Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5001/67. The contractual start date was in March 2013. The final report began editorial review in May 2014 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Historically, chemotherapy treatment for cancer patients was delivered in hospital. More than 10 years ago, a BMJ editorial noted a shift in chemotherapy practice in the UK from inpatient to outpatient ambulatory therapy. 1 The editorial highlighted a small but growing body of evidence suggesting that chemotherapy in the home was both safe and acceptable, but also identified a need for further exploration of patient selection and cost-effectiveness.
Potential benefits of receiving chemotherapy treatment at home include less travelling to hospital facilities, reduced risk of hospital-acquired infection, receiving treatment in the comfort and security of the home, less disruption to family life and an increased feeling of control over treatment and illness. 2 Potential concerns for patients include increased feelings of isolation, decreased contact with hospital staff (such as specialist nurses) and other patients, feelings of insecurity from a perception of reduced support outside the hospital setting, and the possibility of less continuity of care. 3
Safety is often perceived as being a key issue in the delivery of chemotherapy owing to the toxicity of the drugs, the costs of management of preventable toxicities and the need for specialist skills to administer and monitor treatment. The OUTREACH trial report indicated that clinicians were reluctant to refer patients for home or general practice chemotherapy in part because of patient safety concerns. 4
It is important to ensure that the risks of toxicity are managed by a cohesive multiprofessional team, that problems with toxicity are identified promptly and correctly managed and that concordance with treatment is optimised if outcomes are to be maintained. Severe side effects can be very disturbing and may influence a patient’s decision to continue with treatment; this is true in any setting, but may possibly have a longer term impact on patients when experienced at home. Whatever the treatment setting, severe adverse events mostly occur between treatment-days and so even outpatients experience them outside hospital. Appropriate pre-treatment assessment and patient education are key issues that apply to patients in any setting. Health-care professionals involved with the administration and monitoring of treatment need to have the relevant skills and expertise.
Throughout the NHS there has been an increasing focus on making care more centred on the needs and preferences of patients. 5 In the area of cancer services, the Cancer Reform Strategy has pledged that care will be delivered in the most clinically appropriate and convenient setting for patients. 6 The Department of Health Cancer Policy Team has produced guidance to develop chemotherapy services in the community [such as in general practitioner (GP) surgeries or patients’ homes],3 which builds on best practice guidance provided in the National Chemotherapy Advisory Group report published in 2009. 7 These documents promote the consideration of opportunities to devolve chemotherapy from cancer centres and cancer units to community settings while maintaining safety and quality, and delivering an efficient service. However, a report on how effectively strategies laid out in the Cancer Reform Strategy have been utilised to improve cancer services for patients found a lack of activity in the commissioning of services, with only 26% of primary care trusts having undertaken a cost–benefit analysis looking at different ways of delivering cancer services. 8
These initiatives should be considered within the context of plans in England to reduce the number of centres commissioned for specialised services and focus provision in a smaller number of centres. 9 It is currently unclear if chemotherapy will be included within the definition of a specialised service, or if this will depend on the nature of the cancer being treated. Such a policy change may have implications for where cancer chemotherapy is prescribed and administered, including options for delivery closer to home.
It is likely that many outpatient facilities across England and Wales are delivering chemotherapy services at full capacity (assuming a 15% year-on-year growth in demand), and increasing strain to the service is anticipated. 7 Future demand for services is likely to increase further; increasingly early detection of cancer, improving cancer survival and an ageing population are key factors. For hospitals without the resources to appropriately expand their capacity in terms of either staffing levels or physical space, it is likely that future patients will face longer waiting lists or a reduced service. Delivering chemotherapy closer to the home may enable hospitals to relieve the demand for hospital ward services while maintaining patients’ care.
The various chemotherapy delivery practices used in the UK reflect the different challenges of, for example, large cancer centres and district general hospitals. 10 Nurse-led chemotherapy is well established within the outpatient setting but home and community delivery of chemotherapy is not currently widespread. Different geographic challenges exist for provision in remote and rural communities compared with urban centres. The Department of Health lists exemplars of NHS community chemotherapy services in Sunderland, Dorset, West Anglia, East Anglia and East Kent, and there are health-care companies who undertake chemotherapy in the community, offering services to both private and NHS providers, for example Healthcare at Home (HaH), BUPA Home Healthcare, Baxter, Calea and Alcura. 3
Successful services are likely to be closely tailored to the local requirements and available resources, and as such are expected to vary considerably. For example, Leeds Teaching Hospitals NHS Trust does not provide intravenous chemotherapy at home, at least in part because of the logistics of covering a large and diverse catchment area; patients can receive intravenous chemotherapy in the community (at Otley community hospital) and would attend pre-treatment assessment clinics at St James’s University Hospital in Leeds. Conversely, the Sunderland model covers a relatively small urban area, which allows for a range of services to be provided (Box 1).
In the Sunderland model, intravenous chemotherapy is available across three different settings according to patient choice where eligibility criteria are met (drug is given in short infusions lasting < 5 hours, or as bolus treatment that is not associated with a high risk of anaphylaxis). This model of care has been in operation since 2009 and is entirely provided by the local NHS hospital, covering a 15-mile radius from the main hospital. Initial assessments are carried out by the chemotherapy nurse prior to treatment being scheduled.
From initiation of treatment, patients choose their preferred setting. Bookings are made through a single appointment system, which allows flexibility so that patients can move between locations to suit their schedules.
-
Hospital outpatient (one venue, provided 6 days per week with a Saturday clinic 8.30 a.m. to 2.30 p.m., extended working until 7.00 p.m. midweek, approximately 40 patients per day).
-
Outreach service (one venue, primary care centre provided 3 days per week, approximately 15 patients per day).
-
Patient home (try to group geographically, provided 4 days per week, six to eight patients per day).
Chapter 2 Introduction
Aims
This aim of the project was to investigate the impact of the delivery of intravenous chemotherapy in different settings on quality of life, safety, patient satisfaction and costs. Our focus was the provision of intravenous chemotherapy led and managed from the oncology department and delivered in the patient’s home, in the community or in the hospital outpatient department.
Objectives
The project comprised four elements:
-
a systematic review of the clinical and economic literature to bring together and assess the existing evidence
-
a brief survey to gather information about the structure of services and variation in practice across the NHS
-
a description of the general pathway for patients who will be offered chemotherapy
-
the development of a decision model to compare delivery of chemotherapy for an eligible population in three settings.
In addition to the project team, an advisory group of specialist nurses, pharmacists, and patient representation was formed to help to guide each of the elements from the proposal stage through to the final report. This report details the methods and results for each element, draws together and discusses the findings and identifies the implications for health care and future research.
Chapter 3 Systematic review
Introduction
To provide a complete overview of the current published evidence base for the delivery of intravenous chemotherapy closer to home, a series of three interlinked systematic reviews was undertaken. Each review assessed a different type of evidence: comparative clinical effectiveness, cost-effectiveness and qualitative studies. We used the same methodology across reviews except where different types of evidence precluded this; any alternative methods are clearly described and signposted.
Together, the three reviews summarise the totality of the evidence base by addressing particular questions and focusing on the most appropriate type of evidence. The reviews were conducted in parallel within an explicit and pragmatic mixed-methods framework based on principles of complementarity. This approach is based loosely on the approach pioneered by the Evidence for Policy and Practice Information (EPPI) and Co-ordinating Centre (the EPPI approach). 11
The same researchers worked on each of the three reviews to ensure a collaborative approach. Regular team meetings and discussions during study selection, data extraction and analysis promoted cross-fertilisation of ideas. Matrices were used to collate the summary findings from each of the three reviews. Commonalities and divergences between the results were identified and integrated with the findings informing the decision model. Chapter 6 presents the meta-synthesis of all elements from the project.
Methods
Searches
The aim of the literature searches was to systematically identify research on the impact of setting (closer to home) on the delivery and outcomes of intravenous chemotherapy.
The base search strategy was constructed using MEDLINE and then adapted to the other resources searched (Box 2).
Date range: 1946 to week 2 March 2013.
Date of search: 25 March 2013.
1564 records identified.
Search strategy-
exp neoplasms/ (2,406,640)
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (2,073,583)
-
oncologic nursing/ (6088)
-
or/1-3 (2,889,785)
-
drug therapy/ (33,151)
-
Antineoplastic Combined Chemotherapy Protocols/ (97,247)
-
chemotherapy, adjuvant/ or consolidation chemotherapy/ or maintenance chemotherapy/ (27,648)
-
administration, intravenous/ or infusions, intravenous/ (46,068)
-
chemotherapy.ti,ab. (223,465)
-
systemic therapy.ti,ab. (5856)
-
intravenous drug therapy.ti,ab. (39)
-
adjuvant therapy.ti,ab. (14,653)
-
or/5-12 (357,679)
-
home care services/ or home care services, hospital-based/ (28,037)
-
*Outpatients/ (2136)
-
*Ambulatory Care/ (14,592)
-
*ambulatory care facilities/ or *outpatient clinics, hospital/ (13,416)
-
community health services/ or community health nursing/ or community health centers/ (47,800)
-
general practitioners/ or physicians, family/ or physicians, primary care/ (16,406)
-
general practice/ or family practice/ (61,185)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (42,475)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (15,680)
-
or/14-22 (207,550)
-
4 and 13 and 23 (1144)
-
home infusion therapy/ (579)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (719)
-
(chemotherapy adj6 service$).ti,ab. (184)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (19)
-
(self-infusion adj6 home).ti,ab. (21)
-
home infusion.ti,ab. (254)
-
or/25-30 (1591)
-
4 and 31 (751)
-
24 or 32 (1595)
-
exp animals/ not humans/ (3,782,734)
-
33 not 34 (1564)
The search included the following components:
-
cancer terms AND
-
chemotherapy terms AND
-
generic home care/ambulatory care terms.
These terms were combined with (OR), the following terms:
-
cancer terms AND
-
home chemotherapy terms.
No date, language or other limits were applied and, where possible, animal-only studies were excluded.
The strategy was constructed by an information specialist within the Centre for Reviews and Dissemination (CRD) and subsequently peer reviewed by another information specialist prior to use.
Search terms were identified by scanning key papers known at the beginning of the project, through discussion with the review team and the use of database thesauri.
The full strategies from all of the databases are given in Appendix 1.
Sources of both published and unpublished information were identified by an information specialist with input from the project team. MEDLINE and MEDLINE In Process & Other Non-Indexed Citations; Allied and Complementary Medicine Database; British Nursing Index; Cumulative Index to Nursing and Allied Health Literature; The Cochrane Library; Conference Proceedings Citation Index – Science; Dissertation Abstracts; EconLit; EMBASE; Google; Health Management Information Consortium; Inside; Office for Health Economics Health Economic Evaluations Database; PsycINFO; PubMed; Social Policy and Practice; ClinicalTrials.gov and Current Controlled Trials databases and the Google search engine were searched.
Databases were searched from date of inception to March 2013. Update searches were undertaken in October 2013.
Reference searches of all included randomised controlled trials (RCTs) and relevant systematic reviews were undertaken. Where necessary, authors of eligible studies were contacted for further information and experts in the field were contacted to see whether or not they had access to further material. We contacted private providers of home care through the National Clinical Homecare Association (including HaH, Bupa, Baxter, Calea and Alcura) to identify unpublished reports, evaluations or resource information.
We also contacted the Medicines and Healthcare products Regulatory Agency to request information on suspected adverse drug reactions and adverse events for intravenous home chemotherapy drugs. Route of administration was available, but the system does not store information on the setting where the drug was given or the adverse event occurred. Therefore, no data relevant to this project could be collated.
Inclusion criteria
Population
Cancer patients receiving intravenous chemotherapy.
Interventions and comparators
Studies comparing intravenous chemotherapy in two (or more) of the following settings:
-
home setting (includes nursing homes)
-
community-based setting (e.g. GP practice, community clinic, community hospital or mobile units)
-
hospital outpatient setting.
Within-setting comparisons were eligible if the study compared different organisational or management approaches.
Outcomes
Any of the following:
-
safety
-
patient quality of life
-
preference
-
satisfaction (including treatment compliance/adherence)
-
social functioning
-
clinical outcomes
-
patient and carer opinions and experiences
-
costs
-
resource/organisational issues (including access).
The clinical outcomes of interest were self-rated health or measures of performance status.
Study designs
Any type of comparative design was eligible. To obtain information about patient quality of life, satisfaction, preferences and opinions, studies that reported results for only one eligible setting and qualitative research (any of the three settings) were considered, providing that they had a stated aim to evaluate one or more of these outcomes. Given the review focus on home and community settings, and the potential diversity and likely volume of these studies in an outpatient setting, we focused on studies of the home and community settings.
Full economic evaluations that compared two or more eligible settings and considered both costs and consequences (including cost-effectiveness, cost–utility or cost–benefit analyses) were eligible.
Screening and study selection
Two researchers independently screened all titles and abstracts obtained using the predefined eligibility criteria. Discrepancies were resolved by consensus, with recourse to a third researcher where necessary. Full manuscripts of potentially relevant studies were obtained where possible and were screened in duplicate. Studies in any language were eligible for inclusion.
Data extraction and quality assessment
Studies were assessed for quality as part of the data extraction process using criteria relevant to the topic and study designs included. Data were extracted into structured forms using a pre-piloted form in EPPI-Reviewer (EPPI-Centre, Institute of Education, University of London, London, UK). Piloting was undertaken by each researcher involved with the process and refined as necessary prior to full data extraction to ensure consistency. Data extraction and quality assessment was conducted by one researcher and checked by a second researcher for accuracy, with any discrepancies resolved by discussion or by recourse to a third researcher where necessary.
Clinical studies
Data were extracted on details of study methods, country and geographical region in which the study was conducted, whether it was single or multicentre, dates over which the study was conducted, patient characteristics, interventions, comparators where appropriate, all relevant outcome measures and results.
The quality of included comparative studies was assessed using criteria appropriate to the study design, adapted from published checklists. 12
Randomised controlled trials
Randomised controlled trials were assessed using the Cochrane risk of bias tool, which focuses on the domains shown to impact on the trial results in particular (selection, performance and detection biases and attrition). 13 The tool was modified to incorporate assessment of baseline imbalances when we evaluated selection bias. 14
Non-randomised comparative studies
Study quality evaluations were based on recently published papers detailing methodological issues and assessment of bias in non-randomised studies. 15–18 Confounding variables (variables other than the intervention being studied, which might affect study outcomes when groups are compared) are known to be a very important source of bias in non-randomised studies. 19 As there were many potentially important confounders for our review question, we focused our assessment on evaluation of the risk of bias due to confounding. This was done by answering the following questions:
-
How were the groups formed?
-
Were the effects of any confounders taken into consideration during the design and/or statistical analysis stages?
-
What methods were used to control for confounders?
-
Were data on the measured confounders recorded precisely enough?
-
Were any key confounders not controlled for?
The important confounders we considered were type of cancer, stage of cancer, type of chemotherapy, age, performance status [e.g. Karnofsky, Eastern Cooperative Oncology Group (ECOG) or Lansky scores], quality of life, treatment intent (curative or palliative) and distance from hospital. These confounders were chosen for their potential to affect outcomes such as quality of life and patient satisfaction. Some of these confounders are correlated.
Where these confounders were not measured, and not taken into account in the design or analysis of the study, the study results were deemed likely to be at a high risk of bias. Studies where such details were not clear were judged to have an unclear risk of bias. Given the non-randomised nature of the studies and the lack of assurance provided about the methods used, the implications of an unclear risk of bias judgement are similar to those of a high risk of bias judgement and the study results should not be interpreted as being reliable.
Where the answer to question 2 was ‘yes’, the details of which confounders were controlled for were recorded and the remaining questions were answered; the overall risk of bias judgement (from confounding) was then made based on the answers to questions 3, 4 and 5. Where the answer to question 2 was ‘no’ or ‘not reported’, a high or unclear risk of bias judgement was made and the remaining questions were not answered. An assessment of whether or not there was evidence that potential confounders did not actually result in confounding was also made when considering question 2.
For non-randomised studies there is evidence that confounding may not, on average, cause bias in the estimation of adverse effects. 17 We considered this during our assessments according to how likely a given adverse effect (as defined in individual studies) might be affected by confounding.
As cost and resource outcomes were extracted to inform the review’s economic modelling, formal synthesis and quality assessments were not routinely performed for these outcomes.
Non-comparative studies were not extracted or quality assessed, but they are listed for reference in Appendix 2.
Cost-effectiveness studies
Data extracted from economic evaluations included interventions compared study population; dates to which the data related; measures of effect; direct costs (medical and non-medical); currency used; utilities/measure of health benefit; and results and details of any decision modelling applied. The quality assessment of the economic evaluations was informed by use of the Drummond 36-point checklist. 20 The purpose of the review was to provide an overview of the current cost-effectiveness evidence base and help to inform the development of a de novo decision model. Any additional information that could aid development of a de novo decision model was also extracted.
Qualitative studies
Qualitative studies were assessed for methodological quality using criteria based on the work of Mays and Pope, among others. 21–23 As with the quantitative studies, the focus was on those domains which are expected to influence the reliability of the findings. Domains included transparency and documentation of the data collection and analysis processes, description and justification of sampling, validity appropriate to the method being used, reflexivity and clear distinction between data and interpretation.
The results sections from each included study [apart from one Doctor of Philosophy (Ph.D.)] thesis were extracted from portable document format files and entered into NVivo (QSR International, Warrington, UK) as text documents. 24 We used online translation for one paper in Danish. This unconventional approach appeared to generate reasonable approximations of the original meaning and avoided the expense and delay of professional translators; this was balanced against the possibility of losing some meaning in translation.
The Ph.D. thesis24 was read through with the other papers, but not extracted and coded until nearer the end of the process. The thesis was useful as a way of checking for gaps or absences in the data as a whole, but it was too dense and, in places, of less immediate relevance to warrant full extraction.
Synthesis
Clinical effectiveness data
Our detailed narrative synthesis explored the methodology and reported outcomes of included studies. Key study characteristics, patient outcomes and quality assessment were tabulated to provide clear summaries of the included studies. The clinical and statistical heterogeneity of the accumulated evidence was assessed. Differences between studies were discussed in the text and the potential impact of these differences on outcomes was explored. The results were interpreted in the context of the quality of the individual studies.
As anticipated, the available data were too heterogeneous for quantitative synthesis.
Cost-effectiveness data
The findings of the systematic review of full economic evaluations were summarised in a narrative synthesis.
Qualitative data
The qualitative studies were synthesised using meta-ethnography, an approach which searches each primary study and systematically extracts key findings and interpretations. 25,26 These data are then compared using a constant comparison method, which categorises key concepts to look for overlapping themes in order to enable linking of material. Each finding is subsequently assessed for similarity or difference to the other studies, and the goal is to develop, in an iterative manner, this reciprocal translation. Ultimately, new lines of argument can be developed which go beyond the data contained within the original studies.
The focus of the analysis was on themes and ideas relating directly to the provision of intravenous chemotherapy with particular reference to treatment location, rather than the experience of having cancer treatments per se.
Each results section was read closely on multiple occasions and coded line by line using participants’ words where possible. Initially, codes were tagged according to the setting (e.g. code ‘prefer to go home after treatment’ was linked to ‘outpatient setting’). Subsequent readings of the texts and resultant codes led to the reworking of the coding framework into key elements rather than distinguishing by treatment location. Codes were collapsed where possible and a process of diagramming used to explore links and interactions between the key lines of argument and categories.
Results
The electronic database searches identified 4260 references. A further 12 references were found by Google searches or by checking reference lists of included randomised trials and relevant systematic reviews. After screening titles and abstracts, full copies of 245 papers were assessed for inclusion in the review. Figure 1 illustrates the flow of studies through the review process. Fourteen references were of papers related to another reference already included. Sixty-seven eligible studies were identified. Nine of the 25 comparative studies (10 RCTs and 15 non-randomised studies) fully evaluated in the review undertook concurrent full economic evaluations; these were evaluated separately in the review to enable a more detailed assessment. The 42 studies of single-settings are listed in Appendix 2. We identified a larger than expected number of comparative studies, and so we evaluated only those single-setting studies which might usefully add to the synthesis of the comparative studies. Consequently, single-setting studies were used only to inform the evaluation of qualitative data on patient, relative or caregiver experience of intravenous chemotherapy (see Chapter 3, Results, Qualitative studies). As we anticipated there to be a large number of single-setting outpatient studies, we ordered full papers only for studies that appeared likely to report qualitative data.
FIGURE 1.
Flow chart of study selection. N/A, not applicable.

The following sections present a detailed breakdown of the results of the RCTs, non-randomised studies and economic evaluations. For each study design, study characteristics, risk of bias or quality assessment and results are presented.
Clinical effectiveness studies
Randomised trials
Study characteristics
Ten randomised trials investigated the effect of setting for patients receiving intravenous chemotherapy (Table 1). Six trials used a crossover design (where participants act as their own controls, and typically receive all interventions in succession). Three trials used a parallel design (where participants typically receive only one intervention). One study reported only as an abstract appeared to use parallel groups and incorporated elements of a crossover design. 27 Most studies were reported as full published papers; two were reported only as conference abstracts. 27,28 Studies were published between 1999 and 2013. Three studies were conducted in the UK (England),4,29,30 two were conducted in Australia,31,32 and one study was conducted in each of Canada,33 Denmark,28 France,34 Japan27 and Spain. 35
| Study | Country | Sample size | Recruitment ratea | Setting | Outcomes | ||
|---|---|---|---|---|---|---|---|
| Home | Community | Outpatient | |||||
| Corrie et al. 2013 (OUTREACH)4 | England | 97P | 1.7 | ✓ | ✓ | ✓ | Quality of life, anxiety, depression, health status, costs, satisfaction, serious adverse events |
| bChen and Hasuimi 199927 | Japan | 10P | NP | ✓ | ✓ | Quality of life, anxiety, nursing time | |
| bChristiansen et al. 201128 | Denmark | 51C | 1.4 | ✓ | ✓ | Quality of life, adverse effects, time spent receiving chemotherapy, preference, costs | |
| Pace et al. 200929 | England | 42C | 3.2 | ✓ | ✓ | Preference, anxiety, depression, safety, resources | |
| Hall and Lloyd 200830 | England | 15P | 2.5 | ✓ | ✓ | Experience and satisfaction, costs | |
| King et al. 200031 | Australia | 74C | 1.5 | ✓ | ✓ | Preferences and strength of preference, satisfaction, unmet need, quality of life, costs | |
| Rischin et al. 200032 | Australia | 25C | 1.8 | ✓ | ✓ | Preference, satisfaction, complications, costs | |
| Stevens et al. 200633 | Canada | 29C | NP | ✓ | ✓ | Quality of life, social/psychological interactions, adverse events, costs | |
| Remonnay et al. 200234 | France | 52C | 1.6 | ✓ | ✓ | Satisfaction, costs, quality of life, anxiety | |
| Borras et al. 200135 | Spain | 87P | 6.7 | ✓ | ✓ | Toxicity, withdrawals, health-care resources, quality of life, satisfaction, Karnofsky Index | |
Eight studies27,28,30–35 compared chemotherapy in the home setting with chemotherapy in a hospital outpatient setting. One study29 compared a community setting with a hospital outpatient setting. One study4 was a three-armed trial that compared home, community and outpatient settings. Setting details were generally not well reported; for example, aspects such as the number of nurses per patient, the degree of access to parking, and facility details were only occasionally provided. The two community settings studies assessed treatment delivered in GP surgeries and community outreach centres. 4,29 Treatment durations were often not stated explicitly or were expressed in terms of cycles; however, most studies reported chemotherapy durations ranging between approximately 2 and 8 months.
All of the trials except Stevens et al. 33 studied adults, with reported mean (or median) ages ranging from 57 years to 64 years. Around half of the studies were of mixed populations; patients with colon, breast, and pancreatic cancer were the most frequently studied. Studies were also conducted solely in populations with ovarian,27 colon28 or breast cancer. 30 The study in children was of a population with acute lymphoblastic leukaemia. 33
The treatment intention was not always reported. Where reported it varied both within and across studies, with chemotherapy administered with either palliative or curative intent (sometimes as an adjuvant treatment). Few studies reported details on where chemotherapy drugs were prepared: in two trials drugs were prepared in the hospital pharmacy4,29 and in one trial a community pharmacy was used. 33 Full study characteristics are reported in Appendix 3.
Recruitment and participation
In total, 482 participants were randomised across 10 trials. Sample sizes ranged from 10 to 97. Six studies reported a target sample size; three of these achieved or exceeded their small recruitment targets of 30 or fewer patients. Three studies did not achieve their targets: in one study a target of 20 patients was not reached (reasons unclear); one study was terminated early when 52 of a targeted 160 participants had been randomised,34 because a large majority preferred the home setting; and the largest included study (OUTREACH) was stopped owing to the poor recruitment rate when 97 of a targeted 390 participants had been randomised (the decision was made on the advice of the independent data monitoring committee). 4 Despite this early termination, the OUTREACH trial rate of recruitment (estimated at around 1.7 patients per month per centre) was similar to the estimates for many other trials (rates ranging from 1.4 to 2.5 patients per month per centre), except for the Borras et al. 35 trial (around 6.7 patients per month/ per centre) and the Pace et al. 29 trial (around 3.2 patients per month per centre) (see Table 1 for details).
Five of the nine trials where the outpatient setting was the standard care setting (the only routinely available setting) reported details of eligible patients who were not randomised. Between them, these five trials randomised 294 participants, but 100 eligible patients chose not to participate for setting-related reasons. Generally, these participants withdrew from the trial to revert to standard practice (which was their preferred setting). In one trial home chemotherapy was already an option before the trial began – and eligible patients had to be registered on the ‘chemotherapy in the home program’. 32 In this study several patients chose not to participate because they wanted only home treatment. These data highlight the inherent bias (in terms of the types of population recruited) often encountered in trials that evaluate settings (see Chapter 6, Limitations of the evidence and of the review for more discussion of this point).
No consistent trends were found in the setting-related reasons for participants who withdrew or dropped out of trials (some studies reported limited details or none at all).
Risk of bias
Results of the risk of bias assessments are presented in Appendix 4. Even though only the OUTREACH trial clearly reported on both the sequence generation and allocation concealment methods,4 most studies can be judged as being at a low risk of selection bias overall. This is largely because treatment groups had similar characteristics at baseline, a factor which was mainly a result of the use of a crossover design.
All studies were judged to be at high risk of performance bias; study participants and personnel will have been aware of which setting had been allocated, and avoidance of such bias is impossible. Similarly, results for the subjective patient-reported outcomes, such as quality of life and satisfaction, were judged to be at high or unclear risk of bias in all studies. Conversely, the risk of detection bias was judged low for studies reporting adverse events because they are mostly not subjective outcomes.
In four of the 10 trials the risk of attrition bias was judged to be low. There were insufficient details for the remaining six trials; accordingly, these trials were judged to be at an unclear risk. Four trials were found to be at a low risk of reporting bias, two were at a high risk of bias owing to missing results (or result detail), and in the remaining trials the risk was unclear.
Of the six crossover trials, only three clearly reported using appropriate statistical analyses. In three crossover trials the use of a crossover design appeared questionable, because of the number of patients withdrawing or dropping out because of disease progression.
Results and synthesis of randomised clinical effectiveness trials
Quality of life
Seven of the 10 randomised trials reported that they evaluated some measure of quality of life; actual result data were available for only four trials (see Table 1). There were no statistically significant differences in European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (30 items) (EORTC QLQ-C30) self-rated quality of life between any settings (home vs. outpatient;4,28,35 home vs. GP, outpatient vs. GP4). Both home and outpatient settings were associated with statistically significant better results for the EORTC QLQ-30 Emotional Function outcome than in the GP surgery setting in the OUTREACH trial; there were no statistically significant differences between home and hospital settings. 4,35
One trial used Functional Living Index Cancer (FLIC) scores as a measure of quality of life and found that setting (home vs. outpatient) had no effect on either total FLIC scores or any of the seven dimension scores. 31 The remaining trial studied 23 children using the Paediatric Oncology Quality of Life Survey (POQOLS). 33 It found statistically significant improvement associated with the home setting, compared with the outpatient setting, in terms of sensitivity to restrictions in physical functioning and the ability to maintain a normal physical routine. There were no statistically significant differences between the settings in terms of emotional distress and reaction to current medical treatment.
Clinical and psychological outcomes
Two trials reported results for EORTC QLQ-C30 self-rated health (see Table 1). 4,35 Both suggested that there was no difference between the home and outpatient settings. One trial compared GP and outpatient settings and GP and home settings and reported no statistically significant differences. The other study evaluated Karnofsky Index scores and reported identical scores for the home and outpatient settings.
Two trials evaluated participants using the Hospital Anxiety and Depression Scale (HADS). 4,29 Results from one trial suggested that higher levels of both anxiety and depression were associated with the GP setting, compared with the home or outpatient settings, but the results were statistically significant only for the GP versus outpatient comparison (for depression). There was little indication of any meaningful differences between the home and hospital settings. The other trial did not report any data, but stated that there were no significant differences between the community hospital and outpatient settings for both anxiety and depression.
Satisfaction and preferences
Five trials reported quantitative results for satisfaction (and related outcomes). Specific outcomes varied between studies (see Table 1). Two trials reported statistically significant results suggesting satisfaction benefits in terms of nursing care for the home and community settings, compared with the outpatient setting. 29,35 The largest study (OUTREACH) that compared home, GP surgery and outpatient settings in the UK reported that 78% of participants were satisfied with their treatment setting, regardless of location. 4 An Australian trial reported that significantly more patients found the outpatient setting more depressing than the home setting, although no significant differences were found for patient needs. 31 Other studies suggested no differences between groups in terms of global satisfaction,29 or doctor-care outcomes. 35
Five trials reported quantitative results for preferences, but only one of these trials evaluated strength of preference. 31 In the four trials where patients experienced two settings (because a crossover design was used) between 70% and 95% of patients preferred the home setting,31,32,34 and 97% preferred a community outreach setting,29 when compared with the outpatient setting. One trial stopped recruiting participants early owing to the strong preferences expressed for home treatment. 34
Results from the study that considered strength of preference suggested that preferences were not very strong. It found that 34% of the participants who preferred home treatment changed their preference to outpatient treatment if home treatment was to involve waiting an extra hour, and that 27% of participants who preferred outpatient treatment changed their preference to home treatment if faced with an extra hour of waiting. 31 These results suggest that for some patients time is more important than setting. This trial was the only study to consider the issue of recruitment bias. The authors performed an additional analysis of patient preference by also including the 13 patients who chose not to participate in the trial for setting-related reasons, which they interpreted as a preference for the outpatient setting; similarly, this analysis also included the eight patients who chose not to receive home treatment after experiencing outpatient treatment (see Appendix 3). The results indicated that the proportion of patients who preferred home care to outpatient care was 48%.
Safety
Six trials reported on adverse events (see Table 1). Four trials provided some assessment of whether adverse events were related to setting (e.g. in one trial a nurse was unable to cannulate an outreach patient, who was consequently treated at the cancer centre). 4,29,32,33 They found no evidence to suggest significant differences existed between settings for any type of adverse event. Two studies evaluated only toxicity and also found no differences between settings. 28,35
Full result details for all outcomes are presented in Appendix 5.
Non-randomised studies
Study characteristics
Fifteen non-randomised, comparative studies investigated the effect of setting for patients receiving intravenous chemotherapy; they were reported between 1989 and 2013 (Table 2). 24,36–49 Several studies were not easy to identify or access: four were reported only as conference abstracts,42,44,47,48 one was only available as a Ph.D. thesis,24 one was an unpublished internal report36 and one was only available as an online report. 37 Five studies took place in England,24,36,39,44,46 four in the USA,42,43,47,48 two in Denmark38,41 and one each in Wales,40 Australia,45 Canada37 and France. 49
| Study | Country | Sample size | Setting | Outcomes | ||
|---|---|---|---|---|---|---|
| Home | Community | Outpatient | ||||
| Taylor 200824 | England | ≈ 140 | ✓ | ✓ | Qualitative data on provision of care at home from health professionals and patients | |
| NHS Bristol 201036 | England | 848 | ✓ | ✓ | ✓ | Patient experience |
| Pong et al. 200037 | Canada | 435 | ✓ | ✓ | Self-reported health status, costs, satisfaction; reasons for choosing setting | |
| Hansson et al. 201338 | Denmark | 75 | ✓ | ✓ | Patient- and parent-reported health-related quality of life, psychological impact on family, costs | |
| Mitchell 201139 | England | 20 | ✓ | ✓ | Patient experience (qualitative), satisfaction, costs | |
| Barker 200640 | Wales | 14 | ✓ | ✓ | Toxicity, satisfaction | |
| Frølund 201141 | Denmark | 6 | ✓ | ✓ | Qualitative data on experiences of chemotherapy | |
| aGrusenmeyer et al. 199642 | USA | NR | ✓ | ✓ | Costs, satisfaction | |
| Herth 198943 | USA | 80 | ✓ | ✓ | Hope, coping | |
| aIngleby et al. 199944 | England | 25 | ✓ | ✓ | Costs | |
| Lowenthal et al. 199645 | Australia | 179 | ✓ | ✓ | Safety, costs, resource use | |
| Payne 199246 | England | 53 | ✓ | ✓ | Quality of life, Karnofsky performance | |
| aSatram-Hoang and Reyes 201147 | USA | ≈ 2800b | ✓ | ✓ | Time to treatment initiation, duration of treatment, number of cycles delivered, compliance | |
| aSouadjian et al. 199248 | USA | Unclear | ✓ | ✓ | Costs, complications, quality of life, preference | |
| Vergnenègre et al. 200649 | France | 20 | ✓ | ✓ | Adverse events, costs | |
In three studies,24,39,41 the only review-relevant outcomes were qualitative (see Results, Qualitative studies). In the remaining 12 studies, eight compared the home and outpatient settings,38,42–46,48,49 two compared community settings with outpatient settings,37,47 one compared home with community settings40 and one compared all three types of settings. 36 Population sizes were not always clearly reported, but ranged from 14 to around 2800 patients (more than half of the studies were of fewer than 100 patients). Most studies were of mixed populations; most patients had colorectal cancer, breast cancer or lung cancer. Mean ages ranged from 50 years to 75 years, where reported. One study was in children with leukaemia or lymphoma and was the only study which indicated where the chemotherapy drugs were prepared; this study also clearly reported setting details (e.g. home care was provided by one or two nurses, depending on the tasks involved). 38 In other studies the setting details were not generally well reported; exceptions were descriptions of a community mobile chemotherapy unit,39 and community oncology clinics. 37 Full study characteristics are reported in Appendix 7.
Risk of bias
Table 3 details the results of the risk of bias assessment of the non-randomised studies. Most studies were judged to be at a high or unclear risk of bias due to confounding. Although four studies did consider the effect of confounders in their study design and/or analysis plan, they did not investigate all the likely confounders. The two studies which were given a low-risk judgement both reported adverse events as their only review-relevant clinical outcome; the types of adverse event assessed were unlikely to have been affected by any confounding factors in the populations studied.
| Study | 1. How were the groups formed? | 2. Were the effects of any confounders taken into consideration during the design and/or statistical analysis stages? | 3. What methods were used to control for confounders? | 4. Were the data on the measured confounders recorded precisely enough? | 5. Were any key confounders not controlled for? | Risk of bias from confounding |
|---|---|---|---|---|---|---|
| Lowenthal et al. 199645 | Oncologist decision as to which patients were offered a choice (based on having satisfactory home circumstances, and type of chemotherapy) | No | N/A | N/A | N/A | Likely to be low risk, as complications (requiring hospital admission) was the only clinically relevant outcome (unlikely to be affected by confounding) |
| Payne 199246 | Oncologist preference | Yes, diagnostic category, Karnofsky score and age were found not to be related to several quality-of-life variables | Stratified analyses for age. Stepwise multiple regression for Karnofsky score. Unclear for diagnostic category | Yes | Yes, stage of cancer. The authors acknowledged that patients who were more severely ill were more likely to be treated in the outpatient setting. Illness severity was not assessed | High |
| Herth 198943 | Non-random, convenience sample | Yes, stage of disease, age and extent of illness were considered | Matching for stage of disease. No details provided for age and extent of illness, just that they ‘were not identified as confounding variables’ | Yes | Yes, type of cancer, type of chemotherapy, performance status, and quality of life were not considered | High |
| Satram-Hoang and Reyes 201147 | Selection of retrospective cohorts from a database | It appeared so, but the study was only reported as an abstract so details were limited | Stratification and ANOVA | Unclear | Unclear | Unclear |
| Souadjian et al. 199248 | Unclear | Not reported – reported only as an abstract | N/A | N/A | N/A | Unclear |
| Hansson et al. 201338 | Based on distance from home to hospital. Historical controls were also used for outpatient setting | Yes, age, diagnosis, gender and time since diagnosis | Multiple linear regression | Unclear whether or not categories were used for ‘age’, and ‘time since diagnosis’ (rather than treating them as continuous variables) | Yes, stage of cancer, type of chemotherapy, quality of life and distance from hospital | High |
| Pong et al. 200037 | Retrospective random selection of patients from a database | No | N/A | N/A | N/A | High |
| Barker 200640 | Unclear | No | N/A | N/A | N/A | Higha |
| NHS Bristol 201036 | Patient choice | No | N/A | N/A | N/A | High |
| Vergnenègre et al. 200649 | Unclear (beyond home eligibility criteria) | Not reported | N/A | N/A | N/A | Likely to be low risk since incidence of grade III or IV toxicity was the only outcome of interest, unlikely to be affected by confounding |
Non-randomised study results
Three studies evaluated quality of life but their results did little to augment the RCT evidence: two studies were small, with a high risk that confounding would affect the reliability of their results; and one study was reported only as an abstract (making it difficult to interpret the results). 38,46,48 There were similar issues for the four studies of patient satisfaction. 37,39,40,42 One study included 435 patients but they were selected retrospectively, with no consideration made for confounding factors; and the study was in Canada, where the travel time and distances are different from those likely to be encountered in the UK. 37
Only one of the four studies which had a safety outcome yielded informative results;45 this Australian study reported that complications in the home setting were rare, although no comparative data were reported for the outpatient setting for this particular outcome. Two of the three other studies that looked at safety had very small sample sizes,40,49 and the other was only reported as an abstract. 48 Three studies that looked at qualitative patient experience are discussed in Results, Qualitative studies.
Only one comparative study looked at the issue of treatment compliance in any detail. 47 It was conducted in the USA and studied approximately 2800 follicular lymphoma patients receiving chemotherapy [with or without rituximab (Mabthera, Roche)]. The study concluded that patients treated in the outpatient setting tended to have longer times to treatment initiation and fewer cycles across all regimens, and were less likely to receive a compliant dosing schedule than patients treated in a community clinic setting. However, the reliability of these conclusions was unclear as the study was only reported as an abstract (the risk of bias due to confounding was unclear). The results for all the non-randomised studies are presented in Appendix 5.
Cost data
Fourteen comparative studies reported costs as an outcome (see Tables 1 and 2). Cost data were recorded only to help inform the decision modelling part of the report and are presented in Appendix 6.
Clinical results evidence summary
The included studies revealed inherent difficulties in conducting randomised trials of chemotherapy settings. Even trials that were designed appropriately to minimise avoidable biases faced problems not only of patient accrual but also of recruiting a population to enable an unbiased evaluation of the settings. These seemingly unavoidable selection biases might be expected to produce results that favour home (or community) settings. Even so, there was little evidence of clinically relevant differences between settings in terms of quality of life and clinical and psychological outcomes. The only potentially meaningful differences were seen for some patient satisfaction and preference outcomes. However, strength of preference was studied in only one trial, with preferences appearing not to be strong in around one-third of patients. The limited safety evidence available suggested there were no differences between settings.
The non-randomised studies added little to the randomised trial evidence (although community settings were more frequently studied). The main limitations were the small populations and the high risk that study results were biased due to confounding.
