Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 10/2000/61. The contractual start date was in October 2011. The final report began editorial review in January 2014 and was accepted for publication in June 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Reuber et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background to the study: ‘patient choice’ in the National Health Service
The National Health Service (NHS) has made a firm commitment to offering patients more choice. Yet the concept of patient choice is a contested one, used in diverse ways across different academic, political and policy literatures. 1 Even within the context of the NHS, patient choice is taken to mean very different things. As a key part of the (Labour) government-driven public service reforms, for example, the patient choice initiative had a limited, measurable definition: it referred to a set of deadlines by which patients were to be offered choices that were previously unavailable within the NHS (such as which hospital to attend for elective surgery). Laid out clearly in the NHS 2013/14 Choice Framework, there are now a series of such legal rights to choose (with explicit limitations), which all patients are accorded. 2 These include choosing one’s general practitioner (GP) practice, having a right to ask to see a particular GP or nurse, choosing where to go for a first outpatient appointment if referred to secondary care, and which consultant will be in charge of one’s treatment.
Yet a much broader notion of patient choice is also evident within NHS policy. For example, the 2000 NHS Plan3 announced that ‘[t]he relationship between service and patient is too hierarchical and paternalistic’ [p. 30 reproduced from The NHS Plan: A Plan for Investment, a Plan for Reform, © Crown Copyright 2000 (this attribution applies to all quotations from this source)], and promised reforms that would ‘shape [NHS] services around the needs and preferences of individual patients, their families and their carers’ (p. 3). As Sir Nigel Crisp,12 then Chief Executive for the NHS, put it: ‘The ambition for the next few years is . . . to change the whole system so that there is more choice, more personalised care, real empowerment of people to improve their health – a fundamental change in our relationships with patients and the public’ (p. 2). Working towards this goal, the NHS provides a public information service – NHS Choices,5 with the strapline, ‘your health, your choices’ – which is intended to ‘symbolise the type of personalised healthcare that the NHS is moving towards’6 (p. 13). This vision is reflected in the NHS Constitution,7 which states not only that patients have ‘the right to accept or refuse treatment that is offered’ (p. 8), but also that they ‘have the right to be involved in discussions and decisions about [their] health and care . . . and to be given information to enable [them] to do this’ (p. 9). The NHS Constitution includes the following pledge to patients: ‘The NHS also commits: . . . to offer you easily accessible, reliable and relevant information in a form you can understand, and support to use it. This will enable you to participate fully in your own healthcare’ (p. 9). 7
It is this broader notion of patient choice that the present study addresses. Our aim is to examine how the drive to involve patients in decisions about their health care – including treatment decisions and decisions about further investigations – is currently being enacted by practitioners. In so doing, we address a gap both in the empirical literature and in the guidance available to health professionals. As we discuss further in our review of the literature below, fine-grained observational studies of doctor–patient interaction have focused overwhelmingly on how doctors deliver treatment recommendations. There is very little comparable research on how they might seek, instead, to hand the decision over to the patient. Moreover, practitioners are explicitly told, within the NHS Constitution,7 that they should aim ‘to involve patients, their families, carers or representatives fully in decisions about prevention, diagnosis, and their individual care and treatment’ (p. 15), and the NHS Choices website5 assures patients that NHS clinicians are trained to involve them in decision-making.
Yet the available guidance tends to be at a rather general level. For example, the General Medical Council in the UK has issued a guidance document, entitled Consent: patients and doctors making decisions together,8 which came into effect in 2008. This ‘provides a framework for good practice’ and provides health professionals with a set of principles to guide their decision-making with patients, including the requirement to work in partnership with patients and, among other things, to:
(c) . . . share with patients the information they want or need in order to make decisions
(d) maximise patients’ opportunities, and their ability, to make decisions for themselves
(e) respect patients’ decisions
Reproduced from Consent: Patients and Doctors Making Decisions Together. © 2008 General Medical Council (this attribution applies to all quotes from this source), p. 68
Such principles are clearly important. However, we are not aware of any guidelines on how they might be put into practice during interaction with patients. This matters for two reasons. First, as we discuss below, there is growing evidence that small differences in doctors’ talk can make a big difference to patient involvement in the consultation. 9 Directives like those shown above inform doctors about what they ought to do during interaction with their patients, but not how best to do it. Second, as we have argued on the basis of the pilot analysis we conducted for this study:10 ‘our findings suggest that the concept of “choice” in the context of doctor–patient interactions is not as simple as the literature may suggest, and that the simple course of telling clinicians to “offer patients more choice” may not achieve its objective’ (p. 319). This echoes conclusions drawn about choice in the context of interactions in care homes for people with learning difficulties:11 that ‘analyses of real-time interactions reveal the complexities of offering choice’, and imply that the best starting point for understanding the delivery of choice is ‘not from the top down but from the ground up’ (p. 264).
Searching for guidelines on how to ‘maximise patients’ opportunities, and their ability, to make decisions for themselves’ (p. 6),8 we located only the Dr Foster marketing health workbook Patients Choosing their Healthcare. 12 Subtitled, How to Make Patient Choice Work for Patients, GPs, and Primary Care Trusts, this looked promising – and is, indeed, a useful document. However, it focuses entirely on the first, more limited, concept of patient choice, outlined above. Likewise, seeking information on any formal training in shared decision-making (SDM) given to students at the Hull–York, University of Sheffield and University of Glasgow medical schools, we were told that – while there is some training included in the curriculum – these communication skills are predominantly acquired through observation and on-the-job learning. There is a need, then, for empirical research to address the gap in our understanding of how practitioners actually go about offering choice, as a crucial step towards developing well-founded guidelines for use in practice.
Aims and objectives
This report discusses findings from a 2-year study, funded by the UK’s National Institute for Health Research, Health Services and Delivery Research programme. In order to identify what works in practice and from the participants’ perspective, the study depends largely on the fine-grained analysis [using conversation analysis (CA); see Chapter 2] of over 200 audio- and video-recorded consultations. These took place in two neurology clinics (one in Glasgow and one in Sheffield), where decision-making around chronic conditions – such as epilepsy, migraine, non-epileptic attack disorder and multiple sclerosis (MS) – occurs on a regular basis. In the broadest terms, our core research questions are:
-
What communication practices are clinicians using to give patients choice in decision-making processes?
-
How do patients respond to the different practices used by clinicians?
More specifically, in answering these questions, the study aims to address three main objectives. These are:
-
to contribute to the evidence-base about whether or not, and how, patient choice is implemented
-
to identify the most effective communication practices for facilitating patient choice
-
to disseminate these findings to clinicians and patients in order to help translate into practice the policy directives to increase patient choice (where appropriate).
It is worth noting that there are situations in medicine when patients are physically unable to contribute directly to decision-making processes (for instance when they are unconscious or lack competence or when decisions have to be made so quickly that there is no time for deliberation). Our study focuses on the many other situations in which decisions about tests or treatment can reasonably be discussed between clinician and patient.
Setting: why neurology?
Neurology is an ideal setting for addressing these aims because ‘a person-centred service’ (p. 4)13 is an explicit quality requirement for neurological practice. This underpins 10 other requirements listed in the National Service Framework for Long-Term Conditions, which states that ‘People with long-term neurological conditions . . . are to have the information they need to make informed decisions about their care and treatment . . .’ (p. 15). 13 More specifically, two neurological conditions – epilepsy (the most common serious neurological disorder in the UK) and MS (one of the most common disabling central nervous system diseases) – are among seven chronic conditions identified by the UK’s Department of Health as particularly suited to a SDM approach. 14 In part this reflects health professionals’ recognition that patients with chronic conditions often understand their disease as well as (or even better than) they do, but that ‘this knowledge and experience by the patient has . . . been an untapped resource’ (p. 5). 14 The Department of Health14 has advocated a ‘fundamental shift . . . which will encourage and enable patients to take an active role in their own care’ (p. 6), arguing that patients with chronic diseases are able to ‘become key decision-makers in the treatment process’ (p. 5). This increased involvement is argued to be of benefit both to ‘the quality of patients’ care and ultimately their quality of life’ (p. 5). 14
Moreover, because many neurological conditions have an uncertain trajectory, decision-making in this context is seldom cut and dried. Indeed, in our pilot work for this study (discussed further in Building on our pilot work), we found that decisions were frequently made against a backdrop of ongoing diagnostic uncertainty. This means that a simple recommendation for the ‘known to be best’ option is often not possible. Even where the diagnosis is certain, the effects of the different treatment options may not be. For example, McCorry, Chadwick and Marson15 note that antiepileptic drugs (AEDs) have ‘dose-related and idiosyncratic effects’ and that ‘ultimately the choice of an individual AED will be determined by an individual risk-benefit assessment’ (p. 730). Decision-making, then, will at least partly rest on factors that only the patient can assess, such as his or her willingness to risk certain side effects, which side effects are more or less manageable in the context of the patient’s personal circumstances, and the extent to which the patient’s condition is affecting his or her quality of life. As Pietrolongo et al. 16 put it in relation to MS: SDM ‘is especially important in gray-zone situations where available treatments have important risks as well as benefits, and where evidence is lacking’ (pp. 1–2). Similarly, the NHS Choices website5 tells patients that, although health professionals can provide information, advice, and treatment recommendations, only the patient will know what matters to him or her with respect to any decisions that need making.
Yet there is some evidence that neurology patients may experience the decision-making process as clinician dominated. For example, an interview study17 of AED treatment decisions found that most of the patients (42 of 47) attributed ‘decisional ownership’ to the neurologist: ‘He just said, “We’re best leaving them as they are for now,” that was it really’ (p. 213). The authors argue that this may be particularly common for conditions which engender feelings of desperation (e.g. to be seizure free), and hence a willingness to defer to any recommendation. Similarly, a study16 that rated the ways in which physicians at four Italian MS centres spoke with patients about disease-modifying treatments, concluded that SDM was ‘not a prominent part of patient care’ (p. 6). There is also evidence that MS specialists in the UK are exerting a significant influence over patients’ selection between four disease-modifying treatments. Although the same options were available in all 72 prescribing centres, Palace18 found that the relative use of these options varied significantly. Moreover:
. . . the percentage of MS specialists in each centre prescribing the disease[-]modifying drugs to patients with secondary progressive (versus relapsing remitting) MS varied from 0% to nearly 50% These variations are likely to be due to physician preference and opinion and not patient influenced
Palace, p. 618
Such findings indicate a discrepancy between the Department of Health’s vision, and (some) patients’ experience. By identifying the key practices for offering patients choice that are evident in current neurology consultations, this study will be of relevance to any neurologist wishing to engage patients (at least under certain circumstances) in decision-making about their health care. More broadly, because our focus is on communication strategies, our findings should not be assumed to be limited to neurology. Although the content of a choice may be condition specific (e.g. different drug or surgical options), practices for making choice available in interaction with patients are not. The findings from this study should therefore be of relevance to clinicians working in a range of settings.
Models versus practices: our methodological starting point
Three main models of decision-making in medical consultations have been described in the research literature: paternalist, shared and consumerist (or informed). 19,20 These have been summarised as follows:
In a paternalist model, doctors make health care decisions based on what they believe to be the best interests of the patient. At the other end of the scale is the consumerist model, where the patient makes the decisions . . . In the middle is shared decision-making, where [both parties] deliberate together
Murray et al. 2007, p. 18921
These models have been widely adopted in research on doctor–patient interaction and have been particularly useful for unpacking decision-making – conceptualised as a process – into its key components. For example, Charles et al. 20 have influentially distinguished between the following three steps: ‘information exchange, deliberation about treatment options and deciding on the treatment to implement’ (p. 654). In practice, ‘these may occur together or in an iterative process’ (p. 654). 20 Crucially, the naming of these steps makes it possible to distinguish analytically between different forms of patient engagement in decision-making, including information seeking (by asking questions), information provision (e.g. about preferences or values) and actively selecting a course of action to implement. Thus, patients may be analysably engaged in the decision-making process even in consultations where they do not make the final choice.
By their very nature, however, models must smooth over the many subtle differences that are evident in the detail of real interactions. Even when based on observation (as opposed to self-report methods or theory), models are typically derived from code-and-count studies which unavoidably lose interactional detail. This is a problem because there is a growing body of evidence that the precise wording used by clinicians can make a significant difference to patient involvement in the consultation. For example, Heritage and Robinson22 investigated the ways in which primary care physicians in the USA elicited patients’ problem presentations – the only phase in such visits ‘that is institutionally designed to provide patients with interactional space in which to present their concerns in accordance with their own agendas’ (p. 99). They identified a key distinction in physicians’ problem solicitation turns: general enquiry questions, which do not constrain the content, extent, or precise form of patient’s responses (such as what can I do for you today?), and questions that request confirmation either of a general gloss of the patient’s problems (e.g. sounds like you’re uncomfortable) or of specific symptoms.
In the last formats, the physician orients to the patient’s concerns as already (partly) known – a state of affairs which arises in this context because patients typically describe their symptoms first to a nurse, who records this in the patient’s notes, which are handed to the physician. The patient is therefore more constrained – relative to the general enquiry questions – with respect to how he or she may answer this kind of question; the relevant response entails a confirmation or disconfirmation of what has already been stated by the physician, rather than a more open account of the patient’s concerns. Heritage and Robinson22 showed that this has a statistically significant effect not only on the length of patients’ problem presentations, but also on the number of symptoms described. They also showed that, of seven demographic variables assessed in addition to question design, only age was significantly associated with longer problem presentations (but not with numbers of symptoms presented). For the most part, then, it was the design of the physician’s question that best predicted the nature of the patient’s response.
Another study9 investigated two question forms used in US primary care to elicit additional concerns that patients may not have raised during their initial problem presentations: ‘is there ANYthing else’ compared with ‘is there SOMEthing else you want to address today?’. Patients recorded their reasons for the visit in a pre-consultation questionnaire and doctors were randomly assigned to use one or other of the question forms during the consultation. The study found that ‘the multivariate odds of unmet concerns were less than one-sixth as high for the SOME intervention when compared to the non-intervention group (i.e. not asking the question at all)’9 [odds ratio = 0.15, 95% confidence interval (CI) = 0.054 to 0.45; p = 0.001] (pp. 1431–2). By contrast, ‘the ANY intervention could not be significantly distinguished from the non-intervention condition’ (odds ratio = 0.213, 95% CI = 0.030 to 1.5; p = 0.122) (p. 1432). 9 Crucially, the study also showed that neither of the intervention arms had a statistically significant effect on visit length. They conclude that, although, it may seem surprising that a range of demographic factors were not found to have a significant impact on their main outcome, this provides a reminder of the centrality of communication to health care. Moreover, they argue that it is encouraging to discover that interactional factors (such as a minor change of wording) can have such an influence on outcomes, given that these are far more amenable to change than many demographic variables.
The significance for patient participation of how doctors design their turns (i.e. word their contributions to the interaction) has also been demonstrated in health-care settings outside the USA. For example, investigating interactions in Finnish primary care, Peräkylä23 found that patients produced extended responses to doctors’ diagnostic statements in about one-third of his cases. Crucially, he showed that the responses were ‘strongly associated with the way in which the doctor referred to the evidence for the diagnosis: After diagnostic statements in which the evidence is verbally explicated, the patients start to talk about the diagnosis more often than after diagnostic statements in which such explication is not done’ (p. 219). 23 These findings have a close parallel in data collected in secondary care cancer consultations in the UK. Focusing on discussions of test results, Murtagh et al. 24 found evidence of a relationship between the way in which the results were delivered and whether or not patients asked direct questions. ‘Plainer’ announcements, which did not include sharing of the diagnostic evidence (e.g. ‘your scan is fine’), were less likely to receive patient-initiated questions than were more ‘elaborate’ announcements, which did include such evidence. In addition, reference to the scan or X-ray appeared to encourage patient participation. The authors conclude that: ‘Incorporating and explaining the evidence appears to be interpreted by patients as an opportunity to contribute to the consultation and establish their information needs in an environment within which the patient’s queries/opinions are welcomed’ (p. 5). 24 This was of particular significance in the study setting as the authors also found that, in general, patients’ levels of involvement were relatively low, and that patients seemed largely ‘disinclined to ask questions’ (p. 5). 24
These (conversation analytic) findings demonstrate how the same activity (e.g. eliciting the problem presentation, giving patients an opportunity to raise additional concerns or delivering a diagnosis and/or test results) can be done in different ways; and that these differences can have significant consequences for what happens next in the consultation. These are precisely the sorts of details that are lost in ‘code-and-count’ studies, which typically code for whether an activity occurred, rather than how it was performed. By contrast, all of the above findings depend on fine-grained, sequential analysis of recorded consultations in order to identify specific interactional practices and their consequences for patient participation. They did not start with a model of doctor–patient interaction and then seek to code their data in accordance with that model. This is vital because, as we will highlight in Chapter 7, Conclusion there is no one-to-one correspondence between specific practices that doctors use to initiate decision-making in interaction with patients and the three core decision-making models that are so prominent in the literature. Indeed, the very same practice can be used in ways that are analysably paternalist, consumerist or shared. Our aim, then, is not to assess the extent to which neurologists are adopting any of these three styles. Rather, our aim is, in the first instance, a more descriptive one: to identify the practices that neurologists are using to initiate decision-making in a way that is demonstrably oriented to patient choice – whether or not a shared decision is actually achieved.
This distinction between practices and models is crucial here for an additional reason. In searching for practices, we sought to adopt – as our starting point – as neutral a position as possible with respect to the value of patient choice. We wanted to avoid starting from an assumption that being offered a choice is inherently better than not being offered one – an assumption that is evident in much of the current NHS literature, which tends to associate increased patient choice with a shared model of decision-making. For example, the NHS Choices website5 explains that medicine today should be understood as a partnership between doctor and patient.
There is good evidence to support NHS policy on choice. For example, research25 suggests that patients who think they have chosen their treatment have better health outcomes than those who think they have not – despite receiving the same treatment. Patients have also been found to be more likely to follow a treatment plan they have chosen, and increased patient satisfaction with the consultation, confidence in the doctor’s recommendations and greater psychological well-being are all associated with more involvement in decision-making. 26 However, it has also been observed that too many options can leave consumers feeling anxious and overwhelmed,27 with patients at particular risk owing to the nature of clinical decision-making: as a health-care consumer, the patient is, by definition, sick, dependent on the professional for treatment and often lacking the knowledge to make an independent decision and the time to deliberate. 28 Moreover, it is well documented that not all patients want to choose their treatment;29,30 and, as Pilnick and Dingwall31 highlight, reviews of the literature on patient centeredness show mixed results with respect to positive health outcomes (although they suggest increased rates of patient satisfaction).
We did not start, then, from the assumption that choice ought to be more widely offered to patients. We also wanted to avoid the opposite assumption that patient choice must necessarily be conceptualised in terms of the consumerist model and that therefore calls for its increase should be rejected – on the grounds, for example, that these are not really directed towards patient empowerment but rather towards (neo-liberal) competition-based reforms of UK health-care provision, as some critics of the NHS choice agenda have argued. 32–34 Our focus on patient choice does not start from either of these normative positions. Rather, it reflects the recognition that (like it or not) patient choice has become increasingly prominent within NHS policy35 and is, as we outlined above, a feature both of the NHS’s commitment to patients and its directives to health professionals. Yet, as we discuss in What do we already know? A focused review of conversation analysis research into how decisions get made in real clinic encounters and What we already know about practices for offering choice existing research has largely overlooked the ways in which this policy is actually enacted through the interactions that occur in NHS clinics on a daily basis. There is a need, then, for an evidence-based understanding of how, if at all, doctors are orienting to the choice agenda in consultation with patients, as a precursor to assessing the value of such practices.
What do we already know? A focused review of conversation analysis research into how decisions get made in real clinic encounters
Existing CA research – with its fine-grained analysis of recordings of real medical consultations – has clearly demonstrated a range of (often subtle) ways in which patients may take the initiative in order to participate in the various activities that make up these interactions. For example, patients may answer ‘more than the question’ during comprehensive history-taking,36 make inexplicit requests for a diagnostic test,37,38 self-initiate problem presentations during prenatal check-ups39 and resist a doctor’s diagnosis23,40 or treatment recommendation. 41–43 In addition, as we discussed in the previous section, there is a handful of studies that have demonstrated how the turn designs selected by doctors can be more or less conducive to subsequent patient participation. 9,22–24 However, CA studies of decision-making in clinical practice have rarely investigated the ways in which doctors might actively invite patients to participate. Instead, as we discuss next, the focus has been predominantly on doctors’ recommendations.
This focus began with Stivers’43–45 influential work on US primary care consultations. Investigating treatment decision-making between doctors and parents of children presenting with upper respiratory tract infections, Stivers showed that when parents responded to treatment recommendations with a full acceptance (such as ‘OK’ or ‘let’s do that’), doctors typically moved towards closure of the consultation. However, when parents responded with silence or minimal receipts (such as ‘mm’), doctors continued to talk about treatment, justifying their recommendation or revising it. On this basis, Stivers convincingly argued that the doctors were treating such responses as passive resistance and were hence working to secure a full acceptance before moving to close. Where this failed, patients were sometimes prescribed antibiotics even when these were medically unnecessary. Stivers concluded that treatment recommendations are better understood as proposals to be accepted or resisted by parents (and patients) than as directives ‘simply handed over to patients who passively receive them’46 (p. 1036; also see our previous discussion of this work47).
Comparable patterns are evident in other settings. For instance, a study in Finnish primary care41 showed that – even when doctors produce a recommendation in a manner that appears to expect no response – patients sometimes go on to introduce their own views in ways that succeed in initiating negotiation and subsequent shaping of the final decision delivery by the doctor. Maynard and Hudak42 have shown how orthopaedic surgeons (in the USA) pursue acceptance of their recommendations and, like Stivers’45 paediatricians, do not always succeed. By introducing small talk, patients can produce a topic shift away from the recommendation-relevant talk, avoiding the sought-after acceptance. Similarly, Costello and Roberts48 found that, if faced with patient resistance, clinicians in US oncology and internal medicine clinics also pursue acceptance. However, in the former setting they typically work to secure acceptance of the original recommendation; in the latter, they usually reformulate the recommendation. In UK neurology consultations, Monzoni et al. 49,50 have shown that the neurologist may sometimes abandon the treatment recommendation altogether: when patients fail to accept a psychosocial explanation for their symptoms, it becomes difficult for the neurologist to propose psychological treatment. Further, Costello and Roberts48 demonstrated that – whether or not patients display resistance – doctors in both oncology and internal medicine clinics routinely orient to the patient’s right to accept or reject their recommendations through the interactional work they do to justify them. Medical recommendations may be understood, then, as a form of ‘joint social practice’ (p. 241).
Indeed, Koenig51 has shown how even so-called passive resistance from patients (in the form of minimal responses to treatment recommendations) can ‘create an opportunity to actively participate in how a treatment recommendation ultimately emerges as acceptable’ (p. 1112). In an instance which he presents as a candidate example of best practice, Koenig shows how a primary care doctor in the US responds to a patient’s passive resistance with an invitation to discuss the patient’s treatment preferences: ‘uh::m, do you- how do you feel about that?’ (p. 1112). 51 This leads to an understanding that the patient is worried about the cost of the recommended referral to secondary care. Once this is resolved, the patient accepts the recommendation. Koenig concludes that: ‘Including patients into the construction of the treatment recommendation ratifies patient active participation in making treatment decisions’ (p. 1112)51 (see Ijäs-Kallio et al. 41 for a similar conclusion in the Finnish primary care context). There is also evidence that this may be done quite routinely – not only in response to patient resistance. For example, Hudak et al. 46 have shown how orthopaedic surgeons design their recommendations in ways that address what they know – from talking with the patient – about the patient’s preferences (for or against surgery). Even when the surgeon disagrees with those preferences, he or she will typically display a sensitivity to them, designing the recommendation in a way that is ‘fitted’ to the recipient. As a result, it is possible for patients to ‘contribute to the joint accomplishment of recommending not only once a recommendation is given, but also by influencing how recommendations are designed from the beginning’ (p. 1029).
Although such studies have shown convincingly that patients are not simply passive recipients of doctors’ recommendations, there is also compelling conversation analytic evidence that doctors routinely influence or even direct patients’ decisions. For example, in the context of decision-making – in Hong Kong – around whether or not to undergo further testing for Down syndrome following a preliminary high-risk result, Pilnick and Zayts52 showed that doctors could be ‘quite directive . . . both explicitly by appearing to assume that testing will take place, and implicitly through the information which they offer or withhold in particular circumstances’ (p. 270). Similarly, Quirk et al. 53 showed how psychiatrists – working in the NHS in the UK – could design their questions about antipsychotic treatment in such a way that they demonstrated a clear ‘preference’ for agreement to a particular course of action. For example, although the question, ‘can I give you a prescription (for that)?’ (p. 101) ostensibly places the decision in the patient’s domain, it clearly implies that this treatment is the psychiatrist’s recommended option. Likewise, Karnieli-Miller and Eisikovits54 identified eight approaches used by paediatric gastroenterologists in northern Israel to ‘market’ their recommended treatments for inflammatory bowel disease. These included ‘various ways of presenting the illness, treatment and side effects; providing examples from other success or failure stories; sharing the decision only concerning technicalities; and using plurals and authority [e.g. ‘We are in favour of. . .’]’ (p. 1). 54
Taken together, these findings imply that the practices used by clinicians to initiate decision-making are indeed best understood as lying along a continuum with respect to how free the patient is to make a choice – just as the three main models of decision-making suggest. This has been described55 as a ‘spectrum of pressure’, running from ‘pressured decisions’ through ‘directed decisions’, to ‘open decisions where the patient is allowed to decide’ (p. 95). Similarly, a well-cited study of UK primary care consultations about diabetes, and secondary care ear, nose and throat cancer consultations, identified a spectrum of clinician approaches to decision-making, ranging from more ‘unilateral’ (or clinician-determined) to more ‘bilateral’56 (or shared). Moreover, the Finnish study,41 discussed above, showed that unilateral decision deliveries themselves lie along a spectrum, both with respect to the extent to which they incorporate the patient’s perspective and the extent to which they indicate that a response from the patient is relevant and expected. Our pilot study,10 likewise, described a ‘spectrum of openness’ in relation to neurologists’ practices for initiating decision-making. At the same time, as we have argued above, it is clear that patients have their own strategies for getting their voices heard. Concluding his review of the CA literature pertaining to medical authority, Heritage57 argued that the evidence points to more of a balance between authority and accountability on the part of health professionals ‘than is traditionally observed in the sociological literature’ (p. 99). Decisions about treatment and testing should, then, be understood as always negotiated in various ways – regardless of which model might best capture the nature of the decision-making overall.
Nevertheless, as we have demonstrated through our pilot work for this study, recommending as an action does not invite the patient to make a decision. 47 As Pilnick58 has argued, there is a real difference between ‘a decision that simply requires assent to a recommended course of action, [and] the production of an independent choice’ (p. 519). By exploring how clinicians may work to create an explicit slot for the latter, the present study will show that it is not only that medical authority may be subject to ‘a tacit bargaining process’57 (p. 99) triggered by patient resistance; clinicians may actively seek to create space for the patient’s voice during decision-making. In widening our focus beyond the recommendation sequence, then, we will be able to examine the more overt ways in which clinicians might be orienting to current policy directives to give patients ‘more choice’, and to evaluate the impact of these practices on the consultation.
What we already know about practices for offering choice
Quirk et al. ’s55 spectrum of pressure and the unilateral to bilateral spectrum described by Collins et al. 56 both provide a good springboard for our research in that they show how health professionals may work to minimise the extent to which they direct patients’ decision-making. For example, Quirk and colleagues list a range of interactional features that contribute to a more open decision, including that ‘the doctor’s wishes are communicated weakly’, that ‘the doctor allows the patient to reformulate the decision and reconsider it once made, and immediately acquiesces to patient resistance to treatment proposals’, and that ‘the decision is constructed as one that will be easy to reverse if the patient experiences difficulties’ (p. 110). 55 Similarly, Collins and colleagues show how, when taking a more bilateral approach, the health professional ‘actively pursues [the] patient’s contributions, providing places for the patient to join in, and building on any contributions the patient makes: e.g. signposting options in advance of naming them; eliciting displays of understanding and statements of preference from the patient’ (p. 2625). 56
However, we are aware of only two previous studies that have examined practices for offering choice specifically. Neither of these has focused on choices relating to a patient’s own health (at least not directly), but both have sought – like the present study – to understand how policy may be enacted through interaction. Pilnick’s58 study of antenatal, nuchal translucency screening for Down syndrome in the UK addressed the question of how the decision to screen (or not) is made in real interactions between pregnant women and midwives. Pilnick notes that guidelines produced by the National Institute for Health and Care Excellence (NICE) state that the pregnant woman has an explicit right to make this choice and, indeed, Pilnick showed that the existence of a decision to be made was routinely articulated by the midwives. Nevertheless, she also found that they presented the decision in ways that made it more a matter of ‘assent’ than of independent choice on the part of the women. For example, the midwives (sometimes) gave information in a way that required only ‘minimal acknowledgment rather than active participation’ (p. 526),58 treated the lack of an explicit refusal as implicit acceptance, and portrayed the test’s benefits in non-neutral ways. Pilnick concludes that ‘a key difficulty in practice’ (p. 527)58 is moving beyond simply telling women that they have a right to choose, which clearly does happen in this setting, ‘to creating an interactional environment in which that choice can actually be exercised’ (p. 527). 58
Although focusing on a different environment, Antaki et al. 59 reached similar conclusions. Investigating how professionals enact the policy requirement in UK care homes to offer choice to people with intellectual difficulties, they identified six practices for offering choice. These included two-option alternatives in a single question (e.g. ‘Do you want to get the glasses or are you drinking them out of your mugs this morning?’), open questions plus various follow-up types [e.g. open question plus multiple-option alternatives, such as ‘what do you want for pudding=look there’s (0.8) prunes, peaches, . . .’ (p. 1167);59 or open question plus a single option], and closed questions [‘a’we still happy f’Chris to come, (.) and do the filming?’ (p. 1170)59]. Like Pilnick,58 Antaki et al. 59 showed how, despite overt orientations to choice for the service users, the way in which the decision-making process was accomplished often actually made it difficult for residents to choose. Antaki et al. 11 also showed that there is a clear discrepancy between policy-level conceptualisations of choice – emphasising fundamental life decisions, like who to marry and what job to do – and the far more frequent choices of everyday life, like what to eat and what to do for fun. They conclude that ‘an account of what actually happens in utterly mundane, routine examples’ (p. 1166)59 is needed to translate policy into effective practice. Likewise, Pilnick has argued that:
. . . what the offering and exercising of choice actually looks like in practice . . . remains unclear. The potential implications of these interactional processes, though, are immense, since . . . ‘good’ practice is ultimately achieved through interaction rather than through policy or regulation
Pilnick, p. 51258
In the present study, we apply this approach to choice around testing and treatment in routine practice in neurology outpatient clinics.
Building on our pilot work
In addition to building on the literature, the present study also builds on the pilot analysis we carried out – published in Epilepsy & Behavior10 and Sociology of Health & Illness47 – on consultations between 13 seizure patients and one epilepsy specialist. We found that the neurologist introduced a range of possible next steps for the patient using two broad approaches: he either recommended a single course of action, which the patient could accept or reject, or he used a practice which we have termed ‘option-listing’, in which he gave the patient a menu of at least two alternatives from which to choose.
Based on this pilot work, we argued that, even when a menu is offered, the choice can be substantially constrained in a range of ways, including how the options are described (e.g. making a case for or against), when the options are provided (e.g. after a discussion in which the neurologist builds a case for or against one of the options) and which options are included (e.g. some may be ruled out explicitly or simply not listed at all). Analysis of even this small data set indicated, therefore, the complexity of putting into practice the apparently simple directive to offer more choice. The presentation of such an offer does not, in itself, ensure that an independent choice is made; and, conversely, the absence of explicit choice does not necessarily mean the clinician has not taken into account the patient’s preferences, which may be built into the design of a recommendation. 10
The present study extends this preliminary work in a number of ways. We examine decision-making in:
-
two geographical settings (Sheffield and Glasgow)
-
a wide range of neurological complaints (not just seizures)
-
a much larger patient sample (n = 223)
-
a more diverse sample of neurologists (14 participants, including women and men with a range of different subspecialties and years of experience; for more information about the sample see Chapter 3).
Through analysis of this substantial data set, we develop our understanding of option-listing as a practice for offering choice and also examine a second practice that was not identified in our pilot work [i.e. the use of patient view elicitors (PVEs): see Chapters 6–8]. But first we turn to a discussion of our methodology.
Chapter 2 Methodology
Introduction
In this chapter we first describe the approach taken to sampling and data collection. We then provide an overview of the methodological approach taken, including an introduction to our core method, CA, and a description of the main stages of analysis undertaken for this study.
Setting and sampling strategy
To identify the strategies clinicians use to offer patients choice, and the interactional consequences of the different strategies, we recorded and analysed actual clinic appointments. The study was conducted in the outpatient departments of two major clinical neuroscience centres (the Southern General Hospital in Glasgow and the Royal Hallamshire Hospital in Sheffield). The recordings were made between February and June 2012 in Glasgow, and between April and September 2012 in Sheffield.
