Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as award number NIHR130995. The contractual start date was in February 2021. The draft manuscript began editorial review in March 2023 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Gavine et al. This work was produced by Gavine et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Gavine et al.
Chapter 1 Background
Importance of breastfeeding
Breastfeeding has a significantly positive impact on multiple health outcomes across the lifespan. For children, this includes fewer deaths and hospital admissions for infectious diseases1–4 and reduced incidence of obesity, diabetes mellitus and dental disease. 5–7 Breastfeeding has been linked to improved educational and behavioural outcomes. 8–10 For women, breastfeeding is associated with a lower risk of cardiovascular disease, breast and ovarian cancer and diabetes mellitus. 11–13 The impact of breastfeeding on health outcomes applies across settings and population groups, including in high-income countries (HICs) such as the UK. Globally, the scaling up of breastfeeding to near-universal level could prevent 823,000 deaths of children under 5 years old and 20,000 annual deaths of women from breast cancer. 14 To optimise population health, global and UK infant recommendations are that infants should be breastfed (or receive breastmilk) exclusively for about 6 months and that this should continue as part of a mixed diet until 2 years or beyond. 15,16
Increased breastfeeding has the potential to reduce healthcare costs. 17,18 In addition to the important effects on health for women and children, breastfeeding has wider health system and societal impacts, including cost savings for the NHS and environmental benefits. The cost to the global economy of not breastfeeding has been estimated at £242B, and, in the UK, estimates are that £23.6M in additional treatment costs could be saved each year by increased breastfeeding. 17 A further cost to the NHS is the increasing number of prescriptions for specialist formula to treat cow’s milk protein allergy. 19 The environmental impact of not breastfeeding (i.e. feeding with infant formula) is significant, for example from plastics and resources used by the dairy industry. 20,21 Therefore, significant health, societal and environmental gains are to be had from increasing breastfeeding duration and exclusivity.
UK breastfeeding patterns
The UK has low breastfeeding rates. Following the cessation of the quinquennial UK-wide Infant Feeding Surveys, comprehensive, robust data on breastfeeding rates are lacking. For England, the most recent data (2020/21 data), reported by NHS trusts, showed a 72% initiation rate and 49% prevalence of breastfeeding at 6–8 weeks. 22 The comparative figures were 60% initiation and 45% prevalence at 6–8 weeks for Wales (2016 data)23 and 65% initiation and 43% prevalence at 6–8 weeks for Scotland (2018/19 data). 24 In Northern Ireland (2020 data), the initiation rate was 62% and prevalence at 6 weeks was 40%. 25 Rates of exclusive breastfeeding are much lower in all four countries. Throughout the UK, there is a marked social gradient in breastfeeding rates whereby women from socioeconomically deprived groups, those with lower education levels and adolescent women are least likely to breastfeed. 26 For example, in Scotland (2018/19 data),24 breastfeeding prevalence at 6–8 weeks was 62% in the wealthiest quintile compared with 28% in the most deprived quintile. The differences were starker by mother’s age, with breastfeeding prevalence at 6–8 weeks of 58% among mothers aged 40 years and 13% among mothers aged under 20 years. 24 In the UK, women from non-white ethnic groups had higher rates of breastfeeding initiation, prevalence and duration than white women; rates of exclusive breastfeeding after 1 week were similar. 26 Women and babies from the most deprived backgrounds and younger mothers have most to gain from the health benefits conferred by breastfeeding. It has also been reported that around 80% of women in the UK stop breastfeeding before they intended, causing distress26 and potentially leading to poorer mental health. 27,28
Comparing breastfeeding rates between the four countries of the UK, and with countries internationally, is fraught with difficulty, as data are collected in different ways, at different time points and for different years. Nevertheless, rates of breastfeeding in the UK are consistently reported to be lower than those in other European countries. For example, in 2015, a survey of European countries found that breastfeeding initiation rates ranged from 80% in the Netherlands to 98% in Norway and that breastfeeding prevalence at 2 months ranged from 64% in the Netherlands to 89% in Norway (both outcomes were reported by 6 of 11 countries). 29 The exception is Ireland, which has similar rates to the UK with a breastfeeding initiation rate of 64%30 and breastfeeding prevalence at 3 months of 35%. 29
Breastfeeding support
In the UK, formal breastfeeding support, comprising practical, informational, emotional and social support may be provided by healthcare practitioners, voluntary organisations and peer supporters. Women may also receive informal breastfeeding support from families and friends. However, many women report feeling unsupported by healthcare providers and their social networks, especially in the early weeks following birth. 31 This was exacerbated by the impact of COVID-19 on breastfeeding support services, which were already being reduced. 32,33
There is evidence that women living in deprived areas face multiple barriers to breastfeeding and accessing appropriate breastfeeding support. Common barriers include pain, the perception that they do not produce sufficient milk to meet their baby’s needs,34 embarrassment about breastfeeding in public and negative societal attitudes to breastfeeding. 34,35 While these barriers affect all women, they can be particularly challenging in settings where family and friends lack knowledge and experience of breastfeeding. 35 Women from disadvantaged backgrounds may value particularly the experiential knowledge and skills adapted to local contexts provided by peer support. 36 However, survey data suggested that coverage of breastfeeding peer support across the UK was variable and not accessed by socially disadvantaged women. 33 Additional barriers for women from minority ethnic groups, for example Bangladeshi women, include diverse cultural influences of their heritage and their areas of residence in the UK37 and cultural stereotypes held by healthcare providers. 38 There is strong global evidence that, for healthy women and babies, breastfeeding support is effective at increasing partial and exclusive breastfeeding. 39–42 However, these reviews combine evidence from high-, middle- and low-income countries, with most of the HIC evidence coming from the USA. Interventions tested in trials are heterogeneous and generally undertheorised. The extent to which global evidence is transferable to the UK setting is unclear. Previous evidence from UK-based trials is limited and has not demonstrated efficacy of interventions. 43–45 Feasibility studies in the UK show that peer support interventions are acceptable46,47 but effectiveness has not been established.
Women with multimorbidities
The prevalence of maternal chronic conditions is rising,48 which is in part due to increasing maternal age and the improved management of long-term conditions (LTCs). 49 For instance, UK data show that 2.3% of women have been diagnosed with diabetes either prior to or during pregnancy,50 0.5% have a diagnosis of inflammatory bowel disease,51 0.5–1.0% have a diagnosis of epilepsy,52 18.4% have a postnatal diagnosis of anxiety53 and 11.4% have a postnatal diagnosis of depression. 53 Moreover, the rates of gestational diabetes in pregnant women in the UK range from 1.2% to 24.2% depending on maternal characteristics and diagnostic method,54 and this increases the risk of developing type 2 diabetes 10-fold. 55
The prevalence of multiple long-term conditions (MLTC) in the UK is also rising, particularly among working-age adults. 56 Within a general adult population, the onset of MLTC happens 10–15 years earlier in those living in the most deprived areas than in those living in more affluent areas. 57 The MuM-PreDiCT study sought to identify the prevalence of multimorbidities specifically during pregnancy and reported that between 19.8% and 46.2% of pregnant women experience two or more LTCs. 58 LTCs were defined as conditions that had a significant impact on patients, and the specific 79 conditions included in the study were determined in consultation with stakeholders. 59 Unlike in the general adult population, it is not currently clear if the prevalence of MLTC is higher among women from areas of high socioeconomic deprivation. The MuM-PreDiCT study did not find higher odds of multimorbidities in women from areas of high socioeconomic deprivation or in any specific ethnic group. 58 Post hoc analysis explored whether this was being impacted by the health conditions used to define multimorbidity, as some conditions, such as irritable bowel syndrome, anxiety and polycystic ovarian syndrome, were higher in more affluent areas. 58 When a shortened list of conditions was used, socioeconomic deprivation was associated with multimorbidities after adjusting for maternal age and gravidity [adjusted odds ratio (aOR) 1.30, 95% confidence interval (CI) 1.08 to 1.57]. 58 However, this was no longer significant once body mass index (BMI) and smoking status were also adjusted for (aOR 1.05, 95% CI 0.87 to 1.27). MLTC were more common in mothers aged 45–49 years (aOR 1.8, 95% CI 1.0 to 3.20), and this remained significant when adjusted for other characteristics.
Living with MLTC can have a significant impact on mental well-being and can make engaging in other activities difficult. 60 Within the context of maternal health, experiencing a LTC during pregnancy is associated with mental health conditions in the postpartum period such as post-traumatic stress. 61 Mothers with LTCs are also more likely to experience other adverse determinants of health, such as intimate partner violence, smoking, living in poverty and a lack of educational qualifications. 62
There is some evidence for the management of single conditions during pregnancy and the postnatal period, for example diabetes,63 epilepsy,64 and depression,65 that is focused on the treatment modalities for that single condition. However, there is a complete lack of evidence on MLTC in mothers. Postnatal care, in particular, has been universally described as poor due to a lack of follow-up care and help for women to care for their babies. 66 Breastfeeding could present a challenge to women with MLTC, as is evidenced by significantly lower breastfeeding rates among women with single LTCs. 62,67 For instance, a study comparing UK women with lifelong limiting conditions found that breastfeeding rates at 3 months were lower in this group than among women without any conditions (25.6% vs. 33.4%);62 however, rates of initiation were similar. Data from Canada showed that although women with chronic diseases had similar odds of initiating breastfeeding, they were more likely to cease breastfeeding early than women in the general population (aOR 2.48, 95% CI 1.49 to 4.12). 68 Data from other countries also suggest that breastfeeding rates are lower among women with a range of specific conditions such as insulin-dependent diabetes (aOR 0.49, 95% CI 0.27 to 0.89), epilepsy (aOR 0.42, 95% CI 0.26 to 0.68)69 and rheumatoid arthritis (any breastfeeding at 3 months in women with rheumatoid arthritis = 26% vs. 46% of general population). There is currently a complete lack of evidence on breastfeeding rates in women with MLTC. 70
There are several reasons why women with LTCs may have additional difficulties breastfeeding, including a physiological delay in milk release to 72 hours after birth, an increased risk of early separation from the infant as a result of caesarean section and/or requirement for the infant to be placed in neonatal intensive care unit facilities, fatigue, and poor and inconsistent advice about the safety of medications. 68 Anecdotal evidence from the Breastfeeding Network has also identified a lack of joined-up care as a barrier to breastfeeding. As breastfeeding can confer significant health benefits to both mother and infant,14 there is a need for breastfeeding support interventions to provide effective support to all women that is tailored to their individual needs. 71
Economic impact
Breastfeeding in itself is considered a cost-effective intervention. 17,72 Increased breastfeeding has the potential to reduce healthcare costs. 17,18 In addition to the important effects on the health of women and children, breastfeeding has wider health system and societal impacts, including cost savings for the NHS and environmental benefits. The cost to the global economy of not breastfeeding has been estimated at US$570B (£396B) each year, with estimates indicating that 0.75% of gross national income in HICs is lost from not breastfeeding. 73 With a UK gross national income of £2505B in 2022,74 this equates to a value to the UK economy of £18.8B. For the UK health system, estimates are that £23.6M of additional treatment costs each year could be saved by increased breastfeeding. 17 This cost to the NHS is considered a conservative estimate, as a limited number of maternal and child-related illnesses were included in the analysis. A further cost to the NHS is the increasing number of prescriptions for specialist formula to treat cow’s milk protein allergy. 19 For example, an 800g tin of specialised formula (Aptamil Pepti® 1 powder, Nutricia, Trowbridge, UK) prescribed for cow’s milk allergy, which would feed a baby under 6 months old for 1 week, costs the NHS £19.72 at 2023 prices. 75 The environmental impact of not breastfeeding (i.e. feeding with infant formula) is significant. For example, plastics and resources used by the dairy industry have a cost in carbon dioxide emissions equivalent to 50,000–77,500 cars on the road each year and a water footprint of 4700 l/kg. 20,21 Therefore, significant health, societal and environmental gains are to be had from increasing breastfeeding duration and exclusivity. In choosing a breastfeeding support intervention to implement into a health system, policy-makers need to understand not only the evidence of effect and contextual factors that should be considered but also the evidence of cost-effectiveness. With pressure on NHS resources, service managers need to ensure that any investment yields a positive return both in the short term, with increased breastfeeding, and in the long term, with reduced health service resource use and subsequent cost savings.
Why this research is needed
There is a need to find out what works to support women in the UK to meet their infant-feeding goals, to breastfeed for longer, and to increase rates of exclusive breastfeeding. This involves understanding the characteristics and components of breastfeeding support interventions that are likely to be effective and cost-effective in the UK, as well as how to implement and evaluate such interventions. This is particularly the case for populations where breastfeeding rates are low, including young mothers, women of low socioeconomic status, those from marginalised groups, and those with multimorbidities. Although this has been a policy aspiration in the UK for several decades, there is a gap in evidence regarding effective interventions. At a time when the NHS is struggling to meet demand, and life expectancy is stalling, cost-effective public health interventions targeted to disadvantaged communities are vital.
Chapter 2 Research design including stakeholder engagement
Aim and objectives
The aim was to synthesise global and UK evidence to co-create with stakeholders a framework to guide the implementation and evaluation of cost-effective breastfeeding support interventions in the NHS.
Objectives
-
Update the Cochrane review ‘Support for healthy breastfeeding mothers with healthy term babies’41 to identify effective breastfeeding support interventions (see Chapter 3).
-
Conduct a theoretically informed mixed-methods synthesis of process evaluations of UK-relevant breastfeeding support interventions (see Chapter 4).
-
Conduct an economic evaluation of interventions to enable women to breastfeed (see Chapter 5).
-
Conduct a systematic review to identify effective interventions that provide breastfeeding support for women with LTCs (see Chapter 6).
-
Conduct a mixed-methods synthesis of barriers to and facilitators of breastfeeding support in women with LTCs (see Chapter 7).
-
Conduct a systematic review of economic evaluations of breastfeeding support interventions for women with single LTCs (see Chapter 8).
-
Co-create a NHS-tailored implementation and evaluation strategy framework to address contextual barriers and inform transferability of cost-effective interventions to increase breastfeeding rates among healthy women and those with LTCs in the UK (see Chapter 9).
-
Contribute to methodological development of involving stakeholders in co-creation of systematic reviews and synthesising process evaluations to support the transferability and applicability of global evidence to local health service contexts (see Chapter 10).
Objectives 1–3, 7 and 8 were in the original proposal (referred to throughout this report as the main study). Objectives 4–6 were added when additional funding was awarded to address the needs of women with multimorbidities. The focus of objectives 4–6 is on single LTCs because of the lack of evidence relevant to multimorbidities. The primary focus of our work was support for healthy women to breastfeed, addressing inequities in health outcomes. This included women from diverse ethnic and socioeconomic groups. The work on MLTC was an add-on. However, we were also interested in multimorbidities as a contributing factor to health inequities. Objective 7 was modified from the original proposal to incorporate the findings of the additional work. To increase usability, we reframed the main output as a toolkit instead of a framework.
Study design
The study comprised evidence syntheses and economic evaluations with embedded stakeholder engagement, including patient and public involvement (PPI). We used principles of co-creation to ensure that study outputs were relevant to the NHS context. The main study comprised four interlinked work packages with a cross-cutting strand of stakeholder engagement and PPI, as shown in Figure 1. The main study took place over 2 years and the additional work took place over 9 months.
FIGURE 1.
Study flow diagram.
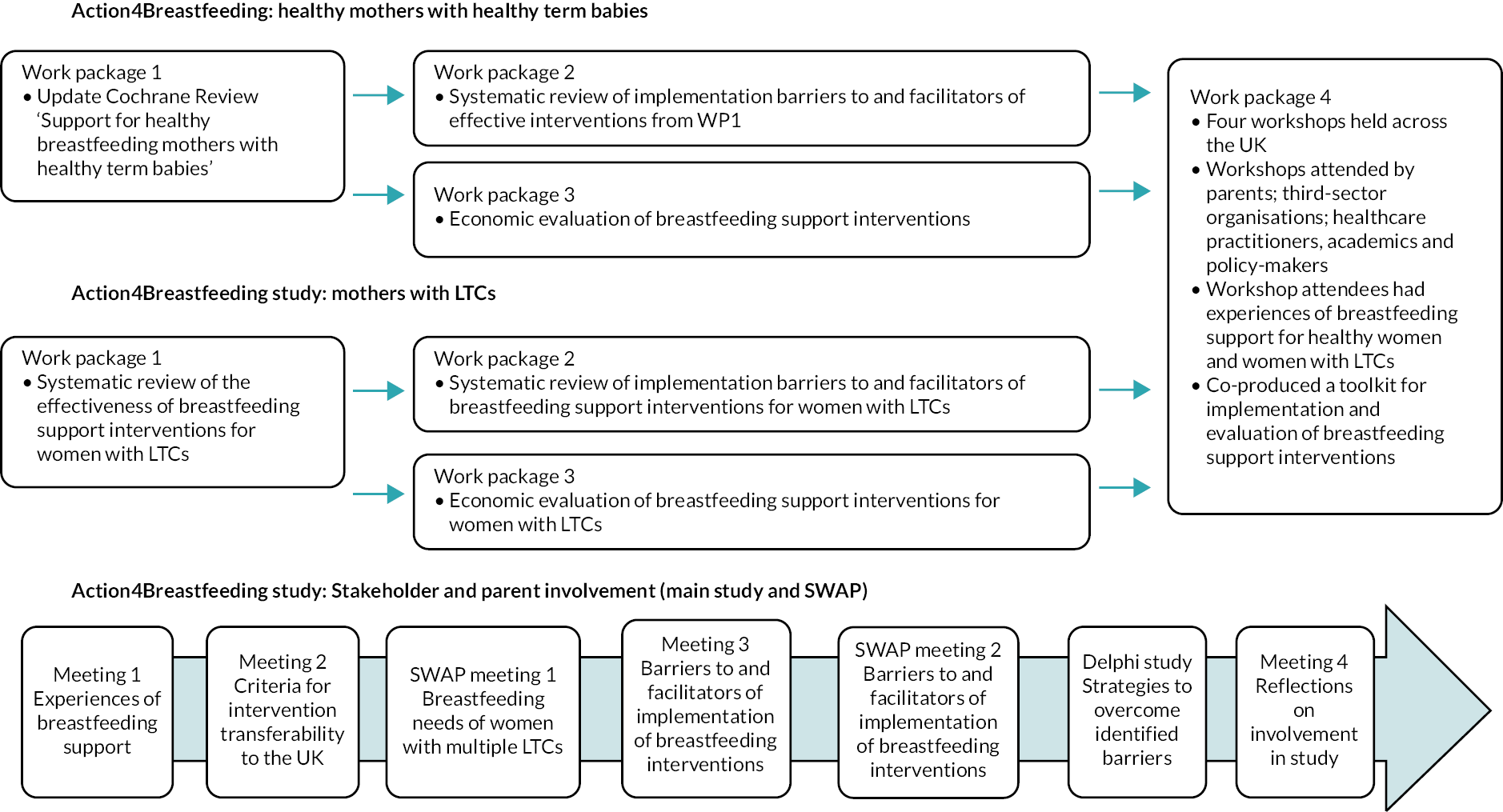
The methods for each evidence synthesis are described in the relevant chapters. In this chapter we present our approach to stakeholder engagement and PPI.
Stakeholder and parent engagement: main study
To ensure joint ownership,76 our approach was ‘active involvement’, defined as ‘the contribution of any person who would be a knowledge user but whose primary role is not research’, throughout the process of evidence synthesis, including planning, production and dissemination. 77 Involvement and co-creation were essential to enhance the quality and relevance of the evidence syntheses. 78,79 Stakeholders and parents were involved in three ways: a co-investigator (PB) from a breastfeeding support organisation represented service user views; the stakeholder working group, parents’ panel and focus group discussions ensured that the experiences of breastfeeding women and service providers were represented in key decisions; and attendees at four workshops co-created the study outputs. Here we describe the participants, activities and outcomes of the stakeholder working group, parents’ panel and focus group discussions. See Chapter 9 for details of the workshops.
Participants
The stakeholder working group comprised 11 members representing third-sector organisations [Breastfeeding Network, Association of Breastfeeding Mothers, La Leche League, National Childbirth Trust (NCT)]; health professionals [general practitioner (GP), midwife, health visitor]; breastfeeding support workers; community breastfeeding support services; national infant-feeding networks; and national policy bodies. Two members also had roles with UNICEF-UK Baby Friendly Initiative. There were representatives from the four nations of the UK. Members of the stakeholder working group were selected to represent areas of high deprivation and/or ethnically diverse populations. For example, the health visitor covered deprived areas in Manchester; the midwife was from the north-east of England, where breastfeeding rates are low; the GP worked in inner-city Glasgow; and the community breastfeeding lead worked in an ethnically diverse area of London.
The parents’ panel comprised nine parents, seven mothers with recent and varied breastfeeding experience and two fathers whose partners had breastfed and who were members of a third-sector organisation. The mothers were recruited via a national third-sector organisation Facebook group. We acknowledge that this approach can lead to the recruitment of parents from higher-income and more educated backgrounds. One member of the parents’ panel was a Gypsy/Traveller, one of the most marginalised and deprived communities in the UK. For this reason, we supplemented the parents’ panel with focus group discussions.
Focus group discussions were held to reach parents from socially disadvantaged backgrounds who were less likely to participate in larger group meetings and who represented groups least likely to breastfeed. The participants were recruited via a not-for-profit organisation providing peer support (not specific to infant feeding) to parents living in economically deprived, ethnically diverse populations in West Yorkshire. Fifteen women participated in the focus group discussions.
Activities and outcomes
The stakeholder working group and parents’ panel each met four times and also participated in an online consensus-building exercise. The consensus-building exercise drew on modified Delphi study methodology. 80 All meetings were held virtually due to COVID-19 restrictions. Focus group discussions were held at three time points, with both a virtual and in-person option provided; there were six focus groups in total. Table 1 shows the main activities at each meeting. Between meetings, a newsletter was circulated to all members to update them on the progress of the study. At the fourth meeting, the stakeholder working group and parents’ panel reflected on their experiences of engaging with the study. Their views are included in Chapter 10.
| Meeting (number of participants) | Description of activity | Outcomes/impact on study |
|---|---|---|
| SWG 1 (11) | Getting to know each other and setting ground rules. Presentation of project and bite-size training on systematic reviews. Assessing the transferability of breastfeeding interventions to the UK (breakout discussions) | Early discussions of criteria for assessing transferability developed for SWG 2 |
| PP 1 (6) | Getting to know each other and setting ground rules. Presentation of project and bite-size training on systematic reviews. Reflections on personal experiences of breastfeeding support | Factors viewed as important to satisfaction with breastfeeding support influenced Cochrane review (review 1) meta-analysis (e.g. selection of outcome time points) |
| FGD 1 (8: 5 online, 3 face to face) | Topic guide covered personal experiences of breastfeeding support, and views of important components of support including who, where, when and how | Factors viewed as important to satisfaction with breastfeeding support influenced Cochrane review (review 1) meta-analysis (e.g. selection of outcome time points) |
| SWG 2 (7) | Interactive exercise to score and rank transferability criteria from the PIET-T process model81 | Top 3 ranked criteria (1, population’s acceptability of the intervention; 2, quality of the primary evidence available; 3, sustainability of the intervention) used to select examples of effective interventions from the Cochrane review (review 1) for discussion of implementation barriers and facilitators |
| PP 2 (4) | The PIE-T model explained. Results of the SWG ranking exercise presented. Discussion of the 12 highest-scoring criteria | Parents’ views of transferability criteria informed decision not to exclude any effective interventions, as any intervention could be transferred to the UK with adaptations and resources |
| FGD 2 (6: 3 online, 3 face to face) | Visual materials in plain language covering the key transferability criteria presented. Participants asked to discuss important factors to take into account when transferring interventions from another country to a UK setting | Discussions of barriers to and facilitators of accessing breastfeeding support and informed consideration of transferability |
| SWG 3 (6) | Five effective interventions from the Cochrane review (review 1) presented and discussed to identify implementation barriers and strategies | Identified barriers and facilitators included in the consensus-building exercise study |
| PP 3 (4) | Five effective interventions from the Cochrane review (review 1) presented and parents discussed positive and negative aspects, barriers to access and strategies to overcome the barriers | Identified barriers to access and strategies included in the consensus-building exercise |
| Consensus-building exercise 1 (10) | Respondents (SWG and PP) presented with 18 barriers (from previous meetings) and asked to recommend strategies from 10 themes from the Expert Recommendations for Implementing Change (ERIC) framework82 | For each barrier, strategy themes with > 70% consensus were taken forward to round 2. Due to lack of consensus on strategies, one barrier was excluded from round 2 |
| Consensus-building exercise 2 (8) | For each of the 17 barriers, respondents asked to rank in order of importance individual strategies from the themes that reached consensus in round 1 (34 strategies) | Due to low response rate (no parents responded) and lack of consensus, 34 strategies were taken forward to the workshops |
| FGD 3 (9: 6 online, 3 face to face) | Five effective interventions from the Cochrane review (review 1) discussed to identify implementation barriers and strategies | Identified barriers and facilitators compared with findings from SWG, PP and workshops to illuminate considerations that might be needed when adapting for communities with low breastfeeding rates |
Stakeholder and parent engagement (multiple long-term conditions)
Participants
The MLTC stakeholder working group comprised 12 members representing third-sector organisations [Breastfeeding Network, La Leche League, Lactation Consultants of Great Britain and the British Human Immunodeficiency Virus (HIV) Association] and a wide range of healthcare professionals (consultant physician, consultant psychiatrist, GP, pharmacist, health visitor, specialist midwife, infant-feeding co-ordinator and diabetes specialist nurse) involved with caring for women with MLTC who may breastfeed. Stakeholder working group members were from England, Scotland and Wales and were selected for their experience in supporting women with a wide range of long-term physical and mental health conditions to breastfeed.
One-to-one discussions with condition-specific experts including a consultant endocrinologist and an HIV breastfeeding specialist were also undertaken.
The MLTC parents’ panel comprised seven parents with MLTC and recent breastfeeding experience. Parents’ panel members were from across the four UK nations and had lived experience of a wide range of physical and mental health conditions, including diabetes, lupus, fibromyalgia, inflammatory bowel disease, multiple sclerosis, hypertension, kidney disease, connective tissue disorders, asthma, chronic fatigue syndrome, anxiety and depression. Parents were recruited via third-sector organisation Facebook groups.
Activities and outcomes
The MLTC stakeholder working group and parents’ panel met twice during the 9-month study. These meetings mirrored the first and third meetings of the main study stakeholder working group and parents’ panel. The first meeting of the parents’ panel was focused mainly on giving parents opportunities to tell their stories of breastfeeding alongside coping with multimorbidities. The first meeting of the stakeholder working groups was focused on participants’ experiences of providing breastfeeding support to women with MLTC and the barriers to and facilitators of providing support. In the second meeting, the stakeholder working group and parents’ panel discussed the same five effective interventions used in the main study, this time focusing on whether and how these interventions could be adapted to meet the needs of women with multimorbidities. The findings from the MLTC stakeholder engagement contributed to the workshop activities as described in Chapter 9.
Role of stakeholder engagement
The main purpose of the stakeholder working groups, the parents’ panel and the focus group discussion was to adapt the international evidence, that is, the findings of the reviews, to ensure relevance to the UK context and the NHS, and to coproduce the toolkit. The stakeholder engagement therefore influenced the interpretation and adaptation to the UK setting of the review findings rather than their methods. The exception to this was in influencing the decision on outcome time points and variables for the meta-regression for review 1.
Chapter 3 Effective interventions for breastfeeding support for healthy women with healthy term babies
Introduction
This chapter contains a summary of the methods and results section from the updated Cochrane review on breastfeeding support for healthy term women with healthy term babies. 83 The full review including table of characteristics, forest plots and risk of bias assessments is published in the Cochrane Library. 83 Parts of this chapter have been reproduced with permission from Wiley. Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Objectives
-
To describe types of breastfeeding support for healthy breastfeeding women with healthy term babies.
-
To examine the effectiveness of different types of breastfeeding support interventions focusing on breastfeeding support provided on its own or breastfeeding support in combination with a wider maternal and child health intervention.
-
To examine the effectiveness of the following intervention characteristics on breastfeeding support:
-
type of support (e.g. face to face, telephone, digital technologies, group or individual support, proactive or reactive)
-
intensity of support (i.e. number of postnatal contacts)
-
person delivering the intervention (e.g. healthcare professional, lay person)
-
to examine whether the impact of support varied between high‐ and low‐ and middle‐income countries.
-
Methods
Criteria for considering studies for this review
Inclusion criteria
Types of studies
All randomised or quasi‐randomised controlled trials (RCTs), with or without blinding, were included. Cluster‐RCTs were also eligible for inclusion.
Types of participants
Participants were healthy pregnant women considering or intending to breastfeed their baby, or healthy women who were breastfeeding healthy babies. Healthy women and babies were considered those who did not require additional medical care. Studies of women requiring additional medical care (e.g. women with diabetes, women with HIV/AIDS, overweight or obese) were excluded. The inclusion criteria were amended in this update to include women undergoing caesarean section.
Types of interventions
We defined breastfeeding support as contact with an individual or individuals (either professional or volunteer) offering support that is supplementary to the standard care offered in that setting. Interventions could be delivered as standalone breastfeeding support interventions (breastfeeding only), or breastfeeding support could be delivered as part of a wider maternal and newborn health intervention (breastfeeding plus) where additional services are also provided (e.g. vaccination, intrapartum care, well-baby clinics).
‘Support’ interventions eligible for this review could include elements such as reassurance, praise, information, and the opportunity to discuss and to respond to the mother’s questions and could also include staff training to improve the supportive care given to women. It could be offered by health professionals or lay people, trained or untrained, in hospital and community settings. It could be offered to groups of women or one‐to‐one, including mother‐to‐mother support, and it could be offered proactively by contacting women directly, or reactively, by waiting for women to get in touch.
This update now also includes support provided via digital technologies as well as support provided over the telephone.
Support could involve only one contact or regular, ongoing contact over several months. Studies were included if the intervention occurred in the postnatal period alone or also included an antenatal component.
Types of outcome measures
-
Stopping any breastfeeding at 6 months postpartum.
-
Stopping exclusive breastfeeding at 6 months postpartum.
-
Stopping any breastfeeding at 4–6 weeks postpartum.
-
Stopping exclusive breastfeeding at 4–6 weeks postpartum.
-
Stopping any breastfeeding at 2, 3–4 and 12 months postpartum.
-
Stopping exclusive breastfeeding at 2 and 3–4 months postpartum.
-
Maternal satisfaction with care.
-
Maternal satisfaction with feeding method.
-
All‐cause infant or neonatal morbidity (including infectious illness rates).
-
Maternal mental health.
Exclusion criteria
Types of studies
Any study that did not involve the random allocation of participants was excluded (non-RCTs; quasi-experimental studies; one group before-and-after studies; cohort studies; case–control studies; case reports; or qualitative studies).
Types of participants
Studies that focused specifically on women or infants with additional care needs were excluded. For mothers this could mean coexisting medical problems (e.g. diabetes, HIV) or pregnancy-related complications (e.g. pre-eclampsia). For infants this could include preterm birth, low birthweight or additional care in a neonatal unit.
Types of interventions
Interventions taking place in the antenatal period alone were excluded from this review, as were interventions described as solely educational or promotional in nature.
Additional limitations
We did not exclude studies based on language or date of publication. Abstracts were eligible for inclusion if they provided sufficient information for data to be extracted. If they did not provide sufficient information, they were recorded as ongoing studies.
Search methods for identification of studies
The Cochrane Pregnancy and Childbirth Group’s Trials Register was searched by its information specialist in May 2021. This includes results of searches of CENTRAL, MEDLINE, EMBASE, CINAHL, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (11 May 2021).
We also searched the reference lists of retrieved studies and the list of excluded studies from the previous version of this review to identify any studies that met the new inclusion criteria. 41
Data collection and analysis
We used standard Cochrane Pregnancy and Childbirth Group methods. Two review authors independently selected trials, extracted data and assessed risk of bias using Covidence software. 84 The certainty of the evidence was assessed by two reviewers using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. 85
We then assessed study trustworthiness using the new approach implemented by the Cochrane Pregnancy and Childbirth Group to identify and manage potentially untrustworthy studies. 86 All full texts meeting the inclusion criteria and studies included in the previous update of this review were evaluated against the following criteria.
Research governance
-
No prospective trial registration for studies published after 2010 without plausible explanation.
-
When requested, trial authors refuse to provide/share the protocol and/or ethics approval letter.
-
Trial authors refuse to engage in communication with the Cochrane review authors.
-
Trial authors refuse to provide individual patient data upon request with no justifiable reason.
Baseline characteristics
-
Characteristics of the study participants being too similar [distribution of mean (standard deviation) excessively narrow or excessively wide].
Feasibility
-
Implausible numbers (e.g. 500 women with severe cholestasis of pregnancy recruited in 12 months).
-
(Close to) zero losses to follow‐up without plausible explanation.
Results
-
Implausible results (e.g. massive risk reduction for main outcomes with small sample size).
-
Unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, for example if they say no blocking was used but still end up with equal numbers, or they say they used blocks of four but the final numbers differ by six.
Any studies classed as potentially high risk for any of these criteria were referred back to the Cochrane Pregnancy and Childbirth Group, who contacted the study authors for more information. If we did not receive adequate information, the study remained ‘awaiting classification’.
Data synthesis
We used methods outlined in the Cochrane Handbook for statistical analysis. 87 In this update of the review, we grouped interventions into two different categories for meta-analysis. The first group, ‘breastfeeding only’, were interventions that only contained breastfeeding support. In the second group, breastfeeding support was one part of a larger intervention that also aimed to provide other health benefits for the mother or her infant (e.g. vaccinations, new-baby care).
We used meta-regression to further assess statistical heterogeneity for the four primary outcomes when a sufficient number of studies were included in the analyses (i.e. at least 10 observations per characteristic modelled). 88 The following four categories were selected for the meta-regression in conjunction with stakeholders:
-
by type of supporter (professional vs. lay person, or both)
-
by mode of support (face to face vs. telephone support vs. digital vs. combination)
-
by intensity of support [low (fewer than four) vs. moderate (four to eight) vs. high (nine or more)]
-
by income status of country [high‐income country vs. low- and middle‐income country (LMIC)].
We performed sensitivity analyses based on risk of bias for allocation concealment and incomplete outcome data. Additionally, sensitivity analyses were conducted to investigate the effect of including cluster‐randomised trials where no adjustment was possible.
Results
A total of 590 trial reports were assessed for inclusion in this update (see Gavine et al. 83 for full details). This included 560 studies from the updated search, 16 trial reports that were awaiting classification in the previous version of the review, 8 studies that were ongoing in the last version of the review and 6 previously excluded studies that were reassessed due to the change in inclusion criteria. Of these, 72 met the inclusion criteria.
All studies (100 previously included and 72 newly identified studies) were assessed against Cochrane’s criteria for trustworthiness. Of the 100 previously included studies, we requested further information for 38, and of the new studies identified in this update, we required clarification for 43. In total, we received satisfactory responses for 27 studies. In total, 54 studies were reclassified to ‘awaiting classification’. The remaining studies were included, and this updated review includes 116 trials, of which 103 contribute data to the analyses.
In total, 249 studies were excluded with reasons (this comprises 139 reports from the updated search and 110 reports from previous versions of the review). The majority of studies (n = 136) were excluded as the intervention was not relevant to the review, for example interventions that were focused on education and/or promotion only and did not offer any support, interventions that were focused on other aspects of postnatal care, and antenatal-only interventions. We excluded any study that was not a RCT (n = 53). A further 49 studies were excluded because they did not focus on healthy mothers (e.g. coexisting medical conditions requiring additional care) or babies (e.g. preterm, low birthweight). Eleven studies were excluded because the comparator was not either standard care or an alternative non‐breastfeeding intervention. Finally, four studies were excluded as they were not research papers. For full details, see Gavine et al. 83 for characteristics of the excluded studies.
Description of included studies
This updated review includes 116 trials, of which 103 contribute data to the analyses. The 116 studies comprise 83 individually randomised trials and 33 cluster‐randomised trials. Most are two‐arm RCTs; however, 20 studies are either three‐ or four‐arm RCTs. In total, 125 interventions with more than 98,816 mother–infant pairs were included. See Gavine et al. 83 for further details and tables of characteristics.
Participants
Participants living in 42 countries are included in the review. Using the World Bank classification of countries by income, 21 of the new included studies in the review were conducted in HICs, 6 in upper-middle‐income countries, 16 in LMICs and 5 in low‐income countries (LICs). Participants were women from the general healthy population of their countries. However, 52 studies recruited women from groups at high risk of health inequalities or health inequities in their country. Most of these studies were conducted in HICs (n = 33). These included women defined as low-income or living in a disadvantaged area (n = 18), women with a non-white ethnic background (n = 9) and young mothers (n = 6).
Interventions
Of the 125 interventions included in the review, 91 interventions comprised only breastfeeding support components. The remaining 34 interventions aimed to increase breastfeeding rates as part of a multicomponent intervention, which aimed to improve other aspects of child health, such as vaccination rates, or sleep.
Women received breastfeeding support proactively in 85 interventions. In 32 studies, women had access to both proactive and reactive support, and in 6 studies only reactive support was offered. Just over half of the studies included an antenatal component.
Most interventions provided one-to-one support (n = 115). However, in 19 of these 115 interventions, additional group support was also available to women. Eight studies consisted of only group support and two studies provided support to partners. The majority of interventions were provided by professionals (n = 74). Thirty-five interventions were provided by a lay person (usually a peer supporter), and 14 had both lay and professional input. The majority of studies reported that the person providing the support had undergone training in breastfeeding (n = 97).
Face-to-face support was a component of the majority of interventions (n = 104). In 64 of the 104 interventions, face‐to‐face support was the only mode of support available. In 36 interventions, face‐to‐face support was complemented with telephone support. Telephone support alone was evaluated in 14 studies. Only five studies used fully digital approaches (e.g. social media, messaging services), and two studies used only two-way text messaging.
Intervention intensity was grouped as follows: low intensity (three or fewer contacts), moderate intensity (four to eight contacts) and high intensity (nine or more contacts). Twenty-one interventions were specified as low intensity, 41 were specified as moderate intensity, and 44 were specified as high intensity. The intensity of the remaining 19 interventions was not specified.
In 97 studies, the control groups were described as receiving the standard care for the study population. However, there are large differences in standard care provision both between and within countries. Thirteen studies compared the study intervention against either an active control arm or a control group that offered participants additional care to the standard care available to non‐participants. In six studies the care received by the control group is either not reported or unclear.
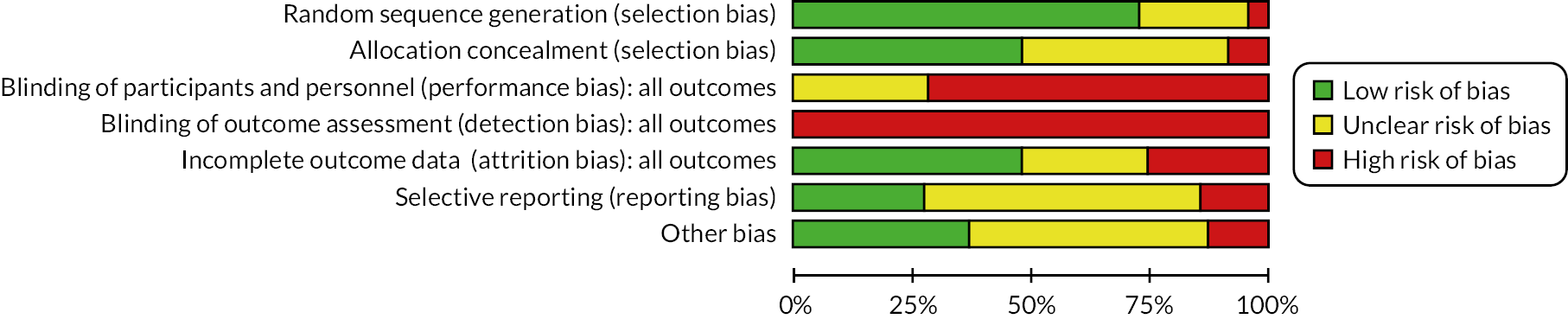
Risk-of-bias assessments
We considered that the overall risk of bias of trials included in the review was mixed. Blinding of participants and personnel is not feasible in such interventions, and, as studies utilised self‐report breastfeeding data, there is also a risk of bias in outcome assessment.
For full details of the risk-of-bias assessments, see Gavine et al. 83 A summary of the judgements is detailed in Figure 2.
FIGURE 2.
Risk-of-bias graph: review authors’ judgements about each risk-of-bias item presented as percentages across all included studies (Gavine et al. 83).
Effects of interventions
Tables 2 and 3 provide the summary of findings. For full details of effects of interventions, including forest plots and funnel plots, see Gavine et al. 83
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with usual care | Risk with support | ||||
| Stopping breastfeeding (any) at 6 months | 600 per 1000 | 558 per 1000 (534 to 582) | RR 0.93 (0.89 to 0.97) | 14,610 (30 RCTs) | +++⃝ Moderateb |
| Stopping exclusive breastfeeding at 6 months | 847 per 1000 | 763 per 1000 (746 to 788) | RR 0.90 (0.88 to 0.93) | 16,332 (40 RCTs) | +++⃝ Moderateb |
| Stopping breastfeeding (any) at 4–6 weeks | 308 per 1000 | 271 per 1000 (244 to 299) | RR 0.88 (0.79 to 0.97) | 11,413 (36 RCTs) | +++⃝ Moderateb |
| Stopping exclusive breastfeeding at 4–6 weeks | 518 per 1000 | 430 per 1000 (394 to 466) | RR 0.83 (0.76 to 0.90) | 14,544 (42 RCTs) | +++⃝ Moderateb |
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with usual care | Risk with support plus | ||||
| Stopping breastfeeding (any) at 6 months | 541 per 1000 | 508 per 1000 (492 to 524) | RR 0.94 (0.91 to 0.97) | 4879 (11 RCTs) | +++⃝ Moderateb |
| Stopping exclusive breastfeeding at 6 months | 685 per 1000 | 541 per 1000 (479 to 616) | RR 0.79 (0.70 to 0.90) | 7650 (13 RCTs) | ++⃝⃝ Lowb,c |
| Stopping breastfeeding (any) at 4–6 weeks | 433 per 1000 | 407 per 1000 (355 to 467) | RR 0.94 (0.82 to 1.08) | 2325 (6 RCTs) | +++⃝ Moderated |
| Stopping exclusive breastfeeding at 4–6 weeks | 542 per 1000 | 396 per 1000 (309 to 515) | RR 0.73 (0.57 to 0.95) | 2402 (6 RCTs) | +⃝⃝⃝ Very lowc,e |
Primary outcomes
Moderate‐certainty evidence indicated that ‘breastfeeding only’ support probably reduced the number of women stopping breastfeeding for all primary outcomes: stopping any breastfeeding at 6 months [relative risk (RR) 0.93, 95% CI 0.89 to 0.97]; stopping exclusive breastfeeding at 6 months (RR 0.90, 95% CI 0.88 to 0.93); stopping any breastfeeding at 4–6 weeks (RR 0.88, 95% CI 0.79 to 0.97); and stopping exclusive breastfeeding at 4–6 weeks (RR 0.83 95% CI 0.76 to 0.90). Sensitivity analyses excluding studies rated as being at high or unclear risk of bias for allocation concealment and incomplete outcome reporting found similar or more beneficial treatment effects.
The evidence for ‘breastfeeding plus’ was less consistent. For primary outcomes there was some evidence that ‘breastfeeding plus’ support probably reduced the number of women stopping any breastfeeding (RR 0.94, 95% CI 0.91 to 0.97, moderate‐certainty evidence) or exclusive breastfeeding at 6 months (RR 0.79, 95% CI 0.70 to 0.90). ‘Breastfeeding plus’ interventions may have a beneficial effect on reducing the number of women stopping exclusive breastfeeding at 4–6 weeks, but the evidence is very uncertain (RR 0.73, 95% CI 0.57 to 0.95). The evidence suggests that ‘breastfeeding plus’ support probably results in little to no difference in the number of women stopping any breastfeeding at 4–6 weeks (RR 0.94, 95% CI 0.82 to 1.08, moderate‐certainty evidence).
We conducted meta‐regression to explore substantial heterogeneity for the primary outcomes using the following categories: person providing care, mode of delivery, intensity of support and income status of country. It is possible that moderate levels (defined as four to eight visits) of ‘breastfeeding only’ support are associated with a more beneficial effect on exclusive breastfeeding at 4–6 weeks and 6 months. ‘Breastfeeding only’ support may also be more effective in reducing women stopping exclusive breastfeeding at 6 months in LMICs than in HICs. However, no other differential effects were found and thus heterogeneity remains largely unexplained. The meta‐regression suggested that there were no differential effects regarding person providing support or mode of delivery; however, power was limited.
Secondary breastfeeding outcomes
Moderate‐certainty evidence indicated that ‘breastfeeding only’ support probably had a beneficial effect on the following: stopping exclusive breastfeeding at 2 months (RR 0.81, 95% CI 0.74 to 0.89), any breastfeeding at 3–4 months (RR 0.87, 95% CI 0.81 to 0.93) and exclusive breastfeeding at 3–4 months (RR 0.81, 95% CI 0.74 to 0.89). Low-certainty evidence suggested that ‘breastfeeding only’ interventions may have a beneficial effect on the number of women breastfeeding at 9 months (RR 0.87, 95% CI 0.78 to 0.97). However, low certainty evidence suggests that ‘breastfeeding only’ interventions have little impact on the number of women doing any breastfeeding at either 2 months (RR 0.93, 95% CI 0.77 to 1.11) or 12 months (RR 0.87, 95% CI 0.90 to 1.00).
‘Breastfeeding plus’ interventions probably had little to no impact on stopping breastfeeding for any of the secondary outcomes: any at 2 months (RR 0.92, 95% CI 0.79 to 1.07, moderate-certainty evidence); exclusive at 2 months (RR 0.90, 95% CI 0.78 to 1.03, very low-certainty evidence); any at 3–4 months (RR 0.97, 95% CI 0.81 to 1.15, low-certainty evidence); exclusive at 3–4 months (RR 0.86, 95% CI 0.75 to 1.00, low-certainty evidence); or any at 12 months (RR 0.96, 95% CI 0.91 to 1.00, moderate-certainty evidence).
Non-breastfeeding outcomes
There were no consistent findings emerging from the narrative synthesis of the non‐breastfeeding outcomes (maternal satisfaction with care, maternal satisfaction with feeding method, infant morbidity and maternal mental health), except for a possible reduction of diarrhoea in intervention infants.
Chapter summary
The update of this Cochrane review on breastfeeding support for healthy term women identified 116 trials, of which 103 contribute data to the analyses. More than 98,816 mother–infant pairs were included. When ‘breastfeeding only’ support is offered to women, the duration and particularly the exclusivity of breastfeeding is likely to be increased. Support may also be more effective in reducing the number of women stopping breastfeeding at 3–4 months than at later time points. For ‘breastfeeding plus’ interventions the evidence is less certain.
There does not appear to be a difference in who provides the support (i.e. professional or non‐professional) or how it is provided (face to face, telephone, digital technologies or combinations). Indeed, various kinds of support may be needed in different geographical locations to meet the needs of the people within that locality.
Chapter 4 Systematic review of implementation research of effective breastfeeding support interventions for healthy women with healthy term babies
Introduction
The Cochrane review update undertaken in our review 1 confirmed that there is ample evidence to know that breastfeeding women need support to be available and to be provided, and that such support is likely to make a difference. Such an evidence base also suggests that one key research question for the future is to identify how such support can best be provided consistently across countries and settings.
Therefore, there is now a need to improve the evidence base around scaling up issues for breastfeeding support interventions, which will require a greater emphasis on implementation and quality improvement approaches rather than effectiveness studies. To enable further advances in this area, it will be fundamental to identify and synthesise available qualitative and process evaluation data on existing interventions. The overall aim of this review was to conduct a theoretically informed mixed-methods synthesis of process evaluations of breastfeeding support interventions identified as effective in review 1.
Objectives
-
To identify qualitative and quantitative data from process evaluation studies linked to breastfeeding support interventions identified as effective in review 1.
-
To synthesise the views and experiences of those involved in receiving or delivering breastfeeding support interventions identified as effective in review 1.
-
To identify the contextual factors (barriers/facilitators) affecting the implementation of breastfeeding support interventions identified as effective in review 1.
Methods
The protocol for this systematic review is registered on PROSPERO (CRD42021229769).
Search strategy
We systematically searched six electronic databases (MEDLINE, CINAHL Plus, PsycInfo, ASSIA, Scopus and Web of Science). Searches were conducted in March 2022 using combinations of index terms and free-text words relating to ‘breastfeeding support’ AND ‘implementation research’ (a sample search strategy for MEDLINE is provided in Appendix 1). No restrictions were applied on publication date and publication language. Reference lists of all included studies and relevant systematic reviews were scanned for eligible studies. Supplementary searches were conducted based on the name of interventions identified in Gavine et al. ,83 included articles’ authors, and forwards and backwards citation checking.
Eligibility criteria
Inclusion criteria
Studies were included if they reported findings of primary research exploring the views and experiences of any participants involved in either delivering or receiving any of the breastfeeding support interventions identified as effective in Gavine et al. ,83 including breastfeeding women and babies and their families, service providers, managers, commissioners and policy-makers.
Qualitative and quantitative studies, either standalone or in mixed-methods designs, were included. Studies reported any type of process evaluation outcome relating to the selected interventions, including any subjective participant-reported outcomes and constructs such as attitudes, views, beliefs, perceptions, understandings or experiences.
There were no restrictions on publication date or language of publication.
Exclusion criteria
Articles only reporting on impact evaluation results of breastfeeding support interventions (i.e. effectiveness of interventions) were excluded.
Studies that focused specifically on women or infants with additional care needs were excluded. For mothers this could mean coexisting medical problems (e.g. diabetes, HIV) or pregnancy-related complications (e.g. pre-eclampsia). For infants this could include preterm birth, low birthweight or additional care in a neonatal unit.
Studies relating to interventions taking place in the antenatal period alone were excluded from this review, as were interventions described as solely educational or promotional.
Selection process
Two reviewers independently screened titles, abstracts and relevant full texts against the predetermined eligibility criteria. Any discrepancies were resolved through discussion and consultation with a third reviewer.
Data extraction and quality appraisal
Data extraction was undertaken independently by two reviewers using a piloted data extraction form. Any discrepancies were resolved through discussion and consultation with a third reviewer. The table of characteristics is presented in Appendix 2, Table 13.
Quality appraisal of included studies was conducted by two reviewers, using a self-developed tool derived from a set of criteria previously used in other National Institute for Health and Care Research (NIHR)-funded work to assess the quality of process evaluations. 89 Studies were not excluded based on the quality/adequacy of the reporting. Instead, the quality of studies was taken into consideration during data synthesis by exploring whether any particular finding or group of findings were dependent, either exclusively or disproportionately, on one or more studies classed as ‘low quality’ or ‘inadequately reported’. Any discrepancies were resolved by discussion and the involvement of a third reviewer where necessary. See Appendix 2, Table 14.
Data synthesis
We adopted a mixed-methods synthesis approach. We first undertook two preliminary syntheses of quantitative (synthesis 1) and qualitative (synthesis 2) process evaluation studies, and then integrated qualitative and quantitative process evaluation data into a theoretically informed cross-study synthesis (synthesis 3).
For synthesis 1 we used narrative methods90 to synthesise quantitative findings from included process evaluations. Two reviewers independently assessed the tabulated characteristics of the included quantitative studies and agreed the criteria to organise the included studies. For synthesis 2 we used a data-driven approach to thematic synthesis91 to synthesise qualitative findings from included process evaluations. This involved three overlapping and interrelated stages: (1) line-by-line coding of findings from primary studies, (2) categorisation of codes into descriptive themes and (3) development of analytical themes to describe or explain previous descriptive themes. To ensure the robustness of the synthesis, various techniques to enhance trustworthiness were undertaken, including audit trail, multiple coding, reviewer triangulation and team discussions. Finally, for synthesis 3, we adopted a theory-driven approach to thematic synthesis91 to synthesise and bring together quantitative and qualitative findings from included primary studies. This synthesis was informed by the Consolidated Framework for Implementation Research (CFIR),92 a comprehensive framework that characterises the contextual determinants of implementation and can be used to inform implementation theory development and verification of what works where and why across multiple contexts.
Results
The searches identified 2894 records, which were assessed against the inclusion criteria. Title and abstract screening resulted in 243 records considered eligible or inconclusive. Full-text articles were then retrieved and assessed for eligibility. Two records could not be retrieved. Of the 241 records screened at full text, 225 were excluded. The main reason for exclusion was studies not being linked to an intervention identified as effective in review 1 (n = 84), followed by standalone studies that were not linked to any intervention (n = 51) and studies not involving implementation research and/or process evaluation data (e.g. pre-implementation or intervention development studies) from eligible interventions (n = 50). Other reasons for exclusion were studies linked to either interventions (n = 26) or populations (n = 6) not eligible for inclusion in review 1, and systematic reviews (n = 4) and other publication types not reporting primary research findings (n = 4). The remaining 16 studies were included in the final synthesis (Figure 3). The 16 studies are linked to 10 RCTs of effective interventions from review 1.
FIGURE 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
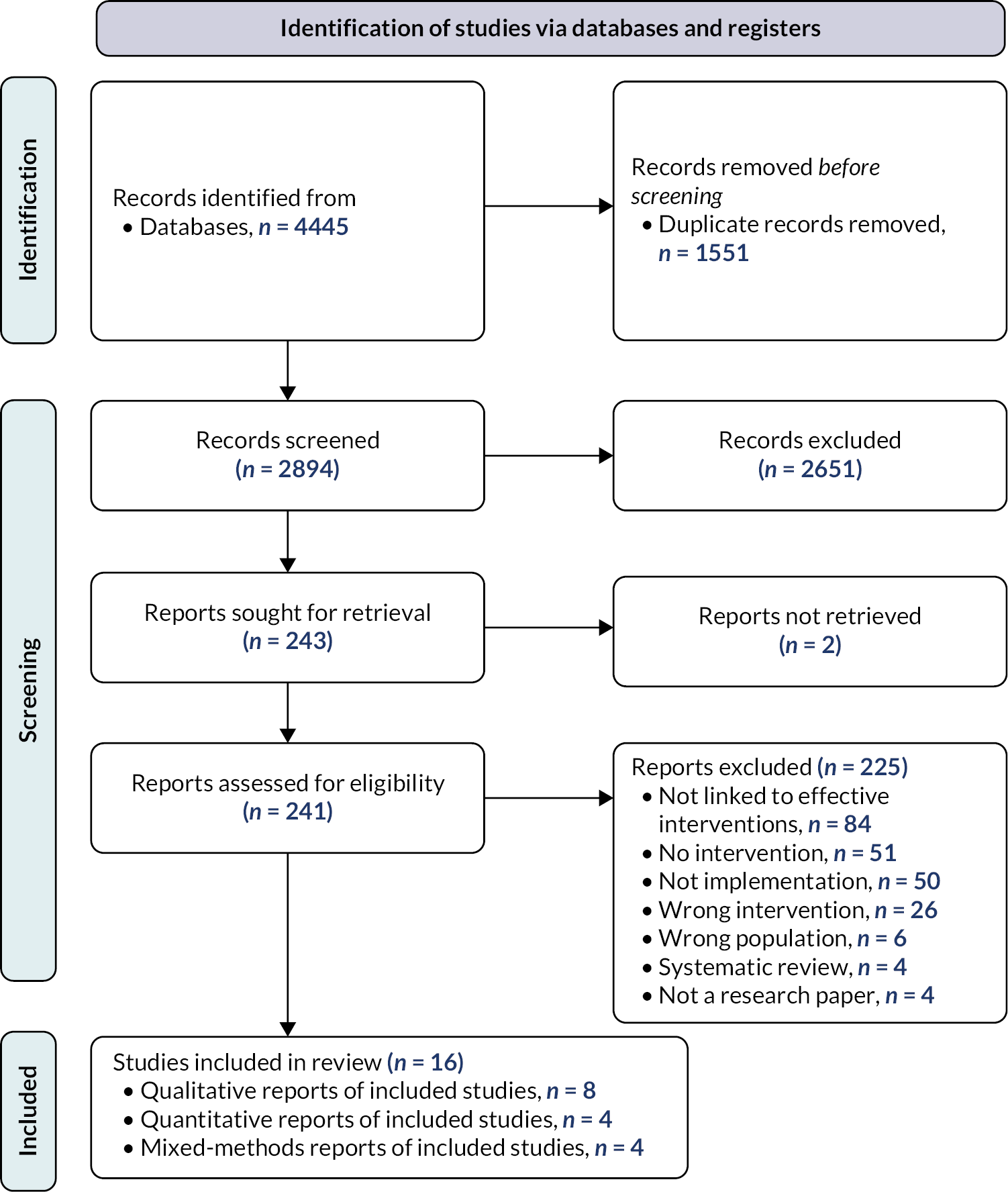
Summary of included studies
A summary of key characteristics of the included studies is presented in Appendix 2, Table 13.
Twelve studies contributed qualitative data to the synthesis, comprising eight qualitative93–100 and four mixed-methods101–104 process evaluation studies; and eight studies contributed quantitative data to the final synthesis, comprising four quantitative105–108 and four mixed-methods studies. 101–104
Studies reported data from ten countries: nine from HICs (five in the USA, two in Australia and one each in Canada and the UK); and seven from LMICs (four in Uganda, two in South Africa and one in Pakistan). All of the studies from Uganda and South Africa were evaluations of aspects of the PROMISE-EBF RCTs. 109
Study settings included rural and urban areas and hospital and community facilities. In eight of the studies in HICs, the target populations were low-income or disadvantaged populations, or those living in areas with low breastfeeding rates.
Study samples ranged from 26 to 130 mothers, 12 to 254 peer counsellors, 13 to 28 healthcare staff and 2 to 409 other stakeholders, including supervisors, programme managers and co-ordinators, and unspecified key informants. Other forms of data included observations, diaries and daily activity logs.
Process evaluations included in this review were linked to effective interventions identified in review 1 (for details, see Appendix 2, Table 13).
The descriptions of linked interventions were coded against a taxonomy of behaviour change techniques. The most commonly identified behaviour change techniques related to social support, goals and planning, and feedback and monitoring. A summary of the behaviour change techniques identified across all the linked interventions is provided in Appendix 2, Table 15.
Quality appraisal
The quality of the 16 process evaluations was mixed (see Appendix 2, Table 14). Seven studies were judged to have made a fairly thorough attempt to increase rigour and minimise bias in sampling, data collection and analysis. 93,96,97,100,101,103,107 A further six studies were assessed to have taken at least a few steps to increase rigour of sampling, data collection and analysis. 94,98,99,102,104,105 For the remaining three studies, judgements for at least one element of sampling, data collection or data analysis were hindered by poor reporting. 95,106,108 All studies’ findings were judged to be at least fairly well supported by the data. The findings of three studies were judged to have limited breadth and/or depth. 93,106,108 In Andaya et al. ,93 the evaluation was based on exit interviews lasting 8–12 minutes. Chapman et al. 106 report only coverage of the intervention. Ridgeway et al. 108 do not report responses to open-ended questions in their survey. Seven studies were judged not to have privileged the perspectives of breastfeeding women. 94,97,98,102,104,106,108 Two studies were judged to have low reliability of findings94,95 and one study was judged to have low usefulness. 94,95
Stakeholders’ perceptions and experiences
Stages 1 and 2 of our mixed-methods synthesis resulted in the categorisation of primary quantitative and qualitative data from included studies into 86 descriptive themes. Building on these findings, further analytical work and team discussion was undertaken, and the initial descriptive themes were grouped around a resulting set of 18 factors affecting the implementation of effective interventions, which in turn informed our preliminary, data-driven synthesis conclusions. These revolved around the following three analytical themes:
-
that qualitative/quantitative monitoring data and feedback are provided for women and/or professionals to reflect on and evaluate the progress, quality and experience of implementing the new breastfeeding support intervention
-
that breastfeeding support needs of women/families served by the implementing organisation (including any barriers to/facilitators of meeting those needs) are known
-
that individuals involved in the new breastfeeding support intervention are appropriately trained, have confidence in their capabilities, and are able to execute the courses of action required to achieve the desired implementation/intervention goals.
