensing) AND (device* or system* or technolog*))):TI 144
-
#17 ((virtual AND (ward* or healthcare or health care or hospital* or monitor*))):TI 18
-
#18 telemonitoring 163
-
#21 ((telecare or telemedicine or telemetry or telehealth* or m-health* or mhealth* or e-health* or ehealth* or electronic health*) AND monitor*):TI 8
-
#22 “assistive technology” AND monitor* 23
-
#23 smart home* AND monitor* 10
-
#24 smart house* AND monitor* 1
-
#27 home automation AND monitor* 1
-
#30 “internet of things” AND monitor* 10
-
#33 gerontechnolog* AND monitor* 4
-
#36 “electronic patient reported outcome” 11
-
#39 eprom or eproms or epro or epros 14
-
#42 #1 OR #2 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #21 OR #22 OR #23 OR #24 OR #27 OR #30 OR #33 OR #36 OR #39 753
OT Seeker (search date 30 March 2022)
Total records: 49
-
Any Field: remote* AND Method: Systematic Review: 12
-
Any Field: wearable* AND Method: Systematic Review: 3
-
Any Field: telemonitoring AND Method: Systematic Review: 4
-
Any Field: telemetry AND Method: Systematic Review 1
-
Any Field: telecare AND Method: Systematic Review: 11
-
Any Field: telemedicine AND monitor* AND Method: Systematic Review: 5
-
Any Field: telehealth* AND monitor* AND Method: Systematic Review 0
-
Any Field: ehealth* AND monitor* AND Method: Systematic review 2
-
Any Field: e-health* AND monitor* AND Method: Systematic Review 3
-
Any Field: mhealth* AND monitor* AND Method: Systematic Review 0
-
Any Field: m-health* AND monitor* AND Method: Systematic Review 6
-
Any Field: virtual AND monitor* AND Method: Systematic Review 2
-
Any Field: virtual AND ward* AND Method: Systematic Review 0
-
Any Field: biosensor* AND Method: Systematic Review 0
PEDro (search date 28 March 2022)
Total: 125 records (not deduplicated)
-
Title/Abstract: remote* AND Method: Systematic Review 47
-
Title/Abstract: wearable* AND Method: Systematic Review: 35
-
Title/Abstract: telemonitoring AND Method: Systematic Review: 12
-
Title/Abstract: telemetry AND Method: Systematic Review 2
-
Title/Abstract: telecare AND Method: Systematic Review 0
-
Title/Abstract: telemedicine monitor* AND Method: Systematic Review 4
-
Title/Abstract: telehealth* monitor* AND Method: Systematic Review 4
-
Title/Abstract: ehealth* monitor* AND Method: Systematic review 7
-
Title/Abstract: e-health* monitor* AND Method: Systematic Review 3
-
Title/Abstract: mhealth* monitor* AND Method: Systematic Review 4
-
Title/Abstract: m-health* monitor* AND Method: Systematic Review 2
-
Title/Abstract: virtual monitor* AND Method: Systematic Review 5
-
Title/Abstract: virtual ward* AND Method: Systematic Review 0
-
Title/Abstract: biosensor* AND Method: Systematic Review 0
ProQuest Dissertations and Theses Global (search date 31 March 2022)
(title(qualitative NEAR/2 synthesis) OR abstract(qualitative NEAR/2 synthesis) OR abstract((mixed-stud* or mixed-method*) NEAR/2 (review)) OR title((mixed-stud* or mixed-method*) NEAR/2 (review)) OR title((scoping or rapid or realist or mapping or umbrella) NEAR/2 (review)) OR abstract((scoping or rapid or realist or mapping or umbrella) NEAR/2 (review)) OR (abstract(evidence NEAR/2 map*) OR title(evidence NEAR/2 map*)) OR (abstract(thematic synthesis) OR title(thematic synthesis)) OR (abstract(evidence synthesis or “review of reviews”) OR title(evidence synthesis or “review of reviews”)) OR abstract((systematically) NEAR/1 (review* or search)) OR abstract((systematic) NEAR/1 (review or overview or search*)) OR title((systematic) NEAR/1 (review or overview or search*)) OR (title(metaanalysis or meta-analysis or metasynthesis or meta-synthesis) OR abstract(metaanalysis or meta-analysis or metasynthesis or meta-synthesis))) AND ((title(remote* OR home* OR digital OR virtual* OR telephon* OR smartphone* OR phone* OR smartwatch* OR “smart watch” OR ambulatory OR app OR apps OR mobile* OR device* OR location* OR GPS OR “global positioning” OR acceleromet* OR gyroscop* OR wearable*) AND (title(monitor* OR sensor* OR sensing OR tracker OR tracking) OR abstract(monitor* OR sensor* OR sensing OR tracker OR tracking))) OR (title(measurement* OR supervision OR surveillance) AND title(remote*)) OR title(sensor* OR sensing OR tracker* OR tracking) OR title(wearable*) OR (abstract(wearable*) AND abstract(device* OR system* OR technolog*)) OR (title(telecare OR telemedicine OR telemetry OR telehealth* OR m-health* OR mhealth* OR e-health* OR ehealth* OR “electronic health” OR “electronic healthcare”) AND title(monitor*)) OR (abstract(telecare OR telemedicine OR telemetry OR telehealth* OR m-health* OR mhealth* OR e-health* OR ehealth* OR “electronic health” OR “electronic healthcare”) AND abstract(monitor*)))
Epistemonikos (search date 30 March 2022)
| Advanced search | All results | Systematic reviews | Broad synthesis |
|---|---|---|---|