Cost-effectiveness studies
Study characteristics
Nine economic evaluations published between 1996 and 2013 met the criteria for inclusion. The key characteristics, methods and results for the nine studies are summarised in Table 4. Details of patient characteristics and treatment regimens can also be found in Table 4. All nine evaluations also met the inclusion criteria and have been assessed independently as comparative studies.
| Study characteristics | Main analytical approaches | Primary outcomes and health-related quality of life | Resource use | Cost analysis |
|---|---|---|---|---|
| Rischin et al. 200032 (full paper) Country: Australia Settings: home; hospital outpatient |
Economic evaluation alongside a RCT (crossover, n = 25 recruited, n = 20 evaluated) Analysis: CEA (CCA) Perspective: hospital Time horizon: two chemotherapy cycles |
Patient preference and satisfaction All patients preferred remaining treatment at home. 0% reported concerns with home treatment, 20% had concerns with hospital. 90% felt that there were advantages to home, 5% hospital |
No resource use data reported Cost categories: nurse time and travel, vehicle costs, one meal in hospital. Unclear if drug costs included |
Price year NR Home associated with average increased cost of AUS$83 per treatment vs. hospital (95% CI AU$46 to AU$120; p = 0.0002) First treatment AUS$57 more expensive than second treatment on average (95% CI AUS$20 to AUS$94, p = 0.0044) |
| King et al. 200031 (full paper) Country: Australia Settings: home; hospital outpatient |
Economic evaluation alongside a RCT (crossover, n = 74 recruited, n = 40 evaluated) Analysis: CEA (CCA) Perspective: health service Time horizon: 4 months |
Patient preference and strength 73% (95% CI 59% to 86%; p = 0.008) preferred home. Strength of preference was low. Preference dropped to 48% (95% CI 35% to 60%; p = 0.61) when accounting for preferences of withdrawn patients (p-value testing if = 50%). No apparent differences in quality of life (FLIC score) between settings |
No resource use data reported Cost categories: nurse cost, travel time, vehicles, equipment, cost of capital and overhead costs. Individual category costs reported |
Price year NR Net additional cost of home vs. hospital: AUS$68.81. Additional cost attributed to extra nurse time Cost of new chemotherapy ward = AUS$70,581. Home chemotherapy less expensive per treatment than a new ward used with up to 50% ward capacity. New ward less expensive above 50% ward capacity |
| Lowenthal 1996 et al.45 (full paper) Country: Australia Settings: home (included workplaces, GP offices, day-care centres); hospital outpatient |
Analysis based on a retrospective non-randomised audit (n = 184 recruited, n = 179 evaluated) Analysis: CEA (CMA) Perspective: hospital Time horizon: 5 years for safety, 1 year for costs |
Safety: single setting (number of major complications at home only) One major complication among visits to 179 patients. Assumed this would be at least as safe as in the hospital (no data for hospital) The authors appeared to assume equal efficacy between settings |
Reported number of visits, duration, travel and preparation time Cost categories: labour, travel, hospital resources, pharmaceuticals and overheads (drug costs assumed the same). Individual cost category costs reported |
Price year: 1994 AUS ($) Cost per home chemotherapy treatment: AUS$49.93. Hospital: AUS$116.00 Annual cost to deliver 345 chemotherapy treatments and additional services for 65 patients in the hospital (extending hours): AUS$38,207. Home: AUS$45,767 |
| Corrie et al. 20134 (full paper) Additional data from personal communication with author Country: UK Settings: home; GP surgery; community (GP + home); hospital outpatient |
Economic evaluation alongside a RCT (n = 97 recruited, n = 57 evaluated) Analysis: CUA Perspective: NHS Time horizon: 12 weeks |
EORTC QLQ-C30 QoL Emotional Function domain: No difference for community vs. hospital. Home vs. GP: 15.2, 95% CI 1.3 to 29.1; p = 0.033 (favoured home). GP vs. hospital: –16.6, 95% CI –31.4 to –1.9; p = 0.028 (favoured hospital) EQ-5D: No significant differences. Unadjusted mean (SD) QALY home: 0.165 (0.036); GP: 0.191 (0.04); hospital: 0.174 (0.034). Based on 14 hospital patients, 15 GP, 19 home (complete-case analysis) Patient preference: 57% of hospital patients preferred future treatment in hospital, 81% GP, 90% home |
Resource use data collected from nurse diaries and Client Service Receipt Inventory Unit costs from PSSRU (included salaries and overheads) Cost categories: inpatient, outpatient, day hospital, A&E visits, community care, medication, and nurse diaries contact. Nurse travel (not patient) included |
Price year: 2010 GBP Home: £2139 (SD £1590); GP: £2497 (SD £1759) Hospital: £2221 (SD £1831) ICER GP vs. hospital = £16,235/QALY gained |
| Pace et al. 200929 (full paper) Country: UK Settings: community hospital; hospital outpatient |
Economic evaluation alongside a RCT (crossover, n = 42 recruited, n = 31 evaluated) Analysis: CEA (CCA) Perspective: NHS and patient Time horizon: completion of treatment |
Patient preference and satisfaction 30/31 (97%) patients chose to receive remaining cycles of treatment in the community hospital and would have preferred to receive all their chemotherapy there |
Service-related resource Average round-trip mileage from main hospital to community centres = 24.2 miles Total time of 104 minutes for each treatment Two nurses for each treatment Patient-related resource Average patient distance from community clinic = 10.25 miles vs. 19 miles to hospital |
Price year NR Service costs: Average cost of round-trip = £12.83 per clinic session. Opportunity cost of travel for each nurse (based on £29,538 salary) was £32.08 (£64.16 for two nurses, £384.96 for six cycles = marginal cost of clinic) Patient costs: Mean cost of travel and parking for patients to outreach = £4.85/treatment vs. £8.77 for hospital. Including private car and public transport cost, average cost to attend outreach = £8.07 vs. £14.99 to hospital |
| Stevens et al. 200633 (full paper) Country: Canada Settings: home; hospital outpatient Paediatric population |
Economic evaluation alongside a RCT (crossover, n = 29 recruited, n = 23 evaluated) Analysis: CEA (CCA) Perspective: societal Time horizon: 1 year |
POQOLS QoL questionnaire Factor 1 (normal physical routine): switching to home led to an improvement, switching to hospital led to a worsening (–10.5 for home vs. + 5.2 for hospital p = 0.023) Factors 2 and 3 (emotion and reaction): no significant differences due to crossover. Comparison using end of 6-month data; children at home had significantly higher emotional distress than at hospital (p = 0.043) Child Behaviour Checklist No significant differences |
No resource use data reported Parents provided resource use data for physician/care provider visits, medications/supplies, babysitting, travel and productivity losses. Cash transfer effects were assessed (unemployment insurance, workman’s compensation, mother’s allowance) Costs excluded health professionals who administered chemotherapy and drug costs |
Price year NR Total societal costs were reported for each setting at three time points. At 1 year (last time point): home (n = 13), median CAD$851 (range $147–8726); hospital (n = 9), median CAD$1050 (range $29–10,278); p = 0.95 Home had higher costs at baseline, and lower costs at 6 and 12 months. No evidence that costs were affected by location of treatment The difference between family costs associated with home vs. hospital was not significant (p = 0.79) (no family costs reported) |
| Vergnenègre et al. 200649 Country: France Settings: home; hospital outpatient |
Analysis based on non-randomised comparative study (n = 20 recruited, n = 20 evaluated) Analysis: CEA (CCA) Perspective: health service Time horizon: NR |
Adverse events Home: two adverse events in 24 cycles Outpatient: seven adverse events in 30 cycles Not statistically significant difference (p = 0.27) |
Resource use for home Home visit nurse time = 130 minutes (€0.25/minute) Administrative costs = 30 minutes (€0.19/minute) Co-ordination costs = 30 minutes (€0.40/minute) Hospital costs were reported as aggregates with/without comorbidities. Home treatment costs included chemotherapeutic drugs, nursing, co-ordination, administration, disposables, transportation, GP visits and non-chemotherapeutic drugs |
Price year NR Average cost per cycle was €2829.51 (95% CI €2560.74 to €3147.02) for hospital infusion, €2372.50 (95% CI €1962.75 to €2792.88) for home-based care (–16.15%). Difference was €–457.01 by cycle (95% CI –€919.74 to €26.82) in favour of home. Real costs by injection for home was €484.42 (95% CI €424.18 to €540.32) vs. a fee of €699.89 (95% CI €643.64 to €750.23) (–30.79%)a |
| Remonnay et al. 200234 (full paper) Country: France Settings: home (managed by an external association – Soins et Santé); hospital outpatient |
Economic evaluation alongside a RCT (crossover, n = 52 recruited, n = 42 evaluated) Analysis: CEA (CCA) Perspective: societal Time horizon: four treatments |
Patient satisfaction 95% of first 52 patients preferred chemotherapy at home, recruitment discontinued after that point |
No resource use data reported Costs categories: personnel, medication, transport, laundering and overhead |
Price year: 1998 USD Marginal cost (i.e. excluding overhead costs) for one treatment home vs. hospital: US$232.5 vs. US$157; p < 0.0001 Average cost (including overheads): $252.6 vs. $277.3; p = 0.002 Category costs reported in paper |
| Hansson et al. 201338 (full paper) Country: Denmark Settings: home; hospital outpatient Paediatric population |
Economic evaluation alongside a non-RCT (n = 89 recruited, n = 75 evaluated) Analysis: CEA (CCA) Perspective: hospital Time horizon: 2 years |
PedsQL scale Trend towards higher home-care QoL Parent Proxy Cancer Module Significantly better physical health and less worry for children in the home-care group Child Self-Reported Cancer Module; Family Impact Module No significant differences |
No resource use data reported Cost of home-care service included nurse wages, car hire, fuel, parking, new nurse uniforms, nursing bags, equipment, safe storage and hospital overhead costs (administration) |
Price year NR Hospital charge for home visit: US$597 Hospital visit: US$600 Disaggregated costs for categories of home-care cost reported in Danish krone in linked study |
Three of the evaluations were conducted in Australia,31,32,45 two in the UK (England),4,29 two in France34,49 and one in each of Canada33 and Denmark. 38 Most studies assessed adult populations; two studies assessed paediatric populations. 33,38 Six evaluations were conducted alongside RCTs, two alongside non-randomised controlled studies,38,49 and one was conducted as part of a retrospective audit. 45 Most studies did not conduct a full incremental analysis i.e. to produce [incremental cost-effectiveness ratios (ICERs)], but instead reported cost and health outcomes separately. Costs and outcomes were generally assessed over a short time horizon (1 year or under).
All of the non-UK studies compared treatment delivered in the home with treatment delivered in a hospital outpatient setting. 31–34,38,45,49 The two UK studies included community settings: Pace et al. 29 compared treatment delivered in a community hospital setting with treatment delivered in a hospital outpatient setting; and OUTREACH4 compared home, GP surgery and hospital outpatient settings. None of the economic evaluations assessed the delivery of chemotherapy by mobile bus units. One study assessed the delivery of home chemotherapy by a third-party charity organisation. 34 In all other studies it was implied that home/community care was delivered by the health service.
As highlighted in the clinical study sections, most studies assessed mixed populations with various cancer types, including breast cancer, colon cancer, lung cancer, gastrointestinal cancer, lymphoma, pancreatic cancer and leukaemia. One study assessed only patients with acute lymphoblastic leukaemia. 33 An array of treatment regimens was used for a mixture of curative, supportive and palliative intent. The populations appeared to be heterogeneous in terms of disease severity.
The nine evaluations conducted alongside clinical studies recruited a total of 593 patients and 487 participants were evaluated in the concurrent economic evaluations. Most of these participants were evaluated in the two non-randomised studies; 179 patients were evaluated in the retrospective audit45 and 75 were evaluated in the controlled study. 38 The six evaluations made concurrently with RCTs were based on small data sets:4,29,31–34 the largest study (OUTREACH)4 had complete primary outcome data (EORTC QLQ-C30 Emotional Function subdomain) on only 57 participants across three treatment arms/settings. Chapter 6 contains an overview of recruitment and participation within the RCTs (see Limitations of the evidence and of the review). The number of participants informing the concurrent cost-effectiveness analysis is presented in Table 4, alongside other study characteristics and findings.
Economic quality assessment
The Drummond checklist was used to assess methods and reporting in the nine included economic evaluations. 20 Clinical studies undertaken alongside the economic evaluations were assessed for risk of bias as part of the quality evaluation of comparative studies (see Table 3 and Appendix 4). All of the evaluations suffered from limitations, as highlighted by the checklist. Many did not undertake appropriate data collection and/or sensitivity analyses. Seven of the nine studies reported no data on resource use outcomes, which significantly reduces the transparency and transferability of the results. Five studies reported disaggregated costs (costs for each of the included cost categories rather than a total cost only), but the usefulness of such costs in terms of informing potential UK NHS cost estimates is limited without resource use data. Reporting of methods used to derive cost and resource use outcomes was limited and this made the validity of the cost estimates unclear. Most studies failed to report the price year or any cost adjustments applied. None of the studies conducted an adequate analysis of the potential impact of uncertainty on the results; all three studies that included sensitivity analyses were limited in scope. Most studies failed to consider the generalisability of their results. Full results for the quality assessment can be found in Table 5.
| Item | Response by study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rischin et al. 200032 | King et al. 200031 | Lowenthal et al. 199645 | Pace et al. 200929 | Stevens et al. 200633 | Vergnenègre et al. 200649 | Remonnay et al. 200234 | Hansson et al. 201338 | Corrie et al. 20134 | |
| Study design | |||||||||
| 1. The research question is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. The economic importance of the research question is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. The viewpoint(s) of the analysis are clearly stated and justified | Yes | Yes | Yes | No | Yes | No | Yes | No | No |
| 4. The rationale for choosing the alternative programmes or interventions compared is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. The alternatives being compared are clearly described | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. The form of economic evaluation used is stated | No | No | No | Yes | No | No | Yes | No | Yes |
| 7. The choice of form of economic evaluation is justified in relation to the questions addressed | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Data collection | |||||||||
| 8. The source(s) of effectiveness estimates used are stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Details of the design and results of effectiveness study are given (if based on a single study) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 11. The primary outcome measure(s) for the economic evaluation are clearly stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Methods to value health states and other benefits are stated | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Yes |
| 13. Details of the subjects from whom valuations were obtained are given | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | No |
| 14. Productivity changes (if included) are reported separately | N/A | N/A | N/A | N/A | No | N/A | N/A | N/A | N/A |
| 15. The relevance of productivity changes to the study question is discussed | N/A | N/A | N/A | No | Yes | Yes | N/A | N/A | N/A |
| 16. Quantities of resources are reported separately from their unit costs | No | No | Yes | Yes | No | Partially | No | No | No |
| 17. Methods for the estimation of quantities and unit costs are described | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| 18. Currency and price data are recorded | No | No | No | No | No | No | Yes | No | No |
| 19. Details of currency of price adjustments for inflation or currency conversion are given | No | No | No | No | No | No | No | No | No |
| 20. Details of any model used are given | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 21. The choice of model used and the key parameters on which it is based are justified | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Analysis and interpretation of results | |||||||||
| 22. Time horizon of costs and benefits is stated | Unclear | Yes | Yes | Unclear | Yes | No | No | Unclear | Yes |
| 23. The discount rate(s) is stated | No | N/A | N/A | No | N/A | N/A | No | No | No |
| 24. The choice of rate(s) is justified | Unclear | Yes | Yes | No | Yes | No | Unclear | Unclear | Yes |
| 25. An explanation is given if costs or benefits are not discounted | No | No | No | No | No | No | No | No | No |
| 26. Details of statistical tests and confidence intervals are given for stochastic data | Yes | Yes | N/A | No | Yes | Yes | Yes | Yes | Yes |
| 27. The approach to sensitivity analysis is given | N/A | Yes | N/A | N/A | N/A | Yes | Yes | N/A | Yes |
| 28. The choice of variables for sensitivity analysis is justified | N/A | No | N/A | N/A | N/A | Yes | No | N/A | No |
| 29. The ranges over which the variables are varied are stated | N/A | Yes | N/A | N/A | N/A | Yes | Yes | N/A | N/A |
| 30. Relevant alternatives are compared | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Partially |
| 31. Incremental analysis is reported | No | No | No | No | No | No | Yes | No | Partially |
| 32. Major outcomes are presented in a disaggregated as well as aggregated form | No | Yes | Yes | Yes | No | Partially | Yes | N/A | No |
| 33. The answer to the study question is given | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 34. Conclusions follow from the data reported | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 35. Conclusions are accompanied by the appropriate caveats | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 36. Generalisability issues are addressed | No | No | Yes | No | Yes | No | Yes | Yes | No |
Overall, the studies were deemed to be of low or uncertain quality due to small sample sizes; limited reporting on resource use, costs and methodology; and lack of robust sensitivity analyses. There is likely to be significant uncertainty around the cost-effectiveness results.
Economic study results
Health outcomes
Several health outcomes were assessed in the nine economic evaluations: EORTC QLQ-C30, FLIC scores, safety and adverse events, patient satisfaction and patient preferences. It was unclear why only one economic evaluation reported quality-adjusted life-year (QALY) outcomes when these would better facilitate modelling.
There was no robust evidence of any meaningful between-setting differences for most health outcomes (EORTC QLQ-C30, FLIC scores, safety and global satisfaction). There was less than robust evidence that emotional functioning, anxiety and depression were improved in the home and outpatient settings compared with a GP surgery (OUTREACH)4 and that satisfaction was higher in the home than in the outpatient setting in terms of nursing care and the depressive nature of the setting. 29,31 Evidence in the child population was limited. Children’s quality of life was improved in the home setting, compared with an outpatient setting. 33 A full description of these health outcome results, as measured in the trials, is presented in Results, Clinical effectiveness studies.
OUTREACH measured patient utility across outpatient, home and community (GP practice) settings in a UK population using the European Quality of Life-5 Dimensions (EQ-5D) questionnaire. 4 The study recruited 97 participants: 57 patients provided data for analysis of the primary end point EORTC QLQ-C30 and 48 provided EQ-5D utility scores; there were no details of why nine patients provided data on the primary outcome but not for EQ-5D.
The OUTREACH publication4 reported total QALYs gained but not baseline values. Mean differences from baseline over a 12-week period showed that the community setting produced the largest change [0.191 QALYs; standard deviation (SD) 0.04 QALYs], followed by hospital outpatient (0.174 QALYs; SD 0.034 QALYs), and home (0.165 QALYs; SD 0.053 QALYs).
The OUTREACH authors provided us with an analysis of the difference in mean differences adjusted for baseline EQ-5D utility values. With adjustment for differences in baseline QALYs, hospital outpatient chemotherapy was found to produce the most QALYs and was used as the reference for other treatment settings. Compared with the outpatient setting, the community setting (GP practice) had a mean difference of –0.009 (p = 0.0471) and the home setting had a mean difference of –0.010 (p = 0.374) (P McCrone, Centre for the Economics of Mental and Physical Health, Institute of Psychiatry, King’s College London, personal communication). These data implied that patients treated in a GP/community and hospital settings had a lower health-related quality of life at baseline, suggesting that they might have been in a poorer health state than participants who were treated at home.
There were significant limitations with these data. In particular, they were based on a small number of patients (19 or fewer in each treatment arm) and both the adjusted and unadjusted QALY results were subject to significant uncertainty. None of the results achieved statistical significance and it would be inappropriate to draw definitive conclusions on the basis of these data.
Resource use and costs
Reporting for resource use and costs across the nine economic evaluations was variable. Cost categories and perspectives were similarly inconsistent. These inconsistencies in resource use and perspectives across the economic evaluations make it difficult to ascertain any objective trend in favour of one treatment setting over another.
Only two studies reported resource use, an Australian study45 and a UK study,29 but details were limited to travel and labour. Both studies reported resource use for nurse travel to community or home settings. 29,45 Pace et al. 29 reported resource use for patient travel, in addition to nurse travel, to a community outreach site, and the number of nurses needed to deliver the service in the community setting. Lowenthal et al. 45 reported hours spent on delivering treatment as well as time spent travelling and preparing treatments.
Costs across the included evaluations varied widely. Most studies were from non-UK settings, which can limit the generalisability of resource use and cost data to the NHS and reduced their usefulness in informing a de novo model for this review. Costs and resource use across the countries vary greatly owing to differences in health-care delivery systems and differences in the prices paid for services.
One of the non-UK studies, Remonnay et al. ,34 compared chemotherapy administered in an outpatient setting with chemotherapy administered at home by a charitable organisation. The care concept has relevance to the UK as several examples of chemotherapy delivered in the community as part of a partnership between a charitable organisation and hospital have emerged in recent years. 50–53 In the Remonnay et al. study,34 the charitable organisation paid higher costs for chemotherapy drugs than the outpatient facility (25% to 121% higher) because of differences in purchasing methods. In sensitivity analysis, drug costs were made equivalent for home administration, which led to home chemotherapy being less costly than outpatient administration. This was primarily due to large overhead costs in the outpatient setting. The study was generally well reported, but did not report resource use, which limits transferability to a UK setting. The authors were contacted for further information, but no response was received.
The review identified two UK economic evaluations, both of which, owing to scope and reporting, were of limited usefulness in informing a de novo model. The study by Pace et al. 29 was concerned only with travel time and did not consider other health-care resources and costs that might differ between settings. The OUTREACH trial4 presented only total costs for each treatment arm in their published paper, with no breakdown of the costs within each intervention arm. Correspondence with the authors led to additional cost data being provided. These data were broad cost categories including inpatient and outpatient costs, day hospital costs, accident and emergency (A&E) visits, non-cancer medications and nurse diary contacts. The review team identified some discrepancies in these data. Queries were raised with the authors, but were not resolved. In brief, for some cost categories the data appeared to be resource use with no total cost provided, for other categories the data were total costs with no resource use data, and for some categories it was not clear which were being presented.
Overall, the evidence on resource utilisation and costs was extremely limited. Data sets were small, results were inconsistent and there was a great deal of between-study heterogeneity for patient characteristics and methodology; these limit the generalisability of the results.
Economic evidence summary
Quality across the economic evaluations was variable, and overall should be considered poor. Biases in the clinical study, the level of reporting on resource use and associated unit costs, and the lack of health-related quality-of-life outcomes were major limitations. Poor reporting of resource use and the use of different perspectives across the different settings made the results difficult to compare. Several economic evaluations used patient preference as an outcome measure, rather than health-related quality-of-life outcomes, which are more widely used to inform decision-making. 29,32,34,45,54 High levels of uncertainty make it difficult to ascertain whether or not costs or outcomes differ between settings.
Qualitative studies
Overview of study characteristics and quality
Seventeen qualitative or mixed-methods studies were included in this review (published between 1984 and 2012). 4,24,29,30,32,39,41,51,55–64 The studies were conducted in Canada (four55–57,60), the USA (one59), the UK (nine4,24,29,30,39,51,62,63), Denmark (two41,61), Iceland (one64) and Australia (one32). UK studies were in England (six4,24,29,30,39,62) and one in each of Wales,51 Northern Ireland63 and Scotland. 58 Table 6 displays a summary of the included study characteristics. Appendix 8 gives full details of the extracted data. Table 7 provides summary quality assessment results. Stevens et al. 200655 and Stevens et al. 200456 are separate publications from the same study, where one paper reported on health-care professional views and the other focused on the experiences of the children and their parents; these two publications have been kept separate for clarity and, therefore, there were 18 sources of data contributing to the synthesis.
| Study | Linked to trial? | Country | Perspectives presented within the data (patients included both children and adults) | Contexts discussed | Data collection | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Carers/partners/parents | Health-care professionals | Home | Community | Outpatient | ||||
| Bakker et al. 200157 | No | Canada | ✓ n = 28 | ✓ | ✓ | Purposive sample | |||
| Interviews | |||||||||
| Butler 198459 | No | California (USA) | ✓ n = ? | ✓ | ✓ | Sample unclear | |||
| Follow-up interviews | |||||||||
| Corrie et al. 20124 | Yes (RCT) | England (UK) | ✓ n = 11 | ✓ n = 15 | ✓ | ✓ | ✓ | Purposive sample | |
| Interviews | |||||||||
| Crisp 201060 | Yes (pilot non-RCT) | Alberta (Canada) | ✓ n = 10 | ✓ | ✓ | Convenience sample | |||
| Interviews | |||||||||
| Frølund 201141 | Yes (case series) | Denmark | ✓ n = 6 | ✓ | ✓ | All case series included | |||
| Interviews | |||||||||
| Hall and Lloyd 200830 | Yes (RCT) | England (UK) | ✓ n = 15 | ✓ | ✓ | All trial patients included | |||
| Interviews | |||||||||
| Hansson 201161 | Yes (non-RCT) | Denmark | ✓ n = 11a | ✓ n = ? | ✓ | ✓ | Purposive sample | ||
| Interviews (with families) | |||||||||
| Hjorleifsdottir et al. 200864 | No | Iceland | ✓ n = 25 | ✓ | Convenience sample | ||||
| Interviews | |||||||||
| Iredale et al. 201151 | No | Wales (UK) | ✓ n = 6? | ✓ | ✓ | Sampling not reported | |||
| Interviews | |||||||||
| Kelly et al. 200462 | No | England (UK) | ✓ n = 5 | ✓ n = 12 | ✓ | ✓ | Targeted convenience sample | ||
| Interviews | |||||||||
| McIlfatrick et al. 200763 | No | Northern Ireland (UK) | ✓ n = 30 | ✓ | Convenience sample | ||||
| Interviews | |||||||||
| Mitchell 201139 | No | England (UK) | ✓ n = 20 | ✓ n = ? | ✓ | ✓ | Convenience sample | ||
| Interviews | |||||||||
| Pace et al. 200929 | Yes (RCT) | England (UK) | ✓ n = 11 | ✓ | ✓ | All patients in trial | |||
| Open-ended questionnaire item | |||||||||
| Rischin et al. 200032 | Yes (RCT) | Australia | ✓ n = 20 | ✓ | ✓ | All patients in trial | |||
| Open-ended items in questionnaire | |||||||||
| Smith and Campbell 200458 | No | Scotland (UK) | ✓ n = 19 | ✓ | ✓ | Purposive sample | |||
| Telephone interviews | |||||||||
| Stevens et al. 200456 | Yes (RCT) | Canada | ✓ n = 33 | ✓ | ✓ | Purposive sample | |||
| Interviews | |||||||||
| Stevens et al. 200655 | Yes (RCT) | Canada | ✓ n = 14a | ✓ n = 24 | ✓ | ✓ | Convenience sample | ||
| Interviews | |||||||||
| Taylor 200824 | No | England (UK) | ✓ n = 9 | ✓ n = 2 | ✓ n ≈ 130 | ✓ | ✓ | Convenience and purposive sample | |
| Focus groups, open-ended questionnaires and interviews | |||||||||
| Study | Author’s position/reflexivity | Sampling | Data collection | Data analysis | Validity/validation | Participants’ voice | Data vs. interpretation | Transferability/generalisability |
|---|---|---|---|---|---|---|---|---|
| Bakker et al. 200157 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Butler 198459 | No | Unclear | Unclear | Unclear | Unclear | Yes | No | No |
| Corrie et al. 20124 | No | Yes | Unclear | Unclear | No | Yes | Unclear | Yes |
| Crisp 201060 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Frølund 201141 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hall and Lloyd 200830 | No | Yes | Unclear | Unclear | No | Yes | Yes | No |
| Hansson 201161 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hjorleifsdottir et al. 200864 | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | No |
| Iredale et al. 201151 | Unclear | Unclear – not reported | Yes | Unclear | No | Yes | Unclear | No |
| Kelly et al. 200462 | Unclear | Yes | Yes | Yes | No | Yes | Yes | Yes |
| McIlfatrick et al. 200763 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Mitchell 201139 | Yes (partial) | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Pace et al. 200929 | No | Yes | Yes | No | No | Unclear | Unclear | No |
| Rischin et al. 200032 | No | Yes | Yes | No | No | Unclear | Unclear | No |
| Smith and Campbell 200458 | No | Unclear | Yes | Yes | Yes | Yes | Yes | No |
| Stevens et al. 200456 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Stevens et al. 200655 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Taylor 200824 | Unclear | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
Participant views represented include chemotherapy patients (16 studies;4,24,29,30,32,39,41,51,55,57,59–64 two included children55,61); carers/partners or children’s parents (four studies24,39,55,61); and health-care professionals (five studies4,24,56,58,62). The study contexts varied, with half the studies taking place within a clinical study. Participants often spoke comparatively about their experiences based on current and prior treatment received or given.
Six papers contributed data on community provision of intravenous chemotherapy. 4,29,39,51,57,58 Eleven papers contributed data on the home setting4,24,30,32,41,55,56,59–61 and one paper was a theoretical discussion of a proposed service. 62 All 18 papers included discussion of outpatient settings and two studies focused exclusively on this. 63,64 The OUTREACH study contributed data on all three settings. 4
As discussed in the summary of the quantitative studies (see Chapter 6, Strengths of the review, Clinical effectiveness studies) study populations are likely to reflect a limited and biased perspective given the nature of the research. All papers dealing with community or home chemotherapy were based around new services, pilots or trials, which implies that the provision may be particularly high quality. It was rare to see any indications of problems with the new services. There was one comment about the chance of the community chemotherapy bus being cancelled but the patient noted that this never occurred. One Canadian study mentioned problems with drug deliveries. 55 There were no studies discussing new community cancer suites or outpatient chemotherapy suites; these facilities tend to offer integrated care in light and airy spaces designed for comfort. 65,66
Almost all patients expressed a preference for treatment location, with varying degrees of strength. The few patients who did not seem to mind about location were clear that the treatment was a necessary inconvenience regardless of the place it occurred.
It is worth noting that although Smith and Campbell58 was a discussion around a proposed community service, the views and comments from participants were essentially the same as those given by patients who had received community chemotherapy in other studies. This suggests that focus group work may be a useful preamble to setting up new services, as both patients and health professionals are likely to identify any potential barriers and benefits, enabling pre-emptive action.
Fifteen studies4,24,30,39,41,51,55–58,60–64 used interviews to gather data (one study used telephone interviews58), and two used open-ended questions on a questionnaire. 29,32 One study collected data through focus groups, open-ended questionnaires and telephone interviews. 24
Most papers did not clearly identify the author’s position, or appear to engage substantially with reflexivity. That is, they did not appear to consider the impact of the researcher on the data collection and analysis. Four papers at least partly addressed these issues including considering the potential impact of the author on the data collection and analyses process. 39,41,60,61 Other papers either did not clearly identify the researcher who collected and analysed the data or did not discuss the implications where the researcher was a cancer nurse. 30
The most common form of sampling was based on convenience; fewer studies used purposive sampling and three studies included all patients who participated in the trial or pilot. 29,30,32 Information on participation rates was scantly reported across the studies making it difficult to draw any general conclusions. In some studies, staff delivering care to study participants were responsible for recruitment or suggesting participation; this may have contributed some bias.
Study methodology was reported infrequently. Analyses were most commonly labelled as content analysis (five studies55,56,58,61,64) or thematic (four studies24,30,51,62). Two studies used framework,4,57 two used phenomenology,39,41 one used the constant comparative method60 and one used narrative analysis. 63 Three studies did not report their methods of analysis. 29,32,59
Validation and the consideration of validity can be a problematic topic within qualitative research. Ten out of 18 studies39,41,55–58,60,61,63,64 in this group reported using some form of validation. This included peer discussion, independent coding by more than one researcher and discussion of preliminary findings. Most studies provided clear quotations from the participants and emphasised the primacy of the interviewee’s voices. Thirteen out of 18 studies24,30,39,41,55–58,60–64 drew clear distinctions between collected data and researcher interpretation. Transferability and generalisability were discussed in some detail by nine studies.
Synthesis
Overall, the data were grouped under three main lines of argument (Table 8). Two were relatively self-contained: one addressed issues around perceived cost and barriers to service use, while the second addressed satisfaction with treatment experience and provision.
| Key lines of argument | |||
|---|---|---|---|
| Barriers to service provision | Satisfaction with intravenous chemotherapy | Making compromises to maintain normality | |
| Example codes | Staff personal safety concerns | Communication | Medical expertise |
| Reluctant to treat | Information provision | Safety | |
| Patient safety | Understanding information | Additional procedures and tests | |
| Lack of professional support | Privacy | Keeping cancer out of the home | |
| Capacity concerns | ‘KFC’-style treatment | Shared experiences with other patients | |
| Cost of the service (patient and staff views) | Rapport and relationship with health professionals | Time: travel time and costs | |
| Lack of communication between health professionals | Security | Time: waiting for treatment | |
| Time: to spend on other activities | |||
| Anxiety | |||
| Fatigue and energy | |||
| Identity | |||
| Control | |||
The third line of argument was more substantial than the other two, and more clearly relates to specific issues of the setting for intravenous chemotherapy. The decisions made by patients, carers and family members were focused on maintaining normality in everyday life. Compromises were required to balance competing factors and patients’ location preferences reflected their individual situations.
The three main lines of argument are not entirely independent; where links were obvious these have been noted in the descriptive text that follows.
Line of argument: perceived barriers
Most data within this concept were contributed by health professionals (oncologists and nurses of varying grades) rather than by the patients (Figure 2).
FIGURE 2.
Line of argument: barriers.
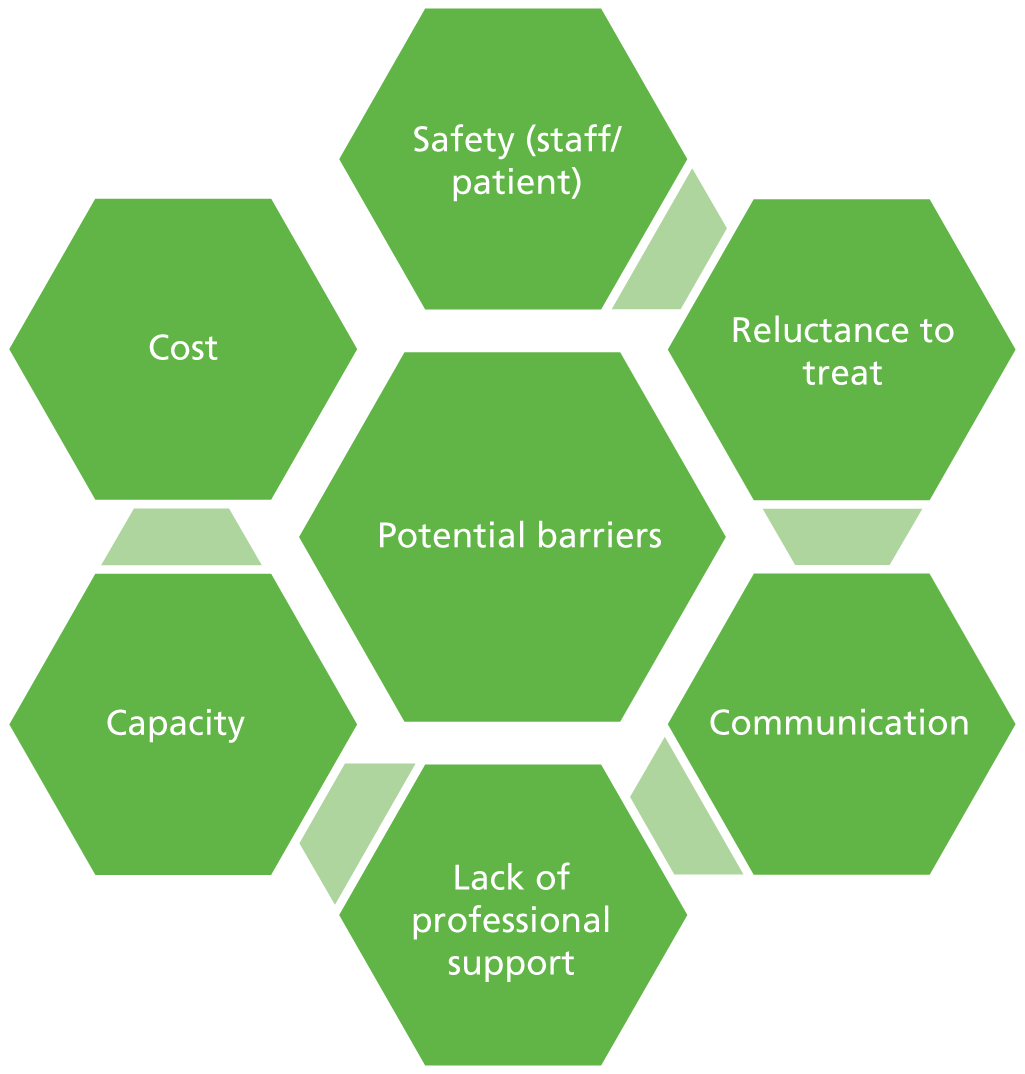
Several patients cast doubt on the cost-effectiveness of home chemotherapy, even where they had reported positive experiences:
It’s just not cost-effective we’re going into a period of austerity, you know cut backs and all the rest of it [patient]
It’s crazy [wife]
It doesn’t make any sense to me [patient]
Patient and wife39
Comments such as this suggest that patients may not take advantage of an offered service where they feel it is a waste of resources, even if they would find it personally useful.
The health professionals from Kelly et al. ’s study62 raised issues of the costs in setting up the service and numbers of patients who could be treated, commenting that it was very difficult to accurately estimate costs for a new service. The consultant questioned whether or not offering home chemotherapy would simply mean that the spare capacity in the outpatient ward would be swallowed up by new demands. Similar views were expressed by health-care professionals in the study by Taylor. 24
You can’t take one bit out and leave a gap and not expect it to fill in very quickly. We’re more like a beach than a building.
Consultant62
Health professionals from the Stevens et al. study55 commented that additional administrative tasks required for home visits were time-consuming. While actual visit time was relatively brief, preparation calls, such as checking drug deliveries or telephoning for blood test results, were described as ‘frustrating’. 55
Both consultants and nurses raised concerns around the personal safety of health professionals travelling alone to patients’ homes. 4,24
If something went wrong you are on your own, you’ve got no back-up whatsoever if anything happened.
Chemotherapy nurse4
One consultant highlighted that working in settings other than outpatient wards meant a potential lack of peer support and guidance for staff.
The nurses will be doing it in isolation, they can’t ask anyone to come and have a look and it’s quite nice often to run things past someone else.
Oncologist4
The potential for lack of communication or inconsistent treatment between settings was also flagged up by health-care professionals in Stevens et al. 56 This was seen as a factor that could damage trust between outpatient and community staff teams, but clarifying responsibilities and increasing communication would reduce this.
Many hospital HPs [health professionals] indicated that inconsistent interaction with the child and family was somewhat distressing and that they would prefer regular updates on the child’s progress . . . The HPs also emphasised the need for treatment procedures to be consistent in the home and hospital. 56
Line of argument: satisfaction
There was a cluster of codes around satisfaction with the treatment overall including facilities and staff (Figure 3). This seemed to sit outside the trade-off being made by patients to maintain normality and is likely to reflect concerns and experiences about NHS treatment in general. Data here include elements specific to home and community treatment.
FIGURE 3.
Line of argument: satisfaction. KFC, Kentucky Fried Chicken.
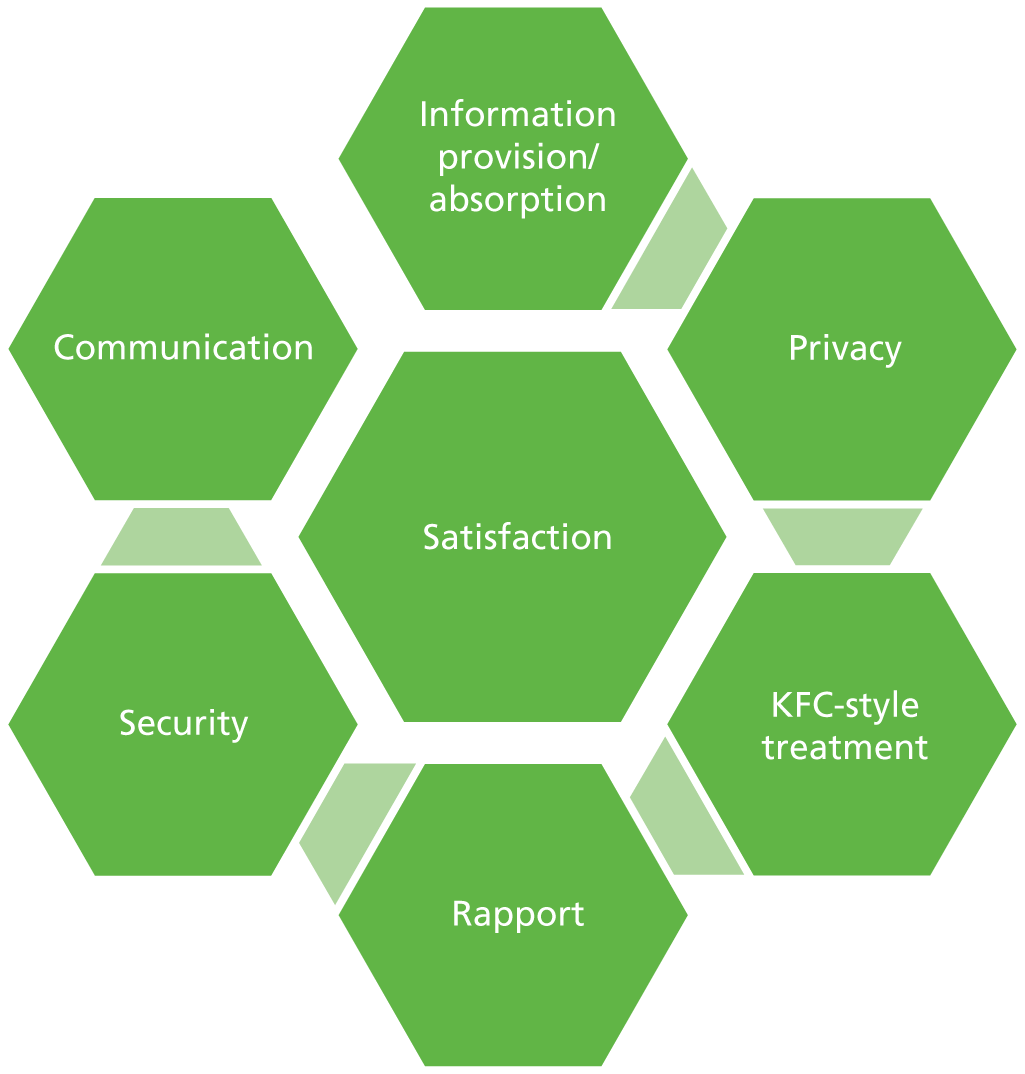
As mentioned earlier, all of these data relate to new community or home services, and to pre-existing outpatient settings. None of these studies looked at the newer, purpose-built chemotherapy suites, and so it is unsurprising that many of the comments about physical facilities reflected unfavourably on the outpatient environment. Mobile buses in particular were described as being relaxed, warm, calm and very unlike a busy hospital. 51 This has clear links into the largest line of argument around maintaining normality: such facilities offer a middle ground between the comfort of home and the barren hospital.
Good communication (e.g. between community chemotherapy settings and the main cancer hospitals) was cited as a factor in increased confidence for patients receiving treatment in other locations. The faxes, e-mails or telephone calls indicated that local staff were in contact with, and supported by, the larger cancer care team. This category links into medical expertise versus normality as discussed in the third line of argument.
The staff at the [regional] cancer center phoned the nurse here and told her what she was to give me and she had everything ready for me the next day. I feel confident that if I called her, she would have the answer . . .
Patient57
This communication also balanced out some of the potential disadvantages in a less technologically developed community setting.
Maybe you don’t have the technology that you would have at the big cancer centers . . . but I believe that in the smaller clinics the comfort you find with the communication compensates for some of the loss in technology.
Patient57
In contrast, poor communication around waiting times and making appointments was felt to be a particular problem in outpatient settings. This is explored in more detail in the following section. Patients were clear about not holding the nurses responsible but complained about the organisational aspects and systems.
You couldn’t fault the staff it’s just that it doesn’t seem to be very logistically organised at all . . . All I can say about it is it just needs leadership. . . . since the new clinic [has been built] the appointments are worse, far worse.
Patient39
Aspects such as the physical layout of the outpatient units, the use of temporary facilities that are still required after 10 years, and logistical difficulties in scheduling appointments all tended to lead to frustration on the part of both health-care professionals and patients. As Kelly et al. 62 reported, staff working in the chemotherapy unit experienced regular difficulties when trying to communicate between these different sites.
There’s just so many links in the chain that almost inevitably one breaks down. So it’s set up to be very difficult to manage.
