Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 08/1809/233. The contractual start date was in January 2009. The final report began editorial review in August 2012 and was accepted for publication in January 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bill Noble has received funding from an academic journal for board membership and has received funding from charitable organisations and the Department of Health to attend conferences. Jane Seymour has received funding from an academic journal for board membership, has undertaken consultancy for international bodies, has received grants from a range of UK and international funders and has received royalties from a UK publisher.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2013. This work was produced by Gott et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Report structure
The report itself is presented in the following nine chapters, which are presented in the order the research took place. Below we outline the content of each chapter, along with selected published outputs arising from each chapter. A full list of our outputs is provided at the end of the report (see Appendix 6). Further publications are currently being prepared or are under review.
Chapter 1 provides the policy background to the study; presents the study aims and objectives; and provides an overview of the study design.
Chapter 2 discusses the role of the Palliative Care Studies Advisory Group, a group of service users that was established to support the project. Extracts of this chapter have been published as follows:
-
Palliative Care Studies Advisory Group. Service user involvement in research: a briefing paper by the Palliative Care Studies Advisory Group. Sheffield: University of Sheffield; 2012.
Chapter 3 presents two systematic reviews of the literature, which informed subsequent data collection and analysis. Extracts of this chapter have been published as follows:
-
Gardiner C, Ingleton C, Gott M, Ryan T. Exploring the transition from curative care to palliative care: a systematic review of the literature. BMJ Support Palliat Care 2011;1:56–63.
-
Gott M, Ward S, Gardiner C, Cobb M, Ingleton C. A narrative literature review of the evidence regarding the economic impact of avoidable hospitalisations amongst palliative care patients in the UK. Prog Palliat Care 2011;19:291–8.
-
Green E, Gardiner C, Gott M, Ingleton C. Communication surrounding transitions to palliative care in heart failure: a review and discussion of the literature. Prog Palliat Care 2010;18:281–90.
Chapter 4 summarises findings from qualitative focus groups and interviews conducted with health-care professionals to explore their views of palliative care transitions within the acute hospital setting. Extracts of this chapter have been published as follows:
-
Gardiner C, Cobb M, Gott M, Ingleton C. Barriers to the provision of palliative care for older people in acute hospitals. Age Ageing 2011;40:233–8.
-
Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: qualitative study. BMJ 2011;342:d1773 (republished as Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: qualitative study. BMJ Support Palliat Care 2011;1:42–8).
-
Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. ‘That’s part of everybody’s job’: the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med 2012;26:232–41.
-
Ryan T, Gardiner C, Bellamy G, Gott M, Ingleton C and nursing staff. Barriers and facilitators to the receipt of palliative care for people with dementia: the views of medical and nursing staff. Palliat Med 2012;26:879–86.
Chapter 5 presents the findings of the survey of palliative care needs and management conducted at the two study sites. Extracts of this chapter have been published as follows:
-
Gardiner C, Gott M, Ingleton C, Cobb M, Seymour J, Ryan T, et al. Extent of palliative care need in the acute hospital setting: a survey of two acute hospitals in the UK. Palliat Med 2013;27:76–83.
-
Gardiner C, Gott M, Ingleton C, Richards N. Palliative care for frail older people: a cross-sectional survey of patients at two hospitals in England. Prog Palliat Care 2013; in press.
-
Gott M, Gardiner C, Ingleton C, Cobb M, Noble B, Bennett M, et al. What is the extent of potentially avoidable admissions amongst hospital inpatients with palliative care needs? BMC Palliat Care 2013;12:9.
-
Gott M, Ingleton C, Gardiner C, Seymour J, Parker C, Ryan T. Prevalence and predictors of transition to a palliative care approach amongst hospital inpatients in England. J Palliat Care 2013;29:146–52.
-
Ingleton C, Gardiner C, Richards N, Seymour J, Gott M. Exploring education and training needs amongst the palliative care workforce. BMJ Support Palliat Care 2013;3:207–12.
-
Ryan T, Ingleton C, Gardiner C, Parker C, Gott M, Noble B. Symptom burden, palliative care need and predictors of physical and psychological discomfort in two UK hospitals. BMC Palliat Care 2013;12:11.
-
Ward S, Gott M, Gardiner C, Cobb M, Richards N, Ingleton C. Economic analysis of potentially avoidable hospital admissions in patients with palliative care needs. Prog Palliat Care 2012;20:147–53.
Chapter 6 considers the findings of the qualitative interviews conducted with patients meeting diagnostic and prognostic criteria for palliative care need. Extracts of this chapter will be published as follows:
-
Richards N, Gardiner C, Gott M, Ingleton C. Awareness contexts revisited: indeterminacy in initiating discussions at the end of life. J Adv Nurs 2013; in press.
Chapter 7 presents the results of the retrospective review of case notes conducted for patients who were present in the hospitals at the time of the survey and who had died in the subsequent 12 months.
Chapter 8 provides a summary of information collected from focus groups convened with key health- and social-care professionals in the two study localities to explore their views of key findings.
Chapter 9 presents the final study conclusions within the context of the existing evidence base and discusses their implications for future research.
Extracts from the following articles have been reprinted with permission from SAGE:
-
Gardiner C, Gott M, Ingleton C, Cobb M, Seymour J, Ryan T, et al. Extent of palliative care need in the acute hospital setting: a survey of two acute hospitals in the UK. Palliat Med 2013;27:76–83 by SAGE Publications Ltd, All rights reserved. © Gardiner et al.
-
Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. ‘That’s part of everybody’s job’: the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med 2012;26:232–41 by SAGE Publications Ltd, All rights reserved. © Gott et al.
-
Ryan T, Gardiner C, Bellamy G, Gott M, Ingleton C and nursing staff. Barriers and facilitators to the receipt of palliative care for people with dementia: the views of medical and nursing staff. Palliat Med 2012;26:879–86 by SAGE Publications Ltd, All rights reserved. © Ryan et al.
Chapter 1 Background to the study
In this chapter we provide a brief background to the study, highlighting the rationale for undertaking research focusing on palliative care management for older people in acute hospital settings in England. The policy context relevant to the study is also discussed, with a particular focus on policy recommendations specific to the acute hospital setting. Finally, this chapter presents the study aims and objectives and gives an overview of the overarching study design.
Introduction
This report was prepared at a time of considerable challenge for those charged with planning the future provision of palliative and end-of-life care services. An almost twofold increase in the number of people dying globally is predicted over the next 40 years, with particularly dramatic rises expected in the proportion of people living to, and therefore dying in, their ninth and tenth decades. 1 Overarching these demographic trends are worries about the future funding of care in the face of a global economic recession2 and gradually emerging recognition that the mid-20th-century model of palliative care is no longer fit for purpose. Indeed, it is now widely acknowledged that older people, who will represent an ever-increasing majority of those requiring end-of-life care in the future, are the ‘disadvantaged dying’3 because they do not fit with classical 20th-century understandings of who needs palliative care and who delivers it. It is within this context that the World Health Organization has identified addressing the ‘substandard care’ that older people receive at the end of life as a key public health concern. 4 In England, improving palliative care provision for older people has been recognised as an NHS priority; however, there are still clear gaps in the evidence base needed to underpin much-needed improvements in service delivery and organisation.
The UK policy context
This project started shortly after the publication in England of a landmark policy document: the End of Life Care Strategy for England. 5 This is a national manifestation of a broader international trend towards resituating ‘palliative care’ within public health policy6 and human rights discourses. 7 Long-standing debates about whether such care should be ‘five star’ care for the few8 or whether its legitimate focus should be confined largely to dying cancer patients have now largely ceded to new debates about the relationship between ‘generalism’ and ‘specialism’ in palliative care;9 how to encourage widespread planning for incapacity;10 and how to aid transitions to palliative care in the face of the rapidly rising incidence of long-term conditions and the overall ageing of the population, which mean that clear designations of ‘terminal illness’ are increasingly rare. 11
Proposed fundamental changes to the NHS, in which primary care trusts (PCTs) will be replaced by clinical commissioning groups12,13 responsible for commissioning services in line with the needs of patients within localities, bring into sharp focus the urgent need to move beyond simple models of palliative care to ensure that patients approaching death are appropriately supported in complex care systems. 14 The National Council for Palliative Care15 has advised that each commissioning group has an end-of-life care lead, precisely because of this complexity.
Palliative care in acute hospitals
An area of particular policy priority recently has been the provision of palliative and end-of-life care in acute NHS hospitals. 16 A number of high-profile reports have concluded that a proportion of patients dying in these settings ‘experience very poor care’ (p. 1). 16 As 90% of people spend time in hospital in their final year of life17 and 56% of all deaths occur in this setting,18 this ‘proportion’ translates into a significant number of patients receiving poor care, the vast majority of whom will be older people. The proportion of deaths in institutionalised settings (including acute hospitals) has been predicted to rise by > 20% in the next 20 years. Although more recent data suggest that there may be the beginnings of a shift towards an increasing proportion of home deaths in the UK,19 the reality is that significant numbers of patients will continue to require care in hospital in their last 12 months of life. Ensuring high-quality palliative care provision in hospital must therefore be prioritised.
There is evidence that the extent of palliative care need amongst hospital inpatients is high. In a survey of palliative care needs in an acute hospital in Sheffield in 2001, medical and/or nursing staff identified that 23% of the 453 inpatients in their care had palliative care needs (according to a standard definition). 20 Three-quarters of these patients were aged > 60 years, with the greatest proportion aged between 81 and 90 years. Only 2% had received specialist palliative care input, and any palliative care that they were receiving was ‘generalist’ in nature (i.e. provided by health professionals with no accredited training in palliative care). A survey conducted in France in 1999 reported that only 13% of total hospital beds were occupied by palliative care patients. 21 A more recent study in 2011 reported that 9.4% of hospital patients in Belgium were identified as having palliative care needs. 22 However, both of these studies used the subjective judgement of ward-based medical and nursing staff to identify patients with palliative care needs, rather than using an objective measure.
Nevertheless, acute hospitals obviously do represent a significant site of palliative and end-of-life care management in England, as reflected in the growing body of policy guidance directed at these settings from professional bodies and the Department of Health (DoH). At the time of designing the study, palliative care policy and practice within acute NHS hospitals focused primarily on the period immediately before death. The Liverpool Care Pathway (LCP),23 promoted by the End of Life Care Programme24 for use on all acute hospital wards, remains the most widely adopted end-of-life care ‘tool’ within this setting. However, it has been increasingly argued that attention needs to be paid to palliative care needs earlier in the disease trajectory. 25,26 Geriatric medicine, for example, has promoted the concept of ‘continuous palliation’, a care approach incorporating palliative care from the point of diagnosis of a life-limiting illness onwards. 27
More recently the AMBER care bundle (see www.ambercarebundle.org; accessed June 2013) has been developed to support clinicians in decision-making about care and treatment of hospital patients who are at risk of dying in the next 1–2 months. Although some early audit data suggest that this initiative is successful in improving patient outcomes, at the time of preparing this report limited data were available on this initiative.
A focus on ‘transitions’ within the acute hospital context
In 2010 the General Medical Council (GMC)28 published guidance on end-of-life care that is particularly pertinent to the focus of the current study. This guidance states that doctors must ensure that death becomes an explicit discussion point when patients are likely to die within 12 months, and is in line with one of the central tenets of the End of Life Care Programme, namely that health professionals recognise when patients are likely to be entering the last year of life and ensure an appropriately managed transition to a palliative approach to care. 5 Midway through the current project, the National End of Life Care Programme29 published specific and comprehensive guidelines regarding how to manage palliative care transitions within the context of acute hospital settings. The guidelines advocate ‘good honest communication’, ‘advanced care planning’ and ‘access to tailored information’ as crucial for optimising the provision of palliative care in the hospital setting.
The following are identified as key steps in ensuring a well-managed palliative care transition: recognising when the patient is in the last 12 months of life, understanding that the patient has palliative care needs and building consensus within the clinical team as to how these should be addressed, effectively communicating the team consensus to patients and their families, and ensuring that patients are offered opportunities to express preferences for end-of-life care that are recorded and subsequently acted on.
It is within this context that the present study aimed to address the need to improve palliative care management within acute hospitals through a focus on ‘transitions’. For the purposes of this study we defined ‘transition’ as a change in the approach to a patient’s care from ‘curative treatment’ (in which the focus is on cure or chronic disease management) to ‘palliative care’ [in which the focus is on maximising quality of life (QoL)]. Transitions in care may or may not be associated with a change of care setting. The transition will not be complete or unproblematic in all cases. Indeed, many experts now recommend that curative and palliative approaches to treatment are adopted concurrently,30 particularly for older people31 for whom adopting a transition late in the disease trajectory can lead to ‘missed opportunities for palliation’ (p. 2). 29 How this process is managed within acute hospitals, however, remains unknown.
The extent to which a transition in care setting to the acute hospital corresponds to a transition in care approach is also unclear, although repeat hospitalisations have been identified as a trigger for moving to a palliative approach in certain conditions. 32
This study aimed to clarify these ‘unknowns’ by examining the extent and nature of palliative care transitions within the acute hospital setting. Adopting a proactive approach to palliative care management through a managed transition in care may result in several tangible benefits for both patients and the wider NHS. These include facilitating patient involvement in advance care planning (when desired) and enabling a proactive care plan to be developed. There is also evidence that many more people are dying in acute hospitals than would wish. 33 Not only does this result in people not having the death that they would have chosen, but it also incurs significant unnecessary financial costs for the NHS. Health-care costs are most significant in the last 3 years of life, with high rates of hospitalisation. As such, this study also explored the economic impact of potentially avoidable hospital admissions amongst patients nearing the end of their life.
Research aim and objectives
Research aim
To examine how transitions to a palliative care approach are managed and experienced in acute hospitals and to identify best practice from the perspectives of older patients and key service providers and commissioners.
Research objectives
-
To explore the extent and current management of palliative care need within acute hospitals.
-
To identify patient factors that are predictive of key aspects of palliative care need and, in particular, physical and psychological symptom load.
-
To examine the circumstances under which transitions to a palliative care approach occur within acute hospitals, with a particular focus on the influence of age and disease type on decision-making.
-
To explore how decisions to move to a palliative care approach are made and who is involved in decision-making.
-
To examine if and how information about a transition to a palliative care approach is communicated to patients and their families and how they are involved in decision-making.
-
To explore the perspectives of patients, service providers and commissioners regarding acute hospital admissions and discharges associated with a transition in care.
-
To identify those hospital admissions amongst people with palliative care needs that were avoidable but which occurred because of a lack of alternative service provision or support in the community.
-
To identify patient factors predictive of avoidable hospital admissions.
-
To quantify the cost of avoidable acute hospital admissions amongst those patients with palliative care needs.
Study design
The research aims were addressed using a mixed-methods approach informed by a pragmatic philosophy. 34 Mixed-methods approaches are recommended for the study of complex issues in which multiple but inter-related objectives are being addressed. In this study we used techniques of both complementarity (in which findings from one method are used to elaborate or clarify findings from another method) and development (in which findings from one method are used to inform the other method). 35 The study was conducted in the following phases:
-
phase 1: two systematic literature reviews were conducted to inform data collection and analysis
-
phase 2: exploratory focus groups and interviews were held with 58 key health- and social-care professionals to determine survey methodology
-
phase 3: a survey of palliative care need and management amongst inpatients at the two settings was conducted, which involved collecting information from patients, the key medical and nursing professionals responsible for their care, and patient notes
-
phase 4: in-depth interviews were conducted with 15 patients identified in the survey as having palliative care needs
-
phase 5: 12 months following the survey a review was conducted of the case notes of all hospital inpatients who were present in the two hospitals on the first day of the surveys but who died in the subsequent 12 months
-
phase 6: interviews and focus groups were convened with key health- and social-care professionals and service commissioners/planners to discuss the implications of the study in the two study settings.
Detailed methodological information about each study phase is provided in the relevant chapter.
Study settings
UK National Health Service
The UK National Health Service (NHS) was launched in 1948 and is the world’s largest publicly funded health service. With the exception of charges for some prescriptions and optical and dental services, the NHS remains free at the point of use for anyone who is resident in the UK, currently more than 62 million people. The Department of Health is responsible for the NHS, which is funded centrally from national taxation. NHS services in England, Northern Ireland, Scotland and Wales are managed separately. The NHS employs more than 1.7 million people. 36
Hospital settings
The settings selected for inclusion in this study were two acute NHS hospitals in England: the Sheffield Northern General Hospital (SNGH) and the Royal Lancaster Infirmary (RLI).
These settings were selected because they serve sociodemographically distinct populations. The RLI serves a predominantly white Caucasian semi-rural/remote rural population. By contrast, the SNGH services a largely urban, more economically disadvantaged and ethnically diverse area. A brief overview of the sociodemographic and clinical characteristics of the patient populations and health services of relevance to the study is provided in the following sections to provide context to our findings.
Sheffield Northern General Hospital
The SNGH is the largest hospital campus within the Sheffield Teaching Hospitals NHS Foundation Trust, with approximately 1100 inpatient beds. The catchment population for tertiary referrals is 1.3 million and the local population is 500,000. The results of the 2010/11 national NHS Inpatient Survey37 placed Sheffield Teaching Hospitals in the top 20% of NHS trusts for patient satisfaction. As part of the national Transforming Community Services programme, adult community services, which were part of NHS Sheffield, became part of Sheffield Teaching Hospitals NHS Foundation Trust from 1 April 2011.
Sheffield is predominantly urban, with 98% of the population classified as living in urban settlements. 38 The city has a diverse population both ethnically and socioeconomically, and the black and minority ethnic population in the city has increased significantly since the 2001 census, from around 11% of the total population to 17% in 2009. 39 The closing of the mining and steel industry in the 1980s has left Sheffield with a legacy of extreme socioeconomic variation; 31.8% of the population are classified as being in the most deprived quintile nationally. Life expectancy for people living in the wealthier suburbs is over 10 years more than for those living in poorer areas,40 where there are also high levels of health risk behaviours such as smoking and obesity. Correspondingly, Sheffield has a high incidence of socioeconomic health-related diseases such as cardiovascular disease and type 2 diabetes. In total, 15.5% of the population are aged > 65 years. 38 These National End of Life Care Intelligence Network data reveal that 67% of deaths in Sheffield occur amongst people aged > 75 years and 36% amongst those aged > 85 years. Causes of death are in line with overall trends for England, with the top three leading causes being respiratory (34%), cardiovascular (29%) and cancer (28%). In terms of place of death, 57% of people are classified as dying in hospital, 18% in their own home, 17% in care homes and 5% in hospices, similar to the average figures for England. An average of 15.3 hospital days for admissions ending in death is reported. Total spend on end-of-life care per death and hospice services per death is higher than the national average at £2803 (national average £525) and £2047 (national average £1096) respectively.
In terms of palliative care provision, the SNGH houses an 18-bed specialist palliative care unit and St Luke’s Hospice, in the south of the city, has 20 beds. There is also a 24/7 on-call palliative care service available throughout the trust. On average, the service cares for 800 new patients per year.
Royal Lancaster Infirmary
The RLI is operated by University Hospitals of Morecambe Bay NHS Foundation Trust (UHMBT), which serves the population of South Cumbria and North Lancashire. The trust operates from three main hospital sites – the RLI, Furness General Hospital in Barrow and Westmorland General Hospital in Kendal – serving a population of circa 363,000, spread across an area of over 1000 square miles. It operates a range of acute and emergency services, including a 24-hour emergency department (A&E). End-of-life care intelligence data are provided by the PCT; data for Lancaster are therefore included within the wider Cumbria region. 41 In Cumbria, the population is slightly older than the average for England, with 20% aged > 65 years and 11% aged > 75 years. A 161% increase in the population aged > 85 years is predicted by 2033. Only 48% of the population are classified as living in urban settlements (compared with 81% for England as a whole) and > 99% are white (compared with 90% for England). The population is more affluent than the national average, with only 11% in the most deprived quintile. In total, 36% of deaths occur amongst those aged > 85 years. Place of death is mainly in line with national averages, although there is a slightly lower proportion of hospital deaths (52% in hospital) and a slightly higher proportion of home deaths (23%). In line with Sheffield and England as a whole, the top three leading causes of death are respiratory (31%), cardiovascular (33%) and cancer (28%). 41 The average number of bed-days per hospital admission ending in death is 12.5 (in line with the national average). The total spend on end-of-life care services and hospice services is below the national average at £498 and £359 respectively (compared with £1096 and £525).
Ethical approval
It is a requirement of all research involving NHS patients or staff that ethics approval is granted through the appropriate research ethics committee. National multisite ethical approval for this study was granted by Nottingham 1 Research Ethics Committee and, where required, from the National Information Governance Board (NIGB) Ethics and Confidentiality Committee (ECC). Research governance approvals were gained from the relevant NHS trusts and honorary contracts were issued to all members of the research team (n = 30).
A number of ethical issues were of particular importance. The main ethical issues with regard to the survey related to the discussion of sensitive issues with patients and the possibility of patients becoming distressed. The research team has considerable experience in palliative care research and extensive expertise discussing sensitive issues with patients. The methodology was developed following consultation with service users (see Chapter 2) and professional academics and clinicians to minimise patient burden. The NHS research ethics committee stipulated that the terms ‘palliative’ or ‘end of life’ were not used on any of the patient/carer study materials and were not mentioned verbally in interactions with patients or carers. This was to avoid the potential distress that use of these terms might evoke.
There were also ethical issues surrounding the inclusion of patients lacking the capacity to consent. Much research in palliative care has neglected to include the views and opinions of patients with dementia and other cognitive impairments and therefore we felt that it was imperative to include these patients to capture their perspectives. The core principles of the Mental Capacity Act 200542 informed the research methods so that patients lacking capacity could be appropriately protected but also be given the opportunity to participate. 10 In addition, the data collection team included researchers with specific and extensive experience in dealing with patients lacking capacity.
Ethical issues: the retrospective case note review (phase 6)
We were unable to obtain patient consent to collect data for the retrospective case note review as we were examining notes of patients who had died. Therefore, additional ethical approval was sought from the NIGB ECC under section 251 of the NHS Act 200643 for use of identifiable patient data without patient consent. We recognised this as an ethically contentious issue and as such undertook significant consultation with our service user group and experienced academics and clinicians to inform our application.
Amendments to the protocol
Amendments to the protocol are listed in full on the ‘Record of Reported Changes in SDO Project’ form and are summarised below.
The original protocol aimed to collect data from the general practitioner (GP) of every patient who participated in the hospital survey through a short telephone interview; however, this was not possible because of difficulties with the recruitment of GPs. Although the majority of patients gave consent for the research team to contact their GP, the response from GPs was extremely poor. After contacting approximately 200 GPs by telephone, only 25 agreed to participate. Therefore, data collection was ceased.
Patients were interviewed on only one occasion post discharge from hospital rather than on two occasions as detailed in the original protocol. This was because the majority of the patients interviewed were in poor health and extremely frail at the time of the interview and it was not felt appropriate to approach them a second time following a 6-month interval.
An extra phase of research (retrospective review of case notes) was added to the protocol following completion of the hospital survey. The original protocol aimed to collect data from the medical notes of all hospital inpatients at the time of the survey; however, this approach was not possible because of ethical restrictions preventing collection of data from patients unable or unwilling to providing consent. As objectives 7, 8 and 9 were dependent on collection of data from a complete population of hospital inpatients, the retrospective review was conducted.
The final focus groups held with NHS staff and managers to disseminate the results of the study did not involve any individual interviews, as stated in the original protocol. Although it was anticipated that senior clinicians and stakeholders would not wish to participate in group discussions and we would therefore offer individual interviews, these staff proved keen to discuss the implications of the research with their teams. Interviews were therefore not necessary.
Chapter 2 Service user involvement
The study was underpinned by service user involvement and collaboration. In this chapter we will showcase some of the work undertaken by our service user group and highlight the important role that it played in the success of the research project in terms of improving research methods, gaining ethical approval and outcomes for the study. We also highlight the future role for the group and its planned activities.
Service user involvement in palliative care research
It is now widely accepted that the involvement of consumers or service users can enhance research and practice in palliative care. 44 In line with other areas of health care, the importance of ensuring effective service user involvement in palliative and end-of-life care research is now widely acknowledged. However, user involvement within this context provides a number of unique challenges, particularly because of the potential vulnerability of the service user group in question. Our team has undertaken a significant body of work over the past decade developing models of effective and safe service user involvement in palliative and end-of-life care research. This has involved comprehensively reviewing the evidence base for service user involvement in health research more broadly and drawing implications for palliative care,45 stimulating debate about the theoretical context informing service user involvement in research46,47 and, most importantly, exploring the views of service users themselves about what optimum models of user involvement in palliative care research should constitute. 48–51 We drew on this previous work, in addition to our collective experience of facilitating service user involvement in research in multiple projects in the area of palliative and end-of-life care, to develop a strategy to ensure appropriate and effective service user involvement in the current study.
User involvement
From inception, a key goal of this study was to facilitate the curative involvement of service users in the research process. Service users have had a significant impact on the study from the design stages, through data collection and project management to dissemination. A dedicated service user group was set up in June 2009, which from mid-2011 has been known as the Palliative Care Studies Advisory Group (PCSAG). The group has provided ongoing support to the study and is now consulted by researchers from across the region on a wide range of palliative and end-of-life care projects.
Demand for the group’s input is such that members intend to continue their regular meetings and maintain their involvement in research, with support from our research team at the University of Sheffield.
Project development and design
Before the study was funded, a group of research partners from the Cancer Experiences Collaborative (CECo) was consulted regarding the preliminary proposal. The group provided important feedback, including outlining key ethical challenges from the perspective of service users, and this feedback was incorporated into the final version of the proposal.
Setting up a dedicated user group
Initial meetings of the project steering group identified a range of significant potential ethical challenges in the design of the study. As such, it was proposed that a dedicated group of service users, consumers and research partners be set up to provide consultation and feedback on all aspects of the project, but particularly to assist in identifying and managing ethical issues.
An initial User Consultation Day was arranged for June 2009. The aims of this full-day event were to provide an introduction to the project and generate feedback on some early issues; encourage involvement from a wide range of service users and consumers; and identify individuals who might like to continue to be involved with the project as part of a formal user group. The User Consultation Day was attended by 17 service users, carers and advocates with an interest in palliative and end-of-life care. Attendees were drawn from a range of local, regional and national organisations including the North Trent Cancer Research Panel, CECo, the Alzheimer’s Association, the Older Lesbian and Gay Association, Darnall Dementia and local care homes. Feedback from the day was excellent, and 16 of the attendees expressed an interest in continuing their involvement in the project through formal user group meetings.
Ongoing involvement of the user group
Following the successful User Consultation Day, members of the dedicated user group have met every 6 months to provide consultation to the project. Additional members have joined the group from an older carers research project at the University of Nottingham and from the volunteer service at the Nightingale Macmillan Unit at the Royal Derby Hospital. The group now has around 20 members, with 8–12 members attending each meeting.
The biannual meetings are chaired by Dr Gardiner or Professor Ingleton and have focused on designing materials for the study, discussing difficult ethical issues, providing feedback on findings and assisting in dissemination plans.
Motivations for getting involved
Many members were motivated to become involved because of personal experiences of health or social care, either their own care or that received by loved ones. The following quotes are illustrative of members’ motivations more generally:
As far as research is concerned, it doesn’t matter what the subject is, if you don’t have continual research into it you don’t move forward, you just stand still. This is why I’m prepared to take part. My thoughts are based on my experiences of looking after my wife. If by influencing research I can help somebody in the future, that’s all to the good. I can put something back into society in return for what I got out of it.
Ken Hall, member of the advisory group
It was at the time my wife was in hospital for the umpteenth time because she was ill over 13 years and I got talking to one of the sisters there and she sort of put my name down [laughing]. I really don’t know how it came about but she said would you be interested and . . . I thought I’ll have a go at it. But I’ve found it highly interesting, highly informative and I’m pleased I did.
John White, member of the advisory group
Well I decided to take part in the advisory group because I did have a personal issue. I was diagnosed with breast cancer, and I didn’t feel ill, and it seemed that I must be made very ill in order to get better and I wanted to question this. Why is cancer treatment so arduous, for one thing? And in the interview with my physician, why were my concerns about other health problems just totally dismissed? That wasn’t right, so now I try and bring that sort of perspective into groups I attend.
Also, at first I did think that the glamorous side of research – the expensive new drugs – was the be-all and end-all, but over the 8 years I’ve been involved in research groups, I find that this sort of research which helps patient comfort is much more important and perhaps people should recognise this.
Jacqui Gath, member of the advisory group
Another member of the group, Simon Cork, wrote a short piece about his motivation for and experiences of being involved in the group for a newsletter produced by the School of Nursing at the University of Sheffield. This short commentary is provided in Appendix 1.
Key contributions of the group to the study
The user group has made a number of important contributions to the project.
Commenting on whether research in this area is important
A discussion was had about the value and the merits that the group members found in the proposed research. Members gave examples of both positive and negative experiences of end-of-life care. It was emphasised that a good death should be seen as a success in medical terms. The group feedback was incorporated into the study’s application for ethical approval, and facilitated the approval process.
Developing project information leaflets for distribution to the public and assisting with distribution
The group designed and agreed content for a project information leaflet. Members encouraged a more positive style of language and pointed out areas in which more explanation about the research was needed. The group identified places where the leaflet could usefully be distributed (e.g. day centres, local council offices and Age UK) and many members were involved in distribution.
Identifying ethical problems with or concerns about the study
One of the most important contributions of the advisory group has been in negotiating the ethical challenges of the study. All ethically contentious issues were discussed with the group to inform the study design and to assist with applications submitted to the research ethics committees. The most ethically challenging issue related to gaining approval from a specialist ethics committee to undertake a retrospective medical case note review of patients who had died. An initial application made by the project researchers was rejected on the grounds of the contentious ethical issues. A dedicated meeting of the advisory group was held to gain detailed feedback on the issues raised, which was then incorporated into a revised application. Additional written support from one member of the group was also supplied and a second application to the ethics committee was approved. It is worth highlighting how crucial the input of the advisory group was in securing this ethical approval; without it it is extremely unlikely that a key component of the project would have been able to go ahead.
Commenting and providing feedback on the project website and suggesting content
The group agreed that a project website (see www.transitionstopalliativecare.co.uk; accessed June 2013) was a very good idea as it would help to disseminate information about the study to a wider audience, especially younger people. Suggestions for content included links to other related websites and regular updates on the input of the advisory group.
Contributing to dissemination and distribution of the research findings
To see the results of the research being implemented, the group was keen to play a part in disseminating findings from the study in different forums. Some wanted to do so informally through face-to-face encounters with their peers and with people they came into contact with on a regular basis: ‘That’s far more effective than reading it in a written report in the funder’s office.’ The group also agreed that it was important to see findings disseminated through the mainstream media.
Advising other research projects
In late 2010 the group was approached by a research group from the University of Nottingham and by a researcher at the School of Health and Related Research at the University of Sheffield. These researchers had become aware of the group and asked if members would consider providing advice to their projects. The group now provides consultation to a wide range of palliative care research studies from a range of universities. Projects that the group has offered advice to include a study exploring palliative sedation, a study on end-of-life care communication for patients with respiratory disease and a study on optimising hospital environments for palliative care. The group has also advised on a PhD research project and on a medical student’s research project, directly contributing to the success of these projects and to the awarding of educational qualifications.
The group has offered valuable feedback for researchers. People who use services are able to offer different perspectives on research from those of professional researchers. They can help to ensure that the issues that are identified and prioritised are important to them and therefore to health-care, public health and social-care services as a whole. For researchers, it is easy to get preoccupied with the more technical aspects of conducting research. The user group was able to remind the professionals of the overall purpose of the research – the ‘nuts and bolts’ as one member called it – and why it matters.
Future role for the user group
User group members have been keen to continue their involvement in research after the end of this study. The group, now titled the PCSAG, is working with researchers on palliative and end-of-life care in Sheffield, Nottingham and even as far away as New Zealand. This includes large- and small-scale projects, PhD projects and projects funded by universities, government departments and the third sector. This project has benefitted greatly from the involvement of the group and it is envisaged that other future projects can consult the group and benefit from its feedback.
To promote itself to academic institutions, health-care organisations and the third sector, the group has produced a briefing paper (Figure 1) outlining the background of the group and the important role that it can play in improving research methods and outcomes (see Appendix 2 for the briefing paper).
FIGURE 1.
Palliative Care Studies Advisory Group briefing paper: front cover.

A Service User Involvement in Research Showcase event was held in Sheffield on the 10 July 2012 and was facilitated by Dr Gardiner, Professor Ingleton and Professor Gott, on behalf of the PCSAG. The aim of this full-day event, which was evaluated positively, was to reflect on the role of patient and public involvement (PPI) in research. It focused on the activities and input of the PCSAG in addition to showcasing the role of PPI in other research areas. The showcase event was attended by a wide range of services users, advocates, academics and researchers. A number of presentations were made about service user involvement in research, and during breakout sessions delegates discussed ways in which to disseminate project findings. Delegates suggested publishing a short document describing project findings in a format appropriate for both users and professionals. This document will be produced following submission of this final report and will be disseminated to a wide audience as part of the project dissemination strategy.
Chapter 3 Exploring the transition from curative care to palliative care: a systematic review of the literature (phase 1)
To inform the study, two systematic reviews were conducted of the relevant health- and social-care literature. This chapter presents findings from the reviews and identifies the research evidence base in the following areas:
-
the transition from curative care to palliative care
-
the economic impact of avoidable hospitalisations amongst palliative care patients in the UK.
A systematic review of literature exploring the transition from curative care to palliative care
Background
The transition to palliative care can be a confusing and traumatic time for patients and their families, and may trigger fears of helplessness and abandonment. 52 Although traditionally a sharp transition point has signalled the beginning of palliative care, more recent therapeutic models have described an approach incorporating gradual transitions, emphasising palliative input and QoL considerations during the active phase. 53 A phased transition or simultaneous care approach recognises that treatment goals evolve and that concurrent curative and palliative care may be most appropriate. 25
A scoping review by Marsella54 identified three key elements that complicate the transition to palliative care. First is the nature of the transition and what it means to patients. Second, transitions can be difficult because of a lack of time to appropriately prepare patients and families. Lastly, a lack of information regarding the goals of palliative care can lead to confusion and complications. Schofield and colleagues55 undertook a literature review as the basis for outlining steps for facilitating the transition to palliative care. Although these steps provide useful recommendations, the authors acknowledge a paucity of research in the area and fail to address the impact of variations in health-care systems and resources in cross-national literature.
Evidence also suggests a lack of concordance with respect to triggers indicating the appropriateness of a transition to palliative care. Although policy guidelines advocate the use of the ‘12 months’ question as an indicator that patients may require palliative care input, recent evidence suggests that this question may not be appropriate for patients with non-cancer diagnoses. 56
The Gold Standards Framework (GSF)57 suggests the use of a prognostic indicator guide to identify patients predicted to be in the final 12 months of life who might be in need of palliative care. However, implementation is variable and the direct impact on patients and carers is not known. 58
Review methods
A systematic review of qualitative and quantitative literature was undertaken to explore evidence relating to transitions to palliative care in the UK. The review was undertaken in the following five stages: (1) search strategy, (2) inclusion criteria, (3) assessment of relevance, (4) data extraction and appraisal and (5) data synthesis using a descriptive thematic model.
Search strategy
The aim of the search was to identify a comprehensive list of published papers that met predefined inclusion criteria. Medical subject headings (MeSH) (‘palliative care’, ‘terminal care’, ‘hospice care’) and keywords (‘supportive care’, ‘end of life care’, ‘transition’, ‘continuity’) were identified and relevant databases were selected and searched in consultation with a health-care information management specialist. The following databases were searched for literature published between 1975 and March 2010: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews (CDSR) and NHS Economic Evaluation Database (NHS EED) (see Appendix 3 for the search strategies). The following journals were hand-searched for relevant articles: Palliative Medicine, Journal of Palliative Care, Supportive Care in Cancer and Journal of Advanced Nursing. Relevant references from bibliographies and through citation indices were followed up. Grey literature searches were conducted through the above databases, through consultation with expert colleagues from the wider study steering group (n = 10) and through internet search engines.
Inclusion criteria
Inclusion criteria were developed by consensus within the research team. Literature had to refer to the transition from active or curative care to care incorporating a palliative approach. Literature also had to refer to an adult population (aged > 18 years) and be UK based (as variations in health-care systems and resourcing worldwide mean that the relevance of international papers to the UK is likely to be limited). All types of published literature were eligible for inclusion, including grey literature.
Assessment of relevance
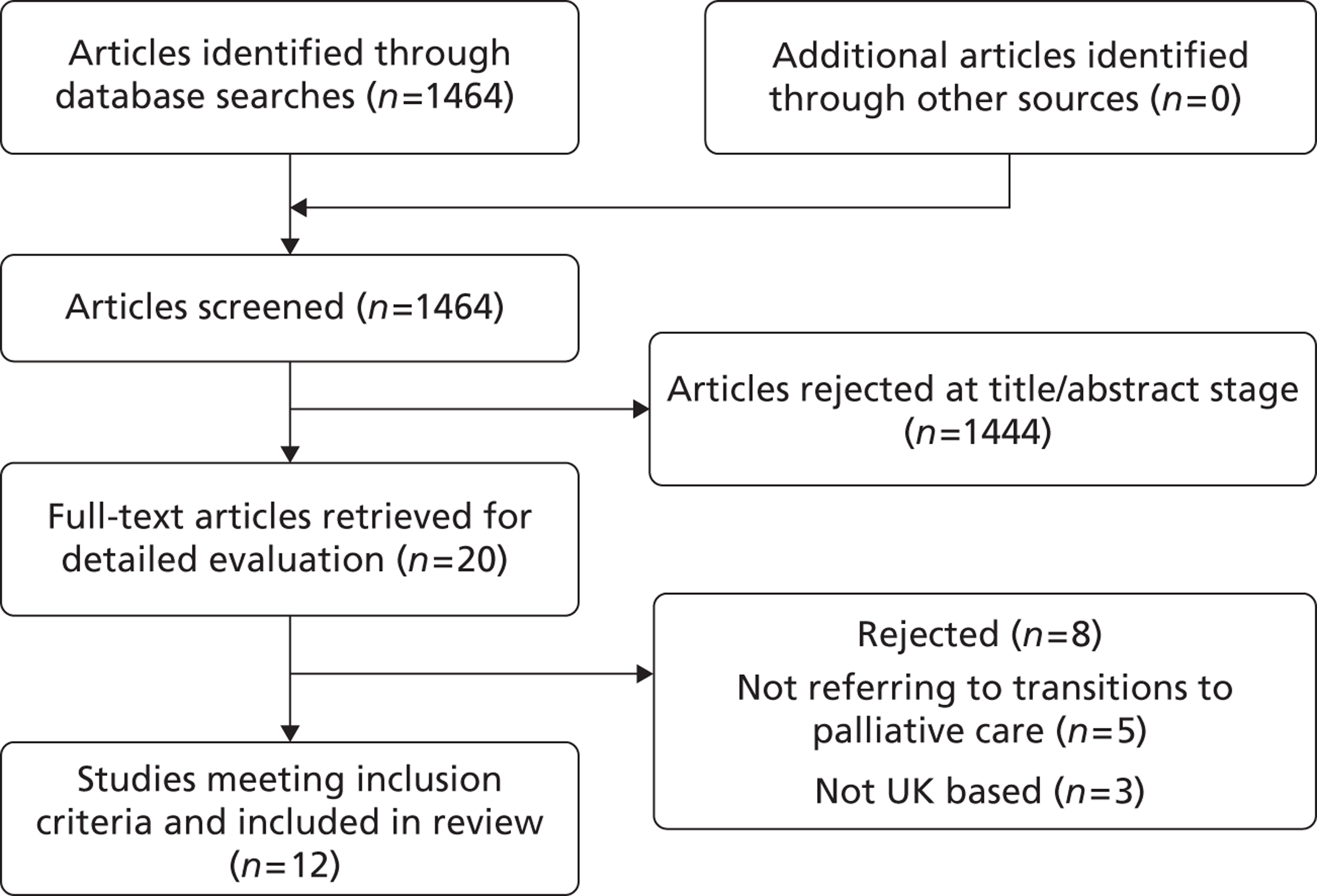
Study selection was conducted in a systematic sifting process over three stages: on the title, abstract and full text. Details of the study identification and selection process are shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart in Figure 2. At each stage studies were rejected that definitely did not meet the inclusion criteria. Each paper was independently assessed by CG and one of the other authors (CI, MG or TR); in cases in which there was disagreement between researchers, consensus was reached by discussion.
