Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 09/2000/37. The contractual start date was in April 2011. The final report began editorial review in December 2012 and was accepted for publication in June 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Simpson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Each year, influenza causes substantial morbidity and mortality, particularly in people aged ≥ 65 years and in those with underlying serious comorbidities. In the USA for example, it has been estimated that influenza is responsible for 186,000 excess hospitalisations and 44,000 excess deaths at a cost of $87B per year. 1,2 In England, influenza-related mortality is estimated to be as high as 8600 deaths per year. 3 National vaccination strategies represent a potentially important approach to reducing both influenza-related illness and death, hence the considerable investment in this preventative strategy in many parts of the world. There is good evidence of the benefits of the vaccine in young healthy adults and children. 4 However, in populations at risk of developing influenza-related complications (e.g. adults aged ≥ 65 years and people with medical conditions such as diabetes, heart or respiratory disease or immune deficiency) there is a paucity of reliable estimates of efficacy from randomised controlled trials, which offer the best opportunity to produce unbiased estimates of vaccine efficacy. This is of particular concern as it is thought that influenza vaccine may be less effective in the oldest age groups due to immune senescence. 5 However, given that influenza vaccination programmes now exist in most developed countries, the randomised controlled trial as a form of study design (for evaluating influenza vaccine effectiveness) is now impractical and, moreover, is viewed by many in the medical community as unethical. 6
Observational studies are an alternative means of investigating the effectiveness of vaccine programmes. However, an individual's decision to attend his or her local general practice surgery for vaccination may be a marker of healthier behaviour generally, as well as identifying more highly educated individuals who are more aware of, and more likely to act on, recommendations for their own health. These individuals may be less likely to die from any cause or be admitted to hospital, thus inducing a spurious relationship between vaccination status and outcome (i.e. positive confounding). Similarly, a ‘healthy vaccine effect’ may occur, whereby patients who are very frail and unable to attend the general practice surgery may be less likely to be vaccinated, but much more likely to die or be admitted to hospital. 7 Standard methods of adjustment for confounders are likely to be inadequate to control for confounding due to the healthy vaccine effect. This can result in excessive estimates of vaccine effectiveness (VE) in observational studies using non-influenza-specific outcomes due to residual confounding, in particular for all-cause mortality where VE greater than the total 5% estimated risk of death during winter has been found. 8,9 A number of methods can be used to try and address this problem including quasi-experimental study designs and advanced statistical methods. In addition, an analysis framework has been proposed to identify residual confounding when undertaking VE studies using observational methods. 5
Quasi-experimental studies are an alternative that can be used to investigate the effectiveness of vaccine programmes, but very few have estimated VE in reducing medically confirmed influenza using reliable methods such as viral culture or reverse transcription-polymerase chain reaction (RT-PCR) testing. 10 Given the ongoing controversy regarding VE, particularly in relation to influenza vaccination in at-risk groups,10 there is further need of information before current policies regarding seasonal vaccine strategies can be altered. We therefore undertook an observational cohort study to determine uptake and VE of the trivalent inactivated influenza vaccine in a quarter of a million people from across Scotland, registered with a sentinel surveillance network of primary care practices. This builds on related work to estimate the effectiveness of the 2009 monovalent pandemic (H1N1) influenza vaccine. 11,12
Objectives
The aim of the Seasonal Influenza Vaccine Effectiveness (SIVE) study was to examine the effectiveness of the seasonal influenza vaccination in individuals registered with a national sample of general practices in Scotland. Our three specific objectives were to evaluate:
-
uptake of the influenza vaccine by the relevant at-risk populations, i.e. patients with relevant comorbidities and those aged ≥ 65 years, as well as by the general population
-
the reduction in the expected incidence of influenza-related morbidity and mortality in these at-risk groups, as this is the major rationale behind current immunisation policies
-
the effectiveness of the influenza vaccine in the population as a whole.
Chapter 2 Methods
Study design
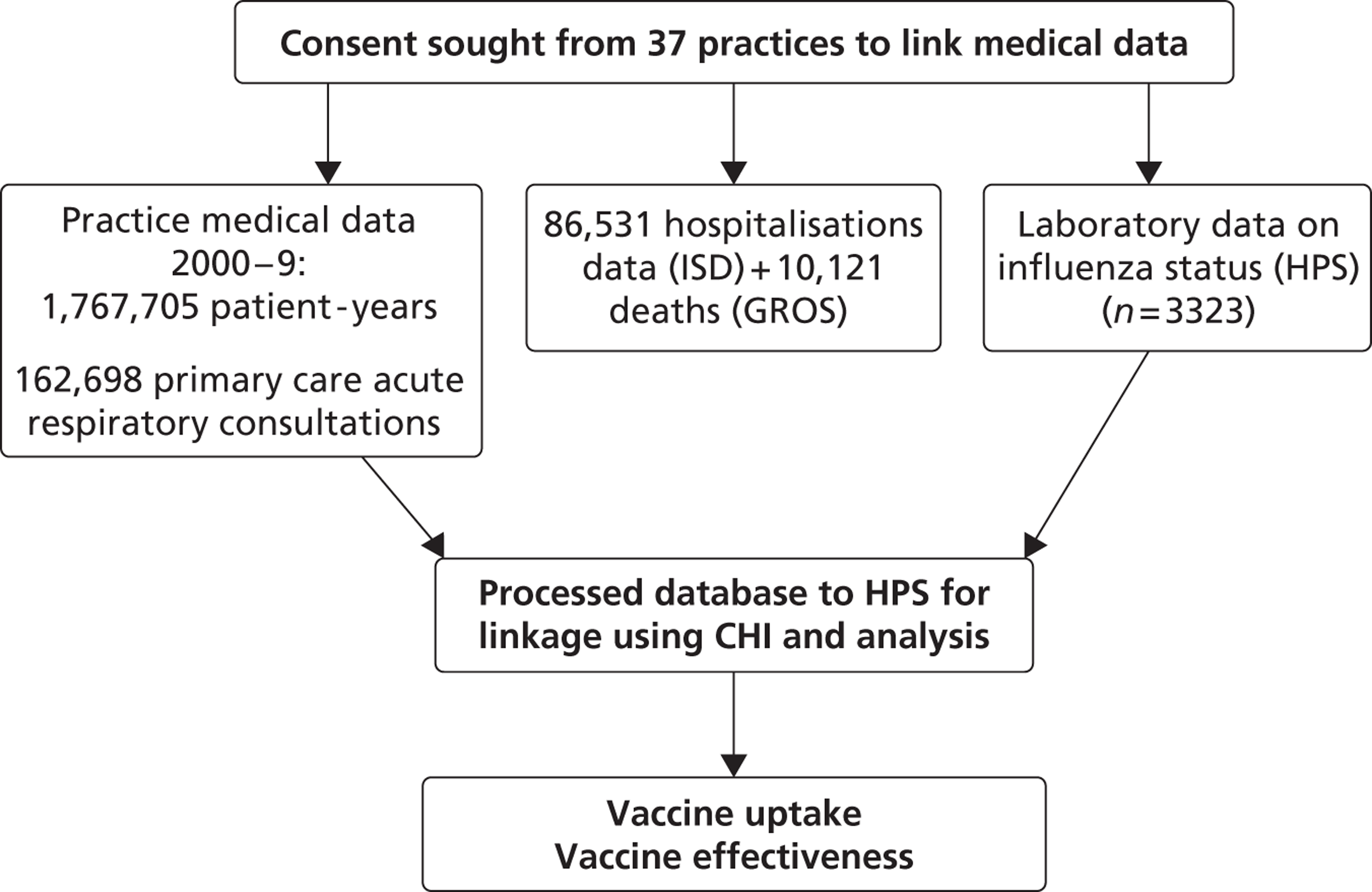
Almost all individuals resident in Scotland are registered with a general practice, which provides a comprehensive array of health-care services, including the issuing of prescriptions for medications. Specialist hospital care services are typically accessed through referral from primary care or, in emergency situations, through patients attending accident and emergency. General practitioners (GPs) also provide and co-ordinate much of the care of patients discharged back into the community after a hospital admission. The Practice Team Information network practices included in this study are a representative sample constituting 5% of Scottish practices. These practices receive an annual financial incentive to electronically record all face-to-face contacts with patients. 13 Data from practices within Scotland are of high quality (the completeness of capture of contacts and accuracy of clinical event coding in primary care has been found to be > 90%14) and their value for epidemiological research has been repeatedly demonstrated. 15 Using each patient's unique Community Health Index (CHI) number, general practice patient-level data were extracted and then linked to the Scottish Morbidity Record (SMR) catalogue, which has information on all inpatient hospital admissions within Scotland as well as information on death certification linked from the General Register Office for Scotland (GROS)16 (Figure 1). Hospital data from 1981 onwards have been shown to be reliable, with completeness and accuracy rates > 90%. 17 Additionally, we used the Health Protection Scotland (HPS) virology data set, which records all laboratory-confirmed cases of influenza in Scotland.
We established the following key characteristics of each identified patient in the cohort: sex; age (0–4, 5–14, 15–44, 45–64, 65–74 or ≥ 75 years); socioeconomic status [Scottish Index of Multiple Deprivation scores18 expressed as quintiles: 1 (most affluent) to 5 (most deprived)]; smoking status (current smoker, ex-smoker, non-smoker or not recorded); urban/rural location (one large urban and six remote rural locations were included in the study); whether or not the patient belonged to any clinical at-risk groups (i.e. those suffering from chronic respiratory, heart, kidney, liver or neurological disease, immunosuppression or diabetes); pregnancy status; Charlson comorbidity index;19 previous pneumococcal and influenza vaccination; and number of previous primary care consultations, prescribed drugs and hospital admissions (e.g. in the year prior to 1 September 2000). No direct measure of functional status existed within the database; to allow this to be taken into account, home consultations by a GP only (vs. practice attendance by the patient) and nursing home residence (including social care support) were also included in our propensity score.
FIGURE 1.
Flow diagram for the SIVE study. ISD, Information Services Division.
Procedures
Clinical conditions (consultations for influenza and other acute respiratory infections), risk group information and prescribing data (e.g. seasonal influenza vaccination and pneumococcal polysaccharide status) were extracted from primary care records. Influenza primarily infects the respiratory tract, which can often result in pneumonia and influenza being grouped together in data recording. 20 We therefore extracted from the SMR and the GROS details on the primary diagnosis of hospitalisation and cause of death from influenza grouped with pneumonia. Furthermore, we also analysed chronic obstructive pulmonary disease (COPD) grouped with influenza and pneumonia, as influenza can account for a significant proportion of COPD exacerbations resulting in hospitalisation and death. 21
A number of secondary analyses were undertaken using other outcomes. The effect of vaccination status on hospital admissions and deaths relating to cardiovascular and cerebrovascular events as a composite outcome was analysed. In addition, exploratory analyses were undertaken to assess the effect of vaccination status on outcomes for which it would not be expected to have an effect, for example appendicitis or trauma. This approach of using an alternative outcome as a negative control has been shown to be a useful method for detecting residual bias. 22
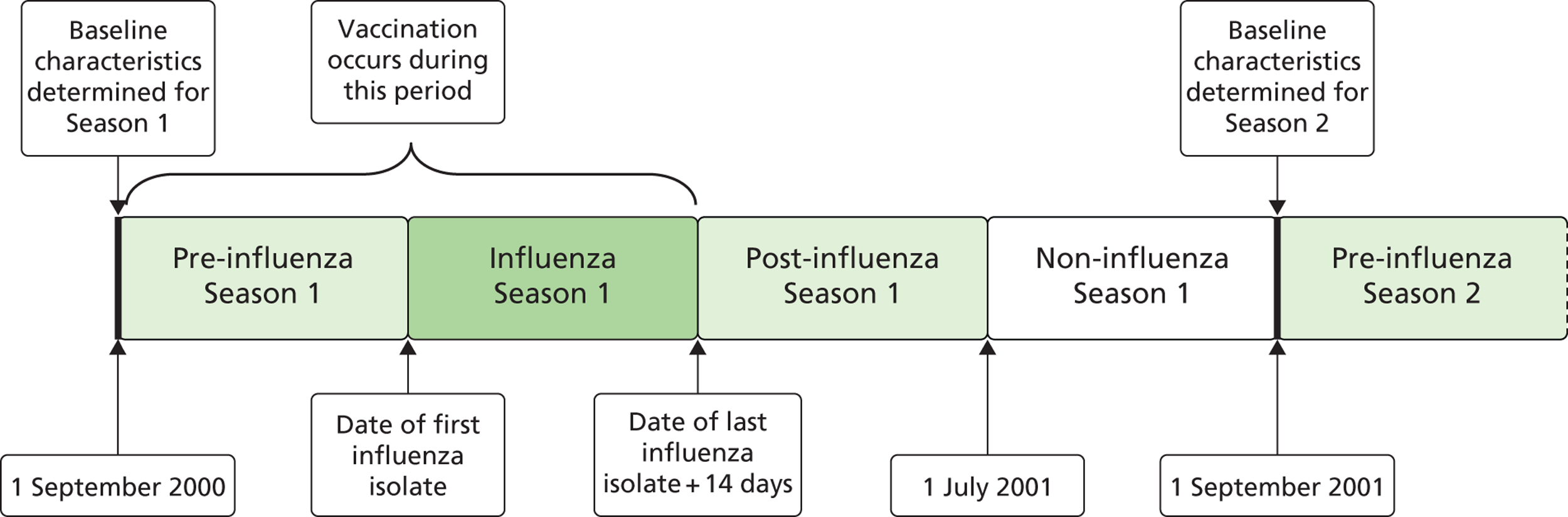
Data from 1 September 2000 to 31 August 2009 were used. This allowed for the analysis of nine influenza seasons (2000/1 to 2008/9), yielding a total of 1,767,919 person-seasons for analysis. The influenza season was defined as the period from the date of the first influenza isolate reported by HPS for each year, in or post week 40 of that year, until the date of the last influenza isolate, pre or in week 20 of the subsequent year (i.e. the period of peak influenza) (Figure 2).
FIGURE 2.
Relationship of first influenza season (2000/1) to pre-, post- and non-influenza season periods. Influenza season was defined as the period from the date of the first isolate ≥ week 40 until that of the last isolate ≤ week 20.
