Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 09/2000/36. The contractual start date was in September 2011. The final report began editorial review in January 2014 and was accepted for publication in July 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Roderick et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction and background
Chronic kidney disease and end-stage kidney failure in older adults
Chronic kidney disease (CKD) is categorised into five stages depending on the estimated glomerular filtration rate (eGFR) and evidence of kidney damage, as recognised by the National Institute for Health and Care Excellence (NICE) and other international guidelines. 1,2 The most severe of these stages, termed end-stage kidney failure (ESKF) is termed stage 5 CKD (CKD5) where the eGFR is < 15 ml/minute/1.73 m2. National guidelines state that renal replacement therapy (RRT) should be considered in all patients with CKD5. The symptoms of ESKF are largely due to the failure of erythropoietin (EPO) production and consequent anaemia, accumulation of toxic metabolites (‘uraemia’), acid base and electrolyte imbalance, and fluid retention. While a lower eGFR is associated with more severe and more frequent symptoms, the effects of a decrease in kidney function can vary between individuals, with some CKD5 presenting as asymptomatic.
The rate of decline of kidney function in CKD5 to a level of eGFR and/or symptoms that would justify starting RRT is variable between patients and can be difficult for clinicians to predict. Clinicians are faced with complex decisions about whether RRT should be started or not and, if so, when. The rationale for initiation of dialysis may be to prevent imminent death from hyperkalaemia (high potassium), fluid overload or uraemic coma, or to maintain quality of life and prevent complications of severe uraemia. These decisions depend on the rate of deterioration of kidney function, on the development and attribution of symptoms and on whether or not clinicians believe that dialysis will improve outcomes. Such decisions are especially difficult in older patients, who often have symptoms related to multiple comorbidities.
Planning for renal services over the past decade has been largely dictated by the National Service Framework for Renal Services published in 2004/5. 3 Services for ESKF (CKD5 which has progressed to a disease state at which dialysis is the default treatment) have been commissioned as specialist rather than general acute services and development of renal services in England has remained centred on teaching hospitals and large district general hospitals. Personalisation of choice in ESKF care has been an explicit policy goal. Increasing emphasis has been given to preparation for ESKF and choice of treatments including conservative kidney management (CKM). The majority of patients with stage 4 chronic kidney disease (CKD4) or CKD5 approaching ESKF are now seen in multidisciplinary renal clinics, where risk factors for progression and major cardiovascular events are managed, advanced CKD metabolic abnormalities and symptoms are treated and preparation for RRT is organised.
The population rate of starting RRT has increased steadily over the last few decades, though with a recent stabilisation. 4,5 This is in part because of the ageing population, the rising prevalence of type 2 diabetes, a decline in competing mortality risk from cardiovascular disease and considerable expansion in the supply of dialysis facilities. 4 This increase in RRT is highest among those over 75 years old, with disproportionate numbers who are frail and have multiple comorbid conditions. 6,7 In 2011, there were over 1500 patients aged 75 years and over who started RRT in the UK. 4 Several small retrospective or prospective cohort studies have raised the possibility that the balance of benefit versus burden in this older frail group may favour non-dialysis (conservative) management,6–12 especially when the patient has a significant burden of morbidity. Those aged 75 years and over with ESKF who have dialysis do have a survival advantage, but this advantage may be small in those with high comorbidity, especially when the effects of establishing access for dialysis, time spent travelling to, receiving and recovering from dialysis three times a week, and complications of dialysis per se, which often result in hospitalisation, are also considered. Moreover, quality of life can be maintained on conservative care pathways. 11 Conservative management may increase the likelihood of dying at home and with input from palliative services. 12,13 Dialysis per se leads to loss of functional status in older frail patients. 14 For selected patients, and in the context of increasing frailty and loss of independence,13,15,16 RRT may therefore be a costly intervention both for patients (in terms of quality of life and treatment burden) and to the NHS (in terms of resource usage).
Providing an alternative to dialysis for end-stage kidney failure patients
We have used the term CKM to describe the management of ESKF without RRT, but with active symptom management, communication and advanced care planning (ACP), interventions to delay progression and minimise complications, psychological support, social and family support, and spiritual care. It is expected that a recent Kidney Disease Improving Global Outcomes (KDIGO) consensus conference will produce a definitive definition.
Such an approach might contribute to a cost-effective strategy for managing the rising number of older people with CKD5. However, there has been a perception that CKM might be seen as rationing care. Rationing means the limiting of access to (usually) expensive interventions from which patients would benefit, in order to control resource utilisation where resources do not permit such patients to be treated. However, consideration of CKM is not to deny dialysis but to recognise that for some patients dialysis may be futile or detrimental to their well-being, and CKM a more appropriate option. Historically the UK RRT programme has had lower uptake rates than in many other countries with comparable populations. It is not easy to disentangle the role of rationing (implicit or explicit) in this, as compared with careful and appropriate use of treatment. CKM is now more accepted in the UK than in many other developed countries; this means that the UK is in a key position to provide the evidence base for its appropriate and effective development.
In recognition of the gap in provision of high-quality care for those dying with ESKF, there have been a number of initiatives to raise the profile of care for kidney patients in the last year of life. The 2005 National Service Framework for Renal Services: Part 23 recommended that people with ESKF receive timely evaluation of prognosis, information about their choices and, for those near the end of life, a jointly agreed palliative care plan, built around individual needs and preferences. 16 Guidelines have been developed for managing the symptoms of kidney patients in the last days of life. 17 The Department of Health introduced policies to improve end-of-life care across conditions, through initiatives such as the End of Life Care Strategy18,19 and, in primary care, the Gold Standards Framework. 20,21 To consolidate and help embed these policies, the Framework for End of Life Care in Advanced Kidney Disease was published22 and piloted across the UK along with advice from NHS Kidney Care. 23
The pathway through ESKF to CKM includes regular CKD management work, dialysis/CKM education to ensure informed decision-making, ongoing discussions about CKM versus dialysis for patients who have chosen CKM, activity to manage sequelae/symptoms of advancing CKD, and when the patient becomes symptomatic escalation of palliative care. It is not simply a ‘no dialysis’ option. 7 Maximum care to slow disease progression,24 management of other comorbidities, assessment and active management of symptoms (e.g. by correcting anaemia and acidosis, maintaining fluid balance and treating troublesome symptoms with drugs) including dietary restrictions,25,26 optimising communication and ACP,27 and improving care at the end of life,28 are all recommended. Services have increasingly been developed to focus on optimising conservative care. 29,30
Potentially, the delivery of such care can be undertaken by renal unit teams in outpatient departments or community outreach, or both, with varying input from specialist palliative care expertise and from primary care. 31 There may be crossover from intended CKM to dialysis and vice versa. Some patients with CKD5 may not be referred to a nephrologist at all or may be referred back to primary care, so receiving all their CKM outside specialist renal services. The physical health of patients and the attitudes of patients and physicians towards the added value of attending kidney clinics for CKM are likely to influence the decision to be referred and then remain in secondary care. Variation at centre level in the spectrum of older patients with CKD5 referred to renal units and managed by dialysis or CKM needs to be taken into account when examining outcomes of CKM patients in renal units.
Assessing optimal delivery of conservative kidney management
Major questions remain unanswered about how to commission and deliver CKM. Analysis of UK Renal Registry data4 suggest there is significant variation between renal units in the mortality rate during the first year of RRT. Although this could reflect variations in the quality of care delivered, variation in case-mix is a more likely explanation. This variation in case-mix is likely to be driven by variation in whether RRT or conservative care is recommended to frail, elderly patients with comorbidities, and by whether or not such patients are referred to the centre at all. There are no recent data on practice patterns for CKM. The last survey, conducted over 5 years ago before the National Service Framework for Renal Services: Part 23 suggested that only half of units even recorded the pathway choice for conservatively managed patients, and only five units had nursing or professions allied to medicine staff devoting over 12 hours per week to CKM. 31 Yet it has been reported that, if late referrals are excluded (patients referred with ESKF who start RRT within 3 months of referral), about 15% of elderly patients in managed nephrology care with CKD5 opt for CKM. 6,8,32 Decisions regarding these choices have been based upon clinical consensus and experience, supported by a very limited number of UK studies. 6–13,33,34 These studies have focused predominantly on survival, and few have captured evidence on other outcomes10 (such as patient preference, symptom burden, quality of life or quality of death) or clarified which patients in the older cohort would or would not benefit from RRT. Given the cost to patients (in terms of quality of life and dialysis treatment burden) and the cost to the NHS (in terms of resource usage), addressing this question has become imperative. The outcomes and costs of different models of care may vary substantially. Currently there is no financial payment for CKM under the Payment by Results tariff scheme and a better understanding of the resources and costs of CKM is needed.
The current study, Conservative Kidney Management Assessment of Practice Patterns Study (CKMAPPS), follows the guidance on developing and evaluating complex interventions. 35 CKM is clearly a complex intervention with multiple components and outcomes, and variable patterns of delivery. We first need to understand the intervention and how it is delivered, before we explore how it can be evaluated.
Study aim
The overall aim of this study was to determine the practice patterns for CKM of older patients with CKD5. This information should inform future service development and the design of a future prospective multicentre study to evaluate the effectiveness, cost-effectiveness and appropriateness of CKM compared with dialysis for treating elderly patients.
Objectives
The main objectives were:
-
to describe the variation between renal units in the extent and nature of CKM, its relative scale compared with dialysis, the factors influencing service developments and future plans (staff interview study, survey)
-
to explore how and when decisions are made in renal units about the main treatment options for older patients with CKD5, and what are the main clinical and patient factors that influence decisions to opt for CKM (staff interview study, survey, patient interview study)
-
to explore clinicians’ willingness to randomise patients with CKD5 to CKM versus dialysis and to assess the feasibility of a subsequent prospective study (survey)
-
to describe the interface between renal units and primary care in managing CKD5 patients [staff interview study, survey, general practitioner (GP) interview study/data linkage]
-
to identify the resources involved and potential costs of CKM (survey, staff interview study).
Methods
The research programme was a mixed-methods study divided into five parts:
-
patient interview study: a qualitative study with patients over the age of 75 years exploring their experiences of choosing between CKM and dialysis for the treatment of CKD5 in a purposive sample of nine renal units
-
staff interview study: a qualitative study across the nine renal units exploring the views and experiences of staff members who provide care for CKD5 patients over the age of 75 years
-
survey: a national survey of all UK renal units assessing the delivery of CKM
-
data linkage: linking routine data on new ESKF patients from the local clinical biochemistry laboratory records of the nine renal units with subsequent referral to the associated renal units
-
GP interview study: a qualitative study of GPs exploring their views and experiences of managing CKD patients and referring patients to secondary care.
Ethical considerations
Ethical approval to conduct the study was obtained from South Birmingham National Research Ethics Committee (11/WM/0240). Various secondary and primary care NHS trusts were involved in recruiting patients and staff in this research. Site-specific approval was obtained from all the relevant trusts. Research staff had NHS research passports and letters of access.
Chapter 2 Patient interview study: making decisions about treatment for stage 5 chronic kidney disease – a qualitative study with older adults
Introduction
This study set out to address objective 2 of the CKMAPPS project by exploring patients’ decisions to opt for CKM.
Few studies have explored why patients opt for CKM. 36–39 Patients who reported making an autonomous decision gave the following reasons: they felt they were too old for dialysis, they thought dialysis was too strenuous for them to undertake, they felt well without dialysis, they did not want to be a burden on their family, they knew other patients who had had bad experiences on dialysis and they found it difficult to travel to dialysis. 36–39 In addition, some patients believed they had no decision to make if they were told dialysis was unsuitable for them. 36 Researchers also identified that some patients were reluctant to think about the future, which meant decision-making about treatment potentially needed in the future was difficult. 38
While these studies give some indication of the reasons patients choose between treatment options, studies have been small and of a single centre. No research has explored, across different units with different CKM policies and practices, the views of patients on choosing between CKM and dialysis, and the reasons for their choice.
This qualitative study aimed to explore the views and experiences of older adults with ESKF, who had chosen different treatments for CKD5, on their treatment decision and reasons for their decision across nine UK renal units.
Methods
Design and setting
Qualitative study with semistructured interviews with patients recruited from nine renal units in England (Birmingham, Heartlands; Bristol, Southmead; Hull; London, King’s College; Manchester; Middlesbrough; Reading; Stevenage, Lister; Stoke-on-Trent). Renal units were purposively sampled to produce a diverse sample in terms of location in England and the scale of CKM delivery. The latter was estimated by responses provided by clinical directors to a previous survey by the UK Renal Registry. 40
Participants
Consultant nephrologists and nurses in each renal unit were asked to identify patients who were 75 years old or older and whose records indicated that they had an eGFR of less than 15 ml/minute/1.73 m2 or were on dialysis. Patients were required to speak English fluently and be judged by their health-care professionals (HCPs) to be sufficiently fit, physically and mentally, to take part in an interview. The researchers aimed to sample patients purposively by stage of illness and management pathway as follows: (1) following the decision to opt for CKM (CKM pathway), (2) following the decision to have dialysis in the future, but before starting dialysis (pre-dialysis pathway) and (3) following the start of dialysis (dialysis pathway). Staff in each unit were responsible for identifying patients in each of these groups who met the inclusion criteria, and patients were invited to take part in the study either by post or in person. Demographic and other information for all patients invited to take part in the study was recorded (gender, age, ethnicity, date first seen at renal unit and date started CKM or dialysis if applicable).
Interviews
Patients were interviewed by an experienced qualitative researcher (ST-C), either face to face, in the patient’s own home or in their renal unit while they were on dialysis, or by telephone. The type of interview was determined by patient preference except for some interviews which had to be carried out by telephone because of the distance between the researcher and participant. The interviewer introduced herself as a non-clinical researcher to explain that she had both no medical training and no specific allegiance to the nephrology field. All patients gave written informed consent, either at the time of interview or before the interview if carried out by telephone. Interviews followed a semistructured interview guide which asked patients about their medical history in relation to their CKD, their contact with their renal unit, their knowledge and understanding about management options and their reasons for their management decision (see Appendix 1). A semistructured format was deemed suitable to ensure relevant questions were asked to all patients but also to allow patients the opportunity to talk about issues which were important to them. Interviews were audio-recorded and transcribed verbatim by an independent transcriptionist. Transcripts were checked by the interviewer to ensure accuracy. Recruitment and interviews continued until the interviewer was satisfied that the data indicated saturation.
Data analysis
Thematic analysis41 allowed an inductive approach to exploring the data, which lessened the likelihood that findings would be influenced by the existing literature or the researchers’ preconceptions. Transcripts were coded line by line, with codes being assigned to each meaningful segment of text. Transcripts were then compared with one another, using a constant comparison approach taken from grounded theory, to search for similarities and differences between interviews. 42 ST-C independently coded 20 interview transcripts and developed an initial set of themes. NVivo 9 (QSR International, Warrington, UK) was used to facilitate coding. Initial themes were discussed with the wider research team, and themes and subthemes were amended and renamed until a consensus was reached. This agreed framework was used to code the remaining 22 transcripts. Any new data occurring in transcripts which did not fit into the existing themes were highlighted and discussed further. New themes and subthemes were added, and existing themes amended, in the light of these new data.
Results
Participant characteristics
Ninety patients were invited to take part in the study and 44 agreed. Eleven patients declined, five patients were unable to take part for health reasons, four patients died after being invited and 26 did not reply. Patients who did not take part were mostly CKM patients, which meant that more CKM patients had to be invited to take part (Table 1).
| Characteristic | Patients who agreed to take part (n = 44) | Patients who refused/did not respond/were unable to take part (n = 46) |
|---|---|---|
| Age (mean, years) | 81.7 | 82.7 |
| Gender (male) | 30 (68%) | 31 (67%) |
| Ethnicity (white British) | 40 (91%) | 44 (96%) |
| Pathway | ||
| CKM | 14 (33%) | 22 (48%) |
| Dialysis | 14 (33%) | 11 (24%) |
| Pre-dialysis | 14 (33%) | 13 (28%) |
Forty-two patients were interviewed, 14 patients in each group (Table 2). All dialysis patients were on hospital haemodialysis (HD) except for two on continuous ambulatory peritoneal dialysis. Interviews ranged from 27 to 87 minutes with a median of 47 minutes. Interviews were carried out between May 2012 and February 2013 and were carried out in person except for five interviews carried out by telephone. There was no indication from the data that interviews carried out by telephone differed substantially from face-to-face interviews in content although interviews were slightly shorter on average. While some interviews took place with a family member in the same room, three of the CKM patients specifically wanted to be interviewed with a family member for support.
| Unit | Pre-dialysis pathway | Dialysis pathway | CKM pathway | Total for unit |
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 5 |
| 2 | 2 | 2 | 2 | 6 |
| 3 | 2 | 1 | 1 | 4 |
| 4 | 1 | 2 | 1 | 4 |
| 5 | 3 | 2 | 1 | 6 |
| 6 | 1 | 3 | 1 | 5 |
| 7 | 2 | 1 | 1 | 4 |
| 8 | 0 | 1 | 3 | 4 |
| 9 | 1 | 1 | 2 | 4 |
| Total | 14 | 14 | 14 | 42 |
Sociodemographic characteristics did not differ substantially between the three groups. Participants’ ages ranged from 74 to 92 years with a mean of 82 years, most were men and most identified themselves as white British. Many patients were married or had a partner (n = 24, 57%) and most were living with their partner (n = 22, 52%). Other patients lived alone (n = 14, 33%), with their children (n = 3, 6%), their friends (n = 1, 2%) or in a care home (n = 2, 4%). Compared with the pre-dialysis and dialysis groups, patients opting for CKM were slightly older, were more likely to be female and were more likely to live alone or in a care home, but group numbers were small (Table 3).
| Characteristic | Pre-dialysis patients (n = 14) | Dialysis patients (n = 14) | CKM patients (n = 14) |
|---|---|---|---|
| Age (mean, years) | 81.3 | 80.4 | 83.5 |
| Gender (male) | 11 (78%) | 10 (71%) | 7 (50%) |
| Living alone | 2 (14%) | 6 (43%) | 6 (43%) |
| Living in a care home | 0 | 0 | 2 (14%) |
At the time of interview, patients had been in contact with their renal unit for a median of 49 months (range from 5 to 131 months). Dialysis patients had been on dialysis for a median of 10.5 months (range from 1 to 120 months) and patients who had opted for CKM had done so a median of 11 months previously (range from 1 to 83 months). Although recruitment had aimed to interview CKD5 patients, some patients reported that their eGFR was between 20 ml/minute/1.73 m2 and 15 ml/minute/1.73 m2 in interviews, indicating that they had CKD4.
Renal unit characteristics
Results from the national survey (see Chapter 4) confirmed variation in the scale of CKM delivery in the nine units sampled (Table 4). Three of the nine renal units reported that, of their patients over 75 years old, under 10% were receiving CKM, as opposed to other units, where up to 50% of over-75-year-olds were opting for CKM. The same three units reported that the terminology used to refer to CKM was usually phrased as ‘non-dialysis’. Based on these data, units were classified into two groups: those with more established CKM pathways (units 1, 2, 5, 6, 8 and 9) and those with less established CKM pathways (units 3, 4 and 7).
| Unit | Percentage of patients over 75 years on CKM | CKM discussed with all patients over 75 years? | Dedicated staff time for CKM patients? | CKM guideline? | Staff training in delivering CKM? | Dedicated CKM clinics? | Funding for CKM? | Terminology used to refer to CKM |
|---|---|---|---|---|---|---|---|---|
| 8 | 40–49 | Yes | Yes | Yes | Yes | Yes | Yes | CC |
| 1 | 20–29 | Yes | Yes | Yes | No, in preparation | Yes | No | CM |
| 2 | 40–49 | Yes | Yes | Yes | Yes | No | No | SC |
| 6 | 20–29 | Yes | Yes | Yes | Yes | No | No | CKM |
| 5 | 10–19 | Yes | Yes | No | Yes | No | No | CC |
| 9 | 20–29 | Yes | Yes | No | No | Yes | No | CM |
| 7 | 1–9 | Yes | No | No, in preparation | Yes | No | No | CM (non-dialysis) |
| 3 | 1–9a | Yes | No | Yes | No | No | No | Non-dialysis care |
| 4 | 1–9 | Yes | No | No | No | No | No | Not for dialysis |
Qualitative findings
Four themes emerged from the analysis of all interview transcripts (Figure 1). All themes were relevant to all patients, but differences within themes emerged between patients who had chosen different treatment options and between patients from different units.
FIGURE 1.
A thematic map of the four themes identified from the analysis of 42 interviews.

The diagram indicates how interaction with staff fed into patients’ conceptualisation of the process between understanding CKD and making (and occasionally revising) a management decision for their own CKD.
Theme 1: patients’ understanding of the management of chronic kidney disease
Patients mentioned that information about management had been provided through discussions with staff, written information and education days (meetings at the unit for patients to come and hear about different types of treatment). Although patients reported being given a large amount of information, some felt overloaded by the amount of information and described feeling unclear about what treatment options entailed, and others felt that the information given to them could be improved.
My wife was enraged with the brochure to such an extent that she brought it home and re-wrote parts of it . . . The pictures were meaningless; a woman sitting at a desk with a tube somewhere didn’t mean anything to anyone. So the information was bad.
Male, 79 years, dialysis, unit 5
Education days seemed to be well received by patients, particularly when they were able to hear about others’ first-hand experiences of dialysis. Education days were not available in all units; however, this appeared to be a popular option among patients and may be of benefit if delivered in more units.
Some pre-dialysis and dialysis patients stressed that they did not want, or had not wanted, to know about the details of dialysis until it was time for them to start.
I wouldn’t come down and see the [dialysis] machines. No, I thought wait till it happens and – I don’t want to know until I have to, you know?
Female, 76 years, dialysis, unit 8
All patients were aware of dialysis and the majority had an idea of the differences between peritoneal dialysis and HD, with most describing them as dialysis ‘at home’ or ‘in hospital’. In most units, knowledge about CKM was not common among patients who had not opted for it.
It was presumed that dialysis would work for me, I presume . . . I can’t remember [unit] ever – ever suggesting to me or saying that there is a third option – of not having dialysis. There were two sorts of dialysis.
Male, 82 years, pre-dialysis, unit 5
However, in units with more established CKM pathways, some dialysis patients were aware of CKM as an option which some patients chose.
[The nurse] was leaving the low clearance to go to people who were having – non-dialysis, I think tablets and things.
Female, 76 years, CKM, unit 8
In relation to this, some patients described how dialysis had, in their view, always been inevitable for them.
Going to low clearance, you saw people go off onto dialysis, so you knew that it would come to you, you know?
Female, 76 years, dialysis, unit 8
Conservative kidney management patients also reflected that, at first, they had also been guided towards dialysis because other patients were preparing for it.
Well initially, because you think that’s the right way to go, you’re on the dialysis track. So you’re going that way, everybody’s going that way . . . So that’s the track you’re on. At some stage – then the conservative management comes into play – and it’s when you realise that the dialysis is perhaps not the best track, but something has to tell you that . . . what told me was [the experience of my friends].
Male, 82 years, CKM, unit 5
Theme 2: patients’ perceptions of their own chronic kidney disease
Most pre-dialysis and CKM patients reported that they had no symptoms from their CKD. Others were unsure whether their symptoms were due to their kidneys, another comorbidity or just ageing. A lack of symptoms seemed to be interpreted by some CKM patients as an indication that their CKD was not serious.
They wanted to put me on dialysis, nearly five months ago, but I didn’t want to go on dialysis. Everything is all right, you know, I don’t have to go on dialysis.
Male, 81 years, CKM, unit 2
In addition, two CKM patients felt they could prevent their kidney function from decreasing through diet and medication.
I think if I try, I can sort of get a bit better than what I am, if I had the right – materials, you know, the medication and all that. And what to eat, I think that’s the main thing, what to eat and what not to eat.
Female, 88 years, CKM, unit 6
It was clear that discussions with staff had helped other patients to realise how serious their CKD was. Most had been told when dialysis would need to be started in terms of the level of their eGFR. In addition, patients from one renal unit all reported that they had been given an estimate of which calendar year they would probably need to start dialysis if chosen.
They’ve been watching this graph going down . . . they talked to me about dialysis and they said that in 2012 it looked as if I would reach the time when I should need it.
Male, 83 years, pre-dialysis, unit 5
However, in other examples, patients reported that they had been told different things by different members of staff about their CKD, which had influenced how serious they felt it was and appeared to influence their treatment decisions.
This nurse came to have a talk with me . . . and she says ‘we’ve given you a score of 6 [eGFR]’. So I thought – 6/10, that’s not bad, I can live with that. Then I thought, 6 out of how many? She said ‘6/100 that’s how poorly you are’ and that brought me down to earth.
Male, 87 years, CKM, unit 9
It went from 6 to 5 and the doctor said, ‘don’t worry, it’s alright’, she says, ‘I’ve got a patient on 4, been on 4 for years and she’s still alive, don’t worry’. I said, ‘oh well, that’s alright’.
Male, 87 years, CKM, unit 9
Theme 3: patients’ experiences of making a management decision for their chronic kidney disease
Patients’ assessments of dialysis
Patients who had opted for different treatments appeared to hold contrasting beliefs about the advantages and disadvantages of dialysis. Some dialysis and pre-dialysis patients believed that dialysis was the only way they could continue to live and described a belief that others who chose not to have dialysis were ‘cutting their lives short’.
I don’t fancy the idea of having to lay on the bed there for 4 hours, but, you know, it’s – the alternative is death, isn’t it, so there’s no choice.
Male, 77 years, pre-dialysis, unit 4
Patients from units with a less established CKM pathway reported that they had been told they would live for many more years on dialysis.
[Consultant] said, ‘well it looks as if you will probably have 6 years [on dialysis]’.
Male, 82 years, dialysis, unit 3
In contrast, some CKM patients, from units with more established CKM pathways, believed that dialysis did not guarantee longer life. This appeared to reflect what they had been told by renal staff.
I decided – after seeing this [dialysis] demonstration – that I didn’t want dialysis. I’m told that’s not terribly unusual – and I was told that – if you say no to dialysis, you don’t necessarily live any longer anyway. And as I’ve no discomfort or pain or anything like that, I thought I’d carry on.
Male, 84 years, CKM, unit 9
Alongside longevity, patients considered quality of life. Many CKM patients believed that they would have a better quality of life without dialysis and prioritised this over living longer. In these situations patients seemed to have formed their own beliefs about dialysis rather than from discussing quality of life with staff.
It did occur to me that [on dialysis] you were, sort of, living for tomorrow, for your next treatment, for tomorrow, for your next treatment, for tomorrow. And it made me think, well, I wonder if it’s better to live as best you can, as you can and let time take its course.
Male, 82 years, CKM, unit 5
In contrast, some pre-dialysis patients believed that dialysis could offer them a better quality of life or help them to maintain their current quality of life.
Well I’ve been promised that I shall be ten times better [on dialysis]. (Laughs) I’m being optimistic.
Male, 76 years, pre-dialysis, unit 9
Conservative kidney management and dialysis patients spoke very differently about the time one would have to spend on dialysis. CKM patients saw this time as a ‘waste’ whereas others felt they were gaining additional days or that it was similar to how they would usually spend their time. Again these views seemed to be based on the patients’ own interpretations rather than on information they had been given by their unit.
I don’t want to waste a week of my life all the time – when I can be sat at home, enjoying myself, you know. I mean, to me, I’m going to lose my life if I’m going to have to be on dialysis.
Female, 82 years, CKM, unit 7
I’m 81 so it really don’t matter to me, I thought 4 hours out of your life twice a week, what difference does it make . . . I mean I would only be sat watching the television anyway.
Female, 81 years, dialysis, unit 7
Additional factors which influence patients’ decisions
Transport to and from dialysis was a major concern for patients and was a reason for some not to have dialysis when home dialysis was not an option for them.
Well, I can’t drive and I live out of town and so it’s relying on hospital transport and I mean you could be waiting hours . . . I just couldn’t cope with it.
Female, 82 years, CKM, unit 7
Many patients talked about how family was a consideration in their decision. Several patients on dialysis had family support which made it possible for them to undertake dialysis. Two patients reported that they were carers for their spouses, which had been the major influence on their decision.
I can understand [people choosing CKM], I can quite easily understand that, but whilst the wife is about, if dialysis helped me along, to keep her out of a nursing home, then that would be a good achievement as far as I’m concerned.
Male, 82 years, pre-dialysis, unit 5
Some CKM patients indicated that they did not want to be a burden to others or the health-care system by having dialysis and indicated that it was of little benefit to have dialysis when they felt they had already reached old age. This appeared to stem from patients’ own beliefs rather than anything they had discussed with staff.
At 80, there is a lot of younger people that could benefit from dialysis which, you know, what’s really the good of dialysis when you reach 80 years old?
Male, 82 years, CKM, unit 5
I could see a big disruption to my husband [if I had dialysis], he’s had quite enough to do without having to get me ready to go in [to hospital]. If I was younger, say 50s I might think about it, but I shall be 87 in a couple of weeks’ time, so what’s the point?
Female, 86 years, CKM, unit 1
Four patients said that they knew of a friend or relative who was, or had been, on dialysis. Some had heard about others’ bad experiences with dialysis, which appeared to have influenced their decisions.
I decided not to have dialysis . . . at that time, actually, two of my close friends had dialysis and I don’t ever want to be involved in that, that’s no way to live . . . There was no fun in their lives, it was just hopeless really. We tried to go on holiday but every 2 . . . every 4 hours you had to go back to change and we didn’t get nowhere at all.
Male, 81 years, CKM, unit 3
Staff influences on patient decision-making
Patients also appeared to have been strongly influenced by staff when making their decision. Many patients talked about how staff had explicitly recommended dialysis or presented it as the best option in most units.
[The staff] said ‘well it’s entirely up to you, you’ve got the choice. You can have dialysis or you can have the other thing . . . But if you want not to have dialysis it’s your choice but you’ve got to realise that it is going to kill you . . . But if you’re on dialysis you could last for 10, 15, 20 years’.
Male, 76 years, dialysis, unit 2
However, in some units with more established CKM pathways, staff appeared to have discussed CKM as an alternative to dialysis with patients, presenting this alongside information on dialysis.
They went to great lengths to tell us about the fact that we could opt in or out of the dialysis and that there was an alternative to dialysis, which is this care path.
Husband of female, 74 years, CKM, unit 8
Theme 4: patients’ experiences of revising management decisions
Only patients who had opted for CKM talked about having the option of changing their decision and most seemed to have been told that this option was always available to them.
Two dialysis patients, from the same unit, reported that they had changed their treatment decision in the past. They reported that they had initially chosen CKM because they felt well but then opted for dialysis when they had felt ill as a result of their CKD.
I said at the time no [to dialysis] and then within a fortnight I’d changed my mind. Because my health wasn’t very good at all.
Male, 88 years, dialysis, unit 4
In three of the 14 interviews with CKM patients, participants implied that they would have dialysis if they ‘had to have it’ or if they ‘got really ill’, although this was not usually revealed until late in the discussion. An example is this discussion thread taken from one interview with a CKM patient.
One of the nurses told us that you had decided not to have dialysis?
No. She said that if I did change my mind – you know – but – I don’t think I will, definitely not.
[later in interview]
And [nurse] said to you more recently that you’re able to change your mind if you decide you want to have dialysis?
Oh yes.
And what do you think about having that – that option still available?
Well it’s nice, I think, that it’s there; whether I’ll ever take it up, I don’t know – but – in a way, I suppose it’s a comfort that I could go back, you know, if I was really ill.
[later in interview]
Yes, so do you think that – you might change your mind then, if you got – if you got more symptoms from it or got quite ill?
Well yes, if I got really ill and I wouldn’t be having any – type of life anyway, would I? If I was that ill, you know, so there wouldn’t be that much choice.
This may indicate that the goals of CKM had not been fully explained to patients. These patients attended both types of units, those with more and less established CKM pathways.
Well if it came to the point, I’d have to do [dialysis], wouldn’t it, you know? But it comes to the stage, you know, that then there’s no alternative. You have to do it.
Male, 81 years, CKM, unit 2
The revision of a decision from CKM to dialysis appeared to be linked to a lack of consideration of what would happen in the future. CKM patients differed in whether or not they had discussed the future with staff. CKM patients from units with more established CKM pathways appeared to have discussed the future with staff more than patients from other units. This included talking about how their CKD would progress and setting up ACP.
I mean I don’t think it’s an agonising death like people suffer with bone marrow, cancer and all that . . . they said ‘you could suddenly start to feel very ill . . . and then ultimately probably go into a coma and just disappear.’ Which doesn’t sound pleasant but it’s not that bad to worry about.
Male, 75 years, CKM, unit 1
[Nurse] arranged care to come – they took her away and put her in a hospice for a couple of weeks, just down the road here. They were marvellous, but she went to the hospice and they discussed the end of life with her and, so, I think [nurse] did as well and I think they spoke about it.
Husband of female, 74 years, CKM, unit 8
Summary
Main findings
While CKD patients consider the same factors when making a treatment decision, patients who choose different treatments hold contrasting beliefs about what dialysis can offer. These beliefs appear to be influenced by the information provided by renal staff, which can differ between units, particularly in regard to CKM. It was noticeable that few patients were aware of CKM as an option if they had not chosen it, although patients from units with a more established CKM pathway appeared to be more aware of CKM. While most acknowledged the severity of their CKD, some CKM patients did not appear to think of their CKD as serious because of its asymptomatic nature, despite information from staff indicating otherwise. There was a distinct divide between CKM patients, and dialysis and pre-dialysis patients in whether people believed they would live longer on dialysis or not and whether they expected their quality of life to get better or worse on dialysis. Information from units with less established CKM pathways appeared to focus on the number of additional years a patient was likely to live on dialysis. Patients from units with more established CKM pathways, however, described being told that living longer on dialysis was not a guarantee and in addition that choosing CKM was ‘not unusual’. Finally, few patients reported speaking to staff about the future, in terms of the consequences of either starting dialysis or opting for CKM. Patients from units with more established CKM pathways appeared to have discussed the future with renal staff and some indicated that they had begun conversations about ACP. For others, being unaware of how their disease was likely to progress added to the misperceptions some patients appeared to hold about the severity of their CKD and the need for dialysis.
