Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 09/1002/38. The contractual start date was in November 2010. The final report began editorial review in November 2012 and was accepted for publication in July 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Kyratsis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
The emergence and increasing popularity of evidence-based medicine (EBM) since the 1990s1 has provided support for ideas advocating the use of research evidence to improve managerial practice and decision-making in health care. 2,3 It is argued that health policy and management, although admittedly different from clinical practice in significant ways, are lagging behind clinical practice in addressing the problems of ‘overuse, underuse and misuse’ of evidence related to management practice and that this has a significant impact on the quality of care and patient outcomes. 2 Under this perspective, health service delivery and organisation, as well as decision-making, could be improved by applying robust and relevant research findings and other forms of knowledge relating to good practice. More recently, discourse espousing the principles of evidence-based management (EBMgt) and the idea of using research evidence to support managerial decisions also emerged in mainstream management and organisation studies literature. 4,5
Following a similar strand of argument, policy-making circles in the UK have been increasingly advocating the merits of using research evidence to inform clinical and managerial practice. This policy discourse has particularly emphasised the need to spread ‘best practices’ and implement innovations within the NHS to help enhance health-care quality and productivity. 6 In recent years, the Department of Health has issued a number of policy reports and has set up agencies with the aim of promoting evidence-based practice and innovation.
The Cooksey report on UK health research funding7 identified a ‘gap’ in translating innovative ideas and products into practice. The Report of the High Level Group on Clinical Effectiveness, established by the Chief Medical Officer, reviewed areas of significant variation in implementing evidence-based practice. Among a number of recommendations on enhancing the effectiveness and efficiency of clinical care, the report emphasised the need for increased understanding of the mechanisms that encourage the adoption of new interventions. 8 The Report of the Clinical Effectiveness Research Agenda Group highlighted the need to develop the capacity of NHS staff to use implementation (and clinical) research in daily practice and the need for greater understanding of the processes by which managers and others access and apply implementation and clinical research when making decisions. 9
A number of agencies were also created, with the NHS Institute for Innovation and Improvement and the NHS Technology Adoption Centre being prime examples. Following the Cooksey report,7 Academic Health Sciences Centres (AHSCs) and Biomedical Research Centres and Units (BRCs and BRUs) were established to facilitate the translation of research knowledge into clinical practice. The National Institute for Health and Care Excellence (NICE) was set up, and in recent years it has become involved in health technology evaluations. With the publication of the latest report, Innovation, Health and Wealth,10 further organisational changes are being envisaged as the NHS seeks to provide more effective support for innovation and adoption.
However, the adoption of new ideas and technologies is regarded as a challenging issue. On the one hand, there is a need to ensure that, once identified, effective new technologies are adopted and disseminated across the NHS, as the policy goals above suggest. The assumption is that relevant and robust evidence of efficacy and cost-effectiveness produced centrally (i.e. via the NICE or the NHS Technology Adoption Centre) will facilitate such dissemination efforts. On the other hand, much recent research suggests that the way in which evidence comes into play during the adoption and system-wide diffusion is a far more situated and context-mediated process. 11,12 Understanding of the actual practice of how evidence is used in organisational decisions within the multiprofessional setting of NHS is limited. We know even less when this process involves non-clinical decisions. This is where our study aims to make a significant contribution to the NHS and to patient benefit.
We empirically focus on infection prevention and control (IPC) in NHS acute care. In this field, relevant NHS policy reports6,13 and legislation14 have highlighted that countermeasures of known effectiveness have not been universally implemented. In addition, the NHS has commissioned large projects in recent years to identify new technologies and products which work best. One example in the field of health-care-associated infections (HCAIs) is the Department of Health’s ‘Showcase Hospitals’ programme, which aimed to evaluate technologies ‘in use’ across a number of NHS hospitals in England and diffuse such learning among health-care practitioners.
Targets for HCAIs are high on the UK Government’s agenda with performance being monitored carefully by regulatory agencies, such as the Care Quality Commission (CQC), with powers to issue warnings and penalty notices to public and private providers. High media attention combined with high public and patient interest in recent years has demanded transparency of investment and resultant benefit to patients and the NHS. This accountability to the public is facilitated by formal channels, such as the patient environment action teams, who assist inspections of NHS sites, as well as the third sector. The complexity of interorganisational contextual influences and the multiprofessional, cross-cutting nature of IPC make our selected empirical setting an information-rich case for investigating the adoption of innovative technologies and the use of evidence in this process.
In summary, despite the interest in EBM in recent decades, which has led to considerable empirical research on the use of clinical evidence by health professionals, there has so far been limited empirical work in health care in relation to the use (or non-use) of management research or other forms of knowledge in decision-making and the adoption of innovations.
Aims and research questions
The main aim of the project is to investigate the use of research-based knowledge in health-care management decisions. A key objective is to explore the construction of what is regarded as evidence by health-care managers when they make organisational decisions. We include general managers (non-clinical staff) and ‘clinical hybrid managers’ (clinicians in a managerial role) to investigate how health-care managers draw upon and make sense of different types and sources of evidence when they make decisions about innovations. Emphasis is also placed on the facilitating or constraining influences of contextual factors on health-care managers’ decision-making processes. The research is empirically set within the context of management decisions relating to HCAIs. In particular, we explored how health-care managers adopt, and implement, innovative technologies to combat HCAIs in NHS acute trusts.
The study design incorporates multiple levels of analysis: (1) it explores the influences of wider ‘macro’-level contextual dynamics on managers’ decision-making, (2) it explores decision-making processes at the ‘meso’ organisational level, and (3) it analyses at a ‘micro’ level the processes by which health-care managers construct meaning of available evidence and how they might use such evidence when deciding on the adoption or rejection of innovations.
The study aimed to address the following key research questions:
-
How do managers (non-clinical and clinical hybrid managers) make sense of evidence?
-
What role does evidence play in management decision-making when adopting and implementing innovations in health care?
-
How do wider contextual conditions and intraorganisational capacity influence research use and application by health-care managers?
Structure of the report
The report is organised as follows. In Chapter 2 we outline a summary of the relevant literature and the research context linked to the aims of this study. Chapter 3 presents our methodology, including the study’s research design and methods.
Overall, Chapters 4 to 9 outline our empirical findings and centre on our research questions, namely:
-
How do managers (with clinical and non-clinical backgrounds) make sense of evidence?
-
What role does evidence play in management decision-making when adopting and implementing innovations in health care?
Chapter 4 presents findings and emergent themes on the challenges to making sense of evidence reported by health-care managers (including both non-clinical and clinical hybrid managers). The chapter sketches out the background for the more detailed exploration of empirical processes presented in later chapters and draws on qualitative interview data from phase 1. Chapters 4 and 5 (drawing mainly on phase 1 data) reflect on what decision-makers ‘say they usually do’ and Chapter 8 (drawing primarily on phase 2 data) investigates in detail ‘what they actually did’ in specific empirical cases of innovation adoption and implementation, thus addressing:
-
How do wider contextual conditions and intraorganisational capacity influence research use and application by health-care managers?
Chapter 5 explores the sensemaking process for individual professionals in context (organisational and macro), using data from the interviews and the structured questionnaires. In this part of the report we review how decision-makers at different levels of the hierarchy within the organisation report on access and use of various sources and types of evidence related to innovation decisions. We also outline key contextual influences at organisational and macro levels with a focus on IPC and the NHS.
Chapters 6 to 8 look at ‘evidence in action’ (how evidence played out in specific empirical cases). In detail, Chapters 6 and 7 set the background for the in-depth exploration of the innovation products’ journeys. Chapter 6 draws principally on secondary data sources to present an overview of the eight ‘macrocases’ (the acute-care NHS trusts included in phase 2). Important characteristics of the trusts, such as size, performance, crises and critical events during the period of the study, the research and innovation activity, communication and espoused values, are presented in a comparative fashion. The aim is to sensitise subsequent analysis and inform the reader of the potential impact of local and historical contexts on the social and organisational processes investigated. Chapter 7 outlines the 27 adoption and implementation journeys of the technology products, as selected by the trusts (microcases), using interview data from phase 2 and complementary secondary data on supporting evidence for efficacy and cost. In this chapter we also provide a typology of the 27 technologies, distinguishing among three important dimensions: (1) the strength of the evidence on efficacy, (2) perceived impact on practice, and (3) expected impact on budget.
Chapter 8 reports on the 27 microcases in depth. We look at each technology product journey in detail along the three key stages of the innovation process, namely initiation, adoption decision and implementation. We present the interplay among stakeholders involved at each stage, associated evidence types and sources, and how these were linked to organisational adoption and implementation outcomes. This is the longest chapter of the report, and the chapter in which the 27 microcases are presented in detail. We purposely followed the same format across cases to facilitate analysis. The detailed evidence presented in this chapter enables the reader to verify the validity of the inferences made in the following chapters. Chapter 9 outlines key themes from the cross-case analysis (looking at relevant patterns across the macrocases – eight trusts – and microcases – 27 technology product journeys). In Chapters 10 and 11 we reflect on what we have learnt and synthesise the relevant findings as to how the collective sensemaking process took place within the multiprofessional empirical setting of our investigation. The report concludes with a discussion on potential implications for policy and practice and suggestions for future research.
Chapter 2 Relevant literature and the research context
Evidence-based medicine and the spread of innovations
The spread and adoption of innovations re-emerged as an important theme in health care with the rise of the EBM movement in the 1990s. 1,15 A central argument in this literature is that clinical practice should be based on rigorous and systematic evidence rather than individual opinion. The EBM movement is evident in a number of health systems, especially in Canada, in the USA and in the UK NHS, with explicit interest in understanding the diffusion of evidence-based innovations. 16–18 One of the central questions in organisational innovation diffusion literature that aligns with the aims of this study is as follows: ‘Why do innovations not readily spread, even if backed by strong (scientifically generated) evidence?’
There has now emerged considerable empirical evidence that argues that the adoption of health technologies and innovations, even if supported by sound research evidence on effectiveness, is a far more dynamic and complex process than previously suggested. 19–22 The classic innovation diffusion model of change, which has been particularly influential in UK health policy, suggests that the adoption of innovative ideas, practices or products is conditioned by the interaction among the attributes of the innovation, the characteristics of the adopter and the environment. 23 However, this early innovation diffusion work was criticised for adopting a simplistic rational view of change that ignores the complexities of the change process: also focusing on individuals rather than organisations. Later work by Rogers24 partly addressed the criticism by explicitly considering the adoption process within organisations.
Recent studies have departed from the linear model of innovation diffusion23 to offer more dynamic and interactive conceptualisations25,26 and respond to a need for context-sensitive, contingent approaches. 19,27 Building on this latter literature stream, it is suggested that innovation adoption is a process which is highly dependent on the interactions among the innovation, local actors and contextual factors. 11,27–31 These factors include the interaction among the attributes of the innovation, the organisational context and leadership;32 an organisational culture encouraging involvement, experimentation and learning; micropolitical factors; support by peer and expert opinion leadership;23,30,33 social networks;23,34 structural organisational characteristics;35 organisational capacity for absorbing new knowledge;36 and the existence of a ‘receptive context for change’. 37
Organisational innovation process and the use of evidence
The innovation process in organisations is complex and involves several stages. Damanpour and Schneider38 suggest that the process can be divided into three broad phases of ‘pre adoption’, ‘adoption decision’ and ‘post adoption’, also referred to in the literature as ‘initiation’, ‘adoption (decision)’ and ‘implementation’. 24 In this report, we use the latter terminology, which is also more commonly applied in the literature. Different concerns are central at the different phases, from an initial focus on innovation awareness and information seeking, through innovation use and application to manage a task or solve a problem, to consequences, and issues of sustainability. Adoption is often viewed as a process in which organisational members examine the potential benefits and costs or potential negative consequences of an innovation on the basis of relevant knowledge. 24 Potential adopters move from ‘ignorance’, through awareness, attitude formation, evaluation, and on to adoption: ‘the decision to make full use of the innovation as the best course of action available’. However, organisations should not be thought of as merely rational decision-making entities and innovation as an ordered sequential process. Rather, the adoption process should be recognised as complex, iterative and organic. 19,26
A key element in the organisational decision-making process that underpins innovation and technology adoption is the availability of supporting evidence of effectiveness. Despite the challenges above, there has been impetus for the development of EBMgt in health care to improve managerial decision-making through the use of the ‘best available scientific evidence’. 2,39 The integration of EBMgt with EBM is advocated to enhance the performance of health-care organisations. 3
However, within a health-care setting the evaluation of a technology can take a number of forms and include technical, economic and social assessments. Adoption decisions involve a number of stakeholders and thus it is important that the evidence used to support adoption is not just sufficient but also relevant and addresses the concerns of all parties. The earlier innovation evaluation stages are concerned with technical assessments of efficiency40 – as well as efficacy and safety in health-care interventions41 – whereas the focus in the later stages includes considerations of ease of use and social acceptance. 42 It, thus, marks a move away from scientific assessment to consideration of the complete value system for technology factors relating to types of evidence supporting adoption and contextual factors that might help or hinder implementation.
Implementation includes local trials and evaluation. The approach taken to implementation needs to vary according to the type and scale of the technology being adopted and the level and type of consequential changes it brings about. 43 For example, some technologies can be procured and put into service, whereas others require strategies such as pilots and phased roll outs. Implementation is linked to trialling and experimentation. For more complex technologies, and for those that require or lead to wider changes, such as changes in practice of health-care staff and changes to a process involving several stakeholders or cutting across departments, or even organisations, or need to be rolled out across many locations, implementation may be more challenging. 27 The end point for successful implementation will normally be the point at which the technology has become integrated into everyday practice.
A different insight on innovation adoption is available in a recent scoping review by Ferlie et al. 44 and Crilly et al. ,45 which conceptually synthesised issues of knowledge mobilisation in the NHS and, in particular, the perceived gaps in the process of translating knowledge from ‘bench to bedside’. The change towards EBMgt raises key questions such as ‘what evidence is considered as credible (and by whom)?’. And what is regarded as a legitimate epistemological basis for validating evidence (what is viewed as legitimate knowledge)? For example, should the evidence base for implementing an innovation into a specific context be exclusively focused on scientific reproducibility? Or alternatively, should the basis of innovation evidence take into account broader forms of evidence and wider concepts of what constitutes relevant and acceptable forms of knowledge?
Sensemaking in organisations
When making decisions, managers need to justify these to themselves and to organisational members. The sensemaking lens allows these two processes to be examined in context. 46,47 Sensemaking theory is a social psychological approach that emphasises cognitions. Sensemaking is about ‘reality’ as ‘an ongoing accomplishment that emerges from efforts to create order and make retrospective sense of what occurs’ (p. 635). 48 According to this perspective, values, beliefs, culture and language are important concepts. Central to this approach is enactment: the important role that people play in creating the environments that impose on them. The implications of a sensemaking lens in the evaluation of critical events is the difference between action as an ‘individual making bad choices’ and action as a result of an individual in a set of circumstances at a given time. 49 The event is therefore reframed ‘where context and individual action overlap’ (p. 410). 47 Thus, this perspective provides an analytical lens that helps understand actions in context.
The sensemaking perspective asks: how does a manager define his or her role? How is this shaped by the organisational culture, by peers, by professionals, by patients? Does his or her educational and professional background draw him or her to a particular paradigm of what constitutes evidence? This perspective is also interested in drawing out differences according to who the decision-maker is, and how individuals influence the sensemaking of others.
The sensemaking lens has been useful because of the nature of health care, with multiprofessional work in complex settings where organisational learning is important. 50 As Fitzgerald and Dopson51 observe, a clinical team is one example of an enactment of negotiated order, in which team members learn to work with each other through repeated interpersonal encounters around joint tasks. Those members with a higher degree of power are able to influence ways in which work roles are enacted. 51 This interplay between professionals is described well through nurses’ accounts in the management of hospitalised babies. 47 The nurse makes her case to the attendant physician that a baby requires immediate attention: ‘the first nurse translates her concerns for the second more powerful nurse, who then rearticulates the case using terms relevant to the Attending [physician]’ (p. 413). 47
Weick and Sutcliffe,52 in their reanalysis of the inquiry into deaths at the Bristol Royal Infirmary in the UK, found an environment in which they could further demonstrate how small actions can enact a social structure that keeps the organisation ‘entrapped in cycles of behavior that preclude improvement’ (p. 74);52 that is, easy explanations of an unusual situation should be challenged – this did not happen in Bristol. In the study of patient safety, sensemaking provides a powerful lens, as ‘the most fundamental level of data about patient safety is in the lived experience of staff as they struggle to function within an imperfect system’ (p. 1556). 53 Greenhalgh and coresearchers54 suggest collective sensemaking (developing shared meaning) as one narrative approach to understanding issues of organisational innovation processes. For proposed changes to be accepted and assimilated by providers and service users, the change ‘must make sense in a way that relates to previous understanding and experience’ (p. 447). 55
Our research questions aimed to explore ‘sensemaking’ in the local and wider contexts; that is, the health-care organisation and the NHS environment. 56 In addition, we explicitly set out to explore how individual and collective sensemaking plays out – which is particularly pertinent when making decisions about innovation adoption and implementation. This lens allows one to focus on an individual’s sensemaking processes and how these iteratively ‘update’ ways of approaching decision-making and use of evidence. This also allows reflection on how this process differs in ‘everyday’, more passive situations compared with those of heightened activity owing to the need for decision-making, either because of funding deadlines or because of external influences relevant to the empirical setting (in this case, infection outbreaks or poor performance in the infection rates). In the latter, sensemaking is usefully applied along Weick’s seven dimensions (grounded in identity construction; retrospective; enactive of sensible environments; social; ongoing; focused on and extracted by cues; driven by plausibility rather than accuracy), and emergent from this framework an appreciation of how ‘sense for self’ and ‘sense for others’ plays out.
Here the concept of ‘making sense for others’ or ‘sensegiving’ is useful. Sensegiving ‘is concerned with the process of attempting to influence the sensemaking and meaning construction of others towards a preferred organisational reality’ (p. 442). 57 The concept first emerged as an explanatory concept in the study of strategic change at an American university. 57 In this ethnographic study, the researchers observed the chief executive officer (CEO) adopt a ‘sensegiving mode’ whereby his actions and cues were used to ‘make sense for others [organisational members]’. This concept relates to previous literature in the study of organisational member behaviour, namely ‘impression management’58,59 and ‘self-monitoring’. 60 (The theory of self-monitoring60 proposes that individuals regulate their own behaviour in order to convey alignment with a preferred behaviour in any given context or situation. High self-monitors monitor and modify their behaviour to fit different situations; low self-monitors are more consistent in behaviour across situations.) Sensegiving describes the more purposeful and explicit action rather than implicit cues. The sense-giver will also make sense of organisational member behaviour and in turn modify sensegiving.
The social production of reality for oneself is a very tacit process which shapes decision-making and influences non-deliberate decisions. Sensemaking as justification to self and the resulting decision is influenced by other factors such as legitimacy and plausibility to others, that is, the publicly accountable decision. This lens pays particular attention to the social construction and coproduction of evidence through the interaction of a range of diverse professional and managerial groups. We engage with this body of literature summarised above, which has been useful in explaining organisational response to critical events in the health-care setting,47,52 as well as to strategic change. 61,62
Gaps in innovation, evidence-based health care and organisational sensemaking literatures
In summary, we note four key gaps in the relevant literature streams on innovation, evidence use and sensemaking in organisations which triggered our empirical exploration in this study.
First, with this study we address a significant gap in evidence-based health-care implementation literature. Namely, we respond to the call for more sustained interpretive work that explores the role and motives of actors and the influence of the organisational context and the social construction of evidence. 63
Second, despite the progress that has been achieved in our understanding of innovation diffusion and adoption processes, a consistent issue raised in high-quality reviews of general innovation diffusion literature26,64–66 and a review of related literature in health care19 is that empirical research has generally been limited to a single level of analysis – individual, organisational or interorganisational – thus failing to provide a holistic explanation of the influence of inter-related factors on innovation adoption and diffusion. Our study aimed to address the aforementioned criticism by exploring the innovation adoption process and by reflecting on influences at various embedded levels of analysis: namely, micro (individual), meso (organisational) and macro (interorganisational) levels.
Third, there are few empirical cases exploring issues of health management decision-making that focus on non-clinical decisions and particularly innovation, which is characterised by inherently high uncertainty and ambiguity. Moreover, little primary research exists that links the use of evidence to adoption decision-making and implementation within service organisations. We currently have a limited understanding of how pluralist evidence bases (and the associated diverse epistemological bases) might be reconciled or not in practice. The construction of shared meanings, or collective sensemaking,46 is key for understanding how new types of evidence may be successfully embedded in certain contexts, or even be rejected under conditions of innovation uncertainty and ambiguity.
Fourth, in sensemaking theory there is less emphasis on empirical studies that deal with the day-to-day processes of sensemaking, rather than crises and critical events, and on the sensemaking that occurs among many and diverse organisational stakeholders as they address a range of issues. 46,62 By applying this theoretical lens to the investigation of managerial decision-making on the adoption and implementation of innovative technologies, we aim to empirically contribute to the field.
Chapter 3 Study design and methods
This study uses a comparative and processual case study design, that is, the study of organisational processes over time in multiple cases. Specifically, the research focuses on nine case sites purposely selected for comprising diverse organisational types, each with a potentially dissimilar level of engagement with research organisations and internal capacity for knowledge production and utilisation. The research also comprises 27 embedded microcases of specific technology products used to investigate the innovation processes over time and the use of evidence in these processes. This chapter provides the rationale for this research design and then considers the operational methods applied.
Study design
We employed a comparative case study approach with mixed methods. The employed study design aimed to develop theory inductively from multiple in-depth case studies combining an inductive search for emerging themes with deductive reason. 67,68 Comparative case studies offer the opportunity for a deeper insight to generate new conceptual and theoretical propositions or extend existing knowledge through comparing, linking and integrating different cases. The main aim of this study has been to produce new understanding of the access and use of research-based and other forms of knowledge by health-care managers in organisational decisions. This can be achieved through detailed descriptions and a rich understanding of contexts across the empirical sites. As our study objectives concerned interpretive ‘how’ and ‘why’ questions, our overarching design drew primarily on qualitative methodology. 68 To retain the richness of unique cases and enhance the generalisability and applicability of findings, individual case studies were followed by cross-case analysis. Our study comprised nine ‘macrocases’ of acute-care organisations, and, for eight of them, we further investigated 27 embedded ‘microcases’ of technology product journeys, which we followed across the stages of the innovation process.
The selection of cases involved theoretical, rather than random, sampling. 69 Nine acute NHS trusts were selected across three broad geographic regions in England: (1) London, (2) northern and central England, and (3) southern England. The nine research case studies were conducted concurrently. By focusing across different localities, we sampled for diversity and aimed to explore the influence of any local network effects if present, for instance, by comparing London-based institutions to non-London-based institutions, bearing in mind the fact that London is a major cosmopolitan city which has many health-care institutions, universities and research centres and in which a plethora of social and professional events take place on a regular basis. We anticipated that this potential ‘regional effect’ might exert influence on the behaviour and perceptions of academics, health professionals and managers.
In our sample of cases, we sampled for diverse organisational types, including examples of research-engaged health-care organisations, such as AHSCs, university/teaching hospitals and ‘ordinary’ health-care service providers, such as district general hospitals ( Table 1 ). To better delineate the impact of contextual factors in research use and the application of various forms of evidence by health-care managers on the same innovation, we included multiple ‘showcase hospitals’ – as selected by the Department of Health – to evaluate the in-use value of HCAI technologies.
| Trust | PFI | Foundation | AHSC | UH/TH | SH | BRC 2007–12 | BRC 2012–17 | BRU 2008–12 | BRU 2012–17 | DGH |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | ✗ | In the process | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| T2 | ✓ | In the process | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ |
| T3 | ✓ | In the process | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| T4 | ✓ | In the process | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| T5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ |
| T6 | ✓ | In the process | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| T7 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ |
| T9 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
The study was conducted in two phases, looking in detail at processes in context. In phase 1 we first systematically examined espoused use of evidence by potential decision-makers in the studied organisations; then, in phase 2 we systematically analysed the use of evidence in practice at the point of decisions in relation to specific technology products. Phase 1 explored perceptions of senior and operational managers and health professionals of different backgrounds in managerial roles across each trust and, specifically, in IPC. Phase 2 explored those organisational members involved in the adoption decisions and implementation of particular technologies in IPC. Eight of the nine trusts in our initial sample participated in phase 2; trust 8 decided to withdraw from the study. This was a result of challenges faced by the trust in service delivery during the study period. Two infection outbreaks impacted significantly on the availability of staff to participate in our study.
A robust, systematic and participatory options appraisal (see Appendix 1 ) was carried out to inform the sampling strategy of phase 2. This involved input from steering group members, expert advice by Professor Sue Dopson, and input from peers within the research team and from NHS colleagues working in IPC. One particular IPC priority area, namely ‘environmental hygiene/cleaning/disinfection’, was finally selected. Other IPC areas considered but not sampled included ‘hand hygiene’, ‘diagnostics’, ‘antibiotic prescribing’, ‘catheter-related care’, ‘training and education’, ‘medical devices/equipment hygiene’, ‘information technology surveillance systems’ and ‘patient hygiene’. Interview respondents at each trust during phase 1 were asked to select three environmental hygiene innovations/technologies considered by the trust from 2007 onwards (2007–12) as follows:
-
A technology that has been selected but not implemented yet.
-
A technology that has been selected and successfully implemented.
-
A technology that has been rejected.
The initial selection of a technology may finally lead to adoption or rejection of organisational decisions. Following earlier work,22,41 by ‘successful adoption’ we refer to the organisational executive decision to introduce and make full use of a technology, which results in procurement. By ‘successful implementation’ we refer to the actual introduction of the new technology in the organisation, meaning that the technology is put into use and operationalised; the extensiveness of implementation may vary from trust-wide use to use in selected wards. The rationale for the selection of environmental hygiene as the IPC area of focus for our empirical investigation in phase 2 and the defined time period of 2007–12 are detailed below.
First, the selected time frame (dimension A in Appendix 1 ) captures the period when major policy initiatives in IPC were implemented or already in place, for example, The Health Act 2006: Code of Practice for the Prevention and Control of Healthcare Associated Infections (known as the ‘hygiene code’);70 the introduction of the evidence-based EPIC-2 guidelines;71 the mandatory reporting of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia and Clostridium difficile infections (April 2001 and January 2004, respectively); the Saving Lives programme 2007;72 and the Clean Safe Care programme launched in 2008. 6 We selected ‘environmental hygiene’ technologies because they represented 50% of selection decisions according to a recent study of innovation adoption in IPC in England. 43,73 In addition, environmental hygiene is a cross-cutting intervention for various HCAIs. Such interventions range from an inexpensive poster and basic cleaning products to expensive cutting-edge technologies including hydrogen peroxide robots. This is a growing area in industry and has gathered particular attention in recent years through regulations, such as the Department of Health’s Deep Clean Programme. More importantly, a wide range of stakeholders could be involved to debate the evidence in this area in contrast to a highly specialised or technical field, such as diagnostics.
Conceptual framework
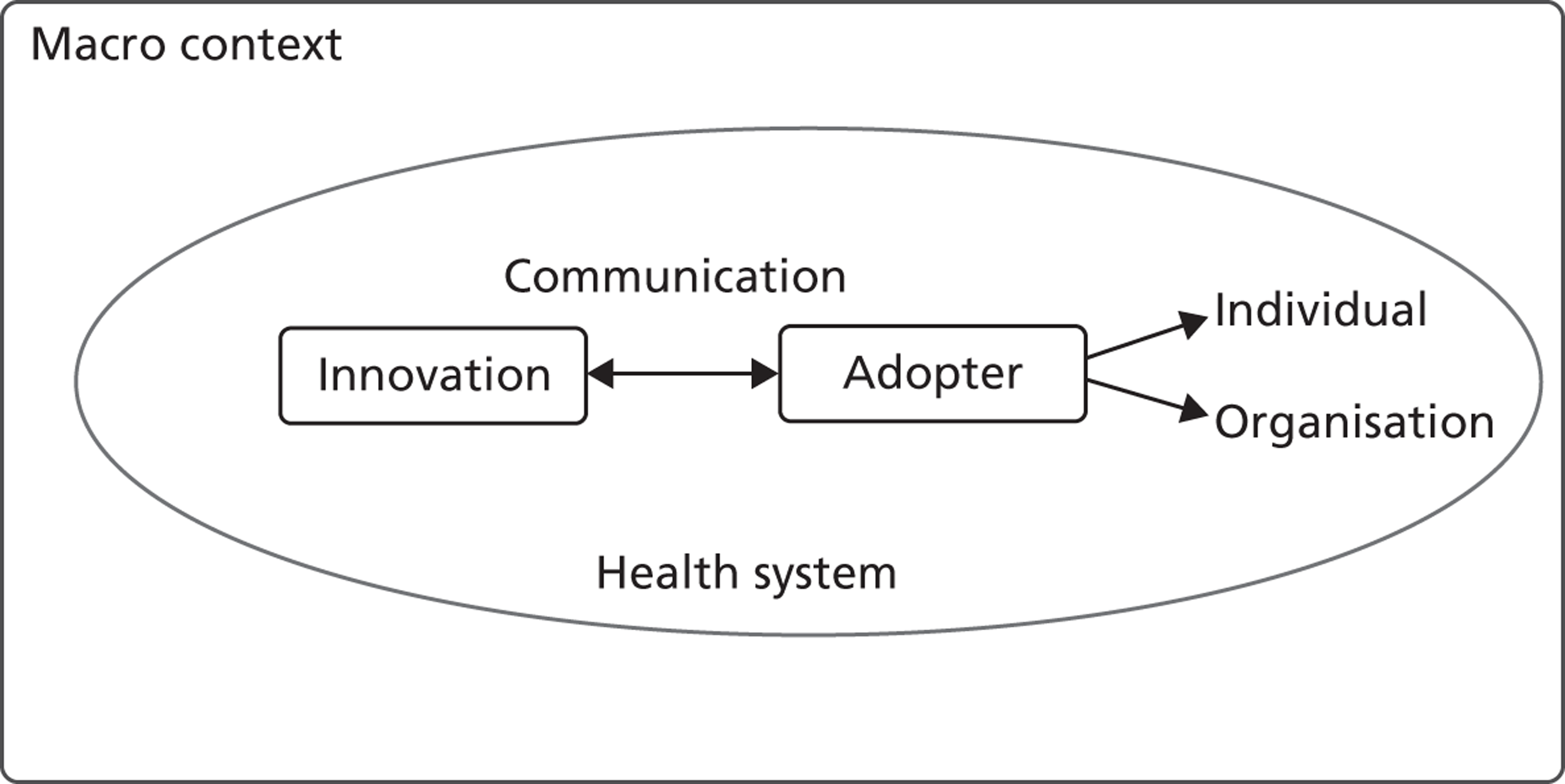
Our approach draws primarily on innovation diffusion theory of change. 24 We specifically focus on four factors ( Figure 1 ) that health-care researchers11,21,22,27,31 broadly agree influence the adoption decision and subsequent implementation of health innovations: (1) the perceived attributes of the innovative technologies, (2) the characteristics of adopters, including both individual health-care managers and their organisations, (3) contextual factors that include the relevant sector (NHS) and wider societal, political, economic and institutional (symbolic, ideational and material) environments, and (4) the communication process.
FIGURE 1.
A conceptual framework for the adoption of complex health innovations.
Data collection strategy and methods
We employed a two-phased approach to the field work. Phase 1 focused upon senior (director level, including trust directors of medicine and nursing), middle-level and operational managers involved in organisational decision-making. We focused on a specific type of organisational decision, the adoption of innovative interventions, which entails uncertainty and the risk of newness, and thus offers great potential for sourcing evidence from the decision-makers. Technology products for phase 2 research were then sampled, which examined in detail the stakeholders involved in specific cases of evidence use in practice.
Primary data
In phase 1 the unit of analysis was the individual manager (non-clinical and clinical hybrid managers) and the level of analysis was each of the nine trusts (macrocases). For phase 1 we used a combination of semistructured interviews with structured questionnaires embedded in the interview schedules (see Appendix 4 ). We employed a multilevel sample of key informants. Informants included senior, middle and operational managers and representatives from different professional groups, including doctors, infection control specialists, clinical microbiologists, nurses, pharmacists, allied health professionals and non-clinical managers with diverse professional backgrounds (i.e. in engineering, science, accounting or finance). The categories of evidence used in these questionnaires were informed by a previous study on HCAI technology adoption funded by the Department of Health which involved 121 interviews with NHS staff from 12 NHS trusts across England. 22,43 The categories were further refined and validated following expert advice from policy-makers in the Health Protection Agency (HPA) and the Department of Health, as well as health professionals in IPC with non-clinical, nursing and medical backgrounds. We further validated and finalised the categories in consultation with the members of our project steering group.
The primary focus of phase 2 was the mobilisation and use of evidence in the decision-making for specific technology products ‘in context’ and in relation to the task of solving an identified problem in IPC. The unit of analysis was the group of stakeholders involved through the innovation journey for each of the selected technology products and the level of analysis was each of the eight trusts that participated in phase 2 (macrocases) and each of the 27 embedded technology journeys (microcases).
The longitudinal, real-time design of the study was intended to give a better understanding than short-term and ‘snap-shot’ methods. As well as these measures of methodological rigour through the study design and methods of analysis, we used measures of acceptability and relevance of the research as defined by key stakeholders, namely professional, managerial and patient groups (e.g. patient environment action teams, the two patient advisors who were members of the project’s steering group).
For both phases we also looked at the wider context through the systematic collection of secondary data (discussed in more detailed below). For example, we considered for each macrocase the profile of the population, the institutional conditions (e.g. legislation and regulatory frameworks, the influence of professional associations, social norms), intraorganisational factors, including practices and organisational culture, and trusts’ history and tradition. For the microcases we additionally considered the capacity and previous experience relating to the technology under consideration and similar innovations.
The research was conducted over a period of 2 years, between November 2010 and October 2012. After ethical approvals were obtained, field work and data collection began in April 2011, and was completed in July 2012. The recruitment of respondents followed closely the plan outlined in Appendix 3 , the study protocol. YK or KH invited potential participants via e-mail to take part in the study and these e-mails were accompanied by a participant information sheet (see Appendix 2 ). Our predefined roles detailed in the study protocol, suggestions by local study leads and snowballing shaped the actual respondents’ sample. In addition, for phase 2 the final sample of respondents was determined by participation of staff in the adoption and/or implementation of the selected technology products studied in each of the eight trusts. Very high respondent recruitment was achieved (> 90% acceptance of invitations with the exception of T8, as outlined above). We used semistructured interview schedules for both phases of the research (see Appendices 4 and 5 ).
Prior to the field visits, interviewers familiarised themselves with contextual information on each trust and information about IPC-related innovations. This enhanced their knowledge on local contexts and enabled them to ask relevant questions to explore areas of further interest. On average, each interview lasted 60–90 minutes. Face- to-face interviews were conducted at trust sites, and we obtained prior consent to audio record interviews (see Appendix 3 ). The total number of interviews was 191, including 126 for phase 1 (with all 126 informants also having completed the embedded structured questionnaires) and 65 semistructured interviews for phase 2. The detailed breakdown per trust and professional background of informant is summarised in Tables 2 (phase 1) and 3 (phase 2).
| Type of informant | Number of respondents per trust | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | ||
| Doctor | 5 | 2 | 3 | 2 | 3 | 2 | 4 | 1 | 2 | 24 |
| Nurse | 10 | 9 | 8 | 5 | 5 | 8 | 6 | 1 | 9 | 61 |
| Non-clinical manager | 3 | 4 | 4 | 3 | 4 | 3 | 3 | 0 | 1 | 25 |
| Allied health professional | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 6 |
| Pharmacist | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 10 |
| Total | 20 | 16 | 17 | 13 | 15 | 14 | 15 | 3 | 13 | 126 |
| Type of informant | Number of respondents per trust | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T9 | ||
| Doctor | – | – | – | – | – | 1 | – | – | 1 |
| Nurse | 7 | 4 | 5 | 3 (6) | 7 | 4 | 7 | 7 | 44 (47) |
| Non-clinical manager | 3 | 4 | 3 | 1 | – | 1 | 1 | 2 | 15 |
| Allied health professional | – | – | – | 1 | – | – | – | – | 1 |
| Pharmacist | – | – | – | – | – | – | – | 1 | 1 |
| Total | 10 | 8 | 8 | 5 (8) | 7 | 6 | 8 | 10 | 62 (65) |
Secondary data
We systematically collected data from secondary sources (both trust specific and global) for each case study site to obtain a detailed contextual description for each trust. The trust-specific sources included the following: trusts’ annual reports and financial accounts (2007/08; 2008/09; 2009/10; 2010/11; 2011/12 where available and applicable), trusts’ quality accounts (2009/10; 2010/11; 2011/12), reports by the director of IPC (DIPC) (where available), trust board meeting minutes (where available), staff magazines, and newsletters and/or bulletins that were published up to spring 2011. We also collected publications from governmental or regulatory agencies, including the CQC (previously the Healthcare Commission), the Audit Commission, the Monitor and the Network of Public Health Observatories, to highlight wider contextual factors that might influence the innovation decision-making processes at the trusts. We systematically reviewed a total of > 800 documents derived from the aforementioned sources. The trust-specific secondary data sources supplemented and/or triangulated other data sources, including global secondary data sources and our primary data originating from interviews with trust managers.
Data analysis
Data analysis comprised six interlinked and, to some extent, overlapping stages: (1) transcribing of qualitative data; (2) initial open coding of interview data focusing around the research questions; (3) systematic coding of interview data; (4) cleaning, error checking, creation of descriptive summaries and tabulation of the questionnaire data; (5) individual case study analyses; and (6) cross-case analysis.
Soon after the completion of interviews, the content of audio recordings was verbatim transcribed by professional transcribers – an independent professional and an agency. The linguistic accuracy of texts was checked by the interviewers themselves, and, whenever transcribers felt unsure, the researchers who conducted the interviews confirmed the accuracy of the text and revised it accordingly by paying close attention to the raw interview data. Interviewees validated most transcripts (the option was offered to obtain a copy their transcribed interview).
Upon completion of transcription, three researchers thoroughly read through the full transcribed texts several times to enable understanding of the meaning of the data in their entirety. 74 The reviewing of data prior to coding helped us identify emergent themes without losing the connections between concepts and the context associated with these concepts. The qualitative data analysis computer software package NVivo 9 (QSR International, Cambridge, MA) was used to systematically code the collected data and assist analysis. In line with recommendations by qualitative methodologists, we used multiple coders (MI, RA, KH, YK) to enhance inter-rater reliability of the qualitative study. 74,75
Our qualitative analysis followed an integrated approach. 76 We employed an inductive approach to open up new lines of enquiry and then agreed a framework for data analysis based on these findings together with our conceptual framework (delineating factors that influence the adoption process of complex health innovations, see also Figure 1 ).
The development of the code structure was finalised when the point of theoretical saturation was reached in each of the empirical cases. 69,77 Secondary data were used as complementary to the preliminary interpretation (based on interview data) of each case study and for triangulation, through cross-checking the validity of claims in interview accounts. Field notes taken by the interviewers during the visits to the trusts were shared with the other members of the research team during analysis of the interview and documentary data. The field notes provided a ‘feeling’ for each trust, allowed for a better understanding of the trust context and included explanatory information about the ‘technologies in use’. In particular, the field notes helped sensitise and ‘acclimatise’ the researchers not familiar with the microcases.
Analysis within cases was followed by a cross-case analysis of emergent themes. The cross-case analysis commenced with a systematic examination of the data based on our research questions. We compared the data on these questions across the macrocases and across the microcases (technology product journeys). Individual case study reports with common formats were produced for each of the eight trusts that participated in phase 2. Summary tables were used to simultaneously compare several categories and dimensions of the content and context of change implied by the adoption and implementation of the innovative technologies across the nine trusts. The above strategies helped us to reduce the volume of primary data. The final interpretation was conducted through comparison and integration of seemingly common or contradictory themes, categories, patterns and cases.
Learning from project challenges
The context of the NHS poses challenges to access to participants. In the field of IPC, this is further exacerbated because critical events can pose high demands on IPC teams and senior management within trusts. We employed a multifaceted approach to gaining access, building on our previous relationship with five of the trusts in the first instance. We found that the nature of the research questions prompted genuine interest and, therefore, engagement in the research. We were able to access a higher than initially anticipated number of respondents. Nonetheless, these very pressures resulted in incomplete participation in the study for one of the trusts, T8.
The initial delays in gaining ethics approval and then local access did make data collection challenging. Internal contingency and a highly flexible approach by the research team was employed to maximise interview opportunities at each research site visit.
We found that the guidance from our expert steering committee and, notably, our patient advisors helped with every stage of the research process, enhancing the quality of the research. Perspectives from health policy, quantitative methods and accounting, organisational management, service users, and IPC and service managers were present and debated. The multidisciplinary make up of this group reflected well the stakeholders we were studying and allowed a reflexive approach to data analysis and will inform dissemination activities beyond the project.
Chapter 4 Challenges in making sense of evidence
In this chapter we summarise some key themes from the qualitative study, which drew on primary data collected through interviews in phase 1. These themes were deemed helpful in providing a conceptual understanding of the main ‘challenges to making sense of evidence’ reported by the informants. The emergent issues discussed in this chapter help place individual sensemaking of evidence by health-care managers in the context of the hospital and wider NHS environment.
Ongoing sensemaking: keeping up with the evolving evidence
The very nature of evidence as emergent, iterative and changing featured in the majority of interviews with respondents, particularly as the context of this research was innovation. The accuracy of evidence therefore had a temporal dimension, irrespective of the source of the evidence, or the audience making sense of the evidence. The need for ‘up-to-date’ evidence, which sometimes needed to be generated locally, was an important theme in respondents’ accounts:
Well-written trials that have been peer reviewed and written up in trusted journals. But I am also now old enough to see that some of the things that we took as facts, 10 years ago, have already been proved incorrect.
T5M4 – doctor
This evolving landscape of evidence posed a challenge, therefore, for individuals and groups when making decisions about adoption and methods of implementation. Further challenges to making sense of evidence reported by the respondents ranged from the individual’s internal capacity to process the scientific data presented to external factors, such as the lack of evidence in the specific areas of innovation and IPC. The lack of ‘high-quality’ evidence was reported by the majority of respondents, although this definition of quality varied across the professional groups and is discussed later when we look at the importance attributed to various sources and types of evidence (see Chapter 5 ):
As a doctor I go to the medical literature, didn’t find a lot. So my nurses came back with a lot of nursing literature evidence. Which I felt was of poorer quality evidence, but there was a large volume of it, so it was put into the mix somewhere.
T3M3 – doctor
In terms of the individual’s internal capacity, respondents across the professional groups cited difficulties in understanding the evidence presented in published papers and reports. Specifically, 75% of medical hybrid managers and 77% of nursing hybrid managers said that they sometimes found ‘the content of presented evidence difficult to understand’. Similarly, the majority in each of these groups found it ‘difficult to relate evidence to practice’: 63% of doctors and 72% of nurses. The non-clinical managers reported a different experience – 60% stated that they sometimes found the content of presented evidence difficult to understand, but only 40% had difficulty in relating this to practice.
There was consistency among the groups in agreeing that different professional groups have access to different sources of evidence because of different needs for evidence. This access and need for different types of evidence was deemed to have direct implications for practice:
If everybody isn’t looking at the same piece of information it can affect how you make the decision because we can all be coming at it from different points of view. I can say generally speaking within this organisation when we are looking to do anything we get the relevant people round the table. It’s not that IPC would make a decision that would impact on the provider without involving, they would involve our actual service provider and we will be involved as well. And I do think we do that well really.
T2M2 – non-clinical manager
Missing research evidence
Looking in greater detail at the gap in evidence or ‘missing evidence’ as identified by the respondents, there is more consensus than variation on this issue across professional groups and across the trusts. Challenges arising from the lack of relevant evidence as well as incomplete evidence were mentioned by the majority of respondents. For example, a laboratory-based/microbiology study may be available for a given product but no studies relating to cost. Implementation studies may be available, but may only report a ward-based small study, which may not be relevant to the hospital-wide context. A lack of product trials was described, the products being either untested in the ‘real-world’ setting or untested in the locally relevant setting. Particularly among doctors, basing decisions within the context of incomplete evidence was reported as not just a challenge but undesirable:
There’s damn all evidence most of the time. So we’re very used to doing what seems sensible from first principles, which may not actually be, so often we do things without a formal level of evidence basically.
T9M2 – doctor
It’s difficult because some of the things we do I must admit they are based on very little evidence.
T1M17 – doctor
It [personalised care plans for renal patients] was a good idea but it wasn’t trialled anywhere, there was no sort of pilot study to demonstrate how much time it was going to take to fill these things out, whether they would actually be useful, did the patients think they were useful, did the doctors think it was useful.
T5M5 – doctor
These findings have an important implication as to what managers currently perceive as ‘incomplete evidence’ in research when making decisions on innovation adoption, and what future research should focus on to meet such local needs.
Types or topics of research studies perceived as missing by the respondents did vary across the respondents and across the trusts, but views converged for three types of research study, which were identified as missing in the following order: behavioural studies, implementation research and organisational studies or management research.
Approximately one-third of respondents in trusts T1, T3, T6 and T9 felt a need for behavioural studies to assist decision-making, implementation and evaluation. Specifically, interest in behavioural studies was driven by the need to overcome ‘non-compliant’ behaviour; insights into bringing about change in the way people work; learning from training and development mechanisms; and better communication. More importantly, the respondents identified a need for better understanding of decision-making across the different levels of hospital staff, from senior management to front-line staff:
I think that there is quite a lot of management research that is missing. Partly because managers don’t tend to do a great deal of research in this organisation. Then again all the behavioural work that is done is linked to nursing or medical, I think this is the first research that I have seen that is linked to managers as well. I would like to see a lot more research based around behaviours and how managers and clinical staff could work much better together, to deliver a health service, because I see models out there where they work so well and yet somehow the NHS cannot get it right across the whole trust.
T1M1 – non-clinical manager
Yeah not enough is done around the whole decision architecture and influencing behaviours in the clinical areas. How do we improve behaviours? Not just in the clinical areas, managerial areas, but how do we improve the way we work which is not around new technologies but how can we stop wasting huge amounts of time repeating the same things over and over again.
T1M10 – doctor
The ‘missing evidence’ highlighted by respondents is interlinked and demonstrated a need by the hospital managers not only for applied, meaningful evidence use in adoption decision-making itself, but also more operational and managerial research. This ranged from effective management to psycho-social research about behaviour change and receptive organisational culture. The following respondent highlighted a shift in evidence needs – highlighting what is most useful to managers:
My research brain has gone exponential in the last year or two. I think there needs to be far more focus on the behavioural and cultural aspects of innovation spread as well as just the subject matter. Because the understanding ‘how’ to challenge the behaviours and ‘how’ to develop the people who are involved in the organisations is far more important than the actual evidence that drives it. Increasingly I’m convinced more and more.
T3M4 – doctor
[. . .] where we’ve got the catheter project, the CAUTI project we’re doing, where we’ve got John who’s our clinical academic, we’re looking at doing some sort of research around people’s decision making as to why they’re putting catheters in. [. . .] Why do people make those decisions to do that or why do people make decisions to move away from the guideline that’s there [. . .] There’s a lot, from an infection prevention point of view there’s a lot of scientific type stuff we could do but that is quite difficult already because we don’t want to inject people with, but [. . .] I find that behaviour really interesting as to why people do make the decisions they do.
T9M1 – nurse
I work with a public health doctor and he was really interested in implementing change, change methodology. And I think as much importance of thinking about that as thinking about the evidence. If the evidence stacks up, or evidence doesn’t stack up particularly well. You could have good evidence and poor implementation and no effect. Poor evidence not even particularly good but with really good implementation will make it improve but almost, I think there is something there. What I would say [is] that even if I’d like to assimilate stuff, actually it’s not what other people want to do, you don’t have the time to do it. Lots of people who are over-committed and busy and sometime go, I’m sure there is something better unless you tell me what you want to do.
T9M8 – doctor
This type of management literature was not accessible to the respondents largely because of the sources used by these professionals, and also because of the time constraints faced by these professionals, who were not able to branch out to wider literature streams.
Of the professionals groups, pharmacists appeared to be more aware of the discrepancy between recommended practice (through national or local guidelines and protocols) and ‘non-adherence’ or ‘deviation’ of behaviour than the other professionals in our study sample. Approximately two-thirds of respondents, despite the small sample size, commented on the importance of behavioural studies and the lack of such studies. One-quarter of nurses and non-clinical managers identified the importance of studies to address ‘non-adherence’ to guidelines, whereas this view was less prevalent in accounts from medical managers and missing in accounts from managers with an allied health professional background. Medical managers were the ‘outliers’ in terms of being less concerned about understanding behavioural change in greater depth.
Making sense of evidence for self and others
Sourcing practice-based evidence was mentioned as being important across professional groups. The practice of learning from other trusts and peers featured across respondents’ accounts. This was because of the locally relevant information in practice-based evidence but also because of the exchange of information which is possible through such means:
A microbiologist in another hospital or someone who has used something in practice and any research or studies they have done, that is usually the most useful. I guess because you are talking to them you can ask questions and get feedback straight away, so you know where you are with it. So that’s a really good source.
T7M3 – doctor
Upon direct questioning, respondents reported a hierarchy of evidence, but this was articulated more as processual rather than as an objective vertical hierarchy, or means to exclude certain forms of evidence. Although the first port of call may be scientific randomised controlled trials (when available), this was assessed in tandem with experiential evidence:
We used that, literature searches for that [Gentamicin (antibiotic) as first line for the treatment of urinary tract infections]. But we also used experience of other hospitals, our own experiences, we drew on that. So actually it was probably a decision which was much more of a pragmatic decision rather than a pure academic-based decision.
T3M17 – pharmacist
The approach described by the majority of respondents was an iterative process of ‘triangulating’ different types of evidence. There were few reports of an evidence dichotomy within professional groups, but rather a more complex picture of synthesis across the professional groups. Paradoxically, many respondents did view other professional groups as having a more dichotomous approach to evidence, as illustrated by this view of non-clinical managers:
[. . .] if they’re an accountant it will be purely based on cost effectiveness without looking at the wider picture of your added value this technique may bring.
T1M19 – doctor
This view was reciprocated by non-clinical managers:
Partly because people spend more time critiquing the research paper than looking at how we can implement it, or not implement it or how we can try it ourselves. That’s how we get stuck sometimes, people spending too much time focusing on their research, was it true was it evidence-based, did it have flaws?
T1M1 – non-clinical manager
A contested ground emerged, with each professional group claiming a more rounded view of evidence and perceiving other groups as taking a one-dimensional approach.
The quest for evidence of doctors was driven primarily by plausibility and accuracy to self. The evidence sought was largely of a biomedical nature. Doctors appreciated that the cost-effectiveness of interventions was important but, as shown above, described non-clinical managers’ approach as too focused on the business case.
Although both the nursing group and the non-clinical managers group reported a relatively balanced multidimensional view to evidence, the motivation for sourcing a diverse evidence base was different for these two groups. Non-clinical managers took a multidimensional view to satisfy the major objective in their organisational role, that is, to improve performance and outcomes. Nurses were driven primarily by the need to ‘make the case’ for others and appreciated that different professionals had different evidence needs:
Most of things I do are evidence-based. I would be looking for things such as standard of construction, standard of validation with processing that sort of thing. I can’t honestly say that I can think of an instance that I did something where I didn’t actually have the evidence.
T2M12 – non-clinical manager
Non-clinical managers were similar to doctors in that the way they made sense of evidence was driven primarily by ‘plausibility and accuracy to self’, although their sensemaking was based on different views of evidence. That is, what came to count as evidence for doctors and non-clinical managers was different, but what counted most was that they themselves were satisfied with the evidence. The nursing group differed markedly in this respect from the doctors and non-clinical managers. The nursing hybrid managers focused on the pursuit of evidence for ‘plausibility and accuracy for self and others’. For the nurses, what counted as evidence to others mattered equally and sometimes more than their own satisfaction with evidence. This shaped the types and sources of evidence used by nurses.
In the nursing group, we found there was a high awareness of different types of evidence being relevant to different organisational members. They appreciated the evidence needs of those working both at the front-line and at more strategic levels, and the needs across professional groups. Nurses were also the only group to make explicit reference to the perceptions of patients. Plausibility to others thus featured highly in accounts by nurses. Nurses made purposeful attempts to frame evidence using language which was meaningful and tailored to the audience. Nurses also were aware of their own professional role and identity and how they were perceived by others – that is, being reflective on their own ‘credibility’ as sources of evidence. This non-clinical manager articulated this varying credibility of the presenter of evidence:
Although it galls me to say it but I think the medical colleagues within the team are better at accessing [evidence] and they may come to a meeting and say I have had a look at the evidence. I don’t think it could necessarily have been a systematic review of the evidence. Stating quite confidently a particular position and that could be quite influential so that is something they are more likely to do than nursing members of the team.
T7M13 – non-clinical manager
Nurses therefore approached sourcing evidence in a systematic and comprehensive way in order to find evidence that was meaningful and accurate for themselves as well as for significant others. There was a convergence towards synthesising diverse forms of evidence, but, ultimately, evidence synthesis was grounded in the biomedical paradigm. This was partly a result of their own training but also reflected a need to resonate with doctors, who were consistently identified as influential stakeholders in organisational decision-making:
You will see it in very specialist nurses that they will do scoping exercises around what the evidence is, systematic review around evidence of implementing a certain thing and clinical evidence to support it. I think the reason why nurses do that is because they know that the doctors, that are going to try and influence [the decision], will ask them for that evidence, so they already do it.
T1M2 – nurse
I think it is the availability of good quality evidence and research something that will convince the senior members and the medical staff that this is a good quality piece of research, peer reviewed etc.
T6M5 – senior nurse
I can remember quite clearly presenting to our anaesthetist body, some 200 odd anaesthetists on one of the clinical audit days, on a topic, [. . .] around line care, and the changes we had made in the organisation. And there was one consultant, a specific consultant who’d been a problem all the way through, he’d not engaged well. We gave the presentation, we demonstrated what we’d achieved in the organisation since we’d introduced our changes in practice, and he actually turned round in front of the other 200, and he said ‘I change my opinion’, he says, ‘I accept what you’ve been championing’. And to be honest that was one of the most powerful moments in my career, to get that individual to, in front of 200 of his colleagues, to turn round and say ‘I’ve seen the light.’ [. . .] And sort of do, do the St Paul’s Damascus moment, it was just, it was tremendous, [. . .] It was strongly presented with good, we used, took an epidemiological approach to demonstrate that the changes we had made had had a significant impact.
T8M1 – nurse
Nurses were aware of the use of evidence for different agendas, but overall perceived that evidence was used primarily for the benefit of patients in the context of financial constraints. This, in turn, led to the need for combining different types of evidence (i.e. clinical effectiveness, cost, usability) to satisfy the perceptions and priorities of key organisational stakeholders – from doctors to managers.
Doctors and non-clinical managers were both mindful of issues of cost-effectiveness, particularly given that our sample in phase 1 comprises senior managers.
The findings from the qualitative interviews are validated by quantitative analysis. The quantitative analysis shows that nurses were aware of, and utilise more widely, the full range of centrally available evidence sources when compared with the other professional groups (see Chapter 5 ). In addition, nurses were more formally engaged across the phases of the innovation process, whereas doctors were more formally involved in the later phases of technology adoption decisions and post-implementation evaluation. The nursing group was, across the trusts, more formally tasked with ‘making the case’ to diverse groups.
Across respondent groups, plausibility to self was closely linked with perceived ‘accuracy’ of the evidence. This was influenced by social and personal identities situated within a wider organisational context. For example, financial considerations were evident in the sensemaking of the majority of respondents. The influences of the local and macro context of financial parsimony added to the challenges of making sense of evidence:
Financial viability [. . .] that has rapidly changed, we have to justify everything that is new in terms of spending.
T1M2 – nurse
Reflection on this chapter
In summary, all respondents reported that they experienced challenges in making sense of evidence. Key issues that contributed to this were reported as a lack of capacity or skills to process presented evidence, a lack of time to thoroughly search for and review the evidence base, unawareness of appropriate literature on management and implementation research and poor perceived quality of available evidence. Professional background and training coupled with differential access to different evidence reinforced some of the divergence in the type of evidence accessed. Pursuit of evidence to satisfy oneself or others was found to guide action and explained some of the complexity in the process of decision-making. Looking across the professional groups, what counted as evidence for doctors and non-clinical managers was different, but what counted most was that they themselves (doctors and non-clinical managers) were satisfied with the evidence. For the nurses, what counted as evidence to others mattered equally and sometimes more than their own satisfaction with evidence. This shaped the types and sources of evidence used by nurses.
As regards perceived missing evidence, three research study types were identified by respondents: behavioural studies, implementation research, and organisational and management research. Pharmacists were particularly mindful of the need to understand behavioural change within organisations, particularly in relation to non-compliance with guidelines.
Chapter 5 Making sense of evidence in the health-care organisational and macro context
In this chapter we summarise findings on how non-clinical and clinical hybrid managers from various professional backgrounds reported on how they make sense of evidence within the health-care context. We review how they access and use different sources and types of evidence related to innovation decisions and outline key contextual influences at organisational and macro levels within IPC and the NHS. This chapter draws on data from, first, the structured questionnaires embedded in the phase 1 interview schedule and, subsequently, from the semistructured interviews themselves.
We outline the espoused use of evidence by the decision-makers. First, we look at the reported use of more general sources of evidence (such as peer-reviewed journals, professional networks, peers, the industry) to inform the decision-making of different professionals. Second, we outline the reported awareness and use of central evidence sources including sources directly linked to IPC. Third, we outline responses on the use of different types of evidence (such as systematic reviews, guidelines, economic cost analyses and expert opinion). In the final sections, we delineate important influences on the use of evidence by health-care decision-makers from the organisational context (main level of analysis) and the wider context.
In the proceeding chapters we find out how this espoused use is actioned.
Innovation decisions: evidence sources
Figure 2 presents the use of more general sources of evidence, such as peer-reviewed journals, professional networks, peers and the industry, in decision-making by different professionals.
FIGURE 2.
Evidence sources – breakdown by professional group (a) Doctors; (b) nurses; (c) non-clinical managers; (d) allied health professionals; and (e) pharmacists. DH, Department of Health.