| Title/abstract: (remote*) and (monitor* or track* or sens*) | 2850 | 395 | 39 |
| wearable AND title/abstract (monitor* or track* or sens*) | 362 | 165 | 8 |
| Title/abstract: telemonit* | 394 | 87 | 6 |
| Title: Telemetry | 47 | 6 | 0 |
| Title: telecare | 112 | 15 | 0 |
| Title: Telemed* | 2640 | 353 | 55 |
| Title: Telehealth* | 1708 | 284 | 46 |
| Title: ehealth* | 385 | 201 | 25 |
| Title: mhealth | 406 | 183 | 16 |
| Title: “mobile health” | 474 | 199 | 13 |
| Title: m-health | 38 | 13 | 0 |
| Title: Virtual and (monitor* or track* or sens*) | 40 | 6 | 0 |
| Title: Virtual AND (ward* or clinic*) | 319 | 36 | 2 |
| Biosens* | 335 | 18 | 0 |
Total: 2171
Duplicates and pre-2012 removed: 376
Copied across: 1795
Google Scholar (search date: 30 March 2022)
-
Searched via Publish or Perish (Harzing)
-
Google Scholar (all in title) using Publish or Perish
-
Remote monitoring and systematic review = 46
-
Wearable and systematic = 164
-
Mobile and health and systematic = 235
-
De-duped in separate library
-
383 records copied to main EndNote library
Appendix 3 List of continuing systematic reviews
Continuing reviews have been categorised according the patient population of focus, using the categories defined in the EGM (reviews with multiple patient populations can be found under ‘Not clearly defined/reported’).
Cardiovascular disease (36)
-
Al-Abdouh A, Mahmoud B, Jabri A. Efficacy of implanted device telemonitoring in patients with heart failure: a meta-analysis of randomized controlled trials. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=238122
-
Azmi Nabila KA, Noor MI, Wibowo RA, Sofro ZM. Evaluating the effectiveness of telemonitoring in primary hypertension management: a systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=268119
-
Brahmbhatt D, Cowie M, Gallagher A. Facilitators and barriers to effective remote monitoring of heart failure patients using cardiovascular implantable electronic devices. PROSPERO 2018. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=100043
-
Calderon EHC. Effect of remote monitoring of implantable cardiac pacemakers. A systematic review and meta-analysis of randomized controlled trials. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=203615
-
Cheong A, Xu F, Wang S. Outcomes in patients with CIEDs followed up via remote monitoring: a systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=277010
-
de Barros K, Martins MAP, Praxedes M, Ribeiro ALP. Effectiveness and usability of mobile health applications for medication adherence in patients with heart failure: a systematic review protocol. JBI Evid Synth 2021;19:2777–82. https://doi.org/10.11124/JBIES-20-00399
-
Fatrin S, Auliani S, Pratama S, Margaret SP, Brunner TM, Siswanto BB. Outcome of telemedicine in heart failure patients. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=271540
-
Hwang M, Aekyung Chang A. The effect of nurse-led digital health intervention for patient with hypertension: a systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=2
-
Igai Y, Negishi Y, Kato E, Ishikawa K, Harada T, Kamei T. Effectiveness of telemonitoring by healthcare providers on health outcomes for patients with chronic heart failure: a systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=237639
-
Jin K, Hafiz N. Evidences on the cardiovascular benefits in the use of wearable devices in adults with cardiovascular disease? PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=162045
-
Kelly S, Wells G. Qualitative synthesis of patient- and healthcare provider-reported barriers to virtual follow-up and care models for patients with cardiovascular implantable electronic devices. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=160533
-
Koo K, Ferguson C, Liang-Han L, Cleland J, Inglis S. Implantable device monitoring versus usual care for managing individuals with heart failure [Cochrane protocol]. PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=148354
-
Lee WL, Syazwani N, Zulfazli I, Suhaimi RA. A systematic review protocol on the use and the effectiveness of wearable electronic activity tracking system (EATs) for patients with coronary heart disease undergoing cardiac rehabilitation program. PROSPERO 2018. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=106366
-
Maximidou T, Mons U. Impact of wearable activity trackers on the prognosis of coronary artery disease – a systematic review and meta-analysis of randomized controlled trials. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=252651
-
McGee M, Ray M, Sverdlov A. Benefits of remote monitoring in patients with cardiac implantable electronic devices who have heart failure: systematic review. PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=129270
-
Mikulski B, De Marchi A. Effects of using mHealth apps on medication adherence in patients with arterial hypertension. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=205973