Comparison with existing research
Particular results mirrored those identified in previous qualitative research. 36–39 CKM patients reported that they had chosen CKM because they felt too old for dialysis, they were worried about being a burden on family/society and they were concerned about travelling to have dialysis. While these factors can be valid reasons for making a decision there is also a sense that some patients may feel dialysis is not really a possibility or that they do not have a right to dialysis. Thinking of oneself as a burden on society, if dialysis is chosen, may lead to feelings of guilt for patients, who may benefit from extra time to talk through their decision with staff. Equally, concerns about whether transport is a possibility or not, rather than a preference to avoid transport, are different thought processes, and equity of access to treatments needs to be assured.
Reluctance to think about the future and feeling well without dialysis have been identified in previous studies37,38,43 and they linked to the current findings about perceptions of CKD severity and revising decisions. Feeling well, with few symptoms, seemed to make some patients think their CKD was less severe, which meant they did not see a reason to consider dialysis. A reluctance to think about the future and possibilities of becoming more ill had led some to choose CKM initially and then change their decision later on. This may be unavoidable for some patients who are unable to understand their CKD until they feel symptoms, although clinicians should be wary about using the term ‘CKM’ to refer to such patients. Patients who are in ESKF often want to know their prognosis but clinicians may feel uncertain or uncomfortable about when to discuss this with patients. 43 Open discussions about prognosis are likely to have a substantial influence on discussions about treatment options and feed into better shared decision-making.
Results also supported previous qualitative and quantitative work which showed how CKM patients may choose quality of life over length of life. 39,44,45 While this indicated different priorities between patients, the current results highlighted how patients held contrasting beliefs about whether dialysis would extend life or not, indicating the importance of patient expectations as well as priorities. Results also supported previous work which indicates that patients may choose CKM if they feel they have achieved everything they have wanted to in life. 39 This contentment and feeling of having had a ‘complete life’ has been suggested to lessen anxiety about death and end of life. 39
In addition to existing research, the current study identified the influence staff, and information from the renal unit, had had on patient decision-making. Patients from different units reported being provided with varying types of information, which presented CKM in a more or less positive light. It appeared that patients from units with a more established CKM pathway were more likely to know what CKM was, had been given more information about CKM and, for those who had chosen it, had discussed the consequences of their decision in more detail. While there may be recall issues with patient reports, results suggest that there are unit variations in the way in which older CKD patients are informed.
Strengths and limitations
This was the first qualitative study to explore patients’ views of choosing between dialysis and CKM across different renal units. Including nine units in the study meant that the sample contained diversity in terms of both patients’ treatment decisions as well as the service delivery experienced. We were able to include units which had more or less established CKM pathways in order to explore variation in what patients had been told.
Qualitative results cannot be generalised to other populations but data gathered can identify important issues and offer conceptual transferability. Indeed, the findings presented resonate with the existing literature. While interviewing methods run the risk of obtaining socially desirable responses, the interviewer presented herself as an impartial observer who had no link to the renal units or staff. Moreover, patients mentioned negative aspects of the care they had received, which suggests that they felt able to speak freely and in confidence. Finally, as with all interview studies, the findings do not provide a window on events as they happened but rather provide insight into how participants construct their experiences of relevant events.
Although the study had clear entry criteria, one participant under 75 years was invited to take part and three participants reported they were CKD4 rather than CKD5. Recruitment was difficult and time-consuming for renal staff and it was not always clear that participants did not match the inclusion criteria until part way through an interview. Data on patients’ eGFR over time were not collected. It is likely that patients with both advanced but stable kidney function and those with a linear, progressive, predictable decline in kidney function were recruited. Such differences may have had implications for how treatment options were discussed because of the clinician’s (un)certainty of how likely a patient was to die from their CKD rather than another condition.
The study excluded participants who did not speak English fluently, in order to ensure that participants were able to provide full responses to questions without the need for a translator. This limitation meant that the study was less likely to recruit patients from black and minority ethnic groups. Recruitment of such patients would have required additional resources which were unavailable and somewhat unfeasible for this study and may be better suited to a more focused qualitative study looking specifically at this population.
Recruitment of advanced CKM patients was particularly difficult. CKM patients most often had an eGFR of over 10 ml/minute/1.73 m2 and few felt they had any symptoms from their CKD. This meant CKM patients who were symptomatic and who otherwise would have started dialysis were not well sampled; interviewing more of this type of patient might have provided additional insights into why CKM is chosen by some and would have allowed more direct comparison of CKM versus dialysis patients. However, such patients may not have been physically and mentally well enough to take part. This is likely to be a limitation of any qualitative work carried out with a chronically ill older population across several centres.
The views of dialysis, pre-dialysis and CKM patients were compared. While all patients were similar in age, patients differed greatly in their physical and mental health, within and between treatment groups. The choice between dialysis and CKM is most relevant to older adults who are frail with other comorbidities. While we tried to recruit such patients, this was not always possible and some CKD patients who were not frail or burdened with multiple comorbidity were recruited to the study. For these patients it may be unsurprising that less emphasis had been placed on CKM as an option by renal unit staff.
The number of renal units in the study captured variety in CKM delivery. However, this influenced the number of patients interviewed per unit, which had to be limited in order to obtain a manageable data set which could be explored in depth. Future qualitative work may benefit from sampling more patients from fewer units in order to follow-up initial data which suggests that patients attending different units are receiving different information about treatment options.
The categorisation of units into two groups representing units with a more or less established CKM pathway was relatively crude. Inevitably there was some overlap in policy and practice between the two groups; however, the categorisation helped us to look at general trends in the data.
Implications
Patient reports indicated that they had been given different types of information, at different time points, by their renal unit and that units with a more established CKM pathway appeared to have provided more detail about the option of CKM than others. While this may have reflected the suitability of dialysis or CKM for the individual patient, it was interesting that patients from units with a more established CKM pathway were aware of both treatments regardless of what their final decision had been. Having fewer resources dedicated to CKM may reflect a general trend in a unit to encourage dialysis for all patients. However, it may also reflect less experience in providing care for CKM patients. Staff with access to fewer resources and less experience in providing a type of care may be less likely to discuss such care with patients, which may bias discussions towards dialysis. Staff may also be less supported in these discussions if they are not provided with materials which help to explain the CKM pathway or with training in how to have such discussion with patients. Finally staff may not feel comfortable discussing CKM with patients if they believe that dialysis will always offer more benefit despite comorbidity or frailty. Research which can provide data on the comparative outcomes of CKM and dialysis patients will help to clarify whether dialysis is beneficial for certain patients or not.
It was interesting to note that some patients had opted for CKM several months before interview, in one instance 7 years previously. It was also apparent that some patients had initially opted for CKM when they were well and changed their mind once they had experienced symptoms from their CKD. Both situations suggest that the label of CKM is being used for a very broad population, arguably one that is much larger and more diverse than the label ‘CKM’ would initially suggest.
Conservative kidney management is an alternative to dialysis and therefore could be strictly defined as applying only to patients who have passed the point at which dialysis would usually have been started, though this point can be difficult to define, especially in patients who may have symptoms related to other comorbidities. While this time point will be different for individual patients and judged differently between clinicians, a consensus based on eGFR or symptoms linked to kidney failure may be possible and would reduce the number of patients with asymptomatic, stable CKD being labelled as receiving CKM. Delaying a label of CKM would allow patients more time to think about their decision. It may lead to patients experiencing more symptoms from their CKD and give them greater awareness of how much their CKD is affecting their life. Having a more standard approach to the labelling of CKM, specifying separately those patients who currently plan to have CKM in the future and those who are currently receiving CKM, would provide a clearer view of the numbers of patients on this pathway and the variation between units. Our interviews emphasise that both categories of patients retain the option of changing their plan or choice of treatment.
Both dialysis and CKM patients reported they had not discussed the future with staff. Regardless of what treatment decision is made, it is important that renal staff discuss the likely trajectory of illness with patients. Previous literature has highlighted this issue and recommended earlier, rather than later, conversations about ACP to promote optimal end-of-life care. 46,47
Finally, quantitative results from the CKMAPPS survey indicate that units use different terminology for CKM and that there is a subgroup who refer to CKM as ‘non-dialysis’. CKM is accepted by most as an alternative to dialysis for a subset of patients, and patients may benefit if the option of CKM is presented in a consistent and unbiased manner across renal units. Units that are organised to support staff in the discussion of CKM options with patients will deliver patient benefit by promoting greater shared decision-making. 48,49 Clinicians should consider the implications of having a pathway framed as a negative option. Consistency in terminology would help establish CKM as a clearly defined and appropriate pathway for some patients.
Conclusion
Our results indicated that older adults with CKD5 who have chosen different treatment options have contrasting beliefs about what dialysis will offer them. Patients’ decisions were influenced by the information provided by their renal units, which differed between units with more and less established CKM pathways. Supporting staff in discussing CKM as a valid alternative for a subset of patients across all renal units will promote informed decision-making and thereby stand to benefit patients.
Chapter 3 Staff interview study: treating older adults with stage 5 chronic kidney disease who opt for conservative kidney management – a qualitative study with renal unit staff
Introduction
This substudy addresses objectives 1, 2 and 4. Semistructured interviews were conducted with renal HCPs to explore the organisation and provision of CKM to patients in their units, and their views and experiences of treating patients who opt for CKM. The primary aim of conducting these interviews was to inform the development of the national survey questionnaire.
Only a few qualitative studies have explored renal staff’s experiences with patients on RRT. One study identified that physicians and nurses found it difficult to meet the complex needs of elderly patients and faced dilemmas when making decisions concerning withholding or withdrawal of dialysis. 50 Another study found that nephrologists working in HD care faced ethical dilemmas where they were forced to make ‘life or death’ decisions, such as whether or not to start or discontinue dialysis. These decisions were made particularly difficult if patients and relatives were in disagreement about a treatment option, and when they were faced with time restraints and professional and personal demands. 51 Nephrologists also found it difficult to explain the complexity of advanced kidney disease to patients with difficulties in managing a disease over which they have little control. Consequently, discussions with patients focused on present clinical status and avoidance of discussions of prognosis and the future. 44
While these studies identify difficulties that renal staff face, they do not give a detailed account of staff’s experiences of discussing alternative treatment options such as CKM with patients. None of these studies was conducted in the UK. This study focused on exploring UK renal staff’s views about CKM and their experiences of the provision and delivery of the CKM option for older adults with ESKF.
Methods
Design and setting
Semistructured interviews were conducted with staff members in nine renal units across England. These units were the same as those involved in the patient interview study (see Chapter 2) and were purposively sampled to obtain a range of locations and scale of CKM practice.
Participants
Consultant nephrologists in each participating unit were approached by the principal investigators and asked to identify a minimum of five staff members who were involved in the care of CKM patients in their unit. For units that had very few CKM patients, staff who cared for patients in low-clearance clinics or for those whose eGFR was less than 20 ml/minute/1.73 m2 were recruited. We stipulated that the staff members interviewed had to include a minimum of one lead nephrologist and one nurse per unit. Allied health professionals were also invited to participate in the study.
Interviews
Participants were interviewed by IO and ST-C, either face to face at their renal unit or by telephone. All participants signed a consent form either at the time of interview or before the interview if they were interviewed over the telephone. Semistructured interviews were conducted following an interview schedule (see Appendix 2). The interview schedule was initially constructed from a literature review and discussion with steering group members and was then developed iteratively as interviews were carried out. The interview schedule asked participants to discuss their views of CKM, their experiences of being involved in decision-making about CKM, how CKM was delivered in units and the role of primary and palliative care services in caring for CKM patients. Interviews were digitally audio-recorded and transcribed verbatim, and interviewers verified the accuracy of transcription by listening to the interview and reading transcripts.
Data analysis
A content analysis of the 60 interviews was carried out to inform the survey questions (see Chapter 4). This consisted of several steps and included rereading the interview transcripts, identifying frequency of words, phrases and themes, and then developing categories of words, phrases or themes with similar meaning. 52 The data from the qualitative study helped to identify the sections of the survey and facilitated the formulation of the survey questions and multiple choice options.
Subsequently, a more detailed thematic analysis41 of the qualitative data was conducted. NVivo 10 was used to facilitate coding of the data. From the 60 staff interviews, 28 interviews were sampled using maximum variation sampling to ensure variation of units and experience of being involved with renal patients. Fifteen interviews were analysed in the first instance using thematic analysis by IO. 41 ST-C independently coded 10 of the 15 interviews. These interviews were selected from four units, which were the first participants recruited to the study. This sample was selected to ensure variation in units, staff roles and their experience of being involved with renal patients. Researchers reread the interview transcripts in order to identify emerging codes and to develop an initial coding framework. A further 13 interviews were selected to ensure variation of units and experience of being involved with renal patients; they were then coded by IO, ST-C and a third researcher, CE, using the preliminary framework, which was refined and further developed into the final thematic framework. Discrepancies were discussed and resolved by consensus. Data from the remaining 32 transcripts also added to the final thematic framework and facilitated saturation of the themes, which was achieved when no new codes or themes emerged from the data.
Results
Participant characteristics
Sixty renal staff members were interviewed between February 2012 and November 2012. Table 5 summarises characteristics of all those interviewed and of the 28 staff whose interviews were analysed using in-depth thematic analysis.
| Characteristic | 60 participants interviewed | 28 participants whose interviews were analysed in depth |
|---|---|---|
| Gender (n) | ||
| Male | 13 | 7 |
| Female | 47 | 21 |
| Age (years) | ||
| Median | 49 | 47 |
| Range | 28–67 | 36–67 |
| Job title (n) | ||
| Nephrologist | 22 | 14 |
| Nurse | 25 | 9 |
| Palliative care consultant | 1 | 0 |
| Allied health professional | 12 | 5 |
| Time at current unit | ||
| Median | 9 years | 10 years |
| Range | 9 months to 40 years | 10 months to 31 years |
Qualitative findings
Three themes emerged from the detailed analysis of 28 interviews and the analysis of the remaining 32 interviews.
Theme 1: providing conservative kidney management to patients
Many staff discussed how they provided CKM, including what services CKM involved and the resources available for the delivery of CKM. They also talked about how they viewed CKM and how it could be improved in their units.
Staff views about providing conservative kidney management as a treatment option
A variety of terms were used by participants to refer to CKM, such as ‘conservative care’, ‘maximum supportive care’, ‘non-dialysis care’ and ‘renal palliative care’. For example, the term ‘maximum supportive care’ was used at unit 8, which had a developed CKM programme with dedicated staff, CKM clinics, CKM guidelines and CKM funding, whereas unit 3, with a less developed CKM programme, used terms such as ‘non-dialysis care’.
We call it maximum supportive care and I’m sure one of the things that you’re coming across is that people have all kinds of different names for this.
So do I call it non-dialysis care or conservative kidney management?
Well you know, we probably . . . we probably use it . . . we probably use non-dialysis care. I think perhaps everywhere . . . I think we may change with the rest of the world where everybody calls it conservative kidney management.
Conservative kidney management was generally accepted by renal HCPs as a treatment option. Many renal staff described CKM as a valuable option for some patients, particularly for those with multiple comorbidities, and recognised it as an active treatment, managing the symptoms of kidney failure without using RRT. Some staff said that not dialysing someone used to be thought of as a failure by medical staff, and talked about how HCPs had recently started to realise that dialysis was not suitable for everyone. Having CKM as an established care pathway also helped staff, as they described how they were able to provide something to patients rather than just not providing dialysis.
When you are dialysing [patients] you know you are doing the most you can, but now because we have got an active conservative management programme my colleagues feel you know although the patient is elderly we can still offer them something. So you know, you feel that you can do something proactive.
Consultant nephrologist, unit 1, participant 1
While many described CKM as a valuable alternative treatment to dialysis, there was one nephrologist who did not hold this view.
There are amazingly few [CKM] patients [in our unit], whereas most people’s conservative care clinic is filled with people who do not currently need dialysis, who may or may not need dialysis at some point in the future, but it has been decided that they will not get dialysis, at that point in the future. And so these are the same people that we are treating in our nephrology clinics for chronic kidney disease, Stage 4 and 5 [. . .] We remain unconvinced that this is a useful concept for – for managing people with kidney disease. It doesn’t add anything to what we do at the moment.
Consultant nephrologist, unit 4, participant 23
Many interviewees recognised that there were some patients who unexpectedly responded well to dialysis, while others did not respond well and experienced complications as a direct consequence of the treatment. Some staff reported that they sometimes found it difficult to assess whether patients were suitable for RRT or CKM and wondered if they had given their patients the right treatment for them.
I think most of the nephrologists will find it very tricky when a GP sends you a 90-year-old lady with multiple comorbidities, with a GFR [glomerular filtrations rate] of 10 – how do you approach that? Do you say – well you are going to die – without dialysis; you are probably going to die with dialysis – and on dialysis and you’re probably going to have worse quality of life; I really don’t know.
Consultant nephrologist, unit 4, participant 22
Resources available for conservative kidney management vary
Resources available for delivering CKM varied across the units. For instance, only unit 8 had funding dedicated to providing CKM. Some of the units without such funding used alternative funding to develop general CKD services, which partly contributed to the delivery of CKM.
We won a bit of money and what we used that to do was to set up the Cause for Concern Register, the two renal resource days and also communications, so myself and [nurse’s name] have done the advanced communication course, but we want to now – for the whole of the renal services so every consultant, every specialist nurse will do the enhanced communication training. So that’s going to happen in the next year; so that’s what we use our funding for.
Consultant nephrologist, unit 7, participant 42
Many staff did not have allocated time for CKM patients, and their time for CKM was integrated into their normal workload. Some units did have dedicated CKM staff and stressed the importance of having staff who had dedicated time to treat CKM patients. Unit 8 had a dedicated palliative care nurse treating CKM patients as part of CKD patients approaching end of life.
We have a palliative care nurse. I mean one of the things that stops people doing [CKM] is that they know that if they sit down and have a conservative care conversation with someone, that takes an hour. One of the things that’s helped us to do [CKM] over the last couple of years is that we have a dedicated palliative care nurse who can spend all that time having those conversations. We have a dedicated palliative care nurse who can then do all the set ups to the hospice and get all the home care organised.
Consultant nephrologist, unit 8, participant 47
Some units provided their staff with training, which was perceived to improve staff’s confidence to deal with patients who were approaching the end of life, including CKM patients.
We have undergone quite an intensive staff education programme, communication skills mainly; we’ve done it across the board from clerical staff to doctors, right the way across nursing staff, and it really just helps patients. It’s dealing with a distressed patient, so say, somebody on dialysis says – I’m fed up with this, I really don’t want to carry on, I’m quite certain some of our nurses beforehand would have said . . . , I don’t know what to say, so I’ll pretend I didn’t hear it. Now they feel more empowered. So we have champions in each area now, who do feel competent in discussing end of life or death and dying.
Consultant nephrologist, unit 8, participant 47
Many staff reported that patients’ CKM decisions were recorded in their medical notes or database. Some also pointed out that having a clear record of the CKM decision not only within the renal unit but also in the primary care record was very important. Adequate recording was thought to minimise inappropriate hospital admissions and/or dialysis.
I think one of the most important things we do is make a clear record of the decision about dialysis in the hospital and in the primary care records . . . certainly in my letters it says please make it very clear for the out-of-hours doctors, that if they become unwell they would rather not be admitted to hospital and rather not be, you know, started on dialysis.
Consultant nephrologist, unit 5, participant 32
Service components of conservative kidney management
All staff mentioned that CKM offered assessment and management of symptoms caused by CKD. However, the way CKM patients were seen and what service they received varied between the units. At some units, especially those without CKM clinics, CKM patients have the same pathway as pre-dialysis patients until they reached the point when they would have started dialysis if it had been their chosen treatment.
[Patients on] conservative care do all the same [as dialysis patients] up to this point; counselled, chosen, coming back to clinic on anaemia treatment, [GFR] 8 to 10 they start getting symptomatic, then the nurses start visiting. They don’t start doing anything between [GFR] 15 and 8–10, they are not sort of having a different pathway at that point from anybody else, so they have got the same symptoms as the people that would start dialysis. They are not different at that point.
Consultant nephrologist, unit 6, participant 36
Unit 5 also did not have CKM clinics and a consultant nephrologist reported that they saw their CKM patients less often than in a hospital with CKM clinics and that they mainly monitor patients rather than actively manage them.
[At (a hospital name)] it looks like they see [CKM patients] much more often, they see people as if it is an active treatment. For us, we bring people back in 4 months and do a blood test, there is not a lot you can do every 4 months when you are seeing somebody and it sends out the message that well, we are just monitoring things.
Consultant nephrologist, unit 5, participants 32
There were units that had a multidisciplinary approach towards CKM patients. Unit 9 had dedicated CKM clinics and a nurse explained that they provided not only medical but also social care to patients.
If they come to the [CKM] clinic and we ask them very generic things about their social life, how things are going, do you need any more carers putting in, how is the carer, are you managing – mum’s not well, she had a fall. We do all that and then [nurse’s name] does a medical assessment; so they get a social assessment if you like, dietetic assessment and a medical assessment, all in one clinic. And then we’ve got our helpline, our phone as well.
Nurse, unit 9, participant 54
All staff discussed that patients on CKM received palliative/supportive care when they started to approach end of life. Some units mentioned the importance of providing patients with a smooth and timely transition to end-of-life care, which usually happened in the community or at a hospice. Staff from unit 2 reported that they monitored CKM patients carefully so that they could plan the end-of-life care for patients proactively.
We do our RAG [red–amber–green] rating, so we have red–amber–green rating for patients who are on conservative management. We are trying to identify those that would you be surprised if they are here in 3 months? [. . .] When the liaison nurses see one of the conservative management patients in clinic, they attend the clinic visit with them, do their RAG rating and come and readjust it on the supportive care register, and then raise those patients that are flagging up and moving and changing on the register, so you’re becoming more concerned about them. You would raise them at that [quality assurance] meeting and then at that point the liaison nurse should not only have highlighted the increasing need but have actioned it. For example, they would have said, ‘We are thinking of introducing them to the hospice’, ‘I’ve been in touch with the community nurses’, ‘I might have had a conversation with the GP’. We have started an advanced care plan, a social worker would often go out and arrange another home visit to see if there was a carer problem.
Consultant nephrologist, unit 2, participant 7
Staff views about organisation of conservative kidney management
Units 1, 8, and 9 had dedicated CKM clinics. Staff in units without dedicated CKM clinics saw CKM patients in low-clearance or general nephrology clinics. Some units had a small number of CKM patients, and staff discussed not needing dedicated clinics because of this.
[CKM patients] aren’t seen separately. We don’t have a non-dialysis care clinic. I suppose in the future, depending on numbers isn’t it, because what will happen to non-dialysis care patients is they will die off. You know so that the numbers don’t grow huge.
Consultant nephrologist, unit 3, participant 13
In contrast, some talked about the need for such clinics, as they would be likely to help provide better quality of care.
We do feel that it is important for us to develop some sort of service which would be dedicated to the conservative care and that’s one of the driving forces for us to probably think that we should have some sort of dedicated clinic. And one of the advantages of having a dedicated clinic for the conservative care, would be that we could at least spend a bit more time [with patients].
Consultant nephrologist, unit 6, participant 38
Some said that setting up dedicated CKM clinics would not be supported by consultants because they would then lose any continuity of care.
They want to see their patients through, whether they’re dialysis patients or conservative patients, or transplant patients. They want to be able to deal with the whole group of patients rather than . . . as soon as they make the decision then another consultant looks after them.
Nurse, unit 5, participant 30
Conservative kidney management patients were also seen by GPs and the community team towards the end of life, and this was mostly as a result of the patient’s preference. The size of geographical area a unit covered also affected how the unit cared for CKM patients. For example, unit 7 encouraged patients to be cared for by primary care teams once patients had chosen CKM, and they cared for patients in collaboration with GPs and community teams. This is mainly because the unit covered a large geographical area and many of their patients needed to travel a long way to come to the renal unit. Consultants from the unit questioned if it was fair to bring CKM patients, who were usually old and frail, to the clinic when the same care could, in theory, be given in the community.
[CKM] patients want to be followed up in the community and I think that suits them. [Patients] are enjoying what life they have and they are more likely to die at home, rather than coming into hospital and relatives having to take days off just for me to check a blood pressure. I don’t add any value at that point and I know some units tend to bring them back and I don’t approve, I don’t agree with that; I think it’s not fair.
Consultant nephrologist, unit 7, participant 42
This approach was not supported by all, as other staff had doubts about the quality of care delivered by units that mainly saw CKM patients remotely.
When we started we learnt a lot from [other unit]. You know I think [other unit] had a good [CKM] programme on paper but when the nurse actually went there, they were doing things by remote access if you know what I mean? Sort of phoning the GP and saying do the bloods, it’s not the same as seeing to the patient. So on paper you could have a lovely conservative management programme but how much of that is . . .
Consultant nephrologist, unit 1, participant 1
How to improve conservative kidney management within units
Many members of staff mentioned that they would need more involvement from primary care and palliative care teams in order to improve the current way of providing CKM in their units. Some described that they needed a more formal process to support GPs by providing information about renal management.
Do you give any sort of notes about symptom control to the GPs?
To be honest, we haven’t got a formal process but the [name] strategy has developed a conservative managed pathway and on that they’ve got a lot of work on symptom control. [. . .] We are in the process of doing our own renal LCP [low clearance pathway]. So I think once the [name] strategy has been agreed, then we can sort of disseminate that or put that on to the PCT [primary care trust] drug website. [. . .] But you’re right, I think we need to perhaps formalise that and get that [name] strategy conservative management out there, more locally and regionally – which is the plan really.
Staff perceived that more resources were needed to improve CKM in their unit. Some mentioned that they would need more nurse time, and others talked about the importance of having a psychologist or a counsellor available. There was also an issue regarding the Payment by Results tariff system, as it was difficult to see CKM patients under the current payment system because CKM patients might require longer consultations than other CKD patients.
I’d like to have a psychologist on board. [. . .] Give a counselling type of psychological service. I’ve heard of a project that one hospital is doing where they have got funding to support a day a week or a day a month at one of the local hospices for, specifically for, renal patients, for conservatively managed patients, to go to.
Nurse, unit 1, participant 6
The hospital gets paid by the number of patients it sees. So if I’ve got a 4-hour clinic – my follow-up slots are 15 minutes each, so I can see about 16 kidney disease patients and 15 minutes for a kidney disease patient is enough. So my manager is very happy because I maximise my income. If I’m seeing conservative care patients, then the whole thing takes a bit longer and the chatting and planning for this is what will happen and involving the palliative care team etc., I may not be able to see 16 patients, I might be able to see only 10. So I get paid the same salary, whether I see 15 patients or 10 patients, so the manager will be wondering why am I paying you more and you are earning less. So the current way – at least in England – the way the tariff system works is that it doesn’t differentiate between the needs of the patient.
Consultant nephrologist, unit 5, participant 27
Theme 2: discussing management options to patients
Informed choice and open discussion with patients were discussed as key issues for treatment decision-making. Many staff also described the involvement of family in decision-making as very important especially at an early stage so that patients and family members have enough time to think about treatment options. Some staff pointed out that deciding whether to have dialysis or CKM could be difficult for some patients; similarly explaining CKM could be difficult for some staff. Staff also recognised that there were patients who later changed their minds to have dialysis after opting for CKM.
Supporting patient informed choice
Most staff said that they discussed CKM as an option with all patients, although some staff explained that discussions about CKM options with patients depended on the consultant’s preference for CKM.
From my point of view, conservative management is very dependent on the consultant in this unit. And some of the consultants will do very good teaching on different options or help the right patients make very good decisions not to have dialysis.
Nurse, unit 5, participant 30
All staff members talked about the importance of informed choice and open discussion with patients, and stressed that if patients wanted to choose CKM they should be fully informed and understand what it involved.
I would have a very open discussion with [patients who chose CKM], just making sure that they understand what they are saying, because you get the odd patient says – oh – I don’t want dialysis – but what they mean is – I don’t want it now, but maybe later. So we want really for them to be sure that they are understanding what they are saying.
Consultant nephrologist, unit 9, participant 57
All staff also acknowledged the importance of patient choice in deciding whether or not to opt for CKM.
They feel that they are having the treatment to please us as much as anything. Important that they know it’s got to be a choice, a choice they have. [If dialysis is] just too much for them then that’s got to be their choice.
Nurse, unit 3, participant 15
Staff explained that they would try to take a neutral stance when discussing treatment options with patients so that patients could make their own choice.
The way that a patient will decide is obviously critically influenced by what we tell them. So it’s very important that we portray things in a neutral way initially because if we start the conversation by saying – you’re very ill, I don’t think dialysis would help you very much – and do you want to have [dialysis]? You are kind of creating a self-fulfilling answer in a sense. So [nurse’s name] spends a lot of time talking about the risks of dialysis as well as the benefits and then brings in the effect of comorbidity on the overall success of things.
Consultant nephrologist, unit 7, participant 46
Other staff, however, explained how they would provide a more guided explanation to help patients make a decision. This usually happened when it was obvious that patients would not benefit medically from dialysis.
[Patients] have numerous comorbidities and you often think, from a medical assessment, what would dialysis offer these patients? I think it’s only over the past few years, maybe, that we are getting more honest with these patients and we are actually saying by having dialysis it might actually shorten your life span, because you’ve already got these problems. And then we talk about quality of life and how dialysis will impinge on what they do now, and all those things are talked about over a period of time and then patients say – it’s not for me. I think some of them are guided by us being honest about their medical condition.
Nurse, unit 9, participant 54
Some staff showed a more flexible approach, changing their stance depending on the situation. For example, the above-mentioned consultant (participant 46) also described how he tailored information for patients who he thought would not medically benefit from dialysis.
If it’s somebody that I think might not benefit from dialysis and might wish to make a conservative decision, once they understand things, I’ll usually say to them something along the lines of – there are some situations and combinations of problems where dialysis isn’t always as successful as in other patients, some sort of fairly soft introduction to it like that – and then say to them we should talk to you more about what dialysis will and will not do for you.
Consultant nephrologist, unit 7, participant 46
Family involvement in decision-making
Interviewees recognised the important role family and carers could play in decision-making, but patients’ wishes were construed as the most important. Many staff mentioned that it was important to discuss treatment options with patients early on in their pathway, giving patients, their family and their carers time to think about the treatment options available.
There are situations where families near the end come and say – I want my father to have dialysis – and you have to say, well, your father has got capacity and it’s what your father wants that guides us, not what you want, because it’s natural that you don’t want to lose people. But that discussion does have to happen sometimes that families can’t demand that their elderly, frail parent gets dialysed against our wishes and the parent’s wishes; you have to explain to them that – and that’s one of the reasons that having a discussion early is so important.
Consultant nephrologist, unit 7, participant 46
Difficulties that staff experienced in discussing treatment options with patients
Many staff reported that there were some patients who had not discounted dialysis as a future available treatment option although they had decided not to have dialysis currently. Staff emphasised that it was very important to distinguish these patients from those who actively chose CKM.
[Patients] don’t want dialysis but actually when you talk to them more they say they don’t want dialysis and then they say some phrase like, ‘But if it came to it I’d consider it,’ and you have got to be really careful that you don’t accidentally put them in the conservative care box because actually they are not conservative care, they are I’m not deciding until I have to box, and you have to make sure you do keep revisiting it with them and trying and get them to understand that.
Consultant nephrologist, unit 6, participant 36
Some staff described how they understood the difficulties that patients had in making their decision and explained that it was also difficult for renal staff to explain CKM. This difficulty was perceived as being due to a decision having to be made about a future treatment option.
We haven’t been very successful in putting them in positions where explaining that we are talking about a decision for when you become symptomatic not about a decision [when you] feel fine at the moment. It’s that abstract . . . that sort of what if, theoretical situation that [patients] might come to in the future.
Consultant nephrologist, unit 5, participant 32
Difficulty in discussing CKM with patients could be amplified by the fact that staff would need to explain CKM positively while making patients understand that they would consequently die of kidney disease.
It is all very well to talk about CKM as a management strategy, but the nitty gritty is [not talked about], [CKM] is appreciated as it’s something you die of? I think that’s sometimes where it falls down. People feel [CKM] is sort of woollily described and very supportive and sort of warm and fuzzy but . . . at the end you must actually understand that it is ok to die from it too. I think sometimes we try to present it in a sort of a very watered down version to make it more palatable to patients when in fact misunderstand upfront that and then you won’t necessarily have the people suddenly changing their minds and when it comes to the point of, ‘Oh am I going to die of this now, I didn’t realise?’
Consultant nephrologist, unit 2, participant 7
A lack of experience in discussing CKM was also perceived as a factor which could mean some junior staff members struggled to discuss CKM with patients.
Some of the more junior nurses do feel very unconfident making a comment about conservative care. They feel very unhappy about it. We’ll try and get [patients] to one of the senior doctors or [name], our most senior nurse, because I think that we are the people who probably have most experience. I’m not saying we are necessarily better but we have the most experience of trying to take people through these decisions.
Consultant nephrologist, unit 8, participant 47
Interviewees reported holding many meetings and discussions between the patient and the renal staff over a period of time to facilitate this decision-making process. Some staff described how a good relationship with patients helped discussions about CKM and treatment options.