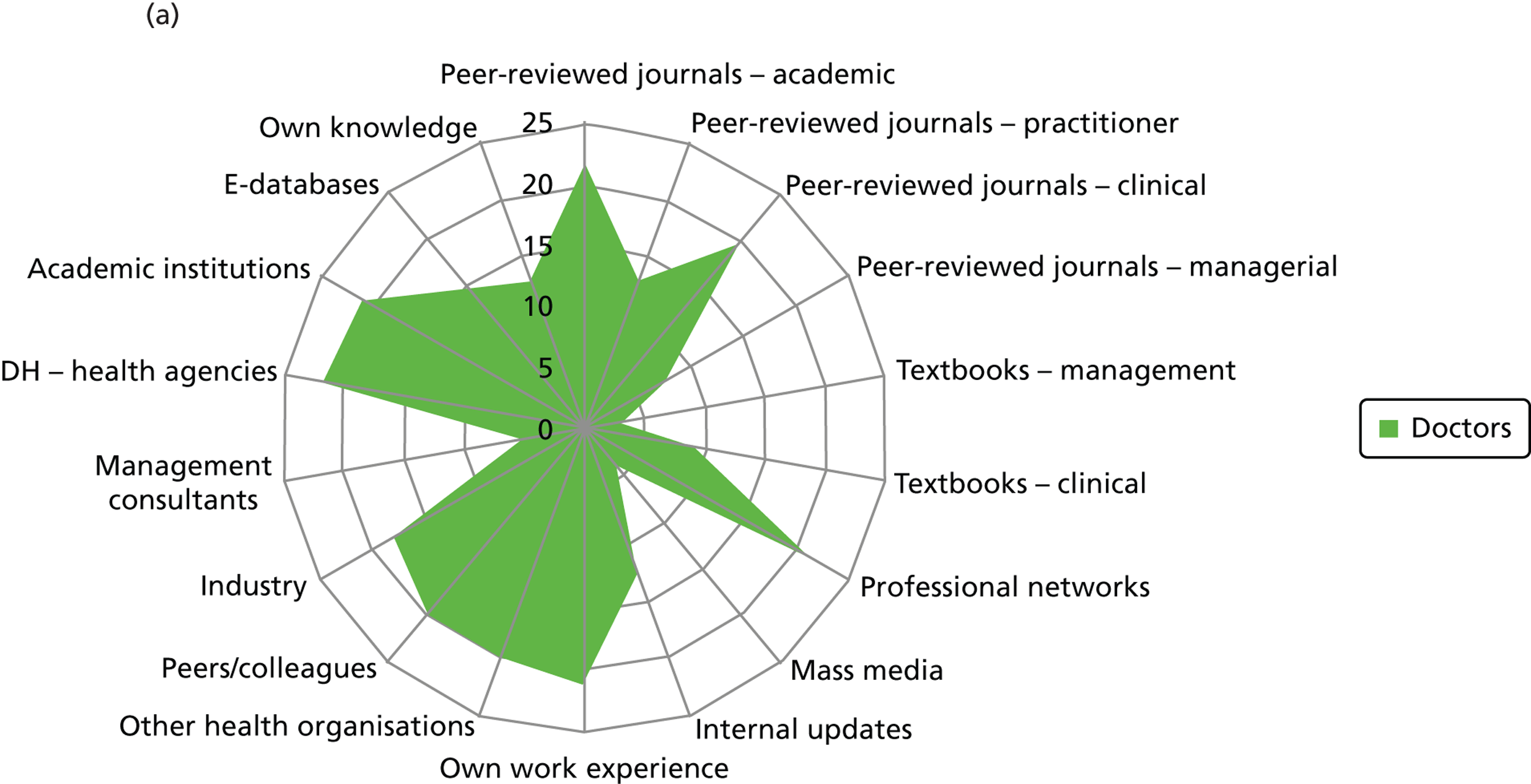
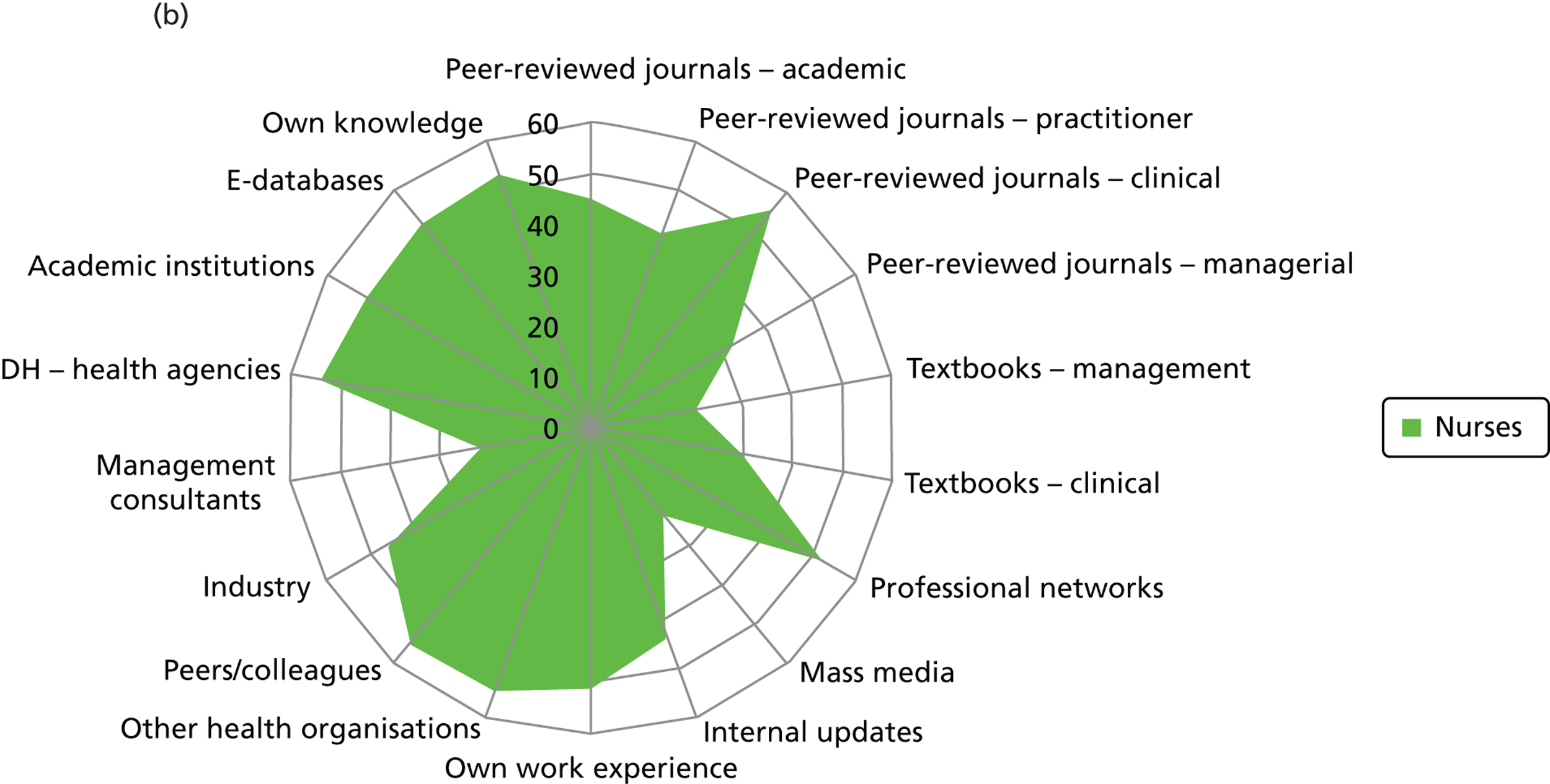
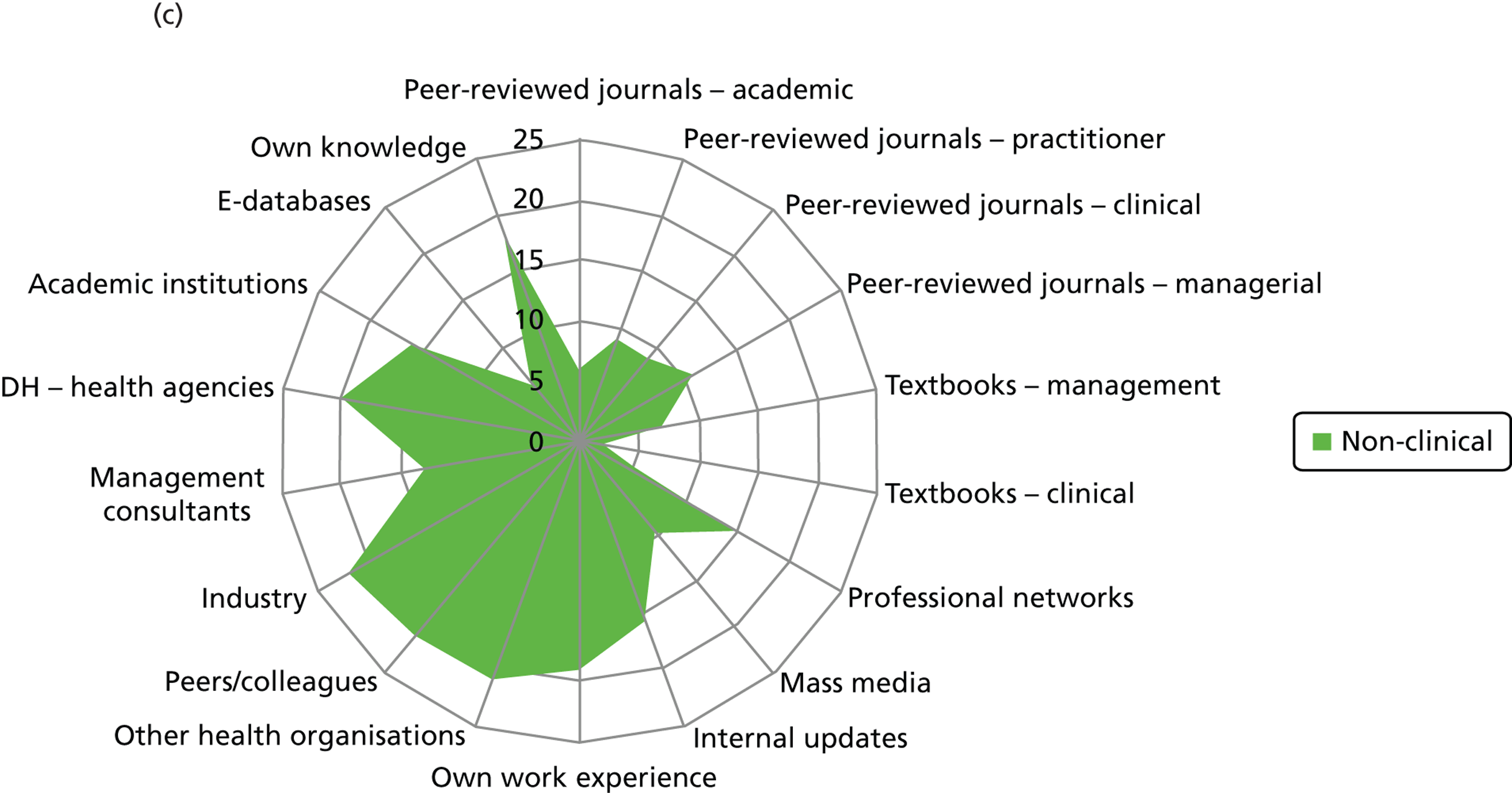
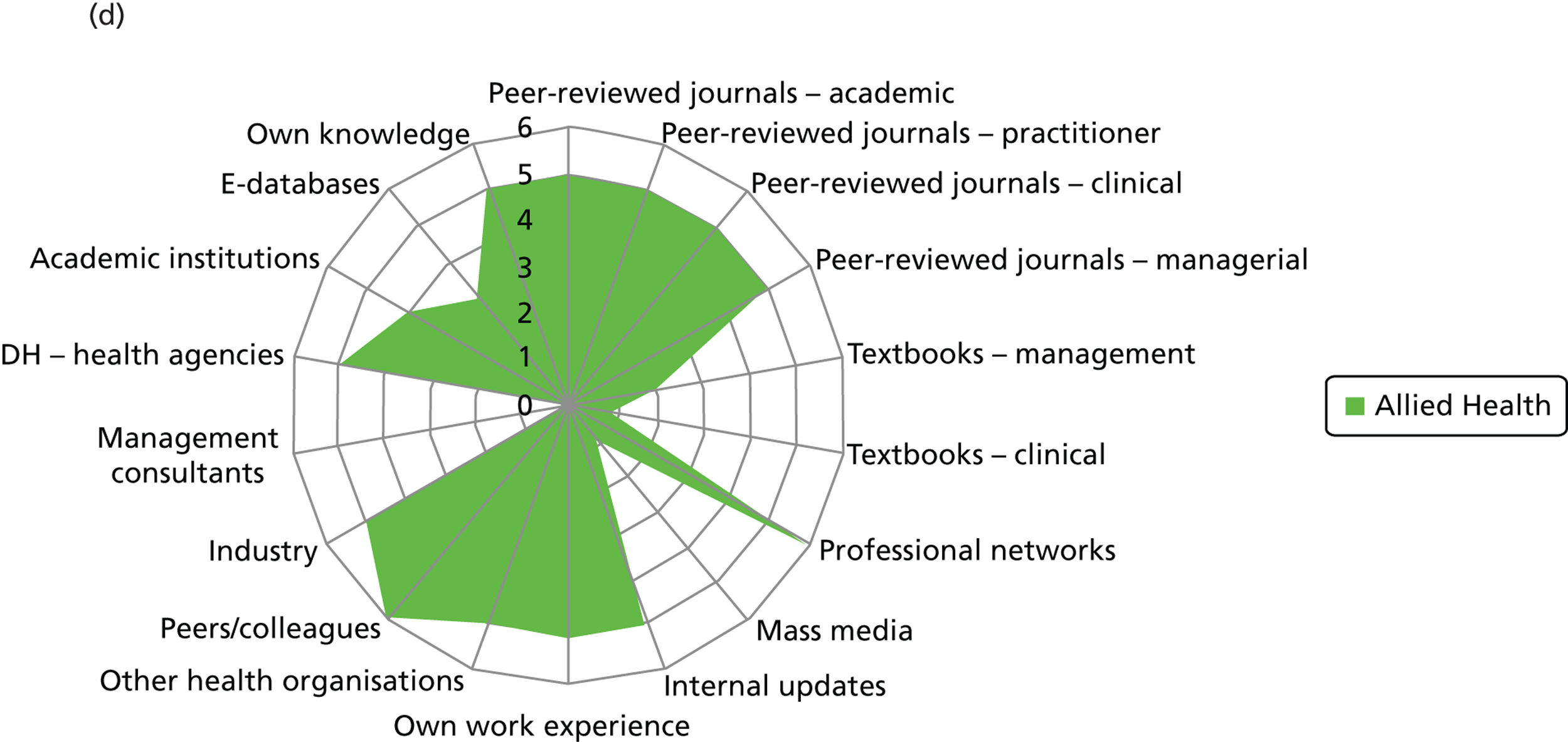
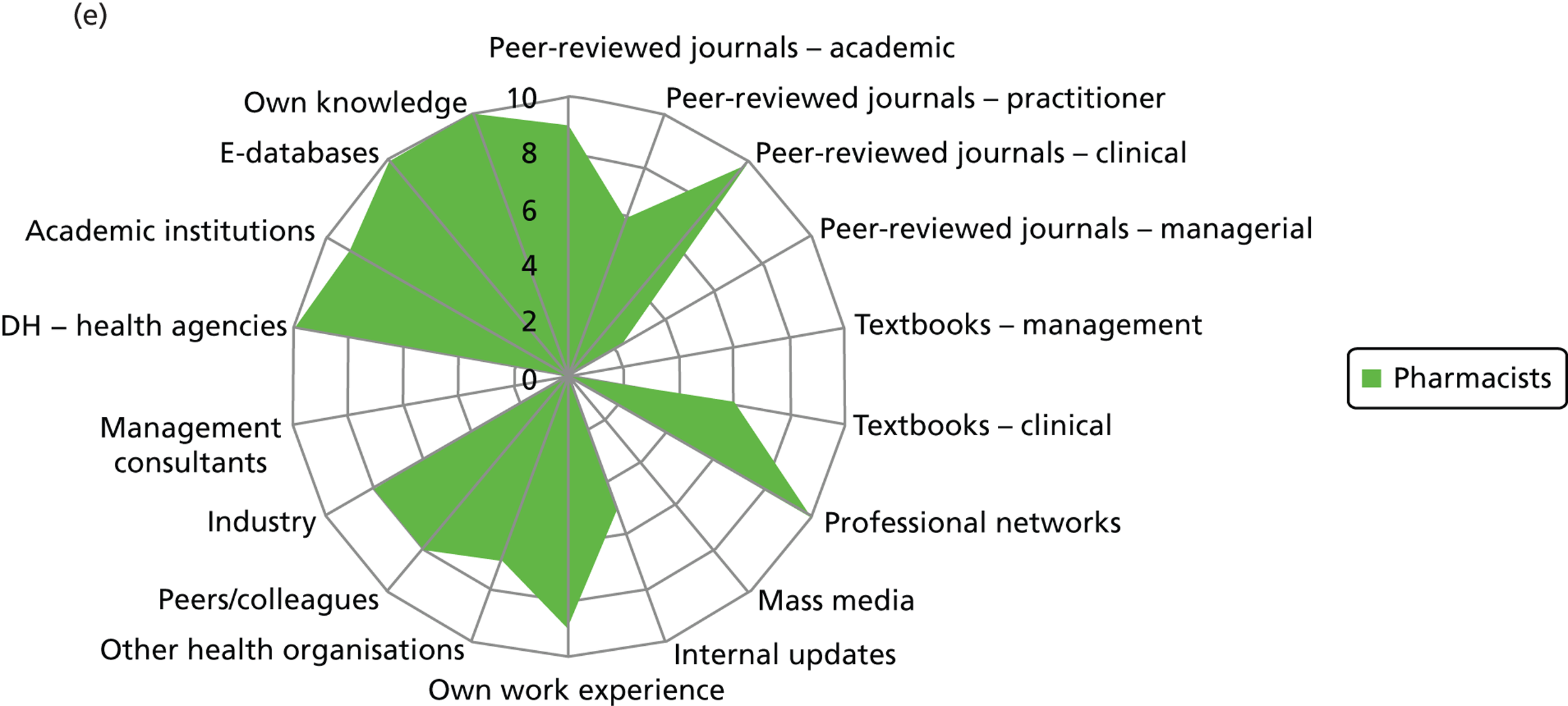
Few non-clinical managers sourced peer-reviewed journals, either management or clinical. This group clearly veered towards centralised and standardised sources of evidence (Department of Health agencies), internal updates and also locally derived evidence from other health-care organisations. They were the only group to source management consultants. These sources align well with the organisational role of non-clinical managers as well as the diversity in professional background of this group. Non-clinical managers were reported to show the least preference (15/25) for accessing evidence through their professional networks out of the different professional groups: doctors (22/24), nurses (53/61), pharmacists (10/10) and allied health professionals (6/6).
Nurses reported a uniform and consensus view within this group, reporting use of a wide range of sources. Doctors, allied health professionals and pharmacists displayed very similar patterns of reported evidence use with a strong preference for professional networks and most making use of academic institutions.
Across the professional groups, and not surprisingly given the context of the interviews was innovation, text books were not reported as an evidence source. Mass media was evident as a source for only a few nurses and non-clinical managers. Peer-reviewed management journals were mentioned as a source by only a few of the allied health professionals.
Innovation decisions: awareness and use of central evidence sources including sources concerning infection prevention and control
Figure 3 details the reported awareness and use of central evidence sources, including sources directly linked to IPC. Central here refers to those sources available across professional groups, generated by the Department of Health or one of the Department of Health’s arm’s length bodies.
FIGURE 3.
Knowledge and use of central evidence sources – breakdown by professional group (a) Doctors; (b) nurses; (c) non-clinical managers; (d) allied health professionals; and (e) pharmacists. NHS PASA, NHS Purchasing and Supply Agency; NPSA, National Patient Safety Agency; SDO, Service Delivery and Organisation programme.
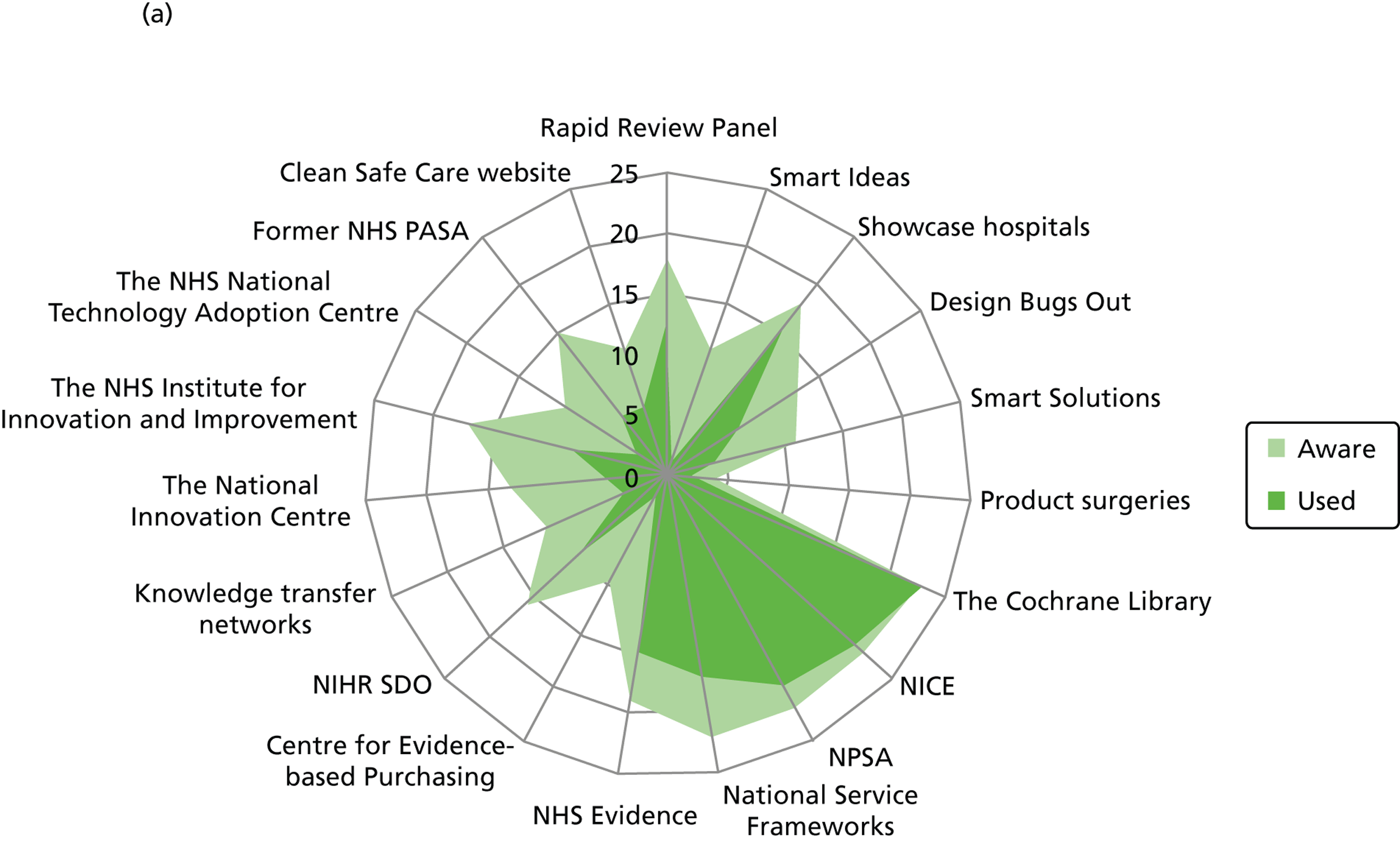
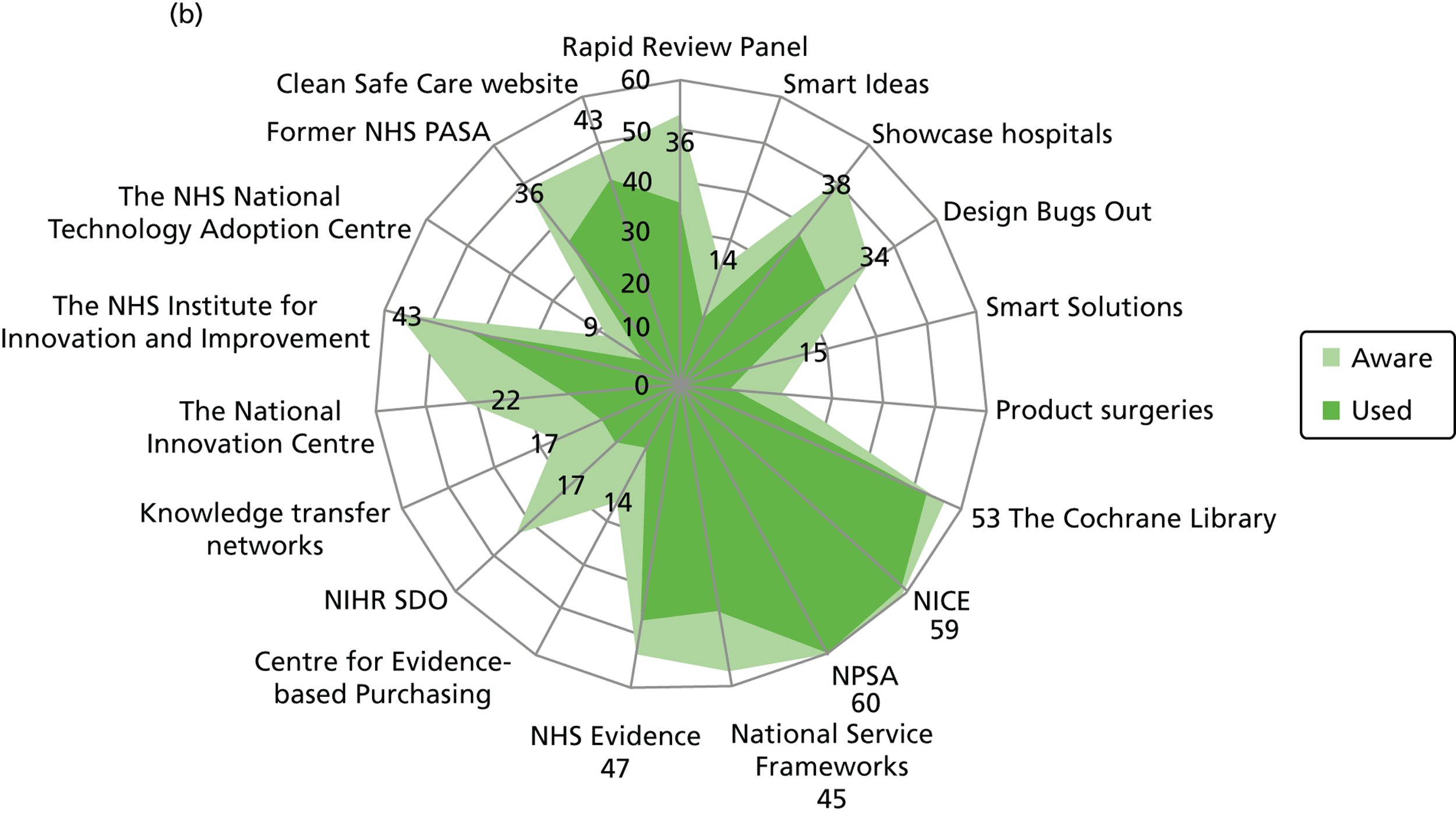
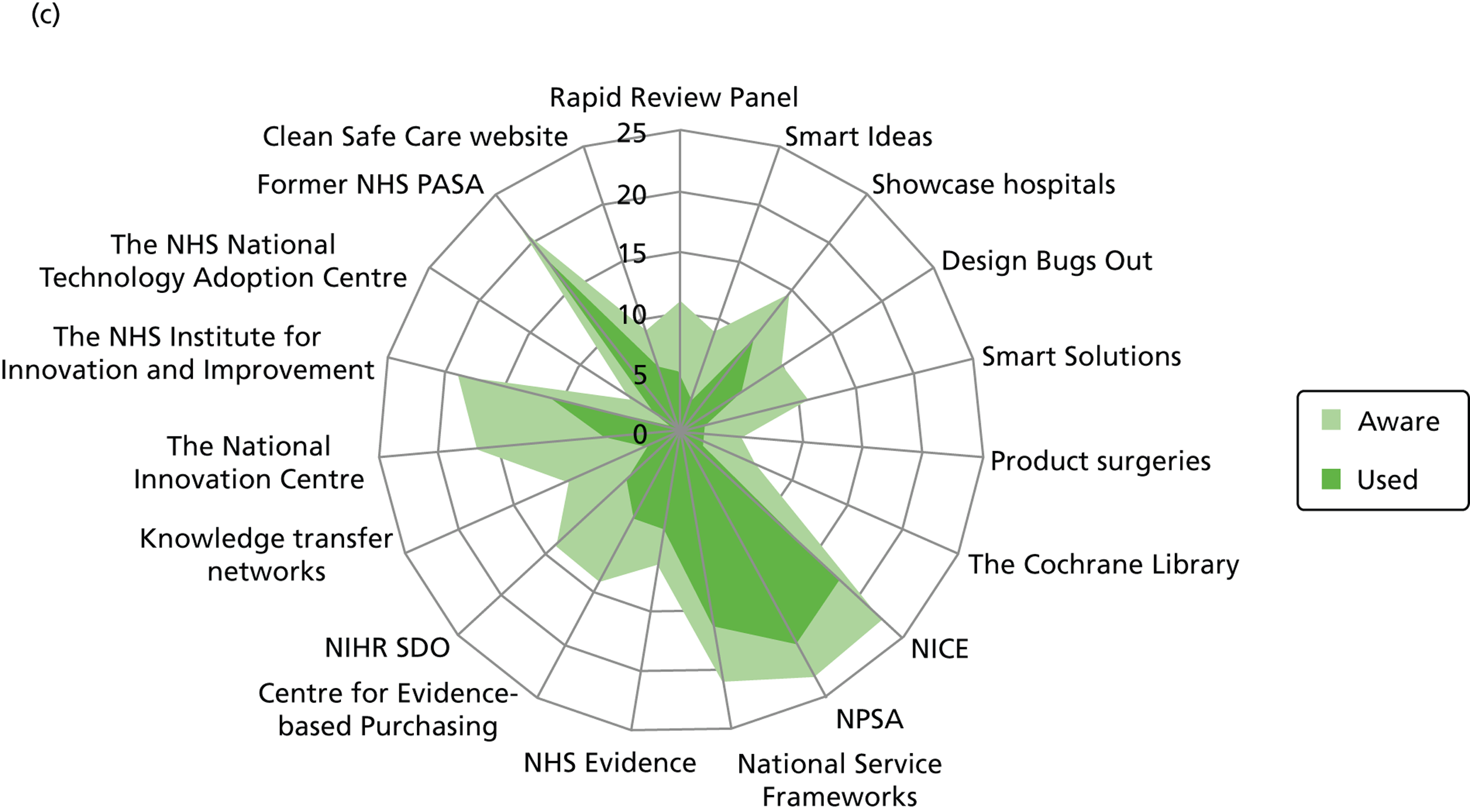
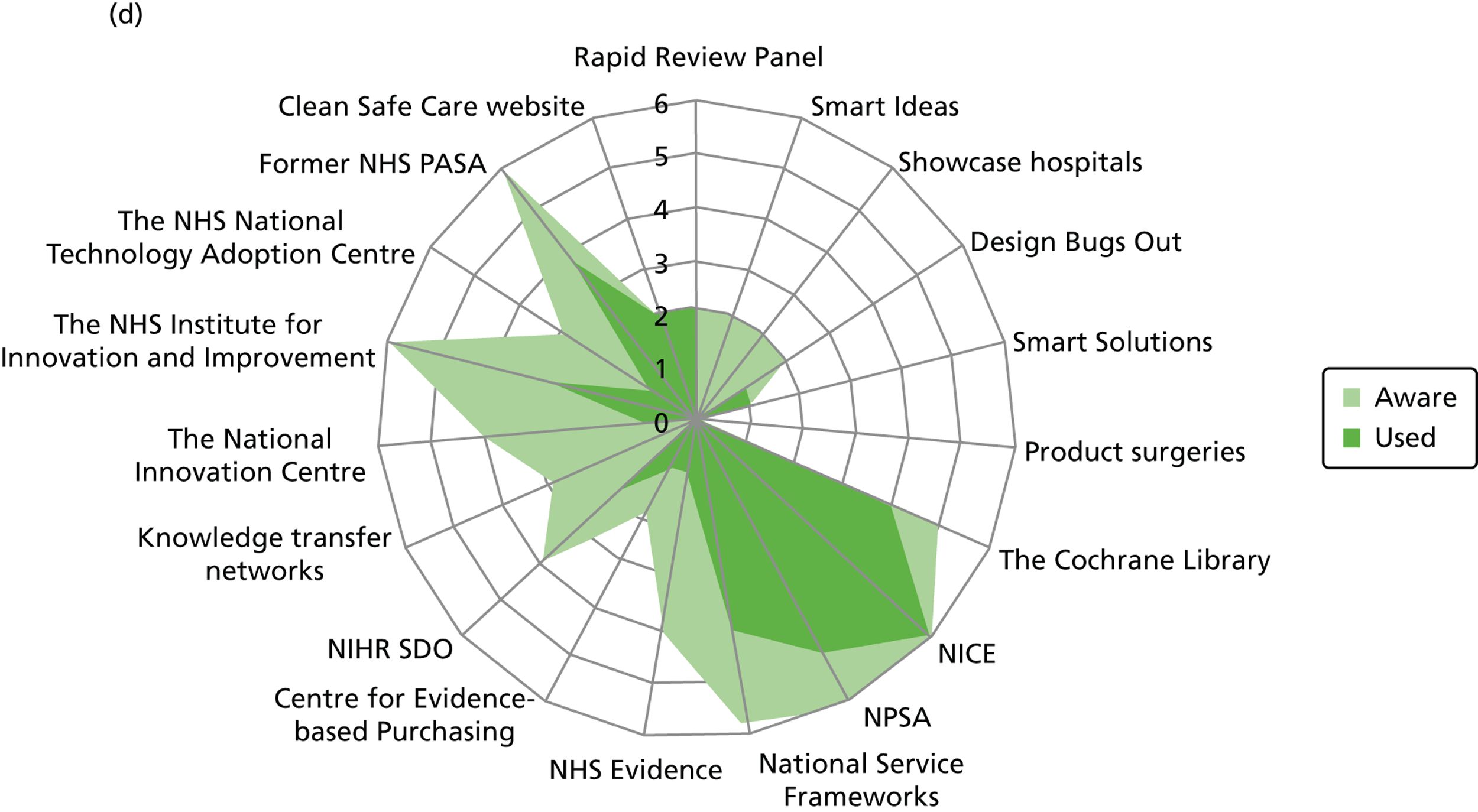
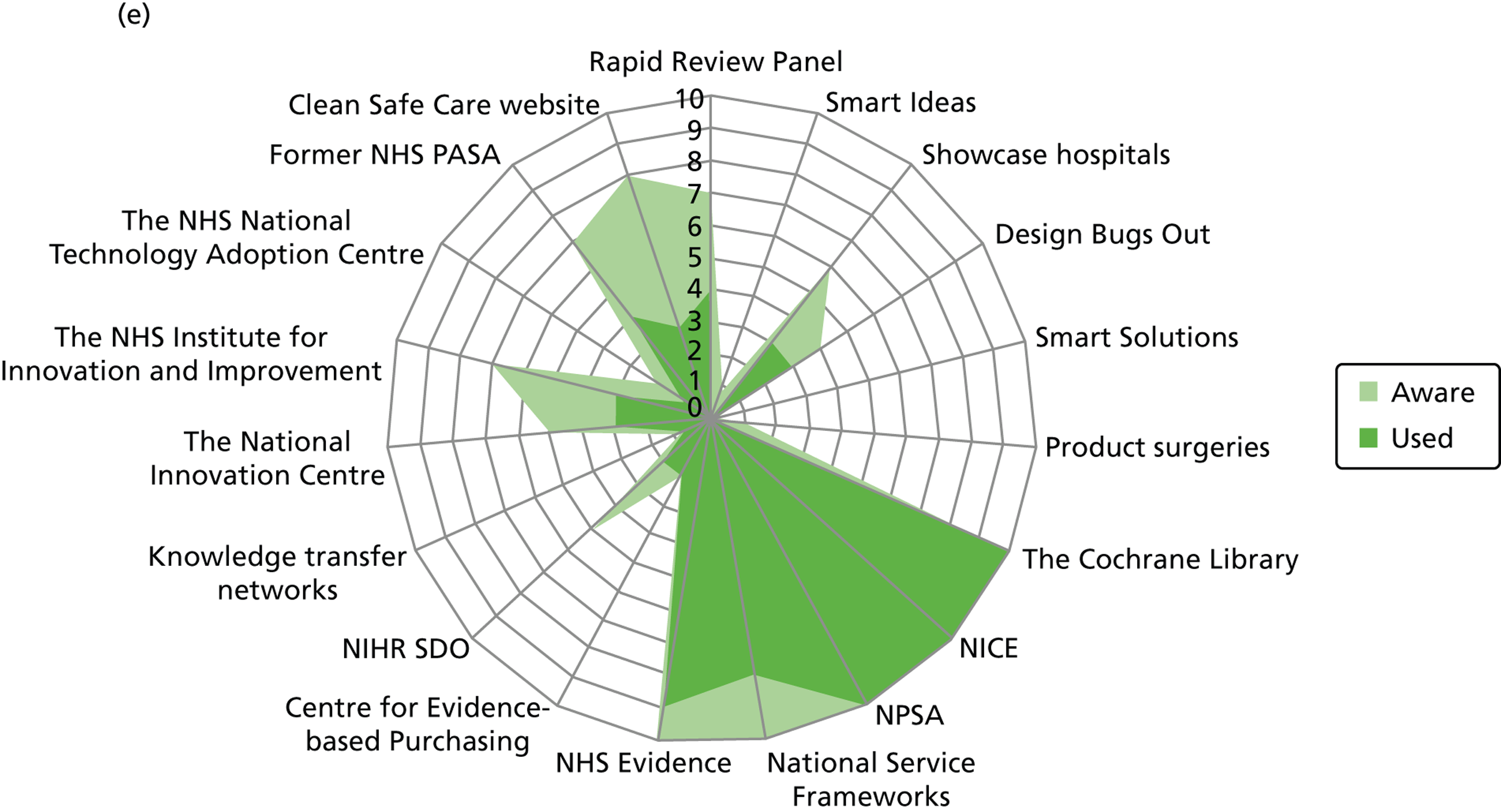
The majority of respondents across the five professional groups reported awareness of the NICE, the National Patient Safety Agency (NPSA) and National Service Frameworks. In addition, the majority reported using these sources and all nurses, pharmacists and allied health professionals reported using NICE guidelines. Comparatively, NHS evidence was less known and, consequently, less used. The majority, with the exception of non-clinical managers, were aware of The Cochrane Library. Non-clinical managers reported use of National Service Frameworks less frequently, which is broadly in line with their non-clinical roles. Overall, pharmacists and allied health professionals displayed very similar patterns of reported awareness and use of central sources.
With regards to central sources specific to IPC, non-clinical managers, allied health professionals and pharmacists were least aware, with the former NHS Purchasing and Supply Agency (PASA) being the only source known, or reported to be used, by these respondents. Half of the doctors were aware of the former NHS PASA, Clean Safe Care website, HPA Rapid Review Panel (RRP) and the Department of Health’s Showcase Hospitals programme, with fewer reporting using these sources. A larger proportion of nurses were aware, and reported use, of these sources. Across the professional groups, a small minority were aware of the Smart Solutions programme, Smart Ideas, Product Surgeries, Centre for Evidence Based Purchasing and the National Technology Adoption Centre.
Innovation decisions: perceived importance of evidence types
Figure 4 details responses on the perceived importance of different types of evidence (such as systematic reviews, guidelines, economic cost analyses and expert opinion).
FIGURE 4.
Perceived importance of evidence types – breakdown by professional group. (a) Doctors; (b) nurses; (c) non-clinical managers; (d) allied health professionals; and (e) pharmacists.
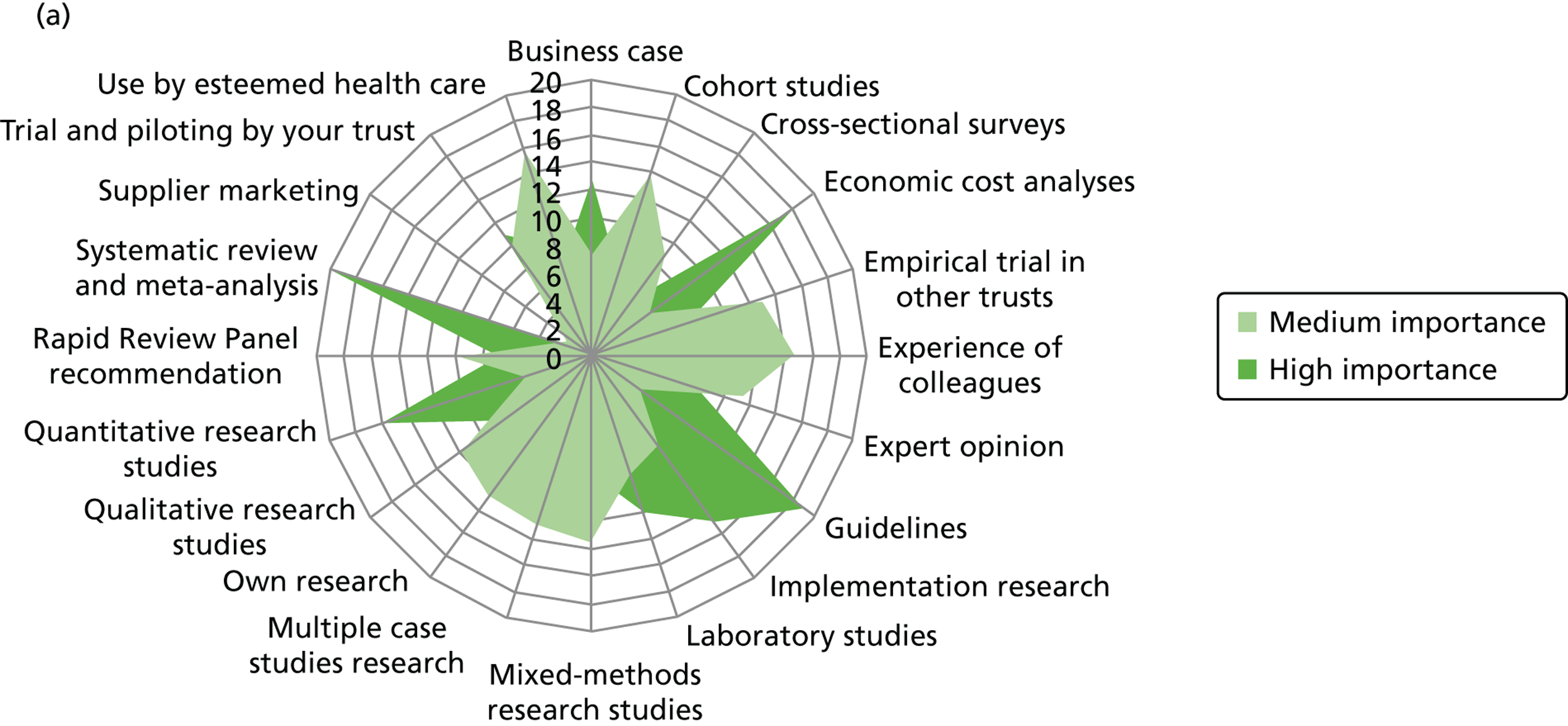
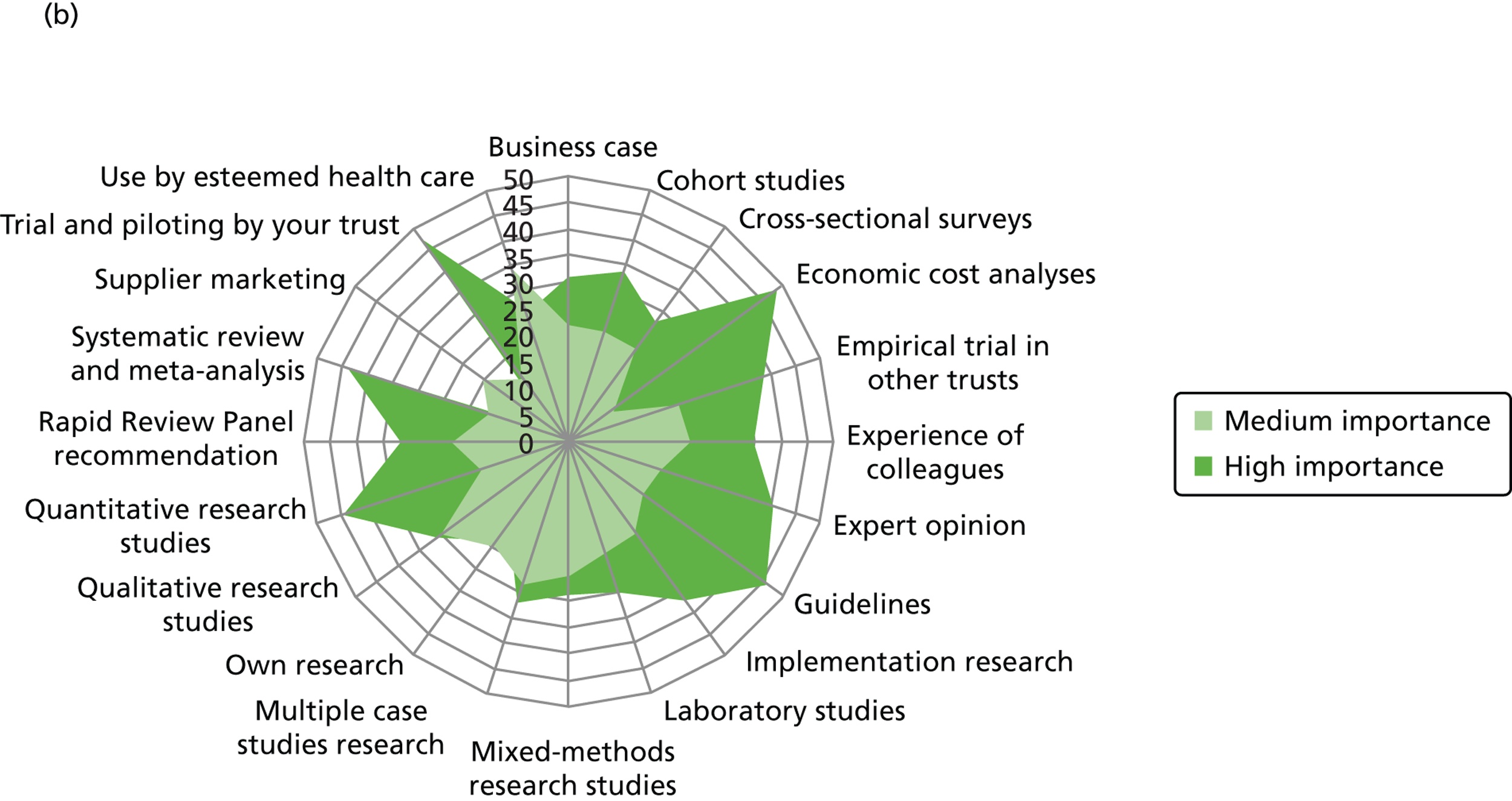
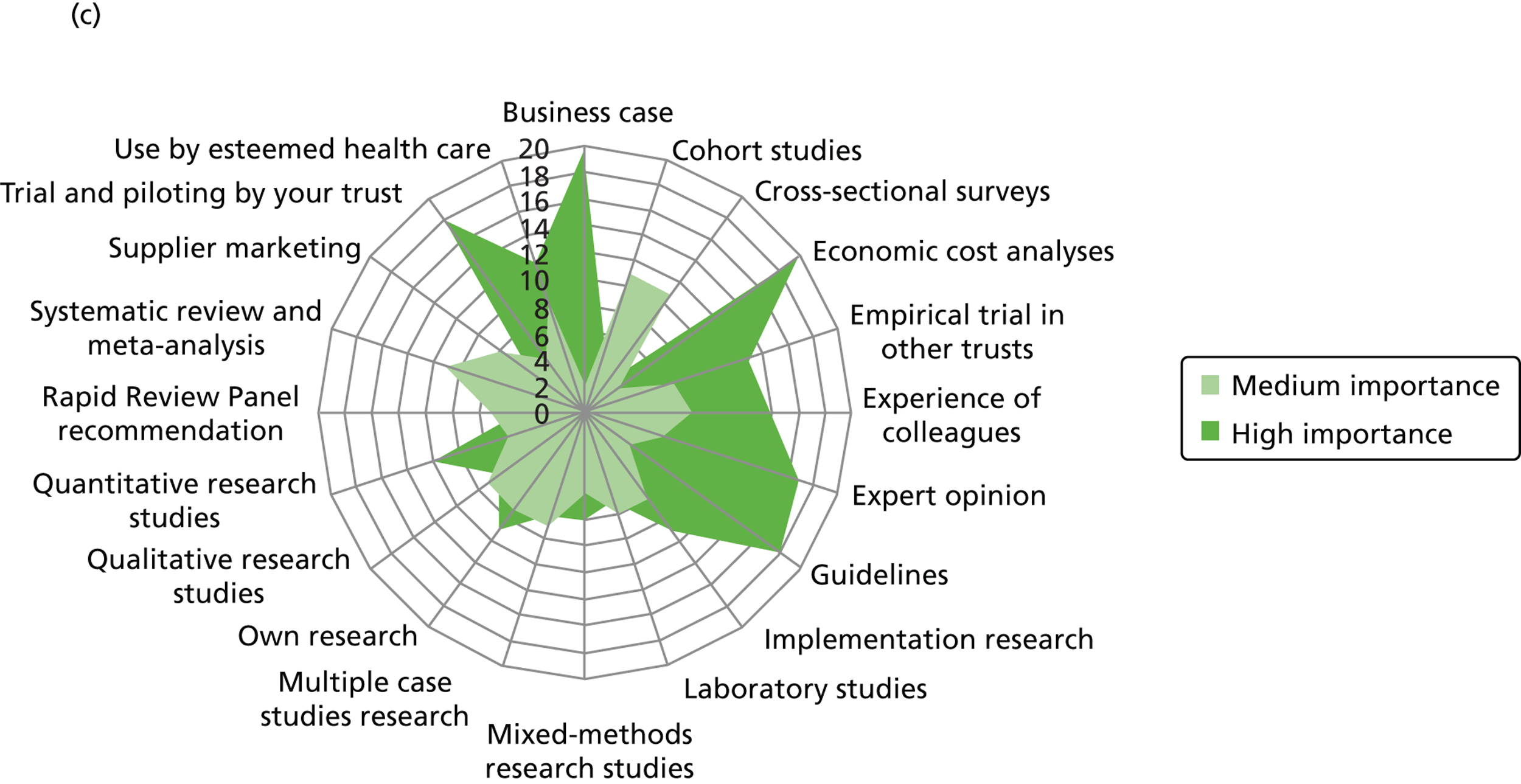
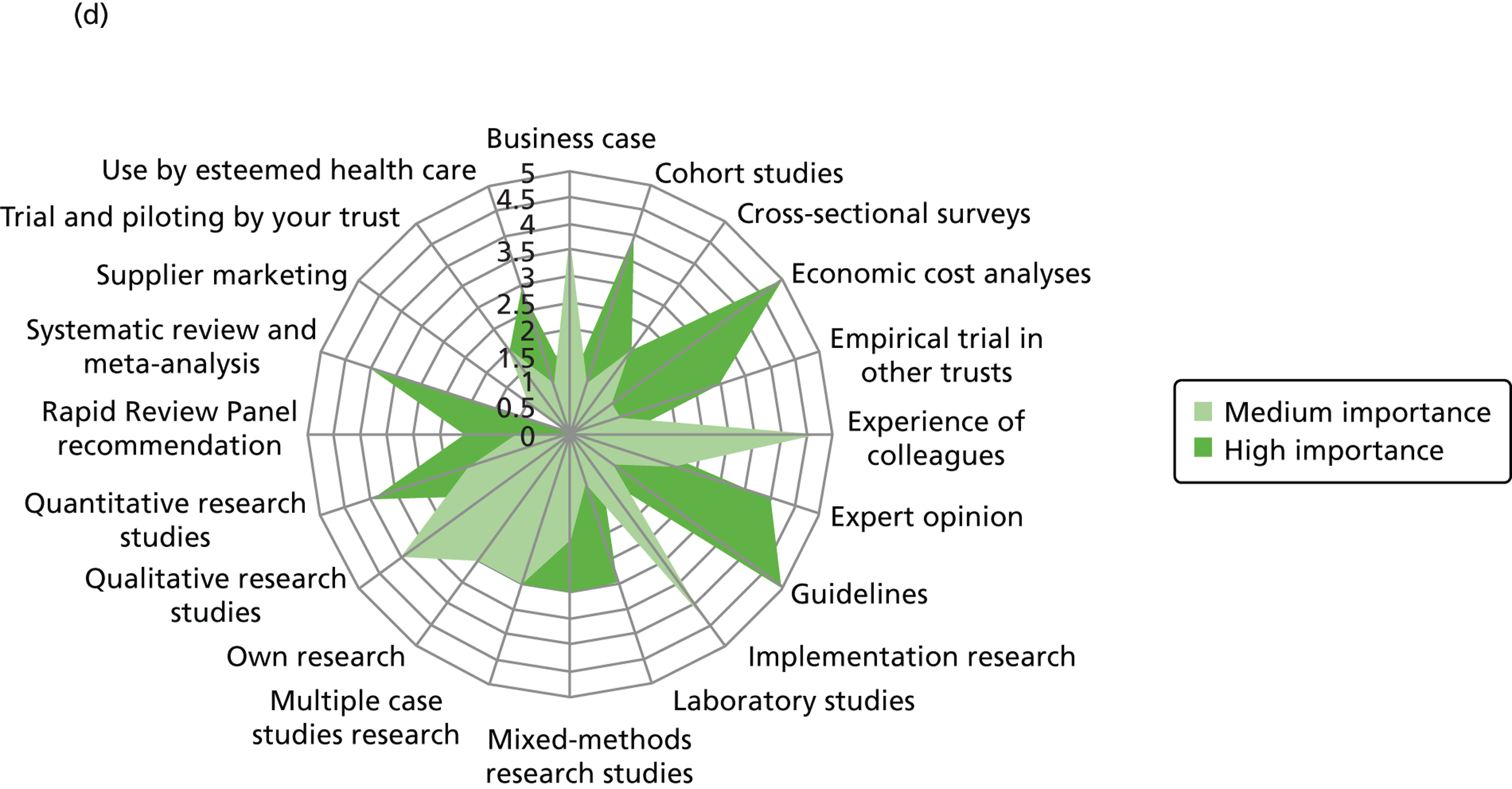
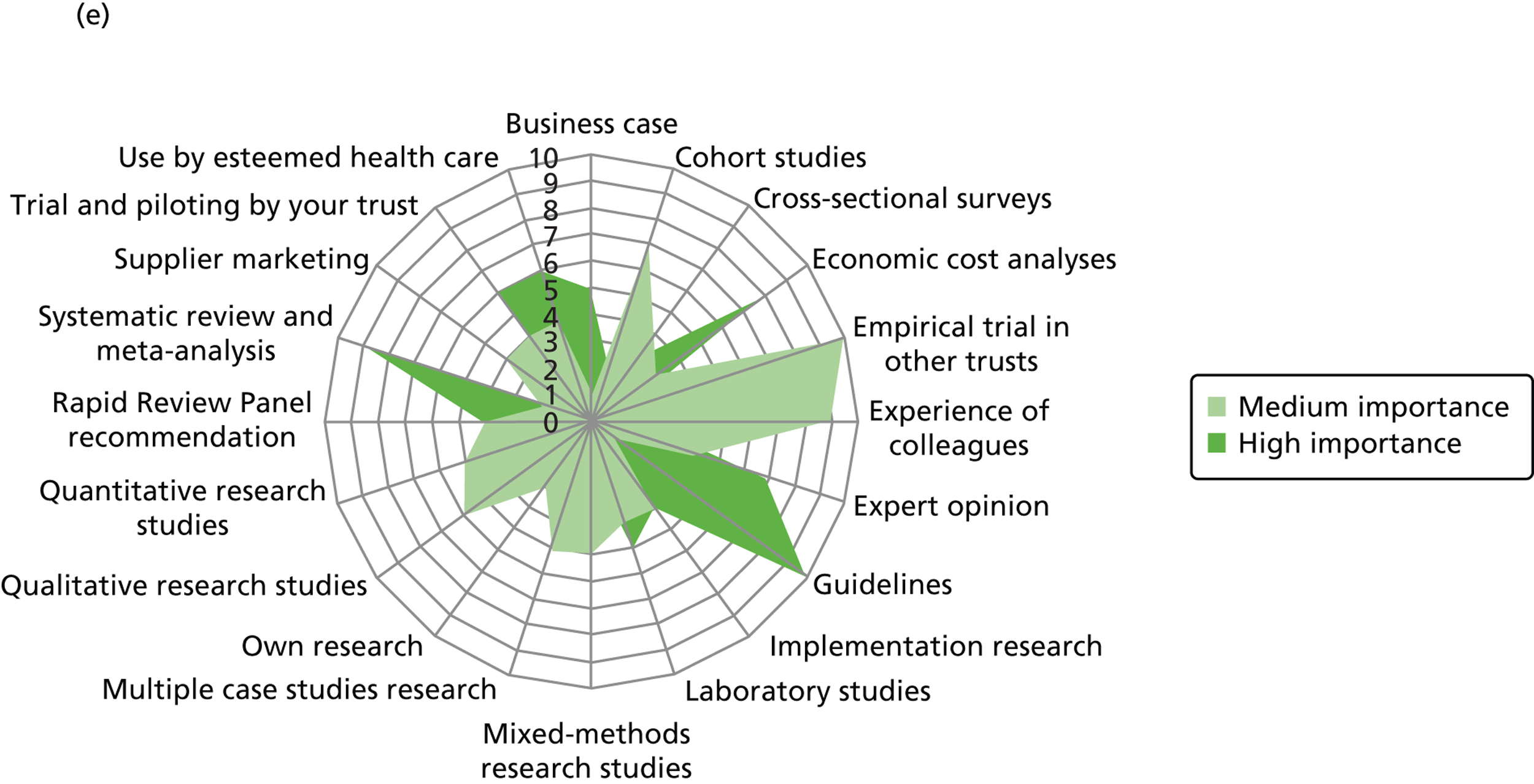
As demonstrated in the qualitative analysis, high importance was accorded to economic cost analysis (including cost-effectiveness, cost–benefit, cost-minimisation and cost–utility analyses) by the majority of respondents across the professional groups. Overall, nurses gave high importance to a wide range of evidence types. Doctors’ responses peaked on guidelines and systematic reviews; quantitative research was accorded high importance by the majority of doctors.
Non-clinical managers were reported to place high importance on the business case and related evidence. They also reported high preference for locally generated evidence, such as empirical trials in other trusts, and the personal experience of colleagues.
Organisational context: influences on the use of evidence
The temporal dimension emerged as a strong theme influencing the use of evidence, but particularly so in the hospital context. The macro environment of infection control in England was widely reported to have affected the nature and speed of decision-making in the hospitals studied. Notably, pressures to make decisions did not always allow the ‘preferred’ evidence synthesis to inform decision-making:
Change is forced upon you from my own personal experience in infection prevention and control. For example, a few years ago we had a huge outbreak of C. difficile, so we had to do something immediately in response, or people’s lives were at risk. You can’t sort of gather evidence and then go through all the processes; you have to make an instant decision. I had to make a decision overnight, what we were going to do to prevent this outbreak escalating. So obviously I was aware of the Department of Health Guidance, other people’s experience. I had to make reasonable rapid decision.
T4M1 – doctor
The use of evidence stemming from policy mandates featured, not surprisingly, in the accounts from non-clinical managers; in addition, similar accounts were reported by other respondent groups with reference to non-clinical managers. The use of this type of evidence was influenced not only by the organisational role of non-clinical managers, but also by the wider policy context and pressures. These pressures, although evident to other respondents to varying degrees, were nonetheless more acutely relevant to non-clinical managers.
Respondents across all groups mentioned that the pressures in IPC were intensified by an organisational and sometimes departmental drive to urgently respond to IPC issues:
Well again it comes, if you have individual areas within an organisation for whom a change is perceived by them as being very important, and so therefore they will, if in a sort of unrefined way a development results in increased patient benefit, the people that practise in that area will undoubtedly push very hard for that. They will not necessarily be concerned about what the organisational consequences of that change will be. And so you require, that’s the role of senior management, is to try and, if you like, harmonise laudable ambition in some areas with what can be practically achieved for the best good of the patients as a whole.
T3M14 – doctor
High importance was reported to be given to meeting the organisational IPC targets, as they constituted one of the key performance indicators for the trusts. In the respondents’ views, such pressures often resulted in staff feeling the necessity to ‘act’ and make decisions rapidly. This had a temporal dimension, narrowing the time frame in which evidence could be gathered, synthesised and used to inform decisions. As the quotes below illustrate, many respondents perceived such pressures to adversely affect the quality and effectiveness of organisational decision-making:
[. . .] I suppose the pressures often force rapid reactions which are often not particularly evidence based. And I think what we often find, is we respond quickly, in a let’s do something way and then we look back that actually after a time the evidence for that wasn’t fantastic, actually we didn’t really look at the evidence what we could have done.
T1M8 – doctor
For IPC, I just think sometimes they (the pressures) can act as a distraction and therefore prevent the proper level of thoroughness we want around looking at plans and seeing.
T7M10 – allied health professional
However, for other respondents a thorough approach to evidence gathering and reviewing was primarily conditioned by intrinsic individual motivation and organisational impetus for continuous improvement and critical thinking. Hence, according to this group of respondents, policy and organisational pressures in IPC incentivised staff to look out for innovations and source evidence in their quest to find solutions to problems:
I would say, [the pressure] incentivises the use of evidence; so if you take C. diff[icile] for example we’ve tried to tap into all sorts of evidence to get on top of our C. diff[icile] rate. So if you take the, hydrogen peroxide, the cleaning, we’ve nearly exhausted all sorts of evidence base now to try and get on top of that figure. So I think it incentivises. You’re always striving, aren’t you? Is there something else out there that we haven’t thought about that tackles a particular infection?
T4M5 – nurse
I think they (the pressures) incentivise. Because we’re constantly under pressure, pressuring ourselves and pressure externally to improve things, it is an incentive to constantly look for, to review our processes and ways of working.
T9M1 – nurse
I don’t think they inhibit I think there is more incentive to look at new products to see what you are doing to critically analyse where you are going and look at the new innovations that are out there and what other trusts are doing. And constantly keep your eye on the ball so to speak. I think 10 years ago it was we have always done this, we’re always going to do it, it has always worked there is no problem and it was a bit like putting your head in the sand. Whereas now you have constantly got to look at what you’re doing and why you’re doing it.
T6M5 – nurse
Data from the research interviews also indicated diverse attitudes towards the use of evidence in decisions between the university-affiliated trusts (especially T1, T5, T7 and T8) and the non-affiliated ones (T4 and T6). The latter are classified as less-research-orientated organisations, as documented in Chapter 6 . It was reported by respondents in all university-affiliated trusts that there was an organisational norm of high expectation to use evidence (or to show the use of evidence to others) to justify innovation decisions and change in practices. This organisational norm was criticised as contributing to slowing down decisions:
If you’re looking at, making change then it has to be justified, and therefore it is the quality of the evidence that supports that change. So without that then it is unlikely you’re going to really make much headway in an organisation, particularly in an organisation like this. The big teaching hospitals, teaching trusts pride themselves on their academic status. The downside of that often is the academic attitude that they want the evidence to the highest degree, whereas intuitively you’re saying this will work and will make an improvement. And sometimes you really have to sort of ‘cross every t [and] dot every i to get there’. [. . .] it slows, it really slows the process down sometimes.
T8M1 – nurse
Financial pressures were viewed by the respondents as a double-edged sword with regards to the use of evidence. On one hand, such pressures were perceived to promote and incentivise the sourcing of evidence:
When you’ve got financial constraints I think if there is a good thing about financial constraints, I think it then actually pushes you to look for the evidence more to make the best of the resource that you have got . . . We have had, like everywhere else, difficult financial terms both within our research centre here and the trust itself over a couple of years. And it does make you look much more carefully at what you do. It is not altogether a bad thing to have financial constraints because it does make you reassess whether what you are doing is really the most valuable and what’s the evidence, and what you are doing works the best and is there a better way of doing it.
T5M2 – allied health professional
A prime example of how financial constraints promote the use of evidence in organisational decision-making was found in T4. The trust experienced significant internal financial turmoil during the study period (see Chapter 6 for more information). This influence from the organisational context was reported to have nurtured the standardisation and formalisation of innovation decision mechanisms, increasing the demand for business cases, as well as requiring a more systematic and thorough assessment of the innovation evidence base:
[. . .] maybe three years ago when I started here it was pretty relaxed in a sense that it was a trust that was in profit, [. . .] now we’re a trust in deficit. We have a large financial deficit and therefore the mechanisms for innovation and change have become more formalised, which isn’t necessarily a bad thing because I think before perhaps they were too informal and perhaps parochial. So, would go up to the medical director and say, I think this is a good thing, and, yes, it seemed a good thing and it would happen. But the process now is more rigorous and therefore there is a financial element, everything has a formal business case, lardy, lardy, lardy la.
T4M2 – pharmacist
On the other hand, organisational financial pressures were also perceived to have inhibited the use of evidence, with a potentially adverse impact on patient safety and care:
It (the pressure) is a disincentive to use evidence when, for example, severe financial targets are likely to reduce the number of stool specimens we send for C. diff[icile] testing and that could ultimately be harmful to patients.
T9M11 – pharmacist
Non-clinical managers were widely reported to focus on performance and tangible organisational outcomes. The non-clinical managers in our sample have a range of educational and professional backgrounds including engineering, management, finance and accounting. More than any other professional group in our study, they reported high preference for local ‘testing’, and the generation of local data to enable them to identify ‘real’ improvement in service performance or cost-savings:
If we were installing a new type of light fitting or new type of control and it would reduce our energy consumption then the evidence would be reduced energy consumption, so evidence is the outcome really.
T1M9 – non-clinical manager
[. . .] Improvement in performance. What I would see as evidence probably wouldn’t be seen, as a scientist would, I want to see the qualitative and quantitative evidence. Or quantitative measures of some improvement in a service that adopts an innovation.
T2M11 – non-clinical manager
A research paper, a presentation, an abstract, something associated with a real outcome – any tangible or real outcome is assessed on the basis of the documentation that goes with it.
T1M1 – non-clinical manager
If you are trying to put a case forward for something in particularly anything that is going to cost money you have to be able to provide fast and hard evidence that is going to make a difference or what the different is whether it is quality and improvement in standards.
T2M2 – non-clinical manager
When asked about the use of evidence for assessing specific new products or activities, non-clinical managers almost unanimously reported that they used as evidence primarily quantitative, ‘hard’ data presented in documentary form. More importantly, they reported that what counted as evidence for them was information demonstrating organisational productivity improvements in their area of service. Their formal organisational accountability and role conditioned the acceptance as evidence of those sources of information that allowed them to identify such efficiency improvements.
Macro context influences on the use of evidence
This section looks at how, and to what extent, the macro context was reported in the research interviews to mediate the use of evidence. The quick pace of change, policy, financial and clinical targets, public expectations and patient fear constituted the widely reported external pressures influencing the use of evidence in IPC.
Some IPC policy interventions, particularly infection targets, were perceived as being ‘imposed’ and, at times gave rise to ‘clinical scepticism’, as this doctor reported:
Imposed targets usually are a disincentive to serve innovation I would say. [. . .] I think with infection control there is a clinical scepticism about the evidence base for a lot of the policy. [. . .] Bare below the elbows which is national policy a lot of the infection diseases experts say there is no evidence for this at all. And this is an example of a national policy that was imposed without providing any evidence.
T1M19 – doctor
Such ‘clinical scepticism’ was also seen among senior doctors towards central guidelines:
I suspect that it, a lot of change that is put down from above in terms of the ways in which hospitals function, the way in which we are expected to do things, often does not seem it, anyway, to come with a massive basis of evidence base behind it, tends to be, we think this would be a good idea, sometimes with the brackets after it, we suspect this is what our politicians would like us to do, and kindly do it. And I think we are sometimes very bad at stopping and saying, let us pilot this and see whether it actually does produce an advantage, although there are downsides that have not yet been thought about, it’s great until we did it would be a word that comes to mind.
T6M3 – senior doctor
I think we are now are being bombarded, is a slightly strong word, but certainly the number of NICE guidelines that appear on a monthly basis is quite substantial and those are pretty much, by the trust anyway, simply accepted at face value. It isn’t really possible to question NICE guidance now . . . I do have some concerns that whatever [the] NICE says is what must happen. I think that can, under certain circumstances, be a bad thing.
T5M5 – doctor
In response to the perceived top-down approach, particularly in relation to policies such as ‘bare below the elbows’ and ‘no tie’ – which were included as part of good practices, for example, in the Department of Health’s 2010 uniform and work wear guidance78 – some doctors insisted on seeing the evidence in support of this guidance, which was often seen by others as a tactic to justify their inaction in following such guidelines:
We convince people that they need to wash their hands. There has been quite a few debates around that. Some of the consultants were saying it was not proven that you have to wash your hands. [. . .] It kind of makes sense, doesn’t it, to wash your hands in between everything that you do. It’s got to be better than not do it. So it’s one of those things you just say, ‘don’t be silly’. But for example, the issues about ties, the consultants were told that they couldn’t wear a tie, so the neurologists all brought in a tie that they regularly used and they all cut the end off it. And they all had them cultured to prove that is no infection on the end of their tie I am quite sure they all washed them in the washing machine before they did it, but anyway.
T5M1 – allied health professional
The following respondent indicated what motivated him to justify local decisions (i.e. stick to local antibiotic policy) through exercising a greater level of local autonomy and not following a national recommendation by the Department of Health at face value:
I suppose they incentivise evidence when we have to justify not following a recommendation from the Department of Health. So for example in certain areas we continue to use cephalosporin and ‘quinolone’ antibiotics which are considered to be quite high risk for C. diff[icile]. We had to research the literature to argue that there were potential to detrimental consequences to switching to low risk antibiotics in certain populations.
T9M11 – pharmacist
In other cases, respondents talked about ‘breathing space’ after some years of intense focus upon the implementation of certain innovations. Revalidation of innovations is seen under these circumstances to justify continuous use or discontinuance:
[. . .] I suppose they (the pressures) often force rapid reactions which are often not particularly evidence based. And I think what we often find, is we respond quickly, in a let’s do something way and then we look back that actually after a time the evidence for that wasn’t fantastic, actually we didn’t really look at the evidence maybe what we could have done. We had an experience of that where we thought maybe we should use this hydrogen peroxide in response to outbreaks of C. diff[icile] and there was a lot of activity around getting access to the machines etc., and then we looked at it after a period of time and thought hang on actually the evidence of this making any long term difference is not robust so let’s not do that.
T1M8 – doctor
External drivers, including sudden financial constraints, appearance of competing alternative innovations or central policy changes, can also act as triggers for rethinking and innovation. The following respondent indicated how ongoing sensemaking of locally produced evidence was used to justify budgeting in T8:
I think it is important when you’re implementing a major change in a trust’s hygiene policy or whatever that you put in place some kind of robust surveillance internally so that you can confirm and so that you know when it comes to the next round of financial budgeting that you’ve actually got data internally that allows you to justify.