These two sites were chosen for a number of reasons. First, access was facilitated by Professor Reuber (in Sheffield) and Professor Duncan (in Glasgow), both of whom have extensive experience with medical communication research and, at the time, were practising in these clinics (Professor Duncan has since relocated to New Zealand) and so were able to manage recruitment directly. Second, each of these sites serves a diverse population, meaning that patients from a range of different backgrounds participated in the study (see Chapter 3, Data collection sites for further details). Third, each site employs a diverse team of neurologists, with different specialties, ensuring a broad range of consultations (see Chapter 3). Sampling at these sites therefore offered a cost-effective, closely managed approach to capturing the communication strategies used by a range of clinicians with different patient groups.
The eligible sample population was all neurologists (20 in Sheffield and 23 in Glasgow) at the two sites and all patients (aged 16+ years) attending the clinics over a 6-month period, provided they were able to give informed consent in English. Each neurologist typically sees around 10–20 new patients, and 10–20 follow-up patients, per week, meaning a target of 200 recordings (100 per site) with at least 10 clinicians was attainable (these targets were in fact exceeded; see Chapter 3, Recruitment figures for each site for details). Although this is a substantially larger sample size than is common in much qualitative research, similar samples are now commonplace in CA studies of medical interaction. 45,56,60 Larger data sets are desirable for CA research because they provide more instances of the same action (e.g. offering choice), making it easier to identify patterns and deviations from these,61 as we discuss further below.
Recruitment and consent
The two collaborating neurologists were responsible for making initial contact with their colleagues and providing written information sheets describing the study and what involvement would entail. The collaborating neurologists then passed on the details of any potentially interested colleagues to the research assistant at each site, who explained the study face to face and obtained written informed consent from participating colleagues. The nature of the sample was, therefore, partially determined by which neurologists were willing to participate.
A full-time research assistant (henceforth, researcher, for short) was employed at each site for a period of 6 months: Fiona Smith (Glasgow) and Zoe Gallant (Sheffield). These researchers liaised with participating neurologists and, for each upcoming clinic, generated a list of eligible patients. The initial approach to these eligible patients was a letter of invitation to participate, plus patient information sheet, enclosed in the appointment reminder letter from the neurology clinic (sent out 2 weeks before patients’ appointments). The purpose of sending the invitation letter (and information sheet) was to give the patient adequate time to think about the study and (potentially) to ask the research team questions (via e-mail, letter or telephone).
Patients were then subsequently approached in the waiting room for each clinic, prior to the start of the patient’s appointment. When approaching patients, the researchers checked if patients had received the information, asked whether or not they were willing to take part and ensured that patients had a chance to discuss the study prior to the start of the appointment. This initial approach also ensured that the appointment itself could proceed as it normally would (without a consent procedure being undertaken at the start by the neurologist). This not only saved on valuable clinic time, but also allowed the neurologists to follow their usual approach to the appointment. This was important for this study given the aim to investigate what happens in ordinary practice.
The clinical encounters (first and follow-up visits) between consenting patients and neurologists were audio- or video-recorded, depending on participants’ preference. Signed consent was obtained from all participating patients prior to administering questionnaires or making any recordings (see Ethical considerations). Patients and neurologists (and any accompanying others, including spouses, partners, carers and friends) were also asked to indicate whether or not they agreed to clips from the recordings being used in presentations. The researchers operated the recording equipment, but did not attend the consultations. They did, however, remain close by in case the recording equipment malfunctioned or the patient or neurologist asked for the recording to be stopped (in no case did the latter occur).
Pre- and post-appointment questionnaires
The main source of data for this study was the audio- and video-recordings of clinic appointments. In addition, quantifiable information was collected by means of questionnaires, which patients completed immediately before and after the consultation, and neurologists completed after (see Appendices 2–7). The researchers administered these. The pre-appointment questionnaires (for patients) captured:
-
demographic details (see Appendix 4)
-
the patient’s agenda (‘Please list your reasons for seeing the doctor today, including the problems you want to talk about and any tests or treatments you hope to receive’; see Appendix 2).
The post-appointment questionnaire captured patients’ impression about whether or not they were given a choice using a short series of questions devised for this study (e.g. were they given a choice; do they think the clinician had a preference; how do they feel about whatever is going to happen next; did they think the clinician had a preference) (see Appendix 3). The clinicians’ questionnaire asked about the diagnosis and any decisions reached (e.g. did the clinician have a preference; did they think the patient had one; do they think the clinician had a preference; how do they feel about whatever is going to happen next;). Clinicians’ levels of diagnostic certainty were self-reported on a scale from 1 for ‘very uncertain’ to 10 for ‘very certain’ (see Appendix 5).
In the data analysis section below, we detail how these self-report data were analysed alongside the recordings. It should be noted that the pre-appointment questionnaire (see Appendix 2) responses were, mostly, not detailed enough to be useful (patients tended not to provide much detail about their agenda prior to the appointment).
In addition, we took the opportunity to collect follow-up patient satisfaction data using the Medical Interview Satisfaction Scale 21(MISS-21), which has been validated in UK patient populations,62 and used in previous CA research to measure patient satisfaction in primary care63 (see Appendix 7).
These data were collected as a pilot for a potential follow-up, quantitative evaluation of the interactional strategies identified in the present study. This is an increasingly common approach when applying CA to medical interactions (i.e. the foundational, fine-grained, qualitative analysis is done first, followed by various statistical measures of evaluation). 61 The purpose of the pilot was to assess the suitability of the above questionnaires for encounters in secondary care, and to provide information for group size calculations for future quantitative or mixed methods studies (see Chapter 9). We also carried out some exploratory analysis on the MISS-21 data within the present study (see Quantitative analysis).
Ethical considerations
The main ethical issue for this study was to ensure that patients and clinicians were able to make an informed choice about participation. As noted above, study information was sent to patients (through the clinics) at least 2 weeks in advance, with an invitation to contact the team with questions. On the day of the patient’s appointment, a researcher with experience of recruiting in the NHS explained the study further (if patients were willing to consider participating) and answered any questions. Clinicians were approached at the start of the study by the two neurologists on the research team, who provided an information sheet. However, to ensure that they did not feel under pressure to participate by virtue of being approached by colleagues, they were subsequently recruited by the researchers, who provided a verbal explanation of the study and answered questions. All participants were assured that they could refuse to participate without any repercussions, and, if they decided to take part, were able to choose between video- and audio-recording. Participants could also ask for the recording to be stopped at any time during the consultation. All who agreed to participate (patients and clinicians) completed a written consent form, which guaranteed the confidentiality of their data.
A further important ethical consideration was the safe storage of all data in accordance with the Data Protection Act (1998). 64 Code numbers (for labelling questionnaires and recordings) and pseudonyms (in transcripts) have been used to ensure that all personal information collected for the study is kept strictly confidential. All data are stored on secure, password-protected servers, encrypted hard drives and/or in locked filing cabinets, as appropriate.
Ethics approval was granted by the National Research Ethics Service Committee for Yorkshire & the Humber (South Yorkshire) on 11 October 2011, following revisions to supporting documentation requested following the Research Ethics Committee meeting held on 29 September 2011 and attended by Dr Toerien on behalf of the research team.
Analytic approach
The core analytic approach for this project was one that allowed for the identification of those interactional practices already being used by neurologists to offer patients choice about their treatment and/or further investigations. The methodology through which we analysed the recorded consultations is known as CA. Because of the specialist nature of this approach, we include, below, an introduction to CA and a description of the main ways in which we applied it. In addition, as we also describe below, we carried out some quantitative analysis of our questionnaire data and sought to link some of these findings with our analysis of the recordings.
An introduction to conversation analysis
As we have also noted elsewhere, CA is a qualitative, microanalytic, systematic method for studying real-life interaction. 65 It is widely recognised as the leading methodology for investigating how doctor–patient communication operates in practice. 66,67 It uses audio- and video-recordings of authentic interactions to enable direct observation and fine-grained analysis, focusing not only on what is said but how it is said (e.g. the exact words used, and evidence of hesitation, emphasis, interruptions, laughter or misunderstanding). Its key advantages are that it does not rely on recall – which can often be incomplete or inaccurate68 – and it investigates how people behave at a level of detail that they could not be expected to articulate (e.g. in a research interview). Although CA originated through the study of everyday interaction, this approach has been applied to numerous institutional settings, including (among others) medical interactions,67 psychotherapy69 beauty therapy sessions,70 helpline calls,71 interviews with state benefits claimants72 and courtroom interactions. 73
In everyday interactions, we accomplish a wide range of social actions with others. We make and accept or reject offers, we agree or disagree with one another, we make suggestions or invitations, we announce news, we apologise, complain and account for our actions, and so forth. In neurology outpatient appointments, neurologists are, similarly, engaged in a range of context-specific activities with their patients, such as history-taking, diagnostic questioning, delivering diagnoses and discussing treatment and investigation options. It is the accomplishment of such activities – specifically, how they are done and how certain approaches appear to influence the ensuing interaction – that forms the focus for a CA study of institutional interaction. The aim is not to uncover people’s hidden meaning (conceptualised as lying beneath their words) or to interpret their underlying intentions, feelings or desires. 66 Rather, CA focuses on participants’ objectively observable, empirical conduct, based on detailed analysis of actual interactions. It offers advantages, therefore, in expanding our knowledge and understanding of decision-making in the context of neurology outpatient services, by providing detailed information about what actually takes place, as a basis for future effective practice recommendations.
Importantly, CA enables us to focus on the patients as much as the neurologists, because:
CA begins from the presumption that physicians and patients, with various levels of mutual understanding, conflict, cooperation, authority, and subordination, jointly construct the medical visit
Heritage and Maynard, p. 36361
As we have argued elsewhere,65 the key advantages of a CA approach may be summarised as follows:
-
It does not rely on patients’ or neurologists’ recall, which may be incomplete or inaccurate.
-
It is less susceptible to ‘socially desirable’ reframing according to what people think they should say.
-
It investigates directly how people talk, in a level of detail that the speakers are unlikely to be aware of and could not possibly recall.
-
It allows us to explicate and give voice to patients’ orientations as well as those of clinicians.
For general introductions to CA, see Drew,74 Sidnell75 and Toerien. 76 For introductions to CA as applied to interactions in institutional settings, see Antaki,77 Drew and Heritage78 and Heritage and Clayman. 79
Analytic methods
In this study, all recordings were transcribed verbatim (and anonymised) by a professional transcriber. All questionnaire data were entered into Excel (version 14.4.4.; Microsoft corporation, Redmond, WA, USA) spreadsheets by the researchers at each site and checked/cleaned by members of the research team. Thereafter, the research team undertook both quantitative and qualitative analyses using IBM Statistical Product and Service Solutions (SPSS; Version 20; IBM Corporation, Armonk, NY, USA).
Quantitative analysis
First, the responses from patient and clinician to the following post-appointment questions were summarised and compared, asking of clinicians ‘Did you give the patient a choice about treatment or further management?’ and of patients ‘Did the doctor give you a choice about any tests or treatment you might have or the next step in the management of your condition?’ (see Chapter 4). This gives an indication of the perception of choice for the recorded consultations and the degree of agreement/disagreement about this across our sample.
Patients had also completed the MISS-21 questionnaire after their appointment with the clinician. For the purpose of our exploration of MISS-21 findings, missing values were replaced by median replacement if less than 10% of responses were missing from a particular patient. Replies with 10% or more of missing data were discounted. Total MISS-21 scores were calculated and analysed and examined.
In our exploration of the data we first compared the data sets collected in the two study centres. Bivariate analyses were conducted using chi-squared statistics for categorical variables and two-tailed t-tests for continuous data (after Levene’s test had revealed equality of variance). Two-tailed p-values of < 0.05 were considered statistically significant. In view of the exploratory character of these statistical analyses, no procedures were undertaken to reduce the risk of type 2 errors.
In the statistical examination of associations of perceptions of choice, we initially carried out univariate tests of differences between categorical variables using chi-squared statistics, and of differences between the means of normally distributed continuous data (age, MISS-21 score) using two-tailed t-tests. If differences between grouping variables were found on univariate tests, forward logistic regression analysis was carried out to determine the predictive value of the findings in the univariate analyses.
Qualitative analysis
Initially, all recordings were viewed/listened to repeatedly in conjunction with their transcripts. The goal was to identify all instances of the interactional activity of making a decision about treatment and/or investigations. These decision points were transcribed in considerable detail, to show not only what was said but how it was said, using symbols to represent features of both the timing of speech and the manner of speaking. For example, transcriptions show overlapping speech between speakers, pauses, and aspects of intonation and prosody (for explanation of transcription symbols, see Appendix 1).
As a means of comparing patient and neurologist reports of choice in the encounter with what took place in the recordings, we also divided the recordings into four subsets based on the self-report findings (those for which participants agreed either that a choice or no choice had been offered, and those for which either patient or neurologist thought a choice had been offered when the other did not). The aim was to discover, inductively, what was hearable in the recordings – for participants – as choice. It was intended to be exploratory and to suggest directions for the central analysis, which followed the standard methodology of CA. That is, as we discussed in Chapter 1, we did not want to approach the data set with preconceptions about what patient choice might look like on the basis of one theory or another. Rather, we analysed, in depth, those consultations for which participants agreed that there was/was not choice, for evidence of what was being heard in this way. In addition, we examined points of disagreement, again to see if this could help us define, inductively, what counts (for participants themselves) as choice. Consultations with missing self-report data were also examined to see if there was anything unusual about them. As we could not see anything that distinguished them as a group, they were excluded from the analysis on the grounds that we were not sure which subset they fell within.
On the basis of the above exploration of the recordings, we built collections of practices that were demonstrably being used by the neurologists to offer patients choice. The most substantive phase of analysis entailed close examination of these practices (and patients’ responses to them) using the techniques of CA. The analysis was systematic (not selective), incorporating all cases of each practice rather than just the most striking ones. Focusing on details of the talk, we looked for patterns of similarity and difference (e.g. in words or phrases used) in the design of neurologists’ turns. A core aim was to see whether or not some practices were more effective at engaging patients in decision-making. This is a complex question, but patient responses provide an important internal indicator of effectiveness. By internal, we mean ‘within the interaction itself’65 – such as forms of patient resistance or alignment with the neurologist’s prior turn or displays of (mis)understanding – as opposed to some kind of correlational assessment of external outcome measures (such as questionnaire scores for patient satisfaction, measures of adherence to treatments or measures of health outcomes following the consultation).
Internal measures are important for two reasons. First, they are measures of the direct, immediate consequences of the clinician’s approach to decision-making – they tell us precisely what happened next in response to practice x compared with practice y. If there are significant patterns to these consequences – such as one approach routinely shutting down the decision-making process compared with another routinely engaging the patient – then we gain strong evidence for the likely effect of using these practices in the future. By contrast, outcome measures are indirect ways of assessing effectiveness. For example, a measure of adherence can allow us to infer the role and significance of the consultation in whether or not the patient went on to take the prescribed treatment, but that consequence will always be at one remove from the exact approach used by the neurologist to initiate decision-making. Moreover, numerous other factors are likely to have been relevant to the patient’s subsequent level of adherence.
Second, clinicians only have direct control over what they do in the consultation itself – not what happens in patients’ lives afterwards. For example, they can influence, but not directly control whether or not the patient goes on to take the prescribed medication. By contrast, if it turns out that practice x routinely blocks patient engagement in decision-making, clinicians can choose to stop using that practice. In so doing, they may be able to directly affect the process of decision-making as it unfolds in future consultations. So, by scrutinising the internal consequences of neurologists’ talk, we sought to provide insights upon which clinicians can act directly.
Patient and public involvement
We set up a service users’ group (SUG) comprising five users of neurological services, recruited through the Royal Hallamshire Hospital in Sheffield (four patients and the husband of one patient). The group met before recruitment for the study commenced and during data collection and analysis periods in order to facilitate engagement with every stage of the research process. In addition, a service user representative took part in the study’s Steering Committee meetings. The service users have been engaged with the study in the following ways:
-
Informing the drafting of the participant invitation letter, information leaflets and consent forms. Concrete changes that occurred as a direct result of discussion with the SUG included substantive amendments to the invitation letter to make it less formal and more explicit about why the study matters.
-
Informing the design of the open-ended questions for the pre- and post-appointment questionnaires. Again, we made concrete changes to our questionnaires on the basis of service user feedback. For example, a service user noted that our list of options relating to educational qualifications on the demographic questionnaire did not include professional qualifications; these were added.
-
Informing our study aims. Discussion of our work in progress with service users – and also with our Steering Committee (which included a service user) – has highlighted an important issue that the language we were using to talk about patient choice (including in our initial grant application) tended to imply that we thought choice was always better than no choice. The service users called this assumption into question. We therefore amended our study aims accordingly (see aims 1 and 3 in Chapter 1). This has also impacted our thinking about choice more broadly throughout our analysis.
-
Providing feedback on the acceptability of the recruitment approach. The group confirmed that they would be happy to be approached regarding study participation in the manner described above.
-
Feeding into the analytic process by reading and commenting on transcripts and work in progress by the study team. Discussion of our work in progress has significantly contributed to our understanding of service user perspectives on decision-making in neurology. For example, lively debates about what constitutes choice and (often poignant) anecdotes about service users’ experiences of their health conditions and engagement with health services have had two kinds of impact on the study: they have helped redirect our thinking about particular data extracts; and, more broadly, have served as powerful reminders of the very real consequences for patients of their interactions with clinicians.
-
Facilitating access to patient groups for presentation of our work in progress. The service users have advised on, and enabled access to, wider patient groups. For example, they have helped arrange opportunities for us to speak at Epilepsy Action meetings in Sheffield, Scarborough and Harrogate and at a Parkinson’s UK meeting in Sheffield.
We are currently in the process of arranging two dissemination workshops on the basis of our findings. Two service users have volunteered to assist with the delivery of these. They are also involved in the ongoing task of commenting on the clarity and accessibility of all publications (including plain English summaries) arising from this research.
Structure of the results sections of this report
The remainder of the report is structured as follows: we begin with an overview of the study data set, including our recruitment figures and sample characteristics (see Chapter 3). We then present a quantitative analysis of the questionnaire data pertaining to choice (see Chapter 4). We also provide a brief overview of the ways in which neurologists in our data set constructed the patient’s condition as involving no choice (see Chapter 5). In Chapters 6 and 7 we analyse option-listing – the only practice demonstrably oriented to offering choice that was only evident (with one exception, which, as we show, proves the rule) in the subset of recordings in which neurologists and patients agreed a choice had been offered. In Chapter 8, we consider two alternative practices for offering choice, this time focused on single-option decisions. Chapter 9 discusses the implications of our findings and our recommendations for future research.
Throughout the report, findings are illustrated with extracts from the transcribed recordings. Some of these extracts may seem rather long. However, we ask the reader to bear with this as it partly reflects the nature of our phenomenon (e.g. option-listing involves information provision and, typically, rather extended discussion between neurologist and patient) and partly reflects the nature of our methodology – its key advantage is the fine-grained analysis of what really happened rather than summaries thereof. We hope that readers will avoid the temptation to skip over the extracts and will see the benefits of the transparency that they allow with respect to evaluating our analysis.
Each transcript is numbered consecutively to make it easier to refer to consultations shown in different chapters. The transcript labels include the following information: the study site (‘G’ for Glasgow, ‘S’ for Sheffield); a three-digit patient number; a two-digit clinician number and the diagnosis that the neurologist reported (in the post-consultation questionnaire) as most likely.
Chapter 3 Overview of the study data set
Introduction
The aim of this chapter is to describe the data set on which this study is based. We start by describing the two data collection sites, then present our recruitment figures, and finally map out the nature of the sample with respect to factors such as the type of appointments recorded and a range of participant demographic variables.
Data collection sites
All data were collected at the outpatient departments of two major clinical neuroscience centres (one in England and one in Scotland). The Royal Hallamshire Hospital in Sheffield, South Yorkshire, is part of Sheffield Teaching Hospitals NHS Foundation Trust and has approximately 800 beds, of which 50 are sited within the neurology department. The Southern General Hospital in Glasgow, Lanarkshire, is a large teaching hospital with an acute operational bed complement of approximately 900 beds. The hospital is sited in the south-west of Glasgow and provides a comprehensive range of acute and related clinical services. The Royal Hallamshire Hospital serves as the clinical neuroscience hub to a population of 2.2 million. The general population served by the Southern General in Glasgow is 2.5 million.
Each site employs a diverse team of consultant neurologists (20 in England and 23 in Scotland) and offers general neurology as well as a range of subspecialty clinics (such as epilepsy, MS, dementia, ataxia, headache, cerebrovascular disease, neuromuscular disorders and movement disorders) ensuring a broad range of consultation types. New patient appointments are usually scheduled to last 30–45 minutes and follow-up appointments 15–20 minutes.
Recruitment figures for each site
Fourteen clinicians (seven in Glasgow and seven in Sheffield) agreed for recordings to be made in their clinics subject to patient consent. As detailed in Chapter 2, the eligible patient population included all patients (aged 16+ years) attending clinics run by the participating clinicians, provided they were able to give informed consent in English. In total, 223 patients agreed to take part (114 in Glasgow and 109 in Sheffield). One appointment per patient was captured. In addition to patients and clinicians, 120 accompanying others (including spouses, parents, carers and friends) consented to contribute to the study (63 in Glasgow and 51 in Sheffield).
Figures 1 and 2 describe the data collection process at Glasgow and Sheffield, respectively. Patients who attended on the day of the clinic (there were a number of non-attenders at each site) were approached in the waiting room, prior to the start of the patient’s neurology consultation. If patients agreed to participate, signed consent was obtained (see Chapter 2 for more information about our approach to recruitment).
FIGURE 1.
Recruitment of participants at the Glasgow site.
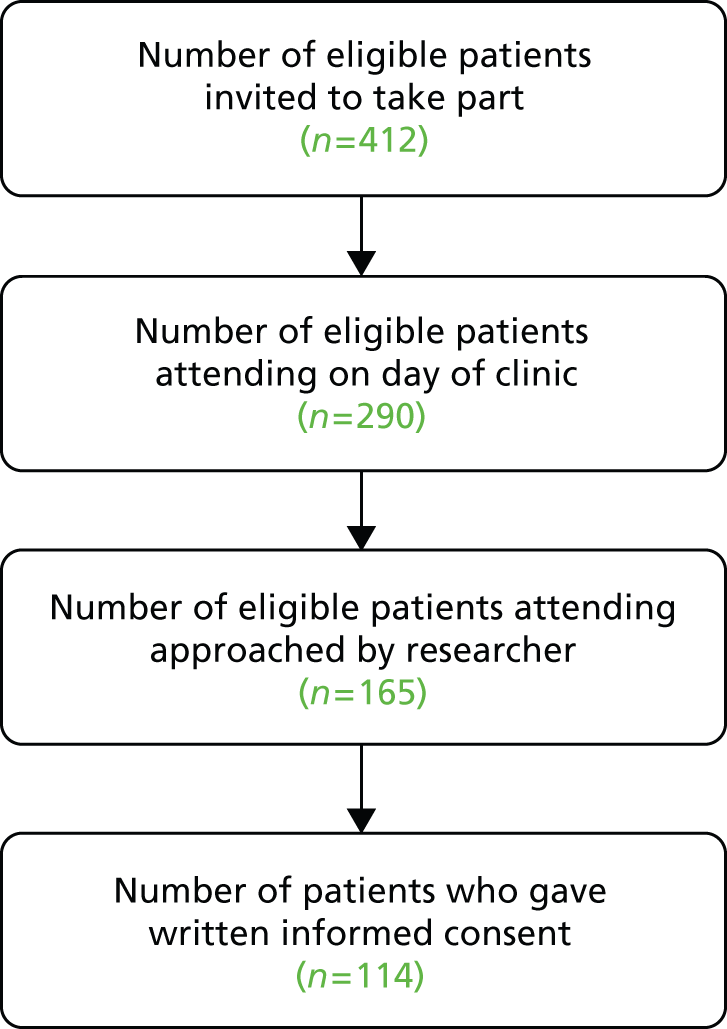
FIGURE 2.
Recruitment of participants at the Sheffield site.
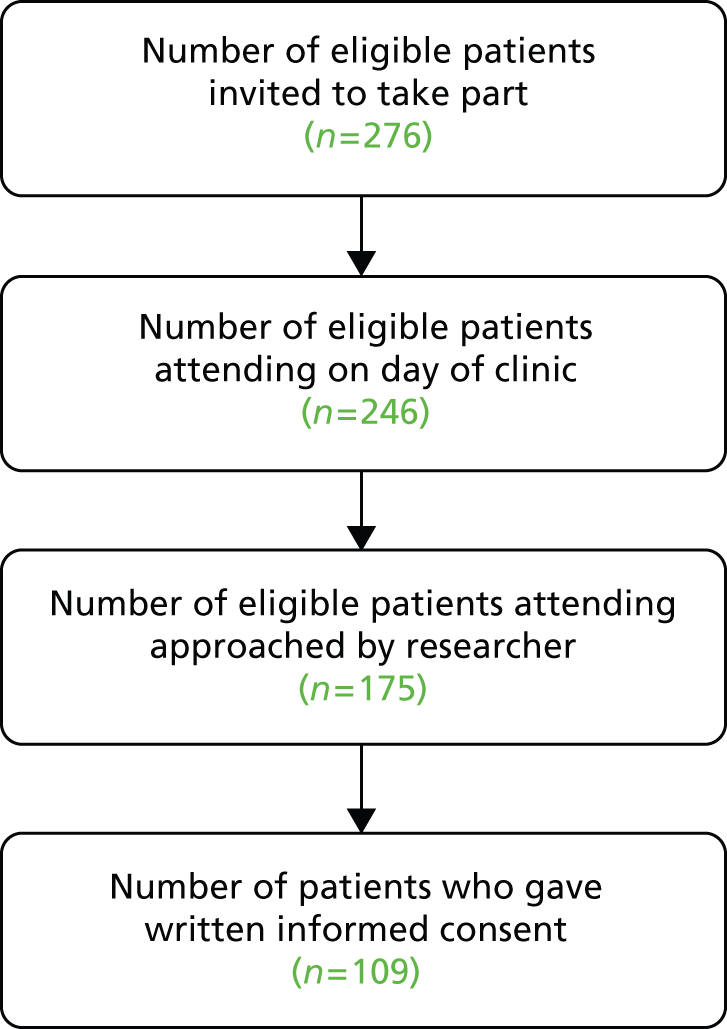
In total, 69% of those approached to consent by a researcher in Glasgow agreed to take part and 62% of those approached in Sheffield agreed to take part – an overall response rate of 66%. It should be noted that researchers were not able to approach all patients attending, as researchers had competing demands on their time (e.g. they were handling the consent procedure with another patient, setting up recording equipment or administering questionnaires).
Participant demographics
The overall sample of patients ranged in age from 17 years to 80 years, with a mean age of 46 years. More than half the patients recruited were female (60%). Ninety-three per cent described themselves as white (English, Welsh, Scottish, Northern Irish or British). Forty-two per cent of the sample was in employment (full- or part-time) or education. Educational qualifications beyond school leaving age (16 years) had not been obtained by 40.5%, while 40% had received a high school or university education. The neurologist sample ranged in age from 31 years to 57 years, with a mean age of 43 years. The neurologists consisted of eight men and six women, 12 consultants and two registrars. Seventy-nine per cent of the neurologists described themselves as white. Table 1 shows the demographic features of the study sample in more detail, including a breakdown per study site. More Glasgow patients were seen in general neurology clinics than Sheffield patients. Glasgow patients were a little older and more likely to be out of work or on sick leave because of their illness or disability.
| Feature | Sheffield (109 patients, seven doctors) | Glasgow (114 patients, seven doctors) | Difference: Sheffield vs. Glasgow | Combined (223 patients, 14 doctors) |
|---|---|---|---|---|
| Consultations | ||||
| Percentage accompanied | 46.8 | 52.6 | N/S | 49.8 |
| Percentage of first appointments | 27 | 32 | N/S | 29 |
| Percentage seen in general clinics | 2.8 | 29.8 | p < 0.0001 | 16.7 |
| Patients | ||||
| Patient age (years) | 49.1 (SD 15.2, range 17–80) | 43.7 (SD 14.9, range 17–80) | p = 0.005 | 46.3 (SD 15.3, range 17–80) |
| Percentage male patients | 46.2 | 34.2 | N/S | 40.1 |
| Percentage white (English, Welsh, Scottish, NI or British) | 89 | 96.5 | N/S | 92.8 |
| Percentage without post-school qualifications | 39.2 | 42.1 | N/S | 40.5 |
| Percentage with university qualification | 35.3 | 25.5 | N/S | 29.9 |
| Percentage in work/education | 45.9 | 37.7 | N/S | 41.7 |
| Percentage on leave/out of work because of sickness/disability | 33.6 | 16.7 | p = 0.005 | 25.3 |
| MISS–21 scores | 101.0 (SD 9.2) | 95.0 (SD 11.9) | N/S | 98.0 (SD 11.0) |
| Neurologists | ||||
| Neurologist age (years) | 43.7 (SD 5.3, range 37–53) | 41.4 (SD 9.86, range 31–57) | N/S | 42.6 (SD 7.7, range 31–57) |
| Percentage male neurologists | 57.1 | 57.1 | N/S | 57.1 |
| Percentage white (English, Welsh, NI or British) | 100 | 57.1 | N/S | 78.6 |
| Level of diagnostic certainty (1 – uncertain to 10 – certain) | 8.3 (SD 1.9) | 8.7 (SD 1.7) | N/S | 8.5 (SD 1.8) |
Type of appointment and type of clinic
There were more than twice as many follow-up appointments as first appointments (as recorded in the clinicians’ post-appointment questionnaires), in both Glasgow and Sheffield (see Table 1). Over 80% of patients were recruited in different subspecialties rather than general neurology clinics (in Glasgow, 80 out of 114 patients attended subspecialty clinics and, in Sheffield, 106 out of 109 patients attended subspecialty clinics – information is missing on one appointment) (Figure 3). It should be noted that this does not necessarily reflect the pattern of attendance at the two outpatient departments more broadly. It is likely to be a consequence of which neurologists agreed to take part and the timetabling of clinics.
FIGURE 3.
Type of clinic. Other includes a sleep clinic, neuro-oncology, movement disorder, memory and transient ischaemic attack.
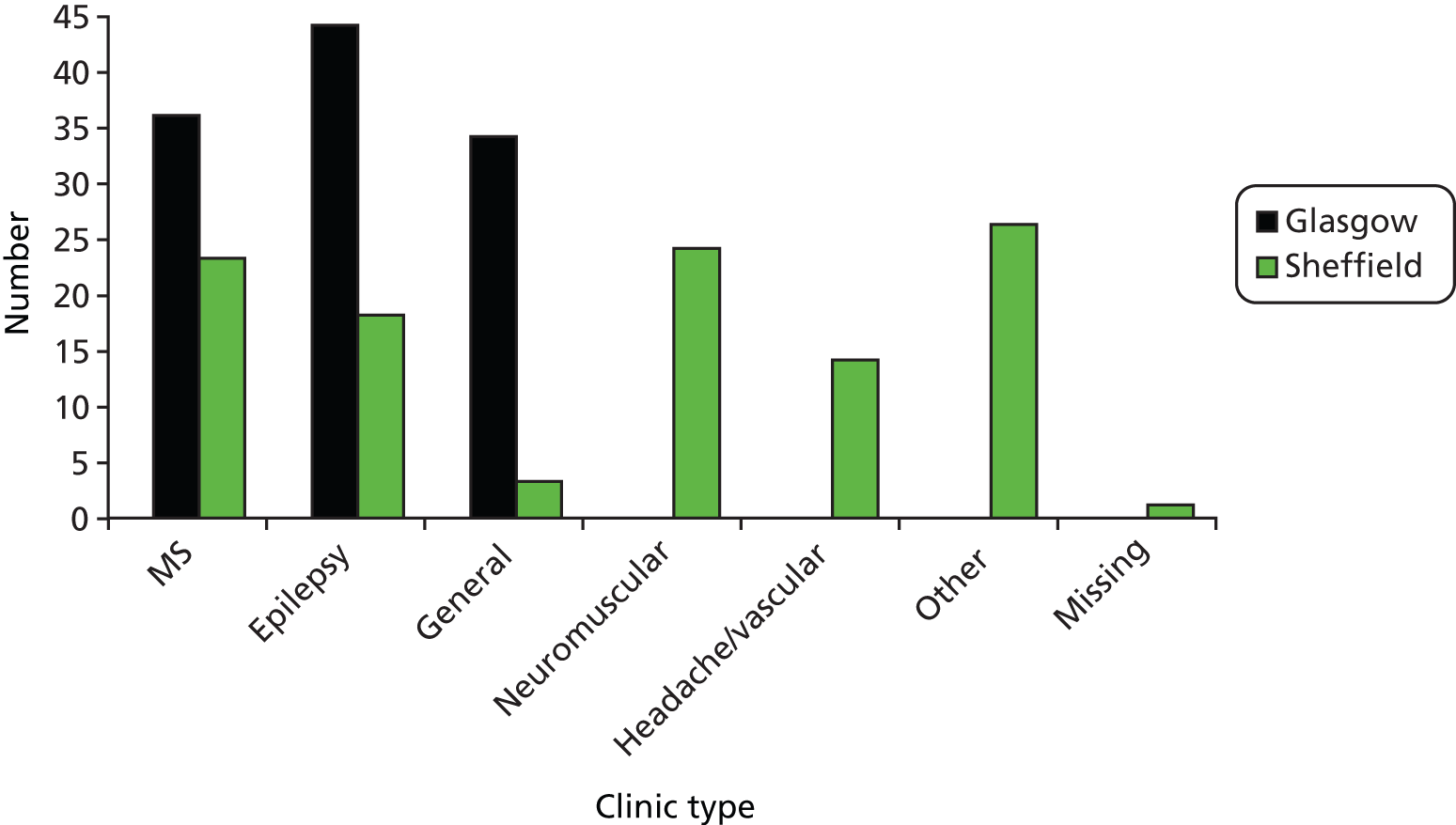
Conclusion
In summary, we did not identify any differences in the demographics or clinical differences between the participants recruited in Glasgow or Sheffield that would suggest that the Sheffield and Glasgow data could not be combined to one larger data set and analysed together. To the neurologists in the research team, the patient (and clinician) mix of participants recruited to this study seems representative of the populations from which the patients and neurologists were drawn.
Chapter 4 Perceptions of choice
Introduction
This chapter presents our (quantitative) analysis of the questionnaire data pertaining to choice. The aim is twofold, first to allow linking – in subsequent chapters – of the self-report data (i.e. perceptions of choice) with participants’ orientations to, and constructions of, choice in the recorded consultations. Second, to start to address objective 1 as outlined in Chapter 1 [i.e. to contribute to the evidence-base about whether or not (or the extent to which) patient choice is implemented in our data set].
Quantitative exploration of prevalence of choice
The findings reported in this chapter derive from responses to the post-appointment questionnaires. In these, clinicians were asked ‘Did you give the patient a choice about treatment or further management?’ and patients were asked ‘Did the doctor give you a choice about any tests or treatment you might have or the next step in the management of your condition?’. Patients also completed the MISS-21. Having concluded in our initial examination of the available quantitative data that there were no meaningful systematic differences, the Glasgow and Sheffield data sets were combined for all subsequent analyses.
In answer to the questions listed above, 67.9% of neurologists answering this question immediately after their encounter with the patient (n = 215) stated that they had offered choice and 71.8% of patients responding to the corresponding question (n = 202) said that a choice had been offered. Neurologists and patients agreed there had been a choice in a total of 53.6% of recorded consultations (105/196; there are missing data for 27 consultations where pre- and/or post-appointment questionnaires were not completed by the neurologist and/or patient and, of these, 13 were from Sheffield and 14 from Glasgow).
Patients without any post-school educational qualifications were less likely to agree with the neurologists that choice had been offered than patients with these qualifications (43.9% vs. 61.2%; p = 0.038, χ2 = 4.748), but higher educational achievements (completion of a university qualification) was not associated with even higher levels of agreement. Encounters after which clinicians and patients agreed that choice had featured did not differ significantly from other encounters in terms of study site (Glasgow or Sheffield), whether patients had attended a general or specialist neurology clinic, a new or follow-up appointment, or were accompanied. There were no differences in terms of patient sex, ethnicity (white or non-white) or whether patients were not working because of illness or disability.
Patients did not report higher satisfaction after encounters in which they and the clinician agreed that choice had been offered {MISS-21 scores 98.0 [standard deviation (SD) 10.9] vs. 98.3 [SD 11.0]; difference not significant}.
After 14.3% of clinic encounters (28/196), patients and clinicians agreed that no choice had been offered. In univariate statistical tests, encounters after which clinicians and patients agreed about the absence of choice did not differ from others in terms of study site, clinic type, appointment type, whether or not patients were accompanied, patient sex, ethnicity, educational achievements or whether patients were unable to work because of their medical condition.
After 31.9% of consultations patients and clinicians disagreed whether or not choice had featured – either patient or clinician thought there was choice (63/196). See Figure 4 for a summary of these findings.
FIGURE 4.
Agreement and disagreement about presence of choice.
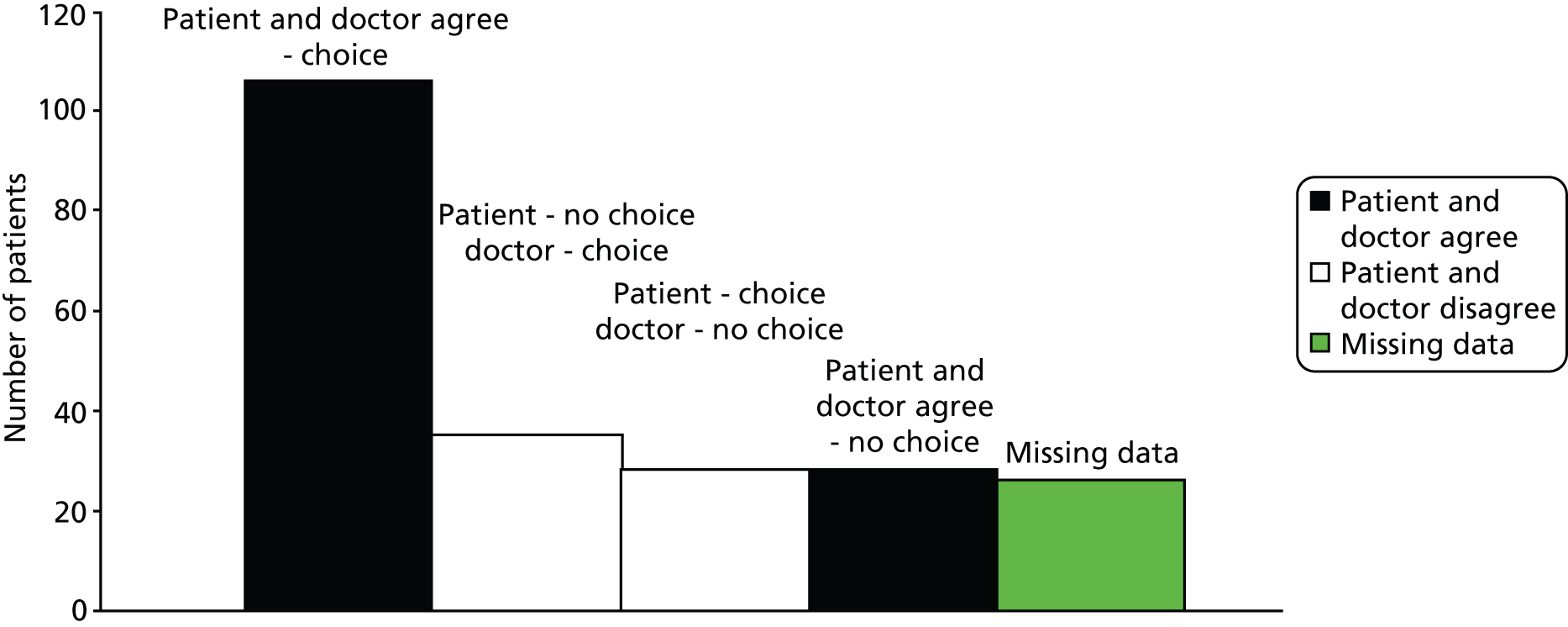
Patients’ perception of whether or not choice was offered
Univariate statistics revealed that patients were more likely to perceive that choice had been offered if they had attended a general neurology clinic appointment rather than a specialist clinic appointment (p = 0.004, χ2 = 9.895). There were no significant relationships between patients’ perception of whether or not choice had been offered and study site, appointment type, whether or not patients were accompanied during the encounter, the duration of the consultation, the clinician’s level of certainty about the diagnosis, patient or clinician sex, patient age, ethnicity, educational achievements and whether they were in employment or not working because of illness or disability. Only general neurology clinic attendance contributed to a logistic regression model of patients’ perception of choice (model significance p < 0.001, Wald 36.771). This model classified 74.7% of cases correctly.
Patients did not report higher levels of satisfaction with the medical interview if they thought that they had been given choice [MISS-21 score 98.6 (SD 11.0) when choice was perceived, MISS-21 score 97.0 (SD 10.4) when choice was not thought to have been given].
Neurologists’ perception of whether or not choice was offered
Univariate statistics revealed that clinicians were significantly more likely to offer choice if they had a higher level of diagnostic certainty [certainty level 8.7 (SD 1.5) vs. 8.0 (SD 2.2); p = 0.006, t = 2.782]. Whether or not clinicians reported that they had offered the patient choice was not significantly related to the type of clinic or appointment attended; consultation duration; whether or not patients were accompanied; patient or clinician sex; patient age, ethnicity, education and whether patients were in employment or not working because of illness or disability.
Patients did not report higher levels of satisfaction with medical interviews after which clinicians reported having given choice [MISS-21 score 98.2 (SD 10.3) when choice had reportedly been offered, MISS-21 score 97.6 (SD 12.7) when choice had not been offered].
Conclusion
Choice was perceived in well over half of the recorded consultations. Neurologists and patients agreed on whether or not choice had been present in over two-thirds of their encounters. Patients with no educational achievements beyond basic school qualifications were less likely than those with higher attainments to agree with neurologists’ reports that choice had been offered. Choice seems to have been offered more often in general than specialist neurology clinics. The reason for this is uncertain. Choice was also more likely to have been offered when neurologists were more certain of the diagnosis, perhaps suggesting that the diagnostic process is more clinician directed, and that clinicians are more likely to involve the patient in decision-making processes when the diagnosis has been made.
We did not identify any significant association between choice and patient-reported satisfaction with the consultation they had just experienced.
Chapter 5 When participants agree that choice is absent
Introduction
The data shown in Chapter 4 (see Figure 4) can be usefully depicted as a two-by-two table, in which two quadrants reflect agreement between neurologist and patient and two reflect disagreement (see Table 2). We will use this as shorthand for linking the self-reported data with our analysis of the recordings. Thus, recordings in subset 1 are those for which there was agreement that a choice has been offered to the patient and so forth, as shown in Table 2.
| Neurologist: choice | Neurologist: no choice | |
| Patient: choice | Subset 1. Agree (n = 105) | Subset 2. Disagree (n = 35) |
| Patient: no choice | Subset 3. Disagree (n = 28) | Subset 4. Agree (n = 28) |
In Chapter 4, we showed that patients and neurologists agreed that choice had featured in over half of all encounters and that participants were almost four times as likely to agree that there was a choice offered as to agree that there was not. The 105 instances in which neurologist and patient agreed that there was choice form the bedrock of this report. However, in order to identify any patterns that might distinguish clear choice cases from the rest, we also examined the full data set, including the 28 consultations for which neurologist and patient agreed that no choice was offered. In this chapter, we provide a brief overview of these, focusing on the ways in which the neurologists constructed the patient’s condition as involving no choice. This analysis serves two purposes: (1) to further address objective 1 – to contribute to the evidence-base about whether or not, and how, patient choice is implemented – by considering the possible limits of choice in these consultations; and (2) to foreground the need to keep an open mind about whether or not choice is always better than its absence. Given the emphasis on choice both within NHS policy and as a cultural value in the Western world, we believe this to be an important counterweight to help avoid any premature normative conclusions about best practice.
Logical consequence rather than a decision to be made
One clear distinction between the choice and no-choice subsets was that, in the latter, the neurologists treated their diagnostic conclusions as leading to a given next step for the patient. In the majority of cases, this next step entailed doing nothing, doing nothing neurological or doing nothing new. In other words, what was to happen next was constructed, not as a matter to be decided, but as a logical consequence of the diagnosis (or, in cases where a diagnosis had not yet been reached, of the diagnostic conclusions thus far). The no-choice set is largely characterised by five types of logical consequence, namely:
-
no treatment required because there is no medical problem
-
nothing this specialty can offer the patient because there’s no neurological problem
-
nothing to be done, although there is a neurological problem, because no treatment exists or because treatment is not part of the protocol for this stage of the condition
-
nothing to be done, although there is a neurological problem, because possibilities have been exhausted
-
nothing (different) to be done because no change to the current treatment regime is required (i.e. ‘continue as you are’).
Each of these consequences is overviewed in turn.
No medical problem
In a number of consultations the patient was not offered a choice about treatment and/or further investigations because the neurologist’s diagnosis was that there was no medical problem as such. The absence of choice was constructed, then, as a logical consequence of a no-problem diagnosis. For example, in Extract 1 the neurologist names the condition (line 1), provides information in a form which emphasises the non-seriousness of fainting (lines 3–6, 10 and 11), and proposes no treatment (line 9). Transcription conventions are given in Appendix 1.

