Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 11/1026/04. The contractual start date was in September 2012. The final report began editorial review in July 2013 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Paton et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
The NHS faces severe funding constraints now and in the medium term. The forecast reduction in resources provides an enormous challenge to NHS organisations and staff. The savings required are substantial and are estimated to equate to an increase in productivity of more than 7%. 1 Service providers can improve productivity by carefully identifying initiatives that produce more value from the finite resources available: ‘doing things right and doing the right things’. 2
NHS managers and clinicians need to make full use of available evidence when considering how best to configure and organise care. Service redesign can save money and improve quality, but much depends on how care is co-ordinated and the way services are implemented in a local setting. 3,4 NHS decision-makers need to consider not only the clinical effectiveness and cost-effectiveness of any initiative but also how best to implement it. Consideration also needs to be given to the likely implications for service delivery, budgets and equity of access.
The need to reduce lengths of stay in secondary care hospital settings provides a key potential productivity opportunity. There has been growing interest over recent years in the use of enhanced recovery programmes [also known as enhanced recovery after surgery (ERAS), fast-track, multimodal, rapid or accelerated recovery programmes]. Such programmes seek to design and implement an optimal pathway (covering the preoperative, intraoperative and postoperative periods) that is focused on rapid recovery and discharge for patients. The approach was pioneered in Denmark in the late 1990s for patients undergoing colorectal surgery and is now spreading to other surgical pathways such as musculoskeletal, urology and gynaecology.
Enhanced recovery programmes for patients undergoing elective surgery involve development of enhanced recovery multidisciplinary teams, agreed basic principles, improved efficiency around the surgical pathway, increased patient awareness about the process, and early discharge planning using agreed criteria. 5 Since 2011, the Department of Health’s Enhanced Recovery Partnership Programme (ERPP) has sought to raise the profile and promote the benefits of enhanced recovery for elective surgical care across the NHS.
The underlying aim of enhanced recovery programmes is to ensure that patients are in optimal condition for treatment (to minimise the risk of surgery being postponed or cancelled because of the patient’s condition), receive innovative care during surgery and experience optimal postsurgical rehabilitation. 5 Programmes differ widely but share common elements such as patient education and involvement in preoperative planning processes, preoperative oral carbohydrates, improved anaesthetic and postoperative analgesic techniques to reduce the physical stress of the operation, early oral feeding and mobilisation. 6,7 Enhanced recovery programmes have been delivered in the UK NHS since the early 2000s. Implementation has to date been variable despite the support of the Department of Health and, more recently, the Royal Colleges. It is likely that this variation reflects both the complexity of enhanced recovery programmes themselves and issues around implementing change in fundamental surgical procedures at a time when the NHS is facing severe funding constraints. Differences in programme implementation may also reflect differences between surgical specialties. For example, enhanced recovery has been more widely implemented in colorectal surgery than in the higher volume field of orthopaedics. 8
This study was commissioned in response to a call for research on initiatives to reduce length of stay in acute hospitals; a key feature of enhanced recovery programmes is that they should reduce hospital length of stay compared with usual operative care (referred to in this report as ‘conventional care’). The ERPP has estimated that national implementation of enhanced recovery programmes in colorectal, gynaecology, urology and musculoskeletal surgery could save 140,000–200,000 bed-days per year. 9 Enhanced recovery programmes have a range of other potential benefits. Some of these, such as reduced exposure to risk of hospital-acquired infections, follow directly from reductions in length of hospital stay. Benefits may also be derived from another important feature of enhanced recovery, namely that it ‘empowers the patient to be a partner in their own care and have greater choice through shared and informed decision making’. 5 This means that enhanced recovery can potentially improve patients’ experience of surgery and subsequent recovery, an important consideration as the NHS seeks to be increasingly patient centred. 10 Commissioners of local NHS services have supported implementation of enhanced recovery programmes through a variety of mechanisms, notably the use of Commissioning for Quality and Innovation (CQUIN) payments to support providers in the establishment of enhanced recovery programmes. 9
Set against the benefits of enhanced recovery programmes are concerns that discharging patients too soon after surgery could increase complications and readmissions, thereby worsening patient experience and increasing pressure on primary and/or secondary health-care services. In many cases, maximising the benefits of enhanced recovery will require integrated working between health and social services. Any assessment of the evidence base for enhanced recovery programmes requires consideration of many outcomes and issues beyond simple reductions in length of stay.
Having potential for productivity gains does not guarantee that any change will deliver gains. Initiatives that look effective in theory may not have the hoped for impact when implemented in practice and on a large scale. 11 Before embarking on large-scale adoption of such a major initiative, NHS managers and clinicians need to be fully aware of the strength of the underlying evidence base to support the use of such programmes. Managers and clinicians need to have a clear understanding of how best to implement enhanced recovery programmes and the likely implications for service delivery within finite budgets and considering the need for equity of access.
As part of the National Institute for Health Research Collaborations for Leadership in Applied Health Research and Care for Leeds, York and Bradford, researchers at the Centre for Reviews and Dissemination (CRD) have been developing a rapid response knowledge translation service aimed at NHS commissioners and senior managers in provider trusts (see www.york.ac.uk/inst/crd/projects/knowledge_translation_service.html). The methods we have employed to assist evidence-informed decision-making at the local level in the NHS are ideally suited for rapid evidence syntheses that focus on high-profile initiatives and that are being widely promoted and advocated. Our approach to evidence synthesis12 highlights the quality and the strength of existing systematic reviews and economic evaluations and goes beyond clinical effectiveness and cost-effectiveness to consider applicability, implications relating to service delivery, resource use, implementation and equity.
There are a substantial number of systematic reviews and economic evaluations that examine the clinical effectiveness and cost-effectiveness of enhanced recovery programmes. This study uses this evidence as the basis of a comprehensive rapid evidence synthesis relating to the clinical effectiveness, cost-effectiveness, implementation, delivery and impact of enhanced recovery programmes and contextualise the findings to secondary care hospital settings in the NHS.
Chapter 2 Aims and objectives
The aim of this project was to conduct a rapid synthesis of the evidence on the clinical effectiveness, cost-effectiveness, implementation, delivery and impact of enhanced recovery programmes in secondary care.
The project addressed three main objectives:
-
Clinical effectiveness and cost-effectiveness: Evaluation of the clinical effectiveness and cost-effectiveness of enhanced recovery programmes designed to improve clinical pathways in acute hospital settings in patients undergoing elective surgery, including the impact on the organisation of care, configuration of workforce and resource utilisation in UK NHS settings.
-
Implementation: Identification and critical description of the key factors associated with successful adoption, implementation and sustainability of enhanced recovery programmes in UK settings.
-
Patient experience: Summary of existing knowledge about patient experience of enhanced recovery programmes in UK settings, including issues surrounding equity of access.
Chapter 3 Methods
The rapid synthesis was undertaken systematically following established principles13,14 and adapted as appropriate to ensure relevance to the current context. We followed a protocol drawn up in advance of the evidence synthesis.
The rapid nature and resource constraints of this project mean that we will focus on the best available evidence. Therefore, the primary sources of evidence about clinical effectiveness and cost-effectiveness will be derived from existing systematic reviews and economic evaluations. We have augmented this evidence with recent randomised trials and studies of implementation and patient experience of enhanced recovery programmes in NHS settings.
Searching
The Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA), NHS Economic Evaluation Database (NHS EED) and Health Economic Evaluations Database (HEED) electronic databases were searched from 1990 to March 2013 to identify systematic reviews, health technology assessments and economic evaluations. The International Prospective Register of Systematic Reviews (PROSPERO) database was searched to identify unpublished and ongoing systematic reviews. National Institute for Health Research (NIHR) HTA, NIHR Health Services and Delivery Research programme and the National Institute for Health and Care Excellence guidelines were screened for further studies.
Randomised controlled trials (RCTs) were identified from MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL) and the ClinicalTrials.gov trials register. Searches were conducted from 1990 to February 2013. Reference lists of retrieved articles, reviews and evaluations were scanned to identify additional studies. No language restrictions were applied. See Appendix 1 for full details of all search strategies.
Evidence from case studies of experiences of patients and clinical teams in implementing and delivering enhanced recovery programmes in UK settings were identified from:
-
Department of Health ERPP
-
ERRP Innovation sites
-
ERAS (UK)
-
NHS Evidence
-
NHS Institute for Innovation and Improvement
-
NHS Improvement – Enhanced Recovery
-
NHS Cancer Action Team.
Relevant individuals were identified and contacted for additional evidence. These include regional leads at the NHS Institute for Innovation and Improvement, ERAS (UK) society members and ERPP Innovation site contacts. We identified NHS trusts with enhanced recovery programmes and established contacts with relevant people. We sent a request (by e-mail) to access any information detailing the experience of clinical teams in implementing and delivering enhanced recovery programmes and/or for documentary evidence of patient experience. We telephoned individuals who responded on behalf of a trust and asked a set of standardised questions and captured their responses using a structured proforma (see Appendix 2 ).
Inclusion criteria
Participants
Patients of any age undergoing any type of elective surgery in an acute hospital setting.
Intervention
Evaluations of enhanced recovery programmes (as defined in the original articles) were considered for inclusion. Eligible interventions could include enhanced recovery combined with other techniques to reduce the impact of any type of elective surgery.
Reviews and studies were assessed to identify which ones encompassed the main components of the approach, including preoperative, intraoperative and postoperative elements. (See Box 1 for an example pathway; this list is not exhaustive and protocols that included different combinations of elements were eligible for inclusion.)
-
Pre-admission counselling.
-
Fluid and carbohydrate loading.
-
No prolonged fasting.
-
No/selective bowel preparation.
-
Antibiotic prophylaxis.
-
Thromboprophylaxis.
-
No premedication.
-
Short-acting anaesthetic agents.
-
Mid-thoracic epidural anaesthesia/analgesia.
-
No drains.
-
Avoidance of salt and water overload.
-
Maintenance of normothermia (body warmer/warm intravenous fluids).
-
Mid-thoracic epidural anaesthesia/analgesia.
-
No nasogastric tubes.
-
Prevention of nausea and vomiting.
-
Avoidance of salt and water overload.
-
Early removal of catheter.
-
Early oral nutrition.
-
Non-opioid oral analgesia/NSAIDs.
-
Early mobilisation.
-
Stimulation of gut motility.
-
Audit of compliance and outcomes.
NSAIDs, non-steroidal anti-inflammatory drug.
Reviews and studies that focused on only one element of an enhanced recovery protocol or that compared different techniques (such as different surgical methods) within an enhanced recovery pathway were excluded from the review.
Comparator
Conventional (usual/standard) care without a structured multimodal enhanced recovery patient pathway (as defined in the included studies). Comparators were only relevant to clinical effectiveness and cost-effectiveness evaluations.
Outcomes
All health- and cost-related outcomes were considered for inclusion; eligible studies had to report at least one outcome. We distinguished between clinical outcomes (mobilisation, mortality and morbidity, pain, readmission rates, reintervention rates, length of hospital stay), patient-reported outcomes (patient experience and satisfaction, quality of life) and resource use in secondary care (workforce utilisation and costs, including involvement of an enhanced programme facilitator and resource implications post discharge).
Initially, our inclusion criteria required patient experience to be assessed using validated questionnaires and surveys (such as 2011 National Inpatient Survey, Picker Institute Europe for the Care Quality Commission). Evidence on patient experience was sparse so we amended our criteria to remove the restriction to validated assessment methods.
Study design
Clinical effectiveness
Systematic reviews of primary studies were considered for inclusion. Primary studies identified in these reviews were noted; additional RCTs not already identified in the systematic reviews were also considered for inclusion. Other synthesised evidence, such as reviews of reviews, were eligible for inclusion but were assessed separately.
Cost-effectiveness
Economic evaluations were eligible for inclusion. UK NHS cost analysis studies identified from HEED were also eligible for inclusion.
Implementation and patient experience
Case studies, impact assessments and surveys of patient experience that documented the experience of implementing enhanced recovery in a UK setting were considered for inclusion.
Study selection
We stored the literature search results in a reference management database (EndNote; Thomson Reuters, CA, USA). Two researchers independently screened all titles and abstracts obtained through the searches for potentially relevant articles. Full manuscripts of potentially relevant articles were ordered and two researchers independently assessed the relevance of each article using the criteria stated in Chapter 3, Study design. Disagreements between reviewers were resolved by discussion or by recourse to a third reviewer where necessary.
Data extraction
Clinical effectiveness data and implementation and patient experience data were extracted into review software (EPPI-Reviewer 4.0; Evidence for Policy and Practice Information and Co-ordinating Centre, University of London, London, UK). Data extraction forms were piloted on approximately four studies and adjusted as necessary; data extraction forms are available on request from the authors. Data were extracted by one researcher and checked by another; discrepancies were resolved by consensus or, where necessary, by recourse to a third researcher.
Economic evaluation study characteristics and results were extracted into a Microsoft Word (Microsoft Corporation, Redmond, WA, USA) template. Data were extracted by one researcher and checked by another; discrepancies were resolved by consensus or where necessary by recourse to a third researcher.
Quality assessment
Quality assessment of systematic reviews and economic evaluations was based on the CRD critical appraisal processes for DARE and NHS EED (see www.crd.york.ac.uk/crdweb/HomePage.asp). Identified RCTs were appraised using criteria based on CRD guidance. 13 Cost analysis studies were not formally quality assessed. Quality assessment was performed by one researcher and checked by a second; discrepancies were resolved by consensus or recourse to a third researcher where necessary.
We did not make a formal quality assessment of studies of patient experience because of a lack of rigorous studies and because the studies identified did not correspond with designs (survey or audit) for which we could identify suitable quality assessment methods.
Our planned quality assessment of case studies of implementation was not possible in most cases because of limited reporting. We did not formally quality assess these case studies, but have commented on quality issues where relevant when discussing these studies.
Data synthesis
Clinical effectiveness and cost-effectiveness
The type and range of evidence and differences in settings and interventions precluded meta-analysis. We performed a narrative synthesis by type of surgery, differentiating between evidence from reviews and additional RCTs.
For economic evaluations, the differences in settings and interventions and variable costing methods precluded pooling. These factors also limited the generalisability and usefulness of studies across settings. We extracted data for all evaluations that met our inclusion criteria and performed a narrative synthesis for those studies perceived to be useful in informing this rapid synthesis.
Implementation and patient experience
Case studies from the innovation sites were analysed separately to published implementation studies from other NHS trusts. Data captured via the structured proforma were anonymised and reported separately. One reviewer identified key themes in relation to implementation and sustainability. A second reviewer checked the emerging themes. Any discrepancies were discussed and where consensus could not be reached they were referred to a third reviewer. We have reported these data narratively. We extracted the limited available data on patient experience and discussed these as a separate narrative.
Chapter 4 Effectiveness
Description of studies
Systematic reviews
Initial screening of titles and abstracts identified 24 potentially relevant reviews. We identified one additional review15 which was published after the last literature search and this is discussed separately from the main synthesis. Full-paper screening resulted in the exclusion of two systematic reviews that did not meet inclusion criteria, as they only compared open versus laparoscopic surgery within an ERAS programme. 16,17 Two reviews did not specifically meet our inclusion criteria as they discussed individual elements of ERAS programmes rather than the effects of a complete ERAS programme; these reviews are discussed briefly in the systematic review results summary. 18,19 Four articles were linked to systematic reviews included in this section (some reviews had multiple publications). Any additional details presented in these four articles were extracted alongside the main review details and are not discussed separately (see Figure 1 for full details).
FIGURE 1.
Study flow diagram.
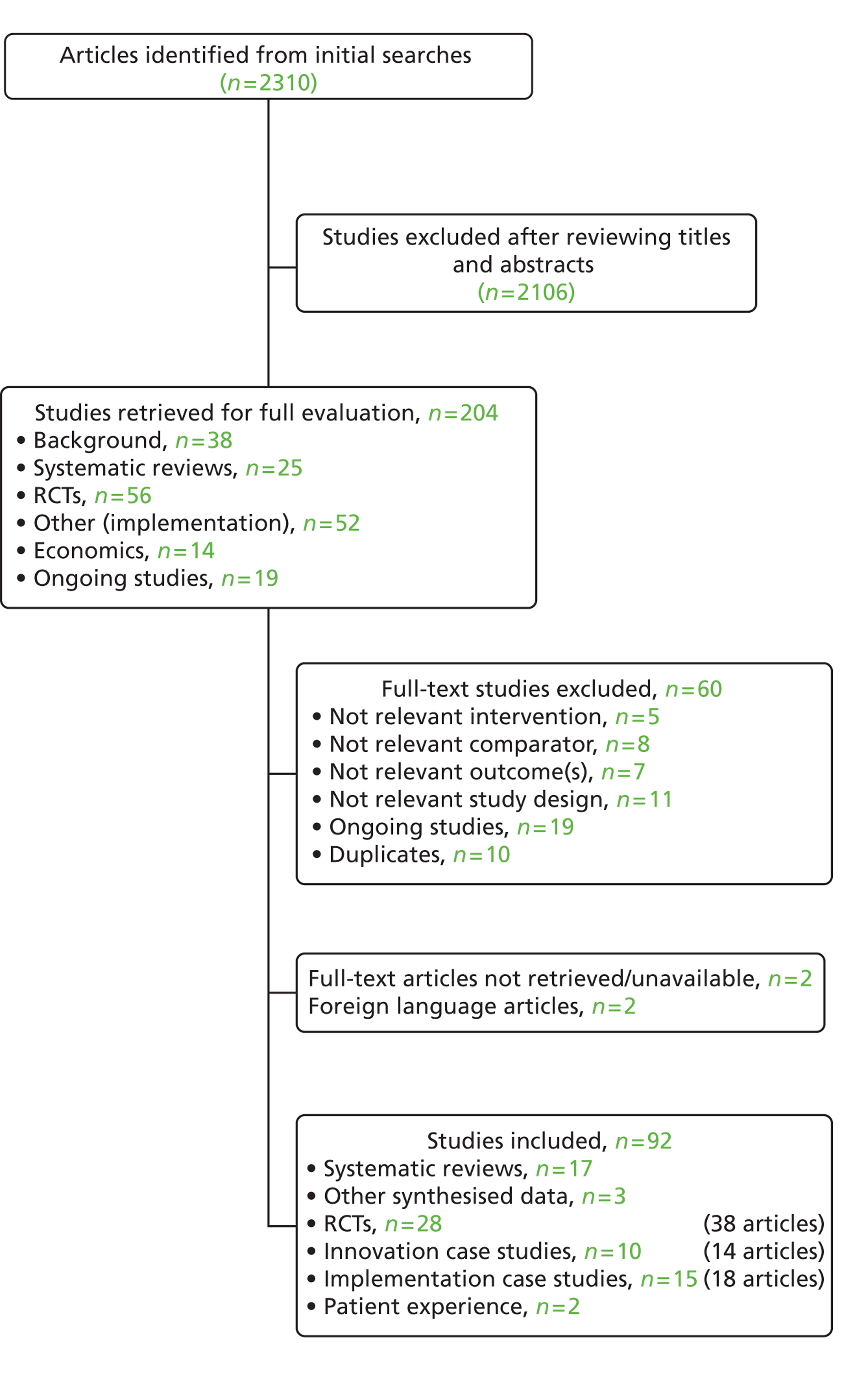
Seventeen systematic reviews that assessed the effects of enhanced recovery programmes were included in this report. 7,20–35 Summary review characteristics are presented in Appendix 3 and full evidence tables are available on request from the authors.
Eleven of the reviews focused on colorectal/colon surgery. 20,21,24,25,27,29–33,35 Of the remaining reviews, one focused on liver surgery,22 one on pancreatic surgery,23 one on liver and pancreatic (hepatopancreatobiliary) surgery26 and one on gynaecological surgery. 34 Sturm and Cameron7 and Lemmens et al. 28 assessed ERAS across various different surgical specialties.
The single Cochrane systematic review34 in gynaecological cancer care did not find any evidence in the form of RCTs. One review assessed compliance with ERAS protocols in colorectal surgery21 and one review assessed the effects of an ERAS protocol on health-related quality of life and satisfaction in patients who underwent colorectal surgery. 27 The remaining reviews assessed the efficacy and safety of ERAS protocols in various surgical specialties. Reviews specified different study inclusion and exclusion criteria. Nine of the 17 reviews stated a minimum number of ERAS elements for studies to be eligible for inclusion, this ranged from four to seven.
The systematic reviews included between 4 and 13 studies conducted in various countries including the UK, Germany, Denmark, Switzerland, Czech Republic and the Netherlands. Six reviews were restricted to RCTs,7,20,24,30,31,35 although Sturm and Cameron7 is a HTA report that also includes the results of a systematic review. 33 One review did not report individual study designs. 21 The remaining reviews included mixed study designs including RCTs, non-randomised studies or observational studies such as case–control studies or case series.
The 11 reviews in colorectal/colon surgery and one of the reviews in various surgical specialties7 presented evidence from different combinations of the same six RCTs ( Table 1). 36–41 The most recent of the systematic reviews35 included these six RCTs plus an additional four-arm RCT42 that compared ERAS with traditional care in both laparoscopy and open surgery. This four-arm trial was the only multicentre trial, the remaining trials were small, single-centre trials. One of the six commonly reported RCTs37 only included postoperative elements and was not considered to represent a comprehensive ERAS protocol as required by our inclusion criteria. Similarly, two reviews included a different RCT43 that was excluded from our synthesis as the intervention did not encompass enough components to represent a comprehensive ERAS protocol (i.e. preoperative or intraoperative and postoperative elements).
| Author | Linked articles | Linked systematic reviews |
|---|---|---|
| Anderson (2003)36 | Adamina (2011);20 Eskicioglu (2009);24 Gouvas (2009);25 Khan (2010);27 Spanjersberg (2011);30 Varadhan (2010);31 Wind (2006);33 Walter (2008);32 Sturm (2009);7 Rawlinson (2011)29 | |
| Delaney (2003)37 | Adamina (2011);20 Eskicioglu (2009);24 Gouvas (2009);25 Khan (2010);27 Spanjersberg (2011);30 Varadhan (2010);31 Wind (2006);33 Walter (2008);32 Sturm (2009);7 Rawlinson (2011);29 Lv (2012)35 | |
| Gatt (2005)38 | Adamina (2011);20 Eskicioglu (2009);24 Gouvas (2009);25 Khan (2010);27 Spanjersberg (2011);30 Varadhan (2010);31 Wind (2006);33 Walter (2008);32 Sturm (2009);7 Rawlinson (2011)29 | |
| Gralla (2007)44 | Sturm (2009)7 | |
| Khoo (2007)39 | Adamina (2011);20 Eskicioglu (2009);24 Gouvas (2009);25 Spanjersberg (2011);30 Varadhan (2010);31 Sturm (2009);7 Rawlinson (2011)29 | |
| Larsen (2008)45 | Sturm (2009)7 | |
| Muehling (2009)46 | Muehling (2008);47 Muehling (2011)48 | Sturm (2009)7 |
| Muehling (2008)49 | Sturm (2009)7 | |
| Muller (2009)40 | Hübner (2010);50 Hübner (2012)51 | Adamina (2011);20 Spanjersberg (2011)30 |
| Petersen (2006)52 | Petersen (2008)53 | Sturm (2009)7 |
| Recart (2005)54 | Sturm (2009)7 | |
| Serclova (2009)41 | Adamina (2011);20 Spanjersberg (2011);30 Varadhan (2010);31 Rawlinson (2011)29 | |
| Vlug (2011)55 | Van Bree (2011);56 Vlug (2011);55 Wind (2006);57 Vlug (2011)42 | Lv (2012)35 |
Where reviews reported the number of included patients, sample sizes ranged between 99 and 5747patients in the ERAS group and between 99 and 1062 in comparator groups. Publication dates of studies included in the systematic reviews ranged from 1998 to 2012. Indications for surgery were rarely reported, but five reviews diagnosed patients as having benign, malignant or inflammatory disease. Where the age of patients was reported, this suggested that all patients were adults within similar age ranges.