FIGURE 2.
Flow chart of included literature.
Data extraction, appraisal and synthesis
The review was conducted using a descriptive thematic method for systematically reviewing and synthesising research that employed different research methods and modes of analysis. The thematic approach was data driven and all data relating to transitions from curative care to palliative care were extracted from papers. A checklist adapted from Hawker and colleagues59 was used as the basis for extraction and appraisal. Double data extraction was performed independently on all included studies by two authors (CG and MG, TR or CI).
Quality assessment was carried out using a range of quality indicators of rigour,60 which varied by study type. A score was calculated for each paper based on the scores for each item on the checklist. Scores ranged from 9 (very poor) to 36 (good) and indicate the methodological rigour for each paper. As each paper was assessed by two researchers, a mean score for each paper was calculated. Because of the diverse nature of the included studies, statistical synthesis or analysis of study findings was not appropriate. Quality assessment for non-empirical papers was undertaken according to the Joanna Briggs Institute (JBI) Narrative, Opinion and Text Assessment and Review Instrument (NOTARI) tool for assessment of expert opinion. This tool does not generate a score for methodological rigour and, although findings from non-empirical papers must be considered with caution, expert opinion papers are included in this review to minimise exclusion of relevant context.
Results
Of 1464 citations initially identified, 12 articles61–72 (relating to 11 studies) met the inclusion criteria. Articles that were excluded (n = 1452) were not relevant to the research aim or were not UK papers (Figure 2). One paper included both UK and European data and was included. 61 Eight of the included articles were qualitative studies of patients, carers or health professionals. 61–67,72 One study was a mixed-methods comparative cohort study,68 one a case study report,69 one a critical discourse analysis70 and one a non-empirical discussion piece. 71 Transition to palliative care was the main focus in only two of the included papers;61,72 the remainder referred to transition only as a minor theme or as a component of the discussion.
Most papers scored satisfactorily on assessment of methodological rigour, with no paper scoring < 28 out of a maximum of 36. One paper71 could not be scored as it was a non-empirical expert opinion piece; therefore, findings from this paper should be considered with caution. Details of the 12 papers and assessment scores are provided in Table 1.
The thematic synthesis of evidence led to the emergence of four main themes: (1) patient and carer experiences of transitions, (2) recognition and identification of the transition phase, and criteria for making transitions, (3) optimising and improving transitions and (4) defining and conceptualising transitions.
Patient and carer experiences of transitions
Of the 12 papers included in the review, nine61–68,72 provided evidence or discussion of patient and carer experiences of transitions. The overwhelming consensus was of fear and uncertainty when making the transition to palliative care. Larkin and colleagues61,72 reported how cancer patients described a variety of emotional responses reflecting fears and losses. Patients found transitions confusing because of mixed messages, poor communication and uncertainty. They described having limited knowledge about the purpose and timing of transitions, feeling uncertainty about who instigated the transition, having limited involvement in decision-making and, once transferred to palliative care services, feeling a sense of waiting for something to happen. Patients also reported that hospitals can provide unrealistic information about the level of service available for patients on transitioning to palliative care. This finding resonated with health professionals who reported that patients’ expectations may be unrealistic regarding the care that can be delivered.
Patient concerns were also identified in a qualitative study of lung cancer patients, who reported that they felt particularly unsafe in periods between curative treatment and follow-up appointments. 63 They also felt ill-prepared for discharge from curative care and detected inadequacies in interprofessional communication. Communication was also highlighted as an important issue in a qualitative interview study of patients receiving specialist palliative care. 64 Patients in this study described uncertainties about the extent and nature of inter- and intraprofessional communication and described having to relay information themselves between different professionals involved in their care. Patients and carers described continuity of care as a key component for improving the experience of transition to palliative care; continuity appears critical to satisfaction with care and services. However, it is clear that complexities may occur and continuity may be disrupted when many agencies are involved in providing care for an individual.
Recognition and identification of the transition phase
Recognition of the palliative care transition phase by health- and social-care professionals was identified as an important factor for facilitating optimum care. O’Leary and colleagues68 reported that early recognition of the palliative care transition point was key to ensuring that end-of-life issues were addressed. In a study by Bestall and colleagues,65 primary care professionals described how late recognition of palliative care need, and referrals at a late stage, could have a negative impact on patients and on their relatives during bereavement. However, it was acknowledged that a clear-cut transition to a palliative care approach was rare. Particular challenges exist when identifying the transition in non-cancer conditions such as heart failure, in which the episodic nature of the condition can lead to a delayed recognition of the palliative transition.
Four papers made suggestions for criteria to identify the transition to palliative care. 61,65,68,70 O’Leary and colleagues68 listed factors defining the palliative transition point in heart failure, including deterioration despite optimum support, increasing fatigue or functional dependence, low ejection fraction, recurring hospitalisations, emotional distress, carer fatigue and patient request. Bestall and colleagues65 explored reasons for referral to specialist palliative care for both cancer and non-cancer conditions and highlight a lack of standardised criteria in the UK to determine when a referral should be triggered. Referral criteria identified in this study included complex symptoms, problems with medication side effects, complex social or practical issues, carer burnout, and emotional distress. Health professionals discussed the use of referral criteria such as the Leeds Eligibility Criteria73 or locally developed guidelines, but most would have liked further guidance about when and how to refer patients to specialist palliative care. Cancer patients interviewed in a study by Larkin and colleagues61 described how a rapid deterioration resulting in loss of independence was a primary reason for a transition to palliative care. Some respondents reported that a decision to move to palliative care was based on an evaluation of their potential burden to others rather than on personal choice.
Optimising and improving transitions
The majority of studies acknowledged that the transition to palliative care could be improved. As early as 1978, researchers identified the importance of continuing care after the cessation of curative treatment. 69 However, only four papers made any specific recommendations or developed any guidelines for improving the transition. 62,67,68,70
O’Leary and colleagues68 discussed how the optimum transition should encompass planned and integrated transfer of patient information, the reiteration of patient preferences and the renegotiation of care goals. Recognition of the transition point was identified as key so that a collaborative care plan can be established, ensuring the most appropriate level of care. In addition, improvements to services such as respite and out-of-hours care were also identified as a requirement for optimum transition. 62 Kendall and colleagues67 developed recommendations for the care of patients with cancer in primary care after discussion with patient groups. Patients and carers outlined an important and unique role for primary care staff throughout the cancer trajectory. Continuity of care and an individualised approach were considered crucial to driving patient-centred care forwards. Recommendations given for managing the recurrence of cancer and the last weeks included letting patients express their concerns, helping with social and practical issues, respecting patients’ values and choices and supporting carers, frequently reviewing and co-ordinating care, and being flexible and responsive. Continuity of care was also highlighted as a crucial factor by patients and staff in a study by Patrick and colleagues. 62 Continuity appeared critical to overall satisfaction and was particularly important during the transition when many agencies were involved in an individual package of care. Researchers in critical care also identified individualised assessment as important, and again highlighted a need for comprehensive collaboration. 70 Although it is acknowledged that transitions in critical care may be very different from transitions in other care settings, many of the care goals and recommendations are similar.
Defining and conceptualising transitions
Defining the concept of a transition to palliative care remains a challenge. In health care, transitions may include changes in the place of care, the caregiver or the goals of care. However, transition in the palliative care literature goes further than just change in place or caregiver. It also relates to the personal meaning of life, life/role changes, perceptions of end of treatment, and likelihood of death. Understanding what this transition entails is necessary for facilitating end-of-life care. 68 A study by Larkin and colleagues61 explored the experiences and meaning of transition for a group of palliative care patients. Although they reported that the successful merging of the curative–palliative interface was beneficial for patients, they suggest that the concept of transition warrants further investigation. The authors describe how transition literature often describes overtly positive outcomes such as resilience, reconstruction, coherence, life purpose, sense of self, transcendence and transformation, whereas interview data from patients do not always match these descriptions. Transience is suggested as an alternative concept and is further explored in a second study. 72
| Author and year | Aim | Participants | Setting | Method | Relevant findings |
|---|---|---|---|---|---|
| Bestall 200465 | To explore the reasons why patients and families are
referred to SPC Assessment score = 31 |
13 patients referred to SPC (cancer and non-cancer diagnoses), 12 professionals working in SPC, 3 GPs, 6 community nurses | North Trent Cancer Network, England | Qualitative semistructured interviews and content analysis | Five key themes reported: reasons for referral to SPC, reasons against referral to SPC, timeliness of referrals, continuity of care, use of referral criteria. Currently no standardised criteria in the UK to determine when a referral to SPC should be triggered. Referral criteria outlined and include complex symptoms, use of referral guidelines. Development of referral criteria may aid transition to SPC |
| Jarrett 200964 | To investigate patients’ perceptions of IIPC in
a SPC setting Assessment score = 32 |
22 patients receiving SPC input (21 cancer patients and 1 multiple sclerosis patient) | Two SPC units, England | Qualitative in-depth interviews, grounded theory analysis of transcripts | Patients largely positive about IIPC when it occurred, some patients uncertain about extent and nature of IIPC, some patients described relaying information between different professionals or care locations and some patients and families very proactive to enhance IIPC and continuity of care |
| Kendall 200667 | To involve patients with cancer and their carers in
designing a framework for providing effective cancer
care in primary care Assessment score = 32 |
18 patients/carers with cancer and 16 professionals involved in cancer care | South-east and south-west of Scotland | Action research model involving two patient/carer discussion groups who met monthly over a year and interviews with professionals | Five key points during cancer trajectory have particular significance: diagnosis, treatment, after discharge, recurrence and the final weeks. Important role for primary care acknowledged throughout cancer trajectory. Support from primary care beneficial during transition from remission to recurrence to final weeks. Continuity of care and an individualised approach are crucial |
| Krishnasamy 200763 | To explore patients’ and family members’
experiences of care provision after a diagnosis of lung
cancer Assessment score = 31 |
23 lung cancer patients and 15 carers | Tayside, Scotland | Qualitative in-depth longitudinal interview study involving three interviews over a 6-month period | Four key domains of need apparent: pathway to confirmation of diagnosis; communication of diagnosis, treatment options and prognosis; provision of co-ordinated care; and support away from acute services including difficulties transitioning between services. Patients felt particularly unsafe in periods in between treatment and follow-up appointments and felt ill prepared for discharge or detected inadequacies in primary/secondary care communication. Many patients relied on relationship with their hospital consultant and found it difficult transitioning into palliative care |
| Larkin 200761 | To document palliative care patients’
experiences at the palliative–terminal
interface; to identify perceived supportive and
inhibitory factors; and to analyse common experiences in
the context of current palliative care development in
European terms as a means to inform
practice Assessment score = 30.5 |
100 advanced cancer patients | Palliative care centres in six European countries (UK, Ireland, Spain, Netherlands, Italy, Switzerland) | Phenomenological approach using semistructured interviews | Transition is a confusing time for patients because of mixed messages and poor communication and uncertainty; physical environment of the hospice offers a place of security to address this. Transition concepts fail to capture the palliative care experience fully and warrant further exploration. Transience is reported as an alternative concept, although more research is needed. Successful merging of curative–palliative interface is beneficial to patients. Clinicians need to ensure a seamless transition as proposed as a key construct of palliative care |
| Larkin 200772 | To support, define and consolidate the emerging concept
of transience and to critically appraise how far
qualitative approaches fit the examination of transience
as a concept and its potential importance to palliative
care Assessment score = 28.3 |
100 advanced cancer patients | Palliative care centres in six European countries (as above) | Qualitative conceptual evaluation using two case examples from interview data (see Larkin57) and a critical review of the literature | Transience is proposed as a preferred concept to transition in relation to palliative care. Transience encompasses attributes such as fragility, impermanence and stasis, which are not adequately explained by the transition concept. More evidence is needed before transience can be described as a well-defined and robust concept for palliative care but data from case studies support concept of transience |
| Murray 200766 | To identify and compare changes in psychological, social
and spiritual needs of people with end-stage disease
during the last year of life Assessment score = 35.5 |
24 patients with lung cancer and 24 with heart failure | Primary and secondary care in south-east Scotland | Data synthesis from two longitudinal, qualitative in-depth interview studies. Thematic and narrative analysis of transcripts | Characteristic social, psychological and spiritual trajectories were discernible. Lung cancer patients reported particular distress at transition points including after treatment – ‘returning to their old life’. They also experienced difficulties at relapse/disease progression – for some engaging in a battle with the cancer gave them some sense of purpose. In terminal phase patients had overwhelming uncertainty, panic attacks, etc. In heart failure, social and psychological deterioration ran in parallel with physical deterioration and were mediated by this. Spiritual distress fluctuated more and was modulated by other factors |
| O’Leary 200968 | To demonstrate whether the palliative care needs of
patients with advanced heart failure receiving
specialist multidisciplinary co-ordinated care are
similar to those of cancer patients deemed to have
specialist palliative care needs Assessment score = 28.5 |
50 heart failure patients (NYHA stage III/IV) and 50 cancer patients (newly referred to SPC) | Outpatient heart failure disease management clinic and SPC home service in England | Cross-sectional comparative cohort study using quantitative and qualitative methods to explore functional status, symptom burden, emotional well-being, QoL and information and communication needs | Heart failure and cancer patients similar in terms of symptom burden, emotional well-being and QoL. Heart failure patients should not be excluded from SPC services; however, many needs can be met at a specialist heart failure unit. Recognition of palliative transition point may be key to ensuring that end-of-life issues are addressed. Various factors defining the transition point in heart failure are listed. Understanding the concept of transition can facilitate end-of-life care |
| Patrick 200762 | To learn about the quality of local services from the
perspective of patients, carers and staff and to develop
an appropriate methodology for future
consultation Assessment score = 32 |
10 palliative care service users/carers and 9 staff | Palliative care services (hospice, community and hospital) in Kent, England | Semistructured interviews with patients and focus groups with staff. Content analysis of transcripts | Continuity of care important and complex when many agencies involved in an individual package of care. Continuity critical to participants’ overall level of satisfaction with the service provided. Staff had concerns that patients’ expectations are beyond what they can deliver. Hospitals give unrealistic expectations about the level of service in the community, e.g. out-of-hours and respite care services. More respite and out-of-hours medication services needed |
| Pattison 200471 | Discussion paper on the integration of critical care and
palliative care at end of life No assessment score (non-empirical paper) |
N/A | UK | Discussion paper drawing from several literature sources. | Discussion of the difficulties faced when patients transition from curative treatment to palliative care. Transition must not emphasize a dichotomy between cure and palliative care. Nurses can potentially be excluded from decisions regarding a transition and may not be in control when the change of goal takes place. Transitions can be fragmented & comprehensive collaboration is required, patients must not be reduced to a prognostic probability |
| Pattison 200670 | To explore written guidelines and documents for critical
care as evidence for the provision of end-of-life care
in critical care Assessment score = 34.2 |
N/A | UK | Critical discourse analysis of four key UK government critical care documents | Little clear guidance about how to provide end-of-life care in critical care. Transitions to end-of-life care in critical care are often discussed within the context of a transition in physical location; this defines a very definite transition point. In addition, patients can deteriorate very quickly and the transition from curative to palliative care may be rapid. Dying in critical care may infringe dignity. Transition away from interventions to comfort measures can improve dignity |
| Wills 197869 | Case study summary of the first year of a Macmillan
continuing care unit for patients with malignant
disease Assessment score = 17.5 |
71 cancer patients referred to the unit in its first year of opening | Continuing care unit at an acute hospital in West Sussex, England | Case study of routinely collected data | The unit has the unique ability to co-ordinate with community- and hospital-based services. Importance of continuing care after cessation of curative treatment acknowledged and achieved by regular home visits. As a consequence, good relationships were built up, inpatient duration was reduced and more effective episode care was made possible |
Conclusions
Recent UK policy has stressed the importance of managing and facilitating the transition from curative care to palliative care. This review of the literature suggests that, within a UK context, little is known about this potentially complex transition, and literature relating to the optimisation of the transition is sparse. As such, the review attempts to identify important issues for the conceptualisation and optimisation of transitions to palliative care. It is clear that such transitions can be a confusing and distressing time for patients and their families. They can leave patients and their families feeling abandoned and lacking a clear understanding of their future care and treatment options. Facilitating a sensitive transition is therefore imperative for improving the experiences of patients and their families at this difficult time.
The current review identified only four papers that include suggested criteria for identifying the transition to palliative care, and none has been formally evaluated or validated. Further indicators have recently been proposed by Boyd and Murray,30 taking into account a review of prognostic models and guidelines. They propose that clinical judgement informed by evidence, rather than more refined prognostic accuracy, is the key to an earlier identification of patients with palliative care needs. There is a clear need for further formal validation of proposed indicators if a model of best practice is to be identified. Internationally developed indicators such as the US National Hospice and Palliative Care Organization tool should also be considered. However, the organisation and resourcing of palliative care services in the USA and elsewhere may define a sharper transition to palliative care accompanied by an immediate cessation of curative care, thus reducing the appropriateness of US models for a UK health-care system.
Recommendations for optimising or improving the transition to palliative care are similarly sparse, despite recognition that patients and their families often experience a poor transition in their care. A key challenge is sensitively managing the often abrupt change in care provider, care location and care goal that has traditionally accompanied a referral to specialist palliative care services. The abruptness of these changes can lead to patients and their families feeling confused and abandoned, and recommendations highlight a need for collaborative working and continuity of care during the transition. 62,67,68 Evidence suggests that continuity of care is crucial to achieving a sensitive and well-managed transition. UK strategies currently place emphasis on improving the palliative care that is delivered by generalist providers (primary care teams, hospital staff, social-care services),30 who are often well placed, because of their long-standing relationships with patients, to provide high-quality palliative care whilst retaining continuity of care. However, it is likely that they will need support from specialist palliative care services and close collaborative working between care providers is necessary so that patients with a need for palliative input are identified whilst disease-modifying treatments continue.
Many patients may stand to benefit from better identification, assessment and management of the transition to palliative care. Research is required to further explore these issues, particularly in light of evidence which suggests that some patients may be reluctant to receive information relating to a poor prognosis or ‘bad news’. 74,75 A phased transition incorporating palliative care in parallel with disease-modifying treatments appears the most appropriate model for optimising transitions. This model is particularly relevant for patients with non-cancer disease, whose condition may be more slowly progressive, or with fluctuating trajectories. Within this phased transition, continuity of care and multidisciplinary collaboration are crucial. An agreed consensus of definition, and potential refinements to the concept of ‘transition’, may also be necessary to enhance consistency. Further research is required, taking into account UK policy and guidance, to maximise current resources and develop appropriate guidelines and care models for managing the transition from curative care to palliative care.
A narrative literature review of the evidence regarding the economic impact of avoidable hospitalisations amongst palliative care patients in the UK
Background
The need to more fully understand the economics of palliative care provision is widely acknowledged. 76 One area in which it has been suggested that costs could be saved, or at least more appropriately distributed, relates to place of care in the last 12 months of life. For example, the second annual report on progress in implementing the end-of-life care strategy for England notes that maximising scarce health-care resources must be a priority given the current economic climate. 77 It is also suggested that, in end-of-life care, such economic rationalisation is linked to improving quality because most people would prefer to die in their own home, and, it is suggested, high-quality community-based services cost no more, and can cost less, than hospital-based care. Currently, around 90% of people spend time in hospital in their final year of life, and the total cost of non-elective final finished consultant episodes ending in death amounts to around £750M per annum. By improving or expanding community services to allow more people to be cared for and to die at home, a proportion of these costs could be avoided.
However, such assumptions should be considered within the context of the increasing body of work being undertaken on ‘avoidable hospitalisations’. In the international health policy literature, the terms ‘preventable hospitalisation’ and ‘avoidable hospitalisation’ are used interchangeably to denote an admission or readmission relating to an exacerbation or acute illness episode associated with a chronic health condition that could otherwise have been avoided through health promotion, preventative measure, or timely access to primary health care. 78
A recent systematic review of the literature aimed at identifying factors predicting preventable hospitalisations within the context of chronic conditions identified a lack of clarity relating to both scope and solutions, therefore limiting its ability to inform practice and policy. 78 However, the evidence base regarding the extent of potentially avoidable admissions for patients in the last year of life, and in particular the link with reducing/redistributing overall costs for end-of-life care, has not previously been systematically evaluated.
With this context in mind, we present a narrative literature review, systematic in nature, of UK evidence regarding the potential economic impact of reducing hospital admissions amongst patients with palliative care needs.
The review aimed to identify studies that estimated or directly calculated the economic impact of avoidable admissions, or which reported the impact on hospital admissions or inpatient usage of introducing new palliative care services to support patients in the community. The review focused on evidence from the UK on the basis that international variations in health systems and funding are likely to influence the economic consequences of avoidable admissions and therefore conclusions drawn from other countries may not be generalisable to the UK setting.
Methods
The literature search included a search of 10 electronic databases using search terms devised in consultation with a specialist librarian; examination of a number of papers that had been recommended by colleagues; and a hand search of reference lists from relevant papers. Databases searched were MEDLINE, EMBASE, CDSR, Cochrane Central Register of Controlled Trials, Health Technology Assessment (HTA), NHS EED, BIOSIS, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO and Web of Science. The databases were searched from inception to April 2010. Key search terms used to identify studies reporting on avoidable hospital admissions included ‘hospitalisation’, ‘length of stay’, ‘hospital stay’ and ‘inpatient’. Palliative care was identified using terms including ‘palliative care’, ‘hospice care’, ‘life support care’, ‘terminal care’ and ‘home care services’. End-of-life care was identified using terms including ‘end of life’, ‘terminally ill’ and ‘last year of life’. These terms were combined with an economics filter to identify studies reporting the economic consequences of avoidable admissions.
Inclusion/exclusion criteria
Papers were included if they were concerned with an adult population with palliative care needs at the end of life. Only English-language articles were considered. Studies were excluded if they did not provide an estimate of the proportion of avoidable hospital admissions or report on the impact on hospital admissions or inpatient usage (in terms of resource use or costs) of the introduction of new palliative care interventions.
Study selection
Study selection was conducted in a systematic sifting process over three stages: on the title, abstract and full text. At each stage, studies were rejected that definitely did not meet the inclusion criteria.
Results
The electronic search identified 346 unique papers of which 339 were excluded at the title and abstract stage. Of the remaining seven papers only one79 met the inclusion criteria. Four studies80–84 were identified through other means. A total of six papers describing five studies were therefore included within the review.
A summary of the studies is given in Table 2. Two80,81 of the five studies were randomised controlled trials (RCTs) comparing the addition of new services to support patients in the community at the end of life with the existing standard service provided for these patients. These papers reported on the impact on hospital admissions and/or inpatient usage of the provision of these additional services for terminally ill patients and hence indicate the scale and economic consequences of admissions avoided following the provision of improved community services. A third study82 was a descriptive analysis of the change in usage and costs of health-care services following the introduction of two community-based services in Boston, Lincolnshire, as part of the Marie Curie Delivering Choice Programme to develop services that allow people to be looked after and die in the place of their choice. The final two studies79,83 were retrospective analyses of patient records for patients who had died in hospital to estimate the proportion of final admissions that could be classified as avoidable.
| Author and year | Setting and intervention | Type of study | Study population | Time frame of analysis | Impact: hospital admissions/length of stay | Impact: NHS costs |
|---|---|---|---|---|---|---|
| RCTs | ||||||
| Grande 1999,84 Grande 200081 | Former Cambridge health district, UK Hospital at home (HAH) service, providing 24-hour nursing care in the last 2 weeks of life (or occasionally for respite care) |
RCT of HAH vs. standard care | Cancer (87%), motor neurone disease and AIDS HAH (allocated) 168, HAH (admitted to service) 113, control group 43 |
2 weeks | HAH and control groups did not differ in amount of input from secondary care service in final 2 weeks of life (no figures reported) | |
| Raftery 199680 | Wandsworth Health Authority, South London, April
1987–June 1990 Two nurse co-ordinators |
RCT of two nurse co-ordinators vs. standard care | Cancer, with < 1 year to
live Co-ordination group 86, control group 81 |
Patients followed until death. Proportion of patients died before end of study: co-ordination group 67%, control group 79% | No. of admissions: intervention 2.5, control 3.3;
p = not
significant Mean no. of inpatient days: intervention 24, control 40; p = 0.002 |
Average cost per patient: reduction of 41% (£4774 vs. £8034) of which 75% was inpatient costs |
| Non-RCTs | ||||||
| Abel 200979 | South West England Hypothetical: full implementation of the End of Life Care Strategy for England5 |
Review of hospital records at district general hospital | Adults with multiple conditions | Final hospital admissions of patients, assessed as being in last year of life, dying in district general hospital between June 2006 and May 2007 | All patients: avoidable admissions: up to 33% (20% clearly,
13% probably) Patients classified as having < 12 months to live: avoidable admissions: up to 80% (59% clearly, 20% probably) |
Potential cost saving per hospital admission avoided: £3173. Mean length of stay 12 days. Implied cost per day of £264 |
| Addicott 200882 | Boston, Lincolnshire, UK, 2006/7 Rapid response team and two discharge community nurses |
Descriptive analysis of before-and-after programme implementation | Adults with cancer | Last 8 weeks of life | No difference in average no. of bed-days per admission
before and after implementation Non-statistically significant fall in average no. of admissions per patient |
No cost savings. Average cost of inpatient admissions: before services: £3066; after introduction of services: £3019 (£3067 for patients who accessed the services) |
| Balance of Care Group 200883 | Sheffield, England Hypothetical: assumed high-quality community services with unlimited capacity |
Review of hospital medical records | Adults with multiple conditions | Patients dying in Sheffield PCT during October 2007 | Avoidable admissions: 40%. Alternative places of care: 50% home, care home; 50% hospice |
Cost saving from avoidable admissions £375,250 in 1
month in Sheffield. Equates to £450M per year for
England Average cost saving of £4690 per patient. Estimated cost saving based on cost of £250 per inpatient day Mean length of stay approx. 19 days |
Evidence regarding the scale of avoidable admissions
Neither RCT demonstrated a significant reduction in the number of hospital admissions following implementation of new community services. Raftery and colleagues80 did, however, report a significantly lower number of total inpatient days for the co-ordinated group than for the control group (mean number of inpatient days: 24 vs. 40, p = 0.002). Grande and colleagues81 reported that the hospital at home group and the control group did not differ significantly in the proportion who spent time at home during their final 2 weeks (77% vs. 82%, p = 0.455) and did not differ in the amount of input from secondary care services in the last 2 weeks of life. The number of admissions and average number of inpatient days were not reported.
The two retrospective studies reported a high level of potentially avoidable admissions. Abel and colleagues79 concluded that up to one-third of final admissions were avoidable; at least 20% were defined as ‘clearly avoidable’ and 13% as ‘probably avoidable’. These assessments were based on the assumption that the End of Life Care Strategy for England5 was fully implemented and services always had capacity. For the subset of patients (n = 152) who were also classified as being in the last year of life, 90 (59%) admissions were identified as ‘clearly avoidable’ and 31 (20%) as ‘probably avoidable’. The Balance of Care Group study83 concluded that 40% of admissions for patients who died in hospital over a 1-month period were avoidable, and that these patients did not have medical needs that required them to be treated in hospital. Potential alternative places of care were identified for these patients and were equally split between home-based alternatives (in a patient’s own home or a care home) and bed-based care (in a hospice).
The final study reported a non-statistically significant decline in average number of admissions per patient following implementation of two new community services compared with the previous 2 years. 82 There was no significant difference in the average number of bed-days per admission compared with previous years. For patients accessing the new services the decline in average number of admissions was greater than when considering all patients, but the average length of stay per admission for patients accessing the new services increased compared with previous years.
Evidence regarding the potential cost impact of avoidable admissions
Of the two RCTs, Grande and colleagues81 reported no change in resource use and hence costs of secondary care services. In contrast, Raftery and colleagues80 reported a 40% lower mean cost per patient receiving co-ordinated care compared with control group patients (£4774 vs. £8034, p = 0.006). Although the number of admissions did not decline significantly, the mean number of days spent in hospital almost halved, and this accounted for most of this cost saving. The costs of inpatient days, outpatient and day-care attendances and home visits were presented, with inpatient care accounting for approximately 75% of costs.
Addicott and Dewar82 reported a neutral cost impact. The average cost of acute hospital care per patient was almost unchanged following implementation of the new services, with post-implementation costs of £3019 compared with £3267 and £3060 in the previous 2 years. The impact on community service costs was also neutral with increased planned community support provided after programme implementation offset by a reduction in unplanned community support resource use, such as GP visits, 999 ambulance journeys and out-of-hours visits. A small proportion of patients with complex needs was found to be very expensive to support in the community. Of the sample of 40 patients who accessed the new services, 25% had community support costs of > £4000, with the most expensive patient costing > £35,000 to support in the community.
The two retrospective studies reported on the savings from avoidable admissions without taking into account the additional costs that might be imposed on other services, including hospice and community services required to support patients in other locations.
Abel and colleagues79 estimated the cost saving per avoidable admission as £3129 per patient, based on a mean length of stay of 12 days and using Healthcare Resource Group (HRG) tariffs (published by the DoH) to estimate the cost per admission. HRG tariffs were available for only 38% of patients but based on these tariffs the mean cost of an admission was £3173, an implied cost of £264 per inpatient day. Mean costs for subgroups based on the classification of appropriateness of staying at home were similar. For the 33% of all admissions classified as avoidable, this amounted to an annual cost saving of up to £612,000 for one district general hospital. This implies a cost saving of around £282,000 in relation to the 152 patients who were classified as being clearly in the last 12 months of life. The Balance of Care Group83 reported a higher cost saving per admission. It estimated costs for the 40% of all admissions classified as avoidable, based on an average cost per inpatient day of £250, in line with the payment by results tariffs for excess bed-days. Based on the average length of stay of around 19 days for avoidable admissions, the potential cost saving from avoidable admissions amounted to £375,250 over a 1-month period, an average of £4690 per patient. By scaling this up to a national basis, the authors concluded that this amounted to avoidable costs of £450M per year for England.
Discussion
The evidence on the economic impact of avoidable hospital admissions for patients at the end of life in the UK is limited and contradictory.
The overall cost impact of avoidable admissions should take into account not only the cost savings from reduced inpatient usage but also the cost of supporting patients in alternative locations (their own home, care homes, etc.). The two retrospective studies considered only the cost savings from admissions labelled as avoidable. 84,85 Of the remaining studies, that by Raftery and colleagues80 was the only study to report a cost saving, a reduction in the mean cost per patient of £3260. In the more recent study by Addicott and Dewar,82 the cost of acute care and the total costs of community care remained largely unchanged. Raftery and colleagues80 reported on a more limited range of costs than Addicott and Dewar,82 reporting only inpatient days, outpatient and day-care attendances and home visits. GP and social services costs were reported as showing no differences between the groups. The difference in findings may in part be because a broader range of costs was collected in the study by Addicott and Dewar,82 including agency day and night cover, health-care assistant support, ambulance discharge and after-hours paramedic services. However, the largest cost reductions in this study were seen in relation to the reduction in GP visits, in direct contrast to the study by Raftery and colleagues,80 which showed no difference in GP costs between the groups.
The cost savings generated by the reduction in inpatient bed-day usage will depend on the total number of admissions avoided, the associated length of stay of these admissions and the complexity of patient needs during the admissions.
The assumed cost per inpatient day was similar between the Balance of Care Group study83 and the study by Abel and colleagues;79 the derived cost savings varied as a result of longer lengths of stay associated with the avoided admissions in Sheffield. 83 The majority of studies did not report the impact on indirect costs, such as lost productivity and informal care provided by family and friends. The only study84 to collect indirect costs for both the patients and their families did not report them on the basis that they were relatively low and did not vary between arms; however, no details are provided.
The identified studies are subject to a number of weaknesses. The quality of the evidence is mixed. The study by Raftery and colleagues80 suffered from high dropout rates between recruitment and first follow-up. This varied between groups and may have led to potential bias as a result of the imbalance amongst the previously randomised groups, as well as creating problems in relation to statistical power because of attrition. Similarly, the study by Grande and colleagues84 suffered from problems of obtaining sufficient statistical power and dilution of the treatment effect.
The significant increase in the likelihood of dying at home in the subsample of patients admitted to the hospital at home scheme may have been due to the characteristics of the patients who were admitted to the scheme, rather than the service itself.
The non-RCT studies are subject to more important limitations. The absence of a control group in the study by Addicott and Dewar82 reduces the ability to draw robust conclusions. Differences in case mix cannot be accounted for and therefore the influence of case mix on differences in service usage between years cannot be ascertained. The two retrospective studies83,84 involved subjective assessments of avoidable admissions, although it is acknowledged that clinical decisions to admit patients to hospital are subjective by nature. Both studies assumed no capacity restrictions in relation to alternatives to hospital care and therefore represent an upper ceiling on the estimate of the proportion of admissions that may be avoided.
Although this review considers only UK literature, the wider international evidence base lends some support to the view that hospital admissions can be avoided, with accompanied cost savings, by improving palliative care support in the community. 85,86 The National Audit Office End of Life Care report86 identified 25 international studies reporting on the effects of palliative care during the last year of life. The majority of studies reported cost savings, although three studies reported increases in the average cost per patient. Other recent international reports suggest that new palliative care interventions do not necessarily lead to reductions in hospital use. For example, in Canada, after the first 12 months, Ontario’s end-of-life care strategy failed to show any impact on individual patients’ use of end-of-life home care and acute care services. 87 The extent of changes in resource use and costs in any particular country or locality is likely to depend on the baseline levels of service use and funding incentives within the health-care system. Differences between the basic health-care service provision in the UK and that in other countries suggest that the results of international studies are unlikely to be generalisable to the UK.
Conclusions
This study has found that the evidence base from the UK relating to the economic impact of avoidable admissions in palliative care is limited. The studies suggest that the economic impact of avoidable admissions may vary according to a range of factors, including the baseline service provision, the type of new community interventions introduced and the complexity of needs of the patients under consideration. Findings from one location need to be interpreted with caution and cannot be assumed to be transferable, either within or between countries.
Further high-quality evidence is needed to provide a more robust estimate of the extent to which the additional costs of providing high-quality community support can be offset, at least to some extent, by a reduction in inpatient usage by palliative care patients. Further studies should be undertaken in the UK to explore the generalisability of the recent retrospective studies reporting high levels of avoidable terminal admissions. The potential for avoiding other (non-terminal) palliative care admissions should also be explored. In addition, more research on the variation in the cost of supporting patients in non-hospital locations would be useful to assist understanding of the economic consequences of avoiding admissions. More importantly, additional prospective studies are needed that seek to demonstrate the scale of admissions that can actually be avoided in clinical practice, when issues such as capacity constraints come into play.
Limitations
Although the first review provides important evidence that, within a UK context, little is known about the potentially complex transition to palliative care, shortcomings in the literature mean that we are unable to provide strong empirical evidence to support practice and policy recommendations. Comprehensive search, retrieval and review strategies were used and address some of the limitations of previous reviews, although only English-language databases were used and hand searches of journals were not exhaustive. Quality assessment for non-empirical papers was undertaken according to the JBI NOTARI tool for assessment of expert opinion. This tool does not generate a score for methodological rigour; however, although findings from non-empirical papers must be considered with caution, this expert opinion paper was included in the review to minimise exclusion of relevant context. We could not undertake statistical synthesis or analysis because of the diverse nature of the studies included. Despite these limitations this review identifies issues of significant importance that warrant further research and discussion. In doing so, we add to the limited body of knowledge surrounding the transition from curative care to palliative care.
The second review was a narrative review to appraise the evidence regarding the economic impact of avoidable hospitalisations among palliative care patients in the UK. Given the small number of studies identified by this review and limitations in the amount and quality of evidence reported, caution needs to be exercised when appraising the costs of avoiding such admissions. The review focused on evidence from the UK setting on the basis that international variations in heath-care systems and funding are likely to influence the economic consequences of avoidable admissions and conclusions drawn from other countries are unlikely to be generalisable to the UK setting. We chose a narrative review method to synthesise the evidence. This approach has been criticised for not adhering to the strict principles underpinning the systematic review method. Although we accept that this could be a limitation, we took the view that the narrow focus and prescribed methods of the systematic review would not allow for comprehensive coverage of the topic. 88
Chapter 4 Qualitative focus groups with health professionals (phase 2)
This chapter focuses on phase 2 of the project and addresses the following study objectives:
-
to explore the extent and current management of palliative care need within acute hospitals
-
to examine the circumstances under which transitions to a palliative care approach occur within acute hospitals, with a particular focus on the influence of age and disease type on decision-making
-
to inform the design of data collection materials for the prospective survey of palliative care need in acute hospitals.
To address these objectives we conducted exploratory qualitative focus groups and interviews with 58 health-care professionals with experience of palliative care management to explore their perceptions of barriers to, and facilitators of, palliative care transitions in hospital.
Background
The literature review in Chapter 3 outlined the limited evidence relating to transitions to palliative care and identified a need to further explore assessment and management of such transitions. It highlighted that more research is required to maximise current resources and develop appropriate guidelines and care models for managing the transition from curative care to palliative care. 89
The GMC guidance on end-of-life care,28 which came into effect on 1 July 2010, states that doctors must ensure that death becomes an explicit discussion point when patients are likely to die within 12 months. This guidance is in line with policy initiatives that identify a need for health professionals to recognise when patients are likely to be entering the last year of life to ensure an appropriately managed transition to palliative care. 5 The National End of Life Care Programme recently published guidelines for end-of-life care in acute hospitals. 29 The guidelines advocate ‘good honest communication’, ‘advanced care planning’, and ‘access to tailored information’ as crucial for optimising the provision of palliative care in the hospital setting.
Implicit in these guidelines is a need to initiate a transition to a palliative care approach with patients earlier in the disease trajectory and to ensure that this information is transmitted to patients by the clinician who is best placed to have this discussion. It is recognised that in many cases this will be a patient’s GP.
The discipline of palliative medicine developed around an oncology model, and, although current definitions of palliative care encompass the care of patients with any life-limiting condition,90 patients with non-malignant disease are disadvantaged in terms of access to palliative care. 91 It has been identified that people with dementia, for example, rarely gain access to palliative or supportive care,92 contrary to evidence that points to the efficacy of such an approach for people with this condition. 93 Several reviews point unequivocally to the importance of adopting palliative approaches in the case of non-malignant diseases such as dementia, particularly in acute settings. 94–96 There is also continuing evidence of inequalities in referral to and use of specialist palliative care services for older people. 20 Older people are proportionally more likely to die from conditions other than cancer, and hence are disadvantaged in access to specialist palliative care services by diagnosis. This is despite demographic trends indicating that the core population of patients requiring palliative care is ageing,1 and evidence suggesting that a palliative care approach is appropriate for older people. 4 Barriers to the provision of palliative care for older people are not well understood and there is a paucity of published literature in this area.