Neu, neurologist; Pat, patient.
The construction here of no choice is predicated on a clear diagnosis, a straight assertion as described by Peräkylä,80 but built on what the patient himself has reported earlier in the consultation (data not shown). In response, the patient (unlike patients identified in earlier literature,81 who were found to be silent or minimally responsive to diagnostic announcements) explicitly agrees with this conclusion (line 2). The neurologist goes on to state (in generalised terms) that ‘this isn’t something you would treat’ (line 9), thereby building the absence of any treatment choice for the patient as a logical consequence of the diagnosis – this would be the case for any person presenting with the same complaint. By describing the complaint as non-serious and fairly common, he also provides a rationale for non-treatment. In other cases, the neurologist also offers advice on how best to deal with symptoms in a non-medical way, but the pattern is otherwise the same (i.e. the construction of no choice with respect to treatment is built off the neurologist’s diagnostic findings).
No neurological problem
In a number of consultations there is a problem diagnosis, but the problem is one that is outwith the remit of neurology. In these instances, the neurologist accounts for the lack of a neurological finding (e.g. ‘all the tests have been negative’) and provides information about possible next steps, in terms of seeking medical help outside of neurology (e.g. ‘there’s nothing we can do, but we can refer you to . . . ’), as displayed in Extract 2.

Neu, neurologist; Pat, patient.
Again, then, the next step is constructed not as a matter for the patient to decide, but as a logical next step given the neurological findings (again, a no-problem diagnosis from a neurological perspective) – a conclusion with which the patient aligns.
Neurological problem, but no treatment required
In some cases, a clear neurological problem is reported, but the neurologist explains that treatment is not part of the protocol at this stage of the condition. For example, patients presenting with a first episode of MS are routinely advised that ‘after a single event we wouldn’t put you on any long-term treatment’. In Extract 3, for example, the patient has presented with ongoing symptoms of tiredness, sensory sensations and headaches, and the neurologist’s diagnosis is that this is a single one-off episode of MS with newer symptoms possibly being the after-effects of the single flare-up. The neurologist’s recommendation is to ‘let it settle’ and in response the patient aligns (minimally).

Neu, neurologist; Pat, patient.
Here, then, the absence of a choice is presented as the logical consequence of being at this point in the disease trajectory. Note also that the neurologist sends the patient for a test and that this, too, is constructed as a foregone conclusion rather than a matter for the patient to decide (see Chapter 9, Option-listing: a practice for tempering doctors’ epistemic and deontic authority and Chapter 6, Component 1: constructing the decision as yet to be made and Component 3: producing a slot for the patient to announce a view on, or selection from, the listed options for further discussion of the construction of recommendations in our data set).
Neurological problem, but exhausted possibilities
In other consultations, there is a clear neurological problem reported, but no (further) treatment is available for the condition. For example, in Extract 4, the neurologist explains that there is ‘not much we can do to prevent the progression’ (of spinocerebellar ataxia type 8). He offers to maintain the current regimen of routine follow-ups, ‘just to keep an eye on things’, but otherwise the proposed plan of action is to ‘unfortunately leave things be’. The patient responds, again, with minimal alignment.

Neu, neurologist; Pat, patient.
Again, then, this is constructed as a no-choice situation as a consequence of the diagnostic conclusions reached.
Continue as you are
The final category of next steps identified occur in follow-up appointments with patients who have a clear diagnosis and are already on a treatment pathway. In these appointments, the neurologist sometimes recommends no change to the current regimen, advising patients to continue or carry on as they are. These are mainly patients living with conditions such as MS and epilepsy which are currently well controlled, or where treatment has started fairly recently and is being well tolerated. For example, Extract 5 is from a follow-up appointment with a patient with well-controlled MS. The neurologist advises the patient to continue with the current treatment, a recommendation that, the patient accepts.

Neu, neurologist; Pat, patient.
In this final category, then, no choice is constructed for the patient in the here and now. However, the basis for this is that the current treatment regimen appears to be working – a regimen that may well have been constructed as a choice for the patient in a previous consultation.
Conclusion
Across the no-choice data set, the various next steps presented by the neurologist are constructed as a logical consequence of the diagnostic conclusions reached. As such, they are treated as entailing no decisions to be made (in the here and now) by the patient. However, that does not necessarily mean that the patient is offered nothing. In all cases where the neurologist is essentially saying there is nothing to be done (for the primary complaint), he or she still goes on to offer some form of support (e.g. referral to other specialties, referral to various forms of allied health support, opportunities to contact the neurologist if help is needed before the next review, opportunities to see a specialist nurse or suggestions about self-help). Extract 6 is a good example – both the patient and neurologist agreed (in the post-appointment questionnaire) that no choice was offered. However, the patient is given an explicit slot to request anything else he needs to help manage his condition (line 10). As a result he gets help via the physiotherapy team (data not shown).

Neu, neurologist; Pat, patient.
The main theme across this subset is the neurologists’ conclusions that there is nothing (or nothing new) to be done from a neurological perspective. It is, of course, a clinical question whether or not this was the appropriate conclusion in each case. In interactional terms, the key finding is that, when this is the main conclusion within a consultation, we get agreement in the self-report data that no choice was offered. Indeed, part of what the neurologists’ turn designs are doing is constructing this as a case involving no decision to make, when in fact that conclusion is dependent on the neurologist having reached a range of decisions himself or herself. Thus, the no-choice subset also highlights the interconnections between diagnostic and treatment decision-making. Where no treatment choice is constructed for the patient, this is premised on – and justified with respect to – the diagnostic decisions made by the neurologist. As Agledahl et al. 28 argue, to avoid making such decisions would often be unethical (e.g. if an available option has no medical justification for a particular patient) or impractical (e.g. where the organisation of the healthcare system does not allow for certain options that might be available elsewhere).
Thus, choice ought not to be assumed to be inevitably the better way to handle decisions in neurology. There are circumstances where it may not be appropriate. Moreover, there are other ways in which neurologists might involve patients (e.g. by giving opportunities to ask questions). The absence of a choice does not necessarily mean the absence of patient participation.
Chapter 6 Option-listing as a practice for offering patients choice
Introduction
A striking finding emerged from our comparison of the four subsets in our sample, divided according to self-report perceptions of choice (see Table 2, Chapter 5). The practice of option-listing – which we first identified in our pilot data set as a practice for giving patients (some) choice10,47 – was present (with just one exception) in only one of those subsets in our full collection of recordings: the consultations for which neurologist and patient agreed that a choice had been offered to the patient. It appears, then, to be a practice that is not only analysable as creating a slot for patient choice, but also one that neurologists and patients perceive as doing so when they reflect on their interactions. Moreover, it was the only practice demonstrably oriented to offering patients choice that occurred (with just one exception) only in this subset of recordings. These findings provide a strong warrant for examining the features of this practice further as part of addressing our primary research question of what practices are neurologists using to (try to) engage patients in decision-making?
We start by describing option-listing, showing the three components that make up its full form. We then demonstrate how these components can be designed in ways that are clearly directed towards giving patients choice. Next, we illustrate the types of response to option-listing – by patients and/or accompanying others – that were evident in our recordings: those that aligned with, deferred or countered the constraints imposed by the neurologist’s turn. We show how all three demonstrate patients’ orientations to option-listing as a practice for handing the decision to them.
Option-listing: an overview
In its full form, option-listing consists of three components:
-
an announcement by the neurologist that there is a decision to be made
-
the formulation of a list of options
-
an invitation to the patient to announce his or her views with respect to the options or (depending on how this is worded) to select an option from the list, a component that we will refer to as a patient view elicitor (PVE; see Chapter 8 for discussion of PVEs used independently of option-listing).
This three-component package is clearly evident in Extract 7a (see highlighted talk). At lines 33–35 the neurologist announces explicitly that there is a choice to make. At lines 46 and 95 he lists the two options, each clearly labelled as such (‘first one’s . . . ’; ‘the other drug’s . . . ’). Finally, at lines 140 and 142, he hands the choice over to the patient, overtly seeking his selection from the two options presented.
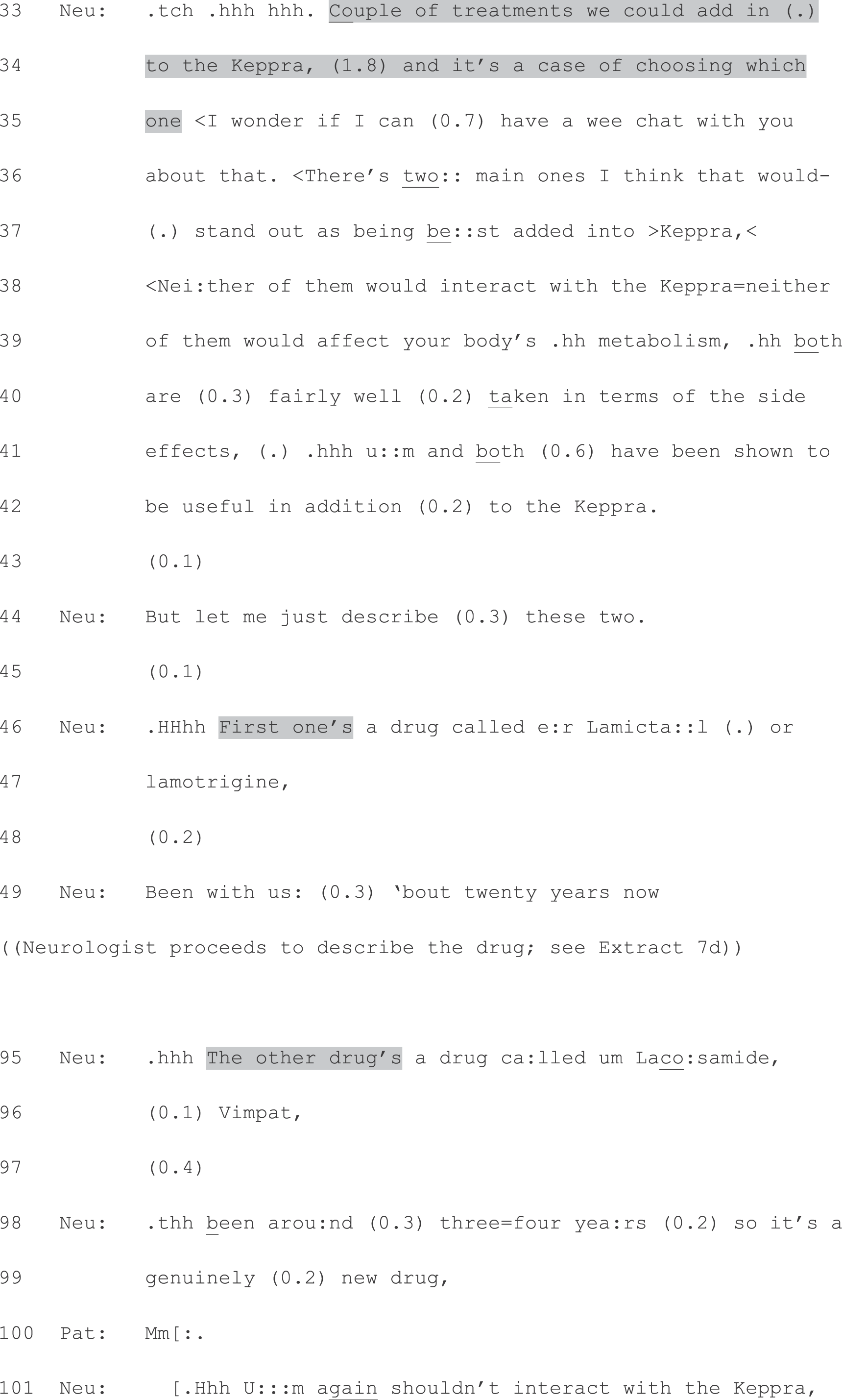