The number and combination of ERAS elements varied considerably across reviews and individual studies, and within and across surgical specialties. The number of ERAS elements in individual pathways ranged from 4 to 14. The elements reported to differ most between reviews were avoidance of mechanical bowel preparation,7,23–25 no premedication,23,25,29,30,33 avoidance of nasogastric tubes or abdominal drains,7,22,25 and prevention of hypothermia. 23,25,29,30,33
The elements most frequently reported as part of an ERAS protocol were preoperative patient information20–26,28,29,31,32 and early postoperative oral nutrition and mobilisation. 7,20–26,28–33
Surgical techniques differed across reviews. One review in colorectal surgery included only patients who underwent major elective open surgery. 31 Another review in colorectal surgery included only patients who underwent open surgery but trials that used minimally invasive techniques were eligible. 30 Some reviews did not mention surgical techniques and some reviews included both open and laparoscopic techniques.
Where reviews reported the type of care received by comparator groups, this was defined as traditional care, conventional care or standard care (herein referred to as conventional care). Most reviews did not provide further details on the content of conventional care and it was not possible to determine the extent to which there was overlap between ERAS and conventional care pathways. One review stated that conventional care included up to four ERAS elements. 30 This had implications for the overall findings as some ERAS protocols included only four elements.
The main end points assessed in the reviews were length of hospital stay, readmission rates and morbidity and mortality rates. Some reviews distinguished between primary and total hospital stay. Primary hospital stay represented the number of days in hospital after surgery. Total length of stay was defined as total days spent in hospital including possible readmissions. Other reviews did not distinguish between the two measures and the inconsistency may, to some extent, explain the variability in length of stay across studies.
There was variability in how other outcomes (such as pain, readmission rates and morbidity) were defined and measured, and the reviews may have been measuring slightly different outcomes. This may, to some extent, explain the inconsistencies between reviews in reported event rates. Where reported, most reviews stated a follow-up duration of up to 30 days.
Several reviews reported findings on outcomes such as lung function, immune system function, gut and pulmonary function that were beyond the scope of this review and are not discussed further.
Other reviews
The systematic review identified after the final literature search included 13 RCTs in colorectal surgery. 15 Ten of the RCTs were identified in the reviews discussed above and three were not. 58–60 These three RCTs were identified in our separate search for RCTs and are discussed in the following section.
Two reviews focused on individual ERAS elements and so did not strictly meet eligibility criteria, but they provided some interesting findings which are reported in the systematic review results summary section. 18,19 Arsalani-Zadeh et al. 18 reviewed ERAS elements in patients who underwent breast surgery. Where evidence was scarce, data were extrapolated from non-breast surgery trials. Hoffmann and Kettelhack19 focused on challenges of postsurgical treatment and the role of translational research elements in ERAS, including investigations on stress, and immune and inflammatory responses after surgery.
Randomised controlled trials
Screening of titles and abstracts identified 56 potentially relevant RCTs; full-text screening identified 28 trials (reported in 38 articles due to multiple publications) that met our inclusion criteria.
Of these, 12 RCTs (21 articles due to multiple publications) were included in published systematic reviews discussed in the systematic reviews section above and will not be discussed here further. 36,38–42,44–57,61 Another 12 RCTs (13 articles) that were not included in the systematic reviews are discussed here separately. 58–60,62–71
Two foreign-language articles were identified: one Russian72 and one Chinese. 73 Time and resource constraints did not permit full translation of these articles, but we mention them briefly under ‘other evidence’ (see Chapter 4, Results) along with two RCTs that were available only in abstract form. 74,75
The 12 RCTs not discussed in the systematic review discussions were all single-centre trials above enrolled patients between 2006 and 2012. Clinical practice may have changed during this time. Inclusion/exclusion criteria varied considerably between the individual trials. Most trials selected patients with independent daily lifestyles and excluded patients with factors (such as comorbidities) that might impede a fast recovery. Therefore, patient populations in the trials should be reflective of patients undergoing enhanced recovery in clinical practice.
Seven RCTs (eight articles) were conducted in China,58–60,62,68–71 two in the Republic of Korea63,66 and one each in Spain,64 Romania65 and New Zealand. 67 Health systems in these countries differ from each other and the NHS in England. Seven RCTs (eight articles) were in colorectal surgery,58–60,64–66,69,71 four were in gastrointestinal surgery62,63,68,70 and one was in bariatric surgery. 67 Most of the trials were in patients with cancer; Lemanu et al. 67 was in obese patients.
All RCTs were in adults and there were no significant differences in mean age or sex proportions between ERAS and conventional care groups. Most trials analysed < 100 patients (range 44–597 patients). One trial did not report follow-up duration65 and a second trial reported follow-up between 3 and 44 months. 58 Follow-up in the other trials was up to 30 days post discharge. We considered 30-day postoperative follow-up sufficient to capture the benefits of enhanced recovery programmes and any complications or readmissions.
Details on health professionals involved in the ERAS programmes were scarce and only briefly mentioned as surgeon, anaesthetist and nurse involvement.
It appeared that individual trials were of fairly comprehensive ERAS programmes that included between 10 and 14 ERAS elements (see Appendix 4 ). All trials included preoperative and postoperative elements; individual elements and their combinations differed between trials regardless of surgical specialty. This may reflect changes over time where additional elements were added into the ERAS clinical pathway model. Similarly, different elements may be used dependent on surgical specialty and local preference. Descriptions and the amount of detail provided on each element varied across trials and made it difficult to determine whether or not elements such as preoperative information, pain management and mobilisation were applied consistently across trials. This highlights a lack of standardisation for ERAS programmes.
The most common preoperative elements were information/counselling (nine RCTs),59,62–68,70 no or selective mechanical bowel preparation (nine RCTs)58,59,62–65,68–70 and no prolonged preoperative fasting (10 RCTs). 58,59,62–64,66–70
The most frequently reported intraoperative elements were avoidance of drains (unless necessary), implemented in seven RCTs,59,60,62,63,67–69,71 and use of mid-thoracic epidural anaesthesia/analgesia, implemented in five RCTs. 58–60,69–71
The only postoperative element consistently implemented across all 12 RCTs was early mobilisation. Eleven RCTs implemented early oral nutrition. 58–60,62,64–71 The next most frequently used postoperative elements were avoidance of nasogastric tubes (unless necessary) in nine RCTs58–60,62,63,65,66,68,70,71 and early removal of drains/catheter in 10 RCTs. 58–60,62,64–66,68–71
Discharge criteria differed across the trials. All patients had to be mobile before they would be discharged from hospital; other criteria were reported inconsistently. Some trials stated a need for patients to be taking oral fluids. 62,67 Other trials stated a need for patients to tolerate soft diets. 64 Some patients were required to be analgesia free in order to be discharged66 and other trials discharged patients with analgesics for pain relief. 62,67 Discharge criteria relating to defecation and normothermia also varied across trials.
Traditionally, conventional care includes some form of bowel preparation, prolonged preoperative fasting, use of nasogastric tubes or catheters, later postoperative mobilisation and oral intake. However, approximately half of the RCTs identified in this report described a conventional care pathway that included at least one ERAS element (such as preoperative carbohydrate loading and no preoperative bowel preparation), which could reflect change in practice over time. Liu et al. 68 described a conventional care pathway that included four ERAS elements [use of antibiotic prophylaxis, avoidance of long-acting opioids, maintenance of normothermia and use of epidural mid-thoracic anaesthetic/non-steroidal anti-inflammatory drugs (NSAIDs)]. As highlighted by the systematic reviews discussed above, some ERAS programmes included only four elements. This further highlights the lack of standardisation across ERAS programmes and agreement on what constitutes an ERAS pathway, and will have implications on the overall findings.
These individual RCTs reported various definitions for length of hospital stay consistent with the evidence presented in the systematic reviews. Some RCTs distinguished between primary and total hospital stay, and others did not. Variability in how other outcomes (such as pain, readmissions and morbidity) were measured was also similar to the systematic reviews.
Other evidence
The two articles available in abstract form included only small numbers of patients. One involved 60 patients who underwent elective colorectal surgery in Egypt74 and the other involved 50 patients who underwent laparoscopic radical prostatectomy in Germany. 75 Migheli et al. 75 appeared to focus only on postoperative care rather than a full ERAS pathway with preoperative and/or intraoperative elements. Without the full article it was not clear whether or not this RCT would have met our inclusion criteria for this report and will not be discussed further. From the information provided in the abstract, it seemed that the trial in colorectal surgery would have met inclusion criteria;74 the results are discussed briefly as ‘other evidence’ under RCT results (see Chapter 4, Results).
The articles in foreign languages represented two small RCTs in two very different countries. It was unclear from the information provided in the abstract whether or not the RCT conducted in Russia would have met inclusion criteria. This RCT included 44 patients who underwent caesarean section but it was unclear whether surgery was elective or emergency, and the study appeared to focus on the anaesthesiologist’s role in fast-track surgery. 72 The results from this trial will not be discussed. The trial in China involved 80 patients who underwent surgery for lung cancer. 73 The limited results provided in the abstract are discussed briefly in the RCT results section on clinical effectiveness.
Nineteen ongoing trials were identified from ClinicalTrials.gov.uk in the initial literature search but given the lack of details and results on the trials, and the time and resource constraints, they were not followed up and will not be discussed further. Details are available on request.
Quality assessment
Systematic reviews
Colorectal/colon surgery
Three reviews in colorectal/colon surgery met all quality criteria and we considered these reviews to be at low risk of bias ( Table 2). 30,32,33 Three other reviews in colorectal surgery met all study quality criteria except accounting for study quality in the analysis and we considered them to be at moderate to low risk of bias. 24,25,31 Three other colorectal surgery reviews were limited as they did not account for study quality in the analysis and did not explore statistical heterogeneity. 20,27,35 These reviews were considered to be at moderate to high risk of bias. The other two reviews in colorectal surgery met only two criteria and both reviews were considered to be at high risk of bias. 21,29 It was unclear whether these last two reviews were poorly conducted or just poorly reported.
| Author | Adequate search | Risk of bias assessed | Quality score accounted for in analysis | Study details reported and differences accounted for | Statistical heterogeneity investigated | Gaps in research identified | Conclusions justified |
|---|---|---|---|---|---|---|---|
| Colorectal/colon surgery | |||||||
| Adamina (2011)20 | ✓ | ✓ | UC | ✓ | UC | ✓ | ✓ |
| Ahmed (2012)21 | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Eskicioglu (2009)24 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Gouvas (2009)25 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Khan (2010)27 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Lv (2012)35 | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Rawlinson (2011)29 | ✓ | ✗ | ✗ | ✓ | UC | ✗ | UC |
| Spanjersberg (2011)30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Varadhan (2010)31 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Walter (2009)32 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Wind (2006)33 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gynaecological surgery | |||||||
| Lv (2012)34 | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ |
| Liver/pancreatic surgery | |||||||
| Coolsen (2012)22 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Coolsen (2013)23 Link to76 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hall (2012)26 | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Various surgical specialties | |||||||
| Lemmens (2009)28 | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Sturm (2009)7 | ✓ | ✗ | ✗ | ✓ | UC | ✓ | ✓ |
Liver/pancreatic surgery
The review in pancreatic surgery23 met all quality criteria and we considered the review to be at low risk of bias. The review in liver surgery did not fulfil two criteria (accounting for quality scores in analysis and exploration of statistical heterogeneity) and we considered the review to be at moderate to high risk of bias. 22 The single review in hepatopancreatic surgery met only three criteria and we considered the review to be at high risk of bias. 26
Other surgical specialties
Both systematic reviews that assessed ERAS in various surgical specialties7,28 were limited by a lack of quality assessment in individual studies and no formal assessment of statistical heterogeneity. We considered these reviews to be at moderate to high risk of bias.
Eleven of the included reviews assessed risk of bias using various measurement tools, including the Jadad scale, U.S. Preventative Services Task Force criteria and the Methodological Index for NOn-Randomised Studies (MINORS). The individual studies included in the reviews had their own limitations and implied some risk of bias. Review authors varied in their judgements on risk of bias but, overall, seemed to conclude that individual studies were at moderate or high risk of bias. The main reason for high risk of bias in the RCTs was lack of blinding. Owing to the nature of the interventions, blinding was not feasible in patients and health professionals, but it should have been possible to blind outcome assessors.
Randomised controlled trials
We considered all RCTs to be at high risk of bias, mainly due to lack of blinding, which, as already mentioned, was not feasible for health-care professionals or patients owing to the nature of the intervention ( Table 3 ). One trial stated that participants were blind to treatment, but it was unclear how this was applied. 62 Quality of reporting was generally poor.
| Author | Adequate random allocation | Adequate allocation concealment | Blinding of health-care professional | Blinding of participants | Blinding of outcome assessor | Unexpected imbalances in drop outs between groups | Imbalances accounted/adjusted for | ITT analysis | ITT appropriate and appropriate methods used to account for missing data |
|---|---|---|---|---|---|---|---|---|---|
| Bariatric surgery | |||||||||
| Lemanu (2013)67 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | NA | UC | UC |
| Colorectal/colon surgery | |||||||||
| Garcia-Botello (2011)64 | UC | ✗ | UC | ✗ | UC | ✗ | NA | UC | ✓ |
| Ionescu (2009)65 | ✓ | ✓ | ✗ | ✗ | UC | ✗ | NA | UC | UC |
| Lee (2011)66 | ✓ | ✓ | UC | ✗ | UC | ✗ | NA | UC | UC |
| Ren (2012)69 | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | NA | UC | UC |
| Wang (2011)59 | UC | UC | UC | ✗ | UC | ✗ | NA | ✓ | ✓ |
| Wang (2012)58 | UC | UC | ✗ | ✗ | ✓ | UC | UC | UC | UC |
| Yang (2012)60,71 | ✓ | UC | ✗ | ✗ | UC | ✗ | NA | ✗ | ✗ |
| Gastric surgery | |||||||||
| Chen (2012)62 | UC | UC | ✗ | ✓ | ✓ | ✗ | NA | UC | UC |
| Kim (2012)63 | UC | UC | ✗ | ✗ | ✗ | ✗ | NA | UC | UC |
| Liu (2010)68 | UC | ✗ | ✗ | ✗ | ✗ | ✗ | NA | UC | UC |
| Wang (2010)70 | UC | UC | ✗ | ✗ | UC | ✗ | NA | ✗ | ✗ |
Four RCTs reported adequate random allocation and allocation concealment, but it was unclear whether or not other criteria were met by these RCTs. 66,67,69 Three trials reported blinding of outcome assessors58,62,69 and one reported intention-to-treat (ITT) analysis59 but, again, other criteria were poorly reported for these RCTs. The overall high risk of bias has serious implications on the findings and reliability of these RCTs.
Results
The results are presented separately for systematic reviews and RCTs and organised according to outcomes and surgical specialty.
Systematic reviews
Table 4 indicates which outcomes were assessed in each systematic review. A more detailed summary of findings is presented in Appendix 5 .
| Author | Length of hospital stay | Mobilisation outcomes | Mortality | Morbidity | Pain | Readmission rates | Reintervention rates |
|---|---|---|---|---|---|---|---|
| Colorectal/colon surgery | |||||||
| Adamina (2011)20 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Ahmed (2012)21 | ✓ | NR | NR | NR | NR | ✓ | NR |
| Eskicioglu (2009)24 | ✓ | NR | ✓ | ✓ | NR | ✓ | ✓ |
| Gouvas (2009)25 | ✓ | NR | ✓ | ✓ | ✓ | ✓ | NR |
| Khan (2010)27 | NR | NR | NR | NR | ✓ | NR | NR |
| Lv (2012)35 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Rawlinson (2011)29 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Spanjersberg (2011)30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR |
| Varadhan (2010)31 | ✓ | NR | ✓ | ✓ | ✓ | ✓ | NR |
| Walter (2009)32 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Wind (2006)33 | ✓ | NR | ✓ | ✓ | ✓ | ✓ | NR |
| Gynaecological surgery | |||||||
| Lv (2012)34 | NA | NA | NA | NA | NA | NA | NA |
| Liver/pancreatic surgery | |||||||
| Coolsen (2012)22 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Coolsen (2013)23 Link to76 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Hall (2012)26 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Various surgical specialties | |||||||
| Lemmens (2009)28 | ✓ | NR | ✓ | ✓ | NR | ✓ | NR |
| Sturm (2009)7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR |
Colorectal surgery
Length of stay
Ten reviews of colorectal/colon surgery reported length of stay. Seven of these reviews performed meta-analyses; we considered all reviews to be at low to moderate risk of bias. All meta-analyses showed a significant mean reduction in primary or total length of stay that ranged from 1.56 days [95% confidence interval (CI) 0.50 to 2.61 days]33 to 2.94 days (95% CI 2.19 to 3.69 days). 30 We considered both Wind et al. 33 and Spanjersberg et al. 30 to be at low risk of bias. Wind et al. 33 measured primary hospital stay and Spanjersberg et al. 30 measured total length of stay, which included extra stay for complications and readmissions.
The few reviews that conducted subgroup analyses found that study design (RCTs vs. observational studies or non-randomised studies) and RCT risk of bias (high vs. low) did not significantly alter the findings. 25,30,33 However, these subgroup analyses were based on studies with small sample sizes. Levels of statistical heterogeneity varied considerably across the reviews in colorectal surgery (I 2 = 0% to I 2 = 75%). Reasons for high levels of heterogeneity were not discussed by the review authors and it was unclear why these differences existed given that the systematic reviews included the same RCTs (albeit in different combinations).
Three colorectal surgery reviews presented limited narrative syntheses; we considered two to be at high risk of bias. One review reported median length of stay that ranged between 2 and 11 days but did not provide further details. 21 The other two reviews reported that most studies showed a significantly shorter length of stay in the ERAS group but, again, did not report further details. 24,29
Surgical techniques differed across individual studies. Any effects of the different methods on the findings were not addressed in the reviews. A single RCT included in the most recent review35 compared open or laparoscopic methods within the two different treatment pathways, but comparisons between all four arms were not reported.
Summary
Reviews in colorectal/colon surgery suggested that length of hospital stay was reduced in ERAS patients compared with patients who received conventional care. Some of the marked differences in length of stay in the reviews and individual studies could be explained by use of different definitions for length of stay. Statistical heterogeneity was inconsistent between reviews and often not formally explored but may have reflected differences in ERAS protocols and surgical populations.
Mortality and morbidity rates
Morbidity and mortality rates were reported in nine reviews in colorectal/colon surgery. Deaths were rare and no significant differences between treatment groups were reported (see Appendix 5 ).
Six of the nine reviews that assessed morbidity reported statistically significant reductions in morbidity in ERAS patients. However, when three of these reviews distinguished between major and minor complications, no statistically significant differences were found between treatment groups. One review29 presented a narrative synthesis that indicated that most individual studies found no significant differences in morbidity in colorectal patients, but this review had substantial methodological limitations.
Two other reviews in colorectal/colon surgery (we considered both to be at low risk of bias) reported conflicting findings. One review showed a significant reduction in morbidity in ERAS patients compared with conventional care patients [relative risk (RR) 0.54; 95% CI 0.42 to 0.69; I 2 = 0%; four studies]. 33 The second showed no significant differences between treatment groups. 32 Both reviews performed subgroup analyses by study design; both showed significant differences between treatment groups in non-randomised studies but not in RCTs. The reason for these differences was unclear, but could be due to confounding in the non-randomised studies or too few patients and events in the RCTs.
Summary
There is no evidence to suggest that ERAS programmes compromise morbidity and mortality in patients who undergo colorectal/colon surgery. The heterogeneity in protocols, patient populations and definitions for morbidity make it difficult to determine the reliability and generalisability of these findings.
Readmission rates
Readmission rates were reported in 10 colorectal/colon surgery reviews and all showed no significant differences in readmission rates between the two treatment groups. Reported readmission rates ranged from 0% to 24% in ERAS patients and from 0% to 20% in conventional care patients.
Two reviews in colorectal surgery performed subgroup analyses to assess the influence of study design (RCTs vs. non-randomised studies) on the findings. 22,33 Both reviews found that there were fewer readmissions in patients receiving conventional care in non-randomised studies compared with the randomised studies.
Subgroup analysis was performed in another colorectal review30 to assess the effect of including ERAS protocols with a limited number of elements. Findings were not significantly altered and continued to favour ERAS (RR 0.57; 95% CI 0.38 to 0.85; I 2 = 0%) but the analysis was based only on two small RCTs.
Summary
The evidence suggests that ERAS protocols do not increase readmission rates in patients who undergo colorectal/colon surgery, but it was unclear how readmissions were defined and measured in the reviews. One review found that the shortest length of stay (2 days) was associated with the highest rate of readmission (22%). 21 However, the review was at high risk of bias and the association was based on one non-randomised controlled study that did not state how readmissions were measured. This association was not explored in other reviews and may need to be addressed in future research.
Pain
Pain was discussed in five reviews25,27,30,31,33 in colorectal surgery. All at moderate to low risk of bias. Different measures of pain were used across reviews and individual studies and this made comparisons difficult. One review33 reported that it was not possible to analyse the data due to heterogeneity, the remaining four reviews reported inconsistent findings across individual included studies.
Summary
Limited evidence, variation in pain measurement tools and inconsistent findings preclude definitive conclusions.
Mobilisation and other clinical outcomes
A single review in colorectal patients reported that mobilisation outcomes were better in ERAS patients than conventional care patients on the day of surgery and postoperative day 1. 30 This review was at low risk of bias but the evidence was based on two small RCTs, mobilisation outcomes were not clearly defined and no quantitative data were reported. Early mobilisation is one of the core elements in an ERAS protocol5,9,77,78 and it was unclear why mobilisation outcome results were rarely reported in the systematic reviews.
One review at low risk of bias reported reintervention rates in patients undergoing colorectal surgery. 24 The review reported no significant differences in reintervention rates between treatment groups, but the evidence was based on one small RCT.
Summary
Limited evidence precludes robust conclusions on the effects of ERAS protocols on mobilisation outcomes and reintervention in patients undergoing colorectal/colon surgery.
Quality of life
Three reviews used various assessment tools to measure quality of life in patients who underwent colorectal/colon surgery. 25,27,33 All three reviews were considered to be at low to moderate risk of bias. Follow-up was up to 30 days post operation.
Results reported by individual studies were sometimes conflicting, but overall the reviews stated that there were no significant differences in quality-of-life measures in patients who underwent colorectal/colon surgery compared with conventional care. 25,27,33
Patient experience and satisfaction
One review (considered to be at moderate risk of bias) assessed patient experience and satisfaction. 27 The review found no significant differences between treatment groups at 30 days in patients who underwent colorectal surgery. The instrument used to assess patient satisfaction was not validated and the evidence was based on one non-randomised study.
Summary
The evidence suggests equivocal findings between ERAS and conventional care for quality of life and patient experience/satisfaction. There were some inconsistencies in findings between individual studies for both outcomes and methods used to assess these outcomes varied across individual studies and reviews. The evidence was based on few studies, outcomes were self-reported and some studies assessed outcomes using non-validated measures. The limitations of the evidence mean definitive conclusions cannot be made.
Resource use
None of the reviews in colorectal/colon surgery assessed workforce utilisation or costs.
Liver/pancreatic surgery
Length of stay
We considered the systematic review in pancreatic surgery23 to be at low risk of bias. This review provided results from comparative and non-comparative studies. Four out of the five comparative studies reported significant differences in length of stay in favour of ERAS. However, length of stay varied across individual studies for both ERAS and conventional care groups, ranging between 6.7 and 13.5 days in ERAS patients and between 8.0 and 16.4 days in conventional care patients. Non-comparative studies reported a length of stay of 10 days. It was unclear whether the number reported were reported as means or medians. 23
The review in liver surgery22 reported mixed findings across comparative studies; two trials reported a significant difference in length of stay between ERAS and conventional care patients (p < 0.001) and one trial reported no significant differences between treatment groups. Length of stay ranged from 5 to 7 days in ERAS patients, and between 7 and 11 days in conventional care patients. Non-comparative studies reflected the length of stay reported by the comparative studies in ERAS patients (range 4–7 days). 22 We considered this review to be at moderate risk of bias.