Methods
Methodological approach
Given the exploratory nature of the enquiry and the limited existing evidence base, a qualitative study design was adopted. Focus groups were used to capitalise on group interactions and to elicit rich experiential data by exploring participants’ knowledge and experiences. 97 Individual interviews were held with consultant-level staff who wanted to participate in the study but were unable to attend a focus group.
Sampling and data collection
Four focus groups were held at general practices (groups comprised four, six, seven and 11 participants). Two focus groups (both comprising five participants) and four interviews were held in acute hospitals, and two focus groups (comprising six and nine participants) were held in hospices. We held individual interviews with consultants who wanted to participate in the study but were unable to attend a focus group.
Focus groups and interviews were held in the two cities – Sheffield and Lancaster – where all components of the research project took place. Staff at acute hospitals and hospices were identified and approached through senior medical and nursing staff with the assistance of researchers. General practices were identified and recruited through local primary care research networks.
Purposive sampling was used to select a diverse range of health professionals and achieve the maximum possible variation of experience and opinion and reflect the diversity within the population (Table 3). However, focus groups were also held with members of existing health-care teams to support the illumination of cultural values informing the work of the team. 98
| Characteristic | n (%) |
|---|---|
| Male | 12 (20.7) |
| Mean (SD) age (years) | 46.3 (9.9) |
| Age range (years) | 28–69 |
| Job title | |
| Consultant | 4 (6.9) |
| Junior doctor | 9 (15.5) |
| GP | 6 (10.3) |
| Practice nurse | 4 (6.9) |
| Clinical nurse specialist | 11 (19.0) |
| Other nurse | 19 (32.8) |
| Allied health professional | 5 (8.6) |
| Place of work | |
| Acute hospital | 10 (17.2) |
| General practice | 28 (48.3) |
| Hospice | 15 (25.9) |
| Specialist palliative care unit | 5 (8.6) |
The focus group and interview guide (Box 1) was developed following a review of the literature (see Chapter 3) and relevant policy and addressed the overall aim of the study, namely to explore how transitions to a palliative approach to care are currently managed in acute hospitals in England. The interview guide covered the following key areas: understanding and experience of palliative and end-of-life care, management and organisation of care and management of transitions to palliative care. The same interview guide was used for all focus groups and individual interviews.
-
What do you understand by the terms ‘palliative care’ and ‘end-of-life care’?
-
Are you regularly involved in providing palliative and end-of-life care?
-
Do you think many inpatients in acute hospitals have palliative and end-of-life care needs?
-
Thinking about the management and organisation of care and the way that palliative care is currently managed in acute hospitals, what are the main barriers to, and facilitators of, providing palliative care in acute hospitals?
-
Do you think that palliative care management is approached differently in older people compared with younger people?
-
Are there issues surrounding the transmission of information about patients with palliative care needs from the acute hospital into the community?
-
What are your thoughts on how transitions to palliative care management within acute hospitals are currently managed?
-
What sort of decisions made in acute hospitals indicate that a palliative care approach to patient management has been adopted?
-
In your experience, is a palliative care approach adopted alongside active curative treatment (a) within primary care; (b) within acute hospitals?
-
Is prognosis routinely discussed with patients in acute hospitals? Do you feel it ought to be?
-
Are decisions about adopting a palliative approach to patient management disclosed to patients and/or their families?
-
What would trigger such discussions? Who would be involved in these discussions?
Data analysis
With the consent of participants, focus groups and interviews were tape-recorded and transcribed verbatim. Three researchers read the transcripts individually (MG, CG and CI) and independently noted down the core themes that emerged. Notes were cross-compared and any discrepancies resolved by consensus. Each researcher then took the lead to identify subthemes. The data analysis programme NVivo version 8 (QSR International, Southport, UK) was used to assist with this process. The coding frame developed was grounded in the data rather than decided a priori. 99 Subthemes identified were then considered in relation to relevant literature. Direct quotations have been selected to illustrate the issues raised by participants and they are indicative both of typical responses and of the diversity of views obtained.
Results
Recognising the palliative transition point
Participants identified that structured transitions to a palliative care approach early in the patient disease trajectory, advocated in policy, are rarely evident in acute hospital settings. Key to changing the focus of care is a discussion of prognosis, and all participants reported that prognostic discussions with patients and their families were not routine:
Researcher: And is prognosis routinely discussed with patients in hospitals?
Participant: We never do that . . . I think for a variety of reasons. We don’t routinely do that. It’s not because we don’t want to provide information but quite often breaking bad news to a patient can be pretty difficult . . . and we take a very different approach which may not be right but unless the patient asks their prognosis we don’t tell them the prognosis.
Secondary care, consultant geriatrician
Participants identified that the failure to appropriately time palliative care transitions could have negative implications for meeting a patient’s end-of-life preferences:
They don’t always recognise that yes they are in the last few days of life and that person wants to go home, until the very last minute. Then they ring up . . . like we had one this morning, he’s coming home tonight . . . they must have known really from the information that they’ve given me that this was going to happen but they leave it until the very last minute.
Primary care, district nurse
Participants also reported that, in their experience, a phased transition in which ‘curative’ and ‘palliative’ approaches to care were adopted concurrently was rarely evident in the hospital setting, apart from amongst cancer patients receiving palliative chemotherapy. Indeed, an ‘either/or’ mentality amongst clinicians about approaches to care was reported:
Some feel that by just doing palliative care we don’t need to cure . . . we can just stop everything and just give pain relief and even sometimes they say it’s debatable whether to give IV [intravenous] fluids or sub fluids.
Secondary care, geriatric specialist registrar
The importance of good communication within and between clinical teams and settings
The role of good communication in supporting decision-making relating to a palliative care transition was identified as key by all participants:
Participant: You have to communicate well to get across that this patient is palliative.
Researcher: You mean communication to the patient?
Participant: To the patient, to the family, to colleagues, you’ve actually got to be able to communicate well.
Primary care, GP
A critical first step in this process was seen to be communication within the hospital setting and, in particular, reaching a consensus amongst all clinicians involved in a patient’s care that a palliative approach was now appropriate. The opinion and approach to treatment of the consultant were seen to be pivotal in this respect:
You’ve got to have some sort of consensus though about how you’re going to treat the patient . . . and sometimes I think what happens in a hospital is that the consultant is seen as the be-all and end-all so their decision is what decides it, whereas actually you need to reach a decree amongst a number of people.
Secondary care, geriatric specialist registrar
Issues of power within the professional hierarchy of the hospital were discussed within this context, both between medicine and nursing and within medicine itself. The need for nursing staff to be provided with opportunities to raise their concerns about the approach being taken to a patient’s care was identified:
I think maybe that point when the nurses start triggering and saying ‘why are we doing this?’, it would be nice for them to be able to, I don’t know, circumvent or put up a flag so that somebody else gets involved, or some kind of mediator. Because I get a lot of nursing staff telling me ‘why are we doing this? Why do you keep doing this?’ And I say ‘why didn’t you ask yesterday when the consultant was coming round because it would be really nice for you to ask somebody more senior than myself what their intentions are in the situation’. But it’s well ‘you’re here now, why aren’t you doing something?’ But actually I am, I’m following the plan that I have available to me and I can question it but I’m still not going to change that unless obviously something significant happens and it’s an acute deterioration but I still feel there’s a lot of . . . I don’t know, stresses in the system.
Secondary care, geriatric specialist registrar
Consultant clinicians participating in the study acknowledged that they found decision-making around the palliative care transition challenging:
I think sometimes the transition from an intervention to palliative care sometimes can be pretty daunting for me as a consultant, I don’t know what experiences you have interviewing others but I certainly find it difficult, and you may have to take a softly-softly approach, one step at a time, and then say look the outlook looks grim so we may have to move from there, we need to discuss it with everyone else, with the relatives and everyone else and see how you can take this forward. I mean it’s very difficult.
Secondary care, consultant geriatrician
Participants also identified the importance of documenting decisions made to adopt a palliative care approach in the patients’ notes. They acknowledged that this rarely happened currently and could represent a barrier to ensuring continuity of care.
The importance of good communication between clinicians and patients/families
The need to improve clinician/patient communication about palliative approaches to care was also discussed at length by many participants:
I think it’s the definition of discussion isn’t it? It’s very different to a consultant than it is to a patient. They go around and say ‘oh you’ve got this and this is what’s going to happen’ and walk off and that’s discussed, whereas what the patient wants is to sit down for a good hour or so and ask loads of questions. So although it’s appropriate that it’s discussed before they come home it’s got to be in the right setting.
Primary care, district nurse
However, many participants challenged the idea that information regarding a palliative care transition should routinely be conveyed to patients within hospital settings, advocating instead for the GP to take a lead in these discussions:
Researcher: Do you think that prognosis is routinely discussed with patients in hospitals?
District nurse: No.
GP1: No.
GP2: If it is it’s not often.
GP1: My evidence to answer that question is from the patients and I would say no.
GP3: Some patients don’t ask and they don’t want to know. Because basically you’re saying that we aren’t on the curative line any more and I think it takes . . . you don’t have the relationship that GPs have with the patient because you haven’t necessarily known them for a long period of time and you don’t know the family whereas often in a community setting you know them and you’ve known them over a period of time, you’ve known their relatives, and I think the context is very difficult in hospital, it’s much more clinical in the hospital.
Primary care
Participants identified particular difficulties in communicating with patients with conditions other than cancer, who, it was recognised, were particularly likely to undergo a very late transition to a palliative care approach. Although they recognised policy guidance regarding the use of the ‘surprise question’ – ‘would you be surprised if this patient were to die in the next 6/12 months?’ – respondents grappled with how to convey this information to patients:
If somebody were to ask you that question – ‘would you be surprised if they were dead in 12 months?’ – well no you wouldn’t but you wouldn’t be amazed if they were alive either, so you can’t communicate that to a patient in a way that’s meaningful to them so I think we don’t discuss it.
Secondary care, consultant geriatrician
Primary care clinicians reported that their ability to inform the patient more fully could be compromised by the failure on the part of the hospital to convey treatment information to them in a timely manner:
I think it should be secondary and primary care working together alongside, alongside each other, and the communication is the biggie and unfortunately we still haven’t got it right. We’re working towards that I know, but I don’t think it’s still there.
Primary care, community matron
The ability to act on expressed preferences regarding place of death
Participants identified that a further barrier to communicating information to patients and their families regarding a palliative care transition was the extent to which any preferences for end-of-life care that were expressed as a result could be acted on:
What I wanted to say is even though we have developments in advanced communications and advanced planning mechanisms that are coming in to help shape some of the decision-making, even when you put patients and the families central to that process and they may express themselves that they want to die at home etc., because of a whole host of issues that we’ve touched on, including resources, that that’s just not always possible and a huge percentage of people die in a place that they would chose not to do so.
Secondary care, hospice nurse
Indeed, many participants reported that, in their experience, patient preferences for place of death, and in particular dying at home, could not always be met for a range of reasons:
People get admitted to hospitals because there’s a deterioration in an illness. And there’s nowhere particularly for them to go . . . they want to go home but that depends on a lot of communications with the family, the person, the carers, the MDT [multidisciplinary team] to make it possible really and so, with the best will in the world sometimes, people will end up dying in hospital.
Secondary care, palliative medicine consultant
Barriers to ensuring appropriate palliative care provision for older patients
Significant barriers were identified to providing palliative care and instigating referrals to specialist palliative care teams for older patients. Several participants acknowledged that an older patient with palliative care needs elicited a different response from them than a younger patient with comparable needs, and that this had implications for clinical practice. For example, a terminal diagnosis in an older person was seen as less ‘shocking’ and more expected than a terminal diagnosis in a younger person:
I think it’s possibly the case that . . . it’s more acceptable in older people . . . it’s the good innings argument . . . you know they’ve had their innings, they’re old so they’ve perhaps got less to live for.
Secondary care, consultant geriatrician
Limited social and family support was also identified as contributing to a lack of palliative care provision for older people. Younger people were seen to have more comprehensive support networks, as well as often having an advocate who could demand best-quality care on their behalf. Limited access to specialist palliative care services was also seen to compound the lack of psychosocial support available to older people more generally, many of whom live alone:
There’s often a lot more support for younger people as far as families and people go. With younger people nearly always family members, friends, neighbours will rally round. Often with old people there’s no one.
Secondary care, hospice nurse
Lack of resources, particularly for older people dying from conditions other than cancer
Both generalist and specialist palliative care provision within acute hospitals were identified as being particularly deficient for the population of predominantly older people dying from conditions other than cancer. It was reported that patients with non-malignant disease were less likely to be referred to specialist palliative cares services, in part because of the historical link between cancer and palliative care:
Practice nurse 1: They don’t admit them to the palliative care unit do they . . . the COPD [chronic obstructive pulmonary disease] and heart failure?
Practice nurse 2: They go on to the general ward.
Primary care
Generalist palliative care provision within acute hospitals was seen as being particularly susceptible to resource restrictions. It was acknowledged that inadequate staffing levels and increased time pressures on generalists impacted on the ability of staff to provide good palliative care. Psychosocial palliative care, in particular, was rarely prioritised under these circumstances:
The staffing levels there [hospital] are often so poor that it isn’t a question of not wanting to do it, it’s not being able to do it. They’re not even able to satisfy the basic requirements, much less go in and listen to people in the way that they’d like to.
Secondary care, hospice social worker
A focus on acute or interventionist care
Participants reported that both generalist palliative care and timely referral to specialist palliative care could be compromised by an inappropriate focus on interventionist care with a curative intent. This was described in some instances as a reluctance of doctors ‘to let patients die’. The widespread public expectation that hospital is a place ‘where ill people go to get better’ was cited as a further justification for the focus on acute care, with physicians not wanting to seem as if they had ‘given up’ on patients:
I think some doctors find it difficult to let go, some doctors find it uncomfortable to admit that the patient is going to die, they feel that they should carry on, doing all they can for them in terms of investigations and treatment.
Secondary care, consultant geriatrician
Palliative care for patients with dementia
Particular difficulties were noted with the provision of timely and appropriate palliative care for patients suffering from dementia. The data suggest that there remain considerable difficulties in the achievement of good-quality end-of-life care in the form of palliative services for people with dementia. There are two notable subthemes: candidature, and ‘rising tide’ and resources.
The notion of candidature refers to the ways in which people with dementia are seen, or not seen, by health-care practitioners to be the potential recipients of palliative care provision. To some participants in the study, the idea that dementia constituted a condition that might on its own be a cause of death was questionable. Reluctance to acknowledge this resulted in a failure to access services of a palliative nature:
Dementia isn’t a disease it’s just something that happens to people.
Secondary care, specialist palliative care team
Dementia is not terminal.
Primary care, GP
I think people with dementia are not considered often in the same league as somebody with heart failure, COPD or cancer. That is what happens to elderly people isn’t it? They get confused.
Primary care, GP
The final quote suggests that other conditions are considered worthy of specialist palliative care whereas dementia is not. Considerable discussion relating to criteria for referral to palliative services was noted and, in particular, the idea that cancer had been recognised for a long time as being the only condition that might warrant such care. Participants in this study reflected on their own experiences of how services inhibit the transition to palliative care for older people, particularly those who have dementia:
Elderly with dementia don’t really get the care. They don’t get the active palliative care, you know what I’m talking about, active treatments, active palliative care.
Primary care, GP
And it’s always been . . . again there’s been this idea that it’s almost like a culture that demented elderly patients belong in, and I hate to use the term, but geriatric units. That’s where they go, they don’t come to hospices or anywhere for palliative care even though they’re just as deserving as anyone else.
Secondary care, hospice nurse
Questions about the resources available to teams to enable them to provide palliative care to a wider population (including people with dementia) were raised. The ideas expressed below, by members of a specialist palliative care team in the community, suggest that existing pressures, combined with an already stretched capacity to deliver to its existing client group, have created tensions over being able to offer its services to people with dementia:
We just couldn’t do that [provide palliative care to people with dementia]. We don’t have the resources but people with dementia are just allowed to die in nursing home or rest home beds.
Primary care, GP
But is [increasing dementia referrals] just reducing the palliative care service available to perhaps other people, I don’t know?
Secondary care, hospice nurse
I would think in terms of beds, we’re struggling as it is for palliative care cancer, if they take [dementia] in it’s going to be absolutely chaotic.
Primary care, nurse
Discussion of a ‘rising tide’ scenario prompted alternative proposals about the function, nature and possible model of palliative care for people with dementia. Focus group participants articulated a reluctance to offer people with dementia palliative care because their difficulties may not be the same as those of cancer patients, particularly in the realm of pain management. This amounted to a very different approach to palliation in dementia, relying on basic nursing skills and activities such as mouth care, pressure care and maintenance of dignity, which could be provided adequately by non-specialist teams:
Good symptom management, good emotional support, good support for the family, psychological and spiritual, social support, should be part and parcel of what everybody does and it should be offered to absolutely everybody I think.
Secondary care, specialist palliative care team
Discussion
This qualitative phase reveals significant barriers to implementing successful structured transitions to palliative care in the hospital setting. It is unsurprising that participants reported difficulties in recognising that a patient has entered the last 12 months of life given previous research regarding clinician barriers to prognostication, particularly in ‘non-cancer’ conditions100–104 in which dying trajectories are typically unpredictable. 105 These are issues that are not unique to the hospital setting. However, the implications that this has for patient care in acute hospitals have not previously been explored in any detail. Our research found that prognosis does not appear to be routinely discussed with hospital inpatients, representing a key barrier to a structured transition to a palliative care approach being initiated. Moreover, an ‘either/or’ approach to care was identified rather than concurrent palliative and curative treatment, as recommended in contemporary models of palliative care. 30
Our research indicates a need for more effective communication within the hospital team, to achieve consensus that a patient has palliative care needs and subsequently to use this information to change the care plan. Two key barriers to this being achieved in practice were identified. First, the internal momentum of the hospital directed towards cure was seen to inhibit clinicians from standing back and thinking about the overall goals that should be informing patient care. Second, decision-making was identified as being consultant led, with more junior members of the team typically having few opportunities for input with regard to decisions about transitions to palliative care. Opportunities for nursing staff to feed into such decisions were identified as being particularly limited.
Even when consensus regarding a palliative care transition is achieved within the clinical team, information regarding such a transition was reported not to be conveyed routinely to patients and their families. That clinicians experience significant difficulties in ‘breaking bad news’ is well known. Uncertainties regarding prognosis, unpredictable illness trajectories and difficulties maintaining hope after such communications were cited as particular barriers to these discussions across all settings. 106 Our data indicate that training courses in communication skills need to be tailored to the acute hospital setting in recognition of the unique problems identified in this study.
This qualitative phase also confirmed that significant barriers exist to the provision of optimum palliative care for older people within acute hospital settings. The finding that older age can act as a barrier to accessing specialist palliative care resonates with some research findings106 but not all. Indeed, a recent study exploring equity of use of specialist palliative care in lung cancer clinic patients found that age was not associated with receipt of such services, and referral was based on need. 107 However, health professionals in our study reported that they often believed older people to be less requiring of palliative care than younger people, as a consequence of death being more expected in older people and the perception that older people find it easier to come to terms with a terminal diagnosis. It seems that, for a proportion of health professionals, the belief that older people have fewer requirements for specialist palliative care may be a factor affecting referral patterns and leading to reduced utilisation of specialist care.
Provision of good palliative care for older people is also crucially mediated by a situation in which the role of the first line of health professionals for older people, specifically geriatricians, is ill-defined in terms of responsibility for providing palliative care. 108 A further debate surrounds the definitional and conceptual issues relating to palliative care for older people. The findings show that, for generalists in particular, palliative care is often equated with dying. This conceptual issue may present a further barrier to optimum care, particularly for patients with non-malignant disease for whom ‘dying’ may not be diagnosed until close to death. Ensuring both the early introduction of palliative care and continuous palliation is central to achieving improvements in the end-of-life experiences of older people. Indeed, overall, there is an urgent need to clarify the terminology used within palliative care to ensure consistency in clinical practice.
Findings from this study indicate that a situation exists in which specialist palliative care services are still inextricably linked with cancer care, despite substantial evidence to suggest that patients with advanced non-malignant disease would benefit from this care. 109 Older people are proportionally more likely to die from conditions other than cancer and hence are disadvantaged by diagnosis in terms of access to specialist palliative care. 3 The findings also suggest that there are particular barriers to making the transition to a palliative care approach for people with dementia.
Clinicians alluded to the notion that dementia is not recognised as a cause of death and that candidature for palliative services is therefore questioned. Mitchell and colleagues110 have demonstrated that practitioners hugely underestimate mortality rates of people with dementia. Poor knowledge amongst some teams is apparent here, but uncertainty about prognostication may account for some of these shortcomings. 11 Inadequacies of a systemic nature may also be a result of the traditional role of hospices (as providers of palliative care to people with cancer) and reliance on cancer charities as a source of funding. Certainly there appear to be concerns about the resources available to provide palliative services to an ageing population, but little critical debate about how these challenges might be overcome using different models of provision. These data also point to limited confidence amongst some health-care teams in the assessment and management of dementia at the end of life. 111
This exploration of the experiences of health-care practitioners in the provision of palliative care for older people and those with dementia has drawn attention to a number of ongoing problems and challenges in clinical practice. Data from these focus groups and interviews with health professionals would support the notion that a transition to good-quality palliative care continues to be the exception and that collaborative working relationships between acute care and primary care, as well as among mental health specialists and others, are not always apparent. The data suggest that teams need to build on the internal resources available to them to be in a position to recognise the needs of people with dementia and meet the challenges they present.
Chapter summary
The findings reported here have significant implications for practice, indicating as they do the level of support that will be needed if current UK policy directives for palliative care management are to be implemented within the context of acute hospital settings. Such support needs to encompass not only education and training for ‘generalist’ palliative care providers tailored specifically to the unique nature of acute hospital settings, but also a critical consideration of how to address the further significant barriers that this study has revealed. Indeed, how to ensure that structured palliative care transitions do happen for patients, something that is critical to enabling end-of-life care preferences to be elicited and enacted, requires significant further attention by researchers.
Limitations
To the best of our knowledge, this study is the first to explore how transitions to a palliative care approach are perceived to be managed in acute hospital settings in a developed country. Purposive sampling was used to maximise the diversity of participants in this qualitative study and two very different hospital settings were also selected.
Data collection was continued until theoretical saturation was reached to maximise the generalisability of our findings to other areas of the UK. However, we need to acknowledge certain limitations. Participants were reporting on their practice and that of their colleagues; this was not directly observed. Caution is also required in interpreting and generalising from these data as it is likely that professionals were more likely to participate if they already had an interest in palliative care. Group dynamics within focus groups may have hindered junior staff from sharing their perspectives amongst senior colleagues. Individual interviews were conducted in cases in which participants were unable to attend a focus group; these interviews lack the interaction of a group setting and may not necessarily reflect the views that individuals may report amongst their peers. Owing to the different methods of data collection used, transferability may be limited. As all data collection was carried out in England, findings may not be generalisable to other countries.
Chapter 5 Survey of palliative care need at Sheffield Northern General Hospital and Royal Lancaster Infirmary (phase 3)
In this chapter we present the background, methods and results from the quantitative cross-sectional survey of hospital inpatients, which was undertaken in two UK hospitals. In doing so we address the following objectives:
-
to explore the extent and current management of palliative care need within acute hospitals
-
to identify patient factors predictive of key aspects of palliative care need and, in particular, physical and psychological symptom load
-
to examine the circumstances under which transitions to a palliative care approach occur within acute hospitals, with a particular focus on the influence of age and disease type on decision-making
-
to examine if and how information about a transition to a palliative care approach is communicated to patients and their families and how they are in involved in decision-making
-
to identify those hospital admissions amongst people with palliative care needs that were avoidable but which occurred because of a lack of alternative service provision or support in the community
-
to identify patient factors predictive of avoidable hospital admissions
-
to quantify the cost of avoidable acute hospital admissions amongst those patients with palliative care needs.
Background
The majority of deaths in developed countries now occur in the acute hospital setting; in England, around 58% of people currently die in acute hospitals. 112 Although recent evidence suggests a slow increase in the proportion of deaths at home in England and Wales,19 other predictions based on past trends estimate that only one in 10 people in the UK will die at home by 2030, and that an expansion of inpatient facilities by one-fifth may be required. 113 The End of Life Care Strategy for England5 has highlighted the delivery of high-quality palliative and end-of-life care in the acute hospital setting as an area of priority, acknowledging that a significant proportion of patients dying in acute hospitals receive very poor care.
The identification of patients who may benefit from palliative care is recognised as problematic. Health professionals have reported differing understandings of what constitutes a ‘palliative care’ patient30 and when a palliative care approach might be appropriate.
This finding is supported by evidence from our qualitative focus groups presented in Chapter 4. There are significant implications of a lack of consensus in identifying which patients have palliative care needs, including poor continuity of care, inadequate service provision and support and excess economic cost. 89,114
Given the challenge that the increasing number of hospitalisations at the end of life presents, coupled with the problem of identifying patients who would benefit from palliative care input, it is now imperative to gain a better understanding of the extent of palliative care need in the hospital setting to more appropriately map services to patient need, define priorities for care and assess the economic impact of palliative care in the acute setting. 2
Methods
Survey methods
A comprehensive survey of hospital inpatients was undertaken in two UK hospitals selected for their sociodemographic diversity. The SNGH has > 1100 beds and serves a largely urban, economically disadvantaged and ethnically diverse area. In contrast, the RLI has approximately 400 beds and serves a predominantly white Caucasian semi-rural/remote rural population. Although the term ‘census’ was originally proposed to describe this phase of data collection, this has been replaced throughout by the term ‘survey’. This was because of concerns that the term ‘census’ implies a collection of a complete population data set. However, this was not possible as the ethics committee stipulated that data collection from patient notes was restricted to those patients who consented to involvement in the study.
The survey of the SNGH was undertaken over an 11-day period in May 2010 and the survey of the RLI took place over a 5-day period in November 2010. All inpatient wards, with the exception of children’s wards and mother and baby units, were included. Each ward was visited by two members of the data collection team at some point during the survey period. Inclusion criteria were age ≥ 18 years and resident on the ward at 0900 on the day that the ward was surveyed. Non-English-speaking patients and deaf patients were excluded because of a lack of translation facilities.
The approach to the inclusion of patients lacking capacity to consent for themselves was developed in line with Mental Capacity Act 2005 guidance. 10 Senior medical and nursing staff, and relatives (when available) were consulted to identify any patients lacking capacity to consent. Personal consultees (relatives or close friends) were identified and, when available, were invited to participate on behalf of patients lacking capacity.
For patients/consultees who consented to participate, the following data were collected:
-
Data from patients’ hospital case notes comprising evidence of palliative care need according to GSF prognostic indicator criteria57 (the GSF prognostic indicator guide provides 11 diagnostic criteria categories, which provide an indication of patients who might benefit from palliative care input); reason for admission; sociodemographic and diagnostic information; details of comorbidities; evidence of adoption of a palliative care approach using a list of predefined indicators (identified by health professionals during a previous qualitative phase of this study; see Chapter 4); number of hospital admissions in the last 12 months; discharge plans.
-
For each consenting patient a member of the medical staff and a member of the nursing staff known to the patient were interviewed. Staff were asked to provide diagnostic and admission information for the patient. They were also asked whether they believed the patient to have palliative care needs according to a standardised definition (a broad and inclusive definition of palliative care was purposively selected to maximise the potential for patient identification),115 whether they would be surprised if the patient died within 12 months; about the appropriateness of the admission to hospital; and whether prognostic discussions had taken place. When possible the member of the nursing staff was the designated ‘named nurse’ for the patient and the member of the medical staff was the junior (Foundation Year 1 and 2) or senior (Specialist Trainee year 1 and year 2) house officer or the registrar.
-
Patient-/consultee-completed questionnaires comprising sociodemographic information; a service use questionnaire developed for use with a palliative care population (Gott M, Barnes S, Payne S, Seamark D, Small N. Department of Health, 2007, unpublished report); and the Sheffield Profile for Assessment and Referral for Care (SPARC). 116 SPARC is a validated holistic self-assessment tool to identify patients who would benefit from palliative care input (see Appendix 4). It provides scores across a range of physical, psychological and social domains. In cases in which consultees participated, they were asked to answer questions as they believed that the person they were acting as consultee for would have done.
All data were collected by a team of 30 researchers with previous experience in health-care research as either an academic or a clinician. Data from hospital case notes were collected by researchers with a clinical background in medicine or nursing. All researchers attended a full-day training session prior to the study commencing, which provided training in approaching patients/staff, the correct use of data collection tools and procedures for problem situations.
Data analysis
All data were recorded onto anonymised paper proformas and were subsequently transferred onto a SPSS database version 20 (SPSS Inc., Chicago, IL, USA) for data cleaning and analysis, with the guidance of the project statistician (CP). Descriptive analyses were used to describe the data from all sources.
Cohen’s kappa measure of chance-corrected agreement was used to assess agreement between sources regarding identification of patients with palliative care needs and appropriateness of admission to hospital. Logistic regression analyses were used to explore the effects of a range of predictor variables on various outcomes.
Economic analysis methods
Two palliative care consultants (BN and MB) undertook a post hoc assessment of appropriateness of admission to hospital for patients who had been identified with palliative care needs according to GSF criteria. They reviewed the survey data collected from the case notes of these patients and made a decision whether the admission was ‘potentially avoidable’ or ‘unavoidable’ or whether there were ‘insufficient data to make a decision’. For those patients whose admission was classed as potentially avoidable, an alternative place of care was suggested by the consultants.
An estimated cost was then attached to each of the admissions identified by the consultants as potentially avoidable. The cost of each hospital admission was estimated using HRG codes. HRG codes are clinically meaningful groupings of patient activity, based on both diagnoses and clinical procedures undertaken. Standard costs are assigned to each HRG code and these are used across the NHS in England to calculate the cost of a hospital admission. 117
Because of ethical restrictions, HRG codes for patients in the surveys could not be obtained retrospectively from the trusts involved. The Sheffield Teaching Hospitals coding team therefore undertook an ad hoc exercise to allocate HRG codes to each admission identified as potentially avoidable across both hospitals using the data from our survey. HRG codes and tariffs for 2010/11 were used.
Admissions for patients who remain in hospital beyond the expected upper length of stay for the HRG code (trim point) would normally be allocated an additional long-stay or excess bed-day payment. In the absence of length-of-stay data for our sample of patients, we were unable to cost for excess bed-days.
We then estimated the costs of the alternative places of care suggested by our consultants for those admissions deemed avoidable. The costs of alternative places of care were taken from published national sources, inflated to 2011 prices when necessary (see Table 11). The cost of nursing home care (£106 per day) was obtained from the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2010. 118 The cost of hospice care (£325 per day) was obtained from research commissioned by the National Audit Office. 119 The cost of home care (£50 per day) was derived from a King’s Fund report,82 which estimated the cost of home care in the last 8 weeks of life. This cost included the following services: GPs, district nursing, Marie Curie and Macmillan nurses, ambulance journeys, hospice at home and hospice (inpatient) and equipment. An average daily cost was calculated from the 8-week figure cited in the report.
These studies all assumed a broad societal perspective120 to take account of costs irrespective of funding source. Although we would have also liked to include informal carer costs and indirect costs – those incurred as a result of lost productivity – in our calculations, this was not possible because of a lack of data. As a point of comparison, we explored a more restrictive cost perspective. This was the NHS and personal social services (PSS) perspective, which considers only those costs that fall within the remit of these two organisations. To do this we used the costs cited in a previous study,121 which indicated that only 43% of nursing home costs and 44% of hospice costs were funded by the NHS or PSS. Costs were inflated to the 2011 price year when necessary.
Results
Survey response rate
A total of 1359 inpatients were eligible for inclusion in the survey (1009 patients in Sheffield and 350 patients in Lancaster). Of the total eligible patient population, 654 (48.1%) patients agreed to participate in the study of whom 616 patients consented for themselves and 38 consented through a consultee. Patient response rates were similar for the two hospitals (SNGH 46.9%, RLI 52.9%). Details of patient recruitment at the two participating hospitals are provided in Figure 3.
FIGURE 3.
Details of patient recruitment at the two participating hospitals.
Of the 654 consenting patients/consultees, complete data sets are available for 514 patients (final response rate 37.8%). A complete data set is defined as containing a case note review and a questionnaire completed by a member of either the medical staff or the nursing staff, but not necessarily both. The analyses presented in this section relate to the 514 patients with complete data sets.
Results of the case note review
Of the 514 patients, just over one-third (n = 185, 36.0%) met one or more of the GSF prognostic indicator criteria for palliative care need. Of the patients identified with palliative care needs according to GSF criteria, 53.8% were female and the median age was 78 years, with an age range of 20–103 years. The majority of these patients were aged ≥ 65 years (77.8%), with a considerable proportion aged ≥ 85 years (23.2%) (Figure 4). Table 4 shows demographic information for the sample of 185 patients with palliative care needs according to GSF criteria.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 85 (45.9) |
| Female | 100 (54.1) |
| Partnership status | |
| Married | 66 (35.7) |
| Single | 19 (10.3) |
| Divorced | 18 (9.7) |
| Widowed | 59 (31.9) |
| Not stated | 23 (12.4) |
| Living arrangements | |
| Cohabits | 89 (48.1) |
| Lives alone | 78 (42.2) |
| Nursing home or residential care | 18 (9.7) |
| Median age (years) | 78 |
FIGURE 4.
Age range of patients with palliative care needs according to GSF criteria (n = 181, no age given for four patients).
The majority of patients (70.8%) met only one GSF criterion for palliative care need; however, just under one-third (29.2%) met two or more criteria (see Table 5). Figure 5 shows the breakdown of GSF prognostic indicators amongst the patient sample.
The most common GSF prognostic indicator was frailty, with almost one-third of patients (27%) meeting this criteria. Heart disease (20.5%), cancer (19.5%), COPD (18.4%) and dementia (17.8%) were the next most common GSF criteria, and were roughly equal in prevalence. Other indicators, including stroke, renal disease and Parkinson’s disease, were less common.
FIGURE 5.
Numbers of patients meeting each of the GSF prognostic indicators for palliative care need (n = 185). a, Other life-limiting illnesses included cystic fibrosis, Huntington’s disease, asbestosis, etc.
Table 5 provides admission and diagnostic information for the patient group. Reason for admission to hospital was ascertained in all but five patients (included in the ‘other’ group). The most common reasons for admission were falls/confusion/general frailty (14.6%), complications relating to cancer (13.0%) and respiratory disease or exacerbation (13.0%). Patients had a median of two comorbid conditions, with over one-third of patients having three or more comorbidities. In the 12 months before the survey, patients had a median of one previous hospital admission and spent a mean of 42 days (lower quartile = 1.8, upper quartile = 53.0) in hospital. For the majority of patients (65.9%) there was no evidence of the adoption of a palliative care approach. Around one-third (28.6%) of patients had a do not attempt resuscitation (DNAR) order in place, but only a small number (8.1%) had been referred to specialist palliative care services.
| Admission and diagnostic data | n (%) |
|---|---|
| Reason for admission | |
| General frailty/fall/confusion or deterioration | 27 (14.6) |
| Cancer or cancer-related problems | 24 (13.0) |
| Respiratory disease or exacerbation | 24 (13.0) |
| Chronic heart disease | 12 (6.5) |
| Dementia | 12 (6.5) |
| Infection | 12 (6.5) |
| Accidental injury | 12 (6.5) |
| Renal failure | 10 (5.4) |
| Stroke | 10 (5.4) |
| Myocardial infarction/acute cardiac event | 10 (5.4) |
| Neurological conditions (excluding dementia) | 5 (2.7) |
| Other | 27 (14.6) |
| Number of comorbid conditions per patient | |
| 0 | 11 (5.9) |
| 1 | 64 (34.6) |
| 2 | 43 (23.2) |
| 3 | 41 (22.2) |
| > 3 | 26 (14.1) |
| Number of GSF prognostic indicator criteria per patient | |
| 1 | 131 (70.8) |
| 2 | 43 (23.2) |
| ≥ 3 | 11 (5.9) |
| Number of hospital admissions in previous 12 months (excluding current admission) | |
| 0 | 31 (16.8) |
| 1 | 52 (28.1) |
| 2 | 26 (14.1) |
| 3 | 18 (9.7) |
| > 3 | 20 (10.8) |
| Missing data | 38 (20.5) |
| Number of days in hospital in last 12 months | |
| 1–20 | 65 (35.1) |
| 21–40 | 27 (14.6) |
| 41–60 | 15 (8.1) |
| > 60 | 28 (15.1) |
| Missing data | 50 (27.0) |
| Indicators of adoption of a palliative care approach | |
| Do not attempt resuscitation order in place | 53 (28.6) |
| Evidence of referral to specialist palliative care | 15 (8.1) |
| Placed on LCP | 2 (1.1) |
| Prescription of long-term opiates/syringe driver | 9 (4.9) |
| Documented advance care plan | 0 (0) |
| No indicators of palliative care approach | 122 (65.9) |
Results of medical and nursing staff assessment of palliative care need
Medical and nursing staff were asked whether they believed patients to have palliative care needs according to the Canadian Palliative Care Association 1997 definition. 115 Nurse questionnaires were completed for 473 patients; of these, nurses stated that 84 (17.8%) had palliative care needs. However, data from patients’ hospital case notes indicated that 174 (36.8%) of these 473 patients were identified as having palliative care needs according to GSF criteria (Table 6). Staff were also asked, ‘would you be surprised if this patient died (1) during this admission and (2) in the next 12 months?’ Nursing staff would not have been surprised if the patient died during the current admission in 74 (15.6%) cases and in the next 12 months in 180 (38.1%) cases. Medical staff questionnaires were completed for 297 patients; of these, doctors stated that 46 (15.5%) had palliative care needs, whereas using the GSF criteria 108 (36.4%) were identified with palliative care needs (see Table 6). Medical staff would not have been surprised if the patient died during the current admission in 50 (16.8%) cases and in the next 12 months in 123 (41.4%) cases.
| Medical and nursing staff assessment | Palliative care need according to the GSF | No palliative care need according to the GSF |
|---|---|---|
| Nursing staff assessment of palliative care need available (n = 473) | 174 | 299 |
| Palliative care need according to nurse | 52 (30%) | 32 (11%) |
| No palliative care need according to nurse | 122 (70%) | 267 (89%) |
| Kappa = 0.22, n = 473 | ||
| Medical staff assessment of palliative care need available (n = 297) | 108 | 189 |
| Palliative care need according to doctor | 32 (30%) | 14 (7%) |
| No palliative care need according to doctor | 76 (70%) | 175 (93%) |
| Kappa = 0.25, n = 297 | ||
Table 6 shows the level of agreement between medical staff, nursing staff and the GSF regarding the identification of patients with palliative care needs. Cohen’s kappa indicates a poor agreement between nursing staff and the GSF (n = 473, kappa = 0.22) in terms of identifying patients with palliative care needs. Agreement between medical staff and the GSF was also poor (n = 297, kappa = 0.25). Agreement between medical staff and nursing staff regarding which patients had palliative care needs was moderate (n = 256, kappa = 0.42). 122
Results of patient self-report data
Self-report questionnaires were completed for all 185 patients identified with palliative care needs according to GSF criteria. Questionnaires were completed by patients in 162 cases (87.6%) and by consultees in 23 cases (12.4%). The SPARC questionnaire provides a self-assessment of palliative care needs and scores variables from 0 to 3; a score of 3 on any variable indicates that the patient merits ‘immediate attention by the attending clinician’. 116 The SPARC questionnaire contains variables in six domains: physical symptoms, psychological symptoms, religious and spiritual issues, independence and activity, family and social issues and treatment issues. The majority of patients (n = 154, 83.2%) scored 3 on at least one variable in one of the six domains. Physical symptoms were most troublesome with 74.6% of patients scoring 3 on one or more variable in this domain. Patients also reported high levels of psychological symptoms (43.2%) but fewer problems relating to the other domains (Figure 6). Consensus between patients and medical staff (kappa = 0.20, n = 107) and between patients and nursing staff (kappa = 0.20, n = 173) was poor regarding identification of palliative care need when a SPARC score of 3 on one or more variable was used as a proxy for self-assessed palliative care need.
