Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 09/2001/04. The contractual start date was in November 2010. The final report began editorial review in December 2013 and was accepted for publication in March 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Raine et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter overview The structure of the report
This project consists of two studies. Study 1 examines the determinants of effective decision-making and areas of diversity across MDT meetings. Study 2 uses a consensus development method to develop a list of indications of good practice for MDT meetings. In this report each of the studies is introduced, presented and discussed in turn. Study 1 encompasses Chapters 1–5, study 2 is reported in Chapter 6, and Chapter 7 brings together the findings from the two studies and highlights issues warranting further investigation.
Chapter 1 Study 1: examining the determinants of multidisciplinary team meeting effectiveness and identifying areas of diversity
Background
Multidisciplinary team (MDT) meetings are central to the management of chronic disease and they have become widely established across the NHS1–4 and internationally. 5 The proliferation of MDT meetings in health care has occurred against a background of increasingly specialised medical practice, more complex medical knowledge, continuing clinical uncertainty and the promotion of the patient’s role in their own care. In this environment, it is believed that MDT meetings ensure higher quality decision-making and improved outcomes,6–8 for example by providing a better assessment of treatment strategies and safeguarding against errors. 9
However, the evidence underpinning the establishment of MDT meetings is limited and mixed, and the degree to which they have been absorbed into clinical practice varies widely across conditions and settings. 10–12 One recent trial found multidisciplinary care is associated with improved survival for patients with breast cancer. 13 However, another trial comparing multidisciplinary memory clinics with general practitioner care found no evidence of improved effectiveness. 14 Several recent systematic reviews of MDTs working in cancer, mental health and other disciplines have concluded that there is insufficient evidence to determine their effectiveness. 15–17 In the light of this ambiguity, there have been calls for empirical research on MDT meeting decision-making in routine practice to understand how and under what conditions MDT meetings produce effective decisions. 11
Team features and group processes
Research into the critical factors which have the greatest impact on team effectiveness has established the importance of certain features including clear leadership,18,19 clearly defined goals with measurable outcomes19 and team reflection. 20,21 Some studies have found team ‘climate’, defined as shared perceptions of policies, practices and procedures,22 to be associated with improved performance;23 however, others have found no association. 24 Taylor and Ramirez25 surveyed cancer MDT members, assessing the extent to which they agreed with an extensive list of statements regarding team features potentially important for effective MDT working. They identified several domains that were considered important, including the physical environment, meeting administration, leadership and professional development. However, virtually all respondents agreed with the majority of the statements, making it difficult to prioritise particular issues, and the narrow distribution of responses makes it uncertain whether they are an accurate representation of views or the result of a design effect. Furthermore, their findings may not be generalisable to non-cancer MDTs.
Team structure and group processes are also likely to influence the effectiveness of teamwork. Professional boundaries and hierarchies have the potential to undermine the benefits of multidisciplinarity. 21,26 Previous research has demonstrated that team diversity can create communication barriers arising from differences in knowledge, skills, ability, professional identity21 and status. 27 The unidisciplinary nature of professional education may hinder collaboration as professional groups struggle to assert the primacy of their own theories of illness. 28,29 A recent survey of community mental health teams (CMHTs) across 67 mental health trusts found that CMHT members felt satisfied with multidisciplinary working, but that there were still cases of ‘silo working’ and concerns about the ‘dilution’ of core professional skills. 30
Hierarchical differentiation and the inability of some MDT members to freely communicate may undermine decision-making. A recent systematic review of cancer MDT meetings found that MDT decisions are typically driven by doctors, with limited input from nurses. However, where there was active involvement of nurses, this improved perceptions of team performance. 17 Howard and Holmshaw31 found that a lack of engagement in MDT discussions and a lack of ownership and follow-up of discussed plans hindered effective teamworking.
Context
Context is the environment or setting in which the MDT meeting is located. This incorporates social, economic, political and cultural influences. 32 Organisational research has demonstrated the influence of context on team behaviour and performance. 33 However, a recent systematic review found that the majority of studies investigating health-care team effectiveness have not addressed the impact of the context in which teams operate. 34 Health-care management research has drawn a distinction between the outer (national, sectoral or health-care issue-based) and the inner (local) context, which includes both ‘hard’ features such as the degree of structural complexity and ‘soft’ features such as culture. 35 Team climate (described in the previous section, Team features and group processes) may be considered an aspect of the local ‘soft’ context. Models of ‘receptive contexts for change’ have suggested that improvement in health care is most likely to occur where relationships are good, learning, teamwork and a patient focus are emphasised, and the larger system and environment are favourable. 36
Comparative assessments of a range of MDT meetings are needed to explore how contexts vary and how MDT meetings respond to both national and local influences. Although MDT working has been endorsed at a high level for chronic disease management,2 each of the disease types under study in this report is underpinned by different policies and guidance, which contain varying levels of detail.
Cancer multidisciplinary teams
Specialist multidisciplinary teams in cancer were initially introduced in response to the Calman–Hine Report published in 1995. 37 This was followed in 2000 by the NHS Cancer Plan,38 which documented the progress made in establishing specialist teams for the most common cancers (breast, colorectal and lung) and recommended the extension of MDTs for other cancer types. Specifically, the NHS Cancer Plan committed to ensuring that all patients were reviewed by tumour-specific cancer MDTs from 2001. 38 Additional resources were made available, and there was significant commitment at both national and local levels to support these reforms.
By the time the Cancer Reform Strategy was published in 2007, approximately 1500 cancer MDTs had been established in England. 39 The strategy confirmed that MDT working was to remain the core model for cancer service delivery. This was reiterated when the coalition government published its national policy document for cancer in 2011, which concluded that MDTs should remain the cornerstone of cancer care, in recognition of support for the model from the National Institute for Health and Care Excellence (NICE) and clinicians. 40
In addition to these national policies, tumour-specific guidance has been published by NICE41 and the National Cancer Action Team (NCAT). 1,42,43 The NCAT guidelines set out measures relating to the composition of teams, attendance at MDT meetings and the frequency of meetings, in addition to stating which patients should be discussed and the need for documentation of MDT decisions. Since 2001, MDTs have been audited annually to ensure they fulfil these requirements. 10
Community mental health teams
In contrast, although MDTs are long established in mental health, their development has been much more ‘bottom up’, more locally varied44 and less well defined than in cancer. Multidisciplinary teams providing mental health services for adults based outside hospitals began to emerge in the 1970s, bringing together social workers from local authorities and NHS clinicians in ‘community mental health centres’. 45 These were criticised, however, for having a lack of clear service objectives, and for providing limited services. 46 The National Service Framework for Mental Health (NSF), published in 1999, provided significant funds to reorganise and improve the quality of mental health services to address the wide geographical variations in provision. 47 However, there are still concerns that mental health services are underfunded. 48 The NSF promoted the use of more specialist teams, including Crisis Resolution and Home Treatment teams as well as teams for those experiencing a first episode of psychosis (Early Intervention Services). Generic CMHTs have retained an important role, however, continuing to care for the majority of mentally ill people in the community and functioning as a key source of referrals to these more specialist teams. 49
In contrast with the detailed tumour-specific guidance for cancer MDTs, there is little guidance on the structure and processes that teams should follow for MDT meetings within community mental health. Recommendations that do exist state that assessments and reviews of patients should be routinely discussed by the whole team in a timetabled weekly meeting where actions are agreed and changes in treatment are discussed, and that these weekly meetings should include the consultant psychiatrist. There is also some guidance about the different professions that should be members of the team, but the guidance explicitly avoids being ‘too prescriptive’ in order to allow local flexibility. 49 Recently, however, the Care Quality Commission has committed to developing definitions of ‘what good looks like’ in mental health and is currently developing an assessment framework of indicators to facilitate quality inspections. 50 It remains to be seen whether or not this will lead to more specific guidance on multidisciplinary working.
Heart failure multidisciplinary teams
The detailed guidance for cancer MDTs is also in marked contrast to heart failure MDTs, where they appear to have evolved in response to local needs. In 2003, NICE recommended that heart failure care should be delivered by a multidisciplinary team. 51 The August 2010 NICE update reiterated the pivotal role of the multidisciplinary team in the continuing management of the heart failure patient. 52 This specified that patients should be referred to the specialist multidisciplinary heart failure team for initial diagnosis of heart failure; management of severe heart failure; heart failure that does not respond to treatment; and heart failure that can no longer be managed effectively in the home setting.
In addition to this national guidance, the NICE commissioning guide, Services for People with Chronic Heart Failure,53 provided a number of best-practice service models for integrated care. It proposed that regular MDT discussion of patients should involve review of people with chronic heart failure covering outcomes, current care plans and possible improvements as well as potential discharge or onward referrals. 53 However, there is no further detail on processes or structures for heart failure MDT meetings.
Memory clinics
In 2001, the National Service Framework for Older People was published, which recommended that all specialist mental health services for older people include memory clinics. 54 Memory clinics specialise in the diagnosis and treatment of people with memory impairment, delivered by a core multiprofessional team. 55 However, existing evidence demonstrates that there are major variations in practice between teams. 56 Guidance on formal team meetings to make diagnosis or treatment decisions is limited, with recommendations on developing a service specification for memory clinics stating that only care providers should draft a care plan for dementia patients ‘in consultation with other relevant disciplines’. 57
Summary
This overview of the different national policies that underpin MDT working highlights the degree of variation in the context within which different MDTs operate. While NHS policy for cancer, and to a more limited extent in mental health, provides guidance on MDT meetings, in other specialties the format is locally determined. It is unknown whether such flexibility is appropriate or undermines the productivity of meetings.
Patient-related factors
The impact of patient-related factors upon MDT meeting decisions also requires examination. Although reducing health-care inequalities is a key aim of government policy,58 there remains evidence of inequalities in health-care use according to socioeconomic circumstances, age, ethnic group and sex, having adjusted for clinical need. 59–61 In heart failure, some studies have found socioeconomic variations in the use of services such as angiography and heart surgery. 62–64 In mental health, there is mixed evidence of an association between socioeconomic circumstances and contact with psychiatric services, with some finding associations between socioeconomic variables, such as low income, low education and unemployment, and contact with psychiatric services65 and other studies finding no such association. 66–68 One of the main aims of the NHS Cancer Plan for England was to tackle inequalities in cancer survival for people from deprived or less affluent backgrounds. 38 Whereas some studies have reported no difference in treatment by socioeconomic circumstances,69 others have demonstrated evidence of lower rates of surgical treatment for patients in more deprived areas70,71 as well as differences in the use of chemotherapy72 and radiotherapy. 73,74
If patients’ sociodemographic characteristics influence MDT effectiveness, then explanations such as the influence of patient preferences and comorbidities should be sought. The involvement of patients in decision-making is now widely advocated75 and guidelines state that patient preferences should be taken into account when managing their care. 40,50,76–78 The influence of patient-centred factors such as patient preference and comorbidity on MDT decision-making has been shown to be partly dependent on variation in the presence and participation of particular professional groups (e.g. specialist nurses). 79,80 There is also some evidence to suggest that MDT decisions that take account of patient preferences are more likely to be implemented. 81,82 There is, therefore, a need to explore how best to obtain and consider patient preferences in MDT meeting decision-making.
Measuring effectiveness
There is debate in the literature concerning the most appropriate outcome for evaluating the effectiveness of MDTs. Measures used include health-care use, patient and team member satisfaction, and well-being, as well as various health outcomes. Each measure has limitations; for example, for patient satisfaction, it is difficult for patients to separate satisfaction with a clinical intervention from the benefits of teamwork. 8
The underlying rationale for MDT meetings is to produce higher-quality decisions that lead to improved health outcomes. However, health improvement is affected by factors other than the quality of care. These factors include subsequent onset of unrelated morbidity, change in patient’s personal circumstances, extent to which patients adhere to treatment and efficiency of care provision. 8,83 Furthermore, intended health outcomes may not be evident for a year or more. Finally, many outcomes are specific to particular illnesses, making it impractical to compare MDTs for different conditions. It is for these reasons that other researchers have robustly defended the use of process measures over outcomes, provided that the process measure clearly lies on the pathway to health improvement. 84
However, the identification of an indicator of high-quality decisions is also difficult. While some decisions can be compared with best practice guidelines, the latter rarely specify recommended courses of action for every management decision made for a patient. Moreover, guidelines rarely consider how decisions should be modified for patients with comorbidities or other factors (such as their social circumstances or preferences) which will influence decision-making. Finally, guidelines are not available for all the conditions considered in MDT meetings. We identified decision implementation as a useful process measure of effectiveness because it lies on the pathway to health improvement and reflects effective team decision-making that has taken account of relevant clinical and non-clinical information. For example, MDT meeting decisions may not be implemented because of a lack of meaningful patient involvement, failure to consider comorbidities, lack of agreement with the decision among those who have to implement it, and incomplete clinical information available. 85,86 Furthermore, decision implementation can be measured and compared across MDTs for different conditions. Investigation of a diverse range of MDT meetings may enable identification of factors associated with effective decision-making that are generalisable across health care. For these reasons, the measure of MDT meeting effectiveness used in this research is decision implementation, and when the term ‘MDT meeting effectiveness’ is used in this report it refers to decision implementation.
Given the widespread presence of MDTs, the opportunity costs for the NHS of unwarranted variations in team membership and processes and the consequences for patients of inequitable care, we undertook a prospective cohort study of chronic disease MDT meetings across the North Thames area (North London, Essex and Hertfordshire).
Study 1 examined the effect of a range of team and patient features on decision implementation. We collected quantitative and qualitative data, including clinical and sociodemographic data, through non-participant observation of MDT meetings, review of patients’ medical records and interviews with professionals and patients.
In study 2, we drew on these quantitative and qualitative findings and used formal consensus methods to derive a series of generalisable recommendations for modifying the structure and process of MDT meetings to improve the quality of decision-making.
Purpose of the research
Main research question
How can we improve the effectiveness of decision-making in MDT meetings for patients with chronic diseases?
Aims
-
To identify the key characteristics of chronic disease MDT meetings that are associated with decision implementation.
-
To derive a set of feasible modifications to MDT meetings to improve effective MDT decision-making for patients with chronic diseases.
Objectives
-
To undertake an observational study of chronic disease MDT meetings to identify factors which influence their effectiveness in terms of decision implementation (study 1).
-
To explore the influence of patient preferences and comorbidity on any socioeconomic variations in implementation found (study 1).
-
To explore areas of diversity in beliefs and practices across MDT meetings (study 1).
-
To use the results from study 1 in an explicit structured formal consensus technique to derive a set of feasible modifications to improve MDT meeting effectiveness (study 2).
Chapter summary
In this chapter we have outlined the background to the study and described the relevance to MDT team effectiveness of the national and local contexts within which MDTs operate, of their distinctive features and group processes, and of patient characteristics. We have discussed the complexity of measuring effectiveness in this context, and outlined our rationale for investigating treatment plan implementation. Finally, we outlined the aims and objectives of the study. In Chapters 2 and 3, we discuss the methods through which these aims and objectives were addressed.
Chapter 2 Study 1 methods
We undertook a prospective cohort mixed-methods (quantitative and qualitative) study of MDT meetings in 12 adult chronic disease MDTs across North Thames between December 2010 and December 2012. We examined one skin cancer, one gynaecological cancer, two haematological cancer, two heart failure, two psychiatry of old age (memory clinic) and four community mental health MDTs (including one early intervention service for psychosis).
Ethics and research governance permissions
This study was approved by East London Research Ethics Committee (10/H0704/68) and the National Information Governance Board (NIGB) for Health and Social Care [Ethics and Confidentiality Committee (ECC) 6-05 (h)/2010]. The NIGB ECC provided permission, under Section 251 of the NHS Act 2006,87 to process patient identifiable information without consent. We gained permissions from the relevant research and development (R&D) departments to collect data following submission of site-specific information forms (using the Integrated Research Application System) at each of the NHS organisations that participated in the study.
Recruitment of multidisciplinary teams
We aimed to include a diverse range of teams and chronic conditions to allow examination of key issues about MDT decision-making and implementation. We sought to include teams which varied in terms of health-care context, professional mix of team, conditions which affected patients from different parts of the adult life range, and fatal versus lifelong conditions. When deciding which and how many chronic diseases to include, we also had to take a pragmatic approach to ensure feasibility, because the data collection phase involved extended periods of observation at multiple sites.
Teams which might have been interested in participating in the study were identified by our clinical coapplicants. The principal investigator (PI) wrote to the lead clinician of each MDT inviting them to take part. This was followed by a discussion with each MDT lead to clarify any issues or concerns.
Following this, the research team visited each MDT meeting to introduce the study. We provided participant information sheets and obtained members’ signed consent to observe, record data from and audiotape MDT meetings (see Appendix 1). Other professionals occasionally attend MDT meetings on an ‘ad hoc’ basis, so we also displayed a printed notice at the entrance to the meeting room during every meeting we recorded. This explained the nature of the observation and provided the researcher’s contact details. We made it clear that if individuals did not wish to take part in the study we would delete their contributions from any transcripts. Nobody requested this.
We initially aimed to recruit 10 teams. This was based on estimates made by our clinical coapplicants of the number of patients discussed in each specialty. In practice, we found considerable differences in the number of patients discussed at each MDT (even within the same specialty), so we had to vary the observation period in each team to maximise data collection in those teams which discussed fewer patients (Table 1). In addition, one mental health team disbanded during the observation period. We therefore asked one of our coapplicants (with a background in mental health) to identify other suitable mental health MDTs. We subsequently recruited an additional two mental health teams (in order to increase our capability of achieving the required sample size), taking the total number of teams in the study to 12. Three field researchers observed a total of 394 meetings (including two meetings for each of the 12 teams where the data collection tools were piloted) and collected quantitative and qualitative data from 370 meetings.
| Team | Observation period (months) | Number of meetings observed |
|---|---|---|
| Gynaecological cancer | 5 | 18 |
| Haematological cancer 1 | 13 | 38 |
| Haematological cancer 2 | 12 | 36 |
| Skin cancer | 11 | 31 |
| Mental health 1 | 5 | 15 |
| Mental health 2 | 8 | 20 |
| Mental health 3 | 17 | 55 |
| Mental health 4 | 8 | 23 |
| Heart failure 1 | 13 | 42 |
| Heart failure 2 | 9 | 24 |
| Memory clinic 1 | 12 | 43 |
| Memory clinic 2 | 9 | 25 |
| Total | 370 | |
| Pilots (two per team) | 24 | |
| Total | 394 |
We also planned to include semiurban and metropolitan teams but, in the event, only one MDT from outside London accepted our invitation to participate.
Quantitative methods
We collected both quantitative and qualitative data. In the rest of this section (Quantitative methods) and the next (Qualitative methods) we first describe how we collected and then analysed the quantitative data, and then describe how we collected and analysed the qualitative data.
Quantitative data collection
Non-participant observation
Non-participant observation included collection of both quantitative and qualitative data. The quantitative data collected are described here (see below under Qualitative methods for a description of the qualitative data).
In collaboration with our clinical coapplicants and patient advisors we designed a standard proforma to collect quantitative data on each patient discussed. This included information on patient features (e.g. presenting problems, comorbidities, medical and social history) mentioned during the discussion. The clinically active members of the research team identified the most important and common comorbidities and other health behaviour risk factors which could have an impact on the MDT decision and/or whether or not it was subsequently implemented. Examples of data collected are provided in Box 1. The proforma also included a space for free text on additional clinical or social factors mentioned during a discussion but not already included in the standard proforma. We also used this proforma to record discussion features (e.g. whether or not patient preferences were mentioned, whether or not the presenter was questioned), and decision features (e.g. decision, whether responsibility for implementing the decision was given to a named individual or not, and whether the agreed decision was written down or verbally summarised) (see Appendix 2).
-
Comorbidities including other chronic diseases, for instance anaemia, diabetes and transient conditions (e.g. broken leg, bladder infection, flu).
-
Medical history (e.g. history of breast cancer, history of substance misuse).
-
Health behaviours (smoking, drinking, physical inactivity).
-
Obesity.
-
Terms that indicate comorbidity without being given a diagnostic name (e.g. back problems, memory problems).
-
Allergies, hypersensitivity to medication/side effects.
-
Learning disabilities.
-
Behavioural problems.
-
Family history (e.g. BRCA gene).
Patient preference included any preference indicated by a patient, even if it was not directly related to the decision ultimately made, for example general mention of the fact that a patient was reluctant to see doctors.
A decision was defined as a resolution about patient management, either with or without discussion (e.g. a decision based on local protocols) between MDT members. This did not include:
-
mention of a decision that had been made outside the MDT
-
when team members provided feedback or updates regarding a case but no decisions were made
-
when a patient was listed for discussion on an agenda but the discussion was deferred until the following week because key information or people needed to make a decision were absent.
We completed the proforma during observation of each team’s weekly MDT meetings. We also audiotaped each meeting and collected data on meeting attendance (number of attendees and their professional category). Following the meeting, a researcher listened to the audio recording to double-check that the information had been recorded accurately.
Follow-up of medical records
We reviewed patients’ medical records to ascertain sociodemographic information, whether or not their MDT decisions had been implemented, and reasons for non-implementation where applicable (Table 2). This involved accessing electronic patient records, paper records, or both, at the hospital or clinic where the MDT meeting took place. On some occasions, files had been moved to other sites for storage. In these instances, we either requested that the specific records be returned to the MDT site or we obtained permission to review the records at the site where they were stored. We followed up each individual decision observed, and we recorded all information directly into an IBM Statistical Product and Service Solutions (SPSS) database, version 20 (IBM Corporation, Armonk, NY, USA).
| Type of information | Data collected |
|---|---|
| Patient details | Information recorded |
| Date of birth | |
| Ethnicity | |
| Postcode (used to derive indicator of deprivation) | |
| Recorded diagnosis | |
| Decision details | Response options |
| Decision implementation | Implemented |
| Partially implemented | |
| Not implemented | |
| Not documented | |
| Patient not identifiable | |
| Records not available | |
| Reasons for non-implementation recorded in notes | Patient choice |
| Carer/family choice | |
| Patient and family/carer choice | |
| Comorbidity present but not mentioned at MDT meeting | |
| Comorbidity deteriorated post MDT meeting | |
| New comorbidity arose post MDT meeting | |
| No reason recorded | |
| Clinician notes decision not implemented | |
| Patient died | |
| Other | |
| Patient did not attend | |
| Change in circumstances | |
| Condition not met | |
| Was implementation rescheduled? | Rescheduled by patient |
| Rescheduled by staff | |
| Not applicable |
These data were collected at least 3 months after each MDT decision was made. The Department of Health national targets for cancer services state that MDT decisions should be implemented within 31 days of patients receiving their diagnosis of cancer. 58 For the other conditions studied, although there is no national standard, MDTs commonly aim to have the investigations complete and the patients and carers offered an appointment for discussion of diagnosis and plan 6 weeks after being seen. Hence, 3 months after the MDT was deemed a reasonable time to check for MDT decision implementation, even allowing for unavoidable delays in beginning treatment. For longer-term decisions, where it was explicit that a decision would not be implemented within 3 months (e.g. follow up in 4 months), we followed up after the requisite time period.
Examples of partially implemented decisions included ‘cancel existing appointment and write to patient confirming no diagnosis of cancer’, where the records showed ‘wrote to patient but still saw in clinic to discharge’, or ‘biopsy of left sided nodules’ where the records showed ‘biopsy attempted but had to be abandoned after patient lost consciousness’. The research team met to review all cases that were recorded as ‘partially implemented’ to ensure the category was used consistently across the data set.
Team Climate Inventory
During the final month of observation, core team members (as defined by the lead clinician or based on a team list, where this existed) were invited to complete the Team Climate Inventory (TCI), which is a 44-item questionnaire. This validated measure88–90 assesses members’ perceptions of team processes in four domains: team vision, participative safety (i.e. a facilitative atmosphere for involvement), task orientation and support for innovation. Responses for each item on the TCI are given on a 5-point Likert scale, with a rating of 1 indicating strong disagreement with the statement, and a rating of 5 indicating strong agreement. 91 Lower TCI scores reflect poorer perceptions of team climate.
Additional questions
In addition to the TCI items, we added two items to the TCI questionnaire. The first asked respondents to rate their agreement with the statement ‘I believe that the [team name] MDT meetings are an effective use of my time’ on a scale of 1 to 5. The second was an open question: ‘Is there anything you would change about these meetings?’
Planned sample size
We calculated the sample size using the conservative assumption (based on published research and clinical experience) that 18% of decisions would not be implemented. 92 Using Peduzzi’s rule of 10, we required 80 non-implemented decisions to estimate eight coefficients in our regression model; this would require approximately 440 patients. 93 To allow for clustering by MDT, the sample size was inflated using estimates of the intracluster correlation coefficient (ICC) and the average cluster size. 94 Directly relevant estimates for the ICC were not available, but published models associating the ICC and outcome prevalence for a range of outcomes in community and health services settings suggested an ICC of between 0.01 and 0.05. 95 Thus, we assumed an ICC of 0.025 and that across MDTs the average number of patients with relevant decisions during the study would be approximately 230. Based on this information we aimed to include 3000 patients with a decision from the 12 MDTs.
The sample size calculation, based on decision implementation, assumed one decision for each patient. Some patients had more than one decision per meeting. We grouped these decisions into a ‘treatment plan’. This is described below under Summary measures.
Summary measures
The data were categorised for analysis using the following summary measures.
Treatment plan
We limited our analyses to the first presentation of each patient at an MDT meeting within our observation period. Discussion of a patient often resulted in more than one decision being made which cannot be assumed to be independent. We therefore grouped decisions into a ‘treatment plan’. This is consistent with cancer guidance, which refers to treatment plans rather than individual decisions. 42
In the majority of cases (88%) implementation of the treatment plan was consistent, that is either all or no component decisions of treatment plans were implemented. In cases where some decisions in the plan were implemented and others were not, we classified a treatment plan as implemented if more than 50% of the component decisions were implemented. This definition was agreed to be reasonable by our study team but was investigated further in sensitivity analyses (see Appendix 3).
Team Climate Inventory
The overall team TCI score was obtained by averaging the scores of team members. Team members with missing responses for more than 25% of items in one of the four dimensions of the TCI were excluded. There is no advice given about an acceptable team completion rate for the TCI. Previous studies using the TCI have ranged from including teams where at least 30% of members responded96 to including any teams where at least two members responded. 97 For this study we included all team responses in the main analysis.
We averaged the scores of team members in response to the statement ‘I believe that the [team name] MDT meetings are an effective use of my time’ and summarised responses to the additional open question about things they would change for use in the qualitative analysis.
Skill mix (Adjusted Teachman’s Index and number of professional categories)
There is no consensus on the best measure of skill mix or team diversity. 98 We therefore considered both the number of professional categories represented and the more complex Adjusted Teachman’s Index (ATI). This index captures both the number of professional categories represented and the evenness of representation within each of these categories. A higher value reflects a more even spread across a greater number of categories. 98 By including both measures we could assess whether or not the more complex index added any predictive value over a count of professional categories. These were classified as diagnostic doctor, surgeon, doctor, MDT co-ordinator, nurse, researcher, social worker, allied health professional and psychologist (Table 3). Both core and occasional members were included. Observers such as students were excluded.
| Professional category | Inclusion criteria |
|---|---|
| Allied health professional | Includes occupational therapists, support workers and Age Concern representatives |
| Diagnostic doctor | Includes radiologists, pathologists, consultants in nuclear medicine, clinical scientists |
| MDT co-ordinator | Includes individuals whose role focuses on facilitating the smooth running of the meeting (MDT co-ordinator may not be their official job title). They may have limited clinical input, prepare notes for patients discussed and take minutes |
| Nurse | Includes clinical nurse specialists, community psychiatric nurses, palliative care nurses and visiting crisis team nurses |
| Doctor | Includes junior doctors, consultants and staff grade doctors (medical students were excluded) |
| Psychologist | Includes assistant psychologists and clinical psychologists |
| Social worker | Includes junior and senior social workers |
| Surgeon | Includes subspecialist trainees, specialist registrars and consultants |
| Researcher | Includes clinical research fellows, research nurses, clinical trials practitioners and clinical trials co-ordinators |
Index of Multiple Deprivation
We used the Index of Multiple Deprivation (IMD) 2010 as an indicator of patient’s socioeconomic circumstances. 99 This index is a widely used area-based measure that combines seven domains into a single deprivation score for each small geographical area (i.e. each ‘lower layer super output area’, which covers about 1500 people) in England. The domains comprise income; employment; health and disability; education; skills and training; barriers to housing and services; and crime and living environment. 99 We grouped IMD scores into quintiles, where quintile 1 encompassed the least deprived and quintile 5 the most deprived areas. The postcode address of patients was linked to the lower layer super output area and hence to the corresponding deprivation quintile.
Health behaviours/other clinical factors
We combined data on comorbidities (physical and mental health), health behaviour risk factors, medical history and family history into a binary variable which we referred to as ‘health behaviours/other clinical factors’ (see Box 1).
Primary statistical analysis
Data were collected on all patients discussed, although only those about whom at least one decision was made were included in the primary quantitative analysis, which aimed to identify factors predicting MDT decision implementation. The factors we investigated were patient characteristics (age, sex and socioeconomic circumstances) and MDT and decision characteristics (team climate, disease type, skill mix, discussion of patient preferences and health behaviours/other clinical factors).
For the primary analysis, the implementation response categories were collapsed into a predefined binary outcome where partially implemented was combined with implemented. We also combined decisions where implementation was not documented with those decisions which were not implemented. This was because, on the basis of our clinical experience, non-implementation is commonly not explicitly recorded in patients’ records.
The influence of MDT and patient-related factors on treatment plan implementation was investigated using random-effects logistic regression models, allowing for clustering by MDT. We fitted unadjusted models for each factor of interest. We then undertook the following predefined selection process to obtain a final adjusted model. Initially we fitted two separate models. Model 1 included patient characteristics (age, sex and IMD quintile) and model 2 considered MDT and decision related characteristics (TCI score, disease type, ATI score, number of professional groups, mention of patient preferences and health behaviours/other clinical factors). Factors identified as having potential importance from these models (p < 0.3)100 were then fitted in the final model (model 3).
The suitability of models was investigated, including considering normality of the random effect, goodness of fit (using a Hosmer–Lemeshow test) and evidence of overfitting (using bootstrapping). 100 We examined the pattern and extent of missing data and considered its potential impact on our results. We also carried out a set of sensitivity analyses to investigate some assumptions made in our main analysis. We refitted model 3 including adjustment for the number of decisions making up the first treatment plan. The impact of using the first recorded treatment plan in analysis was examined by refitting the model based on a randomly chosen treatment plan for each patient. We also refitted model 3 collapsing the five IMD quintiles into two groups (IMD 1–3 and IMD 4–5) in order to produce an eight-coefficient model (as specified in our sample size calculation).
We undertook exploratory investigations to further understand the associations observed in our model and whether these differed by disease type. We extended model 3 to examine interaction terms between the number of professional groups and disease type, and between IMD and guideline-driven cancer compared with non-cancer MDTs.
Quality assurance: clinician validation
The field researchers were not clinicians, but there were several quality assurance procedures in place to safeguard against this potential limitation. Clinical members of the research team (the PI and several of the study coapplicants) were involved throughout data collection and analysis, and were available to respond to any specific queries the field workers had throughout the project (e.g. relating to specialist terminology).
In addition, the clinical members of the research team ensured accurate recording of the primary outcome, decision implementation. During the follow-up period, a clinician from each specialty (SG for heart failure, AL for cancer and GL for dementia and mental health) reviewed a random selection of approximately 20 treatment plans from each of the specialities, and examined the medical records to independently ascertain whether or not the decision had been implemented. Any discrepancies between the outcome recorded by the field researcher and the clinician were discussed with the wider research team. Discrepancies were rare and the process gave us confidence in the accuracy of data collection. We also sought advice from the clinical coapplicants on any other clinical queries that had arisen throughout the medical record follow-up.
Qualitative methods
The qualitative aspect of the study served two purposes: firstly, to provide insights into possible explanations for the quantitative findings, and, secondly, to identify areas where there were diverse beliefs or practices with respect to MDT meetings.
To achieve this we collected qualitative data using three data collection methods:
-
non-participant observation of MDT meetings
-
interviews with MDT members
-
interviews with patients.
The steps taken to collect and analyse the qualitative data are described below.
Non-participant observation
In addition to the quantitative data collected during non-participant observation of MDT meetings, we also developed a qualitative observation coding sheet to obtain a detailed understanding of the meeting context and processes.
Developing the qualitative observation coding sheet
The observation coding sheet (see Appendix 4) was based on an adaptation of an inputs–process–outcome model. 33,34,101,102 It included sections on the meeting environment, mention of national or local policies, features of the team and task, levels of participation, and mediators of team processes and outcomes. This provided a framework to map out the potential factors influencing implementation of MDT decisions.
Data collection
Although we attended a total of 394 meetings, the purpose of the first two meetings we attended at each team was to test the data collection instruments and to enable the team to get used to the presence of the researcher. We took observational field notes at 370 MDT meetings. These notes focused on significant events and interactions observed by the researcher. Within 24 hours of each meeting, the researcher categorised these field notes according to the observation-coding sheet. The researcher also listened to the audio recording of each meeting, adding further notes and noting the timing of key events on the recording for future reference.
Interviews
We conducted semistructured interviews with MDT professionals and with patients to gain an understanding of MDT meetings from different perspectives.
Developing the interview topic guides
Draft topic guides for the MDT professional and patient interviews were developed based on reviews of the literature and the research aims. These were revised based on emerging issues identified during non-participant observation and suggestions from the study steering group.
The MDT professional interview topic guide included open-ended prompts about the purpose of the MDT, areas of diverse beliefs or practices, and what participants saw as their role in the meeting (see Appendix 5). This was piloted with a clinical coapplicant and amended on the basis of their feedback.
The patient topic guide covered issues such as whether or not patients were aware of the MDT meeting and what issues they believed should be considered when the MDT was making decisions about their care. This topic guide was piloted with two patient representatives and amended on the basis of their comments (see Appendix 6).
Recruitment of multidisciplinary team professionals
We purposively selected interviewees to ensure diversity in terms of professional group, seniority and level of participation in MDT meetings (based on the observation data).
Interviewees were approached after observation for each team was completed. This was done either in person or by e-mail, and individuals were provided with an information sheet and consent form. While we suggested that the interview would take approximately 1 hour, some clinicians requested shorter interviews (e.g. 30 minutes) to fit in with their schedules.
The interviews were all conducted at the place of work of the participants and were audiotaped. Researchers wrote reflective field notes following each interview, including comments on setting, the main issues discussed and new perspectives on the research questions prompted by the interview.
Recruitment of patients
We aimed to interview patients or carers across all four disease types and to maximise diversity in terms of sex, age and ethnicity. Patients were recruited from the MDTs under observation. The inclusion and exclusion criteria for these are detailed in Box 2. To increase the likelihood that interviewees would still be under the care of the team, the 30 patients who had been most recently discussed by each team at the end of the observation period were selected. From this group, we gave the relevant lead clinician or team manager a list of the patients we sought to invite for interview. We asked clinicians to highlight any cases where they thought the interview would present a risk to the patient or to the interviewer. These patients were not approached for interview.
Diversity of age, sex and ethnicity.
Under the care of the team.
Summary of exclusion criteriaNon-English speaker.
Clinician deems a risk to interviewer.
Clinician deems too vulnerable for interview.
Not living in England.
Declines to be interviewed.
The relevant key worker was then asked to contact the selected patients, provide them with a study information sheet and consent form, and seek permission for the researchers to contact patients directly to discuss the study further. Patients who agreed to be contacted were telephoned by a member of the research team to confirm participation, address any questions they had and arrange the interview. Interviews took place in either the hospital/clinic or the patient’s home and were audiotaped. As with the professional interviews, researchers wrote reflective field notes directly after each interview.
Qualitative analysis
We devised a strategy for qualitative data analysis to enable us to manage the large volume of data generated. The analysis was an iterative process, using a combination of both inductive and deductive coding and ethnographic methods such as constant comparison and coding frame revisions. Analytic conferences of the field researchers and the wider research team were used to scrutinise and revise codes and themes and to encourage reflexivity.
Analysis of non-participant observation data
This was undertaken in two stages, as described below:
Stage one: initial coding and selective transcription
Verbatim transcription of MDT meetings was not practical given the large number of participants at some meetings, overlapping talk and variable audio quality depending on the layout of the meeting rooms. Instead, we used selective transcription to document relevant parts of the meetings. 103
In the first instance we inductively coded the observation coding sheets (i.e. our field notes) for the first 16 meetings (excluding the initial 2-week ‘test’ period for each team) of each of the teams observed (with the exception of one team, which disbanded after 15 weeks of observation). We used NVivo 9 (QSR International, Warrington, UK) to organise the data. Each observation was labelled with a basic descriptive code, and recurring and salient issues were compiled into an initial coding framework. Exceptions were noted within the relevant codes and some codes were relevant to only specific teams or specialties.
This coding framework was then used to identify and selectively transcribe at least two discussions illustrating each code. This ‘data reduction’ technique allowed us to manage the huge volume of data collected, while still reviewing the excerpts in the context in which they occurred by listening to the audio recordings.
These selective transcripts were imported into the NVivo database alongside the observation coding sheets and were read, reread and further coded using the initial coding framework. Thus, codes included data from both observation notes and transcripts. The coding framework was revised iteratively and additional codes were added where new issues that were relevant to the research questions arose. Code definitions were discussed by the team and revisited regularly throughout the analysis to ensure they were being applied consistently. The list of observation code definitions is provided in Appendix 7. This pool of codes was drawn upon throughout the analysis, as appropriate to the question at hand. For example, for the qualitative investigation of the primary quantitative findings, relevant codes were grouped as shown in Appendix 8.
Stage two: analytic conferences
Conducting qualitative research as a team is recognised to have a number of potential benefits, such as increasing rigour by providing an opportunity to challenge and clarify conflicting understandings and encouraging a richer conceptual analysis. 104 We drew upon the expertise and experience of project team members to devise a strategy for the analysis of the qualitative data that would be both reflexive and rigorous. This involved using analytic conferences to discuss the analysis and interpretation of the findings.
Analytic conferences were attended by the researchers who conducted the observation and senior members of the team with clinical and research experience. For each of the 12 MDTs, the researcher who completed stage one of the observation analysis provided each member of the analytic team (CN, IW, PX, AL, AC) with an audio recording of a different meeting from the same team. Each team member listened to their allocated audio recording, making notes of potential codes, exemplary quotes and deviant cases. Following this, the team met to discuss and review the appropriateness of the codes allocated.
Once this process had been completed for half the teams, an analytic conference was dedicated to refining and integrating the codes across the data set, merging synonymous codes and splitting compound codes to form a coherent framework. Analytic conferences were also held for the remaining six teams to monitor the consistent application of the framework.
Analysis of interviews
All interviews were transcribed verbatim and analysed thematically using NVivo 9. Transcripts were anonymised and identifying details (such as names and specific job titles) were removed where necessary to preserve anonymity.
The initial analysis of professional interviews was conducted independently of the patient interviews.
In order to manage the large volume of data, and to build on our observation analysis (as described above), we adopted a hybrid inductive and deductive approach to analysing the interview data.
The deductive themes were based on our research objectives and our preliminary qualitative analysis of the observation data. These are listed in Box 3. We applied these in the first instance to collate the data relevant to each theme. We then inductively analysed the data to generate subthemes. This allowed us to explore relevant issues identified in the data more fully. Tables 4 and 5 provide illustrative examples of these levels of analysis for the professional and patient interview analyses respectively.
Added value of the MDT meeting, in relation to (1) decision-making, (2) other formal and informal functions of the MDT meeting.
Areas for improvement/weaknesses/problems of the MDT meeting, in relation to (1) decision-making, (2) other formal and informal functions of the MDT meeting.
Patient preferences and decision-making.
Comorbidities and decision-making.
MDT patient interviews: deductive themesPatient preferences.
What should and should not be considered when making a decision in the MDT meeting.
Most appropriate methods for implementing patient preference.
Feedback (what kind of feedback patients received and would want in the future).
| Deductive theme | Inductive subthemes | Codes |
|---|---|---|
| Added value of MDT in relation to decision-making | Improving decision-making | Consistency of decision-making (acting as a ‘check’) |
| Having access to all the information to inform decision-making | ||
| Context within which MDT decision-making is most helpful | When the ‘right’ people attend | |
| When there is good leadership/management | ||
| When people make meaningful, significant contributions | ||
| Difference as strength | Sharing professional knowledge and expertise | |
| Providing a different perspective |
| Deductive theme | Inductive subthemes | Codes |
|---|---|---|
| What feedback do patients want? | Content of feedback | Symptoms/test results |
| Options | ||
| Decisions | ||
| Other | ||
| Life expectancy | ||
| Impact on life: side effects | ||
| Feedback format | No paperwork/a phone call | |
| No feedback wanted | Feedback would not change anything | |
| Best for others to decide | ||
| Too much information can be confusing | ||
| Might not be able to understand | ||
| Need for basic information in order to recover | ||
| Control over feedback | Asking for information that you want to know | |
| Feedback dependent on the person/situation | ||
| Feedback wanted | Full feedback of all the options | |
| Information about the MDT discussion |
Analytic integration and triangulation across data sources
The term ‘triangulation’ has been ascribed several meanings. Two of the most common uses are the corroboration approach (assessing whether or not accounts derived from different data sets are consistent) and complementarity approach (using different methods to assess different aspects of the same issue to gain a more complete picture). 106,107 We adopted a complementarity approach106 using multiple methods to gain a more comprehensive, multidimensional understanding of a complex issue. 107 We integrated our three qualitative data sources with two specific objectives in mind: firstly, to provide insights into possible explanations for the results from the quantitative analysis, and, secondly, to identify areas of diverse beliefs or practices in MDT meetings.