For the final stage of our thematic synthesis, we mapped our descriptive and analytical themes against the domains of the CFIR. Our three analytical themes and subthemes aligned across five subdomains of the implementation process domain (assessing needs, assessing context, tailoring strategies, engaging, and reflecting and evaluating) of the CFIR framework.
Our final three overarching, theoretically informed analytical themes are described below. Table 4 shows the distribution of primary studies underpinning each analytical theme and their mapping against the relevant CFIR subdomains.
| Included studies (n = 16) (first author and year) | Implementation process (consolidated framework for implementation research) mapped sub-domains | ||||||
|---|---|---|---|---|---|---|---|
| 5B – assessing needs | 5C – assessing context | 5E – tailoring strategies | 5F – engaging | 5H – reflecting and evaluating | |||
| 1 – innovation deliverers | 2 – innovation recipients | 1 – implementation | 2 – innovation | ||||
| Theme 1: assessing the needs of those delivering and receiving breastfeeding support interventions | Theme 2: assessing the context and optimising delivery of and engagement with breastfeeding support interventions | Theme 3: reflecting and evaluating the success of implementing and providing breastfeeding support | |||||
| Ahmed 2012101 | • | • | • | ||||
| Andaya 201293 | • | • | |||||
| Bronner 2000105 | • | • | • | • | |||
| Chapman 2004106 | • | ||||||
| Cramer 2017102 | • | • | • | • | |||
| Daniels 201094 | • | ||||||
| Dennis 2002107 | • | • | • | • | |||
| Hoddinott 2012103 | • | • | • | • | • | ||
| Nankunda 200695 | • | • | • | • | |||
| Nankunda 201096 | • | • | • | ||||
| Nankunda 2010104 | • | • | • | ||||
| Nkonki 201097 | • | • | |||||
| Rahman 201198 | • | • | • | ||||
| Ridgway 2016108 | • | • | |||||
| Rujumba 202099 | • | • | |||||
| Teich 2014100 | • | ||||||
Assessing the needs of those delivering and receiving breastfeeding support interventions
Included studies identified several implementation challenges relating to the needs, preferences and priorities of those delivering and receiving breastfeeding support interventions. Nine studies reported on issues from the perspective of intervention deliverers.
Some reported having to deal with feelings of frustration when running breastfeeding support services with low attendance rates. 102 This was a particular challenge for those running services located in small or rural areas. For those juggling a breastfeeding support role with healthcare provider roles, the pressure of the competing demands in the context of low attendance rates could make them feel like their time might have been better spent on other activities. 102
One key strategy reported to both identify and address the needs of breastfeeding support providers was through training. 94–96,98,103,105,107 Studies largely reported that intervention deliverers felt training prepared them well, in terms of both counselling skills and technical competence (e.g. being able to show how to breastfeed correctly). All of this was perceived as key to ensuring consistency in intervention delivery.
Other issues that could be addressed through training were to do with the practical expectations of undertaking the breastfeeding supporter role. Uncertainties about safety, transport and reimbursement while delivering support were among the most reported needs for those delivering community-based interventions,94,95 as well as around more complex issues, such as managing difficult scenarios or the interplay of cultural beliefs and breastfeeding practice. The last was particularly relevant to lay breastfeeding supporters delivering interventions at community level. They noted the importance of acknowledging that trainees themselves belong to a range of communities that might be systematically exposed to certain issues/inequities more than others (e.g. rural isolation, HIV prevalence in the community) and/or might hold cultural beliefs about breastfeeding or breastfeeding-related practices that could act as barriers. These should be identified and addressed in a culturally sensitive manner and without antagonising the communities, enabling lay providers to appropriately and inclusively support breastfeeding women from a range of communities. 94,95,97
Those in implementation leadership roles also emphasised the importance of effective management and supervision. This was reported as a key facilitator of some interventions,94,97 particularly to ensure that certain needs of intervention deliverers continue to be addressed beyond the provision of formal training. For example, for those engaged in interventions relying on peer, lay and/or volunteer supporters, there was an important need to provide them with ongoing emotional support, including mentoring and motivation.
Overall, the breastfeeding supporters felt that their role was important, satisfying and rewarding,15 with implications that were perceived to go beyond the specific breastfeeding support encounters to act as triggers of the wider support network of the breastfeeding women. 95,96
The needs, preferences and priorities of recipients of breastfeeding support interventions were echoed in five studies.
Breastfeeding women perceived the provision of support as positive, important and needed. 99,107 Key to this was being offered the opportunity to ask questions and being allowed to spend enough time to address any issues. 103,104 Also important was accessing support flexibly as needed, rather than having to fit support around fixed working hours or at times that might not be convenient (particularly if receiving support visits at home or after starting paid work after maternity leave). 101,103,104
Assessing the context and optimising delivery of and engagement with breastfeeding support interventions
Some studies reported a range of contextual factors affecting the implementation and delivery of breastfeeding support interventions. These included identification of appropriate settings and accessible, available spaces to deliver breastfeeding support;95,102 consideration of environmental factors that are considered breastfeeding promoting (and avoidance of those that are not) in the intervention delivery settings (e.g. use of breastfeeding promotion leaflets, posters and videos);105 and availability of and alignment with local policies and procedures, as well as with existing practices, in maternity care. 98,105 Studies also reported examples of tailoring implementation strategies to address barriers, leverage facilitators and optimise how breastfeeding support interventions fit the context. These included strategies to promote and encourage engagement, such as ensuring embeddedness within the community,95,96 addressing challenges to recruit breastfeeding supporters,102 favouring lay language;103 teamwork and positive interactions with other breastfeeding supporters and healthcare professionals;96,105 responsiveness of support content and language to address known barriers and common issues;100,103,106,108 and continuity/accessibility of interventions across the continuum of care. 93,103
Reflecting and evaluating the success of implementing and providing breastfeeding support
Included studies reported a broad range of reflective and evaluative accounts about the success of implementation processes and about how impactful breastfeeding support interventions were perceived by women.
Reports about the success of implementation focused on issues relating to key implementation outcomes such as satisfaction,103,104,107 fidelity,103 convenience101,103,104 or usefulness. 101,104,107 Other studies reported on the key drivers that enabled successful engagement between mothers and breastfeeding supporters,97,104,107 including elements of responsiveness/tailoring and content areas addressed in support encounters. 95,97,104,106,108 Some studies reported data on the views and experiences of enacting the role of breastfeeding supporter95,96,98,105,107 and breastfeeding supporter’s supervisor/lead,97,107 all of which documented positive perceptions by those undertaking and/or interacting with those roles. Other studies looked at factors affecting the scale-up of breastfeeding support interventions, including key barriers (e.g. stigma around exclusive breastfeeding, economic barriers and limited resources, health facilities, lack of supportive policies, low male involvement, negative sociocultural beliefs) and facilitators (e.g. promotion at health system level, engagement of professional associations and active collaborations with existing groups, the media and appropriate role models). 98,99
Some studies included reports of perceived meaningfulness and impact of breastfeeding support interventions from women’s perspectives, which can be considered reflective accounts that add to the existing body of evidence about the success of breastfeeding support interventions. Women perceived breastfeeding support interventions as beneficial to women, babies and the wider community;102 and helpful for improving breastfeeding knowledge,93 ensuring the early establishment of breastfeeding93 and enabling women to recognise feeding patterns and problems. 101 Breastfeeding supporters were perceived by women as allies who bolstered their confidence in their decision to breastfeed, particularly for those who were faced with a lack of encouragement from family or hospital staff. 93
The provision of practical information about breastfeeding mechanics and hands-on support were perceived as useful and enabled women to feel reassured and encouraged to continue breastfeeding. 93 The element of responsiveness in terms of support content areas afforded by breastfeeding support interventions helped make interventions meaningful for women in the context of their specific breastfeeding support encounters. 95,97,104,106,108 The most commonly reported issues addressed were reassurance, general breastfeeding information, supply and demand, breastfeeding positioning and attachment, feed frequency, normal infant behaviour, expressing and breast pump use, nipple pain/damage issues and not having enough milk. More interactive intervention components (e.g. monitoring systems, telephone-based support) were appreciated and seen as useful but perceived as a ‘mixed fit’ for breastfeeding support. Women saw these modes of support as an addition to rather than a replacement for face-to-face support. 101,103
Chapter summary
This review comprised 16 studies linked to 10 interventions identified as effective in review 1, which reported the views and experiences of those delivering or receiving breastfeeding support. The quality of the included studies was mixed, but all study findings were judged to be at least fairly well supported by the data.
The synthesis resulted in three overarching themes, theoretically informed by the CFIR: (1) assessing the needs of those delivering and receiving breastfeeding support interventions; (2) assessing the context and optimising delivery and engagement with breastfeeding support interventions; and (3) reflecting and evaluating the success of implementing and providing breastfeeding support.
Included studies identified several implementation challenges relating to the needs, preferences and priorities of those delivering and receiving breastfeeding support interventions. Breastfeeding supporter training was a commonly reported implementation strategy, which also enabled implementation teams to identify and address breastfeeding supporters’ needs. Included studies reported a range of contextual factors (e.g. alignment with local policies) affecting the implementation and delivery of breastfeeding support interventions as well as a range of tailoring strategies (e.g. community involvement, use of lay language, responsive support content/information) to address contextual factors. Reports about implementation success focused on issues relating to key implementation outcomes such as satisfaction, fidelity and usefulness.
Chapter 5 Health economic evaluation
Overview
Previous chapters have identified which support interventions were effective in terms of stopping the drop-off of women breastfeeding, and what contextual factors need to be considered when implementing interventions into healthcare settings in the UK. This chapter builds on this evidence by exploring how well breastfeeding support interventions work in relation to how much they cost health services. A systematic review of economic evidence was conducted to appraise and synthesise what was already known about the cost-effectiveness of breastfeeding support interventions for healthy mothers with healthy babies. This was followed by a model-based economic evaluation, which was informed by the systematic reviews of effect and of cost-effectiveness. The health economic component of the evidence syntheses was designed and interpreted with input and advice from the stakeholder engagement groups, workshops and the study steering committee.
Systematic review of economic evidence
The aim of this review of economic evidence was to gain an understanding of whether breastfeeding support interventions for healthy mothers with healthy babies were considered value for money. The overarching review question was: What are the incremental costs and cost-effectiveness of breastfeeding support interventions in comparison with standard care, no intervention, or an alternative intervention for healthy mothers with healthy babies in the UK? The review objectives were to:
-
identify and synthesise the evidence base for incremental costs and cost-effectiveness of breastfeeding support interventions
-
assess the applicability of the evidence to a UK setting
-
identify limitations and uncertainties in the applicable economic evaluations
-
examine the level of consistency between applicable economic evaluations.
Methods
Eligibility criteria
Guidance on searching for economic evidence and conducting reviews of economic evidence was adhered to,87,110–112 along with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement for reporting systematic reviews. 113 The eligibility criteria for this review mirrored those for the systematic review of evidence of effect, reported in Chapter 3, in terms of the population, intervention and comparator. For the population, studies were included if they related to healthy pregnant women considering or intending to breastfeed or breastfeeding healthy babies. Healthy women and babies were considered those who did not require additional medical care. For the intervention criterion, studies were included if they involved contact with professional(s) or volunteer(s) offering support that was supplementary to the standard care offered in that setting. The support could include elements such as reassurance, praise, information and the opportunity to discuss and to respond to the mother’s questions. Interventions only provided in the antenatal period were excluded. The review planned to include interventions that were deemed suitable and/or potentially transferable for use in UK settings. Understanding of this was to be gained through stakeholder engagement, with discussion and agreement reached through the focus groups outlined in Chapter 2. In relation to the comparator criterion, studies were included if the comparison group received standard care, an alternative intervention or no comparator. In keeping with the systematic review of evidence of effect, it was decided to group studies by whether the intervention was considered a ‘breastfeeding only’ intervention or a ‘breastfeeding plus’ intervention by providing additional broader support targeting a range of health or non-health effects.
The outcomes of interest for the review included the health effects recorded for the systematic review of effect (any and/or exclusive breastfeeding), as well as any outcomes associated with supporting women to breastfeed that were selected and measured in the economic evaluation. These included, but were not limited to, health-related quality of life and healthcare resource use. Economic outcomes of interest were those that were selected, measured and valued, such as incremental costs (cost savings), incremental cost-effectiveness ratios (ICERs), net benefit ratios and quality-adjusted life-years (QALYs). Finally, types of studies included were full economic evaluations (cost-effectiveness, cost–benefit and cost–utility analyses) and partial economic evaluations (cost–consequences analyses, cost analyses, cost descriptions). Economic analyses excluded were non-comparative studies such as cost-of-illness studies, as it was considered that the objectives and results of these study designs would not align with the review question.
Search strategy
A search strategy was developed encompassing three domains: (1) breastfeeding, (2) support and (3) costs/economics, under which relevant index terms and text words were identified and collated. The domain of costs/economics made use of the search filter for economic studies used by the Scottish Intercollegiate Guidelines Network, which was adapted from the search filter designed by the NHS Centre for Reviews and Dissemination at the University of York. Within each domain, search terms were combined with the Boolean operator ‘OR’ and then across domains with the Boolean operator ‘AND’. An example of the list of search terms used for one of the bibliographic database searches can be found in Appendix 1. The full search strategies are available from the corresponding author on request.
Five electronic bibliographic databases were searched using all three search domains: MEDLINE via Ovid, EMBASE via Ovid, CINAHL via EBSCOhost, HMIC via Ovid and MIDIRS via Ovid. Electronic databases for economic literature were searched with a modified search syntax without the need for the search filter for economic studies: American Economic Association’s electronic bibliography (EconLit) via EBSCOhost, NHS Economic Evaluation Database (NHS EED), Paediatric Economic Database Evaluation (PEDE), IDEAS economics database via RePEc and EconPapers via RePEc. The stakeholder working group provided additional advice on relevant sources to facilitate the search. A modified search syntax relating to all three domains was developed and used with the following search engines: ClinicalTrials.gov, WHO International Clinical Trials Registry Platform; the Virginia Henderson International Nursing Library (VHL); GreyNet International; OISter and Google Scholar. For this last search, it was decided to extract the first 500 records from the return, as search results were presented by relevance and this number was deemed sensitive to identifying eligible records. No language or date restrictions were applied other than those inherent in each database; for example, NHS EED contains economic evaluations of health and social care interventions published between 1994 and the end of 2014.
The search was last updated on 2 February 2022. Reference lists of systematic reviews identified during the search and reference lists of eligible studies were consulted to identify any relevant studies missed from the database searches. In addition, eligible studies were forward searched using the ‘cited by’ tab in Google Scholar. This process was completed in July 2022.
Selection process
Returned records from database searches were transferred into the reference management software EndNote version 20.3 (Clarivate Analytics, Philadelphia, PA, USA) and duplicate records were removed. All unfiled references were then transferred into Covidence to be screened for eligibility for inclusion. Two reviewers independently screened titles and abstracts against the inclusion criteria. All potentially relevant records were brought forward for the full-text sift. During the full-text sift, two reviewers independently read all full papers and reports to assess for eligibility. Any conflicts were discussed, and consensus was reached. Any unresolved conflicts were discussed with the broader project team for final consensus to be reached. Reasons for exclusion at this stage were recorded. A PRISMA flow diagram was completed to illustrate the selection process. 113
Data extraction and quality assessment
All studies eligible for inclusion were progressed to data extraction and quality assessment. Two review authors independently extracted and recorded data using a piloted data extraction form in Covidence. The data extraction form for Cochrane reviews was used as a starting point, allowing for relevant data to be extracted from trial-based studies, and modified to include data related specifically to the economic evaluation. These items extracted details on the type of economic evaluation, perspective taken, currency, price year, year of conversion, time horizon, discount rate, data sources, model assumptions, measurement of uncertainty, consideration of heterogeneity, sensitivity analyses, base-case results in terms of incremental costs, cost-effectiveness and/or net-benefit estimates, where available. Data were summarised in tabular form for each included study.
Quality assessment of the economic evaluations was conducted using the checklist provided by the National Institute for Health and Care Excellence (NICE),111 which is separated into two sections. Section 1 assesses the applicability of each included study to the review question. Those judged directly or partially applicable progress to section 2, which assesses the limitations of the economic evaluation. The checklist, which was partly informed by the Evers checklist114 and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist for reporting economic evaluations,115 is used to review economic evaluations and incorporate findings into the development of NICE guidelines. For section 1, economic evaluations were reviewed independently by two authors and rated as directly applicable, partially applicable or not applicable. Disagreements were resolved by discussion until consensus was reached. Those studies judged to be not applicable to the review question did not progress to section 2 of the checklist for quality assessment. For those judged to be directly or partially applicable, section 2 was completed, again independently by two authors. Section 2 allowed for an overall assessment of the methodological quality of the studies, judging them to have minor limitations, potentially serious limitations or very serious limitations. The classification depended on whether the studies met the 11 quality criteria. Studies classified as having very serious limitations had failed to meet one or more quality criteria that would be highly likely to change the conclusions about cost-effectiveness; those with potentially serious limitations failed to meet one or more criteria that could change the conclusions about cost-effectiveness; and those with minor limitations failed to meet one or more criteria, but this would be unlikely to change the conclusions about cost-effectiveness. Quality assessments for each section were summarised separately in tabular form.
Synthesis methods
Economic evidence profiles were created for those studies deemed directly or partially applicable, with limitations and uncertainty summarised for each study, along with incremental costs, incremental effects and ICERs. In terms of the estimates of costs extracted from individual studies, these were adjusted to GBP 2022 prices using the Campbell and Cochrane Economics Method Group – EPPI-Centre Cost Converter web-based tool, which was created by the Campbell and Cochrane Economics Methods Group and is available at https://eppi.ioe.ac.uk/costconversion/. A narrative synthesis summarised the characteristics and results of the applicable economic evaluations grouped by the level of support provided by the interventions (breastfeeding only or breastfeeding plus), in keeping with the systematic review of effect. Inconsistency between results of economic evaluations were considered, with the potential impact of including methodologically weak studies explored as part of the narrative synthesis. If results were available for subgroups of women who were considered socially disadvantaged, inconsistencies between results were also considered.
This review of economic evidence was not registered; however, the review protocol can be accessed via the repository held by the Queen’s University Belfast Research Portal (https://pure.qub.ac.uk/).
Results
Study selection
Following engagement with stakeholders, as reported in Chapter 2, agreement was reached that all breastfeeding support interventions identified as effective were deemed suitable and transferable to a UK setting. Justification for this was based on the consideration that if an intervention was effective and resources were available, then implementation should be supported to adapt services to deliver the intervention. For this review of economic evidence, consideration also needed to be given to whether the system and context of the setting were similar to those of the UK. Subsequently, while no consideration was given to country setting for inclusion, only those studies conducted in Organisation for Economic Cooperation and Development (OECD) settings were assessed for applicability, and only those judged to be directly or partially applicable were assessed for limitations. 111
Figure 4 presents the PRISMA flow diagram for the study selection process. Following the removal of duplicate records, 5699 records were screened at the title and abstract stage. Of these, 5491 were excluded and the full text of 208 records was sought. Nine records could not be retrieved: three were ongoing studies still in the recruitment phase of the aligned RCT, three had no relevant data available and three were awaiting classification with no response from the corresponding authors. Of the 199 records screened for eligibility, 162 were excluded. The main reason for exclusion was the wrong design (n = 116), as on full-text review many studies did not report an economic evaluation. Further reasons for exclusion were the wrong intervention (n = 28), wrong population (n = 15) and wrong outcomes (n = 1). The systematic search, identification and screening process resulted in 39 studies eligible for inclusion.
FIGURE 4.
The PRISMA flow diagram for review of economic evidence for breastfeeding support interventions for healthy mothers with healthy babies.
Study characteristics
Of the 39 studies, 7 were conducted in a UK setting,47,116–120,154 and 14 were conducted in OECD settings, with 7 in the USA,121–127 5 across Australia and/or New Zealand,128–132 and 1 each in Canada133 and Ireland. 134 The remaining 18 studies were conducted in non-OECD settings, with 10 conducted in sub-Saharan Africa,135–144 3 in Asia/South East Asia,145–147 3 in Latin America,148–150 and 2 across multiple countries with high adult and child mortality or undernutrition. 151,152
Studies that assessed ‘breastfeeding only’ support interventions (n = 21) were shorter in duration, lasting from a minimum of 7 days133 to a maximum of 10 weeks postpartum,136 and were delivered by professionals,47,117,133,134,147,148,150 lay providers120,124,136,137,142,145,152 or both. 118,119,121,123,126,135
‘Breastfeeding plus’ support interventions were assessed in 18 of the 39 evaluations, with primary aims being obesity prevention,128–131 nutrition improvement,138,139,151 and maternal and infant care and/or support. 116,120,125,141,144,153 Four studies conducted economic evaluations related to baby-friendly hospital initiative (BFHI) accreditation or Ten Steps to Successful Breastfeeding. 122,132,146,149 The duration of ‘breastfeeding plus’ interventions ranged from a short time frame with hospitalisation for labour and delivery122,141 to a longer time frame from pregnancy to infant age of 2 years. 143,154
A range of methods were used for the economic evaluations. Seventeen studies were partial economic evaluations with a cost analysis comparing two or more alternatives117,120,122–124,126,128,133,153 or a cost/cost-outcome description with one alternative. 47,121,137,139,142,144,145 Full economic evaluations were reported in the remaining 23 studies, with 10 studies reporting a cost-effectiveness analysis,118,129,131,135,140,143,147,149–151 6 studies reporting a cost–benefit analysis, 5 studies reporting a cost-effectiveness and a cost–utility analysis125,127,132,134,138,146 and 2 studies reporting a cost–utility analysis alone. 119,152 Eighteen of the studies were trial-based economic evaluations, with 13 of these aligned with RCTs116–118,123,126,128–130,133,136,138,142,155 reported in the Cochrane review.
Applicability
At this stage of the review process, studies conducted in OECD settings progressed to quality assessment. An evidence table of 21 economic evaluations identified for inclusion that were conducted in OECD settings is presented in Appendix 3, Table 16. Each evaluation is described in terms of the setting, intervention, comparator and participant characteristics. Detailed methods of economic analysis are provided, along with a summary of results and the judgment of applicability to the review question.
In terms of the applicability criteria assessed, all 21 studies fulfilled or partially fulfilled the criteria for the study population. Reasons for a partial judgment for the population stemmed from eligibility for participation that did not specify inclusion/exclusion criteria based on the health status of the mother and infant. All interventions were judged to be relevant to the review question, providing either ‘breastfeeding only’47,103,117–119,121,123,124,126,127,133,134 or ‘breastfeeding plus’ support. 116,120,122,125,128–132,153
Twelve studies were judged not applicable. The use of a payer perspective taken for the costing of the intervention and/or healthcare resource use in an organisational setting was considered too diverse from a UK provider perspective in six of the studies. 121–125,127 In addition, studies that only provided costs for one alternative or a cost comparison were deemed not applicable. 47,117,121,123,127,128,132,134,153 Without data on incremental cost or incremental cost-effectiveness comparing two alternatives, the studies failed to provide enough relevant information for the review question. Failing to meet these criteria for applicability would likely change the conclusions about cost-effectiveness or give rise to no meaningful conclusions; thus, these studies were excluded from further consideration.
Nine of the 21 studies were judged applicable. Two studies were deemed directly applicable,116,119 as they fulfilled all the criteria in terms of the population, intervention, provider perspective for costs and outcomes recorded and reported incremental costs or ICERs with relevant discounting of costs and outcomes where the time horizon was beyond 1 year. The remaining studies were judged to be partially applicable. Either the setting and system where the study was conducted was not the UK126,130,131,133,154 or the limited time horizon and/or scope for the economic evaluation indicated that not all relevant costs and outcomes had been accounted for. 118,126,133,155
Evidence of cost-effectiveness from applicable studies
Tables 5 and 6 present the economic evidence profiles for applicable studies that evaluated ‘breastfeeding only’ support118,119,126,133 and ‘breastfeeding plus’ support. 116,130,131,154,155 Base-case results for incremental costs, incremental effects and incremental cost-effectiveness are provided. Costs have been converted and uplifted to 2022 GBP for ease of comparison. Two of the ‘breastfeeding only’ support studies126,133 provided healthcare costs and outcomes of effect on breastfeeding separately and did not evaluate in terms of incremental costs per additional woman breastfeeding. For illustrative purposes, we estimated ICERs from the events data on breastfeeding (any and exclusive) for these studies.
| Study ID | Applicability | Limitations | Incremental | Uncertainty | ||
|---|---|---|---|---|---|---|
| Cost (£)a | Effect | ICER (£/effect)a | ||||
| Hoddinott et al., 2012118 | Partially applicable Provider perspective, cost per unit BMI avoided reported, within-trial time horizon from discharge following birth up to infant age 8 weeks |
Very serious limitations Limited time horizon of 8 weeks; limited costs and outcomes recorded; no sensitivity analyses conducted |
24.87; 24.87 | 0.23; 0.22 | 107.52 per additional woman breastfeeding; 112.47 per additional woman exclusively breastfeeding | Measures of uncertainty not reported. Alternative intervention costing scenarios suggest costs would be sensitive to varying staff requirements and period of coverage |
| Mavranezouli et al., 2022119 | Directly applicable UK setting, provider perspective, cost per QALY gained reported, time horizon from birth up to 1 year or lifetime, depending on condition |
Minor limitations Economic model undertaken over a long time horizon with deterministic and probabilistic sensitivity analysis. May be limited by the quality of the data from sources for model parameters |
69.94 | 0.001 | 56,074.98 per QALY gained | The value of the ICERs held with the sensitivity analysis. The two-way sensitivity analysis suggested that the cost-effectiveness of the intervention improved as its effectiveness increased and intervention cost decreased |
| Pugh et al., 2002126 | Partially applicable OECD setting, provider and family perspective with costs reported separately, within-trial time horizon from birth to 6 months, incremental costs reported |
Very serious limitations Limited time horizon; intervention costs only from provider perspective with health service use not valued; study reported costs and outcomes separately; no sensitivity analyses conducted |
332.06; 332.06 | 0.136; 0.08 | 2446.22 per additional woman exclusively breastfeeding at 6 months;b 4226.21 per additional woman breastfeeding (any) at 6 monthsb | Measure of uncertainty (standard error) reported around incremental costs. Alternative scenarios suggest incremental costs would be sensitive to change in method of valuing staff time ICER estimated herein without addressing uncertainty |
| Stevens et al., 2006133 | Partially applicable OECD setting, provider and family perspective with costs reported separately, within-trial time horizon from birth to 5–12 days, incremental costs reported |
Potentially serious limitations Limited time horizon; study reported costs and outcomes separately; no sensitivity analyses conducted |
14.55 | 0.216 | 67.36 per additional woman exclusively breastfeeding at 5–12 daysb | Incremental costs were not statistically significant ICER estimated herein without addressing uncertainty |
| Study ID | Applicability | Limitations | Incremental (bootstrapped 95% CI) | Uncertainty | ||
|---|---|---|---|---|---|---|
| Cost (£)a | Effect | ICER (£/effect)b | ||||
| Barnes et al., 2017116 | Directly applicable UK setting, provider perspective, cost per QALY gained reported, time horizon from pregnancy up to infant aged 1 year |
Potentially serious limitations Data not extrapolated beyond study context; broader outcomes not considered which likely would affect cost-effectiveness estimates |
2377.38 (−967.25 to 5723.16) | −0.01 (−0.05 to 0.03) | −283,960.75 per QALY gained | The value of the ICERs held with the sensitivity analysis. The probability of group FNP + usual care being more cost-effective than usual care alone at a WTP threshold of £20,000 per QALY gained ranged from 0% to 3% |
| Hayes et al., 2014129 | Partially applicable OECD setting, provider perspective, cost per unit BMI avoided reported, within-trial time horizon from birth to infant age 2 years |
Potentially serious limitations Trial-based economic evaluation with a limited time horizon of 2 years, retrospective costing used, no sensitivity analyses conducted |
825.95 (487.34 to 1189.91); 825.95 (487.34 to 1189.91) | 0.33 (−0.043 to 0.662); 0.23 (0.026 to 0.475) | 2383.20 per unit BMI avoided; 355.51 per 0.1-BMI z-score reduction | In the scenario analysis, the probability of Healthy Beginnings + usual care being more cost-effective than usual care alone at a WTP threshold of $500 per 0.1-BMI z-score reduction was 66%, compared with the base-case 30% |
| Morrell et al., 2002155 | Partially applicable UK setting, provider perspective, intervention costs reported only, time horizon limited to within-trial (birth to infant age 6 months) |
Potentially serious limitations Cost analysis with intervention activities measured and valued only; limited time horizon of 6 months; limited sensitivity analysis |
287.16 (127.98 to 437.96) | The incremental cost was largely driven by the intervention cost. The sensitivity analysis to explore uncertainty around the cost of the developing service estimated that a reduction in postnatal support workers time spent on home visits would result in a reduction in intervention costs, but this reduction may adversely impact on future health services resource use | ||
| Tan et al., 2020130 | Partially applicable OECD setting, provider perspective, cost per QALY gained reported, modelling was undertaken over a 15-year time horizon |
Potentially serious limitations QALY estimates based on children’s weight status; important outcomes not considered, for example mother’s health-related quality of life; healthcare costs from birth to 5 years omitted, with authors’ assumption that they are unlikely to affect cost-effectiveness results. These are likely to change the conclusions about cost-effectiveness |
297.17 (265.78 to 330.65); 297.17 (265.78 to 330.65); 314.43 (305.02 to 324.90) | 0.006 (−0.007 to 0.017); −0.11 (−0.38 to 0.16); −0.09 (−0.28 to 0.11) | 49,528.15 per QALY gained (age 15 years); 2701.72 per BMI avoided (age 15 years); 3493.81 per BMI avoided (age 5 years) | The ICER for the cost per QALY gained was not considered cost-effective for the combination intervention, which included a breastfeeding advice component. Subsequently, sensitivity analyses were not conducted to measure uncertainty. The combination intervention was more cost-effective over a 15-year than a 5-year time horizon in terms of BMI unit avoided, due in large part to the projected savings in healthcare costs |
| Wen et al., 2017131 | Partially applicable OECD setting, provider perspective, incremental cost per unit BMI avoided reported, time horizon limited to within-trial (birth to infant age 2 years) |
Potentially serious limitations The study did not assess cost-effectiveness with the cost per QALY gained and conducted a within-trial economic evaluation that did not take into account breastfeeding outcomes or longer-term costs and outcomes |
Telephone 266.81 (207.28 to 339.60); SMS 90.37 (53.12 to 134.34) | Telephone −0.05 (−0.35 to 0.23); SMS −0.03 (−0.03 to 0.25) | Telephone 5579.69 per unit BMI avoided; SMS 2696.56 per unit BMI avoided | The value of the ICERs held with the sensitivity analysis. The sensitivity analysis suggested that taking a wider perspective with the inclusion of productivity losses increased the value of the ICER, but the ICER for SMS support remained more favourable than for telephone support when compared with usual care alone |
| Unclear whether either intervention is cost-effective without understanding of the threshold for health providers’ WTP for the prevention of BMI gain | ||||||
The evidence of cost-effectiveness for ‘breastfeeding only’ interventions in terms of incremental cost per QALY gained comes from one well-conducted model-based cost–utility analysis by Mavranezouli et al. 119 At a UK willingness-to-pay (WTP) threshold of £20,000–30,000 per QALY gained, the modelled intervention (+ standard care) was not considered cost-effective in comparison with standard care alone. Three evaluations118,126,133 have estimates of the cost per additional woman exclusively breastfeeding, which ranged from £67 at 5–12 days to £112 at 8 weeks and £2446 at 6 months postpartum. For the cost per additional woman breastfeeding (any), ICERs ranged from £108 at 8 weeks to £4226 at 6 months postpartum, the latter due in large part to a lower effect. However, without understanding of the threshold for health providers’ WTP for an additional woman breastfeeding, exclusively or any, it is unclear whether ‘breastfeeding only’ support is cost-effective.
The evidence of cost-effectiveness of ‘breastfeeding plus’ interventions in terms of incremental cost per QALY gained comes from two evaluations: one trial-based without extrapolation beyond study time frame of infant aged 1 year116 and a second trial- and model-based cost–utility analysis up to child aged 15 years. 130 At a UK WTP threshold of £20,000–30,000 per QALY gained, both interventions (+ standard care) were not considered cost-effective in comparison with standard care alone. None of the studies assessing ‘breastfeeding plus’ interventions estimated the incremental cost per additional woman breastfeeding. Additional ICERs related to the cost per unit BMI averted for interventions that had a broad aim of obesity prevention in children. One study154 provided an Australian WTP threshold of AU$500 (equivalent to £236 at 2012 prices), suggesting that these interventions are cost-effective.
Appraisal of limitations and uncertainty in the results
Methodological limitations were judged as minor,119 potentially serious116,129–131,155 or very serious. 118,126 The last set reflects that the studies were conducted to assess the effect of an intervention with a relatively short duration to support mothers to continue to breastfeed, with the alongside economic evaluation limited to the time horizon of the trial. Few health effects were measured and valued in the analysis, such as the costs of hospitalisations for infant morbidity, which would likely change the conclusions about cost-effectiveness. The time frames were short and reflect the duration of the intervention and the time horizon for the economic evaluation. Mavrnezouli et al. 119 was the only evaluation to model the costs and outcomes over the lifetime. While the model fell back on not having trial-based individual participant data for costing the intervention arms and using estimating baseline probabilities for breastfeeding sourced from England alone, the model parameters were comprehensive, with a wide range of conditions accounted for. The authors note some caution in the sources of model parameters; although priority was given to sourcing data from high-quality systematic reviews and meta-analyses or meta-regressions, the quality of the included studies in these reviews suggested a moderate to high level of risk of bias. Those studies judged to have potentially serious limitations tested the effect of ‘breastfeeding plus’ support. Four of these studies took a within-trial approach, not assessing costs and outcomes beyond the follow-up period. 116,129,131,155 Tan et al. 130 modelled intervention effect up to child aged 15 years; however, QALY estimates were based on the children’s weight status and the authors did not include healthcare resource use from birth to 5 years with the assumption that differences across groups were unlikely to affect conclusions about cost-effectiveness.
In terms of measures of uncertainty, where sensitivity analysis was reported the value of the ICERs held. 116,119,131 The remaining studies either did not handle uncertainty130,133 or made allowance for methodological uncertainty with scenario analyses from the base case. 118,126,129,155 These analyses suggested that incremental costs and ICERs were sensitive to change in alternative intervention costing scenarios, for example changing the costing method for staff time or the grade of staff delivering the service.
Consistency between studies
For ‘breastfeeding only’ support, there appeared consistency in the estimated ICERs for cost per additional woman breastfeeding (any or exclusive); however, without evidence of UK WTP thresholds for this outcome, it is unclear if the intervention would be considered cost-effective by health providers. Only one ‘breastfeeding’ support evaluation estimated the cost per QALY, which indicated an intervention that was unlikely to be cost-effective when compared with usual care.
There was less consistency between studies assessing ‘breastfeeding plus’ interventions. Studies that reported cost per QALY concluded that the interventions were not cost-effective. However, Barnes et al. 116 reported a negative ICER, as the intervention was more costly and less effective than the control, while Tan et al. 130 reported a positive ICER that exceeded the threshold value, similar to findings by Mavranezouli et al. 119 None of the studies in this category reported cost per additional woman breastfeeding as an outcome. This is as expected as most of the studies were obesity prevention interventions with a primary outcome of reducing BMI in children. The beneficial effect of breastfeeding (any or exclusive) for up to 6 months against obesity is recognised,156 hence the support for breastfeeding in these broader interventions. 130,131,154 There was less consistency between studies assessing ‘breastfeeding plus’ interventions.
Chapter summary
Thirty-nine studies were identified that conducted a partial or full economic evaluation of a breastfeeding support intervention for healthy women compared with a control. Nine of these studies were judged to be applicable or partially applicable to the UK setting. Of these, four assessed the cost-effectiveness of ‘breastfeeding only’ support and five assessed the cost-effectiveness of ‘breastfeeding plus’ support.
For 'breastfeeding only’ support, there was limited evidence that interventions were cost-effective. One model-based cost–utility analysis estimated that a hypothetical intervention providing six contacts with a health professional or lay person, starting in the antenatal period and continuing in the early postnatal period, was considered unlikely to be cost-effective in terms of cost per QALY gained (£56,075 per QALY gained). There was limited evidence for the incremental cost per additional woman breastfeeding (any or exclusive), with estimates from cost-effectiveness analyses ranging from £67 at 5–12 days to £2446 at 6 months postpartum. Without WTP thresholds, whether the interventions are cost-effective is unclear. Evidence for breastfeeding plus support was reported in two studies that modelled cost-effectiveness in terms of cost per QALY gained. Both studies identified the interventions as not value for money.
We judged that there was uncertainty in the findings of cost-effectiveness due to the limited number of studies and the lack of good-quality evidence. Limitations of the evaluations centred on a short time horizon, with seven out of nine studies not extrapolating beyond the time frame of the underlying effectiveness study, and a limit to the scope of costs and benefits measured. Five of the nine studies costed the intervention only and did not record the health service resource use of the mother or infant. These limitations suggest uncertainty in the findings. There appeared to be consistency between studies evaluating ‘breastfeeding only’ support in terms of cost per additional woman breastfeeding (any or exclusive). There was less consistency observed between studies assessing ‘breastfeeding plus’ interventions. These inconsistencies may be due to the different time horizons, differing scope of costs and benefits measured and valued and different outcomes of cost-effectiveness estimated, which make comparison difficult.
Chapter 6 Systematic review of interventions to support women with long-term conditions to breastfeed
Introduction
Women with LTCs face additional challenges in breastfeeding. The Cochrane review on breastfeeding support for healthy women with healthy term babies by its nature excludes women with LTCs. By receiving additional study funding, we were able to conduct an additional piece of work that looked at the effectiveness of breastfeeding support for women with LTCs.
Aim and objectives
The aim of this systematic review was to identify the effectiveness of breastfeeding support interventions in women with LTCs.
The objectives were to:
-
identify breastfeeding support interventions that have been designed for women with LTCs
-
describe the characteristics of breastfeeding support interventions –
-
provider
-
intensity of support
-
type of support (e.g. face to face, telephone, digital technologies, group or individual support, proactive or reactive)
-
additional intervention components (i.e. wider child and maternal healthcare)
-
timing of support (antenatal, postnatal)
-
-
determine the effectiveness of breastfeeding support interventions for women with LTCs.
Methods
This systematic review followed the methods for systematic reviews of interventions outlined in the Cochrane Handbook. 87 The protocol is registered on PROSPERO (CRD42022337239).
Eligibility criteria
Inclusion criteria
Types of studies
We included individually randomised and cluster-randomised controlled trials.
We excluded the following types of study designs: non-RCTs, quasi-experimental studies, one-group before-and-after studies, cohort studies, case–control studies, case reports and qualitative studies.
Participants
Studies were included if they included women with a long-term physical or mental health condition who were in one of the following groups: pregnant women, mothers who may initiate breastfeeding, and mothers who are breastfeeding. The LTCs included were based on the list developed as part of the MuM-PreDiCT study. 59
We also included women with gestational diabetes mellitus (GDM) as this group has a 10-fold increased risk of developing type 2 diabetes mellitus. 55 Moreover, women with GDM are less likely to breastfeed exclusively and face similar challenges to women with type 1 and type 2 diabetes, such as delays in lactogenesis, neonatal hyperglycaemia and increased rates of caesarean section. 157,158
Studies were also included if the intervention involved fathers and/or other caregivers in addition to mothers with a LTC.
Studies with mothers whose infants required additional care were also included.
We excluded studies that included only women without LTCs. However, we did include studies that included healthy women and women with LTCs, if the data on women with LTCs were reported separately.
Intervention
To be eligible for inclusion, breastfeeding support interventions had to be two-way between the supporter and the participant. They could include discussing the practical management of breastfeeding (e.g. attachment of the baby, identifying baby’s cues, issues around delayed lactogenesis, separation of mother and infant), symptom management and/or the use of medications when breastfeeding. They could include elements such as reassurance, praise, information and the opportunity to discuss and to respond to the mother’s questions.
We included interventions that were delivered by healthcare professionals and/or peers. Interventions could be delivered antenatally, postnatally or both. Interventions could be delivered in the community or in hospital. Finally, we included interventions that used any mode of delivery (e.g. face to face, telephone, digital technologies, SMS).
We did not include interventions that were purely educational and one-way (i.e. information from a provider with no opportunity for the women to respond).
Comparator
The comparator could be standard care or no breastfeeding support.
Types of outcome measures
We did not exclude studies based on their outcome measures. Our primary outcomes were:
-
number of women who stop any breastfeeding at 4–8 weeks
-
number of women who stop exclusive breastfeeding at 4–8 weeks
-
number of women who stop any breastfeeding at 6 months
-
number of women who stop exclusive breastfeeding at 6 months.
Additional outcomes were:
-
number of women who stop any breastfeeding at 3–4 months
-
number of women who stop exclusive breastfeeding at 3–4 months
-
breastfeeding initiation
-
maternal satisfaction with care
-
maternal satisfaction with feeding method
-
perinatal mental health indicators
-
infant and child morbidity and mortality including neonatal intensive care unit admissions.
Studies that did not measure any of the primary or additional outcomes were included in the review but did not contribute data.
Exclusion criteria
Types of studies
We excluded the following types of study designs: non-RCTs, quasi-experimental studies, one-group before-and-after studies, cohort studies, case–control studies, case reports and qualitative studies.
Participants
We excluded studies that included only women without LTCs (i.e. those that included general populations of healthy women). However, we did include studies that included healthy women and women with LTCs, if the data on women with LTCs were reported separately.
Intervention
We excluded interventions that were purely educational or health promotion and one-way (i.e. information from a provider with no opportunity for the women to respond).
Additional limitations
We did not exclude studies based on date of publication.
Abstracts were eligible for inclusion if they provided sufficient information to extract data. If they did not provide sufficient information, we contacted authors to try to obtain further information.
Studies published in either peer-reviewed journals or the grey literature were eligible for inclusion.
Due to resource constraints, only studies published in English were included.
Searches
Electronic databases
We searched the following databases in August 2022: MEDLINE (via Ovid), CINAHL (via EBSCOhost), MIDIRS (via Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL), PsycInfo (via Ovid) and EMBASE (via Ovid). Searches were based on the following four strings:
-
breastfeeding terms
-
support terms
-
LTC terms based on the list developed as part of the MuM-PreDiCT study59
-
RCT terms.
No limits were placed on language, date or publication type. An example MEDLINE search strategy is available in Appendix 1.
Additional searches
We searched the reference lists of included studies and systematic reviews identified in the search.
We also searched the list of excluded studies in the Cochrane review on breastfeeding support for healthy women with healthy term babies. 83
We also searched for grey literature through a targeted website search of relevant third-sector organisations.
Study selection
We imported all records identified via electronic databases into Covidence, a web-based collaboration software platform that streamlines the production of systematic and other literature reviews. 84 The title and abstract of each record were double-screened by two reviewers (AG, LH, SS, AMcF, FL, PB or FXV). If the two reviewers disagreed, consensus was reached via discussion by AG and LH. The same process was followed for full-text screening. The results of this selection process are shown in a PRISMA flow chart (see Figure 5).
FIGURE 5.
The PRISMA flow diagram illustrating study selection.
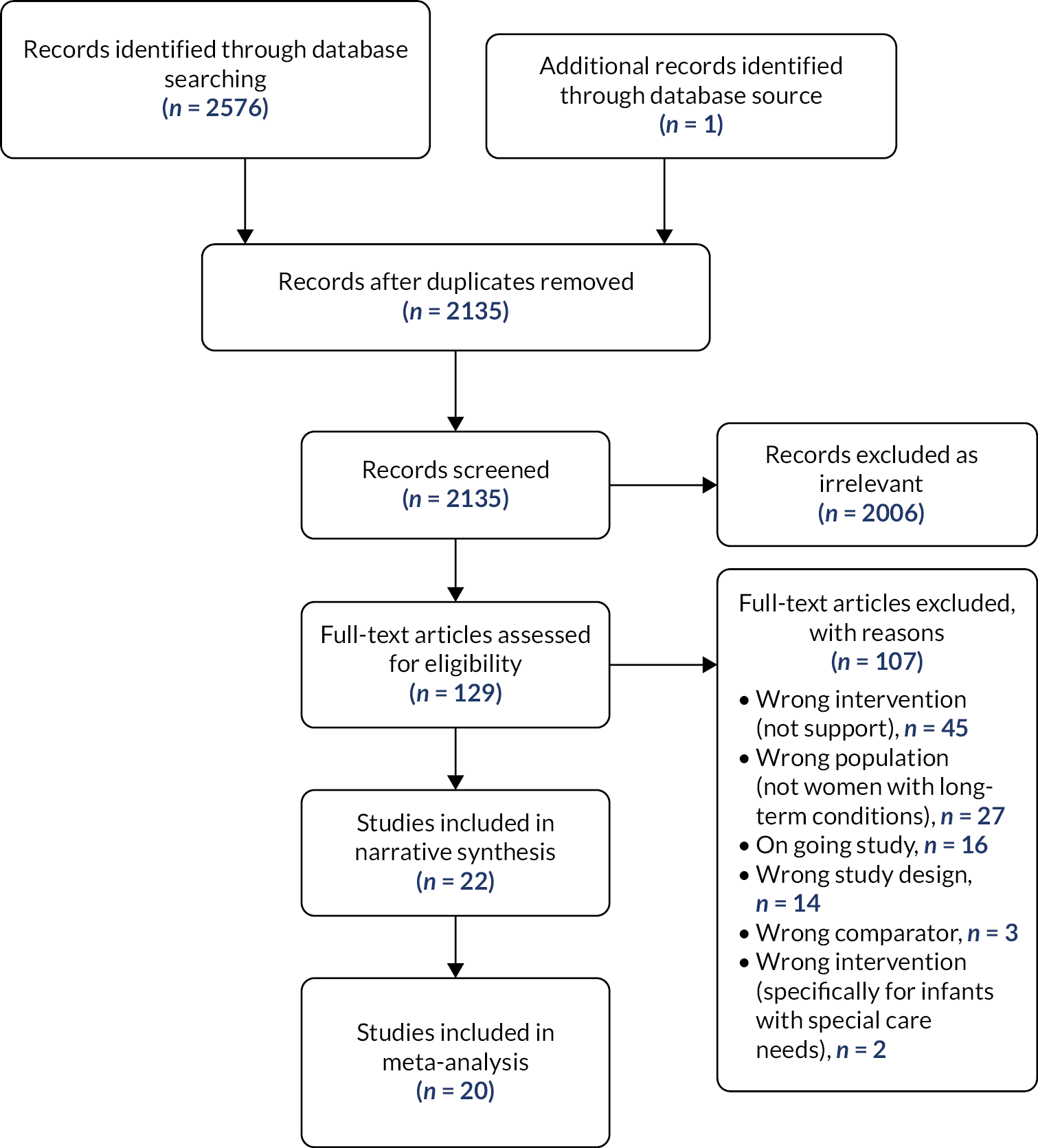
Data extraction and management
We used Covidence to manage information on study characteristics extracted from the study. Two review team members completed the data extraction template separately (AG, AMcF, FXV, PB, SC, SS). AG addressed any conflicts.
We used the template in Covidence to extract data on the following:
-
study details – methods (e.g. cluster or individually randomised trial), funder, conflicts of interest, dates of study, additional linked papers
-
participants – number of participants, description of their LTC, context and baseline characteristics (age, parity, ethnicity, education level, socioeconomic status, details of condition, delivery method)
-
intervention – details of person providing support, delivery method (e.g. face to face, telephone, digital), number of contacts, timing of support (e.g. antenatal, postnatal), description of intervention, theoretical basis.
AG extracted study outcome data into a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet and they were checked by a second reviewer (LH, SS). For the primary outcomes we extracted data on the number of women randomised to each group and the number of women who had stopped breastfeeding at each time point. Due to the high levels of heterogeneity in additional outcomes, we did not plan to do a meta-analysis with these data, and the findings of the individual study were extracted to a spreadsheet.
When study information was not available, we contacted study authors for further details.
Risk-of-bias assessment
We assessed risk of bias using the Cochrane Risk of Bias Tool 1 in Covidence. 159 Two review members conducted this independently (AG, AMcF, FXV, PB, SC, SS) and conflicts were addressed by AG.
Measures of treatment effect
All data for the main outcomes were dichotomous, and we presented the results as summary risk ratios with 95% CIs.
Unit of analysis issues
Cluster-randomised trials
Sample sizes were adjusted using the methods described in the Cochrane Handbook, incorporating an estimate of the intracluster correlation coefficient derived from the trial. 87 For one study there were insufficient data to calculate this adjustment, so we conducted sensitivity analyses to investigate the impact of including this study. 160
Trials with multiple arms
To avoid ‘double counting’ in studies involving one control group and two different intervention groups, we split the control group number of events and participants in half so that we could include two independent comparisons. 87
Dealing with missing data
Analyses were carried out, as far as possible, on an intention‐to‐treat basis (i.e. all participants randomised to each group were included in the analyses). We used one of the approaches in the Cochrane Handbook to deal with missing data,87 whereby all participants randomised were included as the denominator. For missing participants, we imputed an assumed worst‐case outcome (i.e. not breastfeeding). Sensitivity analyses were conducted to investigate the effect of excluding studies with high levels of attrition.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the tau-squared, I-squared and chi-squared statistics. We regarded heterogeneity as substantial if I² was > 30% and either τ² was > zero or there was a low p-value (< 0.10) in the chi-squared test for heterogeneity. The findings of this were interpreted in conjunction with a consideration of clinical heterogeneity (i.e. type of LTC, context, nature of support).
Assessment of reporting biases
For all outcomes where there were at least 10 studies, we generated funnel plots. We examined plots visually to assess if there was asymmetry that might suggest different treatment effects in smaller studies, which may suggest publication bias. 161 If there was funnel plot asymmetry in the presence of high levels of heterogeneity, we compared the findings of our random‐effects model with those of a fixed‐effect model. 162 If the random‐effects model showed a more beneficial effect, we considered this suggestive of the intervention being more effective in smaller studies. If it did not show a beneficial effect, we considered that asymmetry may be a result of high levels of heterogeneity.
Data synthesis
Statistical analysis of the main outcomes was performed using Review Manager 5.4 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). 163 As we anticipated some heterogeneity between studies in terms of the interventions and populations, we used a random-effects model. The appropriateness of combining different LTCs was considered in consultation with the study steering committee. It was agreed that this could be considered appropriate for the breastfeeding outcomes. The rationale for this is as follows. First, breastfeeding support was similar across the interventions, the exception being some of the support for women with HIV. This is because there is a risk of transmission of HIV to the child if breastfeeding is not exclusive, and mixed feeding must be avoided. 164 We explored the impact of this further via sensitivity analysis (see Sensitivity analysis). Second, there is multimorbidity between the conditions. For instance, antenatal depression has been reported to be associated with obesity,165 and obesity is a risk factor for GDM. 166 Finally, the prevalence of some of the LTCs is higher in areas of high socioeconomic deprivation,58 which may increase the similarity of the external factors influencing breastfeeding rates in the studies.
The results were presented as the average treatment effect with 95% CIs, and the estimates of τ² and I².
Subgroup analysis and investigation of heterogeneity
Due to the small numbers of studies for each outcome, we considered that subgroup analysis or meta-regression would not be meaningful. However, post hoc we considered that the studies with women with HIV were considerably different from studies with women with non-communicable diseases. This is because there is a risk of transmission of HIV to the child if breastfeeding is not exclusive, and mixed feeding must be avoided. 164 We therefore conducted a sensitivity analysis to assess whether or not the studies with women with HIV had biased the overall findings.
Sensitivity analysis
In addition to the sensitivity analysis that separated studies with women with HIV from studies with women with non-communicable diseases, we performed sensitivity analyses based on risk of bias. We first removed studies rated as being at high or unclear risk of bias for allocation concealment. We then removed studies rated as being at high or unclear risk of incomplete outcome data to assess the impact of attrition on our findings. As we had several cluster-randomised studies for which we could not calculate a design effect, we also conducted a sensitivity analysis to assess the impact of these studies on our findings.
Summary of findings
We assessed the certainty of the evidence using the GRADE approach for all main outcomes. 85 This approach considers study limitations, consistency of effect, imprecision, indirectness and publication bias. Evidence can be downgraded by one or more levels for issues in these domains. The findings of this process are reported in a summary of findings table (see Effects of interventions).
Results
Description of studies
Results of the search
The database search identified 2134 unique records, and we identified one study from the list of excluded studies in the Cochrane review on breastfeeding support for healthy women with term babies. We excluded 2006 of these based on title and abstract. We then reviewed 129 full texts, and, of these, 107 were excluded for the following reasons: not a breastfeeding support intervention (i.e. solely educational or health promotion and involved one-way contact with women, or no breastfeeding content), n = 45; not women with LTCs, n = 27; ongoing study, n = 16; wrong study design, n = 14; wrong comparator, n = 3; and intervention specifically targeted at infants in neonatal units, n = 2 (Figure 5). We searched MEDLINE and Google for results of any ongoing studies identified in our database search that we could not link to a study in Covidence. In total, 22 studies were included in the review. Several studies linked to additional references in Covidence (e.g. protocol papers, additional findings). For ease of reading, we have referred to just the main paper for each study within the text. We have included additional references in the table of characteristics (see Appendix 4, Table 17).
Included studies
Of the 22 studies included, 18 contributed data to the review. Four studies did not contribute data. First, Lewkowitz et al. 166 did not measure any of the breastfeeding outcomes included in the meta-analyses. Second, Fan et al. 167 did not provide the number of women with each condition randomised to each group. Third, Ijumba et al. 168 did not provide the raw data in a way that could be used in a meta-analysis. Finally, Martin et al. 169 did not report breastfeeding rates by intervention group. Thus, at least 5048 mother–infant pairs were included in the meta-analysis. Three studies provided only partial outcome data, as only some of the relevant outcomes were reported in a way that could be used in a meta-analysis. 170–172
A summary of the included studies is presented in Appendix 4, Table 17.
One study reported on two separate interventions: BIBS 1 and BIBS 2. 173 BIBS 1 investigated the effectiveness of breastfeeding support from a lactation counsellor and we therefore included it. However, BIBS 2 compared the effectiveness of electric and manual breast pumps, which does not meet our eligibility criteria, and we have therefore not included it.
The majority of studies were conducted in the following HICs: the USA (n = 8),160,166,172–177 Australia (n = 3),167,169,178 Denmark (n = 1),179 Ireland (n = 1)180 and the UK (n = 1). 181 A further five studies were conducted in the following upper-middle-income countries: South Africa (n = 3),168,182,183 China (n = 1)184 and Colombia (n = 1). 185 Two studies were conducted in lower-middle-income countries: Kenya (n = 1)186 and India (n = 1). 187 Only one study in a lower-income country was identified: Uganda (n = 1). 171
Methods used in trials
Most studies were individually randomised two-arm trials (=14). Six studies used cluster-randomised designs to compare two interventions. 160,168,182,183,186,187 We were unable to adjust for clustering in one of these studies. 160
Two studies were three-arm studies. For one study we included both interventions. 171 For the other study we included one intervention arm and used the other intervention as the control arm, as it did not contain breastfeeding content and women in the intervention group also received it in addition to their breastfeeding support. 169 We therefore included 23 interventions in the review.
Participants
Long-term conditions
Nineteen of the studies specifically included women with a LTC. However, three studies included both women with and women without a specific LTC and reported findings for these separately. First, two cluster-RCTs included women with and without HIV and analysed these data separately;168,186 however, data from one of these studies were not presented in a way that allowed us to include them in the meta-analysis. 168 Another study analysed breastfeeding rates separately in women with obesity or depression. 167 However, as the denominators were not reported in this conference abstract, we could not include the study in our meta-analysis.
The most common condition was overweight and obesity, with nine interventions focused on this. 166,169,172–174,176,179,180,185 The BMI score required for inclusion ranged from 25 to 30 kg/m2. A further three studies focused on GDM. 160,175,184 With the exception of one,185 these studies were conducted in HICs.
Substance misuse was the focus of two studies. 178,181 Only one study specifically included breastfeeding support for women with depression. 177
In LMICs, HIV was the most common condition, with five studies focused on this. 168,171,182,183,187 All women included in these studies received treatment with antiretrovirals.
Socioeconomic status
Four of the studies aimed at women with overweight or obesity were specifically targeted at low-income women. 166,172,174,176
A further five studies mainly included women who experienced higher levels of socioeconomic deprivation than the national average. 160,169,171,173,178
Parity
Of the 13 studies that reported parity, all included primiparous and multiparous women. No study had an exclusion criterion relating to parity. Rates of primiparity ranged from 15% to 57%.
Interventions
Interventions varied in how much content was directed at breastfeeding support. Breastfeeding support was the sole focus of six interventions for women with overweight/obesity. 167,173,174,180,184,185
Other studies provided additional components to help with the LTC of interest. All the interventions aimed specifically at HIV-positive women included other aspects of prevention of mother-to-child transmission. 171,182,183,186,187 Four studies that focused on either women with GDM or those who were overweight or obese also provided weight loss support (e.g. diet and exercise). 160,169,175,176 Finally, one study of women with depression also provided cognitive–behavioural therapy for management of depression.
Several other studies included additional components to support the following: maternal well-being;176–178 aspects of infant well-being such as growth and immunisations;172,178,179,181,182 and wider parenting skills such as sleep and activities. 166,172,176
Provider
Half of the included studies used an intervention that was provided either exclusively or in part by a lactation consultant160,167,169,172,173,175,177,179,180,184 or certified breastfeeding consultant. 185 Only a few studies involved support from other healthcare professionals, including midwives,178,180 nurses173 and maternity support workers. 181 Two studies of obese women also included dietitian support. 169,175
Ten studies included some form of non-healthcare professional support, which may or may not have been combined with professional support. This mainly took the form of support from trained community members. 166,168,172,174,183,186,187 In two studies it involved online peer support from other breastfeeding mothers with GDM or obesity. 176,184 In another study it involved a family member or friend being nominated as supporter. 171 These studies tended to be conducted in LMICs or areas of socioeconomic deprivation in HICs. 176,184
Several studies also involved a combination of healthcare professional and non-healthcare professional support and the professional’s role tended to focus on training or facilitating sessions. 172,176,182
Mode of delivery
Most studies included at least some face-to-face support. Ten studies only used face-to-face support. 168,169,171,172,178,181–183,186,187 Five studies used a combination of face-to-face and telephone support. 160,170,173–175 Often the calls were used to provide reactive additional support for women with difficulties.
Three studies used a combination of digital, telephone and face-to-face support. In two studies the digital element took the form of online support groups. 167,176 One study was conducted during the COVID-19 pandemic and so face-to-face group clinics were replaced with video calls (or individual face-to-face appointments).