T8M2 – doctor
Some other IPC practices were seen as ‘common sense’ without the expectation of ‘evidence’ to back up action in this highly systematic way demanded by others:
It did seem very logical from our knowledge having an aseptic non-touch technique you can look at the research. But also from our knowledge of an aseptic non-touch technique it did, the methodology it reduced the risk of introducing infections into wounds or introducing infections into lines. You could even say a no brainer this is just common sense that it’s packaged around a methodology.
T9M7 – nurse
The majority of respondents perceived either external or internal pressures to improve IPC performance; however, most respondents identified more ‘external’ rather than ‘internal’ pressures to play an important role. Regarding ‘external’ pressure at the national level, targets or trajectories set by the Department of Health or compliances regulated by the CQC or Monitor were the main source of concern for trust staff, being expressed as a concern by approximately half of respondents; these were often pressures mandating prescriptive action by NHS staff.
The expectations of patients or the public were the second largest pressure, being voiced as a pressure by approximately one-third of respondents. Public expectations are often instigated or voiced through the mass media. The historical context also played a part. Other trusts’ poor performance on infection was publicly severely scolded; as a result, this created a ‘high profile drive’ to maintain or raise the organisational image:
[. . .] a raised profile, I think as people then started to get the education from the infection team that went along with that high profile drive, and I think a lot of this was down to the fact a number of other trusts had been hit quite hard nationally and were being very publicly berated for their poor infection performance.
T9M3 – nurse
The third largest source of perceived ‘external’ pressure was from regional authorities or commissioners, who impose financial penalties if the trust’s performance is not met; this was identified by approximately one-quarter of respondents.
A few respondents also commented that their pre-foundation-trust (FT) status became part of the environmental pressures at the time of the interview. As the trusts’ performance on the main HCAIs can be key for successful application for FT status, this theme was considered to be of importance in relation to other external pressures such as targets.
Some respondents gave qualitative significance to unrealistic targets:
Well MRSA rates were dropped down dramatically some years ago. It’s somewhat irritating really we have a million patient come to us a year because our MRSA absolute number was about six. They said we need to half that. You say hang on a minute there you have given an organisation somewhere else the same size as us a target of 106, how does that stack up. They said it’s about improving on your own performance, that’s right but there is a point you get to when you say actually if we hit three total how we gonna get to one and a half out of a million patient contacts per year. That’s just comes a bit absolute but nobody else accepts the argument but that’s what it’ll be.
T5M15 – doctor
Overall, the pressures in the views of respondents incentivised more than inhibited evidence sourcing for decision-making:
I actually think it has, I think it’s improved the use of evidence because as the pressure’s come on, and it’s got harder and harder to meet the target, you start to look more and more at what the evidence actually is showing you and where you best target your resources.
T4M10 – nurse
The evidence was often required to demonstrate ‘very quick wins’ to persuade and convince others at some trusts:
I think undoubtedly that the targets that we have to meet in terms of achieving certain goals within infection prevention are kind of here and now targets. So any innovations, any changes made have to be very kind of very quickly tangible. And I think it would be more difficult for the trust or departments to introduce changes that have a longer term affect. Because it would be more difficult to kind of demonstrate the evidence potentially or more difficult the justification to making changes that would have effect 12 to 15 months down the line.
T4M11 – nurse
Respondents also reported their reactive organisational attitude through a ‘fire-fighting’ attitude towards problem-solving:
But I think we have to turn our activity to the prevention side. It’s difficult because you need to dedicate the time to the prevention that involves control. The ‘fire-fighting’ stuff is still happening so it’s really difficult to kind of, to balance it. I think we need to invest more in the prevention side of it.
T1M3 – nurse
However, T9 respondents indicated their more proactive organisational attitude towards evidence use:
I think our track record is that we’ve taken a completely systematic approach to try and, to improve the infection control performance within the organisation. And I think that’s been right the way through from ensuring basic practice is improved, so things like the, you know, naked below the elbow, you know, appropriate dress code, hand washing policies, audits and compliance around administration of IV [intravenous] antibiotic. All those, all those things where the evidence points to potential for introduction of sources of infection. Isolation policy, those type of things. So getting some of those fundamentals right, auditing them, and having a sustained programme of education, engagement and performance management. And we’ve done that over several years. So I think the, the state of play in this organisation is one of, you know, long term sustainability in terms of the way that we’ve used evidence.
T9M5 – nurse
Others reported mixed attitudes towards evidence use:
[. . .] we fire-fight at the moment as an interim period but there are people looking forward at the future. So we are kind of taking a two-pronged approach. So we do have people working on the long-term.
T3M9 – non-clinical manager
The attitude of hospital staff was affected by priority shifts at an organisational level:
We have improved a lot recently in our infection control, so the pressure has eased, a lot of it, because that’s what makes you worry sometimes, if the pressure from outside ease then the focus might ease. I’m not saying that here, what will be introduced is complacency, and people are just in status quo, nothing is happening and we’re not going to improve, and there’s a lot of pressure from the financial side because to do something better you need financial support, and you’d be arguing, I need this, I need this and, because they’ve not got priority now, yeah. And then the infection control will move down [. . .], I’m quite sure we have seen a lot of complacency creeping in.
T6M6 – doctor
Thus, the perceived impact of ‘external’ pressures was often viewed to influence evidence use in decision-making either directly or indirectly. Nurses and non-clinical managers linked use of evidence to justification of own decisions or persuading others, frequently referring to the need for generation of local evidence.
Reflection on this chapter
Evidence sources and types were portrayed as variably prioritised and used by decision-makers depending on their professional background. Doctors reported a strong preference for science-based, peer-reviewed and published evidence. Nurses drew upon a wider range of evidence sources and types than all other groups in our study sample. Non-clinical managers tended to prioritise evidence linked to productivity and cost improvements. They also reported a preference for benchmarking information on implementation produced by national-level sources, and from local trials in their own or other hospitals. Doctors and nurses prioritised evidence on the clinical efficacy and effectiveness of innovations. Non-clinical managers relied more on their own or peer experiential knowledge than doctors, who showed a preference for more systematic forms of knowledge. Non-clinical managers and nurses considered evidence on ‘ease of use’, including local trials of innovative technologies, to be of high importance.
The quick pace and high magnitude of change in IPC, policy, financial and clinical targets, high public expectations, the pursuit of creating a positive organisational image, a drive for continuous improvement, intensified media pressures and scrutiny on hospital infections constituted the widely reported organisational and external pressures influencing the use of evidence in IPC. According to the accounts of most respondents, such pressures incentivised and promoted the use of evidence in organisational and clinical decisions. An additional contextual pressure linked to research-engaged organisations was the reported organisational norm of high expectations for the use of evidence, or the public demonstration of engagement with evidence for maintaining credibility.
Chapter 6 Organisational context: the macrocases of the eight NHS trusts studied
In this chapter, our analytic attention focuses mainly on the organisational context (also exploring key influences beyond it) to elicit a historical and local contextual dimension for each empirical site to help better situate contemporary issues during the study period (2007–12). The findings presented in this chapter draw mainly on secondary data sources and are summarised in a cross-case comparative account. Contextual data helped us to outline key characteristics and recent trends of important factors in each of the eight ‘macrocases’, including trust size, locality, resources, espoused values and vision, critical contextual events, research and innovation activity, and communication patterns. Changes in key areas of trust performance, the magnitude of shocks and continuity in leadership are outlined. The aim is to sensitise analysis in subsequent chapters of the potential impact of local and historical contexts on the social and organisational processes related to evidence access and utilisation. In addition, this chapter provides the audience with a means of translating findings to own context by comparing these wider contextual factors.
Trust size and financial and human resources
Table 4 shows basic characteristics of each trust – trust size and number of site(s) and financial and human resources. This information was gathered from trusts’ annual reports and financial accounts for 2007/08, 2008/09, 2009/10, 2010/11 and 2011/12; trusts’ quality accounts for 2009/10; and the CQC inspection reports on the prevention and control of infections for 2009 and 201079.
| Trust | Number of beds (2009/10) | Population covered (M) | Number of staff (2007/08–2011/12) | Financial turnover (£M) (2007/08–2011/12) | Number of sites/campuses | IPC staff : trust staff ratioa (2010/11) |
|---|---|---|---|---|---|---|
| T1 | 1600 | 1.9 (S), 3.0 (T) | 9100–10,500 | 840–940 | Multisite | 0.23% |
| T2 | 1200 | 0.3 (S), > 2.0 (T) | 5500–6000 | 340–420 | Multisite | 0.17% (2008) |
| T3 | 900 | 0.5 (S), 1.0 (T) | 5700–7000 | 350–570 | Multisite | 0.14% |
| T4 | 1400 | 0.5 (S), 2.0 (T) | 5800–6900 | 410–450 | Multisite | N/A |
| T5 | 1400 | 0.2 (S), > 1.0 (T) | 8500–9900 | 570–760 | Single site | 0.12% |
| T6 | 900 | > 0.5 | 3700–4500 | 270–350 | Multisite | 0.31% |
| T7 | 2300 | 0.5 (S), 1.7 (T) | 11,900–13,700 | 690–860 | Multisite | 0.30% |
| T9 | 1100 | 1.3 (S), > 3.0 (T) | 6700–7800 | 440–540 | Multisite | 0.25% |
Based on the number of staff, there appeared to be three different clusters by trust size during the study period: (1) T1 and T7 as relatively large organisations; (2) T5 and T9 as medium; and (3) T2, T3, T4 and T6 as relatively small. This was reflected in the financial turnover.
Regarding the number of sites, T5 is the only trust that operates in a single site; T1 has three main sites and the remaining six trusts have two sites. The last column of the table shows the ratio of the number of staff on the IPC team to the total number of trust staff. These ratios appeared to be associated with the number of sites in each trust to some degree; for example, a single-site trust, T5, had the lowest ratio (0.12%), whereas the trusts that run more hospitals in multiple sites had higher ratios.
Organisational values, vision and aims
In this section, we assess organisational values, vision or aims for each trust, based on largely trust-based secondary sources, but also supplemented by qualitative interview data from phase 1. This is to provide some contextual background regarding principles (espoused and actioned) and perceived legitimacy and creditability.
Organisational values, vision or aims varied across the different trusts. Nevertheless, high-quality, safe and integrated care was noted as the overarching core element of the publicly declared values, visions or aims for the majority of participating trusts. T7 was an exception and did not explicitly articulate these aims but placed emphasis on ‘patient-centred’ care or ‘patient experience’. Value for money or finance-related elements were expressed by T2, T4, T5 and T6.
Research and/or innovation were also a popular value expressed in the majority of trusts. Exceptions were T3 and T6: T3 placed a stronger focus and efforts on teaching as a less mature university-affiliated NHS trust, whereas T6 has shifted towards a focus on innovation but not research. T1 placed importance on innovation, coupled with a focus on pride and achievement; respondents reiterated this desire and pressure to be seen as leaders and a centre of excellence for research.
As part of their vision, values or aims, T1 and T3 included staff attitude/behavioural change elements. T3 collaborates with the local medical school, and had a research strategy including ‘behavioural medicine’. Therefore, these organisations were highly conscious of heterogeneous behaviour in their practice – a crucial element in the pursuit of effective and sustainable implementation of innovations. Staff engagement or teamwork is also an important element for the realisation of behavioural change and enhancing a sense of ownership; notably this theme constituted the values or vision of T2, T6, T7 and T9.
The question arises of whether, and to what extent, these formally set out organisational values, vision or aims were shared or trickled down to individuals or teams and how they then affected the use of evidence in managerial decisions. One senior respondent from phase 1 commented:
[. . .] the culture of the organisation is that we do want to be leading edge and we want to be innovative and we want our practice to be evidence base. But some of them are [. . .] either about personal about me because I’ve an enquiring mind [. . .] but also executive group and the team I work with are also sort of have that culture. So the infection control team for example if we are looking at a solution for something I would work with Dr XXX, the Director of Infection Prevention and Control, and we would talk about something, and then she would look for either an evidence-based or if one didn’t exist we’d set something up. So there is something around the people and the teams you work with and that generates sometimes from the bottom up.
T7M5 – nurse
These informal, personal, as well as formal, ‘rational-policy’ paradigms (which aligned with organisational objectives, aims or values) were important aspects in helping to better understand how staff made sense of evidence within the organisational context. An approach of ‘inquiring minds’ was viewed to be linked to local creativity, local trials or ‘first-line innovation’. In these cases, decision-making did not necessarily involve an externally produced evidence base, but rather local generation of evidence was encouraged. The same respondent articulated further:
Obviously my role is around leading nursing but also leading the clinical operations of the hospital. So when we are making decisions ideally we would look for an evidence base for those decisions which isn’t always possible. And we would try to use innovations from other centres, or the evidence from the research base. [. . .] I wouldn’t always say it was research based or we would look for another innovation that had some evidence of success in another organisation or in another country. Of course that doesn’t always happen and decisions don’t always come with evidence base and some things we try, are sort of first-line innovations. So we think of the idea and we’ll try it.
T7M5 – nurse
Another respondent, with a non-clinical background, suggested that managers’ strategic communication was articulated around relevance to ‘patient experience’, which was one of the organisational aims at T7, as a means of convincing or persuading others (making sense for others):
[. . .] inevitably, we work with front line teams and we choose certain ways of working there. We obviously present at formal executive meetings and that often requires a different type of communication. I suppose going back to an earlier point kind of presenting things from the patients’ experience can be something that unites from there to there. And that for me is an important consistency, it’s about how you use patient experience and patient stories to improve communication.
T7M13 – non-clinical manager
Thus, using relevant language led to effective interconnectedness in engagement across different levels of people, from front-line to senior management, at T7.
Trust performance and patient experience
Table 5 shows the trusts’ recent performance on financial management and degree of quality of care as well as the incidence of major HCAIs. The former was assessed through the Annual Health Check, which was conducted by the Healthcare Commission (replaced by the CQC80 in April 2009). The performance data on the incidence of MRSA bacteraemia and C. difficile infection came from the Healthcare Commission81/CQC82 as part of the Annual Health Check in earlier years (2007/08 and 2008/09), and from the trusts themselves through their annual reports and/or quality accounts in later years (2009/10 onwards). Major HCAIs have been subject to mandatory surveillance, for example, MRSA bacteraemia since April 2001 and C. difficile infection since January 2004. 86 The number of cases for these HCAIs was assessed against their annual reduction targets (under mandatory surveillance) set externally by the Department of Health. There was also a local target for each trust set by their respective strategic health authority or primary care trust (as part of the Commissioning for Quality and Innovation scheme), which was stricter than the national targets set by the Department of Health for each trust. 87,88
| Trust | Annual Health Check – quality of care (2007/08, 2008/09)a | Annual Health Check – use of resources/financial management (2007/08, 2008/09)a | Incidence of MRSA bacteraemiab (2007/08, 2008/09, 2009/10, 2010/11, 2011/12) | Incidence of C. difficile infectionb (2007/08, 2008/09, 2009/10, 2010/11, 2011/12) | Adult inpatient survey results regarding IPC-specific questions (2009, 2010, 2011)c | Overall rating |
|---|---|---|---|---|---|---|
| T1 | Good (2007/08); good (2008/09) | Good (2007/08); good (2008/09) | Achieved (2007/08); achieved (2008/09); achieved (2009/10); underachieved (1.5 times higher) (2010/11);d underachieved (1.4 times higher) (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d underachieved (1.1 times higher) (2011/12)d | ‘Worse’ in (c) and (d); ‘better’ in (e) in 2009. Improved from 2010 onwards – ‘about the same’ for all | 2, 2, 0, 1, 1 = 6† |
| T2 | Good (2007/08); excellent (2008/09) | Excellent (2007/08); excellent (2008/09) | Underachieved (1.7 times higher) (2007/08);e achieved (2008/09); achieved (2009/10); achieved (2010/11);f achieved (2011/12)f | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);f achieved (2011/12)f | Consistently ‘about the same’ for all indicators throughout 2009–11 | 2, 2, 1, 2, 2 = 9††† |
| T3 | Excellent (2007/08); good (2008/09) | Fair (2007/08); good (2008/09) | Underachieved (1.5 times higher) (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);f achieved (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d achieved (2011/12)d | ‘Worse’ in (e) in 2010. ‘About the same’ for remaining indicators during 2009 and 2010 | 2, 1, 1, 2, 1 = 7†† |
| T4 | Excellent (2007/08); excellent (2008/09) | Good (2007/08); fair (2008/09) | Achieved (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);f achieved (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);f achieved (2011/12)d | ‘Worse’ in (c) during 2009 and 2010, but improved in 2011. ‘About the same’ for remaining indicators throughout 2009–11 | 2, 1, 2, 2, 0 = 7†† |
| T5 | Fair (2007/08); good (2008/09) | Good (2007/08); good (2008/09) | Achieved (2007/08); achieved (2008/09); achieved (2009/10); underachieved (1.2 times higher) (2010/11);e achieved (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);e achieved (2011/12)d | Consistently ‘about the same’ for all indicators throughout 2009–11 | 1, 2, 1, 2, 2 = 8†† |
| T6 | Good (2007/08); good (2008/09) | Fair (2007/08); good (2008/09) | Underachieved (1.4 times higher) (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d underachieved (1.4 times higher) (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d achieved (2011/12)d | Consistently ‘about the same’ for all indicators throughout 2009–11 | 2, 1, 0, 2, 2 = 7†† |
| T7 | Excellent (2007/08); excellent (2008/09) | Excellent 2007/08); excellent (2008/09) | Achieved (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d achieved (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d underachieved (1.3 times higher) (2011/12)d | ‘Better’ in (g) in 2010 only. Consistently ‘about the same’ for the rest throughout 2009 and 2011 | 2, 2, 2, 1, 2 = 9††† |
| T9 | Good (2007/08); fair (2008/09) | Good (2007/08); good (2008/09) | Underachieved (1.5 times higher) (2007/08); underachieved (1.1 times higher) (2008/09); achieved (2009/10); achieved (2010/11);d achieved (2011/12)d | N/A (2007/08); achieved (2008/09); achieved (2009/10); achieved (2010/11);d achieved (2011/12)d | Consistently ‘about the same’ for all indicators throughout 2009–11 | 1, 2, 0, 2, 2 = 7†† |
We assessed the last indicator in the table, ‘patient experience’, by using annual inpatient survey data that were reported by the CQC. As shown in the table footnotes, seven IPC-related questions were selected in the following areas: (a) cleanliness of rooms and wards; (b) cleanliness of toilets and bathrooms; (c) posters regarding hand wash gels; (d) availability of hand wash gels; (e) hand cleaning (doctors); (f) hand cleaning (nurses); and (g) having enough nurses on duty. 83–85 Each of these indicators is assessed against the country’s average; in other words, each datum falls into the category of either ‘better’, ‘about the same’ or ‘worse’ than country average. Sample size for the annual inpatient survey (CQC) varied across the trusts, ranging from 292 to 508 patients (average 400 patients) in 2009, 2010 and 2011. 83–85 T1 had the lowest response rate (average 37%, 316 patients) during the aforementioned period, whereas T4 tended to have the highest response rate (average 55%, 469 patients). The sample size in this survey appears to be very small, when compared with the actual number of inpatients (elective and non-elective) treated at each trust; for example, in the case of T3, the sample in the survey represented approximately 0.6% of the inpatients during 2010/11 and this itself has attracted mixed reactions from NHS organisations over the years. Nevertheless, these were the only officially available inpatient survey data that allowed comparison across the trusts.
The overall rating shows the result of the aforementioned indicators around trust performance and how patients thought about the trust in relation to IPC practices. T3, T4, T5, T6 and T9 achieved relatively moderate scores on performance and patient experience. T2 and T7 had a better overall rating, whereas T1 had the worst overall rating among the participating trusts. Notably, T1 appeared to be struggling to meet the targets for MRSA bacteraemia and/or C. difficile infection between 2010/11 and 2011/12.
The results in Table 5 masked some realities and additional pressures faced in IPC. First, the targets for MRSA bacteraemia and C. difficile infection have become tougher in recent years. All trusts had the MRSA bacteraemia target of 10 or less, and half the trust cases (T2, T4, T6 and T9) set MRSA bacteraemia targets of five or less during 2011/12. This embodied an enormous external pressure for these trusts, which was echoed in the interviews with managers in each trust (previously discussed in Chapter 5 ). One respondent explained as follows:
Well they get tighter based on previous year’s performance when you come in under last year’s target and then you find that it has been brought way, way lower.
T7M2 – nurse
These HCAI issues were perceived to be of higher importance in trusts submitting FT applications.
Second, improvements in the reduction of HCAI cases can be masked by setting higher targets; as a result, despite a reduction in HCAI cases the relative performance score against targets might show deterioration.
Third, through technological advancement towards a more sensitive and accurate test, including new assay kits for C. difficile that used improved molecular techniques, more C. difficile cases were detected in the laboratories. This can have negative add-on effects on the trust; in other words, the trust could not meet the target and, therefore, had to seek a more realistic target and negotiate with commissioners.
Fourth, as a result of restructuring, including mergers, the standardisation of processes and systems became all the more important. The delay in the standardisation/streamline of information systems across the different sites, as well as the standardisation of assay methods for microorganisms across the different sites, raised serious concerns in some trusts, as they could not simply merge the data between the sites for analysis and management and, moreover, could not compare the data and spot the negative outliners for action within the trust.
Fifth, in a more globalised world, the emergence and threats of pandemics of new microorganisms have posed a huge challenge to NHS trusts. Finally, other microorganisms were recently added to mandatory surveillance: for example, methicillin-sensitive Staphylococcus aureus (MSSA) bacteraemia since January 2011 and Escherichia coli bacteraemia since June 2011. 86 This imposed extra pressure on trusts. As explained in Chapter 5 , according to the interview data, some trust managers voiced concerns that these new targets, as well as increasingly unrealistic/tougher targets, contributed to significant external pressures.
Magnitude of shocks, crises and critical events
Table 6 gives a picture of the magnitude of shocks, crises and critical events at each trust during the study period. The first (mergers, redevelopments/expansions, FT application/status attainment) and the third (continuity of leadership – CEO and DIPC) indicators represent inner shocks, and the last indicator (health profiles from the Network of Public Health Observatories89) was selected as an outer constraint. The second indicator, a trust’s year-end position, considers both inner and outer financial shocks.
| Trust | Mergers, redevelopments/expansions, FT application/status attainmenta , b | Trust financial crisisa (a trust’s year-end position 2007/08–2011/12) [deficit as percentage of NHS trust turnover (%)] | Continuity of leadershipa (CEO, DIPC) | Health profilec (the Network of Public Health Observatories) (2008–12) | Overall rating |
|---|---|---|---|---|---|
| T1 | Merger of two trusts and integration with a local university in the mid to late 2000s; a series of improvements of infrastructure and equipment, costing £60–70M annually during the late 2000s. Expected to submit a FT application in 2013 | Dramatically fluctuated over recent years, with deficits of £59.3M [7.1%] (2008/09) and £20.5M (2011/12) → adjusted retained deficit of £8.4M [0.9%] (2011/12) | Unstable CEO position (changed twice in the mid to late 2000s and early 2010s); stable DIPC (from the mid to late 2000s onwards) | 0.0 (2008–09), 2.0 (2009–10), 0.0 (2010–11), –3.0 (2011–12) | 3, 2, 1, 3 = 9††† |
| T2 | Merger in the mid-1990s; a series of redevelopment projects over recent years, including a £90M redevelopment of hospitals that was completed in the late 2000s; the new hospital redevelopment project (under PFI scheme) was under way at the time of the study, costing over £400M and to be finished in the mid-2010s. Expected to be approved as a FT shortly after the study | 2009/10 saw a deficit of £15.9M (adjusted retained surplus of £4.0M), followed by a recovery with consecutive surpluses of £4.8M (2010/11) and £6.0M (2011/12) → adjusted retained surpluses of £4.4M (2010/11) and £5.5M (2011/12) | Unstable CEO and DIPC position in the mid to late 2000s and then became stable | –2.6 (2008–09), 4.8 (2009–10), –1.0 (2010– 11), 0.0 (2011–12) | 2, 0, 2, 3 = 7†† |
| T3 | In the late 2000s, the redevelopment, expansion and refurbishment of facilities and buildings occurred through the investment of over £50M altogether. A £400M hospital redevelopment with new state-of-the-art facilities newly approved by a strategic health authority in the early 2010s. The main construction work was expected to begin in 2013. Aimed to submit a FT application in 2013 | After some consecutive years of retained deficits the trust achieved a stable position in the late 2000s, but has experienced difficult financial years recently, with deficits of £12.1M [2.2%] 2010/11 and £16.2M [2.8%] 2011/12 | CEO changed twice in the mid to late 2000s and in the early 2010s; DIPC changed twice in the late 2000s and early 2010s | 2.6 (2008–09), –4.0 (2009–10), 0.4 (2010–11), 1.3 (2011–12) | 2, 2, 2, 3 = 9††† |
| T4 | Through a > £250M major redevelopment under PFI scheme, the creation of a modern and ‘fit-for-purpose’ hospital with state-of-the-art facilities completed in the late 2000s. Established the FT Project Board during study, and aimed to submit its application by 2013 | Plunged into deficits of £6.1M [1.4%] (2008/09) and £77.1M [17.8%] (2009/10); this was followed by a swift recovery with retained surpluses of £6.0M (2010/11) and £1.8M (2011/12) → adjusted retained surplus of £0.1M (2011/12) | Stable CEO (from the mid-2000s onwards); DIPC changed in the early 2010s | 1.3 (2008–09), –1.3 (2009–10), –1.4 (2010–11), –0.3 (2011–12) | 2, 3, 1, 4 = 10††† |
| T5 | Merger of two trusts in the early 2000s. In the late 2000s, many hospitals/services relocated under new hospitals development (£500M under PFI). Became a FT in the late 2000s | The 2009/10 saw a huge deficit of £179.8M [27.2%], followed by a great recovery with a deficit of £3.6M [0.5%] (2010/11) and a surplus of £56.1M (2011/12) | Stable CEO (from the early 2000s onwards) and DIPC (from the late 2000s onwards) | –0.5 (2008–09), 3.1 (2009–10), –1.2 (2010–11), 1.1 (2011–12) | 2, 4, 0, 2 = 8†† |
| T6 | Merger of two trusts in the late 1990s. Became one of the first trusts that breached the Hygiene Code; warned by the Healthcare Commission in the mid to late 2000s. Expected to become a FT shortly after the study | Recorded relatively gradual consecutive falls from 2007/08 to 2009/10, with a deficit of £9.6M [2.9%] in 2009/10; however, 2010/11 saw a healthy recovery with a surplus of £3.1M, followed by an adjusted retained surplus of £2.2M during 2011/12 | Unstable CEO position in the early 2010s; DIPC changed in the late 2000s | 3.5 (2008–09), 0.0 (2009–10), –0.5 (2010–11), 0.0 (2011–12) | 1, 1, 2, 1 = 5† |
| T7 | Merger of two trusts in the early 2000s. Became a FT in the mid-2000s. A series of redevelopment, expansion and refurbishment projects across the hospitals, costing approx. £100M over recent years; the majority of new hospital facilities opened in the late 2000s | Recorded consecutive falls, in particular a deficit of £54.2M [6.9%] in 2009/10; this was followed by a recovery with surpluses of £4.4M (2010/11) and £8.0M (2011/12) | Unstable CEO position in the mid to late 2000s; stable DIPC (from the mid-2000s onwards) | –2.5 (2008–09), 3.0 (2009–10), 0.0 (2010–11), 1.2 (2011–12) | 1, 1, 1, 2 = 5† |
| T9 | The trust was formed in the early 1990s. Major hospital developments began in the late 2000s with the investment of £30M. This was followed by a £60M extension of hospital buildings in the late 2000s. Became a FT during study | Recorded relatively gradual consecutive falls with deficits of £2.3M [0.5%] (2009/10) and £4.3M [0.8%] (2010/11), but demonstrated a slight recovery last year, to a deficit of £0.3M (2011/12) → adjusted retained surplus of £3.9M (6 months ended 31/03/12, adjusted retained surplus) | Stable: CEO (from the mid-2000s onwards) and DIPC (from the mid-2000s onwards) | 6.0 (2008–09), –2.0 (2009–10), 2.0 (2010–11), –1.0 (2011–12) | 2, 2, 0, 3 = 7†† |
T1 was the only trust that went through a merger process during the period observed (from 2007/08 onwards). The majority of trusts (except for T6) had gone, or were going, through hospital (re)development, expansion/extension or refurbishing processes to modernise the hospitals with purpose-built, state-of-the-art technology buildings and facilities. This coincided with the government’s Deep Clean Programme, which aimed at enhancing patients’ hospital experience and improving hospitals’ public image. According to the Department of Health’s Clean, safe care: reducing infections and saving lives, released in January 2008,6 a total of £57.5M was invested in the Deep Clean Programme for all hospitals across the country during 2007/08 and these hospitals were expected to complete the programme by the end of March 2008. IPC team members were often involved in the planning/designing stages of the redevelopment, expansion and refurbishment, and gave advice to ensure that the new buildings and facilities were IPC sensitive/friendly (i.e. T3).
All non-FT hospitals are now attempting to gain foundation status, consistent with government plans. The FT application involves a long process – mobilising local people and key local organisations, setting up governors’ councils, all of which requires much energy and effort from the trust as an organisation. Some trusts seem to have taken a strategic approach to when they should apply for FT status; for example, T3 considered the timing to become a FT, and intentionally delayed its application process, as they were also considering major hospital redevelopment during the same period. It is logical for T3 to have focused on controlling the major HCAIs (MRSA bacteraemia and C. difficile infection) before embarking on the foundation application. An outlier among our sample is T6, which was subject to a significant additional shock as one of the first trusts to breach the Hygiene Code and be warned by the Healthcare Commission.
Regarding the year-end financial position for trusts, the great majority of participating trusts experienced deficits during the observed period. Analysis of the degree of each trust’s financial crisis, by the borrowing indicator ‘deficit as percentage of NHS trust turnover (%)’,90 revealed that T4 and T5 portrayed a more severe degree of deficit, 17.8% (2009/10) at T4 and 27.2% (2009/10) at T5, than other trusts, which showed deficits < 10% of turnover. This was echoed in the interviews with T4 and T5 respondents during phase 1. Moreover, finance as a newly emerged priority was prevalent in the interviews with respondents from T3. Our findings from phase 1 were consistent with the secondary sources.
The continuity of leadership is also a key factor when considering the extent of inner shocks. T5 and T9 enjoyed the continuing leadership of both the CEO and the DIPC during the observed period, whereas T2, T3 and T6 experienced changes of both leaders (CEO and DIPC). The remaining trusts (T1, T4 and T7) had a moderate inner shock, experiencing changes of either the CEO or the DIPC.
Health profile indicators appeared to be associated with the location of each trust. Trusts within central/north England had poorer local population health profiles than their counterparts in south England and London. 89 The health profile indicators were reviewed for changes over recent years (2008–12). We attempted to interpret this as part of the outer shock/constraint in the local area(s) where each trust operated.
According to the overall ratings for each trust, T1, T3 and T4 experienced relatively severe inner and outer shocks, whereas T6 and T7 experienced much milder impacts. T2, T5 and T9 experienced moderate shocks and crises during the observed period.
There are some caveats when interpreting the data in Table 6 . First, treating each indicator in Table 6 equally can be problematic. There is a risk of underestimating a possible tremendous effect: for example, the recent merger in T1, which is currently operating five hospitals across three major hospital sites, may have varying short-term and long-term shocks. A merger of an organisation is a disruptive process, especially when constituent organisations have held strong individual identities and pride. There is a need to consolidate structural and cultural differences, political capital and power clashes. It is also a time-consuming process. For example, T5 underwent a merger in the early 2000s and, during the study period, found itself in a situation in which it had been freed from ‘demarcation’ only recently. One respondent suggested:
A lot of it (demarcation) is broken down now. When the XXX hospital trust merged with the YYY hospital trust in [. . . ], it has taken us probably 8 or 9 years to break down our organisational roles.
T5M3 – non-clinical manager
Second, a combination of certain crises might have an exponential effect rather than simply being aggregated. Third, how quickly the changes in health profile indicators might have an impact on the trust at an organisational level is unknown. Moreover, the Network of Public Health Observatories warns us that ‘a green circle [better than England average] may still indicate an important public health problem’ in its health summary chart for each geographical area. 89 Nevertheless, one can say that each category within the table, to a large extent, illuminates the degree of shocks, critical events and crises internally (within the trust) and externally (in the local areas), and the overall rating can give us a flavour of recent changes of inner (in terms of structural, financial, managerial) and outer (local populations) contextual conditions where each trust has been operating.
Research activity
To assess the research activity domain at each trust, we selected five aspects based on secondary data: (a) university affiliation [with medical and/or nursing school(s)]; (b) formal research organisational structure that looked at the existence of research and innovation divisions, BRC, BRUs, AHSC and other research-related organisational structures; (c) research-supporting infrastructure and research initiatives, such as research forums, tools, networks, research training and access to electronic resources; (d) intensity of research activity reported, as captured by the number of clinical research studies, the number of studies supported by the National Institute for Health Research (NIHR), the number of Research Passports issued and the number of publications; and (e) IPC-related evaluations or trials reported by the trust, for example, a trial of the silver alloy urinary catheter.
The figures in Table 7 show the aggregated number of the data: for example, we counted ‘1’ for ‘formal research organisational structure’ if the trust reported its AHSC status. Regarding ‘intensity of research activity reported’, all the data were not always available from all the trusts, but we attempted to compare the possible maximum available indicators across the trusts.
| Trust | [a] | [b] | [c] | [d] | [e] | Overall rating (total score) |
|---|---|---|---|---|---|---|
| T1 | Medical | 29 | 29 | 6 | 11 | ††† (1 + 3 + 2 + 2 + 3 = 11) |
| T2 | Medical | 17 | 37 | 3 | 3 | †† (1 + 2 + 2 + 2 + 1 = 8) |
| T3 | Medical | 5 | 43 | 1 | 2 | †† (1 + 1 + 3 + 1 + 1 = 7) |
| T4 | N/A | 1 | 24 | 3 | 1 | † (0 + 1 + 1 + 2 + 1 = 5) |
| T5 | Medical | 14 | 48 | 8 | 10 | ††† (1 + 2 + 3 + 3 + 3 = 12) |
| T6 | N/A | 3 | 19 | 0 | 1 | † (0 + 1 + 1 + 0 + 1 = 3) |
| T7 | Medical; nursing | 14 | 45 | 7 | 7 | ††† (2 + 2 + 3 + 3 + 2 = 12) |
| T9 | Medical | 10 | 40 | 2 | 5 | †† (1 + 2 + 3 + 1 + 2 = 9) |
| Key | [a] | [b] | [c] | [d] | [e] | Overall rating |
| Scored 2 when it was affiliated to both medical and nursing schools | Scored 3 when the aggregated number was 21–29 | Scored 3 when the aggregated number was 39–48 | Scored 3 when the score was 7–8: a greater number of relevant activities was reported | Scored 3 when the aggregated number was 9–11 | Assigned ††† when the total score was 10–12: a greater number of research activities | |
| Scored 1 when it was affiliated to either medical or nursing school | Scored 2 when the aggregated number was 10–20 | Scored 2 when the aggregated number was 29–38 | Scored 2 when the score was 3–6: moderate number of relevant activities was reported | Scored 2 when the aggregated number was 4–8 | Assigned †† when the total score was 6–9: moderate number of research activities | |
| Scored 0 when it had no university affiliation | Scored 1 when the aggregated number was 1–9 | Scored 1 when the aggregated number was 19–28 | Scored 1 when the score was 1–2: fewer relevant activities were reported | Scored 1 when the aggregated number was 1–3 | Assigned † when the total score was 3–5: fewer research activities | |
| No. of clinical research studies (2009/10) | No. of studies supported by NIHR (2009/10) | No. of Research Passports issued (2009/10) | No. of publications | [d] | ||
| Scored 3 if the number was 594–870 | Scored 3 if the number was 214–250 | Scored 3 if the number was 81–110 | Scored 3 if the number was 1948–2870 | [d] refers to the aggregated score from all four elements of research activities (no. of clinical research studies; no. of studies supported by the NIHR; no. of Research Passports issued; and no. of publications) reported by each trust | ||
| Scored 2 if the number was 317–593 | Scored 2 if the number was 177–213 | Scored 2 if the number was 50–80 | Scored 2 if the number was 1023–1947 | |||
| Scored 1 if the number was 40–316 | Scored 1 if the number was 140–176 | Scored 1 if the number was 20–49 | Scored 1 if the number was 100–1022 | |||
| Scored 0 if no data were available | Scored 0 if no data were available | Scored 0 if no data were available | Scored 0 if no data were available |
Within this chapter, the quantitative data set for each variable was divided into three groups according to tertile distribution. The cut-off points in each scoring table or ‘the formula’ under each table were calculated based on this approach and each group was given a score accordingly. If it was infeasible to give the equal scale to these three groups, slightly more weight was usually given to the middle tertile and the upper and bottom tertiles were always given equal weight. These scores were aggregated per trust in each domain in order to obtain an overall rating in a similar fashion to scoring through the application of the tertile distribution. For example, when the total scores for all trusts in the ‘Research activity’ domain ran from 3 to 12, we assigned ‘†’ to the bottom tertile (3–5) reflecting ‘fewer research activities’, ‘††’ to the middle tertile (6–9) indicating a ‘moderate number of research activities’ and ‘†††’ to the upper tertile (10–12) reflecting ‘a greater number of research activities’.
There is a significant difference between two clusters of trusts: university-affiliated trusts and non-affiliated trusts. The latter (T4 and T6) were less research orientated, reporting fewer research activities. Among the university-affiliated trusts, T1, T5 and T7 seem to have taken a particularly strong research stride, confirmed through the majority of aspects observed. The fact that T1, T5 and T9 were Showcase Hospital trusts appeared to reflect the higher number of IPC-related evaluations reported by the trust.
Table 7 does not capture the transient nature of formal research structures, and does not include the latest status of BRCs and BRUs, which were renewed in 2012 (see Table 1 in Chapter 3 ). The majority of trusts maintained their status, but T7 lost both BRC and BRU status in 2012. No major research structural change was witnessed at T3, T4 or T6 during the same period. Whereas T3 had a quasiresearch structure, T4 and T6 did not have BRC or BRU status at any point during the study period.
In phase 1 the respondents were asked about research involvement and experiences. With regard to conducting research themselves, a number of prompts/triggers were identified by the respondents – the most cited were as follows: felt the need to improve services or update practice, which was cited by one-third of respondents; this was followed by identification of (recurring) problems, or a gap in knowledge, or problem solving, cited by one-quarter of respondents; and better patient outcomes, benefits, experiences and/or safety, to a lesser degree. Finance as a trigger (i.e. cost-saving, value for money, cost-effectiveness) was considered by only a few respondents in the non-clinical managers and nursing managers groups. Organisational role also featured:
I think again it depends on what role they’re in. For me it’s about improving the patient care and patient flow, so that would be my, you know, that would prompt me to find information, but for others it may be how to run a more cost effective endoscopy service or something like that. So it depends on the individual and what they’re managing.
T6M8 – nurse
[. . .] we do, do a lot of research here and we for example may say. I mean it rarely comes from the management team to be honest. A lot are clinical and clinically driven.
T7M5 – nurse
Other important themes were related to sensemaking for self and others: including verification or validation of existing practice (i.e. verifying something unfamiliar, validating evidence, for proving one’s own (others’) practice, and value judgement); supporting or justifying decisions on the introduction of certain innovations; building up an evidence base for proving the effectiveness/benefits of the innovation; or challenging the product introduced.
The verification of existing practice often requires critical thinking and an inquiring mind in selecting the optimum treatment or innovation as an individual/team. This approach was highlighted by the following respondent, who echoed also the temporal nature of evidence discussed in Chapter 4 :
Either because a new thing has been developed and evidence is needed either way, it is a good or bad thing, does it work, is it effective, or to answer the things we do on a daily basis, we always do it this way, why? I think we are getting better in health care in asking why, challenging ourselves and challenging kind of assumptions we had for long time. H.[elicobacter] pylori causes ulcers, we know that now – 10 to 20 years ago it was stress. You know it is never assuming things and constantly questioning and developing a hypothesis and testing it.
T3M2 – nurse
[. . .] I think it is just an inquiring mind, people want to know or test out and then therefore get the best treatment, so that they can feel assured that they’re doing the right treatment or intervention.
T7M10 – allied health professional
I don’t know [. . .] what makes other people do it. What would make me want to do it is if the evidence isn’t already out there and strong and you’ve worked on historical practice for so long it would be actually nice to know scientifically whether what you are doing is making a difference. Ultimately it’s around patients’ benefits but I think for me, there is a big gap particularly in infection prevention control where we have historically done things one way but there isn’t the evidence to support that one way or another and I think effective practice making practice meaningful I think there needs to be a lot more in that.
T6M4 – nurse
In relation to patient-centred drivers as a source of motivation, T2 and T3 cited this above other drivers, which triangulates with espoused organisational culture and vision. The patient-centred element was more evident among nurses from T2 and T3 (a half of respondents from each trust) than their counterparts (e.g. one-third of respondents from T7 and approximately one-quarter of respondents from T9). Based on secondary data analysis, T2 and T3 were beacons for patient safety – these were the only trusts among our sample to have visited and directly observed international leaders and pioneers of patient safety models in the USA. Further, documentary analysis revealed the successful transfer of lessons to local management practices at hospital and ward level through the development of a safe and supportive culture, and changes in organisational structure and practices.
Innovation activity
This section examines how ‘innovative’ each trust has been, and more specifically how many IPC-related innovations have been reported by the trust over recent years. The figures within Table 8 show the aggregated number of the data based on trust-specific secondary sources: for example, we counted ‘1’ for the first column, [a], if the trust reported IPC- or patient-safety-related innovations that were developed by the trusts themselves, seemingly reflecting local innovation capacity. Similarly, we counted ‘1’ for the second column, [b], if the trust reported the adoption of innovations by using terms such as ‘the first in the UK’ or ‘cutting edge’, ‘ground breaking’ or similar. This has an implication of organisational willingness to become a front runner when it comes to adopting relatively less-established innovations, seemingly reflecting pro-first organisational culture.
| Trust | [a] No. of locally developed IPC-/patient-safety-related innovations | [b] No. of innovations reported as ‘the first in the UK’; ‘cutting edge’ | Overall rating (total score) |
|---|---|---|---|
| T1 | 10 | 13 | ††† (2 + 3 = 5) |
| T2 | 9 | 3 | †† (2 + 1 = 3) |
| T3 | 12 | 10 | ††† (3 + 3 = 6) |
| T4 | 7 | 4 | † (1 + 1 = 2) |
| T5 | 9 | 5 | †† (2 + 2 = 4) |
| T6 | 8 | 1 | † (1 + 1 = 2) |
| T7 | 12 | 7 | ††† (3 + 2 = 5) |
| T9 | 7 | 8 | †† (1 + 2 = 3) |
| Key | [a] | [b] | Overall rating |
| Scored 3 when the aggregated number was 11–12 | Scored 3 when the aggregated number was 10–13 | Assigned ‘†††’ when the total score was 5–6: a greater number of activities that reflect local innovation capacity and pro-first culture | |
| Scored 2 when the aggregated number was 9–10 | Scored 2 when the aggregated number was 5–9 | Assigned ‘††’ when the total score was 3–4: moderate number of activities that reflect local innovation capacity and pro-first culture | |
| Scored 1 when the aggregated number was 7–8 | Scored 1 when the aggregated number was 1–4 | Assigned ‘†’ when the total score was 2: fewer activities that reflect local innovation capacity and pro-first culture |
Based on the above quantitative results, T1, T3 and T7 reported a greater number of activities, seemingly reflecting a greater level of local innovation capacity and pro-first culture during the observed period, and respondents who were aware of this organisational identity:
[. . .] the fact that it [Bioquell vapour hydrogen peroxide (VHP) Room Bio-Decontamination Service (RBDS)] is innovative and we are one of the first trusts in the country to get on board with it, actually quite appeals to the execs because they can then say take a look at us, we’re ahead of the game and make them feel quite proud of themselves, in that respect.
T3M2 – nurse
We can say that these trusts were eager to be perceived as ‘the first’ or ‘cutting-edge’ organisations, willing to take a risk in becoming ‘the first of its kind’; therefore, they have demonstrated an open-minded, pro-change organisational culture. On the other hand, based on the above quantitative results, T4 and T6 reported fewer relevant activities; this might reflect a less proactive attitude towards something very new. T2, T5 and T9 showed a moderate attitude towards developing innovation locally as well as introducing ‘cutting edge’ or relatively less-established innovation imported from outside the organisation. Our primary data (interviews) captured what was evident through secondary sources for T9:
It’s one of degrees, as I say, if we were way behind the ball and someone came back from another trust and say, ‘They’re doing this, why aren’t we doing it?’, then that, you then have perhaps a formal expectation to develop that particular aspect but [. . .] the assumption is that we are at the leading edge for most things or we’ve actively made a decision that we’re not going to be at the leading edge because either it doesn’t suit us or whatever. [. . .] there are no doubt some things we’re at the trailing edge but again the expectation is that that is an active decision that we’re deliberately holding back because we’ve made an assessment and we see some risks associated with it or it doesn’t fit with the overall strategy of the trust.
T9M2 – doctor
The above respondent at T9 reported a risk averse attitude towards introducing ‘cutting-edge’ innovation and his or her decision-making was rather inclined to the ‘active’ rational paradigm. Based on the secondary data analysis, T1 showed a strong organisational appetite to take a risk in the innovation field, whereas T6 seemed to be a risk averse organisation.
We will now look at the number and the type of IPC-related innovations reported by the trusts. We also compare the number of innovations with the number of staff for each trust ( Table 9 ). The innovations identified here are a wide range of IPC interventions or interventions that might directly or indirectly affect IPC. These were reported by the trusts themselves. They ranged from small to large and from technological/technical/product to organisational/administrative/programmatic to process/protocol types of innovations. Based on trust-specific secondary data, the innovation activities and innovation types were analysed for each trust. The sources included the following: trusts’ annual reports and financial accounts (2007/08, 2008/09, 2009/10, 2010/11, 2011/12), trusts’ quality accounts reports (2009/10), DIPC reports (when available), trust board meeting minutes (when available), staff magazines, newsletters, bulletins and other publicly available materials (between 2007/08 and spring 2011).
| Trust | Organisational/administrative/programmatic innovations | Technical/technological/product innovations | Process/protocol innovations | Total number of innovations reported | No. of staff (average between 2007/08 and 2010/11) | Innovations relative to size (no. of innovations/100 staff) | Rating based on ‘Total number of innovations reported’ (total score) |
|---|---|---|---|---|---|---|---|
| T1 | 86 | 46 | 90 | 101 | 9793 | 1.03 | † (1) |
| T2 | 125 | 51 | 128 | 160 | 5690 | 2.81 | ††† (3) |
| T3 | 100 | 46 | 109 | 125 | 6175 | 2.02 | †† (2) |
| T4 | 75 | 26 | 77 | 87 | 6407a | 1.36 | † (1) |
| T5 | 84 | 29 | 76 | 86 | 8831 | 0.97 | † (1) |
| T6 | 126 | 45 | 133 | 156 | 4133 | 3.77 | ††† (3) |
| T7 | 145 | 50 | 134 | 165 | 12,356 | 1.34 | ††† (3) |
| T9 | 69 | 26 | 72 | 84 | 7308 | 1.15 | † (1) |
| Key | Total number of innovations reported | Rating based on ‘total number of innovations reported’ | |||||
| Scored 3 when the number was 139–165 | Assigned ‘†††’ when the score was 3: a greater number of innovations reported by the trust | ||||||
| Scored 2 when the number was 111–138 | Assigned ‘††’ when the score was 2: moderate number of innovations reported by the trust | ||||||
| Scored 1 when the number was 84–110 | Assigned ‘†’ when the score was 1: fewer innovations reported by the trust |
The figures within Table 9 show the aggregated number of the data based on these trust-specific secondary sources: for example, we counted ‘1’ for ‘process/protocol innovations’ if the trust reported the introduction of a new IPC training scheme, as it falls into the category of process innovation. Similarly, we counted ‘1’ for ‘organisational/administrative/programmatic innovations’ if the trust reported the appointment of new IPC staff (i.e. a medical IPC lead, antibiotic prescribing pharmacists, infection prevention link nurses, etc.), as it falls into the category of organisational/administrative innovation. Some innovations were identified as cross-cutting or multifaceted innovations: for example, we considered a Bioquell VHP RBDS (Bioquell UK Ltd, Andover, UK) at T5 as a multifaceted innovation within all three categories. It is a product technology; its evaluation was initiated as part of the Department of Health’s Showcase Hospitals programme; and the innovation process involved collaboration with the trust’s domestic services partner.
The number of innovations reported implies the openness or transparency of the organisation in terms of information sharing, and also a desire to demonstrate to others a pro-innovation culture. T2, T6 and T7 were stronger in this aspect, whereas T1, T4, T5 and T9 were weaker ( Figure 5 ).
FIGURE 5.
Total number of innovations reported 2007/08–spring 2011.
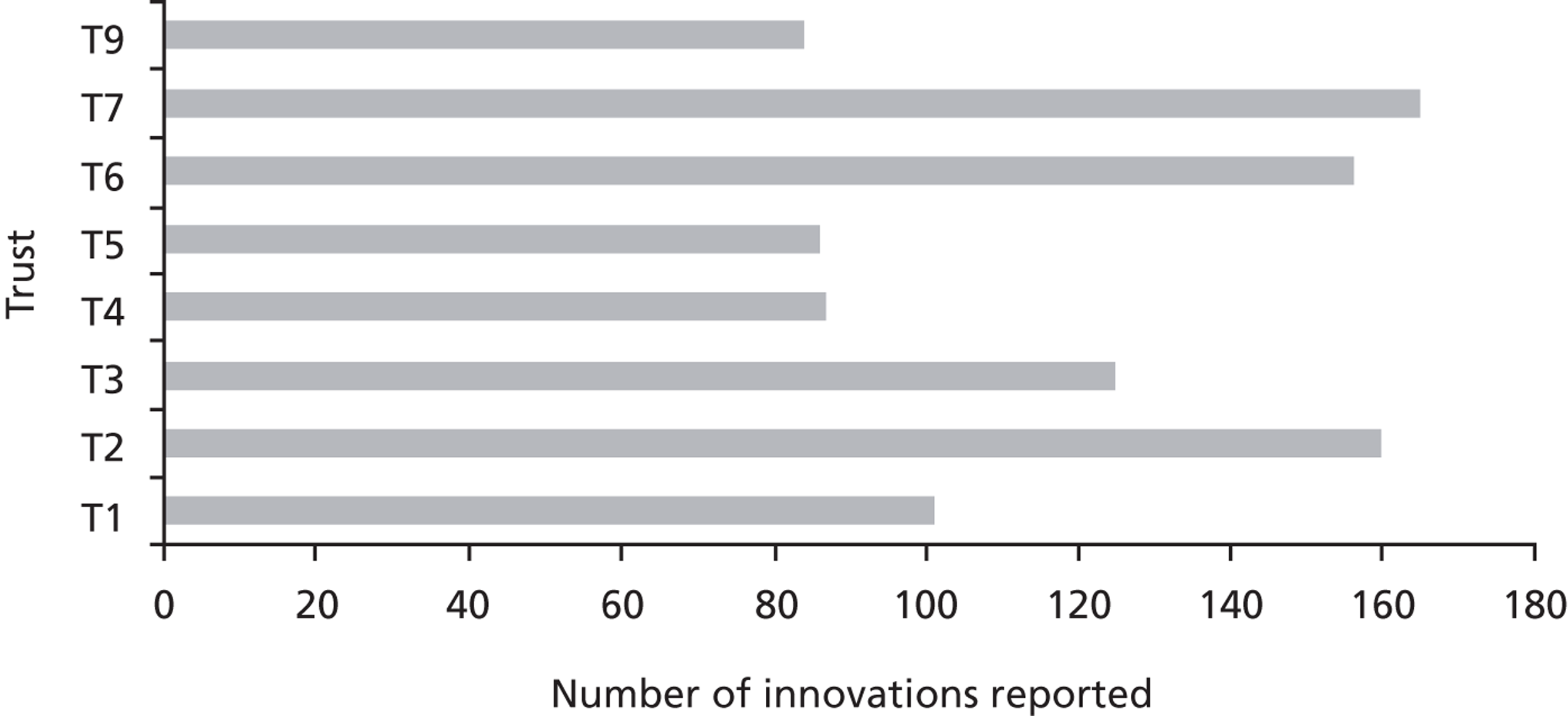
In relation to the type of innovation, all trusts tended to focus on ‘organisational/administrative/programmatic’ and ‘process/protocol’ rather than ‘technological/technical/product’ innovations ( Figure 6 ).
FIGURE 6.
Types of innovations reported by each trust 2007/08–spring 2011.
The data that include termination following adoption decisions or implementation of an innovation are not often disseminated in the public domain. It is therefore difficult to assess the degree of sustainable implementation of innovations from secondary sources.
The results of the comparison concerning the number of innovations relative to size (no. of innovations reported/no. of staff) implied the importance of organisation size ( Figure 7 ). As demonstrated by a U-shaped curve here, relatively extreme trusts, in terms of size (either smaller or larger), reported more innovations. On the other hand, moderate-sized trusts reported much fewer innovations during the observed period.
FIGURE 7.
Relationship between ‘number of staff (average 2007/08–2010/11)’ and ‘number of innovations reported (2007/08–spring 2011)’.
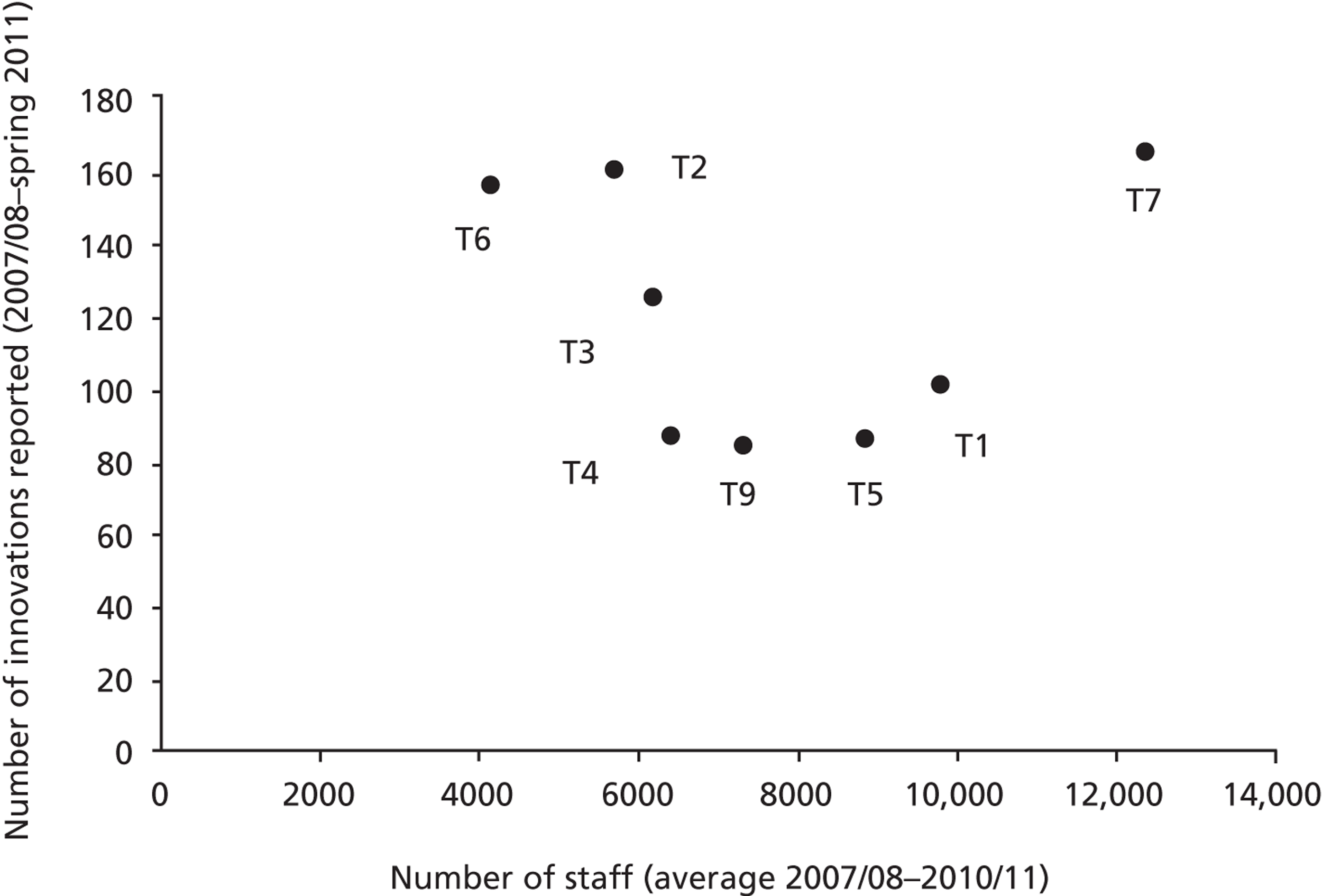
Communication: internal/external
We will now assess the communication domain at each trust, based on secondary sources that were reported from 2007/08 to spring 2011. To examine this domain, we selected four aspects: (1) communication channels, including newsletters, magazines, events, briefings and leadership walk rounds; (2) patient and public involvement initiatives, and related infrastructure; (3) linkage with regional, national and international IPC/patient safety initiatives; and (4) IPC/patient safety training or awareness-raising events ( Table 10 ). The figures in Table 10 show the aggregated number of data; for example, we counted ‘1’ for ‘communication channels’ if there was a regular team brief within that organisation.
| Trust | [a] | [b] | [c] | [d] | Overall rating (total score) |
|---|---|---|---|---|---|
| T1 | 7 | 5 | 14 | 18 | † (1 + 1 + 1 + 2 = 5) |
| T2 | 7 | 6 | 39 | 17 | †† (1 + 1 + 3 + 2 = 7) |
| T3 | 12 | 15 | 35 | 24 | ††† (2 + 2 + 3 + 3 = 10) |
| T4 | 6 | 12 | 25 | 13 | †† (1 + 2 + 2 + 2 = 7) |
| T5 | 4 | 2 | 19 | 12 | † (1 + 1+ 1+ 1 = 4) |
| T6 | 10 | 8 | 30 | 19 | †† (2 + 1 + 2 + 2 = 7) |
| T7 | 13 | 28 | 32 | 8 | ††† (2 + 3 + 3 + 1 = 9) |
| T9 | 19 | 18 | 30 | 14 | ††† (3 + 2 + 2 + 2 = 9) |
| Key | [a] | [b] | [c] | [d] | Overall rating |
| Scored 3 when the aggregated number was 15–19 | Scored 3 when the aggregated number was 20–28 | Scored 3 when the aggregated number was 32–39 | Scored 3 when the aggregated number was 20–24 | Assigned ‘†††’ when the total score was 9–10: higher level of communication | |
| Scored 2 when the aggregated number was 9–14 | Scored 2 when the aggregated number was 11–19 | Scored 2 when the aggregated number was 22–31 | Scored 2 when the aggregated number was 13–19 | Assigned ‘††’ when the total score was 6–8: moderate level of communication | |
| Scored 1 when the aggregated number was 4–8 | Scored 1 when the aggregated number was 2–10 | Scored 1 when the aggregated number was 14–21 | Scored 1 when the aggregated number was 8–12 | Assigned ‘†’ when the total score was 4–5: lower level of communication |
Table 10 reflects the extent to which internal and external communication was reported by each trust during the observed period. Communication channels, [a], and IPC/patient safety training and awareness-raising events, [d], can be considered as internal communication, and the rest as external. However, data within the table do not take account of the quality of the communication channels or of informal communication channels.
The overall ratings in Table 10 show that T1 and T5 seemed to score relatively low on communication, whereas T3, T7 and T9 reported relatively high numbers of communication-related channels and initiatives and high levels of communication-related infrastructure. Notably, T7 showed a significantly higher number of patient and public involvement initiatives and higher levels of related infrastructure than the remaining trusts; this was also demonstrated by the extremely high number of patients (approximately 20,000) who were recruited to participate in research during 2009/10. A similarly research-strong organisation, T1, recruited approximately 13,000 patients during the same period. In fact, T7 was one of the UK’s first trusts to incorporate the public into research decision-making mechanisms.
Summary
Table 11 shows overall ratings for each domain of contextual factors for the eight ‘macrocases’. To sum up, T7 obtained a better overall rating in all of the domains observed. A greater number of research activities were reported by T1, T5 and T7. Activities that reflect local innovation capacity and a ‘pro-first’ culture were more likely to be seen among T1, T3 and T7. A higher number of internal/external communication-related channels/initiatives/infrastructures was reported by T3, T7 and T9.
| Trust | Trust performance and patient experience | Magnitude of shocks, crises and critical events (inner, outer) | Research activity | Communication (internal, external) | Innovation activity | Trust size/resources (no. of staff – average between 2007/08 and 2010/11) | |
|---|---|---|---|---|---|---|---|
| Local innovation capacity and pro-first culture | Innovation reported by the trust | ||||||
| T1 | † | ††† | ††† | † | ††† | † | 9793 |
| T2 | ††† | †† | †† | †† | †† | ††† | 5690 |
| T3 | †† | ††† | †† | ††† | ††† | †† | 6175 |
| T4 | †† | ††† | † | †† | † | † | 6407a |
| T5 | †† | †† | ††† | † | †† | † | 8831 |
| T6 | †† | † | † | †† | † | ††† | 4133 |
| T7 | ††† | † | ††† | ††† | ††† | ††† | 12,356 |
| T9 | †† | †† | †† | ††† | †† | † | 7308 |
| Better overall rating in performance/activity/communication, or relatively low level of internal/external shocks. | |||||||
| Moderate level of overall rating in performance/activity/communication, or moderate level of internal/external shocks. | |||||||
| Worse overall rating in performance/activity/communication, or relatively high level of internal/external shocks. | |||||||
Being research-orientated, having a greater local innovation capacity and a ‘pro-first’ culture, and/or a greater number of internal/external communication-related initiatives/channels did not seem to have played an important role in increasing the number of reported innovations. This was demonstrated in T6, where such attributes were at a lower level than at other trusts. Relatively extreme trusts, in terms of size (either smaller, such as T6 and T2, or larger, such as T7), reported more innovations. Hence, in our organisational sample the trust size was a prominent factor in this regard. Limitations in the classifications and clustering of cases presented in this chapter relate to our reliance upon self-reported data for a number of organisational context dimensions, and the unavoidably reductionist approach we used in analysing and reporting on the large data set at hand.
Chapter 7 Evidence in action: technology products overview and typology
In this chapter we discuss the results of our formal investigation of 18 environmental hygiene technology products. We do this by using interview data from phase 2 and complementary secondary data on product-supporting evidence for efficacy and cost.
We start by presenting an overview of the selected products. We purposefully sampled for three technology product examples in each of the eight trusts fulfilling the following criteria: (1) one technology product currently being considered for adoption, (2) one technology product successfully adopted and implemented, and (3) one technology product rejected or discontinued after initial adoption. All products empirically examined concerned the period 2007–2012. In this chapter we also provide a typology of these 18 products, distinguishing among three important dimensions: (1) expected budgetary impact, (2) perceived impact on practice, and (3) evidence strength on efficacy.
Technology products overview
This section offers an overview and typology of the environmental hygiene technology products studied in each of the trusts. Detailed accounts of their journeys in the eight trusts participating in phase 2 are included.
Overall, 18 environmental hygiene technology products based on 15 unique technologies have been reviewed in a total of 27 individual technology product journeys in the eight trusts. These 18 products constitute tracers for studying the use of evidence in support of the innovation adoption process in each organisation. These products were recommended by participating trusts’ staff to the research team as examples of innovative technologies considered for adoption since 2007, and can be categorised into:
-
Liquid cleaning technology products (three): DIFFICIL-S® (Clinimax Ltd, Bury St Edmunds, UK); Chlor-Clean soluble tablets (Guest Medical Ltd, Aylesford, UK); and Virusolve+® (Amity International, Barnsley, UK).
-
Wipe cleaning technology products (four): clinell® universal sanitising wipes (non-sporicidal, green wipes) (GAMA Healthcare Ltd, London); clinell® sporicidal wipes (red wipes) (GAMA Healthcare); clinell® alcoholic 2% chlorhexidine wipes (GAMA Healthcare); and PDI® Sani-Cloth® CHG 2% alcoholic chlorhexidine gluconate wipes (PDI Inc., Orangeburg, NY, USA).
-
Inspection technology products (four): 3M™ Clean-Trace™ NG luminometer (3M United Kingdom plc, Bracknell, UK); Hygiena SystemSURE II adenosine triphosphate (ATP) hygiene monitoring system (Hygiena, Camarillo, CA, USA); UV (ultraviolet) light inspection torch (UV Light Technology Ltd, Birmingham, UK); and DaRo UV light inspection cabinet (DaRo UV Systems Ltd, Sudbury, UK).