FIGURE 6.
Sheffield Profile for Assessment and Referral for Care questionnaire responses for patients with palliative care needs according to GSF criteria (n = 185).
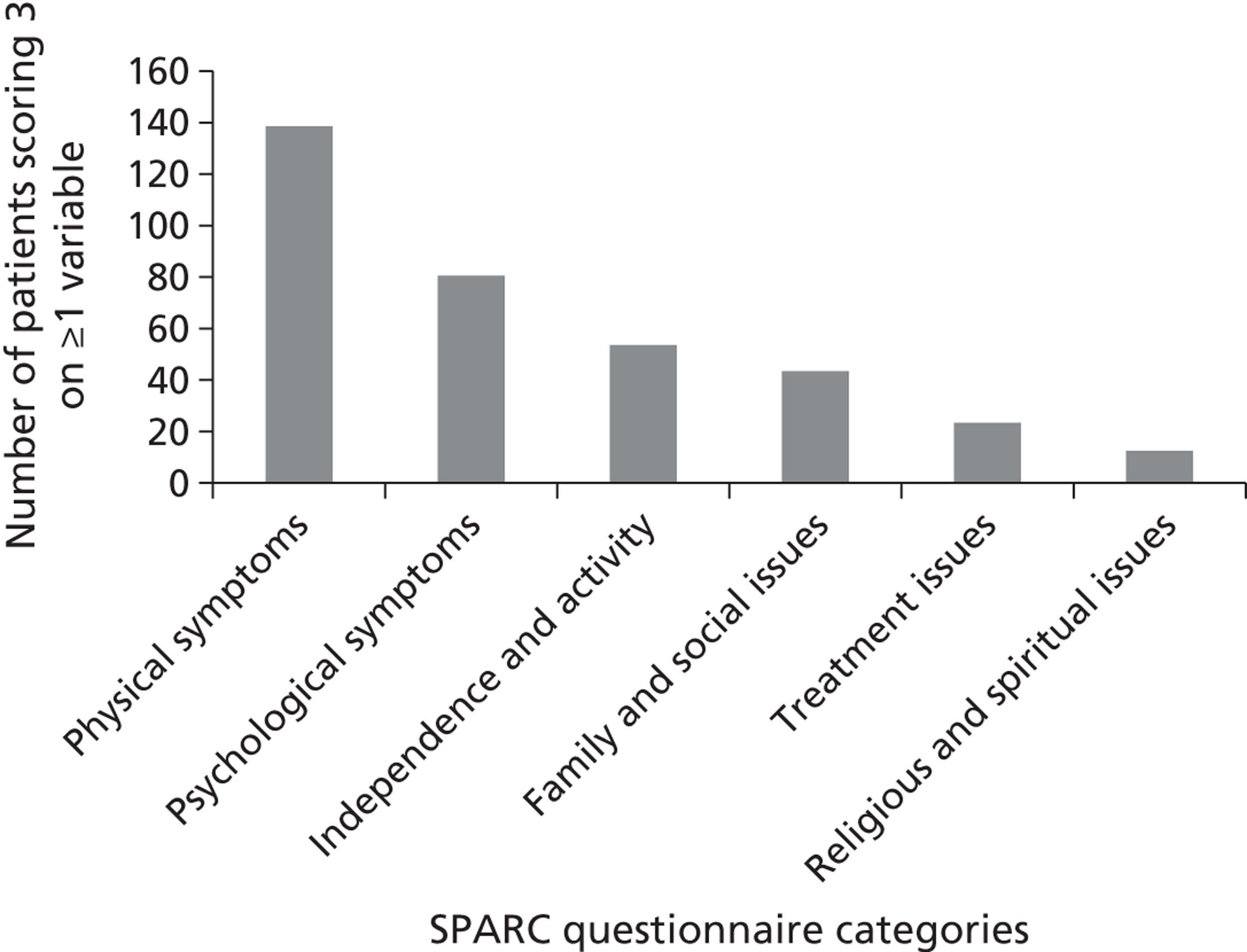
Predictors of symptom burden amongst patients with palliative care needs
Binary logistic regression was performed to assess whether known diagnostic factors (GSF indicator, number of comorbidities) and/or demographic factors (age, sex, living arrangements) were able to predict symptom burden as measured by the SPARC questionnaire. Only 183 of the 185 patients were included in these analyses as two patients had insufficient case note data. As only two SPARC variables included substantial numbers of patients (psychological and physical symptoms; see Figure 6), other SPARC variables were removed as outcome measures from the logistic regression. The two outcome variables indicating symptom burden (physical and psychological symptoms) were entered as binary outcome measures indicating ‘high symptom burden’ (score of 3 on one or more variable) compared with ‘low symptom burden’ (no scores of 3 on any variables) in the logistic regression.
Each predictor variable was entered singly and not adjusted for other variables. The results of these analyses are presented in Table 7. The results indicate that patients diagnosed with heart disease were less likely than those with other diagnoses to have a high physical symptom burden [odds ratio (OR) 0.42]. The results also indicate that patients with dementia were more likely to have a high physical symptom burden (OR 3.94) and a high psychological symptom burden (OR 2.88). Female sex was a significant predictor of psychological burden (OR 2.00), and having three or more comorbidities was also a significant predictor of psychological burden (OR 3.97). Neither age nor living arrangements were significant predictors of symptom burden.
| Predictor | Physical burden | Psychological burden | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| GSF cancer | 1.27 | 0.51 to 3.17 | 0.611 | 0.78 | 0.36 to 1.68 | 0.519 |
| GSF heart | 0.42 | 0.20 to 0.91 | 0.027 | 0.77 | 0.37 to 1.60 | 0.488 |
| GSF COPD | 1.67 | 0.64 to 4.36 | 0.293 | 1.52 | 0.72 to 3.22 | 0.270 |
| GSF renal | 0.93 | 0.28 to 3.09 | 0.908 | 0.43 | 0.13 to 1.39 | 0.158 |
| GSF frailty | 0.77 | 0.37 to 1.61 | 0.486 | 1.61 | 0.83 to 3.12 | 0.156 |
| GSF dementia | 3.94 | 1.14 to 13.64 | 0.030 | 2.88 | 1.29 to 6.41 | 0.010 |
| GSF stroke | 2.76 | 0.61 to 12.56 | 0.190 | 1.90 | 0.69 to 5.23 | 0.216 |
| GSF others combined | 0.78 | 0.31 to 1.92 | 0.586 | 0.93 | 0.41 to 2.09 | 0.854 |
| Age | 1.00 | 0.98 to 1.02 | 0.977 | 1.00 | 0.98 to 1.01 | 0.606 |
| Comorbidities | ||||||
| 0/1 | 1.00 | 1.00 | ||||
| 2 | 1.33 | 0.58 to 3.07 | 0.506 | 1.49 | 0.74 to 3.01 | 0.265 |
| 3+ | 1.68 | 0.35 to 8.18 | 0.520 | 3.97 | 1.01 to 15.69 | 0.049 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.57 | 0.80 to 3.11 | 0.193 | 2.00 | 1.09 to 3.64 | 0.024 |
| Living arrangements | ||||||
| Living with others | 1.00 | 1.00 | ||||
| Living alone | 0.61 | 0.29 to 1.29 | 0.197 | 1.07 | 0.56 to 2.04 | 0.836 |
| Residential/nursing | 1.33 | 0.34 to 5.12 | 0.680 | 1.28 | 0.46 to 3.55 | 0.638 |
Older patients aged ≥ 85 years
A key focus of this study was the care provided to older people at the end of life in hospital. Therefore, additional analyses were undertaken on patients who participated in the survey who were aged ≥ 85 years. Of the 654 patients who agreed to participate in the survey, 127 (19.4%) were aged ≥ 85 years. After data cleaning, complete data sets were available for 110 patients aged ≥ 85 years. Table 8 shows demographic and admission data for these 110 patients. The majority were female and the median age was 89 years. Most of the patients lived alone and all were of white ethnic origin. Reason for admission to hospital was obtained from hospital case notes. The most common reason for admission (25.5%) was general frailty (including falls, confusion or general deterioration).
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| Median | 89 |
| Range | 85–103 |
| Sex | |
| Male | 39 (35.5) |
| Female | 71 (64.5) |
| Living arrangements | |
| Cohabits | 28 (25.5) |
| Lives alone | 69 (62.7) |
| Nursing home or residential care | 12 (10.9) |
| Missing data | 1 (0.9) |
| Reason for admission to hospital | |
| Frailty/fall/confusion or deterioration | 28 (25.5) |
| Heart disease | 15 (13.6) |
| Infection | 13 (11.8) |
| Accidental injury | 10 (9.1) |
| Stroke/transient ischaemic attack | 8 (7.3) |
| Dementia | 6 (5.5) |
| Cancer or cancer-related problems | 6 (5.5) |
| COPD, other chronic lung disease | 4 (3.6) |
| Diabetes | 3 (2.7) |
| Renal failure | 3 (2.7) |
| Other | 14 (12.7) |
The hospital case notes of the patients aged ≥ 85 years were examined for evidence of palliative care need according to GSF prognostic indicator criteria. Forty-four (40.0%) patients met one or more criteria for palliative care need. Frailty (16.4%) and dementia (13.6%) were the most common criteria met (Figure 7). The majority of these patients met just one GSF criterion (n = 30, 68.2%), with smaller numbers meeting two (n = 11, 25.0%) or three (n = 3, 6.8%) criteria.
FIGURE 7.
Gold Standards Framework indicators met by patients aged ≥ 85 years (n = 44).
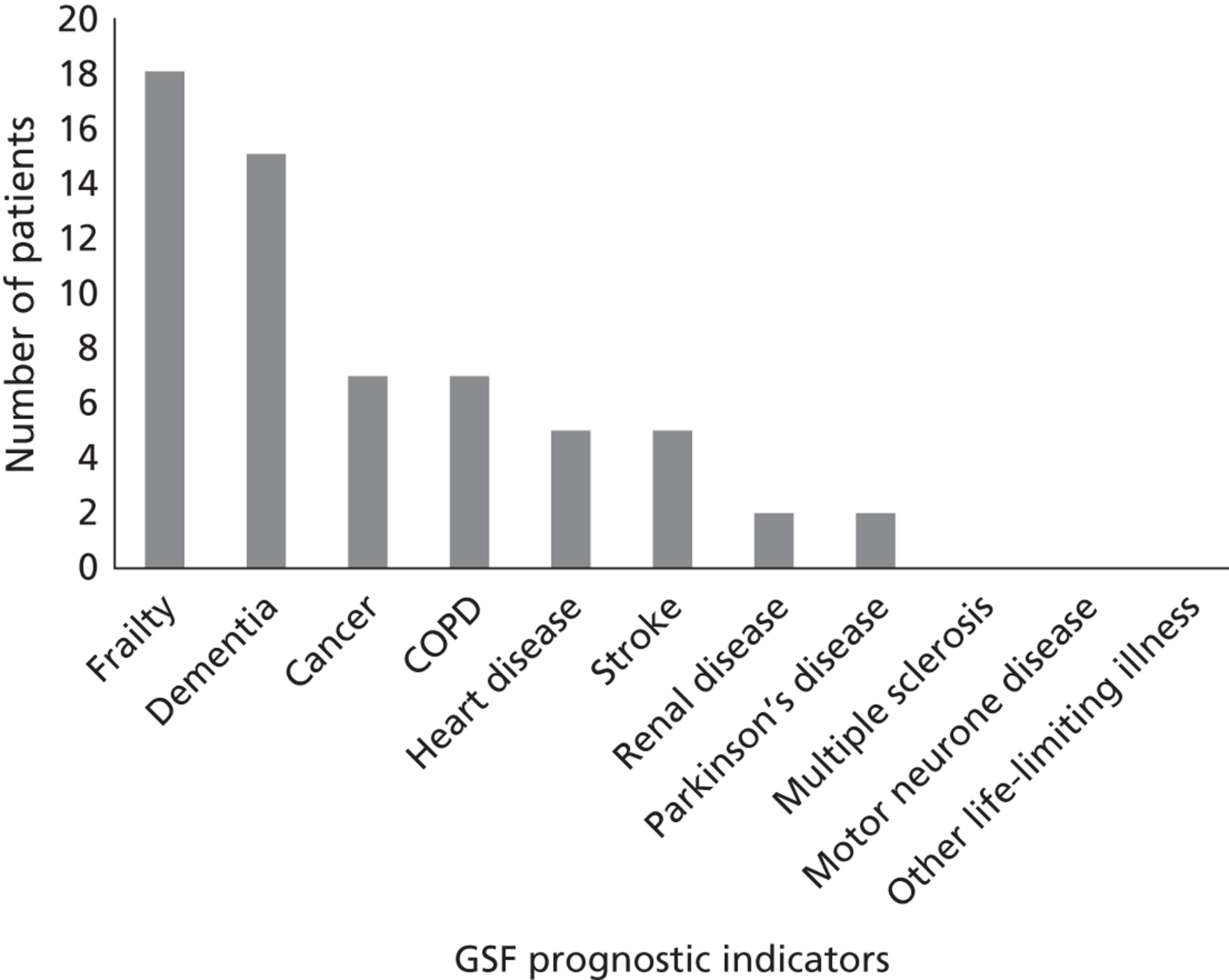
Hospital case notes were also examined for indicators of a transition to a palliative care approach. Half of the patients (50%) who met GSF criteria for palliative care need met one or more indicator of transition to palliative care. Half of the patients had a DNAR order in place; however, there was documentation of discussion with the patient and/or family regarding the DNAR order in only 6 cases (13.6%). Even amongst patients who did not meet GSF criteria, a reasonable number (16.7%) had a DNAR order in place. Only two patients of the 44 who met GSF criteria had been referred to specialist palliative care services; however, one patient was referred to specialist palliative care despite not meeting any GSF criteria. The only patients for whom advanced care plans (1.5%) or use of syringe drivers (3.0%) had been documented did not meet GSF criteria for palliative care need.
Predictors of transitions to palliative care
Analyses relating to transitions to palliative care were undertaken on 183 of the patients with palliative care needs according to GSF criteria (two patients were excluded from the analyses because of missing data on key transition variables). Of the 183 patients, 61 (33.3%) showed evidence of transition to a palliative care approach by meeting one or more indicator of adoption of a palliative care approach (Table 9). Of these, 43 patients met just one indicator, 14 patients met two indicators and four patients met three indicators.
| Indicator | n (%) |
|---|---|
| DNAR order in place | 53 (86.9) |
| Evidence of referral to specialist palliative care | 15 (24.6) |
| Placed on LCP | 2 (3.3) |
| Prescription of long-term opiates/syringe driver | 9 (14.8) |
| Documented advance care plan | 0 (0.0) |
A logistic regression was performed to assess whether various diagnostic and demographic factors were able to predict a transition to palliative care. Data were recorded for five indicators of transition to palliative care. As only one individual indicator of transition to palliative care included substantial numbers of patients (DNAR; see Table 9), a single binary outcome indicating any evidence of a transition to palliative care compared with no evidence was used for the logistic regression. Among the GSF diagnostic indicators, no patients had motor neurone disease and the frequencies for Parkinson’s disease and multiple sclerosis were very low; therefore, these last two indicators were combined with ‘other life-limiting conditions’ to form a combined indicator.
Table 10 shows that, in the unadjusted logistic regression, the significant predictors of a transition to palliative care were the GSF indicators for cancer, heart disease and stroke, together with age and living in a residential or nursing care home. The table also shows the final multivariate predictive model, which comprised three GSF diagnostic indicators (cancer, dementia, stroke) together with age. This model correctly predicted 74% of outcomes and could not be improved significantly by adding the other GSF indicators, number of comorbidities, sex or living arrangements. In this model, the odds of a transition to palliative care were multiplied by an estimated 5.1 for patients with a cancer diagnosis, by 8.0 for a stroke diagnosis and by 2.6 for a dementia diagnosis. The odds were also increased by an estimated 3% for every additional year of age (with no evidence of significant non-linearity in this relationship).
| Predictor | Unadjusted analysis | Multivariate model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| GSF cancer | 2.8 | 1.3 to 5.9 | 0.009 | 5.1 | 2.2 to 12.1 | < 0.001 |
| GSF heart disease | 0.4 | 0.2 to 0.9 | 0.033 | |||
| GSF COPD | 1.3 | 0.6 to 2.8 | 0.502 | |||
| GSF renal disease | 1.0 | 0.3 to 3.1 | > 0.999 | |||
| GSF frailty | 1.2 | 0.6 to 2.3 | 0.639 | |||
| GSF dementia | 1.9 | 0.9 to 4.1 | 0.106 | 2.6 | 1.0 to 6.3 | 0.039 |
| GSF stroke | 5.7 | 1.9 to 17.1 | 0.002 | 8.0 | 2.5 to 25.9 | 0.001 |
| GSF other life-limiting conditions | 0.6 | 0.2 to 1.5 | 0.256 | |||
| Age | 1.03 | 1.01 to 1.06 | 0.010 | 1.03 | 1.00 to 1.06 | 0.038 |
| Number of comorbidities: | ||||||
| 0/1 | 1 | |||||
| 2 | 1.2 | 0.5 to 2.6 | 0.708 | |||
| ≥ 3 | 1.1 | 0.6 to 2.3 | 0.723 | |||
| Sex | ||||||
| Male | 1 | |||||
| Female | 1.0 | 0.5 to 1.8 | 0.956 | |||
| Living arrangements | ||||||
| Cohabiting | 1 | |||||
| Alone | 1.0 | 0.5 to 2.0 | 0.989 | |||
| Nursing/residential care | 3.4 | 1.2 to 9.9 | 0.022 | |||
Potentially avoidable admissions to hospital for patients with palliative care needs
Appropriateness of admission assessments were undertaken for 208 patients; this was the total number of patients who were identified with palliative care needs according to GSF criteria and for whom complete case data were available (no medical/nursing data or patient data were required for this stage of the analysis; this accounts for the slightly larger sample size than that described in Results of the case note review). The admissions of 14 (6.7%) patients were classified by our two palliative medicine consultants as potentially avoidable. Double coding of a random sample of 15% of the notes indicated high levels of agreement between the consultants, using the kappa measure of chance-corrected agreement (kappa = 0.792, n = 30).
Of the 14 patients whose admission was assessed as potentially avoidable, seven were male and seven were female. The median age of the patients was 84 years (range 75–97 years). Cancer was the primary diagnosis for 6 out of the 14 patients; three patients had a primary diagnosis of stroke; and the other patients had a primary diagnosis of encephalopathy, end-stage renal failure, Alzheimer’s disease, general frailty or hypertension. Half of the patients lived in nursing or residential care. Of the remaining patients, 50% lived alone and 50% cohabited. Most patients (n = 12) were admitted to hospital ‘out of hours’ (outside 0900–1700, Monday–Friday). Reasons for admission to hospital were confusion/general deterioration (n = 5), symptom control (n = 3), fall (n = 2), stroke (n = 2), urinary tract infection (n = 1) and intra-abdominal catastrophe (n = 1).
The mean cost of a potentially avoidable admission was estimated to be £2595 (range £451–£5363), based on the HRG tariffs assigned to each admission by the Sheffield Teaching Hospitals coding team. The distribution of costs is shown in Figure 8. The costs fall into three broad groups: < £1000 (n = 4), between £1000 and £4000 (n = 5) and > £4000 (n = 5). We can therefore estimate that the total potentially avoidable hospital cost from potentially avoidable admissions for the period of the survey was £36,334. The mean length of stay, based on the HRG codes assigned to these admissions, was 16.7 days.
FIGURE 8.
Distribution of estimated costs of potentially avoidable admissions.
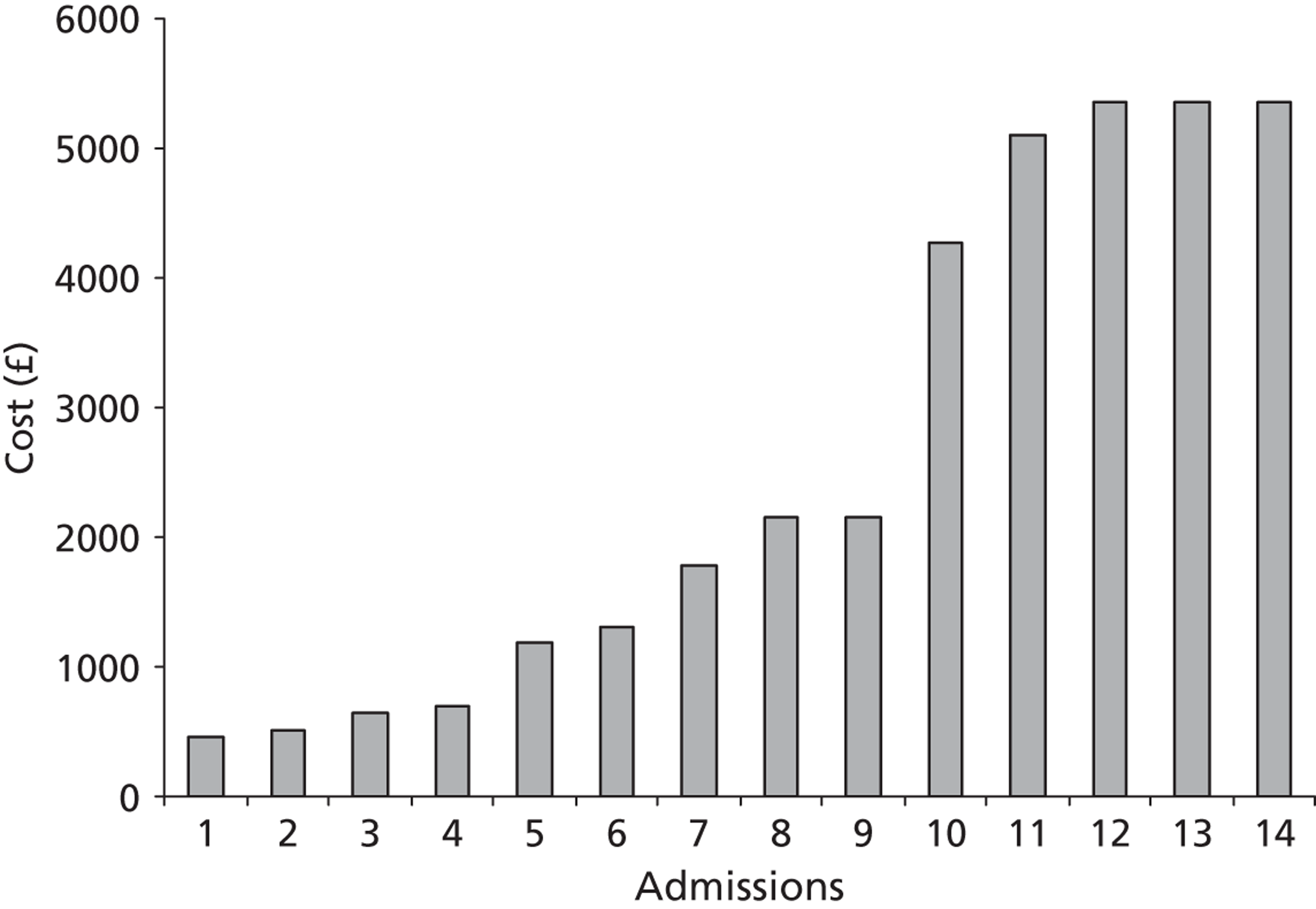
The most commonly recommended alternative place of care for patients was a nursing home (n = 10). In addition, it was considered that three patients could have been appropriately cared for in a hospice, and one in their own home. We used the same estimate of average length of stay in these alternative places of care as that estimated for length of hospital stay (16.7 days). The overall cost of alternative places of care based on this same length of stay was estimated to be £34,807 (Table 11).
| Care setting | Cost per day (£)a | Duration of care (days) | No. of patients | Total (£) |
|---|---|---|---|---|
| Nursing home care | 106 | 16.7 | 10 | 17,657 |
| Hospice care | 325 | 16.7 | 3 | 16,317 |
| Home care | 50 | 16.7 | 1 | 812 |
| Total cost | 34,807 |
Taking into account the avoided hospital costs and the cost of providing support in alternative locations, the estimated economic impact is a potential cost saving of £1527 across both hospitals for the period of the survey. There were 1359 inpatients in the two hospitals over the survey period. This accounted for 0.9% of the total admissions over the year. Assuming that the proportion of potentially avoidable admissions identified during the survey period is indicative of the number who would be identified over the course of a year, the potential annual cost saving for the two hospitals can be estimated at just under £180,000. If the cost perspective is restricted to NHS and PSS costs, only the cost of avoided hospital admissions remains unchanged; the cost of alternative care provision falls to £15,141. Based on a NHS and PSS cost perspective, then, the potential economic impact is predicted to be a cost saving of £21,193 for the census period or £2.5M per annum for the two hospitals.
Discussion
Extent of palliative care need
Our results indicate that, according to the GSF prognostic indicator guide, over one-third of hospital inpatients (36.0%) meet the criteria for palliative care need. This figure is substantially higher than other estimates of palliative care need in the acute hospital population. A French survey in 1999 reported that only 13% of total hospital beds were occupied by palliative care patients. 123 In a census undertaken in 2001, Gott and colleagues124 reported that 23% of hospital inpatients were identified as having palliative care needs. A more recent study in 201122 reported that just 9.4% of hospital patients in Belgium were identified as having palliative care needs (although this study excluded intensive care units and palliative care units). All of these studies used the subjective judgement of generalist medical and nursing staff to identify patients with palliative care need, rather than an objective measure based on diagnostic criteria, as used in this study. Our results show that, when using a systematic and objective measure, the percentage of patients with identified needs is much higher and represents a substantial proportion of the inpatient population.
Lack of consensus between medical staff, nursing staff and Gold Standards Framework prognostic indicators
One of the most significant findings from this survey is the lack of concordance between medical staff, nursing staff and GSF prognostic indicators regarding the identification of patients with palliative care needs. Although it must be acknowledged that medical and nursing staff were using a different definition of palliative care need than that in the GSF, the Canadian definition was selected on the basis that it is one of the broadest and most inclusive definitions, and is not restricted to particular diagnostic groups. Despite this, medical and nursing staff identified far fewer patients with palliative care needs than the GSF (15.5% and 17.4%, respectively, vs. 36.0%). Significantly, for the majority of patients who met GSF criteria for palliative care need (65.9%), there was no evidence of adoption of a palliative care approach.
Even amongst patients who were expected to die within 12 months, recognition of palliative care need was inconsistent. Medical and nursing staff judged that less than half of the patients they expected to die within 12 months had palliative care needs. This is despite the ‘12 months’ question constituting a key component of GSF prognostication and being advocated in policy such as in the End of Life Care Strategy for England. 5 Data from the SPARC tool for self-assessment of palliative care need indicate that, of the 185 patients identified with palliative care needs according to GSF criteria, the majority (83.2%) had problems that ‘warranted immediate attention by an attending clinician’. Despite this, agreement between medical and nursing staff and patients was very poor regarding which patients had palliative care needs.
The identification of patients with palliative care needs presents a recognised challenge. 2,89,114 Recent policy5 recommends that health professionals should be trained to identify patients approaching the end of life and to recognise when patients are dying. However, there is a lack of consensus regarding how these patients should be identified and this has significant implications for quality of patient care. 79,83,125,126
The challenge in agreeing a consensus of definition and identification of palliative care needs has additional implications for generalist palliative care providers. Recent policy and research have sought to engage more effectively with the generalist provider;125,126 however, our survey results show that many generalists are struggling to identify patients who might benefit from palliative care input. Generalist palliative care is increasingly central to hospital-based palliative care provision. Therefore, it is crucial that generalists are provided with opportunities for greater partnership working with specialist palliative care colleagues. More generally, there is also a clear need for a consensus of definition and for standardised validated criteria for the identification of patients with palliative care needs.
Older people with palliative care needs
Data from the survey suggest that older people with frailty conditions constitute a substantial proportion of hospital inpatients with palliative care needs. Although specialist palliative care uptake is low amongst the frail elderly, it is unclear whether a specialist palliative care framework is the most appropriate model for this group. The care and services provided to older people at the end of life may best be provided by generalists such as geriatricians, as part of a comprehensive generalist-led palliative care framework. Given recent concerns about the level of care provided to older people within the acute hospital setting,127,128 priority should be given to considering ways of improving the care that older people receive at the end of life. Improving generalist palliative care to support older patients in the community, improving recognition of palliative care needs amongst older frail patients and implementing models of palliative care that are appropriate for older patients at the end of life are key priorities that need to be addressed.
Extent of transitions to palliative care
Although UK policy5 advocates an early and phased transition to a palliative care approach for any patient thought to be within the last 12 months of life, our findings indicate that only one-third (33.3%) of patients with identified palliative care needs showed evidence of any such transition. Moreover, the majority of these were identified as having made a transition by virtue of having a DNAR order in place and, although a DNAR order prevents cardiopulmonary resuscitation in the event of cardiac and/or pulmonary arrest, it does not presuppose that any other treatments or interventions that the patient receives will be palliative in intent.
The odds of a transition to palliative care were multiplied by an estimated 5.1 for patients with a cancer diagnosis, by 8.0 for a stroke diagnosis and by 2.6 for a dementia diagnosis. It is well recognised that patients with a cancer diagnosis are more likely to receive specialist palliative care than patients with non-malignant disease. 5 Our results are therefore interesting in indicating that patients with a diagnosis of stroke are most likely to have made a transition to palliative care. A number of policy and educational factors may have contributed to this finding. These include the Royal College of Physicians’ guidelines for stroke129 and the DoH’s Stroke-Specific Educational Framework,130 which underlines the importance of assessment and management of end-of-life care. In the case of the dementia finding it is possible to speculate on the impact of recent recommendations that NHS hospital policy should apply the principles of palliative care to patients with dementia. 26,131
However, National Institute for Health and Care Excellence (NICE)/Social Care Institute for Excellence guidelines26 identify that cardiopulmonary resuscitation may not be effective in the case of cardiopulmonary arrest for people with severe dementia. These data may therefore be an artefact of NICE guidance as opposed to a genuine shift towards appropriate palliative transitions for people with dementia.
Guidance from the British Medical Association, the Resuscitation Council (UK) and the Royal College of Nursing132 indicates that, regardless of diagnosis, advance care planning should occur alongside the implementation of a DNAR order. Our data indicate that this is not the case in acute hospitals in England. Indeed, no documented evidence of advance care planning was found for this sample of 183 patients with palliative care needs according to GSF criteria. Various barriers to advance care planning have been identified in the literature103,133 and advanced communications skills training programmes such as Connected have been initiated across some clinical settings (mandatory in oncology settings), including acute hospitals, to address these. Our findings lend weight to the need to address the gap between policy promotion of advance care planning and current practice. They also indicate a need to ensure that any such discussions are recorded in clinical notes, both to facilitate clinical decision-making and continuity of care and to facilitate future audit and research in this area.
There are no recognised definitions of what constitutes a ‘transition to palliative care’, despite this concept being widely used in UK policy. 5 Capturing the timing and presence of a shift in care regime of this nature is inherently problematic, particularly given that there is evidence to suggest that end-of-life care discussions between clinical teams and patients and their families go unrecorded. 134 In addition, notions of phased transitions, incorporating elements of both palliative care and curative care, can further complicate the identification of this shift. In the absence of any formal criteria for identifying a transition to palliative care, we defined a transition on the basis of findings from our earlier qualitative research phase with health professionals. However, it is recognised that the list of indicators used in this study may not be comprehensive and may require validation, and this represents a limitation of this study.
Patient symptom burden
A number of variables were shown to be significant predictors of both physical symptom burden and psychological symptom burden. Patients with heart failure were less likely to have high physical symptom burden, whereas those with dementia were more likely to report high levels of both physical symptom burden and psychological symptom burden. These data provide further evidence of elevated levels of physical symptoms amongst dementia patients, requiring interventions of a supportive and palliative nature, and add to an existing literature. 135
Female sex was shown to be a significant predictor of psychological burden. Although consistent with primary care studies and other evidence on common mental disorders and sex,136 this finding is at odds with previous evidence in relation to psychological difficulties at the end life, which would suggest that the prevalence amongst male patients is higher. 137 A further predictor of psychological burden was the existence of three or more comorbidities. The relationship between multiple conditions and poor psychological status has been demonstrated in community studies. 138 Studies focusing on those patients eligible for palliative forms of care are limited to the relationship between disease-specific conditions, comorbidities and psychological distress. The development of new comorbidities is associated with readmission of palliative care patients. 139
Dementia is again implicated as a significant predictor of psychological burden (OR 2.88) and this adds further credence to the use of palliative regimes in providing care and treatment to address psychological needs for this vulnerable patient group. 140 Furthermore, it has been demonstrated on numerous occasions that this group of patients faces a number of challenges in accessing palliative and supportive regimes of care and that these challenges are both cultural and organisational. 141,142 These data suggest that, in addressing the complex physical and psychological needs of an ageing population, care teams will be increasingly required to adapt integrated palliative approaches to a cognitively frail hospital population.
Economic impact of potentially avoidable hospital admissions
The total cost of hospital admissions in the last year of life for adults admitted with a primary diagnosis indicating palliative care need has been estimated to be in the region of £1.3B. 117 A lack of timely access to services in the community may result in people with palliative care needs being unnecessarily admitted to hospital. 121 It has been suggested that, by improving or expanding community services to allow more people to be cared for and to die at home, there is the potential for a proportion of hospital costs to be avoided. 5 In 2010, the DoH reported that high-quality community-based services cost no more, and can cost less, than hospital-based care. 77 However, a review126 conducted as part of this study found that the evidence base from the UK was presently too limited to support the case for offsetting the additional costs of providing high-quality community support through a reduction in hospital admissions for this patient group.
Only 7% of hospital admissions of patients identified as having palliative care needs were classified as potentially avoidable. The potential cost saving of avoiding these admissions and supporting patients in alternative places of care in the two locations under study was estimated to be just under £180,000 per annum. The proportion of admissions identified as potentially avoidable was low relative to the proportions reported in two recent UK studies. 79,83 This difference is most likely attributable to the fact that, in our study, not only were data relating to admission and patient characteristics such as comorbidities, age and living arrangements considered, we also took into account the availability of local services. If local services were known to be inadequate to support the patient in the community, then the admission was considered appropriate. In contrast, Abel and colleagues79 based their retrospective case note review on the assumption that the End of Life Care Strategy for England5 had been fully implemented and that local services were always available and had capacity. Employing this method, these researchers classified one-third of all deaths in hospital as avoidable, suggesting that they could have occurred at home. The second study was conducted by the Balance of Care Group83 at a hospital in Sheffield and used a similar ‘blue sky’ approach whereby researchers assumed that alternative community facilities were always available and had capacity. In this similarly retrospective study, 40% of admissions for patients surveyed who died in hospital were considered to be inappropriate. The fact that both of these studies adopted a ‘blue sky’ approach to the availability and capacity of alternative places of care means that both face the issue of feasibility. Equally, the fact that both studies undertook a retrospective analysis using data collected after death means that their researchers were judging whether the admission was avoidable given the ‘full story’, whereas our clinicians were exercising their judgement from information available at the point of admission.
Although the 7% of hospital admissions seen as avoidable in our study may appear low, it does reflect the lack of appropriate alternative services to hospital admission in the two study sites for palliative care patients.
Our exploratory analysis of the economic data estimates a mean cost per avoided admission of £2595. Our estimate is lower than that reported by both Abel and colleagues79 and the Balance of Care Group83 and may have resulted in an underestimation of the cost savings generated by avoiding admissions. The difference between the estimates cited in these two studies and that calculated in our study may be attributable, at least in part, to limitations in our costing data. HRG codes were assigned to our patients using a more limited data set than would ordinarily be available. Also, because we did not have length-of-stay data, we were unable to include excess bed-day payments, which Abel and colleagues79 did include in their calculations.
Differences in the patient mix under consideration in each of the studies may also contribute to cost differentials. The adoption of a ‘blue sky’ scenario in the studies by Abel and colleagues79 and the Balance of Care Group83 would have resulted in a greater proportion of patients with more complex and, crucially, more expensive needs having their admissions categorised as avoidable.
Enabling more people with palliative care needs to be cared for and to die at home or in other community settings may result in a proportion of hospital costs being avoided. However, a clearer understanding of the proportion of hospital admissions that could be avoided and the economic impact of avoiding such admissions is needed. Further research is required to explore the relationship between admissions deemed avoidable given the situation at the point of admission and those deemed potentially avoidable in a hypothetical ‘blue sky’ scenario – and to demonstrate the feasibility of avoiding such admissions in clinical practice. In addition, more robust estimates of the cost of supporting patients in the community are also needed, in terms of both the proportion of patients requiring different types of care and the costs associated with such care.
Limitations
Although this study provides important evidence relating to palliative care in acute hospitals, certain limitations must be acknowledged. Although a wide range of experienced health professionals from primary and secondary care, and from specialist and generalist palliative care backgrounds, were asked during the focus group phase (see Chapter 4) to identify indicators of a palliative care transition, we accept that the list of indicators used in this study may not be comprehensive and may require validation. The GSF was developed as a tool for use in primary care and has to date received no formal validation in the hospital setting. Criticisms of the GSF include that it is a poor predictor of mortality; therefore, its use as a tool for identifying patients with palliative care needs in hospital should be further explored. However, amongst the 185 patients identified as meeting at least one GSF criteria, the subjective scores on the SPARC questionnaire suggest that this group had a high level of palliative care need. Validation of the GSF is now warranted by comparing GSF criteria, self-assessment of palliative care need and subsequent survival.
A further limitation that must be acknowledged is the relatively low patient response rate: only 37.8% of the total inpatient population agreed to participate and provided a complete data set. There is also a probable response bias as a result of the self-selected nature of the patient sample. However, as the overwhelming reason given for non-participation was that patients felt too ill, we believe that our sample constituted the ‘most well’ of the inpatient population. As such, the findings presented here may underestimate the true incidence of palliative care need in the acute hospital setting.
In 23 cases, consultees completed questionnaires on behalf of patients who lacked capacity to consent, and responses given by consultees may not be accurate. Therefore, caution is required in interpreting findings from the questionnaire responses and further research should seek to compare self-assessment and consultee assessment measures to explore consensus.
Chapter 6 In-depth post-discharge interviews with patients (phase 4)
The aim of this element of the study was to explore if, and how, information about a transition to a palliative care approach was communicated to patients who had been recently discharged from hospital. Fifteen service users who met criteria for palliative care need and who had been present in the hospital at the time of the survey participated in in-depth interviews exploring their perspectives on communication with health professionals regarding prognosis and goals of care. In this chapter we report on participants’ awareness of their prognosis and of the prospect of their condition deteriorating. We categorise participants’ awareness contexts according to three ‘types’ (open awareness, partial awareness and closed awareness) as an aid to conceptualising the themes that are reported in this chapter.