Only two studies used the telephone as the sole delivery mode. 167,179 One study only used a digital approach, which included online lessons, video calls and messaging. 177
Timing of delivery
Most interventions were delivered in both the antenatal and the postnatal periods. 160,168,169,172–177,180,182–187 Five studies were postnatal only167,171,178,179,181 and one study was antenatal only. 166
Number of contacts
We tried to group intervention intensity as low intensity (three or fewer contacts), moderate intensity (four to eight contacts) or high intensity (nine or more contacts).
Intensity levels
Just over half of the interventions were judged to be of moderate intensity. 167–169,171–173,178,181–185 However, a number of these interventions also offered reactive support as required so the number of contacts may have been greater. Conversely, we judged eight of the interventions to be high intensity. 160,174–177,179,180,186 This may be an overestimation, as for some breastfeeding was not the sole focus or it depended on women engaging with digital content such as support groups. No studies were low intensity and two did not specify. 166,187
Control group care
Most studies compared the intervention with standard care. 167,171–175,179–181,183–187 However, there are considerable differences in what constituted standard care between the studies, for example the provision of lactation consultants or peer supporters or care in a baby-friendly hospital (see Appendix 4).
In four studies the comparator was a non-breastfeeding intervention designed to promote other aspects of infant or maternal health such as weight loss or maternal mental health. 168,170,176,177
Finally, two studies compared the breastfeeding support intervention with limited breastfeeding support. 166,178
Risk of bias in included studies
See Appendix 5, Table 18, for a summary of our risk-of-bias assessments.
Random sequence generation (selection bias)
Most studies were rated as being low risk for this domain (n = 18). Four studies did not provide sufficient information. 167,173,178,183
Allocation concealment (selection bias)
We judged only eight studies as being at low risk of allocation concealment. 169,172,176,178–180,184,185 One study was judged to be high risk. 181 No other studies provided sufficient information, so we judged them as unclear.
Blinding of personnel and participants (performance bias)
Owing to the nature of the intervention, it was not possible to blind participants and/or personnel, so we judged all studies as being at high risk of bias.
Blinding of outcome assessment (detection bias)
As all breastfeeding data were all self-reported by mothers, we judged 21 of the studies as being at high risk of bias in this domain. We judged one cluster-RCT to be at unclear risk of bias as it would potentially have been possible to blind the women in a cluster to allocation, but it is not clear if it was the unblinded service providers who collected the data. 187
Incomplete outcome data (attrition bias)
Half of studies were judged as being at high risk of attrition bias, which we defined as loss to follow-up of > 20%. 160,169,172,174,177,180–183,185,187 Nine studies were judged as having a low risk of bias in this domain. 166,168,171,173,175,176,179,184,186 Finally, two studies were judged to be at unclear risk of bias. One study had higher attrition in the control group (12%) than in the intervention group (4%) and no details were provided. 178 A second, Fan et al. ,167 provided insufficient information to make a judgement.
Selective outcome reporting (reporting bias)
We judged most studies (n = 15) as being at unclear risk of bias for this domain. The primary reason was that studies did not have a published protocol for us to assess this. The remaining seven studies were judged as being at high risk of bias for the following reasons: not reporting outcomes detailed in protocol or methods;160,172,182 not fully reporting outcomes;174 not stating when breastfeeding would be measured;175 and adding in breastfeeding outcomes post hoc. 166,179
Other biases
We judged only two studies to be at low risk of bias in this domain. 166,175 Eleven studies were judged as being at high risk of bias for one or more of the following reasons: insufficient information to adjust for clustering;160 baseline imbalance;172,173,176,182,183 industry funding/support;173,176,182 financial conflicts of interest;187 loss of clusters;182 issues with intervention implementation;173 and reporting errors. 172 For the remaining nine studies there was insufficient information to judge this domain.
Effects of interventions
Table 7 presents the summary of findings for the primary outcomes. The forest plots for all primary and additional breastfeeding outcomes are presented in Appendix 6, Figures 9–15. We have also included tables with the data from the sensitivity analyses (see Tables 19–24) and the funnel plots for studies with at least 10 studies in Appendix 6, Figures 16 and 17.
| Outcomes | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with usual care | Risk with breastfeeding support | ||||
| Not any breastfeeding at 4–8 weeks | 339 per 1000 | 305 per 1000 (261 to 359) | RR 0.90 (0.77 to 1.06) | 1385 (10 RCTs) | +++⃝ Moderateb |
| Not exclusive breastfeeding at 4–8 weeks | 686 per 1000 | 631 per 1000 (570 to 707) | RR 0.92 (0.83 to 1.03) | 2165 (10 RCTs) | ++⃝⃝ Lowb,c |
| Not any breastfeeding at 6 months | 513 per 1000 | 425 per 1000 (343 to 518) | RR 0.83 (0.67 to 1.01) | 1018 (6 RCTs) | +++⃝ Moderateb |
| Not exclusive breastfeeding at 6 months | 820 per 1000 | 779 per 1000 (730 to 820) | RR 0.95 (0.89 to 1.00) | 3206 (12 RCTs) | +++⃝ Moderatec |
Primary outcomes
Stopping any breastfeeding at 4–8 weeks
Ten studies with 1385 participants measured stopping any breastfeeding at 4–8 weeks. 160,172,173,175,177–181,184 Breastfeeding support interventions probably have little to no impact on the number of women stopping any breastfeeding at 4–8 weeks (RR 0.90, 95% CI 0.77 to 1.06; moderate-certainty evidence). There was no evidence of any significant statistical heterogeneity (τ² = 0.01, I² = 16%, χ² = 10.71, p = 0.30). See Appendix 5, Figure 9.
A sensitivity analysis using only studies assessed as having low risk of bias for allocation concealment found very similar effect estimates. A sensitivity analysis excluding studies rating as being at low risk of attrition bias changed the direction of the findings; however, the 95% CI widened and still crossed the line of no effect (RR 1.02, 95% CI 0.62 to 1.67). Similarly, a sensitivity analysis excluding studies with cluster-RCTs, for which we could not calculate a design effect, found similar effect estimates to the main analysis. No studies in this analysis included interventions for women with HIV. See Appendix 5, Table 19.
An assessment of publication bias via funnel plot inspection suggested possible asymmetry; however, given the small number of studies, we would interpret this with caution. See Appendix 5, Figure 15.
Stopping exclusive breastfeeding at 4–8 weeks
Ten studies with 2165 participants measured stopping exclusive breastfeeding at 4–8 weeks. 160,172–174,177,179,180,184,186,187 Breastfeeding support interventions probably have little to no impact on the number of women stopping exclusive breastfeeding at 4–8 weeks (RR 0.92, 95% CI 0.83 to 1.03; low-certainty evidence). There was evidence of substantial statistical heterogeneity (τ² = 0.01, I² = 53%, χ² = 19.32, p = 0.02). See Appendix 5, Figure 10.
A sensitivity analysis using only studies assessed as having low risk of bias for allocation concealment and low risk of attrition bias found similar effect estimates; however, the 95% CI widened. Similarly, a sensitivity analysis excluding studies with interventions for women with HIV found similar effect estimates. Excluding the cluster-RCT for which we could not calculate a design effect changed the effect estimate and 95% minimally (RR 0.94, 95% CI 0.84 to 1.06). See Appendix 5, Table 20.
An assessment of publication bias via funnel plot inspection suggested possible asymmetry; however, given the small number of studies and substantial levels of heterogeneity, we would interpret this with caution. See Appendix 5, Figure 16.
Stopping any breastfeeding at 6 months
Five studies reporting on six interventions in studies with 1018 participants measured stopping any breastfeeding at 6 months. 171,175,178,180,184 Breastfeeding support interventions probably have no impact on the number of women stopping any breastfeeding at 6 months (RR 0.83, 95% CI 0.67 to 1.01; moderate-certainty evidence). There was no evidence of any significant statistical heterogeneity (τ² = 0.00, I² = 0%, χ² = 1.21, p = 0.98). See Appendix 5, Figure 11.
Sensitivity analyses using only studies assessed as having low risk of bias for allocation concealment and for attrition widened the 95% CI. A sensitivity analysis excluding interventions for women with HIV found very similar effect estimates and 95% CIs. There were no cluster-RCTs for which we could not calculate a design effect. See Appendix 5, Table 21.
An assessment of publication bias via funnel plot inspection was not possible because of the small number of studies.
Stopping exclusive breastfeeding at 6 months
Eleven studies171,174,176,178–180,182–184,186,187 reporting on 12 interventions in studies with 3206 participants measured stopping exclusive breastfeeding at 6 months. Breastfeeding support interventions probably have little to no impact on the number of women stopping exclusive breastfeeding at 6 months (RR 0.95, 95% CI 0.89 to 1.00; moderate-certainty evidence). There was evidence of substantial statistical heterogeneity (τ² = 0.01, I² = 84%, χ² = 67.87, p < 0.00001). See Appendix 5, Figure 12.
Sensitivity analyses using only studies assessed as having low risk of bias for allocation concealment and for attrition both widened the 95% CI. Sensitivity analyses excluding interventions for women with HIV found very similar effect estimates and 95% CIs. There were no cluster-RCTs for which we could not calculate a design effect. See Appendix 5, Table 22.
An assessment of publication bias via funnel plot inspection suggested possible asymmetry; however, given the small number of studies and high levels of statistical heterogeneity, we would interpret this with caution. See Appendix 5, Figure 17.
Additional outcomes
We have divided the additional outcomes into breastfeeding and non-breastfeeding outcomes. The additional breastfeeding outcomes were analysed via meta-analysis, and the forest plots are available in Appendix 6. There was considerable heterogeneity in the non-breastfeeding additional outcomes and how they were measured. Meta-analysis was therefore not appropriate, so a narrative summary is provided instead.
Additional outcomes: breastfeeding
Not initiating breastfeeding
Eight studies166,169,173,176–180 with 903 participants measured not initiating any breastfeeding. However, studies varied considerably in their definition of breastfeeding initiation (e.g. within 1 hour, within 24 hours, before discharge, or ever). In addition, some interventions did not commence until breastfeeding had been initiated. This led to some studies having higher rates of breastfeeding at 4–8 weeks than at initiation, which was nonsensical. A post hoc decision was therefore made to exclude this outcome from the review.
Stopping any breastfeeding at 3–4 months
Four studies173,177,180,184 with 522 participants measured stopping any breastfeeding at 3–4 months. Breastfeeding support interventions probably have little to no impact on the number of women stopping any breastfeeding at 3–4 months (RR 0.86, 95% CI 0.53 to 1.38; low-certainty evidence). There was evidence of substantial statistical heterogeneity (τ² = 0.14, I² = 68%, χ² = 9.29, p = 0.03). See Appendix 5, Figure 13.
A sensitivity analysis using only studies assessed as having low risk of bias for allocation concealment and low risk of attrition bias found similar effect estimates; however, the 95% CI widened. No studies in this analysis included interventions for women with HIV or cluster-RCTs for which we could not calculate a design effect. See Appendix 5, Table 23.
An assessment of publication bias via funnel plot inspection was not possible because of the small number of studies.
Stopping exclusive breastfeeding at 3–4 months
Five studies171,175,178,180,184 with six interventions and 785 participants measured stopping exclusive breastfeeding at 3–4 months. Breastfeeding support interventions may have a beneficial effect on the number of women exclusively breastfeeding at 3–4 months (RR 0.77, 95% CI 0.59 to 1.00; low-certainty evidence). There was evidence of substantial statistical heterogeneity (τ² = 0.06, I² = 76%, χ² = 20.89, p = 0.0009). See Appendix 5, Figure 14.
A sensitivity analysis using only studies assessed as having a low risk of bias for allocation concealment widened the 95% CI (RR 0.70, 95% CI 0.48 to 1.02). Removal of the one study with HIV-positive women widened the 95% CI marginally (RR 0.77, 95% 0.59 to 1.01). Conversely, removal of studies at low risk of attrition bias showed a more beneficial effect estimate and narrower 95% CI (RR 0.60, 95% CI 0.46 to 0.80). There were no studies for which a design effect could not be calculated. See Appendix 5, Table 24.
An assessment of publication bias via funnel plot inspection was not possible because of the small number of studies.
Additional outcomes: non-breastfeeding
Fifteen of the included studies non-breastfeeding outcomes between intervention and control groups. We grouped these into the following categories: infant outcomes (seven studies); maternal physical health (six studies); maternal mental health (four studies); maternal satisfaction with feeding method (one study); and measured maternal satisfaction with care (one study).
Infant outcomes
The most frequently measured outcome was infant growth (six studies). Five studies were focused on overweight/obesity or GDM and the aim was to reduce infant weight at follow-up. No differences between intervention and control groups were found in any of these studies. Three studies used weight-for-length or -age z-scores. More specifically, Aldana-Parry et al. 184 calculated scores at 4 months and found no difference between intervention and control groups (0.75 ± 1.3 vs. 0.65 ± 1.7; p = 0.76). Similarly, Reifsnider et al. 172 found no difference in scores at 12 months between intervention and control groups (0.72 ± 1.13 vs. 0.84 ± 1.20, p = 0.66). Fiks et al. 176 reported there was no difference in weight-for-length z-scores (raw data not provided). Carlsen et al. 179 measured infant weight at 6 months and found no differences between the intervention and control groups (8169 g ± 963 vs. 8356g± 959; p = 0.18). Similarly, an additional paper for the study by Steube et al. 188 found no difference in infant length, weight, BMI percentile, biceps circumference or triceps skinfolds at any time point.
However, in LMIC settings where low weight is the concern, intervention infants were more likely to have a slightly larger increase in weight-for-age score between 2 and 12 months [odds ratio (OR) 1.08; p = 0.035]. 183
Only one study,178 which was an intervention for women with substance misuse, measured rates of immunisations at 2, 4 and 6 months and found no differences between the groups at any time point (p = 0.757, p = 0.477, p = 0.283).
Only one study measured rates of hospital admissions and childhood infectious diseases. Chapman et al. 174 found beneficial effects in terms of infant hospitalisations in the intervention compared with the control in the first 3 months (10% vs. 26%; p = 0.03) and 6 months after birth (11% vs. 28%; p = 0.03). There were also higher rates of diarrhoea in control infants at 6 months but not at 3 months (details not provided). There was no difference in rates of otitis media or attendance at the emergency department.
One study that examined support for HIV-positive women included infant mortality as an outcome and did not identify any differences between intervention and control groups (aOR 1.6, 95% CI 0.37 to 6.91). 187
Maternal physical health outcomes
Four studies focused on overweight/obesity or GDM included maternal weight as an outcome and did not identify any differences between intervention and control groups (note that in some studies the comparator included a weight loss component). All studies measured maternal weight using different methods. Aldana-Parry et al. 185 compared the mean maternal weight loss between the first week postpartum and 4 months and found no difference between the intervention (1.9kg ± 4.7 kg) and the control (4.2kg ± 5.1 kg; p = 0.07). Similarly, in the DEBI study,160,188 there were no differences between intervention and control groups in weight, BMI, and skinfolds at 6 weeks, 4 months, 7 months or 10 months. The intervention group had a slightly smaller waist circumference at 7 months than the control group (104.70cm vs. 115.60 cm; p = 0.046). However, there was no difference at any other time points. A linked paper to Ehrlich et al. 189 reported that although women with GDM in the intervention group had higher rates of meeting their postpartum weight loss goals than controls at 6 weeks (20.9% vs. 17.4%; p = 0.54), 7 months (38% vs. 23.9%; p = 0.13) and 12 months (37.5% vs. 21.4%), none of these reached statistical significance. Martin et al. 169 reported no difference in BMI between the intervention and the control group at 3 months (30.6 ± 5.4 vs. 30.7 ± 4.1; p-value not reported) or 6 months (31.2 ± 4.4 vs. 30.6 ± 4.3; p-value not reported).
Two studies included measures related to blood sugar levels and found no differences between groups. The DEBI study188 found no differences in fasting insulin and 2-hour glucose at any time points. Similarly, Martin et al. 169 found no differences in HBA1c, insulin or glucose levels at any time points.
Two studies included maternal physical activity as an outcome and found no differences between groups. The DEBI study188 found no difference in levels of physical activity at 6 weeks (p = 0.92) or 7 months (p = 0.91). Fiks et al. 176 also included number of periods of physical activity per week as an outcome measure and found no difference between intervention and control groups at 6 months (2.2 vs. 2.0; p > 0.05).
Two studies188,189 included measures related to diet (percentage of calories from dietary fat) and found a small reduction in the intervention group compared with the control group at 7 months (8.04% vs. 7.47%; p = 0.002). 189 This was not significant at 6 weeks (7.44% vs. 8.02%; p = 0.54). The DEBI study188 included 23 variables related to diet, which were measured at 6 weeks, 4 months, 7 months and 10 months. There were differences in only four of these, and all with the exception of water consumption favoured the control group: sweetened beverages at 6 weeks (intervention 79.49% vs. control 53.85%; p = 0.03); drinking water at 4 months (intervention 78.57% vs. control 47.83%; p = 0.76); fast food (intervention 88% vs. control 52%; p = 0.01); and using fat for cooking (intervention 100% vs. control 77.78%; p = 0.04).
One study178 measured maternal substance use using the Opiate Treatment Index and found similar scores in the intervention and control groups for the following: heroin (0.22 vs. 0.04; p = 0.084), other opiates (2.0 vs. 0.14; p = 0.72), cannabis (2.0 vs. 1.9; p = 0.56), amphetamines (0.15 vs. 0.11; p = 0.99), benzodiazepines (1.0 vs. 1.5; p = 0.74); alcohol (0.21 vs. 0.36; p = 0.22) and cigarettes (10 vs. 12; p = 0.52). A higher score suggests more use. Findings were similar at 6 months.
Finally, one study187 that provided support for women with HIV measured maternal mortality and found no difference between the intervention and control groups (aOR 0.58, 95% CI 0.23 to 1.34).
Maternal mental health outcomes
Two studies included depression as an outcome, with mixed findings. First, in a study183 for HIV-positive women, women in the intervention group had a larger decrease in depressed mood by 12 months than women in the control group (OR 1.08; p = 0.002). However, Pezley et al. 177 provided cognitive–behavioural therapy for the management of depression and anxiety to both intervention and control groups. Depression scores and anxiety scores remained consistent with baseline in the third trimester and at 6 and 12 weeks postpartum (significance levels not reported).
Two studies included a measure of stress. In a support intervention176 for women with obesity, parental stress was included as an outcome and scores were similar between intervention and control groups (30.2 vs. 29.6; p > 0.05).
The DEBI study188 for women with GDM included stress management as an outcome and found no difference between the groups at 6 weeks, 4 months, 7 months or 10 months.
Maternal satisfaction with feeding method
Only one study measured the mother’s satisfaction with feeding method. In a study with women with obesity, Lewkowitz et al. 166 asked participants if they would be likely to breastfeed again were they to have another child, and there was no difference between the intervention and control groups (RR 1.03, 95% CI 0.86 to 1.25).
Maternal satisfaction with feeding method
Only one study measured satisfaction with care. MacVicar et al. 181 examined support for women receiving opioid substitution and reported that the intervention group felt more satisfied with the support received (mean 9.6 vs. 6.8). However, the number of participants was very small (n = 11) and significance was not tested.
Strengths and limitations
We followed systematic review methods outlined in the Cochrane Handbook;87 however, there is the potential that bias was introduced into the review. First, because of resource constraints we were able to include only studies published in English, so there is a risk of language bias. Second, although we attempted to identify all published and unpublished trials on breastfeeding support for women with LTCs, it is possible that not all existing trials were identified. Funnel plot analyses suggested some possible asymmetry; however, interpretation is limited by the small number of studies. Third, we were unable to adjust for clustering in one of the studies; however, a sensitivity analysis from which that study was removed did not change the effect estimate. Fourth, there was considerable variability in how breastfeeding initiation was measured (e.g. within 24 hours vs. ever), so a post hoc decision was made to exclude this outcome from the meta-analysis. Finally, there is heterogeneity between the studies, which may be a result of differences between interventions and population characteristics, in particular the LTCs.
Chapter summary
Twenty-two studies were identified that examined the effectiveness of breastfeeding support for women with single LTCs. Of these, 20 contributed data to the review. No studies were identified that included women specifically with MLTC.
The most common conditions were overweight and obesity, with nine studies focused on this. A further three studies were for women with GDM. Five studies included women with HIV. Two studies were for women with substance misuse problems and only one was for women with anxiety and depression. Interventions varied in terms of whether they only provided breastfeeding support or if they also provided support for the LTC. The majority of studies had an antenatal component.
We performed meta-analysis for all the primary and additional breastfeeding outcomes. There was little to no difference between intervention and controls for any of these. We judged these outcomes to be low and moderate certainty. When we used a sensitivity analysis to exclude interventions for women with HIV, there was no meaningful change in the effect estimates. We considered the overall risk of bias in the included trials to be mixed. Sensitivity analyses excluding studies rated as being at high or unclear risk of bias for allocation concealment and attrition also did not alter the effect estimates.
Fifteen studies measured secondary non-breastfeeding outcomes, which included infant weight, infant health, maternal weight and health behaviours, satisfaction with care and satisfaction with feeding method. Because of the heterogeneity in outcomes, meta-analysis was not possible and so results were reported narratively. There was little evidence of any beneficial intervention effect on any of the secondary outcomes measured.
To conclude, this review identified that the breastfeeding support interventions for women with LTCs probably had little to no effect on breastfeeding outcomes. There is, therefore, a need for further research to develop breastfeeding support interventions for women with LTCs.
Chapter 7 Systematic review of views and experiences of breastfeeding support for women with long-term conditions
Introduction
As part of our additional funding for MLTC, we sought to complement the evidence on effectiveness from review 4 (see Chapter 6) by undertaking a mixed-methods review looking at what is known about the views and experiences of breastfeeding support in women with LTCs.
Objectives
-
To identify and synthesise the views and experiences of those involved in delivering and receiving breastfeeding support for women with LTCs.
-
To identify the contextual factors (barriers/facilitators) affecting the implementation of breastfeeding support for women with LTCs.
Methods
The protocol for this systematic review is registered on PROSPERO (CRD42022374509).
Search strategy
A comprehensive search strategy was developed employing combinations of search filters, free-text words and index terms relating to breastfeeding support and LTCs. Terms relating to LTCs were derived from the list of LTCs published in the MuM-PreDiCT study. 59 We included permutations and variations of search terms, and no limits were placed on date or language.
The following bibliographic databases were searched for primary studies in October 2022: MEDLINE, EMBASE, CINAHL, PsycInfo and MIDIRS. Citations and references in all included papers and any relevant reviews identified were screened for eligible primary studies. This review was conducted in parallel with a systematic review that aimed to identify the effectiveness of breastfeeding support interventions for women with LTCs (see Chapter 6). Therefore, we conducted additional searches to identify any papers related to the interventions included in that review. We also searched reference lists of included studies and searched websites of organisations related to key conditions (e.g. Diabetes UK, Crohn’s and Colitis UK, Epilepsy Action).
Eligibility criteria
Inclusion criteria
Studies were included if they reported qualitative and/or quantitative findings of primary research exploring the views and experiences of breastfeeding support for women with LTCs, including breastfeeding women and babies and their families, service providers, managers, commissioners and policy-makers.
Qualitative and quantitative studies, either standalone or in mixed-methods designs, were included.
Long-term conditions are defined according to the list published as part of the MuM-PreDiCT study, in addition to others such as GDM that are not included in the MuM-PreDiCT study. However, mothers with GDM can face some similar issues to women with type 1 or type 2 diabetes when breastfeeding (e.g. neonatal hypoglycaemia, delayed lactogenesis, preterm birth).
Studies were included that reported any type of experiences relating to breastfeeding support in women with LTCs. This included breastfeeding support that is delivered/received in any setting (e.g. in hospital, at home or in the community). This may be formal or informal support that has been provided as part of a breastfeeding support intervention, routine care or in the context of women’s personal support networks, including any subjective participant-reported outcomes and constructs such as attitudes, views, beliefs, perceptions, understandings or experiences.
There were no restrictions on publication date.
Exclusion criteria
We excluded articles only reporting on impact evaluation results of breastfeeding support interventions (i.e. effectiveness of interventions).
We excluded studies that included only women without LTCs (i.e. those that included general populations of healthy women).
Due to resource constraints, only studies published in English were eligible for inclusion.
Selection process
Two reviewers independently screened titles, abstracts and relevant full texts against the predetermined eligibility criteria. Any discrepancies were resolved through discussion and consultation with a third reviewer.
Data extraction and quality appraisal
Data extraction was undertaken independently by two reviewers using a piloted data extraction form. Any discrepancies were resolved through discussion and consultation with a third reviewer.
We assessed the quality of qualitative studies and qualitative components of mixed-methods studies using the Critical Appraisal Skills Programme (CASP) tool. 190 We used the Axis tool to assess the quality of cross-sectional surveys. 191 Quality assessments were conducted by one reviewer and checked by a second reviewer (AG or AmcF). Consensus was reached through discussion. No studies were excluded from the review because of poor quality.
Data synthesis
We adopted a mixed-methods synthesis approach. We first undertook two preliminary syntheses of quantitative (synthesis 1) and qualitative (synthesis 2) studies, and then integrated qualitative and quantitative data into a cross-study synthesis (synthesis 3).
For synthesis 1 (qualitative studies) we used an inductive approach to thematic synthesis to synthesise qualitative findings from included studies. 91 This involved three overlapping and interrelated stages: (1) line-by-line coding of findings from primary studies; (2) categorisation of codes into descriptive themes; and (3) development of analytical themes to describe or explain previous descriptive themes. To ensure the robustness of the synthesis, various techniques to enhance trustworthiness were undertaken, including audit trail, multiple coding, reviewer triangulation and team discussions. For synthesis 2 (quantitative studies) we used narrative methods to synthesise quantitative findings from included studies,90 tabulating characteristics of included quantitative studies and developing a conceptual framework to organise the included quantitative studies. The overall data synthesis (synthesis 3) brought together quantitative and qualitative findings from primary studies included in syntheses 1 and 2. First, the conceptual frameworks developed in both syntheses were compared and combined into a comprehensive framework to characterise the views and experiences of breastfeeding support in women with LTCs across multiple contexts/settings. Second, the qualitative and quantitative findings from syntheses 1 and 2 were integrated using the resulting framework. Two reviewers independently reviewed the categorisation of findings and refinements were discussed in review team meetings until a consensus was achieved and the final synthesis results were established.
Results
The searches identified 5058 records, which were assessed against the inclusion criteria. Title and abstract screening resulted in 119 records considered eligible or inconclusive. Full-text articles were then retrieved and assessed for eligibility. Three records could not be retrieved. Of the 116 records screened at full text, 92 were excluded. The main reason for exclusion was not reporting views and experiences of breastfeeding support (e.g. views and experiences of breastfeeding) (n = 37), followed by involving study designs not eligible for inclusion in this review (e.g. effectiveness studies) (n = 22). Other reasons for exclusion were abstract-only records (e.g. conference proceedings) (n = 19), focusing on ineligible populations (n = 8), not reporting on views and experiences (n = 5), and language of publication not being English (n = 1). The remaining 24 studies were included in the final synthesis (see Figure 6).
FIGURE 6.
The PRISMA flow diagram.
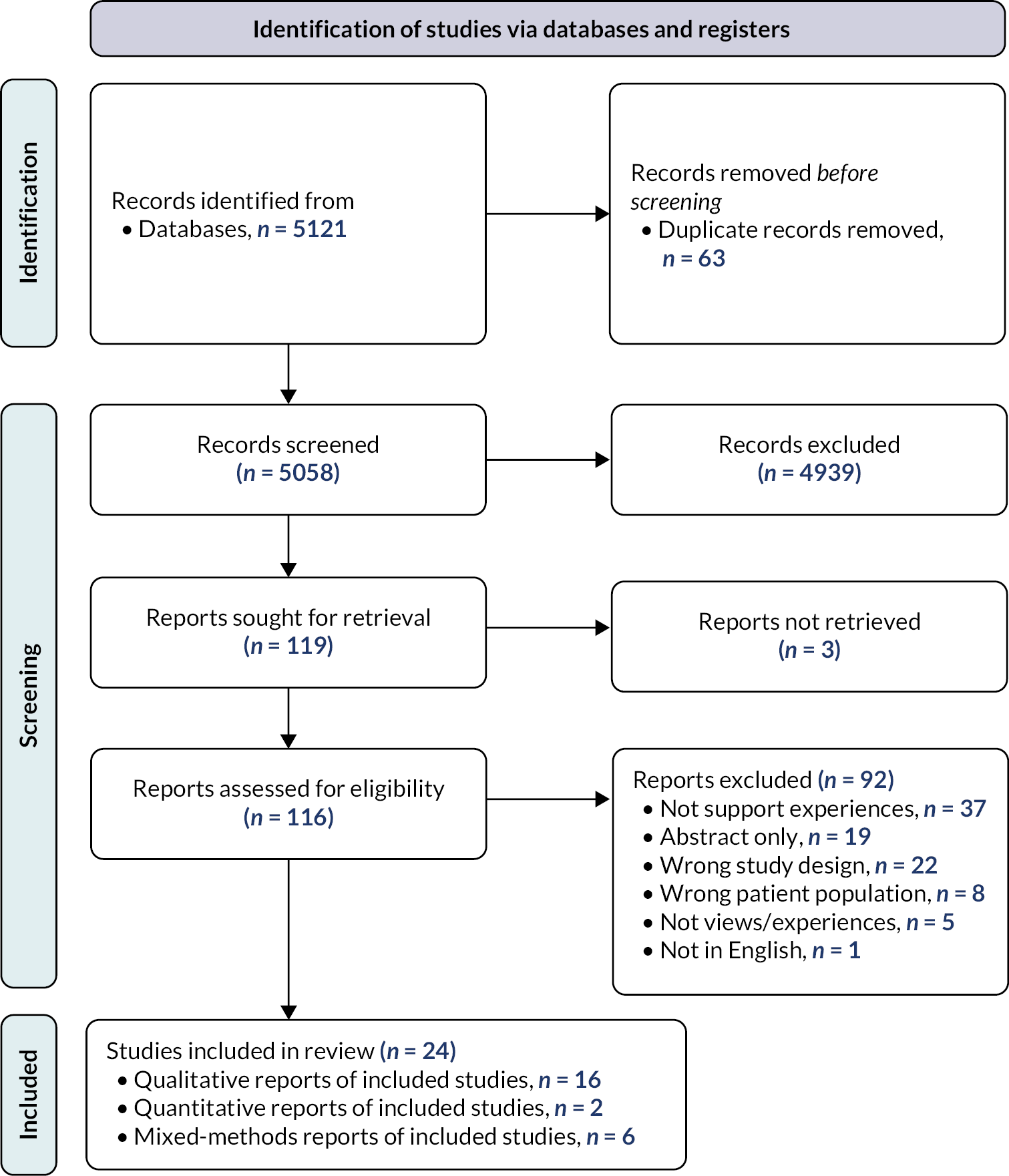
Summary of included studies
A summary of key characteristics of included studies is presented in Appendix 6, Table 25.
Twenty-four studies contributed qualitative data to the synthesis, comprising 16 qualitative,192–206,209 two quantitative208,210 and six mixed-methods181,207,211–214 studies. Studies reported data from 12 countries (Australia, Canada, Ghana, Ireland, Japan, Kenya, Malawi, South Africa, Uganda, UK, USA and Zambia). Study samples in intervention groups ranged from 6 to 296 participants. Study settings included hospitals, community settings and population-based studies. Long-term conditions covered were HIV (eight studies),192,197,199,203–205,212,213 obesity and overweight (five studies),195,196,201,206,210 substance use (five studies),181,194,198,202,207 diabetes in pregnancy (three studies),200,208,214 disabilities (two studies)193,209 and a rare genetic disorder (one study). 211 The eight studies of HIV-positive women were all from LMICs, while studies of women with all other conditions were all from HICs.
Quality appraisal
We assessed 16 qualitative studies192–206,209 and the qualitative components of 5 mixed-methods studies. 181,211–214 Four cross-sectional surveys were assessed. 207,208,210,211 The quantitative components of four mixed-methods studies181,212–214 did not provide data relevant to our review and were not assessed for quality.
The quality of qualitative studies was mixed. Although all studies had clear objectives for which qualitative methodology was appropriate, the specific study design was not always explained or justified (see Appendix 6, Table 26). Three studies192,196,213 provided full details of methods for recruitment, data collection and rigorous analysis; most other studies had at least partially addressed these aspects. Three studies199,211,212 provided insufficient information to assess the rigour of data analysis. O’Reilly et al. 206 was the only study that adequately considered the relationship between researchers and participants. Most studies confirmed ethics approval and at least partially discussed ethical issues. Andrews et al. 193 failed to report ethics approval and provided no discussion of ethical issues other than the use of consent forms. Howard et al. 198 stated that their study was exempt from Institutional Review Board approval without giving reasons and did not discuss any ethical issues. All but one study199 at least partially addressed the credibility and transferability of findings.
The quality of the cross-sectional surveys was weak with poor reporting (see Appendix 6, Table 27). Laws et al. 211 and Rasmussen et al. 210 provided very little information with which to assess quality and did not address key quality criteria. Matsunaga et al. 208 was the only survey for which the sampling strategy was clear, although the low response rate raised concerns about non-response bias. None of the studies used previously tested or published instruments/measurements. Ethics approval was reported for all surveys except Rasmussen et al. 210 Three studies207,208,211 discussed some limitations of their studies, but only Matsunaga et al. 208 presented conclusions that were justified by the results.
Stakeholders’ perceptions and experiences
Stages 1 and 2 of our mixed-methods synthesis resulted in the categorisation of primary quantitative and qualitative data from included studies into 70 descriptive themes. Building on these findings, further analytical work was undertaken to develop analytical themes, resulting in four overarching analytical themes: (1) additional breastfeeding support needs for mothers with LTCs; (2) the availability of breastfeeding support for mothers with LTCs; (3) the role and practice of breastfeeding support for mothers with LTCs; and (4) suggested strategies to improve breastfeeding support for mothers with LTCs. The four themes are described below. Appendix 6, Table 28, illustrates the distribution of primary studies underpinning each analytical theme and provides exemplar data extracts from primary studies.
Additional breastfeeding support needs for mothers with long-term conditions
Included studies highlighted a range of challenges that breastfeeding mothers with LTCs face, which are compounded by more general individual, social and cultural challenges commonly reported as faced by all breastfeeding mothers.
Challenges of specific relevance to breastfeeding mothers with LTCs reported in included studies comprised issues relating to mother and infant health conditions and treatments; stigma, misconceptions and misinformation; and emotional distress.
Health-condition-related barriers included a range of concerns and difficulties with breastfeeding due to the mother’s condition or treatment,196,198,201,208,209 as well as concerns and difficulties relating to any conditions or medical interventions needed for the infant. 200,207,208 These circumstances, either mother or infant related, could also be associated with hospital episodes and hospital stays (e.g. admission to critical care), which raised additional barriers and difficulties in terms of breastfeeding; for example, in one study length of infant hospital stay was inversely correlated with breastfeeding duration.
Concerns relating to stigma, misconceptions and misinformation about the interplay of illness, treatment and breastfeeding (e.g. perceptions of breast milk safety while taking antiretroviral medicine) were reported in several studies. 194,197,198,203,204 These experiences could result in women feeling pressure to stop breastfeeding and adopt other feeding options, with the potential for abrupt weaning and breast complications. 197,198,203,204 In some contexts, breastfeeding practices were reported as more driven by financial or family pressures than by health information. 203
The emotional implications of breastfeeding challenges experienced by those living with a LTC were reported in several studies. 196,205,211,212,214 These impacts included difficulties with contact and bonding,198,201 which were associated with treatment and recovery, birth complications, and mothers’ histories of abuse and trauma. Some of these emotional implications translated into some effects on mothers’ self-efficacy,198 with a few studies reporting issues associated with perceived breast milk insufficiency193,197,200,209 and latching. 193, 209 One study found that emotional comorbidity was linked to perceived failure to breastfeed,211 and two other studies reported that women with LTCs were less likely to fully breastfeed214 and more likely to breastfeed for a shorter duration212 than mothers in the general population.
Availability of breastfeeding support for mothers with long-term conditions
Several studies reported variable or insufficient availability of breastfeeding support for mothers with LTCs,194,200,206,207,209,211,213 particularly when taking multiple healthcare settings beyond maternity care into consideration. For example, one study213 found that health professionals in a mother-to-child HIV transmission programme infrequently advised women on breastfeeding (41% of visits), and in another study207 only 23.3% of women reported that healthcare staff at an opioid-dependence treatment centre had discussed breastfeeding with them. Alongside insufficient breastfeeding support, some of these studies also reported that women perceived a lack of or limited information from professionals or available in hospital settings. 194,199,200,208,209
Health professionals’ training and knowledge on specific issues and risks to breastfeeding success for women with LTCs and their infants can be limited, and not necessarily seen to warrant a tailored approach to breastfeeding support. 203,210,211 Conversely, one study found that specialist breastfeeding clinics were perceived as useful by women; however, these were found to be underused. 202 The hospital environment can be a source of both support and tension for breastfeeding mothers with LTCs,198 and a range of organisational barriers were reported,208 including lack of resources (staffing and time) for breastfeeding support; competing in-hospital systems and policies that hinder the promotion of breastfeeding; and lack of continuous interprofessional support system, particularly following discharge and in terms of collaborating and co-ordinating with other facilities. However, how supportive of breastfeeding hospital settings are perceived to be may depend on women’s own feeding choices; for example, one study found that women who breastfed for shorter amounts of time or not at all were more likely to report that the hospital encouraged breastfeeding. 207 More generally, postnatal care experiences may also influence maternal attitude to and receptiveness of breastfeeding support, particularly on aspects of care that relate to privacy and confidentiality. 181,201
The role and practice of breastfeeding support for mothers with long-term conditions
The experiences of breastfeeding support of mothers with LTCs reported in the included studies involved a wide range of interactions, individuals, settings and factors that could align to impact (positively or negatively) the complex journeys of breastfeeding mothers with LTCs.
Some studies reported a range of positive interactions with breastfeeding supporters,181,192,193,200,202,207 including several strategies and forms of support that had enabled women to successfully breastfeed, such as adaptations (e.g. adapted positioning), equipment/aids (e.g. use of breast pumps), physical assistance from others (e.g. physical help with positioning) and access to peer support (e.g. women with the same health condition). There were examples of positive breastfeeding support accounts that highlighted the element of psychological and emotional support embedded in breastfeeding support. 181,202,205 One study found that women who were encouraged to breastfeed by healthcare staff were more likely to breastfeed for longer durations. 207
Most studies, however, reported support experiences shaped by a range of negative interactions (e.g. communication difficulties)181,192,193,200,202,209 as well as barriers faced by breastfeeding supporters. Breastfeeding support could sometimes be overshadowed by condition-related support. 203,212 The provision of breastfeeding support for women with LTCs was described as requiring more time and effort and being more challenging personally and in terms of competence. 196,208 Some studies reported that breastfeeding supporters lacked specialist training,194,199,203,210 with some women not feeling well understood by health professionals211 and reporting that trust in health professionals as a source of advice was an important factor. 213 Persistent barriers could hinder the effectiveness of breastfeeding support interventions; for example, one study found that several barriers remained after participation in a peer counselling intervention to promote exclusive breastfeeding, which contributed to a preference for mixed feeding. 204
Several studies reported issues relating to perceived pressures or biases in favour of certain feeding options. This was identified in a range of directions: one study reported that women perceived an intense pressure to breastfeed and felt like breastfeeding was characterised as the only acceptable choice, which led to expressions of fear and anxiety about not being able to breastfeed successfully. 193 In another study, health professionals reported that encouraging mothers to practise exclusive breastfeeding was a policy directive, and concluded that mothers were not given an opportunity to weigh the pros and cons of other feeding options;197 there were also examples where avoiding breastfeeding was promoted as the ideal option. 212 Other studies identified inconsistent and inaccurate messaging about complementary199,213 and mixed204,214 feeding options; and some studies identified encouragement of formula supplementation, which some women associated with difficulties in establishing breastfeeding. 200,201,206
Information and knowledge provision was reported as one key aspect of breastfeeding support to help empower informed maternal feeding decisions. 198,206,212 However, within the healthcare community, women obtained information and misinformation about breastfeeding in the context of their health condition. 194,199,200,202,208,209 The understanding of the perceived benefits of breastfeeding was reported as an important driver of successful breastfeeding support,194,212 which could in turn drive the motivation,194 determination,206 self-confidence195 and resilience198 needed to breastfeed in the context of living with an LTC.
Suggested strategies to improve breastfeeding support for mothers with long-term conditions
Studies echoed a range of suggestions from participants regarding potential strategies for improving breastfeeding support, with the most widely reported suggestion being the need to acknowledge the role and influence of other sources of support (e.g. partners, family, friends, peers, external professionals, web-based resources) and involve them in the provision of breastfeeding support. 192,194,195,200,201,205–209,213,214 Another important suggestion was to increase the provision of education and raise awareness among health professionals196,206,210,211 to improve their understanding of the specific breastfeeding support needs of mothers with LTCs and to help them identify feeding problems earlier. One study sought women’s views202 and feasibility tested181 a proposed set of intervention components (including practical skills, emotional support, availability of accurate and accessible information, individualised support provision and a low-stimuli environment) with positive results. Another suggestion for improvement reported across several studies was that breastfeeding support for women with LTCs should be established early on antenatally and carried on postnatally, ensuring continuity and consistency throughout. 196,197,200,214
Chapter summary
This review comprised 24 studies reporting primary research on the views and experiences of breastfeeding women with LTCs and/or support providers.
The health conditions covered were HIV-positive, obesity and overweight, substance use, diabetes in pregnancy, disabilities and a rare genetic disorder. The overall quality of included studies was mixed, with some studies rated as weak and/or with poor reporting.
Four key themes were identified: (1) additional breastfeeding support needs for women with LTCs; (2) availability of breastfeeding support for mothers with LTCs; (3) the role and practice of breastfeeding support for mothers with LTCs; and (4) suggested strategies to improve breastfeeding support for mothers with LTCs.
Included studies highlighted a range of additional support needs for women with LTCs, such as issues relating to treatments or medical interventions for women’s/infant’s health conditions, misconceptions, misinformation or emotional distress. Studies reported variable or insufficient availability of breastfeeding support for mothers with LTCs, particularly when support was needed across multiple healthcare settings beyond maternity care. The data suggest complex breastfeeding journeys involving a wide range of interactions, individuals, settings and factors that could impact women’s experiences.
Chapter 8 Review of economic evidence for women with long-term conditions
Overview
The previous two chapters reported a systematic review identifying (1) which interventions were effective in providing breastfeeding support for women with single LTCs, and (2) the barriers to and facilitators of breastfeeding support to women with LTCs. This chapter builds on this evidence by assessing how well breastfeeding support interventions for women with LTCs work in relation to how much they cost health services. As evidence was expected to be limited, a systematic review of economic evidence was planned to appraise and synthesise what is known about the cost-effectiveness of breastfeeding support interventions for mothers with LTCs.
Aim and objectives
The aim of this review of economic evidence was to gain an understanding of whether breastfeeding support interventions for mothers with LTCs were considered value for money. The overarching review question was: What are the incremental costs and cost-effectiveness of breastfeeding support interventions in comparison to standard care, no intervention, or an alternative intervention for mothers with LTCs? The review objectives were to:
-
identify and synthesise the evidence base for incremental costs and cost-effectiveness of breastfeeding support interventions
-
assess the applicability of the evidence to a UK setting
-
identify limitations of and uncertainties in the applicable economic evaluations
-
examine the level of consistency between applicable economic evaluations.
Methods
Eligibility criteria
The methods for conducting the systematic review of economic evidence followed those reported in Chapter 5, with guidance on searching for economic evidence and conducting reviews of economic evidence adhered to,87,110–112 along with the PRISMA 2020 statement for reporting systematic reviews. 113 The eligibility criteria mirrored those for the systematic review of evidence of effect for women with LTCs, reported in Chapter 6, in terms of the population, intervention and comparator. 113 For the population, studies were included if they related to pregnant women with long-term physical or mental health conditions considering or intending to breastfeed or mothers who were breastfeeding. For the intervention criterion, studies were included if they involved contact with professional(s) or volunteer(s) offering support that was supplementary to the standard care offered in that setting. The support could include elements such as reassurance, praise, information and the opportunity to discuss and to respond to the mother’s questions. Interventions could be provided in the antenatal or postnatal period or both. In relation to the comparator criterion, studies were included if the comparison received standard care, an alternative intervention or no comparator.
The outcomes of interest for the review included the health effects recorded for the corresponding systematic review of effect (any and/or exclusive breastfeeding), as well as any outcomes associated with supporting women with LTCs to breastfeed that were selected and measured within the economic evaluation. These included, but were not limited to, health-related quality of life and healthcare resource use. Economic outcomes of interest were those that were selected, measured and valued, such as incremental costs (cost savings), ICERs, net benefit ratios and QALYs. Finally, the types of studies included were full economic evaluations (cost-effectiveness, cost–benefit and cost–utility analyses) and partial economic evaluations (cost–consequences analyses, cost analyses, cost description). Economic analyses excluded from the review were non-comparative studies such as cost-of-illness studies, as it was considered that the objectives and results of these study designs would not align with the review question.
Search strategy
The search strategy developed for the systematic review of economic evidence reported in Chapter 5 was used for this review. In brief, this encompassed three domains, (1) breastfeeding, (2) support and (3) costs/economics, under which relevant index terms and text words were identified and collated. It was decided that search terms related to LTCs would not be included in the search, as records returned without this domain were manageable for screening. The domain of costs/economics made use of the search filter for economic studies used by the Scottish Intercollegiate Guidelines Network, which was adapted from the search filter designed by the NHS Centre for Reviews and Dissemination at the University of York. Within each domain, search terms were combined with the Boolean operator ‘OR’, and then across domains with the Boolean operator ‘AND’. An example of the list of search terms used for one of the bibliographic database searches can be found in Appendix 1. The full search strategies are available from the corresponding author on request.
Five electronic bibliographic databases were searched using all three search domains: MEDLINE via Ovid, EMBASE via Ovid, CINAHL via EBSCOhost, HMIC via Ovid and MIDIRS via Ovid. Electronic databases for economic literature were searched with a modified search syntax without the need for the search filter for economic studies: American Economic Association’s electronic bibliography (EconLit) via EBSCOhost, NHS EED, Paediatric Economic Database Evaluation (PEDE), IDEAS economics database via RePEc and EconPapers via RePEc. A modified search syntax relating to all three domains was developed and used with the following search engines: ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, the Virginia Henderson International Nursing Library (VHL), GreyNet International and OISter. No language or date restrictions were applied, other than those inherent in each database; for example, NHS EED contains economic evaluations of health and social care interventions published between 1994 and the end of 2014.
The search was last updated on 18 August 2022. Reference lists of systematic reviews identified during the search and reference lists of eligible studies were consulted to identify any relevant studies missed from the database searches. In addition, eligible studies were forward searched using the ‘cited by’ tab in Google Scholar. This process was completed in November 2022.
Selection process
Returned records from database searches were transferred into the reference management software EndNote version 20.3 (Clarivate Analytics, Philadelphia, PA, USA) and duplicate records were removed. All unfiled references were then screened for eligibility for inclusion. Two reviewers independently screened titles and abstracts against the inclusion criteria. All potentially relevant records were brought forward for the full-text sift. During the full-text sift, two reviewers independently read all full papers and reports to assess for eligibility. Any conflicts were discussed, and consensus was reached. Any unresolved conflicts were discussed with the broader project team for final consensus to be reached. Reasons for exclusion at this stage were recorded, with reasons for exclusion at full-text screen noted. A PRISMA flow diagram was completed to illustrate the selection process.
Data extraction and quality assessment
All studies eligible for inclusion were progressed to data extraction and quality assessment. Two review authors independently extracted and recorded data in Microsoft Excel using the data extraction form developed for the review reported in Chapter 5. Items extracted included the type of economic evaluation, perspective taken, currency, price year, year of conversion, time horizon, discount rate, data sources, model assumptions, measurement of uncertainty, consideration of heterogeneity, sensitivity analyses, base-case results in terms of incremental costs, cost-effectiveness and/or net-benefit estimates, where available. Data were summarised in tabular form for each included study.
Quality assessment of the economic evaluations was conducted using the checklist provided by NICE,111 which is separated into two sections. Section 1 assesses the applicability of each included study to the review question. Those judged directly or partially applicable progress to section 2, which assesses the limitations of the economic evaluation. For section 1, economic evaluations were reviewed independently by two authors and rated as directly applicable, partially applicable or not applicable. Disagreements were resolved by discussion until consensus was reached. For those judged to be directly or partially applicable, section 2 was completed, again independently by two authors. Section 2 allowed for an overall assessment of the methodological quality of the studies, judging them to have minor limitations, potentially serious limitations or very serious limitations. Quality assessments for each section were summarised in tabular form.
Synthesis methods
Economic evidence profiles were created for those studies deemed directly or partially applicable, with limitations and uncertainty summarised for each study, along with incremental costs, incremental effects and ICERs. In terms of the estimates of costs extracted from individual studies, these were adjusted to GBP 2022 prices using the Campbell and Cochrane Economics Methods Group – EPPI-Centre Cost Converter web-based tool, which was created by the Campbell and Cochrane Economics Methods Group and is available at https://eppi.ioe.ac.uk/costconversion/. A narrative synthesis summarised the characteristics and results of the applicable economic evaluations. Inconsistency between results of economic evaluations were considered, with the potential impact of including methodologically weak studies explored as part of the narrative synthesis.
This review of economic evidence was not registered; however, the review protocol can be accessed via the repository held by Queen’s University Belfast Research Portal (https://pure.qub.ac.uk/).
Results
Study selection
Figure 7 presents the PRISMA flow diagram for the study selection process. Following the removal of duplicate records, 5732 records were screened at the title and abstract stage. Of these, 5713 were excluded and the full text of 19 records were sought. One record, which was an ongoing study, could not be retrieved (Jacobson, 2020). Of the 18 records screened at full text, 13 were excluded. The main reason for exclusion was the wrong study design (n = 7), followed by wrong population (n = 4) and wrong intervention (n = 2). The systematic search, identification and screening process resulted in five studies eligible for inclusion.
FIGURE 7.
The PRISMA 2020 flow diagram for review of economic evidence for breastfeeding support interventions for women with LTCs.
Study characteristics
An evidence table of the five economic evaluations identified for inclusion is presented in Appendix 7, Table 29. Each evaluation is described in terms of the setting, intervention, comparator and participant characteristics. Detailed methods of economic analysis are provided, along with a summary of results and the judgment of applicability to the review question. Of the five studies, one was conducted in a UK setting and included women with a BMI of > 25kg/m2. 215 Two were conducted in OECD settings of the USA: one that addressed women–infant dyads with prenatal use of opioids216 and a second that presented data for a subgroup of medically high-risk women. 125 The remaining two studies were conducted in South Africa, addressing support for women living with HIV. 217,218
Three studies assessed ‘breastfeeding only’ support interventions. 216–218 Avram et al. 216 assessed the short-term intervention of rooming-in following birth in hospital to support women to breastfeed their infants with neonatal opioid withdrawal. Desmond et al. 217 assessed an intervention to promote exclusive breastfeeding through home and clinic visits from late pregnancy to 6 months postpartum, which were delivered by a lay breastfeeding counsellor. Maredza et al. 218 assessed three infant-feeding strategies to prevent mother-to-child transmission of HIV, which included a strategy of actively supporting breastfeeding with extended nevirapine prophylaxis for 12 months. ‘Breastfeeding plus support’ interventions were assessed in two of the five evaluations, with a broader programme of weight management at 8–16 weeks postpartum,215 and doula support during pregnancy and up to 8 weeks postpartum. 125
A range of methods were used for the economic evaluations. One study reported a partial economic evaluation alongside a feasibility RCT, with a cost-outcome description comparing two alternatives. 215 Full economic evaluations were reported in the remaining four studies, with one study reporting a trial- and model-based cost-effectiveness analysis assessing cost per increased month of exclusive breastfeeding,217 one reporting a trial-based cost–benefit analysis with the return on investment,125 and one study reporting a model-based cost–utility analysis with incremental cost per disability-adjusted life-year averted reported, respectively,216 and a model-based cost–utility analysis with cost per QALY gained. 216
Evidence of cost-effectiveness
Of the four studies that conducted a full economic evaluation, all judged the breastfeeding support interventions assessed for the base case to be cost-effective at given WTP thresholds, when cited,216–218 or reported a positive return on investment. Avram et al. ,216 in assessing rooming-in to support mothers to breastfeed their infant with neonatal opioid withdrawal, concluded that the intervention led to reduced costs and increased effects. The cost savings were largely due to the reduced need for pharmacotherapy from an increase in breastfeeding with rooming-in. When the sensitivity of the ICER was tested to a change in the risk ratio of need for pharmacotherapy, the ICER held.
In assessing peer counselling breastfeeding support for women living with HIV, Desmond et al. 217 calculated ICERs for a range of intervention scenarios. While the base case was considered cost-effective in terms of cost per increased month of exclusive breastfeeding, the ICER was sensitive to a change in the intensity of the intervention. Moving from a basic scenario to a simplified and full scenario increased the intervention cost; however, it was balanced by an increase in effect. The most efficient scenario in terms of cost per increased month of exclusive breastfeeding was judged to be the simplified scenario that combined clinic and home visits. Maredza et al. 218 similarly modelled the cost–utility of various infant-feeding strategies for women living with HIV compared with current practice. The provision of breastfeeding support for those living in an urban setting was a dominant intervention and considered cost-effective in terms of cost per disability-adjusted life-year averted. However, the ICER did not hold in a one-way sensitivity analysis for a range of modelled study parameters. Those living in a rural setting and provided with breastfeeding support had lower estimated costs than those receiving current practice; however, this was offset by an increase in the number of HIV infections.
Mottl-Santiago et al. 125 recruited women from low-income communities and subsequently conducted a subgroup analysis to consider heterogeneity in the results of the return on investment for doula support. The author reported a higher return on investment (US$276:$1) when assessing doula support during pregnancy up to 8 weeks postpartum for women considered medically high risk, compared with full sample’s return on investment (US$18:$1) at 2018 prices. However, the evaluation did not consider health resource use and costs (cost savings) beyond the study time horizon.
Applicability
In terms of the applicability criteria assessed, all the studies fulfilled the criteria of the study population and intervention being relevant to the review question. Two studies were judged not applicable because the system in which the studies were conducted was too different from the UK context, making it difficult to translate findings of cost-effectiveness. 217,218 Two further studies were deemed not applicable due to the payer perspective taken for the costing of the intervention and/or healthcare resource use in an organisational setting that is too diverse from the UK NHS and Personal Social Services. 125,216 Failing to meet these criteria for applicability to the UK would likely change the conclusions about cost-effectiveness; thus, they were excluded from further consideration. One study219 was applicable in terms of the country setting (UK) and the provider perspective taken of the NHS and Personal Social Services; however, with the aim of assessing the feasibility of collecting economic data, the findings were not applicable to the review question to understand the incremental costs and cost-effectiveness of breastfeeding support interventions for mothers with LTCs compared with a control. If at a future date the study progressed to a full trial and conducted a cost–utility analysis as planned, the findings would likely be judged applicable.
Appraisal of limitations
None of the included studies progressed to section 2 of the quality assessment process, to judge study limitations and uncertainty in results, because of their lack of applicability to the UK system and context.
Strengths and limitations
To the best of our knowledge, this is the only systematic review of economic evidence on breastfeeding support interventions for women with LTCs, and it has identified a lack of evidence on incremental cost and incremental cost-effectiveness that is applicable to a UK setting.
We followed methods recommended for identifying, assessing and reviewing economic evidence;87,110,112,220 however, there is a potential for bias. While we attempted to identify all published and unpublished economic evaluations on breastfeeding support for women with LTCs, it is possible that not all studies were identified.
Chapter summary
Five studies were identified that examined the incremental cost and/or cost-effectiveness of breastfeeding support interventions for women with LTCs compared with a control or provided a cost–outcome description. The conditions assessed in the studies were HIV, obesity, prenatal opioid use and medically high risk (maternal hypertension and diabetes prior to birth). Interventions provided only breastfeeding support or also provided support for the LTC or provided care across the continuum. Each of the interventions assessed in the full economic evaluations was deemed cost-effective for the base case. On appraisal, none of the studies was judged to be applicable to the system and context of the UK.
Chapter 9 Co-creating a toolkit for implementation and evaluation of breastfeeding support interventions
Aims
The final stage of the research aimed to develop and refine a toolkit for the implementation and evaluation of effective breastfeeding interventions relevant to the UK, based on all evidence and stakeholder input from the previous work. An additional aim was to elicit stakeholders’ preferences in terms of WTP for a breastfeeding support intervention. This stage of the research included both the main study and additional work for women with LTCs. See Appendix 8 for the draft toolkit.
Methods
The co-creation of the toolkit built on the findings of the evidence syntheses and stakeholder engagement, as described in the previous chapters, as follows:
-
effective breastfeeding support interventions for healthy women with healthy term babies from the updated Cochrane review83
-
barriers to and enablers of implementing breastfeeding support derived from synthesising process evaluations of effective interventions (see Chapter 4)
-
barriers to implementation and strategies to overcome them derived from the main study stakeholder engagement (see Chapter 2)
-
key challenges for women with multimorbidities when accessing support for breastfeeding and for healthcare providers in offering support derived from the MLTC stakeholder engagement (see Chapter 2).
The next stage of developing the toolkit involved a wider group of stakeholders via co-creation workshops. The workshop activities revolved around a prototype breastfeeding support intervention drawn from elements of interventions from the Cochrane review. 83 The interventions that informed the prototype were selected as they were effective in reducing the number of women stopping breastfeeding and were judged to be at low risk of bias using allocation concealment as a proxy for this. This prototype is a composite of the characteristics of these seven interventions and together they provide a range of the ways breastfeeding support could effectively be implemented. 103,221–226
Prototype intervention
The breastfeeding support intervention will be delivered one-to-one by infant-feeding advisors. It consists of one 30-minute antenatal appointment, one 30-minute postnatal visit in hospital, one 30-minute home visit within 48 hours of discharge and regular telephone calls. The antenatal session will focus on rapport building, education and identifying any concerns regarding breastfeeding. The hospital and discharge visits will involve checking latch, helping with positioning and observing a feed if requested by the mother. Infant-feeding advisors will also provide encouragement and reassurance during visits. Women will be given the chance to ask questions and raise any concerns.
Following the initial three contacts, support will be provided remotely unless a face-to-face visit is required. For the first 4 weeks there will be a weekly proactive telephone call, and beyond that support will be provided monthly until 3 months or when breastfeeding ceases. Women can also contact infant-feeding advisors as needed via telephone or SMS during this 3-month period and beyond as new issues arise.
The infant-feeding advisor will also signpost women to the local breastfeeding peer support group, which provides support via WhatsApp and weekly face-to-face support groups and/or one-to-one peer support service. Infant-feeding advisors will receive training on the intervention delivery.
Workshops
Four 1-day workshops were held in November 2022 in Belfast, Birmingham, Cardiff and Edinburgh, representing the four nations of the UK. We aimed to include up to 30 participants in each workshop representing four key groups: (1) service users and their representatives, including third-sector advocacy organisations and lay/peer supporters; (2) health services including frontline practitioners (e.g. midwives, health visitors, doctors, lactation consultants, support workers), and service managers and commissioners; (3) national and local policy-makers, including government bodies, and public health and social care organisations; and (4) academic researchers. Invitations were disseminated via the research team’s networks, members of the stakeholder working groups and third-sector organisations with a focus on participants who represent or work with communities where breastfeeding rates are low to maintain the focus on inequalities.