-
Decontamination technology products (five): Bioquell VHP RBDS; Steris BioGenie VHP decontamination system (STERIS Corporation, Mentor, OH); Advanced Sterilisation Products (ASP®) GLOSAIR™ 400 aerosolised hydrogen peroxide (aHP) system (ASP c/o Johnson & Johnson Medical Ltd, Wokingham, UK); JLA OTEX® laundry system (JLA Ltd, Ripponden, West Yorkshire, UK); and Medixair™ and Medixair™ Meos UV air sterilisation units (Pathogen Solutions Ltd, Solihull, UK).
-
Other infection prevention products with no anti-infective agent (two): Design Bugs Out (DBO) commode (The Kirton Healthcare Group Ltd, Haverhill, UK); and disposable sterile surgical site gowns.
Table 12 shows the different technology products examined in each of the trusts. They have been listed in columns representing the outcome for each at the time of data collection (April 2011–July 2012), with the number in parentheses denoting each individual technology product microcase. Only one example, the Bioquell VHP RBDS, features in all three columns.
| Trust | Products under consideration | Successfully implemented products | Rejected products |
|---|---|---|---|
| T1 | (One) DBO commode | (Two) clinell universal sanitising wipes (2% chlorhexidine, C22H30Cl2N10, 70% ethanol-based alcohol) | (Three) Bioquell VHP RBDS (30% w/w aqueous hydrogen peroxide, H2O2) |
| T2 | (Four) disposable sterile surgical site gowns | (Five) ASP GLOSAIR 400 aHP system (5–6% hydrogen peroxide solution, released in aerosol form) | (Seven) UV Light Technology Ltd inspection torch |
| (Six) 3M Clean-Trace NG luminometer (based on ATP measurement) | |||
| T3 | (Eight) Bioquell VHP RBDS (30% w/w aqueous hydrogen peroxide, H2O2) | (Nine) clinell sporicidal wipes (peracetic acid, CH3CO3H) | (10) Medixair and Medixair Meos UV air sterilisation units |
| T4 | (11) Clinimax DIFFICIL-S (chlorine dioxide, ClO2) | (12) Bioquell VHP RBDS Steris BioGenie VHP system (30% w/w aqueous hydrogen peroxide, H2O2) | (13) Virusolve+ liquid (dodecylamine-based substances, biodegradable detergent) |
| T5 | (14) Bioquell VHP RBDS (30% w/w aqueous hydrogen peroxide, H2O2) | (15) Chlor-Clean tablets (sodium dichloroisocyanurate, C3Cl2N3NaO3) | (16) 3M Clean-Trace NG luminometer, Hygiena SystemSURE II ATP hygiene monitoring system (based on ATP measurement) |
| T6 | (17) Bioquell VHP RBDS (30% w/w aqueous hydrogen peroxide, H2O2) | (18) JLA OTEX laundry system (ozone, O3) | (19) Medixair and Medixair Meos UV air sterilisation units |
| T7 | (20) Clinimax DIFFICIL-S (chlorine dioxide, ClO2) | (21) ASP GLOSAIR 400 aHP system (5–6% hydrogen peroxide solution, released in aerosol) | (22) UV Light Technology Ltd inspection torch |
| (23) UV Light Technology Ltd hand inspection unit | |||
| T9 | (24) clinell alcoholic wipes and PDI Sani-Cloth wipes (2% chlorhexidine, C22H30Cl2N10, 70% ethanol-based alcohol) | (25) clinell sporicidal wipes (red) (peracetic acid CH3CO3H) | (27) Bioquell VHP RBDS (30% w/w aqueous hydrogen peroxide, H2O2) |
| (26) JLA OTEX laundry system (ozone, O3) |
Typology of technologies
This section offers a typology of the environmental hygiene technology products in terms of (1) expected budgetary impact for trusts, (2) perceived impact on practice for trust staff, and (3) evidence strength on efficacy.
The budget and practice impact classifications have been based on trust staff perceptions of product attributes elicited during phase 2. This is in line with qualitative methods used in health technology assessment deployed within the context of the health-care organisation to rigorously examine ‘the objective material conditions; the actors’ prior knowledge, values and experience; and the actors’ working definitions of what kind of event they were engaged in and the ways in which conditions, knowledge, values and experience were relevant’ (p. 46). 91 This approach connects seamlessly with sensemaking theory, and incorporates the innovation studies concepts of compatibility, which is reflected in the perceived budget and practice impact, relative advantage, which is linked to budget impact and evidence strength on efficacy, and complexity, reflected in practice impact.
In this typology, a specific technology product budget impact is classified as ‘high’, ‘medium’ or ‘low’. These classifications are based on trust staff perceptions of (1) the immediate budgetary impact of each product examined in their trust [unit cost price, financial support available for procurement, cost assumed by private finance initiative (PFI) partner], and (2) sustainability costs.
Classifications for the 18 products across the eight trusts are listed in Table 13 . In addition, Appendix 7: Technology products unit cost price list shows the technology products according to their list unit cost price in descending order, from the most to the least expensive. As part of the Department of Health and the former NHS PASA’s ‘HCAI Technology Innovation Programme Award’ for outstanding contributions to fighting infections 2009’, £150,000 was awarded to trusts T3, T4 and T7 and £50,000 to T2. The trusts were given free reign to use the sum to procure technologies that could help reduce HCAIs (awarded in February 2009 and technologies procured within the following year). The award funding was taken into account in the classification of technologies in terms of expected budgetary impact for these four trusts and for the specific technology products procured using this method. When this funding was reported by the respondents to have played a significant role in the adoption and implementation processes, this is discussed in the individual microcases in Chapter 8 . For example, the technology product ASP GLOSAIR was perceived to have a ‘low’-budget impact in T2 and a ‘high’ budget impact in T7, as T2 used the award funding to procure the specific product.
| Technology product | Trust | Budget impact |
|---|---|---|
| ASP GLOSAIR 400 aHP system | T2 | Low |
| T7 | High | |
| Steris BioGenie VHP system | T4 | Low |
| JLA OTEX laundry system (JLA 40 High Spin machine with OTEX system fitted) | T6 | Low |
| T9 | Low | |
| 3M Clean-Trace NG luminometer (with board and swab rods) | T2 | Medium |
| T5 | Medium | |
| Bioquell VHP RBDS (one hospital room) | T1 | High |
| T3 | Medium | |
| T5 | Medium | |
| T6 | Medium | |
| T9 | High | |
| Medixair and Medixair Meos UV air sterilisation unit | T3 | Low |
| T6 | Low | |
| T7 | Low | |
| UV LIGHT Technologies inspection torch | T2 | Low |
| Hygiena SystemSURE II ATP hygiene monitoring system (with swab rods) | T5 | Medium |
| DBO commode | T1 | Medium |
| DaRo UV light inspection cabinet (with gel and accessories) | T7 | Low |
| DIFFICIL-S disinfectant solution (with mixing vessel and four bottles) | T4 | Low |
| T7 | Low | |
| Virusolve+ | T4 | Low |
| Disposable sterile surgical site gowns (box of 30) | T2 | Medium |
| Clinell universal sanitising wipes (non-sporicidal, six 200-wipe packs) | T1 | Medium |
| Clinell sporicidal (red) wipes (pack of 25) | T3 | Medium |
| T9 | Medium | |
| Clinell alcoholic wipes | T9 | Low |
| Chlor-Clean tablets | T5 | Low |
| PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes (pack of 200) | T9 | Low |
Table 14 groups technology products in terms of their associated impact on practice perceived by the trust staff interviewed. The classification has been put together based on qualitative analysis of phase 2 interviews, and includes five classes:
-
Very low – product or technology same or very similar to existing, established products in the NHS.
-
Low – product has new features (e.g. active ingredient) and is used in the same manner as existing, established products in the NHS: limited staff training required.
-
Medium – product has new features and is used differently from existing, established products in the NHS: staff training required.
-
High – new product or technology with precursor method, product or technology.
-
Very high – new product or technology without precursor.
| Very low | Low | Medium | High | Very high |
|---|---|---|---|---|
| Disposable sterile surgical site gowns | DBO commode | DIFFICIL-S | ASP GLOSAIR 400 aHP system | Medixair UV air sterilisation unit |
| Clinell universal sanitising wipes (non-sporicidal) | Clinell sporicidal (red) wipes | Chlor-Clean tablets | Steris BioGenie VHP system | Medixair Meos UV air sterilisation unit |
| Clinell alcoholic wipes | Virusolve+ | JLA OTEX laundry system | 3M Clean-Trace NG luminometer | |
| PDI Sani-Cloth wipes | Bioquell VHP RBDS | |||
| UV LIGHT Technologies inspection torch | ||||
| Hygiena SystemSURE II ATP hygiene monitoring system | ||||
| DaRo UV light inspection cabinet |
Evidence strength data on the efficacy of products have been collected through secondary research: ‘Efficacy refers to the probability of benefit to individuals in a defined population from a medical technology applied for a given medical problem under ideal conditions of use’ (p. 711). 92 Table 15 illustrates the strength of the evidence base for the efficacy of each product based on (1) a HPA RRP recommendation, (2) an evaluation report published as part of the Department of Health’s HCAI Technology Innovation Programme, Showcase Hospitals programme, or other government-sponsored technology assessment or evaluation programmes, and (3) scientific articles published in peer-reviewed journals. A product of RRP recommendation 1, published scientific articles, and technology assessment evaluation reports where available is classified as an ‘established’ product having a ‘high’ evidence strength on efficacy. Products of RRP recommendation 2 which also feature in published scientific articles or technology assessment evaluation reports are classified as ‘emergent’ products, having ‘medium’ evidence strength on efficacy. All other combinations of (1)–(3) result in an ‘emergent’/’low’ classification for the products concerned.
| Technology product | HPA RRP recommendation | Technology assessment evaluation report (UK/international) | Peer-reviewed journal scientific articles | Evidence strength on efficacy (established/emergent) |
|---|---|---|---|---|
| ASP GLOSAIR 400 aHP system | ✓ RRP3 (2008) | ✓ | Medium, emergent | |
| Steris BioGenie VHP system | ✓ RRP2 (2005) | Low, emergent | ||
| JLA OTEX laundry system | ✓ RRP1 (2012) ✓ RRP2 (2008) |
✓ | ✓ | High, established |
| 3M Clean-Trace NG luminometer | ✓ RRP1 | ✓ (multisite) | ✓ | High, established |
| Bioquell VHP RBDS | ✓ RRP1 (2008) ✓ RRP2 (2004) |
✓ | ✓ | High, established |
| Medixair UV air sterilisation unit | ✓ RRP2 | ✓ | ✓ | Low, emergent |
| UV LIGHT Technologies inspection torch | ✓ | ✓ | Medium, emergent | |
| Hygiena SystemSURE II ATP hygiene monitoring system | ✓ RRP1 (2010) | ✓ | High, established | |
| Medixair Meos UV air sterilisation unit | Low, emergent | |||
| DBO commode | ✓ | Low, emergent | ||
| DaRo UV light inspection cabinet | ✓ | Low, emergent | ||
| DIFFICIL-S | ✓ RRP2 (2009) ✓ RRP3 (2008) |
Low, emergent | ||
| Virusolve+ | ✓ RRP5 | Low, emergent | ||
| Disposable sterile surgical site gowns | ✓ | Low, emergent | ||
| Clinell universal sanitising wipes | ✓ RRP2 | ✓ | Medium, emergent | |
| Clinell sporicidal (red) wipes | Low, emergent | |||
| Chlor-Clean tablets | ✓ | Low, emergent | ||
| Clinell alcoholic wipes | ✓ RRP2 | ✓ | Medium, emergent | |
| PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes | ✓ | Low, emergent |
The HPA RRP may publish one of seven recommendation statements for each particular technology product. Products that obtain RRP1 are understood as having a strong evidence base confirming their efficacy, and are normally considered for fast tracking and inclusion in NHS Procurement and NICE work plans. RRP1 is issued as ‘basic research and development, validation and recent in use evaluations [of the product] have shown benefits that should be available to NHS bodies to include as appropriate in their cleaning, hygiene or infection control protocols’ (p. 5). 73 All of the remaining six recommendations describe different levels of a product’s emergent evidence base on efficacy. [Please see the following page of the HPA portal for a full explanation of the RRP recommendations: www.hpa.org.uk/ProductsServices/MicrobiologyPathology/RapidReviewPanel/rapRecommendations/ (accessed 12 November 2012).]93
Chapter 8 Evidence in action: product microcases in eight NHS trusts
This chapter provides an in-depth presentation of the 27 technology product ‘microcases’ across the eight NHS trusts that participated in phase 2. The reader is alerted to the length of the chapter, which details findings from a sizable study with multiple cases. All microcases follow a standardised format to facilitate cross-comparison. Tables 16 – 41 list the specific evidence types used in each microcase alongside the sources from where these were elicited. Figures 8 – 33 depict the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for each of the technology products. Text in light grey shows the evidence type(s) or stakeholder(s) who were not involved in that substage.
We look at each technology product journey in detail along the three key stages of the innovation process: initiation, adoption decision and implementation. We present the interplay among stakeholders involved in each stage, the associated evidence types and sources debated, and how these were linked to organisational adoption and implementation outcomes. What relevant evidence and knowledge bases have the diverse stakeholders in our case sites accessed, debated, used or rejected? What can be learnt from exploring the relationships among such diverse evidence forms? How does the espoused use of evidence by individual professionals reported in phase 1 compare with collective processes of accessing and using evidence for the adoption and implementation of specific products in the context of an organisation explored in phase 2 and outlined in this chapter?
Trust 1 technology microcases
Microcase 1: Design Bugs Out commode
Attributes perceived by stakeholders
The special design features of the DBO commode were perceived by informants as novel, suggesting that a lot of ‘high-tech’ input went into its design and fabrication. It was perceived to be a ‘low-tech’ piece of equipment and one that comes with a process of how to clean it after use. At the time of its introduction, environmental hygiene had still been an area of concern for T1. However, informants suggest that it was viewed as an opportunity to launch a ‘next-generation’ product. Its main advantages are identified to be (1) its modern look and feel; (2) staff time savings owing to ease and speed of cleaning its surfaces; (3) its capacity to save space by placing commodes onto one stack – easy to move and store; and (4) patient and staff safety in terms of reduced infection risk.
Stakeholders, evidence and decision-making
The Showcase Hospitals programme is understood to have provided the initial idea and support for the adoption of the DBO commode in T1. Evidence was also sourced through seminars and presentations available through the Design Council DBO project. The T1 showcase lead played a ‘championship’ role by providing support to the local trial at every stage.
Key stakeholders involved at the start of the DBO commode trial were the DIPC, senior and junior members of the IPC team, ward and domestic contractor staff (domestic services are outsourced in T1), heads of nursing and clinical leads. The local trial was approved at the trust’s infection prevention committee meeting. During the trial, patient feedback on its use was elicited. The strong interest and commitment shown by the DIPC, along with effective communication of the trial intent and benefits by the IPC team to other stakeholders, are viewed as pivotal in the successful conduct of the trial. Senior nurses engaged positively with the trial process, while the clean, modern look and feel of the product served to complement the IPC team communication with shop-floor stakeholder groups.
Evidence sources accessed during the stages of initiation, adoption decision, and implementation included material available through Showcase Hospitals and the DBO programme ( Table 16 , Figure 8 ). Senior IPC team members also accessed evidence through their professional networks in all three stages. Local evidence, in the form of ‘ongoing’ patient and ward staff feedback, was also directly collected during the trial. Key decision-makers are aware of the importance of the perceived cleanliness of bedside products and care environment in enhancing patient confidence when selecting new products. One respondent highlighted:
I think you’ve got to remember when you’re in the patient environment how it looks is very important to patients and visitors. So if you’ve got different commodes and all look tatty and unclean, or difficult to clean, and even you could have the nicest ward but when you’ve got kit around the bedside that doesn’t match . . . .
Senior nurse
| Evidence sources | Evidence types |
|---|---|
| Showcase Hospitals programme |
|
| DBO programme |
|
| Professional networks |
|
| Trust care environment |
|
FIGURE 8.
T1 DBO commode: professionals’ engagement and evidence types in decision-making.
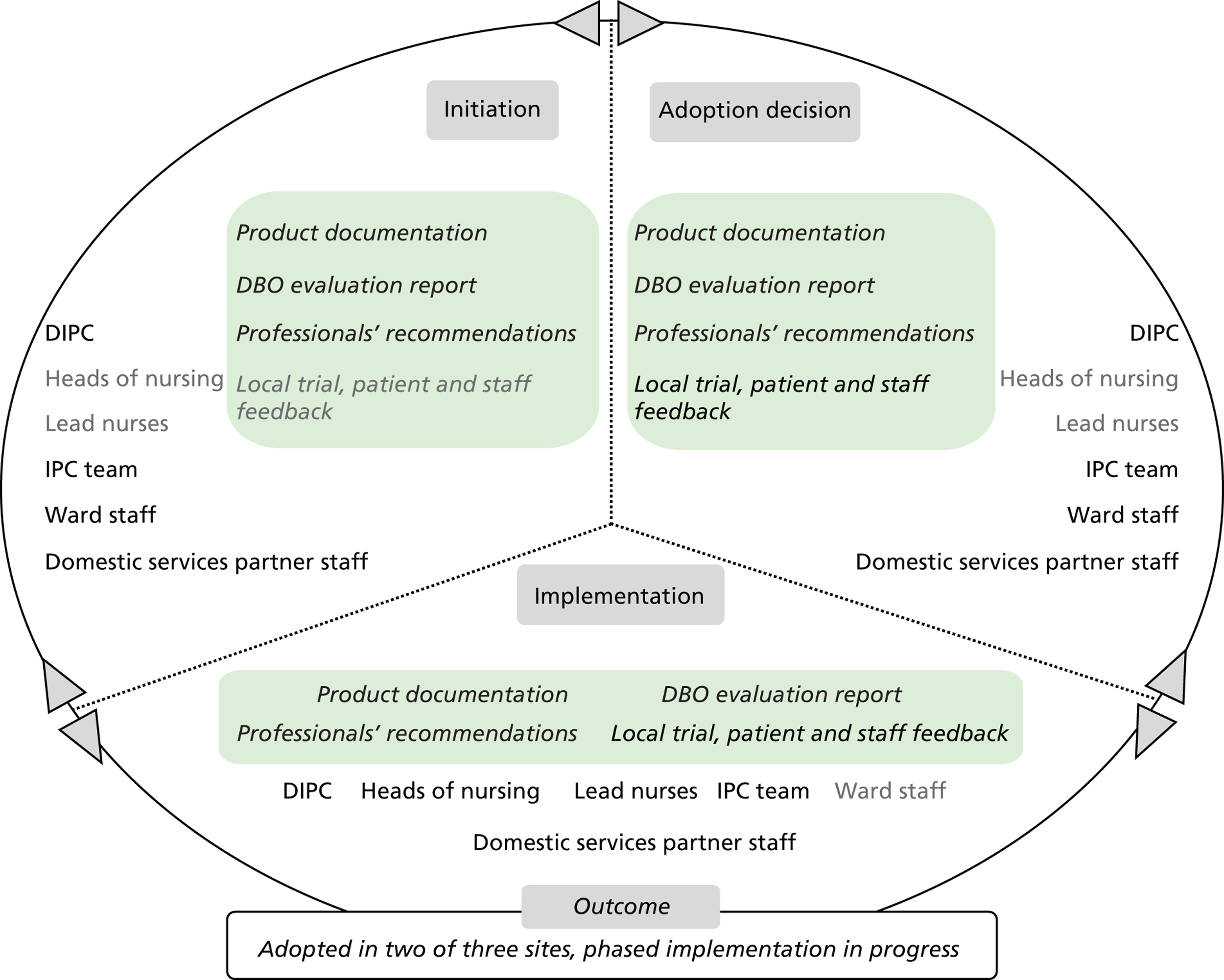
Training materials showing the cleaning process to follow after use of the commode were made available to users.
Informants estimated the innovation adoption process to have lasted a maximum of 2.5 years, including the early supplier involvement phase facilitated by the Department of Health involving discussions among designers, manufacturers and NHS trusts.
Outcome
The outcome was that, following the trial, the DBO commode became available for general purchase in T1 in December 2011. One incompatibility identified prior to the trial was that the commode’s portable pan requires macerator discharge in sluice rooms and only two of the three larger T1 hospital sites have sluice room macerators installed. The product has otherwise generally been considered to be easy to use and in line with the organisation’s culture and values.
Key factors in the commode’s adoption are perceived to be its ease of cleaning, patient comfort and cost neutrality. Sustainability was considered during its design and trial. A requirement shared among external stakeholders and T1 was that the changeover to the new commode had to be cost neutral. Financial pressures have now led the IPC team to collaborate with external stakeholders in assessing product life-cycle cost implications. Some minor damage has been reported to the commodes in the form of, for example, discolouration of the commode arms caused by extensive cleaning and use in the trust care environment.
Microcase 2: clinell universal sanitising wipes
Attributes perceived by stakeholders
This product is perceived as a ‘low-tech’, well-established and validated product. It is also understood as being compatible with the systems, structures and processes underpinning care delivery in the ward environment, as well as with the infection-control-related values and culture of the organisation. The product’s specific advantage is considered to be the time saved compared with using other products and equipment. Its use is simple: staff take it out of the packet, wipe the surface and then dispose of it.
Before the introduction of wipes in T1, cleaning in wards was based on the use of a bucket, a mop and cleaning solution. This solution was harmful to the ward environment and equipment: it was abrasive and would wear down surfaces over time. This cleaning process was also fraught with errors associated with dilution, use of mops, etc. Using a wipe was thought to be a simpler and more effective means of cleaning. Several wipe products had been introduced in the trust. These were withdrawn when it was decided to standardise to and procure one product for use across the trust hospital sites.
Stakeholders, evidence and decision-making
Disinfectant wipes were first introduced in T1 at the start of 2009. Many wipe-based disinfectant products had appeared on the market as general sanitisers or specific to infectious diseases such as C. difficile. As several of these were used in T1 hospitals and the merger of T1’s constituents was progressing, product standardisation emerged as a priority. In this case, the initial focus was on the clinell Sporicidal Wipe. However, a senior IPC team member of nursing background suggested standardising to one universal sanitising wipe across the organisation, gathered the economic and other evidence, and produced a business case for this product.
Visits to other trusts and the knowledge of IPC team members provided a basis for ideation and evidence gathering. Evidence was elicited from three main sources during the initiation, adoption decision and implementation stages: professional networks of individual staff members; peers and colleagues in other trusts; and industry/suppliers ( Table 17 and Figure 9 ). Evidence acted as a facilitator towards adopting this new, innovative cleaning product. Trust members interviewed suggested that their review of the evidence on use in other trusts created a peer pressure effect, as it made them realise that ‘they were behind the times’.
| Evidence sources | Evidence types |
|---|---|
| Trust management |
|
| Industry/suppliers |
|
| Professional networks |
|
| Peers and colleagues |
|
FIGURE 9.
T1 clinell universal wipes: professionals’ engagement and evidence types in decision-making. E&F, estates and facilities; IC, infection control.
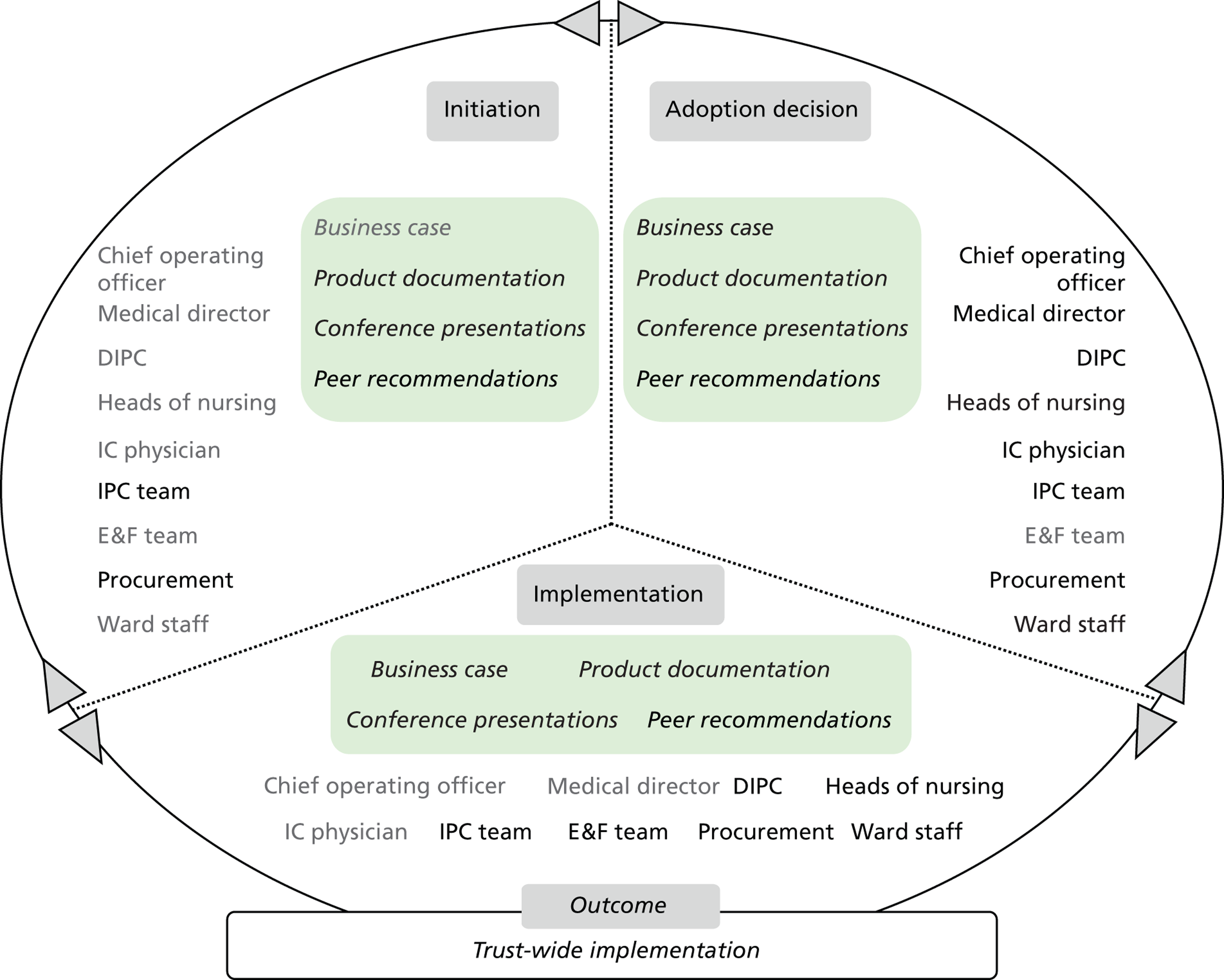
Sustainability was considered in the decision-making process. The procurement department required this product standardisation to be cost neutral or lead to an overall cost reduction. Financial pressures during that time are understood to have been less intense than more recently. The IPC team would later be required to confirm any such change as cost-saving. The selection process itself had a few weeks’ duration.
Pressures and other contextual factors, including high C. difficile infection rates at that time, and the T1 hospitals merger circumstances, triggered a sense of urgency to solve these problems, and created favourable ground for the adoption of a product with low complexity and low cost. One respondent suggested:
I think just at the time of the merger there was just lots going on and this was a quick win and a quick decision really.
Senior nurse
More importantly, external support, including the supplier providing wall mount fittings for the product, was another key facilitating factor in the trust-wide adoption decision across three main sites. One respondent said:
[. . .] clinell® come with wall-dispensers which was a factor so the only consultation with clinell® was whether they could support us to physically come round and fit them all. And so we needed to involve them because they need their maintenance staff to come round and fit it all.
Senior nurse
[. . .] there were points to negotiate and that really came down to fixtures and fittings and did slight changes in areas, slight changes in practice not resistance.
Senior nurse
There was no special project team set up. The product was approved through a committee whose members included all senior stakeholders understood to have a key role by staff members: the DIPC, infection control physician, medical director, chief operating officer. Final approval was granted by the DIPC. No patient views were elicited as part of the process.
The IPC team initially worked with the procurement department to identify a range of products for consideration. Once the product choice was made, other stakeholder groups were invited to take part including the heads of nursing and ward staff. The groups involved in implementation planning included IPC nurses, the heads of nursing, procurement, and estates & facilities department (E&F). The product champion role is identified with the senior IPC nurse who suggested the product initially. IPC nurses were involved in the day-to-day, ward-to-ward implementation of the new cleaning product, working with procurement to streamline supplies, and with E&F to mount new wall racks in wards where wipes are stored and accessed.
Outcome
Some challenges were often heard during early implementation, especially around issues of compatibility with certain equipment and poor engagement with predictable ‘hot spot’ users (i.e. theatres staff), or status quo supporters, who often challenge new practices, processes and/or products as a result of a lack of effective communication. Two respondents commented:
There always is a group of individuals who is saying they’ve done it in this way, this many years, why are we changing?
Senior non-clinical manager
When you make an executive decision about changing products, you could have 99.9% of key stakeholders that are happy and 1% will not be happy. I think most of the people were concerned about it post-implementation. Because as I said what we did was we removed all of the other products that were available through to order and it was kind of an anxiety from particular sets of clinicians and places like theatres that they felt that ought to have alcohol based products to clean their surfaces. They didn’t understand the technology behind it. I suppose on reflection we probably should have communicated that a bit better.
Senior nurse
Nevertheless, a product champion (senior IPC nurse) utilised effective communication channels with senior nurses, including a weekly ‘Back to the Floor Fridays’ meeting, to inform their adoption decision, facilitating a smooth transition from one product to another. Other members of the IPC team followed this paradigm and championed the new product across hospital areas of the trust:
I think to be fair, clinell® [wipes] was something that we all felt very strongly about. We knew it would make life easier on the wards, and we knew that we would make things happen. So I think that is one thing that we can say is that as a team we were probably all championing, and still do when we are doing our audits.
Senior nurse
Standardisation to the new clinell sanitising wipe product was completed in T1 in late 2009. One measure of success identified was ward staff productivity gains in terms of less time needed to clean surfaces. The trust C. difficile rate reduction is perceived by some trust members to be associated with the use of this wipe. However, there has been no formal evaluation to measure and substantiate these claims. Stakeholder groups involved in the innovation adoption decision included IPC nurses, heads of nursing, procurement and ward staff.
Microcase 3: Bioquell vapour hydrogen peroxide Room Bio-Decontamination Service
Attributes perceived by stakeholders
Informants perceive the main attributes of the service to be its efficacy in eliminating pathogens via ‘disinfection’ and ‘decontamination’ of the environment. The service is understood to involve a piece of equipment, a device and a process – operating procedure – and be part of the Showcase Hospitals programme.
The technology is considered to have been novel, ‘high-tech’, at the time of its introduction to the market in 2008/2009. The clear relative advantage, understood as relevant to patients, staff, the trust and the NHS, is the high level of decontamination and hygiene assurance it offers. However, it is thought that it cannot be a substitute for a ‘deep-clean’ process; it is perceived as a complement offering additional assurance, as it also prompts ward staff to conduct a deep clean and de-clutter the ward environment prior to use. The service itself, its operation and its merits are understood as relatively easy to explain to others. However, delivery of the service (portable machine operation, area sealing, etc.) is thought to be quite complex.
Stakeholders, evidence and decision-making
The trust had sporadically used the service during infection outbreaks and viewed the Showcase Hospitals programme as an opportunity to trial a promising environmental hygiene technology. There was also an incentive for the trust to urgently improve C. difficile infection rates at that time. Initially, little resistance was noted by respondents. The trial was supported by the Showcase Hospitals programme and was led by the trust Showcase Hospitals programme team, comprising a project manager and assistant ( Table 18 and Figure 10 ).
| Evidence sources | Evidence types |
|---|---|
| Trust management |
|
| Industry/suppliers |
|
| Showcase Hospitals programme |
|
| Professional networks |
|
| Trust care environment |
|
FIGURE 10.
T1 Bioquell VHP RBDS: professionals’ engagement and evidence types in decision-making.IC, infection control.
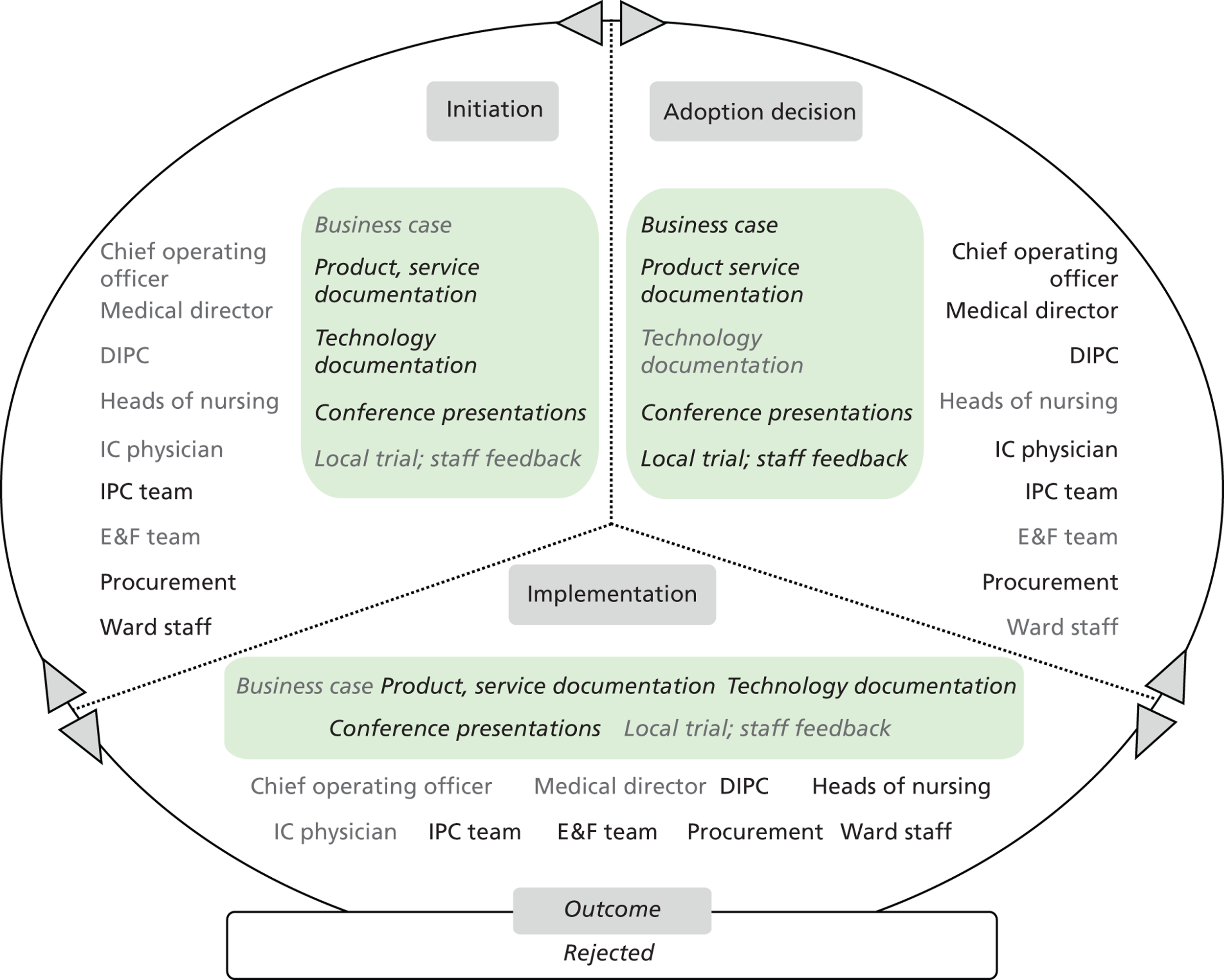
Stakeholder groups involved included the IPC team, E&F, domestic staff, heads of nursing, procurement and clinical leads in areas where the service was trialled. The trial was also on the agenda of two trust C. difficile task force group meetings, which include the medical director and chief operating officer. The showcase lead acted as a champion and promoted use of the technology with enthusiasm.
The service was initially considered as compatible with care structures and systems, as well with T1 values. During the trial the service began to be understood as a complement to other means of infection control, and as a last resort tool of ensuring effective decontamination. This change in perception, coupled with its high cost, resulted in a difference of opinion between clinicians and managers; the former were in favour of its adoption as an effective decontamination means, whereas the latter felt that its routine use came with a high cost.
The service was also understood by some to introduce a time lag between care systems and processes that had to be run concurrently. For example, bays and rooms were becoming available later than anticipated. One respondent highlighted awareness of the complexity of this technology’s use in terms of space and cost (i.e. patient flows, related hidden cost), which seems to have triggered a negative perspective on adoption among the IPC team:
The business case was written but the cost was quite substantial for the organisation. [. . .] when organisations had funding that they could spend but as time went on we thought actually it’s not a good use of funding. So even though the business case was written, almost approved, infection control [team] strongly disagreed with funding a product that they strongly believed would have an impact. [. . .] The C. diff[icile] task force accepted that and partly because actually the use of that product was so complex in terms that you would have to move patients off. Then actually just the cost of all that would have been more than using a piece of equipment. So they were happy with that decision.
Senior non-clinical manager
As the service trial progressed, the two main types of evidence featuring in the decision-making of whether to adopt or reject were (1) the business case prepared by the IPC team, and (2) feedback elicited by stakeholder groups while the trial was ongoing. The business case confirmed the high cost arising from regular use of the service. The E&F department anticipated additional workload to be generated as a result of using this technology in terms of preparing clinical areas for its use. There were some reports of intensive-care clinicians suggesting bed management to have been somewhat adversely affected during the trial.
The stakeholder groups also considered the possibility of ad hoc use. During a lengthy adoption decision-making stage, the decline of infection rates through ‘other initiatives’ was witnessed:
[. . .] there was a lot of discussion for about 6 months afterwards, as to whether we would bring them in on an ad hoc basis and use them. We got as far as having a business case. And we then decided infection rates were going down regardless and we felt it was something that wasn’t really needed but we know it’s there if we do need it.
Senior nurse
Outcome
The final decision not to mainstream use of this service at the present time was reached at trust board level. The trial report suggested that, generally, the service was positively perceived. A business case was put together by the IPC team, the showcase team, managers and procurement. It was presented to the trust’s infection prevention committee group and was then recommended to the trust board. Overall, a key factor facilitating the trial was that the technology became available as a managed service. However, the cost of mainstreaming the use of this particular service across the trust is understood to have played a key part in the decision taken, along with any disruptive effects anecdotally reported during the trial.
Trust 2 technology product microcases
Microcase 1: standardisation of disposable sterile gowns
Attributes perceived by stakeholders
Disposable sterile gowns were understood by T2 informants to be a product that is easy to use, and one that has been available in the NHS for a long time. The objectives behind standardising gowns used in T2 to one gown product, namely to ensure quality and generate savings for T2, were highlighted by informants as important. Several different gowns used in T2 theatres have been found to allow ‘strike-through’, that is, fluid seepage from their exterior into their interior surface, posing a risk to theatre staff.
Gown standardisation was understood to be compatible with care delivery structures and systems, T2 values and the service quality culture. It has been approached through an integrated care perspective that examines the use of gowns in care pathways involving different surgical procedures. It has aimed at selecting one gown product that provides adequate protection against ‘strike-through’ for several hours, ease of use in any procedure and cost savings. Benefits to patients and staff include less risk of having to stop a procedure for a gown change and a more comfortable working routine for staff.
Stakeholders, evidence and decision-making
The need for standardisation was highlighted by T2 senior theatre staff. The procurement specialist nurse then sourced further information and evidence through NHS procurement, professional networks and suppliers ( Table 19 and Figure 11 ). Suppliers were then approached individually with requests for product demonstrations. A special focus group was formed to look at the evidence, as part of the wider T2 cost-saving lean transformation programme. Focus group members were senior nurses, procurement specialists, senior surgery staff and a T2 lean service improvement team member.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Industry/suppliers |
|
| Peers and colleagues |
|
| Trust care environment |
|
FIGURE 11.
T2 standardisation of disposable sterile gowns: professionals’ engagement and evidence types in decision-making.
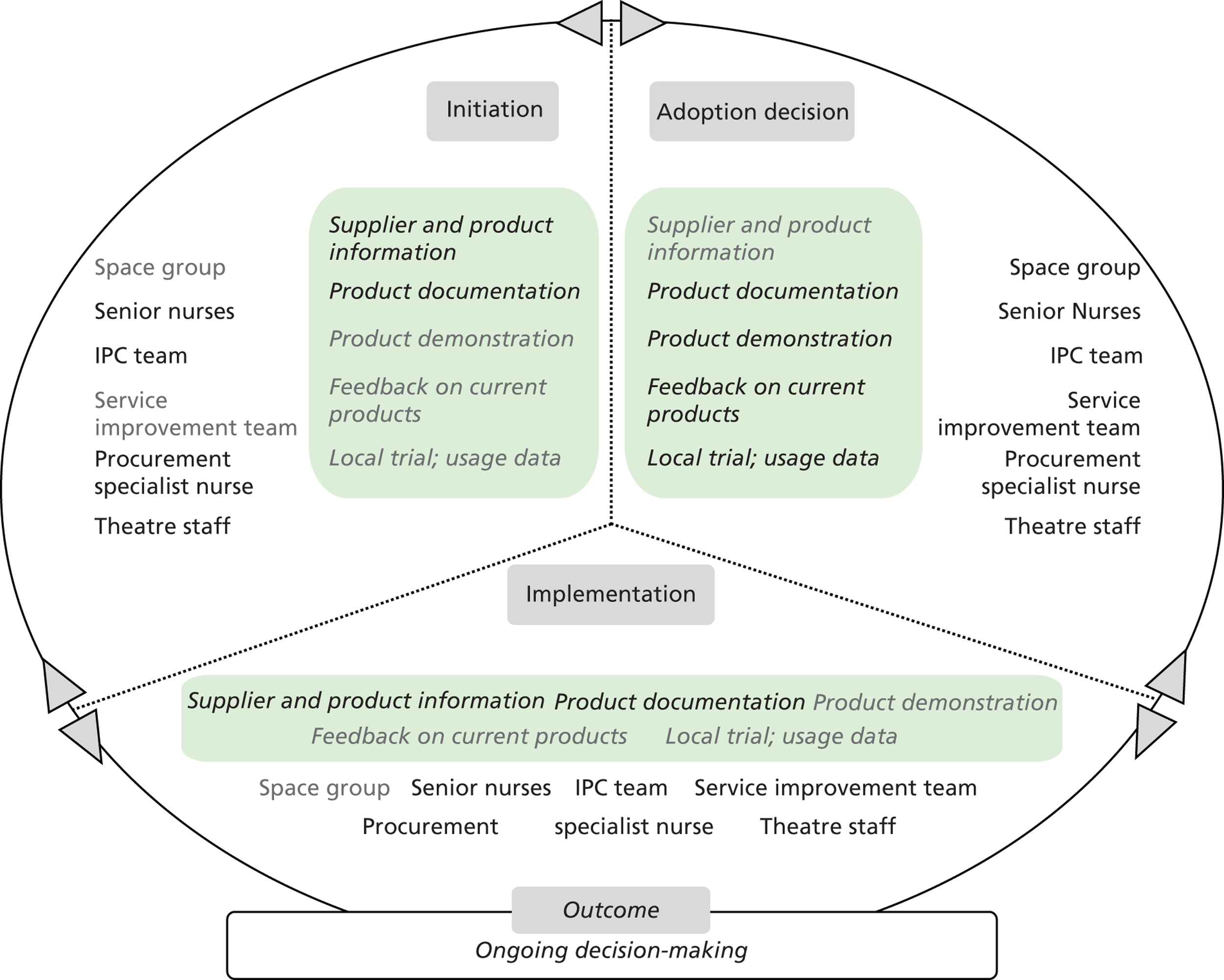
Five products of different suppliers were shortlisted by the focus group within 2 weeks. An evaluation plan was then put together to assess the relative merits of each product regarding highest quality offered for a price acceptable to the trust. The procurement specialist nurse acted as a champion, supported shortlisting and led the evaluation process. The evaluation of the five gown products was organised in early 2012. It was based on a single data entry form agreed a priori with manufacturers. Each of the gowns was evaluated on a particular day within 1 week. Results were collected and fed back to the focus group by the procurement specialist nurse.
Outcome
The results of the trial were fed back to the focus group and the T2 innovations or ‘space’ group. However, the decision process was delayed in the second quarter of 2012, as one of the companies participating in the trial raised a formal complaint against T2.
Microcase 2: 3M Clean-Trace NG luminometer
Attributes perceived by stakeholders
This system was understood by T2 informants to be a medium- to high-technology solution for assessing whether clinical areas and surfaces are cleaned effectively. Prior to its introduction, there were no means of testing whether a previously cleaned surface had been decontaminated of microorganisms and residues. This system was viewed as having a key benefit for patients, staff, the trust and the NHS. T2 staff members suggested patients were reported as feeling more secure when observing use of the device, while levels of staff responsibility vis-à-vis effective cleaning also were raised. It is generally perceived to be compatible with care structures and systems and T2’s culture and values.
Stakeholders, evidence and decision-making
Ideation about the system came through the Showcase Hospitals programme and the T2 DIPC – Executive Nurse. The DIPC put the system forward at the T2 space group. It was then followed up by the IPC nurse consultant, who liaised with ward staff for training and trialling purposes. Stakeholder groups involved included the IPC team and ward staff. ‘Championship’ roles are identified with the nurse consultant and individual matrons in wards where the device was first trialled.
In addition to the Showcase Hospitals programme, further information and evidence became available through the companies involved and other trusts in the region ( Table 20 and Figure 12 ). Additional evidence featuring in the decision-making process included peer-reviewed journal articles on environmental assessment based on ATP, reviewed by T2’s microbiology department.
| Evidence sources | Evidence types |
|---|---|
| Showcase Hospitals programme |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Trust care environment |
|
FIGURE 12.
T2 3M Clean-Trace NG luminometer: professionals’ engagement and evidence types in decision-making.
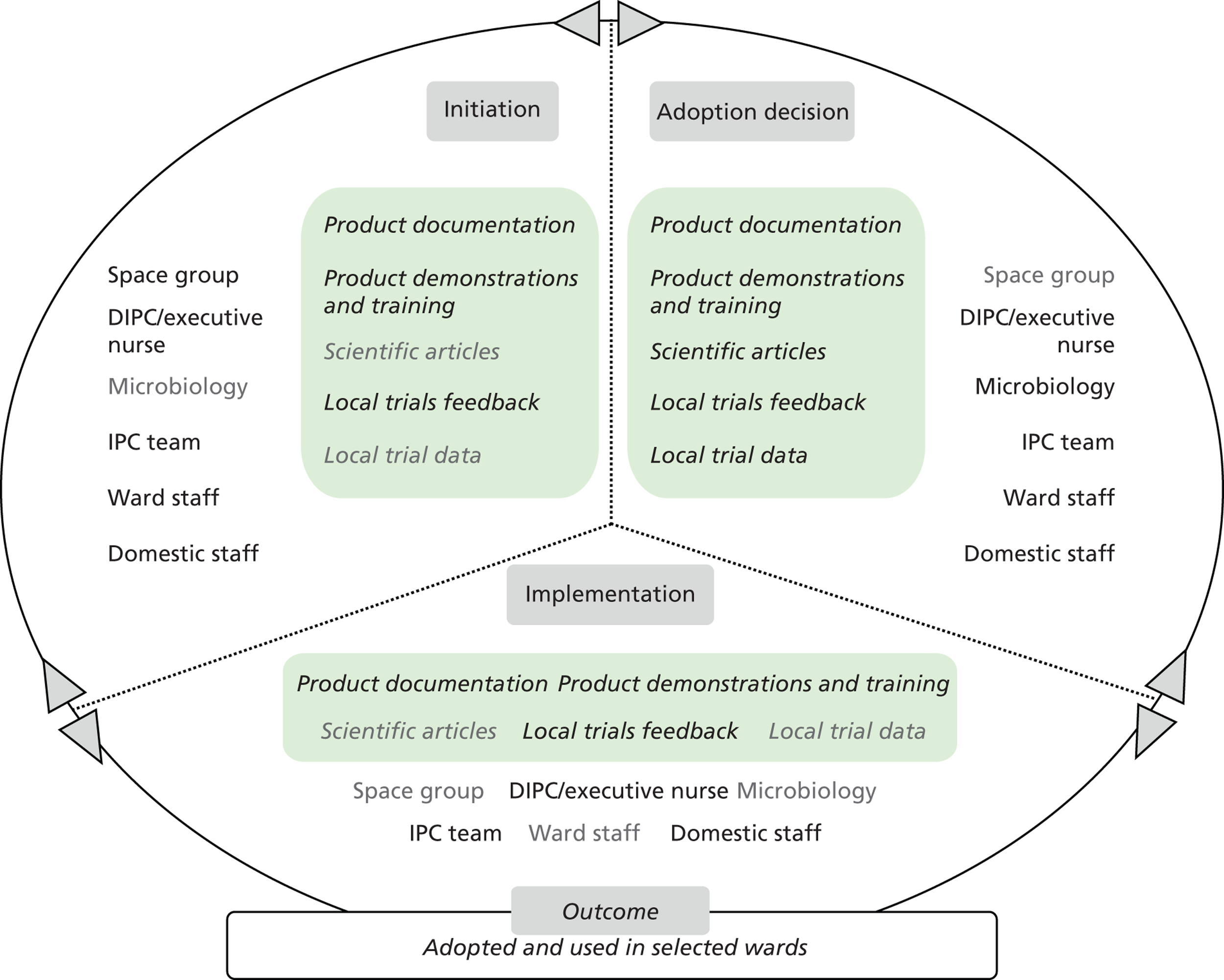
A local evaluation was funded by the Department of Health through collaboration with the Smart Solutions programme. This supported an evaluation of the Clean-Trace system and the UV light torch equipment at the same time, as it was thought that an alternative method of assessing surface cleanliness was needed.
Outcome
Following the trial, the system has continued to be used in selected wards in the main T2 hospital site. Funding and support to sustain its use has been provided by the DIPC. Although a reduction in C. difficile cases was noted in wards where it was used, a formal evaluation was not carried out to establish a link. The main outcomes identified have been raising staff (including matrons’) interest in and motivation for effective cleaning, and improved communication among staff and patients.
The innovation adoption process in this case lasted 6 months. The system’s main advantageous feature is understood to be the direct, online feedback provided to middle and senior management on surface cleanliness in wards. New domestic staff members were appointed for the implementation of this technology, and their culture of collaborative teamwork was also witnessed as leading to successful implementation. ‘Ownership’ of the swabbing process by domestic staff is thought to have been another key facilitating factor, as it helped generate enthused and interested users among them. An alternative scenario would see IPC team members visiting wards and obtaining samples. This has been viewed as potentially intrusive to care delivery in wards. One respondent commented:
[. . .] a practicality. Something that can actually translate down to ward level. Because that was a bit of a challenge because I think some ways of implementing that this would be given to infection control and then they just pop out and do swabbing. But you then go into an area as a stranger and with [domestic staff] using it there was a bit of ownership and you don’t actually get that if you do something and you give somebody feedback that’s fine but you’re very much an inspector. If people can take part in it and do something about getting the readings for themselves and you’ve got somebody who knows the area really well and knows what they are doing, I think you get more involvement.
Senior nurse
Microcase 3: ASP GLOSAIR 400 aHP system
Attributes perceived by stakeholders
The system’s perceived attributes include its decontamination and disinfection capability and its versatility regarding room size. The system is used by the trust’s Deep Clean Team as part of their cleaning regime once patients and staff have been removed from clinical areas before having them sealed. Its use is complementary to and follows the ‘deep clean’, general or terminal cleaning methods practised before its adoption. It is most often used during E. coli, C. difficile and norovirus outbreaks. Although there has been no formal review of its effectiveness and use conducted after adoption, its introduction is considered successful. As one T2 adopter suggested, ‘it seems to be working for us’. Adopters further suggested that a positive communication dividend has been reaped in terms of higher assurance of patients and the public that infection control is a top priority at T2. One respondent said:
[. . .] even patients ask as well now, ‘what’s this machine’ in a roundabout way.
Non-clinical manager
[. . .] we have had feedback from patients. And we have done that by a variety of surveys and actually patients feel very assured when they see us doing that and actually taking cleaning very seriously. And actually we have had a couple of complaints in winter that patients had been in our trust previously. And they had seen the HPV vapour being used on the ward and during the following admission they had not seen it, so they wrote in to say are you still not doing that? I think it does assure patients around the standard of cleaning when they see things like that happening.
Senior nurse
The system is perceived as complex to utilise by senior trust members. Staff members working more closely with the Deep Clean Team suggest that it is relatively easy to use. However, operators need to be trained appropriately, and cleaning prior to its operation is viewed a prerequisite. Bed management issues arising from its use were reported. However, respondents suggested that staff members work together to minimise impact, and that this system is considered quicker to use than related services. The innovation is considered to be in line with the values and culture of T2, as one organisation in which IPC and patient safety are high priority areas.
Stakeholders, evidence and decision-making
Senior trust members first heard about the system at a company presentation in a neighbouring trust. A decision was subsequently taken to trial the system in T2. Trial and purchase were supported through the Department of Health’s Deep Clean programme. The system’s purchasing cost was viewed as prohibitive. A workshop and demonstration were organised by the T2 Hotel Services Team. Stakeholders involved in the process included the T2 IPC team, microbiology department, hotel services and domestic staff. Key individuals with a leading role were the IPC Team Leader, the Consultant Medical Microbiologist (Infection Prevention Doctor) and the DIPC. Microbiology assessed the evidence and highlighted the system’s relative merits to other stakeholders. Some informal challenges towards the evidence gathered by microbiology were witnessed, and the company’s promotion efforts were perceived as insistent by some stakeholders:
I think there was a little bit of [something] about the results of the information, I think there was a bit of resistance, well not resistance, slightly challenged. [. . .] I think it was Microbiology.
Non-clinical manager
[. . .] I think not formally, there have been some reservations because as I said not a huge amount of evidence that this works. Primarily it was the company that was pushing it forward and so there has been some kind of disquiet around is this actually making a difference or is it generally raising the awareness of you know, cleaning and things. It’s never been formally challenged as such.
Senior nurse
Nevertheless, the decision to adopt the system was made at senior management level and was greatly supported by the DIPC. A respondent commented:
It was agreed at a committee an infection control group committee but I think the decision to go with it sat with the DIPC the Director of Infection Prevention, I think the ultimate decision was [theirs].
Senior nurse
Table 21 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 13 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Mass media |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Trust care environment |
|
FIGURE 13.
T2 ASP GLOSAIR 400 aHP system: professionals’ engagement and evidence types in decision-making.
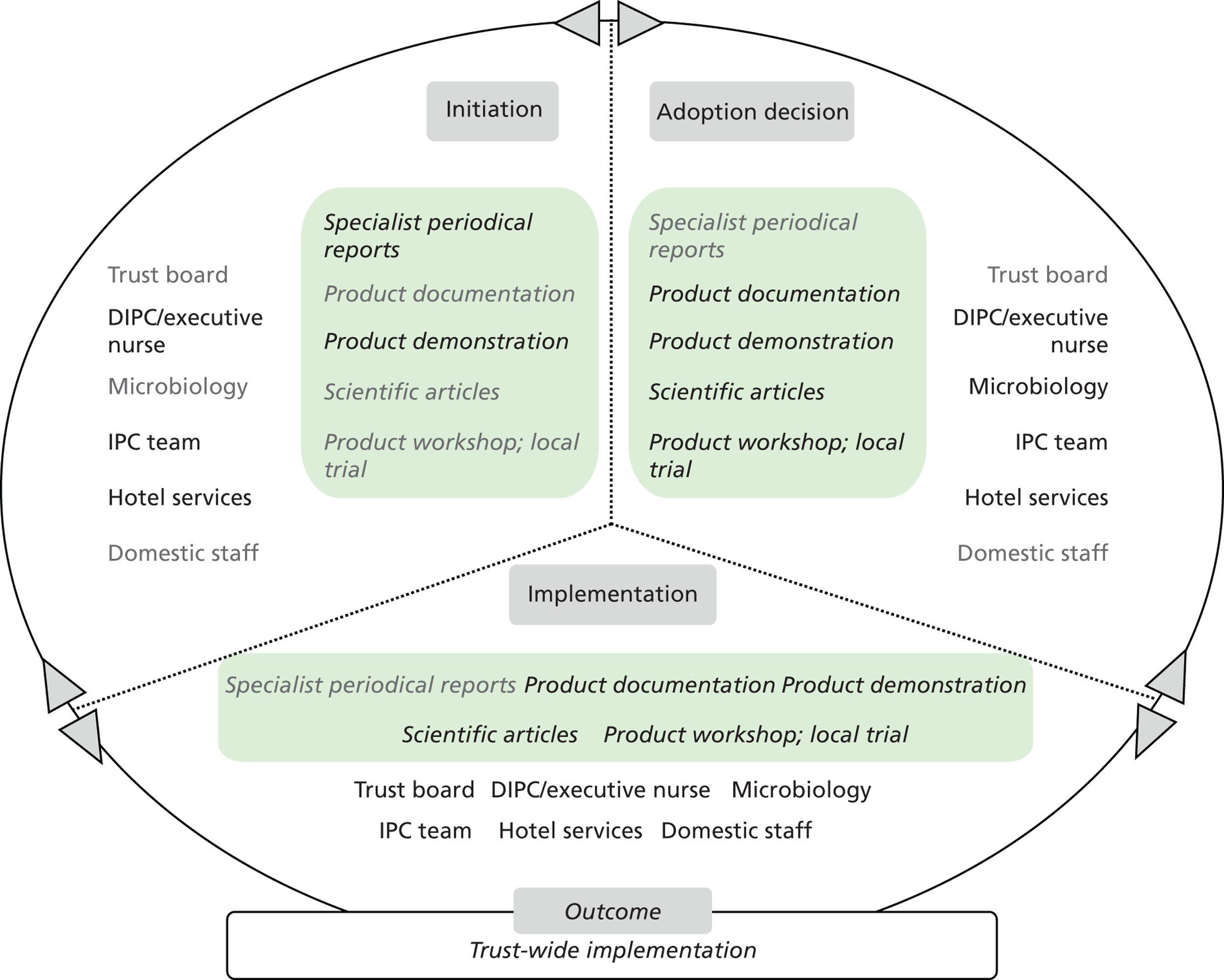
Outcome
The system became available for use across T2 in late 2007. The evidence featuring during the process and multidisciplinary teamwork during implementation – part of T2’s organisational culture – are understood to have facilitated adoption. The technology has been considered an opportunity during a challenging climate of persistently high C. difficile and MRSA bacteraemia case incidence at T2. However, since adoption the system has mostly been used to combat C. difficile and norovirus. Protocols were developed to support its use. The innovation adoption process is understood to have concluded fairly quickly. The company provided training and a new group was formed as part of the T2 Deep Clean Team, the Rapid Response Clean Team; the respondents considered these to have been key enablers.
In T2, use of the system was reported to take 3 hours for a bay or room. A full deep-clean process, including use of this system, was reported to take a maximum of 6–8 hours. Space to host patients becomes available after the deep clean and/or rapid vapour-based clean take place. This is considered very important in alleviating bed management pressures. The system’s adoption is understood to be a success as part of the measures helping T2 report lower infection rates.
Based on T2 interviews from phase 1, this technology product is currently in the re-evaluation process to build up its local evidence base. This was driven by an organisational need for justification of its ongoing use, and a need for evidence felt by stakeholders based on microbiologists’ critical appraisal of the existing evidence available.
Microcase 4: UV LIGHT Technologies inspection torch
Attributes perceived by stakeholders
Staff members of T2 understand the torch to be bulky and heavy when carried within the care environment. The risks of eye damage when directing or reflecting light towards others, and of skin burn when touching the front glass once the torch has been in operation for some time, were also identified by respondents. The torch is viewed as a high-tech item that was recently introduced in the NHS and that is useful in revealing stains and other surface residues that are not readily discernible. However, further training is required for observers to identify the type and content of stains or residues revealed. Its main benefit was thus understood to be that it enables staff to visually assess surface cleanliness rapidly. It was perceived as rather impractical for use in wards, but useful in occasional inspections, and generally as bringing limited benefit to T2’s IPC practice.
Stakeholders, evidence and decision-making
The UV light torch was initially considered as an opportunity at T2 during a time of high C. difficile incidence in 2007–8. Subsequently, the opportunity for a trial came in the form of an evaluation of the UV light torch in tandem with the 3M Clean-Trace luminometer, funded by the Department of Health and Smart Solutions.
One respondent expressed some concerns about the suitability of this evaluation method to assess genuine outcomes:
I think if you have a go with something quite a lot of spin-offs, we didn’t know at the time what kind of spin-offs there was going to be. But a big benefit to us was being able to try the ATP out at the same time. So actually we ended up with two technologies and at the end of it I was actually thinking to myself that maybe we shouldn’t, always be viewing the technologies in isolation because quite often some of these things are complementary.
Senior nurse
Once the risks associated with its use became apparent, a detailed protocol was developed to guide the use of torches, luminometers and rod swabs. All items were placed on a trolley for easier transfer, access, use and reposition by T2 staff members during the trial.
Table 22 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 14 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Industry/suppliers |
|
| Peers and colleagues |
|
| Trust care environment |
|
FIGURE 14.
T2 UV LIGHT inspection torch: professionals’ engagement and evidence types in decision-making.
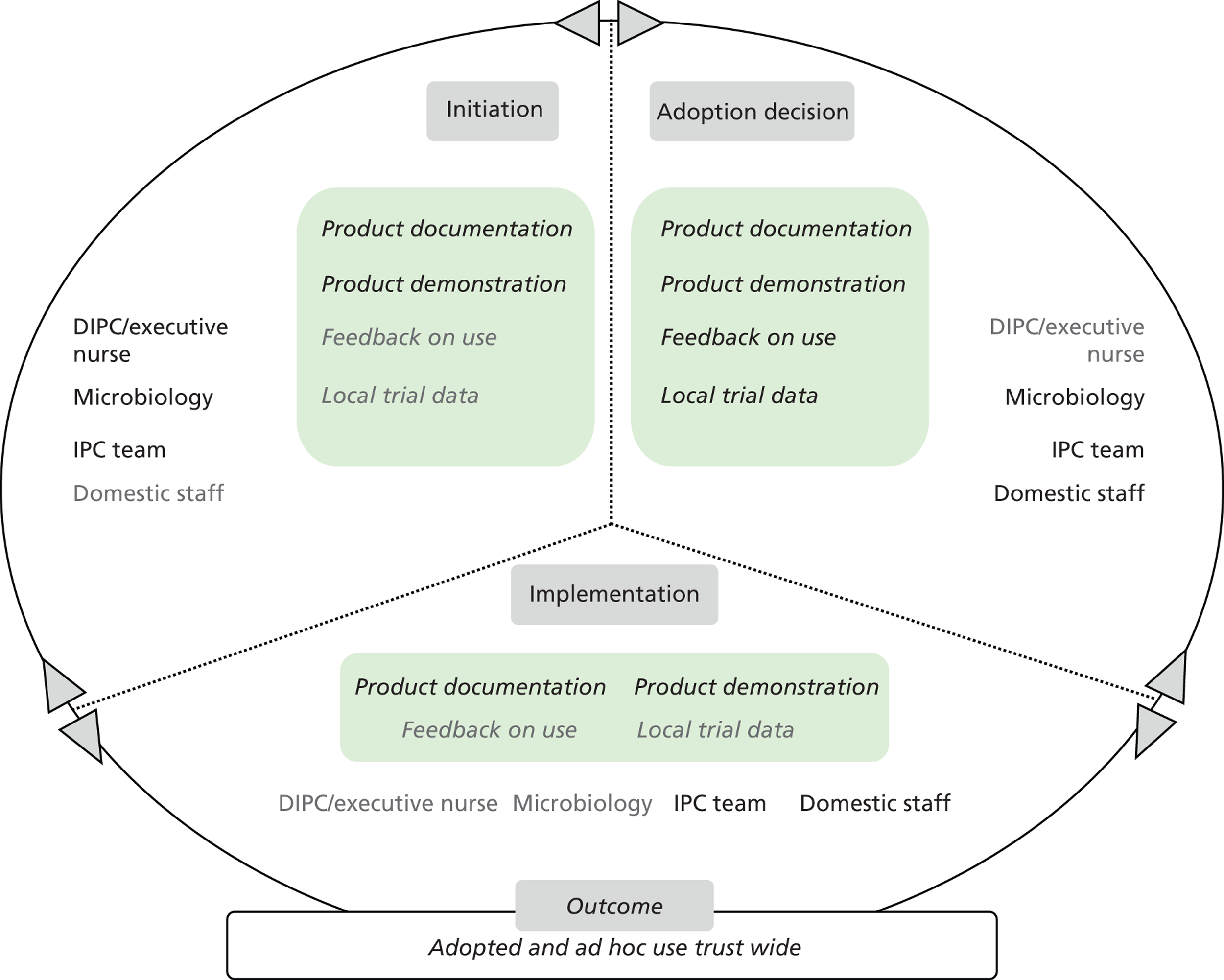
Outcome
Following the trial, consensus among staff centred on not recommending the system for trust-wide implementation, but keeping the unit for use in occasional inspections. A facilitating factor to this end is considered to be the availability of the torch at a reduced price by the supplier. A respondent commented:
[. . .] it would have been better to look at the UV light and its usefulness in moving equipment, for example, into a darker room and examining how clean it was. Rather than try to do it in broad day light at the bed-side. It is useful in the operating theatre.
