Notes
Article history
The research reported in this issue of the journal was joint funded by the HS&DR programme or one of its preceeding programmes and INVOLVE as project number 10/2001/29. The contractual start date was in October 2011. The final report began editorial review in June 2014 and was accepted for publication in March 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Hanley reports personal fees from the Medical Research Council and personal fees from User Involvement in Voluntary Organisations Shared Learning Group, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Gamble et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
What is patient and public involvement?
Public involvement in research is described as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. 1 The role of patients and the public therefore extends far beyond that of a research ‘subject’ or participant. Increased recognition that patients and public are stakeholders in research has led to increasing calls that they be represented within that research process, resulting in a growth of patient and public involvement (PPI) in health research both nationally and internationally, and also within the peer review process of that research. 2–4 This includes the USA, where it is known as stakeholder engagement, and Australia, where it is termed consumer and community participation.
It has been suggested that clinical trials are particularly likely to benefit from PPI. 5,6 Health research funding bodies strongly encourage researchers to implement PPI at every stage of the research process and specifically to include PPI contributors on Trial Steering Committees (TSCs). 7–12 Assimilation of PPI into grant applications is therefore becoming commonplace, with clinical trial funding bodies requiring that plans for PPI be submitted by investigators to ensure trial participants’ needs are respected, and to maximise research quality and relevance. 7,12–15
When should patient and public involvement start?
Patient and public involvement can start at various stages of a trial and may influence many aspects. The ability to have an impact on a trial has to be considered in line with the opportunity to exert influence. Staniszewska et al. 16 discuss the importance of PPI in the design of research to optimise its impact and relevance. During the design stages of a trial, many decisions are made that determine the relevance and conduct of the proposed research: the precise specification of the research question including the outcomes to be measured; visit schedules; methods of data collection; and recruitment and consent procedures. Fudge et al. 17 suggest that decisions made by professional researchers at the outset of a study have a cumulative and significant influence on the potential for PPI to have an impact on a study and that involvement is more difficult to achieve once studies are under way. It is therefore important to consider the stage of the trial at which PPI begins alongside the process of involvement when identifying impact.
Boote et al. 18 reviewed published case examples that focused solely on PPI at the design stage of primary health research; they identified just six peer-reviewed journal articles reporting on PPI in the development of a clinical trial. The PPI methods entailed group discussions. Although the methods of PPI may not be considered representative of the diverse nature of PPI, suggesting the presence of selective reporting, the key contributions identified have been reported from other models of involvement: review of patient information sheets and consent procedures; suggestion of outcome measures; review of acceptability of data collection procedures; and recommendations on the timing of potential participants being approached for the study and timing of follow-up. 5 To understand how frequently these contributions occur and the factors associated with their occurrence there is a need to investigate PPI in an unselected cohort of trials.
The UK Department of Health guidelines, Best Research for Best Health,7 state that PPI must be included in all stages of the research process including priority setting, defining research outcomes, selecting research methodology, patient recruitment, interpretation of findings and dissemination of results.
The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme has encouraged PPI, previously asking researchers to consider the benefits of its incorporation and now requesting evidence of PPI from researchers submitting their proposals. Oliver and Gray19 assessed the impact of public involvement in the HTA research-commissioning programme; however, the impact of public involvement in the research funded by the HTA programme is yet to be assessed.
How should patient and public involvement be implemented?
Challenges to the realisation of plans for PPI include debate regarding its purpose, lack of evidence regarding the impact of PPI, complexities in researchers and contributors sharing power, and difficulties in ensuring sufficient resources for PPI. 5,15,18,20–22 Alongside such challenges are uncertainties regarding how best to plan PPI. Guidance drawing on the opinions and experiences of those involved in PPI activity within trials is available21,23 and a 2011 review examined case studies of PPI in the design and conduct of trials. 6 However, the evidence base is limited in terms of the range of trials, researchers and patients that have informed this previous work, and there has been no systematic evaluation of the extent to which triallists’ intentions for PPI are put into practice.
Little is known about how or when researchers incorporate PPI in clinical trials, or what impact may stem from that involvement. There are indications that PPI can have favourable impacts upon every stage of the research process5,24–28 by helping to ensure that research funds are appropriately prioritised and that research evidence is relevant to patients, by improving recruitment and retention rates and by supporting the uptake of research in practice. Indications that PPI may have unfavourable impacts upon research24,29 or no impact at all30 have also appeared. In this intensely moral and political arena, the rarity of such reports has raised concerns that the benefits of PPI have been selectively reported. 5,31 Concerns have been expressed that the existing literature is selectively reported to make the case for or against PPI, with many reports aiming to make the case or convince the sceptics about PPI, and these concerns have led to questions about the quality of the evidence base for PPI in trials. Furthermore, as much reporting has involved single case studies, generalisability of the PPI literature is limited and may provide a misleading account of how PPI is implemented and its impact. 31 Crucially, these problems make it difficult to predict what type of involvement is most effective and where.
The relationship between the nature of involvement and the control of PPI contributors in the decision-making of the research process has been debated, with higher levels of control often being considered as of higher quality, and lower levels of control being criticised as tokenistic. 32 However, this approach has been critiqued. 33 For example, while it is often assumed that approaches that are described as limited, tokenistic or of low quality are ineffective, they may still achieve valuable impacts; conversely, those considered to be better models of involvement might not.
Who can be a patient and public involvement contributor?
The NIHR states:34
We need people with everyday experience of health, education, social care or services delivered in your home or near where you live. We often look for people who have experience of specific health services as a patient or carer, and who have an interest in research. We welcome individuals and representatives of voluntary organisations and patient groups.
However, there is debate about whether or not it is acceptable for individuals employed within a medical or research capacity to provide PPI. Current NIHR HTA guidance states that ‘To achieve the aim of bringing fresh eyes to the work of the HTA programme a patient or member of the public should not normally be a health practitioner, manager or researcher’. 35 Concerns have also been raised about PPI contributors becoming professionalised. 36 This may happen either as a result of contributing across a number of separate research projects or as a result of their role in facilitating or supporting PPI contributors within the remit of their employment, and may be extended to those undertaking leading roles in charities and patient organisations. The debate on the professionalisation of PPI contributors also has implications for training provisions.
Patient and public involvement contributors may be selected because their attributes or experience are considered to strengthen their ability to contribute to their role in the research. Others may be selected based on the perception of their ability to ‘represent’ the wider population of interest. Little is known about how PPI contributors are identified or the selection process used by researchers. This may affect the training and support needs of the PPI contributor in fulfilling their role.
Whereas the evidence on PPI activity in research is expanding, PPI training has received little research attention. Training demands time and resource, and also has potential to shape the future conceptualisation, implementation and impact of PPI in research. INVOLVE, the UK-based advisory body on PPI in health and social care research, reports that most PPI training courses have been developed within particular organisations or in the context of individual research projects. 37 It defines training broadly as any activity ‘that aims to help members of the public and researchers develop their knowledge, skills and experience to prepare them for public involvement in research’ (p. 5). 37 An examination of training and educational provision of PPI in research confirms the diversity of aims, content and delivery of training. For example, education and training for PPI contributors ranges from a year-long formally assessed and certificated course on the discovery, testing and evaluation of medical products and technologies,38 to one-day informal workshops to help contributors identify suitable research roles and build confidence. Examples of training for researchers are almost as variable, ranging from formal modules on the theory, policy and current practice of PPI within accredited master’s courses39,40 to single ‘awareness raising’ workshops on the aims and implementation of PPI in research. 41
Although this diversity of training may be appropriate, it raises questions about how to ensure training is fit for purpose. INVOLVE proposes that training be provided for both PPI contributors and researchers,37,42 tailored to their needs and roles, delivered on an ongoing basis and in ways that allow contributors and researchers to learn from each other. 37 These principles were drawn from consultations with over 30 stakeholders who had direct experience of PPI training either as providers or recipients. However, few details of the methods of consultation are available and little is known about the perspectives of those researchers and PPI contributors who have not participated in training. A key consideration for any training is that it engages with the diversity of learners’ needs and is meaningful from their perspective. 43 Insights on how members of the clinical trials community perceive PPI training, regardless of whether or not they have had prior experience of training, will help to ensure its relevance and uptake.
Should we assess impact of patient and public involvement?
Robust evidence on the effectiveness of PPI in research is absent. It has been argued that PPI in research is ‘the right thing to do’ and should occur irrespective of impact. 44 However, incorporating PPI within research requires time and resource,5 so we should be expected to learn from both the positive and negative experiences of researchers and PPI contributors to determine facilitators and barriers to impact for the benefit of future research. 45
For PPI contributors, getting involved in research has been reported to lead to ‘personal development’ such as boosting confidence, empowerment and a sense of purpose. 46 Similarly, there can be personal benefits for researchers, who have reported that their attitudes, values and beliefs about the worth of PPI had been heightened as a result of such involvement. 20 However, as well as being a vehicle for improving research validity, there are indications that ‘patient influence’ can pose a potential threat to the validity of research if it is not drawn upon appropriately. 14 For example, PPI in technical decisions may result in worse as opposed to improved project outcomes. 47
It is important that accounts of researchers and PPI contributors be accessed in establishing an evidence base to guide future approaches to the implementation of PPI. Each brings different perspectives and, consequently, the two parties may differ in their views of how PPI impacts on trials. This position is supported by the evaluation of PPI in the UK Clinical Research Collaboration (UKCRC). 48 Indeed, much of the existing literature looks at the experience of PPI representatives or advisory groups, but there is less research on the experiences of professional researchers and clinical trials units (CTUs). An examination of the experience of PPI from all perspectives is needed to strengthen understanding and develop a more robust evidence base for future implementation.
A national questionnaire survey on the role of PPI in designing, conducting and interpreting randomised trials managed by clinical trial coordinating centres concluded that PPI was still uncommon. 49 Since the publication of this survey there have been many changes in the clinical research environment, including those brought about by the establishment of the UKCRC in 2004 and the UKCRC Registered Clinical Trials Units (RCTUs) in 2007. 50 RCTUs are assessed as having the expertise necessary to ensure high-quality, successful and timely trials, and to meet regulatory and governance requirements. There is limited knowledge about the engagement of RCTUs with PPI contributors, and challenges to early PPI for trials competing for public funding have been identified. These include the short time frame for completion of applications, the lack of resources to support PPI and difficulties in identifying appropriate PPI contributors. It is expected that a growing proportion of publicly funded clinical trials will be co-ordinated via a RCTU. Therefore, there is an important need for new work to be conducted within the network of RCTUs to explore the current role they have in determining the process and quality of PPI, and to aid strategic planning for future practices drawing strength across RCTUs.
How should the impact of patient and public involvement be assessed?
The assessment of impact is difficult because of the complexity of PPI. Problems with the conceptualisation and measurement of the impact of PPI have also been identified,51 and few studies have accessed the perspectives of both PPI contributors and researchers. Moreover, much of the literature on the impact of PPI in research has not focused specifically on randomised trials, although these are regarded as particularly likely to benefit from PPI. 5
In assessing impact stemming from PPI in clinical trials, there is a need to consider the empirical evidence on how PPI was actually implemented in its broadest form. For example, direct impact may be observed in terms of suggested improvements to patient information sheets, logistics or the visit schedule, which in turn may be thought to lead to improved recruitment. Direct impact may be observed in relation to the choice of outcomes to be measured, either by suggestions to include outcomes otherwise considered unimportant by health professionals or by suggestions to improve the likely completion rate of participant questionnaires. In turn, these may lead respectively to research that patients are more likely to use to help them make decisions and to research of higher quality. Impact may also be less direct, for example, with PPI providing an opportunity for dialogue between researchers and PPI contributors; the improved awareness of patient perspectives may not only help to guide researchers’ attention towards clinical problems that are most relevant to patients, but also drive their motivation to address these problems.
Despite its importance, there is a lack of well-accepted approaches to the assessment of the impact of PPI in health research. 5,52,53 Staniszewska54 commented that the varied methods used to assess and report the impact of involvement caused difficulties when trying to synthesise evidence across studies. More recently a Public Involvement Impact Assessment Framework (PiiAF)55 has been developed to help researchers at the beginning to identify the issues that could affect the impacts public involvement can have on their research and to develop an approach to assessing these impacts during their research.
Aim
The overall aim of this project, known as EPIC (Evidence base for Patient and public Involvement in Clinical trials), is to increase knowledge of PPI within randomised trials by:
-
establishing an unselected evidence base of how PPI has been implemented within randomised trials
-
identifying associated impact to inform the future optimisation of PPI by systematically describing and critically evaluating the process, challenges and impact of PPI from the perspectives of the PPI representative, chief investigator (CI) and CTU staff.
Chapter 2 Study design and methods
The EPIC project aimed to investigate PPI in a cohort of randomised trials funded by the NIHR HTA programme between 2006 and 2010. EPIC comprised four phases:
-
Phase 1 examined triallists’ plans for PPI as described within their funding applications.
-
Phase 2 was a questionnaire survey of CIs’ and PPI contributors’ opinions and activities concerning PPI.
-
Phase 3 involved semistructured interviews with CIs, PPI contributors and trial managers (TMs).
-
Phase 4 examined the role of CTUs in identifying and supporting PPI needs by means of a questionnaire survey.
Ethical approval was obtained from the University of Liverpool Institutional Ethics Board (reference RETH000489).
Establishing the cohort
The cohort was identified as randomised trials that were actively receiving funding from the NIHR HTA programme between 2006 and 2010. The cohort included randomised trials at different stages, from recently funded applications to randomised trials that had reached the final report stage, providing data on PPI across all stages of the research process.
The NIHR HTA programme has a two-stage application process (Figure 1). In summary, the outline application is considered by the funding board and applicants are asked to address feedback from the board if a full application is requested. The full application is sent for external peer review and considered by the board to determine if it should be rejected or any changes made prior to funding. The full application consists of a completed application form and a detailed project description.
FIGURE 1.
National Institute for Health Research HTA programme application process.
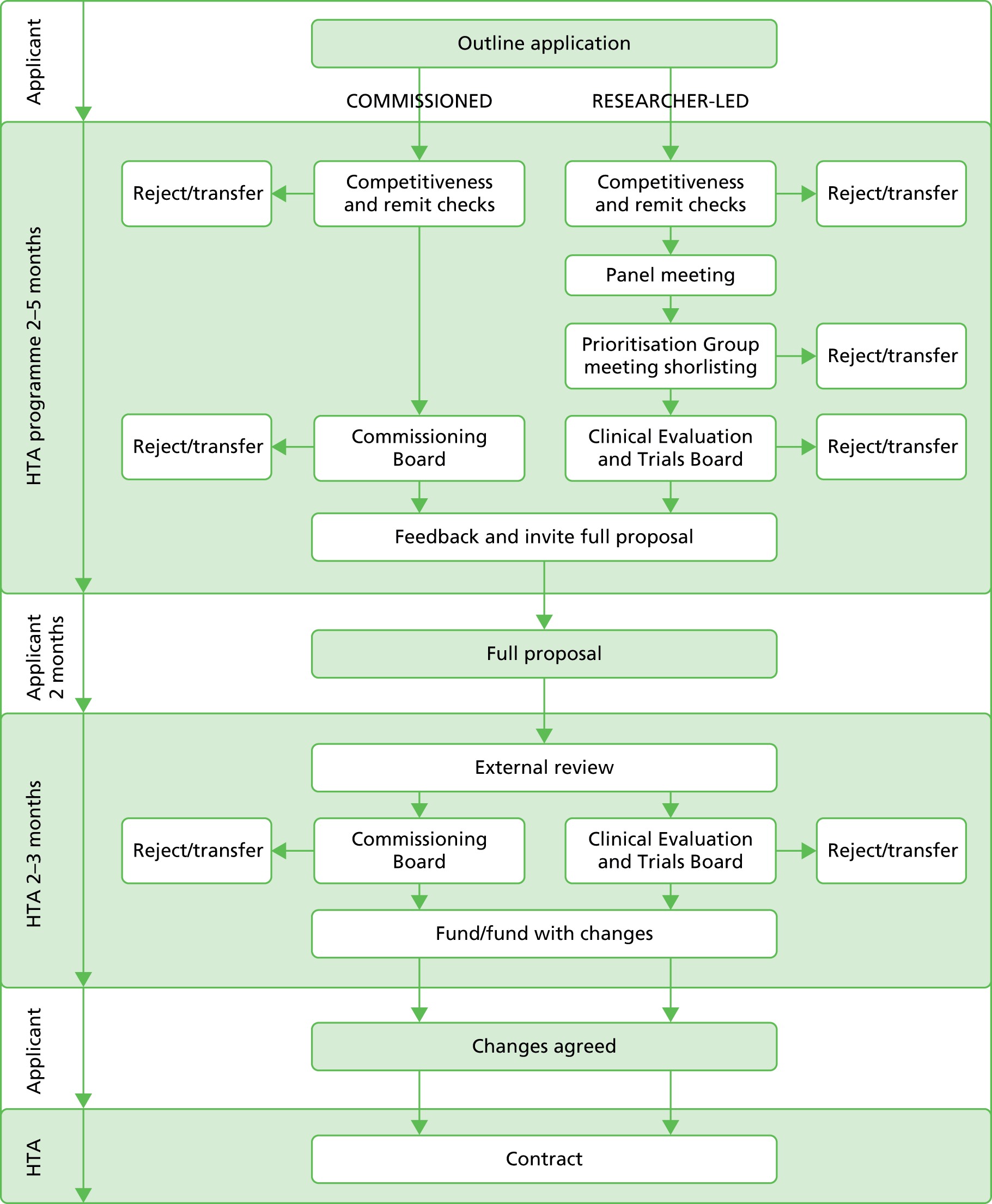
We requested all available documentation relating to the application process. This comprised outline applications; the minutes of the board meetings at which the outline applications were considered and which contained feedback for the applicants to consider in submitting the full application; the full application form and the detailed project description; external referee reports; and the minutes of the board in which the final decision on funding was made. Prior to the release of these documents the NIHR HTA programme contacted the CIs of the trials involved, informing them of the intention to release their names, which were readily available within the public domain. We signed a confidentiality agreement and the NIHR HTA programme redacted sensitive information regarding budget information and names of coapplicants (not available within the public domain) before releasing the documentation.
Phase 1: documentation, data extraction and coding
A Microsoft Access® version 2010 (Microsoft Corporation, Redmond, WA, USA) database was developed to assist in extracting and analysing the information on PPI within the applications. Each application was given a unique identifier within the database and this identifier, rather than the NIHR unique identifier, is used to maintain confidentiality throughout. A PPI advisory group of five members with experience of providing PPI in randomised trials was established for this project (see Appendix 1). The advisory group commented upon the data extraction tool and made recommendations for changes. Data were extracted to characterise the cohort and to describe PPI activity within the two-stage application process and plans for involvement once trial funding was secured. Trial characteristics linked to trial complexity, or thought to be barriers or facilitators to recruitment,56 were also extracted. In brief, the extracted data included characteristics of the trial design and setting, disease or condition under study, type of intervention, participant characteristics, recruitment setting and any text that described or was relevant to PPI. Data were extracted by three reviewers (LD, JP, CG). Extracted text was anonymised by replacing any identifying details with a general term in brackets [term], or using [. . .] to indicate removed text.
Text extracts describing PPI were examined to determine the stage of its actual or planned initiation; for example, the outline application (submitted in the first stage of the application process) may have specified that PPI was planned to occur during the development of the full application (submitted in the second stage of the application process). The stages of initiation were the development of the outline application; the development of the full application; and following a positive funding decision or during the trial.
The text was also examined to determine the role of the PPI contributor’s input. This was categorised as managerial, responsive or oversight. We categorised PPI contributors as managerial if they were described as coapplicants or involved in the management of the trial, for example a member of the Trial Management Group (TMG). We categorised PPI contributors as having a responsive role if descriptions of their input were largely confined or targeted towards a particular aspect of the application or trial, or if PPI contributions were on an ‘as required’ basis. An oversight role was defined by appointment as an independent member of either the TSC or the Data Monitoring Committee (DMC). Descriptions of PPI in the application documentation were often limited, and coding ‘rules’, informed by our knowledge of grant application development and clinical trial implementation processes, were devised to categorise the descriptions. The codes were developed by CG and LD after reading the PPI descriptions and then reviewed by JP. CG and LD independently categorised the PPI descriptions. Disagreements between CG and LD were discussed and agreement reached by referring back to the documentation. All categorisations were cross-checked by JP. The coding rules (Box 1) and the classifications were reviewed by the PPI advisory group and no changes were suggested.
-
If a PPI contributor is described as a member of the research team or ‘lay member’, categorise them as inputting across all future stages of the study.
-
The design of the study is determined within the full application stage so if a PPI contributor is described as inputting in to the design of the study, categorise their input as starting no later than at development of the full application.
-
If a PPI contributor’s role is confined to TSC membership (which is usually agreed by the funders and follows the funding decision), categorise their input as starting after the full application regardless of the tense of the sentences describing their involvement.
-
If a PPI contributor’s role is described as managerial or as a coapplicant, or referred to as a part of the team, categorise their level of involvement as managerial.
-
If a PPI contributor’s role is confined to a panel or advisory group, categorise their contribution as responsive.
-
If a PPI contributor’s role is limited to a specific aspect of the trial, categorise their input as responsive.
The external referee assessment forms that were completed by each referee began requesting referees to comment on any aspect of the proposal that they considered relevant from the perspectives of patients or service users. In addition, within a section on resources and feasibility, referees were asked to consider whether or not there is ‘appropriate representation from all relevant groups in the research team (this might include consumers, researchers from different disciplines, managers and professionals) and is the role of each collaborator/co-applicant clear (particularly important for multi-centre studies)?’ All areas of the form were examined for references to referee assessment of PPI.
Comments from external reviewers and the funding board were coded as positive, negative or factual. Positive comments implied that the referee or board considered the PPI to be satisfactory or sufficient, and did not contain suggestions for adding to the existing PPI plans. Negative comments were those which indicted that PPI was considered weak or unclear, or gave direction on strengthening PPI from that proposed within the application. A comment may indicate the absence of PPI in an area, such as membership of TMG or TSC, but not specifically indicate that this was required. Where this occurred it was interpreted as a negative comment in that it was aiming to highlight a gap in the approach to PPI. Factual statements were those which outlined the importance of service users without commenting on the plans proposed, or identified PPI plans without any indication of the respondent’s view on their appropriateness.
Phase 1 analysis
In considering trends over time, the year the outline application was submitted was used. The cohort was identified as randomised trials that were in receipt of funding from the NIHR HTA programme between 2006 and 2010, but the year the outline applications were made ranged from 2003 to 2008.
The following specific conditions were selected to consider in more detail: mental health, pregnancy and childbirth, human immunodeficiency virus/acquired immunodeficiency syndrome, cancer, stroke, paediatrics, diabetes, and dementias and neurodegenerative diseases. These were identified either by their strong history of PPI or by the establishment of a NIHR clinical research network for a specific condition. 57
Categorical data were summarised using descriptive statistics with numbers and percentages. Chi-squared tests or Fisher’s exact tests were used as appropriate.
Phases 2 and 4: surveys of chief investigators, patient and public involvement contributors, and UK Clinical Research Collaboration Registered Clinical Trials Units
Three surveys were planned as part of the EPIC project targeting CIs, PPI contributors and the network of RCTUs. Each survey was web-based and developed in SurveyMonkey (www.surveymonkey.com), with portable document format (PDF) versions available from the EPIC website. Each survey consisted of both closed and open questions to avoid constraining responses. In addition, further free text was collected when ‘other’ was selected as a closed response. The surveys were initially developed by LD and CG and sent for comments to BY, JP and PRW prior to being considered by the PPI Advisory Group. Surveys were piloted within the Clinical Trials Research Centre, University of Liverpool, prior to being finalised. The CI and PPI survey questions targeted opinions and motivations about PPI, methods of engagement, areas of contribution and level of impact within the cohort of trials. The RCTU survey questions focused on the experience and processes of the RCTUs across trials rather than within the cohort.
The link for the CI survey was emailed to the CI of each trial within the cohort. The e-mail addresses of each CI were obtained from the funding application but were checked against web searches on the CI names to ensure that they were up to date. The invitation e-mail described EPIC in brief, explained why they were being contacted and referred to the initial contact made by the NIHR HTA programme about the project. E-mails to the CIs also contained a link to a website which contained PDF versions of the survey and information sheets. The CI surveys were sent out on 13 March 2013 and two reminder e-mails were sent to non-responders on 17 April 2013 and 28 June 2013. Non-responders known to members of the EPIC research team were contacted to encourage completion.
Names of the PPI contributors for each trial were not available in the public domain. To obtain their contact details we requested the CI of each trial to contact their PPI contributor(s) asking them to contact the EPIC team so that we could send them information about the project and the PPI survey. An additional e-mail was sent to the trial CIs reminding them to contact their PPI contributors about EPIC. In addition an advert was drafted by JP and sent to the PPI Advisory Group for comments. The advert was placed on the websites of Involving People and North West People in Research. Finally we contacted the NIHR HTA programme to ask if it could contact the PPI contributors to inform them about EPIC; however, PPI contact details were not held by the NIHR HTA programme. The NIHR HTA programme contacted the chair of each of the TSCs asking them to contact any PPI contributors known to them.
The directors of the 46 RCTUs were contacted by e-mail requesting them to complete the CTU survey. The names of the fully and provisionally registered CTUs were obtained from the UKCRC website58 following the publication of the results of the 2012/13 Review Process. Websites for each RCTU were identified and contact details of the directors obtained. The survey could have been circulated on our behalf by the UKCRC across the directors using the group UKCRC directors e-mail list, but it was hoped a personal e-mail would encourage response. The initial e-mail was sent on 27 March 2013. The e-mail contained a brief summary of EPIC and the purpose of the survey along with links to the EPIC website, which contained a PDF version of the survey. The RCTU directors were asked to complete or delegate completion of the survey, which targeted PPI processes across trials in their units rather than focusing on individual trials. Reminder e-mails were sent on 29 April 2013 and, if it was unclear whether or not contact had been made, we used their websites to identify an alternative senior person within the RCTU.
Analysis of phase 2 and phase 4 surveys
All surveys were analysed using descriptive statistics, and chi-squared tests were used for cross-tabulations between questions. Recurring themes were identified within the free-text responses and used to group responses provided.
Phase 3: interviews
Within the CI and PPI surveys, respondents indicated if they were willing to be contacted to take part in an interview to further explore their experiences of PPI within the trial.
We initially sampled CIs for maximum diversity based on their survey responses, although we eventually invited all but three of the CIs who had responded to the survey and indicated their willingness to be interviewed. We invited for interview all PPI contributors who returned a survey response and indicated their willingness to take part. Additionally, we invited TMs for all trials for which the CI or PPI contributor had been interviewed. We obtained contact details for TMs from CTUs, trial websites and protocols, or via CIs.
We contacted all potential informants by e-mail and provided an information leaflet inviting them to contact the EPIC research associate to arrange an interview. Non-responders were sent one reminder e-mail. We expected that some PPI contributors might access their e-mail accounts infrequently so we subsequently telephoned those who had not responded. All informants provided signed or audio-recorded verbal consent before being interviewed.
A psychologist, LD, conducted audio-recorded semistructured telephone interviews with informants between April 2013 and November 2013. Before starting interviews, she explained that study data would be anonymised and kept confidential. Interviewing was conversational to allow informants to voice their views and experiences of PPI freely. In order to minimise the risk of idealised accounts, LD adopted a neutral stance in her interviewing. This was to avoid creating a sense that informants had to justify or defend their approach to PPI, which might have inhibited or coloured their accounts. LD familiarised herself with each of the documents for each trial before interviews to tailor questions to specific aspects of the trial. Nevertheless, we used topic guides to steer the interviews (see Appendix 2, Topic guides). We developed three versions to ensure interviews were appropriate for each of the three informant groups (CI, PPI contributor and TM), although the topic guides mirrored one another to ensure core topic areas were explored. Table 1 provides summary topic guides for researchers and PPI contributors. Topic guides were informed by the previous literature, reviewed by EPIC team members and the PPI advisory group, and developed in the light of the ongoing data analysis. In addition, the PPI advisory group read transcripts from early interviews and fed back on the interview with implications for the topic guide. Interviewing paralleled the analysis and continued until theoretical saturation had been reached,59 and additional data ceased contributing to the analysis. Interviews were transcribed using an ‘efficient’ verbatim style that involved transcribing the content of informants’ accounts, rather than detailed features of speech such as subvocalisations and duration of pauses and hesitations. All transcripts were checked for accuracy and anonymised.
| Topic | Researchers | PPI contributors |
|---|---|---|
| Expectations | Understanding of PPI Experience of including PPI in research Goals or plans for PPI in current trial |
Previous experience of being a PPI contributor Expectations about what working on the current trial would be like |
| What happened? | Stage of PPI implementation Identifying and selecting PPI contributors Roles of the PPI contributors Overall experience of including PPI in the current trial |
How did they become involved in the trial? PPI contributor’s role Relationship with research team |
| Impact | Perceived contributions of PPI Challenges of including PPI |
Differences made to the trial as a result of their input Benefits to themselves of being involved Challenges of being involved |
| Training and support | Training or support given to PPI contributors PPI training received by researchers |
Training or support for their role Views on PPI training for researchers |
This qualitative workstream of EPIC allowed us to access CIs’ and PPI contributors’ accounts of PPI in their own words and to analyse them inductively. Given the moral and political expectations surrounding PPI, we thought it was particularly important to adopt an interpretive approach60,61 and consider how informants talked about PPI. Therefore, we focused on the language informants used to describe PPI and on the aspects of PPI they gave little emphasis to in their interviews, as well as what they emphasised. Before each interview we reviewed the documents for each trial on their PPI plans, in order to tailor our questions and identify particular lines of enquiry to pursue. The interviews enabled us to seek clarification and prompt informants to elaborate on their experiences and perspectives. Similarly, informants were able to seek clarification from us, to elaborate on their perspectives and to raise topics that they considered important which we had not foreseen.
Phase 3 analysis
Analysis was informed by the principles of the constant comparative method62,63 with elements of content analysis. 64 We used procedures to support rigour in qualitative research65 and, as we note above, our approach was interpretive. To ensure a contextualised analysis we referred to transcripts as a whole as well as to particular data segments. We initially analysed CI and PPI contributor transcripts at the informant group level for evidence of their beliefs and experiences about the process and impact of PPI as well as their views and experiences of training and support for PPI. Subsequently, we triangulated CI and PPI contributor transcripts within each trial, before comparing them with the TM transcripts within each trial. Where trials did not have a full data set (i.e. did not include all three groups of informants), we compared the two available accounts. Where only one account was available, analysis was at the informant group level.
The analysis was led by LD, who read CI and PPI contributor transcripts several times before developing open codes. BY also read multiple transcripts, and she and LD met regularly to compare interpretations of the data and review the ongoing analysis. Open coding took place at multiple levels, from line-by-line coding of detailed descriptions to the general stance informants took towards PPI. Open codes were grouped into categories and organised into a framework. Coding and indexing of data was assisted by NVivo 9 software (QSR International, Warrington, UK) and we continually compared categories with new data and amended them to ensure that they reflected the data while accounting for deviant cases. For TM transcripts we conducted some open coding of sections relevant to the categories emerging from the CI and PPI contributor analysis. Subsequent discussion and review of detailed analysis reports by other members of EPIC team including DB, who led an analysis of the same data set on the implementation of PPI, helped to refine the analysis and corroborate the findings. To evidence our interpretations we present illustrative extracts from the data. Extract codes indicate informant group (CI; PPI contributor 1 or 2, where more than one were interviewed for the same trial; TM) and trial identification numbers.
To compare what PPI actually happened in the trial with that planned within the application (implementation of PPI), the following primary and secondary data sources were used.
Primary sources of data were trial documentation (full application forms, reviewer comments, detailed project descriptions and study protocols), from which we extracted data about trial teams’ plans for PPI; and CI and PPI contributor interview transcripts, from which we determined whether or not the documented plans were implemented.
Secondary sources of data were outline application forms, CI survey responses and TM interview transcripts. We used the secondary sources in cases of ambiguity, that is where it was unclear from the primary sources whether or not aspects of a particular set of plans had been implemented. We also used the secondary sources to elucidate the illustrative examples that we present in the results.
The implementation of PPI analysis, led by DB, used a thematic analysis approach to analyse the interview data regarding the implementation of plans for PPI, alongside data extracted from trial documentation about written plans for PPI. Thematic analysis is a useful method for identifying, analysing and reporting patterns (themes) within data. 66 DB familiarised herself with the interview data by reading the transcripts several times, and then drew on the Framework technique,67 which is a manual method to develop and apply open codes to the interview data. Codes were grouped into broader categories within the framework and compared with data extracted from the documented plans. Other members of the EPIC team, who were familiar with the interview transcripts and trial documentation, examined the early stages and ongoing refinements of the descriptive coding framework, as well as the tabulated comparisons of planned and implemented PPI, thus providing confidence in the credibility and ‘confirmability’ of the findings. 68
Chapter 3 Patient and public involvement in the application process: results of phase 1
Cohort documentation
One hundred and eleven randomised trials were identified as being in receipt of funding from the NIHR HTA programme between 2006 and 2010, and were therefore eligible for inclusion within the cohort. Complete documentation for each trial was requested. The initial batch of documentation was provided by the NIHR HTA programme and then supplemented by LD visiting the NIHR HTA offices to access missing documentation. All included trials had at least one of the outline application form, the full application form or the detailed project description available, from which we could assess PPI plans.
Cohort summary
Table 2 summarises the completeness of trial documentation available across the cohort. Of the 111 trials eligible for inclusion, 110 were required to submit an outline application. The dates of submission of the associated outline applications were between 2003 and 2008.
| Documentation (N = 111) | n (%) |
|---|---|
| Outline applicationa | 90 (82) |
| Board feedback on outline | 77 (70) |
| Full application form | 106 (95) |
| Detailed project description | 99 (89) |
| Referee comments on full applicationb | 111 (100) |
| Board feedback on full application | 100 (90) |
Table 3 provides a summary of the trial characteristics. Trials were funded across a wide range of clinical conditions, with the most common area of study being trials in mental health (16%). The majority of trials (79%) were aimed at treatment of a condition, with 17% working on prevention. The trials used a wide range of interventions. Over one-third investigated a medicinal product and just under one-fifth each evaluated behavioural interventions (18%), surgical techniques (15%) and devices (16%).
| Condition/intervention (N = 111) | Number of trials (%) |
|---|---|
| Long-term conditiona | 61 (56.5) |
| Rare condition | 2 (1.8) |
| Condition expected to reduce lifespan | 36 (32.4) |
| General shortening | 20 of 36 (55.6) |
| Rapid mortality | 16 of 36 (44.4) |
| Condition under studyb | |
| Mental health | 18 (16.2) |
| Heart disease/condition | 4 (3.6) |
| Haematology/phlebology | 8 (7.2) |
| Infections | 9 (8.1) |
| Musculoskeletal | 7 (6.3) |
| Cancer | 7 (6.3) |
| Renal | 4 (3.6) |
| Childbirth and pregnancy | 4 (3.6) |
| Obstetrics and gynaecology | 2 (1.8) |
| Addiction | 5 (4.5) |
| Dermatology | 3 (2.7) |
| Gastroenterology | 7 (6.3) |
| Diabetes | 2 (1.8) |
| Obesity/nutrition | 3 (2.7) |
| Falls in the elderly | 3 (2.7) |
| Sleep disorders | 3 (2.7) |
| Stroke | 5 (4.5) |
| Dental | 2 (1.8) |
| Degenerative neurological disorders | 4 (3.6) |
| Respiratory | 5 (4.5) |
| Other | 6 (5.4) |
| Aim of intervention | |
| Treatment | 88 (79.3) |
| Prevention | 19 (17.1) |
| Diagnostic | 4 (3.6) |
| Nature of interventions useda | |
| Drug | 44 (39.6) |
| Behavioural | 20 (18.0) |
| Device | 18 (16.2) |
| Surgery | 17 (15.3) |
| Physical, e.g. exercise | 11 (12.2) |
| Educational | 10 (9.0) |
| Community care | 5 (4.5) |
| Other | 4 (3.6) |
| Commissioned brief | 45 (40.5) |
Table 4 describes the characteristics of trial participants and features of the trial designs. Three-quarters of the trials recruited adults only, with paediatric trials accounting for 18% of the cohort. The majority of the trials were not gender specific and approximately a quarter recruited participants at the time of diagnosis. Trial recruitment was most commonly conducted within secondary care (61%). Just under one-quarter of the trials (25 of 111) involved blinding of the treating clinical team or the participants, with 28 of 111 blinding the outcome assessor only. Just over 15% of trials used a placebo, and all participants received an active intervention in one-third of these, indicating the use of a double dummy design.
| Trial participant and design characteristics (N = 111) | n (%) |
|---|---|
| Age group | |
| Adults only | 83 (74.8) |
| Paediatrics only | 20 (18.0) |
| Adults and paediatrics | 7 (6.3) |
| Unclear | 1 (0.9) |
| Gender | |
| Female | 10 (9.0) |
| Male | 1 (0.9) |
| Male and female | 100 (90.1) |
| Recruiting newly diagnosed patients | 27 (24.3) |
| Trial recruitment settinga | |
| Secondary | 68 (61.3) |
| Primary | 24 (21.6) |
| Community | 12 (10.8) |
| Emergency | 8 (7.2) |
| Tertiary | 8 (7.2) |
| Social care | 7 (6.3) |
| Blinded trialb | 53 (47.8) |
| Clinician blind | 19 of 53 (35.8) |
| Participant blind | 24 of 53 (45.3) |
| Trial involves a placebo | 17 (15.3) |
| Placebo involved, but all participants receive an active intervention | 6 of 17 (35.3) |
To develop understanding of PPI within the earliest stages of clinical trial development we placed an emphasis on the outline application process. The specific objectives were to identify if, and how, PPI is described within the early development of a grant application for funding; examine how PPI contributions within the development of the outline application were reviewed by the funding board; consider how applicants describe their proposed PPI plans for the development of the full application and the trial once funded; and describe variations in PPI in relation to time of funding and trial characteristics.