Workshop participants
There were 87 participants across the 4 workshops, and all sectors were represented as shown in Table 8, although there was no policy-maker at the Cardiff workshop. The health service participants included midwives, health visitors, lactation consultants, infant-feeding co-ordinators/leads and support workers. Health service participants made up the largest group, followed by service users/third-sector organisations. There were relatively few policy-makers. Participants were not all from the country in which the workshop was held. It can also be noted that the balance of participants at each workshop was different. For example, at the Edinburgh workshop the largest group of participants was parents and third-sector organisation representatives, whereas at the other three workshops the largest group was health services staff. Each workshop was facilitated by members of the research team.
| Belfast | Birmingham | Cardiff | Edinburgh | Total | |
|---|---|---|---|---|---|
| Service user/third sector | 3 | 4 | 5 | 13 | 25 |
| Health services | 13 | 10 | 12 | 7 | 42 |
| National/local policy-makers | 3 | 1 | 0 | 1 | 5 |
| Academics | 2 | 2 | 3 | 2 | 9 |
| Student midwives | 3 | 3 | |||
| Information missing | 3 | 3 | |||
| Total | 21 | 17 | 26 | 23 | 87 |
Workshop activities
Following an overview of the main study and the additional work for women with LTCs, and an explanation of the prototype intervention, participants worked in small groups of six to eight people on four activities. Each group comprised participants from the four main groups of attendees as described above, and a member of the research team to facilitate and document key discussion points. Next was a description of each of the activities along with a summary of the key findings based on a synthesis from all four workshops. Throughout all the activities, participants were asked to focus on women from communities with low breastfeeding rates.
Activity 1: adaptation of the prototype intervention for women with multimorbidities
Participants were presented with a hypothetical case study of a woman with several LTCs (fibromyalgia, Crohn’s disease and anxiety), which was drawn from the experiences of members of the MLTC parents’ panel. Participants were asked to discuss what, if any, adaptations to the prototype intervention would be needed to meet the needs of breastfeeding women with multimorbidities.
The consensus across the workshops was that the intervention needed significant modifications. The greatest consensus was on three modifications: (1) the antenatal appointment should be longer than 30 minutes; (2) continuity, with the same person delivering the intervention antenatally and postnatally so that women do not have to repeat their stories; and (3) infant-feeding advisors should be included in joint obstetric and medical clinics.
Other modifications mentioned frequently were:
-
The person delivering the intervention should have expertise in medications and breastfeeding, as well as in breastfeeding support.
-
Antenatal appointments of 90 minutes would be more realistic, or several shorter appointments could be helpful.
-
Starting discussions early in pregnancy could be beneficial to take account of the higher risk of preterm birth in women with multimorbidities and to give practitioners more time to find accurate information.
-
Women require a medication review in early pregnancy, and this should involve a pharmacist who is knowledgeable about medications and breastfeeding.
-
Women should be able to see all of their healthcare providers (e.g. midwife, obstetrician, physician, pharmacist) during one appointment to minimise women’s time, effort and costs. Ideally, the appointment would include key members of the women’s support network (e.g. partner, family).
-
The antenatal appointment should focus on practical tips for managing varying levels of fatigue and pain, such as how to find comfortable feeding positions. Content should also be flexible to the women’s needs, adaptable to changing circumstances and consistent across different healthcare providers.
-
30-minute postnatal appointments are too short.
-
For the 3-month follow-up support, women should have the option of telephone or face-to-face contacts, and 24-hour telephone support should be available.
-
Peer support could be offered antenatally, and group antenatal peer support could help normalise breastfeeding for women with LTCs. Women could be offered the choice of one-to-one or group peer support.
-
Third-sector organisations could help with the provision of breastfeeding and emotional support.
-
To be sustainable, peer supporters should be paid.
-
Training is needed to increase knowledge of breastfeeding and multimorbidities in the multidisciplinary team, including GPs. Supporting women with multimorbidities to breastfeed should be included in routine breastfeeding training updates.
-
Services should be co-ordinated, with the infant-feeding advisor as the key point of contact for the multidisciplinary team.
Activity 2: identified barriers to implementation of prototype intervention for healthy women and women with multimorbidities
Participants were asked within their groups to discuss and list barriers to implementing and, for parents, accessing the prototype intervention in their settings. Open discussion was encouraged; however, facilitators were provided with a prompt sheet comprising the domains and constructs adapted from the CFIR227 to stimulate consideration of all aspects of implementation and accessibility. The lists of barriers were collated and, along with the 18 barriers identified by the stakeholder working group and parents’ panel (see Chapter 2), mapped to the updated CFIR. 92 There was a high degree of overlap within and across the workshops and between the workshops and stakeholder and parents’ panel discussions. We present the main themes under each domain of the CFIR,92 while acknowledging that there is overlap between constructs within a domain and between domains. Constructs of the CFIR are denoted in italics in the following text.
Innovation domain: Barriers relating to the innovation, defined as the ‘thing being implemented’,92 mapped mainly to the constructs of adaptability, complexity, design and cost. The most frequently mentioned barrier referred to adaptability in that the schedule and length of appointments lacked flexibility and would need to be tailored to individual women’s needs and circumstances. The next most frequently mentioned barrier was that the design of the intervention did not include the women’s partner and/or other family members who could be important sources of breastfeeding support. Further frequently mentioned concerns with the intervention design were lack of continuity across the intervention and lack of intensity in the first 2 weeks postnatally. Barriers related to cost highlighted concerns that costs to the service could be high and may not represent value for money or be sustainable. Regarding complexity, the intervention was perceived to be multifaceted and to necessitate multiple appointments that may not be convenient for women. The stakeholder working group identified that the intervention may not be perceived to offer relative advantage compared with existing or alternative approaches to breastfeeding support.
Outer setting domain: Barriers related to local attitudes to breastfeeding were discussed by all groups across the four workshops and were one of the most frequently mentioned of all barriers across all domains. Typically, barriers were phrased as ‘negative societal attitudes to breastfeeding’ or the existence of a ‘bottle-feeding culture’. These were said to result in family or peer pressure for women to formula feed based on unhelpful beliefs. Linked to this were external societal pressures, including the impact of social media influencers and formula company marketing. Lack of political priority and/or strategy for breastfeeding and failure to monitor and enforce the International Code of Marketing of Breastmilk Substitutes were common themes that mapped to policies and laws. A further frequently mentioned barrier related to the challenges of developing partnerships and connections between health services and other sectors such as third-sector organisations or local authorities. Outer setting barriers are also related to local conditions, for example lack of good transport and/or childcare, digital poverty and the current cost-of-living crisis. Lack of financing for breastfeeding along with funding targets was mentioned, but it was often unclear when this referred to the outer (funding from external entities) or the inner setting (funding to implement and deliver the innovation). Finally, the impact of COVID-19-related restrictions, particularly on group settings, was seen to be a barrier reflecting critical incidents.
Inner setting domain: There were twice as many barriers identified under the inner setting domain as under any of the other domains. The most frequent themes are linked to work infrastructure, culture and available resources. Workforce challenges such as staff shortages, high turnover of staff and lack of time/protected time were the most frequently mentioned. Other barriers related to work infrastructure included a lack of the right skill mix and overdependency on one or a small group of individuals. Overlapping with work infrastructure were barriers relating to relational connections and communications, for example poor communication and working practices across the multidisciplinary team, fragmented services and challenges to embracing peer support within the health settings. The last of these included peer support not being valued and a reliance on unpaid volunteers. Regarding culture, a very frequent theme (linked to human equality-centredness and recipient-centredness) was barriers relating to the lack of accessibility of services to diverse populations, including lack of language support, sensitivity to women’s backgrounds and stereotyping, as well as the cost of the intervention (e.g. travel costs) to women who have little resource. Also linked to culture were issues of learning-centredness, such as lack of visibility of data to staff (e.g. breastfeeding rates), lack of data sharing and lack of sharing of good practice. Regarding available resources, the most frequently mentioned barriers were lack of funding and lack of space, such as appropriate venues to deliver the intervention with consideration of space for women to breastfeed and accessible locations for groups to meet. Other themes were lack of compatibility of the innovation with existing policies and guidelines or with the practice of early postnatal discharge. Workshop participants and the stakeholder working group identified that the innovation overlapped with current provision and may not fit with existing workflows or system values.
Individuals domain: The most common theme in this domain is mapped to the capability (knowledge, skills, interpersonal competence) of innovation deliverers, resulting in conflicting information for breastfeeding women. The main concern was a lack of experience and training of many of the staff who would be delivering the intervention. This included a lack of access to high-quality education. A frequently mentioned barrier was some staff’s negative attitudes to breastfeeding, which could impact on their interactions with women. Second to the capability of staff was that some staff lacked motivation either because they did not value breastfeeding or due to professional fatigue. Lack of confidence of staff to implement the innovation was identified as a barrier by the stakeholder working group and at two workshops. The second most common theme of barriers related to the buy-in, understanding and valuation (capability and motivation) of the innovation by high- and mid-level leaders, that is key strategic decision-makers and those whose remit is to operationalise strategic decisions, without whose support the implementation would be unlikely to succeed. The stakeholder working group identified a lack of champions and skilled implementation leads as a further barrier. A final theme under individuals related to innovation recipients with barriers to opportunity such as lack of time and lack of knowledge of or access to services.
Implementation process domain: At the workshops, fewer barriers were linked to this domain than to the other domains. The only barriers mentioned by more than one group related to engaging, for example staff lack of engagement or resistance to change, and planning in the lack of management oversight to ensure that the innovation is being implemented as intended. The stakeholder working group identified concerns regarding the lack of feedback to staff to evaluate the quality of the intervention (reflecting and evaluating), the need to assess accurately the needs of parents and families (assessing needs) and poor communication of the goals, policies and procedures related to the innovation (planning).
Activity 3: prioritised strategies to overcome implementation barriers
In this activity, participants were presented with the 34 implementation strategies adapted from the Expert Recommendations for Implementing Change framework,82 derived from the stakeholder and parent consensus-building exercise (see Chapter 2). The task was to select the most relevant strategies to overcome each of the barriers identified in activity 2. Participants selected multiple strategies for each barrier, and each strategy multiple times. Given that so many barriers were related to the inner setting domain and were therefore context driven, we here present those strategies that were most frequently selected, giving examples of the barriers that they might address. Participants were also invited to add any additional strategies they thought were missing from the those provided. A full list of strategies and the number of times each was selected, along with additional strategies suggested by participants, can be found in Report Supplementary Material 1, Table 9 presents the five strategies chosen most frequently, along with examples of the barriers these were selected to overcome.
| Strategy | Number of times selected | Examples of barriers |
|---|---|---|
| Deliver realistic, evidence-based information in multiple formats on how to deliver the breastfeeding support intervention and why it is important | 84 | Lack of staff training, knowledge and skills Lack of consistency of information Lack of continuity of care Challenges to accessing the intervention for women and families Lack of buy-in from senior managers |
| Assign a key practitioner to raise awareness about the intervention to ensure a consistent message | 75 | Challenges to working with sectors outside the health system Poor communication across the multidisciplinary team Lack of joined-up vision and working |
| New or existing funding for breastfeeding support should be a general health investment for local councils and the government, and not just the NHS | 72 | Lack of funding within the health system Cost of the service to the NHS Lack of relationship between the health system and the community Lack of sustainability Cost of the intervention to women Reliance on non-paid peer supporters |
| Create an infant-feeding team in every NHS organisation to lead the intervention, working collaboratively with multidisciplinary practitioners and lay supporters | 72 | Lack of availability of good-quality training Time and capacity issues Professional boundaries – especially working with peer supporters Lack of confidence of those delivering the intervention Lack of integration across the continuum (antenatal/postnatal) and across the multidisciplinary team |
| Revise roles as needed to support the intervention; for example, integrate peer supporters with NHS infant-feeding teams, and consider upskilling maternity staff to specialist lactation training levels | 70 | Barriers to integrating peer support with health services, including lack of valuing peer support Lack of right skill mix Lack of knowledge and skills of staff delivering the intervention Infant-feeding specialists overloaded |
While Table 9 shows the most frequently selected strategies across all four workshops, there were differences between the workshops. For example, the two most frequently selected strategies at the Edinburgh workshop did not feature in the top five strategies across the workshop. They were:
-
start with pilots (in Baby-Friendly Initiative and non-Baby-Friendly Initiative accredited settings) to refine implementation and resources required as a means of phasing in the intervention and change in a sustainable way (#6 in the overall strategy ranking)
-
use new survey and routine data to assess the impact and monitor the quality of the breastfeeding support intervention (#12 in the overall strategy ranking).
The second most frequently selected strategy at the Cardiff workshop was:
-
involve parents, peer supporters and charities in adapting the intervention for the local area and to encourage uptake (#10 in the overall strategy ranking).
The differences between the workshops can most likely be explained by a combination of the different balance of participants at each workshop (with more parents and third-sector representatives at the Edinburgh workshop) and the different policy contexts of the four nations.
Activity 4: considerations for evaluating breastfeeding support interventions
Participants discussed how the prototype intervention could be evaluated and were prompted to consider outcomes that are important to parents, the timing of breastfeeding outcome data collection, the important data related to processes, and how to assess the impact on health inequalities.
Important outcomes for parents were suggested to be meeting their feeding goals and expectations, whether the support and information was helpful, and how confident or empowered a woman felt after the intervention.
With regard to the timing of breastfeeding outcomes data collection, the most frequently mentioned was to collect data on ‘any’ and ‘exclusive’ breastfeeding at the following time points:
-
first feed within 1 hour after birth
-
discharge from hospital
-
6–8 weeks
-
6 months.
Other suggestions with high consensus were 10–12 days (to coincide with discharge from routine midwifery care), 3–4 months and 1 year. Other comments on collecting breastfeeding outcome related to definitions of any and exclusive and whether these needed to be subdivided further.
Other outcomes felt to be important included health outcomes, for example the number of infants admitted to hospital and the reasons for stopping breastfeeding.
Process data
The most frequently mentioned were the views and experiences of those receiving and delivering the intervention (including women, healthcare practitioners and peer supporters), women’s satisfaction, and intervention fidelity (did women receive all components of the intervention). There was discussion that data could be collected early to capture those who cease to engage with the intervention and to gain feedback from those who declined the intervention. Many methods for collecting data were suggested, including digital options such as WhatsApp, and there was high consensus that participants in studies should be offered options for follow-up, for example between online, telephone, e-mail, post or a phone app.
Impact on inequalities
Discussions about evaluating the impact on health inequalities centred around gathering background information such as maternal characteristics (age, ethnicity, socioeconomic status) and making sure that the intervention and evaluation are inclusive, for example by addressing language barriers.
Activity 5: willingness to pay for a breastfeeding support intervention
To evaluate stakeholders’ preferences for a breastfeeding support intervention, participants in the workshop were presented with a stated preference discrete choice experiment (DCE). A DCE is a method of eliciting preferences for a given product or service by presenting a series of scenarios to individuals; each scenario presents two or more alternatives that differ in the attributes of the product/service, and the individual chooses their preferred alternative. 228 The theoretical underpinnings of the experiment are derived from (1) random utility maximisation,229 where it is assumed that individuals’ choice behaviours are made to maximise their satisfaction while allowing for unobserved sources of utility; and (2) Lancaster’s economic theory of value, which posits that an individual’s utility for a whole product or service can be separated into utilities for each component or attribute of that service. 230 If a change thus occurs in one of the attributes of the service, the individual may choose an alternative product if they deem it of greater value, while acting to minimise cost. 231 DCEs have been used increasingly over the last 20 years in health-related research and are useful for informing health policy, providing preferences for clinical outcomes of a service, as well as the process and cost attributes. 232 The aim of the experiment presented during the workshops was to estimate the value of a breastfeeding support intervention to participants, as well as the relative importance of each attribute and attribute level of the intervention.
Guidance on constructing the experimental design for DCEs was followed. 233,234 Careful consideration was given to the selection of attributes and suitable levels to be presented within the DCE. While DCEs present participants with hypothetical scenarios to choose from, it is important that the scenarios reflect practice and are recognisable to participants to ensure that the exercise is capable of deriving preferences. 228 The attributes (n = 7) and attribute levels (range 3–5) that were used to create the alternative choices presented in each scenario are outlined in Table 10.
| Attributes | Attribute levels | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Number of contacts | ≤3 | 4–8 | ≥9 | – | – |
| Provider | Peer supporter | Breastfeeding counsellor | Health professional | Lactation consultant | Combined provision |
| Mode of support | Telephone | Face to face | Online | Hybrid | – |
| Approach to support | Reactive | Proactive | Blended | – | – |
| Reduction in drop-off for any breastfeeding at 6 weeks | 1% | 5% | 10% | 15% | – |
| Reduction in drop-off for exclusive breastfeeding at 6 weeks | No reduction | 1% | 5% | 10% | – |
| Additional cost per woman | £25 | £50 | £100 | £150 | – |
The attributes and levels were informed by the findings from the systematic reviews reported in Chapters 3 and 5, the findings from the stakeholder engagement, which comprised online discussions, the modified Delphi study and face-to-face focus groups, and the resulting prototype intervention. The intervention components included process attributes of the number of contacts between service users and service providers, provider of the intervention, mode of support and approach to support. The clinical outcome attributes were the percentage reduction in drop-off for any, or exclusive, breastfeeding at 6 weeks. Only one of the two clinical outcome attributes was presented in any given experiment to each participant. Finally, the cost attribute indicated the additional cost to the NHS per woman supported.
A fractional factorial design was then used to create the experiment to limit participant fatigue and the length of time required to complete it. An orthogonal main effects plan, using Statistical Package for Social Sciences version 27 (IBM Corporation, Armonk, NY, USA) software, generated profiles for the alternatives and 12 choice sets. Participants were presented with an unlabelled DCE with two alternative intervention options (A and B), which differed in their attribute levels, along with a third alternative of choosing neither intervention. This third alternative provided an unconditional choice set where participants could opt out if they preferred. Figure 8 illustrates an example scenario designed to enable participants to trade across attributes and, thus, identify the relative value of each attribute and level for stakeholders.
FIGURE 8.
Example scenario presented to workshop participants.

An interview-based format was used to administer the experiment to workshop participants, allowing the facilitator to answer any queries and clarify any issues. Before the activity commenced, the DCE was explained, and participants were introduced to each attribute and associated levels. They were informed that the breastfeeding support intervention was additional to current service provision and that several outcomes of effect may occur as a result of the additional support, such as a change in maternal satisfaction with care or a change in breastfeeding initiation rates. However, for the purposes of the exercise they were asked to consider a reduction in drop-off for breastfeeding (any or exclusive) at 6 weeks, which reflected the outcome of effect in the Cochrane review that had recently been updated as part of the study. 83
Data from the experiment were entered into Microsoft Excel. Data entry was carried out using a multiple-line format, whereby data are divided into a number of blocks. Effects coding was used for the levels of the process attributes, while the clinical outcomes and cost attributes were maintained. Each block represented a participant’s choice set and each row within that block corresponded to an alternative within the choice set, effectively clustering the data to allow for multiple observations from respondents to the experiment. The choice outcome was the variable that signified the decision made for each scenario and, as such, was the dependent variable within the model. The discrete choice analysis was undertaken using a random utility model and conducted in R (The R Foundation for Statistical Computing, Vienna, Austria) using guidance provided by Croissant. 235 Modelling the choice sets of participants produced choice probability estimates and an indirect utility function for choosing an alternative, an attribute and an attribute level. Estimated marginal rates of substitution enabled the interpretation of participants’ WTP for each attribute and attribute level.
Results
A total of 87 workshop participants completed the DCE in November 2022. Table 11 presents the results from the discrete choice modelling.
| Attribute and attribute level | Βeta coefficient | Standard error |
|---|---|---|
| Number of contacts | ||
| ≥9 | 0.03 | 0.142 |
| 4–8 | 0.35** | 0.122 |
| ≤ 3a | −0.38 | |
| Provider | ||
| Combined provision | 0.46** | 0.172 |
| Lactation consultant | 0.03 | 0.165 |
| Breastfeeding counsellor | 0.18 | 0.202 |
| Peer supporter | −0.45* | 0.196 |
| Healthcare professionala | −0.22 | |
| Mode of support | ||
| Hybrid | −0.30 | 0.187 |
| Online | 0.11 | 0.187 |
| Telephone | −0.29* | 0.135 |
| Face to facea | 0.48 | |
| Approach to support | ||
| Blended | −0.15 | 0.148 |
| Proactive | 0.16 | 0.117 |
| Reactivea | −0.01 | |
| Reduction in drop-off for any breastfeeding at 6 weeks | 0.26** | 0.018 |
| Reduction in drop-off for exclusive breastfeeding at 6 weeks | 0.52** | 0.070 |
| Additional cost per woman | −0.02** | 0.002 |
| Neither intervention | −1.16** | 0.186 |
| Log-likelihood | −748.03 | |
| Number of iterations | 5 | |
With regard to the estimated beta coefficients, preference formation was as expected a priori and resonated with the findings from the stakeholder engagement activities and the resulting prototype intervention. Stakeholders exhibited statistically significant preference for four to eight contacts over three or fewer (β = 0.35, SE 0.122, p < 0.01) and provision from a range of providers over healthcare professional alone (β = 0.46, SE 0.172, p < 0.01), and valued face-to-face support over telephone support (β = −0.29, SE 0.135, p < 0.05). Although there was a positive value for a proactive approach to support over reactive support, this was not statistically significant (β = 0.16, SE 0.117, p > 0.05), suggesting that stakeholders did not consider the different approaches to support (reactive, proactive, hybrid) in their decision-making process. Both clinical outcome attributes of reducing the number of women stopping any breastfeeding (β = 0.26, SE 0.018, p < 0.01) or exclusive breastfeeding (β = 0.52, SE 0.070, p < 0.01) at 6 weeks postpartum were statistically significant, suggesting that the greater the percentage reduction in drop-off, the greater the value to participants. For the additional cost per woman, participants valued a lower cost intervention over a higher cost (β = −0.02, SE 0.002, p < 0.01), upholding underlying assumptions of individuals acting to minimise cost. 231 The overall preference by stakeholders for introducing an additional breastfeeding support intervention into practice was reiterated by the lack of preference for the status-quo alternative, which displayed a negative beta coefficient (β = −1.16, SE 0.186, p < 0.01).
In terms of WTP for additional breastfeeding support, estimated marginal rates of substitution indicated that participants were willing to pay £67.40 per woman for additional breastfeeding support, regardless of how it was delivered or whether it was effective in reducing the number of women stopping breastfeeding at 6 weeks postpartum. Table 12 presents the WTP for each clinical outcome attribute and each process attribute level valued by participants, which was represented by a statistically significant, positive beta coefficient in the model.
| Attribute valued | Marginal WTP/woman |
|---|---|
| 4–8 contacts | £20.29 |
| Combined provision | £26.83 |
| Face-to-face supporta | £27.89 |
| For each 1% reduction in drop-off for any breastfeeding at 6 weeks | £14.90 |
| For each 1% reduction in drop-off for exclusive breastfeeding at 6 weeks | £30.03 |
As an example, the estimated WTP by stakeholders was £89.91 per woman for a breastfeeding support intervention that realised a 1% reduction in drop-off for any breastfeeding at 6 weeks postpartum, and £105.04 per woman for an intervention that realised a 1% reduction in drop-off for exclusive breastfeeding at 6 weeks postpartum. The WTP thresholds would increase to £149.51 and £225.16 if the interventions realised a 5% reduction in drop-off of any or exclusive breastfeeding at 6 weeks postpartum, respectively.
Finalising the toolkit
Following the workshops, the study team collated the information presented above and synthesised it with the findings from the systematic reviews presented in this report. The findings were then combined to form the toolkit, a draft of which is presented in Appendix 8. The intention is that the toolkit will be developed into a digital version.
Chapter 10 Discussion and conclusions
Summary of findings
The aim of this study was to synthesise global and UK evidence to co-create with stakeholders a framework to guide the implementation and evaluation of cost-effective breastfeeding support interventions in the NHS. The original focus of the study was on women without LTCs; however, we broadened the scope to include women with MLTC when additional funding was awarded. Given the anticipated paucity in evidence for women with MLTC, our review work considered women with single LTCs.
In total, we conducted six systematic reviews:
-
two systematic reviews and meta-analyses examining the effectiveness of breastfeeding support for healthy women and women with LTCs
-
a theoretically informed mixed-methods synthesis of process evaluations of UK-relevant breastfeeding support intervention
-
a mixed-methods synthesis of barriers to and facilitators of breastfeeding support in women with LTCs
-
two economic evaluations of breastfeeding support for healthy women and women with LTCs.
This study also contained embedded stakeholder engagement in the form of stakeholder working groups, parents’ panels, focus group discussions with women from socially disadvantaged groups and four workshops held across the UK.
The first work package was an update of the Cochrane review on breastfeeding support for healthy women with healthy term infants. 83 We included 116 studies in the review and ‘breastfeeding only’ interventions, which included breastfeeding support only (n = 86), and ‘breastfeeding plus’ interventions (n = 30), which included other aspects of maternal and child health such as vaccinations, well-baby clinics, intrapartum care and contraceptive services. We found moderate‐certainty evidence that ‘breastfeeding only’ interventions probably led to a small reduction in the risk of women stopping exclusive breastfeeding at 6 months, 4–6 weeks, 2 months and 3–4 months, and stopping any breastfeeding at 6 months, 4–6 weeks and 3–4 months. Effect estimates ranged from RR 0.93 (95% CI 0.89 to 0.97) for stopping any breastfeeding at 6 months to RR 0.81 (95% CI 0.77 to 0.95) for stopping exclusive breastfeeding at 3–4 months. Effect estimates were generally greater for exclusive breastfeeding than for any breastfeeding.
For ‘breastfeeding plus’ the evidence was less consistent. Support probably reduced the number of women stopping any breastfeeding or exclusive breastfeeding at 6 months. The evidence suggests that ‘breastfeeding plus’ support probably results in little to no difference in any of the other outcomes. Effect estimates ranged from RR 0.94 (95% CI 0.91 to 0.97) for stopping any breastfeeding at 6 months to RR 0.73 (95% CI 0.57 to 0.95) for stopping exclusive breastfeeding at 4–6 weeks. Again, effect estimates were generally greater for exclusive breastfeeding than for any breastfeeding. It is not clear why ‘breastfeeding plus’ interventions tended to have less of an impact on the number of women stopping breastfeeding. The proportion of interventions categorised as low, medium and high intensity was broadly similar for ‘breastfeeding only’ and ‘breastfeeding plus’. However, it is feasible that the time spent providing breastfeeding support was less in the ‘breastfeeding plus’ interventions as other aspects of maternal and infant care were also included. There was a lack of information in the intervention characteristics to explore this issue fully. Moreover, although interventions were categorised into ‘breastfeeding only’ and ‘breastfeeding plus’, there was still substantial heterogeneity in interventions.
Meta‐regression was conducted to further explore heterogeneity. This suggested that moderate-intensity (four to eight visits) compared with low-intensity (three or less), support may be beneficial for reducing the number of women stopping exclusive breastfeeding at 4–6 weeks or 6 months. Additionally, women in LMICs were less likely to have stopped exclusive breastfeeding at 6 months, and this may be explained by the higher background breastfeeding rates at 6 months in LMICs. 14 However, beyond this the meta-regression did not explain the high levels of heterogeneity. As we did not want to increase the likelihood of false positives from the meta-regression, we limited the number of variables to four,88 and these were determined in conjunction with stakeholders. There is, therefore, a possibility that variables not included in the meta-regression may be contributing to the high levels of heterogeneity. For example, just under half of the included studies focused on populations described as high poverty, deprivation and poor health outcomes, and it is possible that this may explain some of the heterogeneity as breastfeeding rates typically are lower in groups of high levels of deprivation in HICs. 14
The second work package comprised a mixed-methods synthesis of process evaluations of effective breastfeeding support interventions identified in work package 1. We included 16 studies linked to 10 effective interventions. The identified 18 factors affecting implementation of interventions, and data-driven analytical themes were mapped to a theoretical implementation framework resulting in three overarching, theoretically informed, analytical themes: (1) assessing the needs of those delivering and receiving breastfeeding support interventions; (2) assessing the context and optimising delivery and engagement with breastfeeding support interventions; and (3) reflecting and evaluating the success of implementing and providing breastfeeding support. Included studies identified implementation challenges relating to the needs, preferences and priorities of intervention providers and recipients. Overall, breastfeeding women perceived support as positive, important and needed. Breastfeeding supporter training was a commonly reported implementation strategy that enabled implementation teams to address breastfeeding supporters’ needs. Studies reported contextual factors (e.g. alignment with local policies) affecting the implementation and delivery of breastfeeding support interventions as well as tailoring strategies (e.g. community involvement, use of lay language, responsive support content/information) to address contextual factors. Reports about implementation success focused on key implementation outcomes such as satisfaction, fidelity or usefulness.
The third work package comprised a review of economic evidence of both trial- and model-based evaluations of the incremental cost and incremental cost-effectiveness of breastfeeding support interventions. Of the 39 studies identified, nine were deemed directly or partially applicable to the UK system. Evidence of cost-effectiveness using the UK-recommended incremental cost per QALY gained was limited and inconsistent. For breastfeeding-only support, one study provided evidence that the estimated ICER for the intervention was not cost-effective, at £56,075 per QALY gained. This ICER held in deterministic and probabilistic sensitivity analyses. However, there were notable limitations to the model, with the exclusion of costs (cost savings) and benefits to infants beyond 1 year of age and clinical conditions that were excluded, such as obesity. A lack of good-quality epidemiological and cost data warranted the exclusion but highlights the uncertainty in the findings and the need for more robust evidence to inform future economic evaluations. There was evidence for the incremental cost per additional woman breastfeeding (any or exclusive) with ICERs ranging from £67 to £112 from 2 weeks up to 8 weeks postpartum and from £2446 to £4226 up to 6 months postpartum. However, we judged the findings to be uncertain because of the limited number of studies and the lack of good-quality evidence. None of these studies extrapolated data beyond the time horizon of the associated trial, and potential costs (cost savings) from health service use were not estimated and valued. Without WTP thresholds, whether or not the findings were cost-effective was unclear. Evidence for ‘breastfeeding plus’ support suggested that this was not cost-effective in terms of cost per QALY gained, with similar inconsistencies in results. The scope of costs and outcomes reported and the time horizon for many of the studies was limited. What is missing from the evidence is a high-quality trial-based economic evaluation that then models costs and outcomes beyond the trial period. If breastfeeding in itself is considered a cost-effective intervention, then the provision of additional effective support to populations or subgroups of women with lower rates of breastfeeding initiation is likely to be worth the investment. Engagement with stakeholders during the workshops elicited a positive value for a breastfeeding support intervention, with a WTP of £89.91/£105.04 per woman for a 1% reduction in drop-off for any/exclusive breastfeeding at 6 weeks postpartum. If policy- and decision-makers are willing to pay this cost to realise this outcome, then such a breastfeeding support intervention, delivering four to eight face-to-face contacts with women by a combination of providers, would be considered value for money.
The first work package of the additional funding aimed to identify effective interventions that provide breastfeeding support for women with LTCs. We identified 22 studies that met the inclusion criteria, all of which were for women with single LTCs. A range of conditions were identified: overweight and obesity (nine studies), HIV (five studies), gestational diabetes (two studies), substance misuse (two studies) and depression (one study). Interventions varied in terms of whether they provided only breastfeeding support or if they also provided support for the LTC. No studies were identified for women with MLTC. In contrast to the Cochrane review of breastfeeding support,83 most studies had an antenatal component. The importance of antenatal support, particularly having a flexible feeding plan, was raised by the stakeholder working group and parents’ panel for women with MLTC. Effect estimates for the primary breastfeeding outcomes were generally small and crossed the 95% CI, which suggests that included interventions probably had little to no impact on the number of women stopping breastfeeding. Effect estimates ranged from RR 0.83 (95% CI 0.67 to 1.01) for stopping any breastfeeding at 6 months to RR 0.95 (95% CI 0.89 to 1.00) for stopping exclusive breastfeeding at 6 months. Findings for the additional breastfeeding outcomes were similar. Due to the small number of studies, meta-regression to explore the impact of the nature of the LTC on breastfeeding rates was not possible. Sensitivity analysis did not find a difference in findings when studies with women with HIV were excluded. Similarly, due to the small number of studies, meta-regression was not possible to explore possible causes of heterogeneity, such as nature of condition or socioeconomic deprivation. Moreover, only a few studies had beneficial intervention effects for at least one outcome. 184,185,187 It is, therefore, not possible to make any conclusions about support being more or less effective for specific conditions. Similarly, a narrative synthesis showed little to no beneficial effect on maternal and infant health outcomes.
The second work package for women with MLTC comprised a mixed-methods synthesis of experiences of breastfeeding support for women with LTCs. The 24 included studies covered health conditions including HIV, obesity and overweight, substance use, diabetes in pregnancy, disabilities and a rare genetic disorder. Key findings were that women with LTCs have additional breastfeeding support needs, but that breastfeeding support can be difficult to access. Women and healthcare providers reported challenges including the overshadowing of breastfeeding support by condition-related support and supporters lacking in knowledge and skills. Suggested strategies to improve breastfeeding support for mothers with LTCs included acknowledging the influence of partners, families and friends and training healthcare providers to improve their understanding of the specific breastfeeding support needs of women with LTCs.
The third work package for women with MLTC conducted a review of economic evidence for breastfeeding support interventions for women with LTCs. Five evaluations were identified that assessed cost and effect for women with a small range of health conditions: HIV, obesity, prenatal opioid use, and women considered medically high risk (maternal hypertension and diabetes prior to birth). There was a lack of evidence of cost-effectiveness from full economic evaluations, with limited scope in the costs and benefits valued. One cost-effectiveness analysis study reported a cost of US$88 per increased month of exclusive breastfeeding to support women living with HIV to breastfeed, while a cost–utility analysis study reported that promoting breastfeeding was less costly and more effective, in terms of disability-adjusted life-years averted, for women living with HIV in rural areas than the current scenario. A third cost–utility analysis study reported less cost and more effect, in terms of QALYs gained, for breastfeeding support using rooming-in after childbirth for women with prenatal opioid use. However, none of the studies met the applicability criteria for the UK system, making it likely that these conclusions of cost-effectiveness would change if tested in a UK setting.
The final phase of the project involved developing and refining a toolkit for implementing and evaluating effective breastfeeding interventions relevant to the UK, based on synthesising the findings of the reviews and stakeholder and parent engagement along with the views of a broader group of stakeholders who attended workshops. The toolkit presents an example intervention based on high-quality evidence on effective breastfeeding support interventions. The intervention comprises structured, proactive antenatal and postnatal components, combines professional and peer support, and offers face-to-face and telephone follow-up. The toolkit proposes the most important considerations when adapting this evidence-based intervention for local services, which are acceptability to the local population, the quality of the primary evidence, and the sustainability of the intervention. Regarding tailoring the intervention for women with LTCs, the most important modifications to be considered are more time for antenatal breastfeeding support, continuity of support, and including infant-feeding specialists in combined obstetric and medical clinics.
The toolkit highlights barriers that may be encountered when implementing breastfeeding support interventions considering the intervention itself, the broader societal setting, the context of local services, the roles and capabilities of those implementing and receiving the intervention and, finally, the process of implementation (see Appendix 8). The toolkit proposes a range of strategies that can be used to address barriers, the most important of which are providing information on how to deliver the intervention and why it is important, assigning roles such as a key practitioner to raise awareness, having an infant-feeding team to lead implementation, integrating peer support with NHS services, and leveraging investment from local councils and government as well as the NHS.
Finally, the toolkit proposes considerations for evaluating the intervention, including whether women meet their infant-feeding goals and expectations and whether the support is helpful. The suggested times to measure breastfeeding outcomes are first feed, discharge from hospital, 6–8 weeks and 6 months. Other important outcomes to consider are infant admissions to hospital and the reason for stopping breastfeeding. Process data to be considered include views and experiences of the intervention deliverers and recipients, women’s satisfaction and intervention fidelity. To assess impact on inequalities, data should be collected on women’s characteristics and the intervention and evaluation should be inclusive, that is accessible to all women.
Agreements and disagreements with other reviews
First, in terms of the Cochrane review, both ‘breastfeeding only’ and ‘breastfeeding plus’ tended to have a greater impact on exclusive breastfeeding. One explanation for this comes from a realist review that suggested that more highly motivated mothers may benefit more from breastfeeding support. 236 In addition, effect estimates tended to be greater at earlier time points, which may be a consequence of support being primarily targeted at the first 1–2 months. At later time points, wider issues around returning to employment influence breastfeeding rates237 and may not be considered in the interventions included. The results of this meta-analysis are similar to effect estimates reported in a review looking at breastfeeding counselling interventions. 238 Other systematic reviews looking at support interventions have shown greater effect estimates;239 however, these reviews identified a much smaller number of studies due to limitations in search strategies and selection processes. In addition, previous systematic reviews have found greater effect estimates for multicomponent breastfeeding support (i.e. providing different aspects of breastfeeding support in a combination of settings such as BFHI). 40,240,241 We did initially aim to categorise the interventions based on breastfeeding support components; however, given the large number and heterogeneity of interventions we were unable to do this in any meaningful way. Interestingly, our review suggested slightly higher effect estimates than did a review looking at breastfeeding support that was provided on a remote basis only. 242 However, in our review, meta-regression did not identify any clear differences between support provided remotely and that provided face to face, but the power to detect any differences was limited. Reviews of alternative methods to increase breastfeeding rates have identified a relatively small number of studies and no clear intervention effects, for example incentives243 or workplace-based strategies. 244
To the best of our knowledge, this is the first systematic review to focus on implementation research linked to breastfeeding support interventions for healthy women with healthy term babies that have shown effectiveness in RCTs. However, some existing reviews have looked more widely at the views and experiences of those delivering and receiving breastfeeding support interventions and have reported findings that are well aligned with our review. These include the importance of key intervention strategies that women perceive as supportive, such as those that rely on the provision of both practical/technical expertise245–247 and emotional support/encouragement245,246,248 and are person centred and socioculturally specific,246,247,249 as well as key implementation issues such as the importance of contextual factors. 249,250 In terms of the review on effectiveness of breastfeeding support for women with LTCs, our findings are consistent with a Cochrane review of support targeted at women with overweight and obesity for which only a few small-scale studies were identified. 251 Meta-analysis likewise identified small effect estimates and imprecision. A further systematic review that included any intervention (e.g. support, breast pumps, education) designed to increase breastfeeding initiation and continuation in women with overweight/obesity also did not appear to show any impact on improving breastfeeding rates. 252 To the best of our knowledge, there are no existing reviews of breastfeeding support for any other form of LTC.
Our mixed-methods synthesis of experiences of breastfeeding support for women with LTCs is consistent with other review findings. This includes that overweight/obese women find breastfeeding challenging. 253,254 Similar to our review, Chang et al. 253 concluded that healthcare professionals require education to enable them to provide tailored, non-judgemental breastfeeding support. Cummins et al. 255 made similar recommendations based on their systematic review of in-hospital support for women with GDM. Tanganhito et al. 256 emphasised the influence of family and friends and professional support for women with postnatal depression.
In terms of the review of economic evidence, our findings resonate with one previous economic evidence review. 257 This review was conducted to inform NICE guidance on postnatal care and comprised seven studies. The authors judged the existing evidence to be inconclusive. While their inclusion criteria had a wider remit of breastfeeding education, advice and support interventions, which included financial incentives, their findings were consistent with the findings in the current review for ‘breastfeeding only’ support. The review highlighted similar limitations and inconsistencies between studies, such as the limited time horizon, the different economic outcomes estimated and the different scope of costs and benefits measured and valued, which have an impact on the strength of any conclusions.
Strengths and limitations
This study has several key strengths. First, a criticism of systematic reviews is a lack of uptake of review findings into policy and practice;258 however, the mixed-methods reviews and stakeholder engagement have enabled to us to understand how interventions could be effectively implemented in practice. To address this, we included two mixed-methods syntheses that aimed to explore how such support could be implemented in the NHS for all women. We believe that this is the first comprehensive synthesis of evidence of effectiveness of breastfeeding support and of barriers to and strategies for implementing breastfeeding support for women with and women without LTCs. Furthermore, our work has been underpinned by implementation frameworks providing theoretically informed recommendations in the form of a toolkit. Finally, and perhaps most importantly, it had extensive PPI and stakeholder involvement that ensured a co-created output grounded in the realities of women’s experiences of breastfeeding, particularly those from socially disadvantaged groups, and NHS context and practice. Hopefully, this gives a sense of ownership to those involved in the project. The toolkit should be relevant and adaptable to the four UK nations. Second, the two effectiveness reviews and meta-analyses followed Cochrane methodology to ensure rigour. Third, the update of Cochrane review of breastfeeding support83 included the use of a new trustworthiness checklist, which helps ensure that the findings of this review are not based on fraudulent data. 86
However, there are several limitations that should be considered. With all systematic reviews there is the potential for bias to be introduced. First, although we did involve two reviewers in all review processes (e.g. study selection, data extraction, critical appraisal, synthesis, GRADE), these judgements are subjective. Second, except for the Cochrane review, studies not published in English were excluded, so there is a risk of language bias. Third, although we attempted to identify all available evidence meeting our inclusion criteria, it is possible that we did not identify all studies, and the Cochrane review in particular showed some evidence of funnel plot asymmetry, which may be suggestive of publication bias. Fourth, issues in reporting meant that there was often insufficient information about intervention characteristics (e.g. person providing the intervention, number of contacts, theoretical basis, definitions of exclusive breastfeeding, nature of standard care). Fifth, the systematic reviews on effectiveness identified a lack of digitally provided interventions. As the COVID-19 pandemic has led to an increase in remotely provided maternity care,242 this evidence is perhaps limited in a post-COVID world. Sixth, our syntheses were limited by the mixed quality and lack of published process evaluations linked to effective interventions, as well as the relative dearth and poor quality of studies of experiences of breastfeeding support for women with LTCs. The latter body of evidence covers a very limited range of conditions, with many being studies of HIV-positive women in LMICs and of obese/overweight women in HICs. We did not find any studies of experiences of breastfeeding for women with mental health conditions. Seventh, there was a lack of evidence from the UK. This is representative of a long-standing problem whereby UK trials have failed to demonstrate benefits for breastfeeding outcomes, possibly due to the interventions tested and the way they were delivered rather than the trial design. 44 Eighth, the search for the Cochrane review on breastfeeding support was conducted in May 2021 and will not have included any studies that look at digital support post COVID-19. Ninth, a post hoc decision was made to exclude breastfeeding initiation from the review on effectiveness of support for women with LTCs. Studies that included this as an outcome used considerably different definitions (e.g. within 1 hour vs. ever), which gave rise to some nonsensical findings, such as in the same study more women breastfeeding at 4–8 weeks than had initiated it. Finally, there is unexplained heterogeneity in both the Cochrane review and the review on breastfeeding support for women with LTCs. In both these reviews, just under half of the studies were targeted at populations at risk of poorer outcomes (e.g. high levels of socioeconomic deprivation, ethnicity, young motherhood). As these factors influence breastfeeding rates,14 it is possible that the impact of support is different in these populations. However, for the Cochrane review this was not included as a variable in our meta-regression and for the LTC review there were insufficient studies to investigate this. In addition, the review for women with LTCs has additional heterogeneity due to the different conditions included.
Strengths and limitations of patient and public involvement and stakeholder involvement
We used the GRIPP2 (Guidance for Reporting Involvement of Patients and Public) checklist to inform our account of PPI in the study. 259 There was significant involvement of stakeholders and PPI in this project. In addition to the research team’s reflections, we sought the views of the main study stakeholder working groups and parents’ panels on their engagement.
First, our research team included a PPI co-applicant (PB) who was involved at all stages from the initial design to writing the final report and disseminating the findings. This ensured the PPI voice in all team meetings, providing valuable advice and feedback and influencing decisions. Furthermore, PB participated in the systematic reviews, including study selection, data extraction, quality appraisal and interpreting the results, and is a co-author of the Cochrane review. 260
Stakeholder engagement and PPI were identified as a cross-cutting theme in the study protocol, co-led by co-applicants PB and JM, ensuring that it was a standing agenda item in all team discussions and study steering committee meetings. A key responsibility of the full-time project manager was co-ordinating the stakeholder working group and PPI meetings ensuring sufficient administration time was dedicated to it.
A considerable strength was the range of individuals involved in the stakeholder working groups and parents’ panels. Members of the parents’ panels had a wide range of breastfeeding experiences, and experiences of breastfeeding with a range of comorbidities. We also included two fathers in the main study parents’ panel. Members of the stakeholder working groups represented the main health professions involved in breastfeeding support as well as the key national breastfeeding support third-sector organisations, and a national policy-maker. This work was enhanced by conducting focus group discussions in an area of high deprivation and ethnic diversity to ensure that we gained perspectives from communities that have low breastfeeding rates and to complement the parents’ panels. A further strength was that 87 people attended the co-creation workshops, covering extensive geographies, NHS and third-sector organisations, and parents.
All parents’ panels and stakeholder working group meetings were held virtually, by necessity at the outset of the project, which removed geographical barriers from inclusion. We worked hard to keep participants engaged in the work, as can be seen from the level of engagement across the 2-year study. Focus group participants were offered a choice of face-to-face or virtual meetings, and we ran both modes at each of the three time points. Holding the workshops face to face was a huge advantage, and participants provided very positive feedback about the activities and the benefits of working with others on such an important topic. For many, it was their first experience of a face-to-face event since the COVID-19 restrictions were lifted.
All those involved have been remunerated for attending meetings, as well as for expenses incurred travelling to the workshops. NHS organisations were reimbursed for releasing staff to attend meetings and workshops.
We were transparent at every stage of the study about how the PPI and stakeholder involvement influenced the study, including in co-creation of the toolkit. Feedback from the main study parents’ panel and stakeholder working group was that they felt proud to be involved and enjoyed seeing how the project evolved, that the meetings were very inclusive, and that the communication from the team both during and between meetings was very informative and clear.
There were several limitations to this component of the study. We acknowledge that recruiting parents via a third-sector organisation could have resulted in participants who were mainly from middle-class backgrounds. We feel that we mitigated this by conducting the focus group discussions. However, we did not collect sociodemographic data from participants in the parents’ panels and focus groups. We did not recruit any women to the parents’ panels and focus group discussions who had exclusively formula-fed their babies, and this could be considered a limitation. However, this was because the focus of our work was supporting women who had chosen to breastfeed in continuing longer and increasing exclusivity. Nevertheless, our parents’ panels and focus group discussion participants included several women who had combined formula feeding and breastfeeding, and those who had breastfed initially but had switched to formula feeding because of the challenges they had faced. We believe that this brought a wide range of views to our work. We had originally intended to conduct the initial meeting of the main study stakeholder working group face to face, but this was not possible due to COVID-19 restrictions. A face-to-face meeting may have helped build rapport and allowed for informal conversations. We were aware that some participants accessed meetings on their mobile phones and tried to plan activities accordingly, but it was still challenging for some. Finding a convenient time for meetings was difficult, and although we offered evening times for the parents’ panels, this option was not taken up. Nevertheless, some parents were disappointed that they could not attend all meetings. Several members of the stakeholder working group changed roles during the study, and offered replacements, but this inevitably resulted in some lost continuity. The university processes for reimbursement were bureaucratic and time-consuming for the participants and the project manager. Although our workshops had good attendance, many more people registered than attended. The workshops were held in November 2022, a time of high levels of winter illnesses (COVID-19 and flu), travel disruption and high demand in the NHS, all of which affected attendance. Some members of the parents’ panels were disappointed that they could not attend a workshop because of distance and full-time employment. One suggestion from the parents’ panel was to have a combined meeting with the parents’ panel and the stakeholder working group. We held the meetings separately to ensure that the parents’ voices were heard but will consider at least one combined meeting in future projects.
Implications for practice
Considering the importance of breastfeeding for public health and the existence of high-quality, moderate-certainty evidence of what works to support healthy women to breastfeed, the key challenge is overcoming the barriers to implementing breastfeeding support interventions. Decision-makers and frontline practitioners can use the toolkit to inform implementation efforts and to overcome barriers specific to their settings. Further co-development work is ongoing with an extended set of stakeholders to refine the draft toolkit and produce a user-friendly output that will support NHS and third-sector organisations to implement evidence-based breastfeeding support for women in the UK. Key to success will be addressing the system barriers and enhancing the skills, knowledge and confidence of practitioners. To reduce inequalities, interventions must be adapted to be accessible to all women, for example by ensuring that venues are accessible at a low cost and that language and cultural barriers are considered. Breastfeeding peer support is lacking across much of the UK. 33 Addressing barriers to integrating peer support with health service support is needed, as suggested by Trickey et al. 236 This requires action by health service strategic and operational decision-makers to adequately resource and value peer support as integral to effective breastfeeding support.
While less research evidence is available on how to provide effective breastfeeding support for women with LTCs, our stakeholder engagement and PPI work highlighted additional support needs and proposed possible strategies for achieving this. Health services could consider implementing proposals to integrate an infant-feeding specialist with the multidisciplinary team to give infant feeding a higher profile in obstetric and medical care.
The lack of knowledge, skills and confidence of those providing breastfeeding support is a frequent theme in research on breastfeeding support. Our stakeholder work suggested that training to UNICEF UK Baby-Friendly Initiative standards261 should be a minimum level for those providing care to mothers and infants. However, our workshop participants also proposed enhancing the training of those delivering breastfeeding support to lactation consultant level. Any upskilling strategies should incorporate the needs of women with LTCs.
The toolkit can be used by those leading breastfeeding support services to guide implementation efforts. This will probably necessitate rethinking existing roles and skill mix and involve finding ways to work with other sectors such as third-sector and community organisations. According to our work, a key to effective implementation is providing feedback to staff through data sharing.
The societal and commercial influences on women’s breastfeeding experiences are well recognised. 17,262 Although this needs a whole-system approach beyond the scope of our work, one strategy emphasised by our project is to involve partners and wider families in breastfeeding support interventions, as found in Bengough et al. 249 Regarding reducing inequalities in breastfeeding, the current economic climate and cost-of-living crisis is likely to exacerbate inequalities and necessitate the consideration of minimising costs to breastfeeding women such as ensuring that venues are accessible and helping with travel costs. Digital poverty must also be considered if the breastfeeding service has a digital component. Exploring the needs and preferences of the local population and working with a wide range of third-sector organisations and local government could address this.
Suggested future research
Crucially, this study found only a small number of studies on breastfeeding support for women with LTCs and a lack of evidence on cost-effectiveness in this group, compared with the large number of studies looking at support for healthy women. Moreover, both reviews identified that effect estimates were generally small. There is therefore a need to develop support interventions that are effective for all women. While further inspection of the Cochrane review findings did identify specific interventions that had larger effects and could form the basis of a NHS intervention, many of the barriers to breastfeeding for women with LTCs identified by our parents’ panel and stakeholder working group, and the mixed-methods synthesis, would not be considered in these interventions. In particular, there is a greater need for antenatal support and development of a feeding plan, consistent communication between healthcare professionals regarding medication safety, and the consideration of breastfeeding as a physical activity. There is therefore a need to develop and test an intervention for women with MLTC that takes account of these aspects. In particular, this work identified a very small number of studies for women with mental health conditions. In addition, while many of our included studies did focus on women with overweight/obesity and GDM, the interventions were generally not effective. Given the prevalence and co-occurrence of these conditions, and the fact they are more likely to affect women from groups least likely to breastfeed, we would suggest these as priority areas.
Both systematic reviews on the effectiveness of breastfeeding support identified a lack of digitally provided interventions. As the COVID-19 pandemic has led to an increase in remotely provided maternity care,242 there is also a need to consider how digital technologies could be utilised. However, both our work with stakeholders and existing research263 suggest that remotely provided support cannot be a replacement for face-to-face support and thus it should be provided alongside face-to-face breastfeeding support.
More research is needed on the experiences of receiving and providing breastfeeding support among women with LTCs and those with multimorbidities.
Evidence for the effectiveness of breastfeeding feeding support interventions in the UK is lacking, and the toolkit can be used to guide evaluation design. This could be via implementation or effectiveness studies or by using quality improvement methodology. Studies could be based on the prototype intervention developed for this study (tailored to local contexts), as described in the draft toolkit, and could test different implementation strategies for effectiveness. Further evidence of value for money in a UK setting is also needed.
Future economic evaluations would need to address the current limitations in the evidence in terms of the short time horizon and limited scope of health service resource use measured and valued. A cost–utility analysis could be conducted alongside an effectiveness study, combining trial-based and model-based evidence with long-term follow-up of mother–child dyads to collect data on resource use and health-related quality of life, and modelling costs and benefits over the lifetime. A societal perspective should also be considered in conjunction with the provider (NHS) perspective to gain a better understanding of the opportunity cost of providing support to women to breastfeed.
Equality, diversity and inclusion
We addressed equality, diversity and inclusion in the following ways:
-
Our work focuses on support for breastfeeding women; women of childbearing age and pregnant women are recognised as underserved groups.
-
The stakeholder working groups included healthcare practitioners serving ethnically diverse and disadvantaged populations, and rural localities across the UK.
-
The parents’ panel for the main study included a Gypsy/Traveller mother (one the most socially marginalised groups in the UK) and two fathers (men are rarely included in breastfeeding research).
-
The parents’ panel for women with MLTC included women with multiple physical and mental health conditions and are a group who face additional challenges in accessing breastfeeding support and are often excluded from breastfeeding research.
-
For the main study, we ensured the voices of women from ethnically diverse and socioeconomically deprived populations were included through conducting focus group discussions in West Yorkshire to supplement the views of the parents’ panel.
-
We ensured all communication was accessible for participants.
-
We offered evening meetings for the parents’ panels.
-
We paid parents and third-sector organisation representatives for their involvement to value their contributions.
-
In our workshops, we focused activities on the needs of populations with low breastfeeding rates.
-
The co-applicant team involved a range of levels of experience, included male and female researchers, and a PPI representative.
-
Our approach to the work was inclusive and everyone had the opportunity to contribute all aspects resulting in co-authorship of the report and development of knowledge and skills in evidence synthesis methods.
-
We also included a wide range of early career researchers including doctoral students in the conduct of the reviews to develop skills and have co-authorship of the resulting publications including the Cochrane review.
Regarding limitations, the research team (co-investigators) was not ethnically diverse, and we will consider this in future research. We acknowledge that recruiting parents to the parents’ panels via a variety of Facebook breastfeeding support groups, including those run by third-sector organisations, somewhat restricted those who engaged with us in terms of diversity of backgrounds. In future work, we will consider different strategies to optimise diversity.
Conclusions
‘Breastfeeding only’ support probably leads to a small reduction in the number of women stopping any and exclusive breastfeeding. ‘Breastfeeding plus’ support and breastfeeding support for women with LTCs probably leads to little or no reduction in the number of women stopping breastfeeding for most outcomes. As the work with stakeholders and mixed-methods review identified that women with LTCs face additional challenges when breastfeeding, more research is needed to develop effective support. In addition, evidence for the effectiveness and cost-effectiveness of breastfeeding feeding support interventions in the UK is lacking.
Additional information
Acknowledgements
We would like to thank all members of our stakeholder working groups, parents’ panels, focus group participants and workshop attendees for their time and insights.
We also thank members of the study steering committee for their invaluable advice and encouragement.
Thanks to the following people for their contribution to the project:
-
Karen Allum
-
Sharon Carstairs
-
Sushila Chowdhry
-
Shadrach Dare
-
Wendy Jones
-
Jayne Samples
-
Jonathan West
-
Flavia Ximenes Vasconcelos.
Contributions of authors
Anna Gavine (https://orcid.org/0000-0003-3750-2445) (Lecturer) led the systematic reviews on effectiveness of breastfeeding support for healthy women [update of Cochrane review (see Chapter 3)] and breastfeeding support for women with long-term conditions (see Chapter 6); participation in tasks related to mixed-methods review (see Chapter 7); design and running of stakeholder working groups, parents’ panels and workshops.
Albert Farre (https://orcid.org/0000-0001-8970-6146) (Lecturer) led the mixed-methods systematic reviews for healthy women (see Chapter 4) and women with long-term conditions (see Chapter 7); design, running and analysis of stakeholder working groups, parents’ panels and workshops; participation in tasks related to update of Cochrane review (see Chapter 3).
Fiona Lynn (https://orcid.org/000-0002-0216-643X) (Senior Lecturer) led the systematic reviews on cost-effectiveness (see Chapters 5 and 8); design and running of stakeholder working groups, parents’ panels and workshops; participation in tasks related to update of Cochrane review (see Chapter 3).
Shona Shinwell (https://orcid.org/0000-0001-9369-9698) (Project Manager) participation in tasks related to update of Cochrane review (see Chapter 3) and mixed-methods systematic review (see Chapter 4); design and running of stakeholder working groups, parents’ panels and workshops.
Phyllis Buchanan (https://orcid.org/0000-0002-1436-4396) (PPI Breastfeeding Network) participation in tasks related to update of Cochrane review (see Chapter 3) and effectiveness of breastfeeding support for women with LTCs (see Chapter 6); design, running and analysis of stakeholder working groups, parents’ panels, focus groups and workshops.
Joyce Marshall (https://orcid.org/0000-0002-2784-1817) (Lecturer) design, running and analysis of focus groups, stakeholder working groups, parents’ panels and workshops; participation in tasks related to update of Cochrane review (see Chapter 3).
Sara Cumming (https://orcid.org/0000-0003-0714-128x) (Clinical Academic Fellow) participation in tasks related to update of Cochrane review (see Chapter 3), mixed-methods reviews (see Chapters 4 and 7) and effectiveness of breastfeeding support for women with LTCs (see Chapter 6); design and analysis of stakeholder working groups, parents’ panels and workshops.
Louise Wallace (https://orcid.org/0000-0003-3770-0580) (Professor) study design and running of workshops.
Angie Wade (https://orcid.org/0000-0002-3944-8908) (Professor) design and analysis of update of Cochrane review (see Chapter 3); statistical advice for Chapter 6.
Elayne Ahern (https://orcid.org/0000-0001-9230-6776) (Lecturer) contributed to tasks related to the cost-effectiveness reviews (see Chapters 5 and 8).
Laura Hay (https://orcid.org/0000-0002-3259-9463) (Project Manager) design of and contribution to systematic review on effectiveness of breastfeeding support for women with LTCs (see Chapter 6); design and running of stakeholder working groups, parents’ panels and workshops.
Marianne Cranwell (https://orcid.org/0000-0003-0605-3923) (Research Assistant) analysis of stakeholder working group and parents’ panels; contribution of tasks related to mixed-methods review (see Chapter 7).
Alison McFadden (https://orcid.org/0000-0002-5164-2025) (Professor) led stakeholder and parent engagement work packages; participated in tasks related to update of Cochrane review (see Chapter 3), systematic review on effectiveness of breastfeeding support for women with LTCs (see Chapter 6), mixed-methods reviews (see Chapters 4 and 7); project oversight.
All authors were involved in drafting and/or commenting on the report.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/DGTP5702.
Primary conflicts of interest: Louise Wallace was a panel member of the following: DH National Institute for Health and Care Research (NIHR) Health and Social Care Delivery Research (HSDR) Development panel (2012–13); NIHR HSDR Researcher Led panel (2013–15); SDO Service Evaluations panel (2009–11); and NIHR Health Services & Research Development panel (Seacole) (October 2020–22). She received no payments for these other than expenses. Louise Wallace was also a paid scientific advisor for the NIHR HSDR Remit and Competitive Committee (2015–19).