[Talking about CKM with patients who do not want to talk about it] is hard, because I think you have to just be so aware and you have to be really clear about what is going on here for this person. [. . .] Because I think that one of the things that is really important is about the relationship you have with someone. And if you’ve been able to build a relationship then I think it enables some difficult things to be discussed.
Clinical counsellor, unit 2, participant 9
Related to building a good relationship with patients, interpersonal continuity by the same staff was mentioned. Unit 9 conducted a patient care survey, which revealed that their patients had been well supported by being seen continuously by the same staff members.
The thing is with that clinic, what I think makes it work is they see the same nurse and the same doctor all the time; there’s not a mixture of people. So we get to know the patients and that comes out in the survey [we conducted with patients about care]; what came back was very positive: they felt supported and that seeing the same people was beneficial.
Nurse, unit 9, participant 54
Patients who change their mind about having dialysis
Many staff reported that some patients who had opted for CKM suddenly changed their minds to have dialysis. This was not considered preferable by renal staff, as it usually involved creation of a temporary catheter, which would increase the possibility of infection and other complications. This happened mainly when patients became symptomatic and deteriorated significantly, or when their family wanted them to have dialysis. The number of CKM patients who changed their mind, however, appeared to vary between units. There were many such patients in unit 5.
Because I had a meeting last week with [name] talking about [hospital’s name] experience, and she talks about no patients changing their mind there and I wonder whether that is, because they have a much more standardised, consistent approach and they are much more focused on low clearance, along with the conservative kidney management work. I do think that within our practice there are more people changing their minds. So I’m surprised that is her finding, but my experience is that quite a lot of people, a substantial proportion, change their minds when they become symptomatic.
Consultant nephrologist, unit 5, participant 32
One staff member from unit 9 reported that they had only six CKM patients who changed their mind over the last 6 years. Several factors that might have helped minimise the number of such patients were talked about.
I think education is important; I think getting the family on board is important. I think having a clinic makes it almost like it’s a treatment, well it is a treatment and I think the thing to remember with these patients is, most of them, if not all of them, would die of something else. They are dying of cardiac or a stroke or of something else, so they don’t get to the point where they might need to change their mind, because they are old and frail, and they die of something else.
Nurse, unit 9, participant 54
Once patients made a decision to opt for CKM, many staff considered it important to revisit the patients’ decision from time to time in order to check their understanding of the treatment and whether or not they would like to change their mind.
I always revisit [patient’s CKM decision] with them, obviously not every single time they come. But, reasonably regularly because it’s important, otherwise they’ll say ‘no one told me . . . I didn’t know I wasn’t going to be on dialysis’ and also people change their mind, that’s the other thing once they become more symptomatic they maybe get a bit frightened and want to think about possibly dialysis. Or they get tipped into end stage for another reason and they then revisit their decisions. You always have to revisit people’s decisions.
Nurse, unit 8, participant 32
Theme 3: working with other health-care professionals to provide care for patients approaching the end of life
Once patients were identified as approaching the end of life, staff described how they then started to talk about issues related to end-of-life and palliative care with patients. Such patients were mostly referred back to the community and cared for by GPs and palliative care teams in collaboration with renal units. Many staff explained their important role for the care of patients approaching end of life.
Discussing end-of-life issues with patients
Staff reported that they would discuss end-of-life issues with patients once they were identified as deteriorating.
Most patients can probably manage till the GFR [glomerular filtration rate] is about 10 or so and when it gets less than that, often even coming to clinic is quite hard – and it’s at that stage where the most likely life expectancy is less than 3 to 6 months – is when we start negotiations with the local palliative service – community palliative service to get involved. Usually around that time we also start talking to them about preferred place of death and have that conversation also – hospice versus hospital, et cetera.
Consultant nephrologist, unit 5, participant 27
When asked about their experiences of discussing end-of-life issues, staff tended to relate their experiences to patients who were withdrawing from dialysis. It was reported that discussing palliative and end-of-life care with patients could be difficult for some renal staff. Some staff explained that, however, open discussion and being honest was important to patients when discussing these issues.
I have to be honest, some of the doctors are still hesitant [to talk about palliative and end-of-life care with patients] and it becomes very difficult because most doctors don’t have a formal training and it becomes especially very difficult if somebody is on dialysis and you see in front of your eyes that they are deteriorating and we encourage the doctors to ask the surprise question*, and then think about if they think that the prognosis is very poor, at least to be honest to them and start planning their care plan, so that at least things are in place.
Consultant nephrologist, unit 6, participant 38
*Would you be surprised if this patient died in the next year?
We are quite open, your kidney function will start to deteriorate, we can start to think about where you want to be, planning where you want to be – we’re not saying it’s going to happen now. No one knows when you’re going to die but you want to make light of it if you can, but we need to be seriously thinking, do you want to be at home, do you want to be at a hospice.
They don’t get upset when you talk about [end-of-life care]?
No, they don’t, I think they prefer when you’re open. I was with a chap yesterday on the ward who wants to stop dialysis and we had this very open conversation about death and dying.
Working with primary, community and palliative care services
All staff reported referring their CKM patients back to their GP at some point, especially towards the end of life. Many stressed that they provided support for the primary, community and palliative care teams to share their care with them. Patients were usually referred back to the community when they preferred to be seen by GPs and/or when staff thought there was no value for patients in coming to the clinic because of their physical circumstances, as GPs could provide the same care in the community.
We tend to continue seeing them in clinics while they feel well enough and able to come. But as soon as they feel it’s just too much we are not going to force them into coming, drag them to clinic. So they will be looked after by their GP. Some patients we’ve gone out and seen at home ourselves. But that’s more of a keeping in touch and keeping continuity for them rather than particularly doing anything. We rely much more on the palliative care and GP by that point.
Nurse, unit 3, participant 15
Providing education and support for other health-care providers
Many staff talked about the importance of providing primary care, community and palliative care teams with education and support to help them care for patients with CKM and to help with the management of ESKF. Some staff also recognised that GPs and palliative care nurses were sometimes hesitant to be involved with renal patients. In order to improve the situation, there were units which provided GPs with written guidelines for managing ESKF held some clinical meetings for GPs and had an exchange education programme between renal nurses and palliative care nurses, where renal staff spent a week at a hospice while hospice staff stayed at the renal unit. Some staff reported that such meetings were well received and were perceived to improve the confidence of GPs and other members of staff.
What we commonly face is once patients are diagnosed with kidney disease, then the GPs are very, very hesitant to get involved, even with the minute amount of issues. So in order to support the GPs, we have conducted a few clinical meetings where we’ve talked about conservative management in those meetings as well. [. . .] It’s a GP forum where we invite all the GPs from the whole catchment area and we do have a good attendance as well and we tend to organise that every year. We are in the process of developing a GP leaflet as well, about symptom management in conservative care, just to help them out.
Consultant nephrologist, unit 6, participant 38
Summary
Main findings
This is the first qualitative study in the UK exploring renal staff’s views and experiences of treating patients who choose CKM. CKM was generally accepted by renal staff as a treatment option; however, they had mixed views concerning CKM, which were reflected in the varied terminology that was used to describe CKM. Most considered that CKM offered a valuable treatment option for patients, while some did not perceive that CKM offered an alternative to standard care. Most staff commented that it was difficult to assess whether patients were suitable for RRT or CKM, but all were very supportive of having open discussions and of informing patients of their treatment options, and ensuring that family members were involved in this decision-making process. While many staff considered that it was important for patients and their family to make their own decisions based on the information that they had been given, some had a more directed approach and would guide patients to a decision, particularly if they felt that patients would not benefit from dialysis. Decision-making about treatment options, including about CKM, was acknowledged as challenging for both patients and staff, as this often concerned future treatment. Explaining CKM to patients was also found difficult because staff would need to discuss the consequence of CKM (death) with patients while explaining the treatment positively. There were also some CKM patients who suddenly changed their minds and opted for dialysis, and many staff emphasised the importance of revisiting patients’ decision over time, implying that the decision-making was a process rather than a one-off event. It was perceived that having a good relationship with the patient and interpersonal continuity would facilitate good decision-making. Towards the end of their life, CKM patients were often referred back to their GP, and it was vital for renal units to work in collaboration with the primary, community and palliative care teams, often supporting them to care for CKM patients by providing them with renal-specific education.
Comparison with existing qualitative research
Only a few qualitative studies have explored renal staff’s experiences with patients on RRT. 44,50–51 None have focused on staff’s experiences of being involved in the care of patients on CKM. Despite this difference in focus, some of the previous literature can be related to our findings.
Much of the previous literature has explored the difficulties renal staff face when making the decision whether or not to initiate dialysis and/or to withdraw dialysis. 50,51 This may be because it was difficult to assess whether or not dialysis would be in the patient’s best interests and good for their quality of life. Consequently, staff members wished to have more time for careful and open discussions with patients and their family. 51 The current study also identified that staff found it difficult to assess whether patients would be more suitable for dialysis or CKM, and many staff recognised the importance of having open discussions with patients and family. Previous work also identified that renal staff tended to avoid discussing prognosis and end-of-life issues, and many believed that such discussions would be interpreted as negative and would diminish the patients’ hope. 44 Discussing end of life at an early stage was also perceived as ‘not practical’ and challenging for renal staff, who tended to engage in such conversations at a later stage or only when prompted by patients. 44,53 Our findings concur, as renal staff held similar views; for example, some staff might face difficulty in discussing the consequence of choosing CKM (i.e. end-of-life issues). It was also recognised in a previous study that having an ongoing relationship between renal staff and patients, and continuity of care was essential to good communication for end-of-life issues,54 and renal staff in this study also held the same views. The current study also demonstrated that the decision whether to opt for CKM or not was a decision patients had to make for their future treatment. Staff discussed that many patients found this difficult, and some who had opted for CKM would consequently change their minds and request dialysis when they became more symptomatic or when their family wanted them to have dialysis. This implies that decision-making about their treatment options is an ongoing process rather than a one-off activity, and is a product of an iterative process of information assessment as suggested by other literature. 55
In previous studies, not having dialysis was perceived as implying imminent death by both patients and renal staff, and this perception made it difficult to discuss issues around not initiating or withdrawing dialysis. 50,51 This perception was not shared by the participants in this current study. Instead they perceived CKM as an active treatment option that could help with the management of symptoms of kidney failure without using RRT. Other studies have suggested that renal staff were also burdened by having sole responsibility and by being the final decision-maker in life-or-death decisions for patients. 50,51 In the current study, however, renal staff emphasised the importance of patients’ informed decisions, which facilitated patient choice in the decision whether to opt for CKM or not. Open discussion was seen to enable patients to make their own choices and also alleviated the burden on the renal staff of making treatment decisions.
Strength and limitations
This is the first study to explore renal HCPs’ views and experiences with patients who opted for CKM. The strengths of this study include the participation of a wide range of renal HCPs from units from a range of locations and a range of CKM practices. However, as the primary aim of this qualitative study was to explore the key issues surrounding CKM practice patterns, we recruited only HCPs who were involved in the care of CKM patients. As a result, it is likely that only individuals with an interest in CKM were interviewed in this study, and levels of renal staff’s keenness or willingness to provide CKM in the UK in general may be much lower than shown in this study. A further limitation is that the researcher’s presence during the data collection might have elicited socially desirable responses from the study participants; however, the researcher presented as an impartial observer rather than being actively engaged with the participants during the data collection. Furthermore, collecting data through interviews does not allow us to open a window on events as they happened but can instead provide insights into how participants construct their experience of the event. These findings resonate with other literature and, although qualitative findings cannot be generalised to other populations, the data can sensitise us to important issues and can offer transferability to other populations and to other settings.
Implications for practice
The findings of this study showed that renal staff faced challenges in discussing CKM and/or end-of-life issues with patients. The provision of education, such as training regarding communication skills, CKM and/or end-of-life issues for renal staff, may be beneficial. Better decision aid may also help facilitate discussion about treatment options. Staff in our study also expressed that they sometimes found it difficult to assess whether patients were suitable for RRT or CKM, and this may make staff feel less comfortable discussing CKM with patients. Research which compares outcomes between patients who receive CKM and similar patients who receive RRT will inform shared and informed decision-making when contemplating the suitability of CKM and RRT for a patient.
Our study also demonstrated that working together with primary, community and palliative care teams was key to the provision of care for CKM patients, emphasising the importance of communication between renal units and those services. A clear record of the CKM decision in the primary care record will be of benefit in minimising inappropriate hospital admissions and/or dialysis. Educational support for primary, community and palliative care teams regarding the care for CKM and renal patients in general can facilitate this process. This may involve developing a formal strategy for knowledge translation that includes better educational tools and measurement of the effectiveness of those tools. 56 In this study some units described providing educational material to other HCPs, which renal staff reported was successful; however, the effectiveness of such education is still not clear. Research is therefore also needed to understand the effectiveness of specific education/interventions.
Currently there is no financial payment for CKM under the Payment by Results tariff scheme and this was pointed out as a barrier to seeing CKM patients in general nephrology clinics. The current Payment by Results system may need to be reviewed to support the provision of CKM. There is a need to increase opportunities for units to obtain funding dedicated to providing CKM.
There is also a need for more standardised terminology for CKM in the renal community, which will enable accurate communication for clinical and research purposes.
Conclusions
Conservative kidney management is generally accepted by renal staff, and perceived as a valuable option for patients who would not medically benefit from dialysis. Patient informed choice and open discussion at an early stage of the patient illness trajectory were considered important, even though staff reported difficulties in discussing CKM as an option with patients. Providing staff with training about communication, CKM and/or end-of-life care would facilitate the decision-making process. A more standard terminology for CKM is needed, as different terminology may lead to different conceptualisations about CKM.
Chapter 4 Conservative Kidney Management Assessment of Practice Patterns Study survey: the delivery of conservative kidney management in UK renal units – a national survey
Introduction
This national survey of UK renal units addresses all the main objectives of the CKMAPPS project, namely (1) to understand the scale and patterns of delivery of CKM; (2) to explore how decisions not to have dialysis are made; (3) to explore clinicians’ willingness to randomise patients with CKD5 to CKM versus dialysis; (4) to describe how renal units and primary care work in managing CKM patients; and (5) to identify the resources involved in delivering CKM.
Only a small number of surveys on practice patterns in renal units have been conducted in the past, and none outside the UK to our knowledge. Gunda et al. 31 surveyed the pattern of provision of palliative care for ESKF in the UK, reporting a significant variation in provision of such care across the country. While lack of resources was identified as a major problem, they indicated that palliative care might be given a low priority in some geographical areas. In order to provide a more adequate service for ESKF, existing palliative care services would need to be made available also to non-cancer patients. 31 Another national survey of renal units in the UK surveyed services for CKD patients in 2004, and found that the organisation and delivery of pre-ESKF care were variable across the units. It also identified some barriers to the development of services for CKD patients, which included lack of a full complement of multiskilled renal team (MSRT) in many units, problems of geographical accessibility to pre-ESKF care and lack of sociopsychological support. 57
These studies provide insights into the practice patterns of provision of services for CKD patients and for those who need palliative care in UK renal units. Their data were, however, published in 2005 and in 2006, and since then CKM has been established as a treatment option in many UK renal units. CKM is one of the fastest-changing areas of renal medicine,58 and there is a need to understand how it is delivered nationally.
Methods
Survey development and distribution
The content of the survey was developed based on existing literature and findings from the staff qualitative study and feedback from the steering group.
Based on the staff qualitative study, the following main themes were identified as areas to investigate: how CKM is provided (service components, resources available for CKM, future improvement); how CKM is discussed with patients; and roles of primary care and palliative care services. Using the staff interview guide, interview questions regarding the above areas were selected and revised to make them more suitable for the survey questions. Data from the qualitative study also helped to organise multiple-choice options under each question.
The draft survey was piloted using cognitive interviews with three nephrologists and one renal nurse specialist. Two forms (web-based and postal) of the survey were used. The survey focused mainly on the management of patients aged 75 years and over with ESKF (CKD5) who did not or had opted not to have dialysis (i.e. who were on CKM). In order to supplement the renal unit data publicly available from the UK Renal Registry, the survey also included general questions regarding the management of patients with CKD (see Appendix 3 for the survey).
Contact details of clinical directors from all 71 UK renal units were obtained from the UK Renal Registry, and both versions of the survey were sent to them in March 2013. Telephone and e-mail follow-ups were conducted after the first mailing in April and May 2013.
Data analysis
Data analysis was conducted using basic statistics. Cross-tabulation was conducted to explore the relationship between practice patterns and selected key factors which we hypothesised might lead to differences in such patterns. These were factors related to the general unit organisation for patients with CKD; the size of units in terms of numbers of CKM patients; and resources available for CKM (e.g. funding). Closed-ended questions related to the above factors in the survey were selected for the analysis (see Appendix 4). Units were categorised into two groups, larger and smaller, based on responses to questions on numbers of CKM patients and numbers of symptomatic CKM patients (see Appendix 5 for details). We related unit size to the number of patients aged 75 years and over on RRT derived from UK Renal Registry data on prevalent RRT in 2012.
We tested for associations using these categorical variables using χ2 and Fishers exact test; given the potential for multiple testing and false positives we report only associations that were significant at p < 0.01. We report some descriptive comparisons of interest in order to measure how much time renal staff were involved in CKM, full-time equivalent (FTE) time was asked. A FTE of 1.0 indicates that a person is equivalent to a full-time worker, or two persons working half-time.
In order to analyse resources and cost to provide services for CKM patients, the following questions from the survey were used.
-
Question 35. Of those, how many were on conservative care and followed up in your unit?
-
(This question was under Q34. In calendar year 2012, approximately how many CKD5 patients aged 75 and over were cared for by your renal service?)
-
Question 37. In 2012, how many patients aged 75 and over in your unit chose to have conservative care, became symptomatic of advanced CKD and did not have dialysis?
-
Question 39. How much time do the following staff have specifically allocated for CKD 5 patients on conservative care? Please enter number of fulltime equivalent (FTE) hours for each discipline.
-
Question 47. How much annual funding was dedicated to providing conservative care in the 2011/12 financial year (April 2011–March 2012)?
Results
Of the 71 renal units in the UK, 67 (94%) responded (50 of 52 units in England, five of five in Wales, eight of nine in Scotland and four of five in Northern Ireland). Thirty-seven units completed the web-based survey and 30 completed the paper one. Of the 67 units, two did not fully complete the survey, and one unit did not have any patients with CKD5 who made an active decision not to undergo dialysis, so most of the survey questions were not applicable for this unit. Therefore the total number of responses was 64 for many questions. Some questions were not answered by all the 64 units so the number of responses varied by question (Appendix 6 shows the full survey results).
Services for chronic kidney disease patients
Almost all responding units (66 of 67, 99%) had an MSRT and, of those, 58 of 66 (87%) had regular MSRT meetings. Half of these teams (33 of 58) met once a week and in 14 units, teams met once a month. When asked which staff members either were involved in their MSRT or usually attended the MSRT meeting, most had a dietitian (51 of 58, 88%) and a pre-dialysis education provider (46 of 58, 79%), but only 18 (31%) and 16 (27%) of responding units had a psychologist and a counsellor respectively (Table 6).
| Staff | Staff involved in MSRT or usually attending MSRT meetings (%) |
|---|---|
| Consultant nephrologists | 100 |
| Renal nurses | 97 |
| Dietitians | 88 |
| Pre-dialysis education providers | 79 |
| Anaemia nurses | 77 |
| Renal registrars | 76 |
| Vascular access co-ordinators | 72 |
| Pharmacists | 59 |
| Social workers | 53 |
| Palliative care consultants | 36 |
| Renal palliative care clinical nurse specialists | 34 |
| Surgeons | 33 |
| Psychologists | 31 |
| Counsellors | 27 |
| Specialty and associate specialist grade doctors | 26 |
| Occupational therapists | 12 |
| Physiotherapists | 10 |
| Palliative care registrars | 7 |
| Diabetes nurses | 3 |
In addition to clinics at their renal unit, the majority of units (59 of 67, 88%) also ran clinics for CKD patients in neighbouring hospitals. The number of neighbouring hospitals they served ranged from one to 12 per unit, with a median of three.
Many units (56 of 67, 84%) had a formal ‘pre-dialysis’ clinic (or equivalent) for managing patients approaching RRT. Of those, however, 15 units responded that not all consultants who had CKD patients used the pre-dialysis clinic. Reasons for this varied: three reported that it was because some consultants thought that long-term continuity of care by the same consultant was more important, while another three said it was because some consultants’ clinics were at neighbouring hospitals but the pre-dialysis clinic was in the main hospital and they wanted their patients to avoid additional travel. Two respondents indicated that both of these factors were important factors and seven gave other reasons: for example, because consultants had different clinics; there were different models across their geographical area; and MSRT members could not support all pre-dialysis patients.
The majority of units (54 of 67, 81%) had a pre-dialysis education day (a group session with other pre-dialysis patients). During the education day, many responding units reported that they covered topics such as transplantation (53 of 53 responding units covered), dietary restrictions (53 of 53), types of dialysis (52 of 53), fluid balance (47 of 53), medicines (46 of 53), side effects (45 of 53), CKD-related anaemia (45 of 53) and psychological support (42 of 53). However, some topics, such as sexual matters (20 of 53), cardiovascular risk factors (35 of 53), and renal bone disease (39 of 53) were less commonly covered. Conservative care was included in most (45 of 53).
How consultants shared responsibility of patients with each other varied between the units: 18 of 66 (27%) units shared responsibility, whereas in 19 units (29%) consultants took sole responsibility for individual patients. Twenty units (30%) indicated that they shared responsibility for most patients but took a lead role for individual patients with particular needs. Nine units (14%) had more a mixed approach: for example, they shared inpatients but not outpatients; and consultants had both dedicated patient caseloads and shared patient caseloads.
Services for patients who chose conservative kidney management
Sixty-six of 67 renal units (99%) had patients with ESKF who made an active decision not to undergo dialysis even when they were symptomatic. The unit without such patients reported that their patients were well informed of the possible limitations and side effects of RRT, and RRT was still their choice.
When asked what was the most commonly used word in their unit to refer to CKM, respondents reported a variety of terms: ‘conservative management’ (30 of 65, 46%), ‘conservative care management’ (8 of 65, 12%), ‘supportive care’ (5 of 65, 8%) and ‘conservative kidney management’ (3 of 65, 5%). Eleven out of 65 units (17%) reported they used more than one term to refer to CKM.
Fifty-one out of 66 units (77%) reported that all consultant nephrologists followed the same practice regarding CKM patients. The remaining units indicated that some consultants acted differently, their practice differing only slightly (10 of 15) or moderately (5 of 15) from one another.
Number of conservative kidney management patients
Thirty-five of 67 units (52%) reported the approximate number of CKM patients aged 75 years and over in their renal unit in 2012. Numbers ranged from 4 to 152 with a median of 45 [interquartile range (IQR), 20.0–83.0] patients per unit.
Thirty-three respondents (49%) provided data on the number of symptomatic patients over 75 years old who received CKM. Numbers ranged from 1 to 50 patients with a median of 8 (IQR 4.5–22.0). Respondents who did not provide data reported that it was difficult to obtain numbers for such patients specifically aged 75 years and over, and they did not collect data on whether CKM patients were symptomatic or not.
Following the unit categorisation criteria shown in Box 1 (see Appendix 5 for more details), 24 and 23 units were categorised as larger and smaller respectively and 20 were unclassifiable because of missing data.
Units were divided into two categories based on their responses to the survey questions regarding the number of CKM patients aged 75 years and over in calendar year 2012 (Question 2.5.1) and the number of patients aged 75 years and over who stayed on CKM after they became symptomatic in the same year (Question 2.6).
Units that had ≥ 25 CKM patients and/or ≥ 20 symptomatic CKM patients were categorised as units with a larger size of CKM (24 units), and the rest of the units were categorised as those with a smaller size of CKM (23 units). (This resulted in including three units that had ≥ 25 CKM patients but had fewer than 20 symptomatic patients in the ‘large’ group.)
The larger CKM units had more patients aged 75 years and over (median 161, IQR 121–200) than the smaller ones (median 54, IQR 44–110); those with missing CKM data were intermediate in size (median 95, IQR 65–164).
The implementation of conservative kidney management
About a third of 66 respondents (23, 35%) had a written guideline (other than a palliative care or symptom control guideline) on how to manage CKM patients. Eighteen (27%) respondents were preparing such a guideline.
Two-thirds (43 of 65, 66%) of units had a single person or small team primarily responsible for CKM. All staff responsible were consultant nephrologists and/or renal nurses. In two units palliative care consultants were also involved.
Only 15 of 65 units (23%) reported having clinics exclusively for CKM patients. Only a minority of the smaller units had CKM clinics (4 of 23), while 10 of 24 larger units had such clinics. Half of the units with dedicated CKM clinics (7 of 15) ran these once a week in their renal unit. Six units ran CKM clinics outside the main renal unit but less frequently (once a fortnight/month). Of the units without dedicated CKM clinics, 22 of 49 most commonly saw CKM patients in pre-dialysis clinics, and 11 in general nephrology clinics. Patients were also seen at home by their GP or a community team (4 of 49) and by the renal team (3 of 49). There was no unit whose CKM patients were followed up solely by primary care or community services.
The availability of dedicated CKM clinics was closely related to whether or not units had staff primarily responsible for CKM (p < 0.001): units without staff responsible for CKM appeared not to have dedicated CKM clinics; however, 64% of the units who had staff responsible for CKM practised CKM without dedicated clinics (Table 7).
| Availability of staff responsible for CKM | n | Availability of CKM clinics | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| Yes | 43 | 36 | 64 |
| No | 22 | 0 | 100 |
| Total | 65 | 23 | 77 |
A higher proportion of units (18 of 43, 42%) with staff responsible for CKM had a written guideline for CKM than those that did not have such staff (4 of 22, 18%).
Symptomatic patients were seen more frequently: 34 of 61 (56%) units said they saw symptomatic patients monthly, while 43 of 63 (69%) units saw asymptomatic patients every 3 months. Nine units indicated that patients were seen as required.
Resources and conservative kidney management funding
Components of conservative kidney management
All units (65 of 65) responded that they provided EPO and iron therapy, and symptom assessment and management. Most units also provided dietary advice (64 of 65, 99%), prescription of medication for renal symptoms (63 of 65, 97%), and clinic consultations (61 of 65, 93%) The four units that did not have clinic consultations provided home visits instead. Seventeen units (26%) reported that an occupational therapist attached to the renal unit or hospital provided advice on the patient’s home environment, just over half of units provided some form of psychological support to patients (38 of 65, 59%) and 36 units (55%) provided home visits by renal staff (Table 8).
| Key components of conservative care | % (n) |
|---|---|
| Provision of EPO and iron therapy | 100 (65) |
| Symptom assessment and management | 100 (65) |
| Dietary advice | 99 (64) |
| Prescription of medication for renal symptoms (fluid retention, itching, etc.) | 97 (63) |
| Clinic consultations | 93 (61) |
| Blood results review | 91 (59) |
| Telephone support for patients | 88 (57) |
| Communication with primary care team for Gold Standards Framework approach | 80 (52) |
| Telephone support for carers | 79 (51) |
| ACP | 77 (50) |
| Social circumstances review by social workers attached to the renal unit or hospital | 63 (41) |
| Psychological support | 59 (38) |
| Home visits by renal staff | 55 (36) |
| Advice on home environment by occupational therapist attached to the renal unit or hospital | 26 (17) |
| Other | 11 (7) |
Staff resources
About half of the units (28 of 65, 45%) reported that they had staff whose time was specifically allocated for CKD5 patients who were on a CKM pathway. The larger units were more likely to have such staff (15 of 24) than the smaller units (8 of 23) (Table 9).
| CKM size | n | Availability of staff whose time is dedicated to CKM | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| Larger | 24 | 63 | 37 |
| Smaller | 23 | 35 | 65 |
| No response | 18 | 28 | 72 |
| Total | 65 | 43 | 57 |
Twenty-four of the 28 units provided data on how much time each staff member had specifically allocated for CKM patients (see Table 10). Sixteen units had renal nurses whose time was specifically allocated for such patients. A median FTE of these 16 units was 0.9 (IQR 0.5–1.0). Twelve units responded that consultant nephrologists had allocated time for CKM patients with a median FTE of 0.2 (IQR 0.1–0.2). Nine units had either pre-dialysis education providers or dietitians whose time was allocated for CKM patients. These units had median FTEs of 0.4 (IQR 0.18–1.0) and 0.2 (IQR 0.1–0.35) respectively. The relationship between number of CKM patients and total staff time dedicated to CKM is shown in Figure 2. Renal registrars, occupational therapists, pharmacists, counsellors and management staff were reported by up to three units for each.
| Staff | n | Median | Minimum | Maximum | Percentiles | |
|---|---|---|---|---|---|---|
| 25 | 75 | |||||
| Consultant nephrologists | 12 | 0.20 | 0.05 | 1.00 | 0.10 | 0.20 |
| Renal nurses | 16 | 0.90 | 0.30 | 2.60 | 0.50 | 1.00 |
| Social workers | 7 | 0.20 | 0.05 | 1.00 | 0.05 | 0.20 |
| Dietitians | 9 | 0.20 | 0.10 | 1.00 | 0.10 | 0.35 |
| Psychologists | 5 | 0.10 | 0.05 | 0.20 | 0.08 | 0.20 |
| Pre-dialysis education providers | 9 | 0.40 | 0.10 | 1.00 | 0.18 | 1.00 |
| Anaemia nurses | 6 | 0.15 | 0.10 | 1.00 | 0.10 | 0.44 |
FIGURE 2.
The relationship between total staff hours dedicated to CKM and the number of CKM patients aged ≥ 75 years per unit.
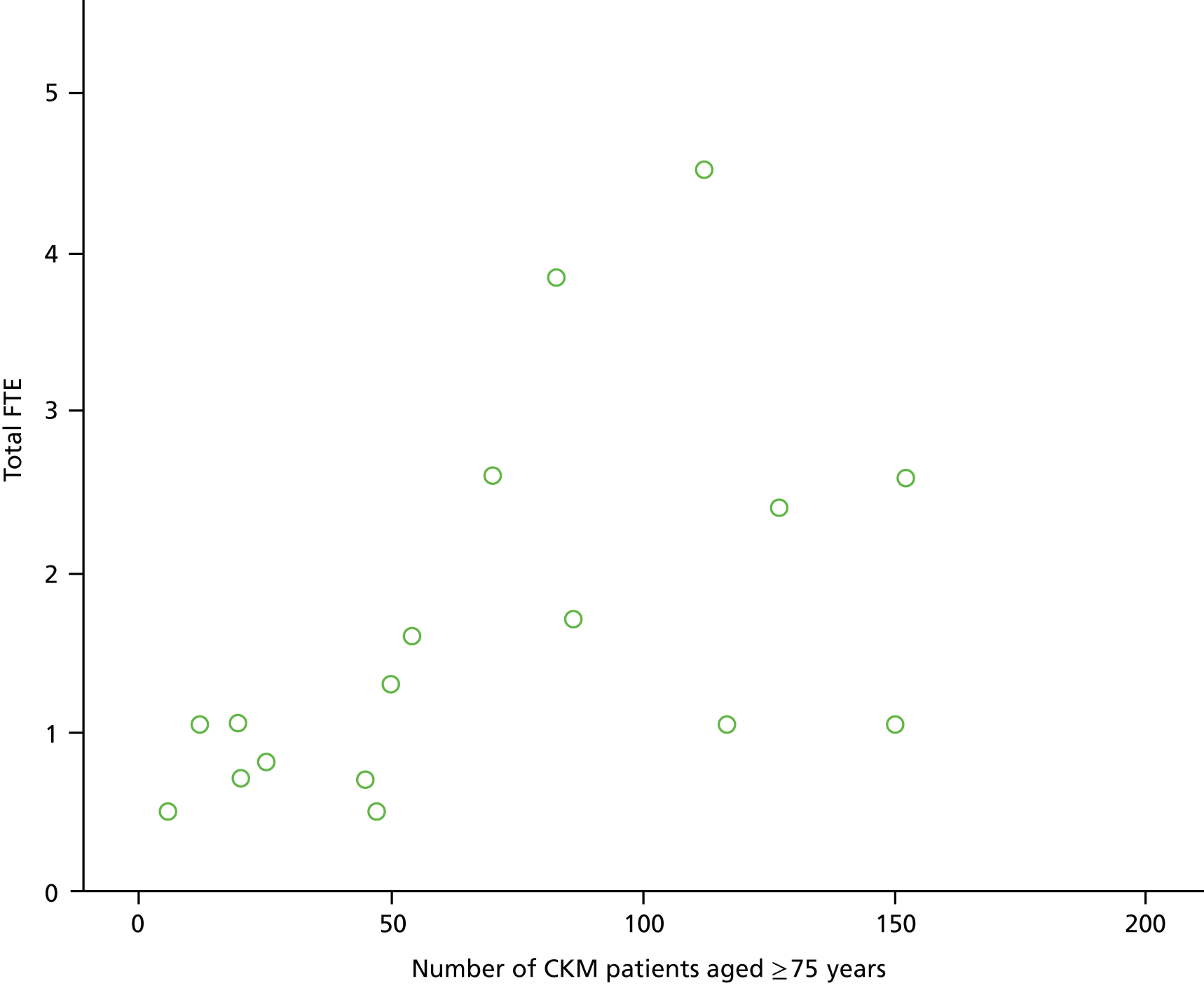
Twenty-two of 28 units (79%) with staff whose time was specifically allocated for CKM patients had staff primarily responsible for CKM, whereas 54% of the units (20 of 37) without such dedicated staff had staff primarily responsible for CKM. Half of the units with dedicated staff (14 of 28) had a written guideline for CKM, whereas fewer than a quarter of the other units (9 of 37) had a guideline. Units with dedicated staff were more likely to have dedicated CKM clinics (10 of 28) than those without (5 of 37). Many units with dedicated staff provided staff with formal training about CKM (20 of 28), whereas only one-third of other units had such training (13 of 37) (p = 0.009). Twenty-one out of 28 units with dedicated staff had a written guideline for renal end-of-life care, whereas only fewer than half of the other units (15 of 37) had such a guideline.