-
Moura Dantas de Lima T, da Silva de Lima e Silva EH, Santos da Costa MG. Systematic review of remote monitoring in patients with implantable cardioverter defibrillator (ICD) with ventricular arrhythmia. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=242864
-
Nagy KV, Hernandez-Montfort J, Al-Hussaini A, Stafylas P. Contemporary non-invasive remote monitoring longitudinal impact in adults with chronic systolic heart failure related hospital admissions: systematic review and meta-analysis. PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=145815
-
Ogbu I, Dota A. Remote pulmonary artery hemodynamic monitoring in chronic heart failure: Systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=261416
-
Patel H, King-Shier K, Hayden A. A systematic review of wearable monitoring technology for heart failure management. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=209743
-
Pei X. The effectiveness of telemedicine interventions in high blood pressure monitoring: a systematic review and meta-analysis. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=211461
-
Rebolledo Del Toro M, Muñoz Velandia OM, García Peña AA, Fernández Ávila DG, Barahona Correa JE, Herrera Leaño NM. Effectiveness of mobile telemonitoring applications in heart failure patients. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=299516
-
Reis L, Mesquita E, Carraro A, Périssé L, Neto N, Rodrigues T, et al. Telemedicine in heart failure during the pandemic: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=224057
-
Scholte N, Gürgöze M, Aydin D, van der Boon R, Brugts J, Boersma E. Effects of non-invasive and invasive telemonitoring on heart failure outcomes: a state-of-the-art systematic review and meta-analysis. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=306677
-
Srigati SA, Aliyah SF, Aryasatya DWB. The impact of telemedicine for heart failure management during COVID-19 pandemic: a systematic review of cohort studies. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=306241
-
Somberg C, Eastland T, Allen J, Schooley A. Effect of nurse-led telehealth on rehospitalization and quality of life among community-dwelling adults with heart failure: a quantitative systematic review protocol. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=313122
-
Thanigaimani S, Golledge J. Systematic review of sensors and wearables to improve walking performance in peripheral artery disease. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=308138
-
Tourais Matos Sousa JM, Moreira E, Sousa Pinto BS, Viana Pinto J, Pinto R, Azevedo LF, et al. Effectiveness of non-invasive home telemonitoring in outpatient care for patients with heart failure: a systematic review and meta-analysis. PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=146396
-
Tourais Matos Sousa JM, Silva Cardoso JS, Azevedo LF, Moreira RPE. The effectiveness of non-invasive home telemonitoring in outpatient care for patients with heart failure: a systematic review. PROSPERO 2018. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=88522
-
Veres B, Schwertner W, Kiss B, Engh M, Kosztin A, Merkely B. Continuous invasive remote monitoring in patients with heart failure compared to regular in-clinic follow-up: A systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=299820
-
Warraich H, Maqsood MH. Telemonitoring in heart failure patients: an updated systematic review and meta-analysis. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=184381
-
Wireklint Sundstroem B, Josephsson H, Olofsson S. Patient participation in self-monitoring in case of heart failure and home-based care, by means of patient experiences: a integrative systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=244252
-
Wu Y, Zhao P, Li W, Cao MQ, Du L, Chen JC. The effect of remote health intervention based on internet or mobile communication network on hypertension patients: protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98:e14707. https://doi.org/10.1097/MD.0000000000014707
-
Yong J. Effects of wearable devices in adults with cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=186489
-
Zhiqiang Wang Z, Jin X, Tang Z, Kang Y. The practical effect of remote cardiac rehabilitation technology on patients with heart disease. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=271283
-
Zito A, Princi G, Romiti GF. Remote monitoring strategies for guided management of patients with heart failure: a systematic review and meta-analysis. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=308167
Neurological conditions (8)
-
de Barros Gonze B, di Paschoale Ostolin TLV. Effectiveness of telehealth oriented to the attention and care of adults with neurological diseases before and during the COVID-19 pandemic: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=250334
-