Data-sharing statement
Further anonymised data are available on request from the corresponding author.
Ethics statement
The stakeholder engagement and PPI components of the study were approved by the University of Dundee School of Health Sciences research ethics committee (UOD‐SHS‐2021‐010) on 24 June 2021.
Information governance statement
The University of Dundee is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Dundee is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here https://www.dundee.ac.uk/corporate-information/data-protection-policy
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HSDR programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publication
Gavine A, Shinwell SC, Buchanan P, Farre A, Wade A, Lynn F, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2022;10:CD001141.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Bowatte G, Tham R, Allen K, Tan D, Lau M, Dai X, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta‐analysis. Acta Paediatr 2015;104:85-9.
- Horta B, Victora C. A Systematic Review on the Benefits of Breastfeeding on Diarrhoea and Pneumonia Mortality. Geneva: World Health Organization; 2013.
- Li R, Ware J, Chen A, Nelson JM, Kmet JM, Parks SE, et al. Breastfeeding and post-perinatal infant deaths in the United States, a national prospective cohort analysis. Lancet Region Health Am 2022;5.
- Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta‐analysis. Acta Paediatr 2015;104:3-13.
- Horta BL, Loret de Mola C, Victora CG. Long‐term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta‐analysis. Acta Paediatr 2015;104:30-7.
- Peres KG, Cascaes AM, Nascimento GG, Victora CG. Effect of breastfeeding on malocclusions: a systematic review and meta‐analysis. Acta Paediatr 2015;104:54-61.
- Tham R, Bowatte G, Dharmage SC, Tan DJ, Lau MX, Dai X, et al. Breastfeeding and the risk of dental caries: a systematic review and meta‐analysis. Acta Paediatr 2015;104:62-84.
- Heikkilä K, Kelly Y, Renfrew MJ, Sacker A, Quigley MA. Breastfeeding and educational achievement at age 5. Matern Child Nutr 2014;10:92-101.
- Heikkilä K, Sacker A, Kelly Y, Renfrew MJ, Quigley MA. Breast feeding and child behaviour in the Millennium Cohort Study. Arch Dis Child 2011;96:635-42.
- Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta‐analysis. Acta Paediatr 2015;104:14-9.
- Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta‐analysis. Acta Paediatr 2015;104:96-113.
- Rameez RM, Sadana D, Kaur S, Ahmed T, Patel J, Khan MS, et al. Association of maternal lactation with diabetes and hypertension: a systematic review and meta-analysis. JAMA Network Open 2019;2.
- Tschiderer L, Seekircher L, Kunutsor SK, Peters SA, O’Keeffe LM, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta‐analysis involving data from 8 studies and 1 192 700 parous women. J Am Heart Assoc 2022;11.
- Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Lancet Breastfeeding Series Group . Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475-90.
- NHS . Benefits of Breastfeeding 2020. www.nhs.uk/conditions/baby/breastfeeding-and-bottle-feeding/breastfeeding/benefits/ (accessed 16 January 2023).
- World Health Organization . Global Strategy for Infant and Young Child Feeding 2003.
- Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, et al. Lancet Breastfeeding Series Group . Breastfeeding 2: why invest, and what it will take to improve breastfeeding practices. Lancet 2016;387:491-504.
- Bartick MC, Schwarz EB, Green BD, Jegier BJ, Reinhold AG, Colaizy TT, et al. Suboptimal breastfeeding in the United States: maternal and pediatric health outcomes and costs. Matern Child Nutr 2017;13.
- van Tulleken C. Overdiagnosis and industry influence: how cow’s milk protein allergy is extending the reach of infant formula manufacturers. BMJ 2018;363.
- Joffe N, Webster F, Shenker N. Support for breastfeeding is an environmental imperative. BMJ 2019;367.
- Karlsson JO, Garnett T, Rollins NC, Röös E. The carbon footprint of breastmilk substitutes in comparison with breastfeeding. J Clean Prod 2019;222:436-45.
- Office for Health Improvement and Disparities . Fingertips Public Health Data: Child and Maternal Health 2022. https://fingertips.phe.org.uk/profile/child-health-profiles/data#page/1/gid/1938133228 (accessed 17 January 2023).
- Welsh Government . A Review of Breastfeeding Support and Practices in the Maternity and Early Years Settings in Wales 2018. https://gov.wales/sites/default/files/publications/2019-03/a-review-of-breastfeeding-support-and-practices-in-the-maternity-and-early-years-settings-in-wales.pdf (accessed 17 January 2023).
- Information Services Division . Infant Feeding Statistics Scotland: Financial Year 2018 19 2017. www.isdscotland.org/Health-Topics/Child-Health/Publications/2019-10-29/2019-10-29-Infant-Feeding-Report.pdf (accessed 17 January 2023).
- Public Health Agency . Health Intelligence Briefing: Breastfeeding in Northern Ireland 2021. www.publichealth.hscni.net/sites/default/files/2021-11/HI%20Brief%20Breastfeeding%202021%20FINAL%20Nov%2021.pdf (accessed 17 January 2023).
- McAndrew F, Thompson J, Fellows L, Large A, Speed M, Renfrew MJ. Infant Feeding Survey 2010. Health and Social Care Information Centre, IFF Research; 2012.
- Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs 2016;72:273-82.
- Gregory EF, Butz AM, Ghazarian SR, Gross SM, Johnson SB. Are unmet breastfeeding expectations associated with maternal depressive symptoms?. Acad Pediatr 2015;15:319-25.
- Theurich MA, Davanzo R, Busck-Rasmussen M, Díaz-Gómez NM, Brennan C, Kylberg E, et al. Breastfeeding rates and programs in Europe: a survey of 11 national breastfeeding committees and representatives. J Pediatr Gastroenterol Nutr 2019;68:400-7.
- Health Service Executive . Irish Maternity Indicator System: National Report 2019 2020.
- UNICEF . Protecting Health and Saving Lives: A Call to Action 2016.
- Brown A, Shenker N. Experiences of breastfeeding during COVID‐19: lessons for future practical and emotional support. Matern Child Nutr 2021;17.
- Grant A, McEwan K, Tedstone S, Greene G, Copeland L, Hunter B, et al. Availability of breastfeeding peer support in the United Kingdom: a cross‐sectional study. Matern Child Nutr 2018;14.
- Cook EJ, Powell F, Ali N, Penn-Jones C, Ochieng B, Randhawa G. Improving support for breastfeeding mothers: a qualitative study on the experiences of breastfeeding among mothers who reside in a deprived and culturally diverse community. Int J Equity Health 2021;20:1-14.
- McFadden A, Toole G. Exploring women’s views of breastfeeding: a focus group study within an area with high levels of socio‐economic deprivation. Matern Child Nutr 2006;2:156-68.
- Hunt L, Thomson G, Whittaker K, Dykes F. Adapting breastfeeding support in areas of socio-economic deprivation: a case study approach. Int J Equity Health 2021;20:1-13.
- Rayment J, McCourt C, Vaughan L, Christie J, Trenchard‐Mabere E. Bangladeshi women’s experiences of infant feeding in the London Borough of Tower Hamlets. Matern Child Nutr 2016;12:484-99.
- McFadden A, Renfrew MJ, Atkin K. Does cultural context make a difference to women’s experiences of maternity care? A qualitative study comparing the perspectives of breast‐feeding women of Bangladeshi origin and health practitioners. Health Expect 2013;16:e124-e35.
- Haroon S, Das JK, Salam RA, Imdad A, Bhutta ZA. Breastfeeding promotion interventions and breastfeeding practices: a systematic review. BMC Public Health 2013;13:1-18.
- Sinha B, Chowdhury R, Sankar MJ, Martines J, Taneja S, Mazumder S, et al. Interventions to improve breastfeeding outcomes: a systematic review and meta‐analysis. Acta Paediatr 2015;104:114-34.
- McFadden A, Gavine A, Renfrew MJ, Wade A, Buchanan P, Taylor JL, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2017;2.
- Kim SK, Park S, Oh J, Kim J, Ahn S. Interventions promoting exclusive breastfeeding up to six months after birth: a systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud 2018;80:94-105.
- Hoddinott P, Britten J, Pill R. Why do interventions work in some places and not others: a breastfeeding support group trial. Soc Sci Med 2010;70:769-78.
- Hoddinott P, Seyara R, Marais D. Global evidence synthesis and UK idiosyncrasy: why have recent UK trials had no significant effects on breastfeeding rates?. Matern Child Nutr 2011;7:221-7.
- Jolly K, Ingram L, Khan KS, Deeks JJ, Freemantle N, MacArthur C. Systematic review of peer support for breastfeeding continuation: metaregression analysis of the effect of setting, intensity, and timing. BMJ 2012;344.
- Clarke JL, Ingram J, Johnson D, Thomson G, Trickey H, Dombrowski SU, et al. An assets-based intervention before and after birth to improve breastfeeding initiation and continuation: the ABA feasibility RCT. Public Health Res 2020;8:1-156.
- Paranjothy S, Copeland L, Merrett L, Grant A, Phillips R, Gobat N, et al. A novel peer-support intervention using motivational interviewing for breastfeeding maintenance: a UK feasibility study. Health Technol Assess 2017;21:1-138.
- NHS England . Better Births. Improving Outcomes of Maternity Services in England. A Five Year Forward View for Maternity Care 2016.
- Jølving LR, Nielsen J, Kesmodel US, Nielsen RG, Beck‐Nielsen SS, Nørgård BM. Prevalence of maternal chronic diseases during pregnancy – a nationwide population based study from 1989 to 2013. Acta Obstet Gynecol Scand 2016;95:1295-304.
- Nishikawa E, Oakley L, Seed PT, Doyle P, Oteng-Ntim E. Maternal BMI and diabetes in pregnancy: investigating variations between ethnic groups using routine maternity data from London, UK. PLOS ONE 2017;12.
- Sultan AA, West J, Ban L, Humes D, Tata LJ, Fleming KM, et al. Adverse pregnancy outcomes among women with inflammatory bowel disease: a population-based study from England. Inflamm Bowel Dis 2016;22:1621-30.
- RCOG . Epilepsy in Pregnancy 2016.
- Fallon V, Davies SM, Silverio SA, Jackson L, De Pascalis L, Harrold JA. Psychosocial experiences of postnatal women during the COVID-19 pandemic. A UK-wide study of prevalence rates and risk factors for clinically relevant depression and anxiety. J Psychiatr Res 2021;136:157-66.
- Farrar D, Simmonds M, Griffin S, Duarte A, Lawlor DA, Sculpher M, et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess 2016;20:1-348.
- Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369.
- Head A, Fleming K, Kypridemos C, Schofield P, Pearson-Stuttard J, O’Flaherty M. Inequalities in incident and prevalent multimorbidity in England, 2004–19: a population-based, descriptive study. Lancet Healthy Longev 2021;2:e489-97.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43.
- Lee SI, Azcoaga-Lorenzo A, Agrawal U, Kennedy JI, Fagbamigbe AF, Hope H, et al. Epidemiology of pre-existing multimorbidity in pregnant women in the UK in 2018: a cross-sectional study. Lancet 2021;398.
- MuM-PreDiCT . List of Conditions 2022. https://mumpredict.org/portfolio/shared-findings/ (accessed 17 January 2023).
- Taskforce on Multiple Conditions . ‘Just One Thing After Another’: Living With Multiple Conditions 2018.
- Harrison S, Ayers S, Quigley M, Stein A, Alderdice F. Prevalence and factors associated with postpartum posttraumatic stress in a population-based maternity survey in England. J Affect Disord 2021;279:749-56.
- Šumilo D, Kurinczuk JJ, Redshaw ME, Gray R. Prevalence and impact of disability in women who had recently given birth in the UK. BMC Pregnancy Childbirth 2012;12:1-6.
- Brown J, Ceysens G, Boulvain M. Oral anti-diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database Syst Rev 2017;1.
- Bromley R, Weston J, Adab N, Greenhalgh J, Sanniti A, McKay AJ, et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev 2014;2014.
- Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev 2013;2.
- Plachcinski R, Moss N. Evidently Cochrane: Contemplating Pregnancy With Long-Term Health Conditions: Cochrane UK 2021. www.evidentlycochrane.net/contemplating-pregnancy-with-long-term-health-conditions/ (accessed 23 February 2023).
- Sokou R, Parastatidou S, Iliodromiti Z, Lampropoulou K, Vrachnis D, Boutsikou T, et al. Knowledge gaps and current evidence regarding breastfeeding issues in mothers with chronic diseases. Nutrients 2023;15.
- Scime NV, Patten SB, Tough SC, Chaput KH. Maternal chronic disease and breastfeeding outcomes: a Canadian population-based study. J Matern-Fetal Neonatal Med 2022;35:1148-55.
- Johnson EL, Burke AE, Wang A, Pennell PB. Unintended pregnancy, prenatal care, newborn outcomes, and breastfeeding in women with epilepsy. Neurology 2018;91:e1031-9.
- Ince-Askan H, Hazes JM, Dolhain RJ. Breastfeeding among women with rheumatoid arthritis compared with the general population: results from a nationwide prospective cohort study. J Rheumatol 2019;46:1067-74.
- Williams D, Webber J, Pell B, Grant A, Sanders J, Choy E, et al. ‘Nobody knows, or seems to know how rheumatology and breastfeeding works’: women’s experiences of breastfeeding whilst managing a long-term limiting condition – a qualitative visual methods study. Midwifery 2019;78:91-6.
- Renfrew MJ, Pokhrel S, Quigley M, McCormick F, Fox-Rushby J, Dodds R, et al. Preventing Disease and Saving Resources: The Potential Contribution of Increasing Breastfeeding Rates in the UK. UNICEF; 2012.
- Ahsan S, Jain S, Walters DD. The New Cost of Not Breastfeeding Tool 2022. www.aliveandthrive.org/en/the-new-cost-of-not-breastfeeding-tool (accessed 5 September 2023).
- Office for National Statistics . Gross National Income: Current Price: Seasonally Adjusted £m. UK Economic Accounts Time Series (UKEA) 2023. www.ons.gov.uk/economy/grossdomesticproductgdp/timeseries/abmz/ukea (accessed 5 September 2023).
- National Institute for Health and Care Excellence . BNF Child Specialised Formulas: Infant and Child 2023. https://bnfc.nice.org.uk/borderline-substances/specialised-formulas/specialised-formulas-infant-and-child/ (accessed 25 August 2023).
- Hickey G, Brearley S, Coldham T, Denegri S, Green G, Staniszewska S, et al. Guidance on Co-producing a Research Project. Southampton: NIHR INVOLVE; 2018.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a scoping review. Syst Rev 2018;7:1-26.
- Boote J, Baird W, Sutton A. Involving the public in systematic reviews: a narrative review of organizational approaches and eight case examples. J Comp Eff Res 2012;1:409-20.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. J Health Serv Res Policy 2019;24:245-55.
- Maxey D, Kezar A. Leveraging the Delphi technique to enrich knowledge and engage educational policy problems. Educ Policy 2016;30:1042-70.
- Schloemer T, Schröder-Bäck P. Criteria for evaluating transferability of health interventions: a systematic review and thematic synthesis. Implement Sci 2018;13:1-17.
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:1-14.
- Gavine A, Shinwell SC, Buchanan P, Farre A, Wade A, Lynn F, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2022;10.
- Covidence systematic review software 2022. www.covidence.org (accessed 4 June 2024).
- GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group; 2013.
- Alfirevic Z, Kellie F, Stewart F, Jones L, Hampson L. on behalf of Pregnancy and Childbirth Editorial Board . Identifying and handling potentially untrustworthy trials in Pregnancy and Childbirth Cochrane Reviews. Cochrane Database Syst Rev 2021;6.
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). London: Cochrane; 2022.
- Deeks JJ, Higgins JP, Altman DG, . Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (Updated February 2022). London: Cochrane; 2022.
- Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al. The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation. Health Technol Assess 2010;14.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Lancaster University; 2006.
- Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8.
- Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated consolidated framework for implementation research based on user feedback. Implement Sci 2022;17:1-16.
- Andaya E, Bonuck K, Barnett J, Lischewski-Goel J. Perceptions of primary care-based breastfeeding promotion interventions: qualitative analysis of randomized controlled trial participant interviews. Breastfeed Med 2012;7:417-22.
- Daniels K, Nor B, Jackson D, Ekström E-C, Doherty T. Supervision of community peer counsellors for infant feeding in South Africa: an exploratory qualitative study. Hum Resour Health 2010;8.
- Nankunda J, Tumwine JK, Soltvedt A, Semiyaga N, Ndeezi G, Tylleskär T. Community based peer counsellors for support of exclusive breastfeeding: experiences from rural Uganda. Int Breastfeed J 2006;1.
- Nankunda J, Tylleskär T, Ndeezi G, Semiyaga N, Tumwine JK. PROMISE-EPF Study Group . Establishing individual peer counselling for exclusive breastfeeding in Uganda: implications for scaling-up. Matern Child Nut 2010;6:53-66.
- Nkonki LL, Daniels KL. PROMISE-EBF study group . Selling a service: experiences of peer supporters while promoting exclusive infant feeding in three sites in South Africa. Int Breastfeed J 2010;5.
- Rahman A, Haq Z, Sikander S, Ahmad I, Ahmad M, Hafeez A. Using cognitive-behavioural techniques to improve exclusive breastfeeding in a low-literacy disadvantaged population: cognitive-behavioural techniques to improve breastfeeding rates. Matern Child Nutr 2012;8:57-71.
- Rujumba J, Ndeezi G, Nankabirwa V, Kwagala M, Mukochi M, Diallo AH, et al. ‘If I have money, I cannot allow my baby to breastfeed only …’ barriers and facilitators to scale-up of peer counselling for exclusive breastfeeding in Uganda. Int Breastfeed J 2020;15.
- Teich AS, Barnett J, Bonuck K. Women’s perceptions of breastfeeding barriers in early postpartum period: a qualitative analysis nested in two randomized controlled trials. Breastfeed Med 2014;9:9-15.
- Ahmed AH, Ouzzani M. Interactive web-based breastfeeding monitoring: feasibility, usability, and acceptability. J Hum Lact 2012;28:468-75.
- Cramer RL, McLachlan HL, Shafiei T, Amir LH, Cullinane M, Small R, et al. Implementation and evaluation of community-based drop-in centres for breastfeeding support in Victoria, Australia. Int Breastfeed J 2017;12.
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. NHS Grampian and the University of Aberdeen FEST project team . Process evaluation for the Feeding Support Team (FEST) randomised controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2.
- Nankunda J, Tumwine JK, Nankabirwa V, Tylleskär T, Group P-ES. ‘She would sit with me’: mothers’ experiences of individual peer support for exclusive breastfeeding in Uganda. Int Breastfeed J 2010;5.
- Bronner Y, Barber T, Vogelhut J, Resnik AK. Breastfeeding peer counseling: results from the national WIC survey. J Hum Lact 2001;17:119-25; quiz 132.
- Association of Degree and Timing of Exposure to Breastfeeding Peer Counseling Services with Breastfeeding Duration. Boston, MA: Springer US; 2004.
- Dennis C-L. Breastfeeding peer support: maternal and volunteer perceptions from a randomized controlled trial. Birth 2002;29:169-76.
- Ridgway L, Cramer R, McLachlan HL, Forster DA, Cullinane M, Shafiei T, et al. Breastfeeding support in the early postpartum: content of home visits in the SILC trial. Birth 2016;43:303-12.
- Tylleskär T, Jackson D, Meda N, Engebretsen IMS, Chopra M, Diallo AH, et al. PROMISE-EBF Study Group . Exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. Lancet 2011;378:420-7.
- Glanville J, Paisley S. Evidence‐Based Decisions and Economics: Health Care, Social Welfare, Education and Criminal Justice. New York, NY: Blackwell Publishing; 2010.
- National Institute for Health and Care Excellence (NICE) . Developing NICE Guidelines: The Manual [PMG20] 2022. www.nice.org.uk/process/pmg20/chapter/introduction (accessed 17 January 2023).
- Shemilt I, McDaid D, Marsh K, Henderson C, Bertranou E, Mallander J, et al. Issues in the incorporation of economic perspectives and evidence into Cochrane reviews. Syst Rev 2013;2:1-10.
- Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372.
- Evers S, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. CHEERS Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care 2013;29:117-22.
- Barnes J, Stuart J, Allen E, Petrou S, Sturgess J, Barlow J, et al. Randomized controlled trial and economic evaluation of nurse-led group support for young mothers during pregnancy and the first year postpartum versus usual care. Trials 2017;18:1-15.
- Hoddinott P, Britten J, Prescott GJ, Tappin D, Ludbrook A, Godden DJ. Effectiveness of policy to provide breastfeeding groups (BIG) for pregnant and breastfeeding mothers in primary care: cluster randomised controlled trial. BMJ 2009;338:a3026-a3026.
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. NHS Grampian and the University of Aberdeen FEST Project Team . The Feeding Support Team (FEST) randomised, controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2.
- Mavranezouli I, Varley-Campbell J, Stockton S, Francis J, Macdonald C, Sharma S, et al. The cost-effectiveness of antenatal and postnatal education and support interventions for women aimed at promoting breastfeeding in the UK. BMC Public Health 2022;22.
- Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A. Costs and benefits of community postnatal support workers: a randomised controlled trial. Health Technol Assess 2000;4:1-100.
- Delgado RI, Gill SL. A microcosting study of establishing a Baby Café® in Texas. J Hum Lact 2018;34:77-83.
- DelliFraine J, Langabeer J, Williams JF, Gong AK, Delgado RI, Gill SL. Cost comparison of baby friendly and non-baby friendly hospitals in the United States. Pediatrics 2011;127:e989-94.
- Frick KD, Pugh LC, Milligan RA. Costs related to promoting breastfeeding among urban low-income women. J Obstet Gynecol Neonatal Nurs 2012;41:144-50.
- Haider SJ, Chang LV, Bolton TA, Gold JG, Olson BH. An evaluation of the effects of a breastfeeding support program on health outcomes. Health Serv Res 2014;49:2017-34.
- Mottl-Santiago J. A Mixed Methods Economic Analysis of Doula-service Enhanced Maternity Care as Compared with Standard Maternity Care. Boston, MA: Boston University; 2020.
- Pugh LC, Milligan RA, Frick KD, Spatz D, Bronner Y. Breastfeeding duration, costs, and benefits of a support program for low-income breastfeeding women. Birth 2002;29:95-100.
- Wouk K, Chetwynd E, Vitaglione T, Sullivan C. Improving access to medical lactation support and counseling: building the case for Medicaid reimbursement. Matern Child Health J 2017;21:836-44.
- Brown V, Tan EJ, Hayes A, Baur L, Campbell K, Taylor R, et al. Cost comparison of five Australasian obesity prevention interventions for children aged from birth to two years. Pediatr Obes 2020;15.
- Hayes A, Lung T, Wen LM, Baur L, Rissel C, Howard K. Economic evaluation of ‘healthy beginnings’ an early childhood intervention to prevent obesity. Obesity 2014;22:1709-15.
- Tan EJ, Taylor RW, Taylor BJ, Brown V, Hayes AJ. Cost‐effectiveness of a novel sleep intervention in infancy to prevent overweight in childhood. Obesity 2020;28:2201-8.
- Wen LM, Baur LA, Rissel C, Flood V, Simpson JM, Hayes A, et al. Healthy Beginnings Trial Phase 2 study: follow-up and cost-effectiveness analysis. Contemp Clin Trials 2017;33:396-401.
- Pramono AY, Desborough JL, Smith JP, Bourke S. The social value of implementing the ten steps to successful breastfeeding in an Indonesian hospital: a case study. Yale J Biol Med 2021;94:429-58.
- Stevens B, Guerriere D, McKeever P, Croxford R, Miller K-L, Watson-MacDonell J, et al. Economics of home vs. hospital breastfeeding support for newborns. J Adv Nurs 2006;53:233-43.
- Hanafin S, O’Dwyer K, Creedon M, Clune Mulvaney C. Social Return on Investment: PHN-facilitated Breastfeeding Groups in Ireland. Research Matters; 2018.
- Chola L, Fadnes LT, Engebretsen IMS, Nkonki L, Nankabirwa V, Sommerfelt H, et al. PROMISE-EBF Study Group . Cost-effectiveness of peer counselling for the promotion of exclusive breastfeeding in Uganda. PLOS ONE 2015;10.
- Chola L, Nkonki L, Kankasa C, Nankunda J, Tumwine J, Tylleskar T, et al. PROMISE-EBF Study Group . Cost of individual peer counselling for the promotion of exclusive breastfeeding in Uganda. Cost Eff Resour Alloc 2011;9.
- George G, Mudzingwa T, Horwood C. The cost of the training and supervision of community health workers to improve exclusive breastfeeding amongst mothers in a cluster randomised controlled trial in South Africa. BMC Health Serv Res 2020;20:1-8.
- Kimani-Murage EW, Kyobutungi C, Ezeh AC, Wekesah F, Wanjohi M, Muriuki P, et al. Effectiveness of personalised, home-based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial. Trials 2013;14.
- Lamstein S, Perez-Escamilla R, Koniz-Booher P, France B, Adeyemi S, Kaligirwa C, et al. The Community Infant and Young Child Feeding Counselling Package in Kaduna State, Nigeria: A Mixed Methods Evaluation. Arlington, VA: SPRING; 2018.
- Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of women’s groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster-randomised controlled trial. Lancet 2013;381:1721-35.
- Manasyan A, Chomba E, McClure EM, Wright LL, Krzywanski S, Carlo WA. Cost-effectiveness of essential newborn care training in urban first-level facilities. Pediatrics 2011;127:e1176-81.
- Nkonki LL, Daviaud E, Jackson D, Chola L, Doherty T, Chopra M, et al. Promise-EBF Study Group . Costs of promoting exclusive breastfeeding at community level in three sites in South Africa. PLOS ONE 2014;9.
- Wynn A, Rotheram-Borus MJ, Leibowitz AA, Weichle T, Roux I, Tomlinson M. Mentor mothers program improved child health outcomes at a relatively low cost in South Africa. Health Aff 2017;36:1947-55.
- Daviaud E, Nkonki L, Ijumba P, Doherty T, Lawn JE, Owen H, et al. South-Africa (Goodstart III) trial: community-based maternal and newborn care economic analysis. Health Policy Plan 2017;32:i53-63.
- Nguyen TT, Hajeebhoy N, Li J, Do CT, Mathisen R, Frongillo EA. Community support model on breastfeeding and complementary feeding practices in remote areas in Vietnam: implementation, cost, and effectiveness. Int J Equity Health 2021;20:1-14.
- Pramono A, Smith J, Desborough J, Bourke S. Social value of maintaining baby-friendly hospital initiative accreditation in Australia: case study. Int J Equity Health 2021;20.
- Patel A, Kuhite P, Puranik A, Khan SS, Borkar J, Dhande L. Effectiveness of weekly cell phone counselling calls and daily text messages to improve breastfeeding indicators. BMC Pediatr 2018;18:1-12.
- Horton S, Sanghvi T, Phillips M, Fiedler J, Perez-Escamilla R, Lutter C, et al. Breastfeeding promotion and priority setting in health. Health Policy Plan 1996;11:156-68.
- Silva OLO, Rea MF, Sarti FM, Buccini G. Cost-effectiveness analysis of Baby-Friendly Hospital Initiative in promotion of breast-feeding and reduction of late neonatal infant mortality in Brazil. Public Health Nutr 2021;24:2365-75.
- Valdes V, Perez A, Labbok M, Pugin E, Zambrano I, Catalan S. The impact of a hospital and clinic-based breastfeeding promotion programme in a middle class urban environment. J Trop Pediatr 1993;39:142-51.
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group . Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet 2013;382:452-77.
- Adam T, Lim SS, Mehta S, Bhutta ZA, Fogstad H, Mathai M, et al. Cost effectiveness analysis of strategies for maternal and neonatal health in developing countries. BMJ 2005;331.
- Spiby H, Green JM, Darwin Z, Willmot H, Knox D, McLeish J, et al. Multi-site implementation of a promising innovation in low income communities: support for childbearing women. Health Serv Deliv Res 2015;3:1-332.
- Hayes A, Tan E, Brown V, Taylor B, Taylor R. A sleep modification programme in early infancy is a cost-effective and affordable approach to obesity prevention in young children. Obes Rev Conf 2020;21.
- Morrell CJ. Postnatal Support Who Wants It, What Is Its Benefit and How Much Does It Cost? PhD Thesis 2002.
- Rito AI, Buoncristiano M, Spinelli A, Salanave B, Kunešová M, Hejgaard T, et al. Association between characteristics at birth, breastfeeding and obesity in 22 countries: the WHO European Childhood Obesity Surveillance Initiative – COSI 2015/2017. Obes Facts 2019;12:226-43.
- Nguyen PTH, Pham NM, Chu KT, Van Duong D, Van Do D. Gestational diabetes and breastfeeding outcomes: a systematic review. Asia Pac J Public Health 2019;31:183-98.
- Finkelstein S, Keely E, Feig D, Tu X, Yasseen A, Walker M. Breastfeeding in women with diabetes: lower rates despite greater rewards a population‐based study. Diabet Med 2013;30:1094-101.
- Higgins J, Altman D, JAC S. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011). London: Cochrane; 2011.
- Stuebe AM, Bonuck K, Adatorwovor R, Schwartz TA, Berry DC. A cluster randomized trial of tailored breastfeeding support for women with gestational diabetes. Breastfeed Med 2016;11:504-13.
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443-57.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343.
- The Cochrane Collaboration . Review Manager 2020.
- Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007;369:1107-16.
- Steinig J, Nagl M, Linde K, Zietlow G, Kersting A. Antenatal and postnatal depression in women with obesity: a systematic review. Arch Womens Ment Health 2017;20:569-85.
- Lewkowitz AK, López JD, Stein RI, Rhoades JS, Schulz RC, Woolfolk CL, et al. Effect of a home-based lifestyle intervention on breastfeeding initiation among socioeconomically disadvantaged African American Women with overweight or obesity. Breastfeed Med 2018;13:418-25.
- Fan WQ, Chan C, Paterson S, Foster K, Morrow M, Bourne D, et al. Weekly lactation consultant led telephone calls in the first month postpartum improves breast feeding rates over standard care – a randomized controlled trial. J Paediatr Child Health 2022;58.
- Ijumba P, Doherty T, Jackson D, Tomlinson M, Sanders D, Swanevelder S, et al. Effect of an integrated community-based package for maternal and newborn care on feeding patterns during the first 12 weeks of life: a cluster-randomized trial in a South African township. Public Health Nutr 2015;18:2660-8.
- Martin J, MacDonald-Wicks L, Hure A, Smith R, Collins CE. Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: a pilot randomised controlled trial. Nutrients 2015;7:1464-79.
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070-6.
- Namale-Matovu J, Owora AH, Onyango-Makumbi C, Mubiru M, Namuli PE, Motevalli-Oliner M, et al. Comparative effects of three methods of promoting breastfeeding among human immunodeficiency virus-infected women in Uganda: a parallel randomized clinical trial. Int Health 2018;10:430-41.
- Reifsnider E, McCormick DP, Cullen KW, Todd M, Moramarco MW, Gallagher MR, et al. Randomized controlled trial to prevent infant overweight in a high-risk population. Acad Pediatr 2018;18:324-33.
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeed Med 2011;6:69-75.
- Chapman DJ, Morel K, Bermudez-Milan A, . Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics 2013;131:e162-70.
- Ehrlich SF, Hedderson MM, Quesenberry CP, Feng J, Brown SD, Crites Y, et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med 2014;31:862-7.
- Fiks AG, Gruver RS, Bishop-Gilyard CT, Suh AW, Kalra GK, DeRusso PA, et al. A social media peer group for mothers to prevent obesity from infancy: the Grow2Gether randomized trial. Child Obes 2017;13:356-68.
- Pezley L, Tussing-Humphreys L, Koenig MD, Maki P, Odoms-Young A, Freels S, et al. Feasibility of a web-based intervention to prevent perinatal depression and promote human milk feeding: randomized pilot trial. JMIR Form Res 2022;6.
- Bartu A, Sharp J, Ludlow J, Doherty DA. Postnatal home visiting for illicit drug-using mothers and their infants: a randomised controlled trial. Aust N Z J Obstet Gynaecol 2006;46:419-26.
- Carlsen EM, Kyhnaeb A, Renault KM, Cortes D, Michaelsen KF, Pryds O. Telephone-based support prolongs breastfeeding duration in obese women: a randomized trial. Am J Clin Nutr 2013;98:1226-32.
- O’Brien E, O’Reilly S, Sheehy L, O’Hagan L, McGuinness D, Coughlan B, et al. Latchon: a multi-centre, randomised controlled trial of perinatal support to improve breastfeeding outcomes in women with overweight and obesity. Arch Dis Child 2019;104.
- MacVicar S, Humphrey T, Forbes-McKay KE. Breastfeeding and the substance-exposed mother and baby. Birth 2018;45:450-8.
- Reimers P, Israel-Ballard K, Craig M, Spies L, Thior I, Tanser F, et al. A cluster randomised trial to determine the efficacy of the ‘feeding buddies’ programme in improving exclusive breastfeeding rates among HIV-infected women in Rural KwaZulu-Natal, South Africa. AIDS Behav 2018;22:212-23.
- Rotheram-Borus MJ, Richter LM, Van Heerden A, Van Rooyen H, Tomlinson M, Harwood JM, et al. A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants. PLOS ONE 2014;9.
- You H, Anjiang L, Jie X, Yan W, Biru L, Juan H, et al. Effects of breastfeeding education based on the self-efficacy theory on women with gestational diabetes mellitus: a CONSORT-compliant randomized controlled trial. Medicine 2020;99:1-7.
- Aldana-Parra F, Olaya G, Fewtrell M. Effectiveness of a new breastfeeding counselling intervention on breastfeeding prevalence, infant growth velocity and postpartum weight loss in overweight women: a randomized controlled trial. J Pediatr Gastroenterol Nutr 2022;74:976-7.
- Samburu BM, Young SL, Wekesah FM, Wanjohi MN, Kimiywe J, Muriuki P, et al. Effectiveness of the baby-friendly community initiative in promoting exclusive breastfeeding among HIV negative and positive mothers: a randomized controlled trial in Koibatek Sub-County, Baringo, Kenya. Int Breastfeed J 2020;15:1-13.
- Suryavanshi N, Kadam A, Gupte N, Hegde A, Kanade S, Sivalenka S, et al. A mobile health-facilitated behavioural intervention for community health workers improves exclusive breastfeeding and early infant HIV diagnosis in India: a cluster randomized trial. J Int AIDS Soc 2020;23.
- Berry DC, Hall EG, Neal MN, Adatorwovor R, Schwartz TA, Stuebe A. Results of the optimizing outcomes in women with gestational diabetes mellitus and their infants, a cluster randomized, controlled pilot study: lessons learned. JNBNA 2016;27:1-10.
- Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34:1519-25.
- Critical Appraisal Skills Programme 2022. https://casp-uk.net/casp-tools-checklists/ (accessed 1 March 2023).
- Downes M, Brennan M, Williams H, Dean R. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011458.
- Acheampong AK, Naab F, Kwashie A. The voices that influence HIV-positive mothers’ breastfeeding practices in an urban Ghanaian society. J Hum Lact 2018;34:176-83.
- Andrews EE, Powell RM, Ayers KB. Experiences of breastfeeding among disabled women. Womens Health Issues 2021;31:82-9.
- Demirci JR, Bogen DL, Klionsky Y. Breastfeeding and methadone therapy: the maternal experience. Subst Abus 2015;36:203-8.
- Dieterich R, Chang J, Danford C, Scott PW, Wend C, Demirci J. She ‘didn’t see my weight; she saw me, a mom who needed help breastfeeding’: perceptions of perinatal weight stigma and its relationship with breastfeeding experiences. J Health Psychol 2022;27:1027-38.
- Garner CD, Ratcliff SL, Devine CM, Thornburg LL, Rasmussen KM. Health professionals’ experiences providing breastfeeding-related care for obese women. Breastfeed Med 2014;9:503-9.
- Hazemba AN, Ncama BP, Sithole SL. Promotion of exclusive breastfeeding among HIV-positive mothers: an exploratory qualitative study. Int Breastfeed J 2016;11:1-10.
- Howard MB, Wachman E, Levesque EM, Schiff DM, Kistin CJ, Parker MG. The joys and frustrations of breastfeeding and rooming-in among mothers with opioid use disorder: a qualitative study. Hosp Pediatr 2018;8:761-8.
- Israel-Ballard K, Waithaka M, Greiner T. Infant feeding counselling of HIV-infected women in two areas in Kenya in 2008. Int J STD AIDS 2014;25:921-8.
- Jagiello KP, Azulay Chertok IR. Women’s experiences with early breastfeeding after gestational diabetes. J Obstet Gynecol Neonatal Nurs 2015;44:500-9.
- Keely A, Lawton J, Swanson V, Denison FC. Barriers to breast-feeding in obese women: a qualitative exploration. Midwifery 2015;31:532-9.
- MacVicar S, Humphrey T, Forbes-McKay KE. Breastfeeding support and opiate dependence: a think aloud study. Midwifery 2017;50:239-45.
- Nieuwoudt S, Manderson L. Frontline health workers and exclusive breastfeeding guidelines in an HIV endemic South African community: a qualitative exploration of policy translation. Int Breastfeed J 2018;13:1-10.
- Nor B, Ahlberg BM, Doherty T, Zembe Y, Jackson D, Ekström E-C. PROMISE-EBF Study Group . Mother’s perceptions and experiences of infant feeding within a community-based peer counselling intervention in South Africa. Matern Child Nutr 2012;8:448-58.
- Nor B, Zembe Y, Daniels K, Doherty T, Jackson D, Ahlberg BM, et al. ‘Peer but not peer’: considering the context of infant feeding peer counseling in a high HIV prevalence area. J Hum Lact 2009;25:427-34.
- O’Reilly SL, Conway MC, O’Brien EC, Molloy E, Walker H, O’Carroll E, et al. Exploring successful breastfeeding behaviors among women who have high body mass indices. J Hum Lact 2022;39:82-9.
- Hicks J, Morse E, Wyant DK. Barriers and facilitators of breastfeeding reported by postpartum women in methadone maintenance therapy. Breastfeed Med 2018;13:259-65.
- Matsunaga M, Kataoka Y, Igarashi Y, Fukui T, Imura M, Horiuchi S. Breastfeeding support and barriers to women with gestational diabetes mellitus: a nationwide cross-sectional survey of hospitals in Japan. BMC Pregnancy Childbirth 2021;21.
- Powell RM, Mitra M, Smeltzer SC, Long-Bellil LM, Smith LD, Rosenthal E, et al. Breastfeeding among women with physical disabilities in the United States. J Hum Lact 2018;34:253-61.
- Rasmussen KM, Lee VE, Ledkovsky TB, Kjolhede CL. A description of lactation counseling practices that are used with obese mothers. J Hum Lact 2006;22:322-7.
- Laws T, Pelentsov L, Esterman A, Steen M. Informing the midwife on the rare genetic disorders and their effects on mothers breastfeeding – a mixed methods study. Evid Based Midwifery 2016;14:11-5.
- Fadnes LT, Engebretsen IM, Moland KM, Nankunda J, Tumwine JK, Tylleskar T. Infant feeding counselling in Uganda in a changing environment with focus on the general population and HIV-positive mothers – a mixed method approach. BMC Health Serv Res 2010;10.
- Flax V, Hamela G, Mofolo I, Hosseinipour M, Hoffman I, Maman S. Infant and young child feeding counseling, decision-making, and practices among HIV-infected women in Malawi’s Option B+ prevention of mother-to-child transmission program: a mixed methods study. AIDS Behav 2016;20:2612-23.
- Misita D, Yamamoto JM, Yuan Y, Donovan LE, Bell RC, Jarman M. An exploration of differences in infant feeding practices among women with and without diabetes in pregnancy: a mixed-methods study. Diabet Med 2021;38.
- Bick D, Taylor C, Bhavnani V, Healey A, Seed P, Roberts S, et al. Lifestyle information and commercial weight management groups to support maternal postnatal weight management and positive lifestyle behaviour: the SWAN feasibility randomised controlled trial. BJOG 2020;127:636-45.
- Avram CM, Yieh L, Dukhovny D, Caughey AB. A cost-effectiveness analysis of rooming-in and breastfeeding in neonatal opioid withdrawal. Am J Perinatol 2020;37:001-7.
- Desmond C, Bland RM, Boyce G, Coovadia HM, Coutsoudis A, Rollins N, et al. Scaling-up exclusive breastfeeding support programmes: the example of KwaZulu-Natal. PLOS ONE 2008;3.
- Maredza M, Bertram MY, Saloojee H, Chersich MF, Tollman SM, Hofman KJ. Cost-effectiveness analysis of infant feeding strategies to prevent mother-to-child transmission of HIV in South Africa. Afr J AIDS Res 2013;12:151-60.
- Bick D, Taylor C, Bhavnani V, Healey A, Seed P, Roberts S, et al. Lifestyle information and access to a commercial weight management group to promote maternal postnatal weight management and positive lifestyle behaviour: the SWAN feasibility RCT. Public Health Res 2020;8:1-210.
- National Institute of Nursing Research . Evaluation of a Community Health Nurse Peer Counselor Program to Help Low-Income Women Breastfeed Longer 2006. https://ClinicalTrials.gov/show/NCT00608088 (accessed 5 September 2023).
- Bonuck K, Stuebe A, Barnett J, Labbok MH, Fletcher J, Bernstein PS. Effect of primary care intervention on breastfeeding duration and intensity. Am J Public Health 2014;104:119-27.
- Cavalcanti DS, Cabral CS, de Toledo Vianna RP, Osório MM. Online participatory intervention to promote and support exclusive breastfeeding: randomized clinical trial. Matern Child Nutr 2019;15.
- Fu I, Fong D, Heys M, Lee I, Sham A, Tarrant M. Professional breastfeeding support for first‐time mothers: a multicentre cluster randomised controlled trial. BJOG 2014;121:1673-83.
- McLachlan HL, Forster DA, Amir LH, Cullinane M, Shafiei T, Watson LF, et al. Supporting breastfeeding In Local Communities (SILC) in Victoria, Australia: a cluster randomised controlled trial. BMJ Open 2016;6.
- Unger J, Ronen K, Perrier T, DeRenzi B, Slyker J, Drake A, et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low‐to middle‐income country setting: a randomised trial. BJOG 2018;125:1620-9.
- Wu Q, Huang Y, Liao Z, van Velthoven MH, Wang W, Zhang Y. Effectiveness of WeChat for improving exclusive breastfeeding in Huzhu County China: randomized controlled trial. J Med Internet Res 2020;22.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:1-15.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. PharmacoEconomics 2008;26:661-77.
- Marschak J. Stanford Symposium on Mathematical Methods in the Social Sciences. Stanford, CA: Stanford University Press; 1960.
- Lancaster KJ. A new approach to consumer theory. J Polit Econ 1966;74:132-57.
- McFadden D. Economic choices. Amer Econ Rev 2001;91:351-78.
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902.
- Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health 2013;16:3-13.
- Street DJ, Burgess L. The Construction of Optimal Stated Choice Experiments: Theory and Methods. John Wiley & Sons; 2007.
- Croissant Y. Estimation of random utility models in R: the mlogit package. J Stat Softw 2020;95:1-41.
- Trickey H, Thomson G, Grant A, Sanders J, Mann M, Murphy S, et al. A realist review of one‐to‐one breastfeeding peer support experiments conducted in developed country settings. Matern Child Nutr 2018;14.
- Alianmoghaddam N, Phibbs S, Benn C. Reasons for stopping exclusive breastfeeding between three and six months: a qualitative study. J Pediatr Nurs 2018;39:37-43.
- McFadden A, Siebelt L, Marshall JL, Gavine A, Girard L-C, Symon A, et al. Counselling interventions to enable women to initiate and continue breastfeeding: a systematic review and meta-analysis. Int Breastfeed J 2019;14:1-19.
- Brockway M, Benzies K, Hayden KA. Interventions to improve breastfeeding self-efficacy and resultant breastfeeding rates: a systematic review and meta-analysis. J Hum Lact 2017;33:486-99.
- Beake S, Pellowe C, Dykes F, Schmied V, Bick D. A systematic review of structured compared with non‐structured breastfeeding programmes to support the initiation and duration of exclusive and any breastfeeding in acute and primary health care settings. Matern Child Nutr 2012;8:141-61.
- Pérez‐Escamilla R, Martinez JL, Segura‐Pérez S. Impact of the Baby‐friendly Hospital Initiative on breastfeeding and child health outcomes: a systematic review. Matern Child Nutr 2016;12:402-17.
- Gavine A, Marshall J, Buchanan P, Cameron J, Leger A, Ross S, et al. Remote provision of breastfeeding support and education: systematic review and meta‐analysis. Matern Child Nutr 2022;18.
- Moran VH, Morgan H, Rothnie K, MacLennan G, Stewart F, Thomson G, et al. Incentives to promote breastfeeding: a systematic review. Pediatrics 2015;135:e687-702.
- Vilar-Compte M, Hernández-Cordero S, Ancira-Moreno M, Burrola-Méndez S, Ferre-Eguiluz I, Omaña I, et al. Breastfeeding at the workplace: a systematic review of interventions to improve workplace environments to facilitate breastfeeding among working women. Int J Equity Health 2021;20:1-21.
- Chang Y-S, Beake S, Kam J, Lok KY-W, Bick D. Views and experiences of women, peer supporters and healthcare professionals on breastfeeding peer support: a systematic review of qualitative studies. Midwifery 2022;108.
- MacVicar S, Kirkpatrick P, Humphrey T, Forbes‐McKay KE. Supporting breastfeeding establishment among socially disadvantaged women: a meta‐synthesis. Birth 2015;42:290-8.
- Schmied V, Beake S, Sheehan A, McCourt C, Dykes F. Women’s perceptions and experiences of breastfeeding support: a metasynthesis. Birth 2011;38:49-60.
- Leeming D, Marshall J, Hinsliff S. Self‐conscious emotions and breastfeeding support: a focused synthesis of UK qualitative research. Matern Child Nutr 2022;18.
- Bengough T, Dawson S, Cheng HL, McFadden A, Gavine A, Rees R, et al. Factors that influence women’s engagement with breastfeeding support: a qualitative evidence synthesis. Matern Child Nutr 2022;18.
- Chesnel MJ, Healy M, McNeill J. Experiences that influence how trained providers support women with breastfeeding: a systematic review of qualitative evidence. PLOS ONE 2022;17.
- Fair FJ, Ford GL, Soltani H. Interventions for supporting the initiation and continuation of breastfeeding among women who are overweight or obese. Cochrane Database Syst Rev 2019;9.
- Reichental ZL, O’Brien VM, O’Reilly SL. Interventions to support women with overweight or obesity or gestational diabetes mellitus to initiate and continue breastfeeding: systematic review and meta‐analysis. Obes Rev 2022;23.
- Chang YS, Glaria AA, Davie P, Beake S, Bick D. Breastfeeding experiences and support for women who are overweight or obese: a mixed‐methods systematic review. Matern Child Nutr 2020;16.
- Lyons S, Currie S, Peters S, Lavender T, Smith DM. The perceptions and experiences of women with a body mass index ≥ 30 kg m2 who breastfeed: a meta‐synthesis. Matern Child Nutr 2019;15.
- Cummins L, Meedya S, Wilson V. Factors that positively influence in-hospital exclusive breastfeeding among women with gestational diabetes: an integrative review. Women Birth 2022;35:3-10.
- Tanganhito DDS, Bick D, Chang Y-S. Breastfeeding experiences and perspectives among women with postnatal depression: a qualitative evidence synthesis. Women Birth 2020;33:231-9.
- National Institute for Health and Care Excellence (NICE) . Postnatal Care [P] Breastfeeding Interventions 2021. www.nice.org.uk/guidance/ng194/evidence/p-breastfeeding-interventions-pdf-326764485980 (accessed 6 September 2023).
- Wallace J, Nwosu B, Clarke M. Barriers to the uptake of evidence from systematic reviews and meta-analyses: a systematic review of decision makers’ perceptions. BMJ Open 2012;2.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358.
- Shin C-N, Reifsnider E, McClain D, Jeong M, McCormick DP, Moramarco M. Acculturation, cultural values, and breastfeeding in overweight or obese, low-income, Hispanic women at 1 month postpartum. J Hum Lact 2018;34:358-64.
- UNICEF . Guide to the UNICEF UK Baby Friendly Initiative Standards 2017.
- Pérez-Escamilla R, Tomori C, Hernández-Cordero S, Baker P, Barros AJ, Bégin F, et al. Breastfeeding: crucially important, but increasingly challenged in a market-driven world. Lancet 2023;401:472-85.
- Lubbe W, Niela-Vilén H, Thomson G, Botha E. Impact of the COVID-19 pandemic on breastfeeding support services and women’s experiences of breastfeeding: a review. Int J Womens Health 2022;14:1447-57.
- Ahmed AH, Roumani AM. Breastfeeding monitoring improves maternal self-efficacy and satisfaction. Am J Matern Child Nurs 2020;45:357-63.
- Gross SM, Caulfield LE, Bentley ME, Bronner Y, Kessler L, Jensen J, et al. Counseling and motivational videotapes increase duration of breast-feeding in African-American WIC participants who initiate breast-feeding. J Am Diet Assoc 1998;98:143-8.
- Chapman DJ, Damio G, Young S, Pérez-Escamilla R. Effectiveness of breastfeeding peer counseling in a low-income, predominantly Latina population: a randomized controlled trial. Arch Pediatr Adolesc Med 2004;158:897-902.
- Dennis C-L, Hodnett E, Gallop R, Chalmers B. The effect of peer support on breast-feeding duration among primiparous women: a randomized controlled trial. CMAJ 2002;166:21-8.
- Sikander S, Maselko J, Zafar S, Haq Z, Ahmad I, Ahmad M, et al. Cognitive-behavioral counseling for exclusive breastfeeding in rural pediatrics: a cluster RCT. Pediatrics 2015;135:e424-31.
- Wen LM, Baur LA, Rissel C, Flood V, Simpson JM, Hayes A, et al. Healthy Beginnings Trial Phase 2 study: follow-up and cost-effectiveness analysis. Contemp Clin Trials 2012;33:396-401.
- Taylor RW, Gray AR, Heath AM, Galland BC, Lawrence J, Sayers R, et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the Prevention of Overweight in Infancy (POI) randomized controlled trial at ages 3.5 and 5y. Am J Clin Nutr 2018;108:228-36.
- Killedar A, Wen LM, Tan EJ, Marshall S, Taki S, Buchanan L, et al. Economic evaluation of the communicating healthy beginnings advice by telephone trial for early childhood obesity prevention. Obesity 2022;30:2256-64.
- Wen LM, Xu H, Taki S, Buchanan L, Rissel C, Phongsavan P, et al. Effects of telephone support or short message service on body mass index, eating and screen time behaviours of children age 2 years: a 3-arm randomized controlled trial. Pediatr Obes 2022;17.
- Aldana-Parra F, Olaya G, Fewtrell M. Effectiveness of a new approach for exclusive breastfeeding counselling on breastfeeding prevalence, infant growth velocity and postpartum weight loss in overweight or obese women: protocol for a randomized controlled trial. Int Breastfeed J 2020;15:1-14.
- Berry DC, Neal M, Hall EG, Schwartz TA, Verbiest S, Bonuck K, et al. Rationale, design, and methodology for the optimizing outcomes in women with gestational diabetes mellitus and their infants study. BMC Pregnancy Childbirth 2013;13.
- Stuebe AM, Bonuck K, Adatorwovor R, Schwartz TA, Berry D. A tailored breastfeeding support intervention for women with gestational diabetes. Am J Obstet Gynecol 2016;214.
- Carlsen EM, Kyhnaeb A, Renault KM, Cortes D, Michaelsen KF, Pryds O. Telephone-based support prolongs breastfeeding duration in obese women: a randomized trial. MIDIRS Midwifery Digest 2014;98.
- Chapman D, Perez-Escamilla R. Exclusive breastfeeding in the first 24 hours postpartum associated with improved breastfeeding outcomes of low-income, overweight and obese women. FASEB J 2013;27:122-3.
- Chapman DJ, Wetzel K, Bermudez-Millan A, Young S, Damio G, Perez-Escamilla R. Effects of breastfeeding peer counselling for obese women on infant health outcomes. FASEB J 2010;24:91-6.
- Morel K, Chapman DJ, Kyer N, Bermudez-Millan A, Young S, Perez-Escamilla R. Peer counselors improve breastfeeding technique among low-income, obese women. FASEB J 2010;24:91-7.
- ClinicalTrials.gov . Breastfeeding Education and Support Trial for Obese Women (BESTOW) 2011. www.cochranelibrary.com/central/doi/10.1002/central/CN-02017604/full (accessed 5 September 2023).
- O’Reilly SL, O’Brien EC, McGuinness D, Mehegan J, Coughlan B, O’Brien D, et al. Latch on: a protocol for a multi-centre, randomised controlled trial of perinatal support to improve breastfeeding outcomes in women with a raised BMI. Contemp Clin Trials Commun 2021;22.
- McCormick DP, Reyna L, Reifsnider E. Calories, caffeine and the onset of obesity in young children. Acad Pediatr 2020;20:801-8.
- Reimers P, Israel-Ballard K, Spies L, Tanser F, Thior I, Scott Gordon W, et al. A protocol for a cluster randomized trial on the effect of a ‘feeding buddy’ Program on adherence to the prevention of mother-to-child-transmission guidelines in a rural area of KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2016;72:130-6.
Appendix 1 Search strategies
MEDLINE search strategy for main study mixed-methods systematic review (see Chapter 4)
| S1 | TI OR AB (“wom#n” OR “mother*” OR “father*” OR “parent*” OR “famil*” OR “midwi*” OR “health professional*” OR “health provider*” OR “service provider” OR “maternity staff” OR “staff” OR “peer supporter*” OR “lay supporter*” OR “volunteer*” OR “manager*” OR “commissioner*” OR “policymaker*” OR “stakeholder*” OR “key informant*” OR “lactation consultant” OR “breastfeeding counsel#or” OR “infant-feeding lead*” OR “infant-feeding specialist*” OR “infant-feeding co-ordinator*”) | 3,268,371 |
| S2 | MH “Breast Feeding+” | 41,413 |
| S3 | TI OR AB (“breastfe*” OR “breast feed*” OR “breast fed” OR “breast-fe*”) | 46,339 |
| S4 | S2 OR S3 | 60,438 |
| S5 | TI OR AB (“support*” OR “help” OR “assist*” OR “education*” OR “class*” OR “workshop*” OR “champion*” OR “promot*” OR “counsel#ing”) | 5,380,883 |
| S6 | S4 AND S5 | 19,542 |
| S7 | MH “Pregnancy+” | 955,061 |
| S8 | MH “Maternal Health Services+” | 54,923 |
| S9 | MH “Maternal-Child Health Services” | 938 |
| S10 | MH “Perinatal Care+” | 11,204 |
| S11 | MH “Postnatal Care” | 6188 |
| S12 | MH “Postpartum Period+” | 70,902 |
| S13 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 988,993 |
| S14 | S6 AND S13 | 7333 |
| S15 | S6 OR S14 | 19,542 |
| S16 | S1 AND S15 | 15,537 |
| S17 | TI OR AB (“questionnaire*” OR “survey*” OR “interview*” OR “focus group*” OR “case stud*” OR “observ*” OR “ethnograph*” OR “hermeneutic*” OR “narrative*” OR “phenomenolog*” OR “grounded theory” OR “process evaluation” OR “implementation study” OR “implementation research”) | 5,274,651 |
| S18 | TI OR AB (“view*” OR “experienc*” OR “opinion*” OR “attitude*” OR “perception*” OR “perceive*” OR “belie*” OR “feel*” OR “know*” OR “understand*” OR “barrier*” OR “facilitator*” OR “enabler*” OR “obstacle*”) | 5,649,064 |
| S19 | MH “Qualitative Research+” OR TI (“qualitative” OR “mixed method*”) OR AB (“qualitative” OR “mixed method*”) | 302,606 |
| S20 | (S17 OR S18) AND S19 | 9,474,588 |
| S21 | S16 AND S20 | 1399 |
MEDLINE search strategy for main study economic evidence review (see Chapter 5)
Ovid MEDLINE® ALL 1946 to 17 August 2022
n = 2911, searched on 2 February 2022
-
exp Breast Feeding/
-
breastfeed*.mp.
-
breastfed.mp.
-
breast-feed*.mp.
-
breast-fed.mp.
-
breast feed*.mp.
-
breast fed.mp.
-
infant feed*.mp.
-
exp Milk, Human/
-
Lactation/
-
lactat*.mp.
-
support.mp.
-
Social Support/
-
advice.mp.
-
advis*.mp.
-
help*.mp.
-
supportive adj2 relationship.mp
-
counsel*.mp.
-
educat*.mp.
-
consult*.mp.
-
Health Promotion/
-
Health Education/
-
Economics/
-
exp “Costs and cost analysis”/
-
“Cost allocation”/
-
Cost-benefit analysis/
-
“Cost control”/
-
“Cost savings”/
-
“Cost of illness”/
-
“Cost sharing”/
-
“deductibles and coinsurance”/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp “fees and charges”/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
or/1-11
-
or/12-22
-
or/23-54
-
55 and 56 and 57
-
exp animals/ not humans.sh.