Senior nurse
Trust 3 technology product microcases
Microcase 1: Bioquell vapour hydrogen peroxide Room Bio-Decontamination Service
Attributes perceived by stakeholders
The Bioquell VHP RBDS was perceived by T3 informants as an effective means of disinfection and decontamination. It is understood that hydrogen peroxide vapour can access those areas that may not be readily accessible by manual cleaning, and that it is more efficacious than its aerosolised variant. It is viewed as a new, high-tech system that is a complement to deep cleaning. Its efficacy in eradicating C. difficile spores has been a particularly attractive feature. The main benefits arising from its use are understood to be a safer care environment for patients, reduced risk of cross-infection and a reduced workload for staff. Opinion seems to differ on whether there would be financial savings to be had from its use, and whether the time taken to use the service amounts to more or less the overall time required to deep-clean care areas to the same effect. Respondents suggested that there is a substantial cost element in using this service and VHP technology more generally. Nonetheless, they would generally recommend the service and technology be taken up more widely in the NHS.
Stakeholders, evidence and decision-making
The technology was reviewed as part of T3’s efforts to mitigate C. difficile incidences. A senior member of the trust IPC team acted as the evidence broker, forming a project team to collect and review evidence in the first instance, and bringing the evidence to other trust fora. These included working groups on C. difficile reduction and meetings with the trust CEO. The technology was also discussed by the trust Investment and Purchasing Group. After a review of the evidence, the trust CEO allowed site surveys to be carried out in the trust. Patient groups also informally demonstrated their interest in this technology.
The trust’s medical microbiologist was involved in the adoption decision. The following respondent suggested that he was exercising ‘expert’ power as a microbiologist over the other stakeholders when reviewing the evidence:
The microbiologist, not challenged it but are you sure that it’s doing, that was almost the thing. Because I would take the evidence to him and go through it with him so that is his nature. If you are going to sit in a meeting with him I need you to be with me. Not then be against me and start challenging. So I was very clear to pick out all the things that I thought were relevant and I think he was reading himself. But actually he left it up to me to do a lot of the stuff and he would actually challenge a lot.
Senior nurse
Moreover, the key decision-makers became confident in making a more concrete decision through a site visit to another trust, where they directly observed this technology in action. One respondent suggested:
Only after we had the practical demonstration in [the other trust] we were cautious before that. They provided us with the evidence when they gave us the seminar here. But we were cautious until we had actually seen exactly what was the process involved. How they sealed all the rooms up, how they controlled it while they gas. It was only after we had actually seen it and had it demonstrated to us, and we had spoken to the operatives, [that we] did we feel completely safe that it was all being controlled in the proper manner.
Senior non-clinical manager
Sustainability has been a factor in the decision-making process, as regular use of the service has been understood to represent a significant outlay for the trust.
Table 23 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 15 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Health agencies |
|
| Showcase Hospitals programme |
|
| Peer-reviewed academic literature |
|
| Professional networks |
|
| Industry/suppliers |
|
| Other trusts |
|
| Trust care environment |
|
FIGURE 15.
T3 Bioquell VHP RBDS: professionals’ engagement and evidence types in decision-making. H&S, health and safety; IC, infection control.
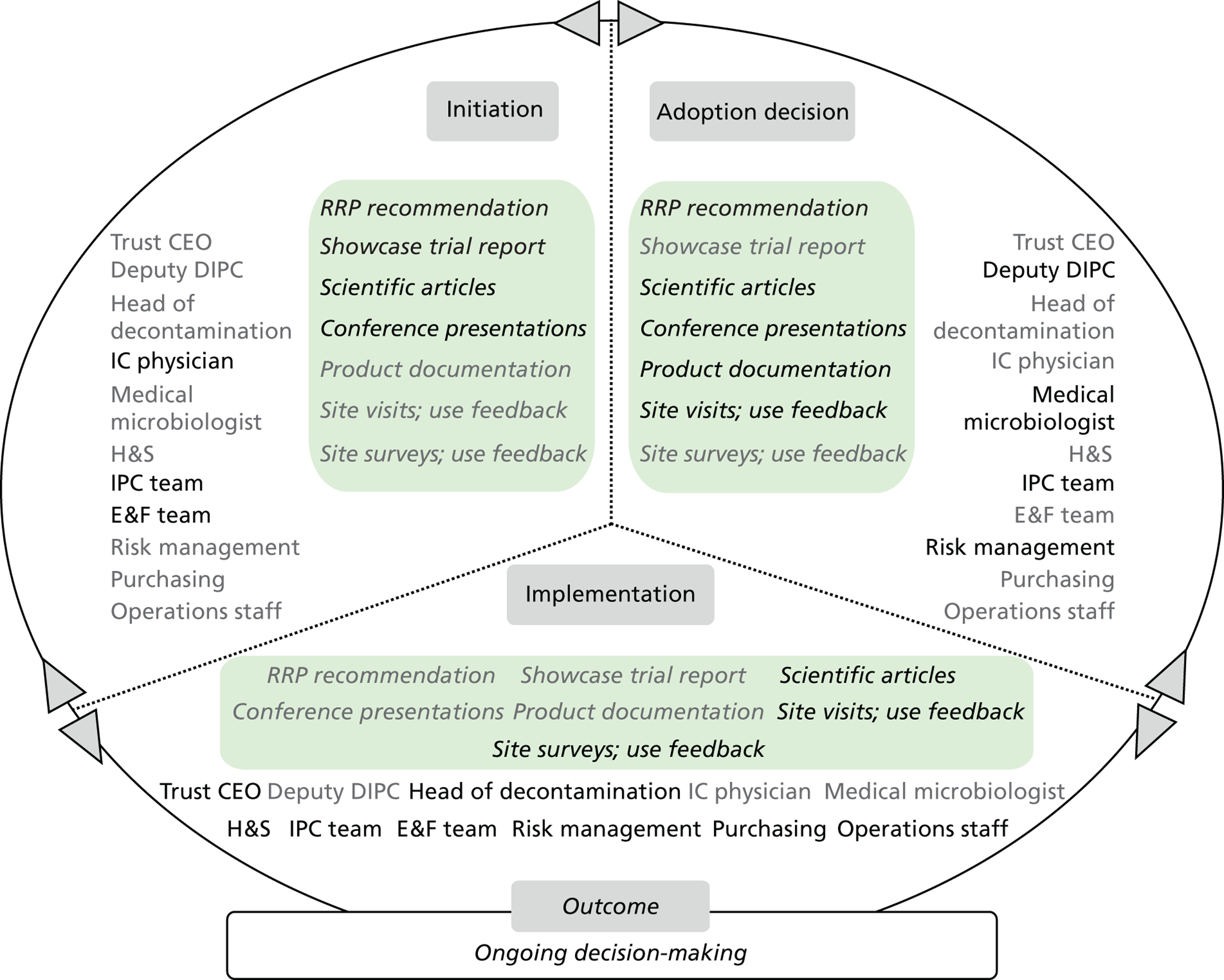
Outcome
Informants from T3 suggest that the technology is positively viewed in the trust. A decision on it is pending owing to perceived costs associated with its use, and also because it cannot be widely used across all of the trust’s sites. The supplier’s engagement with the process, with particular regard to the organisation of local site surveys and delivery of evidence on the service, is considered as a key facilitator of the decision to trial the service and willingness to develop its use further as an active component in the trust’s decontamination approach, particularly when a new planned hospital site is built. The evidence on the technology’s efficacy is also thought to have played a supporting role.
Microcase 2: clinell sporicidal wipes
Attributes perceived by stakeholders
The clinell Sporicidal Wipe is understood by T3 informants as a ‘low-tech’, novel product combining sporicidal action with wipe use. They suggested that the product was introduced as another measure to help reduce infection rates further. Trust members suggested that they were already familiar with using wipes in the trust care environment and welcomed use of this product. The product’s perceived benefits included ease of use, efficacy and a safer care environment for patients and staff. Before its adoption, a spray-based sporicidal product was used. Its use by staff was fraught with problems despite regular training, and its efficacy was understood to be rather low.
Stakeholders, evidence and decision-making
The clinell sporicidal wipes were introduced at a time when the incumbent sporicidal product was understood to be failing. Ideation came from a presentation in a professional conference attended by IPC team members. In a short space of time, small local trials were held in a few hospital areas, where ward staff were given the product to try it out. The decision was channelled through the trust’s product selection committee, whose membership includes care specialists, divisional representatives and matrons. Minutes from formal meetings held were reviewed by trust directors, including finance directors. The trust’s Health and Safety Team were also consulted. The product’s cost was considered favourably during the decision-making process, as the product was proven to be less expensive than the incumbent product, generating small savings for the team and the trust.
Table 24 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 16 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Professional networks |
|
| Other trusts |
|
| Trust care environment |
|
FIGURE 16.
T3 clinell sporicidal wipes: professionals’ engagement and evidence types in decision-making. H&S, health and safety; IPS, Infection Prevention Society.
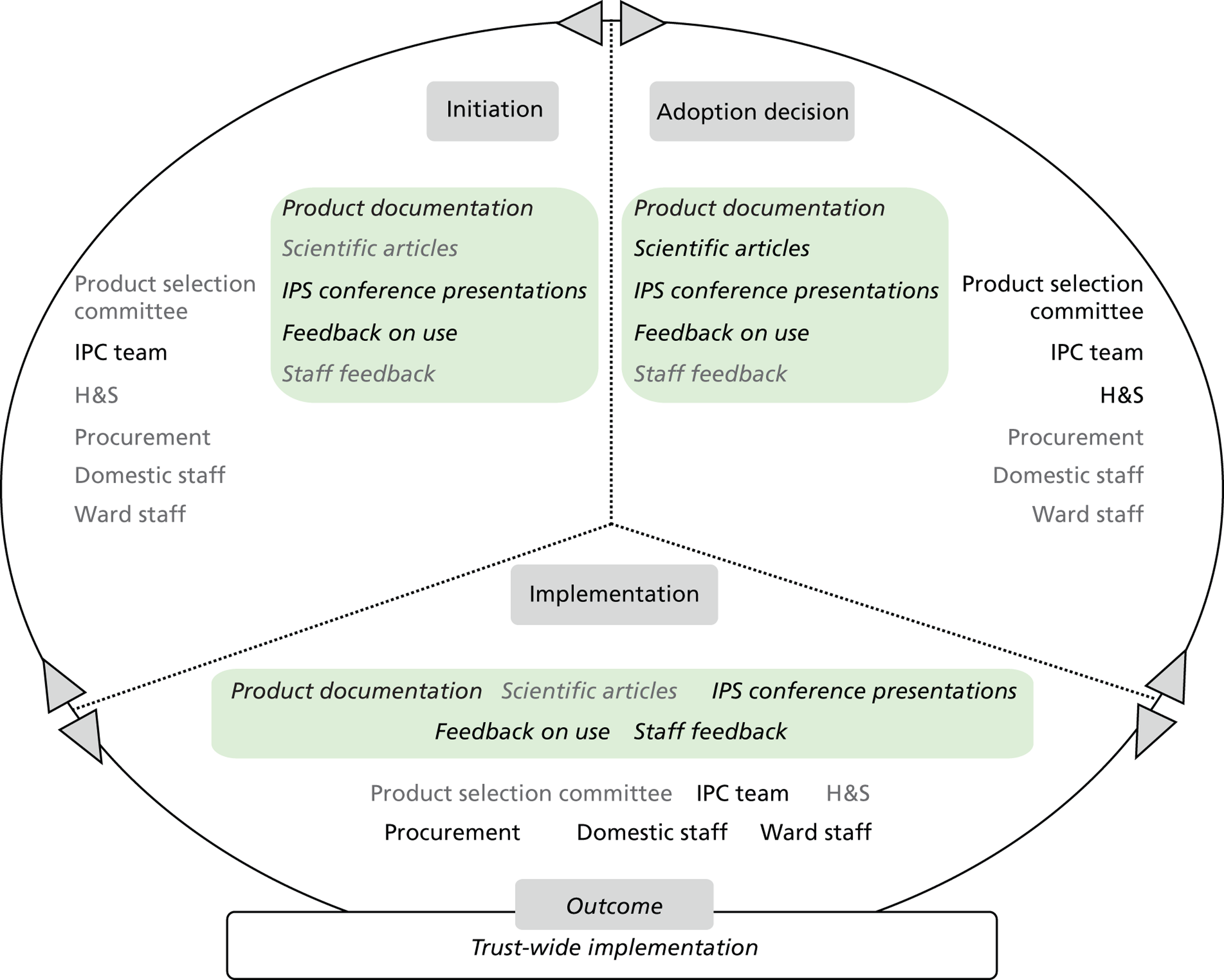
Outcome
The decision to adopt the product was taken by the IPC team, and was approved by the product selection committee. A key factor was the review of the evidence on the product’s cost neutrality. After the decision was made, IPC team members communicated with ward managers via e-mail and visits to wards. The company’s active engagement during product deployment, in terms of product support and trust staff training, is understood to have facilitated implementation. One respondent described this as follows:
[. . .] the company had been very supportive in rolling out to an organisation. But they went round and saw all the staff you know we looked at all the posters and that, these are the sort of measures that we need to give our staff. They did all of that and went round and helped us and the company came round and put all the dispensers up and everything, so I think it’s important.
Senior nurse
No resistance was reported during the implementation stage; this was partly a result of the nursing staff’s experiences with the previous similar products. One respondent commented:
[. . .] we’ve had negative stuff with previous products but I think because that is so negative, the fact that something that would just so easy to use I think they just grasped it so I think they were very happy with it. I think perhaps that helped it. The negative experience helped it to be a positive yeah.
Senior nurse
Clinell sporicidal wipes have been used in the sluice rooms of the trust’s hospital wards since their introduction. The product is understood to be successful because of trust members’ satisfaction, wide adoption by staff, a decrease in cost and the substitution of the earlier problematic product. There is a perception among staff that use of the wipes is associated with a reduction of infection rates and outbreak duration.
Microcase 3: Medixair UV Light Air Sterilisation Unit
Attributes perceived by stakeholders
Informants understand the Medixair systems to claim to eradicate air-borne pathogens by producing pathogen-free air in clinical areas. A collateral effect of keeping environmental surfaces cleaner is understood to be claimed also. They are also understood as a high-tech product and quite new to the NHS, but not compatible with existing trust care systems and structures. Benefits anticipated included a reduction in cross-infection, a cleaner care environment, better patient outcomes, a perception of actively improving care for patients and related benefits for the trust.
Stakeholders, evidence and decision-making
The IPC team was presented with grant funds to procure and use new technology to combat HCAIs.
Use of the devices was trialled in two hospital wards (thought to be ‘hot spots’), in which air samples were collected. Evidence was gathered, reviewed by the IPC team and presented at the trust’s product selection committee. IPC team members noted that the evidence presented on the technology was conflicting. Those IPC team members who had a microbiology background understood the trial results to be inconclusive in proving the efficacy of the technology and devices themselves. The devices were understood by some staff members to be disruptive while in operation in clinical areas. IPC members found it difficult to source spare parts when required. Knowledge about such maintenance requirements and ongoing cost was neither readily identified by the trust from the start nor provided by the supplier:
[. . .] we did pilot again and they were on rental because we started realising there were a lot of issues with the maintenance of them and the ongoing maintenance, and that was going to be a problem, and the ongoing cost really. It wasn’t as it appeared when we first started out with it. So things started to change we weren’t confident in the results. We did air sampling and we weren’t confident with those results either.
Senior nurse
This resulted in staff members losing confidence in making this technology work in reality.
Table 25 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 17 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Industry/supplier |
|
| Showcase Hospitals programme |
|
| Other trusts |
|
| Health agencies |
|
| Trust care environment |
|
FIGURE 17.
T3 Medixair UV Light Air Sterilisation Units: professionals’ engagement and evidence types in decision-making. IC, infection control.
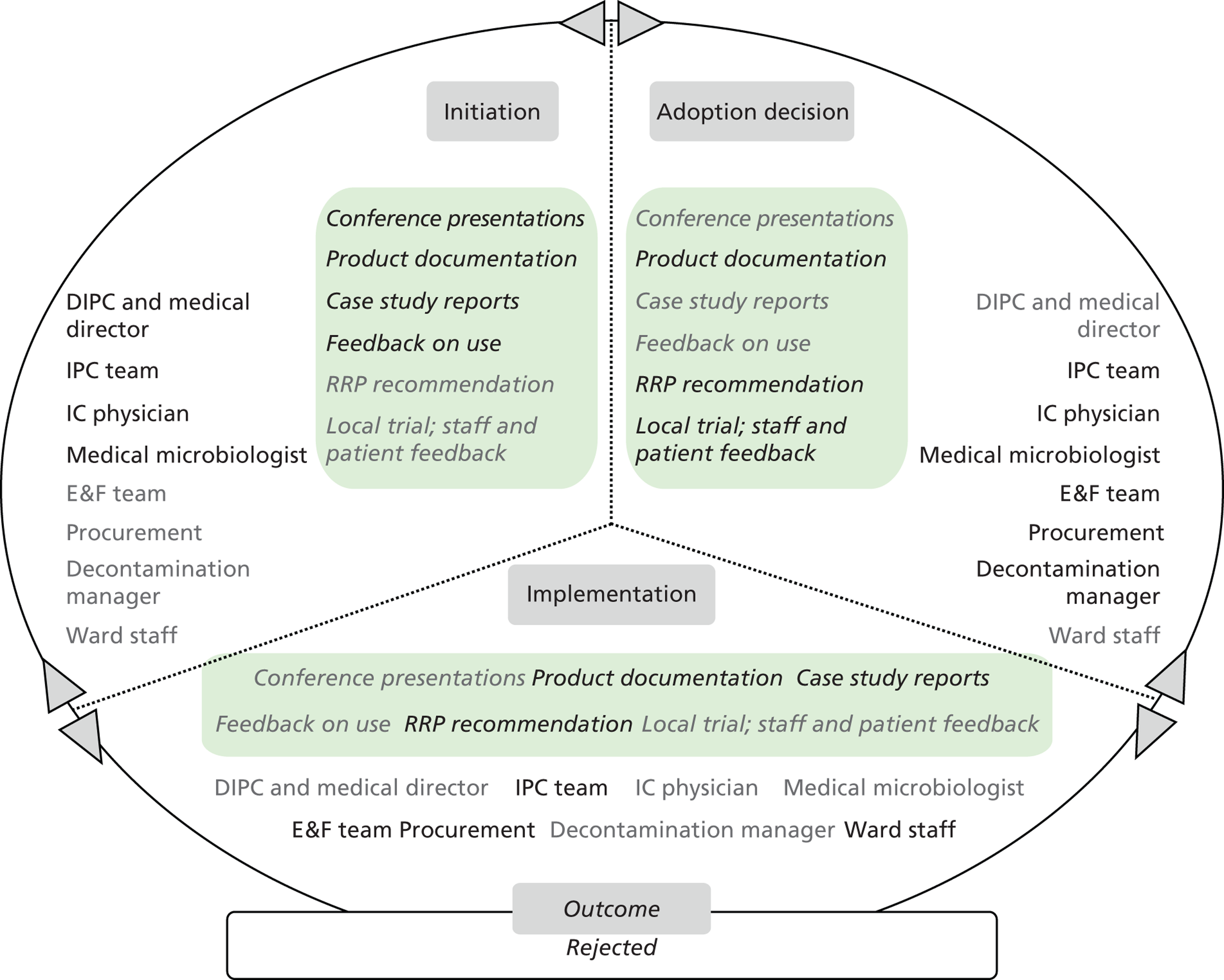
Outcome
The outcome of the evidence review and decision-making process was a rejection of the system’s continued use in the trust. The decision was taken by the IPC team. Local trial results were not suggestive of a high level of efficacy of the product in terms of air purification. Patient and staff feedback included comments on the product being obtrusive as part of the ward environment and noisy at times. T3 informants felt that, overall, the evidence was not robust enough to warrant their further engagement with this technology.
Trust 4 technology product microcases
Microcase 1: DIFFICIL-S disinfectant liquid detergent
Attributes perceived by stakeholders
DIFFICIL-S is understood by T4 informants to be a next-generation cleaning and disinfection product based on chlorine dioxide, which is produced upon mixing. It is considered a step forward from hypochlorite products used, which are understood to cause surface corrosion. Currently, the hypochlorite disinfectant used at T4 is ActiChlor™ Plus (ECOLAB, Swindon, UK) tablets. DIFFICIL-S is thought to be highly effective, as it achieves a very high bacterial load reduction in a short time, and also because of its low risk Control of Substances Hazardous to Health profile. These aspects are considered as key advantages over chlorine dioxide and hypochlorite products alike. Products with chlorine dioxide as their active ingredient have been available on the market for some time, but their use in the NHS seems relatively new.
The fact that the product delivers the required chlorine dioxide concentration means that it is viewed by senior staff members as high tech, whereas the product application generally is viewed as low tech. The product is considered to be in line with existing cleaning practice, care delivery processes and the trust’s culture. However, it is perceived as a product that is less easy to use than detergent wipes, and one for which variable cleaning efficacy due to errors in preparation might still be an issue. Infection rate reduction is suggested as the main benefit. Further benefits for the trust include a longer life of surfaces and equipment and public confidence owing to lower infection rates.
Stakeholders, evidence and decision-making
Members of the IPC team found out about the product at the Infection Prevention Society annual conference. Subsequently, they contacted the HPA for further input, and consulted a scientific paper that reported research results about the product in a specialist peer-reviewed journal.
The recent C. difficile rates and trust management support for efforts mitigating C. difficile incidences were also thought of as favourable conditions for selecting the product as well as launching its local trial. The following respondent noted the external pressure applied from the strategic health authority in relation to trust’s C. difficile performance. The aforementioned scientific paper was published during the period when trust members were actively searching for solutions:
[. . .] our rates haven’t come down as quick as the SHA (Strategic Health Authority) would have liked them to do. [. . .] we still had less cases than last year but they wanted less [than that . . .] we were actively looking for anything that would help us with C. difficile, otherwise it, with new products it’s whether you hear about them through professional networks, you see them at conferences, or a rep letterboxes you and gives you some literature, or in our case it was a mix of we were looking at the time that this was available, so we were thinking what can we do and this paper came out.
Senior nurse
Key stakeholders involved included the IPC team, the medical director and director of nursing, heads of nursing from the oncology hospital areas where the product was trialled, T4’s domestic services partner staff, the finance department and the multiprofessional infection prevention monitoring committee. As T4 is a PFI trust, the decision has involved negotiations between the IPC team and the trust domestic services partner on costing, staff training and PFI contractual aspects.
The trial had an approximate duration of 2 months. It was organised in the oncology areas of the T4 main hospital site, because of their stable patient flow patterns that do not typically include transfers to and stays in other hospital areas. Any effect on infection rates was, thus, thought to have been contained within, and be relevant to, those areas only.
Evidence featuring in the initiation, adoption decision and implementation stages included feedback from peers and colleagues in T4 and other trusts, expert advice from the HPA, supplier product documentation, peer-reviewed journal articles and local data collected during the trial.
Table 26 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 18 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Peers and Colleagues |
|
| Health agencies |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Trust care environment |
|
FIGURE 18.
T4 DIFFICIL-S disinfectant liquid detergent: professionals’ engagement and evidence types in decision-making. IPM, infection prevention monitoring.
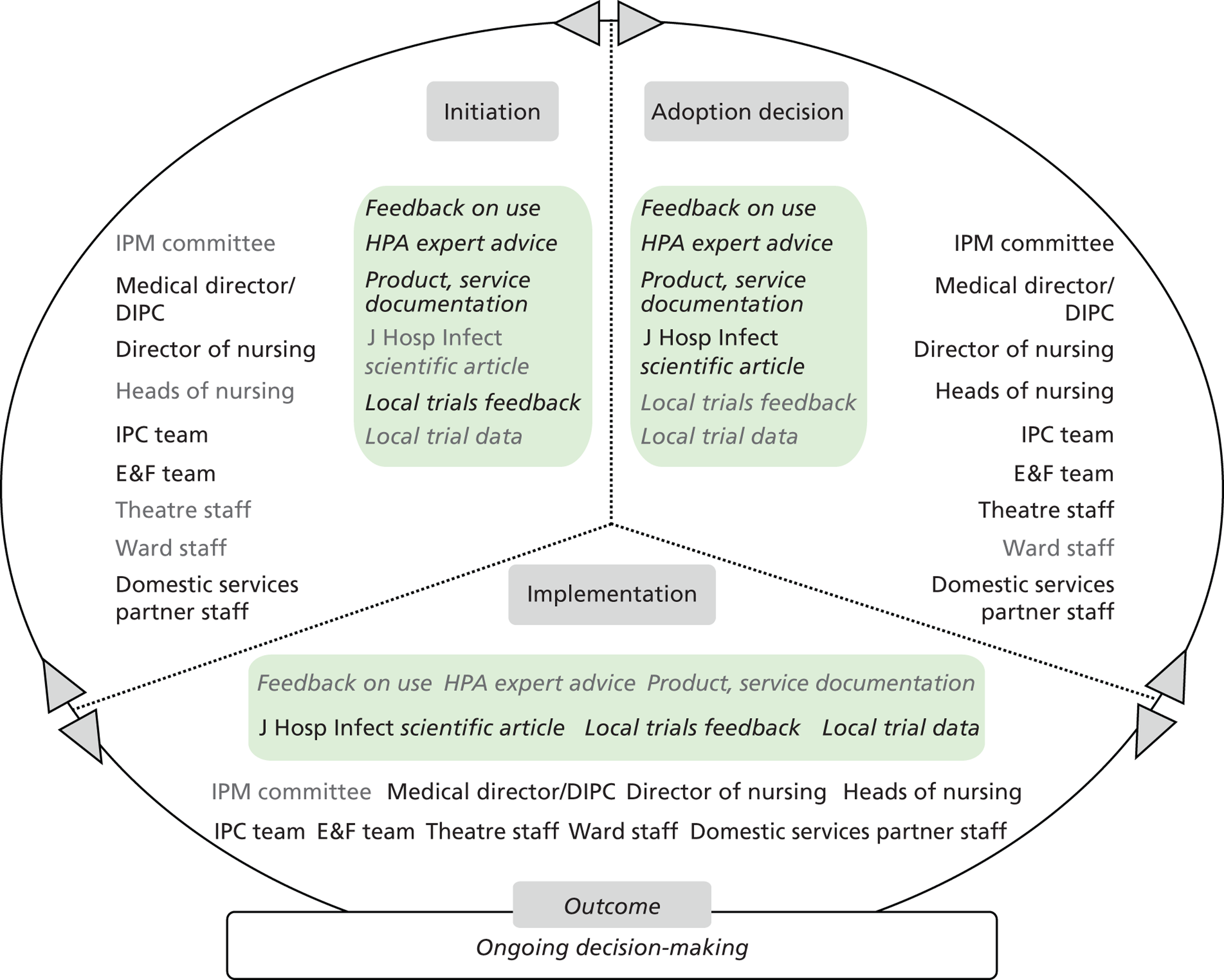
Outcome
The technology was viewed positively by T4 informants. The IPC team took a leading role in organising the trial. A ‘champion’ role is identified with the manner in which a senior IPC team member has led the process. The trial took place in the first quarter of 2012 and data were collected. This locally produced evidence was reviewed in April 2012. A decision is pending regarding adoption of DIFFICIL-S in T4.
Microcase 2: Bioquell vapour hydrogen peroxide Room Bio-Decontamination Service and Steris BioGenie
Attributes perceived by stakeholders
Informants understood this technology to be appropriate for use once manual surface cleaning has been completed by staff. The vapour released is thought to permeate and decontaminate areas not easily accessible by manual cleaning, with the same efficacy each time it is used, unlike manual cleaning. The technology is understood to have been utilised in other industries for many years, and has only recently been made available in health care. It is thus viewed as a low-tech principle whose application in this manner in T4 is understood to be high-tech. This may reflect the attributes of the newer VHP machines bought in T4, which can perform both vapour generation and aeration based on programing that takes into account the volume of the room where the device will operate.
The main benefits perceived to be stemming from the use of this technology are (1) a high degree of decontamination and low infection rates for patients, (2) reassurance and a reduced workload for staff to manage, and (3) a good public perception of the trust.
Stakeholders, evidence and decision-making
Initially, T4 had used Bioquell VHP RBDS to effectively eradicate multiple MRSA bacteraemia strains in its neonatal intensive-care unit. It was also used to decontaminate areas after an incident with sanitation pipelines. One respondent described this as follows:
We had a massive sewage leak in the eye department or an air con failure [. . .], so when the eye department came in on a Monday morning there was water just literally running down the walls and off the ceiling tiles, and in their three operating theatres, and it was like going into a tropical rainforest. But we were then worried about pseudomonas and things like that, so we decided then that we would just peroxide the whole lot, clean it up and peroxide it, so we’d used it a couple of times, we liked it.
Senior nurse
Persisting issues with environmental hygiene at T4 led the IPC team to consider solutions. The team attended a Showcase Hospitals programme conference where they were presented with evidence on VHP technology. The technology was considered easy to use and manage by the IPC team themselves, and very effective in raising environmental hygiene standards. Decision-making was confined to senior members of the IPC team. The stakeholders at the initiation stage did not go through a tender process. A quick decision was taken to purchase this particular product, mainly based on financial incentives and previous positive experience with the technology. This resulted in several problems, dilemmas and internal frictions, and was exacerbated by a lack of ongoing support from the company.
Table 27 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 19 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Showcase Hospitals programme |
|
| Health agencies |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Peers and colleagues |
|
| Trust care environment |
|
FIGURE 19.
T4 Bioquell VHP RBDS and Steris BioGenie: professionals’ engagement and evidence types in decision-making.
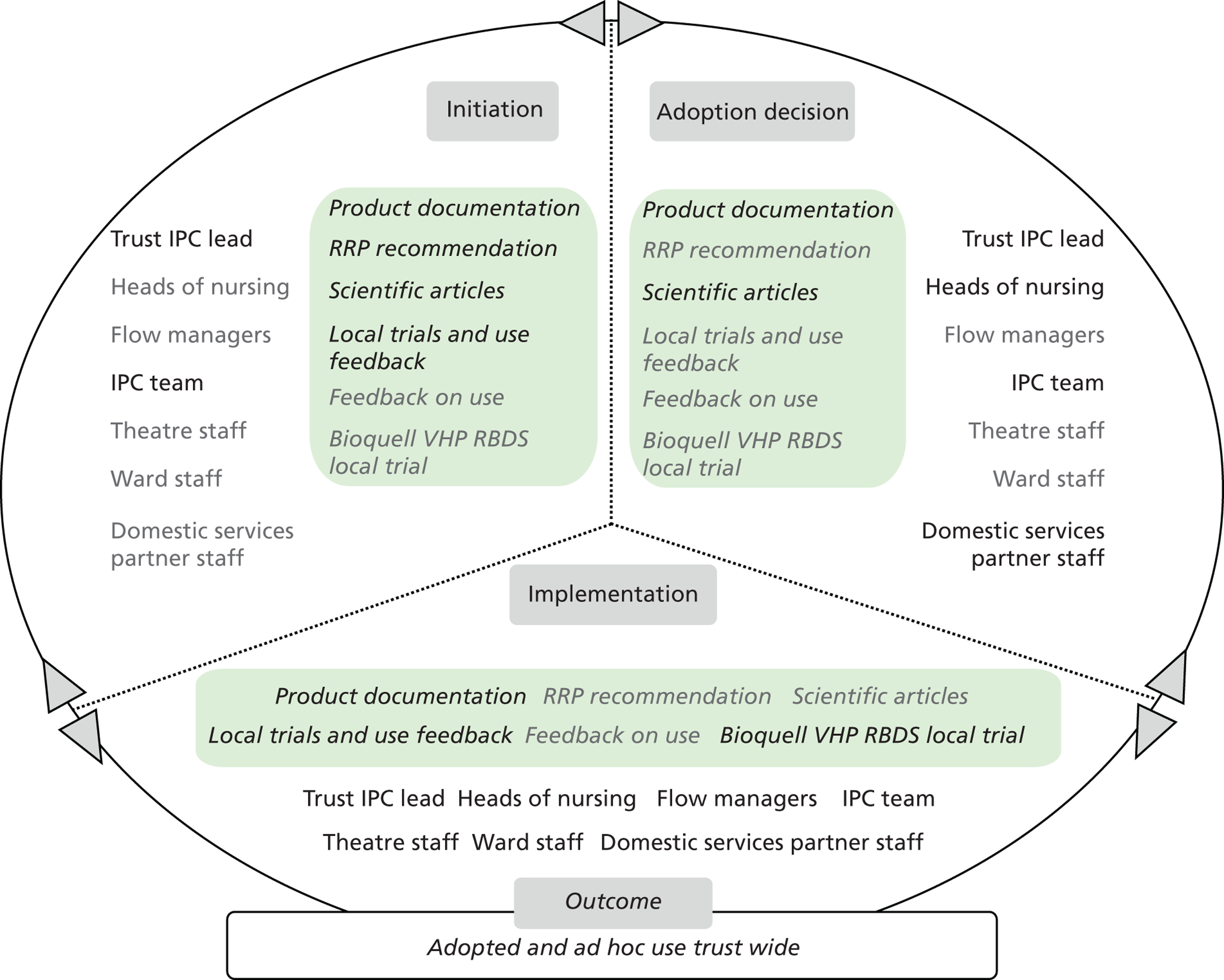
Outcome
Based on these positive experiences and on available external funding [£150,000 was given to T4 as part of the first HCAI Technology Innovation Programme Awards, which is a joint initiative by the Department of Health and the former NHS PASA, which was expected to be used for new technology aimed at further tackling HCAIs (source: trust’s annual report 2008/09)], a decision was made by the trust IPC team to buy four VHP machines, now marketed by Steris BioGenie for use in T4 hospitals as and when required. This decision was implemented through direct contact with the supplier. The product was chosen on the basis of its perceived ease of use, its combined operating modes of vapour generation and aeration/dehumidification, its residual antibacterial efficacy through inclusion of 6 parts per million (ppm) silver iron in the vapour mix and the discount offered by the supplier on the purchase of four machines.
Subsequently, T4 procurement highlighted formal procurement channels and alternative providers offering products with identical attributes. Issues with the after-sales service and support of the supplier, before Steris BioGenie, led the IPC team to seek VHP machine maintenance through the T4 clinical engineering team.
Members of the IPC team are called on by other hospital groups to operate the machines. The IPC members conduct all sealing required within a room, including doors, windows, air vents, etc., and they then operate the machines themselves. Since its introduction, a further hospital unit, the neonatal intensive-care unit, chose to use the machines. IPC team members have provided training to neonatal intensive-care-unit staff. Two of the machines now reside in the neonatal intensive-care unit for immediate use upon incidence of an infection.
Wider use has been met with difficulties. Despite interest from theatre staff in the machines, theatre rooms have been confirmed to require more time and specialist input in order to be prepared for VHP, and, as the IPC team has had a higher than expected staff turnover, it has been unable to provide this additional support. As some bed management and staff working time issues have been reported in this trust of high bed occupancy, it is thought that a dedicated team would help expand service use, but that comes with its own cost implications. It has also been suggested that domestic services partner staff undertake this task; however, a training and cost dimension has been identified that has merited discussion. The IPC team’s intention of establishing a rolling VHP programme across the trust has thus not been realised to date.
Microcase 3: Virusolve+
Attributes perceived by stakeholders
The suggestion to adopt this product came from a theatre infection control lead staff member (IPC theatre link nurse). The product was understood to be very effective against all types of bacteria, including blood-borne pathogens. Its ease of use resulted from it needing to be prepared only once for frequent use thereafter. This was perceived to be a relative advantage over the current disinfectant needing to be made up before each use. The product’s cost was viewed as acceptable to the trust. Virusolve+ is understood by IPC team members to have been used in other trusts, and adopted relatively quickly in T4, without full recourse to and examination of the evidence available.
Stakeholders, evidence and decision-making
Shortly after the product began to be used, the IPC team asked to see the product data sheets and related information. This occurred as part of a wider discussion and evidence review exercise among IPC team members on new disinfectant products, prompted by corrosion effects observed on surfaces, attributed to the currently used, incumbent, product. It resulted in doubts being raised about the active ingredient of the product, its chemical composition and its cleaning efficacy. Theatre staff feedback suggested that the product was deposing a thin layer of substance on equipment, which needed extra rinsing to remove and whose odour was rather unpleasant.
Further IPC team members’ communication with the supplier did not produce satisfactory results. The decision to withdraw the product was taken by the T4 infection control lead.
Table 28 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 20 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Industry/suppliers |
|
| Peer-reviewed practitioner journals |
|
| Health agencies |
|
FIGURE 20.
T4 Virusolve+: professionals’ engagement and evidence types in decision-making. IPS, Infection Prevention Society.
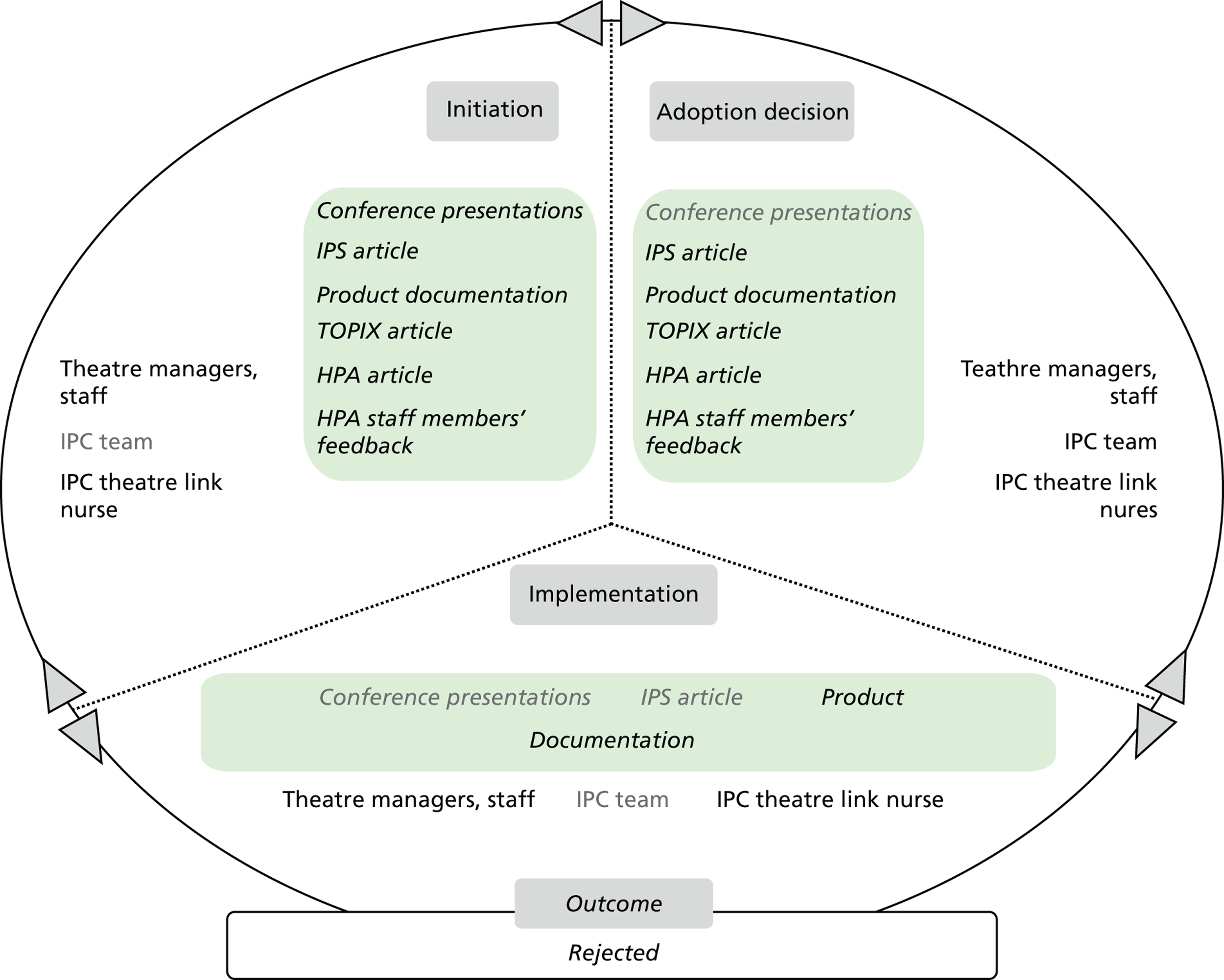
Outcome
During its short time of use, the product was deployed in theatres. T4 staff members are reported to have had a positive view of the product. However, doubts about its efficacy, resulting from a relative lack of evidence and transparency on the product operating principles, have been the main reason for its withdrawal. There were some concerns about a major change in cleaning products impacting on a high number of domestic staff, coupled with an ambiguous evidence base around the advantages of Virusolve+. One respondent commented:
[. . .] logistically there are 600 domestics in this trust, they have all been trained to use hypochlorite, [. . .] that is quite a task, and my lot, especially XXX, she came in nights, she came in weekends [. . .]. And to change over to another product is a big undertaking, and while it’s not a clear picture to me as to whether we should change the Virusolve+, or we should even question about our hypochlorite, I’m not going to change, I’m not in a position to change because my numbers for C. diff[icile] are just hovering above or on the trajectory, and I cannot rock the boat in anyway. So until I have a good body of evidence that there is superior product, whether it’s Virusolve+, or chlorine dioxide, or something else, I’m not going to change.
Senior nurse
This risk averse attitude towards the introduction of HCAI technologies can be partly explained by the fact that T4 is the only trust among the participating trusts that achieved a good performance on both MRSA bacteraemia and C. difficile infection rates during the whole period observed (see Chapter 6 ).
Trust 5 technology product microcases
Microcase 1: Bioquell vapour hydrogen peroxide Room Bio-Decontamination Service
Attributes perceived by stakeholders
The service was understood by T5 informants to involve a piece of equipment, a device and a process or operating procedure. It became available to the trust through the Showcase Hospitals programme in 2008. It is considered to be a high-tech solution. Informants perceive the main attributes of the service to be its efficacy in eliminating pathogens via ‘disinfection’ and ‘decontamination’ of the environment. The equipment itself is considered easy to use; however, there was limited understanding of its principles and review of any evidence, especially among ward staff. Perceived caveats include that it is time-consuming, costly and cumbersome in the form it was offered, that is, a service delivered by an external party rather than by trust operators.
The service’s clear relative advantage is perceived to be the high level of decontamination assurance it offers. Several informants suggest its use seems to coincide with falls in infection rates in wards. However, it is thought that it is not a substitute for cleaning, but rather a complement to standard clinical area cleaning practice. The service and equipment operation are understood to be relatively easy to understand and explain to others. However, delivery of the service (portable machine operation, area sealing, etc.) is thought to be complex and requiring specialist help.
Stakeholders, evidence and decision-making
Key stakeholders involved were the IPC team, including the DIPC, who is the chief nurse, nurse consultant and medical microbiologist, heads of nursing and lead nurses from the surgery, medicine, clinical and scientific hospital divisions, domestic services staff and the T5 domestic services partner.
The trial had an approximate duration of 3 months. Types of evidence featuring in the initiation, adoption decision and implementation stages included product documentation from the Showcase Hospitals programme, feedback on use via professional networks, peers and colleagues in other trusts, the Department of Health’s HCAI Technology Innovation Programme evaluation reports, supplier product documentation, peer-reviewed journal articles and local data collected during the trial ( Table 29 and Figure 21 ).
| Evidence sources | Evidence types |
|---|---|
| Showcase Hospitals programme |
|
| Professional networks/peers and colleagues |
|
| Health agencies |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Trust care environment |
|
FIGURE 21.
T5 Bioquell VHP RBDS: professionals’ engagement and evidence types in decision-making.
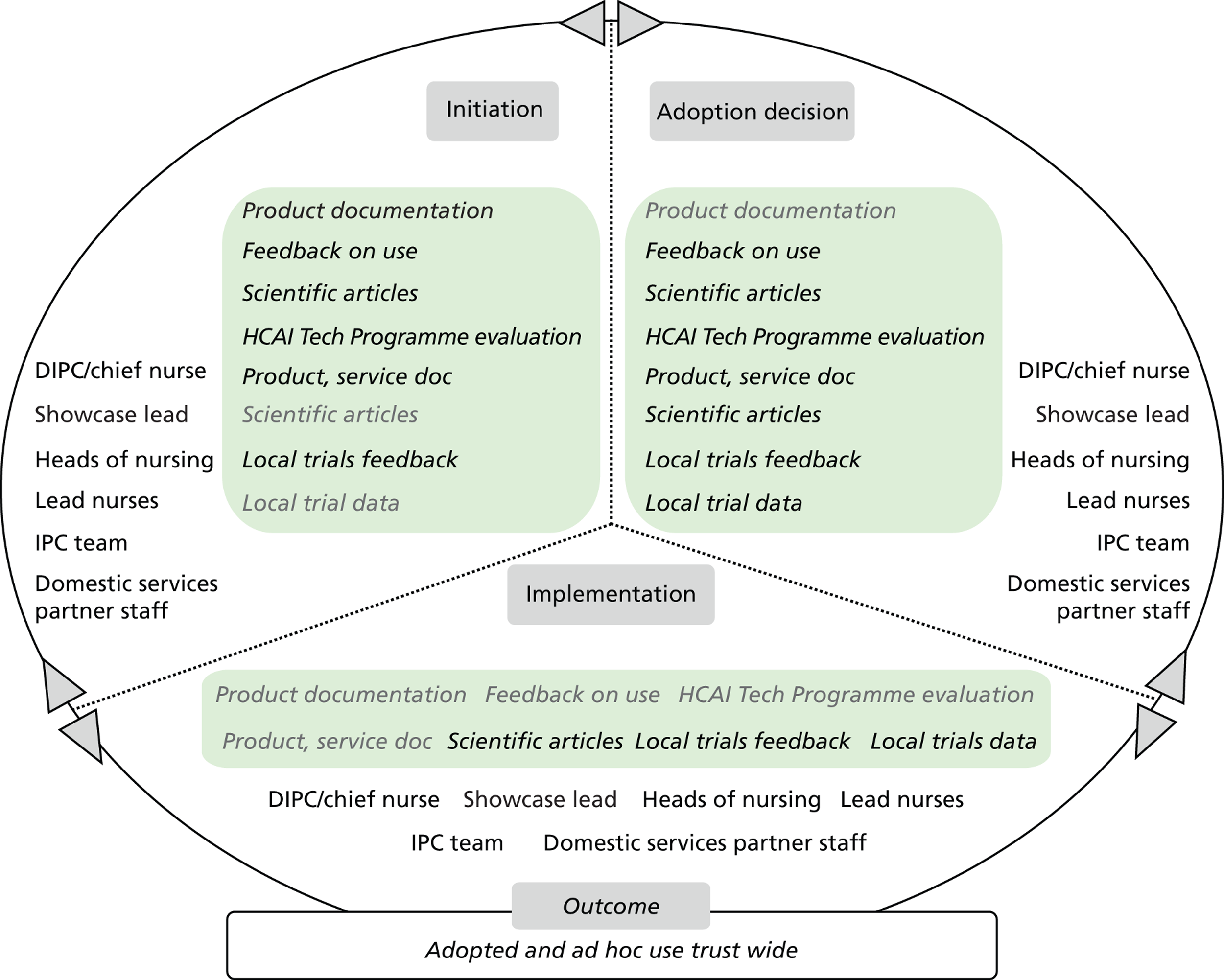
Outcome
The technology was viewed positively by T5 informants. Users’ support during the pilot was evident, in part facilitated through awareness of the Showcase Hospitals programme, and by showcase lead acting as a boundary spanner. One respondent said:
[. . .] we had a showcase lead nurse so they were, she was the one, or he was the one at the time who was involved in about six different projects around the showcase hospitals. And he was sort of liaising with the different teams so [. . .] it was discussed as a division, if you needed it then come down from infection control because they’d get the results, and then it would come to use and say ‘right, where do you want Bioquelling?’ or whatever the need was.
Senior nurse
In addition, anecdotal feedback was suggestive of a positive reception by T5 staff and patients. The service was understood to improve on the inconsistent efficacy of manual cleaning, reduce the cleaning workload for nursing staff and relieve training needs for domestic staff associated with a high turnover. A less positive aspect is identified in the waiting times for rooms to become available for occupancy and bed management issues associated with them. The sustainability dimension was also discussed during decision-making.
The service was again used successfully during the 2011–12 winter months in wards reporting a higher than usual pathogen incidence. The trust has been looking at different options for adopting HPV technology in tandem with its cleaning regime and staffing requirements, in close collaboration with its domestic services partner. This has involved the preparation of a business case for a further HPV evaluation, which has been led by the IPC team in collaboration with hospital divisions.
Overall, a key factor in facilitating the trial has been that the technology became available as a managed service. However, the disruptive effects that emerged during the trial, related to patient flow, staff deployment and bed management, are understood to have played a key part in the decision taken.
Microcase 2: Chlor-Clean tablets
Attributes perceived by stakeholders
Chlor-Clean is perceived to be a low-tech, innovative product which falls within the national guidelines on effective sporicidal cleaning. It is understood to be easy to use. However, attention is required to use it correctly: that is, the tablet has to be diluted in water to achieve the concentration recommended by guidelines. Prior to adopting Chlor-Clean, Haz-Tabs (Guest Medical Ltd, Aylesford, UK) were used comprehensively at T5, along with detergent wipes where appropriate. Haz-Tabs are now used only in cases in which a higher-chlorine-concentration agent is required, for example in cases of bodily fluid spillage. Detergent wipes are used when cleaning with a lesser chlorine concentration is considered appropriate.
Stakeholders, evidence and decision-making
Senior IPC team members collaborated with the manufacturer to develop a protocol detailing how to use Chlor-Clean in clinical areas. The adoption decision was taken by the IPC team. Subsequently, the product was introduced across the trust on the basis of active electronic, verbal communication and a teaching schedule. Ward link nurses liaised with the IPC team members and ward staff with regards to the product on a regular basis.
Evidence was shared with other hospital groups via the intranet IPC team website; however, the exact types and sources of evidence distributed have not been determined. Ward staff seemed unaware of the product’s specific relative advantage, as well as of its cost and sustainability aspects. They seemed to place trust in the work of the IPC team preparing the product’s implementation.
Implementation centred on the use of posters in wards and cascade teaching as part of T5’s routine teaching programme. This also included demonstrations about how to dilute tablets in water to achieve the required concentration, and how to conduct cleaning using the product. Instructions have also been included on the containers and tablet tubes themselves.
Table 30 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 22 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Health agencies |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Peers and colleagues |
|
FIGURE 22.
T5 Chlor-Clean tablets: professionals’ engagement and evidence types in decision-making.
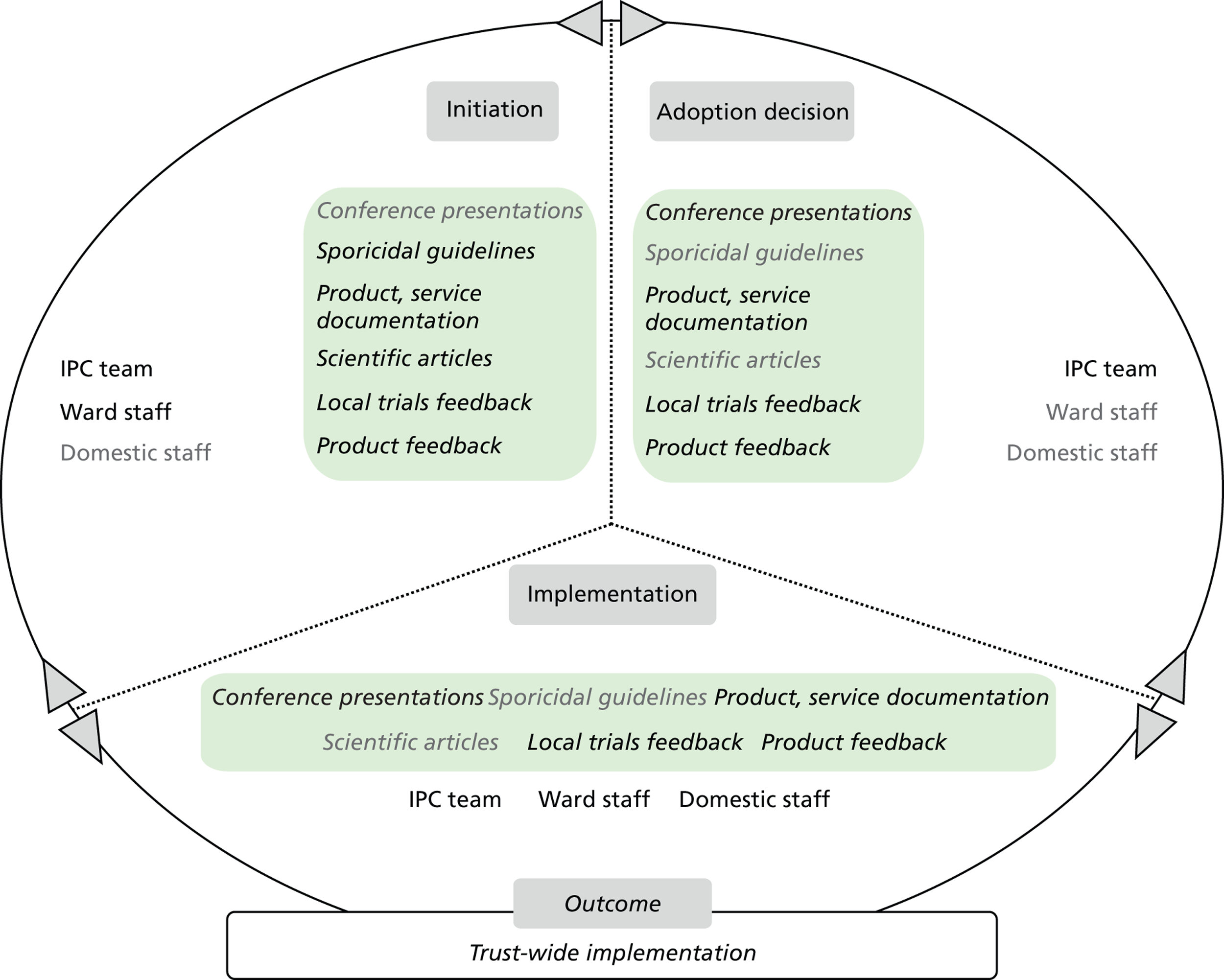
Outcome
Since its introduction, the product has been adopted and widely used by ward staff and domestic staff. The product has been successfully mainstreamed across T5 and used extensively in the last 5 years. Informants suggest that staff are generally happy with using the product and seem inclined to associate it with a reduction in infection rates. Two problems with its use were reported as part of this research. One concerns the undesirable effect on surfaces observed after repeated use: many surfaces start to show signs of breaking down once repeatedly cleaned with Chlor-Clean. A similar concern was raised with regard to using probes for oxygen saturation in areas recently cleaned with the product. Soap and water continue to be used for soft, for example fabric, surfaces, such as mattresses.
Microcase 3: 3M CleanTrace and Hygiena SystemSURE II ATP hygiene monitoring system
Attributes perceived by stakeholders
Informants from T5 understand both systems to be low-tech products, new to the NHS and relatively easy to use; the 3M product is viewed as somewhat easier to handle. Storing results on a server for later viewing is considered a high-tech feature; however, respondents suggested that the graphs and tables generated through the software are not always easy to make sense of. Before their trial, there was no means of determining the efficacy of surface cleaning other than visual inspection. The systems are thought to be in line with T5’s values and culture, emphasising infection prevention and environmental hygiene. However, their introduction was met with some difficulty vis-à-vis systems and structures supporting care delivery at T5.
Stakeholders, evidence and decision-making
The Showcase Hospitals programme provided ideation about this technology. Three local trials supported by the programme were conducted in specific hospital areas of high patient throughput, such as the medical assessment unit, neonatal and paediatric intensive-care units, and renal and gastroenterology wards. Specific surfaces were selected that represented areas of high frequency of use by ward staff. In one ward, patient feedback was sought through questionnaires about their confidence levels. These patients were presented with ATP data collected from their bedside surfaces. In other wards, managers and matrons presented data as they became available.
The trials were supported by academic staff based at Loughborough University, who visited wards to collect data through swabbing. One respondent described this as follows:
[. . .] what we agreed was that every week that the ward managers would get feedback from Loughborough University, from the professor. [. . .] Every week, every Monday morning, OK. And it came to me, and it came to the ward managers because obviously that’s their immediate area, OK. And he actually would highlight for them, this may need a little bit more attention or whatever, but overall the results were very, very good, very good.
Senior nurse
Additional staff members were also hired to work with the system and sample certain surfaces, which were agreed upon beforehand between ward staff and the IPC team. Results, in the form of tables and graphs, were fed back to T5’s staff of those wards and clinical areas where trials took place.
Stakeholder groups involved included the IPC team, ward staff, domestic staff (housekeepers), heads of nursing and the trust’s infection control committee. The T5 showcase lead has been identified as a ‘champion’, as a result of him or her enacting communications about the trials among staff groups, advocating the added value of the systems and the trial to the trust and organising data collection. One respondent commented:
I e-mailed the matrons, the ward managers and the heads of nursing for them areas and said, we’re going to be given the opportunity to do this, I see it as a very positive step forward, I involved people like [. . .] and all these people from Sodexo, and I said if we use it as a positive thing it’s going to be a very good guideline for as to how well we’re doing our cleaning. And as we all know clean hospitals are something that’s very high on the agenda for the Department of Health. So I kind of sold it to them in a very positive way.
Senior nurse
The types of evidence featuring in the adoption process as suggested by informants ( Table 31 ) included product documentation available through Showcase Hospitals, presentations at professional network conferences, RRP recommendation reports, feedback from other trusts, data generated through local trials. Figure 23 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Showcase Hospitals programme | Product documentation |
| Professional networks | Conference presentations |
| Health agencies | Department of Health’s HCAI Technology Programme evaluation reports; HPA RRP recommendations |
| Other trusts | Local trials feedback |
| Trust care environment | Local trial data |
FIGURE 23.
T5 3M CleanTrace and Hygiena SystemSURE II ATP hygiene monitoring system: professionals’ engagement and evidence types in decision-making. IC, infection control.
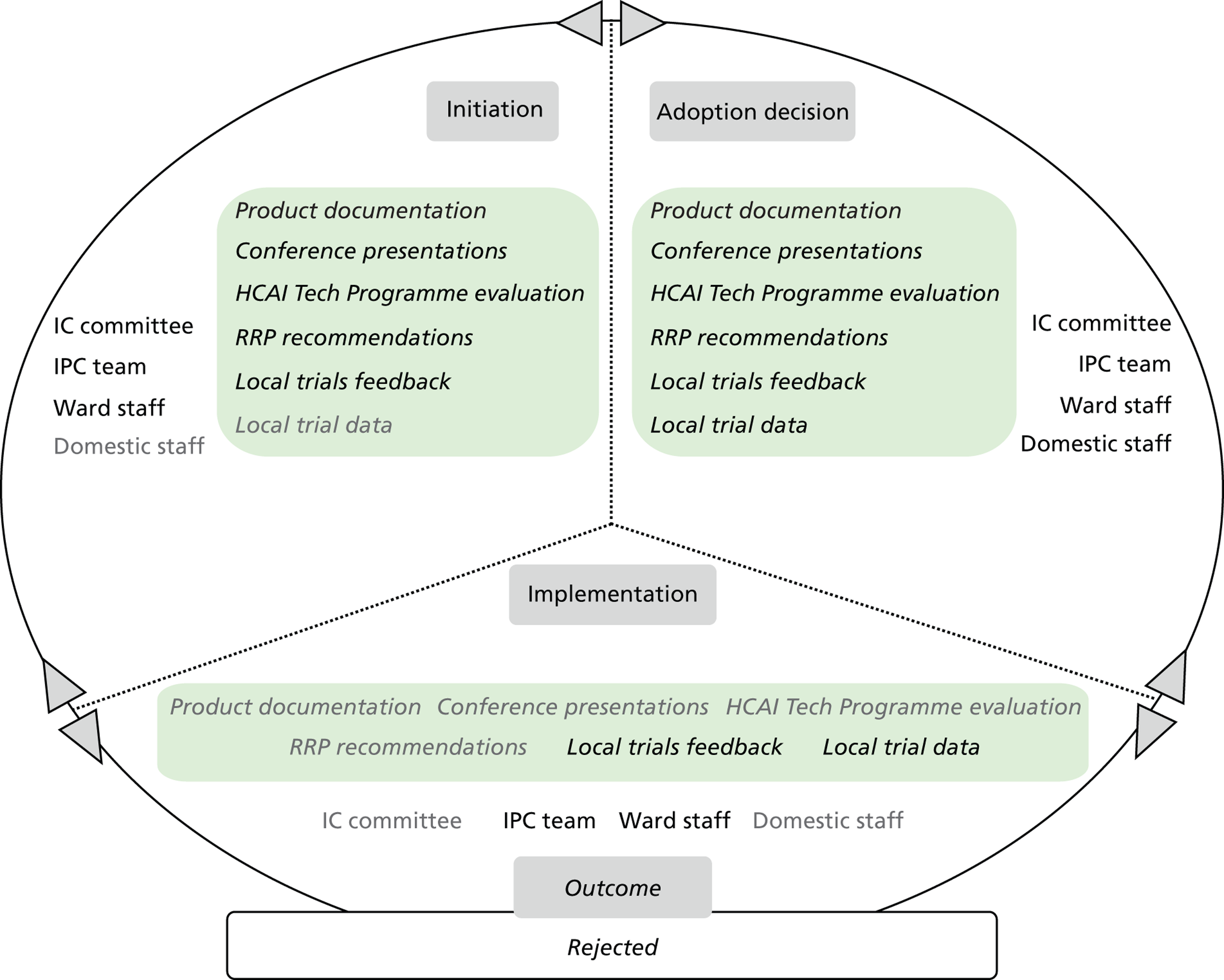
Outcome
The results of the trial suggested to T5’s staff members that the trust’s cleaning regime and practices were generally quite effective. Both systems were viewed positively by most professionals and hospital groups involved in the trials. Domestic and nursing staff members in wards where trials took place were reportedly enthusiastic about the systems; however, information or training does not seem to have been provided systematically.
Following the trials, use of the two systems was discontinued, as there was no interest shown by T5’s senior staff. One incompatibility was identified between the use of Chlor-Clean tablets and ATP swabbing: a particular ingredient of Chlor-Clean altered ATP measurements. Cost implications had become apparent during the trials. In addition, no particular hospital group or team was identified that would utilise the system without adding to the existing workload of staff and disrupting existing care delivery systems and processes. Use of the two systems was thus discontinued.
Trust 6 technology product microcases
Microcase 1: Bioquell vapour hydrogen peroxide Room Bio-Decontamination Service
Attributes perceived by stakeholders
This technology is understood by T6 informants to be an effective means of decontamination, and more effective than manual cleaning, as hydrogen peroxide vapour can access those areas that may not be readily accessible by manual cleaning. It is viewed as a new, ‘high-tech’ system, a departure from the traditional means and methods of cleaning. Prior to its introduction, manual deep clean and steam cleaning were practised. It is, otherwise, thought to be relatively straightforward to explain and train staff members in the use of. However, the time taken to carry out the procedure is understood to be a disadvantage regarding its use in the trust and the NHS more widely. Benefits arising from its use may include a safer care environment for patients and staff, a reduced workload for staff, a higher throughput and better financial position for the trust, and improved quality of care in the NHS.
Stakeholders, evidence and decision-making
The technology was introduced in the trust as an environmental cleaning measure to help alleviate the impact of a prolonged outbreak that caused significant disruption. Since then, it has been used one more time for the same purpose. A respondent discussed how evidence is sourced:
We’ve, oh right OK, obviously from your training. If you’re a microbiologist you would have gone through the contamination training and things like that. You would have gone through conferences, you would have seen it with hydrogen peroxide. The pressure to use it would have come from the outbreak control meetings. . . . So there’s a lot of things that converges through, focus you. And sometimes the drug reps or the manufacturers, if they’re present they might come and talk to you.
Senior doctor
Trust staff members were approached by companies offering VHP-based products. The RRP recommendation of ‘1’ awarded to the service was a key reason for deciding to introduce the technology to the trust. The IPC and E&F teams took part in the evidence review and decision-making, whereas the DIPC led the process. The decision to bring in the Bioquell service was taken by the DIPC and director of E&F.