Neu, neurologist; Pat, patient.
The above extract shows a particular type of decision: a choice between more than one active alternative (drug A vs. drug B), where the option of doing nothing new (i.e. in addition to the drug, Keppra, which the patient was already taking) was not included on the list. However, option-listing was not limited to this type of decision in our data set. As we will show across the range of cases included in Chapters 6 and 7, it was used also for either/or decisions between doing something or nothing (or something new vs. nothing new), and for decisions where the list of three or more options included doing nothing (or nothing new). In addition, a wide variety of other types of future action were included on lists produced by neurologists across our collection, such as a dose increase (see Chapter 7, Extract 14), further monitoring (see Chapter 7, Extracts 20a and b); withdrawing a drug as an outpatient or as an inpatient, arranging a GP follow-up to check if treatment was definitely necessary (see Chapter 6, S03807: amyloid angiopathy cerebral bleed); regular scans or surgery; and antidepressants or counselling. Alternatives were not, then, always of the same type (e.g. drug A vs. drug B, as in Extract 7a); and, as we show in Chapter 7 (The malleability of interactional practices: using option-listing to curtail choice) equipoise (with respect to the options) was not found to be a necessary condition for the use of this practice. Furthermore, because option-listing has multiple components, significant variations on the full form are possible (see Chapter 7, Replacing the patient view elicitor: partial option-listing plus recommending, and Displacing the patient view elicitor: seeking the patient’s view after making a recommendation).
In what follows, we start by examining the full form, showing how it can demonstrably function as a practice for giving patients (some) choice. To illustrate this, we will draw on examples from different neurologists who all used the practice. In addition, we will keep returning to the consultation shown in Extract 7a. This will help to demonstrate how all the formats described may be used in a single interaction. We have selected this as a focal example for several reasons: (1) it consists of all three components of full form option-listing, it includes features that are clearly designed to construct this as the patient’s; the neurologist works hard to formulate the alternatives as equivalent; and it is relatively short owing to the presentation of just two alternatives. It makes, then, for a particularly clear illustration. Our analysis will centre on the design of each of the components of option-listing, showing how each can be produced in ways that, incrementally, construct the decision as the patient’s.
Component 1: constructing the decision as yet to be made
As we have seen in relation to our focal extract, above, full-form option-listing begins with an overt orientation to there being a choice at hand. Box 1 shows additional examples of this component. In each, a different neurologist announces explicitly that there is more than one possible course of action.
Where- where do we go from here? Um well there’s a number of things we could do . . .
G08405
So there are various treatments available which you take on a regular basis . . .
G05201
Um I mean with regards the- the swallowing, so there are options . . .
S07002
Then we move onto the preventative medications and this is where we have to have the do you really want to be taking tablets ((conversation)) . . . There are about four main classes of tablets . . .
S01606
I mean your choice here is . . .
G07504
Thus, right from the start, when option-listing, neurologists are setting up a decision that has yet to be made. To appreciate the significance of this for patient choice, it helps to contrast the first component of option-listing with an alternative approach to decision-making. Extract 8 is taken from the subset of consultations for which patient and neurologist agreed that there was no choice offered to the patient. This extract occurs after the neurologist has completed the verbal examination, culminating in checking the patient’s current address. As Extract 8 shows, when the neurologist moves on to talk about what to do next, he announces this as a conclusion that has already been reached: ‘I think, (0.4) e::r (0.2) need to do a couple=o’ wee tests here.’ (lines 1 and 2). The contrast with the first component of option-listing is most evident in the overt orientation to choice in Extract 7a: ‘it’s a case of choosing which one’ (lines 34 and 35). Any equivalent of this is absent in Extract 8. However, there is also a more subtle contrast in the design of these turns: in Extract 7a, the neurologist uses the conditional (‘we could’, line 33); in Extract 8, he uses the imperative (‘need to’, line 1). The proposed course of action in Extract 8, then, is constructed as a necessary step to achieve a diagnosis – it follows from the lack of diagnostic certainty. No such necessity is constructed in Extract 7a. Instead, possible alternatives are presented from which the patient may choose.

Neu, neurologist; Pat, patient.
Of course, the proposed next steps (treatment vs. further investigations) are very different in Extract 7a and Extract 8, emerging from a certain diagnosis in the former, and the absence of a clear diagnosis in the latter. However, as will become clear across Chapters 6–8, even when the diagnoses and next steps are far more comparable, neurologists may construct the decision very differently. For now, the key point is that neurologists must – whether or not consciously – select from a range of possible practices whenever they initiate decision-making in interaction with a patient. If they are to construct the decision as a choice, they must perform particular kinds of interactional work. A first step towards doing so is to announce that the decision has yet to be made. This avoids, at least in the first instance, the announcement of a recommendation for a particular course of action (as in Extract 8). The first component of option-listing displays, then, the neurologist’s orientation to (the possibility of) patient involvement in the decision-making process.
Component 2: constructing more than one option as a reasonable course of action
Further evidence that option-listing can function as a practice for offering patients choice comes from the ways in which neurologists (may sometimes) construct the options as equally valid – or, at least, as reasonable alternatives. The extent to which this is done across our cases is markedly variable (see also Chapter 7). Our goal in this section is to show how neurologists may construct a relatively level playing field with respect to the alternatives. Again, Extract 7a provides a clear example. Here we focus on the bit of talk that follows the neurologist’s announcement that there is a decision to be made (see highlighted lines in Extract 7b). This serves as a preface to his description of each option, and is designed to convey a state of equipoise between the two antiepileptic drugs he is about to list. The neurologist lays out a set of ways in which the two are equally good options (lines 38–42). Both drugs are described as the ‘best added into Keppra’ (line 37) – the drug that the patient is currently taking – when compared with a wider range of drugs. And the neurologist repeatedly refers to ‘neither’ and ‘both’ in order to highlight their similarities. This preface functions, then, to place both drugs on an equal footing, up front. This allows the neurologist to shift into talking about each one on the understanding that he is not recommending one over the other.

Neu, neurologist.
The neurologist’s description of each option contains further evidence that he is working to present these as neutrally as possible (Extract 7c). He structures his information provision for each in a similar way, naming each drug with its set of names (Lamictal/lamotrigine and lacosamide/Vimpat), informing the patient of how long the drug has been available, explaining that they would build up the dose slowly, then describing potential side effects. A few other features of his talk are notable with respect to his efforts at neutrality. He constructs both as (relatively) new epilepsy drugs despite an age difference that could seem substantial to a lay person (20 years vs. 3 or 4 years). When talking about the slow build up of the dose for the second drug, he also refers back to the first, again highlighting their equivalence (‘same as lamotrigine’, line 104). And for both drugs, he minimises the significance of potential side effects. Bold font in Extract 7c has been used to highlight similarities in how the neurologist presents the two drugs.




Neu, neurologist; Pat, patient.
The neurologist concludes his formulation of the options with another overt orientation to choice, produced again with the conditional form: ‘So we could try either one of these:.’ (line 123, Extract 7d). As he did in the preface shown in Extract 7a, he rounds off his information provision with another clear construction of equipoise: he presents himself as unable to be sure which drug will work best for this patient (lines 125 and 126), but draws on population-level evidence as a basis for considering either of them to be a reasonable choice (lines 128–130).

Neu, neurologist; Pat, patient.
In summary, throughout his production of the second component of full-form option-listing, the neurologist has worked to construct both drugs as medically suitable for this patient. We have shown some of the design features of his turns that have enabled this. In addition, this apparent equipoise is partially achieved by what the neurologist does not do while providing information about the options: he does not produce a recommendation with respect to the two alternatives prior to inviting the patient’s view. Thus, the decision is constructed as lying, quite legitimately, in the patient’s domain; it amounts to a question of which side effects he is willing to risk, rather than a matter of professional judgement.
Component 3: producing a slot for the patient to announce a view on, or selection from, the listed options
The final component of option-listing consists of a turn type that we have identified as a PVE. Box 2 shows how this action – of eliciting the patient’s view on the just completed list – is accomplished in a range of consultations. In all cases this turn occurs after the first two components of option-listing described above.
So what- what- what would you like to do about them?
G08405
So that’s how they work and that’s really my job done at that point . . . I have to sort of pass it back to you and say what do you want?
S01606
So those are kind of the options, any first thoughts?
S07904
Anyway, so what do you think?
S01001
Crucially, the turns in Box 2 all consist of questions that orient explicitly to the patient’s wishes, feelings or thoughts with respect to the just completed list. In this way, they shift the decision-making into the patient’s domain. Moreover, they all use open-ended formats. In other words, they make relevant some kind of narrative or announcement from the patient, rather than a simple yes/no. Again, the significance of this for patient choice becomes more clearly evident when formats like those in Box 2 are compared with a recommendation. Extract 9 shows another case from the subset of data for which neurologist and patient agreed that no choice was offered. As is clear from the brief exchange below, they also agreed on the course of action that was selected: referral for cognitive–behavioural therapy (CBT). However, this decision was managed through a recommendation by the neurologist, rather than through option-listing. In place of listing alternatives and then creating a slot for the patient to announce her view/selection from the list, the neurologist announces what he thinks should be done. Note, as we did for Extract 8, the use of the imperative rather than the conditional form (‘we have to’, line 6 rather than ‘we could’), and the announcement of a conclusion that the neurologist has already reached (‘I think’, lines 6 and 10, followed by a single, proposed course of action).

Neu, neurologist; Pat, patient.
Extract 9 shows, then, how neurologists’ turns can be produced in a relatively closed fashion, creating a slot for a response that needs do nothing more than provide assent to the proposed course of action – as the patient does at lines 14 and 16 through the agreeing turns, ‘yeah’ and ‘OK (then)’. This assent is strongly projected by the design of the neurologist’s turn, which is heavily biased in favour of acceptance (for good reason, given, in data not shown, the prior agreement reached that CBT is a good form of treatment).
By contrast, in Extract 10, we see a patient’s response to one of the open-ended formats shown in Box 2. Note how, although somewhat condensed, all three components of full-form option-listing are present in this case. The shift from ‘I can’ to ‘you can either’ (line 2) foreshadows that an either/or choice is about to be proposed, and places this in the patient’s domain. The neurologist then lays out the two options (starting blood pressure medication right away or only if a future test shows it to be necessary). Although the neurologist goes on to make a recommendation, this is contingent on future testing, rather than a recommendation for the here and now. She hands the current decision explicitly over to the patient at line 30: ‘so it’s up to you’. Through its design, this turn makes relevant something other than a yes/no response. Unlike the recommendation in Extract 9, line 30 in Extract 10 cannot be adequately addressed with a simple token of assent, because it contains no proposal for the patient to accept. Instead, the patient’s announcement of her selection from the neurologist’s just completed list is due next. This is what she provides at lines 31 and 32.


Cou, cousin; Neu, neurologist; Pat, patient.
If we return to our focal extract, we can see the neurologist, likewise, handing the decision explicitly to the patient through the construction of an open-ended turn. Moreover, he works hard to maintain the neutrality that we have seen him constructing throughout the production of the option-listing package. His use of the phrase ‘either one of them’ (Extract 7e, line 140) implies that both drugs are reasonable options in this case. And the informal ‘grab you’ places this decision very much in the patient’s domain (i.e. this is constructed as a matter not of medical knowledge, but of patient preference). When the patient produces no verbal response at line 141 (this is one of the cases for which we do not have video so it is not possible to see if he responds non-verbally), the neurologist pursues one. His reformulated turn (line 142) retains both the emphasis on patient preference – now made even more explicit (‘Any particular preference’) – and the effort to keep both options on the table (‘one or the other’).

Neu, neurologist.
In summary, by constructing the decision as yet to be made, constructing more than one option as a reasonable course of action, and producing a slot for the patient to announce a view/select from the list at a point where the neurologist has not (yet) made a recommendation, neurologists can treat the decision as lying in the patient’s domain. In so doing, they can open up space for patients to engage – to some extent – in the decision-making process. It is to patient responses that we turn next.
Responding to option-listing
As we have shown, full-form option-listing includes a final component which invites the patient’s views on the options listed (or, at its strongest, makes relevant a selection from the list). In our data set, patient responses to this final component clustered into three types, as follows:
-
The first type aligns with the action performed by the neurologist’s turn. By alignment we mean the production of a responsive turn that is fitted to the action being performed in the prior, (i.e. the responsive turn provides the kind of next action which was being sought by the initiating turn). So, for example, a question makes relevant a fitted answer. If that fitted answer is provided, then the responsive turn may be said to align with the prior (note that this does not necessarily mean that the next turn in some way endorses or affiliates with whatever stance may have been embodied in the prior, just that it facilitates – as opposed to obstructs – the action being pursued in the first turn). 82
-
The second type defers83 the relevant response. For example, a patient might, after option-listing, ask the neurologist a question. This evades, at least for a while, the constraint placed on the patient by the neurologist’s turn (i.e. to voice a view). Once the patient’s question has been dealt with, however, that constraint becomes ‘live’ again. Hence, the patient will typically go on to produce another form of response.
-
The third type is one that counters83 the action performed by the neurologist’s turn. For example, if, in response to a question, the recipient turns the question back on the questioner, that is a counter. In so doing, they are not just deferring, but rather are fully resisting, the constraint placed upon them by the prior turn.
In what follows, we will illustrate these response types, our aim being to show how all three demonstrate patients’ orientations to option-listing as a practice for handing the decision to them. Thus, our analysis serves as further evidence in support of the central argument of this chapter: that option-listing can function as a practice for offering patients choice.
Aligning with the neurologist’s turn
At their most straightforward, aligning responses consist of an announcement of the patient’s selection from the neurologist’s list of options. 47 We have already seen a clear case in Extract 10. As we showed above, the neurologist’s turn at line 30 explicitly hands the decision over to the patient. It makes relevant, then, the patient’s selection from the just completed list. The patient’s response aligns with this constraint – she announces her decision about what to do next (lines 31 and 32) – and explicitly demonstrates its alignment in its design. Most significantly, it mirrors the three-part construction used by the neurologist when describing the second option, as shown in Table 3.
| Component of the turn | Neurologist’s turn | Patient’s turn |
|---|---|---|
|
Neu:see your GP (line 6) | Pat:I (will) go back to the GP (line 31) |
|
Neu:and if it’s . . . less on a day when you’re not stressed coming up to the hospital (line 7) | Pat:and have me blood pressure taken (lines 31 and 32) |
|
Neu:But if it’s still a little bit higher than target . . . then I would recommend that we start some treatment (lines 13–18) | Pat:and if it’s high then . . . (line 32) |
In this way, the patient creates a clear link between her response and the neurologist’s list. Although she leaves the upshot of her turn incomplete (see line 32), there are a number of features of her talk that claim a strong commitment to acting on this plan. Again, the three-part formulation of her response is significant because it provides more detail than is strictly necessary to announce her selection. Parts 1 and 2 could have been folded into each other. For example, something like ‘I’ll have my blood pressure taken again and if it’s high then . . .’ would have been sufficient. In spelling out the steps she will take to enact her decision, the patient aligns not only with the neurologist’s prior turn – which gave her the right to choose – but also with the option she has selected. This is further confirmed through her subsequent assenting turns: ‘Yes . . . Yeah. Uhahh’ (lines 34 and 36).
Patients sometimes also coupled their announcement of a selection/preference with an account for that preference. For example, in Extract 11a, the patient explains that his reason for preferring Copaxone® – the preference his wife has just voiced at line 82 – is that he wants to ‘continue to work’ (lines 88 and 89). Although our focus here is on the PVE (at lines 77 and 78) and its response, we have included, in Extract 11a, enough of the prior talk to show that all three components of option-listing are, again, present. These are highlighted in bold font. Note that, just as we saw in Extract 7a, the neurologist prefaces the list with a clear orientation to (a relative state of) equipoise with respect to the alternatives (lines 20–24). Again, then, the neurologist is treating the decision as lying, quite legitimately, in the patient’s domain, rather than being a matter for her professional judgement.



Neu, neurologist; Pat, patient; Wif, wife.
Returning to the patient and wife’s responses, we can see that the wife’s naming of a specific disease-modifying drug (DMD) for MS (at line 82) functions to tie the response back to the listed options, one of which was glatiramer acetate (Copaxone®, TEVA UK Ltd; lines 11 and 56). In this way, her turn operates in a similar way to the patient’s in Extract 10. By first naming a drug from the list, she produces a turn that is well fitted to the practice of option-listing. But notice how the rest of the turn unfolds. This is not produced as a final decision, to be acted on for sure – as was the case in Extract 10. Rather, the design of the wife’s response is exquisitely tailored to the design of the prior PVE. The neurologist has asked for any first thoughts and quickly reassured them that a final decision is not required today (line 80). The wife’s response aligns with this two-part turn by announcing the drug that they have been ‘looking at’ (lines 82–85). In so doing, she constructs this preference as one that is founded on their current thinking, rather than as a definitive selection from the list. This turn aligns with the neurologist’s, then, both by announcing a preference (from the list) and by treating this as a decision in progress, which fits with the neurologist’s orientation to this as an early stage in the decision-making.
As in Extract 10, the patient and his wife display an active engagement in the decision-making process in their response to the PVE. Indeed, in Extract 11a, they show themselves to have already started this process independently, prior to the consultation. This is evident not only in their claim to have already been looking at Copaxone, but in how they produce a joint response. In doing so, they make visible their own SDM as a couple. The wife names the selected treatment, which is confirmed by the patient at lines 83 and 86, and the patient begins his account in overlap with his wife, who is doing something very similar on his behalf at lines 85 and 87. Although not a focus of the present study, this joint response highlights a broader point that treatment decisions are often shared not only between doctor and patient, but between patient and significant others as well. 84 When one or more of those others is present in the consultation, the locus of the decision-making can shift. In Extract 11a, the neurologist includes the patient and his wife through her use of gaze, which she shifts from the patient to his wife during her turn at line 80. Likewise, the patient and his wife co-ordinate their responses via gaze. For example, at line 83, the patient shifts his gaze from the neurologist to his wife and back to the neurologist.
There are several factors in Extract 11a, then, that demonstrate the patient and wife’s orientation to this decision as lying in their domain: their announcement of a (current) preference, their display of joint decision-making, their claim to have been engaged in decision-making prior to the consultation, and the nature of their account for preferring Copaxone. This account clearly links back to the information provided by the neurologist. She has said that the interferons can ‘make you feel fluey’ (line 34) and that Copaxone ‘tends to win on side effects’ (lines 61 and 62). In turn, the patient’s wife alludes to the likelihood of fewer side effects with Copaxone in her version of the account for this preference (lines 85 and 87), and this is implicit in the patient’s version too (the implication of his stated desire to continue working is that he thinks this may not be possible if he experiences too many side effects). At the same time, the concern they raise is one that lies firmly in the lifeworld of the patient, rather than in the medical domain. 85 The patient and his wife have primary rights (relative to the neurologist) to know what (everyday life) factors are important to him in making this decision. By accounting for the decision in terms of his love of work, then, they construct it as one that lies in the patient’s – rather than neurologist’s – domain.
In summary, the responses shown in Extract 10 and 11a align with the design of the prior PVE. Both responses display an understanding that the neurologist’s turn has created a slot for them to express their views. Moreover, both responses align with the wider action that the neurologist was performing through option-listing: they both announce a selection/preference with respect to the items on the just completed list. In so doing, they treat themselves as having a right to choose from among those items.
Deferring a fitted response
By contrast, patients (or those accompanying them) sometimes filled the same slot – following the third component of option-listing – in a way that did not, in itself, meet the constraints of the neurologist’s turn. Instead, they deferred the relevance of that response. This deferral was accomplished through questioning: patients/significant others sought further information from the neurologist. If we return to our first extract, we can see that this is what the patient’s mother does at the highlighted lines shown below (Extract 7f, lines 148–156). The patient has already experienced some difficulty in responding to the neurologist. At line 141 there is over half a second’s silence, after which the neurologist pursues a response. Following this, there is an even longer silence, broken only by some breathiness from the patient which sounds like slight laughter – an indication of trouble in producing a fitted response. The neurologist acknowledges this difficulty, providing an account of why the patient may be struggling to make a selection (lines 145 and 146). After another (very short) pause, the patient’s mother steps in. Notably, however, she does not make a selection on the patient’s behalf. Instead, she seeks further information about a specific concern: the risk of the patient losing weight.


Neu, neurologist; Pat, patient.
The mother’s question is the kind of turn that Schegloff83 calls a ‘pre-second insert expansion’ (p. 106). This means that it ‘look[s] forward, ostensibly to establish the resources necessary to implement the second pair part [i.e. response] which is pending’ (p. 106). In this case, the mother treats herself as lacking some of the resources she needs in order to reach a treatment decision. Just like the account we saw in Extract 11a, this question orients to likely side effects of the treatment options. As such, it pursues matters that are, arguably, lifeworld concerns rather than biomedical ones. Of course, weight loss can become a biomedical problem, but it is not constructed as one in this case. In asking this question, the mother is making available a factor that is important to her in the decision-making process, just as the patient and his wife foregrounded the ability to work in Extract 11a. Thus, the mother’s question, despite deferring a fitted response, nevertheless shows her understanding that the neurologist has given them the right to choose. It seeks the information that the mother treats as necessary for making that choice. Once the neurologist has responded, the announcement of a decision becomes relevant again.
It is worth noting that the mother’s turn does not resist making a selection from the neurologist’s list per se – as do the counters we will discuss shortly. However, it does introduce the potential for resistance: should the neurologist respond in the affirmative for one or both treatments, the mother would have a basis for resisting their selection. Indeed, we know from studies of ordinary conversation that deferral of this kind can often be employed as a strategy for (subtly) indicating some kind of resistance to the prior turn. 83 Deferral and resistance may, then, be closely intertwined.
Extract 12a provides a good example of the relationship between deferral and resistance. Here, the patient produces a turn that seems to be in favour of starting disease-modifying therapy for MS (lines 25–27, 29, 31 and 32). Her husband then does something similar to the mother’s turn in Extract 7f: he asks a question (lines 34–36, 38 and 40). This question, however, seeks information about a drug that is not on the neurologist’s list. In that sense, he is, rather gently, starting to resist the terms of the decision as laid out by the neurologist. In the interests of space, we have not included the whole of the option-listing package in the extract below. As can be seen from lines 1 and 2, however, the neurologist is clearly in the midst of providing information about a set of alternatives. These (in data not shown) closely mirror the options for disease-modifying therapy that we saw described by another neurologist in Extract 11a.


Hus, husband; Neu, neurologist; Pat, patient.
The PVE used in Extract 12a is designed rather differently to the others we have considered so far. It is formatted as a yes/no interrogative,86 rather than an open question; (i.e. the response could, in principle, be a version of ‘yes I have thought about/researched these’ or ‘no I haven’t’). However, it is clear that the patient herself understands the neurologist to be after something more than the format of his turn would suggest; that his turn has, in effect, created a slot – just like the other PVEs we have seen – for her to produce a narrative response. This she does, again showing an orientation to lifeworld factors85 in her no-problem story about the experiences of other DMD-takers she has met. She constructs an almost inevitable trajectory in which the drugs are just a next step (‘that’s what happens’, line 32) and something that can be absorbed into life’s routines (lines 26 and 27). In so doing, she shows herself to be orienting to the fundamental choice of whether or not to start any such therapy, and aligns herself with the broad option of doing so.
Similarly, the question from the patient’s husband is around which treatment to choose, rather than whether to start treatment. His question, however, constitutes a very different kind of response to the patient’s. Like the patient’s mother in Extract 7f, he seeks further information. Again, the question he poses does not resist the terms of the neurologist’s prior turn per se. Indeed, by asking about a drug he has ‘read about’ (line 34), the husband is implicitly answering the neurologist’s yes/no interrogative in the affirmative: he has done some research into the options. However, as we have seen, his question is resistant in the sense that it goes off piste (he is asking about something that was not on the neurologist’s list). Like in the previous extract, by asking a question, he is also claiming not yet to have the resources to provide a fitted response to the neurologist. He is deferring, then, an announcement of a view until he has those resources.
Again, once the neurologist has addressed the question, the matter of what the patient (and her husband) think about the options becomes live again. Extract 12b shows how this is handled. Here the neurologist is just completing his response to the husband’s question, which includes the description of another oral drug that is still in development [BG12 or Dimethyl Fumarate (during the preparation of this manuscript Biogen Idec, Cambridge, MA, USA has registered this drug under the name Tecfidera®)]. A subsequent long silence (line 74) is broken by breathy laughter from the patient (line 75) – much like we saw in Extract 7f. Again, this signals difficulty in producing a fitted response. Importantly, the husband’s turn at line 77 shows his understanding of what kind of turn is due: a selection from among the options listed by the neurologist. Moreover, we see yet again an orientation, in his turn, to lifeworld85 concerns: how the patient feels about daily injections. In effect, he is producing his own version of a PVE, recreating a slot for the action that his question deferred: the production of a decision by the patient. Notably, this turn also implicitly accepts the neurologist’s response to his question. He is no longer pursuing an alternative to the drugs on the neurologist’s list.

Hus, husband; Neu, neurologist; Pat, patient.
In summary, even as they defer fulfilling the constraints placed upon them by the neurologist’s turn, questions by the patient (or accompanying other) after option-listing still show an understanding that the neurologist has handed them a choice. By seeking further information as a basis for decision-making, such questions treat the decision as one for the patient to make. Once the question gets answered, the decision becomes live again. It is important to note that these questions can carry the ‘seeds’ of resistance to one or more of the options just listed. However, they do not resist the overall action being performed by the neurologist through option-listing: placing the decision in the patient’s domain. It is to such resistance that we turn next.
Countering the neurologist’s turn
This third and final type of response was not common in our data set, but is significant because it corroborates the finding from self-report studies that patients do not always want choice. Extract 7g shows what happened next in the consultation we have been following across Extract 7a–f. As we have seen, the neurologist has assured the mother that weight loss is not a side effect of either of the drugs he has listed. The mother accepts this at lines 162 (Extract 7f) and 167 (Extract 7g). Thereafter, a decision about which drug to try becomes relevant again. However, following over half a second’s silence, the patient’s father produces a turn that resists that constraint entirely. Instead of choosing from the list, he produces a counter: he hands the decision back to the neurologist. It is difficult to hear precisely what he says, but it sounds like he is invoking the neurologist’s professional status. Certainly the effect of the turn is to say: ‘you may have placed this decision in our domain, but we’re placing it back in yours’. The neurologist replies in kind, giving his opinion on which of the drugs to select.