Specific funding
Only 10 of 65 units (15%) had funding dedicated to providing CKM in their renal service. Of those, seven units reported that it came as part of routine NHS income, while one answered that it came from non-NHS sources, and two received funding from both NHS and non-NHS sources. Only 5 of 10 units that had CKM funding were able to provide the total amount; the median amount was £40,000 (range £3942–£101,300).
Funding availability was significantly related to whether or not there were staff whose time was specifically allocated for CKM patients and to the number of CKM patients. Units without such funding were significantly less likely to have staff with dedicated time for CKM (p = 0.002), and the proportion of units that had such funding was significantly higher in units with a larger CKM patient population than in smaller units (p = 0.009). However, 68% of units that had dedicated staff did not have any CKM funding (Table 11).
Staff training
Half of the units (33 of 66) provided renal staff with formal training or education regarding CKM, and 12 (18%) of 66 units were developing such training. One-third (21 of 63) did not provide their staff with formal training; common reasons for this were lack of time (11 of 21) and lack of funding (8 of 21). Five units also reported that staff did not need formal training, as CKM was an ingrained culture in the unit.
Development of conservative kidney management
The key influences on the reported development of CKM in units were nurses’ attitudes (57 of 64, 89%), nephrologists’ attitudes (51 of 64, 80%) and patients’/families’ attitudes towards CKM (45 of 65, 69%). Two units (2 of 62, 3%) indicated that the Payment by Results tariff system for dialysis negatively influenced the development of CKM. Both such units were in England.
Most units (43 of 61, 70%) indicated that frequency of late referrals did not affect the development of the CKM programme. Just over half of the units (35 of 64, 55%) reported that the availability of funding specifically for CKM had no influence, while 14 (22%) and 15 (23%) of 65 units respectively indicated that it positively and negatively influenced the development of CKM.
Discussing conservative care with patients and assessing suitability
The majority of units (56 of 65, 86%) reported that they discussed the option of CKM with all CKD5 patients aged 75 years and over. The other nine units reported that consultant nephrologists tended to decide whether or not to discuss CKM with such patients. There was no unit which only discussed the CKM option if a patient or carer asked about the alternative to dialysis.
Various factors influenced staff when they contemplated the suitability of conservative care for a patient (Figure 3). All 65 respondents reported that patients’ preference for CKM influenced staff strongly or very strongly when considering the suitability of conservative care. This factor was followed by the extent and severity of comorbidities (61 of 65, 94%), frailty (58 of 65, 89%), functional status (52 of 65, 80%), patient’s current quality of life (50 of 65, 77%) and cognitive status (48 of 65, 74%). In addition, 12 of 64 (19%) and 11 of 65 (17%) units respectively reported that carer and consultant preferences for CKM strongly or very strongly influenced them. The distance from the dialysis unit to a patient’s home had the least influence on decisions about the suitability of conservative care (2 of 64, 3%). Uraemic symptoms and rate of decline in kidney function did not have a major influence.
FIGURE 3.
Factors likely to influence staff when contemplating the suitability of conservative care for a patient (n = 65). a, ‘Would I be surprised if this patient died in the next year?’ b, n = 64 because of missing responses.
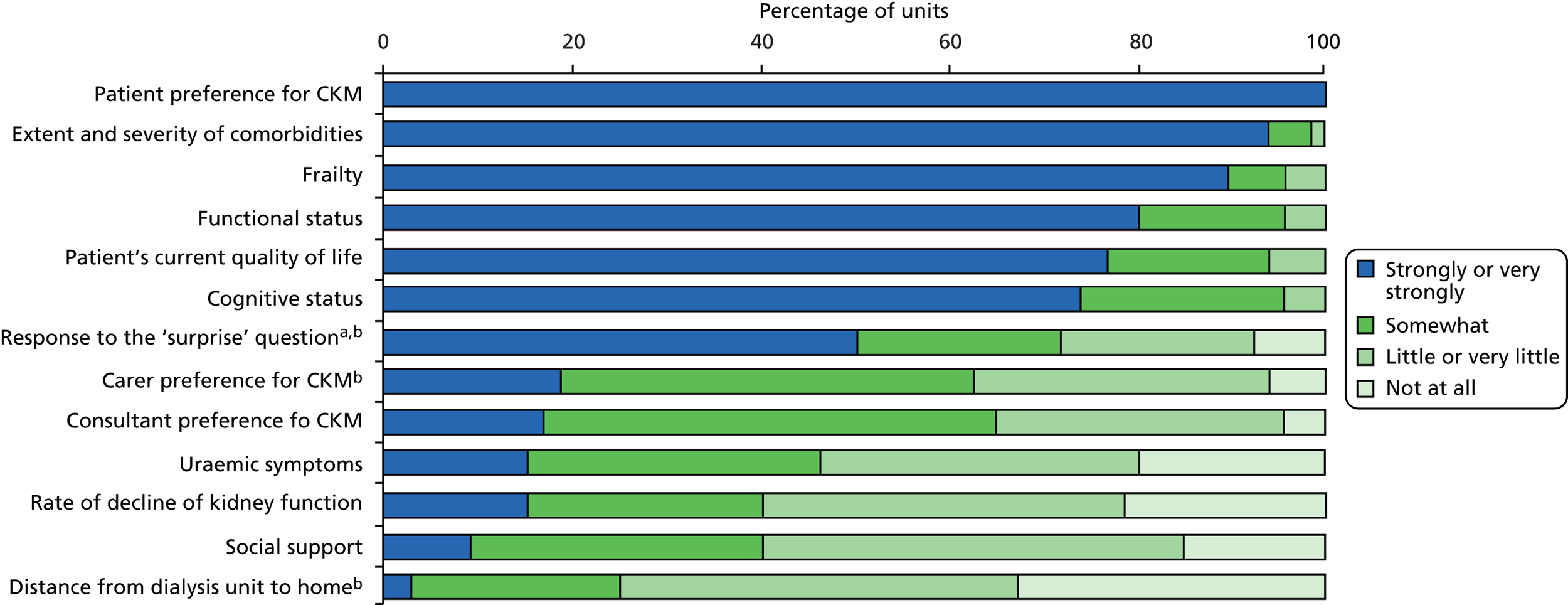
About half of the units (37 of 65, 57%) indicated that the option of CKM was most commonly first raised with a patient when they were referred to the pre-dialysis clinic. Fifteen of 65 units (23%) reported that CKM was first raised when a patient’s eGFR reached a certain level: 10 units when eGFR reached 20 ml/minute/1.73 m2, two units at eGFR level 19 ml/minute/1.73 m2 and three units at eGFR level 15 ml/minute/1.73 m2. In six units (9%) the option of CKM was usually first raised with patients at a specific time before the anticipated start of dialysis (from 3 to 12 months with a median of 9 months).
Family involvement
Family/carers were reported to be actively involved in decision-making about CKM. Almost all units (62 of 65, 95%) encouraged family to attend clinics, and 50 units (77%) involved family in the discussions to review patients’ CKM decision. Patients’ family/carers were also invited to patient education days (44 of 65, 68%), and involved when patients were visited at home (42 of 65, 65%).
Decision aids
The majority of units (54 of 65, 83%) used decision aids when discussing the option of CKM with patients. Forty-four of these units (82%) used booklets or handouts from national organisations; 33 units (61%) used booklets or handouts written by their own renal unit staff; and 22 units (41%) used DVDs from national organisations. The recently developed NHS Right Care Patient Decision Aid was being used by 16 units (30%).
Recording a conservative kidney management decision
All responding units recorded a CKM decision: medical notes were used most commonly. Sixty-one of 65 units (94%) used medical notes in conjunction with other databases and two used medical notes alone. Twenty-two of 65 units (34%) used medical notes and a renal database; 16 units (25%) used medical notes, a renal database and a GP database; six units (9%) used medical notes, a renal database, a GP database and an out-of-hours database.
Reviewing treatment decisions
All 65 respondents indicated that once patients decided not to have dialysis their decision was reviewed by renal staff, and two-thirds of units (43 of 64, 67%) reviewed the decision during each clinic visit. The patient’s decision was also reviewed on their request (9 of 64, 14%) and when they became symptomatic (4 of 64, 6%).
All of the units (63/63) indicated that they had had patients who changed their mind after deciding not to have dialysis. The commonest reason for this was because a patient’s family wanted them to have dialysis (47 of 62 units, 72% – occasionally, frequently or very frequently). Thirty-seven of 62 (60%) units also indicated that they had patients who changed their mind after having had longer to think about their decision (occasionally or frequently). On the other hand, patients’ CKM choice was rarely changed because they were unconscious on hospital admission and they did not have their wishes in writing and the family insisted on dialysis (52 of 61 units, 85% – rarely; very rarely; or never).
Only a minority of units (10 of 65, 15%) reported having any CKM patients who had vascular access created. The main reason provided for CKM patients having vascular access was that they had previously chosen dialysis and then changed their mind to CKM after access had been created (7 of 10 units). Only three units reported that access was made in case a CKM patient changed their mind and later decided to have dialysis; one of them reported that it happened very occasionally.
Working with primary care and general practitioners
No unit reported referring all CKM patients aged 75 years and over back to GPs to be cared for under primary care only. Some units referred patients back to GPs but GPs shared care of patients with the renal unit (12 of 65, 19%), whereas in some units patients were primarily cared for by the renal unit with little GP involvement (7 of 65, 11%). More than half of the units (40 of 65, 62%) indicated that GPs’ involvement was varied in line with the three options above. Thirty-one units indicated that this decision varied by patient/carer preference and nine units indicated that the decision varied by nephrologist preference.
When asked about the role of GPs in the management of CKD5 patients receiving CKM, a majority (59 of 65, 91%) of units reported that GPs liaised with the renal unit for specialist support. Forty-four of 65 units (68%) also reported that GPs and primary care staff were involved by providing/organising palliative care support at the end of life. Half of the units (34 of 65, 52%) reported that GPs also checked patient medication and arranged blood tests for patients but liaised with the renal unit for their interpretation. A minority of units indicated that GPs assessed patients regularly/routinely (not on demand) in the GP surgery (9 of 65, 14%) or via home visits (13 of 65, 20%).
A majority (57 of 65, 88%) of units provided GPs and/or their practice team with information or advice regarding the treatment of CKD5 patients receiving conservative care. Information given to GPs and/or their practice team was mainly written advice/guidelines (55 of 57, 97%) and verbal advice (46 of 57, 81%). One-third (18 of 57, 32%) of units held educational meetings to provide information to GPs. Among the units that did not provide any information/advice to GPs, four of eight units said it was for lack of time and/or funding. One unit reported lack of time and funding as well as the opinions of consultants as a precluding factor.
End-of-life care
Just over half (36 of 65, 55%) of units had a written guideline for renal end-of-life care, and 11 units (18%) were preparing such a document. Thirty-six of 65 units (55%) used a register to identify CKM patients approaching the end of life. The following factors were indicated as influencing very strongly or strongly their decision whether or not to add a patient to the register: frailty (31 of 35, 89%), frequent hospitalisation (30 of 35, 86%), symptoms (28 of 35, 80%), comorbidities (27 of 35, 77%), quality of life (27 of 35, 77%), and the ‘surprise’ question of survival of less than 12 months (26 of 35, 74%). Of those units without a register (29 units), three indicated that they were in the process of developing a register; 11 units used their medical notes or database instead; and other units identified CKM patients approaching end of life based on individual assessment, clinical judgement and discussion with other health professionals, GPs or family.
Fifty-one (79%) of 65 units used ACP in end-of-life care; those who were involved in ACP were mainly consultant nephrologists and nurses (13 units). Eight units indicated that they also had palliative care specialists involved in ACP, and six units had social workers and counsellors as well.
In terms of staff training in palliative/end-of-life care for renal patients, a majority of responding units (57 of 64, 89%) indicated that their staff had had such training; however, 39 of 64 (61%) said only a small number of the staff had received training. Only two units (3%) reported that all staff had had such training, and seven (11%) reported the majority of the staff had.
All 65 responding units appeared to liaise with some services to provide care for patients receiving CKM and approaching the end of life. Specialist palliative care services within the hospital were used most commonly (59 of 65, 91%), followed by a primary care team (58 of 65, 89%). Almost all units (62 of 65, 95%) used more than one service; for instance, 38 of 65 units (58%) liaised with specialist palliative care services within the hospital, those from a local hospice, those in the community and the primary care team. Patients received these services mainly at home (59 of 65, 91%), at a hospice (55 of 65, 85%) or within the hospital as an inpatient (53 of 65, 82%). About half of the units provided specialist palliative care services within the hospital to outpatients (38 of 65, 59%) and at the GP practice (33 of 65, 51%).
The major services that specialist palliative care services provided for renal patients on CKM were symptom management at the end of life (61 of 62, 94%), followed by patient support at home out of hours (54 of 62, 83%) and admission to the hospice, when required (52 of 62, 80%).
More than half of the units (42 of 65, 65%) provided palliative care specialists with training or advice regarding the management of renal patients. Verbal advice was most commonly given (35 of 42, 83%), followed by written advice or guidelines (24 of 42, 57%) and educational meetings (22 of 42, 52%).
Evaluation and future provision of conservative care
Fewer than half (25 of 65, 39%) of responding units reported that the quality of CKM provided in their unit was regularly evaluated. Place of death was most commonly used as information when evaluating CKM (22 of 25 units), followed by symptoms (19 of 25), survival (16 of 25) and quality of life (16 of 25).
When asked about factors that could help improve the provision of CKM in their unit, 52 of 64 units (81%) strongly or very strongly agreed that both providing renal staff members with more CKM education/training and increasing communication/involvement with GPs could help this. These were followed by providing GPs with more education/training regarding CKM (51 of 64, 80%); increasing communication/involvement with community teams (50 of 64, 78%); increasing communication/involvement with palliative care teams (49 of 64, 77%); improving computer systems by integrating primary care data with renal data (48 of 64, 75%); and more funding to develop CKM within the unit (48 of 64, 75%). Forty-two of 63 (67%) units also strongly or very strongly agreed that having funding models specifically designed to reimburse the cost of delivering CKM could help improve the provision of CKM. Fifty-one units (80%) also supported better evidence of the comparative outcomes between patients who receive CKM and those who receive dialysis (Figure 4).
FIGURE 4.
Factors that could help improve the provision of conservative care in renal units. a, n = 63. b, n = 62 because of missing responses.
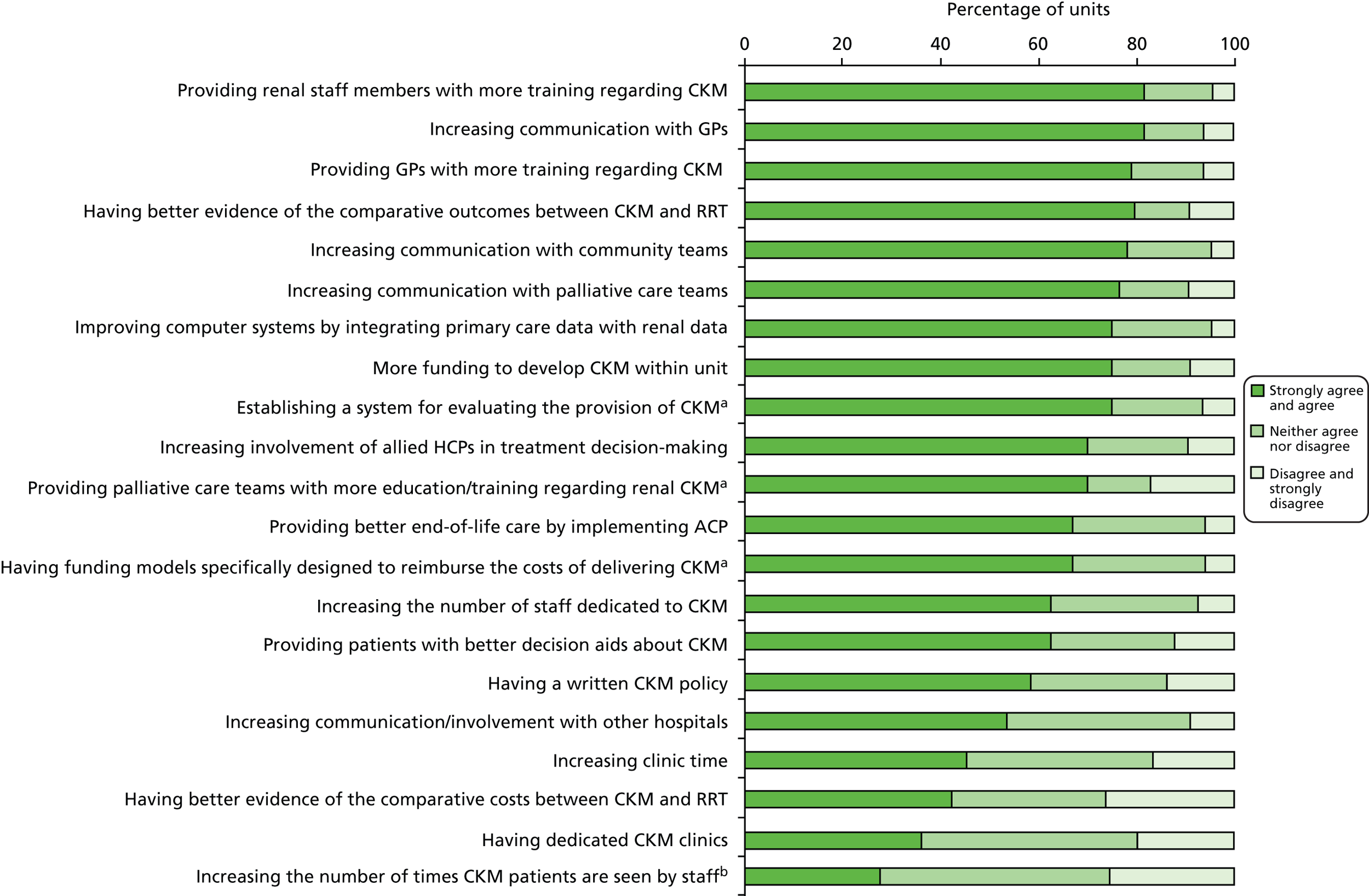
In terms of actual reported plans to improve CKM services, over half of the responding units (36 of 63, 57%) planned to provide renal staff members with more CKM education/training, and to provide better end-of-life care by implementing ACP (33 of 63, 52%). Half of the units (32 of 63, 51%) planned to increase communication/involvement with GPs, 25 units with palliative care teams (40%) and 24 units (38%) with community teams. Other plans were to improve computer systems by integrating primary care data with renal data (21 of 63, 33%), and to establish a system for evaluating the provision of CKM (21 of 63, 33%). Only a quarter of units (16 of 63) planned to increase the number of staff dedicated to CKM (including nine units with dedicated staff and seven without such staff) and to implement a written up CKM policy (only 6 of 25 units without CKM policy planned to have a written policy). Fewer than 20% of units were planning to have dedicated CKM clinics (12 of 63, 19%) and to obtain funding to develop CKM (10 of 63, 16%) (Figure 5).
FIGURE 5.
Planned changes in renal unit regarding the provision of conservative care (n = 63).
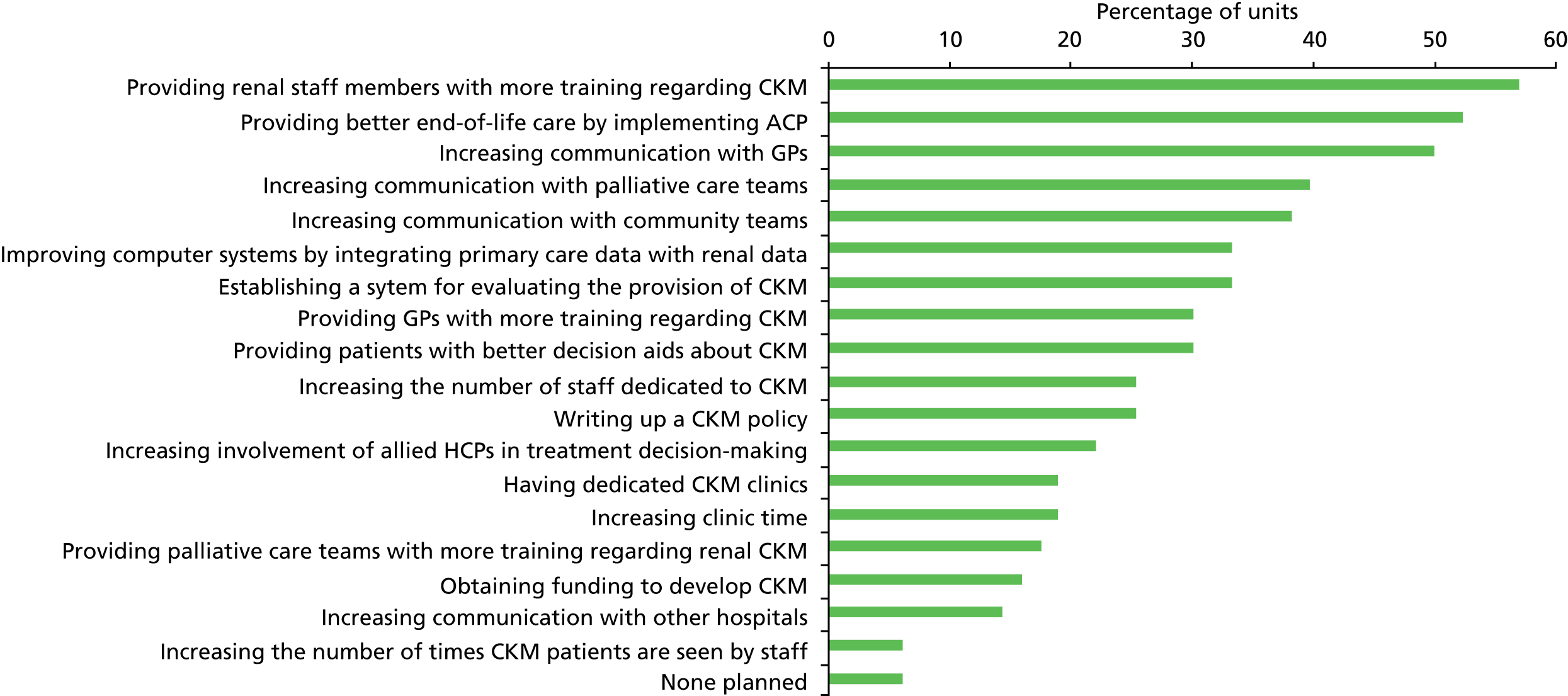
Future research
Participants were presented with theoretical descriptions for a randomised clinical trial (RCT) and an observational study, which could compare conservative care with dialysis (see Appendix 3).
When asked if their unit would consider entering a patient aged 75 years or over with CKD5 into an RCT comparing conservative care with dialysis, more than half of respondents (42 of 65, 65%) indicated that they would consider it for some patients. Of those, 18 units said that their unit would definitely be willing to participate in such a trial, and 20 units said they might consider doing so. Only one unit reported they would be unwilling to participate in such a trial.
When asked if they would consider entering CKD5 patients aged 75 years and over into a prospective multicentre observational study to compare conservative care and dialysis, a majority of units (60 of 65, 92%) said they would consider it for some patients; of those, 28 units indicated that they would definitely be willing to participate in such a study, and another 28 units might like to do so. No unit said it would not be willing to participate.
There was no significant relationship between the factors such as size of CKM patient population, availability of staff primarily responsible for CKM, or availability of staff whose time was specifically allocated for CKM patients and whether or not units would consider entering their patients into an RCT or an observational study (Table 12).
| Unit characteristic | n | RCT | Observational study | |||
|---|---|---|---|---|---|---|
| Yes (%) | No (%) | Yes (%) | No (%) | |||
| CKM size | Larger | 24 | 67 | 33 | 92 | 8 |
| Smaller | 23 | 65 | 35 | 91 | 9 | |
| No response | 20 | 61 | 39 | 94 | 6 | |
| Total | 67 | 65 | 35 | 92 | 8 | |
| Staff responsible for CKM | Yes | 43 | 62 | 38 | 95 | 5 |
| No | 22 | 67 | 33 | 86 | 14 | |
| Total | 65 | 64 | 36 | 92 | 8 | |
| Staff whose time is dedicated to CKM | Yes | 28 | 68 | 32 | 93 | 7 |
| No | 37 | 61 | 39 | 92 | 8 | |
| Total | 65 | 64 | 36 | 92 | 8 | |
Summary
Main findings
This national survey had a very high response rate and, using a detailed structured questionnaire, it provided a robust assessment of the state of CKM in the UK for older patients with CKD5. Several important findings emerged.
All but one unit had a pathway alternative to dialysis. There was no agreed terminology to describe this pathway, although ‘conservative management’ was most frequently used. The lack of agreed terminology means that there is no standard definition for CKM. Although data about numbers of CKM patients were obtained in this survey, the lack of definition for CKM hampered evaluation of such data. CKM is used to characterise a future treatment option as well as non-dialysis care for ESKF (i.e. a disease state equivalent to being on dialysis); the number of patients in the latter group on CKM was relatively small (median 8, IQR 4.5–22) in the units that responded to this question.
In order to assess suitability for CKM for a patient, similar criteria were used across the units, foremost among them being patients’ preference for CKM. The CKM decision-making process was also similar across units: most reported that they undertook informed decision-making with CKD5 patients aged 75 years and over by presenting treatment options including CKM to all such patients, and decision aids were widely used when discussing the options. Not only patients but also family/carers were actively involved in decision-making about CKM. After the initial CKM decision-making, their decision was always reviewed at clinic visits. Family members were often involved when a decision was reviewed. All units had patients who had changed their mind after deciding not to have dialysis; however, from our survey we could not quantify numbers of such patients.
Conservative kidney management practice patterns varied across units; some showed considerable investment of staff time (both nursing and medical) and consequent processes with evidence of dedicated clinics, a written CKM guideline and staff training initiatives. There was also some evidence that having staff responsible for CKM was associated with some of these practice patterns, such as having a dedicated CKM clinics and a CKM guideline. The number of patients was related to the number of patients aged 75 years and over on RRT. The components of CKM varied, for example provision of EPO and iron therapy, and symptom assessment and management were uniform, whereas formal psychological support and use of home visits was more varied. Only a minority of units had funding dedicated to providing CKM. Whether they had funding or not was associated with numbers of CKM patients; however, our survey could not identify whether they had more CKM patients as a result of receiving funding or units with the larger number of CKM patients had more opportunities to apply for and/or to receive such funding. Only a quarter of units had clinics exclusively for CKM patients, and this was closely related to availability of staff responsible for CKM. Again, we could not identify whether they had CKM clinics because they had responsible staff or vice versa.
All responding units worked collaboratively with primary care and palliative care teams, indicating their vital role in the care for patients receiving CKM especially at the end of life. For instance, many units provided GPs and their practice team with information or advice regarding the treatment of CKD5 patients receiving CKM, and all responding units appeared to liaise with some palliative care services in order to provide care for patients receiving CKM and approaching the end of life. Some units involved patients and families in the decision regarding sharing care for CKM with the primary care team.
In relation to palliative care, almost all units appeared to have staff who had training in palliative/end-of-life care for renal patients. There was, however, variation in the proportion of such staff: from two units with all staff trained to 39 with very small proportions of such staff. This may be related to a lack of resources, but may also be because higher priority is given to involving palliative care specialists in providing care to ESKF patients rather than developing the skills of renal staff.
To improve the provision of CKM in future, increasing communication/involvement with GPs, community teams and palliative care teams were thought to be very important by many units. For better collaborative working, information sharing was also thought to be vital, which could be facilitated by the integration of primary care and renal computer-based data. Many units reported that providing more education and training regarding CKM was important not only for renal staff but also for primary care and palliative care teams. However, it was also clear that lack of funding and time was a big issue for many units. Although many units thought that more funding could help develop CKM in their units, only a minority were actually planning to apply for funding. This may indicate that either it is difficult to obtain such funding, or that funding to cover CKM may not be widely available. Moreover, currently there is no financial payment for CKM under the Payment by Results tariff scheme. There is a need for a better understanding of the resources and costs of CKM to improve the commissioning of CKM. In terms of planned changes regarding the provision of CKM, increasing the number of staff dedicated to CKM and setting up dedicated CKM clinics were not prioritised as much as other items. This may be also because of a lack of funding; however, many units delivered CKM without receiving funding. It would be useful to understand how units without funding currently manage this provision.
Comparison with existing research
This is the second national survey to explore the practice patterns of renal units regarding care for older adults who choose not to have dialysis. Our survey showed similar variation in practice patterns to those found by the previous national surveys of the provision of CKD and of palliative care provision to renal patients in UK renal units. 31,57
The previous study regarding CKD practice patterns in the UK demonstrated that the most common perceived staff shortages in the care were of counsellors and psychologists: only 33% and 25% of units respectively had such staff in their MSRT. 57 Figures are similar in the current study. This suggests that formal emotional and psychological support is still considered to be a low priority in many units.
The proportion of units with a pre-dialysis clinic has increased from 50 of 70 (71%) to 56 of 67 (84%). 57 Similarly, regarding geographical accessibility to pre-ESKF care, the proportion of units that run clinics for CKD patients in neighbouring hospitals has increased from 49 of 70 (70%) to 59 of 67 (88%). The previous research showed that, even in units where outreach clinics were already set up, some patients were not receiving the same MSRT input as those being followed up in the main unit57 because these MSRT personnel were not funded to work outside the remit of the employing hospital. Our survey did not investigate whether CKD patients in the neighbouring hospitals could access to MSRT services or not; therefore, the quality of care in neighbouring hospitals remains unclear.
Many units that had a pre-dialysis education day covered CKM and psychological support as topics. From this survey, however, it is not possible to find to what extent they were discussed during such programmes. Compared with the previous study,57 psychological support was more commonly reported.
Many renal units first raised the option of CKM with patients either when they were referred to the pre-dialysis clinics or when their eGFR level was about 20 ml/minute/1.73 m2. Our survey was not set to explore if the timing of referral to pre-dialysis was governed by eGFR or timing before the expected start of dialysis. According to an Australian study conducted by Morton et al. ,59 84% of patients received information about treatment options when their eGFR was < 15 ml/minute/1.73 m2. They suggested that education on treatment was probably best commenced in stage 4 (eGFR < 30 ml/minute/1.73 m2) rather than CKD5. Although our survey did not collect actual patients’ eGFR values at the time of the first discussion on treatment, our results may indicate that many UK renal units provide patients with such information earlier, as suggested by Morton et al. 59 Only a minority of units used timing rather than eGFR level in order to decide when to raise the CKM option with a patient. Many CKD patients have a non-linear eGFR trajectory or a prolonged period of non-progression in contrast to the traditional notion of steady eGFR progression over time. 60 This suggests that careful assessment of kidney disease progression is needed to decide when to discuss CKM with patients rather than solely using an eGFR level.
In the past, UK renal units did not have ready access to palliative care services, although most were willing to provide ongoing care in terms of outpatient follow-up. 31 Our survey, however, showed that all units liaised with specialist palliative care service for CKM patients approaching the end of life. Many liaised with such services not only within the hospital but also from a local hospice. Access to palliative care used to be restricted to patients with malignant disease, and specialist palliative care services were involved only in the minority of ESKF patients’ care. 31 However, data from the more recent study undertaken by Hobson et al. 61 demonstrated that specialist palliative care services accepted more referrals of ESKF patients than before. In our survey, precise figures related to referrals were not collected; however, this survey may support Hobson’s data, suggesting that palliative care services are now more widely available to patients with ESKF. Similarly, the current study showed that a larger number of renal units had more standardised care for renal end-of-life care than before: currently over a half of responding units had a written renal end-of-life care protocol compared with only 20% at the time of the past study. 31
Strengths and limitations
Although we achieved an excellent response rate, some limitations need to be addressed. First, the survey responses were self-reported; therefore, we could not check their validity and they were predominantly completed by clinical directors. Some of these data were necessarily estimates, such as the number of CKM patients aged 75 years and over, and the number who were symptomatic. Such estimates should be regarded with caution. Our division of units into larger and smaller categories was arbitrary and for exploratory purposes. This survey focused on CKM patients aged 75 years and over. There are patients on CKM aged under 75 years;62 therefore, the results do not fully address all CKM patients. While we examined if some selected factors were associated with the use of certain practices regarding CKM, we were unable to determine causal relationships. Furthermore, most questions in the survey were multiple-choice questions where respondents were asked to select the best possible answer (or answers) out of the choices from a list. This may have limited their responses, although a selection of ‘other’ was provided in case the respondent could not find any appropriate items in the list. Finally, these findings represent a current snapshot of CKM practices in the UK, which is one of the fastest-changing areas among the renal community.
Implications for research and practice
There is a need for a standard terminology and more precise definition for CKM, since a non-uniform terminology and definition may lead to both conceptual errors and misinterpretation of data. Our study highlighted a vital role of primary care and palliative care teams, which emphasises the importance of communication between renal units and those services. Improving computer systems by integrating renal data with primary care and out-of-hours databases is essential in order to ensure good continuity of care across different care providers. Lack of resources was seen as a big problem. More opportunities for funding dedicated to providing CKM will be needed, and the current Payment by Results tariff scheme and renal commissioning may need to be revised in order to support CKM. Resources available for delivering CKM varied between the units; however, the value of resources such as dedicated staff and dedicated CKM clinics in terms of quality of service is still not clear. There is a need to quantify the value of such services in order to facilitate development of CKM services.