The review in hepatopancreatic surgery (high risk of bias) showed similar length of stay in liver patients undergoing an enhanced recovery programme as reported by Coolsen et al. 22 (range 4–7 days). Length of stay for pancreatic patients undergoing an enhanced recovery programme compared with controls or historical controls ranged from 10 to 13 days. 26
Summary
Findings were mixed across comparative studies and length of stay differed considerably. Non-comparative studies tended to reflect the length of stay in comparative studies, but these studies were at high risk of bias. The inconsistency across individual studies and limited quality of some of the individual studies makes it difficult to determine the effects of ERAS protocols in patients undergoing liver and pancreatic surgery.
Mortality and morbidity rates
Deaths were rare and no significant differences between treatment groups were found in the two reviews that included comparative studies22,23 (see Appendix 5 ). The review in hepatopancreatobiliary surgery (high risk of bias) reported mortality rates that ranged from 0.0% to 4.9%. 26
The three comparative studies in the review on liver surgery22 reported no significant differences in morbidity between treatment groups. Morbidity was defined in terms of complication rates but complications in the studies were not always reported using validated methods and this made it difficult to make meaningful comparisons across the studies. These were small studies and, hence, were at risk of bias.
By contrast, the review in pancreatic surgery23 indicated significant differences in morbidity in favour of ERAS [risk difference (RD) 8.3%; 95% CI 2.1% to 14.5%; four comparative studies]. There was no evidence of statistical heterogeneity (I 2 = 0%). However, visual inspection of the individual results showed that the largest study influenced the findings: this was the only study to show a significant difference. The non-comparative studies included in these reviews tended to report lower rates of morbidity than the comparative studies.
The review that assessed morbidity in hepatopancreatobiliary surgery did not provide comparative findings but reported rates in pancreatic patients who ranged from 38.6% to 47.6%. Morbidity rates in liver patients ranged between 1.0% and 46.4%. 26 These high morbidity rates are expected for these types of surgery and reflect clinical practice.
Summary
The evidence is inconsistent and insufficient to enable conclusions to be made on morbidity.
Readmission rates
Comparative studies included in the two reviews in liver surgery or pancreatic surgery showed no significant differences in readmission rates between ERAS and conventional care. 22,23 Again, the non-comparative studies tended to report lower rates of readmission than did comparative studies. The review that assessed morbidity in hepatopancreatobiliary surgery did not provide comparative findings. 26
Summary
The evidence is insufficient to enable conclusions to be drawn about readmission rates for liver and pancreatic surgery.
Other outcomes
None of the reviews reported findings on pain, mobilisation outcomes, other clinical outcomes or patient-reported outcomes.
Resource use
The review in pancreatic surgery assessed total hospital costs pre- and post-ERAS pathway. 23 Costs pre pathway ranged from US$26,393 to US$240,242. Costs post pathway ranged from US$22,806 to US$126,566. Three studies reported statistically significant reductions post implementation and one reported no significant differences. The highest pre- and post-ERAS figures were from a single study that reported charges rather than costs; costs reported in other studies were much lower.
Various surgical specialties
The following results relate to findings reported by two reviews in various surgical specialties. We considered both reviews to be at moderate to high risk of bias. 7,28 The reviews covered different specialties and we present them here separately.
Lemmens et al.28
This review was poorly reported and presented mixed findings that may reflect the different populations and surgical specialties included in the individual studies.
Length of stay
Eleven of the 13 included studies reported a significant decrease in length of hospital stay in ERAS patients, the remaining two studies reported no significant differences between treatment groups.
Mortality and morbidity rates
Where deaths were reported, events were rare and no significant differences between treatment groups were found (see Appendix 5 ).
The review reported that most of the individual studies (10 of 13) showed no significant differences in mobilisation rates between treatment groups.
Readmission rates
Most of the included studies that reported readmission rates (10 of 11 studies) found no significant differences between treatment groups.
This review did not report findings on any other outcomes.
Summary
The limited evidence precludes robust conclusions on the effects of ERAS protocols across these various surgical specialties.
Sturm and Cameron7
Length of stay
Mixed findings were reported in individual studies included in the review. This may reflect the different populations and surgical specialties and the different definitions for length of stay. Some studies did not clearly state a definition for length of stay, some results reflected only postsurgical stay and some studies included readmissions. Length of stay for ERAS patients ranged from 2 to 11 days and from 4 to 11 days for conventional care patients.
Mortality and morbidity rates
Deaths were rare (two ERAS patients and five conventional care patients) and no significant differences between treatment groups were reported, regardless of surgical specialty (see Appendix 5 ).
Sturm and Cameron7 reported that five of the seven individual studies that reported statistical data showed no significant differences in morbidity rates between treatment groups.
Readmission rates
Eight trials reported readmission rates. These ranged from 0.0% to 9.7% in ERAS patients and from 0% to 20% in comparator patients. Only one trial reported a significant difference between treatment groups, favouring ERAS.
Pain
Sturm and Cameron7 assessed pain but different measures were used in the individual studies and findings were inconsistent: two trials reported significant reductions in pain in ERAS patients and four trials reported no significant differences between treatment groups.
Mobilisation and other clinical outcomes
Four of the 11 trials assessed mobilisation as an outcome. Two trials were reported to have significantly shorter median time from surgery to unaided mobilisation to toilet in ERAS patients (although the table included in the systematic review suggested that in one trial ERAS patients took longer to mobilise). Two trials reported significantly increased time out of bed in ERAS patients. The review did not assess reintervention rates. 7
Quality of life
The evidence was based on two small RCTs in different specialties that used different quality-of-life measures. One trial reported significantly greater quality of life in ERAS patients who underwent hip and knee replacement. The other trial reported no significant differences between patients who underwent rectal surgery.
Patient experience and satisfaction
Sturm and Cameron7 reported no significant differences between treatment groups in one RCT in patients who underwent rectal surgery. A RCT in patients who underwent nephrectomy reported greater satisfaction in the ERAS groups for pain management (p < 0.05) but not for quality of recovery, which was not clearly defined in the review.
Resource use
This review did not report findings on workforce utilisation or costs.
Summary
Some studies showed benefits in terms of reduced length of stay, morbidity and readmission rates for ERAS patients. Findings were sometimes inconsistent or sparse for other outcomes. There was wide variability between included studies in terms of ERAS protocols, outcome definitions and surgical populations. The included studies had generally low numbers of patients which suggests that the studies may have been insufficiently powered to detect significant differences between treatment groups, particularly for those outcomes that were sparsely reported.
Other reviews
The systematic review in colorectal surgery identified after our last literature search showed similar findings to systematic reviews discussed above. 15 Mean length of primary hospital stay was statistically significantly reduced in ERAS patients [mean difference (MD) −2.44; 95% CI −3.06 to −1.83; 11 RCTs] but with significant statistical heterogeneity (I 2 = 88%). There was no evidence to suggest increased rates of readmissions, complications and mortality. Some of the individual RCT results for primary length of stay did not appear to be consistent with results reported in other systematic reviews and the original primary studies. Therefore, reported reductions may be overstated. 15
Arsalani-Zadeh et al. 18 assessed individual ERAS elements in patients undergoing breast surgery for cancer. The authors recommended 12 core elements. Evidence for some elements was scarce and there was heterogeneity between the included studies. The authors did not attempt to identify the most important elements of an ERAS protocol or assess the effectiveness of a full ERAS protocol in breast surgery. The review, although interesting, is of limited value in the assessment of a full ERAS pathway.
The second review by Hoffman and Kettelhack19 highlighted difficulties in implementing ERAS into clinical practice that included poor compliance with ERAS protocols and constant evolution of treatment strategies. Hoffmann and Kettelhack19 concluded that the lack of standardisation of ERAS protocols meant that the level of evidence remained low.
Randomised controlled trials
A summary of the clinical outcomes assessed in the RCTs is presented according to surgical specialty and outcome ( Table 5 ). Full evidence tables are available from the authors on request.
| Author | Length of hospital stay | Mobilisation outcomes | Mortality | Morbidity | Pain | Readmission rates | Reintervention rates |
|---|---|---|---|---|---|---|---|
| Bariatric surgery | |||||||
| Lemanu (2013)67 | ✓ | NR | NR | ✓ | NR | ✓ | NR |
| Colorectal/colon surgery | |||||||
| Garcia-Botello (2011)64 | ✓ | NR | NR | ✓ | ✓ | ✓ | NR |
| Ionescu (2009)65 | ✓ | ✓ | NR | ✓ | NR | ✓ | ✓ |
| Lee (2011)66 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ren (2012)69 | ✓ | NR | ✓ | ✓ | NR | NR | NR |
| Wang (2011)59 | ✓ | ✓ | ✓ | ✓ | NR | ✓ | NR |
| Wang (2012)58 | ✓ | ✓ | ✓ | ✓ | NR | NR | NR |
| Yang (2012)60,71 | ✓ | NR | NR | ✓ | NR | ✓ | NR |
| Gastric surgery | |||||||
| Chen (2012)62 | ✓ | NR | NR | ✓ | NR | NR | NR |
| Kim (2012)63 | ✓ | NR | NR | ✓ | ✓ | ✓ | ✓ |
| Liu (2010)68 | ✓ | NR | NR | ✓ | NR | ✓ | NR |
| Wang (2010)70 | ✓ | NR | ✓ | ✓ | ✓ | ✓ | NR |
Colorectal surgery (seven randomised controlled trials)58–60,64–66,69,71
Length of hospital stay
Six RCTs of colorectal surgery reported significant reductions in length of hospital stay in patients following an ERAS programme. Lee et al. 66 reported no significant difference in length of stay between ERAS patients (9 days) and conventional care patients (10 days). The reason for this finding may be explained partly by similarities in care elements received by ERAS and conventional care patients. ERAS patients received a rehabilitation programme with elements incorporating early mobilisation and diet, but these were the only differences between the two treatment pathways. 66 Despite the similar hospital length of stay, overall time to recovery (which included patients tolerating diet and being analgesia free) was significantly shorter in the ERAS group.
The other trials reported a mean length of stay in ERAS patients who ranged from 4.1564 to 6.43 days. 65 Mean length of stay in conventional care patients ranged from 6.669 to 11.7 days. 60,71
Mobilisation outcomes
Only four trials reported on patient mobilisation as an outcome and these defined mobilisation differently so it was unclear whether or not the end points were the same. Results were reported in various formats (such as means, medians, percentage of patients), which made it difficult to combine the data. Individual trial authors reported that baseline characteristics were similar between treatment groups. No statistical tests for heterogeneity were reported.
One trial defined mobilisation as ‘time to complete mobilisation’. 65 The mean time to complete mobilisation in ERAS patients was 19.6 hours, compared with 37.1 hours for those in conventional care. Another trial described mobilisation as ‘safe ambulation’. 66 Patients in the ERAS group took a median time of 18 hours to ambulate safely and those in conventional care took 21 hours. Wang et al. 59 reported that a higher proportion of ERAS patients were mobilised on the day of surgery (35% compared with 0% in the conventional care group) up to postoperative day 2, when 85% of ERAS patients were mobilised, compared with 59% of conventional care patients. Wang et al. 58 reported a median time to ambulation of 12 hours in ERAS patients, compared with 18 hours in conventional care patients.
Despite the variations in outcome definitions, all four trials reported significantly quicker times to mobilisation in patients who received ERAS compared with those treated conventionally.
Mortality and morbidity rates
Consistent with the evidence from the systematic reviews, deaths were rare and no significant differences were reported between treatment groups. A total of six deaths were reported in the four trials that assessed mortality in colorectal patients.
All six trials reported morbidity but defined this differently, and trials may have been assessing slightly different outcomes. This was reflected by the inconsistent findings. Two trials reported 50% fewer events in ERAS patients. 59,66 Wang et al. 58 reported a significant reduction in overall complications in ERAS compared with conventional care patients (5.0% vs. 21.1%). A third trial60,71 reported a significant reduction in total infectious complications in ERAS patients but no significant differences in non-infectious complications. The other three trials reported no significant differences between treatment groups.
Pain
Two RCTs assessed pain. 64,66 It was unclear whether or not the same visual analogue scales were used to assess this outcome. Neither trial reported a significant difference in levels of pain.
Readmissions
Five trials reported readmission rates. 59,60,64–66,71 Rates were low (0–5% in ERAS patients and 0–9% in conventional care patients) and no significant differences between treatment groups were reported.
Reintervention rates
Two trials reported reintervention rates. Only one incident was reported in the conventional care group and none in the ERAS group. 65,66
Quality of life
One RCT provided evidence on quality of life and indicated no significant differences between treatment groups at 1 and 4 weeks post discharge. 66
Patient experience and satisfaction
One RCT provided limited evidence on patient experience. 60,71 As would be expected given restrictions on preoperative fluids and later postoperative oral intake in conventional care patients, these patients reported significantly greater thirst and hunger than patients following an ERAS programme. Twenty-three out of 30 conventional care patients (76.7%) reported thirst, compared with 2 out of 32 (6.3%) ERAS patients. Twenty out of 30 (66.7%) conventional care patients reported being hungry, compared with 5 out of 32 (15.6%) ERAS patients.
Resource use
Two trials provided cost information but none related to an NHS setting. 64,69 Garcia-Botello et al. 64 reporteda significant reduction in mean hospital costs in patients following an ERAS programme in Spain(p < 0.001). A trial conducted in China69 showed significant reductions in total cost of procedures in favour of ERAS (p < 0.001). The benefit resulted from reduced postoperative expenses rather than any differences in preoperative and surgical expenses.
Summary
The evidence clearly suggests reduced length of hospital stay in colorectal surgery patients following an ERAS programme. Definitions for length of stay varied considerably across trials, which could reflect different care programmes and different health-care systems in different countries. Lee et al. 66 stated that in the Republic of Korea most patients do not ask to be discharged from hospital even when they are sufficiently recovered as hospital stay is inexpensive because of the medical insurance system. Safe reduction of postoperative stay is not a major focus in the Republic of Korea.
Reductions in length of stay did not appear to be at the detriment of other clinical and patient-reported outcomes, but evidence on outcomes other than morbidity was sparse and different measurement tools were used to assess differently defined outcomes. The evidence was too limited to allow robust conclusions to be drawn on these other outcomes.
Bariatric surgery (one randomised controlled trial)67
Length of hospital stay
Length of stay for ERAS patients was 1 day and for patients receiving conventional care was 2 days. Median lengths of hospital stay (including subsequent readmissions) in this instance were shorter than length of stay in other surgical specialties. We can speculate that these differences reflect the differences in surgical procedures and also the underlying conditions. Patients who undergo bariatric surgery for obesity and patients who undergo surgery for cancer will have different needs and comorbidities. We do not know which antiobesity procedures were undertaken in the review, so it is not possible to conclude whether or not the short hospital stay is generalisable to all bariatric procedures for obesity.
Morbidity
Thirty-day postoperative complication rates were higher in patients following an ERAS programme (25%) than in those receiving conventional care (21%) but the difference was not statistically significant. The proportion of patients who reported complications was much higher than in patients who underwent other surgical procedures; the reasons for this are unclear.
Readmission rates
There were no significant differences in readmission rates within 30 days of surgery (ERAS was 20% and conventional care was 21%) and no difference in median length of readmission stay between the treatment groups. The proportion of patients readmitted after bariatric surgery was much higher than after with other surgical procedures (0–5% for ERAS and 0–9% in conventional care).
Patient experience and satisfaction
There was no significant difference in postoperative fatigue between treatment groups at any time point (measured using a published scale at 1, 7 and 14 days after surgery). The usefulness of this single outcome measure in this patient population is questionable.
Resource use
There was a slight reduction in mean cost per patient in patients following an ERAS programme (€9391) compared with conventional care (€9853) but the difference was not significant.
Other outcomes were not assessed in this surgical population.
Summary
The evidence was based on one small RCT (78 patients) and robust conclusions on the effect of ERAS programmes in bariatric surgery cannot be drawn.
Gastric surgery (four randomised controlled trials)62,63,68,70
Length of hospital stay
Liu et al. ,68 Wang et al. 70 and Kim et al. 63 reported significant reductions in primary length of hospital stay in patients following an ERAS programme (all comparisons p < 0.001). Mean length of stay in ERAS patients was 5.36 days63 and 6.20 days;68 median length of stay was 6.00 days. 70 By comparison, patients who received conventional care reported a mean length of stay of 7.95 days63 and 9.80 days,68 median length of stay was 8.00 days. 70
Chen Hu et al. 62 was a four-arm trial that compared ERAS compared with conventional care in laparoscopic and open surgery. Compared with open surgery plus conventional care (median length of stay 8.75 days) the other three treatment arms had significantly shorter postoperative hospital stay (p < 0.05).
Mobilisation outcomes
None of the trials reported this outcome.
Mortality and morbidity rates
The small trial by Wang et al. 70 reported no deaths in either treatment group. The other trials did not report mortality rates.
All four trials reported morbidity rates but this outcome was defined differently and different complications were measured. Liu et al. 68 and Wang et al. 70 showed conflicting findings. Liu et al. 68 indicated reduced morbidity in ERAS patients (12%) compared with conventional care patients (20%). Wang et al. 70 showed the opposite with fewer events in the conventional care group (14.9%) compared with the ERAS group (20.0%). The higher rate in ERAS patients in Wang et al. 70 seemed to be the result of greater nausea and vomiting in these patients. Despite these conflicting findings, neither trial reported statistically significant differences between treatment groups. The four-armed trial62 and Kim et al. 63 also reported no significant differences between treatment groups.
Pain
Kim et al. 63 and Wang et al. 70 assessed pain using different scales. Kim et al. 63 reported that additional pain control was more frequently needed in conventional care patients but ultimately they reported no differences in pain scores between treatment groups. Wang et al. 70 indicated significantly less pain in ERAS patients up to 5 days after surgery (p < 0.05).
Readmissions
Liu et al. ,68 Kim et al. 63 and Wang et al. 70 reported a total of four hospital readmissions: three in ERAS patients and one in a conventional care patient. Individual RCTs reported no significant differences between treatment groups.
Reintervention rates
None of the trials reported on reintervention.
Quality of life
Wang et al. 70 assessed quality of life and indicated significantly greater quality of life in ERAS patients (p < 0.05). It was unclear whether or not quality of life was measured using a valid tool and was assessed only in the short term at hospital discharge. Kim et al. 63 showed that only 4 out of 20 quality-of-life items were significantly better in ERAS patients.
Patient experience and satisfaction
None of the trials reported this outcome.
Resource use
In the four-armed trial,62 mean costs of different surgical procedures (laparoscopy-assisted radical distal gastrectomy vs. open distal gastrectomy) were lower when following an ERAS programme than with conventional care. 62 Wang et al. 70 also reported significantly reduced costs in patients following an ERAS pathway (p < 0.001). Kim et al. 63 reported no significant differences in treatment costs in a Korean hospital (p = 0.21). It was unclear whether or not costs for readmissions and reinterventions were included in the overall costs. The findings have limited relevance or generalisability to NHS settings.
Summary
Consistent with findings in other surgical specialties, length of hospital stay was reduced in patients who underwent gastric surgery within an ERAS programme. However, the evidence was based on four small RCTs and findings on other outcomes were sparse and sometimes conflicting.
Other evidence
The two articles available in abstract form included only small numbers of patients. A RCT in colorectal surgery74 showed findings consistent with other evidence presented in colorectal surgery in terms of reductions in hospital stay without compromising patient safety.
A trial involving 80 Chinese patients who underwent lung cancer surgery suggested that hospital length of stay, costs and postoperative pain were significantly reduced in the ERAS patient group compared with the conventional care group. 73 This trial is important as evidence in this surgical population is lacking but the trial was small and full details were not obtained so it was not possible to make firm conclusions about the efficacy of ERAS programmes in lung surgery.
Summary of clinical effectiveness
Systematic reviews
Overall, the reviews suggest that length of hospital stay is reduced in ERAS patients compared with patients receiving conventional care. The evidence was based mainly on colorectal surgery, and the applicability of findings to other surgical specialties remains unclear. Most of the reviews, particularly those in areas other than colorectal surgery, were at moderate to high risk of bias and this reduces the reliability of the findings. Individual studies included in the reviews had their own methodological limitations and 11 of the 12 RCTs were conducted in single centres.
The extent to which ERAS and conventional care pathway elements overlapped was unclear from the evidence presented. The review that performed subgroup analysis found that the number of elements did not impact on the findings and still favoured ERAS, but this was based on only two small RCTs. 30 Evidence on the clinical effectiveness of ERAS elements and their combined clinical effectiveness was lacking and requires further investigation.
There were marked differences in length of stay across reviews and individual studies regardless of specialty. These differences may reflect differences in ERAS protocols and health-care systems and/or outcome definitions. Some reviews included readmissions in the number of days in hospital and others measured only days in hospital immediately post surgery. This questions the magnitude of effect of the ERAS protocols on length of stay, which may be overstated in some reviews. The relevance of length of stay has also been questioned and the importance of an outcome to patients has been posed as the most important perspective to identify benefit. 30
Use of different surgical procedures (open surgery vs. laparoscopy) across the two care pathways was assessed in only one trial. 35 The length of stay between the two techniques requires further consideration within an enhanced recovery pathway.
The evidence suggests that ERAS programmes do not compromise patient morbidity, mortality and readmission rates but outcome definitions varied across reviews and individual studies. Slightly different outcomes may have been measured and different measurement tools may have been used. Such differences make it difficult to determine the reliability and generalisability of the findings.
Equivocal findings were reported for quality of life and patient experience/satisfaction, but the evidence was based on few studies and various methods were used to measure these outcomes. The findings on patient satisfaction reported here differ from the evidence presented by case studies in Chapter 7 . The case studies reported high levels of patient satisfaction with ERAS but, again, the evidence was limited and based on only two case studies.
The limited evidence precludes conclusions on the effects of ERAS protocols on pain, mobilisation outcomes and reintervention. No details were provided on resources and it remains unclear what impact enhanced recovery programmes may have on staff and equipment requirements. Data on costs/charges were sparse and none of the evidence was in a NHS setting. These data were insufficient to inform the cost-effectiveness analysis (discussed further in Chapter 5 ).
Data were lacking on reasons for delayed discharge. Where data were reported, these were limited but included concerns about complications or extensive surgery, low patient confidence and transport-related or other social problems. Further exploration of underlying causes of delayed discharge is warranted and should include factors such as the impact of different NHS trust working hours (some trusts practice evening or weekend working), non-compliance with elements (patients and staff), resource limitations and any difficulties in arranging postdischarge care.
Only one review assessed compliance with ERAS elements. 21 This review highlighted considerable variation across studies. Ahmed et al. 21 noted that, in general, compliance fell during the postoperative period in most of the studies (from around 100% to around 20%). Use of epidural analgesia had the highest levels of compliance across all studies (range 67–100%). Use of transverse incisions had the lowest levels of compliance (around 25%). Reasons for differences in compliance and waning of compliance were not measured in the reviews. None of the reviews assessed patient compliance, including adherence to preoperative advice to ensure fitness for surgery.
Some reviews recommended implementation of ERAS programmes as the standard approach for perioperative care in colorectal surgery but the review by Spanjersberg et al. 30 argued that the low quality of the studies and lack of sufficient data on other outcome parameters did not justify this; this review had a low risk of bias. Evidence presented in the systematic reviews in our evidence synthesis does not clarify the situation. Although there is consistent evidence of benefit for length of stay, the lack of evidence on patient outcomes and resource use and costs precludes firm conclusions on the overall clinical effectiveness and cost-effectiveness of enhanced recovery programmes.