Vaccination was used to define exposure status if it was given at a time point between the start of the pre-influenza season (e.g. 1 September) and the end of the influenza season. An individual was defined as vaccinated 14 days after the seasonal influenza vaccine was administered. The time period from the first day of the influenza season to day 14 post-vaccination was defined as ‘unexposed’ and the period from day 14 post vaccination until the end of the influenza season was defined as ‘exposed’. A detailed description of our methods has previously been published. 23
The earliest influenza season began on 26 September and the latest began on 25 November (Table 1). All seasons finished in May.
| Year | Start date | End date |
|---|---|---|
| 2000/1 | 5 October 2000 | 14 May 2001 |
| 2001/2 | 18 October 2001 | 17 May 2002 |
| 2002/3 | 25 November 2002 | 15 May 2003 |
| 2003/4 | 26 September 2003 | 7 May 2004 |
| 2004/5 | 22 October 2004 | 19 May 2005 |
| 2005/6 | 6 October 2005 | 16 May 2006 |
| 2006/7 | 19 October 2006 | 9 May 2007 |
| 2007/8 | 2 October 2007 | 13 May 2008 |
| 2008/9 | 13 November 2008 | 5 May 2009 |
General practitioners in this study were also involved in the HPS sentinel swabbing scheme, whereby practices are encouraged to obtain nasal/throat swabs from patients of all ages who have symptoms suggestive of influenza. Each general practice is requested to submit five swab samples per week to the West of Scotland Specialist Virology Centre in Glasgow, a World Health Organization-accredited National Influenza Centre which participates in a quality assurance programme to maintain this status. Here, RT-PCR testing for a range of respiratory pathogens is carried out. Swabs should be obtained from any patient presenting for consultation with influenza symptoms, across all age groups and regardless of whether the patient has or has not been vaccinated. We also included results for the patients in our study from swabbing carried out for routine diagnostic purposes in primary and secondary care outside the sentinel scheme.
Vaccine effectiveness is expressed as a percentage, and represents a reduction in risk provided by the vaccine for a given outcome. To calculate VE, each patient's swab data were linked using his or her CHI number, allowing patient characteristics such as vaccination status to be established from general practice and hospital admission data.
Framework for detecting residual confounding
We undertook additional analyses to identify the presence of residual confounding. This has been recommended as part of an analytical framework when reporting VE using observational study designs. 9 We assessed the variation in VE using the following criteria:
-
Seasonality. Stratification by season is more important when VE is measured using non-specific outcomes. Each year of observation was partitioned into four periods: the non-influenza period, pre-influenza season, influenza season (when influenza virus is circulating) and post-influenza season (see Figure 2). Maximal VE should be seen during the influenza season. The vaccine should have no effect on outcome in the pre-influenza and non-influenza seasons. The non-influenza season used vaccination status from the previous influenza season. This was to minimise the bias that might occur when vaccination status is applied retrospectively. This retrospective application of vaccine status included patients who died during the preceding non-influenza season as unvaccinated, despite the fact that they would not have survived long enough to be eligible for vaccination.
-
Vaccine match. VE should be lower in years during which the influenza vaccine was a poor match for the circulating virus.
-
Severity of influenza season. VE should be greater in years during which the circulating virus caused a large excess mortality during the influenza season.
-
Age. It is thought that influenza vaccine is less effective in the oldest age groups owing to immune senescence. 10 If this assumption is correct, VE should be lowest in the oldest subgroup. A stratified analysis of age groups was undertaken to assess for this effect.
-
Specificity of outcome measure. VE should be greatest for the most specific outcome (laboratory-confirmed influenza infection) and lowest for the least specific outcome (all-cause mortality). In addition to the primary analysis, in the three non-laboratory databases (primary care, acute hospital discharge and death register) we undertook analyses using the more influenza-specific outcomes (influenza-coded deaths, hospital admissions and primary care attendances) and less specific outcomes (any emergency hospital admissions, all-cause deaths and any primary care attendances).
Statistical methods
We constructed a propensity score model and calculated adjusted odds ratios (ORs) to assess differences in vaccine uptake by patients with each of the characteristics outlined previously. The model was non-parsimonious in order to include a wide range of factors that influence propensity to be vaccinated. 24 Using this model, a probability of receipt of the influenza vaccination was assigned to each individual in the cohort. Unadjusted illness and mortality rate ratios (RRs) were calculated as the ratios of the rate of emergency admission to hospital or death in vaccinated patients to the rate of emergency admission to hospital or death among those who did not receive the vaccine. The unadjusted estimate of VE was (1 − RR) × 100. Adjusted illness and mortality hazard ratios (HRs) were estimated from a Cox proportional hazards model adjusted for all patient characteristics, and the propensity score (as deciles) and VE were calculated as 1 − HR where HR was that of the measured outcome in vaccinated compared with unvaccinated individuals.
For our analysis we pooled data from all nine seasons. This a priori approach gave a more powerful analysis and increased precision, particularly for the less common outcomes (with some patients represented for multiple seasons). In this pooled analysis, we accounted for the within-person correlation resulting from repeated measures on the same individual in different influenza seasons by making an adjustment for clustering using robust standard errors in the model. The effect of time was implicitly considered in the model by using the Cox proportional hazards approach and the inclusion of year as a fixed effect allowed for variation between seasons. In our propensity score matched analysis, only patients with similarly matched propensity to be vaccinated were included. To test the validity of the pooled analysis, heterogeneity between years was determined by testing for interaction between vaccine status and year for the outcomes. Where significant heterogeneity occurred, the pooled analysis was restricted.
For estimates of VE derived from linked virological swab data, we carried out a nested case–control study design. A generalised additive logistic regression model25 was fitted adjusting for the effects of week during the study period, age, sex, deprivation, previous number of primary care consultations and being in a clinical at-risk group. Some of these patients did not receive the influenza vaccine; some received the vaccine, but after they were tested; and others received the vaccine before they were tested. We therefore measured VE by comparing swabs taken after vaccination from individuals who were vaccinated with swabs taken from all those who were not vaccinated at the time the swab was taken (people who were unvaccinated at the time of the swab and who were then subsequently vaccinated counted as unvaccinated in our analysis, as did people who were never vaccinated). We assessed only the first dose when two doses were given. We also stratified our analysis based on patients aged ≥ 65 years and those aged < 65 years who were classified as at risk. Using the ‘epitools’ library in R version 2.14.1 (the R Foundation for Statistical Computing, Vienna, Austria), we calculated 95% confidence intervals (CIs) for the OR and RR by median-unbiased estimation using the ‘odds ratio’ and ‘rateratio’ functions. 26 Tests of the differences were performed using the ‘rate2by2.test’ function.