Patient and public involvement within the first stage of the application process
Outline applications were available for 90 of the 111 randomised trials in the cohort. The trial and participant characteristics restricted to these 90 trials are summarised elsewhere. 69 Of these 90 outline applications, 49 (54%) provided some level of detail on PPI. Table 5 summarises the stage of initiating PPI and role of PPI across the trials. The first row of Table 5 shows that there were 19 applications in which the text provided within the outline described PPI occurring at all three stages (within the outline application, in the full application and once the trial was funded). Of these 19 applications the role was managerial at each stage in 13. In the remaining six there was variation in the roles across the stages, or it was unclear, when a statement indicated that PPI would occur but no details were provided to allow classification. An example of a description that we were unable to categorise is ‘Investigators have, and will continue to, collaborate with service users.’
| Stage | Number of outline applications (%), n = 90 | Role | Number of outline applications | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Outline | Full application | Trial once funded | Outline | Full application | Trial once funded | |||
| Y | Y | Y | 19 (21.3) | M | M | M | 13 | Two had multiple approaches alongside M in the main trial (TSC; TSC plus R) |
| R | M | M | 1 | |||||
| R | R | R | 2 | |||||
| R | U | U | 1 | |||||
| U | R | R | 1 | |||||
| U | U | U | 1 | |||||
| Y | Y | NS | 2 (2.2) | R | R | – | 1 | |
| O | R | – | 1 | O = described as informal contacts | ||||
| Y | NS | Y | 2 (2.2) | R | – | O | 1 | O = scale development |
| R | – | TSC | 1 | Consulted in pilot study | ||||
| Y | NS | NS | 3 (3.4) | O | – | – | 3 | O = clinical studies group; survey; service user forum |
| NS | Y | Y | 8 (9.0) | – | M | M | 3 | In one also TSC and R in trial |
| – | R | R | 2 | In one TSC too | ||||
| – | R | M | 1 | |||||
| – | U | U | 2 | |||||
| NS | Y | NS | 1 (1.1) | – | R | – | 1 | |
| NS | NS | Y | 12 (13.5) | – | – | M | 3 | |
| – | – | R | 1 | |||||
| – | – | TSC | 7 | Two above also listed TSC alongside higher-order approach | ||||
| – | – | O | 1 | O = piloted and then refined with users | ||||
| NS | NS | NS | 41 (45.6) | – | – | – | 41 | |
| NS | U | U | 2 (2.2) | – | U | U | 1 | |
| – | M | M | 1 | Unclear when PPI initiated but when it starts it is at M level | ||||
Twenty-nine per cent (26 of 90) specified a level of involvement within the development of the outline application. This was managerial in 13, on a responsive basis in 7, unclear in 2 and other approaches used in 4 (e.g. a patient survey or pilot feedback). Within the ‘other’ approaches it was difficult to determine conclusively whether this was PPI or they were examples of data collection aimed at ascertaining public opinion. In the three applications that specified use of a survey, the extent of the distribution of the survey was unclear in two. PPI was planned to occur within the full application for 32 trials (36%). This was managerial in 18, responsive in 9 and unclear in 5. Forty-three (48%) applications indicated that PPI was planned after the trial was funded. This was as managerial in 22, responsive in 6, a member of the TSC in 8, unclear in 5 and other in 2. The numbers of outline applications by year with and without details of PPI are displayed in Figure 2, with Figure 3 showing the percentage of applications with PPI. These figures show a general trend for an increasing number of funded applications; however, the proportion of those containing PPI fluctuates. The proportion ranges from approximately half to two-thirds (see Figure 3), with the exception of 2003, for which only one application was available.
FIGURE 2.
Number of outline applications by year in which the application was made.

FIGURE 3.
Percentage of outline applications containing PPI details by the year in which application was made. The number of trials included within each year is indicated at the top of each bar.
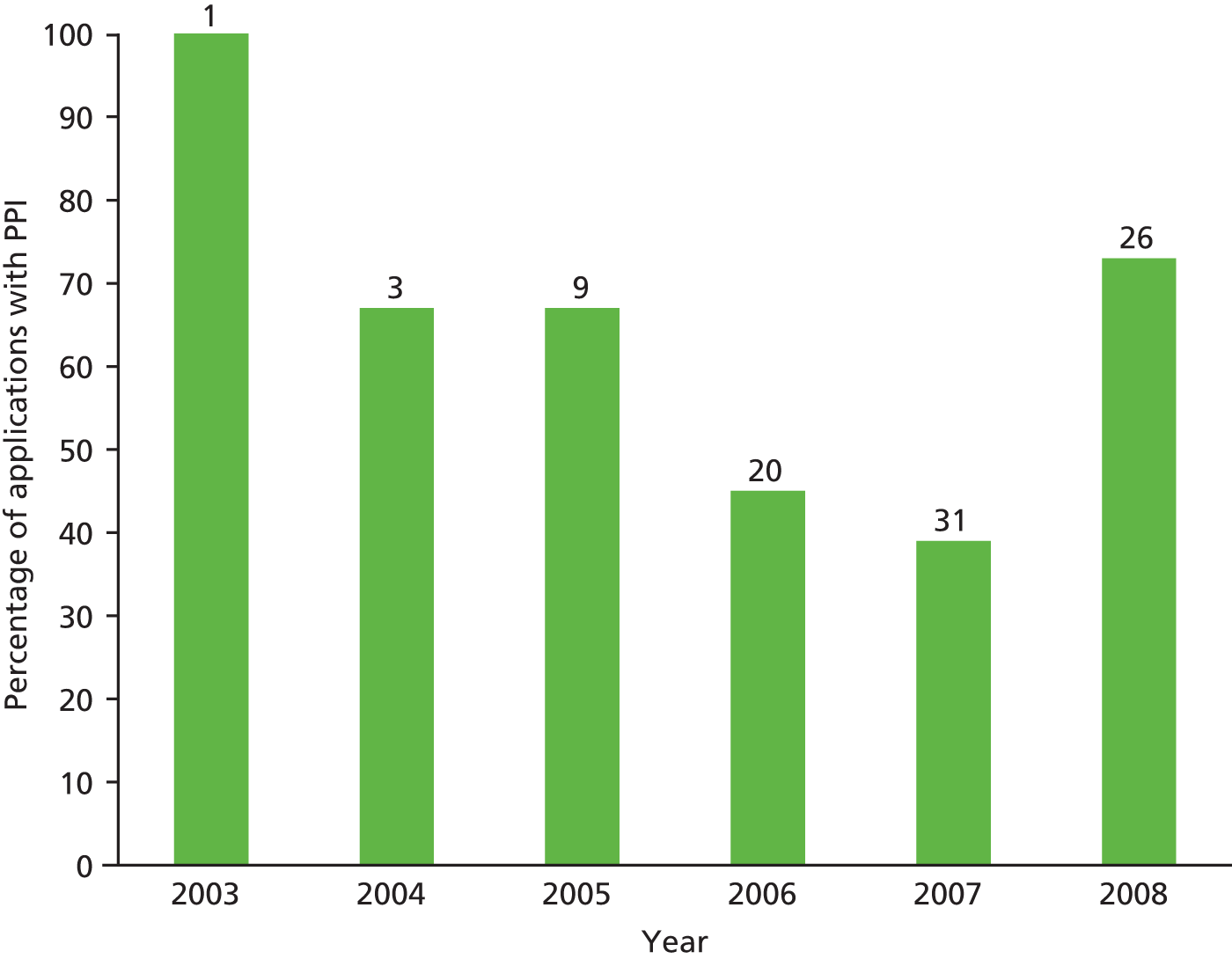
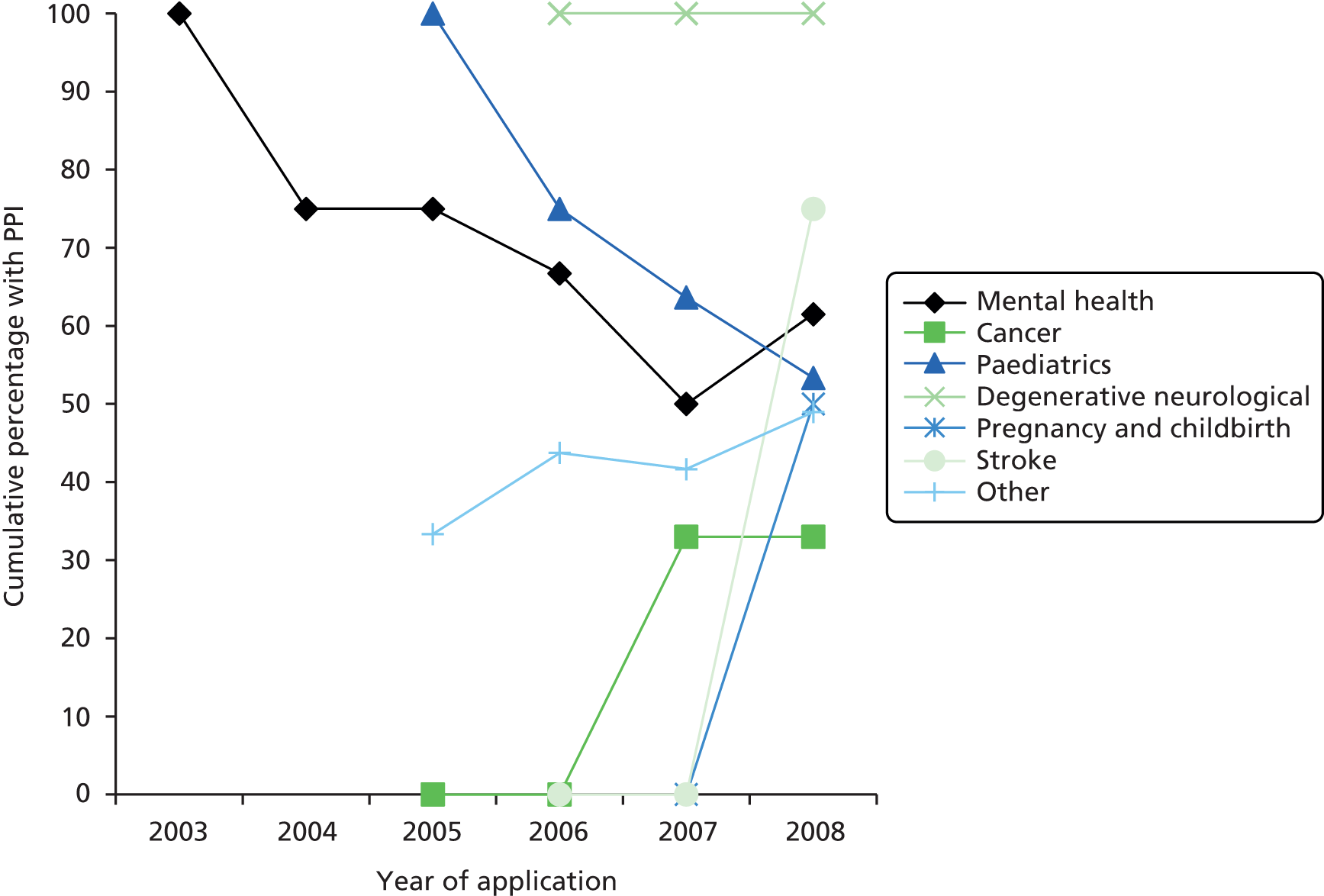
Figure 4 displays the data by year for specific conditions. Both of the diabetes trials were in children and were coded as paediatric, and there were no human immunodeficiency virus/acquired immunodeficiency syndrome trials within the cohort with outline applications available. Figure 4 suggests declining rates of PPI in paediatric and mental health, with other areas, including the general ‘other’ category, demonstrating an increase over time. However, as shown in Table 6, the numbers in some categories were small and therefore limit conclusions based on disease areas. Table 7 suggests an absence of association between specification of PPI within the outline application and disease area (n = 0.51).
FIGURE 4.
Cumulative percentage of outline applications by disease/condition.
| Disease/condition category | Cumulative number of outline applications by year | |||||
|---|---|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |
| Mental health | 1 | 4 | 4 | 6 | 10 | 13 |
| Cancer | 0 | 0 | 1 | 1 | 3 | 3 |
| Paediatrics | 0 | 0 | 5 | 8 | 11 | 15 |
| Degenerative neurological | 0 | 0 | 0 | 1 | 2 | 4 |
| Pregnancy and childbirth | 0 | 0 | 0 | 0 | 1 | 2 |
| Stroke | 0 | 0 | 0 | 1 | 1 | 4 |
| Other | 0 | 0 | 3 | 16 | 36 | 49 |
| Disease/condition category | PPI details in outline text | p-value | ||
|---|---|---|---|---|
| Yes (% of category total) | No (% of category total) | Total (% of overall total) | ||
| Pregnancy and childbirth | 1 (50.0) | 1 (50.0) | 2 (2.2) | 0.51a |
| Cancer | 1 (33.3) | 2 (66.7) | 3 (3.3) | |
| Stroke | 3 (75.0) | 1 (25.0) | 4 (4.4) | |
| Mental health | 8 (61.5) | 5 (38.5) | 13 (14.4) | |
| Paediatricsb | 8 (53.3) | 7 (46.7) | 15 (16.7) | |
| Degenerative neurological | 4 (100.0) | 0 (0.0) | 4 (4.4) | |
| Other | 24 (49.0) | 25 (51.0) | 49 (54.4) | |
| Total | 49 (54.4) | 41 (45.6) | 90 (100.0) | |
Table 8 shows the associations between trial design characteristics, or characteristics of the condition under study, and consideration of PPI within the outline application.
| Intervention | PPI details in the outline application | p-value | ||
|---|---|---|---|---|
| Yes (% of category total) | No (% of category total) | Total (% of overall total) | ||
| Aim of intervention | ||||
| Treatment | 40 (54.1) | 34 (45.9) | 74 (82.2) | 0.66a |
| Prevention | 8 (61.5) | 5 (38.5) | 13 (14.4) | |
| Diagnostic | 1 (33.3) | 2 (66.7) | 3 (3.3) | |
| Nature of interventionb | ||||
| Drug | 16 (51.6) | 15 (48.4) | 31 (34.4) | 0.70 |
| Device | 7 (46.7) | 8 (53.3) | 15 (16.7) | 0.51 |
| Surgery | 4 (25.0) | 12 (75.0) | 16 (17.8) | 0.01 |
| Education | 6 (60.0) | 4 (40.0) | 10 (11.1) | 0.75a |
| Behavioural | 11 (64.7) | 6 (35.3) | 17 (18.9) | 0.35 |
| Physical | 8 (72.7) | 3 (27.3) | 11 (12.2) | 0.19 |
| Settingb | ||||
| Primary care | 10 (47.6) | 11 (52.4) | 21 (23.3) | 0.47 |
| Secondary care | 30 (56.6) | 23 (43.4) | 53 (58.9) | 0.62 |
| Emergency care | 4 (50.0) | 4 (50.0) | 8 (8.9) | 1.00a |
| Community | 6 (50.0) | 6 (50.0) | 12 (13.3) | 0.77 |
| Social care | 4 (66.7) | 2 (33.3) | 6 (6.7) | 0.68a |
| Tertiary | 4 (66.7) | 2 (33.3) | 6 (6.7) | 0.68a |
| Blinding | ||||
| Yesc | 30 (69.8) | 13 (30.2) | 43 (47.8) | 0.01 |
| No | 19 (40.4) | 28 (59.6) | 47 (52.2) | |
| Involved a placebo | ||||
| No placebo | 39 (51.3) | 37 (48.7) | 76 (84.4) | 0.17 |
| Placebod | 10 (71.4) | 4 (28.6) | 14 (15.6) | |
| Received an active intervention | ||||
| Received placebo onlye | 8 (88.9) | 1 (11.1) | 9 (10.0) | 0.04a |
| Received an active intervention | 41 (50.6) | 40 (49.4) | 81 (90.0) | |
| Recruitment at diagnosis | ||||
| Yes | 8 (33.3) | 16 (66.7) | 24 (26.7) | 0.02 |
| No | 41 (62.1) | 25 (37.9) | 66 (73.3) | |
| Long-term conditionf | ||||
| Yes | 31 (60.8) | 20 (39.2) | 51 (58.6) | 0.17f |
| No | 17 (47.2) | 19 (52.8) | 36 (41.3) | |
| Impact of condition on life expectancy | ||||
| None | 36 (57.1) | 27 (42.9) | 63 (70.0) | 0.72 |
| General shortening | 7 (50.0) | 7 (50.0) | 14 (15.6) | |
| Rapid mortality | 6 (46.2) | 7 (53.9) | 13 (14.4) | |
Of the intervention aims, numbers were too small to draw conclusions about involvement in diagnostic studies, but prevention trials described PPI more frequently than treatment trials. Trials involving educational, behavioural or physical interventions were more likely to have provided details of PPI in the outline application than trials involving drugs or devices. Surgical trials were significantly less likely to have provided details of PPI.
The settings for trial recruitment did not appear to affect specification of PPI, whereas recruiting participants at the point of diagnosis was associated with less PPI.
Forty-three trials were described as being blind and these trials were associated with increased frequency of describing PPI. Of these trials 23 involved blinding of the outcome assessor only. Only 14 trials involved a placebo and, of these, participants in five trials all received an active intervention, with the placebo used as a double dummy. The allocation of a placebo only to one arm of the trial was significantly associated with greater frequency of PPI detail.
Only two trials were in rare conditions. Both of these trials provided details of PPI within the outline application. Although there appeared to be some increase in describing PPI when the condition was long term, there did not appear to be any influence based upon impact on life expectancy.
Board comments on outline applications
National Institute for Health Research HTA Board minutes were available for 70% (77 of 110) of the outline applications. Only nine (12% of 77) board minutes gave feedback on PPI and two of these did so indirectly (Table 9). One comment was supportive of the PPI described in the outline, which involved a PPI contributor as a coapplicant.
| Unique identifier | Year outline application submitted | Text from board minutes for outline applications |
|---|---|---|
| 28 | 2007 | The applicants should consider involvement of disadvantaged groups |
| 65 | 2006 | There was no clear service user involvement and this needs to be addressed |
| 70 | 2006 | The Board would be pleased to see letters of support from appropriate PCTS [primary care trusts] & patient groups that the trial is feasible. The Board wish to see patient and public involvement in any full proposal |
| 34 | 2008 | The Board noted it was a well designed study that has received input from patients |
| 92 | 2007 | Ethical aspects including acceptability to parents must be fully considered |
| 39 | 2008 | Consideration should be given to increasing service user involvement |
| 42 | 2008 | Patient representation is required |
| 98 | 2007 | An explanation and demonstration of acceptability to parents must be included |
| 105 | 2007 | The application would benefit from strong patient and public involvement |
Of the 77 trials for which board minutes were available, the corresponding outline applications were also available for 64 (84%). Of these applications 39% (25 of 64) gave no information about PPI.
Eight of the nine sets of board comments that were made about PPI expressed the need for applicants to increase PPI. Six of these contained no detail about PPI within the application. Of these six, two were drug trials, three were exercise interventions and one was comparing an invasive with a non-invasive intervention. Two were recruiting participants with addiction (smoking and alcohol), two were recruiting elderly participants, one was recruiting infants and one was recruiting participants with a long-term, chronic, debilitating condition.
In the two outline applications which had given some details on PPI, comments from the board were to increase PPI. In one application the PPI contributors were employed within a NIHR condition-specific network in roles relating to PPI. These individuals also had relevant experience as carers or patients of the condition. In the other application the PPI contributor was a coapplicant with an unrelated clinical background (midwife) and was the mother of a child with the condition being studied.
Patient and public involvement within the second stage of the application process
Documentation within the second stage of the NIHR HTA application process requires submission of a full application form and a detailed project description. Ninety-five per cent (106 of 111) of the full applications were available and 89% (99 of 111) of detailed project descriptions. The level of information about PPI within the full application forms was limited, with greater detail generally provided within the detailed project descriptions.
Full application forms
Within the full application form only 38% (40 of 106) provided any level of detail about PPI, with 19% (20 of 106) of trials including a PPI contributor as a coapplicant. The classifications used within the outline applications were applied and are summarised in Table 10. In applying the classifications to the text in the full application form the presence of a PPI coapplicant was used.
| Stage | Number of full applications (%), N = 106 | Role | Number of full applications | ||
|---|---|---|---|---|---|
| Full application | Trial once funded | Full application | Trial once funded | ||
| Y | Y | 18 (17) | M | M | 8 |
| M | M/TSC | 2 | |||
| R | R | 1 | |||
| R | R/TSC | 2 | |||
| M/R | M/R | 1 | |||
| M/R | M/R/TSC | 1 | |||
| R | U | 1 | |||
| U | R | 1 | |||
| U | U | 1 | |||
| Y | NS | 5 (5) | R | – | 4 |
| U | – | 1 | |||
| NS | Y | 16 (15) | – | R | 6 |
| – | TSC | 4 | |||
| – | TSC/R | 3 | |||
| – | U | 3 | |||
| U | U | 1 (1) | U | U | 1 |
| NS | NS | 66 (62) | – | – | – |
Twenty-three (22%) applications indicated that PPI had occurred within the development of the full application; 34 (32%) provided some indication that PPI would occur once the trial was funded; and 18 (17%) indicated involvement in the development of the full proposal and once the trial was funded. Among these 18 applications PPI occurred most frequently in a managerial role (12 applications).
A total of 10 trials included a responsive approach within the development of the full application, and 15 planned it once funding had been obtained. Twelve trials planned to include a PPI contributor on the TSC.
In five trials details of involvement in the full application were provided, but continued involvement within the trial post funding was not specified. In four of these trials a responsive approach to PPI was used in the development of the full application.
In 16 applications details of PPI were not specified within the development of the full application, but some text was provided on their plans for implementation in the trial once funded. Nine of these planned a responsive approach, and three of these combined it with a PPI contributor on the TSC. Four planned to include a PPI contributor on the TSC only, and in three applications this was unclear.
In three trials qualitative research was described as an approach to address the objectives of PPI.
We have conducted in depth interviews with 7 patients who have survived [condition/intervention] and their spouses concerning the design of the trial and key outcome measures.
Of the 40 full applications that provided any detail of PPI, the process for recruiting or identifying PPI contributors was frequently unclear; 17 provided no information or used the term ‘service users’ without further detail. In 10 applications use of an already established support group was proposed, while in six representation was proposed to stem from leaders such as directors or chairs of support groups or charities. Three included participants identified from previous trials or feasibility work, and four specified identifying contributors from local patients.
Detailed project descriptions
The detailed project descriptions, submitted alongside the full application, were available for 89% (99 of 111) of the cohort. Ninety-three per cent (92 of 99) provided some text of relevance to PPI. A summary of PPI described in the detailed project descriptions is provided in Table 11.
| Stage | Number of full applications (%), N = 99 | Role | Number of full applications | ||
|---|---|---|---|---|---|
| Full application | Trial once funded | Full application | Trial once funded | ||
| Y | Y | 57 (58) | M | M | 2 |
| M | M/R | 1 | |||
| M | O/M | 5 | |||
| M | O/M/R | 1 | |||
| M | O/M/R/Q | 2 | |||
| M/R | O/M/R | 3 | |||
| M/R | O/M | 3 | |||
| M/R | R | 1 | |||
| M/R | M | 1 | |||
| M/S | M | 2 | |||
| M/R/Q | M/R | 1 | |||
| R | R | 6 | |||
| R | O | 9 | |||
| R | M | 1 | |||
| R | O/R | 5 | |||
| R | O/R/Q/S | 1 | |||
| R | O/M | 1 | |||
| R | O/R/Q | 1 | |||
| R | O/M/R | 1 | |||
| R | M/R | 1 | |||
| R | R/Q | 1 | |||
| R/Q | O | 1 | |||
| R/Q | R | 2 | |||
| R/S | O/R | 1 | |||
| Q | O/R | 1 | |||
| Q/S | R | 1 | |||
| Q | O/R/Q | 1 | |||
| S | O/R | 1 | |||
| Y | NS | 4 (4) | R | – | 4 |
| NS | Y | 24 (24) | – | O | 7 |
| – | M | 1 | |||
| – | R | 3 | |||
| – | O/R | 7 | |||
| – | R/Q | 1 | |||
| – | M/Q | 2 | |||
| – | O/Q | 1 | |||
| – | O/M/R | 1 | |||
| – | O/R/Q | 1 | |||
| N | Y | 3 (3) | – | O | 1 |
| – | O/R | 1 | |||
| – | O/M/R | 1 | |||
| N | N | 4 (4) | – | – | 4 |
| NS | NS | 7 (7) | – | – | 7 |
Within the detailed project description 31% of applications (31 of 99) did not specify whether or not any PPI had occurred within the application development; however, 24 of these described plans for PPI once the trial was funded. Sixty-one (61%) provided some details of PPI during the development, with 57 of these also providing some text of relevance to plans for PPI once the trial was funded. The remaining seven provided reasons for not incorporating PPI within the application development; however, three of these planned to attempt PPI once the trial was funded:
The planned work does not directly focus on patients; [. . .] it will recruit people with and without medical conditions. We are actively considering the involvement one or more members of the general public, possibly someone from [name], but are struggling to identify how he/she could be involved beneficially.
In all our previous [condition] research we have tried to incorporate user involvement although we have met with limited success. Often the biggest challenge has been finding people who wish to be involved and developing this involvement. In writing this application we planned to discuss the content with service users whom we have previously collaborated, but this was not possible due to death and illness. [. . .] we are hoping to develop such a local group (independently from this trial) that will be initiated and run in line with the PPI good practice.
Twenty-two applications described PPI contributions as being managerial within the development of the application, with 44 describing a responsive approach. Responsive PPI contributors were frequently described as ‘lay members’, ‘patient representatives’, ‘patients’, ‘carers’ or ‘user groups’ (42 of 44). Managerial PPI contributors were described as ‘Chairs’ of charities or support groups (7), members of charities (5), patients, carers and patient representatives (10).
Few applications indicated that the approach to incorporating PPI in the development of the application would remain the same during the funded trial. Among those that had PPI contributions within the application development and planned for PPI in the trial, only 14% (8 of 57) indicated that the approach to PPI would continue unchanged in the trial. Forty-seven per cent (27 of 57) planned to increase the variety of approaches used, 25% (14 of 57) would make changes but maintain the same number of approaches and 14% (8 of 57) intended to reduce the routes for PPI input.
Five applications described the use of surveys to inform the development of the application, while seven used qualitative research to obtain patient perspectives and opinions. Although qualitative research and surveys do not fall within the definition of PPI, they do partner some of the objectives of PPI and have therefore been included here. Of the seven applications that used a qualitative approach with their development, three also used a responsive or managerial approach to PPI. Of the 11 that planned to include qualitative research within the trial once funded, only one had used qualitative research in the application development and all planned to include oversight, managerial or responsive approaches. However, the level of detail provided about the qualitative research was low. Many used the terminology ‘focus group’ but in all but one it was unclear that these would be carried out by qualitative researchers:
Towards the end of the study, when the provisional results are available, we will use the expertise and contacts of our panel of commissioners/trainers/users’ representatives to form focus groups to assist in the understanding and dissemination of findings.
It is clear from our contact with the [local topic-specific research network] user group that consumers welcome the proposed study. The members of the group have already contributed to the design of the study, with feedback from qualitative interviews with service users confirming the relevance of [intervention/assessments], and supporting the plan to identify target symptoms for individual participants, with the involvement of the clinical team and participants themselves.
From the [condition] clinics at [name] Hospital and [name] Hospital, 88 [participants] with a history of [condition] were interviewed with a set of open and closed questions to identify their opinions regarding the need for the trial, [intervention delivery], duration of therapy, suitability of [trial processes], and the choice of outcomes.
Service users have already contributed to the design of the study; with feedback from qualitative interviews with service users resulting in our amending our secondary outcome measures by including a measure of well-being.
We have consulted widely, including with patients to seek their views on trial design and relevant outcome measures: [. . .]. We have involved services users (n = 7) in the design of the trial. We used the patient information pack and part of the questionnaire that has been developed and validated in collaborative research with the [name] Institute as a basis for in-depth interviews to identify patient perspectives on trial design and outcomes. We have identified one service user, [name] who will advise the trial management committee on patient perspectives.
This will allow us to convene 1 or 2 focus groups consisting of 5 to 10 individuals selected by us on the basis that they might represent regular users of [service]. Costs of convening that group along with appropriate honoraria will be met from the project budget. These focus groups will be run by an experienced qualitative researcher. The group will have the opportunity to comment on the proposal, suggesting amendments and modification in the light of their lived experience [. . .]. Finally, we will invite two representatives from these groups to sit on the project advisory board.
Changes in patient and public involvement between the first and second stages of application
There were 80 trials in the cohort for which we had the outline application, the full application and the detailed project description.
Plans for patient and public involvement in the development of the full application provided in the outline application
At the outline stage 36% (29 of 80) of the applications indicated plans for PPI during the development of the full application. In the full application 86% (25 of 29) indicated that this had occurred; 13 specified it within the text of both the full application form and the detailed project description, 11 in only the detailed project description, with one application providing detail in the full application form only. Seventy-two per cent (18 of 25) were consistent in their approach to PPI; for example, they had specified within the outline application that a managerial approach would be used within the development of the full application and, judging from the text provided within the full application form or detailed project description, this had occurred.
In six, there were changes or inconsistencies; for example, five specified a managerial approach in the outline but indicated that a responsive approach had been used in the development of the full application. Inconsistency also occurred between the approaches described in the full application form and the detailed project description. One application could not be assessed because of an ‘unclear’ classification at the outline stage.
Of the four outline applications that had planned to include PPI in the development of the full application, but did not provide any text to show that it had occurred, two had planned a responsive approach, one managerial, and in one the approach was unclear.
No plans for patient and public involvement in the development of the full application provided in the outline application
Fifty-one per cent (26 of 51) of outline applications that did not indicate plans for PPI in the development of the full application did obtain PPI input in its development. PPI activity in the development of the full application was reported in both the full application form and the detailed project description for three, in the detailed project description only for 22 and in the full application form only for one. Within these applications a responsive approach was used in 20 and a managerial approach in eight (approaches not mutually exclusive).
Consistency in plans for patient and public involvement for the funded trial between the two stages of the application process
Plans for PPI once the trial was funded were included within 49% (39 of 80) of the outline applications. Of these, 38 also provided plans in the full application to incorporate PPI once the trial was funded.
Of the 18 outline applications that planned to use only a managerial approach in the trial, three continued with these plans in the full application but 10 increased this planned PPI to include other approaches too (oversight in nine and/or responsive in five). Of the five that changed the managerial approach, three planned to use both oversight and responsive, with one using oversight only and one responsive only.
Nine outline applications planned to use oversight PPI only. This was maintained as the only approach in three, and expanded to include responsive in three and managerial in one. In two applications the approach was changed to be responsive (one application) or managerial (one application).
Four of the five outline applications that had planned to use only responsive PPI kept that approach but three also planned to include oversight, while the remaining one planned to use oversight instead of responsive PPI.
Multiple approaches to PPI were specified in three outline applications. All three had specified oversight as an approach in the outline; two dropped it from the full application, leaving managerial and responsive in one trial and only responsive in one. In three the classification was unclear at the outline stage, preventing comparison.
Of the 51% (41 of 80) of outline applications that did not suggest that PPI would occur in the trial once funded, 78% (32 of 41) did provide plans within the detailed project description; the remaining nine provided no mention of PPI plans for the trial.
The single approach to PPI in the main trial was planned to be responsive in six, managerial in three and oversight in seven. In eight, oversight was planned to occur in conjunction with either managerial (two applications) or responsive (six applications), with four applications using all three approaches. Four applications specified plans for qualitative research to obtain patient perspectives but plans for this occurred alongside PPI in responsive, oversight or managerial approaches.
The level of information varied between the full application form and the detailed project description; discrepancies in approaches planned were common.
Within the section of the detailed project description which asked applicants to specify changes between the outline and full applications, only three specified that changes to PPI had occurred and that these changes were in response to board comments.
Referee comments on the full applications
There were 515 sets of referee comments for the 111 trials in the cohort. The minimum number of referees for a trial was one, the maximum was nine and the median was five.
Across all referee comments only 41% (211 of 515) gave a comment in relation to the PPI. The median number of referee comments relating to PPI per trial was two, with a minimum of zero (occurring for 11 applications) and a maximum of six.
Thirty-four per cent (72 of 211) of comments were positive, 51% (107 of 211) were negative and 15% (32 of 211) were factual. Sixty-three applications had more than one set of referee comments that contained text relating to PPI; after factual comments were removed, 52 applications remained, of which 56% (29 of 52) had referee assessments that disagreed in their assessment of PPI. Table 12 provides examples of conflicting referee comments.
| Unique identifier | Referee comments |
|---|---|
| 11 | It is positive to note the extent to which the trial team have gone to attempt to obtain user involvementThe research team acknowledge that they have not consulted with service users in the preparation of this proposal. However they give no indication of what efforts they made to try and remedy this situation or if they consulted with INVOLVE or service user groups who could advise them. The only limitation here is the lack of service user involvement |
| 17 | The service user representation is minimal and this should be enlarged if possible . . . service user representation on the trial is important it is minimal at the moment and the trial will require steering carefully if interim results are positive or notThere is a service user representative on the steering committee and their choice seems appropriate |
| 22 | The applicants have a patient representative on the trial steering group and appear to have strong links with both service users and their parents/carers; they plan to involve members of the [name] Users participation group in the development and piloting of trial materials and outcomesI am not convinced that service users have enough input to this study. Due to the well-recognised concerns around recruitment, a more explicit involvement of service users may maximise sample size – which is the binding factor on this trialHopefully the involvement of service users and carers will help to ensure that the information leaflets will be acceptable to potential participants. The participation of service users and carers seem well established on the team and the team seem clear about the roles they will undertake. The inclusion of a representative from a voluntary sector organisation on the ‘Trial Steering Committee’ is commendable but I would think it advisable in terms of best practice in user involvement to have a service user on both the TSC and the DMEC [Data Monitoring Committee]. Support arrangements to enable them to make a meaningful contribution would also be necessaryI note that this proposal has strong plans to include service users in the [region] area. It would be helpful if the investigators could give some idea of whether or not they plan to involve services in the other centres, namely [region 2] and [region 3]. It may be that the [topic-specific] Research Network could help with the involvement of service users in this project |
| 24 | It was encouraging to note that this application has included a consumer-applicant who has already been integrally involved in the discussions regarding choice of outcome and the drafting of the patient information leaflets. The applicants have also undertaken a small consumer survey to inform their decisionsAs a service user I feel that one parent on the proposal is not enough (are they being paid for their time/expenses?) and that her role in advising on patient sheets and consent forms is minimal. I would like to see more parental or voluntary organisational representation – perhaps some support for parents involved in the study |
| 82 | There appears to have been good communication with patients and families that have helped with study design. The role played in this development is clear within the proposalI strongly recommend (and there is no mention of this in the application) that there is service user representation, adults with [condition] or parents of children with [condition], on the steering committee. There should also be a patient and parent representation on this steering CommitteeThere are no service users in the research team |
Board comments on the full application
Board comments on the full application were available for 90% (100 of 110) of the cohort. Of these, only 14% (14 of 100) made a comment about PPI (Table 13).
| Unique identifier | Text | Code | PPI details |
|---|---|---|---|
| 11 | There should be user involvement in the trial | Negative | None specified within the outline application or the full application form. The detailed project description gave difficulties experienced in obtaining PPI |
| 23 | More service user involvement is required | Negative | None specified within the outline application or the full application form. The detailed project description indicated PPI in oversight, managerial and responsive roles |
| 29 | Consideration should be made as to whether it would be appropriate to include patient views in this study | Neutral | A responsive approach to PPI described in the development of the outline and full applications with further details of this in the detailed project description. PPI was provided by a local user group which would continue to provide responsive PPI once the trial was funded |
| 33 | There were some concerns around [. . .] and the lack of service user involvementIncrease the service user involvement in this trial. The HTA would be expecting to see adequate public/patient contribution | Negative | No PPI specified within the development of the outline or full applications. Plans given for PPI during the trial were based on recruiting ‘a service user’ for responsive approach to PPI |
| 34 | The Board were encouraged by the level of service user involvement in this trial | Positive | Outline application described a responsive approach used across the development stages and the full trial. The full application form gave no details of PPI in its development but gave details of use of responsive and oversight PPI in the trial once funded. The detailed project description described use of a long-standing institutional PPI group providing input, which would continue with a member of the group attending TMG meetings |
| 36 | An enhanced TSC is required that will include at least two service user representatives | Neutral | The outline application described responsive PPI in its development, with further responsive PPI planned for the full application and once the trial was funded. The detailed project description specified a user group providing responsive PPI in the development of the full application, which would continue in the trial once funded, with a PPI contributor on the oversight committee |
| 37 | More consumer involvement is required | Negative | Outline application and full application form described responsive PPI in their development, with responsive PPI planned for the trial once funded. The detailed project description suggested managerial input in the development stage and managerial and oversight PPI once the trial was funded |
| 40 | Further service user involvement is required, a clear plan as to how service users and carers will be involved in conduct of the trial is needed | Negative | No PPI specified in the full application form. The detailed project description mentioned PPI review of the application by a lay member of a CSG, with plans for a ‘focus group’ once the study was funded to provide responsive PPI, together with a member on the oversight committee |
| 42 | Service user involvement is weak and needs further strengthening. The role and purpose of service user involvement needs to be made more explicit | Negative | PPI discussed in detailed project description only. Responsive PPI in review of application, with oversight planned for main trial |
| 52 | User involvement in the study should be boosted | Negative | Outline and full applications used responsive PPI in their development, with responsive PPI planned for the trial once funded. Detailed project description suggested use of qualitative research to gain PPI input via interviews in development of the application alongside responsive PPI. Responsive PPI planned for trial with oversight PPI |
| 58 | Service user involvement is essential, and the applicants should recruit a suitable organisation | Negative | Oversight and responsive PPI within the detailed project description from ‘at least one user’ |
| 72 | Service user involvement should be detailed and active from an early stage of the study. Such individuals or organisations should be actively involved in the conduct of the trial | Negative | Managerial approach to PPI once trial funded specified within the detailed project description. Individual from a charity to join the TMG |
| 78 | Patient representative involvement should be clarified and strengthened if appropriate | Neutral | Detailed project description detailed use of a network group which provided responsive PPI in the development and would continue during the trial in addition to oversight membership |
| 94 | It might be helpful to have a service user (someone who is/was a [. . .]) on the team | Neutral | Outline, full and detailed project descriptions specify oversight PPI once trial funded |
Of these 14 comments, nine (64%) were negative, stating that PPI should be increased, but gave no indication of how this should be achieved or why existing plans were insufficient; four were neutral, with two of these providing some direction for PPI if appropriate; and one was positive.
There were no clear reasons why these 14 applications received comments on their PPI plans in comparison with the other applications within the cohort.
Key points
-
Within this cohort there was no trend evident to suggest that PPI is increasingly being sought within randomised trials.
-
There was no association identified between specification of PPI activity within the first stage of the application and disease area.
-
There was some association between trial characteristics and frequency of PPI.
-
Only half of first-stage applications provided some detail on PPI, with only one-quarter describing PPI activity in their development.
-
A third of first-stage applications described PPI activity plans in the development of the second stage of the application process and half had plans for PPI activity once the trial was funded.
-
The majority of first-stage applications which stated plans for PPI activity in the development of the second-stage application implemented their plans, with over 70% being consistent with the plans they had outlined.
-
Half of the first-stage applications that did not describe plans for PPI in the development of the second stage did obtain PPI in its development.
-
There was inconsistency in the second stage of the application process between the full application form and detailed project description regarding PPI activity, planned or completed, possibly suggesting that applicants were confused about where this should be incorporated, or that it was a consequence of space constraints.
-
Few applications indicated that the approach to incorporating PPI in the development of the application would remain the same during the funded trial.
-
Qualitative research methods were described as an approach to deliver PPI-associated objectives; however, it was often unclear that this would be conducted by people with the necessary skills.
-
Board feedback to applicants rarely commented on PPI.
-
Only two-fifths of referees commented on PPI, and referees often disagreed on the acceptability of PPI plans described.
Chapter 4 Surveys of chief investigators and patient and public involvement contributors: results of phase 2
Chief investigators survey
The survey was sent to 111 CIs, of whom 81 (73%) responded.
To consider the potential for respondent bias, CI response was considered against the inclusion of details about PPI within their outline application. This was chosen as an indicator of baseline motivation and positivity towards PPI. The proportion of CIs responding was higher for those who included PPI in the outline application [PPI detail included, 82% (40 of 49); PPI detail not included, 66% (27 of 41); outline application not available, 67% (14 of 21)] however, the result did not reach statistical significance (p = 0.19).