The incidence of outbreaks, the introduction of this technology in other trusts and trust members’ awareness of that were the other factors leading to this decision. As one respondent commented:
So I think your need, and what’s going on in your organisation at the time also directly influences your willingness to go out there and seek the new innovation and technologies.
Senior nurse
Another respondent said:
There was good consensus, we bought it in after a couple of outbreaks. We were not meeting the objectives and the targets that we were getting from [the Strategic Health Authority], so it was felt that although this was over and above what is recommended nationally, it would be a good decision for the trust to undertake.
Senior nurse
Table 32 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 24 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Health agencies |
|
| Practitioner journals |
|
| Professional networks |
|
| Industry/suppliers |
|
| Other trusts |
|
| Trust care environment |
|
FIGURE 24.
T6 Bioquell VHP RBDS: professionals’ engagement and evidence types in decision-making. IC, infection control.
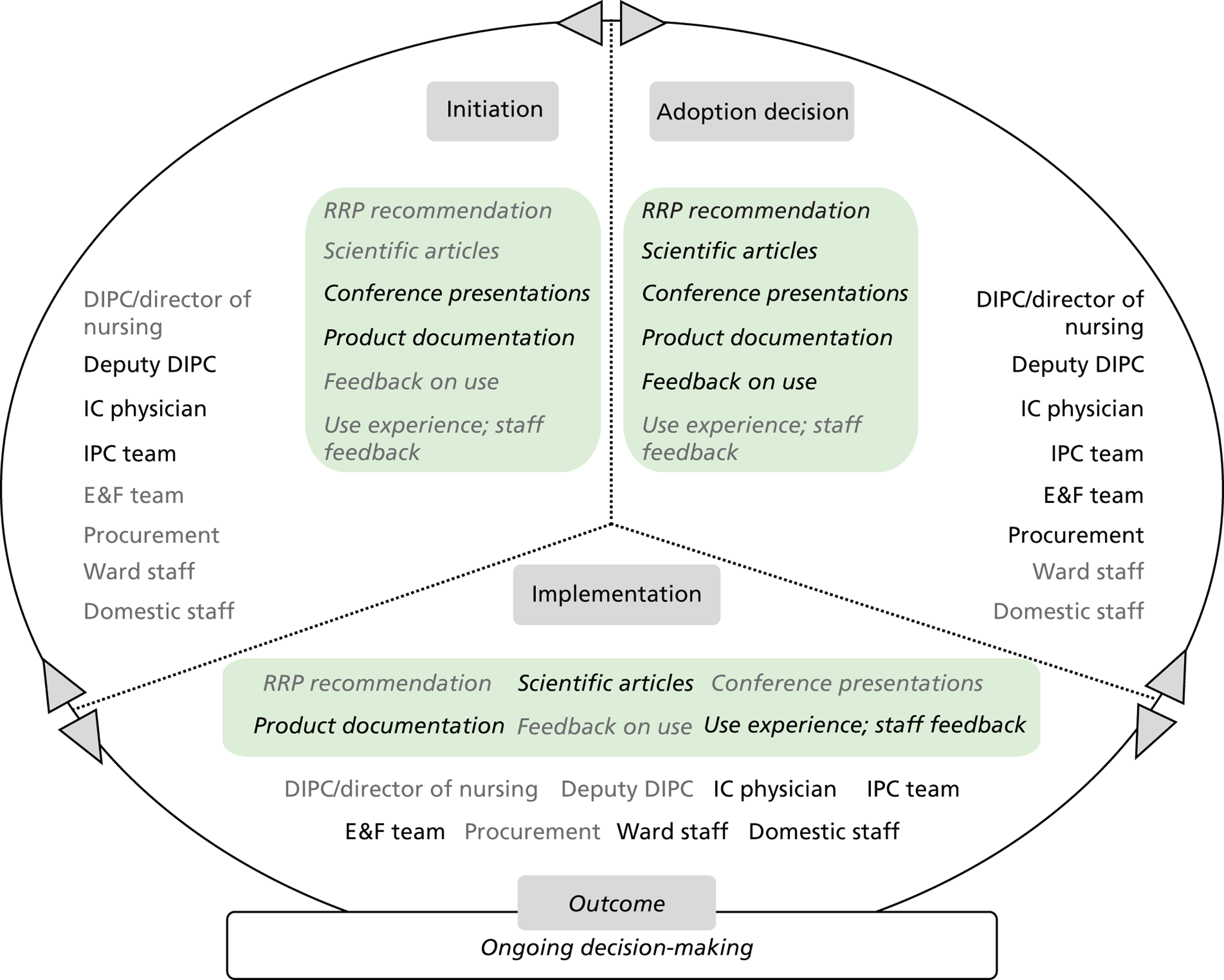
Outcome
Decision-making was channelled through the trust’s outbreak management committee. The final decision to introduce the technology was taken by the DIPC. The DIPC is understood by some respondents to have championed the introduction of VHP technology in the trust. One respondent commented:
So [the DIPC], I would think [the DIPC] was obviously a champion. [the DIPC], not because of the evidence and things, there’s a lot of pressure that comes to the DIPC and the DIPC thinks differently in terms of management.
Senior doctor
The experience of having used the service is thought to be a positive one by the trust’s staff members, with particular regard to the support offered by the supplier. Some reservations have been expressed regarding its use, as it introduces a time lag before bays become available after decontamination. A respondent suggested:
I think the main thing I said they were a barrier is the patient flow and the activities, yeah, disruption to that. That’s the one nobody can swallow.
Senior doctor
Senior trust members wish to establish whether the evidence is strong enough to confirm its efficacy against other methods of cleaning. If it is to be mainstreamed, it should be better only than current cleaning methods such that it can deliver better patient outcomes in tandem with other measures.
Microcase 2: JLA OTEX system
Attributes perceived by stakeholders
OTEX is understood by T6 informants to be a novel way of machine washing fabric-based care and hospital equipment, not by traditional means of water and detergent, but through the release of ozone. Respondents suggest that there is substantial evidence to support the efficacy of this technology, in particular its ability to release dirt from fabric. It is understood as a fairly well-established and validated technology, viewed as simple to explain and quite compatible with T6’s values and culture. Informants’ opinions seemed to differ on whether there is added value from this technology and benefits to patients, staff, the trust and the NHS more widely.
Stakeholders, evidence and decision-making
The deployment of OTEX coincided with the introduction of microfibre mops and cloths in the trust. Following a suggestion to use the product by the trust domestic services partner, a business case was prepared. This was reviewed first by the IPC team and then by the trust’s IPC Committee, whose membership includes the trust’s directors of operations, other senior trust board members and senior domestic services partner staff. The business case was reviewed in tandem with other evidence types, which are shown in Figure 25 , with a view to the new system featuring in the trust PFI contract. As one respondent commented:
I don’t think cost has been an issue, I think the only problem there might have been is [the hospital] being a PFI and getting the agreement of the PFI Group to agree the installation of the machines.
Senior nurse
FIGURE 25.
T6 JLA OTEX system: professionals’ engagement and evidence types in decision-making. IPCC, infection prevention and control committee; SH, Showcase Hospitals.
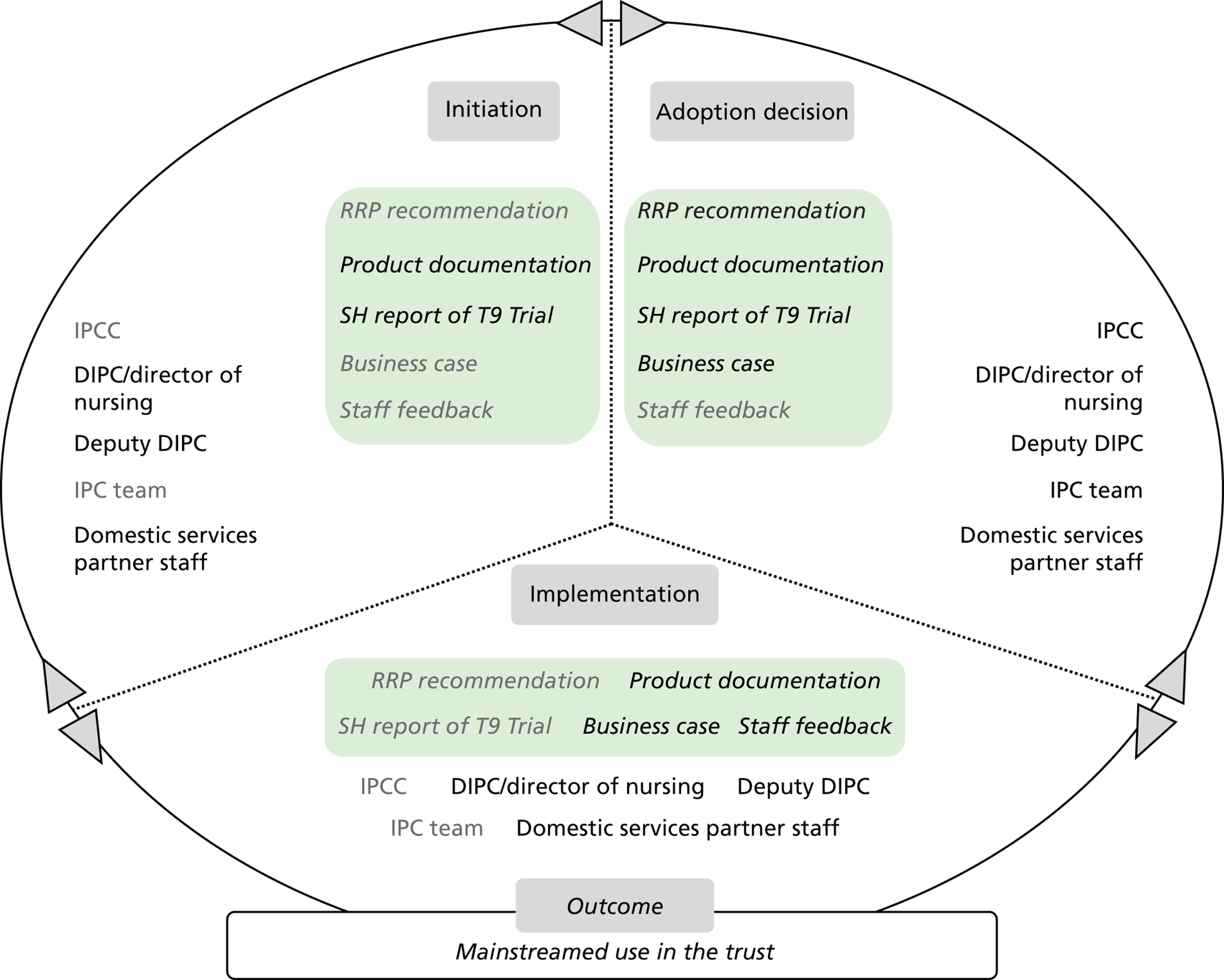
Table 33 lists the specific evidence types used in this microcase alongside the sources from where these were elicited.
| Evidence sources | Evidence types |
|---|---|
| Health agencies |
|
| Industry/suppliers |
|
| Showcase Hospitals programme |
|
| Trust care environment |
|
Outcome
The technology has been mainstreamed in the trust and has become part of the trust’s PFI contract. This is monitored through meetings between T6’s board members and senior staff of the domestic services partner. T6’s domestic services partner has assigned ‘championship’ responsibilities to their staff members deployed in the trust, who perform all tasks related to the system’s operation in collaboration with the trust’s ward staff. This aspect is viewed as important vis-à-vis adoption and continuous use. One respondent commented:
[Domestic service partner staff] works, once they’re on the ward we have what we call devolution, where they become part, they are part of the ward team . . . they’re [domestic service partner] employees, but once they work on the ward they will take a lead from the sister [on that ward].
Senior non-clinical manager
The trust’s domestic services partner also offers courses and product-based training, demonstrations and training sessions with trust members.
Microcase 3: Medixair UV air sterilisation units
Attributes perceived by stakeholders
Informants from T6 understand Medixair to eradicate air-borne bacteria and be appropriate for use in clinical areas. They are viewed as ‘high-tech’, radically new pieces of equipment whose efficacy has yet to be supported by robust evidence. They are also understood to be compatible with existing trust care systems and structures. Benefits anticipated included a reduction in cross-infection, a cleaner care environment and a perception of actively improving care on behalf of patients.
Stakeholders, evidence and decision-making
The technology was considered for introduction in the trust care environment both as an opportunity in itself and as part of a suite of measures to help reduce MRSA bacteraemia colonisation through air-borne bacteria in bays.
Ideation began through a communication by the supplier to the CEO at T6. The supplier forwarded product documentation, a report on the equipment’s scientific principles of operation and case studies of the equipment’s use in other trusts. These were then forwarded to the IPC team for consideration. The HPA RRP recommendation on the product was sourced during the adoption decision stage. The entire evidence review and decision-making process lasted for approximately 1 month. The Deputy DIPC collected all evidence types and information, and distributed it to the other stakeholders.
Table 34 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 26 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Industry/supplier |
|
| Health agencies |
|
FIGURE 26.
T6 Medixair UV air sterilisation units: professionals’ engagement and evidence types in decision-making.
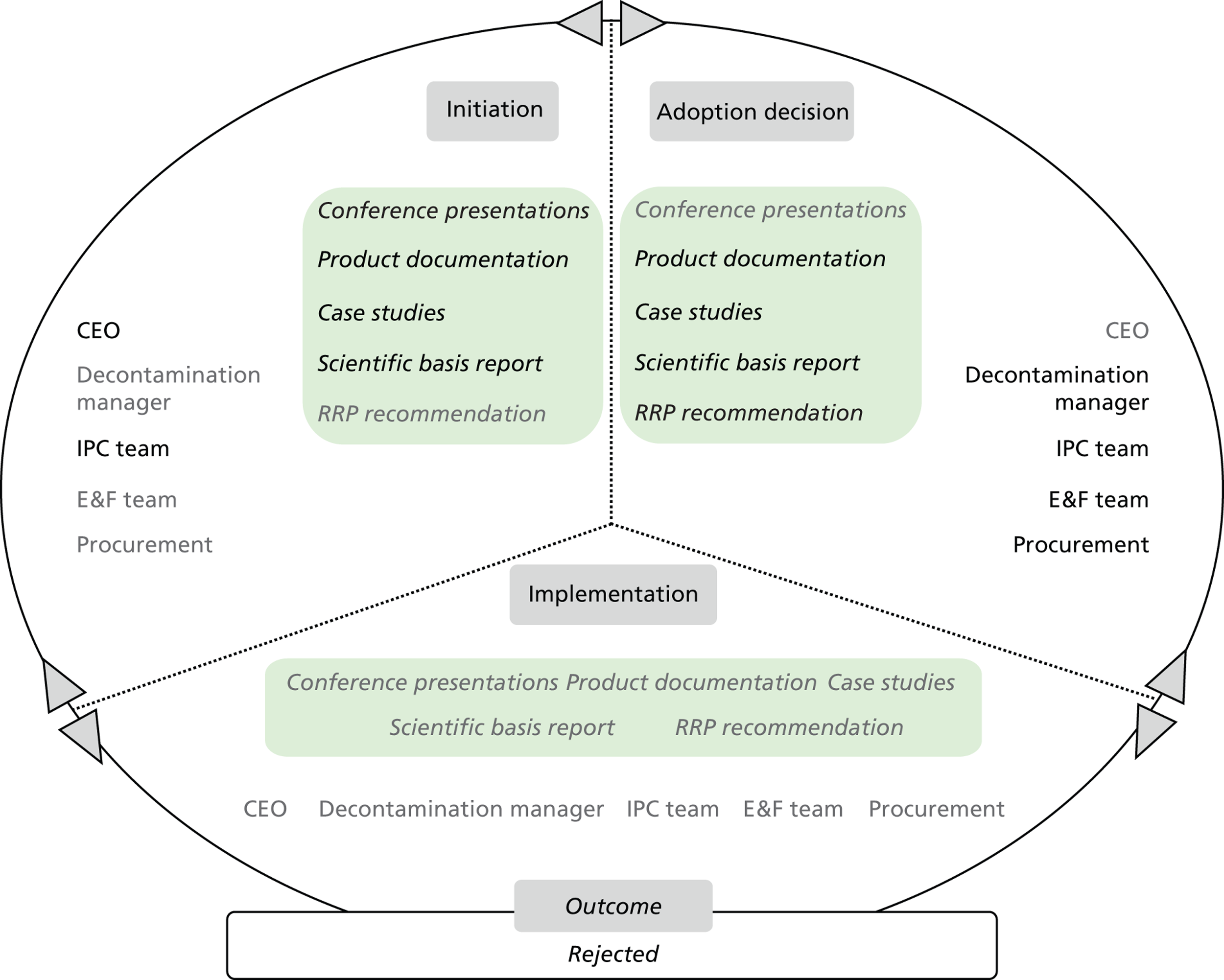
Outcome
The outcome of the evidence review and decision-making process was a rejection of the systems. The trust’s infection control physician and decontamination manager scrutinised the evidence and suggested that the alleged efficacy could not be sustained on the basis of the product’s principles of operation. Informants suggested that this scrutiny and derived conclusion was accepted on the basis of the specialist training of their two senior colleagues. Informants also felt that, overall, the evidence wasn’t robust enough to warrant their further engagement with this technology. A respondent commented:
So we all basically sat round the table, having looked at the information that we’d been given, and came up, had a discussion around whether we felt this was worth pursuing. The outcome was that the evidence really wasn’t very strong, and we probably wouldn’t pursue it.
Senior nurse
Trust 7 technology product microcases
Microcase 1: DIFFICIL-S disinfectant liquid detergent
Attributes perceived by stakeholders
DIFFICIL-S is understood by T7 informants to be a new cleaning and disinfection product based on chlorine dioxide, which is produced upon mixing. It is considered to be particularly effective against C. difficile vis-à-vis other disinfectant products, resulting in a higher level of decontamination and cross-infection mitigation. It is understood to have been evaluated by other trusts and still not widely adopted.
It is viewed as a product that is only somewhat complicated to prepare owing to its mixing requirement. Chlor-Clean, the earlier disinfectant used in T7, also requires mixing with water in a container. Hence, DIFFICIL-S has been viewed to be compatible with cleaning practice and easily adoptable by T7 staff based on their experience with Chlor-Clean. It is also thought to be in line with T7’s values and culture, including a focus on implementing innovative technologies that add value to care delivery. Anticipated benefits are a lower C. difficile incidence, a safer care environment for patients and staff and a shorter patient length of stay. Further positive effects are expected vis-à-vis the trust’s reputation with the public and health-care regulators.
Stakeholders, evidence and decision-making
The trial and use of DIFFICIL-S featured in an action plan towards mitigating C. difficile incidence, which was regularly reviewed by the IPC team and senior managers. This action plan was put together as part of a business continuity risks management suite of measures designed by T7’s senior staff:
. . . the decision to trial it was in response to the increase in C. difficile and there were regular meetings with the Chief Executive.
Senior nurse
A trial was organised on the basis of the product viewed as an opportunity to improve care delivery. The decision to trial the product was taken among the DIPC, lead infection control nurse and the head of domestic services. The trial was organised in four areas of one T7 hospital site and two wards in the T7 main site, and lasted for 10 weeks. The lead infection control nurse organised the trial and led the process, which involved ward visits and meetings with domestic and ward staff and monitoring of progress at the IPC team meeting.
Senior nursing staff were informed of the trial at a special meeting. Ward staff, including link IPC nurses, were briefed on the product by a company representative. Domestic services staff applied the product during the trial, including using swab equipment to check cleaning efficacy. There was no patient involvement. Junior IPC team members attended a demonstration in one ward where the trial was taking place. Company representatives explained the process of producing and then using the product, and responded to questions.
During the trial, complaints were made of a rather unpleasant scent in bays and throat irritation after product use. Other concerns put forward included the somewhat longer time for disinfection to take effect and increased usage of cleaning cloths. In addition, the product came at a significantly higher cost than the incumbent product. Cost, staff and training implications, arising from frequency of use, mainstreaming the product across the trust or introducing the product in areas where the C. difficile incidence was higher than others, were reviewed in the meetings of T7’s infection control committee, which included stakeholders such as patient groups, the local primary care trust and the HPA.
Evidence types featuring at the initiation stage included presentations at professional network conferences, product test data obtained from the Birmingham-based Hospital Infection Research Laboratory, product documentation and demonstrations organised by suppliers ( Table 35 and Figure 27 ). Product principles were examined by recourse to the following types: a peer-reviewed journal article, feedback on use sourced from other trusts, and data and staff feedback from T7’s local trial.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Health agencies |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Peers and colleagues |
|
| Trust care environment |
|
FIGURE 27.
T7 DIFFICIL-S disinfectant liquid detergent: professionals’ engagement and evidence types in decision-making. IC, infection control.
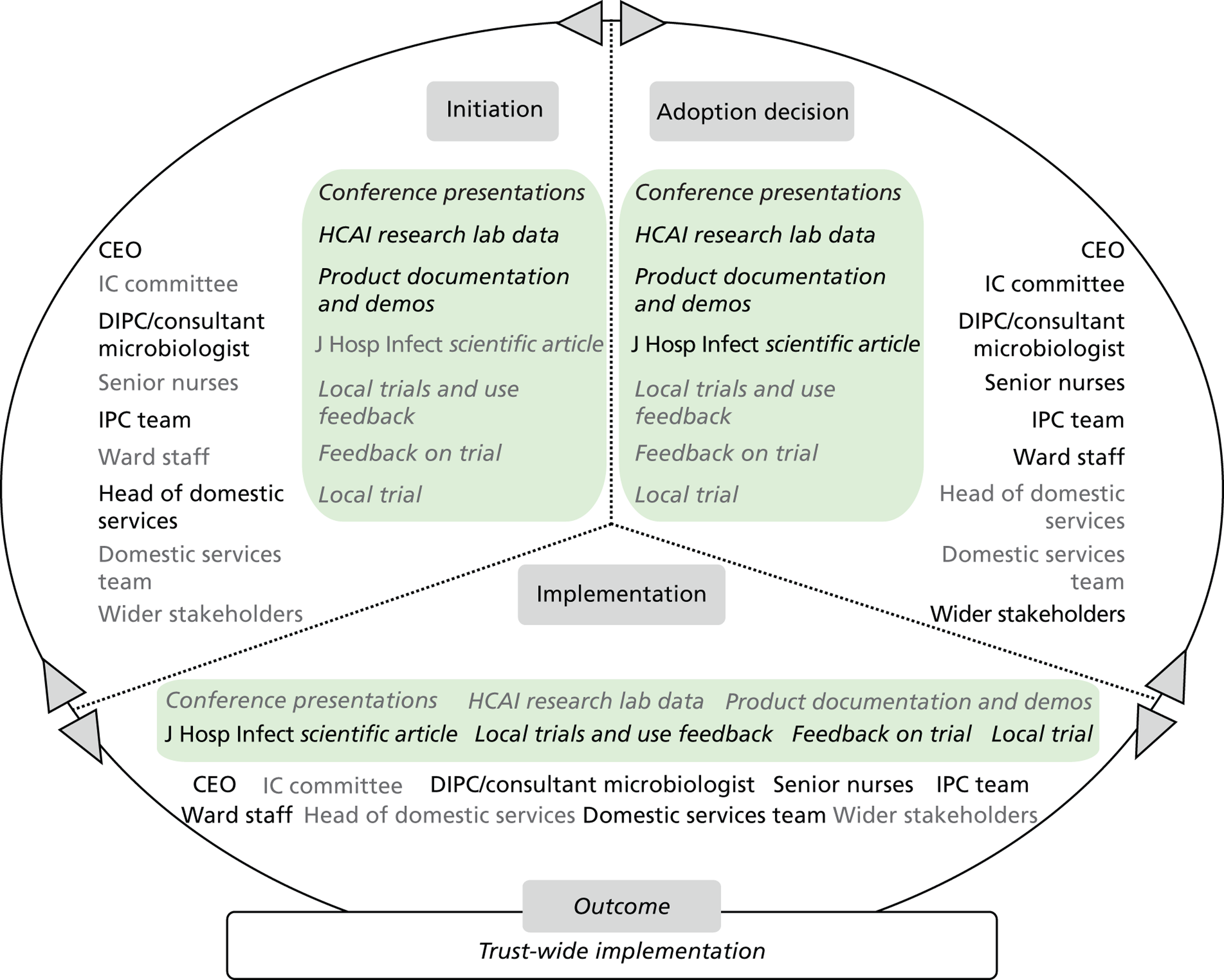
Outcome
The outcome of the trial was positive. A somewhat lower cleaning aesthetic was identified on ceramic and stainless steel surfaces. The use of additional cleaning agents was thought to be required in those cases. The trial and related evidence were reviewed by the T7 Trust Executive Group:
[. . .] the purpose of that group is to do that challenging, it’s to make sure that there is robustness to the decisions that you make. Usually with the things that we’ve done there has been enough evidence to suggest that this is well worth doing and therefore the dissent is lessened.
Senior nurse
Following the trial, DIFFICIL-S was adopted by the trust in December 2011. As part of T7’s C. difficile Action Plan for 2012/13, it is now used in the T7 main hospital’s medical assessment units, surgical assessment centre and haematology unit as well as in another trust hospital. It is also used following any individual cases of C. difficile and in any risk areas identified.
Microcase 2: ASP GLOSAIR 400 aHP system
Attributes perceived by stakeholders
The promise of this particular technology, the C. difficile incidence at the trust and funding availability provided the impetus for the decision to review and trial this product. The choice of technology for the trial was the Bioquell VHP system. Locally generated evidence appeared to be important to trust members of different seniority and professional background. A respondent noted:
Anything that we do, I think we try, we usually do an audit on and give feedback. Sometimes it may just be the infection link nurses that will do it with them. But normally we trial quite a few things on our ward.
Senior nurse
Several issues regarding room closure, hospital air conditioning systems, fire alarm systems, bed management and Control of Substances Hazardous to Health were identified and reviewed by T7’s senior managers. A significant financial impact is reportedly associated with the purchase of the machines and staff training costs. A respondent commented:
Certainly in terms of this technology there is an ongoing cost but within the terms of disposables, in terms of the hydrogen peroxide canisters. But equally . . . maintenance costs for the machines themselves . . . are not inconsiderable. So this will have been considered at a high level within the trust. Agreement has been made about how that’s funded and it is being funded. We will need to review because these things won’t last forever and, but it would not be conceivable [that] the trust would go forward without this technology.
Senior nurse
Stakeholders, evidence and decision-making
The chief nurse, deputy chief nurse and lead IPC nurse are viewed as trust members who championed the technology. Use of aHP was understood to involve a trade-off between bed availability and staff workload, and enhancing patient care. The senior team supporting the adoption of this technology worked through issues identified with colleagues from other hospital groups. A respondent commented on the approach towards evidence:
We became aware [of trials] across the country and we then began to look at those products from our own perspective and to see and test whether the evidence was there. There was some manufacturer evidence, research studies and . . . a rapid review assessment suggested, same as before that these things seemed to have got the science base to use them. By which stage we’d already got nine of them because locally we perceived that there was enough evidence to suggest that they were going to be effective in the way that we wanted to use them.
Senior nurse
Ten machines were then purchased, followed by another 10 after a few months. The two purchases represented a large capital outlay earmarked for adopting this technology. Overall, senior team members’ commitment is understood to have facilitated adoption. A respondent noted:
[. . .] some organisations will have different priorities, the speed of adoption is often about local factors as much as it is about evidence. We were taking a very, I’d call, forthright view at that time, we needed to do something different to get on top of things.
Senior nurse
Table 36 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 28 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Health agencies |
|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Other trusts |
|
| Peers and colleagues |
|
| Trust care environment |
|
FIGURE 28.
T7 ASP GLOSAIR 400 aHP system: professionals’ engagement and evidence types in decision-making. IC, infection control.
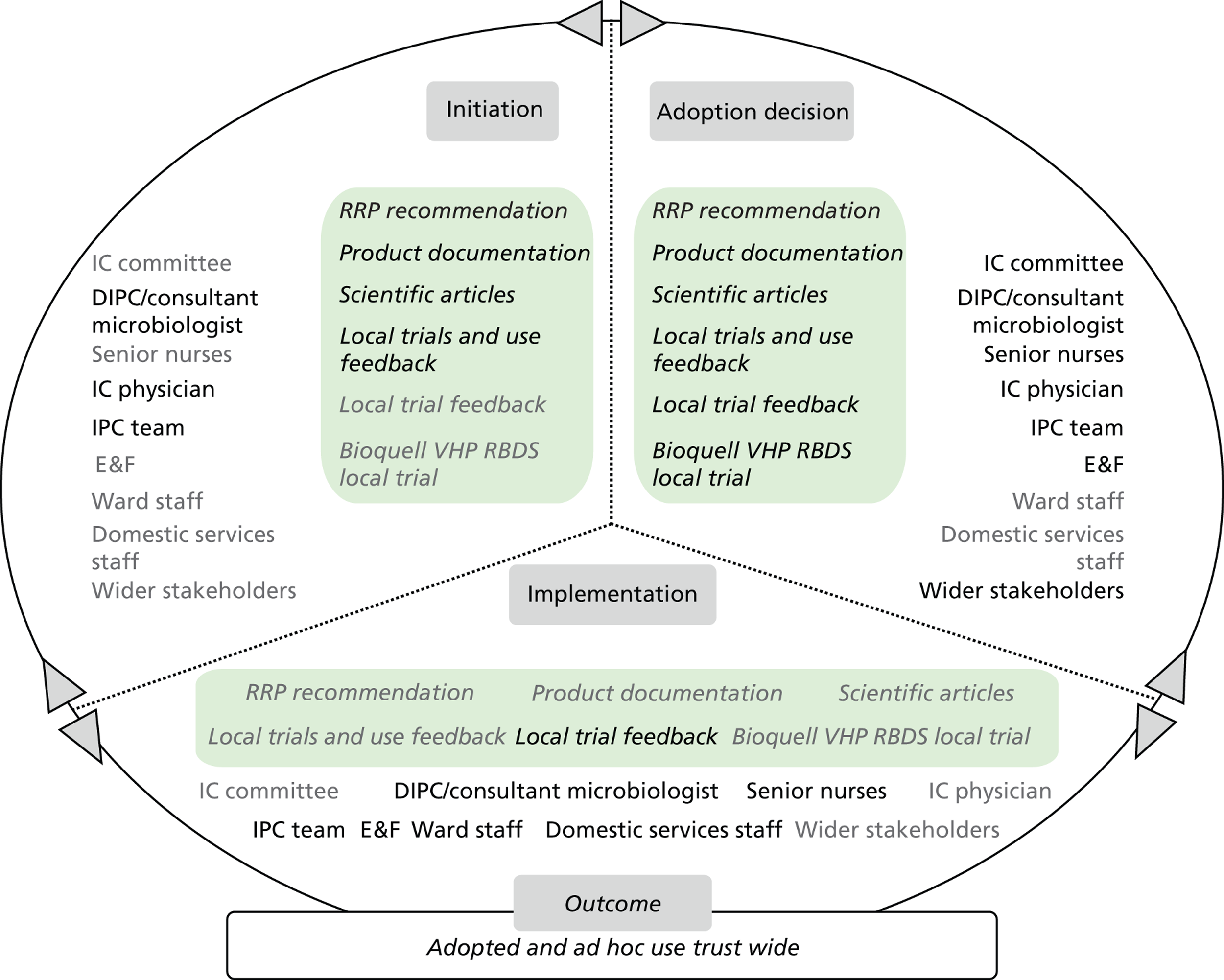
Members of the IPC team were trained to use the machines and initially operated these across the trust, with no implications arising for other hospital groups. Subsequently, the trust appointed infection control assistants as new members of the T7 IPC team, who were trained in how to use the aHP machines for a period of 4 weeks until they became competent. As an additional measure towards mitigating C. difficile incidence in the trust, domestic services and other hospital group staff members were trained and took on use of the machines. Bed management issues have been worked around successfully through liaison among IPC team members, bed managers and ward staff. Effective collaboration was also reported between domestic services and the IPC team. Despite some reluctance to taking on additional workload, the number of users and trained staff has expanded across the trust.
Outcome
Following the introduction of aHP and other measures to mitigate C. difficile at the trust, infection rates were falling throughout the post-implementation period. On this basis, and because of its wider utilisation, including norovirus cases and other pathogens, aHP is understood to be a successful technology, and remains central to the trust IPC strategy. One respondent commented:
I think what was really helpful was, shortly after they got introduced, there was a dramatic fall in the number of cases of C. difficile and so whether it was or not associated with this it would almost be impossible to say directly. But, having achieved such a high level of improvement, I think there would then be a reluctance to take away something which would be seen as an effective tool in the armour.
Senior nurse
Microcase 3: Medixair UV air sterilisation units
Attributes perceived by stakeholders
The Medixair UV air sterilisation units are understood by T7 informants to be new, high-tech products available on the market and to the NHS. Benefits associated with their use include a reduction in cross-infections from air-borne viruses such as norovirus. Air-borne viruses may spread quickly and cause a major operational impact on emergency, planned or elective care, including closure of bays and wards for decontamination purposes, as well as adversely affect trust reputation.
Stakeholders, evidence and decision-making
In their efforts to reduce infection rates further and limit the impact of outbreaks, senior T7 staff members looked at technologies capable of sterilising the air in rooms and stopping cross-contamination of air-borne bacteria and viruses. One respondent noted:
A couple of years ago the hospital was hit by a prolonged, disruptive [outbreak] . . . . It affected multiple wards, lots of bed closures, operational difficulties, a lot of disharmony in the hospital about how we were dealing with it and a frustration that it appeared that there was very little we could do to prevent the spread of this, perhaps. So a number of issues were looked at, one of them was for the potential for these units to, if you like, clean the air.
Senior nurse
The medical assessment units of T7 were identified as areas to deploy the Medixair units, because of the possible onward transmission of viral infections from these units to other hospital areas. During the initiation stage, there were also some tests in the ward environment of using portable UV light inspection devices in tandem with ATP swabbing. This has been viewed as a complement to cleaning conducted by domestic services, and an aid for them to check the efficacy of their cleaning process.
Consultant microbiologists led on evidence gathering and review in collaboration with T7’s deputy chief nurse, IPC team members and T7’s E&F at a subsequent stage. The supplier suggested a clinical trial should be organised; however, a consensus was reached among staff members that the evidence did not seem to support this. A smaller pilot, in the form of a case study on the product devices, was completed:
It was quite a small group really and it largely centred around infection control doctors, DIPC, lead infection control nurse, deputy chief nurse, the chief operating officer.
Senior nurse
The evidence suggested that the air intake and release rates of these particular products were a lot lower than those of similar systems used in theatres. On this basis, their use in busy medical wards proved to be ineffective. The initial and ongoing maintenance costs of these products were also understood to be high. The evidence base put together is understood to have acted as a barrier to adoption. The evidence base was reviewed and a decision to reject adoption was taken by the staff members mentioned above.
Table 37 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 29 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Professional networks |
|
| Industry/suppliers |
|
| Trust care environment |
|
FIGURE 29.
T7 Medixair UV air sterilisation units: professionals’ engagement and evidence types in decision-making.
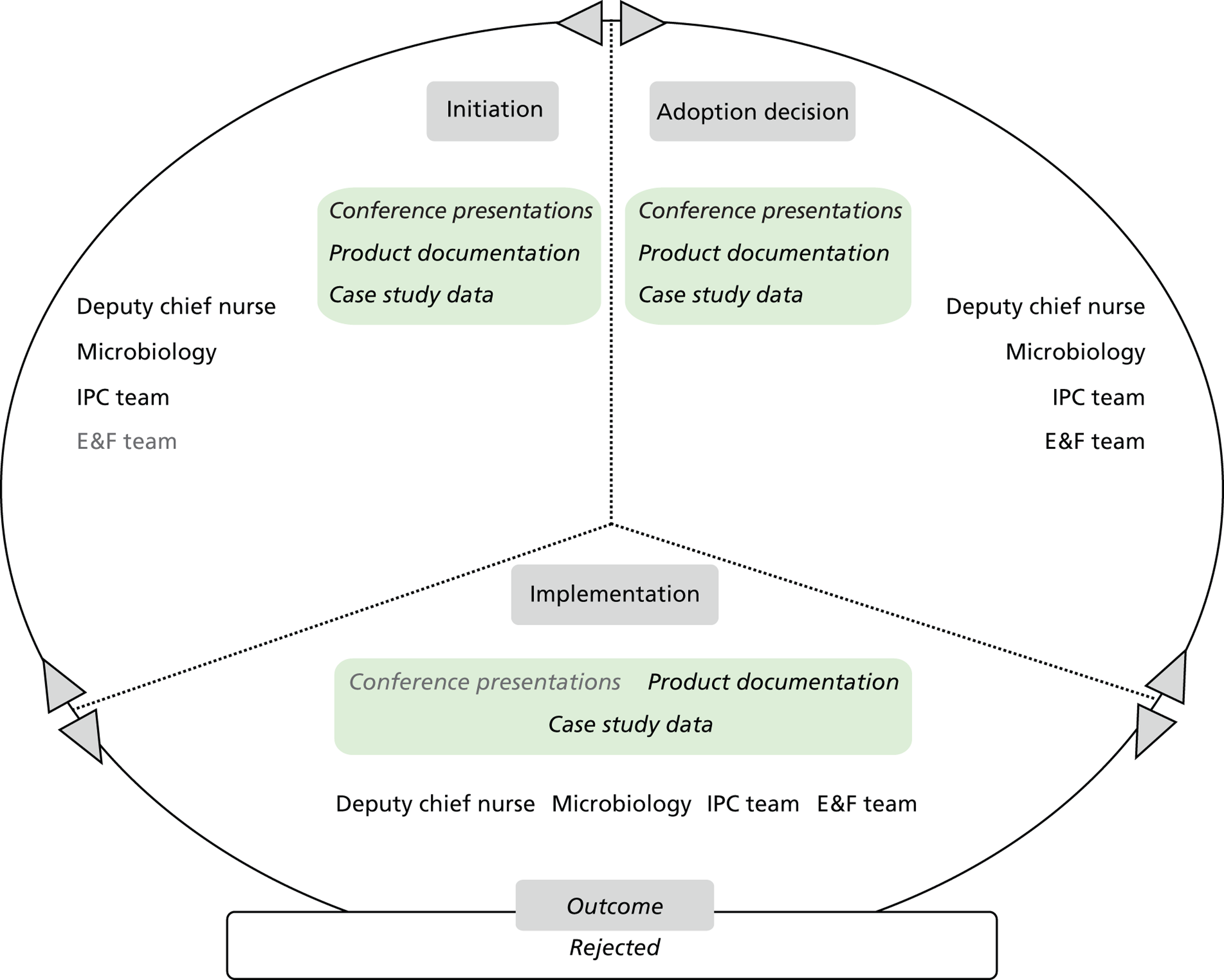
Outcome
Following review and deliberation of the evidence, it was felt that the efficacy of the Medixair units did not match the procurement and maintenance costs involved. The evidence, its examination and the decision to reject the product, which was taken by the group of staff members who looked at the evidence, were documented in a paper presented to the Trust Executive Group. The decision to reject its adoption in the trust was approved by the Trust Executive Group. One respondent noted:
Essentially, the infection control doctors looked very closely at this equipment and they did not concur with the manufacturer’s findings in terms of efficacy. And they did not feel that they could substantiate their claims.
Senior nurse
Microcase 4: DaRo UV light inspection cabinet
Attributes perceived by stakeholders
The UV light inspection cabinet marketed by DaRo UV Systems Ltd has also been trialled at T7. Informants understand this technology product to be based on organic material luminescence becoming visible with UV radiation. The technology is understood to originate from the food industry, and to have only recently been applied in health care.
Stakeholders, evidence and decision-making
DaRo UV light inspection cabinets were also introduced by the IPC team in wards, in collaboration with senior nurses and ward staff, as a complement to the trust’s hand-washing campaign. The product was first viewed at a conference by IPC team members, and was then discussed at a meeting with senior nurses. The decision to buy the product was reached swiftly, and the product was then actively promoted by hand hygiene leads in wards.
Outcome
The UV light inspection cabinet has been viewed as a tool to train staff in hand-washing techniques, to examine the efficacy of these techniques and to prevent cross-infection from inadequately sanitised hands. IPC nurses ran demonstrations of the cabinet for ward staff. The cabinets remain in regular use, including as part of the induction for all new trust staff.
Trust 9 technology product microcases
Microcase 1: clinell and PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes for skin preparation
Attributes perceived by stakeholders
Informants at T9 understand these two wipe products to have been designated to be suitable for use on medical devices, for decontamination and device preparation. The wipes, viewed as low-tech products, are required to be used with the technique termed ‘scrub the hub’, requiring friction to be applied while the hand is used to handle the wipe. They have not been licensed for skin preparation.
The product currently used for skin preparation in the trust and elsewhere in the NHS is an ampoule-based product that contains a solution with a chemical composition and specification and chemical properties very similar to those of the two wipe products. This is the ChloraPrep® (CareFusion Corp., San Diego, CA, USA) preoperative skin preparation antiseptic of 2% chlorhexidine gluconate and 70% isopropyl alcohol. Use of the ampoule also requires an appropriate topical massage routine that generates friction and helps with skin substance absorption. This technique, akin to the aseptic non-touch technique, allows the health-care worker to appropriately use the ampoule to decontaminate the skin before any procedure, while not coming into contact with the patient.
Use of the wipes in this innovative manner is understood to relate to earlier practice, before the introduction of chlorhexidine and ChloraPrep, when alcohol- or iodine-based wipes and solutions were used. The innovation is understood to be in line with the cost-saving culture at the trust. One respondent said:
No one to my knowledge has challenged it because it seems to make sense. We’re in difficult financial times and this is a classic example of what we call a QIPP [QIPP refers to ‘Quality, Innovation, Productivity and Prevention’, which is a Department-of-Health-led programme to transform NHS services to make efficiency savings of £20 billion’. 94] – quality improvement, innovation, innovation productivity, and prevention – where we see something that achieves the same outcomes but for much less financial outlay, so.
Senior pharmacist
Stakeholders, evidence and decision-making
The ideation for using wipe products in this manner originated from the trust’s Child Health Division. It searched for evidence to confirm that the use of ChloraPrep reduces infection rates. Such evidence was not identified; hence, the recommendation to use wipe products was made.
Senior managers reportedly understand ChloraPrep to be more expensive than the wipes, which are viewed as the same product but in a different format. The wipes have been highly efficacious in the trust in disinfecting intravenous devices, resulting in rates of MRSA bacteraemia, and other bloodstream infections related to central or peripheral devices, being close to zero. However, IPC team members also saw a risk in using the wipes in a patient context, as skin may be contaminated or recontaminated through wipe contact. Moreover, the friction required when using a wipe is understood to be considerably larger and applied during a longer time interval, entailing some risk of physical damage, particularly for frail patients. Therefore, an audit in the form of a local trial was decided as a means to collect evidence on this alternative use of the wipes and related staff training requirements. A small working group was formed to organise two trials in paediatrics and general surgery. One respondent commented on clinical engagement during the local trials, and identified surgical areas as ‘hot spots’:
They must have got quite a lot of consensus because as I said, some of the doctors have been a little bit difficult to change, that’s why it’s not fully rolled out in some, in all of the surgical areas.
Senior nurse
The trust’s infection control physician is understood to have led on evidence gathering and evaluation. A paediatrics lead nurse has acted as a local champion, leading the audit in that division and reporting on it. One respondent commented:
It’s very difficult when you’re implementing a change that everybody has to comply with and they don’t have achoice. So ultimately it is the ward nurses who are implementing this, they have to do it, they don’t have a choice, but you have to get them on side. I would imagine that the benefits of it were explained in that we’re reducing the MRSA, we’re saving the trust money, we’re promoting patient safety, patient care, which is ultimately, like I say, we all signed up to be nurses because we want to care for people.
Senior nurse
The way in which the evidence was communicated by senior nurses to the ward nurses, who were the implementers of the innovation, was believed to be rational sensegiving aligned with the ward nurses values and sensitivity to certain forms of evidence.
Table 38 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 30 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Industry/suppliers |
|
| Peers and colleagues |
|
| Trust care environment |
|
FIGURE 30.
T9 clinell and PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes for skin preparation: professionals’ engagement and evidence types in decision-making. DoN, director of nursing.
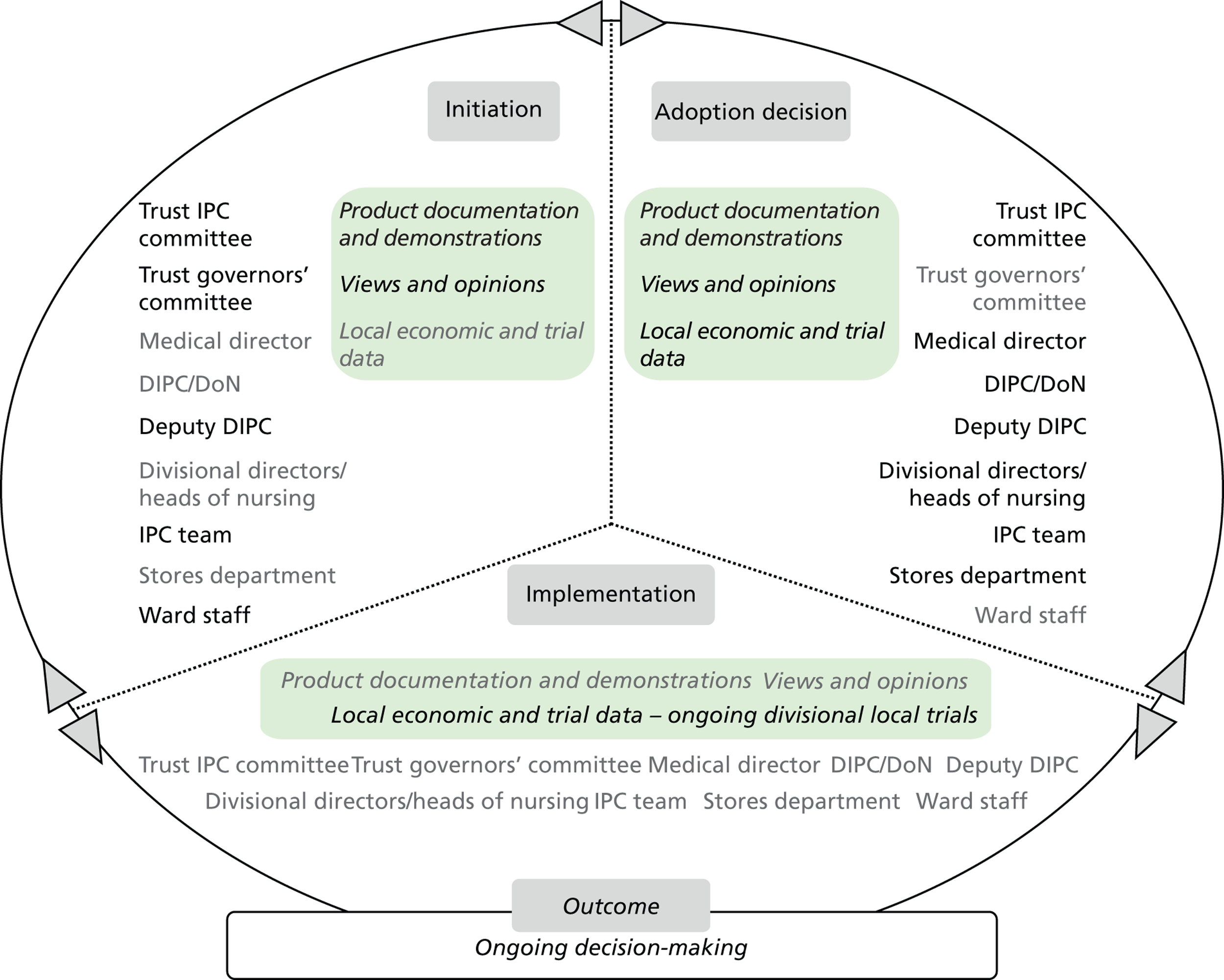
Outcome
The outcome is anticipated following conclusion and evaluation of the trust’s local trials.
Microcase 2: clinell sporicidal wipes (red)
Attributes perceived by stakeholders
Informants from T9 understand this product to be effective against C. difficile spores, which prove hard to remove with cleaning based on soap and water, and cause cross-infection among patients. The wipe’s active ingredient is activated by using tap water to wet the wipe, which then becomes ready for application. The product is understood to have been radically new when it was introduced, because of its special C. difficile sporicidal focus. Before its adoption in the trust, ActiChlor Plus tablets and water were used to produce a chlorine-based solution and clean commodes and other surfaces, causing surface deterioration and damage. The product is understood to be ‘high tech’ because of the perceived high level of research that was required to develop it; its uses are thought of as ‘low tech’. Informants associate it with quicker cleaning practice; a reduction in C. difficile rates; and raising the importance of efficacious commode cleaning in the trust. It is viewed as befitting the trust’s values and culture.
Stakeholders, evidence and decision-making
Clinell sporicidal wipes were introduced at a time of relatively high C. difficile incidence for the trust. Before its introduction, processes were in place to ensure a high level of hygiene, such as cleaning practice audits and spot checks by senior staff. The product was introduced to the trust by the IPC team. Ward staff were informed about it through the infection control link nurses’ meetings. The availability of evidence on its sporicidal efficacy is understood to have facilitated its adoption. The product comes with a relatively high cost of approximately £7.65 per pack of 25 (see Appendix 7: Technology Products Unit Cost Price List); however, this was weighted against the high costs of persistent C. difficile incidence. It was then decided that it would be used to clean only the trust’s commodes and bed pans.
While the product was being rolled out, it was championed by an IPC team member. Appropriate communication during training provided by the supplier, by internal e-mail and posters, is understood to have played a key role in the product’s adoption by ward staff. Carefully worded posters were prepared with specific instructions on how to handle and use the wipe.
Table 39 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 31 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Industry/suppliers |
|
| Peer-reviewed academic literature |
|
| Professional networks |
|
| Trust care environment |
|
FIGURE 31.
T9 clinell sporicidal wipes (red): professionals’ engagement and evidence types in decision-making. DoN, director of nursing.
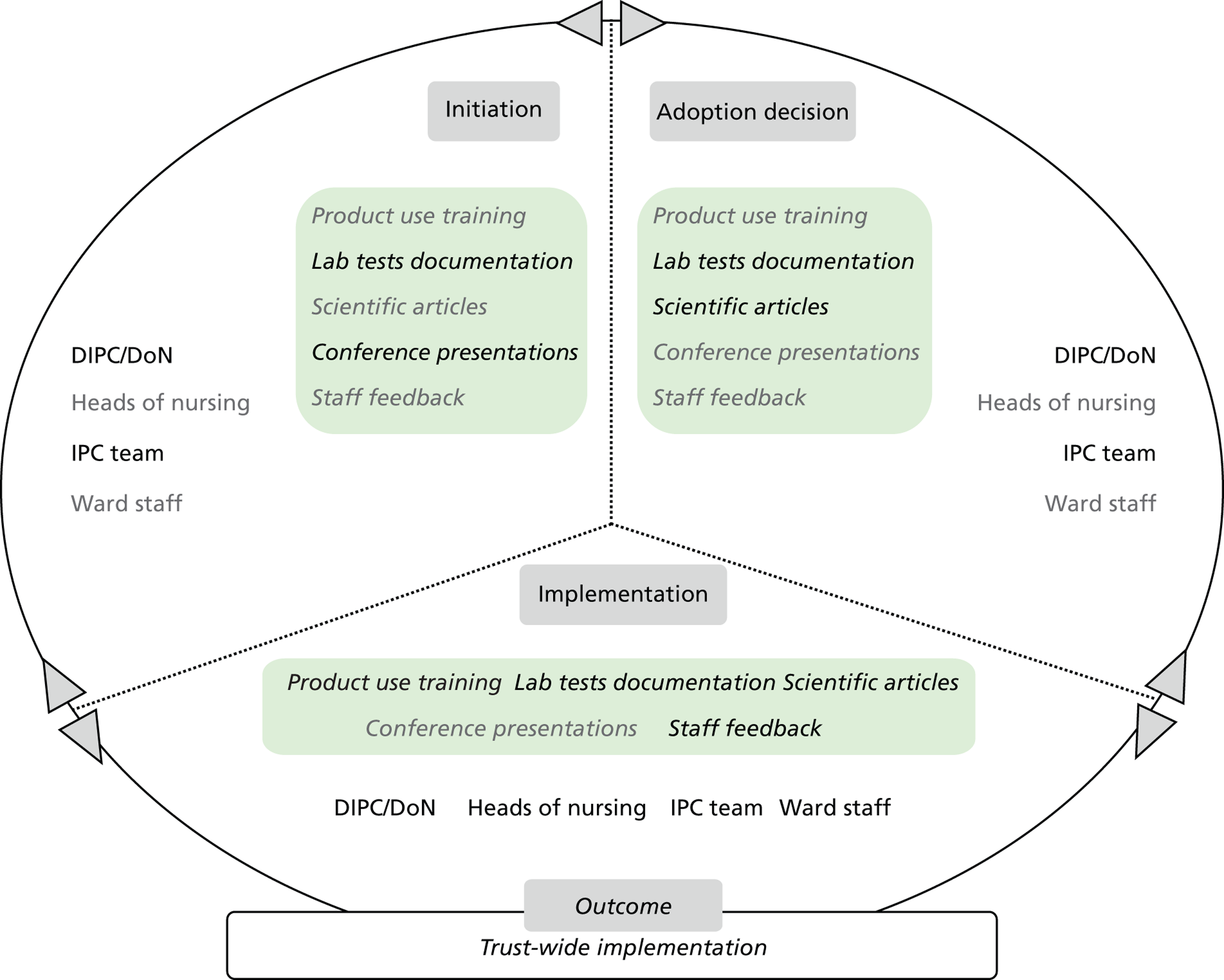
Outcome
Clinell sporicidal wipes have been used in all hospital areas of the trust in the last 2–3 years. The product is understood to be one successful part of a suite of measures introduced to substantially reduce infection rates across T9.
Microcase 3: JLA OTEX system
Attributes perceived by stakeholders
OTEX is understood by T9 informants to be a very effective product for washing and disinfecting fabric-based domestic services equipment (mops, cloths), as well as hotel services equipment (curtains, linen, etc.). Its environmental benefits, using ozone rather than chemical disinfectants and energy and carbon emissions savings, are also recognised. It is viewed as a new concept for the NHS and its environmental benefits, in particular, are thought to be of relevance. It is also viewed as complicated to explain, in part because of the long-standing use of disinfectant-based washing in the NHS. The system is thought of as fully compatible with care delivery systems and processes, as well as with T9’s culture and values.
Stakeholders, evidence and decision-making
The trust was asked to trial OTEX as part of the Showcase Hospitals programme. The trust’s domestic services partner team had been interested in the system for a few years before support for its evaluation became available through the programme. It was involved in all stages (initiation, adoption decision and implementation), as illustrated in Figure 32 . Such user involvement from the initiation stage, together with the partner team’s pre-existing positive perception of the system, played a catalyst role for consensus decision-making among stakeholders. One respondent commented:
I think, because the contract, domestic contractor wanted it anyway, we didn’t, it was a really easy task because we agreed, I think we were asked to evaluate it, we knew that our domestic service were interested in it, so I think that’s partly why we agreed, because actually they, it was something they wanted to do and we’d been asked to do, so that was a, I think that, we came to our consensus of opinion very quickly that we’d go and evaluate it, because we wouldn’t have too many hurdles to cross by trying to persuade someone it was a good idea.
Senior nurse
FIGURE 32.
T9 JLA OTEX system: professionals’ engagement and evidence types in decision-making. DoN, director of nursing.
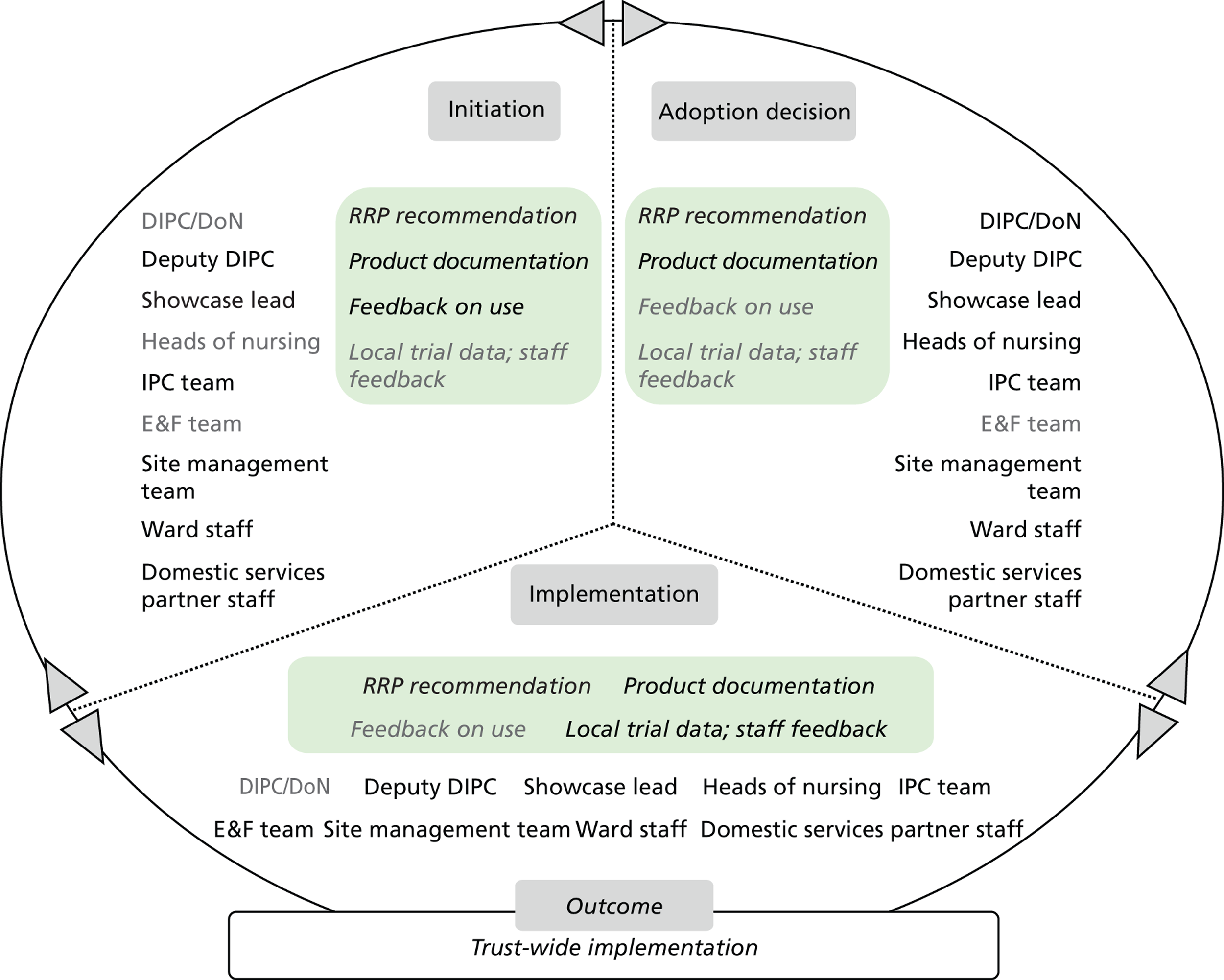
The trust was looking at further environmental hygiene measures at the time, so the trial of OTEX was viewed as a timely opportunity.
The trial was managed by the trust IPC team. Choices were carefully made on both the hospital groups to engage with, and of how to engage with them. These included the trust’s domestic services partner, the trust’s cleaning operational group, including the patient services manager, and the clinical environmental monitoring lead. A close collaboration is reported between the IPC team and the domestic services contractor. The trial lasted for a period of 3 months. The evidence generated from the trial was discussed in the IPC team’s weekly operational meetings. No separate project structures, for example meetings, etc., were put together. Progress was reported at the trust’s infection prevention committee meetings, which occurred every 2 months.
Table 40 lists the specific evidence types used in this microcase alongside the sources from where these were elicited.
| Evidence sources | Evidence types |
|---|---|
| Health agencies |
|
| Industry/suppliers |
|
| Other trusts |
|
| Trust care environment |
|
Outcome
The trial evidence is understood to have demonstrated that the technology had no adverse effects and proved efficacious in eradicating germs and pathogens. Cost savings resulting from using less water, less energy and less detergent were also documented. The change in practice and transition to a new technology also had no adverse effects. Such positive local results changed the views of this technology among IPC team members. One respondent said:
The only resistant was from Infection Control, because they didn’t really, actually I don’t know, they just were very negative against it. But once they saw the results [i.e. onsite infection cases drop], and saw what it offered, they were all for it.
Non-clinical manager
Following the trial, the trust’s IPC team approved further use of the system in the trust. The domestic service partner confirmed that it would be able and willing to continue using the technology. Its use has, thus, now been mainstreamed as part of the trust’s cleaning regime. Use of OTEX was included in the domestic services contract, with no further financial implications for the trust. Adoption was facilitated through collaboration among the showcase lead, domestic services and the supplier’s after-sales staff.
The domestic services partner’s motivation, support from the Showcase Hospitals programme and cost neutrality are understood to be the main factors supporting adoption. Moreover, the supplier is viewed as having a very supportive after-sales service.
Microcase 4: Bioquell vapour hydrogen peroxide Room Bio-Decontamination Service
Attributes perceived by stakeholders
During the trial of the service, patients and staff perceived the service to be highly efficacious, resulting in high levels of disinfection and creating a safe care environment at the trust. Patient and staff opinions were formally documented as part of the service evaluation.
Stakeholders, evidence and decision-making
The Showcase Hospitals programme provided the opportunity to the IPC team to trial the service without cost implications for the trust. The programme supported the availability of Bioquell staff and equipment on-site. These would be requested by trust members who wished to use the equipment for decontamination in specific hospital areas. Trust members were reportedly encouraged to use the service. The Showcase Hospitals programme lead introduced the service throughout T9.
The trust had multiple measures in place to combat HCAIs, and infection rates started to come down. At the same time, use of the service was more often than not exacerbating delays associated with bed availability and waiting times. There was time pressure, arising from competing priorities, and this was exacerbated by the trust’s poor isolation capacity. One respondent commented as follows:
[. . .] you’ve got your competing demands of patients not being able to wait in A&E (accident & emergency) for more than four hours and that, the constant pressure. We have internal targets around how quickly patients need to be isolated, so the turnover of those, so that was I think the biggest hurdle was the whole timeframe.
Senior nurse
After the end of the trial, C. difficile infection rates remained low and continued to fall. This led the trust to take a more cautious approach to adoption. The same respondent described this as follows:
[. . .] We did then get it back in again for a short period when our C. difficile rates went up, [. . .] and we’d managed to secure some funding to do that. C. difficile rates went down but again we’d refocused all our energies within the organisation on the C. difficile so all the practice improved as well. So again it was difficult to see the impact and the rates still have gone down since we stopped using it again. [. . .] we evaluated it in 2008/9 I think it was, and the last two, three years we’ve often had conversations about Bioquell because it’s quoted so much, the evidence is there. However, I think now where our C. difficile numbers have radically reduced quite significantly, [. . .] from a cost justification perspective.
Senior nurse
On this basis of evidence related to infection rates and operational issues, it was considered that the service may not actually make a difference as part of the trust’s suite of measures mitigating HCAI incidence. Nonetheless, the technology was viewed in a positive light by senior trust members, including the DIPC/director of nursing.
Table 41 lists the specific evidence types used in this microcase alongside the sources from where these were elicited. Figure 33 depicts the stakeholders involved and evidence types used in each of the innovation process substages (initiation, adoption decision and implementation), and the related organisational adoption and implementation outcomes for the technology product. Text in light grey shows the evidence types or stakeholders who were not involved in that substage.
| Evidence sources | Evidence types |
|---|---|
| Health agencies |
|
| Showcase Hospitals programme |
|
| Professional networks |
|
| Industry/suppliers |
|
| Trust care environment |
|
FIGURE 33.
T9 Bioquell VHP RBDS: professionals’ engagement and evidence types in decision-making. DoN, director of nursing.