-
To provide insights into possible explanations for the quantitative findings
-
This analysis drew upon each of the different methods (non-participant observation and semistructured interviews) to explore possible explanations for variation in treatment plan implementation. We undertook a deductive analysis of both the observation and interview data, where each finding from the logistic regression formed the basis of a deductive theme (see Appendix 8 for an illustration of how the observation codes were grouped according to the quantitative variables examined). We drew on the entire pool of inductive codes, grouping them according to their relevance to each deductive theme, as illustrated by Table 6. This allowed us to identify the relevant transcripts and field notes to illuminate possible reasons for each finding. Additional analytic conferences were held to facilitate these analyses.
-
-
To identify areas of diverse beliefs or practices in MDT meetings
-
In order to identify themes for study 2, we distinguished issues around which there were differences in beliefs or practices, either within or across teams. This was achieved by synthesising findings from the three qualitative data sources to produce overarching ‘metathemes’ relating to these differences and issues of contention.
-
Throughout the analysis, we drew on the ‘following a thread’ technique108 where key themes and topics identified in the initial analyses of each data source were further explored in the other data sets. Since we gathered the observational data before the interview data were collected, we were able to follow up ‘promising’ findings when analysing the other data sets, as well as in the questions we asked interviewees. This allowed us to collect richer interview data on ‘promising’ codes than would have been possible by integrating the findings in the analytic stages alone.
-
Once we had undertaken preliminary analyses of the three qualitative data sources, we convened an analytic synthesis meeting attended by the chief investigator (RR), the three field researchers (CN, IW, PX), two senior clinical members of the research team (AL, AC) and two patient representatives (DA, MH). At this meeting, we reviewed the outputs from each of the preliminary analyses (observational codes, professional interview themes and patient interview themes) and, taking the comprehensive data set as a whole, we identified all the issues around which there were different opinions or practices (which we called metathemes, e.g. differing opinions about the role of the patient and differing chairing practices). We then collated the relevant themes, codes and quotes from all sources related to each of these issues.
Quality assurance
Non-participant observation
Observation notes and selective transcripts of the meeting discussions were coded and analysed in a constant comparative manner, with repeated inspection of each data source between three researchers, and at regular analytic conferences with other members of the research team. We used multiple coding to address the issues of subjectivity sometimes levelled at the process of qualitative data analysis. 109 As new codes were introduced, they were assigned a working definition to ensure they were used consistently by the different researchers. These definitions were debated and revised repeatedly throughout the process. The analytic conferences allowed the researchers to check whether or not codes were being applied according to the definition, and that definitions were iteratively revised where appropriate. The analytic conferences also facilitated group reflexivity and safeguarded against individual bias by providing opportunities to make each researcher’s assumptions explicit and open to challenge. 110 Together with regular meetings between the field researchers, the chief investigator and other members of the team, these formed a kind of auditing that was ‘built into the research process to repeat and affirm researchers’ observations’. 111
Professional and patient interviews
In order to establish consistency of coding for the interview data, two researchers initially independently coded 20% of the transcripts. Following this, the researchers met to discuss any incongruence, going through each transcript line by line to check for differences in terms of both sections coded and the specific code applied in each case. Differences were resolved by discussing the differing interpretations, identifying any misunderstandings and refining code definitions as necessary. A third researcher was present to give an independent perspective if the two coders failed to reach agreement.
Steering group meetings
Throughout the study, we convened four steering group meetings (between July 2011 and March 2013), which provided a mechanism for peer review and guidance (see Appendix 9 for collaborators and steering group members).
In these meetings, as well as providing general support and advice (e.g. with recruitment), the steering group members discussed methodological issues, reviewed the definitions of variables and outcomes and the interview topic guides, and helped to develop data-auditing strategies, hence providing further quality assurance.
Patient and public involvement
We had two active patient and public involvement (PPI) representatives on our project steering group (MH and DA, coauthors on this report), who were actively involved throughout. They attended steering group meetings and provided in-depth and valuable contributions to our study design and analysis. In particular, they reviewed and amended the information sheets for the patient interviews, to ensure these were clear and informative. They also collaborated in the design of the patient interview topic guide and helped to organise pilot interviews to test and refine them. In addition, they reviewed the anonymised patient interview transcripts and participated fully in the analytic conference where these were discussed.
The collaboration of patient representatives in this research was integral to both its design and delivery. We found that having continuous rather than sporadic PPI engagement was invaluable, because their in-depth knowledge of the background and progress of the study meant that they could respond quickly when their patient perspective on particular aspects of the study was needed.
Chapter summary
We began this chapter by outlining the ethics and recruitment procedures undertaken. We then described the data collection and analysis methods for the quantitative components (i.e. non-participant observation of meetings and medical record review) and the qualitative components (i.e. non-participant observation of meetings, interviews with patients and professionals) of the research. Finally we explained how we integrated these data to address the study aims. Chapters 3 and 4 present the quantitative and qualitative results.
Chapter 3 Study 1 quantitative results
A shorter version of the results presented in Chapters 3 and 4 has been published in Raine et al. 105
Team characteristics
We observed 370 MDT meetings during which 3184 patients were discussed. Characteristics of the 12 teams and their meetings are summarised in Table 7. There was considerable variability among the 12 teams in the number of patients discussed at each meeting. The average for each team ranged from 4 to 49 patients. The average meeting size across the 12 MDTs ranged between 5 and 28 members, with one to seven professional groups represented (an overall median of four). Of the patients discussed, 83% had a least one treatment plan made, but this proportion was notably lower for the mental health teams.
| MDT characteristics | Haematological cancer | Gynaecological cancer | Skin cancer | Memory clinic | Mental health | Heart failure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Team 1 | Team 2 | Team 1 | Team 2 | Team 1 | Team 2 | Team 3 | Team 4 | Team 1 | Team 2 | |||
| TCI score | 4.32 | 3.79 | 3.49 | 4.11 | 4.10 | 3.89 | 4.01 | 3.31 | 3.64 | 4.01 | 4.00 | 3.75 |
| Number of meetings | 38 | 36 | 18 | 31 | 43 | 25 | 15 | 20 | 55 | 23 | 42 | 24 |
| Number of patients discussed per meeting: mean (SD) | 14.5 (4.1) | 14.2 (4.8) | 34.5 (5.0) | 21.7 (5.6) | 11.2 (5.4) | 4.3 (1.3) | 29.1 (11.5) | 14.6 (4.4) | 14.0 (4.9) | 49.3 (12.1) | 8.0 (2.3) | 6.0 (2.2) |
| ATI: mean (SD) | 1.38 (0.14) | 1.29 (0.10) | 1.70 (0.12) | 1.52 (0.12) | 1.75 (0.14) | 0.86 (0.33) | 1.26 (0.17) | 1.34 (0.14) | 1.34 (0.13) | 1.47 (0.08) | 1.36 (0.13) | 0.89 (0.14) |
| Number of professional categories represented: median (25th–75th percentile) | 5 (4–5) | 5 (5–5) | 6 (6–6) | 6 (5–6) | 5 (5–6) | 3 (2–3) | 3 (3–4) | 4 (3–4) | 4 (3–4) | 4 (4–4) | 4 (4–5) | 2 (2–2) |
| Number of MDT members at the meeting:a mean (SD) | 11.79 (1.65) | 28.25 (4.43) | 18.28 (2.89) | 17.48 (2.66) | 9.23 (1.60) | 6.92 (1.75) | 7.73 (2.60) | 8.35 (2.13) | 9.09 (2.21) | 9.70 (1.94) | 15.02 (2.96) | 5.38 (1.58) |
| Number of patients discussed during observation period (at least once) | 390 | 371 | 324 | 384 | 403 | 106 | 231 | 134 | 314 | 169 | 225 | 133 |
| Patients with at least one treatment plan: number (%) | 330 (85) | 321 (87) | 281 (87) | 335 (87) | 356 (88) | 106 (100) | 145 (63) | 71 (53) | 251 (80) | 131 (76) | 197 (88) | 130 (98) |
Team Climate Inventory and additional questions
Of the 211 TCIs issued, 161 were returned, giving a response rate of 76%, ranging from 50% (mental health team that disbanded) to 100% (skin cancer).
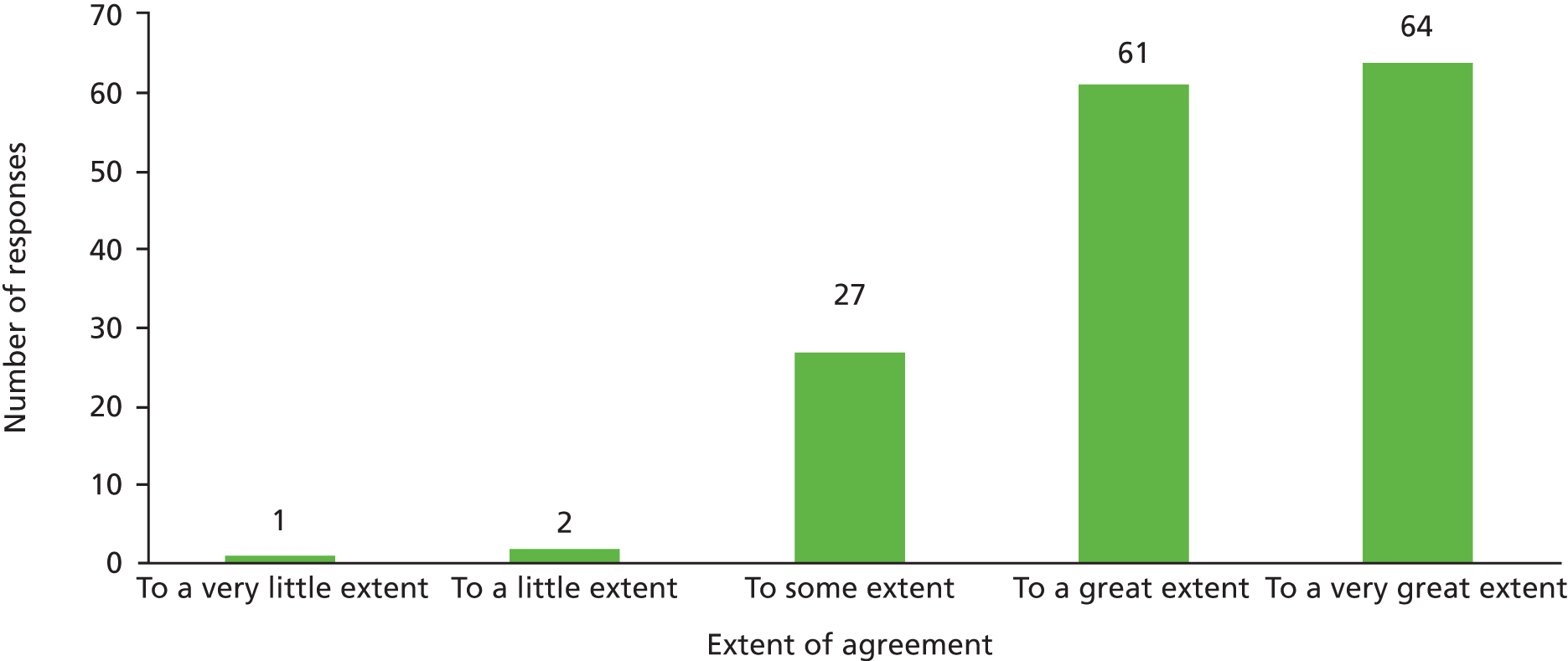
Across all teams, 155 members responded to the additional question, with almost all agreeing to a great or very great extent with the statement, ‘I believe that the [team name] MDT meetings are an effective use of my time’ (Figure 1).
FIGURE 1.
MDT member response to ‘I believe that MDT meetings are an effective use of my time’ across 12 MDTs.
Treatment plan implementation
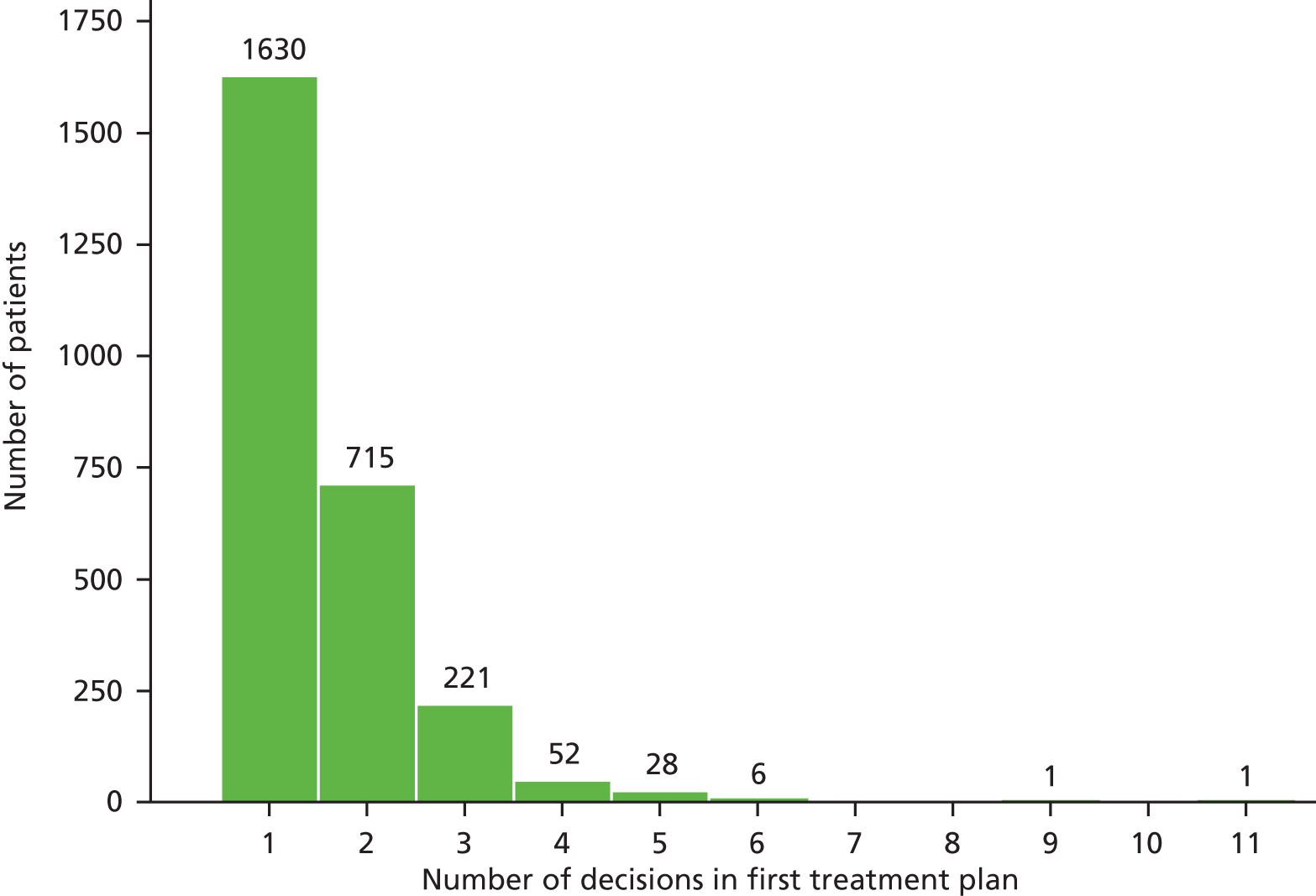
Overall, 2654 patients had a treatment plan. The number of decisions making up the plan ranged between 1 and 11 (Figure 2) with an average of 1.6 decisions per plan.
FIGURE 2.
Number of decisions making up the first treatment plan recorded for each patient. Average number of decisions = 1.6, number of decisions for first treatment plans = 4127.
Of the patients with treatment plans, 2512 (94.6%) had adequate implementation data recorded for implementation status to be classified, and 1967 (78.3%) of these had implemented plans. Treatment plan implementation is summarised by patient, MDT and discussion characteristics in Table 8. By disease type, implementation was highest in the gynaecological cancer team and lowest in the CMHTs, although implementation also varied by team. There was a trend for non-implementation with increasing patient deprivation.
| Characteristic | Treatment plan implemented, n (%) |
|---|---|
| Age (years) (n = 2504) | |
| 20–39 | 355 (73) |
| 40–59 | 488 (79) |
| 60–79 | 739 (81) |
| 80+ | 381 (80) |
| Sex | |
| Male | 945 (78) |
| Female | 1022 (79) |
| IMD quintile (n = 2431) | |
| Least deprived | 197 (85) |
| 2 | 331 (82) |
| 3 | 395 (82) |
| 4 | 541 (76) |
| Most deprived | 442 (73) |
| TCIa,b | |
| < 4 (median score) | 853 (78) |
| ≥ 4 | 1114 (79) |
| Type of diseasea | |
| Haematological cancer | 502 (81) |
| Gynaecological cancer | 228 (84) |
| Skin cancer | 229 (78) |
| Memory clinic | 362 (81) |
| Mental health | 403 (70) |
| Heart failure | 243 (80) |
| Teama | |
| Haematological cancer 1 | 276 (85) |
| Haematological cancer 2 | 226 (76) |
| Gynaecological cancer | 228 (84) |
| Skin cancer | 229 (78) |
| Memory clinic 1 | 263 (76) |
| Memory clinic 2 | 99 (94) |
| Mental health 1 | 106 (77) |
| Mental health 2 | 42 (65) |
| Mental health 3 | 163 (68) |
| Mental health 4 | 92 (70) |
| Heart failure 1 | 148 (81) |
| Heart failure 2 | 95 (79) |
| ATIc | |
| < 1.2 | 349 (82) |
| 1.2–1.4 | 538 (76) |
| 1.4–1.6 | 591 (78) |
| > 1.6 | 489 (78) |
| Number of professional groupsc | |
| 1–3 | 312 (81) |
| 4–5 | 1199 (77) |
| 6–7 | 456 (79) |
| Patient preferences considered | |
| Yes | 361 (75) |
| No | 1606 (79) |
| Health behaviours/other clinical factors mentioned | |
| Yes | 1069 (79) |
| No | 898 (77) |
| Total | 1967 (78) |
Adjusted associations (Table 9) showed no evidence of a relationship between treatment plan implementation and patient’s age or sex, or with discussion of patient preferences or health behaviours/other clinical factors. ATI and number of professional groups were highly correlated (correlation coefficient 0.8) but did not appear to have an association when fitted together (model 2). When included individually in the model, however, each showed a relationship such that an increase in team diversity or number of professional groups was associated with a reduced odds of treatment plan implementation.
| Characteristic | Unadjusted (n = 2512)b | Adjusted model 1 (n = 2431) | Adjusted model 2 (n = 2512) | Adjusted model 3 (n = 2431) | ||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age (at first decision) | 1.00 (0.99 to 1.01) | 0.60 | 1.00 (0.99 to 1.01) | 0.84 | ||||
| Sex | ||||||||
| Male | 1 | 1 | ||||||
| Female | 0.99 (0.80 to 1.21) | 0.89 | 1.01 (0.82 to 1.24) | 0.96 | ||||
| IMD quintile | ||||||||
| Least deprived | 1 | 1 | 1 | |||||
| 2 | 0.83 (0.53 to 1.30) | 0.83 (0.53 to 1.30) | 0.80 (0.52 to 1.25) | |||||
| 3 | 0.91 (0.58 to 1.40) | 0.91 (0.58 to 1.40) | 0.87 (0.56 to 1.34) | |||||
| 4 | 0.64 (0.43 to 0.98) | 0.65 (0.43 to 0.99) | 0.64 (0.42 to 0.97) | |||||
| Most deprived | 0.60 (0.39 to 0.93) | 0.04 | 0.60 (0.39 to 0.93) | 0.04 | 0.60 (0.39 to 0.91) | 0.05 | ||
| TCI (0.1 increase) | 1.05 (0.97 to 1.15) | 0.25 | 1.09 (1.03 to 1.15) | 0.004 | 1.07 (1.01 to 1.13) | 0.01 | ||
| Type of disease | ||||||||
| Haematological cancer | 1 | 1 | 1 | |||||
| Gynaecological cancer | 1.22 (0.62 to 2.38) | 2.76 (1.58 to 4.83) | 2.48 (1.48 to 4.15) | |||||
| Skin cancer | 0.81 (0.42 to 1.56) | 0.97 (0.67 to 1.39) | 0.97 (0.67 to 1.39) | |||||
| Memory clinic | 1.21 (0.67 to 2.20) | 1.11 (0.74 to 1.67) | 1.05 (0.76 to 1.45) | |||||
| Mental health | 0.56 (0.35 to 0.90) | 0.60 (0.37 to 0.99) | 0.57 (0.39 to 0.82) | |||||
| Heart failure | 0.93 (0.52 to 1.54) | 0.03 | 0.78 (0.49 to 1.21) | < 0.001 | 0.75 (0.50 to 1.14) | < 0.001 | ||
| ATI | 0.64 (0.35 to 1.17) | 0.15 | 0.65 (0.24 to 1.76) | 0.40 | ||||
| No. of professional groups | 0.90 (0.77 to 1.06) | 0.21 | 0.84 (0.65 to 1.10) | 0.21 | 0.75 (0.66 to 0.87) | < 0.001 | ||
| Patient preferences considered | ||||||||
| No | 1 | 1 | ||||||
| Yes | 0.86 (0.67 to 1.11) | 0.24 | 0.89 (0.69 to 1.14) | 0.34 | ||||
| Health behaviours/other clinical factors mentioned | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.03 (0.83 to 1.27) | 0.80 | 1.07 (0.86 to 1.32) | 0.55 | ||||
Factors with an association (p < 0.3) in models 1 and 2 were fitted together in the final model (model 3). The adjusted odds of implementation increased by 7% (95% CI 1% to 13%) for a 0.1-unit TCI score increase (indicating improved team climate). In contrast, the adjusted odds of implementation were reduced by 25% for each additional professional group represented, and were lower for patients living in more deprived IMD quintiles. Adjusted odds of implementation also varied by disease type.
We had sufficient data on 2512 (92%) patients to include them in the final model. Data were missing for age (2%), IMD quintile (6%) and treatment plan implementation (5%), mainly because of missing patient notes. The only characteristic associated with ‘missingness’ was MDT disease type. Given the small proportion of missing values, we did not consider it necessary to account for this in our analysis.
Sensitivity analyses for model 3 included adjusting for the number of decisions making up the first treatment plan (which varied between 1 and 11 decisions); examining the impact of using the first recorded treatment plan in analysis by refitting the model based on a randomly chosen treatment plan for each patient; collapsing the five IMD quintiles to two groups (IMD 1–3 and IMD 4–5) to produce an eight-coefficient model; and altering the definition of an implemented treatment plan such that implementation is when > 80% of component decisions are implemented. None of these analyses substantially changed our conclusions (see Appendix 3).
We further explored the observed trend between implementation and number of professional groups to examine whether this was dependent on disease type. Tabulations suggested that this association was mainly apparent in memory clinic and mental health teams, and an interaction term added to model 3 indicated some evidence of a differential effect by type of disease (p = 0.06) (Table 10). We also considered whether the relationship between IMD and implementation differed for cancer and non-cancer specialties. This interaction term was not significant (interaction p = 0.13) (Table 11).
| Disease type | Number of professional categories represented | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Haematological cancer | 60 (85%) | 442 (81%) | |||||
| Gynaecological cancer | 42 (86%) | 151 (82%) | 35 (90%) | ||||
| Skin | 96 (76%) | 133 (79%) | |||||
| Memory clinic | 3 (100%) | 37 (97%) | 34 (94%) | 73 (87%) | 78 (76%) | 137 (74%) | |
| Mental health | 146 (76%) | 257 (67%) | |||||
| Heart failure | 84 (79%) | 8 (72%) | 110 (81%) | 41 (80%) | |||
| Total | 3 (100%) | 121 (84%) | 188 (79%) | 500 (74%) | 699 (80%) | 421 (78%) | 35 (90%) |
| Disease type | IMD quintile | ||||
|---|---|---|---|---|---|
| 1 (least deprived) | 2 | 3 | 4 | 5 (most deprived) | |
| Haematological cancer | 62 (86) | 127 (80) | 144 (85) | 93 (78) | 59 (76) |
| Gynaecological cancer | 32 (94) | 43 (84) | 49 (85) | 59 (82) | 42 (78) |
| Skin cancer | 30 (77) | 45 (74) | 67 (87) | 57 (75) | 23 (72) |
| All cancer | 124 (86) | 215 (79) | 260 (85) | 209 (78) | 124 (76) |
| Memory clinic | 40 (89) | 69 (90) | 64 (74) | 119 (81) | 63 (75) |
| Mental health | 0 (0) | 4 (80) | 15 (63) | 159 (71) | 208 (70) |
| Heart failure | 33 (85) | 43 (83) | 56 (88) | 54 (75) | 47 (77) |
| All non-cancer | 73 (84) | 116 (87) | 135 (78) | 332 (75) | 318 (72) |
| Total | 197 (84) | 331 (82) | 395 (83) | 541 (76) | 442 (73) |
The reasons for non-implementation of treatment plans are summarised in Table 12 by disease type. Although 15% of non-implementations were recorded as for patient, family or carer reasons, ‘other’ reasons for non-implementation were most commonly recorded. The ‘other’ reasons were diverse, and included cases where the decision was implemented outside the 3-month follow-up period or when new information or test results emerged after the MDT meeting, for example where it was subsequently decided that a referral had been inappropriate or that a patient should have been referred to a team in a different catchment area. Patient-led reasons were recorded most frequently in CMHTs (e.g. because of patient non-attendance) while patient death was reported most frequently for heart failure MDTs. We also collapsed the five IMD quintiles to two groups (IMD 1–3 and IMD 4–5) and found that reasons for non-implementation were similar, although patient death was a more frequent reason for non-implementation in the least deprived group while patient non-attendance and changes in circumstances were recorded less for this group (Table 13).
| Reason for non-implementation of treatment decision | Gynaecological cancer (n = 40) | Haematological cancer (n = 109) | Skin cancer (n = 48) | Community mental health (n = 102) | Heart failure (n = 25) | Memory clinic (n = 31) | Total (n = 355) |
|---|---|---|---|---|---|---|---|
| Patient/carer/family choice | 7 (18) | 12 (11) | 10 (21) | 16 (16) | 3 (12) | 6 (19) | 54 (15) |
| Change in circumstancesb | 5 (13) | 12 (11) | 0 | 19 (19) | 4 (16) | 0 | 40 (11) |
| Patient did not attend | 2 (5) | 6 (6) | 3 (6) | 25 (25) | 0 | 0 | 36 (10) |
| Decision was conditional and condition was not met | 6 (15) | 6 (6) | 5 (10) | 4 (4) | 0 | 0 | 21 (6) |
| Patient died | 3 (8) | 7 (6) | 1 (2) | 0 | 8 (32) | 0 | 19 (5) |
| Comorbidity arising post MDT meeting or deteriorated post MDT meeting | 2 (5) | 3 (3) | 1 (2) | 0 | 0 | 0 | 6 (2) |
| Comorbidity not discussed | 2 (5) | 4 (4) | 1 (2) | 0 | 0 | 0 | 7 (2) |
| Otherc | 4 (10) | 36 (33) | 17 (35) | 29 (28) | 4 (16) | 8 (26) | 98 (28) |
| Non-implementation recorded but reason not given | 9 (22) | 23 (21) | 10 (21) | 9 (9) | 6 (24) | 17 (55) | 75 (21) |
| Why was the decision not implemented? | Least deprived (IMD quintile 1–3) (n = 143) | Most deprived (IMD quintile 4 or 5) (n = 204) |
|---|---|---|
| Patient/carer/family choice | 20 (14) | 33 (16) |
| Change in circumstances | 11 (8) | 29 (14) |
| Patient did not attend | 10 (7) | 26 (13) |
| Decision was conditional and condition was not met | 7 (5) | 14 (7) |
| Patient died | 13 (9) | 4 (2) |
| Comorbidity arising post MDT meeting or deteriorated post MDT meeting | 3 (2) | 3 (1) |
| Comorbidity not discussed | 3 (2) | 4 (2) |
| Other | 37 (26) | 58 (28) |
| Non-implementation recorded but reason not given | 39 (27) | 33 (16) |
Chapter summary
In this chapter we have quantitatively described characteristics of the teams studied and presented the results of the primary quantitative analysis, assessing factors associated with decision implementation. We also described some of the reasons for non-implementation. Chapter 4 presents the qualitative results. This includes a qualitative description of team characteristics, a qualitative exploration of the quantitative findings, and a qualitative analysis investigating areas of diversity in beliefs and practices relating to MDT meetings.
Chapter 4 Study 1 qualitative results
A shorter version of the results presented in Chapters 3 and 4 has been published in Raine et al. 105
As described in the methods chapter, the qualitative data served two functions. Firstly, we drew upon the pool of qualitative codes across the three data sets to provide explanations for the main quantitative findings (a deductive analysis where the quantitative findings were the main themes). Secondly, we conducted a thematic analysis (by synthesising the three qualitative data sources) to explore areas where there were diverse beliefs or practices with respect to MDT meetings. These results provided data for study 2.
Data sources
Non-participant observation
We observed 370 meetings in four cancer teams, four mental health teams, two memory clinic teams and two heart failure teams. Of these, we performed an in-depth analysis of 192 meetings that took place during the first 4 months of observations of each team.
Semistructured interview
We conducted 53 semistructured interviews with MDT professionals and with 20 patients (carers were also present and contributed to seven of these). The characteristics of MDT member interviewees in terms of their profession and specialty are shown in Table 14. The characteristics of patients interviewed are shown in Table 15.
| Profession | Cancer | Heart failure | Memory clinic | Mental health | Total |
|---|---|---|---|---|---|
| Allied health professional | 0 | 0 | 1 | 2 | 3 |
| Diagnostic doctor | 3 | 0 | 0 | 0 | 3 |
| MDT co-ordinator | 0 | 0 | 1 | 0 | 1 |
| Nurse | 4 | 4 | 0 | 5 | 13 |
| Doctor | 8 | 2 | 3 | 3 | 16 |
| Psychologist | 1 | 0 | 4 | 1 | 6 |
| Social worker | 0 | 0 | 0 | 8 | 8 |
| Surgeon | 3 | 0 | 0 | 0 | 3 |
| Totals | 19 | 6 | 9 | 19 | 53 |
| Disease type | Males | Females | Total |
|---|---|---|---|
| Cancer | 4 | 3 | 7 |
| Heart failure | 5 | 1 | 6 |
| Memory clinic | 3 | 2 | 5 |
| Mental health | 1 | 1 | 2 |
| Total | 13 | 7 | 20 |
Overview of the multidisciplinary team meetings and their context
Here we present a brief analysis of our observation and interview data to provide an overall description of the teams, and the contexts within which they operated.
In terms of meeting characteristics, overall there was considerable variation between teams, in the size of the teams, the number of patients discussed (as mentioned in Chapter 3) the length of the MDT meetings, the chairing arrangements and the administrative support provided (Table 16).
| Team | Number of patients discussed | Approximate duration of meeting (hours) | Number of professional categories in attendance (min.–max.) | Number of core team members | Chairperson | Administrative support for meeting |
|---|---|---|---|---|---|---|
| Cancer 1 | 35 | 2.5 | 5–7 | 28 | Doctor | MDT co-ordinator |
| Cancer 2 | 15 | 1 | 4–5 | 17 | Doctor | MDT co-ordinator |
| Cancer 3 | 14 | 1 | 5 | 45 | Doctor | MDT co-ordinator |
| Cancer 4 | 22 | 1.5 | 5–6 | 21 | Doctor | MDT co-ordinator |
| CMHT 1 | 29 | 2.5 | 3–4 | 12 | Social worker | None |
| CMHT 2 | 15 | 1 | 3–4 | 12 | Rotating chairperson | Administrator records minutes |
| CMHT 3 | 14 | 1 | 3–4 | 15 | Rotating chairperson | Administrator records minutes |
| CMHT 4 | 49 | 2.5 | 4 | 16 | Social worker | None |
| Heart failure 1 | 8 | 1.5 | 4–5 | 30 | Doctor | None |
| Heart failure 2 | 6 | 2 | 2 | 8 | No formal chairperson – varied throughout discussion | None |
| Memory clinic 1 | 11 | 1 | 4–6 | 15 | Doctor or nurse | Provided by team manager |
| Memory clinic 2 | 4 | 1.5 | 1–4 | 13 | No formal chairperson – varied throughout discussion | None |
The context within which each of the 12 teams operated, and attendance and structural characteristics, are described below.
Cancer teams
Context
All four cancer teams worked closely according to local and national policies and guidelines, which were mentioned frequently in meetings. Thus, references were often made to NICE guidelines and professional guidelines for pathology or radiology reporting, as well as to local discharge policies and team operational policies. It was also apparent that teams were expected to collect and report specific data regularly, for example diagnostic information for Cancer Registries.
National waiting time targets and breach dates were a recurring issue for two of the cancer teams. This added to a sense of constant pressure: ‘we are under scrutiny at the moment’ (cancer surgeon, observation) and ‘we are asking for more [theatre] lists left, right and centre’ (cancer surgeon, observation). Occasionally, in these teams there was also mention of bed shortages, but these comments were made in passing and the discussion did not dwell on these issues. Some members of the MDT believed these pressures were unsustainable, while others did not necessarily think they were a problem. The quotes below illustrate that these differences were apparent even within the same team:
I think we’ve got more clinicians, we’re seeing more patients, we’ve got more patients with complex issues and I would question, it, it feels fragile because I feel that, it feels like you’re in a bubble that’s about to burst. How much more can, how, how much more can you take?
Cancer nurse, interview
In general . . . people are very happy to just have extra patients in clinic.
Cancer doctor, interview
Meeting environment
All the cancer MDT meetings were held weekly in dedicated hospital meeting rooms. These were set up in a lecture-style format, with rows of chairs facing projector screens at the front, which were used to display pathology and radiology images. This was in keeping with the hierarchical nature of the meetings and there was a tendency for consultants to sit towards the front of the room, with junior doctors, nurses and other members of the team further back. All cancer teams used information technology (IT), either to present and access patient information, or to link up with clinicians on different sites.
Attendance and structure
The cancer MDTs were the most heavily attended teams we observed. National guidelines for each tumour type list ‘core’ professions that must be present at MDT meetings, including specialist surgeons (where applicable), oncologists, imaging specialists, pathologists, clinical nurse specialists and MDT co-ordinators. Teams documented attendance at each meeting to demonstrate adherence to the national guidelines. However, in practice, in some teams not all clinicians attended for the duration of the meeting. Further detail about the specific disciplines and structure of the meeting by team are included in Appendix 10.
In two of the teams in particular, there were regular interruptions and members would talk over each other, or in small groups, on occasions taking phone calls during the meeting to discuss other issues.
Decision-making processes
The focus of all the cancer team meetings was on making treatment decisions for patients and there was a fairly standard format to the way that cases were presented. This involved an initial brief presentation of a patient by a consultant or specialist registrar, depending on who had seen the patient. Very occasionally a clinical nurse specialist would present a case, but this would be an exception rather than the norm. The results of investigations were presented by radiologists and pathologists. In addition, some of the teams accessed patient information either electronically or using paper records during the meeting to clarify issues relating to, for example, comorbidities or previous test results. Presentation of cases and discussions were generally structured, with a focus on medical issues. Presentations rarely included reference to patient preferences or social factors.
It was common for decisions to relate to clinical treatment options, for example specific chemotherapy regimens or surgical procedures (depending on the specialty). Most other decisions related to carrying out further investigations or tests (e.g. bone marrow biopsy, chest radiograph, skeletal survey), liaising with or referring to another team, decisions to watch and wait, or to discharge or admit. These decisions were generally quite clearly summarised and then documented so even those who had not attended the meeting were able to see what decision the MDT had made for a specific patient.
Across all cancer teams, and in line with national guidance, all new cancer patients were discussed at the MDT meeting. Where the team did not have the necessary information to make a decision, for example where the pathology results were not yet available, cases were deferred until the following week. Where a patient required urgent treatment and could not wait until the next meeting, a decision would be made outside the meeting, but this was usually then ratified the following week.
Heart failure teams
Context
The two heart failure teams operated in very different contexts from each other. Although there were occasional references to NICE guidelines for diagnosis and treatment in both teams, there was no sense of a clear national policy to provide a framework for the MDT meeting itself (in contrast with the cancer teams).
Although there was an awareness of resourcing issues, as demonstrated for example by reference to procedures being ‘expensive’, ‘cost-effective’ or ‘cheap’, there was less of a sense of financial pressure than in some other teams:
I know we’ve got lots of money to throw at these things, but is that really going to change how we manage him?
Heart failure doctor, observation
Meeting environment
Both heart failure MDT meetings were held weekly on an acute hospital site and used a computer and projector to present and access patient information.
For team 1, the meeting room was set up in a lecture-style format, with rows of chairs facing the projector screens at the front:
It’s got everything we need there and the room’s big enough for the meeting.
Heart failure nurse, interview
Although both heart failure teams were medically dominated, the larger team was also hierarchical in physical terms, with consultants sitting at the front of the room and nurses and others towards the back. Team 2 was much smaller, with members sitting in a large room around a table, in no particular order.
Attendance and structure
Attendance, structure and processes of the two meetings were very different from each other. Team 1 had formal structures and processes, with a wide range of different professionals attending regularly and punctually (further detail about the specific disciplines and structure of the meeting by team is included in Appendix 10). This team was more similar to the cancer teams described above, both in size and in having an agreed patient list used during the meeting, structured presentations, and documented decisions which were later added to patient notes. Team 2 was a newly established team, and was much smaller (usually five attendees), with a less formal structure. There were regular interruptions, with members often taking phone calls, arriving late and leaving early. Meetings were also sometimes cancelled because consultants were not able to attend.
Decision-making processes
Both teams presented patient information electronically for everyone in the room to see, and presentations were heavily dominated by a focus on imaging. In team 1, cases were presented by a junior doctor and in team 2 they were presented by a nurse or a specialist registrar. The format of presentations in team 1 was similar for all patients (information permitting), including symptoms/reasons for the patient being discussed, previous medical history, comorbidities, current drug regimens, blood test results and, finally, a slide at the end with proposals for a treatment plan. Consultants and specialist registrars tended to contribute spontaneously while the imaging was being presented. Following this, decisions were usually made by the consultant cardiologists, with the specialist heart failure nurses occasionally contributing or being invited to comment on particular issues such as social circumstances. In team 2, the presentation of patients varied. In some cases, presentations were brief and limited to short, specific questions. In other cases, the patient history and test results were presented before a decision was made.
The type of decisions made in both heart failure teams tended to relate to medication or surgery, liaison or referral to other teams, including palliative care, further reviews or investigations, or discharge.
Not all patients were discussed in heart failure meetings, and those that were tended to be complex cases, or those where a member of the team had a specific query to be addressed. This meant that there were fewer patients discussed than in the cancer team meetings, even though some meetings ran for a similar length of time.
Memory clinic teams
Context
The two memory clinic MDTs were relatively small teams. In terms of context, it was apparent that both teams were operating under local and national policies and guidelines, but there was no clear national policy to provide a framework for the MDT meeting itself. When these teams did refer to policies or guidelines, these mainly related to diagnostic criteria (i.e. World Health Organization International Classification of Diseases) or treatment protocols:
We have a very clear protocol about prescribing antipsychotics and that behavioural routes should be tried first.
Memory clinic nurse, observation
Caseload and capacity issues were a common theme:
It’s ridiculous! . . . 15 [new referrals] is really bad news because we haven’t got the people here to see them . . . as we’re in complete crisis I’ll take four [new referrals] . . . all hands on deck!
Memory clinic doctor, observation
Meeting environment
Both teams catered to community-based patients, though the meetings took place on hospital sites. Team members sat around a table and discussions were relaxed and friendly. In team 1, there were sometimes problems with the room, such as inappropriate heating and inadequate space:
It actually gets really hot in there in the winter cause the radiator is very fierce so from a heating point of view it can become uncomfortable at times.
Memory clinic team manager, interview
Occasionally it’s a little bit cramped.
Memory clinic doctor, interview
Team 2 did not use any IT during the meeting and relied on paper patient records and individual members’ notes. Team 1 recorded decisions directly into patient records using a laptop.
Attendance and structure
Team 1 had regular attendance by all members and there were very few disruptions to the meeting. In contrast, attendance in team 2 was irregular and members would arrive late or leave early. The consultants would often take phone calls during the meeting.
There were also notable differences between the teams in terms of their purpose and structure. Team 2 had a very specific purpose, which was to diagnose patients. The meeting was medically dominated in terms of both attendance and participation. Discussions were often very long, and there was not always time to discuss all cases that had been brought to the meeting.