The results of the survey are provided in Appendix 3. Fifty-two per cent (42 of 81) of the respondents thought that PPI should always be incorporated in a research study, 43% (35 of 81) felt that PPI could be beneficial but was not always necessary and 5% (4 of 81) were not convinced of its benefits. When stratified against availability of the outline application and the absence or presence of detail about PPI within it, there were no clear differences in the proportions who thought it should always be incorporated [PPI detail included, 55% (22 of 40); PPI detail not included, 52% (14 of 27); outline application not available, 43% (6 of 14)]. Of the four who responded they were not convinced of the benefits of PPI, the outline applications were available for two, neither of which provided any detail of PPI.
Forty respondents had text about PPI in their outline application. Of those that felt PPI should always be incorporated, 45% (10 of 22), 73% (16 of 22) and 91% (20 of 22) respectively provided detail within the outline application about PPI occurring in the development of the outline application, the full application and once the trial was funded, compared with 67% (12 of 18), 61% (11 of 18) and 72% (13 of 18) of those who felt it not always necessary.
Conversely, those who thought PPI should always be incorporated were more likely to report they considered PPI immediately (66.7%, 28 of 42) (question 3) than those who considered it not always necessary (45.7%, 16 of 35) (p = 0.006). Prompting by the CTU occurred more frequently among the group who considered it not always necessary (20%, 7 of 35, vs. 9.5%, 4 of 42).
Ten of the 11 (91%) who were prompted by the CTU provided detail about PPI in the outline application compared with six of the nine (67%) who reported being prompted by the application form (outline application form not available for one respondent to question 3 category 3). This may suggest that CTUs are an effective mechanism for initiating PPI.
The majority (65.8% 52 of 79) of respondents reported more than one motivation for including PPI (question 5). Requirement for funding was the sole reason for four CIs, compared with being the right thing to do for 14 and previous experience of its benefits for nine.
Of the 55 who indicated that their motivation was due to PPI being the right thing to do, 34 (62%) indicated their personal view was that PPI should always be incorporated, with 21 (38%) indicating PPI can be beneficial but not always necessary (cross-tabulation of questions 2 and 5).
Patients, carers and parents were the most common PPI contributors involved within the cohort. Of those studies that involved a charity member, only five reported this as the only PPI contributor involved, whereas 16 also involved patients, 13 involved carers and/or parents, and five involved medical staff.
Of the 11 that reported medical staff as PPI contributors, they all also included patients, parents or carers as contributors, with five also including charity members. An open question (question 7) asked for the reasons behind the selection of PPI contributors. Multiple reasons could be provided within a single response and the coded categories are provided in Table 14. Where it was not clear from the statement provided, information from question 6 (‘Which PPI representatives did you involve?’) was used to support the coding. It was often difficult to determine whether the previous experience referred to related to being a research participant or to providing PPI, so no distinction is made. The most common reason for selection was that the demographics of the PPI contributors were believed to make them representative of the patient population. Other leading factors were their experience and knowledge of providing PPI, experience from an existing or previous role considered to be beneficial, having previously worked with them or their connection to charities or organisations.
| Code | Example of free text provided |
|---|---|
| Recommended by another colleague/person | He was recommended by a collaborator |
| Their previous experience/knowledge of providing PPI or being a research participant | They had contributed to a previous study and were both interested in the topic of this studyShe had been a participant in a previous studyThe patient had supported previous studies and is extremely keen to help |
| Characteristics perceived to be helpful in role | Understanding of what we were trying to achieve. Able to disseminate information to others to get more feedbackVolunteered, keen, articulate and contributed to study development and conduct |
| Relevant demographics to be ‘representative’ | Because these seemed to be the most relevant PPIs for the studyMost appropriateBest able to comment on disease experience and recruitment issues |
| Responded to advert or volunteered | Volunteered |
| Their existing or previous role was considered relevant and beneficial | They also had come from a nursing background and had content knowledge which was useful to the trialPatient was a member of staff in the same department |
| Previous history of working with them | Previous experience working with this individualHe had experience with writing patient information documents and consent forms. Also previous experience showed that he had a great ability to look at the trial design and highlight common sense areas that had been overlooked |
| Their connection with a charity or organisation | Involving a member of a patient charity is my usual way of working |
| Other | The study was their idea! |
The most common way of identifying PPI contributors was opportunistic, for example by patients, parents or carers known or by previous involvement. Charities, patient support groups and voluntary organisations were also frequently used. Advertising and use of the research networks or NHS Patient Advisory Liaison were uncommon.
The majority (54 of 76, 71%) felt that they had provided a clear description to the PPI contributor(s) at the time they joined the trial (question 9). However, the level of detail they had provided to the PPI contributor was not captured by this question and it is likely the level of detail varied considerably from a short introductory e-mail to specific terms of reference.
Over 80% of the CIs indicated they had a PPI contributor on their TSC (question 10). This is specified within the NIHR HTA constitution of TSCs. 11
Thirty-three (43%) CIs reported a single approach to PPI contributions, 26 (34%) reported two, eight (11%) reported three, six (8%) reported four and two (3%) reported five.
In 79% (26 of 33) of the trials where a single approach was used, this was including a member on the TSC. This could suggest ticking the funder’s requirement box or taking the funder’s lead on how to implement PPI.
Considering the number of approaches used in obtaining PPI in relation to the CIs’ personal opinion of PPI (Fisher’s exact test p = 0.005), those who thought PPI should always be included were more likely to have more than one approach to obtaining it (Table 15).
| Opinion | Number of approaches to PPI (%) | Total | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Always | 12 (28.57) | 20 (47.62) | 5 (11.90) | 3 (7.14) | 2 (4.76) | 42 |
| Sometimes | 21 (65.63) | 6 (18.75) | 2 (6.25) | 3 (9.38) | 0 (0.00) | 32 |
| Not convinced | 0 (0.00) | 0 (0.00) | 1 (100.00) | 0 (0.00) | 0 (0.00) | 1 |
| Total | 33 | 26 | 8 | 6 | 2 | 75 |
The number of PPI contributors per trial varied (Table 16), as did the frequency of contact between researchers and contributors (Table 17).
| Number of PPI contributors | Number of trials, n = 72 | Supporting free text |
|---|---|---|
| 0 | 1 | |
| 1 | 25 | |
| 2 | 11 | Two, but we also have patient meetings once every 6 months |
| 3 | 10 | 3 on steering group + (don’t know) re dissemination1 each on TSC, DMC and a separate advisory group |
| 4 | 8 | 1 in the TSC and 3 from previous studies |
| 5 | 2 | |
| 7 | 1 | |
| 9 | 2 | |
| ≥ 10 | 5 | |
| Other | 7 | |
| 1 | One on TSC, but other groups involved & fed back on instruments | |
| 1 | Difficult as stakeholder meetings include many who had small role through to applicant | |
| 1 | 8 sets of child/parents advised on the intervention. 1 [professional] representative is co-applicant, 1 retired [professional] is on TSC, 1 [executive professional] advised on [professional institution] engagement | |
| 1 | 2 on the trial steering committee (together with representative from [charity]), varied on PPI panel | |
| 1 | 10 patients interview, one ex-patient on TSC | |
| 1 | 1 [professional] was our main advisor. During the pilot we had focus groups with 27 stakeholders. This feedback helped us to revise our trial design and intervention content for the main trial | |
| 1 | 1 on the TSC, 7 gave questionnaire feedback, 5 in pilot study |
| Frequency of contact | n (%) |
|---|---|
| Once a month | 12 (15.8) |
| Once every 6 months | 39 (51.3) |
| Once a year | 1 (1.3) |
| Less than once a year | 1 (1.3) |
| Other | 23 (30.3) |
| Once a week | 1 |
| Four times a year | 4 |
| Twice a month | 1 |
| Three times a year | 2 |
| Varied by stage of study | 8 |
| Varied by role | 7 |
Eight described how the frequency of meetings varied with the stage of the trial, with greater frequency occurring during the early stages of the trial:
During study set up and enrolment every couple of months. In follow up once every 6 months.
Initially once per month. Less frequently during the study. More frequent involvement towards the end.
Variation in frequency of contact was also associated with role. Meetings with members of the TSC were most commonly reported to occur every 6 months (71%, 45 of 63); 24% (15 of 63) met more frequently than this and 3% (2 of 63) less frequently; one informant commented only that the frequency varied and did not provide an average.
Of the 20 coapplicants, 40% (8 of 20) met once a month, 50% (10 of 20) met every 6 months and the remaining 10% (2 of 20) specified a frequency greater than once a month.
Of the 23 with TMG members, 43% (10 of 23) met monthly, 22% (5 of 23) met every 6 months, three met three times a year and three met once every week or two.
Of the 10 DMC members, four met monthly, four every 6 months, one yearly and one twice a month. A high frequency of meetings (more often than 6-monthly) is unlikely for a DMC unless it is a high-risk trial, suggesting the potential for confusion among respondents between the different committee titles and remits.
Of the 16 involved as PPI advisory groups, three met monthly, six met every 6 months and the remaining seven described variable frequencies.
The majority (59%, 45 of 76) did not experience any problems related to including PPI in the trial (question 13). The most commonly reported problem was inability of the PPI contributors to attend meetings (24%, 18 of 76). Other problems were related to finding suitable contributors, the contributors not fully undertaking their role, and training and support for the PPI contributors.
Of interest is that 80% of CIs (60 of 76) felt that researchers should be trained to help them support PPI contributors (question 14).
Patient and public involvement by trial stage is provided in Table 18. Few reported involvement in analysis and just over one-third reported it for dissemination.
| Area | Yes (%) | No (%) |
|---|---|---|
| Trial setup | 56 (73.68) | 20 (26.32) |
| Trial conduct | 62 (81.54) | 14 (18.42) |
| Analysis | 9 (11.84) | 67 (88.16) |
| Dissemination | 28 (37.33) | 47 (62.67) |
The most common areas of PPI contribution during trial setup were in considering the burden of patient participation and designing or commenting on the patient information sheets (question 16). Thirteen provided free-text comments describing ‘other’ areas of contribution. These are provided in Table 19 along with the closed response selections.
| Free-text provision | Selections from Q16 categories |
|---|---|
| He provided a very helpful discussion of a number of aspects of the design and on the protocol | 8 |
| . . . contributing to protocol on un-blinding at certain point of follow-up after primary endpoint | 2–5 and 8 |
| The lead PPI rep attended trial development group meetings and so had input on all design issues. The other PPI members were brought in later and mainly advised on the trial materials (particularly PILs) and ongoing issues | 1–6 and 8 |
| Length and spacing of intervention | None selected |
| Had a lot of input in these areas in pilot stage not in the main trial | 2–4, 6 and 7 |
| One charity member was a regular attendee of team meetings and was therefore involved in all planning and monitoring of the trial. It’s difficult to identify any stage in which he was not involved. In addition there was a patient of the relevant age group who also commented on plans | 1–8 |
| The [professional] representative took part in all discussions as a co-investigator, but also we had separate meetings to discuss some of the above points more specifically | 3, 4, 6 and 8 |
| Giving feedback on the intervention during a pilot phase | 2 |
| Developing patient information leaflets and communiques to patients. Explaining the study outcomes to participants | 3, 5 and 6 |
| . . . commenting on the whole study. We added to the study | 3 and 8 |
| Developed research question – not just ‘helped’ | 1–8 |
| . . . helped with ethical application, spoke to commissioners re Excess treatment costs | 1–8 |
| A key member of the trial steering committee | 1, 3, 5, 7 and 8 |
The most common areas of PPI activity during trial conduct (question 19) were troubleshooting recruitment, attending meetings and raising the trial profile. Of the nine respondents who reported PPI in the analysis, four stated they were yet to reach that stage of the trial. The free-text comments of the remaining five are provided below:
Emerging findings were presented to [patient advisory group] whose feedback helped us interpret study results and disseminate study findings.
They read and commented on the analysis, discussed whether the correct assumptions had been made about the data. Helped understand what the data meant.
Was involved in the close out meeting and gave feedback on data quality assurance processes.
Commenting on what they thought the analysis meant.
As a co-applicant.
The CI opinion of impact of PPI in each of the areas of PPI contributions is summarised in Table 20. High impact occurred more frequently in trial setup, with low or no impact being more common during trial conduct, analysis and dissemination.
| Area | High (%) | Moderate (%) | Low (%) | None (%) |
|---|---|---|---|---|
| Q17 setup | 15 (26.79) | 30 (53.57) | 10 (17.86) | 1 (1.79) |
| Q20 conduct | 9 (14.52) | 27 (43.55) | 24 (38.71) | 2 (3.23) |
| Q22 analysis | 1 (12.50) | 3 (37.50) | 3 (37.50) | 1 (12.50) |
| Q24 dissemination | 5 (17.86) | 14 (50.00) | 7 (25.00) | 2 (7.14) |
The free-text responses provided to questions on impact (questions 17, 20, 22 and 24) are provided in Appendix 3 along with the level of impact selected.
Patient and public involvement contributors survey
Thirty-two PPI contributors from 28 trials completed the survey (two respondents each for four trials). Of these, 31 were contacted about the survey via the CI of the study and one was identified as a result of the NIHR HTA programme letter to TSC chairs. All PPI contributors who were successfully contacted to complete the survey did so.
As identification of PPI contributors could largely be achieved only via the CI, we considered respondent bias by whether or not there was an association between a PPI response and the CI opinion on PPI as categorised by CI response to question 2 on the CI survey. The results are provided in Table 21 and no association was evident (p = 0.93).
| Status | PPI should always be incorporated, n (%) | PPI not always necessary, n (%) | Not convinced of the benefits, n (%) |
|---|---|---|---|
| No response | 28 (52.8) | 22 (41.5) | 3 (5.7) |
| Response | 14 (50.0) | 13 (46.4) | 1 (3.6) |
The results of the survey are provided in Appendix 3, Table 30. The majority of contributors were approached personally by a member of the research team (66%). Just over half were involved during the development of the application for funding, a percentage largely consistent with the results of phase 1. The most frequently indicated motivations behind undertaking the role were personal experience of the disease, general interest in the topic or research, wanting to help and involvements with charities. One-quarter of respondents cited their previous experience of providing PPI as a motivating factor (question 4), with 41% indicating that they had previous experience.
Half of the respondents indicated that they were the only person providing PPI (question 6). This is not consistent with the results of the CI survey but may be explained by the level of interaction between PPI contributors in different roles. The majority of respondents (72%) indicated being on the TSC (question 11), with approximately one-fifth of respondents being on TMGs or being coapplicants. Contact (question 12) was once every 6 months for 41%, a frequency consistent with membership on a TSC. Only four respondents indicated receiving any training to assist them in their role (question 15). One-quarter indicated receiving peer support from other PPI contributors (question 18), with 41% indicating this came from a member of the research team. Sixty-nine per cent were not aware of any resources available to them (question 19) and 59% received feedback from the research team on their involvement (question 21).
Over 60% of respondents felt they had the right level of involvement (question 22). No one indicated they would have preferred to be less involved, but 16% wanted increased involvement and 22% wanted a targeted approach to areas of involvement.
The various areas of involvement are summarised within Table 22 for ease of comparison across the areas respondents were interested in undertaking before the trial started (question 13) and during the trial (question 14), the areas in which they would have liked to have received training (question 17) and areas where they felt their contribution made a difference (question 20). There was a general consistency within each area between interest before and during the trial, and where they felt their contribution made a difference. Of interest is that fewer than 60% indicated an interest in outcomes and patient information sheets, areas commonly associated with PPI. A higher proportion of PPI contributors felt their contribution in piloting questionnaires or assessments made a difference, despite the lower proportion who indicated it as an area of interest. This was reversed for data collection, where nearly one-fifth indicated it as an area of interest before the trial but only 3% felt it was an area in which they had made a difference. Although this was also the case for dissemination, a number of respondents had not yet had the opportunity to make a difference because of the stage of the trial.
| Aspect | Q13a (N = 32), n (%) | Q14b (N = 32), n (%) | Q17c (N = 32), n (%) | Q20d (N = 32), n (%) |
|---|---|---|---|---|
| Setting research priorities | 11 (34.4) | 8 (25.0) | 5 (15.6) | 8 (25.0) |
| Developing the research question | 9 (28.1) | 10 (31.3) | 8 (25.0) | 9 (28.1) |
| Outcomes to be measured, including selection and development of questionnaires | 19 (59.4) | 15 (46.9) | 10 (31.3) | 13 (40.6) |
| Piloting of assessments or questionnaires | 7 (21.9) | 10 (31.3) | 6 (18.8) | 12 (37.5) |
| Method of randomisation | 1 (3.1) | 1 (3.1) | 4 (12.5) | 1 (3.1) |
| Designing or commenting on participant information materials | 18 (56.3) | 17 (53.1) | 5 (15.6) | 18 (56.3) |
| Troubleshooting recruitment issues | 8 (25.0) | 8 (25.0) | 3 (9.4) | 7 (21.9) |
| Active involvement in recruitment/consent process | 2 (6.3) | 2 (6.3) | 6 (18.8) | 2 (6.3) |
| Data collection | 6 (18.8) | 4 (12.5) | 4 (12.5) | 1 (3.1) |
| Data analysis | 5 (15.6) | 5 (15.6) | 4 (12.5) | 5 (15.6) |
| Visit schedules (frequency of participant visits to the clinic) | 4 (12.5) | 2 (6.3) | 1 (3.1) | 3 (9.4) |
| Length and nature of follow-up | 7 (21.9) | 4 (12.5) | 5 (15.6) | 7 (21.9) |
| Trial marketing and publicity | 8 (25.0) | 9 (28.1) | 1 (3.1) | 8 (25.0) |
| Dissemination of trial findings to research participants or the wider public | 11 (34.4) | 8 (25.0) | 5 (15.6) | 8 (25.0) |
| Q13: Was not aware of the options | 0 | NA | NA | NA |
| Q14, Q17, Q20: None | NA | 0 | 12 (37.5) | 3 (9.4) |
| Other | 8 (25.0) | 7 (21.9) | 9 (28.1) | 8 (25.0) |
Respondents were asked what they would change about their experience of providing PPI within this trial (question 28). Of the 29 responses, nine stated that they would not make any changes. Three commented on logistical factors that could be improved, for example ‘Have the meeting later in the day given that they are held 80 miles away from my home’. Three would have had earlier involvement:
I would have been more use had I joined the trial at the outset. I feel I am more useful in the new trial, because I have been able to help guide patient information from the start, rather than coming in half way through when documents had already been prepared.
Two commented on increasing the level of involvement: ‘It would have been nice to have been more involved and to feel that this was wanted’. Five suggested training and support: ‘Greater awareness of my role within the trial and some training to aid this’. One individual commented on an aspect related to their ability to ‘represent’ trial participants:
In some ways I was a ‘proxy’ for the voice of the [participant demographic] this trial was targeting. To be genuinely participatory, it would have been good to have [someone within the demographic] as a PPI rep, but that may have involved a change of methodology for the group. I felt I had to fit in with the way the steering group was designed to run, rather than the group being adapted to accommodate PPI.
One commented on financial reimbursement and one on ensuring inclusion in circulation of finalised documentation. Three responses provided were not applicable to the question.
The majority (78%) felt engaged in the research project and valued as a member of the team (question 23), with all respondents recommending the role to others (question 29).
Key points
Chief investigator survey
-
Half of respondents thought that PPI should always be incorporated in a research study, with the majority of the remainder believing that it could be beneficial but was not always necessary.
-
The majority of respondents reported more than one motivation for including PPI; it being ‘the right thing to do’ was the most common reason.
-
Trials included PPI contributors with various backgrounds, with patients, carers and parents being the most common contributors; where charity members or medical staff were involved in a PPI capacity the majority also incorporated other contributors.
-
The most common reason for selecting PPI contributors was that their demographics made them ‘representative’ of the patient population. However, selection was based on prior experience and knowledge, previous engagement, or connections to charities or organisations. These characteristics are unlikely to make them ‘representative’.
-
Nearly half of respondents indicated PPI activity being within a single approach, with the majority of this restricting involvement to membership on the TSC. Membership on TSCs was associated with lower frequency of contact, potentially suggestive of ticking the funder’s requirement box or taking the funder’s lead on how to implement PPI.
-
The most commonly reported problem was an inability of the PPI contributors to attend meetings (24%, 18 of 76). Other problems were related to finding suitable contributors and the contributors not fully undertaking their role.
-
The most common areas of PPI contribution during trial setup were in considering the burden of patient participation, and designing or commenting on the patient information sheets.
-
The most common areas of PPI activity during trial conduct were troubleshooting recruitment, attending meetings and raising the trial profile.
-
High impact occurred more frequently in trial setup, with low or no impact being more common during trial conduct, analysis and dissemination.
-
The majority of CIs felt that researchers should be trained to help them support PPI contributors.
Patient and public involvement survey
-
The majority were approached personally by a member of the research team (66%).
-
Just over half were involved during the development of the application for funding.
-
Over 40% indicated previous experience of providing PPI. Motivating factors included personal experience of the disease, general interest in the topic or research, wanting to help and involvements with charities.
-
Many were the only person providing PPI, commonly as a member of the TSC, and a fifth indicated being on TMGs or being coapplicants.
-
Training was rare, with support from other PPI contributors or a member of the research team being more common. There was a lack of awareness of available resources.
-
Opportunities for contributor peer support may not be being maximised within a trial or between trials.
-
Areas of interest to contributors at the start of their involvement did not always match areas which researchers commonly associate with PPI, such as outcomes and patient information sheets, or match areas in which they later felt they had made a difference.
-
Contributors generally felt they had the right level of involvement; however, some indicated they wanted increased involvement and just over a fifth indicated they wanted a targeted approach to areas of involvement potentially to match their areas of interest.
-
The majority felt engaged in the research project and valued as a member of the team, and all recommended the role to others.
Chapter 5 Interviews: results from phase 3
We recruited informants from 28 trials. For nine trials we interviewed both the CI and a PPI contributor(s), and for five of these trials we interviewed the TM too. The informants interviewed, the trial settings and the intervention types are provided in Appendix 2, Table 33.
The flow diagram in Figure 5 illustrates the recruitment of CIs and PPI contributors. Of those invited for interview we interviewed 21 of 41 (51%) CIs, and 17 of 29 (59%) of PPI contributors participated. Regarding TMs, out of the 28 participating trials, one trial did not have a TM at the time of our study and we were unable to obtain contact details for TMs within three trials. We invited the remaining 24 TMs for interview; of these, nine did not respond, five declined and 10 (42%) were interviewed.
FIGURE 5.
Flow diagram illustrating CI and PPI contributor interview recruitment.
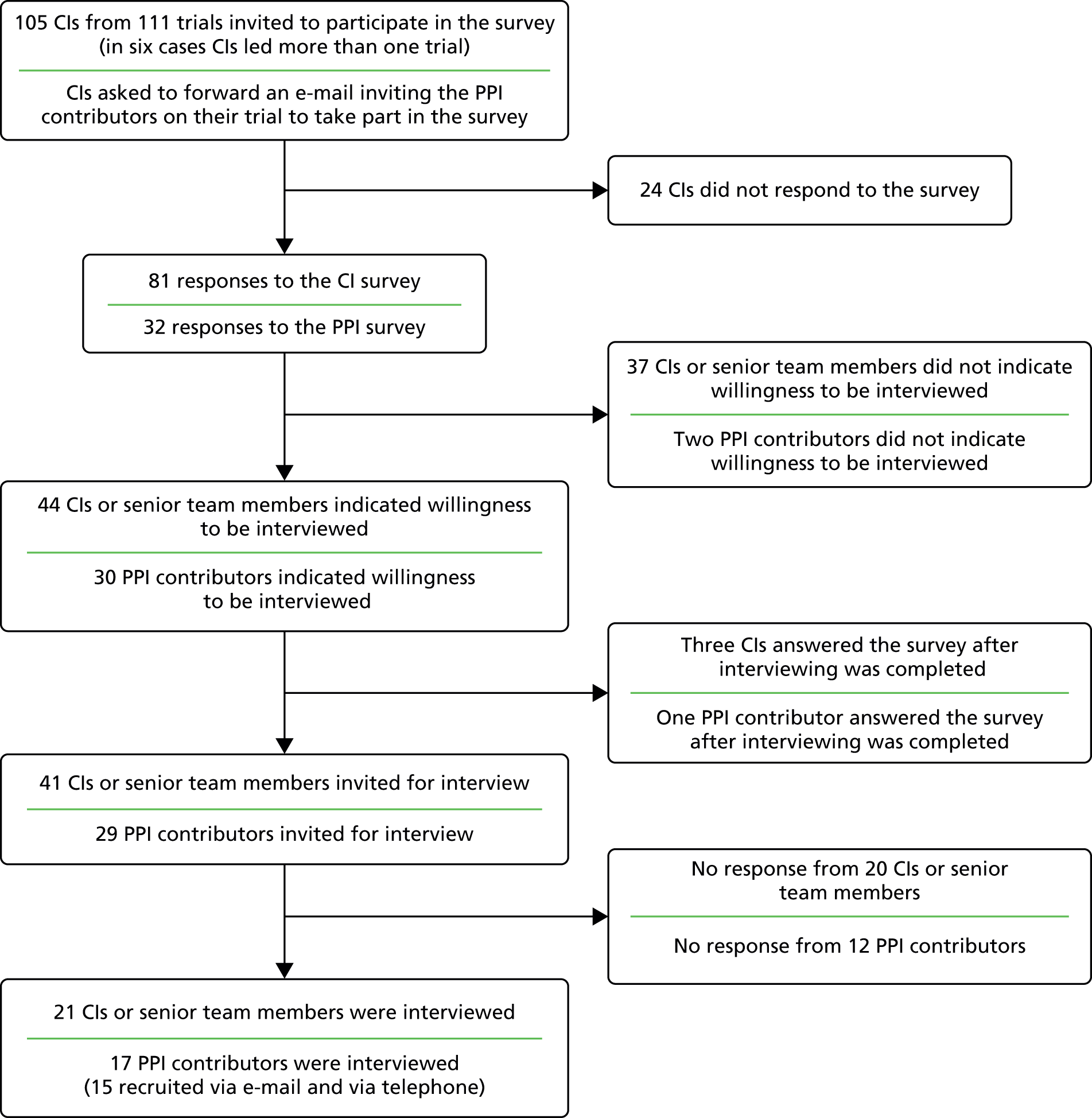
Appendix 2, Table 28 shows the survey responses that informed initial sampling of CIs and compares responses to these questions for the qualitative interview subsample with the wider sample of surveyed CIs. Of the 32 PPI contributors who responded to the survey, 31 were accessed via CIs and one via the chairperson of a TSC. We did not recruit any PPI contributors through the online advert. On average, interviews with informants lasted for 45 minutes. The results of the interviews were used to consider two key topic areas: impact of PPI and training.
Impact
None of our informants identified PPI as having an unfavourable impact on the trial. Of the 21 CIs interviewed, 14 described PPI as having an impact and seven explicitly stated that they felt PPI had not made an impact. Of the 17 PPI contributors, 11 reported that they felt their input had made a difference to the trial, three explicitly indicated that they had made no impact and three could not identify impacts arising from their input. Of the 10 TMs, five described PPI as having made an impact and five did not identify any impacts of PPI. When we triangulated the accounts of researchers and PPI contributors from the same trial, they were largely in agreement about the perceived impact of PPI. Divergences between the groups could largely be attributed to informants being unable to remember specific PPI contributions because a trial had started ‘a long time ago’.
Based on the accounts of informants who perceived PPI as having an impact, we distinguished two main types: focused and diffuse (Table 23). Focused impact comprised PPI contributors’ input that, from the perspective of the informant, changed or influenced an aspect of the trial, whereas diffuse impact comprised PPI contributions that influenced the way researchers thought or felt about the trial. In addition to the examples in Table 23, focused impact included PPI contributors helping to choose the primary outcome for the trial and increase recruitment through their contacts and networks. As Table 23 indicates, diffuse impact largely entailed interactions between researchers and PPI contributors that helped to reassure the research team and increase or maintain their confidence and motivation for the trial. For example, both researchers and PPI contributors described how a PPI contributor’s presence kept the research ‘grounded’ and reminded them ‘what it’s about.’
| Focused impact | Diffuse impact |
|---|---|
| We got rid of whole scales [. . .] it definitely had an effect on our response rates and probably attrition I would thinkTM 3It probably made some of the questions easier to understand [. . .] and therefore it would have improved the data collectionCI 3The intervention acceptability, [. . .] hopefully the effect of the intervention should hopefully be maximised by tailoring it to the needs of the communityCI 1The ethics committee were saying that they didn’t want women to be phoned up [. . .] the professor asked me to provide [. . .] a customer’s point of view [. . .], she came back later to say that on the basis of [my letter] the ethics committee had changed their minds and now agreed that follow-up phone calls could be held [. . .] [it] could have affected the full outcome of the trial had they not agreed to do thatPPI 11-1 | For me the main contribution is that they remind us all the time what it’s about. So we don’t lose touch with what our patient population is or what the trial’s about actuallyCI 20They were great [. . .] it made it sort of like a real, researching something that was quite real and felt like it was importantCI 7I think it does make the academics stop and think [. . .] I think that’s a good thing because I think the academics can get a bit too bogged down in their acronyms and the stats and things and actually forget that there’s people at the end of itTM 18[If] academics go off into a corner developing a piece of research that’s totally irrelevant to the real situation, and they never talk to patients or carers or the public about it [. . .] the original purpose may be lost in a way; what are we doing this for?PPI 22 |
Informants who reported no impact either explicitly stated that they felt PPI had not had an impact, or were unable to identify a PPI contribution that had led to a change in the trial or influenced the researchers:
Within that TSC I can’t remember them making any particular contribution that changed the way we run the, ran the study. And if they had made a substantial contribution I would remember.
CI 10
She reviewed all the paperwork, she came to the meetings, but did she actually change the trial in a meaningful way? Well no um probably not.
CI 7
Informants who explicitly reported no impact from PPI spoke only of the lack of focused impact. None of these informants referred to diffuse impact when speaking about the absence of PPI influence on the trial, whereas many informants who reported that PPI had made an impact identified both the focused and diffuse types and their accounts did not deem one more important than the other. Therefore, we use the term ‘impact’ to refer to both types.
Influences on impact
We identified two main influences on whether or not informants perceived PPI as having an impact: whether or not CIs expressed any personal goals and plans for PPI aside from its perceived role in leveraging funding; and the quality of the relationship between the PPI contributors and the researchers.
Goals and plans
Chief investigators who reported having goals and plans tended to perceive PPI as having an impact, and PPI contributors on the same trials corroborated this by describing the differences that their involvement had made. CIs’ goals and plans for PPI included their ideas about what they wanted to achieve by including PPI in the trial (beyond complying with funders’ requirements), what the contributors’ role would be and what activities PPI contributors would be involved in. For example, one CI expected PPI to:
Input into the choice of measures used to evaluate the intervention [. . .] the tools to make sure that they’re workable, that they capture the things that are important to the service users, and I’d expect it to help with interpreting the findings.
CI 3
Eleven CIs reported having goals and plans for PPI and seven of these felt PPI had had an impact. Nine reported having no goals and plans and only two of these felt PPI had an impact. CIs’ goals and plans also linked to their ideas about how to choose PPI contributors (e.g. if the PPI contributor should be a patient or charity member and how experienced they should be) and what stage(s) of the trial the PPI contributors should be involved in. Researchers who reported impact explained how PPI needed ‘to be done in a way that means it’s central’ (TM 3) to the trial, and emphasised the importance of planning what they wanted to achieve from PPI and how to achieve it.
Planning it from the beginning really. So thinking what do we really want to get out of this, what input do we want to have and how do we want to involve them, um, because if you can involve them in more of the trial [. . .] get more input from them and make it more meaningful, then you’re going to get sort of a better outcome.
TM 27
In contrast, researchers who lacked goals or plans for PPI spoke of their lack of experience of PPI and limited understanding of the function and process of PPI. Their motivation to include PPI was primarily to comply with the requirements of funding and they gave little priority to considering what PPI might achieve or how to implement it. Some also openly reflected on how, by not having any goals or plans for PPI in their trial, they could be accused of ‘tokenism’ or of following a ‘tick box’ approach:
It was a degree of tokenism and I say that completely openly that we felt we should [. . .] actually none of us knew quite why or what the patient’s role would be or how it would work out [. . .] there was a patient who we knew who’d already had the [condition] that we were seeing, so let’s get them involved.
CI 5
If I’m honest, I don’t think there was much planning for PPI. I think it was really more a matter of when we were putting together the trial steering committee, we said, ‘oh we should have a patient representative, let’s see who we can find’.
CI 6
These two CIs did not report any impact from PPI. While commenting that PPI had made little difference within their trials, researchers also acknowledged, vaguely and in some cases reluctantly, that their interactions with PPI contributors had been a ‘positive experience’ or they implied that the research team could have done more to try to ensure that their trial was able to benefit from PPI:
It’s required by the funder and therefore it would help me achieve my goal of getting the study funded. Um, it’s clearly politically required [. . .] we’ve had fairly positive um, a very positive experience of the patient reps [. . .] but I don’t think [they] have added very much. But they’re there, we can say they’re there [. . .] ticking a political box.
CI 2
To be honest, we probably didn’t think about it and discuss it as much as maybe we should have to get the full, utilise it as much as we possibly could. I think our funders, we have to have a lay member as part of our trial steering committee.
TM 5
How CIs spoke of the value of PPI seemed to be related to whether or not they had goals for it. For example, a CI whose goal was for PPI to support recruitment emphasised how important it was to researchers working in a particular disease area to engage with patients with that disease: ‘one of the things that I’ve tried to convey is that I think that when you are studying pathology, to talk to the patients that have that pathology is extremely important’ (CI 21). This account stood in marked contrast to others who described PPI as ‘political correctness’ and reported having no goals beyond using it for ‘getting the study funded’:
It’s almost become an industry, um and I think everybody has just joined on in saying you need PPI for all of these studies. I have to say I’m not convinced.
CI 2
Researchers whose accounts indicated they did not value or have goals for PPI tended to implement it mainly in one way: by including PPI contributors on TSCs. In the next two subsections we elaborate how the roles of PPI contributors and the stage of the trial at which PPI was implemented seemed to mediate the relationship between PPI goals and plans, and the perceived impact of PPI.
Oversight, managerial, and responsive patient and public involvement
Researchers identified several different roles for PPI contributors. We grouped these into three main types: oversight, managerial and responsive. An oversight role usually entailed one PPI contributor having formal involvement in the trial on multiple but infrequent occasions, for example as a member of the TSC or DMC meeting 6-monthly or annually. A managerial role was also usually formal and entailed one PPI contributor being involved on a more regular basis, for example as a coinvestigator or as a member of the TMG. Responsive PPI was often impromptu and more informal than the other two types, and often involved researchers approaching PPI contributors as difficulties arose: ‘when we had a problem, I went back to her and said, can you please comment on these questionnaires because it’s taking too long for people to fill them in’ (CI 3). Responsive PPI also included contributors advising on patient information sheets, troubleshooting recruitment difficulties and tailoring interventions, methods of data collection and follow-up to the needs of patients. Of the 21 trials for which a CI was interviewed, eight implemented one type of PPI, nine implemented two types and four implemented three types, giving 38 instances of PPI across the trials. Each CI described the perceived impact for each type of PPI they implemented.
In general, researchers who had experience of more than one type of PPI tended to favour informal, responsive approaches. One CI spoke of how ‘formal’ PPI, where PPI contributors had an oversight role, did not work as well as ‘informal’ PPI, where researchers approached PPI contributors on a responsive or ‘as required’ basis:
Comparing the two trials that we have, where we have formal and informal, I do think so, the informal arrangements worked very well.
CI 1
Out of the 21 trials, nine included responsive PPI, and researchers from six of these trials felt that this PPI had an impact. While researchers tended to associate responsive PPI with perceived impact, this may be because they tended to have very defined goals for this type of PPI, making it easier to link PPI input and impact. In addition, responsive PPI usually involved more PPI contributors than the other types, and researchers emphasised the importance of accessing a ‘plurality of opinion’ and doing so as and when questions or problems arose. Therefore, it could be that researchers associated responsive PPI with impact because it was particularly helpful in identifying strategies to address problems. It could also be that contributions from this type of PPI carried more weight with the research team because it allowed access to a more diverse range of contributors, who were seen by researchers as more ‘representative’ of the target population, than the PPI contributors who had oversight and managerial roles:
Rather than just having members of the committee [. . .] having more lay reference groups that we can refer to [. . .] you need a broader pool of people to advise on this to make sure you get a really sensible reality check.
CI 10
Patient and public involvement contributors who had oversight and managerial roles sat on committees and attended formal and scheduled meetings. Whereas some contributors were able to recall specific contributions that they made during these meetings, researchers often struggled to pinpoint what particular individuals had contributed in meetings: ‘when you’ve got, um, meetings like that [. . .] unless you did a full analysis, it would be difficult to tell what the impact was of any individual’ (CI 3). Nevertheless, managerial PPI was associated with impact more often than oversight PPI. Of the 23 trials that included oversight PPI, researchers and PPI contributors from seven trials reported that they felt PPI had made a difference. Of the 13 trials that included managerial PPI, researchers and PPI contributors from nine trials reported that they felt PPI had made a difference. Its relative lack of impact could be linked to the infrequent contact between the PPI contributors and the research team, whereas managerial PPI contributors would usually be present when particular problems were discussed and could offer immediate advice.
Stage of patient and public involvement implementation
Both researchers and PPI contributors pointed to how it was important to implement PPI early during the course of a trial before plans were set in stone and so that PPI contributors had opportunities to develop a sense of ownership of the trial. We categorised early involvement as PPI activity that was implemented around the time that the first meetings about a trial were held and before the final funding application was submitted. Of the 28 trials, 16 had PPI contributors that had been involved at an early stage, four had PPI involvement after funding only and eight had a mixture of some PPI contributors being involved at an early stage and others joining after funding was confirmed. Informants were less likely to report impact from PPI that had been implemented after funding. Both researchers and PPI contributors emphasised how PPI contributors were better placed to contribute at an early stage and also towards the end of a trial, whereas opportunities to influence a trial mid-course were seen as limited:
It’s more just that my involvement was probably more useful at the beginning, and I think it will probably be more useful at the end when we get into the interpretation of data and how it’s going to be probably disseminated. But the middle, the middle bit is fairly technical.
PPI 9
Decisions may have been made that are difficult to change [after funding] that, from a patient’s perspective, may be wrong.
PPI 6
So more input at the beginning in the choosing the research question and at the end in terms of dissemination, and less actually in the day-to-day running of the management of the trial, which for most patients is um, I think it was a bit of tokenism.
CI 5
Relationships
Both CIs and PPI contributors spoke of how it was important to invest time and effort in forming a relationship so that PPI contributors felt part of the team. In turn, PPI contributors who reported feeling part of the team tended to report an impact from their involvement, in contrast to those who did not feel part of the team. Of the 17 PPI contributors, seven indicated or implied that they felt part of the team, and all of the seven felt their involvement made a difference. Of 10 PPI contributors who explicitly stated or implied they did not feel part of the team, only four felt their involvement had made a difference. PPI contributors who felt they were part of the team tended to describe their relationship with researchers as a ‘partnership’ and spoke of how they were ‘treated as equals’ (PPI 7). Both CIs and PPI contributors spoke of how feeling part of a team empowered PPI contributors to voice their perspectives in interactions with the research team:
Build a relationship with them a little bit as well so that they are comfortable and confident. Because I think probably it could be a little bit daunting, sitting around a table with a whole pile of professionals and experts.