Neu, neurologist.
In effect, the patient and his parents have resisted the neurologist’s attempt to hand the decision to them. Nevertheless, their response shows as much of an orientation to option-listing as a practice for giving choice as the other types of response we have examined. By countering the neurologist’s turn with a request for a recommendation, the patient’s father is demonstrating his understanding that one has not yet been given. This shows, very powerfully, that participants themselves hear full-form option-listing as a practice that is distinct from recommending.
In summary, patients’ (and accompanying others’) responses to full-form option-listing ranged from an announcement of the patient’s selection from the list through to avoidance of making a choice. All three types of response shown above display an orientation to option-listing as a means of offering patients choice. Coupled with the evidence that neurologists, when option-listing, use formats that construct a choice for the patient, these responses clinch the argument that option-listing is understood as a practice for offering choice by participants themselves (not just by us as analysts).
A deviant case
We noted at the start of this chapter that there was just one case of full form option-listing that occurred outside of subset 1 (i.e. where neurologist and patient agreed a choice had been offered). This was evident in a follow-up consultation for a patient with epilepsy, where the neurologist reported that a choice had been offered, but the patient reported that it had not (i.e. subset 3 in Table 2). In this final analytic section of Chapter 6, we examine this deviant case, showing how the design of the options make them hearable as a non-choice, despite the neurologist’s use of the same ‘machinery’ of option-listing that we have examined in this chapter.
Crucially, the decision-making in this case is not initiated, at first, through the practice of option-listing. Rather, the neurologist starts with a recommendation that is akin to those seen in the no-choice subset (i.e. subset 4, where neurologist and patient agreed that no choice was offered). Specifically, as we showed in Chapter 5, there were cases in subset 4 where the neurologist recommended continuing on the current treatment – either because it was working or because the alternatives had been exhausted. In such cases, no choice was constructed for the patient. Instead, as we argued in Chapter 5, the course of action to be undertaken was treated as a logical consequence of where the patient was at on the trajectory of their condition. This is also true of our deviant case: having been prescribed a range of AEDs over an extended period of time (see lines 2–5), this patient has – the neurologist tells her – few (viable) options left to try (lines 1, 2 and 6–9).

Neu, neurologist; Pat, patient.
It is against this backdrop of little to offer that the neurologist goes on to map out a choice using option-listing. In the following extract we see the now familiar three components. At line 15, the neurologist flags up that there is a decision to be made. He then lists two alternatives by invoking the voices of other patients (lines 15, 16 and 18–22). He places the decision explicitly in the patient’s domain (‘it’s up to you’, line 15 and ‘it depends a wee bit on you . . . what your attitude is’, lines 23 and 24) and, finally, uses a patient view elicitor to hand the decision to the patient: ‘so it’s really up to you whether you want to try them or not’ (lines 38 and 39). When this fails to elicit an immediate response (line 40), the neurologist returns to his original position: that he has little to offer (lines 41–46). Thus, the choice, despite being constructed using all the elements of full form option-listing, is sandwiched between two announcements that alternative treatments are unlikely to be any better at controlling the patient’s seizures than the drug she is already taking.


Hus, husband; Neu, neurologist; Pat, patient.
Given the construction of the new option as unlikely to be helpful – and with the added risk of side effects (line 21 and also in further talk not shown) – it is perhaps unsurprising that the patient reported not having a choice, even though a decision point was explicitly constructed for her by the neurologist. While she was given the right to try alternative drugs, these were, in effect, presented as highly likely to be a waste of effort. Indeed, despite the neurologist’s explicit framing of the decision as the patient’s ‘choice’ (line 99), the patient and her husband ultimately construct it as a non-choice – note the husband’s claim that she has ‘gotta live with the situation’ (lines 103–106) and the patient’s agreement that she ‘need[s] to stick with the situation [she’s] got’ (lines 109–111). Both ‘got to’ and ‘need to’ convey their subsequently reported sense of an absence of choice.


Hus, husband; Neu, neurologist; Pat, patient.
In summary, the use of full form option-listing (and, indeed, even the use of the phrase, ‘it’s your choice’) does not guarantee that patients will experience themselves as having had a choice. However, it should be emphasised that this was the only case in our dataset where full form option-listing was used to generate a decision point (in the here and now) about a test or treatment that was not perceived as a choice by the patient. Indeed, as we will show in Chapter 7, even when option-listing was used in our dataset in a way that effectively curtailed choice, patients still reported having been given a choice. What, then, distinguishes our deviant case from the rest? Its unique feature, we would argue, lies in what it lacks: it lacks the construction of any option as likely to address the patient’s trouble. We would argue, then, that our deviant case amounts to an exception that proves the rule. While choice is there in the formal sense – the patient has the choice to try an alternative drug or not – choice is not there in the sense of there being an option that is (at least as the neurologist constructs it) likely to make any difference. In short, full form option-listing works to give patients the perception of having a choice – as we will show in more detail in the next chapter – with one caveat that there must be at least one option presented as likely to be in some way beneficial to the patient.
Conclusion
We began this chapter by highlighting a striking relationship between the self-report and interactional data collected for this study: that the practice of option-listing was present (with one exception) only in the subset of recordings for which neurologist and patient agreed that a choice had been offered to the patient. It was also the only practice analysably oriented to choice that fell (almost) exclusively in this subset. For example, PVEs used independently of option-listing (discussed in Chapter 8) were commonly found in this subset but were also regularly evident in the subsets of recordings for which neurologist and patient disagreed over whether a choice had been offered. Full-form option-listing appears, then, to be the most overt, and easily recognisable, practice used by neurologists – at least in our data set – to (try to) engage patients actively in the decision-making process. In this chapter we have shown how both neurologists (in their design of the components of option-listing) and patients (in their responses to option-listing) orient to this practice as a means of handing the decision over to the patient. How effective a practice it is will be the subject of Chapter 7.
Chapter 7 Option-listing: an effective practice?
Introduction
In Chapter 6 we showed how option-listing can be used to construct a choice for the patient. We also showed how patients, through their responses to option-listing, routinely displayed a clear understanding that this was what the practice was being used to do. In addition, we found that this was (with just one expection) the only practice for offering choice that occurred exclusively in those consultations for which participants agreed on the self-report questionnaire that a choice had been offered. Taken together, these findings strongly suggest that full-form option-listing works in one particular sense of the word: that it readily generates the perception of choice.
But what about effectiveness in other senses of the word? As we showed in the previous chapter, full-form option-listing evidently also worked, in some cases, in the sense that it resulted in the patient voicing a preference/making the decision. In other cases, however, the practice evidently failed in this regard, (i.e. the patient lobbed the decision back to the neurologist). The question arises, then, whether this kind of differential success is related in some way to how the neurologist designed those turns making up the option-listing package – and particularly the final component (the PVE), which most explicitly sets up what kind of turn the patient can legitimately produce next.
In this chapter, we will begin with a negative observation – a preliminary comment on what we have not found. We have not, at least thus far, been able to demonstrate a link between the specific design of the PVE and whether or not the patient goes on to voice a preference/make the decision. Rather, the crucial factor seems to be whether or not (and if so, when) the neurologist announces his/her view. If, as we will show, the PVE is either replaced with, or displaced by, a recommendation from the neurologist, the slot for the patient’s response to option-listing can be substantively altered.
Moreover, the sequential position of full-form option-listing, as a package, is significant. Thus far, our analysis has focused on option-listing as a practice for opening the decision-making process. However, it can also be used to seek a decision some way into that process, at a point at which one or more other practices have already been tried. Indeed, when we first began work on the pilot analysis for this project, our clinical team members had a theory based on their experience: that they typically used option-listing as a way of dealing with patient resistance to a proposed course of action. They hypothesised that option-listing was, in fact, not really a practice they used (much) for offering choice, but was rather a strategy for getting out of interactional deadlock or bringing an (often problematic) encounter to a close. Although our findings suggest that this use of option-listing is not common, there are a few cases in our data set that support this hypothesis. This demonstrates that the same kind of interactional machinery may be used to achieve very different ends.
In brief, our analysis is structured as follows:
-
First, we show how even PVEs (as the third component of a broader option-listing package) that are demonstrably unconducive in their design tend to be successful in so far as they do elicit the patient’s view.
-
Second, we demonstrate the interactional impact of two hybrid practices – where option-listing is combined with recommending, such that:
-
the PVE is either replaced
-
or displaced by a recommendation.
-
-
Third, we illustrate the malleability of interactional practices, showing how option-listing can be used to curtail choice.
-
And finally, we reflect on possible indicators of effectiveness other than whether or not the patient makes a choice.
The design of the patient view elicitor: a preliminary negative observation
Our hypothesis was that the design of the PVE (the third component of option-listing) would be significant in a similar way to the design of the additional concerns elicitor9 discussed in Chapter 1, Models versus practices: our methodological starting point. It is possible that with more cases and/or by using a different study design (e.g. a randomised trial9) we might find something similar for PVEs after option-listing. However, in our current data set we have found that turn designs that look both more and less conducive to eliciting patient views appear to be similarly effective for doing so.
Extracts 11b and 14 show a clear contrast in how the PVEs are designed. Drawing on Boyd and Heritage’s87 description of the dimensions of questioning and answering, we can see that both PVEs (see shaded turns) set a similar topical agenda: that is, talk about the patient’s ‘thoughts’ on the prior treatment options. Beyond that, they differ markedly, as we show in Table 4.

Neu, neurologist; Pat, patient; Wif, wife.

Neu, neurologist; Pat, patient.
| Features of the PVE | Extract 11b: Any first thoughts? | Extract 14: So what do you think? |
|---|---|---|
| Action agenda The kind of action that is made relevant by the PVE. For example, yes/no response or a discursive answer. Patients can engage or decline to engage with this agenda87 |
Both turns create a slot in which a discursive response could relevantly be produced. However, the format in Extract 11b makes it possible for the patient to produce a fitted response that does not announce a view; e.g. something along the lines of ‘no, we haven’t really thought about it yet’. Such a response would still engage with the action agenda set by the form of the neurologist’s turn (i.e. to say whether or not they have ‘any first thoughts’) | By contrast, the format in Extract 14 makes relevant a response that does announce a view. To avoid doing so, the patient would have to decline to engage with the action agenda of the neurologist’s turn (i.e. to say ‘what’ she thinks) |
| Presuppositions The underpinning assumptions about the patient’s life, health and knowledge embodied in the doctor’s turn. Any or all of the presuppositions in a turn may be confirmed or disconfirmed in a patient’s response87 |
Partly through the inclusion of ‘first’, this turn is designed to avoid the presupposition that the patient will already be able to make a final decision. In addition, by using the negatively valenced ‘any’,88 the turn does not presuppose that the patient will even have ‘first thoughts’ about treatment. This turn design, then, treats the decision-making process as in its early stages. This is made more explicit in the neurologist’s rapid move to reassure them that a commitment to treatment is not required now (line 80) | This turn presupposes that the patient will already have a view on treatment – one that she will be able to announce in next turn. Moreover, while the patient could respond, appropriately, in ways that would not amount to voicing a decision, the turn design does not preclude making a selection from the prior list. Thus, while the format in Extract 11b presupposes a decision process that is likely to extend beyond the consultation, the format in Extract 14 does not |
| Preference structure Turns can be ‘structured to facilitate or “prefer” one response over another and, similarly, patients’ responses are managed so as to be aligned or disaligned with those preferences’87 (p. 160) |
The turn in Extract 11b prefers (is ‘biased’ or ‘tilted’ in favour of) a negative response through the use of the negative polarity item, ‘any’.88 It projects, then, a negative response, along the lines of ‘no, we haven’t really thought about it yet’. Despite being a negative response, this kind of turn would still align with the preference embodied in the design of the PVE | The turn in Extract 14, by contrast, projects a discursive response that entails the patient announcing her thoughts. To decline to voice a view, she would have to disalign with the preference structure of the neurologist’s turn |
In summary, the PVE in Extract 14 creates a much stronger bias or expectation for the patient to produce her own view on the listed treatment options. In Extract 11b, by contrast, the neurologist provides far more ‘wriggle room’ for the patient, in that she explicitly holds open both the possibility that he will not yet have thoughts about the options and that he will not yet be in a position to make a final decision. By design, then, Extract 14 is more conducive to eliciting the patient’s view than is Extract 11b. And yet, in both cases the responsive turns provide a clear announcement in favour of one of the listed treatments over others (‘Copaxone’ in Extract 11b and ‘that medication’, meaning topiramate, in Extract 14). In so doing, the patient (and his wife) in Extract 11b actually disalign with the preference structure of the neurologist’s PVE (i.e. they produce ‘thoughts’ in response to a turn that was tilted in favour of them not being able to do so). They also disconfirm the neurologist’s presupposition that they may be at a very early stage of the decision-making process.
This contrast was chosen for its clarity but it appears to be typical across our collection; we have not been able to isolate design features that routinely distinguish between types of response to a PVE in full-form option-listing. This is, perhaps, unsurprising, since announcing a view on treatment/making a choice may depend also on a range of non-interactional factors (e.g. the patient’s level of confidence and understanding; whether or not they have a preference; how well they know the neurologist; what the options are; the nature and certainty of their condition; and possibly certain demographic factors, such as age, education and socioeconomic status). The relationship between such factors and the design of turns at talk needs further exploration using methods that go beyond the scope of this study (see Chapter 9, Recommendations for future research).
An additional – and compatible – possibility is that the wider action performed through option-listing, as a multicomponent package, sets a stronger constraint on what kind of action patients produce next than does the specific design of the PVE. As we have seen, option-listing sets up a forthcoming decision point for the patient through a series of three components. The PVE is not, then, the only part of the neurologist’s turn responsible for setting the agenda for what the patient ought to do next. Thus, it may not be so surprising that even a relatively unconducive PVE will typically succeed in eliciting the patient’s view. Indeed, it was very rare, in our data set, for full-form option-listing to fail categorically in the sense that the patient avoided voicing any kind of view on the listed options.
But what if a PVE were not produced at all? In Chapter 6, we considered option-listing in its full-form, and treated recommending as an alternative practice for initiating decision-making. However, because full-form option-listing is a multi-component package, it is also possible to combine the two practices in various ways. One such hybrid entails the loss of the third component of option-listing – the PVE – and its replacement with a recommendation. This gives rise to a kind of natural experiment in our data, making it possible to compare the interactional consequences following option-listing plus PVE and option-listing plus recommendation. It is to this comparison that we turn next.
Replacing the patient view elicitor: partial option-listing plus recommending
Extract 15a shows a clear example of this hybrid practice, which combines option-listing with recommending. Just as we saw in the classic cases of full-form option-listing shown in Chapter 6, the neurologist announces that there is a decision to be made (line 9), then lists two options (lines 10–15). Having produced these first two, familiar components, he then deviates from the pattern we have seen thus far. In place of a PVE – which could have been produced at lines 16–18 – he performs a very different kind of action: a recommendation for the second of the just listed alternatives (lines 17 and 18).

Neu, neurologist; Pat, patient.
Drawing on what we have already seen of patient responses to full-form option-listing, we are well placed to assess the interactional import of this variant. The patient goes on to accept the neurologist’s recommendation, so the best comparison is with those cases where the patient aligns with the neurologist’s PVE. As we saw in Chapter 6, Responding to option-listing and in Extracts 11b and 14, such aligning turns are typically finely tuned to the precise design of the PVE. However, in more general terms, we know that aligning responses to full-form option-listing typically consist of an announcement of the patient’s selection from the list or a view on the listed items, with or without an account for the patient’s preference. Crucially, for the patient to align fully with the action agenda of the PVE, they must produce a discursive response. Minimal acknowledgements or simple acceptances do not constitute fitted responses because the neurologist has sought the patient’s view. Consider, then, what happens in Extract 15b, which shows how the patient responds to the option-listing–recommending hybrid presented in Extract 15a.


Neu, neurologist; Pat, patient.
As we have seen, the neurologist first shifts from option-listing to recommending at line 17–18, where he announces his view in a mitigated form (note the uncertainty marker at line 17: ‘probably’). There is no verbal response to this, but we cannot be sure of the patient’s non-vocal response (if any) as we were not granted permission to video record. We do know that the neurologist moves very quickly (note the inbreath showing that he is gearing up to speak immediately after the recommendation) to produce some partial reassurance: the scan does not appear to be showing signs of inflammation in the brain (lines 18–21). Building contrastively on this reassurance, he reformulates his recommendation, providing more detail (spelling out what tests would be involved), and strengthening his bid for this course of action (note the upgrade from ‘investigate’ in line 14 to ‘investigate this fully’ in line 23). In response, the patient produces a series of minimal acknowledgements, which receipt the neurologist’s informing turns (see lines 25, 32, 36, 38, 46 and 51). In addition, he produces minimal turns which accept the proposed course of action: ‘Yeh’ (line 28) and the stronger ‘Okay’ (line 48).
Crucially, these minimal responses are treated as adequate for enacting the neurologist’s plan. Although the patient does (at lines 53 and 54) ask a question (which subsequently proves to be related to concerns about his life assurance policy), he does not voice an independent view on the options listed by the neurologist or on the neurologist’s recommendation. This is not treated as problematic by either the neurologist or patient. Once the life assurance questions have been dealt with, the neurologist starts to draw the consultation to a close (data not shown) by giving the patient a form for a follow-up appointment and informing him that an appointment for the tests will arrive by post.
In the post-appointment questionnaires, both participants stated that the patient had been offered a choice, and the patient described this in a way that directly maps onto what we can see in the recording: ‘none or further tests’. Both also agreed that the neurologist had a preference for the latter course of action. Notably, then, losing the PVE – and replacing it with a hearable recommendation – did not dispel the perception of patient choice in this consultation. In examining the detail of the patient’s responses, however, we have seen a real difference in the interactional consequences of these turn types. As we have argued previously,47 accepting a recommendation is interactionally easy: all the patient need do is go along with the neurologist’s stated view of what is best – as he does in Extract 15b. By contrast, simply going along with the neurologist is not possible when producing a fitted, aligning response to a PVE. By replacing the third component of option-listing with a recommendation, then, the neurologist radically alters the kind of slot created for the patient’s response – and hence, for his engagement in the decision-making process.
Displacing the patient view elicitor: seeking the patient’s view after making a recommendation
In other hybrid cases, all three components of full-form option-listing were still produced. However, the final component (the PVE) was displaced from the first two. Again, the crucial factor was that option-listing was combined with recommending. The PVE was displaced by an announcement of the neurologist’s view on what ought to be done. Extract 16a shows an example. This consultation includes, just like the full-form cases shown in Chapter 6, all three option-listing components. At lines 1 and 2 we see the now familiar orientation to an upcoming choice, followed by an explicit listing of the options (lines 2, 22, 29, 44). In contrast to Extract 15a and b, the neurologist also produces a PVE, at line 103. Before he does so, however, he makes a clear recommendation for a combination of options 2 and 3 (see lines 95–100).




Neu, neurologist; Pat, patient.
Extract 16a contrasts with the classic cases shown in the previous chapter not only with respect to the displacement of the PVE, but also with respect to the design of the options. Recall, in particular, the extended case shown in Extracts 7a–g in Chapter 6. What is especially notable about this case is the trouble taken by the neurologist to construct a state of equipoise with respect to the options. The opposite is evident in Extract 16a. As he lists each option, the neurologist asserts his view on it. At line 5 he expresses his reluctance to go for option 1 and, although he accepts that the patient’s version of ‘doing nothing’ (i.e. withdrawing all medication; see lines 12 and 13) is ‘another thing’ they could add to the list (see line 18), he goes on to reiterate his version (i.e. continuing with the current dose of azathioprine, lines 22–26). He thereby makes it apparent that he is not in favour of the patient’s proposal.
At lines 35–42, he announces a preference for pursuing option 2, that is, increasing the patient’s current dose. This is tentatively worded (note the use of ‘probably’ at lines 35 and 42), suggesting some leeway on whether or not this plan is enacted. However, his account for this preference – the patient’s size – and his use of the imperative (‘we should’) counterbalance this cautiousness. At lines 66–69 and 71, he also provides an account for carrying out option 3. Again, there is some cautiousness in his turn design. He explains that the repeat biopsy can ‘sometimes’ be good practice and uses the depersonalised format (‘in these situations where patients aren’t responding’), which avoids directly claiming that it would be good practice in this particular situation. Nevertheless, he is clearly making a case for the value of performing option 3. By the time he makes his recommendation at lines 95–100, then, it is already apparent from the construction of the list that he has a preference with respect to the options.
It is both the design of the list and the displacement of the PVE that matter in this case. These combine to create a very different sequential environment for the patient’s response. Although the PVE does seek the patient’s view, this is set against the backdrop of a clear recommendation by the neurologist as opposed to the efforts to construct a neutral position that we saw in Chapter 6. Extract 16b shows how the patient responds to this PVE. The first thing to notice is that he prefaces his response with the explicit marker of agreement: ‘yeah’ (line 106). This displays his understanding that he is responding to another’s view and, in this case, is aligning with that view. Thus, although he goes on to produce a more extended turn than we saw in response to the recommendation in Extract 15b – where the PVE was dropped altogether – this differs markedly from responses to PVEs that are not displaced by a recommending turn. As we have seen, they cannot properly be receipted with agreement because the neurologist has not produced a proposed course of action. But when the PVE is produced in a different sequential location – following a recommendation – agreement becomes possible.

Neu, neurologist; Pat, patient.
Clearly, the patient does more than produce the kind of minimal agreement we saw in response to a recommendation alone (in Extract 15b). The presence of the PVE, then, does make relevant a more expansive turn – one that is fitted to the opportunity created for the patient to voice his view. It is notable that the patient attends to this explicitly by referring back to something he said earlier (‘like I say’, line 106), thereby constructing his response as partially independent of the neurologist’s prior recommendation. However, if we track back in the talk, we can see that this assessment of the length of time he has been on the azathioprine (‘like I say. . . it’s only been three or four months’, lines 106–108) is actually at odds with his first assessment of this same time period, shown in Extract 16c. Here, when asked how long he has been on the drug, the patient casts the 3–4 months as a long, rather than short, period of time.

Neu, neurologist; Pat, patient.
In recasting the time period as short, he has shifted from his own version of events to that of the neurologist – who has said, with respect to the azathioprine, that ‘it’s still relatively early days’ (lines 25 and 26). Moreover, in proposing that they give it 6 months, the patient not only defers to the neurologist’s opinion regarding whether or not this is an appropriate amount of time (‘whatever=what you think I don’t know’, lines 108 and 109) but relinquishes his previous bid to stop treatment altogether (lines 7–9, 12 and 13). Thus, although his turn is constructed as more than a simple agreement with the neurologist’s recommendation, it does not amount to an announcement of the patient’s preference. Indeed, in his follow-up questionnaire, the neurologist reported that the patient’s preference was for stopping treatment, thereby showing that this was hearable within the consultation.
This is a complex case since the patient’s immediate response is not entirely clear with respect to what is being accepted. The rather emphatic ‘yeah’ appears to accept all that the neurologist has proposed but his expanded turn does not explicitly accept either the dose increase or the proposal to repeat the biopsy – he simply accepts continuation of treatment. However, as is evident at line 111, he does go on to accept the former and, without any subsequent sign of resistance, he fully accepts the latter later in the consultation. Key to our argument here is that – despite the presence of the PVE – the patient produces a turn that connects back to the neurologist’s prior recommendation, aligning with that even as he constructs a turn that claims to do more than simple agreement. Again, the presence of the recommendation changes the sequential landscape, altering what kind of (fitted) response is possible next.
In summary, even if we turn out to be right that there are, indeed, no clear links between the design of the PVE and whether or not patients go on to announce a choice/view on the options, it is clear that the PVE matters. The PVE – if used in a way that is detached from any announcement of the neurologist’s view – is overwhelmingly successful at eliciting patient views following option-listing.
The malleability of interactional practices: using option-listing to curtail choice
We consider next an exemplar of the way in which the machinery of option-listing may be used to do very different interactional work to that seen so far. Again, the contrast is with those full-form option-listing cases shown in the previous chapter, where the practice was evidently being used to hand the decision over to the patient. Here, the practice is evidently being used to try to direct the patient towards the neurologist’s preferred course of action (while, nevertheless, implicating the patient in the decision-making process). In our analysis, we highlight those features of the talk that make it possible for the same practice to be used in such contrasting ways.
Extract 17a shows the first major difference between this case and those shown in the previous chapter, that is, the decision is first initiated by means of a recommendation, not option-listing. It is important to note that this is the same decision that will subsequently be pursued through option-listing later in the consultation (see Extract 17c). At lines 1 and 2 and again, after an inserted question to the patient, at lines 8–11, we see the neurologist initiating decision-making for the first time in this consultation. To do so, he recommends a particular course of action: trying to record some of the patient’s ‘turns’ using a monitor at home (lines 8–11). In common with the recommendations shown earlier in this chapter, this one is constructed as the neurologist’s opinion (‘I think’, lines 1 and 8). It is also given extra weight through the imperative, treating this course of action as something that is necessary (‘we have to’, lines 1 and 8). Indeed, the neurologist does not, at this stage, raise the option of doing nothing. The matter of whether to try to capture a turn is not on the table; only how to do so is up for discussion. His proposal to try home monitoring is highly mitigated by means of the ‘I wonder if’ construction,89 which tentatively ‘floats’ this as something requiring the patient’s agreement. Nevertheless, even within this cautious turn, the neurologist is clearly positioning this option as a candidate ‘best way’ (line 9) forward.

Neu, neurologist; Pat, patient.
This is a complex case because the patient appears to have a mild form of learning difficulty, which seems at least partially to account for the way in which she and the neurologist pursue parallel agendas during the consultation. Here, she is continuing to consult her seizure diary, which she brought into the discussion earlier as a means of answering the neurologist’s question regarding the longest period of time she can go without having a turn. She continues trying to pursue discussion of her diary as the neurologist tries to move the discussion onto the matter of what to do next (lines 6–13, above). In the absence of a fitted response to his recommendation (which could have been produced at line 12), the neurologist produces a PVE (line 13). It is difficult to make out precisely what the patient says next, but it does not appear to be a response to line 13; indeed, the patient starts talking before the neurologist has completed that turn. Her response seems to entail a further attempt to answer fully (based on her diary entries) the neurologist’s earlier question. Neurologist and patient are, then, talking at cross-purposes at this point in the decision-making process.
The neurologist then seeks to bring the discussion back onto the topic of capturing a turn. At lines 22–24 (Extract 17b) he provides an account for his recommendation, which implicitly counters a possible argument against this course of action: that they have already tried, and failed, to capture one. This again makes relevant an acceptance by the patient of the proposed course of action. It is at this point that she produces explicit resistance (lines 25 and 27). However, it is resistance to an alternative method of capturing a turn to the one currently being proposed. The alternative – which they tried previously – involves staying in a specialist centre, often for an extended period of time. The neurologist accepts that she does not want to do this again (line 28), but goes on, at lines 33–37, to make a very strong bid for his initial recommendation (i.e. that they ‘have to try and record some of these turns’, lines 8 and 9). By this point, they are into something of a disagreement, even if that may well be founded on a lack of understanding by the patient of what exactly is being proposed (home recording, not a stay at the centre). Certainly, the mum’s question at lines 38 and 39 suggests that more information is needed.


Neu, neurologist; Pat, patient.
Having provided the requested information (lines 40 and 41) the neurologist again pursues an agreement to his proposed plan. This time, he designs the PVE in a way that is less open, more ‘tilted’ in favour of agreement that the patient would, indeed, ‘be up for’ home recording (compare with ‘what do you think of that’, line 13). Again, the patient starts to derail the focus on decision-making (lines 48 and 52), seeking to show the neurologist photos of bruises she had previously sustained (presumably as a consequence of one of her turns). Explicitly pursuing a response to his question (line 53), the neurologist makes another strong bid for his recommended course of action, constructing it as likely to be the only way the patient will be able to receive any help from this clinic (lines 57–63).
It is at this point that the neurologist shifts from recommending to an alternative approach that bears the hallmarks of option-listing. From lines 65–70 (Extract 17c), we see a condensed version of the option-listing components shown in Chapter 6. At lines 65 and 66, he flags up that there is a decision to be made, and explicitly places this in the patient’s domain: ‘you need to decide what you would like us to do’. Then, at lines 66–70, he lists two options, formatted as an interrogative, thereby folding the action of listing and seeking the patient’s view into one unit (i.e. ‘d’you want us to x or d’you want to y’ serves as another way of doing, ‘We can do x or we can do y. What do you think?’). The design of these options is clearly not neutral with respect to which the neurologist thinks is best. The use of the extreme case formulation90 (‘completely sure’, line 68) and the possibility of treatment, which is held to be contingent on further attempts to record a turn, make it clear that this is his preference. Indeed, the alternative is set up as unsatisfactory for the patient (i.e. having to soldier on implies coping in the face of adversity – adversity that could, by implication, be lessened if they identified an appropriate treatment).

Neu, neurologist.
This is as close to a case of Hobson’s choice as we have in the data set. What it does, however, is precisely what our clinical team members hypothesised: it functions as a method of dealing with the patient’s prior resistance. Against the backdrop of repeated failure to secure her agreement to carry out his recommended course of action, this shift to option-listing creates (yet another) decision point. This time the terms of the decision are set in a way that makes it very difficult for the patient to bid for anything other than home monitoring (think how it would sound to produce some version of, ‘I’d like to soldier on as I am, thanks’). Here, then, the design of the options is crucial. Although the neurologist has used the machinery of option-listing, he has done so in a way that, in effect, constructs another version of his initial recommendation, while more or less pushing the patient into claiming responsibility for the decision.
As it turns out, this approach ‘works’ (in the sense that the patient ends up agreeing with the proposed course of action) – although not instantly. The mum’s immediate response is well fitted to the design of the neurologist’s turn. She has heard the implications of continuing ‘as they are’, and voices her preference next, just as one would anticipate in an aligning turn after option-listing: she announces that she does not wish to ‘soldier on’ because this is limiting her life (lines 73 and 74). The neurologist then tries again to secure the patient’s agreement, using yet another PVE (Extract 17d, line 80). After clearing up some misunderstanding of her immediate next turn (see lines 82–88), the patient makes it clear that she too does not want to ‘soldier on’ because this involves needing someone to go out with – she wants to be fully independent (lines 96, 97 and 102). The neurologist builds off this to secure, finally, a clear agreement from both patient and mum. At lines 103 and 104 he produces another PVE, this one heavily tilted in favour of an agreement to ‘do some recordings’. In response, the mum produces an emphatic agreement token (line 105) and the patient gives an explicit go-ahead (line 106).