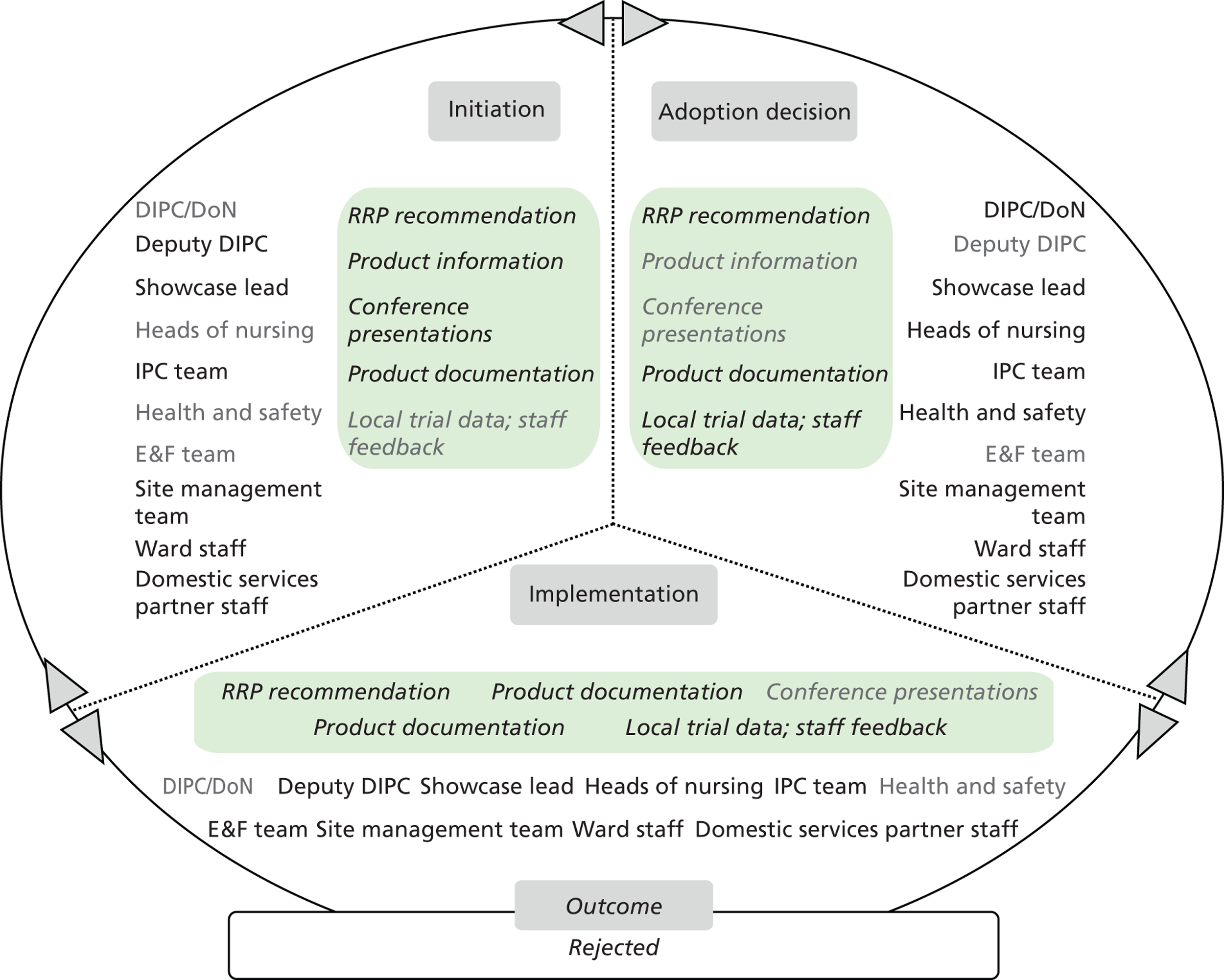
Outcome
The IPC team documented the trial and its outcomes and the evidence was presented to the trust board. The CEO made further enquiries and a consensus developed with regard to the costs related to its permanent adoption and operational use, which outweighed the benefits obtained. One respondent commented:
We then had some quite big discussions around whether we would continue using it and obviously Bioquell as a company were very keen. We could see the advantages in terms of the concept and the technology. The biggest stumbling block for us was cost and the operational issues. So I think we sort of put a provisional case together and it was sat on the shelf in terms of, the chief executive and the trust board were very much, OK, that’s great but what are the benefits? And obviously we then see, or they wanted to know the benefits because it was such a huge cost to the existing service, the existing process.
Senior nurse
A decision was, thus, taken at that level to reject adoption of this technology at the trust.
Chapter 9 Cross-case analysis
This chapter outlines key themes from the cross-case analysis [looking at relevant patterns across the macro- (eight trusts) and microcases (27 technology product journeys)].
Overview of technology microcase outcomes across trusts
In Table 42 the adoption and implementation outcomes for the technology microcases are compared along the dimensions of expected budgetary impact, perceived impact on practice and strength of evidence base on efficacy (discussed in Chapter 7 ). Themes from this comparative analysis are summarised by decision outcome and adoption/implementation processes. The patterns observed are reflective of the eight trusts sampled for this study; they provide important insights into the relevance of perceived technology attributes to this process.
| Technology product | Budget impact | Practice impact | Evidence strength on efficacy | Trusts | Adoption and implementation outcome |
|---|---|---|---|---|---|
| ASP GLOSAIR 400 aHP system | Low | High | Medium, emergent | T2 | Trust-wide implementation |
| DIFFICIL-S disinfectant solution | Low | Medium | Low, emergent | T7 | Trust-wide implementation |
| Chlor-Clean | Low | Medium | Low, emergent | T5 | Trust-wide implementation |
| Clinell universal sanitising wipes | Medium | Very low | Medium, emergent | T1 | Trust-wide implementation |
| JLA OTEX laundry system | Low | Medium | High, established | T6, T9 | Trust-wide implementation |
| Clinell sporicidal (red) wipes | Medium | Low | Low, emergent | T3, T9 | Trust-wide implementation |
| ASP GLOSAIR 400 aHP system | High | High | Medium, emergent | T7 | Adopted and ad-hoc use trust-wide |
| Steris BioGenie VHP system | Low | High | Low, emergent | T4 | Adopted and ad-hoc use trust-wide |
| DBO commode | Medium | Low | Low, emergent | T1 | Adopted in two of three sites; phased implementation in progress |
| DaRo UV light inspection cabinet | Low | High | Low, emergent | T7 | Adopted and ad-hoc use trust-wide |
| Bioquell VHP RBDS | Medium | High | High, established | T5 | Adopted and ad-hoc use trust-wide |
| 3M Clean-Trace NG luminometer | Medium | High | High, established | T2 | Adopted and used in selected wards |
| UV light inspection torch | Low | High | Medium, emergent | T2 | Adopted and ad-hoc use trust-wide |
| DIFFICIL-S disinfectant solution | Low | Medium | Low, emergent | T4 | Ongoing decision-making |
| Disposable sterile surgical site gowns | Medium | Very low | Low, emergent | T2 | Ongoing decision-making |
| Clinell alcoholic wipes | Low | Low | Medium, emergent | T9 | Ongoing decision-making |
| PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes | Low | Low | Low, emergent | T9 | Ongoing decision-making |
| Bioquell VHP RBDS | Medium | High | High, established | T3, T6 | Ongoing decision-making |
| Bioquell VHP RBDS | High | High | High, established | T1, T9 | Rejected |
| Medixair UV air sterilisation unit | Low | Very high | Low, emergent | T3, T6, T7 | Rejected |
| Medixair Meos UV air sterilisation unit | Low | Very high | Low, emergent | T3, T6, T7 | Rejected |
| Virusolve+ | Low | Low | Low, emergent | T4 | Rejected |
| 3M Clean-Trace NG luminometer | Medium | High | High, established | T5 | Rejected |
| Hygiena SystemSURE II ATP hygiene monitoring system | Medium | High | High, established | T5 | Rejected |
Decontamination and inspection products were mostly put forward by trusts to be studied as microcases and most of these also resulted in adoption and trust-wide implementation. Decontamination products, such as liquid cleaning and wipe cleaning, were generally associated with a lower expected budgetary impact and a low to medium perceived impact on practice. The evidence associated with these products was generally of ‘low’ strength. The adoption and implementation outcomes for these products varied considerably across the trusts, from trust-wide implementation, adoption by the trusts and availability for ad hoc use to rejection. By ‘ongoing decision-making’ we refer to those technology products under consideration at the time of the study. The next section examines the decision-making processes and outcomes across these technologies by considering the three dimensions of our typology, namely perceived budgetary impact, practice impact and strength of the evidence base on efficacy.
Decision outcome themes
Evidence strength on efficacy themes
There is no clear observable pattern when we look at outcomes and ‘evidence strength on efficacy’
Table 42 includes products whose evidence base on efficacy ranges from ‘low’ to ‘high’ and is emergent or established. Within these classifications, decision outcomes vary between rejection and trust-wide implementation. For those technologies implemented trust-wide, the evidence base on efficacy spans the range of low, emergent, to medium. Only two microcases, both of the JLA OTEX laundry system, with a ‘high, established,’ evidence base on efficacy resulted in trust-wide implementation, which occurred in both T6 and T9.
High evidence strength on the efficacy of a technology product did not always lead to its adoption and full-scale implementation (as initially planned)
There were four technologies in our sample with a ‘high, established,’ evidence base on efficacy, and these featured in 10 of the microcases. Decision outcomes across these 10 microcases included rejection, adoption and use in selected wards, adoption and ad-hoc use trust-wide, and trust-wide implementation. Two examples of these are Bioquell VHP RBDS, which was adopted for ad-hoc use trust-wide in T5, but rejected in T1 and T9, and the 3M CleanTrace and Hygiena SystemSURE II ATP hygiene monitoring system, which were both rejected in T5.
Low evidence strength on the efficacy of a technology product did not preclude adoption and trust-wide implementation or adoption and ad-hoc use trust-wide
There were 10 technology products classified as having a ‘low, emergent,’ evidence base on efficacy. These were perceived as having variable budget and practice impacts. Three of these products were adopted and implemented trust-wide in four trusts, whereas three more have been adopted for ad-hoc use across three trusts. Specifically, the technologies implemented trust-wide were as follows: DIFFICIL-S in T7; Chlor-Clean in T5; and clinell sporicidal wipes in T3 and T9. Those adopted and used ad hoc include Steris BioGenie VHP system in T4; the DBO commode in T1; and the DaRo UV inspection cabinet in T7.
Perceived impact on practice themes
Technology products associated with a ‘low’ or ‘medium’ perceived impact on practice were less likely to be rejected
In our sample, 10 technology products considered for adoption were perceived as having a very low, low or medium impact on practice. The majority of decisions concerning these products across the trusts (8 out of 13 decisions) led to trust-wide or phased implementation: for example, DIFFICIL-S in T7; Chlor-Clean in T5; clinell universal wipes in T1; JLA OTEX laundry system in T6 and T9; clinell sporicidal wipes in T3 and T9; and the DBO commode in T1.
Technology products associated with a ‘high’ perceived impact on practice were more likely to be rejected
Eight technology products with a high or very high practice impact were considered for adoption in eight trusts. Out of these 16 decisions, there were 7 rejections reported. These included Bioquell VHP RBDS in T1 and T9; Medixair in T3, T6 and T7; and the 3M CleanTrace and Hygiena SystemSURE II ATP hygiene monitoring system in T5. Seven decisions led to products being adopted and implemented trust-wide or used ad hoc, namely the ASP GLOSAIR aHP system in T2 and T7; the Steris BioGenie VHP system in T4; the DaRo UV Inspection Cabinet in T7; Bioquell VHP RBDS in T5; and the 3M CleanTrace luminometer and the UV inspection torch in T2.
Technology products associated with a ‘low’ or ‘medium’ practice impact were more likely to be adopted and implemented trust-wide
In our sample, 10 technology products perceived to involve a very low, low or medium impact on practice were reviewed in 13 technology decisions across the eight trusts. In seven cases, these products were fully implemented. These cases were as follows: DIFFICIL-S in T7; Chlor-Clean in T5; clinell universal wipes in T1; JLA OTEX laundry system in T6 and T9; and clinell sporicidal wipes in T3 and T9. In four cases, the decision to adopt was still pending at the close of the study: these were DIFFICIL-S in T4; disposable gowns in T2; and PDI Sani-Cloth and clinell alcoholic wipes in T9. In T4, Virusolve+ was rejected.
Rejection decisions on technology products with a perceived ‘high’ impact on practice consistently used local sources of evidence (data generated within trusts)
Our technology sample featured four technology products with a ‘very high’ or ‘high’ practice impact that were rejected in seven of the microcases. These were as follows: Bioquell VHP RBDS in T1 and T9; the Medixair units in T3, T6 and T7; and the 3M CleanTrace and Hygiena SystemSURE II ATP hygiene monitoring system in T5. In all seven cases, these rejections were based on staff feedback and local trial or product case study data generated from within the trust care environment.
In the Bioquell RBDS case, in T1, the IPC team conducted a local trial and collected and analysed data on staff experiences during and after the trial, which was reviewed by senior executives and other stakeholders from within the trust including E&F and procurement. In T5, the IPC team conducted a local trial and compared the results with feedback from local trials undertaken in other trusts. In T7, the microbiology team and IPC team conducted a small case study of Medixair units in the ward environment. Similarly, T3’s IPC team conducted a local trial and elicited staff and patient feedback on the use of Medixair devices in wards.
Budget impact theme
There is no clear observable pattern when we look at outcomes and ‘budget impact’
In total, 11 technology products with a ‘low’ perceived budgetary impact were considered for adoption in seven trusts. Out of these 15 decisions, there were 4 rejections reported: Virusolve+ in T4 and the Medixair units in T3, T6 and T7. Five decisions led to trust-wide implementation: the ASP GLOSAIR aHP system in T2, the DIFFICIL-S in T7, the Chlor-Clean in T5 and the JLA OTEX in T6 and T9. Another three technology products were adopted and used ad hoc trust-wide, while for three more the adoption decision was ongoing. Two technology products with a ‘high’ perceived budgetary impact were considered by three trusts: the ASP GLOSAIR aHP system was adopted and used ad hoc trust-wide in T7, while the Bioquell VHP was rejected in T1 and T9. The former technology product, ASP GLOSAIR, was perceived as having a ‘low’ budget impact in T2 (T2 had used externally awarded funding to procure the product) and was adopted for trust-wide implementation, while in T7 it was perceived as having a ‘high’ budget impact, leading also to adoption but with ad hoc use trust-wide.
Technology product microcase themes
A comparative review of the 27 technology product microcases enables the following observations to be made regarding stakeholder involvement and evidence use in the decision-making process.
Nurses were involved in all stages across the trusts for all technology products.
Out of the 27 product cases, 10 featured the involvement of nursing staff and at least one interdisciplinary hospital group including senior trust members beyond the IPC team (trust innovations groups, IPC committees, product selection groups, risk management groups and the trust board). The IPC team was exclusively involved in three microcases. These were Virusolve+ in T4, Chlor-Clean in T5 and clinell sporicidal in T9, and these cases were characterised by a ‘low’ or ‘medium’ budget impact, a ‘low’ practice impact and a ‘low’ evidence strength on efficacy. Nursing staff, doctors and non-clinical specialists were involved in tandem in all three stages in four microcases, namely, DIFFICIL-S in T4 and T7; Bioquell in T3; and the DBO commode in T1. Non-clinical staff were involved in all three stages of 11 microcases, and in at least one stage in 25 microcases.
In the initiation stage, non-clinical staff, for example from domestic services or procurement, were involved in 12 cases of mostly ‘medium’ budget impact; these involved ‘high’ practice impact products whose evidence strength on efficacy varied. Doctors were involved in the initiation stage in 10 cases of ‘medium’ or ‘high’ budget impact and ‘high’ practice impact products of variable evidence strength on efficacy. All three professional groups were involved in the initiation stage for eight products associated with ‘medium’ or ‘high’ budget impact, ‘medium’ to ‘very high’ practice impact and ‘low’, ‘medium’ or ‘high’ evidence strength on efficacy.
Doctors participated in adoption decisions in 14 cases (almost half of the total cases). These were primarily the DIPCs and in some cases the medical director and infection control doctor. They fulfilled a strategic role of endorsement and support to the newly adopted product with regard to its cost and practice implications. This was reflected in the small sample of doctor respondents in phase 2 (i.e. those actively involved in the decision-making process).There were 10 microcases for which senior members of all three professional groups were represented in the adoption decision stage. Non-clinical staff participated in the adoption decision stage in 21 journeys, making them the professional group with the second strongest presence after nurses.
Doctors were also involved in early or full implementation in nine microcases. Non-clinical staff were involved in the implementation stage in 22 cases, again being the second most prevalent group after nurses.
The types and sources of evidence used seemed to vary only slightly depending on whether only one or multiple professional groups were involved. Technology product journeys can be grouped into 14 cases in which all three professional groups were involved in at least one stage and 13 in which this did not happen. The types of evidence used most frequently within the former microcases were product information and demonstrations, sourced from industry and product suppliers, followed by local trial data, scientific articles and conference presentations. In the latter case, the evidence types most often used by those professionals involved – typically nurses, and ward, domestic or procurement staff – were product information and demonstrations provided by industry and suppliers, recommendations by health agencies, local trial data and staff feedback from the trust care environment, scientific articles and conference presentations.
The involvement of medical microbiologists in 13 product journeys led to the production of locally generated evidence through early implementation in the form of a local evaluation trial or case study. The data generated from these studies complemented peer-reviewed academic literature regarding the proposed technologies. This approach supported decisions concerning products with a ‘medium, ‘high’ or ‘very high’ practice impact in 11 cases. Research-active trusts with a substantial research capacity, as defined by our categorisation in Chapter 6 , generated evidence in their local care environment: examples include T1, T5 and T7.
In all cases of ‘high’ practice impact and ‘high’ evidence strength on the efficacy of products, senior organisational executives were involved in adoption decisions.
Senior organisational executives were involved in 14 microcases of products of considerable practice impact and otherwise variable expected budget impact and evidence strength on efficacy. This senior involvement was in the form of a number of organisational roles, including medical director, chief operating officer, director of nursing, deputy chief nurse, DIPC and deputy DIPC, head of nursing, decontamination manager, and senior consultant microbiologist. Senior executives also contributed directly to decisions regarding products of ‘low’ budget impact and ‘medium’ practice impact, such as DIFFICIL-S (low evidence strength on efficacy) in T4 and T7 or OTEX (high evidence strength on efficacy) in T6 and T9.
The role of the director of infection prevention and control
Trust DIPCs were involved in at least one stage of the adoption process of 17 technology products, whose expected budget impacts varied from ‘low’ to ‘very high’. Of these product microcases, 11 featured products of ‘medium’, or ‘high’ practice impact. One-third of the decisions in which DIPCs were involved (10 microcases) concerned products with a ‘medium’ or ‘high’ budget impact and products with a ‘low’ or ‘medium’ evidence strength on efficacy. In 11 cases in which DIPCs were involved, products were either implemented trust-wide (six cases) or adopted and made available for ad hoc use in the trust either in selected wards or trust-wide (five cases). Three decontamination products of ‘high’ or ‘very high’ practice impact were rejected.
Trust DIPCs were involved in the adoption decision stage of 15 product microcases. Only five of these products were classified as ‘low’ for evidence strength on efficacy, whereas seven were identified as having a ‘high’ evidence strength on efficacy. Of these 15 decisions, 10 involved products with a ‘medium’ or ‘high’ budget impact for trusts, and 9 were on products of ‘medium’, ‘high’ or ‘very high’ practice impact.
The involvement of the DIPC at this stage of the process was associated with a positive outcome: 10 decisions resulted in trust-wide implementation, whereas one decontamination product was rejected in two trusts, and, in three cases, decision-making was ongoing. In addition, six of decontamination and inspection products with a ‘medium’, ‘high’ or ‘very high’ practice impact were adopted, two were rejected and one decision was yet to be made.
In nine technology microcases, the DIPC was involved in all three stages. Six of these microcases involved products with a ‘low’ or ‘medium’ evidence strength on efficacy: the DBO commode in T1; clinell sporicidal wipes in T9; ASP GLOSAIR in T2 and T7; and DIFFICIL-S in T4 and T7. Eight of these microcases related to products of ‘medium’ or ‘high’ practice impact, namely Bioquell VHP RBDS in T5; OTEX in T6; ASP GLOSAIR in T2 and T7; DBO commode in T1; clinell sporicidal wipes in T9; and the 3M Clean-Trace and Hygiena SystemSURE II ATP hygiene monitoring system in T2.
In our trust sample, the DIPC was involved in the initiation stage of 11 innovation journeys, and the implementation stage of 12 journeys.
Product adoption and implementation were facilitated by the identification of an appropriate hospital team or group to act as the ‘prime user’ of the new product. For example, OTEX is operated by the trust domestic services partner in T6 and T9, which took ‘ownership of the practice’. In T7, involvement of domestic staff in the decision concerning the aHP system led to improved understanding of the workload implications for domestic staff, which in turn resulted in hiring additional staff to optimise the product’s use. In T2, the 3M Clean-Trace and Hygiena SystemSURE II ATP hygiene monitoring system were successfully adopted for use by domestic staff, and, by contrast, in T5 there was no group identified that could potentially incorporate the routine use of the luminometers into its daily practice. Ownership of the swabbing and data recording process by domestic staff in T5 was widely viewed as a key facilitating factor in the product’s successful adoption and implementation, as it generated enthused and interested users among domestic staff.
In T9, the fit of products with the trust’s care systems and processes was reviewed in a local trial, and emerged as a key determinant of successful adoption and implementation. In T3, active supplier engagement during the product or service trial and implementation also emerged as a factor conducive to products being positively perceived and considered for adoption by staff.
Mobilising sources and types of evidence and innovation stakeholders
Our phase 1 findings show that several types and diverse sources of evidence were variably reported to be accessed and used by non-clinical or clinical hybrid managers. Access to and use of evidence was reported to vary significantly among professional groups. Further, synthesis of phase 1 and 2 analyses allowed the exploration of ‘what managers say they do’ as individual decision-makers and ‘what managers reported to do’ in cases of collective organisational decisions concerning the use of research-based and other forms of evidence. Our analysis revealed the complex interplay between diverse stakeholders, in terms of their professional background and organisational role, and the evolving mix of evidence types accessed from different sources. At each stage of the product innovation journeys, collection, assessment and presentation of multiple types and sources of evidence by different stakeholders to address various audiences and for different purposes were observed.
We found that what managers claimed that they do as individual decision-makers (phase 1 data) was not followed through in the context of collective decision-making processes (phase 2 data). Specifically, in phase 1 three national or central sources of evidence, the NICE, the NPSA and NHS National Service Frameworks, were reported as being used across the professional groups when adopting innovations (see Figure 3 ). An additional source, The Cochrane Library, was also consistently reported as being accessed and used by all clinical staff respondents, but not by those in the non-clinical group. However, respondents also noted that evidence types associated with these sources, such as research-based evidence guidelines or scientific articles, do not immediately or seamlessly relate to the delivery of health care or innovation adoption in their own care environment. Our phase 2 data revealed that, across the technology journeys in all the microcases, none of these sources was actually used when evidence was sourced and reviewed to inform decisions; nor were journals classified as health services research and management, or mainstream organisation and management, used.
Local types of evidence, for example data generated through local evaluation trials, were more likely to be mentioned by informants in phase 1 interviews when non-adoption or rejection decisions were made than other evidence from other trusts, the supplier, and national or international sources. Our phase 2 data illustrate that locally generated evidence was usually produced in order to critically appraise other evidence types, and confirm the innovation’s compatibility, or otherwise, with the local care environment.
Our phase 2 data further suggest that research-engaged organisations in our sample (T1, T5 and T7) tended to conduct local trial evaluations to inform adoption decisions, particularly in cases in which cost and practice impact were perceived to be substantial. This is in contrast to non-research-engaged trusts, for which supplier-sourced product documentation and peer-reviewed scientific articles tended to feature most frequently across the stages of the innovation journeys. University-affiliated organisations showed a pattern of a somewhat wider range of evidence types utilised, frequently drawing on exchanges with other trusts, professional conferences and the HPA.
In phase 1, supplier marketing materials were reported to be of low importance in innovation adoption decisions by respondents across the trusts. However, in our phase 2 technology microcases, product documentation sourced by suppliers featured very frequently in each of the stages of the innovation journeys across our macrocases of eight trusts. There appeared to be a more pronounced relationship with suppliers in less-research-orientated organisations (i.e. T4, T6) than in university-affiliated trusts (i.e. T1, T5, T7), where supplier-sourced evidence was reconciled with other types in the evolving evidence mix.
Overall, research-engaged organisations emerged as those wishing to qualify external evidence vis-à-vis the adoption of innovative technology products by:
-
generating and drawing on local evidence on these products
-
engaging a high number of stakeholders from different professional groups involved in order to support this process of evidence review and customisation.
In less-research-engaged organisations, locally generated data took a minimal role relative to ‘external evidence’ types, which were research-based and sourced from suppliers, professional networks and other health-care organisations.
Several salient aspects of these different evidence types featuring in each of the innovation journey stages in trusts were interwoven in discourses shared among members of different professional groups. In these discourses, locally generated evidence, research-based evidence, professional experience, subject-matter knowledge, supplier-sourced materials and other evidence were other mediating factors. These discourses were again principally framed, and emerged from, within the professional background of stakeholders. This underlies the importance of professional background vis-à-vis evidence plausibility to self and others in collective decisions.
As reported earlier (see Chapter 4 ), what counted as evidence for doctors and non-clinical managers differed, but what mattered most was that they themselves (doctors and non-clinical managers) were satisfied with the evidence. For the nurses, what counted as evidence to others mattered equally and sometimes more than own satisfaction with the evidence. This shaped the types and sources of evidence used by nurses at each stage of the innovation journey.
When professionals came together as stakeholders to review evidence and enact decision-making, the professional background and organisational role of the evidence presenters, and those of members of their audience, were of prime importance. Members of all professional groups recognised that doctors have a unique position, either as presenters or as the audience, when evidence is being considered ( Table 43 ). This, coupled with the relative lack of involvement of this group in the product innovation journeys explored in phase 2, partially explains the slow adoption of innovations, particularly in IPC, as the field is dominated ‘by default’ by the nursing group:
The infection control agenda was almost entirely run through the nursing staff. So it was the nursing staff who knew that if an infection occurred on their ward they were going to be hold up in front of the senior nurse and have to account why it might have happened. The medical staff we are not subject to that sort of view at all [. . .]. ‘Why are the medical staff not part of the analysis of why these things happen?’ And it was really partly because, [. . .] there was a level of hostility amongst consultant staff towards changes. And I think even the senior nurses in the trust felt it would be counterproductive dragging consultants into these meetings. And the result of that was that the infection control agenda became perceived as a nursing agenda. And the consultants were able to stand back from it even more and say, ‘It’s nothing to do with us, it’s all to do with the nurses, it’s nothing we have to be involved in’, and that sort of you could see the implementation of a lot of infection control stuff faltering on that basis.
Doctor
| Theme | Exemplar quote |
|---|---|
| Clinician as presenter | I always find certain individuals within the team to present it, then it is listened to more. Again this is my own personal experiences, I can say the same thing as a clinical member of the team, but what I say is not accepted because I am not clinical. The clinical person can say exactly the same thing, same words, and they would think it is wonderful. The right people. That is a challenge of being a manager in a clinical setting. Non-clinical manager Although it galls me to say it but I think the medical colleagues within the team are better at accessing [evidence] and they may come to a meeting and say I have had a look at the evidence. I don’t think it could necessarily have been a systematic review of the evidence. Stating quite confidently a particular position and that could be quite influential so that is something they are more likely to do than nursing members of the team. Non-clinical manager |
| Clinician as audience | Clinicians seem to be the most powerful group. And if you can get them on-board or at least some of them on-board then generally it makes things a lot easier because the nature of their training they will ask for hard and fast data. Nurse |
There is the added complexity of organisational role versus professional role. For example, non-clinical managers as an organisational group have a diverse ‘professional’ background including, for example, engineers and accountants; hence, the organisational role dominates in evidence-use practices. Nurses focused in our study on experiential and biomedical forms of evidence based on their professional background. However, the organisational role of nurses in IPC appeared to override the professional template as the primary frame of rationality in decisions and was reported to shift according to the audience. In Chapter 4 we detailed the concepts of plausibility for self and others and how the latter was reported to be of greater concern to the nursing group. This variance in reported motivation mediated the span of evidence sourcing along a continuum from ‘narrow to ‘wide’ ( Table 44 ).
| Motivation | Behaviour: span of evidence sourcing | |
|---|---|---|
| Narrow | Wide | |
| Plausibility to self dominates | Medical hybrid manager | Non-clinical manager |
| Plausibility to others dominates | Nurse hybrid manager | |
Chapter 10 Synthesis and inferences
In this chapter we reflect on and synthesise our empirical findings as to how the individual and collective sensemaking processes of health-care managers evolved in practice as regards the use of evidence. Through our comparison of multiple empirical cases, we provide insights into how managers’ sensemaking played out within the multilayered domains of influence: uniprofessional groups; multiprofessional groups; health-care organisations; the field of IPC and the heath-care sector. First, we return to our research questions to summarise key learning from our empirically grounded work. We then reflect with some implications for relevant theory.
Reflecting on our research questions
Considering our original research questions, we note that the process of ‘managers making sense of evidence’ was found, not surprisingly, to be a situated social process of individual and collective ‘cognition in context’. There was retrospective, ongoing interpretation of different types of evidence variably sourced and assessed by a large group of stakeholders with diverse professional identities. Organisational roles further influenced this individual and collective sensemaking by shaping the focus of attention on the evidence debate. We found that organisational members, through ongoing social interactions with both immediate peers and seniors and distant comembers of the organisation, iteratively coconstructed plausible accounts of relevance and credibility to self. In addition, this process involved sensemaking for others. Notably, the processes of decision-making involved tacit and explicit – and sometimes ‘political’ or ‘tactical’ – sensemaking for others. Captured in this study is the more explicit sensemaking whereby ‘making the case’, for or against, at the different stages of the innovation process was played out by diverse organisational members. Regulation and policy mandates, perceived decision urgency and service need, external players, such as the commercial suppliers of technologies, and critical events, such as infection outbreaks, all influenced these social, situated processes of collective cognition. Here we summarise our findings as regards our original research questions.
How do managers (non-clinical and clinical hybrid managers) make sense of evidence?
Managers encountered challenges and constraints in accessing and making sense of evidence relevant to the decision at hand. Managers made sense of evidence by overcoming the conceptual constraints inherent in the nature of evidence as being multifaceted, diffuse, ongoing and constantly updated (or in need of updating).
By being mindful of the temporal nature of evidence, particularly in the context of innovations, ‘high strength’ and ‘established’ scientific evidence about the efficacy of technologies did not override other forms of evidence, such as cost and experiential knowledge about ease of use. Equally, ‘low evidence strength on efficacy’ for a product with an emergent scientific evidence base did not preclude these technologies from being adopted trust-wide (e.g. DIFFICIL-S or Chlor-Clean in T7 and T5, respectively). Managers were faced with and negotiated the map of an ‘incomplete’ evidence base in terms of missing evidence or poor-quality evidence. The definition of ‘quality’ varied to some degree according to professional background.
Managers made sense of evidence in innovation decisions by sourcing evidence which was plausible and accurate to self but also for significant others, that is to convince other members of the organisation for the case at hand. This function fell within the remit of some organisational members (e.g. nurse hybrid managers in the context of IPC) more than others. At the same time the managers justified their own credibility in the decision-making discourse as presenters of this evidence.
What role does evidence play in management decision-making when adopting and implementing innovations in health care?
The articulation and discourse around evidence played a major role in decision-making when adopting innovations given the newness and risk inherent in innovations. Decision-makers felt the urgency for sourcing evidence to reduce the uncertainty and ambiguity associated with the introduction of innovations. Innovation evidence was often perceived as emergent, iterative and changing.
In the early stages of initiation and adoption, those involved drew on a variety of types of evidence, many of which were available from central sources; managers reported that in early technology considerations they were not always clear about what types of evidence they needed. Evidence played a different role in the stage of implementation, as respondents often cited ‘missing evidence’ at this later stage of the innovation process. Credible and relevant evidence generated from systematic research on the topics of implementation and management was identified by the majority of respondents as currently missing from the NHS evidence base. This is despite the fact that a significant body of such research evidence exists in health services research journals [e.g. Implementation Science, BMJ Quality and Safety, Health Services Research & Policy, Healthcare Management Review, NIHR Health Services and Delivery Research (HS&DR) research reports] and mainstream management journals (e.g. Organization Studies, Academy of Management Journal, Organization Science). When probed, most respondents were not aware of these journals, and did not report reading them. As a result, decision-makers often drew on more local evidence, such as evidence from other trusts, or instigated the generation of local trials.
Stakeholder constellations evolved along the innovation stages, shaping the mix of evidence types reviewed and taken forward at each stage. There was consistency in respondents’ accounts that different professional groups accessed different sources of evidence because of dissimilar needs for evidence.
How do wider contextual conditions and intraorganisational capacity influence research use and application by health-care managers?
The presence of standardised evidence from central sources affected all stakeholders involved in decisions in the context of health care. In addition, pressures of patient safety and performance influenced the use of evidence in different ways. In the context of IPC, external pressures, in the form of performance targets, media scrutiny, patient expectations and fear, were reported as incentives for sourcing evidence on innovations that would deliver results in specific organisational contexts. Several decontamination technologies were introduced as a result of these pressures in our trusts sample. These same pressures, however, were seen as a barrier to establishing a well-informed and rigorous process of evidence consideration because of the pressure to act.
Under such intense contextual pressures decision-makers often embarked on a quest for plausible rather than accurate evidence. The consequent pace of decision-making necessitated a focus on plausibility to others. Conversely, sometimes the pace influenced the exclusion of key stakeholders to avoid protracted decision-making processes with wider involvement delayed to the implementation stage.
In this study, trust infrastructure redevelopment projects, a strong emphasis on patient safety, and strong and trustful collaboration (especially between IPC teams and other organisational departments) appeared to widen the scope for the search, and use, of evidence in decision-making. In nearly all phase 2 microcases, the number of evidence types and individuals involved grew as the innovation process progressed from initiation to early trial use, adoption decision and implementation. These evidence types were diverse and came from several sources irrespective of whether one or many professional groups came to be involved. In these discourses research evidence, personal experiences and knowledge, relationships with the suppliers, politics, resources, national performance targets, national and organisational policies, organisational and departmental priorities, and clinical pressures (infection outbreaks) were continuously at play and shaped decision-making outcomes.
Our findings also suggest that research-engaged organisations in our sample were more likely to generate their own evidence, for example conduct local evaluation trials to inform adoption decisions. Research-engaged trusts tended to assess innovations by drawing on a wider range of evidence types, and engaging a high number of stakeholders from different professional groups.
Looking across professional groups, what counted as evidence for doctors and non-clinical managers was different, but what counted most was that they themselves (doctors and non-clinical managers) were satisfied with the evidence. For the nurses, what counted as evidence to others mattered equally and sometimes more than own satisfaction with evidence. This shaped the types and sources of evidence used by nurses.
Engagement with evidence unfolded over time through interaction and negotiation. There were diverse, but closely interlinked, ‘evidence templates’ in circulation and in use, namely ‘biomedical-scientific’, ‘practice-based experiential’ and ‘rational-policy’ (see Sensemaking in organisations below). These templates served as frames of reference for the managers and defined what constituted acceptable and credible evidence in the decision-making process. Informants variably drew on those templates to make sense of the evidence and of the organisational problems identified and this is where micro and macro contextual influences shaped this process.
Implications for theory
In this section, we suggest some theoretical implications that emerge from our empirical findings in relation to the relatively ill-exposed literature we identified in the concluding section of Chapter 2 . We briefly outline some of the implications to the relevant theoretical debate in three literature streams, namely, evidence-based health care, organisational innovation and sensemaking in organisations. The first two are considered conjointly as our study focused on the use of evidence in the context of organisational innovation adoption and implementation decisions.
Evidence-based health care and organisational innovation processes
Much of the current empirical research on innovation adoption and diffusion in health care has generally been limited to a single level of analysis. Our study explored the innovation adoption process following a multilayered analysis at micro (individual managers’ sensemaking), meso (collective sensemaking of evidence in organisational innovation decisions) and macro (interorganisational professional and policy influences, evidence templates) levels to provide a holistic understanding of social processes in context. 95
In organisational innovation literature, there is also insufficient exploration of how pluralist evidence bases (and the associated diverse epistemological bases) might be reconciled (or not) in practice to make the case for or against particular innovations. 96 Throughout our empirical cases, we consistently observed that organisational contexts, and especially the organisational culture and level of research engagement, policy mandates, perceived urgency of issues, physical infrastructure, social interactions and stakeholder engagement, and professional identities, exerted a mediating influence on how decision-makers accessed and used evidence for non-clinical organisational decisions impacting on clinical-care delivery. We explored the role and expressed motives of actors and the influence of context, which mediated the social construction of evidence in practice.
Nature of innovation evidence
The nature of evidence was conceptualised along a continuum of ‘hard’ to ‘soft’. Hard evidence comprised cost and efficacy, whereas evidence on practice impact, usability and patient experience was more often perceived as soft. An emphasis on organisational productivity outcomes was also viewed as linked with ‘hard’ and ‘tangible’ evidence and such claims were prevalent in the evidence discourse of managers with non-clinical backgrounds.
The temporal nature of evidence served as both a motivator to seek and generate new evidence and a constraint. Sourcing evidence for the most up-to-date developments was viewed as part of the innovation process and a means to improve patient outcomes. At the same time, the delay in availability of high-quality evidence was seen as a barrier, with decisions being put on hold in the absence of such evidence. The temporal dimension also emerged as an external pressure generated by performance measures and targets, particularly in the area of IPC, whereby the necessity or perceived urgency to act precluded decision-makers having sufficient time to review evidence in a robust manner.
Many organisations in our study sample (especially research-engaged NHS trusts) sought opportunities to add to the evidence base through local trials, and this may be explained by the high majority of senior managers interviewed in phase 1 who found it difficult to relate evidence from central sources to local practice.
We identified no clear observable pattern between adoption or implementation outcomes and the ‘evidence strength on efficacy’ or ‘expected budget impact’ of the studied technologies when considered in isolation. A low perceived practice impact was more likely to be linked to successful adoption and trust-wide implementation. The combination of all three dimensions of evidence better explained outcomes, and these were consistently considered in tandem by decision-makers across all microcases. These empirical findings help innovation researchers to better map the perceived attributes of innovations (e.g. relative advantage, compatibility, complexity, trialability, observability) and innovation knowledge types (e.g. ‘awareness’, ‘principles’ and ‘how-to’ innovation knowledge)24 along these additional three domains for improved understanding of innovation uptake within organisations.
Situated evidence
If technology adoption were to follow a purely evidence-based approach, then organisational adoption decision-making would be an entirely rational process. Our findings suggest that individual interests, certain critical shocks, external pressures and the trust’s organisational culture and values had a strong impact on the use of evidence in the decision-making processes. This was often linked to a sense of urgency to solve perceived problems, leading to organisational priority shifts in favour of such acute problem solving. For example, outbreaks, financial pressures, performance targets and trusts’ relationships with commercial suppliers often led to reactive or ‘fire-fighting’ attitudes towards problem solving, or a less scrutinised approach to evidence use: decision-makers often relied upon an emergent evidence base adopting a ‘pragmatic’ approach and sourced ‘sensible’ and ‘credible’ evidence; in other cases, a ‘political’ and ‘imperative’ approach dominated the evidence review process.
Extrinsic motivation, such as organisational image and the related pressures (i.e. meeting the expectations of patients and the public, ‘must-have’ innovations, organisational reputation and being seen as NHS leaders in IPC or NHS leaders in quality and innovation), exerted a significant influence on decision-making and was reinforced by certain frames of reference among key decision-makers.
Redevelopment projects, patient-centred organisational values, and relationships of collaboration and teamwork were linked to a broader range of sources and types of evidence accessed, reviewed and used by decision-makers. A narrower span in accessing and using sources and types of evidence was often observed when there was a sudden and drastic change in the magnitude of pressures on the studied trusts – especially performance-related pressures. In other words, the use or non-use of diverse forms of evidence became wider or narrower, depending on multilevel contextual influences.
Innovation adoption decision-making occurs within dynamic and complex contexts (both micro and macro). Our study found that this has had an effect on how actors perceived evidence (i.e. acute/urgent, credible, relevant), and whether they actually utilised it in their decision-making. The temporal nature of evidence per se as well as the diversity of actors’ identities (i.e. professional background, organisational role) can add further complexity. This is because such evidence requires continuous (re)construction, triangulation and interpretation by use of one’s own cognitive ‘template(s)’, which translate evidence to be deemed plausible to self and/or others.
Within our studied cases the nature of the problems being addressed drove the quest for evidence and the amount of resources (time, manpower and expertise) expended. The external forces that have been discussed previously featured as inhibitors or facilitators when considering innovation and supporting evidence. In addition, the nature of the problem as technical and product based or more processual and concerned with service delivery and organisation also impacted on the types and sources of evidence used. For problems of high perceived technical complexity, ‘harder’ evidence was more readily available. Furthermore, although rated as of ‘low importance’ by clinicians and managers interviewed in phase 1, information from suppliers was often the point of first call in the early stages of technology assessments by the trusts in phase 2.
Forms of evidence and sources of influence on decisions
Our findings suggest that there were six main sources of influence on organisational decision-makers’ use of evidence during the various stages of the innovation process, and these are summarised in Figure 34 . The various sources of evidence were used at different times depending on the nature of the organisational problem identified, but also depending on who was involved in the various stages of the innovation process.
FIGURE 34.
Main sources of influence on sourcing evidence in organisational decisions.
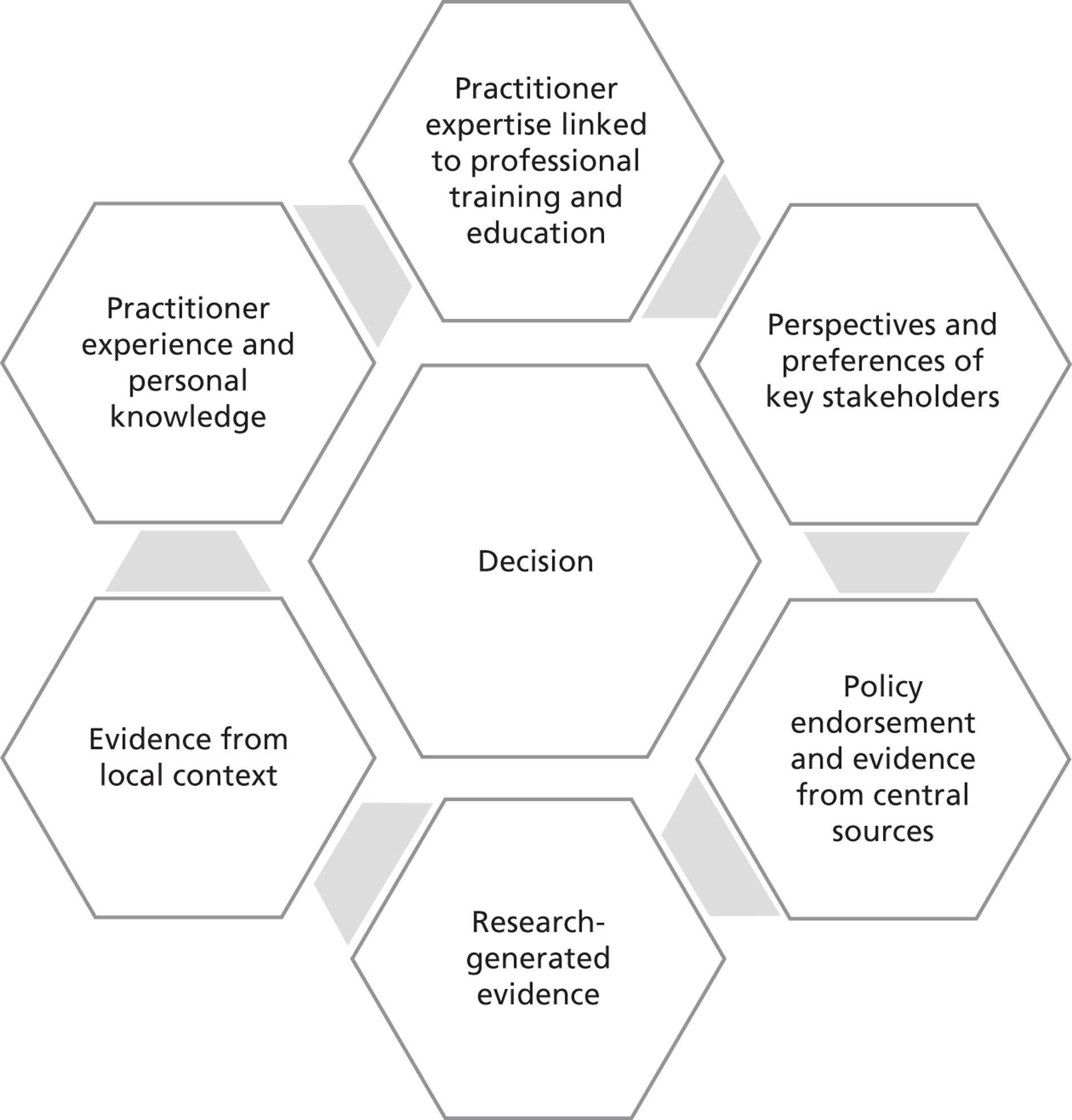
The ‘size’ of each hexagon-like box – and hence the strength of the source of influence – varied with each decision. In some circumstances, evidence from a local trial or the preferences of stakeholders (e.g. patient expectations and safety assurance for the use of the hydrogen peroxide vapour system) were judged by the decision-makers to be more important and relevant than research-generated evidence and, thus, were given much greater emphasis in specific decisions. In other situations, little evidence was available from central sources, and thus their influence on managerial decisions was relatively limited.
This conceptualisation builds and expands on the key elements of EBMgt presented by Briner et al. 97 by also explicitly reflecting on the influence of policy and practitioner embodied knowledge and skills as sources of credible evidence. 98
Sensemaking in organisations
This study has provided insights into the sensemaking processes of diverse professional groups in the context of acute NHS trusts. Although sensemaking is ongoing and iterative by its very nature, this process becomes more pronounced and easier to capture in the context of uncertainty and change. Our study examined decision-making in innovation adoption and implementation processes, which are inherently associated with ‘newness’ and change. The context of IPC is also closely related to issues of operational uncertainty and clinical risk.
Everyday sensemaking in health care
In organisational sensemaking theory, there is less emphasis on empirical studies that deal with the day-to-day processes of sensemaking than on those that deal with crises and critical events. There are fewer inquiries into sensemaking that occurs among many diverse organisational stakeholders as they address a range of issues. 46,62 By applying this theoretical lens to the investigation of managerial decisions on the adoption and implementation of innovative technologies, we empirically contribute to the field.
Collective sensemaking: strategic and operational decision-making
The construction of shared meanings, or collective sensemaking,46 is key for understanding how new types of evidence may be successfully embedded in certain contexts, or even be rejected under conditions of innovation uncertainty and ambiguity. However, less attention has been paid to the social processes that underpin sensemaking at the organisational level. 62 In this study we empirically examined issues in which a large and diverse group of stakeholders was involved, giving rise to differential needs for evidence (i.e. for strategic or operational decision-making) and dissimilar perspectives on its interpretation.
Connecting micro cognitive processes with macro shared templates
Our findings highlight the importance of ‘sensemaking in context’. Drawing on our empirical work, we suggest that sensemaking theorisations that overlook the role of larger social contexts (i.e. in this study the use of evidence being shaped by diverse professional frames of rationality) in explaining cognition are necessarily incomplete. This is in line with recent arguments in conceptual papers, which highlight the lack of an explicit account of embeddedness of sensemaking theory in social space and time. 56 We found that professionals within health-care organisations drew, through sensemaking practices, on existing shared rationalised frames of reference to make sense of issues in decision-making (this is detailed below.) We approached sensemaking as an ongoing communication process by which actions, events, situations and circumstances are talked into existence. 46
The emergence of evidence templates
Although the focus of this study was around perceptions and use of evidence in organisational decisions, this was one dimension of the process. We also note that, although the perceptions and reported use of evidence in phase 1 interviews may have reflected more espoused beliefs rather than what managers actually did (see the works from McGlynn et al. 99 and Runciman et al. 100 for a review of what constitutes evidence by clinicians), this discrepancy is in itself important. What managers think that they ought to be doing, or what they would like to be doing, is important. In phase 2 we were able to partly relay which contextual factors impacted on these espoused beliefs. In addition, phase 2 allowed us to capture part of what happens when the decision-making dynamics change by virtue of those involved, and also to capture influences of local organisational priorities and macro-level policy agendas.
We reflect here on how decision-making processes compared across professional groups. It is useful to reflect on two contextual issues which contributed to the way different organisational members made decisions. First, the issue of ‘who is the decision for?’: this could be the individual/patient, unit or ward, organisational or population level. Second, the issue of ‘who makes the decision?’. Is it essentially an individual or a collective decision? By exploring these issues, we understand more about the ‘usual’ role of evidence in decision-making for these diverse professionals. At one end of the continuum we could position doctors, who largely make clinical decisions about an individual patient on a case-by-case basis, sometimes with immediate feedback of the impact on the patient. At the other end of the continuum we could place non-clinical managers, who make decisions largely for an organisation, usually with longer-term and often unclear direct impacts. How different organisational members make sense of evidence is then interlinked to how they make decisions and how well they translate this ‘usual’ sensemaking when considering organisational decisions, which are carried out collectively. We saw from phase 2 that, with the exception of DIPCs, doctors were not present in collective decision-making.
Given these different professionally dominated ways of doing things, what happens when these members come together to make essentially organisational-level decisions? Making sense of evidence in the context of the organisational decisions described in this study can be conceptualised through the use of evidence templates and the purposeful action of ‘adopting an evidence template’ by different organisational members to make sense either for self or for others. Specifically, we consider three apparent templates: the ‘biomedical-scientific template’, the ‘practice-based template’ and the ‘rational-policy template’ (described in detail below). These constituted a source of interpretive and legitimating resources in the cognitive processes of individual professionals making sense of evidence. We discuss below how the different templates dominated in different contexts and at different times along the innovation process. We found that adoption of an evidence template was shaped by professional background, current organisational role, current decision and the presence of other stakeholders in the decision-making process either explicitly or implicitly.
Tested/proven according to the biomedical-scientific template
The biomedical-scientific approach ‘minimises risk’ to patients in that a thorough level of ‘testing’ has been completed. This template formed the ‘basis’ from which medical and other clinical professionals embarked. Only once this evidence was available would they proceed to look at other levels of evidence. This template played a central role in the earlier stages of the organisational innovation process. The degree to which other levels of evidence were considered was shaped by experience and who else was at the table making the case for any particular decision. Close teamwork among the professional groups and advances in the way nurses sourced evidence required attention to the biomedical-scientific template, but this took more of a balanced, complementary role alongside other evidence templates. The role of nurses, in particular in convincing a wide range of stakeholders, may have shaped their convergence towards the biomedical paradigm, given the reported preference of doctors for this template and their central position in decision-making.
Tested/proven according to the practice-based template
All stakeholder groups gave importance to this approach, but to different degrees and at different times. Accounts about learning from other trusts and peers featured in making sense of the evidence under this template. This template followed a logic of practical action and dominated the focus of attention of the decision-makers in the later stages of the innovation process. Potential adopters wished for extensive information about the technology, its workings and its anticipated impact; it was not easy to access this. Considerable (personal) effort and improvisation was needed and informal networking proved invaluable. However, this type of information retrieval produced information of variable quality and applicability. The extent of industry involvement in the decision-making was surprising but explained by the dearth of both skills and accessible information. Technical training for users, on the whole, was the responsibility of the industrial supplier of the technology and was well organised.
Rational-policy template: tested/proven according to the discourse of bureaucratic rationality
This was determined by the goals of the organisation, which are shaped by the macro environment, particularly relevant regulation and policy. The organisational role of non-clinical managers appeared to be most aligned with this approach, when compared with other organisational members. Their performance requirements and remit of responsibility matched this paradigm. Policy endorsement and mandate shaped cognition and attention of decision-makers to certain forms of evidence. Such issues permeated the whole organisational innovation process.
Our findings suggest there are shortcomings of linear evidence-based conceptualisations of innovation adoption, and a need for context-sensitive, practice and policy contingent approaches. 19 We noted the importance of decision-makers’ and significant others’ understandings of the nature of the perceived problem and the associated risk and safety issues, as well as the perceived need for various forms of evidence. For example, local trials, and the need for ‘pragmatic evidence’, were deemed important by decision-makers in the early stages of innovation adoption. For innovations (and especially for those that require significant changes in practices or processes to be implemented), creating an evidence base will require agreement about what is regarded as a legitimate epistemological basis for verifying and validating evidence and relevant knowledge. For example, should the evidence base for implementing an innovation in a specific context rely exclusively on scientific reproducibility and explicit, codified forms of knowledge? Or, alternatively, should the basis of evidence also account for a wider conceptualisation of valid and relevant knowledge (including practice-based and experiential tacit forms of knowledge)? How might pluralist evidence and knowledge bases be reconciled?
Evidence sources and types appeared to be variably prioritised and used by decision-makers depending on their professional background. Doctors and nurses prioritised evidence on the clinical efficacy and effectiveness of innovations. High importance was accorded to systematic forms of knowledge by doctors, whereas non-clinical managers and nurses relied more on their own or peers’ experiential knowledge. Non-clinical managers and nurses also considered evidence on ‘ease of use’, including local trials of innovative products and technologies, as highly important. Different evidence hierarchies emerged in practice and were reinforced through routine enactment. The antecedents and ongoing sensemaking that shaped interpretive frameworks of different stakeholders were articulated in templates that structured shared cognition.
The confluence of diverse templates
Within a health-care setting, the evaluation of an innovation can take a number of forms and include technical, economic and social assessments. Adoption decisions involve a number of stakeholders, and therefore it is important that the evidence used to support adoption is not just sufficient but also relevant and perceived as appropriate to address the concerns of all parties.
As described above, different professional members had different roles in decision-making in our empirical study. Another differentiating feature is the ‘entry point’ of health-care practitioners when compared with non-clinical managers. Health-care professionals have a well-defined entry point that establishes within these professionals a common ‘frame of reference’ or ‘template’ through formal training and then through socialisation within practice. Non-clinical managers in health care come from a variety of professional backgrounds and may have received no formal management training. 2 The fact that management does not have a commonly shared frame of reference to define quality criteria of evidence has led to the prominence of experiential ‘evidence’. 101
The various professional groups drew variably on co-existing evidence templates to help them make sense of the evidence base. In our empirical cases, nurses drew on all diverse ‘templates in circulation’ and aimed for evidence plausibility to self and others and were the only professional group who explicitly tried to make the case to other stakeholders. Non-clinical managers also drew on all diverse ‘templates in circulation’ but aimed primarily for evidence plausibility to self and then sought to justify to others, based on this evidence template. In contrast, doctors drew primarily on the biomedical-scientific template and were exclusively concerned with evidence plausibility to self. The use of evidence templates by health-care managers aligns with the conceptualisation by Gabbay and Le May102,103 of clinical ‘mindlines’, namely, internalised, collectively reinforced, tacit guidelines for knowledge use by clinical practitioners. We share with these authors the empirical experience and conceptualisation of evidence representing ‘knowledge in practice in context’ rather than accepting linear rational approaches to explain how knowledge is mobilised and used as evidence in practice. Unlike ‘mindlines’, evidence templates are socially embedded frames of cognition collectively shared by groups of organisational decision-makers, rather than being idiosyncratic cognitive guidelines informing clinical decisions. However, we refer to evidence templates as abstractions that help organisational decision-makers reconcile and make sense of complex knowledge that often crosses epistemological paradigms of what constitutes valid knowledge. Clinical decisions taken within the well-established epistemological biomedical paradigm trigger qualitatively different sensemaking processes.
Strengths and weaknesses/limitations of the study
Although our study draws on rich empirical data from a large-scale multisite project, which helped untangle important dynamics on the use of evidence in organisational decisions, more work is needed to expand our understanding. Our qualitative study is limited to the field of IPC; we draw on data from specific organisations and technologies, also reporting on a special type of decision, namely innovation adoption. Generalisations, therefore, from this data set must be treated with caution. We deliberate here some limitations of our methodology and then reflect on some of the strengths of the study, which have been discussed in detail in the methodology section.
First, we need to acknowledge that most of the primary data in this research are drawn from interviews and structured questionnaires that explored views, perceptions and self-reported accounts on activity and practices. The qualitative study is based on our purposeful sample of respondents rather than the trust population. The interviews were conducted in retrospect for most of the microcase examples, and as a result we cannot completely mitigate recall bias. The interviews were semistructured, allowing respondents to elaborate on facilitators, barriers and prevailing conditions at the time of decision-making; however, the accounts were essentially a reconstruction of events. Potential bias owing to selective memory for events that occurred in the past and retrospective and attribution bias might have shaped the responses given by the study participants, and need to be considered when making inferences from the study. One strategy we used to avoid problems of retrospective bias and also to avoid dominance of one particular viewpoint was to interview organisational members at several different levels of seniority, from different parts of the organisations, and from different professional groups. We also used direct questions in each of the phase 2 interviews, such as ‘who made the final decision’; this tactic helped to highlight discrepancies in the accounts and any perceptions of ‘forgone conclusions’ versus ‘debated and transparent decision-making’. We further checked, whenever possible, against documentary and other secondary sources (e.g. websites) regarding timescales and any wider involvement in the decision-making process. We reported as findings only those events and relationships that were corroborated by multiple informants and that were consistent with documentary data, whenever such data were available. We also purposefully limited the timespan of technologies included in the study to the period after 2007 to minimise the potential bias of recall and issues of incomplete accounts as a result of staff turnover.
Second, our study context and questions relate to a wide range of possible factors that potentially impact on evidence use in the organisational innovation processes. We employed an integrated approach to analysis as opposed to pure grounded theory, which may have given rise to alternative explanations. The scope and relevance of the study required a balanced approach between external validity through cross-comparative analysis and an in-depth smaller-scale study employing a more open framework of analysis. For example, a follow-up in-depth study based on ethnographic work may reveal the interplay among evidence, knowledge and power. Alternatively, employing a more deductive framework, and using an explanatory theory such as interprofessional power, would have undoubtedly revealed some findings to support the theory but, at the same time, stifled any new or alternative explanations being discovered.
A third important methodological limitation is due to practical constraints of time and accessibility. We were not able to conduct real-time observations of discussions in meetings and observe instances of decision-making and evidence use ‘in action’ across all participating trusts. We were able to attend only one meeting in T4. Obtaining observational data proved challenging in the high-pressure environment of busy NHS trusts, in part because of the relatively short window of opportunity for data collection; access issues were further exacerbated during the winter months owing to flu emergencies and in summer months owing to staff holidays. Extending the data collection period would have caused important delays to our project. We were fortunate to complete collection of all other data in eight of the nine planned trusts. One trust (T8) decided to drop out because of issues of high operational demands during the course of the study. In some cases, we were not able to observe the final outcome of a decision-making process within the lifespan of this project and these decisions have been reported as ongoing. A careful balance needed to be maintained to avoid ‘closing the door’ for future researchers.
Despite the limitations described above, the study has important strengths that distinguish it from earlier research exploring similar issues. First, the study draws on a large sample of informants and investigates qualitatively multiple and comparable cases, focusing on health innovation technology as the tracer issue. We followed a phased approach that explored individual and collective processes of evidence mobilisation in decisions. In the first phase we investigated accounts of how individuals, from various professional backgrounds and organisational roles situated in diverse organisations, reported accessing and using evidence to accomplish individual tasks in relation to innovation decisions. In the second phase we contrasted such accounts with experiences in collective decision-making processes.
Second, whereas most previous empirical investigations using sensemaking as a theoretical lens focused almost exclusively on either individual or organisational processes, this study employed a multilevel perspective to also account for wider contextual influences, such as the collective frames of rationality or evidence templates, we identified in this research. This aligns well with the nature of complex health-care organisations, such as the NHS, which are distinctively multilayered entities. It also responds to repeated calls for multilevel organisational research. 95,96
Third, the study allowed for tracing the possible relationships between the quality of decisions and final outcomes. We looked at full processes, namely the innovation process from initiation to adoption decision and implementation, and linked this process to adoption and implementation outcomes for specific technology products in specific trusts. We purposefully sampled for innovation rejections and discontinuances, which are rarely empirically studied.
Chapter 11 Implications and suggestions for future research
In this chapter we conclude with a discussion of the potential implications of our findings for policy and practice and suggestions for future research.
Implications for policy and practice
In our empirical findings we presented systematically and in detail a large number of innovation decisions which unfolded across diverse health-care organisations. We described in detail 27 microcases of the ‘what’, ‘who’, ‘how’ and ‘why’ along the decision-making and implementation process. This included the level of involvement of diverse stakeholders at different stages of the process, the issues they encountered, the sources and types of evidence mobilised at each stage and the final outcomes of these decisions. The varied role played by different professionals in how evidence is collectively used has implications for ‘who to involve’ and ‘for what purpose’. An EBMgt approach that inflexibly applies the principles of EBM neglects how evidence is actioned in practice. The nuanced and processual consideration of evidence gives rise to an iterative exchange between codified, systematised knowledge generated from research and other forms of evidence that are also valued by decision-makers. This research demonstrates that experience, personal knowledge and expertise, perspectives and preferences of stakeholders, policy mandates and endorsement, and evidence from the local context all may contribute as credible and relevant evidence sources.
Clinicians and managers were influenced by central or national-level institutions (e.g. The Cochrane Library, NICE, National Service Frameworks, the NPSA), some of which have been active in producing research and disseminating knowledge about the organisation and delivery of health care. There was, however, disconnect between what was perceived as credible (as these sources were) and what was deemed relevant to the decision-making process. How do managers use the research produced by these institutions or influence its production? Our findings showed that the impact of these central institutions differed greatly owing to varied awareness and perceptions of these sources. This leads to the question: is there a need for a central evidence database/depository for managerial practice? Although some informants were aware of NHS Evidence, they rarely used it to source evidence in decisions. The NIHR HS&DR and the NHS Institute for Innovation and Improvement were rarely mentioned and never used (phase 2). There appears currently to be a gap in credible evidence sources relevant to managerial practices in the studied context. The open-access HS&DR journal, which is part of the new online NIHR Journals Library, has the potential to play the role of the management decisions evidence portal, provided that the awareness, credibility and relevance of the journal can be established among practitioners.
The issue of who in organisations searches for, synthesises and presents evidence to others is important. As doctors invariably hold the unique position of being perceived as highly credible at the decision-making table, they need to be engaged. The case of IPC puts nurses at the frontline; in our cases, nurses were the most involved group in innovation processes and charged ‘by default’ with making the case within organisations. The lack of involvement of key stakeholders (e.g. doctors, procurement, the research and development department) was perpetuated in some of our cases to avoid ‘counterproductive interactions’ among professional groups. Nonetheless, the delayed involvement of key stakeholders gave rise to the possibility of decisions being challenged at a later stage. This differential engagement positioned the evidence templates (biomedical-scientific, rational-policy, practice-based) in competition, unless the organisational culture mediated a consensus approach. This lack of involvement of doctors (phase 2) not only contributed to slowing the adoption of innovations, but also curtailed opportunities to draw upon diverse evidence templates.
The NHS and other health systems have explicit policy goals to promote the uptake of innovations and systematise new practices across health-care organisations. 10 Our findings suggest that local processes, and professional and microsystem considerations, play a significant role in adoption and implementation. On the basis of this, and significant other research,16,104–106 this policy goal of systematisation appears to be infeasible, because of the idiosyncrasies of situated circumstances and cultures. This has substantial implications for the effectiveness of large-scale projects and systems-wide policy.
Reported missing research
Respondents in phase 1 reported that areas of missing research comprised behavioural studies, implementation research and organisational studies or management research. They were particularly interested in how to tackle non-conformance behaviour and better understand implementation challenges. In particular, pharmacists reported a lack of research in this area, followed by nurses and non-clinical managers. Doctors were less attuned to this aspect of organisational change. Frequently reported medical resistance towards IPC practices confirm this finding – doctors representing the professional group least aware of behavioural change. T1, T3, T6 and T9 appeared to be more behavioural-change conscious than other trusts. This could be linked to their proactive organisational culture or relevant research strategies set up through collaboration with local universities, as documented in Chapter 6 .
Suggestions for future research
This study has provided original insights into the use of evidence by health-care managers in organisational technology adoption. Whereas we investigated in detail the individual and collective sensemaking processes as managers sourced and applied evidence during the innovation journey, future research can further develop such insights and assess their transferability and relevance to other contexts. We suggest the following ideas that can inform future research (these are not listed in order of priority or importance):
-
While we elicited complex dynamics of the innovation process, from initiation to implementation, our study draws primarily on data derived from self-reported accounts. An understanding of the discourse between professional groups and non-verbal cues would provide further insight into the actual decision-making processes. Direct observation using in-depth ethnographic studies would be the most appropriate approach.
-
Further exploration of the evidence templates and how they link to broader shared cognitive frames of rationality in the form of institutional logics in the field of health care107,108 is needed to address the following questions: what are the constitutive elements of these templates?; what role do the templates play in knowledge production as well as utilisation and what are the consequences for practice?; and how does the interplay of diverse templates occur in practice in different contexts? A longitudinal research design with multiple case studies at the level of the organisational field focusing on evidence use by health-care managers from diverse professional backgrounds can be a fruitful option for this stream of inquiry.
-
An in-depth study looking at the theme highlighted in this research regarding ‘making sense for others’ and a more focused research question about interprofessional power dynamics.
-
In this study we included technology products bounded within NHS acute trusts. We suggest similar dynamics are explored for innovations across different boundaries (sectoral, level of care) and with less clearly defined boundaries (process, organisational innovations). This is particularly relevant given the restructuring of the English NHS, with public health-functions based in local government.