CI 6
It was actually quite an engaged process and I felt very much part of the team, rather than just somebody sitting on the outside who occasionally was asked for their view. So I felt I was very able to sort of shape and steer the project as well.
PPI 3
The stage of the trial at which PPI contributors became involved, and the frequency of their contact with the research team, influenced whether or not PPI contributors felt a part of the team. As noted above, PPI contributors described how they could make more of a contribution when they were involved in the early stages of a trial, whereas becoming involved later in the course of a trial made it hard for PPI contributors to develop a relationship with the research team:
If a patient comes in at a later stage, the group has jelled so [. . .] you could become an outsider because the group is already formed.
PPI 6
One PPI contributor, who did not identify any impacts arising from his role within the trial he was currently involved in, compared that particular trial, where his involvement had not started until after the funding application had been submitted, with other trials, where he had been involved from the beginning:
If you come in late, or pick up from someone else, then it’s maybe not as easy as whether it’s something that you’ve been involved in from the application stage forward, and the relationships are formed.
PPI 9
Similarly, a CI who could not identify any impact of PPI and was ‘never convinced’ that the PPI contributors ‘felt’ part of the team commented:
I think it’s much harder to actually express their views as they go along and it doesn’t feel like a genuine partnership.
CI 12
Training for patient and public involvement contributors in clinical trials
Induction and support: informal and implicit
We wanted to explore how informants conceptualised ‘induction’ and ‘support’ within the context of PPI without constraining their responses, so we did not define what we meant by these terms during the interviews. Most informants characterised induction as a one-off informal ‘conversation’ between PPI contributors and researchers, rather than a ‘formal’ or structured process. Inductions typically involved researchers discussing with PPI contributors what researchers ‘saw their input might be’ (CI 2) and ‘what the study was about’ (TM 8), with PPI contributors being ‘given all the data, the explanation of what the trial was about’ (PPI 6). Therefore, while both researchers and PPI contributors tended to describe ‘induction’ as an informal, unstructured encounter, their accounts implied that it was mostly a one-way exchange, with researchers positioned as providers of inductions and PPI contributors positioned as recipients. Neither party spoke of entering into a negotiation at this stage about what the role of the PPI contributors would entail.
Most informants reported that PPI contributors had received an induction. When PPI contributors had not received an induction from researchers, informants explained the PPI contributor had previous experience in a PPI role. Researchers added they had assumed an induction was superfluous. However, PPI contributors who reported not having received an induction indicated that it would have been useful ‘to get a bit more of a sort of formal induction at the beginning about what the trial was, what my role was going to be’ (PPI 9).
Similarly, informants described a low-key approach to the support for PPI contributors, emphasising how PPI contributors knew they could contact researchers for support and advice if they needed to, although it was rare for them to do so:
We did not say ‘look we will give you support in this if you want it’, but I know that they had free and instant access to me. I would get e-mails and I would always respond, so even though it [support] might not have been explicitly stated, it was implicit.
CI 13
We always organise all her train travel and that kind of thing for her [. . .] she had my e-mail; she had my phone number and things, and the same for the project administrator. So she always knew that she could contact us if she needed to.
TM 27
I haven’t taken up much of it [support] but I know that the team, [name of CI] and his assistants have been there if I’ve needed to contact them, which I haven’t really, but I know that they’ve been there.
PPI 2
Therefore, ‘offers’ of support for PPI contributors from researchers were largely implicit. In their interviews, researchers spoke of their readiness to provide support, and contributors of a sense that support was available if needed, although it seemed that both parties rarely discussed such support overtly during the course of PPI activities. Moreover, most informants saw support largely as synonymous with practical or logistical help, such as assistance with expenses or travel arrangements, rather than ongoing support to contributors in core aspects of their roles. From our analysis of CIs’ accounts, these roles included PPI contributors providing advice on patient recruitment and information materials, choice of outcome measures and the acceptability of the trial design. Although some PPI contributors felt unclear about their role, only one spoke of accessing support to address this:
I was in a learning process myself not knowing exactly what I could do, and I kept returning things to the professor saying, ‘I’ve been a bit pedantic here’ [. . .] and she would say, ‘Pedantic is what I’m looking for, we need to have things pointed out to us that we may not have noticed.’ So it was me learning how to approach the situation.
PPI 11-1
As well as emphasising the informality of the induction process and the implicit nature of support, as we explain in the following sections, both PPI contributors and researchers spoke of their preference for informal ways of learning about PPI over more formal or structured training, particularly for PPI contributors.
Training
Mirroring their accounts of induction, informants often drew a distinction between formal training and informal ‘conversations’ about the trial. Most informants tended to conceptualise ‘training’ as ‘formal’, structured activities. A summary of the training needs that were identified by researchers and PPI contributors can be found in Table 24.
| Reported by | Training for researchers | Training for PPI contributors |
|---|---|---|
| Researchers |
|
|
| PPI contributors |
|
|
Researchers on their own training: useful to a point
Of the 31 researchers, 18 had not had any form of training in PPI, whereas 13 had accessed training or attended presentations or workshops on PPI covering topics such as how to incorporate PPI into research, run focus groups or identify and engage PPI contributors. Researchers spoke of how these activities had been useful in discovering how to do PPI, what had worked for others and how PPI can benefit research. However, one CI commented that his training has been too focused on ‘how PPI should work’ and that insufficient emphasis had been given to ‘the practicalities‘ (CI 5).
Of the 18 researchers who had not received training, nine indicated that they would like to receive training and nine expressed reluctance. CIs who wanted training explained that they would like guidance on how and when to involve PPI contributors, how to optimise their input, what is expected of PPI contributors, how PPI benefits research and payment for PPI. Additionally, one TM wanted to learn about the wider research community’s expectations regarding the implementation of PPI:
A little bit more on how we can work together, patients and researchers, and how we can benefit from having a strong PPI involvement in this study [. . .] I’d like to learn a bit more on how things should be done or how they are expected to be done.
TM 21
Of the 21 CIs, six had no experience of PPI prior to the current trial. Researchers who did not want training commented that they already knew ‘how to do PPI’ because they had learnt about it ‘through experience rather through any particular formal training’ (CI 5).
Although researchers, particularly CIs, generally described one of the main challenges of PPI as finding ‘suitable’ people, they did not identify this as a training need. We did not find any evidence that researchers’ views on the value of training were influenced by their views on the value of PPI. For example, some researchers who were sceptical about the value of PPI felt that training would be useful for certain topics. Conversely, some researchers who spoke of PPI as important were reluctant to receive training because they could not envisage what topics the training would cover, or felt that there was insufficient knowledge of PPI to inform training:
Hopefully your research project will come out with sort of clearer guidelines about when and where PPI input is useful, because I’m never quite sure myself [. . .] I wouldn’t be keen on any training at the moment.
CI 15
Patient and public involvement contributors on training for researchers: useful to address specific difficulties
Of the 17 PPI contributors, nine felt that researchers should receive training in PPI. Contributors pointed to the use of jargon in research as one of the main challenges they faced and, reflecting this, indicated that researchers could benefit from training on the importance of using plain English and avoiding jargon, although one qualified this by emphasising that rather than formal training ‘just a note’ or ‘reminder’ would suffice. Some PPI contributors also pointed to the lack of role clarity as a challenge they faced and felt that researchers would benefit from training in understanding the public mindset, contributors’ roles and how to engage with them:
I’d be told what [. . .] the expectations of me were in the team and what are the things I was supposed to do in the team, and they [researchers] would have that explained to them as well [. . .] It would just be making sure that people understood my role.
PPI 22
Other PPI contributors felt that training researchers was unnecessary, particularly as they saw CIs as individuals who were used to ‘dealing with people’ and expected researchers to have acquired the requisite knowledge and experience or ‘at a level of um ability, shall we say, that shouldn’t need much training’ (PPI 11). A few PPI contributors also felt that interpersonal abilities could not ‘be taught, it’s a question of interface and interaction and experience with one another’ (PPI 7).
There were indications that PPI contributors’ views on training for researchers were linked to their previous PPI experience. Of the nine PPI contributors who felt that researchers should receive training, six had little or no experience of being a PPI contributor. Of the five PPI contributors who felt that researchers did not need training, four had previous experience of being a PPI contributor.
Patient and public involvement contributors on their own training: largely unnecessary and potentially detrimental
Of the 17 PPI contributors, only three had received training for their role within the EPIC cohort trial. They indicated that this training had focused on research processes including ‘basic appraisal of clinical papers’, ‘general research training’ and good clinical practice, rather than the roles of PPI contributors. All three contributors described the training as useful, although the contributor whose training had covered critical assessment of research papers focused on how it had informed her paid employment as a nurse rather than her PPI role:
I learned a lot [. . .] I’m actually able [to] apply it to other aspects of my work now because if we’re looking at products in the area I work in, I can look at the clinical papers attached to them and understand more how to read them, so it has been a help to me.
PPI 2
The remaining 14 PPI contributors did not report having received any training. Of all 17 PPI contributors, 15 indicated that they did not want or need training, with most explaining that a conversation at the beginning of their involvement to clarify their particular role was sufficient.
Our wider analyses had identified three main roles for contributors – oversight, managerial and responsive – and researchers acknowledged that a contributor’s training needs depended on the type of role they had. In oversight roles, contributor’s activities were formal and often entailed being the sole PPI member of the TSC or DMC, and their activities were formal and structured. Managerial roles were also usually formal and usually entailed one PPI contributor acting as a coinvestigator or member of the TMG. Responsive PPI roles were typically more informal and spontaneous than the other two types, with contributors being approached for advice on an ‘as required’ basis. All interviewed contributors had oversight or managerial roles within the EPIC trials and some spoke of not needing training because they had already acquired the necessary skills through their employment or previous experience as a PPI contributor. For example, a contributor who described himself as having a worked as a ‘senior manager in industry’ and whose description of his role within the trial resembled that of a project manager commented that training was unnecessary because he was:
Well used to running meetings and keep people on track [. . .] I’m used to actually making decisions and coming up with proper ways of getting things done.
PPI 22
The account of this PPI contributor stood in contrast to another contributor, who saw her role as rather more limited:
I can use a computer and I can use e-mail and things like that because of my job, [. . .] but as far as training is concerned, because my role is absolutely not to run the trial or anything [. . .] I’m not sure that training per se is necessary.
PPI 11
Despite the differing perceptions of their roles, both contributors talked of how they already possessed the skills their roles required and neither could therefore see a need for training. Some PPI contributors gave passing mention to how training may be useful for ‘other’ contributors who had less experience: ‘I didn’t need it [training]. I would suggest other PPI members [. . .] would need some training and familiarisation in how it works’ (PPI 26).
Two contributors were also concerned that training would be detrimental to providing a patient perspective. For example, training could encourage contributors to look at issues ‘from a clinician’s point of view [. . .] once you’ve learnt too much I don’t believe that you’re a lay person’ (PPI 23/24). Interestingly, these PPI contributors usually spoke of such professionalisation in general or hypothetical terms, rather than as something they struggled with personally. Concern about training leading to the overprofessionalisation of PPI contributors was also a recurring theme in the accounts of researchers and is a topic that we explore further below.
Researchers on training for patient and public involvement contributors: better to select than train
Of the 31 researchers interviewed, six indicated that training would be of value for PPI contributors on their trial, six did not express an opinion and 19 felt that training was unnecessary. Like the contributors, researchers in the third group regarded PPI contributors as already possessing the skills and experience that their roles required:
I don’t think that she [PPI contributor] was in any way reserved about contributing and I think she understood the role on the trial steering committee [. . .] because she had had a role of representing patients before.
CI 4
Informants’ accounts of training were closely bound up with their accounts of how to select PPI contributors, and both researchers and PPI contributors implied that, rather than training PPI contributors, it was often better to select individuals who already possessed the attributes necessary for their role:
It can take a lot of time to bring people up to speed with the principle of trial design. And I’m not saying that’s not necessarily a good thing to do but if somebody’s already got that experience and knowledge you’ve already overcome quite a big hurdle.
CI 9
Six researchers echoed the concerns of the PPI contributors who thought that training could overprofessionalise PPI contributors and therefore be detrimental to their role:
If you train them then I think they’re probably aware of more of what should be happening and they won’t have such an objective view, whereas if they’re coming at it almost totally fresh then they have more of the perspective of if a patient received this information.
TM 18
What you really want from them is for them to be kind of impartial and [. . .] to really represent what the patients and the public think. So if they are too kind of clued up on research they might not actually be representative of our target audience.
TM 13
A few researchers struggled to identify what the content of training would be for PPI contributors:
I would find it difficult to know what you could train people in.
CI 11
Researchers who felt that training would be beneficial for contributors on their trial pointed to general research training such as research methods and the role of PPI contributors as suitable topics. Although researchers generally tended to emphasise that the particular PPI contributors on the EPIC cohort trial did not need training, some pointed to circumstances in which training might be useful. For example, contributors who were less experienced could benefit from training, particularly if they were to have oversight or managerial roles.
Making sure that the person who is the PPI rep is comfortable and confident to ask questions and to query things [. . .] that’s maybe when training actually for the PPI, now I think about it, might be really important.
CI 6
If you’re expecting them to attend the trial steering committee, if they’ve not done that type of thing before, then I think some information and training [. . .] around the topics that are going to be come up.
TM 18
Need for ‘professional’ and ‘lay’ contributors
According to our informants, it was not just training that could lead to the professionalisation of PPI contributors; cumulative experience in a PPI role could also do this. Both groups of informants distinguished between ‘lay’ and ‘professional’ PPI contributors, although the latter were not necessarily individuals with professional employment backgrounds. Researchers in particular described professional contributors as people who ‘went around doing PPI’, implying that such individuals had a level of experience and knowledge that set them apart from other patients, whereas lay contributors were ‘just [. . .] people with the experience of whatever it is you’re researching’ (CI 3).
Indeed, professional contributors were believed to become ‘less and less like the population the more engaged they become’ (CI 16), increasingly influenced by the ‘researcher mind set’ and unable to contribute an authentic patient perspective. Conversely, researchers acknowledged that difficulties could arise when PPI contributors were naive to their role in research. For example, one CI spoke of a PPI contributor on a previous trial who had been ‘preoccupied by getting their personal health care improved’ (CI 7) and other researchers commented on how it was helpful to have contributors who understood the research process and the constraints on researchers:
When it works it works probably because we’ve got people who are able to understand what we want of them and are able to be quite articulate and succinct and able to separate out their own stuff from the problem on the table.
CI 20
Researchers were therefore sometimes torn between wanting the benefits that professional contributors brought and worrying that they were hardly ‘representative’ of target participants. Others spoke of how this tension could be resolved by involving both professional and lay PPI contributors in trials. Whereas professional PPI contributors were believed to be suited to managerial or oversight roles, lay PPI contributors were felt to be suited to responsive roles which required a ‘true’ patient perspective and where it was possible to ‘come along and be your self’.
I was a little bit surprised to find that there were these sort of professional PPI reps. I think it is good because they come with an understanding of research, but I think that’s where it’s really important that you have a mix of people that you’re getting views from, because I think if maybe people are too research-savvy, they’re only going to think like researchers and they’re not actually going to give us a real patient perspective.
CI 6
Selecting patient and public involvement contributors
Most researchers had identified PPI contributors through a charity or patient organisation, or indicated that the PPI contributor was previously known to them, either through earlier PPI work or as participants in a previous trial. Two CIs had PPI contributors who were their own patients. Sometimes CIs had sought or received recommendations regarding the suitability of a potential PPI contributor from other researchers, PPI contributors, patient organisations or personal contacts. Only one CI reported having sent an advert out through a patient organisation inviting people to volunteer for the role of PPI contributor.
Informants from all groups described the importance of selecting PPI contributors to suit their role. As we illustrate in Box 2, all informant groups emphasised the importance of selecting contributors who could be confident and active in meetings if they were to have oversight and managerial roles, and spoke of how PPI contributors should be motivated and have interests in the research or clinical area. Although some informants, as we note above, commented that it was important for PPI contributors to have previous experience of a PPI role, the lack of such experience could be compensated for in other ways, for example if contributors had characteristics or experiences in common with the participants to be targeted for a particular trial, or had an educational or employment background seen as relevant to their role.
You want them to be confident and capable of presenting their view. So you don’t want somebody who’s going to be shy. You don’t want somebody who’s going to be easily intimidated.
CI 9
They’ve [PPI contributors] got to be a bit of a confident person to speak up in these kinds of meetings. You’ve got lots of clinicians, they’re talking about lots of things that the patient probably doesn’t understand.
TM 5
You do have to be confident to make your point. So you’re working with clinicians, they talk quite technical [. . .] being happy to make your point can be challenging, but I do it and I’m comfortable doing it.
PPI 26
Motivation and commitment
Finding the right person who has the time and the commitment and the interest.
CI 20
Someone who’s interested in research [. . .] who’s willing to commit [. . .] So if we wanted someone on one of our steering committees they would have to be able to commit to a certain number of meetings a year and be happy to review documents in time for meetings.
TM 9
They [PPI contributors] should be passionate people that really believe that they, they want to try and make a difference.
PPI 7
Focus on the ‘greater good’
My PPI people were really good because they never, ever [. . .] brought it back down to themselves all the time [. . .] But in another study that I’ve got, we have had that and it’s become quite draining to everybody because the PPI [. . .] person has become very much preoccupied by getting their personal health care improved.
CI 7
An ability to see beyond their own particular experience and maybe draw on the experience of peers, others in the field.
TM 3
They should be people who can look outside of their immediate needs to the greater good [. . .] if I was particularly concerned about my condition then it could over-ride everything in terms of the trial. I might want to steer it towards something I’m particularly keen to have resolved or sorted. So you need to have someone who sees the trial, and understands the concept in the broadest sense. And that the benefits may not indeed help them.
PPI 26
Previous patient and public involvement experience
It’s useful [to have contributors with previous PPI experience] because they have an understanding both of the general world, if you can call it that, and also of the research field, so, and of what their researchers are expecting [. . .] It probably does help if they have had some experience before, but it’s not always necessary.
CI 1
We had people who, who had been [contributors] on other randomised controlled trials so they, they knew what we were getting at but [. . .] you’re bound to get somebody who . . . for whom it’s, it’s new but as long as [. . .] you’re not asking them to think about something that they can’t possibly have any familiarity with, then I think you can overcome the lack of methodological experience.
CI 16
Experience of the target condition, groups or of interventions similar to those being investigated
One of the most important things is that they represent the type of person that the study will be directed at. There’s no point going for a 50-year-old guy when your study is about [condition] and you’re going to be recruiting people who are under 24.
CI 19
We often recruit more broadly than just patients now. So it’s carers and people who work with frail elderly patients who often have greater time and as much insight as the patient themselves in many ways.
CI 5
It’s important that they’ve [. . .] had experience of having the type of operation that you’re investigating, or the disease area that you’re investigating, just so that they’ve got a better perspective of what is important to the patient.
TM 11
[PPI contributors should be] people that have experience of the condition [. . .] it should be condition-specific.
PPI 7
Intelligence
It’s got to be somebody who, is sort of, I don’t know if intelligent is the right word, but who has a lot of common sense basically. Obviously [they] do need to be intelligent and be able to read moderately complex stuff and understand fairly complex things. You don’t want somebody who’s not very bright.
CI 71201
You’ve got to be quite bright. I think some of these documents are quite dense, so I think that that’s important.
PPI 11
Key points
-
Well over half of the informants indicated that PPI had made a difference to the trial or influenced the trial team, and none reported unfavourable impacts from PPI.
-
Chief investigators who described goals for PPI and planned its implementation in the light of these goals tended to report impact, whereas those whose goals for PPI did not extend beyond meeting perceived funding requirements usually reported little or no impact from PPI.
-
Researchers whose accounts indicated they did not value or have goals for PPI tended to implement it mainly in one way: by including PPI contributors on TSCs.
-
Patient and public involvement contributors who spoke of having a good relationship, particularly in terms of feeling part of the team, also tended to report impact from PPI, and both researchers and PPI contributors pointed to the importance of implementing PPI before seeking funding.
-
Whether or not CIs valued PPI seemed to be linked to the goals they described and how they implemented PPI. CIs who expressed scepticism about PPI focused mainly on using PPI to meet funding requirements, whereas those who valued PPI often described in detail how it was of benefit within their trials.
-
Some researchers seem to accord little value to PPI. It also raises the possibility that this may become a self-perpetuating cycle, with such researchers implementing PPI in ways that may provide little opportunity for it to benefit randomised controlled trials and then concluding that PPI made little difference to their trials.
-
Informants had reservations about the need for training in PPI, particularly training for PPI contributors. Very few contributors had received training and many were reluctant to engage in it. Researchers shared this lack of enthusiasm for training PPI contributors, although both groups of informants welcomed informal induction ‘conversations’ to help contributors to understand their roles.
-
Induction seemed to provide little scope for contributors to negotiate their roles, and support for contributors was largely implicit and focused on practical arrangements rather than on helping contributors to function in their roles.
-
Rather than training contributors, researchers used their networks and others’ recommendations to identify and select individuals who already possessed attributes perceived as important for the role. Therefore, informants tended to see training PPI contributors as redundant because, through the way they had been selected, contributors were believed to possess the necessary attributes.
-
Informants were also concerned that training and cumulative experience in PPI roles overprofessionalised contributors and limited their ability to provide an authentic patient perspective. Researchers described a tension between needing contributors who could provide an authentic patient perspective and needing contributors who could function in oversight and managerial roles (e.g. as members of TSCs and TMGs respectively).
-
Some commented that this tension could be resolved by selecting particular PPI contributors for particular roles within a trial. Informants were more receptive to training researchers in PPI than training PPI contributors, and most researchers had either received training or indicated that they would find it helpful. Nevertheless, a sizable minority pointed to how it was sufficient to learn about PPI on the job or that evidence to inform training was lacking. Contributors also saw a fairly limited role for training researchers in PPI, although some pointed to the use of plain English and clarity about PPI contributor roles as areas in which researchers could benefit from training.
Chapter 6 Implementation of patient and public involvement: from plans to actions – results across phases 1 and 3
Challenges to the realisation of plans for PPI include debate regarding its purpose, lack of evidence regarding the impact of PPI, complexities in researchers and contributors sharing power, and difficulties in ensuring sufficient resources for PPI. 5,15,18,20–22 Alongside such challenges are uncertainties regarding how best to plan PPI. Guidance drawing on the opinions and experiences of those involved in PPI activity within trials is available21,23 and a 2011 review has examined case studies of PPI in the design and conduct of trials. 28 However, the evidence base is limited in terms of the range of trials, researchers and patients that have informed this previous work, and there has been no systematic evaluation of the extent to which triallists’ intentions for PPI are put into practice. This is an important gap in view of the above challenges and the increased onus on researchers to build plans for PPI into their grant applications. Such plans run the risk of being uninformed because of the lack of evidence across a range of trial contexts and informant perspectives.
Using the information extracted from phase 1 and phase 3, the extent to which documented PPI plans were implemented are described. Therefore, to be eligible for the current analysis at least one source of interview data was required from either the CI or the PPI contributor, as well as the grant application documents from which we identified and extracted data regarding plans for PPI. To determine the extent to which these documented plans were implemented, we focused equally on the qualitative data from the CI and PPI contributor interview transcripts. In cases of ambiguity we consulted the TM interview transcripts where available. Where multiple sources of interview data were available, for example from a CI and a PPI contributor, there were no major discrepancies between accounts. Although we conducted interviews with 10 TMs, only one TM interview was used in the current analysis to resolve ambiguity regarding whether or not all plans for PPI had been implemented. The results are split into two sections: from intentions to actions; and the challenges of implementing PPI plans.
Intentions to actions
As shown in Table 25, all but three of the 28 trials had documented plans for PPI in their grant application. These documents varied greatly regarding the extensiveness of PPI activity planned and the precision with which plans were described, from vague references to activities that hinted at PPI – ‘We will make use of two primary care research networks and an [intervention-specific] research network’ (trial 115) – to statements that were quite precise: ‘The [society] confirmed their willingness to represent their members through steering committee membership [. . .] and to help in the construction of the MREC [Multicentre Research Ethics Committee] application and patient information leaflets’ (trial 102). Based on informants’ interview accounts, all trials subsequently incorporated some form of PPI and it was clear from the interviews that documented plans were fully implemented in most (20 of 25) instances regardless of whether the plans were vague or precise, minimal or extensive. The three trials without documented plans did proceed to include some PPI activity, perhaps prompted, to an extent, by comments from peer reviewers, who had remarked on the lack of PPI plans in each case. A further three trials expanded on documented plans, giving a total of six trials which had seen addition or expansion of plans for PPI.
| Unique identifier, status (trial ended or ongoing), mode(s) | Summary of planned activitya | PPI plans fully implemented? | Accounts of ‘actual’ PPI activityb |
|---|---|---|---|
| Trials which had a chiefly oversight mode (n = 6) | |||
| 115, ended, O | Unclear whether or not trial had PPI coapplicants although service user contributed to the proposalWe will make use of two primary care research networks and an exercise research network | U | Had PPI membership on TSC but unclear in terms of ‘making use of research networks’. CI had expectations for and prior experience of PPI; no challenges. PPI contributor had prior experience of PPI; challenges (problems getting to meetings because of health) |
| 36, ongoing, O | No PPI coapplicants. Patient representative was named as a member of the TSC. In response to referee comments, applicants stated they would consider increasing the number of PPI contributors on the TSC from one to two ‘to provide mutual support’ | Y | Has two PPI contributors on TSC but CI talked of ‘no direct impact’ and ‘ticking a political box’. CI had no expectations for but had prior experience of PPI; challenges (‘only very minor such as patient rep not having email’). PPI contributor had no prior experience of PPI; challenges (jargon) |
| 65, ended, O | No PPI co-applicantsWe will have lay representation on the TSC. We will use the expertise and contacts of our panel to form focus groups to assist in the understanding and dissemination of findings | U | Had PPI membership on TSC as planned, but unclear whether or not implemented plans regarding the use of the panel/focus groups to understand/disseminate findings. CI felt no direct PPI involvement overall. CI had no expectations for but had prior experience of PPI; challenges (getting the right people engaged; difficult target population; unable to get enough early engagement to inform changes to study design). No PPI contributor interview |
| 2, ongoing, O | No PPI coapplicants. No documented plans. Did refer to PPI that had occurred prior to grant application | n/a | Has PPI membership on TSC. CI had no expectations for but had prior experience of PPI although spoke of initial ‘tokenism’ and ‘ignorance’ about what to expect of PPI in current trial; challenges (‘just the slight feeling that we were taking up her time’). No PPI contributor interview |
| 64, ended, O | No PPI coapplicantsWe have identified two people with [condition] who have agreed to be consumer reps and have advised on the development of this proposal | Y | No CI interview. Had PPI membership on TSC. PPI contributor had no prior experience of PPI; challenges (jargon, unable to attend all the meetings, some team members were felt to lack understanding) |
| 96, ongoing, O | No PPI coapplicantsA patient representative will provide input into the design of patient literature and trial presentations to a general audience as well as providing a patient’s perspective at TSC and [Data Monitoring and Ethics Committee] meetings. TSC will meet two to three times a year | Y | No CI interview. Has PPI membership on TSC. ‘Keep in contact’ approximately twice a year. PPI contributor had no prior experience of PPI; no challenges |
| Trials which included a managerial mode (n = 14)c | |||
| 20, ended, M + R | Had a PPI coapplicantThe research team will convene a steering group of research and service users. This will meet three times during the study and will provide an opportunity for the research team to consult about research design and methods for data collection, choice of outcomes and methods for data analyses. The TSC will have an important role in interpreting initial findings and developing dissemination strategies. Consultation with young people and parents will be carried out in intervention and comparison clinics using focus groups. The views gathered in these groups will inform the development of research procedures (e.g. consent, outcome measures), tools for data collection and the process evaluation. Focus groups will also provide opportunity for young people to contribute to interpretation of study findings. Further consultation with young people will involve piloting all research tools to ensure acceptability and appropriateness | Y | Had input from four PPI contributors at different times. Membership on TSC. Sought additional input when struggling with particular issues. CI had expectations for and prior experience of PPI; challenges (having a contributor who was a patient of the lead CI – ‘conflict of roles’; frustration at inability to integrate contributors’ ideas regarding questionnaire, which was a validated instrument and therefore could not be altered). PPI contributor had no prior experience except as charity member; no challenges |
| 21, ended, O + M + R | Had a PPI coapplicantUser and consumer groups have discussed the application and suggested changes to protocol which we have accepted. In the trial the groups will be asked to help with development of info leaflets, consent forms, letters, questionnaire design. The groups were very keen that a user was a collaborator on grant application. The team includes [name], a consumer representative who is chair of [Consumer Research Group], works with the [condition] Association and the [Research Network] | Y | Had PPI coapplicant. Plans expanded (in terms of recruitment, analysis, interpretation of results, dissemination). CI had expectations for and prior experience of PPI; challenges (‘poaching’ of contributors; stress about funding/paying contributors for their time if in receipt of benefits/pension; disagreement with funders regarding contributor’s activities). PPI contributor had prior experience of PPI; challenges (time; being in demand) |
| 27, ongoing, O + M + R | No PPI coapplicantsWe will include two [condition] patients to act in an advisory capacity. They will be invited to attend all collaborator meetings and quarterly trial management meetings. We will disseminate project information and findings for patients and patient groups | Y | Has PPI membership on trial management, steering and data monitoring groups. CI had expectations for and prior experience of PPI; challenges (finding contributors). Two PPI contributors interviewed had no prior experience of PPI; challenges (some doctors do not want to understand your point of view; jargon; they talk about things you have gone through as a patient in a dispassionate way) |
| 16, ongoing, O + M | Had a PPI coapplicant[Name] is Head of Policy and Research at [name of a national trust]. She has extensive experience of representing the views of the consumer in clinical research and at local and national policy levels. [She] will ensure that the perspective of the consumer remains central during all stages of the trial. Independent user representative(s) will be included on the TSC. The role of user representatives on the Data Monitoring Committee is more difficult because of the complex technical nature of the role of this committee. However, once a Chair of the Data Monitoring Committee has been appointed, we will discuss with the Chair their views about the composition of this committee, and specifically the role of users. User groups at annual [User Group meeting] have commented on the proposal and several groups have agreed to help develop the information and consent process | Y | Has PPI coapplicant. CI had expectations for and prior experience of PPI; challenges (finding the right people; consumer groups with a specific interest and so may be ‘partisan’). PPI contributor had prior experience of PPI; challenges (jargon; infrequent meetings ‘not much to build a relationship on’) |
| 5, ongoing, O + M | Had a PPI coapplicantWe have identified consumer representation from participants in our previous studies, and one, who is a grant applicant, has contributed to the development of the application, trial design and study documentation, particularly the information to be provided about the safety and efficacy of [device]. We have identified a consumer representative to ensure that patients’ views are incorporated into the design from the start. She is a grant applicant and has already contributed to the trial design and the participant information sheet. Consumer groups will ensure all relevant issues are covered, that patient information and survey instruments are acceptable and outcome measures relevant | Y | Has PPI coapplicant. CI had no expectations for but had prior experience of PPI; challenges (finding the right people; finding people without an ‘axe to grind’). Two PPI contributors interviewed had no prior experience of PPI; challenges (jargon; not liking flying) |
| 10, ongoing, O + M + R | Had PPI coinvestigator. No documented plans | n/a | Has coinvestigator (from local authority). Consulted with parents regarding timing of intervention. Has a contributor on TSC. When getting low response, approached education professionals for advice. CI had expectations for PPI; said had no formal PPI experience, ‘only informal’; challenges (sometimes difficult to get in touch with coinvestigator contributor because of other commitments). PPI contributor had prior experience of PPI; challenges (concern about ‘being too pernickety’) |
| 4, ended, M | No PPI coapplicantsA project management steering group [. . .] will include all co-applicants, research assistants and user representatives. User representatives will be involved in the development, implementation and interpretation of the study. This involvement will include: advice on recruiting patients, invitation letters, the design of information leaflets, and research instruments, piloting assessments, helping to assess progress, and contributing to the evaluation of the project, the interpretation of findings and the dissemination of results. User representatives will be invited to project steering group meetings and also provide assistance in each centre | Y | Had two PPI members on the TMG. Involved in most activities as envisaged and, although unclear from CI interview about plans for interpretation of the study, responses to the CI survey indicate that analysis had not yet started. CI had expectations for and prior experience of PPI; no challenges. No PPI contributor interview |
| 7, ongoing, O + M + R | Had a PPI coapplicantWe will include patients and carers as active participants in the research at all stages. [Name] and [name] have taken the role of patient representatives during the preparation of this research proposal. As the relevant service users are highly likely to be frail, we will use innovative methods to allow full involvement. We will not expect attendance at full research team meetings by patients or carers, although our patient representatives may bring their views to the team meetings, following meetings with individual or groups of service users in other forums. We identified service users to be involved in this trial through the [names of two organisations]. Our named co-applicant will attend Trial Management Group meetings throughout the study in order to contribute the service user perspective at all stages. In addition, [name] is a named co-applicant to the study and will play a role in ensuring that a patient focus is maintained throughout the study. We also plan to seek further views through a wider stakeholder group that will feed into the Trial Management Group through a nominated representative | Y | Has PPI coapplicant and membership on trial management, steering and data monitoring groups. Also consult separate panel of service users for specific issues. CI had expectations for and prior experience of PPI; challenges (identifying/engaging the right people; some less able to articulate their views; some wanting to do something impossible; difficulty getting other staff to understand or prioritise PPI). No PPI contributor interview |
| 14, ongoing, M | Had a PPI coapplicantCo-applicant with an academic interest in representing patients’ perspectives in the design and conduct of health care research will advise the research team on the development of processes and materials which take into account patient concerns | Y | Has PPI coapplicant but CI felt it was a ‘tick box’ exercise. CI had no expectations for or prior experience of PPI; challenges (meetings attendance; lack of engagement). No PPI contributor interview |
| 41, ongoing, O + M + R | Had a PPI coapplicantA representative from [charity] has been involved in preparatory work and will be nominated as a member of the TSC. A minimum of two users will be invited to be part of the project team. A virtual user advisory group will be developed to provide further user support as appropriate. User involvement will contribute to: TSC and project management decisions on all stages of the project; project approval; refinement of self-assessment tools and advice package, exercise intervention; training events for health professionals; interpretation of findings; evaluation of user involvement; dissemination | Y | Has PPI coapplicant. Trial has two PPI contributors although CI feels no strong PPI input overall. Unclear whether or not CI had expectations for PPI; had no prior experience of PPI; challenges (contributors with an ‘axe to grind’; contributors lack confidence about contributing at meetings). No PPI contributor interview |
| 55, ended, O + M | Had a PPI coapplicantPatient reps have been very much involved in the preparation of this bid since its inception. The lead service user joined the TSG, will co-ordinate involvement of service users in the consumer panel and report their views to the TSG. Members of the consumer panel have commented on the current proposal and will be asked to comment on specific design and/or management issues during the course of the study. In particular, their views have been, and will continue to be sought during the preparation of patient information leaflets and posters, and in the preparation of study newsletters. They will be asked to help with dissemination of research findings | Y | Had PPI coapplicant. Planned to involve consumer panel in dissemination of the findings. This did not happen but PPI ‘evolved’ because the team disseminated through other partners, i.e. other patients they were ‘working with in the field’ by that time. Other plans were adhered to. CI had expectations for and prior experience of PPI; challenges (not realising how much training the panel might need; not being clear about expectations of the main contributor; panel feeling ostracised; difficulty getting TM to understand importance and use of the patient panel in the early stages). No PPI contributor interview |
| 15, ended, O + M | Had a PPI co-applicant[Name], a former patient and lay member of the advisory panel, has been fully involved in the application process as a co-applicant and will be a full, active and vocal member. The trial will be guided by a group of respected and experienced critical care personnel and trialists as well as a ‘lay’ representative | Y | No CI interview. PPI coapplicant helped to prepare paperwork for funding; also member of TSC. PPI contributor had prior experience of PPI; challenges (jargon) |
| 34,d ended, M | Had a PPI coapplicantThis proposal has been reviewed by our patient service user group and any opinions and comments incorporated. A patient representative will attend TSC meetings and be directly involved in decision making of trial processes and then relay back information to the [user groups] on a regular basis. Our Service Users group will be involved in all aspects of project design, data collection, analysis and dissemination | U | No CI interview. Had PPI coapplicant, who appears to have been involved as intended, but it is not clear whether or not plans to involve the user group in data collection, analysis and dissemination were implemented. PPI contributor had prior experience of PPI; challenges (not being involved from the start) |
| 18,d ongoing, M | Unclear whether or not had PPI coapplicants. Same plans as trial 34d | U | As above except unclear whether or not the informant was a coapplicant on this particular trial |
| Trials which included a responsive mode (n = 14)c | |||
| 20, ended, M + R | Had a PPI coapplicantThe research team will convene a steering group of research and service users. This will meet three times during the study and will provide an opportunity for the research team to consult about research design and methods for data collection, choice of outcomes and methods for data analyses. The TSC will have an important role in interpreting initial findings and developing dissemination strategies. Consultation with young people and parents will be carried out in intervention and comparison clinics using focus groups. The views gathered in these groups will inform the development of research procedures (e.g. consent, outcome measures), tools for data collection and the process evaluation. Focus groups will also provide opportunity for young people to contribute to interpretation of study findings. Further consultation with young people will involve piloting all research tools to ensure acceptability and appropriateness | Y | Had input from four PPI contributors at different times. Membership on TSC. Sought additional input when struggling with particular issues. CI had expectations for and prior experience of PPI; challenges (having a contributor who was a patient of the lead PI – ‘conflict of roles’; frustration at inability to integrate contributors’ ideas regarding questionnaire, which was a validated instrument and therefore could not be altered). PPI contributor had no prior experience except as charity member; no challenges |
| 101, ended, O + R | No PPI coapplicantsWe will convene user group meetings in each locality during the pilot study, we will organise separate focus groups to explore expectations of treatment. We have a commitment from panels of users/experts including representatives from relevant charities to meet annually during the study to advise on its conduct. We will have lay representation on the TSC | Y | Had PPI membership on TSC and consulted with wider groups as planned. CI felt PPI was underutilised and said ‘people above me in the scheme of things may see it as a tick box exercise’. CI had no expectations for PPI; unclear regarding prior experience of PPI; challenges (finding suitable people; ‘pinning people down’; some may find it daunting whereas ‘professional PPI reps’ do not). PPI contributor had prior experience of PPI; no challenges |
| 21, ended, O + M + R | Had a PPI coapplicantUser and consumer groups have discussed the application and suggested changes to protocol which we have accepted. In the trial the groups will be asked to help with development of info leaflets, consent forms, letters, questionnaire design. The groups were very keen that a user was a collaborator on grant application. The team includes [name], a consumer representative who is chair of [Consumer Research Group], works with the [condition] Association and the [Research Network] | Y | Plans expanded (in terms of recruitment, analysis, interpretation of results, dissemination). CI had expectations for and prior experience of PPI; challenges (‘poaching’ of contributors; stress about funding/paying contributors for their time if in receipt of benefits/pension; disagreement with funders regarding contributor’s activities). PPI contributor had prior experience of PPI; challenges (time; being in demand) |
| 27, ongoing, O + M + R | No PPI coapplicantsWe will include two [condition] patients to act in an advisory capacity. They will be invited to attend all collaborator meetings and quarterly trial management meetings. We will disseminate project information and findings for patients and patient groups | Y | Has PPI membership on trial management, steering and data monitoring groups. CI had expectations for and prior experience of PPI; challenges (finding contributors). Two PPI contributors interviewed had no prior experience of PPI; challenges (some doctors do not want to understand your point of view; jargon; they talk about things you have gone through as a patient in a dispassionate way) |
| 10, ongoing, O + M + R | Had PPI ‘co-investigator’. No documented plans | n/a | Consulted with parents regarding timing of intervention. Has a contributor on TSC. When getting low response, approached education professionals for advice. CI had expectations for PPI; said had no formal PPI experience, ‘only informal’; challenges (sometimes difficult to get in touch with coinvestigator contributor because of other commitments). PPI contributor’s challenges: concern about ‘being too pernickety’ |
| 9, ended, O + R | Unclear whether or not there were PPI coapplicantsThe TSC will include a patient representative, [name], who has acted in this capacity in several other large-scale trials and is aware of issues that might be raised from the lay perspective. The patient information leaflet and consent form have been reviewed by potential service users, and their comments taken into account in finalising these documents prior to submission for ethics approval | Y | Unclear whether or not CI had expectations for or prior experience of PPI; no challenges. No PPI contributor interview |
| 102, ended, O + R | No PPI coapplicantsAt the outline proposal stage, this trial was submitted to the [name of funding body] who sought the opinion of the [condition] Society. The [condition] Society unequivocally confirmed their support of the proposed trial. The [condition] Society have also confirmed their willingness to represent their members through steering committee membership of the [name of trial] and to help the trialists in the construction of the MREC [Multicenter Research Ethics Commitee] application and patient information leaflets | Y | Seems to have expanded plans (in terms of dissemination, i.e. press releases and findings for participants). CI had expectations for and prior experience of PPI; no challenges. No PPI contributor interview |
| 6, ongoing, O + R | No PPI coapplicantsThe TSC will include an already identified patient. He will provide an informed patient perspective. He is willing to assist us in the trial, and will be listed as a member of the TSC. We will also work with [charity] to involve service users. This will be done through our links with the [unit], which is co-directed by one of our applicants, [name]. We will begin this process during the protocol set-up period | Y | CI had expectations for but unclear whether or not had prior experience of PPI; no challenges. No PPI contributor interview |
| 7, ongoing, O + M + R | Had a PPI coapplicantWe will include patients and carers as active participants in the research at all stages. [Name] and [name] have taken the role of patient representatives during the preparation of this research proposal. As the relevant service users are highly likely to be frail, we will use innovative methods to allow full involvement. We will not expect attendance at full research team meetings by patients or carers, although our patient representatives may bring their views to the team meetings, following meetings with individual or groups of service users in other forums. We identified service users to be involved in this trial through the [names of two organisations]. Our named co-applicant will attend Trial Management Group meetings throughout the study in order to contribute the service user perspective at all stages. In addition, [name] is a named co-applicant to the study and will play a role in ensuring that a patient focus is maintained throughout the study. We also plan to seek further views through a wider stakeholder group that will feed into the Trial Management Group through a nominated representative | Y | Consulted separate panel of service users for specific issues. CI had expectations for and prior experience of PPI; challenges (identifying/engaging the right people; some less able to articulate their views; some wanting to do something impossible; difficulty getting other staff to understand or prioritise PPI). No PPI contributor interview |
| 41, ongoing, O + M + R | Had a PPI coapplicantA representative from [charity] has been involved in preparatory work and will be nominated as a member of the TSC. A minimum of two users will be invited to be part of the project team. A virtual user advisory group will be developed to provide further user support as appropriate. User involvement will contribute to: TSC and project management decisions on all stages of the project; project approval; refinement of self-assessment tools and advice package, exercise intervention; training events for health professionals; interpretation of findings; evaluation of user involvement; dissemination | Y | Has PPI coapplicant. Trial has two PPI contributors although CI feels no strong PPI input overall. Unclear whether or not CI had expectations for PPI; had no prior experience of PPI; challenges (contributors with an ‘axe to grind’; contributors lack confidence about contributing at meetings). No PPI contributor interview |
| 79, ended, O + R | No PPI coapplicants. No documented plans | n/a | Although no documented plans, the CI wanted PPI to sit on TSC and comment on patient info leaflets. The CI felt that PPI started early. There were two types of involvement: two contributors on the TSC; and then obtained views on information sheets from relevant groups. CI had no previous experience of PPI; no challenges. No PPI contributor interview |
| 76, ongoing, O + R | No PPI coapplicantsThe [organisation] has recently established a Research Advisory Group. This Group, which includes key stakeholders with an interest in the research carried out by [organisation] (patients, charities representing patients’ interests, general practitioners, NHS commissioners, research funding organisations and a regional [medical] network), has been set up to ensure that the clinical research carried out in [organisation] is ethical, important, relevant, appropriately designed to meet the needs of patients and the NHS. We anticipate the Group would have the opportunity to influence important details of the project before recruitment starts. A patient representative (we propose a member of the [advisory group]) will be invited to join the TSC | U | Has PPI membership on TSC as planned; unclear whether or not plans to seek advice of new advisory group prior to recruitment were implemented (although did approach a group of patients from a previous trial about format/comprehensibility of questionnaire). CI talked of a ‘tick box exercise’ but also ensuring participants’ perspective; ‘overseeing the trial – a ‘safeguard’ rather than improving research’. CI had expectations for but no prior experience of PPI; challenges (communication and understanding). No PPI contributor interview |
| 106, ended, O + R | No PPI coapplicantsWe have consulted widely, including with patients to seek their views on trial design and relevant outcome measures. We have involved service users in the design of the trial. We used the patient information pack and part of the questionnaire that has been developed and validated in collaborative research with the [institute] as a basis for in-depth interviews to identify patient perspectives on trial design and outcomes. We have identified one service user, [name], who will advise the trial management committee on patient perspectives | Y | No CI interview. PPI contributor had prior experience of PPI but felt she had made no difference to the trial; no challenges |
| 91, ongoing, O + R | No PPI coapplicantsWe have involved [name] who is a non-executive patient representative member of [hospital trust] and who has co-ordinated consumers’ input into the scientific quality, feasibility and practicality of the proposal. She will continue to participate in the protocol design of the study and be a member of the TSC | Y | No CI interview. Plans expanded (in terms of the PPI contributor obtaining feedback from ‘women’s groups’). PPI contributor had prior experience of PPI; challenges (just being confident enough to make your point) |
Despite informants indicating that most of the documented plans for PPI had been implemented, some revealed no personal expectations for PPI and spoke of using it as a means of ‘ticking the right boxes’. This raises questions about the motivations behind the PPI plans in some grant applications.