Having identified the individual components comprising CKM in renal units around the UK, there is an urgent need to establish the most cost-effective combination of these components in a CKM service and indeed the optimal way to organise a CKM service across primary and secondary care.
At this stage, an observational approach is likely to be the most appropriate next step to achieve this. With practice patterns now defined and measurable, provision/receipt of these individual components of CKM could be captured at a unit level/individual patient level, and novel statistics such as propensity score matching, instrumental variable analysis or marginal structural modelling used to deal with the confounding due to treatment by indication bias. The UK Renal Registry, which has recently had its Section 251 approval extended to pre-dialysis CKD, could provide a very cost-effective infrastructure for obtaining some of these data.
The present study has also highlighted the importance of measuring patient quality of life and satisfaction with treatment when comparing the outcomes achieved with CKM and dialysis, and addressing carer and family burden. Given the age, comorbidity and cognitive function of the patients for whom there is likely to be equipoise for this decision, such matters will be particularly important to consider when planning and designing future observational or interventional studies.
While an observational study may provide high-quality comparative effectiveness evidence for CKM versus dialysis, the gold standard is likely to remain a RCT. Although 65% of centres indicated willingness to participate in such a study, some serious doubts persist among clinicians about whether such a study is really possible or not. A previous attempt to undertake a RCT comparing the outcomes of HD and peritoneal dialysis was unsuccessful because of the limited number of patients who were uncertain over their therapy choice and willing to participate. 63 A feasibility study exploring the willingness of clinicians and patients to be randomised to CKM versus dialysis, with embedded qualitative research to identify recruitment, would likely be a necessary first step. 64 Data from the observational work described above would also be very useful for refining both the hypothesis and the intervention for such a study.
Conclusion
Conservative kidney management is widely practised in UK renal units. Provision and organisation of CKM varies in many ways, such as availability of CKM funding, dedicated clinics, staff whose time is especially allocated to CKM patients, written CKM policy and availability of staff training. Our survey also highlighted the vital role of primary and palliative care teams for the provision of CKM. Palliative care services for renal patients approaching the end of life are more widely available than previously reported. In order to improve the provision of CKM, better understanding is needed of the comparative outcomes and costs between CKM and RRT.
Chapter 5 General practitioner interview study: managing patients with advanced chronic kidney disease in primary care – a qualitative study with general practitioners
Introduction
This study set out to address objective 4 of the CKMAPPS project by exploring GPs’ views and experiences of managing patients with CKD4 and 5.
National Institute for Health and Care Excellence guidelines on the management of CKD and recommendations on CKD in the Quality and Outcomes Framework (QOF) provide GPs with guidelines on how to monitor and treat patients with CKD and, where necessary, refer them to a nephrologist. 2,65 The introduction of these recommendations in 2006 highlights that CKD is a relatively new condition for general practice. Awareness of how to treat CKD has subsequently increased among GPs. 65
Previous quantitative studies have identified that CKD can easily be detected in primary care electronic records,66 offering opportunities for better health promotion behaviours and disease prevention. Others have identified the positive effects of CKD recommendations on patient care and referral rates. 67 Although these benefits exist, other literature has identified that primary care patients may not always be aware of a CKD diagnosis and that GPs may have difficulties discussing the diagnosis with patients, thereby limiting the opportunity for self-management and health promotion. 68–71
Qualitative studies with GPs can help to explore approaches to the management of CKD in primary care and identify barriers which GPs may experience when discussing the diagnosis or treatment with patients. Few such studies have been carried out to date. Studies in the UK and the USA have identified that GPs have varied views on CKD and its treatment, indicating that GPs may be sceptical about guidance and whether or not CKD can be classified as a disease. 66,72,73 GPs also stress the difficulties of diagnosing CKD, particularly in older adults, and explaining a diagnosis to patients. 66,71,72 Other studies have identified how the organisation of primary care influenced these factors, concluding that general practice was probably missing opportunities for health promotion and prevention of disease progression and/or complications such as acute kidney injury. 71,74
To date, few studies have explored GPs’ experiences of managing patients with CKD, particularly in its more advanced stages. This study aimed to explore GPs’ views and experiences of managing patients with CKD4 and 5 and in particular GPs’ decisions to refer such patients to secondary care renal services. A secondary aim was to identify the proportion of older adults with CKD5 not known to a local renal unit by examining laboratory data.
Methods
Design and setting
This was a mixed-methods study involving quantitative data collection from UK renal units and semistructured interviews with GPs. The same nine English renal units were included as in the staff and patient studies. GPs were identified from general practices which were in the catchment area of four of the nine renal units (King’s College, London; Southmead, Bristol; Heartlands, Birmingham; and Lister, Stevenage).
Data linkage
Nine renal units were contacted to identify an information technology (IT) professional who could obtain patient data. Two data sets were required, renal unit data and clinical biochemistry data from their local laboratory. Renal unit data included any patients who were known to the renal unit at the time of the laboratory data extract. Laboratory data included patients, aged 75 years or older, with two eGFR results < 15 ml/minute/1.73 m2 for the first time on record between January 2010 and June 2011. These individuals were therefore new cases of CKD5. Specific data were required in each data set as detailed in Appendix 7. IT professionals sent secure versions of the data to the UK Renal Registry. Approval was obtained from the National Information Governance Board. Laboratory data provided the number of patients with new CKD5 in the 18-month period (as determined by eGFR blood results). Renal unit data provided information on all patients known to a renal unit; in some units, such patients included those who had received phone advice only. Laboratory data were matched with data from renal units to identify patients with new CKD5 who were and were not known to a renal unit.
Participants and interviews
The original approach for identifying GPs to take part in the qualitative section of the study involved inviting the GPs of patients identified through the quantitative data collection. Patients of interest were those who were identified as having new CKD5, but who were not known to local renal units. As only a small number of patients were identified from the quantitative data, an alternative approach was adopted in order to recruit GPs. This approach aimed to invite any GP working in the catchment area of four of the nine renal units of interest. Additional ethical approval was granted for this.
Primary care trusts (PCTs) in the catchment area of the four renal units of interest were identified. General practices within each PCT were identified through the QOF database. One GP in each practice was invited to take part in the study by post. If a GP did not respond or declined to take part, a second GP from the same practice was invited. GPs returned a reply slip and a signed consent form by post to indicate they were happy to take part in the study. GPs were then contacted by telephone or e-mail to arrange a time for interview. Interviews were carried out by telephone by ST-C, an experienced qualitative researcher. Interviews followed a semistructured interview guide which asked about GPs’ previous experience of managing patients with CKD, referring CKD patients to secondary care and providing palliative care to end-of-life CKD patients (see Appendix 8). GPs were reimbursed for their time to take part in interviews at £40 per interview. Interviews were audio-recorded and transcribed verbatim by an independent transcriptionist. Interview transcripts were checked by ST-C.
Qualitative data analysis
Data analysis was carried out using an inductive thematic approach in order to allow results to be driven by the data. 41 This approach minimised the influence of the researchers’ preconceptions or existing knowledge on analysis. Transcripts were analysed once they had been checked and reread by ST-C. Line-by-line coding was used to produce basic codes, and new codes were added as more transcripts were analysed. NVivo 9 was used to organise data and facilitate coding. ST-C independently coded 10 interviews and developed an initial set of themes. Themes were developed by comparing codes and grouping them into similar categories to produce themes and subthemes. Themes and supporting quotes were discussed with MS and revised to produce a consensus framework. This framework was used to code the remaining transcripts. Any new codes were incorporated into the existing framework, which was amended if necessary. Final themes were developed by ST-C and MS and discussed with the wider research team until a consensus was achieved.
Results
Data linkage
Data were linked for only three of the nine pairs of renal units and laboratories (Table 13). In all three, renal units were aware of the majority of CKD5 patients; however, there were patients in all areas who were not known to the local renal unit.
| Unit | Number of CKD5 patients identified from laboratory data | Number of patients identified in laboratory data but not on renal unit database (unknown to renal unit) | % of CKD5 patients unknown to renal unit |
|---|---|---|---|
| 5 | 152 | 1 | 1 |
| 1 | 55 | 5 | 9 |
| 7 | 45 | 8 | 18 |
In the remaining units, four contacts reported that it was not possible to obtain the data required. This was most often because of the computer systems used by the various units and laboratories and the ability to search for the required data. For two units, it was not possible to identify an appropriate IT contact whom we could liaise with, and in one, the CKD5 patients were not broken down by age.
Participant characteristics
A total of 353 GPs were invited to the study across the four areas. Twenty-five GPs responded to say that they were interested in taking part in the study. Other than two responses from GPs who replied to say they did not want to take part, no response was received from any of the other GPs. The overall response rate was 7%, although this varied between areas, with a response rate of 4% in London and 13% in Bristol. It was not possible to obtain characteristics of non-respondents.
Nineteen GPs were interviewed, with three to six GPs being interviewed in each of the four regions. Six GPs who had initially indicated they would like to take part were unavailable to be interviewed. GPs were mostly male (63%), had a mean age of 46 years, a mean of 16 years in practice and worked in practices with five doctors on average. Three GPs worked in inner-city practices, five in rural areas and the remaining 11 in suburban areas. Nine of the GPs said they had no special interest in kidney disease or other associated conditions; the remaining 10 GPs indicated that they were interested in diabetes, palliative care and/or care of the elderly, so there was some selection effect.
Qualitative findings
Five themes were identified from the analysis of transcripts. All themes were relevant to all interviews but GPs occasionally reported different views and behaviours within themes depending on the context in which they worked and their experience.
Theme 1: managing chronic kidney disease in primary care
General practitioners reported that they commonly identified patients with stage 3–5 CKD through regular monitoring for other chronic conditions. Some GPs felt that it was hard to reach a firm diagnosis of CKD because of the difficulty of having to check repeated blood results.
Well, generally as a GP we routinely check people with hypertension, high blood pressure and diabetes for their renal function, so we do that at least once a year.
GP7, Bristol, rural location, 20 years in practice
It is quite hard to actually make the diagnosis [of CKD] and you’ll find that you will get two or three tests with an eGFR of less than 60, and then all of a sudden you get some normal ones when you have done absolutely nothing. So actually making the diagnosis can be difficult.
GP14, London, suburban location, 32 years in practice
While all GPs reported that they had several patients diagnosed with stage 3 chronic kidney disease (CKD3), most reported that they rarely saw patients with CKD4 or 5 and thus had little experience of treating the later stages of kidney disease.
I know some Birmingham areas they have a lot of patients on dialysis because they are from some different [ethnic] backgrounds, so we don’t have that, we have one or two maximum on dialysis in our practice. So it’s not that frequent to go into that stage.
GP8, Birmingham, suburban location, 10 years in practice
General practitioners stressed how new the concept of CKD was to general practice, following the introduction of NICE guidelines in 2008 and QOF targets in 2006. Most GPs reported that they did not feel confident about managing patients who had stage 4 or 5 CKD without input from specialists or colleagues with more experience.
What about when you are referring someone, what sort of stage would you want to refer them?
Ooo again I probably would look it up. I think that . . . no, I’m not going to answer that because I would look it up to be honest because I’m so unfamiliar at the moment that I wouldn’t guess.
In contrast, a few GPs had a particular interest in renal disease or associated conditions (e.g. diabetes) and therefore felt they knew more than the average GP about managing CKD. In addition, GPs with an older population, who saw CKD more often, also reported that they were confident in managing patients with CKD4.
I’ve got an older population. Nine per cent of my population have got an eGFR below 60. That’s nine per cent. Which is why it’s an area, shall I say, I do quite a lot of work in.
GP1, Bristol, rural location, 32 years in practice
Theme 2: explaining chronic kidney disease to patients
Most GPs made reference to the asymptomatic nature of CKD. Many felt it was difficult to explain a diagnosis of CKD to patients because they did not feel unwell and were not familiar with kidney disease as a condition.
I think [patients] don’t understand what [CKD] actually means. Especially those who don’t really have symptoms, there are lots of people with CKD5 that don’t have symptoms . . . it’s ‘life’s all fine, how can my kidneys be failing? I feel fine’ . . . I think because they don’t have symptoms often they don’t really understand the importance of it.
GP4, Stevenage, suburban location, 10 years in practice
It is tough because [patients] don’t feel it . . . and I think that is where the biggest problem is. If you knew your heart was failing you can feel you heart failing, you are becoming more short of breath, but with your kidneys if your kidneys are failing you don’t feel it until literally the last moment.
GP12, Stevenage, suburban location, 13 years in practice
General practitioners stressed that they tried to prevent their patients from becoming distressed or anxious about their diagnosis. Most explained the diagnosis when patients were at CKD3. Some mentioned how they gave information about CKD over several consultations in order not to overwhelm patients with information. Most GPs said that they did not use the terminology ‘CKD’ or ‘kidney failure’ when explaining a diagnosis to patients but instead most explained to patients that their kidneys were not ‘working as well as they should be’ or that their kidneys were ‘ageing faster than you’.
It’s like other things, if you use the word ‘kidney failure’ or ‘heart failure’ people instantly think ‘oh my goodness, I’m going to drop dead tomorrow’.
GP2, Bristol, inner city location, 3 years in practice
I mean if you start talking about renal impairment or kidney disease [patients] sometimes panic. So it’s trying to alleviate the communication gap, it’s just saying that the kidneys are just slightly more leaky than normal which means that they are losing a bit of protein from the body and that can cause problems in the future. It doesn’t mean that the kidneys aren’t working properly; they are just showing signs maybe of a bit of wear and tear.
GP10, Birmingham, suburban location, 19 years in practice
General practitioners reported that all CKD patients who were at stage 4 or 5 would know of their diagnosis but those diagnosed with stage 3 might not always know. This was usually because patients were older, their kidney function was stable and their eGFR indicated they had CKD3a rather than CKD3b.
When making a referral to secondary care, GPs told patients that it would be beneficial to get an expert opinion about their kidneys. GPs reported that patients were generally happy to be referred and understood the need for a referral. All GPs said that they avoided talking about dialysis and left patients to discuss treatments with specialists.
If they are stage 4, I would tell them that their kidney is behaving like a kidney for an eighty-year-old person . . . but at the level that it is at a specialist input is necessary.
GP5, Stevenage, suburban location, 12 years in practice
Theme 3: getting advice on managing chronic kidney disease
All GPs contacted their local renal unit for advice about how to manage patients with CKD4 and 5. Most GPs felt communication with secondary care was good although a minority reported that there were sometimes delays in responding to queries. Some suggested that having a dedicated phone line for renal queries may be a way to improve communication.
I think the nephrology service we’ve got is good, as I say, we have got a lot of liaison with them. Easy access to them.
GP11, Birmingham, suburban location, 8 years in practice
A few GPs were from practices which appeared to have very good links with their local renal unit. These GPs reported that their local unit had provided guidelines for the management of CKD patients and some had received education sessions run by the renal unit.
[The consultant nephrologist] has been pro-active, he’s come out and given talks to us, come to the practice and he’s also given talks to postgraduate meetings.
GP9, Birmingham, suburban location, 8 years in practice
General practitioners mentioned specific areas where they felt they needed guidance. This was most often in assessing whether or not a patient needed to be referred to nephrology and how to manage CKD4 or 5 patients when they did not yet need to be referred or had been sent back from secondary care.
If you are a young person with [CKD] 4 and 5 it’s much more clear cut as to what you are treating and how you manage it compared to an elderly person when there is all this comorbidity, you know, they have all got diabetes, they have all got ischemic heart disease, very few of them have just got renal disease. The care is much more complicated really.
GP14, London, suburban location, 32 years in practice
I think if they are going to discharge more and more people back to GPs there has to be clear guidelines as to when you refer them back [to nephrology].
GP4, Stevenage, suburban location, 10 years in practice
Theme 4: referring patients with chronic kidney disease to secondary care
There appeared to be variation in when GPs referred patients to secondary care. Some reported that they referred all CKD4 and 5 patients whereas most GPs reported that referral depended on the individual. GPs with less experience of managing CKD tended to refer all patients. Several GPs said that they had learnt which patients they needed to refer through having patients referred back from secondary care. Some GPs also mentioned the tension between national guidance to refer all patients with CKD4 or 5 and believing that their renal unit may not wish to see all these patients or may refer them straight back.
I suppose in my very GP brain I have CKD 4 means the renal unit. You know, that’s where I have divided that in my brain.
GP13, London, suburban location, 11 years in practice
At that level [CKD4] um a specialist input is necessary . . . I don’t think I have got enough skills to say that [dialysis] is not going to be beneficial to the patient.
GP5, Stevenage, suburban location, 12 years in practice
That is the dilemma, as an ex-colleague of mine said, you know, ‘I didn’t really want to refer all my patients with CKD stage 4 because one of the nephrologists said ‘we’re not going to do a great deal anyway.’ So even though the guidance says refer everybody with stage 4, you know, [GPs] don’t particularly.
GP7, Bristol, rural location, 20 years in practice
Most GPs made reference to the decline in eGFR, rather than the exact value, and stressed that referral should be based on this rather than the value alone.
I think [referral] depends on rate of decline [of eGFR], I think it depends on other features, I mean if there are other things that we think might be causing it, particularly diabetes, difficult hypertension, yeah, I think rate of decline.
GP6, Bristol, rural location, 20 years in practice
Several GPs talked about assessing the patient holistically when deciding whether or not they needed to be referred. GPs were more likely to refer patients with particular management problems such as poorly controlled hypertension or proteinuria. Some said they avoided referring very elderly patients unless there were particular concerns about their CKD.
I mean if they are sort of over 75, over 80, I think each case is on its own merit in terms of stage 4 really, you know, have we got well controlled hypertension? Um, is it recently um sort of developed? Is it rapidly declining? And if there is a lot of proteinuria as well we would refer that particular stage four, but otherwise some stage 4s in the quite elderly we might just be sort of keeping an eye on.
GP9, Birmingham, suburban location, 27 years in practice
Interviewees gave several reasons for trying not to refer unless absolutely necessary. There appeared to be a general feeling among GPs that it was better for patients to be treated in primary care for as long as possible where appropriate. A few GPs working in rural locations also considered how far patients lived from their closest renal unit and delayed referral where possible to avoid patients having to travel. In addition, GPs commented on the importance of not overloading secondary care services with unnecessary referrals.
[Our] kidney service is fine but it’s a pressurised service. And I don’t like sending people we could have managed better in the community or managed better locally, down to a service which is pushed. But at the same time if needs do, hey, I work with them.
GP1, Bristol, rural location, 32 years in practice
We’ve communicated fairly closely with the [hospital trust] and we’ve been encouraged to be relatively sort of independent and managing as much as possible within the practice . . . mainly [for] the stage 4s.
GP9, Birmingham, suburban location, 27 years in practice
One GP reported that his/her practice preferred not to refer older patients with CKD to secondary care because they had other comorbidities which were being actively treated which might conflict with the management of their CKD.
Well to be honest we don’t send the older [patients] because, as I say, it is all about their other morbidities. [later in interview] Because usually [elderly patients] have got something else, they have got a coronary disease or something else which kind of overrides what is happening in their kidneys . . . what will frequently happen is the cardiologist will start the medication that makes the renal function deteriorate but the cardiologist will say, ‘never mind the renal function, take it.’ They then get to the renal physicians who say, ‘absolutely not, they can’t be taking this’, and they stop it. And so you’ve gone round in a big circle and um nobody has benefitted. That frequently happens.
GP14, London, suburban location, 32 years in practice
Theme 5: managing conservative kidney management patients and patients for palliative care
Once patients were being seen by a nephrologist, GPs reported that they had little involvement in managing CKD patients who were pre-dialysis or who had started dialysis. GPs did, however, continue to care for patients with CKD4 and 5 who had not been referred and, less commonly, patients who had opted for CKM.
General practitioners identified patients who would not be referred as those whom they would not expect to benefit from dialysis. Most often GPs mentioned patients receiving palliative care, patients with dementia and/or patients who were in nursing homes. In most cases, GPs said they would not alert nephrology to such patients, although GPs differed in this decision, with some wanting to check their non-referral decision with specialists.
[I wouldn’t refer a] palliative care patient, with cancer, or a patient who is in a nursing home, or who has severe dementia and therefore is in a nursing home.
Ok. And what would your reasons be for not referring those?
Purely what quality of life would they have? Because they are not mobile and it’s not fair.
If there is still a possibility that [the patient] may well be palliative for several months or so then we may consider [referring], but if it’s going to be a matter of weeks or whatever then I think it would just be a matter of just keeping them comfortable.
GP10, Birmingham, suburban location, 19 years in practice
Generally GPs did not appear to be familiar with the idea of CKM and few had experience of caring for such patients. GPs felt comfortable with the idea of conservative management of CKD if patients could be treated as a palliative care patient, although some worried about patients changing their mind and wanting dialysis in the future.
We have had [CKM patients]. I’m not sure whether they are still with us or not. But, yes, there have been situations where they have declined dialysis.
And how do you feel about managing those patients?
Well, again I suppose it is trying to do it as best you can. I mean obviously with the patients being under the care of the secondary care services at least you feel that there is communication going on and if there is a change of heart on the side of the patient or a deterioration then, you know, you have got someone to call on to and give you some further advice and support.
GPs emphasised that it was very unusual to have patients at ‘end of life’ who had only renal disease. Most CKD patients had several other conditions and when deteriorating were treated as any other palliative care patient.
We do control, we do treat, we do pain, and families and at the end of the day it is end of life, ok, they are not taking medication, they need some pain relief, just like any other palliative care, end-of-life care patient.
GP8, Birmingham, suburban location, 10 years in practice
Palliative care registers helped GPs to identify patients who were approaching end of life and this information was shared with community teams. GPs reported that renal units did not get involved in end-of-life care unless a patient was withdrawing from dialysis, in which case the care was usually co-ordinated by secondary care teams.
Summary
Main findings
Quantitative results indicated that the majority of patients with CKD5 were known to local renal units, indicating that GPs were referring most patients, although it was difficult to obtain and evaluate such data, and our data set was limited to three renal units. The qualitative analysis identified several factors which influenced GPs’ management and referral of patients with CKD4 and 5 to help explain these numbers.
Previous experience of treating patients with CKD was a good indicator of how familiar GPs were with guideline recommendations and when to refer. Some GPs had relatively little experience of managing patients with CKD4 or 5 and of CKM per se and found it harder to comment about their experiences with these patients. GPs with older patient populations felt more comfortable managing patients with CKD, who were often older adults with comorbidities. While some GPs felt that patients with CKD3 might not know of their diagnosis, all felt that patients diagnosed with stages 4 and 5 would be aware of their CKD and that patients were generally happy to be referred. Most GPs reported that they had good communication with their local renal units and particularly sought advice on when to refer patients and how to manage patients if they were not referred, or after they had been sent back from nephrology. There was variation in when individual GPs said they would refer patients with CKD4. They were influenced by the eGFR, decline in eGFR, the general well-being of the patient, the patient’s age and comorbidities, and occasionally the distance to the renal unit. In general, GPs emphasised that it was preferable for patients to stay under primary care where possible and to refer patients based on their individual characteristics. Some GPs identified older adults with comorbidities as complicated cases where referral decisions were more difficult. Finally, GPs identified patients receiving palliative care or with advanced dementia as less likely to be referred to the renal unit and were happy to care for such patients. Most interviewees had little experience of managing palliative care for patients with CKM those who had were comfortable with this as long as the patient had made an informed decision in secondary care and that this had been communicated to primary care.
Comparison with existing research
Qualitative research in this area is limited, with only a few studies having been carried out in the UK and the USA;66,71,72,74,75 however, there were similarities between the results of the current study and such previous research. GPs reported that they felt some anxiety about telling patients with early-stage CKD of their diagnosis71,72 and that GPs felt patients and the public had little understanding of kidney disease. 66,72 In addition, most GPs accepted that they did not know a great deal about the management of CKD4 and 5 because they had relatively few patients with these and they welcomed any guidance or additional advice that they could get to inform their practice. 75
Other studies have found that GPs can be sceptical about using the measure of eGFR to make a diagnosis of CKD. 66,76 It was interesting to note that GPs did not seem to have this concern in the current study. Although a few GPs described using eGFR as a factor when deciding whether or not to refer, it was most often the decline in eGFR rather than the single reading that was referred to. This may have meant that GPs were more confident using a decline in eGFR as evidence of impaired renal function or that GPs had had longer to get used to referring to eGFR results since the introduction of NICE guidelines.
Only one previous study mentioned GPs’ approaches to referring patients. 75 Its results were similar to the current study, showing that GPs varied in when they made a referral and on which factors a referral was based. The study also indicated that GPs working in more rural locations tried to manage patients themselves as much as possible to avoid unnecessary travel for patients. 75
Patients who have opted for CKM are not currently identified and recorded by the UK Renal Registry or any other national registry. While our data suggest that most are known to nephrology services, it is likely that some are kept in primary care and are never referred. A report by Kidney Health Australia identified the number and proportion of patients with ESKF who did not start RRT by age, based on death certificates and registry data. 77 The results identified a substantial proportion of patients who did not receive RRT, with a higher proportion being seen in older age groups. 77 More data that links prevalent CKD cases to dialysis is needed in the UK, and linking primary care databases to the UK Renal Registry may help with the identification of patients with CKD and an assessment of the proportion of patients treated without dialysis.
Strengths and limitations
This was the first study to identify the proportion of CKD patients known to renal services through the collection of laboratory data. It was difficult to obtain the data needed to identify the proportion of patients with CKD5 not known to renal services, and only three of nine units could provide the full information. Moreover, we accessed the clinical biochemistry laboratory of only the hospital in which the renal unit was based; this might have led to an overestimation of the referral proportion referred. This also meant that GPs could not be sampled based on individual patient cases and that some GPs had to talk hypothetically in some instances because they had not treated a patient with CKD5 recently. Despite this, GPs were able to answer all questions posed to them and there was a similarity between those who had specific patients in mind when answering questions and those speaking hypothetically, indicating that the approach taken by GPs was consistent between practices and areas.
General practitioner participation was voluntary; this probably led to sampling GPs who had a particular interest in CKD or a related specialty (half the volunteers). Data did indicate that there were differences in GPs’ knowledge of guidelines and confidence in managing patients between groups who had a special interest and those who did not. This may mean findings have less relevance to all the GPs in the UK.
There was a particularly low response rate from GPs invited to take part in the qualitative study. Study materials emphasised that GPs did not have to have specialist knowledge in the management of CKD. However, GPs may have been reluctant to participate because it was something they did not commonly see in practice. Previous research has identified low response rates in general practice research and the difficulties of recruiting GPs to research studies involving surveys. 78 Postal invitations, FreepostTM envelopes for replies and reimbursement for time spent taking part in the study have been shown to help increase participation by GPs and were all used in the current study design. 79
Unlike previous studies, GPs were recruited from practices in specific areas of the UK, rather than from practices which were taking part in a larger research study. 66,71 Invitations sent to individual GPs, rather than practice managers, avoided recruiting only from those practices that were research-focused and probably added to the relevance of findings to GPs in general.
Additional insight into primary care practice might have been obtained by including practice nurses or district nurses in the sample. Practice nurses might have provided insights into the management of CKD patients and discussions with CKD patients when diagnosed, and district nurses might have been able to offer insight into palliative care for patients with CKM, although these are likely to form a small minority of their workload. It was not possible to obtain data on nurses working in all practices and this would have limited recruitment. In addition, nurses were less likely to have views on the decision to refer patients to secondary care, as this was something most commonly discussed between GP and patient.
Implications for practice
General practitioners distinguished between younger and older adults with CKD, of whom many had comorbidities. Some mentioned that there was a conflict between treatment for CKD and treatment for another chronic condition and others felt unsure about referring older adults because of the many other conditions they had. It appeared to be difficult for GPs to judge which condition was most detrimental to a patient and, given contrasting advice from specialists, there may have been a preference to keep the patient under primary care. While this approach may avoid anxiety and multiple hospital appointments for patients, there may be missed opportunities to educate patients about their CKD and prevent further decline. Guidelines which focus on single conditions are particularly problematic in CKD, where so many patients have comorbidities. Further advice on managing common comorbidities would be helpful for GPs. 80
General practitioners appeared to be comfortable managing patients with CKD and were confident that they could obtain advice from renal specialists when necessary, for example about medication. Some GPs suggested that current communication with secondary care could be improved by the provision of a dedicated telephone helpline to answer renal queries. GPs reported that this already existed for other conditions and that it would make it easier for them to obtain fast responses to their queries. GPs felt patients needed to be seen by secondary care when they needed to talk about future options for treatment.
Some GPs reported that they had already received specific input from their local renal units through local guidelines or educational meetings. These had been positively received and appeared to have offered clarity on issues about referral. There is potential for other renal teams to emulate such approaches and to initiate better communication strategies with primary care professionals, which will probably improve care for renal patients in local areas.
General practitioners used decline in eGFR as a key factor in the decision if and when to refer to a nephrologist. Highlighting trends in eGFR in pathology reports by clinical chemistry laboratories would aid this decision process. 81
Conclusion
General practitioners make decisions about referrals to nephrology services on an individual patient basis. A substantial majority of CKD5 patients are known to renal units. GPs have access to guidelines on management of CKD and, although they sometimes found it difficult to refer all patients suggested in national guidelines, they generally felt supported by additional information from local nephrology services. GPs may benefit from advice delivered from their local renal unit and clinical chemistry laboratory to clarify referral criteria and additional guidance on the management of older adults with multiple comorbidities. Most had little direct experience of managing palliative care for patients with CKM.
Chapter 6 Discussion and conclusions
This mixed-methods study sought the views of renal unit staff, patients and GPs about the management of ESKF in older people and the potential role of a conservative care pathway, and undertook a national survey of renal units to identify current practice. Several important issues have emerged from the individual study components and from triangulating the findings:
-
terminology and definition
-
informed decision-making in multimorbid patients
-
communication, education and training
-
role of primary care
-
commissioning and funding.
These are considered under the five objectives.
Objective 1: to describe the differences between renal units in the extent and nature of conservative kidney management
This was the primary objective and was met largely by the national survey, which in turn was informed by the qualitative studies with staff and patients. The national survey had a very high response rate. It demonstrated the widespread acceptance of a conservative pathway as an active alternative to dialysis by UK nephrology services and the development of such a programme in virtually all renal units over the past 10 years. CKM patient numbers, practice patterns, components of care and terminology varied markedly across units. Patient numbers were hard to obtain for some units and differed markedly for the pre-dialysis phase of CKM and postdialysis equivalent, for the latter numbers were small with a median of only eight at any one time, partly reflecting short survival. There was some evidence that units with larger populations of patients aged 75 years and over on RRT had more CKM patients. Some units showed considerable investment in staff time (both medical and nursing) and consequent processes such as dedicated clinics, guidelines and staff training programmes, and this bore some relation to patient numbers.
A key issue underlying practice patterns is the terminology and definition of CKM. We chose the term CKM to underpin our study. However, there was considerable variation in the terms used by different units. An agreed terminology and definition would assist the further development of CKM.
The pathway from advanced CKD to a symptomatic state where dialysis could be offered or a supportive conservative pathway followed is complex and unpredictable. This pathway may be described using a staging classification which reflects its progressive nature, though individual subjects may move from stage to stage in either direction and at variable rates.
-
Early conservative care pathway option: the time before clinical manifestations of the ESKF occur; the focus here is education, decision-making and standard CKD care, with the exception of dialysis access and transplant work-up.
-
The alternative to dialysis phase, when dialysis would have been commenced if opted for, characterised by a low eGFR, rising serum urea, increasing uraemic symptoms and/or symptoms related to comorbidity; the focus here is revisiting of options, clarification of pathway chosen, symptom assessment and management and ACP. There may be periods of stability, punctuated by crisis episodes that need intensive management, followed by a period of instability, increasing symptom burden, revisiting decision-making and discussion about preferred place of death.
-
End-of-life phase, when prognosis is short (days) and previous plans for end-of-life care need confirming and implementing, including provision of anticipatory medicines for the end of life.
-
Bereavement support.
Most units did not report the numbers of patients receiving CKM, indicating that these data were not captured prospectively. In units that did report a number of patients designated as receiving CKM, the wide variation suggests that units differed in when in the course of a patient’s CKD they applied the term. While guidance promotes early discussion with patients and decision about future conservative care (the ‘early conservative care pathway’ option above), for many patients there may be a long period before the decision about whether or not to opt for dialysis is faced (the ‘alternative to dialysis’ phase). A significant proportion of patients die from their other comorbidities before reaching this later phase. 32 It may be that two designations are needed to capture these key points on the pathway: (1) a decision to opt for conservative care and not to prepare for dialysis and (2) a decision to opt for conservative care rather than dialysis despite symptoms.
It is expected that the recent KDIGO consensus conference on renal supportive care will produce a definitive definition, although the definition produced will need to include other elements relating to situations in countries in which CKM is obligated by the lack of resources for RRT. Adoption of an agreed definition has considerable implications for clinical practice, research and policy: in clinical practice, a common understanding is necessary to aid communication with patients and carers and to share best practice; in research, evidence can be developed only once there is recognition and an accurate definition of this CKM population; and for policy, decisions, guidance and direction can be achieved only if the population is clarified. Any definition must be clear for patients, and their carers and families. A clear definition would facilitate derivation of key quality indicators.
In this study we focused on patients aged 75 years and older, as the majority of patents opting for CKM are in this age band. However, we recognise that CKM may be a mode of choice for some patients who are aged under 75 years.