Further statistical methods to adjust for confounding
Instrumental variable analysis
An instrumental variable is a factor related to exposure status (i.e. vaccination status), which does not have an independent effect on outcome other than by ways mediated through the exposure. 27 Furthermore, an instrumental variable should not be related to any variables that confound the relationship between exposure and outcome. If an association with confounders is demonstrated, it is assumed that the instrumental variable is associated with unmeasured confounders and is therefore not valid. If an instrumental variable fulfils these criteria, it can be used in analyses to produce unbiased estimates of VE by accounting for unmeasured confounding. 27 We assessed the suitability of five potential instrumental variables to estimate VE: antacid prescription, thyroxine prescription, depression, gout and participation in any health screening programme.
Modelling an unmeasured confounder
Hospital admission and death rates are likely to be highest in the frailest members of the study population. As these patients are less likely to seek vaccination, it has been suggested that inadequately measured frailty may explain some of the VE measured in observational studies. 7,28 As we may not have fully accounted for frailty, which has been defined in recent studies,29,30 we used estimates from published data to model this potentially inadequately measured confounder in a sensitivity analysis. 31 We assumed that prevalence of frailty varied from 5–20% in those aged ≥ 65 years, that frail individuals were two to four times more likely to be hospitalised or die32 and that frail individuals had a 50% lower probability of being vaccinated. 33
Chapter 3 Results
Vaccine uptake
In total, during the 1,767,705 person-seasons of observation over nine influenza seasons, 274,071 seasonal influenza vaccinations were administered to the whole population, of which most (93.6%; n = 256,474) were distributed to at-risk patients targeted for vaccination. There was 69.3% uptake of the vaccine among those aged ≥ 65 years (178,754 vaccinations during 258,100 person-seasons). For at-risk patients aged < 65 years there was a 26.2% uptake (77,264 vaccinations during 295,116 person-seasons).
High vaccine uptake was found among the oldest age group (≥ 75 years), care home residents, those previously vaccinated (Table 2), those with chronic diseases (except for chronic respiratory disease) and those being issued many prescriptions (Table 3). After adjustment, the OR for uptake was lower for individuals who smoked and those with no recorded smoking status (see Table 2) and for those with five or more comorbidities when compared with those with no comorbidities (see Table 3). There were similar findings for at-risk patients and those aged ≥ 65 years, although for the latter, an OR (with 95% CI) < 1 was also found among people whose contacts with primary care were by home consultation only (unadjusted OR 0.53; 95% CI 0.50 to 0.56) and, after adjustment for covariates, among those admitted multiple times to hospital when compared with those not admitted (> 10 hospitalisations: adjusted OR 0.71; 95% CI 0.53 to 0.95).
| Characteristic | Vaccinated (% total) (n = 274,071 seasons) | Unvaccinated (% total) (n = 1,493,634 seasons) | Uptake (%) | Uptake adjusted OR (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Female | 55.69 | 49.22 | 17.19 | 1.00 |
| Male | 44.31 | 50.78 | 13.80 | 1.03 (1.01 to 1.06) |
| Age group (years) | ||||
| 0–4 | 0.23 | 5.36 | 0.80 | 1.00 |
| 5–14 | 1.59 | 13.49 | 2.12 | 1.39 (1.23 to 1.56) |
| 15–44 | 9.21 | 49.84 | 3.28 | 1.21 (1.07 to 1.36) |
| 45–64 | 23.59 | 26.02 | 14.26 | 2.59 (2.30 to 2.91) |
| 65–74 | 35.74 | 3.04 | 68.36 | 16.10 (14.28 to 18.15) |
| 75+ | 29.63 | 2.25 | 70.72 | 10.54 (9.33 to 11.89) |
| Deprivation quintile | ||||
| 1a | 24.91 | 23.71 | 16.16 | 1.00 |
| 2 | 24.51 | 22.38 | 16.73 | 0.97 (0.94 to 1.01) |
| 3 | 20.17 | 19.23 | 16.14 | 1.00 (0.97 to 1.04) |
| 4 | 16.97 | 18.72 | 14.26 | 1.00 (0.96 to 1.03) |
| 5 | 13.43 | 15.95 | 13.38 | 1.08 (1.04 to 1.12) |
| Urban/rural score | ||||
| 1 | 13.83 | 18.39 | 13.80 | 1.00 |
| 2 | 57.31 | 56.72 | 18.54 | 1.07 (1.04 to 1.11) |
| 3 | 10.49 | 9.08 | 21.20 | 1.27 (1.22 to 1.33) |
| 4 | 2.11 | 1.73 | 22.44 | 1.53 (1.41 to 1.66) |
| 5 | 10.72 | 9.82 | 20.03 | 1.29 (1.24 to 1.35) |
| 6b | 5.52 | 4.25 | 23.85 | 1.19 (1.12 to 1.26) |
| Care home resident | 0.66 | 0.05 | 70.05 | 2.10 (1.69 to 2.60) |
| Primary care home consultations only | 2.48 | 0.77 | 37.28 | 1.01 (0.87 to 1.17) |
| Pneumococcal vaccine | 43.82 | 1.41 | 85.08 | 1.61 (1.56 to 1.67) |
| Previous influenza vaccine | 76.89 | 2.04 | 87.38 | 24.14 (23.48 to 24.81) |
| Smoking status | ||||
| Non-smoker | 44.37 | 37.93 | 17.67 | 1.00 |
| Ex-smoker | 30.44 | 9.31 | 37.50 | 1.06 (1.03 to 1.09) |
| Current smoker | 22.07 | 23.57 | 14.66 | 0.87 (0.85 to 0.90) |
| Not recorded | 3.12 | 29.20 | 1.92 | 0.44 (0.46 to 0.48) |
| Characteristic | Vaccinated (% total) (n = 274,071 seasons) | Unvaccinated (% total) (n = 1,493,634 seasons) | Uptake (%) | Uptake adjusted OR (95% CI) |
|---|---|---|---|---|
| Chronic kidney disease | 6.35 | 0.38 | 75.59 | 0.96 (0.90 to 1.04) |
| Chronic respiratory disease | 19.71 | 9.00 | 28.68 | 1.74 (1.68 to 1.80) |
| Chronic heart disease | 27.71 | 2.43 | 67.68 | 1.32 (1.27 to 1.37) |
| Dementia | 2.67 | 0.24 | 67.36 | 0.93 (0.84 to 1.03) |
| Diabetes | 15.33 | 1.30 | 68.43 | 1.98 (1.88 to 2.08) |
| Impaired immune function | 1.62 | 0.21 | 58.49 | 2.98 (2.59 to 3.43) |
| Liver disease | 1.36 | 0.36 | 41.22 | 1.25 (1.12 to 1.40) |
| Neurological disease | 12.66 | 1.62 | 58.90 | 1.08 (1.03 to 1.14) |
| Charlson comorbidity | ||||
| 0 | 28.49 | 80.15 | 6.12 | 1.00 |
| 1–2 | 51.21 | 18.26 | 33.97 | 1.65 (1.60 to 1.70) |
| > 5 | 5.03 | 0.29 | 75.87 | 0.79 (0.71 to 0.87) |
| Primary care consultations | ||||
| 0 | 9.67 | 36.98 | 4.58 | 1.00 |
| 1 | 8.01 | 14.60 | 9.14 | 1.17 (1.03 to 1.34) |
| 2 | 7.67 | 11.06 | 11.29 | 1.26 (1.11 to 1.44) |
| 3 | 7.32 | 8.11 | 14.21 | 1.35 (1.18 to 1.53) |
| 4 | 6.91 | 6.23 | 16.92 | 1.35 (1.18 to 1.53) |
| 5 | 6.53 | 4.76 | 20.10 | 1.40 (1.23 to 1.60) |
| > 5 | 53.90 | 18.26 | 35.13 | 1.54 (1.36 to 1.75) |
| Prescriptions | ||||
| 0 | 10.03 | 70.85 | 2.53 | 1.00 |
| 1 | 7.41 | 11.03 | 10.97 | 1.91 (1.85 to 1.97) |
| 2 | 9.66 | 6.59 | 21.20 | 2.67 (2.58 to 2.77) |
| 3 | 9.70 | 3.77 | 32.08 | 3.12 (3.00 to 3.24) |
| 4 | 9.63 | 2.33 | 43.11 | 3.35 (3.21 to 3.49) |
| 5 | 9.04 | 1.54 | 51.83 | 3.75 (3.58 to 3.93) |
| 11–15 | 10.61 | 0.62 | 75.71 | 3.79 (3.55 to 4.03) |
| > 15 | 3.68 | 0.19 | 77.83 | 3.46 (3.12 to 3.84) |
| Hospital consultations | ||||
| 0 | 76.48 | 90.92 | 13.37 | 1.00 |
| 1 | 12.84 | 6.52 | 26.56 | 1.11 (1.08 to 1.15) |
| 2 | 5.16 | 1.44 | 39.67 | 1.12 (1.06 to 1.18) |
| 3 | 2.23 | 0.51 | 44.77 | 0.98 (0.90 to 1.07) |
| 4 | 1.18 | 0.23 | 48.90 | 1.01 (0.88 to 1.15) |
| 5 | 0.71 | 0.12 | 51.68 | 1.05 (0.88 to 1.26) |
| 6–10 | 1.04 | 0.20 | 48.96 | 1.04 (0.90 to 1.20) |
| > 10 | 0.34 | 0.07 | 48.05 | 1.17 (0.91 to 1.51) |
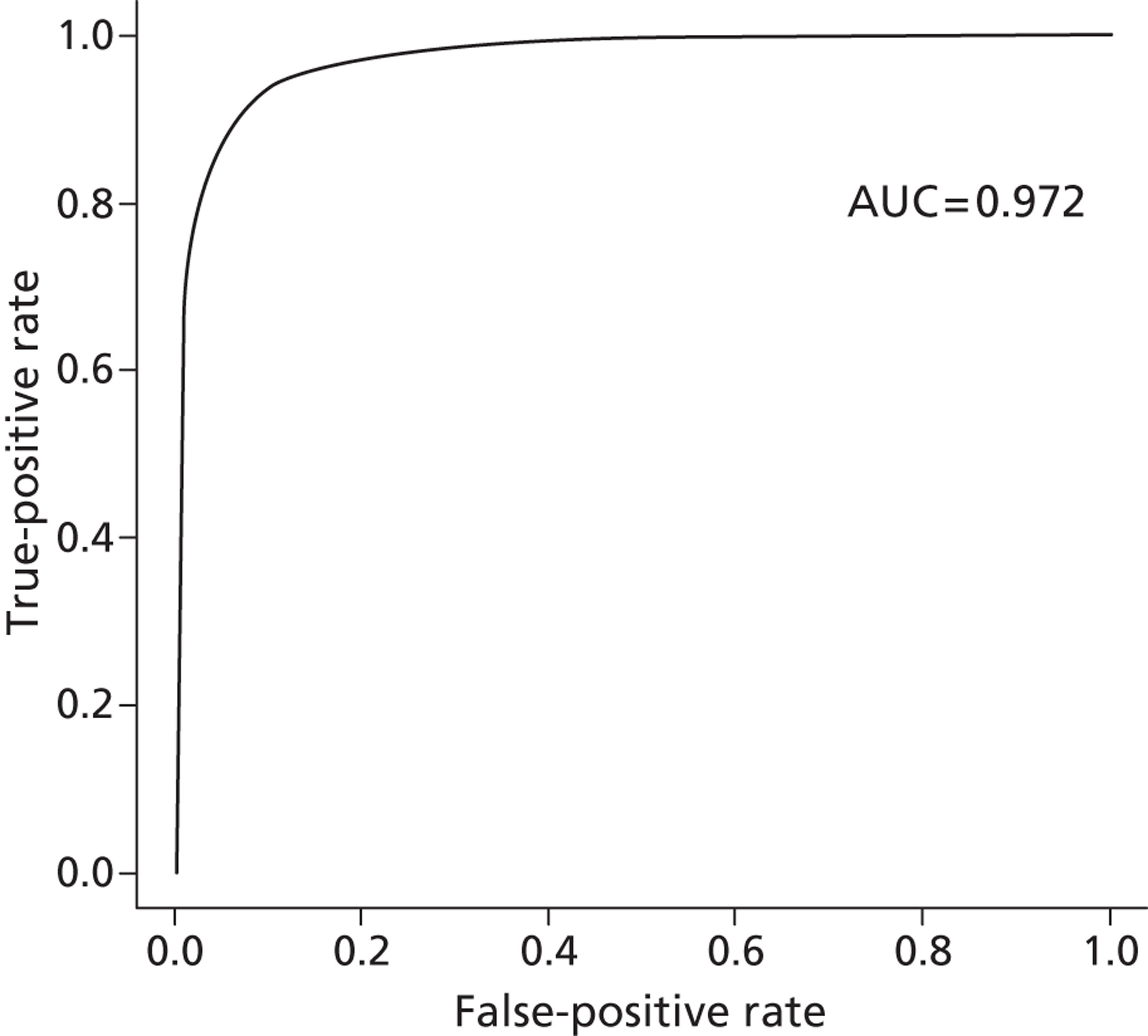
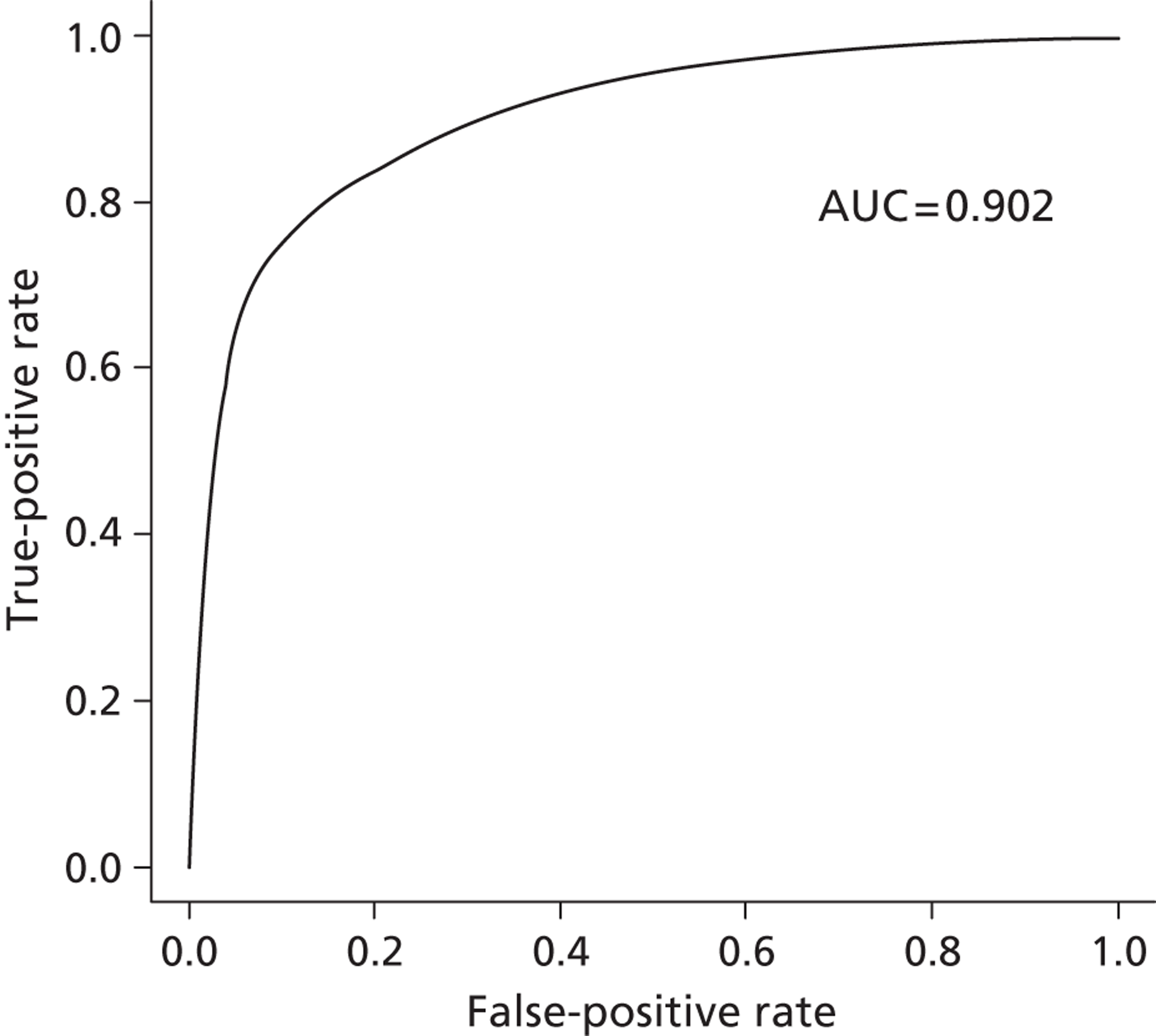
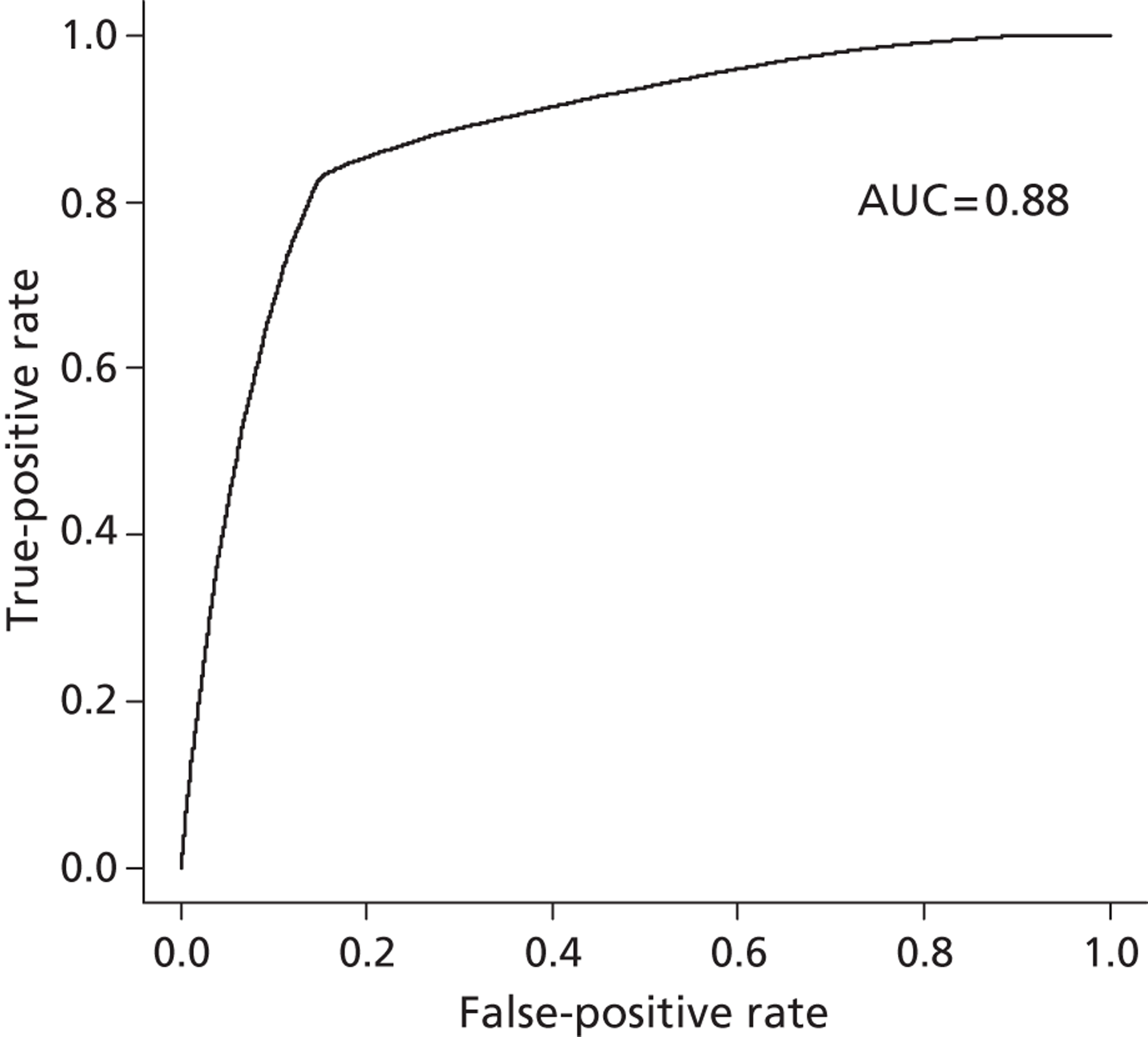
For our propensity score, our prediction model showed a high discriminatory power to identify patients who did and did not receive influenza vaccine (see Appendix 1) [all patients: receiver operating characteristic – area under the curve (ROCAUC) = 0.97; patients aged ≥ 65 years: ROCAUC = 0.88; at-risk patients aged < 65 years: ROCAUC = 0.90].
Laboratory-confirmed influenza
A total of 3323 swabs were taken from 3016 patients over the nine seasons and then tested with RT-PCR for evidence of influenza infection. Although all subgroups were represented, patients from whom swabs were taken were more likely to be younger (aged < 75 years), female and relatively affluent (Table 4). During our study, male patients and the socioeconomically affluent were more likely to test positive for influenza. Of all swabs taken, 13.9% tested positive for RT-PCR-confirmed influenza. This included one-quarter of swabs from school-aged children.