Based on informants’ accounts it appeared that six trials largely confined PPI to an oversight mode of involvement, although some had hinted at other modes in their applications. We begin by examining what happened in these trials.
Oversight-mode trials (n = 6)
Oversight-mode trials were those which confined PPI input to membership of TSCs. Based on informant interview accounts, there were six trials that constrained PPI to this mode of involvement, although three of these had hinted at other modes in their applications. A further application had been too vague to discern the mode of planned PPI, and another had no documented plans for PPI (see Table 25).
Based on informants’ accounts, all trials which had documented plans for PPI membership on their TSC had implemented this aspect of the plans. Researcher interviews were available for four of these six oversight trials and, of this four, only one researcher divulged any personal expectations for PPI in the trial. Moreover, informants’ accounts raise concerns about the motivations for including PPI in their applications and the danger of assuming that contributors know what is expected of them. For example, trial 36 had named a ‘patient representative’ as a member of the TSC at the application stage and subsequently, in direct response to peer reviewer comments, the team had indicated that it would consider increasing the number of ‘patient representatives’ on the TSC from one to two, in order to provide ‘mutual support’. The team proceeded to include two PPI contributors on the TSC, thereby achieving its documented plans. Despite having prior experience of PPI, however, the researcher divulged no personal expectations for PPI and referred to it as a ‘tick box’ exercise:
It was a requirement of. . . that we had representation on our steering committee and therefore I went through that [. . .] We can say [the PPI contributors] are there and therefore it’s, if you like, ticking a political box.
CI 36
The documentation for trial 2 included no plans for PPI during the trial but did state that there had been ‘several stages of user involvement’ prior to the grant application, ‘to confirm that the research question is pertinent to both the needs of the NHS and the NIHR programme of research development’. Two grant reviewers commented on the lack of ‘service user representation’ on the team and suggested membership ‘on the research team or steering group’. The TSC did include PPI membership but during the interview the researcher spoke of his ‘tokenism’ and ‘ignorance’ about how PPI ‘should and could work’. When asked about the expectations of their role, the PPI contributors in two other oversight trials (115 and 96) implied similar uncertainties when they spoke of not knowing what was expected of them and of feeling ‘bewildered’ in meetings:
I can’t understand why they use me . . . they seem to find me useful but I just sit there bewildered. I’m there as a sort of grey background while the others do all the sparky stuff.
PPI 115
In the next section we describe planned and implemented PPI in 14 trials which incorporated a managerial role of PPI. Unlike the six trials with a mainly oversight mode, many of the managerial-mode trials had utilised more than one form of PPI.
Beyond oversight: into managerial mode (n = 14)
Fourteen trials indicated some type of managerial involvement in the documented plans, usually including PPI contributors as coinvestigators (see Table 25). Two trials (4 and 27) did not have PPI contributors as coinvestigators but planned to include PPI contributors on the TMG, and interviews with informants indicated that this had been implemented. It was unclear in one ongoing trial whether or not there was a PPI coinvestigator, but documented plans stated that a named PPI collaborator would be ‘directly involved in decision making of trial processes and then relay back information to user groups’; according to the PPI contributor interview these plans were being implemented (trial 18). Trial 10 had no documented plans for PPI but the interview with the CI indicated that there was a PPI coinvestigator.
Informants’ accounts indicated that all trials which had planned a managerial mode of PPI did implement it (see Table 25). This included trial 21, which had a PPI coapplicant and documented plans to involve user groups in developing information leaflets, consent forms, letters and questionnaire design. There was a budget for PPI travel and expenses, which is perhaps indicative of careful planning. The documented plans stated that ‘user and consumer groups were very keen that a user was a collaborator on the grant application’. The applicants also planned and included oversight PPI (TSC membership) and expanded beyond their plans to include contributors in recruitment, in the analysis and interpretation of results, and in dissemination. Although we could not pinpoint from the informant interviews exactly what prompted these additional PPI activities, the PPI contributor whom we interviewed described his extensive previous experience in similar roles and noted that his role in the trial had ‘evolved’. He also explained that ‘I’m there because I want to change things’ (PPI 21) and this proactive approach may have contributed to the expansion of PPI in this particular trial. Correspondingly, the CI spoke of wanting the PPI contributors to ‘feel welcomed and valued as part of the group’, and had personal expectations for PPI that included PPI contributors helping with ‘running the study’ and ‘disseminating the results’, and that ‘they would stay involved’ and ‘feel able to speak out and have their own opinion’:
We wanted them to offer to do things that they felt they could do and feel happy to say if they didn’t feel they could do certain things that might come their way.
CI 21
There were several examples akin to this among trials incorporating a managerial mode of PPI, in which CIs reported having personal expectations for PPI or in which PPI contributors appeared to be integral members of the research team. However, one of two exceptions was trial 14, in which documented plans had been to involve a PPI coapplicant ‘with an academic interest in representing patients’ perspectives in the design and conduct of health care research’, adding that this individual would advise on ‘the development of processes and materials which take into account patient concerns’. Responses to the CI survey described the PPI contributor as ‘a serial patient representative’. When interviewed, the CI divulged no personal expectations regarding PPI contribution, describing it as a ‘tick box exercise:
The funders were insistent on having patient representation and wanted to know what that representation was on your grant submission.
CI 14
In summary, most trials which planned a managerial mode of PPI implemented it. However, as trial 14 shows, simply having a PPI coinvestigator is not necessarily a guarantee of meaningful contribution if researchers have no expectations for PPI or if contributors are unable to provide the input that a particular trial requires, for example because they are selected out of convenience rather than to match trial needs. In the next section we focus on the less formal, responsive, form of PPI in which researchers ‘reach out’ for specific PPI input as and when needed.
‘Reaching out’: responsive modes (n = 14)
Fourteen trials embraced some form of responsive involvement, although trial documents for two (10 and 79) had not indicated any plans for PPI (see Table 25). The remaining 12 had stated in their documented plans that they would, or already did, engage with PPI groups or panels rather than just with the one or two individuals that was typical of oversight and managerial PPI. Data from application forms, project descriptions and informant interviews showed that this responsive activity sometimes entailed seeking advice from PPI groups prior to the application for funding. Informants noted that many triallists continued to seek advice from such groups during the trial regarding specific issues. Other trials began a responsive approach once the trial had commenced, often as and when particular problems arose. Most trials implemented all aspects of their documented plans but in one case (trial 76) it was unclear from the CI interview whether or not specific plans to seek advice of a new advisory group before recruitment were implemented.
Trial 20 used responsive alongside managerial PPI, including having a PPI coapplicant. The trial had ended at the time of the interviews, and the researcher stressed that the responsive PPI had been ‘crucial’ when faced with specific problems. The CI explained that one PPI contributor would attend research team meetings:
. . . but I then reached out to other people in addition when we needed more help [. . .] I think what was crucial was being able to get input, not in terms of regular intervals but [. . .] when you’ve got a problem.
CI 20
Further illustrating the flexibility that responsive PPI allows, in her interview one of the PPI contributors on the same trial (managerial role) advised researchers to ‘have some understanding’ of the needs of PPI contributors. She then went on to refer to another contributor on the same trial who never attended project meetings but operated in a more responsive mode outside meetings. It appeared this arrangement had evolved to accommodate the needs of the latter contributor, who, it seemed, found meetings difficult:
She didn’t really know what to do, so I think it was much more a one-to-one conversation which is what she was happy with rather than sitting in a committee.
PPI 20
Documented plans for trial 7 involved a combination of oversight, managerial and responsive modes. This trial was collecting outcome data at the time of the researcher interview, and PPI plans were being implemented, including consultation with a panel of service users who advised on issues such as how to increase participant response rates to the outcome questionnaire, and on the promotional material that accompanied it. When interviewed, the researcher spoke of her personal expectations that PPI would help to maximise recruitment, ensure the right outcomes were measured and help in interpreting the findings. There was no PPI contributor interview but the researcher also spoke of having to tailor ‘different ways of involving people’ in PPI depending on the ‘population of interest’:
It might be children, people from disadvantaged groups or older people [. . .] so you probably have to find other tailored ways of including people to make it effective. So it’s not a one size fits all.
CI 7
The majority of those researchers interviewed who described such ‘as and when’ contributions (10 of 12) spoke of expectations for PPI, and tended to view responsive modes as constructive. Only in one case (trial 101) did the researcher allude to the PPI within their trial as a ‘tick box’ exercise.
Three trials undertook additional responsive PPI activity that had not been specified in their documented plans. Trials 21 and 102 expanded on their plans by involving PPI contributors in a broader range of activities than initially indicated, namely advising on recruitment, and interpretation and dissemination of study findings. As with trial 21 (described in Beyond oversight: into managerial mode, above), we could not determine from the CI interview why plans for trial 102 had been expanded upon, and there was no PPI contributor interview for trial 102 to help illuminate this issue. The PPI contributor for the third trial (trial 91) mentioned that she sought the views of ‘women’s groups’. This was additional to the documented plans for her to be involved in ‘protocol design of the study’. As with trial 21, this PPI contributor had previous PPI experience and appeared to be a particularly active member of the research team, and with considerable knowledge of the relevant health condition.
In summary, most applicants implemented their documented plans for PPI regardless of the mode of planned involvement. In five cases we were unable to discern whether or not PPI plans were fully implemented, although some PPI was achieved in these trials. Regardless of whether PPI was implemented as planned or evolved, most trial teams faced challenges and learnt lessons about implementing PPI as they went along. We now turn to their accounts of this learning and then use these to derive practical advice for planning and implementing PPI.
Researchers on the challenges of patient and public involvement, and lessons learnt
Most CIs spoke of the challenges they encountered in implementing PPI (Table 26) and things they would do differently as a result. The involvement of trial investigators’ own patients as contributors was perceived to lead to a ‘conflict’ (CI 20) between an investigator’s research and clinical roles. This brought a risk that research would ‘cross over into clinical care’ (CI 6) and that such contributors would be ‘out of their depth’ (CI 20) and find it difficult to ‘say something which might imply a criticism of their clinician’ (CI 20). CIs talked about the problems of failing to engage PPI contributors fully or early enough to inform changes in study design, and ‘underutilising’ (CI 101) PPI contributors by not involving them in the planning stages, thereby making PPI less ‘robust’ (CI 101). They reflected on the potential detrimental consequences of such failings on the relationship between researcher and PPI contributors, for example being less likely to ‘form a bond and get loyalty’ (CI 14). Finding and engaging the right people with an interest in and understanding of the research, with enough confidence, commitment and impartiality, was another major stumbling block:
You hear that some consumers get involved [. . .] because they have a particular point of view or axe to grind [. . .] in those circumstances it could be very detrimental to a trial, to be driven by somebody who has had a bad experience [. . .] and those are the ones you don’t want on your team.
CI 5
You’ve got triallists in the [meeting] who are trained to run clinical trials. And then you’ve got one lay representative who may be slightly intimidated by everyone else, who’ll not be able to truly give their views, may be slightly overawed.
CI 14
| CI interviews (n = 21) | PPI contributor interviews (n = 17)a |
|---|---|
| Challenges common to researchers and PPI contributors | |
| Failure to engage contributors fully or early | Not being involved from the start; infrequent meetings |
| Contributors overawed/lacking confidence | Feeling unqualified or overwhelmed |
| Failing to clarify to contributors what was expected of them | Role expectations (being unsure what was expected of you) |
| Worry about taking up contributors’ time | Time constraints |
| Contributors being ‘poached’ | Being in demand from other research teams |
| Meeting attendance by PPI contributors | Getting to meetings |
| Challenges unique to researchers or PPI contributors | |
| Finding the right people Own patient as a PPI contributor (can lead to conflict between clinical and research roles) Communication difficulties due to age Change of PPI personnel Getting other team members to understand/prioritise PPI Underestimating training needs of contributors Worry that contributors may lose payment if receiving state pension/benefits Disagreement with funders about implementing contributors’ suggestions |
Jargon Interactions within team and being listened to Concern about appearing confrontational Concern about appearing too ‘pernickety’ Remembering ‘what side you are on’ |
Researchers also pointed to the practical difficulties that contributors experienced in attending meetings due to geographical distance or time constraints. They emphasised how teleconferences could be less conducive to forming a relationship with PPI contributors than face-to-face meetings. They also reported problems relating to communication and mutual comprehension between themselves and PPI contributors. Some described PPI contributors as struggling to understand the nature of research, or the distinction between research and clinical practice, and one CI referred to his own ‘naivety’ (CI 55) in underestimating how much training PPI contributors might need. CIs described difficulties getting other staff such as TMs to understand or prioritise PPI. This included one CI who noted that some investigators are unable to ‘cope’ with having a ‘working relationship with service users’ and ‘can’t let go of the fact that [they] are people they study’:
It’s a mindset [. . .] an attitude where you have an equal partnership. You’re working together, not studying these people. You’re asking for their expertise and I’ve found that some people who’ve worked with me, that comes easily and some people absolutely never get it.
CI 20
Chief investigators remarked that they were unclear about what to expect in relation to PPI and worried about taking up the contributors’ time. External forces also played a part in some cases; for example, one CI described PPI contributors being ‘poached’ by other studies, a ‘fight’ with the university regarding paying a PPI contributor for his time, and disagreement with funders when a contributor wanted to add to the patient information sheet that he was a PPI contributor on the project (CI 21).
Chief investigators spoke of how they had learnt as the trial went along, revealing that their ‘practice had evolved’ (CI 14) and their skills had:
. . . changed beyond recognition [. . .] now we’re much better equipped [. . .] but at the time when [trial] started we had very little idea at all about what PPI involved or how it would help or how it would work.
CI 2
In the light of these challenges, CIs spoke of how in future they would involve more than one PPI contributor, in particular by using focus groups or panels of contributors rather than individual contributors, enlist the help of relevant charities, and conduct surveys or use social media when there was a ‘burning question’ (CI 55). Use of responsive PPI rather than individual contributors was described as ‘gold standard’ PPI (CI 14), as this avoided ‘the danger of having a single opinion’ (CI 76), provided structure for all parties and helped to enhance the confidence of individual contributors:
I would certainly have more involvement and some kind of framework around it [. . .] a small user group and set boundaries [. . .] try to agree how often we should meet and what people’s roles and responsibilities are [. . .] and provided more structure [. . .] to make them feel that their views are important and their involvement is very important, I think that would go a long way to easing the process.
CI 41
Many CIs indicated that they would extend PPI in future by asking contributors to lead in the dissemination of findings to relevant groups and help in the development of research questions and study design, and involve PPI contributors as coinvestigators. CIs placed particular emphasis on how ‘crucial’ it was to have ‘early input’ (CI 14):
The most useful things are [. . .] the design stage [. . .] RCTs [randomised controlled trials] you’ve got to plan ahead [. . .] after the development phase you shouldn’t really be changing anything [. . .] it is during that development phase when decisions are being made.
CI 115
Early engagement and appreciation that their input into the question is really important [. . .] with retrospect and for the future studies [. . .] more involvement at the front end, less in the middle and more at the end.
CI 2
Finally, CIs reflected on the importance of ‘thinking through’ plans and being clear about whether or not, what and why PPI is needed for individual trials:
Be clear about the link between particular methods [of PPI] and particular benefits and challenges [. . .] it’s not all the same, there are so many ways of doing it but you have to have good reasons for choosing how to do it.
CI 20
I don’t think it should be automatic that there must be PPI involvement in every study, and different types of involvement are necessary for different parts of study. Having a core group is not necessarily the right thing because at different points there are different types of people and types of involvement that would be useful.
CI 10
Contributors on the challenges of patient and public involvement, and suggestions for improvement
Most PPI contributors mentioned challenges or difficulties linked to their involvement in the trial which may inform future research teams in planning and implementing PPI. Some of the contributors’ challenges paralleled CIs’ accounts whereas others were unique to the contributors (see Table 26). Whereas researchers referred to problems they had experienced in their communication with contributors, a prominent issue exclusively mentioned by contributors related to the problems they experienced with ‘jargon’ and the technical language that was used in trials, such as statistical or medical terminology and acronyms. Several contributors suggested remedies such as supplying a list of acronyms or a booklet of research terms, or simply ‘if they’re going to use jargon, explain it’ (PPI 64). A further idea was that the person chairing meetings could try to ensure that discussion about statistical issues or other areas of technical expertise was translated and summarised adequately. Contributors talked about difficulties in interacting with researchers, including not always feeling listened to by everyone. One contributor who had been invited by her consultant and had previous experience of PPI implied that ‘some doctors’ were unwilling to understand the perspectives of patients (PPI2 27). Another felt that female researchers were more understanding than males regarding problems with travelling or feelings of insecurity, and a further contributor alluded to how in meetings the team sometimes talked about patient experiences in a ‘dispassionate’ way and, although this was not a problem for that particular contributor, she felt it might be for others (PPI1 27).
Some of the challenges that contributors described echoed those raised by the CIs. These included lack of clarity about roles, and the difficulties contributors experienced in attending meetings, for instance because of a health condition. Such practical difficulties could give rise to additional complexities. For one contributor, infrequent meetings meant ‘not much to build a relationship on’ and, while academics worked closely together, she had to ‘work quite hard to keep up’ (PPI 16). Contributors also talked about wanting to be more involved in between annual meetings, to take part in ‘shaping the bid’ (PPI 20) so that it was less focused on the primary clinical outcome, to see the intervention itself and to have initial briefing meetings at the outset of their involvement. Finally, one contributor described it as a ‘downfall’ that he was not receiving feedback or ‘thank yous’ and commented on how important it was to make PPI contributors ‘feel valued’ (PPI 34).
Key points
-
Most triallists are putting their plans into action, although in some cases the plans were minimal and relatively easy to execute.
-
Many trials implemented multiple modes of PPI, which is both surprising and encouraging given that PPI was less prominent when the proposals for the trials in this cohort were being developed.
-
Regardless of statements about PPI in their funding application, some triallists had no expectations of what PPI might achieve, and their only motivation for including PPI was a belief that it was necessary or would help to secure funding for their trial.
-
Chief investigators encountered complications from which they learnt valuable lessons, suggesting that it is perhaps necessary to learn by experience in this area, including what to expect of PPI. Difficulties in finding and retaining suitable contributors, and engaging in PPI ‘too little too late’, led triallists to say they would do things differently in future, including seeking involvement from a more diverse source such as patient panels or focus groups.
-
Patient and public involvement contributors themselves mentioned that becoming involved after the trial had begun, or infrequently, resulted in missed opportunities for them to contribute. Some referred to uncertainty about their role and many struggled with jargon, an enduring problem despite the availability of apparently straightforward solutions.
Chapter 7 Survey of registered clinical trials units: results of phase 4
The RCTU survey was sent to each of the 46 CTU directors, with an 85% (39 of 46) response rate (Table 27). Two of these stated that there was no PPI involvement at their CTU, so they were not required to respond to subsequent questions, and a further two CTUs started but did not complete the survey.
| Question | Response, n (%) |
|---|---|
| 2. What types of trials do you predominantly run in your CTU? (Please tick all that apply) | 39 |
| Cancer | 16 (41.0) |
| Mental health | 9 (23.1) |
| Obstetrics/gynaecology | 2 (5.1) |
| Paediatrics | 5 (12.8) |
| Varied | 18 (46.2) |
| Other (please specify) | 19 (48.7) |
| Non-pharmalogical, surgical; Urology, physical activity; Cardiovascular; Cancer prevention; Complex interventions including surgery; Diabetes; emergency and critical care; Musculoskeletal and arthritis; Stroke, complex interventions, skin, Cardiovascular, MSK [musculoskeletal]; Diabetes and Cardiovascular Disease; Expanding to other disease areas; Respiratory; Diabetes, nutrition, cardiology, orthopaedics, gastro, endocrine; Obstetric and neonatal; Dementia, Rehab and muskloeskeletal; Vaccines and Primary Care; Complex intervention; Primary Care; Oncology | |
| 3. In your trials where do you most frequently recruit patients from? | 39 |
| GP surgery | 5 (12.8) |
| Hospital | 23 (59.0) |
| Community | 4 (10.3) |
| Other (please specify) | 7 (17.9) |
| All of above – varied, depends on study; All of above – reasonable balance across all; Care homes; Varied; All of above and emergency ambulance services; All of above, mostly GP surgeries and hospitals | |
| 4. Do you have any PPI in the trials co-ordinated in your CTU? | 39 |
| Yes | 37 (94.9) |
| No (please explain why) | 2 (5.1) |
| Under review – but primarily via the CI; Not specifically in our CTU. All of our projects have PPI representation though | |
| 5. Do you have a formalised PPI standard operating procedure or guidance document? | 36 |
| Yes | 9 (25.0) |
| No | 17 (47.2) |
| In development | 10 (27.8) |
| 6. What model of PPI do you use? | 35 |
| A panel of representatives who provide input across multiple trials | 9 (25.7) |
| Individuals who are generally attached to a single trial | 18 (51.4) |
| Other (please specify) | 8 (22.9) |
| Ad-hoc – depends on trial/team; Both of the above, mostly a group of representatives; Both of the above; We use a combination of different models tailored to the national and local resources available in each topic area. Includes a topic specific network for the skin portfolio, involvement of reps from patient charities, local and national PPI networks as well as individuals working on specific trials; Our multi ethnic community prefer different methods of engagement. We have individuals who are willing and able to be part of investigator committees, community groups who are keen to be consulted for specific studies, and a well-established group with a history of collaboration across numerous studies in PPI; It varies. On trials in subject areas where there are vibrant patient panels in the area (Emergency medicine and critical care being a notable example) then those panels would be used. More often, we will convene a special-purpose patient panel, usually of 2–4 individuals, in advance of the outline submission of a researcher-led proposal. Where we are responding to an NIHR commissioning brief, there is not always time to do this before the outline. We would try to get at least one PPI rep involved to read the flow diagram and lay summary and have other input to the study (sanity and burden of the outcome assessments and other procedures), and we would try to convene a more varied panel before the full application. Often, we would seek RDS [research design service] professional and financial support to do all of the above.; PPI input comes from the following sources: Consumer Research Panels associated with the Cancer Research Networks; patient advocate members of NCRI [National Clinical Research Institute] Clinical Studies Groups, patient advocate members of open membership groups such as the UKBI [UK Breast Intergroup] the Independent Cancer Patient’s Voice (ICPV) organisation. Individual patient advocates are usually identified from these groups and become attached to individual trials as a member of the Trial Management Group; Both | |
| 7. Whose responsibility is it to identify PPI representatives for trials that you run? | 35 |
| CI | 9 (25.7) |
| CTU staff | 10 (28.6) |
| Research design service (RDS) contact | 1 (2.9) |
| Other (please specify) | 15 (42.9) |
| Both CI and CTU staff (n = 7)7; PPI co-ordinator or liaison officer with the CI and CTU team (n = 3); Early dialogue and planning at grant app stage: Plan is developed in partnership and responsibility depends on topic and resource; Depends on the individual study; Not on all trials, TMG discuss at setup | |
| 8. Do you specify/agree with the person(s) providing PPI what is expected from them and what they should expect in return? | 35 |
| Yes – verbally | 16 (45.7) |
| Yes – written | 15 (42.9) |
| Remit currently in development | 3 (8.6) |
| No | 1 (2.9) |
| 9. How do you incorporate PPI representatives input? (Please tick all that apply) | 35 |
| Grant co-applicant | 29 (82.9) |
| TMG | 24 (68.6) |
| TSG | 30 (85.7) |
| Data Monitoring Committee | 11 (31.4) |
| Contacted as required (consultancy basis) | 13 (37.1) |
| Other (please specify) | 10 (28.6) |
| For ethics applications; Forum meetings to look at new trial ideas and issues for the patient population that can be addressed – as and when needed e.g. if there’s a grant call; Separate advisory group depending on the trial; PPI meetings/workshops which focus on the patient perspective, this has included interpretation of data, tool development and trial design; We try to involve PPI representatives throughout the research process, including initial design; MCRN [Medicine for Children Research Network] YPAG [Young Persons Advisory Group] and through MCRN CSG parent members; Dissemination;Input is incorporated via the Trial Management Group but patient advocates are contacted more informally by the trial team and CI for general advice; Qualitative research component of the trial;Local management groups and local reference groups in multi-centre trials | |
| 10. Have you had any PPI representation through a research network or charity? | 35 |
| Yes – research network | 2 (5.7) |
| Yes – charity | 12 (34.3) |
| Yes – both of the above | 18 (51.4) |
| No | 3 (8.6) |
| 11. How do you determine the level of PPI required for a particular trial? | 35 |
| Same approach generally used across all trials | 8 (22.9) |
| Determined by trial characteristics | 19 (54.3) |
| Availability of suitable representatives and their preferences/experience | 2 (5.7) |
| Other (please specify) | 6 (17.1) |
| Determined by trial characteristics and patient population; Combination of 2 and 3; Determined by trial characteristics and trial team; A mix of trial characteristics and the existence of a service user group or network; Both determined by trial characteristics and availability; Same approach generally but can be modified determined by characteristics of trial and who’s available, capacity to be involved, experience of involvement and their desires, practicalities and their health | |
| 12. Which factors have motivated you to carry out PPI in clinical trials? (Please tick all that apply) | 35 |
| Requirement of funder | 23 (65.7) |
| Requirement of ethics committee | 11 (31.4) |
| Have involved PPI representatives in the past and have valued their contribution | 25 (71.4) |
| Other (please specify) | 10 (28.6) |
| Has heard examples from others about how PPI can improve research; Ethical issues – for example if there’s is vulnerable patient population such as paediatrics or brain surgery; Deemed as a positive thing to have in unit, improves quality of study; PPI benefits recruitment; Led by chief investigator; CIs sometimes very passionate about getting PPI; Important to get documents, recruitment strategy correct before you submit it as later you’ll just have to amend it; Involving PPI is the right thing to do as they are the recipients of the care – improves the quality and relevance of research; Improves the quality of trials; Moral issue – they have a right to be involved. Strengthens research | |
| 13. Which of the following activities have your PPI representatives been involved in? (Please tick all that apply) | 33 |
| Prioritising an area as a research topic | 15 (45.5) |
| Developing a research protocol | 25 (75.8) |
| Commenting on patient information literature/websites/newsletters | 33 (100.0) |
| Developing qualitative research on trial experience | 13 (39.4) |
| Identifying which outcomes are important to measure | 19 (57.6) |
| Providing insight in recruitment issues/promoting trial | 31 (93.9) |
| Developing mechanisms for feeding back the study results to the trial participants | 15 (45.5) |
| Commenting on public/patient friendly reporting of study results | 15 (45.5) |
| Presenting study results | 9 (27.3) |
| Other (please specify) | 9 (27.3) |
| Read the lay summary at the design stage. Help with finding interview people for focus groups or interviews with participants as part of the trial; Seek advice on retention issues; Co-authoring, presenting research at funder visits; interpretation, tool development; Providing feedback to general public on experience of being involved in research as PPI representatives; Developing the research question; Acceptability of research; Providing input and advice regarding trial design at the grant application stage (and throughout the life of the trial for some trials) mainly concerning the acceptability of trial procedures to patients e.g. taking additional research biopsies.; Recruiting other service users to be involved | |
| 15. Do you have a payments policy for PPI? | 33 |
| Yes | 12 (36.4) |
| No | 14 (42.4) |
| In development | 7 (21.2) |
| 16. Do you use a costing model to estimate costs of PPI in clinical trials? | 33 |
| Yes | 13 (39.4) |
| No | 15 (45.5) |
| In development | 5 (15.2) |
| 17. What training do the PPI representatives receive? [By training we mean formal instruction through training resources, as opposed to informal mentoring (support) and advice provided by a member of staff/peer PPI representative] (Please tick all that apply) | 33 |
| A general induction to your unit and the role of PPI in your trials | 11 (33.3) |
| Introduction to clinical research (e.g. understanding the value of research, how trials are developed, different trial designs and the research process) | 10 (30.3) |
| Communication skills/meeting skills | 5 (15.2) |
| Critical appraisal skills | 2 (6.1) |
| Plain English skills | 0 (0.0) |
| Ethics in clinical trials | 1 (3.0) |
| Training on the condition being researched | 3 (9.1) |
| Good clinical practice | 5 (15.2) |
| Qualitative research methods in trials | 1 (3.0) |
| They are provided with INVOLVE resources | 7 (21.2) |
| Training under development | 5 (15.2) |
| No training currently offered | 12 (36.4) |
| Other (please specify) | 15 (45.5) |
| Ad hoc; Haven’t got the expertise to offer this; Unsure (PPI is through CI); Training on the trials we run and up to date information. Also ask for feedback from PPI reps on what training they want, such as clinical trials training for if they want to be involved in other areas of the research; Lab tour. Mentorship programme offered. Told where to go for more information. Attendance at conferences; Team working skills. What is a systematic review workshop; varies depending on the individual involved and the topic; We have used a recent NIHR PDG grant to develop PPI. Our first training session is scheduled for early Autumn in 2013; Unsure what training is provided – it’s given through the group who work closely with Involve; No training routinely offered as we want a lay member not a trained lay member; training delivered through NISCHR [National Institute for Social Care and Health Research] Involving People; Unknown; PAGs [Patient Advisory Group] receive much of the above training but not via [CTU] i.e. via training organised for the NCRI Consumer Groups and by the Cancer Network Groups, IPCV, etc. We are always willing to contribute to these training programmes if requested.; Introduction to research methods; Training if they are on a trial committee and how it fits into the research structure | |
| 18. How do you provide support to your PPI representatives? (Please tick all that apply) | 33 |
| A specified member of staff at the CTU | 21 (63.6) |
| The PPI representatives are linked together for peer support | 10 (30.3) |
| The chair of the oversight committee provides support | 6 (18.2) |
| INVOLVE website and publications | 10 (30.3) |
| No formal support is offered as yet | 5 (15.2) |
| Other (please specify) | 14 (42.4) |
| Mentoring *3; trial team/trial coordinator*3; PPI liaison officer/group in the CTU*3; Informal support is offered for example pre-meeting with PPI before the TSC meeting; Unsure (PPI is through CI); Provide lay summaries; varies depending on the individual involved and the topic; They are part of the CSGs and Research Network Consumer Panels | |
| 19. Do you evaluate the experience of the PPI representatives? | 33 |
| No | 22 (66.7) |
| Yes (please specify) | 11 (33.3) |
| Ask for their feedback and incorporate this; We have ongoing discussion with PPI reps; Informally ask for feedback; An evaluation form for written feedback from PPI – needs assessment on training/workshops; varies but has included debriefing and evaluation of specific PPI events via interviews; Although we provide opportunities for feedback, both formal and informal, this is an area in development for us.; currently piloting an evaluation; Annual meeting to ask if they need any support; We do not formally ‘evaluate’ the experience of the PPI but their relevant experience is often recorded and noted in grant applications; Feedback form that they are asked to complete at the end of every meeting; Regular slot on TMG | |
| 20. Have there been any recent developments or changes in how you plan to approach PPI in the future? | 33 |
| No | 12 (36.4) |
| Yes (please specify) | 21 (63.6) |
| Appointment of a PPI coordinator *4; Becoming more structured – having a formal training program, don’t current have own PPI panel – more aware of this. Becoming more formal.; Want to set up PPI but haven’t done this yet; Looking into having PPI more tied to a specific trial rather than across trials so they see more areas of one trial rather than be general about the advice they give. Also expanding into other trials – surgery and rheumatology; Increasing involvement; A more formal policy is being developed; Developing an induction pack for new PPI reps and researchers who want PPI. Set of 15 guides for PPI representatives, putting together support workshops for members of TSCs; recent update of PPI policy; Owing to the development work around PPI carried out by colleagues as part of a NIHR PDG grant, there are a number of new initiatives taking place, or planned, to strengthen our PPI activities across our research team, including the CTU.; Starting to look at involving PPI in presenting study results. Also getting PPI involved earlier as it’s a requirement of NIHR to involve them during the stages of development.; Formalising PPI; Beginning to discuss how PPI can be linked/networked across trials for peer support. Starting to develop a more unit wide policy for PPI, including payment for PPI, has set up an advisory group on how to do this that includes a PPI rep.; We have plans to create a PPI group that can be a resource used by supported studies, ensuring involvement of patient and the public across all the stages of the research process; Changed from having a panel in the unit from which 2 representatives will be involved on each trial. This was difficult to manage as no specific member of staff was employed to look after the group so this changed. Now, if a trial comes to them without PPI they go through RDS processes to find PPI, e.g. advertising through posters, charities; Keen to formalise and evaluate use of PPI in trials and to develop guidance and training for PPI representatives. Challenge is doing this across a wide variety of conditions and research groups; Wants to increase the involvement of PPI as co-applicants. Have set up a meeting with the James Lind Alliance to make trials more patient-centred, this will involve PPI. Wants to set up an annual ‘thank you’ meeting for PPI representatives. Wants to develop a way of ensuring how to help/support PPI representatives who are not contributing the way they would like.; Planning to start evaluating the experiences of the PPI reps. Want to start recording what they’re doing with PPI so they’ve got models to build on in the future.; Moving from individual PPI on a specific trial to a group/panel. The department is setting up a PPI health services research group which as a trials unit we’re feeding into that via the working group to development the terms of reference and SOP [standard operating procedure] for that group. Ensuring the PPI panel is costed into the application for each trial | |
| 21. Which factors have been challenging in involving patient and public representatives in the design and conduct of clinical trials? (Please tick all that apply) | 33 |
| Identifying PPI representatives | 22 (66.7) |
| Lack of funding to carry out PPI appropriately | 11 (33.3) |
| Difficulties with PPI members’ expectations | 7 (21.2) |
| Maintaining PPI representatives throughout the trial | 12 (36.4) |
| Time taken to support PPI representatives | 6 (18.2) |
| Other (please specify) | 15 (45.5) |
| Getting people to understand why we are asking their opinions about things. Some members of the PPI group ‘get it’, some are there because they want to say thank you or are interested in finding out more about the clinical side, they haven’t even grasped that this is about research necessarily. When doing the qualitative research the participants find it difficult to separate their experience of being part of the research and being a patient in hospital;Researchers expectations of PPI and PPI expectations have been a miss-match [sic]. This could be improved by training – what areas can PPI best contribute? Also turnover – doing a trial where there’s a significant disease e.g. cancer, a PPI rep can become unwell;How to implement PPI, difficult to find out how to do this. Time taken to get them involved;Retention of PPI and getting regular contribution;Patients that just talk about their experiences not wider patient perspective;PPI’s understanding of clinical trials, e.g. design and interpretation of results;The overlap in roles between ICTU and chief investigator;We are aware that, particularly in our [ethnic minority] groups, there are strong preferences for how PPI should be conducted which are at odds with funders expectations. For example, in our [ethnic minority] PPI groups, there is a definite aversion to belonging to committees, and a strong desire to be consulted as a group by our PPI liaison colleagues;How to get them involved in data analysis;No challenges;When you have funding the payment regulations are now a [. . .] nightmare. We can’t make direct payments without making giving PPI reps honorary contracts with the University. I don’t think we can even give them high street shopping vouchers any more. There’s a direct conflict between what INVOLVE want us to do and what the treasury demands of HEIs – and no one’s interested in sorting things out. Fortunately, most of our PPI reps are willing to do it on a pro bono basis – we try to pay them but it’s become too much of a drag for them;PPI not contributing as they would like – how to address this? What to do when PPI ‘is not working’ either for the PPI or the organisation;None; Finding people with the right skills/attitude. Difficulties with academics expectations of PPI. Getting PPI input that is ‘real’ and not token input. Informed input – that they understand what the issues of the trial are and their input is really relevant;Difficulties in PPI reps understanding – e.g. understanding randomisation | |
Key motivating factors for PPI were previous positive experience (71%) and requirement of the funder (66%) (question 12). The CI identified PPI contributors for one-quarter of RCTUs, with the majority of remaining RCTUs indicating shared responsibility between the CI and the RCTU (question 7). Identification of PPI contributors was highlighted as being a challenging factor for two-thirds of RCTUs (question 21). Half of the RCTUs adopted a model of PPI where individuals were attached to a single trial, and one-quarter used a panel who contributed across multiple trials (question 6).