Background
Glaser and Strauss’s143 original typology of awareness incorporated a movement in any direction between a context of ‘closed’ awareness, in which staff kept from patients information about their prognosis, and one of ‘open’ awareness, in which all parties, the patient and family included, acknowledged that the patient was dying. The rationale for not telling patients of their prognosis was that it would better preserve their hope of a recovery and thereby prevent them from experiencing the fear and despair long associated with a knowledge of dying. 144 However, the expansion of modern hospice provision in the 1970s was accompanied by a ‘revival’ of notions of the ‘good death’,145 which could be facilitated only when there was open acknowledgment of the imminence of death. 146 From the outset, then, the modern hospice movement perceived a clear link between encouraging people to talk about their experiences of dying and an improvement in that dying experience. 147
One of the continuing barriers to entering a context of open awareness is the difficulty of prognostication, particularly for patients with conditions other than cancer, whose illness trajectories are more unpredictable. 100 Another barrier is that clinicians, whose responsibility it is to break bad news, continue to find doing so difficult and thus avoid it, or fail to reach a consensus about whose role it is to do so. 114,148 People’s interpretation or absorption of bad news when they are given it is not straightforward. There is often a discrepancy between the information that the professional perceived was given and the message received by the patient. 149
At a societal level, open awareness appears to accord with dominant Anglophone cultural values emphasising responsible care of the self and preparation for death as an extension of the highly individualised project of identity construction. 150 However, at an individual level, research evidence shows that some older people114,151 and hospice patients152,153 have stated a preference for a sudden rather than an aware death, which shows the still-limited reach of the espoused ideal.
Method
Participants were recruited during the survey of inpatients (see Chapter 5). Participants were asked to indicate whether they would be interested in participating in a follow-up interview after discharge from hospital. At the time of the survey, 131 participants agreed to be contacted (by letter) about an interview. Fifteen of those 131 participants then agreed to be interviewed 3–6 months after discharge. It is possible to attribute the low response rate to patients having died in the intervening 3–6 months; patients being too sick to participate in an interview; or patients still being in hospital or no longer residing at the contact address supplied (e.g. because of moving to a nursing home), which meant that we were unable to contact them.
All 15 interviewees were living with life-limiting conditions and met one or more of the GSF prognostic indicators for advanced disease. Participants’ ages ranged from 20 to 90 years and there were no age restrictions on this phase of the study. Table 12 details the medical conditions that participants were living with.
| Pseudonym | Age | Sex | GSF criteria | Other medical conditions |
|---|---|---|---|---|
| Mary | 87 | F | Heart disease, COPD | Musculoskeletal disease |
| Liz | 96 | F | Frailty | Duodenal lesion, peripheral vascular disease, cerebrovascular disease |
| John | 56 | M | Cancer | Peripheral vascular disease |
| Judith | 85 | F | Heart disease | Osteoporosis, peripheral vascular disease |
| Frank | 82 | M | Cancer | |
| Michael | 64 | M | COPD | Previous cancer |
| Mavis | 90 | F | Heart disease | Chronic renal disease |
| Peter | 85 | M | Frailty | Heart disease, osteoarthritis |
| Paul | 64 | M | Cancer, COPD | Heart disease, history of substance abuse |
| Beverley | 73 | F | Cancer | Heart disease, peripheral vascular disease |
| Elsie | 84 | F | COPD | Diabetes, peripheral vascular disease |
| Clare | 20 | F | Other life-limiting condition (tetraplegia) | |
| Henry | 57 | M | Other life-limiting condition (paraplegia) | Heart disease |
| Simon | 42 | M | Other life-limiting condition (tetraplegia) | |
| Mandy | 27 | F | Multiple sclerosis |
In-depth interviews were conducted (by CG) either in participants’ own homes or over the telephone and lasted approximately 1 hour.
In these interviews participants were asked about (1) the communication that they had with health professionals about their illness and about their ‘care plan’; (2) how their life was affected by their condition; (3) their satisfaction generally with the health and social care that they had received; and (4) their thoughts about the future (Box 2). All interviews were tape-recorded and transcribed verbatim. Family members/carers were present for three interviews at the request of the participant.
Two researchers (CG and NR) read the transcripts individually, and independently noted down the core themes that emerged. Notes were cross-compared and any discrepancies resolved by consensus. The data analysis programme NVivo version 8 was used to assist this process. The coding frame developed was grounded in the data rather than decided a priori. 96 Subthemes identified were then considered in relation to relevant literature. Direct quotations have been selected to illustrate the issues raised by participants and they are indicative both of typical responses and of the diversity of views obtained.
-
1. Can you tell me why you were admitted to hospital recently?
-
2. What have the doctors and nurses told you about what’s wrong with you and why you were admitted to hospital?
-
3. What have your doctors or nurses told you about what might happen with your health in the future?
-
4. Have any of your doctors or nurses given you an opportunity to discuss a care plan?
-
5. Is there any other information you would like to receive about your health?
-
6. What is bothering you most about your health at the moment?
-
7. Do you think the quality of your life has been affected by your condition?
-
8. Do you think the doctors and nurses looking after you could do anything more to make your condition easier to live with?
-
9. Is there someone who helps you with personal care or with physical tasks on a regular basis?
-
10. What worries/concerns or thoughts do you have, if any, about your future?
-
11. What type of future do you think you might have?
-
12. Do you have any thoughts or preferences on where you would like to die?
-
13. Have your doctors and nurses spoken to you about how your condition might mean you have a limited future?
-
14. Is there anything else you would like to share with me about your experience?
-
15. Were you satisfied with the care you received whilst in hospital?
-
16. Can you tell me what care you are currently receiving from your doctors and any other health- or social-care professionals?
-
17. Are you satisfied with the care you receive outside of hospital, e.g. from your GP, social services?
-
18. Could anything more be done to help you?
Results
In the following analysis we report on participants’ awareness of their prognosis and of the prospect of their condition deteriorating. We build up a picture of their awareness at a particular point in time, although we are mindful that this is a static representation that may be subject to change. We categorise participants’ awareness contexts according to three ‘types’ as an aid to conceptualising the themes that arose.
Although all but one of the participants were aware of his or her diagnosis, knowledge of prognosis was far less complete. None of the participants mentioned ‘palliative care’ and, although this is not altogether surprising given a poor lay understanding of the medical specialism,154 one might have anticipated more discussion from participants about ‘non-curative’ care, along with some indication that they had engaged with advance care planning, for example by completing a DNAR order or an advance decision. Only two participants had DNAR forms, and one existed seemingly without the participant’s knowledge. One participant did mention that his only option would be ‘remedial care’ if his current treatment failed. However, it is significant that this participant saw this as something that lay in the future, rather than something which applied to him now in his current state of poor health. Although other participants admitted that ‘there’s not that much [the doctors] can do’, this was a comment on the lack of possibilities for curative treatment, rather than an admission that their palliative care needs were being met, or that they had been informed of, and helped to come to terms with, their approaching death.
‘I don’t want no shilly shallying’: ‘open’ awareness
Some participants did seem fully aware of their prognosis and acknowledged that they might be approaching the end of their life. For one of these participants, Beverley (all names are pseudonyms), the experience of developing cancer 40 years earlier when she was in her 30s, and recovering from it, had the effect of reducing her fear of dying from her present illness, because she already believed that she had been granted additional life – ‘every day was a bonus’. This influenced the approach that she took when asking for information from her doctor:
I’d rather they just said to me ‘we don’t know how long you’ve got, but . . .’. And I would say ‘well, can you give me what normally is the length of time, you know, I know you haven’t got a crystal ball’.
Beverley was actively trying to open up a conversation with her doctors about her future, without looking for false certainty (the elusive ‘crystal ball’). Her awareness context was radically different to that during the first time that she had been treated for cancer, when ‘they didn’t tell you anything. All I was told was that it was something nasty.’
Another participant, Paul, a 64-year-old man living with heart disease and severe COPD, was similarly aware of his limited life expectancy. He had signed a DNAR order – evidence that some discussion of future deterioration had occurred. To indicate to his doctor that he preferred a situation of open awareness, Paul encouraged his doctor to be ‘straight’ with him:
[The doctor] says to me, ‘I’m glad you’re upfront about it’, so I said, ‘well, it’s daft lying isn’t it’. I said, ‘well, I want you to do the same, if you think I’ve got about 6 months to live, don’t hang back’.
Here we infer that Paul is giving his doctor permission to engage in open and honest communication. According to his son, also his live-in carer, Paul had been admitted to hospital eight or nine times in the past 2 years for exacerbation of his COPD. Paul and his son both openly acknowledge that his disease is life-limiting:
Paul: [The doctor] said ‘it can’t be cured, you know that don’t you’ and I said ‘well, aye’.
Son: They’ve not gone into details, like, [that the attacks] are going to get more frequent or they’re going to get worse, but they’ve said, you know, it’s terminal. He’s come to terms with that, he does know, he’s not shirking, he knows what’s what.
The use of the term ‘shirking’ here is suggestive of a moral imperative to take responsibility for one’s own awareness context and, therefore, one’s own dying. In spite of Paul’s request for ‘straight’ talking, his son’s comment about a ‘lack of details’ reveals that they were still lacking information about what they could expect from the disease trajectory. This is something that, had Paul been referred to specialist palliative care services during one of his many hospital admissions, would have been discussed. One of the reasons given by Paul and his son for not wanting ‘no shilly shallying’ was that they had recently experienced the death of Paul’s other son.
In both of these accounts the participant entered a context of ‘open’ awareness only because he or she gave explicit indication to the doctor that he or she wanted to know his or her prognosis.
‘I’ll bounce back’: reluctance to face the future
Other participants we identify as displaying more reluctance in accepting the life-limiting nature of their condition. Although some participants made no mention of conversations with health professionals about their prognosis, others acknowledged conversations but they appeared not to have fully internalised the information communicated to them. This latter scenario is what Timmermans155 classified as a ‘context of uncertain open awareness’.
Michael, aged 64 years, who was interviewed with his wife, had experienced four admissions to hospital in the last year for COPD exacerbations resulting from his cancer. Together, Michael and his wife gave their joint account of the multiple hospital visits, the extensive treatments undertaken, the complications resulting from bacterial infections while in hospital and the contact that they had with health-care staff in the different hospital departments. Despite being told by his doctor that ‘you’re not going to get any better’, both Michael and his wife sounded hopeful that his health had finally stabilised:
Wife: He’s slowly getting a little bit stronger and I’m not as anxious because I can see we’re on that plateau and that’s a good place to be.
And later:
Wife: Because of the damage the cancer had left in his lungs we were thrown into a terrible year, so we haven’t had . . . we’ve never had a plateau, we’ve always been on that, well, turbulent sea, we’ve been tossed around in it. But we’re . . .
Michael: We know where we are at the moment [. . .] I know that I’ve got to take my medication every morning and I have to take it every night and that keeps me on an even keel.
Talk of a ‘plateau’ and an ‘even keel’ indicates a belief that Michael’s condition had stabilised, pushing to the background thoughts about the inevitable progression of his symptoms. To an outside observer, his compounding conditions, and the chronic nature of his COPD, make their expectations seem unrealistic. However, in the context of long hospital stays and repeated admissions it is evident that ‘recovery’ becomes a relative term and that patients can read hopeful signs when doctors’ indications are to the contrary.
Another participant, John, who had lung cancer, could also be identified as being in a context of ‘uncertain open awareness’. Reflecting on being told of his prognosis by his surgeon, he said: ‘I don’t think he held back, he just told me how it was and that’s accepting it. It’s just how it’s got to be.’
John was the only participant who made reference to having been referred to a specialist palliative care professional (a community Macmillan nurse). However, he said he didn’t really rate the service because the nurse had attempted to contact him only twice. Later in the interview he said he ‘could have done with talking to somebody’ because he felt he was ‘going through it all on his own’: ‘[I could have benefited from] a bit more info, a bit more care.’
John clearly wanted more opportunities to talk through his fears. Later in the interview he signalled that he was still hopeful of a recovery. Although his doctor ‘hadn’t held back’ in his truth-telling, John still did not want to abandon his residual hope that he would recover, or that things would get back to normal: ‘It were a close shave, a couple of close shaves for me [. . .] but I’ll bounce back.’
Other participants we identify as being in a state of conditional awareness because of their avoidance of specific questions about the future. One younger participant, Clare, who had been left tetraplegic following a road traffic accident, commented: ‘a lot of the things you don’t really want to ask you avoid, you avoid it’.
Despite Clare’s noted dependence on the internet for information about her condition and insistence that ‘there’s a lot more information out there than what you’re told by your immediate consultants’, she still wanted to ‘avoid’ discussions about the future so that she could continue to believe that she could ‘do everything that I wanted to do in the first place’. People who have severe spinal cord injuries can often experience respiratory problems, which can result in ventilator dependency during a severe episode. 156 Once on a ventilator there is a chance of not being able to breathe independently again. Advance care planning in such circumstances can enable people to make decisions in advance about such interventions.
For some participants there was a reluctance to accept that future curative treatment was likely to be ineffective. They expressed an understandable ambivalence towards fully accepting the life-limiting nature of their condition and towards making the types of preparations that a palliative approach to care would have encouraged.
‘As long as I can cope, I’m not interested’: ‘closed’ awareness
Some participants did not seem to be aware of their prognosis at all in that they did not mention any discussions with health-care professionals about expected future deterioration in their health and reported no desire for information. These patients were all aged ≥ 85 years. Some registered a reluctance to ask questions of health-care professionals, either in primary care or in secondary care. As one woman, aged 90 years, commented:
I’m not bothered, I don’t care. As long as they know what’s wrong with me [. . .] they can tell you all these things and frighten the living daylights but if you don’t know what’s wrong and you’re just quite happy to lay there and let them do their worst. That’s all you can do really, isn’t it?
This woman’s comments reveal her preference for doctors, with their technical expertise, to make decisions on her behalf. This has been termed the ‘other culture’ perspective, stemming from trust being placed in others to make decisions on the person’s behalf. 157,158 If a person is reluctant to ask questions or to hear bad news, then this begs the question of how far health-care professionals should persist in giving this information.
Other participants’ lack of awareness of the life-limiting nature of their condition could be detected in the way that they spoke about their condition. Liz, aged 96 years, living with various comorbidities, said that she thought she had been ‘cured’ for now:
They’re going to operate if it gets desperate but not otherwise [. . .] the surgeon said if they operated I should probably be bedfast so it was up to me so I turned it down and said I’d wait [. . .]. They put it all down to old age [laughing].
Liz’s comments suggest that it was presented as her choice whether or not to opt for more surgery. She declined the intervention because she valued her mobility over her longevity. However, the disclaimer – ‘if it gets desperate’ – indicates a reluctance to let go altogether of the possibility of further medical interventions at some future point, if they might ‘add time’. 159 Her comments indicate her lack of awareness that she is nearing the end of her life. The reference she makes to her age suggests that, in her mind, it was a factor influencing the doctors’ diagnosis of her symptoms and their advice against opting for further treatment, rather than a factor influencing her own decision to turn down further medical and nursing interventions.
Another participant, Judith, aged 85 years, who had a new stent put in her heart during her recent hospital admission, spoke of her age as a factor influencing her own decisions about opting for treatment in the future:
Well, I’ve still got angina right, but as for the other [heart disease], no it should be all right because they joked about it and said ‘oh, you’ll be all right now for another 10 years’ – what! I’ll be 96 then [laughing]. Too old. I shan’t bother.
Judith distinguished between the age at which she is now – when she opted for a new stent to prolong her life – and her age in 10 years time – when she predicts she will consider herself ‘too old’ to consider further treatment. The tension between awareness of old age and yet seeming lack of awareness of one’s own dying was a common theme.
In general terms, although these participants showed an awareness of the influence of old age on decisions about treatment, and on their expectations of their health, this did not signal a concomitant awareness of the way that their own old age brought them closer to death. In this way, age was a factor that might have made their impending deaths ‘timely’ or ‘natural’ to others, but not to them, and not right now.
Discussion
In their formative research into people’s awareness of dying in a hospital context, Glaser and Strauss143 came to the conclusion that a context of ‘open’ awareness was preferable as it gave the patient ‘an opportunity actively to manage his own dying’ (p. 135). As the evidence base for palliative care has grown, so too has the conviction that, if a person is given insight into the probable trajectory of their condition, both they and their families will have more opportunity to participate in decisions about end-of-life care. Evidence suggests that a person’s lack of awareness of his or her disease stage is likely to result in unnecessary and often unwanted hospital admissions160 leading to an increased likelihood of dying in hospital;161,162 a lower likelihood of referral to specialist palliative care services;163 and less end-of-life planning that would enable them to use ‘time left’ in a conscious way to ‘achieve their personal goals’. 164 Such evidence has combined with culturally informed assumptions about a desire for self-determination to offer a cue to policy-makers to create guidance5,28,165 that is unequivocal in its promotion of an ‘open’ awareness context as a person approaches his or her death.
The 15 participants in this study were all identified as being potentially in need of palliative care according to standardised diagnostic criteria (GSF) that indicate a prognosis of ≤ 12 months. Despite indications, then, that participants were approaching the end of their life, participants’ testimonies show that awareness varied and even fluctuated within individual patients. When prognosis had been discussed with health professionals, participants did not always interpret this information to mean that they were nearing death. The overall impression given by participants’ accounts was that their expectations about the future trajectory of their illness were open, vague and uninformed.
The participants who we suggest were openly aware of their prognosis spoke of having to encourage their doctors to be ‘straight’ with them. These were people for whom death was ‘experience near’, either because of the death of close kin, or through surviving previous life-threatening illnesses, perhaps making death appear less ‘other’. Comments made by one participant’s son that his father ‘wasn’t shirking’ indicate that, for him, a context of open awareness was an ideal state and one that indicated that a person was taking responsibility for his or her own dying. Care of the self and improvement of the self are culturally valued attributes150 and Paul’s son shows his support for this normative imperative, although the fact that he was the only participant to suggest a moral virtue in preparedness for death shows the limited reach of this aspect of the ‘good death’ ideal amongst our participants.
Other participants who we describe as being in a context of ‘uncertain open awareness’155 had been made aware of their prognosis but were hopeful of an improvement in their condition or at least that it could be made stable. Others were more focused on ‘living with’ rather than ‘dying of’ their illness and did not encourage forthrightness about their prognosis. This finding is contrary to expectations laid out in guidance to doctors that ‘patients whose death from their current condition is a foreseeable possibility are likely to want the opportunity to decide what arrangements should be made to manage the final stages of their illness’ (emphasis added). 28
Other participants we identified as being in a context of ‘closed’ awareness. It is significant that all of these participants were aged ≥ 85 years, if only because it challenges the commonly held belief that death in old age is ‘timely’ or ‘natural’166 and thus more easily anticipated and accepted by those advancing in years. This was not the case for our participants, who spoke of their age in terms of its influence on the judgements of the health professionals responsible for their care – ‘they put it all down to old age’ – rather than in terms of its influence on their relationship with their own death. As Gott and colleagues75 write, ‘timely may always be at an age older than you are now’ (p. 1119). The slow decline that many in the fourth age of life experience means that older people do not reach a ‘dramatic moment of dying’ and are thus left out of the ‘heroic script of aware dying’ (p. 612). 167 In terms of our broader study, we found that a large proportion of the patients surveyed in the hospitals who met GSF criteria were frail older people (aged > 85 years) with multiple comorbidities and multiple previous admissions to hospital. 168 However, there is evidence that older people are less likely to be referred to specialist palliative care services,106 a move that would be likely to ‘force’ awareness of their proximity to death.
Overall, findings from this phase of the study show that communication between health professionals and patients and their family is not easily mapped onto ‘ideal-type’ scenarios. Although medical staff are responsible for disclosing a patient’s prognosis, nursing staff hold the day-to-day information about a patient’s circumstances on which judgements about how best to open up end-of-life discussions can be based. 169
Nurses are also the ‘practical managers’ of events that follow disclosure of prognosis169 and so must, by all accounts, be involved in the multidisciplinary effort that keeps patients informed, in unambiguous language, about the likely trajectory of their disease. Knowledge of prognosis is often viewed as uncertain by health professionals and this is used as a reason to defer difficult conversations requiring emotional labour. 170 Nonetheless, as Murray and colleagues’171 work on illness trajectories shows, patients with specific diseases and their carers often have common experiences, symptoms and needs as the illness progresses, which supports the notion of a typical or characteristic trajectory. Often there are other reasons why patients develop a false optimism about their chances of recovery, such as patients’ collusion through ‘gratefully accepting every opportunity to “forget” the future’ (p. 1380). 164
Although patients have been found to want honesty, they also want ambiguity in the information that they receive, rather than it being too definitive or unequivocal. 172 As this study shows, patients are not always clamouring for information about their prognosis. In fact, some displayed a reluctance to acquire the wrong type of knowledge; knowledge that would require them to face the imminence of death.
Chapter summary
Given the complexity of the social interactions and subjectivities that determine the context in which a person becomes aware that they are dying, it is unsurprising that UK regulatory guidance and policy fails to capture the all-too-human ways in which people do not conform to the autonomy paradigm of a self-directed life followed by a self-directed death. Although awareness of dying can lead to better preparedness for death for both individuals and the wider social group, this study shows that, at the level of communication between individual patients, their families and health professionals, considerable barriers exist to ensuring that patients are aware. To reduce people’s suffering at the end of life by ensuring timely access to palliative care for those who need it, we suggest that, alongside doctors, whose overall responsibility it is to initiate end-of-life discussions, nurses must play their part within a multidisciplinary team to come up with ‘novel ways of delivering palliative care’173 that do not rely on patients acknowledging that they are dying, rather than relying on the false assumption that awareness can and should be encouraged in all cases.
Limitations
The main limitation of this part of the study is the relatively low response rate from participants. For ethical reasons we were unable to explore reasons for non-participation in interviews; however, we have suggested reasons for non-participation earlier in this chapter (see Methods). This is a limitation commonly experienced by researchers attempting to gather data from people who are nearing the end of their life. Because of the small sample size we cannot be certain that we reached thematic saturation. However, for the data collected, two researchers engaged in a constant comparative exercise to check that the interpretation offered was internally valid. A second limitation was the research ethics committee’s stipulation about the language to be used in the interviews (see Chapter 1, Ethical approval). As a consequence of the indirect line of questioning adopted, there is a possibility that participants possessed knowledge of their prognosis that remained unexpressed during the interviews. This potential for ‘missed’ data is an unavoidable consequence of adopting an ethically cautious approach, which, in light of the finding that participants’ knowledge of prognosis was indeed limited, proved to be warranted.
Chapter 7 Retrospective case note review (phase 5)
In this chapter we present the methods and results from a retrospective case note review of patients who died following an admission to the SNGH or the RLI. In doing so we address the following objectives:
-
to identify those hospital admissions amongst people with palliative care needs that were avoidable but which occurred because of a lack of alternative service provision or support in the community
-
to quantify the cost of avoidable acute hospital admissions amongst those patients with palliative care needs.
As noted in Chapter 1 (see Amendments to protocol), this phase was added to the original protocol as a result of changes stipulated by the research ethics committee. Data collection was undertaken during a 6-month no-cost extension (January–July 2012). In agreement with the Health Services and Delivery Research (HS&DR) programme we present preliminary findings only. Further analyses are currently being conducted and prepared for publication.
Background
Around 90% of people spend time in hospital in their final year of life. 17 Findings from Chapter 5 indicate that 36.0% of all hospital inpatients have palliative care needs. The total cost of UK hospital admissions in the last year of life for adults admitted with a primary diagnosis indicating palliative care needs has been estimated to be in the region of £1.3B. 2 A lack of timely access to services in the community may result in people with palliative care needs being unnecessarily admitted to hospital. 86 It has been suggested that improving and expanding community services may reduce avoidable hospital admissions amongst patients with palliative care needs, thus reducing a proportion of hospital costs. 5 However, the review reported in Chapter 2 found that the evidence base from the UK was presently too limited to support the case for offsetting the additional costs of providing high-quality community support through a reduction in hospital admissions. 174 Findings reported in Chapter 5 present preliminary evidence which suggested that the estimated cost saving of preventing potentially avoidable hospital admissions amongst patients with palliative care needs was £1527 for both hospitals over the survey period. This extrapolates to savings of around £180,000 per annum. However, limitations of this data set were acknowledged, in particular the potential bias amongst the patient sample and limitations in the costing approach. 175
Therefore, the retrospective case note review was undertaken to provide robust evidence from a complete population sample of hospital inpatients who died as a consequence of life-limiting illness on the economic cost of potentially avoidable hospital admissions amongst patients with palliative care needs.
Ethical approval
As this phase of the study required access to patient-identifiable data without consent, support under section 251 of the NHS Act 200643 was required. This was provided by the NIGB ECC (22 November 2010).
Retrospective review methods
Data collection
Data queries were sent to Information Services at the two participating hospitals requesting lists of inpatients present in the two hospitals during day 1 of their respective survey periods (10 May 2010 for SNGH and 15 November 2010 for RLI) and who had died by that date 1 year later. Data from Information Services were generated from locally managed databases that derive information from hospital patient administration systems. Data returned from Information Services comprised the following: NHS number, sex, date of birth, date of death, HRG code for survey admission and length of stay (days) for survey admission. HRG codes are clinically meaningful groupings of patient activity, based on both diagnoses and clinical procedures undertaken. HRG codes are allocated locally by ‘coding teams’ whose remit is to generate and allocate the most appropriate code for each admission. Standard costs are assigned to each HRG and these are used across the NHS in England to calculate the cost of a hospital admission and generate reimbursement through a system known as payment by results. 117
Patient lists generated by Information Services were submitted to the medical records department of the relevant hospital and the complete hospital case notes of each patient on the list were recalled. Case notes were reviewed by two researchers (senior nurse academics) and data were recorded onto paper proformas. The range of data collected from hospital case notes is shown in Box 3.
-
Living arrangements at time of survey admission.
-
Sex.
-
Ethnicity.
-
Partnership status at time of survey admission.
-
Cause of death.
-
Place of death.
-
Age at death.
-
Any GSF criteria that the patient met at the time of the survey admission.
-
Reason for survey admission.
-
Time/date of survey admission.
-
Length of stay for survey admission.
-
Number of comorbidities at time of survey admission.
-
Medical and nursing staff admission plans for survey admission.
-
Evidence of transition to palliative care in the 12 months before death (using indicators outlined in Chapter 5).
-
Evidence of cognitive impairment in the last 12 months before death.
-
Number of hospital admission in the 12 months prior to death.
-
Number of days spent in hospital in the 12 months prior to death.
Recording of cause of death and place of death data in hospital case notes was variable and not available for all patients. Therefore, these data were also collected using Office for National Statistics Hospital Episode Statistics (ONS-HES)-linked mortality data, from the Medical Research Information Service (MRIS) of the NHS Information Centre for Health and Social Care. Patient lists from the two hospitals were uploaded via a secure data-sharing system to MRIS. MRIS undertook data matching and lists were returned for successfully matched patients with data on the following variables: place of death, cause of death, underlying cause of death (up to four iterations) and coroner’s verdict when an inquest was held.
Appropriateness of admission to hospital
Data collected from hospital case notes and the MRIS were reviewed by two palliative medicine consultants (BN and MB) to assess appropriateness of admission to hospital for the survey admission. Consultants reviewed the notes from the hospital where they worked, as knowledge of local service provision and organisation was considered critical for informing decision-making. Data collected from case notes and the MRIS were considered in addition to knowledge of local services and a decision was made whether the admission was ‘appropriate’, ‘potentially avoidable’ or ‘insufficient data to make a decision’. An ‘appropriate admission’ was defined as an admission that was necessary because of clinical need, taking into account the patient’s circumstances and the availability of local community services at the time of the admission. For admissions deemed potentially avoidable, an alternative to hospital admission was suggested. To ensure consistency in clinical decision-making between the two consultants, they double-reviewed a random sample of 10% of the notes. The level of agreement between the clinicians was assessed using a Cohen’s kappa statistic.
Data analysis
All data were recorded onto paper proformas and entered onto an SPSS database. For each of the hospital admissions identified by the consultants as potentially avoidable, the cost of the admission was calculated using the assigned HRG code. HRG codes and tariffs for 2010/11176 were used except in three cases in which the code was not identifiable and the code and tariff for 2011/12 were used. 117 For patients whose hospital length of stay exceeded the expected upper length of stay for the HRG (trim point), an additional long-stay or excess bed-day payment was added. This was derived by multiplying the per-day long-stay payment for the relevant HRG code by the number of days that the hospital spell exceeded the HRG trim point.
The costs of the alternative places of care suggested by our consultants were estimated, based on costs taken from published national sources and inflated to 2011 prices when necessary (Table 13). The cost of nursing home care was obtained from the Personal and Social Service Research Unit’s Unit Costs of Health and Social Care 2010. 118 The cost of hospice care was obtained from research commissioned by the National Audit Office. 121 The cost of home care was derived from a King’s Fund report that estimated the cost of home care in the last 8 weeks of life. 82 This cost included the following services: GPs, district nursing, Marie Curie and Macmillan nurses, ambulance journeys, hospice at home and hospice (inpatient), and equipment. An average daily cost was calculated from the 8-week figure cited in the report.
Results
Patient recruitment
At the SNGH, 302 patients who were present in the hospital on 10 May 2010 had subsequently died by 10 May 2011. In total, 279 sets of notes were reviewed; 23 sets of notes were not available despite repeated requests to the medical records department. At the RLI, 211 patients who were present in the hospital on the 15 November 2010 had subsequently died by 15 November 2011. In total, 205 sets of notes were reviewed; six sets of notes were not available. In total, notes were reviewed for 484 patients (response rate 94.3%). Data from the two hospital Information Services departments were returned for all 484 patients. ONS-HES-linked mortality data were returned for 480 patients; matching was not possible in four cases. For these four patients, cause and place of death data were retrieved from hospital case notes.
As the focus of this study was hospital admissions in patients with palliative care needs before death, cause of death was examined for all patients. Those patients who did not die from causes relating to chronic or life-limiting conditions (i.e. who died from accidental, or sudden unpredictable causes) were excluded. Only one patient was excluded, for whom a coroner’s verdict of suicide was recorded. The final analyses were therefore undertaken on 483 patients (Figure 9).
FIGURE 9.
Details of recruitment for hospital inpatients who died within 1 year of a hospital admission.
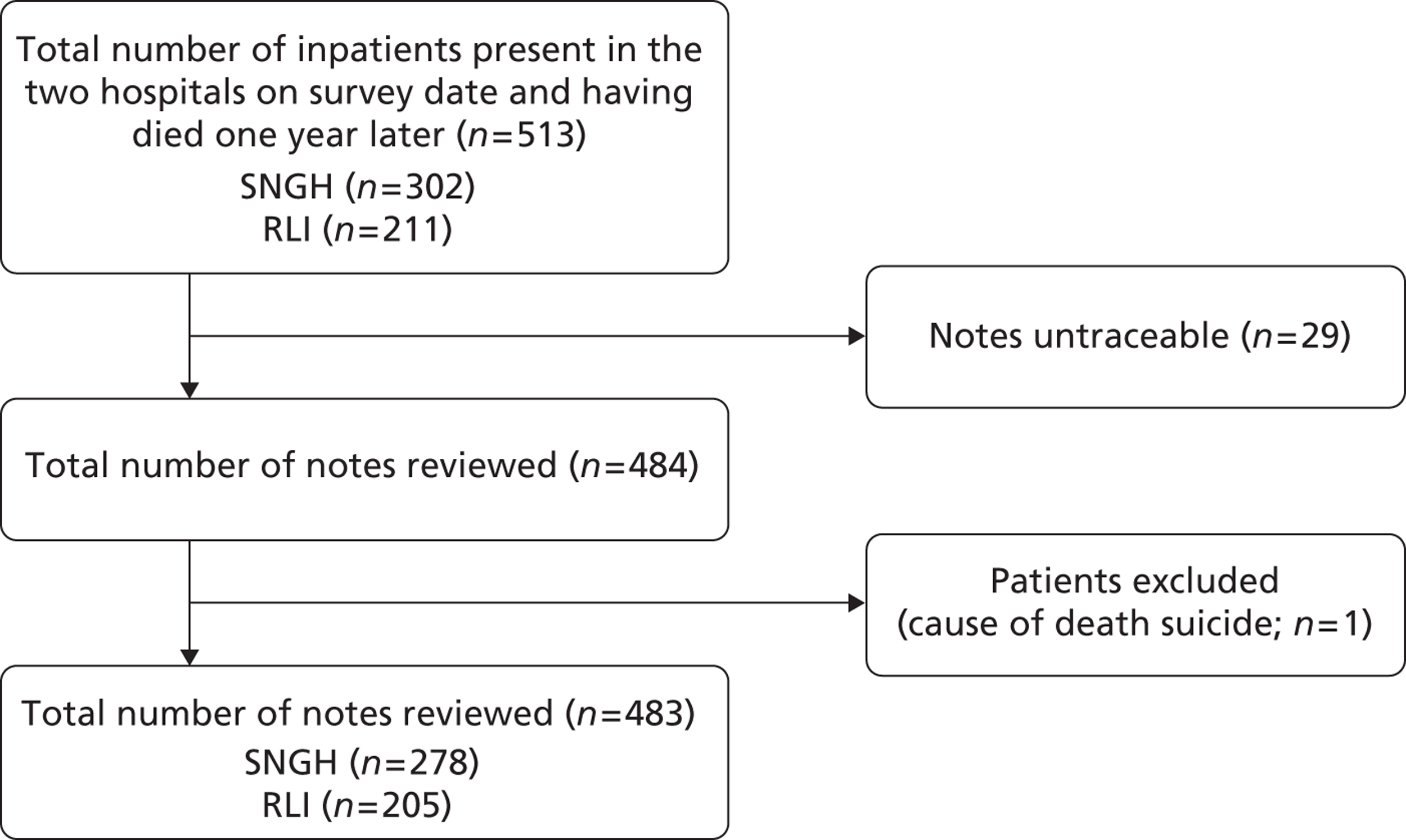
Demographic data for all patients
Table 14 presents demographic data for the total patient sample. Just over half of patients were female (52.2%) and the majority were of white ethnic origin (87.8%). Most of the patients lived independently at the time of their admission, either living alone or cohabiting (70.4%). Demographic information was poorly recorded in hospital case notes and there was a substantial amount of missing data relating to demographic variables.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 231 (47.8) |
| Female | 252 (52.2) |
| Ethnicity | |
| White | 424 (87.8) |
| Asian/Asian British | 11 (2.3) |
| Black/black British | 4 (0.8) |
| Chinese | 1 (0.2) |
| Not stated | 43 (8.9) |
| Living arrangements | |
| Cohabits | 172 (35.6) |
| Lives alone | 168 (34.8) |
| Nursing home or residential care | 91 (18.8) |
| Not stated | 52 (10.8) |
| Median age (years) | 82 |
| Age range (years) | 23–103 |
The majority of patients died in hospital (65.6%). Although only 8.7% of patients died in their own home, this figure increased to 30% when including all patients who died in their usual place of residence (Table 15).
| Place of death | n (%) |
|---|---|
| Hospital | 316 (65.4) |
| Hospice | 22 (4.6) |
| Nursing home | 88 (18.2) |
| Residential home | 15 (3.1) |
| Own home | 42 (8.7) |
The most common cause of death was bronchopneumonia (27.5%), followed by cancer (18.2%). Cause of death is documented in Table 16.
| Cause of death | n (%) |
|---|---|
| Bronchopneumonia | 133 (27.5%) |
| Cancer/carcinoma | 88 (18.2%) |
| Heart disease (including cardiac arrest) | 42 (8.7%) |
| Congestive heart failure | 34 (7.0%) |
| Septicaemia | 29 (6.0%) |
| Frailty/old age | 23 (4.8%) |
| Renal failure | 22 (4.6%) |
| Dementia | 20 (4.1%) |
| Stroke | 19 (3.9%) |
| Multiple organ failure | 14 (2.9%) |
| COPD/pulmonary fibrosis | 12 (2.5%) |
| Cerebral haemorrhage | 6 (1.2%) |
| Liver disease | 4 (0.8%) |
| Other | 37 (7.7%) |
To identify patients who had made a transition to a palliative care approach before death, case notes were explored for indicators of transition. Of the 483 patients who had died, just over half (n = 255, 52.8%) had evidence of a transition to a palliative care approach before death. Nearly half of the patients (47.4%) had a DNAR order in place in hospital before they died, 14.1% of patients had been placed on the LCP in hospital before death and 9.1% of patients had evidence of a referral to specialist palliative care services before death (Table 17).
| Indicator of transition | n (%) |
|---|---|
| Advanced refusal of resuscitation | 229 (47.4) |
| Advanced decision to refuse treatment | 4 (0.8) |
| Placed on LCP | 68 (14.1) |
| Referred to specialist palliative care services | 44 (9.1) |
| Prescription of long-term opiates | 48 (9.9) |
| Use of syringe driver | 16 (3.3) |
| No indicators of a transition to palliative care | 228 (47.2) |
Potentially avoidable admissions
Amongst the 483 patients included in the case note review, 35 (7.2%) admissions were classified by our two palliative medicine consultants as potentially avoidable. Double coding of a random sample of 10% of the notes indicated moderate levels of agreement between the consultants, using the kappa measure of chance-corrected agreement (kappa = 0.474, n = 52). 122 Of the potentially avoidable admissions, 21 (60.0%) were of male patients and 14 (40.0%) were of female patients. The majority lived in nursing or residential care at the time of their hospital admission (n = 26, 74.3%). Nine patients (25.7%) lived independently; of these, five (14.3%) lived alone and four (11.4%) cohabited.
The most commonly recommended alternative place of care was a nursing home (n = 28). In addition, it was considered that three patients could have been cared for in a hospice and four in their own home with appropriate support (Table 18).
| Alternative place of care | n (%) |
|---|---|
| Hospice | 3 (8.6) |
| Own home | 4 (11.4) |
| Nursing home | 28 (80.0) |
Cause of death data were obtained from ONS-HES-linked mortality data and are shown in Table 19. The most common cause of death was bronchitis/pneumonia (n = 11), followed by frailty/old age (n = 8).
| Cause of death | n (%) |
|---|---|
| Bronchitis/pneumonia | 11 (31.4) |
| Frailty/old age | 8 (22.9) |
| Dementia | 4 (11.4) |
| Renal failure | 3 (8.6) |
| Cancer | 3 (8.6) |
| Multiple organ failure | 2 (5.7) |
| Congestive heart failure | 1 (2.9) |
| Cardiac arrest | 1 (2.9) |
| Upper airway obstruction | 1 (2.9) |
| Clostridium difficile infection | 1 (2.9) |
Economic impact of potentially avoidable admissions
The mean cost of a potentially avoidable admission was estimated to be £6068 (range £1571–£27,343). The mean length of stay of these admissions was 40.4 days. For 13 of the 35 admissions, the length of stay of the hospital spell exceeded the HRG trim point and costs relating to the excess bed-days were included. The total hospital cost for the 35 potentially avoidable admissions was £212,397.
The recommended alternative place of care for patients was a nursing home for 28 patients, a hospice for three patients and own home for four patients. Based on the mean length of stay of 40.4 days the overall cost of alternative places of care was estimated to be £167,110 (Table 20).
| Care setting | Cost per day (£)a | Duration of care (days) | No. of patients | Total cost (£) |
|---|---|---|---|---|
| Nursing home care | 106 | 40.4 | 28 | 119,588 |
| Hospice care | 325 | 40.4 | 3 | 39,469 |
| Home care | 50 | 40.4 | 4 | 8054 |
| Total cost | 167,110 | |||
Taking into account the avoided hospital costs and the cost of providing support in alternative locations, the estimated economic impact is a potential cost saving of £45,287 across both hospitals for inpatients with palliative care needs on a single day. Data from the surveys detailed in Chapter 5 provide a snapshot of hospital activity and indicate that the total number of resident inpatients at the two hospitals was 1359 per day. This accounted for 0.9% of total admissions over the year. Assuming that 1359 inpatients is representative of the total number of patients in the hospital on any given day, the proportion of patients with palliative care needs is estimated to be 35.5%. Further, assuming that the proportion of potentially avoidable admissions identified during the survey period is indicative of the proportion who would be identified over the course of a year, the potential annual cost saving for the two hospitals can be estimated at just under £5.3M.