Vaccine effectiveness for the trivalent inactivated influenza vaccine was 57.1% (95% CI 31.3% to 73.3%) (Table 5). VE was 59.6% (95% CI 22.0% to 79.1%) for at-risk patients aged < 65 years and 18.8% (95% CI – 103.7% to 67.6%) for patients aged ≥ 65 years. Peak VE was found in the 2007/8 influenza season.
| Description | Total samples (rate per 1000 person-seasons) | No. vaccinated at test (% of total) | No. of positive swabs (% of total) | Swab-positive adjusted ORa | Adjusted OR 95% CI |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 1995 (2.25) | 310 (15.54) | 248 (12.43) | 1.00 | – |
| Male | 1328 (1.51) | 188 (14.16) | 214 (16.11) | 1.35 | 1.07 to 1.69 |
| Age group (years) | |||||
| 0–4 | 390 (4.83) | 5 (1.28) | 60 (15.38) | 1.00 | – |
| 5–14 | 433 (2.10) | 15 (3.46) | 104 (24.02) | 1.56 | 1.05 to 2.32 |
| 15–44 | 1405 (1.82) | 69 (4.91) | 196 (13.95) | 0.89 | 0.63 to 1.27 |
| 45–64 | 741 (1.63) | 174 (23.48) | 79 (10.66) | 0.71 | 0.47 to 1.06 |
| 65–74 | 244 (1.70) | 154 (63.11) | 18 (7.38) | 0.67 | 0.36 to 1.28 |
| 75+ | 110 (0.96) | 81 (73.64) | 5 (4.55) | 0.41 | 0.15 to 1.13 |
| Deprivation quintile | |||||
| 1b | 961 (3.49) | 189 (19.67) | 100 (10.41) | 1.00 | – |
| 2 | 789 (2.42) | 135 (17.11) | 97 (12.29) | 1.18 | 0.85 to 1.63 |
| 3 | 735 (2.15) | 79 (10.75) | 116 (15.78) | 1.55 | 1.13 to 2.12 |
| 4 | 519 (1.92) | 59 (11.37) | 96 (18.50) | 1.94 | 1.39 to 2.71 |
| 5 | 309 (0.73) | 36 (11.65) | 51 (16.50) | 1.86 | 1.24 to 2.79 |
| Primary care consultations | |||||
| 0–2 | 1133 (1.13) | 42 (3.71) | 206 (18.18) | 1.00 | – |
| 3–4 | 785 (3.10) | 63 (8.03) | 103 (13.12) | 0.69 | 0.52 to 0.92 |
| ≥ 5 | 1405 (2.76) | 393 (27.97) | 153 (10.89) | 0.87 | 0.66 to 1.15 |
| Description | Influenza-positive (cases) | Influenza-negative (controls) | % total positive | Adjusted VE (95% CI) | ||
|---|---|---|---|---|---|---|
| No. vaccinated/total | % vaccinated | No. vaccinated/total | % vaccinated | |||
| Age group (years) | ||||||
| All ages | 27/462 | 5.84 | 471/2861 | 16.46 | 13.90 | 57.13 (31.26 to 73.26) |
| < 65 | 14/439 | 3.19 | 249/2530 | 9.84 | 14.79 | 66.23 (39.29 to 81.21) |
| < 65 at risk | 14/117 | 11.97 | 209/788 | 26.52 | 12.93 | 59.60 (21.95 to 79.08) |
| ≥ 65 | 13/23 | 56.52 | 222/331 | 67.07 | 6.50 | 18.82 (– 103.66 to 67.64) |
| Season | ||||||
| 2000/1 | 0/59 | 0.00 | 53/404 | 13.12 | 12.93 | NA |
| 2001/2 | 1/55 | 1.82 | 25/310 | 8.06 | 7.67 | 77.26 (– 116.76 to 97.61) |
| 2002/3 | 1/21 | 4.76 | 22/220 | 10.00 | 10.55 | 68.19 (– 310.30 to 97.53) |
| 2003/4 | 4/56 | 7.14 | 12/269 | 4.46 | 5.18 | 49.14 (– 57.81 to 83.61) |
| 2004/5 | 5/49 | 10.20 | 60/351 | 17.09 | 19.40 | 43.59 (– 66.17 to 80.85) |
| 2005/6 | 6/141 | 4.26 | 52/470 | 11.06 | 10.49 | 28.68 (– 108.83 to 75.64) |
| 2006/7 | 2/26 | 7.69 | 23/228 | 10.09 | 10.92 | 22.29 (– 374.58 to 87.28) |
| 2007/8 | 3/43 | 6.98 | 55/214 | 25.70 | 29.15 | 80.19 (20.64 to 95.06) |
| 2008/9 | 4/40 | 10.00 | 50/254 | 19.69 | 22.50 | 38.04 (– 135.74 to 83.71) |
Clinical outcomes
In total, during the nine influenza seasons, 5591 of 162,698 (3.4%) primary care acute respiratory consultations (acute respiratory infections including influenza-like illnesses) were for influenza-like illnesses, 3096 of 86,531 (3.6%) emergency hospitalisations (all cause) were due to influenza and pneumonia and 2096 of 10,121 (20.7%) deaths (all cause) were due to influenza and pneumonia. One-fifth of hospitalisations due to influenza or pneumonia that occurred during the nine influenza seasons were in at-risk patients aged < 65 years (n = 502/2835 hospitalisations; 17.7%) and most occurred in older patients aged ≥ 65 years (n = 1700/2835; 60.0%). Almost all deaths from influenza or pneumonia occurred in those groups targeted for vaccination (n = 2001/2096; 95.5%); most occurred in people aged ≥ 65 years (n = 1860/2096; 88.7%).
In Tables 6 and 7, we report the incidence rate of clinical outcomes (and unadjusted rates) for unvaccinated versus vaccinated individuals as well as VEs for the whole cohort. In Table 8 we report results for one subgroup, clinically at-risk patients aged < 65 years; Table 9 presents the results for people who are aged ≥ 65 years. In Tables 6 and 7, where results for the whole population are reported, it should be noted that unadjusted rates among the vaccinated are far higher than among the unvaccinated. This is largely due to the majority of the vaccinated being older people (aged ≥ 65 years) who are more likely to have clinical outcomes (particularly hospitalisation and death) than younger, unvaccinated individuals.
| Outcomes of interest | Vaccination status | Events (rate per 1000 person-seasons) | Unadjusted RR (95% CI) | Adjusted VE (95% CI)a | Matched VE (95% CI)b |
|---|---|---|---|---|---|
| Primary care ILI | No | 2998 (2.60) | 1.00 | – | – |
| Yes | 599 (2.69) | 1.03 (0.95 to 1.13) | 9.96 (– 3.81 to 21.90) | 16.44 (5.70 to 25.96) | |
| Primary care ARI | No | 62,751 (54.40) | 1.00 | – | – |
| Yes | 22,434 (100.67) | 1.95 (1.92 to 1.98) | 2.73 (0.20 to 5.19) | – 33.00 (– 39.90 to – 26.40) | |
| Hospitalisation IP | No | 1188 (1.03) | 1.00 | – | – |
| Yes | 1090 (4.89) | 4.77 (4.39 to 5.18) | 8.53 (– 3.59 to 19.24) | 19.32 (8.25 to 29.05) | |
| Hospitalisation IPC | No | 2251 (1.95) | 1.00 | – | – |
| Yes | 3289 (14.76) | 7.66 (7.26 to 8.08) | 12.22 (4.36 to 19.44) | 26.66 (19.82 to 32.92) | |
| Hospitalisation CVD | No | 3659 (3.17) | 1.00 | – | – |
| Yes | 5944 (26.67) | 8.61 (8.26 to 8.98) | 9.26 (3.71 to 14.49) | 22.60 (17.53 to 27.35) | |
| Hospitalisation all | No | 32,598 (28.26) | 1.00 | – | – |
| Yes | 17,672 (79.29) | 2.96 (2.91 to 3.02) | 7.08 (4.14 to 9.94) | 12.39 (9.10 to 15.57) | |
| Hospitalisation trauma | No | 5427 (4.70) | 1.00 | – | – |
| Yes | 2115 (9.49) | 2.03 (1.93 to 2.13) | 11.35 (3.74 to 18.37) | 18.10 (10.10 to 25.39) | |
| Hospitalisation appendicitis and hernia | No | 700 (0.61) | 1.00 | – | – |
| Yes | 402 (1.80) | 2.98 (2.63 to 3.36) | 0.70 (– 21.27 to 18.69) | – 4.20 (– 21.40 to 10.61) |
| Outcomes of interest | Vaccination status | Events (rate per 1000 person-seasons) | Unadjusted RR (95% CI) | Adjusted VE (95% CI)a | Matched VE (95% CI)b |
|---|---|---|---|---|---|
| Deaths IP | No | 789 (0.68) | 1.00 | – | – |
| Yes | 833 (3.74) | 5.48 (4.97 to 6.04) | 29.86 (19.47 to 38.91) | 37.91 (29.47 to 45.35) | |
| Deaths IPC | No | 1228 (1.06) | 1.00 | – | – |
| Yes | 1385 (6.21) | 5.87 (5.43 to 6.34) | 19.47 (22.00 to 37.50) | 41.78 (33.94 to 48.70) | |
| Deaths CVD | No | 1832 (1.59) | 1.00 | – | – |
| Yes | 2110 (9.47) | 6.01 (5.64 to 6.40) | 32.96 (26.55 to 38.81) | 41.13 (35.60 to 46.19) | |
| Deaths all | No | 3961 (3.43) | 1.00 | – | – |
| Yes | 3807 (17.08) | 5.04 (4.82 to 5.27) | 33.60 (29.00 to 37.90) | 41.63 (31.10 to 45.83) |
| Outcomes of interest | Vaccination status | Events | Rate per 1000 person-seasonsa | Unadjusted RR (95% CI) | Adjusted VE (95% CI) |
|---|---|---|---|---|---|
| Primary care ILI | No | 600 | 3.51 | 1.00 | – |
| Yes | 215 | 3.32 | 0.94 (0.81 to 1.10) | 6.39 (– 15.00 to 23.81) | |
| Primary care ARI | No | 20,127 | 117.81 | 1.00 | – |
| Yes | 9858 | 152.01 | 1.34 (1.31 to 1.38) | – 0.69 (– 2.76 to 4.02) | |
| Hospitalisation IP | No | 247 | 1.45 | 1.00 | – |
| Yes | 167 | 2.58 | 1.78 (1.46 to 2.17) | 6.56 (– 20.53 to 27.55) | |
| Hospitalisation IPC | No | 539 | 3.16 | 1.00 | – |
| Yes | 574 | 8.85 | 2.82 (2.51 to 3.17) | 9.81 (– 7.60 to 24.40) | |
| Hospitalisation CVD | No | 1211 | 7.09 | 1.00 | – |
| Yes | 1031 | 15.90 | 2.26 (2.08 to 2.46) | 13.15 (2.76 to 22.43) | |
| Hospitalisation all | No | 7691 | 45.02 | 1.00 | – |
| Yes | 4030 | 61.14 | 1.41 (1.35 to 1.46) | 9.54 (4.31 to 14.47) | |
| Deaths IP | No | 67 | 0.39 | 1.00 | – |
| Yes | 43 | 0.66 | 1.69 (1.15 to 2.48) | 34.14 (– 7.50 to 59.63) | |
| Deaths IPC | No | 144 | 0.84 | 1.00 | – |
| Yes | 103 | 1.59 | 1.89 (1.46 to 2.43) | 35.68 (9.52 to 54.27) | |
| Deaths CVD | No | 211 | 1.24 | 1.00 | – |
| Yes | 146 | 2.25 | 1.82 (1.48 to 2.25) | 30.04 (7.19 to 47.26) | |
| Deaths all | No | 549 | 3.21 | 1.00 | – |
| Yes | 307 | 4.73 | 1.48 (1.28 to 1.70) | 33.02 (18.78 to 44.77) | |
| Hospitalisation trauma | No | 964 | 5.64 | 1.00 | – |
| Yes | 372 | 5.74 | 1.02 (0.90 to 1.15) | 6.36 (– 10.28 to 20.50) | |
| Hospitalisation appendicitis and hernia | No | 134 | 0.78 | 1.00 | – |
| Yes | 62 | 0.96 | 1.22 (0.90 to 1.65) | 24.36 (– 12.80 to 49.26) |
| Outcomes of interest | Vaccination status | Events | Rate per 1000 person seasonsa | Unadjusted RR (95% CI) | Adjusted VE (95% CI) |
|---|---|---|---|---|---|
| Primary care ILI | No | 103 | 1.79 | 1.00 | – |
| Yes | 348 | 2.40 | 1.35 (1.08 to 1.68) | 19.29 (– 3.25 to 36.91) | |
| Primary care ARI | No | 2558 | 44.43 | 1.00 | – |
| Yes | 11,927 | 82.43 | 1.93 (1.85 to 2.02) | 4.17 (– 0.33 to 8.46) | |
| Hospitalisation IP | No | 453 | 7.87 | 1.00 | – |
| Yes | 905 | 6.25 | 0.79 (0.71 to 0.89) | 11.57 (– 2.42 to 23.65) | |
| Hospitalisation IPC | No | 1130 | 19.62 | 1.00 | – |
| Yes | 2687 | 18.57 | 0.95 (0.88 to 1.01) | 15.85 (7.04 to 23.83) | |
| Hospitalisation CVD | No | 1888 | 32.79 | 1.00 | – |
| Yes | 4883 | 33.75 | 1.03 (0.98 to 1.09) | 12.77 (6.45 to 18.67) | |
| Hospitalisation all | No | 5716 | 99.27 | 1.00 | – |
| Yes | 13,114 | 90.63 | 0.90 (0.88 to 0.93) | 13.14 (9.51 to 16.62) | |
| Deaths IP | No | 657 | 11.41 | 1.00 | – |
| Yes | 781 | 5.40 | 0.47 (0.42 to 0.52) | 32.50 (21.94 to 41.63) | |
| Deaths IPC | No | 991 | 17.21 | 1.00 | – |
| Yes | 1270 | 8.78 | 0.51 (0.47 to 0.55) | 32.75 (24.38 to 40.20) | |
| Deaths CVD | No | 1428 | 24.80 | 1.00 | – |
| Yes | 1955 | 13.51 | 0.54 (0.50 to 0.58) | 34.35 (27.65 to 40.42) | |
| Deaths all | No | 2749 | 47.74 | 1.00 | – |
| Yes | 3437 | 23.75 | 0.49 (0.46 to 0.51) | 36.93 (32.25 to 41.30) | |
| Hospitalisation trauma | No | 841 | 14.60 | 1.00 | – |
| Yes | 1681 | 11.62 | 0.79 (0.73 to 0.86) | 19.27 (10.15 to 27.46) | |
| Hospitalisation appendicitis and hernia | No | 116 | 2.01 | 1.00 | – |
| Yes | 333 | 2.30 | 1.14 (0.92 to 1.41) | – 16.22 (– 51.24 to 10.69) |
After adjustment for confounding factors (including age in the model), recipients of influenza vaccine were less likely to have acute respiratory illnesses, less likely to require hospitalisation for influenza, pneumonia, COPD or cardiovascular disease, and less likely to die from all outcomes studied in the whole population (see Tables 6 and 7). In the at-risk population aged < 65 years, analyses were less precise due to lower event rates for most of the outcomes. However, significant VE was found for hospitalisations and deaths from cardiovascular disease and deaths due to influenza, pneumonia or COPD (see Table 8). Significant VE was found for most hospitalisation and death outcomes in the population aged ≥ 65 years (see Table 9).