Gebrye T, Fatoye F, Anazodo C. Effect of tele-rehabilitation on quality of life in stroke patients: a systematic review and meta analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=295888
-
Harris P. Factors influencing physical activity sensor use for self-management in stroke patients and older at-risk adults – a systematic review and thematic synthesis. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=211472
-
Quel de Oliveira C, Scianni A, Vehagen A. Telerehabilitation to improve functional outcomes in individuals with neurological conditions. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=160327
-
Seri E, Bilotta F. Technology-assisted clinical management of brain injured patients after hospital discharge. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=255515
-
Wade R, Simmonds M, Meader N, Fulbright H. Devices for remote continuous monitoring of people with Parkinson's disease: a systematic review and economic analysis. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=308597
-
Wilchesky M, Guseva E, Iaboni A, Hermann N, Kumar S, Seitz D, et al. Wearable sensor technology for assessment and monitoring of neuropsychiatric symptoms of dementia. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=219917
-
Yan Y, Chan ML, Kwok JYY, Jung Jae Lee J. The effect of mobile health interventions on hyperlipidaemia control among stroke patients: a systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=281946
Diabetes (10)
-
Adams D, Zheng H, Sinclair M, Murphy M, McCullough J. Wearable technologies in type one diabetes pregnancy: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=261671
-
Alvarez SD, Sculley D, Santos D, Acharya SH, Garcia XG, Wynne K-J, Coda A. The role of smartwatch technology in the provision of care for type 1 or type 2 diabetes mellitus or gestational diabetes: a systematic review. PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=136825
-
Colley J, Dambha-Miller H, Stuart B, Bartholomew J, Price H. Home monitoring of HbA1c in diabetes mellitus: A protocol for systematic review and narrative synthesis on reliability, accuracy, and patient acceptability. medRxiv 2021. https://dx.doi.org/10.1101/2021.12.15.21267851
-
Colley J, Price H, Dambha-Miller H, Stuart B, Bartholomew J. Home monitoring of HbA1c in diabetes mellitus: a systematic review and narrative synthesis on reliability, accuracy and patient acceptability. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=225606
-
Endo M, Yamamoto Y, Kamei T. Effect of telehome-monitoring-based telenursing in people with type 2 diabetes: A systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=273579
-
Jones P, Webb D, Davies M, Khunti K, McCarthy M. Attitudes to wearable technology to prevent foot ulceration in people with diabetes and neuropathy: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=164449
-
Luz S, Henriques H, Ferraz I, Lapão L, Guerreiro M, Emilia M, et al. The effects of telehealth on the health literacy of people with type 2 diabetes: a systematic review. PROSPERO 2018. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=94910
-
Siddiqui S, Gillies C, Gray L. The effectiveness of telemedicine interventions for glycaemic control in patients with Type 2 diabetes: A systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=255164
-
Sun J, Cai J, Jiang S, Xu H, Broadley S. Effectiveness of telemonitoring intervention using resilience theory based patient self care approach on glycaemic outcomes in patients with type 2 diabetes mellitus. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=265979
-
Yong J. Effect of wearable devices on diabetes in adults: a systematic review and meta-analysis of RCT. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=152297
Respiratory conditions (13)
-
Alghamdi SM, Janaudis-Ferreira T, Alhasani R, Ahmed S. Acceptance, adherence and dropout rates of individuals with COPD approached in telehealth interventions: a protocol for systematic review and meta-analysis. BMJ Open 2019;9:e026794. http://dx.doi.org/10.1136/bmjopen-2018-026794
-
Anand R, McLeese R, Stewart J, Busby J, Clarke M, Bradley J. Unsupervised remote spirometry vs supervised clinic spirometry: a protocol for a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=272816
-
Gaveikaite V, Fischer C, Schonenberg H, Pauws S, Kitsiou S, Chouvarda I, et al. Telehealth for patients with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis protocol. BMJ Open 2018;8:e021865. http://dx.doi.org/10.1136/bmjopen-2018-021865
-
Gaveikaite V, Fisher C, Schonenberg H, Kitsiou S, Chouvarda I, Pauws S, et al. Systematic review and meta-analysis of telehealth for COPD patients. PROSPERO 2018. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=8367
-
Harris K, Grigg J. The use of electronic monitoring devices in adherence with asthma medications, and their impact on patient outcomes: a systematic review. PROSPERO 2019. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=127361
