Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 09/1006/25. The contractual start date was in April 2011. The final report began editorial review in August 2013 and was accepted for publication in March 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Padmanabhan Badrinath co-ordinates the Clinical Priorities Group in Suffolk. Claire Beynon is a member of South West Commissioning Support. Christine E Hine was a consultant in public health employed by NHS South Gloucestershire and NHS Bristol to provide independent public health advice to health services commissioning during the research period. She was a member of the Bristol, North Somerset and South Gloucestershire Commissioning Advisory Forum. Hayley E Jones reports personal fees from Novartis AG and personal fees from the British Thoracic Society, outside the submitted work. All other authors declare no competing interests.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Hollingworth et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
National pressures on health-care spending
Health-care expenditure in the UK more than doubled from £42.8B (5.4% of gross domestic product) in 1996/7 to £94.7B (7.0% of gross domestic product) in 2006/7. 1 However, health-care spending in the UK NHS and many other high-income countries has stagnated since the economic crisis of 2007. The 5% annual increases in real expenditure that existed before the crisis have been replaced by flat-line funding, which is projected to persist in coming years. 2 This financial constraint is already pushing some NHS budgets to breaking point. 3 These pressures are thought to arise from ever-increasing demands on health services due to population growth, increasing population health needs and rising patient expectations of health care in wealthier societies. Supply-side factors are also clearly important, with factor costs (e.g. staff salaries) and technological progress often cited as drivers of increasing health-care costs. 4 Studies that have tried to unpick the relative contribution of these factors have identified technological change as a predominant cause of increased health-care spending. 5 Therefore, one natural response to the current financial pressures is for health-care policy-makers to revise the methods that they use to evaluate and regulate new and existing health-care technologies.
The policy context
The aftermath of the economic crisis coincided with a major reconfiguration of NHS care in England, as laid out in the 2012 Health and Social Care Act (HSCA). 6 Prior to the Act, local commissioning of most health-care services was the responsibility of 151 primary care trusts (PCTs) across England. One key legislative aim of the 2012 HSCA was to promote clinically led commissioning by abolishing PCTs and transferring most local health service commissioning to 211 newly established Clinical Commissioning Groups (CCGs) supported by 23 commissioning support units (CSUs) before April 2013. 7 CCGs are responsible for approximately £65B (70%) of NHS funding and are required to plan, commission and monitor services such as elective and emergency hospital care, community, and mental health services. 8 NHS England now directly commissions primary care and specialised services while Public Health England and local authorities are responsible for public health services.
Clinical Commissioning Groups must pay for new technologies mandated by the National Institute for Health and Care Excellence (NICE) and find the necessary savings from elsewhere in their budgets. CCGs have a statutory duty to ensure their annual expenditure does not exceed their budget. Primary care doctors [general practitioners (GPs)] play a leading role in the new CCGs; the HSCA envisages that by giving more budgetary responsibility to front-line clinicians it will encourage them to redesign health-care provision more efficiently in their locality. It is too early to judge how CCGs will differ from PCTs in commissioning local health care. However, it is clear that they have taken on the role of commissioning in extremely challenging financial circumstances and that appropriate diffusion of new cost-effective health technologies and discontinuance of existing inefficiently applied health technologies will remain high on their list of priorities. 9
Theories of technology diffusion and discontinuance
Rogers identified seams of diffusion and discontinuance theory in anthropology, sociology, economics, communication and marketing. 10 He argues that discontinuance of inefficient or inappropriately applied technologies will depend on characteristics of the technology (e.g. perceived relative disadvantage), characteristics of individuals who use it (e.g. training and receptiveness to change), systems within which they operate (e.g. financial incentives) and interactions among each component. Rogers distinguishes between replacement discontinuance, which occurs when more efficient technology displaces the existing technology (e.g. radiography replaced by computerised tomography in head trauma) and disenchantment discontinuance, which results when new information indicates that the benefits of the existing technology do not justify the costs or adverse effects (e.g. the decline in tonsillectomy rates in the 20th century).
Discontinuance can be spontaneous or managed (i.e. disinvestment, see Figure 1). Reliance on spontaneous discontinuance will fail if there are imperfections in the market for health care. In particular, imperfect evidence about the costs, effects and safety of existing interventions or lack of communication of this evidence to clinicians and patients may delay optimal discontinuance.
FIGURE 1.
Schema of health-care technology adoption and withdrawal.
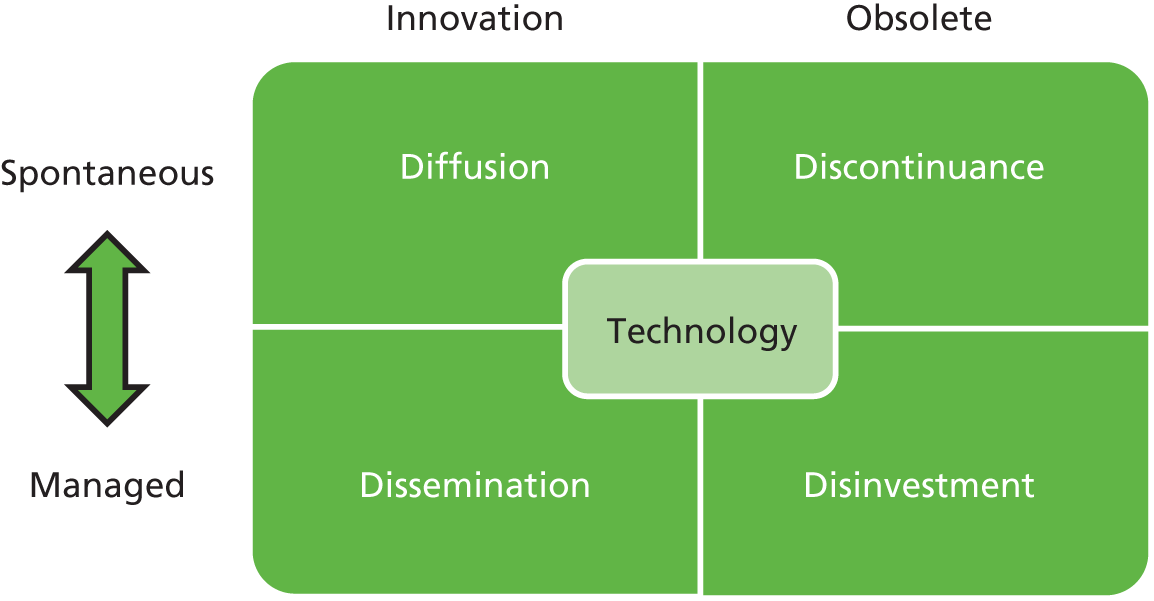
Overview of discontinuance and disinvestment in the health sector
In an extensive systematic review of the diffusion of innovations in health care and other service organisations, Greenhalgh et al. 11 build on theoretical work from 13 multidisciplinary research traditions to build a conceptual model of innovation diffusion and dissemination in health service delivery and organisation. The model depicts the interactions between the innovation characteristics (e.g. relative advantage), system antecedents (e.g. absorptive capacity) and readiness (e.g. innovation–system fit) for innovation, adopter characteristics (e.g. motivation), communication channels (e.g. peer opinion) and the outer context (e.g. incentives and mandates). Where active dissemination or disinvestment is the goal, the linkage (e.g. shared meanings and mission) between the agency promoting change and the target audience is essential to achieve sustained implementation. Greenhalgh et al. 11 note that, in the context of medicine, ‘the evidence base for particular technologies and practices is often ambiguous and contested and must be continually interpreted and reframed in accordance with the local context and priorities, a process that often involves power struggles among various professional groups’. However, they found little empirical work in the service sector on internal politics, such as doctor–manager power balances.
Very little is known about the rate of spontaneous health technology discontinuance, managed disinvestment or factors that facilitate them. The Greenhalgh et al. review included more than 400 studies, but identified only one study that explicitly and prospectively studied discontinuance. 11 Therefore, while there is growing recognition of the importance of disinvestment, there is little theoretical foundation or empirical evidence to inform practice. However, there are good reasons to believe that health-care disinvestment may be considerably more challenging than dissemination.
Elshaug, who has done much to reinvigorate research interest in this area, defines disinvestment in health care as ‘processes of (partially or completely) withdrawing health resources from any existing health-care practices, procedures, technologies or pharmaceuticals that are deemed to deliver little or no health gain for their cost’. 12 By stating that disinvestment may be only partial, this definition acknowledges that existing health-care technologies typically do not suddenly become completely obsolete. Instead this process is more likely to be gradual and incomplete as evidence emerges that a new intervention is more clinically effective and cost-effective for some clinical subgroups. Alternatively, it might reflect growing disenchantment if an existing intervention is revealed to have been overdiffused based on inadequate or outdated evidence. The economic concept of opportunity cost is central to this view of disinvestment. 13 Full or partial disinvestment from inefficient health care in one area of medicine gives health policy-makers the opportunity to spend the money to achieve larger improvements in patients’ health in another area.
In order to be evidence-based, disinvestment in health care will rely on a programme of health technology reassessment (HTR)14 analogous to the technology appraisal process implemented by NICE to evaluate new medicines and treatments in England and Wales. HTR has been defined as ‘A structured, evidence-based assessment of the clinical, social, ethical and economic effects of a technology currently used in the healthcare system, to inform optimal use of that technology in comparison to its alternatives’. 14 Elshaug et al. 15 identified five key challenges to health-care disinvestment: (1) lack of resources to support disinvestment policy mechanisms; (2) lack of methods to identify and prioritise technologies with uncertain cost-effectiveness; (3) political, clinical and social challenges to changing established practice; (4) lack of evidence on the efficiency of many existing technologies; and (5) lack of funding for research into disinvestment methods. This lack of evidence on the cost-effectiveness of existing technologies is an example of what have been described as the first (from knowledge need to discovery) and second (from discovery to clinical application) translation gaps in health-care knowledge. 16 Policy-makers and commissioners have a need for more extensive knowledge about particular clinical subgroups of patients for whom a given intervention is cost-effective and, as importantly, those for whom it is either less cost-effective or not cost-effective at all. However, high-quality evidence is often lacking because randomised controlled trials (RCTs) are scarce and are not large enough to provide robust evidence on subgroups. The third translation gap (from clinical application to action) is also likely to be particularly challenging for disinvestment even when robust evidence is available. Lack of familiarity with the evidence, scepticism about the evidence or its applicability, and external pressures (such as patient expectations or financial and professional rewards for procedure use) are just a few of the powerful influences on clinicians towards inertia rather than disinvestment. 17
The quantitative analyses described in this report are based on the hypothesis that high geographical variation in clinical procedure rates is an indicator of interventions where clinicians are uncertain of the diagnostic threshold or the clinical value in particular patient groups and therefore may be using the procedure inappropriately or inefficiently. As NHS commissioners can easily benchmark procedure rates, this is a potentially valuable method of addressing the second key challenge described by Elshaug et al. The qualitative components of our project explore some of the barriers to disinvestment emphasised by Elshaug et al. ’s third key challenge.
Geographical variation in procedure rates and clinical uncertainty
Glover, comparing pre-1945 tonsillectomy rates, found such high geographical variations that he concluded that it was ‘a prophylactic ritual carried out for no particular reason with no particular result’. 18 Wennberg has developed this into the ‘professional uncertainty hypothesis’. 19 That is the theory that geographical variations occur because of differences among physicians in their diagnostic thresholds or in their belief in the value of the procedure, rather than any differences in clinical need. For example, hip fracture repair, where the diagnosis is clear cut and consensus on the value of surgery is high, has a low geographical coefficient of variation (CV), indicating little variation between regions of the USA. In contrast, for lumbar spine fusion surgery, where there is less agreement on the indications for surgery or the benefit of surgery, the CV is much higher. 20
Geographical variation remains after adjustment for demographic factors and is unlikely to be due to differences in disease prevalence or patient preferences. Small area variations are prevalent in the UK. 21,22 It is thought that variations build up over time as clinicians arriving in a region conform to existing practice patterns, because of local opinion leaders and educational forums that generate enthusiasm (or lack thereof) for a procedure. 23 These local patterns may become entrenched as more hospital diagnostic, specialist and surgical resources are devoted to a particular procedure and may be further exacerbated by hospital reimbursement or surgeon prestige which encourages more intensive care. 23 Bisset24 observed that, as Scottish appendectomy rates declined (from 2.89 per 1000 in 1973 to 1.47 per 1000 in 1993), there was a concurrent decrease in the amount of variation in procedure rates between the 12 health boards. She concluded that the reduced variation ‘supports the view that improved management policies may have helped reduce “professional uncertainty”, unnecessary operations and variation in surgical practice’. Where there is marked practice variation, there is potential for evidence synthesis to identify current gaps in knowledge to guide the national research agenda and to inform local commissioning to standardise current practice around current best evidence.
Structure of the project report
This project was funded by the National Institute for Health Research (NIHR) Service Delivery and Organisation (SDO) programme in response to a call for research on ‘NHS responses to financial pressures’. Specifically, we studied whether or not clinical practice variations can be used by commissioners and clinicians to identify and priorities opportunities for disinvestment in health care. We used six interlinked projects using quantitative and qualitative methods to address a series of related research objectives described in Chapter 2.
At the national level, we assessed whether or not high geographical variation in procedure rates between PCTs is associated with uncertainty about the clinical value of the procedure and therefore might be used by research funders to identify topics where better evidence can lead to more appropriate use of resources (see Chapter 3). At the local level, we worked with two PCTs to evaluate the potential of benchmarking procedure rates against other PCTs to identify procedures that might be overutilised in their area and that were potential candidates for disinvestment (see Chapter 4). In collaboration with both PCTs we selected one high-utilisation procedure to explore if existing evidence could help commissioners work with providers to establish appropriate rates of procedure use, potentially leading to partial disinvestment. In PCT1 we conducted a rapid systematic review of carpal tunnel release (CTR) surgery to synthesise the evidence on the clinical effectiveness and cost-effectiveness of this procedure for patients with carpal tunnel syndrome (CTS) and discuss potential options for the PCT to regulate the procedure rate based on the evidence (see Chapter 5). In PCT2 we conducted a rapid systematic review of laser capsulotomy to synthesise the evidence on the clinical effectiveness and cost-effectiveness of this procedure for patients with posterior capsule opacification (PCO) following cataract surgery (see Chapter 6). We then discuss the probable causes of the high national variation in rates and high local use of this procedure.
In qualitative work we used observations of local commissioning advisory group meetings and semistructured interviews with group members and other stakeholders to understand the facilitators of and/or barriers to disinvestment at the local level (see Chapter 7). We used carpal tunnel surgery as a case study and, through semistructured interviews with patients and surgeons, explored their perspectives on the role of local commissioners in regulating access to surgery in order to regulate procedure rates and contain costs (see Chapter 8). Each of these chapters addresses different aspects of the challenges facing disinvestment. 15 Chapters 3 and 4 evaluate the potential for clinical practice variations to identify and prioritise existing technologies with uncertain cost-effectiveness. Chapters 5 and 6 examine the role of evidence in guiding appropriate use of existing procedures and preventing overutilisation. Chapters 7 and 8 highlight some of the political, clinical and practical challenges to changing established local practice.
In the final chapter we synthesise the main findings of our work and discuss the potential for research funders, commissioners and clinicians to use clinical practice variations and benchmarking to identify and priorities opportunities for disinvestment in health care. We highlight the most important barriers to local commissioners in achieving disinvestment and discuss the implications for the health service and for future research (see Chapter 9).
Chapter 2 Research objectives
The overall aim of this project is to develop and refine the process by which NHS policy-makers identify existing clinical procedures where there is uncertainty about appropriate use and the process by which local commissioners identify procedures that might be over-utilised in their area and are potential candidates for disinvestment.
Our specific objectives are:
-
To use routine inpatient data [Hospital Episode Statistics (HES)] to identify procedures with the highest inter-PCT variation in use. We explore whether or not high inter-PCT variance is a marker of clinical uncertainty about the value of the procedure in some patient subgroups or in some settings (see Chapter 3).
-
To work with two PCT commissioning groups to use benchmarking against the national average procedure rate to select two procedures that might be overutilised by their local NHS trusts (see Chapter 4).
-
To conduct rapid systematic reviews and assemble national and local guidelines for these two procedures to summarise the current evidence on clinical effectiveness and cost-effectiveness. In the light of these technology appraisals, we discuss the likely causes of high local utilisation and the options available to commissioners to regulate local procedure rates to achieve disinvestment (see Chapters 5 and 6).
-
To use qualitative research methods to understand obstacles and solutions to local commissioners achieving evidence-based disinvestment (see Chapter 7) and to explore patient and surgeon perspectives on regulating access to care (see Chapter 8).
Over the course of the project our objectives have evolved to some extent. In part this has been because of the fallout from the large-scale reconfiguration of the NHS and, in particular, PCTs that took place during our project. One of our original objectives had been to evaluate the effectiveness of existing PCT commissioning criteria in reducing the volume of procedures of uncertain value. However, our freedom of information requests to PCTs to access historical threshold policies resulted in insufficient detail about threshold policies and in particular the dates when they were introduced and modified. Previous work25 has found that PCTs have lower response rates to freedom of information requests than other health-care agencies and our complex request coincided with a period of huge upheaval due to the introduction of the HSCA. We therefore decided to drop this original objective in favour of a more detailed exploration of the association between geographical variation and clinical uncertainty described in Chapter 3.
The original objective of the qualitative components of this study was to investigate how two PCT commissioning groups implemented disinvestment from procedures with high local utilisation. However, one of the key findings to emerge from the benchmarking and rapid systematic review process (described in Chapters 4–6) was the dilemma faced by commissioners who are aware of high and unexplained procedure rates locally but lack sufficient evidence to regulate procedure rates through local commissioning policies. In light of this, the revised aim of our qualitative study was to investigate how disinvestment currently works at the local level of health-care commissioning. Specific objectives included investigating the processes underlying local disinvestments, identifying barriers to successful implementation of disinvestment decisions and investigating patients’ and surgeon’s perspectives on disinvestment processes.
Chapter 3 Understanding the causes of national variation in hospital procedure rates
Introduction
In 2011/12 there were over 17 million inpatient and day case episodes in the English NHS,26 costing the tax payer £22.5B. 27 Just under 60% of these episodes involved some form of procedure or intervention. 26 There have been substantial increases in both the number of episodes (92% increase) and the number of episodes involving a procedure (68% increase) since 2001/2. 26 These trends have been accompanied by concerns that some inpatient admissions and procedures are inappropriate or avoidable. 28,29
Current disinvestment initiatives at the national level
The National Institute for Health and Care Excellence was established to provide ‘guidance on the use of new and existing medicines, treatments and procedures’ (emphasis added). 30 In fact, the first technology appraisal published by NICE, on wisdom teeth removal, concluded with a disinvestment message that ‘The practice of prophylactic removal of pathology-free impacted third molars should be discontinued in the NHS’. 31 However, the focus of NICE technology appraisals quickly shifted away from reassessment of existing medical technologies towards appraisals of new interventions, particularly new and expensive pharmaceuticals. By 2006, NICE had published 113 technology appraisals, of which only two (wisdom teeth extraction and electroconvulsive therapy) targeted existing technology rather than innovations.
In 2008, NICE was strongly criticised by the House of Commons Health Committee, which recommended ‘that more be done to encourage disinvestment. No evaluation of older, possibly cost ineffective therapies has taken place to date; . . . it is not acceptable that NICE continues to ignore this recommendation’. 32 Since then, NICE has developed numerous tools to help the NHS respond to efficiency challenges. These include the ‘Cost saving guidance’ and ‘Spending to save’ initiatives, which identify investments in, for instance, optimal prescribing of drugs for hypertension, which are expected to save money through preventing subsequent events (e.g. heart attacks and strokes) and therefore GP and hospital visits. NICE has also developed a number of commissioning guides (e.g. surgical management of otitis media in children) to help PCTs/CCGs commission local services in line with best evidence on clinical effectiveness and cost-effectiveness outlined in NICE clinical guidelines. Of most relevance to disinvestment from existing procedures, NICE has developed a database of more than 850 ‘do not do’ recommendations since 2007. 33 Drawn predominantly from NICE clinical guidelines, technology appraisals and interventional procedures guidance, these recommendations aim to stop premature diffusion or achieve disinvestment from procedures which are not supported by the evidence (e.g. ‘scleral expansion surgery for presbyopia should not be used’). 33 However, these recommendations are derived ad hoc, NICE does not have a formal HTR process for judging the cost-effectiveness of existing medical procedures, analogous to the technology appraisals process for new pharmaceuticals. NICE has tried to launch a more formal HTR programme in 2006 to ‘reduce spending on [existing] treatments that do not improve patient care’. However, this programme faced immediate difficulties, as, ‘in conversations with its stakeholders, NICE has received enthusiastic backing for the idea of appraising existing technologies to seek opportunities for disinvestment; but, when followed by requests for specific suggestions, the subsequent silence has been striking’ (p. 162). 34
The current economic downturn has reignited international interest in how publicly funded health services might manage disinvestment from health care that no longer represents, or perhaps never represented, value for money. 12,35–39 Many countries have emulated the NICE process for evaluating new technologies to ensure that they are safe, clinically effective and cost-effective before diffusion. However, most are struggling to develop any structured process for identifying medical technologies which might be overutilised in patient groups where they offer little benefit. Leggett et al. 14,40 conducted a survey of international HTR initiatives to evaluate the cost-effectiveness of existing non-drug technologies. They concluded that HTR was in its infancy and, although HTRs were being conducted in a few nations, there was no standardised approach.
The HTR process involves the identification and prioritisation of potential candidates for reassessment and potentially disinvestment, a fair and transparent HTR of the evidence that has accumulated over years of use, and robust systems for implementing decisions and monitoring compliance. 14 The process faces challenges at every stage. 15 Unlike innovation, there is rarely a commercial, professional or patient group lobbying for existing practices to be re-evaluated with a view to disinvestment. In fact, hospitals and clinicians often have a vested interest in maintaining the status quo to protect income or prestige. 41 Therefore, the first barrier is to identify technologies where there is clinical uncertainty about appropriate use.
One promising approach is to monitor variations in clinical practice in routinely collected data to help identify where best practice is uncertain and overtreatment may be prevalent. 21,41 Wennberg’s professional uncertainty hypothesis19 postulates that many geographical variations in care are unwarranted and occur because of differences among doctors in their diagnostic thresholds or in their belief in the value of the procedure, rather than any differences in clinical need. This idea has historical precedent. Glover demonstrated 20-fold variations in tonsillectomy utilisation among English boroughs in 1938. 18 In the same decade, a study in the USA (published in the 1940s) found minimal agreement among physicians in judging which children would benefit from tonsillectomy. 42 These initial doubts belatedly led to dramatic declines in tonsillectomy rates on both sides of the Atlantic43 and RCTs to better define the small subgroup of children where tonsillectomy is effective. 44
The interpretation of observed geographical variation in routine data is not straightforward. 21 Variance may be spurious, merely a reflection of random fluctuations in care or inconsistent coding. Furthermore, variation in care may be warranted, caused by differences in clinical need or patient preferences. Despite these considerations, the large and persistent variation brought to light by the publication of documents such as The NHS Atlas of Variation in Healthcare45 and international equivalents46 suggests that some variation reflects more than simple differences in population health need. It is possible that high variation in practice may help policy-makers identify existing health care where HTR is needed and partial disinvestment might be appropriate. In exploring this issue further, we need a better understanding of the causes of observed variation in health care. In this chapter we use routinely collected data on day case and inpatient procedures performed by the NHS in England to quantify the extent of variation across all commonly performed procedures and explore potential causes of ‘high-variance’ procedures.
Methods
Identifying and categorising procedures
We used the HES26 admitted patient care data set to identify inpatient procedures. HES is a routinely collected data set that records all episodes of care provided to all patients (NHS funded and privately insured) admitted, as a day case or inpatient, to NHS hospitals and NHS-funded patients treated in independent sector hospitals. We extracted pseudoanonymised individual episode records on all admissions contained in the HES data set between the financial years 2007/8 and 2011/12. Up to 24 clinical procedures per episode may be recorded using Office of Population, Censuses and Surveys (OPCS) (fourth revision) codes. OPCS codes are hierarchical and include more than 9000 four-character codes defining procedures at the finest level of detail. However, most of these codes are used infrequently, so we elected to define procedures using the three-character OPCS codes (n ≈ 1500).
We focused on the most clinically and economically consequential procedures by including only the 264 most widely used procedure codes, which accounted for over 90% of all inpatient procedures, in our analysis. OPCS codes include some very minor procedures (e.g. blood withdrawal) and diagnostic procedures (e.g. diagnostic echocardiography), which were predominantly not the primary reason for hospital admission. We decided to focus on more major therapeutic procedures, which were thought more likely to be recorded accurately and consistently between hospitals. We excluded diagnostic (n = 53), minor (n =38) and obstetric (n = 5) procedures from our analysis to focus on major therapeutic procedures. We removed a further three procedures because postcode of residence was missing in more than 10% of episodes. We excluded 11 procedures from all analyses because of changes in OPCS procedure codes between years (version 4.4 used in 2007/8 to version 4.6 introduced in 2011/12) which may have led to inconsistent clinical coding. This left 154 therapeutic procedures for analysis. These include procedures that are predominantly elective and those that are more frequently performed as an emergency procedure. A single stay in hospital may comprise more than one episode as patient care is transferred from one consultant to another. Therefore, a hospital stay may contain many procedures, all of which were eligible for inclusion in our analysis. When multiple records of the same procedure were recorded on the same patient with the same admission date, we included only the first record in our analysis in order to avoid the risk of double-counting procedures which were recorded more than once as a result of coding errors. 47
Estimating variation in procedure rates
Geographical variation was measured using PCT boundaries. Until April 2013, PCTs were responsible for commissioning most NHS services for their resident population; they represent geographically contiguous areas of England. In 2007/8 there were 152 (which decreased to 151 in April 2010) PCTs in England; on average each PCT was responsible for approximately 340,000 residents. Since April 2013, PCTs have been replaced by 212 CCGs.
We denote the observed number of utilisations of procedure j on residents of PCT i by ‘Observedij’ (i = 1, . . . , 152 PCTs; j = 1, . . . , 154 procedures). We used a two-stage approach to quantifying variation. In stage 1 we calculated expected numbers of procedure utilisations for procedure j on residents of PCT i based on demographic factors and factors that might affect clinical need for NHS care, denoted by ‘Expectedij’. These expected numbers were calculated using indirect standardisation48 for age and sex (using quinary age groups and gender for England as the standard population49), to account for differences in the size and the age/sex composition of PCT populations. Then Poisson regression was used to further adjust rates for the ethnic50 and socioeconomic composition51 of PCTs, the prevalence of chronic diseases (asthma, atrial fibrillation, coronary heart disease, chronic kidney disease, dementia, diabetes, hypertension, stroke, all-cause cancer),52 and markers of unhealthy lifestyle (binge drinking, smoking and obesity). 52 We also adjusted for the prevalence of private medical care, which might substitute for NHS treatment, based on the number of private hospital beds within a 30-mile radius of the PCT headquarters,53 using mean imputation for the 29% of private hospitals where bed numbers were not recorded.
In stage 2 of our statistical analysis we quantified the residual inter-PCT variability in utilisation rates by using the expected counts as a covariate in random effects Poisson regression models:
The crucial parameter is σ2j, which quantifies the remaining variability in utilisation of procedure j across PCTs, having adjusted for all factors reflected in the expected counts. Importantly, focusing attention on the inter-PCT standard deviation (SD) from a random effects model (or equivalently on a function of it, as we describe in the next paragraph) also appropriately adjusts for chance variability. In what follows, we refer to this parameter as the inter-PCT SD.
Models were fitted within a Bayesian framework using the WinBUGS software (version 1.4.3, MRC Biostatistics Unit, Cambridge, UK),54 which allowed us to estimate the probability of this unexplained variation in utilisation of each procedure exceeding a given threshold. We estimated the probability that a procedure was ‘very highly’ or ‘highly’ variable, which we arbitrarily defined as an inter-PCT SD greater than three or two times the median variability across all therapeutic procedures respectively. To improve interpretability, we transformed each model-based inter-PCT SD estimate into a ‘utilisation ratio’ (UR), which we defined as the rate in a high-utilisation PCT (at the 90th centile of the random effects distribution) divided by the rate in a low-utilisation PCT (at the 10th centile).
Estimating temporal changes in geographical variation and exploring factors associated with high variation
If variation is a proxy for clinical uncertainty about the value of a procedure, then temporal growth in variation could be an indicator of procedures where evidence is evolving, creating either local enthusiasm or disillusionment about the value of a procedure. In order to explore this possibility, for each procedure we performed a linear regression of the log of the estimated inter-PCT SDs on year, to quantify the change in inter-PCT variation between 2007/8 and 2011/12. The log transformation was applied to the SDs because of the large positive skew in the distribution and unequal variation in the error terms. We also conducted exploratory analyses to examine five factors that might be associated with high geographical variation in hospital procedure rates: (1) coding uncertainty; (2) variation in the quality of community care; (3) uncertainty about the appropriate setting for the procedure; (4) urgency and invasiveness of the procedure; and (5) evolving or uncertain evidence. The eight variables selected as potential proxies for these five factors, their definition and our rationale for their potential association with high inter-PCT variation are documented in Tables 1 and 2. We conducted a multivariable linear regression of the log of the inter-PCT SD for each of the 154 procedures on these eight variables.
| Variables used in the multivariate regression | Variable definition | Rationale |
|---|---|---|
| Coding uncertainty | ||
| Miscellaneous procedure code | Catch-all OPCS codes (e.g. other operations on the mouth) | Variation may be higher among miscellaneous procedure codes because of coder uncertainty about when to use them |
| Variation in community care | ||
| Procedure often performed in elderly patients | % of patients receiving the procedure who are ≥ 70 years old | Variation may be higher among procedures commonly used in elderly or chronically ill patients because of variations in community care that might have prevented the need for the procedure |
| Procedure often performed in patients with chronic disease | % of patients receiving the procedure who have chronic disease (see Table 2) recorded in the episode record | |
| Uncertainty about the setting of care | ||
| Procedures that could be performed in the outpatient setting | Procedures where ≥ 10% are performed in the hospital outpatient departmenta | Variation in admitted patient procedure rates may be high if some hospitals have switched to providing the procedure in an outpatient setting (and therefore it is not included in the admitted patient care data set that we analyse) |
| Urgency and invasiveness of the procedure | ||
| Less invasive procedures | Median length of stay for episodes where the procedure was performed | Variation may be higher in less invasive procedures, where there is less potential harm for the patient and therefore may be more leeway for clinical discretion about the need for the procedure |
| Emergency procedures | % of patients receiving the procedure classed as emergency rather than admitted from a waiting list | Variation may be lower in predominantly emergency procedures, where there may be less leeway for clinical discretion about the need for the procedure |
| Evolving or uncertain evidence | ||
| Procedures with rapidly increasing or decline utilisation | Procedures with ≥ 3% or ≤ –3% growth since 2007/8 | Variation may be higher in procedures with rapid diffusion or discontinuance, where uncertainty about appropriate use exists |
| Procedures with one or more substitute procedure codes | Procedure dyads (or triads, etc.) within the same OPCS chapter that have an intra-PCT correlation of ≤ –0.15b | Variation may be higher if two (or more) procedure codes are substitutes for each other (e.g. hip replacement with cement, hip replacement without cement) and there is uncertainty about which procedure is preferable |
| Condition | ICD-10 codes included (three character) |
|---|---|
| Acquired immunodeficiency syndrome/human immunodeficiency virus | B20, B21, B22, B23, B24 |
| Arthritis | M0X, MX1, M20, M21, M22, M23, M24, M25 |
| Asthma | J45 |
| Cancer | CXX |
| Cerebral infarction | I6X |
| Cerebrovascular disease | I6X |
| Chronic liver disease | K70, K73, K74 |
| Chronic obstructive pulmonary disease | J40, J41, J42, J43, J44, J47 |
| Diabetes | E10, E11, E12, E13, E14 |
| Heart failure | I50 |
| Ischaemic heart disease | I20, I21, I22, I23, I24, I25 |
Results
During the analysis period there were 17.8 million episodes which contained one or more of the 154 therapeutic procedures (for a total of 20.6 million procedures) included in our analysis. In 2011/12 this ranged from prosthesis of lens (1.6 million procedures) to other total prosthetic replacement of knee joint (5560 procedures). The degree of inter-PCT variation in procedure rates differed vastly from procedure to procedure. The median estimated UR among the five procedures with the highest inter-PCT variance was 13.0, indicating that the procedure rate was 13 times higher in the PCT at the 90th percentile than the PCT at the 10th percentile (Figure 2). In contrast the median estimated UR among the five procedures with least variation was 1.3. High variation is not solely driven by a small number of PCT outliers but, instead, reflects a spread across all PCTs.
FIGURE 2.
Comparison of high, median and low variance procedures in 2011/12. (a) Graph displaying the median UR within the group of five procedures with the highest, median and lowest intra-PCT SD; (b) key of procedure label.
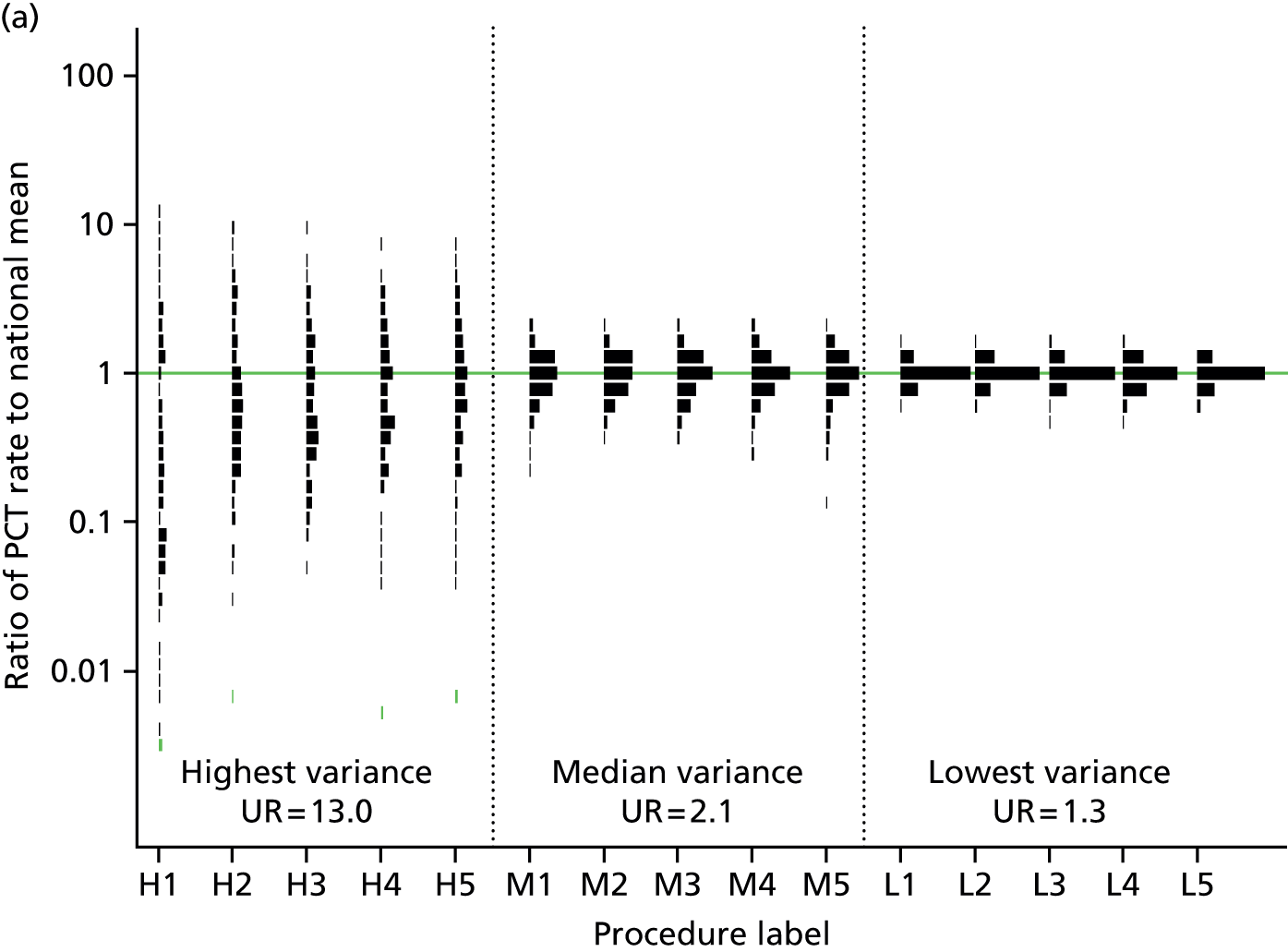
Further analysis of the 20 procedures with the highest estimated inter-PCT variability (Table 3) demonstrates that they represent a range of clinical specialties and include both relatively uncommon (e.g. denervation of spinal facet joint) and common (e.g. destruction of lesion of retina) and both minor (e.g. excision of vas deferens) and major (e.g. hip replacement) procedures. Many of the procedures with highest variance were those which could be performed in the outpatient setting (e.g. incision of capsule of lens) or procedures which were potential substitutes for one another (e.g. transluminal versus combined varicose vein procedures). A full listing of all procedures is provided in Appendix 1.
| Procedure | Number of procedures | Inter-PCT SD (95% CI) | Inter-PCT SD, age/sex (95% CI) | UR (95% CI) | Probability ‘highly variable’ | Probability ‘very highly variable’ |
|---|---|---|---|---|---|---|
| Incision of capsule of lens | 15,131 | 1.8 (1.6 to 2.0) | 1.9 (1.7 to 2.1) | 109.1 (59.8 to 196.1) | 1.00 | 1.00 |
| Neurostimulation of peripheral nerve | 7983 | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.2) | 16.7 (12.0 to 23.2) | 1.00 | 1.00 |
| Curettage of lesion of skin | 13,046 | 1.0 (0.9 to 1.1) | 1.1 (1.0 to 1.3) | 13.0 (9.6 to 17.7) | 1.00 | 0.99 |
| Excision of vas deferens | 10,192 | 0.9 (0.8 to 1.1) | 1.2 (1.0 to 1.3) | 12.1 (8.9 to 16.7) | 1.00 | 0.96 |
| Hybrid prosthetic replacement of hip joint using cemented femoral component | 7882 | 0.9 (0.8 to 1.1) | 1.2 (1.0 to 1.3) | 11.3 (8.3 to 15.5) | 1.00 | 0.91 |
| Transluminal operations on varicose vein of leg | 10,262 | 0.9 (0.8 to 1.0) | 1.2 (1.1 to 1.4) | 10.7 (7.9 to 14.7) | 1.00 | 0.82 |
| Prosthetic replacement of head of femur not using cement | 6287 | 0.9 (0.8 to 1.0) | 1.0 (0.9 to 1.1) | 9.7 (7.2 to 13.3) | 1.00 | 0.61 |
| Denervation of spinal facet joint of vertebra | 10,168 | 0.9 (0.8 to 1.0) | 1.0 (0.9 to 1.1) | 9.4 (7.2 to 12.6) | 1.00 | 0.56 |
| Restoration of tooth | 14,720 | 0.8 (0.7 to 1.0) | 1.1 (1.0 to 1.3) | 8.9 (6.9 to 11.8) | 1.00 | 0.39 |
| Destruction of lesion of retina | 23,007 | 0.8 (0.7 to 0.9) | 0.9 (0.8 to 1.0) | 8.1 (6.4 to 10.5) | 1.00 | 0.17 |
| Other vaginal operations on uterus | 13,256 | 0.7 (0.6 to 0.8) | 0.9 (0.8 to 1.1) | 6.6 (5.2 to 8.4) | 1.00 | 0.01 |
| Combined operations on varicose vein of leg | 10,269 | 0.7 (0.6 to 0.8) | 0.8 (0.7 to 1.0) | 6.2 (5.0 to 7.8) | 1.00 | 0.00 |
| Operations on vitreous body | 101,606 | 0.7 (0.6 to 0.8) | 0.8 (0.7 to 0.9) | 5.8 (4.8 to 7.2) | 1.00 | 0.00 |
| Excision of cervix uteri | 15,886 | 0.7 (0.6 to 0.7) | 0.7 (0.6 to 0.8) | 5.6 (4.6 to 6.9) | 1.00 | 0.00 |
| Intramuscular injection | 28,293 | 0.7 (0.6 to 0.7) | 0.7 (0.7 to 0.8) | 5.7 (4.7 to 6.9) | 1.00 | 0.00 |
| Other operations on bladder | 64,605 | 0.7 (0.6 to 0.7) | 0.7 (0.6 to 0.8) | 5.3 (4.4 to 6.5) | 0.98 | 0.00 |
| Other excision of appendix | 14,360 | 0.6 (0.6 to 0.7) | 0.6 (0.6 to 0.7) | 5.1 (4.2 to 6.4) | 0.94 | 0.00 |
| Other operations on urethra | 9672 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 5.0 (4.2 to 6.1) | 0.91 | 0.00 |
| Destruction of haemorrhoid | 27,387 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 4.7 (4.0 to 5.8) | 0.79 | 0.00 |
| Other operations on sympathetic nerve | 6978 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 4.8 (3.9 to 5.9) | 0.79 | 0.00 |
Six procedures (incision of capsule of lens, neurostimulation of peripheral nerve, curettage of skin lesion, excision of vas deferens, hybrid hip replacement and transluminal operations on varicose veins) had estimated URs in excess of 10. These procedures all have a probability of 1 of meeting our definition of a ‘high variance’ procedure, and a probability of at least 0.82 of being ‘very high variance’. Sixteen procedures had a greater than 95% probability of being ‘high variance’ procedures according to our definition (see Appendix 1). Estimates of procedure variation were similar between the age- and sex-adjusted model and the model adjusted for all markers of clinical need (see Table 3).
Among all 154 procedures included in the variation trend analysis, the mean annual change in the variation between 2007/8 and 2011/12 was –2.3% [95% confidential interval (CI) –3.7% to –1.8%], indicating that variation in utilisation of procedures decreased on average during the study period. Substantial increases (Table 4) and decreases (Table 5) in geographical variation were observed for some procedures. Procedures with increasing geographical variability included those where utilisation was generally declining (e.g. combined operations on varicose vein) and those with more stable (e.g. hip replacement using cement) and increasing (operations on vitreous body) trends in use.
| Procedure | Number of procedures | Inter-PCT SD | Estimated annual % inter-PCT SD increase (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 2007/8 | 2009/10 | 2011/12 | 2007/8 | 2009/10 | 2011/12 | ||
| Operations on vitreous body | 35,076 | 69,058 | 101,606 | 0.333 | 0.619 | 0.693 | 14.2 (8.5 to 19.5) |
| Combined operations on varicose vein of leg | 20,081 | 15,486 | 10,269 | 0.567 | 0.592 | 0.699 | 4.2 (0.1 to 8.0) |
| Repair of recurrent inguinal hernia | 6066 | 5893 | 6221 | 0.128 | 0.165 | 0.176 | 3.5 (–2.0 to 8.9) |
| Combined operations on muscles of eye | 6834 | 6721 | 7082 | 0.205 | 0.244 | 0.270 | 3.4 (–1.6 to 7.6) |
| Other total prosthetic replacement of hip joint | 10,633 | 10,492 | 9830 | 0.349 | 0.406 | 0.416 | 3.3 (–0.3 to 7.0) |
| Drainage through perineal region | 12,046 | 12,431 | 12,656 | 0.044 | 0.050 | 0.098 | 3.1 (–2.2 to 9.0) |
| Drainage of middle ear | 45,760 | 45,448 | 40,680 | 0.203 | 0.188 | 0.244 | 2.7 (–1.5 to 6.6) |
| Total prosthetic replacement of hip joint using cement | 33,182 | 29,167 | 32,020 | 0.370 | 0.441 | 0.422 | 1.9 (–3.8 to 5.5) |
| Release of entrapment of peripheral nerve at wrist | 56,037 | 58,150 | 54,093 | 0.249 | 0.223 | 0.298 | 1.7 (–1.8 to 4.8) |
| Primary repair of umbilical hernia | 21,547 | 22,129 | 24,529 | 0.131 | 0.116 | 0.149 | 1.7 (–3.4 to 7.5) |
| Procedure | Number of procedures | Inter-PCT SD | Estimated annual % inter-PCT SD increase (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 2007/8 | 2009/10 | 2011/12 | 2007/8 | 2009/10 | 2011/12 | ||
| Clearance of external auditory canal | 8116 | 6787 | 7406 | 0.732 | 0.365 | 0.379 | –14.6 (–17.6 to –10.7) |
| Endoscopic operations to increase capacity of bladder | 3472 | 4629 | 10,946 | 0.729 | 0.597 | 0.463 | –9.8 (–13.4 to –5.8) |
| Debridement and irrigation of joint | 10,999 | 16,141 | 16,835 | 0.545 | 0.493 | 0.374 | –8.8 (–12.5 to –5.2) |
| Transluminal operations on varicose vein of leg | 4609 | 10,347 | 10,262 | 1.553 | 1.147 | 0.889 | –8.7 (–12.2 to –3.8) |
| Intramuscular injection | 28,695 | 26,860 | 28,293 | 1.034 | 0.919 | 0.664 | –8.4 (–12.1 to –5.3) |
| Orthodontic operations | 7518 | 8020 | 8649 | 0.343 | 0.212 | 0.228 | –8.4 (–12.6 to –4.0) |
| Cardioverter defibrillator introduced through the vein | 4621 | 6933 | 8443 | 0.332 | 0.254 | 0.201 | –8.0 (–13.0 to –3.1) |
| Percutaneous transluminal balloon angioplasty and insertion of stent into coronary artery | 53,173 | 60,340 | 65,673 | 0.237 | 0.184 | 0.173 | –7.8 (–11.2 to –4.7) |
| Extracapsular extraction of lens | 305,669 | 330,161 | 324,345 | 0.207 | 0.135 | 0.153 | –7.1 (–10.3 to –3.8) |
| Excision of lung | 4505 | 5621 | 6815 | 0.258 | 0.201 | 0.176 | –7.1 (–11.3 to –2.6) |
The multivariable analysis (Table 6) provided strong evidence of a U-shaped relationship between procedure diffusion/discontinuance and geographical variation in use. Variation in PCT procedure rates was high where the diffusion or discontinuance of a procedure was most rapidly evolving. Variation in PCT procedure rates was also higher for procedures where alternative procedures were available. We also found evidence that variation was higher for procedures which were predominantly performed in elderly patients, had a length of stay less than 1 day, were more likely to be elective and could be performed in an outpatient setting.
| Variable | Average ratio of inter-PCT SD (95% CI) | p-value |
|---|---|---|
| Miscellaneous code | ||
| No | 1.000 | |
| Yes | 1.006 (0.891 to 1.137) | 0.443 |
| Elderly patientsa | ||
| < 50% | 1.000 | |
| > 50% | 1.320 (1.016 to 1.586) | 0.012 |
| Patients with chronic disease | 1.000 (0.998 to 1.002) | 0.500 |
| Outpatient procedureb | ||
| No | 1.000 | |
| Yes | 1.454 (1.260 to 1.645) | < 0.001 |
| Median length of stay | ||
| ≥ 1 day | 1.000 | |
| < 1 day | 1.430 (1.247 to 1.595) | < 0.001 |
| % elective admission | 1.002 (1.000 to 1.004) | 0.008 |
| Relative increase since 2007 | ||
| < –3% decrease | 1.570 (1.125 to 2.085) | < 0.001 |
| –3% to 3% increase | 1.000 | |
| > 3% increase | 1.390 (1.274 to 1.499) | < 0.001 |
| Substitute | ||
| No | 1.000 | |
| Yes | 1.881 (1.534 to 2.390) | < 0.001 |
Discussion
Main results
There is a high degree of geographical variation in procedure rates for many commonly performed procedures that cannot be explained by proxies of clinical need. For six procedures, rates in PCTs at the upper and lower deciles still differed by more than 10-fold, after adjustments for chance variation and proxies of clinical need. Variation was most pronounced in procedures where utilisation was increasing or decreasing most rapidly and those where a substitute procedure was available. Policy-makers could use geographical variation as a starting point to identify procedures where HTR or new RCTs might be needed to inform investment and disinvestment decisions.
Strength and weaknesses
The main strengths of this study lie in the large sample, novelty of the research question and breadth of procedures considered. Our model-based approach accounts appropriately for statistical chance, and the UR provides a simple summary measure of disparity between PCTs. In order to make valid comparisons of procedure rates between PCTs we have adjusted for a number of indicators of clinical need. However, bias may still occur because of unmeasured aspects of clinical need or if the accuracy of demographic and morbidity measures varies by PCT. In particular, local variations in the quality of community and primary health services could plausibly cause variation in procedure rates for ambulatory care sensitive conditions. 28 Future work could use proxies for ambulatory care quality such as community health service expenditure or the primary care Quality and Outcomes Framework to assess their association with procedure rates. We used the number of private hospital beds as a proxy measure for the number of private hospital procedures which may be inappropriate, particularly for some conditions (e.g. excision of appendix), where private treatment is less likely. It is also possible that some of the residual variation in procedure rates observed in our study actually reflects differences in local PCT criteria for allowing access to procedures paid for by the NHS. We do not have the data needed to test this hypothesis, but, as PCTs typically did not have access criteria for many of the procedures included in our study, we do not think it would be a predominant factor. Arguably, such PCT criteria ‘lotteries’ still reflect uncertainty about the value of care that might be reduced by better evidence informing more uniform criteria. PCTs have now been superseded by CCGs. We cannot use the CCG as the unit of analysis because demographic and morbidity statistics are not currently available for CCGs. Although it is unlikely that boundary changes will have any immediate impact on geographical variation of procedure rates, new CCG approaches to commissioning will have an impact in the medium term. 9
Our analyses are reliant on the OPCS coding framework, which was not developed for this purpose. The OPCS codes are reviewed annually, but contain anomalies whereby some substitute procedures can be distinguished by the primary OPCS three-character code, for example cemented versus uncemented joint replacement, whereas others cannot, for example open versus endoscopic carpal tunnel decompression. Furthermore, some OPCS procedure codes span more than one diverse clinical group, for example adenoidectomy in children with chronic tonsillitis or sleep apnoea. Methodological research is needed to create an OPCS/International Classification of Diseases (ICD) diagnosis code algorithm that would define a clinically nuanced matrix of distinct procedure/diagnosis group pairings. As both coding frameworks contain thousands of codes, such research would be time-consuming and was beyond the scope of this study. It is important to recognise that different levels of procedure code aggregation will be useful in different circumstances. By using three-character OPCS codes we were able to identify high inter-PCT variation in how varicose vein procedures are conducted (e.g. transluminal or combined), but could not explore variation between PCTs in whether or not to do perform varicose vein procedures.
We have restricted our analysis to major therapeutic procedures to reduce the likelihood of inconsistent coding of minor medical and diagnostic procedures affecting our results. However, inaccurate coding of procedures is still a concern which may contribute to high variation. As hospitals have moved to ‘payment by results’ there is a stronger incentive for complete coding of procedures, but also a possibility of up-coding to increase revenues. 56 We have focused on inpatient and day case procedures because outpatient procedures and those performed in other settings tend to be poorly recorded in routine data. An ideal analysis would include procedures across all settings to provide a more comprehensive picture.
Comparison with other studies
Geographical variation in hospital procedure rates has long been documented in the UK21,22,57 and internationally. 58,59 Indeed, its persistence between countries and through time may give the impression that it is an intractable issue. However, practice variation can be reduced;60 in Scotland, a 50% decline in appendectomy rates between 1973 and 1993 was accompanied by increasing uniformity of procedure rates between health boards. 24 Our findings demonstrate that there is now relative uniformity in tonsillectomy rates between PCTs.
There has been relatively little work exploring the potential causes of geographical variation in health care. Westert and Groenewegen23 and Weinstein et al. 61 describe how an individual clinician’s conservative or liberal use of a procedure might be transferred to colleagues, creating local signatures of procedure use that persist over time. They also stress that the development of these practice patterns may be intensified by fee for service clinician and hospital payments. Birkmeyer et al. 58 and others have demonstrated that geographical variation was higher in discretionary procedures, such as back surgery, where indications are fuzzy and the appropriateness is debated than for procedures, such as hip fracture repair, where the diagnosis and the need for surgery is clear cut. Our analysis also found that procedures that were predominantly performed electively had higher variation.
Unlike most other studies on variation, we did not begin with a list of procedures where we suspected clinical uncertainty existed. Instead, we selected all commonly performed NHS procedures and allowed the data to identify extreme variation. Many of the usual suspects for clinical uncertainty did not appear at the top of our list (e.g. excision of tonsil had a low estimated UR of 1.5; excision of lumbar intervertebral disc had a modest estimated UR of 2.5), underlining the potential of this approach to identify emerging areas of clinical uncertainty.