Neu, neurologist; Pat, patient.
This is a very extended decision-making process, in large part because of the misalignment between neurologist and patient. Had the patient accepted the recommendation in the first instance, the decision could have been made as early as line 15. For our purposes, there are four key take-home points:
-
Practices for initiating decision-making are not necessarily mutually exclusive. If one approach fails to secure an agreement on what to do next, another may be used.
-
As a result, option-listing as a whole package (and not just the PVE as we saw in the previous section) may be ‘displaced’ in the sense that it may not be the first approach used to initiate decision-making. As such, it may be produced against the backdrop of a recommendation.
-
The design of the options themselves is crucial to the nature of the work done by the option-listing package. As we have seen here, the options can be designed in such a way as to amount to a recommendation.
-
Depending on sequential location (e.g. after a recommendation) and turn design, option-listing may, then, be used to do very different kinds of work.
In summary, although, as we showed in the previous chapter, full-form option-listing stands as the canonical practice for offering choice (at least in our data set), it can be used to do the opposite. This ‘malleability’ of interactional practices means that they cannot be taught, employed or evaluated mechanistically (e.g. ‘to offer a patient choice, use option-listing’). As we will discuss further in Chapter 9, assessing the effectiveness of neurologists’ approaches to decision-making with patients is complex because the conclusions drawn depend on the question: ‘effective for what?’ And, as we have argued elsewhere, the answer to that question is not purely an empirical matter. Rather, it depends on a range of ethical, political – and in this context, clinical – judgements. 91
In the final section of this chapter, we consider effectiveness from a different angle. Instead of focusing on whether or not patients go on to make a decision/announce their view or preference, we consider the possibility that option-listing may be effective for enabling other forms of patient participation (such as asking questions or raising concerns).
Option-listing as a mechanism for enabling other forms of patient participation
It is clear from patients’ responses to full-form option-listing that, even where patients struggle to reach an independent decision, they regularly do engage in the decision-making process in various other ways when option-listing is used. As we have shown, asking a question about the options is a common first response to the PVE (see Chapter 6). It was also fairly common for patients to ask questions or voice their views on options across the course of the option-listing package (i.e. patients do not necessarily wait until the final component – the PVE – has been produced to take an active turn).
For example, in Extract 18, the patient asks a question (line 14) during the neurologist’s explanation of the first option and even cuts him off to ask a question (lines 94 and 96) when it becomes apparent that he is moving to initiate decision-making without having provided information about the side effects of the third option. In response to the first of these – which is structured as a yes/no interrogative86 – the neurologist goes beyond the terms of the question, providing not only a fitted response (‘Yeah . . . Uhuh’, lines 16 and 18) but also additional information (lines 20–25 and beyond, in data not shown). It is impossible to know whether this information would have been delivered without the patient’s question, but it is notable that this generates talk by the neurologist that could well be consequential for the patient’s assessment of whether he wants to try this drug. The significance of the second question is even more striking in that the patient seeks (and subsequently receives) information that the neurologist appears to have skipped over.


Neu, neurologist; Pat, patient.
In Extract 19, we see another neurologist mid option-listing. He is describing preventative treatments for migraine and has listed the antidepressant tablet, amitriptyline as an option. At lines 1–4, he describes its possible side effects. The patient first acknowledges the information (line 6) and then goes on to produce another unit of talk that indirectly assesses the risk of this drug in her specific case: if, as she says, things tend to knock her out, then the side effects just reported by the neurologist are likely to apply to her. The neurologist, at this point, has not explicitly invited her view. The simple receipt that she produces at line 6 would have been – and in many other cases is treated as – sufficient for the neurologist to move on. In this case, when he does not do so immediately (note the brief silence at line 7), the patient takes the opportunity to say more. In effect, she raises a possible barrier to adopting this particular course of action, based on her prior experience (line 8 and then continued at lines 10, 11 and 13).


Neu, neurologist; Pat, patient.
Moreover, in describing her typical behaviour with regard to hay fever tablets (taking them only when really essential, lines 18 and 19) she implies that she may not be the best candidate for preventative migraine treatment (at least not if it leads to ‘sleepiness and tiredness’, lines 3 and 4), as these tablets need to be taken every day – a fact that the neurologist has made clear (data not shown). Just as we saw the neurologist in Extracts 17a and b assessing the options from his point of view as he listed them, here we see the patient doing the same from hers. By the time the neurologist completes the list, then, he will already have some idea of which option she might be most inclined to choose. The neurologist displays his understanding of her concerns by anticipating the effect of her hay fever tablets before she announces it explicitly (line 14).
Extract 20a also shows a consultation in which the neurologist uses full-form option-listing. Here we see him presenting the third option (in data not shown, he listed two other courses of action: trying to capture one of the new kinds of ‘turn’ that the patient is experiencing or trying a change of antiepileptic medication). Again, even before the neurologist explicitly invites the patient’s participation, she does more than simple acknowledgement in her turn at lines 13 and 14. Responding to the neurologist’s third option (watchful waiting), she minimises the significance of her new symptom, implying that she may well also be ‘happy to hang fire and see how things go’ (as the neurologist put it at line 1). The neurologist pursues this possibility through his PVE at lines 16 and 17, which seeks to establish more explicitly how significant these new ‘turns’ are from her point of view.

Neu, neurologist; Pat, patient.
Responding to the patient’s claim that these new ‘turns’ are not a ‘big thing’ in her life, the neurologist then seeks a definite decision from her about what to do next – with another PVE: ‘So what would you like to do about them’ (Extract 20b, lines 24 and 25). What is particularly interesting about this case is that the patient neither quite aligns with, nor disaligns from, the neurologist’s second PVE. On the one hand, she does not do what is asked of her: announce a preference with respect to what they do about her turns. On the other, she does clarify further what matters to her in this decision-making process: avoiding a fall (lines 27–32). This sets in motion two additional history-taking questions (lines 33, 34 and 40–44), the responses to which lead to advice-giving by the neurologist at lines 54–60. This amounts to a decision in favour of the third option: watchful waiting. But although this decision is voiced by the neurologist, it is clearly predicated on a mutual ‘working through’ – via a series of questions and answers following the option-listing – of the patient’s concerns and experiences relating to her new symptom. In effect, then, one could argue that option-listing, in this case, has successfully generated an extended sequence of SDM, even though the patient has not directly answered the question of what she would like to do about her ‘turns’.


Neu, neurologist; Pat, patient.
In summary, even if patients don’t make a choice following option-listing, they typically do ask questions or voice some kind of opinion beyond just assenting. It appears, then, that option-listing can be effective for enabling a variety of forms of patient participation even in cases where the patient may not wish to make the final decision about treatment/further tests.
Conclusion
In this chapter we have considered patient responses as an internal marker of effectiveness – of whether or not option-listing works. We have argued that option-listing is clearly effective for generating the perception of choice. With respect to whether or not patients actually go on to make a choice, however, it only works some of the time. We considered the possibility that this could be explained by the design of the third component of option-listing (the PVE). Thus far, however, we have been unable to show a link between outcome (whether or not the patient makes a choice) and the specific wording of the PVE. Rather, the crucial factor seems to be whether or not (and if so, when) the neurologist announces his/her view. If the PVE is either replaced with, or displaced by, a recommendation from the neurologist, the slot for the patient response to option-listing can be significantly altered. Moreover, we showed that, depending on when and how the machinery of option-listing is used, it can have the opposite effect to that shown in Chapter 6: instead of promoting patient choice, it may curtail it. 10
This evidence of the malleability of interactional practices highlights the absence of a direct, one-to-one correspondence between, on the one hand, the specific practices that doctors use to initiate decision-making in interaction with patients and, on the other, the more general decision-making models that are so prominent in the literature20 (see Chapter 1). Rather, the same practice can be used in ways that might be analysed as an example of any of these three approaches: for example, one could argue that option-listing, in Extracts 17a–d, is being used to paternalist ends (getting the patient to do as the neurologist sees fit); that, in Extracts 11a and b, it is being used to initiate SDM (both sides sharing relevant information, discussing the options, and plenty of time allowed for further information seeking and final decision-making); and that, in Extract 10 – where the neurologist provides the relevant information but then says ‘it’s up to you’ – a consumerist model is in evidence. These are, of course, arguable characterisations. But the fact that the same practice can be used to such different ends demonstrates the need to take seriously the detail of the talk.
Chapter 8 Patient view elicitors as a practice for offering patients choice
Introduction
As we emphasised in Chapter 6, option-listing was the only practice demonstrably oriented to giving patients choice that occurred (with just one exception) only in those consultations for which there was agreement (between neurologist and patient) that a choice had been given to the patient. However, not all consultations in the subset in which neurologists and patients agreed there was choice contained option-listing. In this final analytic chapter, we examine another practice that was common within this subset: the use of PVEs. We have already examined these in the context of full-form option-listing, as they comprise the third component of that practice. Here we consider how they may be used independently of option-listing – as an alternative approach to initiating decision-making. Crucially, the decisions initiated with PVEs are not constructed as a matter of selecting from a menu of alternatives. Rather, they involve making a decision for or against one possible course of action.
Our collection of such single-option PVEs divides into two distinct groups: those where the possible course of action was introduced prior to the use of the PVE and those where it was introduced through its use. Extracts 21 and 22 show this distinction. In Extract 21, the neurologist, having established with a preliminary query (lines 1 and 2) that the patient is not currently taking treatment for MS-related fatigue, provides information relevant to deciding whether or not to start such treatment (see turns in bold font). This includes making explicit the implication of his question at lines 1 and 2 that ‘there are certain drugs that can help with fatigue’ (lines 6–8) and also providing further information about MS that ties back to what the patient has said about her difficulties when working, which she is currently not doing (i.e. that she used to get particularly tired if she had to concentrate a lot; data not shown). The treatments he is describing, then, are positioned as a possible solution to these difficulties. It is only after providing this information that he seeks the patient’s view on this possible course of action (i.e. starting treatment) at lines 22 and 23 ‘is that something that would interest you’.


Neu, neurologist; Pat, patient.

Neu, neurologist; Pat, patient.
In Extract 22, by contrast, the neurologist’s turn just prior to the PVE has not been directed towards informing the patient about a possible treatment. Rather, she has been describing some of the possible triggers for migraines (lines 1–9). Having concluded with a diagnostic claim: that the patient’s prior description of her symptoms ‘sounds very typical for a migraine aura’ (lines 7 and 9), the neurologist shifts from informing the patient to seeking her view. Here, the PVE itself does the work of informing the patient about the option on offer (a preventative drug for her migraines). This information is built into the design of the neurologist’s question to the patient: ‘Are those kind of often enough that you would be wanting to try a drug to try and reduce how often it’s happening’ (lines 9–11). Again, the information provided by the neurologist about the possible course of action is in bold font. This is the first mention in this consultation of preventative migraine treatment.
Both uses of PVEs – with or without prior information provision about a possible course of action – function to place the decision in the patient’s domain. This is evident in their design. For example, in Extract 21, the neurologist makes trying treatment for fatigue contingent on whether or not doing so would be of interest to the patient; and, in Extract 22, the neurologist makes trying preventative medication contingent on the patient’s assessment of whether her migraines are frequent enough for her to want treatment.
In this chapter, we will develop this analysis of PVEs as a practice for offering patients choice. We will show that:
-
PVEs with prior information provision function to cast that informing as a matter of laying out an option, as opposed to telling the patient what to do
-
PVEs without prior information provision function to avoid constructing a recommendation to the patient altogether, foregrounding, instead, the matter of the patient’s wishes.
In conclusion, however, we will also argue that each of these raises a potential difficulty for patient choice.
Patient view elicitors after information about a possible course of action
As seen in Extract 21, PVEs can be used after the neurologist has informed the patient about a possible course of action. In some cases, there is evidence that the neurologist is working to present this possible option (even before producing the PVE) with only a very weak claim to having any right to direct the patient’s future action – or, to use Stevanovic and Peräkylä’s92 term, with minimal deontic force (see Chapter 9, Implications for patient involvement in decision-making for further discussion of the concept of deontic authority). Extract 23 provides a good example. The neurologist leads up to the topic of disease-modifying therapy for MS by reminding the patient of a previous discussion about ‘the nature of [her] illness’ (lines 1 and 2), then providing a summary of her condition (lines 2–6), followed by another reminder of a previous discussion about treatment (lines 8 and 9). He then justifies initiating discussion of treatment again on the grounds that she has had a further relapse (lines 11 and 12). This initial information provision by the neurologist, then, orients the patient to the relevant facts that she is likely already to know. Its effect is to (rather gently) indicate that the patient is, in the here and now, a candidate for disease- modifying therapy. Moreover, the neurologist’s claim that they ‘need’ to revisit this discussion (line 12) hints at his view – stated in the post-consultation questionnaire – that the best course of action would be to start such treatment. However, he constructs his informing turns in a way that heavily mitigates any implied recommendation about what the patient ought to do. Notably, he only suggests that they revisit the treatment discussion, not that she should start treatment. Indeed, the patient, in her post-consultation questionnaire, stated that the neurologist did not have a treatment preference. When the neurologist produces the PVE, then, he has already laid a relatively neutral foundation for the decision-making.

Neu, neurologist; Pat, patient.
The relative neutrality achieved in the informing is reinforced by the design of the PVE (line 14), which is fully open ended, creating a slot in which the patient has lots of leeway in the kind of response she can give. As it turns out, she decides against treatment at this stage (lines 16, 17, 19 and 22), and the neurologist accepts this decision (at lines 20 and 24 and further, in data not shown). Here, then, we see the design of the neurologist’s informing working hand in hand with the PVE to offer the patient choice. In such cases, the informing plus PVE function in a similar way to option-listing plus PVE: the neurologist first gives the patient the resources she needs to make the decision, then hands that decision to her.
However, just as we saw with respect to option-listing, the information delivered about single courses of action prior to the use of a PVE may be designed in a non-neutral way; it may be designed to do recommending. Extracts 24a and b show a clear case. Here, the neurologist has clearly proposed a two-step course of action, using language that is typically associated with recommending. First, he names the action he is performing as a suggestion (line 2); then he announces what he thinks they ‘should do’ first (lines 3 and 4), and what the likely next step will be (lines 15 and 16). Certain features of his turn function to make his recommendation less directive than it could be. For example, in constructing the two steps as a suggestion, he implies that the patient has the right to agree or refuse to go along with this plan. Moreover, he describes the test as offering ‘some guidance’ (line 11), rather than as an absolute necessity, and he softens the force of the second step with the addition of ‘probably’ (line 16). Nevertheless, there are elements of his talk that suggest relatively little room for patient choice in this decision. For example, in saying: ‘they [a]re gonna give you some exercises’ (lines 6 and 7), ‘that [wi]ll give us some guidance’ (line 11), and ‘my suspicion is we [wi]ll withdraw the drug and not a lot will happen’ (lines 39 and 40), the neurologist is casting this as a decision that has already been made – the tests ‘are going’ to happen and the drug withdrawal ‘will’ take place. Thus, the information here is delivered with far more deontic force than in Extract 23.


Hus, husband; Neu, neurologist; Pat, patient.


Hus, husband; Neu, neurologist; Pat, patient.
It is only after the patient’s husband’s question (lines 44 and 46) has been dealt with – which somewhat diverts them from the decision at hand – that the neurologist produces a PVE (lines 83–86). This not only returns them to the decision, but also recasts that decision in different terms. Having first produced a recommendation, the neurologist uses the PVE to indicate that that decision can, in fact, be made on the basis of what the patient wants to do.
In effect, then, the PVE in Extract 24b serves to decrease the deontic force of the prior recommendation. In seeking the patient’s view, the neurologist diminishes the extent to which he is claiming to have a right to tell the patient what to do, and places the decision explicitly in the patient’s domain. Subsequently, on hearing the patient’s reluctance (lines 88–91) to try the second step of his plan (withdrawing the drug), the neurologist pulls back further from the original force of his recommendation. Whereas he first said that withdrawing the drug would probably be the next step ‘whatever [the test] shows’ (lines 14 and 15), after the patient’s response to the PVE he constructs this second step as more heavily contingent on the test: ‘we’ll do this test first . . . and if it shows any evidence that you do have epilepsy then . . . we’ll have another wee chat’ (lines 104–110).
Just as we saw in option-listing, then, the PVE creates a slot where the patient’s view is relevantly due next; unlike with simple recommendations, that view is actively invited. As a consequence, the patient can (as in Extract 23) announce his/her own decision or (as in Extract 24b) at least influence the decision by raising a concern in next turn. However, unless the neurologist does special work to avoid being heard in this way – as he does in Extract 23 – this kind of PVE works in conjunction with a prior recommendation. Thus, when the patient responds to the PVE, the backdrop is not neutral. As we showed for option-listing cases where the PVE was displaced by a recommendation, agreement with the neurologist’s view becomes a possible response here (a response which, as we showed in the previous chapter, would not be properly fitted to a PVE that did not follow a recommendation).
Extract 25 illustrates this point. Here the neurologist makes a bid to prescribe the AED phenytoin, as a short-term solution while surgical assessment proceeds. As in Extract 24a, the conversation gets diverted onto a related, but separate, topic (matters relating to the patient’s possible surgery). Again, the PVE (line 81) functions as a means of bringing the conversation back to the immediate decision at hand. It also functions to place this decision in the patient’s domain: it is contingent on what she ‘thinks’ about the drug. However, the patient’s response (line 82) does not quite conform to the design of the prior turn. She has been asked for her view on phenytoin and she responds with a decision to try it that is explicitly formulated as an agreement – note, particularly, the turn-initial ‘yeah’, which we saw in a similar sequential context in Chapter 7, Extract 16b, when the patient was responding to a PVE (as part of an option-listing package) that had been displaced by a recommendation.

Neu, neurologist; Pat, patient.
Again, we should be clear that we are not making any prescriptive arguments here. There is plenty of evidence across this consultation that the patient (and her partner) is highly involved in the decision-making process, that the neurologist invites this involvement, and that the patient is happy to try the suggested AED as a short-term solution. Indeed, the patient recorded ‘happy’ in response to the questionnaire item asking how she felt after the consultation. Our point here is to highlight, again, the difference a recommendation makes to the interactional landscape. Patient view elicitors clearly reduce the deontic force of recommending turns. But the recommendation remains hearable, and is something to which patients may demonstrably orient even when responding to a PVE.
Stand-alone patient view elicitors
Stand-alone PVEs, by contrast, do not follow an informing about a proposed course of action. Rather than being susceptible to responses that are oriented to the neurologist’s preference, then, these PVEs carry another risk: that patients will lack the resources to make the proffered decision. Extracts 26 and 27 illustrate this. Both follow the same pattern shown in Extract 22: in neither is there prior information provision about the course of action raised via the PVE. In Extract 26, the neurologist seems set to make a recommendation for an occupational therapist (at line 1), but shifts instead to a preliminary question about the patient’s daily functioning (lines 1 and 2). Despite the patient’s claim not to struggle around the house (line 3), the neurologist pursues the possibility of a visit by the occupational therapist through his subsequent PVE (lines 4 and 5). Although the PVE functions to offer help, the neurologist has not, at this point, provided any information about what exactly could such help might entail. In response, the patient seeks that information (line 7).

Neu, neurologist; Pat, patient.

Neu, neurologist; Pat, patient.
In Extract 27, the neurologist and patient reach an agreement about the likely nature of her current MS-related symptoms (lines 1–10). The neurologist then moves straight to a PVE about possible treatment (line 12). No information about this possible course of action is provided prior to the PVE. Again, the patient seeks that information in next turn (line 15).
Extracts 28a and b show a more extensive example. Here the discussion about the patient’s concern about her speech is triggered by the neurologist’s question at line 1. Responding to the patient’s problem presentation (see lines 3–17), the neurologist first produces a diagnostic question: ‘Is that just when you’re fatigued?’ (lines 18 and 19). However, instead of stopping to allow the patient to reply, she constructs a double-barrelled question which raises two possibilities: that the problem can be explained purely by fatigue and hence does not need professional help, or that the problem is sufficiently significant for the patient to ‘need to see a speech therapist’ (lines 19 and 20). This question functions in the same way as the PVE in Extract 27, placing the matter of treatment in the patient’s domain; it is contingent on her assessment of her symptom’s severity.

Neu, neurologist; Pat, patient.


Neu, neurologist; Pat, patient.
The patient clearly has difficulty in assessing whether she needs a speech therapist but at first her difficulty appears to lie in judging whether her condition is serious enough to warrant intervention. This is the question that the patient pursues for some time (via consideration of what others have said to her). When the patient explicitly claims an inability to assess whether she needs a speech therapist (‘I don’t know’, lines 22 and 35), the neurologist avoids giving her opinion, keeping the decision-making firmly in the patient’s domain, again based on the question of severity: ‘it depends how much of a problem it is for you’ (line 36). After further deliberation by the patient, the neurologist seeks to close the topic by producing a candidate upshot of what the patient has been saying: that she does not want ‘any intervention at the moment’ (lines 65 and 66). It is only then that it becomes apparent that another difficulty underpins the patient’s indecision: she lacks information about the proposed option (lines 67–69). Indeed, in data not shown, she also acknowledges having already asked a speech therapist (who she knows from the school where she teaches) about her condition, and reports having been told: ‘Well I wouldn’t know’.
As is also the case in Extracts 26 and 27, the neurologist goes on to provide the missing information (data not shown). So the issue is not that patients (necessarily) remain uninformed. Indeed, it appears that the PVE, in creating an explicit slot for the patient to speak next, opens up space for information-seeking questions to be raised. Rather, these examples highlight a key challenge of engaging patients in decision-making about a single option. If they go straight for a PVE, neurologists can avoid the risk of being heard to be making a recommendation through their information provision. Thus, the stand-alone PVE carries very little deontic force. However, in the absence of a prior informing, patients may be left ill-equipped to respond to that PVE.
Conclusion
Unlike decisions initiated through option-listing, those initiated with PVEs are not constructed as a matter of selecting from a ‘menu’ of alternatives. Rather, they involve making a decision for or against one possible course of action. We have shown how our collection of these single-option PVEs divides into two distinct groups and have argued that both forms of PVE serve to place the decision in the patient’s domain. However, we have also suggested that each raises a potential difficulty for patient choice: PVEs produced after the neurologist has informed the patient about a possible course of action run the risk of being treated by patients as recommendations (and not a matter of choice) because the information may be heard as an indication of what the neurologist thinks ought to happen next (rather than a neutral informing). By leaving out the prior information, neurologists may avoid this risk. However, they may, instead, leave the patient ill-equipped to respond to the PVE. Ironically, the patient may, on hearing a stand-alone PVE, recognise that s/he has a choice, but be unable to exercise it.
Chapter 9 Discussion
Introduction
Based primarily on the fine-grained analysis of 223 consultations recorded in neurology outpatient clinics in Glasgow and Sheffield, this study has investigated the communication practices used by 14 neurologists to involve patients in decision-making. We sought to contribute to the evidence-base about whether or not, and how, patient choice is implemented in neurology, and to identify the most effective practices for doing so. This matters for three main reasons: first, patient choice is central to NHS policy, and the UK’s General Medical Council8 has made it a requirement that doctors ‘maximise patients’ opportunities, and their ability, to make decisions for themselves’ (p. 6). Second, in neurology ‘a patient-centred service’ is upheld as a quality requirement, and two neurological conditions (epilepsy and MS) have been identified by the UK’s Department of Health13 as well suited to SDM. Third, despite the value placed on patient choice, there is very little detailed, evidence-based guidance for doctors on how to implement the policy directives in practice.
In this final chapter, we first summarise our key findings, after which we then explore their implications for patient involvement in decision-making, clinical practice and medical sociology. We consider the study’s strengths and limitations, reflect on our experience of patient and public involvement, make recommendations for future research, and consider our approach to addressing objective 3 (dissemination of our findings to help clinicians, where appropriate, to deliver patient choice in practice).
Summary of key findings
From the self-report data
This study combined a CA approach – which entails systematic, qualitative analysis of interaction – with a quantitative analysis of post-appointment questionnaires administered to neurologists and patients. This allowed us to investigate participants’ perceptions of choice as well as the practices used to offer patients choice in the clinic. A key finding from the self-report data was that neurologists and patients agreed that choice had featured in over half of all recoded consultations. The agreed presence of choice was four times as common as the agreed absence of choice (i.e. 53.6% vs. 14.3% of consultations or 105/196 vs. 28/196, with data missing for 27 cases; see Chapter 4, Quantitative exploration of prevalence of choice). In an additional 31.9% of consultations, either patient or neurologist thought the patient was offered a choice when the other did not. Perception of choice, then, was relatively high in our data set, and there was agreement about presence or absence of choice between neurologist and patient in just over two-thirds of all recorded cases.
From the no-choice subset (n = 28)
Seeking to link these self-report findings with what happened in the recordings, we examined the full data set for patterns in how decision-making was conducted. We found a distinction between those cases for which neurologist and patient agreed that choice was absent and present. In the former – but not the latter – the neurologist constructed what was to happen next, not as a matter to be decided, but as a logical consequence of the diagnostic conclusions reached (thus far). The key finding from the no-choice subset was that when the main conclusion of the consultation was that nothing (or nothing new) could be done from a neurological perspective, then neurologist and patient typically both reported that no choice was offered (see Chapter 5, Logical consequence rather than a decision to be made).
From the choice subset (n = 105): analysis of option-listing and patient view elicitors
Comparison of all four subsets of recordings (i.e. agreement there was a choice, agreement there was not, neurologist reported there was a choice but patient did not and vice versa) produced a second, striking finding: that option-listing – identified in our pilot data set as a practice for giving patients choice10,47 – was present (with just one exception) only in those consultations for which neurologist and patient agreed that a choice had been offered. It appears, then, to be a practice that is not only analysable as creating a slot for patient choice, but also one that neurologists and patients perceive as doing when they reflect on their interactions. This was the only practice demonstrably oriented to offering patients choice that we could identify as being (with just one exception, which as we show, proves the rule) unique to this subset. We therefore subjected all instances of this practice to extensive analysis using CA.
In Chapter 6 we described the three components that make up option-listing in its full form:
-
an announcement by the neurologist that there is a decision to be made
-
the formulation of a list of options
-
an invitation to the patient to announce their views with respect to the options or select an option from the list.
Focusing on the design of each component, we showed how they can be produced in ways that, incrementally, construct the decision as the patient’s. Examining patients’ responses, we also showed how each of three response types – those that align with the action performed by the neurologist’s turn, those that defer the production of a relevant response, and those that counter the prior action – all demonstrate patients’ orientations to option-listing as a practice for handing the decision to them.
In Chapter 7 we examined option-listing further, with a focus on our second objective: to identify the most effective practices for facilitating patient choice. We argued that this practice works in one sense (i.e. it readily generates the perception of choice). With respect to whether or not patients actually go on to make a choice, however, it clearly works only some of the time. We considered the possibility that this could be explained by the design of the third component – the PVE. Thus far, however, we have been unable to show a link between outcome (whether or not the patient makes a choice) and the specific wording of the PVE. Rather, the crucial factor seems to be whether or not (and if so, when) the neurologist announces his/her view. If the PVE is either replaced with, or displaced by, a recommendation from the neurologist, the slot for the patients response to option-listing can be significantly altered. Moreover, we showed that, depending on when and how the machinery of option-listing is used, it can have the opposite effect to that shown in Chapter 6: instead of promoting patient choice, it may curtail it.
In our final analytic chapter (see Chapter 8), we examined an additional practice that was common in (but not exclusive to) the subset of consultations for which neurologists and patients agreed that a choice was offered: PVEs used independently of option-listing. Decisions initiated with PVEs are not constructed as a matter of selecting from a ‘menu’ of alternatives. Rather, they involve making a decision for or against one possible course of action. Our collection of these single-option PVEs divides into two groups: those where the course of action was introduced prior to the PVE, and those where it was introduced through its use. We showed that:
-
PVEs with prior information provision function to cast that informing as a matter of laying out an option, as opposed to telling the patient what to do
-
PVEs without prior information provision function to avoid constructing a recommendation to the patient altogether, foregrounding, instead, the patient’s wishes.
Thus, both forms of PVE – like option-listing – place the decision in the patient’s domain. However, we suggested that each raises a potential difficulty for patient choice: PVEs produced after the neurologist has informed the patient about a possible course of action run the risk of being treated by patients as recommendations because the information may be heard as an indication of what the neurologist thinks ought to happen next. By leaving out the prior information, neurologists may avoid this risk. However, they may, instead, leave the patient ill-equipped to respond to the PVE. On hearing a stand-alone PVE, then, patients may recognise they have a choice, but be unable to exercise it.
Implications for patient involvement in decision-making
Running through these findings are two themes: the related concepts of epistemic and deontic authority. Drawing on these across the following sections, we will reflect on the implications of our findings for patient involvement in decision-making.
Option-listing: a practice for tempering doctors’ epistemic and deontic authority
Neurologists’ use of option-listing and PVEs is, we would argue, an indicator of their orientation (and one kind of response) to the radical shift in expectations of the doctor–patient relationship that has occurred over the last half-century or so. There has been a sustained critique – in the literature and through patient activism – of the paternalist model, in which doctor knows best and has the right simply to tell the patient what to do. Nevertheless, as Pilnick and Dingwall31 argue, ‘asymmetry lies at the heart of the medical enterprise: it is, in short, founded in what doctors are there for’ (p. 1374).
In large part this asymmetry lies in the epistemic – or knowledge-related – differences between doctor and patient. As Heritage93 has shown, doctors and patients are typically held to have unequal epistemic access to health-care-related information: ‘They occupy different positions on an epistemic gradient (more knowledgeable [K+] or less knowledgable [K–]), which. . . may vary in slope from shallow to deep’ (p. 32) (see Figure 5). Heritage94 argues that even though relative epistemic access is an inherently relational matter, open to manipulation and challenge, it tends to be treated as predominantly agreed upon for particular domains. For instance, individuals are usually treated as knowing more about their ‘relatives, friends, pets, jobs and hobbies than others’ (p. 6), and socially sanctioned ‘experts’ are usually treated as knowing more about their specialist area than ‘amateurs’. As Heritage94 asserts in relation to clinical diagnosis: ‘My doctor and I may both look at an X-ray of my foot, but mere observation will not provide me with the epistemic resources to contest her diagnostic conclusion’ (p. 5). The same can be said for treatment. As we have argued elsewhere: ‘Doctors have, relative to patients, both a greater epistemic right (as qualified professionals) to knowledge about biomedical treatments and (typically) greater epistemic resources (for example, clinical or statistical evidence) to justify their conclusions’47 (p. 6).
FIGURE 5.
Reducing the epistemic gradient through information provision.
Nevertheless, our study suggests that not all approaches to decision-making in clinical practice invoke the same degree of epistemic asymmetry. If we compare option-listing to recommending a course of action, the former sets up a shallower epistemic gradient than the latter. This is because the three components of option-listing – at least as used in cases like our exemplar (see Chapter 6, Extracts 7a–f ) – function to avoid the ‘normative dimension’95 (p. 368) that is embedded in making a recommendation. In other words, option-listing can be used to avoid claiming to know what the patient should do, claiming, instead, only to know what the options are. Recommendations, by contrast, claim a preference on the neurologist’s part and are often justified in ways that suggest a best option for the patient.
Epistemic rights comprise one dimension of medical authority. 47 A related dimension is ‘deontic authority’ or ‘the right to determine others’ future actions’92 (p. 297). Like epistemic authority, deontic authority is relational, but appears to be broadly linked to particular domains, such that ‘the deontic (as well as epistemic) rights of a person vary from domain to domain. A person may therefore have more rights to decide about future courses of action in some areas of action than in others’92 (p. 298). Just as doctors are socially sanctioned experts with respect to biomedical knowledge, so they are widely held to have a legitimate right to decide how patients ought to act with respect to that domain.
Nevertheless, the deontic stance that the design of a particular turn encodes may vary. Thus, a neurologist may construct a stronger or weaker claim to deontic authority vis à vis the patient’s future health-related action. The use, for example, of ‘we need to’ and ‘we have to’ in the recommendations shown in Chapter 6, Extracts 8 and 9, compared with ‘we could add in’, ‘we could try’ (see Chapter 6, Extract 7a), and ‘you can either’ (see Chapter 6, Extract 10) in some option-listing cases, illustrates this. In the former, the neurologists treat themselves as having a stronger right to determine what action is to be taken. In the latter, they weaken their claim to deontic authority, distributing such rights (somewhat) more evenly between themselves and the patient. Moreover, PVEs like, ‘so it’s up to you’ (see Chapter 6, Extract 10), ‘either one of them grab you’ (see Chapter 6, Extract 7a), ‘any first thoughts’ (see Chapter 6, Extract 11a, and Chapter 7, Extract 11b), ‘I have to sort of pass it back to you and say what do you want’ (see Chapter 6, Box 2) and ‘what would you like to do about them’ (see Chapter 6, Box 2), all avoid claiming the right even to propose a course of action for the patient. Instead, a slot is created for the patient to announce a decision/view. The right to decide has thus been relinquished by the neurologist.
This, as we have seen, has significant implications for what patients can legitimately do next. Although previous research has shown that patients have strategies for getting their (dissenting) views heard after a doctor’s recommendation,45,51 recommending itself is not designed to invite their views. By contrast, full-form option-listing is. Moreover, although the neurologist is (largely) in control of the list – which options are included and how each is presented – option-listing generates more room for manoeuvre by the patient than does recommending. As we have argued elsewhere,47 this is partly because, ‘in epistemic terms, the patient need not work with (or against) an expert opinion of what is best’ (p. 14), as the neurologist has not announced such a view. We can now add another dimension: in deontic terms, patients have been given the right to make the decision (or, at least, to add their view into the mix). In option-listing, then, we see one way in which neurologists appear to be working to meet the challenge posed by decades of critique of ‘paternalistic’ medicine. In tempering their claims to epistemic and deontic authority (at least a little), neurologists can actively seek to create a space for the patient’s voice during decision-making.
The concepts of epistemic and deontic authority also offer a convincing explanation for our finding that the presence/absence and sequential placement of the PVE during option-listing can significantly alter the slot for the patient’s response. As we showed in Chapter 7, the PVE – if detached from any announcement of the neurologist’s view – is overwhelmingly successful at eliciting patient views following option-listing. If, however, the PVE is replaced or displaced by a recommendation, the patient may respond in a way that takes the recommendation into account. In the hybrid cases shown in Chapter 7, Extracts 15a and b and 16a and b, the claim to know what the patient should do next is made, despite the use of the wider machinery of option-listing. Thus, the relative levelling of the epistemic and deontic gradients that may be achieved by option-listing is (partially) reversed. This becomes particularly clear if we consider the neurologists’ explicit ownership of the proposed plan in Chapter 7, Extracts 15a–b and 16a. In Chapter 7, Extracts 15a–b, he frames the plan as his recommendation/suggestion (‘what I would recommend doing’, lines 17 and 18; and ‘that’s what I would suggest we do’, line 47) and also as something he wants to do (‘what I’d like to do is’, line 24). In Chapter 7, Extract 16a, we see very similar formulations: ‘I probably would want to do’ (lines 35 and 36) and ‘my preference would be’ (line 95). In both, the neurologists are – however tentatively – announcing their view on what should be done; and they are doing so before the patient has announced his/her view.
Given that neurologists, speaking about treatment/investigation options in a clinical setting, occupy a default position of greater epistemic and deontic authority than do patients, these views carry special weight. They are hearable as professional, expert opinions, rather than personal ones. To go against the neurologist’s preference, the patient must work against the claim of an expert to know what is best for him/her. Thus, even if a PVE is subsequently produced, the patient is responding in the context of a very different interactional landscape: she/he is responding not only to the PVE, but with reference to the neurologist’s view.
The single-option dilemma: balancing deontic and epistemic gradients
Drawing on the concepts of deontic and epistemic authority, we would argue that the two forms of PVE discussed in Chapter 8 indicate something of a dilemma facing neurologists when seeking to give patients choice about a single option. On the one hand, there is typically an epistemic gradient between neurologist and patient, and part of the neurologist’s task is to provide the patient with information – and thereby decrease the gradient. This can be represented as shown in Figure 5.
On the other hand, as we have shown, when there is just one reasonable option, the informing is liable to be heard as something more: as a recommendation for that option. Thus, we would argue, a PVE plus prior informing will tend to carry more deontic force than a stand-alone PVE (i.e. those not preceded by an informing) since, unless special work is done, the informing is likely to be heard as conveying a view on what the patient ought to do, as depicted in Figure 6.
FIGURE 6.
Stand-alone PVE vs. informing plus PVE: a difference in deontic force.