-
Igai Y, Otomo S, Minami K, Kamei T. Effectiveness of telemonitoring by healthcare providers on health outcomes for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=236505
-
Isernia S, Pagliari C, Baglio F, Banfi P, Rossetto F, Borgnis F. Telerehabilitation for people with chronic obstructive pulmonary disease. A systematic review and meta-analysis. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=277381
-
Valenza MC. eHealth assessment tools in COPD patients: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=213189
-
Martin-Valero R, Ortíz-Ortigosa L, Viñolo-Gil MJ. Telerehabilitation and telemonitoring interventions programs used to improving quality of life in people with cystic fibrosis: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=257647
-
Sarasmita MA, Lo A, Yin Chen HY. Effectiveness of mobile health-based self-management interventions to reduce exacerbation and improve health-related quality of life in patients with COPD: a systematic review and meta-analysis. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=181157
-
Shah A, Althobiani M, Saigal A, Hurst J, Mandal S. Home wearable technology in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=299706
-
Shehraj S. The feasibility and effectiveness of digital technology for monitoring cough in chronic respiratory illness: a systematic review. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=306474
-
Ward C, Ha J, Lewis A, Conway J, Parrott H. The remote monitoring of inhaler adherence and technique in asthma: a systematic review. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=299468
Cancer survivors (0)
Kidney disease (3)
-
Ali H, Hamer R. Effect of remote patient monitoring among peritoneal dialysis population on clinical outcomes. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=277329
-
Berg RC, Nygaard H, Nguyen L. Effect of remote patient monitoring for patients with chronic kidney disease who perform dialysis at home: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=281779
-
Okpechi IG, Muneer S, Tinwala MM, Zaidi D, Hamonic LN, Braam B, et al. Impact of home telemonitoring and management support on blood pressure control in non-dialysis CKD: a systematic review protocol. BMJ Open 2021;11:e044195. http://dx.doi.org/10.1136/bmjopen-2020-044195
Others (5)
-
Arumalla N, Patel S, Gibson M, Norton S, Galloway J, Garrood T. The clinical impact of electronic patient-reported outcome measures in the remote monitoring of inflammatory arthritis: a systematic review. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=312762
-
Beeren F, van der Lecq Pepijn B, Reinier van Linschoten T, Meertens-Gunput S, West R, Römkens T. The effect of telemonitoring on disease control and quality of life in inflammatory bowel disease patients: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=255487
-
Nigam GB, John Kuzhiyanjal AJ, Antoniou GA, Limdi JK. Role of telemedicine in the management of inflammatory bowel disease: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=192428
-
Saengpromma P, Jirasakulsuk N. The effectiveness of home-based exercise with tracking and home-based exercise alone for patients with knee osteoarthritis. PROSPERO 2022. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=302699
-
Saunders A, Hill L, Popov J, Kalantar M, Farbod Y, Tewari P, et al. The role of telemedicine in the management of inflammatory bowel disease: a systematic review protocol. Minerva Gastroenterol. 2021. https://dx.doi.org/10.23736/S2724-5985.21.02939-9
Not clearly defined/reported (11)
-
Dermody G, Glass C, Dunham M, Fritz R. The factors affecting clinician readiness to adopt smart home technology for remote health monitoring: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=195989
-
Facchinetti G, Petrucci G, Piredda M, Matarese M, Grazia De Marinis M. The impact of smart home technology on chronic diseases in older people: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=137480
-
Gonçalves RL, Pagano AS, Reis Z, Afagbedzi SK, Head M, Kenny Brackstone K, et al. Telehealth usability evaluation by healthcare professionals in post-pandemic non-communicable disease (hypertension and diabetes) treatment: a systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=296887
-
Harrison C, Porter I, Valderas J, Sidey-Gibbons C. Remote monitoring using patient reported outcomes: the effect on survival. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=178753
-
Mattison G, Forrester D, Sullivan C, Dobbins C, Toyras J. The influence of wearable technology on healthcare outcomes in chronic disease – A systematic review. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=244562
-
Muller AE, Berg RC. A flexible protocol for a systematic review of remote patient monitoring. Prim Health Care Res Dev 2020;21:e45. http://dx.doi.org/10.1017/S1463423620000262