Implications
Our finding that variation is high in rapidly diffusing or declining procedures, and procedures where a substitute is available, supports the theory that variation is a marker for clinical uncertainty. This suggests that regular monitoring of geographical variation might help NHS regulators and commissioners identify procedures ripe for HTR. However, the appropriate HTR question may take many forms. For some of the ‘high variance’ procedures identified, the question is likely to be ‘which of two procedures (e.g. traditional versus various endovenous varicose vein procedures)62 is more cost-effective and for which patient subgroups)?’63 In other cases, the question may be ‘is this procedure (e.g. radiofrequency denervation of spinal facet joints) more cost-effective than conservative care and if so in which patients?’64 Answering these questions and disseminating the findings is vital if patients across the NHS are to receive best care. However, it is important to realise that, while this should lead to disinvestment from procedures that are not being used cost-effectively, it will not necessarily save the NHS money if they are replaced by more effective but more expensive procedures.
We can only speculate on whether or not our data contain a modern-day equivalent of tonsillectomy, where the clinical indications will narrow drastically in the future. The rise of evidence-based medicine should reduce this risk, but large high-quality RCTs of surgical procedures are still not commonplace and many longstanding procedures have never been fully evaluated in rigorous RCTs. 65 Lack of evidence about which clinical subgroups benefit from a procedure and which do not is the major challenge for commissioners and clinicians. 15 A natural response from commissioners with high local procedure rates is to try to enforce convention through criteria-based access (CBA) and referral management, and many new CCGs have adopted this approach. 9 However, the convention might be wrong66,67 and a more far-sighted response would be for research funders to use geographical variation to prioritise procedures where primary research assessing value in specific clinical subgroups is most needed.
It is also likely that the approach outlined in this chapter, being reliant on coding accuracy, will identify some red herrings. In early iterations of our analysis, in vitro fertilisation (IVF) procedures had extremely high apparent inter-PCT variation with a substantial number of PCTs documenting zero procedures. Media reports have highlighted variations in access to IVF across England;68 however, on closer inspection of the data, we felt that the zero rates in some PCTs were in fact more probably due to variable practice in recording the postcode of patient residence for potentially sensitive fertility treatments. 69
Unanswered questions and future research
Similar studies of unplanned admissions,70 diagnostic tests,71 referral, prescription rates72 and other process measures45 will be useful in identifying other aspects of health care where uncertainty manifests through variation. We chose hospital procedures because there are a nationwide routine data set and a financial incentive for hospitals to document procedures. The integration of routine primary and secondary care data sets has the potential to provide much richer analyses of the intersectorial causes of variation. However, such analyses are dependent on complete and accurate coding and may be jeopardised by any future fragmentation of data sets between NHS and independent sector providers. Additional qualitative research in areas with extreme procedure rates would help us gain a better understanding of the causes of observed variations.
Conclusions
The widespread geographical variations in hospital procedure rates in England are not solely due to variance in clinical need and are likely to reflect clinical discretion regarding appropriate procedure use and setting (e.g. day case or outpatient). NICE and NHS England might, with appropriate caution, use geographical variation to identify candidates for HTR and potential disinvestment. These variations should also be used to set a research agenda for investment in RCTs in surgery.
Chapter 4 Benchmarking for local commissioners to identify potential candidates for disinvestment
Introduction
After a sustained period of growth, health-care spending in many high-income countries has stagnated since the economic crisis of 2007. The 5% annual increases in real NHS expenditure in England that existed before the crisis have now disappeared. In the meantime, chronic diseases in ageing populations with high expectations of new health technologies continue to pressurise these constrained budgets. 2
Around £65B (70%) of NHS funding is allocated to local commissioners, formerly called PCTs but recently reconfigured and named CCGs. 8 CCGs are responsible for planning, commissioning and monitoring services such as elective and emergency hospital care and community and mental health services. CCGs must pay for new technologies mandated by NICE and find the necessary savings from elsewhere in their budgets. Since the formation of the NHS internal market in the early 1990s, local commissioners have been challenged with translating economic evidence into cost-effective pathways of health-care provision by hospitals, GPs and community health services. A survey of NHS decision-makers conducted by Drummond et al. 73 in the mid-1990s revealed that key barriers to greater use of economic evaluation included mistrust of the validity of economic evaluations and an inability to move resources from secondary to primary care to achieve efficiencies. 73 A more recent systematic review of local commissioners’ decision-making echoed some of these findings and reported a number of institutional, political, cultural and methodological obstacles to greater use of evidence on cost-effectiveness. 74 Annual budgets allocated in silos make it difficult for commissioners to reallocate resources from secondary to primary care or to invest this year in the expectation of savings in future years, even when the economic case for reallocation is clear cut. National political objectives, for example waiting list targets, can deflect attention from achieving cost-effective care for the local population. In addition, lack of high-quality evidence on cost-effectiveness and lack of time to consider the available evidence have both been cited as barriers to more efficient local health-care commissioning. 74
Primary care doctors (GPs) play a key role in the new CCGs; one goal of the reconfiguration is to give more budgetary responsibility to front-line clinicians to encourage them to redesign health-care provision more efficiently in their locality. However, identifying opportunities to save the NHS money is challenging, particularly at the local level. It is very difficult to investigate or challenge historical levels of provision for the vast majority of services commissioned. Furthermore, since local commissioners purchase the majority of secondary care services from a very small number hospital trusts, maintaining positive and balanced working relationships is vital. Collaborative approaches to disinvestment in some services to provide an optimal mix of health care for the local population can easily degenerate into mutual mistrust, with hospital trust representatives suspecting commissioners of unthinking cost cutting and commissioners presuming that hospital trusts primarily want to protect income.
There are a variety of options available to local commissioners who wish to regulate procedure rates in line with the best available evidence. Referral management strategies that target the transfer of care from GP to specialist include guidelines, structured referral sheets, GP financial incentives, peer review and feedback, and referral management centres. 75 Other strategies aim to direct surgeon decision-making by introducing CBA, also known as threshold policies, for surgical care, which may be reinforced by retrospective audit or a prospective prior approval process. These criteria aim to target medical procedures on the patient subgroups most likely to benefit from them, based on current evidence. At the extreme, procedures deemed to be of very little or no health benefit can be designated as ‘not routinely funded’. Contracting or putting contracts out to tender can also be used to provide financial incentives to hospitals and surgeons to modify their practice. Other approaches focus on increasing the patient’s role in the process through shared decision-making and decision aids. By informing patients of the potential benefits and harms of surgery, these tools could redress the information asymmetry between surgeon and patient. 76
Most local commissioning groups have developed some form of regulation process for procedures commonly viewed as relatively ineffective or largely cosmetic. 77 However, these ‘usual suspects’ might not be most relevant locally. Instead, benchmarking39 a broad range of local procedure rates against the national norms might provide a more flexible way to identify areas where local service provision is inappropriately high or inefficient. The recent development of the NHS Atlas of Variation in Healthcare45 and similar tools allow commissioners to readily benchmark local health-care provision. However, benchmarking might be counterproductive if the data on which it is based misrepresent actual care patterns, adjustment for clinical need is inadequate or evidence on the appropriate procedure rate is lacking. 67
In this chapter we used routinely collected HES data, adjusting for proxies of clinical need, to identify local variations in 181 procedure rates. In collaboration with two local PCT commissioning groups, we benchmarked local procedure rates against national rates. One case study procedure was selected with each PCT commissioning group and, in the next chapters, we conducted rapid systematic reviews to summarise the evidence on clinical effectiveness and cost-effectiveness and explore potential reasons for high local use. Our aim is to explore the potential usefulness and pitfalls of using benchmarking and technology assessment to inform local commissioning and potentially disinvestment.
Methods
Benchmarking inter-PCT variation in procedure rates
The methods for identifying inter-PCT variation in procedure rates and adjusting for proxies of clinical need are explained in detail in the previous chapter. However, as the number of procedures, study dates and method for adjusting for private health-care provision are different for the benchmarking work, we briefly recap those methods in this section.
For the benchmarking work, we extracted anonymised individual episode records from HES between 1 April 2007 and 31 March 2010. All interventions and procedures performed during each episode are recorded using OPCS fourth revision codes. OPCS codes include more than 9000 four-character codes defining procedures at the finest level of detail. However, most of these codes are used infrequently, so we categorised procedures using the three-character OPCS codes (n ≈ 1500). We focused on the most clinically and economically consequential procedures by including only the 269 most widely used procedures, which accounted for over 90% of all inpatient procedures. We excluded minor (n = 45) and diagnostic (n = 34) procedures and procedures where codes changed between OPCS version 4.4 (2007/8) and version 4.5 (used since 2009/10), (n = 9). This left 181 major therapeutic procedures for analysis. The number of included procedures differs from Chapter 3, as prior to the analysis in Chapter 3 we tightened our procedure exclusion criteria to focus on major therapeutic procedures and because of coding changes in 2011/12. Up to 24 procedures can be recorded during each episode and a single stay in hospital may comprise more than one episode as patient care is transferred from one consultant to another. All procedures from both admission and subsequent episodes were eligible for inclusion in our analysis.
Crude annual procedure rates (per 100,000) were calculated by dividing the number of procedures undertaken on PCT residents by the total PCT population. 49 We counted the observed number of each procedure within every PCT, ‘Observedij’, and estimated the expected number, ‘Expectedij’, indirectly standardising48 for age and sex and accounting for differences in PCT ethnic and socioeconomic composition and the prevalence of chronic disease and unhealthy lifestyle as described in the previous chapter. However, in the local benchmarking work we used data on the regional prevalence of private medical insurance, which might substitute for NHS treatment, from the British Household Panel Survey to adjust for private health-care provision. 78 The observed number of procedures within each PCT, ‘Observedij’, was divided by the expected, ‘Expectedij’, and multiplied by the national rate of procedure use (i.e. rate in the standard population) to yield adjusted PCT rates.
Benchmarking
For the work in this chapter, we studied procedure utilisation rates in two PCT commissioning groups in more detail. The first PCT commissioning group (henceforth PCT1) served a region that was relatively rural, with a predominantly white and comparatively wealthy population. The second PCT commissioning group (henceforth PCT2) served a predominantly urban region, consisting of areas with high proportions of ethnic minority populations and pockets of high deprivation. Although we label them as a PCT throughout, one of the commissioning groups supported more than one neighbouring PCT. For each procedure, we quantified the difference between the individual PCT’s rate and the national rate using both the absolute and the relative difference. For each of the two PCTs we presented the 20 procedures with the largest estimated absolute difference as candidates for further study. We calculated a summary measure of the national geographical variability of each of these 20 procedures (described in more detail in Chapter 3). We defined procedures as ‘very high’ variance if there was a greater than 0.95 probability that inter-PCT variation in procedure utilisation was more than three times as high as the median inter-PCT variation for all 181 procedures. Similarly we identified ‘high’ (> 2 times as high as the median), ‘average’ (> 0.5 times as high as the median) and ‘low’ (> 0.33 times as high as the median) variance procedures. The remaining procedures were classified as ‘very low’ variance procedures. In addition, for each procedure, we present the estimated national rank of the specific PCT, where a value of 1 would indicate that the PCT had the largest (of all 152 PCTs) estimated absolute difference in rate from the national rate.
We further explored intra-PCT variation by tabulating procedure numbers by hospital, geographical variation at the sub-PCT (i.e. local borough) level and temporal variation in local procedure rates since 2001/2. A list of 20 procedures with the highest estimated absolute differences between the local PCT rate and national rate was discussed at a project team meeting in October 2011 with public health and commissioning representatives from the two PCT commissioning groups. As a result of the project team meeting we selected one procedure for rapid technology assessment at each PCT. These were ‘release of entrapment of peripheral nerve at wrist’ (OPCS code A65) at PCT1 and ‘incision of capsule of lens’ (OPCS code C73) at PCT2. The choice of procedures was a joint one between the research team and representatives of the two PCTs. These procedures were selected because procedure rates were substantially higher than the national average after adjustment for clinical need and were thought worth further investigation by the PCT representatives. Both procedures were thought likely to have had an important financial impact on PCT spending. Additionally, each procedure code was thought to represent a relatively well-defined procedure and patient population which would allow a subsequent technology appraisal to be undertaken.
Results
Benchmarking: PCT1
The adjusted rate of procedure use is much greater in PCT1 than the national average for a large number of procedures (Table 7). In 2009/10 the procedure with the largest estimated absolute difference from the national rate was ‘operations on spinal nerve root’, where adjusted local use was 62 (95% CI 55 to 70) procedures higher per 100,000 residents than the national mean. If PCT1 reduced its rate of this procedure to the national rate, a reduction of around 370 procedures per annum would be made, leading to substantial cost savings. For three procedures PCT1’s utilisation is in the top 10% of all PCTs. Furthermore, three of the 20 procedures with the largest absolute differences between PCT1 rate and the national rate also exhibit high or very high national variation, suggesting that there may be uncertainty about the appropriate procedure rate across England. For 17 of the procedures (the exceptions being ‘excision of gall bladder’, ‘excision of tonsil’ and ‘endoscopic resection of outlet of male bladder’) utilisation in PCT1 remained above the national median for each year between 2007/8 and 2009/10.
| Procedure name (OPCS code) | Adjusted local rate (95% CI)a | National rate (95% CI) | Absolute difference (95% CI) | Relative difference (95% CI) | 2009/10 estimated national rank(2007/8 rank) | National procedure variabilityb |
|---|---|---|---|---|---|---|
| Operations on spinal nerve root (A57) | 110 (103 to 118) | 48 (47 to 48) | 62 (55 to 70) | 2.3 (2.1 to 2.5) | 7 (1) | Average |
| Combined operations on varicose vein of leg (L84) | 60 (54 to 66) | 30 (30 to 31) | 29 (23 to 35) | 2.0 (1.8 to 2.2) | 11 (36) | Average |
| Release of entrapment of peripheral nerve at wrist (A65) | 141 (133 to 150) | 113 (112 to 114) | 28 (20 to 37) | 1.2 (1.2 to 1.3) | 19 (25) | Average |
| Total prosthetic replacement of hip joint using cement (W37) | 83 (77 to 89) | 55 (55 to 56) | 27 (21 to 33) | 1.5 (1.4 to 1.6) | 20 (21) | Average |
| Excision of gall bladder (J18) | 145 (136 to 154) | 119 (118 to 120) | 23 (15 to 32) | 1.2 (1.1 to 1.3) | 7 (86) | Very low |
| Excision of cervix uteri (Q01) | 59 (54 to 65) | 41 (40 to 41) | 19 (13 to 24) | 1.5 (1.3 to 1.6) | 37 (36) | High |
| Other therapeutic transluminal operations on vein (L99) | 39 (36 to 44) | 23 (23 to 24) | 16 (12 to 20) | 1.7 (1.5 to 1.9) | 20 (19) | High |
| Therapeutic ureteroscopic operations on ureter (M27) | 48 (44 to 53) | 35 (35 to 36) | 13 (8 to 18) | 1.4 (1.2 to 1.5) | 22 (52) | Average |
| Other operations on outlet of male bladder (M70) | 72 (67 to 79) | 60 (60 to 61) | 12 (6 to 18) | 1.2 (1.1 to 1.3) | 51 (71) | High |
| Prosthetic replacement of head of femur using cement (W46) | 37 (32 to 41) | 25 (25 to 26) | 11 (7 to 15) | 1.4 (1.3 to 1.6) | 21 (71) | Average |
| Therapeutic spinal puncture (A54) | 37 (33 to 43) | 27 (27 to 28) | 10 (5 to 15) | 1.4 (1.2 to 1.5) | 25 (19) | Average |
| Primary repair of inguinal hernia (T20) | 137 (129 to 146) | 127 (127 to 129) | 9 (1 to 17) | 1.1 (1.0 to 1.1) | 38 (27) | Very low |
| Operations on prepuce (N30) | 76 (69 to 83) | 67 (66 to 68) | 8 (2 to 15) | 1.1 (1.0 to 1.2) | 33 (43) | Average |
| Other operations on lacrimal apparatus (C29) | 22 (19 to 25) | 13 (13 to 14) | 8 (5 to 12) | 1.6 (1.4 to 1.9) | 16 (13) | Average |
| Abdominal excision of uterus (Q07) | 69 (63 to 77) | 60 (60 to 61) | 8 (2 to 14) | 1.1 (1.0 to 1.2) | 23 (41) | Low |
| Excision of tonsil (F34) | 105 (97 to 114) | 97 (96 to 98) | 7 (0 to 16) | 1.1 (1.0 to 1.2) | 42 (95) | Low |
| Destruction of lesion of cervix uteri (Q02) | 21 (18 to 23) | 14 (13 to 14) | 7 (4 to 9) | 1.5 (1.3 to 1.7) | 26 (38) | Average |
| Primary closed reduction of traumatic dislocation of joint (W66) | 42 (37 to 48) | 35 (35 to 36) | 7 (2 to 12) | 1.2 (1.1 to 1.3) | 37 (26) | Average |
| Other operations on spine (V54) | 148 (138 to 159) | 142 (140 to 143) | 7 (–4 to 17) | 1.0 (1.0 to 1.1) | 62 (63) | Average |
| Endoscopic resection of outlet of male bladder (M65) | 57 (52 to 63) | 50 (50 to 51) | 7 (1 to 12) | 1.1 (1.0 to 1.2) | 40 (86) | Average |
The procedure ‘release of entrapment of peripheral nerve at wrist’ (i.e. CTR surgery) was selected for further evaluation in PCT1. The adjusted local rate was 28 (95% CI 20 to 37) procedures higher than the national rate per 100,000 residents. PCT1 had the 19th-largest estimated absolute difference from the national rate. Nationally there was ‘average’ variation between PCTs in the use of this procedure, which suggests reasonable agreement in appropriate procedure rates across England.
PCT1 had a higher rate of CTR procedures than each of three neighbouring PCTs, although with overlapping 95% CIs (Table 8). About 60% of CTR procedures in PCT1 were performed at hospital A and 30% were performed at hospital B. Analysis of the rates of CTR surgery by local authority area of residence demonstrated substantial differences of CTR surgery rates across the PCT (Table 9). The residents of local authority areas predominantly served by hospital A had the highest rates, for example local authority areas U, V and W all had crude CTR surgery rates 62% higher than the national average, whereas districts X, Y and Z, which were more commonly served by hospital B, had crude CTR surgery rates between 22% and 37% higher than the national average.
| PCT | Number of procedures | Adjusted local rate (95% CI) | National rate (95% CI) | Absolute difference (95% CI) | Relative difference (95% CI) | Estimated national rank |
|---|---|---|---|---|---|---|
| PCT1 | 1004 | 141 (133 to 150) | 113 (112 to 114) | 28 (20 to 37) | 1.2 (1.2 to 1.3) | 19 |
| Neighbour PCTA | 404 | 130 (118 to 144) | 113 (112 to 114) | 16 (4 to 29) | 1.1 (1.0 to 1.3) | 39 |
| Neighbour PCTB | 881 | 86 (81 to 92) | 113 (112 to 114) | –26 (–32 to –21) | 0.8 (0.7 to 0.8) | 129 |
| Neighbour PCTC | 635 | 104 (96 to 112) | 113 (112 to 114) | –9 (–17 to –2) | 0.9 (0.9 to 1.0) | 97 |
| Local authority area | Number of procedures | Unadjusted rate (95% CI) | Hospital: number (%) of procedures |
|---|---|---|---|
| U | 237 | 187.2 (164.8 to 212.6) | A: 228 (96.2) B: 3 (1.3) Other: 6 (2.6) |
| V | 231 | 186.1 (163.6 to 211.8) | A: 215 (93.1) Other: 16 (6.9) |
| W | 173 | 183.7 (158.2 to 213.2) | A: 94 (54.3) B: 61 (35.3) Other: 18 (10.4) |
| X | 133 | 155.01 (130.78 to 183.73) | A: 62 (46.6) B: 62 (46.6) Other: 9 (6.8) |
| Y | 87 | 139.9 (113.4 to 172.6) | B: 64 (73.6) C: 22 (25.3) Other: 1 (1.2) |
| Z | 143 | 138.2 (117.3 to 162.8) | B: 109 (76.2) C: 29 (20.3) Other: 5 (3.5) |
Crude CTR surgery rates in PCT1 have been higher than the national average since 2004/5 (Figure 3), indicating that high utilisation is not a temporary phenomenon, which might occur if capacity for CTR surgery had temporarily been boosted to meet a waiting list target. Although a reduction in the crude CTR surgery rate was observed between 2004/5 and 2006/7, substantial increases have taken place since then, despite only moderate increases in the national rate.
FIGURE 3.
Temporal trends in CTR surgery in PCT1 and in England.
The gender and age distribution of patients admitted for CTR surgery in PCT1 was similar to the national picture (Table 10), as was the high proportion of procedures (≈ 95%) that were done as day cases. Revision CTR surgery, which is one potential explanation of the high procedure rate in PCT1, is not recorded more frequently. Patients treated in PCT1 were slightly more likely to have bilateral CTR surgery coded. As this finding is based on a supplemental laterality procedure code being recorded, it is possible that this difference represents a variation in coding rather than in clinical practice.
| Characteristic | PCT1 | Other PCTs | |
|---|---|---|---|
| n | 1004 | 57,663 | |
| Diagnosis, n (%) | |||
| Mononeuropathies of upper limb (G56) | 958 (95.42) | 54,665 (94.80) | |
| Fracture of forearm (S52) | 10 (1.00) | 650 (1.13) | |
| Fibroblastic disorders (M72) | 4 (0.40) | 83 (0.14) | |
| Other | 32 (3.19) | 2265 (3.93) | |
| Male, n (%) | 331 (32.97) | 19,057 (33.05) | |
| Mean age (SD) (years) | 58.39 (15.80) | 58.08 (15.70) | |
| Age (years), n (%) | |||
| 0–19 | 3 (0.30) | 178 (0.31) | |
| 20–39 | 125 (12.45) | 6799 (11.79) | |
| 40–59 | 404 (40.24) | 24,774 (42.96) | |
| 60–79 | 356 (35.46) | 19,847 (34.42) | |
| 80+ | 116 (11.55) | 6054 (10.50) | |
| Missing | 0 (0.00) | 11 (0.02) | |
| Day case, n (%) | 961 (95.72) | 53,072 (92.04) | |
| Further details, n (%) | |||
| Revision | 14 (1.39) | 1176 (2.04) | |
| Right | 531 (52.89) | 31,235 (54.17) | |
| Left | 409 (40.74) | 24,123 (41.83) | |
| Bilateral | 75 (7.47) | 1545 (2.68) | |
Benchmarking: PCT2
The rate of procedure use is much greater in PCT2 than the national average for a large number of procedures (Table 11). The greatest absolute difference is seen in the ‘destruction of lesion of retina’ procedure, where PCT2 carries out an additional 57 (95% CI 50 to 64) procedures per 100,000 residents than the national average. If PCT2 reduced its rate of this procedure to the national rate, a reduction of around 516 procedures per annum would be made, leading to substantial cost savings. For five procedures, PCT2’s utilisation is in the top 10% of all PCTs. Furthermore, 5 of the 20 procedures with the largest absolute differences between PCT2 rate and the national rate also exhibit high or very high national variation, suggesting that there may be clinical uncertainty about the appropriate procedure rate across England. For the 20 high-utilisation procedures, there was a positive correlation between the procedure rates of neighbouring PCTs (Figure 4). For 18 of the procedures (the exceptions being ‘other vein related operations’ and ‘other therapeutic transluminal operations on vein’), utilisation in PCT2 remained above the national median between 2007/8 and 2009/10.
| Procedure name (OPCS code) | Adjusted local rate (95% CI)a | National rate (95% CI) | Absolute difference (95% CI) | Relative difference (95% CI) | 2009/10 estimated national rank (2007/8 rank) | National procedure variabilityb |
|---|---|---|---|---|---|---|
| Destruction of lesion of retina (C82) | 109 (103 to 115) | 52 (51 to 52) | 57 (50 to 64) | 2.1 (2.0 to 2.2) | 27 (35) | High |
| Simple extraction of tooth (F10) | 248 (238 to 259) | 192 (191 to 193) | 56 (46 to 66) | 1.3 (1.2 to 1.3) | 40 (47) | Average |
| Other vein-related operations (L91) | 281 (270 to 292) | 232 (231 to 233) | 48 (37 to 59) | 1.2 (1.2 to 1.3) | 39 (83) | Average |
| Incision of capsule of lens (C73) | 75 (71 to 80) | 32 (32 to 33) | 43 (39 to 47) | 2.3 (2.2 to 2.5) | 26 (41) | Very high |
| Excision of cervix uteri (Q01) | 75 (69 to 82) | 41 (40 to 41) | 34 (28 to 41) | 1.8 (1.7 to 2.0) | 24 (38) | High |
| Endoscopic extirpation of lesion of lower bowel using fibreoptic sigmoidoscope (H23) | 71 (66 to 77) | 38 (37 to 38) | 33 (28 to 39) | 1.9 (1.7 to 2.0) | 11 (22) | Average |
| Primary open reduction of fracture of bone and intramedullary fixation (W19) | 92 (86 to 98) | 59 (58 to 59) | 33 (27 to 39) | 1.6 (1.5 to 1.7) | 10 (25) | Average |
| Extirpation of lesion of eyelid (C12) | 103 (96 to 111) | 72 (71 to 73) | 31 (23 to 39) | 1.4 (1.3 to 1.5) | 22 (20) | Average |
| Other operations on outlet of male bladder (M70) | 90 (84 to 96) | 61 (60 to 61) | 29 (22 to 35) | 1.5 (1.4 to 1.6) | 38 (11) | High |
| Destruction of haemorrhoid (H52) | 78 (71 to 86) | 51 (50 to 51) | 27 (19 to 35) | 1.5 (1.4 to 1.7) | 26 (13) | Average |
| Other therapeutic transluminal operations on vein (L99) | 55 (51 to 60) | 31 (31 to 32) | 24 (20 to 29) | 1.8 (1.6 to 1.9) | 16 (126) | High |
| Operations on adenoid (E20) | 70 (65 to 76) | 48 (48 to 49) | 21 (16 to 27) | 1.4 (1.3 to 1.6) | 14 (12) | Average |
| Other operations on eyelid (C22) | 33 (29 to 38) | 16 (16 to 16) | 17 (13 to 21) | 2.1 (1.8 to 2.3) | 13 (4) | Average |
| Other operations on tongue (F26) | 28 (25 to 31) | 12 (12 to 13) | 15 (12 to 19) | 2.2 (2.0 to 2.5) | 11 (71) | Average |
| Excision of tonsil (F34) | 113 (106 to 121) | 98 (97 to 99) | 14 (7 to 22) | 1.1 (1.1 to 1.2) | 32 (42) | Low |
| Other internal fixation of bone (W28) | 111 (105 to 117) | 96 (95 to 97) | 14 (8 to 20) | 1.1 (1.1 to 1.2) | 32 (42) | Low |
| Other operations on fallopian tube (Q41) | 38 (34 to 42) | 25 (25 to 26) | 12 (8 to 16) | 1.5 (1.3 to 1.6) | 18 (46) | Average |
| Prosthetic replacement of head of femur using cement (W46) | 41 (37 to 46) | 29 (29 to 30) | 12 (8 to 16) | 1.4 (1.3 to 1.6) | 26 (15) | Average |
| Vaginal excision of uterus (Q08) | 44 (40 to 48) | 32 (32 to 33) | 11 (7 to 15) | 1.3 (1.2 to 1.5) | 20 (17) | Average |
| Extracorporeal fragmentation of calculus of kidney (M14) | 48 (43 to 53) | 37 (37 to 38) | 10 (6 to 15) | 1.3 (1.2 to 1.4) | 29 (61) | Average |
FIGURE 4.
Comparison of utilisation in three neighbouring PCTs. (a) Graph displaying PCT procedures rates compared to the national procedure rate; (b) key of procedure label.
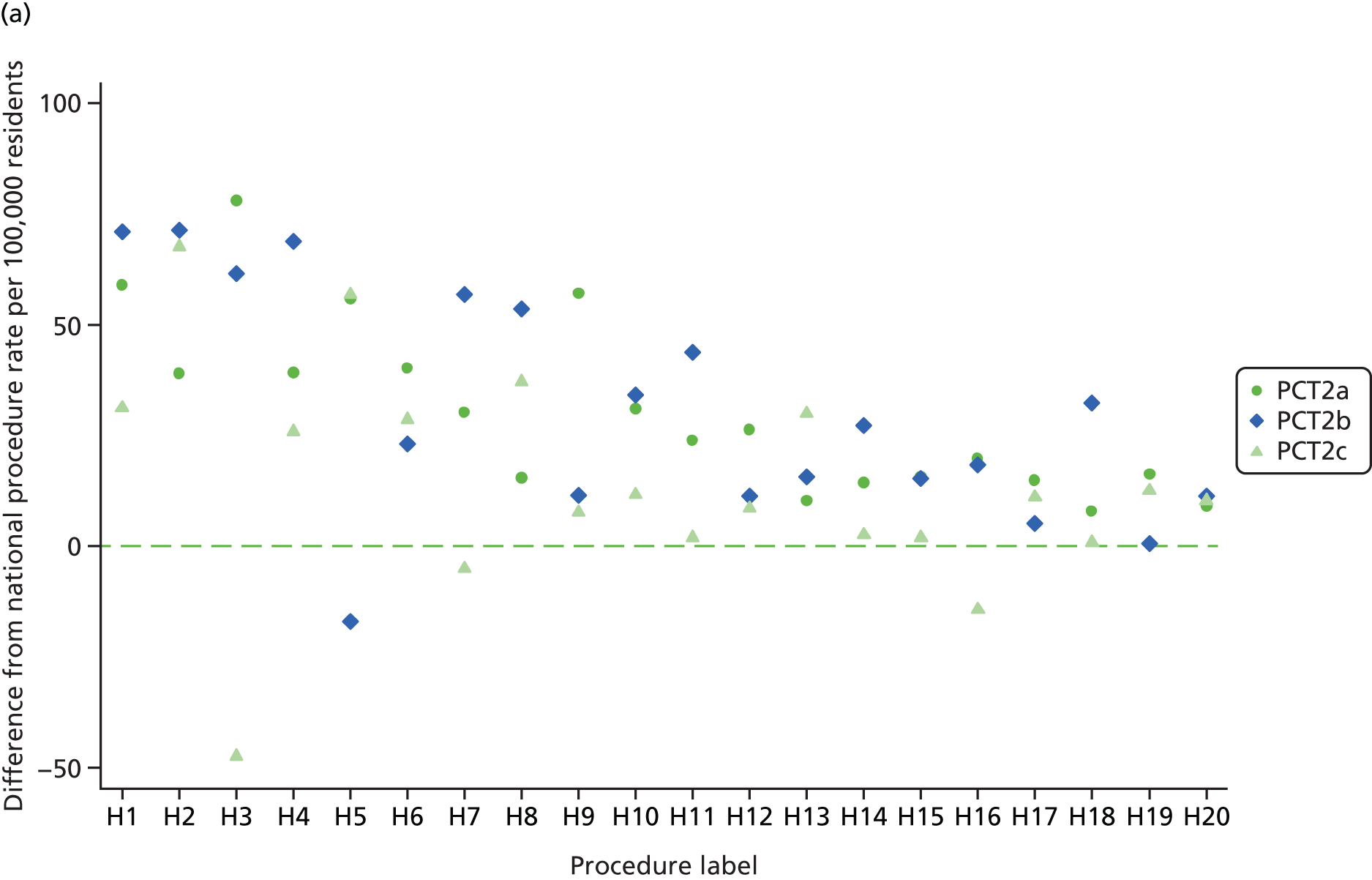

The ‘incision of capsule of lens’ procedure was selected for further evaluation and technology assessment. The adjusted local rate was 43 (95% CI 39 to 47) procedures higher than the national rate per 100,000 residents. PCT2 had the 26th largest estimated absolute difference from the national rate. Nationally there was ‘very high’ variation between PCTs in the use of this procedure, which suggests that there might be a high degree of clinical uncertainty about the appropriate use of this procedure. The procedure, which is more commonly known as capsulotomy, is typically performed in people who have developed PCO following extracapsular cataract extraction and implantation of an intraocular lens for age-related cataract. Neighbouring PCTs, all of which commissioned ophthalmology services from the same hospital trust, had capsulotomy procedure rates well above the national average (Table 12). Capsulotomy procedure rates in PCT2 have been higher than the national average since at least 2001/2 (Figure 5), indicating that high utilisation is not a temporary phenomenon.
| PCT | Number of procedures | Adjusted local rate (95% CI) | National rate (95% CI) | Absolute difference (95% CI) | Relative difference (95% CI) | Estimated national rank |
|---|---|---|---|---|---|---|
| PCT2a | 588 | 72 (66 to 78) | 32 (32 to 33) | 40 (34 to 46) | 2.2 (2.1 to 2.4) | 25 |
| PCT2b | 400 | 101 (92 to 112) | 32 (32 to 33) | 69 (59 to 80) | 3.1 (2.8 to 3.5) | 15 |
| PCT2c | 280 | 59 (52 to 66) | 32 (32 to 33) | 26 (20 to 34) | 1.8 (1.6 to 2.0) | 30 |
FIGURE 5.
Temporal trends in capsulotomy in PCT2 and in England.
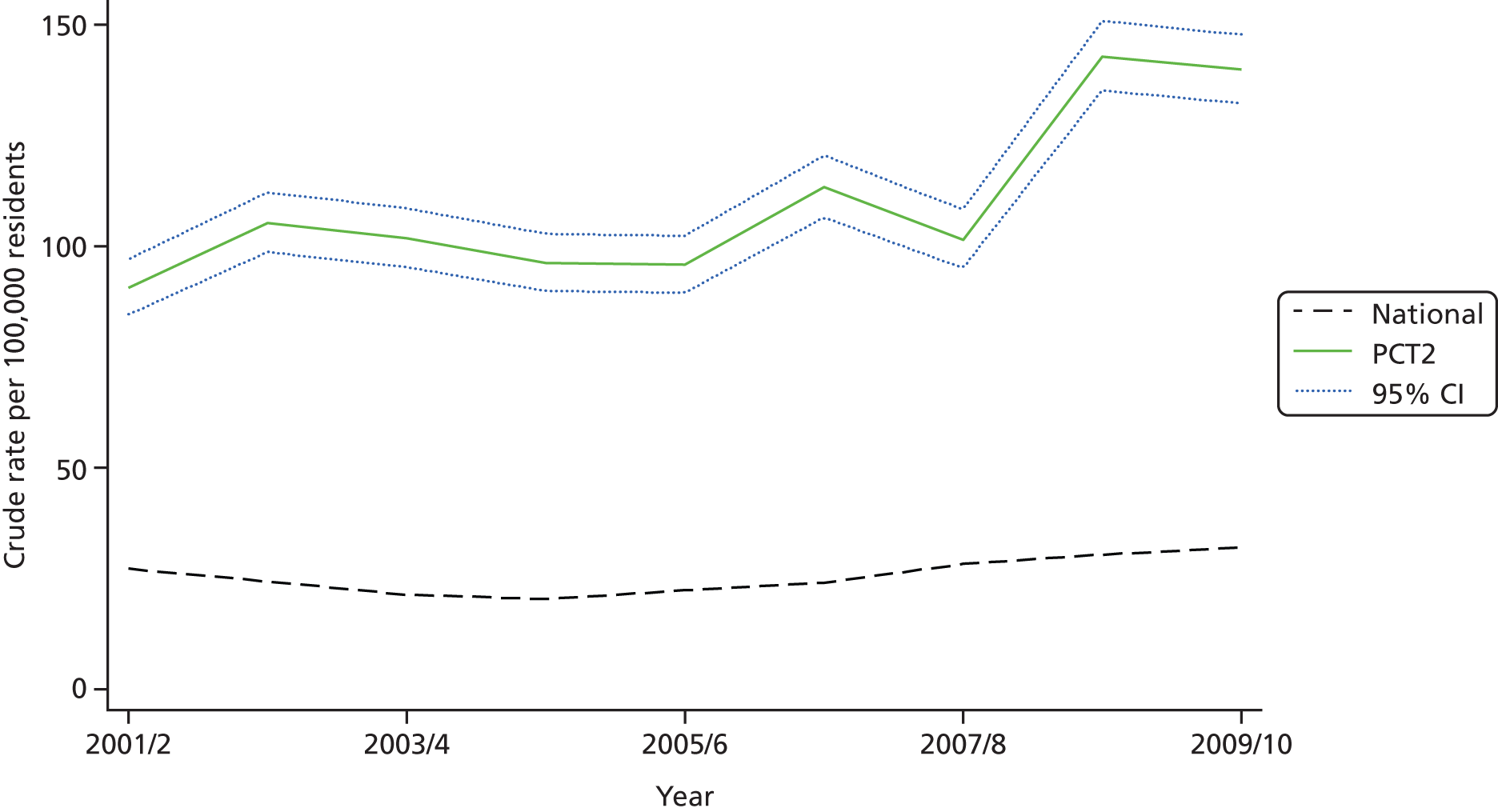
The gender and age distribution of patients admitted for capsulotomy in PCT2 was similar to the national picture (Table 13), as was the high proportion of procedures that were recorded as day cases (≈ 95%). Patients in PCT2 were more likely to have the specific diagnosis code of ‘complications of other internal prosthetic device’ whereas nationally ‘other cataract’ or other diagnosis codes were more commonly used. However, this may simply reflect local differences in diagnosis coding.
| Characteristic | PCT2 | Other PCTs |
|---|---|---|
| n | 1268 | 15,537 |
| Diagnosis, n (%) | ||
| Other cataract (H26) | 1057 (83.36) | 13,791 (88.76) |
| Complications of other internal prosthetic devices, implants and grafts (T85) | 176 (13.88) | 426 (2.74) |
| Congenital lens malformations (Q12) | 7 (0.55) | 112 (0.72) |
| Other | 28 (2.21) | 1208 (7.77) |
| Male, n (%) | 460 (36.28) | 5853 (37.67) |
| Mean age (SD) (years) | 74.79 (13.50) | 73.97 (14.65) |
| Age (years), n (%) | ||
| 0–39 | 27 (2.13) | 460 (2.96) |
| 40–59 | 94 (7.41) | 1346 (8.66) |
| 60–69 | 212 (16.72) | 2436 (15.68) |
| 70–79 | 410 (32.33) | 4963 (31.94) |
| 80+ | 525 (41.40) | 6332 (40.75) |
| Day case, n (%) | 1206 (95.11) | 14,690 (94.55) |
Discussion
Main findings
We used benchmarking of geographical variations in procedure rates to help health-care commissioners identify procedures with high local utilisation which might be candidates for disinvestment. In both PCT commissioning groups, the rate of procedure use was much greater than the national average for a large number of procedures. For some procedures (e.g. operations on spinal nerve root in PCT1 and destruction of lesion of retina in PCT2), rates were more than 50 procedures per 100,000 residents in excess of the national average. In many cases the high local use procedures were not ones that are commonly cited on ‘low value’ procedure lists.
Strengths and weaknesses
The benchmarking approach that we describe does not start from any preconceptions about ‘low value’ procedures and it does not rely on clinician or commissioner introspection to identify areas where local health funds might be used more efficiently. Instead, the data are used to identify procedures where the PCT has a high utilisation rate and then HTR can be employed to explore whether or not the high utilisation is justified. While several procedures identified in PCT1, including varicose vein and carpal tunnel surgery, have appeared on lists of ‘low value procedures’, others have not. The high rate of cholecystectomy is an example where PCT1 is an outlier despite very low inter-PCT variation in procedure rates nationally, suggesting general agreement about the appropriate use of this procedure.
Benchmarking procedure codes does not necessarily lead to straightforward technology reassessment questions. In PCT2, adenoidectomy was initially flagged as having locally high utilisation rates. However, as this procedure may be used in combination with other procedures to treat otitis media, sleep apnoea or chronic tonsillitis, any resulting technology appraisal will be complex. Benchmarking also needs to be conducted carefully to avoid false conclusions. During the course of our work with the PCTs we found apparently high procedure rates due to double counting of procedures where the patient was admitted via an NHS hospital but transferred to an independent sector treatment centre for surgery. This highlights the importance of commissioners working with clinicians from the outset, with willingness from both parties to identify procedures with genuinely high local utilisation and explore the potential causes of this and responses to it.
We used the number of private hospital beds as a proxy measure for the number of private hospital procedures which may be inappropriate, particularly for some conditions (e.g. excision of appendix) where private treatment is unlikely. We have ranked PCTs according to how frequently they used each procedure, relative to national utilisation rates. However, ranks should be interpreted with caution since they are themselves subject to sampling variation. 79 More statistically rigorous methods are available for quantifying whether or not a PCT is truly ‘unusual’ in terms of its rate of utilisation of a procedure. 80,81
Comparisons with other studies
Benchmarking health care in an attempt to improve performance is not a new phenomenon,82 although current financial constraints have brought renewed interest. Benchmarking tools for the NHS in England are now more publicly available than ever, most prominently through the NHS Atlas of Variation in Healthcare45 series, NHS comparators,83 payment by results benchmarker84 and similar tools. However, while the majority of PCTs are aware of the NHS Atlas data, only 34% report using the data to explore causes of variation and potential responses to it. 85 Schang et al. 85 pinpoint one key barrier to the real-world applicability of variations data as the ‘essential ambiguity over the meaning of observed variations’. PCTs can compare their performance with the national norm, but not with any ‘gold standard’ procedure rate.
There is also international interest in the potential of benchmarking to improve health-care efficiency. The seminal work of the Dartmouth group in the USA demonstrated that elderly Medicare patients in higher spending regions did not have better health outcomes or satisfaction with care despite receiving approximately 60% more care,86,87 raising the possibility that substantial savings could be made without detriment to care quality. In Tuscany, a benchmarking exercise including GP and pharmaceutical expenses, inappropriate hospitalisation rates, hospital length of stay and readmission rates estimated that savings equivalent to 2–7% of the health-care budget could be achieved if all health authorities achieved the regional average. 39 However, this finding is based on the questionable assumption that the regional average is desirable and has best outcomes for patients.
Benchmarking should inform and complement other local disinvestment initiatives, such as programme budgeting and marginal analysis (PBMA) and sociotechnical allocation of resources (STAR) approaches. 38,88 In both of these approaches, stakeholder groups discuss and evaluate the evidence supporting numerous investment and disinvestment options within a programme, or programmes, of health care. The aim is to reconfigure care pathways and packages to offer better value care for patients. The NHS spend and outcomes tool89 is designed to help local commissioners identify which health-care programmes are in most need of reconfiguration.
Implications
If there is compelling evidence that a high local use procedure is no longer cost-effective in some or all of the clinical subgroups in which it is currently used, then there is a clear case for local commissioners to intervene to reduce procedure rates. However, when evidence is not compelling, local variation may simply represent legitimate differences in patient preferences for a procedure or unimportant eclectic differences in practice by clinicians. 67 The limited evidence available suggests that patient preferences are unlikely to be a major driver of the large variations in procedure rates observed. One study found that urologists focus more on clinical parameters than patient views when recommending prostatectomy. 90
It has been argued that variation due to eclectic clinical practice may be preferable to an enforced consensus around the prevailing, non-evidence-based, norm,67 provided that the variation is used as a natural experiment to generate evidence comparing patient costs and outcomes between regions of the country. In practice, however, variation is seldom used as an opportunity for research. Furthermore, inpatient procedures are known to be initially costly and in many cases carry known risks to patients; it is the long-term health benefits and potential NHS savings which are more often in doubt. Therefore, it seems reasonable, from the perspective of a local commissioner, that the onus should be on clinicians with high procedure rates to demonstrate that their approach results in better patient outcomes at an acceptable cost, rather than on the commissioners to demonstrate otherwise. Using benchmarking to identify and reduce inappropriately high procedure rates will be only a small part of the response to the ‘Nicholson challenge’ to find £20B in productivity improvements by 2015. 91 For example, if PCT1 did reduce the carpal tunnel surgery rate to the national average it would commission approximately 200 fewer procedures each year and could potentially save approximately £200,000 per annum in inpatient costs. This potential saving will only actually be achieved if, over time, the number of theatre slots and hospital beds are reduced to reflect changing patterns of care. Furthermore, any savings in inpatient costs may be offset to some extent if patients not treated surgically require additional non-surgical care. The relatively small size of the potential savings and the difficulties of intervening to reduce procedure rates might well discourage individual PCTs from investing more time and resources in benchmarking and intervening to regulate procedure rates. The NHS may need to harness economies of scale to address the Nicholson challenge by encouraging nationwide initiatives, such as shared decision-making tools or national guidance on appropriate criteria for surgery, rather than rely on initiatives by individual local commissioners.
There is a real danger that by benchmarking in just one setting (i.e. inpatient care) and on what is recorded routinely (i.e. therapeutic procedures) commissioners will miss the bigger picture and possibly larger opportunities for disinvestment. For example, high rates of carpal tunnel surgery might reflect ‘upstream’ factors such as referral pathways from primary to secondary care or the availability of hand therapy or steroid injections. Therefore it is important that benchmarking lead to a broad conversation about the pathway of care and service configuration, not just a narrow focus on surgeons and the eligibility criteria for surgery.
In 1999, NICE was established to help reduce inequality in access to new treatments across England and Wales. 92 It has attempted to do so through establishing a rigorous, transparent and mandatory technology appraisal process for selected new technologies. However, our benchmarking work reveals the extent to which the postcode lottery of access to treatments in the NHS remains for more established interventions. Indeed the NICE technology appraisal process for new technologies may indirectly exacerbate this by forcing PCTs to cut back in other areas of health care without a transparent or consistent process for making these disinvestment decisions.
Conclusions
Benchmarking can help local decision-makers identify procedures where local utilisation appears to be substantially higher than the national average, even after adjusting for proxies of clinical need. This might represent local overutilisation and an opportunity to provide better value care for the local population. However, benchmarking is vulnerable to inaccurate coding of clinical activity and does not necessarily lead commissioners to an understanding of why local rates are high or, more importantly, what the optimal procedure rate should be based on the available evidence. It is important to recognise that the national average represents a norm and may not be an evidence-based gold standard or provide good clinical outcomes for the patient. We explore these questions further in the next two chapters, which review the evidence on surgical interventions for treating CTS (PCT1) and interventions for treating PCO (PCT2).
Chapter 5 Case study 1: surgical intervention for treating carpal tunnel syndrome – a rapid systematic review
Background
As a result of the project team meeting we selected ‘release of entrapment of peripheral nerve at wrist’ (OPCS code A65) for a rapid technology appraisal in PCT1. This procedure was selected by the PCT representatives and the research team because procedure rates were substantially higher than the national average after adjustment for clinical need and the procedure had an important financial impact on PCT spending. In addition, the procedure code was thought to represent a relatively well-defined procedure and patient population. Through previous benchmarking work, PCT1 was already aware that carpal tunnel surgery rates were high locally and wanted to address this issue.
Overview of carpal tunnel syndrome
Carpal tunnel syndrome results from pressure on the median nerve as it passes through the carpal tunnel of the wrist. Pressure is much higher in patients with CTS than in patients with asymptomatic wrists and is raised by wrist or finger flexion. 93 Chronic CTS has insidious onset and is related to several prognostic factors including pregnancy, diabetes mellitus, hypothyroidism, osteoarthritis, gout, overweight and occupation. 94 The annual incidence of CTS in UK primary care is approximately 88 per 100,000 males and 193 per 100,000 females. 95 Incidence peaks in the late fifties for women and the late seventies for both men and women. 96 Although most people present with CTS in one hand, more than 50% subsequently develop bilateral CTS. 96
Compression of the median nerve produces pain, paraesthesia (pins and needles) and numbness in the hand. Mild CTS presents with intermittent symptoms often at night. As symptoms progress, paraesthesia may become constant and interfere with daily activities such as holding objects. Severe CTS may cause permanent atrophy of the thenar muscles and permanent loss of sensation in the hand. There is no gold standard for diagnosing CTS. In practice, diagnosis can be by clinical examination and careful history-taking, alone or in combination with electrophysiological testing (i.e. nerve conduction and/or electromyography). 97 Specific diagnostic signs and symptoms such as Phalen’s manoeuvre, Tinel’s sign, and the Durkan test and the patient-completed Katz hand diagram may contribute to the diagnosis. 94 Electrophysiological tests stimulate the median nerve and measure the muscle or sensory response at a point further along the nerve pathway94 with the aim of quantifying the severity of the damage and define the pathophysiology. Prognosis varies with the duration and severity of symptoms. 98,99 A cohort study of 196 patients (274 hands) with CTS observed symptomatic improvement in 34% after 10–15 months of non-surgical care. However, a further 21% reported worsening of symptoms. 98 People with mild to moderate symptoms may respond well to non-surgical interventions. 100 In more severe cases CTR surgery is often performed.
Overview of evidence on non-surgical interventions
There are many putative non-surgical treatments for CTS, of which relatively few are supported by high-quality evidence. 100 The most commonly described treatments for CTS are hand splinting, injection of steroids and oral steroids. 93 A Cochrane systematic review100 of non-surgical treatment for CTS found limited evidence, from one small trial at high risk of bias, that nocturnal hand splinting was better than control (no treatment) at improving symptoms and function in the short term. The Cochrane review also found moderate evidence (consistent findings in more than one RCT) to support the short-term benefit of oral steroids101–103 and therapeutic ultrasound,104,105 and limited evidence (findings from one RCT) for the effectiveness of yoga106 and carpal bone mobilisation. 107
Evidence from a second Cochrane systematic review108 indicated that local corticosteroid injections provided greater improvement in clinical symptoms after 1 month than placebo injections. Two RCTs109,110 (141 participants) at low risk of bias demonstrated a significant increase [risk ratio (RR) = 2.58; 95% CI 1.72 to 3.87] in the incidence of short-term (2–4 weeks) clinical improvements after steroid injection. These two studies recruited patients whose symptoms were refractory to prolonged periods of conservative care (> 3 months110 and 6 weeks of hand splinting109). In both trials symptomatic improvement was achieved in ≥ 70% of patients who had steroid injection, but in only 20–34% of patients in the placebo arm. The Cochrane review authors concluded that, while local corticosteroid injection provided greater clinical improvement in the short term, relief beyond 1 month had not been demonstrated.
Overview of surgical interventions and guidelines for surgery
The number of CTR procedures performed by the NHS has increased since the early 1990s. 96 HES data document a 41% increase in NHS CTR surgery in England in the decade between 2001/2 (n = 39,724 procedures) and 2010/11 (n = 55,957). CTR surgery is usually preformed as a day case procedure under local anaesthesia. 111 Open decompression of the transverse carpal ligament involves an incision in the wrist and then the roof of the carpal tunnel is cut. Surgery can also be done endoscopically; the endoscope is passed through the incision and the transverse carpal ligament is cut from within. Comparisons of endoscopic and open surgical techniques indicate that success and complication rates are broadly similar112,113 and selection of technique is largely based on surgeon preference. 93,113 Potential complications of surgery include infection, bleeding, nerve injury, scarring, persistence or return of CTS symptoms and, rarely, complex regional pain syndrome. 111
The British Society for Surgeons of the Hand (BSSH) recommends surgery for patients with severe symptoms (constant numbness or pain, wasting and/or weakness of thumb muscles) or in mild and moderate cases where conservative treatment has failed (unchanged or increasing severity of symptoms after 3 months). 114 The BSSH suggest that nocturnal wrist splints, activity/workplace modification, hand therapy and/or steroid injections should be considered first in mild and moderate cases of CTS. The BSSH also recommends that electrophysiological testing be used only in equivocal cases (e.g. atypical symptoms, suspected neuropathy or medico-legal issues).
Specific aims of this chapter
The aims of this chapter are:
-
to conduct a rapid systematic review of the evidence on the clinical effectiveness and cost-effectiveness of CTR surgery compared with conservative care
-
to discuss the potential causes of and responses to the high rate of CTR surgery in PCT1.
Methods
Defining the research question
We first mapped the A65 procedure code to a clinical question by defining the patient group, intervention, comparators and outcomes of interest. The vast majority of patients (≈ 95% nationally and in PCT1) had ICD-10 diagnosis codes indicating ‘mononeuropathy of upper limb’ while the remainder had diagnoses of forearm fracture or fibroblastic disorders. Almost all procedures (> 99% nationally and in PCT1) were coded as ‘carpal tunnel release’. The procedure code includes both open and endoscopic procedures and was not strongly negatively correlated (i.e. Pearson’s rho < –0.15) with any other OPCS procedure, which indicates that the high rate in PCT1 was not due to the substitution of one surgical procedure for another. Based on these factors the inclusion criteria for our rapid systematic review and technology appraisal were (1) types of studies – RCTs or systematic reviews of RCTs; (2) participants – patients with a clinical diagnosis of CTS with or without electrophysiological confirmation; (3) intervention(s) – any surgical intervention; (4) comparator(s) – any non-surgical intervention, including no treatment; and (5) outcome measures – any reported outcome at 1 month or more after randomisation.