Significant heterogeneity was found in 2000/1 and 2001/2 when compared with other years (test for interaction: p < 0.0001) and these years were excluded from the final pooled analysis. After propensity score matching was performed (for seven seasons from 2002/3 to 2008/9) there were 78,048 matched pairs of patients. Influenza vaccine recipients were less likely to be hospitalised or to die from influenza, pneumonia, COPD or cardiovascular disease (see Tables 6 and 7).
In our sensitivity analysis using negative controls, we found significant VE for trauma hospitalisation with the exception of at-risk patients aged < 65 years. However, further sensitivity analyses (see Table 10) revealed that VE for trauma during the post season was greater than that during peak influenza season, and this suggests that it may be a poor choice of negative control. No significant VE was found for appendicitis and hernia hospitalisation.
Sensitivity analyses
Seasonality
Analyses performed to identify residual confounding found that, with the exception of all-cause hospitalisations, maximal VE for our clinical outcomes were found during the influenza season (Table 10). However, pre-season VEs were higher for primary care acute respiratory disease. In the post-season analysis, positive estimates of VE were found for hospitalisation due to trauma and all causes. In the non-influenza season, positive estimates of VE were found for all-cause hospitalisation and for hospitalisation due to influenza, pneumonia or COPD. In addition, the influenza-positive estimates were found for all-cause mortality and cerebrovascular and cardiovascular disease-related mortality during the non-influenza season period.
| Outcomes of interest | Pre-season adjusted VE (95% CI) | Post-season adjusted VE (95% CI) | Non-season adjusted VE (95% CI) |
|---|---|---|---|
| Primary care ILI | – 36.05 (– 169.04 to 31.21) | – 18.14 (– 100.95 to 30.53) | 17.75 (– 33.81 to 49.44) |
| Primary care ARI | 13.07 (3.56 to 21.65) | – 38.41 (– 46.48 to – 30.79) | – 26.46 (– 33.67 to – 19.64) |
| Hospitalisation IP | – 26.22 (– 129.43 to 30.56) | 11.64 (– 10.18 to 16.07) | 12.59 (– 13.82 to 32.88) |
| Hospitalisation IPC | – 21.38 (– 129.43 to 30.56) | – 12.11 (– 31.66 to 4.53) | 16.35 (1.00 to 29.33) |
| Hospitalisation CVD | – 7.46 (– 34.87 to 14.38) | 1.15 (– 10.58 to 11.64) | 5.84 (– 4.51 to 15.16) |
| Hospitalisation all | – 16.83 (– 170.93 to 49.64) | 6.42 (0.74 to 11.78) | 8.12 (3.01 to 12.96) |
| Deaths IP | –a | 2.22 (– 34.59 to 28.96) | – 12.57 (– 52.28 to 16.24) |
| Deaths IPC | –a | – 9.62 (– 42.19 to 15.47) | – 4.04 (– 31.14 to 17.46) |
| Deaths CVD | –a | 10.07 (– 9.49 to 26.13) | 18.71 (2.70 to 32.09) |
| Deaths all | –a | 8.91 (– 5.62 to 21.43) | 13.35 (1.65 to 23.66) |
| Hospitalisation trauma | 4.73 (– 40.53 to 35.41) | 20.61 (5.34 to 33.42) | 6.56 (– 9.42 to 20.22) |
| Hospitalisation appendicitis and hernia | – 16.81 (– 170.93 to 49.64) | 2.91 (– 42.43 to 33.82) | 11.90 (– 24.45 to 37.63) |
Vaccine match and severity of influenza season
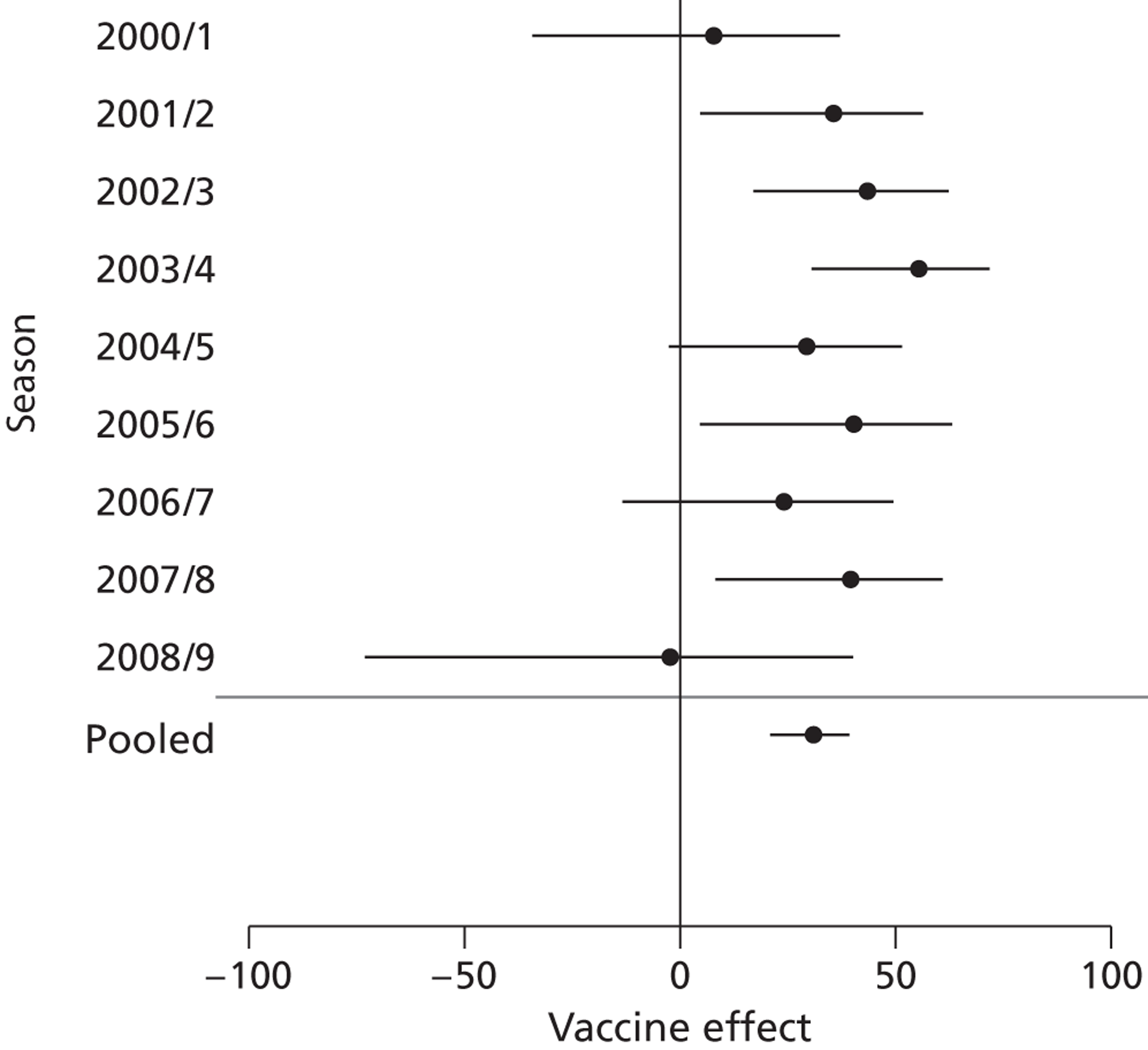
Poor precision resulted in difficulty comparing VE by season. Using the laboratory-confirmed influenza data (see Table 5) the most severe season was 2007/8, with 29.15% of positive tests. This year (2007/8) also had the highest VE in our clinical outcomes (Figure 3: deaths from influenza and pneumonia for people aged ≥ 65 years are shown here as an example). During the season 2005/6, where the influenza B component of the vaccine was regarded as poorly matched, VE was the second lowest for laboratory-confirmed influenza in the nine seasons (see Table 5). However, no obvious decline in VE for this poorly matched year was found in our clinical outcomes.
FIGURE 3.
Death from influenza and pneumonia by season in patients aged ≥ 65 years.
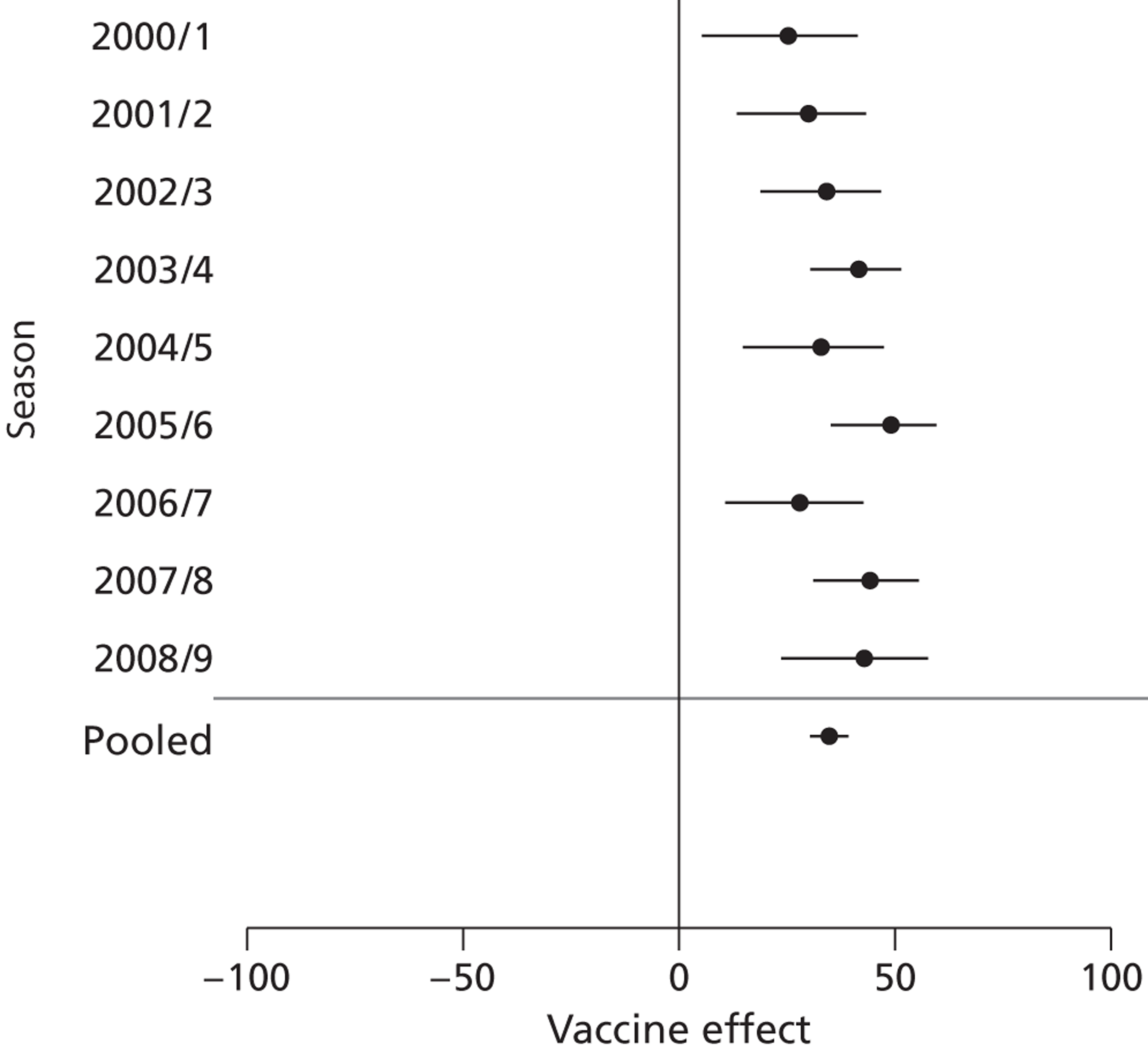
Where all-cause death was used as an outcome, VE was similar between seasons (Figure 4).
FIGURE 4.
All-cause death by season in patients aged ≥ 65 years.
Specificity of outcome measure
The highest VE was for our most specific outcome, laboratory-confirmed influenza (see Table 5). For primary care clinical outcomes, VE for acute respiratory diseases was lower than for the more specific influenza-like illnesses (see Table 6). For hospitalisation and death, however, VE was lower in the most specific outcome, influenza and pneumonia (see Tables 6 and 7).
Presence of immune senescence by age
Vaccine effectiveness was higher for our clinical outcomes in people aged ≥ 65 years compared with younger at-risk patients (see Tables 8 and 9).