-
Prats Vila A, Chabrera C. The use of mHealth for the monitoring and control of patients with chronic diseases: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=198461
-
Ruben D, de Rezende B, Andrade I, Leonardo N, Carvalho C. Better outcomes for monitoring, evaluation and rehabilitation through telehealth for adults and aged with chronic diseases, in randomized controlled trials. PROSPERO 2021. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=247886
-
Shahid N, Rac VE, Bielecki J, Berta W. Understanding factors critical to the implementation of ehealth in chronic disease management: a realist review protocol. BMJ Open 2021;11:e048250. http://dx.doi.org/10.1136/bmjopen-2020-048250
-
Wagle NS, Schueler J, Engler S, Fields S, Kum HC. Patient-perceived barriers and facilitators of using remote health technology to manage diabetes and cardiovascular disease in underserved adult populations: a systematic review. PROSPERO 2020. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=161474
-
Welleman O, Prins-van Gilst G. Telemonitoring in the ambulatory care: a systematic review. PROSPERO 2018. URL: www.crd.york.ac.uk/prospero/display_record.php?RecordID=105589
Appendix 4 Adaptations to and results of AMSTAR 2 assessment
| Quantitative comparative outcome evaluations (e.g. RCTs) | Other quantitative studies (e.g. single-arm evaluations, survey studies) | Qualitative | |
|---|---|---|---|
| 1 | Did the research questions and inclusion criteria for the review include the components of PICO? | Did the review have a clear research question and inclusion criteria? | |
| 2 ** | Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? | ||
| 3 | Did the review authors explain their selection of the study designs for inclusion in the review? | ||
| 4 ** | Did the review authors use a comprehensive literature search strategy? | ||
| 5 | Did the review authors perform study selection in duplicate? | ||
| 6 | Did the review authors perform data extraction in duplicate? | ||
| 7 | Did the review authors provide a list of excluded studies and justify the exclusions? | ||
| 8 ** | Did the review authors describe the included studies in adequate detail? | ||
| 9 ** | Did the review authors use a satisfactory technique for assessing RoB in individual studies that were included in the review? | Did the review authors use a satisfactory technique for assessing the methodological limitations of individual studies that were included in the review? | |
| 10 | Did the review authors report on the sources of funding for the studies included in the review? | ||
| 11 | If a synthesis was performed, did the review authors use appropriate methods to combine the results of individual studies? | ||
| 12 | If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | Not applicable | |
| 13 | Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? | Did the review authors account for methodological limitations in individual studies when interpreting/discussing the results of the review? | |
| 14 ** | Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | Did the review authors provide a satisfactory explanation for, and discussion of, variations in study characteristics and outcomes observed in the results of the review? | Did the review authors provide a satisfactory explanation for, and discussion of, variations in perspective observed in the results of the review? |
| 15 | Did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? Partial Yes – where reviews of quantitative studies (with or without meta-analysis) have discussed the likelihood and impact of publication bias |
Not applicable | |
| 16 | Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | ||
Rating overall confidence in the results of the review
High
No or one non-critical weakness: the systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest.
Moderate
More than one non-critical weakness: the systematic review has more than one weakness but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review. (Multiple non-critical weaknesses may diminish confidence in the review and it may be appropriate to move the overall appraisal down from moderate to low confidence.)
Low
One critical flaw with or without non-critical weaknesses: the review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest.
Critically low
More than one critical flaw with or without non-critical weaknesses: the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
List of abbreviations
- COPD
- chronic obstructive pulmonary disease
- CVD
- cardiovascular disease
- EGM
- evidence and gap map
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SMS
- short messaging service
- WHO
- World Health Organization
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/BVCF6192).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