Search strategy
We searched the Cochrane Library and Database of Abstracts of Reviews of Effects (DARE) databases to identify relevant systematic reviews using the term ‘carpal tunnel syndrome’. To find RCTs we searched MEDLINE and EMBASE on Ovid and Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library. We combined terms for CTS and CTR surgery with a sensitive RCT filter, recommended by the Cochrane Handbook115 (see Appendix 2). Databases were searched from inception to January 2012 and all searches were performed in January 2012. Studies published in languages other than English, letters to journals and abstracts of conference papers were excluded.
Titles and abstracts identified by the searches were screened independently by two reviewers (TM and JB). The full text of potentially eligible studies was assessed for inclusion and data were extracted by one reviewer (TM) and checked by a second (JB). At both stages, disagreements were resolved by a third reviewer (WH). Two reviewers (TM and WH) independently assessed the studies using The Cochrane Collaboration’s tool for assessing risk of bias. 116
Effect sizes were calculated based on the groups to which patients were randomised including all available cases reported by the authors. For dichotomous outcomes, RRs were computed. The published reports did not always contain sufficient information on the outcomes of interest. We contacted the authors of two trials117,118 and subsequently received unpublished data from one117 to supplement our analyses. We also used unpublished data from an additional RCT119 that had been previously obtained by a Cochrane review. 120
We used a forest plot to graphically display the results of primary studies. Visual inspection of the graphs and the I2 statistic121 were used to investigate statistical heterogeneity between primary studies. Because of substantial differences in the non-surgical interventions (e.g. splinting, injection or other) used as comparators and high variance in study findings, we elected to provide a narrative summary of results rather than pool results in a meta-analysis. All analyses were completed using Review Manager software (RevMan version 5.1, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
The search strategy identified 3677 references after we removed duplicates. We found 26 titles and abstracts relevant to this review. On examination of the full text of these papers we excluded 12 reports that did not meet our inclusion criteria (Figure 6). Full details of excluded studies are provided (see Appendix 3). Fourteen papers that described the results of six RCTs met our inclusion criteria (see Appendix 4).
FIGURE 6.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for selection of RCTs.

Four trials118,119,122,123 recruited from a single centre while two trials117,124 were multicentre. All participants had a clinical diagnosis of CTS including electrophysiological diagnostic criteria although electrophysiological parameters measured and diagnostic thresholds varied between RCTs (see Appendix 5). Patients with severe CTS (e.g. thenar atrophy) or atypical aetiology (e.g. recent wrist trauma) were excluded from all but one trial122 and most trials excluded patients who had undergone previous wrist surgery. The duration of CTS symptoms at baseline, where reported, was highly variable, ranging from a mean of just over 30 weeks123 to a mean of 3.5 years,122 and a large proportion of patients had bilateral symptoms. Although many studies reported electrophysiological measurements at baseline, it was difficult to compare findings between studies because of the plethora of different protocols used and measures reported.
There were three comparisons between surgery and splinting;118,122,124 two comparisons of surgery versus corticosteroid injections;119,123 one comparison of surgery versus splinting and injection;118 and one comparison of surgery versus a combination of non-steroidal anti-inflammatory drugs (NSAIDs), hand therapy and exercises. 117 Open surgery was used in five trials,118,119,122–124 with the sixth allowing either open or endoscopic surgery. 117 Splint use varied: the oldest RCT122 used a plaster splint completely immobilising the hand for 1 month; such splinting is no longer advocated. 114 Other studies describe splinting at night for 6 weeks124 up to 3 months. 118 The type of steroid and frequency of injection also varied. Two trials evaluated a single injection of methylprednisalone119 or triamcinolone,118 while the third evaluated one or two (if symptoms persisted) injections of paramethasone. 123 Participants in the non-surgical arm of the final trial117 had a multimodal intervention including NSAIDs, hand therapy, splint use and, if symptoms did not improve, therapeutic ultrasound.
Follow-up varied between 20 weeks119 and 18 months124 and there was little consistency in the measures of symptoms and function at follow-up (see Appendix 5). Secondary outcomes measured in some trials included electrophysiological test results, sleep disruption, grip strength, days of lost work, days of limited activity, patient satisfaction and the need for further surgery or other treatment.
Most trials were judged at low risk of selection bias but at high risk of performance bias as patients, clinicians and in some cases outcome assessors were aware of the treatment received (Table 14). Loss to follow-up rates varied between 0%119 and 25%123 at final follow-up. In some trials the reasons for excluding patients from follow-up appear to be inappropriate, relating to lack of compliance with randomly allocated treatment or lack of satisfaction with treatment results. In two of the otherwise higher quality studies, between 16% and 23% of patients randomised to surgery did not receive it and approximately 40% of patients randomised to non-surgical care had surgery at some point during the trial follow-up. 117,124
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | |
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | ||
| Garland et al. 1964122 | ? | ☹ | ☹ | ☹ | ☺ | ? | ☹ |
| Gerritsen et al. 2002124 | ☺ | ☺ | ☹ | ☹/☺a | ☹ | ☺ | ? |
| Hui et al. 2005119 | ☺ | ☺ | ☹ | ☹/☺a | ☺ | ? | ☺ |
| Jarvik et al. 2009117 | ☺ | ☺ | ☹ | ☹ | ☺ | ☺ | ? |
| Ly-Pen et al. 2005123 | ☺ | ☺ | ☹ | ☹ | ☹ | ? | ☺ |
| Ucan et al. 2006118 | ? | ☺ | ☹ | ☹ | ☹ | ? | ☺ |
Four trials117,119,123,124 provided data on symptom improvement at 3, 6 or 12 months (Figures 7–9). Gerritsen et al. 124 found that patients in the surgery group were more likely to have a successful outcome (‘much improved’ or ‘completely recovered’) at 3 months than the non-surgery group (RR = 1.49, 95% CI 1.18 to 1.86). Jarvik et al. 117 also found that a higher proportion of patients in the surgery group had a 50% or greater improvement in symptom scores at 3 months than in the non-surgical group (RR = 7.69, 95% CI 1.89 to 32.19). Similarly Hui et al. 119, who measured outcomes at 20 weeks, report that a higher proportion of patients in the surgery group had a 50% or greater improvement in global symptom scores than in the steroid injection group (RR = 2.18, 95% CI 1.39 to 3.42). Two trials with longer follow-up found that the relative effect of surgery diminished over time, but was still evident at 12 months. 117,124 In contrast, Ly-Pen et al. 123 found that fewer patients in the surgical group had a 50% or greater improvement in nocturnal paraesthesiae at 3 months than in the group randomised to steroid injection (RR 0.70, 95% CI 0.66 to 0.96), but outcomes were better in the surgical group by 12 months.
FIGURE 7.
Clinical improvement in functioning or symptoms after 3 months. M–H, Mantel–Haenszel. a, Data from Hui et al. 2005119 measured at 20 weeks (5 months). b, Data from Jarvik et al. 2009117 include unpublished data from the authors. c, Data from Ly-Pen et al. 2005:123 the authors report results based on a denominator of the number of patients randomised, assuming that all patients not followed up did not have clinical improvement. To be consistent with the analyses in the other RCTs we calculated the denominator at each follow-up to be the number of patients followed up plus treatment failures documented by the authors.
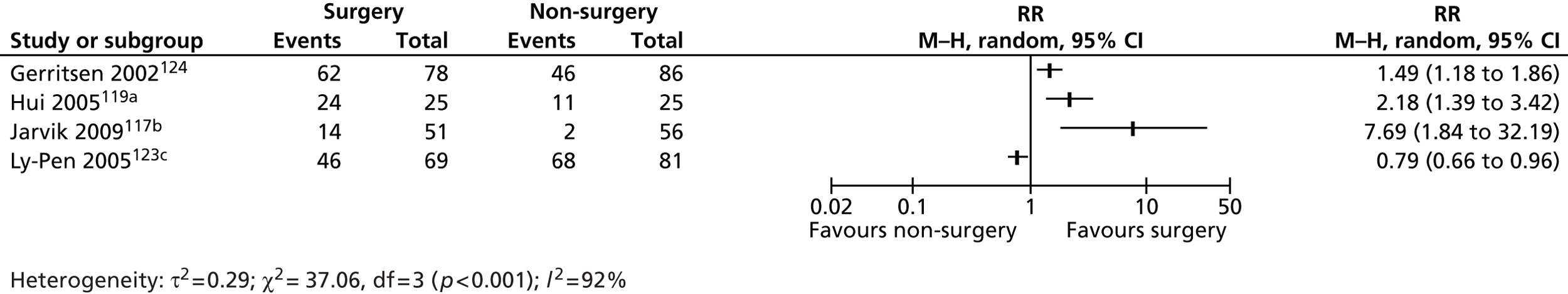
FIGURE 8.
Clinical improvement in functioning or symptoms after 6 months. M–H, Mantel–Haenszel. a, Data from Jarvik et al. 2009117 include unpublished data from the authors. b, Data from Ly-Pen et al. 2005:123 the authors report results based on a denominator of the number of patients randomised, assuming that all patients not followed up did not have clinical improvement. To be consistent with the analyses in the other RCTs we calculated the denominator at each follow-up to be the number of patients followed up plus treatment failures documented by the authors.

FIGURE 9.
Clinical improvement in functioning or symptoms after 12 months. M–H, Mantel–Haenszel. a, Data from Jarvik et al. 2009117 include unpublished data from the authors. b, Data from Ly-Pen et al. 2005:123 the authors report results based on a denominator of the number of patients randomised, assuming that all patients not followed up did not have clinical improvement. To be consistent with the analyses in the other RCTs we calculated the denominator at each follow-up to be the number of patients followed up plus treatment failures documented by the authors.

One RCT117 reported that, in post-hoc subgroup analysis, surgery was more effective than the non-surgical intervention in patients with worse (> 5 ms) median motor latency times on nerve conduction studies at baseline. However, in patients with better (< 5 ms) median motor latency times there was little or no long-term benefit of surgery. One RCT also included an economic evaluation. 125 Total costs in the surgery and splinting treatment groups were €2126 and €2111 respectively. The estimated cost of per quality-adjusted life-year (QALY) was €353 (£285), suggesting that surgery was a cost-effective intervention.
Discussion
Main findings
Based on a case study of CTR surgery, where local use was 20% higher than the national average, we conducted a rapid technology assessment. Six RCTs which randomised approximately 600 patients have provided evidence on the clinical effectiveness and cost-effectiveness of CTR surgery compared with a variety of non-surgical therapies. All RCTs were vulnerable to detection bias and/or performance bias due to lack of blinding. We elected not to pool study findings because of the differences in comparison groups and outcome measures. Three of four RCTs that reported findings at 3 months concluded that clinical improvement in function or symptoms was better in patients randomised to surgery. In two of these three RCTs the benefit persisted, albeit attenuated, at 6 and 12 months. One RCT concluded that CTR surgery was more cost-effective than wrist splinting. However, the RCTs provide very little information to help surgeons or commissioners identify the patient subgroups where the costs and risks of surgery counterbalance the health benefits.
Strengths and weaknesses
We elected to conduct a rapid systematic review in order to complete it in a short time frame, close to that which would be needed in a real-world commissioning setting. We chose a narrow research question and limited our search to a small number of key electronic bibliographic databases, excluding papers not published in English. Rapid reviews risk exacerbating publication bias if studies in the grey literature or foreign language journals are missed. 126 However, our search identified all RCTs included in a previous Cochrane review on the topic. 120 We also retained the Cochrane risk of bias tool in order to describe the quality of the RCTs included in our review.
Our review was based on aggregate data provided in the published report of RCTs rather than individual patient data (IPD). IPD have several potential advantages including the ability to assess how covariates (e.g. patient age, and symptom duration and severity) modify the aggregate treatment effect. 127 These nuances, which remain hidden in aggregate data, would be very valuable for commissioners and clinicians wishing to tailor threshold policies to target the clinical subgroups likely to benefit most from surgery. However, IPD analyses tend to be more time-consuming and potentially introduce bias if IPD can be obtained from only a proportion of all RCTs. By reviewing evidence from randomised trials we have focused on higher quality evidence. Prospective cohort studies of patient outcomes after surgical and non-surgical care128,129 can provide useful information to help predict the types of patients who will have poor outcomes after treatment. However, cohort studies are vulnerable to selection bias.
Implications
The benchmarking exercise and rapid technology assessment leave PCT1 with a dilemma. It is evident that over a number of years the CTR procedure rate, adjusted for proxies of clinical need, has been significantly higher than the national average. However, evidence from the best available RCTs suggests that surgery is more effective and cost-effective than many forms of non-surgical care in the average patient with mild to moderate CTS. Apart from one post-hoc subgroup analysis, there is no trial evidence available to help refine the indications for surgery.
The benchmarking indicates that PCT1 is an outlier, but commissioners cannot be certain whether the local rate is too high, appropriate or even too low. Although the evidence suggests that CTR is likely to be cost-effective in the average patient with mild to moderate CTS, it might still be overutilised locally in patients where the benefit does not justify the cost and risks. If it wants to act to reduce procedure rates, the PCT has several options available to it. It could tighten up the existing CBA thresholds required before surgery will be funded (the thresholds for PCT1 and neighbours are provided in Table 15); for example by demanding longer trials of splinting or corticosteroid injections or requiring prior authorisation from the PCT before surgery. Emerging evidence indicates that the newly established CCGs frequently use this approach in an attempt to trim costs. 9 However, PCT1 already stipulated a relatively lengthy 6-month trial of conservative care before surgery was allowed in mild to moderate cases. Furthermore, for procedures such as CTR where some criteria are subjective (e.g. symptoms significantly interfere with daily activities) this approach can easily be undermined by patients or clinicians who wish to game the system. Alternatively, the PCT could introduce tougher referral management systems ranging from light touch peer review of GP referrals through to new referral management centres and prior approval to triage patients. However, neither approach is cheap and there is a dearth of evidence that referral management centres are cost-effective. 75
| PCT | Criteria for CTR surgery |
|---|---|
| PCT1 (October 2011) | Severe symptoms at presentation (e.g. sensory blunting, muscle wasting or symptoms significantly interfere with daily activities) OR No improvement after 6 months of conservative management (nocturnal splinting and steroid injection) of mild to moderate symptoms Nerve conduction studies are NOT generally needed to confirm the diagnosis |
| Neighbour PCTA (April 2011) | Acute severe symptoms uncontrolled by conservative measures, particularly in pregnancy, significantly interfere with daily activities OR Neurological deficit, i.e. sensory blunting or weakness of thenar abduction (wasting or weakness of abductor pollicis brevis) OR Mild to moderate symptoms with failure of conservative management (4 months) Nerve conduction studies are NOT generally needed to confirm the diagnosis |
| Neighbour PCTB (June 2011) | Neurological deficit is present (e.g. sensory blunting or weakness of thenar abduction) OR Symptoms not resolved to patients satisfaction after 6 months of conservative treatment (e.g. joint injections, splints, tendon gliding exercises, NSAIDs) from the date of first consultation Prior approval required |
| Neighbour PCTC (May 2012) | Severe neurological symptoms (e.g. constant numbness or disabling pain with wasting of thenar muscles and/or weakness of thumb muscles) OR Moderate symptoms (paraesthesia interferes with activities of daily living or causes constant night waking, etc.) AND Has not responded to a minimum of 3 months of conservative management (including complaint with trial of nocturnal splints, consideration of one corticosteroid injection) Electrophysiological testing is usually reserved for equivocal diagnoses |
A further option would be for the PCT to implement a CTR surgery patient decision aid to ensure that treatment choices are based on a shared decision between patient and clinicians. By providing standardised and balanced information on the disease and the potential benefits and harms of treatment options, these aids should, in theory, increase the patient’s role in the decision and reduce the potential for procedure rates to be driven predominantly by GP or surgeon judgements about the value of the procedure. Trial evidence demonstrates that decision aids typically do decrease the proportion of patients selecting more invasive surgery. 76 The introduction of decision aids is not cheap; it requires staff training and access to the aids plus good support and potentially longer primary care sessions to support decisions. Finally, the PCT might use contractual levers to bring about a change in practice patterns. This might involve cost and volume contracts to discourage providers from exceeding a given volume of procedures or opening up the service to tender from other providers.
PCT1 decided to put CTR surgery out to tender at a locally agreed price and set up a prior approval process. The tender was awarded to providers operating at two local independent sector hospitals. Patients still had the option to have surgery at the local NHS hospitals, which continued to provide surgery to patients although a reduced number. The likely effect of this on the local procedure rate is unclear. If providers consider the price per procedure too low or the prior approval system too cumbersome, this will act as a disincentive for them to offer surgery, particularly in complex cases. It is clear, however, that it will change the relative balance between costs to the NHS and the benefits and risks to patients. Despite having a relatively high procedure rate, PCT1 may achieve more cost-effective care for its patients with CTS than other PCTs, by keeping the cost of surgery low. However, this does assume that the quality of the service, which was monitored by the PCT clinical quality team, was not affected by transferring more care to the independent sector.
Unanswered questions
Existing RCT publications on the average (cost-)effectiveness of a procedure have a limited value to commissioners and clinicians who are interested in identifying marginal subgroups of patients where the costs and risks of surgery may outweigh the benefits. It has been argued that open sharing of IPD from completed RCTs should be mandated to facilitate subsequent subgroup analyses, potentially pooling data across similar trials to increase statistical power. 130 For example, if the preliminary finding that carpal tunnel surgery is ineffective in patients with least impairment in median motor latency times is replicated in other existing trials, then surgical criteria could be adjusted accordingly. An alternative is to commission new trials with eligibility criteria targeting patient subgroups where the benefit of surgery is believed to be most questionable. However, selecting eligibility criteria which maintain sufficient clinical equipoise for surgeons to participate in the trial will be difficult in established procedures such as CTR. A further approach is to use geographical variation as a natural experiment67 and conduct observational research to compare processes and outcomes of care between high and low procedure rate regions and explore whether or not higher intensity of care is associated with better outcomes. 87 However, these approaches do not provide a short-term solution to the dilemma faced by commissioners.
Conclusions
Benchmarking can help local decision-makers identify procedures where local utilisation is substantially higher than the national average, even after adjusting for proxies of clinical need. This might represent local overutilisation and an opportunity to provide better value care for the local population through reinvestment of funds in other, more cost-effective, interventions. However, evidence is often not sufficient to identify the appropriate indications for the procedure. This leaves local commissioners with a dilemma: they can either allow clinicians to continue to operate at a higher rate than their peers elsewhere in the country or try to enforce standardisation around a norm that is not fully evidence based but might help them meet their duty to balance the budget.
Chapter 6 Case study 2: interventions for treating posterior capsule opacification – a rapid systematic review
Background
As a result of the project team meeting we selected ‘incision of capsule of lens’ (OPCS code C73) at PCT2. This procedure was selected by the PCT representatives and the research team because procedure rates were substantially higher than the national average after adjustment for clinical need and the procedures had an important financial impact on PCT spending. In addition, the procedure code was thought to represent a relatively well-defined procedure and patient population.
Overview of posterior capsule opacification
Capsulotomy is typically performed in people who have developed PCO following implantation of a posterior chamber intraocular lens (IOL) for age-related cataract. The natural lens is held in place in the eye by a structure called the capsule. During cataract surgery the anterior part of the capsule is removed (or partially removed) to facilitate removal of the natural lens but the posterior capsule of the lens is left intact and in place. The new synthetic IOL is placed on the posterior capsule, which acts as a scaffold or structure to support the new lens. 131 Lens epithelial cells (LECs) are the cause of PCO and can affect vision in two ways. Pearl-type PCO is caused by LECs from the anterior chamber migrating to the lens over the axis of vision, proliferating and then becoming cloudy as collagen is laid down and lens fibre regeneration occurs. Fibrosis-type PCO is caused by proliferation and migration of LECs which transform to myofibroblasts, causing wrinkling and contraction of the posterior capsule, deformation of the capsule bag, and decentralisation of the newly inserted lens, thus reducing visualisation of the peripheral retina. Both processes cause vision to degenerate, light becomes scattered and patients suffer from glare from lights at night and reduction in visual acuity (VA). 131–133 PCO or ‘secondary cataract’ is the most common complication of cataract surgery;134 incidence is reported to be 12%, 21% and 28% at 1, 3 and 5 years respectively. 135
Overview of capsulotomy
Posterior capsule opacification is most often treated using a neodymium:yttrium–aluminium–garnet (Nd:YAG) laser. 132,136 Quick pulses of the laser make precise ablations in the posterior capsule and create a small circular opening in the visual axis. The treatment is usually done using topical anaesthesia with non-sedated patients. The procedure takes approximately 15 minutes, without the need for surgical cuts or stitches and patients can return to normal activities straight away. 137 Early large case series (n = 2110) supported the effectiveness of the Nd:YAG laser procedure, with 98% of procedures successfully opening the posterior capsule. 138 VA was dramatically improved in 84% of cases, with 81% improved to at least 20/40 vision. 138 In a second case series (n = 595), VA improved by at least two Snellen lines in 75% of cases at 6–12 months after the procedure. 139 Improvement in VA was swift: 30% within 24 hours; 75% within 1 week. 139 Contrast sensitivity and glare sensitivity in patients with PCO also improved for all visual angles tested after capsulotomy. 140
Neodymium:yttrium–aluminium–garnet laser capsulotomy may cause complications. Data reported in initial safety studies in the 1980s suggest that, at that time, operative complications included (1) damage (pitting or marking) to the IOL in 20% of cases; (2) rupture of the hyaloid face in 19% of cases; and (3) other, rarer, operative complications such as corneal oedema, bleeding and iris damage in less than 1% of cases. Postoperative complications included (1) raised intraocular pressure – 39% of cases experienced a > 5-mmHg increase in pressure 2–6 hours after the procedure and in 28% of cases the pressure was greater than 30 mmHg; 1% of cases had persistent elevation of pressure at 3–6 months – (2) other, rarer, postoperative complications such as cystoid macular oedema, retinal detachment, pupillary block glaucoma, retinal haemorrhage, iritis and vitritis (which occurred in up to 1.2% of cases). 138 Improved cataract surgery and capsulotomy techniques have reduced the incidence of many of these complications. 141 To further minimise these risks the Royal College of Ophthalmologists (RCOphth) guidelines recommend the use of minimal laser energy to avoid pitting the IOL and note that some clinicians routinely give a hypotensive agent immediately after treatment. 142 Nevertheless a recent survey of UK practice identified considerable variation in yttrium–aluminium–garnet capsulotomy technique and postcapsulotomy management, and highlighted the need for evidence-based guidelines. 143
Other preventative measures focus on improving cataract surgery to prevent the development of PCO and the need for capsulotomy. A meta-analysis of 13 studies of 1456 eyes found strong evidence that fewer eyes fitted with sharp-edge IOLs went on to have laser capsulotomy than those with round-edge IOLs. The review authors also concluded that there was no clear evidence of a difference in outcomes between optic materials, although some studies found that silicone IOLs resulted in less PCO. Few studies evaluated the choice of post-operative anti-inflammatory treatment, but, in those that did, there was little evidence of an effect on PCO development. 144
Specific aims of this chapter
The aims of this chapter are to:
-
conduct a rapid systematic review of the evidence on the clinical effectiveness and cost-effectiveness of capsulotomy and current UK guidelines on the use of this procedure
-
discuss the potential causes of the high rate of day case or inpatient capsulotomy among PCT2 patients.
Methods
Defining the research question
Before embarking on the rapid systematic review, we translated the procedure (capsulotomy) into a critical appraisal question by defining the patient group, intervention, appropriate comparators and outcomes of interest. From work described in Chapter 4 (see Table 13), the predominant reasons for performing capsulotomy were coded as ‘other cataract’ (89% nationally versus 83% locally) and ‘complications of . . . implants’ (3% nationally and 14% locally). The gender and age distribution of local patients was similar to the national picture, as was the high proportion of procedures (≈ 95%) that were done as a day case rather than inpatient. Despite the discrepancy between diagnosis codes, which may well be due to local coding practices, Table 13 suggests that patients treated locally are broadly similar to those treated nationally. The OPCS-4 three-character code for capsulotomy (C73) is a broad grouping of six subcodes, giving the opportunity for coders to describe the procedure in more detail. We examined the HES data for this code (C73) and found that, in PCT2 during 2009/10, more than 99% of these procedures were coded as ‘C73.3 Capsulotomy of the posterior lens capsule’ (Table 16). This is quite different from the national situation, where 30% of procedures are coded as ‘C73.4 Capsulotomy of lens not elsewhere classified’. It is unclear whether this represents actual differences in the types of procedure being performed locally or merely variation in coding preferences across the country. Nevertheless, it is clear that it is posterior capsulotomy that dominates in PCT2 and elsewhere.
| Procedure | PCT2 (%) | Other PCTs (%) |
|---|---|---|
| Capsulotomy of posterior lens capsule (C733) | 1259 (99.29) | 10,208 (65.70) |
| Capsulotomy of anterior lens capsule (C732) | 6 (0.47) | 312 (2.01) |
| Capsulotomy of lens not elsewhere classified (C734) | 3 (0.24) | 4808 (30.95) |
| Other | 0 (0.00) | 209 (1.35) |
High use of day case or inpatient posterior capsulotomy in PCT2 may result from a number of reasons. One possibility is that an alternative surgical intervention is used in other PCTs to treat PCO. However, our scoping of the literature did not suggest that this was likely. Given this, in our rapid systematic review, we considered any other non-surgical intervention or watchful waiting as suitable comparator interventions for our technology assessment. Outcomes included VA and measures of vision-related quality of life. As our scoping searches indicated very little trial evidence on the effectiveness of capsulotomy for PCO, we decided not to restrict our review to those studies that measured specific outcomes. Based on the preliminary steps described above, we systematically reviewed the literature to assess the effects of capsulotomy for treating PCO. The inclusion criteria are described in Table 17.
| Criterion | Inclusion criteria |
|---|---|
| Types of studies | 1. RCTs. 2. Systematic reviews |
| Types of participants | Adults who have undergone cataract surgery for age-related cataract and who have been diagnosed with PCO |
| Types of interventions | Any surgical (including laser) intervention |
| Comparator interventions | Any non-surgical intervention, including no treatment or delayed procedure |
| Types of outcome measures | Any reported outcome |
Search strategy
Existing systematic reviews were sought through searches of DARE and The Cochrane Library. Clinical guidelines produced in the UK were also sought to identify the current consensus on the use of capsulotomy among ophthalmologists. To find primary studies (RCTs) we searched MEDLINE, EMBASE and Cochrane CENTRAL using methods and filters described in the Cochrane Handbook in November 2011. 115 We combined terms for PCO, capsulotomy and Nd:YAG laser with a sensitive RCT filter, recommended by the Cochrane Handbook for use in MEDLINE, on Ovid and in EMBASE (see Appendix 6). We searched The Cochrane Library and DARE database to identify relevant systematic reviews in November 2011 (see Appendix 6). Studies published in languages other than English, letters to journals and abstracts of conference papers for which no full study publication was available were excluded. We searched for UK guidelines on the websites of NHS Evidence, NICE, the Scottish Intercollegiate Guidelines Network and the RCOphth. We used simple terms for ‘posterior capsule opacification’; if no relevant records were identified we used the broader term ‘cataract’.
Titles and abstracts identified by the searches were screened for relevance independently by two reviewers (TM and JB). Disagreements were resolved through referral to a third reviewer (WH). Studies that appeared potentially relevant were ordered and full-text papers were assessed for inclusion by one reviewer (TM) and checked by a second (JB). For lack of trial evidence we did not pool RCT data and instead provide a narrative summary of clinical guideline recommendations.
Results
Results of search for systematic reviews and randomised controlled trials
We found no reviews of interventions for treating PCO. The search of DARE found 10 references: one was on the incidence of PCO and the rest were on cataract surgery techniques or interventions aimed at preventing PCO. The searches for RCTs identified 1249 references (Figure 10) after we removed duplicates. However, there were no RCTs comparing Nd:YAG laser surgery with no treatment or delayed capsulotomy. Details of excluded articles are provided in Appendix 7. The literature on this topic tended to focus on interventions employed during cataract surgery to prevent PCO; use of corticosteroids to prevent LECs from proliferating; exploring in vitro methods to prevent PCO; case series of patient outcomes following Nd:YAG laser surgery; preventing or treating elevated intraocular pressure following Nd:YAG laser surgery; comparison of the effects of different Nd:YAG lasers; and the effect of different sizes of hole cut during capsulotomy.
FIGURE 10.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for selection of PCO RCTs.
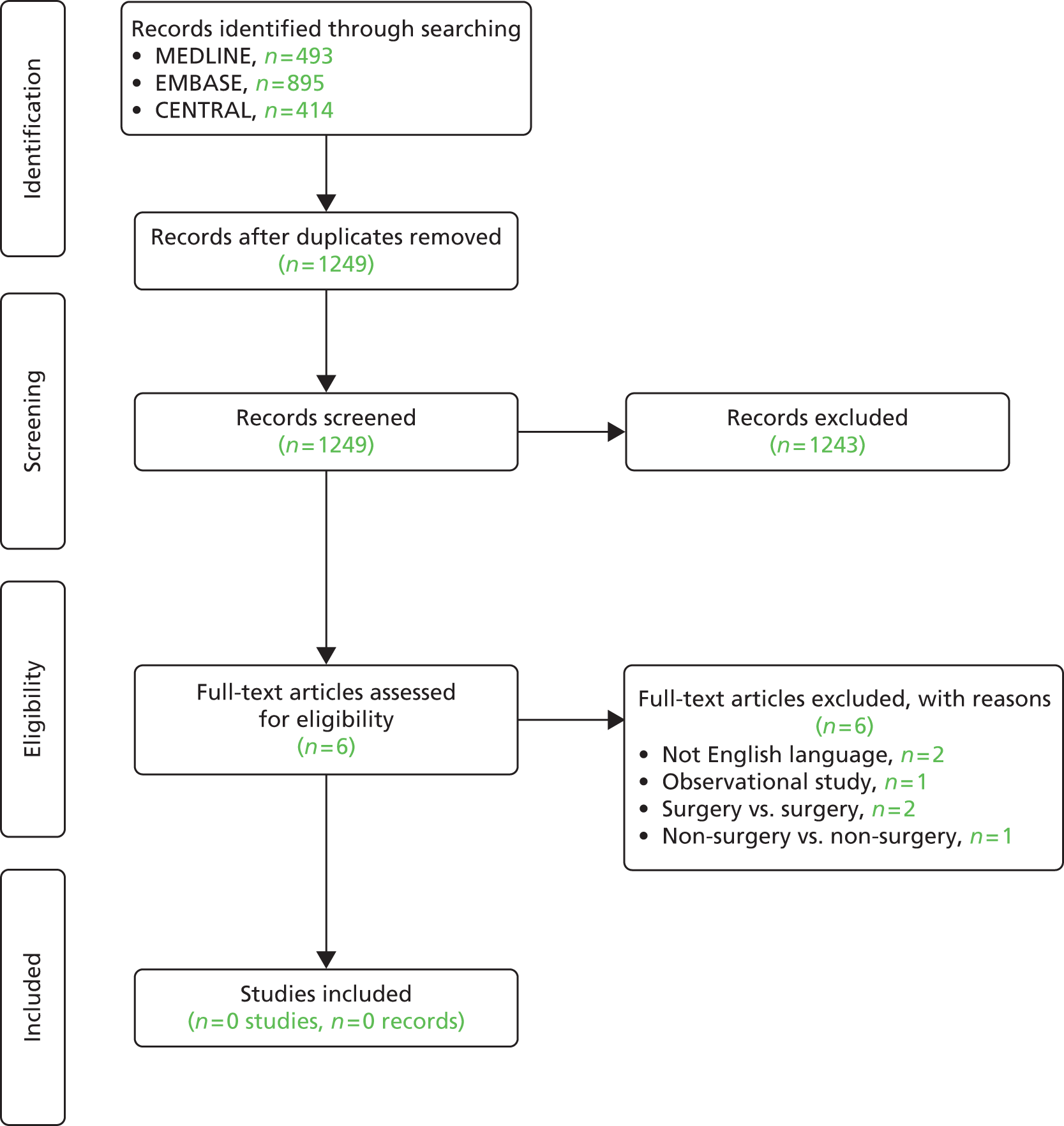
Results of the search for guidelines
The RCOphth guidance on the pathogenesis of PCO draws attention to the effects of different IOL shape, edge profile, haptics (side struts of the IOL that hold it in place) and the material from which they are made on development of PCO. The evidence on these topics was graded 1a (the highest – systematic reviews and RCTs). 142 Specifically, square-shaped IOLs with a sharp optic edge profile are known to inhibit migration of LEC.
The RCOphth recommends that, in treating PCO, a Nd:YAG laser be used and that a more invasive surgical approach is required only rarely. The guidelines do not provide references or a level of evidence for these recommendations. The RCOphth notes that in some units laser treatment is performed by appropriately trained paramedical staff. The RCOphth does not discuss VA thresholds or referral criteria for capsulotomy but does state that PCO should be confirmed by presence of characteristic signs visible on slit lamp examination and that symptoms are more important than tests of visual function: severity of PCO correlates poorly with high-contrast VA, and blurred vision, glare, dysphotopsia and reduced contrast in the presence of PCO on slit lamp examination are the common symptoms. We found no national guidance on referral for intervention for PCO,142 although local referral criteria have been developed. 145
Further exploration of the causes of high variation
Capsulotomy rates will, to some extent, be dependent on rates of the initial cataract surgery. However, the evidence suggests that the rate of cataract surgery between 2007/8 and 2009/10 was only slightly higher in PCT2 than the rest of England (Table 18). The only clear difference evident in this table is in the proportion of patients readmitted for inpatient or day case capsulotomy within 1 or 2 years of cataract surgery. The PCT2 rate is approximately 5% per year compared with approximately 1% per year elsewhere. This raises the possibility that some aspect of the initial cataract procedure in PCT2 (e.g. the IOL design or pharmacological agents used to prevent PCO) or the thoroughness of follow-up (e.g. frequency of screening for PCO) may be a cause of the high capsulotomy rate. However, the PCT2 readmission rate for capsulotomy is still below the literature estimate of the annual incidence of PCO following cataract surgery, which is estimated to be 12% at 1 year. 135
| Characteristic | PCT2 | Other PCTs |
|---|---|---|
| n | 15,382 | 957,121 |
| Rate per 100,000 residents | 640.12 | 618.9 |
| Diagnosis, n (%) | ||
| Senile nuclear cataract (H251) | 6269 (40.76) | 156,760 (16.38) |
| Cataract, unspecified (H269) | 4693 (30.51) | 616,825 (64.45) |
| Other senile cataract (H258) | 2979 (19.37) | 33,819 (3.53) |
| Other | 1441 (9.37) | 149,717 (15.64) |
| Procedure, n (%) | ||
| Insertion of prosthetic replacement for lens NEC (C751) | 15,227 (98.99) | 948,685 (99.12) |
| Removal of prosthetic replacement for lens (C753) | 83 (0.54) | 1180 (0.12) |
| Revision of prosthetic replacement for lens (C752) | 45 (0.29) | 3116 (0.33) |
| Other | 27 (0.18) | 4140 (0.43) |
| Male, n (%) | 6186 (40.22) | 390,013 (40.75) |
| Mean age (SD) (years) | 75.16 (11.17) | 74.55 (10.97) |
| Age (years), n (%) | ||
| 0–39 | 142 (0.92) | 8805 (0.92) |
| 40–59 | 1119 (7.27) | 74,407 (7.77) |
| 60–69 | 2544 (16.54) | 168,180 (17.57) |
| 70–79 | 5499 (35.75) | 360,417 (37.66) |
| ≥ 80 | 6078 (39.51) | 345,319 (36.08) |
| Day case, n (%) | 14,023 (91.16) | 920,983 (96.22) |
| Median episode length (IQR) | 1 (1,1) | 1 (1,1) |
| Readmission, n (%) | ||
| Capsulotomy within 1 year | 248 (5.04) | 2922 (0.96) |
| Capsulotomy within 2 years | 554 (11.26) | 5647 (1.85) |
| Median days to readmission for capsulotomy (IQR) | 506 (270,721) | 515 (189,734) |
In our view the most likely cause of the apparently high rate of inpatient or day case capsulotomy in PCT2 is the uncertainty about whether the procedure should be performed (and coded) as a day case or outpatient procedure. Outpatient procedures are not included in the HES admitted patient data set and are therefore excluded from our analysis. Department of Health reference cost data in 2009/10146 indicate that the majority of capsulotomy procedures (57%) are classified as outpatient rather than day case procedures. It therefore seems quite probable that it is the clinical coding of the setting of care that causes the high rate of inpatient or day case capsulotomy in PCT2. This theory is supported by evidence that many PCTs (see Figure 11) have close to zero capsulotomy procedures recorded in HES admitted patient care data sets. The number of day case or inpatient capsulotomies per cataract procedure performed in PCT2 (Figure 11) was among the top 10 of all PCTs in 2009/10. It seems very likely, therefore, that in many PCTs the vast majority of capsulotomies are recorded as outpatient procedures. This theory was borne out by subsequent investigations in PCT2 that revealed in 2009/10 no (0%) capsulotomy procedures were recorded as outpatient procedures.
FIGURE 11.
Distribution of the proportion of day case or inpatient capsulotomy to cataract procedures.
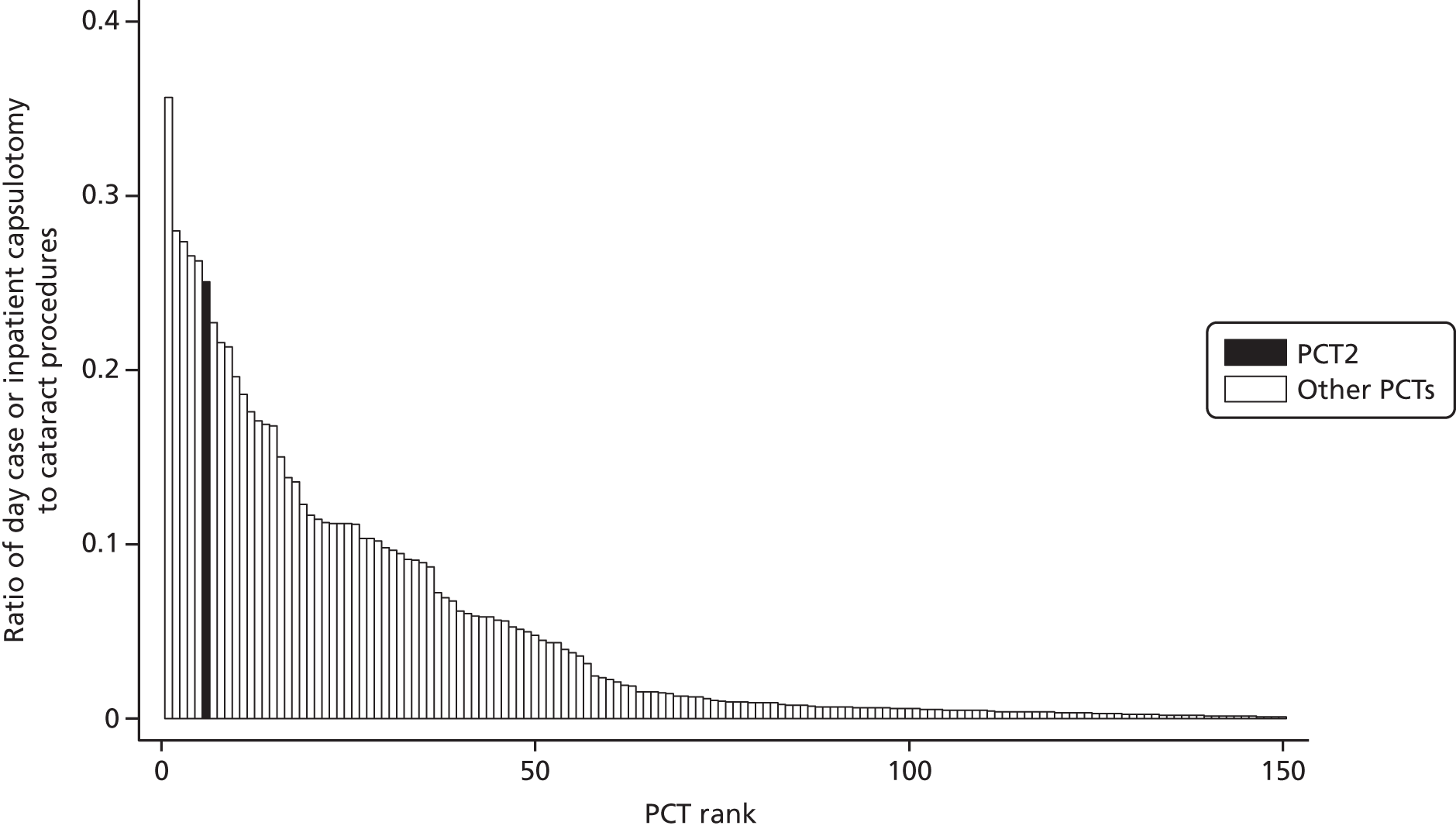
Discussion
Main findings
In 2009/10 PCT2 had 43 (95% CI 39 to 47) more inpatient or day case capsulotomy procedures per 100,000 residents than the national average. We found no systematic reviews or RCTs of surgical versus non-surgical interventions for treating PCO. Despite the lack of RCT evidence, Nd:YAG laser capsulotomy is a well-established method for treating PCO, with more than 14,000 inpatient or day case procedures recorded annually. There is limited national guidance on appropriate thresholds for capsulotomy and most PCTs appear to have no policy in place to guide its use beyond clinical discretion.
Strengths and weaknesses
Working with routine data entails a number of challenges for analysis and interpretation. The analysis relies on accurate coding of clinical activity which is consistent between NHS trusts. This means that, rather than assuming that clinical differences drive the headline procedure rates, the first area for exploration should be the potential for coding anomalies to be the cause of variation. Capsulotomy provides a good example of this. Nationally there is very high variance in day case and inpatient capsulotomy rates between PCTs, and PCT2 is at the upper extreme of the distribution. However, as discussed, this is probably purely because of the setting of care (i.e. other NHS trusts performing capsulotomy as an outpatient procedure rather than a day case) rather than variation in the total number of capsulotomies performed. The HES outpatient data set, available since 2004/5, provides the potential for benchmarking analyses to span settings and address this problem. However, in practice, despite improvements over recent years, the sporadic recording of outpatient diagnosis and procedure codes has limited the value of such cross-setting analyses. 147,148
Our findings in context
Advances in medical technologies mean that over time many more procedures will move from inpatient to day case or day case to outpatients. 149 The Department of Health defines day surgery as ‘day case patients who require full operating theatre facilities and/or a general anaesthetic’. 149 By this definition, most capsulotomy procedures might be more appropriately classified as outpatient procedures. According to NHS reference costs,146 the national average unit cost of performing this procedure (Healthcare Resource Group code BZ04Z) in the outpatient setting is £144 (18,950 procedures in 2009/10) compared with £328 in a day case setting (14,174 procedures in 2009/10). Between 2009/10 and 2011/12, PCT2 achieved a complete transformation in the proportion of capsulotomy procedures recorded as being performed in the outpatient setting (0% to 100%), a potential annual saving of £233,000 [1268 × (£328 – £144)] in hospital reimbursements. Potentially then there are substantial savings for commissioners in moving this procedure from the day case to the outpatient setting, although such a move could have a detrimental impact on provider finances. Aggregate HES data indicate that the number of ‘inpatient/day case’ capsulotomy procedures did not decline substantially between 2007/8 (n = 14,218) and 2012/13 (n = 11,562) suggesting that many local commissioners may still be paying a premium for day case procedures. Even though capsulotomy procedures in PCT2 have been transferred to the outpatient setting, there is still very little evidence on the most appropriate indications for the procedure and therefore little to guide commissioners on the evidence-based referral criteria or appropriate procedure rates.
A recent report by the Audit Commission noted widespread inconsistencies in the way short stay procedures were recorded in the NHS. 150 The report concluded that, while NHS coding guidance could be clarified, the main cause of coding inconsistencies was the reluctance of NHS trusts to code procedures as outpatient procedures because of the negative financial implications of doing so. They also noted that valuable data describing a patient’s diagnoses and treatment are poorly recorded when a service moves from an inpatient to an outpatient setting and lost entirely when a service moves to the community. The Audit Commission emphasised the vital role that new NHS bodies (e.g. NHS England and Monitor) should play in improving coding accuracy and consistency.
Conclusions
PCT2 has a high rate of day case or inpatient capsulotomy, predominantly performed as a day case procedure. This rate cannot be explained by statistical chance or by greater clinical need leading to higher rates of cataract surgery and therefore a higher incidence of PCO. Observational studies suggest that a high proportion of patients have improvements of VA after Nd:YAG laser capsulotomy and the procedure is endorsed in guidelines. However, we found no RCT evidence that demonstrated the effectiveness of Nd:YAG laser capsulotomy compared with conservative treatment or delayed surgery and there was very little information on the appropriate clinical thresholds for performing capsulotomy.
The discrepancy between PCT2 and other PCTs is probably due to the coding of capsulotomy as a day case rather than outpatient procedure. There are substantial potential savings for commissioners in moving this procedure from the day case to the outpatient setting. Coding inconsistencies distort PCT differences in capsulotomy procedure rates and distract attention from a debate about appropriate procedure use and rates.
Chapter 7 Disinvestment in practice: ‘I won’t call it rationing as such . . .’
Introduction
The initial aim of this qualitative study was to investigate how the proposed disinvestment method worked in practice, at the level of two local commissioning groups. There was an intention to use ethnographic methods to understand the barriers and facilitators to the method’s success, thus helping to reform it for future use.
Flexibility was fundamental throughout the conduct of the qualitative study, especially in the light of the recent NHS reforms. It became apparent that the two procedures identified in the benchmarking work (capsulotomy and CTR) would not lead to straightforward interventions for disinvestment, such as tightening of CBA, because of the limited evidence available. This prevented us from road testing the proposed disinvestment method with practising commissioning groups. This realisation came about once data collection had started, but unforeseen insights from concurrent analysis raised new research questions. These new lines of enquiry were pursued for the remainder of the study. In the light of this, the revised aim of this qualitative study was to investigate how disinvestment currently works within naturalistic contexts, at the local level of health-care commissioning. Specific objectives included investigating the processes underlying local disinvestments, and identifying the barriers to successful implementation of disinvestment decisions. Although our focus shifted from the evaluation of a disinvestment method to the investigation of current practices in the field, the qualitative objective of identifying potential barriers to the proposed disinvestment process was still addressed through considering barriers to implementing disinvestment decisions more generally.
This chapter outlines the main findings to emerge from observation and interview data. It begins with a brief summary of the published literature on this area, followed by an overview of the research context and the methodology adopted. Main findings in relation to the following broad areas will then be summarised:
-
Disinvestment in theory: this section will concentrate on interview informants’ understandings of the term ‘disinvestment’.
-
Disinvestment in practice: this section will consider disinvestment practices within the study areas, with a focus on the types of disinvestment encountered/initiated; methods of identifying candidates for disinvestment; commissioners’/providers’ perspectives on experienced disinvestments; and approaches to working through disinvestment processes.
-
Barriers to disinvestment: this section will outline informants’ perceived barriers to disinvestment, and barriers emerging from our analysis of observation and interview data.
The chapter concludes within specific recommendations on how the disinvestment agenda can be progressed in the light of our findings.
What is already known about disinvestment in practice?
Disinvestment has been a topic of considerable discussion within published commentaries and editorials from around the world. 36,151,152 Despite consensus that disinvestment is vital to the survival of health-care systems, there is little empirical evidence on how to approach it, from identification of potential areas for ‘cutting back’ to successful implementation of disinvestment decisions. Commentators from Canada, the UK and Australia have suggested guidance on specific features of the disinvestment process (e.g. identifying candidates). 153,154 Others have proposed full-blown guidance on the entire process. 155 These suggestions remain untested, and disinvestment in practice remains an elusive subject. While disinvestment has featured within previous priority-setting programmes, these have been ‘one off’ exercises, usually introduced by researchers themselves. 156–159 Disinvestment was not the prime focus of any of these studies, most of which report on ‘Programme Budgeting and Marginal Analysis’ exercises that consider investment and disinvestment jointly. Discussion on the disinvestment aspect of these exercises has tended to be brief, if not absent. Overall, these exercises have yielded mixed success in achieving disinvestment, although the reasons behind this have seldom been explored. Some authors have commented that disinvestment was not pursued, or not viewed as a necessity at the time. 157,158 Others report failure at the stage of implementation. 156,158 The context of these previous studies need to be borne in mind, not just in terms of the potentially different economic landscape, but in terms of study design. The exercises tended to include external training and/or facilitation from academic experts, or represented pilot projects. These studies therefore do little to further our understanding of how disinvestment is approached in day-to-day practice (i.e. within a naturalistic context).
Qualitative methods have been applied to investigate health priority setting in practice in a variety of contexts. Some have had a specific focus (e.g. new resource allocation,160 use of technical approaches161). Others, such as recent research by Robinson et al. 162,163 explore more general priority-setting processes. This later body of work used survey and interview methods to gauge the types of priority-setting processes used by local decision-making groups across the English NHS, evaluating these processes based on key criteria derived from the literature (e.g. use of tools, wider engagement and involvement, leadership). The authors reported that disinvestment had not featured heavily on decision-makers’ agendas, but was beginning to receive attention. While there were tools and models to inform priority setting for new resource allocation, these had not been adapted for disinvestment purposes. Commissioners perceived engaging clinicians in priority-setting processes to be difficult, especially when it came to disinvestment, which has potential to reduce revenue. It is not clear whether the above difficulties were based on participants’ actual experiences or theories. Nonetheless, the types of barriers raised support previous (theoretical) commentaries on the challenges of disinvestment. 12
The above studies have paved the way for more in-depth research into disinvestment in practice. Health decision-makers are clearly beginning to turn their attention to disinvestment, but little is known about how disinvestment is understood and negotiated by commissioners and stakeholders. This calls for in-depth, qualitative methods that use inductive approaches to generate evidence. Gaining an initial insight into how disinvestment works in practice will help to uncover detailed and specific barriers to service change. A comprehensive understanding of these issues requires in-depth examination of specific examples and consideration of multiple stakeholder perspectives.
In the light of this research gap, we conducted a qualitative investigation that sought to understand disinvestment as it occurs at the local level of health-care decision-making. Specific objectives of our study were to inductively determine the facilitators and/or barriers to disinvestment, using this to inform recommendations for progressing the disinvestment agenda. An ethnographic approach was taken, with interview and observational data collected from health decision-making groups and stakeholders over a 14-month period. This approach allowed the researcher (LR) to adopt a flexible and iterative approach to data collection, while being as close to the phenomenon under investigation as possible.
Methods
Design and methodology
The study adopted a qualitative design using an ethnographic approach. Two ‘commissioning advisory groups’ situated within separate PCTs were studied over a year. CCGs replaced PCTs following NHS reforms that took place during data collection. The remit of PCTs and CCGs were similar for the purposes of this investigation. Data collection methods included observations of routine commissioning advisory group meetings, combined with semistructured interviews with individuals affiliated with these groups, and individuals who had the potential to be affected by the groups’ decisions.
Observations helped to immerse the researcher (LR) into the field, promoting an in-depth understanding of the context within which those assigned responsibility to disinvest were operating. Interviews allowed further exploration of select topics, from the perspectives of individual members of stakeholder groups involved in or affected by disinvestment. Document analysis of policies and meeting minutes or agendas complemented observations and interviews. As the study progressed, a case study of a specific example of disinvestment was conducted for a more comprehensive view of disinvestment in practice. The case study looked at provision of a given procedure within each region. Data collection consisted of interviews with clinicians whose practice would be potentially influenced by disinvestment, combined with document analysis and informal discussion with commissioning leads from each study site.
Settings and participants
Each commissioning advisory group was responsible for developing commissioning and disinvestment recommendations to the PCTs (replaced by local CCGs as data collection progressed). The PCT/CCG had authority to implement these recommendations locally. Preliminary contact with commissioning group leads checked that the commissioning advisory group meetings were relevant forums for disinvestment decision-making. The groups were situated in sociodemographically contrasting regions of England. Group A served a region that was more rural, with a predominantly white and comparatively wealthy population (PCT1). Group B served a predominantly urban region, consisting of areas with high proportions of ethnic minority populations and pockets of high deprivation (PCT2). Each commissioning advisory group consisted of an array of professional commissioners, including public health consultants, pharmacists, medical directors and directors of finance. Group B also included lay members. Each group hosted regular meetings, attended by representatives from acute NHS trusts and the PCT/CCG in the area.