Objective 2: to explore how decisions are made about the main treatment options for older patients with stage 5 chronic kidney disease
This was addressed first by the qualitative interviews with staff and patients, and then elements of the process were quantified in the survey. Further insights were obtained from the GP interview study. The CKM decision-making process was similar across units: most reported that they undertook shared informed decision-making with CKD5 patients aged 75 years and over by presenting treatment options including CKM to all such patients, and decision aids were widely used.
Stage 5 CKD patients held contrasting beliefs about what dialysis can offer in terms of survival and quality of life, which appeared to be influenced by the information provided by renal staff. This can differ between units, particularly in regard to CKM. Few patients reported speaking to staff about the future, in terms of the consequences either of starting dialysis or of opting for CKM. The staff interviews identified that lack of knowledge regarding the likely impact of dialysis on individual patient’s quality of life and survival made discussion about prognosis difficult. Difficulty discussing poor prognosis and end-of-life issues was also related, partly from a well-intentioned desire to maintain patients’ hope. While most renal units were addressing treatment options including conservative care and were using a variety of resources including decision aids, patients in some units reported not being aware of a conservative option. It was recognised that decision-making about CKM and dialysis options is iterative and time-consuming (and hence needs to be appropriately resourced) and may be facilitated by continuity of care. The assessment and impact of cognitive impairment, dementia and depression, which are not uncommon in these patients, also need to be considered.
Better information on the benefits and risks of both dialysis and conservative care according to patient characteristics (e.g. degree of morbidity and frailty) and including information on quality of life and carer burden would enhance shared decision-making. The patient interviews highlighted the complex trade-offs for patients in terms of survival and quality of life, and the importance of the latter. Renal professionals are likely to see quality of life issues somewhat differently from patients. For instance, in accord with the work of Morton et al. ,45 we identified that transport to and from dialysis was a major concern for patients, and, for some, strongly influenced their CKM decision. Existing and new evidence is needed to better inform decision-making. Such data could come from a synthesis of the existing studies that have compared dialysis and conservative care, along with future research (see below).
The fact that the initial decision to opt for the conservative pathway may often be made when patients are asymptomatic, along with the uncertain trajectory in ESKF, would indicate that (1) more detailed and realistic consideration of what might happen in the future is often needed and (2) regular review of the decision is needed and flexibility over a change of course. This has implications for establishing timely access for dialysis.
We did not specifically address the information needs of patients from ethnic minorities, who will form an increasing proportion of the older people with ESKF given the ageing of these younger populations and their higher rates of ESKF. Not only is language a potential barrier but so are cultural and religious differences in attitudes to death and in the responsibility for decision-making.
It is clear from all four strands of this research project that excellent communication is a cornerstone in delivery of high-quality care to people with advanced CKD and frailty/multiple morbidity. Of course, good communication is essential for the delivery of much of health care, but for such patients it plays an even more important role, supporting the patient’s understanding and perceptions of their kidney disease, providing a solid foundation for their decision about their management pathway, and enabling their subsequent palliative and supportive care.
One of the main findings of our work was the importance of education and training of renal professionals. It is clear that renal unit staff need more training and resources to help them discuss conservative care with patients with ESKF and their carers/families.
This was evident from the patient interviews, where their perceptions and understanding of their kidney disease, as informed by renal staff, were paramount in driving the management decisions, and in the staff interviews, where both the decision-making and the discussion of palliative and end-of-life issues were acknowledged as highly challenging. Because of the very specific challenges entailed, renal-specific training may be useful, such as the training in advanced communication skills tailored to advanced kidney disease developed by Bristowe et al. 82
The marked variation across the UK, both in the numbers of staff with dedicated CKM time and in the amount of training in renal palliative care, indicates that there are a variety of models of care (who delivers care and how), as well as variety in their training (affecting the quality of the care they deliver). There is urgent need for consistent measurement of patient experience in clinical practice, as well as formal research evaluations of education and training programmes, to identify both the effectiveness and the cost-effectiveness of these approaches. This evaluation is an important prior step to sharing best practice. If there is robust evaluation of different models and education/training methods, then there may be scope for informal or formal local and regional networks to share best practice. This would support smaller renal units which had fewer ESKF patients opting for CKM, and hence limited experience and dedicated resources for CKM.
Objective 3: to explore clinicians willingness to randomise patients with stage 5 chronic kidney disease to conservative kidney management versus dialysis
This was covered by a series of questions included in the survey. There was strong support from our respondents for further evidence of the comparative benefits and costs of CKM and dialysis, and willingness to support further prospective studies and to a lesser degree a RCT. We did not specify the details of such studies, so these data are only preliminary indications of the scope for further research. Work is needed to assess the feasibility of a RCT, specifically if there is wider clinician and patient equipoise.
Objective 4: to describe the interface between renal units and primary care in managing stage 5 chronic kidney disease patients
The survey had a section on the primary care interface, informed by the staff interview study, and with further information provided by the GP interview study.
In contrast to CKD3 patients, individual GPs had relatively little experience of managing patients with CKD4 or 5 and of CKM per se, which limited the insights into GP role for CKM patients. Our limited analysis of referral suggested that most ESKF patients were known to nephrology services, and that GPs were making considered judgements about referral based on prognosis and morbidity, although it was difficult to obtain and evaluate such data and our data set was limited to three renal units. More systematic national and local data are needed on patterns of occurrence of advanced CKD and referral.
It is recognised that GPs and community teams including social care professionals have a central co-ordinating role in the end-of-life phase but with a need for training in renal-specific elements. The engagement of GPs in the pre-terminal palliative phase of conservative care varied between units, partly reflecting GP experience and geographical factors. There is scope for sharing good practice and further evaluation of different models. There is also a need for better communication between GPs and renal units about specific patients; integrated primary and secondary care data systems would facilitate this. Other methods such as a dedicated phone line were also suggested.
Objective 5: to identify the resources involved and potential costs of conservative kidney management
This objective was only partly met. We were unable to undertake detailed fieldwork of processes of care either at the level of staff or of patients to enable bottom-up costing. However, detailed analyses of the actual resource use and cost would have been challenging. A patient-focused client inventory approach would have had problems of recruitment of the critical CKM patients on an alternative to dialysis. From the staff perspective we captured ‘dedicated’ staff time; a staff diary approach would have been complex given the number of potential contacts a CKM patient would have had. Analysis of routine unit data on CKM resources and treatments received would be limited by the under-recording of the CKM pathway decision. In essence, the first stage of the CKM pathway in most units generally seems similar to pre-ESKF care without the vascular access; the second stage, after the dialysis decision, will be considerable cheaper than the costs of dialysis.
We were able to identify from the survey the variation in resources reported to be dedicated to CKM patients and, from some units, details of funding sources. Most units had few patients under the ‘post-dialysis equivalent’ and more intensive phase of CKM. Some did have dedicated staff, largely nurses and to a lesser extent medical staff, and only a minority reported receiving funding, which was modest in amount (median £40,000). Most CKM care is subsumed into the overall renal unit budget.
In England, preparation for RRT (or ‘renal assessment’) and RRT per se are commissioned as specialist services and there are specific Payment by Results tariffs. However, CKM (or supportive care, the term used in Payment by Results service specification) is mentioned within the renal assessment pathway but it is commissioned locally. This is based on the rationale that CKM does not need RRT infrastructure, and services should be available locally. However, CKM care can last from months to even a few years (i.e. it is not just end-of-life care), and we were unable to distinguish the resources and costs for CKM for the pre- and postdialysis equivalent phases. In some units the former pathway is equivalent to advanced CKD care before dialysis (other than access formation).
Most units reported no dedicated funding for conservative care. The lack of a tariff for conservative care under Payment by Results was seen as a barrier to developing these services. It remains to be seen if the inclusion of the renal multiprofessional tariff will effectively reimburse at least some of the work undertaken within the CKM pathway. More evidence is needed to compare the effectiveness, acceptability and cost-effectiveness of different approaches to delivering conservative care, and also to understand the comparative benefits and costs between different pathways. This is not, as discussed earlier, in order to ration dialysis but rather to better inform rational or appropriate approach to maximise benefit for resource utilisation.
Once CKM definitions are clarified, there needs to be a consensus about what resource elements should be included. In the early phases of the conservative pathway the input is similar to other ESKF pre-dialysis patients other than the provision of vascular access. The end-of-life component of conservative care also applies to the larger pool of patients undergoing RRT. Detailed costing analysis of CKM pathway in a variety of units would be valuable. The second stage, after the dialysis decision, will be considerably cheaper than the costs of dialysis.
Summary of study strengths and limitations
The study used mixed methods and had several complementary components. The multicentre qualitative phase using a purposive sample of renal units was robust. We were able to obtain multidisciplinary perspectives by interviewing a range of relevant HCPs. For patients, the interviews were cross-sectional rather than sequential within individuals over the CKM pathway and, as alluded to, there were problems with recruiting later-stage CKM patients, and patients from ethnic minorities.
The survey had a very high response rate and a high question completion rate, though with a single respondent at each unit and focusing only on patients aged over 75 years. Determining the size of the CKM population was, however, difficult given variation in definition and recording. Our definition of size of CKM programme was arbitrary.
We had problems linking laboratory biochemistry data on CKM5 referral, and GPs’ limited experience of managing such cases meant that they had less to say about their care of such patients. An alternative strategy of identifying older CKD5 patients who were referred, and contacting their GP, might have been more illuminating. Recruiting GPs to such an interview study was difficult. Our major shortfall was in the expertise and resource required to undertake detailed costing studies using patient and/or staff diaries, as discussed above.
Summary
Clinical/service implications
-
Conservative kidney management is widely recognised and delivered across the UK, but through differing models of care and sizes of CKM programmes.
-
An agreed terminology and definition of CKM are needed to enable future evaluation. The designation of a patient as having CKM must recognise the two key points on the CKM pathway: (1) stating a preference or intention to opt for conservative care rather than have dialysis in the future, made at an unspecified level of kidney function and an unspecified time before dialysis is indicated – ‘the early conservative care pathway’ – and (2) a decision to opt for CKM made at a level of kidney function or despite symptoms that would otherwise justify starting dialysis – the ‘alternative to dialysis pathway’. The systematic routine recording of the CKM pathway within renal data systems would provide a foundation for evaluation of CKM patterns and outcomes.
-
Communication and information given to patients with ESKF who are older and or multimorbid needs to (1) support patients’ understanding and perceptions of their kidney disease and their other comorbidities; (2) routinely include details of the CKM pathway, settings of care and codependencies with other pathways of care that individuals are receiving; (3) include realistic discussions (according to preferences) of what is likely to happen in the future; (4) recognise that shared decision-making in this setting is a process rather than an event and that decisions made need to be periodically reviewed; (5) recognise the importance of identifying and where possible ameliorating cognitive impairment and ensuring that plans are in the best interests of individuals.
-
In order to address the challenging nature of decision-making, and communication about end-of-life issues, renal staff need education and training in (1) advanced communication skills, adapted to include the specific issues around dialysis decision-making, and (2) how to discuss and address palliative and supportive care needs, including end-of-life care.
-
Better communication and information sharing is needed with primary care teams and training in the renal-specific elements of CKM care.
-
End-stage kidney failure is a unique kind of organ system failure in that a replacement therapy is potentially available for the majority. As a consequence, planning for care occurs earlier than in other conditions, such as heart failure. However, a better understanding of patient and their carers’ wishes, needs and values in advancing CKD and of the optimum service for CKM is likely to have wide interest across the health-care system. This is, first, because multimorbidity, frailty and functional decline in those with long-term conditions is a major system challenge, and, second, because the appropriate balance between advanced technology and palliative care is also relevant for other conditions.
Research implications
Research is required to measure the benefits and costs of CKM and dialysis and to inform decision-making by staff, patients and their families. There is some support in principle for a randomised trial, which would be the most robust method in terms of internal validity given the problem of selection bias in observational designs. A feasibility study exploring the willingness of clinicians and patients to be randomised to CKM versus dialysis is likely to be needed before embarking on a definitive interventional study. Data from such observational research would be very useful for refining both the hypothesis and the intervention for such an interventional study.
-
An alternative would be a prospective observational study, and there was strong support for that approach. The fact that there is variation in the scale of conservative care between units suggests that similar patients are being treated by these two approaches across the UK, so it should be possible to undertake such a study.
-
Nested within the CKM versus dialysis question is the need to establish the most cost-effective combination of various components in a CKM service and the optimal way to organise a CKM service across primary and secondary care. An observational approach could capture the provision/receipt of individual components of CKM at a unit level/individual patient level.
-
In observational studies, propensity score matching, instrumental variable analysis or marginal structural modelling could be used to deal with the confounding caused by treatment indication bias.
-
Given the age, comorbidity and cognitive function of patients for whom there is likely to be equipoise with regard to CKM versus dialysis, patient and carer quality of life and satisfaction with treatment will need to be carefully measured in studies comparing outcomes.
-
Given the underascertainment of non-English speakers, a focused qualitative study is needed looking specifically at this population.
Acknowledgements
We would like to thank all the patients, staff and GPs who volunteered to take part in this project and who gave their time to contribute their views. A number of clinical staff within each of the nine renal units helped to invite participants to take part in the patient interview study and we would like to thank them for their enthusiasm and dedication to this task. We would also like to thank all clinical directors of the renal units who responded to the survey and to the additional staff members who helped to fill in the questionnaire.
We would like to thank the additional members of our steering group who were involved in the initial set-up of the study and who provided comments on the project at various stages: Dr Richard Fluck (National Clinical Director for Kidney Care) and Dr Natasha McIntyre (Renal Nurse). We would also like to thank Dr Lynn Josephs (Academic GP) for providing comments on the write-up of the GP study, and Dr David Clancy (Research Fellow), who conducted initial literature reviews. We are very grateful to the IT and clinical biochemistry staff who provided us with the lists of incident CKD5 patients for merging and to David Bull for undertaking data linkage at the UK Renal Registry.
We are very grateful to our patient and public involvement representatives, Jan Cooper, John Fulton, Dick Cooke and Kirit Modi, who provided feedback on the patient and staff interview guides, the plain English summary and/or the write-up of the final report.
We would like to pay tribute to our colleague Professor Donna Lamping, who was a co-applicant but who sadly died before the study commenced.
The study was funded by the National Institute for Health Research Health Services and Delivery Research programme.
Contribution of authors
Professor Paul Roderick (Professor, Public Health) was coprincipal investigator, designed the study, oversaw all aspects of the study including the final report and chaired the study steering group.
Dr Hugh Rayner (Consultant Nephrologist) was coprincipal investigator, designed the study and led clinical aspects of all aspects of the study.
Dr Sarah Tonkin-Crine (Research Fellow, Qualitative Research) conducted interviews with patients, staff and GPs, led on the qualitative analysis of patient and GP interviews, contributed to the analysis of staff interviews and wrote the initial drafts of Chapters 2 and 5.
Dr Ikumi Okamoto (Research Fellow, Qualitative Research) conducted interviews with staff, designed the national survey, led on the qualitative analysis of staff interviews and quantitative analysis of survey data and wrote the initial drafts of Chapters 3 and 4.
Dr Caroline Eyles (Research Fellow, Qualitative Research) contributed to the qualitative analysis of staff interviews and Chapter 3.
Dr Geraldine Leydon (Reader, Qualitative Research) led on the design of the qualitative studies and contributed to the qualitative analysis of patient and staff data.
Dr Miriam Santer (National Institute for Health Research Research Fellow, General Practice) contributed to the qualitative analysis of GP interviews and Chapter 5.
The following authors made substantial contributions to the design of the work and the drafting of the final report:
Dr Jonathan Klein (Senior Lecturer, Management Science)
Dr Guiqing Lily Yao (Reader, Health Economics)
Dr Fliss Murtagh (Clinical Senior Lecturer, Palliative Care)
Professor Ken Farrington (Professor, Renal Medicine)
Dr Fergus Caskey (Consultant Nephrologist)
Dr Charles Tomson (Consultant Nephrologist)
Ms Fiona Loud (Advisor for British Kidney Patient Association, Patient and Public Involvement)
Dr Emma Murphy (Lecturer, Renal Medicine)
Dr Robert Elias (Consultant Nephrologist)
Dr Roger Greenwood (Consultant Nephrologist)
Dr Donal O’Donoghue (Consultant Nephrologist, past National Clinical Director for Kidney Care).
Publications
Okamoto I, Tonkin-Crine S, Rayner H, Murtagh FEM, Farrington K, Caskey F, et al. Conservative care for ESRD in the United Kingdom: a national survey [published online ahead of print November 11 2014]. Clin J Am Soc Nephrol 2014; pii:CJN.05000514.
Tonkin-Crine S, Okamoto I, Leydon GM, Murtagh FE, Farrington K, Caskey F, et al. Understanding by older patients of dialysis and conservative management for chronic kidney failure [published online ahead of print October 7 2014]. Am J Kidney Dis 2014; pii:S0272-6386(14)01166-4.
Tonkin-Crine S, Santer M, Leydon GM, Murtagh FEM, Farrington K, Caskey F, et al. Managing advanced CKD in primary care: a qualitative study with GPs. Br J Gen Pract 2015; in press.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- National Kidney Foundation . KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification 2002. www.kidney.org/Professionals/Kdoqi/guidelines_ckd/toc.htm (accessed 6 December 2013).
- Early Intervention and Management of Chronic Kidney Disease in Primary and Secondary Care. London: NICE; 2008.
- National Service Framework for Renal Services: Part 2. London: Department of Health; 2005.
- The Fourteenth Annual Report, UK Renal Registry 2011. www.renalreg.com/Reports/2011.html (accessed 10 November 2014).
- Feest T, Rajamahesh J, Byrne C, Ahmad A, Ansell D, Burden R, et al. Trends in adult renal replacement therapy in the UK: 1982–2002. Q J Med 2004;98:81-8. http://dx.doi.org/10.1093/qjmed/hci007.
- Smith C, Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 2003;95:40-6. http://dx.doi.org/10.1159/000073708.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007;22:1955-62. http://dx.doi.org/10.1093/ndt/gfm153.
- Burns A, Carson R. Maximum conservative management: a worthwhile treatment for elderly patients with renal failure who choose not to undergo dialysis. J Palliat Med 2007;10:1245-7. http://dx.doi.org/10.1089/jpm.2007.0009.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease?. Clin J Am Soc Nephrol 2009;4:1611-19. http://dx.doi.org/10.2215/CJN.00510109.
- Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grunfeld JP, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 2003;14:1012-21. http://dx.doi.org/10.1097/01.ASN.0000054493.04151.80.
- Da Silva-Gana M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K. Quality of life and survival in patients with advanced kidney failure managed conservatively by dialysis. Clin J Am Soc Nephrol 2012;7:2002-9. http://dx.doi.org/10.2215/CJN.01130112.
- Hussain JA, Mooney A, Russon L. Comparison of survival and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 2013;27:829-39. http://dx.doi.org/10.1177/0269216313484380.
- Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 2011;26:1608-14. http://dx.doi.org/10.1093/ndt/gfq630.
- Tamura MJ, Covbinsky KE, Chertow GM, Yaffe K, Landefeld S, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009;361:1539-47. http://dx.doi.org/10.1056/NEJMoa0904655.
- Jassal SV, Watson D. Dialysis in late life: benefit or burden. Clin J Am Soc Nephrol 2009;4:2008-12. http://dx.doi.org/10.2215/CJN.04610709.
- Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 2009;361:1612-13. http://dx.doi.org/10.1056/NEJMc0905289.
- Douglas C, Murtagh FE, Chambers EJ, Howse M, Ellershaw J. Symptom management for the adult patient dying with advanced chronic kidney disease: a review of the literature and development of evidence-based guidelines by a United Kingdom Expert Consensus Group. Palliat Med 2009;23:103-10. http://dx.doi.org/10.1177/0269216308100247.
- End of Life Care Strategy. London: Department of Health; 2008.
- End of Life Care Strategy: Quality Markers and Measures for End of Life Care. London: Department of Health; 2009.
- National Gold Standards Framework Centre . The Gold Standards Framework 2014. www.goldstandardsframework.org.uk (accessed 10 November 2014).
- Gold Standards Framework Programme . The Gold Standards Framework Prognostic Indicator Guidance 2006. www.qrp.pops.net/iha_wg/d_coping_with_end_of_life/prognostic_indicators_guidance_paper.pdf (accessed December 2013).
- End of Life Care in Advanced Kidney Disease: A Framework for Implementation. London: Department of Health; 2009.
- Getting It Right: End of Life Care in Advanced Kidney Disease. London: Department of Health; 2012.
- Burgess E. Conservative treatment to slow deterioration of renal function: evidence-based recommendations. Kidney Int Suppl 1999;70:S17-S25. http://dx.doi.org/10.1046/j.1523-1755.1999.07003.x.
- Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 2007;10:1266-76. http://dx.doi.org/10.1089/jpm.2007.0017.
- Murphy EL, Murtagh FE, Carey I, Sheerin NS. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract 2009;111:74-80. http://dx.doi.org/10.1159/000183177.
- Davison SN, Simpson C. Hope and advance care planning in patients with end stage renal disease: qualitative interview study. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38965.626250.55.
- Douglas C, Ellershaw J, Murtagh F, Chambers J, Meyer M, Howse M, et al. Adapting the Liverpool Care of the Dying Pathway for Patients Dying With End-Stage Renal Disease n.d.
- Noble H, Chesser A, Go J. Developing a renal supportive care service for patients opting not to dialyse. End Life Care J 2007;1:51-5.
- Murtagh FE, Murphy E, Shepherd KA, Donohoe P, Edmonds PM. End of-life care in end-stage renal disease: renal and palliative care. Br J Nurs 2006;15:8-11. http://dx.doi.org/10.12968/bjon.2006.15.1.20301.
- Gunda S, Smith S, Thomas M. National survey of palliative care in end-stage renal disease in the United Kingdom. Nephrol Dial Transplant 2004;20:392-5. http://dx.doi.org/10.1093/ndt/gfh619.
- Rayner HC, Baharani I, Dasgupta I, Suresh V, Temple RM, Thomas ME, et al. Does community-wide chronic kidney disease management improve patient outcomes?. Nephrol Dial Transplant 2014;29:644-9. http://dx.doi.org/10.1093/ndt/gft486.
- Dasgupta I, Rayner HC. Dialysis versus conservative management of elderly patients with advanced chronic kidney disease. Nat Clin Pract Nephrol 2007;3:480-1. http://dx.doi.org/10.1038/ncpneph0569.
- Ellam T, El-Kossi M, Prasanth KC, El-Nahas M, Khwaja A. Conservatively managed patients with stage 5 chronic kidney disease: outcomes from a single center experience. QJM 2009;102:547-54. http://dx.doi.org/10.1093/qjmed/hcp068.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Noble H, Meyer J, Bridges J, Kelly D, Johnson B. Reasons renal patients give for deciding not to dialyze: a prospective qualitative interview study. Dialysis Transplant 2009;38:82-9. http://dx.doi.org/10.1002/dat.20288.
- Johnston S, Noble H. Factors influencing patients with stage 5 chronic kidney disease to opt for conservative management: a practitioner research study. J Clin Nurs 2012;21:1215-22. http://dx.doi.org/10.1111/j.1365-2702.2011.04001.x.
- Visser A, Dijkstra GJ, Kuiper D, de Jong PE, Franssen CFM, Gansevoort RT, et al. Accepting or declining dialysis: considerations taken into account by elderly patients with end-stage renal disease. J Nephrol 2009;22:794-9.
- Seah AST, Tan F, Srinivas S, Wu HY, Griva K. Opting out of dialysis: exploring patients’ decisions to forego dialysis in favour of conservative non-dialytic management for end-stage renal disease. Health Expect 2013. http://dx.doi.org/10.1111/hex.12075.
- Castledine C, Gilg J, Rogers C, Ben-Shlomo Y, Caskey F. UK Renal Registry 13th Annual Report (December 2010): Chapter 15 UK renal centre survey results 2010: RRT incidence and use of home dialysis modalities. Nephron Clin Pract 2011;119:c255-67. http://dx.doi.org/10.1159/000331783.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Glaser BS. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine De Gruyter; 1967.
- Fine A, Fontaine B, Kraushar MM, Rich BR. Nephrologists should voluntarily divulge survival data to potential dialysis patients: a questionnaire study. Perit Dial Int 2005;25:269-73.
- Schell JO, Patel UD, Steinhauser KE, Ammarell N, Tulsky JA. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis 2012;59:495-503. http://dx.doi.org/10.1053/j.ajkd.2011.11.023.
- Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, et al. Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 2012;184:E277-E283. http://dx.doi.org/10.1503/cmaj.111355.
- Tong A, Sainsbury P, Chadban S, Walker RG, Harris DC, Carter SM, et al. Patients’ experiences and perspectives of living with CKD. Am J Kidney Dis 2009;53:689-700. http://dx.doi.org/10.1053/j.ajkd.2008.10.050.
- Davison SN, Torgunrud C. The creation of an advance care planning process for patients with ESRD. Am J Kidney Dis 2007;49:27-36. http://dx.doi.org/10.1053/j.ajkd.2006.09.016.
- Germain MJ, Tamura MK, Davison SN. Palliative care in CKD: the earlier the better. Am J Kidney Dis 2011;57:378-80. http://dx.doi.org/10.1053/j.ajkd.2010.12.001.
- Tamura MK, Periyakoil VS. The patient perspective and physician’s role in making decisions on instituting dialysis. Nephrol Dial Transplant 2013;28:1-4.
- Halvorsen K, Slettebo A, Nortvedt P, Pedersen R, Kirkevold M, Nordhaug M, et al. Priority dilemmas in dialysis: the impact of old age. J Med Ethics 2008;34:585-9. http://dx.doi.org/10.1136/jme.2007.022061.
- Grönlund CECF, Dahlqvist V, Soderberg AIS. Feeling trapped and being torn: physicians’ narratives about ethical dilemmas in hemodialysis care that evoke a troubled conscience. BMC Med Ethics 2011;12. http://dx.doi.org/10.1186/1472-6939-12-8.
- Weber RP. Basic Content Analysis. Newbury Park, London: Sage; 1990.
- Russ AJ, Shim JK, Kaufman SR. The value of ‘life at any cost’: talk about stopping kidney dialysis. Soc Sci Med 2007;64:2236-47. http://dx.doi.org/10.1016/j.socscimed.2007.02.016.
- Berzoff J, Swantkowski J, Cohen LM. Developing a renal supportive care team from the voices of patients, families, and palliative care staff. Palliat Support Care 2008;6:133-9. http://dx.doi.org/10.1017/S1478951508000217.
- Rapley T. Distributed decision making: the anatomy of decisions-in-action. Sociol Health Illn 2008;30:429-44. http://dx.doi.org/10.1111/j.1467-9566.2007.01064.x.
- Levin A. Ongoing gaps in CKD and CVD care: re-evaluating strategies for knowledge dissemination. Nephrol Dialysis Transplant 2012;27:1282-4. http://dx.doi.org/10.1093/ndt/gfr765.
- Ahmad A, Roderick P, Ward M, Steenkamp R, Burden R, O’Donoghue D, et al. Current chronic kidney disease practice patterns in the UK: a national survey. QJM 2006;99:245-51. http://dx.doi.org/10.1093/qjmed/hcl029.
- Alston H. Conservative care for end-stage kidney disease: joint medical conference with the Renal Association, British Geriatrics Society and Association for Palliative Medicine. Clin Med 2013;13:383-6. http://dx.doi.org/10.7861/clinmedicine.13-4-383.
- Morton RL, Howard K, Webster AC, Snelling P. Patient INformation about Options for Treatment (PINOT): a prospective national study of information given to incident CKD stage 5 patients. Nephrol Dialysis Transplant 2011;26:1266-74. http://dx.doi.org/10.1093/ndt/gfq555.
- Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 2012;59:504-12. http://dx.doi.org/10.1053/j.ajkd.2011.12.009.
- Hobson K, Gomm S, Murtagh F, Caress AL. National survey of the current provision of specialist palliative care services for patients with end-stage renal disease. Nephrol Dialysis Transplant 2011;26:1275-81. http://dx.doi.org/10.1093/ndt/gfq530.
- Morton RL, Turner RM, Howard K, Snelling P, Webster AC. Patients who plan for conservative care rather than dialysis: a national observational study in Australia. Am J Kidney Dis 2012;59:419-27. http://dx.doi.org/10.1053/j.ajkd.2011.08.024.
- Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PMM, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003;64:2222-8. http://dx.doi.org/10.1046/j.1523-1755.2003.00321.x.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. http://dx.doi.org/10.1136/bmj.325.7367.766.
- 2013/14 General Medical Services (GMS) Contract Quality and Outcomes Framework (QOF). London: NHS Employers and BMA; 2013.
- Crinson I, Gallagher H, Thomas N, de Lusignan S. How ready is general practice to improve quality in chronic kidney disease? A diagnostic analysis. Br J Gen Pract 2010;60:403-9. http://dx.doi.org/10.3399/bjgp10X502100.
- Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007;72:92-9. http://dx.doi.org/10.1038/sj.ki.5002273.
- Phillips LA, Donovan KL, Phillips AO. Renal quality outcomes framework and eGFR: impact on secondary care. QJM 2009;102:415-23. http://dx.doi.org/10.1093/qjmed/hcp030.
- Abdi Z, Gallagher H, O’Donoghue D. Telling the truth: why disclosure matters in chronic kidney disease. Br J Gen Pract 2012;62:172-3. http://dx.doi.org/10.3399/bjgp12X635958.
- McIntyre NJ, Fluck R, McIntyre C, Taal M. Treatment needs and diagnosis awareness in primary care patients with chronic kidney disease. Br J Gen Pract 2012;62:e227-32.
- Blakeman T, Protheroe J, Chew-Graham C, Rogers A, Kennedy A. Understanding the management of early-stage chronic kidney disease in primary care: a qualitative study. Br J Gen Pract 2012;62:e233-42.
- Greer RC, Crews DC, Boulware LE. Challenges perceived by primary care providers to educating patients about chronic kidney disease. J Renal Care 2012;38:174-81. http://dx.doi.org/10.1111/j.1755-6686.2012.00323.x.
- Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4298.
- Blakeman T, Harding S, O’Donoghue D. Acute kidney injury in the community: why primary care has an important role. Br J Gen Pract 2013;63:173-4. http://dx.doi.org/10.3399/bjgp13X664207.
- Fox CH, Brooks A, Zayas LE, McCellan W, Murray B. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an upstate New York practice-based research network (UNYNET) study. J Am Board Fam Med 2006;19:54-61. http://dx.doi.org/10.3122/jabfm.19.1.54.
- Smith DH, Schneider J, Thorp ML, Vupputuri S, Weiss JW, Johnson ES, et al. Clinician’s use of automated reports of estimated glomerular filtration rate: a qualitative study. BMC Nephrol 2012;13. http://dx.doi.org/10.1186/1471-2369-13-154.
- End-Stage Kidney Disease in Australia: Total Incidence, 2003–2007. Cat. no. PHE 143. Canberra, NSW: AIHW; 2011.
- Kellerman SE, Herold J. Physician response to surveys: a review of the literature. Am J Prev Med 2001;20:61-7. http://dx.doi.org/10.1016/S0749-3797(00)00258-0.
- VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: a systematic review. Eval Health Prof 2007;30:303-21. http://dx.doi.org/10.1177/0163278707307899.
- Reeve J, Blakeman T, Freeman GK, Green LA, James PA, Lucassen P, et al. Generalist solutions to complex problems: generating practice-based evidence – the example of managing multi-morbidity. BMC Fam Pract 2013;14. http://dx.doi.org/10.1186/1471-2296-14-112.
- Kennedy DM, Chatha K, Rayner HC. Laboratory database population surveillance to improve detection of progressive chronic kidney disease. J Renal Care 2013;39:23-9. http://dx.doi.org/10.1111/j.1755-6686.2013.12029.x.
- Bristowe K, Shepherd K, Bryan L, Brown H, Carey I, Matthews B, et al. The development and piloting of the REnal specific Advanced Communication Training (REACT) programme to improve advance care planning for renal patients. Palliat Med 2014;28:360-6. http://dx.doi.org/10.1177/0269216313510342.
Appendix 1 The semistructured interview guide followed during patient interviews
Part one: you and your illness – a brief biography
-
Please could you tell me briefly about your kidney disease?
-
Probe: when were you diagnosed?
-
How was your kidney disease diagnosed?
-
Can you tell me what you think caused your kidney disease?
-
What impact does your kidney disease have on your life now?
-
How do you think your kidney disease may impact on your life in the future?
-
-
-
Where are you now with your kidney disease in terms of its management?
-
Probe: CKM versus wait for RRT or on RRT.
-
How long since CKM decision or since RRT start?
-
-
Part two: how decisions were made about management and views on pathway
-
3. How were the options for the management of your kidney disease presented to you?
-
Probe: who discussed the options with you?
-
How did you feel about this discussion?
-
When were the options discussed with you?
-
How did you feel about the timing of this discussion?
-
What information was shared with you/were you given?
-
– How did you feel about the information you were given?
-
– What sort of questions did you have about your options for management?
-
-
-
-
4. Who made the decision about whether or not to have dialysis?
-
Probe: when was the decision made?
-
How much time was spent thinking about the decision?
-
– By you? By your doctor?
-
– What factors influenced your final choice of management?
-
– Have you changed your initial choice?
-
– Do you think you will change your choice in the future?
-
-
-
-
5a. [For those who chose CKM] What did choosing CKM mean to you?
-
Probe: what were the reasons you chose CKM?
-
What did you think the benefits might be?
-
What other implications did you think there would be?