The dilemma, then, is this: as the epistemic gradient is reduced (by means of information provision) so the deontic force may be increased (by virtue of the informing being hearable as a recommendation). But if the neurologist decreases the deontic force without also decreasing the epistemic gradient (by handing the decision to the patient without prior information), the patient may be unable to act on his/her increased right to decide (owing to a lack of resources). There is, then, a potential trade-off with regard to epistemic and deontic authority. In the next section, we examine a single case in detail to highlight some ways in which this dilemma might be addressed.
Addressing the single-option dilemma: some pointers from an illustrative case
The case shown in Extracts 29a–d does not quite fit the key patterns described in this report. In action terms, however, the neurologist achieves the same effect as achieved with a PVE: the patient not only has an opportunity to voice her view on treatment but actually makes the final decision. Moreover, we have chosen this case because there is evidence, within the interaction, that the neurologist is successful both in informing the patient about a single treatment option and in avoiding being heard to make a recommendation.
In Extract 29a, the neurologist sets up the matter of ‘considering treatment’ (lines 4 and 5) virtually from the outset of the consultation, when he seeks clarification of the patient’s reason for attending. Moreover, responding to the patient’s implicit query about whether a treatment exists (line 7), the neurologist answers affirmatively (line 9), but starts to avoid advocating a course of action by highlighting the fact that any treatment will have drawbacks (lines 9–12). Before describing any options, then, the neurologist has begun to indicate that there is a decision to be made. In questioning the patient, he also considers a decision-making factor that is firmly in her domain: how embarrassed she feels about her symptom (line 21). Although preliminary to the decision-making proper, this question is a kind of PVE, in that it seeks her view on the effect of the condition as a basis for deciding whether or not treatment is likely to be worthwhile.

Neu, neurologist; Pat, patient.
Having received a relatively ‘no problem’ type of response, the neurologist delivers information about treatment (Extract 29b, lines 25–33). This turn is designed in a way that avoids being hearable as a straightforward recommendation. By announcing first that there is an ‘issue’ (line 25), the neurologist again implies that there is some kind of deliberation required. Moreover, this preface indicates something of what it will take for the neurologist to complete his turn. Lines 26, 27 and 29 are insufficient to deliver on that preface, because the neurologist has not yet described an ‘issue’. Thus, the preface foreshadows the downside – the ‘but’ – which is produced after the initial informing about the type of treatment on offer (lines 32 and 33). From the start of this informing, then, the neurologist has worked to minimise the likelihood that the patient will hear this as a recommendation, aiming to construct it, instead, as information provision about the pros and cons of what is on offer (akin to many of the informing turns we have seen in option-listing).

Neu, neurologist; Pat, patient.
There is evidence that the neurologist’s attempts to do plain informing have been successful (i.e. at line 48, the patient positions herself as being the one to decide). Having established that this is definitely a one-option decision (lines 43–46), she announces her uncertainty about what to do (line 48). Although at this point she has not yet announced a decision, this turn provides clear evidence that she has heard the neurologist as handing the decision to her. Even without the explicit go-ahead provided by a PVE, she is orienting to this as a slot in which her decision is relevantly due. By including the word ‘then’ in her turn, she displays that her uncertainty is the consequence of now being fully informed about what is on offer. She is not treating the prior as a recommendation to be accepted or resisted, but as information on the basis of which to reach a decision.
On hearing her indecision, the neurologist provides further information about the treatment and her condition (Extract 29c, lines 50–56). He makes explicit the relevance of his prior question about the patient’s degree of embarrassment, firmly placing the decision in her domain – it is a question of what (emotional) effect the symptom is having. Again, he does not produce a PVE in so many words, but by positioning this as a personal decision, and reflecting on how he might feel personally, the neurologist further constructs this as the patient’s choice. She responds as if to a PVE at lines 67–75, providing an account for deciding to try the treatment. She thereby displays an understanding of the information just provided. Arguing that the injection could be seen as an experiment, she implicitly seeks confirmation that this is a legitimate decision. The neurologist provides this assurance at lines 70, 74 and 76.


Neu, neurologist; Pat, patient.
The final decision is overtly owned by the patient, when she responds rather literally to the neurologist’s invitation to move seats to receive the injection. As he starts speaking at line 101 (Extract 29d), he shifts towards the other seat. During his invitation, he gestures towards that seat and starts standing up, embodying his understanding that a decision has been made. Nevertheless, the design of her response –‘I do’ (line 104) – constructs this as an affirmation of her preference96 (i.e. that she does want the treatment), rather than a simple agreement to move (e.g. ‘yeah’) – as if the prior turn was itself a PVE.

Neu, neurologist; Pat, patient.
This example shows how neurologists can work to give patients information about a single option (thereby decreasing the epistemic gradient between neurologist and patient) without simultaneously increasing the deontic force of their turn. In this consultation, the neurologist:
-
makes it clear that there is a decision to make
-
is successful in delivering information that is not treated as a recommendation
-
allows space for the patient to voice her view and, ultimately, to make the decision.
These three features map closely onto the more structured approach evident in option-listing. We suggest that part of the effectiveness of option-listing as a practice for offering choice may be the way in which it provides a ready solution to the potential difficulty of balancing epistemic and deontic gradients when talking with patients about decisions.
Revisiting the components of full-form option-listing: a solution to the single-option dilemma?
As we have shown, full-form option-listing opens with an announcement, flagging up that there is more than one option for what to do next. In addition to the work this does in setting up an opportunity for the patient to make a choice, it achieves another function: clearing the way for an extended turn at talk. Interactionally, this is important, since the ordinary turn-taking system typically accords each speaker just one turn at a time before transition to another speaker becomes relevant. 97 To break from this norm, speakers can signal that a more extended telling is forthcoming. For example, speakers might use a story preface (e.g. ‘a funny thing happened . . .’) to gain the right to complete a storytelling. 98 Likewise, the first component of option-listing – for example, ‘there’s a number of things we could do’ (G08405 Chapter 6, Box 1) – sets up a more extended telling to come: in order to fulfil the promise of this announcement, the neurologist must talk about more than one course of action.
This first component serves, then, as a mechanism for delivering (often extensive) information in an orderly fashion. Moreover, it not only alerts the patient to the fact that his/her view is not expected until the list is complete, it also signals that what is to come is not necessarily to be heard as a recommendation. Rather, it is to be heard as information about a set of options. The success of option-listing with respect to participants’ perceptions of choice may thus lie partly in its ability to resolve the dilemma outlined above. With its structured slot for information provision on two or more alternatives, option-listing can help to reduce the epistemic gradient between neurologist and patient, giving the patient resources for decision-making. With this informing topped and tailed by (1) an opening announcement of upcoming options, and (2) a PVE, neurologists can reduce the deontic force of that informing. This is accomplished by making it clear both that the upcoming information is not (necessarily) to be heard as a recommendation and that the patient will have a slot for contributing to/making the decision.
If this line of argument is correct, what are the implications for single-option decisions? One solution might be to adapt the three-part format from option-listing, along the lines of:
-
Component 1: an announcement that there is a decision to be made (e.g. ‘There’s an option to take a drug for this symptom. Let me describe the drug and its likely pros and cons first before we think about whether or not this is a good option for you’).
-
Component 2: information provision.
-
Component 3: PVE.
This would offer a more structured approach to achieving the same ends accomplished in Extracts 29a–d. We did not find a clear example in our data set of this kind of structured approach to offering choice about single options. Indeed, as we have shown, in those cases where the machinery of option-listing was used to initiate a single-option decision – e.g. having further tests or not – the neurologist also produced a clear recommendation (see Chapter 7, Extracts 15a and b and 17a–d). Thus, unlike Extracts 29a–d, these do not stand as examples of a strategy for solving the above dilemma. Nevertheless, there is no principled reason that option-listing could not be adapted in this way. For example, if we return to Chapter 7, Extract 15a (partially reproduced as Extract 30), it is clear that – if the neurologist had so chosen – the recommendation at lines 17 and 18 could have been replaced with a PVE, thus making this a case of full-form option-listing regarding a single option. An alternative here (lines 17 and 18) could have been something like, ‘What are your thoughts on this?’, as we have seen elsewhere.

Neu, neurologist; Pat, patient.
Implications for the other three subsets: no-choice cases and those about which neurologist and patient disagreed
Although neurologists’ recommendations were not central to this study, in searching our full data set for interactional patterns it became clear that recommending turns can be conceptualised as lying along a deontic continuum. As this carries implications for understanding the other subsets, we will illustrate this briefly here. Extracts 31 and 32 illustrate its extreme ends, ranging from recommendations that take for granted that the patient will comply (claiming a strong right to determine what the patient does) through to such mitigated forms that the patient is, ultimately, left to make the decision in his/her own time (claiming a weak right to determine what the patient does).

Neu, neurologist; Pat, patient.