-
58 not 59
MEDLINE search strategy for LTCs effectiveness review (see Chapter 6)
Ovid MEDLINE® ALL 1946 to 17 August 2022
n = 1144, searched on 18 August 2022
-
exp Breast Feeding/ 42,543
-
(breastfeed* or breast-feed* or breast feed*).ab. 38,704
-
(breastfed or breast-fed or breast fed).ab. 12,845
-
lactation.ab. 35,254
-
infant feed*.ab. 4936
-
exp Lactation/ 46,504
-
exp Breast Milk Expression/ 385
-
exp Milk, Human/ 21,849
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 129,346
-
((support* or help or assist* or class* or workshop* or champion* or promot*) adj5 (breastfeed* or breast feed* or breastfed or breast fed or lactation or infant feed*)).ab. 7340
-
exp Social Support/ 78,157
-
anticipatory guidance.mp. 1527
-
exp Counseling/ 47,862
-
counsel*.mp. 152,131
-
exp Directive Counseling/ 4838
-
exp Health Promotion/ 83,692
-
exp Health Education/ 259,211
-
peer support.mp. 6270
-
professional support.mp. 2017
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 478,908
-
exp Chronic Disease/ 598,162
-
chronic disease*.mp. 339,068
-
chronic illness*.mp. 18,795
-
chronic condition*.mp. 23,438
-
(long term condition* or long-term condition*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2409
-
exp Comorbidity/ 124,635
-
(comorbid* or co-morbid*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 306,350
-
exp Multimorbidity/ 2332
-
(multimorbid* or multi-morbid*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 8950
-
(multidiseas* or multi-diseas*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 265
-
cancer.mp. or exp Neoplasms/ 4,276,838
-
atrial fibrillation.mp. or exp Atrial Fibrillation/ 98,446
-
cardiomyopathy.mp. or exp Cardiomyopathies/ 141,244
-
heart failure.mp. or exp Heart Failure/ 240,900
-
exp Hypercholesterolemia/or exp Hyperlipidemias/ 69,579
-
(hypercholesterol?emia or hyperlipid?emia).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 75,870
-
exp Hypertension/or hypertension.mp. 541,419
-
exp Myocardial Ischemia/ 461,609
-
(isch?emic heart disease or myocardial infarction).mp. [mptitle, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 296,886
-
peripheral vascular disease.mp. or exp Peripheral Vascular Diseases/ 64,594
-
exp Stroke/or stroke.mp. 371,135
-
exp Ischemic Attack, Transient/ 21,493
-
transient isch?emic attack.mp. 12,427
-
congenital heart disease.mp. or exp Heart Defects, Congenital/ 176,482
-
valvular heart disease.mp. or exp Heart Valve Diseases/ 135,662
-
rheumatic heart disease.mp. or exp Rheumatic Heart Disease/ 14,973
-
exp Heart Diseases/ 1,235,397
-
(heart disease or cardiac disease).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 211,888
-
alopecia areata.mp. or exp Alopecia Areata/ 5330
-
vitiligo.mp. or exp Vitiligo/ 8844
-
exp Eczema/or eczema.mp. 23,857
-
psoriasis.mp. or exp Psoriasis/ 59,218
-
acne.mp. or exp Acne Vulgaris/ 20,890
-
hidradenitis suppurativa.mp. or exp Hidradenitis Suppurativa/ 3821
-
lichen planus.mp. or exp Lichen Planus/ 11,081
-
rosacea.mp. or exp Rosacea/ 4650
-
seborrheic dermatitis.mp. or exp Dermatitis, Seborrheic/ 3404
-
allergic rhinitis.mp. or exp Rhinitis, Allergic/ 31,279
-
allergic conjunctivitis.mp. or exp Conjunctivitis, Allergic/ 4460
-
exp Hearing Loss/ 75,766
-
(hearing loss or deaf*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 110,959
-
exp Addison Disease/ or addison* disease.mp. 5996
-
exp Adrenocortical Adenoma/ or adren* adenoma.mp. 3139
-
exp Pheochromocytoma/ 16,444
-
ph?eochromocytoma.mp. 23,761
-
exp Cushing Syndrome/ or cushing* syndrome.mp. 15,868
-
exp Diabetes Mellitus, Type 2/ or exp Diabetes, Gestational/ or exp Diabetes Mellitus/ or exp Diabetes Mellitus, Type 1/ 485,761
-
(diabetes or diabetic$1).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 796,593
-
exp Parathyroid Diseases/ or (parathyroid dis* or hyperparathyroid* or hypoparathyroid*).mp. 46,457
-
exp Thyroid Diseases/ or thyroid dis*.mp. 165,219
-
(hyperthyroid* or hypothyroid* or thyroiditis or graves disease).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 100,159
-
exp Pituitary Diseases/ or pituitary dis*.mp. 64,970
-
exp Endocrine System Diseases/ 1,088,901
-
exp Vision Disorders/ 77,172
-
(visual* impair* or blindness).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 58,699
-
exp Cataract/or cataract.mp. 71,192
-
exp Diabetic Retinopathy/ 28,274
-
(diabetic retinopathy or diabetic eye dis*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 38,358
-
glaucoma.mp. or exp Glaucoma/ 78,243
-
scleritis.mp. or exp Scleritis/ 2363
-
episcleritis.mp. 619
-
exp Uveitis/or uveitis.mp. 40,674
-
retinal detachment.mp. or exp Retinal Detachment/ 28,643
-
exp Eye Diseases/ 619,560
-
alcoholic liver disease.mp. or exp Liver Diseases, Alcoholic/ 18,468
-
autoimmune hepatitis.mp. or exp Hepatitis, Autoimmune/ 7214
-
sclerosing cholangitis.mp. or exp Cholangitis, Sclerosing/ 7796
-
primary biliary cirrhosis.mp. or exp Liver Cirrhosis, Biliary/ 10,926
-
chronic hepatitis.mp. or exp Hepatitis, Chronic/ 73,061
-
exp Hepatitis B, Chronic/ or hepatitis B.mp. 108,026
-
exp Hepatitis C, Chronic/ or hepatitis C.mp. 98,802
-
liver cirrhosis.mp. or exp Liver Cirrhosis/ 111,811
-
non-alcoholic fatty liver disease.mp. or exp Non-alcoholic Fatty Liver Disease/ 26,127
-
exp Liver Diseases/ 608,267
-
chronic pancreatitis.mp. or exp Pancreatitis, Chronic/ 16,961
-
c?eliac disease.mp. or exp Celiac Disease/ 26,258
-
food allergy.mp. or exp Food Hypersensitivity/ 25,939
-
cholelithiasis.mp. or exp Cholelithiasis/ 40,132
-
gallstones.mp. 20,398
-
inflammatory bowel disease.mp. or exp Inflammatory Bowel Diseases/ 112,247
-
exp Crohn Disease/ or crohn* disease.mp. 62,081
-
ulcerative colitis.mp. or exp Colitis, Ulcerative/ 55,469
-
proctitis.mp. or exp Proctitis/ 4698
-
irritable bowel syndrome.mp. or exp Irritable Bowel Syndrome/ 16,561
-
lactose intolerance.mp. or exp Lactose Intolerance/ 3642
-
peptic ulcer.mp. or exp Peptic Ulcer/ 88,760
-
chronic pelvic inflammatory dis*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 135
-
exp Pelvic Inflammatory Disease/ 11,366
-
dysmenorrhea.mp. or exp Dysmenorrhea/ 7265
-
endometriosis.mp. or exp Endometriosis/ 31,546
-
infertility.mp. or exp Infertility/ 103,527
-
assisted reproduction.mp. or exp Reproductive Techniques, Assisted/ 80,694
-
(in vitro fertili#ation or IVF).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 40,047
-
leiomyoma.mp. or exp Leiomyoma/ 25,329
-
fibroids.mp. 5638
-
exp Menopause/ or menopause.mp. 74,996
-
menorrhagia.mp. or exp Menorrhagia/ 6281
-
exp Urinary Incontinence/ or exp Pelvic Floor Disorders/ or exp Pelvic Organ Prolapse/ or exp Fecal Incontinence/ 54,690
-
(pelvic floor dysfunction or pelvic floor disorder*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2942
-
(urinary incontinence or f?ecal incontinence).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 54,435
-
pelvic organ prolapse.mp. 7750
-
exp Polycystic Ovary Syndrome/ or polycystic ovar* syndrome.mp. 21,740
-
recurrent miscarriage.mp. or exp Abortion, Habitual/ 9651
-
exp Blood Coagulation Disorders/ or coagulation disorder.mp. 103,883
-
h?emophilia.mp. 28,903
-
exp Anemia, Sickle Cell/or sickle cell.mp. 31,815
-
thalass?emia.mp. or exp Thalassemia/ 30,118
-
thrombophilia.mp. or exp Thrombophilia/ 30,397
-
pernicious an?emia.mp. or exp Anemia, Pernicious/ 7005
-
exp Thrombocytopenia/ or primary thrombocytopenia.mp. 52,195
-
venous thromboembolism.mp. or exp Venous Thromboembolism/ 28,924
-
deep ve* thrombosis.mp. 31,151
-
pulmonary embolism.mp. or exp Pulmonary Embolism/ 59,166
-
HIV.mp. or exp HIV Infections/ 431,237
-
AIDS.mp. or exp Acquired Immunodeficiency Syndrome/ 231,823
-
exp Immunocompromised Host/ 27,296
-
exp Immunosuppression Therapy/ or exp Immunosuppressive Agents/ 386,330
-
immunosuppress*.mp. 247,166
-
exp Transplantation/ 558,640
-
transplant*.mp. 821,998
-
exp Alcohol-Related Disorders/ 119,529
-
(alcohol misuse or alcohol abuse or alcohol dependence or alcoholism).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 102,278
-
exp Substance-Related Disorders/ 303,321
-
(substance misuse or substance abuse or substance dependence).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 61,758
-
exp Anxiety/ or exp Anxiety Disorders/ 179,995
-
anxiety.mp. 287,258
-
panic disorder.mp. or exp Panic Disorder/ 11,686
-
phobic disorder.mp. or exp Phobic Disorders/ 12,239
-
phobia.mp. 9375
-
exp Stress Disorders, Post-Traumatic/ 39,160
-
(post traumatic stress disorder or post-traumatic stress disorder or PTSD).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 36,261
-
exp Mood Disorders/ 132,929
-
exp Depression/ or exp Depression, Postpartum/ 148,840
-
(depression or depressive disorder or mood disorder).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 504,128
-
dementia.mp. or exp Dementia/ 245,230
-
eating disorder.mp. or exp “Feeding and Eating Disorders”/ 38,341
-
(anorexia nervosa or bulimia nervosa).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 21,280
-
bipolar disorder.mp. or exp Bipolar Disorder/ 54,684
-
exp “schizophrenia spectrum and other psychotic disorders”/ 159,357
-
(schizophrenia or psychosis or psychotic disorder or schizoaffective disorder).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 182,203
-
dissociative disorder.mp. or exp Dissociative Disorders/ 4820
-
obsessive compulsive disorder.mp. or exp Obsessive-Compulsive Disorder/ 21,228
-
personality disorder.mp. or exp Personality Disorders/ 49,154
-
self harm.mp. or exp Self-Injurious Behavior/ 83,262
-
exp Mental Disorders/ 1,386,917
-
(mental disorder or psychiatric disorder).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 21,591
-
(serious mental illness or severe mental illness).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 9002
-
exp Attention Deficit Disorder with Hyperactivity/ 33,335
-
(attention deficit hyperactivity disorder or ADHD).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 36,926
-
exp Autism Spectrum Disorder/ 39,314
-
(autism or autistic).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 64,227
-
cerebral palsy.mp. or exp Cerebral Palsy/ 30,646
-
intellectual disabilit*.mp. or exp Intellectual Disability/ 113,325
-
exp Down Syndrome/ or down* syndrome.mp. 32,403
-
exp Brain Injuries/ or acquired brain injury.mp. 79,525
-
exp Headache Disorders/ 38,752
-
(cluster headache or tension headache or chronic headache or migraine).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 47,340
-
exp Epilepsy/ 122,463
-
(epilepsy or epileptic).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 167,049
-
idiopathic intracranial hypertension.mp. or exp Pseudotumor Cerebri/ 5202
-
multiple sclerosis.mp. or exp Multiple Sclerosis/ 94,760
-
peripheral neuropathy.mp. or exp Peripheral Nervous System Diseases/ 171,846
-
exp Parkinson Disease/ or parkinson* disease.mp. 126,506
-
exp Neurodegenerative Diseases/ 351,658
-
(huntington* disease or huntington* chorea).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 20,268
-
motor neurone disease.mp. 1091
-
exp Sleep Wake Disorders/ 104,157
-
(sleep disorder or narcolepsy or obstructive sleep apn?ea).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 41,903
-
spina bifida.mp. or exp Spinal Dysraphism/ 12,050
-
exp Fatigue Syndrome, Chronic/ 6142
-
(chronic fatigue syndrome or myalgic encephalomyelitis).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6471
-
fibromyalgia.mp. or exp Fibromyalgia/ 13,310
-
exp Chronic Pain/ 20,443
-
exp Complex Regional Pain Syndromes/ 5884
-
exp Myofascial Pain Syndromes/ 6762
-
(chronic pain or pain syndrome).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 66,066
-
exp Back Pain/ or chronic back pain.mp. 44,337
-
osteoarthritis.mp. or exp Osteoarthritis/ 104,568
-
exp Osteoporosis/ 60,974
-
(osteoporosis or osteopenia).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 101,668
-
scoliosis.mp. or exp Scoliosis/ 28,225
-
exp Spinal Diseases/ 136,461
-
exp Fractures, Compression/ 2943
-
(compression fracture or collapsed vertebra*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2462
-
intervertebral disc degeneration.mp. or exp Intervertebral Disc Degeneration/ 8378
-
sciatica.mp. or exp Sciatica/ 7190
-
spinal stenosis.mp. or exp Spinal Stenosis/ 9544
-
spondylosis.mp. or exp Spondylosis/ 11,155
-
spondylolisthesis.mp. or exp Spondylolisthesis/ 7445
-
amputation.mp. or exp Amputation/ 52,172
-
amputee.mp. 2992
-
paralysis.mp. or exp Paralysis/ 116,571
-
exp Hemiplegia/ or hemiplegia.mp. 16,538
-
exp Paraplegia/ or paraplegia.mp. 22,118
-
quadriplegia.mp. or exp Quadriplegia/ 10,093
-
exp Disabled Persons/ 71,645
-
(disabled or disabilit*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 362,858
-
chronic kidney disease.mp. or exp Renal Insufficiency, Chronic/ 162,815
-
h?emodialysis.mp. or exp Renal Dialysis/ 146,472
-
exp Urinary Calculi/ 37,823
-
(urinary tract stone* or kidney stone*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 7369
-
exp Asthma/ or asthma.mp. 191,929
-
exp Lung Diseases/ or chronic lung disease.mp. 1,147,038
-
bronchiectasis.mp. or exp Bronchiectasis/ 15,082
-
exp Pulmonary Disease, Chronic Obstructive/ 64,208
-
(chronic obstructive pulmonary disease or COPD).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 75,276
-
cystic fibrosis.mp. or exp Cystic Fibrosis/ 55,910
-
interstitial lung disease.mp. or exp Lung Diseases, Interstitial/ 87,153
-
pulmonary fibrosis.mp. 34,100
-
pulmonary hypertension.mp. or exp Hypertension, Pulmonary/ 56,630
-
exp Sarcoidosis/ or sarcoidosis.mp. 33,019
-
exp Tuberculosis/ or tuberculosis.mp. 272,422
-
exp Ehlers-Danlos Syndrome/ or ehlers-danlos.mp. 4661
-
rheumatoid arthritis.mp. or exp Arthritis, Rheumatoid/ 160,931
-
exp Sjogren’s Syndrome/ 14,126
-
(sjogren* syndrome or sjogren* disease).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 19,617
-
exp Raynaud Disease/ or raynaud*.mp. 10,156
-
systemic sclerosis.mp. or exp Scleroderma, Systemic/ 26,894
-
scleroderma.mp. 29,230
-
primary systemic vasculitis.mp. or exp Systemic Vasculitis/ 18,109
-
marfan* syndrome.mp. or exp Marfan Syndrome/ 8617
-
spondyloarthritis.mp. or exp Spondylarthritis/ 31,153
-
psoriatic arthritis.mp. or exp Arthritis, Psoriatic/ 12,337
-
ankylosing spondylitis.mp. or exp Spondylitis, Ankylosing/ 21,066
-
systemic lupus erythematosus.mp. or exp Lupus Erythematosus, Systemic/ 79,628
-
autoimmune disease.mp. or exp Autoimmune Diseases/ 540,341
-
frailty.mp. or exp Frailty/ 22,139
-
exp COVID-19/ or long covid.mp. 181,547
-
post COVID syndrome.mp. 213
-
exp Obesity/ 247,988
-
(obese or obesity).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 411,960
-
polypharmacy.mp. or exp Polypharmacy/ 12,571
-
turner* syndrome.mp. or exp Turner Syndrome/ 9685
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 or 146 or 147 or 148 or 149 or 150 or 151 or 152 or 153 or 154 or 155 or 156 or 157 or 158 or 159 or 160 or 161 or 162 or 163 or 164 or 165 or 166 or 167 or 168 or 169 or 170 or 171 or 172 or 173 or 174 or 175 or 176 or 177 or 178 or 179 or 180 or 181 or 182 or 183 or 184 or 185 or 186 or 187 or 188 or 189 or 190 or 191 or 192 or 193 or 194 or 195 or 196 or 197 or 198 or 199 or 200 or 201 or 202 or 203 or 204 or 205 or 206 or 207 or 208 or 209 or 210 or 211 or 212 or 213 or 214 or 215 or 216 or 217 or 218 or 219 or 220 or 221 or 222 or 223 or 224 or 225 or 226 or 227 or 228 or 229 or 230 or 231 or 232 or 233 or 234 or 235 or 236 or 237 or 238 or 239 or 240 or 241 or 242 or 243 or 244 or 245 or 246 or 247 or 248 or 249 or 250 or 251 or 252 or 253 14,314,773
-
randomized controlled trial.pt. 575,118
-
controlled clinical trial.pt. 94,989
-
randomi#ed.ab. 684,071
-
placebo.ab. 230,860
-
drug therapy.fs. 2,521,208
-
randomly.ab. 389,335
-
trial.ab. 612,686
-
groups.ab. 2,394,830
-
255 or 256 or 257 or 258 or 259 or 260 or 261 or 262 5,455,224
-
exp animals/ not humans.sh. 5,037,553
-
263 not 264 4,753,806
-
9 and 20 and 254 and 265 1144
MEDLINE search strategy for LTCs mixed-methods systematic review (see Chapter 7)
Ovid MEDLINE® ALL 1946 to 23 November 2022
n = 2187, searched on 24 November 2022
-
exp Breast Feeding/ 42,954
-
(breastfeed* or breast-feed* or breast feed*).ab. 39,367
-
(breastfed or breast-fed or breast fed).ab. 13,012
-
lactation.ab. 35,775
-
infant feed*.ab. 4996
-
exp Lactation/ 46,965
-
exp Breast Milk Expression/ 385
-
exp Milk, Human/ 22,053
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 130,890
-
((support* or help or assist* or class* or workshop* or champion* or promot*) adj5 (breastfeed* or breast feed* or breastfed or breast fed or lactation or infant feed*)).ab. 7474
-
exp Social Support/ 78,607
-
anticipatory guidance.mp. 1545
-
exp Counseling/ 48,142
-
counsel*.mp. 154,556
-
exp Directive Counseling/ 4882
-
exp Health Promotion/ 84,136
-
exp Health Education/ 260,144
-
peer support.mp. 6522
-
professional support.mp. 2070
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 483,065
-
exp Chronic Disease/ 602,943
-
chronic disease*.mp. 342,009
-
chronic illness*.mp. 19,105
-
chronic condition*.mp. 24,060
-
(long term condition* or long-term condition*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2474
-
exp Comorbidity/ 125,267
-
(comorbid* or co-morbid*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 312,919
-
exp Multimorbidity/ 2454
-
(multimorbid* or multi-morbid*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 9346
-
(multidiseas* or multi-diseas*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 278
-
cancer.mp. or exp Neoplasms/ 4,329,926
-
atrial fibrillation.mp. or exp Atrial Fibrillation/ 100,464
-
cardiomyopathy.mp. or exp Cardiomyopathies/ 143,152
-
heart failure.mp. or exp Heart Failure/ 245,181
-
exp Hypercholesterolemia/ or exp Hyperlipidemias/ 69,913
-
(hypercholesterol?emia or hyperlipid?emia).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 76,793
-
exp Hypertension/or hypertension.mp. 547,959
-
exp Myocardial Ischemia/ 464,415
-
(isch?emic heart disease or myocardial infarction).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 299,852
-
peripheral vascular disease.mp. or exp Peripheral Vascular Diseases/ 65,062
-
exp Stroke/ or stroke.mp. 378,102
-
exp Ischemic Attack, Transient/ 21,618
-
transient isch?emic attack.mp. 12,655
-
congenital heart disease.mp. or exp Heart Defects, Congenital/ 177,949
-
valvular heart disease.mp. or exp Heart Valve Diseases/ 136,981
-
rheumatic heart disease.mp. or exp Rheumatic Heart Disease/ 15,031
-
exp Heart Diseases/ 1,245,391
-
(heart disease or cardiac disease).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 214,566
-
alopecia areata.mp. or exp Alopecia Areata/ 5456
-
vitiligo.mp. or exp Vitiligo/ 8980
-
exp Eczema/ or eczema.mp. 24,134
-
psoriasis.mp. or exp Psoriasis/ 60,070
-
acne.mp. or exp Acne Vulgaris/ 21,180
-
hidradenitis suppurativa.mp. or exp Hidradenitis Suppurativa/ 3951
-
lichen planus.mp. or exp Lichen Planus/ 11,203
-
rosacea.mp. or exp Rosacea/ 4729
-
seborrheic dermatitis.mp. or exp Dermatitis, Seborrheic/ 3431
-
allergic rhinitis.mp. or exp Rhinitis, Allergic/ 31,617
-
allergic conjunctivitis.mp. or exp Conjunctivitis, Allergic/ 4493
-
exp Hearing Loss/ 76,292
-
(hearing loss or deaf*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 112,157
-
exp Addison Disease/ or addison* disease.mp. 6034
-
exp Adrenocortical Adenoma/ or adren* adenoma.mp. 3177
-
64exp Pheochromocytoma/ 16,529
-
ph?eochromocytoma.mp. 23,936
-
exp Cushing Syndrome/ or cushing* syndrome.mp. 15,980
-
exp Diabetes Mellitus, Type 2/ or exp Diabetes, Gestational/ or exp Diabetes Mellitus/ or exp Diabetes Mellitus, Type 1/ 492,036
-
(diabetes or diabetic$1).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 809,517
-
exp Parathyroid Diseases/ or (parathyroid dis* or hyperparathyroid* or hypoparathyroid*).mp. 46,799
-
exp Thyroid Diseases/ or thyroid dis*.mp. 166,479
-
(hyperthyroid* or hypothyroid* or thyroiditis or graves disease).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 100,991
-
exp Pituitary Diseases/ or pituitary dis*.mp. 65,344
-
exp Endocrine System Diseases/ 1,099,839
-
exp Vision Disorders/ 77,726
-
(visual* impair* or blindness).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 59,580
-
exp Cataract/ or cataract.mp. 71,946
-
exp Diabetic Retinopathy/ 28,637
-
(diabetic retinopathy or diabetic eye dis*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 39,000
-
glaucoma.mp. or exp Glaucoma/ 79,199
-
scleritis.mp. or exp Scleritis/ 2393
-
episcleritis.mp. 624
-
exp Uveitis/ or uveitis.mp. 41,131
-
retinal detachment.mp. or exp Retinal Detachment/ 28,930
-
exp Eye Diseases/ 624,904
-
alcoholic liver disease.mp. or exp Liver Diseases, Alcoholic/ 18,612
-
autoimmune hepatitis.mp. or exp Hepatitis, Autoimmune/ 7336
-
sclerosing cholangitis.mp. or exp Cholangitis, Sclerosing/ 7900
-
primary biliary cirrhosis.mp. or exp Liver Cirrhosis, Biliary/ 10,999
-
chronic hepatitis.mp. or exp Hepatitis, Chronic/ 73,739
-
exp Hepatitis B, Chronic/ or hepatitis B.mp. 109,029
-
exp Hepatitis C, Chronic/ or hepatitis C.mp. 99,589
-
liver cirrhosis.mp. or exp Liver Cirrhosis/ 113,060
-
non-alcoholic fatty liver disease.mp. or exp Non-alcoholic Fatty Liver Disease/ 27,321
-
exp Liver Diseases/ 613,804
-
chronic pancreatitis.mp. or exp Pancreatitis, Chronic/ 17,127
-
c?eliac disease.mp. or exp Celiac Disease/ 26,440
-
food allergy.mp. or exp Food Hypersensitivity/ 26,232
-
cholelithiasis.mp. or exp Cholelithiasis/ 40,302
-
gallstones.mp. 20,545
-
inflammatory bowel disease.mp. or exp Inflammatory Bowel Diseases/ 114,088
-
exp Crohn Disease/ or crohn* disease.mp. 62,863
-
ulcerative colitis.mp. or exp Colitis, Ulcerative/ 56,412
-
proctitis.mp. or exp Proctitis/ 4741
-
irritable bowel syndrome.mp. or exp Irritable Bowel Syndrome/ 16,860
-
lactose intolerance.mp. or exp Lactose Intolerance/ 3661
-
peptic ulcer.mp. or exp Peptic Ulcer/ 88,964
-
chronic pelvic inflammatory dis*.mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 135
-
exp Pelvic Inflammatory Disease/ 11,423
-
dysmenorrhea.mp. or exp Dysmenorrhea/ 7392
-
endometriosis.mp. or exp Endometriosis/ 31,997
-
infertility.mp. or exp Infertility/ 104,859
-
assisted reproduction.mp. or exp Reproductive Techniques, Assisted/ 81,518
-
(in vitro fertili#ation or IVF).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 40,612
-
leiomyoma.mp. or exp Leiomyoma/ 25,551
-
fibroids.mp. 5762
-
exp Menopause/ or menopause.mp. 75,624
-
menorrhagia.mp. or exp Menorrhagia/ 6342
-
exp Urinary Incontinence/ or exp Pelvic Floor Disorders/ or exp Pelvic Organ Prolapse/ or exp Fecal Incontinence/ 55,156
-
(pelvic floor dysfunction or pelvic floor disorder*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 3030
-
(urinary incontinence or f?ecal incontinence).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 54,896
-
pelvic organ prolapse.mp. 7917
-
exp Polycystic Ovary Syndrome/ or polycystic ovar* syndrome.mp. 22,165
-
recurrent miscarriage.mp. or exp Abortion, Habitual/ 9737
-
exp Blood Coagulation Disorders/ or coagulation disorder.mp. 104,538
-
h?emophilia.mp. 29,136
-
exp Anemia, Sickle Cell/ or sickle cell.mp. 32,225
-
thalass?emia.mp. or exp Thalassemia/ 30,381
-
thrombophilia.mp. or exp Thrombophilia/ 30,589
-
pernicious an?emia.mp. or exp Anemia, Pernicious/ 7018
-
exp Thrombocytopenia/ or primary thrombocytopenia.mp. 52,629
-
venous thromboembolism.mp. or exp Venous Thromboembolism/ 29,575
-
deep ve* thrombosis.mp. 31,577
-
pulmonary embolism.mp. or exp Pulmonary Embolism/ 59,857
-
HIV.mp. or exp HIV Infections/ 434,875
-
AIDS.mp. or exp Acquired Immunodeficiency Syndrome/ 233,377
-
exp Immunocompromised Host/ 27,383
-
exp Immunosuppression Therapy/ or exp Immunosuppressive Agents/ 388,928
-
immunosuppress*.mp. 250,351
-
exp Transplantation/ 562,203
-
transplant*.mp. 829,484
-
exp Alcohol-Related Disorders/ 120,079
-
(alcohol misuse or alcohol abuse or alcohol dependence or alcoholism).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 102,885
-
exp Substance-Related Disorders/ 305,479
-
(substance misuse or substance abuse or substance dependence).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 62,218
-
exp Anxiety/ or exp Anxiety Disorders/ 182,212
-
anxiety.mp. 293,708
-
panic disorder.mp. or exp Panic Disorder/ 11,740
-
phobic disorder.mp. or exp Phobic Disorders/ 12,318
-
phobia.mp. 9483
-
exp Stress Disorders, Post-Traumatic/ 39,810
-
(post traumatic stress disorder or post-traumatic stress disorder or PTSD).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 37,152
-
exp Mood Disorders/ 133,816
-
exp Depression/ or exp Depression, Postpartum/ 151,065
-
(depression or depressive disorder or mood disorder).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 512,446
-
dementia.mp. or exp Dementia/ 249,440
-
eating disorder.mp. or exp “Feeding and Eating Disorders”/ 38,866
-
(anorexia nervosa or bulimia nervosa).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 21,488
-
bipolar disorder.mp. or exp Bipolar Disorder/ 55,305
-
exp “schizophrenia spectrum and other psychotic disorders”/ 160,377
-
(schizophrenia or psychosis or psychotic disorder or schizoaffective disorder).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 184,089
-
dissociative disorder.mp. or exp Dissociative Disorders/ 4851
-
obsessive compulsive disorder.mp. or exp Obsessive-Compulsive Disorder/ 21,507
-
personality disorder.mp. or exp Personality Disorders/ 49,545
-
self harm.mp. or exp Self-Injurious Behavior/ 84,338
-
exp Mental Disorders/ 1,400,314
-
(mental disorder or psychiatric disorder).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 22,024
-
(serious mental illness or severe mental illness).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 9183
-
exp Attention Deficit Disorder with Hyperactivity/ 33,703
-
(attention deficit hyperactivity disorder or ADHD).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 37,625
-
exp Autism Spectrum Disorder/ 40,143
-
(autism or autistic).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 65,738
-
cerebral palsy.mp. or exp Cerebral Palsy/ 31,036
-
intellectual disabilit*.mp. or exp Intellectual Disability/ 114,299
-
exp Down Syndrome/ or down* syndrome.mp. 32,674
-
exp Brain Injuries/ or acquired brain injury.mp. 80,562
-
exp Headache Disorders/ 39,158
-
(cluster headache or tension headache or chronic headache or migraine).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 47,919
-
exp Epilepsy/ 123,445
-
(epilepsy or epileptic).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 168,992
-
idiopathic intracranial hypertension.mp. or exp Pseudotumor Cerebri/ 5277
-
multiple sclerosis.mp. or exp Multiple Sclerosis/ 96,126
-
peripheral neuropathy.mp. or exp Peripheral Nervous System Diseases/ 173,401
-
exp Parkinson Disease/ or parkinson* disease.mp. 128,663
-
exp Neurodegenerative Diseases/ 356,554
-
(huntington* disease or huntington* chorea).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 20,536
-
motor neurone disease.mp. 1097
-
exp Sleep Wake Disorders/ 105,644
-
(sleep disorder or narcolepsy or obstructive sleep apn?ea).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 42,731
-
spina bifida.mp. or exp Spinal Dysraphism/ 12,140
-
exp Fatigue Syndrome, Chronic/ 6267
-
(chronic fatigue syndrome or myalgic encephalomyelitis).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6552
-
fibromyalgia.mp. or exp Fibromyalgia/ 13,499
-
exp Chronic Pain/ 21,039
-
exp Complex Regional Pain Syndromes/ 5902
-
exp Myofascial Pain Syndromes/ 6785
-
(chronic pain or pain syndrome).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 67,401
-
exp Back Pain/ or chronic back pain.mp. 44,796
-
osteoarthritis.mp. or exp Osteoarthritis/ 106,483
-
exp Osteoporosis/ 61,530
-
(osteoporosis or osteopenia).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 102,970
-
scoliosis.mp. or exp Scoliosis/ 28,584
-
exp Spinal Diseases/ 137,686
-
exp Fractures, Compression/ 3022
-
(compression fracture or collapsed vertebra*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2523
-
intervertebral disc degeneration.mp. or exp Intervertebral Disc Degeneration/ 8566
-
sciatica.mp. or exp Sciatica/ 7238
-
spinal stenosis.mp. or exp Spinal Stenosis/ 9721
-
spondylosis.mp. or exp Spondylosis/ 11,295
-
spondylolisthesis.mp. or exp Spondylolisthesis/ 7552
-
amputation.mp. or exp Amputation/ 52,820
-
amputee.mp. 3034
-
paralysis.mp. or exp Paralysis/ 117,463
-
exp Hemiplegia/ or hemiplegia.mp. 16,665
-
exp Paraplegia/ or paraplegia.mp. 22,286
-
quadriplegia.mp. or exp Quadriplegia/ 10,148
-
exp Disabled Persons/ 72,228
-
(disabled or disabilit*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 368,071
-
chronic kidney disease.mp. or exp Renal Insufficiency, Chronic/ 165,323
-
h?emodialysis.mp. or exp Renal Dialysis/ 147,868
-
exp Urinary Calculi/ 38,075
-
(urinary tract stone* or kidney stone*).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 7526
-
exp Asthma/ or asthma.mp. 193,826
-
exp Lung Diseases/ or chronic lung disease.mp. 1,172,810
-
bronchiectasis.mp. or exp Bronchiectasis/ 15,266
-
exp Pulmonary Disease, Chronic Obstructive/ 65,000
-
(chronic obstructive pulmonary disease or COPD).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 76,565
-
cystic fibrosis.mp. or exp Cystic Fibrosis/ 56,480
-
interstitial lung disease.mp. or exp Lung Diseases, Interstitial/ 88,055
-
pulmonary fibrosis.mp. 34,669
-
pulmonary hypertension.mp. or exp Hypertension, Pulmonary/ 57,397
-
exp Sarcoidosis/ or sarcoidosis.mp. 33,257
-
exp Tuberculosis/ or tuberculosis.mp. 274,528
-
exp Ehlers-Danlos Syndrome/ or ehlers-danlos.mp. 4735
-
rheumatoid arthritis.mp. or exp Arthritis, Rheumatoid/ 162,479
-
exp Sjogren’s Syndrome/ 14,277
-
(sjogren* syndrome or sjogren* disease).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 19,871
-
exp Raynaud Disease/ or raynaud*.mp. 10,222
-
systemic sclerosis.mp. or exp Scleroderma, Systemic/ 27,173
-
scleroderma.mp. 29,499
-
primary systemic vasculitis.mp. or exp Systemic Vasculitis/ 18,247
-
marfan* syndrome.mp. or exp Marfan Syndrome/ 8688
-
spondyloarthritis.mp. or exp Spondylarthritis/ 31,559
-
psoriatic arthritis.mp. or exp Arthritis, Psoriatic/ 12,555
-
ankylosing spondylitis.mp. or exp Spondylitis, Ankylosing/ 21,291
-
systemic lupus erythematosus.mp. or exp Lupus Erythematosus, Systemic/ 80,550
-
autoimmune disease.mp. or exp Autoimmune Diseases/ 545,468
-
frailty.mp. or exp Frailty/ 23,160
-
exp COVID-19/ or long covid.mp. 199,512
-
post COVID syndrome.mp. 288
-
exp Obesity/ 251,605
-
(obese or obesity).mp. [mp=title, book title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 419,142
-
polypharmacy.mp. or exp Polypharmacy/ 12,907
-
turner* syndrome.mp. or exp Turner Syndrome/ 9757
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 or 146 or 147 or 148 or 149 or 150 or 151 or 152 or 153 or 154 or 155 or 156 or 157 or 158 or 159 or 160 or 161 or 162 or 163 or 164 or 165 or 166 or 167 or 168 or 169 or 170 or 171 or 172 or 173 or 174 or 175 or 176 or 177 or 178 or 179 or 180 or 181 or 182 or 183 or 184 or 185 or 186 or 187 or 188 or 189 or 190 or 191 or 192 or 193 or 194 or 195 or 196 or 197 or 198 or 199 or 200 or 201 or 202 or 203 or 204 or 205 or 206 or 207 or 208 or 209 or 210 or 211 or 212 or 213 or 214 or 215 or 216 or 217 or 218 or 219 or 220 or 221 or 222 or 223 or 224 or 225 or 226 or 227 or 228 or 229 or 230 or 231 or 232 or 233 or 234 or 235 or 236 or 237 or 238 or 239 or 240 or 241 or 242 or 243 or 244 or 245 or 246 or 247 or 248 or 249 or 250 or 251 or 252 or 253 14,494,082
-
(questionnaire* or survey* or interview* or focus group* or case stud* or observ* or ethnograph* or hermeneutic* or narrative* or phenomenolog* or grounded theory or process evaluation or implementation study or implementation research or view* or experienc* or opinion* or attitude* or perception* or perceive* or belie* or feel* or know* or understand* or barrier* or facilitator* or enabler* or obstacle*).tw. 9,977,739
-
exp Qualitative Research/ or (qualitative or mixed method*).tw. 332,065
-
255 or 256 10,059,034
-
9 and 20 and 254 and 257 2187
MEDLINE search strategy for LTCs economic evidence review (see Chapter 8)
Ovid MEDLINE® ALL 1946 to 17 August 2022
n = 3077, searched on 17 August 2022
-
exp Breast Feeding/
-
breastfeed*.mp.
-
breastfed.mp.
-
breast-feed*.mp.
-
breast-fed.mp.
-
breast feed*.mp.
-
breast fed.mp.
-
infant feed*.mp.
-
exp Milk, Human/
-
Lactation/
-
lactat*.mp.
-
support.mp.
-
Social Support/
-
advice.mp.
-
advis*.mp.
-
help*.mp.
-
supportive adj2 relationship.mp
-
counsel*.mp.
-
educat*.mp.
-
consult*.mp.
-
Health Promotion/
-
Health Education/
-
Economics/
-
exp “Costs and cost analysis”/
-
“Cost allocation”/
-
Cost-benefit analysis/
-
“Cost control”/
-
“Cost savings”/
-
“Cost of illness”/
-
“Cost sharing”/
-
“deductibles and coinsurance”/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp “fees and charges”/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
or/1-11
-
or/12-22
-
or/23-54
-
55 and 56 and 57
-
exp animals/ not humans.sh.
-
58 not 59
Appendix 2 Study characteristics, risk-of-bias assessments and behaviour change techniques for mixed-methods synthesis (see Chapter 4)
| Author year | RCT paper(s) | Country setting/target population | Intervention description | Methods | ||
|---|---|---|---|---|---|---|
| Study objective | Participants and data collection | Data analysis | ||||
| Ahmed et al. 2012101 | Ahmed and Roumani 2020264 | USA, hospital | Interactive web-based breastfeeding monitoring system | To develop an interactive web-based breastfeeding monitoring system (LACTOR) and examine its feasibility, usability and acceptability among breastfeeding mothers | Convenience sample of women (n = 26) Online survey incorporating the System Usability Scale and a perception survey with open-ended questions |
Descriptive statistics; Fisher’s exact tests; content analysis |
| Andaya et al. 201293 | Bonuck et al. 2014221 | USA Urban Primary healthcare venue Low-income population |
Two intervention arms (1) Lactation consultant and electronic prompts; (2) lactation consultant only Lactation counselling and electronic pumps |
To examine women’s perceptions and reported effects of routine, primary-care-based interventions to increase breastfeeding | Quantitative Prenatal and 1-month follow-up questionnaires (number not reported) Qualitative Semi structured exit interviews at 6 months (n = 67 women) |
Interview data coded and analysed in MAX.qdA |
| Teich et al. 2014100 | To examine women’s perceptions of early infant-feeding experiences and identified early postpartum barriers to breastfeeding | |||||
| Bronner et al. 2001105 | Gross et al. 1998265 | USA Urban Community Low-income women enrolled in WIC |
Three intervention arms (1) Motivation video; (2) peer support; (3) motivational video and peer support |
To examine breastfeeding peer counselling within the context of the organisational structure of state and local WIC agencies | Convenience sample of programme managers/co-ordinators (n = 409) and peer counsellors (n = 254) Survey |
Descriptive statistics |
| Chapman et al. 2004106 | Chapman et al. 2004266 | USA Urban Hospital and community Low-income Latina women |
Peer counsellors – hospital and home visits and telephone contact | To report a process evaluation focusing on coverage | Peer counsellor contact logs (number not reported) | Cox regression Descriptive statistics |
| Cramer et al. 2017102 | McLachlan et al. 2016224 | Australia Urban and rural community Areas with low breastfeeding rates |
Two interventions One early home-based visiting by a maternal and child health nurse to women identified at risk of breastfeeding cessation Two home-based visiting and access to a drop-in centre |
To describe drop-in centres established during the trial; and the profile of women who accessed them To explore the views and experiences of the drop-in centre staff, and the challenges faced in establishing and maintaining a breastfeeding drop-in centre in the community |
Quantitative Survey of nurses running drop-in centres (n = 7) Visitor logbooks Qualitative Focus groups with nurses running drop-in centres (n = 6) Semi structured interviews with drop-in centre co-ordinators (n = 4) Observational visits, nurses’ reflective diaries and visitor comments |
Quantitative data analysed using Stata version 11 (no further details reported) Inductive thematic analysis |
| Ridgway et al. 2016108 | To describe the content of the home visits | Quantitative Pre-coded data collection forms completed at each home visit (n = 1043 forms) |
Descriptive statistics | |||
| Dennis et al. 2002107 | Dennis et al. 2002267 | Canada Urban Community hospitals |
Telephone support by volunteer with breastfeeding experience | To describe maternal and peer volunteer perceptions of their experience while participating in a breastfeeding peer support trial | Quantitative Questionnaires – mothers (n = 130) Peer supporter weekly activity logs (n = 78) Questionnaires – peer supporters (n = 30) |
Descriptive statistics Content analysis for open-ended questions |
| Hoddinott et al. 2012103 | Hoddinott et al. 2012118 | UK Urban and rural Community Disadvantaged population |
Proactive telephone calls daily for 1 week following hospital discharge | To assess the feasibility, acceptability and fidelity of a feeding team intervention of team-initiated (proactive) and woman-initiated (reactive) telephone support after hospital discharge | Quantitative Telephone call log and workload diaries Qualitative Interviews with women (n = 40) with follow-up (n = 11) and staff (n = 17) Ward observations Recorded telephone calls (n = 16) Steering group meetings notes (n = 9) Trial case notes (n = 69) Telephone interviews (n = 372) |
Descriptive statistics Framework analysis |
| Nankunda et al. 200695 | Tylleskar et al. 2011109 | Uganda Rural Community and healthcare settings |
Peer counselling Minimum of five home visits form late pregnancy up to 6 months postnatal Intervention delivered in three areas and adapted to local circumstances |
To assess the feasibility of training community-based peer counsellors to support exclusive breastfeeding in a rural district in Uganda | Focus group discussions with peer counsellors (n = 2 groups); mothers (n = 2 groups) and men (n = 2) groups) | Transcripts were used to develop general impressions |
| Nankunda et al. 201096 | To describe the experience of establishing individual peer counselling including training and retaining peer counsellors for exclusive breastfeeding | Pre-test and post-test questionnaire (n = 12) Observation, field notes and records of interactions |
Descriptive analysis Thematic analysis |
|||
| Nankunda et al. 2010104 | To describe women’s experiences of peer counselling for exclusive breastfeeding | Interviews guided by a structured questionnaire with closed and open-ended questions | Chi-square or Fisher’s exact test Coding of open-ended responses |
|||
| Rujumba et al. 202099 | To explore the barriers to, facilitators of and solutions to scaling-up of peer counselling support for exclusive breastfeeding in Uganda | Key informant interviews (n = 15) Focus groups with peer counsellors (n = 7 groups with 6–8 participants in each) |
Content thematic approach | |||
| Daniels et al. 201094 | Tylleskar et al. 2011109 | South Africa Community Poor areas with high HIV prevalence |
Peer counselling Minimum of five home visits form late pregnancy up to 6 months postnatal Intervention delivered in three areas and adapted to local circumstances |
To report the experience of three community health worker supervisors who were responsible for supporting infant-feeding peer counsellors | Semi structured interviews (n = 3) | Framework analysis |
| Nkonki et al. 201097 | To describe the experiences of peer supporters who promote exclusive infant feeding | Focus group discussions with peer supporters (n = 19) | Thematic analysis | |||
| Rahman et al. 201298 | Sikander et al. 2015268 | Pakistan Rural community Low literacy, with low rates of exclusive breastfeeding |
Seven psycho-educational sessions integrated into the routine work of lady health workers | To explore the integration of cognitive-behavioural therapy in the routine breastfeeding counselling practice of community health workers | Quantitative Lady health worker questionnaires (n = 40) Qualitative Focus group discussions with lady health worker trainers (n = 28) Interviews with managers (n = 2) |
Quantitative– not reported Qualitative Coding and themes based on inductive and a priori theory |
| Study | 1. Were steps taken to increase rigour/minimise bias and error in the sampling? | 2. Were steps taken to increase rigour/minimise bias and error in the data collected? | 3. Were steps taken to increase rigour/minimise bias and error in the analysis of the process data? | 4. Were the findings of the process evaluation grounded in/supported by the data? | 5. Please rate the findings of the process evaluation in terms of their breadth and depth | 6. To what extent does the process evaluation privilege the perspectives and experiences of breastfeeding women? | 7. What weight would you assign to this process evaluation in terms of the reliability of its findings? | 8. What weight would you assign to this process evaluation in terms of the usefulness of its findings? |
|---|---|---|---|---|---|---|---|---|
| Ahmed et al. 2012101 | Yes – a few steps were taken | Yes – several steps were taken | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair breadth but very little depth | A lot | Medium | Medium |
| Andaya et al. 201293 | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Reasonably well grounded/supported | Limited breadth or depth | Somewhat | Medium | Medium |
| Bronner et al. 2000105 | Yes – several steps were taken | Yes – several steps were taken | Yes – several steps were taken | Fairly well grounded or supported | Good/fair breadth but very little depth | Not at all | High | Medium |
| Chapman et al. 2004106 | Yes – several steps were taken | Unclear | Yes – a few steps were taken | Reasonably well grounded/supported | Limited breadth or depth | A little | Medium | Medium |
| Cramer et al. 2017102 | Yes – several steps were taken | Yes – several steps were taken | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair depth but very little breadth | Not at all | High | Medium |
| Daniels et al. 201094 | Yes – fairly thorough attempt | Yes – a few steps were taken | Yes – several steps were taken | Reasonably well grounded/supported | Good/fair depth but very little depth | Not at all | Low | Low |
| Dennis et al. 2002107 | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Fairly well grounded/supported | Good/fair breadth but very little depth | Somewhat | Medium | Medium |
| Hoddinott et al. 2012103 | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair breadth and depth | A lot | High | High |
| Nankunda et al. 200695 | Unclear | Yes – fairly thorough attempt | Unclear | Fairly well grounded/supported | Good/fair breadth and depth | Somewhat | Low | Medium |
| Nankunda et al. 2010a96 | Yes – several steps were taken | Yes – several steps were taken | Yes – fairly thorough attempt | Fairly well grounded/supported | Good/fair breadth but very little depth | Not at all | Medium | Medium |
| Nankunda et al. 2010b104 | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair breadth and depth | A lot | High | High |
| Nkonki et al. 201097 | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Fairly well grounded/supported | Good/fair depth but very little breadth | Not at all | High | High |
| Rahman et al. 201198 | Yes – a few steps were taken | Yes – a few steps were taken | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair depth but very little breadth | Not at all | High | Medium |
| Ridgway et al. 2016108 | Unclear | Yes – a few steps were taken | Yes – a few steps were taken | Reasonably well grounded/supported | Limited breadth or depth | Not at all | Medium | Medium |
| Rujumba et al. 202099 | Yes – several steps were taken | Yes – several steps were taken | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair breadth and depth | A lot | High | High |
| Teich et al. 2014100 | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Yes – fairly thorough attempt | Reasonably well grounded/supported | Good/fair breadth but very little depth | A lot | Medium | High |
| Linked intervention study included in Cochrane review (WP1) (first author and year) | Implementation research articles included in WP2 review (first author and year) | Behaviour change techniques identified in Cochrane study articles |
|---|---|---|
| Ahmed 2020264 | Ahmed 2012101 | 1.2. Problem-solving 2.2. Feedback on behaviour 2.3. Self-monitoring of behaviour 2.4. Self-monitoring of outcome(s) of behaviour 2.7. Feedback on outcome(s) of behaviour 3.1. Social support (unspecified) 3.2. Social support (practical) 4.1. Instruction on how to perform the behaviour 4.2. Information about antecedents 7.1. Prompts/cues 9.1. Credible source 10.4. Social reward |
| Bonuck 2014221 | Andaya 201293 | 1.2. Problem-solving 3.1. Social support (unspecified) 4.1. Instruction on how to perform the behaviour 5.1. Information about health consequences 10.4. Social reward |
| Chapman 2004266 | Chapman 2004106 | 1.2. Problem-solving 1.3. Goal setting (outcome) 1.4. Action planning 1.5. Review behaviour goal(s) 2.1. Monitoring of behaviour by others without feedback 2.2. Feedback on behaviour 2.7. Feedback on outcome(s) of behaviour 3.1. Social support (unspecified) 3.2. Social support (practical) 7.1. Prompts/cues 7.2. Cue signalling reward 9.1. Credible source 10.4. Social reward 16.3. Vicarious consequences |
| Dennis 2002267 | Dennis 2002107 | 1.2. Problem-solving 3.1. Social support (unspecified) 3.2. Social support (practical) |
| Gross 1998265 | Bronner 2001105 | 1.1. Goal setting (behaviour) 1.2. Problem-solving 1.3. Goal setting (outcome) 3.1. Social support (unspecified) 3.2. Social support (practical) 3.3. Social support (emotional) 4.1. Instruction on how to perform the behaviour 5.1. Information about health consequences 5.6. Information about emotional 6.1. Demonstration of the behaviour 7.1. Prompts/cues 7.2. Cue signalling reward 9.1. Credible source 9.2. Pros and cons |
| Hoddinott 2012118 | Hoddinott 2012103 | 2.2. Feedback on behaviour 2.7. Feedback on outcome(s) of behaviour 3.1. Social support (unspecified) 3.2. Social support (practical) 3.3. Social support (emotional) 4.1. Instruction on how to perform the behaviour 6.1. Demonstration of the behaviour |
| McLachlan 2016224 | Cramer 2017102 Ridgeway 2016108 |
1.1. Goal setting (behaviour) 1.2. Problem-solving 3.1. Social support (unspecified) 15.1. Verbal persuasion about capability |
| Sikander 2015268 | Rahman 201298 | 1.1. Goal setting (behaviour) 1.2. Problem-solving 1.4. Action planning 1.5. Review behaviour goal(s) 2.1. Monitoring of behaviour by others without feedback 2.2. Feedback on behaviour 2.3. Self-monitoring of behaviour 3.1. Social support (unspecified) 3.2. Social support (practical) 4.1. Instruction on how to perform the behaviour 5.1. Information about health consequences 8.2. Behaviour substitution 9.1. Credible source 9.2. Pros and cons 9.3. Comparative imagining of future outcomes 12.2. Restructuring the social environment 13.2. Framing/reframing 13.5. Identity associated with changed behaviour |
| Tylleskar 2011109 | Nankunda 200695 Rujumba 202099 Nankunda 201096 Nankunda 2010104 Nkonki 201097 Daniels 201094 |
1.2. Problem-solving 1.4. Action planning 2.1. Monitoring of behaviour by others without feedback 2.2. Feedback on behaviour 2.3. Self-monitoring of behaviour 2.4. Self-monitoring of outcome(s) of behaviour 3.1. Social support (unspecified) 3.3. Social support (emotional) 4.1. Instruction on how to perform the behaviour 4.2. Information about antecedents 5.1. Information about health consequences 12.2. Restructuring the social environment |
Appendix 3 Characteristics of included economic evaluation studies
| Study ID and setting | Intervention | Comparator | Participant characteristics | Methods of economic analysis | Summary of results | Applicability |
|---|---|---|---|---|---|---|
| Barnes 2017 (Barnes et al., 2017116) England Seven study sites: London (two sites), the Midlands (two sites), the north-east (one site) and the north-west of England (two sites) Community healthcare setting |
Group family-nurse partnership (FNP) + usual care Support: Breastfeeding plus Description: Content aimed to improve maternal health and pregnancy outcomes, improve child health and development by helping parents provide more sensitive and competent care; and to improve parental life course by helping parents develop effective support networks, plan future pregnancies, complete their education and find employment |
Usual care Description: Offers every family a programme of screening tests, immunisations, developmental reviews, and information and guidance to support parenting and healthy choices. There are core universal elements provided for all families with additional progressive, preventive elements for those at medium or high risk. The universal programme includes a neonatal examination, a new-baby review at about 14 days, a 6- to 8-week baby examination and a review by the time the child is 1 year old and at 2–2.5 years old |
Inclusion criteria: Expectant mothers at 16–20 weeks’ gestation, with expected delivery date within ≈ 10 weeks, aged either (1) < 20 years at last menstrual period with one or more previous live births, or (2) 20–24 years at last menstrual period with no previous live births and with low educational qualifications | Type of economic evaluation: CUA and CEA (trial-based) Perspective: Provider (NHS and PSS) Currency, price year: GBP, 2014–15 Time horizon: Pregnancy to infant aged 12 months Discount rate: 3.5% for costs and benefits accrued beyond the first 12 months of follow-up Primary outcome: Incremental cost per QALY gained Secondary outcomes: Incremental cost per gain in AAPI-2 score (attitudes to parenting), incremental cost per gain in CARE Index score (maternal sensitivity) |
Base-case results: ICER = −£247,485 per QALY gained, ICER = £111,334 per gain in AAPI-2 score, ICER = −£2382 per gain in CARE Index score For the primary outcome, intervention dominated (more costly and less effective than usual care) with a 2.3% probability of being cost-effective at a threshold of £20,000 |
Directly applicable: UK setting, provider perspective, cost per QALY gained reported, time horizon from pregnancy up to infant aged 12 months |
| Provider: Professional (two FNPs) Mode of delivery: Face to face with groups Intensity: High (44 contacts) Duration: From first trimester of pregnancy until infants were 12 months old |
Exclusion criteria: Women who had previously received FNP and those with psychotic mental illness Sample size: Total, N = 166 (IG, n = 99; CG, n = 67) Baseline characteristics: Baseline characteristics of participants appear balanced. Participants appear in keeping with target population |
Data sources: Outcome of effect: Within trial (EQ-5D-5L valued using UK tariffs) Resource use: Within trial Unit costs: National sources Measurement of uncertainty: 10,000 replications of incremental costs and benefits generated to determine level of sampling uncertainty around the mean ICERs Consideration of heterogeneity: Subgroup analyses by (1) completers (attended ≥ 17 sessions) and (2) programme phase (1, 2, 3) to examine effects of organisational learning Sensitivity analyses: (1) Adopting a wider societal perspective, (2) restricting analyses to complete cases and (3) recalculating the average cost per group FNP session per attending woman by varying (a) mean number of group FNP sessions attended to the highest and lowest observed mean values across all groups across all sites and (b) number of group FNP group participants to the greatest and smallest number of observed values across all groups and sites |
Findings from subgroup analyses: No evidence that the subgroups had a positive effect on the ICER Findings from sensitivity analyses: Little effect on the results, with the mean ICER holding in the north-west quadrant and the probability of cost-effectiveness remaining < 20% at a threshold of £20,000 |
|||
| Brown 2020 (Brown et al., 2020128) Australia and New Zealand Urban areas Community settings |
Obesity prevention interventions + usual care Support: Breastfeeding plus Description: Five early obesity prevention interventions, three of which fulfilled the eligibility criteria: (1) Healthy Beginnings (HB) trial – see Hayes 2014 for description; (2) Communicating Healthy Beginnings Advice by Telephone (CHAT) trial – see Wen 2017 for description; (3) Prevention of Overweight in Infancy (POI) trial – see Tan 2020 for description |
Usual care Description: HB trial – see Hayes 2014; CHAT trial – see Wen 2017; POI trial – see Tan 2020 |
Inclusion criteria: HB trial – see Hayes 2014; CHAT trial – see Wen 2017; POI trial – see Tan 2020 Exclusion criteria: HB trial – see Hayes 2014; CHAT trial – see Wen 2017; POI trial – see Tan 2020 Sample size: HB trial – see Hayes 2014; CHAT trial – see Wen 2017; POI trial – see Tan 2020 |
Type of economic evaluation: Cost comparison of intervention delivery costs across five trials (three eligible for this review) Perspective: Provider/funder Currency, price year: AUD, 2018 Time horizon: Birth to infant age 2 years Discount rate: 5% for costs Primary outcome: Intervention cost Secondary outcomes: Not applicable Data sources: Outcome of effect: Not applicable Resource use: Within trial Unit costs: National sources |
Base-case results: From most to least costly: HB AU$1135 (AU$1059–1189); POI-combined AU$602 (AU$577–624); POI-FAB alone AU$429 (AU$409–449); CHAT-Telephone AU$394 ($373–382); CHAT-SMS AU$80 (AU$77–82) Interventions varied widely in terms of resource use and costs |
Not applicable: Intervention costs reported with a comparison, OECD setting. Provider perspective, time horizon from birth up to infant age 2 years |
| Provider: Professional Mode of delivery: Face to face and/or telephone/SMS Intensity: Moderate to high (8–10 contacts) Duration: From late pregnancy/birth to infant age 2 years (POI 18 months; 2 years on request) |
Baseline characteristics: HB trial – see Hayes 2014; CHAT trial – see Wen 2017; POI trial – see Tan 2020 | Measurement of uncertainty: Estimated 95% uncertainty intervals around mean costs for the base case and sensitivity analyses using Monte Carlo simulation (2000 iterations) Consideration of heterogeneity: Not reported Sensitivity analyses: (1) Adopting a wider perspective with inclusion of family costs, (2) discount rate of 3% |
Findings from sensitivity analyses: Little effect on the results of the cost comparison, with the sensitivity analyses demonstrating similar variance and the same order of most to least costly | |||
| Delgado 2018 (Delgado et al., 2018121) USA One study site in city of San Antonio, Texas Community setting |
Baby Café Support: Breastfeeding only Description: Authors state: The Baby Café breastfeeding support model was developed in the UK with the primary purpose of working with mothers for the first 8 weeks after birth … located in a facility of easy access to mother ‘Partners’, where weekly demonstration sessions take place in a relaxed environment conducive to open discussions on breastfeeding approaches |
No comparator Description: Not applicable |
Inclusion criteria: No inclusion criteria – personnel promoted the Baby Café to low-income pregnant women and postnatal mothers Exclusion criteria: Not applicable |
Type of economic evaluation: Cost description Perspective: Provider (state funder) Currency, price year: USD, 2010 Time horizon: Birth to infant age 8 weeks Discount rate: 3.5% for delivery costs across 5 years Primary outcome: Cost per mother Secondary outcomes: Cost per session |
Base-case results: Cost per mother = US$105; cost per session = US$22.53 Findings from sensitivity analyses: Cost per mother ranged from US$65–247 suggesting results sensitive to weekly number of baby sessions and number of mothers attending |
Not applicable: Cost of one alternative reported, time horizon of intervention costed from birth to 8 weeks; OECD setting, provider perspective |
| Provider: Professional (lactation specialist) and lay person (peer counsellor) Mode of delivery: Face to face in groups Intensity: Low to moderate (2–8 contacts) Duration: From birth to infant age 8 weeks |
Sample size: A total of 95 mothers visited the café during the 1-year data collection period Baseline characteristics: Not applicable; however, 95% of mothers attending came from the WIC clinic catchment area, indicating low-income status |
Data sources: Outcome of effect: Not applicable Resource use: Programme data Unit costs: Programme data Measurement of uncertainty: Not reported Consideration of heterogeneity: Not reported Sensitivity analyses: ‘A two-way sensibility [sic] analysis was completed, varying the weekly number of baby sessions and number of mothers attending each Baby Café session’ |
||||
| DelliFraine 2011 (DelliFrane et al., 2011122) USA Nationwide Hospital setting |
BFHI-accredited hospitals Support: Breastfeeding plus Description: BFHI steps 1–9 for organisations to promote successful breastfeeding |
Usual care Description: Non-BFHI accredited hospitals. No further information provided |
Inclusion criteria: All baby-friendly hospital and birthing sites in the USA in 2009 with data available in the public data files (intervention group) and matched with similar size and type non-baby-friendly hospitals in the same city (control group) | Type of economic evaluation: Cost analysis of two alternatives Perspective: Payer Currency, price year: USD, 2007 Time horizon: 1 year Discount rate: Not applicable Primary outcome: Mean cost per nursery plus labour and delivery Secondary outcomes: Not applicable |
Base-case results: Differential cost of US$35 per nursery plus labour and delivery For the primary outcome, no statistically significant difference in mean cost per delivery identified (US$2205 vs. US$2170) for baby-friendly hospitals compared with non-baby-friendly hospitals |
Not applicable: Payer perspective, differential cost reported and limited to one category of resource use with gross costing methods used; OECD setting, time horizon 1 year |
| Provider: Professional Mode of delivery: Face to face Intensity: Not reported, but organisational level intervention focused on nursery, labour and delivery Duration: Hospitalisation for labour and delivery |