Discussion
In this retrospective study, 7.2% of hospital admissions amongst patients who died in the subsequent 12 months of causes relating to chronic or life-limiting conditions were classified as potentially avoidable. The potential cost saving of avoiding these admissions and supporting patients in alternative places of care in the two locations under study was estimated to be around £45,000 for patients in hospital on a single day.
The proportion of admissions identified as potentially avoidable is in close agreement with that reported in our previously reported exploratory study. 175 It is, however, low relative to the proportions reported in two other UK studies,79,83 which both used a ‘blue sky’ approach in which researchers assumed that alternative community facilities were always available and had capacity. The low level of potentially avoidable admissions in both of our studies may well reflect the lack of appropriate alternative services in the two study sites to hospital admission for palliative care patients. More admissions may be potentially preventable but this would require significant expansion of existing services and greater resources directed to community, respite and social care. Investment in advance care planning may also reduce hospitalisations,5 although evidence is currently limited.
The mean cost per potentially avoided admission of £6068 is considerably higher than the estimate of £2595 from our original exploratory analysis. 175 The costing methodology employed in this analysis is considerably more robust than that used in our exploratory analysis. In the exploratory analysis the HRG codes were assigned to our patients using a more limited data set than would ordinarily be available and we did not have length of stay data and so were unable to include long-stay payments. The impact of including long-stay payments in our current analysis is to increase the mean cost per avoided admission from £3179 to £6068; this provides a more complete picture of the total cost of admissions for all patients, including these long-stay patients. Our estimate of £6068 is much higher than the costs reported by both Abel and colleagues79 (£3173 per patient, based on a mean length of stay of 12 days) and the Balance of Care Group83 (£4690 per patient, based on a mean length of stay of around 19 days for avoidable admissions). This reflects the higher lengths of stay in our study.
Our study suggests that the scope for cost savings by avoiding admissions may be relatively limited given current service configuration. This suggests that a greater emphasis should be on discharging patients from hospital more rapidly; this is where recognising transitions is key and where costs savings are likely to be greater. It is significant that, in this retrospective study, 13 (37.1%) of the potentially avoidable admissions included hospital stays that exceeded the maximum HRG trim point, driving up further the cost of avoidable admissions.
The mean per-day long-stay payment for the 35 avoidable admissions in this analysis is £191. If it is assumed that this is a reasonable estimate of the cost per day for all palliative care admissions, then reducing the length of stay for all 483 patients in this current analysis by 2 days or 3 days would result in an estimated saving in hospital costs of £184,865 or £277,297 respectively. A 2-day reduction in length of stay would result in a cost saving of £21.6M, and a 3-day reduction a cost saving of £32.4M if this is extrapolated to an annual cost (using the same rationale as reported in Economic impact of potentially avoidable admissions). This compares with the hospital cost saving of £212,380 for the 35 potentially avoidable admissions. Increasing resources within hospitals (more palliative care teams, etc.) could increase the speed of transition to palliative care and improve early discharge of patients. Aiming to reduce hospital costs by reducing length of stay may be more achievable than avoiding admissions. Capacity issues may, however, still remain, including the extent to which there is capacity to accommodate large volumes of additional patients in community locations such as nursing homes.
Study limitations
There are recognised limitations to this study. The first relates to the subjective assessment of whether an admission was potentially avoidable. We relied on highly experienced clinicians to make this decision; clinical decisions to admit patients to hospital are, by definition, subjective and will vary geographically because of differing community service provision. We tried to minimise bias by double coding a random sample, which indicated a reasonable level of agreement between clinicians. Given the different community service configurations in each locality and the approach that we adopted of considering the appropriateness of admissions within each specific local context, perfect agreement would not be expected. The cost of supporting patients in other locations was derived from other published studies and these studies themselves have limitations. In particular, the cost of supporting people at home was taken from a study for patients in the last 8 weeks of life. 82 Not only did these costs vary significantly, they are not necessarily representative of patients in our survey who were in the last year of life. More research is required to determine valid costings for generalist and specialist community palliative care services in England.
Chapter summary
Our estimate of the proportion of admissions classified as potentially avoidable provides support to the estimate from our previous exploratory study. 175 It is, however, lower than those reported in two other UK studies,79,83 both of which assumed that alternative places of care were always available.
Further research is still required to explore the relationship between admissions deemed avoidable given the situation at the point of admission and those deemed potentially avoidable in a hypothetical ‘blue sky’ scenario – the approach adopted in the other UK studies – and to demonstrate the feasibility of avoiding such admissions in clinical practice.
Finally, our findings indicate significant potential cost savings from reducing the length of stay for inpatients with palliative care needs. This requires further examination. In addition, more robust estimates of the costs of supporting patients in the community are still needed, in terms of both the proportion of patients requiring different types of care and the costs associated with such care.
Chapter 8 Focus groups with key health- and social-care professionals (phase 6)
In this chapter we present findings from focus groups that explored the perspectives of service providers and commissioners regarding acute hospital admissions and discharges associated with a transition to palliative care.
Following analysis of the survey data, focus groups were planned within each locality with service commissioners/planners and key health- and social-care providers involved in the care of people with palliative care needs, within hospital and community settings. The aim of this phase of the research was to explore current practice in palliative care management, with a particular focus on issues identified as priorities during the survey. In addition, the results of the surveys were to be fed back to participants, as a trigger to applying a SWOT analysis (strengths, weaknesses, opportunities and threats) to develop models for best practice.
Methods
Focus groups were selected as the most suitable method for this phase of the research as they allowed researchers to capitalise on group interactions and to elicit rich data by exploring participants’ knowledge and experiences in relation to key findings from the hospital surveys. 97 Focus groups were arranged with the assistance of local NHS trusts and Comprehensive Local Research Networks (CLRNs) in each locality.
Four focus groups were held with a total of 83 participants. One focus group (n = 15) was held during a regular meeting of the UHMBT End of Life Group, a locality group with a special interest in end-of-life care. Participants included members of the clinical directorate, PCT commissioning leads, chaplaincy representatives and Macmillan nursing staff. One focus group (n = 15) was held during a regular meeting of the South Yorkshire Cutler Group, a consortium of GP practices that support research activity. Participants included GP practice research leads, GP commissioners, GP clinical academics, community occupational therapists and a representative from the local Primary Care Research Network. One focus group (n = 23) was held during a monthly research seminar at Rotherham Hospital; the hospital organises monthly seminars to disseminate relevant research to hospital staff. Participants included a wide range of medical, nursing and allied health professional staff from across the hospital. The final focus group (n = 30) was held during a clinical management board meeting of the Sheffield Teaching Hospitals NHS Foundation Trust. The clinical management board meet monthly to discuss issues of relevance to the clinical management of the trust. Participants included the trust chief executive, senior clinical management staff and trust directorate leads.
Focus groups were purposively arranged during planned meetings of existing groups because of feasibility issues to do with drawing together large numbers of senior staff for standalone focus groups. Two focus groups were facilitated by CG, one by CI and one by BN and MC. Each focus group began with a 10-minute Microsoft PowerPoint 2010 presentation (Microsoft Corporation, Redmond, WA, USA) highlighting key findings from the project, tailored to the local interests of each group. Any questions about the project or the data were answered following the presentation. A focus group guide was developed following analysis of the survey data from the two participating hospitals, and this was used to guide discussion subsequent to the presentation (Box 4).
Prompts:
-
What are the implications of the study findings for local service provision and management?
-
What do you think are the prioritisations for palliative care provision of services across and between different providers, given these findings?
-
What are the priorities and challenges for commissioning in light of these findings and the changing environment for commissioning?
-
Feasibility of implementing study recommendations in relation to management and delivery of palliative care.
Prompts:
-
How appropriate do you think current education and training provision is regarding palliative care provision in the acute hospital setting?
-
What are the priorities for education and training for hospital staff (pre-registration and learning beyond registration)?
-
What are your thoughts regarding the feasibility and acceptability of education and training for the palliative care workforce?
Prompts:
-
In your opinion, what are the future priorities in light of these findings and the proposed changes to commissioning and the structure of the NHS (practice-based commissioning, personalised health budgets: commissioning for outcomes)?
-
In your opinion, what are the future priorities in light of these findings and local and regional structure and organisation of services?
Two focus groups were digitally recorded and transcribed verbatim; for the other two focus groups recording was not possible and so detailed notes were taken and written up in full afterwards. A thematic analysis of the data was completed by NR, who synthesised key points. Direct quotations and transcribed notes have been selected to illustrate the issues raised by participants and they are indicative both of typical responses and of the diversity of views obtained.
Results
Throughout, participants made reference to their own experiences of specialist and generalist palliative care provision and how they thought services could potentially be improved to provide a better standard of care for patients at the end of life.
Response to project findings: ‘We’re sitting on a bit of a time bomb . . .’
In general, there was some noted surprise when those in attendance were informed of the finding that 36.0% of patients surveyed in hospital met one or more GSF prognostic indicator criteria – ‘I was expecting something a fraction of that’. Some participants said that this high figure showed that ‘we massively under-identify’ people with palliative care needs. The fact that the majority of patients identified in the hospital survey as having palliative care needs were frail, older people with multiple comorbidities and multiple previous admissions to hospital indicated to one respondent that ‘we are sitting on a bit of a time bomb’. Some health professionals acknowledged difficulties in recognising frail patients as having palliative care needs: ‘You recognise the people who have got “palliative terminal cancer”, but you don’t say “she’s the person who has got palliative frailty”.’
It was also recognised that frail older patients might not perceive their condition as necessitating a palliative care approach:
Does the patient perceive themselves as being palliative? . . . People who have terminal cancer can also see themselves as palliative but not so for, say, heart disease or chronic lung disease.
Participants were unsurprised that many patients with frailty were hospitalised:
We in general practice are much better at trying to keep people out of hospital who have palliative care related to cancer, but we’re not very good at keeping people out of hospital who have palliative care related to frailty, for instance.
The project finding that 6.7% of patients with palliative care needs were admitted to hospital ‘inappropriately’ was also commented on by some participants: ‘For me, if 36% have palliative care needs . . . that’s 36% that’s inappropriately in hospitals.’
It was acknowledged that hospital was unlikely to be the preferred place of death for many people, and that more should be done to provide alternative places of care: ‘That’s a huge scandal in this country that people die in hospital who shouldn’t do and against their wishes.’
Some participants were also concerned that, if the palliative care need was as significant as the findings from the project showed, then effectively and appropriately caring for these patients was likely to have resource implications: ‘Resources are more stringent now in the hospitals as well as in the community and people are stretched.’
As one participant emphasised, implementing good palliative care was time-consuming in that it involved negotiating complex family dynamics.
Development of local policy and strategy in response to the findings
A number of participants expressed a desire to see the results of the study communicated to health service managers so that local policy could be changed in accordance with the findings. There was a criticism from one participant that, all too often, health service managers were interested only in meeting targets, rather than in allowing doctors the time needed to meet patients’ palliative care needs: ‘The biggest barrier is time. Targets are what the managers are interested in.’
Both clinicians and health service managers were present during a meeting of the clinical management board of the Sheffield Teaching Hospitals NHS Foundation Trust. The impact of the study findings was seen as significant within the trust. The group endorsed the proposed development of a supportive and end-of-life care strategy group for commissioning, co-ordinating and educating as a result of hearing the findings of the study. This group is intended to provide an overall vision for supportive, palliative and end-of-life care within the trust. Key priorities for the group were discussed and included horizontal integration between directorates to aid identification and care; vertical integration between primary care and secondary care to aid timely discharge; and a focus on improving care at the end of life in Sheffield, both in hospital and at home. The strategy group will be led by two palliative medicine consultants (including BN) and will meet quarterly.
During the meeting with the Cutler Group of GPs there was some discussion, with representation from the CLRN, around the importance of the issues raised. In acknowledgement of the weight of the findings, the CLRN representative proposed considering the development of a local specialty group in palliative care to capitalise on research expertise and drive up the quality of local research. Members of our study steering group are currently involved in setting up this group.
In other focus groups it was hoped that a change in policy and strategy could reduce the ‘fragmentation’ in services, which hindered local palliative care provision. Some participants spoke about the need for a more ‘holistic’ and long-term approach to be taken to a patient’s care:
We’re used to seeing patients for that [acute] episode of care and [we need] to try and get people to think about what’s been happening in the run up to this admission and what needs to happen beyond, when the patient goes home.
There were also calls for a ‘culture change’ in terms of both shifting the definition of palliative care (to encompass more than just terminal or cancer care) and altering health professionals’ overall approach to caring for people at the end of life. A number of participants thought that education and training was the best way to achieve a change in attitudes and philosophy. The fact that medical and nursing staff identified far fewer patients as having palliative care needs than they did people who they thought were likely to die within the next 12 months indicated to one person that there was a ‘gap which needed to be bridged’. It was suggested that training health professionals to ask the ‘surprise’ question first and then cross-referencing that with prognostic indicators might be a step towards bridging this gap, and this could stem from a change in policy. Also, improving the skills of generalists, for whom ‘palliative care is our bread and butter’, was identified as a priority in terms of policy. Similarly, developing a policy that helped to ‘empower’ more inexperienced hospital doctors and nurses who lacked confidence in identifying patients who might be approaching the end of life was also suggested as a way of initiating a ‘culture change’ that would help to ‘get patients home, and quickly’. One initiative that was mentioned which could inform such a policy was to encourage consultants and registrars to identify palliative care patients to more junior doctors and nurses during their medical and surgical rounds. This policy was found to have had a positive effect when implemented at UHMBT.
Another participant spoke about some of the tools associated with end-of-life care initiatives, including palliative care registers and DNAR orders. Although GPs were instructed to use these in care homes, this did not necessarily effect a ‘culture change’:
We were left with the feeling of we know where they’re coming from, and we know why we’re being encouraged to do it in this way, but actually it’s just creating more paperwork rather than actually changing the mindset.
Other participants were also doubtful about whether tools such as the GSF and the LCP actually worked to instil ‘culture change’: ‘We do have GSF and LCP but at the end of the day that is a tick box, an aid memoire, it doesn’t address the core belief system.’
Developing local policy and strategy was identified as key to increasing palliative care education and training opportunities for health professionals in the local area. In addition, a review of the way in which the various end-of-life tools available – GSF, LCP, advance care planning – are used locally may help to assess their role in initiating the ‘culture change’ that was deemed essential for improving patient care. Indeed, at Westmorland General Hospital there was a view presented that if advance care planning could be made more ‘routine’ then patients could ‘actually become part of the discussion’ and it would enable more informed patient choice: ‘If we feel it is routine as practitioners, then you communicate it as routine and patients take it as such.’
Impact of the findings for service provision and commissioning
One participant felt that the findings from the project could help to inform GP commissioning in terms of highlighting issues resulting specifically from an ageing population:
That population graph you showed, that’s our world now and these are the people popping in and out of hospital sucking in resources that we shouldn’t be using. What you’re telling us is the information that backs up what we sort of know already but we’re looking at it from a financial point of view . . . we can take this information now and we match it with the management information and say ‘look here’s a plan’.
Given the project finding that 36.0% of hospital inpatients had palliative care needs and that only 8.1% had been referred to specialist palliative care services, some participants expressed the view that more specialist palliative care was needed: ‘Their expertise is needed and we need more of it.’
Others were concerned about the resource and staffing implications if more patients in hospital had palliative care needs than previously thought:
If we get better at identifying those with palliative care needs what impact does that have in the acute setting? [. . .] we’re going to have to have a different set of people working in the hospitals to do that.
Specialists are already squeezed and more referrals means more work for them.
Anybody who is identified as having palliative care needs ought to have involvement from the chaplaincy if they choose . . . I haven’t got the resources to respond to that.
Another issue raised was whether GPs could be more effective in preventing potentially avoidable admissions to hospital for people who were at the end of life. It was noted that GPs did not always use the GSF tool and put patients on a palliative care register when it would have been appropriate to do so. Similarly, out-of-hours GPs were reported to not routinely check patients’ notes for mention of the GSF and so this information was not then passed on to hospital staff: ‘They do have access to the [palliative care register] . . . it’s not that they’ve not got the information, it’s just that they’re not passing it on.’
One proposal for improving palliative care provision in line with the findings from the study was to nominate a GP within each practice who could be designated as a lead for palliative care, or frailty. Practice leads could then be responsible for encouraging other GPs within the practice to identify patients’ palliative care needs.
A further significant issue identified in relation to service provision and commissioning was the lack of alternative venues to hospital for patients whose requirements for care could not be met in their own homes:
Quite often we admit people who are frail elderly to hospital because we have no other option of keeping them safe in the community . . . they don’t want to be in hospital and we don’t want to put them in hospital but we’ve got absolutely no other option.
There was a call made by some for more community hospitals and community services, which, again, could help to keep people approaching the end of their life in the community: ‘What we’re all screaming about is that if we had some community-based facilities then we could avoid hospital admission almost at a stroke.’
There was also a demand for improved continuity of care for patients in residential and nursing care – ‘one practice looking after one nursing [home]’ – as a way of preventing hospital admissions. Further research in primary care that could help to identify what services could usefully be commissioned to help avoid unnecessary admissions was also requested. For one GP commissioner, the fact that ‘the last 6 months of life cost the NHS 50% of its budget’ meant that finding alternative places of care for the 36.0% of patients approaching the end of life, rather than high-cost hospital care, was a key priority. It was suggested that case studies should be accrued to provide evidence of the value of community hospitals and the non-acute services attached to them as an alternative to acute hospitals.
Suggestions for further research and comments on proposed research
There were some interesting suggestions for further research. One participant suggested that, as palliative care was closely related to the self-management of long-term conditions, there might be the potential to undertake research on the intersection of the two in the next Collaboration for Leadership in Applied Health Research (CLAHRC) application as a ‘natural follow on’ (Cutler Group). This suggestion is being taken forward by members of our steering group in collaboration with other colleagues from Sheffield University. Another participant suggested that some secondary analysis could be performed on the original data set using GPs rather than palliative care consultants to assess whether admissions for patients with palliative care needs were ‘potentially avoidable’ or not. This participant argued that GPs might make a different assessment given that they might have better knowledge of the community-based services available to patients. A third participant asked whether the data could be further analysed to assess the difference that specialist palliative care made to those patients who received it.
Participants were also asked to comment on a new intervention being developed by our research team that might support staff in initiating a transition to palliative care in inpatient settings. One participant thought that, if such an intervention encouraged individual patient management plans, it would be a good idea. It was suggested that such plans should be overseen by a lead clinician but with involvement from GPs, non-hospital specialists and other therapists. This participant said that there was too much ‘fragmentation’ in the NHS and not enough knowledge of the services available outside of hospitals.
Another participant also advised that any new intervention should be ‘based around the team’: ‘Lots of times people are working in silos and there is lots of fragmentation.’
Chapter summary
Many participants were surprised by the high percentage of patients who were found to meet one or more of the GSF criteria for palliative care need in the two hospitals surveyed. A number of participants thought that hospital was an inappropriate place to care for patients who were approaching the end of life, but that alternative places of care within the community would need to be available to reduce referrals to hospitals. Concerns were expressed about the resource and staffing implications if such large numbers of hospital patients were found to have palliative care needs, but an improved strategy for education and training of generalists and better use of existing tools (GSF, palliative care registers, advance care planning) was suggested as a way forward.
Outcomes
As a direct consequence of the focus group at the Sheffield Teaching Hospitals NHS Foundation Trust’s clinical management board meeting a new supportive and end-of-life care strategy group has been set up. The remit of the strategy group is to consider effective commissioning of palliative care services, provide guidance on co-ordination of care pathways and develop and deliver education for professionals outside the specialist palliative care service. The overarching aim of the strategy group is to optimise patients’ experiences at the end of life within the trust.
As a consequence of the Cutler Group meeting, a CLRN Local Specialty Group in Palliative Care is being set up, led by BN, CG and MC.
Chapter 9 Conclusions
Introduction
This chapter draws together findings from all phases of the study and summarises them in relation to the existing literature and wider policy context. Study strengths and limitations are summarised and recommendations for future research directions established.
Summary of the main findings in relation to research aims and objectives
What proportion of hospital inpatients have palliative care needs?
-
Of the 514 patients in the sample, just over one-third (n = 185, 36.0%) met one or more of the GSF prognostic indicator criteria for palliative care need.
-
The majority (77.8%) of these patients were aged ≥ 65 years, with a considerable proportion (23.2%) aged ≥ 85 years.
-
The most common GSF prognostic indicator was frailty, with almost one-third of patients (27.0%) meeting this criteria. Heart disease (20.5%), cancer (19.5%), COPD (18.4%) and dementia (17.8%) were the next most common GSF criteria and were roughly equal in prevalence.
-
Amongst the 185 patients meeting criteria for palliative care need, a self-completed needs assessment identified that physical symptoms were most troublesome, with 74.6% reporting a symptom that merited ‘immediate attention by the attending clinician’. Patients also reported high levels of psychological symptoms, with 43.2% of patients reporting a symptom that merited ‘immediate attention by the attending clinician’.
-
When medical and nursing staff were asked to identify patients with palliative care needs according to a standardised definition, nursing staff identified 17.4% of patients surveyed whereas medical staff identified 15.5% of patients surveyed. Agreement between medical staff and nursing staff and the GSF with respect to identifying patients with palliative care needs was poor (Cohen’s kappa = 0.22 and 0.25 respectively).
Under what circumstances do transitions to a palliative care approach occur? What is the influence of age and disease type on decision-making? Who is involved in decision-making?
-
Of the 183 patients who met GSF criteria for palliative care need and for whom complete data were available, 61 (33.0%) showed evidence of transition to a palliative care approach by meeting one or more indicator of adoption of a palliative care approach (DNAR order 29.0%, referral to specialist palliative care 8.2%, prescription of long-term opiates/syringe driver 4.9%, on LCP 1.1%, documented advance care plan 0.0%).
-
The significant predictors of a transition to palliative care were the GSF indicators for cancer, heart disease and stroke, together with age and living in a residential or nursing care home.
-
The retrospective case note review identified that 255 out of 483 patients (52.8%) who had died following an admission to hospital showed some evidence of a transition to a palliative care approach before death (DNAR order 47.4%, placed on LCP 14.1%, referral to specialist palliative care 9.1%, prescription of long-term opiates 9.9%, use of syringe driver 3.3%, advanced decision to refuse treatment 0.8%).
-
Health professionals reported difficulties in recognising that a patient had entered the last 12 months of life and reported that prognosis was not routinely discussed with hospital inpatients, representing a barrier to a structured transition to palliative care being initiated. However, they were comfortable in identifying individuals with palliative care needs.
-
An either/or approach to care was identified among health professionals, rather than concurrent palliative and curative treatment as recommended in contemporary models of palliative care.
-
Older age was perceived by health professionals to act as a barrier to accessing specialist palliative care because older people were seen to have less need for specialist input as a consequence of death being more expected and the perception that older people find it easier to come to terms with a terminal diagnosis.
-
There was a persistent assumption among health professionals that specialist palliative care services are inextricably linked with cancer.
-
No patients who were interviewed mentioned ‘palliative care’.
-
Patients are not routinely offered the opportunity to make decisions about the care and treatment that they receive at the end of life.
How is information about a transition to a palliative care approach communicated to patients and their families and how are they involved in decision-making?
-
Most patients who were interviewed were unaware of their prognosis and showed little insight into what they could expect from the trajectory of their disease. None reported having held discussions about goals of care during their hospital admission; some patients expressed a reluctance to hold such discussions, preferring to live ‘day to day’.
What proportion of hospital admissions amongst people with palliative care needs is avoidable given the current local configuration of health- and social-care services?
-
Two palliative medicine consultants identified that hospital admission was potentially avoidable for 6.7% of patients (n = 14) who might be in need of palliative care according to GSF indicators. In the retrospective case note review, 7.2% of admissions (n = 35) were classified as potentially avoidable.
What patient factors predict potentially avoidable admissions?
-
The numbers of potentially avoidable admissions were too small to conduct multivariate analyses to identify predictors, but it is notable that the majority of these patients (n = 33 out of 49) in both the survey and the retrospective case note review were elderly and resident in nursing or residential care.
What is the cost of potentially avoidable acute hospital admissions amongst patients with palliative care needs?
-
An exploratory analysis estimated that the cost of these admissions for the period of the survey was £36,334, but the cost of alternative places of care, based on this same length of stay, was estimated to be £34,807. The estimated economic impact was therefore a potential cost saving of £1527 across both hospitals for the period of the survey. The potential annual cost saving for the two hospitals was estimated at just under £180,000. Restricting the cost perspective to NHS and PSS costs increased the cost saving to £2.5M per annum as the costs of self-funded care home places and non-NHS contributions to hospice funding are excluded.
-
The retrospective case note review examined the appropriateness of admission for 483 patients who had been present in the hospital at the time of the survey but who had died (excluding sudden deaths) in the 12 months subsequently. Thirty-five admissions (7.2%) were classified by our two palliative medicine consultants as potentially avoidable. Taking into account the avoided hospital costs and the cost of providing support in alternative locations, the estimated economic impact is a potential cost saving of £45,287 across both hospitals for the inpatients with palliative care needs on the first day of the survey. The potential annual cost saving of preventing admissions amongst these patients for the two hospitals was estimated to be approximately £5.3M.
-
The mean per-day long-stay payment for the 35 avoidable admissions from the retrospective case note review was £191. If it is assumed that this is a reasonable estimate of the cost per day for all palliative care admissions, then reducing the length of stay for the 483 patients in this current analysis by 2 days or 3 days would result in an estimated saving in hospital costs of £184,865 or £277,297 respectively. The annual cost saving for both hospitals per annum would be £21.6M for a 2-day reduction and £32.4M for a 3-day reduction.
Study strengths
To the best of our knowledge this is the first study to explore the extent, and nature, of transitions to a palliative approach to care within the acute hospital setting internationally. Using multiple sources of data strengthened our ability to draw robust findings. We used techniques of triangulation to draw together data from patients, family members, doctors, nurses, clinical notes and the hospital information system to examine the issue of ‘transitions’ from multiple perspectives.
Additional strengths of the study include:
-
The integral involvement of a service user group in the study design and conduct, as described in Chapter 2.
-
Investing time and resources to provide rigourous training of the team of 30 researchers involved in conducting the survey, as described in Chapter 5.
-
The involvement of a multidisciplinary research team from a range of clinical and/or social science backgrounds.
-
Persistence, and the support of the service user group, to secure ethical approval for the retrospective case note review reported in Chapter 7.
-
A concurrent study being conducted in New Zealand with similar aims and objectives; data from the two studies are now being analysed together to examine commonalities and differences between the two countries.
Study limitations
Limitations specific to each research phase have been presented in the relevant chapter. To summarise:
-
The change in the original study protocol stipulated by the ethics committee meant that a ‘census’ of palliative care need could not be conducted as originally planned. Although the survey achieved a response rate of 37.8%, economic analyses were contingent on a complete 100% sample of the patient population. Therefore, the retrospective case note review (although not part of the original study protocol) was conducted to address this limitation.
-
The appropriateness of admission decisions, defined by two experienced clinicians, were subjective. This was necessitated by our approach, which required detailed knowledge both of palliative care and of local community services that could prevent admissions.
-
In common with many studies involving interviews with patients at the end of life, the response rate to the patient interviews was relatively low. We were unable to collect reasons for non-response but believe that a significant number of potential participants had died, or had become too unwell to participate, in the time between the initial invite during their hospital admission and the interview date.
Summary of findings in relation to the wider literature
Within each chapter we have discussed specific findings in relation to the wider research literature. To summarise, here we present new and novel data regarding palliative care management in the acute hospital setting, extending current knowledge and understanding. In most areas our findings resonate with the existing, albeit limited, evidence base, for example:
-
It is now well established that prognostic discussions are not routinely held with people with life-limiting conditions other than cancer. Our findings, from clinicians and patients, are in line with the growing body of research in this area. 177
-
The failure of clinicians to initiate goal of care discussions with patients in hospital, a key finding from our study, was also evident in recent audits of the LCP,16 in which two-thirds of people on the LCP had no recorded discussion of end-of-life care planning in their clinical notes.
-
Some (albeit limited) previous empirical work confirms our finding that not everyone prefers ‘open awareness’ when living with a life-limiting condition,74,172,178 despite this being a key tenet of the philosophy informing palliative care provision. However, more research in this area is required, as reported below.
In some areas our findings do not concord with published research, for example:
-
We report a higher proportion (36%) of hospital inpatients meeting criteria for palliative care need than previous studies. We acknowledge that this may be partly to do with response bias (although this seems unlikely given that the most common reason for non-participation was feeling too unwell). However, another factor is likely to be our inclusion of frailty as a condition indicating palliative care need, in line with current UK policy.
-
The proportion of potentially avoidable hospitalisations that we identified both in the survey and in the retrospective case note review was significantly lower than in the published literature. As discussed in Chapters 5 and 7, we attribute this to our novel approach, which involved considering admissions within the context of current community palliative care service provision, rather than the ‘blue sky’ approach previously adopted in other published studies.
Specific recommendations for further research in the area of palliative care transitions
The primary aim of this study, which was to explore the extent and nature of transitions to a palliative approach to care within the acute hospital setting, identified a significant gap between policy recommendations and current practice.
Crucially, we have identified that ensuring patient involvement in decision-making during the last 12 months of life, a central tenet of the End of Life Care Strategy for England,5 is not currently being achieved. Patients are not routinely offered the opportunity to make decisions about the care and treatment that they receive at the end of life. If transitions to a palliative approach to care are initiated within the acute hospital setting, it is too late for meaningful involvement to be achieved. With this in mind, we suggest that a significant body of research is urgently needed to inform initiatives that can close the gap between policy and practice. Our findings confirm that focusing efforts on improving transition management within the acute hospital setting is fruitful because of the high proportion of inpatients meeting criteria for palliative care need. Specific steps required to ensure that this is achieved have been identified, including (1) clarification of definitions and terms, (2) education of the hospital-based generalist palliative care workforce, (3) initiatives to support team decision-making in collaboration with the patient and his or her family and (4) further understanding of patient and family preferences for involvement in end-of-life decision-making. Further research is required in all of these areas, as described in more detail below.
In relation to our secondary aim, we have concluded that the extent of potentially avoidable admissions amongst hospital inpatients with palliative care needs is not as high as previous estimates suggest. Crucially, we defined these within the context of current service provision; previous studies have adopted a ‘blue sky’ approach, assuming that a full range of community services is available and accessible to support patients with palliative care needs outside of the inpatient setting. Future initiatives to prevent admissions would be most fruitfully targeted to the nursing and residential care sector. Of more economic consequence may be reducing the length of time that patients with palliative care needs spend in hospital; this requires further evidence of optimum discharge planning amongst patients with palliative care needs, as well as an understanding of how increased community support can be provided. The need to grow the evidence base regarding the health economics of palliative care provision is well established;179 our study points to a need to develop improved means of costing community service provision as well as, crucially, costs incurred by family carers.
Preventing hospitalisations, or reducing their duration, only minimises costs placed on statutory services. Further research is urgently needed to fully understand the economic implications of life-limiting illnesses for patients and their families; this will require the development of rigorous and acceptable tools to capture this information. Specific recommendations in relation to this aim are presented below.
To implement the DoH guidance29 on initiating palliative care transitions within the acute hospital setting, high-quality evidence is required in the following areas:
-
Initiatives to educate hospital-based clinicians about palliative care management. Robust interventions are required to educate hospital-based clinicians regarding the meaning and remit of palliative care. There is a particular need to clarify definitions of ‘palliative care’ and ‘end-of-life care’ and raise awareness of the range of initiatives promoted by the End of Life Care Programme. 29 Furthering understanding of palliative care beyond cancer and, in particular, in relation to frail older people with multiple comorbidities, potentially including dementia, is required. Research is needed to identify the optimum means of delivering palliative care education in this setting. Our findings indicate that team-based initiatives are needed to promote team approaches to palliative care management; however, the practicalities of achieving this are obviously complex. There is also a need for more applied research on methods of implementing and sustaining culture change. Such research should build in a systematic way on the insights provided by this study and actively involve staff, patients and family carers in any further work aimed at further developing and testing educational interventions and associated change methodologies. This would lend itself to a programme of research culminating in a large-scale trial of an intervention based on the principles identified in this study.
-
Interventions to support the identification of patients with palliative care needs. Our participants identified significant challenges in identifying patients likely to be in the last 12 months of life. The GSF is being implemented within acute hospital settings. Research is needed to validate the GSF as an instrument for identifying palliative care need.
-
Interventions to promote palliative care transitions tailored to the acute hospital setting. A robust intervention is required to support clinicians in initiating palliative care transitions within the acute hospital setting and ensuring that patients and families are involved in discussions about goals of care. A complex intervention of this nature would likely involve multiple components and would require rigorous development and testing within the Medical Research Council framework for the development and testing of complex interventions. 179 Crucially, our data indicate that it would have to target the whole clinical team and have built-in mechanisms to ensure that discussions are recorded and conveyed to patients’ GPs. Means of ensuring appropriate support for patients and families following discussions about goals of care would also need to be developed. Links to further policy initiatives would also need to be ensured. Such discussions could provide a good lead into advance care planning, of which there was no evidence in our study.
-
Patient and family preferences and experiences. The extent of palliative care transitions within our study was so limited that little information could be gathered regarding patient and family experiences at this time. However, our findings did confirm that patients do not always want detailed information about, or to be made aware of, the life-limiting nature of their condition. Further research exploring patient information needs is urgently required. The evidence base for prognostic discussions for people with conditions other than cancer is particularly limited.
The following are specific recommendations for further research in the area of potentially avoidable hospitalisations:
-
Evidence-based initiatives targeted at the nursing and residential care sectors. Our findings indicate a need for further research to identify which community supports targeted to nursing and residential care settings could prevent the small proportion of potentially avoidable hospitalisations currently evident amongst patients with palliative care needs. Previous research79,83 has found that many more admissions could be prevented by the full implementation of the End of Life Care Strategy,5 which will require not only additional research evidence but also significant investment in community services. We have also identified that current admission lengths amongst patients with palliative care needs are relatively high and that significant cost savings could be achieved by earlier supported discharge.
-
Earlier supported discharge from hospital. Further research to identify optimum discharge planning for patients with palliative care needs, in addition to identifying the community supports required to prevent readmission, is needed.
-
Family costs of caring. Preventing hospitalisations, or reducing their duration, only minimises costs placed on statutory services. Further research is urgently needed to fully understand the economic implications of life-limiting illnesses for patients and their families; this will require the development of rigorous and acceptable tools to capture this information.
Acknowledgements
We are indebted to a great many people who, over the last 3 years, have contributed much to this project.
We thank the National Institute for Health Research under the Health Services and Delivery Research (HS&DR) programme for funding the project. Specifically, at the HS&DR programme, Lisa O’Rourke has provided guidance at key stages of the project.
We are particularly grateful to patients and members of their families who agreed to be interviewed. Without the generosity of these people, who gave their valuable time and shared their thoughts and experiences, we would not have been able to proceed.
Service users from a range of local, regional and national organisations have done sterling work on the project. They provided the research team with excellent and sustained support throughout the project for which we would like to offer our thanks.
We would like to extend our gratitude to the 30-strong team of researchers and clinicians who carried out the census surveys in Sheffield and Lancaster. They made a daunting task less so because of their professionalism and commitment to the project.
We also would like to acknowledge the part played by Gerry Marvell, Anthony Ingleton and Jane Ryan in carrying out the large task of data collection for the case note review and inputting that data.
We received help from many health-care professionals who took the time to complete questionnaires and participate in interviews in spite of their busy schedules. We also received invaluable support from the Sheffield Teaching Hospitals HRG coding team, who took the time to undertake retrospective coding.
Our final and most heartfelt thanks go to Kate Chadwick, our project administrator, who has provided excellent assistance to all of the team and has been a continuing source of support throughout the project. She was a model of the mixture of sensitivity and punctiliousness called for in carrying out this role.
Contributions of authors
Professor Merryn Gott (Professor of Health Sciences) was involved in conceiving the study and all aspects of study design, implementation and dissemination, and had overall responsibility for the study.
Professor Christine Ingleton (Professor of Palliative Care Nursing) was involved in conceiving the study and all aspects of study design, implementation and dissemination, and had overall responsibility for the study.
Dr Clare Gardiner (Lecturer in Public Health) was project manager with responsibility for overall management of the study, including data collection, analysis and dissemination.
Dr Naomi Richards (Postdoctoral Research Fellow) was project manager with responsibility for overall management of the study, including data collection, analysis and dissemination.
Rev. Mark Cobb (Clinical Director) was involved in conceiving the study and participated in decisions regarding study design, implementation and dissemination.
Dr Tony Ryan (Senior Lecturer) was involved in conceiving the study and participated in decisions regarding study design, implementation and dissemination.
Dr Bill Noble (Senior Lecturer in Palliative Medicine) was involved in conceiving the study and participated in decisions regarding study design, implementation and dissemination.
Professor Mike Bennett (Professor of Palliative Medicine) was involved in conceiving the study and participated in decisions regarding study design, implementation and dissemination.
Professor Jane Seymour (Professor in Palliative and End of Life Studies) was involved in conceiving the study and participated in decisions regarding study design, implementation and dissemination.
Ms Sue Ward (Senior Operational Research Analyst) conducted the health economic analysis and participated in dissemination.
Mrs Chris Parker (Medical Statistician) conducted the statistical analysis and participated in dissemination.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
Publications
Gott M, Ingleton C, Gardiner C, Ryan T, Noble B, Seymour J, et al. How to improve end of life care in acute hospitals. Nurs Older People 2009;21:26–9.
Green E, Gardiner C, Gott M, Ingleton C. Communication surrounding transitions to palliative care in heart failure: a review and discussion of the literature. Prog Palliat Care 2010;18:281–90.
Gardiner C, Gott M, Ingleton C, Cobb M, Green E. Barriers to providing palliative care for older people in acute hospitals. Age Ageing 2011;40:233–8.
Gardiner C, Gott M, Ingleton C, Ryan T. Exploring the transition from curative care to palliative care: a systematic review of the literature. BMJ Support Palliat Care 2011;1:56–63.
Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: a qualitative study. BMJ 2011;342:d1773.
Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: a qualitative study (reprinted). BMJ Support Palliat Care 2011;1:42–8.
Gott M, Ward S, Cobb M, Gardiner C, Ingleton C. A narrative literature review of the evidence regarding the economic impact of avoidable hospitalizations amongst palliative care patients in the UK. Prog Palliat Care 2011;19:291–8.
Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. ‘That’s part of everybody’s job’: the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med 2012;26:232–41.
Palliative Care Studies Advisory Group. Service user involvement in research: a briefing paper by the Palliative Care Studies Advisory Group. Sheffield: University of Sheffield; 2012.
Ryan T, Gardiner C, Bellamy, G, Gott M, Ingleton C and nursing staff. Barriers and facilitators to the receipt of palliative care for people with dementia: the views of medical and nursing staff. Palliat Med 2012;26:879–86.
Ward S, Gott M, Gardiner C, Cobb M, Richards N, Ingleton C. Economic analysis of potentially avoidable hospital admissions in patients with palliative care needs. Prog Palliat Care 2012;20:147–53.
Gardiner C, Gott M, Ingleton C, Cobb M, Seymour J, Ryan T, et al. Extent of palliative care need in the acute hospital setting: a survey of two acute hospitals in the UK. Palliat Med 2013;27:76–83.
Gardiner C, Gott M, Ingleton C, Richards N. Palliative care for frail older people: a cross-sectional survey of patients at two hospitals in England. Prog Palliat Care 2013; in press.
Gott M, Gardiner C, Ingleton C, Cobb M, Noble B, Bennett M, et al. What is the extent of potentially avoidable admissions amongst hospital inpatients with palliative care needs? BMC Palliat Care 2013;12:9.