Neu, neurologist; Pat, patient.
In Extract 31, the neurologist places the decision in his own domain from the outset through the question–answer structure he employs, which poses a question (to himself) about what he wants to do (lines 18–21). The decision about whether or not to conduct another scan is not even partially handed to the patient through this turn design. The neurologist goes on to build a contrast, designed to offer reassurance: the current scan does not worry him, but he will monitor the patient’s health with a follow-up scan. Again, this is not constructed as a matter for the patient to decide. At lines 31 and 32, the neurologist treats this as an informing: telling the patient about a course of action that will take place. Note that the brief check at line 44 (‘alright?’) refers back to the neurologist’s reassurance that he does not expect the scan to alter his current diagnosis. Moreover, at lines 46 and 47, when he confirms his decision (to re-scan), he again announces this as something he will do.
Across Extract 31, the factors relevant to making this decision are cast as lying in the neurologist’s domain. This is a matter of his clinical expertise (the decision is based the conclusions he has drawn from reading the scan). By contrast, Extract 32 shows a case where – even though the neurologist is clearly recommending that the patient try a mandibular advancement device to help with his snoring – he places the decision itself in the patient’s domain. He starts by giving information about the device (lines 1–27). In so doing, he is clearly advocating its use: it ‘can reduce snoring quite significantly’ – a key problem affecting the patient’s sleep. However, he is relatively cautious in his ‘sales pitch’. Note the mitigated recommendation at line 26: ‘can be effective sometimes’. More significantly still, he raises a question that only the patient can answer: whether or not he can ‘tolerate’ the device in his mouth (line 29). The conditional (‘if’) at the start of line 29 recasts the recommendation as contingent on the patient’s reaction to the device. Moreover, through some self-disclosure (lines 32, 34, 36 and 38–40), the neurologist implies that an inability to use the device is not unreasonable. In so doing, he holds open the option of not doing as he recommends. The neurologist’s final, highly mitigated recommendation – that the patient see if he can ‘find a way to make it work’ for him (line 46) – is explicitly cast as being conditional on the device proving beneficial for the patient in practice (lines 42–44).
In summary, Extracts 31 and 32 both show recommendations, but they represent opposing ends of the deontic continuum. The central points of distinction are:
-
whether or not the course of action is introduced as if it is already a foregone conclusion
-
whether the factors to take into account are constructed as lying in the neurologist’s or patient’s domain
-
whether an opportunity is given to the patient to reach (or have a hand in) the final decision to go ahead, or not, with the recommended course of action.
In Extract 31, the neurologist treats himself as having the right simply to announce what will happen to the patient. In Extract 32, the neurologist treats himself as having the right only to suggest that the patient try the recommended device.
This difference in turn design is clearly occasioned by the different kinds of recommendation being made in each extract. We should be clear that we are not making a normative argument here in favour of one approach to initiating decision-making. Each may be suitable for a particular purpose. Rather, this continuum helps make sense of our findings regarding participants’ perception of choice. In the no-choice cases discussed in Chapter 5, neurologists were producing recommendations on the strong end of the deontic continuum, such as: ‘This isn’t something you would treat’ (Extract 1); ‘From my point of view at this point I wouldn’t be doing anything extra’ (Extract 3); ‘And I think (.) you kno:w you just stay with the treatment=at the moment’ (Extract 5). Thus, even though we know that patients can, and do, resist recommendations51 – and, in that sense, have a choice – the deontic force of such turn designs means, we suggest, that they are not heard as offering choice.
Although this needs further analysis, our preliminary view is that those cases for which there was disagreement about whether or not a choice was offered tended to include recommendations on the weaker end of the deontic continuum. One explanation for the disagreement between neurologist and patient, then, might be that these recommendation formats are subject to being interpreted either way (as offering choice because they are so highly mitigated or as directing the patient to take a particular course of action because they are, nevertheless, designed to perform the action of recommending).
Implications for clinicians
The above discussion of patient choice is, in effect, a discussion of implications for clinical practice and should be of clear relevance to clinicians. Here we focus more explicitly on how clinicians might be able to make use of our findings. It is crucial to recognise that the implications of our findings for practice (and training) are profoundly contingent: they depend, first and foremost, on clinical judgement regarding whether or not patient choice is appropriate. As we argued in Chapters 1 and 5, there are circumstances in which it would be unethical or impractical for doctors to offer patients options – although of course patients are, except under very particular circumstances, free to refuse. Whether or not choice is the better way to initiate a decision is also dependent on the doctor’s assessment of a range of non-clinical factors, including the patient’s preferences regarding decision-making. 29 In short, the answer to the question of how to initiate decision-making most effectively is heavily dependent on the answer to a primary question: ‘effective for what?’91
If there is reason to want to make sure the patient has understood that there is a choice to be made, and that this choice is theirs, then our study indicates that full-form option-listing provides an effective strategy. But if there is reason to want to ensure the patient has understood that the neurologist believes there is a best course of action, then our study suggests that a recommendation is likely to be more effective. Moreover, since option-listing can also be used to direct a patient’s choice, it may be effective for addressing patient resistance and/or for pursuing patients’ commitment to a proposed course of action. For example, in reflecting on the consultation shown in Extracts 17a–d, the clinical members of our team commented that the conflicting agendas evident are typical of consultations with patients with psychogenic (or non-epileptic) seizures. For the neurologist, the (implicit) goal may be to achieve a diagnosis that can be defended with a test result; for the patient, the (implicit) goal may be to avoid being told, definitively, that the symptoms are non-biomedical in origin. These are not, then, like other choice conversations in so far as the neurologist may be consciously trying both to ‘back the patient into a corner’ (so that a diagnosis can be achieved), and to involve the patient in the decision-making process in an effort to shift him/her out of the helplessness and avoidance that this patient group often displays. 49,50 Moreover, if the patient is accompanied (as in Extracts 17a–d), much of what is said may be partially directed at the relative/partner, whose agenda may be more akin to the neurologist’s than the patient’s.
Given this malleability of interactional practices – demonstrated by the way in which option-listing can be used to accomplish different ends – our findings suggest that it is inappropriate to try to implement such practices mechanistically. Indeed, the contrast between Extracts 29a–d and the possibility of using a more structured approach to delivering single-option choice highlights a broader point: that, although a more structured approach might be useful as a training resource, achieving the kinds of interactional outcomes discussed in this report is not dependent on – and indeed, cannot be achieved by – merely following a script. However, our findings show very clearly that there are consequences to the selection between interactional practices that neurologists must inevitably make whenever initiating decision-making in the clinic. By being aware of these, neurologists (and other clinicians) can make more informed choices themselves, depending on the particular situation and the outcome they hope to achieve.
An important lesson from this study is that simply asking doctors to adopt a particular interactional practice (like option-listing) will not automatically lead to a patient-centred approach. Instead, our findings show that precisely how a practice is implemented is crucial. Small differences in how options are presented, for example, may turn a potentially patient-centred method into a tool for doctor-dominated advice-giving. Our findings should, then, encourage doctors to focus more on the micro level of talk if they want to interact most effectively. Listening to recordings or studying transcripts of real consultations, and reflecting on the small differences in interactional practices that CA describes, can allow doctors to improve their communication skills, as has been demonstrated previously. 77,99
The research team has a number of short-term plans for disseminating the findings reported here. An immediate step is to develop a leaflet for clinicians and patient groups (e.g. Epilepsy Action, which has taken a strong interest in this research) summarising our findings and their implications for practice. Two workshops for clinicians will provide hands-on opportunities to work with recordings and transcripts from the study to increase awareness of the difference small changes in wording can make and to illustrate (more vividly than one can in writing) the practices reported here. We will adopt the approach shown to be effective by Jenkins and Reuber,99 who demonstrated that even a ‘one-day CA-based intervention can influence the way in which doctors solicit problems and descriptions from patients’. The workshops will emphasise the practical ways in which clinicians might deliver, where appropriate, patient choice in practice, and demonstrate what makes choice – as opposed to recommending – hearable in talk. In addition, the deontic/epistemic dilemma, discussed above, provides a useful theoretical lens that can help clinicians increase their awareness of the likely consequences of how they put things to patients. It should be noted that we have consent, for many of our recordings, to use clips for training purposes. They offer, then, a rich resource.
Implications for medical sociology
Our findings carry two key implications for medical sociology. The first is for those studies that take a conversation analytic approach. As we have proposed elsewhere,47 the standard CA account of the treatment phase needs revision. This takes the recommendation sequence as given. For example, key texts67,79 discussing the application of CA to clinical encounters carry summaries of Stivers’45 work (see Chapter 1) as the sole discussion of how treatment decisions are reached. Likewise, recent CA work in this area46,51 takes the recommendation sequence as its starting point, and this equation between recommending and the treatment phase is encoded in Robinson’s100 important analysis of the structural organisation of acute primary care visits. Robinson identifies four key activities making up these consultations, the last of which he labels ‘treatment recommendations’ (p. 27), thereby conflating the action of recommending with the phase in which doctor and patient consider what to do next. Widening the focus beyond recommending will allow more extensive investigation both of the ways in which clinicians are orienting to policy directives to give patients ‘more choice’, and of their impact on the consultation. 47
With respect to the wider medical sociology literature, this study not only adds substantive detail to models of SDM – based on actual practices evident in doctor–patient interaction, rather than summary coding or reports thereof – but it indicates possible difficulties with some of the criteria listed in such models. For example, Charles, Gafni and Whelan’s20 model (one of the most widely cited101) includes the following as a necessary characteristic of SDM: ‘both the physician and the patient take steps to participate in the decision-making process by expressing treatment preferences’ (p. 652). Our findings do not contradict this claim. They do, however, indicate a crucial nuance: if clinicians express treatment preferences before or in place of using a PVE, they make it more likely that patients will respond as if to a recommendation; if they hold off voicing their view until the patient has done so, it is more likely that patients will announce a treatment preference or decision. As we have shown, the challenge for doctors in voicing a preference is that they are typically viewed as holding greater epistemic and deontic authority than patients with respect to treatment and investigations. Thus, there is no ‘level playing field’ when it comes to expressing preferences. To meet the criterion described by Charles and colleagues,20 then, it is insufficient simply for both to express a preference. When and how those preferences are expressed is crucial.
Strengths and limitations
A key strength of our study is its focus on real clinical interactions. In so doing, we have been able to explicate – at a fine-grained level of detail – those features of the talk that demonstrably function to offer patients choice. We were concerned primarily with internal indicators of effectiveness (i.e. within the interaction itself as opposed to some correlational assessment of external outcome measures). As we described in Chapter 2, internal indicators (such as patient responses) are important because they assess the direct, immediate consequences of a clinician’s approach – they tell us precisely what happened next in response to practice x compared with practice y. This is important both because clinicians only have direct control over what they do in the consultation itself – not what happens in patients’ lives afterwards – and because there is growing evidence that small differences in wording can make a big difference to what happens next9 (see Chapter 1, Models versus practices: our methodological starting point). This study contributes substantively to our understanding of the interactional consequences of initiating decision-making one way rather than another.
The focus on the detail of interaction is also important because guidance based on self-report data, coding and/or theory may prove to be too general to help much in practice. For example, the General Medical Council’s8 directive to doctors to ‘maximise patients’ opportunities, and their ability, to make decisions for themselves’ (p. 6) fails to acknowledge the many complexities that have been demonstrated in this study. Moreover, as we suggested above, in the absence of more detailed analysis, guidance that has obvious face validity may not function as expected when acted upon in the moment-by-moment reality of interaction.
This study has taken seriously the recognition that CA – although the method par excellence for studying how interaction works in practice – does not give us access to people’s perceptions. 102 A further strength of this study has been our attempt to link an analysis of the recordings with self-report assessments of the presence/absence of choice therein. This gives a measure of both participants’ perceptions and their orientations to choice.
For a predominantly qualitative study, we obtained a relatively large sample, spanning two geographical locations and a range of subspecialties. We cannot, however, make claims on the basis of our sampling strategy to generalisability across neurology or beyond. Given that the neurologists who participated were self-selected, our sample may be biased in favour of those with a particular interest in communication. However, the nature of our primary aim – to identify practices for offering choice – means that our findings are likely to apply beyond our sample. Such practices are evidently not condition-specific; although an epilepsy patient will be offered different options to, say, a breast cancer patient, there is a range of interactional activities a clinician might perform appropriately in either setting. Thus, our findings should be of practical value to clinicians working in different settings.
The key limitation of our study is that it is largely exploratory. We hope to have shown how crucial such foundational, descriptive work is. We now know, for example, that option-listing is a robust phenomenon – evident across multiple neurologists and clinics. We also know that it is identifiable, by patients and clinicians themselves, as a practice for offering choice. We know its contours and some deviations from these. This makes it both a codeable practice for future studies and a trainable practice. Indeed, we have collected recordings that will allow us to train clinicians in how best to offer patients choice (or to produce readily identifiable recommendations if this seems more appropriate). However, this study raises some questions that cannot be answered by the methodology employed here. Our recommendations for future research are, then, also a reflection on what we take to be the limitations of the present study.
In addition, this study has focused primarily on overt orientations to choice because this was largely missing from the literature. However, previous self-report findings have suggested that patients may associate greater choice with a range of interactional practices that are not overtly designed to offer patients a chance to make a decision per se (e.g. answering questions and indicating an interest in the patient’s perspective). 103 A limitation of this study, then, is that we have not considered a wider range of indicators of patient engagement, which might be more significant markers of effectiveness than whether or not patients voice a view/make a decision. Perhaps more importantly, as we discuss below, we have not examined the outcome – beyond the consultation – of the decisions made. We do not know whether or not recommendations are less likely to be followed than patient-made choices.
Reflections on patient and public involvement
As discussed in Chapter 2, we have been struck by the extent to which the Service Users’ Group (SUG) for this study has influenced the direction of our analytic thinking, particularly regarding the extent to which patient choice should be conceptualised, necessarily, as a ‘good’ thing. We learnt an enormous amount from the SUG and the Steering Committee – which involved discussion between patients, neurologists and academics, who rarely seemed to be coming from the same starting point. This was challenging but very productive. A key learning point occurred at the first SUG meeting: it was only halfway through this 2-hour session that we realised that some members thought we were asking them to participate in the study – not in a group to support the study. This caught us by surprise since we had previously explained the purpose of the group in writing and by telephone. It was a stark reminder to attend to our own communication practices!
Subsequently, we sought to structure discussions across three activities to clarify the group’s purpose: (1) presenting our findings-in-progress for feedback; (2) giving space for members to raise issues and experiences that had a bearing on the study; and (3) asking members to comment on data extracts to help direct our analysis. This proved fruitful and we continue to be in regular, very encouraging, contact with two group members who have volunteered to contribute to the planned dissemination workshops.
Reflections on our pilot use of the Medical Interview Satisfaction Scale 21
Our exploratory examination of an association of patient perceived choice with higher levels of satisfaction with the medical interview using the MISS-21 did not produce a positive finding. This questionnaire has previously been used in studies examining presentations with new clinical problems. It is possible that this questionnaire simply does not work for follow-up appointments which may be relatively brief and which may not contain all the elements of clinic conversation the MISS-21 asks about. In any case, a questionnaire such as the MISS-21 can offer only a relatively superficial estimate of a complex concept such as patient satisfaction with a medical encounter, which may perhaps need to be unpicked with a post-encounter interview and analysed qualitatively. Having said this, it may be that the lack of an observed association of perceived choice and satisfaction with the interview reflects the observation made already that choice is neither appropriate from a clinical point of view nor desired by patients in some clinical settings. For reflections from the study’s research assistants on their experience of administering the MISS-21 in the neurology clinic, see Appendix 7.
Recommendations for future research
On the basis of this study, we would recommend three directions (in order of priority) for future research:
-
A follow-up study designed to investigate links between the practices identified here and one or more relevant outcome measures (e.g. patient satisfaction, treatment adherence or a measure of health status). The findings of our study allow us to place the interactional methods used in clinical decision-making processes on a spectrum of (patient-centred) choice and (doctor-centric) recommendation. A naturalistic study in which clinical decision-making processes would be placed on this spectrum by conversation analytic methods and then related to clinical outcomes (for instance AED adherence in patients with epilepsy) would provide additional insights into the objective effectiveness of different methods of clinical decision-making.
-
The question of whether giving patients choice is better or worse than making a recommendation (regarding what the doctor thinks is best) could be explored further with a data set that includes more demographic factors and clinical parameters than examined here, perhaps also involving post-consultation interviews with patient and doctor. This is important because, while it has been argued that patient choice will reduce inequalities in healthcare provision,6 there is a risk that the opposite might occur. 103 For example, patients with more ‘capital’ (e.g. better education, greater confidence about asking questions/voicing a view, etc.) might benefit from being given a choice, while those with less ‘capital’ might find it too daunting.
-
Our methodological approach could also be employed fruitfully in other settings. As we highlighted in Chapter 1, there has been minimal CA work focused on how the choice agenda is translated into practice on the ‘frontline’. Those studies that have been done, have agreed that there is a significant gap between policy and practice. 11,58 Further research could test the generalisability of our findings and help build a more complete picture of the ways in which professionals are seeking to engage in SDM.
-
Finally, in the more immediate future, our team could conduct further analysis on the data set reported here. In common with most qualitative materials, the data are so rich that we could not possibly mine their full potential within a single study. The following are just some matters that could be addressed without collecting further data:
-
teasing apart the following three factors for all decisions in the data set:
-
–whether or not the patient is offered a choice
-
–who makes the decision
-
–whether the patient/doctor gets what they want
-
-
specifying some of the less explicit ways in which patients may be engaged in decision-making
-
further analysis of the subset of consultations for which there was disagreement about whether or not choice was offered
-
further analysis of the deontic continuum apparent in our set of recommending turns.
-
Conclusion
Choice featured in the majority of our recorded consultations. Whether or not offering choice is the best way to initiate decision-making is contingent on clinical, ethical and practical considerations. However, if doctors want to ensure a patient knows she/he has a choice, our study shows how option-listing can provide an effective strategy. Crucially, our findings imply that simply asking doctors to adopt a practice (like option-listing) will not automatically lead to a patient-centred approach. Precisely how a practice is implemented is crucial. Our findings should, then, encourage doctors to focus more on the micro level of talk if they want to interact most effectively. Listening to recordings or studying transcripts of real consultations and reflecting on the small differences in interactional practices that CA describes, can allow doctors to improve their communication skills in a way that adopting a script can never achieve. In addition to our analytic findings, a lasting resource provided by this study is our collection of recordings and transcripts, which can be used for practical training. Unlike more generalised ‘good practice’ frameworks or models of the doctor–patient relationship, our findings can be used to provide detailed, evidence-based guidelines for clinicians, grounded in a clear understanding of how decision-making works in the real-time interactions lying at the heart of clinical practice.
Acknowledgements
We would like to thank the members of the Steering Committee for their valuable contributions and guidance: Professor Paul Drew (conversation analytic adviser), Dr Richard Grunewald and Dr Richard Metcalfe (neurology advisers), and Mr Andrew Myers (patient representative).
We would like to thank the members of the SUG for their significant insight, gentle challenging and support, and for establishing contact between the research team and wider patient organisations: Mr Andrew Myers, Mrs Jo Morton, Mr Chris Morton, Mr David Statham and Mr Robert Wilks.
Thanks to Ms Zoe Gallant and Ms Fiona Smith, the research assistants at the Sheffield and Glasgow sites, responsible for recruitment and data collection. This study would not have happened without their hard work and expertise.
Thanks to Ms Sara Christensen for her hard work and skill in producing timely and careful transcripts of all the recordings.
We are also grateful to the many administrators and support staff at the Universities of Sheffield, York and Glasgow, and the Royal Hallamshire and Southern General Hospitals, who helped ensure the smooth running of the study. Thanks to those at the Sheffield Institute for Translational Neuroscience (SITraN) for providing us with a venue, refreshments and other support for our services users’ group and Steering Committee meetings.
Many thanks also to Ruth Saw for her wise and cheerful guidance across the life of the study. And much gratitude to Elizabeth Fenwick and Wayne Britcliffe for informal statistical, technical and moral support!
Finally, our very special thanks to all patients and neurologists who agreed to have their consultations recorded. This study would not have been possible without them.
Contributions of authors
All the authors contributed to the design of the study and to the analysis of the data.
Professor Markus Reuber (Consultant Neurologist and Professor of Clinical Neurology, specialising in seizures) conducted the statistical analysis and contributed to the write-up of the report – including the CA findings – from a clinical perspective. He also supervised the recruitment of participants for the study (in Sheffield).
Dr Merran Toerien (Lecturer in Sociology, specialising in CA) conducted the CA of the recorded consultations and took primary responsibility for preparing the main CA chapters of the report for publication as well as the introductory and discussion chapters.
Dr Rebecca Shaw (Lecturer in Social Sciences, specialising in CA) conducted the CA of the recorded consultations and helped prepare the main CA chapters of the report for publication as well as taking primary responsibility for the methods, statistical and no-choice chapters. She also contributed to the statistical analysis.
Professor Roderick Duncan (Consultant Neurologist and Associate Professor of Neurology, specialising in seizures) conducted the statistical analysis and contributed to the write-up of the report – including the CA findings – from a clinical perspective. He also supervised the recruitment of participants for the study (in Glasgow).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
Publications
Toerien M, Shaw R, Reuber M. Initiating decision-making in neurology consultations: ‘recommending’ versus ‘option-listing’ and the implications for medical authority. Sociol Health Illn 2013;35:873–90.
Toerien M, Shaw R, Duncan R, Reuber M. Offering patients choices: a pilot study of interactions in the seizure clinic. Epilepsy Behav 2011;20:312–20.
References
- Fotaki M. Choice is yours: a psychodynamic exploration of health policymaking and its consequences for the English national health service. Hum Relat 2006;59:1711-44. http://dx.doi.org/10.1177/0018726706072871.
- NHS 2013/14 Choice Framework. London: HMSO; 2013.
- The NHS Plan: A Plan for Investment, a Plan for Reform. London: HMSO; 2000.
- Creating a Patient-led NHS: Delivering the NHS Improvement Plan. London: Her Majesty’s Stationery Office; 2005.
- NHS England . NHS Choices 2013. http://www.nhs.uk/Pages/HomePage.aspx (accessed 17 October 2014).
- Choice Matters: 2007–08 Putting Patients in Control. London: HMSO; 2007.
- NHS Constitution: The NHS Belongs to Us All. London: HMSO; 2013.
- Consent: Patients and Doctors Making Decisions Together. London: General Medical Council; 2008.
- Heritage J, Robinson JD, Elliott MN, Beckett M, Wilkes M. Reducing patients’ unmet concerns in primary care: the difference one word can make. J Gen Intern Med 2007;22:1429-33. http://dx.doi.org/10.1007/s11606-007-0279-0.
- Toerien M, Shaw R, Duncan R, Reuber M. Offering patients choices: a pilot study of interactions in the seizure clinic. Epilepsy Behav 2011;20:312-20. http://dx.doi.org/10.1016/j.yebeh.2010.11.004.
- Antaki C, Finlay WML, Walton C. Choices for people with intellectual disabilities: official discourse and everyday practice. J Policy Pract Intellect Disabil 2009;6:260-6. http://dx.doi.org/10.1111/j.1741-1130.2009.00230.x.
- Patients Choosing Their Healthcare: How to Make Patient Choice Work for Patients, GPs and Primary Care Trusts. London: Dr Foster Ltd; 2005.
- The National Service Framework for Long-Term Conditions. London: HMSO; 2005.
- The Expert Patient: A New Approach to Chronic Diease Management for The 21st Century. London: HMSO; 2000.
- McCorry D, Chadwick D, Marson A. Current drug treatment of epilepsy in adults. Lancet Neurol 2004;3:729-35. http://dx.doi.org/10.1016/S1474-4422(04)00935-4.
- Pietrolongo E, Giordano A, Kleinefeld M, Confalonieri P, Lugaresi A, Tortorella C, et al. Decision-making in Multiple Sclerosis consultations in Italy: third observer and patient assessments. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0060721.
- McCorry D, Marson T, Jacoby A. Understanding routine antiepileptic drug desicions: a qualitative analysis of patients’ accounts of hospital consultations. Epilepsy Behav 2009;14:210-14. http://dx.doi.org/10.1016/j.yebeh.2008.10.010.
- Palace J. Partnership and consent in MS treatment choice. J Neurol Sci 2013;335:5-8. http://dx.doi.org/10.1016/j.jns.2013.09.001.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681-92. http://dx.doi.org/10.1016/S0277-9536(96)00221-3.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999;49:651-92. http://dx.doi.org/10.1016/S0277-9536(99)00145-8.
- Murray E, Pollack L, White M, Lo B. Clinical decision-making: physicians’ preferences and experiences. BMC Fam Pract 2007;8. http://dx.doi.org/10.1186/1471-2296-8-10.
- Heritage J, Robinson JD. The structure of patients’ presenting concerns: physicians’ opening questions. Health Commun 2006;19:89-102. http://dx.doi.org/10.1207/s15327027hc1902_1.
- Peräkylä A. Agency and authority: extended responses to diagnostic statements in primary care encounters. Res Lang Soc Interact 2002;35:219-47. http://dx.doi.org/10.1207/S15327973RLSI3502_5.
- Murtagh GM, Furber L, Thomas AL. Patient-initiated questions: how can doctors encourage them and improve the consultation process? A qualitative study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003112.
- England SL, Evans J. Patients’ choices and perceptions after an invitation to participate in treatment decisions. Soc Sci Med 1992;32:1217-25. http://dx.doi.org/10.1016/0277-9536(92)90314-G.
- Edwards A, Elwyn G. Shared Decision-Making in Health Care: Achieving Evidence-Based Patient Choice. Oxford: Oxford University Press; 2009.
- Schwartz B. The Paradox of Choice: Why More is Less. New York, NY: HarperCollins; 2004.
- Agledahl KM, Førde R, Wifstad Å. Choice Is not the issue: the misrepresentation of healthcare in bioethical discourse. J Med Ethics 2011;37:212-15. http://dx.doi.org/10.1136/jme.2010.039172.
- Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns 2012;86:9-18. http://dx.doi.org/10.1016/j.pec.2011.02.004.
- Flynn KE, Smith MA, Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med 2006;63:1158-69. http://dx.doi.org/10.1016/j.socscimed.2006.03.030.
- Pilnick A, Dingwall R. On the remarkable persistence of asymmetry in doctor/patient interaction: a critical review. Soc Sci Med 2011;72:1374-82. http://dx.doi.org/10.1016/j.socscimed.2011.02.033.
- Appleby J, Dixon J. Patient choice in the NHS. BMJ 2004;329:61-2. http://dx.doi.org/10.1136/bmj.329.7457.61.
- Cribb A, Owens J. Whatever suits you: unpicking personalization for the NHS. J Eval Clin Pract 2010;16:310-14. http://dx.doi.org/10.1111/j.1365-2753.2010.01390.x.
- Fotaki M. Patient choice in healthcare in England and Sweden: from quasi-market and back to market? A comparative analysis of failure in unlearning. Public Adm 2007;85:1059-76. http://dx.doi.org/10.1111/j.1467-9299.2007.00682.x.
- Greener I. Towards a history of choice in UK health policy. Sociol Health Illn 2009;31:309-24. http://dx.doi.org/10.1111/j.1467-9566.2008.01135.x.
- Stivers T, Heritage J. Breaking the sequential mold: answering ‘more than the question’ during comprehensive history taking. Text 2001;21:151-85. http://dx.doi.org/10.1515/text.1.21.1-2.151.
- Gill VT. Patient ‘demand’ for medical interventions: exerting pressure for an offer in a primary care clinic visit. Res Lang Soc Interact 2005;38:451-79. http://dx.doi.org/10.1207/s15327973rlsi3804_3.
- Gill VT, Halkowski T, Roberts F. Accomplishing a request without making one: a single case analysis of a primary care visit. Text 2001;21:55-81. http://dx.doi.org/10.1515/text.1.21.1-2.55.
- Nishizaka A. Response expansion as a practice for raising a concern during regular prenatal checkups. Commun Med 2011;8:247-59.
- Ijäs-Kallio T, Ruusuvuori J, Peräkylä A. Patient resistance towards diagnosis in primary care: implications for concordance. Health 2010;14:505-22.
- Ijäs-Kallio T, Ruusuvuori J, Peräkylä A. ‘Unilateral’ decision making and patient participation in primary care. Commun Med 2011;8:145-55.
- Maynard DW, Hudak PL. Small talk, high stakes: interactional disattentiveness in the context of prosocial doctor–patient interaction. Lang Soc 2008;37:661-88. http://dx.doi.org/10.1017/S0047404508080986.
- Stivers T. Parent resistance to physicians’ treatment recommendations: one resource for initiating a negotiation of the treatment decision. Health Commun 2005;18:41-74. http://dx.doi.org/10.1207/s15327027hc1801_3.
- Stivers T. Non-antibiotic treatment recommendations: delivery formats and implications for parent resistance. Social Soc Sci Med 2005;60:949-64. http://dx.doi.org/10.1016/j.socscimed.2004.06.040.
- Stivers T. Prescribing Under Pressure: Physician-Parent Conversations and Antibiotics. Oxford: Oxford University Press; 2007.
- Hudak PL, Clark SJ, Raymond G. How surgeons design treatment recommendations in orthopaedic surgery. Soc Sci Med 2011;73:1028-36. http://dx.doi.org/10.1016/j.socscimed.2011.06.061.
- Toerien M, Shaw R, Reuber M. Initiating decision-making in neurology consultations: ‘recommending’ versus ‘option-listing’ and the implications for medical authority. Sociol Health Illn 2013;35:873-90. http://dx.doi.org/10.1111/1467-9566.12000.
- Costello BA, Roberts F. Medical recommendations as joint social practice. Health Commun 2001;13:241-60. http://dx.doi.org/10.1207/S15327027HC1303_2.
- Monzoni CM, Duncan R, Grünewald R, Reuber M. How do neurologists discuss functional symptoms with their patients: a conversation analytic study. J Psychosom Res 2011;71:377-83. http://dx.doi.org/10.1016/j.jpsychores.2011.09.007.
- Monzoni CM, Duncan R, Grünewald R, Reuber M. Are there interactional reasons why doctors may find it hard to tell patients that their physical symptoms may have emotional causes? A conversation analytic study in neurology outpatients. Patient Educ Couns 2011;85:e189-200. http://dx.doi.org/10.1016/j.pec.2011.07.014.
- Koenig CJ. Patient resistance as agency in treatment decisions. Soc Sci Med 2011;72:1105-14. http://dx.doi.org/10.1016/j.socscimed.2011.02.010.
- Pilnick A, Zayts O. ‘Let’s have it tested first’: choice and circumstances in decision-making following positive antenatal screening in Hong Kong. Sociol Health Illn 2012;34:266-82. http://dx.doi.org/10.1111/j.1467-9566.2011.01425.x.
- Seale C, Chaplin R, Lelliott P, Quirk A. Sharing decisions in consultations involving anti-psychotic medication: a qualitative study of psychiatrists’ experiences. Soc Sci Med 2006;62:2861-73. http://dx.doi.org/10.1016/j.socscimed.2005.11.002.
- Karnieli-Miller O, Eisikovits Z. Physician as partner or salesman? Shared decision-making in real-time encounters. Soc Sci Med 2009;69:1-8. http://dx.doi.org/10.1016/j.socscimed.2009.04.030.
- Quirk A, Chaplin R, Lelliott P, Seale C. How pressure is applied in shared decisions about antipsychotic medication: a conversation analytic study of psychiatric outpatient consultations. Sociol Health Illn 2012;34:95-113. http://dx.doi.org/10.1111/j.1467-9566.2011.01363.x.
- Collins S, Drew P, Watt I, Entwistle V. ‘Unilateral’ and ‘bilateral’ practitioner approaches in decision-making about treatment. Soc Sci Med 2005;61:2611-27. http://dx.doi.org/10.1016/j.socscimed.2005.04.047.
- Heritage J, Duchan J, Kovarsky D. Diagnosis as Cultural Practice. New York: Mouton De Gruyter; 2005.
- Pilnick A. ‘It’s something for you both to think about’: choice and decision making in nuchal translucency screening for Down’s syndrome. Sociol Health Illn 2008;30:511-30. http://dx.doi.org/10.1111/j.1467-9566.2007.01071.x.
- Antaki C, Finlay W, Walton C, Pate L. Offering choices to people with intellectual disabilities: an interactional study. J Intellect Disabil Res 2008;52:1165-75. http://dx.doi.org/10.1111/j.1365-2788.2008.01101.x.
- Robinson JD, Heritage J. The structure of patients’ presenting concerns: the completion relevance of current symptoms. Soc Sci Med 2005;61:481-93. http://dx.doi.org/10.1016/j.socscimed.2004.12.004.
- Heritage J, Maynard DW. Problems and prospects in the study of physician–patient interaction: 30 years of research. Annu Rev Sociol 2006;32:351-74. http://dx.doi.org/10.1146/annurev.soc.32.082905.093959.
- Meakin R, Weinman J. The ‘Medical Interview Satisfaction Scale’ (MISS-21) adapted for British general practice. Fam Pract 2002;19:257-63. http://dx.doi.org/10.1093/fampra/19.3.257.
- Robinson JD, Heritage J. Physicians’ opening questions and patients’ satisfaction. Patient Educ Couns 2006;60:279-85. http://dx.doi.org/10.1016/j.pec.2005.11.009.
- Data Protection Act 1998: Elizabeth II. London: The Stationery Office; 1998.
- Drew P, Toerien M, Irvine A, Sainsbury R. A Study of Language and Communication Between Advisers and Claimants in Work Focused Interviews. Department for Work and Pensions Research Report 633. Norwich: HMSO; 2010.
- Drew P, Chatwin J, Collins S. Conversation analysis: a method for research into interactions between patients and health-care professionals. Health Expect 2001;4:58-70. http://dx.doi.org/10.1046/j.1369-6513.2001.00125.x.
- Heritage J, Maynard DW. Communication in Medical Care: Interactions Between Primary Care Physicians and Patients. Cambridge: Cambridge University Press; 2006.
- Waitzkin H. Information giving in medical care. J Health Soc Behav 1985;26:81-101. http://dx.doi.org/10.2307/2136599.
- Peräkylä A. Making links in psychoanalytic interpretations: a conversation analytical perspective. Psychother Res 2004;14:289-307. http://dx.doi.org/10.1093/ptr/kph026.
- Toerien M, Kitzinger C. Emotional labour in action: navigating multiple involvements in the beauty salon. Sociology 2007;41:645-62. http://dx.doi.org/10.1177/0038038507078918.
- Shaw R, Kitzinger C. Memory in interaction: an analysis of repeat calls to a Home Birth helpline. Res Lang Soc Interact 2007;40:117-44. http://dx.doi.org/10.1080/08351810701331307.
- Toerien M, Sainsbury R, Drew P, Irvine A. Putting personalisation into practice: Work-Focused Interviews in jobcentre plus. J Soc Policy 2013;42:309-27. http://dx.doi.org/10.1017/S0047279412000980.
- Atkinson JM, Drew P. Order in Court: The Organisation of Verbal Interaction in Judicial Settings. New Jersey: Humanities Press; 1979.
- Drew P, Fitch KL, Sanders RE. Handbook of Language and Social Interaction. New Jersey: Lawrence Erlbaum; 2005.
- Sidnell J. Conversation Analysis: An Introduction. Chichester: Wiley-Blackwell; 2010.
- Toerien M, Flick U. The SAGE Handbook of Qualitative Data Analysis. London: Sage; 2013.
- Antaki C. Applied Conversation Analysis: Intervention and Change in Institutional Talk. Basingstoke: Palgrave Macmillan; 2011.
- Drew P, Heritage J. Talk at Work: Interaction in Institutional Settings. Cambridge: Cambridge University Press; 1992.
- Heritage J, Clayman S. Talk in Action: Interactions, Identities, and Institutions. Chichester: Wiley-Blackwell; 2010.
- Peräkylä A. Authority and accountability: the delivery of diagnosis in primary health care. Soc Psychol Q 1998;61:301-20. http://dx.doi.org/10.2307/2787032.
- Heath C, Drew P, Heritage J. Talk at Work: Interaction in Institutional Settings. Cambridge: Cambridge University Press; 1992.
- Stivers T. Stance, alignment, and affiliation during storytelling: when nodding is a token of affiliation. Res Lang Soc Interact 2008;41:31-57. http://dx.doi.org/10.1080/08351810701691123.
- Schegloff EA. Sequence Organization in Interaction: A Primer in Conversation Analysis. Cambridge: Cambridge University Press; 2007.
- Robson C, Drew P, Reuber M. Duration and structure of unaccompanied (dyadic) and accompanied (triadic) initial outpatient consultations in a specialist seizure clinic. Epilepsy Behav 2013;27:449-54. http://dx.doi.org/10.1016/j.yebeh.2013.03.008.
- Mishler EG. The Discourse of Medicine: Dialectics of Medical Interviews. Norwood, NJ: Ablex; 1984.
- Raymond G. Grammar and social organization: yes/no interrogatives and the structure of responding. Am Sociol Rev 2003;68:939-67. http://dx.doi.org/10.2307/1519752.
- Boyd E, Heritage J, Heritage J, Maynard DW. Communication in Medical Care: Interactions Between Primary Care Physicians and Patients. Cambridge: Cambridge University Press; 2006.
- Heritage J, Freed A, Ehrich S. ‘Why Do You Ask?’: The Function of Questions in Institutional Discourse. New York, NY: Oxford University Press; 2010.
- Curl TS, Drew P. Contingency and action: a comparison of two forms of requesting. Res Lang Soc Interact 2008;41:129-53. http://dx.doi.org/10.1080/08351810802028613.
- Pomerantz A. Extreme case formulations: a way of legitimizing claims. Human Studies 1986;9:219-29. http://dx.doi.org/10.1007/BF00148128.
- Toerien M, Irvine A, Drew P, Sainsbury R, Antaki C. Applied Conversation Analysis: Intervention and Change in Institutional Talk. Basingstoke: Palgrave Macmillan; 2011.
- Stevanovic M, Perakyla A. Deontic authority in interaction: the right to announce, propose, and decide. Research on Language and Social Interaction 2012;45:297-321. http://dx.doi.org/10.1080/08351813.2012.699260.
- Heritage J. The epistemic engine: sequence organization and territories of knowledge. Res Lang Soc Interact 2012;45:30-52. http://dx.doi.org/10.1080/08351813.2012.646685.
- Heritage J. Epistemics in action: action formation and territories of knowledge. Res Lang Soc Interact 2012;45:1-29. http://dx.doi.org/10.1080/08351813.2012.646684.
- Heritage J, Sefi S, Drew P, Heritage J. Talk at Work: Interaction in Institutional Settings. Cambridge: Cambridge University Press; 1992.
- Schegloff EA. Confirming allusions: toward an empirical account of action. Am J Sociol 1996;102:161-216. http://dx.doi.org/10.1086/230911.
- Sacks H, Schegloff EA, Jefferson G. A simplest systematics for the organization of turn-taking for conversation. Language 1974;50:696-735. http://dx.doi.org/10.2307/412243.
- Jefferson G, Schenkein J. Studies in the Organization of Conversational Interaction. New York, NY: Academic Press; 1978.
- Jenkins L, Reuber M. A conversation analytic intervention to help neurologists identify diagnostically relevant linguistic features in seizure patients’ talk. Res Lang Soc Interact 2014;47:266-79. http://dx.doi.org/10.1080/08351813.2014.925664.
- Robinson JD. An interactional structure of medical activities during acute visits and its implications for patients’ participation. Health Communication 2003;15:27-59. http://dx.doi.org/10.1207/S15327027HC1501_2.
- Makoul G, Clayman M. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006;60:301-12. http://dx.doi.org/10.1016/j.pec.2005.06.010.
- Drew P, Smith JA. Qualitative Psychology: A Practical Guide to Research Methods. London: Sage; 2003.
- Ogden J, Daniells E, Barnett J. The value of choice: development of a new measurement tool. Br J Gen Pract 2008;58:614-18. http://dx.doi.org/10.3399/bjgp08X330735.
- Jefferson G, Lerner GH. Conversation Analysis: Studies from the First Generation. Philadelphia, PA: John Benjamins; 2004.
- Atkinson JM, Heritage J. Structures of Social Action: Studies in Conversation Analysis. Cambridge: Cambridge University Press; 1984.
Appendix 1 Transcription conventions
Transcription conventions used here follow Jeffersonian transcript conventions. 104,105
| A. Some aspects of the relative timing of utterances | |
|---|---|
| [] square brackets | Overlapping talk |
| = equals sign | No discernible interval between turns |
| (0.5) time in parentheses | Intervals within or between talk (measured in tenths of a second) |
| (.) period in parentheses | Discernible interval within or between talk but too short to measure (less than two-tenths of a second) |
| < | ‘Jump’-started talk |
| B. Some characteristics of speech delivery | |
| Punctuation symbols are designed to capture intonation, not grammar and are used to describe intonation at the end of a word/sound, at the end of a sentence or some other shorter unit: | |
| . period | Closing intonation |
| , comma | Slightly rising intonation (a little hitch up on the end of the word) |
| ? question mark | Fully rising intonation |
| - dash | Abrupt cut off of sound |
| : colon | Extension of preceding sound – the more colons the greater the extension |
| here underlining | Emphasised relative to surrounding talk |
| .tch or.t | Tongue click |
| hhh. | Audible outbreath (number of ‘h’s indicates length) |
| .hhh | Audible inbreath (number of ‘h’s indicates length) |
| >Talk< | Speeded up talk |
| <Talk> | Slowed down talk |
| # | Croaky or creaky voice |
| £ or $ | Smiley voice |
| Hah hah or huh huh etc. | Beats of laughter |
| () empty single brackets or words enclosed in single brackets | Transcriber unable to hear words or uncertain of hearing |
| ((word)) words enclosed in double brackets | Transcribers’ comments |
| ↑↓ | Marked change in pitch |
| CAPITALS | Louder speech |
| °° Degree signs | Softer speech |
Appendix 2 Patient pre-appointment questionnaire
Appendix 3 Patient post-appointment questionnaire
Appendix 4 Patient demographic questionnaire
Appendix 5 Clinician post-appointment questionnaire
Appendix 6 Clinician demographic questionnaire
Appendix 7 The Medical Interview Satisfaction Scale 21, annotated
As discussed in Chapter 2, we piloted the use of the follow-up patient satisfaction questionnaire (the MISS-21) use in neurology outpatient clinics. The following, annotated, copy of the form, shows common difficulties that the researchers encountered when administering this questionnaire in this context.
List of abbreviations
- AED
- antiepileptic drug
- CA
- conversation analysis
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- DMD
- disease-modifying drug
- GP
- general practitioner
- MISS-21
- Medical Interview Satisfaction Scale 21
- MS
- multiple sclerosis
- NHS
- National Health Service
- PVE
- patient view elicitor
- SD
- standard deviation
- SDM
- shared decision-making
- SUG
- Service Users’ Group
Transcription conventions are shown in Appendix 1.