Instrumental variable analysis
Participation in a screening programme was the variable most closely correlated with influenza vaccination (correlation coefficient 0.71) and was also associated with one of the main study outcomes (death due to influenza or pneumonia; HR 2.6, 95% CI 2.4 to 2.9, p < 0.001). However, this association with outcome was not exclusively mediated through vaccination status (HR adjusted for vaccination status 1.4, 95% CI 1.3 to 1.6, p < 0.001). Furthermore, participation in a screening programme was also strongly associated with a number of confounding variables (e.g. number of previous hospital admissions, comorbidity, age, sex and socioeconomic status). Similarly, each of the remaining four variables was unlikely to fulfil criteria for use as an instrumental variable to produce unbiased estimates of VE. Our findings are in keeping with those of other investigators who have attempted to assess influenza VE using instrumental variables in routine health-care data. 34 We were therefore unable to use this method to estimate VE accounting for unmeasured confounding.
Modelling an unmeasured confounder
We used estimates from published data to model the effect of inadequately measured confounders on VE in our matched population.
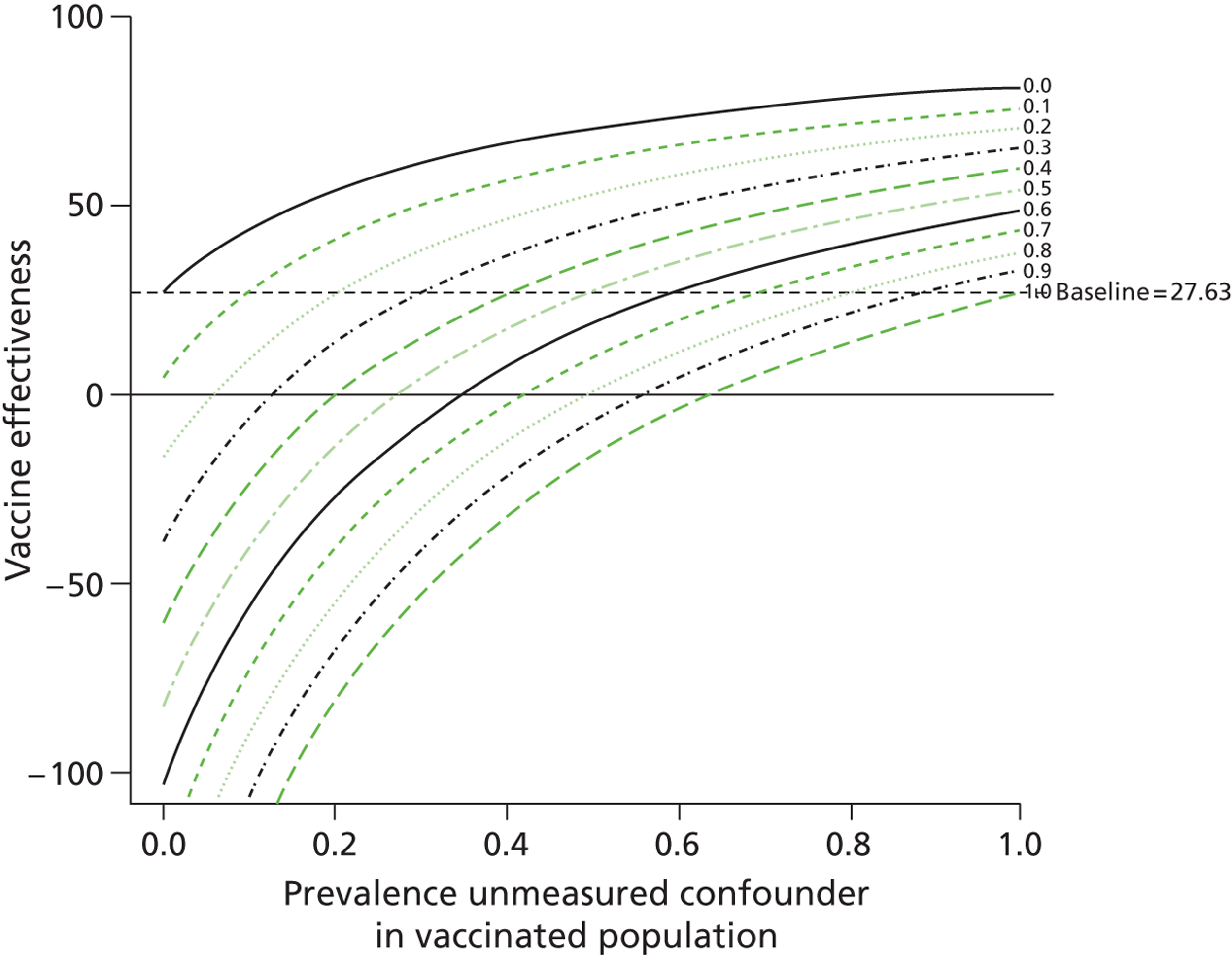
We found that for one outcome, death due to influenza or pneumonia, a scenario of 20% prevalence of frailty among unvaccinated individuals resulted in the vaccine no longer being effective (VE 13.2%, 95% CI 2.2% to 22.3%). 9 Frailty resulted in a twofold increase in the risk of death and was not present in the vaccinated population. By varying the prevalence of frailty and its risk on outcome, we modelled the change in VE estimates for a number of scenarios (Table 11). We also found that this model can be displayed effectively in graphical form (Figures 5 and 6). In Figures 5 and 6 the VE is estimated given levels of the unmeasured confounder in the vaccinated population (x-axis) and unvaccinated population (each of the lines representing a different prevalence of the unmeasured confounder in the vaccinated population). At points where the prevalence of the unmeasured confounder is the same in both unvaccinated and vaccinated individuals, the baseline result is achieved, as there is no difference in the level of the unmeasured variable in the two groups.
| Increase in the risk of outcome on account of the confounder | Prevalence of confounder | Death from pneumonia or influenza: adjusted VE (95% CI) | |
|---|---|---|---|
| In unvaccinated individuals (%) | In vaccinated individuals (%) | ||
| – | 0 | 0 | 27.63 (18.51 to 35.73) |
| Doubled | 5 | 0 | 24.01 (14.43 to 32.51) |
| Doubled | 15 | 5 | 20.73 (10.74 to 29.61) |
| Doubled | 20 | 5 | 17.29 (6.86 to 26.55) |
| Quadrupled | 5 | 0 | 16.77 (6.28 to 26.09) |
| Quadrupled | 15 | 5 | 8.75 (– 2.75 to 18.96) |
| Quadrupled | 20 | 5 | – 0.69 (– 13.38 to 10.58) |
FIGURE 5.
Unmeasured residual confounding and VE (doubling of risk of death from influenza and pneumonia).
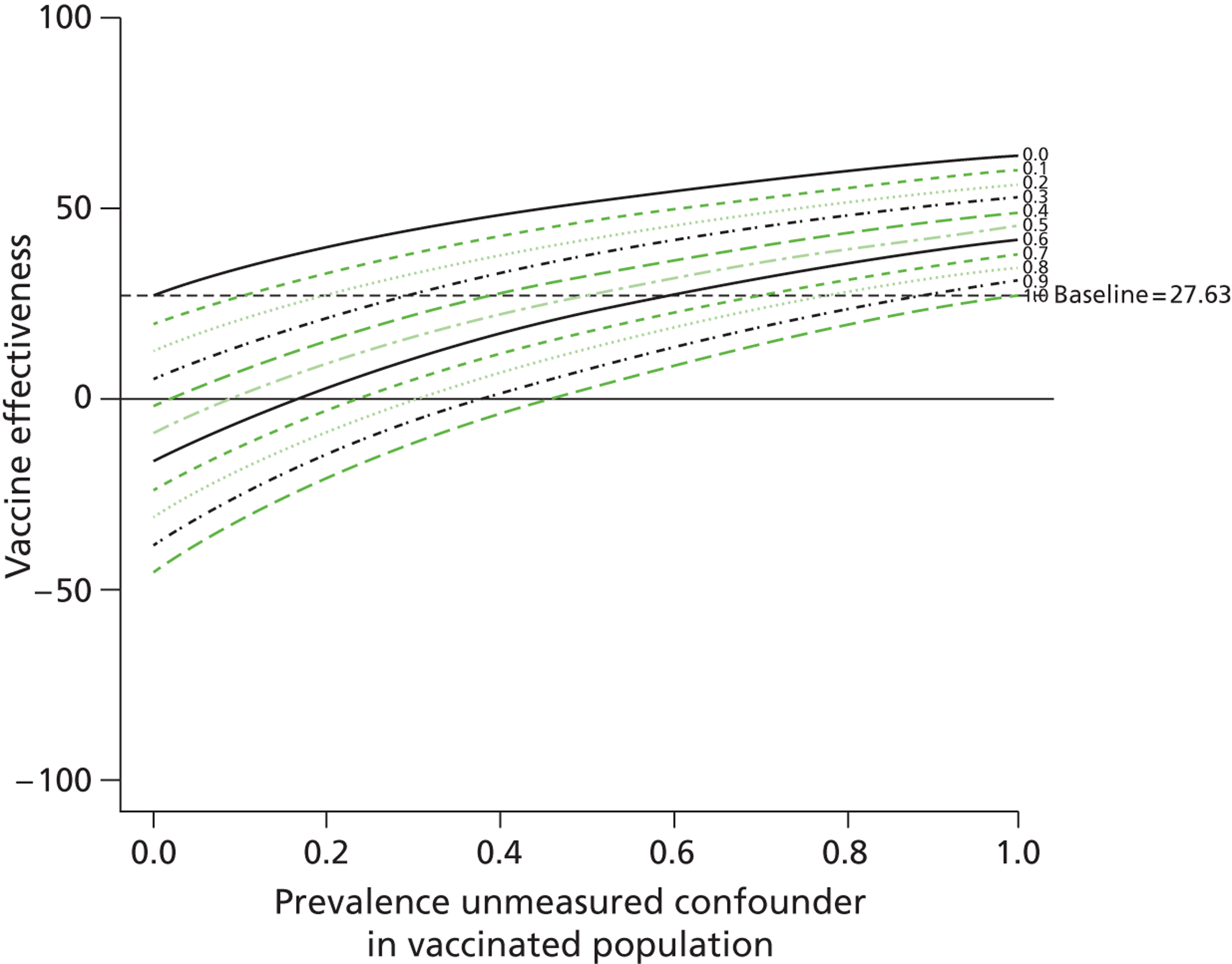
FIGURE 6.
Unmeasured residual confounding and VE (quadrupling of risk of death from influenza and pneumonia).
Chapter 4 Discussion
The majority of the trivalent inactivated influenza vaccines were distributed to at-risk patients targeted for vaccination. There was high uptake of the vaccine among those aged ≥ 65 years. However, during the nine seasons, only one-quarter of at-risk patients aged < 65 years received the vaccine. The trivalent inactivated influenza vaccine was effective in reducing RT-PCR laboratory-confirmed influenza, as well as hospitalisations and deaths from influenza and pneumonia.
The modest vaccine uptake among at-risk patients aged < 65 years was similar to that found using the QRESEARCH database (2000/1: 20.6%; 2004/5: 29.3%),35 but lower than that found using questionnaire surveys carried out in a more recent year (2007/8: 56.0%). 36
Our finding of 57.1% trivalent influenza VE, using RT-PCR in symptomatic medical attenders of all ages presenting over nine seasons, is similar to the pooled efficacy of 59% found in a meta-analysis of randomised studies (albeit in a younger, healthier population). In the 2007/8 season, when vaccine and circulating virus were well matched (A/H1N1 Solomon Islands virus), our VE for laboratory-confirmed influenza was higher than that of a US study which used a study design similar to ours (80% vs. 52%). 37 We also compared our results with those of two case–control effectiveness studies in older age groups which used a similar study design to ours. 38,39 One of these, carried out in the USA in 2010/11 using RT-PCR, found a VE of 36% (95% CI – 22% to 62%) but suffered from poor precision due to low numbers of cases. 38 The other was a pilot using RT-PCR and culture in Spain in 2008/9, which found a very high effectiveness (78%; 95% CI 26% to 93%) but with very low numbers of unvaccinated control subjects. 39
Rates of hospitalisation for influenza and pneumonia were identical to those reported for people aged ≥ 65 years in the USA (0.7% in unvaccinated and 0.6% in vaccinated individuals). 32 However, our all-cause death rates were higher (4.6% vs. 1.6% in unvaccinated and 2.4% vs. 1.0% in vaccinated patients) although our rates of death for influenza and pneumonia were lower (1.0% in unvaccinated and 0.5% in vaccinated individuals; no comparative figures available from Nichol et al. 32). This may be partly attributable to differences between the Scottish and US health-care systems.
Although criticised for bias,28 previous observational studies have found a high VE for preventing clinical outcomes such as hospitalisation for influenza and pneumonia (27%) and all-cause death (48%) in people aged > 65 years. 32 More recently, estimates made during periods when influenza was not circulating estimated far lower VE. 40,41 Our study, which included detailed patient information from the primary care electronic health record linked to other health-care data sources, found that in people aged ≥ 65 years, VE estimates were higher than those reported by Fireman et al. 40 for emergency admissions for influenza and pneumonia (4.6%) and all-cause death (8.5%).
Quasi-experimental studies can be used to assess the effects of health-care interventions without influencing the care provided or the patients who receive it. 4 When used in the assessment of vaccination programmes they therefore have high external validity and broad generalisability. However, the non-randomised nature of studies such as this are limited by the extent to which there may be dissimilarities between vaccinated and unvaccinated individuals, in both their likelihood of receiving vaccination and in their subsequent care and follow-up, leading to overestimated mortality benefits. The retrospective ascertainment of vaccination status is necessarily less reliable than prospective clarification, but our use of data derived from health records has been found to be more reliable than self-reporting methods,42 as is the electronic recording of uptake rates in this sample of the Scottish population.