The selected case study for each region was disinvestment from surgical management of CTS (referred to from here on as ‘CTS surgery’). CTS surgery is a minor orthopaedic procedure, generally conducted under local anaesthetic (also known as CTR or carpal tunnel decompression). Case study clinicians were all hand surgeons who provided this procedure to NHS patients within the study regions. Labels have been used to categorise the various groups of informants participating in this study. Professionals employed by the PCT/CCG have been labelled as ‘commissioners’ (C). Representatives from acute trusts affiliated with commissioning groups are referred to as ‘providers’ (P). Case study clinicians have been labelled as ‘clinicians’ (Clin). Ethical approval for the observation of commissioning group meetings and interviews with individual group members was granted by the University of Bristol, Faculty of Medicine and Dentistry Committee for Ethics (application 111210, January 2012). Ethical approval for the patient and clinician interviews on access to CTS surgery was granted by the National Research Ethics Service Committee South Central – Southampton B (Research Ethics Committee reference 12/SC/0418; August 2012).
Sampling and recruitment
Observations
The researcher carried out non-participant, overt observations of all scheduled commissioning advisory group meetings. Observations continued for each group until LR was satisfied that no new analytical insights would emerge from additional analysis. Group leads circulated study information sheets to group members and meeting attendees 2 weeks prior to the first observation. The chairs of each group introduced LR at the start of the first observed meeting at each site. Group leads were asked to dispatch study information sheets to any new attendees as and when they circulated the agendas for upcoming meetings (usually 1 week prior to meetings).
Interviews
Interview participants were initially purposefully selected with an intention of capturing a wide selection of professionals, from the full range of health-care organisations represented in meetings. This information was gathered from previous minutes of meetings. The group leads were asked to review our selections and suggest other potential participants affiliated with the groups. Additional participants were also selected throughout the study period based on their contributions within observed meetings. A degree of snowball sampling also occurred, whereby interview participants were asked to suggest other potential participants from within their organisations. Participants were also selected with an intention of testing emerging theories towards the later stages of data collection. Recruitment efforts ceased at the point of data saturation, defined as the point at which two consecutive additional interviews produced no new themes, and had no impact on emerging theories.
We intended to recruit any NHS clinician whose practice was potentially influenced by the disinvestment case study. Key informants were initially identified by conducting Dr Foster® web searches for NHS secondary care specialists within the study regions. Additional identification of potential participants proceeded on the basis of snowball sampling. All potential interview informants received an information sheet and invitation letter by e-mail and post, and were asked to return a reply slip indicating whether or not they wished to take part. Reminders were sent if no response had been received within 2 weeks of dispatch.
All but one of the individuals invited to participate in this research agreed to take part. The single individual who declined agreed to participate in observations, but declined a face-to-face interview because of time constraints.
Data collection
Observations
Observations took place between February to December 2012. Group A meetings occurred monthly, with each meeting lasting between 1 and 2 hours. Group B meetings occurred on an irregular basis, with each meeting lasting between 2.5 and 3 hours. All meetings took place in a standard boardroom. The location and physical characteristics of rooms differed for each of group B’s meetings. The setting for group A’s meetings remained constant throughout the observation period. LR sat among the meeting attendees during all observations. Written informed consent was obtained from each individual present at meetings. Meetings were audio-recorded in full and field notes were taken, recording details such as body language, individual/group reactions to speakers and initial analytical thoughts. Detailed accounts and reflections were written after each observed meeting.
Interviews
Interviews were conducted between February 2012 and April 2013. Face-to-face interviews took place within NHS organisations. One interview took place by telephone because of access issues. Each interview lasted between 20 minutes, and 1 hour and 15 minutes. Topic guides (see Appendix 8) were used to maintain consistency in the broad areas covered across interviews. Commissioner/provider topic guides explored understandings of the role of the commissioning advisory groups; views on the groups’ disinvestment decision-making processes; experiences of local disinvestments; and perceptions of the types of challenges experienced during disinvestment. Clinician topic guides explored experiences of disinvestment processes, rationalisations of disinvestment and views on any changes brought about through disinvestment. These topics were all explored in the context of the chosen case study. Topic guides for interviews were revised during data collection on the basis of unforeseen issues being raised by participants, and emerging analytical insights. Interviews were audio-recorded in full once written informed consent had been obtained.
Analysis
Audio recordings of interviews and meetings were transcribed in full using standard notation. Transcripts were analysed thematically using the constant comparison method derived from grounded theory methodology. 164 This involves line-by-line coding of transcripts, categorising codes into themes, and developing codes and themes as transcripts are reread in the light of newly collected data. Analysis was primarily conducted by LR, and supported through use of NVivo (version 9, QSR International, Warrington, UK). A sample (10%) of transcripts from interviews and observations was independently analysed by AO-S midway through data collection. Any differences in coding and thematic interpretations were discussed, and additional areas suggested for addition to the topic guide. Descriptive accounts of observation and interview findings were written once analysis was complete. Matrices for major themes from interviews were drawn up and populated with individuals’ quotes. Informants were grouped according to their role to visualise patterns of meaning within each group’s accounts (i.e. commissioners, providers, clinicians). These differences had become apparent throughout the study, but mapping data in this manner helped to identify ‘negative’ cases that conflicted with emerging theories. These exceptions were revisited by rereading transcripts, and described accordingly in reported findings.
Results
Final sample
Eight meetings were observed in total: five from group A and three from group B (Table 19). Twenty-eight individuals took part in interviews. The breakdown of commissioners, providers, lay members and clinicians interviewed in each region can be seen in Table 20.
| Source | PCT1 (commissioning advisory group A) | PCT2 (commissioning advisory group B) | Total |
|---|---|---|---|
| Meetings observed | 5 | 3 | 8 |
| Informant role | PCT1 (commissioning advisory group A) | PCT2 (commissioning advisory group B) | Total |
|---|---|---|---|
| Commissioner | 5 | 5 | 10 |
| Provider | 5 | 2 | 7 |
| Lay member | 2 | 0 | 2 |
| Clinicians | 4 | 5 | 9 |
| All | 16 | 12 | 28 |
Presentation of data
Quotations from interviews and observations have been selected on the basis of how clearly and succinctly they illustrate the dominant themes to emerge from this research. Tensions and inconsistencies have been discussed, and quotations from divergent cases presented where relevant. Some quotations have been edited to ease comprehension and/or protect individuals’ anonymity.
Disinvestment in theory
Individuals’ interpretations of the term ‘disinvestment’ were investigated prior to delving into questions about local practices. The intention here was to establish how disinvestment was understood in theory. Three categories of disinvestment definitions emerged from commissioners’ and providers’ responses: reducing/stopping currently funded health-care activity; refraining from investment; and finding cheaper ways to deliver health care. One individual – a lay member from group A – was not able to provide a definition.
Reducing/stopping activity
Most informants defined disinvestment as the reduction or cessation of activity, and/or the removal of funding from an area of health care. Reducing activity and reducing spend are connected, and this clearly came through in all interviews. Some informants mentioned these concepts together in their initial definitions. One commissioner and three providers mentioned finances alone:
What does disinvestment mean to you, or how would you define disinvestment?
Um . . . reducing the amount of money that’s spent on a service.
Interview, group A, PCT1
Other informants focused solely on cessation or restriction of activity (one lay member and a mix of providers and commissioners):
It means that there are certain procedures which will perhaps no longer be done.
Interview, group A, PCT1, lay member 1
To either decommission (treatments/procedures), or try to increase the threshold so that some people do get it, but not all of them. So that is what I think, in very broad terms, disinvestment means.
Interview, group A, PCT1, C1
Reducing activity: is disinvestment something more?
The presentation of informants’ theoretical understandings of disinvestment was partly based on their accounts of the types of activities they felt constituted disinvestment. Of the informants who described disinvestment as the reduction of activity/spend, most showed an awareness that this could be prompted by any number of reasons, from financial constraint to fears for patient safety. To these informants, it was this end result (i.e. activity reduction/removal), rather than the motivations behind this, that marked a process as ‘disinvestment’. There were, however, a few exceptions, where informants delineated disinvestment by more specific criteria. This tended to be expressed indirectly, during discussion of local examples of activity reduction.
One commissioner felt disinvestment implied reduction or cessation of activity on the grounds of cost alone. This emphasis on finance may explain why this commissioner was reluctant to label local examples of activity reduction as ‘disinvestments’:
Um I guess, from my side, I would probably call it decommissioning of activity.
Is there a difference between disinvestment and decommissioning?
Well I guess disinvestment is quite an economic sort of term, isn’t it? You know, you’ve got money or you haven’t got money; you invest or you don’t invest. Whereas the commissioning of services is about which services are provided and which ones aren’t.
Interview, group B, PCT2
Disinvestment was understood by some as a managed process of searching for opportunities to reduce/stop activity in response to financial constraint. The idea of disinvestment being an active process with a financial motivation was what distinguished it from other examples of stopping/reducing activity. This distinction was important, having implications for one informant’s response when asked if they could think of any local examples of disinvestment.
Finally, interviews and observations revealed examples where disinvestment was suggestive of denying or withholding access to health care. This interpretation was based on some informants’ insistence that locally produced ‘threshold policies’ were not forms of disinvestment. Threshold policies set out criteria that needed to be fulfilled for a patient to access a given treatment or service. In theory, implementation of a threshold policy could reduce the number of patients accessing a treatment/service if no prior thresholds had been in place. Whereas most commissioners and providers saw these policies as forms of disinvestment, two commissioners disagreed, thereby suggesting a different interpretation of the term:
Instead of a disinvestment, it’s a ‘let’s have a better portal for a decision made’. It doesn’t say ‘we won’t fund those forms of ophthalmic surgery,’ it says ‘well let’s just look at a sensible mode for making decisions’.
Interview, group A, PCT1, C3
The first commissioner (C9, above) tended to understand disinvestment as the complete removal of a service, practice or health-care organisation. Another commissioner (C11, below) had very strong views that disinvestment was an undesirable practice, suggestive of denying services on the basis of cost. Based on this theory, policies that were implemented on the basis of clinical evidence were not deemed to be disinvestments:
But you see it’s [disinvestment] a really bad word, in some ways. I don’t want to disinvest in any care – I want to change the way you do it.
[Later]
With these threshold policies, would you call them forms of disinvestment?
No, I think the threshold policies are a way of making sure. Because, you know – first do no harm. So let’s be sure that – sometimes I think [. . .] they might have to do an operation, and the patient sometimes isn’t any better off. So by making it a sensible decision, you know, have you thought this, this and this? . . .
Interview, group B, PCT2
The scope for public accounts, and thus bias, was a prime consideration throughout the conduct of this study, and commissioners’ portrayal of previous local practices was no exception. Even if the above commissioners rejected the term ‘disinvestment’ in a public context, the mere desire to dissociate local practices from ‘disinvestment’ suggests some level of concern that disinvestment carries negative connotations.
There was one example of disinvestment being presented differently in individual (interview) versus group (meeting) contexts. Despite describing threshold policies as examples of disinvestment within their interview (particularly the ‘cataract policy’), the commissioner below tried to dissociate these policies from ‘disinvestment’ when addressing the group:
There are various grades of disinvestment . . . threshold policies are a kind of disinvestment.
Interview, group A, PCT1, C1
So I’m going to share some of the work we do, what our system is, how we involve the providers, how we work collaboratively . . . and also a couple of [threshold] policies, [. . .] and also the cataract policy. So – not disinvestment, but a proper use of resources.
Observation, group A, PCT1, C1
In summary, although the majority of informants defined disinvestment in terms of cessation/reduction of activity or spend, there were subtle distinctions in how the term was understood and used, especially among commissioners.
Choosing not to invest
Two commissioners defined disinvestment in terms of choosing to avoid commissioning new activity:
Disinvestment means there is something there that you’ve identified as an activity that you really want to do [. . .]. But because of conflicting priorities disinvestment means that you’re not going to be able, or you potentially are not going to be able to find the money to do that.
Interview, group A, PCT1, C7
One of the two commissioners (C12) also demonstrated an understanding of disinvestment that was in line with the majority of informants, but clearly felt ‘not investing’ was a component of disinvestment. The statement below was made while discussing the events of a meeting that both LR and C12 had recently attended. C12 had enthusiastically supported an application to fund a new drug, although this was eventually rejected by the wider group. The commissioner’s comment below was in reference to this experience:
I suppose there’s many forms of disinvestment, isn’t there? It’s like making it so difficult to get funding for something that people don’t bother – they either get better or die, you know?
Interview, group B, PCT2, C12
Finding cheaper alternatives
Most informants who discussed funding in their definitions implied, or directly stated, that this would be coupled with reductions or cessations of activity. In contrast, the commissioner below talked about finding cheaper ways of providing a service, with no mention of reduced activity:
Ideally it means that this is a service that we can – or an issue that we can handle in a different way that is cheaper.
Interview, group A, PCT1, C3
Later in the interview, it became apparent that this informant’s idea of disinvestment was more aligned with cessation or restriction of activity. Nonetheless, it was interesting that their initial response, prior to probing, was distinct from that of others.
Summary: disinvestment in theory
This section has unravelled various ways in which individuals understood the term ‘disinvestment’. Whereas there was some confusion about what disinvestment meant, most informants saw it as a term used to denote a reduction of activity or funding within an area of health care. Within this broad group, there were some individuals who emphasised additional criteria as being necessary to classify an activity as disinvestment. Individuals’ interpretations of local disinvestment practices will be discussed in the next section. Once informants had offered their initial definitions, disinvestment tended to be framed in very particular and distinct ways throughout the remainder of interviews, with clear patterns emerging within provider and commissioner accounts. This suggests that, although there can be general agreement over what disinvestment is in theory (i.e. reducing or stopping activity), disinvestment in practice can be interpreted in different ways.
Local disinvestment practices
Observed examples of disinvestment
Observed meetings were dominated by new requests for funding and implementation of NICE mandates. Disinvestment decisions were rarely discussed, despite a constant undertone of concern about the affordability of newly commissioned activity:
The business case before us is very clear about how much money this is going to cost, and I’m sure the Director of Finance will say, ‘Well, what should we stop doing instead?’ Um, which is very challenging, and we don’t have any proposals before us to do that.
Observation, group A, PCT1, C2
Disinvestment initiatives, when mentioned in meetings, related to ‘threshold policies’ or ‘not routinely funded’ policies written for specific treatments or services. As mentioned earlier, ‘threshold policies’ set out eligibility criteria patients needed to fulfil prior to access the treatment/service in question. ‘Not routinely funded’ policies stated that the treatments/services would not normally be provided through the NHS. These will jointly be termed ‘restrictive policies’ throughout this chapter. When mentioned, restrictive policies tended to be included on meeting agendas if they were pre-existing and undergoing review. There was no discussion or reference to new disinvestment decisions being made in Group A (PCT1). Group B (PCT2) implemented eight new restrictive policies, although all but one of these new policies were written to establish a formal line of what is and what is not currently funded in the local health-care community. The single example of disinvestment came in the form of a threshold policy designed to ‘control the volume’ of referrals to orthopaedic knee surgeons. A near finalised version of the policy had been developed by a public health consultant outside of the meeting. This draft was brought to the group to obtain final approval of the criteria, and decide on its mode of implementation. A clear process of ‘policy development’ was not observed within the meetings attended by the researcher.
Overall, meetings did not provide much opportunity to observe how disinvestment decisions are made, on account of the rare occurrence of such activities. However, observing these meetings provided valuable insight that helped to place previous disinvestment initiatives (discussed in interviews) in context. Furthermore, meetings provided an opportunity to observe some of the difficulties with implementing disinvestment decisions, even though these decisions had been made prior to the period of observations. These difficulties will be covered in the next main section, Barriers to disinvestment. Finally, our observations provided an insight into the context within which commissioners are expected to engage in disinvestment activity. This allowed us to speculate on some of the reasons underlying the lack of disinvestment decision-making observed. These reasons are presented in the section, subsection Tools and capacity.
Interview informants’ experiences: examples of disinvestment
Category 1: restrictive policies
Commissioners and providers were directly asked to talk about their experiences of local disinvestment (using the term ‘disinvestment’). Most commissioners and providers who discussed disinvestment experiences referred to restrictive policies that had been implemented in recent years. These policies were, in many cases, the sole examples of disinvestment provided.
Clinician interviews were focused largely around local disinvestment from CTS surgery. This procedure had been subjected to a threshold policy within both regions, where patients needed to fulfil certain criteria before being granted access to surgery (e.g. undergo 3–6 months of conservative therapy). Clinicians therefore spoke at length about their perspectives on threshold policies, often referring to other clinical areas that had been subjected to similar forms of disinvestment. There were clear patterns in the way restrictive policies were portrayed and rationalised by commissioners and clinicians/providers. These patterns were apparent in both regions.
Restrictive policies: a tool for minimising waste
Commissioners tended to portray restrictive policies as tools for minimising wasteful use of NHS resources. They reported achieving this by ensuring the provision of treatment led to evidence-based clinical benefit. The following portrayal of ‘not routinely funded’ policies was typical of commissioners; here, the decision to stop funding activity is presented as logical and non-contentious:
It’s pretty much a no-brainer to say that something doesn’t work, therefore we shouldn’t be doing it [. . .]. You get a body of experts to look at the evidence base and say, ‘That’s rubbish, don’t do it’. That’s fine.
Interview, group A, PCT1, C2
Threshold policies were also typically presented by commissioners as indisputable choices that made intuitive sense. Most commissioners avoided any suggestion that policies denied care, emphasising that criteria ensured that patients who would benefit from treatment would still be granted access:
We’re just turning back the tide of the ones that shouldn’t be [receiving treatment].
Interview, group B, PCT2, C10
Only one commissioner (C1, group A, PCT1) suggested that decisions were not quite so clear cut, with the degree of benefit being an important consideration. This commissioner often made reference to threshold policies restricting access to patients who ‘benefit the most’. These activities were distinguished from ‘rationing’ on the assertion that patient safety would never be compromised through implementation of restrictive policies:
As long as it is not harmful or it is not going to lead to some disastrous consequence – so, I won’t call it as rationing as such, but I will call it ‘people who are really in need’, and that’s quite difficult. That’s why we have local deliberations.
Interview, group A, PCT1, C1
As addressed in the discussion about theoretical understandings of the term ‘disinvestment’, two commissioners did not view restrictive policies as forms of disinvestment, owing to the term having negative connotations or being suggestive of services being withheld. Both of these informants shared other commissioners’ views that restrictive policies sifted out activity that should not be occurring in the first place:
If we didn’t have this financial pressure on us now, were we therefore working in a way that wasn’t as efficient? [. . .] Um, possibly yeah.
Interview, group B, PCT2, C11
Restrictive policies: a form of rationing
All providers and clinicians viewed restrictive policies as rationing or cost-cutting exercises, implemented with an intention of saving money. Restrictive policies were seen as a direct consequence of having limited resources:
I remember there’s a bit of a hoo-ha at the moment, where they’re not going to pay to have grommets done [. . .]. But everybody knows they need to save money.
Interview, group B, PCT2, P4
One provider had come to expect restrictive policy implementation to follow on from any large investments:
At the last meeting when they agreed to fund that eye treatment [. . .] it’s 450,000 for that. It’s going to be 450,000 taken from somewhere else [. . .]. It’s not really explicit at the time where that might be, but obviously another battle ground will probably open up around something else – and that’s why you begin the other discussion about [restrictive policies].
Interview, group A, PCT1, P2
The focus on the financial element of decision-making was in stark contrast to commissioners’ emphasis on stopping non-evidence-based activity. Importantly, these different interpretations were based on exactly the same policies; they had simply been framed differently. Providers and clinicians had picked up on the distinct way in which commissioners tended to frame restrictive policies, often expressing frustration that the term ‘rationing’ was never used:
Do I think that rationing is necessary, and do I think the introduction of the word ‘rationing’ is appropriate? Then I do. Because I think that’s a more honest term.
Interview, group A, PCT1, P6
And I can understand why. Er, you know, ultimately one is – we’re going to have to ration health services. It’s being honest about it though. We need to be honest and say, ‘This is what we’re doing, we’re rationing it’. Not to turn up and say, ‘You’re operating on too many patients, we think you – you shouldn’t be operating on all these patients’.
Interview, PCT1, Clin4
Similar to the informants above, a number of other providers and clinicians used the term ‘rationing’ when referring to restrictive policies throughout the duration of their interviews:
So do you have any experiences of the [commissioning group] making a disinvestment decision?
Only in terms of the raft of rationing, if you like, or the, what they call ‘low priority procedures’ which they’re not funding.
Interview, group A, PCT1
Category 2: service reconfiguration
Although service configuration as a form of disinvestment was not apparent during the period of observation, a mix of commissioners and providers from both regions discussed examples of it. Past initiatives included changing the way in which treatments were delivered, and closure of satellite health centres in favour of centralising services:
So it could happen at an organisational level. So there’s been examples where services have been centralised . . . so all the clinical evidence would suggest that the more of something that someone does, the better the outcomes will be.
Interview, group A, PCT1, P3
One participant talked about changes in service delivery that represented more efficient use of resources (lay member 1). In particular, this informant discussed changes to the types of professionals that administer care:
I think chiropody was actually flagged up as something that we were spending a lot of money on. There was a huge waiting list, and we weren’t actually delivering a decent service. And so health-care assistants and so on are now delivering this [instead of clinicians].
Interview, group A, PCT1, lay member 1
At the heart of the examples of service reconfiguration was emphasis that quality and patient outcomes were either maintained or improved:
I suppose you could call it a disinvestment, because we wanted to reduce the number of people going into hospital for pain clinics and then use the consultants in a different way. So the GPs could do a lot of the work [. . .] and then put the money into the psychology approaches and everything else for the more complicated [cases] . . . that makes a lot of sense to me.
OK so actually nobody is losing out at all, it’s . . .
Well people are potentially gaining – exactly, exactly.
Interview, group B, PCT2
The quotation above was taken from one of the commissioners who tended to associate disinvestment with denial of treatment/services. The above example was the only form of disinvestment they provided within the interview; clearly, though, this activity is presented in very positive terms. Service reconfiguration was generally presented as a positive step by all informants who discussed these changes.
Category 3: responding to evolving health care
Two commissioners talked about shifts in practice as a form of disinvestment they had experienced, whereby newer treatments came to replace older ones. The underlying suggestion here was that disinvestment had been a passive process whereby older treatments were gradually replaced with improved versions. One commissioner provided an example where clinicians had been encouraged to change to more cost-effective materials:
There are a number of things where our policies have shifted clinical practice away from something which is less cost-effective. And we are in the process of developing guidance from our orthopaedic colleagues about which particular prosthesis to use when they’re doing major joint replacements.
Interview, group A, PCT1, C2
As described earlier, another commissioner (C9) discussed a similar idea, but felt the term ‘decommissioning’ was a better representation of what the PCT typically did. C9 felt that ‘disinvestment’ referred to removing or stopping for financial reasons, whereas ‘decommissioning’ suggested that this would be followed by investment in the same area:
It may be a swap [for] something that’s more – a treatment that’s more current or more clinically effective. Or, you know, new trials come out and there’s a new drug on the horizon that means that an older one drops off.
Interview, group B, PCT2, C9
Limited experiences
Seven informants (five commissioners and two providers) from both regions reported little or no experiences of disinvestment. One commissioner from group A (PCT1) felt disinvestment had not been part of the culture of local decision-making, as there had been little to no attempt to actively seek opportunities to withdraw funding from existing health-care pathways. Here, the informant’s initial definition of disinvestment had important implications for their perceived lack of local disinvestment practices. Others similarly felt that disinvestment had been largely absent from the NHS agenda. This was presented by one commissioner as an effect of successfully achieving efficiency savings:
If you’re more productive, and you’re getting more for the same money, it allows you not to have to disinvest very much. So we haven’t done tons of disinvestment, because we’ve achieved what we’ve needed to do.
Interview, group A, PCT1, C3
Two providers were initially unable to think of any local examples of disinvestment. Each provider (one from each region) felt that previously implemented restrictive policies either tended to target treatments/procedures that were rarely carried out or reflected current practice:
I don’t think there’s been any change in the way we are operating on patients, we’re just now better at ticking the box.
Interview, group A, PCT1, P3
Here, informants’ definitions of disinvestment entailing a reduction in spend may have had implications for their perceived lack of disinvestment activity. One of these providers felt that previous initiatives’ failure to release money had contributed to the financial struggles the health-care community was currently facing:
That’s why we’re in some of the mess we’re in now – we haven’t disinvested.
Interview, group B, PCT2, P5
Identifying candidates for disinvestment
The meetings provided little insight into how disinvestment opportunities were identified. This was largely owing to the lack of disinvestment decision-making occurring within the observed meetings. Interview informants were probed to discuss the approaches used to identify candidate areas for disinvestment, based on previous local experiences. What was notable across all interviews was the absence of any description of a systematic process that could be routinely used. Informants’ responses were based on specific examples of disinvestment recalled, and the routes by which these had been identified. Some responses, particularly those involving benchmarking, tended to be triggered by questions from other areas of the topic guide (i.e. initiated by LR).
Central influence
One commissioner stated how occasional guidance from ‘the centre’ could play a role in triggering disinvestments in certain areas of health care. Two commissioners made direct reference to the NHS ‘Right Care’ work stream: part of the Quality, Innovation, Productivity and Prevention (QIPP) programme. QIPP had played a role in initiating benchmarking exercises in specific areas of health care:
[The QIPP Right Care stream] came out with a list of 160-ish interventions that were of low – could, in some circumstances, be of low clinical value [. . .]. So we did use that as a starting point for looking at what activity we’d got around those things. And over the last few years we have introduced policies in some of those areas
Interview, group B, PCT2, C9
Providers frequently assumed that central bodies informed areas for disinvestment. One provider from PCT2 assumed that central bodies, such as the Department of Health, provided guidance on identifying areas for disinvestment:
I think there is probably sufficient noise from the centre guiding PCTs and commissioners as to the areas that they should be considering for assuring themselves that appropriate treatment and appropriate thresholds are being made.
Interview, group A, PCT1, P6
Another provider made a brief reference to the QIPP programme, assuming that this must have played a role in triggering disinvestments. A third provider was certain that QIPP had brought about some degree of disinvestment, but considered this to be insignificant in the grand scheme of the local health-care budget:
I couldn’t tell you any area that has been disinvested, apart from the ones I mentioned about the small ones that came from the Muir Grey document on lower-value things, and in the scheme of things they were tiny.
Interview, group B, PCT2, P5
Only one commissioner (PCT1) made reference to NICE’s published list of ‘do not do’ procedures. This list was not thought to have led to substantial savings because it had little local relevance:
I think – I don’t know – when we looked through the disinvestment list, they had some huge figures on it, but in reality it just didn’t really pan out to anything substantial.
Interview, group B, PCT2, C12
Benchmarking
Three commissioners talked about benchmarking exercises without prompting. Two of these informants related benchmarking to national tools, such as the NHS Atlas of Variation in Healthcare,45 and the QIPP work streams that had triggered specific benchmarking exercises. Based on these accounts, benchmarking did not appear to be routinely carried out. Commissioners from both regions put this down to capacity issues:
So, one is, we can do benchmarking, but again, it goes back to capacity. Do you regularly benchmark every month and see which procedures are high, which are low? We don’t have capacity to do that. So, from time to time, interested people, like me, can look at it.
Interview, group A, PCT1, C1
So we get high-level data that says, you know, we’ve got X many cataracts happening, but we wouldn’t have the time to go into how is that different from everywhere else, and why is it different from everywhere else?
Interview, group B, PCT2, C9
Three commissioners discussed benchmarking as an activity they had ‘done before’. These statements all followed on from LR raising the topic of benchmarking as part of the proposed disinvestment process this study had intended to evaluate. These accounts also supported the notion that benchmarking tended not to be conducted routinely. The prospect of benchmarking was explored in more depth during discussions about the proposed disinvestment process. This evoked a range of responses, most of which aligned with concerns about the value of benchmarking. These have been outlined in more depth in Appendix 9. In summary, informants had little confidence in the reliability of data used in benchmarking exercises. Reasons for this included concern about data artefacts, and perceived inconsistencies in how activity was coded. Consequently, there were doubts surrounding the validity of the conclusions that could be drawn from making national comparisons:
So the big issue with health service data is problems of comparability [. . .]. If you look at the rate of emergency admissions and look at [acute trust 1] and [acute trust 2], you’ll get a difference. And the difference is largely driven by the way in which activity is counted.
Interview, group A, PCT1, P3
Yeah a lot of it’s down to training, or a lot of it’s down to complexity. [. . .] Without demeaning them – they’re just an admin clerk in the Finance Section, and they’re being told to put these 100 operations on the system. I wouldn’t be able to do that without making mistakes. I’d be looking at it and thinking, ‘Tonsillectomy, yeah, the first one there will do, that’ll do’.
Interview, group B, PCT2, C10
The capacity constraints expressed are likely to be a further explanation for the seemingly infrequent use of benchmarking in practice.
Targeting high-volume elective procedures
One commissioner stated that high-volume elective procedures were prime candidates for disinvestment, regardless of their activity rates relative to other regions:
Basically commissioning managers tend to look for commonest elective procedures, things that are high volume, and say, ‘Could we – you know, is there scope for cutting back here?’
Interview, group B, PCT2, C8
This precursor to potential disinvestment was not mentioned by any other commissioners. A second commissioner (C10), from the same commissioning group as C8, made reference to C8’s emphasis on focusing disinvestment efforts on areas that could deliver considerable savings. C10 mentioned how disinvestment had sometimes occurred on the basis of observed ‘mistakes’ that had occurred in other regions of the country, where policies had been developed to prevent similar issues occurring within the local region. However, there appeared to be disagreements within the commissioning group surrounding what areas warranted policy development:
we’ve got a policy on tongue tie coming to the next [meeting]. So that will restrict children like that getting it in future. But we don’t necessarily – and this is where [C8] gets quite irked because [he/she] says, ‘Well where is your evidence that this is a real problem?’ I say, ‘Well, we have isolated cases, and if this isolated case stops another child spending 3 weeks in hospital next year, then it’s worth doing’. It might not have a major financial impact, but, you know.
Interview, group B, PCT2, C10
Providers tended not to discuss volume of activity, but one of the case study clinicians spoke generally of how they felt disinvestment opportunities were identified by looking for large areas of spend within the budget:
I think that they’re focusing on things that they can identify which form a large part of the budget. And where the numbers are big enough to make it worth their while looking at them.
Interview, PCT1, Clin1
Case study clinicians’ views on why CTS surgery had been subjected to disinvestment are reported in Chapter 8.
Looking to other health-care organisations
Providers and commissioners from both regions discussed looking to other health-care organisations for guidance on potential areas for disinvestment:
quite a few PCTs have got so many policies, more than us, so you can look at that.
Interview, group A, PCT1, C1
Observations of meetings broadly supported this idea that commissioning groups monitored the practices and policies of other health-care organisations, although this was evident in decision-making surrounding provision of new treatments.
‘Soft intelligence’/local knowledge
Commissioners from both regions talked about the less technical routes to identifying candidates for disinvestment. At times, ideas had been suggested based on an individual’s observations, or local knowledge that would filter through to the commissioning groups:
The other thing is, some ‘soft intelligence’, because sometimes somebody will just drop something. They will say ‘Oh, we are doing – I just heard that we people can just walk in and get a cataract done,’ or ‘drive through and get a cataract done’.
Interview, group A, PCT1, C1
One commissioner’s comments gave the impression that these types of suggestions for disinvestment – if initiated by providers – can be thought of as reactions to commissioners’ suggestions:
Sometimes clinicians themselves come forward saying, ‘I think we’re doing too many of procedure X or Y, and it’s something you could cut back on,’ so yeah.
Oh OK that’s interesting. Has that happened very often?
Well we do ask the trusts and the clinicians to suggest to us areas of health care that they think would be the better candidates, perhaps compared to things that we might suggest.
Interview, group B, PCT2
Difficulties with identifying candidates for disinvestment
Generally, interviews suggested a lack of systematic tools and training to identify opportunities for disinvestment. The above approaches described by informants were non-sustainable, reliant on chance or not conducive to independently identifying local opportunities for disinvestment. These insights from interviews supported the apparent lack of disinvestment decision-making observed within meetings. A handful of commissioners and providers raised the issue of ‘identifying’ areas for disinvestment as being particularly challenging:
It’s one of those sort of things people say, ‘Well I’m sure there’s loads of things you’re doing out there that you should stop,’ and you think, ‘Well come and let’s have a look then and see if you can point them out to me’.
Interview, group B, PCT2, C12
Often, comments related to the challenge of identifying candidates were made in response to LR asking about how disinvestment opportunities were identified:
I don’t know that there’s been a solution to that problem.
Interview, group A, PCT1, P3
Case study clinicians’ rationalisation of disinvestment
Taking CTS surgery case study, we explored clinicians’ perspectives on why commissioners had enforced threshold policies. Commissioners from both regions explained this in terms of its high benchmarking data, yet only two clinicians (both PCT1) spoke of this:
I think someone told me [. . .]. So I was aware that our rates of [CTS surgery] for the population are higher.
Interview, PCT1, Clin1
Most clinicians, from both regions, felt that CTS surgery had been an easy target for restriction, because of its high volume of activity, and subsequent high levels of spend. These clinicians made no mention of procedures rates being high relative to other regions:
They’ve picked [CTS surgery] because it’s a big number. In terms of numbers of patients it’s probably one of the four biggest operations that an orthopaedic department does.
Interview, PCT1, Clin2
Oh that’s an easy one. They have chosen operations that are most frequent.
Interview, PCT2, Clin7
Another suggestion was that CTS surgery was easily identifiable thanks to the clear recording of activity. This was compared with other procedures, which could be coded under multiple categories and thus would not be easily identified:
the PCT don’t know anything about [comparative procedure], or they don’t look at it because it’s just a procedure. I expect it gets absorbed into some other HRG [Healthcare Resource Group] code.
Interview, PCT1, Clin1
Three clinicians felt orthopaedic procedures in general were easy targets for ‘rationing’ exercises, as there was flexibility for manipulating thresholds, and the indications for treatment were not perceived to be life-threatening:
There’s no secret that there is an agenda to reduce costs in the health service. And orthopaedics is seen as one prime area where you can reduce the costs.
Why do you think that is?
Well because most orthopaedic conditions are not life-threatening . . . if somebody has got arthritis in their hip, whether they have an operation this year or next year is a matter of their tolerance of their symptoms, their social circumstances and all the rest of it. And you can quite easily juggle your decision according to how you want to prioritise it.
Interview, PCT1
Well I think they targeted lots of things, and they’ve picked out some things which they think are simple and easy, and have tried to reduce the cost on that. And I think, you know, more and more they will find actually [CTS surgery] is not simple and easy.
Why do you think they do think it’s simple?
Because it’s not a life-threatening thing.
Interview, PCT2
Working through a disinvestment process
Interviews provided an opportunity to investigate how previous disinvestment decisions had been made. Most accounts, based largely on restrictive policy formation, were vague and limited in detail. Interview accounts, supported by observations, led to the impression that there was no clear disinvestment agenda in place in either region. There was a very broad process in place (within both regions) for determining the criteria stated within restrictive policies. This process was similar for both regions. First, individuals working within commissioning groups would conduct ‘evidence syntheses’ of published literature to inform draft criteria. These drafts would then be sent to local expert clinicians for comment and feedback (a process commonly referred to as ‘consultation’), although there was no guarantee that this feedback would be taken into account in finalised policies. Implemented policies were placed on the local commissioners’ website, but rarely proactively disseminated to local clinicians. Although interview informants were not aware of any clear disinvestment processes, two broad topics were discussed by commissioners and providers/clinicians from both regions: the process of evidence synthesis and the process of consultation. Each of these topics was the source of disagreement within observed meetings, and a clear point of contention revealed through interview accounts.
Evidence synthesis
A clear concern, expressed by numerous providers/clinicians, related to the process by which evidence was reviewed during disinvestment decision-making. This theme clearly came through during observed meetings and within interview accounts. One of group A’s meetings involved a lengthy exchange between providers and commissioners regarding the apparent omission of evidence in a previously implemented restrictive policy:
No, no, I think it’s just if [clinician from trust] highlighted some evidence that hasn’t been considered as part of the process, then we need to understand why [numerous voices: Yes, yes, overlap from commissioners and providers] that evidence wasn’t considered in the process [. . .]. So, somehow there’s been something that’s gone amiss, um, and we can use this as an example to maybe tighten things up for the future.
Observation, group A, PCT1, P3
Providers contributing to these meetings also raised issue with commissioners’ tendencies to allow junior doctors or non-clinical managers to conduct literature reviews on highly specialist areas of health care. This was particularly difficult to accept when the outcome of reviews was presented to clinical experts in the given field:
It’s just sheer frustration at what he [a clinician] would consider a committee like this sitting round making important decisions about things they don’t really know about.
Observation, group A, PCT1, P3
The above issue was raised by other providers within the region who did not attend the meeting from which the above quote was taken:
We’ve got some foundation doctors, F2 doctors, the second year qualified, are sent away to do little projects and look up some evidence, and then they come up with some theories. And some of it is just a pile of tosh . . . it’s nothing to do with them [. . .] but instead of getting GPs and specialist secondary care doctors to sit around a table and say, ‘How could we do this and what is sensible and which patients really benefit from such and such a treatment, you know, which patients don’t?’
Interview, group A, PCT1, P7
Both clinicians and providers used the above issues as a platform to promote their greater involvement in the formation of disinvestment outcomes (as shown above). One provider discussed the problems in relying on published evidence rather than expert opinion to guide disinvestment decisions, as some clinical areas had a limited literature that was unlikely to grow.
[If] it’s an area where there’s never likely to be a clinical trial, it’s not a drug that makes a drug company very much money [. . .] so for a drug such as [X], you’re unlikely to ever get really good clinical trial evidence. Which doesn’t necessarily mean it doesn’t work, of course.
Interview, group A, PCT1, P1
These concerns all contributed to an overall desire for clinical experts to play a much more active role in the process of conceiving and developing disinvestment ideas.
While providers expressed general concern about the quality of evidence synthesis, the clinicians interviewed were more precise in their criticisms of the evidence underlying the restrictive policies they had encountered. One clinician raised issue with the scientific quality of ‘evidence’ synthesised, as well as the various biases inherent in the ‘stance’ clinical papers were written from:
You’ll find a lot of the papers written about conservative management of carpal tunnel will be written by people who don’t actually operate on carpal tunnel. They’re rheumatologists or they’re neurologists, people like that. So they will see one spectrum and I’ll see perhaps a different spectrum.
Interview, PCT1, Clin4
The above clinician also suggested that the evidence incorporated into reviews represented a selection bias, where evidence had been selected to support premeditated outcomes:
I can’t comment on what they’ve considered. I’m sure they’ve looked at it carefully but – you know, it depends what direction you’re wanting to push the thing [. . .] I can’t remember the papers.
Interview, PCT1, Clin4
On a more general level, clinicians expressed concern about relying solely on published evidence, regardless of the quality of that evidence. Clinical practice was thought to be a complex mix of following evidence-based guidelines in conjunction with using tacit knowledge, and considering patients on a case-by-case basis:
They [commissioners] say they are evidence based, but the randomised control trials, they’re only appropriate for the patients who absolutely match the entry criteria for that trial, but anybody else slightly different you’ve got to have personal evidence for that. And that’s going right back to how we used to make clinical decisions for that individual. That’s the difficulty the PCT has.
Interview, PCT1, Clin2
Clinicians from both regions emphasised the limited evidence base in their specific area of practice, further highlighting the dangers of relying on this evidence alone:
I suspect given the literature’s in general rubbish, you are probably not going to come up with a system that is err, sufficiently sensitive and specific. And so whilst the population remains relatively uneducated we will get away with it. But some patients will get denied access and will have irreversible neurological damage and will then sue somebody – and then your cost–benefit analysis will go out the window.
Interview, PCT2, Clin9
The process of consultation
Both commissioning groups routinely sent any form of restrictive policy out to the local clinical community for consultation. Commissioners described this as an attempt to work collaboratively with clinical experts through sending out policies for feedback. This process was sometimes described as arduous, with frequent experiences of delay or feedback not being provided at all. Commissioners assumed this was due to time limitations, administrative hurdles or clinicians’ failure to recognise that policies will impact their practice:
So there may not always be a furore about a policy when it’s being implemented and when it’s going through the [commissioning advisory group B] process . . . but once you start implementing that policy and sticking to it, then I think people can realise, ‘Actually this is having an impact on my clinical behaviours’.
Interview, group B, PCT2, C8
Unfortunately, people don’t . . . because either they don’t have time, or . . . and sometimes we don’t hear . . . it’s maybe something with the trust, it doesn’t get through to the system.
Interview, group A, PCT1, C1
The commissioner above went on to express how there had been difficulties in the way consultation was undertaken, in that acute trust medical directors preferred commissioners to go directly to the specialists relevant to the procedure/treatment:
The engagement has been not that great, and they have been telling us that ‘You should come and engage with us individually,’ which we don’t because we simply don’t have the resources.
Interview, group A, PCT1, C1
Providers’ perspectives on the process of policy formation revealed that engagement was impeded by issues of communication and their perceived lack of influence over the decision-making process. One of group A’s meetings involved lengthy discussions about the receipt of a complaint letter sent in by a local orthopaedic surgeon. The letter expressed concern that one of the previously implemented restrictive policies had ignored the feedback from clinical consultation, and was thus having implications for patient welfare:
Now, there is a threatening line at the end [of the letter], where it says that the GMC [General Medical Council] code of medical practice requires us to raise concerns where we feel the patients are being compromised. And it . . . hmm, and it’s suggesting that we have taken no notice of the comments made in the consultation process.
Observation, group A, PCT1, C1
This letter opened up discussions, spanning two meetings, about the process of restrictive policy formation. Commissioners voiced concern that policies sent for feedback were not given an appropriate level of priority:
I think what this issue has demonstrated is that clinicians need to take very seriously our first approach to them about a change in the policies, particularly with respect to low-priority treatments. [. . .] And I think what’s happened in the past is perhaps there’s been a sort of um, ‘Well here’s a policy, well it’s totally impractical, so we’ll just ignore it and hope it’ll go away’.
Observation, group A, PCT1, C1
Based on another provider’s comments, it appeared that this issue may have been related to clinicians’ misinterpretations of how ‘finalised’ the circulated policies actually are:
I think you’re quite right though, I think there needs to be more proactive engagement.
And there is urgency about it.
And an understanding that, when a policy comes through, it’s not actually policy, it’s for consultation. And I think that’s sometimes where people can be slightly confused: you’re sent something which you think is, at first sight, is absolute nonsense, but there’s nothing you can do about it, and it just makes you irritated and frustrated.
Observation, group A, PCT1
Providers’ sense of exclusion from the decision-making process was clearly apparent within interviews. The assumption that the consultation process presented ‘finished’ policies compounded this:
If they had included us more in the drafting rather than once it had been [completed], they could have come up with better ideas [. . .]. Some of the secondary care consultants have just been banging their – you know, phew yeah, incredibly frustrated by the process. Because we are presented with: here is the document, have you any – you know, we might tweak it a bit, but basically it’s fait accompli. And they don’t always seem to listen to us when we say this actually doesn’t make sense.
Interview, group A, PCT1, P7
Although providers who attended commissioning group meetings had similar ideas in relation to the process of consultation, their concerns were voiced on behalf of clinicians whose practice had been influenced by disinvestment. The interviews with orthopaedic clinicians provided an ideal opportunity to directly investigate this key stakeholder group’s perspectives on the consultation process. Accounts from each region revealed slightly different levels of acceptance surrounding their involvement in the decision-making process. Clinicians from PCT2 sensed complete exclusion from the process. Some clinicians did not know who had formed the policy, or how the policy had come into fruition:
OK and so presumably if you don’t know who wrote them, you don’t know how they formed this policy?
No, absolutely nobody knows. In fact it’s done on purpose because they don’t want to put themselves forward, because then they’ll get some proper good medical questions thrown at them. Yeah because remember it’s all done for financial gain, you know. But er, I’d love to find the name of these people.
Interview, group B, PCT2
Two clinicians from PCT2 reported trying to express their views to the PCT, though this did not appear to translate into action. These two clinicians conducted the bulk of CTS surgery in PCT2:
And essentially they said, ‘OK fine, we’re hearing this,’ and then the pathway that’s sent, which was about a couple of months later, completely ignored it, absolutely 100%. And I replied back to everyone again saying, ‘What’s going on? You know, why are you inviting us and asking us? I can see you have completely ignored it’. And then they redid another pathway, put it in, but that hasn’t been in place now.
Interview, group B, PCT2, Clin5
I gave them evidence of um cost-effectiveness of treatment versus other treatments, of my opinions and er, yeah, they were pretty much – pretty much ignored.
Interview, group B, PCT2, Clin7
In contrast, clinicians from PCT1 reported high levels of involvement, to the point where some clinicians asserted that they had written the policies themselves:
OK so you feel that you’re able to practise fully as you desire?
[Nod] There is a reason for that. I wrote the criteria.
Oh right, so you actually made the thresholds?
Yeah when they first started coming out with thresholds, [CTS surgery] was one of the first things that they looked at, and they actually did invite us to come, and we had a big meeting about it, and we wrote them in a way that I felt was sort of liveable with.
Interview, group A, PCT1
Other clinicians from PCT1 explained how the threshold policy had emerged based on consensus, but were particularly confident that their input lent them the flexibility to work in accordance with their usual practice:
Well the redrafted thresholds were a sort of consensus. But, as I say, we argued for a long time about this 6-month period.
Ah so that was one of the changes that . . . ?
I can’t remember what it was before, to be honest. By and large it was pretty much in line with what I would do anyway.
Interview, group A, PCT1
Barriers to disinvestment
Collaboration issues
The issue of providers and clinicians feeling excluded from the decision-making process threatened the sense of collaboration between commissioners and providers/clinicians. With poor collaboration came greater potential for turbulence in implementing disinvestment decisions. This was deep rooted, extending beyond the process of policy formation. An environment of mistrust was apparent through the accounts of commissioners and providers/clinicians from both regions.
Five commissioners from across both regions expressed doubt over providers’ and clinicians’ capacity to remain unbiased in the face of disinvestment decisions. The potential reduction of paid activity was often the backdrop to this:
Because . . . that is the hidden agenda. Because people won’t tell you openly that ‘I’m getting hit’, but they might use other things, some true, some untrue. That is my view, but they may say something different . . . they may use patient safety.
Interview, group A, PCT1, C1
Not all commissioners discussing these ideas were as direct as the informant above. Concerns were sometimes expressed more subtly:
There is a mechanism for making sure the consultants aren’t, um . . . overtreating, shall I say.
Interview, group A, PCT1, C4
Providers and clinicians raised the issue of commissioners’ mistrust as a felt phenomenon. Over half of the providers and most of the clinicians interviewed raised concerns that commissioners had very set views on their motives:
I mean I don’t think doctors want to operate on people for the sake of operating. They always want to make people better. And I don’t think that’s always thought of by the commissioners: it’s almost as though we’re dragging in people off the street for A&E [accident and emergency] and to operate on, and actually, we don’t want to do that.
Interview, group B, PCT2, P4
One commissioner from group B (PCT2) shared others’ views that clinicians have difficulties viewing disinvestment impartially, but was exceptional in that they attributed this to clinicians’ genuine belief that the treatments they conduct are of clinical value, even if this does not hold up to external scrutiny by commissioners.
There was evidence from both regions that providers and clinicians felt particularly uncomfortable with the idea that some restrictive policies had pre-set criteria that defined whether or not a patient was eligible for treatment. This was seen as a reductionist approach to what should be a deliberative decision-making process, based on each individual patient:
That [threshold policies] suggests that it’s black and white, that a person with such and such a scar and such and such a condition is fully deserving of an operation, and this person who has got a real problem, but it doesn’t fit that particular tick box, doesn’t deserve an operation.
Interview, group A, PCT1, P6
That’s why medicine is a long process – it’s an art essentially [. . .], because no books can tell you exactly what to do, for that very reason. And now you’ve been through all of that, and overnight a criteria tick box exercise has been introduced? It just doesn’t agree with medicine, full stop.
Interview, group B, PCT2, Clin5
Commissioners from both regions were aware that clinicians were uncomfortable with the loss of clinical freedom to judge need; however, the issues of mistrust generally prevailed:
We’re challenged on a regular basis, probably weekly, where people are saying, ‘You’re not trusting my clinical judgement,’ or, ‘You’re thinking I’m lying’. [Later] And it’s been said to me, ‘Well I’m a GP, I have a relationship with our patient, I know that they are truly suffering from tonsillitis and they need this procedure, and you should trust me as the clinical lead to refer them in for this treatment’. That’s fine, but for every one GP who is aware of the procedure, and is aware they can only refer in properly, there’s another five who aren’t . . .
Interview, group B, PCT2, C10
Other practices and procedures set by commissioners contributed to providers’/clinicians’ sense of exclusion from decision-making processes. For instance, the auditing or ‘policing’ of compliance with restrictive policies had created tension in PCT1. Providers felt commissioners had not been explicit about their intentions to withhold payment in the event of administrative tasks not being completed correctly:
But I think that the way they went about it wasn’t very clever, and they didn’t really take me into their confidence with what they were trying to achieve. I don’t like that style of saying, ‘Oh just do a clinical audit, just so we know everything is OK,’ and then turning round and saying, ‘And now we’re not going to pay you for 15% of the work’. So that was something that seriously angered me.
Interview, group A, PCT1, P6
The sense of being ‘penalised’ reinforced the sense of division between the two stakeholder groups in the face of disinvestments:
It is very much more the sensation of, as an acute secondary care doctor, is that it [disinvestment] is something that is done to us.
Interview, group A, PCT1, P7
Finally, group A’s approach to finalising decisions clearly marked out the divisions and seemingly unequal distribution of power among commissioners and providers. Group A tended to make decisions on the basis of a voting system, but providers around the table did not share this right to vote. One provider from group A expressed how this reinforced feelings of exclusion from the group:
it doesn’t feel as if the secondary care providers are equal members of that committee.
Interview, group A, PCT1, P1
Reluctance to be explicit about financial constraints
A number of clinicians, providers and commissioners commented on the culture of discomfort in being explicit about cost in the context of health care. Providers and clinicians from both regions were conscious of commissioners’ reluctance to associate disinvestment practices with a desire to save money. Commissioners’ tendencies to portray local disinvestments as ‘waste minimisation’ implied that activity had been conducted unnecessarily. This notion sat uncomfortably with providers and clinicians, promoting disengagement rather than collaboration:
[I think]: ‘don’t turn round and tell me that I am unnecessarily operating on people. Because that’s not ever going to engage me in um – in trying to work through a rationing or disinvesting process.’
Interview, group A, PCT1, P6
One commissioner from PCT2 was exceptional in referring to similar issues touched on by providers:
Yeah and you just think, well, I think, if we’re going to say no to something, then let’s be honest about it. Because these days you can say, ‘OK, you know, it’s got some evidence base, perhaps not a lot of safety, but basically can’t afford it’. At least you’ve been honest then.
Interview, group B, PCT2, C12
Providers’ and clinicians’ impressions that money was the key instigator to previous restrictive policy implementation were paramount. Some provided additional anecdotes supporting their views. For instance, the auditing of compliance with restrictive policies was seen as a covert money-saving exercise, littered with opportunities for commissioners to withhold payment. This was demonstrated through providers’ and clinicians’ accounts:
I mean it’s become a game [. . .]. I mean it’s, ‘How many different ways can we find to not pay you for doing the work, or fine you for doing the work too slowly, too quickly, in the wrong way or whatever?’
Interview, group A, PCT1, Clin4
The use of the term ‘game’ was apparent in two separate clinicians’ accounts of commissioners’ auditing exercises. This use of language suggested that the process was not only a waste of time, but one that could be learnt and manipulated:
It was a way where the [commissioners] could stop paying for stuff even though it was clinically necessary. And we’re now better at ticking the box that says ‘this patient is in pain’. It’s a financial game that’s being played.
Interview, group A, PCT1, P3
Evidence of commissioners’ reluctance to talk about ‘cutbacks’ or financial difficulties was apparent during observations. One of group B’s meetings involved discussion about implementation of a policy restricting access to a cosmetic surgical procedures; although they were not currently provided, commissioners wished to establish a formal line by developing a policy. The discussion that ensued was fraught with concerns that the wording of the policy implied that ‘cost’ was the underlying cause for restriction. This was not deemed to be defensible, with the group concluding that the policy would require substantial reworking:
Yeah because I’m inclined to agree with [commissioner] in that it does look like it’s possibly money, you know, a money thing, when obviously that’s against the spirit . . . but I appreciate it’s challenging if you have lots of people who want to access it.
Observation, group B, PCT2, C9
The culture of disassociating ‘affordability’ from health care in the public arena was seen to be counterproductive to making disinvestment decisions. One commissioner explained how the fear of public outcry or media response had prevented disinvestment initiatives in the past:
If you get somebody who is dissenting – which might be the Local Medical Committee, or it might be a local consultant making a lot of noise in the paper – that’s where your chief exec goes very wobbly, certainly in this organisation it’s happened in the past, and you go back from it and decide that actually you’re not going to be the big brave people to disinvest.