Exclusion criteria: Baby-friendly hospital and birthing sites in the USA in 2009 without data available in the public data files Sample size: Total, N = 124 (IG, n = 62; CG, n = 62) Baseline characteristics: Hospitals matched on city, state, bed size and number of deliveries to minimise differences. No other differences observed in length of stay, case-mix index, and percentage Medicaid and self-pay deliveries |
Data sources: Outcome of effect: Not applicable Resource use: Data from the 2007 American Hospital Association Unit costs: National sources Measurement of uncertainty: Not reported Consideration of heterogeneity: Not reported Sensitivity analyses: Not reported |
||||
| Frick 2012123 USA Two hospitals (one university and one community hospital) serving urban areas in Baltimore, MD, USA |
Usual care plus a structured programme of education and support + usual care Support: Breastfeeding only Description: In addition to usual care, a structured programme of education and support comprising postnatal visits by a breastfeeding team (community nurse and peer counsellor) |
Usual care Description: Usual care included access to a lactation consultant in hospital and phone access after discharge home |
Inclusion criteria: Mother English-speaking, with telephone access and living within 25 miles of the hospital, intending to breastfeed, family eligible for WIC programme, singleton term infant (> 37 weeks’ gestation) Exclusion criteria: Infants or mothers with positive drug screen, infants with craniofacial abnormalities, infants admitted to NICU |
Type of economic evaluation: CA Perspective: Societal perspective – limited to payer and family Currency, price year: USD, 2009, adjusted to 2011 prices Time horizon: Infant age 12 weeks Discount rate: N/A Primary outcome: Base-case per-person costs of the programme (personnel and transportation costs only) Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: Within trial and national sources Measurement of uncertainty: Varied labour costs to upper and lower CI limits for time (assuming maximum and minimum expenditures, respectively) |
Base-case results: Cost = US$296.54 (US$274.12–320.97) Findings from sensitivity analyses: (1) Cost at upper limit = US$320.97, (2) cost at lower limit = US$274.12 |
Not applicable: Cost of one alternative reported, time horizon from birth to infant age 12 weeks; OECD setting, payer and family perspective with costs presented separately |
| Provider: Professional and paraprofessional with a community nurse and peer counsellor Mode of delivery: Face to face and telephone Intensity: High Duration: Birth to 24 weeks postpartum |
Sample size: Total, N = 328 (intervention, n = 168; control, n = 160) Baseline characteristics: Baseline variables were measured using established valid instruments and were used as covariates to adjust for differences between randomisation groups in some of the analyses in the paper |
Consideration of heterogeneity: None reported Sensitivity analyses: (1) Costs at upper limit, (2) costs at lower limit |
||||
| Haider 2014 (Haider et al., 2014124) USA 22 counties in the state of Michigan Community healthcare setting |
Peer counsellor breastfeeding support programme Support: Breastfeeding only Description: Breastfeeding education and support to low socioeconomic status women using mothers recruited from the community to serve as peer counsellors. Support includes breastfeeding advice, access to technical advice from lactation consultants, and advice regarding nutrition, health and other local services for which the mothers are eligible |
Unclear Description: Women had requested the peer counsellor support programme but did not receive it due to high demand and low capacity |
Inclusion criteria: Women who requested the breastfeeding support programme, individuals for whom Medicaid claims data were available and who were recruited into the programme prenatally Exclusion criteria: Not reported Sample size: Total, N = 846 (IG, n = 274; CG, n = 572) |
Type of economic evaluation: Cost outcome description of two alternatives Perspective: Payer Currency, price year: USD, price year not reported Time horizon: From birth to infant age 12 months Discount rate: Not applicable Primary outcome: Mean expenditures on health utilisation per infant Secondary outcomes: Programme cost per mother Data sources: Outcome of effect: Breastfeeding rates Resource use: Data from Medicaid administrative data |
Base-case results: Adjusted differential expenditure of US$770 (−US$927, US$2467) on health utilisation per infant For the primary outcome, no statistically significant difference in mean expenditures on health utilisation per infant for women receiving peer counsellor support compared with those who requested support but did not receive it |
Not applicable: Payer perspective, differential cost reported with gross costing methods used; OECD setting, time horizon from birth to infant age 12 months |
| Provider: Lay person (peer counsellor) Mode of delivery: Face to face and telephone Intensity: High – aim for monthly home visits or telephone calls, depending on type of support needed Duration: Third trimester of pregnancy up to maximum infant age 12 months |
Baseline characteristics: Appear balanced at baseline | Unit costs: Not applicable (total expenditure from Medicaid administrative data) Measurement of uncertainty: 95% CIs reported Consideration of heterogeneity: Regression model adjusted for potential confounders Sensitivity analyses: Not reported |
||||
| Hanafin 2018134 Ireland Country-wide Community healthcare setting |
PHN-facilitated breastfeeding groups + usual care Support: Breastfeeding only Description: The PHN-facilitated breastfeeding groups aimed to provide support, knowledge and advice to breastfeeding mothers, maternal confidence and capacity to breastfeed. Mothers also have opportunities to meet other mothers and develop social networks |
No comparator Description: Not applicable |
Inclusion criteria: N/A Exclusion criteria: N/A Sample size: N/A Baseline characteristics: N/A |
Type of economic evaluation: CBA – SROI Perspective: Societal Currency, price year: Euros, price date and year of conversion unclear Time horizon: Costs and benefits are calculated and presented in terms of average annual figures for a group Discount rate: Outcomes beyond 1 year were discounted at 5% for those 2–5 years Primary outcome: Net present value SROI ratio in euros per annum for the PHN-facilitated breastfeeding groups Data sources: Outcome of effect: Within study and literature Resource use: Within study and literature Unit costs: Within study and literature |
Base-case results: SROI ratio in euros per annum for the PHN-facilitated breastfeeding groups €15.85:1 Findings from sensitivity analyses: SROI ratio per annum with prolongation of breastfeeding doubled to 2.58 months, SROI = €31.71:1; SROI ratio per annum with a social value for additional life years gained from a medical intervention estimated at €114,000, SROI = €15.95:1 |
Not applicable: Cost of one alternative provided, no ICER reported OECD setting, societal perspective, time horizon from birth to lifetime |
| Provider: Professional (lactation consultants) Mode of delivery: Face to face Intensity: High Duration: Antenatal through to postnatal duration of breastfeeding |
Measurement of uncertainty: More optimistic assumptions related to prolongation of breastfeeding, and the value of lives saved due to lower incidence of invasive breast cancer and ovarian cancer Consideration of heterogeneity: No consideration of heterogeneity Sensitivity analyses: Sensitivity analysis assessed changes to valuations of key benefits: increased intelligence, improved lifetime earnings, reduced cancer incidence |
|||||
| Hayes 2014 (Hayes et al., 2014;129 Wen et al., 2012269) Australia Socially and economically disadvantaged areas of Sydney Community setting with home visits |
Healthy beginnings + usual care Support: Breastfeeding plus Description: Specifically trained research nurse delivered a staged home-based intervention in the antenatal and postnatal period. At each visit the research nurse spent 1–2 hours with the mother/infant and addressed four key areas: infant-feeding practices, infant nutrition and active play, family physical activity and nutrition, as well as social support) |
Usual care Description: Usual childhood nursing service, comprising one home visit by a community nurse within a month of birth, if needed, plus visits to the local clinic. The control group also received home safety information sent by mail at five time points up to 18 months |
Inclusion criteria: ≥ 16 years old, expecting first child, between weeks 24 and 34 of pregnancy, able to communicate in English, and lived in the local area Exclusion criteria: Women were excluded from the study if they had severe medical conditions as evaluated by their physicians |
Type of economic evaluation: CEA (trial-based) Perspective: Provider (health funder) Currency, price year: AUD, 2012 Time horizon: Within trial – pregnancy to infant aged 2 years Discount rate: 5% for costs and benefits accrued beyond the first 12 months of follow-up Primary outcome: Incremental cost per unit BMI avoided Secondary outcomes: Incremental cost per 0.1 BMI z-score reduction Data sources: Outcome of effect: Within trial |
Base-case results: ICER = AU$4230 per unit BMI avoided; ICER = AU$631 per 0.1 BMI-z score reduction Difficult to gauge cost-effectiveness, as no understanding of health providers’ WTP for the prevention of BMI gain Findings from scenario analyses: A reduction in travel and administration time for the community nurse reduced intervention costs and led to a higher probability of Healthy Beginnings being cost-effective (66% vs. 30%) at a suggested WTP threshold of AU$500 for a 0.1 BMI z-score reduction |
Partially applicable: OECD setting Provider perspective, cost per unit BMI avoided reported, within-trial time horizon from birth to infant age 2 years |
| Provider: Professional (community nurse) Mode of delivery: Face to face with individuals via home visits Intensity: Moderate (eight contacts) Duration: From pregnancy until infant age 2 years |
Sample size: Total N = 667 for the trial; subsample consenting to phase 2 with complete data available included in the economic evaluation [N = 324 (IG, n = 166; CG, n = 158)] Baseline characteristics: Baseline characteristics appear balanced for age, household income and education level, excepting marital status (p = 0.046) with a lower percentage (90% vs. 96%) of women being married/de facto in the intervention group |
Resource use: Retrospective costing of trial data Unit costs: National sources Measurement of uncertainty: Bootstrapping was used to estimate a distribution around costs and health outcomes; CEAC was produced to examine uncertainty around the probability of being cost-effective at decision-makers’ WTP Consideration of heterogeneity: Not reported Sensitivity analyses: No sensitivity analysis reported, a scenario analysis was conducted to examine costs in a ‘real world’ setting with travel and administration time reduced from 90 minutes to 20 minutes |
||||
| Hoddinott 2009117 UK 14 localities (of 66) in Scotland Community-based, primary care setting – GP practices |
BIG + usual care Support: Breastfeeding only Description: A policy intervention aimed at locality areas rather than at individual women. The policy aimed to double the number of local breastfeeding support groups and to make weekly support groups open to all pregnant women and breastfeeding mothers, aiming to provide breastfeeding support and social interaction for women |
Usual care Description: Control localities received no additional intervention; however, breastfeeding support postnatal groups existed in some control areas |
Inclusion criteria: Pregnant women and breastfeeding mothers Exclusion criteria: Not stated Sample size: 14 clusters randomised, birth records supplied data for n = 9747 in intervention group and n = 9111 in control group |
Type of economic evaluation: CA Perspective: Provider (e.g. NHS and PSS), patient (i.e. mother) Currency, price year: GBP, 2005/6 Time horizon: Not reported. Assume within-trial: Cost per year evaluated for the health service costs; costs per woman attending weekly group sessions evaluated, with attendance at a median of four times |
Base-case results: Intervention cost per woman attending = £143; intervention cost per attendance at a group = £36 | Not applicable: Cost of one alternative, time horizon within trial UK setting, provider and family perspective with data presented separately |
| Provider: Health professional group facilitator Mode of delivery: Face to face Intensity: Low |
Baseline characteristics: Localities varied in size, baseline breastfeeding rates, the number of pre-existing groups and how pregnancy and postnatal care were organised. The authors reported matching them in pairs by mean breastfeeding rate at 6–8 weeks in 2002 and 2003, rural classification, and existing number of breastfeeding groups per 1000 births. Considered intervention and control groups to be comparable |
Discount rate: Not reported Primary outcome: Intervention cost per woman attending Secondary outcomes: Intervention cost per attendance at a group Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: Not clear how unit costs were established Measurement of uncertainty: N/A Consideration of heterogeneity: N/A |
||||
| Hoddinott 2012118 Scotland Disadvantaged areas with a mix of urban and rural Hospital and community setting |
Feeding support team with proactive telephone support + usual care Support: Breastfeeding only Description: Proactive telephone calls Provider: Professional and paraprofessional staff (two band 4 staff: a nursery nurse and a maternity care assistant) and a band 7 (midwife) team leader |
Feeding support team with reactive telephone support + usual care Description: Reactive telephone calls; women could telephone the feeding team at any point over the 2 weeks following discharge. Text and answerphone messaging available |
Inclusion criteria: Women admitted to the ward who lived in three most disadvantaged postcode area quintiles for the Scottish Index of Multiple Deprivation in 2009 and who were breastfeeding Exclusion criteria: Women aged < 16 years with serious medical or psychiatric problems or with insufficient spoken English to communicate by telephone |
Type of economic evaluation: CEA (trial-based) Perspective: Provider (NHS) Currency, price year: GBP, unclear but likely 2010 Time horizon: within-trial (from discharge up to 6–8 weeks postpartum) Discount rate: Not applicable Primary outcome: Incremental cost per additional woman breastfeeding at 6–8 weeks Secondary outcomes: Incremental cost per additional woman exclusively breastfeeding at 6–8 weeks |
Base-case results: ICER = £87 per additional woman breastfeeding at 6–8 weeks, ICER = £91 per additional woman exclusively breastfeeding at 6–8 weeks Findings from scenario analyses: Unclear how the scenario analyses may impact on ICER, due to reporting of total annual cost of each scenario |
Partially applicable: UK setting, provider perspective, cost per additional woman (exclusively) breastfeeding at 6–8 weeks reported, within-trial time horizon from discharge to infant age 6–8 weeks |
| Mode of delivery: Telephone Intensity: Moderate (median of eight contacts) Duration: From hospital discharge up to 14 days post discharge |
Sample size: Total, N = 69 (IG, n = 35; CG, n = 34) Baseline characteristics: Concerns about baseline imbalances with women in IG a year older on average, more living in the most disadvantaged postcode areas (SIMD 1), and half a day longer hospital stays. Otherwise, groups were similar for parity, method of delivery, gestation and admission to the neonatal special care unit |
Data sources: Outcome of effect: Within-trial data Resource use: Within-trial data Unit costs: Unclear, but states ‘standard sources were used to assign costs’ Measurement of uncertainty: Not reported Consideration of heterogeneity: Not reported Sensitivity analyses: Not reported, a scenario analysis was conducted to examine alternative intervention costing scenarios, varying staff requirements, using band 4 and band 5 grade nurse support, and period of coverage by varying hours of coverage per day |
||||
| Mavranezouli 2022 (Mavranezouli et al., 2022119) UK Nationwide Hospital and community healthcare setting |
Antenatal and postnatal education and support intervention + standard care Support: Breastfeeding only Description: Authors state ‘an intervention for women that comprised education, advice or support from a peer or professional, provided postnatally and initiated antenatally or within the first 8 weeks after birth’ |
Standard care Description: Standard care variable across England. Authors state it: may include provision of written material, antenatal breastfeeding educational programmes, and postnatal breastfeeding support groups run by peers and/or health professionals; in most settings breastfeeding information and support is provided by midwives and health visitors as part of routine postnatal care visits |
Inclusion criteria: Pregnant women and women who have given birth to a healthy baby at term (or to healthy twins or triplets), from the birth of the baby to 8 weeks after birth, and their partners Exclusion criteria: Not reported Sample size: Not applicable |
Type of economic evaluation: CUA with decision-analytic modelling Perspective: Provider (NHS and PSS) Currency, price year: GBP, 2018 Time horizon: From initiation up to 16–26 weeks postpartum, 1 year or lifetime, depending on the outcome Discount rate: 3.5% for costs and benefits accrued beyond the first 12 months of follow-up Primary outcome: Incremental cost per QALY Secondary outcomes: Not applicable Data sources: Outcome of effect: Age- and gender-specific UK population-based EQ-5D-derived utility values used |
Base-case results: ICER = £51,946 per QALY, which suggests it is not cost-effective at the current lower NICE threshold of £20,000/QALY Findings from sensitivity analyses: Results of deterministic and probabilistic sensitivity analysis were similar. The two-way sensitivity analysis suggested that the cost-effectiveness of the intervention improved as its effectiveness increased and intervention cost decreased. Using a discount rate of 1.5% had the greatest impact on the value of the ICER (£22,667/QALY), which was explained by greater maternal benefits several years after breastfeeding takes place, for example incidence of breast cancer |
Directly applicable: UK setting, provider perspective, cost per QALY gained reported, time horizon from birth up to 1 year or lifetime, depending on condition |
| Provider: Lay person and professional Mode of delivery: Face to face Intensity: Moderate (six contacts: four individual and two group-based) Duration: Initiated antenatally and provided postnatally. No indication of duration |
Baseline characteristics: For modelling purposes, maternal mean age was 30 years for both groups | Resource use: Expert advice for the intervention, systematic review evidence for probability estimates on healthcare resource use Unit costs: National sources Measurement of uncertainty: 10,000 iterations of incremental costs and effects generated to determine level of sampling uncertainty around the mean ICER Consideration of heterogeneity: Sensitivity analysis considered scenario of different starting age (25 and 35 years) to examine effects on the ICER Sensitivity analyses: (1) Two-way sensitivity analysis for intervention cost (£20–100) and intervention effect (1.05–2.00), (2) one-way sensitivity analysis performed for (a) 1.5% discount rate, as recommended for public health interventions, (b) inclusion of post-mortem examination cost for baby deaths, (c) intervention effect retained for future births |
||||
| Morrell 2000 (Morrell et al., 2000120 and 2000155) England Recruitment from one maternity hospital Community healthcare setting |
Community postnatal support worker + usual care Support: Breastfeeding plus Description: Community postnatal support worker with 8 weeks’ training provided home-based practical and emotional social support |
Usual care Description: Standard UK care includes postnatal home visits from midwives and health visitors |
Inclusion criteria: English-speaking women, aged ≥ 17 years, who gave birth at the study hospital Exclusion criteria: Baby spent > 48 hours on the SCBU Sample size: Total, N = 623 (IG, n = 311; CG, n = 312) |
Type of economic evaluation: Cost analysis (conducted alongside a RCT) Perspective: Provider (NHS and PSS) Currency, price year: GBP, 1996 Time horizon: From birth up to infant aged 6 months Discount rate: Not applicable Primary outcome: Mean incremental costs at 6 months Secondary outcomes: Mean incremental costs at 6 weeks |
Base-case results: Mean incremental costs at 6 months £178.61 (£79.60–272.40); Mean incremental costs at 6 weeks £179.58 (£125.85–232.34) Authors note ‘There were no savings to the NHS over 6 months after the introduction of the community postnatal support worker service’ |
Partially applicable: UK setting, provider perspective, intervention costs reported only, time horizon limited to within trial (birth to infant age 6 months) |
| Provider: Lay (postnatal support workers) Mode of delivery: Face to face Intensity: High (up to 10 contacts) Duration: The first 28 days after birth (maximum of 3 hours/visit) |
Baseline characteristics: There were no significant differences between groups at baseline across 114 birth and socioeconomic variables, except for incidence of twins, use of transcutaneous electrical nerve stimulation machines during labour, and adults living with the mother | Data sources: Outcome of effect: Within trial (but not included in economic evaluation) Resource use: Within trial Unit costs: Local and national sources Measurement of uncertainty: Non-parametric bootstrap centile CIs were estimated for the difference in mean scores between the groups Consideration of heterogeneity: Not as part of the economic evaluation. Sensitivity analyses: No formal sensitivity analysis reported, although there was reference in the discussion to reducing the postnatal support workers time spent in the mother’s home |
Findings from sensitivity analyses: Authors state that reducing the postnatal support workers time spent in the mother’s home to 120 minutes would reduce intervention costs from £179 to £151 at 6 weeks | |||
| Mottl-Santiago 2020125 USA Boston, MA Community healthcare setting |
Birth Sisters Best Beginnings for Babies program (doula support) + usual care Support: Breastfeeding plus Description: Birth Sisters Best Beginnings for Babies provided doula support, health promotion and education for low-income women, connecting them with social and medical services that improve perinatal and maternal outcomes |
Usual care Description: Usual prenatal, intrapartum and postpartum maternity care |
Inclusion criteria: Being a pregnant woman at 16–24 weeks’ gestational age, first-time mother, singleton, public insurance, no known fetal anomaly. Described as ‘a healthy population of nulliparous pregnant women’ | Type of economic evaluation: CBA (study-based) Perspective: Payer Currency, price year: USD, 2018 Time horizon: From mid-pregnancy to 6–8 weeks postpartum Discount rate: N/A Primary outcome: Average incremental cost per additional person served over the 3 years Secondary outcomes: Return on investment |
Base-case results: Incremental cost=US$433, ROI: 18% Findings from subgroup analyses: Variation in target population, ROI changed for social risk (70%), medical risk (276%), medical and social risk (471%) Findings from sensitivity analyses: Variations in wages, programme costs ranged from US$769 to US$1604 |
Not applicable: Payer perspective taken OECD setting, incremental costs reported and return on investment |
| Provider: Lay (doula peer support) Mode of delivery: Face to face Intensity: High – up to 12 contacts Duration: 24 weeks’ gestation through to 6–8 weeks postpartum |
Exclusion criteria: < 18 years of age, high risk pregnancy defined by care in the high-risk prenatal clinic Sample size: Total, N = 411 (intervention, n = 207, control, n = 204) Baseline characteristics: No group differences observed at baseline |
Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: Local sources Measurement of uncertainty: Payments were winsorised to address outliers Consideration of heterogeneity: Variations in impact for different populations Sensitivity analyses: One-way sensitivity analyses were conducted for differences in wages and benefits. Data for labour input sensitivity analyses for the program were derived from the Bureau of Labor Statistics |
||||
| Paranjothy 201747 UK (England and Wales) Community healthcare setting |
Mam-Kind intervention + usual care Support: Breastfeeding only Description: Mam-Kind is a motivational interviewing-based breastfeeding peer-support intervention to support breastfeeding maintenance |
No comparator Description: Not applicable |
Inclusion criteria: Pregnant women considering breastfeeding Exclusion criteria: Women who did not plan to breastfeed, who had a clinical reason that precluded breastfeeding continuation or who were unable to consent were excluded |
Type of economic evaluation: Cost–outcome descriptions Perspective: Societal Currency, price year: GBP, 2016 Time horizon: Bottom-up approach: pregnancy up to 10 weeks’ postpartum; top-down approach: 6 months Discount rate: N/A Primary outcome: Total intervention costs |
Base-case results: Total intervention costs = £33,595, intervention cost per participant = £480 | Not applicable: UK-based study, societal perspective, costs for one alternative reported |
| Provider: Lay (Mam-Kind buddy) Mode of delivery: Face to face Intensity: High – mean 16 contacts (0–44) Duration: Birth to 6 weeks postpartum |
Sample size: Total, N = 70 (no control group) Baseline characteristics: N/A as no control group. Differences with population – ‘women who were recruited may not be representative of the study sites’ (94% white) |
Secondary outcomes: Intervention cost per participant Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: Within trial and national sources Measurement of uncertainty: N/A Consideration of heterogeneity: N/A |
||||
| Pramono 2021146 Australia Canberra Hospital-based – one maternity unit |
Implementation of BFHI in a maternity unit + usual care Support: Breastfeeding plus Description: BFHI focuses on providing a high standard of maternity services to enable every infant to attain the best nutrition standards available. BFHI status is awarded to hospitals that implement consistent high-quality and ethical maternity care through the Ten Steps to Successful Breastfeeding policy, while remaining independent from formula companies and their affiliates |
No comparator Description: Not applicable |
Inclusion criteria: N/A Exclusion criteria: N/A Sample size: One maternity hospital Baseline characteristics: N/A |
Type of economic evaluation: CBA – SROI Perspective: Societal Currency, price year: AUD, 2019 Time horizon: 15 years Discount rate: 3.8%; adjusted to 6% for the sensitivity analysis Primary outcome: SROI ratio in AUD per annum at the maternity hospital Data sources: |
Base-case results: SROI = AU$55.38:1 Findings from sensitivity analyses: SROI range AU$16–111:1 |
Not applicable: OECD setting, societal perspective, no cost per QALY gained reported |
| Provider: Professional Mode of delivery: Face to face Intensity: High |
Outcome of effect: Within study and literature Resource use: Within study and literature Unit costs: Within study and national sources Consideration of heterogeneity: SROI approach enabled estimation of outcomes for mothers and infants separately, but no further consideration of heterogeneity Sensitivity analyses: A sensitivity analysis was conducted to check changes for estimates of deadweight, attribution, displacement, drop-off and discount rate, value of SIDS risk reduction, value of type 2 diabetes, value of ovarian cancer risk reduction and birth type |
|||||
| Pugh 2002126 USA City of Baltimore, Maryland Community healthcare setting |
Breastfeeding support programme + usual care Support: Breastfeeding only Description: Breastfeeding support visits by community health nurse/peer counsellor team. Support offered daily in hospital, and at home during weeks 1, 2 and 4 and at team’s discretion. Telephone support from peer counsellor twice weekly through to week 8 and monthly through to month 6 |
Usual care Description: Usual breastfeeding support consisted of support from hospital nurses, assistance by means of a telephone ‘warm line’ and if mothers gave birth on a weekday, one hospital visit from a LC |
Inclusion criteria: Low-income women receiving financial medical assistance Exclusion criteria: Not reported Sample size: Total, N = 41 (intervention, n = 21; control, n = 20) Baseline characteristics: Authors state ‘The intervention and usual care groups were not significantly different in major characteristics, including age, ethnicity, education, marital status, and breastfeeding goals’ |
Type of economic evaluation: Cost–outcome description of two alternatives Perspective: Provider and family Currency, price year: USD, not reported – used cost data from the National Compensation Survey, which was authored in 1998 and accessed on 25 January 2002. November 1999 was used as reference point when valuing the cost of concentrate/powder for formula feeding Time horizon: Birth to 6 months postpartum Discount rate: N/A Primary outcome: Incremental cost per mother (contact time and travel) Secondary outcomes: Incremental cost per mother (formula milk and Intervention + mother’s time to feed) |
Base-case results: Incremental cost per mother (contact time and travel) = US$646 (SE US$251); Incremental cost per mother (formula milk and intervention + mother’s time to feed) = US$646 (SE US$251) Findings from SA: Alternative costing scenario suggest incremental costs would be sensitive to change in method of valuing staff time |
Partially applicable: OECD setting, provider and family perspective with costs reported separately, within-trial time horizon from birth to 6 months, incremental costs reported |
| Provider: Professional and lay (community health nurse/peer counsellor team) Mode of delivery: Face to face and telephone Intensity: High Duration: From birth to infant age 6 months |
Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: Within trial, local and national sources Measurement of uncertainty: Measure of uncertainty (standard error) reported around incremental costs Consideration of heterogeneity: N/A Sensitivity analyses: Calculation of project costs using project records to ascertain what staff were paid, taking into account training and in-service education |
|||||
| Spiby 2015153 UK Five study sites, city Community healthcare setting |
Volunteer doula service + usual care Support: Breastfeeding plus Description: Volunteer doula service Provider: Lay person Mode of delivery: Face to face Intensity: High Duration: Pregnancy to 6 weeks postpartum |
Usual care Description: Not reported |
Inclusion criteria: Mixed-methods study, so differed depending on method Exclusion criteria: As above Sample size: As above Baseline characteristics: N/A |
Type of economic evaluation: CCA Perspective: Provider (NHS and PSS) Currency, price year: GBP, 2011–12 Time horizon: Antenatal up to 6 weeks postpartum Discount rate: N/A Primary outcome: Average cost to the doula service per woman supported |
Base-case results: Average cost to the doula service per woman supported = £2438.85, cost differential = –££6.66 | Partially applicable: UK-based study, provider perspective, intervention costs and cost differentials only provided |
| Secondary outcomes: Cost differential for exclusive breastfeeding outcomes and potential NHS costs per birth per annum: doula service vs. comparators Data sources: Outcome of effect: Within study and literature Resource use: Within study and literature Unit costs: National sources Measurement of uncertainty: Not reported Consideration of heterogeneity: Not reported |
||||||
| Stevens 2006133 Canada City of Toronto Hospital and community setting |
Home breastfeeding support Support: Breastfeeding only Description: Planned early discharge from hospital (24–36 hours postpartum) and up to three home visits by community nurse LCs. Content of support unclear Provider: Professional (LC) Mode of delivery: Face to face with individual with home visits Intensity: Low (three contacts) Duration: From birth until infants age 1 week |
Standard care Description: Standard discharge from hospital (48–60 hours postpartum) with in-hospital breastfeeding support |
Inclusion criteria: Live, singleton, term or near-term infant delivered in 12 hours before recruitment; women aged ≥ 21 years residing in defined study area, intending to breastfeed and with satisfactory home circumstances (assessed by postpartum nurses) Exclusion criteria: Non-English-speaking women, caesarean delivery, postpartum complications, morbidities, chronic illness or disabilities; infants with congenital abnormalities or morbidities |
Type of economic evaluation: Cost analysis (conducted alongside a RCT) Perspective: Healthcare provider and family Currency, price year: CAD, 2002 Time horizon: Birth to 5–12 days postpartum Discount rate: Not applicable Primary outcome: Incremental cost for term infants Secondary outcomes: Incremental cost for near-term infants |
Base-case results: Healthcare provider and family (incremental cost for term newborns = CA$119, incremental cost for near-term newborns = CA$1352) Healthcare provider only (incremental cost for term newborns = CA$17, incremental cost for near-term newborns = –CA$309) |
Partially applicable: OECD setting, provider and family perspective with costs reported separately, within-trial time horizon from birth to 5–12 days, incremental costs reported. Outcomes reported separately, but allowed for an estimation of the cost per additional infant exclusively breastfed at 5–12 days |
| Sample size: Total, N = 138 (IG, n = 72; CG, n = 66) Baseline characteristics: Outcomes were not assessed at the same time in the intervention and control groups (mean day of follow-up was 8.4 days in the intervention group vs. 7.8 days for controls) and there was high attrition (26% overall, with 33% loss to follow-up in the control group) |
Data sources: Outcome of effect: Within trial, % of mothers exclusive breastfeeding in past 24 hours (not incorporated into economic evaluation but used herein to estimate cost per additional infant exclusively breastfed at 5–12 days taking a provider perspective) Resource use: Within trial Unit costs: National sources Measurement of uncertainty: Not reported Consideration of heterogeneity: Incremental costs and outcomes reported separately for term infants and near-term infants Sensitivity analyses: Not reported |
Estimated ICER = CA$78.70 per additional term infant exclusively breastfed at 5–12 days, ICER for additional near- term infant exclusively breastfed at 5–12 days was dominant for home breastfeeding support due to lower healthcare costs and greater effect | ||||
| Tan 2020 (Tan et al., 2020;130 Taylor et al., 2018270) New Zealand City of Dunedin Community setting |
Combination of sleep + Food Activity Breastfeeding (FAB) programme + usual care Support: Breastfeeding plus Description: Participants received infant sleep education and advice on food, physical activity and breastfeeding |
Usual care Description: Standard maternity care and well-child care from a maternity care professional and a well-child provider of their choice |
Inclusion criteria: All mothers booked into the maternity hospital invited to participate at 28–30 weeks’ gestation with an ‘opt out’ recruitment strategy Exclusion criteria: Before birth, home address outside greater Dunedin area, planning to move away in next 2 years, unable to communicate in English or Te Reo Maori. After birth, identification of a congenital abnormality likely to affect feeding or growth, or infant born < 36.5 weeks’ gestation |
Type of economic evaluation: CUA and CEA (trial-based and modelled) Perspective: Provider (health sector) Currency, price year: AUD, 2018 Time horizon: Extrapolation of 5 years. Within trial data to 15 years Discount rate: 5% for costs and benefits accrued beyond 1 year Primary outcome: Incremental cost per QALY gained Secondary outcomes: Incremental cost per BMI avoided at age 15 years, incremental cost per BMI avoided at age 5 years Data sources: Outcome of effect: QALYs to age 15 years modelled using utility weights associated with child weight status |
Base-case results: ICER = AU$94,667 per QALY gained, ICER = AU$5164 per BMI avoided at age 15 years, ICER = AU$6678 per BMI avoided at age 5 years For the primary outcome, the intervention was not considered to be cost-effective Findings from sensitivity analyses: Sensitivity analyses not conducted, as the ICER for the combination intervention was not considered cost-effective |
Partially applicable: non-OECD setting, provider perspective, cost per QALY gained reported |
| Provider: Professional (lactation consultant provided the FAB intervention) Mode of delivery: Face to face with individuals Intensity: Moderate for breastfeeding support (five sessions) but overall high for the broader combination intervention at 10 parent contacts Duration: Not reported |
Sample size: Total, N = 405 (IG, n = 196; CG, n = 209) Baseline characteristics: No baseline imbalance apparent |
Resource use: Assumed same resource use for children under 5 years in the intervention and control groups; healthcare costs modelled from 5 years up to age 15 years using the EPOCH microsimulation model, which predicts healthcare costs using a top-down method Unit costs: Local and national sources of programme costs Measurement of uncertainty: 10,000 replications of incremental costs and benefits generated to determine level of sampling uncertainty around the mean ICERs Consideration of heterogeneity: Not reported Sensitivity analyses: One-way sensitivity analyses planned to determine whether the uncertainty in model and health economic parameters had any impact on shifting the calculated ICERs beyond the cost-effective threshold |
||||
| Wen 2017 (Killedar et al., 2022;271 Wen et al., 2017;131 Wen et al., 2022272) Australia Recruitment from seven hospitals in four health districts in the metropolitan area of Sydney Community setting |
Telephone (IG1) or SMS (IG2) support + usual care Support: Breastfeeding plus Description: The intervention was informed by the Health Belief Model providing six staged intervention booklets corresponding to key stages of child feeding and movement. The booklets were mailed to the intervention groups IG1: 1 week after mailing, a child and family health nurse called the participant to provide support, talk about the booklet and discuss issues raised. Each call was approximately 30–60 minutes long |
Usual care Description: Usual care plus home safety promotion materials and a newsletter on ‘kids’ safety’ were sent to the control group at the third trimester and at 3, 6 and 9 months of infant age. Usual care involved universal child and family health services provided by local health districts comprising one nurse home visit, multiple visits up to 2 years for high-risk families, or attendance at child and family health centres available to all families |
Inclusion criteria: Women aged ≥ 16 years, 24–34 weeks’ gestation, able to communicate in English, with a mobile telephone, and living in recruitment areas Exclusion criteria: Severe medical condition, could not give informed consent, expecting multiple births and had babies with known major fetal anomalies Sample size: Total, N = 1155 (IG1, n = 386; IG SMS, n = 384; CG, n = 385) |
Type of economic evaluation: CEA (trial based – conducted alongside a three-arm RCT) Perspective: Provider (local government) Currency, price year: AUD, 2018 Time horizon: Pregnancy to infant age 2 years Discount rate: 5% for costs and benefits accrued beyond the first 12 months of follow-up Primary outcome: Incremental cost per unit BMI avoided Secondary outcomes: Incremental cost per 0.1 BMI z-units avoided |
Base-case results: ICER = AU$10,664.89 per unit BMI avoided (IG1 vs. CG), ICER = AU$5154.14 per unit BMI avoided (IG2 vs. CG) SMS + usual care was more cost-effective than telephone support + usual care when compared with usual care alone. Difficult to gauge cost-effectiveness, as no understanding of health providers’ WTP for the prevention of BMI gain |
Partially applicable: OECD setting Provider perspective, incremental cost per unit BMI avoided reported. Time horizon within trial from birth to infant age 2 years |
| IG2: 1 week after mailing, a set of SMS messages was sent to participants twice per week for 4 weeks to reinforce the information Provider: Professional (child and family health nurse) Mode of delivery: Telephone calls (IG1) or SMS (IG2) with individuals Intensity: Moderate (six contacts) Duration: From third trimester of pregnancy until infants were 10 months old |
Baseline characteristics: No baseline imbalance. Participants in the economic evaluation (n = 662), who completed the 2-year follow-up with BMI measurements, appeared similar to baseline sample Maternal age grouped: < 24 years (IG1, 9%; IG2, 9%; CG, 8%), 25–34 years (IG1, 59%; IG2, 63%; CG, 64%), > 35 years (IG1, 32%; IG2, 28%; CG, 29%); primiparous (IG1, 54%; IG2, 56%; CG, 52%); married or de facto partner (IG1, 91%; IG2, 94%; CG, 94%); education – up to HSC to TAFE or diploma (IG1, 33%; IG2, 33%; CG, 37%), university degree (IG1, 67%; IG2, 67%; CG, 63%) |
Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: National sources Measurement of uncertainty: Joint uncertainty in costs and outcomes was determined using bootstrapping with replacement Consideration of heterogeneity: Not reported Sensitivity analyses: Adopted a limited societal perspective with the inclusion of productivity losses for the mother |
Findings from sensitivity analyses: Adopting a limited societal perspective increased the ICER, but the ICER for SMS support remained more favourable than for telephone support | |||
| Wouk 2017127 USA State of North Carolina |
Lactation consultant service + usual care Support: Breastfeeding only Description: IBCLC support Provider: Professional (IBCLC) Mode of delivery: Face to face Intensity: Low (average 1.3 contacts) Duration: Between birth and 1 week postpartum |
Usual care Description: Not reported |
Inclusion criteria: Low-income mothers Exclusion criteria: Not reported Sample size: 174 maternity centres/WIC agencies Baseline characteristics: Overall characteristics reported |
Type of economic evaluation: CBA (alongside geospatial analysis) Perspective: Payer Currency, price year: USD, 2010 data Time horizon: 1 year Discount rate: N/A Primary outcome: Cost savings for averted cases of lower respiratory tract infection, gastroenteritis, necrotising enterocolitis Secondary outcomes: Cost of service Data sources: Outcome of effect: Literature and state/national sources Resource use: Expert opinion for costing the intervention; literature and state sources for health service use Unit costs: Not reported Measurement of uncertainty: Not reported Consideration of heterogeneity: Not reported Sensitivity analyses: Not reported |
Base-case results: Cost savings of US$7.1M; cost of service = US$4.77M | Not applicable: OECD setting, payer perspective, lack of detail with aggregate costs (cost savings) reported |
Appendix 4 Study characteristics and risk-of-bias assessments of breastfeeding support interventions for women with long-term conditions (see Chapter 6)
Characteristics of included studies
| Study | Country | Participant characteristics | Participants’ condition | Total sample (n) | Intervention | Comparator |
|---|---|---|---|---|---|---|
| Aldana-Parra et al.185,273 | Colombia | Not reported | Obesity (defined as BMI of ≥ 28.1 kg/m2) and no diabetes | 90 | EBF counselling by certified counsellor; antenatal and postnatal; at least four contacts; face to face | Based on the institutional and national policy for breastfeeding |
| Bartu et al.178 | Australia | Median age SG 27 (IQR 17–39) years Median age CG 25 (IQR 18–41) years Ethnicity SG: 68 (90%) Caucasian, 8 (10%) other (not further specified) Ethnicity CG: 67 (88%) Caucasian, 9 (12%) other (not further specified) |
Substance misuse (mainly heroin) | 152 | Home visiting by research midwife; antenatal and postnatal; eight contacts; face to face; also included mental health and stress management support, and immunisation discussion | A telephone contact at 2 months and a home visit at 6 months |
| Steube et al.160,188,274,275 | USA | Mean age (SD) SG 30.3 (6.6) years Mean age (SD) CG 30.0 (6.0) years Ethnicity SG: Hispanic 5 (10%); non- Hispanic 45 (90%); Black/African American 22 (44%). Ethnicity CG: Hispanic 6 (12%); non- Hispanic 44 (88%); Black/African American 30 (60%) |
GDM (excluding women with overt diabetes, indexed by a baseline A1c of ≥ 6.5mg/dl) | 100 | Group sessions which included some breastfeeding support by IBCLC; 13 contacts but IBCLC was only available at four; antenatal and postnatal; face to face; telephone, SMS; also included nutritional advice and exercise classes to control GDM | Usual care, which included access to breastfeeding peer counsellors and inpatient consultation with IBCLC |
| Carlsen et al.179,276 | Denmark | Mean age SG 31.3 (SD 4.5) years Mean age SG 31.8 (SD 4.1) years Ethnicity not reported |
Obesity. Women had a pre-pregnancy BMI of at least 30 kg/m2 | 226 | Phone-based advisory support by a IBCLC; at least nine contacts; postnatal only; telephone | Standard care which included support in hospital and a contact with a health visitor or midwife within first week after birth |
| Chapman et al.174,277–280 | USA | Median age SG 23 years (IQR 21–28 years) Median age CG 25 years (IQR 22–31 years) Ethnicity SG: Hispanic 80.3%; African American 13.2%; white 5.3%; other 1.3% Ethnicity CG: Hispanic 83.3%; African American 7.7%; white 5.1%; other 3.8%; Hispanic 83.3%; African American 7.7%; white 5.1%; other 3.8% |
Low-income, overweight/obese women with a BMI of ≥ 27.0kg/m2 | 206 | Specialised peer counsellors by peers who received additional training on breastfeeding and obesity; at least 15 contacts; antenatal and postnatal; face to face and telephone; women also received breast pumps and a sling | BFHI hospital. Routine care included prenatal education, assistance during hospital from nurses and IBCLC. Post-discharge access to a ‘warm line’ for advice |
| Ehrlich et al.175,189 | USA | Age SG: 21–24 years 3.1%; 25–29 years 18.8%; ≥ 30 years 78.1% Age CG: 21–24 years 4.0%; 25–29 years 20.8%; ≥ 30 years 75.3% Ethnicity SG: non-Hispanic white 19.8%; Black/African American 5.2%; Asian or Pacific Islander 49.0%; Hispanic origin 18.8%; other 4.2%; missing 3.1% |
GDM | 197 | Diet, breastfeeding and exercise support by dieticians and IBCLCs; 15–26 contacts antenatal and postnatal; face to face and telephone; also provided advice and support to lose weight via diet and exercise | Usual care including printed material on GDM and infant safety |
| Ethnicity CG: non-Hispanic white 18.8%; Black/African American 4.0%; Asian or Pacific Islander 54.5%; Hispanic origin 18.8%; other 2.0%; missing 2.0% | ||||||
| O’Brien et al.180,281 (also includes unpublished data provided by author) | Ireland | Not reported | Overweight and obesity defined as BMI of ≥ 25kg/m2 | 224 | Multicomponent intervention that targets prospective mothers and their support partner. Included antenatal education class; postnatal group clinics and video calls all by an IBCLC; at least eight contacts antenatal and postnatal | Oral and written information on antenatal and postnatal support for breastfeeding that is available in the study site hospital and community and receive routine antenatal care |
| Fan et al.167 | Australia | Not reported | Not specifically targeted intervention for LTC but includes data for obese mothers and mothers with depression | 765 | Weekly lactation consultant telephone call; four contacts; postnatal | Standard postnatal care (no details) |
| Fiks et al.176 | USA | Mean age SG 25.8 (SD 5.2) years Mean age SG 27.3 (SD 5.6) years Ethnicity SG: Hispanic/Latina 5%; Black/African; American 84%; white 7%; other 7% Ethnicity CG: Black/African American 93%; Hispanic/Latina 0%; white 5%; other 7% |
Low-income women with obesity at start of pregnancy (defined as BMI of > 25kg/m2) | 87 | Private peer Facebook group facilitated by a psychologist with two face-to-face group sessions; antenatal and postnatal; also considered sleep obesity, wellbeing and wider infant-feeding topics | Text message reminders for recommended infant primary care visits |
| You et al.184 | China | Median age SG 33.0 (30.0–37.0) years Median age CG 34.0 (31.0–37.0) years Ethnicity SG: Han 95.3%; minority 4.7% Ethnicity CG: Han 96.2%; minority 3.8% |
GDM (women with type 2 diabetes were excluded) | 226 | Education and counselling from an IBCLC, written materials and WeChat peer support group; at least six contacts; antenatal and postnatal; face to face, telephone and digital | Usual care for lactation support during the antenatal and postnatal period (no details) |
| Ijumba et al.168 | South Africa | Not reported by HIV status. Overall median age 23 years Ethnicity not reported |
HIV | At least 3957 but not all were HIV positive | CHW who visited women to support infant feeding and other aspects of antenatal and newborn care; 7–9 visits; face to face; access to social welfare grants | CHWs who visited women to provide information on obtaining social welfare grants and not breastfeeding |
| Lewkowitz et al.169 | USA | Age SG: aged 18–34 years 93%; aged ≥ 35 years 7% Age CG: aged 18–34 years 91.5%; aged ≥ 35 years 8.5% Women had to be African American to take part |
Socioeconomically disadvantaged African American women with overweight or obesity defined as BMI of 25.0–45.0 kg/m2 | 118 | Home-based visits by parent educators with additional breastfeeding training, support and development of breastfeeding plan; bi-weekly antenatal only; also provided general parenting support and education | Standard home-based visits by parent educators who had one session on breastfeeding. Additional breastfeeding support was available on request |
| MacVicar et al.181 | UK | Age SG: 20–35 years, 5 (71%); > 35 years, 2 (29%) Age CG: 20–35 years, 4 (57%); > 35 years, 3 (43%) All participants were Caucasian |
Substance misuse | 14 | Support worker trained in BF in neonatal abstinence syndrome provided daily support during first 5 days of hospital stay; postnatal only; five contacts; face to face; also had a low-stimuli environment | Standard postnatal care of the newborn at risk of abstinence syndrome. Feeding advice was provided by ward staff and underpinned by the UNICEF ten steps to successful breastfeeding |
| Martin et al.170 | Australia | Mean age SG 31.6 (SD 5.1) years Mean age SG 29.5 (SD 7.8) years Ethnicity SG: born in Australia 100% Control SG: born in Australia 91% |
Overweight and obese mothers with a BMI of 25–35kg/m2 | 24 | Lactation support from IBCLC; at least three contacts; antenatal and postnatal; telephone and face to face; dietary intervention included antenatal sessions by a dietitian | Dietary intervention and standard antenatal care (no details) |
| Reifsnider et al.172,260,282 | USA | Age was not reported All women were Hispanic. SG mother’s nation of birth: Mexico 57.4%; USA 42.6%; other 0%. CG mother’s nation of birth: Mexico 56.9%; USA 39.7%; other 3.5% |
Low-income Hispanic women with obesity: pre pregnant BMI of > 25kg/m2 | 174 | Home visiting from promotors and support from lactation consultant if needed; antenatal and postnatal; at least seven contacts; also included infant growth and development; sleep; and play/exercise | Standard WIC services |
| Namale-Matovu et al.171 | Uganda | All groups had a mean age of 34 years Ethnicity not reported |
HIV positive and undergoing appropriate antiretroviral treatment | 218 | Arm B: enhanced peer support. Family members and a hospital-based peer supported women to EBF; postnatal; five training sessions plus peer support as needed; also considered wider PMTCT | Standard PMTCT messages on HIV and infant feeding with counselling and support from PMTCT counsellors face to face; postnatal; five sessions |
| Arm C: enhanced peer support + clinic-based coaching by an infant-feeding counsellor; face to face; postnatal; five sessions; also considered wider PMTCT and suitable foods for infants | ||||||
| Pezley et al.177 | USA | Mean age SG 30.9 (3.3) years Mean age SG 29.7 (4.7) years Ethnicity SG: non-Hispanic 100% Ethnicity SG: non-Hispanic 89% |
Mild-moderate depression (as defined with PHQ score of 5–14) but not medicated | 22 | Sunnyside Plus, which was web-based lesson, text support and video calls with a lactation consultant; antenatal and postnatal; six lessons and at least two video calls; also received Sunnyside for anxiety and depression | Sunnyside web-based programme to manage mood before and after pregnancy; web-based; antenatal and postnatal; nine sessions; no breastfeeding support |
| Rasmussen et al.173 | USA | Mean age SG 27.3 (8.6) years Mean age CG 26.6 (9.1) years Ethnicity not reported |
Obesity (defined as BMI of > 29 kg/m2 pre-pregnancy) | 50 | Breastfeeding support from nurses in hospital plus pre- and postpartum calls with lactation consultant; visiting restrictions in hospital; at least four contacts; face to face and telephone; women also encouraged to move about after delivery | Routine care where women room-in with their infants and are observed using the Mother-Baby Assessment tool during at least one breastfeeding episode session. One prepartum call from a lactation consultant |
| Reimers et al.182,283 | South Africa | Median age SG 28.4 (27.5–29.2) years Median age CG 28.8 (27.5–30.0) years Ethnicity not reported |
HIV positive | 619 | Feeding buddy to help with adherence to PMTCT guidelines. Mothers selected the buddy and they were trained together including in EBF; face to face; antenatal and postnatal; four training sessions and ongoing buddy support; also considered compliance with treatment, immunisations and baby monitoring | No details provided |
| Rotheram-Borus et al.183 | South Africa | Mean age SG 26.5 (5.5) years Mean age CG 26.5 (5.5) years Ethnicity not reported |
HIV positive | 1200 | Peer mentor meetings, which included CBT, PMTCT, wider child support and breastfeeding; face to face; antenatal and postnatal; eight contacts | Standard clinic care of PMTCT services (does not seem to include breastfeeding) |
| Samburu et al.186 | Kenya | Mean age SG 22.5 (0.5) years Mean age CG 22.4 (0.5) years Ethnicity not reported |
HIV positive | 52 (NB this is a subsample from a larger cluster-RCT that also included HIV-negative women) | Home-based counselling from community health visitors based on Baby Friendly Community Initiative. Included EBF and PMTCT; face to face; antenatal and postnatal (no. of contacts not defined). Also mother support groups, community gatherings, breastfeeding rooms at the primary care centre | Routine services including antenatal and postnatal care, delivery, general nutrition, hygiene and nutrition. Routine visits by community health workers |
| Suryavanshi et al.187 | India | Median age SG 25 (IQR 22–29) years Median age CG 25 (IQR 22–29) years Ethnicity not reported |
HIV positive | 1191 | COMBIND. Counselling based on scripts by outreach workers on breastfeeding and PMTCT; face to face; antenatal and postnatal; no. of contacts not specified; also includes HIV testing and treatment | India’s national PMTCT programme that includes promotion of EBF, HIV testing and treatment |
Risk-of-bias assessments
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other bias | |
|---|---|---|---|---|---|---|---|
| Fiks et al.176 | Low | Low | High | High | Low | Unsure | High |
| Stuebe et al.160 | Low | Unsure | High | Unsure | High | Unsure | High |
| Suryavanshi et al.187 | Low | Unsure | High | Unsure | High | Unsure | High |
| Samburu et al.186 | Low | Unsure | High | High | High | High | Low |
| Rotheram-Borus et al.183 | Low | Unsure | High | High | Low | Unsure | High |
| Reimers et al.182 | Unsure | Unsure | High | High | High | High | High |
| Rasmussen et al.173 | Low | Unsure | High | High | High | High | High |
| O’Brien et al.180 | Unsure | Unsure | High | High | Low | Unsure | High |
| Pezley et al.177 | Low | Low | High | High | High | Unsure | Unsure |
| Namale-Matovu et al.171 | Low | Unsure | High | High | High | Unsure | High |
| Reifsnider et al.172 | Low | Unsure | High | High | Low | Unsure | High |
| Martin et al.170 | Low | Low | High | High | High | High | High |
| MacVicar et al.181 | Low | Low | High | High | High | Unsure | Unsure |
| Lewkowitz et al.169 | Low | High | High | High | High | Unsure | Unsure |
| Ijumba et al.168 | Low | Unsure | High | High | Low | High | Unsure |
| You et al.184 | Low | Unsure | High | High | Low | Unsure | High |
| Fan et al.167 | Low | Low | High | High | Low | Unsure | Unsure |
| Ehrlich et al.175 | Unsure | Unsure | High | High | Unsure | Unsure | Unsure |
| Chapman et al.174 | Low | Unsure | High | High | Low | High | Low |
| Carlsen et al.179 | Low | Unsure | High | High | High | High | Unsure |
| Bartu et al.178 | Low | Low | High | High | Low | High | Unsure |
| Aldana-Parra et al.273 | Unsure | Low | High | High | Unsure | Unsure | Unsure |
Appendix 5 Data and analysis for breastfeeding support interventions for women with long-term conditions (see Chapter 6)
Forest plots for interventions examining effectiveness of breastfeeding support for women with long-term conditions
Primary outcomes
FIGURE 9.
Breastfeeding support vs. usual care, outcome: not any breastfeeding at 4–8 weeks.
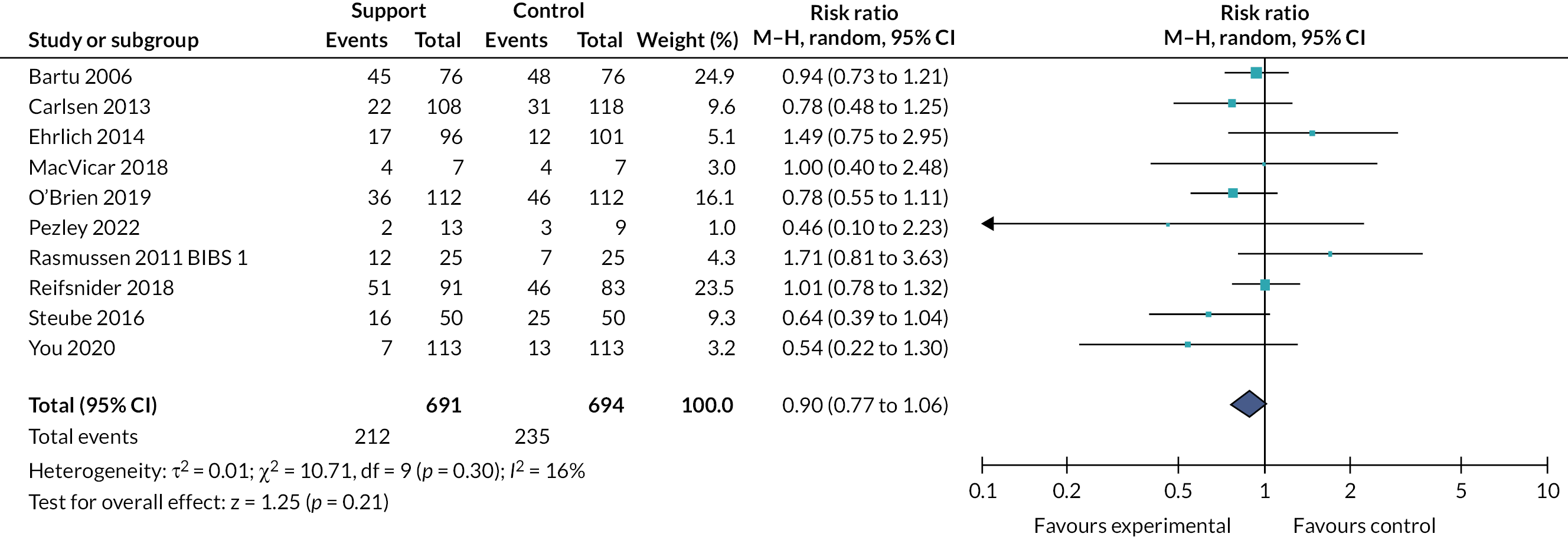
FIGURE 10.
Breastfeeding support vs. usual care, outcome: not exclusive breastfeeding at 4–8 weeks.
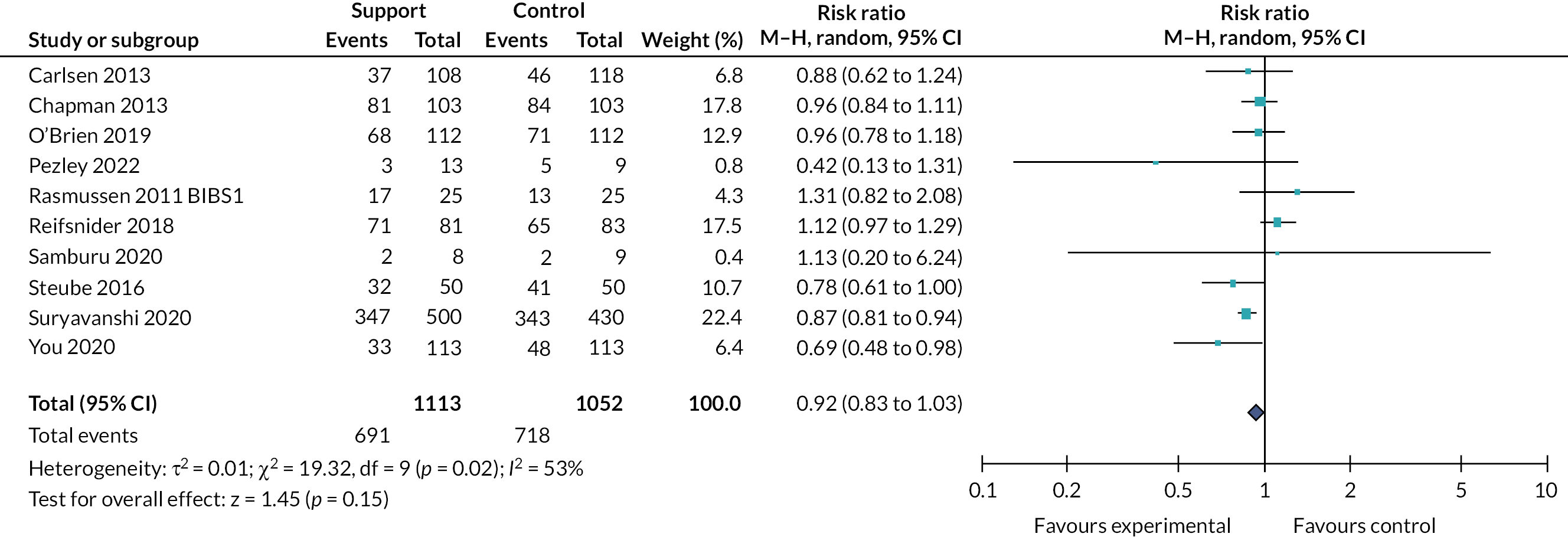
FIGURE 11.
Breastfeeding support vs. usual care, outcome: not any breastfeeding at 6 months.
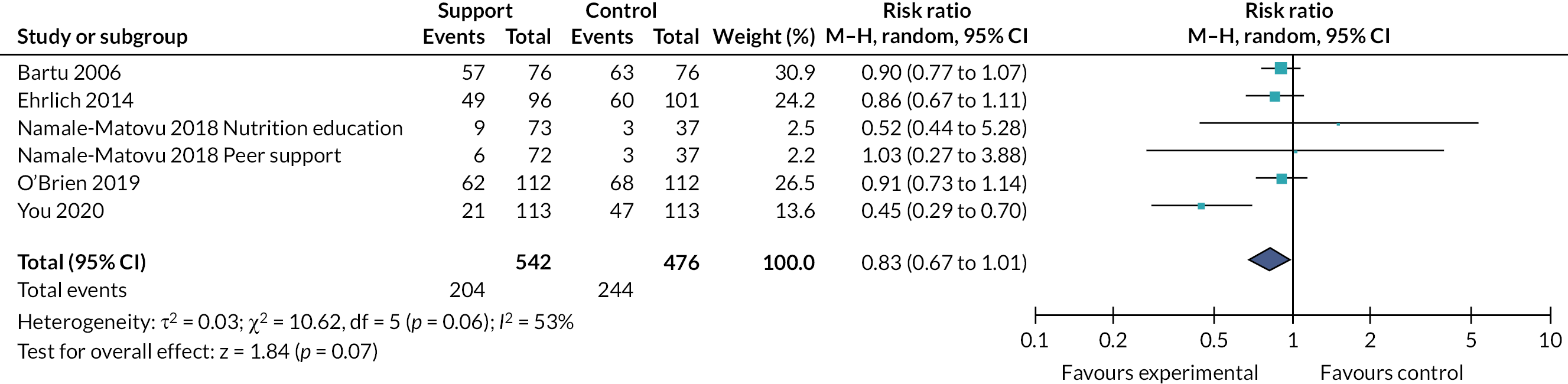
FIGURE 12.
Breastfeeding support vs. usual care, outcome: not exclusive breastfeeding at 6 months.

Additional outcomes
FIGURE 13.
Breastfeeding support vs. usual care, outcome: not any breastfeeding at 3–4 months.

FIGURE 14.
Breastfeeding support vs. usual care, outcome: not exclusive breastfeeding at 3–4 months.