Team 1 had a broader remit, managing and treating patients as well as determining their diagnosis. Reflecting this broader function, this team had more disciplines attending and more multiprofessional participation (further detail about the specific disciplines and structure of the meeting by team is included in Appendix 10). Discussions were clearly structured and all cases brought to the meeting were discussed.
Decision-making processes
Patients were generally presented by the member of the team who was working with them, after which other team members would ask questions and there was an opportunity for discussion. In both teams, there was extensive discussion about patient characteristics and the needs of family and/or carers. Consultant psychiatrists usually made the decisions although others had the opportunity to contribute. In team 1, it was common for the consultant psychiatrist to have made decisions about their own patients before the MDT meeting, and these were then fed back to the team. This was not usually the case for other team members although occasionally some members would present a plan they had already implemented, with other team members suggesting additional actions.
Decisions in team 1 related to diagnosis, further assessments or investigations, medication, reviews or follow-up appointments, discharge and contact with other services in the community including GPs. Decisions also concerned practical or social issues, for example organising a smoke alarm to be fitted or giving advice about state benefits. In contrast, decisions made by team 2 were clearly focused on making a diagnosis, and patients were subsequently discharged back to the GP or followed up in clinic. Occasionally, decisions were made about further investigations or recruitment to trials but this was much less common.
Decisions were clearly recorded and summarised in both memory teams.
Mental health teams
Context
All four mental health teams were undergoing major organisational restructuring. During the observation period, team 1 disbanded and teams 2 and 3 were preparing for major changes. Generic CMHTs were being abolished in favour of triage teams that would redirect referrals into specialist teams dealing with specific kinds of mental illness (e.g. psychosis, personality disorders). As part of the restructuring, staff were being transferred to different teams, many had to re-interview for their jobs and redundancies were expected.
This context had a major influence on the attitudes and morale of staff, and many felt it was negatively impacting on the quality of care:
There’s some redundancies and people re-applying for their jobs, and uncertainty, and breakdown of teams, and merging of teams, and people going . . . that has affected morale and it’s also, I think, had an impact. I think some of our structures within the team and the direction has loosened a little . . . tightness of discharge criteria, regularity of meetings . . . all of these things have sort of been eroded a bit you know.
Mental health doctor, interview
People are far less able to engage and reflect on the way that they’re making decisions and empathise with people . . . if they’re in a state of fear. I am absolutely sure it has a direct influence [on care].
Mental health social worker, interview
Team members frequently discussed resource constraints such as restricted treatment options due to service closures, a lack of appointment slots and difficulty finding a physical space to hold appointments. It was apparent that members of the teams had to make difficult choices balancing ‘the clinical ideal versus the financial reality’ (mental health psychologist, observation). Two of the mental health teams had no psychologist, and reference was made to a waiting list of 18 months for referral to primary care psychology (further detail about the specific disciplines and structure of the meeting by team is included in Appendix 10).
Meeting environment
The meeting rooms were just large enough to accommodate the team and members would sit in a circle around a table. Meetings were described as having a relatively ‘relaxed and open atmosphere’ (mental health social worker, interview) and discussions were inclusive, with all attendees contributing each week. Case presentations were relatively informal and unstructured. Only team 4 used a computer (to take minutes).
Attendance and structure
The whole team usually attended, with occasional visitors such as researchers, students or members from related teams (forensic, home treatment or crisis services). Meetings were structured into sections which varied slightly by team. All teams had sections for feeding back on new assessments and raising ongoing cases of concern. Other sections, found in some teams but not all, included recent/planned Mental Health Act assessments, clients under Home Treatment Services, clients under Crisis Services, and new referrals. Generally, the chairperson announced each section and members volunteered if they had anything relevant to report. The meeting served a range of functions (see The purpose and functions of MDT meetings) and discussions did not always result in a specific treatment plan for each patient.
Decision-making processes
The CMHT meetings focused on members of the team feeding back about recent appointments and challenges or noteworthy incidents such as patients’ relapsing or having crises. Discussions were not usually framed in terms of specific queries or deciding between different options, and did not always result in a treatment plan. Rather, members of the team often provided a range of suggestions and advice to the patient’s key worker without explicitly agreeing a particular course of action.
Sometimes team members explicitly sought advice about particular options (‘I wondered if he’d be a candidate for cognitive behavioural therapy, I don’t know what anybody else thinks?’, mental health social worker, observation), while on other occasions they would describe a general problem (‘I don’t know what to do. I’m stuck’, mental health social worker, observation) and the team would use the discussion to generate ideas.
Where decisions were made, they often related to arranging further appointments with patients and attempts to resolve ongoing issues, for example trying to persuade patients to take medication or to attend their appointments. There were also decisions about social issues, including arranging accommodation or checking a patient’s asylum status as well as liaising with other services in primary and community care such as forensic, housing, and drug and alcohol services.
Of the four teams, CMHT 1 used the weekly MDT meeting to share the contents of new referral letters and allocate them to a key worker (also known as a care co-ordinator). In CMHTs 2, 3 and 4, cases were allocated by the team manager. In CMHT 4, a patient list was distributed at the beginning of the meeting listing all patients under the care of the team, organised by key worker. The team would read through the names and the relevant key worker would comment, sometimes briefly, on each patient. In the other teams (CMHT 1, 2 and 3), members were prompted to feed back on new assessments but there were no patient lists and it was up to individual team members to raise patients about whom they had particular concerns.
In addition to the weekly MDT meetings, CMHTs 2 and 3 had brief morning handover meetings to make everyone aware of any crises or emergencies that needed to be attended to.
Qualitative exploration of the quantitative results
The main findings from the quantitative analysis formed the basis for the following five deductive themes, which we explored further using our qualitative data:
-
theme 1: influence of disease specialty on implementation
-
theme 2: influence of multidisciplinarity on implementation
-
theme 3: influence of team climate on implementation
-
theme 4: influence of socioeconomic characteristics on implementation
-
theme 5: roles that patient preference, health behaviours and other clinical factors may have in explaining the socioeconomic variations in implementation.
Theme 1: influence of disease specialty on implementation
Our quantitative analysis demonstrated significant differences in implementation rates across specialties. However, we also found considerable variation within specialties (e.g. memory clinic 1 implemented 76% of the decisions made, while memory clinic 2 implemented 94%). The qualitative data indicated that this may be because the two memory clinics tended to carry out different functions:
I think the main function . . . it’s discussion about clinical cases and making decisions about clinical cases. Be that assessment, intervention, you know, that would be the main role, and I think there are other roles, like information giving, teaching, sharing, support role, for other practitioners. I think, but mainly it’s clinical discussion, you know, around cases, and planning around what you’re going to do with the case.
Memory clinic 1 psychologist, interview
It [the main aim of the meeting] tends to be making a diagnosis for the client. It is like a diagnostic clinic.
Memory clinic 2 psychologist, interview
High-implementing teams, regardless of disease specialty, more frequently referred to diagnostic or treatment protocols and national guidelines. In the cancer MDTs, for example, all new patients were discussed, many of whom were treated according to standard protocols. This contributed to a higher throughput of patients and higher implementation rates than for those non-cancer teams which focused on discussing only complex cases where the appropriate treatment plan was less clear:
We have protocols for melanoma here, so that’s easy. The same with squamous cell and basal cell carcinoma there are national guidelines about optimal treatment. And so often the decision is obvious.
Cancer doctor, interview
High-implementing teams also had clear goals, and members shared the view that the main purpose of the MDT meeting was to make treatment recommendations for patients. In contrast, in lower-implementing teams, members identified a range of diverse objectives and some stated that there was a lack of clarity of purpose:
I am never quite sure what the purpose of the meetings are . . . It was the thing that was done and therefore I did not have any say on whether it was done or not done.
Mental health nurse, interview
All four cancer teams adhered closely to national guidelines, so they had the dedicated administrative support members considered to be essential for meeting preparation and facilitation:
[The MDT co-ordinator] she’s brilliant. She really is. She holds the meeting together very well I think . . . She’s really, really important. Like she’s not medical in the slightest, but she knows where the patients are, when they should be treated.
Cancer nurse, interview
High-implementing teams also tended to have permanent and authoritative chairpersons who maintained a focus on decisions, which were clearly recorded.
I think it is the best-run meeting I have attended . . . the person who runs it, he runs it very well in the sense that he is very strict in starting the meeting on time, going through cases promptly.
Cancer diagnostic doctor, interview
In teams with rotating chairpersons, there was some concern that meetings were often left without a confident leader to direct and manage discussions. Occasionally time was spent at the start of the meeting trying to establish whose turn it was to chair that week:
I think one of the weaknesses is everyone chairing and therefore everyone being lumbered two or three times a year . . . seniors chairing the meeting, that makes a little bit more sense, because then that person can deliver their control of the group a bit more . . . [it] was the idea of having everyone as equals but not everyone was as equals.
Mental health nurse, interview
High-implementing teams also assembled lists of patients for discussion in advance of the meeting. This stimulated case presenters to consider the management options they wished to discuss before, rather than during, the meeting and led to more explicit decisions. The other MDTs included either none or some of these features, and this varied by team rather than by disease type.
Theme 2: influence of multidisciplinarity on implementation
Contrary to policy-makers’ expectations that multidisciplinarity increases the quality of decisions and thus the likelihood of implementation, the quantitative analysis found that there were increased odds of implementation when there were fewer professional groups in attendance. However, this trend was not consistent across teams and was mostly accounted for by mental health and memory teams. In these teams, when meetings were attended by more professional groups there was a tendency for very diverse issues to be raised in an ad hoc manner, with abrupt changes of subject. For example, the discussion might move from medication to housing problems to employment issues, without a clear summary of what was decided in relation to each issue. There was a lack of focus on specific questions and documentation was inconsistent. Thus, if a key worker was unclear about which of the suggestions had been ultimately agreed, there was rarely a comprehensive record of the discussion to refer to.
In addition, some MDT members found decision-making more difficult when a range of professionals were attending and teams struggled to bring the diverse viewpoints into alignment to agree a plan:
Sometimes I used to think too many cooks . . . you could have too many opinions.
Mental health social worker, interview
Sometimes it feels a bit like they feel like they’re in two camps [social work and nursing] . . . social workers very much do try to defend, you know a person’s right to be mad. I mean the nurse view is ‘you’re not well so we need to get you on the path and let’s make the decisions you would make’ . . . well social workers, they come from a different point of view.
Mental health nurse, interview
Across all teams, participants emphasised the value of disciplinary diversity and considered multidisciplinary participation a key benefit of MDT meetings:
All of those people bring different things into that mix.
Cancer doctor, interview
In practice, however, there was considerable variation in the degree of multidisciplinary input into discussions. Some complained that meetings were overly dominated by the medical profession in terms of both the numbers of each discipline represented and the power dynamics involved:
It’s rarely a multidisciplinary meeting. It is in many ways, it often consists of a surgeon talking to the radiologist.
Cancer doctor, interview
It is quite medically dominant. There can be one psychologist and assistant and then a lot of doctors, it can be a very strong medical dominance in terms of what is more likely to be the outcome.
Memory clinic psychologist, interview
Even where disciplines were represented in the team, some felt it was difficult for them to be effective if they were largely outnumbered:
Doing it on your own is very hard. I think MDTs don’t work where you’ve got overwhelming numbers of one set of professional and just one of another.
Mental health social worker, interview
Several non-medical MDT members commented that they did not feel a sense of ownership at the meeting and did not always feel able to contribute freely about patient management or the design of the meeting:
The room is full, there are other people in there . . . however they don’t have a voice. I notice that nobody else speaks. It’s just the consultants that are around the table that discuss among themselves . . . nobody else has any involvement.
Heart failure nurse, interview
In some teams there was a physical divide with consultants sitting at the front and nurses at the back:
The discussion is very much focussed on the people who are sitting at the front and information is invited from other people if it’s required . . . sometimes does make it difficult to hear and/or be involved in a discussion.
Cancer nurse, interview
However, some of the doctors found it frustrating that the other professionals did not involve themselves more:
I’m always amazed how very able staff can be so passive . . . it would be nice if the non-medical people were a little bit more volunteering, didn’t have to draw out, but I’ve given up trying to expect that.
Heart failure doctor, interview
The principle of multidisciplinary input, therefore, was highly valued by professionals across all disease specialties. However, there was dissatisfaction about the way that it played out in practice.
Theme 3: influence of team climate on implementation
The team meeting was considered important for many aspects of team functioning. Health professionals emphasised that team meetings served a range of social functions in addition to decision-making, including opportunities for peer support, team-building and bonding:
I probably go there because it’s valuable for education, for team building, for giving a sense of responsibility, for influencing other people’s approach to things, for building up team culture, that’s the reasons I go.
Heart failure doctor, interview
The MDT meeting was also perceived to be important for understanding the roles of other team members:
An opportunity for I think all the staff to be aware of the sort of work that other members of the team do and I think that’s you know, sort of all helps with sort of cohesiveness of the team and also you know awareness.
Memory clinic doctor, interview
In this way, MDT meetings were a forum where team climate was cultivated, providing an opportunity to develop the team’s culture, share learning and develop clear understandings of one another’s roles. These positive aspects of the meeting were considered key to the general effectiveness of the team.
Theme 4: influence of socioeconomic characteristics on implementation
The odds of implementation was lower for more socioeconomically disadvantaged patients. Socioeconomic information was only occasionally mentioned in cancer and heart failure teams. In contrast, it was routinely discussed in memory clinic and mental health teams: ‘he lives with his wife no financial concerns at all’ (memory clinic doctor, observation).
Has been claiming on and off for seventeen years of his life . . . he’s trapped really I think with his kind of ideology and the reality that’s he’s on housing benefit. He’s on DLA [disability living allowance] and he’s on, he couldn’t remember if it’s income support or incapacity erm, and he’s on these benefits so and he’s taking up our time and resources as a team . . . he’s very bright so he’s in no denial about that, the reality of that he’s receiving them. And he can’t seem to manage without them. You know, he can’t hold down a job.
Mental health social worker, observation
Although socioeconomic circumstances were discussed within the context of services provided by mental health and memory clinic teams, it was unclear how, if at all, these influenced decision-making or implementation.
Theme 5: roles that patient preference, health behaviours and other clinical factors may have in explaining the socioeconomic variations in implementation
We explored the roles that patient preference, health behaviours and other clinical factors may have in explaining the socioeconomic variations in implementation that we found in the quantitative analyses. However, although there was marked team variation in discussion of these factors, most members considered that the extent to which ‘health behaviours and other clinical factors’ were discussed was appropriate:
I think we discuss it when it’s important . . . yes we do bring in physical problems as well which we need to if necessary.
Memory clinic allied health professional, interview
However, it was acknowledged that information on comorbidities was not always available when making decisions in the MDT meeting:
There may be some instances where we don’t know enough about the comorbidities to make the decision and have come back to clinic and found that actually the patient’s not fit for a particular treatment.
Cancer doctor, interview
There were mixed views from patients, with many indicating that they were happy for clinicians to use their judgement when presenting their cases, and that there was nothing specific about them that they would want mentioned. Others felt strongly that their comorbidities were crucial to their quality of life and therefore should be discussed:
What is the point in mending a man’s heart, well not mending it but keeping it going on a really nice basis, and lungs, if every day he has got wind up to his ear holes, he can’t eat and he feels awful. It just seems bad, it seems wrong.
Heart failure patient, interview
With regard to patient preferences, some clinicians argued that these should be central to MDT decision-making:
If you don’t take patients’ views into account that does create a lot of problems for the team in the long run, so I think it’s important for both the patient and the team, so that’s become a big part of your decision-making.
Memory clinic doctor, interview
Others preferred to elicit patient preferences after the MDT, when treatment options could be shared with patients. This was attributed partly to the fact that patient preferences can change over time, but also to the importance of being fully informed before discussing treatment options with a patient in clinic:
One of the values of the MDT meeting is to allow the clinician to actually go into a consultation [after the meeting] with a patient and tell them what the options are, tell them how the decision has been reached and what the advantages and disadvantages are, and I think that that’s more useful to a patient than actually giving patients a list of options beforehand . . . and then having the MDT meeting decide that half those options are off the table anyway.
Cancer doctor, interview
In summary, although patients from more deprived areas are less likely to have their treatment plans implemented, consideration of patient preference, comorbidities or other health-related factors did not seem to explain this.
Additional information in regards to discussion of comorbidities is presented in Content of discussion in multidisciplinary team meetings, theme 11. Further discussion of patient preferences in MDT discussions is explored in Content of discussion in multidisciplinary team meetings, theme 13.
Qualitative investigation of areas of diversity in beliefs and practices
Our synthesis of the observation, professional and patient interview data resulted in 16 metathemes relating to areas of diverse beliefs or practices in MDT meetings (Box 4) (see Chapter 2, Summary measures, for a description of this analysis). Each of the 16 metathemes is discussed below.
-
Theme 1: the purpose of MDT meetings.
-
Theme 2: teaching and peer-to-peer learning as a function of MDT meetings.
-
Theme 3: recruitment to trials in MDT meetings.
-
Theme 4: attendance and participation in the MDT meeting.
-
Theme 5: administrative support and the role of the MDT co-ordinator.
-
Theme 6: chairing the MDT meeting.
-
Theme 7: agreeing which patients should be discussed in MDT meetings.
-
Theme 8: preparing and presenting cases for discussion.
-
Theme 9: recording and reviewing MDT decisions.
-
Theme 10: the role of research and evidence in MDT meetings.
-
Theme 11: discussing comorbidities at MDT meetings.
-
Theme 12: discussing patients holistically.
-
Theme 13: incorporating patient preferences about treatment into MDT discussions.
-
Theme 14: patient awareness of MDT meetings.
-
Theme 15: patient attendance at MDT meetings.
-
Theme 16: providing feedback to patients on the outcome of MDT discussions.
The purpose and functions of multidisciplinary team meetings
Theme 1: the purpose of multidisciplinary team meetings
Our analysis demonstrated that MDT meetings served a variety of functions, including decision-making, information-sharing, peer support and education. When asked what the primary function of the MDT meeting was, interviewees provided a wide range of responses, although there were some similarities across specialties (Table 17). While ‘agreeing treatment plans’/‘decision-making’ and ‘diagnosis’ were cited by cancer, mental health and memory clinic teams, the heart failure and mental health teams also reported that ‘ensuring quality’ was a primary function.
| Cancer | Memory clinic | Mental health | Heart failure |
|---|---|---|---|
| Agreeing treatment plans | Agreeing treatment plans | Decision-making | Improving care through consensus |
| Meeting audit requirements | Validation of earlier decisions | Ensuring quality and consistency | Ensuring quality |
| Holistic multidisciplinary review | Liaising with peers | Liaising with peers | Coordinating different specialties |
| Diagnosis | Diagnosis | Information-sharing | Information-gathering |
| Checking trial eligibility | Seeking advice | Seeking advice | |
| Team cohesiveness | Raising concerns and providing support | ||
| Feedback on ongoing work (sharing responsibility) |
For cancer teams, the responses centred around decision-making regarding treatment, diagnosis and eligibility of patients to participate in research: ‘the overt aim is to review all the patients and their treatment decisions’ (cancer psychologist, interview).
In contrast, mental health interviewees highlighted broader communication functions such as liaising with peers, advice and peer support, and providing feedback to ensure continuity of care:
It’s a useful thing, well, in all sorts of ways, it’s information-sharing, it’s an opportunity for the team to actually meet as a group and know each other and know what everyone’s doing in a way. It’s a, it’s quite a containing function . . . it can be quite a supportive thing for members of the team as well . . . a nurturing thing, an educational thing, people can learn from what others say.
Mental health doctor, interview
The wide range of explicit and implicit functions was also clear from the observation data. For example, meetings in all specialties were also sometimes used to carry out management functions (e.g. reminding staff of administrative deadlines, training opportunities, etc.), and some mental health teams regularly used the meeting to discuss the ongoing service restructuring.
The time dedicated to decision-making as opposed to other functions varied across teams and specialties. The more treatment decision-focused meetings tended to have a structured format designed to support decision-making. This included a precirculated agenda listing patients for discussion, prepared case presentations and standard forms for documenting each patient’s treatment plan. Teams with more wide-ranging functions tended to raise patients in a less structured manner, taking turns to discuss clients of concern, usually without prepared presentations or paperwork and with details documented in free-form minutes.
Though almost all mental health professionals endorsed the team meetings as valuable, some believed their purpose needed to be revisited:
I think with things that evolve organically, I think we, probably don’t step back and think as readily as we should about what’s the purpose that it solves . . . we probably haven’t actually talked about the function of the team meeting probably for about 3 years at least.
Mental health doctor, interview
Go back to the basics and revisit [the objectives] . . . if you interview some people here they might say, ‘Well, I don’t see the purpose of them’. I see the importance in them but it’s just to refresh.
Mental health nurse, interview
In contrast to the desire for a more clearly defined purpose in mental health, some members of other teams reported that they would like to use the meeting in a more flexible way, with more time for feedback and peer support: ‘that’s another thing that could be improved is feedback [about outcomes of previous decisions]’ (heart failure doctor, interview).
I think that sometimes the team don’t talk about the impact of what’s it’s like to work with difficult clients in our MDT . . . I do hear people say in our team meeting, you know, ‘I’m really frustrated because the wife never does what I say’, but I don’t think we always have enough time to think with them about how could they manage their response to that.
Memory clinic psychologist, interview
Theme 2: teaching and peer-to-peer learning as a function of multidisciplinary team meetings
Teaching
There was evidence of teaching in some teams, but not all. This was not specific to teams of the same disease type: teaching was a feature in most cancer teams, but not all, and in one heart failure team but not the other. For some, teaching was argued to be an opportunity to make meetings more inclusive and stimulating:
If they tried to genuinely include everyone, made it a teaching session as well, you know, talk to young doctors about things, and medical students about things and so on and so forth. It would be a more stimulating environment, less parochial, less excluding I suppose.
Cancer surgeon, interview
Teaching included junior doctors presenting patients to the MDT meeting and being questioned about specific drugs or therapies, and individuals sharing knowledge from their particular specialty as a form of interdisciplinary education:
This is just to show you a beautiful classical Reed–Sternberg cell you don’t often see that.
Cancer diagnostic doctor, observation
There were also examples of team members being given advice on how to present cases to the rest of the team:
Just stop you there to give you feedback . . . so the problem is if you present history with all of that then you forgotten the punch lines . . . so you know try to summarise.
Heart failure doctor to heart failure nurse, observation
However, concerns were raised about the practicalities of teaching in MDT meetings, particularly given time constraints:
It’s always a bit of a challenge I think with MDT meetings . . . how much you use them as an opportunity for learning.
Memory clinic doctor, interview
In addition, some MDT members also reported that students were not always able to keep up with the pressurised pace of the meeting.
Peer-to-peer learning
For some members peer learning, particularly between different professions or specialties, was perceived to be a valuable part of the meeting:
I actually think it, it was always an opportunity to learn from, from colleagues.
Mental health social worker, interview
Others argued that in reality there was little scope for professional development in MDT meetings: ‘I personally don’t feel that I benefit anything professionally from being there’ (cancer nurse, interview).
Theme 3: recruitment to trials in multidisciplinary team meetings
There was wide variation between MDT meetings in how frequently recruitment to clinical trials was considered. Formal mechanisms for recruiting patients to clinical trials were evident in some teams, for example some cancer and heart failure teams had dedicated research nurses or clinical trials practitioners at the MDT meeting. In other teams reference to eligibility of a specific patient for a clinical or research trial was made on an ad hoc basis by a member of the team, most commonly the lead consultant. Some mental health, heart failure and memory teams occasionally had an external speaker attend the meeting to tell the team about a new study (either to encourage the team to recruit patients or to disseminate findings), and in cancer teams members occasionally gave brief reminders to the team about specific trials they were involved with to raise awareness within the team: ‘people should know about the EuLITE [European Trial of Free Light Chain Removal by Extended Haemodialysis in Cast Nephropathy] trial, it’s a real breakthrough’ (cancer doctor, observation).
Some members found it helpful to discuss trial recruitment in the MDT meeting to raise awareness of trials and eligibility criteria, and to ensure that patients were identified at an early stage:
When you sit in the clinic talking to the patient, you don’t necessarily consider, or you may not be aware of the trials that the patient might be eligible for and just discussing it at the meeting . . . have you considered giving them the option of participating in this trial or that trial . . . all of these things are good.
Cancer surgeon, interview
One interviewee however cautioned that becoming overly research focused could be at the expense of the individual patient under discussion, for example where there is conflict between clinical needs and research needs:
So say for instance that the clinic has a research orientation but it is dealing with clinical cases. So sometimes there are conflicts between clinical needs and research needs. So I guess that. Sometimes it doesn’t work well, the clinical needs are outweighed by the research needs.
Memory clinic psychologist, interview
The structure of multidisciplinary team meetings
Theme 4: attendance and participation in the multidisciplinary team meeting
Attendance
The MDT meetings for memory and mental health were usually attended by the whole team, and were characterised by discussions in which most members participated. Those for cancer and one of the heart failure teams tended to be larger and were often attended by additional people who were not part of the core team. These included observers such as visiting members of staff and students, and professionals from other clinical teams who had come to discuss specific patients.
It was common in cancer meetings for some members to attend only specific parts of the meeting:
One of the radiologists will quite often leave the meeting part way through, the pathologists don’t turn up till near, till part way through the meeting, the oncologists’ attendance is flaky.
Cancer doctor, interview
Punctuality was also an issue in some teams, with delays occurring while waiting for all team members to arrive. On several occasions mental health team members had to send someone back to the office to ‘round up’ the remaining team members and encourage them to attend. This indicated a certain reluctance to attend the meetings among some members and resentment among those who felt the meeting should be prioritised:
Is this us? Four people? It’s pathetic!
[They discuss which staff are on annual leave and note that several team members had been seen in the office.]
Did they look like they were moving?
I don’t know, shall I go and –
Yes, give them a hard poke. You know the sooner we start the sooner it’s over.
Mental health team, observation
I’m going to send out an e-mail [about attendance at meetings] because I think we’re going to lose the sense of a team meeting if we don’t, I know it’s hard and everyone’s flat out, but it’s just the only opportunity to think about, you know, the difficulties we have with work that we do and if we don’t come together and really prioritise that time.
That will make it worse.
Yeah.
Mental health team, observation
In some teams, poor punctuality regularly delayed the start of the meeting and often a meeting would be disrupted by latecomers because the layout of the room would need to be rearranged and chairs retrieved from other rooms to accommodate them.
Participants cited conflicting work commitments and practical difficulties getting to the location as reasons for poor and late attendance:
It’s just other commitments, just busy-ness; generally we all work in various different places. On the wards, here, in outpatient clinics, on other hospital sites.
Heart failure doctor, interview
We’re all pretty busy and for some of us, by the time we get to the meeting, have the meeting, that’s a couple of hours out of the day.
Heart failure nurse, interview
Participation
As outlined in Theme 2: influence of multidisciplinarity on implementation, participants emphasised the value of multidisciplinary participation in MDT meetings. However there was some dissatisfaction with how this played out in practice.
There was also evidence that not all members found the meetings to be a valuable use of their time. Though the very senior members tended to participate in all discussions, most attendees contributed only when discussing patients directly under their care and many rarely spoke at all:
There were fifteen, sixteen, seventeen people are sitting doing nothing because other people’s patients were being discussed. You’re actually using up about 14 personnel in one of those meetings, that’s the equivalent of having another worker on the team and that felt like an enormous waste at the time.
Memory clinic doctor, interview
Tedious . . . we are talking about people that, you haven’t got a clue what they look like, who they are.
Mental health nurse, interview
In addition, some interviewees complained that the team lacked key professional groups; for example, two of the mental health teams did not have a psychologist. Several mentioned that it would be useful to have involvement from other disciplines such as general practice and palliative care.
Theme 5: administrative support and the role of the multidisciplinary team co-ordinator
There was wide variation in how MDT meetings were organised in different specialties. All cancer teams studied had dedicated MDT co-ordinators, while in heart failure, memory and mental health, administrative duties were undertaken by managers, health-care professionals and general administrators.
Cancer MDT co-ordinator responsibilities included distributing lists of patients for discussion, operating IT equipment for imaging and video-links, recording attendance, ensuring that medical records and test results were available at the meeting, and monitoring patient review periods and compliance with waiting time targets. Some MDT co-ordinators also recorded the outcome of each MDT discussion.
In teams without MDT co-ordinators, administrative support was more limited and MDT related administration was largely undertaken by the professionals themselves. There was evidence that some professionals resented having to perform administrative functions, as it detracted from their ability to perform their professional roles:
It’s a bit stressful . . . doing an admin role as well as participating as a professional.
Mental health nurse, interview
What is the manager’s role? Is it just to be the admin person who produces the minutes and sort of chairs the meeting meekly on behalf of the psychiatrists so they don’t have to? Or is it to say no, this is where we’re going with this team?
Mental health team manager, interview
In teams without MDT co-ordinators, meetings were often disrupted by people having to leave to get documents which were necessary for the discussion (e.g. test results, medical records). There was occasionally confusion about whether or not particular patients had already been discussed at previous meetings:
We don’t have the form, what are we going to do? Isn’t it on a memory stick? . . . I’ll just go quickly upstairs.
Memory clinic doctor 1, observation
I think I’ve already discussed him . . . I don’t know, I haven’t brought him, erm I am not sure . . . I can’t remember . . . have I discussed him?
Memory clinic doctor 2, observation
In contrast, in cancer teams, MDT co-ordinators ensured that relevant documents were brought to the meeting and kept track of which cases had been discussed previously and which were due for discussion. Even in these teams, however, missing information was a relatively common problem, particularly when the team was reliant on information being transferred from another hospital:
I don’t have the stains.
The histology’s not ready yet, do you want to leave it?
Yep so we’ll have to move that to another week.
Cancer team, observation
It was clear from observations that, while there were similarities across teams in their administrative needs (e.g. having access to relevant documents), there were also differences. For example, an important part of the MDT co-ordinator role in cancer related to monitoring waiting times against national targets and co-ordinating pathology and radiology results. However, not all MDTs in other disease specialties routinely used radiology, pathology or other diagnostic information during their decision-making.
It is noteworthy that, among those teams which did not have dedicated co-ordinators, some managed administration more effectively than others. For example, one of the memory teams brought a laptop to the meeting which allowed them to access electronic medical records on demand. This meant that there was rarely any missing information or disruption due to people leaving to retrieve documents. Conversely, those teams with MDT co-ordinators were not immune to administrative and procedural problems (e.g. raising patients for discussion before necessary test results were available and providing out-of-date information):
We’re not good at updating the dialogue box about the patient which describes their history, comorbidities, etc. and sometimes you’ll find that those comments relate to six months before and the MDT co-ordinator has just cut and pasted them on to the sheet and I don’t think that’s good . . . if it’s not accurate that’s very upsetting.
Cancer doctor, interview
Multidisciplinary team meeting processes
Theme 6: chairing the multidisciplinary team meeting
The observation data showed that chairing varied considerably between teams. Most meetings were formally chaired by a member of the team: in cancer teams, one memory clinic team and two mental health teams, this was a designated person who chaired the meeting each week (either a consultant or the team manager). In two mental health teams, the chairperson rotated between team members on a weekly basis. The remaining three teams did not have a predefined chairing system and different senior members took the lead on different occasions, sometimes changing during a meeting.
Some members argued that it was a significant responsibility for one person to chair the meeting every week, and rotating between team members was seen as a way to reduce this burden. In addition, it was suggested that rotating this role provided an opportunity for everyone in the team to acquire chairing skills:
I liked the idea of having a rotating chair to give the others the opportunity to take on that skill.
Mental health doctor, interview
However, in teams with a rotating chairperson there were several occasions during the observation period when members did not know who was due to chair specific meetings, resulting in delays to the start of the meeting. In addition, some members acknowledged the specific skills needed to chair a meeting effectively, and argued that not everyone had the necessary authority or training to keep presentations succinct and discussions focused:
We rotate it, and some people chair better than others, and it depends on their sense of authority.
Mental health psychologist, interview
I think one of the weaknesses is everyone chairing and therefore everyone being lumbered two or three times a year . . . [Senior members chairing] makes a little bit more sense because then that person can deliver their control of the group a bit more. I think one of the reasons why they pass it around . . . was the idea of having everyone as equals, but not everyone was as equals.
Mental health nurse, interview
Theme 7: agreeing which patients should be discussed in multidisciplinary team meetings
Teams had different approaches to selecting patients to be discussed in MDT meetings. In the cancer teams, for example, all new patients were routinely discussed to agree an initial treatment plan, and patients tended to be discussed again only if a clinician had specific concerns. One mental health team went through the whole team caseload at each meeting. In contrast, heart failure team meetings focused only on specific groups of patients, for example all inpatients, and in most mental health and memory clinic meetings team members were given the opportunity to discuss patients if, or as and when, they thought it was necessary.
It became clear that there were different opinions among MDT members about which patients should be discussed in MDT meetings. Some argued that only complex cases should be raised because in many cases the same decision would be made even if that patient was not discussed in the meeting:
I think for most, the decision, the treatment decision is the treatment decision that most people would have come to conclude just through their knowledge base or an informal chat in clinic.
Cancer doctor, interview
There were also concerns about time constraints in the meeting, and prioritising patients according to complexity was seen as one way of ensuring that there was sufficient time to discuss those patients who would most benefit from multidisciplinary input:
Sometimes there are too many patients which means that some patients are not discussed or the discussion might be a bit rushed about some patients.
Cancer diagnostic doctor, interview
Another member warned of the dangers of creating a ‘tick box exercise’ (cancer nurse, interview) if too much emphasis was placed on discussing all new patients.
However, in spite of these concerns, many acknowledged the practical difficulties of stratifying patients according to complexity, for example how to determine in advance which patients would benefit the most from an MDT discussion. As one interviewee pointed out:
In the majority of cases you can pick up the ones that need more discussion, but there are always the surprise patients who throw up something unusual, who you can’t identify beforehand, and that’s the problem.
Cancer doctor, interview
It was also suggested that patients who were not discussed might miss out on the benefits of a multidisciplinary discussion, including consideration of different options and a decision-making process that was transparent and subject to scrutiny because ‘you have to robustly defend your decision in the MDT’ (cancer doctor, interview).
In teams which focused on complex cases, there was concern that devoting too much time to the most anxiety-provoking or risky cases could lead to the neglect of others:
The quiet clients might not get discussed, the ones who aren’t causing worry . . . not being very tightly looked after.
Mental health psychologist, interview
Some members also believed that patients could ‘only’ benefit from being discussed in an MDT: ‘it certainly can’t make anything worse I don’t think’ (heart failure doctor, interview).
It was suggested that one solution would be to prioritise patients in advance of the meeting to allow for more time for complex patients, without necessarily excluding other patients completely:
I think we discuss a lot of follow ups that maybe don’t need to perhaps see the images or actually formally discuss them. You could say, you know, ‘End of treatment scans show complete response, you know, and treatment ended.’ You could save maybe a bit of time in that way.
Cancer nurse, interview
Finally, another perspective was provided by a patient, who pointed out that not all patients want to be discussed in an MDT meeting, because they do not like the thought of being discussed by people they have never met:
These are people who I’ve never met who are reading abbreviations and in my case I find that very dangerous . . . I don’t really agree with some cases being a multidisciplinary discussion.
Memory clinic patient, interview
Theme 8: preparing and presenting cases for discussion
Preparing patient lists for discussion
In order to determine which patients were to be discussed at the MDT meeting, cancer teams and one of the heart failure teams had a patient list which was circulated to members in advance of the meeting (either by an MDT co-ordinator or by a clinical member of the team). Any member of the MDT could add to this list in advance of the meeting. In some cases, members also had to record the specific reason for bringing each patient for discussion.
The observation data highlighted a number of benefits to using a formal structured approach to determine which patients were discussed each week. This included the opportunity to collate all the necessary material needed to discuss a patient before the meeting:
They [reference to another MDT] are so organised and you literally go from one patient to the next to the next very smoothly, at a fair speed and still get all the information that you want.
Cancer nurse, interview
Patient lists were also used by some chairpersons to manage the meeting, because they knew in advance how many patients needed to be discussed and could prioritise cases on this basis:
There are around seven cases left so if there were those that have to be presented before we end the meeting we could talk about those.
Cancer team doctor, observation
Using a patient list also encouraged MDT members to define a specific query in advance of the meeting, leading to improved focus during the presentation.
Most importantly, however, having a patient list agreed in advance of the meeting was a means of ensuring that patients were not forgotten about. Teams which did not have a structured approach to selecting cases for discussion in the MDT meeting relied on members presenting patients as and when they thought it necessary. There was a risk with this approach that some patients would be forgotten about:
Because you’re so busy you end up at that meeting and there probably are things but you haven’t kind of thought about it and then you’re thinking at the end ‘oh no I wanted to talk about . . .’.
Mental health social worker, interview
This was also illustrated in a meeting where the chairperson asked if there were any Mental Health Act assessments. Someone replied that there was one last week that ‘I completely forgot to mention’ (mental health professional, observation).
However, there were a number of other arguments put forward that suggested that some flexibility in determining which patients were to be discussed was a positive thing. One issue related to the resources need to collate a patient list, because this relied on a member of the team co-ordinating this every week. In addition, some felt that an informal approach where all team members were asked in the meeting if there was anyone they wanted to discuss could be more inclusive, creating the expectation that all individuals should participate in the discussions:
You would literally go round the table . . . which I think at least gives a sense of equal importance to each member of the team.
Mental health social worker, interview
It was also suggested that sticking too rigidly to a list can disadvantage some groups of patients, as the order of patients on the list is likely to impact on the length of time available for their discussions:
Because of the time pressures on that meeting often what happens is . . . everyone else is rushed at the end. So I would potentially either you have to curtail the cases a bit or you have to allow the meeting to go on longer and maybe mix up the order a bit . . . maybe one week you start with a different [patient group] just so that there’s, just more room for some other, some other cases.
Cancer nurse, interview
Presenting cases for discussion
Again, differences were apparent between teams in the way that patients were presented in the MDT meeting. A relatively structured approach was used in the cancer and heart failure teams. This involved a brief case presentation of predominantly clinical information, followed by review of any relevant imaging or pathology results, and discussion and/or decision-making.
This contrasted with the approach taken in mental health and memory teams, where case presentations were less structured but generally more holistic, and focused on the patient and their personal and social circumstances as well as (and at times instead of) biomedical information. Specifically, some MDT meetings were characterised by lengthy presentations in which information was not prioritised according to its relevance for decision-making – ‘sometimes they go in unbelievable detail into incredibly irrelevant things’ (memory clinic doctor, interview) – and, in some cases, MDT members were unclear why a patient had been presented because no queries were raised: ‘Thanks for sharing that story, why did we hear it?’ (Mental health doctor, observation.)
The need for more focus in presentations was raised by MDT members from all types of team (cancer, mental health, memory and heart failure). A clearly structured presentation was seen as beneficial for a number of reasons, including the fact that it could open up a discussion to all team members, by ensuring that everyone had enough information to comment constructively on the case: ‘unless you know the background to the case well it’s very difficult to disagree’ (cancer doctor, interview).
It was also argued that well-structured presentations kept the meetings focused and more interesting, because otherwise:
People tend to dwell on cases and then everyone loses interest.
Cancer diagnostic doctor, interview
However, there were barriers to achieving focused and structured presentations, including the fact that some members were unsure of the best way to do this, particularly for complex cases:
Because I find myself it’s hard to discuss inpatients in a meeting when they’re very complex and there’s an awful lot going on with them . . . I did find it difficult at the beginning. And it’s also getting to know how people work and how people like you to present things as well.
Heart failure nurse, interview
In addition, some members stated that they valued the flexible nature of case presentations to stimulate more creative solutions to complex problems.
I’ve found a lot of times when I’m going to the doctors with my different opinion . . . you say what you have to say quickly . . . whereas in the meeting . . . at least you have that time to go back and forth and go back and forth.
Mental health nurse, interview
It was also apparent that different members within teams prioritised different types of information as important for discussion. This meant there was potential for certain types of information to be overlooked if there was too much emphasis on presenting information in a structured format.
Theme 9: recording and reviewing multidisciplinary team decisions
Recording multidisciplinary team decisions
The process, completeness and accuracy of documentation varied widely across teams and specialties. In some teams decisions or treatment plans were typed directly into patients’ electronic records, on a screen visible to all members of the team. In other teams, handwritten records were made and subsequently filed in the patient’s notes. In others, actions were noted as part of the minutes of the meeting, or recorded in a notebook. In this last case, patient names were often misspelled or omitted, making it impossible to link decisions and action points to particular patients (Table 18).
| Team | Recorded electronically | Recorded manually | Visible to others during meeting | Systematically recorded in/transferred to patient notes | Recorded by clinician | Recorded by administrator |
|---|---|---|---|---|---|---|
| Cancer 1 | ✓ | ✓ | ✓ | ✓ | ||
| Cancer 2 | ✓ | ✓ | ✓ | |||
| Cancer 3 | ✓ | ✓ | ✓ | ✓ | ||
| Cancer 4 | ✓ | ✓ | ✓ | |||
| Mental health 1 | ✓ | ✓ | ||||
| Mental health 2 | ✓ | ✓ | ||||
| Mental health 3 | ✓ | ✓ | ||||
| Mental health 4 | ✓ | ✓ | ||||
| Heart failure 1 | ✓ | ✓ | ✓ | |||
| Heart failure 2 | ✓a | ✓ | ||||
| Memory clinic 1 | ✓ | ✓ | ✓ | |||
| Memory clinic 2 | ✓a | ✓ | ✓ |
There was also variation in how easily accessible the minutes were to team members after the meeting, for example whether the outcome of the MDT discussion was recorded in a patient’s notes or in a hardcopy book kept by a single member of the team.