Interview, group A, PCT1, C4
Lack of central support
As indicated earlier, some informants referred to one-off central guidance on potential areas for disinvestment. However, there was general agreement across interviews that central bodies did little to publicly progress the disinvestment agenda, while some informants suggested that central bodies actually hindered disinvestment. For example, NICE mandates to fund new treatments represented a substantial pressure on the groups, and were rarely accompanied with disinvestment suggestions. The way in which these mandates were presented in group A’s meeting was suggestive that NICE was out of touch with local financial pressures:
This is a classic example of somebody making a decision at the centre without any regard to the resources or anything – and then we of course, in future it’ll be the CCGs, to pick up the bill – because the bill is going to be enormous.
Observation, group A, PCT1, C1
Perceptions of the government’s silence when it came to disinvestment made these decisions all the more challenging. Informants from both regions had a strong sense that the government attempted to distance itself from disinvestment, which was thought to be a ‘vote loser’ (interview, PCT1, P1). One provider expressed that devolving these decisions to local bodies had the potential to introduce ‘postcode lotteries’:
And they [government] run a mile from being held responsible for that [. . .]. Politicians want to devolve these decisions to a local level [. . .]. And so, almost by definition, they are introducing a postcode lottery.
Interview, group A, PCT1, P7
Concern over public response over postcode lotteries was raised by numerous commissioners, demonstrating a further hurdle to engaging in disinvestment locally. Nationally applied disinvestment decisions were thought to be more palatable to the public:
Say for instance [the commissioners] don’t pay for homeopathy, so we’ve made a decision, and that’s supported nationally, that’s easy. But where it’s a much more localised decision it’s much more difficult to support.
Interview, group B, PCT2, C10
Overall, the government’s reluctance to become embroiled in disinvestment was suggestive of a more general avoidance of being explicit about financial constraints within the NHS. This was commonly referred to as the main barrier to engaging in disinvestment:
I think it’s very difficult to disinvest when the whole front case for the government is that every time a patient and a doctor sit down and talk about something, then the PCT, the CCG shall somehow magic it up. Until there is some restraint put forward to the public – I mean it’s like, you’ve got to expect treatment whenever you want it, wherever you want it, whatever you want, whatever is in the Daily Mail you ought to be able to have it. Expectations are cranked right up.
Interview, group B, PCT2, C11
Tools and capacity
The lack of systematic tools to identify opportunities for disinvestment was a clear barrier to engaging in disinvestment. As discussed earlier, commissioners and providers were aware of this deficiency. One commissioner went on to point this out as a substantial barrier to disinvestment:
The bit that’s challenging is where you’ve got embedded practice which is not um – does not have a sufficient evidence base behind it. Then you’ve got to identify that practice and disinvest in it. And identifying it is the issue.
Interview, group A, PCT1, C2
Three commissioning group members (two commissioners, one lay member) identified a lack of time and resource capacity as a barrier to pursuing disinvestment:
We haven’t had the opportunity and the time, I think it’s probably as simple as that – having the resources.
Interview, group B, PCT2, C9
The issue of limited capacity clearly emerged through our observations of meetings. Both commissioning groups were inundated with investment proposals. Observations revealed the extensive time and attention invested in working through these funding requests, especially treatments that had been recommended by NICE (mainly group A). Implementing NICE guidance was a process of considering relevance to local practice and fine tuning the details of how national policies could be implemented into specific local systems. These processes were presented as complex and time-consuming in observed discussions:
This is really a piece of work that ties together quite a lot of NICE guidance on the use of antiplatelet drugs. And it was extraordinarily difficult to work out from all these different bits of guidance exactly how we should be using antiplatelet drugs.
Observation, group A, PCT1, C4
Observations also revealed occasional cases where NICE guidance was perceived to lack specificity, thereby creating additional work:
There’s other NICE guidance which gives a little bit – I mean some of the NICE guidance is really unhelpful: ‘It is an option’. You know, I mean and, ‘It is an option,’ is really quite an unhelpful thing.
Observation, group A, PCT1, P2
The NICE guidance is unbelievably woolly.
Observation, group B, PCT2, P5
Discussion
This study has provided a novel, in-depth insight into how disinvestment is experienced at the local level of health-care decision-making. Investigating disinvestment within a naturalistic context has allowed us to identify barriers to disinvestment that have not yet been reported, as well as confirm and further develop previously suggested barriers. Most of the identified barriers are interconnected, playing a role in reinforcing one other.
The qualitative study, originally proposed as an evaluative process of the proposed disinvestment method, evolved into an ethnography of how disinvestment works in naturalistic contexts. One of the merits of qualitative methods is the potential to adapt and uncover new research questions. We found that commissioning groups were far less involved in disinvestment decision-making than we had expected while designing the study. It was therefore imperative that the qualitative study adapt, shifting focus to ask fundamental questions about how disinvestment is currently approached (if at all), and the reasons underlying the apparent limited discussions around disinvestment. The findings presented in this chapter provide novel insights into the naturalistic setting within which the project’s proposed disinvestment method would be applied. We found there was a clear need for tools and guidance for identifying opportunities for disinvestment, such as the methodology proposed in this project. However, we also uncovered a host of wider barriers to implementing disinvestment decisions that would be applicable regardless of the methodology used to identify disinvestment candidates. In this sense, the barriers uncovered (summarised in the next section) are relevant to implementing disinvestment proposals that might emerge through the methodology proposed in the wider project.
Main findings in relation to existing literature
The barriers to disinvestment identified in this report were practical and ideological in nature. Practical barriers included limited knowledge, tools and capacity to engage in disinvestment. Ideological barriers related to reluctance to be seen to ration health care, and difficulties in collaboration between commissioners and health-care providers. Interestingly, the practical barriers identified are similar to previously reported obstacles to use of economic evaluation in local settings, as reported by Eddama and Coast. 74 We also noticed parallels between the ideological barriers to disinvestment we identified and Greenhalgh et al. ’s11 reports of professional ‘power struggles’ hindering diffusion of knowledge and innovations. Each of the specific barriers to disinvestment identified in this report has been summarised below, and considered in the light of existing literature.
Definitions of disinvestment
Our research reveals a lack of clarity surrounding the definition of disinvestment. This has implications for interpreting previous and future research in this area. While most informants understood this to refer to reduction or cessation of activity, some felt the term referred to limiting the funding of new treatments. Even within the broad group that focused on currently funded activity, tendencies to label actions as ‘disinvestment’ were inconsistent. At one extreme, disinvestment was thought to exclusively entail complete removal of a service; at another extreme, substitution of one activity for an improved version was thought to constitute disinvestment. It is perhaps unsurprising that such mixed interpretations of disinvestment exist, given the inconsistencies in how the concept is framed in the literature. 165 We also found that the term ‘disinvestment’ can carry negative connotations, suggestive of denial of care due to financial cutbacks. Disinvestment can thus be framed depending on perspective: some focus on the end result (e.g. activity reduction/cessation); others look more closely at the motivations behind this reduction/cessation.
Our finding that the term ‘disinvestment’ is poorly demarcated has implications for how the disinvestment process proposed in the wider project may be received in practice. The proposed method involves identifying high-variance/high-use procedures, and presenting these as candidates for ‘disinvestment’. However, the process also promotes open discussion with commissioners to identify the reasons underlying the apparently high-volume procedure rates, and potential solutions for regulation. Our qualitative findings imply that, depending on the action taken, the term ‘disinvestment’ may or may not be an appropriate label from the perspective of stakeholders.
The ambiguity of the term ‘disinvestment’, with its potential negative connotations, supports the promotion of new terminology. ‘Resource optimisation’ and ‘resource reallocation’ are but two alternative phrases that may carry different implications and promote more consistent understanding.
The lack of tools and guidance to inform disinvestment
Commentaries from the literature suggest that the absence of tools and guidance are key barriers to engaging in disinvestment. 15 In support of Robinson et al. ’s findings,163 we found little evidence of any tools or frameworks to support disinvestment decision-making. Our observations and interviews gave insight into the strategies health-care decision-makers had adopted in the absence of established tools and methods. While commissioners were able to develop disinvestment policies, these had been identified through unsystematic processes that are not sustainable, and not guaranteed to make worthwhile savings. Interview informants expressed difficulty with identifying relevant, high-impact opportunities for resource release. Similar challenges have been reported during pilot priority-setting exercises that have sought to incorporate disinvestment. 166 The absence of a clearly defined process also threatened the transparency, and thus acceptability, of disinvestment decisions that had been made. For instance, case study clinicians were not clear on why procedures had been targeted for disinvestment. Having a transparent process could promote discussions between stakeholder groups, thus minimising risk of miscommunication. The prospect of a well-defined process to guide disinvestment would be welcomed by commissioners, as shown through qualitative interviews undertaken in England, Australia and Canada. 12,163,167
The literature is sparse in terms of offering empirically supported tools to guide disinvestment. Academic commentators and experts in the field have suggested frameworks and models to guide disinvestment, although these remain untested. 152,155,168–170 There is, however, a growing body of empirical evidence to aid in the identification of disinvestment candidates (e.g. looking at clinical practice variations). 39 There have also been suggestions that the criteria for assessing disinvestment candidates could be adapted from established criteria used to assess new technologies (e.g. the criteria used by NICE). 152 If the latter were adopted, criteria would need to reflect the different context of evaluating currently provided technologies. For instance, engagement with patient user groups may need to feature more heavily in the reassessment of technologies. One area of potential contention is the use of cost per QALY thresholds, and whether or not these should be the same for investment and disinvestment decision-making. 171,172
Finally, research needs to be undertaken on how best to implement disinvestment decisions. NICE, for instance, has most of its disinvestment guidance built in to clinical guidelines. 173 Our research revealed frequent implementation of disinvestment through restrictive policies. Robinson et al. ’s survey on priority-setting practices suggested that policy formation is likely to be a common form of implementing disinvestment decisions. 162 Further research is needed to investigate the best ways of presenting and enforcing these policies, taking into account different stakeholder perspectives (e.g. patients, clinicians and commissioners). This is an area we have started to explore by considering two forms of policy enforcement – a major theme covered in the next chapter.
Collaboration issues
Issues of collaboration were key barriers to successful implementation of disinvestment decisions. This was a multifaceted issue, with many factors contributing to creating and reinforcing division between commissioners and health-care providers. These include underlying assumptions about providers’ agendas, problems with the process of clinician engagement, and the absence of a shared language when discussing disinvestment.
The environment of mistrust observed among commissioners and providers is not a new concept. 174 Concern about provider resistance to disinvestment is rooted in assumptions that reducing or withdrawing health services is countercultural to providers accustomed to being rewarded for activity. 159,163 We are not in a position to comment on the extent to which financial matters motivated providers, but we are able to show how the mere existence of these ideas can threaten a sense of collaboration. Commissioners in this study were very much mindful of what they perceived to be conflicting agendas, perceiving this as the basis for providers’ and clinicians’ resistance to disinvestment. Other studies have reported similar beliefs among commissioners and budget holders. 163,175 Providers and clinicians from our study were acutely aware of these assumptions. This awareness threatened collaboration, instead reinforcing providers’ sense of exclusion from resource allocation decisions. Providers had no sense of ownership over the local health-care budget, and lacked a sense of belonging within the decision-making groups.
Collaboration with clinical and provider stakeholders has been cited as an important theoretical facilitator to disinvestment within qualitative interviews with professionals at different levels of health-care decision-making. 40,167,176 We found similar emphasis was placed on the importance of engagement among our informants, but went on to show the difficulties of clinical engagement in practice. Although the commissioning meetings we observed had wide representation, commissioners were most influential in decision-making processes. Providers’ sense of exclusion suggested that the process of engagement was ineffective. This can partly be explained by the lack of a clearly defined disinvestment process. Providers were unsure of what the consultation process was designed to achieve, and unclear on the extent of power they held in changing policies. Providers/clinicians also desired input in determining initial drafts of policies, rather than commenting on these once formed. These findings suggest that exercises designed to promote wider stakeholder engagement need to be transparent in their aims, well thought out, and agreed upon by commissioners and the targeted stakeholders. There are still wider questions about stakeholder engagement that need to be addressed, such as the ideal timing and frequency of consultation. Commissioners from our study emphasised the importance of timely decision-making, yet providers clearly felt consultation was too little and too late. There needs to be a balance struck, where consultation is both effective and manageable.
Finally, collaboration was further impeded by differences in how local disinvestments were framed by commissioners and providers/clinicians. Cooper and Starkey highlight the importance of having a shared dialogue when it comes to disinvestment in this area. 151 The language used by commissioners was often suggestive of clinicians’ practices being unnecessary or wasteful. This implication did little to engage clinicians/providers, who attributed disinvestment purely to financial factors. Providers were particularly frustrated by this divide, stressing that commissioners failed to cast local disinvestments in an honest light. A recent commentary on rationing in the US health system talked of a shift from ‘rationing’ terminology to ‘waste avoidance’, but, in doing so, runs the risk of suggesting deliberative fraud on providers’ part. 177 Previous priority-setting exercises, led by researchers, also anticipated this issue in relation to disinvestment, and thus avoided presenting candidates for resource release as being unnecessary or excessive. 153
Being explicit about financial constraint
Cutting back in health care is undeniably an unpalatable subject. Informants’ perceived barriers to disinvestment were all based on the contentious issue of reducing or removing health care, regardless of how broad informants’ definitions of disinvestment were. There was a general discomfort surrounding the notion of ‘taking away’ health care: a theme that recurred throughout our findings, from commissioners’ careful framing of previous disinvestment exercises to the frequently expressed concern of public and media outcry. There were accounts of commissioners refraining from disinvesting for these very reasons. Social attitudes surrounding the removal of health care are an important barrier to progressing the disinvestment agenda. The failure to identify opportunities for disinvestment within previous PBMA exercises have been attributed to group leaders’ reluctance to discuss disinvestment, for fear this would ‘scare off’ stakeholders and sacrifice other useful elements of the PBMA exercise. 157 This has led to discussions remaining in the safe zone of resource-neutral and resource investment proposals. 158
The providers/clinicians we interviewed were not only aware of resource constraints, but appeared to welcome open discussion about sacrifices that would need to be made on the grounds of scarcity. Failure to talk openly about these issues led to frustration, promoting a culture of distrust. Similarly to previous studies,163 we found that commissioners themselves desired to be open with the public about what the NHS could realistically afford, but there had been no action taken towards achieving this. Patient and public perspectives on disinvestment are an important area in need of research attention, particularly during times of austerity, when cuts to public services are likely to be well publicised. The next chapter will set out our findings surrounding patients’ acceptance of disinvestment, although this type of research will need to be conducted across a broad range of health services.
Capacity issues and limited central support
Limited capacity was an observed obstacle to engaging in disinvestment. Local decision-making groups’ agendas were packed with proposals for investment and implementation of central guidelines and mandates. Other qualitative studies exploring priority setting have also found that disinvestment tends to be left off local decision-making groups’ agendas. 160,161 Central organisations responsible for health technology appraisals have similarly quoted limited capacity as an obstacle to reassessing existing technologies, especially when the invested time and effort are not guaranteed to produce ‘worthwhile’ savings. 40 Underlying the capacity barrier are questions about how decision-makers’ time is prioritised. It has been argued that disinvestment should now be top of decision-makers’ lists, in the light of the current state of health economies around the world. 36 Some have proposed that these apparent capacity issues may reflect a lack of political will to harness the time and expertise required to prioritise disinvestment. 15 Health technology appraisal organisations identify the lack of ‘champions’ for disinvestment and absence of political drive as key obstacles to developing a disinvestment agenda. 40 It is unclear how much room local decision-making groups have to manoeuvre: they are legally obliged to implement NICE mandates within 3 months, and could face appeals and legal backlash if funding proposals are not considered carefully. These pressures can indirectly work against local decision-making groups’ capacity to engage in disinvestment. 178
The lack of central support when it comes to disinvestment was strongly conveyed by participants across all sectors, regardless of their commissioner or provider status. The government was not only seen to refrain from talk of disinvestment, but thought to continually raise public expectations of what the NHS can deliver. This made local disinvestment decision-making all the more difficult, especially given the desire to avoid postcode prescribing. One solution to this issue of devolved disinvestment is for NICE to take the lead with disinvestment recommendations. NICE has produced some ‘do not do’ recommendations, but has still been criticised for its failure to couple mandates for investment with suggestions for disinvestment. 169,178 This has led to concern that local bodies will make haphazard disinvestments whilst under pressure to fund mandated technologies. 178,179 We did not find any evidence to support this: our observations instead suggested that decision-makers tended to engage in fire-fighting behaviour when faced with requests for new resource allocation, rather than strategically look for disinvestment opportunities. The barriers to disinvestment can thus have significant practical and ethical implications for the provision of new treatment.
Strengths and limitations
Our study was unique in that it focused on disinvestment as it is experienced in practice. A key strength of the study was its reliance on inductive approaches to formulate findings that are grounded in actual experiences and events. We sought to approach the research topic with a blank slate, asking questions that might be taken for granted or preconceived prior to this work. Empirical evidence that has been inductively generated is crucial for forming a foundation for subsequent research, and any interventions that seek to push forward the disinvestment agenda. The economic difficulties faced by health-care systems worldwide make this an important area of research, especially given that disinvestment is widely viewed as a challenging prospect. The methods we used not only enabled us to put forward novel barriers to effective disinvestment, but also confirmed and added dimensions to obstacles that have previously been suggested.
Adopting multiple data sources helped to lend credibility to reported findings. Using observations and interviews helped to view the complex issue of disinvestment from different angles, helping to build a more comprehensive view of the phenomenon. Where similar themes have emerged, this has lent weight to findings. Contradictions have also been reported accordingly. These added richness to the findings, and re-emphasised the complex social factors underpinning disinvestment. For instance, the fact that disinvestment was explained differently in group versus individual contexts relates to wider issues, such as the reluctance to be explicit about rationing. We were able to uncover some of the barriers to disinvestment by adopting source triangulation. Comparison of different stakeholder groups’ responses to the same questions illuminated issues of division and threats to collaboration. In some instances, commissioners’ and providers’ responses converged, lending credibility to reported themes. For instance, the lack of central guidance when it came to disinvestment appeared to be a substantial and widely felt issue, highlighting it as an easy starting point for intervention. Our findings will be relevant to health-care decision-makers operating at the local level of resource allocation, in that we have identified broad, fundamental issues that are likely to be transferable. We can confidently report findings with the knowledge that themes were not specific to any of the two sociodemographically contrasting research sites. Individual decision-making groups operating in different geographical and political contexts are likely to experience their own set of barriers, and some of the more specific barriers we cover may not be applicable.
There were a number of limitations to this study. First, as with any controversial subject discussed by public figures, socially desirable responses could have acted as a substantial source of bias. Questions must also be raised about the influence of the NHS reforms that were occurring throughout the period of data collection. The plans to restructure the NHS have been controversial, and could have impact on professional roles. These factors may have indirectly influenced informants’ accounts, particularly if they felt threatened or aggrieved by the reforms. Similarly, disinvestment may be approached very differently, or have a different level of priority, once the reforms have settled in. The provider/commissioner divisions discussed may be retained, but could be altered in light of GPs taking the helm in commissioning. GPs may also be better placed to identify areas for disinvestment, given their experience and knowledge of patient preference and care pathways.
We were able to continue sampling interview informants from each site until we were satisfied that saturation had been achieved. Making claims of saturation for the observed meetings is more problematic, as we had less control over when and how often data collection could occur. While we were able to make a choice to stop data collection at one site (on grounds of saturation), the second site’s meetings were less frequent and often cancelled. Data collection had to end because of timing constraints, although the three meetings observed replicated the same themes, and were broadly similar to the themes emerging from the first site. Assessment of this site’s meeting agendas that were received after the close of data collection reassured us that no new themes were likely to emerge had we continued observations.
As with all qualitative research, the extent to which our findings can extend to other settings is questionable. Further research in this area could adopt survey methodology to investigate the generalisability of some of the views or perspectives to emerge from our study.
Finally, discussions about current disinvestments that were in the process of implementation were absent from meetings. It is possible that disinvestment discussion took place away from the observed commissioning meetings, although the research team established at the outset that the commissioning advisory group meetings were key forums where disinvestment decisions would be discussed. However, the lack of discussion within meetings reflects one of the key issues our research highlights: disinvestment still does not appear to be adopted as a routine exercise. Our adoption of interview methods was an alternative means of investigating how disinvestment is tackled in practice, and, importantly, allowed in-depth exploration of the underlying reasons for the lack of disinvestment decision-making observed. The various practical challenges to disinvestment to emerge through interview, and our observations of the competing demands commissioners face within meetings, offer some explanations of why disinvestment does not occupy a more substantial proportion of commissioners’ agendas.
Conclusion/recommendations
Disinvestment at the local level of health-care decision-making is fraught with difficulties, owing to a lack of tools and capacity to engage in the complex decision-making process. Implementation of disinvestment decisions would benefit from greater inclusion of provider groups. This will require promotion of a shared dialogue, and greater transparency in the process of identifying and negotiating opportunities for disinvestment. Local decision-making groups will either need more guidance from central organisations when it comes to disinvestment, or promote democratic processes in disinvestment decision-making, whereby the public has greater involvement. This may help to tackle some of the social factors underlying the reluctance to take responsibility for disinvestment decisions. Finally, ‘disinvestment’ is a poorly demarcated term that sparks a range of different understandings. Any guidance to improving engagement with disinvestment will need to take account of this, and consider promotion of a less ambiguous term that reduces inconsistent interpretations.
Chapter 8 Case study of carpal tunnel release surgery: patient and clinician perspectives
Introduction and aims
The previous chapter presented disinvestment from the perspective of professional groups working within the health-care sector, from commissioners to front-line clinicians. The patient and public voice on such matters was not considered. Citizen involvement in priority setting has been long promoted,180 particularly in the face of contentious decision-making. Given this, there is a clear need to consider the patient and public voice in the ongoing development of the disinvestment agenda, both in general and in relation to specific cases of disinvestment. Research has explored patients’ perspectives on rationing health interventions that were not routinely funded at the time of investigation. 181–183 The underlying principles of disinvestment – to ‘remove, reduce or change currently provided services’ – presents a different research context. Citizens’ and patients’ attitudes to the concept of ‘disinvestment’, both generally and in relation to one’s own care, have not yet been the subject of systematic investigation.
The CTS surgery case study introduced in the previous chapter was an ideal platform to begin investigating some of the above issues. A qualitative substudy was designed with the aim of investigating patients’ perspectives on disinvestment processes that had potential to influence their care. Specific objectives of the substudy were to investigate (in the context of CTS surgery):
-
patients’ awareness of disinvestment issues
-
patients’ rationalisations of the steps taken throughout their care pathways
-
patients’ perspectives on the use of threshold policies for regulating access to surgery
-
patients’ views on who should gate-keep access to surgery.
In addition, clinician views on the provision of CTS surgery, and their perceptions of the impact disinvestment could have on their practice, are also presented in this chapter.
Methods
Study design and methodology
The qualitative substudy of patient views was a component of the wider ethnographic study described in the previous chapter. Methodological details relating to the clinician interviews can also be found in the previous chapter. Semistructured interviews were conducted with patients over a 3-month period (January–March 2013). Interviews were conducted face to face wherever possible, but telephone interviews took place if it was not feasible to meet in person.
Sampling and recruitment
The sample of patients was drawn from three NHS acute hospital trusts (all three acute trusts were participating in the wider ethnographic study, described in the previous chapter) situated within the two study regions introduced in the previous chapter (i.e. PCT2 and PCT1). Eligible patients needed to have attended an outpatient appointment in relation to a working diagnosis of CTS within 12 months preceding the date of recruitment. The aim was to achieve a sample of maximum variation on the basis of age, gender and outcome of the most recent outpatient appointment (i.e. admitted for CTS surgery, not admitted for CTS surgery). Clinicians and research nurses identified eligible patients. Clinicians leading this process were participants in the wider ethnographic study. Hospital administrative staff posted recruitment packs to potential participants from December 2012 to March 2013. Each pack consisted of a letter introducing the study; a participant information sheet; a pre-paid postage envelope; and instructions on what to do if the recipient did not speak English. Instructions were translated into the top five most widely spoken non-English languages within each region (based on local council statistics). Patients interested in participating were asked to contact the researcher (LR). Once the researcher had confirmed eligibility in relation to the criteria above, a mutually convenient interview time and location were agreed on. Recruitment packs were sent within time periods to control the volume of responses received. The aim was to stop recruitment at the point of data saturation. This was achieved in PCT1, but recruitment in PCT2 ceased 1 month prior to the study’s official close (to allow time for analysis). Data saturation was considered separately for each region on account of the different contexts of CTS provision.
Data collection: patients
Consent process
Written consent was obtained from all participants on the day of interview. For telephone interviews, consent forms were posted with a pre-paid return envelope in advance. Interviews did not take place until the researcher had received participants’ signed consent forms.
Interview conduct
All interviews took place in participants’ homes (or by telephone), and were audio recorded with permission. An interview schedule was used to ensure topics were consistently covered with all participants. Questions either explored patients’ experiences, or required patients to consider scenarios posed by the interviewer. Topics of interest included patients’ understanding of diagnostic tests and prescribed treatments; acceptability of their care pathways; knowledge of/perspectives on local disinvestment initiatives; and views on who is best placed to gate-keep access to CTS surgery. Seventeen patients were interviewed in total. Table 21 shows the breakdown of patients interviewed per NHS trust/region.
| Region | NHS trust | Number of informants interviewed |
|---|---|---|
| PCT2 | NHS trust 1 | 6 |
| PCT1 | NHS trust 2 | 7 |
| NHS trust 3 | 4 |
Analysis and presentation of data
The approach to analysis and presentation of data consistent with methods described in the previous chapter.
Background/context: disinvestment from carpal tunnel syndrome surgery
Clinician interviews provided an overview of each region’s standard patient pathway, defined as the typical route from initial GP consultation to admission for surgery. Clinicians were also asked about the nature of the disinvestment(s) that had occurred in relation to CTS within their regions. Separate meetings with commissioning leads were arranged to confirm the accuracy of clinicians’ descriptions, with the emphasis being on factual detail, rather than rationalisation/interpretation of actions taken. Commissioners’ and clinicians’ accounts were consistent, establishing a reliable framework within which patient experiences could be placed.
Threshold policies: PCT1 and PCT2
Threshold policies for CTS surgery were implemented within both regions (edited versions shown in Box 1). The eligibility criteria set out in these policies were broadly similar, although subtle differences in wording were apparent. Both regions’ policies included a statement conveying eligibility for surgery once conservative therapy has been attempted for a specified period (3 or 6 months). Both regions allowed patients to bypass conservative therapy if they had clinically specified symptoms indicating severity, or if symptoms were having a significant impact on patients’ day-to-day lives.
PCT2 will fund CTS surgery, if:
-
symptoms persist despite at least 3 months of conservative therapy with either nocturnal splinting and/or local corticosteroid injection, OR
-
there is neurological deficit, e.g. sensory blunting, muscle wasting or weakness of thenar abduction, OR
-
the patient is suffering from significant functional impairment, defined as symptoms that prevent the patient fulfilling vital work, educational, domestic or carer responsibilities.
PCT1 will fund CTS surgery, if:
-
there is no improvement in symptoms after 6 months of conservative management (defined within a footnote as ‘nocturnal splinting and local corticosteroid injection’), OR
-
there is evidence of severe symptoms at presentation (including but not limited to sensory blunting, muscle wasting, weakness on thenar abduction), OR
-
symptoms interfere significantly with daily activities. This includes all individuals whose symptoms are severe where 6 months’ conservative management would be detrimental to the management of the condition.
Differences between each region’s policy include (1) different required durations of conservative therapy (3 vs. 6 months); (2) different requirements in the number/types of conservative therapies used (‘nocturnal splinting and/or local corticosteroid injection’ vs. ‘nocturnal splinting and local corticosteroid injection’); (3) different specified outcomes in relation to conservative therapy (symptom persistence vs. lack of improvement in symptoms); (4) different levels of flexibility in clinical definitions of symptom severity (mentioning/not mentioning that that clinical signs of severity are ‘not limited to’ those listed); and (5) differences in specification of non-clinical criteria for assessing impact on life (defined in PCT2, not defined in PCT1).
The issues of ‘clinical consultation’ and transparency of the disinvestment decision-making process were discussed in the previous chapter. In summary, clinicians from PCT2 had little awareness of how threshold policies were formed, or the identity of individuals making prior approval decisions. These clinicians expressed frustration that their views had not been acknowledged prior to, or after, implementing these policies. By contrast, clinicians from PCT1 knew the creators of the threshold criteria by name, and were actively involved the formation of these policies.
Prior approval process: PCT2 only
Although each region had broadly similar thresholds for funding CTS surgery, their implementation differed. Trusts in PCT1 were paid for activity, provided that clinicians had documented how patients satisfied the threshold criteria, retrospectively assessed by periodic audit. In contrast, trusts in PCT2 were paid only for activity that had been approved by the PCT in advance. Patients’ fulfilment of threshold criteria was assessed by the PCT’s ‘exceptional funding panel’. This panel was responsible for assessing eligibility for all clinical activity that carried a ‘prior approval’ status. Clinicians were required to submit a prior approval application to this panel for each patient they intended to refer for surgery. The PCT had the right to withhold payment for any CTS surgery that had not been approved in advance, regardless of the fulfilment of threshold criteria.
Introduction of intermediary services: PCT2 only
PCT2 had introduced a physiotherapy-like intermediary service between general practice and the orthopaedic secondary care. Physiotherapists and related clinical experts working in these centres provided conservative care and/or submitted prior approval applications to the PCT.
Standard patient pathways
The standard patient pathway to receiving CTS surgery in each region is summarised in Figure 12. The pathway for PCT1 was relatively simple: GPs were able to directly refer to secondary care, and surgery could take place without prior approval. There was no formal direct route between general practice and orthopaedic services in PCT2. GPs were expected to either refer patients to the intermediary services, or apply for prior approval themselves if they felt surgery was indicated. Clinician interviews revealed that patients were sometimes referred to secondary care orthopaedic clinics from other secondary care departments. In these instances, consultants were required to apply for prior approval themselves.
FIGURE 12.
Typical routes to surgery for PCT1 (left) and PCT2 (right).

Carpal tunnel syndrome surgery out to tender: PCT1 and PCT2
Both regions had put CTS surgery out to tender, allowing other health-care providers in the region (some non-NHS) to compete for contracts. CTS surgery provision was thus fragmented across different providers within each region.
Results
This section begins with an overview of clinicians’ perspectives on the implementation of threshold policies and the prior approval process described above. Patients’ experiences of these systems/processes follows.
Clinician perspectives
Clinicians’ views on threshold criteria (PCT1 and PCT2)
Looking at threshold criteria alone, clinicians across both regions felt these had little impact on their clinical practice. For clinicians in PCT1, this was largely because of their involvement in writing the threshold criteria. Those consulted during policy formation explained how they had included a ‘catch all’ clause that preserved their ability to act in accordance with their own clinical opinion:
We haven’t rigidly defined ‘severe symptoms’. So there’s a clause that says you can operate on them if they’ve got severe symptoms . . . it’s the ones I want to operate on.
Interview, group A, PCT1, Clin2
Careful framing of patients’ symptoms also allowed clinicians to demonstrate eligibility if they felt the patient warranted surgery. Across the board, clinicians from PCT1 demonstrated that they were very much in control when it came to making decisions about surgery:
Well um you don’t want to um – you can’t invent symptoms, you can’t invent something that isn’t there, and one shouldn’t do it, because that’s a very slippery slope if you do that. But of course, you can guide the patient to give the answers that enable you to put them on a waiting list, giving them questions that will lead them to – um – perhaps make their symptoms more intrusive than they possibly are, if you feel strongly.
Interview, group A, PCT1, Clin3
Clinicians in PCT2 expressed similar views with regard to the threshold policies, but the intermediary services and prior approval system largely removed clinicians’ responsibility of assessing patients against these criteria. By the time a patient had progressed to seeing a consultant, their eligibility for surgery will have been determined by the PCT:
But now they [patients] don’t get to me, because someone else is making some sort of decision that they need steroid injections or they don’t need carpal tunnel surgery or they do.
Interview, group B, PCT2, Clin7
Despite this, patients still filtered through the system without funding approval – usually when referred from other secondary care specialists (e.g. rheumatologists, neurologists). In these situations, the consultant would apply for prior approval, using the threshold criteria to justify why a patient required surgery. One clinician described this process, maintaining that they had never allowed threshold criteria to prevent them from operating on a patient they felt truly required surgery:
It’s like everything else: you find ways around the system. [. . .] I have a patient in front of me who needs treatment. And if I think the best thing for them is carpal tunnel surgery then quite clearly, on paper, they will meet the criteria. So the whole thing is a nonsense.
Interview, group B, PCT2, Clin9
The above perspective was similar to that of a clinician from PCT1:
I mean, I feel I’m being interfered and manoeuvred, but actually when a patient is sitting in front of me, if I need to operate on that patient, a lack of funding or anything like that has not stopped me from doing it – I’ve always been able to do that.
Interview, group A, PCT1, Clin4
One clinician from PCT2 expressed concern that threshold criteria were inappropriate if adopted as the sole decision-making tool. Whereas clinical specialists could draw on tacit knowledge and experience, others (i.e. PCT commissioners) would be fully reliant on an overly reductionist tick-box system:
I fully appreciate we’re in a time of austerity, but you cannot pick a condition and put criteria for treatment. That’s why medicine is a long process – it’s an art essentially [. . .], because no books can tell you exactly what to do, for that very reason. And now you’ve been through all of that, and overnight a criteria tick-box exercise has been introduced? It just doesn’t agree with medicine, full stop.
Interview, group B, PCT2, Clin5
Clinicians’ views on the prior approval process/intermediary services (PCT2)
Most clinicians from PCT2 commented on how the intermediary services and prior approval system delayed patients’ progression to surgery. These delays were thought to increase the risk of poorer surgical outcomes. One clinician felt there had been a notable increase in the proportion of patients seen with late stage CTS since the introduction of the disinvestment initiatives:
So I now see people who have had carpal tunnel syndrome for years, have permanent nerve injury, and several years ago would have been referred a lot earlier by their GP, and would have had surgery, and would not have had the permanent nerve injury which they definitely now have as a result of the delay coming through for surgery.
Interview, group B, PCT2, Clin8
Clinicians from PCT2 were adamant that the intermediary service and prior approval system would not prove cost-effective in the long term, pointing out the possible financial repercussions if patients with poor surgical outcomes attributed this to delays brought about by the convoluted care pathway:
Whilst the population remains relatively uneducated we will get away with it. But some patients will get denied access and will have irreversible neurological damage and will then sue somebody – and then your cost–benefit analysis will go out the window.
Interview, group B, PCT2, Clin8
Another clinician commented on how the administrative effort demanded by the prior approval process was a threat to cost-effectiveness in its own right:
So I can tell you that my secretary’s job, and she’s very competent, now more than 50% of her time she spends doing administration with respect to prior approval.
Interview, group B, PCT2, Clin9
There was a common view among clinicians that most CTS cases would eventually require surgery. One clinician held the more extreme opinion that surgery was the only permanent treatment option for CTS (with the exception of cases related to pregnancy). Delaying surgery was thus viewed as delaying the inevitable, having clear implications for long-term cost-effectiveness:
Because eventually patients with carpal tunnel syndrome need surgery. [Later] They think they’re saving money, but they’re not, it’s costing them a lot more money in the long run.
Interview, group B, PCT2, Clin7
Explaining disinvestment processes to patients (PCT1 and PCT2)
Most clinicians from PCT1 had no experience of discussing disinvestment with CTS patients, on the basis that they had been able to maintain standard practices (by their accounts). There was one exception to this, where one clinician acknowledged that he had, on occasion, had to explain to patients that their surgery would need to wait until they had tried conservative therapy. This clinician reported being very open about the lack of control he had over the situation, diverting responsibility to the PCT:
Yes I have told them that um the reason why they are having this treatment, and not an operation which they possibly expected to get, is that they don’t fulfil the criteria set up by the PCT.
Interview, group A, PCT1, Clin3
Another clinician from PCT1 echoed the above ideas, although this was in reference to other ‘low priority’ procedures:
I mean we blame it squarely and fairly on the PCT; we take no responsibility for it. We say, ‘Look, this is the health authority’s rules,’ and if we’re feeling jaded we do tell them, ‘Yes, it’s just a form of rationing’.
Interview, group A, PCT1, Clin2
Most clinicians from PCT2 saw patients only once their prior approval for funding had been granted. Two clinicians reported seeing patients without secured approval. In these cases, they too reported having no qualms in being explicit about the PCT’s control over the provision of surgery. Although the prior approval forms required clinicians to explain the possibility that funding might not be granted, both clinicians felt they would have explained the process to patients regardless of this obligation:
Oh God yeah, you have to. On the form it says you need to explain to the patient that the funding might get turned down. So, I mean, it’s absolutely clear. I do anyway, because I feel obliged to. They need to understand why they’re waiting for their surgery.
Interview, group B, PCT2, Clin5
One PCT2 clinician who had never needed to explain the prior approval process still suggested a readiness to be open about the loss of clinicians’ control in decision-making. This was reflected in his comment that he would readily support a patient if they were to challenge the PCT on legal grounds:
I suspect at some point someone will successfully sue somebody preventing access. That has not occurred yet as far as I am aware, but I would support that if they ask me as an expert witness.
Interview, group B, PCT2, Clin8
Patient perspectives
Overview of patients’ pathways
Patients’ pathways from initial consultation to their final secondary care appointments varied considerably, in terms of both the treatments received and the settings in which these treatments were delivered. Patients were divided into two groups based on the types of treatments received. These groups are summarised in Box 2. There was an even mix of patients who had received their CTS diagnosis from primary and secondary care clinicians.
Over half of the patients interviewed had tried some form of conservative treatment (n = 9). Most of these patients were from PCT1 (n = 7). Five of the nine patients had tried nocturnal splinting followed by corticosteroid injections (all from PCT1). Other patients had tried only splinting (n = 4). It was notable that no patient from PCT2 had been prescribed corticosteroid injections for their CTS symptoms.
All patients who underwent conservative treatment were eventually offered surgery, but two opted against this (both from PCT1). Reasons for this included patient concern about the long recovery period, and patient-perceived improvement in symptoms following corticosteroid injection.
‘Straight to surgery group’A considerable number of patients did not undergo any conservative therapy prior to surgery (n = 8, evenly spread between both regions). Five of these patients had comorbidities in the upper limb region; one patient had experienced symptoms for 12 years, and two patients had opted for surgery, having been offered a choice of treatment (both from PCT1).
The summary in Box 2 shows how patient choice may have had a role to play in treatment provision. Reports of shared decision-making tended to come from patients from PCT1. Beyond patient choice, reasons underlying the structure or content of treatment pathways could not be reliably ascertained on the basis of patients’ accounts alone. Such information needed to be derived by analysing patient notes or interviewing clinicians about specific patient cases. Thus, our focus was not so much on the extent to which clinicians complied with threshold criteria, but more on patients’ rationalisations of their treatment pathways. We were particularly interested in elucidating patients’ awareness of rationing/disinvestment initiatives, and the extent to which different rationing approaches were acceptable.
Patients’ expectations for surgery
All but a few patients reported that they had been familiar with CTS and its management prior to receiving their diagnosis. Most patients had an expectation that surgery was a certainty or possibility. Lay experiences played an important role in building patients’ knowledge of the condition and its treatment options. When asked to explain their expectations, most patients commented that they were familiar with at least one other person who had received surgery for CTS:
I belong to a craft class and several ladies there have had it done.
Interview, PCT1, P1
Similarly, three patients’ personal experiences of having CTS in the past shaped their expectations for surgery. Two patients had come to expect surgery having conducted their own research:
I was expecting to be referred for surgery, which I was. I think that was the . . .
What created that expectation?
I think a bit of research and everything else as much as anything.
Interview, PCT2
Patients without expectations reported being unfamiliar with CTS prior to their diagnosis, having little knowledge of the condition or its treatment options.
Patients’ rationalisation of pathway stages
Nerve conduction studies
Nerve conduction studies (NCSs) are a form of diagnostic test sometimes used to diagnose and evaluate the severity of CTS. NCSs featured prominently within most patients’ pathways, occurring at various stages prior to and during conservative therapy regimens. Patients rationalised the NCS in different ways. Some saw it primarily as a diagnostic tool, while others focused on its potential to gauge severity:
He just said that he was going to send me to see what was causing the problem.
Interview, PCT2, P6
I think they can tell how bad it is.
Interview, PCT1, P1
Three patients saw the NCS as a test to ‘prove’ that they had CTS and were worthy of treatment. All of these patients received surgery after their NCS:
She [GP] didn’t think I [had CTS]. So then I went off to have the electrical test done – and I passed that.
Interview, PCT2, P5
Well I think they’ve got to prove that you’ve, that’s what it is, haven’t they. I can only imagine that’s what it was.
Interview, PCT1, P16
Conservative therapy
Patients who underwent conservative therapy tended to view this as an expected, acceptable step that served a clinical or practical purpose. There was no single overarching theme that represented patients’ understandings of why they had undergone conservative treatment, but four subthemes emerged. Patients’ accounts sometimes incorporated more than one of these themes.
Patient choice
Two patients were very vocal about their sense of control over what treatments they underwent. Both patients had tried splinting, and then opted to try an injection when offered a choice of injection or surgery. Both had a strong preference to avoid surgery:
He said I’ve got that choice. Anyway, I said well obviously I didn’t want an operation if it was avoidable.
Interview, PCT1, P13
When asked why she was averse to surgery, the patient above explained how their preferences had been shaped by an interaction with another patient in the outpatient clinic:
This man I was talking to in the waiting room – he said ‘no way’. He was going back again for treatment of something and he said ‘No way – I wouldn’t have surgery again,’ he said. So of course naturally that put me off didn’t it?
Interview, PCT1, P13
The second patient’s decision to avoid surgery was based on their perceived ability to cope with their symptoms. Their assessment of the impracticalities of surgery tipped the balance in favour of coping with symptoms, which had considerably improved following steroid injection. The patient recalled feeling reassured that there was an option to contact the consultant directly if they changed their mind:
Um because I feel I’ve got to try and live with it a bit longer [. . .] It is painful all the time, but I know I can get around it, and I know there’s a lot of people worse off than me. I said to him that I was in no position really to actually take that time out, and I would like to sort of live with it for a little while longer. And um – but I didn’t feel that I wanted to not have contact with him any more.
Interview, PCT1, P3
Potential to resolve symptoms/avoid surgery
Three patients suggested that conservative therapies served a clinical purpose, with potential to improve or cure symptoms:
I looked it up on the web first and basically they said that sometimes a splint will help. [Later] Obviously an injection is much cheaper than an operation and for some of the people it cures it, apparently.
Interview, PCT1, P8
The chance of potentially avoiding surgery was viewed by most patients as a clear justification for trying conservative therapy. Patients tended to rationalise the use of conservative treatment under the premise that surgery was always perceived as a last resort – often normalising this as a widespread societal attitude:
Yeah, oh yeah, because nobody wants surgery. I know I didn’t really want it. It was a last resort, to be honest.
Interview, PCT2, P5
Financial considerations
Two patients made unprompted reference to financial factors underpinning the stepwise progression from conservative therapy to surgery. One of these patients had been made aware of threshold-based access to surgery through their GP. This patient assumed that surgery may have been a more immediate solution to treating CTS in the past. This was thought to have changed in light of the financial constraints the NHS was now facing:
They did make it clear that um, you know, with all the various cutbacks, I guess, I don’t know if it’s due to cutbacks, that um they’re not going to operate unless they’ve tried all the other routes first. So he made it clear to me about that.
Interview, PCT1, P2
The second patient to discuss financial factors saw conservative therapy as an expected prerequisite to surgery, having conducted his own research. This patient had not been explicitly informed of any cost-related explanations or rationing processes, but assumed that the ‘standard’ steps he had read about on the internet had been formulated on the basis of financial considerations:
I think that the reason for that is quite simple, because that injection must be much, much cheaper than the operation. Whatever it is they are putting into your body has got to be less than all the theatre costs, etcetera, etcetera, costs of an operation.
Interview, PCT1, P8
Temporary relief
Finally, three patients explained how they had tried conservative therapy (injections or splints) while waiting to receive surgery/referral to secondary care. These patients saw conservative treatment as a measure to bring about temporary symptomatic relief, not an alternative to surgery:
I think they did tell me that this [injection] would just sort of tide me over. So – because I knew there would be quite a long wait to have the um [surgery]
Interview, PCT1, P1
Straight to surgery
Patients who were referred straight for surgery were aware, at the time of interview, that alternative treatments existed. Bypassing conservative therapy was rationalised in a number of ways.
Unlikely to benefit from conservative therapy
Two patients, both from PCT2, believed they had bypassed conservative treatment on account of the advanced nature of their CTS:
I think because I’d had it such a long time. I think I’d passed the stage of any sort of therapy before that. So really I’m not your typical case – further down the line, aren’t I?
Interview, PCT2, P5
Patient choice
Two patients attributed their bypassing of conservative therapy to personal choice, having been offered an option of surgery or injection:
Yeah whereas this [surgery] would last a lot longer. ‘It’s not guaranteed,’ he said, ‘but it’ll last certainly a lot longer, and it’s a better um – not recovery rate – a better success rate,’ he said. And we said, ‘Right, we’ll go for the better success rate’.
Interview, PCT1, P4
The above patient had experienced a long history of failed medical interventions for numerous non-related health conditions. This may shed light on the patient’s primary concern of avoiding further unsuccessful treatment:
I said, ‘I want my hands to work,’ I said, ‘everything else in my body is knackered,’ I said, ‘I need my hands,’ I said, ‘I need something’. [Consultant said] ‘Oh no, it’ll work, it’ll work, make a nice job, quick snip and do it up – bosh, away.’
Interview, PCT1, P4
The second patient had preconceptions about injections, perceiving these to have only temporary benefit:
Well that’s more my decision – I heard things about cortisone injections. I don’t know – I just didn’t think the cortisone injection would have worked the first time.
Interview, PCT1, P15
In contrast to the patients above, one of the ‘straight to surgery’ patients reported little involvement in the decision-making process:
Do you feel it was more in line with him saying ‘I think you need this,’ or did he ask you the way you want to be managed?
No not at all. I was told what I was going to have and that was it. Yes, yes, very much so.
Interview, PCT1
Carpal tunnel syndrome surgery as an adjunct
Two patients who had bypassed conservative care commented that their consultant had recommended they have CTS surgery as an adjunct to another upper limb surgical procedure. Both of these patients were from PCT2:
I told him that I’d had this one done quite a while ago – well 25 years I think it is – well he said I may as well do that [CTS surgery] when I’m doing the [other surgical procedure].
Interview, PCT2, P11
Provision of the ‘other surgical procedures’ was not restricted in any capacity (no threshold policies or prior approval process). It was not clear whether treating CTS at the same time was for clinical reasons or due to the prior approval status of CTS surgery.
Patient involvement in decision-making
As demonstrated above, some patients felt they had influenced the treatment they received by expressing a preference when given a choice. All of these patients were from PCT1. Other patients conveyed their personal involvement or control over their care in more subtle terms. The following accounts suggested that patients had a clear idea of what they wanted, seeing the clinicians as providers of this service. This was suggestive of a very consumerist style of doctor–patient interaction:
Well that’s what I wanted [surgery], and that’s what I said, and that’s what I was expecting.
Interview, PCT1, P2
At the opposite end of the spectrum, a handful of patients indicated a willingness to go along with whatever their clinician recommended:
I thought, ‘I’d come to you and that’s what you recommended was what was best for me so, but I’m not a surgeon and I don’t know what’s what’.
Interview, PCT1, P9
The findings relating to patient involvement and doctor–patient communication should be interpreted with caution. Patients tended to recall the outcomes of appointments, rather than how these outcomes were determined. It may not be surprising that patients who actually chose their treatments were better able to explain how treatment decisions were reached. Preferences of other patients may also have been explored in consultation without the patient being aware of or recalling this.
Patient awareness and understanding of rationing
The focus for other stakeholder group interviews was on ‘disinvestment’, implying a change in CTS surgery provision from how it once was. The actual changes implemented (i.e. introducing thresholds, prior approval systems) will be referred to as ‘rationing’ processes for the purposes of this section. Patients’ awareness of rationing processes were explored through different approaches as the interviews progressed: initially through open-ended questions that encouraged narrative accounts, and later through more structured questioning. For ethical reasons, the interviewer made no suggestion that rationing had influenced patients’ care. This was discussed only if initiated by the participant. Table 22 summarises patients’ awareness of (1) the existence of threshold criteria (PCT1 and PCT2) and (2) the prior approval process (PCT2 only). Most patients were not aware of rationing in any capacity. Of the patients who were aware (n = 5), most were from PCT2 (n = 4). This section focuses on the five patients’ views on the rationing processes that they had become aware of in relation to CTS surgery. These patients perceived that their care pathway had been influenced by factors outside their GP or consultant’s control. In all cases, this knowledge emerged unprompted.
| Patient identifier | Awareness of prior approval process? | When/how knowledge of prior approval acquired? | Aware of threshold criteria? | When/how knowledge of thresholds acquired? | Satisfied with care pathway? |
|---|---|---|---|---|---|
| PCT2 | |||||
| P5 | No | N/A | No | N/A | Yes |
| P6 | Yes | GP: mid-pathway, after recommendation for surgery | Yes | GP: mid-pathway, after recommendation for surgery | No |
| P7 | Yes | GP: first consultation of pathway | No | N/A | Yes |
| P10 | Yes | Consultant: mid-pathway, after recommendation for surgery | Somewhat: GP discussed threshold criteria, but patient did not appear to recall/understand the details of this | GP: first consultation of pathway | No |
| P11 | No | N/A | No | N/A | Yes |
| P17 | Yes | Consultant: mid-pathway, when recommendation for surgery first received | No | N/A | Yes |
| PCT1 | |||||
| P1 | N/A | N/A | No | N/A | Yes |
| P2 | N/A | N/A | Yes | GP: first consultation of pathway | Yes |
| P3 | N/A | N/A | No | N/A | Yes |
| P4 | N/A | N/A | No | N/A | Yes |
| P8 | N/A | N/A | No but suspects there is a ‘standard pathway’ influenced by cost | N/A | Yes |
| P9 | N/A | N/A | No | N/A | No |
| P12 | N/A | N/A | No | N/A | Yes |
| P13 | N/A | N/A | No | N/A | Yes |
| P14 | N/A | N/A | No | N/A | Yes |
| P15 | N/A | N/A | No | N/A | Yes |
| P16 | N/A | N/A | No | N/A | Yes |
Patients receiving care in PCT2
Patient awareness
Four of the six PCT2 patients were informed of the prior approval process by clinicians overseeing their care (i.e. their GP or consultant). Most patients alluded to the idea of disinvestment in their descriptions of the prior approval process, focusing on recent health-care cuts or the present state of CTS surgery provision:
She said, ‘Well there’s no funding now for carpal tunnel’.
Interview, PCT2, P7
P7 had a baseline for comparison, having been admitted for CTS surgery 12 years before. P10 clearly saw the prior approval process as a form of disinvestment, drawing parallels between their experience of CTS and another procedure the NHS no longer routinely funded:
The doctor said ‘Ah we just had funding cuts, we are not allowed to do this any more,’ and said, ‘We are not allowed to treat [condition] any more on the National Health’.
Interview, PCT2, P10
One patient suggested that the prior approval process was the standard approach to commissioning CTS surgery, making no reference to any changes in treatment provision:
He said they don’t normally get funding for carpal, you’ve got to ask permission. [Later] They just said it was one of the things they don’t do as a matter of course.
Interview, PCT2, P17
Patients’ prior experiences, and the context within which they had learnt of the prior approval process, had potential to influence their perspectives on this system. P7 had been informed of the prior approval process during their first consultation in primary care, at the beginning of their pathway. They reported feeling little surprise on hearing that CTS surgery was restricted, viewing this as an example of ‘cuts within the NHS’. Other factors contributing to P7’s reaction was their existing awareness that not all treatments are routinely funded by the NHS. They had become well acquainted with this through witnessing a friend’s experience of applying to an exceptional funding panel for treatment for a potentially terminal condition. Restricted access to CTS – a fairly low-priority procedure in the patient’s eyes – did not come as a surprise.
Well no, she just said with the cuts, this is one of the non-emergency procedures that have been cut. I suppose there’s lots that have been cut, aren’t there?
Interview, PCT2, P7
P17 had been informed of the prior approval process during their first consultation with the orthopaedic consultant. P17 recalled being told that, while there were funding issues for CTS surgery, this did not apply to the surgical procedure recommended for treating their comorbidities:
He said they don’t normally get funding for carpal, you’ve got to ask permission, and I said well, if I could have the [other surgical procedure] done, that would do me. He said ‘No, have it both done together’. It wasn’t actually very long.