Nurse62
There was often a large amount of information to take in before and after chemotherapy treatment. One study indicated that patients struggled to access the desired information from their doctors in the outpatient setting which reduced control and increased anxiety. 63 Data from Crisp,60 and Hall and Lloyd30 suggested that patients found it easier to understand the information in the home setting rather than in an outpatient clinic. Patients commented that they felt more able to ask questions of the nurses when at home and that the extra time made it easier to ask about the niggling concerns that might be missed in an appointment with the doctor. In one example, the nurse telephoned the patient later on to answer a specific query, indicating a high level of ongoing communication.
I was able to really talk to the nurses and the nurses had a lot more time with me. It was one on one time. So I got way more information from my treatment at home meetings than with my doctor.
Patient60
The mobile bus services were mentioned in relation to privacy, both positively and negatively. Iredale et al. 51 reported that patients felt it was like having a private room, whereas Mitchell’s39 participants reported that the close proximity of the treatment chairs meant very little privacy in relation to physical aspects of treatment. However, this was not perceived to be an unresolvable problem once, for example, patients ‘get used to knowing what to wear’.
Home settings were associated with increased privacy, which patients valued in relation to the side effects of the chemotherapy. Crisp reported patients preferring to use ‘their own bathrooms for vomiting or diarrhoea or taking steps to make intravenous insertion easier’. 60 Home chemotherapy was described as more private also because no-one was listening in on patients’ conversations. 30
A mixture of experiences was reported across settings in relation to forming good relationships with the health professionals. There was no clear indication that one setting resulted in better rapport than any of the others and some patients were particularly keen to highlight the excellent care they received from both outpatient and community or home chemotherapy staff.
Hospital staff appeared to be under more pressure than their home or community counterparts; however, they were still delivering excellent care in most of the accounts included in this synthesis. Patients valued the human contact as highly as the actual treatment itself.
This also included the importance of receiving treatment, which was given in a warm and sensitive way, and caring encounters were seen as closely intertwined with the treatment itself.
Author interpretation64
In one of the studies which included interviews with children, they commented that they liked the hospital sessions particularly because of getting to see their favourite regular nurses. 55
Community chemotherapy settings were most often described as personal, friendly and more relaxed than outpatient clinics, which allowed more time to ask questions. This included the input of the mobile bus driver, who made patients a cup of tea and showed them where to wait for treatment. 39,51
The patient who said that they preferred outpatient over community treatment cited ‘the gloomy décor and lack of atmosphere at the outreach location’,29 which may suggest that physical environments are influential in decision-making. Alternatively, this may highlight the particularly subjective nature of such experiences and judgements. It was not clear from the data how such preferences interacted with travel time and waiting times.
Consistency of nursing staff was mentioned most often in relation to chemotherapy delivered at home. Having the same nursing team for each visit combined with the feeling of having their ‘undivided attention’ to increase satisfaction levels. 30,41,60,61 Patients did not have to repeat themselves to multiple new nurses and there was an improved understanding of their circumstances, which led to an easier exchange of information. Seeing the same well-qualified and punctual nurses was particularly important for parents in the Hansson et al. study, who felt this increased security. The children enjoyed showing their home-care nurses around the home. 61
I like to have the same nurse who is my main nurse, it is the relationship with my nurse, she knows everything about me and I know a lot about her, this is like a friendship.
Paediatric patient64
In contrast, Frølund reported that patients felt that it was more important to be treated by an experienced nurse than to see the same nurse on every visit. This was not reflected in other data but remains a valid comment on treatment preferences. 41
Despite these generally positive comments, one couple reported that they felt that the home-care nurse had less time for questions and discussion than the hospital nurses. 67 The only negative comments from health-care professionals reflected the reduced contact with parents and children who were having treatment at home. While the hospital nurses valued having the extra time to spend with other patients, they worried about how the home treatment children were coping with their treatments. 56
Line of argument: compromise to maintain normality
The degree to which patients expressed a preference about where they received chemotherapy appears to depend on which location offered the best possible compromise between a range of factors which are discussed in the following pages. The balancing act can be seen as between factors that favoured outpatient treatment and those that favoured an alternative to outpatient. That is, there was no clear distinction between home and community settings (mobile or other location), but a collection of factors which might sway a patient towards preferring non-outpatient treatment.
Themes which seemed to push patients and carers/parents towards outpatient treatment included medical expertise, safety, scheduling non-chemotherapy treatments, keeping cancer out of the home and shared experiences with other patients (Figure 4).
FIGURE 4.
Factors pushing patients towards outpatient treatment.
Medical expertise and safety was mentioned in all of the included study results and was clearly an important factor when patients and health-care professionals were thinking about preferred treatment locations. Community clinics were generally characterised as being less technologically advanced, although this was described as a ‘small disadvantage’ rather than a major concern in one study. 57
There was a mixture of experiences across those studies where patients experienced more than one setting. As described earlier, for some patients the evidence of clear communication and support between community and outpatient settings was sufficient reassurance,57 while for others they preferred to be treated in hospital with immediate access to the expertise. 4,39,64 These patients tended to have already experienced outpatient care and were unwilling to try a service such as the mobile chemotherapy unit even when assured it would be delivered by the same nurse team.
. . . it was exactly the same treatment as Cheltenham, . . . the reason I didn’t opt for it, and it isn’t really logical, . . . was simply because I was confident with the treatment I had at Cheltenham and it was the feeling of not wanting to put that at risk.
Patient39
Concerns about safety and being unwilling to take chances were most strongly expressed in one study of paediatric cancer patients, although this was not consistently demonstrated in all child-related data. In Stevens et al. 2006,55 some patients felt more secure in the hospital setting, particularly where there was a concern about negative reactions to the chemotherapy.
Some studies highlighted specific concerns relating to the medical equipment such as catheters, pumps and intravenous devices. Again, there was a mix of views between patients who were worried about what might happen in the home setting,59 and those who found that it was easier to prepare for intravenous insertions in the warmth of their own home60 and so worried less about the associated procedures.
In all of this data set, patients still had to attend the outpatient clinics for review meetings or monitoring via blood tests or other procedures. The logistical arrangements prevented some patients from being able to have community chemotherapy owing to the need for co-ordinating tests and treatments, which resulted in disappointment. 39 In Stevens et al. ’s Canadian study,55 the children receiving home chemotherapy visited a local laboratory to have bloods taken while the children having outpatient treatment had bloods taken in the hospital. This caused some problems as the laboratory used venous sampling which could be more painful than the finger-stick sampling children were accustomed to from the hospital. Although this was not seen as a major disadvantage, it was an unpleasant experience which reduced the appeal of home chemotherapy treatments. 55 There was no evidence in the data that patients would prefer outpatient treatment in order to enable all of the tests and procedures to be carried out in one location or visit.
For some patients and parents of children, one of the key benefits to having their chemotherapy in the outpatient unit (as opposed to at home) was the ability to keep the cancer segregated from everyday life. In these situations, being able to go home after treatment was seen as a relief and allowed the cancer to be left in the hospital. 4,56,61,63
I know I sound a bit weird, but there is also the thing that if you are treating the cancer at home, then the cancer is at home.
Patient4
The fact that the chemotherapy treatment was carried out as an outpatient was also seen as helpful; being able to walk in and walk out reduced the feeling of being ill. 63 Where there were other children in the family, treatment in the hospital allowed the home to remain as a safe refuge, although treatment at home was felt in some cases to reduce sibling anxiety about the whole process. 61
Opinions among the patients and families were mixed when it came to the benefits of being treated among those experiencing similar illnesses. 4,32,41,60,61,63 Health professionals were also divided in their opinions. 58,60 Positive aspects included being able to talk to other patients, which was described as cathartic.
You’re talking to patients out in the waiting area, the person sitting beside you. Not talking in any morbid way, but you’re exchanging experiences. It’s a great therapy.
Patient63
It also allowed the conversation to stay within the hospital setting.
It’s like a support group in its own. Once you do that, you don’t need to talk about it outside anymore; you feel part of some kind of exclusive club.
Patient63
For some parents of children being treated with chemotherapy, being able to form relationships with other families was seen as a useful source of support. 61 One study reported that an adult patient felt that it was beneficial to see others who were worse off. 32
These benefits were also reported in relation to community chemotherapy settings, where smaller groups of patients were often being treated simultaneously. The chance to share experiences was seen as part of the treatment.
When I first went in I thought well this isn’t very private at all . . . actually as I sat and watched, I thought no, these people are sharing conversation with each other . . . there was a kind of bonding that went on between the patients.
Patient4
Some of the patients who had mainly received chemotherapy at home suggested that they might have preferred the chance to meet other people in the same situation, although this was not particularly strongly expressed. 30,60
Themes which pulled patients and carers away from outpatient treatment and towards the alternative (regardless of what form that might take) included specialist expertise, normal life, energy, travel, waiting time, identity, control, support network and anxiety about treatment. These were more broadly grouped under the idea of patients desiring control over a range of factors (Figure 5).
FIGURE 5.
Factors pulling patients away from outpatient treatment.
Chemotherapy in the outpatient setting was frequently associated with pre-treatment anxiety, nausea and other unpleasant side effects. Four studies also reported that following a return to hospital treatment (either for routine reasons or due to the end of pilot service provision), patients experienced anxiety-related problems. 55,57,60,61
In contrast, community chemotherapy was seen as less stressful owing to reduced travel and easier parking, while home chemotherapy was mentioned in relation to reduced treatment-related anxiety. 32,51,57,60 For one patient this was particularly pertinent as she also suffered from irritable bowel syndrome:
Helen suffered from Irritable Bowel Syndrome (IBS), and described her difficulties travelling back and forth to the hospital. At home, she had the luxury of using her own washroom and reported having less frequent attacks as a result of reduced anxiety. 60
The shared experiences and contact with other patients or families in the same situation, mentioned earlier as a beneficial aspect of outpatient chemotherapy, were sometimes seen as a disadvantage. 4,41,60,61,63 Interestingly, none of the respondents mentioned these comments in relation to community settings, although they would seem to be equally applicable.
Attending the hospital and seeing others who were more or less sick than them was quite difficult for some patients. Seeing others who were more ill added to the anxiety and fear of future deterioration, and in some cases emphasised their identity as a sick person. 41,60
I know that death is coming, but I don’t need to be reminded of it several times a week.
Patient41
The whole experience of visiting hospital for chemotherapy was identified as potentially pulling patients back into the sick role that many were trying hard to escape from.
Although health care professionals strive to promote patient independence and self-care, there are still aspects of treatment that seem to pull the patient back into the traditional sick role.
Author summary60
The formation of friendships during treatment in outpatient hospital settings was discussed earlier as a positive aspect, but loss of these friends when they died was difficult. For some participants this led to a deliberate withdrawal from social contact.
I saw a lot of people dying, I found that very off-putting. I say to myself now, I’m not going to get friendly with anybody else, because when something happens, and then, you know, it sort of unnerves you.
Patient63
While for some patients being treated as an outpatient made it easier to keep cancer away from the home, for others being treated at home minimised the impact of the disease. This was particularly obvious in data gathered from mothers with cancer who had young children. For example, the patient below describes how the disruption to daily routines caused by outpatient treatment was more difficult than the shorter home chemotherapy appointments.
My husband’s mom lives on Vancouver Island and she came over to help look after us and she was really worried that the kids were going to associate her with Mom being sick. Because she would only come when I was having treatments or when I needed help.
Patient60
In some situations home chemotherapy offered the chance for the children to see the benefits in reduced stress and anxiety, which improved the family experience in general, while for other parents the home treatments were more easily scheduled when children were at school, which made it easier to hide them.
I mean, we have such a close family. And we’re so involved with each other and I think for my children to see me happy and settled is a gift for them.
Patient60
Overall, treatment at home was associated with having greater control over how much or how little other family members living either at home or elsewhere were exposed to the chemotherapy processes.
Time also influenced this theme; as one participant eloquently stated, reducing the time spent on treatment made the illness less invasive for the whole family.
Home care diminishes the invasion in one’s life that the illness represents. It simply makes that invasion smaller: you don’t feel that affected by the illness as a family, when it means 20 minutes in your own home compared to when it means 6 hours at the hospital.
Patient61
Being able to perform normal daily tasks as a result of receiving chemotherapy at home in turn reduced the impact of the disease on their identity.
I have a deadly cancer, I know. It is there, and it will always be there . . . Home treatment makes me feel normal . . . It helps that I can live as before I got the disease.
Patient41
In contrast to some of the positive views expressed within the qualitative synthesis (see Line of argument: satisfaction), seeing different doctors in the outpatient setting who were not necessarily up to speed on the case notes was described as disjointed and frustrating. 39
This lack of individualised and tailored understanding was reflected in the comments from participants in McIlfatrick et al. ’s study. 63 The outpatient setting was described as factory-like and dehumanising, with little chance to discuss more general concerns or the person outside their cancer treatment.
It’s quick. It’s like a KFC [Kentucky Fried Chicken] cancer ward . . . you can see they have to, with, I mean, with the amount of people that comes through. But they still try to keep a personal touch to it, but it is hard for them.
Patient 763
The time taken to travel to and from outpatient centres for chemotherapy was frequently mentioned across the included studies, in terms of duration of travel, costs for patients and carers and the energy expended. 4,29,32,39,51,55–57,60–62,64
For most patients the journey to the hospital took considerable time, with associated parking difficulties and costs. This in itself might not have been such a problem, but when combined with the often long waits for treatment, patients were spending almost a full day on the chemotherapy treatment. 51
These long days were particularly difficult as patients were already feeling ill, and children might be nauseous and anxious.
Traveling is difficult, especially when you know you’re going to be sick and it’s very tiring.
Parent of paediatric patient57
It was exhausting for the parents and the child to get up in the morning and go to the hospital and they experienced it as stressful to leave the home with a child who was plagued by nausea and vomiting.
Author summary61
Travel and parking costs were often mentioned as adding to the anxiety about chemotherapy treatment, particularly for families with children and single parents who struggled to both find the funds and take the time off work. 55,61,62 Arranging childcare when the parent was required to attend hospital for treatment incurred additional financial burdens, and in some cases significantly impacted on family life. 60
The importance of reducing travel time lies in the ability of the patient (and their carer) to make ‘better use’ of this time, and money, such that they might be able to continue to work, go to school and participate in normal activities. 55,60,61
Community and home chemotherapy settings benefited from reduced travel times, but participants also commented frequently and favourably on the absence of waiting to be treated. Community chemotherapy was highlighted for the short waiting time prior to treatment; patients reported that they were able to arrive just before their appointment and be seen almost immediately. This appeared to reflect a more organised system which was much appreciated by patients as it meant the total time spent travelling and being treated was perhaps 2 hours rather than the full day required for outpatient chemotherapy.
A typical day at the clinic is as long as a normal working day, whereas treatment at home is just over an hour.
Patient41
In Mitchell’s study,39 waiting time and travelling time were some of the most important factors in every patient’s choice to have treatment on the community chemotherapy bus.
Outpatient chemotherapy accounts frequently mentioned the unpredictable nature of waiting times. 39,56,60–62,64 Health professionals also mentioned the difficulty in managing waiting times, referring to organisational factors such as drug ordering systems.
The way that the present service was configured contributed to this problem. For instance, delays occurred between the ordering of chemotherapy drugs and their arrival in the clinic. 62
Some participants commented that waiting itself was a tiring activity made more difficult by the lack of facilities or the lack of heating, which made subsequent intravenous insertion more problematic. 39,60,64
The waiting room was described as uncomfortable, and having to wait there was tiring for people with little strength and sometimes in pain.
Author summary64
Despite this issue, this same patient understood and was sympathetic to the possible reasons for the unexpected delays.
Home settings generally involved no waiting time and the nurses were praised for their punctuality. The decreased waiting time in home and community settings meant that the portion of the day spent on treatment was significantly smaller, thus impacting on attempts to maintain normality. Some of the health professionals from the Stevens et al. study56 commented that the increased flexibility within home chemotherapy benefited their teenage patients, but could be more stressful for the staff, who were trying to accommodate patient requests.
I found that we were juggling a lot. Trying to work around the teenagers’ schedules because you would end up calling them to say that you were going to come to do the chemo and they would say ‘Oh no I’m off to something or other tonight’.
Nurse56
Waiting and travel time somewhat naturally clustered together within the data set. McIlfatrick et al. ’s paper63 adds a useful aspect by highlighting that, for patients, this meant more than just travelling to and waiting for treatment.
Whenever you have the treatment, your life does revolve around it . . . you are marking time.
Patient63
In many of the studies where home or community chemotherapy was available, patients commented favourably on being able to do things (such as bathe and eat) when they wanted rather than have those activities ‘dictated by the convenience of hospital routine’. 59 The time which was freed up by not having to travel such long distances or wait as long for treatment could then be used for jobs around the house or going back to work (adult patients) or, for children, being able to continue attending school. 30,41,55,57,60
This additional time allowed patients to stay in touch with their own personal support networks, whether these were family or friend oriented. Outpatient care was associated with the loss of these networks,59,60 although for some patients the relationships formed with other patients might have been some compensation.
When patients are diagnosed with cancer, they are displaced from their homes to a contrived, heavily scheduled setting such as a hospital. Routines are lost, and the patient becomes bound by ‘the system’.
Author summary60
Patients were keenly aware of having a limited amount of time to spend with friends and family, particularly in cases where the treatment was not likely to be curative. Parents valued the opportunity to spend more time with partners and children.
Savouring positive experiences as a family frequently becomes paramount to cancer patients and their families.
Author summary59
Home chemotherapy was most often mentioned in relation to being able to spend more of the available time with key people.
Time is a great opponent . . . An invincible opponent. I know that I have an incurable disease, and therefore it is very important that I can be with family and friends. For me, home treatment has given me this opportunity to a greater extent.
Patient41
Chemotherapy was universally described as a difficult process resulting in fatigue caused by both the treatment side effects and the travelling and waiting time.
I found even driving (to the hospital) and getting my chemo, by the time I got home, I’d have to have a nap. I found myself getting tired from it really quick.
Patient60
This was important both to patients and to their carers, who were also affected. For example, parents of children receiving chemotherapy commented on the beneficial effects of home care for their children, and also for themselves.
[W]ith home care the children could sleep as much as they needed . . . a great burden had been lifted from their shoulders in a period when they didn’t have much energy due to their child’s life-threatening disease and their lack of a normal everyday life.
Parent of paediatric patient61
Patients talked about having a finite budget of energy, with home and community chemotherapy leaving more resources for them to spend as they chose after treatment.
In the past I put a cross in calendar. The whole day was devoted to the treatment . . . when I receive treatment at home; I have the energy to do other activities.
Patient41
Qualitative summary
The synthesis incorporated data from 18 papers and over 450 participants including health professionals, patients (adults and children), parents, carers, siblings and partners. Most of the available data focused on experiences of outpatient and home settings for intravenous chemotherapy, with some data on community settings including mobile bus units. Overall, the quality of the included studies was moderate to good.
Maintaining normality was the important overarching theme which tied much of the data together. Across the data set, the importance of maintaining normality throughout a difficult illness/treatment was seen as key to being able to survive and look forward. Exactly what normal life constituted varied from patient to patient. Most patients were clearly making explicit trade-offs to maximise their resources (e.g. time, money or energy). Looking at the data set, it was rarely as simple as saying that one setting maintained normality and alleviated any safety-related concerns. Health professionals recognised and referred to this when talking about their patients, indicating a shared understanding.
Normality was more easily maintained when family life was minimally interrupted, the impact of cancer on daily life and family members was controllable, and patients were able to participate in activities of value. The time and energy consumed by chemotherapy underpinned much of this category: time spent travelling and waiting for treatment meant less time for normal life. The energy expended on treatment (including travel and waiting time) could leave patients unable to participate in important activities. Although treatment- and setting-related anxiety or side effects were mentioned, these seemed to be important because dealing with the anxiety, stress and nausea took up valuable time and energy.
While the outpatient settings were most often associated with increased confidence in the staff’s ability to deal with adverse reactions, there was some evidence that good, visible communication between an expert centre and an outreach location could ameliorate some of the safety concerns. Based on the available data, the time and energy consumed by outpatient treatment reduces overall quality of life and this is a sufficient driver for patients to prefer alternative treatment locations. These themes were particularly evident in the accounts from patients receiving palliative treatment and from parents of children with cancer.
Chapter 4 Identifying current provision
Introduction
Based on the scoping work undertaken to facilitate this research and discussions with our advisory group, we were aware that there appeared to be variation in chemotherapy delivery practices throughout the UK. This variation was expected to include the likely existence of a variety of systems, reflecting the different challenges of large cancer centres and district hospitals, for example. Nurse-led chemotherapy is established in some centres but home delivery of chemotherapy is not widespread. Different geographic challenges exist for provision in remote and rural communities compared with more urban-based centres. 3,10 Some hospitals elect to utilise private providers to deliver services, while some elect to deliver these ‘closer to home’ services using their own NHS resources. To gain insight into the variation in current practice in the NHS, we undertook a survey canvassing views from relevant professionals about their experience of the delivery of home and community chemotherapy. The survey was not intended to be comprehensive; rather, it was intended to provide a general overview that would help to describe the patient pathway and inform the development of the decision model.
Methods
Owing to the likely variation in private provision and NHS provision of services, we designed two questionnaires on current provision of intravenous chemotherapy administered at home and in the community. Both were administered using an internet-based survey programme (Survey Monkey: www.surveymonkey.com) between June 2013 and September 2013. One was circulated to NHS trust organisations providing chemotherapy services and the other was sent to commercial organisations identified as providing home care or community services on behalf of the NHS.
Invitations to participate in the NHS provision questionnaire were sent via e-mail to stakeholders across England and Wales. Individuals were identified via the Cancer Network websites (where still available) and their replacement clinical groups, and through contacts provided by members of the advisory group. The survey was administered between June 2013 and September 2013. Invited participants were encouraged to disseminate the questionnaire to colleagues. Briefly, the questionnaire asked participants whether or not their hospital offered chemotherapy at home and/or in the community, how long their service had run for, who delivered it, what type of pharmacy was use and staffing details, as well as setting characteristics for the community setting. The full survey is available in Appendix 9.
The private provider questionnaire was sent to HaH, Calea UK Ltd, Bupa Home Healthcare, Baxter, Polar Speed Distribution Ltd, Alcura UK, Evolution Homecare Services Ltd, B. Braun Medical Ltd and MedCo. These providers were identified via the advisory group who provided contact details for the National Clinical Homecare Association, an industry body representing companies providing clinical home care services to NHS patients along with charitable and independent sectors within the UK. In brief, the survey asked whether the organisation delivered intravenous chemotherapy in the home or community setting on behalf of the NHS, what aspects of home or community chemotherapy were provided, who was involved in administering and overseeing the service, and whether or not any unpublished information was available. For organisations that provided the service, follow-up questions were sent to gather further details about provision of home- and community-based intravenous chemotherapy. The full survey is available in Appendix 10.
Responses
The aim of the surveys was to provide a general overview of current provision to inform this project. In the following sections we describe and summarise the main responses to the surveys. Full details of all responses to both surveys are available on request.
NHS provision survey
Respondents and services provided
We sent 65 e-mails inviting stakeholders to participate and sent a reminder e-mail to non-respondents after 2 months. Respondents were encouraged to forward the survey to other contacts; this increased the number of responses at the expense of making the percentage response unclear.
In total, 26 people from 22 organisations provided usable survey responses to the NHS survey. Sixteen of the 22 organisations were in the north of England and six were in the south. Organisations included trusts, specific hospitals and one commissioning body. Respondents were in various roles including commissioners, pharmacists, cancer nurses, oncologists, regional managers, haematologists, directors and administrators. All survey respondents were in England.
Ten organisations provided chemotherapy at home or in the community: three delivered intravenous chemotherapy only in the home, three delivered only in a community setting and four delivered treatment in both settings.
Figure 6 provides a flow chart demonstrating the services provided by respondents.
FIGURE 6.
Flow chart of current NHS provision of home and community intravenous chemotherapy. a, Herceptin®, Roche.
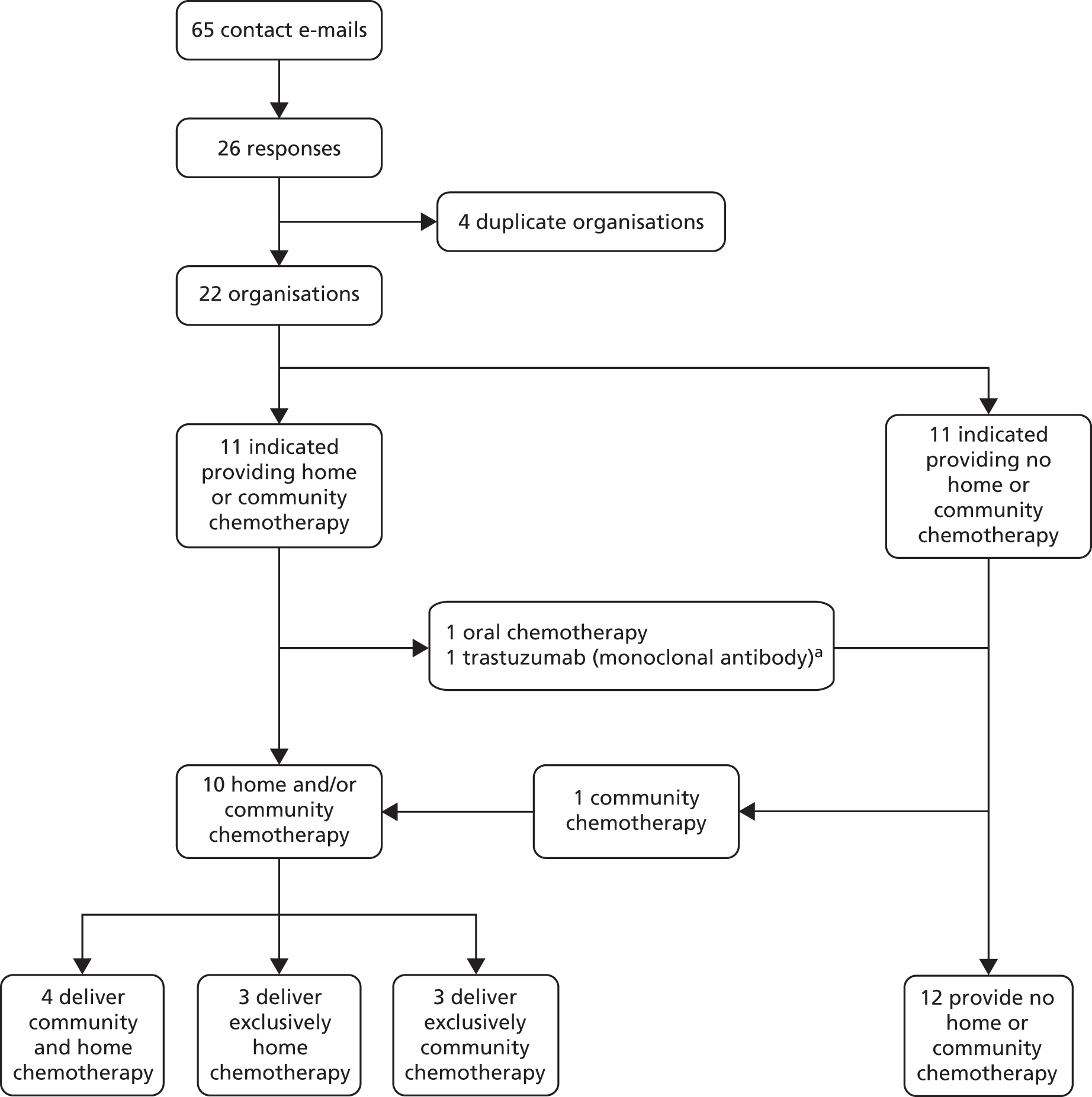
Aspects of the service and pharmacy use
Two of the seven organisations that provide chemotherapy at home indicated that the NHS delivered all aspects of the service, one failed to respond and four indicated that they used a private provider to deliver some or all of the service. Two of those using a private provider indicated that they used a hospital pharmacy for home chemotherapy; both of these deliver treatment in the home and community setting. The other two organisations which deliver in both settings were both NHS-provided services, including pharmacy.
Two of the three organisations providing treatment only in a community setting indicated that the NHS provided all aspects of the service, including hospital pharmacy, and one did not respond. Table 9 provides service provision details for settings offered, pharmacy use and training/recruitment requirements for services in the home or community.
| Organisation identifier | Services provided | Home services | Community services | Additional training | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Home chemotherapy | Community chemotherapy | NHS delivers all aspects of service | Private provider delivers some/all of service | Hospital pharmacy used | Private pharmacy used | NHS delivers all aspects of service | Private provider delivers some/all of service | Hospital pharmacy used | Private pharmacy used | Hire additional staff | Required additional training | |
| 1 | ✓ | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | – | NR | Yes |
| 2 | – | ✓ | – | – | – | – | ✓ | – | ✓ | – | Yes | Yes |
| 3 | ✓ | – | NR | NR | NR | NR | – | – | – | – | NR | NR |
| 4 | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | NR | Yes |
| 5 | ✓ | – | – | ✓ | – | ✓ | – | – | – | – | NR | NR |
| 6 | – | ✓ | – | – | – | – | ✓ | – | ✓ | – | Yes | Yes |
| 7 | ✓ | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | – | Yes | Yes |
| 8 | ✓ | – | – | ✓ | - | ✓ | – | – | – | – | No | No |
| 9 | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | – | No | No |
| 10 | – | ✓ | – | – | – | – | NR | NR | NR | NR | NR | NR |
Staff and training necessary for administration of chemotherapy at home or in the community
Staff involved in administration of chemotherapy in the home or the community included oncologists, nurses, haematologists and pharmacists. Five organisations responded that additional training and/or additional recruitment was required for their service, two indicated that no additional training was required and three did not respond. Nurses were the focus of additional training, which included training nurses to higher certification levels, training them on how to use mobile chemotherapy units (chemotherapy bus), and lone-worker training. Three of the five organisations that indicated additional training was required delivered in both settings (two NHS and one private provider). Two organisations delivered only in a community setting using a NHS service.
Three organisations indicated that hiring additional staff was required, while two indicated that it was not and five failed to respond. All of those that indicated that they would need to hire additional staff also indicated that additional training would be required; two of these were community NHS-delivered services and the other a NHS-delivered service across both settings. The two organisations that indicated no additional hiring also indicated no additional training requirements; both services had home services that were delivered by private providers.
The number of nurses involved in delivering intravenous chemotherapy in a home or community setting varied between organisations. Two organisations used one nurse at their community locations and three organisations used two nurses. Two organisations did not report how many nurses they used for community chemotherapy locations. The number of nurses who administered each individual treatment at home and community locations was fairly consistent. All five organisations that provided a response reported that one nurse was involved in each intravenous treatment.
The details of services provided, by whom and where, plus additional staff hiring and training requirements for each of the organisations, are presented in Table 9.
Eligibility for participation in home chemotherapy
Various eligibility policies were described for home chemotherapy. One of four organisations that provided home chemotherapy via a private provider reported an eligibility requirement of ‘a few’ cycles delivered in hospital; another reported that eligibility decisions were based on regimens; and two did not report eligibility requirements. There were few responses regarding eligibility requirements for chemotherapy; three organisations provided no response to eligibility requirements for chemotherapy at home and one reported that drug regimen suitability determined patient eligibility for chemotherapy at home.
Three of the seven organisations that provided chemotherapy at home reported that patients were referred to the service by consultants, and two indicated that consultants and specialist nurses could refer patients to the service. No other organisations provided referral details.
Three organisations provided estimates for the proportion of patients eligible for the home service and the proportion of patients accepting the service. There was a mix of proportions eligible and accepting, with some eligibility levels of < 5%. Both organisations that indicated < 5% eligibility also indicated that they used a home-care provider, with HaH named by one. Both organisations that had home services completely delivered by the hospital reported eligibility levels higher than those using a service delivered through a home-care provider (Table 10).
| Organisation identifier | Services provided | Private provider delivers some or all aspects of service | Eligibility requirements for home | Eligibility requirements for community | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Provides chemotherapy at home | Provides chemotherapy in the community | Home | Community | Number of cycles in hospital | Regimen | Number eligible | Number accept | Number of cycles | Regimen | Distance to hospital | Per cent eligible | Per cent accept | |
| 1 | ✓ | ✓ | – | – | – | – | 40 | 20 | – | – | – | 60 | 80 |
| 2 | ✓ | – | – | – | – | – | – | 2 | ✓ | – | NQ | NQ | |
| 3 | ✓ | – | – | – | – | – | – | – | – | – | – | – | – |
| 4 | ✓ | ✓ | ✓ | ✓ | – | – | NQ | NQ | – | – | – | – | – |
| 5 | ✓ | – | ✓ | – | 3 | – | < 5 | NQ | – | – | – | – | – |
| 6 | – | ✓ | – | – | – | – | – | – | – | ✓ | ✓ | 10–15 | 10–15 |
| 7 | ✓ | ✓ | – | – | – | ✓ | 15 | 50 | – | ✓ | – | 50 | 30 |
| 8 | ✓ | – | ✓ | – | – | – | < 5 | 70 | – | – | – | – | – |
| 9 | ✓ | ✓ | ✓ | – | – | ✓ | – | – | – | ✓ | ✓ | – | – |
| 10 | – | ✓ | – | – | – | – | – | – | – | – | – | – | – |
Eligibility for participation in community chemotherapy
In the community setting, patient eligibility criteria focused primarily on suitability of regimens and patient distance from their hospital. Only one organisation expressed eligibility limitations based on which cycle of chemotherapy was being administered; they reported that patients must be fit and have had two cycles in hospital. Two indicated that distance of patient travel was a factor in eligibility, but did not quantify what distances were acceptable. Three quantified the percentage of patients eligible for chemotherapy in the community: one reported that 60% of patients were eligible and 80% of eligible patients accepted; another reported that 10–15% were eligible and 10–15% accepted; and a third reported that 50% of patients were eligible, with 30% accepted (see Table 10).
Provision of chemotherapy services at home and in the community
Three organisations delivered chemotherapy in the community using mobile chemotherapy units. The other four organisations that delivered intravenous chemotherapy in a community setting used different locations: two in community hospitals, one in a satellite unit in a primary care centre and another used a room in a local hospice.
There were several similarities between home and community chemotherapy administration. Patients in both settings were cared for between chemotherapy treatments at their regular institutions and given access to standard 24-hour advice telephone lines, and patients were referred to both services by consultants and specialist nurses.
Organisations that indicated they did not provide chemotherapy at home or in the community
There were 12 organisations that did not provide intravenous chemotherapy at home or in the community; six indicated that they were interested in providing a service and three indicated that they may be interested in providing a service in the future. One organisation had indicated that they provide home chemotherapy, but only provided home trastuzumab. No organisations said that they would not consider providing a community or home service. Of the six organisations that said they would consider providing a service, one organisation was working on a proposal for a service, and another indicated that a service was not offered yet but was at an advanced stage of planning.
Barriers to service delivery
One of the aims of the survey was to identify barriers to service provision in the community and at home from those who provided services, and those who did not provide services. Common concerns existed in both groups. The full set of responses to these questions is available on request. Some commonly perceived barriers highlighted by responders were:
-
costs of running a service
-
value-added tax (VAT) savings, which were driving which drugs were offered at home rather than the suitability of the drugs for administration at home
-
the fact that there might have been less expensive ways to deliver chemotherapy in the community than delivering at home, but current regulations did not allow or incentivise more efficient community delivery
-
issues with consultant support for home services
-
poor strategic planning
-
broad geographical area that would be difficult to serve
-
interest in delivering a service but lack of a commissioned service
-
limited numbers of eligible chemotherapy regimens
-
lack of nursing resources
-
lack of suitably trained staff
-
a need to convince patients to use the service.
There were several limitations to the survey: the sample was small; the information provided by respondents was generally not very detailed; the survey requested recollections and descriptions from providers rather than service data; and questions were not always interpreted as intended. However, the aim was to provide a picture of current provision and add clarity to the patient pathway, where possible. The survey highlights the wide variation in current provision.
Private provider survey
All nine groups that we contacted responded to the survey but only HaH, Bupa Home Healthcare and Calea Ltd currently provided chemotherapy closer to home. None of the respondents described providing intravenous chemotherapy services in a community setting. However, the survey did not have differentiated questions regarding home and community services, and so a description for a home service did not necessarily preclude the provision of intravenous chemotherapy in a community setting. Both HaH and Bupa ran comprehensive home chemotherapy services that included patient registration; prescription, preparation and delivery of cytotoxic drugs; supply of nurses; patient counselling, and telephone support for adverse reactions; and logistics and waste removal for a variety of chemotherapy regimens (specific regimens were considered commercially sensitive and not disclosed). Calea provided off-the-shelf and compounded methotrexate to NHS trusts in the Yorkshire region inclusive of nurses where necessary. HaH indicated that they provided chemotherapy in the home or community setting to more than 40 NHS trusts. Bupa did not provide information on how many NHS organisations they provided with home or community chemotherapy services.
Further follow-up with the organisations yielded limited results. Questions about customer satisfaction, quality-of-life data, cost data and resource use were generally unanswered. Some providers said that information on these outcomes was commercially sensitive; other organisations might have been non-responsive for similar reasons. HaH responded to some queries, and instructed our team to seek answers related to quality of life, patient satisfaction, adverse events and resource use from their health informatics service, Sciensus Ltd. On investigation it was apparent that information was not freely available from Sciensus. We did not pursue paying for information.
Healthcare at Home provided some useful descriptions of their service via personal communication (S McAndrew HaH, 12 September 2013, personal communication). They indicated that their home chemotherapy service uses a regional hub for northern Europe, with support services for adverse reactions and general patient counselling provided from this facility. They also indicated that next-day service was available to the UK mainland, major islands and northern Europe using their own vehicle fleet from their regional hub. According to HaH, nurse travel to patients and between patients averaged 1 hour. HaH used their own private pharmacy, which might have made them eligible for a zero VAT rating on drugs they delivered in the home, under current UK legislation. 68
Bupa did not respond to additional requests for information but their website was very informative and included thorough service descriptions and a full list of chemotherapy regimens eligible for home delivery. 69,70 Bupa provided a business example on their website where the cost of a drug administered at home is reduced by 20% compared with NHS administration. This appeared to indicate that private providers were providing drugs with zero VAT liability. 69
From the private provider survey, it is clear that the consolidated nature of the private providers enabled them to serve larger regions. All private organisations that provided home chemotherapy to the NHS did so across multiple trusts.
Summary of current provision
There was great variety in service provision, with differences in the total number of staff involved, who provided services and how they were provided. The total number of nurses involved in delivering home and community services varied across providers, but the numbers administering each individual treatment were consistent: one at home, and one or two in the community setting.
Private providers were often used for administering home chemotherapy; this usually entailed using a private pharmacy. These private providers appear to have very selective eligibility criteria to their programmes and only accept patients after two or more cycles have been delivered in hospital. The percentage of patients eligible in privately provided programmes was lower than that provided in services that were administered completely by the NHS. Outside private provider requirements for a certain number of cycles in hospital, regimen appeared to be the most important determinant of eligibility, followed by patient performance.
Community settings included three mobile units (chemotherapy bus), two community hospitals, a satellite unit in a primary care centre and a room in a local hospice. Most community providers indicated that the NHS provided all aspects of the service. Regimen and patient fitness for treatment appeared to be the most important determinants of eligibility for home chemotherapy. More patients were eligible for chemotherapy in the community than at home.
Private providers were found to offer a potentially wide variety of regimens70 and provide comprehensive chemotherapy services to a large number of trusts (HaH), but it was unclear what effect their services had on patient quality of life or patient satisfaction. Private providers were able to take advantage of VAT exemptions for drugs, and provide services across multiple providers, both of which could lead to less expensive and more efficient service capabilities.
Chapter 5 Patient pathway
Overall pathway
Using the evidence identified through the systematic review and survey, and with advice from our advisory group, we have outlined the general pathway that an intravenous chemotherapy patient may follow through the NHS. The pathway involves many steps, some of which are outside the scope of the decision problem this research is addressing. They are outlined, however, to give context to the question that will be addressed.
-
Step 1: access and referral to an oncologist.
-
Step 2: assessment and decision to treat, patient consent.
-
Step 2a: assessment of decision to treat with chemotherapy.
It is anticipated that these three steps (1 to 2a) will be the same regardless of the setting in which the treatment will be delivered. On completion of these three steps a group of patients eligible and willing to receive intravenous chemotherapy have been identified. Figure 7 depicts the pathway for this population. In addition to the figure, the following is a summary of some of the considerations made at each step in the pathway. The evidence collated from the questionnaires, outlined earlier in Chapter 4, suggests that the eligibility criteria for treatment at home may be stricter than the criteria for treatment in the community. Therefore, a proportion of the total population eligible for intravenous chemotherapy will be eligible to receive that treatment in a community setting and a subgroup of those will be eligible to receive their treatment at home.
FIGURE 7.
Patient pathway. i.v., intravenous.
-
Step 2b: assessment of eligibility for treatment at community and/or home.
In any organisation delivering treatments within these settings, individual patient eligibility criteria will be defined [step 2b (i)]. Eligibility criteria are expected to consist of the following elements:
-
patient fitness status (ECOG performance status, comorbidities)
-
drug regimen suitability (drug stability, length of infusions, low-risk adverse event profiles)
-
clinical perception of individual patient probability of being hypersensitive to drugs (this can be mitigated through having one or two cycles in hospital)
-
clinical perception of individual patient susceptibility to severe adverse events
-
cancer type (breast, lung, colon, etc.)
-
cancer stage/grade
-
line of treatment (first, second, third)
-
neoadjuvant/adjuvant versus primary treatment
-
patient age (children, young adults, adults, elderly).
Once patient eligibility is determined, it is expected that an assessment [step 2b (ii)] of the patient’s home environment and location would be necessary unless the patient expresses a preference for treatment not to take place at home. The elements considered in any such assessment are likely to include:
-
distance from home to hospital or drug preparation unit
-
whether the patient lives alone or has support
-
whether or not the patient has pets and if they can be removed for the treatment duration
-
whether or not the patient has children and if they will be present at treatment delivery
-
whether or not the home is of an acceptable cleanliness
-
whether or not there is a place for the nurse to wash their hands
-
whether or not there is a workspace available.
These assessments may be undertaken by a multidisciplinary team (led by the oncologist and specialist cancer nurse) or by the specialist chemotherapy nurse. These assessments ensure that patients and their homes are suitable. There is also the issue of patient choice. Assuming that the health service organisation is in a position to offer all three treatment settings, there are still a number of considerations for the patients and uptake of treatment in alternative settings is likely to be variable. Across regions, choice and preference may depend on socioeconomic variation, geographical differences and the overall demographics of the regional population.