In one heart failure team, the lack of documentation was attributed to the fact that there was ‘no admin support, no real guidelines’ (heart failure nurse 1, interview).
We don’t take official MDT meeting notes as such, I imagine that’s something we should do . . . it would be a huge extra work load for us if we were to you know start typing up minutes and everything . . . might be beneficial because we don’t keep a very accurate list of people who’ve been discussed . . . if we were to be audited we probably wouldn’t do very well.
Heart failure nurse 2, interview
Review of multidisciplinary team decisions
Variation in the quality of documenting decisions has implications for the ability to undertake reviews and audits. In most teams there was no formal audit of the outcomes of the MDT meeting, although some MDT members stated that they would like to know how their MDT performed:
It would be interesting to audit the MDT decision against what happens . . . if the MDT decision is consistently not the one that gets done, then that tells you that either people don’t listen to the MDT in which case why are you doing it? Or they listen but the MDT’s missing something.
Cancer doctor, interview
MDT members overall thought it was a good idea to review the MDT outcomes, and, in one MDT, a member stated that there were plans to audit outcomes but that this was not the case at the moment:
I think that there is a plan to try and audit outcomes of the first two hundred odd patients who were discussed here to see whether it is possible to demonstrate improved care.
Heart failure doctor, interview
Members also suggested that MDT processes and discussions should be audited:
Perhaps record the discussions and some sort of audit to make sure that all the right things have been discussed, the right course of treatment options have been considered and to work out if we are doing things as we should be, according to best evidence based practice. And that would also have the benefit of course of allowing audit of what we do rather than having to go back and retrospectively audit everybody.
Heart failure doctor, interview
However, some were concerned that having too many audit procedures can be unhelpful:
Maybe [auditing outcomes] is something we should do, but then we keep other audits and it’s just how many? Is it, would it be useful?
Heart failure nurse, interview
Content of discussion in multidisciplinary team meetings
Theme 10: the role of research and evidence in multidisciplinary team meetings
There was variation across teams in how frequently they referred to scientific evidence and research in MDT meetings. There were a number of contexts in which research was discussed. During specific patient discussions, teams sometimes mentioned evidence in favour of particular treatment options or debated whether or not it would be appropriate to recruit a patient to a particular trial:
I was just thinking with the trial if he was encouraged.
Oh right, ok no I don’t think, I don’t think he’s somebody who’d be particularly suitable for that.
Mental health team, observation
Do we know from the [trial]? Has anyone published data on using stress echo to.
We have, we are using, I mean there’s no publication but we are doing stress echoes on many of such patients.
And they’re following them up and seeing what . . . because I think it would be very useful for this chap, obviously we’ll have a chat with him and see what he would like, but if he is agreeable to going ahead for the assessment, we can . . . discuss him with one of the [trial] people.
Heart failure team, observation
Referencing evidence in relation to specific patient discussions was most common in cancer and heart failure, followed by memory and then mental health meetings, where research and evidence were mentioned only occasionally.
Research was also mentioned as a point of learning:
There’s an interesting paper in this week’s BMJ looking at the radiation risk from CT scans and you know I think that’s something that’s going to be increasing topic of how we are following up patients.
Cancer doctor, observation
For some this was seen as an important function of the meeting:
I think it is educational because everybody keeps up to date with the latest research . . . it’s a cascading way of information giving.
Cancer doctor, interview
Research was generally mentioned by consultant doctors. The reliance on senior consultants making the decisions in cancer and heart failure teams was attributed to the fact that they have ‘the experience and the evidence base’ (cancer doctor, interview). For non-medical team members, using research evidence was seen as a way of capturing the attention of the rest of the team when they perceived that they were not being listened to:
The times where you feel you just had to pull something from your ragbag of research knowledge, you just go bat, bat and they go ‘oh, that’s very interesting’.
Cancer psychologist, interview
While evidence was sometimes used as a means of resolving disagreements, at other times discussions of research turned into long debates that were not always relevant to the case under discussion.
There was also concern that an overzealous focus on research evidence could lead to neglect of the specific needs of individual patients, and the more holistic aspects of care:
I think cardiologists are very focussed on treatment but they don’t always look at the whole person and I suppose that’s where nurses step in and try and treat the patient holistically as well as all the lovely evidence based practice and all the rest of it.
Heart failure nurse, interview
Theme 11: discussing comorbidities at multidisciplinary team meetings
As described in Theme 5: roles that patient preference, health behaviours and other clinical factors may have in explaining the socioeconomic variations in implementation, there was variation across teams in the extent to which patient comorbidities were mentioned during case discussions. No team had a formal process for discussing information on comorbidities, although some discussed this information regularly (mental health, memory and one cancer MDT). Most, however, discussed this on an ad hoc basis, ‘not routinely done, is done case by case depending on the circumstances’ (memory clinic manager, interview).
Overall, MDT members and patients considered discussion of comorbidities to be important. MDT professionals highlighted the importance of considering how different conditions interact, and that discussion of comorbidities helped to determine suitable management options and informed differential diagnosis (e.g. cognitive impairment and depression):
Some of the cancers are, you’re at a higher risk if you’ve got a pre-existing comorbidity so obesity, diabetes.
Cancer nurse, interview
Some of our service users smoke a lot, smoke too much so we have to be mindful of the fact that a physical illness can impact on mental illness and vice versa . . . you can’t have one in isolation from the other, it’s a human being we’re talking about.
Mental health social worker, interview
The majority of professional interviewees believed that comorbidities were appropriately considered where relevant:
We send people off for exercise tolerance tests and you know think very carefully about, how best we can manage their comorbidities.
Cancer nurse, interview
It was argued that the presentation of comorbidities encouraged a holistic approach to treatment, for example, by highlighting issues relevant to patient engagement and adherence:
Comorbidities . . . they obviously impact on the whole treatment and particularly cognitive impairment and how they [patients] co-operate and comply.
Heart failure nurse, interview
Patients also stated that they wanted their comorbidities to be considered and included in treatment plans, as they were important for their quality of life.
However, MDT members also identified a number of barriers to the inclusion of comorbidities during MDT meetings. They stated that information on comorbidities was not always available in advance of an MDT meeting, for example when teams were discussing patients for the first time, ‘discussing people you’ve never seen’ (cancer doctor, interview). Information on comorbidities could also be inaccurate or incomplete (e.g. as a result of incomplete assessment): ‘often the letters you get give you no indication’ (cancer surgeon, interview). MDT members also stated that clinicians did not always capture this information appropriately:
They’re clearly very important and the problem is these patients are normally seen by the surgeons . . . as a rule surgeons don’t take detailed histories. So if you don’t take a history you can’t capture a comorbidity.
Cancer doctor, interview
Another issue identified in discussing comorbidities routinely was that it added to the volume of potentially irrelevant information to be considered by the team, which could hamper decision-making: ‘we already discuss things in sometimes in too great a detail’ (cancer doctor, interview). This was supported by analysis of the observation data, where one team in particular allocated significant time to the presentation of health behaviours, comorbidities and medical and family history, which were often irrelevant to the presenting complaint: ‘It is just not relevant for somebody aged 75 if they had a normal delivery’ (memory clinic doctor, interview).
Theme 12: discussing patients holistically
Our analysis revealed differences between specialties in the degree to which discussions focused on psychosocial issues in addition to biomedical complaints.
Mental health and memory MDT members placed much more emphasis on describing the patient’s social circumstances in depth:
He is in some respects a product of his environment. Very, very dysfunctional family. The mother is regarded to be an inadequate parent, an inadequate person, not being derogatory . . . failed to establish any boundaries whatsoever in the household . . . his sister was under children’s services under child protection on the basis that mum was unable to keep her safe. There was lots of sibling rivalry between himself and the sister, there was lots of antisocial behaviour from him and his lifestyle that was taking place at the family home.
Mental health social worker, observation
Cancer and heart failure teams spent significantly less time on holistic discussions and focused instead on the presenting biomedical complaint: ‘[patient name] came in [on date] with bilateral leg cellulitis and fluid overload’ (heart failure nurse, observation).
These teams did mention psychosocial issues, but only briefly, usually as part of a structured presentation – ‘he’s a 75 year old retired printer’ (heart failure doctor, observation) – or where particularly relevant to a biomedical treatment:
She’ll be very concerned about hair loss I suspect. She’s a hairdresser, she’s, she’s, I had to write her a letter to say it was ok for her to go for a botox injection and she’s quite into her aesthetics so she wouldn’t be too keen to go for chemo from that perspective I’d imagine.
Cancer doctor, observation
Most MDT members believed that they included information on patients’ psychosocial circumstances where relevant. However, some thought that discussions were overly focused on clinical factors to the exclusion of important psychosocial factors:
I think one always has to be mindful not to forget the individual details of the individual patient that one’s discussing. So when you come to consensus decision there is a danger of losing some of the individual characteristics or comorbidities of that particular patient.
Cancer doctor, interview
It would be good to discuss the holistic patients – this is what I thought an MDT was supposed to do – discuss the case as a whole . . . but that’s not discussed there.
Heart failure nurse, interview
Patients also highlighted the need to be recognised as individuals, not as ‘presenting complaints’:
I would like them to, perhaps [name] the heart nurse there, to just describe what sort of a person I am . . . When they are discussing me I would like someone, like [nurse] or someone like that, just to stand up and say ‘Mr X is so and so . . .’ so they have got a picture of someone who they can then discuss.
Heart failure patient, interview
However, MDT members also highlighted a number of barriers to discussing patients in a holistic way. One reason was the limited time available:
There are a lot of people to be discussed in it, that must be discussed in it and that’s always a challenge between giving enough time to have a full discussion versus getting everyone discussed.
Cancer nurse, interview
In addition, some MDTs discussed patients who were based at different hospitals so it was not always feasible to gather psychosocial information in time for the MDT meeting:
Because they’re discussing so many people that are not in this hospital, we don’t necessarily know the social situation.
Heart failure nurse, interview
Theme 13: incorporating patient preferences about treatment into multidisciplinary team discussions
As well as different perspectives on the most appropriate time to consider patient preferences (outlined in Theme 5: roles that patient preference, health behaviours and other clinical factors may have in explaining the socioeconomic variations in implementation), we found differences between disease specialties both in terms of the frequency with which patient treatment preferences were mentioned, and in the nature of the information that was discussed. This was most striking when comparing mental health and cancer MDTs. In mental health, discussion of patient preferences often revolved around whether patients wanted to avail themselves of services or not. Treatment options were often restricted by resource issues (e.g. unavailability of psychologists), and discussions of choice tended to focus on practicalities such as the timing and mode of delivery of care, rather than choices between different treatments:
Making sure that they have got a bit of choice about who, where, what, when that kind of thing.
Mental health social worker, interview
In contrast, discussions of patient preferences in cancer MDTs tended to focus on treatment alternatives, and were most likely to be mentioned if the patient’s preference differed from the recommendation of the MDT:
I think the standard of care would be to assess his cardiac status to see if he was fit for [chemotherapy] or if he wasn’t . . . consider other agents . . . but this man does not want intravenous chemotherapy and this was something that he was adamant about 18 months ago and he has reiterated, so I would like to consider for the patient [alternative treatment option,] he knows it is not the standard of care . . . it’s against my better judgement but that is what he would like.
Cancer doctor, observation
Options around the practicalities of care were dealt with outside of the cancer MDT (e.g. through choice of appointment time).
Across teams, certain professionals (clinical nurse specialists in cancer and heart failure, and care co-ordinators in mental health) were perceived to be advocates for patients and best placed to know their preferences:
I mean as a nurse, you know, you should sort of represent the patients and their preferences.
Mental health nurse, interview
However, other team members also acknowledged the benefits of taking patient preferences into account when making decisions:
I mean I think it’s important for patients, it’s also important for teams because if you don’t take patients’ views into account that does create a lot of problems for the team in the long run.
Memory clinic doctor, interview
Some felt that patient preferences were not adequately considered in the meetings:
Sometimes I think the patients’ understanding of the disease and their beliefs about treatment and what not are swept under the carpet a little bit.
Heart failure doctor, interview
I think it may be that patients’ preferences aren’t systematically sort of brought into decision-making enough.
Mental health social worker, interview
In contrast, others argued that, although patient preferences were important, the MDT meeting was not always the best place to discuss them. One of the reasons given for this was that it was more appropriate to have an informed discussion with the patient after the risks and benefits of different treatments had been discussed at the MDT meeting:
I think one of the advantages of the MDT is it’s there to provide them with a degree of certainty about their treatment, and wherever possible, I think if there are options they need to be put to the patient. But at least having gone through the MDT they’re going to be realistic options.
Cancer doctor, member interview
It was argued that the patient was the final decision maker and could choose from the options identified following the MDT meeting:
If all the options are equal, then you need to discuss with the patient and see what they wish to do, I think that’s the right thing.
Cancer doctor, interview
Some patients agreed with the idea that clinical decisions should initially be independent of patient input:
What the teams ought to be doing is coming to the best clinical decisions . . . and then take my preferences into consideration.
Cancer patient, interview
Other explanations given for not considering patient preferences in the MDT meeting were that patient preferences could change over time, and that preferences are not always known, for example when a new patient was being presented or when a patient was added to the list at very short notice.
It was also noted that it was not always appropriate to allow patient preferences to influence decision-making (e.g. when considering compulsory admissions or preferences based on racist or homophobic beliefs). Members also suggested that the ‘patient choice agenda’ could result in raised expectations when resources were not available:
People’s expectations are sometimes such that what they want is not particularly what we can provide necessarily.
Heart failure nurse, interview
From a patient perspective, it was clear that different patients wanted different levels of choice. Many patients did want their treatment preferences to be considered (including treatment type and location for receipt of treatment):
I think it’s really important to have involvement in whatever treatment you’re offered.
Mental health patient, interview
The major decisions I think you should be consulted or be able to put your point of view forward.
Heart failure patient, interview
However, a desire for choice depended on, among other things, the severity of illness. For example, for critical decisions, survival was paramount and patients did not always think that their treatment preferences needed to be discussed:
Well I knew I had to have it [chemotherapy], I didn’t have much choice it was either that or die . . . there does come a point where you don’t really have . . . perhaps at the stage my cancer was I didn’t really have a choice.
Cancer patient, interview
Patients also acknowledged that the team could not always know all the factors that would influence their personal choice in advance of the MDT meeting:
I suspect actually that most of, most of the real issues that would make me make a decision, you know, based on what I’ve been given are things that the hospital couldn’t possibly really know about.
Cancer patient, interview
Finally, some patients believed that the doctor was the expert, and they should follow the advice they were given:
I think they know what they are doing and yes you know it is all very well saying ‘I could do this’ and ‘I could do that’ but I think if you’ve got faith in them then yes you go along with what they say.
Cancer patient, interview
I don’t try to interfere. First I know that they are doing what’s best for me . . . I’m confident they are doing the right thing.
Heart failure patient, interview
This was quite common among cancer and heart failure patients who stated that they trusted their clinicians and believed they would make the best decision: ‘it has got to be in my interest healthwise rather than what I want’ (heart failure patient, interview). In contrast, some memory clinic and mental health patients expressed a lack of trust in the decision-making process and felt that they had not been involved:
They’re based too much on a very narrow erm, there’s no decision, if you have a problem, it’s a tablet, that’s the answer.
Memory clinic patient, interview
I don’t really know how they’re [decisions] made to be honest. I don’t know, I don’t believe I have any part in that.
Mental health patient, interview
The role of the patient in multidisciplinary team meetings
Theme 14: patient awareness of multidisciplinary team meetings
Not all patients were aware that their cases were being discussed at MDT meetings. All cancer patients interviewed were aware of the MDT meetings: ‘I knew there’d been a group of people discussing it’ (cancer patient, interview). In contrast, patients in other specialties were often unaware of the existence of an MDT meeting:
The only person that makes that decision about my welfare and my state is Dr [name].
Heart failure patient, interview
[I] don’t know anything about any of that. Like behind the scenes stuff . . . I don’t know whether the psychologist meets and discusses my case with the multidisciplinary team or whether she discusses my case with an individual supervisor, I have no idea . . . I have no idea who I’m discussed with. I’d quite like to know.
Mental health patient, interview
Patients who were aware of the MDT meeting reported that they felt more confident when a decision was made by an MDT rather than one person:
When we’re in that kind of interim period like we are now and you see one of the less senior doctors, you feel quite confident because you know it’s a team.
Cancer carer, interview
Although most patients did not object to being discussed by a team, a small number of mental health and memory clinic patients were uncomfortable with the idea because of concerns about confidentiality and how they would be portrayed. Some patients did not want people they did not know discussing their care:
I think it’s only a useful forum if it’s a multidisciplinary team meeting with team members who are working with that client. But if you’ve just got lots of extra team members who have never met them, got nothing to do with them then where’s the relevance in them even being there?
Mental health patient, interview
There was a concern that patients may be misrepresented at MDT meetings:
Come to my home address or have me in here or whatever rather than asking a member of a team who you might not get on with that good, you might not have a good rapport with them.
Mental health patient, interview
Some patients also felt that the MDT meeting was not a good use of resources:
If they’re not working with you directly, it just seems like a waste of time and resources . . . these are people who you’re potentially never going to meet, don’t even know the names of, are like just an invisible team in the background, it’s like, they could be using those hours actually going out and working with people and providing the care that people need instead of having so much discussion.
Mental health patient, interview
Theme 15: patient attendance at multidisciplinary team meetings
The option of inviting patients to attend the meeting was not discussed in any of the MDT meetings we observed. Patients’ views and experiences were fed into MDT discussions in an ad hoc rather than a systematic manner. A small number of patients said that they would like to attend the meetings to ensure that they were represented accurately, and to provide additional information:
I think the only way they can be represented is by the patients being there really isn’t it.
Memory clinic patient, interview
Overall, however, most patients stated that they would not want to attend MDT meetings as they felt it would be confusing, impractical and potentially upsetting:
Well I don’t think I would like to go to the meeting because they talk in words I wouldn’t understand and then I would come back here and I would sit and worry.
Heart failure patient, interview
It wouldn’t be very constructive to have to keep on stopping the meeting to ask well what does that entail or what, you know that figure is that high, is it low or is it normal?
Cancer carer, interview
Most patients were happy to be represented by a professional who knew them:
People that we know like [doctor] and [nurse] it’s somehow easier than just sitting there hearing it . . . discussed as a case.
Cancer patient, interview
Patients also suggested that they could be involved in other ways, such as adding to their own notes or providing written or audio statements:
They were going to allow service users to add to their own notes, and like obviously not be able to read what was written about them but they could actually update their notes and sort of like have a little bit of input.
Mental health patient, interview
They could speak in the privacy of their home on maybe a phone and, and give a recording over of, of what they, what they feel.
Heart failure patient, interview
Almost all MDT members felt that it would be inappropriate for patients to attend the meeting, citing the reasons mentioned above, as well as the fact that the tone of discussions may be inappropriate for patients to hear:
I think some of the decisions we take for granted . . . might seem quite bullish to people who are just listening.
Cancer nurse, interview
They also argued that it would often be infeasible for patients to attend, as they are sometimes discussed before they meet the clinician or key worker and in urgent cases there might be no time to arrange for patients to attend.
The observation analysis revealed a number of aspects of the meeting which would most likely have to change were patients to attend. In mental health teams, discussions frequently centred around managing relationships with patients, ethical dilemmas, challenging behaviours and peer support:
She denied stuff that we know to be the case. She denied the email, she denied the knocking on neighbour’s door, she denied ringing police repeatedly.
Mental health doctor, observation
So how can we support [social worker] with [patient]?
I’d say that’s the only thing you can work with, feelings he generates. You know, your role is to try and help him. And that’s the problem. You’ve got to try and stop helping him. ’Cause if you take it in, then you just get completely crushed and feel rubbish yourself . . . you’re just gonna feel crap the whole time, and that you’re somehow responsible for keeping this man alive and you’re not.
Mental health, observation
Discussions often involved criticising other services and the use of dark humour, which could be problematic were patients to attend:
‘Please could you and your team take on this woman with whom I’ve been fighting a losing battle’ [laughter] Well that’s encouraging . . . Why didn’t he start her on [medication]?
Because we’ve got magical powers! [laughter]
At least we’ll be kept in work. So GPs don’t treat anyone any more.
Mental health, observation
It’s a combination of difficult patient and difficult GP.
Heart failure doctor, observation
Theme 16: providing feedback to patients on the outcome of multidisciplinary team discussions
Patients’ experiences of feedback from MDT discussions varied, in terms of both the format and the level of detail. Some were aware of when they were being discussed and expected that outcomes would be fed back to them:
I know there was a discussion a couple of weeks ago at the, at the meeting to discuss whether the stent should come out and I’m waiting to hear the results of that in fact.
Cancer patient, interview
Others did not receive any feedback on outcomes of the MDT discussions: ‘at the moment there’s no information fed back to clients at all’ (mental health patient, interview).
Patients varied in the levels of detail they wanted fed back to them with some wanting minimal information for various reasons:
I think sometimes ignorance is bliss, but not all patients feel that . . . I don’t think I need to know every single detail.
Cancer patient, interview
The chances are I might not recognise what they’re talking about.
Heart failure patient, interview
Others wanted to know about all treatment options discussed, including those that were ruled out by the team:
There may be some wonderful treatment that actually stopped [dementia] in its tracks, but it costs £20,000 a week and I’m sorry we can’t have it. And it would be interesting to know.
Memory clinic carer, interview
There were varied preferences even when it came to highly significant information:
Tell me when I’ve got like a month to live.
Heart failure patient 1, interview
If they say well maybe you’ve only got say six months to live or something like that, I don’t think I would want to know that.
Heart failure patient 2, interview
Some MDT members emphasised that they use their professional judgement to decide the level and content of feedback appropriate for each patient at a given time (e.g. according to patient anxiety levels and comprehension). There were several occasions when the team decided not to disclose diagnoses to patients if they felt it would make them unnecessarily anxious: ‘I don’t think there’s any point actually giving her feedback and making her anxious’ (memory clinic doctor, observation). However, there were mixed views from patients on the withholding of information by the team:
I mean some people can’t handle the situation and some people can.
Heart failure patient, interview
A person has a right to know everything no matter how ill they are.
Memory clinic patient, interview
Patients’ preferences regarding the mode of feedback also varied, with some saying they would prefer a phone call and others preferring a written record.
Chapter summary
In this chapter we have provided an overview of the teams studied and their different contexts. We then presented findings from our qualitative exploration of the quantitative findings, and from our investigation of areas of diversity in beliefs and practice across the teams. In Chapter 5 we discuss the findings from study 1, consider its limitations and draw conclusions.
Chapter 5 Study 1 discussion
In this large study, the first to include MDTs for different conditions, we have identified specific team and patient features which impact on decision implementation, and we have delineated 16 key areas where there is substantial diversity in beliefs and practices across MDT meetings.
Identifying factors that influence decision implementation
Rates of implementation varied between specialties, with gynaecological cancer having the highest implementation rate and CMHTs the lowest. Examining the policy context within which these teams operate provides some insights into these differences. Mental health policy and guidance is much less detailed than that in cancer, in terms of the expected attendance, structure and administrative support at MDT meetings. Cancer teams use clear guidelines regarding the MDT purpose and processes, which translated into clearly structured MDT meetings. These include guidelines on documentation,112 attendance,42 chairing113 and administrative support. 1
The clearly structured, guideline-driven cancer MDT meetings are in no small part a consequence of the historic priority given to cancer research, which has produced a robust evidence base for the care of cancer patients. In contrast to mental health, which received 3.5% of Medical Research Council (MRC) funding in 2008/9, cancer research received 14.6% of MRC funds. 114 This is despite the fact that mental illness accounts for 23% of the overall disease burden in the UK, while cancer accounts for 16%. 48 There have been recent efforts to redress this balance, with MRC spending in 2012/13 rising to 21.5% for neurological and mental health research, and falling to 6.2% for cancer. 115 In time, this shift is likely to strengthen the evidence base for decision-making by mental health MDTs.
Another factor which could be argued to influence rates of implementation for teams focusing on different conditions is the type of patient outcome which the teams focus on. The International Classification of Functioning, Disability and Health (ICF) identifies three levels of human functioning: functioning at the level of body or body part (body functions and structures), the whole person (engagement in activities) and the whole person in a social context (participation in society). 116 The clinical conditions which we studied could be argued to vary with respect to the extent to which the MDT meetings would focus on managing and treating diseases and on facilitating patients’ meaningful participation in society. For those teams whose primary focus is to improve physical functioning, universally relevant treatment guidelines are more commonly available. Teams which also place a heavy emphasis on assisting patients’ participation in society may need to develop more individualised treatment plans. Treatment plans relating to an individual’s participation in society may also be more inherently difficult to implement, because they are more likely to rely on multiagency collaboration and complex social and cultural factors.
Contrary to previous reports and expectations, greater multidisciplinarity was not necessarily associated with more effective decision-making. This has important implications, as a major assumption underpinning MDT meetings is that more multidisciplinary input leads to better and more informed decisions for patients, with each team member bringing relevant information for the development of a cohesive care plan. 117 Our data suggest that the relationship between multidisciplinarity and MDT decision implementation is mediated by other factors such as clarity of purpose and agreed processes. This is supported by evidence which points to the benefits of shared objectives to guide and structure communication,118 focused leadership119 and team reflexivity. 21
It is also noteworthy that the teams which implemented the most decisions tended to be the most medically dominated in terms of both attendance and participation. Higher implementation rates among more homogeneous (medically qualified) groups are perhaps unsurprising given a higher likelihood of a shared biomedical perspective which drives the decisions made and implemented. However, the inclusion of additional perspectives in the decision-making process may improve decision quality with respect to patient experience, choice and patient-centred outcomes. It was beyond the scope of this study to examine such components of decision-making, but it remains an important area for future investigation.
Effective teamwork is therefore necessary for the potential benefits of multidisciplinarity to be achieved. MDT members identified difficulties in working across disciplinary boundaries, in terms of both status differences and differing professional perspectives. In line with other research, we found that not all professional groups participated equally in MDT discussions. 120 Cancer and heart failure teams in particular were characterised by a strong medical dominance which was manifest both in the degree of participation and in the seating arrangements. We found some evidence that conflicting disciplinary perspectives made multidisciplinary working a challenge, an issue that has been identified in previous research. 21,121 For example, mental health professionals identified differences in how social workers and nurses viewed their roles (e.g. as healers or as human rights advocates), which sometimes made it difficult to collaborate. In the absence of clear national guidance, team reflection on the assumptions underpinning each disciplinary perspective and clarity regarding the role of each professional group may be necessary to develop a shared perspective and agreement on the purpose of the meetings.
We also found that a positive team climate was an important predictor of treatment plan implementation across teams. This finding corroborates previous research which has demonstrated that a good team climate can translate to better decisions. 23,122 It also adds to the body of evidence demonstrating the effects of local context on team performance34 and supports research that has highlighted the importance of positive relationships, teamwork and supportive systems to effectiveness. 36 It was clear from our qualitative data that local context, in this case restructuring in mental health teams, had a significant impact on staff morale and many believed it hindered team performance. In examining the different team structures and processes in the light of their varied national guidance and standards, our data also give an indication of how the broader policy context impacts on effectiveness.
Patient preferences, comorbidities and socioeconomic variation
Despite clear policy commitments to promote equality in the NHS58 we found that there were variations in treatment by patients’ socioeconomic circumstances. This was evident in all specialties and could not be explained by consideration of patient preference, comorbidities or other health-related factors in team meetings. Previous research reporting socioeconomic inequalities in use of adjuvant therapy for cancer suggests that non-implementation is due to non-uptake of care for cancer patients. 72 This is likely to partly explain the continuing socioeconomic variations in cancer survival. 123 There is little research on treatment inequalities for people with dementia, although a small study indicates that these may occur124 and the evidence in mental health has been mixed. 65,67 Recent models have demonstrated equitable provision of cardiovascular care which translated to similar proportions of deaths averted across deprivation quintiles. 125 However, non-implementation may impact upon morbidity rather than mortality, which was not addressed in these cardiovascular models.
Cancer policy states that patients’ comorbidities, preferences and circumstances are important for decision-making and that MDTs should take these into consideration wherever possible. 113 Guidelines for mental health, memory and heart failure state that patient preferences should be taken into account when managing their care, though they do not relate this specifically to the MDT meeting. 49,77,126 Research in this area has called for routine discussion of patient preferences and comorbidities, as evidence suggests that MDT decisions are often not implemented because of a failure to consider patient preferences during the meeting. 127,128 Inadequate information on comorbidities in MDT meetings has also been associated with failure to make decisions. 129,130
Contrary to these previous studies, we found no association between discussion of patient preferences or comorbidities and treatment plan implementation. However, the qualitative results showed that these were complex issues and there was diversity in how readily this information was available, and that there were differing opinions regarding the most appropriate way of incorporating this information into decision-making. Overall, both MDT members and patients stated that patient preferences and comorbidities were very important for decision-making and that they should be included in MDT discussions when possible. However, some suggested that treatment recommendations should initially be independent of patients’ preferences, which was, in line with previous research,131 partly attributed to the fact that patient preferences can change during the management journey. The belief that patients’ views should be sought after the MDT meeting (to ensure clarity and feasibility of options) rather than beforehand has also been highlighted in a previous study. 120
Areas of diversity in beliefs and practices
We identified substantial diversity across teams in the purpose, structure, processes and content of MDT meetings, and in levels of patient involvement. While such diversity may be appropriate to the various needs of different teams, it may also indicate a lack of clarity regarding best practice and a need for further guidance.
Meetings served a range of explicit and implicit functions, from decision-making to team bonding and emotional support. Clarity of purpose has been identified as an essential to effective teamworking and high quality patient care. 132 In line with this previous research, we found that in mental health teams the purpose of meetings was implicit rather than explicit and that there was rarely a written agenda. Though almost all interviewees found the meetings valuable, several mental health interviewees noted the need for greater clarity around the purpose of meetings. Previous research has also identified the potential benefits of using the meeting for functions such as teaching and recruiting patients to trials. 25,133 We identified mixed views regarding these functions. While generally these were considered beneficial, there were practical issues relating to time constraints and resource issues that limited their potential.
The structure and processes of meetings varied widely between specialties. This partly reflects the different policy contexts described above in which the teams operate. We also found wide variation within specialties where there were no national guidelines for MDT meetings, particularly memory clinic and heart failure teams. These variations highlighted the importance of certain team features, including the availability of administrative support.
The importance of administrative support for MDT meetings was clear from both interviews and observations. Previous research in cancer has emphasised the essential role of the MDT co-ordinator role in MDT decision-making134,135 and they were resourced in the cancer teams we observed. In heart failure, memory clinics and mental health, administrative duties were undertaken by managers, health-care professionals and administrators. Many MDT members in these teams highlighted difficulties in having to undertake administrative tasks while participating as professionals. This had an impact on how patients were selected for discussion, and how decisions were documented, among other things. Previous research has found that meeting processes are crucial to quality-monitoring and effectiveness, for example in terms of the documentation and follow-up of meeting outcomes. 31,113,136 Previous research has also identified advantages in collating lists of patients for discussion in advance of the meeting. Registering patients for MDT discussions can form part of a monitoring system that can reduce delays to treatment. 137
The content of discussions also varied by specialty. There was diversity between teams in how much time they spent discussing patients’ psychosocial issues and preferences. This was much more common in memory clinic and mental health teams (both staffed by mental health professionals), while cancer and heart failure meetings were more focused on biomedical information. This was despite the fact that previous research in cancer has found that 98% of professionals surveyed believed that psychosocial care issues should always be considered in MDT meetings. 25 Where patient preferences were mentioned, there were differences between specialties in the kinds of preferences considered. For example, in cancer, discussions of preferences focused on different treatment alternatives, while, in mental health, discussions of choice tended to relate to the timing and delivery of treatment rather than choosing between treatments. Again, this may reflect contextual differences, as lack of resources was a common issue in mental health team discussions.
There was variation in the role of the patient in MDT meetings. While cancer patients are routinely informed of MDT processes, several patient interviewees from other specialties were unaware of the MDT meeting. Policy variations are also noteworthy here. Cancer policy states that patients should be given a written description of the MDT meeting at which they are discussed,42 and should be informed of the purpose of the MDT, when it meets,113 its membership and the responsibilities of each team member. 41 It also states that patients should be informed of the outcome of discussions within a locally agreed timeframe. 113 In contrast, policy in mental health, heart failure and memory clinics does not specify information that should be given to patients on MDT meetings. The idea that their treatment plans were discussed by a team gave some patients confidence, while others were concerned that they might be misrepresented at meetings. In line with previous research, most patients and staff believed it would be impractical for patients to attend the meetings25 and patients varied in their preferences regarding the format and content of the information they would like fed back to them after the meeting. While previous research has found that clinicians tend to underestimate the amount of information that patients require,138,139 it has also been found that these preferences vary at different stages of illness,140 meaning an ongoing dialogue is required to determine a patient’s current informational needs.
Study limitations
The 12 participating teams were based in a single geographical area and, with the exception of one, were all in London. This may limit the generalisability of our results. However, when designing this research, we had to address the issue of feasibility. The researchers attended weekly MDT meetings and spent long periods of time at hospital sites collecting interview and medical record data. They therefore needed to be located within a reasonable distance of the study sites. It would have been difficult to provide close and constructive supervision if researchers were based in geographically dispersed parts of the country. The alternative would have been for the researchers to travel from London, but this would have impacted on the time and cost of the study.
The use of decision implementation as an indicator of effectiveness raises several issues. Firstly, we recognise that measuring treatment plan implementation does not address the issue of whether or not the ‘right’ decisions have been made. It is possible that poor decisions are implemented. In order to examine whether or not implemented decisions are ‘good decisions’ that do indeed lead to patient benefit, it would be necessary to analyse carefully identified disease-specific health outcomes that can be plausibly and directly related to the MDT decision. Alternatively, a subset of decisions about which there are specific best practice guidelines could be assessed according to whether or not they conform to this guidance.
Secondly, the factors we considered in our analyses were identified as potentially important based on previous research. We appreciate that there may be other, unmeasured factors that could also have influenced implementation.
Thirdly, we did not directly explore determinants of decision implementation or the extent to which ‘good’ decisions were made during our interviews with health professionals. This was because interviews took place towards the end of the observation period for each team and making participants aware that implementation was under investigation may have influenced their decision-making processes and practice.
Fourthly, in our regression analysis, we combined decisions where implementation was ‘not documented’ with those decisions that were ‘not implemented’. This was because, on the basis of our clinical experience, non-implementation is commonly not explicitly recorded in patients’ records. Our conclusions are therefore limited by the accuracy of record-keeping.
Finally, multiple treatment plans recorded for the same patient over the observation period could not be satisfactorily included in our models. Therefore, our main analysis considered only the first treatment plan recorded for each patient. We examined the impact of this in our sensitivity analysis.
It is also possible that team members behaved differently when under observation. However, this was mitigated by the 2 weeks of observation before data were collected. In addition, MDT members were aware that decision-making was being studied, but not that implementation was being examined.
The field researchers were not clinicians, but there were several quality assurance procedures in place to safeguard against this potential limitation, as described in Chapter 2, Quality assurance: clinician validation.
We did not achieve the target sample size of 3000 individual patients with treatment plans. This is because fewer patients than expected had treatment plans formulated at each meeting. However, even with our smaller sample, investigation into model overfitting did not raise concerns.
There is no consensus on the best measure of skill mix or team diversity. 98 We therefore categorised professionals into coherent groups on the basis of our clinical expertise. It was necessary to group allied health professionals together as a result of low numbers. We did not account for the seniority or qualifications of members in the different professional groups, which may have had a bearing on the degree to which each profession participated in discussions.
We also used a well-established area-based measure of socioeconomic characteristics, commonly used where individual level indicators are not available. This method rests on the assumption that individuals conform to the socioeconomic profile of their residential area. We recognise that misclassifications can cause under- or overestimates of the relation between socioeconomic circumstances and implementation.
Mental health patients are under-represented in the interview component of our research because, despite strenuous attempts by the research team, we were able to recruit only two mental health patients. Their experiences were distinct from the other patients we interviewed; for example, neither was aware that their care was being discussed in MDT meetings. An in-depth comparison of our interview data from cancer and heart failure patients with mental health and memory clinic patients further explores patients’ experiences. 141
Finally, we did not collect cost or waiting time data and so we cannot comment on the implications of MDT diversity with respect to MDT cost-effectiveness or consequences for patients.
Conclusions
Multidisciplinary team meetings for the management of chronic diseases are widely established in the NHS and internationally. This study builds on previous research in a number of important ways. It is the first to include MDTs for a range of different conditions, generating findings with relevance across health services for a diverse range of patients. We found that the effectiveness of MDT meetings (as indicated by decision implementation) depends on a range of contextual factors, team features and patient characteristics. Having a clear purpose, adequate administrative support and a team atmosphere that facilitates involvement, task orientation and support for improvement is key to ensuring team decisions are implemented. The benefits of multidisciplinarity per se should not be assumed; clear goals and procedures are crucial. These findings corroborate previous work which points to the benefits of shared objectives to guide and structure communication,118 focused leadership119 and team reflexivity. 21
We believe this is the first study to explore the influence of patients’ socioeconomic circumstances on MDT decision implementation. We found that patients from more deprived areas are less likely to have their MDT treatment plans implemented, highlighting continuing inequalities in NHS care provision. This occurred despite the routine reference to treatment guidelines by cancer teams, and did not seem to be explained by consideration of patient preference, comorbidities or other health-related factors. Further research is necessary to understand this. Routine monitoring of decision implementation is one approach that could be used to ensure the equitable provision of care.
We also found many areas in which there is a diversity of views and practices across MDT meetings. While this diversity may reflect appropriate flexibility with respect to the purpose of meetings and their value for team members, it may also indicate uncertainty regarding the most successful ways to conduct MDT meetings.
Chapter summary
In this chapter we have discussed the findings from study 1 and related them to previous research. We have also considered the limitations of the study. The next chapter describes how we used these data, in combination with other research and policy documents, to derive a series of recommendations that would be generalisable across MDTs for different chronic diseases.
Chapter 6 Study 2: application of a formal consensus method to develop recommendations to improve the effectiveness of multidisciplinary team meetings
Introduction
The aim of study 2 was to apply a modified formal consensus technique to derive a set of feasible recommendations for improving the effectiveness of MDT meetings for patients with chronic diseases. This chapter sets out how the research team, under the guidance of the steering group, used the quantitative and qualitative findings from study 1 to derive a series of recommendations that would be generalisable across MDTs for different disease types.
Formal consensus methods are structured facilitation techniques that explore levels of consensus among a group of experts by synthesising their opinions. 142 They are designed to minimise some of the limitations associated with group decision-making. In contrast to informal decision-making groups such as committees, they follow explicit methodological steps that can be replicated. They have been used to formulate clinical practice guidelines143 and to establish national research priorities. 144 They are particularly useful in defining levels of agreement where there is an insufficient or contradictory evidence base,145 and where there is uncertainty about the value of different options. 146 These methods therefore constitute a valuable tool for agreeing recommendations for improving MDT meetings, where the empirical evidence informs but does not clearly stipulate the action to be taken to improve effectiveness.
The three consensus methods most commonly used in health-care research are the nominal group technique (NGT), the Delphi method and the RAND/UCLA appropriateness method (RAM). 147 In the NGT, experts independently generate ideas, meet to discuss each of the ideas generated and then privately rank them in order of preference. 148 In contrast, the Delphi method does not require the participants to meet. It involves two or more rounds of questionnaires and may start with the generation of ideas. Responses from each round are aggregated and fed back, giving participants the opportunity to revise their answers in the light of the responses of the other participants. 149 The RAM was developed to determine the appropriateness of particular health-care interventions by combining the best available evidence with collective expert judgements. 150 This method involves sending a literature review and list of possible indications for intervention to participants, who independently rate each item. They then meet to discuss areas of discrepancy, with the aid of a second-round questionnaire showing both their own initial rating and the distribution of all first round ratings. Following this, they rerate the items privately and individually, in the light of the discussion. In practice, formal consensus studies often adapt elements from each of these methods to optimally address specific research objectives. 146
Methods
We focused on those areas where the research evidence did not provide clear recommendations on practice to improve effectiveness and where we identified diversity in both MDT practice and in beliefs about best practice. We used a two-round process illustrated in Figure 3 and described in detail below.
FIGURE 3.
Overview of the consensus development method.
We use the term ‘recommendation’ throughout the report because this was the terminology used in discussion with the expert panel members. We have therefore continued to use the term throughout the methods and results sections of this report. However, for our final conclusions, we have named the recommendations that were scored with strong agreement and low variation in agreement as ‘indications of good practice’. This reflects the fact that they are designed to be helpful pointers, rather than policy recommendations.
Generating statements for the expert panel to rate
We used the following sources to generate statements for the expert panel to rate.