-
We also point out that the dissemination of such research needs to transcend mainstream management and organisational literature. Respondents cited a lack of relevant empirical studies in peer-reviewed management journals largely because there is a discord between where such literature is published and the sources used by these decision-makers.
-
A large-scale population-based survey of the structured questionnaire. Such a survey would help determine the extent to which the reported differential preferences on access and use of evidence sources and types by professional groups in our purposeful sample can be generalised.
-
Although this study allowed for investigating the full innovation process from initiation to adoption decision and implementation, as a result of time constraints we were not able to study the later stages of assimilation and routinisation for all technologies. Moreover, because of the study’s focus on sensemaking processes, emphasis was given to decision-making rather than practice adaptation and assimilation. Future research could investigate in more detail how front-line users implement and assimilate technologies into their established day-to-day routines, which are issues that have received limited attention in current empirical studies.
Acknowledgements
We acknowledge the input of Professor Sue Dopson as expert academic advisor on the research.
We acknowledge the valuable advice received from members of the Project Steering Group, namely Professors Chris Chapman, Sue Dopson and Martin McKee, the patient advisers Mr Tim Sims and Mr Roy Oliver, the NHS clinical manager Mr Darren Nelson and Mr Chris Gush for his former involvement in the Department of Health’s HCAI Innovation Technology Programme.
We would like to thank Anthony Sewell for his support in the primary data collection for two trusts, support in secondary data collection for one trust and triangulation, and Siddharth Mookerjee for his support in secondary data collection.
We thank the academic and administrative staff at NIHR HS&DR for their support, especially Sue Pargeter.
We would also like to deeply thank the clinicians and managers who generously gave their time to be interviewed. We are particularly grateful to the nine NHS organisations that hosted the case studies in phase 1 and the eight of them that also hosted the case studies in phase 2.
Finally, we are grateful to four anonymous reviewers of the first draft, whose insight improved this report.
Contributions of authors
Yiannis Kyratsis (Lecturer in Health Management and Leadership) was the co-principal investigator responsible for oversight of the field work, study design, cross-case analysis and writing of the report, and contributed to data collection.
Raheelah Ahmad (Senior Lecturer in Public Health) led on the empirical chapters on sensemaking, co-led (with Yiannis Kyratsis) on study design, cross-case analysis and writing of the report, and contributed to data collection.
Kyriakos Hatzaras (Senior Research Solutions Analyst, Healthcare Innovation and Technology) led on the typology of technologies and empirical chapters on microcases, contributed to cross-case analysis, project management of fieldwork research and data collection.
Michiyo Iwami (Research Associate, Healthcare Organisation) led on secondary data collection and analysis, contributed to the cross-case analysis, led on empirical chapters on contextual influences and commented on drafts of the final report.
Alison Holmes (Professor, Infectious Diseases) was chief investigator liaising with NIHR HS&DR, led on IPC themes, supported access to empirical sites and commented on drafts of the final report.
Publications
1. Kyratsis Y, Ahmad R, Holmes A. Making sense of evidence in management decisions: the role of research-based knowledge on innovation adoption and implementation in health care. Study protocol. Implement Sci 2012;7:22.
2. Kyratsis Y, Hatzaras K, Iwami M, Ahmad R, Holmes A. Making Sense of Evidence Behind Innovations: a Multisite Comparative Study in Infection Prevention and Control in the NHS. Poster presented at the eighth International Healthcare Infection Society (HIS) conference and Federation of Infection Societies (FIS) annual conference, Liverpool, November 2012.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence Based Medicine: How to Practice and Teach EBM. Edinburgh: Churchill Livingstone; 2000.
- Walshe K, Rundall TG. Evidence based management: from theory to practice in health care. Milbank Q 2001;79:429-57. http://dx.doi.org/10.1111/1468-0009.00214.
- Shortell SM, Rundall TG, Hsu J. Improving patient care by linking evidence-based medicine and evidence-based management. JAMA 2007;298:673-6. http://dx.doi.org/10.1001/jama.298.6.673.
- Rousseau DM. Is there such a thing as ‘evidence-based management’?. Acad Manag Rev 2006;31:256-69.
- Pfeffer J, Sutton RI. Hard Facts, Dangerous Half-Truths, and Total Nonsense: Profiting from Evidence-Based Management. Boston, MA: Harvard Business School Press; 2006.
- Clean, Safe Care: Reducing Infections and Saving Lives. London: Department of Health; 2008.
- Cooksey D. A Review of UK Health Research Funding. Norwich: HMSO; 2006.
- Report of The High Level Group on Clinical Effectiveness. London: Department of Health; 2007.
- The Report of the Clinical Effectiveness Research Agenda Group. London: Department of Health; 2008.
- Innovation, Health and Wealth: Accelerating Adoption and Diffusion in the NHS. Leeds: Department of Health; 2011.
- Dopson S, FitzGerald L, Ferlie E, Gabbay J, Locock L. No magic targets! Changing clinical practice to become more evidence based. Health Care Manag Rev 2002;27:35-47. http://dx.doi.org/10.1097/00004010-200207000-00005.
- Contandriopoulos D, Lemire M, Denis J-L, Tremblay É. Knowledge exchange processes in organizations and policy arenas: a narrative systematic review of the literature. Milbank Q 2010;88:444-83. http://dx.doi.org/10.1111/j.1468-0009.2010.00608.x.
- Winning Ways: Working Together to Reduce Healthcare Associated Infection in England. London: Department of Health; 2003.
- The Health and Social Care Act 2008 (c. 14). London: HMSO; 2008.
- Sacket DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312:71-2.
- Ferlie E, Fitzgerald L, Wood M, Hawkins C. The non spread of innovations: the mediating role of professionals. Acad Manag J 2005;48:117-34.
- Lavis JN, Ross SE, Hurley JE, Hohenadel JM, Stoddart GL, Woodward CA, et al. Examining the role of health services research in public policymaking. Milbank Q 2002;80:125-54. http://dx.doi.org/10.1111/1468-0009.00005.
- Berwick DM. Disseminating innovations in health care. JAMA 2003;289:1969-75. http://dx.doi.org/10.1001/jama.289.15.1969.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. http://dx.doi.org/10.1111/j.0887-378X.2004.00325.x.
- Fleuren M, Wiefferink K, Paulussen T. Determinants of innovation within health care organizations: literature review and delphi study. Int J Qual Health Care 2004;16:107-23. http://dx.doi.org/10.1093/intqhc/mzh030.
- Robert G, Greenhalgh T, MacFarlane F, Peacock R. Adopting and assimilating new non-pharmaceutical technologies into health care: a systematic review. J Health Serv Res Policy 2010;15:243-50. http://dx.doi.org/10.1258/jhsrp.2010.009137.
- Kyratsis Y, Ahmad R, Holmes A. Technology adoption and implementation in organisations: comparative case studies of 12 English NHS Trusts. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-000872.
- Rogers EM. Diffusion of Innovations. New York, NY: The Free Press; 1995.
- Rogers EM. Diffusion of Innovations. New York, NY: Simon & Schuster; 2003.
- Williams F, Gibson D. Technology Transfer: A Communication Perspective. Beverly Hills, CA: Sage; 1990.
- Van de Ven AH, Polley DE, Garud R, Venkataraman S. The Innovation Journey. Oxford: Oxford University Press; 1999.
- Denis J-L, Hébert Y, Langley A, Lozeau D, Trottier L-H. Explaining diffusion patterns for complex health care innovations. Health Care Manag Rev 2002;27:60-73. http://dx.doi.org/10.1097/00004010-200207000-00007.
- Ferlie E, Fitzgerald L, Woods M. Getting evidence into clinical practice: an organisational behaviour perspective. J Health Serv Res Policy 2000;5:96-102.
- Timmons S, Ashburner L. Organisational Behaviour and Organisational Studies in Health Care: Reflections on the Future. Basingstoke: Palgrave; 2001.
- Fitzgerald L, Ferlie E, Wood M, Hawkins C. Interlocking interactions, the diffusion of innovations in health care. Hum Relations 2002;55:1429-49. http://dx.doi.org/10.1177/001872602128782213.
- Atun RA, Kyratsis I, Jelic G, Rados-Malicbegovic D, Gurol-Urganci I. Diffusion of complex health innovations – implementation of primary health care reforms in Bosnia and Herzegovina. Health Policy Plan 2007;22:28-39. http://dx.doi.org/10.1093/heapol/czl031.
- Meyer AD, Goes JB. Organizational assimilation of innovations: a multilevel contextual analysis. Acad Manag J 1988;31:897-923. http://dx.doi.org/10.2307/256344.
- Locock L, Dopson S, Chambers D, Gabbay J. Understanding the role of opinion leaders in improving clinical effectiveness. Soc Sci Med 2001;53:745-57. http://dx.doi.org/10.1016/S0277-9536(00)00387-7.
- Coleman JS, Katz E, Menzel H. Medical Innovation: A Diffusion Study. Indianapolis, IN: Bobbs-Merrill Co.; 1966.
- Zaltman G, Duncan R, Holbek J. Innovations and Organizations. New York, NY: Wiley; 1973.
- Ferlie E, Gabbay J, Fitzgerald L, Locock L, Dopson S, Ashburner L. Organisational Behaviour and Organisational Studies in Health Care: Reflections on the Future. Basingstoke: Palgrave; 2001.
- Pettigrew AM, Ferlie E, McKee L. Shaping Strategic Change. London: Sage; 1992.
- Damanpour F, Schneider M. Phases of the adoption of innovation in organizations: effects of environment, organisation and top managers. Br J Manag 2006;17:215-36.
- Kovner AR, Rundall TG. Evidence-based management reconsidered. Front Health Serv Manag 2006;22:3-22.
- Tolbert PS, Zucker LG. Institutional sources of change in the formal structure of organizations: the diffusion of civil service reform, 1880–1935. Admin Sci Quart 1983;28:22-39.
- Westphal JD, Gulati R, Shortell SM. Customization or conformity? An institutional and network perspective on the content and consequences of TQM adoption. Admin Sci Quart 1997;42:366-94. http://dx.doi.org/10.2307/2393924.
- Abrahamson E. Managerial fads and fashions: the diffusion and rejection of innovations. Acad Manag Rev 1991;16:586-612. http://dx.doi.org/10.2307/258919.
- Ahmad R, Kyratsis Y, Holmes A. When the user is not the chooser: learning from stakeholder involvement in technology adoption decisions in infection control. J Hosp Infect 2012;81:163-8. http://dx.doi.org/10.1016/j.jhin.2012.04.014.
- Ferlie E, Crilly T, Jashapara A, Peckham A. Knowledge mobilisation in healthcare: a critical review of health sector and generic management literature. Soc Sci Med 2012;74:1297-304. http://dx.doi.org/10.1016/j.socscimed.2011.11.042.
- Crilly T, Jashapara A, Trenholm S, Peckham A, Currie G, Ferlie E. Knowledge Mobilisation in Healthcare Organisations: Synthesising the Evidence and Theory Using Perspectives of Organisational Form, Resource Based View of the Firm and Critical Theory 2013. www.netscc.ac.uk/hsdr/files/project/SDO_FR_09-1002-13_V07.pdf (accessed 11 July 2013).
- Weick KE. Sensemaking in Organizations. Thousand Oaks, CA: Sage Publications; 1995.
- Weick KE, Sutcliffe K, Obstfeld D. Organizing and the process of sensemaking. Organ Sci 2005;16:409-21. http://dx.doi.org/10.1287/orsc.1050.0133.
- Weick KE. The collapse of sensemaking in organizations: the Mann Gulch disaster. Admin Sci Quart 1993;38:628-52. http://dx.doi.org/10.2307/2393339.
- Snook S. Friendly Fire. Princeton, NJ: Princeton University; 2001.
- Schwandt D. When managers become philosophers: integrating learning with sensemaking. Acad Manag Learn Educ 2005;4:176-92. http://dx.doi.org/10.5465/AMLE.2005.17268565.
- Fitzgerald L, Dopson S, Dopson S, Fitzgerald L. Knowledge to Action? Evidence-based Health Care in Context. Oxford: Oxford University Press; 2005.
- Weick KE, Sutcliffe KM. Hospitals as cultures of entrapment: a re-analysis of the Bristol Royal Infirmary. Calif Manag Rev 2003;45:73-84. http://dx.doi.org/10.2307/41166166.
- Battles JB, Dixon NM, Borotkanics RJ, Rabin-Fastmen B, Kaplan HS. Sensemaking of patient safety risks and hazards. Health Serv Res 2006;41:1555-75. http://dx.doi.org/10.1111/j.1475-6773.2006.00565.x.
- Greenhalgh T, Robert G, Bate P, Macfarlane F, Kyriakidou O. Diffusion of Innovations in Health Service Organisations: A Systematic Literature Review. London: Blackwell; 2005.
- Greenhalgh T, Russell J, Swinglehurst D. Narrative methods in quality improvement research. Qual Saf Health Care 2005;14:443-9. http://dx.doi.org/10.1136/qshc.2005.014712.
- Weber K, Glynn MA. Making sense with institutions: context, thought and action in Karl Weick’s theory. Organ Stud 2006;27:1639-60.
- Gioia DA, Chittipeddi K. Sensemaking and sensegiving in strategic change initiation. Strat Manag J 1991;12:433-48. http://dx.doi.org/10.1002/smj.4250120604.
- Goffman E. The Presentation of Self in Everyday Life. Garden City, NY: Doubleday & Co.; 1959.
- Chatman JA, Bell NE, Staw BM, Sims HP, Gioia DA. The Thinking Organization: Dynamics of Organizational Social Cognition. San Francisco, CA: Jossey-Bass; 1986.
- Snyder M. The self-monitoring of expressive behaviour. J Pers Soc Psychol 1974;30:526-37. http://dx.doi.org/10.1037/h0037039.
- Isabella LA. Evolving interpretations as a change unfolds: how managers construe key organizational events. Acad Manag J 1990;33:7-41. http://dx.doi.org/10.2307/256350.
- Maitlis S. The social processes of organizational sensemaking. Acad Manag J 2005;48:1-49. http://dx.doi.org/10.5465/AMJ.2005.15993111.
- Ferlie E, Dopson S, Dopson S, Fitzgerald L. Knowledge to Action? Evidence-based Health Care in Context. Oxford: Oxford University Press; 2005.
- Downs GW, Mohr LB. Conceptual issues in the study of innovation. Admin Sci Quart 1976;21:700-14. http://dx.doi.org/10.2307/2391725.
- Van de Ven AH, Rogers EM. Innovations and organizations: critical perspectives. Commun Res 1988;15:632-51. http://dx.doi.org/10.1177/009365088015005007.
- Wolfe R. Organizational innovation: review, critique and suggested research directions. J Manag Stud 1994;31:405-31. http://dx.doi.org/10.1111/j.1467-6486.1994.tb00624.x.
- Eisenhardt KM. Building theories from case study research. Acad Manag Rev 1989;14:532-50. http://dx.doi.org/10.2307/258557.
- Yin RK. Case Study Research: Design and Methods. Newbury Park, CA: Sage; 1994.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- The Health Act 2006: Code of Practice for the Prevention and Control of Healthcare Associated Infections. London: Department of Health; 2006.
- Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS Hospitals in England. J Hosp Infect 2007;65:S1-64. http://dx.doi.org/10.1016/S0195-6701(07)60002-4.
- Saving Lives: Reducing Infection, Delivering Clean and Safe Care. London: Department of Health; 2007.
- Kyratsis Y, Ahmad R, Holmes A. Understanding the Process of Innovation Adoption in 12 NHS Trusts – Technology Selection, Procurement and Implementation to Help Reduce HCAIs. London: Department of Health; 2010.
- Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ 2000;320:114-16. http://dx.doi.org/10.1136/bmj.320.7227.114.
- Sofaer S. Qualitative methods: what are they and why use them?. Health Serv Res 1999;34:1101-18.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758-72. http://dx.doi.org/10.1111/j.1475-6773.2006.00684.x.
- Patton MQ. Qualitative Research and Evaluation Methods. Thousand Oaks, CA: Sage Publication; 2002.
- Uniforms and Workwear: Guidance on Uniform and Workwear Policies for NHS Employers. Leeds: Department of Health; 2010.
- Inspection Reports on the Prevention and Control of Infections 2009/10 [A to Z list]. London: Care Quality Commission; 2009.
- 2008/09 NHS Performance Ratings: Overall and Component Level Scores. London: Care Quality Commission; 2009.
- Healthcare Commission . Data Set for the 2007 2008 New National Targets Assessment 2008. http://webarchive.nationalarchives.gov.uk/20100402140226/http:/www.cqc.org.uk/publications.cfm?fde_id=1255 (accessed 7 December 2013).
- 2008/09 NHS Performance Ratings: Existing Commitments and National Priorities Indicator Scores. London: Care Quality Commission; 2009.
- 2009 Inpatient Survey – National Summary Results for England. London: Care Quality Commission; 2010.
- 2010 Adult Inpatient Survey – Results. London: Care Quality Commission; 2011.
- 2011 Adult Inpatient Survey – Results. London: Care Quality Commission; 2012.
- Health Protection Agency . Results for Mandatory Surveillance Data 2012. www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1244763936373 (accessed 30 August 2012).
- The NHS in England: The Operating Framework for 2008/09. London: Department of Health; 2007.
- Department of Health . The Operating Framework for 2009 10 for the NHS in England 2008. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_091446.pdf (accessed 17 April 2012).
- Health profiles 2008, 2009, 2010, 2011, 2012. The Network of Public Health Observatories; 2008.
- NHS Financial Year 2011/12. London: Audit Commission; 2012.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Brook RH, Lohr KN. Efficacy, effectiveness, variations, and quality: boundary-crossing research. Med Care 1985;23:710-22. http://dx.doi.org/10.1097/00005650-198505000-00030.
- Recommendation Statements by the Rapid Review Panel. Health Protection Agency; 2010.
- Public Health, Adult Social Care, and the NHS – QIPP. Department of Health; 2012.
- House R, Rousseau DM, Thomas-Hunt M, Cummings LL, Staw B. Research in Organizational Behavior. Greenwich, CT: JAI; 1995.
- Rye CB, Kimberly JR. The adoption of innovations by provider organizations in health care. Med Care Res Rev 2007;64:235-78. http://dx.doi.org/10.1177/1077558707299865.
- Briner RB, Denyer D, Rousseau DM. Evidence-based management: concept clean-up time?. Acad Manag Perspect 2009;23:19-32.
- Maitlis S, Lawrence TB. Triggers and enablers of sensegiving in organizations. Acad Manag J 2007;50:57-84. http://dx.doi.org/10.5465/AMJ.2007.24160971.
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med 2003;348:2635-45. http://dx.doi.org/10.1056/NEJMsa022615.
- Runciman WB, Hunt TD, Hannaford NA, Hibbert PD, Westbrook JI, Coiera EW, et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med J Aus 2012;197:100-5. http://dx.doi.org/10.5694/mja12.10510.
- Rousseau DM, Rousseau DM. The Oxford Handbook of Evidence-based Management. Oxford: Oxford University Press; 2012.
- Gabbay J, Le May A. Evidence based guidelines or collectively constructed ‘mindlines’? Ethnographic study of knowledge management in primary care. BMJ 2004;329.
- Gabbay J, Le May A. Practice-based Evidence for Healthcare: Clinical Mindlines. Abingdon: Routledge; 2011.
- Swan J, Clarke A, Nicolini D, Powell J, Scarbrough H, Roginski C, et al. Evidence in Management Decisions (EMD) – Advancing Knowledge Utilization in Healthcare Management 2012. http://www.nets.nihr.ac.uk/__data/assets/pdf_file/0019/85060/FR-08-1808-244.pdf (accessed 5 April 2013).
- Dopson S, FitzGerald L, Ferlie E, Gabbay J, Locock L. No magic targets! Changing clinical practice to become more evidence based. Health Care Manag Rev 2010;35:2-12. http://dx.doi.org/10.1097/00004010-200207000-00005.
- Dopson S, Fitzgerald L, Ferlie E. Understanding change and innovation in health care settings: reconceptualizing the active role of context. J Change Manag 2008;8:213-31.
- Scott WR, Rueff M, Mendel PJ, Caronna CA. Institutional Change and Healthcare Organizations. Chicago, IL: University of Chicago Press; 2000.
- Reay T, Hinings CR. Managing the rivalry of competing institutional logics. Organ Stud 2009;30:629-52. http://dx.doi.org/10.1177/0170840609104803.
- Fu TY, Gent P, Kumar V. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J Hosp Infect 2012;80:199-205. http://dx.doi.org/10.1016/j.jhin.2011.11.019.
- Clostridium difficile infection: how to deal with the problem. London: Department of Health; 2008.
Appendix 1 Sampling options appraisal (9 May 2011)
| Dimension and options | Identified strengths | Identified risks | Selection |
|---|---|---|---|
| Temporal | To be guided by trusts | ||
| Option 1: bound innovations/technologies to be considered by time 2007–12 |
|
|
Possible – if trust staff and records available Particularly useful for ‘successful’ implementation of innovations/technologies |
| Option 2: bound innovations/technologies to be considered by time X–2012 (where X > 2007) |
|
|
Possible – to be guided by trust – (2010–2012). Going back earlier if trust staff and records available |
| HCAI | Dimension not to be used as inclusion/exclusion criterion | ||
| Option 1: bound innovations/technologies to be considered by one type of infection, such as MRSA or C. difficile |
|
|
Innovations/technologies are not mutually exclusive to infections – for example, hand hygiene and environmental hygiene prevent MRSA and norovirus |
| Option 2: consider innovations/technologies for all mandatory reported HCAI microorganisms in that time frame (MRSA and C. difficile) |
|
|
|
| Option 3: consider innovations/technologies for wider selection of HCAI microorganisms (MRSA and C. difficile and norovirus, and VRE, and Acinetobacter, etc.) | |||
| High impact interventions (HII) | Dimension not to be used as inclusion/exclusion criterion | ||
| Option 1: bound technologies to be considered by one HII aimed at prevention of SSI or CAUTIs, etc. |
|
|
These technologies may not be found across the trusts making comparisons difficult and increasing the degrees of freedom Narrow scope of technologies excluding wider stakeholder groups May feature in second step of sampling for ‘highly innovative’ technology |
| Option 2: consider technologies for all HIIs aimed at prevention of SSI and CAUTIs and VAP, etc. |
|
|
|
| IPC priority area | Option 1 selected | ||
| Option 1: bound innovations/technologies to be considered by one particular IPC area including hand hygiene, diagnostics, environmental hygiene/cleaning/disinfection, antibiotic prescribing, catheter-related care, training and education, medical devices/equipment hygiene, information technology surveillance systems, patient hygiene |
|
|
IPC priority area: environmental hygiene
Strengths and rationale:
|
| Option 2: Consider all relevant IPC priority areas |
|
|
|
| Number of innovations/technologies | For further consideration | ||
| Option 1: one innovation/technology per trust |
|
|
Final decision to be made once we have the innovations/technologies according to dimension D |
| Option 2: all relevant innovations/technologies generated by applying the selected dimensions and options |
|
|
Potential to cluster by common innovation/technology may be greater with dimension D |
| Option 3: one common innovation/technology across the trusts |
|
|
First, explore all environmental hygiene innovations/technologies considered by each trust within a defined time period Second, select from these the following cases:
|
| Option 4: all common innovations/technologies across the trusts |
|
|
Appendix 2 Participant information sheet and consent form
Participant information sheet and consent form (PDF download)
Participant information sheet and consent form (PDF download)
Appendix 3 Study protocol
SDO Protocol – project ref: 09/1002/38
Version: 2
Date: 19 September 2011
Making sense of evidence in management decisions – the role of research-based knowledge on innovation adoption and implementation in healthcare
Chief investigator
Professor Alison Holmes
Sponsor
Imperial College London
Funder
NIHR/SDO
NIHR Portfolio number
SDO 09/1002/38
ISRCTN registration (if applicable) n/a
Making sense of evidence in management decisions – the role of research-based knowledge on innovation adoption and implementation in healthcare
1. Aims/Objectives:
The study aims to investigate how healthcare managers draw upon and make sense of different types and sources of evidence when they make decisions about innovations. We include general managers and ‘hybrid managers’ (clinicians in a managerial role). Special attention is placed on the role of scientifically produced knowledge and its use by these managers during the decision making process under conditions of innovation uncertainty. The study design incorporates multiple levels of analysis as follows: (a) explores the influences of wider ‘macro’ level contextual dynamics on managers’ decision making, (b) explores decision making processes at the ‘meso’ organisational level, (c) analyses at a ‘micro’ level the processes by which healthcare managers construct meaning of available evidence and how they might use such evidence when deciding on the adoption or rejection of innovations.
Our key research questions are:
-
How do managers make sense of evidence?
-
What role does evidence play in management decision-making when adopting and implementing innovations in healthcare?
-
How do wider contextual conditions and intra-organisational capacity influence research use and application by healthcare managers?
2. Background:
Health service delivery and organisation as well as clinical practice can be improved by applying research findings relating to good practice. While there are many evidence-based healthcare innovations available, new knowledge disseminates slowly, if at all. As a result, health research findings are not always translated appropriately into healthcare practice. This reality also raises the pressing question of how to spread best practices and implement promising innovations within healthcare and specifically in the NHS.
Our empirical study is theoretically based and grounded in the practical experience of healthcare managers dealing with innovation processes. The study focuses primarily on the ‘meso’ organisational level and largely draws on the diffusion of innovations literature. One of the central questions in this body of literature that aligns with the scope of the proposed project is as follows: ‘Why do innovations not readily spread, even if backed by strong evidence?’ There is a growing body of evidence, drawing on examples from healthcare settings, which argues that the adoption of health technologies and practices supported by sound research evidence is a far more dynamic and complex process than previously suggested. The classic innovation diffusion model of change has influenced much healthcare policy and suggests that the adoption of innovative ideas, practices, or artefacts is conditioned by the interaction among the attributes of the innovation, the characteristics of the adopter and the environment (Rogers, 1995). This early innovation diffusion work has been criticised however for adopting a simplistic rational view of change, ignoring the complexities of the change process and also focusing on individuals rather than organisations. Later work by Rogers (2003) partly addressed the criticism by having explicitly considered the adoption process within organisations. Recent studies have departed from the linear model of innovation diffusion (Rogers, 1995) to offer conceptual notions that are more dynamic and interactive (Williams & Gibson, 1990; Van de Ven,et al, 1999). Building on the latter model it is suggested that innovation adoption is a process which is highly dependent on the interactions between the innovation, local actors and contextual factors (Ferlie et al. , 2000; Timmons, 2001; Dopson et al. , 2002; Fitzgeraldet al. , 2002; Denis et al. , 2002, Atun, Kyratsiset al. 2007).
In addition, the nature or definition of ‘evidence’ related to particular innovative technologies or practices is often ambiguous and contested (Greenhalghet al, 2004; Fitzgerald & Dopson, 2005). Managers make decisions relying on experience, personal expertise, judgement, inference, advice, and do not passively receive new knowledge even if presented as evidence which is scientifically produced and validated. Research-based knowledge has to be constantly interpreted and reframed along with the local context and clinical or managerial priorities, a process that often involves power struggles among various professional groups (Ferlieet al. 2001). Different professional and managerial groups may interpret evidence differently, or they may prioritise dissimilar types of evidence partly as a result of their disparate professional role, training and professionalisation processes. We employ a sensemaking perspective to gain insight to this inter and intra professional level and how this plays out in the context of decision making and implementation (Weick, 1995). This lens pays particular attention to the social construction and co-production of evidence through the interaction of a range of diverse professional and managerial groups. We will contribute to this body of literature which has been useful in explaining organisational response to critical events in the heath care setting (Weicket al. , 2005; Weick & Sutcliffe, 2003).
Our work addresses a significant gap in evidence-based healthcare implementation literature. We respond to the call for more sustained interpretive work, which explores the role and motives of actors and the influence of the organisational context and the social construction of evidence (Ferlie & Dopson, 2005). Overall, we aim to address issues that permeate many stages of the research innovation pathway and more specifically will investigate processes that relate to the stages of evaluation, adoption and diffusion. By contributing to the debate on the aforementioned areas our study will add to the current NHS policy and practice body of knowledge as articulated in the NIHR/SDO Research Brief which this proposal responds to. Our work also complements recent and ongoing research commissioned by NIHR research programmes; in particular, it fits well the NIHR/SDO 2008 call for proposals that also focused on issues of knowledge utilisation in healthcare management. We complement and add to this work by looking at different types of decisions, in different healthcare settings.
3. Need:
This research is important from practice, policy and theoretical perspectives. Below we outline how this research: (a) addresses a significant health need, (b) responds to call for research supported by sustained intent, (c) has potential to generate new knowledge.
(a) Health need
Infection control is one of the biggest challenges facing the NHS today. In England 8.19% of all patients within the NHS acquire an infection (Smythet al, 2008). The reporting of MRSA blood stream infection and C. difficile are mandatory and there are national and local targets for reduction. New technologies and interventions have the potential to make a real difference in helping reduce levels of Health Care Associated Infections (HCAIs) by completing good clinical practice. However, their adoption and implementation too often proves challenging and slow. Budgets, competing priorities, and monitoring procedures all play a part in the decision making process when organisations select novel interventions. In addition, professionals and managers may have differing views of what is the optimum intervention given access to evidence, as well as perceptions of this evidence by self and others. To provide sustainable reductions in HCAIs we need to know what has worked, under what conditions, and why. In addition, we need to learn from those settings where an intervention has not worked.
(b) Expressed need for the research supported by sustained interest and intent
Relevant NHS policy reports (DH, 2003; DH, 2008) and legislation (Health & Social Care Act, 2008) have highlighted that countermeasures of known effectiveness have not been universally implemented. In addition, the NHS has commissioned large projects to identify new technologies and products which work best in the fight against HCAIs. One such example is the Department of Health ‘Showcase Hospitals’ Programme. We build on this work by understanding how and why technologies are adopted and disseminated across the NHS. This is where our proposed work would make a significant contribution to the NHS and to patient benefit. Our proposed research is supported by sustained intend also due to the strong political drivers surrounding the control of HCAIs both nationally and internationally. Public and Patient interest in this issue will continue demand for transparency of investments and resultant benefit to patients and the NHS.
(c) Capacity to generate new knowledge
The theoretical basis of the research draws on three main streams from the management and organisational behaviour literature, namely, diffusion of innovations, sensemaking in organisations and neo-institutional theory in organisations. The potential for learning and creating new knowledge from this in depth, multi-method study is substantial due to its theoretical grounding, incorporating micro and macro level perspectives. We will be able to provide a holistic understanding of the phenomenon under consideration
4. Methods:
Setting & Design
The study aims to build theory inductively from multiple in-depth case studies (Eisenhardt, 1989; Yin, 2003). Nine acute NHS Trusts have been selected across three broad geographic regions in England. Our selected NHS Trusts are equally distributed in three regional clusters: (a) London, (b) Northern and Central England, (c) Southern England. The nine research case studies will be conducted concurrently.
The selection of cases involved theoretical, rather than random sampling (Yin, 1995). In our sample of cases we include examples of research-engaged healthcare organisations – such as Academic Health Science Centres (AHSC), University/Teaching Hospitals – and ‘ordinary’ healthcare service providers – such as District General Hospitals. To better delineate the impact of contextual factors in research use and application by healthcare managers on the adoption and implementation of the same innovation we include more than one ‘showcase hospitals’ (as selected by the Department of Health to evaluate the in-use value of Health care associated infection related technologies) for comparative reasons.
As well as individual case studies across the nine acute NHS Trusts, we will conduct cross-case analysis to identify patterns of convergence and divergence, which will enable us to generate new theoretical propositions as well as replicate and extend emerging ones ( Figure 1 ).
FIGURE 1.
Study design diagram.
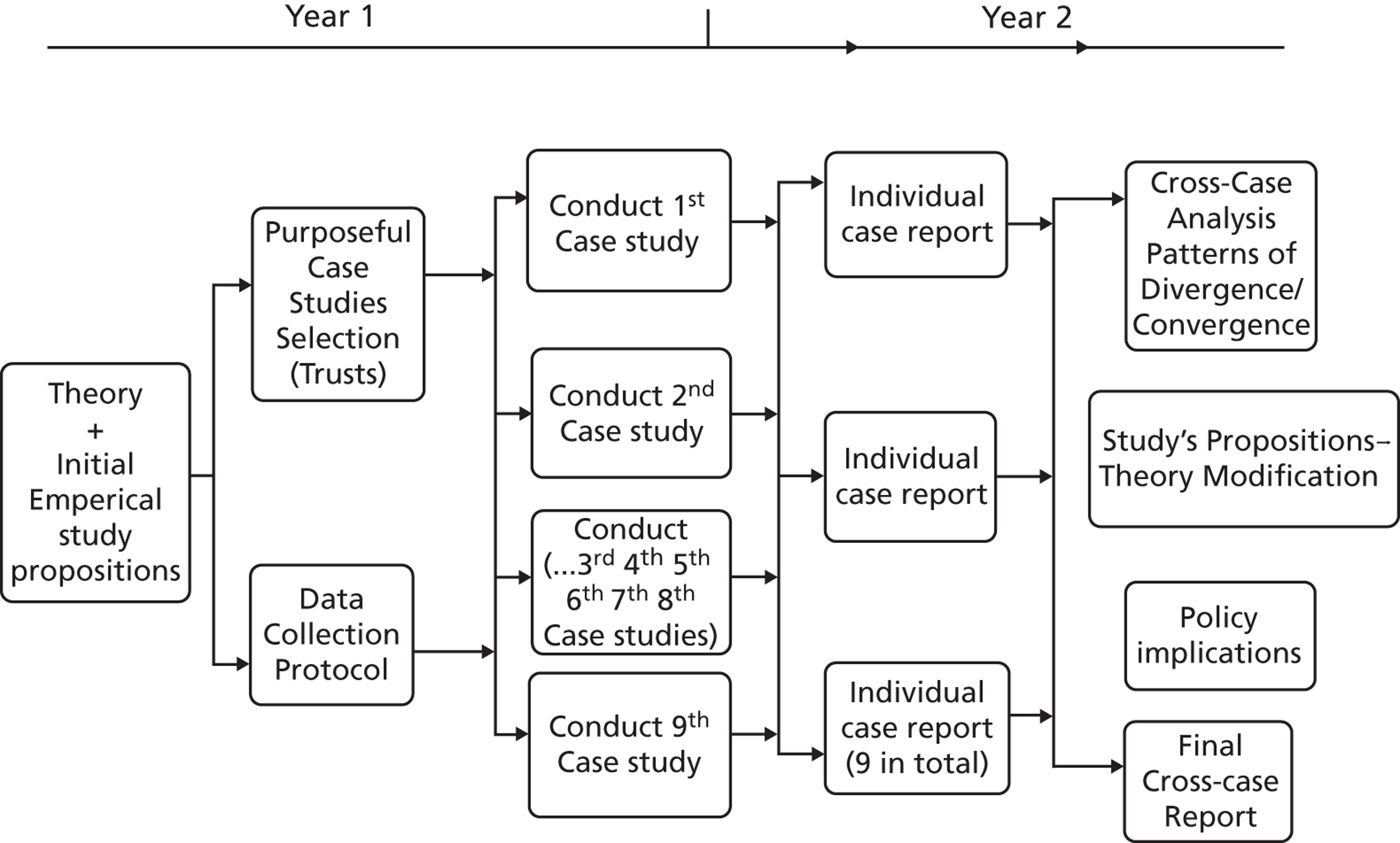
Data collection
Contextual data collection
Detailed templates have been developed and applied to capture and summarise important contextual influences for each of the trusts participating in the study.
Primary data collection: participants recruitment and sampling
Primary data comprise semistructured research interviews and research field notes. The study will last two years and data collection will be longitudinal. Hence the research participants will be involved in the study for two years.
Inclusion criteria for respondents: Informants will include senior, middle and operational managers, representatives from different professional groups including medical doctors, infection control specialists, clinical microbiologists, nurses and allied health professionals, patient representative groups, and administrative personnel.
Inclusion criteria for events: Trust board meetings, infection control team meetings, procurement action group meetings, trust based presentation events for new technologies, interventions adopted in the realm of HCAIs currently or since 2007
Exclusion criteria for respondents: Individuals who are not directly or indirectly involved in the decision making processes of the interventions under study.
Exclusion criteria for events: interventions adopted in the realm of HCAIs prior to 2007.
Total number of interviews will vary according to the size of the Trust and type and span of the innovation (selected to be studied in phase 2). A sample of 6–10 respondents per trust is planned, with follow up interviews and further snowballing to address gaps in the emerging ‘story’. Hence, it is estimated that approximately 90–100 respondents will be interviewed overall.
Semistructured interview schedules, including short questionnaires with a more structured format, have been developed and applied for the two phases of primary data collection. All data collection tools are qualitative in nature. The interview accounts are being audio recorded once consent is given by participants.
The direct involvement of participants to the study primarily involves their participation in an initial and follow up interview with one of the researchers, each lasting approximately between 45 to 60 minutes. Once identified, potential participants are approached locally via the Director of Infection Prevention & Control (DIPC) in the trust or another identified key local collaborator. Either the Chief Investigator Prof Holmes or Co-PI Dr Kyratsis write to potential participants via e-mail inviting them to take part in the study and this e-mail is accompanied by a participant information sheet. The scheduling of interviews allows the collection of primary data with opportunity for further snowballing (and subsequent inclusion into the study sample of additional potential participants) and follow-up interviews with the same respondents where appropriate. This process of participant recruitment is illustrated in detail in Figure 2 .
FIGURE 2.
Participant recruitment process.
Data analysis
Soon after the completion of interviews the content of audio recordings is verbatim transcribed. Upon completion of transcription, four researchers thoroughly read through the full transcribed text several times to enable understanding of the meaning of data in its entirety (Pope et al, 2000). The Qualitative Data Analysis computer software package NVivo 9 (QSR International) is used to systematically code the collected data and assist analysis. In line with recommendations by qualitative methodologists we will use multiple coders to enhance interrater reliability of the qualitative study. (Soafer, 1999 Pope et al, 2000).
Our qualitative analysis follows an integrated approach (Bradley et al, 2007). We will employ an inductive approach to open up new lines of enquiry and then agree a framework for data analysis based on these findings together with our theoretical framework (delineating factors which influence the adoption process of complex health innovations) and our previous work in 12 NHS Trusts looking at adoption processes for new technologies. Hence, we will employ both an ‘inductive and ground up’ development of codes as well as a ‘deductive organising framework as a start up list’ (Bradley et al, 2007: 1762).
Based on the typology suggested by Bradley et al (2007: 1763), the code types employed in the study are the following:
-
Conceptual codes and sub-codes: to identify key concept domains and essential dimensions of these domains;
-
Relationship codes: to identify links between other concepts coded with conceptual codes;
-
Participant perspective codes: to identify whether the participant was positive, negative, or indifferent in attitude about a particular experience or part of an experience;
-
Participant characteristic codes: based on professional/occupational group, hierarchical position, functional role;
-
Setting codes: including rural urban setting, hospital site, particular geographic region, type of trust, Strategic Health Authority
The development of the code structure will be finalised when the point of theoretical saturation will be reached in each of the empirical cases (Glaser and Strauss 1967; Patton 2002).
Analysis within cases will be followed by the cross case analysis across emergent themes but also against the more formal organisational ‘type’ used in our purposeful sampling of sites. Individual case study reports with common formats will be produced as an intermediate research output for each of the nine trusts studied. Summary tables will be used to simultaneously compare several categories and dimensions of the content and context of change implied by the adoption and implementation of the innovations across the 9 trusts.
5. Plan of Investigation:
Individual case studies are being conducted in parallel across all nine participating NHS Trusts. Data collection started in February 2011 and is planned to be completed by end of March 2012.
-
Development of contextual template for each participating trust
-
Phase 1: development and application of an interview topic guide targeting senior and middle managers in the wider trust and within the infection prevention & control team
-
Phase 2: identification of specific examples of innovations in the field of environmental hygiene. Development and application of an interview topic guide (different from phase 1) targeting members of the infection prevention & control team to investigate the decision making and implementation processes of the selected innovation examples.
-
Within & cross-case analysis (started in March 2011 and planned to be completed by end August 2012)
-
Report writing
-
Ongoing dissemination of interim & final research findings
6. Project Management:
Our study is being overseen by a project Steering Group which brings together broad expertise from theoretical and practical perspectives.
-
Darren Nelson – clinical, managerial and operational issues on infection prevention and control and expertise in change management and service re-design; also bringing in the perspective of managing healthcare service delivery in the NHS. In addition his professional nursing background provides specialist insight to the infection prevention context;
-
Professor Martin McKee – translating evidence to policy; also bringing in extensive experience on European and UK public health interventions;
-
Roy Oliver and Tim Sims – patient advisors to critique and invite comment from existing patient groups on all processes of the research (from study inception to dissemination of findings);
-
Professor Christopher Chapman – organisational performance indicators; his expertise lies in the nature and role of performance evaluation and control systems. He explores the construction of organisational and inter-organisational performance indicators from both a qualitative and quantitative perspective; these are relevant methods of inquiry for our research context. For NHS trusts, reporting of clinical and non-clinical performance indicators are important due to mandatory infection prevention and control, quality of services, as well as financial viability and capacity at organisational level.
-
Professor Sue Dopson – knowledge mobilisation in the public sector, specifically in healthcare. Professor Dopson provides additional input through dedicated expert advice to the co-PI Dr Yiannis Kyratsis and fulltime senior researcher Dr Raheelah Ahmad. Ad hoc specialist guidance is complimented by a formal arrangement of quarterly meetings between Professor Dopson and the project researchers.
-
Chris Gush – formerly from the Health Care Associated Infections Technology Innovation programme of DH. He provides insight of central DH innovation and evidence dissemination structures.
FIGURE 3.
Project Governance Structure.
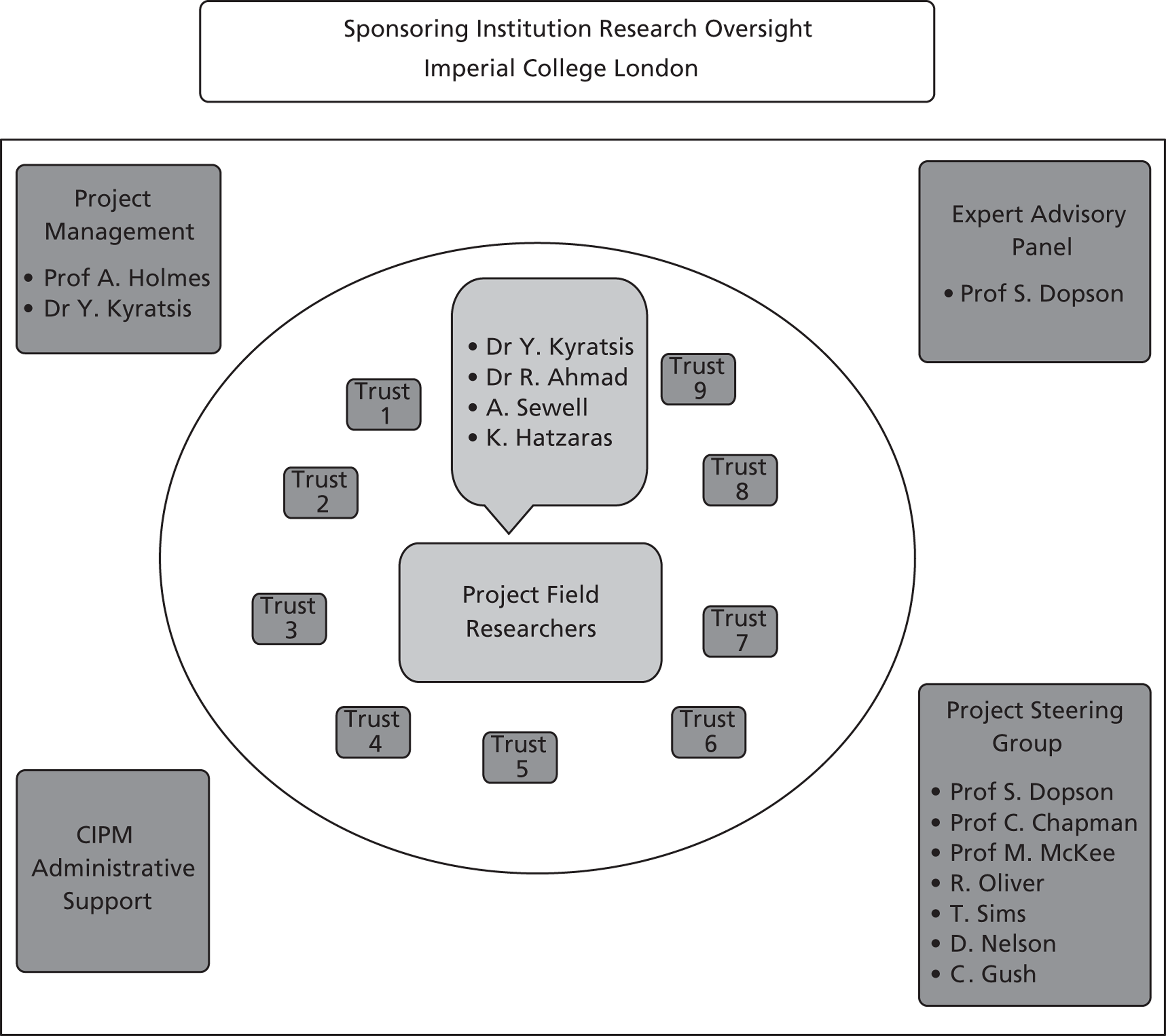
7. Service users/public involvement:
Active public involvement of two patient advisors through Steering Group membership informs the research team to identify and ask the right questions in the right way, making sure that research is relevant to patients and people using services and the public at large. Activities of patient advisors include informing the study design, study management, contribution to dissemination methods as well as providing diverse perspectives from two different localities and trust types (one an academic health sciences centre, the other a district general hospital). We also have one service provider on the steering group (Darren Nelson) bringing in the perspective of managing health care service delivery within the context of a busy NHS trust.
8. References:
- Atun RA, Kyratsis I, Jelic G, Rados-Malicbegovic D, Gurol-Urganci I. Diffusion of complex health innovations--implementation of primary health care reforms in Bosnia and Herzegovina. Health Policy Plan 2007;22:28-39.
- Department of Health . Winning Ways Working Together to Reduce Healthcare Associated Infection in England 2003.
- Department of Health . Clean, Safe Care – Reducing Infections and Saving Lives 2008.
- Dopson S, Locock L, Gabbay J, Ferlie E, Fitzgerald L, Dopson S, et al. Knowledge to action? Evidence-based health care in context. Oxford University Press; n.d.
- Ferlie E, Gabbay J, Fitzgerald L, Locock L, Dopson S, Ashburner L. Organisational Behaviour and Organisational Studies in Health Care: Reflections on the future. Palgrove: Basingstoke; 2001.
- Ferlie E, Fitzgerald L, Wood M, Hawkins C. The Nonspread of Innovations: The mediating role of professionals. Acad Manag J 2005;48:117-34.
- Fitzgerald L, Ferlie E, Wood M, Hawkins C. Interlocking interactions, the diffusion of innovations in health care. Hum Relations 2002;55:1429-49.
- Fitzgerald L, Dopson S, Dopson S, Fitzgerald L. Knowledge to action? Evidence-based health care in context. Oxford University Press; 2005.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of Innovations in service organizations: Systematic review and recommendations. Milbank Q 2004;82:581-629.
- Isabella LA. Evolving interpretations as change unfolds: How managers construe key organizational events. Acad Manag J 1990;33.
- Rogers EM. Diffusion of innovations. New York: The Free Press; 1995.
- Rogers EM. New York: The Free Press; 2003.
- Smyth ETM, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al. Four Country Healthcare Associated Infection Prevalence Survey 2006: overview of the results. J Hosp Infect 2008;69:230-48.
- Timmons S, Ashburner L. How does professional culture influence the success or failure of IT implementation in health services?. Organisational Behaviour and Organisational Studies in Health Care: Reflections on the Future 2001.
- Van de Ven A, Polley ED, Garud R, Venkataraman S. ‘The Innovation Journey’. Oxford University Press; 1999.
- Weick KE. Sensemaking in Organizations 1995.
- Weick KE, Sutcliffe KM, Obstfeld D. Organizing and the Process of Sensemaking. Organ Sci 2005;16:409-21.
- Weick KE, Sutcliffe KM. Hospitals as Cultures of Entrapment: A Re-analysis of the Bristol Infirmary. Calif Manag Rev 2003;45:73-84.
- Williams F, Gibson D. Technology Transfer: A Communication Perspective. Beverly Hills, CA: Sage; 1990.
- Yin RK. Case study research: Design and methods. Sage; 2003.
This protocol refers to independent research commissioned by the National Institute for Health Research (NIHR). Any views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the SDO programme or the Department of Health.
Appendix 4 Interview schedule phase 1
Appendix 5 Interview schedule phase 2
Appendix 6 Brief technology product descriptions
Design Bugs Out commode
The DBO commode has been co-developed by the Department of Health, Design Council, Anglia Ruskin University, industrial partners and participating trusts. The aim was to redesign the portable patient toilet seat traditionally used in the NHS and deliver a specially designed and constructed product that prevents growth of pathogens on its surfaces and saves space in wards. It became available to participating trusts for trial in mid-2009. Commode use is a long-established practice in the NHS.
Clinell universal sanitising wipes (non-sporicidal)
The clinell green, boxed wipe is designed for universal cleaning. It contains 2% chlorhexidine and 70% ethanol-based alcohol. It is currently licensed for use on medical equipment surfaces. Its use is based on a protocol presented on a diagrammatic card, detailing the wipe’s use and application on surfaces.
Bioquell VHP RBDS and Steris BioGenie VHP decontamination system
The Bioquell VHP RBDS involves use of a portable vapour generator, an instrumentation module used to programme the generator and an aeration unit. The generator and aeration unit are placed in a fully sealed (including doors, windows, air conditioning outlets, etc.) bay or ward. Vapour of 5% concentration of hydrogen peroxide, a highly oxidising compound that eliminates air-borne and surface pathogens, is then released within the sealed area. Items need to be positioned in the area in such a way to ensure good vapour exposure. The service can be used as and when required or form part of a hospital site cleaning regime.
A similar device (but not a similar service) is also marketed by Steris BioGenie.
Disposable sterile surgical site gowns
Sterile gowns are a key part of surgical clothing used to prevent contamination or cross-infection between patients and care staff in operating theatres. They are made with special materials that ensure microorganisms and any organic residues do not penetrate them. They must be used according to the manufacturer’s instructions to ensure cross-infection does not occur.
3M Clean-Trace NG and Hygiena SystemSURE II ATP hygiene monitoring system
The Clean-Trace NG luminometer manufactured and supplied by 3M involves the use of a hand-held test monitor and a disposable sampling rod. The disposable rod is used to swab a surface and is then inserted into the hand-held monitor for testing. Results are shown on the screen and are transmitted to a dedicated company server for access by users and later examination. The luminometer measures adenosine phosphatase, a compound found in bacteria, yeast and mould, to assess the surface level of environmental cleanliness. In addition to microorganisms, the device detects the presence of any organic residues left after ineffective cleaning or decontamination.
Another system whose operation is based on exactly the same principles, including the use of data analysis software offering remote monitoring of results, is the Hygiena SystemSURE II ATP hygiene monitoring system.
ASP GLOSAIR 400 aerosolised hydrogen peroxide system
This is an area decontamination system based on hydrogen peroxide, formerly known as Sterinis SR2 and presently supplied by ASP, a Johnson & Johnson company. Its dimensions are 1080 mm (height) × 512 mm (width) × 620 mm (depth) and it has a net weight of 48.8 kg. It creates and releases a gaseous biocide mix, described as ‘dry mist hydrogen peroxide’ or ‘aHP’, of 5–6% hydrogen peroxide concentration and silver ions, from a water-based solution. According to Fu et al. ,109 this solution bears the name STERUSIL® (Gloster Santé Europe®, Toulouse, France) and contains a mix of hydrogen peroxide (∼ 5%), silver ions (< 50 ppm) and orthophosphoric acid (< 50 ppm). The product is recommended for use in patient wards, emergency areas and infectious disease units ranging from 10 m3 to 200 m3. It is thought by some to be capable of accessing area sections (i.e. ceiling, small openings, etc.) not easily reached by normal cleaning.
UV light inspection torch
The UV 35-W torch model marketed by UV Light Technology Ltd is a very high-intensity hand-held torch designed for area irradiation. It weighs 0.75 kg and is powered by means of a rechargeable battery. It produces full UV light output in 15 seconds after power-on, its battery can withstand approximately 300 charging cycles and its bulb has an operating time span of approximately 2000 hours. It emits UVA light radiation with the wavelength range of 315–405 nm. It is, thus, suitable for fluorescent inspection applications, but not for disinfection, as it is not capable of emitting UVC radiation.
Clinell sporicidal (red) wipes
The clinell sporicidal wipe product is said to be highly efficacious, eliminating C. difficile spores within 1 minute, at a 6-log reduction scale or greater. It has been proven to eradicate all microorganisms, including spores. Its active ingredient is peracetic acid, which is understood to be a safe alternative to chlorine, and is activated just by adding water. It is promoted as a simple and easy-to-use wipe product, which requires no dilution, increases compliance and reduces errors, and a wipe that is environmentally friendly and leaves no persistent toxic or carcinogenic residuals, such as any fumes posing risks to staff or patients. It has been tested to a number of European standards and is recommended for use with body fluid spills.
Medixair UV air sterilisation unit
The Medixair UV air steriliser and Medixair Meos compact UV air steriliser units have been marketed by their supplier, Pathogen Solutions Ltd, as devices that sterilise air in the environment. Air sterilisation is conducted by means of air intake at the lower end of the unit, application of UVC radiation at a wavelength of 253.4 nm on that air stream in the unit’s vertical cavity and release of air from the top of the unit. The devices operate at a standard 110 W power rating, provide coverage of 75 m3 and 60 m3 and are of 90 cm and 45 cm in height (Medixair and Medixair Meos, respectively). The supplier featured as a winner in the 2009 Smart Solutions for HCAI awards. The products were issued with a HPA RRP recommendation 2 in December 2008.
DIFFICIL-S disinfectant solution
DIFFICIL-S is a disinfectant solution product advertised as ‘a supremely powerful broad-spectrum disinfectant cleaner developed to target and kill pathogens within 5 minutes, achieving a 5-log reduction of 99.999%’ (Clinimax Ltd, http://www.clinimax.net/difficil-s-clinical.php, accessed 28 January 2014). It is produced by filling a mixing container with water to a specified line marked on the container. Sachet A is then emptied into the container, followed by sachet B. A plastic rod (supplied) is used to stir the contents for 10 seconds. The mix is then ready to be dispensed to bottles by means of a pump and hose. It can be used with a cloth to clean bedside and other surfaces, or diluted at a ratio of two pumpings per litre to clean floor space. It retains its efficacy for 14 days; labels are supplied for staff to note the mixing date and attach to containers. Any liquid left after 14 days can be disposed of at the sink. The container and bottles need to be replaced after 6 months.
Virusolve+
Virusolve+ is advertised by its suppliers, Amity International (http://www.amityinternational.com/product/virusolve/), as an environmental disinfectant cleaning product whose chemical composition includes advanced dodecylamine-based structures, solvents and a fully non-toxic, biodegradable detergent. It does not contain any hazardous aldehydes or chlorine-generating components. It eliminates viruses, bacteria, fungi, yeasts and moulds, including MRSA bacteraemia, C. difficile, hepatitis B, human immunodeficiency virus agents, E. coli extended-spectrum beta-lactamase and NDM1 (New Delhi metallo-beta-lactamase 1) and non-enveloped-type viruses, such as polio, adenovirus and norovirus, by disrupting the microorganism ribonucleic acid and preventing resistance. It is odourless, colourless and safe to use on any surface.
Chlor-Clean
Chlor-Clean is a cleaning product combining surface cleaning and disinfection in one operation. Most detergents, when used in tandem with chlorine, deactivate some of the chlorine’s disinfecting properties. Therefore, a three-step process is required for cleaning and disinfection. Surfaces would first be cleaned with a detergent. They would then be washed with water to remove any detergent residues, before a chlorine-based solution is applied. Chlor-Clean tablets utilise sodium dichloroisocyanurate to produce a dilution of 1000 ppm chlorine, as per the recommendations of the Department of Health and the HPA of using a hypochlorite cleaning agent of appropriate ppm concentration (2008). 110
JLA OTEX laundry system
The JLA OTEX laundry system is a machine washing system suitable for cleaning and disinfecting hotel-type (sheets, towels, etc.) and domestic equipment (mops, cloths). Its active ingredient is ozone. The system first takes in and converts air to 90% pure oxygen gas. An electrical charge is then applied to the gas, splitting oxygen molecules into atoms. These then recombine to form ozone molecules. A patented interfusor chamber pump system collects the ozone and delivers it without interruption into the wash. Ozone disinfects and destroys bacteria, mould, viruses and yeast by disentangling and fully opening up linen fibres. Enhanced cleaning, quicker drying and fresh fully disinfected laundry is thus delivered. The system comprises a specially built washing machine and an ozone safety and validation monitor that stops operation in cases in which the ozone concentration in the air is measured to be higher than the safety limit (lower than the Health and Safety Executive limit of 0.2 ppm). JLA suggests those parts needing regular maintenance are the oxygen concentrator intake filters and the ozone generator. These need to be checked on a daily basis and cleaned when necessary.
UV light inspection cabinet
The UV light inspection cabinet marketed by DaRo UV Systems Ltd has also been trialled at T7. This is a metal cabinet of approximately 15 cm × 12 cm × 8 cm. An enclosed light source shines UVA light on objects positioned inside the cabinet. Use of a special hand rub gel illuminates hands and helps demonstrate the efficacy of correct, or otherwise, hand washing, scrubbing and disinfecting techniques.
Clinell and PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes
The clinell and PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes are designed for universal cleaning. They are presently licensed for use on medical devices only. Each wipe contains 2% chlorhexidine and 70% ethanol-based alcohol.
Appendix 7 Technology products unit cost price list
The table below lists the technology products according to their list unit cost price in descending order, from the most to the least expensive.
Prices are extracted from the NHS supply chain portal. Certain prices include costs of add-ons and accessories required for use. All prices include value added tax at 20%.
These represent the initial capital outlay or upfront costs that trusts would incur when adopting these technology products. They do not include any maintenance or ongoing costs.
| Product | Cost (£) |
|---|---|
| ASP GLOSAIR 400 aHP system | 17,955.00 |
| Steris BioGenie VHP decontamination systema | 12,500.00 |
| JLA OTEX laundry systema (JLA 40 high-spin machine with OTEX system fitted)b | 8756.40 |
| 3M Clean-Trace NG luminometera (with board and swab rods) | 3027.45 |
| Bioquell VHP RBDSa (one hospital room) | 2100.00 |
| Medixair UV air sterilisation unita | 1800.00 |
| UV light inspection torcha | 1380.00 |
| Hygiena SystemSURE II ATP hygiene monitoring system (with swab rods) | 1078.80 |
| Medixair Meos UV air sterilisation unita | 600.00 |
| DBO commode | 352.58 |
| UV light inspection cabineta (with gel and accessories) | 254.40 |
| DIFFICIL-S disinfectant solution (with mixing vessel and four bottles) | 114.25 |
| Virusolve+ | 102.62 |
| Disposable sterile surgical site gowns (box of 30) | 33.20 |
| Clinell universal sanitising wipes (non-sporicidal, six 200-wipe packs) | 22.19 |
| Clinell sporicidal (red) wipes (pack of 25) | 7.65 |
| Chlor-Clean | 7.50 |
| PDI Sani-Cloth CHG 2% alcoholic chlorhexidine gluconate wipes (pack of 200) | 2.07 |
Glossary
- Academic Health Sciences Centre (AHSC)
- A partnership between one or more universities and health-care providers focusing on research, clinical services, education and training.
- Biomedical Research Centre (BRC)
- It was set up by the National Institute for Health Research in 2007, and based in ‘the most outstanding’ university teaching NHS trusts across the country. It promotes translational biomedical research and innovation in the NHS. Currently there are 11 BRCs (some continued from the period 2007–12, and some newly established since April 2012). See URL: www.nihr.ac.uk/infrastructure/Pages/infrastructure_biomedical_research_centres.aspx (accessed 10 October 2012).
- Biomedical Research Unit (BRU)
- It was set up by the National Institute for Health Research in 2008, and based in the UK’s leading university teaching NHS trusts. It conducts translational clinical research, focusing upon seven high-priority areas. Currently there are 20 BRUs (some continued from the period 2008–12, and some newly established since April 2012). See URL: www.nihr.ac.uk/infrastructure/Pages/infrastructure_biomedical_research_units.aspx (accessed 10 October 2012).
- Care Quality Commission (CQC)
- The independent regulator of all health and social-care services in England.
- Department of Health
- The department of the UK government with responsibility for government policy for England on health, social care and the NHS.
- Director of infection prevention and control (DIPC)
- He or she has authority and is responsible for the reduction of health-care-associated infections in a health-care organisation. This includes reporting directly to the chief executive and the trust board, producing an annual report on the state of health-care-associated infections in the organisation, and local control and implementation of infection prevention and control policies.
- Evidence-based management (EBMgt)
- A term adopted from medical science (particularly evidence-based medicine) to describe the practice of management based on empirical evidence.
- Evidence-based medicine (EBM)
- A scientific approach that aims at applying the best available evidence gained from scientific methods to clinical decision-making.
- Foundation trust (FT)
- Organisational type of some hospitals in NHS England. Foundation trusts have a significant amount of managerial and financial freedom compared with other NHS hospitals.
- Health-care-associated infection (HCAI)
- An infection caused by any infectious agent associated with a person’s medical treatment, or acquired by health-care workers in the course of their duties. A hospital HCAI is one that is neither present nor incubating on admission to hospital.
- Health Protection Agency (HPA)
- A non-departmental public body set up in 2003 to offer specialist support and expert advice to local authorities and the NHS for the protection of the health and well-being of the population of the UK in relation to infectious diseases and environmental hazards. It became part of Public Health England (new executive agency of the Department of Health) on 1 April 2013. See URL: www.hpa.org.uk/AboutTheHPA/ (accessed 10 October 2012).
- Health Protection Agency Rapid Review Panel recommendations (HPA RRP)
- An independent panel, set up by the Department of Health in 2004, that offers ‘prompt’ evaluations of new product technologies to tackle HCAIs.
- Infection prevention and control (IPC)
- In relation to health care, the term is generally used with reference to preventing patients from acquiring those infections most often associated with the provision of health care and preventing the transmission of microorganisms from one patient to another (referred to as cross-infection).
- National Institute for Health and Care Excellence (formerly the National Institute for Health and Clinical Excellence) (NICE)
- It was set up as a Special Health Authority in 1999, and initially named the National Institute for Clinical Excellence, to offer guidance on best practice (i.e. current health technologies and clinical management of specific conditions) to the NHS. On 1 April 2005, it became the National Institute for Health and Clinical Excellence, and on 1 April 2013 NICE became the National Institute for Health and Care Excellence, at which point it changed its status to a non-departmental public body and began offering guidance to ensure quality and value for money. See URL: www.nice.org.uk/aboutnice/about_nice.jsp; www.nao.org.uk/wp-content/uploads/2000/02/9900230.pdf (accessed 10 October 2012).
- National Institute for Health Research (NIHR)
- An organisation developed with the aim of creating a health research system through which the NHS can support outstanding individuals, working in world-class facilities, conducting cutting-edge research focused on the needs of patients and the public.
- National Patient Safety Agency (NPSA)
- An organisation set up as a Special Health Authority to monitor patient safety incidents in the NHS. On 1 June 2012, its key functions were transferred to the NHS Commissioning Board Special Health Authority. See URL: http://npsa.nhs.uk/ (accessed 10 October 2012).
- NHS
- The publicly funded health-care system in England.
- NHS Institute for Innovation and Improvement
- A body whose purpose is to support the transformation of the NHS, through innovation, improvement and the adoption of best practice.
- Private Finance Initiative (PFI)
- A public–private partnership that seeks private capital to fund public sector infrastructure projects and service developments.
List of abbreviations
- aHP
- aerosolised hydrogen peroxide
- AHSC
- Academic Health Sciences Centre
- ATP
- adenosine triphosphate
- BRC
- Biomedical Research Centre
- BRU
- Biomedical Research Unit
- CEO
- chief executive officer
- CQC
- Care Quality Commission
- DBO
- Design Bugs Out
- DIPC
- director of infection prevention and control
- E&F
- estates and facilities
- EBM
- evidence-based medicine
- EBMgt
- evidence-based management
- FT
- foundation trust
- HCAI
- health-care-associated infection
- HPA
- Health Protection Agency
- HPA RRP
- Health Protection Agency Rapid Review Panel
- HS&DR
- Health Services and Delivery Research
- IPC
- infection prevention and control
- NHS PASA
- NHS Purchasing and Supply Agency
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPSA
- National Patient Safety Agency
- PFI
- private finance initiative
- RBDS
- Room Bio-Decontamination Service
- UV
- ultraviolet
- VHP
- vapour hydrogen peroxide