Other determinants of uptake may be significantly different. It is expected that an individual’s choice may be influenced by the following aspects:
-
time constraints
-
labour force participation
-
distance to the hospital
-
availability of transport
-
cost of transport and parking
-
ease of hospital access
-
appreciation by some people of a change of location and social interaction
-
availability of childcare
-
symptom severity
-
clinical advice and enthusiasm of health-care workers
-
society’s acceptance of home chemotherapy
-
wish to keep treatment out of the home
-
personal aversion/fear of hospitals
-
personal relationship with hospital staff
-
quality of past hospital care
-
provision of safety information
-
family agreement and support.
The main focus of this report is on the later part of the pathway, wherein patients eligible for home chemotherapy receive treatment in their chosen location. The decision problem addressed in the economic modelling will not take account of eligibility criteria and how clinical staff might identify people to be offered home intravenous chemotherapy as a treatment option. Instead, it commences with an eligible population and addresses the question of which setting is most cost-effective for this population.
Decision model
One of the aims of the systematic review was to identify relevant data for a decision model. The systematic review produced little relevant evidence to facilitate answering the decision problem robustly. In an attempt to illuminate the issues surrounding the cost-effectiveness of delivering community-based chemotherapy, we have taken the best available evidence identified during the review and used this to undertake exploratory decision modelling.
The systematic review identified nine economic evaluations that compared chemotherapy in an outpatient hospital setting with chemotherapy administered in a home and/or community setting. The limitations of these economic evaluations are described in Chapter 3 (see Cost-effectiveness studies). This report is targeted for a UK audience and so the most relevant economic evaluations for model inputs were the OUTREACH trial4 and Pace et al. 29 Both of these studies were set in the UK and provided some, albeit limited, information on costs and outcomes for a relevant UK population.
The systematic review identified eight studies which were not full economic evaluations but reported costs and/or resource use. Six studies were not from the UK; two only provided cost information42,48 and the remaining four were not included for reasons of generalisability. 27,28,35,37 The two UK studies were not sufficiently detailed to provide any additional information above that presented in the OUTREACH trial. 30,71
In the UK, cost–utility analyses (CUAs) are generally considered the most relevant type of economic evaluation to help to inform decision-making. 72 Cost–utility studies represent intervention costs and intervention benefits measured in QALYs. QALYs measure a person’s remaining quantity and also quality of life. These analyses usually produce ICERs, calculated as the costs of intervention A minus the costs of intervention B, divided by the benefits of intervention A minus the benefits of intervention B. These analyses can contain multiple interventions, in which case each intervention is compared with the next least costly intervention. Because the OUTREACH trial produced a CUA in line with UK methodological guidelines, the base-case model replicates data from the OUTREACH trial with some augmentations made where necessary. The following sections outline the model structure, parameters and results.
Model structure
A simple decision model was developed in Excel (Microsoft Excel 2010, Microsoft Corporation, Redmond, WA, USA) to assess the cost-effectiveness of intravenous chemotherapy delivered in the home, community or outpatient setting in a population considered eligible for home treatment. Figure 8 shows this patient pathway and how it relates to the broader issues around the implementation of chemotherapy at home or in the community. Many aspects of the implementation of community and home chemotherapy are not part of the decision problem addressed by the model and these are shaded green.
FIGURE 8.
Modelling patient pathway. i.v., intravenous.
All of the patients in the model are assumed to be eligible for home chemotherapy. They then choose where they receive their treatment, and incur costs and accumulate QALYs over the time horizon of the decision-tree model.
The patient population of the model is assumed to replicate patient characteristics from the OUTREACH trial: patients were above the age of 18 years with an ECOG performance status of 0–2, lived within a 30-minute drive of the recruiting hospital, and either were about to commence or had commenced a course of cancer treatment with standard infusions lasting < 4 hours for a minimum of 12 weeks with intent to treat, cure or provide palliative care. Patients were required to have a life expectancy > 6 months and be capable of independent transportation to the hospital. Patients were not taking any unlicensed drug as part of their treatment. Patients randomised between the three settings were broadly similar in ECOG performance, treatment intent and gender. There were small differences in cancer types in each arm.
We ran the model for a cohort of cancer patients receiving intravenous chemotherapy. The distribution of patients across the model arms was derived from anticipated patient choices based on data from the OUTREACH trial. Cost and utility weights are applied dependent on the treatment setting. Owing to limitations with the trial data, we elected not to extend the time horizon of the model beyond that of the trial (12 weeks) to avoid introducing more uncertainty through the use of extrapolation. The time horizon was 12 weeks and, as a consequence, the cost and utilities were not discounted in the base case. Costs are reported in 2012 pounds sterling.
Model parameters
Patient choice
The initial distribution of patients to different settings within the model was determined by patient choice assumptions derived from OUTREACH. 4 As highlighted in the systematic review of clinical effectiveness, patients may participate in a trial because they want the new treatment; others may elect not to participate to ensure that they are not exposed to unwanted treatment. In OUTREACH, patients who wanted outpatient hospital chemotherapy or did not want to receive treatment in a particular setting could simply opt not to participate in the trial. The proportion of patients in the model choosing each setting was, therefore, adjusted for recruitment bias, based on methodology from King et al. 31
Although OUTREACH reported preference data, it did not utilise a crossover design and patients were asked whether or not they would prefer to continue treatment in their allocated setting. These patients experienced only one setting during the trial and so it is unclear if their decision was informed by past experience of chemotherapy in the hospital outpatient setting or if patients were naive to chemotherapy. Owing to this, it was unclear in which setting any given patient would choose to have treatment had they experienced multiple settings.
To adjust for this limitation of the OUTREACH data we assumed that the patient population had had weak preferences as in King et al. 31 Assuming indifference to treatment settings may be considered conservative as we cannot know the motivation of patients who chose to participate in the trial; they might have participated because of preference for treatment settings other than the outpatient default. Patients who chose not to participate in the trial owing to a stated preference were by definition not indifferent to the setting and so we needed to adjust for this potential bias. Our adjustments took into account patients’ expressed reasons for preferring one setting over another as described in Chapter 3 (see Qualitative studies) and the analysis in King et al. ,31 where patients who had declined to participate in the trial due to strong preferences were reintegrated into the data to adjust preferences; this resulted in 48% of patients preferring chemotherapy at home, as outlined earlier (see Chapter 3, Clinical effectiveness studies and Satisfaction and preferences). 31,54
In OUTREACH, 33 patients were randomised to home, 32 were randomised to community (GP practice) and 32 were randomised to outpatient. Fifty-three patients declined to take part in the trial: 35 wanted an outpatient setting; 16 did not want chemotherapy in a GP practice; and two preferred chemotherapy at home. Following the King et al. 31 methodology, these 53 patients excluded owing to preference were added to those who chose to participate in the OUTREACH trial with the assumption that patients with a stated preference for outpatient would receive outpatient and those with a preference against the GP or home setting would be split evenly between the alternative settings. As the results from the King et al. 31 study suggested that overall patient preference may not be particularly strong, we assumed patients who stated no preference to be indifferent to treatment setting allocation.
After adjustments, the resultant probabilities suggested that 27.3% of patients would choose to have their chemotherapy at home, 22.0% would choose to have it in the community and 50.7% would choose the hospital outpatient setting. In a probabilistic sensitivity analysis, a Dirichlet distribution was used to reflect the uncertainty surrounding choice. Table 11 shows the transition probabilities between states used within the model and the mortality rate applied to all states.
| Intervention | Mean | n | SE | Distribution | Source |
|---|---|---|---|---|---|
| Patients choose home | 0.273 | 43 | Dirichlet | OUTREACH,4 King et al.31 | |
| Patients choose community | 0.22 | 33 | Dirichlet | OUTREACH,4 King et al.31 | |
| Patients choose outpatient | 0.507 | 76 | Dirichlet | OUTREACH,4 King et al.31 | |
| Probability of death | 0.06 | 1,278,602 | 0.0018 | Beta | ONS Cancer Survival in England 201273 |
At this point, including patient choice does not affect OUTREACH results because any benefits and costs in a setting are divided by the number of people in that setting and so they are cancelled out. However, if throughput is introduced to the model, and this throughput determines fixed costs and potential savings due to economic efficiency, then patient choice will impact the results of the model.
The model allows for the possibility that patients may die based on Office for National Statistics mortality statistics for 20 common cancers. 73 Although there were no mortalities recorded in the OUTREACH trial, it is possible that, in practice, some patients receiving chemotherapy will die over a 12-week or longer period. The mortality rate was assumed to be the same for each setting and so had no effect on the model outcomes. The mortality parameter was included to allow for longer model lengths and to improve the face validity of the model.
Patient quality of life
The OUTREACH trial reported the number of QALYs gained over the 12-week length of the trial. Utility values were derived using the EQ-5D questionnaire as completed by the patients in the trial. Questionnaires were completed at baseline and 4, 8 and 12 weeks; only outcomes at 12 weeks were analysed. The model uses the 12-week QALYs derived from OUTREACH. These data are treated as utility scores, assuming constant utility over the time period, implying that any QALY gains are equivalent to multiplying the utility score at 12 weeks by 12/52. The QALYs in OUTREACH were reported without baseline values and so the base-case model has no adjustment for baseline values. Table 12 presents the utility values in the model derived from OUTREACH. The uncertainty in the underlying utility data was represented using beta distributions in probabilistic sensitivity analysis. The utility value for the death state was assumed to be the customary value of zero.
| Intervention | Mean EQ-5D | n | SD |
|---|---|---|---|
| Outpatient | 0.754 | 14 | 0.147 |
| Home | 0.715 | 15 | 0.230 |
| Community (GP practice) | 0.828 | 19 | 0.173 |
Costs
The original OUTREACH publication reported total costs with no details of what these comprised. We obtained additional cost category data from the authors.
Total costs were divided into the following categories: inpatient, outpatient, day hospital, A&E visits, community care, medication (excluding cancer drugs) and nurse contact. Follow-up data provided by the authors were reported as average costs per patient for each category in each setting, with no reported resource use. However, included data suggested that A&E visits, as a cost category, cost on average £3–4 per patient. Clarification was sought from the authors whether the value they reported was the average number of visits, as their table appeared to indicate, or the total number of visits to A&E for each treatment arm, but no additional information was provided. Therefore, we have assumed that this is an error and that the number represented total visits to A&E per arm, rather than the average costs of those visits. In order to produce the average cost of each emergency room visit, we multiplied the NHS reference cost for A&E visits by the number supplied from the OUTREACH trial and divided this by the number of participants in each arm. The cost of one A&E visit without any follow-up visits according to NHS reference costs was £122 in 2011–12. 74
Cost data provided on request from the OUTREACH trial did not contain SDs or standard errors; aggregate costs reported in the published study provided SDs for total costs in each study arm. We assumed that the proportion of the SD to the mean for cost categories would be similar to the proportion of the SD to the mean for total costs. The proportions of the SD of costs to mean costs are shown in Table 13. We assumed that the proportion of the SD to costs for all arms would be the average of the three, that is 75%.
| Setting | SD (£) | Mean cost (£) | Proportion of SD to mean cost |
|---|---|---|---|
| Hospital outpatient | 1831 | 2221 | 0.82 |
| Community (GP practice) | 1759 | 2497 | 0.70 |
| Home | 1590 | 2139 | 0.74 |
In a probabilistic sensitivity analysis, gamma distributions were used to represent costs, as the skew of the gamma distribution and bounding between zero and positive infinity make it a good choice for representing costs. Table 14 incorporates all updated costs from the OUTREACH trial inclusive of our assumptions modifying A&E costs and inflating prices using 2011–12 Personal and Social Services Research Unit price indices for medical services. 75 The cost of an A&E visit was derived from 2011–12 NHS reference costs;74 all other costs were derived from the OUTREACH trial. 4
| Intervention | Mean (£) | n | SD (£) |
|---|---|---|---|
| A&E visit costs | 122.00 | 150,041 | 41.30 |
| Outpatient | |||
| Inpatient | 321.12 | 13 | 240.84 |
| Outpatient | 880.74 | 13 | 660.56 |
| Day hospital | 716.57 | 13 | 537.43 |
| A&E visits | 37.54 | 13 | 28.15 |
| Community care | 210.64 | 13 | 157.98 |
| Medication (non-cancer) | 8.26 | 13 | 6.20 |
| Nurse diaries | 151.78 | 13 | 113.84 |
| Total costs: outpatient | 2326.65 | 13 | 1744.99 |
| Home | |||
| Inpatient | 215.80 | 20 | 161.85 |
| Outpatient | 588.54 | 20 | 441.40 |
| Day hospital | 292.20 | 20 | 219.15 |
| A&E visits | 18.30 | 20 | 13.73 |
| Community care | 242.64 | 20 | 181.98 |
| Medication (non-cancer) | 11.36 | 20 | 8.52 |
| Nurse diaries | 857.00 | 20 | 642.75 |
| Total costs: home | 2225.84 | 20 | 1669.38 |
| Community (GP) | |||
| Inpatient | 367.58 | 17 | 275.68 |
| Outpatient | 617.45 | 17 | 463.09 |
| Day hospital | 214.77 | 17 | 161.07 |
| A&E visits | 21.53 | 17 | 16.15 |
| Community care | 284.98 | 17 | 213.73 |
| Medication (non-cancer) | 16.52 | 17 | 12.39 |
| Nurse diaries | 1073.83 | 17 | 805.37 |
| Total cost: community | 2596.65 | 17 | 1947.49 |
Cost-effectiveness results
The base-case deterministic model results are presented in Table 15. These results do not account for uncertainty; in order to do this, a probabilistic sensitivity analysis was undertaken varying all model parameters within their assigned distribution for 10,000 simulations. The results of the probabilistic analysis are presented in Table 16 and in graphical form in Figures 9 and 10.
| Intervention | Costs (£) | QALY | Incremental costs (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| Home | 2225.84 | 0.165 | – | – | – |
| Outpatient | 2326.65 | 0.174 | 100.81 | 0.009 | 11,200.89 |
| Community | 2596.65 | 0.191 | 270.00 | 0.017 | 15,882.39 |
| Setting | Threshold values (£/QALY) | ||||
|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |
| Home | 0.462 | 0.351 | 0.260 | 0.196 | 0.128 |
| Outpatient | 0.383 | 0.376 | 0.340 | 0.300 | 0.218 |
| Community | 0.155 | 0.273 | 0.400 | 0.504 | 0.655 |
FIGURE 9.
Cost-effectiveness acceptability curves.

FIGURE 10.
Cost-effectiveness acceptability frontier.
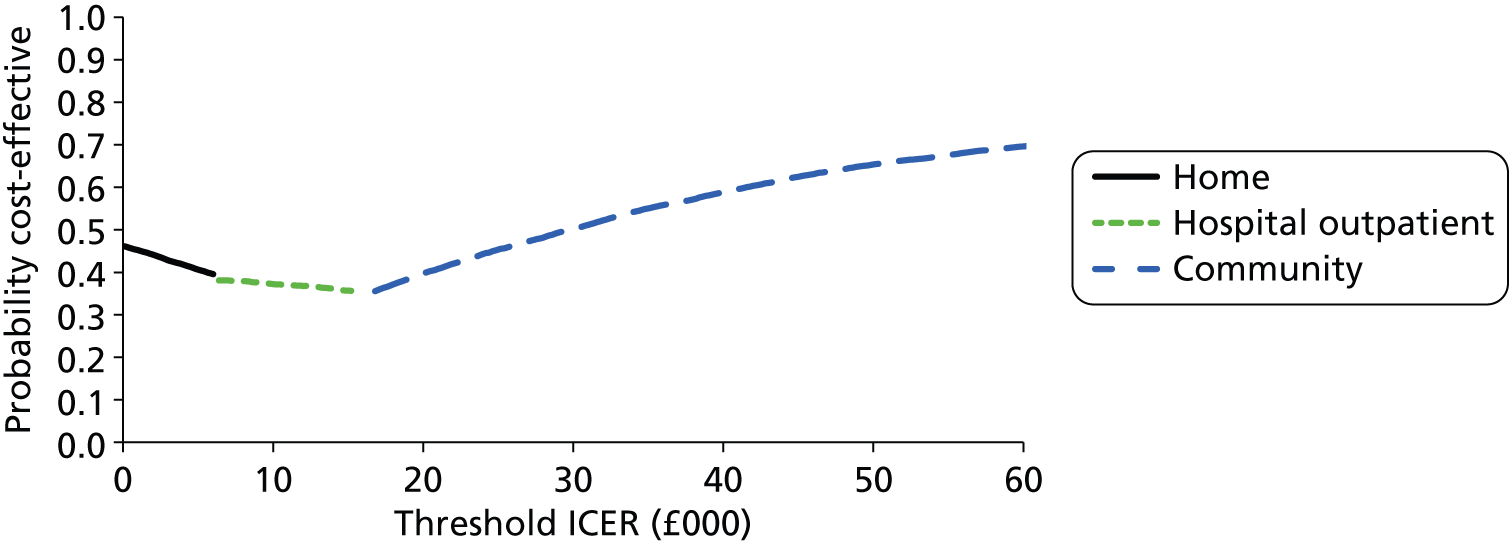
In the base case, home chemotherapy is both the least costly and the least effective treatment setting, followed by outpatient chemotherapy, which has an ICER of £11,201 per QALY compared with the home setting. Assuming the standard National Institute for Health and Care Excellence (NICE) cost-effectiveness threshold willingness-to-pay range of £20,000–30,000 per QALY gained, the ICER of £15,882 per QALY for community chemotherapy compared with outpatient chemotherapy indicates that delivering chemotherapy in a community setting is cost-effective. 72
Table 16 shows the probability of cost-effectiveness of the different settings at several values. NICE guidance also advises that ICERs greater than £30,000 per QALY may be considered for treatments that meet criteria for end-of-life care,72,76 which may be important for specific chemotherapy regimens that meet end-of-life care criteria.
Table 16 and Figures 9 and 10 clearly demonstrate the high level of uncertainty surrounding any decision based on the OUTREACH trial data. There are no treatments that have a high probability (> 60%) of being the most cost-effective treatment setting between a £0 and £30,000 per QALY willingness-to-pay threshold. Only at a threshold of £50,000 per QALY does the likelihood of community chemotherapy being the most cost-effective option rise above 60%. Home and outpatient chemotherapy never have a probability of being the most cost-effective treatment of much more than 45%, and this only occurs at a willingness to pay of £0 per QALY with the treatment setting being home chemotherapy. Figure 10 shows that up to a threshold ICER of £7200 per QALY chemotherapy delivered in the home setting is the preferred option, up to an ICER of £16,400 per QALY outpatient chemotherapy is the preferred setting, and above this value community chemotherapy is preferred.
While this model based on the OUTREACH trial found that the most cost-effective option across accepted willingness-to-pay thresholds in the UK was chemotherapy delivered in the community, as previously stated its purpose is exploratory and the results should not be considered robust or used to inform decisions. Model limitations include:
-
The OUTREACH trial had very small numbers of patients returning health economic outcomes for the CUA (no more than 20 for cost outcomes, and no more than 19 for QALYs).
-
No differences in health-related quality of life were statistically significant.
-
The precise details of each of the settings used in OUTREACH may be different from those used in the same setting in a different location.
-
The model is simplistic and may not represent important nuances in delivering chemotherapy.
-
The generalisability of the patient population is unclear.
Sensitivity analyses
We used sensitivity analyses to further explore impacts of assumptions and small adjustments on data. The limitations of the model meant that an exhaustive suite of sensitivity analyses might not be helpful and could possibly mislead readers. For this reason we performed only three formal sensitivity analyses:
-
Scenario 1: utilities adjusted for baseline.
-
Scenario 2: the mean cost of community care in the home setting was made equal to the mean cost of community care in the community (GP) setting (£285) and the mean nurse diary costs for the community (GP) setting were made equal to those in the home setting (£857).
-
Scenario 3: mean inpatient stay costs in the home setting were made equal to community (GP) setting costs (£368).
These analyses highlight the uncertainty in the outcomes and the impact on the cost-effectiveness of each setting as a result of making small plausible changes to the data. The results of all sensitivity analyses are reported in Table 17. In addition to these analyses, the potential implications of using different analytical perspectives are discussed, as well as potential effects of VAT exemptions on provision of chemotherapy services in the home.
| Intervention setting | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Scenario 1 | |||||
| Home | 2225.84 | 0.164 | – | – | – |
| Outpatient | 2326.65 | 0.174 | 100.81 | 0.010 | 10,080.80 |
| Community | 2596.65 | 0.165 | 270.00 | –0.009 | Dominated |
| Scenario 2 | |||||
| Home | 2268.17 | 0.165 | – | – | – |
| Outpatient | 2326.65 | 0.174 | 58.47 | 0.009 | Dominated |
| Community | 2379.82 | 0.191 | 111.64 | 0.026 | 4294.03 |
| Scenario 3 | |||||
| Outpatient | 2326.65 | 0.174 | – | – | – |
| Home | 2377.62 | 0.165 | 50.97 | –0.009 | Dominated |
| Community | 2596.65 | 0.191 | 270 | 0.017 | 15,882.39 |
Adjusting for baseline imbalances in utility scores
Information provided from the OUTREACH trial indicated that the authors had adjusted QALY gains for baseline imbalances. Because no baseline values were reported and only a difference in difference analysis was provided, this sensitivity analysis assumes that the difference in baseline values is maintained in the difference in final QALYs gained. To maintain consistency, these values were converted to utility scores for use in the model. Table 18 shows the utility adjustments made in the OUTREACH data.
| Intervention | Base-case QALYs | Base-case utility score | Adjusted QALYs | Adjusted utility score |
|---|---|---|---|---|
| Home | 0.165 | 0.754 | 0.164 | 0.711 |
| Outpatient | 0.174 | 0.715 | 0.174 | 0.754 |
| Community | 0.191 | 0.828 | 0.165 | 0.715 |
Using these adjusted values in the model changes the ICER of the chemotherapy settings. In the base case, chemotherapy administered in a community setting was the most effective option at a threshold of £20,000. In this sensitivity analysis, chemotherapy in the community is both more costly and less effective than administration in the outpatient setting. The ICER for outpatient chemotherapy compared with chemotherapy in the home setting also improves.
This analysis emphasises the uncertainty surrounding the utility values used in the model. Given the small sample size in the OUTREACH trial and the suggestion of baseline differences, the utility data cannot be considered reliable. It is likely that the quality of life of patients depends on their disease, treatment status (palliative, supportive or curative), age, and other factors that the OUTREACH data may not have captured. Clarification was sought from the authors on whether or not the data were adjusted for patient characteristics other than baseline EQ-5D scores, but none was received. Given the small patient numbers, it is unlikely that any such adjustment would have been plausible or would reduce the uncertainty. Further research is necessary to confirm or refute differences in patient quality of life between settings.
In scenario 2, making the mean cost of community care in the home setting equal to the mean cost of community care in the community (GP) setting, and the mean nurse diary costs for the community (GP) setting equal to those in the home setting, resulted in outpatient care being extendedly dominated; this means that a hypothetical combination of home and community chemotherapy services would be more cost-effective than outpatient chemotherapy on its own. In scenario 2, chemotherapy in the community setting is the most cost-effective option with an ICER that is lower than the base-case analysis. Similarly, scenario 3 makes home more costly than outpatient and it remains less effective. In scenario 3, home is dominated and community is the most cost-effective option.
Other areas of interest for exploration
The perspective of all our modelling analyses is the NHS and Personal and Social Services perspective (only costs to the NHS and Personal and Social Services are included). Other perspectives could be analysed and may produce different results.
Some economic evaluations included in our systematic review included patient or carer costs: patient travel costs;29 parent travel costs, payments for physician/care provider visits, medication, babysitting, lost productivity and government transfer payments;33 and patient travel costs. 34 These are valid perspectives, but their relevance to NHS decision-makers may be limited even though they are interesting.
The Centre for Health Economics, University of York, published a contingent valuation study of patient time. 77 This study asked patients to value different usages of their time such as time to start of treatment (between scheduling an appointment and receiving treatment), travel time, time waiting at a health facility for treatment and time receiving treatment.
In our model, time to start of treatment and time receiving treatment are expected to be independent of the setting and so only values related to travel time and waiting time are reported. The Centre for Health Economics study measured Dutch patients’ valuations of travel and waiting time for patients in three treatment areas (radiotherapy, orthopaedics and rehabilitation) and found that they valued each hour of travel time at £11.54 and each hour of waiting time at £34.76. 77 Chemotherapy patients were keenly aware of how time spent travelling to hospital and waiting for treatment had negative impacts on their time and energy. Including valuations of patient time could have significant impacts on the cost-effectiveness of treatment settings for chemotherapy. However, our systematic review identified only limited information on time spent travelling to and waiting in hospitals in the UK.
Several respondents in our survey of provision mentioned that drugs delivered at home were exempt from VAT (current rate 20%) and that these savings were used to finance home chemotherapy services. This view was reflected by the advisory group. Private providers have used VAT savings in trying to market their home services to NHS providers. 69 HM Revenue & Customs has indicated that medicines delivered in the home may be eligible for a zero rating for VAT where requirements are met regarding drug use and provision. 68
Considering that cost differences between the settings were small, reducing drug costs by 20% could alter cost-effectiveness decisions on home provision of specific chemotherapeutic drugs. Drug costs were not included in our model as they were not included in the OUTREACH data. However, it is likely that drug costs make up a significant proportion of the total cost of treatment. This will be particularly true in the case of newer chemotherapy drugs where the cost of the drug may represent most of the total treatment cost. In these instances the impact of VAT exemptions may have an impact on where these drugs are prepared and delivered.
Some of the issues outlined will not be relevant across the whole NHS, but local service configurations may make them worthy of further investigation should data become available.
Chapter 6 Discussion
The aim of this project was to investigate the impact of the delivery of intravenous chemotherapy in different settings on quality of life, safety, patient satisfaction and costs.
We performed three systematic reviews to provide a complete overview of the available published evidence base. We supplemented the published evidence with a survey of current practice to better understand the variation in chemotherapy deliver practices in the UK and facilitate the structuring of an economic decision model. In this section we present a summary of those findings and their strengths and limitations and bring together the elements of this mixed-methods project to draw summary conclusions and highlight the implications for practice and further research.
Key findings
The results of this study highlight avoidable study design and reporting limitations, and inherent and sometimes unavoidable difficulties, which arise when conducting primary studies to compare chemotherapy settings. Although several studies were appropriately designed to minimise avoidable biases, conducting randomised trials of chemotherapy settings nevertheless appears difficult in terms of both patient accrual and recruiting a population to enable an unbiased evaluation of the settings. Consequently, few robust conclusions can be made about the clinical effectiveness and cost-effectiveness of the different settings. However, a prevalence of qualitative data enabled a broad evaluation of patient, relative and caregiver experiences, with additional input from health-care professionals.
We identified eligible randomised trials and economic evaluations but there was a lack of useful data to inform and populate a decision model, and little evidence of clinically relevant differences between settings in terms of quality of life, and clinical and psychological outcomes (even though the biases when recruiting patients would have been expected to favour the home or community settings). The synthesis of qualitative studies indicated that decisions and preferences about intravenous chemotherapy treatment setting are strongly influenced by a desire to maintain normality. The modelling developed in our study was necessarily exploratory in nature owing to the limitations of the existing evidence base.
There was little evidence to indicate any effects of setting on quality of life. Trial samples sizes were likely too small to detect any such effects. It was unclear whether or not the quality-of-life assessment tools used were sensitive enough to detect differences between settings. The only potentially meaningful differences evident from the clinical effectiveness review were for some patient satisfaction and preference outcomes. However, strength of preference was studied in only one trial and preferences appeared not to be strong in around one-third of the patients who said that they preferred home chemotherapy. These results indicated that time was more important than setting for those patients. 31 There were no comparative studies of how preferences might change with other factors (such as distance from hospital, financial costs, outpatient environment and nurse–patient relationship). The limited adverse event evidence available gave no indication that there need be any safety concerns when delivering intravenous chemotherapy in either the home or the community setting.
The qualitative studies provided evidence from health professionals, patients (adults and children), parents, carers, siblings and partners. The data focused on experiences of outpatient and home settings for intravenous chemotherapy and included some data on community settings including mobile bus units. The range of participants and experiences suggests that these data provide relatively comprehensive coverage.
We have presented a line of argument around barriers and perceived costs associated with non-outpatient settings. Barriers to service provision centred on costs/resources, lack of support from key referring staff and the need for more training or additional nurses. Staff expressed concerns about their personal safety in relation to home chemotherapy services.
These issues were mirrored in the survey data presented in Chapter 4 and summarised in Table 19. The second line of argument focused on factors influencing patient satisfaction with intravenous chemotherapy across locations. Elements such as communication, information, privacy, rapport and individuality of treatment are unlikely to be unique to chemotherapy treatments, but they indicate key areas where small changes could result in substantially improved satisfaction.
| Qualitative synthesis | Survey data |
|---|---|
| Staff personal safety concerns | |
| Reluctance to treat in other locations | Lack of consultant support for the service |
| Difficulty convincing patients | |
| Adverse event concerns | Limited eligible treatment regimes |
| Lack of professional support | Regulations interfere with cost-effective provision |
| Capacity concerns | Practical difficulties |
| Lack of nursing staff | |
| Cost of the service (patient and staff views) | Concerns about costs |
| Lack of communication between health professionals |
The key line of argument derived from the qualitative data states that decisions and preferences about intravenous chemotherapy setting are rooted in attempts to maintain normality. Various factors push patients towards preferring hospital outpatient settings (mostly safety and expertise) while others pull patients towards other settings (in order to have some control over resources, such as time or energy). Medical expertise was the largest component which favoured outpatient treatment; however, this was seen as a trade-off against other settings which facilitated more normality in everyday life. Time was one of the largest factors that drew patient preference away from outpatient settings; it is important to understand that this is about more than waiting times, travel time and length of appointment. Cancer patients and their families were very conscious of the limited resources at their disposal in terms of time and energy. When chemotherapy treatment absorbed too much of these resources, the resulting fatigue impacted heavily on every other aspect of their life. This suggests that complaints about waiting time should be seen in a broader context.
Limitations of the evidence and of the review
Clinical studies: recruiting a representative and unbiased population
A key issue to consider when interpreting the results of the randomised trials included in this review is their generalisability. Although many studies (but not all) invited all eligible patients to participate, the nature of the interventions in question (the settings) meant that potential participants were likely to have pre-trial perceptions (opinions and likely preferences) about the interventions they might receive. This is an uncommon problem during randomised trial recruitment because patients typically have limited information on which to base prior perceptions (for at least one of the interventions being studied). In most intervention trials it would be difficult for invited patients to attempt to compare the likely benefits and harms of the interventions for them as individuals. Exceptions would include trials evaluating chemotherapy settings, some behavioural intervention trials,78 and trials of participative interventions (such as self-monitoring, rehabilitation and counselling interventions). 79
It is unsurprising that many of the patients who declined to participate in the trials in this review did so because they wanted to receive their chemotherapy in a hospital outpatient setting (or did not want the home setting). By declining to participate, patients would guarantee that they receive chemotherapy in their preferred setting. Conversely, patients who wanted to receive chemotherapy in the home (or community) setting may be likely to accept their invitation to participate in a trial if it were the only route by which they may receive chemotherapy at home or the only way of avoiding the hospital outpatient setting. Consequently, the trial populations in this review are likely to over-represent hospital-averse (or home-inclined) patients, and under-represent patients who are keen to receive chemotherapy in a hospital environment. It is important that the results of these trials are interpreted with this in mind; the trial populations are likely to prefer, to be more satisfied with, and to have a better quality of life, with home or community chemotherapy than with hospital outpatient chemotherapy. Only one trial considered the implications of this issue by performing additional analyses. 31
Problems are also often encountered in recruiting sufficient numbers of patients into oncology trials, although the reasons for limited accrual are not always easy to identify. A study of 82 oncology trials found that therapeutic trials achieved sufficient accrual more often than non-therapeutic trials, but that shorter consent forms, fewer exclusion criteria and simplicity of trial design were not associated with achieving sufficient accrual. 80 Many of the 82 trials were stopped early because of insufficient accrual. The authors noted a need for research to better inform patient accrual prediction practices (a trend towards accrual sufficiency was observed for trial protocols containing documentation supporting predicted accrual goals). All the trials in our review randomised fewer than 100 participants and almost all had slow rates of recruitment.
A systematic review which assessed studies of patients’ attitudes and barriers to participation in cancer trials found that barriers to participation were protocol-related, patient-related or physician-related. The most common reasons given as barriers included concerns with the trial setting; a dislike of randomisation; general discomfort with the research process; complexity and stringency of the protocol; presence of a placebo or no-treatment group; potential side effects; being unaware of trial opportunities; the idea that clinical trials are not appropriate for serious diseases; fear that trial involvement would have a negative effect on the relationship with their physician; and their physician’s attitudes towards the trial. 81
Cost-effectiveness studies, exploratory economic modelling and brief survey
Studies in the cost-effectiveness review were generally poor quality; most were from outside the UK and most did not report resource use or unit costs, and so their generalisability to the UK was limited. Given these limitations, the evidence identified was of limited usefulness for informing a de novo economic model. Sample sizes were generally small and studies were subject to broad uncertainty.
Data from the OUTREACH trial were used to structure and populate a model to enable exploration of cost-effectiveness. However, these data were derived from a small number of trial participants; no more than 20 and as few as 13 patients contributed cost or QALY data for any trial arm. We addressed some of the data limitations using assumptions; where possible these were validated with the authors, but this was not possible in all instances.
The three settings used in OUTREACH may not be representative of all available settings. There appears to be large variation in how UK services are delivered and it remains unclear that the three options modelled would be viable for all organisations. Many will not be in a position to offer more than one alternative to outpatient setting. Owing to capacity constraints, geographical location or other organisational structures, the alternative(s) offered may not constitute a choice. It is possible that some patients may not have multiple options for how they receive their treatment and may not be given a choice that includes settings. Further, it was clear from the survey that third-party providers and chemotherapy buses are already providing treatment closer to home and in the home, but owing to data limitations we were not in a position to include these options as comparators in the model.
The brief survey of NHS and private providers was not intended to be comprehensive and so provided only limited information. The number of responses was relatively low and might not have been representative of the entire NHS, but nevertheless provided a rapid overview of the variation evident in current practice.
Qualitative studies
All of the studies evaluated a new or proposed service against an existing, perhaps struggling, hospital outpatient setting. As discussed previously, participants were drawn from a biased sample. Methodologically, the most common weakness was lack of reflexivity and consideration of the authors’ impact on the data, which reduces the reliability of the findings.
In the review it would have been ideal for us to involve another member of the review team in the synthesis process (rather than an external colleague) but this was not possible owing to limited experience of qualitative data. Our use of online web-based translation services for one paper rather than an experienced translator might have resulted in some loss of meaning or mistranslation.
Strengths of the review
This is the first systematic review to compare the effects of home, community and hospital outpatient settings. The only other systematic review in this area compared only the home and hospital outpatient settings and was broader in terms of the eligible populations (studies of patients taking oral chemotherapy or other intravenous cancer therapies were included). 82 This 2010 review concluded that there was no current evidence that home therapy has any beneficial effect on quality of life or response to chemotherapy, although the trials were not powered to detect these outcomes.
Our review was performed according to CRD guidance and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Our comprehensive searches to identify studies located several unpublished studies. A further strength is that we included non-randomised and single-setting studies.
The qualitative review followed an established method (meta-ethnography), using qualitative data analysis software (NVivo) to clearly track which studies contributed to each code; there was oversight from a second reviewer with qualitative expertise and there was discussion of code and line of argument development. Quality assessment was carried out based on clearly defined criteria that are linked to the reliability of the findings rather than fidelity to a particular method.
Our cost-effectiveness review represented a wide variety of potentially relevant outcomes including NICE’s preferred measurement of health-related quality of life (EQ-5D) and cost and resource use data from the UK. The model has limitations, but was based on UK data from a trial that compared three different settings. The trial used QALYs and UK costs from the NHS perspective, both of which are in line with NICE methodological guidance. By presenting the modelling we aimed to highlight the uncertainty in the data and the impact of different assumptions when undertaking decision modelling in areas of such variability in service delivery and data uncertainty.
Patient and public involvement
To ensure patient and public involvement (PPI) we recruited two representatives to the advisory group for this project. One representative had received chemotherapy treatment for cancer and the other has a wealth of experience in PPI. They were involved from the proposal stage helping to shape discussions around choice of outcome measures and inform the researchers on issues of importance to patients. As active members of the advisory group, both representatives were invited to review drafts and to comment on the full report before submission. Comments from the PPI representatives indicated that they found the process interesting but felt unable to contribute in detail as the focus was on discussing existing research rather than directly shaping the treatment itself.
Patient and public involvement within the framework of a systematic review is often less about directly influencing the methodology itself, but rather can help to sensitise the researchers to the outcomes of importance to patients. Within this project, PPI helped to emphasise the importance of patient choice and preference rather than just concentrating on clinical outcomes. Such outcomes are more likely to be reported in mixed-methods or qualitative studies, and so it was important to ensure that our searches and inclusion criteria were able to capture such studies.
Chapter 7 Conclusions
Few robust conclusions can be made about the clinical effectiveness and cost-effectiveness of the different settings in which intravenous chemotherapy is administered. This is largely a consequence of the difficulties encountered during the clinical trials. There was a lack of useful data to inform and populate an economic decision model, and little evidence of clinically relevant differences between settings in terms of quality of life, and clinical and psychological outcomes. The results from qualitative studies indicated that decisions and preferences about treatment setting are strongly influenced by a desire to maintain normality.
Implications for research
Study design
Recruitment bias and patient accrual problems are likely to be difficult to overcome in randomised trials that use conventional study designs. A more useful and representative study design might be to nest a RCT within a larger observational cohort of patients; ambivalent patients could be randomised and patients with preferences could receive their preferred setting. Such a study might also make use of the qualitative data themes identified in our review, and incorporate them into questionnaires for use before any chemotherapy is initiated and after chemotherapy in a particular setting is completed. Efficacy estimates would result from the randomised component of the study and any additional influence of motivational factors could be studied by comparing patients randomised to the home setting with those who chose the home setting.
The results from this design should also indicate any clinical or demographical differences between the different populations at baseline and produce estimates of likely rates of uptake of the different settings to help inform future service provision. This study design would more clearly identify setting-related safety issues (which appear to be one of the key concerns about the implementation of a home or community chemotherapy service). Larger and more generalisable data sets would enable analyses to be made of whether or not setting-related issues that are important to patients, such as waiting times, anxiety or transport, vary according to patient characteristics.
This approach to study design is very similar to one advocated for trials of counselling following mastectomy described in a paper about patient preference and randomisation; this discussion paper also suggested a change-from-baseline approach when analysing some outcomes of non-randomised groups. 79
Other similar designs include the randomised consent (Zelen) design83 and the cohort multiple RCT. 84 However, in addition to the lack of patient consent issues to be considered when using these designs, neither design uses a crossover component which, in this area of research, would appear to be the most appropriate option for two reasons.
First, when compared with other designs, fewer patients would need to be randomised to obtain the same number of observations and fewer observations are needed to obtain the same precision in estimation. This is a consequence of patients acting as their own controls and between-patient variation being eliminated. 85 This should help to minimise patient accrual problems.
Second, each patient should experience both settings for an adequate period to enable a more accurate estimate of preference.
Crossover trials have some disadvantages in this area of research: patients must have relatively stable disease states; and dealing with dropouts can be more problematic than with parallel designs. Lack of disease stability was an issue for some of the crossover trials in this review. 29,31,34
The many methodological issues identified in this review suggest that, whichever design is chosen for future studies, a feasibility study should first be performed (with feasibility outcomes); any resulting larger study should begin with a pilot phase.
The issues identified in this review which led to these recommendations for future research highlight the importance of using systematic reviews to inform the design of new studies. 86
Another recommendation for further research would be studies of within-setting comparisons; these are absent from the evidence base and it is unclear how a new outpatient facility affects quality of life unless it can be compared with an old facility. Similarly, no studies exist which compare different types of community setting (e.g. community bus vs. GP facility).
Outcome measures
Many studies in this review used validated tools; such tools are often preferred because non-validated scales may produce larger and less reliable effects. Existing validated tools tend to comprise a core set of questions (20 to 30 items) and a number of cancer-specific add-on modules such as the EORTC QLQ-C30, FLIC and Functional Assessment of Cancer Therapy. 87–89 These measures tend to focus heavily on physical functioning, particularly in the case of the EORTC QLQ-C30 and the FLIC, and so may not be responsive to the kind of issues highlighted in the qualitative synthesis (e.g. available time and energy, control over impact of the disease on daily life). Quality-of-life measures sensitive to these issues are needed to ensure that future trials are equipped to detect significant differences where these exist. It may be important to differentiate between patients receiving palliative versus curative treatment; some existing tools offer separate modules to accommodate this. Part of the challenge is that many of these outcome measures were developed to capture the burden of disease and not the burden of treatment.
As an alternative to developing new outcome measures, researchers may find it helpful to consider patient-generated outcome measures. Specifically, Measure Your own Concerns And Wellbeing was developed from the Measure Your Medical Outcome Profile tool for use in cancer treatment centres that provided integrative treatment. 90,91 Validation is ongoing. The innovative use of a patient-generated format, Likert scales and questions about concerns or problems rather than symptoms may make this tool a useful addition to future trials. MYMOP itself has previously been compared with the Short Form questionnaire-36 items, Medical Outcomes Study – 6-item sale, Dartmouth COOP Functional Health Assessment charts and the EQ-5D, and was shown to be both reliable and more sensitive to small changes than the other outcome measures. 91–93
Similar problems face the assessment of quality of life in paediatric populations; however, a well-validated and relatively sensitive tool is available for use by researchers and was used by one study in this review. The Paediatric Quality of Life (PedsQL) scale is a rigorously developed package of quality-of-life outcome measures that includes the generic core scales (parent and child forms),94 a family impact module,95 and several condition-specific add-on modules (including cancer). 96 There has been some work to develop a paediatric-oriented version of the EQ-5D, although this is expected to be less sensitive. 97
Implications for practice
Considering the difficulties we have identified and reported in assessing the evidence base and developing a decision model, commissioners should consider the issues we have described, alongside more bespoke guidance and support. For example, the C-PORT Chemotherapy Capacity Planning Tool is a web-based tool (owned and operated by the NHS: www.cport.co.uk/Home.aspx?ReturnUrl=%2fdefault.aspx) that simulates the activity of an adult chemotherapy day case service. Within its many functions it provides users with a visual picture of how their particular service operates in terms of capacity and demand, resource utilisation and patient delays, and enables users to model the effects of potential change (to help to avoid bottlenecks) and plan for the introduction of new services. Our survey suggests that some organisations use settings other than outpatient for the delivery of treatment and that the mechanisms for delivering treatment in those different settings are variable.