The most frequent approaches to involvement were as grant coapplicants (83%) or members of the TSC (86%) (question 9); only one-third used more responsive modes of engagement whereby the contributors were contacted in response to specific issues. The most common activities of PPI were commenting on patient information sheets, websites or newsletters (100%), and providing insight in recruitment issues (94%) (question 13).
One-quarter of RCTUs reported using the same approach to PPI across their portfolio of trials, with the majority indicating that the approach taken was determined by trial characteristics and availability of contributors including user groups (question 11). This is perhaps suggestive of an informal risk-based approach to PPI.
Half of the RCTUs had a standard operating procedure for PPI either in use or in development, which illustrates that engagement is being recognised as a core activity of RCTUs. However, only one-third are requesting PPI feedback on their experience, suggesting that there may be a gap in developing procedures and learning from the contributors engaged across their trials.
The majority of RCTUs identified and managed expectations of PPI contributors, providing details verbally or in writing (question 8). However, a fifth of RCTUs reported managing expectations of PPI contributors as a challenge of involving PPI members (question 21).
Only one-fifth of RCTUs used INVOLVE resources to train PPI contributors, and one-third currently offer no training (question 17). In terms of support, just under one-third linked PPI contributors together for peer support and over 60% provided support by a specified member of staff. INVOLVE materials were used as support by under one-quarter of respondents.
Responses indicated PPI as an area of development within RCTUs. Sixty-four per cent indicated recent developments or changes in how they plan to approach PPI in the future (question 20) and 28%, 21%, 15% and 15% of RCTUs were developing standard operating procedures, payment policies, costing models and training respectively (questions 5, 15, 16 and 17). Employment of a PPI co-ordinator or support officer appeared to be an approach increasingly taken.
Key points
-
UK Clinical Research Collaboration Registered Clinical Trials Units are proactively considering PPI requirements within their trials portfolios, as demonstrated by the significant proportions that engage in identifying contributors and the increasing numbers that are developing standard operating procedures.
-
The implications of the level of activity within CTUs to support PPI need to be considered in line with core funding requirements.
-
UK Clinical Research Collaboration Registered Clinical Trials Units are determining PPI requirements within individual trials based on the trial characteristics. This presents an opportunity to consider whether or not a formal risk assessed approach to PPI could be developed and evaluated within this network.
-
Targeted engagement between INVOLVE and the UKCRC network of RCTUs should be considered to benefit PPI activity from both researcher and contributor perspectives.
Chapter 8 Discussion and conclusions
This is the first study to systematically examine PPI in a large and varied cohort of randomised trials by investigating plans for PPI; the acceptability of those plans to funders and their assessment by funders; the extent to which those documented plans for PPI were realised; the impact achieved; and the challenges and lessons learnt along the way.
Summary of main findings
Patient and public involvement in grant applications for funding
A minority of early-stage grant applications described PPI activity within their development. Although plans for PPI activity increased within later-stage applications, and once funding had been achieved, a key finding from this project was the need to instigate early PPI and the benefit of doing so. Although funding board comments rarely concerned PPI, a greater proportion of external referees commented on PPI, frequently requesting that PPI be increased but often without elaborating why or how. Disagreements on the acceptability of PPI within a trial were common between referees. This may indicate the difficulty faced by referees in assessing PPI given the absence of a robust evidence base, the low level of detail in the applications and, for the second stage of the application process, the discrepant information within the two separate pieces of documentation.
There was some evidence to suggest that the further the trial deviates from routine clinical practice the more likely the application is to describe PPI, and PPI was particularly frequent in applications for blinded trials or trials allocating participants to placebo only. This may indicate the beginning of a risk-based approach to PPI.
Implementation of patient and public involvement: from plans to actions
This is the first study to examine whether or not plans for PPI, as documented in randomised controlled trial grant applications, are being implemented. Based on the accounts of researchers and PPI contributors we found that most triallists are indeed putting their plans into action, although in some cases the plans were minimal and relatively easy to execute. There were a few trials for which we were unable to confirm whether or not plans were implemented in full, but all did incorporate some PPI. Many trials implemented multiple modes of PPI, which is both surprising and encouraging given that PPI was less prominent when the proposals for the trials in this cohort were being developed. CIs encountered complications from which they learnt valuable lessons. Difficulties finding and retaining suitable contributors, and engaging in PPI ‘too little too late’, led triallists to say they would do things differently in future. Many reflected on how they would aim for earlier engagement next time and seek involvement from a more diverse source such as patient panels or focus groups. PPI contributors themselves mentioned that becoming involved after the trial had begun, or infrequently, resulted in missed opportunities for them to contribute. Some referred to uncertainty about their role and many struggled with jargon, an enduring problem despite the availability of apparently straightforward solutions.
Regardless of statements about PPI in their funding application, some triallists had no expectations of what PPI might achieve, and their only motivation for including PPI was a belief that it was necessary or would help to secure funding for their trial. Such strategic minimalism may be an inevitable side effect of policies to promote or require PPI in trials. It may also reflect researchers’ professed inexperience of PPI. A small number of trials did not have documented plans for PPI but all did nevertheless include some PPI, possibly influenced by reviewer and panel comments. However, one of these trials had been through several stages of PPI prior to the grant application and was requested to implement further PPI over the course of the trial. This highlights the potential predicament of researchers whose trial may have benefited from considerable PPI prior to funding (e.g. in feasibility and pilot work) and who forecast that they would need relatively little PPI during the trial itself, only to find that funders insist on PPI at all stages. Many informants believed formative PPI prior to funding was one of the most useful, credible aspects of PPI. Particularly in cases where there has been extensive PPI prior to the main trial, it is important for all members of the research community to consider whether or not plans for ongoing PPI match the needs of a particular trial and at what stage(s) further PPI would be appropriate.
Pathways to impact
Our study is the first to provide insights from a diverse sample of researchers and PPI contributors about the pathways to impact for PPI within randomised trials. Well over half of the informants indicated that PPI had made a difference to the trial or influenced the trial team and none reported unfavourable impacts from PPI. CIs who described goals for PPI and planned its implementation in the light of these goals tended to report impact, whereas those whose goals for PPI did not extend beyond meeting perceived funding requirements usually reported little or no impact from PPI. PPI contributors who spoke of having a good relationship, particularly in terms of feeling part of the team, also tended to report impact from PPI, and both researchers and PPI contributors pointed to the importance of implementing PPI before seeking funding. Despite the frequent practice and policy recommendation11,12 to include PPI contributors on steering committees, researchers and PPI contributors often reported that such oversight roles made little or no difference within a trial. Whether or not CIs valued PPI seemed to be linked to the goals they described and how they implemented PPI. CIs who expressed scepticism about PPI focused mainly on using PPI to meet funding requirements, whereas those who valued PPI often described in detail how it was of benefit within their trials. CIs who were sceptical of the value of PPI tended to implement it only by including PPI contributors on TSCs. Our study confirms that some researchers seem to accord little value to PPI. It also raises the possibility that this may become a self-perpetuating cycle, with such researchers implementing PPI in ways that may provide little opportunity for it to benefit randomised controlled trials and then concluding that PPI made little difference to their trials.
Training
Informants involved in the interviews had reservations about the need for training in PPI, particularly in relation to training PPI contributors. Very few contributors had received training for their roles and many were reluctant to engage in it. Researchers shared this lack of enthusiasm for training PPI contributors, although both groups of informants welcomed informal induction ‘conversations’ to help contributors to understand their roles. There were, nevertheless, indications that current approaches to induction and support for PPI contributors were a problem. Induction seemed to provide little scope for contributors to negotiate their roles, and support for contributors was largely implicit and focused on practical arrangements rather than on helping contributors to function in their roles. Rather than training contributors, researchers used their networks and others’ recommendations to identify and select individuals who already possessed attributes perceived as important for the role. Therefore, informants tended to see training PPI contributors as redundant because, through the way they had been selected, contributors were believed to possess the necessary attributes.
Our findings raise questions about the selection of PPI contributors. Researchers described how they worked to select PPI contributors who were educated and articulate, despite recognising that this raised questions about contributors’ abilities to provide the patient perspective. Individuals who are educated and articulate have been found to be particularly likely to volunteer as PPI contributors. 70
As alluded to by many of our informants, such PPI contributors may struggle to understand the perspectives of patients who are less articulate or educated. There is also a danger that such selection practices, if reproduced across many studies, could mould research to the preferences of advantaged groups. 70
Informants were also concerned that training and cumulative experience in PPI roles overprofessionalised contributors and limited their ability to provide an authentic patient perspective. Researchers described a tension between needing contributors who could provide an authentic patient perspective and needing contributors who could function in oversight and managerial roles (e.g. as members of TSCs and TMGs respectively). Some commented that this tension could be resolved by selecting particular PPI contributors for particular roles within a trial. Indeed, informants in our study pointed to the importance of involving both professional and lay PPI contributors, the former in managerial or oversight roles, and the latter in responsive roles via patient advisory panels. Such mixed models of PPI could help to avoid the selection difficulties that our participants identified and address the multiple functions required of PPI within clinical trials.
Although few of our informants identified the selection of PPI contributors as a training need, our findings indicate that it warrants consideration as a topic for training.
Informants were more receptive to training researchers in PPI than training PPI contributors, and most researchers either had received training or indicated that they would find it helpful. Nevertheless, a sizable minority pointed to how it was sufficient to learn about PPI ‘on the job’ or that evidence to inform training was lacking. Contributors also saw a fairly limited role for training researchers in PPI, although some pointed to the use of plain English and clarity about PPI contributor roles as areas in which researchers could benefit from training.
Results in the context of previous research
To our knowledge this is the largest study of PPI in randomised trials to date. Several of our observations receive support from previous studies and reviews of PPI, although most of this work has not concentrated on PPI in randomised trials. Compared with other forms of health and social care research, randomised trials are highly structured and intensively regulated entities. This limits the relevance of PPI studies conducted outside the context of a randomised trial for understanding how PPI can make a difference within trials. For example, it will usually be harder to change aspects of a randomised trial after it has started than other types of studies.
Our findings are timely given the 2014 announcement71 of a strategic review of PPI in research within the UK and the increased emphasis on stakeholder involvement internationally.
Patient and public involvement in grant applications
The impact of public involvement in the research commissioning programme of the HTA has been previously assessed;72 however, this is the first study to look at PPI within a cohort of the research funded by the HTA and at funding board and referee assessments of PPI plans contained within applications. Our findings point to the difficulties of implementing PPI prior to funding and consequently the difficulties that funding panels and reviewers face in assessing the quality of a trial team’s plans for PPI, beyond identifying potential ‘red flags’ such as PPI contributors being limited to steering group membership or their involvement being sought only after funding has been awarded.
In England, a PPI bursary scheme has recently been launched by some of the NIHR Research Design Service, but is limited to those in receipt of advice from the Research Design Service although it has been shown to be beneficial. 73,74 However, the funding available is often small and inaccessible to those working within the tight timescales of a typical funding call. 69 Increasing the availability and scale of resource to provide an infrastructure to support researchers and contributors to initiate PPI at the pre-funding stage would help to facilitate earlier implementation.
Implementation of patient and public involvement: from plans to actions
We found no previous reports on the extent to which documented plans for PPI within trials were subsequently implemented. Nevertheless there have been several accounts of challenges involved in implementing PPI which, while not in a trials context, endorse our findings. For instance, recent reports have referred to tokenism,75,76 or highlighted the potential challenges in identifying suitable individuals who are impartial and able to understand research methodologies, retain an interest and commit in the long term;16,18,20–22 of researchers having little experience of PPI and being uncertain about what to expect;20,22,77 and of jargon-related problems. 18,55,74 INVOLVE suggests that PPI contributors would benefit from a ‘glossary of technical terms’,21 again something reflected in the suggestions from contributors within our study.
Staley5 refers to the challenge of ensuring that involvement is meaningful and not simply tokenistic. As described within this report, a tokenistic or minimalistic approach aimed at meeting funder requirements, rather than meeting goals and objectives set by researchers for PPI, may be a self-fulfilling prophecy.
Pathways to impact
Previous work has pointed to the different types of impact that we identified, both focused24–27,28,44 and diffuse. 5 Previous work has also identified the role of researchers’ values,3 the quality of the relationship between researchers and PPI contributors45 and the importance of implementing PPI through the ‘life course’ of a project5 in facilitating the impact of PPI. Our findings concur with the PiiAF,55 which was informed by a large mixed-methods study of the views and experiences of members of the UK health and social care research community. This emphasised the importance of careful planning in implementing PPI and encouraged researchers to be explicit in thinking about how their approach to PPI will lead to the impacts they seek. 3,55 International guidance has also emphasised the importance of having PPI from an early stage, and having wider involvement than PPI on a steering committee,78 although such guidance has lacked an evidence base until now. Many countries now encourage or require PPI to be included in research,7–10,78 so our findings can be applicable internationally. The opportunity to compare our findings with previous evidence beyond this is limited because, as we note above, few studies have specifically investigated the impact of PPI on trials5 and we are not aware of any studies that have examined influences of PPI across multiple randomised trials. As the first evidence to indicate the ineffectiveness of limiting the involvement of PPI contributors to oversight roles on steering committees, our findings indicate that some recommendations on PPI in TSCs need to be amended to acknowledge the limitations of this type of PPI as the sole means of engagement. This recommendation will be of interest to research funders as well as PPI contributors and researchers. The trials we studied often combined two or more approaches to PPI, and our informants described the importance of having the freedom to tailor PPI to the emergent needs of their trial.
Training
Findings from the EPIC project regarding PPI training needs suggest that, although informants were more receptive to PPI training for researchers, there was considerable reluctance regarding the training of PPI contributors, with a preference for ‘informal inductions’. The health service researchers in a previous qualitative interview study varied in how they interpreted PPI policy and in their PPI ‘working practices’ and referred to how PPI brought a ‘fear of the unknown’. 76 This study also points to a ‘know–do’ gap, whereby researchers’ talk of the importance and value of PPI in the ‘ideal’ world stood in contrast to their experiences of ‘the reality’ of implementing PPI in practice. 79
There have been few previous empirical studies of PPI training for either researchers or PPI contributors, although training has generally been recommended for both groups. 3,36,37 In showing that the appetite for PPI training is limited, our findings diverge from previous research that indicates more enthusiasm for PPI training among researchers and PPI contributors. 3,80–82 This may reflect differences in sampling between previous work and ours, and particularly our focus on clinical trials. Previous research has also tended to seek informants’ views on training in general, whereas we explored informants’ views about training specifically for themselves and for the researchers or PPI contributors with whom they worked. Interestingly, we found informants became more receptive to training when their focus shifted to generalised ‘other’ researchers or contributors outside their trial.
Informants were particularly concerned that training could hamper contributors’ ability to provide a patient perspective. Although it is possible to envisage ways in which training could support rather than detract from this ability, such concerns need to be taken seriously. The pronounced reluctance that we identified regarding training for PPI contributors, and informants’ preferences for ‘conversation’ over ‘training’, align with the emphasis informants gave to establishing good relationships between the PPI contributors and the research team. In a context where good working relationships are prioritised, informants may see conversational approaches to learning as more conductive to successful PPI than the type of practical or technical instruction that is usually associated with training. Indeed, the type of learning needed for PPI may be more wide-ranging and better supported by more discursive types of educational provision such as action learning sets83,84 and coaching. 85 Our study indicated the areas of learning or training need identified by researchers and PPI contributors (see Table 24). These are similar to those previously identified,3 although informants in our study tended to speak of training for PPI contributors as comprising ‘how to do research’ whereas training for researchers was seen as comprising ‘how to do PPI’. Given that some PPI contributors felt they lacked clarity about their roles, training that helps both researchers and PPI contributors to learn how to do PPI would be beneficial. Our informants also described training as something that was delivered separately for PPI contributors and researchers. However, reflecting our informants’ emphasis on the need to develop good relationships and mutual understanding of roles, we support previous suggestions that training which allows contributors and researchers to learn from each other in joint sessions would be beneficial. 37
Networks
Hanley et al. 49 reported on a national questionnaire survey on the role of PPI in designing, conducting and interpreting randomised controlled trials of clinical trial co-ordinating centres and concluded that PPI was still uncommon. Since the publication of that survey there have been many changes in the clinical research environment, including those brought about by the establishment of the UKCRC in 2004 and the RCTUs in 2007. RCTUs are assessed as having the expertise necessary to ensure high-quality, successful and timely trials, and to meet regulatory and governance requirements. Within the first wave of CTU applications to the UKCRC for registration status, each of the RCTUs agreed to the best-practice principle of ‘an organisational commitment to patient/public involvement’. 86 However, in later registration rounds there has been no mention of a commitment to PPI. Nonetheless, this project demonstrates a widespread commitment from the CTUs achieving UKCRC registration status. This commitment needs resourcing and may require core funding support to be sustainable. With respect to this, it could therefore be of benefit for future UKCRC registration calls to be explicit about requirements for PPI. This study also identified a need for greater engagement between RCTUs, INVOLVE and funders of research to benefit PPI activity.
Study strengths and limitations
Our study had some limitations and our findings should be regarded carefully, particularly as PPI is a field where policy has tended to outpace evidence. We used a historical cohort of trials that had been funded between 2006 and 2010. Even in the short time since then, the emphasis on PPI has grown and our findings may not reflect the planning and implementation of PPI in trials funded more recently.
Our sample was limited to trials within one UK-based research funding stream, the NIHR HTA programme. Although this may limit the transferability of our findings, as one of the world’s leading funders of health research, NIHR’s research activity is substantial.
To be eligible for inclusion within the cohort, applicants were required to have received funding for the trial between 2006 and 2010. Documentation could not be made available for unsuccessful applications. Consequently this study does not link details of PPI within the application to the successful award of the grant. However, while the HTA programme encouraged PPI during this period, via its guidance notes to applicants and web information, and allowed it to be budgeted for, PPI was not mandatory within the period of the cohort, and that on its own would not have led to a decision that the outline application should not progress. As the cohort was identified by receipt of funding during the period rather than on the year when the outline application was submitted for consideration, fewer trials were available dating from the beginning and the end of the period.
The period of the cohort should be considered when generalising the findings to the present day, given revisions to the guidance for applicants and application forms in relation to PPI. From 2012, HTA boards have started to include PPI membership, and the standard NIHR application form has been introduced, with revised sections requiring applicants to define their PPI involvement clearly, and guidance notes to applicants clearly stating that there is now an ‘expectation’ of ‘active involvement’ of patients and the public in the research it supports. 87 This may lead to increased descriptions but may not be associated with better involvement: a view which has some support from the current guidance, which states, ‘Whilst patient and public involvement (PPI) may not always be needed for all types of research, it is always relevant for HTA trials. Many PPI sections on the application are unconvincing to our consumer referees and Board members’. 88
Some of the trials in our sample were also initiated and completed some time before the interviews. However, this limitation is offset somewhat by the inclusion of ongoing trials in which PPI activity was more recent and therefore easier to recollect. In some cases informants clearly struggled to recall events for trials which had ended several years previously or where researchers were involved in a number of trials simultaneously. We explored with informants how PPI contributors were involved in the trials but did not directly quiz CIs about why certain plans within their application were not implemented. This was intentional, as we did not want to pose questions which might have seemed accusatory and had a detrimental impact on the rapport between informant and interviewer, or risk informants becoming defensive. Whereas some triallists seem to have expanded on their plans for PPI once the trial was under way there may, conversely, have been instances in which plans were not fully documented within the grant application.
Like most other studies exploring the impact of PPI in research, it was limited to investigating researchers’ and PPI contributors’ reports of their views and experiences. 5 Objective techniques for evaluating impact and its influences remain elusive in a process that is inherently relational, subjective and socially constructed. 45 For example, some informants reported that PPI contributors’ input helped to improve response rates by reducing the length of questionnaires, yet there is the possibility that valuable information was lost in the process. Participants in Barber et al. ’s45 mixed-methods Delphi survey and qualitative interview study questioned the feasibility of objectively evaluating the impact of PPI on most research processes and outcomes. In this regard, a strength of our study is that we triangulated the accounts of multiple informants in half the sampled trials. Linked to our study’s retrospective design, however, informants struggled to recall particular examples of PPI input. In addition, as others have noted,45 there are inherent difficulties in attributing impact to the contributions of particular individuals, when the actions to address many difficulties within trials are likely to be the product of a series of complex interactions among research team members.
Although our informants were drawn from a cohort of trials, we could interview only those who opted to do so. The response rate to the CI survey, which was our main route for accessing interview informants, was high (73%) whereas the response rate for the CI interviews was lower (51%). In addition, all of the PPI contributors interviewed were involved in managerial or oversight roles, as we were unable to access those in responsive roles, because most researchers did not hold contact details for contributors in such roles. Our access to the information about PPI was limited in some cases; for example, where the only informant from a trial was the PPI contributor in an oversight role, we were unable to ascertain the other types of PPI within that trial.
Because our interview sample was drawn from a cohort study and survey we are able to provide more information about our sample than is typical for qualitative studies. Although we aimed to purposively sample survey respondents to access a diversity of views, we eventually invited almost all of those who indicated willingness to be interviewed. The survey responses of the subsample who were interviewed were more favourable in their views of PPI than the wider sample of surveyed CIs, although, as we report, interviewed CIs expressed a diversity of views about PPI and some were sceptical about its value. Our access to PPI contributors was limited to those whose contact details were provided by CIs responding to the survey and one identified by the chair of a TSC.
The survey responses cannot be taken as a fixed or true point from which to assess the adequacy of our interview sample. 69 In their interviews participants described their experiences in detail and we were able to consider how they talked about PPI, as well as what they said about it. In this context, it is notable that, while all of the CIs in the survey reported some impact from PPI, the interview accounts of CIs told a rather different story, with one-third describing PPI as having no impact. This indicates the usefulness of qualitative approaches for investigating complex processes such as PPI, which are subject to moral, reputational and other sanctions. The diversity of perspectives that we accessed also suggests that we had some success in minimising the selectivity that has been a difficulty in some previous work on PPI.
To our knowledge this is the first study to report the accounts of researchers and PPI contributors who have not previously engaged with PPI training as well as those who have. Our study provides insights about how PPI training can be developed to enhance its relevance from the perspective of both groups. By exploring training needs in the context of informants’ wider experiences of PPI, our study has also identified some potential topics for training beyond those articulated by our informants. As this illustrates, individuals are not necessarily able to identify potential deficits in their own knowledge and skills,89 and a limitation of our study is that we did not formally assess informants’ knowledge and understanding of PPI.
Future research
Given the difficulties for some informants in recalling PPI contributions, future research in this area that takes a prospective approach would be valuable. PPI is an area of rapidly evolving practice, and prospective research would also be valuable to explore how such changes are influencing how PPI is interpreted and implemented. In view of the difficulties for informants in attributing impact, and the relational and subjective nature of PPI activity, ethnographic research that combines observation and multi-informant interviews is likely to be informative. Many will also regard future prospective investigation of the impact of PPI on trial outcomes, such as recruitment, retention and participant experience of trials, as essential to further optimise PPI. The role of funders and the UKCRC network of registered trials units in monitoring change and subsequent impact should be explored.
The research community needs to give further consideration to processes for selecting PPI contributors and models of implementing PPI. Randomised trials may benefit from a diversity of patient perspectives and have the potential for benefit from ‘professionalised or experienced’ PPI contributors as well as those who are research naive.
However, one or two specially selected individuals cannot represent the perspectives of diverse groups of patients. Indeed, there was evidence that PPI contributors were selected for their atypical characteristics that facilitated their ability to provide input and reduced the need for training. PPI via patient advisory groups or panels enables the voices of multiple and diverse groups of patients to be heard. The findings we report indicate that this type of PPI is more powerful in terms of its perceived impact on research than oversight and managerial PPI. However, when one or two individuals are selected from such groups the process or mechanism for how they will actively engage with the population of interest about the trial needs to be clear. Effective mechanisms for achieving this, including social media, should be explored, as should the role of qualitative researchers with the skills necessary to explore and summarise diverse opinions.
Achieving consensus on essential and desirable attributes, skills and experience of PPI contributors in relation to trial-specific roles may facilitate researchers in selection processes, thereby helping PPI contributors to achieve impact. Further consideration should be given to training requirements, ensuring they are evidence based and evaluated in relation to implementing PPI and subsequent impact. Further research is needed on the role and value of jointly training researchers and PPI contributors in ‘how to do PPI’ rather than separately training PPI contributors on research and researchers on ‘how to do PPI’. In addition, the RCTU network, INVOLVE and funders should consider developing agreed packages of materials to be distributed to CIs, CTUs and PPI contributors that could be used to assist them in developing and supporting PPI activity.
This project has highlighted the strength of the funders in shaping the approach to PPI. However, further changes are needed to the approach funders use to request information about PPI in their application forms. In a bid to move away from a tick box approach to PPI it is a paradox to include a section of tick boxes within this section of the application form. Currently a list of tick boxes is provided to identify the areas of PPI activity. Requesting specific goals for PPI, and assessing the methods and costs for achieving such goals, may promote more considered approaches and improve the ability of reviewers and funders to assess such plans.
Further consideration is needed on how funds can be made available to researchers to support development of PPI plans and activity prior to grant submission. There was some evidence to suggest variation in practice relating PPI activity to some trial characteristics. Many trial activities are now considered in a risk-proportionate approach. Evidence from this project suggests that informally this may be being applied to PPI activity in randomised trials. The acceptability, applicability and cost-effectiveness of such an approach should be considered along with identification of pertinent factors.
Implications and tips for the trials community
We have used the insights of informants to generate practical tips which may help future triallists and PPI contributors (Box 3). We envisage that these be considered alongside previously published guidance for PPI in trials21,23 and consensus principles for PPI in health research. 90,91 The tips generated from evidence in our study cover the importance of early planning, of timely and flexible PPI, and of communication and clarification of roles. They also stress the need to consider the difficulties posed by the use of ‘jargon’, and problems contributors experience in understanding certain aspects of the research process. The difficulties contributors experience with specialist or technical terminology have been widely reported. 16,18,77 Our data suggest that this problem has existed for some considerable time, and we outline the practical solutions suggested by PPI contributors. The tips in Box 3 could be used to inform PPI training and could be helpful in other types of health research. Given that the usefulness of the points in Box 3 depends on researchers’ willingness to engage genuinely with PPI, the tips we present might also assist funding bodies and grant reviewers in determining whether or not submitted plans are fit for purpose.
They should be passionate people that really believe that they want to try to make a difference.
-
Consider the qualities of the contributors in relation to the role that they will fulfil in terms of their confidence, motivation, commitment and any previous experience of contributing.
-
Ensure that they are able to see beyond their own particular experience and draw on the experience of their peers.
It is important to ensure that the persons aiming to champion the patient perspective have the qualities necessary to fulfil the role. Particular consideration should be given to attributes that could not be reasonably achieved by training.
Early patient and public involvement
You’ve got to plan ahead.
-
Begin planning PPI and consulting with contributors when starting to plan the trial.
-
Consider including PPI contributors in responsive and managerial roles, for example as coinvestigators.
Researchers and PPI contributors emphasised how early and regular involvement allowed contributors to input more effectively. PPI prior to the trial (e.g. in contributions to grant writing, trial design and feasibility studies) was a key aspect of PPI, and in some cases the most important one.
Flexible patient and public involvement
One size does not fit all.
Reaching out was crucial.
-
‘Reach out’ and make use of responsive modes of PPI.
-
Consider whether or not oversight PPI (e.g. on a TSC) is sufficient to meet trial needs.
-
Involve more than one or two PPI contributors, more than once or twice a year.
Patient and public involvement is context-specific so it is important to tailor PPI to the emergent needs of trials and be creative to encourage active engagement. In terms of responsive PPI, liaison with relevant patient panels or groups may be particularly helpful when more diverse perspectives or wider consensus is needed; consider whether or not surveys (e.g. of support group members) would be useful in gaining wider opinion on ‘burning questions’ or qualitative research to gain deeper understanding. Researchers felt that managerial or responsive capacity helped to foster meaningful PPI.
Communication, clarification and interaction
I can’t understand why they use me. I just sit there bewildered.
-
Negotiate with contributors at an early stage about what they can bring to the trial and what they want to bring.
-
Determine whether or not this matches the trial’s needs and clarify roles and expectations.
-
Be sensitive to contributors’ needs and preferences.
Communication between researchers and PPI contributors is crucial at the outset to clarify roles and expectations, and throughout the trial to optimise engagement and provide feedback about contributions. It may be that particular contributors do not have the insights a trial needs, or that triallists need to rethink their plans for PPI in the light of experience. Researchers should avoid seeming ‘dispassionate’ during meetings when discussing a particular illness or condition that impacts on the lives of PPI contributors, and make a genuine effort to understand contributors’ points of view.
Language of research
Break it down into a language everybody understands.
-
Minimise and explain jargon.
-
Provide glossaries and ‘translations’ where applicable.
Researchers and contributors should discuss their written and verbal communication preferences and how to minimise and explain jargon. Suggestions for minimising jargon included lists of acronyms or glossaries of research terms. PPI contributors should be prepared to speak up if there is a problem and, with the help of researchers, be willing to acquaint themselves with specialist terms over time.
Budgeting for patient and public involvement
University didn’t want to pay him the money.
We had money in the pot but only for one PPI.
-
Budget for PPI: think about contributors’ time plus expenses.
-
Explore opportunities for pre-trial support for PPI.
Well thought-through plans will help inform how much to ‘cost in’ for PPI. Consult with administrators in your organisation at an early stage to iron out processes for payments to PPI contributors. Talk to contributors to make sure they will be happy to accept reimbursement beyond expenses. Find out whether or not there are any local or national resources to support PPI prior to funding applications.
Fit-for-purpose patient and public involvement
The person we chose had very little engagement, it struck me as a complete waste of time.
-
Agree what types of PPI would be appropriate and understand why.
-
Consider benefits of involving those with experience of the condition.
-
Recognise the drawbacks of involving those under current care of the researcher.
Think through plans for PPI and centre them round the aims and needs of the trial. Agreement about and understanding of what and why PPI is needed will help in planning it. Involving people with experience of the condition, intervention or service where applicable may be particularly germane in identifying research priorities and enhancing trial design. However, the inclusion of patients under the current care of a team member may lead to difficulties for both researchers and contributors.
A study of the UK health and social care research community has recently informed the development of a PiiAF, which emphasises the value of well thought-through planning before implementing PPI as well as the subsequent evaluation of its impact,77 and INVOLVE21 has emphasised the importance of clear guidance about roles. However, researchers also need some scope for flexibility and contingency in planning PPI: our finding that some triallists expanded their sometimes already detailed plans supports the need for flexible and iterative approaches to PPI in order to accommodate the unexpected and respond to opportunities and difficulties as they arise.
Our findings add fuel to recent drives and initiatives to promote the assessment and reporting of PPI processes3,8,92,93 including the GRIPP (Guidance for Reporting Involvement of Patients and Public) checklist. 94 The CONSORT (Consolidated Standards of Reporting Trials) Statement, which was established specifically to encourage adequate reporting of randomised trials, does not cover PPI. We suggest that consideration be given to incorporating advice on reporting of PPI in the main CONSORT checklist, so that reference to PPI is incorporated within the main reports of trials, alongside separate detailed reports on PPI, in line with the GRIPP checklist. If, in planning their PPI, triallists are prepared to consider and report its outcomes in terms of not only what happened and how, but also how this matched the needs of the trial, whether or not any complications arose or adaptations were made, and what lessons were learnt, then the evidence base will grow and the research community as a whole can learn. The EPIC project has highlighted the value of listening to the accounts of PPI contributors as well as researchers, and this should feed into the evaluation and reporting of PPI.
Implications for funders
Many researchers believed that funding would not be forthcoming unless they included PPI. Although this might be regarded as indicating the success of policies to promote PPI, it was clear that some circumvented these policies by adopting a minimal approach to PPI. In the light of our findings, research funders might want to consider how their policies could be refined to address this difficulty. Our study points to the inadvisability of applying ‘one size fits all’ methods to the implementation and evaluation of PPI in research, and underlines the importance of funding panels conducting nuanced assessments of a research team’s goals for PPI in the context of a particular trial. This might encompass scrutiny of a research team’s account of how their proposed trial stands to benefit from PPI and assessing the suitability of their plans in the light of these goals. It might also involve accepting that PPI should be proportionate to the needs of a particular trial and that a minimal approach to PPI may be legitimate in some cases. Researchers who can adequately justify such an approach should not fear that their chances of funding success will automatically be jeopardised by being candid about this. A sizable minority of informants did not report any impact from PPI. Although our findings point to problems in the implementation of PPI as contributing to this lack of perceived impact, it is conceivable that some trials will have little to gain from extensive and elaborate forms of PPI.
Our findings endorse recent revisions to the NIHR’s standard application form, which now require applicants to clearly define their proposed PPI activity. Asking researchers to specify the type of involvement is a step in the right direction. However, we would suggest further changes should be implemented as described within the further research section, as the risk of strategic minimalism remains if plans are not afforded careful, context-specific consideration by funders and reviewers. Equally, there is a risk of inadvertent PPI profligacy, that is the encouragement of elaborate plans for PPI that are disproportionate to the needs of a trial. Ticking several boxes rather than just one box could equally be a token gesture, as well as an expensive one. Therefore, researchers might be encouraged to think just as much about why, how and when PPI will be useful as about what and how much PPI.
Although funding body policies support PPI, this support was not usually evident in HTA Board feedback to applicants at the outline stage. This may be because of the difficulties in assessing PPI given the lack of detail provided within applications. When feedback about PPI was given, this did not provide any guidance on how it should be addressed; that aspect is important given that it is dominated by opinion rather than evidence. Statements about the need to improve PPI should be supported with guidance on what PPI contributions would be appropriate within the trial being considered. Peer reviewers and board members who are asked to comment on PPI should be supported in doing so. Adoption of critical appraisal guidelines may be beneficial in achieving this. 95
Funding is available to support pre-application PPI; for example, the UK-based NIHR Research Development Service offers very small grants, which others have found to be helpful. 73,74 However, these grants are not easily or quickly accessible, particularly for those working to the typically tight deadlines of funding calls. Paradoxically, this renders pre-application PPI the most difficult to implement, even though our findings indicate that PPI is often most useful at this stage. Innovative organisations that involve patients at a metatrial level in research priority setting96 and in schemes such as COMET (Core Outcome Measures in Effectiveness Trials),97 which promotes the involvement of patients in developing ‘core outcome sets’, are providing knowledge and resources that individual trials can use. However, at the level of individual trials, infrastructural support for early PPI is also needed. Although there have been innovations in this area – for example, the US-based Patient-Centred Outcomes Research Institute has recently announced a number of ‘Pipeline to Proposals’ Engagement Awards8 – such moves are relatively novel, and similar steps by other organisations would be beneficial.
As well indicating the need for structures and resources to support PPI, our findings point to the importance of PPI that is fit for purpose, realistic and proportionate. We found that triallists who fully implemented a primarily oversight mode of PPI perceived little value in this involvement. Although oversight PPI seemed limited in terms of its practical impact, arguably it may serve important ethical and moral functions. However, in order to avoid inadvertently promoting PPI that is devoid of any function for both researchers and contributors, as we note above, funders should take full account of any PPI which has taken place prior to funding applications as well as encourage applicants to justify future plans for involvement. The NIHR HTA programme states: ‘While patient and public involvement (PPI) may not always be needed for all types of research, it is always relevant for HTA trials’. 98 Even if there is consensus that PPI is relevant for all trials, it may not be relevant at all stages of all trials. Equally, funders may wish to contemplate how ‘contingency’ resources could be made available for those trials that encounter unexpectedly intensive needs for PPI over the course of their implementation.
Accessing the perspectives of both PPI contributors and researchers is a strength of the EPIC project; however, this was limited by the difficulty in contacting PPI contributors. For future prospective assessments of PPI, both researchers and PPI contributors should be involved in its independent evaluation. Problems with the conceptualisation and measurement of the impact of PPI have been identified making meaningful evaluation problematic. However, funders could help by leading on prospective evaluation of PPI, utilising their strength to document and evaluate PPI processes rather than in the specification of the approach that should be used. Such specification may discourage researchers from considering what PPI is really needed or even act as a constraint.
Funders need to establish a register of contact details for PPI contributors to ensure contact is not required to be via CIs. In addition, progress reports requested by funders during trial conduct now request information on what PPI has occurred since previous reports. Requesting such progress reports from PPI contributors as well as researchers would be beneficial and allow funders to consider both researcher and contributor perspectives and provide communication between contributors and those funding their participation.