Sensitivity analysis framework
There are two types of confounding which have a role when estimating VE: (i) confounding by indication, or negative confounding, which will underestimate VE and will include people with underlying chronic diseases who are more likely to be vaccinated (and are at greater risk of disease and only moderately elevated risk of death); and (ii) positive confounding, which will overestimate VE and will include healthier individuals who are more readily vaccinated (as they may be more mobile and actively seek the vaccine) and, conversely, very frail people who are less likely to be vaccinated and may die soon thereafter. 43
First, to try and account for residual confounding, we used the most detailed electronic health information available (in particular that from primary care, which uses the granular Read coding system44) to include in our model a score determining propensity to be vaccinated. We found variation in uptake between groups. Among care home residents, for instance, uptake was high and this reflects what is a common practice in the UK of vaccinating residents en masse by visiting nursing home facilities. We also found that with increased use of primary care, uptake increased and this may be due to the increased opportunity to vaccinate. We did, however, find a curvilinear relationship between vaccination and Charlson comorbidity. In particular, for patients aged ≥ 65 years, there were higher ORs for uptake in the presence of at least one Charlson comorbidity or hospitalisation, but lower ORs (than in those with no morbidity) were found among those with a Charlson score of five or more and > 10 hospitalisations. By matching individuals in our cohort on a propensity score we were able to effectively compare people with a similar propensity to be vaccinated. This should have limited the effect of positive confounding, which will overestimate VE. We feel that the use of our propensity score has reduced bias for our estimates of VE. However, our VE for all-cause deaths was still higher than the estimated excess mortality attributed to influenza of 5–10%. 9
We also attempted to explore and account for residual confounding using the framework suggested by Simonsen et al. 8 The influenza vaccine should be not be effective for outcomes unrelated to infection with seasonal influenza and reassuringly no VE was found for hospitalisation due to appendicitis and hernia. A positive VE (which continued in the post-influenza season), however, was found for hospitalisation due to trauma (e.g. for wounds and fractures). However, further sensitivity analyses revealed that VE for trauma during the post-influenza season was greater than during peak influenza seasons, and this suggests that it may be a poor choice of negative control. It is of interest though that the unvaccinated group was more likely to be hospitalised than the vaccinated group for this type of admission. When analysing VEs stratified by the four periods of each year (influenza season and non-, pre- and post-influenza season), maximal VE, determined by use of viral surveillance information, was generally seen for most of our clinical outcomes during the influenza season. VEs outside the influenza season were usually close to zero with wide 95% CIs owing to the relatively small exposure periods outside the main influenza season (at most 2.5 months) and a lack of influenza/pneumonia outcomes. Positive VEs were, however, found for less specific outcomes such as hospitalisations and deaths for any cause, or for outcomes that can be exacerbated or caused by factors other than influenza, for example, acute respiratory disease, COPD hospitalisation or death from cerebrovascular and cardiovascular disease. Our VE for less specific outcomes, however, was found to continue during post- and non-influenza seasons when influenza was not circulating, suggesting some residual bias still exists. Sophisticated seasonality techniques such as case-centred logistic regression, which attempt to account for deaths that take place when influenza is not circulating, have found that without vaccination, excess mortality during influenza season would be 9.8%, and estimated VE 4.6% (half of all deaths being prevented by vaccine). 40
Our unique cohort allowed VE to be derived for a range of outcomes in the same population over multiple influenza seasons. However, although the modest size of our cohort made it feasible to collate centrally almost all cases of influenza-related disease, allowing for completeness of reporting, any analysis by subgroups or by individual season resulted in poorer precision and wide CIs. It was therefore less than straightforward to draw conclusions regarding the validity of our VE estimates using Simonsen's framework. 8 However, we were able to determine that during the most severe season for our cohort (2007/8, as determined from swabs), VE was highest for our outcomes. Although there was no protective effect for the outcome appendicitis and hernia, a protective effect was found for trauma, which is unrelated to influenza. The significant heterogeneity in the matched analysis for the years 2000/1 and 2001/2 may have been caused by accuracy and completeness issues (found in 2000), which were resolved by the Information Services Division (ISD) in subsequent years. 45
We attempted to use further methods to assess the degree of bias in our VE estimates. We were unable to use instrumental variables for this purpose as none were found to be valid. However, we modelled the effect of an unmeasured confounder, such as frailty, on our VE estimates. We found that VE for our primary outcome, death due to influenza or pneumonia, was robust to modelling a high prevalence of unmeasured frailty. This is despite the fact that we included a number of variables in our models which would have captured some degree of frailty, such as residence in a nursing home, provision of social care, number of previous hospital admissions, number of GP consultations and number of repeat prescriptions.
Implications for practice
Using VE estimates for our most specific outcome, RT-PCR-confirmed influenza over a 9-year period, the seasonal influenza programme was found to be effective, particularly in preventing influenza in younger, clinically at-risk groups of patients.
Research recommendations
Although the modest size of our cohort made it feasible to collate centrally almost all cases of influenza-related disease, allowing for completeness of reporting, the analysis of subgroups (in particular, older age groups) or analysis by individual season resulted in poorer precision and wide CIs. Any future work should therefore aim to address this issue by ensuring adequate power to test VE in these subgroups of patients, while minimising the effect of bias, such as health-seeking behaviour. While work is being undertaken to produce better vaccines, continued monitoring and a strong international evidence base for the effectiveness of seasonal influenza vaccination programmes is necessary.
Chapter 5 Conclusions
Few countries' health systems allow for the integrated and accessible data recording that made this study possible and made it feasible to collate centrally almost all hospitalisations and deaths attributed to influenza, allowing for completeness of reporting. By making best use of the integrated and accessible Scottish data available to us, we found most influenza vaccines were distributed to those at risk of serious complications from influenza. Influenza vaccination was associated with a significant decrease in the risk of laboratory-confirmed influenza and complications arising from influenza in a nationally representative cohort.
Acknowledgements
The authors would like to thank staff at the Primary Care Clinical Informatics Unit (PCCIU), HPS and the ISD; the general practices and virus laboratories that contributed data to the study; and members of the Independent Steering Committee overseeing this work. This study was funded by a grant from the National Institute for Health Research Health Services and Delivery Research Programme as study 09/2000/37. The authors also acknowledge the financial support of the European Centre for Disease Prevention and Control (ECDC) through their IMOVE seasonal influenza vaccination project led by Epiconcept, which allowed virology data cleaning for the 2008/9 season.
Contributions of authors
Dr Colin Simpson (Reader in Population Health Sciences) and Dr Nazir Lone (Clinical Fellow, Intensive Care) were principal investigators and led the writing of this report.
Professor Lewis Ritchie (Professor of Primary Care), Professor Aziz Sheikh (Professor of Primary Care Research & Development) and Dr Jim McMenamin (Consultant Epidemiologist) helped design the study and commented on drafts of the paper.
Professor Chris Robertson (Professor of Statistics) and Dr Kim Kavanagh (Research Fellow, Statistics) helped to design the study, carry out the analyses and write the paper.
References
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179-86. http://dx.doi.org/10.1001/jama.289.2.179.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007;25:5086-96. http://dx.doi.org/10.1016/j.vaccine.2007.03.046.
- Baguelin M, Jit M, Miller E, Edmunds WJ. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine 2012;30:3459-62. http://dx.doi.org/10.1016/j.vaccine.2012.03.019.
- Smith S, Demicheli V, Di Pietrantonj C, Harnden AR, Jefferson T, Matheson NJ, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2006;1. http://dx.doi.org/10.1002/14651858.CD004879.pub2.
- Simonsen L. Commentary: observational studies and the art of accurately measuring influenza vaccine benefits. Int J Epidemiol 2007;36:631-2. http://dx.doi.org/10.1093/ije/dym084.
- Hak E, Verheij TJM, Grobbee DE, Nichol KL, Hoes AW. Confounding by indication in non-experimental evaluation of vaccine effectiveness: the example of prevention of influenza complications. J Epidemiol Community Health 2002;56:951-5. http://dx.doi.org/10.1136/jech.56.12.951.
- Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine 2009;27:6300-4. http://dx.doi.org/10.1016/j.vaccine.2009.07.008.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007;7:658-66. http://dx.doi.org/10.1016/S1473-3099(07)70236-0.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case–control study. Lancet 2008;372:398-405. http://dx.doi.org/10.1016/S0140-6736(08)61160-5.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis 2012;12:36-44. http://dx.doi.org/10.1016/S1473-3099(11)70295-X.
- Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: a retrospective observational cohort study. Lancet Infect Dis 2012;12:696-702. http://dx.doi.org/10.1016/S1473-3099(12)70133-0.
- Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Vaccine effectiveness in pandemic influenza – primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine. Health Technol Assess 2010;14.
- Whitelaw FG, Nevin SL, Milne RM, Taylor RJ, Taylor MW, Watt AH. Completeness and accuracy of morbidity and repeat prescribing records held on general practice computers in Scotland. Br J Gen Pract 1996;46:181-6.
- Information Services Division . General Practice – Practice Team Information. National Services Scotland. 2005. www.isdscotland.org/pti (accessed November 2012).
- Jhund PS, MacIntyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population survey of 5.1 million people. Circulation 2009;119:515-23. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.812172.
- General Register Office for Scotland . General Register Office for Scotland Statistics Library (population Projections) 2005. www.gro-scotland.gov.uk/statistics/theme/population/projections/small-area-population-projections/index.html (accessed July 2012).
- Harley K, Jones C. Quality of Scottish Morbidity Record (SMR) data. Health Bull 1996;54:410-17.
- Scottish Index of Multiple Deprivation. 2009 General Report. Edinburgh: Scottish Government National Statistics Publications; 2009.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40:373-83. http://dx.doi.org/10.1016/0021-9681(87)90171-8.
- Fleming DM, Elliot AJ. Estimating the risk population in relation to influenza vaccination policy. Vaccine 2006;24:4378-85. http://dx.doi.org/10.1016/j.vaccine.2006.02.053.
- Mallia P, Johnston SL. Influenza infection and COPD. Int J Chron Obstruct Pulmon Dis 2007;2:55-64. http://dx.doi.org/10.2147/copd.2007.2.1.55.
- Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383-8. http://dx.doi.org/10.1097/EDE.0b013e3181d61eeb.
- Lone N, Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Seasonal Influenza Vaccine Effectiveness in the community (SIVE): exploitation of a unique national linked dataset. BMJ Open 2012;15.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55. http://dx.doi.org/10.2307/2335942.
- Friedman J, Hastie T, Tibshirani R. Additive logistic regression: a statistical view of boosting. Ann Stat 2000;28:337-40. http://dx.doi.org/10.1214/aos/1016218223.
- Graham PL, Mengersen K, Morton AP. Confidence limits for the ratio of two rates based on likelihood scores: non-iterative method. Stat Med 2003;22:2071-83. http://dx.doi.org/10.1002/sim.1405.
- Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2000;29:722-9. http://dx.doi.org/10.1093/ije/29.4.722.
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006;35:337-44. http://dx.doi.org/10.1093/ije/dyi274.
- Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Sayer AA. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing 2010;39:197-203. http://dx.doi.org/10.1093/ageing/afp204.
- Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerentol A Biol Sci Med Sci 2009;64A:675-81. http://dx.doi.org/10.1093/gerona/glp012.
- Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948-63. http://dx.doi.org/10.2307/2533848.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. New Engl J Med 2007;357:1373-81. http://dx.doi.org/10.1056/NEJMoa070844.
- Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case–control study. Lancet 2008;372:398-405. http://dx.doi.org/10.1016/S0140-6736(08)61160-5.
- Groenwold RHH, Hak E, Klungel OH, Hoes AW. Instrumental variables in influenza vaccination studies: mission impossible?!. Value Health 2010;13:132-7. http://dx.doi.org/10.1111/j.1524-4733.2009.00584.x.
- Coupland C, Harcourt S, Vinogradova Y, Smith G, Joseph C, Pringle M, et al. Inequalities of influenza vaccine by deprivation and risk group: Time trends analysis. Vaccine 2007;25:7363-71. http://dx.doi.org/10.1016/j.vaccine.2007.08.032.
- Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect 2009;58:446-58. http://dx.doi.org/10.1016/j.jinf.2009.04.001.
- Belongia E, Kieke B, Coleman L, Donahue J, Irving S, Meece J, et al. Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine – Marshfield, Wisconsin, 2007–2008 influenza season. Morb Mortal Wkly Rep 2008;57:393-8.
- Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012;55:951-9. http://dx.doi.org/10.1093/cid/cis574.
- Savulescu C, Valenciano M, de Mateo S, Larrauri A. Estimating the influenza vaccine effectiveness in elderly on a yearly basis using the Spanish influenza surveillance network – Pilot case–control studies using different control groups 2008–9 season, Spain. Eurosurveillance 2010;28:2903-7. http://dx.doi.org/10.1016/j.vaccine.2010.01.054.
- Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol 2009;170:650-6. http://dx.doi.org/10.1093/aje/kwp173.
- Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine 2010;28:7267-72. http://dx.doi.org/10.1016/j.vaccine.2010.08.088.
- Mangtani P, Shah A, Roberts JA. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect 2007;135:139-43. http://dx.doi.org/10.1017/S0950268806006479.
- Kissling E, Moren A, Hanquet G. Current challenges and new methodological approaches to assess vaccine effectiveness and vaccination impact. Veyrier-du-Lac: EPIConcept; 2008.
- Simpson CR, Anandan C, Fischbacher C, Lefevre K, Sheikh A. Will SNOMED-CT improve our understanding of the disease burden posed by allergic disorders?. Clinical Exp All 2007;37:1586-93. http://dx.doi.org/10.1016/j.jaci.2007.12.690.
- Information Services Division . Investigation of Anomalies Highlighted by COPPISH. 2000. www.isdscotland.org/Products-and-Services/Data-Quality/Previous-Projects/Anomalies%20report.pdf (accessed November 2012).
Appendix 1 Propensity scores
All people
Cohort spilt into two to create model (n = 1,179,201) and test predictions (n = 588,718). From the model output a propensity score based on the model coefficients is created and a score is assigned to each individual in the cohort.
Model output
FIGURE 7.
Propensity to be vaccinated score in deciles. (a) Vaccinated; and (b) unvaccinated.

FIGURE 8.
Receiver operating characteristic curve for all individuals. AUC, area under the curve.
At-risk patients aged under 65 years
At-risk patients aged under 65 years (PDF download)
FIGURE 9.
Propensity to be vaccinated score in deciles for age < 65 years. (a) Vaccinated; and (b) unvaccinated.

FIGURE 10.
Receiver operating characteristic curve for at-risk patients aged < 65 years. AUC, area under the curve.
Patients aged 65 years and over
Patients aged 65 years and over (PDF download)
FIGURE 11.