Study 1: qualitative findings
We drew upon the 16 metathemes which described areas of diversity in beliefs or practices (see Chapter 4, Qualitative investigation of areas of diversity in beliefs and practices) to generate a list of issues and questions which could be turned into single-item statements. This took place during an analytic conference attended by the field researchers, the chief investigator, two coapplicants and two patient representatives. When exploring these issues we focused on:
-
identifying specific practices or procedures that were considered effective in some teams but not used in others
-
identification of specific arguments for and against current MDT practices, issues relating to the content of discussion, and reflections on team structure that were made by MDT professionals and patients
-
proposals made by MDT professionals and patients for improving MDT meetings and the experience of patients
-
suggestions made by MDT professionals and patients for incorporating patient preferences into MDT discussions.
Other published research and guidance
We conducted a review of UK-based research literature published between 1995 and May 2013 and of national policy and guidance (for cancer, heart failure, old age psychiatry and mental health MDT teams) on issues related to the 16 metathemes.
Study 1: quantitative findings
We also drew upon information from our quantitative results, for example we found no association between discussion of patient preferences and treatment plan implementation.
Using these data sources we developed an initial series of statements describing potential ways to improve the effectiveness of MDT meetings. These statements were refined in further analytic conferences, with all members of the research team present to ensure that they were supported by the data. We also ensured that each statement was clear and precise and included only one issue for consideration.
In total we generated 68 statements for the expert panel to rate and four open-ended questions where we did not have enough evidence to propose a statement. In these cases we asked the panellists to provide suggestions on feasible methods to improve effectiveness, for example how to incorporate teaching into MDT meetings.
The questionnaire
The final questionnaire was divided into 16 sections, each relating to a theme (a sample theme is available in Appendix 11; all questionnaire items are presented in the results tables in Appendices 12–15 and the entire questionnaire pack is available from the authors on request). Each section summarised the information outlined above, that is relevant policy and guidance, published research literature and our results. This was followed by a series of statements. We instructed panellists to rate their level of support for each statement by drawing on their own knowledge and experience in addition to the information provided. 151 Ratings were on a Likert scale, from 1 to 9, where a rating of 1 indicated that the panellist strongly disagreed with the statement, a rating of 5 indicated neither agreement nor disagreement (i.e. depends on circumstances), a rating of 9 indicated strong agreement, and ‘don’t know’ indicated that participants did not think they were informed enough to answer the question. An example of a statement is provided in Figure 4.
FIGURE 4.
A questionnaire item from round 1.
We piloted the questionnaire with a member of the steering group to ensure that the information was presented coherently and clearly and to provide an estimate of the time required to complete it.
Identification and establishment of formal consensus panel
There is no agreed standard regarding the optimal size of consensus groups. 150,152 Smaller groups provide less reliable decision-making, while bigger groups tend to be more difficult to co-ordinate and have less equal participation. 153 Following guidance146 we aimed for a group of 12–16 participants to represent the range of professional groups and disease specialties under study.
We purposively sampled health-care professionals, policy-makers and patient/carer representatives with MDT experience of each of the different disease types under study. Potential participants were identified by consulting the project’s steering group and relevant professional organisations (e.g. the British Society for Heart Failure). This approach helped to ensure that participants had credibility as experts in their field and were representative of their profession. 146 Three patient representatives were included to bring an additional perspective to the discussion and to ensure that any recommendations reflected what patients value in their care. We did not invite members of any of the MDTs observed in study 1 or members of the project’s steering group to participate.
The chief investigator contacted potential panellists by e-mail to introduce the study, explain the process and likely workload that it would entail, and invite them to participate. Of 22 individuals invited, 16 agreed to participate. The professional background of panellists is shown in Appendix 16.
Formal consensus development process
Expert panel round 1: consensus development questionnaire
Ten weeks before the consensus meeting, all members of the panel were sent the first round questionnaire pack with instructions and a stamped addressed envelope. They were asked to return the completed questionnaire within 1 month (see Appendix 17 for the invitation and instructions).
We contacted panellists who returned questionnaires with missing data and asked them to complete the remaining questions.
Expert panel round 2: consensus development meeting
The ratings from round 1 were used to develop a personalised version of the questionnaire for each panel member (Figure 5). These showed the participant’s own responses (in green) and the distribution of responses for all panellists for each item (in italics above the Likert scale).
FIGURE 5.
A questionnaire item from round 2 showing the distribution of round 1 responses in italics above the Likert scale, and the respondent’s own round 1 rating in green.
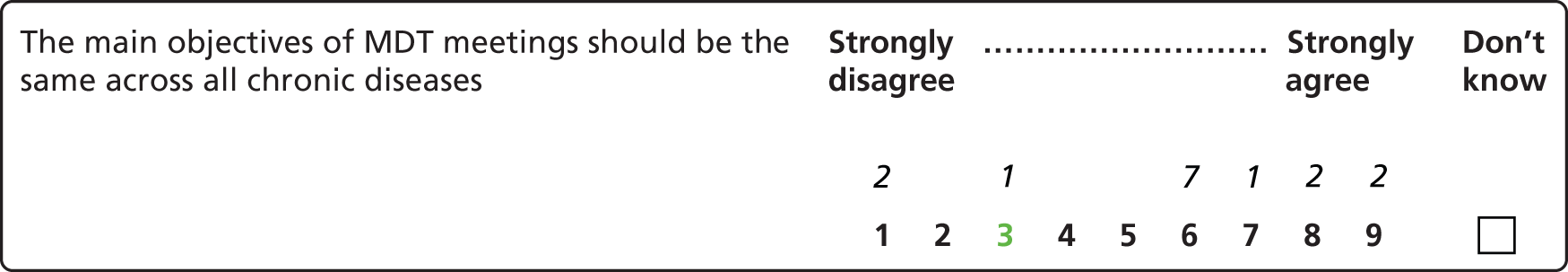
We grouped together all the responses from the four open-ended questions, and included a summary of these in the second-round questionnaire. For one of the questions, we used the suggestions to amend an existing statement, and flagged this up to participants so they could rate it during the consensus development group meeting.
This information was distributed to the panellists when they attended the consensus meeting in central London, chaired by the chief investigator, who is experienced at facilitating formal consensus meetings. The purpose of the meeting was to discuss those statements where there was a lack of consensus, to explore causes of divergent responses and to identify any statements where lack of consensus was secondary to different interpretations of the statements. Therefore, statements were ordered for discussion so that those with the least consensus (defined using RAND guidelines, see Data entry and analysis below) were discussed first, and those which had strong consensus were considered last. Thus, during the meeting, priority was given to discussing the statements where there was most variation in scoring among expert panel members. For these statements, panellists were encouraged by the facilitator to discuss reasons for differences in ratings before rerating each item privately. The facilitator ensured that all participants had an opportunity to contribute during the meeting, and made it clear that participants did not need to conform to the group view. 145
Where it became apparent that differences in first-round ratings had resulted partly from ambiguity in the wording of the statement, the group agreed a revised wording before making their second-round rating. Where there was consensus in the first-round ratings, statements were not discussed individually, although the broader discussion usually touched on the themes in these statements and panellists were given the opportunity to comment on any of these statements before rerating.
With the consent of all the panellists, the meeting was audiotaped and field notes were taken to ensure we could correctly identify the specialty associated with each justification for variations in agreement with statements.
The meeting lasted 3.5 hours. At the end of the meeting, we ensured that panellists had completed the entire questionnaire.
Data entry and analysis
Data entry
Ratings were entered into a database using IBM SPSS V21. At each round, we double-checked 25% of the data entered against the original paper questionnaires to ensure accuracy. No errors were detected.
Analysis of round 1 ratings
We prioritised statements for discussion at the meeting using RAND guidelines for a panel of 16 members. 150 ‘Consensus’ was defined as four or fewer panellists rating outside the three-point region containing the median (1–3.5, 4–6.5, 7–9). These statements were grouped together, and were not discussed individually at the meeting. We ordered the remaining statements according to the number of panellists rating in each extreme (1–3 and 7–9). The RAND guidelines define ‘lack of consensus’ as five or more panellists rating in each extreme (1–3 and 7–9) so statements meeting this criterion were ranked first, followed by those where four or more panellists rated in each extreme, and so on.
Analysis of round 2 ratings: quantitative analysis
For each item, we examined:
-
the strength of agreement with each recommendation and
-
the variation in extent of agreement among panellists.
The strength of the group’s agreement with each item was indicated by the median. 147 Medians between 7 and 9 indicated agreement with the statement, medians between 4 and 6.5 indicated uncertainty, and medians between 1 and 3.5 indicated disagreement. These ranges cover all possible medians for a 16-member panel.
The group’s variation in extent of agreement was indicated by the mean absolute deviation from the median (MADM). 147 This was categorised into low, moderate and high variation according to thirds of the observed MADM scores (low < 1.11, moderate 1.11–1.75 and high > 1.75).
We defined a statement as a final ‘recommendation’ for improving the effectiveness of MDT meetings when both strong agreement (median between 7–9) and low variation in extent of agreement (MADM < 1.11) were present.
Where recommendations were rated ‘uncertain’ (medians 4–6.5) or ‘disagree’ (medians 1–3.5), we categorised the panellists into cancer, mental health and heart failure groups and calculated the median score for each group. This allowed us to assess whether uncertainty or disagreement might be a result of differences in the nature of MDT meetings for different diseases. However, these results are limited by the small numbers represented within each specialist category.
Analysis of round 2 ratings: qualitative analysis
We transcribed the meeting in full. We conducted a thematic analysis of the meeting transcript, coding the panellists’ comments regarding each statement to highlight the range of views about each item discussed and to identify possible explanations for differences in ratings.
Feedback of ratings
The final list of recommendations was shared with the expert panel and the steering group for final comments.
Patient and public involvement
Our PPI representatives assisted in identifying and recruiting patients to participate in the consensus development meeting.
Results
Results from the second round of ratings are summarised here and presented in full in Appendices 12–15, 18 and 19. Of the 68 potential modifications to improve the effectiveness of MDT meetings, only one needed to be reworded before rerating.
There were 21 statements for which there was both strong agreement (median ≥ 7) and low variation in the extent of agreement (MADM score of < 1.11). This included six recommendations relating to the purpose of the meetings, ten relating to meeting processes, two relating to content of the discussion and three relating to the role of the patient. Panellists from all specialties agreed that these recommendations were desirable and feasible. The list of these final indications of good practice is provided in Box 5.
We applied the RAM to determine consensus on 68 statements derived from a mixed-methods study of 12 MDTs, a review of other research evidence and key national policies and guidance. Sixteen expert panellists rated the statements individually, met to discuss their ratings and then rerated them. Statements for which there was strong agreement and low variation in extent of agreement form the basis of the following 21 indications of good practice.
The purpose and functions of multidisciplinary team meetings-
The primary objective of MDT meetings should be to agree treatment plans for patients. Other functions are important but they should not take precedence.
-
MDT discussions should result in a documented treatment plan for each patient discussed.
-
MDT meeting objectives should include locally (as well as nationally) determined goals.
-
The objectives of MDT meetings should be explicitly agreed, reviewed and documented by each team.
-
Explaining the function of the MDT meeting should be a formal part of induction for new staff.
-
There should be a formal mechanism for discussing recruitment to trials in MDT meetings (e.g. having clinical trials as an agenda item).
-
All new patients should be discussed in an MDT meeting even if a clear protocol exists.
-
All chairpersons should be trained in chairing skills.
-
Teams should agree what information should be presented for patients brought for discussion in an MDT meeting.
-
All new team members should be told what information they are expected to present on patients they bring for discussion in an MDT meeting.
-
The objectives of the MDT meeting should be reviewed yearly.
-
Once a team has established a set of objectives for the meeting, the MDT should be audited against these goals (e.g. every 2 years).
-
All action points should be recorded electronically.
-
Implementation of MDT decisions should be audited annually.
-
Where an MDT meeting decision is changed, the reason for changing this should always be documented.
-
There should be a named implementer documented with each decision.
-
Comorbidities should be routinely discussed at MDT meetings.
-
Patients’ past medical history should routinely be available at the MDT meeting.
-
The MDT should actively seek all possible treatment options, and discuss these with the patient after the meeting.
-
Patients should be given verbal feedback about the outcome of the MDT meeting.
-
Where it would be potentially inappropriate to share the content of an MDT discussion with the patient (e.g. where it may lead to unnecessary anxiety or disengagement from services), the decision not to feedback should be formally agreed and noted at the meeting by the team.
Recommendations that the expert panel agreed with
In total, there were 38 recommendations that panellists agreed with (median ≥ 7). This included the 21 final recommendations (see Appendix 12) as well as recommendations where there was agreement (i.e. medians between 7 and 9) but high or moderate variation in the extent of the agreement (see Appendix 13). Of these 38 recommendations, 14 related to MDT meeting processes, including the role of the chairperson, the need for agreed objectives which are reviewed and audited annually, and the formal documentation of decisions made. Seven recommendations were about the purpose of MDT meetings, three were about the structure of the team, six were about the content of MDT discussions and eight were about the role of the patient in the MDT meeting.
Where there were differences in opinion, the main concern among panellists was feasibility: ‘that’s going to be tricky’ (doctor, cancer: recommendation 34, Appendix 13), ‘it would be nice in an ideal world’ (doctor, cancer: recommendation 24, Appendix 13) or ‘I disagree . . . we can’t actually commit to sending everyone written feedback . . . because of time constraints’ (team manager, mental health: recommendation 38, Appendix 13).
Recommendations rated as ‘uncertain’ (see Appendix 14)
There were 17 recommendations where the strength of agreement was ‘uncertain’ (i.e. the median rating was between 4 and 6.5). Most of these related to ensuring patient-centred care, such as how information on patients’ comorbidities and psychosocial issues should be managed and the best ways of facilitating patient input into discussions.
There were also uncertainties regarding who should be required to attend MDT meetings and whether or not patients should be discussed even where a standard protocol for treatment exists. Panellists from different specialties expressed strong differences of opinion with respect to the creation of local (as opposed to nationally) prescribed attendance lists (recommendation 44, Appendix 14). However there were opposing views within the same disease specialty and professional group with respect to patient discussions. For example, one oncologist stated that ‘all patients should be discussed . . . it’s a way of ensuring that absolutely every patient has that safety net of quality assurance’ while another argued that ‘I think that if you’ve got a clear protocol I’m not quite sure of the benefit of going through the protocol again’ (recommendation 42, Appendix 14).
Recommendation 39 (Appendix 14), about whether or not the objectives of team meetings should be the same across all chronic diseases, was rated as ‘uncertain’. Panellists pointed out that MDTs for different diseases ‘are very, very different animals’ (policy-maker, cancer).
When we calculated the median score for each group of panellists with expertise in cancer, mental health or heart failure, we found that, although 17 recommendations were rated ‘uncertain’ by the group overall, every one of these was rated ‘agree’ or ‘disagree’ by at least one disease group (see Appendix 18). For seven of the recommendations, at least one disease group disagreed, while another agreed with the proposal (recommendations 39, 42, 44, 48, 51, 53, and 55, Appendix 18). For example, the cancer and heart failure participants did not think that patients should be given the option to provide audio-recorded input to the meeting (recommendation 55, Appendix 18), while the mental health panellists agreed with the proposal. Mental health participants also agreed that patients should be discussed only when their psychosocial characteristics could be presented (recommendation 48, Appendix 18). Heart failure panellists disagreed with this proposal and cancer panellists were uncertain.
Recommendations the expert panel disagreed with (see Appendix 15)
There were 13 recommendations that the panellists disagreed with (median < 4). Most disagreements centred on the role of the patient in MDT decision-making. For example panellists argued that it was unfeasible to always obtain patients’ treatment preferences before discussing their case: ‘we have to make a diagnosis . . . so . . . before the MDT it’s almost irrelevant because we don’t know what we’re actually going to be talking to her about’ (doctor, cancer: recommendation 61, Appendix 15). They also pointed to practical and cognitive barriers to asking patients before the MDT about how much they wish to be involved in decision-making (recommendation 63, Appendix 15): ‘if you are the tertiary centre . . . you don’t know the patient’ (policy representative, cancer); ‘I’m not sure it’s a question that many patients might necessarily be able to deal with . . . especially . . . if you are presenting with something that you don’t suspect, you’ve got so many things to think about’ (patient representative, cancer). However, one patient representative did point out that: ‘I’ve had those conversations at various stages of my own treatment, including from the very earliest stages . . . It’s not always going to be relevant but I think it can be done’ (patient representative, heart failure and cancer).
Panellists also disagreed with proposed recommendations about the purpose and structure of MDT meetings (recommendations 56, 57, 58 and 60, Appendix 15).
For eight of the 13 potential recommendations that the panel as a whole disagreed with, at least one of the disease group medians fell into the ‘uncertain’ range (recommendations 58, 59, 61, 62, 64, 66, 67 and 68; Appendix 19). For example, although the panel as a whole did not think that there should be time within MDT meetings to discuss current and emerging research and evidence which is not specifically related to an individual case (recommendation 59, Appendix 19), the mental health panellists were uncertain about this. Both the mental health and cancer panellists were uncertain whether or not patients should be discussed only if their treatment preferences were known (recommendation 61, Appendix 19), something that the heart failure panellists strongly disagreed with.
Discussion
We used formal consensus development methods to produce a list of indications of good practice for ensuring the effectiveness of MDT meetings, which expert panellists agreed were both feasible and desirable. Of 68 potential modifications proposed, almost one-third (31%), were rated with ‘strong agreement’ by experts from all three disease types (cancer, heart failure and mental health). While the research to date on improving the effectiveness of MDT meetings has focused on teams within one disease type,9,17,154 our findings illustrate that there is scope for learning between specialties and the potential to determine a significant number of indications of good practice that are applicable in the varied contexts within which MDTs operate. Our group-level findings with respect to those 17 potential recommendations with an overall panel rating of ‘uncertain’ suggest that, in these areas, disease-specific recommendations may be more appropriate.
The largest category of indications of good practice where there was cross-specialty agreement related to MDT processes (10 of the 21 recommendations). Our findings concur with other studies that have demonstrated the importance of clear documentation of meeting outcomes (recommendations 13, 15 and 16, Box 5)113,136 and regular review of meeting objectives (recommendations 11 and 12, Box 5). 21,118 While cancer MDTs already follow, and are audited against, national guidelines that explicitly address these issues by requiring, for example, recording of patients’ treatment plans, other MDTs do not. 10,42 This clarity of structure and processes is reflected in our findings from study 1, with similarities between the cancer teams but greater variation between teams in other disease specialties. Our findings from study 2 demonstrate agreement that it is feasible and desirable for other chronic disease MDTs to adhere to a number of these processes too. Notably, many of these indications of good practice require minimal additional financial resources, indicating that improvement is possible even in resource-stretched teams.
Our findings from study 1 highlighted clarity of purpose as an important area of diversity in MDT practices. Clarity of purpose has also been identified in previous research as a key feature of effective teamworking in health care. 21,154 In study 2 we showed that, while consensus was achieved about the primary objective of MDT meetings, and about the need to include both locally and nationally determined goals, there was uncertainty or disagreement about other functions such as teaching.
Previous research has also emphasised the importance of considering patient preferences, arguing that they are associated with MDT decision implementation rates. 120,127,128 The expert panel discussed the importance of knowing patient preferences before MDT meetings and of shared decision-making. Much of the discussion highlighted the complexity of these issues in terms of the most appropriate and sensitive times to involve patients, practical constraints (such as urgent, last-minute inclusion of patients in MDT discussions where there has been no time to obtain their preferences) and disease-specific concerns (such as difficulties faced by some mental health patients in making decisions about their treatment). The panel therefore recommended that, when making a decision, the MDT should actively seek all possible treatment options and discuss these with the patient after the meeting.
The feasibility and acceptability of giving patients the option to attend MDT meetings has been explored in the Australian healthcare system. 155,156 However the consensus panel rejected this proposal because of logistical difficulties and because these meetings were not considered to be an appropriate forum for breaking bad news.
The calculation of condition-specific medians highlighted 17 areas where disease-specific recommendations may be required. For example, while the cancer panellists argued strongly for national agreement on at least a common core of required professionals at every MDT meeting, the heart failure panellists suggested that it was more appropriate for their MDT attendees to reflect the flexibility inherent in the variable composition of MDTs across the country. Similarly, mental health panellists considered it to be imperative for someone with personal knowledge of a patient be present when that patient is discussed by the MDT, but this was believed to be unnecessary in cancer and heart failure MDT meetings.
Study limitations and strengths
Important strengths of our study include our calculation of both the strength and extent of agreement for each recommendation and our ability to understand the reasons given for each rating by conducting a thematic analysis of the panel discussions. Our consideration of results by disease group allowed us to distinguish between recommendations which might be applied generically across conditions and those which might be more appropriately dealt with on a disease-specific basis. However, these results should be treated with caution, as they represent the views of small numbers of disease-specific participants.
The three consensus methods most commonly used in health-care research are the Delphi method, the NGT and the RAM. In practice, formal consensus studies often adapt elements from each of these methods to optimally address their specific research objectives. 146 In the Delphi method, participants rate recommendations over a series of rounds using questionnaires. They do not meet to discuss their responses, but a summary of previous ratings is provided in each successive round to allow them to take them into consideration into their ratings. In contrast, the NGT convenes a panel of participants who list their ideas on a particular subject and then share them with the group in a structured meeting. After discussion, participants individually rank-order each topic. 146,148 We chose to use the RAM because the forum for detailed discussion allowed us to gain valuable data on panellists’ reasoning, and their ratings allowed us to statistically summarise both the strength and extent of agreement for each recommendation discussed.
Another strength of our study was the size and diversity of our consensus group. The inclusion of 16 participants meant that we were able to include a range of representatives from each disease speciality. Had we convened a larger panel, it is likely that the ability of every panellist to contribute fully to the discussion of each recommendation would have been diminished. However, we recognise that a consensus panel of this size raises questions around the reliability of the results. Another group of different experts might have produced different recommendations. 150 This is particularly relevant for mental health and heart failure MDTs, which, unlike cancer MDTs, are not required to follow guidance about core membership. 41,42 We therefore aimed to achieve a balance between including as many stakeholders as possible (including patient and policy representatives) and ensuring that the size of the panel allowed all participants sufficient opportunities to be heard. In the event, not all the professionals whom we approached accepted our invitation to participate, which meant that we were unable to represent all relevant professional groups (e.g. psychologists). We could have improved within- and between-group reliability by using the results of this study as the basis for a large-scale Delphi survey. 157 However, we did not have the resources to do this.
In terms of patient representatives (of which there were three), the larger proportion of health-care professionals in attendance may have acted as a barrier and prevented the views of patient representatives from being heard. Given the practical challenges described above relating to the size and diversity of the panel, the chairperson sought to overcome this limitation by actively involving all panellists in discussion. It was also made clear throughout the meeting that there were no ‘right’ or ‘wrong’ answers. In addition, by reporting variation in agreement as well as strength of agreement, we were able to take account of differences between panellists, and our final 21 indications of good practice included only those where there was agreement and low variation in agreement.
It is also possible that the framing of the evidence and recommendations could influence judgements. 158 We attempted to address this by using the meeting to identify any differing interpretations of the evidence and recommendations. This resulted in the rewording of just one of the recommendations to clarify its meaning (recommendation 19, see Box 5).
Finally, the 68 recommendations were derived from research evidence and policy guidance. Had we chosen the NGT, in which panellists begin by listing their ideas, additional and perhaps more innovative potential recommendations might have been discussed. However, this approach might also have led to less consensus.
Conclusions
Multidisciplinary team meetings for patients with chronic diseases are well established across the NHS. However, evidence for their effectiveness is mixed and their structures and processes vary widely. Given that MDT meetings are resource-intensive their value to the NHS and patients should be maximised. Previous research on improving MDT meetings has examined specific diseases in isolation,9,17,154 limiting the potential for learning across different specialties. In addition, much of the evidence regarding features of effective teamworking in health care has not been translated into practical recommendations. We used consensus development methods to build on our findings from study 1, and our expert panel identified 21 feasible and desirable indications of good practice to improve the effectiveness of MDT meetings which are applicable to a range of chronic diseases. These can be directly applied in NHS practice and quality-monitoring initiatives. The fact that no single team from the 12 teams that we observed in study 1 met all of the indications agreed suggests that there is room for improvement in all specialties, even those that already have clear policies and procedures. We also identified uncertainty and disagreement regarding a number of issues, including the best ways to involve patients, which patients should be discussed and meeting membership. These issues require further investigation, and disease-specific or locally determined guidance may be necessary.
Chapter summary
This chapter has presented study 2 in its entirety, describing how we used a formal consensus development method to derive a set of feasible recommendations for improving the effectiveness of MDT meetings for chronic diseases. In Chapter 7 we discuss the project as a whole, bringing studies 1 and 2 together to consider the key findings and implications for future research.
Chapter 7 Overall conclusions and future research directions
This research is innovative and important in examining and comparing MDT meetings for different chronic diseases. Examining decision implementation, a proxy measure of effectiveness, allowed us to identify determinants of effectiveness applicable across disease types. A qualitative investigation of differences between disease types highlighted the potential for learning across specialties.
The use of a formal consensus development method allowed us to build on these data to identify indications of good practice that are applicable across chronic diseases. We have also identified areas where disease-specific or locally determined guidance may be required, in particular regarding the best ways to involve patients in multidisciplinary decision-making.
Our key results are summarised below. These findings raise a number of issues which merit further investigation. Many of these issues can be investigated through further in-depth analyses of our rich data sets, some of which is under way. Others will require the collection and analysis of additional data. Having summarised our key findings below, we discuss possible avenues for future research to enhance our understanding of MDT processes and thereby promote improved care.
Finally, we have included a reflexive discussion of key challenges and learning points from our experience of conducting this large multisite mixed-methods study (see Appendix 20). We hope that this will provide valuable lessons for researchers embarking on studies of a similar nature.
Summary of key findings
-
We found that greater multidisciplinarity is not necessarily associated with more effective decision-making; rather it is mediated by having a clear purpose, agreed processes and a team atmosphere that facilitates inclusion and improvement.
-
Overall, 78% of MDT decisions across the 12 chronic disease MDTs under study were implemented.
-
CMHTs implemented fewer decisions than did other teams. Staff in these teams reported a wide array of functions of MDT meetings in addition to decision-making; however, some reported that meetings lacked clarity of purpose.
-
Teams differed widely in relation to the format and structure of meetings, documentation and audit procedures, choice of patients for discussion, the content of discussions and the use of technology.
-
While team members reported that they valued hearing a range of disciplinary perspectives, not all disciplines were perceived to have an equal ‘voice’.
-
Some teams were characterised by a strong medical dominance in terms of attendance and participation. While these teams typically made and implemented high numbers of treatment plans, those plans were less likely to have incorporated the full range of disciplinary perspectives.
-
Patients from more deprived areas were less likely to have their treatment plans implemented and this occurred despite the routine reference to treatment guidelines by cancer teams. Consideration of patient preference, comorbidities or other health-related factors did not seem to explain this, and we were unable to account for these findings.
-
Stakeholders with expertise in cancer, mental health and heart failure agreed on 21 indications of good practice, which were applicable to all the chronic diseases considered. They included recommendations relating to the purpose of the meetings (e.g. that agreeing treatment plans should take precedence over other objectives); meeting processes (e.g. that MDT decision implementation should be audited annually); content of the discussion (e.g. that information on comorbidities and past medical history should be routinely available); and the role of the patient (e.g. concerning the most appropriate time to discuss treatment options). Panellists from all specialties agreed that these recommendations were both desirable and feasible.
-
No single team from the 12 teams that we observed in study 1 met all of the recommendations agreed on by the expert panel. Our findings illustrate that there is scope for learning between specialties and the potential to agree a significant number of indications of good practice that are applicable in the varied contexts within which MDTs operate.
Future research priorities
Our results highlight the following areas for further study.
The effectiveness of a modified multidisciplinary team
Further research is needed to examine whether or not the 21 indications of good practice identified in this study will lead to better decision-making (in terms of decision implementation or appropriate outcomes). This requires the incorporation of the indications into a modified MDT. Effectiveness and cost-effectiveness in comparison with ‘usual care’ can then be experimentally tested in a pragmatic randomised controlled trial.
Although there was agreement that the most appropriate time to discuss treatment options with patients is after the meeting, we did not achieve clarity on the most appropriate methods for ensuring that patient preferences are accurately and systematically incorporated into the decisions made during the various stages of treatment. Our findings suggest that disease-specific approaches are needed. It is crucial for patients to be integrally involved in the design of interventions to improve their role in decision-making.
The value of multidisciplinarity
Although the principle of multidisciplinary input was highly valued by professionals across all disease specialties, considerable shortcomings existed with respect to the extent of effective multidisciplinary input into discussions. This may be important if it results in relevant determinants of decision-making (e.g. patient preferences) being omitted from discussions, or alternative perspectives being lost. We are therefore planning further analysis of our qualitative data to explore why multidisciplinarity is valued and by whom. We will also explore ways in which different professional groups share information and contribute to decision-making, possible determinants of these different approaches and the ways in which genuine multidisciplinary contributions add value to MDT decision-making.
Reasons for low implementation in community mental health teams
Community mental health teams had the lowest implementation rates. Our results suggest that this may, in part, be because members of these teams believe that MDT meetings fulfil a range of functions, but that these functions are not always clear. This opacity may be compounded by a tendency for these teams to lack administrative support, and to be hampered by variable chairing skills and a lack of a structured approach to presentations of patient cases. Taken together, our results suggest that these factors impede the ability to focus on treatment plan formulation. We are embarking on additional analysis of our qualitative data to further explore CMHT members’ beliefs about what the functions and benefits of MDT meetings are and should be; the rationale for these beliefs and how these may vary among different professional groups; and the levers and barriers to achieving key goals.
Inequalities in treatment plan implementation
We were unable to elucidate the reasons for our finding that patients from more deprived areas were less likely to have their treatment plans implemented than those from the least deprived areas. Given the continuing commitment of the NHS to provide care solely on the basis of clinical need, it is vital that further research be conducted in this area. Issues for further study include the extent to which there are systematic socioeconomic differences in choices made by patients and their carers. Certain groups of patients may have systematic variation in their preferences159 and in their capacity to articulate them. 160 There may also be systematic differences in the ways that clinical uncertainty and risk/benefit ratios are discussed with patients. Such variations may be influenced by the professional background of the patient’s key worker and the patient–key worker relationship. The possible influence of these and other factors on patient preferences and their association with treatment received needs further study.
Examination of the most appropriate composition of multidisciplinary team meetings
Although we identified examples of teams that did not meet best practice recommendations (e.g. two of the mental health teams lacked a permanent psychologist), it was beyond the scope of our study to address the issue of whether or not the professional composition of each team influenced decision-making.
Further research in this area would entail a quantitative comparison of a large number of MDTs for one condition (e.g. heart failure) to assess the association between professional composition and decision-making (or implementation).
Examination of the association between multidisciplinary team decision implementation and improvements in patient outcomes
We have explained our rationale for using decision implementation as a proxy measure of effectiveness. In order to examine whether or not implemented decisions are ‘good decisions’ that do indeed lead to patient health benefits, one approach would be to identify disease-specific measures of patient benefit that have a plausible direct relationship with defined decisions (within specified time intervals for each disease) and ideally which can be extracted from medical records. For example, for cancer patients, there are widely accepted international definitions of response of the tumour to the chosen therapy (complete/partial remission, stable/progressive disease) and of adverse events which are readily measurable within 6 months from the start of therapy. For the other conditions, outcomes may need to be measured at 3–6 months (as appropriate). In heart failure, data are routinely collected on New York Heart Association Functional Classification and on weight; in psychiatry of old age clinics, mini mental state assessments are routinely undertaken; and, in mental health, the Health of the Nation Outcome Scales (which are being introduced by some mental health teams) could be used. These would, however, be relevant to only a subset of MDT decisions (i.e. major decisions about treatment, rather than more ‘minor’ decisions, e.g. regarding the best time or method to contact a particular patient, or decisions to raise particular issues for discussion with patients or carers. These ‘minor’ decisions may also influence patient outcomes, e.g. they influence the extent to which patients engage with their care.).
The role of technology in supporting multidisciplinary team meetings
Our research focused on current practice in MDT meetings. While it was not a focus of our study, we found considerable variation in the use of technology (e.g. electronic presentations and documentation, and the use of video-conferencing) across teams. Further investigation of the role of technology in supporting MDT meetings is warranted. 161 Future studies may investigate whether possible limitations associated with using technologies such as video-conferencing (e.g. the need for IT support, less naturalistic participation, difficulties in turn-taking) are offset by potential advantages such as convenience and cost savings associated with less travel. Such research could also examine the scope for existing and emerging technologies and ways of working (e.g. those used in design technology or the aviation industry)162,163 to transform the way that MDTs work together to achieve better outcomes.
Acknowledgements
We are indebted to all the North Thames MDT teams and patients who participated in this research and made it possible, and to the NHS staff who assisted with recruitment and access to medical records. We are grateful to Natalie Austin-Parsons, who assisted with the literature review and data collection, Dr Sophie Bostock, who assisted during early preparation of the project, Dr Khadija Rantell, who assisted with data-cleaning, Dr Mike Galsworthy, who assisted with database-building, cleaning and preliminary analysis, and Dr William O’Driscoll, Dr Manonmani Manoharan and Rowan Calloway, who assisted with the patient interviews.
We would also like to acknowledge the support of the Comprehensive Clinical Research Network.
Contributions of authors
Rosalind Raine, Anne Lanceley, Archie Prentice, Jane M Blazeby and Michael King conceived the idea for this study.
Rosalind Raine led the funding application and all components of the study. As the chief investigator and guarantor, she managed the study overall and had final responsibility for the analysis and report content.
Rosalind Raine, Penny Xanthopoulou, Isla Wallace, Caoimhe Nic a’ Bháird and Julie Barber prepared the first draft of this report. All other authors contributed to subsequent drafts and approved the final draft.
Isla Wallace, Caoimhe Nic a’ Bháird and Penny Xanthopoulou contributed to study design, review of the literature, recruitment of participants, data collection, analysis and interpretation.
Julie Barber collaborated in the funding application and was responsible for the statistical aspects of the study, including involvement in the study design, analysis and interpretation.
Jane M Blazeby, Alex Clarke, J Simon R Gibbs, Michael King, Anne Lanceley, Gill Livingston, Susan Michie and Archie Prentice collaborated in developing the idea for the research and in the funding application. They contributed to study design and provided clinical expertise throughout the project.
David Ardron, Miriam Harris, Alex Clarke, Anne Lanceley, Gill Livingston and Archie Prentice contributed to participant recruitment.
Anne Lanceley and Alex Clarke contributed to the qualitative analysis and interpretation of the findings.
David Ardon and Miriam Harris contributed to study design and provided guidance from a patient perspective throughout the project.
Ewan Ferlie contributed to the funding application and provided expertise on the influence of context.
Publications
Raine R, Xanthopoulou P, Wallace I, Nic a’ Bháird C, Lanceley A, Clarke A, et al. Determinants of treatment plan implementation in multidisciplinary team meetings for patients with chronic diseases: a mixed-methods study [published online ahead of print June 9 2014]. BMJ Qual Saf 2014. http://dx.doi.org/10.1136/bmjqs-2014-002818
O’Driscoll W, Livingston G, Lanceley A, Nic a’ Bháird C, Xanthopoulou P, Wallace I, et al. Patient experience of MDT care and desicion-making. Mental Health Review Journal 2014; in press.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- National Cancer Peer Review Programme Manual for Cancer Services: Haemato-oncology Cancer Measures. London: National Cancer Action Team; 2013.
- Improving Chronic Disease Management. London: Department of Health; 2004.
- Mental Health Policy Implementation Guide: Community Mental Health Teams. London: Department of Health; 2001.
- Management of Stable Angina. London: National Institute for Health and Clinical Excellence; 2012.
- Harrison JD, Choy ET, Spillane A, Butow P, Young JM, Evans A. Australian breast cancer specialists’ involvement in multidisciplinary treatment planning meetings. Breast 2008;17:335-40. http://dx.doi.org/10.1016/j.breast.2008.03.001.
- Wagner E. Effective teamwork and quality of care. Med Care 2004;42:1037-9. http://dx.doi.org/10.1097/01.mlr.0000145875.60036.ed.
- Haward R, Amir Z, Borrill C, Dawson J, Scully J, West M, et al. Breast cancer teams: the impact of constitution, new cancer workload, and methods of operation on their effectiveness. Br J Cancer 2003;89:15-22. http://dx.doi.org/10.1038/sj.bjc.6601073.
- Mickan SM. Evaluating the effectiveness of health care teams. Aust Health Rev 2005;29:211-17. http://dx.doi.org/10.1071/AH050211.
- Head SJ, Kaul S, Mack MJ, Serruys PW, Taggart DP, Holmes DR, et al. The rationale for heart team decision-making for patients with stable, complex coronary artery disease. Eur Heart J 2013;34:2510-18. http://dx.doi.org/10.1093/eurheartj/eht059.
- An Overview of the Findings from the 2011/12 National Cancer Peer Review of Cancer Services in England. London: National Cancer Action Team; 2012.
- Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK?. Lancet Oncol 2006;7:935-43. http://dx.doi.org/10.1016/S1470-2045(06)70940-8.
- Norell M. Decision by consensus: more political correctness or a genuine improvement in care?. Br J Cardiol 2012;19:162-4.
- Kesson EM, Allardice GM, George WD, Burns HJG, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2718.
- Meeuwsen EJ, Melis RJF, Van Der Aa GCHM, Golüke-Willemse GAM, De Leest BJM, Van Raak FHJM, et al. Effectiveness of dementia follow-up care by memory clinics or general practitioners: randomised controlled trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3086.
- Malone D, Marriott S, Newton-Howes G, Simmonds S, Tyrer P. Community mental health teams (CMHTs) for people with severe mental illnesses and disordered personality. Cochrane Database Syst Rev 2010;3.
- Ke KM, Blazeby J, Strong S, Carroll F, Ness A, Hollingworth W. Are multidisciplinary teams in secondary care cost-effective? A systematic review of the literature. Cost Eff Resour Alloc 2013;11. http://dx.doi.org/10.1186/1478-7547-11-7.
- Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol 2011;18:2116-25. http://dx.doi.org/10.1245/s10434-011-1675-6.
- Haward RA. The Calman–Hine report: a personal retrospective on the UK’s first comprehensive policy on cancer services. Lancet Oncol 2006;7:336-46. http://dx.doi.org/10.1016/S1470-2045(06)70659-3.
- Grumbach K, Bodenheimer T. Can health care teams improve primary care practice?. JAMA 2004;291:1246-51. http://dx.doi.org/10.1001/jama.291.10.1246.
- Borrill C, West M, Dawson J, Shapiro D, Rees A, Richards A, et al. Team Working and Effectiveness in Health Care: Findings from the Health Care Team Effectiveness Project. Birmingham: Aston Centre for Health Service Organisation Research; 2002.
- Fay D, Borrill C, Amir Z, Haward R, West MA. Getting the most out of multidisciplinary teams: a multi-sample study of team innovation in health care. J Occup Organ Psychol 2006;79:553-67. http://dx.doi.org/10.1348/096317905X72128.
- Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav 1998;19:235-58. http://dx.doi.org/10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C.
- Gonzalez-Roma V, Gamero N. Does positive team mood mediate the relationship between team climate and team performance?. Psicothema 2012;24:94-9.
- Goh T, Eccles M, Steen N. Factors predicting team climate, and its relationship with quality of care in general practice. BMC Health Serv Res 2009;9:1-11. http://dx.doi.org/10.1186/1472-6963-9-138.
- Taylor C, Ramirez A. Multidisciplinary Team Members’ Views About MDT Working: Results from a Survey Commissioned by the National Cancer Action Team. London: National Cancer Action Team; 2009.
- Powell AE, Davies HTO. The struggle to improve patient care in the face of professional boundaries. Soc Sci Med 2012;75:807-14. http://dx.doi.org/10.1016/j.socscimed.2012.03.049.
- Borrill C, Carletta J, Carter A, Dawson J, Garrod S, Rees A, et al. The Effectiveness of Health Care Teams in the National Health Service. Birmingham: University of Aston; 2000.
- McHugh P, Byrne M. The teamworking challenges of care planning. Ir J Psycholog Med 2012;29:185-9.
- Singh SP. Running an effective community mental health team. Adv Psychiatric Treat 2000;6:414-22. http://dx.doi.org/10.1192/apt.6.6.414.
- Wilberforce M, Tucker S, Abendstern M, Brand C, Giebel CM, Challis D. Membership and management: structures of inter-professional working in community mental health teams for older people in England. Int Psychogeriatr 2013;25:1485-92. http://dx.doi.org/10.1017/S104161021300077X.
- Howard V, Holmshaw J. Inpatient staff perceptions in providing care to individuals with co-occurring mental health problems and illicit substance use. J Psychiatr Mental Health Nurs 2010;17:862-72. http://dx.doi.org/10.1111/j.1365-2850.2010.01620.x.
- Kaplan HC, Brady PW, Dritz MC, Hooper DK, Linam WM, Froehle CM, et al. The influence of context on quality improvement success in health care: a systematic review of the literature. Milbank Q 2010;88:500-59. http://dx.doi.org/10.1111/j.1468-0009.2010.00611.x.