Randomised controlled trials
There is a large body of evidence available on the impact of enhanced recovery pathways in acute settings, most of which focuses on colorectal surgery. Findings from individual RCTs were consistent with findings from systematic reviews, and indicate that ERAS programmes are safe, feasible and efficient; shortening length of hospital stay without compromising patient safety. As to be expected, benefit varied across different surgical specialties and patient populations, although evidence in surgical specialties other than colorectal surgery was lacking.
Despite the observed benefits, the reliability of the evidence from the RCTs was limited by small sample sizes and high risk of bias. In addition, all 12 RCTs were single-centre trials. Substantial differences between trials precluded meta-analyses. Lack of information on factors that may contribute to the success or failure of ERAS programmes prevented further inference on the factors necessary to optimise ERAS pathways and improve clinical outcomes and patient experience. Other limitations, as discussed for systematic reviews in the section above, were also applicable to the individual RCTs. The following list reflects some of the factors that may warrant further research, and implications for research will be discussed in greater detail in Chapter 8 :
-
Exploration into the effect that varying levels of surgical volume and surgical experience might have on the success of an enhanced recovery pathway and subsequent outcomes.
-
Exploration into the effect that different discharge protocols might have on length of stay and other associated outcomes.
-
Issues with implementation and compliance were rarely discussed. Two RCTs reported high compliance (over 80%) with ERAS elements included in the enhanced recovery programmes, with the exception of patient-controlled analgesia. One trial reported non-compliance by the department of anaesthesiology, which resulted in epidural analgesia not being implemented. Adherence/compliance to elements by staff and patients therefore requires further investigation.
Another limitation of the evidence was the relevance of the findings to the NHS as most studies were conducted in countries where health-care systems differ to NHS England. One article highlighted that postoperative rehabilitation is slower in China compared with Western countries and clinicians in China have little information about ERAS. 68 The single trial in Romania reported that all patients were admitted to a high-dependency unit. 65 The reason for this was unclear and it should be noted that this is not usual practice for established ERAS programmes in the UK. These differences may have increased the length of hospital stay.
Evidence from systematic reviews and RCTs is consistent and confirms the benefit of enhanced recovery programmes in terms of length of stay, but the evidence from the RCTs does not add sufficient evidence to allow firm conclusions on the overall clinical effectiveness of these programmes.
Chapter 5 Cost-effectiveness
Description of studies
Ten economic evaluations published between 2005 and 2011 met our inclusion criteria and were considered eligible for data extraction (see Appendix 6 ). 79–88
Three of the evaluations were undertaken in the UK,79,82,87 two in the USA,80,86 two in Denmark83,85 and one each in New Zealand,81 China84 and Japan. 88 All 10 evaluations were based on single clinical studies; no modelling studies were identified. Three evaluations were in colorectal surgery,80–82 two in hip/knee arthroplasty,79,85 two in cardiovascular surgery,87,88 one in liver surgery,84 one in lumbar83 and one in ileoanal pouch surgery. 86
Quality assessment
All of the evaluations included adult populations and all evaluated costs and outcomes over short time horizons (most were less than 6 months and the longest was 2 years). The clinical studies on which the evaluations were based varied from RCTs to retrospective chart reviews. Few evaluations presented sufficient data to enable full assessment of the validity of the clinical data. Time horizons varied from 30 days to 2 years and in some instances were unclear. 81 Most did not present a summary result and instead presented results of costs and outcomes separately. Four of the evaluations considered health-related quality of life as an outcome. 79,82,83,85 The other six studies focused on length of stay, complications and readmissions as outcomes. The level of reporting varied greatly: only three studies presented full details of resource use,81,83,85 the other studies mostly presented total costs.
Results
The generalisability of the results of these evaluations is limited by a lack of transparency in reporting, different settings, different populations and varying methodology used in analyses. All of the evaluations suggest that programmes that achieve a reduction in length of stay are cost saving. The results also suggest that the programmes are not to the detriment of patients in terms of complication rates, readmission and health-related quality of life. The disparity in standard protocols and what has been evaluated across the settings makes it unfeasible to select a cost-effective programme or programme components based on the existing economic evidence.
Chapter 6 Implementation
Innovation site case studies
In 2011 the ERPP established 14 UK innovation sites to act as pathfinders for implementation; some sites were self-selecting and others were encouraged to join. The aim was to raise the profile, promote the benefits and inform the uptake of enhanced recovery for elective surgical care across the NHS. These sites had little or no experience in enhanced recovery pathways.
Case studies and practical examples are publicly available at http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/healthcare/electivecare/enhancedrecovery/index.htm. There was no up-to-date data available to determine how well these sites are currently performing, or indeed whether or not the enhanced recovery programmes have been sustained.
Ten innovation sites provided adequate data for inclusion in this section. Summary data are presented in Appendices 7–13 . Four innovation sites did not provide relevant outcome data and were excluded from the report. It was not possible to assess the quality of the included case studies.
Three innovation sites89–92 implemented an ERAS programme across all four surgical specialties (gynaecology, musculoskeletal, urology and colorectal surgery). One innovation site89,93,94 implemented an ERAS programme across three specialties (colorectal, musculoskeletal and gynaecology). These innovation sites may therefore provide multiple documentation (see Appendix 7 ). Seven sites reported implementation in only one surgical specialty (colorectal or musculoskeletal).
Eight innovation sites introduced ERAS in colorectal surgery. 89–93,95–99 Six innovation sites had implemented a programme in musculoskeletal surgery. 89–92,94,100,101 Four trusts introduced ERAS in gynaecology. 89–91 Three innovation sites had implemented ERAS in urology. 89–91
The most frequently reported reasons for starting an ERAS programme were to reduce patient length of stay or improve patient experience/quality of care. Two innovation sites91,92,96 reported that ERAS programmes had previously been introduced but did not provide details on why they were not sustained (see Appendix 8 ).
Consistent with evidence from systematic reviews and RCTs, descriptions of health professionals involved in the ERAS programmes were limited (see Appendix 9 ). All 10 innovation sites that provided adequate data reported involvement of surgical specialty leads, seven referred to nurses and five mentioned anaesthetists. None of the innovation sites made any reference to primary care or social services involvement. Only one site mentioned a patient representative. 91,92 Three innovation sites mentioned physiotherapist/occupational therapist involvement. 93,94,96,101
Individual innovation sites stated that they introduced between 1 and 14 ERAS elements as part of an ERAS programme (see Appendix 10 ). It was unclear whether or not sites that reported only a small number of changes were already practising other ERAS elements as part of conventional care; if other elements were not being practised, we would question whether or not these programmes were representative of a complete ERAS pathway.
All innovation sites except Colchester Hospital University NHS Foundation Trust95 implemented pre-admission information/counselling and early postoperative mobilisation as elements of the ERAS pathway. The next most frequently used element was fluid and carbohydrate loading implemented by six trusts. 89–93,96,98,99
None of the innovation sites made any reference to five ERAS elements: use of preoperative antibiotic prophylaxis; use of thromboprophylaxis; no preoperative medication; postoperative prevention of nausea and vomiting; and stimulation of gut motility.
Consistent with published data, the innovation site case studies highlighted inconsistency in avoiding selective bowel preparation and use of drains and nasogastric tubes (see Appendix 10 ).
Some innovation sites mentioned changes in the form of structured education programmes for staff (one site),100 same day admission (seven innovation sites),89–92,94–96,101 use of oesophageal Doppler (threeinnovation sites),90–92,94 laparoscopy/minimal invasive techniques (eight innovation sites),89–92,94–99 Kehlet-style postoperative protocol (one site),91,92 and introduction of follow-up procedures (six innovation sites). 89–92,94,96,97 The last of these (follow-up) is important as this can alleviate patient worries/concerns over the telephone, avoiding patients returning to their GPs and potentially being readmitted to hospital. Only Yeovil Foundation Trust98,99 mentioned auditing of compliance and outcomes as part of the pathway.
These variations in practices illustrate the lack of standardisation across ERAS programmes and highlight variability across NHS settings.
Critical success factors
Based on self-reported experiences, the innovation sites identified factors they believed acted as barriers or facilitators to implementing and ERAS programme (see Appendices 11 and 12 ). These personal opinions provide only anecdotal evidence, but they are based on real-world experience.
Barriers
Two innovation sites89,91–94 stated that resistance to change from patients could act as a barrier to the success of an ERAS programme. We consider that patient engagement is necessary for successful ERAS programmes as lack of patient compliance could contribute to delayed recovery and ultimately longer length of hospital stay. The innovation sites also stated that continual education of staff was a factor for success of an ERAS pathway but also a barrier to change.
Several other challenges were identified from the case studies: lack of funding or support from management,89–92 staff turnover; problems arising from poor documentation; and the time required to complete documentation. Other practical issues included securing space for preoperative education and arranging team meetings. Unnecessary bureaucracy was mentioned as a barrier to change by twoinnovation sites,89,91,92 but no further details were provided.
Facilitators
Five innovation sites highlighted a need for a dedicated ERAS project lead/nurse to co-ordinate and sustain multidisciplinary working and continuity of the pathway. 89,91,94,95,98 Four innovation sites mentioned a need for a multidisciplinary team approach. 89–95 This may be particularly important as one of the challenges to implementing an ERAS programme was resistance by some health professionals to change their longstanding traditional views.
Other elements highlighted as critical for success of an ERAS pathway included a need for preoperative patient information (four sites) and continual education (this could refer to both patients and staff; four sites). These were identified as a success factors across six innovation sites. 89,91,92,96–99,101 A need for patient engagement/representation was identified as important in the success of ERAS programmes.
One innovation site (Salford Royal Hospital)96 mentioned that it did not offer a 7-day service for enhanced recovery because staff resources were insufficient. Whether or not trusts provide a 7-day or evening service may influence length of hospital stay. Patients operated on towards the end of the week may have to wait until after the weekend to be discharged if they need to be seen by any health-care professionals or social services.
These themes are consistent with findings from the implementation case studies discussed in Implementation case studies.
Supporting evidence
All 10 innovation sites reported reduced length of stay but few provided supporting evidence and it was unclear whether or not benefits were sustained over a long period of time (see Appendix 13 ). Evidence on other outcomes was limited. Positive experiences were reported by patients, but evidence was generally based on quotes from a small number of patients and reflected the scant published evidence on patient experience highlighted by the evidence presented on systematic reviews and RCTs. Financial gains were suggested by one innovation site94 but, again, no supporting evidence was provided. The scant evidence on cost-effectiveness of ERAS programmes is reflected in the lack of published data.
Innovation site case studies suggest that information on implementation and relevant outcomes is collected but not in a structured format and not in a way that is being shared with others and used to improve or refine the pathway.
Implementation case studies
We included 15 case studies of implementation of ERAS in UK NHS settings ( Table 6 ). Only three of these were reported in published peer-reviewed journal articles;8,102,103 the rest were on websites or in non-peer-reviewed journals, or were supplied to us by the authors. One site (Queen Elizabeth the Queen Mother Hospital) published two separate case studies that covered the periods 2006–7104,105 and 2010–11. 106 Single case studies from Salisbury NHS Foundation Trust,107,108 Sandwell and West Birmingham Hospitals NHS Trust109,110 and Queen Elizabeth the Queen Mother Hospital104,105 were reported from multiple sources.
| Reference | Setting | Surgical specialty | Facilitators of implementation | Barriers to implementation |
|---|---|---|---|---|
| Colorectal | ||||
| Sandwell (2007)109,110 | Teaching hospital (Sandwell and West Birmingham Hospitals NHS Trust) | Colorectal surgery | ER now seen as standard care; required a change of culture among staff working in the area | Barriers included reconfiguration of wards (further details not reported); some patients not being eligible for ER because of comorbidities; and discharge being delayed for social reasons |
| Payne (2008)111 | District general hospital (Queen Mary’s Sidcup NHS Trust) | Colorectal resection | Senior management support. All stakeholders involved from the planning stage. ERAS link nurses. ERAS multidisciplinary training days; communications. Continued audit and evaluation | Delays in discharge due to stoma issues/abnormal tests |
| Parker (2008)112 | NHS hospital (Darent Valley) | Colorectal surgery | NR | NR |
| Elwood (2008)113 | London Teaching Hospital (Guys & St Thomas) | Major elective colorectal surgery | Repeated teaching sessions with all new nurses and doctors with regular feedback to all involved. A sense of ownership from the whole team. Audit of individual care from the first day. ER nurse to co-ordinate the programme | Difficulties in getting nurses and doctors to complete integrated care pathway documentation Time restrictions on the ward |
| Hodder (2012)114 | NHS hospital (Walsall Manor) | Colorectal surgery | NR | NR |
| Smart (2012)103 | Yeovil District Hospital | Laparoscopic colorectal surgery | NR | NR |
| Gynaecological | ||||
| East (2007)116 | UK gynaecological cancer centres and units | Gynaecological cancer surgery | NR | Obtaining consent before admission remains a problem; joint assessment with social services a problem in some centres; lack of nursing home beds and delays with support packages delay discharge. Other clinical teams (delay in referral to gynaecological oncology, delay in requests for input in gynaecological oncology cases); inpatient services (capacity issues); outpatient clinic capacity to review early discharges |
| Schmid (2008)104,105 | Gynaecological Oncology Centre (Queen Elizabeth the Queen Mother Hospital, East Kent; 2006–07) | Major gynaecological surgery | NR | Need for admission the day before surgery and delay in the arrangement of social services Restrictions on space and funding |
| Letton (2011)106 | Gynaecological Oncology Centre (Queen Elizabeth the Queen Mother Hospital, East Kent; 2010–11) | Gynaecological oncology (total abdominal hysterectomy, bilateral salpingo-oophorectomy) | NR | Difficult to implement the pathway as intensive care unit and surgical consultants have their own documentation |
| Bowen-Rampling (2012)117 | Gynaeoncology cancer centre (Musgrove Park Hospital, Taunton) | Gynaecological oncology surgery (laparotomy or laparoscopy) | NR | NR |
| Orthopaedic | ||||
| Schneider (2009)102 | Orthopaedic surgery department (Edinburgh Royal Infirmary) | Orthopaedics: total hip and knee replacement | NR | Reasons for delayed discharge were mainly medical, social or organisational |
| Wainwright (2010)8 | NHS district general hospital (The Royal Bournemouth) | Orthopaedics (hip and knee replacements) | Combined approach of a clinician and manager working together to implement the pathway was crucial due to the large volumes of patients to be treated and the complex organisational issues associated with introducing change and new ways of working | The organisational effort required in large orthopaedic units to implement ER programmes is significant |
| Hibberd (2012)107,108 | NHS Foundation Trust (Salisbury) | Orthopaedics; hip replacements | Tailoring of Joint School and preoperative assessment; revised anaesthetic and analgesic protocols. Therapy outreach including telephone contact, home visit, 2-week postoperation clinic | Geography; primary care and social services; ≥ 5 days; standardisation; trauma demands; resources (no additional funding available). No further details reported |
| McGeehan (2013)115 | Teaching Hospital (Sheffield) | Orthopaedics; elective hip and knee replacements | NR | Challenging when the ward moved hospital sites to protect beds for elective surgery. Concern from anaesthetic staff over patients being in pain but issues being addressed |
| Other | ||||
| Zamoyski (2011)118 | NHS Bariatric Service (North Tyneside General Hospital) | Primary bariatric surgery; Roux-en-Y gastric bypass, gastric bands, gastric balloons, sleeve gastrectomy | Optimal preoperative assessment and getting early planning correct. Specialist bariatric nurses and pharmacists play vital roles in ER programmes | NR |
Case studies focused on colorectal surgery (six studies),103,109–114 orthopaedic surgery (four studies),8,102,107,108,115 gynaecological surgery (four studies)104–106,116,117 and bariatric surgery (one study). 118 Studies were performed in various secondary and tertiary care settings between 2002 and 2012. Numbers of patients ranged from 17 to ≥ 2000. In most cases the stated objective was to reduce length of stay and/or improve quality of care. Two of the formally published studies sought to develop criteria for selecting patients for ERAS102 or for identifying patients at risk of delayed discharge in the setting of an ERAS programme. 103
Reductions in length of hospital stay compared with baseline or historical controls were specifically reported for colorectal surgery in four case studies,109,111–113 gynaecological surgery in two case studies104,117 and for orthopaedic surgery in three case studies. 8,107,115 Improvements in other outcomes such as patient satisfaction were reported in some case studies (full data extraction tables are available from the authors on request).
Critical success factors
Facilitators
Six case studies reported something about factors associated with successful implementation of ERAS in their setting (see Table 6 ). 8,107,109,111,113,118 The factors mentioned most often were a need to involve all stakeholders from an early stage to gain support for changes in practice, the importance of multidisciplinary training and teaching, optimising planning and preoperative assessment and the role of specialist ERAS nurses in co-ordinating care and sustaining change. The sample of case studies was too small to enable conclusions about similarities and differences across surgical specialties or types of hospital.
Perhaps the most robust case study described implementation of an ERAS pathway for orthopaedic surgery in a district general hospital based on results for 2391 consecutive patients treated since August 2007. 8 The authors identified co-operation of a clinician and a manager as crucial to successful implementation of the pathway because of the large number of patients and complex organisational issues associated with introducing the pathway. Other aspects seen as important were a team approach, standardised procedures, a highly organised logistical framework and a commitment to change from all involved. The authors reported that following implementation of the ERAS pathway 80% of patients who underwent hip or knee replacements were discharged home on or before postoperative day 4. 8
Barriers
Ten case studies reported barriers to implementation of ERAS. 8,102,104,106,107,109,111,113,115,116 Four of these mentioned that issues with social services (organising assessment and support) could delay discharge of patients from an ERAS programme. 104,108,109,116 Other general barriers were issues with or concerns of other clinical teams (e.g. from anaesthetic staff over patients being in pain); specific documentation issues such as reluctance of staff to complete ERAS-related documentation or differences between clinical teams; time and space restrictions; and difficulties implementing ERAS without additional funding. Some case studies referred to barriers specific to the local setting such as problems associated with relocating a ward between hospital sites. 115
Other evidence
In addition to the case studies listed above, a further 34 UK NHS trusts were identified as having established ERAS programmes (mostly in colorectal surgery). We identified and contacted key individuals and 11 responded on behalf of their trust. None of them were able to provide documentary evidence suitable for inclusion in the review but all provided responses to a standardised set of questions that we captured using a structured proforma. These responses were personal opinions gathered from a convenience sample, but they offer insight into real-world implementation of ERAS programmes.
Critical success factors
Most respondents highlighted the importance of a multidisciplinary team approach to include all key professional groups from the outset and sustain engagement over the longer term. Clinical and managerial leadership, regular team meetings and data sharing to monitor performance were considered key to sustainability.
Respondents highlighted the role of an enhanced recovery facilitator not only to assist with getting the programme started but also for ongoing co-ordination and collection of metrics. However, one respondent considered that not having a facilitator encouraged a clear expectation that enhanced recovery would become routine.
A need for baseline data was highlighted. One respondent indicated that length of stay was already declining in their trusts and it would be harder to prove an impact without baseline data. One respondent mentioned that a lack of protected beds for elective procedures can act as a barrier.
Summary
Most case studies were uncontrolled or compared patient outcomes and/or experience before-and-after introduction of an ERAS programme. Unlike the RCTs discussed above that were conducted in other countries with differing health-care systems, all case studies were conducted in the UK and are therefore highly relevant. However, they represent experiences of a sample of centres that chose to report their data and their outcomes may not be representative of those achieved elsewhere in the NHS. Their main value as evidence is the light they shed on NHS clinicians’ perceptions of requirements for successful implementation and barriers to implementation of ERAS. We attempted to assess their quality as implementation studies but the required information was almost never reported; for example, implementation fidelity was only measured in two of the case studies. 103,114
Chapter 7 Patient experience
Description of studies
We included two published studies of patient experience of ERAS. 119,120 Each study involved patients who underwent colorectal surgery at an English NHS foundation trust (Yeovil District Hospital119 and St Mark’s Hospital, Harrow120). Some of the case studies on implementation of ERAS (see Chapter 6, Implementation of case studies) also included information about patient experience but this is not considered here because of small sample sizes, lack of analytical rigour and lack of information about how patients were recruited and data collected.
Study characteristics are summarised in Table 7 .
| Reference | Survey details | Administration | ER intervention | Patient details |
|---|---|---|---|---|
| Blazeby (2010)119 | ||||
| Type of health-care system | Type of instrument used | Number of elements | Indication | |
| NHS foundation trust | Semistructured home interviews | Approximately seven | Colorectal cancer | |
| Setting | Copy of instrument available? | Brief summary of ER elements | Mean age (years) | |
| Yeovil District Hospital | Semistructured interview schedule included as appendix in published paper | Pre-admission counselling and information; fluid and carbohydrate loading; thoracic epidural anaesthesia; avoidance of drains; early oral nutrition; early mobilisation; discharge follow-up | 73.8 (SD 8.2) | |
| Surgical specialty/type of operation | Date of survey/audit | Description of ER team | Sex | |
| Colorectal (laparoscopy vs. open surgery) | Between 3 and 6 weeks after discharge (original RCT January 2003 to March 2004) | Two dedicated research nurses (no other details reported) | Male: 10 (50%) | |
| Country | Synthesis methods | Ethnicity | ||
| UK | Verbatim transcription of audiotaped interviews using grounded theory | NR | ||
| How was the sample obtained? | ||||
| Patients participating in a single-centre RCT were invited to participate | ||||
| Number of patients | ||||
| 20 | ||||
| Taylor (2011)120 | ||||
| Type of health-care system | Type of instrument used | Number of elements | Indication | |
| NHS foundation trust | Patient discharge preparations satisfaction questionnaire and semistructured focus groups | NR | NR | |
| Setting | Copy of instrument available? | Brief summary of ER elements | Mean age (years) | |
| Tertiary colorectal unit | No | NR | NR | |
| Surgical specialty/type of operation | Date of survey/audit | Description of ER team | Sex | |
| Colorectal resection | May and June 2009 | NR | NR | |
| Country | Synthesis methods | Ethnicity | ||
| UK | Transcription of audiotaped discussions and analysis using a process described by Holloway and Wheeler (1996)121 | NR | ||
| How was the sample obtained? | ||||
| Patients who entered an ER programme between September 2008 and February 2009, and lived within 1 hour’s drive of the hospital, were invited to participate | ||||
| Number of patients | ||||
| 50 patients invited; 10 patients and three relatives provided feedback |
Information about participant recruitment and response rates was reported in each study. In the Yeovil study,119 20 out of 27 patients invited to participate did so, but it was unclear how these 27 were selected from the 66 patients in the original randomised trial. The St Mark’s study identified 50 potentially eligible participants; 12 confirmed their willingness to attend and 10 (together with three relatives) attended a focus group. 120 Both studies recruited a relatively low proportion of potentially eligible participants; this issue was discussed in the publication of the St Mark’s study120 but less so in the report of the Yeovil study. 119
Both studies recruited patients who underwent colorectal surgery but it was unclear how similar or different they were in several important respects due to the limited reporting. We could not compare the two studies as the St Mark’s study did not report participant details such as age and sex and did not describe the ERAS programme. The St Mark’s patients were treated more recently (September 2008 to February 2009) than those in the Yeovil study (January 2003 to March 2004).
Each study used qualitative research methods to analyse audiotaped material: the Yeovil study gathered data through individual semistructured interviews and the St Mark’s study involved three focus groups. Participants in the Yeovil study were interviewed between 3 and 6 weeks after discharge. The period between treatment and assessment of patient experience was longer in the St Mark’s study (treatment between September 2008 and February 2009; focus groups in May and June 2009).