Interview, PCT2, P17
Relying on patient recollections of how they reacted to previous events poses reliability issues. We were mindful that patients’ accounts may be inaccurate, and reports of how they felt or reacted at a particular time could have been influenced by subsequent events. It was notable that P7 and P17 went on to experience particularly smooth pathways, without being conscious of the prior approval application process or having to waiting for a decision:
I didn’t even know he’d got permission to do it – I just had this phone call to say he’d had a cancellation and could I go in. I didn’t even know you were going to be able to go in and do it.
So you didn’t get a letter from the PCT saying, ‘We‘ve approved?’
No, no.
Interview, PCT2
The seemingly smooth pathway to surgery described by P7 and P17 was in direct contrast to the remaining two PCT2 patients’ reported experiences (P6 and P10). P6 and P10 perceived the prior approval process as a significant setback that slowed progression to surgery. News of the prior approval process had come midway through these patients’ pathways, by which point they had come to expect surgery, following clinical recommendation:
Obviously they [hand clinic physiotherapists] did various tests and everything else, by which time I had complete numbness [. . .], and he said they were getting me in as soon as possible to have the right side done [. . .]. In any case, I then get a phone call from my GP in December saying that they weren’t going to fund it. He had to apply for special funding because the PCT doesn’t fund it regularly.
Interview, PCT2, P6
Knowledge of decision-making criteria
All four patients were aware that the prior approval process would entail some form of application, but lacked insight into how, and by whom, these decisions were made. Only one patient, a retired nurse (P6), assumed that the PCT made the final decision to approve or reject an application. Others were unsure:
Well this is what I can’t understand, you know when they applied for this funding, I mean I don’t know who they apply to and that.
Interview, PCT2, P10
With the exception of the retired nurse (P6), patients were unaware of the threshold criteria by which eligibility for CTS surgery was determined. P6 had been informed of the threshold criteria at the same time as receiving the news about the prior approval process from their GP. The patient had then conducted their own research by browsing through the PCT’s low-priority procedure policy list on the internet. One other patient’s account (P10) suggested that their GP may have explained the threshold criteria, but the patient’s report made it difficult to gauge the details of what had been discussed. P10 was not aware of the specific threshold criteria, but gave the sense that they knew referral to secondary care was dependent on fulfilling requirements that extended beyond the GP’s control (as the patient understood it, a 3-month waiting period within primary care).
While most of the PCT2 patients were unfamiliar with threshold criteria, some patients had formulated their own theories about factors that may promote a favourable decision from the prior approval panel. P7, unlike other patients, had been asked to submit a letter to the PCT to accompany the application submitted by her GP. Their description of the letter produced gave insight into the types of factors they felt they should emphasise:
Why do you think they’ve approved your case?
I must have written a good letter.
So what do you think has been the selling point, if you like?
Probably that I put if I don’t get myself sorted my mum will have to go into care. I didn’t actually put it like that but, you know, I said she’s in her own home and I’ve got to look after her with my sister, and if I haven’t got the use of two good arms then, you know, what’s going to happen? [. . .] So I did sort of play on my mother more so than me, I think, to be quite honest.
Interview, PCT2
P10 explained how they had wondered if a patient’s working status influenced the likelihood of being granted approval. P17 did not directly discuss the decision-making process, but assumed that the severity of their case (due to their comorbidities) had been the key to their approval.
Rationalisation of the prior approval process
All patients explained the prior approval process as a money-saving concept. Patients assumed that CTS had been targeted because of its presumed low-priority status. One patient conveyed their understanding and acceptance of this justification:
Because, let’s be fair, it’s not life-threatening is it? It will just enhance my quality of life basically, won’t it? Because I’m old, or getting older. So um I can see why they – why, you know, why it wouldn’t be prioritised.
Interview, PCT2, P7
P6 and P10, both of whom had felt the impact of the prior approval process on their care, questioned how decisions about priority status were made:
I don’t know really, I suppose they’re trying to prevent operations I mean that aren’t necessary, but then again you’ve got to look at quality of life as well as quantity of life.
Interview, PCT2, P6
Patients receiving care in PCT1
One of eleven patients from PCT1 was aware that access to CTS surgery was rationed. This patient had been informed of PCT1’s threshold criteria by their GP, within the initial consultation of their pathway:
I go to see the doctor and he explained, ‘Well what we’ll do is we’ll try a number of things in order, because this is one of the operations that the NHS don’t want to just do unless you’ve gone through every other route to make sure that it’s severe before they do it’. And in fact he told me everything. He said, ‘We’ll try the splint, that’s the first course of action. If it doesn’t work then we’ll try cortisone’.
Interview, PCT1, P2
P2 saw the application of thresholds as a change from how CTS surgery was provided in the past, thus alluding to disinvestment:
Oh well, no, I’m just assuming, when he said to me, ‘This is an operation which we don’t just do’. You know, I got the impression that maybe years ago if someone was suffering from it they’d say, ‘Oh well let’s get your carpal tunnel sorted out,’ that’s the impression I got – I might be wrong.
Interview, PCT1, P2
Similarly to patients from PCT2, P2 had assumed the threshold system was in place because of current NHS financial constraints, and that CTS had been targeted because of its low-priority status:
So I think it’s quite good, because obviously everything costs money. Um if it doesn’t really – you know, if I wasn’t a motorcyclist I would probably not have noticed it.
Interview, PCT1, P2
Patients’ perceived acceptability of rationing/disinvestment approaches
Patients’ perspectives on the threshold policies and prior approval processes were explored across all interviews. As most patients had been completely unaware of any rationing practices influencing their care, this concept often had to be introduced by the interviewer in hypothetical terms. As a lead-in, patients were probed to discuss their awareness of the financial state of the NHS, and any previous experiences that may have provoked them to consider costs in the context of health care. Patients were very much aware of constraints, but quick to add that these had never come through to their care. Generally, the topic of financial constraint provoked patients to express their personal satisfaction and sense of privilege in having a health service that was free at the point of care. Financial constraints were often associated with wider austerity measures occurring at the time:
I mean, obviously they are doing various cutbacks. I mean interestingly enough, I only went to the surgery to have the stitches removed yesterday, and I picked up a leaflet on the counter that they were giving to everybody, which said they were encouraging people to do telephone appointments now.
Interview, PCT1, P2
A few patients touched on the idea that the health system, by its own nature, would always be under financial pressure:
They need [to have money], don’t they? And people are living longer so obviously they’re needing more.
Interview, PCT2, P7
Once the topic of finance had been raised, patients were asked for their views on a threshold-based system in place for accessing elective procedures. The CTS case study was used to gauge patients’ views on such a system, by presenting conservative therapy as a prerequisite for accessing surgery. This was presented as a hypothetical scenario for patients who had no awareness of rationing processes in their region. Most patients had been informed by their own clinicians that conservative treatments generally had a lower success rate than surgery, but those not aware of this were provided with this information within the interview. Patients were also asked to assume that CTS surgery was an expensive procedure relative to other approaches to managing CTS.
Patients’ views on the prior approval process were also explored. Those unfamiliar with the prior approval system were asked to comment on who they felt was best placed to make decisions about patients’ eligibility to access elective procedures such as CTS. Some patients found it difficult to engage with this question and required additional probing; in these instances, they were given a list of examples (i.e. GP, NHS managers, consultants, the patients) and asked to provide reasons for their responses.
Acceptability of a threshold-based system
Patients’ views on threshold policies were scrutinised for patterns on the basis of region, history of conservative therapy use and knowledge of the prior approval process. The themes emerging from patients’ accounts did not appear to change according to these factors. Divergent or negative cases still emerged, but did not appear to be associated with any particular characteristics. Two patients’ accounts were considered separately, however, as their interviews were heavily focused on the prior approval process. This made it difficult to disentangle views on threshold policies from views on the prior approval system.
Generally, patients interpreted a threshold system to be a fair and sensible approach to controlling access to CTS surgery. A number of benefits to such a system were identified by patients themselves.
Financial benefits
Some patients showed support for a threshold-based system on account of the financial benefits this entailed:
I think it’s a good idea. One: because I’d originally looked into having it done privately and the cost I was told was going to be over a thousand pounds to get it done privately, so when you equate that to having to have a hospital theatre and everything, even in a normal hospital, it is costing the National Health Service a lot of money to do it.
Interview, PCT1, P16
Many of these patients had undergone conservative treatment, but to no avail. Their personal poor outcomes bore no influence over their assertion that conservative therapy should be considered prior to surgery:
I personally – if I were going through this again, I would go through exactly the same steps.
Interview, PCT1, P8
A number of patients conveyed a wider concern about the public’s attitude to the NHS, expressing a desire for the public to take more responsibility over their own health resource use. Some condemned the behaviour of other members of the public for their wasteful use of NHS resources. These patients supported the idea of requiring service users to demonstrate that they had put effort in to improving their condition before granting access to expensive surgery:
There are certain people who assume that because they are nearly 70 and their friends have had something, then they can go on the list for it. Sounds awful saying it. [. . .]. So, by doing a proper recognised procedure that you’ve got to go through, one step before you go through the next, it’s proving that: one, you’re really needing the last step and, two, that you’re prepared to make some sort of effort as well.
Interview, PCT1, P16
Treatment preferences and priority status
The potential to avoid surgery was a commonly cited benefit of a threshold system. Patients across the board had a strong preference for non-invasive procedures for CTS management, despite the lower success rate than surgery. The perceived ‘low priority’ of CTS, as highlighted throughout this chapter, went hand in hand with this. Most patients knew or suspected they had CTS from the early stages of experiencing symptoms, but rarely consulted immediately. Symptoms were presented as ‘non-emergency’ and manageable in these early stages, exerting a trivial impact on patients’ lives. Patients who felt the prior approval process had substantially affected their care pathways were exceptions to this. These patients presented their symptoms, and the impact on their lives, in a different light. These cases will be considered separately. In the grand scheme of treatable conditions, patients across the board placed CTS as low priority:
I think serious and curable illnesses in my opinion should take the first, take the first . . . certainly with children, and then, going down from there, I would say that then we need to look at the lesser illnesses in the working population [. . .], and lastly I would say that the minor things like I’ve got really, like I’ve had [CTS]. I would put that as definitely on the backburner.
Interview, PCT1, P8
None of the patients interviewed expressed any concern that trying conservative therapy would delay surgery:
Surely the time frame is a fairly long time frame? So if you’ve left it too long you’ve left it too long, and another 6 months is not going to make that much difference, and surely the injection you should be able to know fairly soon.
Interview, PCT1, P16
The fact that the option of surgery would still be available once other avenues had been exhausted contributed to patients’ acceptance of a threshold-based system. In this sense, patients felt they had nothing to lose:
Yeah well if it will work, yeah, I don’t mind trying it and seeing if it will. And if it doesn’t work, it’s nothing – it doesn’t matter really then, does it? Nothing ventured.
Interview, PCT2, P7
Even in the context of CTS, some patients acknowledged the subjective nature of assessing severity, challenging their own assessment of CTS being a low priority condition. Some acknowledged that symptoms could be particularly severe or debilitating, and/or explained how the magnitude of severity would depend on the individual’s life context. This called for a degree of flexibility in applying a threshold policy:
There are people whose jobs depends on their hands. So I think you have to take each case individually – which makes it very difficult.
Interview, PCT1, P16
Concerns surrounding thresholds
Three patients expressed concern about a threshold-based system. Two of these passively accepted the idea of trying conservative therapy but appeared to have reservations. The first patient’s acceptance was based on his assessment that he would comply with whatever the clinician recommended. On encouragement to voice his own views, the patient expressed doubt about whether or not an injection with lower success rate would be financially preferable to a ‘straight to surgery’ approach:
I think it’s go straight on, otherwise you’re going to have – I can’t see how you’re saving money if you only get small per cent likelihood of benefit with injection. You might just as well do the operation, because it can’t be that expensive.
Interview, PCT1, P14
The second patient felt a threshold system would simply delay the inevitable:
If it’s going to get worse, I would say go for it, you know.
Interview, PCT1, P1
P6 and P10: prior approval patients’ view on thresholds
P6 and P10 both agreed that meeting thresholds for CTS surgery would be acceptable, but emphasised the need for clinicians to maintain control over a patient’s treatment pathway:
Do you think there should be criteria?
I suppose, to a certain extent, yes and no, because I think if you’ve seen a registrar who says you need to have that surgery done then yes, I think there should be, that they should accept what somebody has said. I mean I’ve had a consultant and a registrar both saying I needed surgery.
Interview, PCT2
P6 and P10s acceptance of thresholds was also somewhat passive. Both agreed that thresholds were appropriate for non-severe cases of CTS. However, these patients’ accounts were distinct from others, in that they placed greater emphasis on the potentially debilitating effects of CTS in the absence of surgery:
It seems to me with all the cutbacks they go for what is the easiest option, you know which, if I only had a little bit of tingling yeah then I would argue that I am the easiest option but when I lost, basically lost the use of my hand I don’t think that is an option.
Interview, PCT2, P10
Acceptability of a prior approval system
Perspectives on a prior approval system were based on hypothetical questions for most patient interviews. Only four patients from PCT2 had experienced the prior approval process and, of these, only two perceived an impact on their care (P6 and P10). Some of the viewpoints surrounding the prior approval process are specific to the patients who were able to draw from personal experiences.
Timing of information provision and delay
The notable difference between P6/P10 and other patients who were aware of the prior approval process was the stage at which they had been informed about the system. P6 and P10 first heard about the process after they had been advised that they would (1) need surgery and (2) be put on the waiting list. The introduction of this unexpected application-based system suggested there was a possibility that they might not be granted the treatments they had already been told they needed:
There’s a surgeon who’s done 5 or 7 years’ training and has decided I am in a position that I need an operation.
Interview, PCT2, P10
Despite having their applications accepted, P6 and P10 sensed that their care had been compromised by issues of delay. Both patients felt issues of bureaucracy and multitiered decision-making had been responsible for their perceived delay in receiving surgery:
What I’m saying is the time factor is money, they could be doing something else or whatever. I feel that there’s a lot of (pause) ones that sort of up high, making decisions, there’s too many tiers.
Interview, PCT2, P6
Loss of clinician control
The single most contentious aspect of the prior approval system, as perceived by all patients aware of the system, was the removal of control from clinicians overseeing their care. Patients questioned why the expert opinions of consultants were not simply accepted:
Well I can’t see why if you’ve gone and seen a specialist and he says that you need an operation why they have to doubt his opinion or his diagnosis?
Interview, PCT2, P10
Patients with no awareness or experiences of a prior approval process were asked to comment on who they felt should have the most influence over determining eligibility for CTS surgery. Most patients felt consultants should have this power, given their expertise and specialist knowledge:
Well I would say the specialist really wouldn’t it? Not the GP, definitely not the GP, no. Even though I’m not very happy with mine, I mean there are other GPs, no I definitely wouldn’t say the GP, no because they don’t know enough about it do they?
Interview, PCT1, P13
A few patients commented that their GPs would be in the most ideal position to determine need for treatment, given their knowledge of the patient’s medical history and life context:
Well I would have said the GPs, because they know that patient more than what um a consultant does. A consultant can only look at what the GP has sent you, a few words, whereas the GP, he gets to know.
Interview, PCT1, P4
Generally, the prospect that anybody other than clinicians overseeing a patient’s care could influence treatment decisions was alien to patients who had not experienced the prior approval system.
Discussion
Summary of findings
Based on clinicians’ accounts, the introduction of threshold policies posed little intrusion or disruption to their standard practices. Clinicians were confident of their ability to ensure that patients’ symptoms fitted with the criteria if necessary. Patients in true need of surgery were thought to be able to receive this, as long as clinicians themselves assessed eligibility against threshold criteria. In contrast, clinicians operating under a prior approval system expressed a loss of control over decision-making. The introduction of an intermediary service and prior approval system was thought to be detrimental to patient outcomes. Clinicians suggested these approaches would eventually impose greater long-term financial penalties on the NHS. Clinicians across both regions expressed little hesitation in being open with patients about rationing/disinvestment processes. Their personal objections to disinvestment, and ability to dissociate themselves from decision-making processes, may have facilitated this.
Patients were very often unaware of rationing/disinvestment measures that had the potential to influence their care. They tended to explain their own care pathways in terms of clinical justification, clinicians’ recommendations and their own treatment preferences. Patients were more likely to be aware of rationing or disinvestment initiatives if this took the form of a prior approval process. Even within this subgroup, knowledge of how decisions were made was limited. Patients showed support for a threshold-based approach to controlling access to CTS, seeing this as a sensible and fair system. Patients emphasised the financial benefit of potentially avoiding surgery by exploring conservative options first, and showed little concern for the potential delay this would impose on receiving surgery. However, this acceptance was connected with the perceived low-priority status of CTS, and clear patient preferences for non-invasive treatment. Patients strongly disagreed with a prior approval process, largely because of the loss of clinician control this implied. Similarly to clinicians’ views, there was also some expressed concern that the prior approval system compromised patients’ potential to benefit from surgery. The only patients to express dissatisfaction with their care had been informed of the prior approval process midway through their care pathways, despite having already been informed that they were in need of surgery.
Our findings suggest that the timing of introducing disinvestment processes may influence patient satisfaction, but this theory will need further exploration. Our findings also raise ethical issues of when and how patients should be informed about disinvestment decisions. Previous commentary on these issues suggests that patients are likely to view clinicians as their advocates. Based on this, care informed by CBA policies – when under the control of clinicians – could still be perceived by patients as being in their best interest. Patients may be more likely to be aware of health resource concerns/rationing if access criteria are seen to be enforced by non-clinicians (e.g. managers, commissioners). 184 Our findings also highlight the importance of effective diffusion of commissioning policies to front-line clinicians. This is particularly important in the case of disinvestment decisions, as policies represent a change in what clinicians might be accustomed to. This distinguishes research in this area from wider rationing issues, where the discussion may be focused on treatments that never were routinely available.
The findings from this case study have implications for refining the disinvestment process proposed in the wider project. The process could benefit from the addition of a step that exclusively considers how disinvestment proposals, once identified and fine tuned, can be disseminated to patient groups and communicated to those receiving care. This step could involve open discussion between commissioners and clinicians involved in the patient pathways under consideration. Key points for discussion could include how, when and by whom disinvestment plans are disclosed to patients.
Strengths and limitations
Our findings provide a detailed insight into patients’ awareness of implemented disinvestment initiatives that had the potential to have substantial influence over their care. We present findings on patient’s views on the acceptability of rationing approaches in a particular clinical context, focusing on people who were able to draw on actual experiences. The literature on qualitative accounts of disinvestment is sparse, especially in relation to the patient/public perspective. A key strength of this research is its detailed exploration of different policies as experienced by clinicians and patients, and the potential it lends to formulating more focused future research questions.
There were a number of limitations to this study, most of which centre around the case-specific nature of each patient’s interview. The individual pathways to surgery challenged our ability to gauge when data saturation had been achieved. The research could have benefited from additional interviews, potentially from other sites that have implemented threshold or prior approval approaches to controlling the volume of CTS surgery. We have conducted exploratory work that provides a good foundation for further research. It is likely that the extent to which patients noticed any policy-derived impact on their care was partially dependent on the specific nature of their case. For example, some of the prior approval patients may not have noticed any significant impact on their care if they were cases that clearly exceeded eligibility criteria. This may have affected how quickly/easily their application was processed by the prior approval panel. We did not observe the prior approval decision-making process, and are thus unable to comment on whether certain patients are moved through the system quicker than others. A notable limitation of this work was the absence of patients who had been denied access to surgery. Sampling from secondary care clinics alone immediately excluded patients at earlier stages of the pathway. This group may have had greater experience of being denied access to surgery on the basis of disinvestment policies. This is especially true for PCT2, where the prior approval process and intermediary treatment centres could have significantly filtered the population of patients we had access to. This raises questions about the transferability of findings to the wider CTS population.
The findings from patient interviews may be limited by recall bias and patients’ interpretations of what had occurred during their care pathways. This was not so much of a problem in terms of exploring patient perspectives on rationing approaches, but had implications for our reports of shared decision-making and disclosure of rationing processes within consultations. Further research in this area may overcome these issues by observing/recording actual consultations within which issues of disinvestment may be discussed. Furthermore, the study focused on one procedure (CTS) in one specialty (orthopaedics). The patient informants perceived the procedure to be low priority in general, particularly relative to life-saving treatments. These views may have also been influenced by informants’ perceptions of symptoms having little to no impact on their quality of life. Further research is needed to explore how patient perspectives vary in relation to disinvestment from a range of health interventions, with a focus on how attitudes vary according to the nature of the intervention (e.g. life-extending treatments versus interventions that improve quality of life). Similar work has already been undertaken in relation to rationing interventions for chronic obesity versus breast cancer. 181 Further work could also investigate how patient attitudes vary within a single procedure according to symptom severity. The present study selected CTS in line with the wider project’s aims. This work was successful in highlighting avenues for potential further research, while future studies will be in a better position to purposefully select clinical specialities best suited for exploring these new lines of enquiry. It is important to acknowledge that we interviewed secondary care clinicians who potentially had a conflict of interest in maintaining provision of health services they provide. The trusts these clinicians were operating within may have experienced a significant loss of revenue. The clinicians’ accounts presented need to be interpreted with these factors in mind.
Findings in relation to the wider literature
Like other studies,181 we found that patients were generally aware of constrained resources in the context of health care, but often failed to relate this to their own care. Drawing conclusions about awareness of disinvestment from their own care is problematic: we cannot be certain if individual patients’ care was actually shaped by disinvestment policies (and, if so, to what extent). This limitation is particularly evident from PCT1 clinicians’ portrayal of thresholds, which were deemed to have little effect on their standard practices. We can be more confident that disinvestment had an impact on some prior approval patients’ pathways.
Prior research in this area suggests an overall desire for openness about rationing with regard to an individual’s care, from both public185,186 and patient181 points of view. However, reasons underlying this desire have been centred around the prospect of not being aware of the type of services that could be available, thus missing out on opportunities to access this care via alternative means (e.g. appeals, private care, etc.). Whether or not these issues are transferable to a disinvestment context will depend on the nature of the disinvestment in question, and patients’ pre-existing awareness of the services that would have been available prior to disinvestment. Previous research suggests clinicians have a desire to be open with patients about rationing, but find this challenging in practice. 181,187 Practising clinicians’ views on being explicit about financial issues have not been investigated within the unique context of disinvestment. We found that clinicians who sensed a loss of control in making treatment decisions reported having no qualms in being open with patients about the reasons for service restriction. Clinicians felt able to divert responsibility to the PCT whenever explaining rationing processes. Views on such matters were more difficult to elicit from clinicians in PCT1, as informants felt they had retained control over treatment decisions, and so any restriction of treatment was effectively their clinical decision.
Future research
This exploratory research suggests a number of further research questions. There is a clear need to investigate how disinvestment decisions are communicated to patients, and patients’ subsequent acceptance of such approaches. This research would benefit from observations of actual consultations, and paired analyses of clinical and patient perspective for specific cases. This emerging area of research would also benefit from application to a range of conditions with different treatment pathways, comparing how responses vary by the nature of the condition and the treatment options available. Although our sample was limited, we found evidence to suggest that clinicians are not always aware of disinvestment decisions made by commissioners. Our findings thus support further research into the best ways to communicate disinvestment decisions to practising clinicians, especially GPs, who often create or maintain patient expectations of what their care pathway is likely to entail. Further research also needs to consider the appropriateness of prior approval systems, and particularly whether or not a check-list operated by health-care managers can safely replace specialist clinical opinion. The implications of such systems on clinical outcomes and patients’ quality of life (as well as cost-effectiveness) need to be urgently investigated, as indicated by the patients and clinicians in this study.
Chapter 9 Discussion
The tight financial constraints on the NHS are likely to keep disinvestment high on the agenda of CCGs and NHS England as they establish their roles within the reconfigured health service. Austerity has focused attention on the challenges faced by clinicians and policy-makers in ensuring optimal use of health-care practices and technologies which may deliver little or no health gain for their cost in some of the clinical subgroups in which they are currently used. Key challenges include the lack of methods to identify technologies with uncertain cost-effectiveness, lack of evidence on the efficiency of many existing health technologies, and political, clinical and social challenges to changing established practice. 15 Unlike the rigorous process for identifying and evaluating new health technologies established by NICE and emulated by other countries, international efforts to establish HTR programmes have been much more haphazard. 14
The aims of this project were to (1) establish whether or not geographical variations in procedure rates are a reliable marker of clinical uncertainty about the value of procedures which might be used to identify candidates for HTR; (2) explore obstacles and solutions to local commissioners in achieving evidence-based disinvestment; (3) work with two PCT commissioning groups to use local benchmarking and rapid technology assessment to identify procedures that might be locally overutilised and consider options to regulate local procedure rates; and (4) explore patient and surgeon perspectives on regulating access to surgery. Over the course of the project our objectives have evolved to some extent. In part, this has been because of the fallout from the large-scale reconfiguration of the NHS and, in particular, PCTs that took place during our project.
Statement of principal findings
Analysis of routinely collected data on 154 commonly used day case and inpatient procedures revealed widespread geographical variation that could not be explained by proxies of clinical need. For six procedures, rates in PCTs at the upper and lower deciles differed by more than 10-fold, which approaches the extent of variation in tonsillectomy rates observed by Glover in the 1930s. 18 Variation was most pronounced in procedures where utilisation was increasing or decreasing most rapidly. This suggests that national policy-makers could use geographical variation as a starting point to identify procedures where HTR or new RCTs might be most needed to inform and standardise practice. However, appropriate caution is needed to distinguish real variations in practice from illusory variations caused by the vagaries of coding medical practice.
At the local level, the qualitative research observing commissioning group meetings demonstrated that their agendas were dominated by new requests for funding and implementation of NICE mandates. Disinvestment decisions were rarely discussed, despite a constant undertone of concern about the affordability of newly commissioned activity. Interviews with commissioners, providers and lay representatives revealed a lack of clarity about the meaning of disinvestment and whether it exclusively entailed complete removal of a service, reduction of activity or replacement with a more efficient service.
Interviewees strongly conveyed limited capacity and lack of central support as key obstacles to engaging in disinvestment. We found little evidence of any local use of tools or frameworks to support disinvestment decision-making. Interviewees expressed difficulty with identifying relevant, high-impact opportunities for resource release. Consequently, our observations suggested that decision-makers tended to engage in fire-fighting behaviour in relation to requests for new resource allocation, rather than looking strategically for disinvestment opportunities or considering how such decisions might be made. Collaboration between commissioners and hospital providers were seen as a key barrier to successful implementation of disinvestment decisions. These stemmed from a perception of conflicting agendas, problems with the process of clinician engagement, and the absence of a shared language when discussing disinvestment. These factors threatened collaboration and reinforced providers’ sense of exclusion from resource allocation decisions.
In the research on benchmarking with two local PCT commissioning groups, we were able to identify a large number of procedures where the local rate of procedure use was much higher than the national average after adjustment for proxies of clinical need. For some procedures, local rates were more than twice the national average. In many cases the high local use procedures were not ones that are commonly cited on ‘low value’ procedure lists. However, because PCTs were benchmarked against a national norm but not to any gold standard procedure rate, interpretation is not straightforward. Furthermore, benchmarking procedure codes does not necessarily lead to straightforward HTR questions. For example, a procedure such as adenoidectomy may be used in combination with other procedures to treat otitis media, sleep apnoea or chronic tonsillitis, resulting in numerous potential HTR topics. Our initial benchmarking work also revealed some coding anomalies such as double counting of procedures where the patient was admitted via an NHS hospital, but transferred to an independent sector treatment centre for surgery. This highlights the importance of commissioners collaborating with clinicians from the outset to identify procedures with genuinely high local utilisation and explore the potential causes of this.
The two case study technology appraisals illustrated some of the challenges in using information on practice variations to inform disinvestment. The first case study in PCT1 concerned CTR, where rates of surgery were persistently high and localised predominantly around one hospital trust. The small body of trial evidence (six RCTs involving approximately 600 patients) was vulnerable to bias and compared surgery with a wide variety of non-surgical interventions. Nevertheless, the bulk of this evidence demonstrated that surgery was more clinically effective and more cost-effective than non-surgical care for patients with mild to moderate CTS in the short term. However, the RCTs provide very little information to help surgeons or commissioners identify the patient subgroups where the costs and risks of surgery counterbalance the health benefits. This leaves PCT1 with a dilemma if it wants to act: it could tighten up the existing CBA thresholds required for surgery, perhaps including a Delphi process to reach consensus on optimal thresholds, or explore alternative measures such as prior authorisation or shared decision-making tools. The fundamental lack of evidence on the optimal thresholds for surgery will hamper whichever approach it adopts.
The second case study focused on capsulotomy for PCO following cataract surgery, where variation in practice was probably more illusory than real. The hospital providing this service to PCT2 coded it as a day case procedure, whereas in many other PCTs it is performed as an outpatient procedure. Therefore, the uncertainty may be as much about the appropriate setting of care rather than the appropriateness threshold for intervention. A shift of activity and coding from a day case to outpatient procedure could save commissioners money without impairing patient care. The technology appraisal for this case study found no trial evidence or national guidelines on the appropriate clinical thresholds for performing capsulotomy. Therefore, while the immediate cause of high variation might be in care setting and coding, it is possible that some variation in capsulotomy procedure rates might be due to clinical uncertainty about the appropriate use of the procedure.
Our exploration of patient and surgeon perspectives on disinvestment and regulation of access to CTR surgery revealed that their views were influenced by the type of regulation measures used. Unless this took the form of an overt prior approval process, patients were very often unaware of rationing measures that had the potential to influence their care. Patients showed support for a threshold-based approach to controlling access to surgery, seeing this as a sensible, fair and efficient system of potentially avoiding surgery by exploring conservative options first. Clinicians reported that the introduction of threshold policies posed little intrusion or disruption to their standard practices, as they were confident of their ability to ensure symptoms fitted the criteria. In contrast, clinicians operating under a prior approval system expressed a loss of control over decision-making, and patients also strongly disagreed with the loss of clinician control it implied. The introduction of an intermediary service and prior approval system were thought to be detrimental to patient outcomes, and clinicians also suggested these approaches would eventually impose greater long-term financial penalty on the NHS because of the likelihood that surgery would eventually be undertaken following unsuccessful conservative treatments.
Strengths and limitations of the project
The key strengths of our approach to quantifying geographical variation in procedure rates include the inclusion of a broad sample of the most frequently used procedures in the NHS. These procedures will be of clinical importance to the thousands of patients who receive them each year and of economic importance to local commissioners. The approach does not rely on a preconceived list of procedures thought likely to have high variation and therefore can identify unexpected candidates for HTR. The method can be easily repeated at regular intervals to scan the horizon for procedures with growing clinical uncertainty as they emerge. The large nationwide sample allows precise estimation of inter-PCT variation. This novel measure of variation is more robust than some conventional crude measures of variation such as the extremal quotient or CV. 21,188 We adjusted for a wide range of variables likely to be associated with clinical need, including the demographic composition of PCTs and the prevalence of chronic disease and markers of unhealthy lifestyles. The remaining, unexplained, geographical variation in procedure rates is therefore unlikely to be solely due to random fluctuations on practice or variation in clinical need. However, bias may still occur because of unmeasured aspects of clinical need or if the accuracy of demographic and morbidity measures varies by PCT.
The benchmarking approach that we used with the two PCT commissioning groups is a transparent and readily available method for local commissioners to identify procedures for more in-depth analysis. This analysis can open up a debate with health-care providers and patients about appropriate procedure use. Combining benchmarking with evidence reviews should centre the debate on the available evidence and minimise the risk of mutual suspicions that the process is purely about cost-cutting (on the part of commissioners) or income preservation (on the part of hospital providers).
There are also a number of limitations to our approach. Our analysis of geographical variation relies on existing coding frameworks and complete and accurate coding of procedures in data routinely collected by the NHS. The three-character OPCS codes used in our analysis may mask variation in distinct procedures that are grouped together under the same primary procedure code (e.g. open and endoscopic CTR). The coding framework may also create spurious variation if there is more than one plausible way to code the same procedure. We identified several potential sources of illusory variation in procedure rates during the course of this project. These include double counting of NHS procedures contracted out to independent treatment centres, variable coding of patient residence for some sensitive procedures, inaccurate coding of procedure type and inconsistency in coding the setting of care. Some of these problems may increase if the ‘any qualified provider’ provision in the HSCA leads to a broader mix of NHS and independent sector hospitals offering therapeutic procedures to NHS patients. 189
Despite the large numbers of procedures performed nationally, the number performed within any individual PCT or CCG is actually relatively small. For example, PCT1 commissioned approximately 1000 CTR procedures per year, about 200 procedures more than the national average. Therefore the costs of benchmarking and the difficulties of intervening to regulate procedure rates may be difficult to justify at the level of local commissioners. The NHS may need to harness economies of scale by encouraging nationwide initiatives, such as shared decision-making tools or national guidance on appropriate criteria for surgery, rather than rely on initiatives by individual local commissioners. There is also a danger that by focusing on one setting (i.e. inpatient care) and on what is measured well (i.e. therapeutic procedures) commissioners will miss the bigger picture and possibly larger opportunities for disinvestment in areas such as follow-up clinic visits, diagnostic tests or medications. The evidence reviews that we conducted are an important part of understanding the potential reasons for high local use of a given procedure and opening up a balanced debate on appropriate procedure use. However, even these streamlined reviews are time-consuming and, as noted in our qualitative work, lack of resources is highlighted by commissioners as an important barrier to undertaking disinvestment initiatives. It remains to be seen whether or not the more centralised CSUs created by the NHS reconfiguration will be better placed and resourced to do this. 190 In addition, the evidence identified, as demonstrated in our cases studies, rarely provides a simple case for disinvestment or set of criteria to regulate procedure rates. Therefore, while the methods that we applied move the debate forwards by identifying procedures where local use is unexplainably high, they do not resolve the issue of how to implement and monitor and disinvestment decisions.
The observations and interviews employed in our qualitative work provided rich accounts of the complex theoretical and practical issues of disinvestment from commissioner and provider perspectives. However, as disinvestment is a controversial subject, the public accounts provided by the public figures interviewed might have reflected socially desirable responses. We also present preliminary findings on the public’s views on the acceptability of rationing approaches in a particular clinical context, focusing on patients who are able to draw on actual experiences. This part of the research could have benefited from additional interviews with patients denied access to surgery and from other sites that have implemented threshold or prior approval approaches to control the volume of CTS surgery.
One recurring limitation encountered in all aspects of our research is that it was conducted at a time when the NHS was entering a state of flux and reconfiguration brought about by the HSCA. The shift in boundaries from PCTs to CCGs is unlikely to substantively alter the geographical variation in procedure rates observed in our study. However, the uncertainty and staff turnover that accompanied it undoubtedly distracted attention away from disinvestment initiatives and might have indirectly influenced informants’ accounts in interviews.
Comparison with other studies
Since Glover’s groundbreaking work in the 1930s, there has been a long line of research demonstrating large and unexplained geographical variations in procedure rates in the UK. 21,191–197 This body of literature demonstrates that geographical variation is a persistent, but not intractable, problem. For example, the more than 20-fold variations in tonsillectomy rates reported by Glover have reduced over the last century as acceptable indications for the operation have become more defined, but sevenfold variations at the extremes still exist and guidelines are still based on imperfect evidence. 191 Our work demonstrates that many of the usual suspects no longer top the list of high-variation procedures, and underlines the importance of monitoring clinical practice variations regularly to identify new procedures where evolving evidence is creating clinical uncertainty about appropriate use. Our broad approach allows us to describe the spectrum of geographical variations in procedure rates from procedures (e.g. excision of kidney) where the interdecile UR was close to one up to procedures (e.g. excision of cervix uteri) where the ratio exceeded five. By tracking geographical variation over time we were also able to identify procedures (e.g. combined operations on varicose veins) where discord in procedure rates has increased recently. To our knowledge, our study is the first to systematically explore the procedure characteristics associated with geographical variation and to demonstrate the link between high variation and rapidly diffusing and declining health technologies.
Our benchmarking work adds to growing international interest in the potential of benchmarking to improve health-care efficiency. 39,46 Much recent work in the UK has focused on benchmarking hospitals on outcomes such as mortality or surrogate outcomes such as reoperation rates in an attempt to identify low-quality care. 198,199 This outcomes benchmarking is fundamentally different from the process measure benchmarking we undertook on procedure rates. The methods of benchmarking hospital mortality rates are complex and controversial,198,200 but the goal of identifying outlier hospitals with high mortality rates caused by low-quality care is not contested. In this context, outcomes benchmarking is conceived as a ‘dial’ measuring actual performance against an achievable target. In contrast, benchmarking procedure rates, where low procedure rates may be as concerning as high procedure rates, is more likely to serve as a ‘tin opener’ indicating potential areas of overutilisation leading to more in-depth analysis potentially resulting in disinvestment. 85 Benchmarking procedure rates has been used by local NHS commissioners previously to identify opportunities for savings. However, approaches, such as the London PCTs ‘Save to Invest’ programme,201 have typically started with a list of low-value procedures rather than the broader approaches that we have used.
Qualitative studies that have explored issues in rationing and priority setting have also found that disinvestment tends to be avoided by local decision-making groups160,161 and that commissioners struggle to routinely apply tools or frameworks to support disinvestment decision-making. 163 The difficulty with identifying relevant, high-impact opportunities for resource release reported in our interviews is consistent with other qualitative work identifying the barriers to disinvestment. 166 While the benchmarking and evidence review approach we have adopted could help commissioners identify topics for potential disinvestment, there remain wider issues about how best to formulate these topics into disinvestment initiatives and implement them within existing and developing commissioning structures. It is also important to consider the resources that would be needed to undertake disinvestment and how to deal with the lack of evidence that can directly support disinvestment decisions and monitor their outcomes.
Implications for practice
The implications for practice are split between implications for local commissioners and implications for national policy-makers and research funders. The implications are listed in descending order of importance.
Implications for local commissioners
Increasing the focus on opportunities for disinvestment
One of the most consistent findings from our observations of commissioning advisory group meetings and interviews with group members was the imbalance between the time and resources spent considering investment initiatives and time spent on exploring disinvestment possibilities. In part, this reflects the steady stream of national technology appraisal mandates and clinical guidelines that demand commissioners’ attention and the central control exercised in the NHS. It may also reflect some scepticism among commissioners about the potential to achieve big money-saving changes by introducing CBA policies for individual hospital procedures. However, it was clear that many commissioners felt they spent most of their time fire-fighting and unable to be strategic about reviewing existing care and achieving disinvestment. In fact there were many different interpretations of the term ‘disinvestment’ and many commissioners reported limited experience of disinvestment. This situation will not be simple to rectify, but a better balance is required if new investments in health care are to continue without real increases in NHS expenditure. Part of the solution may be better training for commissioners on what disinvestment is, case studies of successful disinvestment and tools to facilitate it. However, it also requires local commissioning groups to consider disinvestment strategically. If commissioning advisory group meetings are swamped with investment requests, it may require ring-fenced time with the meeting time split between considering requests for new funding and reviewing existing care pathways to increase efficiency.
Transparent disinvestment processes based on the evidence with early stakeholder engagement
The reconfiguration of NHS commissioning to primary care-led CCGs supported by more centralised support units provides an opportunity to develop a more collaborative, transparent and democratic process for considering disinvestment at the local level. Tensions are inevitable in a system which divides commissioners and providers, giving them divergent incentives and inviting power struggles based on limited evidence about cost-effective care. However, tensions can be minimised by early and meaningful engagement of all stakeholders, including patients, and hospital clinicians and managers, to try to create open communication channels and shared meanings and mission. Adopting transparent mechanisms for identifying potential disinvestment topics (e.g. benchmarking or PBMA) and focusing the debate on the available evidence should foster collaboration on a joint goal to provide the most appropriate care to the local population. However, this process requires resources and central political and NHS support for local decisions that result in disinvestment. Development of these approaches also requires stability in the commissioning structures, organisations and staff.
Focusing on the setting of care
In many ways the example of laser capsulotomy for PCO demonstrated the limitations of benchmarking with routine hospital data. The apparent differences in day case procedure rates were probably due to vagaries in outpatient and day case coding between hospitals and there was no high-quality evidence to guide commissioners on appropriate procedure rates or clinical criteria. However, it also demonstrated the possibility of using benchmarking as a ‘tin opener’ to uncover differences in care pathways and settings between different areas of England. The revelation can have important financial repercussions. More than 11,000 capsulotomy procedures were still recorded as day cases in 2012/13; a switch to the outpatient setting would reduce hospital reimbursements by more than £2M. This emphasises the need for commissioners to have access to data sets that accurately reflect health care provided across the secondary, primary and community care settings.
Focusing on pathways of care
The dilemma for PCT1 faced with persistently high rates of CTR underscores the importance of commissioners focusing broadly on the entire pathway of CTS care, rather than narrowly on what is easily measured (i.e. surgery). Benchmarking revealed the anomaly of high procedure rates, but the evidence was limited and did not support further tightening of existing access criteria. By focusing on the entire pathway of care, the local commissioners, working with patients and colleagues in primary and secondary care, might consider whether shared decision-making, referral management systems or contractual levers are the most effective way of making the care pathway more efficient. Local commissioners will need to use an array of tools to manage the provision of health care for their populations.
Filling the knowledge translation gaps
In both case studies the high-quality evidence needed by commissioners on the clinical subgroups of patients where intervention is most clinically effective and cost-effective did not exist. However, the introduction of NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRCs) provide an opportunity for local commissioners and research communities to begin to bridge this gap from knowledge need to discovery. CLAHRCs aim to conduct applied health research that links those who conduct the research to all those who use it in practice across the health community. Therefore, if there is clinical uncertainty about the appropriate criteria for a given procedure, then providers, commissioners and researchers can use the variation in procedure rates as a natural experiment to learn more about the cost-effectiveness of divergent pathways of care.
Implications for national policy-makers and research funders
Using geographical variation to prioritise health technology reassessment
We found a high degree of geographical variation in procedure rates for many commonly performed procedures that cannot be explained by proxies of clinical need. Variation was most pronounced in procedures where utilisation was rapidly increasing or decreasing or where potential substitute procedures were available. Policy-makers and research funders, such as NICE, NHS England and the NIHR, might use geographical variation to identify procedures where HTR or new RCTs are needed to inform investment and disinvestment decisions. Awareness and understanding of the high geographical variations in NHS care has been reinvigorated by resources such as The NHS Atlas of Variation in Healthcare. 45 Continued NHS investment in these tools is vital to enable commissioners to optimally use routinely collected data to improve care for their local populations.
A more proactive national HTR process for health technologies suspected to deliver little or no health gain for their cost in some patient subgroups could help local commissioners identify and implement disinvestment. This would be analogous to the HTA process for expensive new pharmaceuticals and could use geographical variations in care to identify topics for review. The current more passive approach adopted by NICE, whereby ‘do not do’ recommendations are predominantly drawn from periodically updated clinical guidelines may overlook emerging areas of over-diffusion or obsolescence. It is also clear from our qualitative work that NICE’s technology appraisals of new pharmaceuticals dominate the agenda of local commissioners to the detriment of discussions about disinvestment. A more proactive and high-profile approach to HTR could redress this imbalance.
Investment to promote accurate clinical coding
Accurate recording of activity is important for any large organisation such as the NHS which is committed to monitoring and improving the quality and efficiency of the services it provides. Through the course of this project we have identified a number of instances of inconsistent, incomplete or inaccurate coding of clinical activity which undermine activities such as benchmarking which aim to improve care. It is vital that the NHS more regularly updates and improves the coding systems that it uses to track clinical activity, provides better training, guidance and support to hospital coders in appropriate coding and closely audits routine data provided by hospitals to identify weaknesses.
Promote sharing of randomised controlled trial data to provide better evidence for commissioners and clinicians
The finding in our case studies and elsewhere that published evidence is often of limited use in informing disinvestment decisions has implications for public funders of research. One implication is to prioritise high-quality trials of the clinical effectiveness and cost-effectiveness of new or existing procedures. As importantly, funders might require that triallists share IPD from completed RCTs so that others can pool data across trials and identify marginal subgroups of patients where the costs and risks of surgery begin to outweigh the benefits.
Recommendations for future research
The recommendations for future research are listed in descending order of importance.
Developing tools for commissioners to work with stakeholders to prioritise disinvestment
Commissioners are faced with an array of tools which aim to help them identify, prioritise and implement investment and disinvestment initiatives. These include a variety of benchmarking tools and various multicriteria decision analysis methods such as PBMA, sociotechnical allocation of resources and the Portsmouth scorecard. 202 These methods vary in the extent of stakeholder input and the level of complexity and time required. Research comparing these methods as means of identifying and implementing disinvestment initiatives and exploring their optimal design in order to engage clinicians and the public in a collaborative and transparent decision-making process would help commissioners establish sustainable local investment and disinvestment procedures.
Evaluate the costs and consequences of commissioner interventions to regulate procedure rates
It is already clear that CCGs will continue, and possibly ramp up, the methods used by PCTs (e.g. CBA, prior approval, referral management centres) to regulate access to medical procedures on the NHS. However, there is very little evidence on which methods are effective and some concern that the costs and bureaucracy of more intensive methods may defeat their objective of providing more efficient care, and have the potential to lead to poorer patient outcomes. Alternatives to these criteria-based approaches include patient-centred approaches, such as shared-decision-making aids, and contractual approaches (e.g. cost and volume contracts and tendering with the independent sector), all aimed at efficiently providing appropriate levels of care. Research on the relative merits and drawbacks of these approaches is needed to guide commissioners. This includes the research that we initially planned to evaluate the effectiveness of local commissioners’ policies for CBA in reducing the volume of procedures of uncertain value.
Evaluate the costs and outcomes of care in regions with high and low procedure rates
Observational and qualitative research on procedures with high geographical variation in localities with ‘high’ and ‘low’ procedure rates will help better our understanding of the reasons for variation and appropriate responses to it. This research should focus on not just those patients who receive surgery (e.g. individuals who receive carpal tunnel surgery), but the wider cohort (e.g. individuals with CTS) seen in primary and secondary care. This would help tease apart high procedure rates caused by high primary care consultation and referral rates from those caused by lower thresholds for surgery. Measuring the costs and outcomes of care for this wider cohort could help determine if high-cost care is associated with better outcomes. 86,87
Methodological work to develop a procedure/diagnosis code matrix
We have highlighted the limitations of working with the OPCS coding framework, which was developed for clinical rather than research purposes. For some purposes, the level of aggregation provided by OPCS three-character codes was too crude, while for other purposes the level of aggregation was too detailed. In all cases, procedure rates had to be cross-matched with diagnosis codes to account for procedures that were used for multiple distinct clinical groups (e.g. tonsillectomy and adenoidectomy for chronic tonsillitis and sleep apnoea). In order to make these coding frameworks more amenable for research, work is needed to develop a clinically coherent matrix of commonly occurring procedure/diagnosis group pairings. Given that there are thousands of OPCS procedure codes and ICD diagnosis codes this would be a major undertaking. It would also need to occur at different levels of aggregation so that it could be used at a high level (e.g. to identify the number of total hip replacements for patients with osteoarthritis) and also at a more detailed level (e.g. to identify the number of cemented, cementless and hybrid hip replacements for patients with osteoarthritis).
Conduct benchmarking on patient-reported outcomes collected in routine data
Our geographical variations analysis and local benchmarking was conducted on the process measure of procedure rates. However, now that patient-reported outcome measures are routinely collected for four surgical procedures (hip and knee replacements, hernia and varicose vein repair), there are opportunities to examine interhospital variations and benchmarking on the costs and outcomes of surgery. 203 Estimating the counterfactual (e.g. the costs and outcomes that would have occurred if surgery had not been performed) is problematic, but preliminary research in this area has found quite large variation in estimated costs and QALYs following hip replacement. This work could be expanded particularly if patient-reported outcome measures are introduced more widely in NHS routine data.
Acknowledgements
We are grateful to the NIHR SDO for funding this project. We thank all the members of the two commissioning groups who allowed us to observe and record their meetings and all the commissioners, providers, lay members, surgeons and patients who generously gave their time to participate in interviews.
The search strategies for the technology appraisals were supported by Wendy Marsh at NHS Suffolk and the benchmarking analysis was supported by Laura Geddes at the Avon Information Management and Technology Consortium and we are very grateful to both of them for their help. We thank Professor Jerry Jarvik and Bryan Comstock for providing access to IPD from their RCT of CTR surgery. We finally thank the academic and administrative staff at NIHR SDO for their help and support throughout the project.
Public and patient involvement
Service users were involved in the design of the study. Lay members of a PCT commissioning group were key informants for the qualitative study, as were patients who had recently received CTR surgery.
Contributions of authors
Professor William Hollingworth (Professor of Health Economics) led the conception and design of the project, provided oversight for the data collection and analysis and finalised the draft report.
Dr Leila Rooshenas (Research Assistant in Qualitative Research) conducted the observations of commissioning advisory groups and the semistructured interviews with commissioners, clinicians and patients. She conducted the qualitative analyses and drafted the chapters summarising the qualitative methods and findings.
Mr John Busby (Research Assistant in Medical Statistics/Health Economics) conducted the quantitative analysis examining national variation in procedure rates, the local benchmarking of procedure rates and also contributed to the data extraction and analysis for the rapid systematic reviews. He contributed to the drafting of the chapters detailing the quantitative methods and results.
Dr Christine E Hine (Consultant in Public Health Medicine) contributed to the conception and design of the project, provided advice on the benchmarking and selection of procedures for further review and critically revised the final report.
Dr Padmanabhan Badrinath (Consultant in Public Health Medicine and Associate Clinical Lecturer) contributed to the conception and design of the project, provided advice on the benchmarking and selection of procedures for further review and critically revised the final report.
Dr Penny F Whiting (Reviews Manager) contributed to the conception and design of the project, supervised the rapid systematic reviews and critically revised the final report.
Ms Theresa HM Moore (Research Associate Systematic Reviews) conducted the rapid systematic reviews and drafted the chapters summarising the review methods and findings.
Dr Amanda Owen-Smith (Research Fellow in Qualitative Research) contributed to the conception and design of the project, supervised the qualitative analyses, contributed to the drafting of the chapters summarising the qualitative methods and findings and critically revised the final report.
Professor Jonathan AC Sterne (Professor of Medical Statistics and Epidemiology) contributed to the conception and design of the project, provided advice on the quantitative analyses and critically revised the final report.
Dr Hayley E Jones (Research Fellow in Medical Statistics) supervised the quantitative analyses assessing national variation in procedure rates, contributed to the drafting of the chapters summarising the quantitative methods and findings and critically revised the final report.
Ms Claire Beynon (Head of Threshold Management and Individual Funding Requests) provided advice on the benchmarking and selection of procedures for further review and critically revised the final report.
Professor Jenny L Donovan (Professor of Social Medicine) contributed to the conception and design of the project, provided advice and supervision on the qualitative analyses and critically revised the final report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Harker R. NHS Funding and Expenditure 2012. www.nhshistory.net/parlymoney.pdf (accessed 20 February 2015).
- Appleby J. Spending on Health and Social Care over the Next 50 Years: Why Think Long Term?. London: King’s Fund; 2013.
- Mooney H. More than 30 NHS organisations end 2011–12 in debt totalling £356m. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4648.
- Astolfi R, Lorenzoni L, Oderkirk J. Informing policy makers about future health spending: a comparative analysis of forecasting methods in OECD countries. Health Policy 2012;107:1-10. http://dx.doi.org/10.1016/j.healthpol.2012.05.001.
- Smith S, Newhouse JP, Freeland MS. Income, insurance, and technology: why does health spending outpace economic growth?. Health Aff 2009;28:1276-84. http://dx.doi.org/10.1377/hlthaff.28.5.1276.
- Ham C. What will the Health and Social Care Bill mean for the NHS in England?. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2159.
- Edwards N. Implementation of the Health and Social Care Act. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2090.
- Naylor C, Curry N, Holder H, Ross S, Marshall L, Tait E. Clinical Commissioning Groups: Supporting Improvement in General Practice?. London: King’s Fund; 2013.
- Iacobucci G. GPs put the squeeze on access to hospital care. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4432.
- Rogers EM. Diffusion of Innovations. New York, NY: Free Press; 2003.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. http://dx.doi.org/10.1111/j.0887-378X.2004.00325.x.
- Elshaug AG, Hiller JE, Moss JR. Exploring policy-makers’ perspectives on disinvestment from ineffective healthcare practices. Int J Technol Assess Health Care 2008;24:1-9. http://dx.doi.org/10.1017/S0266462307080014.
- Palmer S, Raftery J. Economic notes: opportunity cost. BMJ 1999;318:1551-2. http://dx.doi.org/10.1136/bmj.318.7197.1551.
- Leggett L, Noseworthy TW, Zarrabi M, Lorenzetti D, Sutherland LR, Clement FM. Health technology reassessment of non-drug technologies: current practices. Int J Technol Assess Health Care 2012;28:220-7. http://dx.doi.org/10.1017/S0266462312000438.