Implications for the networks
It was clear that CIs learnt from their experience of implementing PPI. The majority of NIHR-funded clinical trials are now supported by the RCTU network. This offers the potential to harness the lessons learnt from implementing PPI across a diversity of clinical trials rather than relying on the lessons learnt by a CI in a single trial being implemented in any successive trials in which that CI may be involved.
Clinical trials units are individually working to address PPI within the trials they support. The potential increase in experience, knowledge and efficiency from utilising the UKCRC network should be considered. There needs to be greater engagement between the network of CTUs, funders and INVOLVE to ensure that existing resources to support PPI are being used and address the difficulties CTUs face in accessing and funding PPI activity in the early stages of trial development. Engagement between INVOLVE and registered CTUs could ensure that, instead of a minority, a majority of CIs, PPI contributors and CTUs are aware of and use INVOLVE guidance. INVOLVE could lead on a package of materials that CTUs could provide to PPI contributors upon their engagement in a clinical trial. Any such package would need to be developed in collaboration with CTUs and allow for diversity in resources and processes. In addition, as noted above, the UKCRC should consider formally incorporating requirements for registered CTUs to support PPI activity; however, the implications for core support need to be considered.
Conclusion
We conclude that if researchers, PPI contributors and research funders wish to enhance PPI in trials they should consider how PPI can inform or benefit a trial and plan PPI to suit these goals, work to develop good relationships between PPI contributors and researchers, involve PPI contributors at an early stage, and favour responsive and managerial roles for PPI contributors in preference to roles that involve only oversight.
Effective mechanisms to obtain diversity of PPI contributors need to be explored. Selection of contributors has been identified as a training need and the use of mixed models has been suggested, to allow benefit from experienced contributors on oversight or trial management committees and research-naive contributors on responsive groups. However, where the aim of PPI is to gain a wide spread or diverse opinions, the role of qualitative researchers to support PPI in delivering such goals should be considered.
We recommend that funders remove PPI tick box sections from their forms and instead request a PPI-specific protocol separately requesting goals, methods and costs of PPI; that approach should enable reviewers to appraise the relevance and appropriateness of such plans. We would also advise funders against specifying the nature of PPI activity, to avoid minimalistic approaches intended solely to comply with funder requirements. We recommend increased availability and levels of funding to support pre-application PPI, and the identification of contingency funds to support PPI in response to unplanned need.
We also recommend that PPI contributors be enabled to report on their activities directly to the funders, and that the UKCRC formalise requirements for registered CTUs to support PPI activity. CTUs are ideally placed to lead on the development of a risk-based approach to PPI and of resources to evaluate PPI. They would also be central to encouraging greater peer support between PPI contributors both within and between clinical trials.
Collaboration between funders, INVOLVE and the UKCRC network of registered CTUs should be increased to ensure that they are aware of each other’s available resources, expectations and constraints. Such collaboration could be used to identify core materials that should be packaged for CTUs to provide to researchers and PPI contributors engaging on a trial to enable role negotiation, manage expectations and identify training needs to enable PPI contributors to function in their role.
Acknowledgements
The authors would like to acknowledge the help and support of the NIHR HTA programme in establishing the cohort documentation.
Department of Health disclaimer
This report presents independent research commissioned by NIHR. The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, the NHS, NIHR, the Medical Research Council, Central Commissioning Facility, NIHR Evaluation, Trials and Studies Coordinating Centre, the Health Services and Delivery Research programme or the Department of Health.
Contribution of authors
Carrol Gamble, professor of medical statistics, conceived the idea for the research and led the development of the grant application and project. She developed the data extraction tool, developed the database, extracted data, analysed data, developed and analysed the surveys, developed the interview schedules, commenting on the ongoing analysis and cowrote the report.
Louise Dudley, psychology research assistant, was involved in the development of the data extraction tool, completed data extraction, developed the surveys and was involved in developing the interview schedules, recruiting informants to the study, conducting interviews, conducting qualitative data analysis and interpretation, and writing the report.
Alison Allam, Philip Bell, Heather Goodare and Alison Walker formed the Public Advisory Group. A full description of the Public Advisory Group activities is provided in Appendix 1.
Deborah Buck was involved in conducting qualitative data analysis and interpretation, and writing the report.
Bec Hanley, director, TwoCan Associates, contributed to the project specification, commented on the data extraction items, surveys and manuscript, and co-led the co-ordination of the Public Advisory Group.
Jennifer Preston, consumer liaison manager, contributed to the project specification, commented on the data extraction items, extracted data, commented on the surveys and manuscript, and co-led the co-ordination of the Public Advisory Group.
Paula R Williamson, professor of medical statistics, contributed to the project specification, commented on the data extraction items and the surveys, and provided comments on each phase of the report.
Bridget Young, professor of psychological sciences, contributed to the project specification, commented on the data extraction items and surveys, led the qualitative phase of the report, including the interview schedules and qualitative data analysis and interpretation, and co-wrote the report.
Publications
Buck D, Gamble C, Dudley L, Preston J, Hanley B, Williamson PR, et al. From plans to actions in patient and public involvement: qualitative study of documented plans and the accounts of researchers and patients sampled from a cohort of clinical trials. BMJ Open 2014:4:e006400.
Gamble C, Dudley L, Allam A, Bell P, Goodare H, Hanley B, et al. Patient and public involvement in the early stages of clinical trial development: a systematic cohort investigation. BMJ Open 2014;4:e005234
Dudley L, Gamble C, Preston J, Buck D, Hanley B, Williamson P, et al. What difference does patient and public involvement make and what are its pathways to impact? Qualitative study of patients and researchers from a cohort of randomised controlled trials. PLOS ONE 2015;10:e0128817.
Dudley L, Gamble C, Allam A, Bell P, Buck D, Goodare H, et al. A little more conversation please? Qualitative study of researchers’ and patients’ interview accounts of training for patient and public involvement in clinical trials. Trials 2015;16:190.
Data sharing statement
The report is based on data extracted from NIHR HTA reports, survey responses and qualitative interview data. Anonymised data is available on request from the author for survey responses and HTA report extracts. Data from qualitative interviews is not available because of the difficulty in making interviews anonymous. We have provided relevant anonymised data excerpts from qualitative interview transcripts in the main body of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- INVOLVE . What Is Public Involvement in Research? n.d. www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/ (accessed 19 June 2014).
- Saunders CG, Girgis A. Status, challenges and facilitators of consumer involvement in Australian health and medical research. Health Res Policy Syst 2010;8. http://dx.doi.org/10.1186/1478-4505-8-34.
- Snape DK, Kirkham J, Preston J, Britten N, Collins M, Frogatt K, et al. Exploring areas of consensus and conflict around values underpinning public involvement in health and social care research: a modified Delphi study. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004217.
- Godlee F. Towards the patient revolution. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1209.
- Staley K. Exploring Impact: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2009.
- Boote J, Baird W, Sutton A. Public involvement in the design and conduct of clinical trials: a review. Int J Interdiscip Soc Sci 2011;5:91-111.
- Best Research for Best Health: A New National Health Research Strategy. London: Department of Health; 2006.
- Selby JV, Lipstein SH. PCORI at 3 years: progress, lessons, and plans. N Engl J Med 2014;370:592-5. http://dx.doi.org/10.1056/NEJMp1313061.
- National Institute for Health Research . Patient and Public Involvement n.d. www.nets.nihr.ac.uk/ppi (accessed 5 December 2014).
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada and Social Sciences and Humanities Research Council of Canada . Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans 2010. http://pre.ethics.gc.ca/pdf/eng/tcps2/TCPS_2_FINAL_Web.pdf (accessed 1 May 2014).
- TSC Data Monitoring Committee Guidance: NIHR. Southampton: NIHR; 2014.
- Guidelines for Good Clinical Practice in Clinical Trials. 1998.
- Hull D, Barton D, Guo K, Russell C, Aucott B, Wiles D. Patient and public involvement to support liver disease research. Br J Nurs 2012;21:972-6. http://dx.doi.org/10.12968/bjon.2012.21.16.972.
- Stewart R, Liabo K. Involvement in research without compromising research quality. J Health Serv Res Policy 2012;17:248-51. http://dx.doi.org/10.1258/jhsrp.2012.011086.
- Robinson L, Newton J, Dawson P. Professionals and the public: power or partnership in health research?. J Eval Clin Pract 2010;18:276-82. http://dx.doi.org/10.1111/j.1365-2753.2010.01572.x.
- Staniszewska S, Jones N, Newburn M, Marshall S. User involvement in the development of a research bid: barriers, enablers and impacts. Health Expect 2007;10:173-83. http://dx.doi.org/10.1111/j.1369-7625.2007.00436.x.
- Fudge N, Wolfe C, McKevitt C. Involving older people in health research. Age Ageing 2007;36:492-500. http://dx.doi.org/10.1093/ageing/afm029.
- Boote J, Baird W, Beecroft C. Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy 2010;95:10-23. http://dx.doi.org/10.1016/j.healthpol.2009.11.007.
- Oliver S, Gray J. A Bibliography of Research Reports about Patients’, Clinicians’ and Researchers’ Priorities for New Research. London: James Lind Alliance; 2006.
- Mockford C, Staniszewska S, Griffiths F, Herron-Marx S. The impact of patient and public involvement on UK NHS health care: a systematic review. Int J Qual Health Care 2012;24:28-3. http://dx.doi.org/10.1093/intqhc/mzr066.
- Public Involvement in Clinical Trials: Supplement to the Briefing Notes for Researchers. Eastleigh: INVOLVE; 2012.
- Vale C, Thompson L, Murphy C, Forcat S, Hanley B. Involvement of consumers in studies run by the Medical Research Council Clinical Trials Unit: results of a survey. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-9.
- Evans B, Bedson E, Bell P, Hutchings H, Lowes L, Rea D, et al. Involving service users in trials: developing a standard operating procedure. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-219.
- Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect 2012;17:637-50.
- Barber R, Beresford P, Boote J, Cooper C, Faulkner A. Evaluating the impact of service user involvement on research: a prospective study. Int J Consum Stud 2011;35:609-15. http://dx.doi.org/10.1111/j.1470-6431.2011.01017.x.
- Edwards V, Wyatt K, Logan S, Britten N. Consulting parents about the design of a randomized controlled trial of osteopathy for children with cerebral palsy. Health Expect 2011;14:429-38. http://dx.doi.org/10.1111/j.1369-7625.2010.00652.x.
- Szmukler G, Staley K, Kabir T. Service user involvement in research. Asia-Pacific Psychiatry 2011;3:180-6. http://dx.doi.org/10.1111/j.1758-5872.2011.00145.x.
- Boote J, Baird W, Sutton A. Public involvement in the design and conduct of clinical trials: a review. Int J Interdiscip Soc Sci 2011;5:91-112.
- Bengtsson-Tops A, Svensson B. Mental health users’ experiences of being interviewed by another user in a research project: a qualitative study. J Ment Health 2010;19:234-42. http://dx.doi.org/10.3109/09638230903531084.
- Guarino P, Elbourne D, Carpenter J, Peduzzi P. Consumer involvement in consent document development: a multicenter cluster randomized trial to assess study participants’ understanding. Clin Trials 2006;3:19-30. http://dx.doi.org/10.1191/1740774506cn133oa.
- Bastian H. Consumer and researcher collaboration in trials: filling the gaps. Clin Trials 2005;2:3-4. http://dx.doi.org/10.1191/1740774505cn060ed.
- Arnstein S. A ladder of citizen participation. J Am Inst Planners 1969;35:216-24. http://dx.doi.org/10.1080/01944366908977225.
- Tritter JQ, McCallum A. The snakes and ladders of user involvement: moving beyond Arnstein. Health Policy 2006;76:156-68. http://dx.doi.org/10.1016/j.healthpol.2005.05.008.
- NIHR . Public and Patient Involvement n.d. www.nets.nihr.ac.uk/ppi (accessed 9 April 2014).
- NIHR . Health Technology Assessment (HTA) Programme n.d. www.hta.ac.uk/PPIguidance/section1_2.shtml (accessed 26 September 2013).
- Ives J, Damery S, Redwod S. PPI, paradoxes and Plato: who’s sailing the ship?. J Med Ethics 2013;39:181-5. http://dx.doi.org/10.1136/medethics-2011-100150.
- Developing Training and Support for Public Involvement in Research. Eastleigh: INVOLVE; 2012.
- European Patients’ Academy on Therapeutic Innovation . EUPATI Training Course: Patient Experts in Medicines Research &Amp; Development – A Guide for Applicants n.d. www.patientsacademy.eu/index.php/en/joomdoc/eupati-training-course/267-eupati-guide-for-applicants/file (accessed 1 December 2014).
- INVOLVE . Training Case Study Three: Patient and Public Involvement Module for Researchers in MSc at King’s College, London University n.d. www.invo.org.uk/training-case-study-three-2/ (accessed 1 December 2014).
- INVOLVE . Training Case Study Two: Patient and Public Involvement Module in an MSc Clinical Research Programme n.d. www.invo.org.uk/training-case-study-two-2/ (accessed 1 December 2014).
- INVOLVE . Training Case Study One: Training for Researchers – a Workshop Designed by a Virtual Working Group n.d. www.invo.org.uk/training-case-study-one-2/ (accessed 1 December 2014).
- Examples of Training and Support for Public Involvement in Research: Sharing Innovative Practice Workshop. Eastleigh: INVOLVE; 2010.
- Noe RA, Tews MJ, McConnell Dachner A. Learner engagement: a new perspective for enhancing our understanding of learner motivation and workplace learning. Acad Manag Ann 2010;4:279-315. http://dx.doi.org/10.1080/19416520.2010.493286.
- Gradinger FB N, Wyatt K, Froggatt K, Gibson A, Jacoby A, . Values associated with public involvement in health and social care research: a narrative review [published online ahead of print December 10 2013]. Health Expect 2013. http://dx.doi.org/10.1111/hex.12158.
- Barber R, Boote JD, Parry GD, Cooper CL, Yeeles P, Cook S. Can the impact of public involvement on research be evaluated? A mixed methods study. Health Expect 2012;15:229-41. http://dx.doi.org/10.1111/j.1369-7625.2010.00660.x.
- Simpson A, Jones J, Barlow S, Cox L. Adding SUGAR: service user and carer collaboration in mental health nursing research. J Psychosocial Nurs Mental Health Ser 2014;52:22-30.
- Khwaja AI. Is increasing community participation always a good thing?. J Eur Econ Assoc 2004;2:427-36. http://dx.doi.org/10.1162/154247604323068113.
- TwoCan Associates . An Evaluation of the Process and Impact of Patient and Public Involvement in the Advisory Groups of the UK Clinical Research Collaboration n.d. www.twocanassociates.co.uk/perch/resources/files/2384_PPI+_Evaluation_Report%20(4).pdf (accessed 4 October 2010).
- Hanley B, Truesdale A, King A, Elbourne D, Chalmers I. Involving consumers in designing, conducting, and interpreting randomised controlled trials: questionnaire survey. BMJ 2001;322:519-23. http://dx.doi.org/10.1136/bmj.322.7285.519.
- McFadden E, Bashir S, Canham S, Darbyshire J, Davidson P, Day S, et al. The impact of registration of clinical trials units: the UK experience. Clin Trials 2015;12:166-73. http://dx.doi.org/10.1177/1740774514561242.
- Staniszewska S, Adebajo A, Barber R, Beresford P, Brady LM, Brett J, et al. Developing the evidence base of patient and public involvement in health and social care research: the case for measuring impact. Int J Consum Stud 2011;35:628-32. http://dx.doi.org/10.1111/j.1470-6431.2011.01020.x.
- Boote J, Telford R, Cooper CL. Consumer involvement in health research: a review and research agenda. Health Policy 2002;61:213-36. http://dx.doi.org/10.1016/S0168-8510(01)00214-7.
- Minogue VB, Boness J, Brown A, Girdlestone J. The impact of service user involvement in research. Int J Health Care Qual Assur 2005;18:103-12. http://dx.doi.org/10.1108/09526860510588133.
- Staniszewska S. Patient and public involvement in health services and health research: a brief overview of evidence, policy and activity. J Res Nurs 2009;14:295-8. http://dx.doi.org/10.1177/1744987109106811.
- Popay J, Collins M. PiiAF Study Group . The Public Involvement Impact Assessment Framework Guidance 2014 n.d. www.piaf.org.uk (accessed 23 July 2015).
- Kaur G, Smyth R, Williamson P. Developing a survey of barriers and facilitators to recruitment in randomized controlled trials. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-218.
- National Institute for Health Research . Clinical Research Network 2015. www.nihr.ac.uk/documents/about-NIHR/Briefing-Documents/4.1-Clinical-Research-Network.pdf/ (accessed 3 August 2015).
- UKCRC Registered Clinical Trials Units Network n.d. www.ukcrc-ctu.org.uk/ (accessed 18 June 2014).
- Bowen G. Naturalistic inquiry and the saturation concept: a research note. J Qual Res 2008;8:137-52. http://dx.doi.org/10.1177/1468794107085301.
- Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 2004.
- Lincoln YK, Lincoln SA, Guba EG, Denzin NS, Lynham YS. The Landscape of Qualitative Research. London: Sage; 2013.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- Strauss A, Corbin J. The Basis of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: Sage; 1998.
- Seale C, Silverman D. Ensuring rigour in qualitative research. Eur J Public Health 1997;7:379-84. http://dx.doi.org/10.1093/eurpub/7.4.379.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Ritchie J, Spencer L. The Qualitative Researcher’s Companion. London: Routledge; 2002.
- Shenton A. Strategies for ensuring trustworthiness in qualitative research projects. Educ Info 2004;22:63-75.
- Gamble C, Dudley L, Allam A, Bell P, Goodare H, Hanley B, et al. Patient and public involvement in the early stages of clinical trial development: a systematic cohort investigation. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005234.
- El Enany N, Currie G, Lockett A. A paradox in healthcare service development: professionalization of service users. Soc Sci Med 2013;80:24-30. http://dx.doi.org/10.1016/j.socscimed.2013.01.004.
- National Institute for Health Research . Breaking Boundaries Review of Public Involvement in the NIHR – Update 2014. www.nihr.ac.uk/newsroom/latest-news/?postid2469 (accessed 3 April 2014).
- Oliver S, Armes D, Gyte G. Evaluation of Public Influence of the NHS Technology Assessment Programme. London: Institute of Education, University of London; 2006.
- Walker D-M, Pandya-Wood R. Can research development bursaries for patient and public involvement have a positive impact on grant applications? A UK-based, small-scale service evaluation. Health Expect 2013. http://dx.doi.org/10.1111/hex.12127.
- Boote JD, Twiddy M, Baird W, Birks Y, Clarke C, Beever D. Supporting public involvement in research design and grant development: a case study of a public involvement award scheme managed by a National Institute for Health Research (NIHR) Research Design Service (RDS). Health Expect 2013. http://dx.doi.org/10.1111/hex.12130.
- Buck D, Gamble C, Dudley L, Preston J, Hanley B, Williamson PR, et al. From plans to actions in patient and public involvement: qualitative study of documented plans and the accounts of researchers and patients sampled from a cohort of clinical trials. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-006400.
- Thompson J, Barber R, Ward PR, Boote JD, Cooper CL, Armitage CJ, et al. Health researchers’ attitudes towards public involvement in health research. Health Expect 2009;12:209-20. http://dx.doi.org/10.1111/j.1369-7625.2009.00532.x.
- Hewlett S, de Wit M, Richards P, Quest E, Hughes R, Heiberg T, et al. Patients and professionals as research partners: challenges, practicalities, and benefits. Arthritis Care Res 2006;55:676-80. http://dx.doi.org/10.1002/art.22091.
- Statement on Consumer and Community Participation in Health and Medical Research. Canberra, ACT: Australian Government; 2002.
- Ward PR, Thompson J, Barber R, Armitage CJ, Boote JD, Cooper CL, et al. Critical perspectives on ‘consumer involvement’ in health research: epistemological dissonance and the know–do gap. J Sociology 2010;46:63-82. http://dx.doi.org/10.1177/1440783309351771.
- Staley K. Empowering Researchers: Summary of Responses to an Email Consultation. Eastleigh: INVOLVE; 2008.
- UKCRC . Patient and Public Involvement in Strategic Decision Making: Report of a Workshop Held on 11th March 2009 2009. www.ukcrc.org/patients-and-public/patient-and-public-involvement-in-clinical-research/ppi-strategic-plan (accessed 12 August 2015).
- North West Users Research Advisory Group Working Group . The Collaboration: Supporting Patient and Public Involvement and Public Engagement in Health and Social Care Research in the North West 2009. www.rds-nw.nihr.ac.uk/public-involvement (accessed 4 September 2015).
- Christiansen A, Prescott T, Ball J. Learning in action: developing safety improvement capabilities through action learning. Nurse Educ Today 2014;34:243-7. http://dx.doi.org/10.1016/j.nedt.2013.07.008.
- McGill I, Brockbank A. Action Learning Handbook. London: Kogan Page; 2003.
- Coulter A, Parsons S, Askham J. Where Are the Patients in Decision-Making about Their Own Care?. Copenhagen: World Health Organization Regional Office for Europe; 2008.
- UKCRC Registered Clinical Trials Units Network . Best Practice Guidelines n.d. www.ukcrc-ctu.org.uk/CTURegistrationProcess/Pages/BestPracticePrinciples.aspx (accessed 7 October 2010).
- NIHR n.d. www.nets.nihr.ac.uk/__data/assets/pdf_file/0005/67685/OutlineGuidanceNotesSAF.pdf (accessed 28 April 2014).
- NIHR n.d. www.nets.nihr.ac.uk/funding/hta-researcher-led/Preparing-a-full-application-for-the-Clinical-Trials-and-Evaluation-Board.pdf (accessed 20 November 2013).
- Dunning D, Heath C, Suls JM. Flawed self-assessment implications for health, education, and the workplace. Psychol Sci Public Interest 2004;5:69-106. http://dx.doi.org/10.1111/j.1529-1006.2004.00018.x.
- Boote J, Barber R, Cooper CL. Principles and indicators of successful consumer involvement in NHS research: results of a Delphi study and subgroup analysis. Health Policy 2006;75:280-97. http://dx.doi.org/10.1016/j.healthpol.2005.03.012.
- Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expect 2004;7:209-20. http://dx.doi.org/10.1111/j.1369-7625.2004.00278.x.
- Mathie E, Wilson P, Poland F, McNeilly E, Howe A, Staniszewska S, et al. Consumer involvement in health research: a UK scoping and survey. Int J Consum Stud 2014;38:35-44. http://dx.doi.org/10.1111/ijcs.12072.
- National Institute for Health Research . Information for Authors n.d. www.journalslibrary.nihr.ac.uk/_data/assets/pdf_file/0006/25476/Information-for-Authors.pdf (accessed 24 July 2015).
- Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care 2011;27:391-9. http://dx.doi.org/10.1017/S0266462311000481.
- Wright D, Foster C, Amir Z, Elliott J, Wilson R. Critical appraisal guidelines for assessing the quality and impact of user involvement in research. Health Expect 2010;13:359-68. http://dx.doi.org/10.1111/j.1369-7625.2010.00607.x.
- James Lind Alliance . Establishing Priority Setting Partnerships n.d. www.lindalliance.org/Patient_Clinician_Partnerships.asp (accessed 24 July 2015).
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-132.
- Lamb S, Hewison J. Preparing a Full Application for the Clinical Trials and Evaluation Board n.d. www.nets.nihr.ac.uk/__data/assets/pdf_file/0003/77160/Preparing-a-full-application-for-the-Clinical-Trials-and-Evaluation-Board.pdf (accessed 9 March 2014).
Appendix 1 Patient and public involvement
The EPIC advisory group
Introduction
Central to the EPIC study was the involvement of patients and members of the public to inform each phase of study design. PPI has been embedded in the study from conception, a PPI contributor was a coapplicant of the study and there were two PPI advisors with a considerable amount of experience in PPI and a PPI lead (also coapplicant). To enhance PPI even further, a PPI advisory group was established to inform each phase of study design working alongside the PPI coapplicants team. A separate report focusing on the evaluation of PPI within this methodological study is under development.
The EPIC advisory group
Members of the EPIC advisory group were recruited through an open and transparent process. Advertisements for members of the group were placed on various PPI websites, including those of INVOLVE and Involving People, and via local patient forums such as North West People in Research. Applicants who expressed an interest in joining the group were sent an application pack which included a detailed remit and role description. Applicants were then shortlisted and invited to take part in an informal interview over the phone, and had the opportunity to ask further questions about the role of the group. Five applicants were appointed to the advisory group. Given the methodological nature of the EPIC project, applicants were recruited on the basis of:
-
their current knowledge and understanding of clinical trials and experience of active involvement as a public contributor in a trial (e.g. as a member of a TSC)
-
their understanding of confidentiality in relation to research, ability to travel to Liverpool for approximately four meetings during the lifetime of the project and ability to work effectively in a group situation
-
willingness to act as an ambassador for the project (including being named on the project website and telling others about the project, where appropriate)
-
good communication skills with an ability to listen to others and constructively express a lay view beyond their own personal experience.
The EPIC advisory group was responsible for advising on each phase of the study from contributing to the data extraction and developing the content of the CI and PPI contributor survey, to commenting on the analysis of both survey and interview data, and reviewing papers.
Setting and context
During the lifespan of the study the advisory group met face to face twice and three times by teleconference. E-mail conversations took place on a regular basis. Sadly, shortly after the first meeting one of the members became seriously ill and died 6 months into the project. The remaining four members continued until the end of the project.
Each meeting was chaired by the PPI advisor and supported by the PPI co-ordinator. Each meeting was constructed around various phases of the research project. The face-to-face meetings included presentations by the research team about the study and its progress.
Members of the group were provided with papers in advance of the meetings. All received reimbursement of travel expenses and received payments for their time based on the INVOLVE rate.
Evaluation of EPIC advisory group meetings
Prior to the first PPI advisory group meeting the PPI co-ordinator carried out telephone interviews regarding members’ knowledge and experience in the field of PPI to get a basic understanding of their training and support needs before their membership commenced. Each meeting was then evaluated using a feedback form that was sent to members electronically after that meeting. The purpose of the evaluation form was threefold:
-
to improve the quality of the lay experience as a member of the EPIC advisory group
-
to record the processes and impact of lay involvement
-
to identify factors that enhance or inhibit involvement within the project.
Key feedback from the evaluation
Feedback from PPI members after the first meeting focused on further clarity about the role of the group and project, and highlighted the importance of establishing ground rules for each meeting. Given that this was a methodological project that did not focus on a specific condition to which the PPI members were accustomed, this feedback did not surprise the team. Further clarification of the project was discussed and ground rules were produced and agreed by the group.
Booking accommodation and travel in advance of meetings enhanced involvement in the project, as it was important not to put members out of pocket. The biggest issue throughout the duration of the project was obtaining payments for members’ time. The issue of tax deductions was a problem and it took several months and repeated attempts to resolve this matter. As a result of these issues the university finance team has put a system in place to speed up PPI payments and to avoid members paying tax.
Contributions of the EPIC advisory group
Patient and public involvement members contributed to all phases of the EPIC project. This included:
-
reviewing and commenting on the data extraction fields and categories used to extract data about how PPI was implemented within the cohort
-
contributing to the design of the CI and PPI contributor survey questionnaires
-
piloting the PPI contributor survey
-
reviewing the semistructured interview questions
-
reviewing the transcripts from three interviews and considering emerging themes and direction of questioning
-
contributing to the design of a PPI advert to raise awareness about the EPIC study and to recruit additional PPI contributors from trials included in the cohort that completed the survey questionnaire
-
two members of the group presenting at two different PPI conferences (Involving People and INVOLVE)
-
three members contributing to two papers
-
two members attending the EPIC dissemination meeting.
Appendix 2 Interviews
| Question | CIs who were interviewed, n (%) | Response distribution within CI survey, n (%) |
|---|---|---|
| 1. In general what is your personal view on PPI? | ||
| PPI should always be included in a research study | 12 (57) | 42 (52) |
| PPI can be beneficial but is not always necessary | 8 (38) | 35 (43) |
| I am not convinced of the benefits of PPI | 1 (5) | 4 (5) |
| 2. What motivated you to include PPI in your trial?a | ||
| I think including PPI is the right thing to do | 15 (76) | 55 (70) |
| I have previous experience of the benefits of PPI | 12 (57) | 45 (57) |
| PPI was a requirement for research funding | 8 (38) | 39 (50) |
| A PPI contributor offered their help | 1 (5) | 4 (5) |
| Other | 2 (10) | 2 (3) |
| 3. Which PPI contributor/s did you involve?a | ||
| Patient | 14 (67) | 54 (68) |
| Carer | 2 (10) | 15 (19) |
| Parent | 2 (10) | 13 (16) |
| Charity member | 10 (48) | 24 (30) |
| Medical staff | 4 (19) | 11 (14) |
| Other | 3 (14) | 9 (11) |
| Trial | CI or senior team member interviewed? | PPI interviewed? | TM interviewed? | Settinga | Intervention |
|---|---|---|---|---|---|
| 1 | Y | Y | N | Community | Education and exercise |
| 2 | Y | Y | N | Tertiary | Device |
| 3 | Y | Y | Y | Secondary | Education |
| 4 | Y | N | N | Tertiary | Drug |
| 5 | Y | N | Y | Secondary | Surgical |
| 6 | Y | Y | N | Secondary | Exercise |
| 7 | Y | Y | N | Primary | Community care |
| 8 | Y | Y (two PPI contributors) | Y | Tertiary | Drug |
| 9 | Y | Y | Y | Secondary | Device |
| 10 | Y | N | N | Social care | Exercise |
| 11 | Y | Y (two PPI contributors) | Y | Secondary | Surgical |
| 12 | Y | N | N | Secondary | Device |
| 13 | Y | N | Y | Secondary | Drug |
| 14 | Y | N | N | Secondary | Surgical |
| 15 | Y | Y | Y | Primary | Exercise |
| 16 | Y | N | N | Primary | Exercise |
| 17 | Y | N | N | Secondary | Surgical |
| 18 | Y | N | Y | Primary and secondary | Exercise and community care |
| 19 | Y | N | N | Primary | Other |
| 20 | Y | N | N | Emergency | Community care |
| 21 | Y | N | Y | Secondary | Device |
| 22 | N | Y | N | Secondary | Surgical |
| 23 | N | Y | N | Secondary | Device |
| 24 | N | Y | N | Tertiary | Drug |
| 25 | N | Y | N | Emergency | Surgical |
| 26 | N | Y | N | Secondary and tertiary | Surgical |
| 27 | N | Y | Y | Emergency | Drug |
| 28 | N | Y | N | Secondary | Device |
Topic guides
The topic guides for PPI contributors and TMs mirrored that for the CIs.
Introduction
-
The purpose of this study is to generate a detailed understanding of patient and public involvement (PPI) in clinical trials. While there is now more known about how and why this should happen, less is known about how it does happen and the impact that it may have in the context of clinical trials. It is important to understand the experiences of those involved to help future researchers and members of the public who will engage in PPI. During this interview I will ask you questions about your experiences of PPI for (name of trial). I am open-minded about PPI. I know it’s not necessarily a straightforward process so I’m interested in both positive and negative experiences of PPI in your clinical trial.
-
I’d like to remind you that this interview is being audio recorded, how long are you able to talk to me about your experiences of PPI? Do you have any questions before we start?
Chief investigator questions
Can you tell me what the trial is about? [Is this an on-going or completed trial? Approximately how long has this trial been running for/did this trial run for? What stage are you up to in the trial?/What were the key findings from the trial?]
What is your previous trials experience?
Expectations
-
There are different views about what to call patient and public involvement. In our study we’re using the term ‘PPI representative’, what term would you usually use? Would you prefer we use that in our interview?
-
What is your understanding of patient and public involvement in research?
-
Before (name of trial) did you have any experience of PPI in research? [Prompt: could you tell me more about this?]
-
What were you hoping that PPI would achieve in (name of trial)? [Prompt: can you describe your expectations or why were you unsure what to expect?]
-
Did you have any goals for PPI in (name of trial)? [Prompt: what were they, why did you have these goals?]
-
Did you have any uncertainties about how PPI would contribute to (name of trial)?
What happened?
-
At what stage in developing the trial did you start to think about PPI?
-
At what stage did you start to implement PPI?
-
What influenced you in planning for PPI in (name of trial)? [Prompt: who first suggested PPI to be included in the trial, who has been the main research team member coordinating PPI? Prompt for trial specific factors, condition specific factors, costs of PPI.]
-
How did you identify a PPI representative(s)? [Prompts: who identified the PPI rep/s, where were they identified from? Did you have any PPI through a research network? Who was the PPI rep/s (use their name from now on)?] Why did you choose to have that PPI representative? If charity member, did they consult with other members of the charity about the trial?
-
What personal, experience or qualities did you consider important when identifying a PPI representative? – if appropriate, how do you feel about having PPI representatives with previous experience of PPI?
-
Some people who I’ve interviewed have expressed the opinion that there may be problems having a CI’s patient as a PPI representative, what is your opinion on this?
-
How have you tried to do/implement PPI in the context of (name of trial)? [Prompt: what role did the PPI representative/s have in this trial? Did you consider having a PPI representative on the TMG instead of the TSC (if applicable)?] Did you have funds to support PPI in (name of trial)? [Prompt: what was your thinking behind including that level of funding/not including funding? What were the funds used for (e.g. expenses, appreciation for PPI rep’s time)? Were there any changes over the course of the trial in how you used the funds?]
-
Was there an opportunity to consult with (name of PPI rep/s) about their role? [Prompt: tell me about this, was there a formal agreement, written or verbal? Did this change, how and why? Was there a code of conduct for PPI rep/s and research team members to follow? Was this followed? Why/not?]
-
What has been your experience of involving (name of PPI rep/s) in this trial? [Prompt: how easy/difficult has it been, and for specific examples, has there been anything that surprised you about the process?]
-
How often did the research team keep in contact with the PPI rep/s? [Prompt: do you feel this was too little/enough/too much, why? How did you keep in contact e.g. email?]
-
How well would you say you and the research team got along with the PPI representative/s?
-
Did your plans for PPI change over the course of the trial? [Prompt: how did it differ from plans at the time of PPI initiation? Why did it change?]
-
If you were to start the trial over, would you make any changes to PPI? [Prompt: if yes what changes? Why would you make these changes?]
Impact
-
Has PPI contributed anything to the trial? [Prompt: what has it contributed/how useful was that?]
-
Have you experienced any (other) positive impact of PPI upon (name of trial)? [Prompt: would you tell about these; direct benefits such as getting funding, patient burden, recruitment, outcomes, identifying risks/benefits to participants, trial promotion/dissemination. Indirect benefits such as PPI rep/s confirming that the research team are doing the right thing.]
-
Have you experienced and negative impacts of PPI upon the trial? [Prompts: would you tell me about these; negatively effecting recruitment/consent process.]
-
What (if anything) have you experienced to be the challenges of involving patients and the public in (name of trial)? [Prompt: difference of opinion with PPI representative or PPI rep not being constructive or being disruptive. PPI rep/s dropping out. How did you manage this situation?]
-
Do you feel that the level of funding for PPI (depending on answer from funding question) influenced how much the PPI representative/s contributed to the trial? [Prompt: how/did this impact their ability to contribute to the trial? E.g. PPI reps not attending meeting due to lack of payment.]
-
So looking about on your experience of PPI and the time and effort involved would you say it was/wasn’t (depending on responses to previous questions) worth it?
Information needs, training and support
-
Were the PPI rep/s given an induction as part of preparation for their role? [Prompt: meeting with the CI or research team, information/documents about the trial?]
-
Have the PPI representative/s in this trial had/needed formal/organised training for their role? [Prompt: If YES – what training/information, for example INVOLVE resources or research methods or communication skills training, what’s your opinion on this? What, if anything, has been useful from the training? What other things, if any, would have been useful? If NO – why? Do you think training would have been useful? Did you know where to find training/information for PPI rep/s?]
-
Has the PPI representative/s had/needed any informal support? [Prompt: what support? Buddying/mentoring from other PPI rep/s, clinical/research staff? What other types of support or advice has been provided for PPI rep/s?]
-
Have you been offered any training in ‘how to do PPI’ yourself? [Prompt: yes – could you tell me about that? What’s your opinion on this training? What if anything has been useful from the training? What other things if any would have been useful? Prompt about what other types of support or advice about PPI reps they’ve used and how helpful this has been. No – what, if any, training would you like to receive? How would this be beneficial?]
-
What feedback, if any, did you provide to the PPI representative/s on their input into the trial? [Prompt: did you provide any feedback on input to any other PPI rep/s, for example those consulted through a research network?]
-
Why do you think funders require PPI? [What is your opinion of this requirement? Do you think all trials need PPI; can you think of any circumstances in which a trial would need lots of PPI/need very little or no PPI?]
-
How important would you consider PPI within research? [Prompt: why/why not is PPI important?]
-
What advice would you give to researchers about PPI?
-
What advice would you give to PPI representatives about it?
-
What advice would you give research funders about PPI?
ASK FOR PPI DETAILS.