Results
Patients in both studies expressed a high level of satisfaction with the ERAS ( Table 8 ). In the Yeovil study, participants particularly valued being discharged from hospital after a few days and being able to recover at home. 119 Nine patients stated that they would recommend the programme to others. Overall satisfaction with the ERAS programme was identified as a major theme in the St Mark’s study, particularly the level of preoperative preparation and multidisciplinary team support after surgery. 120
| Reference | Patient outcome(s) | Additional comments |
|---|---|---|
| Blazeby (2010)119 | ||
| Response rate | Limitations of the study include the small single-centre sample which means findings may not be generalisable or representative. Interviews were conducted by a member of the ER team. Nocomparison group | |
| 27 of 66 patients invited to participate; 20 of 27 participated | ||
| Patient satisfaction | ||
| Many participants spoke highly of the ER programme and several reported being pleased with the unexpected good recovery and discharge from hospital after just a few days; mainly due to being at home where they could eat and drink when it suited them, relax and avoid infection. Nine patients would recommend the programme to others or repeat the experience themselves. Conversely, four patients felt they would not want to repeat the experience or recommend this approach to others; mainly because of adverse outcomes and difficulties in obtaining expert advice after discharge. Patients with complications felt vulnerable or nervous at home | ||
| Patient quality of life | ||
| See patient satisfaction. No formal measurement of quality of life | ||
| Other patient outcome(s) | ||
| Some participants felt that discharge from hospital was too soon and they were being hurried out of hospital, and this put undue pressure on their carers | ||
| Taylor (2011)120 | ||
| Response rate | In response to the focus groups, the authors stated that greater postdischarge nursing support is now offered to patients in the fortnight following discharge by the ER programme facilitator. A trial of different pain control methods was commenced to try to address adequacy of pain control throughout the postoperative period. Serving of food was changed to ensure hotter food and greater choice in portion sizes and nutritional drinks | |
| 12 patients confirmed attendance, two did not turn up | ||
| Patient satisfaction | ||
| Main theme was overall satisfaction with the ER programme Preoperative: preoperative preparation was rated very highly and enabled them to form realistic expectations and encouraged more personal responsibility for recovery and compliance with programme Postoperative: level of multidisciplinary team support improved participants’ confidence in the rehabilitation process, helping them achieve independence more quickly. Overall, patients were able to meet daily goals of the programme and they considered the elements for earlier eating, mobilisation and discharge to be feasible, acceptable and preferable |
||
| Patient quality of life | ||
| No formal evaluation of quality of life. Swift resumption of usual role functions brought great relief to patients and reduced convalescent demands on their families | ||
| Other patient outcome(s) | ||
| Three other main themes considered issues for service improvement: food; pain control; and follow-up after discharge (need for more accessible source of specialist support to offer prompt reassurance and pertinent information at a time of vulnerability for many patients) |
Some negative aspects of experience of ERAS and areas for improvement were identified in both studies. Four patients in the Yeovil study felt that they would not want to repeat the experience or recommend the programme to others. 119 Negative aspects included difficulties in obtaining expert advice after discharge, patients with complications felt vulnerable at home and some felt that they had been discharged too soon and this put pressure on their carers. 119 Participants in the St Mark’s study identified a need for accessible specialist support after discharge. 120 Other areas for improvement were pain control and food choice.
Summary
The two studies included in this section provided limited evidence that those patients who were willing to provide feedback took a positive view of their experience of treatment in an ERAS programme. The studies suggested that patients were willing to comment on their experience in a way that can help health-care providers to identify areas for improvement. Patient feedback was reported to have led to changes in the ERAS programme at St Mark’s Hospital. 120
The patient experience evidence had many limitations. Among these were small samples of uncertain representativeness, being based on experience at single centres, being limited to colorectal surgery and collecting data weeks or months after treatment. The St Mark’s study120 provided limited details of participants and the ERAS programme. Both studies were uncontrolled, so it was unclear how the patient experience of an ERAS programme might compare with that of conventional care with a longer hospital stay. The studies used staff associated with the ERAS programmes to facilitate focus groups and conduct interviews. This may have made participants less willing to express criticism of the programme; this was acknowledged as a potential source of bias in the Yeovil study. 119
Neither study used a validated survey instrument, such as those developed by the Picker Institute Europe (www.pickereurope.org), to explore patient experience, and neither study included a formal evaluation of patient quality of life. The limited evidence base meant that we could not address questions about patient experience in the way we specified in the review protocol. Further research should aim to develop a more robust understanding of patients’ experience of ERAS in a variety of different types of surgery using both quantitative and qualitative methods and to compare experiences of ERAS and conventional care.
Chapter 8 Discussion
Service redesign can save money and improve quality but much depends on how care is co-ordinated and how services are implemented in the local setting. There has been growing interest in the NHS over recent years in the use of enhanced recovery programmes to deliver productivity gains through reduced length of stay, fewer postoperative complications, reduced readmissions and improved patient outcomes. This rapid synthesis represents to our knowledge the most comprehensive overview of the evidence relating to the clinical effectiveness, cost-effectiveness, implementation, delivery and impact of enhanced recovery programmes in secondary care.
Seventeen systematic reviews of varying quality were included in this rapid review. Twelve additional RCTs were included; all were considered at high risk of bias. Most of the evidence focused on colorectal surgery and, with the exception of one RCT, were conducted in single settings. Twenty-nine case studies undertaken in NHS settings were identified and provide descriptions of factors critical to the success of an enhanced recovery programme. Ten relevant economic evaluations were identified evaluating costs and outcomes over short time horizons.
Despite the plethora of studies robust evidence was sparse. Evidence for colorectal surgery suggests that enhanced recovery programmes can reduce hospital stays by 0.5–3.5 days compared with conventional care. The mean length of stay in enhanced recovery ranged from 4.15 to 6.43 days. For conventional care, length of stay ranged from 6.6 to 11.7 days. Other surgical specialties showed greater variation in reported reductions in length of stay but this greater uncertainty reflects the more limited evidence base for these specialties.
We found consistent evidence that enhanced recovery programmes can provide some benefit to adults undergoing elective surgery. In particular, there is a suggestion that optimising conditions before, during and after elective surgery can reduce length of patient hospital stay without increasing readmission rates. This finding is consistent with systematic reviews that evaluate other structured multidisciplinary care pathways in hospital settings where clinical care is predictable. 122–125
Our overall results suggest that enhanced recovery programmes do not compromise patient safety but this evidence was based on variably defined outcomes. Differences in morbidity rates between enhanced recovery and conventionally treated patients were observed but these were not consistent. Reviews and trials provided limited data on levels of patient pain. Data on reintervention rates and patient-reported outcomes were limited but did not suggest significant differences between enhanced recovery and conventional care patients.
Early mobilisation is one of the core elements in an enhanced recovery protocol, yet results were rarely reported in the systematic reviews. What each intervention actually entailed is obscured by very limited reporting, but it is clear that there is variation in the type and duration of mobilisation elements delivered. Owing to the limitations in reporting, informed judgements on both the cost and the optimal offering for mobilisation are difficult to make.
Patient experience was reported very rarely and what few data were provided were anecdotal in nature. The ERPP that set up the innovation sites proposed use of the 2010 National Inpatient Survey to measure patient experience. Innovation sites may have followed this instruction but no evidence of use is presented in the case study reports. Data from national initiatives such as the Friends and Family Test are too limited to provide meaningful measurement of patient experience. Patient experience is an important outcome and more needs to be done to help facilitate its measurement. Using questions from the National Inpatient Survey [or another validated survey instrument, such as those developed by the Picker Institute Europe (www.pickereurope.org)] would enable standardised and comparative reporting of meaningful performance data across the NHS.
Over the last few years, lengths of stay for elective procedures have been in decline in the NHS. Given this, if real benefits from implementing enhanced recovery programmes are to be realised, much will depend on the length of stay being achieved via the existing conventional care pathway. The selection of patients for enhanced recovery programmes should also be given consideration as patients are usually relatively fit and may be more likely to have shorter lengths of hospital stay compared with less fit/high-risk patients. However, this reflects practice as enhanced recovery programmes would not be offered to patients who are not fit enough to be candidates for such programmes.
Managers and clinicians need to weigh up potential benefits from enhanced recovery against likely implementation costs of service redesign, the potential impact on budgets and on equity of access. To do this well and build a robust business case access to reliable information on benefits and costs is required. Our review of the cost-effectiveness literature suggests that enhanced recovery programmes that achieve a reduction in length of stay may save costs without detrimental effects on complication rates, readmission and health-related quality of life. These findings are also supported by systematic reviews and trials focusing on clinical effectiveness. However, generalisability of the results of the economic evaluations is limited by a lack of transparency in reporting, use of different settings and populations and variable methodology in analyses. Data were lacking for resource use and costs and could not usefully inform the review of economic evaluations. In addition, the clinical effectiveness of some of the programmes considered in economic evaluations was not based on robust evidence. The implementation evidence included resource use in terms of the professionals involved in enhanced recovery programmes but details were very limited and did not add to the evidence synthesis. The impact of surgical experience and surgical volume on clinical outcomes was not explored and any implications of differences in these areas remain unknown.
Strengths and limitations
The main strength of this study was our use of multiple approaches to acquire and synthesise evidence relevant to different aspects of enhanced recovery programmes. The main limitations were poor methodological quality and reporting of much of the evidence, and the inherent difficulty of reviewing a complex intervention such as enhanced recovery in different health-care systems and surgical specialties. Current methods for synthesising such complex interventions are limited. The methodological limitations and are not discussed here as this was outside the scope of this project, but have been addressed in previous publications (e.g. Noyes et al. 126). Another complication is that elements of early enhanced recovery programmes have become accepted within conventional care. This evolution makes combining studies over different periods and interpreting results of earlier studies in relation to the current context more difficult.
We used existing synthesised evidence in systematic reviews and HTA reports to get a rapid overview of the evidence base for enhanced recovery programmes. Systematic reviews vary in quality of conduct, reporting and inclusion criteria. Our primary sources were DARE (which includes only reviews that meet specified minimum criteria) and Cochrane reviews (which use standard methods and undergo peer review before publication). We made our own risk of bias assessment of the included reviews.
We found a large number of systematic reviews, but there was substantial overlap in the included studies and evidence was not as abundant as the existence of multiple systematic reviews might suggest. For example, the 10 systematic reviews in colorectal surgery included different combinations of the same trials with little fresh insight added by each review. Relatively few trials were conducted in the UK and this may limit the generalisability of evidence to NHS settings.
We searched for further RCTs not included in systematic reviews and located trials published up to March 2013. These trials extended our list of types of surgery for which enhanced recovery has been shown to reduce length of stay and included four RCTs for gastric surgery. Most of the RCTs were small and not high quality. With the exception of one RCT, the remainder were single-centre trials and, therefore, appear to have been undertaken to support implementation of an enhanced recovery programme in a specific setting rather than being planned as research studies. No more trials of this type are needed. There is, however, a shortage of robust evidence evaluating the relative advantage of individual or combinations of components included in an enhanced recovery pathway. The degree to which success is dependent on the delivery of all, or just some, combinations of preoperative, intraoperative and postoperative elements commonly described in pathways is not yet known. Lack of evidence on important outcomes including pain and quality of life is also an issue for research in this field. Trials tended not to report on adherence to the planned enhanced recovery programme. The clinical and methodological differences between individual trials meant that we could not perform a meta-analysis.
An important feature of our review is the implementation of enhanced recovery programmes in the NHS. This evidence has not been synthesised previously. By summarising it we have ensured that the main findings continue to be publicly available. The original programme websites are archived and future access is not assured. We sought evidence on the experience of health professionals and patients of a broad range of sources and study types. Important themes emerged from this evidence that may be of value for implementing and sustaining enhanced recovery programmes in NHS settings. Owing to the rapid nature of the evidence synthesis, the list of sources searched to identify data on implementation and delivery of enhanced recovery programmes was not exhaustive and we acknowledge that relevant evidence may have been missed. Indeed, evidence from Scotland has been noted and eligible case studies have been identified from the NHS Scotland Quality Improvement Hub website. It should be noted that these are as limited as those included in the review.
Case studies are susceptible to risk of bias. Those that are enthusiastic about an intervention may publish results that are unrepresentative of typical practice. The ERPP innovation sites included in our review were a mixture of early adopters and NHS trusts encouraged to participate by their Strategic Health Authority. Use of a standard reporting format was a potential strength of the case studies but variation in what each site reported (particularly in terms of evidence of benefit from the introduction of enhanced recovery programmes) reduced the usefulness of the evidence.
We sought to incorporate published and unpublished evidence on patient experiences and views of enhanced recovery programmes. Only one review27 attempted to address this aspect of enhanced recovery programmes. Evaluation of patient experience of care is increasingly important for the NHS, especially in view of unacceptable failures of care such as those highlighted in the Francis report. 127 Though the evidence was generally positive for enhanced recovery, it was limited by a shortage of studies that used validated measures of patient experience and by study designs that could bias results in favour of enhanced recovery.
A further strength of this study was the consideration of cost-effectiveness evidence, using evidence from the NHS EED database and other sources. Our review highlighted weaknesses in existing studies in this field. None of the three economic evaluations conducted in the UK provided a summary of costs compared with benefits [e.g. cost per quality-adjusted life-year (QALY) gained], which makes it difficult to compare programmes. There is a clear need to capture better data on costs and benefits of enhanced recovery programmes from a clearly stated perspective. Such evaluations would need to fully take into account the resources used in the delivery of the whole enhanced recovery pathway and subsequent follow-up. This would include areas – such as the role of primary care in preparing patients before surgery and in supporting them after discharge from hospital – where our study found little or no evidence.
Critical factors
Evidence from implementation case studies highlights crucial elements for the success of implementation of an enhanced recovery programme and for barriers to uptake. The most frequently reported facilitators included a need for a project lead or champion to drive the process and the adoption of a multidisciplinary team approach from the outset. Other success factors were development and delivery of good-quality preoperative patient information, continued education for staff and patient engagement/representation. The main barriers to change were staff resistance to change, staff organisation, turnover and administrative limitations. This information was based on self-report and no firm data were available to support this.
The most frequently implemented programme elements in the case studies were pre-admission information/counselling and early postoperative mobilisation. It was unclear from the evidence whether or not complexity of some programmes with more elements affected their implementation. Available evidence did not address which enhanced recovery elements and combinations of elements were most clinically effective. Substantial variation in what constitutes an enhanced recovery programme within and between different surgical specialties suggest that the enhanced recovery pathway may be used as a framework and adapted to suit local situations.
All 10 of the innovation case studies reported reduced length of stay with enhanced recovery, but it is unclear whether or not these benefits were sustained over the longer term. Available evidence suggests that there was routine data collection on relevant outcomes but it is difficult to confirm this. It is unclear whether or not a standardised approach was taken and whether or not data were produced in a format that could be shared easily with other interested parties.
Evidence on compliance/adherence to enhanced recovery programmes was lacking. The limited data available highlighted elements for which compliance was poor, but it was unclear how representative this information was. None of the reviews assessed patient compliance, including adherence to preoperative advice to ensure fitness for surgery. It was also unclear whether or not compliance changes over the length of the pathway (i.e. tapering off during the postoperative period) and whether or not it changed over time and with experience.
The need to sustain multidisciplinary working means that, in the absence of 24/7 working for elective procedures, enhanced recovery programmes will tend to be front loaded into the start of the working week (typically Monday to Thursday). Recent evidence suggests a higher risk of death for patients who have elective surgical procedures carried out later in the working week and at the weekend. 128 As enhanced recovery invariably targets the fitter, more likely to be mobilised patient, frailer patients may not receive parity of access to what may be considered to represent optimal treatment and management. Managers and clinicians considering implementing such programmes should consider the likely implication on equity of access. Whether or not inequity is an unintended outcome of enhanced recovery, merits further investigation.
Implications for health care
Overall, there is consistent, albeit limited, evidence that enhanced recovery programmes may reduce length of patient hospital stay without increasing readmission rates. Data on reintervention rates and patient-reported outcomes were limited but did not suggest significant differences between enhanced recovery and conventional care.
Optimal care is certainly the right thing to do, but the evidence does not identify which enhanced recovery programme elements and combinations of elements are most clinically effective. As such, conclusions on the core set of elements that will provide greatest gains and how best to implement them cannot be made. The extent to which managers and clinicians considering implementing enhanced recovery programmes can realise reductions and cost savings will depend on length of stays achieved under their existing care pathway. Consideration of potential benefit also needs to take account of the costs of service redesign, the resource use associated with programmes of this nature, the potential for improvement in patient outcomes and the impact on equity of access.
Enhanced recovery programmes are complex interventions. Knowledge of how well the intervention has been implemented (fidelity) is essential for understanding how and why the intervention works and, hence, how outcomes can be further improved. Assessing fidelity may involve considering not only adherence to the requirements of the programme but also potential moderating factors such as strategies used to assist delivery of the intervention (e.g. programme facilitators), quality of delivery and participant responsiveness to new practices. 129 It would be helpful if future innovation programmes used standardised reporting. Case studies (and any overarching synthesis) need to be written up in sufficient detail to allow those not immediately involved to assess the extent to which the innovation programme has achieved its objectives. This would also help identify elements that may be consistently implemented poorly and enable modifications to be made to ERAS programmes to improve administration and implementation. For multisite programmes, a formal synthesis of findings from all participating sites should be undertaken as part of the evaluative process. This would ensure that the insights and contextual information which can inform the wider spread and adoption (or indeed discontinuation) would be systematically captured in a generalisable format.
Rigorous data on patients’ experiences of enhanced recovery programmes are lacking. Validated tools should be used and administered independently of those providing the service. Efforts should be made to obtain data from representative samples of patients receiving conventional care as well as those treated with enhanced recovery protocols.
Implications for research
Randomised controlled trials comparing an enhanced recovery programme with conventional care continue to be conducted and published. Given the available evidence, further single-centre RCTs of this kind are not a priority. Rather, what is needed is improved collection and reporting of how enhanced recovery programmes are implemented, resourced and experienced in NHS settings. Further multicentre RCTs may also provide additional insight into the effectiveness of enhanced recovery programmes. RCTs assessing the efficacy of different enhanced recovery programme elements and different combinations of elements may also be more beneficial. The cost of these types of studies would need to be taken into account, but would enhance our understanding of the core set of elements applicable to most settings and provide evidence to support local decision-making about whether or not to adopt and how best to implement.
The two groups of implementation case studies included in our synthesis, although all conducted in the UK, provide very limited information on how enhanced recovery programmes have actually been implemented in NHS settings. The standard reporting format originally proposed by The ERPP would enhance the value of future case studies if adhered to. Further research in this area is warranted and could involve small-scale local analyses of routinely collected data as well as larger, more ambitious case study initiatives. Data on the implementation and success of enhanced recovery programmes in the NHS are often collected as part of local initiates, but we were unable to obtain such data for this review. As with case studies, there is a need to standardise both collection and reporting methods. The recent review on the application plan-do-study-act methods offers a timely overview of the reporting limitations that undermine wider understanding. 130
Evidence on the experience of patients in relation to ERAS programmes is also lacking and further research on the experiences of patients and their families/carers is required.
Evidence relating to the cost-effectiveness of enhanced recovery programmes in NHS settings is lacking. Whereas enhanced recovery programmes have the potential to deliver cost savings, improved measurement of costs and benefits of programme elements is crucial to help decision-makers decide how best to make optimal use of limited resources.
Conclusions
Enhanced recovery programmes have been adopted with some enthusiasm by the NHS as a means to achieving productivity gains and cost savings. The evidence base to support widespread implementation is limited but does suggest possible benefits in terms of reduced length of hospital stay, fewer postoperative complications, reduced readmissions and improved patient outcomes.
Acknowledgements
This project was funded as part of a programme of research funded by the NIHR Health Services and Delivery Research programme (project reference: 11/1026/04).
We would like to thank all the NHS staff that took the time to respond and help us identify grey literature considered for inclusion in this review.
Contribution of authors
Fiona Paton Involved in all stages of the rapid synthesis from development of the protocol, through screening studies and data extraction to analysis and synthesis and production of the final report.
Duncan Chambers Involved in all stages of the rapid synthesis from development of the protocol, through screening studies and data extraction to analysis and synthesis and production of the final report.
Paul Wilson Involved in the development of the protocol, provided input on all stages of the review, and involved in the production of the final report. Took overall responsibility for the rapid synthesis.
Alison Eastwood Provided input at all stages of the review and commented on drafts of the report.
Dawn Craig Involved in all stages of the economic evaluation from development of the protocol, study selection, and production of the final report.
Dave Fox Conducted literature searches and contributed to the methods section of the report.
David Jayne Provided clinical advice throughout the rapid synthesis process and commented on the draft report.
Erika McGinnes Provided advice throughout the rapid synthesis process and commented on the draft report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Dixon J. Making Progress on Efficiency in the NHS in England: Options for System Reform. London: The Nuffield Trust; 2010.
- Appleby J, Ham C, Imison C, Jennings M. Improving NHS Productivity. More With the Same Not More of the Same. London: King’s Fund; 2010.
- Øvretveit J. Does Improving Quality Save Money? A Review of Evidence of Which Improvements to Quality Reduce Costs for Health Service Providers. London: The Health Foundation; 2009.
- Øvretveit J. Does improving Care Coordination Save Money: A Review of Research. London: The Health Foundation; 2011.
- Delivering Enhanced Recovery – Helping Patients to Get Better Sooner After Surgery. London: Department of Health; 2010.
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. http://dx.doi.org/10.1016/S0002-9610(02)00866-8.
- Sturm L, Cameron AL. Brief Review: Fast-Track Surgery and Enhanced Recovery After Surgery (ERAS) Programs. Melbourne: Australian Safety and Efficacy Register of New Interventional Procedures – Surgical (ASERNIP-S) 2009. http://docs.health.vic.gov.au/docs/doc/1177A3B11FBE1D2ACA2579280016A633/$FILE/enhanced_patient_recovery_programs.pdf (accessed 29 April 2014).
- Wainwright T, Middleton R. An orthopaedic enhanced recovery pathway. Curr Anaesth Crit Care 2010;21:114-20. http://dx.doi.org/10.1016/j.cacc.2010.01.003.
- Fulfilling the Potential: A Better Journey for Patients and a Better Deal for the NHS. London: NHS Enhanced Recovery Partnership; 2012.
- Liberating the NHS: No Decision About Me, Without Me – Government Response to the Consultation. London: Department of Health; 2012.
- Marshall M, Øvretveit J. Can we save money by improving quality?. BMJ Qual Saf 2011;20:293-6. http://dx.doi.org/10.1136/bmjqs.2010.050237.
- Chambers D, Wilson P. A framework for production of systematic review based briefings to support evidence-informed decision-making. Syst Rev 2012;1. http://dx.doi.org/10.1186/2046-4053-1-32.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: A meta-analysis of randomised controlled trials. Dis Colon Rectum 2013;56:667-78. http://dx.doi.org/10.1097/DCR.0b013e3182812842.
- Li MZ, Xiao LB, Wu WH, Yang SB, Li SZ. Meta-analysis of laparoscopic versus open colorectal surgery within fast-track perioperative care. Dis Colon Rectum 2012;55:821-7. http://dx.doi.org/10.1097/DCR.0b013e31824bd31e.
- Vlug MS, Wind J, van der Zaag E, Ubbink DT, Cense HA, Bemelman WA. Systematic review of laparoscopic vs open colonic surgery within an enhanced recovery programme. Colorectal Dis 2009;11:335-43. http://dx.doi.org/10.1111/j.1463-1318.2008.01679.x.
- Arsalani-Zadeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg 2011;98:181-96. http://dx.doi.org/10.1002/bjs.7331.
- Hoffmann H, Kettelhack C. Fast-track surgery – conditions and challenges in postsurgical treatment: a review of elements of translational research in enhanced recovery after surgery. Eur Surg Res 2012;49:24-3. http://dx.doi.org/10.1159/000339859.
- Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimise health outcomes and resource utilisation: a meta-analysis of randomised controlled trials in colorectal surgery. Surgery 2011;149:830-40. http://dx.doi.org/10.1016/j.surg.2010.11.003.
- Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols – compliance and variations in practice during routine colorectal surgery. Colorectal Dis 2012;14:1045-51. http://dx.doi.org/10.1111/j.1463-1318.2011.02856.x.