- Elshaug AG, Hiller JE, Tunis SR, Moss JR. Challenges in Australian policy processes for disinvestment from existing, ineffective health care practices. Aust N Z Health Policy 2007;4. http://dx.doi.org/10.1186/1743-8462-4-23.
- Pearson A, Jordan Z, Munn Z. Translational science and evidence-based healthcare: a clarification and reconceptualization of how knowledge is generated and used in healthcare. Nurs Res Pract 2012;2012.
- Lang ES, Wyer PC, Haynes RB. Knowledge translation: closing the evidence-to-practice gap. Ann Emerg Med 2007;49:355-63. http://dx.doi.org/10.1016/j.annemergmed.2006.08.022.
- Glover JA. The incidence of tonsillectomy in school children. 1938. Int J Epidemiol 2008;37:9-19. http://dx.doi.org/10.1093/ije/dym258.
- Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med 1982;16:811-24. http://dx.doi.org/10.1016/0277-9536(82)90234-9.
- Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine 2006;31:2707-14.
- Appleby J, Raleigh V, Frosini F, Bevan G, Gao H, Lyscom T. Variations in Health Care. London: King’s Fund; 2011.
- McPherson K, Wennberg JE, Hovind OB, Clifford P. Small-area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med 1982;307:1310-14. http://dx.doi.org/10.1056/NEJM198211183072104.
- Westert GP, Groenewegen PP. Medical practice variations: changing the theoretical approach. Scand J Public Health 1999;27:173-80. http://dx.doi.org/10.1177/14034948990270030801.
- Bisset AF. Appendicectomy in Scotland: a 20-year epidemiological comparison. J Public Health 1997;19:213-18. http://dx.doi.org/10.1093/oxfordjournals.pubmed.a024612.
- Fowler AJ, Agha RA, Camm CF, Littlejohns P. The UK Freedom of Information Act (2000) in healthcare research: a systematic review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002967.
- Health and Social Care Information Centre . Hospital Episode Statistics: Admitted Patient Care 2011–12: Summary Report 2012. www.hscic.gov.uk/hes (accessed 31 March 2013).
- Payment by Results Team: Reference Costs 2011–12. London: Department of Health; 2012.
- Purdy S. Avoiding Hospital Admissions: What Does the Research Evidence Say?. London: King’s Fund; 2010.
- Black N, Browne J, van der Meulen J, Jamieson L, Copley L, Lewsey J. Is there overutilisation of cataract surgery in England?. Br J Ophthalmol 2009;93:13-7. http://dx.doi.org/10.1136/bjo.2007.136150.
- Contributing to NICE Interventional Procedure Guidance: A Guide for Patients and Carers. London; 2006.
- Guidance on the Extraction of Wisdom Teeth. London: NICE; 2000.
- National Institute for Health and Clinical Excellence: First Report of Session 2007–08. London: House of Commons Health Committee; 2008.
- NICE ‘Do Not Do’ Recommendations. London: NICE; 2013.
- Pearson S, Littlejohns P. Reallocating resources: how should the National Institute for Health and Clinical Excellence guide disinvestment efforts in the National Health Service?. J Health Serv Res Policy 2007;12:160-5. http://dx.doi.org/10.1258/135581907781542987.
- Cook S. Disinvestment: experts’ guide to saving money in health. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1281.
- Donaldson C, Bate A, Mitton C, Dionne F, Ruta D. Rational disinvestment. QJM 2010;103:801-7. http://dx.doi.org/10.1093/qjmed/hcq086.
- Gutierrez-Ibarluzea I. Evidence-based disinvestment in Spain. Aten Primaria 2011;43:3-4.
- Mitton C, Dionne F, Damji R, Campbell D, Bryan S. Difficult decisions in times of constraint: criteria based resource allocation in the Vancouver Coastal Health Authority. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-169.
- Nuti S, Vainieri M, Bonini A. Disinvestment for re-allocation: a process to identify priorities in healthcare. Health Policy 2010;95:137-43. http://dx.doi.org/10.1016/j.healthpol.2009.11.011.
- Leggett LE, Mackean G, Noseworthy TW, Sutherland L, Clement F. Current status of health technology reassessment of non-drug technologies: survey and key informant interviews. Health Res Policy Syst 2012;10. http://dx.doi.org/10.1186/1478-4505-10-38.
- Mulley AG. Inconvenient truths about supplier induced demand and unwarranted variation in medical practice. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4073.
- Bakwin H. Pseudodoxia pediatrica. N Engl J Med 1945;232:691-7. http://dx.doi.org/10.1056/NEJM194506142322401.
- Grob GN. The rise and decline of tonsillectomy in twentieth-century America. J Hist Med Allied Sci 2007;62:383-421. http://dx.doi.org/10.1093/jhmas/jrm003.
- Burton MJ, Glasziou PP. Tonsillectomy or adeno-tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev 2009;21.
- NHS Right Care . The NHS Atlas of Variation in Healthcare: Reducing Unwarranted Variation to Increase Value and Improve Quality 2011. www.rightcare.nhs.uk/atlas/qipp_nhsAtlas-LOW_261110c.pdf (accessed 31 March 2013).
- Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC, Bronner KK. Trends and Regional Variation in Hip, Knee, and Shoulder Replacement: A Dartmouth Atlas Surgery Report. Dartmouth, NH: Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- Readmission Rates and HES. Leeds: The Health and Social Care Information Centre; 2013.
- Anon . Standardization: a classic epidemiological method for the comparison of rates. Epidemiol Bull 2002;23:9-12.
- Office for National Statistics . Primary Care Organisations Population Estimates (Experimental), 2011 2011. www.ons.gov.uk/ons/rel/sape/pco-pop-est-exp/index.html (accessed 31 March 2013).
- Current Estimates, Population Estimates by Ethnic Group Mid-2009 for Primary Care Organisations (Experimental). Newport: Office for National Statistics; 2011.
- East Midlands Public Health Observatory . Summary IMD 2010 Scores for Non-LSOA Geographies 2011. www.apho.org.uk/resource/item.aspx?RID=111280 (accessed 20 February 2015).
- National Centre for Health Outcomes Development . Compendium of Population Health Indicators 2011. www.hscic.gov.uk/indicatorportal (accessed 20 February 2015).
- Dr Foster Health . Hospital Guide 2013. http://myhospitalguide.drfosterintelligence.co.uk/downloads/report/Report.pdf (accessed 20 February 2015).
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37. http://dx.doi.org/10.1023/A:1008929526011.
- Health and Social Care Information Centre . Hospital Episode Statistics for England: Outpatient Statistics, 2011–12 2012. www.hscic.gov.uk/hes (accessed 31 March 2013).
- Steinbusch PJ, Oostenbrink JB, Zuurbier JJ, Schaepkens FJ. The risk of upcoding in casemix systems: a comparative study. Health Policy 2007;81:289-99. http://dx.doi.org/10.1016/j.healthpol.2006.06.002.
- Majeed A, Eliahoo J, Bardsley M, Morgan D, Bindman AB. Variation in coronary artery bypass grafting, angioplasty, cataract surgery, and hip replacement rates among primary care groups in London: association with population and practice characteristics. J Public Health Med 2002;24:21-6. http://dx.doi.org/10.1093/pubmed/24.1.21.
- Birkmeyer JD, Sharp SM, Finlayson SRG, Fisher ES, Wennberg JE. Variation profiles of common surgical procedures. Surgery 1998;124:917-23. http://dx.doi.org/10.1016/S0039-6060(98)70017-0.
- Westert GP, Faber M. Commentary: the Dutch approach to unwarranted medical practice variation. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d1429.
- Westert GP, Groenewegen PP, Boshuizen HC, Spreeuwenberg PM, Steultjens MP. Medical practice variations in hospital care: time trends of a spatial phenomenon. Health Place 2004;10:215-20. http://dx.doi.org/10.1016/j.healthplace.2003.07.002.
- Weinstein JN, Bronner KK, Morgan TS, Wennberg JE. Trends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spine. Health Aff (Millwood) 2004.
- Nesbitt C, Eifell RK, Coyne P, Badri H, Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus conventional surgery for great saphenous vein varices. Cochrane Database Syst Rev 2011;10.
- Gohel MS, Epstein DM, Davies AH. Cost-effectiveness of traditional and endovenous treatments for varicose veins. Br J Surg 2010;97:1815-23. http://dx.doi.org/10.1002/bjs.7256.
- van Wijk RM, Geurts JW, Wynne HJ, Hammink E, Buskens E, Lousberg R, et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain: a randomized, double-blind, sham lesion-controlled trial. Clin J Pain 2005;21:335-44. http://dx.doi.org/10.1097/01.ajp.0000120792.69705.c9.
- Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC, et al. Challenges in evaluating surgical innovation. Lancet 2009;374:1097-104. http://dx.doi.org/10.1016/S0140-6736(09)61086-2.
- Keyhani S, Falk R, Bishop T, Howell E, Korenstein D. The relationship between geographic variations and overuse of healthcare services: a systematic review. Med Care 2012;50:257-61. http://dx.doi.org/10.1097/MLR.0b013e3182422b0f.
- Lilford RJ. Should the NHS strive to eradicate all unexplained variation? No. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4809.
- Hughes D. MPs Condemn NHS Regional Variation in IVF Provision 2011. www.bbc.co.uk/news/health-13670615 (accessed 31 March 2013).
- The Health and Social Care Information Centre . Security Issues and Patient Confidentiality 2013. www.datadictionary.nhs.uk/web_site_content/cds_supporting_information/security_issues_and_patient_confidentiality.asp (accessed 31 March 2013).
- Simmonds RL, Shaw A, Purdy S. Factors influencing professional decision making on unplanned hospital admission: a qualitative study. Br J Gen Pract 2012;62:e750-6. http://dx.doi.org/10.3399/bjgp12X658278.
- Busby J, Schroeder K, Woltersdorf W, Sterne JA, Ben-Shlomo Y, Hay A, et al. Temporal growth and geographic variation in the use of laboratory tests by NHS general practices: using routine data to identify research priorities. Br J Gen Pract 2013;63:e256-66. http://dx.doi.org/10.3399/bjgp13X665224.
- Davis P, Gribben B, Scott A, Lay-Yee R. The ‘supply hypothesis’ and medical practice variation in primary care: testing economic and clinical models of inter-practitioner variation. Soc Sci Med 2000;50:407-18. http://dx.doi.org/10.1016/S0277-9536(99)00299-3.
- Drummond M, Cooke J, Walley T. Economic evaluation under managed competition: evidence from the U.K. Soc Sci Med 1997;45:583-95. http://dx.doi.org/10.1016/S0277-9536(96)00398-X.
- Eddama O, Coast J. A systematic review of the use of economic evaluation in local decision-making. Health Policy 2008;86:129-41. http://dx.doi.org/10.1016/j.healthpol.2007.11.010.
- Imison C, Naylor C. Referral Management: Lessons for Success. London: King’s Fund; 2011.
- Knops AM, Legemate DA, Goossens A, Bossuyt PM, Ubbink DT. Decision aids for patients facing a surgical treatment decision: a systematic review and meta-analysis. Ann Surg 2013;257:860-6. http://dx.doi.org/10.1097/SLA.0b013e3182864fd6.
- Reducing Spending on Low Clinical Value Treatments. London: Audit Commission; 2011.
- British Household Panel Survey . Wave 18 2009. http://discover.ukdataservice.ac.uk/catalogue/?sn=6340&type=Data%20catalogue (accessed 20 February 2015).
- Marshall EG, Spiegelhalter DJ. Reliability of league tables of in vitro fertilisation clinics: retrospective analysis of live birth rates. BMJ 1998;316:1701-4. http://dx.doi.org/10.1136/bmj.316.7146.1701.
- Jones HE, Spiegelhalter D. The identification of ‘unusual’ health-care providers from a hierarchical model. Am Stat 2011;65:154-63. http://dx.doi.org/10.1198/tast.2011.10190.
- Ohlssen DI, Sharples LD, Spiegelhalter DJ. Flexible random-effects models using Bayesian semi-parametric models: applications to institutional comparisons. Stat Med 2007;26:2088-112. http://dx.doi.org/10.1002/sim.2666.
- Northcott D, Llewellyn S. Benchmarking in UK health: a gap between policy and practice?. Benchmarking: An International Journal 2005;12:419-35.
- NHS Comparators 2013. www.nhscomparators.nhs.uk/NHSComparators/Login.aspx (accessed 20 February 2015).
- Audit Commission . PbR National Benchmarker 2013. https://pbrbenchmarker.audit-commission.gov.uk/ (accessed 31 March 2013).
- Schang L, Morton A, Dasilva P, Bevan G. From data to decisions? Exploring how healthcare payers respond to the NHS Atlas of Variation in Healthcare in England. Health Policy 2014;114:79-87. http://dx.doi.org/10.1016/j.healthpol.2013.04.014.
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med 2003;138:273-87. http://dx.doi.org/10.7326/0003-4819-138-4-200302180-00006.
- Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med 2003;138:288-98. http://dx.doi.org/10.7326/0003-4819-138-4-200302180-00007.
- Learning Report: Looking for Value in Hard Times. London: Health Foundation; 2012.
- NHS Right Care . CCG Spend and Outcomes Tool 2013. www.rightcare.nhs.uk/index.php/resourcecentre/ccg-spend-and-outcomes-tool/ (accessed 31 March 2013).
- Clarke MG, Wilson JR, Kennedy KP, MacDonagh RP. Clinical judgment analysis of the parameters used by consultant urologists in the management of prostate cancer. J Urol 2007;178:98-102. http://dx.doi.org/10.1016/j.juro.2007.03.029.
- Torjesen I. NHS is unlikely to meet Nicholson challenge to deliver £20bn in efficiency savings, says King’s Fund. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6496.
- NICE 10 Years On: Past, Present and Future. London: NICE; 2010.
- Bland J. Carpal tunnel syndrome. BMJ 2007;335:343-6. http://dx.doi.org/10.1136/bmj.39282.623553.AD.
- Lewis C, Mauffrey C, Newman S, Lambert A, Hull P. Current concepts in carpal tunnel syndrome: a review of the literature. Eur J Orthop Surg Traumatol 2010;20:445-52. http://dx.doi.org/10.1007/s00590-010-0585-9.
- Latinovic R, Gulliford MC, Hughes RAC. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry 2006;77:263-5. http://dx.doi.org/10.1136/jnnp.2005.066696.
- Bland J, Rudolfer S. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991–2001. J Neurol Neurosurg Psychiatry 2003;74:1674-9. http://dx.doi.org/10.1136/jnnp.74.12.1674.
- Bachmann LM, Jüni P, Reichenbach S, Ziswiler H-R, Kessels AG, Vögelin E. Consequences of different diagnostic ‘gold standards’ in test accuracy research: carpal tunnel syndrome as an example. Int J Epidemiol 2005;34:953-5. http://dx.doi.org/10.1093/ije/dyi105.
- Padua L, Padua R, Aprile I. Multiperspective follow-up of untreated carpal tunnel syndrome: a multicentre study. Neurology 2001;56:1459-66. http://dx.doi.org/10.1212/WNL.56.11.1459.
- Ortiz-Corredor F, Enriquez F, Diaz-Ruiz J, Calambas N. Natural evolution of carpal tunnel syndrome in untreated patients. Clin Neurophysiol 2008;119:1373-8. http://dx.doi.org/10.1016/j.clinph.2008.02.012.
- O’Connor D, Marshall Shawn C, Massy-Westropp N, Pitt V. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev 2003;1.
- Chang MH, Chiang HT, Lee SS, Ger LP, Lo YK. Oral drug of choice in carpal tunnel syndrome. Neurology 1998;51:390-3. http://dx.doi.org/10.1212/WNL.51.2.390.
- Herskovitz S, Berger AR, Lipton RB. Low-dose, short-term oral prednisone in the treatment of carpal tunnel syndrome. Neurology 1995;45:1923-5. http://dx.doi.org/10.1212/WNL.45.10.1923.
- Hui AC, Wong SM, Wong KS, Li E, Kay R, Yung P, et al. Oral steroid in the treatment of carpal tunnel syndrome. Ann Rheum Dis 2001;60:813-14. http://dx.doi.org/10.1136/ard.60.8.813.
- Ebenbichler GR, Resch KL, Nicolakis P, Wiesinger GF, Uhl F, Ghanem AH, et al. Ultrasound treatment for treating the carpal tunnel syndrome: randomised ‘sham’ controlled trial. BMJ 1998;316:731-5. http://dx.doi.org/10.1136/bmj.316.7133.731.
- Oztas O, Turan B, Bora I, Karakaya MK. Ultrasound therapy effect in carpal tunnel syndrome. Arch Phys Med Rehabil 1998;79:1540-4. http://dx.doi.org/10.1016/S0003-9993(98)90416-6.
- Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR. Yoga-based intervention for carpal tunnel syndrome: a randomized trial. JAMA 1998;280:1601-3. http://dx.doi.org/10.1001/jama.280.18.1601.
- Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man Ther 2000;5:214-22. http://dx.doi.org/10.1054/math.2000.0355.
- Marshall SC, Tardif G, Ashworth NL. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2007;2.
- Armstrong T, Devor W, Borschel L, Contreras R. Intracarpal steroid injection is safe and effective for short-term management of carpal tunnel syndrome. Muscle Nerve 2004;29:82-8. http://dx.doi.org/10.1002/mus.10512.
- Dammers JW, Veering MM, Vermeulen M. Injection with methylprednisolone proximal to the carpal tunnel: randomised double blind trial. BMJ 1999;319:884-6. http://dx.doi.org/10.1136/bmj.319.7214.884.
- NHS Choices . Carpal Tunnel Syndrome Treatment 2012. www.nhs.uk/Conditions/Carpal-tunnel-syndrome/Pages/Treatment.aspx (accessed 31 March 2013).
- Boeckstyns MEH, Sorensen AI. Does endoscopic carpal tunnel release have a higher rate of complications than open carpal tunnel release?. J Hand Surg Br 1999;24:9-15. http://dx.doi.org/10.1016/S0266-7681(99)90009-8.
- Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev 2007;4.
- BSSH Evidence for Surgical Treatment (BEST); Carpal Tunnel Syndrome. London: British Society for Surgery of the Hand; 2011.
- Lefebvre C, Manheimer E, Glanville J, Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration; n.d.
- Higgins J, Altman D, Sterne J, Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration; n.d.
- Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet 2009;374:1074-81. http://dx.doi.org/10.1016/S0140-6736(09)61517-8.
- Ucan H, Yagci I, Yilmaz L, Yagmurlu F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and open carpal tunnel release outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2006;27:45-51. http://dx.doi.org/10.1007/s00296-006-0163-y.
- Hui ACF, Wong S, Leung CH, Tong P, Mok V, Poon D, et al. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology 2005;64:2074-8. http://dx.doi.org/10.1212/01.WNL.0000169017.79374.93.
- Verdugo RJ, Salinas RA, Castillo JL, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database of Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD001552.pub2.
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Garland H, Langworth EP, Taverner D, Clark JMP. Surgical treatment for carpal tunnel syndrome. Lancet 1964;1:1129-30. http://dx.doi.org/10.1016/S0140-6736(64)91807-0.
- Ly-Pen D, Andreu J-L, de Blas G, Sanchez-Olaso A, Millan I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum 2005;52:612-19. http://dx.doi.org/10.1002/art.20767.
- Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. J Am Med Assoc 2002;288:1245-51. http://dx.doi.org/10.1001/jama.288.10.1245.
- Korthals-de Bos I, Gerritsen AA, van Tulder MW, Rutten-Van Molken MP, Ader HJ, de Vet HC, et al. Surgery is more cost-effective than splinting for carpal tunnel syndrome in the Netherlands: results of an economic evaluation alongside a randomized controlled trial. BMC Musculoskelet Disord 2006;7. http://dx.doi.org/10.1186/1471-2474-7-86.
- Ganann R, Ciliska D, Thomas H. Expediting systematic reviews: methods and implications of rapid reviews. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-56.
- Riley RD, Lambert PC, Staessen JA, Wang J, Gueyffier F, Thijs L, et al. Meta-analysis of continuous outcomes combining individual patient data and aggregate data. Stat Med 2008;27:1870-93. http://dx.doi.org/10.1002/sim.3165.
- Katz JN, Keller RB, Simmons BP, Rogers WD, Bessette L, Fossel AH, et al. Maine Carpal Tunnel Study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg 1998;23:697-710. http://dx.doi.org/10.1016/S0363-5023(98)80058-0.
- Turner A, Kimble F, Gulyas K, Ball J. Can the outcome of open carpal tunnel release be predicted? A review of the literature. ANZ J Surg 2010;80:50-4. http://dx.doi.org/10.1111/j.1445-2197.2009.05175.x.
- Ross JS, Lehman R, Gross CP. The importance of clinical trial data sharing: toward more open science. Circ Cardiovasc Qual Outcomes 2012;5:238-40. http://dx.doi.org/10.1161/CIRCOUTCOMES.112.965798.
- Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res 2002;74:337-47. http://dx.doi.org/10.1006/exer.2001.1153.
- Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol 2009;127:555-62. http://dx.doi.org/10.1001/archophthalmol.2009.3.
- Wormstone IM, Wang L, Liu CSC. Posterior capsule opacification. Exp Eye Res 2009;88:257-69. http://dx.doi.org/10.1016/j.exer.2008.10.016.
- Powe NR, Schein OD, Gieser SC, Tielsch JM, Luthra R, Javitt J, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Arch Ophthalmol 1994;112:239-52. http://dx.doi.org/10.1001/archopht.1994.01090140115033.
- Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology 1998;105:1213-21. http://dx.doi.org/10.1016/S0161-6420(98)97023-3.
- Raja H, Raja D, Roy H. Nd-YAG Laser Capsulotomy n.d. http://emedicine.medscape.com/article/1844140-overview#aw2aab6b4 (accessed 27 January 2012).
- NHS Choices . Health A–Z, Complications of Age-Related Cataracts 2010. www.nhs.uk/Conditions/Cataracts-age-related/Pages/Complications.aspx (accessed 31 March 2013).
- Stark WJ, Worthen D, Holladay JT, Murray G. Neodymium:YAG lasers: an FDA report. Ophthalmology 1985;92:209-12. http://dx.doi.org/10.1016/S0161-6420(85)34051-4.
- Bath PE, Fankhauser F. Long-term results of ND:YAG laser posterior capsulotomy with Swiss laser. J Cataract Refract Surg 1986;12:150-3. http://dx.doi.org/10.1016/S0886-3350(86)80031-1.
- Hayashi K, Hayashi H, Nakao F, Hayashi F. Correlation between posterior capsule opacification and visual function before and after neodymium: YAG laser posterior capsulotomy. Am J Ophthalmol 2003;136:720-6. http://dx.doi.org/10.1016/S0002-9394(03)00425-2.
- Aslam T, Dhillon B. Neodymium:YAG laser capsulotomy: a clinical morphological analysis. Graefe’s Arch Clin Exp Ophthalmol 2002;240:972-6. http://dx.doi.org/10.1007/s00417-002-0578-4.
- Cataract Surgery Guidelines: September 2010. London: Royal College of Ophthalmologists; 2010.
- Gomaa A, Liu C. Nd:YAG laser capsulotomy: a survey of UK practice and recommendations. Eur J Ophthalmol 2011;21:385-90. http://dx.doi.org/10.5301/EJO.2010.6085.
- Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev 2010;2.
- 2012–2013 Tariff Information Spreadsheet, Payment by Results (PbR). London: Department of Health; 2013.
- Department of Health . NHS Reference Costs 2009–2010 n.d. www.gov.uk/government/publications/nhs-reference-costs-2009-2010 (accessed 27 March 2015).
- Audit Commission . Improving Data Quality in the NHS: Annual Report on the PbR Assurance Programme, 2010 n.d. http://archive.audit-commission.gov.uk/auditcommission/sitecollectiondocuments/Downloads/26082010pbrnhsdataqualityreport.pdf (accessed 31 March 2013).
- Spencer A. Hospital Episode Statistics (HES): Improving the Quality and Value of Hospital Data. Leeds: Health and Social Care Information Centre; 2011.
- Day Surgery: Operational Guide. Waiting, Booking and Choice. London: Department of Health; 2002.
- By Definition: Improving Data Definitions and Their Use by the NHS. London: Audit Commission; 2012.
- Cooper C, Starkey K. Disinvestment in healthcare. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1413.
- Elshaug AG, Moss JR, Littlejohns P, Karnon J, Merlin TL, Hiller JE. Identifying existing health care services that do not provide value for money. Med J Aust 2009;190:269-73.
- Cohen D. Marginal analysis in practice: an alternative to needs assessment for contracting health care. BMJ 1994;309:781-4. http://dx.doi.org/10.1136/bmj.309.6957.781.
- Elshaug AG, Watt AM, Mundy L, Willis CD. Over 150 potentially low-value health care practices: an Australian study. Med J Aust 2012;197:556-60. http://dx.doi.org/10.5694/mja12.11083.
- Ibargoyen-Roteta N, Gutierrez-Ibarluzea I, Asua J. Guiding the process of health technology disinvestment. Health Policy 2010;98:218-26. http://dx.doi.org/10.1016/j.healthpol.2010.06.018.
- Ball H, Kemp L, Fordham R. Road testing programme budgeting and marginal analysis: Norfolk Mental Health pilot project. Psychiatr Bull 2009;33:141-4. http://dx.doi.org/10.1192/pb.bp.108.020396.
- Bohmer P, Pain C, Watt A, Abernethy P, Sceats J. Maximising health gain within available resources in the New Zealand public health system. Health Policy 2001 n.d.;55:37-50. http://dx.doi.org/10.1016/S0168-8510(00)00107-X.
- Kemp L, Fordham R, Robson A, Bate A, Donaldson C, Baughan S, et al. Road Testing Programme Budgeting and Marginal Analysis (PBMA) in Three English Regions: Hull (Diabetes), Newcastle (CAHMS), Norfolk (Mental Health). York: Yorkshire and Humber Public Health Observatory; 2008.
- Mitton C, Donaldson C, Waldner H, Eagle C. The evolution of PBMA: towards a macro-level priority setting framework for health regions. Health Care Manag Sci 2003;6:263-9. http://dx.doi.org/10.1023/A:1026285809115.
- Williams IP, Bryan S. Cost-effectiveness analysis and formulary decision making in England: findings from research. Soc Sci Med 2007;65:2116-29. http://dx.doi.org/10.1016/j.socscimed.2007.06.009.
- Eddama O, Coast J. Use of economic evaluation in local health care decision-making in England: a qualitative investigation. Health Policy 2009;89:261-70. http://dx.doi.org/10.1016/j.healthpol.2008.06.004.
- Robinson S, Dickinson H, Freeman T, Rumbold B, Williams I. Structures and processes for priority-setting by health-care funders: a national survey of primary care trusts in England. Health Serv Manage Res 2012;25:113-20. http://dx.doi.org/10.1258/hsmr.2012.012007.
- Robinson S, Williams I, Dickinson H, Freeman T, Rumbold B. Priority-setting and rationing in healthcare: evidence from the English experience. Soc Sci Med 2012;75:2386-93. http://dx.doi.org/10.1016/j.socscimed.2012.09.014.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. London: Weidenfeld and Nicolson; 1967.
- Rumbold G, Allen K, Harris C. Disinvestment of Technologies and Clinical Practices in Health Services: Conceptual and Policy Perspectives. Melbourne, VIC: Centre for Clinical Effectiveness, Southern Health; 2008.
- Mitton C, Patten S, Donaldson C. Listening to the decision makers: sustainability of PBMA in Alberta. Appl Health Econ Health Policy 2004;3:143-51. http://dx.doi.org/10.2165/00148365-200403030-00005.
- Mitton C, Donaldson C. Resource allocation in health care: health economics and beyond. Health Care Anal 2003;11:245-57. http://dx.doi.org/10.1023/B:HCAN.0000005496.74131.a0.
- Garner S, Docherty M, Somner J, Sharma T, Choudhury M, Clarke M, et al. Reducing ineffective practice: challenges in identifying low-value health care using Cochrane systematic reviews. J Health Serv Res Policy 2013;18:6-12. http://dx.doi.org/10.1258/jhsrp.2012.012044.
- Hughes DA, Ferner RE. New drugs for old: disinvestment and NICE. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c572.
- Karnon J, Carlton J, Czoski-Murray C, Smith K. Informing disinvestment through cost-effectiveness modelling: is lack of data a surmountable barrier?. Appl Health Econ Health Policy 2009;7:1-9. http://dx.doi.org/10.1007/BF03256137.
- Mortimer D. Reorienting programme budgeting and marginal analysis (PBMA) towards disinvestment. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-288.
- Paulden M. Investment and disinvestment of health technologies: the need for two cost-effectiveness thresholds. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.03.188.
- Garner S, Littlejohns P. Disinvestment from low value clinical interventions: NICEly done?. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d4519.
- Ruta D, Donaldson C, Gilray I. Economics, public health and health care purchasing: the Tayside experience of programme budgeting and marginal analysis. J Health Serv Res Policy 1996;1:185-93.
- Mitton C, Donaldson C. Health care priority setting: principles, practice and challenges. Cost-Effectiveness and Resource Allocation 2004;2. http://dx.doi.org/10.1186/1478-7547-2-3.
- Henshall C, Schuller T, Mardhani-Bayne L. Using health technology assessment to support optimal use of technologies in current practice: the challenge of ‘disinvestment’. Int J Technol Assess Health Care 2012;28:203-10. http://dx.doi.org/10.1017/S0266462312000372.
- Brody H. From an ethics of rationing to an ethics of waste avoidance. N England J Med 2012;366:1949-51. http://dx.doi.org/10.1056/NEJMp1203365.
- Cookson R, McDaid D, Maynard A. Wrong SIGN, NICE mess: is national guidance distorting allocation of resources?. BMJ 2001;323:743-5. http://dx.doi.org/10.1136/bmj.323.7315.743.
- Walker S, Palmer S, Sculpher M. The role of NICE technology appraisal in NHS rationing. Br Med Bull 2007;81–2:51-64. http://dx.doi.org/10.1093/bmb/ldm007.
- Abelson J, Eyles J, McLeod CB, Collins P, McMullan C, Forest PG. Does deliberation make a difference? Results from a citizens panel study of health goals priority setting. Health Policy 2003;66:95-106. http://dx.doi.org/10.1016/S0168-8510(03)00048-4.
- Owen-Smith A, Coast J, Donovan J. The desirability of being open about health care rationing decisions: findings from a qualitative study of patients and clinical professionals. J Health Serv Res Policy 2010;15:14-20. http://dx.doi.org/10.1258/jhsrp.2009.009045.
- Owen-Smith A, Coast J, Donovan J. ‘I can see where they’re coming from, but when you’re on the end of it . . . you just want to get the money and the drug’: explaining reactions to explicit healthcare rationing. Soc Sci Med 2011;68:1935-42. http://dx.doi.org/10.1016/j.socscimed.2009.03.024.
- Owen-Smith A, Coast J, Donovan J. Are patients receiving enough information about healthcare rationing? A qualitative study. J Med Ethics 2010;36:88-92. http://dx.doi.org/10.1136/jme.2009.033241.
- Harris J. The survival lottery. Philosophy 1975;50:81-7. http://dx.doi.org/10.1017/S0031819100059118.
- Coast J. Who wants to know if their care is rationed? Views of citizens and service informants. Health Expect 2001;4:243-52. http://dx.doi.org/10.1046/j.1369-6513.2001.00147.x.
- Wailoo A, Anand P. The nature of procedural preferences for health-care rationing decisions. Soc Sci Med 2005;60:223-36. http://dx.doi.org/10.1016/j.socscimed.2004.04.036.
- Ayres PJ. Rationing health care: views from general practice. Soc Sci Med 1996;42:1021-5. http://dx.doi.org/10.1016/0277-9536(95)00213-8.
- Ibanez B, Librero J, Bernal-Delgado E, Peiro S, Lopez-Valcarcel BG, Martinez N, et al. Is there much variation in variation? Revisiting statistics of small area variation in health services research. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-60.
- McKee M. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1733.
- Walshe K. Reinventing clinical commissioning groups. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4980.
- Suleman M, Clark MPA, Goldacre M, Burton M. Exploring the variation in paediatric tonsillectomy rates between English regions: a 5-year NHS and independent sector data analysis. Clin Otolaryngol 2010;35:111-17. http://dx.doi.org/10.1111/j.1749-4486.2010.02086.x.
- Michaels JA, Browse DJ, McWhinnie DL, Galland RB, Morris PJ. Provision of vascular surgical services in the Oxford Region. Br J Surg 1994;81:377-81. http://dx.doi.org/10.1002/bjs.1800810318.
- McComb JM, Plummer CJ, Cunningham MW, Cunningham D. Inequity of access to implantable cardioverter defibrillator therapy in England: possible causes of geographical variation in implantation rates. Europace 2009;11:1308-12. http://dx.doi.org/10.1093/europace/eup264.
- Keenan T, Rosen P, Yeates D, Goldacre M. Time trends and geographical variation in cataract surgery rates in England: study of surgical workload. Br J Ophthalmol 2007;91:901-4. http://dx.doi.org/10.1136/bjo.2006.108977.
- Galland RB, Whatling PJ, Crook TJ, Magee TR. Regional variation in varicose vein operations in England 1989–1996. Ann R Coll Surg Engl 2000;82:275-9.
- Black N, Langham S, Petticrew M. Coronary revascularisation: why do rates vary geographically in the UK?. J Epidemiol Community Health 1995;49:408-12. http://dx.doi.org/10.1136/jech.49.4.408.
- Bevan G, Hollinghurst S, Benton P, Spark V, Sanderson H, Franklin D. Using information on variation in rates of supply to question professional discretion in public services. Financ Account Manage 2004;20:1-17. http://dx.doi.org/10.1111/j.1468-0408.2004.00183.x.
- Freemantle N, Richardson M, Wood J, Ray D, Khosla S, Sun P, et al. Can we update the Summary Hospital Mortality Index (SHMI) to make a useful measure of the quality of hospital care? An observational study. BMJ 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002018.
- Burns EM, Bottle A, Aylin P, Darzi A, Nicholls RJ, Faiz O. Variation in reoperation after colorectal surgery in England as an indicator of surgical performance: retrospective analysis of Hospital Episode Statistics. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d4836.
- Mohammed MA, Lilford R, Rudge G, Holder R, Stevens A. The findings of the Mid-Staffordshire Inquiry do not uphold the use of hospital standardized mortality ratios as a screening test for ‘bad’ hospitals. QJM 2013;106:849-54. http://dx.doi.org/10.1093/qjmed/hct101.
- Malhotra N, Jacobson B. Save to Invest. London: London Health Observatory; 2007.
- Dionne F, Mitton C, Macdonald T, Miller C, Brennan M. The challenge of obtaining information necessary for multi-criteria decision analysis implementation: the case of physiotherapy services in Canada. Cost Effectiveness Resour Allocation 2013;11. http://dx.doi.org/10.1186/1478-7547-11-11.
- Appleby J, Poteliakhoff E, Shah K, Devlin N. Using patient-reported outcome measures to estimate cost-effectiveness of hip replacements in English hospitals. J R Soc Med 2013;106:323-31. http://dx.doi.org/10.1177/0141076813489678.
- Gerritsen AA, de Vet HC, Scholten RJ, Barbano R. Surgery was associated with greater long term treatment success than wrist splinting in carpal tunnel syndrome: commentary. Evid Based Med 2003;8. http://dx.doi.org/10.1136/ebm.8.2.55.
- Gerritsen AA, Scholten RJ, Assendelft WJ, Kuiper H, de Vet HC, Bouter LM. Splinting or surgery for carpal tunnel syndrome? Design of a randomized controlled trial [ISRCTN18853827]. BMC Neurol 2001;1. http://dx.doi.org/10.1186/1471-2377-1-8.
- Gerritsen AAM, de Vet HCW, Scholten R, Bertelsmann FW, De Krom M, Bouter LM. Greater clinical effects on carpel tunnel syndrome with surgery than with splinting; results of a randomised clinical trial. Ned Tijdschr Geneeskunde 2002;146:2153-6.
- Martin BI, Levenson LM, Hollingworth W, Kliot M, Heagerty PJ, Turner JA, et al. Randomized clinical trial of surgery versus conservative therapy for carpal tunnel syndrome [ISRCTN84286481]. BMC Musculoskelet Disord 2005;6. http://dx.doi.org/10.1186/1471-2474-6-2.
- Andreu JL, Ly-Pen D. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology 2006;66:955-6. http://dx.doi.org/10.1212/01.wnl.0000218667.40662.4d.
- Andreu JL, Ly-Pen D. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome: author reply. Neurology 2006;66:955-6. http://dx.doi.org/10.1212/01.wnl.0000218667.40662.4d.
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg 1993;75:1585-92.
Appendix 1 Inter-PCT variation in all procedures rates in 2011/12
| Procedure | Number of procedures | Inter-PCT SD (95% CI) | Inter-PCT SD to age (years)/ sex (95% CI) | UR (95% CI) | Probability highly variable | Probability very highly variable |
|---|---|---|---|---|---|---|
| Incision of capsule of lens | 15,131 | 1.8 (1.6 to 2.0) | 1.9 (1.7 to 2.1) | 109.1 (59.8 to 196.1) | 1.00 | 1.00 |
| Neurostimulation of peripheral nerve | 7983 | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.2) | 16.7 (12.0 to 23.2) | 1.00 | 1.00 |
| Curettage of lesion of skin | 13,046 | 1.0 (0.9 to 1.1) | 1.1 (1.0 to 1.3) | 13.0 (9.6 to 17.7) | 1.00 | 0.99 |
| Excision of vas deferens | 10,192 | 0.9 (0.8 to 1.1) | 1.2 (1.0 to 1.3) | 12.1 (8.9 to 16.7) | 1.00 | 0.96 |
| Hybrid prosthetic replacement of hip joint using cemented femoral component | 7882 | 0.9 (0.8 to 1.1) | 1.2 (1.0 to 1.3) | 11.3 (8.3 to 15.5) | 1.00 | 0.91 |
| Transluminal operations on varicose vein of leg | 10,262 | 0.9 (0.8 to 1.0) | 1.2 (1.1 to 1.4) | 10.7 (7.9 to 14.7) | 1.00 | 0.82 |
| Prosthetic replacement of head of femur not using cement | 6287 | 0.9 (0.8 to 1.0) | 1.0 (0.9 to 1.1) | 9.7 (7.2 to 13.3) | 1.00 | 0.61 |
| Denervation of spinal facet joint of vertebra | 10,168 | 0.9 (0.8 to 1.0) | 1.0 (0.9 to 1.1) | 9.4 (7.2 to 12.6) | 1.00 | 0.56 |
| Restoration of tooth | 14,720 | 0.8 (0.7 to 1.0) | 1.1 (1.0 to 1.3) | 8.9 (6.9 to 11.8) | 1.00 | 0.39 |
| Destruction of lesion of retina | 23,007 | 0.8 (0.7 to 0.9) | 0.9 (0.8 to 1.0) | 8.1 (6.4 to 10.5) | 1.00 | 0.17 |
| Other vaginal operations on uterus | 13,256 | 0.7 (0.6 to 0.8) | 0.9 (0.8 to 1.1) | 6.6 (5.2 to 8.4) | 1.00 | 0.01 |
| Combined operations on varicose vein of leg | 10,269 | 0.7 (0.6 to 0.8) | 0.8 (0.7 to 1.0) | 6.2 (5.0 to 7.8) | 1.00 | 0.00 |
| Operations on vitreous body | 101,606 | 0.7 (0.6 to 0.8) | 0.8 (0.7 to 0.9) | 5.8 (4.8 to 7.2) | 1.00 | 0.00 |
| Excision of cervix uteri | 15,886 | 0.7 (0.6 to 0.7) | 0.7 (0.6 to 0.8) | 5.6 (4.6 to 6.9) | 1.00 | 0.00 |
| Intramuscular injection | 28,293 | 0.7 (0.6 to 0.7) | 0.7 (0.7 to 0.8) | 5.7 (4.7 to 6.9) | 1.00 | 0.00 |
| Other operations on bladder | 64,605 | 0.7 (0.6 to 0.7) | 0.7 (0.6 to 0.8) | 5.3 (4.4 to 6.5) | 0.98 | 0.00 |
| Other excision of appendix | 14,360 | 0.6 (0.6 to 0.7) | 0.6 (0.6 to 0.7) | 5.1 (4.2 to 6.4) | 0.94 | 0.00 |
| Other operations on urethra | 9672 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 5.0 (4.2 to 6.1) | 0.91 | 0.00 |
| Destruction of haemorrhoid | 27,387 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 4.7 (4.0 to 5.8) | 0.79 | 0.00 |
| Other operations on sympathetic nerve | 6978 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 4.8 (3.9 to 5.9) | 0.79 | 0.00 |
| Operations on bursa | 12,417 | 0.6 (0.5 to 0.7) | 0.6 (0.6 to 0.7) | 4.6 (3.9 to 5.7) | 0.73 | 0.00 |
| Operations on spinal nerve root | 35,678 | 0.6 (0.5 to 0.7) | 0.6 (0.6 to 0.7) | 4.5 (3.8 to 5.5) | 0.65 | 0.00 |
| Therapeutic epidural injection | 73,079 | 0.6 (0.5 to 0.7) | 0.6 (0.6 to 0.7) | 4.5 (3.8 to 5.5) | 0.65 | 0.00 |
| Other operations on peripheral nerve | 24,705 | 0.6 (0.5 to 0.6) | 0.6 (0.5 to 0.7) | 4.5 (3.8 to 5.3) | 0.61 | 0.00 |
| Other operations on eyelid | 8012 | 0.6 (0.5 to 0.7) | 0.7 (0.6 to 0.8) | 4.4 (3.7 to 5.3) | 0.47 | 0.00 |
| Extracorporeal fragmentation of calculus of kidney | 19,750 | 0.5 (0.4 to 0.6) | 0.5 (0.5 to 0.6) | 3.7 (3.1 to 4.4) | 0.03 | 0.00 |
| Soft tissue operations on joint of toe | 13,271 | 0.5 (0.4 to 0.6) | 0.6 (0.5 to 0.6) | 3.6 (3.0 to 4.3) | 0.02 | 0.00 |
| Other reconstruction of joint | 11,951 | 0.5 (0.4 to 0.5) | 0.6 (0.5 to 0.7) | 3.5 (3.0 to 4.1) | 0.01 | 0.00 |
| Simple extraction of tooth | 99,217 | 0.5 (0.4 to 0.5) | 0.6 (0.5 to 0.6) | 3.5 (3.0 to 4.0) | 0.00 | 0.00 |
| Total prosthetic replacement of hip joint not using cement | 29,987 | 0.5 (0.4 to 0.5) | 0.5 (0.5 to 0.6) | 3.4 (2.9 to 3.9) | 0.00 | 0.00 |
| Endoscopic operations to increase capacity of bladder | 10,946 | 0.5 (0.4 to 0.5) | 0.5 (0.4 to 0.6) | 3.3 (2.9 to 3.9) | 0.00 | 0.00 |
| Endoscopic extirpation of lesion of lower bowel using fibreoptic sigmoidoscope | 22,616 | 0.5 (0.4 to 0.5) | 0.5 (0.4 to 0.6) | 3.2 (2.8 to 3.7) | 0.00 | 0.00 |
| Secondary open reduction of fracture of bone | 8926 | 0.5 (0.4 to 0.5) | 0.5 (0.5 to 0.6) | 3.2 (2.8 to 3.8) | 0.00 | 0.00 |
| Therapeutic endoscopic operations on peritoneum | 20,748 | 0.4 (0.4 to 0.5) | 0.5 (0.4 to 0.5) | 3.1 (2.7 to 3.6) | 0.00 | 0.00 |
| Release of entrapment of peripheral nerve at other site | 6596 | 0.4 (0.4 to 0.5) | 0.6 (0.5 to 0.7) | 3.0 (2.6 to 3.6) | 0.00 | 0.00 |
| Surgical removal of tooth | 115,536 | 0.4 (0.4 to 0.5) | 0.5 (0.4 to 0.5) | 3.0 (2.6 to 3.4) | 0.00 | 0.00 |
| Total prosthetic replacement of hip joint using cement | 32,020 | 0.4 (0.4 to 0.5) | 0.6 (0.5 to 0.7) | 3.0 (2.6 to 3.4) | 0.00 | 0.00 |
| Extirpation of lesion of eyelid | 29,818 | 0.4 (0.4 to 0.5) | 0.5 (0.5 to 0.6) | 2.9 (2.6 to 3.3) | 0.00 | 0.00 |
| Other total prosthetic replacement of hip joint | 9830 | 0.4 (0.4 to 0.5) | 0.4 (0.4 to 0.5) | 2.9 (2.6 to 3.4) | 0.00 | 0.00 |
| Release of contracture of joint | 11,426 | 0.4 (0.4 to 0.5) | 0.5 (0.4 to 0.6) | 2.8 (2.5 to 3.3) | 0.00 | 0.00 |
| Other therapeutic endoscopic operations on ureter | 28,843 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.7 (2.4 to 3.1) | 0.00 | 0.00 |
| Prosthetic replacement of head of femur using cement | 18,118 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.7 (2.4 to 3.1) | 0.00 | 0.00 |
| Other operations on soft tissue | 8759 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.7 (2.3 to 3.1) | 0.00 | 0.00 |
| Clearance of external auditory canal | 7406 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.6 (2.3 to 3.0) | 0.00 | 0.00 |
| Puncture of joint | 120,955 | 0.4 (0.3 to 0.4) | 0.5 (0.4 to 0.6) | 2.6 (2.4 to 3.0) | 0.00 | 0.00 |
| Transluminal operations on femoral artery | 18,916 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.7 (2.4 to 3.1) | 0.00 | 0.00 |
| Therapeutic endoscopic operations on urethra | 18,597 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.6 (2.3 to 2.9) | 0.00 | 0.00 |
| Debridement and irrigation of joint | 16,835 | 0.4 (0.3 to 0.4) | 0.5 (0.4 to 0.5) | 2.6 (2.3 to 3.0) | 0.00 | 0.00 |
| Extirpation of lesion of lip | 10,137 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.6 (2.3 to 2.9) | 0.00 | 0.00 |
| Filtering operations on iris | 7996 | 0.4 (0.3 to 0.4) | 0.5 (0.4 to 0.6) | 2.6 (2.3 to 3.0) | 0.00 | 0.00 |
| Operations on external nose | 25,796 | 0.4 (0.3 to 0.4) | 0.5 (0.4 to 0.5) | 2.5 (2.3 to 2.9) | 0.00 | 0.00 |
| Therapeutic transluminal operations on heart | 6538 | 0.4 (0.3 to 0.4) | 0.5 (0.4 to 0.5) | 2.6 (2.3 to 3.0) | 0.00 | 0.00 |
| Therapeutic spinal puncture | 15,184 | 0.4 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.5 (2.3 to 2.9) | 0.00 | 0.00 |
| Excision of ganglion | 11,557 | 0.4 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.5 (2.2 to 2.9) | 0.00 | 0.00 |
| Primary excision of lumbar intervertebral disc | 9542 | 0.4 (0.3 to 0.4) | 0.5 (0.4 to 0.5) | 2.5 (2.2 to 2.9) | 0.00 | 0.00 |
| Other therapeutic fibreoptic endoscopic operations on oesophagus | 6212 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.5) | 2.5 (2.1 to 2.8) | 0.00 | 0.00 |
| Division of bone of foot | 20,532 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.4 (2.2 to 2.7) | 0.00 | 0.00 |
| Endoscopic bilateral occlusion of fallopian tubes | 9957 | 0.3 (0.3 to 0.4) | 0.7 (0.6 to 0.8) | 2.5 (2.2 to 2.8) | 0.00 | 0.00 |
| Therapeutic endoscopic operations on uterus | 35,342 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.4 (2.1 to 2.7) | 0.00 | 0.00 |
| Arteriovenous shunt | 12,804 | 0.3 (0.3 to 0.4) | 0.5 (0.4 to 0.5) | 2.4 (2.1 to 2.7) | 0.00 | 0.00 |
| Endoscopic extirpation of lesion of colon | 112,452 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.4 (2.1 to 2.7) | 0.00 | 0.00 |
| Emergency excision of appendix | 35,681 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.4 (2.1 to 2.6) | 0.00 | 0.00 |
| Repair of muscle | 14,275 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.4 (2.1 to 2.7) | 0.00 | 0.00 |
| Other operations on anus | 11,737 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.3 (2.1 to 2.6) | 0.00 | 0.00 |
| Therapeutic endoscopic operations on cavity of knee joint | 12,167 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.3 (2.1 to 2.6) | 0.00 | 0.00 |
| Total excision of bone | 6319 | 0.3 (0.3 to 0.4) | 0.5 (0.4 to 0.5) | 2.3 (2.0 to 2.6) | 0.00 | 0.00 |
| Other operations on nail bed | 11,203 | 0.3 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 2.3 (2.0 to 2.5) | 0.00 | 0.00 |
| Fusion of joint of toe | 14,262 | 0.3 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | 2.2 (2.0 to 2.5) | 0.00 | 0.00 |
| Other excision of lesion of skin | 204,386 | 0.3 (0.3 to 0.4) | 0.4 (0.4 to 0.4) | 2.2 (2.0 to 2.4) | 0.00 | 0.00 |
| Other primary fusion of other joint | 7081 | 0.3 (0.3 to 0.4) | 0.3 (0.3 to 0.4) | 2.2 (2.0 to 2.5) | 0.00 | 0.00 |
| Release of entrapment of peripheral nerve at wrist | 54,093 | 0.3 (0.3 to 0.3) | 0.4 (0.3 to 0.4) | 2.1 (2.0 to 2.4) | 0.00 | 0.00 |
| Microtherapeutic endoscopic operations on larynx | 5022 | 0.3 (0.2 to 0.3) | 0.4 (0.3 to 0.4) | 2.2 (1.9 to 2.5) | 0.00 | 0.00 |
| Other plastic operations on breast | 7012 | 0.3 (0.2 to 0.3) | 0.4 (0.4 to 0.5) | 2.1 (1.9 to 2.4) | 0.00 | 0.00 |
| Endoscopic retrograde placement of prosthesis in bile duct | 9298 | 0.3 (0.3 to 0.3) | 0.3 (0.3 to 0.4) | 2.1 (1.9 to 2.4) | 0.00 | 0.00 |
| Open excision of prostate | 5481 | 0.3 (0.2 to 0.3) | 0.4 (0.3 to 0.5) | 2.1 (1.9 to 2.4) | 0.00 | 0.00 |
| Therapeutic ureteroscopic operations on ureter | 19,066 | 0.3 (0.3 to 0.3) | 0.3 (0.3 to 0.4) | 2.1 (1.9 to 2.3) | 0.00 | 0.00 |
| Surgical arrest of bleeding from internal nose | 9927 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.0 (1.9 to 2.3) | 0.00 | 0.00 |
| Primary closed reduction of traumatic dislocation of joint | 9852 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.1 (1.9 to 2.3) | 0.00 | 0.00 |
| Operations on eyebrow | 5682 | 0.3 (0.2 to 0.3) | 0.4 (0.3 to 0.5) | 2.0 (1.8 to 2.3) | 0.00 | 0.00 |
| Transluminal operations on iliac artery | 7372 | 0.3 (0.2 to 0.3) | 0.4 (0.3 to 0.5) | 2.1 (1.8 to 2.3) | 0.00 | 0.00 |
| Vaginal operations to support outlet of female bladder | 14,220 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.0 (1.9 to 2.3) | 0.00 | 0.00 |
| Other therapeutic transluminal operations on heart | 9057 | 0.3 (0.2 to 0.3) | 0.4 (0.4 to 0.5) | 2.0 (1.9 to 2.3) | 0.00 | 0.00 |
| Repair of eardrum | 10,471 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.1 (1.9 to 2.3) | 0.00 | 0.00 |
| Operations on septum of nose | 24,486 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.3) | 2.0 (1.9 to 2.2) | 0.00 | 0.00 |
| Correction of deformity of eyelid | 13,590 | 0.3 (0.2 to 0.3) | 0.4 (0.3 to 0.4) | 2.0 (1.8 to 2.2) | 0.00 | 0.00 |
| Open drainage of bladder | 8034 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.0 (1.8 to 2.3) | 0.00 | 0.00 |
| Operations on canthus | 7141 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.0 (1.8 to 2.3) | 0.00 | 0.00 |
| Combined operations on muscles of eye | 7082 | 0.3 (0.2 to 0.3) | 0.4 (0.4 to 0.5) | 2.0 (1.8 to 2.3) | 0.00 | 0.00 |
| Reduction of fracture of other bone of face | 11,792 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 2.0 (1.8 to 2.2) | 0.00 | 0.00 |
| Operations on adenoid | 24,723 | 0.3 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 1.9 (1.8 to 2.1) | 0.00 | 0.00 |
| Excision of lesion of anus | 14,286 | 0.3 (0.2 to 0.3) | 0.3 (0.2 to 0.3) | 1.9 (1.8 to 2.1) | 0.00 | 0.00 |
| Vaginal excision of uterus | 16,611 | 0.2 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 1.9 (1.7 to 2.1) | 0.00 | 0.00 |
| Extirpation of lesion of external ear | 16,974 | 0.2 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 1.9 (1.7 to 2.1) | 0.00 | 0.00 |
| Drainage of middle ear | 40,680 | 0.2 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 1.9 (1.7 to 2.0) | 0.00 | 0.00 |
| Excision of haemorrhoid | 10,016 | 0.2 (0.2 to 0.3) | 0.3 (0.2 to 0.3) | 1.8 (1.7 to 2.0) | 0.00 | 0.00 |
| Other repair of prolapse of vagina | 28,309 | 0.2 (0.2 to 0.3) | 0.3 (0.3 to 0.3) | 1.8 (1.7 to 2.0) | 0.00 | 0.00 |
| Correction of ptosis of eyelid | 5722 | 0.2 (0.2 to 0.3) | 0.3 (0.2 to 0.3) | 1.8 (1.6 to 2.0) | 0.00 | 0.00 |
| Primary open reduction of fracture of bone and intramedullary fixation | 29,312 | 0.2 (0.2 to 0.3) | 0.3 (0.2 to 0.3) | 1.8 (1.7 to 1.9) | 0.00 | 0.00 |
| Orthodontic operations | 8649 | 0.2 (0.2 to 0.3) | 0.3 (0.3 to 0.4) | 1.8 (1.6 to 2.0) | 0.00 | 0.00 |
| Endoscopic resection of outlet of male bladder | 25,431 | 0.2 (0.2 to 0.3) | 0.3 (0.2 to 0.3) | 1.8 (1.7 to 1.9) | 0.00 | 0.00 |
| Other operations on sheath of tendon | 15,310 | 0.2 (0.2 to 0.3) | 0.2 (0.2 to 0.3) | 1.7 (1.6 to 1.9) | 0.00 | 0.00 |
| Fibreoptic endoscopic extirpation of lesion of upper gastrointestinal tract | 20,003 | 0.2 (0.2 to 0.2) | 0.3 (0.3 to 0.3) | 1.7 (1.6 to 1.9) | 0.00 | 0.00 |
| Other therapeutic fibreoptic endoscopic operations on upper gastrointestinal tract | 28,294 | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 1.7 (1.6 to 1.9) | 0.00 | 0.00 |
| Therapeutic endoscopic operations on semilunar cartilage | 83,241 | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 1.7 (1.6 to 1.8) | 0.00 | 0.00 |
| Endoscopic incision of sphincter of Oddi | 22,680 | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 1.7 (1.6 to 1.9) | 0.00 | 0.00 |
| Connection of thoracic artery to coronary artery | 16,191 | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 1.7 (1.6 to 1.8) | 0.00 | 0.00 |
| Cardioverter defibrillator introduced through the vein | 8443 | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 1.7 (1.6 to 1.9) | 0.00 | 0.00 |
| Other reconstruction of ligament | 15,044 | 0.2 (0.2 to 0.2) | 0.3 (0.3 to 0.4) | 1.7 (1.6 to 1.8) | 0.00 | 0.00 |
| Excision of thyroid gland | 11,143 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.7 (1.5 to 1.8) | 0.00 | 0.00 |
| Attention to artificial opening into ileum | 6807 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.7 (1.5 to 1.8) | 0.00 | 0.00 |
| Other drainage of peritoneal cavity | 43,055 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Other operations on tonsil | 6720 | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 1.6 (1.5 to 1.8) | 0.00 | 0.00 |
| Other closed reduction of fracture of bone | 26,391 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Cardiac pacemaker system introduced through vein | 41,796 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.2) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Other operations on perianal region | 13,466 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Excision of lung | 6815 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.3) | 1.6 (1.4 to 1.7) | 0.00 | 0.00 |
| Repair of recurrent inguinal hernia | 6221 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.6 (1.4 to 1.8) | 0.00 | 0.00 |
| Excision of tonsil | 46,855 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Percutaneous transluminal balloon angioplasty and insertion of stent into coronary artery | 65,673 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.2) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Excision of vulva | 7882 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.3) | 1.5 (1.4 to 1.7) | 0.00 | 0.00 |
| Other internal fixation of bone | 49,935 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.3) | 1.5 (1.5 to 1.6) | 0.00 | 0.00 |
| Primary open reduction of fracture of bone and extramedullary fixation | 45,541 | 0.2 (0.2 to 0.2) | 0.2 (0.2 to 0.2) | 1.6 (1.5 to 1.7) | 0.00 | 0.00 |
| Other operations on internal nose | 14,647 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.5 (1.4 to 1.6) | 0.00 | 0.00 |
| Operations on prepuce | 33,275 | 0.2 (0.1 to 0.2) | 0.3 (0.2 to 0.3) | 1.5 (1.4 to 1.6) | 0.00 | 0.00 |
| Other excision of breast | 40,570 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.5 (1.4 to 1.6) | 0.00 | 0.00 |
| Prosthesis of lens | 326,756 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.5 (1.4 to 1.5) | 0.00 | 0.00 |
| Extracapsular extraction of lens | 324,345 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.5 (1.4 to 1.5) | 0.00 | 0.00 |
| Endoscopic extirpation of lesion of bladder | 42,163 | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.5 (1.4 to 1.6) | 0.00 | 0.00 |
| Primary repair of umbilical hernia | 24,529 | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | 1.4 (1.4 to 1.5) | 0.00 | 0.00 |
| Excision of salivary gland | 5397 | 0.1 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.4 (1.3 to 1.6) | 0.00 | 0.00 |
| Opening of skin | 41,869 | 0.1 (0.1 to 0.2) | 0.2 (0.2 to 0.3) | 1.4 (1.4 to 1.5) | 0.00 | 0.00 |
| Elective caesarean delivery | 65,316 | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | 1.4 (1.4 to 1.5) | 0.00 | 0.00 |
| Other exteriorisation of colon | 12,983 | 0.1 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.4 (1.3 to 1.6) | 0.00 | 0.00 |
| Excision of other fascia | 14,913 | 0.1 (0.1 to 0.2) | 0.3 (0.3 to 0.4) | 1.4 (1.3 to 1.5) | 0.00 | 0.00 |
| Unilateral excision of adnexa of uterus | 16,515 | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | 1.4 (1.3 to 1.5) | 0.00 | 0.00 |
| Plastic repair of aortic valve | 9333 | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | 1.4 (1.3 to 1.5) | 0.00 | 0.00 |
| Operations on hydrocele sac | 7251 | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | 1.4 (1.3 to 1.5) | 0.00 | 0.00 |
| Other operations on pilonidal sinus | 7625 | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | 1.4 (1.3 to 1.5) | 0.00 | 0.00 |
| Excision of pilonidal sinus | 5603 | 0.1 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 1.4 (1.3 to 1.6) | 0.00 | 0.00 |
| Simple excision of inguinal hernial sac | 7309 | 0.1 (0.1 to 0.2) | 0.2 (0.2 to 0.3) | 1.4 (1.2 to 1.5) | 0.00 | 0.00 |
| Abdominal excision of uterus | 31,615 | 0.1 (0.1 to 0.1) | 0.2 (0.2 to 0.2) | 1.4 (1.3 to 1.4) | 0.00 | 0.00 |
| Puncture of pleura | 49,824 | 0.1 (0.1 to 0.1) | 0.2 (0.1 to 0.2) | 1.3 (1.3 to 1.4) | 0.00 | 0.00 |
| Other placement of testis in scrotum | 6537 | 0.1 (0.1 to 0.1) | 0.1 (0.1 to 0.2) | 1.3 (1.2 to 1.5) | 0.00 | 0.00 |
| Bilateral excision of adnexa of uterus | 30,474 | 0.1 (0.1 to 0.1) | 0.2 (0.2 to 0.2) | 1.3 (1.3 to 1.4) | 0.00 | 0.00 |
| Primary repair of incisional hernia | 9652 | 0.1 (0.1 to 0.1) | 0.1 (0.1 to 0.2) | 1.3 (1.2 to 1.4) | 0.00 | 0.00 |
| Primary repair of inguinal hernia | 68,526 | 0.1 (0.1 to 0.1) | 0.1 (0.1 to 0.2) | 1.3 (1.3 to 1.4) | 0.00 | 0.00 |
| Other caesarean delivery | 95,278 | 0.1 (0.1 to 0.1) | 0.2 (0.2 to 0.2) | 1.3 (1.3 to 1.4) | 0.00 | 0.00 |
| Total excision of breast | 17,750 | 0.1 (0.1 to 0.1) | 0.2 (0.1 to 0.2) | 1.3 (1.2 to 1.4) | 0.00 | 0.00 |
| Excision of gall bladder | 70,993 | 0.1 (0.1 to 0.1) | 0.2 (0.2 to 0.3) | 1.3 (1.3 to 1.4) | 0.00 | 0.00 |
| Primary repair of tendon | 22,474 | 0.1 (0.1 to 0.1) | 0.1 (0.1 to 0.2) | 1.3 (1.2 to 1.4) | 0.00 | 0.00 |
| Excision of rectum | 15,341 | 0.1 (0.1 to 0.1) | 0.2 (0.1 to 0.2) | 1.3 (1.2 to 1.4) | 0.00 | 0.00 |
| Drainage through perineal region | 12,656 | 0.1 (0.1 to 0.1) | 0.2 (0.1 to 0.2) | 1.3 (1.2 to 1.4) | 0.00 | 0.00 |
| Total excision of kidney | 6735 | 0.1 (0.1 to 0.1) | 0.1 (0.1 to 0.2) | 1.2 (1.1 to 1.4) | 0.00 | 0.00 |
| Other excision of right hemicolon | 11,165 | 0.1 (0.0 to 0.1) | 0.1 (0.0 to 0.1) | 1.0 (1.0 to 1.2) | 0.00 | 0.00 |
Appendix 2 Search terms used for carpal tunnel release review
MEDLINE
URL: http://ovidsptx.ovid.com/
Date range searched: 1950 to present.