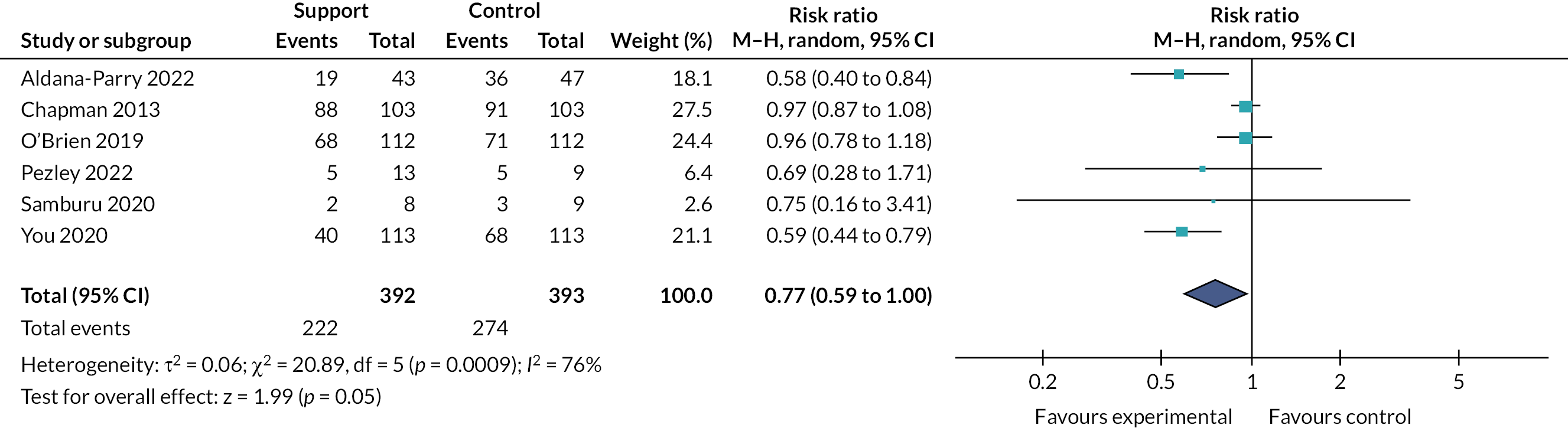
Sensitivity analysis
Primary outcomes
| Outcome | Risk ratio | 95% CI |
|---|---|---|
| Studies excluded due to high risk of bias for allocation concealment | 0.90 | 0.77 to 1.04 |
| Studies excluded with > 20% loss to follow-up | 1.02 | 0.62 to 1.67 |
| Cluster-RCTs for which a design effect could not be calculated excluded | 0.94 | 0.80 to 1.09 |
| Studies for women with HIV excluded | N/A | N/A |
| Results of primary analysis | 0.90 | 0.77 to 1.06 |
| Outcome | Risk ratio | 95% CI |
|---|---|---|
| Studies excluded due to high risk of bias for allocation concealment | 0.93 | 0.75 to 1.16 |
| Studies excluded with > 20% loss to follow-up | 0.90 | 0.67 to 1.20 |
| Cluster-RCTs for which a design effect could not be calculated excluded | 0.94 | 0.84 to 1.06 |
| Studies for women with HIV excluded | 0.97 | 0.85 to 1.10 |
| Results of primary analysis | 0.92 | 0.83 to 1.03 |
| Outcome | Risk ratio | 95% CI |
|---|---|---|
| Studies excluded due to high risk of bias for allocation concealment | 0.76 | 0.54 to 1.07 |
| Studies excluded with > 20% loss to follow-up | 0.75 | 0.45 to 1.23 |
| Cluster-RCTs for which a design effect could not be calculated excluded | N/A | N/A |
| Studies for women with HIV excluded | 0.80 | 0.64 to 1.01 |
| Results of primary analysis | 0.83 | 0.67 to 1.01 |
| Outcome | Risk ratio | 95% CI |
|---|---|---|
| Studies excluded due to high risk of bias for allocation concealment | 0.92 | 0.79 to 1.08 |
| Studies excluded with > 20% loss to follow-up | 0.84 | 0.54 to 1.32 |
| Cluster-RCTs for which a design effect could not be calculated excluded | N/A | N/A |
| Studies for women with HIV excluded | 0.94 | 0.86 to 1.03 |
| Results of primary analysis | 0.95 | 0.89 to 1.00 |
Additional outcomes
| Outcome | Risk ratio | 95% CI |
|---|---|---|
| Studies excluded due to high risk of bias for allocation concealment | 0.69 | 0.42 to 1.13 |
| Studies excluded with > 20% loss to follow-up | 0.87 | 0.28 to 2.73 |
| Cluster-RCTs for which a design effect could not be calculated excluded | N/A | N/A |
| Studies for women with HIV excluded | N/A | N/A |
| Results of primary analysis | 0.86 | 0.53 to 1.38 |
| Outcome | Risk ratio | 95% CI |
|---|---|---|
| Studies excluded due to high risk of bias for allocation concealment | 0.70 | 0.48 to 1.02 |
| Studies excluded with > 20% loss to follow-up | 0.60 | 0.46 to 0.80 |
| Cluster-RCTs for which a design effect could not be calculated excluded | N/A | N/A |
| Studies for women with HIV excluded | 0.77 | 0.59 to 1.01 |
| Results of primary analysis | 0.77 | 0.59 to 1.00 |
Funnel plots
FIGURE 15.
Funnel plot of comparison: 1 Breastfeeding support vs. usual care, outcome: not any breastfeeding at 4–8 weeks.
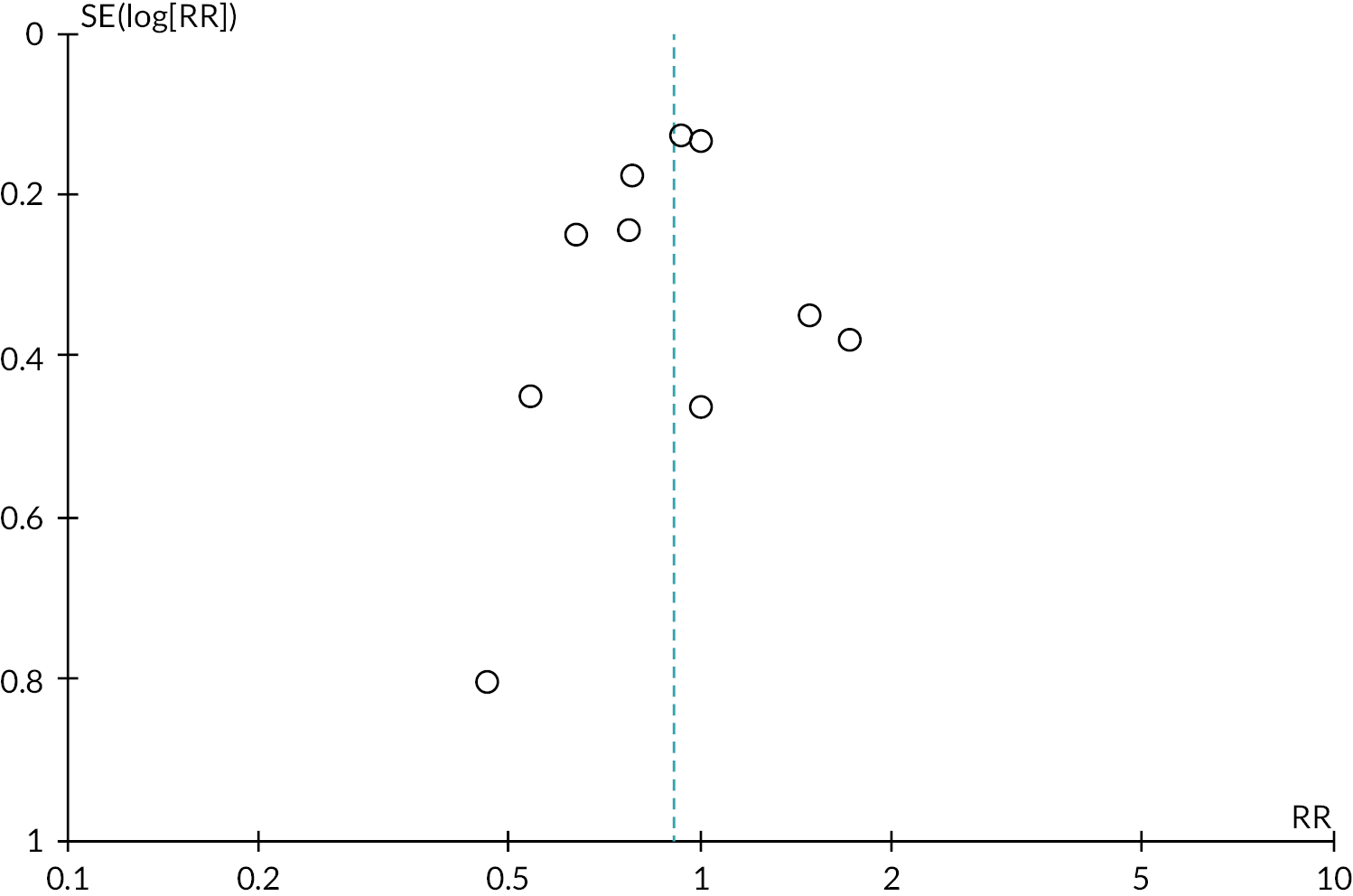
FIGURE 16.
Funnel plot of comparison: 1 Breastfeeding support vs. usual care, outcome: not exclusive breastfeeding at 4–8 weeks.
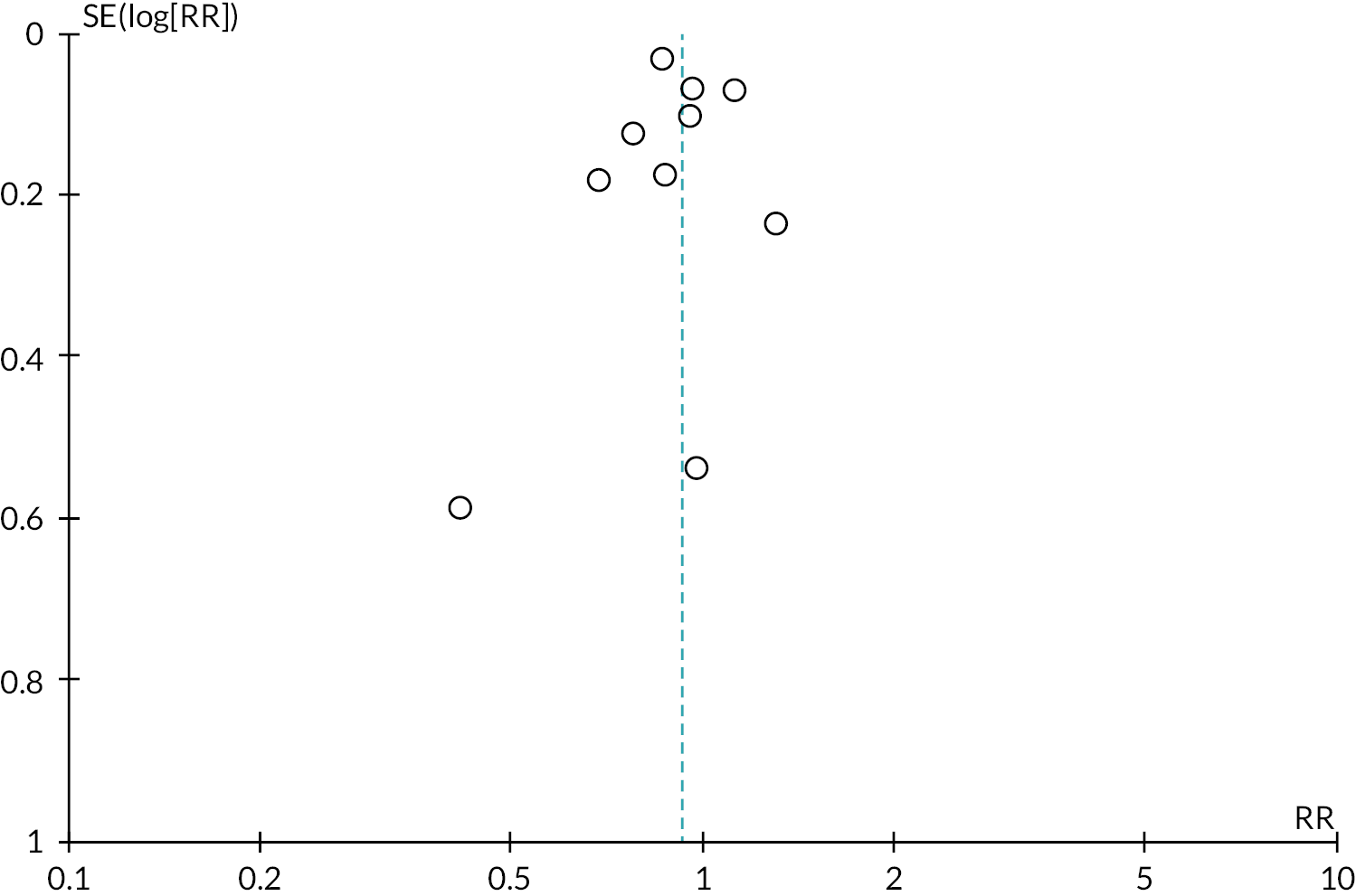
FIGURE 17.
Funnel plot of comparison: 1 Breastfeeding support vs. usual care, outcome: not exclusive breastfeeding at 6 months.
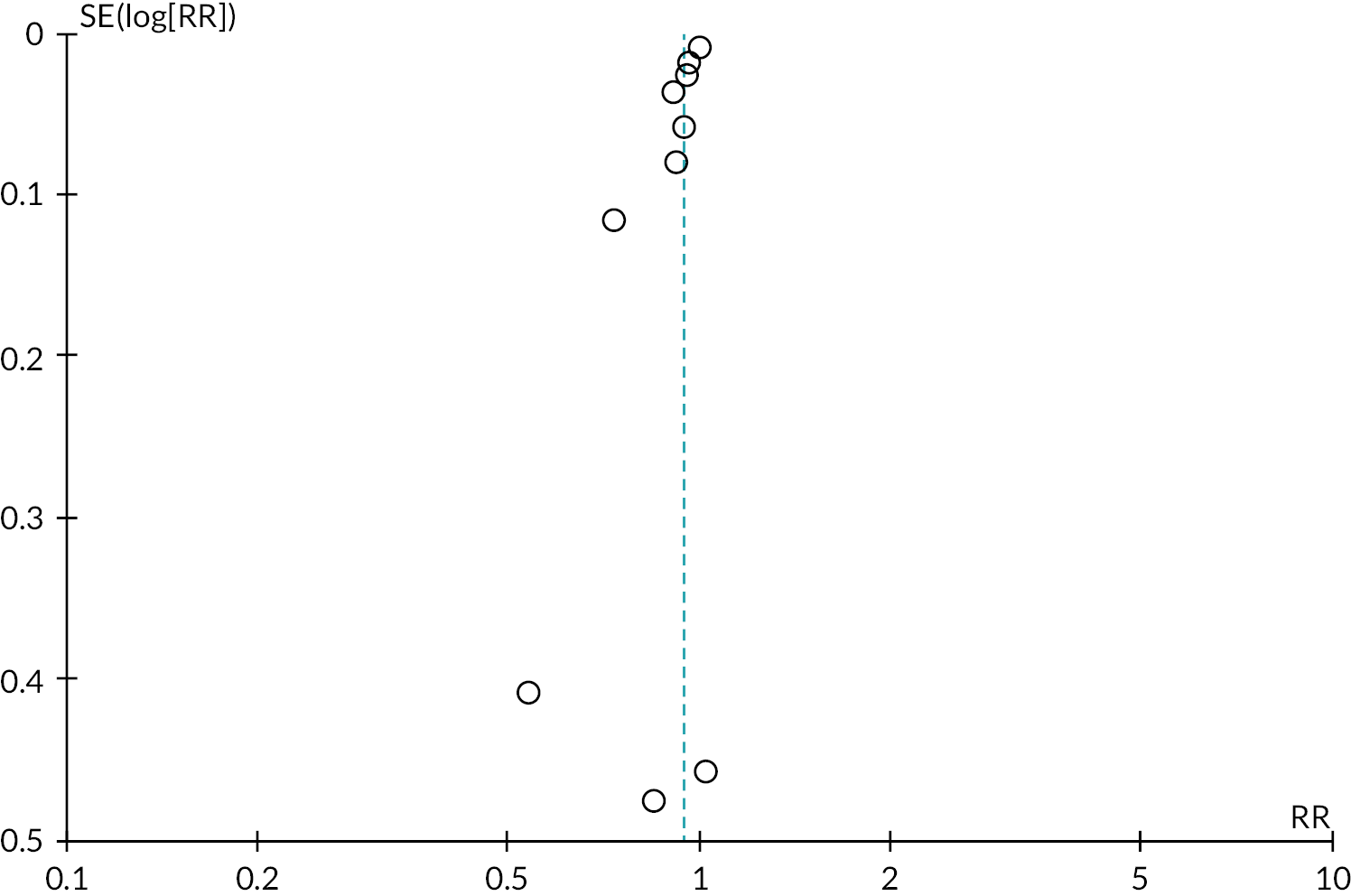
Appendix 6 Study characteristics and risk-of-bias assessments of long-term conditions mixed-methods synthesis (see Chapter 7)
Study characteristics for long-term conditions mixed-methods synthesis (see Chapter 7)
| Study ID, country, setting | Aims | Study design, data collection and analysis method(s) | Sample size, population description | Main study conclusions |
|---|---|---|---|---|
| Acheampong et al. 2018192 Ghana, hospital based |
To describe HIV-positive, lactating women’s perceptions of the role that social persuasion plays in their breastfeeding decisions and practices | Qualitative research In-depth, one-on-one interviews were conducted using a semi-structured interview guide Thematic content analysis |
13 Breastfeeding mothers living with HIV, receiving ART in the public referral hospital, with infants younger than 1 year |
Influential people in the lives of breastfeeding mothers with HIV should be involved during interventions by HIV counsellors to promote breastfeeding practices |
| Andrews et al. 2021193 USA, population based |
To qualitatively explore the lived experiences of disabled women related to breastfeeding | Qualitative research Semi structured interview Descriptive content analysis |
24 Mothers with a disability who have at least one child under the age of 18 years |
Our findings suggest that disabled women should be better supported in their breastfeeding decisions and require greater access to disability-affirmative and informative clinical resources and accessible communication |
| Demirci et al. 2015194 USA, hospital based |
Describe the experiences and perceptions impacting breastfeeding decisions among pregnant and postpartum women taking methadone | Qualitative research Interviews and focus groups following semi structured interview guides Content analysis |
11 Pregnant and postpartum women expressing an interest in breastfeeding their child while taking methadone |
Interventions to increase the prevalence of breastfeeding among women taking methadone should address identified logistical, educational and psychological barriers and consider inclusion of women themselves, partners, peers and clinicians. Clinicians who care for methadone-exposed mothers and infants should be educated on therapeutic communication, up-to-date breastfeeding contraindications and the health benefits of breastfeeding in this population |
| Dieterich et al. 2022195 USA, clinic |
To solicit experiences, perspectives and concerns from postpartum individuals with overweight and obesity who intended to breast-feed and explore if and how they perceived weight stigma impacted their breastfeeding counselling, decisions and experiences | Qualitative research Interview following semi structured guide Content analysis |
18 Pregnant women 28–40+ weeks who had a pre-pregnancy BMI of ≥ 25 kg/m2 that were planning to breastfeed or express milk for their infant |
While participants in this sample recognised the existence of weight stigma in other settings, they did not perceive it during encounters with perinatal healthcare professionals. Additionally, individuals did not perceive weight stigma in any setting as influential on their breastfeeding experiences or practices |
| Fadnes et al. 2010212 Uganda, various study settings, hospitals, community and population setting |
To assess how infant-feeding counselling was done and experienced among counsellors and mothers in eastern Uganda in the context of previous guidelines | Mixed-methods research Interviews and focus group discussions Cross-sectional surveys Inductive thematic analysis |
Sample size not reported Key informant health workers who work with child health and infant-feeding guidance; health workers in the public hospital, health clinics and non-governmental organisations working with people living with HIV; mothers from general population and HIV-positive mothers |
Health workers were faced with challenges related to workload, resources, scientific updating, and also a need to adjust to frequent changes in programs, recommendations and guidelines. The clients were faced with difficult choices, poverty, lack of education and stigma. Feasibility of the recommendations was a major concern. Systematic approaches to update health workers should be a priority |
| Flax et al. 2016213 Malawi, community based |
Study aims were to (1) document the type and frequency of IYCF counselling offered to HIV-infected women during postnatal PMTCT visits; (2) examine IYCF knowledge and practices of HIV-infected mothers in Option B+ with children ranging from 0 to 23 months; and (3) study HIV-infected women’s IYCF decision-making and their perceptions of factors related to their IYCF practices | Mixed-methods research Survey In-depth interviews and observations Descriptive statistics Thematic analysis |
224 (160 survey; 32 in-depth interviews; 32 observations) HIV-infected women participating in PMTCT Option B+, aged ≥ 18 years who had a child aged < 24 months |
This represents a missed opportunity for health workers to support optimal IYCF practices within Option B+ |
| Garner et al. 2014196 USA, community based |
To describe health professionals’ experiences providing breastfeeding care for obese women during the prenatal, peripartum and postpartum periods | Qualitative research Semi structured in-depth interviews using interview guide Content analysis |
34 Health professionals who provide care for obese women during pre-, peri- and postnatal periods |
Health professionals identified multiple challenges that obese women encounter with breastfeeding, as well as their own challenges with providing care |
| Hazemba et al. 2016197 Zambia, health facilities |
The aim of this study was to explore factors that influence the decision to exclusively breast-feed in the context of preventing mother-to-child transmission of HIV | Qualitative research Semi structured interviews Participant observation Framework analysis guided by social constructivism theory |
36 HIV-positive mothers on treatment regimen and have attended health promotion talks on infant feeding and who opted to exclusively breastfeed. Key informants from the prevention of mother-to-child transmission programme, including nurses, nutritionists and clinical officers |
In order to enhance feeding practices for HIV-exposed infants, our study suggests a broader health campaign supporting all mothers to exclusively breastfeed |
| Hicks et al. 2018207 USA, hospital based |
This study aimed to capture the infant-feeding practices and barriers to exclusive breastfeeding for women in methadone maintenance therapy | Mixed-methods research A qualitative and quantitative interview-based survey – 47-item instrument incorporated questions from Infant Feeding Survey and adapted questions anchored by Bandura’s triadic reciprocal causation model Content analysis Descriptive statistics |
30 Women who delivered their baby while in treatment at an opioid dependence treatment centre |
Women in treatment for opioid dependence both desire and attempt to establish breastfeeding, but encounter significant challenges, including long NICU stays and lack of support and education, that compromise their success. These findings should inform the development of future programs or interventions geared toward increasing breastfeeding initiation, support and duration among women who give birth to babies while in treatment for opioid addiction |
| Howard et al. 2018198 USA, hospital based |
To investigate perspectives of mothers with opioid use disorder regarding breastfeeding and rooming-in during the birth hospitalisation and identify facilitators and barriers | Qualitative research In-depth semistructured interviews utilising interview guide Grounded theory analysis |
25 Mothers with opioid use disorder enrolled in a treatment programme |
Future interventions aimed at increasing breastfeeding and rooming-in during the birth hospitalisation should focus on education regarding the benefits of breastfeeding and rooming-in, supporting mothers’ autonomy in caring for their infants, minimising stigma and maximising resilience |
| Israel-Ballard et al. 2014199 Kenya, community based |
To assess how counsellors, who provide infant-feeding counselling to HIV-positive women, deal with challenges they face in two Kenyan provinces | Qualitative research Post-counselling exit interviews Observations and key informant interviews Analysis not reported |
Unclear 80 post-counselling interviews; 22 counselling session observations; 11 key informant interviews HIV-positive women pregnant or with child 3, 6, 9 or 12 months of age Local stakeholders, including district and provincial nutritionists and nursing officers |
Implementing the new WHO guidance will reduce the need for AFASS assessments, greatly simplifying both the government’s and counsellor’s tasks |
| Jagiello and Azulay Chertok 2015200 USA, hospital based |
The purpose of the study was to gain insight into the breastfeeding challenges that women with GDM face in the early postpartum period | Qualitative research Phenomenological approach using focus groups and interviews Thematic analysis |
27 Women with GDM and had initiated breastfeeding following birth |
Participants identified breastfeeding facilitators and barriers, many of which could have been modified. The women expressed a need for consistent lactation advice, education, assistance and strategies to address breastfeeding challenges and milk supply issues |
| Keely et al. 2015201 UK, population based |
To explore the views and experiences of obese women who initiated breastfeeding when their babies were born and intended to continue exclusively breastfeeding until at least 16 weeks later, but who were no longer exclusively breastfeeding, or had stopped breast-feeding 6–10 weeks later | Qualitative research Semistructured face-to-face interviews Thematic analysis |
28 Women who had given birth to a single baby at > 37 weeks’ gestation, breastfeeding at first feed but no longer exclusively breast-feeding at 6–8 weeks postnatal, and BMI at the start of pregnancy of > 30kg/m2 |
Midwives should be mindful of the presence of additional factors alongside maternal obesity, such as caesarean delivery, physical difficulties when breastfeeding, poor body image and lack of confidence about sufficient milk supply. Scope for innovation within hospital policies with regard to both the facilitation of early skin-to-skin contact and privacy in postnatal accommodation could be explored in future research. Women should be provided with information about the provision and specific purpose of breastfeeding support groups and services and encouraged to access these services when appropriate |
| Laws et al. 2016211 Australia, population based |
The aim of the study was to report on the experiences of some mothers attempting to breastfeed when they or their infant have the rare genetic disorder ectodermal dysplasia | Mixed-methods research Focus group discussions Survey questionnaires Content analysis |
149 (23 included in focus groups, 126 survey unclear) Parents caring for a child with ectodermal dysplasia. Also includes parents who had themselves been diagnosed with ectodermal dysplasia |
While genetic screening is offered to pregnant women who have a known family history of a genetic disorder, many genetic orders are rare and go undetected. Newly birthed mothers with a genetic disorder may encounter difficulties when attempting to establish breastfeeding. More genetic education is needed to assist midwives in gaining a better understanding of how physiological problems, associated with a genetic disorder, may be a root cause of breastfeeding difficulties |
| MacVicar et al. 2017202 UK, hospital based |
The aim of this, study was to explore the views of women with opiate dependence on, proposed elements for inclusion in a breastfeeding support intervention | Qualitative research Qualitative think aloud interviews with contextual notes Stepwise approach particular to the think aloud technique Framework analysis |
6 Opiate-dependent women within 6 months of giving birth; were enrolled on opiate medication treatment during their pregnancy; had initiated breastfeeding and accessed in-hospital breastfeeding support |
There are distinct facilitators of, modifiers of and barriers to breastfeeding within the context of opiate exposure. Using this awareness to underpin the key features of the design should enhance maternal receptiveness, acceptability and usability of the support intervention |
| MacVicar et al. 2018181 UK, hospital based |
This study explored the feasibility of in-hospital, tailored breastfeeding support for the substance exposed mother and baby | Mixed-methods research A RCT and maternal questionnaire Descriptive statistics |
14 Mothers who were on opioid substitution medication therapy during pregnancy, had an intention to breastfeed, were > 36 weeks’ gestation and > 16 years of age |
The findings highlight the feasibility of tailored breastfeeding support for the substance exposed mother and baby and endorse the promotion and support of breastfeeding for this group. Future research of a statistically powered RCT to evaluate clinical efficacy is recommended |
| Matsunaga et al. 2021208 Japan, hospital based |
This study aimed to examine the current levels of implementation of breastfeeding support to women with GDM in Japan and to clarify barriers to promoting breastfeeding among this population | Quantitative, cross-sectional study 25-item questionnaire Descriptive statistics Content analysis |
296 Senior midwife or nurse, who was familiar with the hospital’s practices and services for women with GDM |
In Japan, most hospitals that responded provided general breastfeeding support from the antenatal to postpartum periods. However, the benefits of breastfeeding in terms of preventing the incidence of type 2 diabetes following GDM were insufficiently communicated to women with GDM. Furthermore, there were numerous barriers to promoting breastfeeding among women with GDM |
| Misita et al. 2021214 Canada |
(1) To determine the likelihood of full breastfeeding at 3 months postpartum in women with and without DiP; (2) to explore associations between diabetes management practices and infant-feeding practices in those who had DiP; and (3) to examine women’s experiences of feeding their infants after having DiP | Mixed-methods research Infant feeding questionnaires, prospective breastfeeding diaries and medical chart data Semistructured interviews Chi-squared tests, two-sample t-tests Thematic analysis |
261 (62 quantitative cohort matched to 175 participants without diabetes, 24 qualitative interviews) Women who had diagnosis of diabetes in pregnancy 8 months postpartum, > 18 years of age |
Women with diabetes in pregnancy may require additional prenatal and postnatal infant feeding support to be better prepared to overcome feeding challenges they may face |
| Nieuwoudt et al. 2018203 South Africa, community based |
To explore how health workers attached to community health clinics understood and were implementing the new infant-feeding guidelines. The study explored (1) health workers’ knowledge of the declaration; (2) how formula removal and training influenced their counselling; and (3) their impressions about changes in breastfeeding practices. Drawing on health workers to share and reflect on their upbringing, experiences of infant feeding, and values as these related to their experiences | Qualitative research Semistructured interviews using interview guide Thematic content analysis |
11 Health workers from four community health clinics, who had counselled mothers on infant feeding before and after the policy change |
Some participants believed that breastfeeding practices were driven by finance or family pressures rather than the health information they provided. Health workers generally lacked training on the policy’s evidence base, particularly the health benefits of exclusive breastfeeding for non-exposed infants. They wanted clarity on their counselling role, based on individual risk or to promote exclusive breastfeeding as a single option. If the latter, they needed training on how to assist mothers with community-based barriers. Infant feeding messages from health workers are likely to remain confusing until their uncertainties are addressed. Their insights should inform future guideline development as key actors |
| Nor et al. 2009205 South Africa, community based |
The aim of the study was to explore the perceptions and experiences of infant-feeding peer counselling in three diverse settings in South Africa | Qualitative research Individual interviews Participant observations Review of records Informal interviews taken during observations Thematic analysis using interpretative description framework |
27 Women, both HIV-infected and uninfected, enrolled in an exclusive breastfeeding intervention study who had been offered peer counselling |
The findings underline the contextual barriers facing peer counsellors and show that these challenges could have important implications for the effectiveness of infant-feeding counselling in high HIV prevalence countries |
| Nor et al. 2012204 South Africa, community based |
To explore mothers’ perceptions and experiences of infant feeding within a community-based peer counselling intervention promoting exclusive breast or formula feeding. Of particular interest was whether peer counselling on infant feeding helped the mothers to negotiate existing systems of beliefs and traditions | Qualitative research Semistructured interviews using interview guide Qualitative interpretative description |
17 HIV-positive and negative mothers who were participating in the PROMISE-EBF peer counselling intervention cluster |
Efforts to reduce barriers to EBF need to be intensified and further take into account the strong cultural beliefs that promote mixed feeding |
| O’Reilly et al. 2022206 Ireland, population and hospital based |
This study aimed to (a) explore the barriers and enablers to breastfeeding in women with high BMIs, and (b) map specific behaviours suitable for intervention across the antenatal to postpartum periods | Qualitative research Semistructured interviews Reflexive thematic analysis |
61 Women with a BMI of > 25kg/m2 who had exclusively breastfed for ≥ 6 months within the previous 2 years Partners who were main support for a woman who had breastfed successfully for 6 months or more within the previous 2 years; healthcare professionals involved in providing breastfeeding support |
The barriers and enablers identified for participants with high BMIs were similar to those for the broader population; however, the physicality and associated social bias of high BMIs mean that additional support is warranted. Antenatal and postpartum breastfeeding services need a multifaceted, inclusive and high-quality program to provide the necessary support to women with higher BMIs |
| Powell et al. 2018209 USA, population based |
This study aimed to explore the facilitators of and barriers to breastfeeding among women with physical disabilities | Qualitative research Semistructured telephone interviews Content analysis |
25 Women who had a physical disability or condition that affected their ability to walk or use of arms or hands at the time of pregnancy, and had delivered a child within the past 10 years |
The need for greater supports for women with physical disabilities who desire to breastfeed as well as information for women and their clinicians about facilitating breastfeeding |
| Rasmussen et al. 2006210 USA, population based |
The purpose of this study was to describe the experience and attitudes about BF of those who provide care to lactating women about BF and to evaluate how they counsel obese mothers about breastfeeding | Quantitative, cross-sectional study Questionnaire survey conducted via e-mail or telephone interview Chi-squared tests |
120 Healthcare providers (including lactation consultants, physicians, midwives, nurses) who counsel mothers about breastfeeding |
Given the excess risk for premature lactation failure among obese women, these findings suggest that those who care for such women need to be made aware of this risk so that they can develop and provide appropriate services |
Critical Appraisal Skills Programme qualitative summary
| Study | 1. Was there a clear statement of the aims of the research? | 2. Is a qualitative methodology appropriate? | 3. Was the research design appropriate to address the aims of the research? | 4. Was the recruitment strategy appropriate to the aims of the research? | 5. Was the data collected in a way that addressed the research issue? | 6. Has the relationship between researcher and participants been adequately considered? | 7. Have ethical issues been taken into consideration? | 8. Was the data analysis sufficiently rigorous? | 9. Is there a clear statement of findings? | 10. How valuable is the research? |
|---|---|---|---|---|---|---|---|---|---|---|
| Acheampong et al.192 | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes | Yes | Partial |
| Andrews et al.193 | Yes | Yes | Partial | Yes | Partial | Partial | No | Yes | Yes | Yes |
| Dieterich et al.195 | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Partial | Partial | Yes |
| Demirci et al.194 | Yes | Yes | Can’t answer | Partial | Partial | Partial | Partial | Yes | Partial | Yes |
| Fadnes et al.212 | Yes | Yes | Yes | Yes | Can’t answer | No | Yes | Yes | Yes | Yes |
| Flax et al.213 | Yes | Yes | Yes | Yes | Yes | No | Partial | Yes | Yes | Yes |
| Garner et al.196 | Yes | Yes | Partial | Yes | Yes | Partial | Partial | Yes | Yes | Partial |
| Hazemba et al.197 | Yes | Yes | Partial | Yes | Partial | No | Yes | Yes | Partial | Partial |
| Howard et al.198 | Yes | Yes | No | Yes | Yes | No | No | Partial | Yes | Partial |
| Israel-Ballard et al.199 | Yes | Yes | Can’t answer | Yes | Partial | No | Partial | No | No | No |
| Jagiello and Azulay Chertok200 | Yes | Yes | Yes | Yes | Partial | No | Yes | Yes | Yes | Yes |
| Keely et al.201 | Yes | Yes | Yes | Yes | Yes | No | Partial | Partial | Partial | Yes |
| Laws et al.211 | Yes | Yes | Partial | Partial | Partial | No | Partial | No | Partial | Partial |
| MacVicar et al.202 | Yes | Yes | Yes | Yes | Partial | Partial | Yes | Partial | Partial | Yes |
| MacVicar et al.181 | Yes | Yes | Yes | Partial | Yes | No | Partial | Partial | Partial | Yes |
| Misita et al.214 | Yes | Yes | Yes | Yes | Yes | No | Partial | Partial | Partial | Partial |
| Nieuwoudt and Manderson203 | Yes | Yes | Partial | Partial | Yes | Partial | Yes | Partial | Partial | Yes |
| Nor et al.205 | Yes | Yes | Partial | Partial | Partial | Partial | Partial | Yes | Partial | Yes |
| Nor et al.204 | Partial | Yes | Partial | Partial | Partial | No | Partial | Yes | Partial | Partial |
| O’Reilly et al.206 | Yes | Yes | Partial | Partial | Yes | Yes | Yes | Yes | Partial | Yes |
| Powell et al.209 | Yes | Yes | Partial | Partial | Yes | No | Yes | Yes | Yes | Yes |
AXIS summary
| Hicks et al.207 | Laws et al.211 | Matsunaga et al.208 | Rasmussen et al.210 | ||
|---|---|---|---|---|---|
| 1 | Were the aims/objectives of the study clear? | Yes | No | Yes | Yes |
| 2 | Was the study design appropriate for the stated aim(s)? | Yes | No | Yes | Yes |
| 3 | Was the sample size justified? | No | No | Yes | No |
| 4 | Was the target/reference population clearly defined? (Is it clear who the research was about? | Yes | No | Yes | Yes |
| 5 | Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | No | Don’t know | Yes | Don’t know |
| 6 | Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation? | No | Don’t know | N/A | Don’t know |
| 7 | Were measures undertaken to address and categorise non-responders? | No | No | No | No |
| 8 | Were the risk factor and outcome variables measured appropriate to the aims of the study? | Yes | Don’t know | Yes | Don’t know |
| 9 | Were the risk factor and outcome variables measured correctly using instruments/measurements that had been trialled, piloted or published previously? | Don’t know | No | Don’t know | No |
| 10 | Is it clear what was used to determined statistical significance and/or precision estimates? (e.g. p-values, CIs) | Yes | No | Don’t know | Yes |
| 11 | Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | No | No | Yes | No |
| 12 | Were the basic data adequately described? | Yes | No | Yes | No |
| 13 | Does the response rate raise concerns about non-response bias? | Don’t know | Don’t know | Yes | Yes |
| 14 | If appropriate, was information about non-responders described? | No | No | No | No |
| 15 | Were the results internally consistent? | N/A | Don’t know | Don’t know | Don’t know |
| 16 | Were the results presented for all the analyses described in the methods? | Yes | Don’t know | Yes | Don’t know |
| 17 | Were the authors’ discussions and conclusions justified by the results? | No | No | Yes | No |
| 18 | Were the limitations of the study discussed? | Yes | Partial | Yes | Don’t know |
| 19 | Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results? | No | Don’t know | No | No |
| 20 | Was ethical approval or consent of participants attained? | Yes | Yes | Yes | No |
Primary studies underpinning synthesis themes
| Included studies (n = 24) | Additional breastfeeding support needs for mothers with LTCs | Availability of breastfeeding support for mothers with LTCs | The role and practice of breastfeeding support for mothers with LTCs | Suggested strategies to improve breastfeeding support for mothers with LTCs |
|---|---|---|---|---|
| Acheampong 2018 | • | • | ||
| Andrews 2021 | • | • | ||
| Demirci 2015 | • | • | • | • |
| Dieterich 2022 | • | • | ||
| Fadnes 2010 | • | • | ||
| Flax 2016 | • | • | • | |
| Garner 2014 | • | • | • | |
| Hazemba 2016 | • | • | • | |
| Hicks 2018 | • | • | • | |
| Howard 2018 | • | • | • | |
| Israel-Ballard 2014 | • | • | ||
| Jagiello 2015 | • | • | • | • |
| Keely 2015 | • | • | • | • |
| Laws 2016 | • | • | • | • |
| MacVicar 2017 | • | • | ||
| MacVicar 2018 | • | • | • | |
| Matsunaga 2021 | • | • | • | |
| Misita 2021 | • | • | • | |
| Nieuwoudt 2018 | • | • | • | |
| Nor 2009 | • | • | • | |
| Nor 2012 | • | • | ||
| O’Reilly 2022 | • | • | • | |
| Powell 2018 | • | • | • | • |
| Rasmussen 2006 | • | • | • |
Appendix 7 Characteristics of included economic evaluation studies
| Study ID and setting | Intervention | Comparator | Participant characteristics | Methods of economic analysis | Summary of results | Applicability |
|---|---|---|---|---|---|---|
| Avram 2020216 USA Nationwide Hospital setting |
Rooming-in + usual care Support: Breastfeeding only Description: Rooming-in newborns with families encourages parental involvement and promotes breastfeeding, thereby decreasing the need for opioid replacement and shortening hospitalisation Provider: Professional Mode of delivery: Face to face Intensity: Not reported Duration: Hospital stay after birth |
Usual care Description: Not reported |
Inclusion criteria: Women/infant dyads with prenatal use of opioids and infants with neonatal opioid withdrawal Exclusion criteria: Not reported Sample size: Not applicable, as model-based Baseline characteristics: Not applicable |
Type of economic evaluation: CUA (model-based) Perspective: Societal Currency, price year: USD, 2018 Time horizon: Lifetime Discount rate: 3% Primary outcome: Cost per QALY gained Secondary outcomes:N/A Data sources: Outcome of effect: Literature-based (systematic reviews and retrospective cohort studies Resource use: Literature-based costs Unit costs: Not reported Measurement of uncertainty: Not reported Consideration of heterogeneity: Not reported Sensitivity analyses: Univariate sensitivity analyses conducted on model inputs across a range of parameters |
Base-case results: Rooming-in resulted in cost savings of US$509,652,728 and 12,333 additional QALYs per annual cohort Findings from sensitivity analyses: The largest driver of the model was the risk ratio of pharmacotherapy associated with rooming-in compared with not rooming-in. The model was also sensitive to the probability of developing severe neurological impairment in neonates whose withdrawal symptoms did not warrant pharmacotherapy |
Not applicable: OECD settings, societal perspective, cost savings and additional QALYs reported, time horizon from birth up to lifetime |
| Bick 2020219 UK Inner-city unit, south England Community healthcare setting |
Slimming World + usual care Support: Breastfeeding plus Description: Programme of weight management Provider: Lay person Mode of delivery: Face to face Intensity: High (12 weekly sessions) Duration: From 8 to 16 weeks postpartum until infants are 12 months old |
Usual care Description: Standard NHS maternity care to 6–8 weeks postpartum, including routine midwife, health visitor and GP contacts |
Inclusion criteria: Women 18 years +, able to speak/read English, singleton pregnancy, BMI of > 25kg/m2 at pregnancy booking or normal BMIs (18.5–24.9kg/m2) with excessive gestational weight gain Exclusion criteria: Not stated Sample size: Total, N = 193 (intervention, n = 98; control, n = 95) Baseline characteristics: Baseline characteristics appear balanced |
Type of economic evaluation: Cost–outcome description (alongside a feasibility study) Perspective: Provider Currency, price year: GBP, 2000 Time horizon: Within feasibility trial Discount rate: N/A Primary outcome: Feasibility of collecting economic data Secondary outcomes: Not reported Data sources: Outcome of effect: Within study Resource use: Within study Unit costs: National sources Measurement of uncertainty: N/A Consideration of heterogeneity: N/A Sensitivity analyses: N/A |
Base-case results: Data collection tools were suitable | Not applicable: minimal economic data reported; UK setting, provider perspective, time horizon up to infant age 1 year |
| Desmond 2008217 South Africa KwaZulu-Natal province Community healthcare setting |
Vertical Transmission Study (VTS) + usual care Support: Breastfeeding only Description: A breastfeeding intervention strategy, designed to promote exclusive breastfeeding from birth to 6 months Provider: Lay breastfeeding counsellor Mode of delivery: Face to face Intensity: High (minimum 14 visits) Duration: From late pregnancy to 6 months postpartum |
Usual care Description: Not reported |
Inclusion criteria: Women living with HIV Exclusion criteria: Not reported Sample size: not reported, suggested hypothetical sample Baseline characteristics: Not reported |
Type of economic evaluation: CEA (within trial and model-based) Perspective: Provider Currency, price year: USD, 2000 Time horizon: 7 months Discount rate: N/A Primary outcome: Cost per increased month of EBF Secondary outcomes: Not reported Data sources: Outcome of effect: Within-trial Resource use: Within-trial Unit costs: Local and national sources Measurement of uncertainty: N/A Consideration of heterogeneity: N/A Sensitivity analyses: Scenario analyses reported for different levels of intervention |
Base-case results: ICER = US$88 per increased month of EBF Findings from scenario analyses: Simplified scenario US$29 per increased month of EBF, full scenario US$48 per increased month of EBF |
Not applicable: non-OECD setting; provider perspective, cost per DALY averted reported, time horizon from birth up to 7 months |
| Mottl-Santiago 2020125 USA Community healthcare and hospital setting |
Birth Sisters Best Beginnings for Babies program (doula support) + usual care Support: Breastfeeding plus Description: Birth Sisters Best Beginnings for Babies provided community doula services with consultation from the Medical Legal Partnership when indicated Provider: Lay (doula peer support) Mode of delivery: Face-to-face Intensity: High – Participants receive up to eight 2-hour prenatal home visits; continuous support through labour and birth, and up to four 2-hour postpartum home visits through 6–8 weeks postpartum Duration: From 24 weeks gestation up to 8 weeks postpartum |
Usual care Description: Usual prenatal, intrapartum and postpartum maternity care |
Inclusion criteria: Subgroup of medically high-risk women (hypertension or diabetes in pregnancy) Exclusion criteria: < 18 years of age, high-risk pregnancy defined by care in the high-risk prenatal clinic Sample size: Total, N = 411 (intervention, n = 207; control, n = 204) Baseline characteristics: No group differences observed at baseline |
Type of economic evaluation: CBA (study-based) Perspective: Payer Currency, price year: USD, 2018 Time horizon: From mid-pregnancy to 6–8 weeks postpartum Discount rate: N/A Primary outcome: Return on investment Secondary outcomes: N/A Data sources: Outcome of effect: Within trial Resource use: Within trial Unit costs: Local sources Measurement of uncertainty: Payments were winsorised to address outliers Consideration of heterogeneity: Variations in impact for different populations, with the focus here on medically high-risk mothers Sensitivity analyses: N/A |
Base-case results: ROI 276% | Not applicable: OECD setting, payer perspective, time horizon from mid-pregnancy up to 8 weeks postpartum |
| Maredza 2013218 South Africa Rural and urban settings Community healthcare setting |
Infant feeding strategies + usual care Support: Breastfeeding only Description: Strategy of actively supporting breastfeeding with extended nevirapine prophylaxis for 12 months Provider: Paraprofessional (skilled care workers and community health workers) Mode of delivery: Face-to-face Intensity: Unclear Duration: From first trimester of pregnancy until infants are 12 months old |
Usual care Description: Not reported |
Inclusion criteria: Women living with HIV Exclusion criteria: Not reported Sample size: Not reported Baseline characteristics: Not reported |
Type of economic evaluation: CUA (model-based) Perspective: Health provider Currency, price year: USD, 2000 Time horizon: Discount rate: Annual rate of 3% Primary outcome: Incremental cost per DALY averted Secondary outcomes: N/A Data sources: Outcome of effect: Literature and expert opinion Resource use: Literature Unit costs: Local and national sources Measurement of uncertainty: 95% CI estimated Consideration of heterogeneity: Not reported Sensitivity analyses: Univariate sensitivity analyses conducted in certain urban settings |
Base-case results: ICER = Cost per DALY averted dominant with a 95% CI of dominant, 13,000 Findings from sensitivity analyses: ICER for actively supporting breastfeeding was less costly and less effectively for all one-way SA, with the exception of proportion of HIV-diagnosed breastfeeding women on HAART, where the ICER was dominant |
Not applicable: non-OECD settings Provider perspective, cost per DALY averted reported, time horizon from birth up to lifetime |
Appendix 8 Draft toolkit
Introduction
This draft toolkit outlines the proposed toolkit structure and contents resulting from the co-development process described in Chapter 9. Further co-development work, write-up and refinement of the toolkit output is ongoing.
Section 1 of the toolkit describes a proposed set of evidence-based intervention components recommended for breastfeeding support services. Section 2 summarises the key criteria for those considering adopting and adapting the proposed intervention components for delivery in UK settings and/or to meet the needs of breastfeeding women with MLTC. Section 3 provides recommendations to support the planning of the implementation and roll-out stages of the proposed intervention components in UK settings. Finally, section 4 sets out recommendations for the evaluation of breastfeeding support interventions in UK settings, including a range of suggested outcomes and practical considerations.
-
Evidence-based recommendations for breastfeeding support services.
Based on the most recently available high-quality evidence on effectiveness of breastfeeding support interventions, the most effective intervention components have been identified and used to develop a comprehensive breastfeeding support programme prototype. These components were selected from interventions in the Cochrane review83 meeting two key criteria: (1) identified as effective in reducing the number of women stopping breastfeeding; and (2) judged to be at low risk of bias, using allocation concealment as a proxy indicator. Thus, the proposed set of intervention components is underpinned by seven interventions103,221–226 and together they provide a range of ways most likely to effectively support breastfeeding women.
The proposed programme involves the following components and activities.
The breastfeeding support package will be delivered one to one by infant-feeding advisors. It consists of one 30-minute antenatal appointment, one 30-minute hospital visit, one 30-minute home visit within 48 hours of discharge and regular telephone calls. The antenatal session will focus on building rapport, providing education and identifying any concerns regarding breastfeeding. The hospital and discharge visits will involve checking latch, helping with positioning and observing a feed if requested by the mother. Infant-feeding advisors will also provide encouragement, praise and reassurance during visits. Women will be given the chance to ask questions and raise any concerns.
Following the initial three contacts, support will be provided remotely unless a face-to-face visit is required. For the first 4 weeks there will be a weekly proactive telephone call and beyond that support will be provided monthly until 3 months or when breastfeeding ceases. Women can also contact infant-feeding advisors as needed by telephone or SMS during this 3-month period and beyond as new issues arise.
The infant-feeding advisor will also signpost women to the local breastfeeding peer support group, which provides support via WhatsApp and weekly face-to-face support groups. Infant-feeding advisors will receive training on the intervention delivery.
-
Adapting the evidence-based recommendations to your local services.
-
Prioritised criteria to consider to the adoption and adaptation of the proposed intervention in UK settings.
-
These criteria were developed in collaboration with our stakeholders and PPI members through interactive exercises to facilitate the discussion, tailoring and prioritisation of a readily available set of general criteria to evaluate the transferability of health interventions. 81
The resulting set of prioritised criteria were:
-
population’s acceptability of the intervention
-
the quality of the primary evidence available
-
sustainability of the intervention
-
service providers’ perception and support of the intervention
-
conditions of health service provision
-
existence of a knowledge translation process for the intervention
-
quality of communication in multidisciplinary work and teams
-
the utility/usefulness of the primary evidence available
-
the structure of the healthcare system and relevant services
-
co-operation between intervention providers and recipients
-
sociodemographic characteristics of the population
-
the conception of the intervention.
-
Adaptations to meet needs of breastfeeding women with MLTC.
-
These criteria were developed in collaboration with our stakeholders and PPI members, based on the experiences of those who took part in our PPI meetings and in our stakeholder engagement workshops. The suggested adaptations to the proposed intervention components to meet the needs of breastfeeding women with MLTC are the following:
-
The antenatal appointment should be longer than 30 minutes.
-
Continuity is needed with the same person delivering the intervention antenatally and postnatally so that women do not have to repeat their stories.
-
Infant-feeding advisors should be included in joint obstetric and medical clinics.
-
-
Other adaptations to consider:
-
The person delivering the intervention should have expertise in medications and breastfeeding, as well as in breastfeeding support.
-
Antenatal appointments of 90 minutes would be more realistic, or several shorter appointments could be helpful.
-
Starting discussions early in pregnancy could be beneficial to take account of the higher risk of preterm birth for women with multimorbidities and to give practitioners more time to find accurate information.
-
Women require a medication review in early pregnancy, and this should involve a pharmacist who is knowledgeable about medications and breastfeeding.
-
Women should be able to see all their healthcare providers (e.g. midwife, obstetrician, physician, pharmacist) at one appointment to minimise the woman’s time, effort and costs. Ideally the appointment would include key members of the women’s support network (e.g. partner, family).
-
The antenatal appointment should focus on practical tips for managing varying levels of fatigue and pain, such as how to find comfortable positions for breastfeeding. Content should also be flexible to meet the women’s needs, adaptable to changing circumstances and consistent across different healthcare providers.
-
30-minute postnatal appointments are too short.
-
For the 3-month follow-up support, women should have the option of telephone or face-to-face contacts, and 24-hour telephone support should be available.
-
Peer support could be offered antenatally, and group antenatal peer support could help normalise breastfeeding for women with LTCs. Women could be offered the choice of one-to-one or group peer support.
-
Third-sector organisations could help with provision of breastfeeding and emotional support.
-
To be sustainable, peer supporters should be paid.
-
Training is needed to increase knowledge of breastfeeding and multimorbidities in the multidisciplinary team including GPs. Supporting women with multimorbidities to breastfeed should be included in routine breastfeeding training updates.
-
Services should be co-ordinated with the infant-feeding advisor as the key point of contact for the multidisciplinary team.
-
-
Implementing your new breastfeeding support service.
These recommendations to support the planning of the implementation (part 1) and roll-out (part 2) of the proposed intervention components were developed in collaboration with our stakeholders and PPI members through a range of meetings and engagement activities with the research team. Sessions were informed by the barriers to/enablers of implementing breastfeeding support interventions derived from synthesising process evaluations of effective interventions (see Chapter 4) that were discussed, validated and/or refined and adapted based on the views and experiences of participating stakeholders.
The combined recommendations resulting from this process are:
-
3.1. Part 1: considering the barriers to and enablers of implementing your new service.
-
Key enablers to address:
-
Training – counselling skills and technical competence, practical expectations of undertaking the breastfeeding supporter role (e.g. uncertainties about safety, transport and reimbursement while delivering support, managing difficult scenarios, interplay of cultural beliefs and breastfeeding practice).
-
Effective management and supervision.
-
Ongoing emotional support, including mentoring and motivation for peer, lay or volunteer supporters.
-
Offering women the opportunity to ask questions and being allowed to spend enough time to address any issues.
-
Provide support flexibly as needed, rather than having to fit support around fixed working hours or at times which might not be convenient for women.
-
-
Key barriers to address:
-
-
Intervention
-
Schedule and length of appointments lack flexibility and would need to be tailored to individual women’s needs and circumstances.
-
The intervention does not include the women’s partner and/or other family members who could be important sources of breastfeeding support.
-
Lack of continuity across the intervention.
-
Lack of intensity in the first 2 weeks postnatally.
-
Costs to the service.
-
Multiple appointments may not be convenient for women.
-
Intervention may not be perceived to be better than existing or alternative approaches to breastfeeding support.
-
-
External barriers
-
Negative societal attitudes to breastfeeding/bottle-feeding culture.
-
Pressure from families/social networks.
-
Impact of formula marketing.
-
Challenges to developing partnerships between health services and other sectors (local authorities, third-sector organisations).
-
Socioeconomic and structural factors, for example lack of transport, lack of childcare, digital poverty, cost-of-living crisis.
-
Lack of external financing.
-
-
Health system barriers
-
Workforce challenges – staff shortages, high staff turnover, lack of staff time, lack of right skill mix.
-
Overdependency on individuals or small groups of staff.
-
Poor communication within the multidisciplinary team.
-
Fragmented services.
-
Lack of valuing peer support services and barriers to integrating professional and peer support.
-
Reliance on unpaid volunteers to provide peer support.
-
Lack of tailoring of services to diverse populations, for example lack of language support, lack of accessible venues, staff attitudes (stereotyping).
-
Lack of feedback to staff, for example data sharing, sharing good practices.
-
Lack of resources – appropriate venues to deliver the intervention considering space for women to breastfeed and accessible locations for groups to meet.
-
Lack of compatibility of the innovation with existing policies and guidelines.
-
Early postnatal discharge following birth.
-
Overlap of the innovation with existing breastfeeding support services.
-
-
Individuals
-
For those delivering the intervention – lack of knowledge, practical and interpersonal skills, lack of experience and training, lack of motivation, lack of confidence.
-
For strategic and operational managers – lack of buy-in, lack of understanding of the value of breastfeeding, lack of commitment, lack of champions and skilled implementation leads and teams.
-
For intervention recipients – inaccessible services, lack of awareness of services, lack of time.
-
-
Implementation process
-
Lack of engagement of staff/resistance to change.
-
Lack of management oversight to ensure innovation implemented as intended.
-
Lack of feedback to staff concerning the quality of the intervention.
-
-
3.2. Part 2: Planning the implementation strategy to successfully roll out your new service.
-
Overview of most relevant strategies linked to the key barriers they can address.
-
| Implementation strategies | Barriers addressed |
|---|---|
| Deliver realistic, evidence-based information in multiple formats on how to deliver the breastfeeding support intervention and why it is important | Lack of staff training, knowledge and skills Lack of consistency of information Lack of continuity of care Challenges to accessing the intervention for women and families Lack of buy-in from senior managers |
| Assign a key practitioner to raise awareness about the intervention to ensure a consistent message | Challenges to working with sectors outside the health system Poor communication across the multidisciplinary team Lack of joined-up vision and working |
| New or existing funding for breastfeeding support should be a general health investment for local councils, and the government, and not just the NHS | Lack of funding in the health system Cost of the service to the NHS Lack of relationship between the health system and the community Lack of sustainability Cost of the intervention to women Reliance on non-paid peer supporters |
| Create an infant-feeding team in every NHS organisation to lead the intervention, working collaboratively with multidisciplinary practitioners and lay supporters | Lack of availability of good-quality training Time and capacity issues Professional boundaries – especially working with peer supporters Lack of confidence of those delivering the intervention Lack of integration across the continuum (antenatal/postnatal) and across the multidisciplinary team |
| Revise roles as needed to support the intervention, for example integrate peer supporters with NHS infant-feeding teams, and consider upskilling maternity staff to specialist lactation training levels | Barriers to integrating peer support with health services including lack of valuing peer support Lack of right skill mix Lack of knowledge and skills of staff delivering the intervention Infant-feeding specialists overloaded |
-
Evaluating your new breastfeeding support service.
This section sets out recommendations for the evaluation of breastfeeding support interventions in UK settings, including a range of suggested outcomes and practical considerations, based on the views and experiences of those attending our PPI and stakeholder meetings and workshops.
-
Practical considerations for evaluation strategies:
-
Collect data early to capture those who cease to engage with the intervention.
-
Gain feedback from those who declined the intervention.
-
Use digital options for data collection.
-
Collect data on participant characteristics.
-
Consider using quality improvement approaches or comparative studies.
-
-
Recommended outcomes:
-
Parental feeding expectations and goals met.
-
Satisfaction with support and information received.
-
Confidence after the intervention (self-efficacy).
-
Views and experiences of intervention deliverers and recipients.
-
Intervention fidelity.
-
Breastfeeding rates – exclusive and any with clear definitions and consider further subdivisions at:
-
-
First feed within 1 hour after birth
-
Discharge from hospital
-
6–8 weeks
-
6 months
-
(consider adding to above 10–12 days, 3–4 months, 12 months)
-
Number of infants admitted to hospital.
-
Reasons for stopping breastfeeding.
-
Future plans
Further co-development work, write-up and refinement is ongoing, with a view to produce a user-friendly toolkit that will support NHS and third-sector organisations to implement evidence-based breastfeeding support for women in the UK.
Following this, the research team will seek further funding to undertake a robust evaluation of the implementation and effectiveness of our proposed, adapted composite intervention in UK settings.
List of abbreviations
- aOR
- adjusted odds ratio
- BFHI
- baby-friendly hospital initiative
- BMI
- body mass index
- CFIR
- Consolidated Framework for Implementation Research
- CI
- confidence interval
- DCE
- discrete choice experiment
- GBP
- Great British pounds
- GDM
- gestational diabetes mellitus
- GP
- general practitioner
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluation
- HIC
- high-income country
- HIV
- human immunodeficiency virus
- ICER
- incremental cost-effectiveness ratio
- LMIC
- low- and middle‐income country
- LTC
- long-term condition
- MLTC
- multiple long-term conditions
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OECD
- Organisation for Economic Cooperation and Development
- OR
- odds ratio
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- WHO
- World Health Organization
- WTP
- willingness-to-pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/DGTP5702).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