It is not clear what the drivers are that determine the settings that organisations elect to offer. However, these drivers are likely to be a key influence in the way in which services are delivered. Capacity is clearly an issue across the NHS. Dealing with capacity through service reconfiguration is a local issue, dependent on local needs and current service configuration. Patient choice may be a driver in some areas, but it is unclear from the evidence we identified where choice fits into service configuration. In some situations, patients will have choices regarding setting; in others, choice will be dictated by which service is available.
It is likely that service configuration is driven by capacity in areas such as outpatient clinics and pharmacy. Patient choice may then be made available, if feasible, within the service configuration. Other factors, such as VAT exemption on drugs prepared and delivered outside the hospital setting, loss of outpatient tariffs should a service be moved to the home/community and the increasing number of oral treatments, will all impact on local service configuration but are difficult to unpick at a national level.
Research and practice summary points
-
Recruitment biases, and problems of recruiting enough willing participants, are unlikely to be overcome in future randomised studies which use conventional study designs. However, randomised crossover trials which are nested within much larger groups of patients (who can choose their preferred setting) are likely to provide results which are relevant to patients seen in clinical practice. Such studies would also help to inform decisions on future service provision.
-
Any future studies should begin with feasibility and pilot phases, and aim to use patient questionnaires to record qualitative data. Care should be taken to ensure that the tools used to record other study data are sensitive enough to detect changes in outcome measures which are important to patients.
-
Capacity and patient waiting times are among the key issues to consider when evaluating service configuration.
Acknowledgements
We thank Jane Dalton (research fellow, CRD) for assistance with the qualitative synthesis at inclusion, data extraction and analysis stages; Alison Booth for writing the plain English summary; and John Jackson for proofreading the final report. We are grateful to the individuals who helped to translate studies during study selection and data extraction: Yumi Nixon, Nick Meader, Alexis Llewellyn, Jennifer Brown, Ana Duarte, Rocio Rodriguez Lopez and Troels Kristensen.
Advisory group
We would like to thank members of the advisory group who contributed to development of the scope and protocol, answered our questions, attended meetings and reviewed relevant sections of the draft final report. Not all of those listed below were able to participate in all stages of the review.
Dave Ardron, PPI representative.
Pippa Corrie Ph.D. FRCP, Consultant and Associate Lecturer in Medical Oncology, Cambridge University Hospitals NHS Foundation Trust.
Jane Kelly, Pharmacy Procurement Project Manager, Leeds Teaching Hospitals NHS Trust.
Professor Una Macleod, Primary Care Medicine, Hull York Medical School.
Professor Gillian Parker, Social Policy Research Unit, University of York.
Melanie Robertson RLN, City Hospitals Sunderland – Nurse Consultant Cancer Services.
Saskia Syms, PPI representative.
Contributions of authors
All CRD authors contributed to all stages of the systematic review from the development of the protocol to the production of the report.
Mark Corbett (research fellow, CRD) managed the EPPI project software, led on the clinical effectiveness component and shared day-to-day responsibility for the project.
Morag Heirs (research fellow, CRD) took responsibility for project software and co-ordination of the final report, maintained contact with the advisory group members, devised the current provision surveys, led on the qualitative synthesis component and shared day-to-day responsibility for the project.
Micah Rose (research fellow, CRD) analysed the current provision surveys and contributed to the modelling section.
Alison Smith (research fellow, CRD) contributed to the review of economic evaluations.
Lisa Stirk (information specialist, CRD) devised the search strategy, carried out the literature searches and wrote the search methodology sections of the report.
Gerry Richardson (senior research fellow, Centre for Health Economics) provided expertise and advice for the economic components of the project as part of the extended project group, and commented on the protocol and drafts of the report.
Daniel Stark (senior lecturer in cancer medicine at the University of Leeds) provided clinical expertise and advice as part of the extended project group. He also commented on the protocol and drafts of the report.
Daniel Swinson (consultant medical oncologist, St James’s Institute of Oncology) provided clinical expertise and advice as part of the extended project group. He also commented on drafts of the report.
Dawn Craig (research fellow, CRD) contributed to all stages of the review, commented on drafts of the report and took overall responsibility for the economic components of the project.
Alison Eastwood (senior research fellow, CRD) contributed to all stages of the review, commented on drafts of the report and took overall responsibility for the project.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Young AM, Kerr DJ. Home delivery: chemotherapy and pizza? Evidence on safety and acceptability of home chemotherapy is growing. BMJ 2001;322:809-10. http://dx.doi.org/10.1136/bmj.322.7290.809.
- Boothroyd L, Lehoux P. Home-Based Chemotherapy for Cancer: Issues for Patients, Caregivers and the Health Care System. Montreal, QC: Agence d’Evaluation des Technologies et des Modes d’Intervention en Sante (AETMIS); 2004.
- Chemotherapy Services in the Community – A Guide for PCTs. London: Department of Health; 2010.
- Corrie PG, Moody AM, Armstrong G, Nolasco S, Lao-Sirieix S, Bavister L, et al. Is community treatment best? A randomised trial comparing delivery of cancer treatment in the hospital, home and GP surgery. Br J Cancer 2013;109:1549-55. http://dx.doi.org/10.1038/bjc.2013.414.
- NHS 2010–2015: From Good to Great. Preventative, People-Centred, Productive. London: The Stationery Office; 2009.
- Cancer Reform Strategy. London: Department of Health; 2007.
- Chemotherapy Services in England: Ensuring Quality and Safety. London: National Chemotherapy Advisory Group; 2009.
- Delivering the Cancer Reform Strategy. London: The Stationery Office; 2010.
- NHS England . The Latest on NHS England’s Five-Year Strategy for Specialised Services – James Palmer 2014. www.england.nhs.uk/2014/03/03/james-palmer (accessed 3 March 2014).
- Clive S, Baird R, Waterston A, Darby S, Newsom-Davis T, Swinson D. Differing Methods of Delivering Chemotherapy and Managing Toxicities across the UK: Summary Paper from the Association of Cancer Physicians New Consultants Group Workshop n.d. http://conference.ncri.org.uk/abstracts/2011/abstracts/B109.html (accessed 26 February 2014).
- Pope C, Mays N, Popay J, Pope C, Mays N, Popay J. Synthesising Qualitative and Quantitative Evidence: A Guide to Methods. Maidenhead: Open University Press; 2007.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: CRD, University of York; 2009.
- Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Corbett MS, Higgins JPT, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Syn Meth 2014;5:79-85. http://dx.doi.org/10.1002/jrsm.1090.
- Reeves BC, Higgins JPT, Ramsay C, Shea B, Tugwell P, Wells GA. An introduction to methodological issues when including non-randomised studies in systematic reviews on the effects of interventions. Res Syn Meth 2013;4:1-11. http://dx.doi.org/10.1002/jrsm.1068.
- Higgins JPT, Ramsay C, Reeves BC, Deeks JJ, Shea B, Valentine JC, et al. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res Syn Meth 2013;4:12-25. http://dx.doi.org/10.1002/jrsm.1056.
- Valentine JC, Thompson SG. Issues relating to confounding and meta-analysis when including non-randomized studies in systematic reviews on the effects of interventions. Res Syn Meth 2013;4:26-35. http://dx.doi.org/10.1002/jrsm.1064.
- Wells GA, Shea B, Higgins JPT, Sterne J, Tugwell P, Reeves BC. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Syn Meth 2013;4:63-77. http://dx.doi.org/10.1002/jrsm.1077.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org (accessed 26 February 2014).
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Mays N, Pope C. Qualitative research in health care: assessing quality in qualitative research. BMJ 2000;320:50-2. http://dx.doi.org/10.1136/bmj.320.7226.50.
- Mays N, Pope C, Mays N, Pope C. Qualitative Research in Health Care. London: BMJ Books; 2000.
- Spencer L, Ritchie J, Lewis J, Dillon L. Quality in Qualitive Evaluation: A Framework for Assessing Research Evidence. London: Cabinet Office; 2003.
- Taylor HJ. Multimethod Analysis of NHS Policy for the Development of New Services for Ambulatory Cancer Care 2008.
- Noblit G, Hare R. Meta-Ethnography: Synthesising Qualitative Studies. Newbury Park: Sage; 1988.
- Britten N, Campbell R, Pope C, Donovan J, Morgan M, Pill R. Using meta ethnography to synthesise qualitative research: a worked example. J Health Serv Res Policy 2002;7:209-15. http://dx.doi.org/10.1258/135581902320432732.
- Chen JT, Hasuimi K. Differences in the patient anxiety, quality of life (QOL) and nursing efficacy at the first course of chemotherapy infused at home and in hospital. Int J Gynecol Cancer 1999;9.
- Christiansen B, Rishoj A, Jensen BV, Larsen FO, Nielsen D. A randomised crossover trial to determine safety, quality of life and economic consequences of home-based chemotherapy. Eur J Cancer 2011;47. http://dx.doi.org/10.1016/S0959-8049(11)71317-X.
- Pace E, Dennison S, Morris J, Barton A, Pritchard C, Rule SA. Chemotherapy in the community: what do patients want?. Eur J Cancer Care 2009;18:209-15. http://dx.doi.org/10.1111/j.1365-2354.2008.01051.x.
- Hall M, Lloyd H. Evaluating patients’ experiences of home and hospital chemotherapy. Cancer Nursing Practice 2008;7:35-8. http://dx.doi.org/10.7748/cnp2008.02.7.1.35.c6469.
- King MT, Hall J, Caleo S, Gurney HP, Harnett PR. Home or hospital? An evaluation of the costs, preferences, and outcomes of domiciliary chemotherapy. Int J Health Serv 2000;30:557-79. http://dx.doi.org/10.2190/CY03-EV15-K38Y-X4AA.
- Rischin D, White MA, Matthews JP, Toner GC, Watty K, Sulkowski AJ, et al. A randomised crossover trial of chemotherapy in the home: patient preferences and cost analysis. Med J Aust 2000;173:125-7.
- Stevens B, Croxford R, McKeever P, Yamada J, Booth M, Daub S, et al. Hospital and home chemotherapy for children with leukemia: a randomized cross-over study. Pediatr Blood Cancer 2006;47:285-92. http://dx.doi.org/10.1002/pbc.20598.
- Remonnay R, Devaux Y, Chauvin F, Dubost E, Carrere MO. Economic evaluation of antineoplastic chemotherapy administered at home or in hospitals. Int J Technol Assess Health Care 2002;18:508-19.
- Borras JM, Sanchez-Hernandez A, Navarro M, Martinez M, Mendez E, Ponton JLL, et al. Compliance, satisfaction, and quality of life of patients with colorectal cancer receiving home chemotherapy or outpatient treatment: a randomised controlled trial. BMJ 2001;322:826-8. http://dx.doi.org/10.1136/bmj.322.7290.826.
- Evaluation Report of the Choice of Chemotherapy Pilot. London: Department of Health; 2010.
- Pong RW, Irvine A, McChesney C, DesRochers C, Blanco H, Valiquette A. Impact Assessment of an Outreach Chemotherapy Program in Northeastern Ontario. Sudbury, ON: Centre for Rural and Northern Health Research, Laurentian University; 2000.
- Hansson H, Kjaergaard H, Johansen C, Hallstrom I, Christensen J, Madsen M, et al. Hospital-based home care for children with cancer: feasibility and psychosocial impact on children and their families. Pediatr Blood Cancer 2013;60:865-72. http://dx.doi.org/10.1002/pbc.24474.
- Mitchell T. Chemotherapy Closer to Home: Patients’ Perspectives of Receiving Chemotherapy in Outpatient Clinic and/or a Unique Mobile Chemotherapy Unit. Bristol: University of the West of England; 2011.
- Barker C. A toxicity audit for patients receiving chemotherapy at a clinic or at home. Cancer Nurs Pract 2006;5:33-9. http://dx.doi.org/10.7748/cnp2006.11.5.9.33.c7588.
- Frølund J. Home chemotherapy for bone marrow cancer patients – a quantitative study of how patients experience treatment at home. Sygeplejersken 2011;111:69-72.
- Grusenmeyer PA, Gralla RJ, Trapani MM, Bateman MM. Alternatives to inpatient care: a cost comparison of home versus outpatient clinic care for chemotherapy administration and supportive care measures. Proc Am Soc Clin Oncol 1996;15.
- Herth KA. The relationship between level of hope and level of coping response and other variables in patients with cancer. Oncol Nurs Forum 1989;16:67-72.
- Ingleby S, Barnwell J, Baker J, Harvey P, Kerr D, Law V, et al. A pilot study of a feasibility and economic analysis of home based chemotherapy in ad-vanced colorectal cancer. Eur J Cancer 1999;35. http://dx.doi.org/10.1016/S0959-8049(99)80647-9.
- Lowenthal RM, Piaszczyk A, Arthur GE, O’Malley S. Home chemotherapy for cancer patients: cost analysis and safety. Med J Aust 1996;165:184-7.
- Payne SA. A study of quality of life in cancer patients receiving palliative chemotherapy. Soc Sci Med 1992;35:1505-9. http://dx.doi.org/10.1016/0277-9536(92)90053-S.
- Satram-Hoang S, Reyes C. Is there a difference in treatment patterns among follicular lymphoma patients receiving care in the outpatient hospital versus the community clinic setting?. Blood 2011;118.
- Souadjian JV, Bhaskar B, Briscoe KE, Opfell RW, Agopian JM. Home chemotherapy (CT): safety and cost-effectiveness. Proc Am Soc Clin Oncol 1992;11.
- Vergnenègre A, Decroisette C, Vincent F, Dalmay F, Melloni B, Bonnaud F, et al. Economic analysis of home based versus hospital outpatient chemotherapy in stage IV non small cell lung cancer. Rev Mal Respir 2006;23:255-63. http://dx.doi.org/10.1016/S0761-8425(06)71575-3.
- Mitchell T. Patients’ experiences of receiving chemotherapy in outpatient clinic and/or onboard a unique nurse-led mobile chemotherapy unit: a qualitative study. Eur J Cancer Care 2013;22:430-9. http://dx.doi.org/10.1111/ecc.12044.
- Iredale R, Hilgart J, Hayward J. Patient perceptions of a mobile cancer support unit in South Wales. Eur J Cancer Care 2011;20:555-60. http://dx.doi.org/10.1111/j.1365-2354.2011.01247.x.
- Mobile Chemotherapy – Transforming the Lives of Cancer Patients. Manchester: The Christie Charitable Fund; 2014.
- Hope for Tomorrow: Bringing Cancer Treatment Closer to Home. Upton Lodge: Hope for Tomorrow; n.d.
- King MT, Hall JP, Harnett PR. A randomised crossover trial of chemotherapy in the home: patient preferences and cost analysis. Med J Aust 2001;174.
- Stevens B, McKeever P, Law MP, Booth M, Greenberg M, Daub S, et al. Children receiving chemotherapy at home: perceptions of children and parents. J Pediatr Oncol Nurs 2006;23:276-85. http://dx.doi.org/10.1177/1043454206291349.
- Stevens B, McKeever P, Booth M, Greenberg M, Daub S, Gafni A, et al. Home chemotherapy for children with cancer: perspectives from health care professionals. Health Soc Care Community 2004;12:142-9. http://dx.doi.org/10.1111/j.0966-0410.2004.00480.x.
- Bakker DA, DesRochers C, McChesney C, Fitch M, Bennett J. Community cancer clinics: patients’ perspectives. Support Care Cancer 2001;9:234-40. http://dx.doi.org/10.1007/s005200000226.
- Smith SM, Campbell NC. Provision of oncology services in remote rural areas: a Scottish perspective. Eur J Cancer Care 2004;13:185-92. http://dx.doi.org/10.1111/j.1365-2354.2003.00472.x.
- Butler MC. Families’ responses to chemotherapy by an ambulatory infusion pump . . . at home. Nurs Clin North Am 1984;19:139-44.
- Crisp N. Chemotherapy at Home: Keeping Patients in Their ‘Natural Habitat’ 2010.
- Hansson H. Hospital-Based Home Care for Children with Cancer. Ph.D. thesis. Copenhagen: University of Copenhagen; 2011.
- Kelly D, Pearce S, Butters E, Stevens W, Layzell S. Achieving change in the NHS: a study to explore the feasibility of a home-based cancer chemotherapy service. Int J Nurs Stud 2004;41:215-24. http://dx.doi.org/10.1016/j.ijnurstu.2003.05.002.
- McIlfatrick S, Sullivan K, McKenna H, Parahoo K. Patients’ experiences of having chemotherapy in a day hospital setting. J Adv Nurs 2007;59:264-73. http://dx.doi.org/10.1111/j.1365-2648.2007.04324.x.
- Hjorleifsdottir E, Hallberg IR, Gunnarsdottir ED, Bolmsjo IA. Living with cancer and perception of care: Icelandic oncology outpatients, a qualitative study. Support Care Cancer 2008;16:515-24. http://dx.doi.org/10.1007/s00520-007-0333-9.
- Heatherwood and Wexham Park Hospitals NHS Foundation Trust . New Chemo Suite Officially Opened by Its Namesake 2013. www.heatherwoodandwexham.nhs.uk/news/new-chemo-suite-officially-opened-by-its-namesake (accessed 4 March 2014).
- Walker L. Singer Joe McElderry Opens New Shotley Bridge Chemo Unit. The Chronicle 2013. www.chroniclelive.co.uk/news/health/singer-joe-mcelderry-opens-new-4711701 (accessed 4 March 2014).
- Hansson H, Kjaergaard H, Schmiegelow K, Hallstrom I. Hospital-based home care for children with cancer: a qualitative exploration of family members’ experiences in Denmark. Eur J Cancer Care 2012;21:59-66. http://dx.doi.org/10.1111/j.1365-2354.2011.01280.x.
- Notice 701/57 Health Professionals and Pharmaceutical Products. London: HM Revenue and Customs; 2012.
- Bupa . Chemotherapy at Home n.d. www.bupa.co.uk/healthcare-providers/home-healthcare/what-we-do/home-chemotherapy (accessed 23 January 2014).
- Bupa . Home Oncology Service: Supported Regimes and Treatments n.d. www.bupa.co.uk/jahia/webdav/site/bupacouk/shared/Documents/PDFs/healthcare-professionals/bupa-home-healthcare/A-Z%20Supported%20treatments.pdf (accessed 23 January 2014).
- Ingleby S, Baker J, Young A, Kerr D. Health economic analysis of home based vs. hospital based chemotherapy: discussion of results. Br J Cancer 1999;80.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Office for National Statistics . Cancer Survival in England: Patients Diagnosed 2007–2011 and Followed up to 2012 2013. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition = tcm%3A77–320365 (accessed 26 November 2013).
- NHS Reference Costs: Financial Year 2011 to 2012. London: Department of Health; 2012.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the Estimation of the NICE Cost-Effectiveness Threshold: Revised Report Following Referees’ Comments. York: Centre for Health Economics, University of York; 2013.
- van den Berg B, Gafni A, Portrait F. Attributing a Monetary Value to Patients’ Time: A Contingent Valuation Approach. York: Centre for Health Economics, University of York; 2013.
- McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. J Clin Epidemiol 2014;67:247-53. http://dx.doi.org/10.1016/j.jclinepi.2013.09.004.
- Brewin CR, Bradley C. Patient preferences and randomised clinical trials. BMJ 1989;299:313-15. http://dx.doi.org/10.1136/bmj.299.6694.313.
- Schroen AT, Petroni GR, Wang H, Gray R, Wang XF, Cronin W, et al. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clin Trials 2010;7:312-21. http://dx.doi.org/10.1177/1740774510374973.
- Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 2006;7:141-8. http://dx.doi.org/10.1016/S1470-2045(06)70576-9.
- Home Chemotherapy. London: Bazian Ltd; 2010.
- Homer CS. Using the Zelen design in randomized controlled trials: debates and controversies. J Adv Nurs 2002;38:200-7. http://dx.doi.org/10.1046/j.1365-2648.2002.02164.x.
- Relton C, Torgerson D, O’Cathain A, Nichol J. Rethinking pragmatic randomised controlled trials: introducing the ‘cohort multiple randomised controlled trial’ design. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1066.
- Senn S. Cross-Over Trials in Clinical Research. Chichester: Wiley; 2002.
- Clarke M, Hopewell S, Chalmers I. Clinical trials should begin and end with systematic reviews of relevant evidence: 12 years and waiting. Lancet 2010;376:20-1. http://dx.doi.org/10.1016/S0140-6736(10)61045-8.
- European Organisation for Research and Treatment of Cancer . EORTC QLQ-C30 n.d. http://groups.eortc.be/qol/eortc-qlq-c30 (accessed 27 March 2014).
- Schipper H, Clinch J, McMurray A, Levitt M. Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol 1984;2:472-83.
- FACITOrg . Functional Assessment of Cancer Therapy (FACT-G) n.d. www.facit.org/FACITOrg/Questionnaires (accessed 31 March 2014).
- Paterson C, Thomas K, Manasse A, Cooke H, Peace G. Measure Yourself Concerns and Wellbeing (MYCaW): an individualised questionnaire for evaluating outcome in cancer support care that includes complementary therapies. Complement Ther Med 2007;15:38-45. http://dx.doi.org/10.1016/j.ctim.2006.03.006.
- Paterson C. Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey. BMJ 1996;312:1016-20. http://dx.doi.org/10.1136/bmj.312.7037.1016.
- Paterson C. Seeking the patient’s perspective: a qualitative assessment of EuroQol, COOP-WONCA charts and MYMOP. Qual Life Res 2004;13:871-81. http://dx.doi.org/10.1023/B:QURE.0000025586.51955.78.
- Paterson C, Langan CE, McKaig GA, Anderson PM, Maclaine GD, Rose LB, et al. Assessing patient outcomes in acute exacerbations of chronic bronchitis: the measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6 A) and EuroQol (EQ-5D). Qual Life Res 2000;9:521-7. http://dx.doi.org/10.1023/A:1008930521566.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. http://dx.doi.org/10.1097/00005650-200108000-00006.
- Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: preliminary reliability and validity. Health Qual Life Outcomes 2004;2. http://dx.doi.org/10.1186/1477-7525-2-55.
- Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002;94:2090-106. http://dx.doi.org/10.1002/cncr.10428.
- Hennessy S, Kind P, Group OR. Measuring Health Status in Children: Developing and Testing a Child-Friendly Version of EQ-5D. York: Centre for Health Economics, University of York; 2002.
- Corrie PG, Moody M, Wood V, Bavister L, Prevost T, Parker RA, et al. Protocol for the OUTREACH trial: a randomised trial comparing delivery of cancer systemic therapy in three different settings: patient’s home, GP surgery and hospital day unit. BMC Cancer 2011;11. http://dx.doi.org/10.1186/1471-2407-11-467.
- Corrie P. Is Hospital the Best Location to Deliver Cancer Therapy? Interim Results of the Outreach Randomised Clinical Trial. Liverpool: 7th NCRI Cancer Conference; 2011.
- Corrie P, Moody M, Wood V, Nolasco S, Armstrong G, Lao-Sirieix S, et al. Community Cancer Treatment: Final Results of the Outreach Randomised Clinical Trial. Liverpool: 8th NCRI Cancer Conference; 2012.
- Caleo S, King M, Cameron S, Hall J. An Evaluation of Domiciliary Chemotherapy n.d.
- Pace E, Dennison S, Morris J, Rule S, Pritchard C, Barton A, et al. A study to compare patient satisfaction with location of chemotherapy: community hospital versus cancer centre. EJC Suppl 2007;5. http://dx.doi.org/10.1016/S1359-6349(07)71684-1.
- Remonnay R, Devaux Y, Chauvin F, Dubost E, Carrere MO. Developing home-care hospitalization: cost evaluation and assessment criteria. The case of anticancer chemotherapy. Rev Epidemiol Sante Publique 2003;51:649-52.
- Breitfeld PP. Belief in home chemotherapy. Pediatr Blood Cancer 2006;47:237-8. http://dx.doi.org/10.1002/pbc.20679.
- Hansson H, Johansen C, Hallstrom I, Kjaergaard H, Schmiegelow K. Hospital-based home care for children with cancer. Pediatr Blood Cancer 2010;55:838-9.
- Hansson H, Johansen C, Hallstrom I, Kjaergaard H, Schmiegelow K. Hospital-based home care for children with cancer – health related quality of life and the psychosocial impact on the family. Pediatr Blood Cancer 2012;59.
- Herth KA. The Relationship Between the Level of Hope and the Level of Coping Response in Cancer Patients 1987.
- Payne SA. Coping with palliative chemotherapy. J Adv Nurs 1990;15:652-8. http://dx.doi.org/10.1111/j.1365-2648.1990.tb01887.x.
- Hjorleifsdottir E, Rahm Hallberg I, Agren Bolmsjo I, Gunnarsdottir ED. Icelandic cancer patients receiving chemotherapy or radiotherapy: does distance from treatment center influence distress and coping?. Cancer Nurs 2007;30:E1-10. http://dx.doi.org/10.1097/01.NCC.0000300161.06016.a9.
- Taylor H, Ireland J, Duggan C, Bates I. Home chemotherapy – a feasibility study. Br J Home Healthc 2007;3:4-5.
Appendix 1 Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
Date range: 1946 to week 2 March 2013.
Date searched: 25 March 2013.
Records found: 1564.
Update
Date range: 1946 to week 3 October 2013.
Date searched: 29 October 2013.
Records found: 1748.
Search strategy
| Cancer terms |
|
| Chemotherapy terms |
|
| Home care terms |
|
| Cancer + chemo + home care |
|
| Home chemo terms |
|
| Cancer + home chemo |
|
| Set 24 OR Set 31 |
|
| Exclude animal-only studies Final results set |
|
Key:
/ = indexing term (MeSH heading)
exp = exploded MeSH heading
* = major MeSH heading
$ = truncation
.ti,ab. = terms in either title or abstract fields
Adj6 = terms within six words of each other (any order).
Other strategies
Allied and Complementary Medicine Database
Date range: 1985 to March 2013.
Date searched: 25 March 2013.
Records found: 44.
Search strategy
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (13,694)
-
chemotherapy.ti,ab. (1083)
-
systemic therapy.ti,ab. (30)
-
intravenous drug therapy.ti,ab. (0)
-
adjuvant therapy.ti,ab. (76)
-
or/2-5 (1167)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (2940)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (1167)
-
7 or 8 (3782)
-
1 and 6 and 9 (26)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (21)
-
(chemotherapy adj6 service$).ti,ab. (8)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (1)
-
(self-infusion adj6 home).ti,ab. (0)
-
home infusion.ti,ab. (8)
-
or/11-15 (34)
-
1 and 16 (31)
-
10 or 17 (44)
Key:
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
British Nursing Index
URL: http://proquest.com
Date range: 1994 to March 2013.
Date searched: 25 March 2013.
Records found: 65.
Search strategy
S1 TI,AB(cancer* or neoplas* or tumor* or tumour* or malignan* or oncolog* or carcinoma*) 12003
S2 TI,AB(chemotherapy) (1121)
S3 TI,AB(“systemic therapy”) (15)
S4 TI,AB(“intravenous drug therapy”) (6)
S5 TI,AB(“adjuvant therapy”) (39)
S6 S2 or s3 or s4 or s5 (1167)
S7 TI,AB((service* or therapy or treatment*) NEAR/6 (home or community or outreach or “out-reach” or ambulatory or domicil*)) (2719)
S8 TI,AB(“hospital at home” or “hospital in the home” or “own home*” or “home care” or homecare or “closer to home”) (1084)
S9 S7 or s8 (3566)
S10 s1 and s6 and s9 (22)
S11 TI,AB(chemotherapy NEAR/6 (home or community or outreach or “out-reach” or ambulatory or domicil*)) (56)
S12 TI,AB(chemotherapy NEAR/6 service*) (37)
S13 TI,AB(chemotherapy NEAR/6 (“general practitioner*” or “family practitioner*” or “family doctor*” or “primary care physician*”)) (0)
S14 TI,AB(“self-infusion” NEAR/6 home) (0)
S15 TI,AB(“home infusion”) (2)
S16 s11 or s12 or s13 or s14 or s15 (82)
S17 s1 and s16 (61)
S18 s10 or s17 (65)
Key:
TI,AB = terms in either title or abstract fields
* = truncation
NEAR/6 = terms within six words of each other (any order)
“ ” = phrase search.
Cumulative Index to Nursing and Allied Health Literature
Date range: 1982 to March 2013.
Date searched: 25 March 2013.
Records found: 884.
Update
Date range searched: 1982 to March 2013.
Date searched: 29 October 2013.
Records found: 1069.
Search strategy
S1 (MH “Neoplasms+”) (162,398)
S2 cancer* or neoplas* or tumor* or tumour* or malignan* or oncolog* or carcinoma* (201,467)
S3 (MH “Oncologic Nursing”) OR (MH “Pediatric Oncology Nursing”) (10,675)
S4 S1 OR S2 OR S3 (216,493)
S5 (MH “Chemotherapy, Adjuvant”) OR (MH “Chemotherapy, Cancer”) (11,453)
S6 (MH “Administration, Intravenous”) OR (MH “Infusions, Intravenous”) (6862)
S7 chemotherapy (21,389)
S8 “systemic therapy” (967)
S9 “intravenous drug therapy” (45)
S10 “adjuvant therapy” (1311)
S11 S5 OR S6 OR S7 OR S8 OR S9 OR S10 (29,231)
S12 (MH “Home Health Care”) (13,640)
S13 (MH “Home Nursing, Professional”) (6375)
S14 (MH “Community Health Nursing”) OR (MH “Community Health Services”) OR (MH “Community Health Centers”) (29,922)
S15 (MH “Outpatients”) (28,597)
S16 (MH “Ambulatory Care”) (5611)
S17 (MH “Ambulatory Care Facilities”) OR (MH “Ambulatory Care Nursing”) (3924)
S18 (MH “Family Practice”) (9893)
S19 (MH “Physicians, Family”) (7696)
S20 (service* or therapy or treatment*) N6 (home or community or outreach or “out-reach” or ambulatory or domicil*) (32,720)
S21 “hospital at home” or “hospital in the home” or “own home*” or “home care” or homecare or “closer to home” (14,801)
S22 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 (120,856)
S23 S4 AND S11 AND S22 (745)
S24 (MH “Home Intravenous Therapy”) (1197)
S25 chemotherapy N6 (home or community or outreach or “out-reach” or ambulatory or domicil*) (214)
S26 chemotherapy N6 service* (77)
S27 chemotherapy N6 (“general practitioner*” or “family practitioner*” or “family doctor*” or “primary care physician*”) (4)
S28 “self-infusion” N6 home (3)
S29 “home infusion” (263)
S30 S24 OR S25 OR S26 OR S27 OR S28 OR S29 (1476)
S31 S4 and S30 (320)
S32 S23 OR S31 (884)
Key:
MH = indexing term (MeSH heading)
* = truncation
“ ” = phrase search
N6 = terms within six words of each other (any order)
ClinicalTrials.gov
Date searched: 27 March 2013.
Relevant records found: 0.
Search strategy
chemotherapy AND home 154 - none relevant
“ambulatory chemotherapy” (0)
“chemotherapy in the community” (0)
“domiciliary chemotherapy” (0)
“home intravenous therapy” (0)
“self-infusion at home” (0)
“home infusion” 5 - none relevant (0)
“outreach chemotherapy” (0)
“out-reach chemotherapy” (0)
chemotherapy AND “general practice” (9) - none relevant
chemotherapy AND “primary care” (76) - none relevant
Key:
“ ” = phrase search.
The Cochrane Library
URL: http://onlinelibrary.wiley.com
Date searched: 25 March 2013.
Cochrane Database of Systematic Reviews, Issue 2 of 12, February 2013.
Records found: 2.
Update
Date searched: 29 October 2013.
Cochrane Database of Systematic Reviews, Issue 10 of 12, October 2013.
Records found: 3.
Search strategy
#1 MeSH descriptor: [Neoplasms] explode all trees
#2 cancer* or neoplas* or tumor* or tumour* or malignan* or oncolog* or carcinoma*:ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Oncologic Nursing] this term only
#4 #1 or #2 or #3
#5 MeSH descriptor: [Drug Therapy] this term only
#6 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] this term only
#7 MeSH descriptor: [Chemotherapy, Adjuvant] this term only
#8 MeSH descriptor: [Consolidation Chemotherapy] this term only
#9 MeSH descriptor: [Maintenance Chemotherapy] this term only
#10 MeSH descriptor: [Administration, Intravenous] this term only
#11 MeSH descriptor: [Infusions, Intravenous] this term only
#12 chemotherapy or “systemic therapy” or “intravenous drug therapy” or “adjuvant therapy”:ti,ab,kw (Word variations have been searched)
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 MeSH descriptor: [Home Care Services] this term only
#15 MeSH descriptor: [Home Care Services, Hospital-Based] this term only
#16 MeSH descriptor: [Outpatients] this term only
#17 MeSH descriptor: [Ambulatory Care] this term only
#18 MeSH descriptor: [Ambulatory Care Facilities] this term only
#19 MeSH descriptor: [Outpatient Clinics, Hospital] this term only
#20 MeSH descriptor: [Community Health Services] this term only
#21 MeSH descriptor: [Community Health Nursing] this term only
#22 MeSH descriptor: [Community Health Centers] this term only
#23 MeSH descriptor: [General Practitioners] this term only
#24 MeSH descriptor: [Physicians, Family] this term only
#25 MeSH descriptor: [Physicians, Primary Care] this term only
#26 MeSH descriptor: [General Practice] this term only
#27 MeSH descriptor: [Family Practice] this term only
#28 service* near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*):ti,ab,kw (Word variations have been searched)
#29 therapy near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*):ti,ab,kw (Word variations have been searched)
#30 treatment* near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*):ti,ab,kw (Word variations have been searched)
#31 “hospital at home” or “hospital in the home” or “own home*” or “home care” or homecare or “closer to home”:ti,ab,kw (Word variations have been searched)
#32 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31
#33 #4 and #13 and #32
#34 MeSH descriptor: [Home Infusion Therapy] this term only
#35 chemotherapy near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*):ti,ab,kw (Word variations have been searched)
#36 chemotherapy near/6 service*:ti,ab,kw (Word variations have been searched)
#37 chemotherapy near/6 (“general practitioner*” or “family practitioner*” or “family doctor*” or “family physician*” or “primary care physician*”):ti,ab,kw (Word variations have been searched)
#38 “self-infusion” near/6 home:ti,ab,kw (Word variations have been searched)
#39 “home infusion”:ti,ab,kw (Word variations have been searched)
#40 #34 or #35 or #36 or #37 or #38 or #39
#41 #4 and #40
#42 #33 or #41
Key:
MeSH descriptor = indexing term (MeSH heading)
* = truncation
“ “ = phrase search
:ti,ab,kw = terms in title, abstract or keyword fields
near/6 = terms within six words of each other (any order).
The Cochrane Library
Date searched: 25 March 2013.
-
Database of Abstracts of Reviews of Effects: Issue 1 of 4, January 2013.
-
Records found: 9.
-
-
Health Technology Assessment Database: Issue 1 of 4, January 2013.
-
Records found: 2.
-
-
NHS Economic Evaluation Database: Issue 1 of 4, January 2013.
-
Records found: 67.
-
-
Cochrane Central Register of Controlled Trials: Issue 2 of 12, February 2013.
-
Records found: 161.
-
-
Cochrane Methodology Register: Issue 3 of 4, July 2012.
-
Records found: 9.
-
Update
Date searched: 29 October 2013.
-
Database of Abstracts of Reviews of Effects: Issue 3 of 4, July 2013.
-
Records found: 9.
-
-
Health Technology Assessment Database: Issue 3 of 4, July 2013.
-
Records found: 2.
-
-
NHS Economic Evaluation Database: Issue 3 of 4, July 2013.
-
Records found: 67.
-
-
Cochrane Central Register of Controlled Trials: Issue 9 of 12, September 2013.
-
Records found: 161.
-
-
Cochrane Methodology Register: Issue 3 of 4, July 2012.
-
Records found: 9.
-
Search strategy
#1 MeSH descriptor: [Neoplasms] explode all trees
#2 cancer* or neoplas* or tumor* or tumour* or malignan* or oncolog* or carcinoma* (Word variations have been searched)
#3 MeSH descriptor: [Oncologic Nursing] this term only
#4 #1 or #2 or #3
#5 MeSH descriptor: [Drug Therapy] this term only
#6 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] this term only
#7 MeSH descriptor: [Chemotherapy, Adjuvant] this term only
#8 MeSH descriptor: [Consolidation Chemotherapy] this term only
#9 MeSH descriptor: [Maintenance Chemotherapy] this term only
#10 MeSH descriptor: [Administration, Intravenous] this term only
#11 MeSH descriptor: [Infusions, Intravenous] this term only
#12 chemotherapy or “systemic therapy” or “intravenous drug therapy” or “adjuvant therapy” (Word variations have been searched)
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 MeSH descriptor: [Home Care Services] this term only
#15 MeSH descriptor: [Home Care Services, Hospital-Based] this term only
#16 MeSH descriptor: [Outpatients] this term only
#17 MeSH descriptor: [Ambulatory Care] this term only
#18 MeSH descriptor: [Ambulatory Care Facilities] this term only
#19 MeSH descriptor: [Outpatient Clinics, Hospital] this term only
#20 MeSH descriptor: [Community Health Services] this term only
#21 MeSH descriptor: [Community Health Nursing] this term only
#22 MeSH descriptor: [Community Health Centers] this term only
#23 MeSH descriptor: [General Practitioners] this term only
#24 MeSH descriptor: [Physicians, Family] this term only
#25 MeSH descriptor: [Physicians, Primary Care] this term only
#26 MeSH descriptor: [General Practice] this term only
#27 MeSH descriptor: [Family Practice] this term only
#28 service* near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*) (Word variations have been searched)
#29 therapy near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*) (Word variations have been searched)
#30 treatment* near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*) (Word variations have been searched)
#31 “hospital at home” or “hospital in the home” or “own home*” or “home care” or homecare or “closer to home” (Word variations have been searched)
#32 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31
#33 #4 and #13 and #32
#34 MeSH descriptor: [Home Infusion Therapy] this term only
#35 chemotherapy near/6 (home or community or outreach or “out-reach” or ambulatory or domicil*) (Word variations have been searched)
#36 chemotherapy near/6 service* (Word variations have been searched)
#37 chemotherapy near/6 (“general practitioner*” or “family practitioner*” or “family doctor*” or “family physician*” or “primary care physician*”) (Word variations have been searched)
#38 “self-infusion” near/6 home (Word variations have been searched)
#39 “home infusion” (Word variations have been searched)
#40 #34 or #35 or #36 or #37 or #38 or #39
#41 #4 and #40
#42 #33 or #41
Key:
MeSH descriptor = indexing term (MeSH heading)
* = truncation
“ ” = phrase search
near/6 = terms within six words of each other (any order).
Conference Proceedings Citation Index – Science (Web of Knowledge)
URL: http://wokinfo.com
Date range: 1990 to March 2013.
Date searched: 25 March 2013.
Records found: 135.
Update
Date range: 1990 to October 2013.
Date searched: 29 October 2013.
Records found: 141.
Search strategy
# 1 266,359 Topic=(cancer* or neoplas* or tumor* or tumour* or malignan* or oncolog* or carcinoma*)
# 2 27,630 Topic=(chemotherapy)
# 3 625 Topic=(“systemic therapy”)
# 4 3 Topic=(“intravenous drug therapy”)
# 5 1960 Topic=(“adjuvant therapy”)
# 6 29,227 #5 OR #4 OR #3 OR #2
# 7 5659 Topic=((service* or therapy or treatment*) NEAR/6 (home or community or outreach or out-reach or ambulatory or domicil*))
# 8 1213 Topic=(“hospital at home” or “hospital in the home” or “own home*” or “home care” or homecare or “closer to home”)
# 9 6636 #8 OR #7
# 10 61 #9 AND #6 AND #1
# 11 119 Topic=(chemotherapy NEAR/6 (home or community or outreach or “out-reach” or ambulatory or domicil*))
# 12 13 Topic=(chemotherapy NEAR/6 service*)
# 13 2 Topic=(chemotherapy NEAR/6 (“general practitioner” or “family practitioner*” or “family doctor*” or “family physician*” or “primary care physician*”))
# 14 2 Topic=(“self-infusion” NEAR/6 home)
# 15 11 Topic=(“home infusion”)
# 16 146 #15 OR #14 OR #13 OR #12 OR #11
# 17 91 #16 AND #1
# 18 135 #17 OR #10
Key:
TS= topic tag; searches terms in Title, Abstract, Author Keywords and Keywords Plus fields
* = truncation
“ ” = phrase search
NEAR/6 = terms within six words of each other (any order).
Current Controlled Trials
URL: http://controlled-trials.com/mrct/search.html
Date searched: 27 March 2013.
Relevant records found: 0.
Search strategy
chemotherapy AND home 254 - none relevant
‘ambulatory chemotherapy’ 0
‘chemotherapy in the community’ 1 - none relevant
‘domiciliary chemotherapy’ 0
‘home intravenous therapy’ 0
‘self-infusion at home’ 0
‘home infusion’ 6 - none relevant
‘outreach chemotherapy’ 0
‘out-reach chemotherapy’ 0
chemotherapy AND ‘general practice’ 6 - none relevant
chemotherapy AND ‘primary care’ 66 - none relevant
Key:
‘ ’ = phrase search
Dissertation Abstracts
URL: www.dialog.com
Date range: 1861 to March 2013.
Date searched: 27 March 2013.
Records found: 24.
Search strategy
S1 47073 (CANCER? OR NEOPLAS? OR TUMOR OR TUMORS OR TUMOUR OR TUMOURS OR MALIGNANT OR MALIGNANCY OR ONCOLOGY OR CARCINOMA?)