- Cohen SG, Bailey DE. What makes teams work: group effectiveness research from the shop floor to the executive suite. J Manage 1997;23:239-90. http://dx.doi.org/10.1177/014920639702300303.
- Lemieux-Charles L, McGuire WL. What do we know about health care team effectiveness? A review of the literature. Med Care Res Rev 2006;63:263-300. http://dx.doi.org/10.1177/1077558706287003.
- Petticrew M, Ferlie E, McKee L. Shaping Strategic Change. London: Sage; 1992.
- McNulty T, Ferlie E. Reengineering Health Care. Oxford: Oxford University Press; 2002.
- A Policy Framework for Commissioning Cancer Services: A Report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales. London: Department of Health; 1995.
- The NHS Cancer Plan: A Plan for Investment A Plan for Reform. London: Department of Health; 2000.
- Cancer Reform Strategy. London: Department of Health; 2007.
- Improving Outcomes: A Strategy for Cancer. London: Department of Health; 2011.
- Guidance on Cancer Services: Improving Outcomes in Haematological Cancers: The Manual. London: National Institute for Clinical Excellence; 2003.
- National Cancer Peer Review Programme Manual for Cancer Services: Gynaecology Measures. London: National Cancer Action Team; 2013.
- National Cancer Peer Review Programme Manual for Cancer Services: Skin Measures. London: National Cancer Action Team; 2013.
- Burns T, Lloyd H. Is a team approach based on staff meetings cost-effective in the delivery of mental health care?. Curr Opin Psychiatry 2004;17:311-14. http://dx.doi.org/10.1097/01.yco.0000133835.26920.ff.
- Sayce L, Craig TKJ, Boardman AP. The development of community mental health centres in the UK. Soc Psychiatry Psychiatr Epidemiol 1991;26:14-20. http://dx.doi.org/10.1007/BF00783575.
- Onyett S. Teamworking in Mental Health. Basingstoke: Palgrave Macmillan; 2003.
- The National Service Framework for Mental Health: Modern Standards and Service Models. London: Department of Health; 1999.
- How Mental Illness Loses Out in the NHS. London: London School of Economics; 2012.
- Mental Health Policy Implementation Guide. London: Department of Health; 2001.
- A Fresh Start for the Regulation and Inspection of Mental Health Services. Newcastle upon Tyne: Care Quality Commission; 2013.
- Chronic Heart Failure: Management of Chronic Heart Failure in Adults in Primary and Secondary Care. London: National Institute for Clinical Excellence; 2003.
- Chronic Heart Failure: Management of Chronic Heart Failure in Adults in Primary and Secondary Care. London: National Institute for Health and Clinical Excellence; 2010.
- Services for People with Chronic Heart Failure. London: National Institute for Health and Clinical Excellence; 2011.
- National Service Framework for Older People. London: Department of Health; 2001.
- Jolley D, Moniz-Cook E. Memory clinics in context. Ind J Psychiatr 2009;51:S70-S76.
- Szymczynska P, Innes A, Forrest L, Stark C, Mason A. Best Practice Review: Diagnostic and Post-diagnostic Service Provision to People with Dementia and Their Carers with Particular Interest in Remote and Rural Populations. NHS Highland: Dementia Services Development Centre; 2010.
- Dementia Commissioning Pack: Service Specification for Dementia: Memory Service for Early Diagnosis and Intervention. London: Department of Health; 2011.
- The NHS Constitution for England. London: Department of Health; 2013.
- Dixon A, Le Grand J, Henderson J, Murray R, Poteliakhoff E. Is the British National Health Service equitable? The evidence on socioeconomic differences in utilization. J Health Serv Res Policy 2007;12:104-9. http://dx.doi.org/10.1258/135581907780279549.
- Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441-7. http://dx.doi.org/10.1001/jama.291.20.2441.
- Raine R. Does gender bias exist in the use of specialist health care?. J Health Serv Res Policy 2000;5:237-49.
- Vallejo-Torres L, Morris S. Income-related inequity in healthcare utilisation among individuals with cardiovascular disease in England: accounting for vertical inequity. Health Econ 2013;22:533-53. http://dx.doi.org/10.1002/hec.2821.
- MacLeod MC, Finlayson AR, Pell JP, Findlay IN. Geographic, demographic, and socioeconomic variations in the investigation and management of coronary heart disease in Scotland. Heart 1999;81:252-6.
- Hawkins NM, Scholes S, Bajekal M, Love H, O’Flaherty M, Raine R, et al. Reducing socioeconomic inequality in coronary disease treatments: the NHS finally triumphs?. J Epidemiol Comm Health 2011;65:A20-A21.
- Soomro GM, Burns T, Majeed A. Socio-economic deprivation and psychiatric referral and admission rates: an ecological study in one London borough. Psychiatr Bull 2002;26:175-8. http://dx.doi.org/10.1192/pb.26.5.175.
- Drukker M, Krabbendam L, Driessen G, van Os J. Social disadvantage and schizophrenia – A combined neighbourhood and individual-level analysis. Soc Psychiatry Psychiatr Epidemiol 2006;41:595-604. http://dx.doi.org/10.1007/s00127-006-0081-z.
- Bebbington P, Brugha T, Meltzer H, Jenkins R, Ceresa C, Farrell M, et al. Neurotic disorders and the receipt of psychiatric treatment. Int Rev Psychiatry 2003;15:108-14. http://dx.doi.org/10.1080/0954026021000046010.
- Shepherd G, Beadsmoore A, Moore C, Hardy P, Muijen M. Relation between bed use, social deprivation, and overall bed availability in acute adult psychiatric units, and alternative residential options: a cross sectional survey, one day census data, and staff interviews. BMJ 1997;314:262-6. http://dx.doi.org/10.1136/bmj.314.7076.262.
- McMahon M, Barbiere JM, Greenberg DC, Wright KA, Lyratzopoulos G. Population-based trends in use of surgery for non-small cell lung cancer in a UK region, 1995–2006. Thorax 2011;66:453-5. http://dx.doi.org/10.1136/thoraxjnl-2011-200039.
- Raine R, Wong W, Scholes S, Ashton C, Obichere A, Ambler G. Social variations in access to hospital care for patients with colorectal, breast, and lung cancer between 1999 and 2006: retrospective analysis of hospital episode statistics. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.b5479.
- Riaz SP, Luchtenborg M, Jack RH, Coupland VH, Linklater KM, Peake MD, et al. Variation in surgical resection for lung cancer in relation to survival: population-based study in England 2004–2006. Eur J Cancer 2012;48:54-60. http://dx.doi.org/10.1016/j.ejca.2011.07.012.
- Lyratzopoulos G, Barbiere J, Gajperia C, Rhodes M, Greenberg D, Wright K. Trends and variation in the management of oesophagogastric cancer patients: a population-based survey. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-231.
- Downing A, Prakash K, Gilthorpe MS, Mikeljevic JS, Forman D. Socioeconomic background in relation to stage at diagnosis, treatment and survival in women with breast cancer. Br J Cancer 2007;96:836-40. http://dx.doi.org/10.1038/sj.bjc.6603622.
- Lyratzopoulos G, Newsome H, Barbiere J, Bolton K, Wright K, Kitchener H, et al. Trends in the surgical management of epithelial ovarian cancer in East Anglia 1995–2006. Eur J Surg Oncol 2011;37:435-41. http://dx.doi.org/10.1016/j.ejso.2011.02.004.
- Stevens S, Oliver A. Equity in Health and Healthcare. London: Nuffield Trust; 2003.
- Dementia: Supporting People with Dementia and Their Carers in Health and Social Care. London: National Institute for Clinical Excellence; 2006.
- Walker D, Kemp E. A Guide for Review and Improvement of Hospital Based Heart Failure Services. Leicester: NHS Improvement; 2011.
- No Health without Mental Health: A Cross-Government Mental Health Outcomes Strategy for People of All Ages. London: Department of Health; 2011.
- Lanceley A, Savage J, Menon U, Jacobs I. Influences on multidisciplinary team decision-making. Int J Gynecol Cancer 2008;18:215-22. http://dx.doi.org/10.1111/j.1525-1438.2007.00991.x.
- Kidger J, Murdoch J, Donovan J, Blazeby J. Clinical decision-making in a multidisciplinary gynaecological cancer team: a qualitative study. BJOG 2009;116:511-17. http://dx.doi.org/10.1111/j.1471-0528.2008.02066.x.
- Shortell SM, Marsteller JA, Lin M, Pearson ML, Wu SY, Mendel P, et al. The role of perceived team effectiveness in improving chronic illness care. Med Care 2004;42:1040-8. http://dx.doi.org/10.1097/00005650-200411000-00002.
- Blazeby J, Wilson L, Metcalfe C, Nicklin J, English R, Donovan J. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann Oncol 2006;17:457-60. http://dx.doi.org/10.1093/annonc/mdj102.
- Entwistle VA, Sowden AJ, Watt IS. Evaluating interventions to promote patient involvement in decision-making: by what criteria should effectiveness be judged?. J Health Serv Res Pol 1998;32:100-7.
- Lilford RJ, Brown CA, Nicholl J. Use of process measures to monitor the quality of clinical practice. BMJ 2007;335:648-50. http://dx.doi.org/10.1136/bmj.39317.641296.AD.
- Wood JJ, Metcalfe C, Paes A, Sylvester P, Durdey P, Thomas MG, et al. An evaluation of treatment decisions at a colorectal cancer multi-disciplinary team. Colorectal Dis 2008;108:769-72. http://dx.doi.org/10.1111/j.1463-1318.2007.01464.x.
- Jalil R, Ahmed M, Green JSA, Sevdalis N. Factors that can make an impact on decision-making and decision implementation in cancer multidisciplinary teams: an interview study of the provider perspective. Int J Surg 2013;11:389-94. http://dx.doi.org/10.1016/j.ijsu.2013.02.026.
- National Health Service Act 2006. London: The Stationery Office; 2006.
- Agrell A, Gustafson R. The Team Climate Inventory (TCI) and group innovation: a psychometric test on a Swedish sample of work groups. J Occup Organ Psychol 1994;67:143-51. http://dx.doi.org/10.1111/j.2044-8325.1994.tb00557.x.
- Kivimaki M, Elovainio M. A short version of the Team Climate Inventory: development and psychometric properties. J Occup Organ Psychol 1999;72:241-6. http://dx.doi.org/10.1348/096317999166644.
- Ragazzoni P, Baiardi P, Zotti AM, Anderson N, West M. Research note: Italian validation of the team climate inventory: a measure of team climate for innovation. J Manage Psychol 2002;17:325-36. http://dx.doi.org/10.1108/02683940210428128.
- Anderson N, West M. The Team Climate Inventory: User’s Guide. Windsor: NFER-Nelson Publishing Company; 1999.
- Gilligan D, Goodrum L, Magee L, Harris S. Do decisions made at lung cancer multi-disciplinary team meetings get carried out. Lung Cancer 2005;49. http://dx.doi.org/10.1016/S0169-5002(05)80195-3.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. http://dx.doi.org/10.1016/S0895-4356(96)00236-3.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- Gulliford MC, Adams G, Ukoumunne OC, Latinovic R, Chinn S, Campbell MJ. Intraclass correlation coefficient and outcome prevalence are associated in clustered binary data. J Clin Epidemiol 2005;58:246-51. http://dx.doi.org/10.1016/j.jclinepi.2004.08.012.
- Proudfoot J, Jayasinghe UW, Holton C, Grimm J, Bubner T, Amoroso C, et al. Team climate for innovation: what difference does it make in general practice?. Int J Qual Health Care 2007;19:164-9. http://dx.doi.org/10.1093/intqhc/mzm005.
- Bosch M, Dijkstra R, Wensing M, van der Weijden T, Grol R. Organizational culture, team climate and diabetes care in small office-based practices. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-180.
- Harrison DA, Klein KJ. What’s the difference? Diversity constructs as separation, variety, or disparity in organizations. Acad Manage Rev 2007;32:1199-228. http://dx.doi.org/10.5465/AMR.2007.26586096.
- The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- Harrell FE. Regression Modelling Strategies. Springer; 2001.
- McGrath JE. Social Psychology: A Brief Introduction. New York, NY: Holt, Rinehart & Winston; 1964.
- Mathieu J, Maynard T, Rapp T, Gilson L. Team effectiveness 1997–2007: a review of recent advancements and a glimpse into the future. J Manage 2008;34:410-76. http://dx.doi.org/10.1177/0149206308316061.
- Emerson R, Fretz R, Shaw L, Emerson R, Fretz R, Shaw L. Writing Ethnographic Fieldnotes. Chicago: University of Chicago Press; 1995.
- Barry CA, Britten N, Barber N, Bradley C, Stevenson F. Using reflexivity to optimize teamwork in qualitative research. Qual Health Res 1999;9:26-44. http://dx.doi.org/10.1177/104973299129121677.
- Raine R, Xanthopoulou P, Wallace I, Nic a’ Bháird C, Lanceley A, Clarke A, et al. Determinants of treatment plan implementation in multidisciplinary team meetings for patients with chronic diseases: a mixed-methods study [published online ahead of print June 9 2014]. BMJ Qual Saf 2014. http://dx.doi.org/10.1136/bmjqs-2014-002818.
- Hammersley N, Richardson JTE. Handbook of Research Methods for Psychology and the Social Sciences. Leicester: BPS Books; 1996.
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341:1147-50. http://dx.doi.org/10.1136/bmj.c4587.
- Moran-Ellis J, Alexander VD, Cronin A, Dickinson M, Fielding J, Sleney J, et al. Triangulation and integration: processes, claims and implications. Qual Res 2006;6:45-59. http://dx.doi.org/10.1177/1468794106058870.
- Barbour RS. Checklists for improving rigour in qualitative research: a case of the tail wagging the dog?. BMJ 2001;322:1115-17. http://dx.doi.org/10.1136/bmj.322.7294.1115.
- Delaney W, Ames G. Integration and exchange in multidisciplinary alcohol research. Soc Sci Med 1993;37:5-13. http://dx.doi.org/10.1016/0277-9536(93)90311-Q.
- Cohen DJ, Crabtree BF. Evaluative criteria for qualitative research in health care: controversies and recommendations. Ann Fam Med 2008;6:331-9. http://dx.doi.org/10.1370/afm.818.
- Intercollegiate Cancer Committee Educational Initiatives to Improve the Effectiveness of Cancer Multidisciplinary Teams. London: Academy of Medical Royal Colleges; 2009.
- The Characteristics of an Effective Multidisciplinary Team. London: NHS National Cancer Action Team; 2010.
- Cyhlarove E, McCulloch A, McGuffin P, Wykes T. Economic Burden of Mental Illness Cannot be Tackled without Research Investment. London: Mental Health Foundation; 2010.
- Medical Research Council Annual Report and Accounts 2012/1213. Swindon: Medical Research Council; 2013.
- Towards a Common Language for Functioning, Disability and Health. Geneva: World Health Organization; 2002.
- Nembhard IM, Edmondson AC. Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organ Behav 2006;27:941-66. http://dx.doi.org/10.1002/job.413.
- van Knippenberg D, Dawson JF, West MA, Homan AC. Diversity faultlines, shared objectives, and top management team performance. Human Relations 2011;64:307-36. http://dx.doi.org/10.1177/0018726710378384.
- West MA, Borrill CS, Dawson JF, Brodbeck F, Shapiro DA, Haward B. Leadership clarity and team innovation in health care. Leadersh Q 2003;14:393-410. http://dx.doi.org/10.1016/S1048-9843(03)00044-4.
- Kidger J, Murdoch J, Donovan JL, Blazeby JM. Clinical decision-making in a multidisciplinary gynaecological cancer team: a qualitative study. BJOG 2009;116:511-17. http://dx.doi.org/10.1111/j.1471-0528.2008.02066.x.
- Borrill CS, Carletta J, Carter A, Dawson JF, Garrod S, Rees A, et al. The Effectiveness of Health Care Teams in the National Health Service. Birmingham: University of Aston; 2000.
- Bower P, Campbell S, Bojke C, Sibbald B. Team structure, team climate and the quality of care in primary care: an observational study. Qual Saf Health Care 2003;12:273-9. http://dx.doi.org/10.1136/qhc.12.4.273.
- Rachet B, Ellis L, Maringe C, Chu T, Nur U, Quaresma M, et al. Socioeconomic inequalities in cancer survival in England after the NHS Cancer Plan. Br J Cancer 2010;103:446-53. http://dx.doi.org/10.1038/sj.bjc.6605752.
- Cooper C, Blanchard M, Selwood A, Livingston G. Antidementia drugs: prescription by level of cognitive impairment or by socio-economic group?. Aging Ment Health 2010;14:85-9. http://dx.doi.org/10.1080/13607860902918256.
- Bajekal M, Scholes S, Love H, Hawkins N, O’Flaherty M, Raine R, et al. Analysing recent socioeconomic trends in coronary heart disease mortality in England, 2000–2007: a population modelling study. PLoS Med 2012;9. http://dx.doi.org/10.1371/journal.pmed.1001237.
- Dementia: Supporting People with Dementia and Their Carers in Health and Social Care. London: National Institute for Clinical Excellence; 2006.
- Blazeby JM, Wilson L, Metcalfe C, Nicklin J, English R, Donovan JL. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann Oncol 2006;17:457-60. http://dx.doi.org/10.1093/annonc/mdj102.
- English R, Metcalfe C, Day J, Rayter Z, Blazeby J. A prospective analysis of implementation of multi-disciplinary team decisions in breast cancer. Breast J 2012;18:459-63. http://dx.doi.org/10.1111/j.1524-4741.2012.01270.x.
- Lamb BW, Sevdalis N, Benn J, Vincent C, Green JS. Multidisciplinary cancer team meeting structure and treatment decisions: a prospective correlational study. Ann Surg Oncol 2013;20:715-22. http://dx.doi.org/10.1245/s10434-012-2691-x.
- Stalfors J, Lundberg C, Westin T. Quality assessment of a multidisciplinary tumour meeting for patients with head and neck cancer. Acta Otolaryngol 2007;127:82-7. http://dx.doi.org/10.1080/00016480600740589.
- Mallinger JB, Shields CG, Griggs JJ, Roscoe JA, Morrow GR, Rosenbluth RJ, et al. Stability of decisional role preference over the course of cancer therapy. Psychooncology 2006;15:297-305. http://dx.doi.org/10.1002/pon.954.
- West M, Alimo-Metcalfe B, Dawson J, El Ansari W, Glasby J, Hardy G, et al. Effectiveness of Multi-Professional Team Working (MPTW) in Mental Health Care: Final Report 2011.
- McNair A, Choh C, Metcalfe C, Littlejohns D, Barham C, Hollowood A, et al. Maximising recruitment into randomised controlled trials: the role of multidisciplinary cancer teams. Eur J Cancer 2008;44:2623-6. http://dx.doi.org/10.1016/j.ejca.2008.08.009.
- Jalil R, Lamb B, Russ S, Sevdalis N, Green J. The cancer multi-disciplinary team from the co-ordinators’ perspective: results from a national survey in the UK. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-457.
- Whelan J, Griffith C, Archer T. Breast cancer multi-disciplinary teams in England: much achieved but still more to be done. Breast 2006;15:119-22. http://dx.doi.org/10.1016/j.breast.2005.02.010.
- Field KM, Rosenthal MA, Dimou J, Fleet M, Gibbs P, Drummond K. Communication in and clinician satisfaction with multidisciplinary team meetings in neuro-oncology. J Clin Neurosci 2010;17:1130-5. http://dx.doi.org/10.1016/j.jocn.2010.03.001.
- Nouraei S, Philpott J, Nouraei S, Maude D, Sandhu G, Sandison A, et al. Reducing referral-to-treatment waiting times in cancer patients using a multidisciplinary database. Ann R Coll Surg Engl 2007;89:113-17. http://dx.doi.org/10.1308/003588407X155455.
- Fallowfield L, Ford S, Lewis S. No news is not good news: information preferences of patients with cancer. Psychooncology 1995;4:197-202. http://dx.doi.org/10.1002/pon.2960040305.
- Degner LF, Kristianson LJ, Bowman D, Sloan J, Carriere K, O’Neil J, et al. Information needs and decisional preferences in women with breast cancer. JAMA 1997;277:1485-92. http://dx.doi.org/10.1001/jama.1997.03540420081039.
- Leydon G, Boulton M, Moynihan C, Jones A, Mossman J, Boudioni M, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ 2000;329:909-13. http://dx.doi.org/10.1136/bmj.320.7239.909.
- O’Driscoll W, Livingston G, Lanceley A, Nic a’ Bháird C, Xanthopoulou P, Wallace I, et al. Patient experience of MDT care and decision-making. Mental Health Review Journal 2014.
- Campbell SM, Braspenning J, Hutchinson A, Marshall MN. Research methods used in developing and applying quality indicators in primary care. BMJ 2003;326:816-19. http://dx.doi.org/10.1136/bmj.326.7393.816.
- Rolls KD, Elliott D. Using consensus methods to develop clinical practice guidelines for intensive care: the Intensive Care Collaborative project. Aust Crit Care 2008;21:200-15. http://dx.doi.org/10.1016/j.aucc.2008.08.003.
- Vella K, Goldfrad C, Rowan K, Bion J, Black N. Use of consensus development to establish national research priorities in critical care. BMJ 2000;320:976-80. http://dx.doi.org/10.1136/bmj.320.7240.976.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80. http://dx.doi.org/10.1136/bmj.311.7001.376.
- Murphy M, Black N, Lamping D, McKee C, Sanderson C, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Hutchings A, Raine R, Sanderson C, Black N. An experimental study of determinants of the extent of disagreement within clinical guideline development groups. Qual Saf Health Care 2005;14:240-5. http://dx.doi.org/10.1136/qshc.2004.013227.
- Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci 1971;7:466-92. http://dx.doi.org/10.1177/002188637100700404.
- Dalkey N, Helmer O. An experimental application of the Delphi Method to the use of experts. Manage Sci 1963;9:458-67. http://dx.doi.org/10.1287/mnsc.9.3.458.
- Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979-83. http://dx.doi.org/10.2105/AJPH.74.9.979.
- Halcomb E, Davidson P, Hardaker L. Using the consensus development conference method in healthcare research. Nurs Res 2008;16:56-71. http://dx.doi.org/10.7748/nr2008.10.16.1.56.c6753.
- Black N, Murphy M, Lamping D, McKee M, Sanderson C, Askham J, et al. Consensus development methods: a review of best practice in creating clinical guidelines. J Health Serv Res Policy 1999;4:236-48.
- West M, Alimo-Metcalfe B, Dawson J, El Ansari W, Glasby J, Hardy G, et al. Effectiveness of Multi-Professional Team Working (MPTW) in Mental Health Care: Final Report 2011.
- Butow P, Harrison JD, Choy ET, Young JM, Spillane A, Evans A. Health professional and consumer views on involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Cancer 2007;1109:1937-44. http://dx.doi.org/10.1002/cncr.23007.
- Choy ET, Chiu A, Butow P, Young J, Spillane A. A pilot study to evaluate the impact of involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Breast 2007;16:178-89. http://dx.doi.org/10.1016/j.breast.2006.10.002.
- Raine R, Sanderson C, Black N. Developing clinical guidelines: a challenge to current methods. BMJ 2005;3317:631-3. http://dx.doi.org/10.1136/bmj.331.7517.631.
- Loblaw DA, Prestrud AA, Somerfield MR, Oliver TK, Brouwers MC, Nam RK, et al. American Society of Clinical Oncology Clinical Practice Guidelines: formal systematic review-based consensus methodology. J Clin Oncol 2012;30:3136-40. http://dx.doi.org/10.1200/JCO.2012.42.0489.
- Suarez-Almazor ME, Souchek J, Kelly PA, O’Malley K, Byrne M, Richardson M, et al. Ethnic variation in knee replacement: patient preferences or uninformed disparity?. Arch Int Med 2005;165:1117-24. http://dx.doi.org/10.1001/archinte.165.10.1117.
- Katz JN. Patient preferences and health disparities. JAMA 2001;286:1506-9. http://dx.doi.org/10.1001/jama.286.12.1506.
- Kane B, Luz S. Information sharing at multidisciplinary medical team meetings. Group Decis Negot 2011;20:437-64. http://dx.doi.org/10.1007/s10726-009-9175-9.
- Catchpole KR, De Leval MR, McEwan A, Pigott N, Elliott MJ, McQuillan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit-stop and aviation models to improve safety and quality. Paediatr Anaesth 2007;17:470-8. http://dx.doi.org/10.1111/j.1460-9592.2006.02239.x.
- Catmull E. How Pixar fosters collective creativity. Harvard Business Review 2008;86:64-72.
- Department of Health. Choosing Health: Supporting the Physical Needs of People With Severe Mental Illness: Commissioning Framework 2006.
- Department of Health. Living Well With Dementia: A National Dementia Strategy 2009.
- National Collaborating Centre for Mental Health. A NICE-SCIE Guideline on Supporting People With Dementia and Their Carers in Health and Social Care 2007.
Appendix 1 Participant consent forms
Participant consent form to observe
Participant information leaflet
[hospital logo]
VERSION 2, 3 AUGUST 2010
PARTICIPANT information leaflet
Improving the effectiveness of Multi-Disciplinary Team Meetings (MDMs) for patients with chronic diseases
We would like to invite you to take part in a research study. Please read this leaflet which tells you about the study and what it involves and ask one of our team if there is anything that is not clear. Take time to decide whether or not you wish to take part.
What is the purpose of the study?
Multi-Disciplinary Team Meetings (MDMs) are widely used across the NHS for managing chronic diseases. We want to find out more about the factors that influence clinical decision-making at MDMs. The findings will be used to improve treatment decisions in your MDM and in other MDMs across the NHS by recommending possible improvements in the way that MDMs work.
Why have I been chosen?
We wish to examine a number of MDMs covering a wide range of clinical conditions affecting a diverse range of patients. All members of your multi-disciplinary team have been provided with this information sheet because we wish to observe your MDM and to invite some MDM members to participate in an interview. We will invite MDM members from each core professional group represented on the MDM, including both regular and infrequent attenders, to be interviewed.
What does the research involve?
If all multi-disciplinary team members consent, a researcher will attend and observe a number of consecutive MDMs. She will not be an active participant in these meetings, but will both audiotape the meeting and take notes, using a structured form. She will collect information on the structure of the meeting (including the number of patients discussed, the professional mix of members attending); processes (including the roles of each of the members); patient related factors that are discussed and the clinical decision made. We expect to attend and observe between 22 and 41 meetings, depending on the number of patients discussed. The first eight meetings will be audiotaped. In this part of the study, you will not be required to do anything outside of, or in addition to your normal day to day activities.
Patients discussed at each MDM will be given the opportunity to ‘opt out’ of having their medical information included in the study. We will ask each patient’s key worker/treating clinician to give their patients an ‘opt out’ form at the earliest appropriate and feasible opportunity. These forms will be available at every MDM where patients eligible for inclusion in this research are discussed.
In addition, the researcher will conduct face-to-face interviews with a selection of multi-disciplinary team members. The purpose of these interviews is to explore members’ perceptions of MDM strengths and weaknesses; factors influencing MDM decisions; their professional role and value to the MDM. The questions will be flexible and open-ended, to allow you the chance to raise the issues that you feel are important. If you are approached to be interviewed, we will ask you to sign a further consent form. All information given during these interviews will be kept strictly confidential and no names will be attached to the information provided. The interview will be conducted at a convenient time and place of your choosing. The interview will last between 30 minutes and one hour and may be ended by you at any time. It will be tape-recorded, if you consent, but the tapes will be destroyed after analysis has been completed.
Do I have to take part?
It will be entirely up to you to decide whether or not to take part in the study and you can withdraw from the study at any time without having to give a reason. If you decide to participate, you will be asked to sign a consent form, and given a copy to keep. A decision not to take part or a decision to withdraw from the study will not affect your work in any way.
What are the possible disadvantages of being interviewed?
It is expected that this study does not have any disadvantages, but the interview will take between thirty and sixty minutes of your time.
What are the possible benefits of being interviewed?
The information we get from this study will improve the MDM decision-making process by highlighting areas of excellence and possible weaknesses. If you take part in an interview, your anonymised views will contribute to our findings and any resulting recommendations for change.
Will what I say be confidential?
Yes. We will follow ethical and legal practice and all information about you will be handled in confidence. If you are interviewed, this will take place in private, and the recording will not contain your name or any personal information, only a study identification number. Recordings will be encrypted and held in a computer in the Department of Epidemiology and Public Health, UCL. Only those members of the research team who are directly involved in analysing the information will have access to the recordings. In publications and reports, the identity of participating MDMs will not be revealed, only basic descriptive information on the conditions covered and regional location of the MDM will be given. Professor Rosalind Raine is the Chief Investigator and she has overall responsibility for confidentiality and data security.
What will happen to the results of the study?
Once the study has finished the results will be analysed and conclusions drawn about how treatment decisions are reached, and how this process might be improved. Findings will be published in scientific journals, but the MDM and all individuals will be referred to in anonymised form. Quotes from the interviews may be used, but again will be anonymised. Any quotes where the individual concerned could be identified by another team member, or anyone else, will not be used. We will also visit your MDM and provide a summary of our findings. Again, interview quotes will only be used as long as the speaker’s anonymity can be preserved. MDM members will have the opportunity to discuss the findings and give their views on the recommendations.
Who is organising and funding the research
Professor Rosalind Raine is the Chief Investigator and the study is funded by the National Institute of Health Research. No payments are made to the researchers conducting this study.
Who has reviewed this study?
All research in the NHS is looked at by an independent group of people, called a Research Ethics Committee, to protect your interests. This study has been reviewed and given a favourable opinion by East London Research Ethics Committee.
What do I do if I wish to make a complaint about the research?
If you wish to complain about any aspect of the research, you should contact the Chief Investigator, Professor Rosalind Raine or the researcher. If you feel you do not receive a satisfactory response and you wish to take the matter further you should contact the Complaints Manager (see below) giving the project title and the Principal Investigator’s contact details.
Contact details
Please contact [researcher] if you would like to ask questions about the study or for any other reason:
By telephone:
By email:
By post:
You can also contact:
Professor Rosalind Raine on [telephone] or by email [email address]
Contact details for the Complaints Manager are:
[local service address and telephone]
Thank you very much for taking the time to read this information about the study.
Appendix 2 Observation proforma
Quantitative data collection
Appendix 3 Models from sensitivity analyses
Sensitivity analysis 1
| Sensitivity analysis | Final model: adjusted model 3 (n = 2431) | Sensitivity analysis 1: adjusting for number of decisions making up first treatment plan (n = 2431) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio: (95% CI) | p-value | |
| IMD quintile | ||||
| Least deprived | 1 | 1 | ||
| 2 | 0.80 (0.52 to 1.25) | 0.80 (0.51 to 1.24) | ||
| 3 | 0.87 (0.56 to 1.34) | 0.86 (0.56 to 1.32) | ||
| 4 | 0.64 (0.42 to 0.97) | 0.64 (0.42 to 0.96) | ||
| Most deprived | 0.60 (0.39 to 0.91) | 0.05 | 0.59 (0.38 to 0.90) | 0.05 |
| TCI (0.1 increase) | 1.07 (1.01 to 1.13) | 0.01 | 1.89 (1.12 to 3.22) | 0.02 |
| Type of disease | ||||
| Haematological cancer | 1 | 1 | ||
| Gynaecological cancer | 2.48 (1.48 to 4.15) | 2.36 (1.40 to 3.95) | ||
| Skin cancer | 0.97 (0.67 to 1.39) | 0.94 (0.65 to 1.36) | ||
| Memory clinic | 1.05 (0.76 to 1.45) | 1.07 (0.78 to 1.48) | ||
| Mental health | 0.57 (0.39 to 0.82) | 0.56 (0.39 to 0.81) | ||
| Heart failure | 0.75 (0.50 to 1.14) | < 0.001 | 0.83 (0.55 to 1.26) | < 0.001 |
| No. of professional groups | 0.75 (0.66 to 0.87) | < 0.001 | 0.76 (0.66 to 0.87) | < 0.001 |
| No. decisions in first plan | 0.87 (0.78 to 0.98) | 0.02 | ||
Sensitivity analysis 2
| Sensitivity analysis | Final model: adjusted model 3 (n = 2431) | Sensitivity analysis 2: model based on randomly chosen treatment plan for each patient (n = 2433) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| IMD quintile | ||||
| Least deprived | 1 | 1 | ||
| 2 | 0.80 (0.52 to 1.25) | 0.77 (0.50 to 1.20) | ||
| 3 | 0.87 (0.56 to 1.34) | 0.83 (0.54 to 1.27) | ||
| 4 | 0.64 (0.42 to 0.97) | 0.66 (0.44 to 0.99) | ||
| Most deprived | 0.60 (0.39 to 0.91) | 0.05 | 0.59 (0.39 to 0.91) | 0.09 |
| TCI (0.1 increase) | 1.07 (1.01 to 1.13) | 0.01 | 1.05 (1.00 to 1.11) | 0.05 |
| Type of disease | ||||
| Haematological cancer | 1 | 1 | ||
| Gynaecological cancer | 2.48 (1.48 to 4.15) | 2.39 (1.41 to 4.01) | ||
| Skin cancer | 0.97 (0.67 to 1.39) | 0.88 (0.62 to 1.26) | ||
| Memory clinic | 1.05 (0.76 to 1.45) | 1.08 (0.78 to 1.49) | ||
| Mental health | 0.57 (0.39 to 0.82) | 0.54 (0.38 to 0.78) | ||
| Heart failure | 0.75 (0.50 to 1.14) | < 0.001 | 0.81 (0.54 to 1.21) | < 0.001 |
| No. of professional groups | 0.75 (0.66 to 0.87) | < 0.001 | 0.80 (0.70 to 0.92) | < 0.001 |
Sensitivity analysis 3
| Sensitivity analysis | Final model: adjusted model 3 (n = 2431) | Sensitivity analysis 3: model with 5 IMD quintiles collapsed to 2 groups | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| IMD quintile | ||||
| Least deprived | 1 | Group 1–3: | ||
| 2 | 0.80 (0.52 to 1.25) | 1 | ||
| 3 | 0.87 (0.56 to 1.34) | |||
| 4 | 0.64 (0.42 to 0.97) | Group 4–5: | ||
| Most deprived | 0.60 (0.39 to 0.91) | 0.05 | 0.72 (0.57 to 0.90) | 0.004 |
| TCI (0.1 increase) | 1.07 (1.01 to 1.13) | 0.01 | 1.95 (0.15 to 3.31) | 0.01 |
| Type of disease | ||||
| Haematological cancer | 1 | 1 | ||
| Gynaecological cancer | 2.48 (1.48 to 4.15) | 2.50 (1.49 to 4.18) | ||
| Skin cancer | 0.97 (0.67 to 1.39) | 0.98 (0.68 to 1.41) | ||
| Memory clinic | 1.05 (0.76 to 1.45) | 1.05 (0.76 to 1.45) | ||
| Mental health | 0.57 (0.39 to 0.82) | 0.56 (0.39 to 0.81) | ||
| Heart failure | 0.75 (0.50 to 1.14) | < 0.001 | 0.76 (0.50 to 1.14) | 0.004 |
| No. of professional groups | 0.75 (0.66 to 0.87) | < 0.001 | 0.75 (0.65 to 0.86) | < 0.001 |
Sensitivity analysis 4
| Sensitivity analysis | Final model: adjusted model 3 (n = 2431) | Sensitivity analysis 4: alternative definition for implemented treatment plan: > 80% of component decisions implemented (n = 2425) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| IMD quintile | ||||
| Least deprived | 1 | 1 | ||
| 2 | 0.80 (0.52 to 1.25) | 0.99 (0.67 to 1.49) | ||
| 3 | 0.87 (0.56 to 1.34) | 1.07 (0.72 to 1.58) | ||
| 4 | 0.64 (0.42 to 0.97) | 0.74 (0.51 to 1.07) | ||
| Most deprived | 0.60 (0.39 to 0.91) | 0.05 | 0.74 (0.50 to 1.08) | 0.05 |
| TCI (0.1 increase) | 1.07 (1.01 to 1.13) | 0.01 | 1.07 (1.02 to 1.13) | 0.006 |
| Type of disease | ||||
| Haematological cancer | 1 | 1 | ||
| Gynaecological cancer | 2.48 (1.48 to 4.15) | 2.87 (1.74 to 4.72) | ||
| Skin cancer | 0.97 (0.67 to 1.39) | 1.05 (0.74 to 1.50) | ||
| Memory clinic | 1.05 (0.76 to 1.45) | 0.93 (0.69 to 1.26) | ||
| Mental health | 0.57 (0.39 to 0.82) | 0.51 (0.36 to 0.73) | ||
| Heart failure | 0.75 (0.50 to 1.14) | < 0.001 | 0.40 (0.28 to 0.58) | < 0.001 |
| No. of professional groups | 0.75 (0.66 to 0.87) | < 0.001 | 0.71 (0.63 to 0.81) | < 0.001 |
Appendix 4 Observation coding sheet
Qualitative data collection
INPUTS – Systems, organisational
-
Mention of national policy directives or guidelines:
-
Mention of local guidelines/rules/regulations:
-
Mention of resource issues (staff, time, money). How do these factors impact on decision making?
-
Mention of other individuals/services/teams within organisation that impact on options/decision made:
-
Other broader contextual factors influencing decision making:
INPUTS – Team and task
-
What information is shared in advance? How does this influence decision-making?
-
Meeting environment (size of room/seating arrangement/light/acoustics):
-
What use is made of technology? e.g. access to test results, patient notes, virtual team. How does this influence decision making?
-
How structured is the meeting process? (e.g. following agenda, protocol):
-
Who presents cases? How? Use of structured proforma? Framing of decisions to be made:
-
Patient factors: who mentions patient preferences? Who, if anyone, mentions carer or family preferences? How do attendees react? Any variation by patient characteristics:
-
Socio-demographic (age, gender)
-
Socioeconomic (education, poverty)
-
Social (marital status, employment, family)
-
Health literacy (understanding about condition and navigation of healthcare services)
-
-
Mention of missing information? (test results, attendees) Impact on decision making:
-
Other team, task and patient inputs influencing decision making:
MEDIATORS – Team processes, emergent states
-
Participation/Communication: who dominates? Who has least involvement? Is there a hierarchical pattern of participation or a relatively even distribution?
-
Any misunderstandings/lack of clarity – between whom? Who asks questions? To whom? Is there dissent or conflict? Who disagrees? How is it dealt with?
-
Leadership style: clear role or several competing leaders? Does the leader dominate discussion or decision making, or take a back seat? Do they encourage involvement or limit contributions (e.g. because of time)? Do they checking understanding or proceed at their own pace?
-
Cohesion: Any social conversation immediately pre/post meeting? Is everyone focused on the task – anyone chatting/disinterested? Mood/affect – Relaxed? Pressured? Formal? Focused on task?
-
Decision mechanisms: Is consensus sought? How? (Verbal, eye contact) Are decisions made in the absence of consensus?
-
Other mediators, processes influencing decision making:
OUTCOMES
-
OUTCOME: Clarity of recommendations; Who records the decision? Is there a verbal summary and rationale? Is responsibility for implementation discussed?
Other:
Appendix 5 Multidisciplinary team member interview topic guide

MDT STUDY: Participant interview TOPIC GUIDE
Version 1, 28.3.12
INTRODUCTION FOR PARTICIPANTS
The purpose of this interview is to explore your views on the multidisciplinary team meetings that you attend for [team name], and to discuss some of the factors influencing MDM decision-making.
The questions will be flexible and open-ended, to allow you the chance to raise the issues that you feel are important. Your responses will be confidential, and anonymised in any findings we publish. They will not be shared with your team. There are no ‘right’ or ‘wrong’ answers and I stress that this is not about individuals, but about the system. There is conflicting evidence in the literature about whether MDTs are effective or not, and this is what we would like to explore.
Interviewer will then talk through patient information sheet if needed and answer any questions
Warm Up
-
This interview is about your experience of the MDT meeting, in general, how do you find working in this way?
-
What do you think works well about your MDT meeting?
-
Is there anything you would change about your MDT meeting?
-
If there was no MDT meeting, what difference would it make?
-
Is the MDT a good use of your time? What is most/least valuable?
-
What would you describe as the primary role of the MDM? Does it have any other functions?
-
-
What factors are most important to the success of an MDT meeting?
Team Processes – I’d like to focus now on the wider team
-
What is the atmosphere like in the team meetings? What do you think creates this atmosphere?
-
What would make it better?
-
Do you think people are able to speak freely during the meetings? Why?
-
How do different professional groups interact in the team?
-
Are some professions more or less relevant to the meeting than others?
-
What do you see as your role in the meeting?
-
What kind of issues do you bring to the meeting? Do you think the MDM is the best way to do this?
-
-
What happens when people disagree?
-
Can you give example of time you disagreed?
-
Decision-making: can I get you to think about decision making in the MDM?
-
How does the team come to a decision about a case/patient?
-
What information do you need to make a decision?
-
Probe re barriers to accessing information?
-
Are there times when the team doesn’t come to a decision? Why is this?
-
-
Can you talk a bit about patient preferences in relation to decision making?
-
How much do you tend to know about patient’s preferences? To what extent do they influence decision making?