Propensity to be vaccinated score in deciles for age ≥ 65 years. (a) Vaccinated; and (b) unvaccinated.

FIGURE 12.
Receiver operating characteristic curve for all individuals aged ≥ 65 years. AUC, area under the curve.
Appendix 2 Study protocol
1. PROJECT TITLE
Seasonal Influenza Vaccine Effectiveness (SIVE): exploitation of a unique community-based national linked dataset
2. CHANGES SINCE THE PROTOCOL WAS SUBMITTED
We are now in a position to undertake analysis on individual patient virological swab data, which will be linked to the primary-secondary care dataset. These data will be made available by Health Protection Scotland (HPS) for all eight influenza seasons for which data are potentially available (i.e. 2000-2008). Access to these individual patient data should provide more robust estimates of vaccine effectiveness (VE).
3. PLANNED INVESTIGATION
3.1 Research aims and objectives
Building on prior work [1,2], we aim to use a previously successfully employed approach to determine influenza vaccine uptake and effectiveness over eight influenza seasons in the Scottish population. This will involve interrogation of data from a sentinel surveillance network of 41 general practices (yielding a total of 1,020,000 patient years), the Practice Team Information network (PTI), linked to the Information Services Division (NHS Scotland) hospital and mortality records (Scottish Morbidity Record and General Register of Scotland death certification – SMR01) as part of the National Institute of Health Research VIPER project (Ref: 09/84/90) [2].
Our three specific objectives are to evaluate the:
-
Uptake of the influenza vaccine by the relevant at risk populations i.e. patients with relevant co-morbidities and those aged > 65 years and the general population;
-
Reduction in the expected incidence of influenza-related morbidity and mortality in these at risk groups, since this is the major rationale behind current immunisation policies;
-
Effectiveness of the influenza vaccine in the population as a whole.
3.2 Existing research
Each year, influenza is responsible for considerable potentially avoidable morbidity and mortality; much of this disease burden falls on people aged 65 and over and/or those with a range of pre-existing long-term conditions. In the USA, it has been estimated that influenza is responsible for 186,000 excess hospitalisations and 44,000 excess deaths [3]. National vaccination strategies represent a potentially important approach to reduce both influenza-related illness and death, hence the considerable investment in this approach in many parts of the world. Although vaccination rates in those over 65 in Scotland are reasonable (for example, 76.3% for the 2008/9 season), despite widely promulgated guidelines and incentivised vaccination programmes, the rates of vaccination in ‘at risk’ groups under 65 remains poor (47.8% in the 2008/9 season). This may partially be due to the scarcity of reliable estimates of the benefits of the vaccine from randomised controlled trials and limited evidence from observational research which has only shown effectiveness of vaccine in selected groups of patients (i.e. those aged over 65) [4] or for those in ‘at-risk’ groups for single influenza seasons [5]. Furthermore, these studies have been prone to bias, in particular, with confounding from the ‘healthy vaccine effect’ whereby more healthy individuals have been vaccinated [6].
VE has also been previously estimated using information collected by swabs independent of routinely collected clinical information. However, such studies have been limited in that they have either employed the less reliable ‘screening method’ (calculated from aggregated as opposed to patient level data) [7], limited analysis to only a few seasons [8] or to specific groups only (such as > 65 years) [9], and were unable to distinguish whether subjects were at risk of complications from influenza-like illness [8,9]; furthermore, many of these studies were unable to determine vaccination status in a large proportion of their subjects. Further evidence using whole population primary care data linked to hospitalisation, swab and death data is therefore clearly required. Also, this project will significantly build on the Pandemic Influenza Primary Care Reporting (PIPeR) project [10], which has been ongoing since 2006 and also VE work using consultations for acute respiratory and influenza like symptoms estimated using the cohort method within the European Union EpiConcept Programme [11]. CR and JM are collaborating on both projects. Information generated from this and ongoing projects will be important for informing policy makers, clinicians and the public of the relative benefits of the vaccination programme.
3.3 Research methods
3.3.1 Design
We plan to undertake a large national retrospective observational cohort study using a unique community-based linked dataset.
3.3.2 Setting
The PTI network of 41 general practices covers a five per cent representative sample of the Scottish population (n = 240,000). These practices have received annual financial incentives since 1998 to record all of their practice data electronically [12]. Data from general practices within Scotland have shown to be of high quality and useful for epidemiological research [13]. The completeness of capture of contacts and accuracy of clinical event coding in primary care (using Read codes) has been found to be above 91% [14]. Using the unique Community Health Index (CHI) number, general practice patient level data were extracted and linked to the Scottish Morbidity Record (SMR) catalogue which has information on all in-patient hospitalisations within Scotland (as well as information on death certification linked from the General Register Office for Scotland (GROS) [15] from 1981. Hospital data are reliable, with completeness and accuracy rates exceeding 90% [14]. We also wish to link general practice information (using CHI) with the HPS virological swab dataset, which consists of all laboratory-confirmed cases of influenza (since 2000) from the general practices.
3.3.3 Target population
240,000 people of all ages registered with participating practices throughout Scotland.
3.3.4 Recruitment
This general practice and hospital linked dataset was previously used to estimate H1N1 influenza A VE [2] and is currently held at the University of Aberdeen and HPS.
3.4 Planned inclusion/exclusion criteria
All registered patients will be studied so there are no exclusion criteria.
3.5 Planned interventions
The study will involve a quantitative evaluation of the winter influenza vaccination programmes implemented through general practice over eight influenza seasons: 2000/1 to 2007/8. Primary care practices are given financial incentives by NHS National Services Scotland to record and code additional data electronically, over and above that routinely recorded for clinical care or as part of the PTI project (e.g. age, out of hours contacts and socioeconomic status), including influenza vaccination status. Virological swab data have been collected from these practices by HPS. Information generated on VE is important for informing policy makers, clinicians and the public of the relative benefits of the vaccination programme throughout the study period.
3.6 Ethical and privacy arrangements
Permission to use the database for research purposes has provisionally been obtained from the PCCIU Research Governance Group. Further permission has been granted by the MREC West of Scotland 2 Ethics Committee. Permissions from the general practices that have contributed data to this project are being sought, but based on previous experiences we do not foresee any major objections.
3.7 Risks and anticipated benefits for trial participants and society, including how benefits justify risks
As this study is seeking to assess VE using previously collected and anonymised data, this work will not add any known additional risks or benefits for the individual patients present in the databases. Informing patients as to the potential risks and benefits of vaccination will remain at the discretion of the appropriate primary care clinician. There are however potentially large societal benefits from assessing the effectiveness and impact of the vaccination programmes for Scotland and the UK as a whole, but also for the international scientific community.
3.8 Proposed sample size
Patient level data from 41 practices with a combined list size of 240,000 patients have been collected. Approximately 15% (n = 36,000) aged over 65 years, and 30% (n = 72,000) under 65 years will be deemed to be in the at-risk category on the basis of existing illnesses.
A power calculation was carried out to determine the numbers needed to detect a difference in the relative risk of death between the vaccinated and unvaccinated groups in one flu season. Among people over 65 the daily death rate from all causes in Scotland is 0.000133. Assuming that 55–75% of the age group are vaccinated against seasonal influenza then this study has a power in excess of 80% to detect a difference of 30% or more in the proportions of vaccinated and unvaccinated individuals dying over a period of three months. Hospitalisation rates among the at risk groups are of the order of 20 per 100,000 per day and over the period of the four month influenza season the whole cohort will have a power of over 95% to detect a difference of 10% in hospitalisation rates between the vaccinated and unvaccinated, assuming that 50% of the at risk groups are vaccinated. Consultation rates for influenza-like illnesses are of the order of 30 per 100,000 per day and over the period of four months the whole cohort will have a power of over 95% to detect a difference of 8% in consultation rates between the vaccinated and unvaccinated. We anticipate therefore that the numbers of individuals being studied through the PTI dataset will provide high power of assessing clinical effectiveness of the seasonal influenza vaccination programmes in each of the flu seasons from 2000/01 to 2007/08.
3.9 Statistical analysis
Odds ratios (adjusted for age, sex and deprivation) will be calculated for differences in vaccine uptake rates between different groups of patients (sex, age, Carstairs deprivation categories and at-risk groups) and for investigating trends in vaccine uptake. For VE using information from linked virological swab data, a logistic regression model will be fitted adjusting for the effects of gender, age, socioeconomic status and being in an at-risk morbidity group. VE will be measured by comparing swabs taken after vaccination with swabs taken before vaccination for all vaccinated individuals, and secondly by comparing swabs taken after vaccination among those vaccinated to swabs taken among those never vaccinated. A delay of fourteen days after vaccination will be used to establish the protective effect of the influenza vaccine. Propensity scores, such as vaccinations, consultations and hospitalisation in the previous flu season, and the effect modifiers will be used to control for the healthy vaccine effect [6]. In addition, using the cohort method the proportion of influenza-like illness, acute respiratory disease, and other adverse outcomes between vaccinated and unvaccinated cases will be ascertained. Confidence intervals for the rate ratio and tests of the differences between two rates will be carried out using the ‘midp method’ in the ‘rate ratio’ (RR) function and rate2by2.test function respectively using the ‘epitools’ package in R [17]. For small samples, confidence intervals for the RR will be estimated using the Excel workbook [18].
Illness RRs, i.e. the ratio of the rate of first admission to hospital or general practice (GP) consultation in the vaccinated compared to the rate of first admission to hospital or GP consultation among those who did not receive the vaccine will be calculated in both the ‘at-risk’ populations and the general population. This is a direct measure of VE. The unadjusted estimate of VE = (1 – RR)*100. Adjusted RRs of VE for prevention of first hospitalisation/GP consultation will be derived from Poisson regression models, adjusting for gender, age, deprivation and clinical risk group.
3.10 Proposed outcome measures
For the eight influenza seasons, we will calculate:
-
The vaccination uptake in the relevant ‘at risk’ populations (patients < 65 with ‘at-risk’ co-morbidities and those > 65 years) and the general population recorded by general practices;
-
Consultation for influenza-related morbidity (e.g. influenza, pneumonia, COPD and cardiac related consultations) from general practice data in vaccinated and unvaccinated patients stratified by ‘at-risk’ populations, age, sex and socio-economic status;
-
Mortality and influenza-related serious morbidity (e.g. influenza, pneumonia, COPD and cardiac related death and hospitalisation from SMR01 records) in vaccinated and unvaccinated patients stratified by ‘at-risk’ populations, age, sex and socioeconomic status; and
-
Influenza positivity from virological swab data in vaccinated and unvaccinated patients stratified by ‘at-risk’ populations, age, sex and socioeconomic status.
3.11 Research governance
Data are held by HPS and PCCIU. The University of Edinburgh will act as the sponsor for this study. We will, as with previous similar work, convene an Independent Steering Group, comprising of leading academics and policy makers to oversee this work.
4. KEY REFERENCES
- Mooney JD, Weir A, McMenamin J, Ritchie LD, Macfarlane TV, Simpson CR, et al. The impact of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infectious Disease 2008;8.
- Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Vaccine effectiveness in pandemic influenza - primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine. Health Technol Asses n.d.
- Thomson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality Associated With Influenza and Respiratory Syncytial Virus in the United States. JAMA 2003;289:179-86.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. NEJM 2007;14:1373-81.
- Hak E, Buskens E, Nichol KL, Verheij TJM. Do recommended high-risk adults (18-64 years) benefit from a first influenza vaccination?. Vaccine 2006;24:2799-802.
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006;35:337-44.
- Legrand J, Vergu E, Flahault A. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine 2006;24:6605-11.
- Fleming DM, Andrews NJ, Ellis JS, Bermingham A, SebastianPillai P, Elliot AJ, et al. Estimating influenza vaccine effectiveness using routinely collected laboratory data. JECH 2009.
- Kelly H, Carville K, Grant K, Jacoby P, Tran T, Barr I. Estimation of influenza vaccine effectiveness from routine surveillance data. PLoSOne 2009;4.
- Information Services Division . PIPER n.d. URL: http://www.isdscotland.org/isd/3566.html (accessed July 2009).
- Kissling E, Valenciano M, Falcao J, Larrauri A, Widgren K, Pitigoi D, et al. “I-MOVE” towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multi-centric case-control study in Europe, 2008-9. Euro Surveill 2009;14.
- Whitelaw FG, Nevin SL, Milne RM, Taylor RJ, Taylor MW, Watt AH. Completeness and accuracy of morbidity and repeat prescribing records held on general practice computers in Scotland. Br J Gen Pract 1996;46:181-6.
- Ekins-Daukes S, Helms PJ, Taylor MW, Simpson CR, McLay JS. Utility of routinely acquired primary care data for epidemiology and pharmacoepidemiology. Brit J Clin Pharmacol 2005;59:684-90.
- Information Services Division: General Practice – Practice Team Information . National Services Scotland n.d. URL: www.isdscotland.org/pti (accessed November 2009).
- GRO Scotland: General Register Office for Scotland Statistics Library n.d. URL: http://www.gro-scotland.gov.uk/statistics/publications-and-data/populationestimates/ index.html (accessed July 2005).
- Harley K, Jones C. Quality of Scottish Morbidity Record (SMR) data. Health Bull 1996;54:410-7.
- Graham PL, Mengersen K, Morton AP. Confidence limits for the ratio of two rates based on likelihood scores: non-iterative method. Stat Med 2003;22:2071-83.
- Newcombe R. Confidence intervals for proportions and related quantities n.d. URL: http://www.cardiff.ac.uk/medic/aboutus/departments/primarycareandpublichealth/ourresearch/resources/index.html (accessed January 2010).
List of abbreviations
- CHI
- Community Health Index
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- GP
- general practitioner
- GROS
- General Register Office for Scotland
- HPS
- Health Protection Scotland
- HR
- hazard ratio
- ISD
- Information Services Division
- OR
- odds ratio
- PCCIU
- Primary Care Clinical Informatics Unit
- ROCAUC
- receiver operating characteristic – area under the curve
- RR
- rate ratio
- RT-PCR
- reverse transcription-polymerase chain reaction
- SIVE
- Seasonal Influenza Vaccine Effectiveness
- SMR
- Scottish Morbidity Record
- VE
- vaccine effectiveness