Date of search: 18 January 2012.
Search strategy
-
randomized controlled trial.pt. (316,214)
-
(randomized or randomised).ab,ti. (282,509)
-
placebo.ab,ti. (130,897)
-
dt.fs. (1,490,078)
-
randomly.ab,ti. (160,982)
-
trial.ab,ti. (274,934)
-
groups.ab,ti. (1,082,267)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (2,773,556)
-
exp animals/ (15,618,000)
-
exp humans/ (11,977,239)
-
9 not (9 and 10) (3,640,761)
-
8 not 11 (2,353,235)
-
Carpal Tunnel Syndrome.mp. or Carpal Tunnel Syndrome/ (7170)
-
(carp$ tunn$ or tunn$ syndrom$ or carp$ syndrom$).mp. (8672)
-
(nerve entrapment or nerve compression or entrapment neuropath$).mp. (10,579)
-
median nerve entrapment.mp. (92)
-
nerve compression syndromes/ or nerve compression syndrom$.mp. (9137)
-
or/13-17 (17,977)
-
epineurotomy.mp. (35)
-
reconstruct$.mp. (152,873)
-
release.mp. (372,093)
-
SURGERY/ or surgery.mp. (679,668)
-
neurosurgery/ (11,727)
-
SURGICAL PROCEDURES, OPERATIVE/ or surgical.mp. (789,562)
-
operat$.mp. (670,857)
-
19 or 20 or 21 or 22 or 23 or 24 or 25 (2,014,211)
-
18 and 26 (6826)
-
12 and 27 (797)
EMBASE on Ovid
Date range searched: 1980 to 2012 Week 2.
Date of search: 18 January 2012.
Search strategy
-
Carpal Tunnel Syndrome.mp. or Carpal Tunnel Syndrome/ (9992)
-
(carp$ tunn$ or tunn$ syndrom$ or carp$ syndrom$).mp. (12,406)
-
(nerve entrapment or nerve compression or entrapment neuropath$).mp. (11,509)
-
1 or 2 or 3 (21,802)
-
epineurotomy.mp. or carpal tunnel release/ (81)
-
nerve compression/ (9898)
-
surgical approach/ or surgical technique/ or nerve surgery/ (264,182)
-
(surgery or surgical or operation or reconstruct$).mp. (1,968,769)
-
5 or 6 or 7 or 8 (1,974,934)
-
Randomized Controlled Trial/ (295,607)
-
Clinical Trial/ (823,118)
-
Multicenter Study/ (86,014)
-
Controlled Study/ (3,671,242)
-
Crossover Procedure/ (31,644)
-
Double Blind Procedure/ (102,550)
-
Single Blind Procedure/ (14,668)
-
exp RANDOMIZATION/ (55,456)
-
Major Clinical Study/ (1,563,506)
-
PLACEBO/ (191,051)
-
Meta Analysis/ (58,505)
-
phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ (42,895)
-
(clin$ adj25 trial$).tw. (259,385)
-
((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. (138,179)
-
placebo$.tw. (164,030)
-
random$.tw. (677,054)
-
control$.tw. (2,484,239)
-
(meta?analys$ or systematic review$).tw. (40,103)
-
(cross?over or factorial or sham? or dummy).tw. (119,732)
-
ABAB design$.tw. (104)
-
or/10-29 (6,557,827)
-
human/ (12,776,609)
-
nonhuman/ (3,772,499)
-
31 or 32 (15,850,473)
-
30 not 33 (864,912)
-
30 and 31 (4,084,918)
-
34 or 35 (4,949,830)
-
4 and 9 and 36 (3065)
Cochrane Central Register of Controlled Trials
URL: www.thecochranelibrary.com
Date range searched: from inception until 19 January 2012.
Date of search: 19 January 2012.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor Carpal Tunnel Syndrome explode all trees | 342 |
| #2 | “carpal tunnel syndrome” | 512 |
| #3 | (carp* tunn* ) or (tunn* syndrom*) or (carp* syndrom*) | 955 |
| #4 | (nerve entrapment) or (nerve compression) or (entrapment neuropath*) | 422 |
| #5 | (median nerve entrapment) | 30 |
| #6 | MeSH descriptor Nerve Compression Syndromes explode all trees | 441 |
| #7 | (nerve compression syndrom*) | 235 |
| #8 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) | 1304 |
| #9 | (epineurotomy) | 11 |
| #10 | (reconstruct*) | 4100 |
| #11 | (release) | 21,405 |
| #12 | MeSH descriptor General Surgery explode all trees | 266 |
| #13 | MeSH descriptor Surgical Procedures, Operative explode all trees | 81,940 |
| #14 | MeSH descriptor Neurosurgery explode all trees | 81 |
| #15 | (surgical) | 41,845 |
| #16 | surgery | 94,100 |
| #17 | (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) | 151,411 |
| #18 | (#8 AND #17) | 825 |
Appendix 3 Details of studies excluded from the carpal tunnel release review
| Reference | Reason for exclusion |
|---|---|
| Carpal tunnel syndrome: splinting versus surgery. Med Today 2002;3:7 | Commentary |
| Steroid injections vs. surgery for treating carpal tunnel syndrome. Mayo Clin Womens Healthsource 2005;9:3 | Commentary |
| Al-Khuraibet AJ. Response. Evid Based Med 2008;13:38 | Not a RCT |
| Anonymous. Steroid injection equivalent to surgery for carpal tunnel syndrome. J Fam Pract 2005;54:401 | Commentary |
| Demirci S, Kutluhan S, Koyuncuoglu HR, Kerman M, Heybeli N, Akkus S, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33–7 | Groups not allocated at random |
| Elwakil TF, Elazzazi A, Shokeir H. Treatment of carpal tunnel syndrome by low-level laser versus open carpal tunnel release. Lasers Med Sci 2007;22:265–70 | Groups not allocated at random |
| Kiylioglu N, Bicerol B, Ozkul A, Akyol A. Natural course and treatment efficacy: one-year observation in diabetic and idiopathic carpal tunnel syndrome. J Clin Neurophysiol 2009;26:446–53 | Groups not allocated at random |
| Pomerance J, Zurakowski D, Fine I. The cost-effectiveness of nonsurgical versus surgical treatment for carpal tunnel syndrome. J Hand Surg 2009;34:1193–200 | Not a RCT |
| Roitberg B. A randomized trial of splinting vs. surgery for carpal tunnel syndrome. Surg Neurol 2003;59:5–6 | Commentary |
| Sorensen AI, Boeckstyns M, Nielsen NS, Haugegard M. Carpal tunnel release, a comparison of 3 methods: a preliminary report [Abstract]. Acta Orthop Scand 1997;68(Suppl. 277):24–5 | Open vs. endoscopic surgery |
| Stanek IEJ, Pransky G. Unilateral vs. bilateral carpal tunnel: challenges and approaches. Am J Ind Med 1996;29:669–78 | Not a RCT |
| Weber RA, DeSalvo DJ, Rude MJ. Five-year follow-up of carpal tunnel release in patients over age 65. J Hand Surg 2010;35:207–11 | Not a RCT |
Appendix 4 Table of studies included in the carpal tunnel release review
| Study ID | References to published papers |
|---|---|
| Garland 1964 | Garland H, Langworth EP, Taverner D, Clark JMP. Surgical treatment for carpal tunnel syndrome. Lancet 1964;1:1129–30122 |
| Gerritsen 2002 | aGerritsen AAM, de Vet HCW, Scholten RJPM, Bertelsmann FW, de Krom MCTFM, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245–51124 Gerritsen AA, de Vet HC, Scholten RJ, Barbano R. Surgery was associated with greater long term treatment success than wrist splinting in carpal tunnel syndrome: commentary. Evid Based Med 2003;8:55204 bGerritsen AA, Scholten RJ, Assendelft WJ, Kuiper H, de Vet HC, Bouter LM. Splinting or surgery for carpal tunnel syndrome? Design of a randomized controlled trial [ISRCTN18853827]. BMC Neurol 2001;1:8205 Gerritsen AAM, de Vet HCW, Scholten R, Bertelsmann FW, De Krom M, Bouter LM. [Greater clinical effects on carpal tunnel syndrome with surgery than with splinting; results of a randomised clinical trial.] Ned Tijdschr Geneeskunde 2002;146:2153–6206 Korthals-de Bos IBC, Gerritsen AAM, Van Tulder MW, Rutten-Van Molken MPMH, Ader HJ, de Vet HCW, et al. Surgery is more cost-effective than splinting for carpal tunnel syndrome in the Netherlands: Results of an economic evaluation alongside a randomized controlled trial. BMC Musculoskelet Disord 2006;7:86125 |
| Hui 2005 | aHui ACF, Wong S, Leung CH, Tong P, Mok V, Poon D, et al. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology 2005;64:2074–8119 |
| Jarvik 2009 | aJarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet 2009;374:1074–81117 bMartin BI, Levenson LM, Hollingworth W, Kliot M, Heagerty PJ, Turner JA, et al. Randomized clinical trial of surgery versus conservative therapy for carpal tunnel syndrome [ISRCTN84286481]. BMC Musculoskelet Disord 2005;6:2207 |
| Ly-Pen 2005 | aLy-Pen D, Andreu J-L, de Blas G, Sanchez-Olaso A, Millan I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum 2005;52:612–19123 Andreu JL, Ly-Pen D. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome [7]. Neurology 2006;66:955–6208 Andreu JL, Ly-Pen D. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome: author reply. Neurology 2006;66:955–6209 |
| Ucan 2006 | aUcan H, Yagci I, Yilmaz L, Yagmurlu F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and open carpal tunnel release outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2006;27:45–51118 |
Appendix 5 Details of studies included in the carpal tunnel release review
| Study ID | Setting and sample size | Inclusion criteria/exclusion criteria | Baseline characteristics | Interventions (number of arms) | Follow-up and key outcomes |
|---|---|---|---|---|---|
| Garland et al. 1964122 | UK n = 22 patients |
Inclusion criteria: suspected CTS; electromyography median nerve conduction times > 4.5 ms; clinical assessment by neurologist Exclusion criteria: none described |
Age: mean 47 years Female: 100% female Duration of symptoms: mean 3.5 years Bilateral symptoms: 73% |
Two treatment arms Surgery: open operation of the anterior carpal ligament performed by one surgeon Splinting: plaster of Paris splinting of the hand, wrist and arm for 1 month |
Follow-up: ‘Reviewed at regular intervals for up to 1 year’ (p. 1129) Outcomes: ‘completely relieved of symptoms’; ‘motor and sensory nerve conduction . . . returned to normal’ (p. 1129) |
| Gerritsen et al. 2002124 | Netherlands n = 176 patients |
Inclusion criteria: clinically suspected CTS; pain, paraesthesia and or hypoesthesia in the area innervated by the median nerve; electrophysiological confirmation of CTS (median SCV ≤ 41.9 m/s or ≤ 37.3 m/s in patients < 55 years or > 55 years respectively or median DSL ≥ 3.5 ms or median ulnar DSL difference of >0.4 ms or median DML of ≥ 4.34 ms); ≥ 18 years old; able to complete questionnaires Exclusion criteria: previous surgery or splinting; wrist trauma; underlying causes of CTS (e.g. diabetes mellitus or pregnancy); signs or symptoms of disorders that could mimic CTS (e.g. cervical radiculopathy polyneuropathy); or severe thenar muscle atrophy |
Age: mean 49 years (surgery); 49 years (splint) Female: 76% (surgery); 87% (splint) Duration of symptoms: median 40 weeks (surgery); 52 weeks (splint) Bilateral symptoms: 55% (surgery); 63% (splint) DSL (index finger): mean 4.2 ms (surgery); 4.1 ms (splint) Median ulnar DSL difference: mean 1.7 ms (surgery); 1.8 ms (splint) DML: mean 5.6 ms (surgery); 5.7 ms (splint) |
Two treatment arms If bilateral the hand with more severe symptoms was treated Surgery: open CTR. The transverse carpal ligament was released and no concomitant procedures were performed (e.g. flexor tenosynovectomy, internal neurolysis, epineurotomy,) Sutures were removed after 2 weeks Splinting: custom-made soft-cast splint or prefabricated splint that immobilised the hand in a neutral position. To be worn at night for at least 6 weeks. Optional to also wear during the day Concomitant pain medication was allowed |
Follow-up: 1, 3, 6, 12 and 18 months Outcomes: patient self-assessed ‘completely recovered’ or ‘much improved’ on a six-point scale of general improvement; severity of symptoms on 11-point scale; number of nights awoke because of symptoms in past week; Symptom Severity Scale; Functional Status Scale; physiotherapist assessment of severity of CTS on 11-point scale; nerve conduction studies |
| Hui et al. 2005119 | Hong Kong n = 50 patients |
Inclusion criteria: diagnosis of CTS of > 3 months but < 1 year; median ulnar palmar sensory latency difference > 0.5 ms OR median DML > 4 ms Exclusion: thenar atrophy or unelicitable DML; ulnar, radial neuropathy or proximal median neuropathy; coexisting disorders that mimic CTS (e.g. brachial plexopathy, cervical radiculopathy); contraindication to steroid use; diabetes mellitus, wrist trauma, rheumatoid arthritis, acromegaly, hypothyroidism and pregnancy |
Age: mean 51 years (surgery); 48 years (injection) Female: 96% (surgery); 96% (injection) Duration of symptoms: not reported Bilateral symptoms: not reported DML: mean 5.4 ms (surgery); 4.8 ms (injection) SCV: mean 34.2 m/s (surgery); 37.3 m/s (injection) |
Two treatment arms Surgery: open operation of the anterior carpal ligament performed by one surgeon under local anaesthetic Injection: one injection of steroid delivered by one physician. 15 mg methylprednisalone acetate was injected into the carpal tunnel via a 25-mg needle. Other interventions such as oral NSAIDS and splinting were discouraged |
Follow-up: 6 and 20 weeks Outcomes: global symptom score; electrophysiological measures; grip strength Additional data from this study were obtained from the authors by a previous Cochrane review120 |
| Jarvik et al. 2009117 | USA n = 116 patients |
Inclusion criteria: symptoms ≥ 2 weeks; classic probable or possible CTS on hand pain diagram; failure of ≥ 2 weeks of non-surgical treatment, including wrist splints; night pain and a positive flick test or electrophysiological criteria [median DML wrist ≥ 4.4 ms, median ulnar DSL (14 cm) difference > 0.4 ms, median ulnar DSL (8 cm) difference > 0.3 ms, median radial DSL difference > 0.5 ms, or combined sensory index ≥ 1.0 ms]; able to complete questionnaires and telephone interviews; plan to stay in the region for 12 months; age ≥ 18 years; willingness to undergo surgery soon after randomisation Exclusion criteria: severe CTS (thenar atrophy or abnormal two-point discrimination); previous CTR surgery on the study hand; any wrist or hand surgery < 6 months ago; moderate to severe arthritis of study hand; tumour or deformity of wrist; severe trauma to the study hand; current pregnancy or lactation; diffuse peripheral neuropathy or cervical radiculopathy If participant had two affected hands, the hand with more severe symptoms was randomised into the trial |
Age: mean 50 years (surgery); 51 years (non-surgical) Female: 49% (surgery); 58% (non-surgical) Duration of symptoms: 3.2 years (surgery); 3.4 years (non-surgical) Bilateral symptoms: 67% (surgery); 53% (non-surgical) DML: mean 5.2 ms (surgery); 5.3 ms (non-surgical) Median ulnar DSL difference: mean 0.6 ms (surgery); 1.0 ms (non-surgical) Median motor amplitude: mean 7.8 mV (surgery); 7.9 mV (non-surgical) |
Two treatment arms Surgery: open or endoscopic dependent on the surgeon’s preference. All surgeons had at least 10 years’ experience and practices focusing on CTS. Surgery was followed by routine hand therapy Non-surgical therapy: patients were offered NSAIDS (ibuprofen 200 mg three times per day), customised hand-therapy sessions (six visits over 6 weeks), hand booklet, hand exercises. Hand therapy sessions on ligament stretching, tendon gliding, review of splint use. Advice to splint at night and during the day as tolerated. Advice to modify work and other hand-related activities. Patients were asked to return 6 weeks after randomisation and if symptoms had improved no changes were made to therapy. Ultrasound, at 1 MHz 1.0 W/cm2 in pulsed mode 1 : 4, was offered to those whose symptoms had not improved (up to 12 sessions, two to four per week for up to 6 weeks). Patients were asked not to have surgery for 3 months. At 3 months after randomisation, if patients were improved they were asked to continue the regime; if they had not improved they were offered surgery |
Follow-up: 6 weeks and 3, 6, 9, and 12 months Outcomes: function and symptoms on the CTS Assessment Questionnaire; wrist pain intensity on an 11-point scale; days of work lost; days of limited activity; SF-36 |
| Ly-Pen et al. 2005123 | Spain n = 163 wrists |
Inclusion criteria: ≥ 18 years old; CTS ≥ 3 months; Unresponsive to 2 weeks of oral NSAIDs and splinting; (median DML > 4.2 ms; median ulnar DML difference > 1.4 ms; median SCV < 44 m/s; or median ulnar SCV difference > 0.7 m/s) Exclusion criteria: thenar atrophy, previous CTR surgery or injection of steroid, pregnancy, hypothyroidism, inflammatory arthropathy or polyneuropathy |
Age: mean 51 years (surgery); 53 years (injection) Female: 92% Duration of symptoms: mean 31 weeks (surgery); 33 weeks (injection) Bilateral symptoms: 43% (surgery); 69% (injection) |
Two treatment arms Surgery: open operation. Limited palmar incision. All performed by the same surgeon, n = 80 Injection of steroid: one or two injections of steroid. All injections delivered by one investigator. Steroid (paramethasone acetonide 20 mg in 1 ml) injected beneath the transverse carpal tunnel ligament from the ulnar side of the wrist. Second injection after 14 days if night parasthesias have not completely gone |
Follow-up: 3, 6 and 12 months Outcomes: symptom (nocturnal paraesthesia, pain and functional impairment) severity on a 100-mm visual analogue scale |
| Ucan et al. 2006118 | Turkey n = 67 hands |
Inclusion criteria: CTS > 6 months; nerve conduction studies indicating mild or moderate CTS Exclusion criteria: advanced CTS; thenar atrophy, underlying disorders (e.g. diabetes); distal radial fracture; pregnancy; prior CTS treatment |
Age: mean 45 years (surgery); 45 years (splint); 44 years (splint/injection) Female: approximately 93% Duration of symptoms: 21 months (surgery); 15 months (splint); 19 months (splint/injection) Bilateral symptoms: unclear DML: mean 4.5 ms (surgery); 4.1 ms (splint); 4.1 ms (splint/injection) SCV: mean 33.5 m/s (surgery); 35.4 m/s (splint); 34.1 m/s (splint/injection) |
Three treatment arms Surgery: open surgery. Flexor retinaculum was sectioned completely with a short incision. The epineurium was left intact if it was not hypertrophic Splinting: hands were splinted in neutral position with standard cotton-polyester splint for use at night and where possible during the day for 3 months Splinting and steroid injection: splinting as above plus injection of 20 mg triamcinolone acetonid and 20 mg lidocaine mixture into the transverse carpal ligament |
Follow-up: 3 and 6 months Outcomes: Boston Questionnaire;210 electrophysiological measures; patient satisfaction |
Appendix 6 Search terms used for the capsulotomy review
MEDLINE
URL: http://ovidsptx.ovid.com/
Date range searched: 1950 to present (from inception to 25 November 2011).
Date of search: 25 November 2011.
Search strategy
-
randomized controlled trial.pt. (322,599)
-
controlled clinical trial.pt. (84,057)
-
randomized.ab. (227,373)
-
placebo.ab. (130,354)
-
drug therapy.fs. (1,520,338)
-
randomly.ab. (163,568)
-
trial.ab. (235,465)
-
groups.ab. (1,082,545)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (2,816,687)
-
exp animals/ not humans.sh. (3,722,514)
-
9 not 10 (2,390,384)
-
exp cataract extraction/ (25,710)
-
(phacoemulsificat$ or capsulorhexis).tw. (5506)
-
((extract$ or surg$) adj3 cataract$).tw. (16,486)
-
exp lenses, intraocular/ (11,795)
-
exp lens implantation, intraocular/ (6718)
-
exp lens capsule crystalline/ (3514)
-
12 or 13 or 14 or 15 or 16 or 17 (35,557)
-
(posterior adj3 capsul$ adj3 opaci$).tw. (1323)
-
PCO.tw. (3164)
-
aftercataract.tw. (20)
-
secondary cataract$.tw. (402)
-
capsulotom$.tw. (2078)
-
((Nd or Neodymium) adj3 YAG).tw. (2188)
-
19 or 20 or 21 or 22 or 23 or 24 (7817)
-
11 and 18 and 25 (493)
EMBASE on Ovid
Date range searched: from inception to 25 November 2011.
Date of search: 25 November 2011.
Export format to EndNote (Thomas Reuters, CA, USA).
Import EMBASE (Ovid SP).
Database: Embase 1980 to 2011 Week 46.
Search strategy
-
exp randomized controlled trial/ (292,701)
-
exp randomization/ (54,986)
-
exp double blind procedure/ (101,701)
-
exp single blind procedure/ (14,442)
-
random$.tw. (665,175)
-
1 or 2 or 3 or 4 or 5 (779,881)
-
(animal or animal experiment).sh. (3,122,387)
-
human.sh. (12,650,241)
-
7 and 8 (504,084)
-
7 not 9 (2,618,303)
-
6 not 10 (707,167)
-
exp clinical trial/ (872,064)
-
(clin$ adj3 trial$).tw. (215,171)
-
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (132,889)
-
exp placebo/ (187,497)
-
placebo$.tw. (161,437)
-
random$.tw. (665,175)
-
exp experimental design/ (6601)
-
exp crossover procedure/ (31,195)
-
exp control group/ (27,882)
-
exp latin square design/ (189)
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (1,452,360)
-
22 not 10 (1,370,140)
-
23 not 11 (671,160)
-
exp comparative study/ (881,702)
-
exp evaluation/ (164,837)
-
exp prospective study/ (176,527)
-
(control$ or prospectiv$ or volunteer$).tw. (2,872,425)
-
25 or 26 or 27 or 28 (3,725,532)
-
29 not 10 (3,106,375)
-
30 not (11 or 23) (2,495,266)
-
11 or 24 or 31 (3,873,593)
-
exp cataract extraction/ (30,724)
-
(phacoemulsificat$ or capsulorhexis).tw. (6653)
-
((extract$ or surg$) adj3 cataract$).tw. (18,706)
-
lens implantation/ (4322)
-
lens implant/ (15,141)
-
exp lens capsule/ (2937)
-
33 or 34 or 35 or 36 or 37 or 38 (41,455)
-
exp aftercataract/ (315)
-
(posterior adj3 capsul$ adj3 opaci$).tw. (1566)
-
PCO.tw. (2554)
-
aftercataract.tw. (49)
-
secondary cataract$.tw. (462)
-
capsulotom$.tw. (2301)
-
((Nd or Neodymium) adj3 YAG).tw. (2824)
-
40 or 41 or 42 or 43 or 44 or 45 or 46 (8317)
-
32 and 39 and 47 (895)
Cochrane Central Register of Controlled Trials on The Cochrane Library
URL: www.thecochranelibrary.com/view/0/index.html
Date range searched: from inception to 16 November 2011.
Date of search: 16 November 2011.
Search strategy
#1 (MeSH descriptor Cataract Extraction) 91
#2 phacoemulsificat* or capsulorhexis 1610
#3 cataract* NEAR/3 (extract* or surg*) 3374
#4 MeSH descriptor Lenses, Intraocular explode all trees 777
#5 MeSH descriptor Lens Implantation, Intraocular explode all trees 779
#6 MeSH descriptor Lens Capsule, Crystalline explode all trees 223
#7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) 3924
#8 MeSH descriptor Posterior Capsule of the Lens explode all trees 3
#9 posterior NEAR/3 capsul* NEAR/3 opaci* 228
#10 PCO 363
#11 aftercataract 25
#12 secondary cataract* 386
#13 Nd near3 YAG 502
#14 Neodymium near3 YAG 101
#15 capsulotom* 247
#16 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) 1369
#17 (#7 AND #16) 564
Database of Abstracts of Reviews of Effects
URL: www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 14 November 2011.
Date of search: 15 November 2011.
Search strategy
-
MeSH DESCRIPTOR cataract extraction EXPLODE ALL TREES 21
-
MeSH DESCRIPTOR lenses, intraocular EXPLODE ALL TREES 9
-
MeSH DESCRIPTOR lens implantation, intraocular EXPLODE ALL TREES 9
-
(phacoemulsificat*) OR (capsulorhexis) 19
-
(extract* adj3 cataract) OR (surg* adj3 cataract) 19
-
#1 OR #2 OR #3 OR #4 OR #5 45
-
(posterior adj3 capsule* adj3 opaci*) 8
-
(PCO) 5
-
(aftercataract) 0
-
(secondary cataract) 0
-
#7 OR #8 OR #9 OR #10 10
-
#6 AND #11 10
-
#6 AND #11 10
Appendix 7 Details of studies excluded from the capsulotomy review
| Study ID | Reason for exclusion |
|---|---|
| Mansour AM. Treatment of dense aftercataract with a new intraocular drill. Ann Ophthalmol 1988;20:78–9 | Observational |
| Sergienko, NM, Pavliuchenko KP. [A method of treatment of secondary cataract in artiphakia.] Vestn Oftalmol 1989;105:23–5 | Not English |
| Chen F, Li RC, Wang J. [Effect of the different diameter of posterior capsulotomy on visual field.] Zhonghua yan ke za zhi 2003;39:294–7 | Not English |
| Alpar JJ. Experiences with the neodymium: YAG laser: interruption of anterior hyaloid membrane of the vitreous and cystoid macular edema. Ophthalmic Surg 1986;17:157–65 | RCT compares two different YAG lasers |
| Yilmaz S, Ozdil MA, Bozkir N, Maden A. The effect of Nd:YAG laser capsulotomy size on refraction and visual acuity. J Refract Surg 2006;22:719–21 | Compares two different types of surgery |
| Neumayer T, Buehl W, Findl O. Effect of topical prednisolone and diclofenac on the short-term change in morphology of posterior capsular opacification. Am J Ophthalmol 2006;142:550–6 | Non-surgery vs. non-surgery |
Appendix 8 Topic guides used in the semistructured interviews with commissioners, providers and patients
Skeleton topic guide: commissioners’ and providers’ interviews
Researcher explains research process (including reminding participant of confidentiality), takes consent, verbally confirms permission to record, and switches on recorder.
Initial preamble: background of participant
Could we start by you just telling me a little bit about yourself, and what you do?
Probe: what’s their educational background/what roles have they had in the past/how did they become a member of the commissioning group/what is their role within the commissioning group.
The role of the commissioning group, and the impact of funding constraints
Can you explain what the CAF [Commissioning Advisory Forum]/CPG [clinical priorities group] does? Explore their definitions of priority setting and the processes involved.
Are there any challenges involved in this? Explore: can these be overcome? Do they overcome these? How could they be overcome?
(If not yet raised) Has the current economic climate had an impact on the way funding decisions are made? Explore: how; if answer ‘no’, probe further about NHS funding constraints, and what it has meant for decision-makers/how they have responded.
Do you think there will be any commissioning challenges in the coming years? Explore: what, how, why?
Will commissioners need to do things differently over the coming years? Explore: why.
[If so] What do you think they/you should do?
Previous experiences of/attitudes towards disinvestment
What does disinvestment mean to you?
How do you feel about disinvesting from health procedures of low clinical value? Explore: is this a viable solution to being able to fund new treatments? What other methods could be used?
Do you have any experience of disinvesting? If so, what processes/methods have you used? Explore methods: what do they involve, etc. (Partial disinvestment, full, etc.)
What factors/which groups did you need to consider whilst engaging in disinvestment? Probe: patient perspectives sought? Where was evidence obtained from? Other stakeholders, etc.
Did the methods work? Explore: why/why not/what should be done differently?
What do you think are the best ways of saving money? Explore alternatives; probe for other ways of saving money (e.g. changing the way services are delivered, where delivered, by whom, etc.)
Expectations of the proposed disinvestment method
Have you heard of the disinvestment method suggested by the UoB [University of Bristol] research team? If no, introduce the method in lay terms
What do you understand from it? If necessary, explain the method in lay terms
Do you think variations in procedure rates are a suitable tool for identifying candidates for disinvestment? Explore: why/why not.
How would you define a successful disinvestment method? Can they give examples? Explore: ideas surrounding saving money, ‘fairness’, quality of healthcare, clinical consequences.
Do you expect this disinvestment method to succeed? Explore: why/why not?
What do you think the barriers to success might be? Probe: difficulties of changing established practice, etc.
How do you think we can overcome these barriers?
What impact do you think this disinvestment process will have on patients, GPs/other stakeholders/the PCT?
Wind down and closure
Researcher thanks informant for their time, asks if there are any additional comments they would like to make. Some of the main points of the interview may be summarised, if appropriate. The researcher may use this opportunity to check and confirm certain ideas/impressions built up during the interview.
Skeleton topic guide for clinician interviews
Researcher explains research process (including reminding participant of confidentiality), takes consent, verbally confirms permission to record, and switches on recorder.
Initial preamble/background
Could we start by you just telling me a little bit about yourself? Professional role.
Could you give me a brief overview of what surgery for carpal tunnel syndrome (CTS) involves?
Referral
How do you think GPs reach decisions about which CTS patients to refer to secondary care? Are referrals well thought out? Guidelines/policies? Any influence from pat preferences/demands?
What happens after a GP refers a patient to the outpatient clinic?
Do you find that patients have expectations of what may happen? Is this common? Do they expect surgery?
Accessing treatments
How often do patients go on to receive surgery for CTS?
How do you decide on which patients are admitted for surgery?
What happens to patients who don’t get admitted for surgery?
Are there any limitations on your ability to provide this service for patients who need it (in PCT 1/2)? Explore.
What might affect whether a patient gets access to surgery? Explore.
Clinical variation
Do you, or your clinical team, ever look to other trusts to compare clinical practice?
Have you/your team, everlooked at activity rates for CTS surgery? Explore: internal benchmarking or external? Trends over time? Differences btw [between] trusts? Where do they think PCT1/2 might rate in terms of level of activity?
(LR:) The study I am working on has done some benchmarking work to identify secondary care elective procedures that show variation in activity, where this variation is unlikely to be a result of differences in clinical need. We are not sure why variation exists, and do not know what the optimum level of activity is, but we found that the rates of CTS surgery in PCT1/2 are higher than the national average. I was interested in exploring some of your thoughts on this . . .
Does this surprise you? Do you feel there are any explanations as to why PCT1/2’s activity rates for CTS surgery appear to be higher than the national average? Is this acceptable?
Do you think variations in clinical activity matter? Should we be trying to level out activity to a ‘norm’? Explore why/why not.
Role of commissioners
Do you feel commissioners influence demand for CTS surgery in any way? (If yes) How? Why do you think they do this?
If threshold policies mentioned, continue. If not mentioned, ask if clinician is aware these exist. If yes, proceed with following questions. If not, ask if they have heard of threshold policies for any procedures, and adapt following questions.
When did you first become aware of these policies?
What do the policies say?
What do you think the policies are designed to do?
How are the policies enforced? Explore auditing.
Do you believe these policies have impacted your clinical practice in any way? Explore. Also, do policies influence rates of activity?
Do you believe these policies are appropriate? Clinically appropriate? Fair? Would clinician change the policies in any way? Explore.
Do you ever come across situations where the parent wants the child to have surgery, but you have had to say no for any reason? Examples. Explore how clinician felt. Patient reaction. How clinician ended consultation.
(If no to the above) What about any other clinical procedures that may have policies attached?
General views on gate-keeping
Who do you think should have a role in determining which elective surgical treatments NHS patients receive?
On what factors should the decision to have surgery be made?
Draw on some of the issues raised, and ask whether these issues will change with NHS reforms.
Wind down and closure
Researcher thanks informant for their time, asks if there are any additional comments they would like to make. Some of the main points of the interview may be summarised, if appropriate. The researcher may use this opportunity to check certain ideas/impressions built up during the interview.
Skeleton topic guide for patient interviews
Researcher explains research process (including reminding participant of confidentiality), takes consent, verbally confirms permission to record, and switches on recorder.
Background
When did you first start to have problems with your wrist/hand(s)?
How did you deal with this initially? Did they know what was wrong? How? Did they seek any information or advice? Did they try any treatments?
At what stage did you decide to seek medical care for your symptoms? Explore triggers to consult.
Pathway leading to outpatient appointment
Do you remember your first consultation with a GP? Can you talk me through what happened?
If not covered above:
- What did you expect the doctor would do?
- What were you hoping the doctor would do?
- What was the outcome? (including recommendations/treatments)
- Did you feel this decision was made WITH you? (Probe for their involvement/did they express what they wanted/preferences? Why/why not?)
- Did you understand why this decision was made?
What happened after this consultation?
- Did you follow recommendations/advice/take prescribed treatment? Did this help?
- Did the types/intensity/frequency of symptoms change? If so, how?
Thinking about the GP consultation that resulted in your outpatient referral to hospital . . .
- What triggered this consultation?
- Can you talk me through what happened during the consultation? Allow participant to describe the consultation feely.
If not covered above:
- What were you hoping for?
- Why do you think you were referred? Why now? Why not before? (if relevant)
- Did you expect to be referred? Explore what may have created these expectations.
- How did you feel about being referred? Explore.
- Did you feel your views and preferences were taken into account before reaching this outcome?
Outpatient appointment
How many outpatient appointments have you had?
What did you expect from the (first) hospital outpatient appointment? Distinguish between what they expected would happen during appointment, and what outcome they expected. Explore what might have influenced these expectations.
Can you talk me through what actually happened at the hospital outpatient appointment?
Did the hospital doctor discuss with you any tests on your wrist which might help decide which treatment was best?
Did the hospital doctor discuss with you what types of treatment might be best for you? What was discussed? Probe: Surgery? Different types of surgery? Injections? Hand exercises? Other?
What was the outcome? If no surgery, explore if consultant made recommendations. Did patient understand reasons behind decision, and what was going to happen?
Were you happy with this decision? Why/why not?
Did you feel your views and preferences were taken into account?
If multiple outpatient appointments, repeat.
Post-outpatient appointment
(If receiving/received surgery)
How long is it since you received surgery?
How do you feel the surgery went?
Are you glad you received surgery?
Have there been any down sides to undergoing surgery?: Do the benefits outweigh the disadvantages? Informed of disadvantages?
(If not receiving surgery)
How did you feel about not being offered surgery?
What did you do after your outpatient appointment? Did they follow recommendations? Access other avenues of care?
Do you still experience symptoms? If not, when did symptoms reside? What does patient attribute this to? If yes, have symptoms changed in intensity, type, etc.?
(If so) Do you have any plans to seek medical care again/try something different? Explore expectations and what participant hopes to achieve.
Views surrounding access to care
Who do you think was the most influential when it came to deciding whether you would receive surgery? Probe: acceptable? Who should have this responsibility?
If you were to decide who should be offered carpal tunnel surgery, how do you think you would decide?
Generally speaking, do you think there is a need to make decisions in the NHS about who is eligible for treatment? Why? Explore if patient is aware of finite NHS resources.
(If yes) Who do you think should make these decisions? What kinds of criteria should be considered?
Wind down and closure
Researcher thanks informant for their time, asks if there are any additional comments they would like to make, and ensures the conversation has returned to a topic the patient seemed relatively comfortable discussing prior to interview closure.
Appendix 9 Additional details from the qualitative interviews
Perspectives on using clinical practice variations to inform disinvestment
The following is based on interviews conducted with commissioners and providers from PCT1 and PCT2.
Participants were generally positive about looking at high-variation/high-use procedures as a starting point to exploring avenues for disinvestment:
It is absolutely right that we should look at those areas that do too much.
Interview, group B, PCT2, P6
Perceived barriers to using clinical practice variations were usually methodological in nature. In particular, participants questioned how useful (and therefore valid) clinical practice variation data can be. Capacity issues also emerged, because of the expected resource-intensive nature of benchmarking exercises.
Methodological barriers
The most commonly cited barrier to looking at clinical practice variations was the validity of benchmarking data. Participants questioned whether or not a PCT’s comparatively high levels of a particular activity reflected reality. Commissioners and providers from both regions discussed the lack of meaningful information that can be derived from coded activity due to inconsistencies in coding and a lack of detail:
If you – if the data was all perfect it would be a really easy world. But the data is not perfect, and sometimes you’re comparing apples with pears.
Interview, group B, PCT2, C8
One [trust] may count pre-admission attendances as outpatients, the others won’t count them at all. Therefore you’ve immediately got a massive discrepancy in the number of outpatient appointments between organisations. Chemotherapy is counted in half the hospitals in the country as a day case, and the other half count them as outpatients. So if you look at the admission rate for cancer, you’ve got a binomial distribution that doesn’t indicate a difference in the way in which patients are treated, but a difference in the way activity is counted
Interview, group A, PCT1, P3
I think [PCT2] was a very high performer of amputations for diabetes patients. What it didn’t show was whether we are chopping away the odd toe at the outset so that they don’t lose the whole of their legs, you know.
Oh I see. It’s not detailed.
It doesn’t give us that detail.
Interview, group B, PCT2
One provider (P3) felt that these inconsistencies in coding exercises made the concept of ‘external benchmarking’ completely redundant. P3 felt there was more that could be learnt from ‘internal benchmarking’ exercises, where one would be comparing ‘like with like’:
So if you start to look at variation between organisations or between health communities, you’ve got a massive problem on the comparability of data. Which is why it’s always more valuable to start by looking internally, where you’re at least assured of the comparability.
Interview, group A, PCT1, P3
Need for in-depth analysis: explanations for variation
There was acceptance that differences in referral guidelines could lead to variations in activity. This, as expressed by some participants, was not surprising given the subjective nature of local decision-making:
We don’t know whether it is historical practice, or whether they have got a different threshold, or they have a different culture in the particular organisation that they’re in.
Interview, group A, PCT1, C1
[T]here will always be a level of subjectivity in decision-making. Otherwise we wouldn’t have variations in PCT decision-making, would we?
Interview, group A, PCT1, C1
However, there were numerous other explanations for variation. One provider suggested that a locality displaying high activity may have a higher concentration of certain clinical skills:
Because it’s quite easy for them to pull off data and say ‘Why are you doing this so much, when other places aren’t?’ That’s probably partly related to what clinical skills you have available.
Interview, group A, PCT1, P2
Another commissioner suggested that high activity levels could be explained by specific local factors that facilitate cheaper ways of performing activity (C9). For example, one of the study sites used trainee dentists (at a teaching hospital) to perform a proportion of wisdom tooth extractions. Differences in settings of care were also thought to contribute to variation. As pointed out by one commissioner, this explanation for variation would still raise questions over the optimal setting for providing the service in question:
For example, if you’re looking at a hospital only procedure, they will say ‘Oh, this is all different because we are doing – we have no community services at all, there are some other places they are doing a lot, but you are not picking them up because you are only picking up the hospital things, you are not picking up the community ones’. Because, that could be a very valid reason why we are high on that. Having said that, that might not be the right place to do it.
Interview, group A, PCT1, C1
Finally, differences in policy implementation were thought to contribute to variation. Speaking from experience, the following commissioner was able to recall examples where the same policy had been implemented in different ways by acute trusts, leading to intra-PCT variation:
When I chaired the subgroup, I tried very hard to get [acute trust 1] and [acute trust 2] to put in joint business cases for Technology Appraisals. And I think we did it once. There was always a reason why they couldn’t work together to do it, for some reason. So I think that is a barrier, where you perhaps get a policy implemented in a different way. So you’ve got somebody who sits on the board between east and west [of region], and that side is implemented in one way, and that side is implemented in a slightly different way. So you’ve got this postcode lottery starting to appear.
Interview, group A, PCT1, C7
This suggests that variations in clinical activity could be explained by differences in interpretation of policies, rather than different referral thresholds.
Should high variation/high activity be addressed?
The real challenge, according to some participants, lies in deciding whether or not variation is warranted. In some cases, benchmarking highly in a particular field of activity was deemed acceptable. For example, participants from both regions (one lay and two commissioners) felt it important to consider the outcomes associated with activity rates that appear to be ‘above average’:
So if you go back to cardiovascular, for example, you might look at mortality under 75 for cardiac disease, and then you’ll spend on cardiac interventions. So on the basis of a single outcome, you might say: well actually our mortality rate is low, so we could get away with spending a bit less here. But you could turn that around and say: well the reason it’s low is because we’re spending so much. So that, you know, that’s a difficulty.
Interview, group A, PCT2, C8
Three participants suggested that there is no way of knowing what the ‘correct’ level of activity is (P3, PCT2; C1, PCT2; C9, PCT1). One provider (P3) felt pointing out inter-PCT differences in activity was not conducive to progress; providers would simply defend their own practices/activity rates:
Although there is a value to external benchmarking, but any organisation and any clinician will – their first response to a benchmarking will be to ‘dis’ the data from other organisations. And you’ll spend your time criticising the data rather than driving the required change in operational practice.
Interview, group A, PCT1, P3
In contrast to the above perspective, other participants felt that highlighting regional variations could encourage clinicians to question and develop their own practices through comparison with other experts in the field:
And it’s been quite surprising that there are some of these variations in what you think ought to be a fairly standard procedure. So yes, I think clinicians, and clinicians especially, can learn from each other. I think everybody gets a bit cloistered in their own comfort zone.
Interview, group A, PCT1, C7
I mean, it always amazes me that we, from an income point of view, from an all sorts of things point of view, we just sort of put up with the system that we have in [PCT], and we maybe complain about the system we have in [PCT], and we – but we don’t really – we don’t very often go off and see what other places do, see what other systems do.
Interview, group A, PCT1, P1
List of abbreviations
- BSSH
- British Society for Surgeons of the Hand
- CBA
- criteria-based access
- CCG
- Clinical Commissioning Group
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CLAHRC
- Collaborations for Leadership in Applied Health Research and Care
- CSU
- commissioning support unit
- CTR
- carpal tunnel release
- CTS
- carpal tunnel syndrome
- CV
- coefficient of variation
- DARE
- Database of Abstracts of Reviews of Effects
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HSCA
- Health and Social Care Act
- HTR
- health technology reassessment
- ICD
- International Classification of Diseases
- IOL
- intraocular lens
- IPD
- individual patient data
- LEC
- lens epithelial cell
- NCS
- nerve conduction study
- Nd:YAG
- neodymium:yttrium–aluminium–garnet
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- OPCS
- Office of Population, Censuses and Surveys
- PBMA
- programme budgeting and marginal analysis
- PCO
- posterior capsule opacification
- PCT
- primary care trust
- QALY
- quality-adjusted life-year
- QIPP
- Quality, Innovation, Productivity and Prevention
- RCOphth
- Royal College of Ophthalmologists
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SDO
- Service Delivery and Organisation
- UR
- utilisation ratio
- VA
- visual acuity