-
– Have you experienced anything which you did not expect since starting on the CKM pathway?
-
-
What do you think the benefits of CKM are now?
-
Who do you think CKM is most appropriate for?
-
-
-
5b. [For those who chose dialysis but not yet started] What did choosing dialysis mean to you?
-
Probe: what were the reasons you chose to have dialysis in the future?
-
What do you think the benefits might be?
-
What other implications do you think there will be?
-
Who do you think dialysis is most appropriate for?
-
-
-
5c. [For those already on dialysis] What did choosing dialysis mean to you?
-
Probe: what were the reasons you chose to have dialysis?
-
What did you think the benefits might be?
-
What other implications did you think there would be?
-
– Have you experienced anything which you did not expect since starting dialysis?
-
-
What do you think the benefits of dialysis are now?
-
Who do you think dialysis is most appropriate for?
-
-
-
6. Can you tell me about any concerns that you had when the decision about your kidney disease management was made?
-
Probe: were there any challenges that you anticipated?
-
Were there any opportunities that you thought you may miss out on?
-
Do you feel you understood fully what was being agreed?
-
-
-
7. Can you tell me about any concerns that you have now about the decision that was made?
-
Probe: how well do you feel you understood the consequences at the time?
-
How well do you feel you understood what was involved at the time?
-
Did you feel you were involved enough in the decision?
-
Do you feel you had enough clinical input from the doctors?
-
-
-
8. Overall, how do you feel about the way the decision was managed?
-
Probe: how did you feel about the amount of time you were given to think about the decision?
-
Did you feel you were able to ask questions?
-
– Did you feel your family/significant others were able to contribute to the decision?
-
-
How do you think the decision-making process could be improved?
-
Should anything be changed about the decision-making process?
-
-
Conclusion
-
Are there any other relevant issues we haven’t covered that you would like to mention?
-
Are there any questions you that would like to ask me?
Your demographic information
-
Gender.
-
Age.
-
Ethnicity.
-
Occupation prior to retirement.
-
Marital status.
-
Religion.
-
Living arrangements.
Thank you very much for taking the time to speak with me.
Appendix 2 The semistructured interview guide followed during staff interviews
Part one: your role and experience
-
Could you tell me more about your role and your responsibilities within your unit?
-
Could you tell me what conservative management means in your renal unit?
-
What acronym or name do you use for conservative management?
-
-
How much time in your role is spent with CKM patients?
-
Is your role dedicated to CKM patients only?
-
-
Can you tell me what training you have had about assessing and addressing palliative and supportive needs of kidney patients?
-
If none, do you feel the need to receive such training? Why?
-
If some, how do you feel about this training?
-
-
Can you tell me about any communication skills training you have had?
-
If none, do you feel the need to receive such training? Why?
-
If some, how do you feel about this training?
-
Part two: your views on CKM in general
-
Could you tell me your views on the value of CKM in general?
-
Prompts:
-
Advantages?
-
Disadvantages?
-
Reasons why?
-
-
-
In your view what are the components of CKM that are likely to make a difference to outcome?
-
Prompts:
-
Psychological.
-
Physical.
-
-
-
For which types of patient do you think CKM is most appropriate?
-
Prompts:
-
Dilemmas.
-
Comorbidity number and severity, age, other.
-
Social circumstances.
-
-
-
In general, how would you describe the key barriers and facilitators to the implementation of CKM?
-
Prompts:
-
Late referral.
-
Family/carer expectation.
-
Family/patient lack of knowledge.
-
Expectation of clinical staff.
-
Lack of experience of CKM.
-
Lack of resources.
-
-
Part three: how decisions are made about chronic kidney management
-
How do you identify patients who have advanced kidney disease in your unit?
-
Prompts:
-
Do you encourage referrals in any way?
-
Do you identify patients from their laboratory biochemistry reports?
-
-
-
Does your nephrology team also take part in the general/acute medicine service in your hospital?
-
Prompts:
-
How does this affect your access to patients with advanced kidney disease?
-
-
-
Are you aware of any specific guidelines on shared decision-making and withdrawal from dialysis? (e.g. NHS kidney care)
-
If you are, do you refer to these when making decisions?
-
-
In your unit how is the decision made to raise the issue of CKM with a patient?
-
Prompts:
-
Roles of:
-
Renal consultant?
-
MDT [multidisciplinary team] meeting? Participants?
-
Patient/carer?
-
-
-
Is a decision to consider CKM typically ‘distributed’ or ‘shared’ by a number of people?
-
What factors are likely to influence a consideration of CKM?
-
Prompts:
-
Frailty?
-
Extent and severity of comorbidities?
-
Social support?
-
Cognitive function?
-
Patient/carer preference?
-
Use of the ‘surprise’ question?
-
Consultant preference?
-
Age of patient?
-
-
-
At what point in a patient’s illness trajectory will a decision for CKM typically be made?
-
Prompts:
-
eGFR level (for stable patients in the clinic).
-
Iterative nature of decision-making.
-
What about patients presenting unwell and as an emergency?
-
-
-
Once a decision is made to follow CKM pathway, what does a typical patient pathway look like?
-
How does this differ from pre-dialysis and dialysis patients?
-
-
During the CKM pathway, is there any phase where patients are symptomatic and difficult to manage?
-
To what extent do they differ from dialysis patients? (e.g., a longer symptomatic phase for CKM patients?)
-
-
Are you involved in discussing CKM with patients and families?
-
If yes, on the basis of your clinical training, how well prepared do you feel to do so?
-
-
Do you use any decision aids when discussing CKM with a patient?
-
Prompts:
-
Pamphlets? Videos? Web-based tools?
-
-
-
How do you feel patients find making the decision about CKM?
-
Prompts:
-
How much time do patients spend thinking about the decision to have CKM?
-
Do you ever ‘double-check’ patients’ decisions about CKM?
-
– If yes, why? At what point? How often?
-
-
Do patients ever change their mind and opt for dialysis?
-
– If yes, why? At what point?
-
-
Do you find there are any differences in those patients who change their mind?
-
– Patients with families who want them to have dialysis.
-
– Patients from an ethnic minority background.
-
– Patients who were scared of the idea of dialysis/were less well informed.
-
-
-
-
How do you feel patients find making the decision to have dialysis?
-
How much time do patients spend thinking about the decision?
-
Do patients ever change their mind?
-
How do patients find dialysis once they’ve started?
-
– Is there anything which you think surprises patients or that they don’t expect?
-
– Is there anything which patients struggle with?
-
– Are there any other benefits of having dialysis aside from the medical benefits?
-
-
What happens if patients don’t do well on dialysis?
-
-
Do any of CKM patients have access ready for dialysis (i.e. fistula)? If so, to what extent does this ‘access’ decision determine whether they remain CKM or not?
-
If CKM patients change their mind and have dialysis when they become symptomatic, what kind of problems would this cause?
-
How does your renal service link up with general medical teams and general practices in the medical care of elderly patients (> 75 years) with CKD5 for whom dialysis is not considered appropriate?
-
Prompts:
-
Do you keep them under your care?
-
Do you discharge them?
-
Do you share follow up in any way?
-
How do you decide whether to keep or discharge them?
-
-
-
What is the role of GP regarding CKM patients?
Part four: end-of-life care
-
Could you define what the term ‘Palliative care/end-of-life care’ means to you?
-
How do you identify patients approaching end of life?
-
Do you use a register of these patients within your unit to facilitate delivery of palliative and supportive care?
-
What are the criteria used for identification for the register?
-
-
Do you use advance care planning to provide end-of-life care sensitive to an individual’s requirements?
-
If YES, what is the component of ACP?
-
-
How do you support patient’s family and carers?
-
Through end of life and beyond?
-
-
Do any of your staff have specialist training in the delivery of palliative care for patients with end stage disease?
-
What end of life services does your unit typically liaise with for CKM, and how?
-
Prompts:
-
End-of-life/bereavement care?
-
Specialist palliative care services within or outside the hospital?
-
Hospices?
-
What is the role of the GP regarding patients at the end of life?
-
-
-
Are there any palliative care specialists involved in your unit? Role?
-
Are there any Macmillan Nurses involved in your unit? Role?
Part five: the development of chronic kidney management in your unit
-
Is there a policy for CKM?
-
Prompts:
-
Do you have anything written?
-
Who was responsible for drawing up the policy?
-
Who is responsible for its implementation?
-
What are the key components?
-
Strengths/weakness/problems?
-
-
-
Can you describe how CKM has developed in your unit? (e.g. relationships with GPs, other practitioners).
-
Any key players in developing CKM in your unit?
-
Any barriers to overcome in developing CKM in your unit?
-
Part six: chronic kidney management resources
-
What is the size of the CKM programme in your unit?
-
Patients on dialysis versus CKM.
-
Number of CKM patients who would have started dialysis by now if chosen.
-
-
Approximately what percentage of patients aged > 75 years with progressive CKD5 opt for CKM in your unit?
-
Do you have staff dedicated to CKM patients?
-
Prompts:
-
If yes, ask FULL time or PART time.
-
How many and who they are?
-
-
-
How much time do non-dedicated staff spend on CKM patients?
-
In your unit who tends to get involved in the care of patients treated conservatively?
-
Do you have dedicated clinic space for CKM patients in your unit?
-
How often do CKM patients come to your unit?
-
Can you tell us in particular about the key service components in relation to CKM?
-
Prompts:
-
Clinic consultations – follow ups?
-
Blood result review [specifically haemoglobin, potassium, calcium, phosphate, bicarbonate (acidosis), albumin]?
-
EPO and Fe [iron] (what percentage require?)
-
Multidisciplinary meetings?
-
Liaison with GPs? Links to 4.6.
-
Telephone support for patients?
-
Home visits?
-
Liaison with other non-renal palliative care services or hospices?
-
Training/educating staff and patients/careers?
-
-
-
Do you record CKM decisions? If so, how/where is this information recorded?
-
How is CKM funded? Funded separately or as part of renal unit budget?
Part seven: future of chronic kidney management in your unit
-
Is implementation of your unit’s CKM policy evaluated/audited/monitored?
-
How?
-
Details?
-
-
What are the good things about having the CKM pathway in your unit?
-
How could the CKM pathway be improved in your unit?
-
How has the development of your unit influenced the growth in numbers of staff resources (and the number of CKM patients)?
-
Can you tell me about what changes are planned in your unit?
-
Why are these happening? Why are there no changes?
-
For renal clinicians and senior nurses to answer: willingness to randomise
There is a lack of high quality evidence for patients and clinicians to consider when deciding whether to have dialysis or CKM.
-
Would you consider it appropriate to enter any patients aged > 75 years with progressive CKD5 into a RCT of CKM versus dialysis?
-
Prompts:
-
More detail on types of patient that might be considered?
-
Reasons against/for a trial?
-
Would your unit be willing to participate in such a trial?
-
-
If no, willingness to enter into a prospective observational non-randomised study comparing outcomes of patients with CKM with similar patients starting RRT.
Part eight: conclusion
-
Are there any other relevant issues we haven’t covered that you would like to mention?
-
Are there any questions that you would like to ask me?
Your demographic information
-
Gender.
-
Age.
-
Ethnicity.
-
Profession/grade/specific post (e.g. Consultant Nephrologist).
-
How many years of experience do you have in your current role?
-
How many years have you worked within this unit?
Appendix 3 Conservative Kidney Management Assessment of Practice Patterns Study national survey
Appendix 4 Factors used in analysis
Group 1: factors related to chronic kidney management resources/size
CKM size (small or large).
Availability of staff responsible for CKM.
Availability of staff whose time is specifically allocated for CKM patients.
Group 2: factors related to the general unit organisation for patients with chronic kidney disease
Whether or not to have regular MSRT meetings.
Availability of pre-dialysis clinics.
Availability of pre-dialysis education day.
Whether or not consultants share responsibility for patients with each other.
Group 3: factors related to chronic kidney management practices
Whether or not same practice regarding CKM is provided.
Availability of written CKM guideline.
Availability of dedicated CKM clinics.
Group 4: factors related to chronic kidney management resources
Availability of funding for CKM.
Group 5: factors related to chronic kidney management decision-making
Whether or not CKM is discussed with all CKD5 patients aged 75 years and over.
Use of decision aids.
Group 6: factors related to training
Provision of formal CKM training to renal staff.
Training in palliative/end-of-life care for renal patients for staff.
Provision of training about management of renal patients to palliative care specialists.
Group 7: factors related to general practitioner involvement
How are GPs involved with CKM patients.
Provision of information/advice regarding treatment of CKM patients to GPs.
Group 8: factors related to end-of-life care
Availability of written guideline for renal end-of-life care.
Use of register to identify CKM patients approaching end of life.
Use of advance care planning.
Group 9: factors related to future research
Whether or not to consider RCT for CKD5 patients.
Whether or not to consider observational study for CKD5 patients.
Appendix 5 Conservative kidney management unit size
| Renal unit | Q2.5.1a: no. of CKD5 patients aged 75 years and over on CKM | Q2.6a: no. of patients aged 75 years and over who became symptomatic and still on CKM | CKM size used in the reportb |
|---|---|---|---|
| 1 | – | – | No response |
| 2 | – | 11 | Small |
| 3 | 9 | – | Small |
| 4 | 11 | 3 | Small |
| 5 | – | – | No response |
| 6 | 50 | 25 | Large |
| 7 | – | 30 | Large |
| 8 | – | – | No response |
| 9 | – | – | No response |
| 10 | 47 | – | Large |
| 11 | 35 | – | Large |
| 12 | 152 | – | Large |
| 13 | 70 | – | Large |
| 14 | 57 | – | Large |
| 15 | – | – | No response |
| 16 | – | 5 | Small |
| 17 | – | 5 | Small |
| 18 | 112 | – | Large |
| 19 | 60 | 6 | Large |
| 20 | 45 | – | Large |
| 21 | – | – | No response |
| 22 | 6 | 6 | Small |
| 23 | – | – | No response |
| 24 | 117 | – | Large |
| 25 | – | – | No response |
| 26 | 25 | 5 | Small |
| 27 | – | – | No response |
| 28 | – | 6 | Small |
| 29 | 4 | 1 | Small |
| 30 | – | 4 | Small |
| 31 | 5 | 2 | Small |
| 32 | – | – | No response |
| 33 | 100 | – | Large |
| 34 | – | – | No response |
| 35 | – | – | No response |
| 36 | 54 | 29 | Large |
| 37 | 70 | 14 | Large |
| 38 | 83 | 28 | Large |
| 39 | 127 | – | Large |
| 40 | 35 | 20 | Large |
| 41 | – | – | No response |
| 42 | 25 | 24 | Large |
| 43 | – | – | No response |
| 44 | 86 | – | Large |
| 45 | – | – | No response |
| 46 | – | – | No response |
| 47 | – | – | No response |
| 48 | – | 2 | Small |
| 49 | 26 | 26 | Large |
| 50 | – | – | No response |
| 51 | 20 | – | Small |
| 52 | 52 | 40 | Large |
| 53 | – | 3 | Small |
| 54 | 18 | 2 | Small |
| 55 | 128 | 15 | Large |
| 56 | 150 | 50 | Large |
| 57 | – | 20 | Large |
| 58 | 12 | 5 | Small |
| 59 | 20 | 8 | Small |
| 60 | 20 | – | Small |
| 61 | – | – | No response |
| 62 | – | – | No response |
| 63 | – | 10 | Small |
| 64 | 5 | 3 | Small |
| 65 | – | 15 | Small |
| 66 | 25 | 5 | Small |
| 67 | – | 13 | Small |
Questions in the survey
Question 2.5.1. Of those, how many were on conservative care and followed up in your unit? If you don’t know the number, please answer the next question instead.
Question 2.6. In 2012, how many patients aged 75 and over in your unit chose to have conservative care, became symptomatic of advanced CKD and did not have dialysis?
Chronic kidney management size categorisation process
Units were divided into two categories based on their responses to the survey questions regarding the number of CKM patients aged 75 years and over in calendar year 2012 (Question 2.5.1) and the number of patients aged 75 years and over who stayed on CKM after they became symptomatic in the same year (Question 2.6).
Units that had ≥ 25 CKM patients and/or ≥ 20 symptomatic CKM patients were categorised as units with a larger size of CKM (24 units), and the rest of the units were categorised as those with a smaller size of CKM (23 units). (This resulted in including three units that had ≥ 25 CKM patients but had fewer than 20 symptomatic patients in the ‘large’ group.)
Twelve units that provided only the number of symptomatic CKM patients were looked at; two units with ≥ 20 symptomatic CKM patients were added to the large category, and the rest were added to the small category, which made the total numbers of units with ‘large’ CKM and ‘small’ CKM 24 and 23 respectively. (There was no response from 20 units.)
Appendix 6 Conservative Kidney Management Assessment of Practice Patterns Study survey: results tables
Questions regarding chronic kidney disease in your unit
| 1. How many FTE (full time equivalent) consultants (including CKD, dialysis and transplant) do you have working in nephrology in your unit? ___ FTE | ||
|---|---|---|
| Statistics | ||
| n | Valid | 65 |
| Missing | 2 | |
| Mean | 7.35 | |
| Median | 6.60 | |
| Range | 39 | |
| Minimum | 1 | |
| Maximum | 40 | |
| Percentiles | 25 | 3.50 |
| 50 | 6.60 | |
| 75 | 9.20 | |
| 2. Do you have a Multi-Skilled Renal Team (MSRT) available to manage patients approaching RRT in your unit? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 98.5 | 66 |
| No | 1.5 | 1 |
| Answered question | 67 | |
| Skipped question | 0 | |
| 2.1. Do you have regular MSRT meetings? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 87.9 | 58 |
| No | 12.1 | 8 |
| Answered question | 66 | |
| Skipped question | 1 | |
| 2.1.1. If yes, how often do you have the meetings? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Once a week | 56.9 | 33 |
| Once a fortnight | 8.6 | 5 |
| Once a month | 24.1 | 14 |
| Other (please specify) | 10.4 | 6 |
| Answered question | 58 | |
| Skipped question | 9 | |
| 2.2. Which of the following staff members are involved in your MSRT and usually attend the MSRT meeting? Please tick all that apply in each column below | |||
|---|---|---|---|
| Answer options | Staff involved in MSRT | Staff who usually attend MSRT meeting | Response count |
| Consultant nephrologists | 52 | 45 | 58 |
| Renal registrars | 40 | 30 | 44 |
| Renal nurses | 51 | 43 | 56 |
| Palliative care consultants | 20 | 5 | 21 |
| Palliative care registrars | 3 | 1 | 4 |
| Renal palliative care clinical nurse specialists | 19 | 10 | 20 |
| Surgeons | 19 | 3 | 19 |
| SAS grade doctors | 15 | 8 | 15 |
| Diabetes nurses | 2 | 1 | 2 |
| Social workers | 27 | 18 | 31 |
| Occupational therapists | 7 | 2 | 7 |
| Physiotherapists | 5 | 2 | 6 |
| Dietitians | 46 | 31 | 51 |
| Pharmacists | 30 | 14 | 34 |
| Psychologists | 15 | 9 | 18 |
| Pre-dialysis education providers | 44 | 29 | 46 |
| Anaemia nurses | 43 | 27 | 45 |
| Vascular access coordinators | 42 | 24 | 42 |
| Counsellors | 16 | 7 | 16 |
| Other | 4 | 2 | 5 |
| Please specify | 9 | ||
| Answered question | 58 | ||
| Skipped question | 9 | ||
| 3. Do you run clinics for CKD patients in neighbouring hospitals? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 88.1 | 59 |
| No | 11.9 | 8 |
| Answered question | 67 | |
| Skipped question | 0 | |
| Statistics | 3.1. How many neighbouring hospitals do you serve? | 3.2. In how many of the neighbouring hospitals do you have renal clinics? |
| n | ||
| Valid | 58 | 58 |
| Missing | 9 | 9 |
| Median | 3.00 | 3.00 |
| Standard deviation | 2.4 | 2.1 |
| Range | 11 | 10 |
| Minimum | 1 | 1 |
| Maximum | 12 | 11 |
| Percentiles | ||
| 25 | 1.00 | 1.00 |
| 50 | 3.00 | 3.00 |
| 75 | 5.00 | 4.25 |
| 4. Do you have a pre-dialysis clinic or equivalent for managing patients approaching RRT? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 83.6 | 56 |
| No | 10.4 | 7 |
| No, but we are planning to set up similar clinics | 6.0 | 4 |
| Answered question | 67 | |
| Skipped question | 0 | |
| 4.1. Do all consultants who have CKD patients use the pre-dialysis clinic? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 73.2 | 41 |
| No | 26.8 | 15 |
| Answered question | 56 | |
| Skipped question | 11 | |
| 4.1.1. Why don’t all consultants who have CKD patients use the pre-dialysis clinic? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Because some consultants think that long-term continuity of care by the same consultant is more important | 20.0 | 3 |
| Because some consultants’ clinics are at one of a neighbouring hospitals and the pre-dialysis clinic is in the main hospital. They don’t want their patients to travel to the main hospital | 20.0 | 3 |
| Other (please specify) | 60.0 | 9 |
| Answered question | 15 | |
| Skipped question | 52 | |
| 5. What percentage of the outpatients under follow up in your renal clinic, who are approaching dialysis, receive the following? | |||||
|---|---|---|---|---|---|
| Answer options | ≤ 25% | 26–50% | 51–75% | 76–100% | Response count |
| Nurse-led education | 0 | 0 | 3 | 64 | 67 |
| Home visit | 21 | 10 | 9 | 24 | 64 |
| Trained counsellor/psychologist input | 51 | 10 | 2 | 2 | 65 |
| Occupational therapist and/or social work input | 38 | 16 | 9 | 2 | 65 |
| Answered question | 67 | ||||
| Skipped question | 0 | ||||
| 6. How is pre-dialysis education delivered in your unit? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Consultant/registrar consultation | 86.6 | 58 |
| DVD education materials to take home | 73.1 | 49 |
| Written material to take home | 95.5 | 64 |
| Translated (if appropriate) written material (except Welsh) | 41.8 | 28 |
| Computer-based education programme | 31.3 | 21 |
| Group session with other pre-dialysis patients | 76.1 | 51 |
| Talk from a patient on conservative care | 13.4 | 9 |
| Talk from a patient on centre HD | 58.2 | 39 |
| Talk from a patient on home HD | 50.7 | 34 |
| Talk from a patient on peritoneal dialysis | 61.2 | 41 |
| Talk from a patient with functioning transplant | 50.7 | 34 |
| Cultural/language-matched nurse educators | 16.4 | 11 |
| Flexibility to allow extra education time for those who need it | 76.1 | 51 |
| Visit to an HD unit | 95.5 | 64 |
| Formal case-by-case MSRT discussion | 41.8 | 28 |
| Other (please specify) | 32.8 | 22 |
| Answered question | 67 | |
| Skipped question | 0 | |
| 7. Do you have a pre-dialysis education daya? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 80.6 | 54 |
| No | 19.4 | 13 |
| Answered question | 67 | |
| Skipped question | 0 | |
| 7.1. Which of the following topics are usually covered during the pre-dialysis education day? Please tick all that applya | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Types of dialysis | 98 | 52 |
| Transplantation | 100 | 53 |
| Conservative care | 85 | 45 |
| Side effects | 85 | 45 |
| Medicines | 87 | 46 |
| Dietary restrictions | 100 | 53 |
| Fluid balance | 89 | 47 |
| CKD-related anaemia | 85 | 45 |
| Renal bone disease | 74 | 39 |
| Cardiovascular risk factors | 66 | 35 |
| Sexual matters | 38 | 20 |
| Psychological support | 79 | 42 |
| Other (please specify) | 26 | 14 |
| Answered question | 53 | |
| Skipped question | 14 | |
| 8. Do your consultants share responsibility for patients with each other? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes, they share responsibility for all patients | 27.3 | 18 |
| No, they work on a named-patient basis | 28.8 | 19 |
| They share responsibility for most patients but take a lead role for individual patients with particular needs | 30.3 | 20 |
| Other (please specify) | 13.6 | 9 |
| Answered question | 66 | |
| Skipped question | 1 | |
1. Availability of an alternative to dialysis
| 1.1. Does your unit ever have patients with CKD5* where an active decision is made not to dialyse even when they are symptomatic? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 98.5 | 66 |
| No | 1.5 | 1 |
| Answered question | 67 | |
| Skipped question | 0 | |
| 1.1.1. How does your unit follow up patients with CKD5 where a decision is made not to dialyse? Please indicate the approximate percentages followed up as specified below. Totals do NOT need to add up to 100% | ||||||
|---|---|---|---|---|---|---|
| Answer options | ≤ 25% | 26–50% | 51–75% | 76–100% | N/A | Response count |
| In a dedicated programme with its own clinic for those patients | 14 | 1 | 3 | 9 | 38 | 65 |
| In a pre-dialysis clinic/low clearance clinic | 9 | 15 | 4 | 24 | 13 | 65 |
| In a general nephrology clinic | 22 | 10 | 8 | 7 | 17 | 65 |
| Patients are referred back to primary care and unit provides care in collaboration with GPs | 40 | 5 | 2 | 5 | 11 | 64 |
| Other | 11 | 1 | 3 | 3 | 13 | 32 |
| (Please specify and indicate percentage) | 16 | |||||
| Answered question | 66 | |||||
| Skipped question | 1 | |||||
| 1.1.2. What words do you most commonly use in your unit when referring to the care of patients with CKD5 where a decision is made not to dialyse? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Conservative kidney management | 4.6 | 3 |
| Conservative management | 46.2 | 30 |
| Conservative care management | 12.3 | 8 |
| Maximum conservative management | 3.1 | 2 |
| Non-dialysis care | 3.1 | 2 |
| Supportive care | 7.7 | 5 |
| Palliative care | 0.0 | 0 |
| Other (please specify) | 23.1 | 15 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 1.1.3. Do all consultant nephrologists follow the same practice regarding patients with CKD5 where a decision is made not to dialyse? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 77.3 | 51 |
| No | 22.7 | 15 |
| Answered question | 66 | |
| Skipped question | 1 | |
| 1.1.3.1. How much do they differ? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Slightly | 66.7 | 10 |
| Moderately | 33.3 | 5 |
| Greatly | 0.0 | 0 |
| Other (please specify how) | 0.0 | 0 |
| Answered question | 15 | |
| Skipped question | 52 | |
| 1.1.3.2. How do they differ? | |
|---|---|
| Answer options | Response count |
| 4 | |
| Answered question | 4 |
| Skipped question | 63 |
2. The development and implementation of conservative care in your unit
| 2.1. Is there a written guideline for how to manage patients on conservative care (other than a palliative care/symptom control guideline)? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 34.8 | 23 |
| No, but in preparation | 27.3 | 18 |
| No | 37.9 | 25 |
| Answered question | 66 | |
| Skipped question | 1 | |
| 2.1.1. Which staff member(s) predominantly led the development of this policy? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Consultant nephrologist | 70.0 | 28 |
| Consultant in palliative care | 37.5 | 15 |
| Renal nurse | 75.0 | 30 |
| Palliative care nurse within the renal unit | 15.0 | 6 |
| Palliative care nurse from community team/other hospital department | 12.5 | 5 |
| Other (please specify) | 17.5 | 7 |
| Answered question | 40 | |
| Skipped question | 27 | |
| 2.2. Is there a single person or team primarily responsible for conservative care in your unit? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 66.2 | 43 |
| No | 33.8 | 22 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 2.2.1. What is their position? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Consultant nephrologist(s) | 58.1 | 25 |
| Palliative care consultant(s) | 11.6 | 5 |
| Nurse(s) | 79.1 | 34 |
| Other (please specify) | 14.0 | 6 |
| Answered question | 43 | |
| Skipped question | 24 | |
| 2.3. Does your unit provide renal staff with formal training or education regarding conservative care? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 50.0 | 33 |
| No, in preparation | 18.2 | 12 |
| No | 31.8 | 21 |
| Answered question | 66 | |
| Skipped question | 1 | |
| 2.3.1. Approximately what percentage of the following staff members have received the training? | ||||||
|---|---|---|---|---|---|---|
| Answer options | ≤ 25% | 26–50% | 51–75% | 76–100% | N/A | Response count |
| Consultant nephrologists | 9 | 1 | 10 | 10 | 0 | 30 |
| Renal registrars | 7 | 8 | 7 | 5 | 1 | 28 |
| Renal nurses | 5 | 11 | 8 | 7 | 0 | 31 |
| Diabetes nurses | 6 | 0 | 0 | 1 | 19 | 26 |
| Social workers | 6 | 3 | 3 | 7 | 9 | 28 |
| Occupational therapists | 6 | 0 | 0 | 2 | 19 | 27 |
| Physiotherapists | 7 | 0 | 1 | 1 | 18 | 27 |
| Dietitians | 5 | 3 | 5 | 6 | 8 | 27 |
| Pharmacists | 6 | 3 | 1 | 6 | 10 | 26 |
| Psychologists | 2 | 2 | 3 | 11 | 10 | 28 |
| Pre-dialysis education providers | 3 | 1 | 2 | 22 | 2 | 30 |
| Anaemia nurses | 3 | 2 | 2 | 15 | 8 | 30 |
| Vascular access coordinators | 8 | 1 | 2 | 5 | 13 | 29 |
| Counsellors | 3 | 0 | 2 | 8 | 14 | 27 |
| Management/administrative staff | 10 | 0 | 0 | 2 | 15 | 27 |
| Other | 1 | 0 | 1 | 3 | 5 | 10 |
| (Please specify and indicate percentage) | 5 | |||||
| Answered question | 33 | |||||
| Skipped question | 34 | |||||
| 2.3.2. Why is formal training or education regarding conservative care not provided for your staff? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Lack of funding | 38.1 | 8 |
| Lack of time | 52.4 | 11 |
| Lack of appropriate person to organise the training | 23.8 | 5 |
| Consultants’ lack of interest in the training | 0.0 | 0 |
| Clinical director’s lack of interest in the training | 0.0 | 0 |
| Other staff members’ lack of interest in the training | 0.0 | 0 |
| We do not need formal training as conservative care is an ingrained culture in the unit | 23.8 | 5 |
| Other (please specify) | 42.9 | 9 |
| Answered question | 21 | |
| Skipped question | 46 | |
| 2.4. How did each of the factors listed below influence the development of the conservative care programme in your unit? Please indicate if each of the factors below positively or negatively influenced the development of the conservative care programme | ||||
|---|---|---|---|---|
| Answer options | Positively influenced | Negatively influenced | No effect | Response count |
| Frequency of late referrals | 12 | 6 | 43 | 61 |
| Nephrologists’ attitudes towards conservative care | 51 | 5 | 8 | 64 |
| Nurses’ attitudes towards conservative care | 57 | 1 | 6 | 64 |
| Other unit staff’s attitudes towards conservative care | 38 | 2 | 22 | 62 |
| Patient/family/carers’ attitudes towards conservative care | 45 | 3 | 17 | 65 |
| Attitudes of people from different ethnicity/culture towards conservative care | 14 | 5 | 44 | 63 |
| Availability of staff experienced in conservative care | 33 | 9 | 21 | 63 |
| Availability of funding specifically for conservative care | 14 | 15 | 35 | 64 |
| Payment by Results tariff for dialysis | 1 | 2 | 59 | 62 |
| Other | 0 | 2 | 11 | 13 |
| (Please specify) | 6 | |||
| Answered question | 65 | |||
| Skipped question | 2 | |||
| 2.5. In calendar year 2012, approximately how many CKD5 patients aged 75 and over were cared for by your renal service? (Please exclude patients with a failing kidney transplant) | |||
|---|---|---|---|
| Answer options | Response average | Response total | Response count |
| Please enter number | 65 | ||
| Answered question | 65 | ||
| Skipped question | 2 | ||
| 2.5.1. Of those, how many were on conservative care and followed up in your unit? If you don’t know the number, please answer the next question instead | |||
|---|---|---|---|
| Answer options | Response average | Response total | Response count |
| Please enter number | 35 | ||
| Answered question | 35 | ||
| Skipped question | 32 | ||
| 2.5.2. Of those, approximately what % were on conservative care and followed up in your unit? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| 0% | 0.0 | 0 |
| 1–9% | 15.2 | 7 |
| 10–19% | 17.4 | 8 |
| 20–29% | 17.4 | 8 |
| 30–39% | 4.3 | 2 |
| 40–49% | 10.9 | 5 |
| 50–59% | 8.7 | 4 |
| 60–69% | 0.0 | 0 |
| 70–79% | 2.2 | 1 |
| 80–89% | 6.5 | 3 |
| 90–99% | 2.2 | 1 |
| 100% | 4.3 | 2 |
| Don’t know, please tell us why not | 10.9 | 5 |
| Answered question | 46 | |
| Skipped question | 21 | |
| 2.6. In 2012, how many patients aged 75 and over in your unit chose to have conservative care, became symptomatic of advanced CKD and did not have dialysis? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Please enter number | 41 | |
| If you don’t know, please tell us why not | 26 | |
| Answered question | 65 | |
| Skipped question | 3 | |
| 2.7. Does your unit have staff whose time is specifically allocated for CKD5 patients on conservative care? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 45.2 | 28 |
| No | 54.8 | 37 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 2.7.1. How much time do the following staff have specifically allocated for CKD5 patients on conservative care? Please enter number of full-time equivalent (FTE) hours for each discipline. (e.g. If you have two nurses with 0.5 FTE, enter 1.0) | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Consultant nephrologists | 52 | 12 |
| Renal registrars | 12 | 3 |
| Renal nurses | 64 | 16 |