S2 3395 CHEMOTHERAPY
S3 54 SYSTEMIC(W)THERAPY
S4 2 INTRAVENOUS(W)DRUG(W)THERAPY
S5 119 ADJUVANT(W)THERAPY
S6 3523 S2 OR S3 OR S4 OR S5
S7 9903 (SERVICE? OR THERAPY OR TREATMENT?)(6N)(HOME OR COMMUNITY - OR OUTREACH OR OUT(W)REACH OR AMBULATORY OR DOMICIL?)
S8 1689 HOSPITAL(W)AT(W)HOME OR HOSPITAL(W)IN(W)THE(W)HOME OR OWN(- W)HOME? OR HOME(W)CARE OR HOMECARE OR CLOSER(W)TO(W)HOME
S9 11189 S7 OR S8
S10 11 S1 AND S6 AND S9
S11 18 CHEMOTHERAPY(6N)(HOME OR COMMUNITY OR OUTREACH OR OUT(W)REACH OR AMBULATORY OR DOMICIL?)
S12 3 CHEMOTHERAPY(6N)SERVICE?
S13 0 CHEMOTHERAPY(6N)(GENERAL(W)PRACTITIONER? OR FAMILY(W)PRACTITIONER? OR FAMILY(W)DOCTOR? OR PRIMARY(W)CARE(W)PHYSICIAN?)
S14 0 SELF(W)INFUSION(6N)HOME
S15 6 HOME(W)INFUSION
S16 27 S11 OR S12 OR S13 OR S14 OR S15
S17 16 S1 AND S16
S18 24 S10 OR S17
Key:
? = truncation
(W) = terms adjacent to each other (same order)
(6N) = terms within 6 words of each other (any order).
EconLit
Date range: 1961 to February 2013.
Date searched: 25 March 2013.
Records found: 1.
Search strategy
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (1014)
-
chemotherapy.ti,ab. (50)
-
systemic therapy.ti,ab. (3)
-
intravenous drug therapy.ti,ab. (0)
-
adjuvant therapy.ti,ab. (2)
-
or/2-5 (54)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (893)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (345)
-
7 or 8 (1175)
-
1 and 6 and 9 (1)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (1)
-
(chemotherapy adj6 service$).ti,ab. (0)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (0)
-
(self-infusion adj6 home).ti,ab. (0)
-
home infusion.ti,ab. (1)
-
or/11-15 (2)
-
1 and 16 (1)
-
10 or 17 (1)
Key:
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
EMBASE
Date range: 1974 to 22 March 2013.
Date searched: 25 March 2013.
Records found: 2719.
Update
Date range: 1974 to 28 October 2013.
Date searched: 29 October 2013.
Records found: 2940.
Search strategy
-
exp neoplasm/ (3,205,629)
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (2,642,872)
-
exp oncology nursing/ (5654)
-
or/1-3 (3,707,658)
-
chemotherapy/ or adjuvant chemotherapy/ or cancer chemotherapy/ or combination chemotherapy/ or consolidation chemotherapy/ or induction chemotherapy/ or maintenance chemotherapy/ or multimodal chemotherapy/ (252,289)
-
antineoplastic agent/ (211,787)
-
chemotherapy.ti,ab. (314,754)
-
systemic therapy.ti,ab. (8828)
-
intravenous drug therapy.ti,ab. (53)
-
adjuvant therapy.ti,ab. (20,039)
-
or/5-10 (580,292)
-
home care/ (45,243)
-
*outpatient/ (6050)
-
*ambulatory care/ or *ambulatory care nursing/ (11,954)
-
*outpatient department/ (11,572)
-
community care/ or community health nursing/ (68,672)
-
general practitioner/ or general practice/ (115,882)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (54,756)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (18,147)
-
or/12-19 (289,243)
-
4 and 11 and 20 (2138)
-
home intravenous therapy/ (17)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (1000)
-
(chemotherapy adj6 service$).ti,ab. (254)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (28)
-
(self-infusion adj6 home).ti,ab. (27)
-
home infusion.ti,ab. (350)
-
or/22-27 (1624)
-
4 and 28 (954)
-
21 or 29 (2733)
-
animals/ or nonhumans/ (1,821,160)
-
humans/ (14178823)
-
31 not (31 and 32) (1,363,257)
-
30 not 33 (2719)
Key:
/ = indexing term (EMTREE heading)
* = focussed EMTREE heading
exp = exploded EMTREE heading
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
URL: www.google.com
Date searched: 10 September 2013.
Additional records found: 16.
Checked first 100 hits for all search strings for relevancy.
Search strategy
(“home infusion therapy” AND chemotherapy AND (cancer OR neoplasms)) filetype:pdf = 21,000 hits
(“home chemotherapy” AND (cancer OR neoplasms)) filetype:pdf = 1160 hits
(“chemotherapy at home” AND (cancer OR neoplasms)) filetype:pdf = 4360 hits
(“outreach chemotherapy” AND (cancer OR neoplasms)) filetype:pdf = 177 hits
(“ambulatory chemotherapy” AND (cancer OR neoplasms)) filetype:pdf = 1190 hits
(“hospital at home” AND chemotherapy AND (cancer OR neoplasms)) filetype:pdf = 27,000 hits
(“closer to home” AND chemotherapy AND (cancer OR neoplasms)) filetype:pdf = 22,800 hits
Health Management Information Consortium
Date range: 1979 to January 2013.
Date searched: 25 March 2013.
Records found: 44.
Search strategy
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (12,171)
-
chemotherapy.ti,ab. (542)
-
systemic therapy.ti,ab. (9)
-
intravenous drug therapy.ti,ab. (1)
-
adjuvant therapy.ti,ab. (35)
-
or/2-5 (579)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (9283)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (2469)
-
7 or 8 (10,981)
-
1 and 6 and 9 (24)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (30)
-
(chemotherapy adj6 service$).ti,ab. (37)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (0)
-
(self-infusion adj6 home).ti,ab. (0)
-
home infusion.ti,ab. (0)
-
or/11-15 (55)
-
1 and 16 (35)
-
10 or 17 (44)
Key:
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
Inside Conferences
URL: www.dialog.com
Date range: 1993 to March 2013.
Date searched: 27 March 2013.
Records found: 25.
Search strategy
S1 113738 (CANCER? OR NEOPLAS? OR TUMOR OR TUMORS OR TUMOUR OR TUMOURS OR MALIGNANT OR MALIGNANCY OR ONCOLOGY OR CARCINOMA?)
S2 10350 CHEMOTHERAPY
S3 97 SYSTEMIC(W)THERAPY
S4 0 INTRAVENOUS(W)DRUG(W)THERAPY
S5 408 ADJUVANT(W)THERAPY
S6 10815 S2 OR S3 OR S4 OR S5
S7 2387 (SERVICE? OR THERAPY OR TREATMENT?)(6N)(HOME OR COMMUNITY - OR OUTREACH OR OUT(W)REACH OR AMBULATORY OR DOMICIL?)
S8 656 HOSPITAL(W)AT(W)HOME OR HOSPITAL(W)IN(W)THE(W)HOME OR OWN(- W)HOME? OR HOME(W)CARE OR HOMECARE OR CLOSER(W)TO(W)HOME
S9 2938 S7 OR S8
S10 12 S1 AND S6 AND S9
S11 24 CHEMOTHERAPY(6N)(HOME OR COMMUNITY OR OUTREACH OR OUT(W)REACH OR AMBULATORY OR DOMICIL?)
S12 3 CHEMOTHERAPY(6N)SERVICE?
S13 0 CHEMOTHERAPY(6N)(GENERAL(W)PRACTITIONER? OR FAMILY(W)PRACTITIONER? OR FAMILY(W)DOCTOR? OR PRIMARY(W)CARE(W)PHYSICIAN?)
S14 2 SELF(W)INFUSION(6N)HOME
S15 12 HOME(W)INFUSION
S16 40 S11 OR S12 OR S13 OR S14 OR S15
S17 18 S1 AND S16
S18 25 S10 OR S17
Key:
? = truncation
(W) = terms adjacent to each other (same order)
(6N) = terms within 6 words of each other (any order).
Inspec
Date range: 1969 to week 10 2013.
Date searched: 25 March 2013.
Records found: 4.
Search strategy
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (63,025)
-
chemotherapy.ti,ab. (2100)
-
systemic therapy.ti,ab. (40)
-
intravenous drug therapy.ti,ab. (0)
-
adjuvant therapy.ti,ab. (54)
-
or/2-5 (2176)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (5829)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (1339)
-
7 or 8 (6986)
-
1 and 6 and 9 (3)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (4)
-
(chemotherapy adj6 service$).ti,ab. (2)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (0)
-
(self-infusion adj6 home).ti,ab. (0)
-
home infusion.ti,ab. (4)
-
or/11-15 (9)
-
1 and 16 (3)
-
10 or 17 (4)
Key:
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
OHE HEED
URL: http://onlinelibrary.wiley.com/book/10.1002/9780470510933
Date searched: 14 May 2013.
Relevant records found: 29.
Search strategy
chemotherapy AND home 52 - 19 relevant
‘ambulatory chemotherapy’ 2
‘chemotherapy in the community’ 0
‘domiciliary chemotherapy’ 1
‘home intravenous therapy’ 3 - 2 relevant
‘self-infusion at home’ 0
‘home infusion’ 13 - 5 relevant
‘outreach chemotherapy’ 0
‘out-reach chemotherapy’ 0
chemotherapy AND ‘general practice’ 3 - 2 relevant
chemotherapy AND ‘primary care’ 12 - 1 relevant
Key:
‘ ’ = phrase search.
PsycINFO
Date range: 1806 to week 3 2013.
Date searched: 25 March 2013.
Records found: 72.
Search strategy
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (46,619)
-
chemotherapy.ti,ab. (3347)
-
systemic therapy.ti,ab. (369)
-
intravenous drug therapy.ti,ab. (0)
-
adjuvant therapy.ti,ab. (246)
-
or/2-5 (3906)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (24,037)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (4691)
-
7 or 8 (27,793)
-
1 and 6 and 9 (46)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (42)
-
(chemotherapy adj6 service$).ti,ab. (17)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (2)
-
(self-infusion adj6 home).ti,ab. (1)
-
home infusion.ti,ab. (6)
-
or/11-15 (61)
-
1 and 16 (44)
-
10 or 17 (72)
Key:
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
PubMed
URL: www.ncbi.nlm.nih.gov/pubmed
Date range: all dates to 27 March 2012.
Date searched: 27 March 2013.
Records found: 975.
Update
Date range: all dates to 29 October 2012.
Date searched: 29 October 2013.
Records found: 1007.
Search strategy
#1 Search neoplasms[MeSH Terms] 2,415,714
#2 Search ((((((cancer*[Title/Abstract]) OR neoplas*[Title/Abstract]) OR tumor*[Title/Abstract]) OR tumour*[Title/Abstract]) OR malignan*[Title/Abstract]) OR oncolog*[Title/Abstract]) OR carcinoma*[Title/Abstract] 2,032,899
#3 Search oncologic nursing[mh:noexp] 6072
#4 Search ((#1) OR #2) OR #3 2,923,865
#5 Search drug therapy[mh:noexp] 33,188
#6 Search antineoplastic combined chemotherapy protocols[mh:noexp] 96,554
#7 Search ((chemotherapy, adjuvant[mh:noexp]) OR consolidation chemotherapy[mh:noexp]) OR maintenance chemotherapy[mh:noexp] 27,399
#8 Search infusion, intravenous[mh:noexp] 45,706
#9 Search chemotherapy[Title/Abstract] 225,942
#10 Search “systemic therapy”[Title/Abstract] 5969
#11 Search “intravenous drug therapy”[Title/Abstract] 40
#12 Search “adjuvant therapy”[Title/Abstract] 14,837
#13 Search (((((((#5) OR #6) OR #7) OR #8) OR #9) OR #10) OR #11) OR #12 359,727
#14 Search (home care services[mh:noexp]) OR home care services, hospital based[mh:noexp] 27,991
#15 Search outpatients[majr:noexp] 2120
#16 Search ambulatory care[majr:noexp] 14,543
#17 Search (ambulatory care facilities[majr:noexp]) OR outpatient clinics, hospital[majr:noexp] 13,413
#18 Search ((community health services[mh:noexp]) OR community health nursing[mh:noexp]) OR community health centers[mh:noexp] 47,623
#19 Search ((general practitioners[mh:noexp]) OR physicians, family[mh:noexp]) OR physicians, primary care[mh:noexp] 16,302
#20 Search (general practice[mh:noexp]) OR family practice[mh:noexp] 60,996
#21 Search (((((((((((((((((“home service*”[Title/Abstract]) OR “home therapy”[Title/Abstract]) OR “home treatment*”[Title/Abstract]) OR “community service”[Title/Abstract]) OR “community therapy”[Title/Abstract]) OR “community treatment”[Title/Abstract]) OR “outreach service*”[Title/Abstract]) OR “outreach therapy”[Title/Abstract]) OR “outreach treatment*”[Title/Abstract]) OR “out-reach service*”[Title/Abstract]) OR “out-reach therapy”[Title/Abstract]) OR “out-reach treatment*”[Title/Abstract]) OR “ambulatory service*”[Title/Abstract]) OR “ambulatory therapy”[Title/Abstract]) OR “ambulatory treatment*”[Title/Abstract]) OR “domicil* service*”[Title/Abstract]) OR “domicil* therapy”[Title/Abstract]) OR “domicil* treatment*”[Title/Abstract] 5808
#22 Search (((((“hospital at home”[Title/Abstract]) OR “hospital in the home”[Title/Abstract]) OR “own home*”[Title/Abstract]) OR “home care”[Title/Abstract]) OR homecare[Title/Abstract]) OR “closer to home”[Title/Abstract] 14,748
#23 Search ((((((((#14) OR #15) OR #16) OR #17) OR #18) OR #19) OR #20) OR #21) OR #22 180,529
#24 Search ((#4) AND #13) AND #23 845
#25 Search home infusion therapy[mh:noexp] 580
#26 Search (((((“home chemotherapy”[Title/Abstract]) OR “community chemotherapy”[Title/Abstract]) OR “outreach chemotherapy”[Title/Abstract]) OR “out-reach chemotherapy”[Title/Abstract]) OR “ambulatory chemotherapy”[Title/Abstract]) OR “domicil* chemotherapy”[Title/Abstract] 171
#27 Search “chemotherapy service*”[Title/Abstract] 11
#28 Search ((((“general practitioner chemotherapy”[Title/Abstract]) OR “family practitioner chemotherapy”[Title/Abstract]) OR “family doctor chemotherapy”[Title/Abstract]) OR “family physician chemotherapy”[Title/Abstract]) OR “primary care physician chemotherapy”[Title/Abstract] 0
#29 Search “self-infusion at home”[Title/Abstract] 0
#30 Search “home infusion”[Title/Abstract] 259
#31 Search (((((#25) OR #26) OR #27) OR #28) OR #29) OR #30 890
#32 Search (#4) AND #31 238
#33 Search (#24) OR #32 975
Key:
[MeSH Terms] = indexing term (MeSH heading)
[mh:noexp] = non-exploded MeSH heading
[Title/Abstract] = terms in either title or abstract fields
“ ” = phrase search
* = truncation.
Social Policy and Practice
Date range: all dates to January 2013.
Date searched: 25 March 2013.
Records found: 3.
Search strategy
-
(cancer$ or neoplas$ or tumor$ or tumour$ or malignan$ or oncolog$ or carcinoma$).ti,ab. (1854)
-
chemotherapy.ti,ab. (42)
-
systemic therapy.ti,ab. (58)
-
intravenous drug therapy.ti,ab. (0)
-
adjuvant therapy.ti,ab. (0)
-
or/2-5 (100)
-
((service$ or therapy or treatment$) adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (10,540)
-
(hospital at home or hospital in the home or own home$ or home care or homecare or closer to home).ti,ab. (4276)
-
7 or 8 (13,575)
-
1 and 6 and 9 (0)
-
(chemotherapy adj6 (home or community or outreach or out-reach or ambulatory or domicil$)).ti,ab. (2)
-
(chemotherapy adj6 service$).ti,ab. (3)
-
(chemotherapy adj6 (general practitioner$ or family practitioner$ or family doctor$ or family physician$ or primary care physician$)).ti,ab. (0)
-
(self-infusion adj6 home).ti,ab. (0)
-
home infusion.ti,ab. (0)
-
or/11-15 (4)
-
1 and 16 (3)
-
10 or 17 (3)
Key:
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj6 = terms within six words of each other (any order).
Appendix 2 Studies which investigated only one setting
Home studies (single setting)
Alfieri P, Bertocchi M, Petocchi B, Torelli G, Favale E. Intravenous chemotherapy at home in hematology patients: a report from the A.I.L. hematology home care service in Modena. Haematologica 2009;94:179.
Anderson H, Addington-Hall JM, Peake MD, McKendrick J, Keane K, Thatcher N. Domiciliary chemotherapy with gemcitabine is safe and acceptable to advanced non-small-cell lung cancer patients: results of a feasibility study. Br J Cancer 2003;89:2190–6.
Bassot V, Brasnu D, Lacau-St-Guily J, Fabre A, Menard M, Jacquillat C, et al. [Chemotherapy at home. A new methodology.] Ann Otolaryngol Chir Cervicofac 1986;103:77–81.
Brown DF, Muirhead MJ, Travis PM, Vire SR, Weller J, Hauer-Jensen M. Mode of chemotherapy does not affect complications with an implantable venous access device. Cancer 1997;80:966–72.
Butler MC. Families’ responses to chemotherapy by an ambulatory infusion pump . . . at home. Nurs Clin North Am 1984;19:139–44.
Chen JT, Ida K, Hasumi K, Masubuchi K. [Maintenance of housewife activity and the quality of familiar interactions by home continuous infusion chemotherapy in patients with recurrent gynecological cancer.] Gan To Kagaku Ryoho 1991;18:2517–22.
Close P, Burkey E, Kazak A, Danz P, Lange B. A prospective, controlled evaluation of home chemotherapy for children with cancer. Pediatrics 1995;95:896–900.
Crisp N. Chemotherapy at home: keeping patients in their ‘natural habitat’. Ph.D. thesis. Edmonton, AB: University of Alberta; 2010.
D’Andrea B, Belliveau D, Birmingham J, Cooper D. High-dose chemotherapy followed by stem cell transplant: the clinic/home care experience. J Care Manag 1997;3:46–52.
Dahan M, Paule B, Vordos D, Larre S, Salomon L, Yiou R, et al. [Home hospitalisation in urological cancer: assessment of the first 5 years and a satisfaction survey.] Prog Urol 2007;17:855–9.
DeMoss CJ. Giving intravenous chemotherapy at home. Am J Nurs 1980;80:2188–9.
Frohmüller S, Schlag P, Leucht R, Ophof J, Ruoff G. [Oncologic therapy at home: a trial model.] Deutsche Medizinische Wochenschrift 1989;114:1055–8.
Guillevic S, Comont T, Khalifa J, Recher C, Adoue D, Ollier S, et al. Home azacitidine administration in high risk myelodysplastic syndromes: favorable results of a pilot study in 48 patients. Blood 2011;118:749.
Hansson H, Kjaergaard H, Johansen C, Hallström I, Christensen J, Madsen M, et al. Hospital-based home care for children with cancer: feasibility and psychosocial impact on children and their families. Pediatr Blood Cancer 2013;60:865–72.
Harrison DE, Fitch MI. Toronto-Sunnybrook Regional Cancer Centre Home Oncology Model Evaluation (H.O.M.E.) pilot program. Can Oncol Nurs J 1995;5:85–92.
Hooker L, Kohler J. Safety, efficacy, and acceptability of home intravenous therapy administered by parents of pediatric oncology patients. Med Pediatr Oncol 1999;32:421–6.
Jayabose S, Escobedo V, Tugal O, Nahaczewski A, Donohue P, Fuentes V, et al. Home chemotherapy for children with cancer. Cancer 1992;69:574–9.
Joo EH, Rha SY, Ahn JB, Kang HY. Economic and patient-reported outcomes of outpatient home-based versus inpatient hospital-based chemotherapy for patients with colorectal cancer. Support Care Cancer 2011;19:971–8.
Lange BJ, Burroughs B, Meadows AT, Burkey E. Home care involving methotrexate infusions for children with acute lymphoblastic leukemia. J Pediatr 1988;112:492–5.
Lashlee M, O’Hanlon Curry J. Pediatric home chemotherapy: infusing ‘quality of life’. J Pediatr Oncol Nurs 2007;24:294–8.
Lewden-Bernadac B, Courant-Menanteau M, Perrocheau G, Barbarot V, Thomare P. [Outpatient chemotherapy and oncology network: Onco Pays-de-la-Loire experiment.] Bull Cancer 2008;95:543–9.
Liston B, Meenaghan T, Wilson H. Home chemotherapy service from University College Hospital Galway. EJHP Pract 2009;15:45.
Lüthi F, Fucina N, Divorne N, Santos-Eggimann B, Currat-Zweifel C, Rollier P, et al. Home care – a safe and attractive alternative to inpatient administration of intensive chemotherapies. Support Care Cancer 2012;20:575–81.
Midorikawa Y, Suzuki K, Kasuga T, Takemura A. [Our outpatient cases for home anti-cancer chemotherapy.] Gan To Kagaku Ryoho 2005;32(Suppl. 1):1–3.
Oakley C, Wright E, Ream E. The experiences of patients and nurses with a nurse-led peripherally inserted central venous catheter line service. Eur J Oncol Nurs 2000;4:207–18.
Ranuzzi M. Home-care in advanced breast cancer: results in 144 patients. Ann Oncol 2009;20:ii69.
Ranuzzi M, Taddei A, Brunetti S, Gentile S, Vercelloni R, Scarpati S. 10 years experience in home-care with cancer patients. Support Care Cancer 2009;17:973.
Raphael R, Yves D, Giselle C, Magali M, Odile CM. Cancer treatment at home or in the hospital: what are the costs for French public health insurance? Findings of a comprehensive-cancer centre. Health Policy 2005;72:141–8.
Richardson J, Townsend C, Holland D. Outreach Clinics, Ipswich, Anglia Cancer Network. A patient Survey of a Nurse-Led Outreach Service. URL: www.ukchemotherapypartnership.org.uk/service_models/closer_to_home (retrieved 21 November 2013).
Sato K, Takahashi T, Ashikaga K, Hanyu N, Aoiki T. [Clinical study of home hepatic arterial infusion chemotherapy for liver metastasis from colorectal cancer.] Gan To Kagaku Ryoho 1996;23(Suppl. 3):252–5.
Sawada K, Sawada M, Suzuki Y, Nakamura M. [Experience of cancer home chemotherapy with cooperation between hospital and clinic.] Gan To Kagaku Ryoho 1998;25(Suppl. 4):625–30.
Sawada K, Tan M, Kanno M, Saitou K, Oikawa K, Shibuya S, et al. [Home chemotherapy for peritoneal carcinomatosis.] Gan To Kagaku Ryoho 1994;21(Suppl. 4):463–9.
Schlag P, Feil H, Ruoff G, Hohenberger P, Hölting T, Buhl K. [Home treatment of cancer: experiences with ambulatory intra-arterial chemotherapy of liver metastases.] Schweiz Med Wochenschr 1987;117:1342–6.
Taylor H, Ireland J, Duggan C, Bates I. Home chemotherapy – a feasibility study. Br J Home Healthcare 2007;3:4–5.
Watty K, White MA, Matthews JP, Buchanan L, Clarke JL, Sulkowski AJ, et al. There’s no place like home: a prospective evaluation of chemotherapy in the home. Aus J Cancer Nurs 2003;4:18.
Yamada M, Hasegawa A, Matsuura C, Chen JT. [A study of quality of life for cancer chemotherapy patients: a comparison between hospital treatment and home treatment.] Kango Kenkyu 1997;30:425–34.
Community studies (single setting)
Bakker DA, DesRochers C, McChesney C, Fitch M, Bennett J. Community cancer clinics: patients’ perspectives. Support Care Cancer 2001;9:234–40.
Gordon J, Gruber M. An innovative off-campus infusion suite designed to improve experiences of patients with cancer. Clin J Oncol Nurs 2012;16:354–9.
Iredale R, Hilgart J, Hayward J. Patient perceptions of a mobile cancer support unit in South Wales. Eur J Cancer Care 2011;20:555–60.
Turner C, Pateman B. A study of district nurses’ experiences of continuous ambulatory chemotherapy. Br J Community Nurs 2000;5:396–400.
Outpatient studies (single setting, with qualitative outcomes)
Hjörleifsdóttir E, Hallberg IR, Gunnarsdóttir ED, Bolmsjö IA. Living with cancer and perception of care: Icelandic oncology outpatients, a qualitative study. Support Care Cancer 2008;16:515–24.
McIlfatrick S, Sullivan K, McKenna H, Parahoo K. Patients’ experiences of having chemotherapy in a day hospital setting. J Adv Nurs 2007;59:264–73.
Appendix 3 Randomised controlled trial study details
| General details | Population and treatment | Setting | Recruitment and participation | Outcomes reported/comments |
|---|---|---|---|---|
| Main reference: Borras et al. 2001,35 full published paper Linked references: none Design: RCT (parallel group) Country: Spain Recruitment period: October 1997 to October 1998 Number of recruiting centres: one Assessment time points: ‘Treatment completion’. This was approximately 6–8 months for palliative patients and 12 months for adjuvant patients |
Key characteristics of recruited population: Inclusion criteria: adults (aged 18–75 years) with colorectal cancer were eligible Mean age: 60 years Gender: 45/87 male (52%) Cancer type: colon 40/87 (46%); rectum 27/87 (31%); advanced disease 20/87 (23%) Mean Karnofsky index score: 83 Treatment intention: curative (adjuvant) 70/87 (80%) or palliative 17/87 (20%) Chemotherapy used: fluorouracil |
Setting details: home Delivered by a trained nurse (no further details) Hospital (outpatient) Standard care in outpatient clinic (no further details) Preparation of chemotherapy: NR |
Target sample size: NR Number actually randomised: 87 Estimated monthly rate of randomisation, per centre: 6.7 Number of eligible participants who were not randomised because of setting preference: NR (only one eligible patient was not randomised) Did it appear that all eligible patients were invited to participate? NR Withdrawals and dropouts 31/87 (36%) patients did not complete chemotherapy 6/42 (14%) outpatients and 1/45 (2%) home patient withdrew voluntarily (no reason details provided) 13/42 (31%) outpatients and 11/45 (24%) home patients withdrew because of toxicity, disease progression or doctor advice |
Primary outcome(s): not specified Other outcomes: treatment toxicity (ECOG); withdrawals; use of health-care resources; quality of life (EORTC QOL-C30); satisfaction with health care; Karnofsky Index Qualitative data reported? No Economic data reported? Yes Comments |
| Main reference: Chen and Hasuimi 1999,27 conference abstract Linked references: none Design: randomised trial Country: Japan Recruitment period: NR Number of recruiting centres: one Assessment time points: days 1, 3 and 5 of first course of chemotherapy |
Key characteristics of recruited population: the only details reported were that patients had been operated on for ovarian cancer Treatment intention: NR Chemotherapy used: all patients received cisplatin 15 mg per square metre (days 1–5), doxorubicin 35 mg per square metre (day 1), cyclophosphamide 350 mg per square metre (day 1) |
Setting details: home; outpatient Preparation of chemotherapy: NR |
Target sample size: NR Number actually randomised: 10 Estimated monthly rate of randomisation: cannot be calculated Number of eligible participants who were not randomised because of setting preference: NR Did it appear that all eligible patients were invited to participate? NR Withdrawals and dropouts: NR |
Primary outcome(s): not specified Other outcomes: quality of life, State Trait Anxiety Inventory, mean nursing time Qualitative data reported? No Economic evaluation: no Comments: the study aimed to compare the effect of setting on outcomes following the first course of chemotherapy. One group received the first course at home (followed by outpatient chemotherapy) and the other received the first course in the outpatient setting (followed by home chemotherapy) |
| Main reference: Christiansen et al. 2011,28 conference abstract Linked references: none Design: RCT (crossover) Country: Denmark Recruitment period: November 2007 to November 2010 Number of recruiting centres: one Assessment time points: Quality of life: at baseline and before each treatment (total of eight treatments including initial outpatient clinic treatment) Preference: at baseline, change of treatment setting, and end of treatment |
Key characteristics of recruited population: Inclusion criteria: patients with colon cancer who were eligible to receive adjuvant treatment with oxaliplatin and capecitabine Median age: 64 years Gender: 27/51 (53%) female Treatment intention: curative (adjuvant) Chemotherapy used: oxaliplatin and capecitabine every 3 weeks |
Setting details: home Hospital (outpatient): all patients received first infusion at the outpatient clinic before randomisation (for safety reasons) No further details for either setting were reported Preparation of chemotherapy: NR |
Target sample size: NR Number actually randomised: 51 Estimated monthly rate of randomisation, per centre: 1.4 Number of eligible participants who were not randomised because of setting preference: NR Did it appear that all eligible patients were invited to participate? NR Withdrawals and dropouts: 14 patients did not complete all eight treatments |
Primary outcome(s): not specified Other outcomes: quality of life (EORTC QLQ-C30); adverse effects; time spent receiving chemotherapy; patient preference; costs Qualitative data reported? No Economic evaluation: no Comments |
| Main reference: Corrie et al. 2013,4 full published paper Linked references: protocol;98 conference posters;99,100 project report form (Corrie, 2013, Cambridge University Hospitals NHS Foundation Trust, unpublished document) Design: RCT (parallel group) Country: England Recruitment period: January 2009 to May 2011 Number of recruiting centres: two Assessment time points: 4, 8 and 12 weeks. Optional 24-week (or treatment cessation) assessment |
Key characteristics of recruited population: Inclusion criteria: adults with an ECOG status of 0–2, scheduled to receive at least 12 weeks of treatment, living within a 30-minute drive of the recruiting hospital, and infusion lasting no more than 4 hours Exclusion criteria: life expectancy under 6 months, participation in a clinical trial (of unlicensed drug) Gender: 33/97 male (34%) Cancer type: breast 36/97 (37%); lung 27/97 (28%); pancreatic 21/97 (22%); other 13/97 (13%) ECOG status: 0 = 65/97 (67%); 1 = 26/97 (27%); 2 = 6/97 (6%) Prior cancer drug: no = 50/97 (52%), yes = 47/97 (48%) Treatment intention: curative (33%), palliative (53%) or supportive care (14%) Chemotherapy used: no details on drugs or regimes used, but delivered based on standard operating procedures |
Setting details: Home: chemotherapy delivered by a single nurse in the patient’s home GP surgery: offered a choice of three local surgeries all with free parking and conveniently located with respect to the two recruiting hospitals Hospital: outpatient and day unit Preparation of chemotherapy: chemotherapy drugs prepared by oncology pharmacists in the two key hospitals, then dispensed and collected by nurse for delivery in the community |
Target sample size: 390 Number actually randomised: 97 Estimated monthly rate of randomisation, per centre: 1.7 Number of eligible participants who were not randomised because of setting preference: 53. 16 patients were reluctant to receive treatment at a GP surgery, two did not want home treatment and 35 wanted to be treated in hospital Did it appear that all eligible patients were invited to participate? The authors indicated that clinicians were somewhat reluctant to refer patients into the trial, citing concerns about patient and nurse safety and resource use. Also see ‘Comments’ Withdrawals and dropouts: data for 57 patients could be analysed at end of trial. Six patients failed to start treatment, 17 patients did not complete 12 weeks of treatment Home: 33 allocated, 33 started, five stopped, five incomplete data sets GP: 32 allocated, 29 started, eight stopped, four incomplete data sets Hospital: 32 allocated, 29 started, four stopped, right incomplete data sets |
Primary outcome(s): patient-reported quality of life, using the Emotional Function domain of the EORTC QLQ-30 questionnaire Other outcomes: EORTC QLQ-C30 (self-rated health); HADS Anxiety; HADS Depression; EQ-5D; costs; satisfaction; serious adverse events Economic evaluation? Yes Type of economic evaluation: CUA Currency (price year): GBP (£) NR Study perspective: not explicitly reported, appears to be NHS perspective Qualitative data reported? Yes Comments: the trial stopped prematurely due to poor accrual rate, on the advice of the independent data monitoring committee Results reported only as differences (between groups) |
| Main reference: Hall and Lloyd 2008,30 full paper Linked references: none Design: RCT (parallel) Country: UK (England) Recruitment period: 6 months (no dates reported) Number of recruiting centres: one Assessment time points: after the 4th treatment cycle |
Key characteristics of recruited population: Inclusion criteria: breast cancer patients intending to receive a minimum of four cycles of anthracycline-based chemotherapy Cancer type: breast cancer No other data regarding patient characteristics were provided Treatment intention: treatment used either to prevent recurrence or metastatic spread in early-stage disease, or for palliation of symptoms in advanced disease cases Chemotherapy used: anthracycline-based chemotherapy |
Setting details: Home: no details reported Hospital (outpatient): no details reported Preparation of chemotherapy: NR |
Target sample size: 20 Number actually randomised: 15 Estimated monthly rate of randomisation, per centre: 2.5 Number of eligible participants who were not randomised because of setting preference: NR Did it appear that all eligible patients were invited to participate? Patients were identified as being suitable for the study by oncologists or breast cancer nurse specialists at their oncology appointments, and were recruited by the nurse consultant Withdrawals and dropouts: NR |
Primary outcome(s): not specified Other outcomes: patient experience and satisfaction; costs Qualitative data reported? Yes Economic data reported? No Comments: primarily a qualitative study |
| Main reference: King et al. 2000,31 full published paper Linked references: King et al. 2001,54 letters to editor; Caleo et al. 1996,101 conference abstract Design: RCT (crossover) Country: Australia Recruitment period: 1993–5 Number of recruiting centres: two Assessment time points: Patient quality of life: recruitment to study and at each chemotherapy treatment session Preference and satisfaction: after 2 and 4 months (end of setting periods) Unmet needs: NR |
Key characteristics of recruited population: Inclusion criteria: patients who lived in a ≈ 20 km radius of the respective hospital and whose planned treatment consisted of one of the trial chemotherapy regimens Mean age: NR Gender: NR Cancer type: Early colon cancer: 27/74 (36%) Early-stage breast cancer: 21/74 (28%) Metastatic breast cancer: 25/74 (34%) Head and neck cancer: 1/74 (1%) Treatment intention: adjuvant (colon and early breast cancer, 65%) or palliative (metastatic breast cancer, 34%); NR for head and neck cancer Chemotherapy used: 5-flourouracil and levamisole (colon cancer); intravenous CMF, methotrexate and 5-fluorouracil (CMF; early breast cancer); oral CMF (metastatic breast cancer); and methotrexate (head and neck cancer) |
Setting details: Home: treatment provided by existing hospital-based oncology nursing staff. Nurses travelled from the medical oncology unit closest to the patient’s home Hospital (outpatient): no details provided Preparation of chemotherapy: NR |
Target sample size: NR Number actually randomised: 74 Estimated monthly rate of randomisation, per centre: 1.5 Number of eligible participants who were not randomised because of setting preference: 13. Four patients felt safer in hospital; two thought that their home was unsuitable owing to social problems; one did not want to associate home with chemotherapy; and six thought that being in the study would be more inconvenient than regular hospital care Did it appear that all eligible patients were invited to participate? Patient recruitment to the trial was dependent on the medical oncologist or oncologist nurses’ judgement of the patient’s applicability to the trial Withdrawals and dropouts: 34 (46%) patients did not complete both home and hospital treatments. Eight patients revoked consent to home treatment after receiving hospital treatment in the run-in period. Four patients developed conditions (mostly poor venous access) which meant that chemotherapy was technically too difficult to administer at home. Two patients moved residence outside the treatment zone |
Primary outcome(s): not specified Other outcomes: patient and carer preferences and strength of preference; patient and carer satisfaction; unmet patient needs; patient quality of life (FLIC); costs Qualitative data reported? No Economic evaluation? Yes Type of economic evaluation: CEA Currency (price year): AUD ($) NR Study perspective: health service Comments: one ineligible patient was recruited at a time when participation rates were low |
| Main reference: Pace et al. 2009,29 full published paper Linked references: Pace et al. 2007102 Design: RCT (crossover) Country: UK (England) Recruitment period: August 2005 to August 2006 Number of recruiting centres: one Assessment time points: Based on cycles: 1, 3, 4, 5 and completion for most outcomes, toxicity measured at each cycle |
Key characteristics of recruited population: Inclusion criteria: patients ≥ 18 years without previous chemotherapy treatment, scheduled to receive standard chemotherapy for at least six cycles suitable for day-case administration, without other uncontrolled medical illness. WHO status of 0, 1, 2 Median age: 57 years (range 40–80 years) Gender: male 7/42 (17%); female 35/42 (83%) Disease stage: early 29/42 (69%); advanced 13/42 (31%) Cancer type: breast 32/42 (76%); pancreas 2/42 (5%); prostate 2/42 (5%); melanoma 2/42 (5%); other 4/42 (9%) Treatment intention: ‘standard chemotherapy’; no further details given Chemotherapy used: anthracycline-based regimes 31/42 (74%) gemcitabine; carboplatin-based regimes; docetaxel; dacarbazine; COIN study treatment |
Setting details: Community (OUTREACH centre): four centres none previously having delivered chemotherapy. Facilities included a waiting area, emergency call facilities and resuscitation equipment. Only chemotherapy was delivered during sessions in these areas Located 6, 13, 20 and 25 miles from the cancer centre Hospital outpatient: dedicated chemotherapy suite within an oncology unit, including dedicated patient support and information centre. All chemotherapy was delivered by members of the hospital chemotherapy team both in the cancer centre and within the community settings Preparation of chemotherapy: chemotherapy was made to prescription for individual patients and delivered to the oncology day unit. From there it was collected and then taken to the community hospital by a member of the team |
Target sample size: 30 Number actually randomised: 42 Estimated monthly rate of randomisation, per centre: 3.2 Number of eligible participants who were not randomised because of setting preference: five, all female, reasons relating to safety concerns or convenience Did it appear that all eligible patients were invited to participate? Participants were recruited from a consecutive series, estimated 98 were eligible, most reasons for non-entry were unknown Withdrawals and dropouts: 38 (90.5%) completed the first two cycles of chemotherapy at the first location; 31 (73.8%) completed the first four cycles of chemotherapy and therefore crossed over from one location to the second; and 28 (66.7%) completed six cycles of chemotherapy Reasons for withdrawal: disease progression and cessation of chemotherapy |
Primary outcome(s): patient preference for location of treatment (measured via questionnaire) Other outcomes: HADS; C-SAS (toxicity scale); CPSQ (patient satisfaction questionnaire); resource use; safety Qualitative data reported? Yes Economic data reported? Yes Type of economic evaluation: CEA Currency (price year): GBP £ NR Economic perspective: NHS and patient (not explicitly reported) Comments: authors comment that the preference for the outreach location was not mirrored in global CPSQ and HADS scores. They suggest that the CPSQ and HADS may be missing aspects of the chemotherapy experience that people consider important, and determine their treatment location preferences Project was not pursued further due to lack of funding; however, a local patient-led initiative has established a charity and funds the OUTREACH project. See www.chemooutreachproject.co.uk for more details |
| Main reference: Remonnay et al. 2002,34 full paper Linked references: Remonnay et al. 2003,103 full published paper (French) Design: RCT (crossover) Country: France Recruitment period: October 1995 to June 1998 Number of recruiting centres: one Assessment time points: patients were assessed after two courses of chemotherapy, switched from home to hospital or vice versa, and then were assessed after two further courses of chemotherapy |
Key characteristics of recruited population: All patients had previously been hospitalised at the recruiting centre Inclusion criteria: the patient must have not received chemotherapy in the previous 2 months, the patient lived in the geographical area covered by Soins et Santè, the patient lived with a family member, the patient’s personal physician consented, and the patient had permanent access to a vein or an implantable venous access system (Port-A-Cath) Mean age: 60 years (SD 11 years) Gender: 17% male Type of cancer: mostly breast cancer (81%). Some patients had non-small cell lung cancer Treatment intention: unclear Chemotherapy used: most frequently used chemotherapy regimens were cyclophosphamide and doxorubicin, cyclophosphamide, methotrexate, and 5-fluorouracil and navelbine |
Setting details: Home: no details reported Outpatient: no details reported Preparation of chemotherapy: it was unclear where the chemotherapy was prepared, but costs for drugs were higher for home care due to not being provided by the hospital |
Target sample size: 160 Number actually randomised: 52 Estimated monthly rate of randomisation, per centre: 1.6 Number of eligible participants who were not randomised because of setting preference: 10 participants; six refused due to lack of confidence in home delivery and four due to not wanting to impose on loved ones Did it appear that all eligible patients were invited to participate? NR Withdrawals and dropouts: 10 dropouts; six died and four reported deterioration requiring change of treatment |
Primary outcome(s): patient satisfaction Other outcomes: costs, quality of life (FLIC, MADRS), Hamilton Anxiety Scale Quality of life and anxiety results NR, no response to author contact Qualitative data reported? No Economic evaluation? Yes Type of economic evaluation: CEA Currency (price year): US$ (1998) converted using purchasing power parities Economic perspective: societal Comments: care administered by external organisation Soins et Santé. Trial was terminated early because 95% of the first 52 patients expressed a preference for home administration of chemotherapy. The authors assumed that there were no costs for administration in the outpatient setting. Of outcomes listed for the trial, only costs were reported in this study |
| Main reference: Rischin et al. 2000,32 full published paper Linked references: King et al. 2001,54 letters to editor Design: RCT (crossover) Country: Australia Recruitment period: February 1996 to March 1997 Number of recruiting centres: one Assessment time points: after one treatment in each setting |
Key characteristics of recruited population: Inclusion criteria: patients aged ≥ 18 years, who had not received chemotherapy in the preceding 12 months, whose planned first two treatments were identical, and who lived in an area that was geographically suitable for treatment at home Median age: around 60 years Gender: 5/20 (25%) male Cancer type: breast 10/20 (50%); colon 8/20 (40%); non-Hodgkin’s lymphoma 1/20 (5%); pancreatic 1/20 (5%) Treatment intention: NR Chemotherapy used: cyclophosphamide, methotrexate and 5-fluorouracil ± prednisolone [CMF(P)] 50%; 5-FU ± folinic acid or levamisole 45%; cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) 5% |
Setting details: Home: a chemotherapy nurse specialists who also worked in the chemotherapy day ward at the hospital administered all home chemotherapy treatments Hospital (outpatient): chemotherapy administered by hospital chemotherapy nurse specialist. No further details reported Preparation of chemotherapy: NR |
Target sample size: 20 Number actually randomised: 25 Estimated monthly rate of randomisation: 1.8 Number of eligible participants who were not randomised because of setting preference: seven patients wanted home treatment only and 12 were ‘overlooked’ Did it appear that all eligible patients were invited to participate? No: 12 patients were overlooked in the recruitment stage (no further details were provided). In addition, patients were selected from patients registered on the chemotherapy-in-the-home program, for which eligibility criteria were NR Withdrawals and dropouts: in the ‘hospital first’ arm three patients withdrew – one patient did not go on to receive chemotherapy, one received chemotherapy at home and one decided to have all chemotherapy in hospital after the first treatment In the ‘home first’ arm, two patients withdrew: one person did not go on to receive any chemotherapy, and one patient had a change of chemotherapy regimen due to toxicity after cycle 1 |
Primary outcome(s): patient preferred site for remaining treatments Other outcomes: patient preference; patient satisfaction; complications; costs Qualitative data reported? Yes Economic evaluation? Yes Type of economic evaluation: CEA Currency (price year): AU$ NR Study perspective: hospital Comments: all eligible patients were ‘registered on the chemotherapy in the home program’. It appears that home chemotherapy was already an option outside of the trial setting. The targeted trial population seems to exclude those patients more likely to prefer the hospital setting Only one treatment per setting was studied |
| Main reference: Stevens et al. 2006,33 full published paper Linked references: full published paper Stevens et al. 2006;55 full published paper Stevens et al. 2004;56 editorial104 Design: RCT (crossover) Country: Canada Recruitment period: NR Number of recruiting centres: one Assessment time points: baseline, 3 months, 6 months (prior to crossover) and then at 9 months (first assessment after crossover) and 12 months |
Key characteristics of recruited population: Inclusion criteria: children age 2–16 years of age, diagnosed with acute lymphoblastic leukaemia, being treated by standard protocol in the greater metropolitan area of the study Exclusion criteria: children with other major congenital illnesses and those who did not have a patent central venous catheter Age: NR Gender: male 22/29 (75.9%) Phase of chemotherapy: III 1/29 (3.4%) interim maintenance; IV 4/29 (13.8%) reinduction; V 24/29 (82.8%) maintenance Treatment intention: curative Chemotherapy used: intrathecal methotrexate, intravenous cyclophosphamide, vincristine, intravenous methotrexate, cytosine arabinoside (Ara C) |
Setting details: Home: some chemotherapy drugs were delivered in hospital for safety reasons; these included intrathecal methotrexate, intravenous cyclophosphamide, and vincristine. These drugs were delivered by standard protocol. Intravenous methotrexate and Ara C were delivered in patients’ homes by a trained community health services agency nurse. Blood samples were taken at a community laboratory the day prior to administration of chemotherapy. The primary oncology nurse was the main support nurse Hospital (outpatient): patients received chemotherapy by standard hospital protocol. Blood samples were taken during scheduled visits Preparation of chemotherapy: home – chemotherapy drugs were prepared by a community pharmacy and delivered to patients’ homes at pre-arranged times |
Target sample size: 22 Number actually randomised: 29 Estimated monthly rate of randomisation: not possible to calculate Number of eligible participants who were not randomised because of setting preference: 21 eligible patients declined to participate; 16 preferred hospital treatment; three preferred to keep home as a safe haven; two provided no reason Did it appear that all eligible patients were invited to participate? Yes Withdrawals and dropouts: Home followed by hospital chemotherapy: two discontinued (relapse) Hospital followed by home chemotherapy: four discontinued (two withdrew, two relapse). No reason was given for withdrawals |
Primary outcome(s): patient quality of life was measured using the POQOLS questionnaire, and the Child Behaviour Checklist was used to measure social/psychological interactions of children Other outcomes: caregiver burden as measured with the Caregiving Burden Scale; adverse events; costs Qualitative data reported? Yes Economic evaluation: yes Economic evaluation type: CEA Currency (price year): $CAN NR Economic perspective: societal Comments |
Appendix 4 Randomised controlled trial risk of bias
| Type of bias | Judgement (high, low or unclear risk) | Support for judgement |
|---|---|---|
| Borras et al. 200135 | ||
| Random sequence generation | Low | Random numbers were selected in blocks of eight, stratified according to type of tumour |
| Allocation concealment | Unclear | Does not report who performed the randomisation or any details of concealment process |
| Similarity at baseline | Low | Groups very similar for gender, age, tumour site, toxicity, treatment type or radiotherapy use |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High | Patient-reported outcomes subjective (patients not blinded) |
| Low | Adverse events/toxicity unlikely to be affected | |
| Low | Use of health-care resources (unplanned hospitalisation, or primary care or emergency department visits) unlikely to be affected | |
| Incomplete outcome data | Unclear | 6/42 (14%) outpatients and 1/45 (2%) home patient withdrew voluntarily. This difference was statistically significant. However, no details were provided |
| Selective reporting | Low | Could not locate trial protocol, although it appears from the paper that all collected outcomes were reported (and in sufficient detail) |