-
How could patient preferences be incorporated into decision making?
-
What about other factors, for example, other illnesses or conditions: how do these influence decision making?
-
-
Is there anything you think doesn’t get discussed enough during the meeting?
-
Is there anything you think less time should be spent on?
-
Can changes happen (realistically) to improve it? How (how can x or y happen)?
-
-
How do MDT meetings affect the quality of clinical decisions?
-
Do you think MDT meetings lead to better clinical decisions for patients?
-
Prompt for a specific example
-
Are there times when poor/sub-optimal decisions are made? Why do you think this happens?
-
Prompt for a specific example
-
-
Can you describe a time when a decision made at the meeting was changed? Why did this happen?
-
How much of decision-making happens outside the meeting? How does the quality of these decisions compare?
-
To conclude this section, could you reflect on what could be improved about the way decisions are made?
External influences
-
How is decision-making influenced by resourcing issues? (e.g. time-constraints, funding, staff shortages)
-
Thinking about the physical environment, are there any aspects of the room or layout that influence the way the team interacts?
-
At the moment, there are a lot of changes going on in the NHS . . .
-
How would you describe the impact of this on your organisation?
-
And do you think this has impacted on the team?
-
-
Finally, is there anything else you think is relevant?
Appendix 6 Patient interview topic guide

MDT STUDY: Patient interview TOPIC GUIDE
VERSION 1, 28.3.12
INTRODUCTION FOR PATIENTS
This research aims to understand clinical decision-making processes through the eyes of the patient. There will be three sections to the interview. First, we’d like to hear about your experience of your care so far. Then we’ll move on to how you and your healthcare team make decisions about your care. Finally, we’ll discuss more generally how you feel patients should be involved in these kinds of decisions.
We would like to emphasise that the study is being conducted by UCL and we are not employed by your Trust or the NHS.
We wish to reassure you that you do not have to answer any question you feel uncomfortable with and you can stop the interview at any time. This will in no way affect your care.
This is about getting your views across. Your responses will be confidential: they will not be shared with your healthcare team. Quotes from the interviews may be used in our findings, but it will not be possible to identify you or your healthcare team from these.
Interviewer will then talk through patient information sheet if patient/carer wishes
Warm up - I’d like to start by asking you about your condition and your care
-
Could you briefly tell me about your [condition] and the most important ways that it affects you?
-
Encourage focus on main condition (focus of MDM) & impact on quality of life
-
-
What types of care do you currently have for your [condition]?
-
Encourage brevity & focus on major health (+/– social care) interventions
-
-
Can you describe how decisions were/are made about your care?
-
Who are the main people involved in making decisions about your care? i.e. are respondents aware of multidisciplinary team (MDM) vs. individual consultant making decision?
-
What involvement did you have in making decisions/planning care?
-
What were your preferences/wishes regarding your care?
-
Can you describe any times when you were invited to discuss or choose any aspects of your care?
-
Did you want to make choices? Does it depend on the type of decision?
-
-
Please can we talk in more detail about a particularly important decision that was made about your care? This may include a treatment, diagnostic procedure, something about the timing, setting (inpatient/outpatient) or anything else such as a second opinion.
-
Can you describe how you made your decision to/not to receive this care? Can you tell us the factors that influenced the decision?
-
Any perceived concerns/benefits e.g. side effects; time in hospital; convalescence time; enormity of procedure; quality of life etc.
-
Where they received information from (e.g. family, friends, reading, clinical staff)
-
Which of these factors made a difference to the decision made? Why?
-
Extent to which their choice/decision was influenced by personal characteristics e.g. belief systems (fatalism, faith, ‘willing to try anything’, autonomy [want to retain personal control], delegation [want their Dr to make important decisions] etc.)
-
Extent to which personal characteristics interacted/were mediated by context e.g. their relationship with key health professionals / the importance of the decision/uncertainty surrounding management options etc.
-
-
Is there anything about your care that you know now, that you would like to have known before?
-
Side effects, time in hospital, recuperation issues, specified risks etc.
-
How might this information have changed your care preferences/decision?
-
MDT meetings - increasingly in the NHS, patient care is managed by a team of professionals with different skills, rather than one person. For example, [insert relevant mix of professionals] may meet together once a week to discuss different cases. Patient care decisions are often made in these in ‘multidisciplinary team meetings’, by the whole (multidisciplinary) team.
-
What do you know about these meetings?
-
Do you know that your care is discussed?
-
Were decisions fed back to you?
-
What do you think about this way of working?
-
How do you feel about your care being discussed in these meetings?
-
-
What were/are the important things about you that you would want the multidisciplinary team to consider when they meet to discuss your care/management?
-
What were/are the important things about your care that you would want the multidisciplinary team to consider when they meet to discuss your care/management?
-
Defined benefits and concerns e.g. side effects; time in hospital; convalescence time; enormity of procedure; quality of life etc.; importance of short versus long term effects
-
Timing; setting etc.
-
-
When is the most appropriate time to discuss this information with you?
-
How should it be done & how often? e.g. before or after the MDT
-
Who would you want to represent your views at a multidisciplinary meeting (key health professional; patient advocate; patient/carer)?
-
-
Are there issues that you would wish to remain confidential (not to be shared) when the team is discussing management options for you?
-
As appropriate, probe, with sensitivity:
-
Comorbidities; risky behaviours; personal circumstances (do NOT press to define these if the patient does not volunteer the information).
-
In this final section, I’d like to talk more generally about how you think multidisciplinary teams should work
-
Do you think it is important for multidisciplinary teams to always consider patients’ views when making a decision about care?
-
As appropriate, probe the influence of:
-
Context e.g. major decisions (to have a major intervention or not etc.) versus decisions about process (scheduling, setting etc.);
-
Intervention specific issues e.g. where there is clinical uncertainty about options; quality of life (pain, impact on mobility/independence etc.)
-
-
What information should be fed back to patients about the multidisciplinary team meeting decision-making process about their care?
-
As appropriate, probe:
-
How the MDT decided what to recommend e.g. whether it was protocol/evidence based; the specialties involved in making the decision; options discussed; factors which influenced the decision etc.
-
-
Do you have any suggestions for ways for patients’ views to be represented at the multidisciplinary team meeting?
-
As appropriate, probe:
-
Some suggestions include: staff known to patient attend; patient advocate attends; preparatory meeting with patient; patient preferences written down in advance; patient submits a written statement; patient/carer attends meeting; formalised feedback after the meeting; decision options discussed with patient after meeting etc.
-
How would this work in practice? e.g. would they find it upsetting, would they understand the discussion, would it be intimidating; is it feasible (work & family commitments)?
-
What about for patients who feel unable to express their view/ask questions?
-
Finally, is there anything else about how patients are involved in decisions about their healthcare that you would like to add?
Appendix 7 Observation code definitions
| Code | Code name | Definition |
|---|---|---|
| 1 | Administration | |
| 1a | Admin support as enabling factor in decision-making | Demonstrates where administrative support, including the MDT co-ordinator role, is instrumental in facilitating decision-making (e.g. by providing information or reminders of previous discussions) |
| 1b | Use of IT as an enabling factor | Examples of members of the team using IT to access information to help them make a decision |
| 1c | Administrative/IT problems | Illustrates how administrative (including IT/technical issues) problems can impact on decision-making |
| 2 | Role of carers | |
| 2a | Carer preferences | Mention of carer preferences in relation to a patient’s care |
| 2b | Carer well-being | Team discussing the well-being of the carer |
| 2c | Negative carer influence | Discussing concerns that carers are negatively influencing patients, for example by interfering in their treatment or colluding in their illnesses |
| 2ci | obstructive carer interference | |
| 2cii | Obstructive carer collusion | |
| 2d | Positive carer influence | Examples of carer influence being described in a positive light by the team (e.g. supportive of patient) |
| 3 | Patient experience of services | When team acknowledges the impact of services/treatment on patient(s) |
| 4 | Empathy toward patient/carer | Examples of the team showing empathy towards the patient or carer because of their symptoms or social situation |
| 5 | Positive comments about patients | Where staff make positive comments about patients, or positive judgements about patient personality |
| 6 | Casual talk | Staff expressing opinions or sharing information that does not appear to be relevant or helpful to the discussion |
| 7 | Advocacy/Patient centred care | Where staff consider the perspective of patients and advocate on their behalf |
| 8 | Labels and stigma | Discussion of stigma around illness and the effects of ‘labelling’ patients |
| 9 | Comorbidities | Discussion of comorbidities |
| 10 | Treatment decisions based on impact of symptoms on patient | When the members report patient and carer complaints about their condition to decide whether to treat or not |
| 11 | Withholding information or treatment from pt in “pt’s best interest” | When the team decide it is best for the patient not to know certain information, e.g. a diagnosis or treatment alternative |
| 12 | Attendance/punctuality | Reference to a member of the team being absent or late to the meeting |
| 13 | Awareness of researcher | Where reference is made by someone in the team to the fact that the meeting is being recorded |
| 14 | Challenging an earlier decision | Discussions where someone questions the rationale for or challenges a decision made previously by the team |
| 15 | Challenging or difficult cases | |
| 15a | Complex diagnoses | Cases where there are complexities in diagnosing a patient, also when the ‘set’ criteria do not fit but teams ‘have’ to diagnose within specific codes |
| 15b | Lack of clear options for patient | Discussions where clinicians express that they do not feel they have any more treatment options to try or are unsure of what to do with the patient |
| 15c | Challenging behaviour | Discussion of patient behaving in a way that the team finds challenging (e.g. non-compliance, violence, etc.) |
| 15d | Patient disputing illness | When the patient denies/does not agree that they are unwell |
| 15e | Questioning pt truthfulness | Discussions of patients being deliberately untruthful to staff |
| 15f | Conflict between information by patient and other sources | Discussion of patient/carer giving inaccurate information (but no suspicion of deliberate deception) |
| 15h | Opportunistic or inappropriate service use | Where clinicians question the patient’s motives for seeking care, e.g. to claim benefits or to garner mitigating circumstances for criminal behaviour |
| 16 | Cohesion: humour | Highlights examples of the group using humour |
| 17 | Cohesion: personal and social communication | Personal/social exchanges between team members |
| 18 | Consensus | Clear examples of team making a decision by explicit consensus agreement |
| 19 | Criticising other services | Negative perceptions or comments about other teams or services |
| 20 | Content of discussion | |
| 20a | Decision framing | Examples of discussions culminating in a decision |
| 20b | Information giving – presenting patient information | Presenting patient characteristics or clinical history before discussion and/or a decision is made |
| 20c | Information giving – imaging | Radiology/imaging presentation of information to shape discussion and/or decision |
| 20d | Information giving – pathology | Pathology or histology review feeding into discussion and/or decision |
| 20e | Ownership of case in MDT | Establishing responsibility for presenting the case/‘ownership’ of case in MDT |
| 20f | Implementation responsibility | Discussing who will implement the decision – or whether this is not discussed but assumed, etc. |
| 21 | Decision making outside of meeting | Explicit acknowledgement from the team that a decision has been or will be made outside the meeting, e.g. in clinic or in the operating theatre |
| 22 | Directives/Requests | One person instructing or asking another to do something |
| 23 | Discharge | Examples of team deciding to discharge a patient |
| 24 | Ethical dilemmas | Using the meeting to discuss ethical dilemmas or issues |
| 25 | Distinction between “illness” and “behaviour/personality” | Discussions where members distinguish between issues attributed to patient’s ‘illness’ and those attributed to their ‘behaviour’ or ‘personality’ |
| 26 | Divided responsibility between patient and safety of staff | Discussions highlighting conflicting responsibilities towards patient and staff |
| 27 | Divided responsibility between patient and family | Discussions highlighting conflicting responsibilities towards the patient and the patient’s family |
| 28 | Divided responsibility between patient and society | Discussions highlighting conflicting responsibilities towards patient and society at large |
| 29 | Documentation/audit function | Explicit reference to something being done in order to create an audit trail |
| 30 | Emotional expression function | Members expressing emotions during meeting (e.g. in relation to patient or occupational stressors) or acknowledgement of emotional burden related to work. This is separate from ‘Empathy towards patient’, which focuses on acknowledging how the patient feels, rather than how members of the team feel. Some quotes may be coded as both |
| 30a | Resignation/failure | Expressions of resignation regarding a failed treatment attempt |
| 30b | Positive emotion | Expressions of positive emotions relating to work, e.g. successful treatment or engagement |
| 31 | Feedback function | Making the team aware of an issue without seeking to make a decision or seeking input |
| 32 | Hierarchy | Evidence of status differentials between professionals |
| 33 | Holistic discussion of patients | Consideration of a patient’s psychosocial situation |
| 34 | Dominance of clinical information | Focus on clinical information without considering patient’s psychosocial situation or preferences |
| 35 | Multidisciplinary participation and multidisciplinary decision-making | Where people from different professions contribute to a decision or discussion |
| 36 | Legalities and professional safeguarding | Mention of legal issues or potential for legal action |
| 37 | Links with other services | Examples of interaction between team members and other services |
| 38 | Local policy/guidance | Reference to policy or guidance at a local level (i.e. not national or international; usually trust policies) |
| 39 | Meeting disruption | Examples of meeting disruption, e.g. answering mobile phones, responding to pagers |
| 40 | Meeting management | Examples illustrating how the meeting is conducted and procedural issues regarding the meeting, e.g. discussions being moved along quickly, clarifications of who has the final say in decision-making, or decisions regarding whether a patient is discussed or not |
| 40a | Selection of patients for discussion | Demonstrates how the team decides which patients are discussed during or before the meeting |
| 41 | Missing information | Relates to both missing team members and missing information, e.g. scans, pathology results |
| 42 | National policy/guidance | Any reference to external policy or guidance which influences the discussion, e.g. waiting times |
| 43 | Non-clinical disagreement | Disagreements or sharp exchanges between members of the team that are not clinical in nature |
| 44 | Pathways of care | Mention of pathways into or out of the service – demonstrates complexity and ‘bigger picture’ within which the teams are making decisions |
| 45 | Patient awareness of MDT | Reference to a patient being aware that the ‘team’ are making a decision, rather than an individual clinician |
| 47 | Patient treatment preferences | Mention of patient preferences regarding treatment |
| 48 | Patient persuasion, negotiation and management | Mention of trying to persuade or negotiate with patients |
| 49 | Questioning/clarifying | Members of the MDT asking a specific question to another member |
| 50 | Reflecting on therapeutic alliance | Acknowledgement of the quality of the patient/team member relationship |
| 51 | Research/evidence | Explicit reference to research or evidence during the meeting |
| 52 | Resources: funding | Mention of money or funding |
| 53 | Resources: system capacity | Mention of capacity within the team or system (e.g. waiting lists and theatre slots) |
| 54 | Resources: time | Comments reflecting the impact of a lack of time on team behaviour |
| 55 | Responding to errors | Team response to errors – clinical or administrative |
| 56 | Searching for information | Delays in discussion while the team look for information or wait for someone to respond (after meeting has started, so different from punctuality/attendance) |
| 57 | Seeking opinions or advice | Examples of team members explicitly asking the rest of the group for advice |
| 58 | Sense of team responsibility | Where a sense of joint responsibility for the patient is evident |
| 59 | Service improvement | Examples of the team using the meeting as a place to recommend wider improvements to the service or to raise concerns about existing processes |
| 60 | Service restructuring/higher management | Discussions of management issues at levels higher than the team manager, e.g. service-restructuring |
| 61 | Sharing local knowledge of organisational systems (function) | Sharing information regarding the organisation, e.g. policies, services, trials |
| 62 | Sharing local knowledge of patients (function) | Someone with previous knowledge of a patient sharing information relevant to that specific patient with someone who is now working with him or her |
| 63 | Support or guidance (function) amongst team members | Expressions of support and/or encouragement between team members, including continuing professional development or reflective practice |
| 63a | Teaching function | Examples of the meeting being used to educate or teach other team members |
| 64 | Team management (function) | Using the meeting for management duties or management queries, e.g. manager instructing team to update their paperwork, or doctor asking manager for details of the policy on information-sharing |
| 65 | Validation | Relates to the team acting as a ‘check’ on individual members, e.g. correcting errors or misconceptions, or attempting to rectify a mistake made by a member of the team |
| 66 | Meeting environment | Describes the physical space where the meeting takes place |
| 67 | Team Characteristics | Descriptions of features of the team, i.e. what professions attend usually, etc. |
| 68 | Clinical conflict | Exchanges between members of the team that demonstrate a clinical or professional disagreement or challenge |
| 69 | Collateral | Verifying patient’s account with third party |
| x | Recommendations | Potential recommendations noted by the observing researchers |
Appendix 8 Observation codes clustered for qualitative exploration of quantitative results
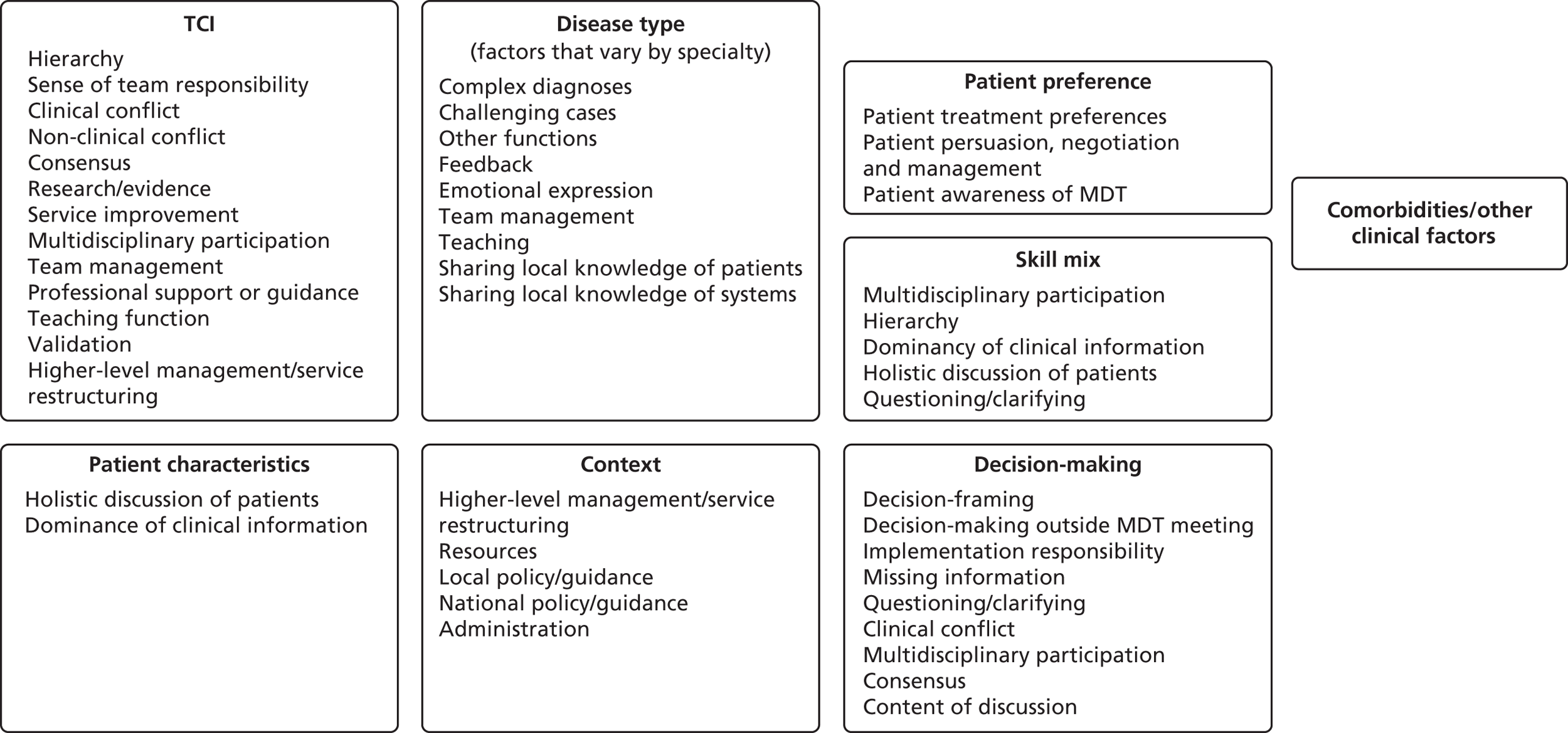
Appendix 9 Collaborators and steering group members
| Name | Profession |
|---|---|
| Coapplicants | |
| Professor Gill Livingston | Professor of Psychiatry of Older People |
| Professor Susan Michie | Professor of Health Psychology |
| Dr Julie Barber | Lecturer in Statistical Science |
| Dr Archie Prentice | President of the Royal College of Pathologists |
| Dr Alex Clarke | Consultant Clinical Psychologist and Nurse |
| Dr Anne Lanceley | Senior Lecturer, Women’s Cancer Research |
| Professor Jane Blazeby | Professor of Surgery |
| Dr Simon Gibbs | Reader in Pulmonary Hypertension |
| Professor Ewan Ferlie | Professor of Public Services Management |
| Professor Michael King | Professor of Primary Care Psychiatry |
| Expert advisory panel | |
| Dr Simon Woldman | Consultant Cardiologist |
| Dr Mark Exworthy | Reader in Public Management and Policy |
| Professor Michael West | Professor of Organizational Psychology |
| Miriam Harris | PPI representative |
| Professor Adrian Newland | Professor of Haematology |
| David Ardron | PPI representative and carer representative |
| James Green | Consultant Urological Surgeon |
| Charles Vincent | Professor of Clinical Safety Research |
| Nick Sevdalis | Senior Lecturer, Faculty of Medicine, Department of Surgery and Cancer |
| Sarah Harvey | Assistant Professor, Department of Management Science and Innovation |
Appendix 10 Summary of the 12 multidisciplinary teams
We aimed to include a diverse range of teams in this study to highlight important issues about MDT decision-making and implementation. The 12 MDTs we observed included four cancer teams, four mental health teams, two heart failure teams and two memory clinic teams. A brief description of each of the teams is provided below.
Cancer team 1
This team meeting was one of the longest MDT meetings we observed, often running for almost 3 hours. Between 35 and 50 patients were discussed each week. It was a large team, with over 30 core members, including surgeons, oncologists, psychologists, palliative care specialists, pathologists, radiologists, research nurses, a clinical trials manager, a physiotherapist and an MDT co-ordinator. There were also often a number of observers present each week who did not participate in discussion, for example medical students or visiting academics.
The meeting was chaired by the MDT lead, who was a surgeon. On occasion, other surgeons would be asked to chair the meeting on their behalf.
A full list of patients to be discussed was provided at the start of the meeting by the MDT co-ordinator. The list was divided into different groups for discussion:
-
radiology and integrated pathology patients (these tended to be lengthy discussions)
-
complex cases (these tended to be a small number of lengthy discussions)
-
new patients referred to the centre (these were brief updates, often feedback only)
-
pathology cases (this tended to be a long list but covered quickly).
During the meeting, the chairperson updated an ‘MDT sheet’ for each patient discussed. This recorded the findings presented by the radiologist and/or pathologist, and captured the plan agreed for each patient. These records were stored on a team shared drive, and were also subsequently uploaded to individual electronic patient files.
Cancer team 2
There were 17 members in this team, including haematologists, an oncologist, a pathologist, a radiologist, clinical nurse specialists, chemotherapy nurses, specialist registrars, clinical trials practitioners and an MDT co-ordinator. Most team members attended the weekly MDT meeting regularly. Three or four members of the team were connected via video link from a second hospital site. The meeting lasted for 1 hour, with between 10 and 20 patients discussed each week. A consultant haematologist chaired the meeting.
A patient list was circulated to all team members a day or two in advance of the meeting. This was used as an agenda to structure the meeting.
Decisions were clearly summarised for the MDT co-ordinator, who made notes of the treatment plan agreed for each patient. These notes were subsequently checked by the MDT lead before being uploaded onto the electronic records system.
At the start of each meeting an IT representative set up the equipment for the video link. However, there were frequent problems with IT, which disrupted the meeting or delayed the start time.
Cancer team 3
This was the largest team we observed, with as many as 35 to 40 people attending some meetings. There was wide input from a number of those present, although many people attending did not contribute at all. Attendees included haematologists, clinical oncologists, pathologists, radiologists, specialists in nuclear medicine, cytogenetics and molecular biology, research nurses, trials co-ordinators, pharmacists, specialists registrars and an MDT co-ordinator. A consultant haematologist chaired the meeting.
The meeting was clearly structured and a patient list was circulated in advance which grouped patients by disease subtype (e.g. lymphoma, myeloma). This was used as an agenda to guide the meeting.
Decisions were recorded for each patient directly into an electronic patient record, which was projected onto a screen for other team members to see. The decision was usually recorded by a specialist registrar or a junior doctor and checked by the chairperson.
Cancer team 4
This team included both medical and clinical oncologists, dermatologists, plastic surgeons, a clinical nurse specialist, pathologists, a radiologist, a nuclear medicine specialist and a research nurse. The pathologists and the radiologist were more actively involved in discussion and decision-making than in the other cancer teams.
A patient list was circulated by e-mail to the team in advance of each meeting. This included information on patient’s sociodemographic characteristics; the consultant responsible for their care; imaging or histology to be reviewed; and a note of the issue for discussion.
There was a clear structure to the meeting, during which between 35 and 50 patients were discussed, in the following order:
-
melanoma cases (radiology done first, allowing the radiologist to leave afterwards)
-
patients from another hospital (done via video link)
-
squamous cell carcinoma cases
-
basal cell carcinoma cases.
The meeting was chaired by a consultant oncologist, with a surgeon taking the lead in their absence.
There was a wide pattern of participation during this meeting, as the focus shifted between different specialties depending on the cases being discussed. Most people attending presented at least one case (with the exception of the clinical nurse specialist, who rarely presented cases), although the consultants usually made the decisions.
Clinical decisions were recorded on a paper proforma by the presenting clinician. They were subsequently photocopied by the MDT co-ordinator at the end of the meeting and filed in both the paper and electronic notes. During the observation period the team began to complete this information electronically.
Heart failure team 1
This was a large team which included cardiologists, surgeons, palliative care specialists, specialist registrars and junior doctors, heart failure specialist nurses, research nurses and imaging consultants. In addition, a small number of observers often attended, but they did not participate in discussion.
There was a patient list, distributed before the meeting, which consisted of inpatients and any other patients flagged by a member of the team as needing discussion. This list was collated by a Senior House Officer (SHO) and included the patient’s name and hospital number, and sometimes included the reason for discussion, for example ‘review of echo’. Each patient was presented to the meeting by the SHO using a PowerPoint presentation (Microsoft Corporation, Redmond, WA, USA). The electrocardiogram, echo and other test results were also projected on a large screen.
These presentations provided the structure for the meeting. While the matter of chairing was not explicitly discussed, discussions tended to be moved along by one of the consultant cardiologists or a specialist registrar.
A specialist registrar made hand-written notes on each case and wrote up a summary of each patient discussion, including a list of named members who had contributed to the discussion. After the meeting this was given to an administrator, who added it to the patient’s electronic record.
Heart failure team 2
This was a new team and attendance changed over the period of observation when additional professionals were asked to join the team. Initially the meeting was attended by six to eight members; however, after the fourth week of observation the team was regularly attended by just three to five members. This coincided with the MDT meeting moving from its initial location to a different building. The meeting was attended by cardiology consultants, specialist registrars and heart failure nurses regardless of the number of attendees. On some occasions meetings were cancelled because the consultant was unable to attend. In response to this the lead consultant suggested that other consultants should attend the meetings.
The meeting ran for approximately 1 hour with an average of six patients discussed each week. The heart failure nurses decided which patients to bring to the meeting, and presented these, sometimes in conjunction with a specialist registrar. Patients’ cases were presented on a computer screen and the discussion focused mainly on the echo findings.
Decisions were not always clear, but when they were made they tended to be very specific, for example medication or surgery. Usually the nurses presented their suggestions and then confirmed with the doctor that the treatment they had chosen was correct.
Memory clinic team 1
Between 8 and 12 staff attended every meeting. Attendance was regarded as compulsory for all members unless there was a good reason for not attending. Team members included nurses, psychiatrists, psychologists, a specialist registrar, a team manager, social worker, an occupational therapist and a dementia support worker. The meeting was chaired by the team manager, who was a senior nurse.
At the beginning of the meeting all new referrals received that week were allocated to team members. Following this, between five and eight existing patients were discussed in the hour-long meeting, during which the results of an assessment were fed back to the team, specific queries were raised or patient updates were provided.
There was no predetermined patient list for this meeting. Instead, all team members were given the opportunity to raise cases on an ad hoc basis. Decisions made by the team were typed directly into electronic patient records during the meeting by the chairperson.
Memory clinic team 2
This team consisted of psychiatrists, psychologists, specialist registrars and a memory specialist nurse. It was a relatively small team with three to eight people attending each week to discuss three or four patients. Specialist registrars would usually attend only if they had a case to present. Not all cases were discussed during the meeting, in which case they were either brought back the following week or discussed outside the meeting by one of the consultants and another member of the team. The meeting was chaired by a consultant psychiatrist.
As with the other memory team, there was no predetermined patient list for the meeting, and team members were given the opportunity to raise patients they had seen most recently. The structure of discussion was uniform, with full presentation of test results, including cognitive assessments and a medical assessment made by a specialist registrar. Discussions would then focus on making a diagnosis based on these assessments. Decisions were recorded informally by the team member who raised the case.
Community mental health team 1
This team was observed for 14 weeks, before it disbanded. It included a consultant psychiatrist, specialist registrar, nurses and social workers, and a team manager. A locum psychologist joined the team for the last few weeks before the team disbanded. The meeting lasted for between 2.5 and 3 hours, during which approximately 30 patients were discussed. The meeting was chaired by the team manager (a social worker).
The structure of the meeting was usually not made explicit, but tended to cover:
-
discussion and allocation of new referrals
-
feedback from assessments (outpatient or community assessments)
-
discussion of patients shared with other teams, if the inpatient/home treatment consultant was in attendance
-
ongoing cases, where the team was given the opportunity to discuss any issues they wanted to raise.
A patient list was not distributed in advance of the meetings. Instead, team members raised patients as they remembered them. They fed back assessments from memory, and did not use IT or patient notes during the meeting.
At the start of the observation period, the team began recording handwritten notes into a hard-backed A4 copybook to capture team discussions and decisions made during the meeting.
Community mental health team 2
The team included nurses, social workers (one of whom was the team manager), a psychologist, a psychiatrist (who did not always attend), a specialist registrar and a junior doctor. Other occasional attendees included a rotating specialist registrar, an SHO, an administrator, who took minutes but did not contribute to discussions, and a representative from the crisis team. The nurses, social workers and psychologists took turns to chair the meeting. The meeting lasted 1 to 1.5 hours, with the team discussing around 25 patients.
The rotating chairperson tended to go through a format of:
-
matters arising from the minutes of the last meeting
-
crisis team liaison
-
feedback from assessments
-
clinical liaison (taking turns to raise issues of concern)
-
any other business (e.g. new staff arriving or updates on the service-restructuring plan).
In common with CMHT 1, a patient list was not distributed in advance of the meetings; instead, team members raised patients as they remembered them. In addition, they did not use IT or patient records during the meeting and fed back assessments from memory.
During the observation period there was considerable anxiety within the team due to service line-restructuring, and many of the team were being re-interviewed for their jobs. There were sometimes extended discussions about this restructuring during MDT meetings, when people spoke about their anxieties. These discussions often ended with a reminder that restructuring issues should be discussed at the weekly ‘business meeting’ held on a different day of the week.
Minutes of the meeting were hand-written by the team administrator (or another team member, if the administrator was absent), who later typed them up. Details of discussions were not systematically entered into patients’ electronic medical notes, although some team members occasionally mentioned the MDT discussion in the notes (e.g. ‘discussed with the team and decided to discharge’). Members did not usually bring paper with them or write their own action points down during the meeting.
Community mental health team 3
This team included a consultant psychiatrist, community psychiatric nurses, social workers (including the team manager) and a clinical psychologist. Other attendees at the meeting included a rotating specialist registrar and/or SHO, an administrator, who took minutes but did not contribute to discussions, a representative from the crisis team and occasionally trainee psychologists or student nurses. The nurses, social workers and psychologists took turns to chair the meeting.
The weekly meeting lasted approximately 1 hour, though this ranged from 30–90 minutes. The team discussed around 25 clients per week. Because the job of chairperson rotated, the style of chairing varied from week to week, though it loosely followed the structure:
-
apologies
-
minutes of last meeting
-
Mental Health Act assessments (mention of any patients sectioned or plans to do so this week)
-
feedback from new assessments
-
crisis team liaison
-
clinical liaison (taking turns to raise any issues of concern).
Like the other CMHTs, there was no patient list made in advance of the meeting. Instead, team members raised patients about whom they had concerns (due, for example, to relapse, crisis or risky behaviour). They also brought up specific medical queries (e.g. asking the psychiatrist about medication dosages). In addition, when the chairperson went through the previous week’s minutes, senior members occasionally asked for feedback (e.g. ‘how did that appointment go?’, team manager, observation). Mental Health Act assessments planned for the following week were mentioned.
Minutes of the meeting were hand-written by the team administrator, who later typed them up. Details of the discussions were not systematically entered into the patient’s electronic medical notes, although some team members occasionally mentioned the MDT discussion in the notes (e.g. ‘discussed with the team and decided to discharge’). Members did not usually bring paper with them or write their own action points down during the meeting.
Community mental health team 4
The team consisted of a consultant psychiatrist and specialist registrar, social workers (including the team manager), community psychiatric nurses and occupational therapists. In addition, a forensic psychiatrist and a forensic nurse attended on a monthly basis and there were other occasional attendees including student nurses and student psychologists. Approximately 16 team members met each week for between 2 and 3 hours, during which approximately 49 patients were discussed. Discussions were guided by a list distributed at the start of meetings by the manager, which listed all patients currently on the team’s caseload. Most team members attended every week, although a small number did not attend for the full duration of the meeting.
The meeting was usually chaired by the team manager (a social worker with no caseload) or the deputy manager (a social worker with a reduced caseload), and was structured around the following categories:
-
inpatients
-
new assessments (discussion of patients in their initial 6-week assessment period while the team decided whether or not to ‘take them on’)
-
clients of concern (each team member read through their list of patients and commented on anyone they were concerned about).
On one occasion the team went through a team member’s whole caseload. They made reference to the fact that they had intended to go through a team member’s whole caseload each week but there was rarely time for this.
During the ‘clients of concern’ session, the team members used their discretion to decide whom to mention, although sometimes the manager asked them about particular clients whom they had not mentioned of their own accord.
There was a computer in one corner, where one member of the team typed notes directly into the patient electronic records system or into a Word (Microsoft Corporation, Redmond, WA, USA) document to be uploaded later. This was usually done by the deputy manager or a nurse.
Appendix 11 Example of a theme from the consensus development group questionnaire pack
Theme: Discussing comorbidities at MDT meetings
Key points from policy and guidance
-
Cancer policy states that patients’ demographic profiles and comorbidities should always be considered at MDT meetings. 113
-
Guidelines for Mental Health, Memory Clinic and Heart Failure MDTs all emphasise the importance of identifying and managing comorbidities but there are no specific references to whether or how this information should be incorporated into MDT discussions. 77,164–166
Key points from the research literature
-
99% of MDT members responding to an online survey agreed that patient comorbidities should always be considered when making a decision in the MDT meeting. 25
-
Inadequate information on comorbidities has been associated with failure to make decisions in MDT meetings. 129,130
-
Having insufficient information on comorbidities has been reported to be an important cause of MDT decision non-implementation. 127
Our research findings
Quantitative findings
-
Our study found no association between discussion of comorbidities and decision implementation.
Qualitative findings
-
There was diversity within and between specialties with respect to the extent to which comorbidities were discussed. No team discussed this information routinely.
-
Team members considered discussion of comorbidities to be valuable.
Arguments in favour of including comorbidities in multidisciplinary team discussions
-
Discussion of comorbidities helps to determine suitable treatment options and informs differential diagnosis (e.g. cognitive impairment and depression).
-
Presentation of comorbidities encourages a holistic approach to treatment, for example, by highlighting the need to consider both physical and mental health issues, and issues relevant to patient engagement and adherence.
-
Patients wanted their comorbidities to be considered and included in treatment plans.
Arguments against including comorbidities in multidisciplinary team discussions
-
Information on comorbidities is not always available (e.g. when discussing patients for the first time), and not always accurate (e.g. as a result of incomplete assessment).
-
When comorbidities are discussed routinely this can add to the volume of potentially irrelevant information to be considered by the team, which can hamper decision-making.
-
Patients may be stigmatised or stereotyped because of their comorbidity (e.g. obesity).
-
It is not always possible to identify in advance of an MDT meeting, and therefore to have the information available on, comorbidities which might be relevant to decision-making.
Questions
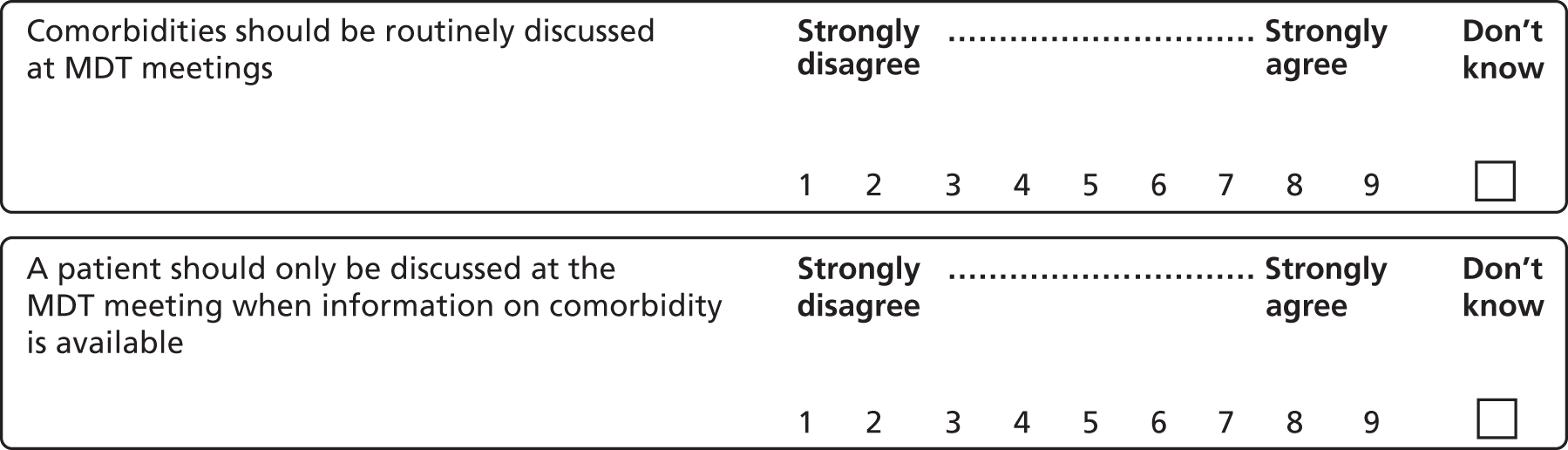
Approaches to incorporate information on comorbidities in the MDT meeting:
Do you have any other suggestions about how to incorporate comorbidities into MDT meeting discussions?
Appendix 12 Consensus development results from round 2: recommendations for which strength of agreement was strongly agree (median ≥ 7) and variation in extent of agreement was low
The 68 recommendations are grouped into four tables by strength of agreement. Appendix 12 shows those recommendations where there was strong agreement (median ≥ 7) and low variation in the extent of agreement (the 21 final recommendations). Appendix 13 shows recommendations where there was strong agreement (median ≥ 7) and moderate or high variation in extent of agreement. Appendix 14 shows those where there was uncertainty (median ≥ 4 and ≤ 6.5) and Appendix 15 shows those recommendations where there was disagreement (median < 4). The quotes in the final column of the tables reflect each of the distinct points made by panellists in order to explain the causes of variation in extent of agreement.