Gott M, Ingleton C, Gardiner C, Seymour J, Parker C, Ryan T. Prevalence and predictors of transition to a palliative care approach amongst hospital inpatients in England. J Palliat Care 2013;29:146–52.
Ingleton C, Gardiner C, Richards N, Seymour J, Gott M. Exploring education and training needs amongst the palliative care workforce. BMJ Support Palliat Care 2013;3:207–12.
Richards N, Gardiner C, Gott M, Ingleton C. Awareness contexts revisited: indeterminacy in initiating discussions at the end of life. J Adv Nurs 2013; in press.
Ryan T, Ingleton C, Gardiner C, Parker C, Gott M, Noble B. Symptom burden, palliative care need and predictors of physical and psychological discomfort in two UK hospitals. BMC Palliat Care 2013;12:11.
References
- Gomes B, Cohen J, Deliens L, Higginson IJ, Gott M, Ingleton C. Living with ageing and dying: palliative and end of life care for older people. Oxford: Oxford University Press; 2011.
- Hughes-Hallett T, Craft A, Davies C, Mackay I, Nielsson T. Creating a fair and transparent funding system; the final report of the palliative care funding review. London: Palliative Care Funding Review; 2011.
- Seymour J, Witherspoon R, Gott M, Ross H, Payne S. Dying in older age: end of life care. Bristol: Policy Press; 2009.
- Better palliative care for older people. Copenhagen: WHO; 2004.
- The end of life care strategy for England. London: Department of Health; 2008.
- Kellehear A. Compassionate cities: public health and end-of-life care. London: Routledge; 2005.
- Human Rights Watch . Palliative Care and Access to Controlled Medicines 2011. www.hrw.org/en/node/86033 (accessed 12 December 2011).
- Field D. Palliative medicine and the medicalisation of death. Eur J Cancer Care 1994;3:58-62.
- Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. ‘That’s part of everybody’s job’: the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med 2012;26:232-41. http://dx.doi.org/10.1177/0269216311408993.
- Mental Capacity Act 2005: code of practice. London: The Stationery Office; 2007.
- End-of-life care. London: The Stationery Office; 2008.
- Equity and excellence: liberating the NHS. London: The Stationery Office; 2010.
- Government response to the NHS Future Forum Report. Cm 8113. London: The Stationery Office; 2011.
- Issues facing commissioners of end-of-life care. London: King’s Fund; 2011.
- Sam E, Chapman S, Hayes A, Smith T. Commissioning end-of-life care; act & early. London: National Council for Palliative Care; 2011.
- Richards M. National care of the dying audit – hospitals: foreward. London: Marie Curie Palliative Care Institute Liverpool in collaboration with the Royal College of Physicians and Clinical Effectiveness and Evaluation Unit; 2007.
- Dixon T, Shaw M, Frankel S, Ebrahim S. Hospital admissions, age, and death: retrospective cohort study. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38072.481933.EE.
- Gomes B, Higginson I. Where people die (1974–2030): past trends, future projections and implications for care. Palliat Med 2008;22:33-41. http://dx.doi.org/10.1177/0269216307084606.
- Gomes B, Calanzani N, Higginson IJ. Reversal of the British trends in place of death: time series analysis 2004–2010. Palliat Med 2012;26:102-10. http://dx.doi.org/10.1177/0269216311432329.
- Gott M, Ahmedzai SH, Wood C. How many inpatients at an acute hospital have palliative care needs? Comparing the perspective of nursing and medical staff. Palliat Med 2001;15:451-60. http://dx.doi.org/10.1191/026921601682553932.
- Morize V, Nguyen DT, Lorente C, Desfosses G. Descriptive epidemiological survey on a given day in all palliative care patients hospitalized in a French university hospital. Palliat Med 1999;13:105-17. http://dx.doi.org/10.1191/026921699672866065.
- Desmedt MS, Kethulle Y, Deveugele M, Keirse EA, Paulus DJ, Menten JJ, et al. Palliative inpatients in general hospitals: a one day observational study in Belgium. BMC Palliat Care 2011;10. http://dx.doi.org/10.1186/1472-684X-10-2.
- Liverpool Care Pathway n.d. www.liv.ac.uk/mcpil/liverpool-care-pathway/ (accessed January 2012).
- NHS Improving Quality . National End of Life Care Programme n.d. www.endoflifecare.nhs.uk (accessed July 2012).
- Ahmedzai SH, Walsh D. Palliative medicine and modern cancer care. Semin Oncol 2000;27:1-6.
- Clinical guidelines CG42. London: NICE; 2006.
- O’Neil D, Knight PV, Michel JP. Improving during-life palliative care will improve end-of-life care. BMJ 2008. www.bmj.com/cgi/eletters/337/oct01_1/a1720 (accessed 19 June 2012).
- Treatment and care towards the end of life: good practice in decision-making. London: GMC; 2010.
- The route to success in end of life care – achieving quality in acute hospitals. Leicester: National End of Life Care Programme; 2010.
- Boyd K, Murray SA. Recognising and managing key transitions in end of life care. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4863.
- Jerant AF, Azari RS, Nesbitt TS, Meyers FJ. The TLC model of palliative care in the elderly: preliminary application in the assisted living setting. Ann Fam Med 2004;2:54-60. http://dx.doi.org/10.1370/afm.29.
- Thomas K. The GSF prognostic indicator guidance. End Life Care 2010;4:62-4.
- Higginson I, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. Palliat Med 2000;3:287-300. http://dx.doi.org/10.1089/jpm.2000.3.287.
- Morgan DL. Paradigms lost and pragmatism regained: methodological implications of combining qualitative and quantitative methods. J Mix Methods Res 2007;1:48-76. http://dx.doi.org/10.1177/2345678906292462.
- Bryman A. Integrating quantitative and qualitative research: how is it done?. Qual Res 2006;6:97-113. http://dx.doi.org/10.1177/1468794106058877.
- National Health Service . About the National Health Service (NHS) n.d. www.nhs.uk/NHSEngland/thenhs/about/Pages/overview.aspx (accessed 22 November 2012).
- Picker Institute Europe . NHS Adult Inpatient Survey 2010 n.d. www.nhssurveys.org/surveys (accessed July 2013).
- National End of Life Care Intelligence Network . National End of Life Care Intelligence Network Profiles 2013. www.endoflifecare-intelligence.org.uk/profiles.aspx (accessed July 2013).
- State of Sheffield 2013. Sheffield: Sheffield First Partnership; 2013.
- Sheffield NHS health data. Sheffield: NHS Sheffield; 2012.
- National End of life Care Intelligence Network . End of Life Care Local Authority Profiles n.d. www.endoflifecare-intelligence.org.uk (accessed July 2013).
- Mental Capacity Act 2005. London: The Stationery Office; 2007.
- National Health Services Act 2006. London: The Stationery Office; 2006.
- Involving the public in NHS, public health and social care research: briefing notes for researchers. London: INVOLVE; 2012.
- Gott M, Payne S, Seymour J, Ingleton C. Palliative care nursing: principles and evidence for practice. Buckingham: Open University Press; 2004.
- Small N, Sargeant A, Gott M, Ingleton C. Living with ageing and dying: palliative and end of life care for older people. Oxford: Oxford University Press; 2011.
- Stevens T, Payne S, Seymour J, Ingleton C. Palliative care nursing: principles and evidence for practice. Buckingham: Open University Press; 2008.
- Gott M, Stevens T, Small N, Ahmedzai S. Bristol: Policy Press; 2000.
- Gott M, Stevens T, Small N, Ahmedzai S. User involvement in cancer care. Br J Clin Govern 2002;7:81-5.
- Bellamy G, Gott M, Frey R. ‘It’s my pleasure?’: The views of palliative care patients about being asked to participate in research. Prog Palliat Care 2011;19:159-64. http://dx.doi.org/10.1179/1743291X11Y.0000000008.
- Sargeant A, Payne S, Gott M, Small N, Oliviere D. User involvement in palliative care: motivational factors for service users and professionals. Prog Palliat Care 2007;15:126-32. http://dx.doi.org/10.1179/096992607X196060.
- Baile WF, Glober GA, Lenzi R, Beale EA, Kudelka AP. Discussing disease progression and end-of-life decisions. Oncology 1999;13:1021-31.
- Meyers FJ, Linder J, Beckett L, Christensen S, Blais J, Gandara DR. Simultaneous care: a model approach to the perceived conflict between investigational therapy and palliative care. J Pain Symptom Manage 2004;28:548-56. http://dx.doi.org/10.1016/j.jpainsymman.2004.03.002.
- Marsella A. Exploring the literature surrounding the transition into palliative care: a scoping review. Int J Palliat Nurs 2009;15:186-9.
- Schofield P, Carey M, Love A, Nehill C, Wein S. ‘Would you like to talk about your future treatment options?’ Discussing the transition from curative cancer treatment to palliative care. Palliat Med 2006;20:397-406. http://dx.doi.org/10.1191/0269216306pm1156oa.
- Small N, Gardiner C, Barnes S, Gott M, Payne S, Seamark D, et al. Using a prediction of death in the next 12 months as a prompt for referral to palliative care acts to the detriment of patients with heart failure and chronic obstructive pulmonary disease. Palliat Med 2010;24:740-1. http://dx.doi.org/10.1177/0269216310375861.
- The Gold Standards Framework . Improving Care for All People Near the End of Life Provided by Front Line Generalist Staff in Any Setting 2008. www.goldstandardsframework.org.uk/ (accessed 14 December 2011).
- Shaw KL, Clifford C, Thomas K, Meehan H. Review: improving end-of-life care: a critical review of the Gold Standards Framework in primary care. Palliat Med 2010;24:317-29. http://dx.doi.org/10.1177/0269216310362005.
- Hawker S, Payne S, Kerr C. Appraising the evidence: reviewing disparate data systematically. Qual Health Res 2002;12:1284-99. http://dx.doi.org/10.1177/1049732302238251.
- Dixon-Woods M, Fitzpatrick R. Qualitative research in systematic reviews. Has established a place for itself. BMJ 2001;323:765-6.
- Larkin PJ, Dierckx de Casterlé B, Schotsmans P. Transition towards end of life in palliative care: an exploration of its meaning for advanced cancer patients in Europe. J Palliat Care 2007;23:69-7.
- Patrick H, Taylor F, Schwenke M, Jones E. Consultation with users, carers and staff in order to improve local palliative care services – how to do it and views on current services. Prog Palliat Care 2007;15:177-81. http://dx.doi.org/10.1179/096992607X196114.
- Krishnasamy M, Wells M, Wilkie E. Patients and carer experiences of care provision after diagnosis of lung cancer in Scotland. Support Care Cancer 2007;15:327-32. http://dx.doi.org/10.1007/s00520-006-0129-3.
- Jarrett N. Patients’ experiences of inter- and intra-professional communication (IIPC) in the specialist palliative care context. Int J Disabil Hum Dev 2009;8:51-8. http://dx.doi.org/10.1515/IJDHD.2009.8.1.51.
- Bestall JC, Ahmed N, Ahmedzai SH, Payne SA, Noble B, Clark D. Access and referral to specialist palliative care: patients’ and professionals’ experiences. Int J Palliat Nurs 2004;10:381-9.
- Murray SA, Kendall M, Grant E, Boyd K, Barclay S, Sheikh A. Patterns of social, psychological, and spiritual decline toward the end of life in lung cancer and heart failure. J Pain Symptom Manage 2007;34:393-402. http://dx.doi.org/10.1016/j.jpainsymman.2006.12.009.
- Kendall M, Boyd K, Campbell C, Cormie P, Fife S, Thomas K, et al. How do people with cancer wish to be cared for in primary care? Serial discussion groups of patients and carers. Fam Pract 2006;23:644-50. http://dx.doi.org/10.1093/fampra/cml035.
- O’Leary N, Murray NF, O’Loughlin C, Tiernan E, McDonald K. A comparative study of the palliative care needs of heart failure and cancer patients. Eur J Heart Fail 2009;11:406-12. http://dx.doi.org/10.1093/eurjhf/hfp007.
- Wills LA. Continuity of care for patients with malignant disease. J Postgrad Med 1978;54:391-4. http://dx.doi.org/10.1136/pgmj.54.632.391.
- Pattison N. A critical discourse analysis of provision of end-of-life care in key UK critical care documents. Nurs Crit Care 2006;11:198-20. http://dx.doi.org/10.1111/j.1362-1017.2006.00172.x.
- Pattison N. Integration of critical care and palliative care at end of life. Br J Nurs 2004;13:132-6.
- Larkin PJ, Dierckx de Casterlé B, Schotsmans P. Towards a conceptual evaluation of transience in relation to palliative care. J Adv Nurs 2007;59:86-9. http://dx.doi.org/10.1111/j.1365-2648.2007.04311.x.
- Bennett MI, Adam J, Alison D, Hicks F, Stockton M. Leeds eligibility criteria for specialist palliative care services. Palliat Med 2000;14:157-8. http://dx.doi.org/10.1191/026921600669491513.
- Gardiner C, Gott M, Small N. Living with advanced chronic obstructive pulmonary disease: patients’ concerns regarding death and dying. Palliat Med 2009;23:691-7. http://dx.doi.org/10.1177/0269216309107003.
- Gott M, Small N, Barnes S. Older people’s views of a good death in heart failure: implications for palliative care provision. Soc Sci Med 2008;67:1113-21. http://dx.doi.org/10.1016/j.socscimed.2008.05.024.
- Gomes B, Harding R, Foley K, Higginson IJ. Optimal approaches to the health economics of palliative care: report of an international think tank. J Pain Manag 2009;38:4-10. http://dx.doi.org/10.1016/j.jpainsymman.2009.04.008.
- End of life care strategy: second annual report. London: Department of Health; 2010.
- Muenchberger H, Kendall E. Predictors or preventable hospitalisation in chronic disease: priorities for change. J Public Health Policy 2010;31:150-63.
- Abel J, Rich A, Griffen T, Purdy S. End-of-life care in hospital: a descriptive study of all inpatient deaths in 1 year. Palliat Med 2009;23:616-22. http://dx.doi.org/10.1177/0269216309106460.
- Raftery JP, Addington-Hall JM, MacDonald LD, Anderson HR, Bland JM, Chamberlain J, et al. A randomized controlled trial of the cost-effectiveness of a district co-ordinating service for terminally ill cancer patients. Palliat Med 1996;10:151-61. http://dx.doi.org/10.1177/026921639601000210.
- Grande GE, Todd CJ, Barclay SIG, Farquhar MC. Does hospital at home for palliative care facilitate home death? A randomised controlled trial. BMJ 1999;319:1472-5. http://dx.doi.org/10.1136/bmj.319.7223.1472.
- Addicott R, Dewar S. Improving choice at end of life. A descriptive analysis of the impact and costs of the Marie Curie delivering choice programme in Lincolnshire. London: King’s Fund; 2008.
- Identifying alternatives to hospital for people at the end of life. London: National Audit Office; 2008.
- Grande GE, Todd CJ, Barclay SIG, Farquhar MC. A randomised controlled trial of a hospital at home service for the terminally ill. Palliat Med 2000;14:375-85.
- Smith TJ, Cassel JB. Cost and non-clinical outcomes of palliative care. J Pain Symptom 2009;38:32-44. http://dx.doi.org/10.1016/j.jpainsymman.2009.05.001.
- National Audit Office . End of Life Care 2008. www.nao.org.uk/publications/0708/end_of_life_care.aspx (accessed August 2012).
- Seow H, Barbara L, Howell D, Dy SM. Did Ontario’s end-of-life care strategy reduce acute care service use?. Healthc Q 2010;13:93-100.
- Integrative approaches to qualitative and quantitative evidence. London: NHS Health Development Agency; 2004.
- Gardiner C, Ingleton C, Gott M, Ryan T. Exploring the transition from curative to palliative care: a systematic review of the literature. BMJ Support Palliat Care 2011;1:56-63. http://dx.doi.org/10.1136/bmjspcare-2010-000001.
- World Health Organization . Better Palliative Care for Older People 2004. www.eapcnet.org/download/forProjects/Elderly/BetterPC.Older%20People.pdf (accessed 13 December 2007).
- Traue DC, Ross JR. Palliative care in non-malignant diseases. J R Soc Med 2005;98:503-6. http://dx.doi.org/10.1258/jrsm.98.11.503.
- Gove D, Sparr S, Doe Santos Bernardo A, Cosgrave M, Jansen S, Martensson B, et al. Recommendations on end of life care for people with dementia. J Nutr Health Aging 2010;14:136-9. http://dx.doi.org/10.1007/s12603-009-0229-0.
- Sampson E, Ritchie C, Lai R, Raven P, Blanchard M. A systematic review of the scientific evidence for the efficacy of a palliative care approach in advanced dementia. Int Psychogeriatr 2005;17:31-40. http://dx.doi.org/10.1017/S1041610205001018.
- Hughes J, Jolley D, Jordan A, Sampson E. Palliative care in dementia: issues and evidence. Adv Psychiatr Treat 2007;13:251-60. http://dx.doi.org/10.1192/apt.bp.106.003442.
- Robinson L, Hughes J, Daley S, Keady J, Ballard C, Volicer L. End-of-life care and dementia. Rev Clin Gerontol 2006;15:135-48. http://dx.doi.org/10.1017/S0959259806001833.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008;17:1144-63. http://dx.doi.org/10.1111/j.1365-2702.2007.02220.x.
- Kitzinger J. Qualitative research: introducing focus groups. BMJ 2005;311. http://dx.doi.org/10.1136/bmj.311.7000.299.
- Morgan DL. Focus groups as qualitative research. London: Sage; 1997.
- Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. London: Sage; 2006.
- Murray SA, Sheikh A. Palliative care beyond cancer: care for all at the end of life. BMJ 2008;336:958-9.
- Barnes S, Gott M, Payne S, Parker C, Seamark D, Gariballa S, et al. Communication in heart failure: perspectives from patients and primary care professionals. Health Soc Care Community 2006;14:482-90.
- Selman L, Harding R, Beynon T, Hodson F, Coady E, Hazeldine C, et al. Improving end-of-life care for patients with chronic heart failure: ‘let’s hope it’ll get better, when I know in my heart of hearts it won’t’. Heart 2007;93:963-7. http://dx.doi.org/10.1136/hrt.2006.106518.
- Gott M, Gardiner C, Small N, Payne S, Seamark D, Barnes S, et al. Barriers to advance care planning in chronic obstructive pulmonary disease. Palliat Med 2009;23:642-8. http://dx.doi.org/10.1177/0269216309106790.
- Bennett M, Davies E, Higginson IJ. Delivering research in end-of-life care: problems, pitfalls and future priorities. Palliat Med 2010;24:456-61. http://dx.doi.org/10.1177/0269216310366064.
- Gott M, Barnes S, Payne S, Parker C, Seamark D, Gariballa S, et al. Dying trajectories in heart failure. Palliat Med 2007;21:95-9. http://dx.doi.org/10.1177/0269216307076348.
- Burt J, Raine S. The effect of age on referral to and use of specialist palliative care services in adult cancer patients: a systematic review. Age Ageing 2006;35:469-76. http://dx.doi.org/10.1093/ageing/afl001.
- Burt J, Plant H, Omar R, Raine R. Equity of use of specialist palliative care by age: cross sectional study of lung cancer patients. Palliat Med 2010;24:641-50. http://dx.doi.org/10.1177/0269216310364199.
- BGS Best Practice Guide 4.8. London: British Geriatrics Society; 2009.
- Addington-Hall J, Fakhoury W, McCarthy M. Specialist palliative care in non-malignant disease. Palliat Med 1998;12:417-27.
- Mitchell SL, Keily DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004;164:321-8. http://dx.doi.org/10.1001/archinte.164.3.321.
- Ryan T, Nolan M, Ingleton C, Gott M, Gardiner C. Supporting older people with dementia to die with dignity. Nurs Older People 2009;21:18-23.
- Variations in place of death in England: inequalities or appropriate consequences of age, gender and cause of death. National End of Life Care Intelligence Network; 2010.
- Gomes B, Higginson IJ. Where people die (1974–2030): past trends, future projections and implications for care. Palliat Med 2008;22:33-41. http://dx.doi.org/10.1177/0269216307084606.
- Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: a qualitative study. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d1773.
- Canadian Palliative Care Association Board of Directors . News and notes. Prog Palliat Care 1997;5.
- Ahmed N, Bestall JC, Payne SA, Noble B, Ahmedzai SH. The use of cognitive interviewing methodology in the design and testing of a screening tool for supportive and palliative care needs. Support Care Cancer 2009;17:665-73. http://dx.doi.org/10.1007/s00520-008-0521-2.
- Department of Health . Payment by Results Tariff 2011–12 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_124356 (accessed 22 May 2012).
- Curtis L. Unit costs of health and social care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Hatziandreu E, Archontakis F, Daly A. The potential cost savings of greater use of home- and hospice-based end of life care in England. A report for the National Audit Office. Cambridge: RAND Europe; 2008.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- End of life care. London: National Audit Office; 2008.
- Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991.
- Morize V, Nguyen DT, Lorente C, Desfosses G. Descriptive epidemiological survey on a given day in all palliative care patients hospitalized in a French university hospital. Palliat Med 1999;13:105-17. http://dx.doi.org/10.1191/026921699672866065.
- Gott M, Ahmedzai SH, Wood C. How many inpatients at an acute hospital have palliative care needs? Comparing the perspectives of nursing and medical staff. Palliat Med 2001;15:451-60. http://dx.doi.org/10.1191/026921601682553932.
- Detering KM, Reade A, Hancock M, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1345.
- Gott M, Ward S, Gardiner C, Cobb M, Ingleton C. A narrative literature review of the evidence regarding the economic impact of avoidable hospitalizations amongst palliative care patients in the UK. Prog Palliat Care 2011;19:291-8. http://dx.doi.org/10.1179/1743291X11Y.0000000014.
- We’ve been listening, have you been learning?. Middlesex: The Patients Association; 2011.
- www.parliament.uk . Parliamentary Health Committee – Fourteenth Report: Social 2012. www.publications.parliament.uk/pa/cm201012/cmselect/cmhealth/1583/158302.htm (accessed 8 February 2012).
- National clinical guidelines for stroke. London: Royal College of Physicians; 2008.
- Stroke-specific education framework. London: Department of Health; 2009.
- Living well with dementia: a national dementia strategy. London: The Stationery Office; 2009.
- British Medical Association, the Resuscitation Council (UK) and the Royal College of Nursing . Decisions Relating to Cardiopulmonary Resuscitation; A Joint 2007. www.bma.org.uk/images/DecisionsRelatingResusReport_tcm41–147300.pdf (accessed 1 August 2012).
- Seymour J, Almack K, Kennedy S, Froggatt K. Peer education for advance care planning: volunteers’ perspectives on training and community engagement activities [published online ahead of print 25 May 2011]. Health Expect 2011. doi:10.1111/j.1369–7625.2011.00688.x.
- Cox K, Moghaddam N, Almack K, Pollock K, Seymour J. Is it recorded in the notes? Documentation of end of life care and preferred place to die discussions in the final weeks of life. BMC Palliat Care 2011;10. http://dx.doi.org/10.1186/1472-684X-10-18.
- Mitchell S, Teno J, Kiely D, Shaffer M, Jones R, Prigerson H, et al. The clinical course of advanced dementia. N Engl J 2009;361:1529-38. http://dx.doi.org/10.1056/NEJMoa0902234.
- NHS Information Centre . Adult Psychiatric Morbidity in England 2007. www.ic.nhs.uk/webfiles/publications/mental%20health/other%20mental%20health%20publications/Adult%20psychiatric%20morbidity%2007/APMS%2007%20%28FINAL%29%20Standard.pdf (accessed 10 August 2012).
- Hayes R, Lee W, Rayner L, Price A, Monroe B, Hansford P, et al. Gender differences in prevalence of depression among patients receiving palliative care: the role of dependency [published online ahead of print 20 July 2011]. Palliat Med 2011. doi:10.1177/026921631141603.
- Walker A. Multiple chronic diseases and quality of life: patterns emerging from a large national sample, Australia. Chronic Illn 2007;3:202-18. http://dx.doi.org/10.1177/1742395307081504.
- Grim R, McElwain D, Hartmann R, Hudak M, Young S. Evaluating the causes of unplanned hospital admissions of palliative care patients. Am J Hosp Palliat Med 2010;27:526-31.
- Hughes J, Robinson L, Volicer L. Specialist palliative care in dementia. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.330.7482.57.
- Ryan T, Gardiner C, Bellamy G, Gott M, Ingleton C. Barriers and facilitators to the receipt of palliative care for people with dementia: the views of medical and nursing staff [published online ahead of print 3 October 2011]. Palliat Med 2011. doi:10.1177/0269216311423443.
- Harrison-Denning K, Greenish W, Jones L, Mandal U, Sampson E. Barriers to providing end-of-life care for people with dementia: a whole-system qualitative study. BMJ Support Palliat Care 2012;2:103-7.
- Glaser BG, Strauss AL. Awareness of dying. Chicago, IL: Aldine; 1965.
- Becker E. The denial of death. New York, NY: Free Press; 1973.
- Walter T. The revival of death. London: Routledge; 1994.
- Clark D. Between hope and acceptance: the medicalisation of dying. BMJ 2002;324:905-7. http://dx.doi.org/10.1136/bmj.324.7342.905.
- Field D. Awareness and modern dying. Mortality 1996;1:255-65. http://dx.doi.org/10.1080/713685838.
- Barclay S, Maher J. Having the difficult conversations about the end of life. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4862.
- Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers and clinicians. J Am Geriatr Soc 2003;51:1398-403. http://dx.doi.org/10.1046/j.1532-5415.2003.51457.x.
- Giddens A. Modernity and self-identity: self and society in the late modern age. Cambridge: Polity; 1991.
- Williams R. A Protestant legacy: attitudes to death and illness among older Aberdonians. Oxford: Clarendon Press; 1989.
- Lawton J. The dying process: patients’ experiences of palliative care. London: Routledge; 2000.
- Payne S, Hillier R, Langley-Evans A, Roberts T. Impact of witnessing death on hospice patients. Soc Sci Med 1996;43:1785-94. http://dx.doi.org/10.1016/S0277-9536(96)00077-9.
- Public perceptions of hospices: market research commissioned by Help the Hospices on behalf of PROMARK. London: Help the Hospices; 2001.
- Timmermans S. Dying of awareness: the theory of awareness contexts revisited. Soc Health Illn 1994;16:322-39. http://dx.doi.org/10.1111/1467-9566.ep11348751.
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003;82:803-14. http://dx.doi.org/10.1097/01.PHM.0000078184.08835.01.
- Seymour J, Gott M, Bellamy G, Ahmedzai SH, Clark D. Planning for the end-of-life: the views of older people about advance care statements. Soc Sci Med 2004;59:57-68.
- Young M, Cullen L. A good death: conversations with East Londoners. London: Routledge; 1996.
- Kaufman S. Time, clinic technologies, and the making of reflexive longevity: the cultural work of time left in an ageing society. Soc Health Illn 2010;32:225-37. http://dx.doi.org/10.1002/9781444391541.ch5.
- Beynon T, Gomes B, Murtagh FE, Glucksman E, Parfitt A, Burman R, et al. How common are palliative care needs among older people who die in the emergency department?. Emerg Med J 2011;28:491-5. http://dx.doi.org/10.1136/emj.2009.090019.
- Aabom B, Kragstup J, Vondeling H, Bakketeig LS, Stovring H. Defining cancer patients as being in the terminal phase: who receives a formal diagnosis, and what are the effects. J Clin Oncol 2005;23:7411-16. http://dx.doi.org/10.1200/JCO.2005.16.493.
- Seale C, Addington-Hall J, McCarthy M. Awareness of dying: prevalence, causes and consequences. Soc Sci Med 1997;45:477-84. http://dx.doi.org/10.1016/S0277-9536(96)00379-6.
- Johnson C, Paul C, Girgis A, Adams J, Currow DC. Australian general practitioners’ and oncology specialists’ perceptions of barriers and facilitators of access to specialist palliative care services. Palliat Med 2011;14:429-35. http://dx.doi.org/10.1089/jpm.2010.0259.
- The AM, Hak T, Koëter G, van der Wal G. Collusion in doctor–patient communication about imminent death: an ethnographic study. BMJ 2000;321:1376-81.
- The code: standards of conduct, performance and ethics for nurses. London: General Nursing Council; 2008.
- Howarth G. ‘Just live for today’: living, caring, ageing and dying. Age Soc 1998;18:673-89. http://dx.doi.org/10.1017/S0144686X98007132.
- Seale C. Heroic death. Sociology 1995;29:597-613. http://dx.doi.org/10.1177/0038038595029004003.
- Gardiner C, Gott M, Ingleton C, Cobb M, Seymour J, Ryan T, et al. Extent of palliative care need in the acute hospital setting: a survey of two acute hospitals in the UK [published online ahead of print 22 May 2012]. Palliat Med 2012. doi:10.1177/0269216312447592.
- May C. Disclosure of terminal prognoses in a general hospital: the nurse’s view. J Adv Nurs 1993;18:1362-8. http://dx.doi.org/10.1046/j.1365-2648.1993.18091362.x.
- Christakis N. Death foretold: prophecy and prognosis in medical care. Chicago, IL: University of Chicago Press; 1999.
- Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ 2005;330:1007-11. http://dx.doi.org/10.1136/bmj.330.7498.1007.
- Innes S, Payne S. Advanced cancer patients’ prognostic information preferences: a review. Palliat Med 2009;23:29-3. http://dx.doi.org/10.1177/0269216308098799.
- Zimmermann C, Rodin G. The denial of death thesis: sociological critique and implications for palliative care. Palliat Med 2004;18:121-8. http://dx.doi.org/10.1191/0269216304pm858oa.
- Gott M, Seymour J, Ingleton C, Bellamy G, Gardiner C. Challenges to achieving the policy rhetoric of universal palliative care provision: lessons from England and New Zealand [published online ahead of print 15 June 2011]. Palliat Med 2011. doi:10.1177/0269216311408993.
- Ward S, Gott M, Gardiner C, Cobb M, Richards N, Ingleton C. Economic analysis of potentially avoidable hospital admissions in patients with palliative care needs. Prog Palliat Care 2012;20:147-53. http://dx.doi.org/10.1179/1743291X12Y.0000000018.
- Department of Health . National Tariff 2010–11 n.d. http://data.gov.uk/data/resource_cache/b11b7061–3e58–4f22-ba53-e7151aa0a643/dh_113900.xls#Index!A1 (accessed 21 March 2012).
- Hancock K, Clayton J, Parker S, Wal der S, Butow P, Carrick S, et al. Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review. Palliat Med 2007;21:507-17. http://dx.doi.org/10.1177/0269216307080823.
- Higginson I, Gott M, Ingleton C. Living with ageing and dying: palliative and end of life care for older people. Oxford: Oxford University Press; 2011.
- Medical Research Council . Developing and Evaluating Complex Interventions: New 2008. www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC004871 (accessed 21 March 2012).
Appendix 1 Palliative Care Studies Advisory Group
This group has met every 6 months and Simon Cork, one of the founder members, reflects on the experiences of the members below:
It was June 2009 when a group of around 30 people attended a ‘User Consultation Day’ in the centre of Sheffield. Professor Merryn Gott introduced the research team that was to carry out the study entitled Transitions to Palliative Care for Older People in Acute Hospitals.
Reflecting back, from the lofty viewpoint of the lectern, we must have looked an unlikely bunch of individuals; however, our task that day was to provide a layperson’s point of view on some aspects of the subject of palliative care, in order that a balanced viewpoint be achieved. Proof copies of leaflets were read and closely studied, changes to font styles and colour discussed. Academia can sometimes concentrate on clinical excellence to the detriment of simple word usage or sentence structure and any of these finer points were considered. We left the meeting a lot the wiser about palliative care.
In September we received a follow up letter asking for further participation in the ‘User Consultation Group’ which resulted in around 12 people opting to continue their involvement. The next three meetings saw further discussions on how the study was continuing with information and feedback going in both directions. One phase of the study received quite a lot of discussion; this was regarding some of the ethical dilemmas with case notes. As laypeople, the group was both interested and involved in this aspect of the study. Ethical issues in the later stages of life are important; charitable organisations and funding partners are keen to ensure that all opinions and points of view are carefully considered. So it was appropriate to receive positive feedback from Dr Clare Gardiner that our comments had been passed further along the chain and had resulted in ethical approval being given to a difficult phase of the study.
At some meetings, other research activities or applications would be covered by the group. A typical example was in June 2011 when a study from the University of Nottingham on anticipatory prescriptions in the last days of life was discussed. All good experience for the group and feedback, as always, going in both directions.
The data from the palliative care project has now been collected and analysed. The results made very interesting reading so the group await with great interest any positive reaction from health professionals further up the chain of command. Reflecting back over two years I hope I speak for the rest of the group when I say that we have all learnt a lot, not just about palliative care, but also about the inner workings of medical research studies.
Appendix 2 Palliative Care Studies Advisory Group briefing paper
The following are excerpts from the PCSAG briefing paper.
For a copy of the paper please contact Clare Gardiner (c.gardiner@sheffield.ac.uk) or Kate Chadwick (k.chadwick@sheffield.ac.uk).
Palliative Care Studies Advisory Group briefing paper (PDF download)
Palliative Care Studies Advisory Group briefing paper (PDF download)
Appendix 3 Search strategies
Ovid MEDLINE(R) <1950 to August week 1 2009>
-
palliati$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (47,738)
-
terminal care.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (17,267)
-
“end of life care”.mp. (2526)
-
hospice.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (6964)
-
4 or 1 or 3 or 2 (64,728)
-
transition$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (144,385)
-
continuity.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (25,495)
-
6 or 7 (168,872)
-
8 and 5 (1144)
-
limit 9 to english language (977)
-
from 10 keep 1-977 (977)
EMBASE <1980 to week 33 2009>
-
palliati$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (36,944)
-
terminal care.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (5183)
-
“end of life care”.mp. (1601)
-
hospice.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (3279)
-
4 or 1 or 3 or 2 (41,542)
-
transition$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (139,442)
-
continuity.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (12,431)
-
6 or 7 (151,529)
-
8 and 5 (520)
-
limit 9 to english language (458)
-
[from 10 keep 1-977] (0)
-
from 10 keep 1-458 (458)
NHS Economic Evaluation Database (NHS EED) <1980–2009>
Searched: 1 March 2009.
Results: 16.
[palliati* or terminal care or end of life care or hospice] and [transition* or continuity]
Cochrane Database of Systematic Reviews (CDSR) <1980–2009>
Searched: 1 March 2009.
Results: 13.
[palliati* or terminal care or end of life care or hospice] and [transition* or continuity]
Appendix 4 Sheffield Profile for Assessment and Referral for Care questionnaire
Sheffield Profile for Assessment and Referral for Care questionnaire (PDF download)
Appendix 5 Protocol
Transitions to palliative care for older people within acute hospitals
Principal Investigators: Prof Christine Ingleton1, Prof Merryn Gott5
Project Manager: Dr Clare Gardiner1
Project Administrator: Kate Chadwick1
Grant Holders: Prof Jane Seymour2, Prof Mike Bennett3, Rev Mark Cobb4, Dr Bill Noble1, Dr Tony Ryan1.
Project Consultants: Sue Ward1, Chris Parker
1University of Sheffield, 2University of Nottingham, 3Lancaster University, 4Sheffield Teaching Hospitals Trust, 5The University of Auckland
Funder: National Institute of Health Research, Service Delivery and Organisation Funding Stream. Reference no: 08/1809/233.
www.transitionstopalliativecare.co.uk
Lay summary
The UK Cancer Tsar has identified that ‘a proportion of . . . dying patients receive very poor care’ in hospitals. This is reflected in the fact that half of patient complaints to the NHS relate to the end of life. As 90% of people spend time in hospital in their final year of life and 56% of people die in hospital, this ‘proportion’ translates into a significant number of patients receiving poor care, most of whom are older.
The study will examine the potential to improve care for older people at the end of life by exploring need for, and provision of, palliative care at two hospitals in England. Using a method developed by one of the study applicants, a census will be carried out of all inpatients present in the hospitals during the two week study period.
Medical and nursing staff will be asked to answer questions about each inpatient to help identify whether they have palliative care needs. For patients with palliative care needs, further questions will explore whether the patient’s care incorporates a palliative element and whether this information has been transmitted to the patient. We will also explore whether the current hospital admission could have been prevented. Where possible, patients will be asked to complete a short questionnaire about their health to identify palliative care needs from their perspective.
Interviews will then be conducted to explore these issues in more detail with patients and their families and professionals involved in planning and delivering services.
The proposal has been refined in the light of comments made by service users/research partners with experience of palliative and end of life care. Four service users will be invited to sit on the project steering group and be involved in all aspects of the study.
Introduction, aims and objectives
Introduction
A recent World Health Organisation (WHO) report concluded that addressing the ‘substandard care’ older people receive at the end of life is a key public health concern (WHO, 2004). An area of particular policy priority is palliative care provision in acute NHS hospitals (Richards, 2007: 1), where ‘a proportion of . . . dying patients receive very poor care’. As 90% of people spend time in hospital in their final year of life (Dixonet al. , 2004) and 56% of all deaths occur in this setting (Gomes and Higginson, 2008), this ‘proportion’ translates into a significant number of patients receiving poor care, the vast majority of whom will be older people.
This proposal addresses this identified need to improve palliative care management within acute hospital through a study focusing on ‘transitions’. Within this context, a transition is defined as a change in the approach to a patient’s care from ‘active treatment’ (where the focus is on cure or chronic disease management) to ‘palliative care’ (where the focus is on maximising quality of life). Transitions in care may or may not be associated with a change of care setting. The transition will not be complete or unproblematic in all cases. Indeed, it is recommended that curative and palliative approaches to treatment are adopted concurrently (Ahmedzai and Walsh, 2000), particularly for older people (Jerantet al. , 2004: 2) where adopting a transition late in the disease trajectory can lead to ‘missed opportunities for palliation’. How this process is managed within acute hospitals, however, remains unknown. The extent to which a transition in care setting to the acute hospital corresponds with a transition in care approach is also unclear, although repeat hospitalisations have been identified as a trigger to moving to a palliative approach in certain conditions (http://www.goldstandards%20framework.nhs.uk/). This study will enable these ‘unknowns’ to be clarified and a model of best practice about managing transitions within this setting to be developed. Adopting a proactive approach to palliative care management through a managed transition in care may result in several tangible benefits for both patients and the wider NHS. These include facilitating patient involvement in advance care planning (where desired) and enabling a proactive care plan to be developed. Current practice in palliative care management in the UK is predicated upon the identification of a time when palliative care should begin (http://www.goldstandards%20framework.nhs.uk/). However, the acceptability of a palliative care transition to patients with conditions other than cancer remains unexplored within the context of UK acute hospitals, the setting where most people will die.
There is evidence that many more people are dying in acute hospitals than would wish (Higginson, 2004). Not only does this result in people not having the death they would have chosen, but it also incurs significant unnecessary financial cost for the NHS. Health care costs are most significant in the last three years of life (Dixonet al. , 2004), with high rates of hospitalisation.