| Diabetes nurses | 0 | 0 |
| Social workers | 32 | 7 |
| Occupational therapists | 12 | 2 |
| Dietitians | 40 | 9 |
| Pharmacists | 16 | 3 |
| Psychologists | 24 | 5 |
| Pre-dialysis education providers | 40 | 9 |
| Anaemia nurses | 4 | 6 |
| Vascular access coordinators | 0 | 0 |
| Counsellors | 12 | 2 |
| Management/administrative staff | 8 | 2 |
| Other (please specify and enter number of FTE hours) | 12 | 3 |
| Answered question | 25 | |
| Skipped question | 42 | |
| 2.8. Do you have clinics exclusively for CKD5 conservative care patients? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 23.1 | 15 |
| No | 76.9 | 50 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 2.8.1. How often do you run conservative care clinics in your renal unit and outside the main renal unit? Please tick one for each row | ||||||
|---|---|---|---|---|---|---|
| Answer options | Once a week | Once a fortnight | Once a month | Other | N/A | Response count |
| In your renal unit | 7 | 1 | 3 | 2 | 1 | 14 |
| Outside the main renal unit | 0 | 2 | 3 | 1 | 6 | 12 |
| If other is chosen please give details | 5 | |||||
| Answered question | 15 | |||||
| Skipped question | 52 | |||||
| 2.8.2 Where are CKD 5 patients receiving conservative care most commonly seen or followed up by clinical staff? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| In a general nephrology clinic | 22.4 | 11 |
| In a pre-dialysis clinic/low clearance clinic | 44.9 | 22 |
| In own home by renal team | 6.1 | 3 |
| In own home by GP/community team | 8.2 | 4 |
| At GP surgery | 0.0 | 0 |
| Telephone clinics run by renal unit | 0.0 | 0 |
| Other (please specify) | 18.4 | 9 |
| Answered question | 49 | |
| Skipped question | 18 | |
| 2.9. How often are your CKD5 conservative care patients most commonly seen? Please tick one for each row | ||||||
|---|---|---|---|---|---|---|
| Answer options | Weekly | Monthly | 3-monthly | 6-monthly | Other | Response count |
| Symptomatic patients | 4 | 34 (56%) | 10 | 0 | 13 | 61 |
| Asymptomatic patients | 0 | 2 | 43 (69%) | 8 | 9 | 62 |
| If other is chosen please give details | 20 | |||||
| Answered question | 63 | |||||
| Skipped question | 4 | |||||
| 2.10. What are the key components of conservative care provided to patients in your renal service? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Clinic consultations | 93.8 | 61 |
| Blood results review | 90.8 | 59 |
| The provision of EPO (erythropoietin) and iron therapy | 100.0 | 65 |
| Symptom assessment and management | 100.0 | 65 |
| Prescription of medication for renal symptoms (fluid retention, itching, etc.) | 96.9 | 63 |
| Telephone support for patients | 87.7 | 57 |
| Telephone support for carers | 78.5 | 51 |
| Home visits by renal staff | 55.4 | 36 |
| Dietary advice | 98.5 | 64 |
| Social circumstances review by social workers attached to the renal unit or hospital | 63.1 | 41 |
| Advice on home environment by occupational therapist attached to the renal unit or hospital | 26.2 | 17 |
| Advanced care planning | 76.9 | 50 |
| Communication with primary care team for Gold Standards Framework approach | 80.0 | 52 |
| Psychological support | 58.5 | 38 |
| Other (please specify) | 10.8 | 7 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 2.11. Do you have any funding dedicated to providing conservative care in your renal service? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 15.4 | 10 |
| No | 84.6 | 55 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 2.11.1. Is the funding part of routine NHS income or from non-NHS sources? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Routine NHS income | 70.0 | 7 |
| Non-NHS sources | 10.0 | 1 |
| Both | 20.0 | 2 |
| Answered question | 10 | |
| Skipped question | 57 | |
| 2.11.2. How much annual funding was dedicated to providing conservative care in the 2011/12 financial year (April 2011–March 2012)? Please enter number | ||
|---|---|---|
| Breakdown of the responses regarding funding | ||
| Funding sources | Amount | |
| Routine NHS income | £29,464 | |
| £101,300 | ||
| £69,959 | ||
| £40,000 | ||
| Money for 0.8 WTE nurses | ||
| No response | ||
| No response | ||
| Non-NHS sources | Part of palliative care consultant’s salary | |
| Both | 0.5 band 7 nurse | |
| £3942 | ||
| Statistics | ||
| Overall £ | ||
| n | ||
| Valid | 5 | |
| Missing | 60 | |
| Mean | 48,333 | |
| Median | 40,000 | |
| Standard deviation | 38,050.755 | |
| Range | 97,358 | |
| Minimum | 3942 | |
| Maximum | 101,300 | |
| Percentiles | ||
| 25 | 15,203 | |
| 50 | 40,000 | |
| 75 | 85,629.50 | |
3. Discussing conservative care with patients
| 3.1. In your unit, is the option of conservative care discussed with all CKD5 patients aged 75 years and over? (excluding emergency patients) | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 86 | 56 |
| No | 14 | 9 |
| I don’t know (please tell us why not) | 0 | 0 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 3.1.1. If the option of conservative care is not discussed with all CKD5 patients aged 75 years and over, please tell us how the decision is made whether or not to discuss conservative care with a patient? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Consultant nephrologist in charge of patient decides alone | 33.3 | 3 |
| Consultant nephrologist in charge of patient decides with input from other consultants | 33.3 | 3 |
| Consultant nephrologist in charge of patient decides with input from other professionals during an MSRT meeting | 44.4 | 4 |
| Clinical nurse specialist/consultant nurse in charge of patient decides alone | 0.0 | 0 |
| Clinical nurse specialist/consultant nurse in charge of patient decides with input from consultants | 22.2 | 2 |
| Clinical nurse specialist/consultant nurse in charge of patient decides with input from other professionals during an MSRT meeting | 11.1 | 1 |
| The decision-making is a reactive process during the consultation | 33.3 | 3 |
| Only if patient/carer asks about alternatives to dialysis | 0.0 | 0 |
| Other (please specify) | 0.0 | 0 |
| Answered question | 9 | |
| Skipped question | 57 | |
| 3.2. Which of the following factors are likely to influence staff when contemplating the suitability of conservative care for a patient? Please indicate how strongly each would influence a decision to discuss conservative care with a patient/carer. Please answer on behalf of all staff members | |||||||
|---|---|---|---|---|---|---|---|
| Answer options | Not at all | Very little | Little | Somewhat | Strongly | Very strongly | Response count |
| Response to the ‘surprise’ question | 5 | 7 | 8 | 12 | 23 | 9 | 64 |
| 20 | 12 | 32 | |||||
| Frailty | 0 | 0 | 3 | 4 | 37 | 21 | 65 |
| 3 | 4 | 58 | |||||
| Extent and severity of comorbidities | 0 | 0 | 1 | 3 | 34 | 27 | 65 |
| 1 | 3 | 61 | |||||
| Cognitive status | 0 | 0 | 3 | 14 | 25 | 23 | 65 |
| 3 | 14 | 48 | |||||
| Functional status | 0 | 0 | 3 | 10 | 35 | 17 | 65 |
| 3 | 10 | 52 | |||||
| Uraemic symptoms | 13 | 6 | 16 | 20 | 9 | 1 | 65 |
| 35 | 20 | 10 | |||||
| Rate of decline of kidney function | 14 | 11 | 14 | 16 | 8 | 2 | 65 |
| 39 | 16 | 10 | |||||
| Social support | 10 | 9 | 20 | 20 | 5 | 1 | 65 |
| 39 | 20 | 6 | |||||
| Distance from dialysis unit to home | 21 | 10 | 17 | 14 | 2 | 0 | 64 |
| 49 | 14 | 2 | |||||
| Patient’s current quality of life | 0 | 2 | 2 | 11 | 35 | 15 | 65 |
| 4 | 11 | 50 | |||||
| Patient preference for conservative care | 0 | 0 | 0 | 0 | 18 | 47 | 65 |
| 0 | 0 | 65 | |||||
| Carer preference for conservative care | 4 | 8 | 12 | 28 | 10 | 2 | 64 |
| 24 | 28 | 12 | |||||
| Consultant preference for conservative care | 3 | 4 | 16 | 31 | 9 | 2 | 65 |
| 23 | 31 | 11 | |||||
| Other | 1 | 0 | 0 | 0 | 0 | 2 | 3 |
| (Please specify and rate) | 2 | ||||||
| Answered question | 65 | ||||||
| Skipped question | 2 | ||||||
| 3.3. When is the option of conservative care most commonly first raised with a patient? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| When estimated GFR reaches a certain level | 23.1 | 15 |
| When they are referred to the pre-dialysis/low clearance clinic | 56.9 | 37 |
| When dialysis access needs to be performed | 1.5 | 1 |
| When symptoms start | 0.0 | 0 |
| At a specific time prior to the anticipated start of dialysis | 9.2 | 6 |
| Other (please specify) | 9.2 | 6 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 3.3.1. Please specify estimated GFR | |
|---|---|
| Statistics | |
| eGFR | |
| n | |
| Valid | 15 |
| Missing | 50 |
| Mean | 18.8667 |
| Standard error of mean | 0.52433 |
| Median | 20.0000 |
| Standard deviation | 2.03072 |
| Variance | 4.12400 |
| Minimum | 15.0000 |
| Maximum | 20.0000 |
| Percentiles | |
| 25 | 19.0000 |
| 50 | 20.0000 |
| 75 | 20.0000 |
| 3.3.2. Please specify when (months) | |
|---|---|
| Statistics | |
| Months | |
| n | |
| Valid | 6 |
| Missing | 59 |
| Mean | 8.5000 |
| Standard error of mean | 1.62788 |
| Median | 9.0000 |
| Standard deviation | 3.98748 |
| Variance | 15.900 |
| Minimum | 3.00 |
| Maximum | 12.00 |
| Percentiles | |
| 25 | 5.2500 |
| 50 | 9.0000 |
| 75 | 12.0000 |
| 3.4. How are patients’ family/carers involved in decision making about conservative care? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| They are invited to patient education day | 67.7 | 44 |
| They are encouraged to attend clinics with patient | 95.4 | 62 |
| They are involved in home visits | 64.6 | 42 |
| They are involved when patient is revisited regarding conservative care decision | 76.9 | 50 |
| Other (please specify) | 4.6 | 3 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 3.5. Do any renal staff members use practical tools (see below for examples) when discussing the option of conservative care with a patient? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 83.1 | 54 |
| No | 16.9 | 11 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 3.5.1. What do they use when discussing the option of conservative care with a patient? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Booklets/handouts from national organisation(s) | 81.5 | 44 |
| Booklets/handouts written by own renal unit staff | 61.1 | 33 |
| DVDs from national organisations(s) | 40.7 | 22 |
| NHS Right Care Patient Decision Aid | 29.6 | 16 |
| Other (please specify) | 13.0 | 7 |
| Answered question | 54 | |
| Skipped question | 13 | |
| 3.6. If a decision is made not to have dialysis, where is this information recorded? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Medical notes | 96.9 | 63 |
| Renal database | 90.8 | 59 |
| GP database | 47.7 | 31 |
| Out of hours (ambulance service) database | 16.9 | 11 |
| Other (please specify) | 18.5 | 12 |
| Answered question | 65 | |
| Skipped question | 2 | |
| Breakdown of the responses above (showing all how each database was used in conjunction with others) | ||
| Medical notes and renal database | 33.8 | 22 |
| Medical notes, renal database, and GP database | 24.6 | 16 |
| Medical notes, renal database, and other | 10.8 | 7 |
| Medical notes, renal database, GP database, and out of hours database | 9.2 | 6 |
| Medical notes and GP database | 6.2 | 4 |
| Medical notes, renal database, GP database, out of hours database, and other | 4.6 | 3 |
| Medical notes, renal database, and out of hours database | 3.1 | 2 |
| Medical notes | 3.1 | 2 |
| Renal database and GP database | 1.5 | 1 |
| Medical notes, renal database, GP database, and other | 1.5 | 1 |
| Renal database and other | 1.5 | 1 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 3.7. If a decision is made not to have dialysis, is this decision reviewed at any time? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 100 | 65 |
| No | 0 | 0 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 3.7.1. When is the decision reviewed? | |
|---|---|
| Answer options | Response count |
| 64 | |
| Answered question | 64 |
| Skipped question | 3 |
The table below was made by grouping the text answers to Question 3.7.1.
| Answer options | Response per cent | Response count |
|---|---|---|
| Clinic visit | 67.2 | 43 |
| On patient’s/carer’s request | 14.1 | 9 |
| When patient becomes symptomatic | 6.2 | 4 |
| Others | ||
| Answered question | 64 | |
| Skipped question | 3 | |
| 3.8. Do patients who decide not to have dialysis ever change their mind and start dialysis? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 98.5 | 63 |
| No | 0 | 0 |
| Answered question | 63 | |
| Skipped question | 4 | |
| 3.8.1. How frequently is the change of mind due to the following reasons? Please indicate how frequently each of the reasons listed below cause the change of mind | |||||||
|---|---|---|---|---|---|---|---|
| Answer options | Never | Very rarely | Rarely | Occasionally | Frequently | Very frequently | Response count |
| Because patients change their mind after having had longer to think about their decision | 2 | 9 | 14 | 32 | 5 | 0 | 62 |
| 25 | 32 | 5 | |||||
| Because a patient’s family wants them to have dialysis and a patient agrees | 0 | 9 | 6 | 38 | 8 | 1 | 62 |
| 15 | 38 | 9 | |||||
| Because patients are acutely admitted to hospital and dialysis is started without time for a full discussion between family and clinical team | 6 | 9 | 16 | 21 | 4 | 5 | 61 |
| 31 | 21 | 9 | |||||
| Because patients present unconscious without having recorded their wishes in writing and the family insist on dialysis | 14 | 16 | 19 | 10 | 2 | 0 | 61 |
| 49 | 10 | 2 | |||||
| Because patients have symptoms that cannot be controlled with conservative treatment | 12 | 9 | 12 | 21 | 5 | 2 | 61 |
| 33 | 21 | 7 | |||||
| Other | 1 | 0 | 0 | 4 | 4 | 0 | 9 |
| (Please specify) | 13 | ||||||
| Answered question | 62 | ||||||
| Skipped question | 5 | ||||||
| 3.9. Is vascular access ever created for patients who have opted for conservative care? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 15.4 | 10 |
| No | 84.6 | 55 |
| Answered question | 65 | |
| Akipped question | 2 | |
4. Working with primary care and general practitioners
| 4.1. Once a decision has been made that a patient aged 75 years and over with CKD5 will not have dialysis, how are GPs involved in their care? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Patients are primarily kept under the care of the renal unit with little GP involvement | 11.3 | 7 |
| Patients are referred back to GPs but care of patients is shared between GPs and the renal unit (e.g. patients are seen by GPs who liaise with the renal unit regarding renal symptom control) | 19.4 | 12 |
| Patients are referred back to GPs and cared for under primary care only | 0.0 | 0 |
| Mix of all three as it varies between nephrologists | 14.5 | 9 |
| Mix of all three as it varies by patient/patient preference | 47.7 | 31 |
| Other (please specify) | 9.2 | 6 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 4.2. What is the role of GPs in the management of CKD5 patients receiving conservative care? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| GPs liaise with the renal unit for specialist support | 90.8 | 59 |
| GPs arrange and interpret blood tests | 26.2 | 17 |
| GPs arrange blood tests but liaise with renal unit for their interpretation | 52 | 34 |
| GPs check patients’ medication | 52.3 | 34 |
| GPs regularly (not on demand) assess patients in the GP surgery | 13.8 | 9 |
| GPs regularly (not on demand) assess patients via home visits | 20.0 | 13 |
| GPs/primary care staff provide/organise palliative care support at the end of life | 67.7 | 44 |
| GPs discuss ACP with patients | 26.2 | 17 |
| Other (please specify) | 18.5 | 12 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 4.3. Do you provide GPs and/or their practice team with information or advice regarding the treatment of CKD5 patients receiving conservative care? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 87.7 | 57 |
| No | 12.3 | 8 |
| Answered question | 65 | |
| Skipped question | 3 | |
| 4.3.1. What do you provide to GPs regarding the treatment of CKD5 patients receiving conservative care? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Verbal advice | 80.7 | 46 |
| Written advice/guidelines | 96.5 | 55 |
| Educational meetings | 31.6 | 18 |
| Other (please specify) | 15.8 | 9 |
| Answered question | 57 | |
| Skipped question | 10 | |
| 4.3.2. Please tell us why information/advice regarding conservative care is not provided to GPs and/or their practice team. Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Lack of time | 62.5 | 5 |
| Lack of funding | 25.0 | 2 |
| Opinion of consultants | 12.5 | 1 |
| Opinion of clinical directors | 0.0 | 0 |
| Opinion of other staff members | 0.0 | 0 |
| GPs do not wish to have any information/advice from the renal unit | 0.0 | 0 |
| Other (Please specify) | 37.5 | 3 |
| answered question | 8 | |
| skipped question | 59 | |
| Breakdown of responses to Question 4.3.2 | ||
| Lack of time | 38 | 3 |
| Lack of time and funding | 12 | 1 |
| Lack of time, funding, and opinion of consultants | 12 | 1 |
| Other | 38 | 3 |
| Total | 100 | 8 |
5. End-of-life care
| 5.1. Does your unit have a written guideline for renal end of life care? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 55.4 | 36 |
| No, but in preparation | 16.9 | 11 |
| No | 27.7 | 18 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 5.2. Do you identify conservative care patients approaching end of life through use of a register? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 55.4 | 36 |
| No | 44.6 | 29 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 5.2.1. How likely are the following factors to influence a decision to add a patient to the end of life register? Please indicate how strongly each of the factors listed below influence this decision | |||||||
|---|---|---|---|---|---|---|---|
| Answer options | Not at all | Very little | Little | Somewhat | Strongly | Very strongly | Response count |
| Surprise question | 3 | 1 | 1 | 4 | 15 | 11 | 35 |
| 5 | 4 | 26 | |||||
| Estimated GFR level | 1 | 4 | 1 | 10 | 13 | 6 | 35 |
| 6 | 10 | 19 | |||||
| Measured GFR level | 10 | 7 | 0 | 6 | 4 | 4 | 31 |
| 17 | 6 | 8 | |||||
| Comorbidities | 0 | 1 | 1 | 6 | 19 | 8 | 35 |
| 2 | 6 | 27 | |||||
| Frailty | 0 | 0 | 0 | 4 | 19 | 12 | 35 |
| 0 | 4 | 31 | |||||
| Unexpected weight loss | 0 | 2 | 3 | 8 | 15 | 7 | 35 |
| 5 | 8 | 22 | |||||
| Quality of life | 0 | 1 | 2 | 5 | 19 | 8 | 35 |
| 3 | 5 | 27 | |||||
| Symptoms | 1 | 0 | 0 | 6 | 17 | 11 | 35 |
| 1 | 6 | 28 | |||||
| Frequent hospitalisation | 0 | 0 | 2 | 3 | 15 | 15 | 35 |
| 2 | 3 | 30 | |||||
| Other | 0 | 0 | 0 | 1 | 2 | 2 | 5 |
| (Please specify and rate) | 5 | ||||||
| Answered question | 36 | ||||||
| Skipped question | 31 | ||||||
| 5.3. Is ACP used in end of life care by renal staff? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 78.5 | 51 |
| No | 21.5 | 14 |
| Answered question | 65 | |
| Skipped question | 3 | |
| 5.3.1. Who is involved in advance care planning in your unit? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Consultant nephrologist(s) | 80.4 | 41 |
| Nurse(s) | 94.1 | 48 |
| Palliative care specialist(s) | 49.0 | 25 |
| Social worker(s) | 31.4 | 16 |
| Counsellor(s)/psychologist(s) | 23.5 | 12 |
| Other (please specify) | 13.7 | 7 |
| Answered question | 51 | |
| Skipped question | 16 | |
| Breakdown of responses to Question 5.3.1 | ||
| Consultant nephrologist(s) and nurse(s) | 25 | 13 |
| Consultant nephrologist(s), nurse(s) and palliative care consultant(s) | 16 | 8 |
| Consultant nephrologist(s), nurse(s), palliative care consultant(s), social worker(s) and counsellor(s) | 12 | 6 |
| Consultant nephrologist(s), nurse(s), palliative care consultant(s), and social worker(s) | 6 | 3 |
| Other combinations | 41 | 21 |
| Total | 100 | 51 |
| 5.4. Have any of your staff had any training in palliative/end of life care specifically for renal patients? Please tick one | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes, everyone has | 3.1 | 2 |
| Yes, the majority of the staff have | 10.9 | 7 |
| Yes, about half of the staff have | 14.1 | 9 |
| Yes, but only the small number of the staff have | 60.9 | 39 |
| No | 10.9 | 7 |
| Answered question | 64 | |
| Skipped question | 3 | |
| 5.5. With which services does your unit liaise for patients receiving conservative care approaching end of life? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Specialist palliative care services within the hospital | 90.8 | 59 |
| Specialist palliative care services from local hospice | 78.5 | 51 |
| Specialist palliative care services in the community (e.g. Macmillan nurses) | 84.6 | 55 |
| Primary care team | 89.2 | 58 |
| None | 0.0 | 0 |
| Other (please specify) | 6.2 | 4 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 5.5.1. Where do patients receive these services? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Within the hospital as inpatients | 81.5 | 53 |
| Within the hospital as outpatients | 58.5 | 38 |
| At home | 90.8 | 59 |
| At hospice where patient is admitted at end of life | 84.6 | 55 |
| At GP practice | 50.8 | 33 |
| Other (Please specify) | 4.6 | 3 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 5.5.2. What services do they provide for renal patients receiving conservative care in your unit? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| They help to write guidelines on how to treat patients receiving conservative care | 32.3 | 21 |
| They provide symptom management at the end of life | 93.8 | 61 |
| They support patients at home out of hours | 83.1 | 54 |
| They discuss ACP with patients | 64.6 | 42 |
| Admission to the hospice as required | 80.0 | 52 |
| Other (please specify) | 10.8 | 7 |
| Answered question | 62 | |
| Skipped question | 3 | |
| 5.6. Do you provide palliative care specialists with training or advice regarding the management of renal patients? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 64.6 | 42 |
| No | 35.4 | 23 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 5.6.1. What do you provide? Tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Verbal advice | 83.3 | 35 |
| Written advice/guidelines | 57.1 | 24 |
| Educational meetings | 52.4 | 22 |
| Other (please specify) | 14.3 | 6 |
| Answered question | 42 | |
| Skipped question | 25 | |
6. The evaluation of the provision of conservative care in your unit
| 6.1. Is the quality of conservative care provided in your unit regularly evaluated? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes | 38.5 | 25 |
| No | 61.5 | 40 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 6.1.1. What measures or information do you use? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Symptoms | 76.0 | 19 |
| Survival | 64.0 | 16 |
| Hospitalisation | 56.0 | 14 |
| Quality of life | 64.0 | 16 |
| Carer burden | 24.0 | 6 |
| Place of death | 88.0 | 22 |
| Survey with patients/carers about their experience of conservative care | 36.0 | 9 |
| Other (please specify) | 16.0 | 4 |
| Answered question | 25 | |
| Skipped question | 42 | |
| 6.2. Which factors do you think could help improve the provision of conservative care in your unit? Please indicate how strongly you agree or disagree with each of the following | ||||||
|---|---|---|---|---|---|---|
| Answer options | Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree | Response count |
| Increasing the number of staff dedicated to conservative care | 21 | 19 | 19 | 3 | 2 | 64 |
| 40 | 19 | 5 | ||||
| Increasing the number of times conservative care patients are seen by staff | 5 | 12 | 29 | 14 | 2 | 62 |
| 17 | 29 | 16 | ||||
| Increasing clinic time | 9 | 20 | 24 | 9 | 2 | 64 |
| 29 | 24 | 11 | ||||
| Providing better end of life care by implementing ACP | 13 | 30 | 17 | 3 | 1 | 64 |
| 43 | 17 | 4 | ||||
| Improving computer systems by integrating primary care data with renal data | 24 | 24 | 13 | 2 | 1 | 64 |
| 48 | 13 | 3 | ||||
| Increasing involvement of allied health-care professionals (e.g. social worker) in treatment decision-making | 15 | 30 | 13 | 5 | 1 | 64 |
| 45 | 13 | 6 | ||||
| Increasing communication/involvement with GPs | 16 | 36 | 8 | 2 | 2 | 64 |
| 52 | 8 | 4 | ||||
| Increasing communication/involvement with community teams | 17 | 33 | 11 | 2 | 1 | 64 |
| 50 | 11 | 3 | ||||
| Increasing communication/involvement with other hospitals | 9 | 25 | 24 | 4 | 2 | 64 |
| 34 | 24 | 6 | ||||
| Increasing communication/involvement with palliative care teams | 13 | 36 | 9 | 5 | 1 | 64 |
| 49 | 9 | 6 | ||||
| Providing renal staff members with more education/training regarding conservative care | 22 | 30 | 9 | 2 | 1 | 64 |
| 52 | 9 | 3 | ||||
| Providing GPs with more education/training regarding conservative care | 13 | 38 | 9 | 3 | 1 | 64 |
| 51 | 9 | 4 | ||||
| Providing palliative care teams with more education/training regarding renal conservative care | 10 | 34 | 8 | 10 | 1 | 64 |
| 44 | 8 | 11 | ||||
| Providing patients with better decision aids about conservative care | 10 | 30 | 16 | 7 | 1 | 64 |
| 40 | 16 | 8 | ||||
| More funding to develop conservative care within unit | 21 | 27 | 10 | 5 | 1 | 64 |
| 48 | 10 | 6 | ||||
| Having funding models specifically designed to reimburse the costs of delivering CKM | 24 | 18 | 17 | 2 | 2 | 63 |
| 42 | 17 | 4 | ||||
| Having a written conservative care policy | 15 | 22 | 18 | 7 | 2 | 64 |
| 37 | 18 | 9 | ||||
| Having dedicated conservative care clinics | 9 | 14 | 28 | 9 | 4 | 64 |
| 23 | 28 | 13 | ||||
| Establishing a system for evaluating the provision of conservative care | 12 | 35 | 12 | 4 | 0 | 63 |
| 47 | 12 | 4 | ||||
| Having better evidence of the comparative outcomes between patients who receive conservative care and those who receive dialysis | 22 | 29 | 7 | 3 | 3 | 64 |
| 51 | 7 | 6 | ||||
| Having better evidence of the comparative costs between patients who receive conservative care and those who receive dialysis | 10 | 17 | 20 | 7 | 10 | 64 |
| 27 | 20 | 17 | ||||
| Other | 2 | 0 | 2 | 0 | 1 | 5 |
| (Please specify and rate) | 3 | |||||
| Answered question | 64 | |||||
| Skipped question | 3 | |||||
| 6.3. What, if any, of the following changes are planned in your unit regarding the provision of conservative care? Please tick all that apply | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Increasing the number of staff dedicated to conservative care | 25.4 | 16 |
| Increasing the number of times conservative care patients are seen by staff | 6.3 | 4 |
| Increasing clinic time | 19.0 | 12 |
| Providing better end of life care by implementing ACP | 52.4 | 33 |
| Improving computer systems by integrating primary care data with renal data | 33.3 | 21 |
| Increasing involvement of allied healthcare professionals (i.e. social worker) in treatment decision-making | 22.2 | 14 |
| Increasing communication/involvement with GPs | 50.1 | 32 |
| Increasing communication/involvement with community teams | 38.1 | 24 |
| Increasing communication/involvement with other hospitals | 14.3 | 9 |
| Increasing communication/involvement with palliative care teams | 39.7 | 25 |
| Providing renal staff members with more education/training regarding conservative care | 57.1 | 36 |
| Providing GPs with more education/training regarding conservative care | 30.2 | 19 |
| Providing palliative care teams with more education/training regarding renal conservative care | 17.5 | 11 |
| Providing patients with better decision aids about conservative care | 30.2 | 19 |
| Obtaining funding to develop conservative care | 15.9 | 10 |
| Writing up a conservative care policy | 25.4 | 16 |
| Having dedicated conservative care clinics | 19.1 | 12 |
| Establishing a system for evaluating the provision of conservative care | 33.3 | 21 |
| None planned | 6.3 | 4 |
| Other (please specify) | 12.7 | 8 |
| Answered question | 63 | |
| Skipped question | 4 | |
7. Future research
| 7.1. Would your unit consider it appropriate to enter a patient aged 75 and over with CKD5 into a randomised clinical trial comparing conservative care versus dialysis? (An abstract of the proposed design is provided below) | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes, for some patients | 64.6 | 42 |
| No, never | 35.4 | 23 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 7.1.1. Would your unit be willing to participate in such a trial? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes, definitely | 42.9 | 18 |
| Maybe | 47.6 | 20 |
| No | 2.4 | 1 |
| Other (please specify) | 7.1 | 3 |
| Answered question | 42 | |
| Skipped question | 25 | |
| 7.2. Would your unit consider entering CKD5 patients aged 75 and over into a prospective multicentre observational study to compare conservative care and dialysis, which addresses the major selection bias? (The same abstract shown previously is provided below again) | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes, for some patients | 92.3 | 60 |
| No, never | 7.7 | 5 |
| Answered question | 65 | |
| Skipped question | 2 | |
| 7.2.1. Would your unit be willing to participate in such a study? | ||
|---|---|---|
| Answer options | Response per cent | Response count |
| Yes, definitely | 46.7 | 28 |
| Maybe | 46.7 | 28 |
| No | 0.0 | 0 |
| Other (please specify) | 6.7 | 4 |
| Answered question | 60 | |
| Skipped question | 7 | |
Appendix 7 The specific data required from both laboratory and renal units sources
| Laboratory data required | Renal data required |
|---|---|
| Date of birth Gender NHS number Date of index eGFR (i.e. first of the two eGFRs used to define CKD5) Most recent eGFR and date |
Date of birth Gender NHS number Date referred to renal unit eGFR at date of referral to renal unit Current treatment status (i.e. CKD, CKM, dialysis, transplant, deceased) Date of first dialysis/transplant Date of discharge to GP Date of death Most recent eGFR and date |
Appendix 8 The semistructured interview guide used for general practitioner telephone interviews
Topic guide for general practitioner structured telephone interviews
A. Seeing chronic kidney disease patients in practice
-
Can you tell me what experience you have in managing patients who have chronic kidney disease? And patients who have kidney failure?
-
Can you tell me a little bit about patients with chronic kidney disease stage 5 that you currently look after or that you have looked after most recently?
-
How old? What comorbidities? Seen how often? For what?
-
-
For patients with kidney failure, where are they in terms of management? (e.g. transplant, on dialysis, likely to be on dialysis in the future, conservative care)
-
Can you tell me approximately how many patients you have seen with established kidney failure in the last 6 months?
-
How often would you/your practice tend to see patients with CKD stage 5?
B. Referring chronic kidney disease patients to secondary care
-
How would your practice normally identify patients with chronic kidney disease or kidney failure? What is the most common way kidney disease is identified?
-
If you identified a patient with new CKD5 what action(s) would you/your practice routinely take?
-
Can you tell me about a time when you told someone they had chronic kidney disease? How did you tell them? What words did you use? What about stage 5?
-
What questions do patients have about CKD? What do they think of when they are told?
-
What proportion of the patients, with CKD5, on your practice list get referred to secondary care?
-
What are the reasons for referral?
-
What are the reasons for non-referral?
-
Do you have any guidelines about when to refer? National or local?
-
-
How is referral to secondary care discussed with the patient?
-
How is non-referral to secondary care discussed with the patient?
-
Do you notify the renal unit about patients who are not being referred? How? What is their reaction?
-
If a patient is not referred, how is this recorded in their notes? (e.g. secondary care if admitted to hospital).
C. Managing patients with stage 5 chronic kidney disease
-
What role do you as a GP play in the management of CKD5 patients who are under nephrology?
-
Do you have a systematic approach to following-up patients with chronic kidney disease?
-
What are the components of patient care if they are not under nephrology?
-
How do you feel about CKD5 patients being referred back to primary care if they opt not to have active treatment/opt for conservative treatment?
-
What agencies/health care professions are involved in CKD management/treatment? How were those connections made? GP referral?
-
How are the palliative care needs of CKD5 patients addressed?
-
Do you have any concerns about managing CKD patients? Do you think you need any training in managing CKD? Would you like any training?
-
What, in an ideal world, would you like to see happen with the management of these patients? What role would GPs play? What are the barriers to achieving this?
Conclusion
-
Are there any other relevant issues we haven’t covered that you would like to mention?
-
Are there any questions you that would like to ask me?
Demographic questions
-
Gender.
-
Age.
-
Years in practice.
-
Years in current surgery.
-
Special interests (e.g. kidney disease, palliative care, care of the elderly).
List of abbreviations
- ACP
- advanced care planning
- CKD
- chronic kidney disease
- CKD3
- stage 3 chronic kidney disease
- CKD4
- stage 4 chronic kidney disease
- CKD5
- stage 5 chronic kidney disease
- CKM
- conservative kidney management
- CKMAPPS
- Conservative Kidney Management Assessment of Practice Patterns Study
- eGFR
- estimated glomerular filtration rate
- EPO
- erythropoietin
- ESKF
- end-stage kidney failure
- FTE
- full-time equivalent
- GP
- general practitioner
- HCP
- health-care professional
- HD
- haemodialysis
- IQR
- interquartile range
- IT
- information technology
- KDIGO
- Kidney Disease Improving Global Outcomes
- MSRT
- multiskilled renal team
- NICE
- National Institute for Health and Care Excellence
- PCT
- primary care trust
- QOF
- Quality and Outcomes Framework
- RCT
- randomised clinical trial
- RRT
- renal replacement therapy