No participant used the ‘don’t know’ option in the second round of ratings.
| Item | Recommendation | Mediana | Mean absolute deviation from the median | Variation in extent of agreementb | Quotes illustrating variability in agreement (when discussed)c |
|---|---|---|---|---|---|
| Recommendations relating to the purpose and functions of MDT meetings | |||||
| 1 | MDT meeting objectives should include locally (as well as nationally) determined goals | 8 | 0.63 | Low | Not discussed |
| 2 | The primary objective of MDT meetings should be to agree treatment plans for patients. Other functions are important but they should not take precedence | 8 | 0.88 | Low | |
| 3 | The objectives of MDT meetings should be explicitly agreed, reviewed and documented by each team | 8 | 0.94 | Low | |
| 4 | Explaining the function of the MDT meeting should be a formal part of induction for new staff | 9 | 0.44 | Low | |
| 5 | MDT discussions should result in a documented treatment plan for each patient discussed | 9 | 0.56 | Low | |
| 6 | There should be a formal mechanism for discussing recruitment to trials in MDT meetings (e.g. having clinical trials as an agenda item) | 8 | 0.81 | Low | |
| Recommendations relating to MDT meeting processes | |||||
| 7 | All chairpersons should be trained in chairing skills | 7 | 0.81 | Low | Not discussed |
| 8 | All new patients should be discussed in an MDT meeting even if a clear protocol exists | 8.5 | 0.94 | Low | |
| 9 | Teams should agree what information should be presented for patients brought for discussion in an MDT meeting | 9 | 0.56 | Low | |
| 10 | All new team members should be told what information they are expected to present on patients they bring for discussion in an MDT meeting | 9 | 0.38 | Low | |
| 11 | The objectives of the MDT meeting should be reviewed yearly | 9 | 1 | Low | |
| 12 | Once a team has established a set of objectives for the meeting, the MDT should be audited against these goals (e.g. every 2 years) | 7.5 | 0.94 | Low | Probably in an ideal world it probably should beNurse (heart failure) |
| I’m not sure what we would auditDoctor (heart failure) | |||||
| 13 | All action points should be recorded electronically | 9 | 0.81 | Low | Not discussed |
| 14 | Implementation of MDT decisions should be audited annually | 8 | 1 | Low | Discussed and rated together with the recommendation: ‘Once a team has established a set of objectives for the meeting, the MDT should be audited against these goals (e.g. every 2 years)’ |
| 15 | Where an MDT meeting decision is changed, the reason for changing this should always be documented | 9 | 0.19 | Low | Not discussed |
| 16 | There should be a named implementer documented with each decision | 9 | 0.38 | Low | |
| Recommendations relating to the content of discussion in MDT meetings | |||||
| 17 | Comorbidities should be routinely discussed at MDT meetings | 8 | 0.94 | Low | Not discussed |
| 18 | Patients’ past medical history should routinely be available at the MDT meeting | 8.5 | 0.56 | Low | |
| Recommendations relating to the role of the patient in MDT meetings | |||||
| 19 | The MDT should actively seek all possible treatment options, and discuss these with the patient after the meeting | 9 | 0.44 | Low | This question was reworded. It initially read as: ‘Patient preferences should not be routinely discussed, but when making a decision, the MDT should actively seek all possible treatment options, and discuss these with the patient after the meeting.’ The panel agreed to reword so it was a single-issue recommendation |
| 20 | Patients should be given verbal feedback about the outcome of the MDT meeting | 8.5 | 0.94 | Low | Not discussed |
| 21 | Where it would be potentially inappropriate to share the content of an MDT discussion with the patient (e.g. where it may lead to unnecessary anxiety or disengagement from services), the decision not to feedback should be formally agreed and noted at the meeting by the team | 9 | 0.63 | Low | |
Appendix 13 Consensus development results from round 2: recommendations for which strength of agreement was agree (median ≥ 7) and variation in extent of agreement was moderate or high
| Recommendation | Mediana | Mean absolute deviation from the median | Variation in extent of agreementb | Quotes illustrating variability in agreement (when discussed)c | |
|---|---|---|---|---|---|
| Recommendations relating to the purpose and functions of MDT meetings | |||||
| 22 | MDT meetings should be a forum for recruiting patients to clinical trials | 8 | 1.19 | Moderate | Not discussed |
| Recommendations relating to the structure of MDT meetings | |||||
| 23 | All MDTs should have a designated (rather than a rotating) chairperson for MDT meetings | 7 | 1.75 | Moderate | It’s quite a difficult skill to necessarily chair an MDT well . . . the MDT becomes temporarily dysfunctional for three months whilst someone is thrown into the position of chair who doesn’t really want to be doing it, and doesn’t necessarily have the skills to do it. So I think it’s quite liberating actually for there to be an appointed positionDoctor (cancer) |
| Having a designated chair and some sort of succession planPatient representative (heart failure and cancer) | |||||
| Perhaps you don’t want to be considered heavily reliant on one . . . so it would be nice to have two or three people that are taken throughDoctor (heart failure) | |||||
| I don’t think it has to be designated, ours rotates and it works fine. I think we should learn and have those skillsTeam manager (mental health) | |||||
| 24 | All teams should have a designated person at each MDT meeting to help identify suitable patients for clinical trials | 7 | 1.88 | High | I would believe that the entire MDT should be doing that. But we would always have . . . trials nurses are always seen in the MDT, and you know, so every patient who was a potential candidate on the basis of their pathology for a clinical trial should be identified as a candidateDoctor (cancer) 1 |
| It’s not necessary to have it a designated person but it would be nice in an ideal worldDoctor (cancer) 2 | |||||
| It’s impractical, the number of people that would potentially be identified for trials across the whole of the mental health serviceDoctor (mental health) | |||||
| 25 | All MDTs should have a dedicated MDT co-ordinator/administrator | 9 | 1.31 | Moderate | I don’t think there are many oncology MDTs that don’t have itDoctor (cancer) |
| I think it would be great to have one actually, because certainly we struggle sometimes getting people to the MDT, and struggle to get things moving when everybody’s quite busy. So actually having a dedicated person who co-ordinates and makes sure everything is in the right place would actually be really beneficial. But certainly in my experience that role doesn’t exist in cardiac careNurse (heart failure) | |||||
| I suppose I think clinicians end up doing a lot of work which could easily be done by someone else, and free up their time to do [other things]Nurse (mental health) | |||||
| If a service has a manager then you’d expect the manager to be co-ordinating and gathering the team and addressing the members of their team . . . I see that as my role in my serviceTeam manager (mental health) | |||||
| Well I don’t know, I’m not sure that we need them . . . because we’re talking about people who we know . . . the person who’s seen them needs to be able to pull all that together succinctly and report back to the team, why do you need someone to co-ordinate that?Doctor (mental health) | |||||
| Recommendations relating to MDT meeting processes | |||||
| 26 | MDT chairpersons should attend at least one other MDT meeting to identify approaches to improve their chairing skills | 8 | 1.56 | Moderate | |
| 27 | A patient list should be available for all team members to view in advance of an MDT meeting | 8.5 | 1.31 | Moderate | |
| 28 | Presentations should be explicitly framed in the light of a specific query or issue to be discussed | 8 | 1.13 | Moderate | |
| 29 | All MDTs should be audited through external peer-review | 8.5 | 1.13 | Moderate | |
| Recommendations relating to the content of discussion in MDT meetings | |||||
| 30 | There should be time within MDT meetings to discuss current and emerging research and evidence only in relation to the case discussed | 7.5 | 1.25 | Moderate | Discussed and rated together with recommendation: ‘There should be time within MDT meetings to discuss current and emerging research and evidence which is not specifically related to an individual case’ |
| 31 | Relevant psychosocial issues for patients presented to each type of MDT should be identified and agreed by the MDT | 7.5 | 1.44 | Moderate | |
| 32 | The MDT member who presents the case should routinely consider psychosocial factors and ensure that relevant information is available at the meeting | 8 | 1.19 | Moderate | |
| 33 | Teams should be explicit about the research evidence that they are drawing on when making a decision in the MDT meeting | 7 | 1.25 | Moderate | |
| Recommendations relating to the role of the patient in MDT meetings | |||||
| 34 | Patients should be given feedback on which professional groups were present when they were discussed at the MDT meeting | 7.5 | 1.69 | Moderate | It’s good practice to let people know, and again it’s an opportunity to explain that care is multidisciplinary and what that might meanDoctor (mental health) |
| We’re able to tell them if they wish to know, but we wouldn’t routinely necessarily tell themDoctor (cancer) 1 | |||||
| On the hand-out it will say you were discussed in MDT which will involve pathologist, gynaecologist, clinical oncologist, so there’s that, but each individual, that’s going to be trickyDoctor (cancer) 2 | |||||
| They should be aware that the radiotherapist wasn’t there on that day, is that what it means, that’s the problem for meDoctor (cancer) 3 | |||||
| 35 | Patients should be given feedback every time they are discussed at an MDT meeting | 8 | 1.25 | Moderate | Not discussed |
| 36 | Patients should be given feedback on all treatment options, even those rejected by the MDT | 7 | 2.25 | High | I think in cancer . . . where there’s sort of three or four options; surgery, radiotherapy, chemotherapy, endocrine therapy . . . there’s such a high profile that the patients are all very aware of the options, they very much want to know, you know, why in my case am I not being recommended chemotherapy. But that was almost as important as the why you are being recommended radiotherapy etcDoctor (cancer) |
| I just thought it was impractical. There may be a number of treatment options for, you know, schizophrenia that are impractical because of the person’s engagement with treatment or ability to use some of those interventions . . . if you were to have to write that down and explain that to your patient, I think you would be entering into very very very long conversation creating a lot of conflict, instead of it being the treatment you think is practical and will hopefully workDoctor (mental health) | |||||
| 37 | Patients should be given written feedback about the outcome of the MDT meeting | 7 | 1.63 | Moderate | If it’s practical to do so for every single person then you can do . . . if you’ve got a co-ordinator and it’s an extra job . . . verbal is the more effective way of doing itNurse (heart failure) |
| Written feedback, that’s pretty much our modelDoctor (cancer) 1 | |||||
| It’s impractical, and I think it’s different in different trusts. But we will see the patient, tell them of the MDT, do a letter at the outpatientDoctor (cancer) 2 | |||||
| 38 | Patients should be able to choose the mode of MDT meeting feedback (e.g. written, phone call, in clinic) | 7.5 | 2.19 | High | In our situation this is not an issue at all, and yet all the other issues would have been pressed with time constraints. That’s not a challenge at all for usDoctor (cancer) |
| It’s just whether this is aspirational or pragmatic isn’t it, so of course in an ideal world of course, but there’s 10,000 heart failure patients . . . this is pragmatic real world MDTs rather than aspirationalDoctor (heart failure) | |||||
| People always have an identified care co-ordinator meeting with them regularly, if there is a decision or not out of an MDT meeting that feedback should be done in the usual way at their meetingDoctor (mental health) | |||||
| I disagree because I thought we can’t actually commit to sending everyone written feedback about what’s happened in the meeting, because of time constraints. So as long as we give feedback that they understand, I thought that was okayTeam manager (mental health) | |||||
Appendix 14 Consensus development results from round 2: recommendations for which strength of agreement was uncertain (median ≥ 4 and ≤ 6.5)
| Recommendation | Mediana | Mean absolute deviation from the median | Variation in extent of agreementb | Quotes illustrating variability in agreement (when discussed)c | |
|---|---|---|---|---|---|
| Recommendations relating to the purpose and functions of MDT meetings | |||||
| 39 | The main objectives of MDT meetings should be the same across all chronic diseases | 6.5 | 1.88 | High | In a mental health MDT, there might well be more discussion about the complexity of a case and the fact that er, people need some emotional support in managing a patient who’s quite risky. That might be very different to er, a more clinically orientated team who are really checking that an algorithm has been followedDoctor (mental health) |
| There are different forms of MDTs, which are very, very different animals . . . and for that reason I don’t think all those different animals can have the same objectivesPolicy (cancer) | |||||
| Generically they should all have the same purpose, which is better care for patientsDoctor (heart failure) | |||||
| 40 | Teaching should be a function of MDT meetings provided it does not add to the length of meetings | 6.5 | 2.31 | High | Discussed and rated together with question: ‘Teaching should be a function of MDT meetings even if it means meetings will be longer’ |
| 41 | Teaching should be a function of MDT meetings even if it means meetings will be longer | 5 | 1.94 | High | I think teaching should happen somewhere else where people can attend to being taught, with the exception of . . . you know, there is something very pertinent to an individual case that’s being discussed, you might well mention that in your team meeting because it relates directly to their treatment. I don’t think the MDT meeting is a teaching forumDoctor (mental health) |
| I felt very strongly, because it’s probably more to do with the fact that for our trainees it’s inherent within the MDT. These are going to be the . . . future consultants, and there is a teaching/learning process in there, irrespective. And if it means you have to extend your MDT slightly, that’s fineDoctor (cancer) | |||||
| 42 | All treatment plans for existing patients should be agreed in an MDT meeting even if a clear protocol exists | 5 | 2.06 | High | All patients should be discussed. Some of our patients can be discussed quite quickly once we talk about the diagnostic process; it’s a way of ensuring that absolutely every patient has that safety net of quality assuranceDoctor (cancer) 1 |
| I think that if you’ve got a clear protocol I’m not quite sure of the benefit of going through the protocol again for the patientDoctor (cancer) 2 | |||||
| There’s no need to bring every patient to an MDT meeting, we would only bring patients we’re struggling to make progress withDoctor (mental health) | |||||
| It would be impossible for us to discuss every recurrenceDoctor (cancer) | |||||
| Recommendations relating to the structure of MDT meetings | |||||
| 43 | Members should be allowed to not attend as long as someone from their discipline is attending and the member does not have a case to present | 5 | 1.75 | Moderate | Not discussed |
| 44 | A list of people who are required to attend the MDT meeting should be decided locally by the team | 5 | 2.44 | High | I agree strongly with this because I think every department, every trust, different parts of the country, they have different make-ups and that needs to be reflected in the teamsNurse (heart failure) |
| I strongly disagreed with this. I think that to have a consensus, and again, coming from the cancer world, we need certain people there as at least a skeleton, and if you leave it locally, what’ll happen then is the Chief Executive will have an MDT with one person in it if they can get their way to save moneyDoctor (cancer) | |||||
| Recommendations relating to the content of discussion in MDT meetings | |||||
| 45 | A patient should only be discussed at the MDT meeting when information on comorbidity is available | 4.5 | 2.19 | High | |
| 46 | A designated MDT member should speak to the patient about comorbidities before the patient is discussed at an MDT meeting | 4 | 2.38 | High | If it had said information about comorbidities should be available for the MDT then that would be a definite yes, you have to incorporate processes into our systems so that we have that information when the patients are discussed. But having a designated member of the team discussing it with the patient is a different questionDoctor (cancer) |
| I want that, you know, my, a discussion about my comorbidities to be in there and brought to the MDTPatient representative (mental health) | |||||
| Somebody speaks to the patient before the patient is discussed at the MDT, again, practically very difficult to doDoctor (heart failure) | |||||
| 47 | Each MDT should identify the most appropriate methods for presenting complete information on comorbidities | 5 | 1.13 | Moderate | We have usually a PowerPoint presentation of the history of the patient, and their other medical conditions listed fairly standardlyDoctor (heart failure) |
| It’s an aspirational counsel of perfection, which is difficult to define, and I don’t think has very much value. Sounds fantastic but I don’t think . . . it’s just not something you can put into a protocol or a methodology I don’t thinkPolicy (cancer) | |||||
| Assessment and measurement of comorbidities, it remains a massive challenge, and there are various ways of trying to get proxy measures . . . but it’s incredibly difficult to get rightDoctor (cancer) | |||||
| 48 | Case presentation should routinely include a brief introduction of the patient and relevant psychosocial characteristics, otherwise the case should not be discussed | 6 | 2.38 | High | You can’t discount a case just because you’re not looking at every single characteristic and every single issue for that patientNurse (heart failure) |
| Only a relatively small proportion of our cases would it be relevant and appropriate . . . to have a multidisciplinary discussion on the psychosocial aspectsDoctor (cancer) | |||||
| Patients are people, and I think it’s relevant as to whether their treatment is likely to impact on their social life erm, their quality of life and in the other direction whether or not their context is having an impact on their treatmentDoctor (mental health) | |||||
| Recommendations relating to the role of the patient in MDT meetings | |||||
| 49 | Any MDT member who presents a case should discuss treatment preferences with the patient before the MDT meeting | 5.5 | 2.00 | High | Not discussed |
| 50 | Patient preferences regarding available treatment options should be discussed with the patient after (rather than before) the MDT meeting | 5.5 | 1.63 | Moderate | Not discussed |
| 51 | Patients should not be presented at the MDT meeting unless there is someone present who has met with them at least once before the meeting, even if this postpones discussion of that patient | 5 | 2.63 | High | The MDT should function automatically anyway, irrespective of whether the person is there or notDoctor (cancer) |
| It’s different [in mental health] You can’t say anything before you’ve met the patientDoctor (mental health) | |||||
| 52 | Patients should be given the opportunity to provide information in advance of the MDT meeting to ensure the information presented is accurate and 7comprehensive | 5 | 2.13 | High | Not discussed |
| 53 | Patients should be able to provide information by having direct access and the ability to modify their medical records | 5 | 2.69 | High | Strongly disagreed with this, it struck to me of potential for falsifying informationDoctor (heart failure) |
| Having the record that represents what the patient’s perspective is, is very helpfulDoctor (mental health) | |||||
| I think access is one thing, but the ability to modify your records is a horrendous conceptDoctor (cancer) | |||||
| 54 | Patients should be given the option to provide a written summary for the meeting | 5 | 1.88 | High | In a practical sense . . . a patient comes in and is told she’s got cancer, I’m going to tell her about the MDT, would you like to provide me with a summary, the meeting’s on Friday, today is Thursday. I’m struggling, I understand the principle of it but it’s the practicalities of it as wellDoctor (cancer) |
| Allowing them the opportunity to present their viewpoints . . . it will be further evidence of their state of mind at the timePatient representative (mental health) | |||||
| 55 | Patients should be given the option to provide audio-recorded input to the meeting | 4.5 | 2.50 | High | It would be . . . it just wouldn’t be relevant to the discussions in what is largely a diagnostic . . .Doctor (cancer) |
| I strongly disagreed with it as well for practical purposesPolicy (cancer) | |||||
| The opportunity to maybe record something that would then be used in the meeting would be er, very welcomeNurse (mental health) | |||||
| From a mental health point of view, to have the service user’s view on the situation I think would reduce the time in terms of making decisions about the treatment of the person with a mental health problem, just to hear what their . . . their choices are, their voiceTeam manager (mental health) | |||||
| It shouldn’t require audio input if you’re being a good doctor or a good nurseDoctor (heart failure) | |||||
| We maybe need to change the system . . . to allow the person to feature in some way . . . then maybe that is something that that we need to think about. Sometimes just because we’ve always done it that way, it doesn’t make it rightNurse (cancer) | |||||
Appendix 15 Consensus development results from round 2: recommendations for which strength of agreement was disagree (median < 4)
| Question | Mediana | Mean absolute deviation from the median | Variation in extent of agreementb | Quotes illustrating variability in agreement (when discussed)c | |
|---|---|---|---|---|---|
| Recommendations relating to the purpose and function of MDT meetings | |||||
| 56 | MDT meetings should be a forum for brainstorming and giving advice without necessarily reaching a decision | 3 | 1.25 | Moderate | There are other forums for brainstorming and I don’t think the multidisciplinary meeting is that forumDoctor (heart failure) |
| It seems to open the possibility that you can brainstorm and give each other advice, but then it’s fine not to reach a decision about the patient, which just seems . . . what’s the thing [the MDT meeting] for then?Policy (cancer) | |||||
| Sometimes the decisions are not that clear, so having to say a range of options that come out of that discussion – I’d call it a discussion rather than a brainstormDoctor (mental health) | |||||
| It’s important teams have a space on an occasional basis where there’s a possibly to perhaps brainstorm, explore wider issues about how the team’s working and stuff, not on a routine basisNurse (mental health) | |||||
| I slightly sat on the fence . . . if that’s the only forum, which it quite often would be, where you might be able to bring something where it isn’t appropriate to make a decision at that time, then it might be that that’s the place because that’s where you’ve got the expertise, so it might happenNurse (cancer) | |||||
| 57 | Only complex cases should be discussed in the MDT meetings (regardless of whether they are new or existing patients) | 3 | 1.31 | Moderate | Some of our patients can be discussed quite quickly once we talk about the diagnostic process, it’s a way of ensuring that absolutely every patient has that safety net of quality assurance. So I really believe we shouldDoctor (cancer) |
| I think there’s a lot of straightforward cardiology that really doesn’t need discussing, where you’d ask, you’d have 100 MDTs you’d always get the same decision, you’d always follow the protocolsDoctor (heart failure) | |||||
| 58 | It is more important to discuss all patients, even if superficially, than it is to discuss a smaller number of patients in more depth | 3.5 | 1.69 | Moderate | I think there’s a lot of straightforward cardiology that really doesn’t need discussingDoctor (heart failure) |
| I actually agree that all patients should be discussed, even if they are what we consider to be a relatively low-risk cancer or whateverDoctor (cancer) | |||||
| Recommendations relating to the content of discussion in MDT meetings | |||||
| 59 | There should be time within MDT meetings to discuss current and emerging research and evidence which is not specifically related to an individual case | 3.5 | 2.38 | High | It’s just not really the forum unless it’s pertinent to the patient careDoctor (cancer) 1 |
| It’s not a CPD [continuing professional development] forumDoctor (heart failure) | |||||
| Knowing what’s up to date, current, and changing the thinking on care is actually really, really important for me to help make decisions with the patients about the way forwardNurse (heart failure) | |||||
| That’s useful to be brought to the meeting . . . may not always be relevant to a specific case, but just . . . [being] aware that this [research] has come outDoctor (cancer) 2 | |||||
| Recommendations relating to the structure of MDT meetings | |||||
| 60 | Members should be allowed to join the meeting for cases that are relevant to them and leave after the discussion of these | 3 | 1.19 | Moderate | There’s nothing worse than seeing people coming and going through an MDTDoctor (cancer) |
| You have your core team and then you can invite other people in to discuss one particular case, maybe a dietitian or physiotherapist. To make them sit through two hours of the rest of the cases that are irrelevant seems a bit harshDoctor (heart failure) | |||||
| Recommendations relating to the role of the patient in MDT meetings | |||||
| 61 | Patients’ treatment preferences should be routinely discussed at the MDT meeting and if not available the case should not be discussed | 3 | 1.94 | High | The horrifying bit is this case should not be discussed . . . we have a defined timeframe in which we have to make a diagnosis and . . . recommendations . . . so the MDT for us, the large part of the MDT is a diagnostic process getting the diagnosis, stage, treatment options correct, and then we sit down with the patient and go through it. And, you know, the patient can say look, I don’t want any of that. That’s fine but before the MDT it’s almost irrelevant because we don’t know what we’re actually going to be talking to her aboutDoctor (cancer) 1 |
| Trying to do that in a practical sense is not really going to happenDoctor (cancer) 2 | |||||
| I think a better model is you decide it’s medically the right treatment and then you spend half an hour with the patient discussing it. Why put them through the worry then go back and say, we’re not going to do that?Doctor (heart failure) | |||||
| People with mental health problems don’t always have a strong preferenceDoctor (mental health) | |||||
| It depends a little bit on whether you’ve got someone who’s totally new to the condition or new to the treatment, they may have already had certain treatments and have quite strong viewsNurse (mental health) | |||||
| 62 | Patient preferences regarding available management options should be reported to the MDT meeting only if the clinician responsible for their care thinks it will alter the decision | 3 | 2 | High | Not discussed |
| 63 | Patients should be asked before the MDT how much they want to be involved in decision-making about their treatment | 3 | 1.88 | High | I’m not sure it’s a question that many patients might necessarily be able to deal with . . . especially . . . if you are presenting with something that you don’t suspect, you’ve got so many things to think aboutPatient representative (cancer) |
| If you are the tertiary centre . . . you don’t know the patient either . . . you don’t have that link with the patient, so I think probably quite difficultPolicy (cancer) | |||||
| The nature of many heart failure MDTs is you’re discussing complex patients much further down the pathway when you know them well and you’ve had many conversations with them. Therefore, it didn’t seem relevant. They’re already involved in the decision-making before thenDoctor (heart failure) | |||||
| In mental health, a lot of service users have difficulties making decisions, so it’s much easier to take it to the MDT, discuss the different options and then go back, as opposed to asking them to kind of make decisions before the options have been discussed in the MDTTeam manager (mental health) | |||||
| What are you going to do with the answer, unless they say no, nothing at all, in which case you’ll persuade them that they should be. You know, it’s just . . . I don’t think it’s a practical questionPolicy (cancer) | |||||
| I’ve had those conversations at various stages of my own treatment, including from the very earliest stages . . . It’s not always going to be relevant but I think it can be donePatient representative (heart failure and cancer) | |||||
| 64 | All patients should be told if they are going to be discussed at an MDT meeting before the meeting otherwise they should not be discussed | 2 | 1.88 | High | I just think it’s good practice to explain that you’re working as part of a multidisciplinary team and that their case will be discussed in that setting . . . it’s not giving people the option to say well, I don’t want you to . . . if people express a concern then you can explain why it’s necessaryNurse (mental health) |
| In the cancer world . . . there will be an explanation that the MDT is routine practice. It’s kind of coveredDoctor (cancer) | |||||
| 65 | All patients should be explicitly given the choice of whether or not to be discussed at the MDT meeting | 1.5 | 1.19 | Moderate | We have a lot of ‘at risk’ clients and if there are risks that they may harm someone else . . . then there’s actually . . . there isn’t an optionDoctor (mental health) |
| If they’re going to be under our care then this is an integral part of our processDoctor (cancer) | |||||
| 66 | Patients should not be given an explicit choice, but if they express concern about being discussed at the MDT meeting they should be allowed to opt out | 2 | 1.25 | Moderate | We couldn’t do our jobs if patients could opt out. It’s not uncommon for patients to not want anything to do with usDoctor (mental health) |
| If then, once you’ve explained it fully to them, they then say they don’t really want to be involved, then that’s a different matter, but I think you need to make sure they fully understand what you’re talking about firstNurse (heart failure) | |||||
| 67 | Patients should be given the option of attending MDT meetings | 1 | 1.19 | Moderate | The logistics of it wouldn’t workPolicy (cancer) |
| It’s not the forum for breaking bad newsDoctor (cancer) | |||||
| 68 | Patients should be given MDT meeting feedback only when decisions are made about their care | 3 | 1.06 | Low | Accepting that there is a scenario where an MDT may meet and not apparently made a decision, feedback, you know, feedback would be harmless wouldn’t it?Policy (cancer) |
| Maybe it’s important to acknowledge to that patient that they were discussed, but decisions weren’t reached, so that they know that they weren’t left outTeam manager (mental health) | |||||
| Why have an MDT and not have a decision?Doctor (cancer) | |||||
Appendix 16 Consensus development group panellists
| Profession | Area of expertise | Location |
|---|---|---|
| Nurse | Heart failure | London |
| Nurse | Heart failure | London |
| Doctor | Heart failure | London |
| Doctor | Heart failure | London |
| Patient/carer representative | Heart failure and cancer | Hertfordshire |
| Nurse | Mental health | Sheffield |
| Nurse | Mental health | London |
| Occupational therapist | Mental health | London |
| Doctor | Mental health | London |
| Patient/carer representativea | Mental health | West Yorkshire |
| Nurse | Cancer | London |
| Nurse/policy | Cancer | London |
| Doctor/policy | Cancer | Sheffield |
| Doctor | Cancer | Birmingham |
| Doctor | Cancer | Kent |
| Patient/carer representative | Cancer | East Sussex |
Appendix 17 Consensus development group information for panellists
Appendix 18 Consensus development results from round 2: medians for each disease group – recommendations rated as ‘uncertain’ overall
| Recommendation | Overall median | Median among mental health panellists (n = 5) | Median among cancer panellists (n = 6) | Median among heart failure panellists (n = 5) | |
|---|---|---|---|---|---|
| 39 | The main objectives of MDT meetings should be the same across all chronic diseases | 6.5 | 3 | 7 | 7 |
| 40 | Teaching should be a function of MDT meetings provided it does not add to the length of meetings | 6.5 | 8 | 6 | 5 |
| 41 | Teaching should be a function of MDT meetings even if it means meetings will be longer | 5 | 7 | 6 | 4 |
| 42 | All treatment plans for existing patients should be agreed in an MDT meeting even if a clear protocol exists | 5 | 7 | 5 | 2 |
| 43 | Members should be allowed to not attend as long as someone from their discipline is attending and the member does not have a case to present | 5 | 7 | 4.5 | 6 |
| 44 | A list of people who are required to attend the MDT meeting should be decided locally by the team | 5 | 6 | 2 | 7 |
| 45 | A patient should only be discussed at the MDT meeting when information on comorbidity is available | 4.5 | 2 | 6 | 6 |
| 46 | A designated MDT member should speak to the patient about comorbidities before the patient is discussed at an MDT meeting | 4 | 6 | 3.5 | 3 |
| 47 | Each MDT should identify the most appropriate methods for presenting complete information on comorbidities | 5 | 7 | 5 | 5 |
| 48 | Case presentation should routinely include a brief introduction of the patient and relevant psychosocial characteristics, otherwise the case should not be discussed | 6 | 7 | 4 | 3 |
| 49 | Any MDT member who presents a case should discuss treatment preferences with the patient before the MDT meeting | 5.5 | 7 | 4.5 | 7 |
| 50 | Patient preferences regarding available treatment options should be discussed with the patient after (rather than before) the MDT meeting | 5.5 | 5 | 6 | 8 |
| 51 | Patients should not be presented at the MDT meeting unless there is someone present who has met with them at least once before the meeting, even if this postpones discussion of that patient | 5 | 8 | 2 | 3 |
| 52 | Patients should be given the opportunity to provide information in advance of the MDT meeting to ensure the information presented is accurate and comprehensive | 5 | 7 | 4.5 | 6 |
| 53 | Patients should be able to provide information by having direct access and the ability to modify their medical records | 5 | 7 | 2 | 5 |
| 54 | Patients should be given the option to provide a written summary for the meeting | 5 | 6 | 3.5 | 3 |
| 55 | Patients should be given the option to provide audio-recorded input to the meeting | 4.5 | 7 | 1.5 | 3 |
Appendix 19 Consensus development results from round 2: medians for each disease group – recommendations rated ‘disagree’ overall
| Question | Overall median | Median among mental health panellists (n = 5) | Median among cancer panellists (n = 6) | Median among heart failure panellists (n = 5) | |
|---|---|---|---|---|---|
| 56 | MDT meetings should be a forum for brainstorming and giving advice without necessarily reaching a decision | 3 | 3 | 3 | 2 |
| 57 | Only complex cases should be discussed in the MDT meetings (regardless of whether they are new or existing patients) | 3 | 3 | 2 | 3 |
| 58 | It is more important to discuss all patients, even if superficially, than it is to discuss a smaller number of patients in more depth | 3.5 | 2 | 5 | 2 |
| 59 | There should be time within MDT meetings to discuss current and emerging research and evidence which is not specifically related to an individual case | 3.5 | 6 | 3.5 | 3 |
| 60 | Members should be allowed to join the meeting for cases that are relevant to them and leave after the discussion of these | 3 | 3 | 3 | 3 |
| 61 | Patients’ treatment preferences should be routinely discussed at the MDT meeting and if not available the case should not be discussed | 3 | 5 | 4 | 2 |
| 62 | Patient preferences regarding available management options should be reported to the MDT meeting only if the clinician responsible for their care thinks it will alter the decision | 3 | 5 | 3.5 | 2 |
| 63 | Patients should be asked before the MDT how much they want to be involved in decision-making about their treatment | 3 | 3 | 2.5 | 3 |
| 64 | All patients should be told if they are going to be discussed at an MDT meeting before the meeting otherwise they should not be discussed | 2 | 5 | 1 | 2 |
| 65 | All patients should be explicitly given the choice of whether or not to be discussed at the MDT meeting | 1.5 | 2 | 1 | 1 |
| 66 | Patients should not be given an explicit choice, but if they express concern about being discussed at the MDT meeting they should be allowed to opt out | 2 | 2 | 2 | 5 |
| 67 | Patients should be given the option of attending MDT meetings | 1 | 5 | 1 | 1 |
| 68 | Patients should be given MDT meeting feedback only when decisions are made about their care | 3 | 3 | 3 | 5 |
Appendix 20 Challenges and learning points: reflections on conducting a large multisite mixed-methods study
Throughout the 3 years of this project we faced a number of unforeseen challenges. This is inevitable in any prospectively conducted health services research project. These difficulties required us to be flexible in our approach and derive innovative solutions. Here we describe some of the challenges and share learning points that may be helpful for researchers embarking on studies of a similar nature.
Conducting a study across different NHS organisations
Conducting the study across eight NHS trusts was not a streamlined process. The hurdles we describe are familiar to every researcher undertaking multisite research in the NHS. Though the field researchers had NHS Research Passports, trusts varied in the approvals they required (e.g. honorary contracts, letters of access, licences to attend) and in the procedures for obtaining them. One site required a site-specific agreement to be drawn up which involved extensive liaison between the researchers, representatives of the study’s sponsoring organisation (University College London) and the contracts team of the trust in question.
Similarly, it was time-consuming to find and follow the local procedures for acquiring the necessary security passes and IT access privileges at each trust. Some trusts required that the researchers go on specific training before granting access to certain computer programs used to access parts of medical records (e.g. software for viewing magnetic resonance imaging scans). This occurred even where the training was not relevant to the researcher’s role – for example, in order to gain access to certain medication records, one of our researchers was obliged to undergo training on electronic prescribing, even though this was clearly not a task she was going to undertake.
Difficulties in reaching the planned sample size for the quantitative analysis
We originally recruited 10 MDTs in eight NHS trusts. The necessary ethical and R&D approvals were obtained by the chief investigator before the project start date to allow us to begin data collection immediately. For a number of reasons, however, it became apparent that these teams would not provide sufficient data for us to reach our target sample size of 3300 patients. Although we drew on the experience of all our clinical coapplicants, the idiosyncratic nature of MDT meetings soon became apparent: there were extensive differences in the number of patients discussed both within and between teams concerned with different chronic conditions. We encountered particular problems in identifying heart failure teams with sufficient patient throughput to allow us to achieve the necessary sample size in the required time frame. Having met with heart failure consultant leads across London we identified two teams, but with low patient throughput. We therefore identified a third heart failure team, which agreed to participate in the study. Unfortunately, despite our efforts and timely production of all relevant documentation, delays in the R&D procedures at the site meant that after 6 months of liaison it was no longer going to be possible to recruit the required number from that site. Instead, we compensated for the small numbers discussed in some teams by observing these teams for longer than originally planned and we also observed extra meetings at the three MDTs discussing the highest numbers of patients.
One of the mental health teams disbanded shortly into the data collection phase because of service-restructuring within their trust. We were unaware of any plans to disband when we started data collection. We therefore recruited another team. As there was not enough time to go through the process of acquiring R&D approval from a new NHS trust, we recruited a new team from a trust which had already approved the study in relation to another team. This allowed us to recruit the new team and begin data collection relatively quickly.
Another challenge related to the nature of discussions in different teams. In our clinical coapplicants’ experience, each patient discussion resulted in a management decision. This information was used to calculate the sample size required. While this did occur in some teams, in practice several teams did not make decisions in every patient discussion, particularly in mental health teams. This meant that, even where teams were discussing sufficient patients, they were not making enough decisions to allow us to reach the target sample size. We therefore recruited a fourth mental health team to compensate for this.
Another unanticipated and time-consuming task related to quality assurance of our data. We originally planned to audiotape the first six meetings observed for each team. However, because meetings often involved team members talking simultaneously, we found that it was necessary to listen to the recordings of all meetings observed to ensure accurate completion of the observation proformas (i.e. to make sure we had captured all decisions and other relevant information). This meant listening to all 370 meetings at least twice (once in person and once on the recording). We amended the participant information sheet to reflect this.
Because of the recruitment of additional teams, we needed to employ an additional research assistant to attend and collect data from these teams. We applied for and were awarded service support costs from the Central and East London Comprehensive Local Research Network to fund this.
The NHS National Research Ethics Service granted approval for these minor amendments to the study.
Challenges relating to the interviews
The need to extend the data collection period in order to achieve a sufficient sample size had consequences in terms of our ability to undertake the planned number of interviews. We considered focusing only on the professional interviews; however, on the advice of our funders and members of our steering group, we proceeded with both professional and patient interviews as planned, though with a smaller sample and with carefully targeted recruitment to retain a diversity of disciplinary perspectives in the professional interviews.
We were assisted in the conduct of some of the interviews with mental health patients by a medical student and two specialist registrar psychiatrists (currently under the supervision of one of our coapplicants). The specialist registrar psychiatrists performed additional analyses of these interviews, and these have resulted in a research article which is currently under review. We invested considerable time to ensure that the medical student and trainee psychiatrists were fully cognisant of the study. Crucially, we trained them in qualitative interview techniques and observed their technique in pilot interviews with volunteer patients identified by our coapplicant PPI representatives before they began data collection. We also helped them to obtain the necessary approvals and submitted a substantial amendment (concerning the additional researchers) to the Research Ethics Committee.
Recruitment of mental health service users for interviews proved very challenging for a number of reasons. We recruited patients who had been recently discussed at MDT meetings, purposively sampling for a diversity of age, sex and ethnicity. For each selected patient, we asked their key worker to contact the patient and, if the patient agreed, we then made direct contact with the patient to explain the study, respond to their questions and arrange the interview. We agreed exclusion criteria with MDT members. These were patients who were acutely unwell, who had been discharged and were no longer contactable, and who were in prison.
The MDT teams from which these mental health patients were drawn were undergoing restructuring. Though only one team disbanded during the observation period (no patients from this team were interviewed), the others were preparing for staff changes and morale was very low because many staff members were concerned about losing their jobs. Thus, the research was understandably not a high priority for them. The key workers were also very busy and did not always have time to speak to the patients they had agreed to contact about the study. Several key workers did not respond to our e-mail and voicemail reminders.
Even where patients had been approved as appropriate by their key workers, by the time they had contacted them to explain the study and let us know, many patients had experienced deterioration in their health or had moved address and were no longer contactable.
Several patients agreed to be interviewed during our initial conversation but we were subsequently unable to contact them. One patient agreed to participate but then rescheduled the date of the interview and subsequently changed their mind and declined. One patient did not attend two appointments that we had agreed for the interview and so we decided not to pursue them for a third appointment. Both of the mental health patients we did interview had rescheduled at least once.
Managing large qualitative data sets
The qualitative aspect of the study involved 73 interviews and observation of 370 meetings (6053 patient discussions and approximately 530 hours of audiotape). Managing this volume of data was challenging, in terms of confidential storage, data reduction, ensuring consistency of data analysis across researchers and reporting the results concisely. At one point we unexpectedly ran out of electronic storage space and had to reduce the size of our audio files. This had a detrimental effect on the sound quality. The large volume of data also significantly slowed the running of the analytic software.
We adopted a number of strategies to facilitate the processing and analysis of the data. We outsourced transcription of the interviews to an external company that was recommended by qualitative researcher colleagues and that signed a confidentiality contract. We did not outsource transcription of the audio recordings of the meetings because they were too complex, with large numbers of speakers and variable audio quality.
Ensuring that each of the three field researchers coded the data reliably was a learning process necessitating close and regular communication between the researchers. Each researcher had different expectations regarding a ‘normal’ number of codes, how broadly or narrowly to interpret the research question, how to divide the task between them and which data management software to use, if any. Each code is open to different interpretations and it was a slow and laborious process to agree unambiguous definitions which coders could use consistently.
However, we found that the benefits of performing the analysis as a group far outweighed the costs. We believe it improved the robustness of the analysis because we needed to scrutinise, challenge and defend our ideas throughout. It led to more accurate and standardised coding and gave us insights into the assumptions underlying our interpretations of events. It allowed us to learn from each other and provided more ideas for a richer conceptualisation. Finally, it allowed us to analyse far more data than an individual could have managed alone.
We began to code data from the different sources as they became available, rather than waiting for the data collection phase to be complete before beginning the analysis. Observation of the different teams finished at different times. Thus we began the basic inductive coding and selective transcription of the observational data for the first team when it was ready, rather than waiting until observation of all the teams had finished. This meant that one field researcher could work on the coding framework and feed back to the team before the next researcher used it on the next ‘batch’ of data. This avoided the potential problem of researchers simultaneously coding different sections of the data and then meeting to find that their coding frames were incommensurable or redundant. This phased approach also meant that we were already very familiar with many of the data by the time data collection was finished and we could proceed relatively quickly to the integration stages.
Conclusions
Studies of this nature, involving collaboration across multiple sites, are inevitably subject to factors that are beyond the control of the research team. Responding to these challenges requires a willingness to be flexible and responsive to unanticipated events. Maintaining an open dialogue with our funders throughout and proactively seeking the advice of our steering committee were also essential to successfully navigating these challenges.
Glossary
There is much debate regarding the term that is used to describe those under the care of mental health services, with a range of terms including ‘service user’, ‘client’ and ‘survivor’ being advocated (Simmons P, Hawley CJ, Gale TM, Sivakumaran T. Service user, patient, client, user or survivor: describing recipients of mental health services. Psychiatrist 2010;34:20–3). For the sake of consistency in comparing a range of teams we use the term ‘patient’. However, we recognise that some prefer other terms.
List of abbreviations
- ATI
- Adjusted Teachman’s Index
- CI
- confidence interval
- CMHT
- community mental health team
- ECC
- Ethics and Confidentiality Committee
- ICC
- intracluster correlation coefficient
- IMD
- Index of Multiple Deprivation
- IT
- information technology
- MADM
- mean absolute deviation from the median
- MDT
- multidisciplinary team
- MRC
- Medical Research Council
- NCAT
- National Cancer Action Team
- NGT
- nominal group technique
- NICE
- National Institute for Health and Care Excellence
- NIGB
- National Information Governance Board
- NSF
- National Service Framework for Mental Health
- PI
- principal investigator
- PPI
- patient and public involvement
- R&D
- research and development
- RAM
- RAND/UCLA appropriateness method
- SHO
- Senior House Officer
- SPSS
- Statistical Product and Service Solutions
- TCI
- Team Climate Inventory