| Other bias (crossover trials only): N/A | ||
| Chen and Hasuimi 199927 | ||
| Random sequence generation | Unclear | ‘Random’ |
| Allocation concealment | Unclear | Study reported as a brief conference abstract |
| Similarity at baseline | Unclear | Study reported as a brief conference abstract |
| Blinding of participants and researchers | Unclear | Study reported as a brief conference abstract |
| Blinding of outcome assessment | Unclear | Study reported as a brief conference abstract |
| Incomplete outcome data | Unclear | Study reported as a brief conference abstract |
| Selective reporting | High | No actual data reported for quality-of-life (and related) outcomes |
| Other bias (crossover trials only): Crossover a suitable design? N/A Appropriate statistical analysis used (to allow for pairing)? N/A |
||
| Christiansen et al. 201128 | ||
| Random sequence generation | Unclear | ‘Randomised’ only details given |
| Allocation concealment | Unclear | Not reported |
| Similarity at baseline | Low | Crossover trial: within participant comparisons |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High | Patient-reported outcomes subjective (patients not blinded) |
| Incomplete outcome data | Unclear | 14 patients did not complete treatment, no further details reported |
| Selective reporting | Unclear | Protocol not available, and unclear from abstract whether or not other outcomes were assessed |
| Other bias (crossover trials only): Crossover a suitable design? Unclear – abstract only available Appropriate statistical analysis used (to allow for pairing)? Unclear – abstract only available |
||
| Corrie et al. 20134 | ||
| Random sequence generation | Low | Randomised by independent centre using minimisation |
| Allocation concealment | Low | Central allocation – participants were allocated a unique trial number and the treatment setting defined. The randomisation outcome information was provided to the investigator within 24 hours |
| Similarity at baseline | Low | Well balanced in terms of key characteristics due to use of minimisation |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High Low Low |
Patient-reported outcomes and service use data, for example family care and travel time (patients not blinded) Adverse events unlikely to be affected |
| Incomplete outcome data | Low | Data for 57 patients could be analysed at end of trial. Six patients failed to start treatment, 17 patients did not complete 12 weeks of treatment Home: 33 allocated, 33 started, five stopped, five incomplete data sets GP: 32 allocated, 29 started, eight stopped, four incomplete data sets Hospital: 32 allocated, 29 started, four stopped, eight incomplete data sets |
| Selective reporting | Low | Protocol available and checked |
| Other bias (crossover trials only): not relevant | ||
| Hall and Lloyd 200830 | ||
| Random sequence generation | Unclear | ‘Randomly allocating’ patients |
| Allocation concealment | Unclear | No details reported |
| Similarity at baseline | Unclear | No details reported |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High | Patient-reported outcomes subjective (patients not blinded) |
| Incomplete outcome data | Unclear | Appear to have full follow-up data (although not explicitly stated) |
| Selective reporting | Unclear | Could not locate trial protocol, appears from the paper that all collected outcomes were reported |
| Other bias (crossover trials only): N/A | ||
| King et al. 200031 | ||
| Random sequence generation | Low | Random number table, stratified by cancer type |
| Allocation concealment | Unclear | Mentions use of sealed envelopes but not whether they were opaque or sequentially numbered |
| Similarity at baseline | Low | Crossover trial: within-participant comparisons |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High | Patient-reported outcomes subjective (patients not blinded) |
| Incomplete outcome data | Low | Eight participants withdrew from receiving home treatment after experiencing outpatient treatment. However, additional analyses were conducted which assumed such patients preferred outpatient treatment |
| Selective reporting | Unclear | Protocol not available |
| Other bias (crossover trials only): Crossover a suitable design? Overall, yes, although seven patients dropped out because of disease progression (using only the early breast cancer population may have been preferable) Appropriate statistical analysis used (to allow for pairing)? Yes, used paired analyses and checked for period effects (data from both periods were used), interactions and carryover effects |
||
| Pace et al. 200929 | ||
| Random sequence generation | Unclear | ‘Telephone randomisation, in blocks of 10, to local Research Support Unit’, but unclear exactly how sequence was generated |
| Allocation concealment | Low | Although this is assuming the blocks of 10 were used only by the local research support unit and not by the investigators |
| Similarity at baseline | Low | Crossover trial: within-participant comparisons |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High | Patient-reported outcomes subjective (patients not blinded) |
| Incomplete outcome data | Unclear | 43% attrition throughout the study due to disease progression and cessation of chemotherapy, all outcomes reported for completing patients. Attrition not reported by treatment location so difficult to assess if differential loss of data |
| Selective reporting | Low | Could not locate trial protocol, but appears from the paper that all collected outcomes were reported |
| Other bias (crossover trials only): Crossover a suitable design? Probably not – too many patients withdrew due to disease progression and cessation of chemotherapy Appropriate statistical analysis used (to allow for pairing)? Unclear, although data from both time periods were used |
||
| Remonnay et al. 200234 | ||
| Random sequence generation | Unclear | ‘Order of passage was selected at random’ only reported in English paper |
| Allocation concealment | Unclear | Not reported |
| Similarity at baseline | Low | Crossover trial: within-participant comparisons |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | Unclear | Not reported |
| Incomplete outcome data | Unclear | No numerical data (only a percentage) reported for preference data |
| Selective reporting | High | No protocol available; only economic data reported although quality of life also measured |
| Other bias (crossover trials only): Crossover a suitable design? No – six patients died and four reported deterioration requiring change of treatment (total n = 52) Appropriate statistical analysis used (to allow for pairing)? Details not reported |
||
| Rischin et al. 200032 | ||
| Random sequence generation | Low | Computer-generated randomisation chart using an allocation scheme based on a biased coin design |
| Allocation concealment | Unclear | Not described, mention of a chart suggests may not have been concealed |
| Similarity at baseline | Low | Crossover trial: within-participant comparisons |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High Low |
Patient-reported outcomes subjective (patients not blinded) Serious adverse events unlikely to be affected |
| Incomplete outcome data | Low | Three withdrawals in hospital first arm, and two in home first arm with reasons given |
| Selective reporting | Unclear | Protocol not available, and unclear from paper whether or not other outcomes were assessed |
| Other bias (crossover trials only): Crossover a suitable design? Yes, no withdrawals due to disease progression Appropriate statistical analysis used (to allow for pairing)? Yes, used paired analyses using data from both time periods, and checked for period and carryover effects |
||
| Stevens et al. 200633 | ||
| Random sequence generation | Low | Table of random numbers |
| Allocation concealment | Unclear | Allocation performed by study site manager but unclear if group identity was concealed |
| Similarity at baseline | Low | Crossover trial: within-participant comparisons |
| Blinding of participants and researchers | High | Neither participants nor researchers were blinded |
| Blinding of outcome assessment | High Low |
Patient-reported outcomes subjective (patients not blinded) Adverse events unlikely to be affected |
| Incomplete outcome data | Low | 23/29 children completed both phases and appear to have recorded all outcome data; relapse similar in both arms but reasons for withdrawal (n = 2) not reported |
| Selective reporting | Low | Could not locate trial protocol; appears from the paper that all collected outcomes were reported |
| Other bias (crossover trials only): Crossover a suitable design? Appears reasonable, disease course relatively stable Appropriate statistical analysis used (to allow for pairing)? Paired analyses (data from both periods) were used and checked for period and programme effects or interactions |
||
Appendix 5 Results
Results for quality-of-life outcomes
| Study | Outcomes |
|---|---|
| Randomised trials | |
| Corrie et al. 20134 | EORTC QLQ-C30 self-rated QoL Community (n = 39) vs. outpatient (n = 17): –0.01 (95% CI –0.87 to 0.86; p = 0.99) Home (n = 23) vs. GP (n = 16): –0.06 (95% CI –0.99 to 0.88; p = 0.90) Home (n = 23) vs. outpatient (n = 17): –0.03 (95% CI –0.99 to 0.93; p = 0.95) GP (n = 16) vs. outpatient (n = 17): 0.03 (95% CI –0.99 to 1.05; p = 0.96) |
| EORTC QLQ-C30 Emotional Function domain Community (home and GP, n = 38) vs. outpatient (n = 17): –7.2 (95% CI –19.5 to 5.2; p = 0.25) Home (n = 23) vs. GP (n = 15): 15.2 (95% CI 1.3 to 29.1; p = 0.033) Home (n = 23) vs. outpatient (n = 17): –1.5 (95% CI –14.5 to 11.5; p = 0.82) GP (n = 15) vs. outpatient (n = 17): –16.6 (95% CI –31.4 to –1.9; p = 0.028) |
|
| EQ-5D Utility scores: not reported |
|
| QALYs Home: 0.174 (SD 0.034) Community (GP): 0.191 (SD 0.040) Hospital: 0.165 (SD 0.053) |
|
| Borras et al. 200135 | EORTC QLQ-C30 self-rated QoL Home (n = 33): 71 (SD 17); outpatient (n = 23): 68 (SD 20); ‘no difference’, nor in changes from baseline |
| EORTC QLQ-C30 Emotional Function domain Home (n = 33): 76 (SD 24); outpatient (n = 23): 79 (SD 19); ‘no difference’, nor in changes from baseline |
|
| EORTC QLQ-C30 Results were also presented for the individual items of the functional and symptom domains of EORTC QOL-C30 |
|
| King et al. 200031 | FLIC (self-administered questionnaire) Overall total FLIC score: 76 (SD 14, interaction p = 0.23). Setting-specific scores were not reported. The ‘location effect’ was –0.49 (p = 0.79), i.e. treatment location (home or hospital) did not have a significant impact on quality of life Note: ‘period effects’ also reported FLIC Emotional Function subscore: overall average score of 72 (SD= 19, interaction p = 0.30). The location effect was –0.09 (p = 0.98) Note: other FLIC subscores were also recorded (role function, pain, hardship, current health, sociability and nausea) |
| Christiansen et al. 201128 | EORTC QLQ-C30 self-rated QoL There was no significant difference between hospital-treated and home-treated patients’ QoL scores. No raw results were reported |
| Stevens et al. 200633 | POQOLS; Child Behaviour Checklist POQOLS Factor 1 (sensitivity to restrictions in physical functioning and the ability to maintain a normal physical routine): Crossover to outpatient led to a 5.2 increase (n = 13 before switch, n = 13 after, lower is better). Crossover to home led to a 10.5 decrease (n = 14 before switch, n = 10 after). The difference between the two groups was significant (p = 0.023). There were 13 patients with baseline measurements in the home group, with a maximum of 14 observations at any follow-up period, and 12 at the final follow-up. For the hospital group, there were 14 patients at baseline, and 10 at final follow-up Factor 2 (emotional distress): No significant difference due to crossover. Patients starting at home had statistically significantly higher scores (lower QoL) at 6 months than those starting in outpatient setting (6.8 difference, p = 0.043) Factor 3 (reaction/response to current medical treatment) Non-statistically significantly higher scores in home group (8.3 difference, p = 0.61) Long-term trends appeared to indicate little difference between treatment locations in any POQOLS factor |
| Remonnay et al. 200234 | FLIC (self-administered questionnaire) Results not reported |
| Chen and Hasuimi 199927 | Unclear QoL measures The authors stated that, by day 5 of the first course, quality-of-life items (such as mood, smell, appetite and satisfaction) were ‘significantly decelerated’ in the home-first group compared with the hospital-first group, but no data were reported Intergroup analysis in the hospital first group indicated that the second infusion at home significantly improved QoL status in appetite, taste, mood and satisfaction |
| Non-randomised studies | |
| Hansson et al. 201338 | PedsQL Scale Generic Core Child self-reported and parent proxy (0–100 scale); PedsQL Cancer Module child self-reported and parent proxy (seven dimensions, 0–100 scale); PedsQL Family Impact Module (eight dimensions) PedsQL Generic Core: child self-reported Home care n = 13; hospital n = 26 at T1 (recruitment), n = 25 at T2 (3 months). At T1 and T2 all home-care group results were higher than standard care. At T2 self-reported mean scores were statistically significantly higher in the home-care group vs. outpatient in the dimensions of total score (75.3 vs. 61.1; p = 0.02), psychosocial health (74.6 vs. 62.4; p = 0.03) and emotional functioning (78.1 vs. 62.2; p = 0.04). The crude mean difference between settings in global total score between T1 and T2 was 14.2 (95% CI 2.0 to 26.3; p = 0.02). The adjusted mean difference was 14.8 (95% CI –0.4 to 30.1; p = 0.06). Subscores across time points were reported (see paper). The only dimension statistically significant for the adjusted mean difference value was social functioning: mean difference = 15.5 (95% CI 0.0 to 31.1; p = 0.05) (Variables adjusted for age, diagnosis, gender and time since diagnosis) Several children did not attend school, which affects the mean score in the school dimension PedsQL Generic Core: parent proxy Home care T1 n = 40, T2 = 41; outpatient T1 n = 62, T2 n = 66. All home scores were higher than outpatient care scores at both time points. The crude mean difference for total score between settings was 7.7 (95% CI 0.4 to 14.9; p = 0.04). The adjusted mean difference was 7.7 (95% CI 0.6 to 16.1; p = 0.07). For the adjusted scores, only the physical health score was significant: mean difference = 14.2 (95% CI 3.3 to 25.2; p = 0.01) (full domain scores reported in paper) Cancer Module: child self-reported There were no statistically significant differences between the home-care group and outpatient group across any of the items Cancer Module: parent proxy For the adjusted results there were two statistically significant domains: nausea (mean difference = 9.9 (95% CI –0.2 to 19.5; p = 0.04) and worry (mean difference = 10.5 (95% CI –0.4 to 20.6; p = 0.04) (higher scores are better, scores favoured home setting). Full domain scores in paper Family Impact Module The mean scores were similar overall between groups (no result detail reported) |
| Payne 199246 | Domains of a bespoke quality of life implement developed for the study (not detailed elsewhere) All results reported as (mean; SD). HADS Anxiety and Depression form the Psychological Stress domain (see psychological results table). The number of patients for each component was not reported Only gastrointestinal complaints (p < 0.01) and housework (p = 0.01) had statistically significant differences Physical complaints:
|
| Souadjian et al. 199248 | Activities of Daily Life Home patients reported an improvement in quality of life after compared with outpatient chemotherapy. Data were not reported, nor was the method of measurement |
Results for clinical outcomes
| Study | Outcomes |
|---|---|
| Randomised trials | |
| Corrie et al. 20134 | EORTC QLQ-C30 self-rated health Community (n = 39) vs. outpatient: 0.30 (95% CI –0.51 to 1.12; p = 0.46) Home (n = 23) vs. GP (n = 16): –0.07 (95% CI –0.97 to 0.83; p = 0.88) Home (n = 23) vs. outpatient (n = 17): 0.28 (95% CI –0.62 to 1.17; p = 0.54) GP (n = 16) vs. outpatient: (n = 17) 0.34 (95% CI –0.64 to 1.33; p = 0.49) |
| Borras et al. 200135 | EORTC QLQ-C30 self-rated health Home(n = 33) 71 (SD 17) vs. outpatient (n = 23) 68 (SD 20). Difference not significant Karnofsky Index Home (n = 33) 85 (SD 11) vs. outpatient (n = 23) 85 (SD 11) |
| Stevens et al. 200633 | Caregiving Burden Scale No evidence of effect from location |
| Non-randomised studies | |
| Payne 199246 | Karnofsky Performance Scale Home: 45.7 (SD 5.3) vs. outpatient: 41.9 (SD 9.4) (p = 0.19) |
| Pong et al. 200037 | Self-rated health status, seven-category Likert sale – survey posted questionnaire Categories 2 and 3, and 5 and 6 were combined Community (COCN) patients (n = 153):
NB: the hospital sample included both patients who could have taken part in the community (n = 55) programme and those who could not |
Results for psychological outcomes
| Study | Outcomes |
|---|---|
| Randomised trials | |
| Corrie et al. 20134 | HADS Anxiety community (n = 40) vs. outpatient (n = 17) 0.97 (95% CI –0.97 to 2.9; p = 0.32) Home (n = 23) vs. GP (n = 17): –1.97 (95% CI –4.10 to 0.17; p = 0.07) Home (n = 23) vs. outpatient (n = 23): 0.13 (95% CI –1.97 to 2.23; p = 0.90) GP (n = 23) vs. outpatient (n = 17): 2.10 (95% CI –0.16 to 4.35; p = 0.07) HADS Depression community (n = 40) vs. outpatient (n = 17): 2.10 (95% CI –0.02 to 4.22; p = 0.05) Home (n = 23) vs. GP (n = 17): –2.01 (95% CI –4.31 to 0.27; p = 0.08) Home (n = 23) vs. outpatient (n = 23): 1.28 (95% CI –1.00 to 3.55; p = 0.27) GP (n = 23) vs. outpatient (n = 17): 3.29 (95% CI 0.81 to 5.77; p = 0.01) |
| Pace et al. 200929 | HADS Anxiety No raw data reported; text states no significant difference between arms although tendency for anxiety to decrease over time in both groups HADS Depression No raw data reported; text states no significant difference between arms although both groups reported rise in depression mid-way in trial; final depression scores lower than mean baseline scores |
| Stevens et al. 200633 | Child Behaviour Checklist No significant differences between treatment groups at any follow-up point |
| Remonnay et al. 200234 | Hamilton Anxiety Scale Results not reported Montgomery–Åsberg Depression Rating Scale (MADRS) Results not reported |
| Payne 199246 | HADS Anxiety Home 35.6 (SD = 22) vs. outpatient 44.2 (SD = 32.2) (p = 0.42) HADS Depression Home 28.7 (SD = 20) vs. outpatient 33.5 (SD = 18.3) (p = 0.51) |
| Non-randomised studies | |
| Herth 198943 | Herth Hope Scale (score range 0–32) Jalowiec Coping Scale (score range 90–200) Outpatients had significantly higher levels of coping response and hope Hope: home (n = 20) 24.1 vs. outpatient (n = 20) 27.0 (p < 0.01) Coping: home (n = 20) 121.9 vs. outpatient (n = 20) 140.2 (p < 0.01) |
Results for satisfaction outcomes
| Study | Outcomes |
|---|---|
| Randomised trials | |
| Corrie et al. 20134 | Questionnaire 78% of patients expressed satisfaction with their treatment setting, whatever their location |
| Borras et al. 200135 | Questionnaire (score range 1–100, larger scores equate to greater satisfaction) There were significant differences between groups in:
There were no significant differences reported for the remaining three types of care (availability of doctor, continuity of care and communication with doctor). Home n = 33 and outpatient n = 23 (for all) |
| Hall and Lloyd 200830 | Individual semistructured interviews See qualitative data extraction |
| King et al. 200031 | Questionnaire (interview) on patient satisfaction The only statistically significant (setting-related) difference in satisfaction with home vs. outpatient care related to the depressing nature of the place of treatment: 15/40 patients found hospital, but not home, a depressing treatment option; one patient found home, but not hospital, depressing; 24 patients found both depressing. The location effect on this item was –1.23; p = 0.00 A significant period effect was found: patients felt significantly less secure during period 2, regardless of setting (p = 0.04) Questionnaire (interview) on patient perception of unmet need There were no statistically significant location effects on the five patient needs dimensions for the 34 patients who completed the questionnaire Self-administered questionnaire (carers) There were no statistically significant location effects on the carer satisfaction scores |
| Pace et al. 200929 | Chemotherapy Patient Satisfaction Questionnaire (CPSQ) Seven dimensions, each item scored from 1–5, low scores = greater satisfaction Patients were significantly more satisfied with the outreach location for ease of access and environment (p < 0.001), and there was greater satisfaction with the outreach centre for interpersonal and technical aspects of nursing care (p < 0.01) There were no significant differences in anxiety and global satisfaction More complete data in full paper for each dimension |
| Rischin et al. 200032 | Questionnaire (patients) None of the patients reported concerns with chemotherapy being given in their home; 4/20 (20%) reported concerns with treatment in hospital, relating to transport difficulties and waiting times (no raw data given) 18/20 (90%) of patients felt there were advantages with treatment in the home. The reasons given included convenience; avoidance of travel and parking problems (particularly not having to travel while feeling unwell); reduction in treatment-associated anxiety; not burdening their carers and family; and being able to continue their duties such as caring for their dependents. One (5%) patient thought that there were specific advantages to chemotherapy in the hospital: being able to see other people who were worse off |
| Stevens et al. 200655 | Qualitative patient satisfaction interviews See qualitative data extraction |
| Non-randomised studies | |
| Grusenmeyer et al. 199642 | 0–100-mm visual analogue scale (VAS) Outpatient satisfaction was 98 mm on the VAS; home satisfaction was not reported |
| Lowenthal et al. 199645 | Number of patients choosing to discontinue home treatment 2 of 424 patients |
| Barker 200640 | Participant comments to the author See qualitative results |
| Pong et al. 200037 | Questionnaire: posted survey (7-point sale: 7 = strongly agree, 4 = neutral, 1 = strongly disagree) Patient satisfaction: Community patients (n = 153) were asked a series of questions about patient satisfaction and acceptance of the community programme. Hospital patients (n = 114) were asked similar questions in order to provide a basis for comparison Generally satisfaction was high About half of those who had received their first chemotherapy treatment at hospital said that the treatment skills and knowledge of those at hospital were superior to those at the community clinics. Hospital patients tended to have more access to sources of information, but did not necessarily use these sources more frequently than community patients. About 85% of the hospital patients 68% of community patients felt that there were sufficient supportive care services Concerning the overall quality of care received, 12.5% of community patients felt that their care had been extremely bad or less than average, compared with 0% of hospital patients; 4.6% of community patients thought that the care had been average, compared with 0% for hospital; and 83% of community patients felt that their care had been good or extremely good, compared with 100% of hospital patients Detailed results reported in paper Community respondents who were dissatisfied tended to be patients in smaller and low-volume clinics Also reported satisfaction with physician and chemotherapy nurses, satisfaction with amount of information provided before first hospital visit, availability of information, suggestions for improving the community programme |
| NHS Bristol 201036 | Questionnaire When patients were asked if felt they were given sufficient privacy, 62% of outpatients said always, compared with 76% at the community health centre and 97% at home When asked if they received caring and sensitive nurse care 85% of outpatients said always, compared with 100% at the community health centre and 97% at home When asked about having sufficient opportunity to ask the chemotherapy nurse a question, 78% of outpatients said always, compared with 100% at the community health centre and 82% at home; 78% of outpatients said they always received an understandable reply, compared with 86% at the community health centre and 78% at home 87% of outpatients said they always had a clear explanation of impending medical procedures, compared with 95% at the community health centre and 87% at home Top five reasons for choosing treatment location: Outpatient:
|
Results for preference outcomes
| Study | Outcomes |
|---|---|
| Randomised trials | |
| Corrie et al. 20134 | Questionnaire 82% of patients expressed a preference for future treatment in the community: the proportions that would prefer any future treatment in the same location were as follows: outpatient 57%, GP surgery 81% and home 90% |
| King et al. 200031 | Questionnaire (interview) on patient preference Including only patients who completed both home and outpatient treatments: 29/40 (73%) of patients preferred treatment at home compared with treatment at hospital (95% CI 59% to 86%; p = 0.01). 11/40 (27.5%) of patients preferred hospital treatment 10/29 (34%) patients who preferred home treatment changed their preference to outpatient treatment if home treatment meant waiting another hour. 3/11 patients who preferred outpatient treatment changed their choice to home treatment if they had to wait an hour longer at hospital Including the 13 patients who chose not to be randomised because they preferred the outpatient setting, and the eight patients who dropped out after receiving outpatient chemotherapy during run-in (because they felt more secure at hospital): 29/61 (48%) of patients preferred home care (95% CI 35 to 60; p = 0.61) For trial recruits only (i.e. including the eight who dropped out during run-in): 29/48 (60%) of patients preferred home care (95% CI 47% to 74%; p = 0.19) Self-administered questionnaire (carers) Of 25 carers who completed the questionnaires, 17/25 (68%) preferred treatment at home (95% CI 50% to 86%; p = 0.11) |
| Pace et al. 200929 |
Two preference questions:
|
| Christiansen et al. 201128 | Questionnaire (patients) Not reported |
| Rischin et al. 200032 | Questionnaire (patients) All 20 patients (100%; 95% CI 83% to 100%) preferred to have their remaining therapy given at home When asked where they would have preferred to receive their first two treatments if they had had their time again, 14/20 (70%) patients said they would have preferred both treatments at home [7/9 (78%) in the hospital-first group, 7/11 (64%) in the home-first group]; 2/20 (10%) said they would prefer the first treatment at home and the second in hospital [0 in the hospital-first group; 2/11(18%) in the home-first group]; 2 (10%) said they had no preference [1/9 (11%) in the hospital-first group, 1/11 (9%) in the home-first group]; and 0 patients said they would prefer both treatments at hospital |
| Stevens et al. 200655 | Qualitative patient preference interviews See qualitative data extraction |
| Remonnay et al. 200234 | Questionnaire 95% of all patients asked preferred chemotherapy treatment at home. Number of patients asked is not clear owing to dropouts |
| Non-randomised studies | |
| Souadjian et al. 199248 | Questionnaire Authors reported that home was the preferred option for patients |
| Pong et al. 200037 | Questionnaire: posted survey n = 153 For the first treatment community patients chose whether to receive their first chemotherapy treatment at the hospital or community clinic. Almost half said that they had received their first treatment at the hospital Reasons for choosing community care: Community respondents (n = 153). Top three reasons from a given list were weighted on basis of choice. Top five reasons (in order) were:
Similarly, community patients were asked to pick the top three disadvantages of community care. 37% did not answer, many of whom said that they believed there were no disadvantages in participating in the programme. Top five responses:
Hospital patients’ (n = 114) reasons for not participating in community programme:
|
Results for compliance outcomes
| Study | Outcomes |
|---|---|
| Randomised studies | |
| Corrie et al. 20134 | Proportions of patients receiving compliant dosing schedules Unclear (17 did not complete 12 weeks of treatment mainly due to disease progression) |
| Borras et al. 200135 | Proportions of patients receiving compliant dosing schedules Unclear |
| Non-randomised studies | |
| Satram-Hoang and Reyes 201147 | Proportions of patients receiving compliant dosing schedules Rituximab + chemotherapy compliance with dosing schedule (once every 3 weeks for up to eight cycles): 34% in outpatient setting (n = 541) vs. 54% in community clinics (n = 3149); p < 0.001 Number of cycles of chemotherapy delivered
|
| Frølund 201141 |
Number of scheduled chemotherapy treatments completed (completed/scheduled):
|
Results for safety outcomes
| Study | Outcomes |
|---|---|
| Randomised studies | |
| Corrie et al. 20134 | Adverse events Four of 39 SAEs recorded during this study were assessed as being related to treatment setting |
| Borras et al. 200135 | Adverse events No data other than withdrawal details (see study characteristics) Chemotherapy toxicity No differences between groups for withdrawals due to toxicity (16 cases in total) |
| Pace et al. 200929 | Adverse events There were no adverse reactions during chemotherapy at any location. On one occasion a nurse was unable to cannulate a patient in the outreach centre; patient returned to cancer centre and was treated there Chemotherapy toxicity Measured with C-SAS The most common side effects were nausea, vomiting, fatigue, feeling weak and difficulty sleeping. These showed no significant differences between the locations (Fisher’s exact test) No further data reported |
| Christiansen et al. 201128 | Chemotherapy toxicity There was no significant difference in treatment toxicity between the two groups (p = 0.10). 14.8% of patients treated at home had to be seen at the outpatient clinic for toxicity evaluation and prescription of chemotherapy, in particular due to difficulties in precise evaluation of hand-foot skin reactions by telephone interviews Mode of measurement not reported Hospitalisation 14.8% of patients treated at home had to be seen at the outpatient clinic (see toxicity) |
| Rischin et al. 200032 | Complications There were no major complications with the chemotherapy administration |
| Stevens et al. 200633 | Adverse events No statistically significant differences in overall adverse events. Regardless of treatment allocation, change of location resulted in significantly more adverse effects in patients who had no previous adverse events |
| Non-randomised studies | |
| Lowenthal et al. 199645 | Adverse events ‘Infrequent difficulties with venous access’ ‘One serious complication’ – patient had dystonic reaction to drug (metaclopramide, an antiemetic), transferred to hospital for treatment but not admitted. The authors assumed equal effectiveness due to insignificant numbers of adverse events |
| Souadjian et al. 199248 | Adverse events 4/83 home chemotherapy patients had chemotherapy related adverse events requiring hospitalisation: two thrombosed catheters; one accidental catheter removal by patient; and one sepsis Data were not presented for the outpatient setting |
| Barker 200640 | Chemotherapy toxicity NB: seven outreach patients received 86 cycles of chemotherapy; seven patients received 112 cycles of home chemotherapy WHO toxicity grading scale:
|
| Pong et al. 200037 | Postal questionnaire (hospital n = 114, community n = 153): 40% of hospital respondents and 45% of community respondents reported that they had called for medical help concerning their chemotherapy. 29% of hospital patients who had called for help said they had encountered difficulty vs. 46% of community patients who said they also had encountered problems (p < 0.01) |
| Vergnenègre et al. 200649 | Adverse events Home: two adverse events in 24 cycles Hospital: seven adverse events in 30 cycles No statistically significant difference (p = 0.27) |
Appendix 6 Costs data
| Randomised studies | |
|---|---|
| Corrie et al. 20134 | Home: £2139 (SD £1590) GP: £2497 (SD £1759) Hospital: £2221 (SD £1831) ICER: GP vs. hospital = £16,235/QALY |
| King 200031 | (CEA study: cost consequences) Costs The net additional cost of home chemotherapy (per treatment) was AUS$68.81 This figure was the sum of:
A range of different estimates (reported in table) for the additional cost of a new ward per treatment were calculated, based on a range of treatments per annum, projected increase in workload (assuming full capacity of new ward is 1560 treatments per annum, based on current ward capacity), annual capital costs per treatment, and additional overhead and joint cost per treatment (assuming incremental cost is 20% of existing overhead allocation) On this basis it was estimated that home chemotherapy would be a less expensive means of expanding the service by up to 50% of the ward capacity (additional cost = $65.54 per treatment for establishing a new ward at 50% capacity is less than the cost of $68.81 per treatment for home care). So, if capacity could be expected to increase by 50% or more it would be less costly to set up a new ward The interest rate in this calculation was varied from 3% to 10%, which made no difference to the break-even point because the opportunity cost of capital accounted for only 7% of the costs of capital and overhead |
| Pace et al. 200929 | Service costs: Mileage cost per session = £12.83 Mileage costs per six-session cycle = £76.98 (assumes £0.53 per mile) Opportunity cost of travelling time for each nurse was £32.08 per clinic (using average pay for a specialist oncology nurse outside London = £29,538 per annum. Plus on-costs, this amounts to £15.11 per hour) Marginal cost per clinic session = £64.16 Marginal cost per cycle of six clinic sessions = £384.96 There was no charge for running the clinics in the community hospitals during the study Patient costs: Mean cost of travel to outreach centre = £4.85 Mean cost of travel to cancer centre = £8.77 Based on travel costs per mile (£0.40 per mile, inland revenue rate) plus parking charges Patient-borne costs of travelling (including public transport costs) to:
|
| Stevens et al. 200633 | Costs were reported as median (n, range) in CAN$ Home (time 3, 6 months, before crossover): 1318 (n = 14, 298 to 6302) Home (time 5, 12 months, after crossover): 851 (n = 13, 147 to 8726) Hospital (time 3, 6 months, before crossover): 1409 (n = 11, 419 to 7342) Hospital (time 5, 12 months, after crossover): 1050 (n = 9, 29 to 10,278) No differences were statistically significant but there was a trend towards lower costs in the home group. The home group also had higher baseline costs (1795, n = 15, 327 to 7227) than the hospital group (1374, n = 14, 98 to 4381) |
| Rischin et al. 200032 | (CEA study: cost consequences) Costs Perspective: treating hospital Home chemotherapy was associated with an estimated average increased cost of $83 (95% CI $46 to $120; p = 0.0002) for each chemotherapy treatment, relative to the cost of chemotherapy in the hospital The average cost of the first course of treatment was estimated to be $57 more than the cost of the second (95% CI $20 to $94; p = 0.004) (non-setting specific). There was no carry-over effect (p = 0.16) |
| Remonnay et al. 200234 | Costs were reported in 1998 US$. Statistical significance was measured using Wilcoxon tests Costs of personnel for one chemotherapy administration Health-care personnel: home = 69.10 (SD 19.20) vs. outpatient = 51.70 (SD 10.60) (p < 0.0001) Co-ordination: home = 20.20 (based on assumption) vs. outpatient = 0 (assumption of no hospital costs) Other: home = 6.60 vs. outpatient = 3.30 Total: home = 95.80 (SD 19.20) vs. outpatient = 55.00 (SD 10.60) (p < 0.0001) Additional costs for health-care personnel at home were driven by the costs of physician home consultations ($19.8 for home vs. $7.8 for consultation) Medication costs All chemotherapy drugs: home = 136.70 (SD 81.90) vs. outpatient = 74.00 (SD 52.80) (p < 0.0001) Three most common regimens: AC: home = 169.10 vs. outpatient = 76.50 CMF: home = 76.20 vs. outpatient = 22.10 Navelbine: home = 140.5 vs. outpatient = 111.90 Other marginal costs for outpatient chemotherapy (per administration) Transportation 21.80 Laundering 6.10 Overhead costs Home = 20.10 vs. outpatient = 120.30 Total costs (marginal costs do not include overhead costs) Marginal: home = 232.50 (SD 81.80) vs. outpatient = 157.0 (SD 62.00) (p < 0.001) Average: home = 252.60 (SD 81.80) vs. outpatient = 277.30 (SD 62.00) (p = 0.0002) Sensitivity analysis of total costs assuming equal medication costs (home-care access to hospital pharmacy) Marginal: home = 170.00 (SD 58.20) vs. outpatient = 157.00 (SD 62.00) (p < 0.001) Average: home = 190.00 (SD 58.20) vs. outpatient = 277.30 (SD 62.00) (p < 0.001) Costs per hour or per tariff activity were provided in the paper |
| Non-randomised studies | |
| Grusenmeyer et al. 199642 | Home care:
|
| Lowenthal et al. 199645 | Home treatment costs: Total cost per treatment: $49.93 Salary $36.39 (including 15% overhead, and travel time, at $24.38 per hour salary) Special equipment $9.11 (106 computer-activated drug-delivery devices at $29.66 each) Car expenses $4.43 (petrol, maintenance, insurance, registration and depreciation) Hospital treatment costs: $116.00 ($165.71 multiplied by the percentage of time spent administering chemotherapy) Marginal cost of delivering treatments at home The estimated costs of adding hospital capacity equivalent to the number of patients receiving home treatment was compared with the cost of delivering treatment at home. Chemotherapy was not evaluated independently for this analysis. Expanding capacity for the hospital assumed extending hours to 20.30 from 17.00 and paying a higher night wage for nurses ($29 per hour) Hospital $38,207 Home $45,767 Further details for the marginal analysis were provided |
| Souadjian et al. 199248 | All costs are in US$/treatment-day Home:
|
| Hansson et al. 201338 | Feasibility study (home setting only): The daily hospital charge for a home visit was US$597 vs. $600 for an outpatient visit (The home-care operational costs, payroll costs and overheads were compared with the costs billed for an outpatient visit) The home-care cost included the following items: wages, fuel, uniforms, nursing-bags, parking, car, mobile telephone, various expenses, leasing of car, and medication. Costs (no resource use) were given for each of these items for 2 years |
| Pong et al. 200037 | Survey posted questionnaire: COCN, n = 153; NEORCC, n = 55 Costs reported in CA$ Transport costs Respondents were asked to estimate the total amount they spent on transportation for a typical trip to and from the hospital. Transport costs included costs of gasoline, car rental, bus fare, etc. Community patients: $44 (range $0–160) Hospital patients: $33 ($0–140) When removing individuals who did not incur any travel costs (e.g. driven by volunteers): Community: $49 (range $15–160) Hospital: $39 ($12–140) Total travel expenses (less any subsidies received from government travel grants), including cost of gas/car rental/bus fare, transportation and accommodation, food, lost wages and other expenses, vehicle operating and ownership expenses: Community patients: $294 (range $58–1010) Hospital patients: $188 (range $40–495) (All costs items reported disaggregated; see paper for more detailed costs) Travel time to community clinics was not considered as community patients lived within a relatively short distance from a clinic and so it was assumed the travel costs would be minimal NB: A budget impact model was also conducted. This has not been extracted as it was a non-comparative assessment (only looked at the cost impact at the hospital site and included non-unique costs) |
| Ingleby et al. 199944 | Costs in GBP (£) Mean weekly costs: Hospital: £230 (DeGramont), £92 (Lokich), £35.39 (Tomudex) Home: £135.27 (DeGramont), £41.77 (Lokich), £33.01 (Tomudex) Home setting additional costs: DeGramont: £689 Lokich: £311 Tomudex: £143 |
| Vergnenègre et al. 200649 | Average cost per cycle was €2829.51 (95% CI €2560.74 to €3147.02) for hospital infusion, €2372.50 (95% CI €1962.75 to €2792.88) for home-based care (–16.15%). Difference was €–457.01 by cycle (95% CI –€919.74 to €26.82). Real costs by infusion for home was €484.42 (95% CI €424.18 to €540.32) vs. a fee of €699.89 (95% CI 643.64; 750.23) (–30.79%) |
Appendix 7 Non-randomised study characteristics
| General details | Population and treatment | Setting | Recruitment and participation | Outcomes reported/comments |
|---|---|---|---|---|
| Main reference: Barker 2006,40 published paper Linked references: none Design: comparative audit Country: Wales Recruitment period: June 2004 to December 2004 Number of recruiting centres: unclear Assessment time points: over 6 months (no specific time points reported) |
Key characteristics of recruited population: Inclusion criteria: colorectal cancer patients No other data reported Treatment intention: adjuvant Chemotherapy used: not reported |
Setting details Home: no details reported Community: outreach chemotherapy clinic, no details reported Preparation of chemotherapy: not reported |
Target sample size: not reported, but 14 patients were included Withdrawals and dropouts: not reported |
Primary outcome(s): toxicity (WHO toxicity grading scale) Other outcomes: patient satisfaction Qualitative data reported? Yes Economic evaluation? No Comments: the pilot provided the background to the CHOICE project – Centre, Home and Outreach: Investigating Chemotherapy Environments. This project never got under way due to lack of funding |
| Main reference: Grusenmeyer et al. 1996,42 conference abstract Linked references: none Design: not reported Country: USA Recruitment period: not reported Number of recruiting centres: not reported Assessment time points: not reported |
Key characteristics of recruited population: colon, breast and lung cancer patients with short, medium and long treatment durations Treatment intention: unclear Chemotherapy used: 5-FU + leucovorin Cyclophosphamide + doxorubicin Cisplatin + etoposide Cisplatin, mitomycin + vinblastine |
Setting details: Home Outpatient Preparation of chemotherapy: not reported |
Target sample size: not reported Withdrawals and dropouts: not reported |
Primary outcome(s): costs, patient satisfaction Other outcomes: none Qualitative data reported? No Economic data reported? No Comments: costs were not broken down by the type of cost. Patient satisfaction was reported only for outpatient chemotherapy |
| Main reference: Hansson et al. 2013,38 full paper Linked references: Hansson et al. 2010105 abstract; Hansson 201161 full paper; Hansson et al. 201267 full paper; Hansson et al. 2012106 abstract Design: controlled, non-randomised study Country: Denmark Recruitment period: August 2008 to December 2009. Historical control patients: December 2007 to August 2008 Number of recruiting centres: one Assessment time points: at inclusion to the study and after 3 months |
Key characteristics of recruited population: Inclusion criteria: Children aged < 18 years who had been diagnosed with any cancer at least 1 month prior to inclusion, with one parent fluent in Danish Gender male ≈ 50% Cancer type: acute lymphoblastic leukaemia/acute myeloid leukaemia/lymphoma (≈70%); central nervous system tumour (≈10%); solid tumour (≈20%) Treatment intention: curative treatment Chemotherapy used: vincristine and dactinomycin. Chemotherapy lasted for no more than 10 minutes |
Setting details: Home: home care was provided by two nurses who were employed specifically for home care. Visits lasted 15–19 minutes and, depending on the task performed, included one or both nurses. All preparations were made at the paediatric oncology ward Outpatient: hospital care consisted of standard care at the paediatric oncology ward, day-care unit or outpatient clinic, i.e. according to Nordic treatment protocols or European and international treatment protocols Preparation of chemotherapy: the authors reported that ‘all preparations were made at the paediatric oncology ward’ |
Target sample size: not reported but 28 children received home chemotherapy and 47 children received outpatient treatment (35 historical, 12 concurrent) Withdrawals and dropouts: < 5% of the nurse referrals for home care were refused by the paediatric oncologist based on medical evaluation. For three out of 54 children home care was declined: two preferred to have treatment at the hospital as the treatment protocol only included a few hospital visits; one preferred to keep the home free from treatments 134 patients were eligible for the study. 45 home-care group children were approached and 31 (66%) initially participated, and 28 (90%) of those completed the 3-month questionnaires. 86 hospital-care group (concurrent and historical control) children were approached and 58 (68%) initially participated and 47 (81%) of those completed 3-month questionnaires None of the reasons for non-participation (n = 38) were setting-related |
Primary outcome(s): not specified Other outcomes: patient and parent reported health-related quality of life (PedsQL Generic Core Scale and PedsQL Cancer Module); psychological impact on family (PedsQL Family Impact Module); cost Qualitative data reported? Yes (but only for home setting, reported in a separate study) Economic evaluation? Yes Type of economic evaluation: CEA Currency (price year): US$ and DKK (not reported) Economic perspective: hospital (not explicitly reported) Comments: home-care patients had to live within 50 km of the hospital. Concurrent outpatient care patients had to live more than 50 km from the hospital (but historical controls did not) The study also included a case series feasibility study which assessed safety and parent and child satisfaction and preference of treatment for home-care families The controlled study included two control groups: (1) historical standard-care control group (8-month period before the home-care programme started, regardless of distance to hospital); and (2) concurrent standard-care control group. Controls (1) and (2) were combined (to increase the sample size) for statistical analysis The authors stated that, of the home-care visits, 86% replaced an outpatient visit and 14% replaced an inpatient visit |