- Coolsen MME, Wong-Lun-Hing EM, van Dam RM, van der Wilt AA, Slim K, Lassen K, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford) 2013;15:245-51. http://dx.doi.org/10.1111/j.1477-2574.2012.00572.x.
- Coolsen MME, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CHC. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 2013;37:1909-18. http://dx.doi.org/10.1007/s00268-013-2044-3.
- Eskicioglu C, Forbes SS, Aarts M-A, Okrainec A, McLeod RS. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg 2009;13:2321-9. http://dx.doi.org/10.1007/s11605-009-0927-2.
- Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis 2009;24:1119-31. http://dx.doi.org/10.1007/s00384-009-0703-5.
- Hall TC, Dennison AR, Bilku DK, Metcalfe MS, Garcea G. Enhanced recovery programmes in hepatobiliary and pancreatic surgery: a systematic review. Ann R Coll Surg Engl 2012;94:318-26. http://dx.doi.org/10.1308/003588412X13171221592410.
- Khan S, Wilson T, Ahmed J, Owais A, MacFie J. Quality of life and patient satisfaction with enhanced recovery protocols. Colorectal Dis 2010;12:1175-82. http://dx.doi.org/10.1111/j.1463-1318.2009.01997.x.
- Lemmens L, van Zelm R, Borel Rinkes I, van Hillegersberg R, Kerkkamp H. Clinical and organizational content of clinical pathways for digestive surgery: a systematic review. Dig Surg 2009;26:91-9. http://dx.doi.org/10.1159/000206142.
- Rawlinson A, Kang P, Evans J, Khanna A. A systematic review of enhanced recovery protocols in colorectal surgery. Ann R Coll Surg Engl 2011;93:583-8. http://dx.doi.org/10.1308/147870811X605219.
- Spanjersberg Willem R, Reurings J, Keus F, van Laarhoven Cornelis JHM. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011;16. http://dx.doi.org/10.1002/41651858j.CD007635.pub2.
- Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010;29:434-40. http://dx.doi.org/10.1016/j.clnu.2010.01.004.
- Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta-analysis. Colorectal Dis 2009;11:344-53. http://dx.doi.org/10.1111/j.1463-1318.2009.01789.x.
- Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 2006;93:800-9. http://dx.doi.org/10.1002/bjs.5384.
- Lv D, Wang X, Shi G. Perioperative enhanced recovery programmes for gynaecological cancer patients. Cochrane Database Syst Rev 2012;12.
- Lv L, Shao YF, Zhou YB. The enhanced recovery after surgery (ERAS) pathway for patients undergoing colorectal surgery: an update of meta-analysis of randomized controlled trials. Int J Colorectal Dis 2012;27:1549-54. http://dx.doi.org/10.1007/s00384-012-1577-5.
- Anderson AD, McNaught CE, MacFie J, Tring I, Barker P, Mitchell CJ. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg 2003;90:1497-504. http://dx.doi.org/10.1002/bjs.4371.
- Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 2003;46:851-9. http://dx.doi.org/10.1007/s10350-004-6672-4.
- Gatt M, Anderson AD, Reddy BS, Hayward-Sampson P, Tring IC, MacFie J. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. Br J Surg 2005;92:1354-62. http://dx.doi.org/10.1002/bjs.5187.
- Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre-Brook IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007;245:867-72. http://dx.doi.org/10.1097/01.sla.0000259219.08209.36.
- Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. Zurich Fast Track Study Group . A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009;136:842-7. http://dx.doi.org/10.1053/j.gastro.2008.10.030.
- Serclová Z, Dytrych P, Marvan J, Nová K, Hankeová Z, Ryska O, et al. Fast-track in open intestinal surgery: prospective randomized study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr 2009;28:618-24. http://dx.doi.org/10.1016/j.clnu.2009.05.009.
- Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868-75. http://dx.doi.org/10.1097/SLA.0b013e31821fd1ce.
- Hendry PO, van Dam RM, Bukkems SF, McKeown DW, Parks RW, Preston T, et al. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg 2010;97:1198-206. http://dx.doi.org/10.1002/bjs.7120.
- Gralla O, Haas F, Knoll N, Hadzidiakos D, Tullmann M, Romer A, et al. Fast-track surgery in laparoscopic radical prostatectomy: basic principles. World J Urol 2007;25:185-91. http://dx.doi.org/10.1007/s00345-006-0139-2.
- Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop 2008;79:149-59. http://dx.doi.org/10.1080/17453670710014923.
- Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder-Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J Surg 2009;33:577-85. http://dx.doi.org/10.1007/s00268-008-9892-2.
- Muehling BM, Halter G, Lang G, Schelzig H, Steffen P, Wagner F, et al. Prospective randomized controlled trial to evaluate “fast-track” elective open infrarenal aneurysm repair. Langenbecks Arch Surg 2008;393:281-7. http://dx.doi.org/10.1007/s00423-008-0284-8.
- Muehling BM, Ortlieb L, Oberhuber A, Orend KH. Fast track management reduces the systemic inflammatory response and organ failure following elective infrarenal aortic aneurysm repair. Interact Cardiovasc Thorac Surg 2011;12:784-8. http://dx.doi.org/10.1510/icvts.2010.262337.
- Muehling BM, Halter GL, Schelzig H, Meierhenrich R, Steffen P, Sunder-Plassmann L, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. http://dx.doi.org/10.1016/j.ejcts.2008.04.009.
- Hübner M, Müller S, Schäfer M, Clavien PA, Demartines N. Impact of the nutritional risk score in fast-track colon surgery. Dig Surg 2010;27:436-9. http://dx.doi.org/10.1159/000313692.
- Hübner M, Schäfer M, Demartines N, Müller S, Maurer K, Baulig W, et al. Impact of restrictive intravenous fluid replacement and combined epidural analgesia on perioperative volume balance and renal function within a Fast Track program. J Surg Res 2012;173:68-74. http://dx.doi.org/10.1016/j.jss.2010.08.051.
- Petersen MK, Madsen C, Andersen NT, Søballe K. Efficacy of multimodal optimization of mobilization and nutrition in patients undergoing hip replacement: a randomized clinical trial. Acta Anaesthesiol Scand 2006;50:712-17. http://dx.doi.org/10.1111/j.1399-6576.2006.01040.x.
- Petersen MK, Andersen NT, Søballe K. Self-reported functional outcome after primary total hip replacement treated with two different periopera-tive regimes: a follow-up study involving 61 patients. Acta Orthop 2008;79:160-7. http://dx.doi.org/10.1080/17453670710014932.
- Recart A, Duchene D, White PF, Thomas T, Johnson DB, Cadeddu JA. Efficacy and safety of fast-track recovery strategy for patients undergoing laparoscopic nephrectomy. J Endourol 2005;19:1165-9. http://dx.doi.org/10.1089/end.2005.19.1165.
- Vlug MS, Bartels SA, Wind J, Ubbink DT, Hollmann MW, Bemelman WA, et al. Which fast track elements predict early recovery after colon cancer surgery?. Colorectal Dis 2012;14:1001-8. http://dx.doi.org/10.1111/j.1463-1318.2011.02854.x.
- van Bree SH, Vlug MS, Bemelman WA, Hollmann MW, Ubbink DT, Zwinderman AH, et al. Faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology 2011;141:872-80. http://dx.doi.org/10.1053/j.gastro.2011.05.034.
- Wind J, Hofland J, Preckel B, Hollmann MW, Bossuyt PM, Gouma DJ, et al. Perioperative strategy in colonic surgery; LAparoscopy and/or FAst track multimodal management versus standard care (LAFA trial). BMC Surg 2006;6. http://dx.doi.org/10.1186/1471-2482-6-16.
- Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Dis 2012;14:1009-13. http://dx.doi.org/10.1111/j.1463-1318.2011.02855.x.
- Wang G, Jiang ZW, Xu J, Gong JF, Bao Y, Xie LF, et al. Fast-track rehabilitation program vs conventional care after colorectal resection: a randomized clinical trial. World J Gastroenterol 2011;17:671-6. http://dx.doi.org/10.3748/wjg.v17.i5.671.
- Yang DJ, Zhang S, He WL, Huang WQ, Cai SR, Chen CQ, et al. Fast-track surgery accelerates the recovery of postoperative humoral immune function in elective operation for colorectal carcinoma: a randomized controlled clinical trial. Chin Med J 2012;92:1112-15.
- Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg 2012;255:216-21. http://dx.doi.org/10.1097/SLA.0b013e31824336e2.
- Chen Hu J, Xin Jiang L, Cai L, Tao Zheng H, Yuan Hu S, Bing Chen H, et al. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1830-9. http://dx.doi.org/10.1007/s11605-012-1969-4.
- Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, Noh SH. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg 2012;36:2879-87. http://dx.doi.org/10.1007/s00268-012-1741-7.
- Garcia-Botello S, Cánovas de Lucas R, Tornero C, Escamilla B, Espí -Macías A, Esclapez-Valero P, et al. Implementation of a perioperative multimodal rehabilitation protocol in elective colorectal surgery. A prospective randomised controlled study. Cir Esp 2011;89:159-66. http://dx.doi.org/10.1016/j.ciresp.2010.12.004.
- Ionescu D, Iancu C, Ion D, Al-Hajjar N, Margarit S, Mocan L, et al. Implementing fast-track protocol for colorectal surgery: a prospective randomized clinical trial. World J Surg 2009;33:2433-8. http://dx.doi.org/10.1007/s00268-009-0197-x.
- Lee TG, Kang SB, Kim DW, Hong S, Heo SC, Park KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: a prospective randomized controlled trial. Dis Colon Rectum 2011;54:21-8. http://dx.doi.org/10.1007/DCR.0b013e3181fcdb3e.
- Lemanu DP, Singh PP, Berridge K, Burr M, Birch C, Babor R, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg 2013;100:482-9. http://dx.doi.org/10.1002/bjs.9026.
- Liu XX, Jiang ZW, Wang ZM, Li JS. Multimodal optimization of surgical care shows beneficial outcome in gastrectomy surgery. JPEN J Parenter Enteral Nutr 2010;34:313-21. http://dx.doi.org/10.1177/0148607110362583.
- Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg 2012;36:407-14. http://dx.doi.org/10.1007/s00268-011-1348-4.
- Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg 2010;14:620-7. http://dx.doi.org/10.1007/s11605-009-1139-5.
- Yang DJ, Zhang S, He WL, Chen HY, Cai SR, Chen CQ, et al. Fast track surgery accelerates the recovery of postoperative insulin sensitivity. Chin Med J 2012;125:3261-5.
- Antipin EE, Uvarov DN, Svirskii DA, Antipina NP, Nedashkovskii EV, Sovershaeva SL. Realization of fast track surgery principles during cesarean section. Anesteziol Reanimatol n.d.:33-6.
- Zhao G, Huang Y, Chen X, Duan L, Ma Q, Lei Y, et al. Research on fast track surgery application in lung cancer surgery. Zhongguo Fei Ai Za Zhi 2010;13:102-6. http://dx.doi.org/10.3779/j.issn.1009-3419.2010.02.04.
- El-Sheikh S, El-Sayed S, Mostafa A, Hussein H. Enhanced recovery program safely improves the outcome of elective colorectal surgery. Egypt J Anaesth 2010;26:229-39.
- Magheli A, Knoll N, Lein M, Hinz S, Kempkensteffen C, Gralla O. Impact of fast-track postoperative care on intestinal function, pain, and length of hospital stay after laparoscopic radical prostatectomy. J Endourol 2011;25:1143-7. http://dx.doi.org/10.1089/end.2011.0020.
- Coolsen MME, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CHC. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies: Supplementary Material [published online April 9 2013]. World J Surg 2013. http://link.springer.com/article/10.1007%2Fs00268–013–2044–3.
- Fearon K, Lobo D, Dejong C. ESPEN Congress 2010 Highlight Topics: Section 7: The Enhanced recovery After Surgery (ERAS) Protocol. Eur Soc Clin Nutr Metab 2012. www.jspen.jp/doc6/sec7.html (accessed 2 November 2012).
- Khan S, Gatt M, Horgan A, Anderson I, MacFie J. Guidelines for Implementation of Enhanced Recovery Protocols. London: Association of Surgeons of Great Britain and Ireland; 2009.
- Reilly KA, Beard DJ, Barker KL, Dodd CA, Price AJ, Murray DW. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty: a randomised controlled trial. Knee 2005;12:351-7. http://dx.doi.org/10.1016/j.knee.2005.01.002.
- Archibald LH, Ott MJ, Gale CM, Zhang J, Peters MS, Stroud GK. Enhanced recovery after colon surgery in a community hospital system. Dis Colon Rectum 2011;54:840-5. http://dx.doi.org/10.1007/DCR.0b013e31821645bd.
- Sammour T, Zargar-Shoshtari K, Bhat A, Kahokehr A, Hill AG. A programme of Enhanced Recovery After Surgery (ERAS) is a cost-effective intervention in elective colonic surgery. N Z Med J 2010;123:61-70.
- King PM, Blazeby JM, Ewings P, Longman RJ, Kipling RM, Franks PJ, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis 2006;8:506-13. http://dx.doi.org/10.1111/j.1463-1318.2006.00963.x.
- Nielsen PR, Andreasen J, Asmussen M, Tønnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-209 (accessed June 2013).
- Jakobsen DH, Sonne E, Andreasen J, Kehlet H. Convalescence after colonic surgery with fast-track vs conventional care. Colorectal Dis 2006;8:683-7. http://dx.doi.org/10.1111/j.1463-1318.2006.00995.x.
- Larsen K, Hansen TB, Thomsen PB, Christiansen T, Soballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg 2009;91:761-72. http://dx.doi.org/10.2106/JBJS.G.01472.
- Kariv Y, Delaney CP, Senagore AJ, Manilich EA, Hammel JP, Church JM, et al. Clinical outcomes and cost analysis of a “fast track” postoperative care pathway for ileal pouch-anal anastomosis: a case control study. Dis Colon Rectum 2007;50:137-46. http://dx.doi.org/10.1007/s10350-006-0760-6.
- Salhiyyah K, Elsobky S, Raja S, Attia R, Brazier J, Cooper GJ. A clinical and economic evaluation of fast-track recovery after cardiac surgery. Heart Surg Forum 2011;14:E330-4. http://dx.doi.org/10.1532/HSF98.20111029.
- Yanatori M, Tomita S, Miura Y, Ueno Y. Feasibility of the fast-track recovery program after cardiac surgery in Japan. Gen Thorac Cardiovasc Surg 2007;55:445-9. http://dx.doi.org/10.1007/s11748-007-0162-2.
- Enhanced Recovery Partnership Programme Case Studies 2011 . Gynaecology: Cambridge University Hospitals NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Colorectal, Gynaecology, Urology, MSK: Medway NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Colorectal Surgery (All Elective Procedures) and Most Major Emergencies from Decision to Treat Surgically; Urology: Radical Prostatectomy, Cystectomy, Nephrectomy; MSK: 1 Hip and Knee Replacement; Gynaecology: Hysterectomy (Vaginal, Abdominal and Laparoscopic) Moving to All Majors: Royal Berkshire Hospital n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Enhanced Recovery After Colorectal Surgery: Royal Berkshire Hospital n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115737.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Colorectal Surgery: Hillingdon Hospitals NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Musculo-Skeletal – Hip and Knee Joint Arthroplasty: Mount Vernon Hospital n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_126174.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . ERP: Colchester Hospital University NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115726.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Colorectal; Starting Implementation in Urology Gynaecology MSK: Salford Royal Hospital n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_126169.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Enhanced Receover Program for All Elective Resectional Colorectal Surgery: Colorectal Surgery, Department of General Surgery, Salisbury NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115738.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Enhanced Recovery Programme: Yeovil District Hospital NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115747.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Enhanced Recovery for Colorectal Surgery: Yeovil District Hospital NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115748.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Enhanced Recovery Hip and Knee Joint Replacement: Northumbria Healthcare NHS Foundation Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_117531.pdf (accessed 29 April 2014).
- Enhanced Recovery Partnership Programme Case Studies 2011 . Orthopaedic Enhanced Recovery (TKR): Wrexham Maelor Hospital North Wales NHS Trust n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115746.pdf (accessed 29 April 2014).
- Schneider M, Kawahara I, Ballantyne G, McAuley C, MacGregor K, Garvie R, et al. Predictive factors influencing fast track rehabilitation following primary total hip and knee arthroplasty. Arch Orthop Trauma Surg 2009;129:1585-91. http://dx.doi.org/10.1007/s00402-009-0825-9.
- Smart NJ, White P, Allison AS, Ockrim JB, Kennedy RH, Francis NK. Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis 2012;14:e727-e34. http://dx.doi.org/10.1111/j.1463-1318.2012.03096.x.
- Winning Principle 2: Improving Patient Care with Shorter Hospital Admissions for Gynaecological Patients. London: NHS Improvement; 2007.
- Schmid KJ, Tewari R, Nordin AJ. Improving Patient Care With Shorter Hospital Admissions: East Kent Gynaecological Oncology Centre 2007. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115730.pdf (accessed 29 April 2014).
- Letton C, Cheung C, Nordin A. Does an enhanced recovery integrated care pathway (ICP) encourage adherence to prescribing guidelines, accelerate postoperative recovery and reduce the length of stay for gynaecological oncology patients?. J Obstet Gynaecol 2013;33:296-7. http://dx.doi.org/10.3109/01443615.2012.758693.
- Hibberd G, Wilcocks K, Cox D. Enhanced Recovery Programme for elective total hip replacement – the Salisbury experience n.d.
- Hibberd G, Wilcocks K. Presentation for Service Improvement Awards. Salisbury NHS Foundation Trust; 2012.
- Winning Principle 2: Reducing Length of Stay for Colorectal Surgery Patients Using Enhanced Rcovery Techniques. London: NHS Improvement; 2007.
- 4 Winning Principles: Cancer Inpatients Case Series: Reducing Length of Stay for Colorectal Surgery Patients Using Enhanced Recovery Techniques. London: NHS Improvement; 2007.
- Payne J, Beazley SM, Hannah. 4 Winning Principles: Cancer Inpatients Case Series: Enhanced Recovery After Surgery Program – Improving Patient Outcomes for Colorectal Surgical Patients. London: NHS Improvement; 2008.
- Parker M. Recovery Times Slashed by Three Weeks 2008. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_115755.pdf (accessed 29 April 2014).
- Elwood M. Implementing an Enhanced Recovery Programme in Colorectal Surgery 2008. http://www.nursingtimes.net/implementing-an-enhanced-recovery-programme-in-colorectal-surgery/1247153.article (accessed 29 April 2014).
- Hodder M, Ratnayaka N, Ali A, Hutton S. An Audit to Assess Compliance with the Enhanced Recovery After Surgery (ERAS) Programme in Colorectal Surgery at Walsall Manor Hospital. Walsall: Walsall Healthcare NHS Trust; 2012.
- McGeehan R. Enhanced Recovery in Orthopaedics – The Journey. Sheffield: Sheffield Teaching Hospitals; 2013.
- 4 Winning Principles: Cancer Inpatients Case Series: Enhanced Recovery for Gynaecological Patients. London; 2007.
- Bowen-Rampling E, Carty S, Coffin K, Donovan A, Golby S, Hopwood H, et al. Enhanced Recovery Summit. London: NHS Improvement on behalf of the Enhanced Recovery Partnership; 2012.
- Zamoyski T, Khera G, Simpson A, Wilson M, Woodcock S, Seymour K. Enhanced Recovery Program Within a Bariatric Service. Department of General Surgery, North Tyneside General Hospital; 2011.
- Blazeby JM, Soulsby M, Winstone K, King PM, Bulley S, Kennedy RH. A qualitative evaluation of patients’ experiences of an enhanced recovery programme for colorectal cancer. Colorectal Dis 2010;12:e236-42. http://dx.doi.org/10.1111/j.1463-1318.2009.02104.x.
- Taylor C, Burch J. Feedback on an enhanced recovery programme for colorectal surgery. Br J Nurs 2011;20:286-90.
- Holloway I, Wheeler S. Qualitative Research for Nurses. Oxford: Blackwell Science; 1996.
- Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010;3.
- Rotter T, Kugler J, Koch R, Gothe H, Twork S, van Oostrum JM, et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs and patient outcomes. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-265.
- Allen D, Gillen E, Rixson L. The effectiveness of integrated care pathways for adults and children in health care settings: a systematic review. JBI Database Syst Rev Implementation Rep 2009;7:80-129.
- Allen D, Rixson L. How has the impact of ‘care pathway technologies’ on service integration in stroke care been measured and what is the strength of the evidence to support their effectiveness in this respect?. Int J Evid Based Healthcare 2008;6:78-110. http://dx.doi.org/10.1111/j.1744-1609.2007.00098.x.
- Noyes J, Gough D, Lewin S, Mayhew A, Michie S, Pantoja T, et al. A research and development agenda for systematic reviews that ask complex questions about complex interventions. J Clin Epidemiol 2013;66:1262-70. http://dx.doi.org/10.1016/j.jclinepi.2013.07.003.
- Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry. London: The Stationery Office; 2013.
- Aylin P, Alexandrescu R, Jen MH, Mayer EK, Bottle A. Day of week of procedure and 30 day mortality for elective surgery: retrospective analysis of hospital episode statistics. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2424.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-40.
- Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMJ Qual Saf 2013. http://dx.doi.org/10.1136/bmjqs-2013-001862.
- Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 2006;93:800-9. http://dx.doi.org/10.1002/bjs.5384.
Appendix 1 Search strategies
The Cochrane Library (includes Cochrane Database of SystematicReviews, Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database, Health Technology Assessment and Cochrane Central Register of Controlled Reviews)
Searched 12 December 2012 via http://onlinelibrary.wiley.com/cochranelibrary/search/advanced.
Limited to 1990 onwards.
Search strategy
-
ERAS:ti,ab
-
((enhanced or early or accelerated or fast track or fast-track or rapid) near/1 (recover* or rehabilitat* or convalesc* or mobil* or ambulat* or walk* or feed* or nutrition* or eat*) near/3 (surger* or program* or protocol* or pathway*)):ti,ab
-
((multimodal or optimised or optimized) near/1 (recover* or rehabilitat* or convalesc*)):ti,ab
-
#1 or #2 or #3
-
Medical subject heading (MeSH) descriptor: [Receptors, Endothelin] explode all trees
-
#4 not #5
One thousand and thirty-three total results included 19 from Cochrane Database of Systematic Reviews, 18 from DARE, 32 from NHSEED, 2 from HTA and 707 from CENTRAL.
Centre for Reviews and Dissemination databases (Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database and Health Technology Assessment)
Searched 24 January 2013 via www.crd.york.ac.uk/crdweb in addition to The Cochrane Library search above to ensure any recent additions captured.
Searched all fields, no date limits applied.
Search strategy
-
(ERAS) (27)
-
(((((enhanced or early or accelerated or fast track or fast-track or rapid) NEAR1 (recover* or rehabilitat* or convalesc* or mobil* or ambulat* or walk* or feed* or nutrition* or eat*) NEAR3 (surger* or program* or protocol* or pathway*))))) (34)
-
((((multimodal or optimised or optimized) NEAR1 (recover* or rehabilitat* or convalesc*)))) (3)
-
#1 OR #2 OR #3 (55)
-
MeSH DESCRIPTOR Receptors, Endothelin EXPLODE ALL TREES (7)
-
#4 NOT #5 (55)
Fifty-five total results included 26 from DARE, 27 from NHSEED, and 2 from HTA.
Health Economic Evaluations Database
Searched 19 December 2012 via http://heed.onlinelibrary.wiley.com.
Compound search of ‘All Data’- all database fields.