A key aim of this study will be to examine the ‘appropriateness’ of inpatient admissions amongst people with palliative care needs using the Appropriateness Evaluation Protocol Criteria, a validated methodology recommended for use by PCT commissioners as a basis for service planning (DoH, 2006a). As the applicability of this protocol within a palliative care context has not been examined, findings from this study component will be reviewed by the two grant applicants who are Palliative Medicine Consultants. The value of the approach is that it enables the ‘inevitability’ of an admission to be determined within a particular health locality (for example, an admission may be ‘appropriate’, but not ‘inevitable’, i.e. it could have been prevented by additional community services; DoH, 2006a). Local cost data will be obtained and combined with the admissions data to estimate the scale of the economic impact of avoidable inpatient admissions. Information will be collected directly from patients with palliative care needs regarding their preferences for, and experiences of, transitions in place of care, such as admission to acute hospital, as well as their current use of health care resources. This would enable an economic case to be explored for expanding community services to reduce avoidable acute hospital admissions amongst people with palliative care needs which takes into account the views and preferences of older people themselves. The palliative care provision being explored within this study will primarily be ‘general’ palliative care, defined as ‘palliative care provided by the patient and family’s usual professional carers as a vital and integral part of their routine clinical care’ (NCHPCS, 2001:1). This is because the vast majority of older people will not receive specialist palliative care, particularly if they are dying from conditions other than cancer (NCPC, 2007).
Research aim
To examine how transitions to a palliative care approach are managed and experienced in acute hospitals and to identify best practice from the perspective of older patients and key service providers and commissioners.
Research objectives
-
To explore the extent and current management of palliative care need within acute hospitals.
-
To identify patient factors predictive of key aspects of palliative care need and, in particular, physical and psychological symptom load.
-
To examine the circumstances under which transitions to a palliative care approach occur within acute hospitals, with a particular focus upon the influence of age and disease type on decision-making.
-
To explore how decisions to move to a palliative care approach are made and who is involved in decision-making.
-
To examine if and how information about a transition to a palliative care approach is communicated to patients and their families and how they are involved in decision making.
-
To explore the perspectives of patients, service providers and commissioners regarding acute hospital admissions and discharges associated with a transition in care.
-
To identify those hospital admissions amongst people with palliative care needs that were avoidable but occurred because of a lack of alternative service provision or support in the community.
-
To identify patient factors predictive of avoidable hospital admissions.
-
To quantify the cost of avoidable acute hospital admissions amongst those patients with palliative care needs.
Relevance to SDO call for proposals
This proposed work will address and build on a number of the recommendations arising from the SDO-commissioned scoping exercise which was undertaken to determine priorities for improving generalist end of life care for adults (Higginsonet al. , 2007). This review found that the majority of studies on generalist care at the end of life were concerned with service delivery organisational issues and health professionals’ perspectives. Fewer were concerned with patients’ experiences and the majority of studies were located in community settings; few were set in hospital. A central focus of this study will be to identify best practice for older people in managing the transition to palliative care within an acute hospital setting from their perspective. Higginsonet al. 2007 advocate mixed method designs for research in palliative care and the use of case studies in this study will facilitate a good understanding of the context in order to see how the findings might apply elsewhere. Resource and health economic evaluation was identified as a cross-cutting theme and ‘one that should be an important component of future commissioned research’ (Higginsonet al. 2007:2). A key aim of this study will be to measure economically the proportion of hospital patients with palliative care needs where the admissions can be termed ‘avoidable’ and identify where gaps in community services have resulted in acute hospital admissions. This proposal addresses the final question of the SDO research brief (REF: PCC198) theme B, topic 4. We will examine the transition from ‘active treatment’, aimed at cure or chronic disease management to palliative care in the context of two acute hospitals, the care setting that currently delivers end of life care for the majority of the population.
Background, including NHS context and relevant literature
In the summer of 2008, the new End of Life Care strategy will be unveiled as part of Lord Darzi’s review of the health service. It is clear from preliminary reviews at a regional level that identification of this activity within the NHS will require the ability to identify the point at which end of life care becomes appropriate. Policy is changing to allow health professionals to respond to patients needs and preferences in a more timely fashion than is customary at present. Any initiatives in the organization or delivery of clinical care will depend on a transition of care such as that which we intend to study. We have chosen the context of the acute hospital sector, the most common care setting for UK patients at the end of their lives.
Research in the UK has identified that older people are disadvantaged at the end-of-life, particularly if they are dying from chronic conditions other than cancer. However, there are still clear gaps in the evidence base to underpin improvements in service delivery and organisation in this area, despite improved palliative care for older people having been identified as a key challenge for the NHS (DoH, 2001; Philp, 2006). A recent review (Higginson et al. , 2007) identifies that little is known about how general palliative care is, or should, be provided in acute hospitals, particularly from the perspective of patients dying from diseases other than cancer (who make up the majority of these patients and who are primarily older people). However, there is evidence that the extent of palliative care need amongst inpatients is high. A census of palliative care need in one acute hospital in Sheffield identified 23% of the 453 inpatients as having palliative care needs (according to a standard definition) by medical and/or nursing staff responsible for their care (Gottet al. , 2001). Three quarters of these patients were over 60, with the greatest proportion aged between 81 and 90 years. Only 2% had received specialist palliative care input and any palliative care they were receiving was ‘general’ palliative care. Current palliative care policy and practice within acute NHS hospitals focuses primarily upon the period immediately prior to death. The Liverpool Care Pathway (LCP), promoted by the End of Life Care Programme for use on all acute hospital wards, is argued to be effective at changing practice (Ellershaw, 2007), but focuses exclusively on the ‘last days of life’. However, as discussed in the Introduction, it is increasingly being argued that attention needs to be paid to palliative care needs earlier in the disease trajectory (Ahmedzai and Walsh, 2000; NICE, 2006). Finally, providing care closer to home and preventing inappropriate hospital admissions is a current NHS priority (DoH, 2006b). This study will explore the extent to which it is in line with the views and preferences of older people with palliative care needs and will explore the economic impact of avoidable hospital admissions within an end of life context.
Plan of investigation
The research aims will be addressed through use of a case study design in two contrasting acute NHS hospitals in England. The case study will comprise the following stages:
-
Census of palliative care needs amongst inpatients at the two settings collecting information from patients, medical and nursing professionals working in primary and secondary care.
-
Case note review; medical notes of all consenting hospital inpatients will be examined to identify (and collect additional information about) patients with palliative care needs according to standardised criteria.
-
Interviews and focus groups with key health and social care professionals and service commissioners/planners.
-
Interviews with older people identified in the census as having palliative care needs on two occasions, six months apart; and
-
An on-going literature review conducted in conjunction with data collection to ensure the findings are interpreted within the context of best international evidence and policy guidance for palliative care management in acute hospitals.
-
Retrospective case note review: twelve months after the census, a retrospective case note review will be undertaken of all inpatients present in the hospital at the time of the census who have died in the 12 months following their hospital admission.
Rationale for study design
Organisational case studies have been found to offer pragmatic solutions to real-problems in palliative and end-of-life research (Payneet al. , 2007; Ingleton, 2007). They are an appropriate design for examining processes and outcomes in dynamic healthcare organisations, where it is important to obtain multiple perspectives, and are suitable for exploring practically and ethically complex situations where flexibility is desirable. Detailed insights from well constructed case studies also have an explanatory potential (Seale, 1999).
Selection of cases
The case studies selected for inclusion in this study are two acute NHS hospitals in England: Sheffield Northern General Hospital and Royal Lancaster Infirmary. These settings have been selected as they serve socio-demographically distinct populations. Royal Lancaster Infirmary serves a predominantly white Caucasian semi-rural/remote rural population. By contrast, Sheffield Northern General hospital services a largely urban, more economically disadvantaged and ethnically diverse area. There are 1,100 in-patient beds at the Northern General Hospital Sheffield and 550 beds at the Royal Lancaster Infirmary.
Methods (including the plan of analysis)
Exploratory focus groups to determine census methodology (n = 8)
A review of the literature and discussions with clinical colleagues identified complexity and lack of concurrence regarding ‘indicators’ that a transition to palliative care has occurred in acute hospital settings. An initial list of indicators was drawn up, including: 1) any evidence of advance care planning (this may include consideration and/or completion of a DNAR form; 2) entry on a GP palliative care register; 3) referral to any palliative care service; 4) decision not to perform investigations or treatments aimed at cure because of QOL issues; 5) prescription of certain key drugs; and 6) decision to withhold or withdraw any treatment on grounds of futility. However, it is apparent that medical and nursing staff working in primary and secondary care are likely to identify additional/alternative indicators. Therefore, 8 preliminary focus groups (4 at each research site) will be held with medical and nursing staff to explore these issues. Half of these focus groups will be held with acute hospital staff and half with community staff. This preliminary research phase will enable an initial exploration of how transitions to palliative care are made and, crucially, what impact this has upon care and treatment. The exact indicators used in the census will be determined by this preliminary work (to be undertaken during the first 6 months of the project).
Census methods
Inpatients aged > 18 years on all hospital wards during the two-week census period will be eligible for inclusion in the census. Although the focus of the study is on older adults, patients of all ages will be included to enable an exploration of the effect of age on palliative care need and management (DoH, 2001; Ahmedet al. , 2004; Burt and Raine, 2006; Grande, Addington-Hall and Todd, 1998). A list of patients present on the ward will be obtained from the ward manager or designate. A member of medical and nursing staff most involved in day-to-day patient care will then be interviewed for each patient (expected to be the named nurse and the Foundation Training doctor). Previous work suggests an approximate achievable patient sample of 1,200 across both hospitals. Interviews will gather socio-demographic and diagnostic information, identify patient ability to consent to the study, examine self-assessed training needs regarding palliative care management and then explore professional perception of palliative care need according to multiple standardised criteria. The remaining questions will only be asked of patients with identified palliative care needs according to any of the one definitions used. However, during data analysis a more in-depth exploration of palliative care need will be made and decisions made on the ground that a patient did have palliative care needs may be over-ridden. Subsequent questioning will explore perceived appropriateness of the current admission, discharge plans, current approach to care (palliative/active or a mixture of both), whether prognosis and care approach have been discussed with the patient, resuscitation status, any psychological/spiritual assessment of patient and carer needs, and any referral to specialist palliative care. Additional indicators of a palliative care transition as identified during the preliminary focus group work will also be examined. All patients resident in the ward and identified as not having capacity issues by the medical/nursing staff will also be asked to complete the Sheffield Profile for Assessment and Referral to Care (SPARC) (Ahmedet al. , 2004), and an 18-item service use questionnaire developed for use with a palliative care population (Gottet al. , 2007). They will also be asked to indicate willingness to participate in a future interview. The word ‘palliative’ will not feature on any patient information (see ethical issues section). In order to include patients with dementia and cognitive impairment in the study, where ward staff identify patients are unable to consent, the patient’s relative/friend/carer will be asked to complete the SPARC questionnaire on the patient’s behalf. The exact process by which this occurs will be discussed with an NHS ethics committee to ensure compliance with the MCA.
Brief telephone administered questionnaires will also be conducted with the GPs of patients identified in the survey as having palliative care needs (following approval to contact them from the patient). The view of the GP regarding the goal of treatment for the patient will be explored, as will whether the patient has been registered on a general practice end of life register in line with the Gold Standards Framework. In cases where the GP is unable to participate, for example due to time constraints, a primary care nurse (e.g. practice nurse, district nurse, community matron) will be approached in their place. The Practice Administrator will also be asked to inform the research team when patients participating in the study die (the Research Secretary will contact each Practice 3 months before the end of the study to ensure this information is complete).
Case Note Review
Medical notes will be reviewed for all consenting hospital inpatients during the 2 week census. Notes will be examined for evidence of palliative care need according to the Gold Standards Framework prognostic indicators (GSF, 2005). For those patients with identified palliative care needs, supplementary information will be gathered including number of hospital admissions, evidence of resuscitation status and evidence of an advanced decision to refuse treatment or lasting power of attorney for health and social care (MCA, 2005). Clinical information regarding the current admission will be considered in relation to the ‘Appropriateness Evaluation Protocol Criteria’ for hospital admission (DoH, 2006a). Final ‘appropriateness’ and ‘inevitability’ of the admission will be reviewed by the two grant applicants who are Palliative Medicine Consultants.
Retrospective case note review
Twelve months after the census has been undertaken, researchers will retrospectively identify census patients who have died in the preceding 12 months i.e. patients who died within 12 months of their hospital admission. Lists of inpatients obtained at the time of the census will be matched against hospital death records (from the medical records department) in order to identify those patients who have died. Reason for patient death will be examined in the first instance and patients who died an accidental death (i.e. road traffic accident) will be excluded. For the remaining patients, case notes will be examined and information recorded concerning various aspects of their care (see retrospective case note proforma v1). Case notes will be obtained from medical records, where notes are kept for up to 4 years after patient discharge. Notes will be examined on hospital premises away from patient areas, by experienced clinical academics. Cause of death and place of death data will be extracted from the Medical Research Information Service at the NHS Information Centre, as this data may not be fully recorded in hospital notes.
The census method is adapted from a study developed to survey palliative care needs in an acute hospital in Sheffield (Gott, Ahmedzai and Woods, 2001). In this research, a structured interview was conducted with ward nursing staff for 99% of inpatients (n = 449) and medical staff for 81% of inpatients (n = 367). In the proposed study, a similar method will be used as it elicited a very high response rate, high quality data, and has been identified as a template for studying palliative care need in acute hospitals (Boydet al. , 2006).
To ensure the census element of the study is effective, experienced health researchers with clinical experience will be required. They will comprise employees at the University of Sheffield with a PhD or equivalent experience in a relevant discipline who will be released from their usual duties for the data collection periods and additional training. Eleven researchers will support the existing project team to conduct the census in Sheffield, and approximately four in Lancaster (as bed numbers are lower). Training will be provided by grant applicants (JS and CI conducted the previous census; MG conducted and managed it), with input from our research partners.
Interviews (n = 40) and focus groups (n = 6) with key health and social care professionals and service commissioners/planners
Following initial analysis of the census data, individual interviews and focus groups will be conducted within each locality with service commissioners/planners and key health and social care providers involved in the care of older people and people with palliative care needs within the hospital setting and in the community. Current practice in palliative care management for older people within the locality will be explored, with a particular focus upon transitions to palliative care. The results of the census will be fed back to all interviewees as a trigger to applying a SWOT analysis (strengths, weaknesses, opportunities and threats) to develop a model for best practice.
Interviews with older people (n = 40)
Approximately 20 patient interviews will be conducted at each research site. Purposive sampling of all patients who return a reply slip indicating willingness to participate in an individual interview will be utilised to ensure a diverse sample in terms of key characteristics (gender, age, diagnosis, family carer status). Interviews will focus on satisfaction with health and social care received and communication with health professionals regarding diagnosis and prognosis. Self-defined need for palliative care will be explored according to key patient centred factors underpinning Steinhauser’s1 multi-dimensional definition of palliative care (symptom management, support of autonomy and function, advance care planning and desired levels of participation in decision-making, patient satisfaction, patient-provider communication, quality of life, patient education and provider continuity).
Preferences for end-of-life care (including place of care) will be initiated where appropriate (Gottet al. , 2007) and views of (current and best practice) in transitions to a palliative care approach explored, again if appropriate. Repeat interviews will be conducted at six months to look at changing experiences of, and preferences for, care (identified as a priority by Higginsonet al. , 2007). Where patients have died during this period, bereaved family carers will be invited to participate in an interview to explore the circumstances of death and their bereavement support needs (Gottet al. , 2007).
Data analysis
Analysis of the qualitative data will adhere to the principles of grounded theory and follow the National Centre for Social Research ‘Framework’ approach, involving a structured process of ‘sifting, charting and sorting material’ according to key issues (Ritchie & Spencer, 1994). Recurring themes and concepts will be identified to make up a thematic framework or index which will then be systematically applied to the transcripts. Analyses will be conducted by the Research Fellow, together with two study applicants (MG and CI) who will work together to ensure data quality (for example, using double-coding and participant validation). Comparison of the findings from each case will then take place, to aid the process of identifying a transferable set of insights from the project. Quantitative data will be coded onto SPSS and analysed on the advice of a medical statistician with expertise in research with older people in a palliative care context (Chris Parker). Key multivariate analyses to be undertaken will include an identification of the main predictors of avoidable hospital admissions amongst patients with palliative care needs (including age, diagnosis, living arrangements etc) and the main predictors of physical and psychological symptom load as measured by the POS.
Economic analysis
An experienced health economist who worked on the NICE guidelines for Cancer and Palliative Care will support this area of work (Sue Ward). This study will measure the proportion of hospital patients with palliative care needs where the admissions can be termed ‘inappropriate’ and identify where gaps in community services have resulted in ‘inevitable’ admissions using the Appropriateness Evaluation Protocol Criteria described above (DoH, 2006). The length of the hospital admission amongst patients with palliative care needs will be obtained by returning to the hospital at a later date; the exact mechanism to do this will be negotiated with the individual Trusts. The cost of an average inpatient day for palliative care patients will be obtained from the local institutions and used to estimate the scale of the economic impact of inappropriate inpatient admissions within this patient group. There is an urgent need to develop methodologies for the economic analysis of palliative care use (Higginsonet al. , 2007). A description of resource use will be built up using data gathered from patients in the Census. For those patients who participate in an individual interview, this will be explored in more detail and reviewed by Sue Ward (Health economist). She will explore the potential to use this information as a basis to economically cost resource use amongst palliative care patients. This exercise will inform the development of a further grant application to explore the economic aspects of resource use in palliative care.
Benefits of research to NHS
This project will improve insights into the delivery of general palliative care in acute hospitals and will specifically identify suggestions for best practice for older patients in this context. This will comprise guidance on when and where discussions about palliative care should occur, by whom, with what information and how these decisions should be communicated. These elements have the potential to form a care pathway to fit alongside other organisation-wide strategies and practices on palliative care, including end of life care. Adopting a proactive approach to palliative care and facilitating appropriate transitions between active to palliative care is likely to result in several tangible benefits. First, patients and their families will be more engaged in decisions affecting their healthcare, particularly those that surround withdrawal or with-holding of burdensome treatments. This will contribute to the appropriate and effective use of healthcare resources. Second, decisions about place of care may be communicated earlier leading to greater chance of the patient being in a place of their choosing. This is likely to be at home or other community based facility and not within the acute hospital. The study will enable an economic case to be examined for moving a proportion of care for people with palliative care needs out of the acute hospital and into the community, something which fits with wider NHS policy. Thirdly, communication of these decisions with primary healthcare teams will improve and enable better planning of future care by such teams, particularly at times of crisis when re-admission to hospital might be avoided. Fourthly, complaints brought by patients and families regarding lack of communication on management decisions, exposure to un-necessary treatments, and poor quality of death are likely to be reduced. Tailored reports of recommendations will also be provided to each research site which it is hoped will bring specific benefits to the NHS within these localities.
Proposals for the involvement of stakeholders
At least two service users from each location (Sheffield and Lancaster) will be represented on the study steering group, drawn from the Cancer Experiences Collaborative research partner’s forum, a collaborative group of academics, clinicians and service users from five UK Universities with expertise in supportive and palliative care research. A preliminary consultation with the CECo research partner’s group identified that they did feel this was an important and worthwhile project with key ethical challenges (summarised below). One member of the group (who is in her 80s) has already agreed to sit on the project steering group. Key roles for these individuals will include helping to train the census researchers, having input into how patients are approached within the hospital, and designing the interview and focus group schedules. They will also be asked to comment on all study materials, as well as emergent findings. It is hoped they will participate in study dissemination.
On-going training and support will be provided to service user members of the research team. This will include appropriate research training and any other support they identify as appropriate and necessary. A highly experienced educator (Maddie Welton) with a clinical background in specialist palliative care practice and management and experience of working with service users in similar research projects will help facilitate these sessions. She will also provide psychological support to the service users, and any other members of the research team who feel they require it, in line with best practice in palliative and end of life care research. Those who plan, manage and deliver services will be included in the study through targeted interviews and focus groups. In addition, one representative from the Acute Trust and one from the PCT within each locality will be invited to sit on the project steering group.
Plans for dissemination of results
There are four main audiences to consider in disseminating the lessons learnt from this project: (1) The local health care trusts that participate in the project and the wider communities of policy makers and practitioners responsible for the governance and planning of local health and social care for those with palliative and end of life care needs, especially among older people. (2) Policy makers and practitioners at national level. (3) Academics. (4) The wider public. We propose to use a range of methods of dissemination to engage with these audiences, drawing on our networks of contacts including the NHS End of Life Care Programme, The Department of Health, The National Council for Palliative Care, Help the Hospices, Help the Aged and other similar statutory and third sector organisations. Elements in our dissemination strategy will include: (1) The preparation of a short summary of findings, including key recommendations for policy, practice and research, written in accessible language. (2) The development of a project website which will outline the project’s aims and objectives and list details of publications and presentations arising from the research as they become available. (3) Presentations will be made by the applicants at relevant national and international conferences to professional and academic audiences. (4) Dissemination of findings via publication in relevant professional, clinical and lay journals. (5) Mention of the study in invited seminars and lectures and teaching activities. (6) The preparation of a press release with the help of media experts based at the University of Sheffield. Applicants have considerable experience in managing media dissemination and conducting interviews on radio and television. (7) The publication of a leaflet summarising the main findings to disseminate to the general public.
Project timetable
The project will run from January 2009–December 2011. Ethical and research governance approval will be secured for the exploratory focus groups prior to the start of the project to save costs to the SDO.
Retrospective case note proforma v1
References
- Ahmed N, . Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. Palliative Medicine 2004;18:525-42.
- Ahmedzai SH, Walsh D. Palliative medicine and modern cancer care. Semin Oncol 2000;27:1-6.
- Boyd H, . The in-Hospital Palliative Care Strategy 2006. URL: www.capc.org/tools-for-palliative-care-programs/co mp-bus-plans/waitemata-business-plan.pdf (accessed 13th December, 2007).
- British Geriatric Society . Palliative and End of Life Care of Older People 2004. URL: http://www.bgs.org.uk/Publications/Publication%20Downloads/Compend_4–8palliative.doc (accessed 12th December, 2007).
- Burt J, Raine S. The effect of age on referral to and use of specialist palliative care services in adult cancer patients: a systematic review. Age and Ageing 2006;35:469-76.
- Costello J. Nursing older dying patients: findings from an ethnographic study of death and dying in elderly care wards. Journal of Advanced Nursing 2001;35:59-68.
- Costello J. Dying well: nurses’ experiences of ‘good and bad’ deaths in hospital. Journal of Advanced Nursing 2006;54:594-601.
- National Service Framework for Older People. London: DoH; 2001.
- Department of Health . Care and Resource Utilisation 2006. URL: http://www.improvementfoundation.org/documents/Care_Resource_Utilisation_Dec_06.pdf (accessed 14/04/2008).
- Our Health, Our Care, Our Say. London: DoH; 2006.
- Dixon T, . Hospital admissions, age, and death: retrospective cohort study. BMJ 2004;328.
- Edmonds P, Rogers A. ‘If only someone had told me …’ A review of the care of patients dying in hospital. Clinical Medicine 2003;3:149-52.
- Ellershaw J. What a difference a LCP makes!. Palliat Med 2007;21:365-8.
- Gomes B, Higginson I. Where people die (1974–2030): past trends, future projections and implications for care. Palliat Med 2008;22:33-41.
- Gomes B, Higginson I. Home or hospital? Choices at the end of life. J R Soc Med 2004;97:413-4.
- Gott M, Ahmedzai SH, Wood C. How many inpatients at an acute hospital have palliative care needs? Comparing the perspective of nursing and medical staff. Palliat Med 2001;15:451-60.
- Gott M, Stevens T, Small N, Ahmedzai SH. Involving users. Improving services. The example of cancer. British Journal of Clinical Governance 2002;7:81-5.
- Gott M, . A Multicentre, Longitudinal Study to Explore the Palliative Care Needs of Older People With Heart Failure and Their Carers 2007.
- Grande G, Todd C, Addington-Hall JM. Variables related to place of death and access to specialist palliative care. Soc Sci Med 1998;47:565-79.
- Healthcare Commission 2006. URL: Http://www.healthcarecommission.org.uk/_db/_%20documents/spotlight_on_complaints.pdf (accessed 15 May 2007).
- Hearn, Higginson . Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Quality in Health Care 1999;8:219-27.
- Higginson I, McCarthy M. Validity of the support team assessment schedule: do staffs’ ratings reflect those made by patients or their families?. Palliative Medicine 1993;7:219-28.
- Higginson I, . Scoping Service on Generalist Services for Adults at the End of Life: Research, Knowledge, Policy and Future Research Needs 2007.
- Ingleton C, Addington-Hall J, Bruera E, Higginson I, Payne S. Research Methods in Palliative Care. Oxford University Press; 2007.
- Jerant AF, . The TLC Model of Palliative Care in the Elderly: Preliminary Application in the Assisted Living Setting. Ann Fam Med 2004;2:54-60.
- Mental Capacity Act 2005. URL: http://www.opsi.gov.uk/ACTS/acts2005/ukpga_20050009_en_1 (accessed 14/04/2008.MCPIL, 2007).
- Murray S. Palliative care in chronic illness. BMJ 2005;330:611-2.
- Strategic Agenda for 2001 to 2004. NHPCS; 2001.
- Minimum Data Sets (MDS) Project Update including 2006/07 data. London: NCPC; 2007.
- NICE . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care 2006. URL: http://www.nice.org.uk/guidance/index.jsp?action=byID&r=true&o=10998 (accessed 12th December 2007).
- ONS Office of National Statistics 2005. URL: http://www.statistics.gov.uk/Nov.
- Payne S, . Case study research methods in end-of-life care: reflections on three studies. Journal of Advanced Nursing 2007;58:236-45.
- Philp I. A New Ambition for Old Age: Next Steps in Implementing the NSF for Older People. London: DoH; 2006.
- Ramirez AJ, Addington-Hall J, Richards MA. BMJ. London: DoH; 1998.
- Richards M. National Care of the Dying Audit – Hospitals. London: MCPCIL; 2007.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Seale C. The quality of qualitative research. London: Sage; 1999.
- Seymour J, Witherspoon R, Gott M, Ross H, Payne S, Owen T. End-of-life Care: promoting comfort, choice and well-being for. older people. Bristol: Policy Press; 2005.
- Steinhauser KE. Measuring end-of-life care outcomes prospectively. Journal of Palliative Medicine 2005;8:S30-S41.
- Thomas K, Noble B. Improving the delivery of palliative care in general practice: an evaluation of the first phase of the Gold Standards Framework. Palliat Med 2007;21:49-53.
- WHO . Better Palliative Care for Older People 2004. URL: www.eapcnet.org/download/forProjects/Elderly/BetterPC.Older%20People.pdf. (accessed 13th December 2007).
Appendix 6 Outputs
Publications summary: transitions to palliative care for older people in acute hospitals
| Papers – under review | ||
|---|---|---|
| Gardiner C, Ward S, Gott M, Ingleton C. Economic impact of potentially avoidable hospital admissions amongst patients with palliative care needs: a retrospective case note review (under review). Palliat Med | ||
| Papers – published/in press | ||
| Gott M, Ingleton C, Gardiner C, Ryan T, Noble B, Seymour J, et al. How to improve end of life care in acute hospitals. Nurs Older People 2009;21:26–9 | ||
| Gardiner C, Gott M, Ingleton C. Failing research in the NHS: research passports don’t help. BMJ 2010;341:10 | ||
| Green E, Gardiner C, Gott M, Ingleton C. ‘Communication surrounding transitions to palliative care in heart failure: a review and discussion of the literature. Prog Palliat Care 2010;18:281–90 | ||
| Gardiner C, Cobb M, Gott M, Ingleton C. Barriers to the provision of palliative care for older people in acute hospitals. Age Ageing 2011;40:233–8 | ||
| Gardiner C, Ingleton C, Gott M, Ryan T. Exploring the transition from curative care to palliative care: a systematic review of the literature. BMJ Support Palliat Care 2011;1:56–63 | ||
| Green E, Gardiner C, Gott M, Ingleton C. Exploring the extent of communication in heart failure: the perspectives of health care professionals. J Palliat Care 2011;27:107–16 | ||
| Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: qualitative study. BMJ 2011;342:d1773 | ||
| Gott M, Bennett M, Ingleton C, Gardiner C. Transitions to palliative care in acute hospitals in England: qualitative study. BMJ Support Palliat Care 2011;1:42–8 | ||
| Gott M, Ward S, Gardiner C, Cobb M, Ingleton C. A narrative literature review of the evidence regarding the economic impact of avoidable hospitalisations amongst palliative care patients in the UK. Prog Palliat Care 2011;19:291–8 | ||
| Ryan T, Gardiner C, Bellamy G, Gott M, Ingleton C and nursing staff. Barriers and facilitators to the receipt of palliative care for people with dementia: the views of medical and nursing staff. Palliat Med 2012;26:879–86. | ||
| Gardiner C, Gott M, Ingleton C. Hospital deaths are on decline, but change is slow, says new report. BMJ 2012;344:e3307 | ||
| Gardiner C, Gott M, Ingleton C, Cobb M, Seymour J, Noble B, et al. Extent of palliative care need in the acute hospital setting: a survey of two acute hospitals in the UK. Palliat Med 2013;27:76–83. | ||
| Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. ‘That’s part of everybody’s job’: the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med 2012;26:232–41 | ||
| Ward S, Gott M, Gardiner C, Cobb M, Richards N, Ingleton C. Economic analysis of potentially avoidable hospital admissions in patients with palliative care needs. Prog Palliat Care 2012;20:147–53 | ||
| Gardiner C, Gott M, Ingleton C, Richards N. Frailty and palliative care in older people: a cross-sectional survey of in-patients at two hospitals in England. Prog Palliat Care 2013; in press | ||
| Gott M, Gardiner C, Ingleton C, Cobb M, Noble B, Bennett M, et al. What is the extent of potentially avoidable admissions amongst hospital inpatients with palliative care needs? BMC Palliat Care 2013;12:9 | ||
| Gott M, Gardiner C, Ingleton C, Seymour J. Prevalence and predictors of transition to a palliative care approach amongst hospital inpatients in England. J Palliat Care 2013; in press | ||
| Ingleton C, Gardiner C, Gott M, Seymour J. Palliative care education and training needs of medical and nursing staff in hospitals. BMJ Support Palliat Care 2013;3:207–12 | ||
| Richards N, Gardiner C, Gott M, Ingleton C. Awareness contexts revisited: indeterminacy in initiating discussions at the end of life. J Adv Nurs 2013; in press | ||
| Ryan T, Gardiner C, Gott M, Ingleton C, Parker C. Symptom burden, palliative care need and predictors of physical and psychological discomfort in two UK hospitals. BMC Palliat Care 2013;12:11 | ||
| Conference presentations | Conference | Presentation type |
| Gott M, Ingleton C, Gardiner C, Ryan T, Bennett M, Cobb M, et al. Transitions to palliative care for older people in acute hospitals in the UK | 11th Congress of the European Association for Palliative Care, Vienna, Austria, May 2009 | Poster |
| Gardiner C, Gott M, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care for older people in acute hospitals | Palliative Care Research Society Annual Scientific Meeting, London, UK,November 2009 | Oral |
| Gott M, Gardiner C, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care for older people in acute hospitals in the UK | Australian Association of Gerontology Conference, Canberra, Australia, November 2009 | Oral |
| Gardiner C, Gott M, Ingleton C, Ryan T, Seymour J, Bennett M, et al. ‘Transitions to palliative care in acute hospitals: methods and ethical challenges’ | Eighth Palliative Care Congress, Bournemouth, UK, March 2010 | Poster |
| Gott M, Ingleton C, Gardiner C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care for older people in acute hospitals | Eighth Palliative Care Congress, Bournemouth, UK, March 2010 | Oral |
| Gardiner C, Gott M, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care in acute hospitals: findings from a qualitative study with medical and nursing staff in the UK | Sixth Research Congress of the European Association of Palliative Care, Glasgow, UK, June 2010 | Poster |
| Gardiner C, Gott M, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Management of palliative care in acute hospitals: findings from a qualitative study with medical and nursing staff in the UK | Sixth Research Congress of the European Association of Palliative Care, Glasgow, UK, June 2010 | Oral |
| Gardiner C, Cobb M, Gott M, Ingleton C. Barriers to providing palliative care for older people in acute hospitals | International Association of Geriatrics and Gerontology VIIth European Region Congress, Bologna, Italy, April 2011 | Poster |
| Gardiner C, Gott M, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care in acute hospitals: findings from a qualitative study with medical and nursing staff in the UK | CECo Final Conference, Manchester, UK, April 2011 | Poster |
| Gardiner C, Ingleton C, Gott M, Ryan T. Exploring the transition from curative care to palliative care: a systematic review of the literature | 12th Congress of the European Association for Palliative Care, Lisbon, Portugal, May 2011 | Poster |
| Gott M, Ingleton C, Gardiner C. ‘There’s a specialism as well which makes things difficult sometimes’: challenges in negotiating ‘generalist’ and ‘specialist’ palliative care provision in England and New Zealand | 12th Congress of the European Association for Palliative Care, Lisbon, Portugal, May 2011 | Oral |
| Ryan T, Ingleton C, Gott M, Gardiner C, Bellamy G. Transitions to palliative care for people with dementia | 12th Congress of the European Association for Palliative Care, Lisbon, Portugal, May 2011 | Poster |
| Gardiner C, Ingleton C, Gott M. Palliative care in the acute hospital setting: a qualitative interview study | SDO Network Annual Conference, Liverpool, UK, June 2011 | Oral |
| Gott M. Transitions to palliative care: challenges and opportunities | Te Omanga ‘Changing Minds’ Conference, New Zealand, July 2011 | Oral |
| Gott M. Integrating palliative care and chronic disease management | Ninth Asia Pacific Hospice Conference, Penang, Malaysia, July 2011 | Oral |
| Gott M. Transitions to palliative care in acute hospitals | Palliative Care Nursing New Zealand Annual Conference, Wellington, New Zealand, October 2011 | Oral |
| Gott M. Living with ageing and dying: international perspectives on palliative and end of life care for older people | New Zealand Stroke and Applied Neurosciences Conference, Auckland, New Zealand, November 2011 | Oral |
| Gardiner C, Gott M, Ingleton C. Showcasing ageing research: what’s happening in South Yorkshire? | What’s happening in South Yorkshire, Sheffield, UK, November 2011 | Oral |
| Ingleton C. Conducting research with older people: lessons from a recent research study (workshop) | Second International Nursing Research Conference, Kuala Lumpur, Malaysia, February 2012 | Oral |
| Ingleton C, Gott M. Living with ageing and dying: international perspectives on palliative and end of life care for older people | Second International Nursing Research Conference, Kuala Lumpur, Malaysia, February 2012 | Oral |
| Ingleton C, Richards N, Gardiner C, Gott M. Awareness contexts revisited | Second International Nursing Research Conference, Kuala Lumpur, Malaysia, February 2012 | Oral |
| Ingleton C, Richards N, Gardiner C, Gott M. Transitions to palliative care in acute hospitals | Second International Nursing Research Conference, Kuala Lumpur, Malaysia, February 2012 | Oral |
| Ingleton C, Richards N, Gardiner C, Gott M. Transitions to palliative care in acute hospitals | Second Research and Development Conference, University of Newcastle, Newcastle, UK, January 2012 | Oral |
| Gardiner C, Gott M, Ingleton C, Cobb M, Bennett M, Seymour J, et al. Extent of palliative care need in the acute hospital setting: a census of two UK hospitals | Ninth Palliative Care Congress, Gateshead, UK, March 2012 | Oral |
| Richards N, Gardiner C, Gott M, Ingleton C. Awareness contexts revisited: indeterminacy in initiating discussions at end-of-life | Ninth Palliative Care Congress, Gateshead, UK, March 2012 | Poster |
| Gardiner C, Gott M, Ingleton C, Cobb M, Bennett M, Seymour J, et al. Extent of palliative care need in the acute hospital setting: a census of two UK hospitals | Seventh World Research Congress of the European Association for Palliative Care, Trondheim, Norway, June 2012 | Poster |
| Richards N, Gardiner C, Gott M, Ingleton C. Awareness contexts revisited: indeterminacy in initiating discussions at the end of life | Seventh World Research Congress of the European Association for Palliative Care, Trondheim, Norway, June 2012 | Poster |
| Richards N, Gardiner C, Gott M, Ingleton C. Awareness contexts revisited: indeterminacy in initiating discussions at the end of life | University of Sheffield Medical School Research Day, Sheffield, 11 June 2012 | Poster |
| Gardiner C, Gott M, Ingleton C. Extent of palliative care needs in the acute hospital setting: a survey of two acute hospitals in the UK | International Palliative Care Network Conference, virtual online conference, September 2012 | Poster |
| Published conference proceedings | ||
| Gott M, Ingleton C, Gardiner C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care for older people in acute hospitals in the UK. Australas J Ageing 2009;28 (Suppl. 2):A58 | ||
| Gardiner C, Gott M, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care in acute hospitals: ethical and methodological challenges. Palliat Med 2010;24:221 | ||
| Gott M, Gardiner C, Ingleton C, Ryan T, Seymour J, Bennett M, et al. Transitions to palliative care for older people in acute hospitals. Palliat Med 2010;24:215 | ||
| Gardiner C, Gott M, Ingleton C, Cobb M, Bennett M, Noble B, et al. Extent of palliative care need in the acute hospital setting: a prospective study of two acute hospitals in the UK. Palliat Med 2012;26:522 | ||
| Richards N, Gardiner C, Ingleton C, Gott M. Palliative care in the acute hospital setting: a qualitative interview study. Palliat Med 2012;26:537 | ||
| Other publications | ||
| PULSE, Newsletter, Spring 2010, Project Focus, p. 2 | ||
| Gardiner C, Gott M, Ingleton C, Bennett M. Transitions to palliative care in the acute hospital setting: reflections on a census at Royal Lancaster Infirmary. Morecambe Bay Med J Spring 2011;6:106–9 | ||
| Cork S. Two years on: palliative care project. Research, Endeavours And Dissemination (READ) Newsletter, School of Nursing and Midwifery, University of Sheffield, Autumn 2011, p. 6 | ||
| Palliative Care Studies Advisory Group. Service user involvement in research: a briefing paper by the Palliative Care Studies Advisory Group, 2012 | ||
| Sue Ryder Care Centre for the Study of Supportive, Palliative and End of Life Care, University of Nottingham, Newsletter, Spring 2012, p. 2 | ||
| Sheffield Teaching Hospitals NHS Foundation Trust. Research identifies differences over palliative care sources. Research & Innovation Bulletin, May 2012 | ||
| Palliative Care Research Society Newsletter, July 2012, no. 34, pp. 8–9 | ||
| Book chapter | ||
| Gardiner C, Barnes S. Improving environments for care at the end of life in hospitals. In Gott M, Ingleton C, editors. Living with ageing and dying: palliative and end of life care for older people. Oxford: Oxford University Press; 2011. pp. 237–44 | ||
List of abbreviations
- CDSR
- Cochrane Database of Systematic Reviews
- CECo
- Cancer Experiences Collaborative
- CLRN
- Comprehensive Local Research Network
- COPD
- chronic obstructive pulmonary disease
- DNAR
- do not attempt resuscitation
- DoH
- Department of Health
- ECC
- Ethics and Confidentiality Committee
- GMC
- General Medical Council
- GP
- general practitioner
- GSF
- Gold Standards Framework
- HRG
- Healthcare Resource Group
- JBI
- Joanna Briggs Institute
- LCP
- Liverpool Care Pathway
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIGB
- National Information Governance Board
- ONS-HES
- Office for National Statistics Hospital Episode Statistics
- OR
- odds ratio
- PCSAG
- Palliative Care Studies Advisory Group
- PCT
- primary care trust
- PPI
- patient and public involvement
- PSS
- personal social services
- QoL
- quality of life
- RCT
- randomised controlled trial
- RLI
- Royal Lancaster Infirmary
- SNGH
- Sheffield Northern General Hospital
- SPARC
- Sheffield Profile for Assessment and Referral for Care
- UHMBT
- University Hospitals of Morecambe Bay NHS Foundation Trust
- WHO
- World Health Organization