| Main reference: Herth 1989,43 full published paper Linked references: Herth 1987107 (unobtainable Ph.D. thesis) Design: non-randomised trial Country: USA Recruitment period: not reported Number of recruiting centres: not reported Assessment time points: not reported |
Key characteristics of recruited population: Eligibility criteria: Adults (> 21 years) currently receiving (appropriate) chemotherapy and English literate were eligible Gender: around 44% were male (based on whole study population, which included some inpatients) Cancer type: varied, but little detail given – breast (28%) and lung cancer (19%) were the most common (based on whole population) Mean age: around 50 years (range 21–85 years) Mean time since diagnosis: around 19 months Treatment intention: 50% of patients had local disease (curative) and 50% had metastases (palliative) Chemotherapy used: not reported |
Setting details: Home: no details reported Outpatient: no details reported Preparation of chemotherapy: no details reported |
Target sample size: unclear, but appeared to be 40 in each setting Withdrawals and dropouts: unclear whether there were any |
Primary outcome(s): hope (Herth Hope Scale); coping (Jalowiec Coping Scale) Other outcomes: none Qualitative data reported? No Economic evaluation? No Comments: two patients declined to take part Age and extent of illness were not identified as confounding variables |
| Main reference: Ingleby et al. 1999,44 conference abstract Linked references: Ingleby et al. 1999,71 abstract Design: cohort study (with retrospective control group) Country: England Recruitment period: not reported Number of recruiting centres: not reported Assessment time point: 12 weeks |
Key characteristics of recruited population: Patients with advanced colorectal cancer Treatment intention: not reported Chemotherapy used: Lokich 5-FU; DeGramont 5-FU and folinic acid; Tomudex© |
Setting details: Home: one senior oncology nurse was responsible for treatment and assessment of the patient with back-up from medical staff as required Hospital outpatient: preparation of chemotherapy: not reported |
Target sample size: 25 patients were recruited Withdrawals and dropouts: 16 patients completed 12 weeks of treatment |
Primary outcome(s): costs Other outcomes: patient preference (though no results presented in abstracts) Qualitative data reported? No Economic evaluation? No Comments |
| Main reference: Lowenthal 1996,45 published paper Linked references: N/A Design: retrospective audit of service over 5 years Country: Australia Recruitment period: 1989–94 (cost analysis 12 months only) Number of recruiting centres: one Assessment time points: clinical effectiveness was not formally assessed Cost analysis was conducted for the 12 months before 30 November 1994. Running costs for the outpatient hospital ward were calculated from 1 July 1994 to 31 December 1994 and extrapolated to a full year |
Key characteristics of recruited population: All patients with ‘satisfactory home circumstances’ and receiving chemotherapy which non-platinum based chemotherapy were eligible Patient characteristics were reported only for diagnosis, and only for the 12-month cost-comparison period Gastrointestinal: 47/184 (25%) Lymphoma: 49/184 (27%) Breast: 35/184 (19%) Lung: 11/184 (6%) Myeloma: 19/184 (10%) Non-malignant/autoimmune: 7/184 (4%) Miscellaenous solid tumours: 16/184 (9%) Treatment intention: not stated, cytotoxic chemotherapy given for cancer and blood disorders Chemotherapy used: flourouracil with/without folinic acid; intravenous methotrexate; cyclophosphamide, methotrexate and fluorouracil with/without prednisolone; cyclophosphamide, vincristine and prednisolone; cyclophosphamide, doxorubicin, vincristine and prednisolone; mitozantrone; hoelzer protocol; interferon; doxorubicin and cyclophosphamide; epirubicin and cyclophosphamide; fluorouracil, epirubicin and cyclophosphamide; vinblastine; cyclophosphamide alone; other |
Setting details: Home: counselling, education and support provided at hospital, received same follow-up as hospital patients. Note that ‘home’ also included patient workplaces, GP offices and day-care centres Outpatient: hospital day patients: no details reported Preparation of chemotherapy: no details reported |
Target sample size: not reported. 179 patients were included Withdrawals and dropouts: not reported |
Primary outcome(s): safety (major complications requiring patient be transferred and admitted to hospital), costs, resource use Other outcomes: N/A Qualitative data reported? No Economic evaluation? Yes Type of economic evaluation: CEA (CMA) Currency: AU$ (1994) Economic perspective: hospital Comments: there was a lack of clear reporting on participant numbers and treatments for the 5-year audit The 1-year cost comparison was more clearly reported (65 patients were treated at home (1486 visits); 119 patients were treated in the hospital day ward) No comparative data in terms of safety were reported for the hospital patients; this was stated to mean treatment at home is safe |
| Main reference: Mitchell 2011,39 full report on interview element Linked references: Mitchell 2013,50 published paper Design: comparative audit of service provision Country: England, UK Recruitment period: not reported (data collected January to October 2010, service initiated in 2007) Number of recruiting centres: one Assessment time points: survey given out at fourth treatment and returned by stamped addressed envelope |
Key characteristics of recruited population: Eligible patients lived more than 15 miles from the oncology centre, required treatment from an approved list that would take less than 3 hours to administer, and had not reacted adversely to the first treatment (given in centre) Male: 6/20 (30%) Mean age: 61 years (range 46 to 76 years) Cancer type:
Chemotherapy used:
|
Setting details: Community: mobile chemotherapy unit (bus) where treatment is nurse-led. Bus is driven to five community hospitals (one per day of the week) for administration of treatment by experienced oncology nurses with additional chemotherapy training. Patients travel directly to the venue nearest to their home Five-patient capacity on board Two nurses per bus. Treatment capacity: 12 patients intravenous chemotherapy and four patients oral chemotherapy per day If fewer than four patients are booked then the service is cancelled (or due to staff sickness) Hospital (outpatient): outpatient clinic All patients had the first treatment in clinic so that any adverse reactions could be observed Preparation of chemotherapy: appears to be prepared at the general hospital and collected along with the staff by the mobile unit on the day of treatment |
Target sample size: 20 (first 20 to respond were interviewed as planned) Withdrawals and dropouts: not reported for the survey. All patients who indicated an interest in being interviewed took part |
Primary outcome(s): qualitative data from in-depth interviews focusing on experience of having treatment in different locations Other outcomes: questionnaire measuring: service satisfaction; transport details and costs; companion costs; cost of childcare/missed work Qualitative data reported? Yes Economic evaluation? No Comments: the survey component does not appear to have been reported or published as yet; author has been contacted Bus was donated by a charity ‘Hope for Tomorrow’ and they also maintain the unit. NHS funds the nurses, driver and fuel |
| Main reference: NHS Bristol 2010,36 unpublished report of pilot study Linked references: none Design: non-randomised trial Country: England Recruitment period: March 2009 to February 2010 Number of recruiting centres: one Assessment time points: not reported |
Key characteristics of recruited population: A wide range of patients were treated, though most had breast, lung, ovarian or gastrointestinal cancer Treatment intention: mixed Chemotherapy used: only treatments lasting up to 4 hours. A wide range was listed in an appendix |
Setting details: Home: given by a private provider Community: a community health centre (care delivered by the hospital team) Outpatient: Bristol Haematology and Oncology Centre Preparation of chemotherapy: not reported |
Target sample size: 220 home patients Report written when 165 patients had received home treatment 556 had outpatient treatment and 127 had community treatment Withdrawals and dropouts: only a random sample of patients were sent a questionnaire regarding patient experience. 118 questionnaires were returned – ‘a return of 45%’. Therefore, the total random sample was 262 patients |
Primary outcome(s): not reported Other outcomes: patient experience Qualitative data reported? No Economic data reported? No Comments: the community setting had also been evaluated in an earlier 2008 study (further details not available) |
| Main reference: Payne 1992,46 published paper Linked references: Payne 1992,108 published paper Design: non-randomised trial Country: England Recruitment period: April 1986 to September 1987 Number of recruiting centres: one Assessment time points: monthly, for 6 months |
Key characteristics of recruited population: consecutive patients with advanced breast or ovarian cancer were referred to the centre Mean age: approximately 57 years Cancer type: breast 36/53 (68%); ovarian 17/53 (32%) Treatment intention: palliative Chemotherapy used: hospital patients received standard dose chemotherapy, while home patients received low-dose intermittent palliative chemotherapy. Specific drug regimens were not described |
Setting details: Home: patients allocated to home chemotherapy had a central venous catheter installed for home treatment Outpatient: no further details of delivery were provided for either setting Preparation of chemotherapy: no details of chemotherapy drug preparation provided |
Target sample size: not reported; 53 participants were recruited Withdrawals and dropouts: four at home withdrew due to illness; eight in hospital withdrew owing to illness Seven at home and six in hospital died during, or immediately after the 6-month study period |
Primary outcome(s): Quality of life: a bespoke tool was constructed with four domains – Psychological Stress, Physical Complaints, Marital Satisfaction and Activity Other outcomes: individual subcomponents of the four domains were reported, including HADS, physical symptoms, and Karnofsky Perfomance Scale Qualitative data reported? No (see linked paper; however, focus on coping strategies without distinction between settings) Comments: six patients refused to participate, mostly due to feeling too ill. The authors noted that any QoL differences may be due to differences in disease severity |
| Main reference: Pong 2000,37 full published report Linked references: none Design: non-randomised retrospective comparative cohort study Country: Canada (north-eastern Ontario) Recruitment period: May 1999 Number of recruiting centres: one Assessment time points: unclear (but appeared to be after treatment course) |
Key characteristics of recruited population: Inclusion criteria for community clinic: Patients must usually live at least 50 km from the hospital, but other factors such as health status and treatment complexity were considered ‘Recent’ patients were selected in order to minimise recall bias (no further details given) Male ≈ 44% Mean age ≈ 58.5 years Cancer type: Gastrointestinal (≈ 40%) Breast (≈ 30%) Haematological (≈ 10%) Lung (≈ 6%) Gynaecological (< 5%) Genitourinary (< 5%) Head and neck (< 2%) Skin (< 1%) Other (≈ 5%) *apart from marital status there were no significant differences between groups on any baseline characteristic Treatment intention: unclear Chemotherapy used: no specifics reported. Included drugs taking < 1 to > 4 hours to administer |
Setting details Community: clinic [Community Oncology Clinic Network (COCN) Program, launched 1994] Medical assessments were undertaken by local physicians and chemotherapy by community chemotherapy nurses at 19 community oncology clinics. Patients attended the hospital or one of three designated peripheral community clinics for follow-up visits Hospital outpatient: North-eastern Ontario Regional Cancer Centre (NEORCC) No details reported All patients had their first appointment at the hospital after diagnosis. Patients were given the option to have their first chemotherapy treatment at the hospital (given directly) or community clinic (if applicable). Treatment setting was dependent on patient distance from hospital and patient choice) Preparation of chemotherapy: not reported |
Target sample size: random sample of 210 community patients and 225 hospital patients from the OPIS database Withdrawals and dropouts: for the survey, the initial samples were reduced to 180 community patients and 190 hospital patients due to incorrect mailing addresses, deaths, etc. Questionnaires were completed by 153/180 (85%) of the community sample and 114/190 (60%) of the hospital sample |
Primary outcome(s): not specified Other outcomes: self-reported health status (seven-category Likert scale); patient travel patterns and costs; patient satisfaction; availability and use of education and supportive care services; reasons for choosing community/hospital setting and main perceived disadvantages; information issues; staff workload and time; budgetary impact; staff opinions Qualitative data reported? No Economic data reported? No Comments: the programme aimed to enable patients in remote communities to receive chemotherapy closer to home (unlikely to generalise to UK context) Very detailed report |
| Main reference: Satram-Hoang and Reyes 2011,47 conference abstract Linked references: none Design: retrospective database cohort study Country: USA Recruitment period: patients diagnosed between 1 January 1998 and 31 December 2007 Number of recruiting centres: N/A Assessment time points: not reported |
Key characteristics of recruited population: Inclusion criteria: Patients aged > 66 years with follicular lymphoma enrolled in Medicare Part A and B Mean age: 75 years Gender: male 45% Disease stage: stage IV: 39% (hospital); 34% (community clinic) Treatment intention: not reported Chemotherapy used: not reported. Only 74% of patients received any chemotherapy (others were treated with rituximab alone) |
Setting details: Community (clinic): no details reported Hospital (outpatient): no details reported Preparation of chemotherapy: not reported |
Target sample size: unclear, but around 3500 patients were studied Withdrawals and dropouts: not reported |
Primary outcome(s): not specified Other outcomes: time to treatment initiation; duration of treatment; number of cycles delivered; compliance Qualitative data reported? No Economic evaluation? No Comments |
| Main reference: Souadjian et al. 1992,48 conference abstract Linked references: none Design: unclear, but observational Country: USA Recruitment period: not reported Number of recruiting centres: one Assessment time points: not reported |
Key characteristics of recruited population: not reported Treatment intention: not reported Chemotherapy used: 5-FU (continuous infusion) CDDP (continuous infusion) CDDP + ETOP + FUDR (continuous infusion) CDDP + ETOP + BLEO (rapid infusion) CARBO + ETOP + IFOS/mesna (continuous infusion) |
Setting details: Home Hospital (outpatient) No details were given about either setting Preparation of chemotherapy: not reported |
Target sample size: not reported; 83 patients had home chemotherapy; the number of outpatients was not stated Withdrawals and dropouts: not reported |
Primary outcome(s): costs Other outcomes: complications; QoL; setting preference Qualitative data reported? No Economic evaluation? No Comments: it was not clear how the costs for outpatient chemotherapy were calculated |
| Main reference: Vergnenègre 2006,49 full published paper Linked references: none Design: non-randomised trial (assignment criteria not reported) Country: France Recruitment period: not reported Number of recruiting centres: one Assessment time points: not reported |
Key characteristics of recruited population: Grade IV non-small cell lung cancer Sufficient life expectancy for three cycles Age (mean): 58 (home 54; outpatient 62) Eligibility for home treatment: within 30 minutes’ drive from hospital; carer/family able to support home treatment; agreement from GP Treatment intention: unclear Chemotherapy used: cisplatine and gemcitabine |
Setting details: Home (day 8): Within 30 minutes’ drive from hospital First dose in outpatient setting on day 1, second dose at home on day 8 Outpatient (days 1 and 8): no details reported Preparation of chemotherapy: no details reported |
Target sample size: 20 Outpatient: 10 Home: 10 Withdrawals and dropouts: not reported |
Primary outcome(s): mean cost per cycle Other outcomes: adverse events (grade III or IV toxicity) Qualitative data reported? No Economic evaluation? Type of economic evaluation: CEA Currency: EUR€ (price year not reported) Economic perspective: health service Comments |
Appendix 8 Qualitative data extraction summary
| General details | Aim/methodology | Population/data collection | Analysis | Summary of findings |
|---|---|---|---|---|
| Main reference: Bakker et al. 200157 Canada Linked references: an additional comparative quantitative paper was mentioned but unobtainable Perspectives: patients |
Aim: to gain an understanding of cancer patients’ experienced of receiving chemotherapy at community clinics Methodology: not reported Sampling: participants were purposively sampled to cover each of the 13 community chemotherapy clinics |
Population: all patients living in the area were given the choice of treatment at the regional cancer centre or at a community clinic, of these, 28 were interviewed Data collection: unstructured interviews conducted in the patient’s home with a research assistant. Interviews were taped and transcribed Patients were asked about receiving treatment at the community clinic, and were asked to compare this experience with visits or appointments at the regional centre including examples of the advantages or disadvantages |
Demographic data were collected and summarised Thematic analysis of the interviews using transcripts. First three interviews reviewed by full research team, subsequently analysed by two researchers. Emergent themes and coding categories were described and line-by-line coding used Coding framework and examples were discussed with the wider research team |
Of the 28 who began community treatment, two chose to return to the regional cancer centre to finish their treatment Two key themes emerged: Balancing gains and losses: Perceived differences in cancer treatment were grouped under QoL (travel time, lifestyle management, disruption) and biomedical care (technical competence, access to information, interaction with other patients) and participants seemed to trade these off when making the decision about where to have their treatment Communication links: This referred to communication between patients and health-care providers, as well as between health-care professionals at the different treatment locations. Patients felt it was easier to establish rapport with community staff. Although regional centres were associated with greater expertise, patients were willing to trade off so long as clear evidence of communication links with cancer specialists (e.g. telephone, fax, computer) Most patients preferred gains in QoL over medical expertise |
| Main reference: Butler 198459 California, USA Linked references: none Perspectives: patients and their family |
Aim: unclear, appears to be an audit of a new service providing home care Methodology: not reported Sampling: unclear, 15 patients were mentioned as receiving the new treatment and all were asked about their experiences |
Population: n = unclear although 15 patients are mentioned, some of whom received outpatient treatment and others experienced treatment at home Equal numbers of men and women. Ages ranged from 30 years to 65 years Data collection: questions about experience of treatment were included in routine outpatient review appointments with the oncology clinical nurse specialist |
Not reported | Patients indicated that their QoL, was improved when they had control over their daily activities Hospitalisation for chemotherapy was disruptive and prevented a sense of normality. Access to regular support systems was interrupted by hospitalisations Home chemotherapy improved many of these aspects but patients were concerned about the functioning of the catheter and pump |
| Main reference: Corrie et al. 20134 UK Linked references: Corrie et al.,98 Corrie,99 Corrie et al.,100 Corrie (2013, Cambridge University Hospitals NHS Foundation Trust, unpublished document) Perspectives: patients; health-care professionals |
Aim: to assess experience of the treatment from patient and staff perspectives Methodology: framework (no further details reported) Sampling: patients purposively sampled for maximum variation, staff sampling not reported Protocol mentions selecting one in every 10 patients plus their carer, but unsure if this was successful or how many took part |
Population: patient participants were taking part in a three-arm RCT comparing home, community and outpatient treatment. Included curative, palliative and supportive care (see Appendix 3 for more details) 11 patients; five consultant oncologists; three GPs; five chemotherapy nurses; two hospital pharmacists and two senior managers Data collection: semistructured interviews conducted before treatment and after 12 weeks of treatment with patients. Interviews were recorded and transcribed |
Matrix-based framework analysis approach | Most patients expressed support for their treatment location regardless of setting. Most patients expressed a preference for future treatment in the community. Notably, many patients declined to be randomised due to strong prior preferences Clinical staff were concerned about patient and staff safety, particularly in relation to the home environment. The hospital and GP settings were seen as more secure Although attitudes to safety concerns improved during the trial, this was not reflected in increased patient referrals All groups felt that community treatment offered patients convenience but raised concerns about affordability |
| Main reference: Crisp 201060 Canada Linked references: none Perspectives: patients |
Aim: to explore and describe the perspectives of cancer patients receiving active treatment who chose to receive or refuse home chemotherapy Methodology: interpretive description Sampling: convenience sampling by nurses from the Cancer Treatment at Home pilot programme conducted by Alberta Health Services |
Population: plan was to interview five who accepted home treatment and five who refused it, but impossible to recruit those who declined treatment at home and so additional accepters were recruited for a total of 10 participants Vignettes of all participants presented, context was large metropolitan area Length of treatment ranged from months to 4 years. Both curative and palliative treatments were given Data collection: semistructured interviews, recorded and transcribed plus field notes. Took place in patient’s home or at the Cross Cancer Institute Nine interviews analysed (one lost due to technical problems) |
Constant comparative analysis and ongoing engagement with the data, focused on inductive analysis. Use of transcripts, research notes, in-process diagrams and audio recordings Analysis followed each interview with key concepts being added to a master board. Researcher and supervisor both involved in process Summaries of research results offered to participants |
Home was identified as being a ‘natural habitat’ in which they were better able to adapt to their circumstances. Patients were better able to redistribute their resources including time, energy and finances in ways that were meaningful. They felt that the care provided was enhanced and they were more receptive to teaching. Lastly patients viewed themselves as less ill and were better able to cope with their treatments |
| Main reference: Frølund 201141 Denmark Linked references: Frølund 2011,41 full published paper, quant results Perspectives: patients |
Aim: to examine how patients experience chemotherapy at home, and how it affects their everyday lives Methodology: not reported Sampling: all patients in the case series were included |
Population: six patients (50% male, aged between 63 years and 73 years) taking part in the case series, all with experience having treatment both in hospital and at home All were diagnosed with bone marrow cancer in 2009 Supportive care treatment Patients were scheduled to receive either 16 or 24 treatments; home treatment was given between 3 and 11 times Data collection: semistructured interviews recorded and transcribed Refers to Steinar Kvale |
Paul Ricoeurs hermeneutic phenomenology
|
Six main themes identified: patients preferred home treatment over hospital treatment; patients are less fatigued and stressed; home treatment has less adverse impact on patients’ daily lives; patients have more energy left over for social relationships; patients feel less medicalised and accordingly home treatment increases quality of life Having experienced nurses from the oncology department provides a sense of security |
| Main reference: Hall 200830 UK Linked references: none Perspectives: patients |
Aim: to compare the experience of receiving chemotherapy at home vs. in hospital Methodology: humanistic approach/phenomenology Sampling: all patients in the trial were included |
Population: 15 patients were randomly allocated to treatment groups: 10 received treatment at home and five in the hospital. All patients were interviewed. See Appendix 3 for more details Data collection: semistructured interviews (timing unclear) were recorded and transcribed |
Thematic analysis (Bowling 1997) | Quality of life was an important factor in how care was perceived by patients. Both groups expressed satisfaction with the quality of their care; however, variations were also present in service provision Theme: comfort and security Theme: privacy Theme: practicalities Theme: relationships Patients treated at home were more strongly positive about their experiences and felt that it should be an option offered to all patients |
| Main reference: Hansson 2011,61 Ph.D. thesis Denmark Linked references: Hansson et al.,38 non-randomised trial report; Hansson et al.,67 qualitative paper; Hansson 2010,105 abstract; Hansson 2011, Hansson 2012,106 abstract Perspectives: patients (children) and family members |
Aim: to describe family members’ experiences of a hospital-based home-care programme for children with cancer Methodology: descriptive inductive method Sampling: purposively sampled families from a pool of 53 children. Sample was based on differences in diagnosis, family constellation, parents’ occupation, number of home-care visits and duration of treatment programme |
Population: 14 parents representing 12 families were invited, of which two declined. 11 interviews conducted, recorded and transcribed Detailed sample characteristics and eligibility given in page 61 of full paper Number of home-care visits 9–66. Duration of treatment 3–16 months Data collection: unstructured interviews were recorded and transcribed Location and family member involvement in interview was chosen by the parents (six at home, five in hospital) Both parents = three families One parent = six families Both parents individually = one family Child and sibling = five families |
Transcriptions were analysed using Graneheim and Lundman (2004) methods. Content analysis used as an interpretative process for analysing written communication. Focused on differences and similarities in the text Analysed using concepts of meaning units, codes, subthemes and themes Four steps: All authors read each interview several times Transcript divided into meaning units by first authors, each meaning unit then condensed into a description Condensed meaning units are labelled with codes, abstracted and compared for similarities and differences by all authors Each subtheme critically read, compared and a main theme formulated with is thread of underlying meaning Preliminary interpretations and themes presented to peers for discussion and credibility checking |
Main theme: supporting the family to remain intact throughout the childhood cancer trajectory Subthemes: ‘Decreasing the strain on the family and ill child’ ‘Maintaining normality and an ordinary life’ ‘Fulfilling the need for safety and security’ |
| Main reference: Hjorleifsdottir et al. 200864 Iceland Linked references: Hjorleifsdottir et al. 2007109 Perspectives: patients |
Aim: to explore perceptions of care and service provided in an outpatient setting (also looked at experience of having cancer and coping strategies) Methodology: inductive qualitative Sampling: oncology nurses selected patients to be approached for the study, more than one hospital clinic included |
Population: n= 25 Having radiotherapy or chemotherapy in an oncology outpatient clinic 16 women, nine men (mean age = 55 years, SD 13 years). 60% receiving chemotherapy, 40% receiving radiotherapy Curative (n = 16), symptom control (n = 8), palliation (n = 1) Data collection: semistructured interviews carried out in preferred location (most chose private room at oncology clinic). Recorded and transcribed Interview questions reported |
Manifest and latent content analyses used. Detailed descriptions provided. Most analysis carried out by one authors working from transcripts using verbatim quotations and pre-established categories. Three other authors also read the analysis during the process and contributed ideas and discussion | Findings: Satisfaction in the outpatient clinic depended on delivery of drugs, caring attitudes of the health professionals and the caring encounters Negative factors included waiting times, difficulty parking and the clinic environment however there was tolerance around other patients sometimes needing more time with a doctor Other sections of the results focused on the impact of the diagnosis, coping strategies including attempting to maintain normality and keeping the uncertainty at a distance (only those findings relating to care received and satisfaction with outpatient setting extracted) |
| Main reference: Iredale et al. 201151 UK (Wales) Linked references: none Perspectives: patients |
Aim: to determine who was using the bus (mobile cancer support unit) and explore their perceptions of having treatments on the bus Methodology: not reported (part of a mixed-methods project with a quantitative survey plus interviews) Sampling: all patients were invited to participate in interviews |
Population: six chemotherapy patients and four social care patients took part. The bus provided both chemotherapy and social care services Data collection: bus visitors were given a survey to complete and also invited to attend follow-up interviews Semistructured interviews with questions informed by prior interviews with Tenovus (cancer charity) and Velindre (hospital) staff took place in patients’ homes. Recorded and transcribed Aimed to capture first impressions, experiences of treatment and comparison with previous treatment elsewhere |
Thematic analysis (no further details) | Most patients spoke very highly of the bus both based on first impressions and interior appearance and cleanliness Bus was said to be more convenient, more personal and more organised in comparison to previous treatment experiences Treatment on the bus was reported to save time and money, with reduced levels of stress and anxiety As confirmed by author, all quotations and comments on service were from chemotherapy patients |
| Main reference: Kelly et al. 200462 UK Linked references: none Perspectives: patients; health-care professionals |
Aim: examine the existing outpatient chemotherapy provision and assess the feasibility of providing a home-based chemotherapy service Methodology: not reported Sampling: targeted convenience sampling of patients with colorectal cancer as their treatment likely to be more amenable to home chemotherapy Purposive sampling of health-care professionals based on role and experience |
Population: five patients with colon cancer currently undergoing outpatient chemotherapy with 5-FU 12 health-care professionals; consultant oncologists, chemotherapy nurses, pharmacist, nursing directorate and financial managers and local commissioner of cancer services Data collected between February and April 2000 Data collection: semistructured interviews recorded and transcribed Patient interview topics included: experiences of outpatient service (travel, side effects, general satisfaction), financial impact of illness/treatment and views on the proposed home chemotherapy service Health-care professionals were asked about opinions on current service, contracting and cost issues and feasibility of a home service |
Transcripts were analysed thematically on a line-by-line basis by two researchers working independently Exemplar quotes were identified and interview themes were then integrated with contract and cost data from the rest of the paper (mixed methods) |
Views on current service provision: All patients were generally satisfied with their care. Any negative comments related to waiting and journey times (all patients reported being delayed by up to 5 hours on at least one visit) Health-care professionals expressed concerns about waiting times and current service configuration, physical set-up contributed to problems Views on home-based chemotherapy: Patients reported a mixture of views; some felt that it would be a good idea and would reduce travel time with treatment given in a familiar and private setting. Some patients expressed concerns about safety and need for expertise from staff Health-care professionals were interested at least in theory in the provision of home chemotherapy. They raised concerns about funding, practicalities of patient numbers and increased demand Specific points relating to changes were made including the need for structured development and reference to local Cancer Networks |
| Main reference: Mitchell 201139 UK Linked references: Mitchell 2013,50 published paper Perspectives: patients and partners |
Aim: to explore experiences of people with cancer who received chemotherapy treatment in outpatient clinic and/or on board the MCU Methodology: interpretive phenomenological approach Sampling: convenience sampling, first 10 to respond to invitation in each setting were interviewed |
Population: n = 20 Ten patients attending the mobile cancer unit, 10 attending the outpatient clinic Data collection: in-depth interviews in patient’s home (n = 19) and in researcher’s home (n = 1). In some cases spouses or partners were also involved All interviews were recorded and transcribed. Interviews lasted between 1 hour and 3 hours An interview journal was also kept to record notes on context and body language |
Thematic phenomenological analysis involving three readings of the transcripts to familiarise, code words/sentences/paragraphs, and then to allocate codes to new or existing categories Exemplary statements for each category were collected. Analyses were verified through discussion with a colleague Themes were developed from the categories |
Only the findings relating to the process of receiving chemotherapy were presented, although participants told of their full journey through symptoms, diagnosis, referral and treatment Theme: in it together Theme: car parking and travel Theme: waiting for treatment in clinic Theme: having chemotherapy on the MCU Theme: privacy, dignity and safety The cancer and chemotherapy journey was described as being undertaken by the participant and their significant other. Available car parking and travelling impacted on quality of life, as did the environment and accessibility of nurses to discuss issues with participants. The most important, distinguishing feature between receiving chemotherapy in outpatient clinic and the MCU was the amount of time spent waiting. Having treatment on the MCU was perceived to be less formal and, therefore, less stressful. Participants reported significant savings in time spent travelling, waiting and having treatment, expenditure on fuel and companion time and costs |
| Main reference: McIlfatrick et al. 200763 Northern Ireland, UK Linked references: none Perspectives: patients |
Aim: to explore patients’ experiences of having chemotherapy in a day-hospital setting Methodology: not reported, based on Meleis’s theory of nursing transitions Sampling: convenience sample of patients who had experienced at least one cycle of chemotherapy as an outpatient and as an inpatient |
Population: n = 30 Ages ranged from 21 years to 77 years; seven different cancers included; 50% had ovarian cancer; range of chemotherapy treatments given Data collection: semistructured interviews conducted in a private room in the day hospital, recorded and transcribed. Topic areas were reported |
Narrative analysis was used, specifically Polkinghorne’s two-stage process. Generalised paradigmatic analysis of narratives followed by an in-depth analysis of the narratives Member checks were used to establish ‘trustworthiness’, eight patients were asked to comment on themes following analysis Analysis was carried out by one researcher and checked by another and coding was performed by two blinded researchers. Agreement on themes and narratives was generally reported |
Findings: Four key themes were identified: Facing the situation Perceptions of the day hospital (positive sense of normality vs. negative dehumanising) System issues (environmental and organisational) Looking ahead The themes in italics above relate directly to this review and these results were extracted in full |
| Main reference: Pace et al. 200929 UK (England) Linked references: Pace et al.102 Perspectives: patients |
Aim: a randomised comparison (crossover) of outpatient and community chemotherapy that included a satisfaction outcome Methodology: not reported Sampling: all participants selected for the trial (see Appendix 3) |
Population: two patients took part in the trial, of which 11 provided additional information on their experiences Data collection: Chemotherapy Patient Satisfaction Questionnaire which includes an open-ended question |
Examples of feedback were provided from four patients | Patients mentioned less waiting time, ability to maintain normality and reduced tiredness following treatment as a consequence of community-based treatment |
| Main reference: Rischin et al. 200032 Australia Linked references: King 200154 Perspectives: patients |
Aim: to determine patient preferences between hospital- and home-based chemotherapy Methodology: not reported Sampling: all eligible randomised patients who completed treatment were included |
Population: 20 participants who completed the RCT and returned their questionnaires. (See Appendix 3 for more details) Data collection: questionnaire administered after two treatments (crossover design ensured all patients would have experienced both settings) to 20 patients Questionnaire included open-ended questions about ‘any perceived difficulties or advantages of treatment in hospital or home’ |
Not reported; summary lists of reasons and comments were provided | No concerns relating to chemotherapy in the home were reported, some patients reported problems with hospital treatment (transport difficulties and waiting times) Almost all of the patients listed advantages to being treated at home; only one patient felt that there were advantages to being treated in the hospital |
| Main reference: Smith and Campbell 200458 Scotland, UK Linked references: none Perspectives: health professionals |
Aim: to explore how key health professionals view current models of outreach cancer care. (Study also collated data on current schemes serving remote rural cancer patients) Methodology: mixed methods (quantitative survey plus semistructured interview) Sampling: outreach clinics defined as more than 1 hour’s drive from one of five cancer centres. Key agents were identified for interview (no further details) |
Population: n = 19 Oncologists (5), clinical nursing manager (1), liaison sister (1), lead cancer nurse (1), specialist nurses (11) covering a total of 23 geographical locations of which seven did not provide chemotherapy Data collection: semistructured telephone interviews recorded and transcribed |
Findings: Widely varying practices in the delivery of cancer care were reported. Health professionals felt the main issues are expertise, travelling, accessibility for patients, communication (between cancer centres and outreach clinics) and expansion of the rural service Professionals were generally keen to see an expansion of the rural services if expertise and communication issues could be addressed |
|
| Main reference: Stevens et al. 200456 Canada Linked references: Stevens et al.,33 RCT; Breitfield,104 editorial; Stevens et al.,55 patient views Perspectives: health-care professionals |
Aim: to explore the views and experiences of health-care professionals involved in a crossover RCT comparing home vs. outpatient chemotherapy for children Methodology: not clearly reported Sampling: purposive sampling to include range of experience, education and roles |
Population: 33 health-care professionals were interviewed after 6 months of delivering the home chemotherapy programme Clinic nurses, community nurses, paediatrician, care co-ordinator, programme administrator, laboratory manager and pharmacist Data collection: individual semistructured interviews including topics on strengths and limitations of the programme, resource/training/education implications, extending the programme, impact of the programme on their role Three experienced interviewers collected the data in a private room. All interviews recorded and transcribed |
Mayring’s content analysis was used. Transcriptions read line by line; memos used to record developing insights. Inductive reasoning used to organise data into categories and emergent themes Inter-rater checks carried out by independent researcher coding sections of transcripts; analysis discussed between two researchers to check for discrepancies and agree consensus NVivo used to display and manage the data including participant characteristics Thirteen broad categories developed then collapsed into three key categories Data from community and hospital-based staff were initially analysed separately and then compared |
Perceived family benefits
Impact on role of health-care professionals: Hospital staff
|
| Main reference: Stevens et al. 200655 Canada Linked references: Stevens et al.,56 Stevens et al.33 Perspectives: patients (children) and parents |
Aim: to examine the perspectives of children with cancer (and their parents) on a home chemotherapy programme Methodology: not reported Sampling: convenience sampling within a crossover RCT to recruit parents and children to the study following a 6-month period of home chemotherapy (some chemotherapy treatments had to be delivered in hospital; blood work took place in community laboratory) |
Population: 23 families, of which five were single-mother families 24 individual parent and 14 individual child interviews took place Overall: 19 mothers, 5 fathers, and 14 children over 6 years took part. Average child age was 12 years Demographics reported in full paper Data collection: semistructured interviews asking about advantages and disadvantages of home chemotherapy, patient preference and how setting affected daily life were recorded and transcribed Place of interview was chosen by the participants and most took place in a private office in the hospital |
Descriptive exploratory content analysis. Transcripts read line by line by two researchers. Memos created independently to record analytic insights, coding ideas and key points Data collected in a table according to common topics of discussion – using exact wording from participants Using inductive reasoning, data organised into categories that reflected emerging themes Compared raw data with themes to note similarities/differences and make comparisons Discussion and consensus was used to merge the categories from each researcher |
Five main categories:
|
| Main reference: Taylor 2008,24 Ph.D. thesis UK (England) Linked references: Taylor et al.,110 full paper Perspectives: patients; carers; health-care professionals |
Aim: to evaluate the perspectives of health-care professionals, patients and carers on ambulatory cancer care developments Methodology: mixed-methods approach using triangulation, underpinned by postmodern and social constructionist approaches Sampling: participants were identified through a mixture of convenience and purposive sampling |
Population: Health-care users: Home chemotherapy users (n = 3) Nurse-/pharmacist-led clinic receiving capecitabine (n = 3) Chemotherapy day-unit users (n = 3) Carers (n = 2) Focus groups including patients and carers (n = 2 groups) Health-care professionals: Pharmacists; commercial home-care company; nurses (district and specialist); GPs; consultants; multidisciplinary group Data collection: focus groups and interviews conducted by the practitioner-researcher Most were recorded and transcribed, field notes taken during focus groups. Focus groups were conducted early on and used to develop the research |
Thematic framework analysis: familiarisation; identification of thematic framework; indexing; charting and mapping; interpretation. Focus group data used to develop framework for analysis and structure the interviews | There was a continued drive by the government to move treatments from day-case hospital settings to outreach ambulatory care settings including cancer. Patients required flexibility throughout their journeys, hospital infrastructure and a collective experience with other patients. These could be lost through outreach ambulatory care developments. Health-care professionals identified a lack of professional and physical capacity to deliver ambulatory care as expressed in the policies |
Appendix 9 NHS survey
Appendix 10 Private survey
List of abbreviations
- A&E
- accident and emergency
- CRD
- Centre for Reviews and Dissemination
- CUA
- cost–utility analysis
- ECOG
- Eastern Cooperative Oncology Group
- EORTC
- European Organisation for
- QLQ-C30
- Research and Treatment of Cancer Quality of Life Questionnaire (30 items)
- EPPI
- Evidence for Policy and Practice Information
- EQ-5D
- European Quality of Life-5 Dimensions
- FLIC
- Functional Living Index Cancer
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HaH
- Healthcare at Home
- ICER
- incremental cost-effectiveness ratio Profile
- NICE
- National Institute for Health and Care Excellence
- PedsQL
- Paediatric Quality of Life Scale
- Ph.D.
- Doctor of Philosophy
- POQOLS
- Paediatric Oncology Quality of Life Survey
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- VAT
- value-added tax