Search strategy
ERAS or ‘enhanced recovery’ or ‘enhanced rehabilitation’ or ‘enhanced convalescence’ or ‘early recovery’ or ‘early rehabilitation’ or ‘early convalescence’ or ‘early mobilisation’ or ‘early mobilization’ or ‘early ambulation’ or ‘early walking’ or ‘early nutrition’ or ‘early eating’
or
‘accelerated recovery’ or ‘accelerated rehabilitation’ or ‘accelerated convalescence’ or ‘accelerated mobilisation’ or ‘accelerated mobilization’ or ‘accelerated ambulation’ or ‘accelerated walking’ or ‘accelerated nutrition’ or ‘accelerated eating’ or ‘fast track recovery’ or ‘fast track rehabilitation’ or ‘fast track convalescence’ or ‘fast track mobilisation’ or ‘fast track mobilization’ or ‘fast track ambulation’ or ‘fast track walking’ or ‘fast track nutrition’
or
‘fast-track rehabilitation’ or ‘fast-track convalescence’ or ‘fast-track mobilisation’ or ‘fast-track mobilization’ or ‘fast-track ambulation’ or ‘fast-track walking’ or ‘fast-track nutrition’ or ‘rapid recovery’ or ‘rapid rehabilitation’ or ‘rapid convalescence’ or ‘rapid mobilisation’ or ‘rapid mobilization’
or
‘rapid ambulation’ or ‘rapid walking’ or ‘rapid nutrition’ or ‘multimodal recovery’ or ‘multimodal rehabilitation’ or ‘multimodal convalescence’ or ‘optimised recovery’ or ‘optimised rehabilitation’ or ‘optimised convalescence’ or ‘optimized recovery’ or ‘optimized rehabilitation’ or ‘optimized convalescence’
Seventy-nine total results saved to Endnote library.
MEDLINE
Searched 12 December 2012 via Ovid interface. RCT filter used and limited to 1990 onwards.
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to present>.
Search strategy
-
ERAS.ti,ab. (1420)
-
((enhanced or early or accelerated or fast track or fast-track or rapid) adj (recover$ or rehabilitat$ or convalesc$ or mobil$ or ambulat$ or walk$ or feed$ or nutrition$ or eat$) adj3 (surger$ or program$ or protocol$ or pathway$)).ti,ab. (888)
-
((multimodal or optimised or optimized) adj (recover$ or rehabilitat$ or convalesc$)).ti,ab. (133)
-
1 or 2 or 3 (2339)
-
exp Receptors, Endothelin/ (7243)
-
4 not 5 (2303)
-
randomized controlled trial.pt. (342,813)
-
controlled clinical trial.pt. (85,716)
-
randomized.ab. (259,597)
-
placebo.ab. (142,189)
-
clinical trials as topic.sh. (163,816)
-
randomly.ab. (189,206)
-
trial.ti. (111,701)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 (824,313)
-
6 and 14 (259)
-
limit 15 to yr=“1990 – 2013” (251)
ClinicalTrials.gov
Searched 14 December 2012 via http://clinicaltrials.gov.
Single-line strategies run independently then de-duplicated.
Search strategy
-
((enhanced OR early OR accelerated OR fast track OR fast-track OR rapid) AND (recovery OR rehabilitation OR convalescence OR mobility OR walking OR feeding OR nutrition OR eating) AND (surgery OR program OR programme OR protocol OR pathway)) (1081)
-
“multimodal recovery” OR “multimodal rehabilitation” OR “multimodal convalescence” OR “optimised recovery” OR “optimised rehabilitation” OR “optimised convalescence” OR “optimized recovery” OR “optimized rehabilitation” OR “optimized convalescence” (87)
-
#1 OR #2 (1138)
PROSPERO
Searched 12 December 2012 via www.crd.york.ac.uk/PROSPERO.
Search terms (all nil results unless marked):
ERAS (2)
enhanced recovery (2)
enhanced rehabilitation
enhanced convalescence
early recovery
early rehabilitation
early convalescence
early mobilisation (1)
early mobilization
early ambulation
early walking
early nutrition
early eating
accelerated recovery
accelerated rehabilitation
accelerated convalescence
accelerated mobilisation
accelerated mobilization
accelerated ambulation
accelerated walking
accelerated nutrition
accelerated eating
fast track recovery
fast track rehabilitation
fast track convalescence
fast track mobilisation
fast track mobilization
fast track ambulation
fast track walking
fast track nutrition
fast-track rehabilitation
fast-track convalescence
fast-track mobilisation
fast-track mobilization
fast-track ambulation
fast-track walking
fast-track nutrition
rapid recovery
rapid rehabilitation
rapid convalescence
rapid mobilisation
rapid mobilization
rapid ambulation
rapid walking
rapid nutrition
multimodal recovery
multimodal rehabilitation
multimodal convalescence
optimised recovery
optimised rehabilitation
optimised convalescence
optimized recovery
optimized rehabilitation
optimized convalescence
Of five total results three were unique.
Following discussion after the initial searches it was decided to run broader searches for RCTs in MEDLINE and for economic evaluations in NHS EED.
MEDLINE
Searched 15 February 2013 via Ovid interface. Randomised controlled filter used and limited to 1990 onwards.
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>.
Search strategy
-
ERAS.ti,ab. (1413)
-
((enhanced or early or accelerated or rapid or multimodal or multi-modal or optimi$) adj3 (recover$ or rehabilitat$ or convalesc$ or preoperative or preoperative or intraoperative or intra-operative or perioperative or peri-operative)).ti,ab. (20,528)
-
((multimodal or multi-modal) adj (optimisation or optimization)).ti,ab. (14)
-
(fast track or fast-track).ti,ab. (1684)
-
1 or 2 or 3 or 4 (23,306)
-
exp Receptors, Endothelin/ (7145)
-
5 not 6 (23,256)
-
randomized controlled trial.pt. (340,616)
-
controlled clinical trial.pt. (85,208)
-
randomized.ab. (257,971)
-
placebo.ab. (140,769)
-
clinical trials as topic.sh. (162,479)
-
randomly.ab. (188,501)
-
trial.ti. (110,031)
-
8 or 9 or 10 or 11 or 12 or 13 or 14 (818,611)
-
7 and 15 (2683)
-
limit 16 to yr="1990 - 2013" (2503)
-
exp animals/ not humans/
-
17 not 18 (2330)
Two thousand three hundred and thirty results deduplicated against previous searches, leaving 2085 records.
The Cochrane Library (NHS Economic Evaluation Database)
Searched 19 December 2012 via http://onlinelibrary.wiley.com/cochranelibrary/search/advanced.
Limited to 1990 onwards.
Search strategy
-
#1 ERAS:ti,ab
-
#2 ((enhanced or early or accelerated or rapid or multimodal or multi-modal or optimi*) near/3 (recover* or rehabilitat* or convalesc* or preoperative or preoperative or intraoperative or intra-operative or perioperative or peri-operative)):ti,ab
-
#3 ((multimodal or multi-modal) near/1 (optimisation or optimization)):ti,ab
-
#4 ("fast track" or fast-track):ti,ab
-
#5 (clinical near/1 pathway*):ti,ab
-
#6 #1 or #2 or #3 or #4 or #5
-
#7 MeSH descriptor: [Receptors, Endothelin] explode all trees
-
#8 #6 not #7
One hundred and six results de-duplicated against previous searches, leaving 72 records.
Centre for Reviews and Dissemination databases (NHS Economic Evaluation Database)
Searched 19 February 2013 via www.crd.york.ac.uk/crdweb in addition to The Cochrane Library search above to ensure any recent additions captured.
Searched all fields, no date limits applied.
Search strategy
-
(ERAS) IN NHSEED (17)
-
(((enhanced or early or accelerated or rapid or multimodal or multi-modal or optimi*) near3 (recover* or rehabilitat* or convalesc* or preoperative or preoperative or intraoperative or intra-operative or perioperative or peri-operative))) IN NHSEED (67)
-
(((multimodal or multi-modal) near1 (optimisation or optimization))) IN NHSEED (0)
-
((“fast track” or fast-track)):TI IN NHSEED (14)
-
((clinical near1 pathway*)) IN NHSEED (118)
-
#1 OR #2 OR #3 OR #4 OR #5 (210)
-
MeSH DESCRIPTOR Receptors, Endothelin EXPLODE ALL TREES (7)
-
#6 NOT #7 (210)
Two hundred and ten results deduplicated against previous searches, leaving 116 records.
Appendix 2 Enhanced recovery structured proforma
| Name and details of ER contact (including address, e-mail, telephone no.): |
| Is ER your main job role or is it in addition to your core job requirements? |
| When was the current ER programme started? |
| Were incentives received to implement the ER programme? E.g. Commissioning for Quality and Innovation (CQUINS), Best Practice Tariffs |
| Were elements of the ER pathway fully or partly implemented? (Primary care/pre-hospital admission, pre-operative, intra-operative, post-operative, and discharge elements) |
| Have you evaluated the programme? If so, do you have any findings you can share with us (e.g. improved clinical outcomes, reduced length of stay, improved pathway/process, improved staff satisfaction) |
| Have you surveyed patients about their experience – how did you sample – what questions did you ask – what were the findings? |
| Do you have details of costs associated with ER programme and implementation. (Approximate costs of ER including staff time/costs, overheads, etc.) |
| Which elements of the ER programme have been most successful? Including list of benefits (e.g. benefits to patients, staff, and local health community, improvements in quality and productivity) |
|
Was an ER facilitator appointed? If so, was their role temporary, seconded or permanent? If temporary or secondment – was this to get the programme started? Was there a clear expectation that ER would become ‘core business’ and spread to additional specialties?
What is/was the primary role of the ER Facilitator within your organisation? |
| Were there any barriers to implementing and/or sustaining ER programmes? E.g. reluctance to change, lack of support, lack of funding, lack of incentives (e.g. engagement of an Executive Sponsor or involvement of the Trust Board) |
| Are you aware of any other specialties within your organisation currently adopting ER? |
| What are the key lessons you have learned that you would pass on to others? |
Appendix 3 Systematic review characteristics
| Author | Surgical specialty | Number of included studies | Included study designs | Follow-up duration |
|---|---|---|---|---|
| Colorectal/colon surgery | ||||
| Adamina (2011)20 | Colorectal | 6 | RCTs | 30 days |
| Ahmed (2012)21 | Colorectal | 11 | NR | NR |
| Eskicioglu (2009)24 | Colorectal | 4 | RCTs | NR |
| Gouvas (2009)25 | Colorectal | 11 | 4 RCTs, 7 non-randomised case–control studies | 10 to 14 days or 30 days |
| Khan (2010)27 | Colorectal | 10 | 4 RCTS, 6 non-randomised comparative studies | 0 to 6 days after surgery, 7 to 21 days after surgery, > 30 days after surgery |
| Lv (2012)35 | Colorectal | 7 (one RCT analysed as two RCTs) | RCTs | 30 days post operation |
| Rawlinson (2011)29 | Colorectal | 13 | 6 RCTs and 7 non-randomised clinical trials | 30 days in 12 studies, 14 days in 1 study |
| Spanjersberg (2011)30 | Colorectal | 6; although 2 did not meet inclusion criteria and were not included in primary analyses | RCTs | 10 to 30 days |
| Varadhan (2010)31 | Colorectal | 6 | RCTs | 30 days (data obtained from authors for one trial) |
| Walter (2009)32 linked to CRD DARE abstract (accession no. 12009106957) | Colorectal | 4 | 2 RCTs, one quasi-randomised trial, 1 cohort | 30 days |
| Wind (2006)33 linked to CRD DARE record131 | Colon | 6 | 3 RCTs, 3 CCTs | 30 days, where reported |
| Gynaecological surgery | ||||
| Lv (2012)34 | Gynaecological | 0 | NA (RCTs and quasi-randomised trials were eligible but none were found) | NA |
| Liver/pancreatic surgery | ||||
| Coolsen (2012)22 | Liver resection, including hemi-hepatectomy, metastasectomy, sectionectomy, central resection and repeat hepatectomy | 6 | 3 case–control, 2 RCTs (both arms ERAS elements; equivalent to prospective case series), 1 retrospective case series | 30 days to 6 months (5 studies) |
| Coolsen (2013)23 Link to76 | Pancreatic resection | 8 | 5 case–control (historical controls receiving traditional care); 2 retrospective case series; 1 prospective case series | 30 days |
| Hall (2012)26 | Hepatopancreatobiliary | 10 | 2 studies with a single intervention in 1parameter of perioperative care but within an ERAS programme (including one RCT); 6 prospective case series comparing ERAS programmes vs. historical controls, 1 retrospective case study, and 1 multicentre study | NR |
| Various surgical specialties | ||||
| Lemmens (2009)28 | Colonic/colorectal, pancreatic, gastric | 13 | 1 RCT, 3 controlled clinical trials, 2 case–control, 1 retrospective case series, 6 pre- and post-pathway studies | NR |
| Sturm (2009)7 | Various | 11 RCTs plus 1 systematic review (Wind 2006)33 | RCTs and systematic review | Range from 3 to 90 days |
Appendix 4 Individual enhanced recovery after surgery elements
| Pathway elements | Bariatric surgery | Colorectal surgery | Gastric surgery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lemanu (2013)67 | Garcia-Botello (2011)64 | Wang (2011)59 | Wang (2012)58 | Yang (2012)60,71 | Ren (2012)69 | Ionescu (2009)65 | Lee (2011)66 | Chen (2012)62 | Liu (2010)68 | Wang (2010)70 | Kim (2012)63 | |
| Preoperative | ||||||||||||
| Pre-admission info/counselling | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Fluid and carbohydrate loading | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| No prolonged fasting | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| No/selective bowel preparation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Antibiotic prophylaxis | ✓ | |||||||||||
| Thromboprophylaxis | ✓ | |||||||||||
| No pre-medication | ✓ | ✓ | ✓ | ✓ | ||||||||
| Intraoperative | ||||||||||||
| Short-acting anaesthetic agents | ✓ | ✓ | ||||||||||
| Mid-thoracic epidural anaesthesia/analgesia | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| No drains (unless necessary) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Restricted fluids | ✓ | ✓ | ✓ | ✓ | ||||||||
| Maintenance of normothermia (body warmer/warm intravenous fluids) | ✓ | ✓ | ✓ | ✓ | ||||||||
| Postoperative | ||||||||||||
| No nasogastric tubes/reduced use | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Prevention of nausea and vomiting | ✓ | ✓ | ✓ | ✓ | ||||||||
| Avoidance of salt and water overload | ✓ | |||||||||||
| Early removal of catheter/drains | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Early oral nutrition | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Non-opioid oral analgesia/NSAIDs | ✓ | ✓ | ✓ | ✓ | ||||||||
| Early mobilisation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Stimulation of gut motility | ✓ | ✓ | ✓ | ✓ | ||||||||
| Audit of compliance and outcomes | ✓ | |||||||||||
| Other | Standardised anaesthesia; multimodal analgesia | High levels of O2; early removal of nasogastric tube | Plan discharge; minimally invasive surgery | Early removal of nasogastric tube | Follow-up calls | Multimodal analgesia | Preoperative risk assessment/continual infusion of PCA | Minimally invasive incision | Hospital telephone numbers provided | Additional pain relief; O2 inhalation | ||
Appendix 5 Systematic reviews: clinical outcomes
| Author and no included studies | Length of hospital stay (days) | Mobilisation outcomes (hours/days) | Mortality (N/%) | Morbidity (condition/N/%) | Pain (measure/N/%) | Readmission rates (N/%) | Reintervention rates (N/%) |
|---|---|---|---|---|---|---|---|
| Colorectal/colon surgery | |||||||
| Adamina (2011)20
6 studies |
Primary LOS: ERAS reduced stay by 2.5 days (95% CrI −3.92 to −1.11 days) | NR | 30-day: four deaths (two ERAS, one control, one unclear) | 30-day: ERAS halved morbidity (RR 0.52, 95% CrI 0.36 to 0.73); for every 4.5 patients (95% CrI 2.9 to 9.3) following ERAS, one complication was avoided | NR | ERAS did not increase readmission rates (RR 0.59, 95% CrI 0.14 to 1.43) | NR |
| Ahmed (2012)21
11 studies |
211 days (10 studies) | NR | NR | NR | NR | 0% to 22% (eight studies). Shortest LOS (2 days) associated with highest readmission rate (22%) | NR |
| Eskicioglu (2009)24
4 studies |
Trials were not pooled because of reporting limitations. Three out of four trials reported a significantly shorter length of primary hospital stay in the ERAS group. Two trials reported overall hospital stay, both of which found a significantly reduced LOS in the ERAS group | NR | Postoperative mortality: four deaths (1/68 ERAS, 3/66 control); no significant difference between groups (RR 0.53, 95% CI 0.12 to 2.38; three trials, I 2 = 0%) | Total complications: 28/99 ERAS, 46/99 control; significantly reduced in ERAS group (RR 0.61, 95% CI 0.42 to 0.88; four trials, I
2 = 0%) Major complications: 6/99 ERAS, 20/99 control; no significant difference between groups (RR 0.40, 95% CI 0.06 to 2.59; four trials, I 2 = 63%) Minor complications: 24/99 ERAS, 37/99 control; no significant difference between groups (RR 0.67, 95% CI 0.37 to 1.23; four trials, I 2 = 41%) |
NR | 7/99 ERAS, 11/99 control; no significant difference between groups (RR 0.67, 95%CI 0.20 to 2.19; four trials, I 2 = 24%) | No significant difference between groups (RR 0.35, 95% CI 0.04 to 3.23; one trial) |
| Gouvas (2009)25
11 studies |
Significantly reduced primary hospital stay with fast track: 3.3 to 6.7 days/5.8 to 10.0 days (WMD −2.35, 95% CI −3.24 to −1.46; nine studies, I 2 = 75%). Similar results in subgroup analysis. Significantly reduced total hospital stay with fast track: 4.0 to 5.5 days/6.5 to 13.0 days (WMD −2.46, 95% CI −3.43 to −1.48; five studies, I 2 = 0%). Similar results for subgroup analysis | NR | ERAS 0% to 5%; control 0% to 9%; difference NS (seven studies). NB small numbers, therefore, % deceptive | ERAS 4% to 47%; control 8% to 75%. Significantly reduced with fast track: (RR 0.56, 95% CI 0.45 to 0.69; nine studies, I 2 = 0%). Similar for subgroup analysis | Not possible to analyse due to heterogeneity | 0% to 24%/0% to 20%: NS (RR 1.37, 95% 0.97 to 1.92; 10 studies, I 2 = 0%). Subgroup analysis showed that non-RCTs had significantly lower readmission rates in the control group | NR |
| Khan (2010)27
10 studies |
NA | NA | NA | NA | Significantly increased immediate postoperative pain in conventional surgery (one RCT, one non-randomised study). Significantly more pain in ERAS patients at discharge (one RCT), three other studies (two RCTs and one non-randomised study) reported NS differences between groups. No difference in pain levels at 2 to 3 weeks after discharge (five studies) | NA | NA |
| Lv (2012)35
7 studies (7 RCT analysed as 2 RCTs) |
Total LOS significantly shorter for ERAS-treated patients (MD −1.88 days, 95% CI −2.91 days to −0.86 days; seven RCTs/eight comparisons, I 2 = 75%). Sensitivity analysis did not significantly alter the results | NR | No significant differences between treatment groups: (RR 1.02, 95% CI 0.40 to 2.57; seven RCTs/eight comparisons, I 2 = 0%). NB I 2 = 0% but forest plot looks heterogeneous | ERAS patients experienced significantly less complications (RR 0.69, 95% CI 0.51 to 0.93; seven RCTs, I 2 = 59%). There were no statistically significant differences between groups for rates of major or minor complications | NR | No statistically significant differences between groups (RR 0.90, 95% CI 0.52 to 1.53; seven RCTs/eight comparisons, I 2 = 0%) | NR |
| Rawlinson (2011)29
13 studies |
11 studies reported on primary hospital stay, of which 10reported a significantly shorter stay in the ERAS group | NR | Mortality ranged from 0% to 5% in ERAS groups and 0% to 9% with traditional care; eight studies; no significant difference between groups | Morbidity (postoperative complications) ranged from 4% to 47% with ERAS, and from 8% to 75% with traditional care; 10 studies. TwoRCTs and one CCT reported significant differences favouring ERAS; other studies found no significant difference between groups | NR | Readmissions ranged from 0% to 24% with ERAS and from 0% to 20% with traditional care; 12 studies; no significant difference between groups | NR |
| Spanjersberg (2011)30
6 studies (2 did not meet inclusion criteria and were not included in primary analyses) |
Statistically significantly reduced in ERAS patients (MD −2.94 days, 95% CI −3.69 days to −2.19 days; four RCTs, I
2 = 0%) Subgroup analyses including the two RCTs involving limited number of ERAS elements did not significantly alter the findings |
Mobilisation better in ERAS patients on postoperative day 0 (one study) and day 1 (one study) | ERAS 1 (0.4%); control 3 (1.3%) No statistical difference between groups (RR 0.53, 95% CI 0.12 to 2.38; four RCTs, I 2 = 0%) |
Overall complications ERAS 34 (28.5%); control 67 (56.8%) ERAS patients showed statistically significantly less complications (RR 0.52, 95% CI 0.38 to 0.71; four RCTs, I 2 = 0%) Subgroup analyses including the twoRCTs involving limited number of ERAS elements did not significantly alter the findings Major complications (including mortality) ERAS 6 (8.8%); control 14 (21.2%). No significant difference between groups (three RCTs, I 2 = 57%). Subgroup analyses including the two RCTs involving limited number of elements resulted in statistically significantly fewer major complications in ERAS patients (RR 0.50, 95% CI 0.28 to 0.92; I 2 = 21%) Minor complications ERAS 17 (25%); control 26 (39.4%). No significant difference between groups (three RCTs, I 2 = 0%). Subgroup analyses including the two RCTs involving limited number of elements resulted in statistically significantly fewer major complications in ERAS patients (RR 0.57, 0.38 to 0.85; I 2 = 0%). Undefined complications (not serious) ERAS 11 (22%); control 27 (52%). Statistically significant difference in favour of ERAS (RR 0.42, 95% CI 0.23 to 0.75; 1 RCT) Subgroup analyses by trial quality did not significantly alter the findings |
1 RCT using epidurals only for ERAS patients showed a significant increase in pain post operatively in control patients compared with no increase in ERAS patients. One RCT showed no significant differences in the amount of opiates used or in pain scores between groups. Four RCTs used epidural analgesics, three did not report pain scores, one RCT reported statistically significantly lower VAS scores for ERAS patients between postoperative days 0 and 5 (p = 0.001) | ERAS 4 (3.3%); control 5 (4.2%). No significant difference between groups (I 2 = 59%, four RCTs). Subgroup analyses including the two RCTs involving limited number of ERAS elements did not significantly alter the findings | NR |
| Varadhan (2010)31
6 studies |
Primary hospital stay was significantly shorter in the ERAS group (WMD −2.51 days, 95% CI −3.54 days to −1.47 days; six trials, I 2 = 55%) | NR | 30-day mortality: four deaths (1/226 ERAS, 3/226 control); no significant difference between groups (RR 0.53, 95% CI 0.09 to 3.15; three trials with deaths, I 2 = 0%) | Total complications: 56/226 ERAS, 108/226 control; significantly reduced in ERAS group (RR 0.53, 95% CI 0.41 to 0.69; six trials, I 2 = 0%) | Postoperative pain was an outcome in three studies but quantitative results were not reported | 10/226 ERAS, 13/226 control; no significant difference between groups (RR 0.80, 95% CI 0.32 to 1.98; four trials with events, I 2 = 9%) | NR |
| Walter (2009)32
4 studies |
Total LOS [mean (SD) days]. Statistically significant reduction in ERAS compared with control groups (WMD −3.75 days, 95% CI −5.11 days to −2.40 days; two RCTs, I 2 = 0%). Primary LOS [mean (SD) days]. Statistically significant reduction in ERAS compared with control groups (WMD −3.64 days, 95% CI −4.98 days to −2.29 days; twoRCTs, I 2 = 0%) | NR | No statistically significant difference between groups (RR 0.92, 95% CI 0.15 to 5.83; two RCTs, I 2 = 16.6%) and (RR 2.00, 95% CI 0.51 to 7.83; two CCTs, I 2 = 0%) | No statistically significant differences between groups (RR 0.63, 95% CI 0.39 to 1.02; two RCTs, I 2 = 0%). Statistically significantly less 30-day morbidity in ERAS patients; RR 0.44 (95% CI 0.32 to 0.61; two CCTs, I 2 = 0%) | NR | No statistically significant difference between groups (RR 0.26, 95% CI 0.03 to 2.25; one RCT) and (RR 1.73, 95% CI 1.00 to 3.01; two CCTs, I 2 = 0%). (p = 0.05 which the authors consider significant) | NR |
| Wind (2006)33
6 studies |
Primary hospital stay (mean) statistically significantly lower in the ERAS group (WMD −1.56, 95% CI −2.61 to −0.50, three RCT, three CCTs; I 2 = 52.9%). Subgroup analyses showed similar results for RCTs and CCTs. Overall hospital stay (mean). All three trials showed statistically significantly shorter overall hospital stay in ERAS patients (p < 0.05) | NR | ERAS 0 to 6 (0% to 5%); control 0 to 4 (0% to 9%) (two RCTs, 2 CCTs). No statistically significant differences between groups | ERAS 3 to 33 (8% to 47%); control 4 to 72 (11% to 75%) (two RCTs, two CCTs). Statistically significantly less morbidity in ERAS patients (RR 0.54, 95% CI 0.42 to 0.69, two RCTs, two CCTs; I 2 = 0%). Subgroup analyses showed similar results for CCTs, but RCTs indicated no statistically significant difference between groups (RR 0.67, 95% CI 0.44 to 1.02, three RCTs; I 2 = 0%) | Measures: VAS, McGill Pain Score Questionnaire. Pain and fatigue was significantly higher in the traditional care group in one RCT, but no differences were reported in a second RCT. Another RCT reported no significant differences in pain scores between groups. One CCT reported no significant differences between groups in pain scores, but fatigue was increased in the traditional care group on the first 2 postoperative days | No statistically significant differences between groups (RR 1.17, 95% CI 0.73 to 1.86, two RCTs, three CCTs; I 2 = 23.6%). Subgroup analyses showed similar results in favour of ERAS in RCTs, but in favour of traditional care in CCTs | NR |
| Gynaecological surgery | |||||||
| Lv (2012)34
0 studies |
NA | NA | NA | NA | NA | NA | NA |
| Liver/pancreatic surgery | |||||||
| Coolsen (2012)22
6 studies |
Three comparative studies: ERAS 5–7 days; control 7–11 days: difference (NS one study, p < 0.001 two studies). Non-comparative studies: 4 to 7 days | NR | Three comparative studies: ERAS 0.0% to 1.8%; control 0% to 2%: difference (NS threestudies). Three non-comparative studies: 0 to 2% | Three comparative studies: ERAS 15.3% to 46.4%; control 15.3% to 43.3%: difference (NS three studies). Three non-comparative studies: 16.6% to 19.0% | NR | Three comparative studies: ERAS 0.0% to 13.0%; control 0.0% to 10.0%: difference (NS threestudies). Three non-comparative studies: 0% to 5% | NR |
| Coolsen (2013)23 Link to76
8 studies |
It was unclear whether results were mean or median number of days. Comparative studies ERAS 6.7 to 13.5 days; control 8.0 to 16.4 days (four of five studies reported statistically significant differences in favour of ERAS). Non-comparative studies 10 days (range 4–115 days), three studies | NR (mobilisation achieved according to protocol in approximately 70% to 85% of patients; three studies) | No significant differences between treatment groups (RD 0.2%, 95% CI −1.7% to 2.1%; four studies, I 2 = 0%) | Significant difference in favour of ERAS patients (absolute RD 8.3%, 95% CI 2.1% to 14.5%; four studies, I 2 = 0%). (Looking at individual studies, only the largest study showed a significant difference.) Sensitivity analyses indicated that results for overall complications were mainly influenced by good-quality studies (MINORS score of ≥ 13) and studies with ≥ 13 ER elements | NR | No significant differences (RD 0.8%, 95% CI −2.6% to 4.1%; four studies, I 2 = 0%) | NR |
| Hall (2012)26
10 studies |
Reduced with ERAS programme: Pancreatic 10 to 13 days (range 4–115 days; four studies); liver 4.0–7.2 days (range 2 to 82 days; five studies) | NE | Pancreatic 2.0% to 4.9% (four studies); liver 0% to 3% (six studies) | Pancreatic 38.6% to 47.6% (four studies); liver 1% to 46.4% (six studies) | NR | Pancreatic 3.5% to 14.6% (four studies); liver 0% to 13% (five studies) | NR |
| Various surgical specialties | |||||||
| Lemmens (2009)28
13 studies |
Statistically significant decrease in clinical pathway group in 11 studies; mean number of days decreased from between 5.9 days and 21.7 days to between 3.3 days and 18.5 days (nine studies). Median number of days decreased from between 5 and 13 days to between 2 and 7 days (four studies). Two studies reported no significant difference between groups | NR | Four studies NR; nine studies NS | Decrease in complications statistically significant in clinical pathway group in three studies (21% to 45% reduced to 8.5% to 25.0%); 10 studies NS | NR | One study reported statistically significant reduction (13% to 6%); study studies NR; 10 studies NS | NR |
| Sturm (2009)7
11 RCTs plus 1 systematic review |
Reporting of LOS varied between trials. LOS was clearly significantly shorter in the ERAS group in six trials (three colorectal, three other). There was no significant difference in one trial (lung surgery). In the remaining trials, significance depended on the analysis or was NR | Time from surgery to unaided mobilisation to toilet was significantly shorter in the ERAS group in two trials (p = 0.043 and p < 0.001), with no significant difference in one trial (all in colorectal surgery). (NB table suggests longer time to mobilisation in ERAS group in one trial). In the hip replacement trial, the ERAS group ‘fulfilled mobilisation goals to a greater extent than did the control group’ (p < 0.001) | Two deaths in ERAS groups, five in controls (five trials reported at least one death) | Two RCTs reported significantly fewer complications in the ERAS group, five reported no significant difference between groups and four did not present statistical analyses | Pain was reported in six trials, of which two reported differences favouring the ERAS group on ≥ 1 measures/time points. The other trials reported no significant differences | Eight trials reported on readmission rates. Rates ranged from 0.0% to 9.7% in the ERAS groups and 0% to 20% in the control groups. Only one trial reported a statistically significant difference and this favoured the ERAS group (p = 0.022) | NR |
Appendix 6 Economic evaluations meeting the inclusion criteria
| Study details | Study comparators | Main analytical approaches | Primary outcome(s) and health-related quality of life | Direct and productivity costs | Incremental cost-effectiveness ratio estimates and decision uncertainty |
|---|---|---|---|---|---|
| Salihiyyah (2011)87
UK Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Cardiac surgery inpatients |
FT transfer post surgery to an independent theatre recovery unit 1–2–1 nursing (n = 84) | Economic evaluation based on a single study | LOS duration of intubation | Total expenditure of unit divided by number of patients | The costs and benefits were not synthesised Mean cost FT: £4182 (SD: £2284) Mean cost C: £4553 (SD: £1355) (p < 0.001) Total LOS NSD 8 patients failed FT and were transferred to ICU 5 patients (4 FT and 1 C) required readmission |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| 6 months | Transfer post surgery to hospital ICU (n = 52) | Hospital | NA | One-way and multiway sensitivity analysis demonstrated robustness in result that FT costs less than C | |
| Lin (2011)84
China Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Liver resection inpatients |
Multidisciplinary team, streamlining of preoperative evaluation, education of patients and families, earlier oral feeding, earlier discontinuation of IV, no drains or nasogastric tubes, early ambulation, urinary catheter < 24 hours, planned discharge 6 days post surgery (n = 56) | Economic evaluation based on a single study | LOS complications, mortality andreadmission | Hospital charges: operation and anaesthesia; pharmacy; auxiliary examination; other | The costs and benefits were not synthesised Mean charge pre-pathway RMB 26,626 Mean charge postpathway RMB 21,004 (p < 0.05) LOS reduced from 11 to 7 days (p < 0.005) Complications, mortality and readmissions NSD |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| NR | Conventional pathway (limited reporting) (n = 61) | Hospital | NA | NA | |
| Kariv (2006)86
USA Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Patients undergoing open ileoanal pouch surgery | Presurgery patients provided with FT protocol and documentation of postsurgery milestones. Epidural or analgesia were not used; early food and mobilisation (day of surgery/anaesthesia), patients who lived 100 to 150 miles from hospital discharged to hotel for 1–3 days. Success defined as discharge within 5 days (n = 97) | Economic evaluation based on a single study | LOS, readmission, reoperation | Total costs for each of the categories were presented: per case of hospitalisation; operating room; radiology; anaesthesia; pharmacy; laboratory; ICU; and nursing care | The costs and benefits were not synthesised Total per case cost FT US$5692 Total per case cost C US$6672 Difference US$980 (p = 0.001) Median postoperative LOS FT = 4 days, C = 5 days (p = 0.012) NSD in readmission outcomes |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| 30 days | Based on professional preferences of surgeon; no supporting documentation; sat out of bed on POD 1, walked POD 2; food withheld until stool or flatus (n = 97) | Hospital | NA | NA | |
| Yanatori (2007)88
Japan Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Cardiovascular surgery (cardiac arrest requiring cardiopulmonary bypass) | Admitted 4 days prior to surgery, preoperative education by nurses, surgeons and rehab staff; discharge at day 7 post surgery | Economic evaluation based on a single study | LOS, complications | Only total costs were presented | The costs and benefits were not synthesised Total mean cost for FT YEN712,545 Total mean cost for C YEN383,268 (p = 0.038) Mean postoperative LOS FT = 15 (12.4) C = 36.7 (6) (p = 0.01) |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| 2 years | Conventional protocol – details not reported | Health-care provider/hospital | NA | NA | |
| Larsen (2009)85
Denmark Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| All patients for elective primary total hip/knee arthroplasty or unicompartmental knee arthroplasty | Patients receive information pre hospitalisation; separate ward; one nurse in charge of multidisciplinary nurses, occupational therapists, and physiotherapists; nutrition screening and special focus on daily consumption of 1.5 L fluid (including two protein beverages); mobilisation of patients and exercise started on day of surgery; intensive mobilisation of patients in teams; 8 hours of mobilisation daily (n = 45: 28 total hip; 15 total knee; 2 unicompartmental knee) | Economic evaluation based on a single study | LOS, adverse events (first 3 months) |
Patients followed over 1 year. Resource use: based on patient level mix of activity based costing and step down methods. Discharge to 3 months cost diary | Accelerated intervention was both more effective and less costly than the comparator Average total cost for I DKK90,227 (+/− 47,475) Average total cost for C DKK71,344 (+/− 39,958) Average QALYs was 0.83 for the intervention and 0.78 in the comparator Average QALY gain for hip patients I vs. C = 0.08 (CI: 0.02 to 0.05) (p = 0.006) Average QALY gain for knee patients was not significant |
| Time horizon | Comparator | Perspective | Health-related quality of life | Productivity costs | Uncertainty |
| 1 year | Patients receive info on day of admission; patients randomly among wards, various nurses in charge of care; and various occupational therapists and physiotherapists responsible for mobilisation; mobilisation of patients and exercise started on first postoperative day; individual and gradual mobilisation according to patient tolerance; 4 hours’ mobilisation daily (n = 42: 28 total hip; 12 total knee; 2 unicompartmental knee) | Societal | QALYs (European Quality of Life-5 Dimensions) (baseline to 3 months) | Average wage rate for age-specific groups | Bootstrapping, uni and multivariate |
| Sammour (2010)81
New Zealand Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Elective colonic resection patients >1 5 years old | Emphasised structured nursing care pathways within an environment focusing on early recovery and various perioperative strategies to improve patient functional recovery (n = 50) | Economic evaluation based on a single study | LOS, complications and readmissions | Total cost of protocol development, inpatient stay, outpatient appointments, treatment costs, readmission and complication costs were all considered. Data on patient resource use was collected from their records. Readmission costs and complication costs were based on hospital records/costs | The costs and benefits were not synthesised The implementation of the intervention protocol cost approximately NZ$102,000 for the first 50 patients (set-up costs included) Cost/patient with NZ$16,052.35 Cost/patients without NZ$22,929.74 Cost-saving NZ$6900/patient Postoperative LOS ERAS: 4 (3 to 34); C: 6.5 (3 to 18) (p < 0.001) Total LOS ERAS: 4 (3 to 34); C: 8(4 to 29) (p < 0.001) Readmissions NS Complications – overall 54% in ERAS ≥ 1 compared with 66% comp |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| Unclear | Conventional non-structured perioperative care (n = 50) | Health-care provider | NA | NA | |
| King (2006)82
UK Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Surgery for colorectal cancer | Preoperative counselling, epidural analgesia, early feeding and mobilisation, predetermined discharge aim (n = 60) | Economic evaluation based on a single study | Postoperative LOS, complications and readmissions |
Resource use data were reported to be individual patient level, but not reported. Direct costs included: theatre (including pre and recovery), hospital (including ICU), postoperative (including re-operation), chemotherapy and radiotherapy, follow-up at 3 months | The costs and benefits were not synthesised otal costs of care for patients receiving the intervention: £7327.47; for those receiving comparator: £7998.18 Postoperative LOS significantly reduced, intervention cohort staying 49% as long as comparator (95% CI 39% to 61%; p < 0.001) No significant difference in quality of life, readmissions, reoperations or complications |
| Time horizon | Comparator | Perspective | Health-related quality of life | Productivity costs | Uncertainty |
| 2 years | Conventional care (fully reported) included no epidural, no formal mobilisation plan, no predetermined discharge (n = 86) | UK NHS stated by author, although inclusion of productivity costs suggests wider societal perspective | European Organisation for Research in the Treatment of Cancer QLQ-C30 | Average earnings based on employment status at commencement of trial | NA |
| Nielsen (2008)83
Denmark Hospital setting |
|||||
| Study population | Intervention | Primary outcome | Direct costs | Results | |
| Lumbar fusion patients with degenerative lumbar disease | Integrated programme including: information and education, optimal operation technique, better pain reduction, early nutrition and aggressive postoperative mobilisation (n = 28) | Economic evaluation based on a single study | Measured using 15D-score (self-reported at inclusion, day of surgery, day of discharge, and 1, 3 and 6 months post operation | Three categories of cost considered: staff resources, equipment and purely bed costs Bed costs included salary of nurses/porters, food, clothes, laundry and cleaning. Post discharge for 3 months GP visits, physiotherapy appointments and emergency room contact was registered and included |
The costs and benefits were not synthesised Intervention direct cost €1174/patient compared with €1668/patient for standard care Intervention productivity costs were €8021 compared with €9152 for standard care No significant difference in health-related quality-of-life scores |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| 6 months | Standard care, not including components above (n = 32) | Societal | Based on return to work rates and Danish average daily wage | Optimistic and pessimistic scenarios varying individually preoperative costs, postoperative hospital costs, direct costs, and productivity costs | |
| Reilly (2005)79
UK Hospital setting |
|||||
| Study population | Intervention | Primary outcome | Direct costs | Results | |
| Patients undergoing unicompartmental knee arthroplasty | Accelerated discharge: aim to discharge day after surgery (n = 20) | Economic evaluation based on a single study | Oxford Knee Assessment | Fixed costs (surgical staff, anaesthetics, prosthesis, pharmacy), outpatient appointment, specialist registrar time | The costs and benefits were not synthesised Intervention resulted in a 6 month Oxford Knee Assessment score of 43.7 (SD 3.7) compared with 42.2 (SD 7.1) for standard care (NS) Total costs for intervention per patient £3391 compared with £4634 for standard care |
| Time horizon | Comparator | Perspective | Productivity costs | Uncertainty | |
| Unclear | Standard discharge: approximately 5 days post surgery (n = 21) | Hospital | NA | NA | |
| Archibald (2011)80
USA Hospital setting |
|||||
| Study population | Intervention | Primary outcomes | Direct costs | Results | |
| Colorectal surgery patients | The availability of patient education, fluid managements, opioid-sparing strategies, tube and drain protocols, ambulation, feeding protocol, and discharge criteria. All based on surgeon’s choice. (n = 1358; 588 enrolled in ERAS and 770 not enrolled) | Economic evaluation based on a study comparing two time periods, where ERAS was available in one and not in the other | LOS, POD and readmission |
Hospital costs (total direct and indirect costs identified via hospital billing system) | The costs and benefits were not synthesised Mean LOS for the intervention was 8.4 days compared with 6.9 days for the comparator (p < 0.0001) Mean POD for the intervention was 7.6 days compared with 6.3 days (p < 0.0001) Mean hospital cost for the intervention population was US$18,741 compared with US$16,978 for the comparator |
| Time horizon | Comparator | Productivity costs | Uncertainty | ||
| Unclear | Standard care historical baseline (n = 1673) | NA | NA |
Appendix 7 Enhanced recovery programme implementation, by surgical specialty (number of sites)
| Specialty | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust89,93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colorectal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Urology | ✓ | ✓ | ✓ | 3 | |||||||
| Musculoskeletal | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | |||||
| Gynaecology | ✓ | ✓ | ✓ | ✓ | 4 |
Appendix 8 Reason for starting enhanced recovery programmea
| Reason | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust89,93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous experience (state where ER not previously sustained) | ✓ (Not sustained – no details provided) | ✓ Consultants had previously tried to implement | 2 | ||||||||
| Improve patient experience/quality of care | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Improve patient length of stay | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | |
| Reduce complications | ✓ | 1 | |||||||||
| Other (state) | ✓ Consultant – advisor for enhanced recovery; benefits for staff and trust | ✓ Early mobilisation of patients and gut function | 2 |
Appendix 9 Brief details on enhanced recovery team and rolesa
| Team roles | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust89,93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary care GP | 0 | ||||||||||
| Clinical lead/ER facilitator | ✓ | ✓ | ✓ ERP nurse | ✓ | 4 | ||||||
| Steering group/management (including commissioning manager, performance manager, service improvement manager, etc.) | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Surgical specialty leads/surgeons | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| Anaesthetist | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Nurse (including specialist nurses) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Physiotherapist/occupational therapist | ✓ | ✓ | ✓ | 3 | |||||||
| Patient panel/representative | ✓ | 1 | |||||||||
| Administrator | ✓ | 1 | |||||||||
| Social services representative | 0 | ||||||||||
| Other (state) | ✓ Dietitian | ✓ Stoma therapists | 2 |
Appendix 10 Changes made/enhanced recovery elements introduceda
| Pathway elements implemented | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust89,93,100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | |||||||||||
| Pre-admission info/counselling | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | |
| Fluid & carbohydrate loading | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| No prolonged fasting | ✓ | ✓ | 2 | ||||||||
| No/selective bowel preparation | ✓ | ✓ | 2 | ||||||||
| Antibiotic prophylaxis | 0 | ||||||||||
| Thromboprophylaxis | 0 | ||||||||||
| No pre-medication | 0 | ||||||||||
| Intraoperative | |||||||||||
| Short-acting anaesthetic agents | ✓ | 1 | |||||||||
| Mid-thoracic epidural anaesthesia/analgesia | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| No drains | ✓ | ✓ | 2 | ||||||||
| Avoidance of salt and water overload | ✓ | ✓ | 2 | ||||||||
| Maintenance of normothermia (body warmer/warm intravenous fluids) | ✓ | ✓ | 2 | ||||||||
| Postoperative | |||||||||||
| No nasogastric tubes/reduced use | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Prevention of nausea and vomiting | 0 | ||||||||||
| Avoidance of salt and water overload | ✓ | ✓ | 2 | ||||||||
| Early removal of catheter | ✓ | ✓ | 2 | ||||||||
| Early oral nutrition | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Non-opioid oral analgesia/NSAIDs | ✓ | ✓ | 2 | ||||||||
| Early mobilisation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | |
| Stimulation of gut motility | 0 | ||||||||||
| Audit of compliance and outcomes | ✓ | 1 | |||||||||
| Total | 5 | 1 | 5 | 3 | 2 | 2 | 8 | 7 | 7 | 14 | |
Appendix 11 Barriers to changea
| Barriers to change | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust89,93,100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staff – resistance to change, lack of dedicated ER programme nurse | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Patients – resistance to new programme | ✓ | ✓ | 2 | ||||||||
| Lack of funds/support | ✓ | ✓ | ✓ | 3 | |||||||
| Other (state) | ✓ Staff turnover; unnecessary bureaucracy | ✓ Difficulties in arranging interdisciplinary meetings | ✓ Lack of space for joint school; poor documentation | ✓ Changes need to be ‘cost neutral’; increased impact on physiotherapists/OTs; goodwill of staff not sustainable | ✓ Need for better integration between pre- and postoperative staff; managerial bureaucracy | ✓ Poor documentation and documentation time-consuming | ✓ Staff changes and need for ongoing deviation from pathway | 7 |
Appendix 12 Critical success factors/lessons learneda
| Critical success factors | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust89,93,100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Board approval/management engagement | ✓ | ✓ | 2 | ||||||||
| Multidisciplinary approach essential | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| ER programme project lead and ER nurse to ensure implementation and sustainability even with staff change | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Staff engagement/change in culture | ✓ | ✓ | ✓ | 3 | |||||||
| Robust plan for data collection and implementation | ✓ | ✓ | ✓ | 3 | |||||||
| Other (state) | ✓ Continual education essential | ✓ Regular meetings; feedback from patients | ✓Preoperative information/joint school; Good patient representative | ✓ Continual education essential; sufficient staff to mobilise patients | ✓ Regular meetings; contact other trusts for support; Preoperative information | ✓ Preoperative information; Continual education essential; patient engagement | ✓Preoperative information; Continual education essential | 7 |
Appendix 13 Evidence on improvementsa
| Evidence on improvement | Cambridge University Hospitals NHS Foundation Trust89 | Colchester Hospital University Foundation Trust95 | Hillingdon Hospitals NHS Foundation Trust93,94 | Medway NHS Foundation Trust90 | Northumbria Healthcare NHS Foundation Trust89,93,100 | North Wales NHS Trust101 | Royal Berkshire NHS Foundation Trust91,92 | Salford Royal NHS Foundation Trust96 | Salisbury NHS Foundation Trust97 | Yeovil Foundation Hospital Trust98,99 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved patient experience | ✓ | ✓ | ✓ (Three patients) | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Improved clinical outcomes (including reduced readmissions and complications) | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Reduced length of stay | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| Improved pathway/process | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Improved staff satisfaction | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Other (state) | ✓ Financial gains | Improvements supported by referencesb | 1 |
Glossary
- Care Quality Commission
- Service to ensure health care in England provides people with safe, effective and high-quality care.
- Commissioning for Quality and Innovation
- A framework to secure improvements in quality of services and better outcomes for patients, while also maintaining strong financial management. Incentives and rewards are available to commissioners to drive improvements in care quality.
- Conventional care
- Also referred to as standard, usual or traditional care. Defined differently between studies or not defined.
- Enhanced recovery after surgery
- Also referred to as fast-track recovery, multimodal recovery, rapid recovery and accelerated recovery programmes.
- Gastrectomy
- Procedure to remove all or part of the stomach.
- Salpingo-oophorectomy
- Surgical removal of fallopian tube and ovary.
List of abbreviations
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination, University of York
- DARE
- Database of Abstracts of Reviews of Effects
- ERAS
- enhanced recovery after surgery
- ERPP
- Enhanced Recovery Partnership Programme
- HEED
- Health Economic Evaluations Database
- HTA
- Health Technology Assessment
- ITT
- intention to treat
- MeSH
- medical subject heading
- MINORS
- Methodological Index for NOn-Randomised Studies
- NHS EED
- NHS Economic Evaluation Database
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- PROSPERO
- International Prospective Register of Systematic Reviews
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RD
- risk difference
- RR
- relative risk