Appendix 3 Surveys
Chief investigator
| Question | Response, n (%) |
|---|---|
| 2. In general what is your personal view on PPI, irrespective of funding requirements? | 81 |
| PPI should always be incorporated in a research study | 42 (51.9) |
| PPI can be beneficial but is not always necessary | 35 (43.2) |
| I am not convinced of the benefits of PPI | 4 (4.9) |
| 3. During the preparation of your grant application, when did you consider PPI? | 81 |
| Immediately – before contact with the clinical trials unit (if involved) | 45 (55.6) |
| When prompted by the clinical trials unit (if involved) | 11 (13.6) |
| When I read the relevant questions on the funding application form | 10 (12.3) |
| Cannot remember when I considered PPI | 7 (8.6) |
| Did not consider PPI as far as I can remember | 4 (4.9) |
| Other (please explain) | 1 (1.2) |
| Unclear | 3 (3.7) |
| 4. Did you include PPI at any stage of the trial (from design to dissemination)? | 81 |
| Yes | 79 (97.5) |
| No (please explain why you chose not to include PPI) | 2 (2.5) |
| It was long ago and far away. We included a PPI perspective by another name within a research stream. Perhaps not how I would do it nowNo benefit in this case, the user voice (as well as that of various other stakeholders) had been obtained in previous extensive focus group study | |
| 5. What motivated you to include PPI in your trial? (Tick all that apply) | 79 |
| I think including PPI is the right thing to do | 55 (69.6) |
| I have previous experience of the benefits of PPI | 45 (57.0) |
| PPI was a requirement for research funding | 39 (49.4) |
| A PPI representative offered their help | 4 (5.1) |
| Other (please explain) | 2 (2.6) |
| Comments indicated ‘can’t remember’ | |
| 6. Which PPI representative/s did you involve? (Tick all that apply) | 79 |
| Patient | 54 (68.4) |
| Carer | 15 (19.0) |
| Parent | 13 (16.5) |
| Charity member | 24 (30.4) |
| Medical staff | 11 (13.9) |
| Other (please explain) | 9 (11.4) |
| Members of research networks | 3 (3.5) |
| Public/lay member | 4 (5.1) |
| Teachers | 2 (2.5) |
| 7. Why did you choose to involve this representative? (free text) | 79 |
| Recommended by another colleague/person | 3 (3.8) |
| Their previous experience/knowledge of providing PPI or being a research participant | 15 (19.0) |
| Characteristics perceived to be helpful in the role | 6 (7.6) |
| Relevant demographics to be ‘representative’ | 43 (54.4) |
| Responded to advert or volunteered | 8 (10.1) |
| Their existing or previous role was considered relevant and beneficial | 15 (19.0) |
| Previous history of working with them | 12 (15.2) |
| Their connection with a charity or organisation | 23 (29.1) |
| Other | 1 (1.3) |
| 8. How was the PPI representative/s approached/identified? (Tick all that apply) | 79 |
| Through charities related to the disease or condition under study | 23 (29.1) |
| A patient, parent or carer known to me | 39 (49.4) |
| Through previous involvement in the trial as a participant of the research | 21 (26.6) |
| Through the People in Research website or INVOLVE (a national advisory group that supports greater public involvement in NHS, public health and social care research) | 1 (1.3) |
| Through contacting the Patient Advisory Liaison Officer based at my local NHS Trust | 1 (1.3) |
| Through PPI leads in National Institute for Health Research Clinical Research Networks | 9 (11.4) |
| Through local or national patient support groups and voluntary organisations | 19 (24.1) |
| Through advice from health and social care professionals | 9 (11.4) |
| Through advertising in GP surgeries, outpatients, local newspapers and radio | 0 (0.0) |
| A PPI representative offered to be involved | 5 (6.3) |
| Other (please explain) | 4 (5.1) |
| Through colleagues/collaborators | 1 (1.3) |
| Known previously through research/work | 3 (3.8) |
| 9. Did you provide a clear description to the PPI representative/s at the time they joined the trial, outlining their role and expectations? | 76 |
| Yes | 54 (71.1) |
| No | 22 (28.9) |
| 10. In what capacity was the PPI representative/s associated with the trial? (Tick all that apply) | 76 |
| Co-applicant | 20 (26.3) |
| A member of the Trial Steering Committee | 63 (82.9) |
| A member of the Trial Management Committee | 23 (30.3) |
| A member of the independent Data Monitoring Committee | 10 (13.2) |
| Part of a separate PPI advisory group | 16 (21.1) |
| Other (please explain) | 11 (14.5) |
| One off consultancy or focus group | 7 (9.2) |
| On-going consultancy | 4 (5.3) |
| 12. On average, how often did a member of the research team have contact with the PPI representative/s? | 76 |
| Once a month | 12 (15.8) |
| Once every six months | 39 (51.3) |
| Once a year | 1 (1.3) |
| Less than once a year | 1 (1.3) |
| Other (please explain) | 23 (30.3) |
| 13. Did you experience any problems which you feel were related to including PPI in the trial? (Tick all that apply) | 76 |
| Insufficient budget set aside | 2 (2.6) |
| Problems or clashes between the PPI representatives and members of the research team | 2 (2.6) |
| Problems or clashes between PPI representatives | 0 (0.0) |
| The PPI representative not undertaking their role fully | 6 (7.9) |
| Confidentiality issues | 0 (0.0) |
| Inability of the PPI representatives to attend meetings | 18 (23.7) |
| Difficulty finding suitable PPI representatives | 8 (10.5) |
| Insufficient support for the PPI representative (for example training to develop their research skills and knowledge) | 2 (2.6) |
| Insufficient training for the PPI representatives (for example training to develop their research skills and knowledge) | 6 (7.9) |
| Lack of clarity of the PPI representatives role | 5 (6.6) |
| None | 45 (59.2) |
| Other (please explain) | 3 (3.9) |
| I was not involved at this stageNAIt was early days for us and we’ve now been able to solve many of these problems by designating a PPI lead in the department and establishing a patient panel that receives regular support and training | |
| 14. Do you feel that training should be available to researchers to help them to support PPI representatives? | 76 |
| Yes | 60 (78.9) |
| No | 16 (21.1) |
| 15. Was the PPI representative/s involved in the set-up of the trial? | 76 |
| Yes | 56 (73.7) |
| No | 20 (26.3) |
| 16. How was the PPI representative/s involved in the set-up of the trial? (Tick all that apply) | 56 |
| Helping to develop the research question | 15 (26.8) |
| Determining outcomes to measured, including selection and development of questionnaires | 26 (46.4) |
| Considering patient burden of participation | 45 (80.4) |
| Considering visit schedules (frequency of participant visits to the clinic) | 24 (42.9) |
| Considering length and nature of follow-up | 20 (35.7) |
| Contributing to the recruitment process | 23 (41.1) |
| Helping to pilot assessments | 21 (37.5) |
| Designing or commenting on participant information sheets | 47 (83.9) |
| Other (please explain) | 13 (23.2) |
| 17. Overall, how much impact do you think PPI had upon the set-up of the trial? | 56 |
| High impact | 15 (26.8) |
| Moderate impact | 30 (53.6) |
| Low impact | 10 (17.9) |
| No impact | 1 (1.8) |
| 18. Was the PPI representative/s involved in the conduct of the trial? (Once the trial had started to recruit) | 76 |
| Yes | 62 (81.6) |
| No | 14 (18.4) |
| 19. How was the PPI representative/s involved in the conduct of the trial? (Tick all that apply) | 62 |
| Trouble-shooting recruitment issues | 36 (58.1) |
| Actively involved in recruitment/consent process | 4 (6.5) |
| Data collection | 4 (6.5) |
| Participant identification (screening process) | 3 (4.8) |
| Advertising (raising trial profile) | 17 (27.4) |
| Other (please explain) | 31 (50.0) |
| Revising documentation trial materials/processes | 6 (9.7) |
| Attending meetings | 22 (35.5) |
| No explanation provided | 3 (4.8) |
| 20. Overall, how much impact do you think PPI had upon the conduct of the trial? | 62 |
| High impact | 9 (14.5) |
| Moderate impact | 27 (43.5) |
| Low impact | 24 (38.7) |
| No impact | 2 (3.2) |
| 21. Was the PPI representative/s involved in the data analysis? | 76 |
| No | 67 (88.2) |
| Yes (please explain how they were involved) | 9 (11.8) |
| 22. Overall, how much impact do you think PPI had upon the data analysis? | 8 |
| High impact | 1 (12.5) |
| Moderate impact | 3 (37.5) |
| Low impact | 3 (37.5) |
| No impact | 1 (12.5) |
| 23. Was the PPI representative/s involved in disseminating findings (either to trial participants or the wider public)? | 75 |
| Yes | 28 (37.3) |
| No | 47 (62.7) |
| 24. Overall, how much impact do you think PPI had upon the dissemination of findings? | 28 |
| High impact | 5 (17.9) |
| Moderate impact | 14 (50.0) |
| Low impact | 7 (25.0) |
| No impact | 2 (7.1) |
| 25. Do you have any more PPI ongoing or planned in this trial? | 75 |
| No | 41 (54.7) |
| Yes (please explain) | 34 (45.3) |
| 26. As a result of your experience with PPI in this trial, would you want to include PPI again in future trials? | 75 |
| Yes, but only if it was a requirement of research funding | 1 (1.3) |
| Yes, if adequate resources are available | 3 (4.0) |
| Yes, PPI makes a valuable contribution to the research process | 60 (80.0) |
| If it was considered appropriate. I don’t believe it is always necessary | 10 (13.3) |
| No | 1 (1.3) |
| 27. Do you advertise to potential trial participants that PPI representatives have contributed to the trial (for example by telling them in the participant information sheet)? | 75 |
| Yes | 17 (22.7) |
| No | 58 (77.3) |
| 28. Have you contacted your PPI representative(s) for this trial and asked them to contact us so they may be sent information about taking part in EPIC? | 75 |
| Yes | 19 (24.1) |
| No | 60 (75.9) |
| 29. Are you willing to be contacted to take part in an interview to further explore your experiences of PPI in this trial? (Answering ‘yes’ does not commit you to taking part in the interview) | 74 |
| Yes | 41 (55.4) |
| No | 33 (44.6) |
| Text responses to question 17 | Impact |
|---|---|
| Helped improve the consent forms considerably and helped advise on consenting process | High |
| The teacher and pupils provided important information that helped us to modify our main trial | High |
| We had a highly supportive organisation working with us. The study was seen to be controversial and having the support of the PPI group was instrumental in ensuring engagement with clinicians and patients | High |
| Excellent engagement with process of trial development and helpful suggestions for PROMs [patient reported outcome measures] | High |
| PPI has significantly contributed to our understanding of the level of trial participation that is considered to be acceptable to patients and therefore increased compliance rates | High |
| Helped to give very good insight into issues that are important to the patient population | High |
| Crucial to have help from PPI – the TMG would not have had confidence in the protocol without PPI | High |
| The PPI on the PMG/grant applicant was a highly motivated and committed lay person who had happened to have the condition in the past, but without this causing any bias from her. She has continued her enthusiastic support onto a new trial too | High |
| They were crucial to ensure the intervention we had in our minds was something that other smokers may be interested in. They were given a real sense that they were making a contribution and they responded accordingly with their valuable feedback | High |
| PPI helped ease the relationship with the CTU, they provided insights for the ethical application, they worked with stroke groups to make sure the study was accessible to as many stroke patients as possible | High |
| Very active engagement at all stages of the process of the trial, sometimes more so that other members of the co-investigator group. Was instrumental in helping us decide outcome measures which were relevant to women | High |
| We are now actively working together to get research into practice | High |
| PPI input to our patient recruitment materials no doubt contributed to our successful recruitment for [trial acronym] by making the invitation letter, trial information, the study questionnaire and reply sheets easily understandable | Medium |
| Shaping trial design and effective delivery of trial | Medium |
| We were more mindful of the burden of the month diaries that the patient had to fill in | Medium |
| Made suggestions and changes that were helpful but did not alter the basic research project outline | Medium |
| He helped keep us focussed on the importance of the project for helping the participants with depression | Medium |
| . . . informed some key aspects of protocol, but most of protocol was fixed by HTA in Brief | Medium |
| Having a PPI rep at all of our initial meetings where we discussed the trial design and set-up ensured that patients’ needs were always in the forefront of our minds. This role was not limited just to PPI though – this person was encouraged to comment on the science and research design according to his own knowledge base | Medium |
| Our PPI was a great advocate for the non-operative programme and pushed for it to be made available outside of the trial | Medium |
| Difficult to be specific, but the PPI member acted as a full team member in the planning process | Medium |
| Helped us develop our message to parents of participants and involved in trial publicity (videos) | Medium |
| Clear-thinking, involved throughout. Experienced in understanding trial patient involvement | Medium |
| Extended original intervention to have a maintenance phase | Medium |
| The advice from the lay experts was useful in planning the battery of measures used and the frequency of follow-up questionnaires, and in solving early recruitment problems | Medium |
| Our thinking on PPI is evolving this trial was set up 3 years ago and we would do things differently now | Medium |
| I think we would have achieved our goals without the patient input but we were reassured by having independent verification | Medium |
| I am not sure that our co-investigator should really be classed as PPI – she is a member of the team and brings specific expertise in her own right. On the other hand, she is not working in an academic setting and so is perhaps a member of the public. We included her in the team as she had expertise that we lacked – we could have consulted her as a separate collaborator, but being part of the team has allowed her to be fully involved in all decisions | Medium |
| Trial design was based on pilot study, limiting scope for involvement | Medium |
| Aspects of consent, patient information were designed and refined based on PPI feedback. Some aspects of study conduct were informed by PPI | Medium |
| Very helpful on TSC – patient advocate to be honest not much input with trial design – did ratify and assess outcomes. I have since submitted another trial to MRC [Medical Research Council] and I used her from inception and she is a trial co-applicant so definitely got more value | Medium |
| This is a trial involving recruitment of sick and incapacitated patients who have been admitted acutely to hospital. PPI advice was particularly valuable in ensuring a balance between rigorous research procedures/data collection, and an appropriate level of intrusion | Medium |
| Helpful in providing lay perspective, thorough in assessing the trial methodology and ethics | Medium |
| Feedback from support group member helped us to choose the primary outcome measure. Feedback from the patients during the pilot phase resulted in changes to the intervention | Medium |
| Very challenging to make this work, we fill seats and put resource into funding PPI throughout the study. However, researchers do not always understand the benefits of PPI, and our patients and patient representatives are often not able to contribute appropriately as they may not understand the research requirements; or be comfortable in meetings | Low |
| Helpful in the set-up but didn’t change the overall plan | Low |
| We made their changes but they were not central | Low |
| Some of these aspects had been refined in earlier pilot work | Low |
| This study was developed between 2007 and 2008 and involvement structure for PPI was very different even though we used our contacts to contribute. At that time and from that experience, the impact on the project set up overall was low | Low |
| The trial was conducted to follow a formula for . . . trials which has had considerable PPI involvement and qualitative/questionnaire research over 20 years to streamline study design, information sheets and approaches to parents. For these formulaic studies PPI involvement from a few individuals is often too individual/opinionated to replace the larger scale feedback and experience which provides a more objective measure – we have received feedback from about 20,000 parents we have worked with over the last decade and this has driven current trial design and information | Low |
| May have been person dependent. Slightly cynically suspect that this was a business development opportunity . . . he is a serial patient representative! | Low |
| . . . minimal use and contribution | None |
| Text responses to question 20 | Impact |
|---|---|
| Without testing out the intervention material, we would not have ironed out problems, or identified problems with the material. We refined, simplified and sometimes altered the intervention material as a result of the discussions and pilot run with the children and their parents | High |
| Therapists appreciated the opportunity to ask patients questions about care, any what the patient liked or didn’t like in this forum | High |
| See answer to section 18 | High |
| We had access to the age group of patients that we needed to recruit and this gave us insight into their motivations to get involved with research of this kind, helped us to prepare suitable messages and patient information for this age group and to feedback to practice staff that this age group would be willing to help if asked | High |
| Helped to raise the profile of the trial, since the medical condition usually has a low profile. Patient population also complains about lack of research, and funding | High |
| Helpful in maintaining profile of the study | High |
| They helped to shape the content and structure of the intervention and identified ways in which we could recruit from the community | High |
| see last box | High |
| Excellent engagement. Helpful to take investigator group through the various scenarios of recruitment (in labour) to minimise burden but maximise recruitment | High |
| The trial ultimately was unsuccessful due to lack of recruitment. This was not part of the role for the PPIs | Medium |
| Very helpful. Always available | Medium |
| See previous explanation | Medium |
| The study is quite patient-focussed as it deals with a trade-off between early blister control and later morbidity and mortality from oral steroids | Medium |
| The representatives on the committees were reliable and insightful. A community organisation helped raise the profile of the trial amongst [minority ethnic group] patients | Medium |
| Patient perspectives considered by TSC and TMG to help with recruitment and follow up | Medium |
| TSC | Medium |
| We sought additional input when it was clear that the baseline questionnaires completed at recruitment were too long for patients and carers to complete in clinics. Comments on the questionnaires revealed some unnecessary duplication of data collection by patients and carers and that some questions could not be understood by UK participants because the instruments had been developed in the USA. The unnecessary duplication was removed. However, the questions not applicable to UK participants were not removed from the questionnaires because the instruments had been ‘validated’, albeit not in the UK | Medium |
| The study was driven with PPI in mind in that our study has a primary outcome that is patient reported | Medium |
| Very helpful to have comments & opinions of lay members | Medium |
| Good suggestions re: incentives. Constructive contributions to increasing the number of participating centres to enhance recruitment | Medium |
| Helpful in suggesting ways to improve impact, and rasing awearness vis the [condition specific] trust | Medium |
| Most strategies for trial conduct were constrained by logistics for rapid trial set up and deployment | Low |
| The steering committe members contributed very little. The dissemination materials were excellent | Low |
| In reality recruitment depends on PIs [principal investigators] identifying appropriate patients at the time of admission | Low |
| Not many problems | Low |
| Primarily, because of the nature of the trial | Low |
| This area had less impact as we didn’t require so much input at this stage. In hind sight, I think we could have used our PPI panel far more effectively throughout the whole trial (as we now do in more recent trials) | Low |
| Recruitment was slow for a number of reasons, and PPI advice and publicity was helpful | Low |
| I am not sure we changed very much in our recruitment process | Low |
| That is the way it was; the PPI contributed as much as she was able to – both intellectually and physically (and this is not meant to sound or be patronising!) | Low |
| The main challenge was recruitment and PPI wasn’t able to help much with this | Low |
| See previous response | Low |
| Only involved one individual on Management Group – ideally would ahve needed much widely consultation/involvement of community members in communities where we were recruiting | Low |
| The PPI on the TSC had little effect and was usuall absent. The focus group in school to consider ways of improving recruitment was very productive but we were unable to implement the advice as the trial was closed by HTA for poor recruitment | Low |
| The study was quite straight forward and unproblematic so more input may have been required if this had not been the case | Low |
| Minimal use and contribution because of limited methodological understanding by PPI | None |
| If you mean, actually conducting the trial, then she was not involved with that but contributed to regular PMG meetings | None |
| Text responses to question 22 | Impact |
|---|---|
| They took a pragmatic and wider view of the data. They supported qualitative findings at meetings when quantitative data was getting too much attention | High |
| . . . difficult to answer this at this stage | Medium |
| The data analysis process was already predetermined before the start of the trial. The PPI contributed modestly to the data analysis process during discussions with the other members of the research team, ensuring that data was analysed as per plan | Medium |
| Too soon to tell | Low |
| again, the analysis has not happened yet | Low |
| Not at analysis stage | None |
| Text responses to question 24 | Impact |
|---|---|
| Freedom of information requests to all UK hospitals to see if they have implemented. Surveys of use published in the Lancet Lobbying WHO [World Health Organization] and other agencies. And we are still working on it | High |
| We had to communicate with our study participants on several occassions to provide information that was complex. The PPI representative along with other members of her organisation were exremely \helpful in ensure that the information was communicated in a simple, direct and straightforward manner | High |
| This is an ongoing process. However, they have communicated directly with trial participants | High |
| On going | High |
| They wrote the dissemination leaflet for participants, GPs [general practitioners] and trial sites | High |
| The trial was promoted a lot via PPI contacts at the [condition-specific local support group] – who Tweet regularly on our behalf. National [condition] Society also helped with dissemination of results via their website and through articles in their Newsletter | Medium |
| As mentioned previously – patient was strong driver for making the non-operative programme available outside of the trial – once it was finished | Medium |
| For the reason already stated | Medium |
| Helped draft the newsletter to participants and ensure it was easily accessible to a lay reader | Medium |
| Ongoing – our trial is about to complete the long term follow up, the patient group will convene to help disseminate th results to our participants | Medium |
| PPI was able to discuss the findings among peers and in a regional meeting of local patient’s network group | Medium |
| High impact re dissemination to service users. No impact in relation to other stakeholders | Medium |
| Presentations at patient sleep apnoea meetings in Wales, London and Oxford | Medium |
| He wrote a piece for his charity newsletter | Low |
| Comments from PAG [Patient Advisory Group] members on a draft summary of study findings which was sent to study participants | Low |
| All these remaining question are not applicable as the study still has 18 months to run | Low |
| Don’t know yet! | Low |
| They checked the letter we sent to participants to make sure it was easy to understand | Low |
| All members of the Steering Committee were given the opportunity to comment on the proposed patient report | Low |
| They haven’t been disseminated yet | None |
| Comment also applies to last question – can’t have impact as trial has not reached that stage yet but anticipate they will be in the future and am sure it will have an impact | None |
| Question | Response, n (%) |
|---|---|
| 2. How did you become a patient and public involvement (PPI) representative for this trial? | 32 |
| Personally approached by a member of the research team | 21 (65.6) |
| Approached as a member of a patient support group or charity | 7 (21.9) |
| Replied to an advert | 0 (0.0) |
| I found out about the trial and I approached the research team myself | 0 (0.0) |
| Other (please explain) | 4 (12.5) |
| A general approach was made by the research team to the Consumers Forum of which I am a member. I repliedAsked as I was about to retire by a member of the [name] unit. They had been asked to suggest someoneI am a PPI for [condition] trialsI was initially in discussions with the PI and others to do with a separate piece of research relating to my PhD [doctor of philosophy] thesis. In discussions it became clear that the PI and her team were planning to apply for funding for the trial and through my supervisor were put in touch with the trials team in my university department | |
| 3. At what stage of the trial did you become involved as a PPI representative? | 32 |
| During the application for funding | 17 (53.1) |
| Before the first participant was randomised | 4 (12.5) |
| During participant recruitment to the trial | 9 (28.1) |
| During the data analysis stage | 0 (0.0) |
| During the dissemination stage | 0 (0.0) |
| Other (please explain) | 2 (6.3) |
| Prior to applicationThey had funding and were setting up the various committees required | |
| 4. What motivated you to become involved as a PPI representative for this trial? (Tick all that apply) | 32 |
| Personal experience of the disease under study (either self or relative) | 24 (75.0) |
| Involved in a charity or foundation concerned with the disease under study | 14 (43.8) |
| Interest in the topic that the trial was investigating | 20 (62.5) |
| Interest in research generally | 17 (53.1) |
| The funding attached to the role | 3 (9.4) |
| Previous experience of providing PPI | 8 (25.0) |
| Previous experience of being on an ethical panel or advocacy group | 4 (12.5) |
| I have previously been involved in a clinical trial as a research participant | 1 (3.1) |
| A relative of mine has previously been involved in a clinical trial as a research participant | 1 (3.1) |
| Wanting to help | 14 (43.8) |
| Other (please explain) | 6 (18.8) |
| A wish to advance improvements in [demographic] health careI am a PPI for [condition] trialsIt would be difficult to attend without fundingMy name was initially passed to the leader from a team in which I was already a PPI (I was in the System!). I have always accepted such kind invitations if they provide potential positive long term help for the [condition] (as opposed to being purely academic)The topic is obviously one of great relevance to every [demographic] and can have a major effect on confidence and personal relationships. Identifying the best types of operations will therefore improve [demographic] quality of life and make best use of NHS services and resourcesInvolved in support group | |
| 5. Did you have previous experience of being a PPI representative on a different research project? | 32 |
| No | 19 (59.4) |
| Yes (please explain) | 13 (40.6) |
| At [name] University and at least one other at [name] UniversityChairman of a patient group who look at P.I.S. [patient information sheets] and relevance of research from a lay point of viewCharity is involved in a number of other projectsI am a PPI for [condition] trialsI am involved in [name 1], [name 2], and [name 3] all specifically look at ensuring research is targeted, appropriate and improves healthI am the Chairman of the [name] patient group at [name] and have been ask to view many consent forms and have been asked for my opinion on many research projectsI have 13 and a half years’ experience of being involved in Patient partnership working and PPI, at local Regional & national level generally, but specifically related to [condition]. I currently hold a honorary position with the University of [name] as Ambassador for [condition] and am Joint Chair of the [local condition] Research Consumer GroupI have been a PPI member at INVOLVE, MRC, [condition] Foundation, [condition] Society, [condition] UK, Royal CollegeI was involved in [study name]I worked in various capacities with the Cochrane [name] GroupInvolved in northwest London clinical networks. Chair of local support groupsMember of other studies such as [study name 1], [study name 2], [study name 3], [study name 4]Previous [condition] studies | |
| 6. Where you the only person providing PPI representation for this trial? | 32 |
| Yes I was the only person | 16 (50.0) |
| No – there were other PPI reps contributing at the same time I was | 16 (50.0) |
| No – there had been other contributors who I replaced | 0 (0.0) |
| 8. Did you have any initial questions or concerns about being a PPI representative for this trial? | 32 |
| No | 28 (87.5) |
| Yes (please explain) | 4 (12.5) |
| A number of questions about what was required of me; what my role would be, time involved, procedures etc etc – the normal questions anyone would ask. I had no specific queries about the trial itselfI was concerned about the workload and the travelling to meetingI had questions about how participating patients would be cared for e.g. timing of blood tests and what would happen to the patient in between testingOnly the commitment involved and any help covering costs of participation | |
| 9. At the time of making the decision to be involved, was your role made clear to you? | 32 |
| Yes | 31 (96.9) |
| No | 1 (3.1) |
| 10. Did your understanding of your role change during your involvement? | 31 |
| No | 23 (74.2) |
| Yes (please explain) | 8 (25.8) |
| As I found out more about the trial and met the professionals involved I was able to see where my role fitted into the bigger pictureAs the trial proceeded I had to quickly learn how to interpret the data produced. The [study name] Newsletter was very useful in setting out in simple terms how recruitment was proceeding in each of the hospitalsI became more confident as we met and felt able to ask questions when I did not understand somethingI was invited to participate at all levels and encouraged to be involved with all aspects of the trial from a patient point of viewI was responsible for looking at the trial from a patient view point and to raise any concerns regarding the quality and relevance of the researchIt became easier as I got to know study staffOnly in that the trials developed in unexpected ways so more judgement was requiredIn the initial recruitment phase I was able to offer advice & support. I now see my role as representing the interests of [organisation] rather than that of a simple observer | |
| 11. In what way were you associated with the trial? (Tick all that apply) | 32 |
| As a co-applicant on the trial | 7 (21.9) |
| As a member of the Trial Steering Committee (a group that provides overall supervision of the trial and ensures that it is being conducted in accordance with the relevant regulations) | 23 (71.9) |
| As a member of the Trial Management Group (a group that monitors all aspects of the conduct and progress of the trial, ensures that the protocol is adhered to and takes appropriate action to safeguard participants and the quality of the trial itself) | 6 (18.8) |
| As a member of the Data Monitoring Committee (a group that reviews accumulating data in a clinical trial and advises on the future of the trial) | 2 (6.3) |
| As part of a separate PPI advisory group | 2 (6.3) |
| Unsure | 1 (3.1) |
| Other (please explain) | 4 (12.5) |
| Advising on recruitment materials aimed at young peopleI also helped develop written material used within the trialI may have been a co applicant and I may have been part of a separate PPI advisory group. It’s rather a long time ago now for me to rememberas Consultant | |
| 12. On average, how often did you have contact with a member of the research team? | 32 |
| Once a month | 9 (28.1) |
| Once every six months | 13 (40.6) |
| Once a year | 1 (3.1) |
| Less than once a year | 0 (0.0) |
| Other (please explain) | 9 (28.1) |
| About one every three monthsAs and when necessary. Could be monthly or quarterly but I was always kept informed and I attended all meetingsBetween 1–6 monthsI was only involved in the initial stagesIn the initial setting up stages of the trial I seem to remember that there was quite a lot of contact. After that point there were around 4 meetings per yearIt is a long time ago so I cannot remember how often the steering group met but probably every two months with Newsletters and data sheetsRoutinely every six months, but with other contact in between when there was things that they wanted my input onTypically quarterly and more often where critical aspects needed close monitoringwhenever there is a meeting or workshop | |
| 13. Before the trial started, which of the following aspects of the trial were you interested in contributing to? (Tick all that apply) | 32 |
| Setting research priorities | 11 (34.4) |
| Developing the research question | 9 (28.1) |
| Outcomes to be measured, including selection and development of questionnaires | 19 (59.4) |
| Piloting of assessments or questionnaires | 7 (21.9) |
| Method of randomisation | 1 (3.1) |
| Designing or commenting on participant information materials | 18 (56.3) |
| Trouble-shooting recruitment issues | 8 (25.0) |
| Active involvement in recruitment/consent process | 2 (6.3) |
| Data collection | 6 (18.8) |
| Data analysis | 5 (15.6) |
| Visit schedules (frequency of participant visits to the clinic) | 4 (12.5) |
| Length and nature of follow-up | 7 (21.9) |
| Trial marketing and publicity | 8 (25.0) |
| Dissemination of trial findings to research participants or the wider public | 11 (34.4) |
| Was not aware of the options | 0 (0.0) |
| Other (please explain) | 8 (25.0) |
| At times I helped with editing other materials such as the trial ProtocolI was particularly interested in how the research would provide psychological and practical support for sufferers from [condition] and their carers. Hands on stuff!Improving the patient’s opportunity for repairThe lead investigator gave a presentation to a large group of the public at the end of the trial. I organised the public meetingThe medical aspects associated with randomisation is explored but the process is one which I do not believe is appropriateThe trial had already started when I became involvedThe trial had already started when I joined it | |
| 14. Which aspects did you feel able to contribute to during the trial? (Tick all that apply) | 32 |
| Setting research priorities | 8 (25.0) |
| Developing the research question | 10 (31.3) |
| Outcomes to be measured, including selection and development of questionnaires | 15 (46.9) |
| Piloting of assessments or questionnaires | 10 (31.3) |
| Method of randomisation | 1 (3.1) |
| Designing or commenting on participant information materials | 17 (53.1) |
| Trouble-shooting recruitment issues | 8 (25.0) |
| Active involvement in recruitment/consent process | 2 (6.3) |
| Data collection | 3 (9.4) |
| Data analysis | 5 (15.6) |
| Visit schedules (frequency of participant visits to the clinic) | 2 (6.3) |
| Length and nature of follow-up | 4 (12.5) |
| Trial marketing and publicity | 9 (28.1) |
| Dissemination of trial findings to research participants or the wider public | 8 (25.0) |
| None | 0 (0.0) |
| Other (please explain) | 8 (25.0) |
| Asking questions on ethical issues as an outsider. Helping in management of the meetings. I also deputised for the Chairman in his absence. I feel a strong sense of involvement with the teamAssisting with the process of editing (including offering minor design changes) other trial documents, which were not necessarily within the patient’s domainBeyond being instrumental in bringing together the team my input ended up being minimalI see my role in such projects as getting the team to understand the sufferer perspective and why what looks simple on paper may not be so in practice (researchers often do understand the mind set of those they are researching!), i.e. Looking at the issue from ‘outside the box’It really is too long ago to remember all of these details. I sat on the Steering Committee not only someone with [condition] but also as a qualified biochemist and as someone with experience of evidence based medicine through my work with the Cochrane Collaboration. I remember being particularly interested in the technical aspects and around the questions concerning the use of [intervention] and how to blind/or not, to their use! I also questioned the use of [intervention]. Not ideal, but of course such a study could not really be controlled in any other wayMy role is simply to represent the patient. I have no involvement in the running of the trial, other than to assess if it is being carried out with patient interest and patient safety/outcomes as the most important element. My particular speciality is in the area of patient information documents and patient information documents, to ensure that those consenting to take part have the clearest possible understanding of what is involved and why they are being approachedPPIs are essentially rubber stamps | |
| 15. Did you receive any training to help you provide input into the trial? | 32 |
| Yes | 4 (12.5) |
| No | 28 (87.5) |
| 16. On which aspects did you receive training to facilitate your contribution? (Tick all that apply) | 4 |
| Setting research priorities | 0 (0.0) |
| Developing the research question | 0 (0.0) |
| Outcomes to be measured, including selection and development of questionnaires | 1 (25.0) |
| Piloting of assessments or questionnaires | 0 (0.0) |
| Method of randomisation | 0 (0.0) |
| Designing or commenting on participant information materials | 0 (0.0) |
| Trouble-shooting recruitment issues | 0 (0.0) |
| Active involvement in recruitment/consent process | 0 (0.0) |
| Data collection | 0 (0.0) |
| Data analysis | 1 (25.0) |
| Visit schedules (frequency of participant visits to the clinic) | 1 (25.0) |
| Length and nature of follow-up | 1 (25.0) |
| Trial marketing and publicity | 0 (0.0) |
| Dissemination of trial findings to research participants or the wider public | 2 (50.0) |
| None | 0 (0.0) |
| Other (please explain) | 4 (100.0) |
| Outcome to be measured, Data analysis, Visit schedules, length and nature of follow up, Dissemination. The training I received was ongoing through the length of the project. Any questions I had regarding data collected etc. were answered fully and I count this as part of trainingMental capacity actTraining was more about the procedures under review and how surgeons come to use specific treatmentsDissemination | |
| 17. On which aspects would you have liked to have received training to facilitate your contribution? (Tick all that apply) | 32 |
| Setting research priorities | 5 (15.6) |
| Developing the research question | 8 (25.0) |
| Outcomes to be measured, including selection and development of questionnaires | 10 (31.3) |
| Piloting of assessments or questionnaires | 6 (18.8) |
| Method of randomisation | 4 (12.5) |
| Designing or commenting on participant information materials | 5 (15.6) |
| Trouble-shooting recruitment issues | 3 (9.4) |
| Active involvement in recruitment/consent process | 6 (18.8) |
| Data collection | 4 (12.5) |
| Data analysis | 4 (12.5) |
| Visit schedules (frequency of participant visits to the clinic) | 1 (3.1) |
| Length and nature of follow-up | 5 (15.6) |
| Trial marketing and publicity | 1 (3.1) |
| Dissemination of trial findings to research participants or the wider public | 5 (15.6) |
| None | 12 (37.5) |
| Other (please explain) | 9 (28.1) |
| Because things are changing all the time, need training to get the best outcome. People who are new to PPI need to have training in order to know their roleI and a professional journalist with more than four decades’ experience in communicating complex and sometimes difficult messages to the general public. I was recruited because of that experience and also because, as a repeat [condition] patient, I have had first-hand experience of the effects of poorly written, unclear information documents. My role in the trial is to ensure that the [demographic] involved know what will take place if they agree to take part; why the study is being done; and to make sure their voice is heard if, during the course of the study, the clinicians report significant worries or concernsI think that I received enough support for my role at the time from other members of the steering group.I would not have wanted training because I wanted to take part from a clear mind from my own unique perspective (I am a post graduate [subject] so I know a bit about research)In the early stages understanding of terminology usedRe Patient materials: As it was the first time I had been involved in such a project, I remember that when my involvement began, I sometimes felt that I may be exceeding my remit by being too forceful with my suggestions, or of ‘stepping on toes’ and so on. When I queried these issues with [name], she always reassured me that I was on the right path, and so I gained confidence as the work progressed. I am not sure if ‘training’ as such would have helped, and it may be that someone newly involved in a project simply needs to go through the learning curve. It would have helped me at the start of the project to have been given a list, say, of all the information passed on in the form of acronyms i.e. various organisations, committees, departments etc., which were familiar to the Project team, but unknown to meTraining was not necessary, having had 8 years as a Non-Executive and a similar period working within the NHS at management levelWhen they were talking at the meetings about statistical analysis especially, it was like a foreign language. A little training/info kit would have helped | |
| 18. What type of support did you receive during the trial? (Tick all that apply) | 32 |
| Peer support from other PPI representatives | 8 (25.0) |
| Personal/professional development from a member of the research team (a review of performance where feedback and encouragement is provided and any skill development needs are established) | 2 (6.3) |
| Practical support from a member of the research team (for example help with making travel arrangements) | 13 (40.6) |
| Financial advice from a member of the research team (for example how PPI payments may affect benefits) | 2 (6.3) |
| Emotional support from a member of the research team (for example help with coping with any distress that may arise as a direct consequence of being involved in the trial) | 4 (12.5) |
| None | 8 (25.0) |
| Other (please explain) | 7 (21.9) |
| Can contact any research team member at any timeGood e-mail support from several members of the teamI had nothing but friendship and encouragement from the team who made me feel really valued. It was pure joy working with them and a great privilegeMy contribution is given freely, and I work as an equal with the trial member due to past experience and knowledgeThe doctors who are involved with the trials committee are helpful and willing to explain. There was nothing in the project that was likely to cause me any emotional problems | |
| 19. Were you aware of any resources available to PPI representatives? (Tick all that apply) | 32 |
| INVOLVE | 6 (18.8) |
| A PPI liaison officer to support my role | 0 (0.0) |
| None | 22 (68.8) |
| Other (please explain) | 8 (25.0) |
| All PPI representatives should be made aware of theseIt is important to stress how fully I was drawn into the process by [name] and his research team at the [institute name]I have been aware of INVOLVE recommendations for a few years but I think this research study was probably prior to INVOLVE’s recommendations. I was encouraged to apply for expensesI knew I could ask the team for help whenever I needed it – I didn’tNo, but have now asked that payments be paid for travel deemed excessive, my time is given freely but this will not necessarily be the case for others who would like to become involvedOur own particular consumer GroupSRN [definition unknown] supportTravelling expenses have been paid | |
| 20. On which aspects of the trial do you feel your contribution made a difference? (For example a difference could be maintaining a focus on patient needs by recommendations you made that led to changes, or your ability to support the views of the research team) (Tick all that apply) | 32 |
| Setting research priorities | 8 (25.0) |
| Developing the research question | 9 (28.1) |
| Outcomes to be measured, including selection and development of questionnaires | 13 (40.6) |
| Piloting of assessments or questionnaires | 12 (37.5) |
| Method of randomisation | 1 (3.1) |
| Designing or commenting on participant information materials | 18 (56.3) |
| Trouble-shooting recruitment issues | 7 (21.9) |
| Active involvement in recruitment/consent process | 2 (6.3) |
| Data collection | 1 (3.1) |
| Data analysis | 5 (15.6) |
| Visit schedules (frequency of participant visits to the clinic) | 3 (9.4) |
| Length and nature of follow-up | 7 (21.9) |
| Trial marketing and publicity | 8 (25.0) |
| Dissemination of trial findings to research participants or the wider public | 8 (25.0) |
| None | 3 (9.4) |
| Other (please explain) | 8 (25.0) |
| Bringing the research team togetherClearly demonstrating the patients perspective in all areas of contributionFirst, raising issues which are important to the patient. Second, having had strategic responsibility in my professional life, it may have helped to contribute approaches to certain problems and outcomesMeeting managementOne of the major questions I had during the study was about the failure of some of the machines used to process the blood samples. It is difficult for me to remember other concerns after four years interimPersonal experience of the illness and situation that they were studying. They were looking at it from the professional point of view and could enlighten them about the patients viewpointPresenting the patient perspective and the problems they have. If I contributed anything beyond that (apart from enthusiasm!) then I am very flattered! | |
| 21. Did you receive any feedback on your involvement from the research team? | 32 |
| No | 13 (40.6) |
| Yes (please explain) | 19 (59.4) |
| Designing the leaflets. Constant feedbackGeneral positive comments on the value of my inputI always felt part of the tem and was encouraged to contribute. I felt that my input as a past patient was valued and acted uponI always was made to feel a very active part of the team – and had positive feedback as to my involvement and input, especially in that suggestions I made were taken on board abs my ‘expert opinion’ was regularly asked forI received feedback during the trial from the Chair and other members. I was given a copy of the trial report. The lead investigator spoke o a group of the public about the findingI was accepted as a member of the Steering Committee and any input I made were acknowledged. Any comments that I made on reviewing documents, including reports, were acknowledgedMinuted actions from a number of suggestions and up dates from the research team from time to timeMy comments and observations were discussed at Team meetings and I was encouraged to remind members of the necessity to listen to and understand the patient’s and relative’s views and concerns. These views and concerns were always considered carefullyOn many occasions there is direct feedback as forms and aspects of the research is modified as a result of my input, and persistenceOne of consultants gave me positive feedback and recommended me as a patient representative of another research project which I am now currently involved withParticipation with the teamPeople have been appreciative of my input and their comments have led me to believe I have done a good job. As a result of my work with this trial, I was approached about another trial, and I am now a member of its TSCPositive comments and many thanks[Name] always made me aware of areas in which my input had been ‘useful’ or ‘very useful’Verbal acknowledgment of the value of my comments at meetingsI was contacted by e-mail by the team at [name] universityMeeting minutes and future meetings | |
| 22. How do you feel about your level of involvement in the trial? | 32 |
| I would like to have been more involved | 5 (15.6) |
| I would like to have been less involved | 0 (0.0) |
| I would have liked a targeted approach related to areas in which I felt able to contribute | 7 (21.9) |
| I feel I had the right level of involvement | 20 (62.5) |
| 23. Do you feel the research team engaged you in the research project and valued you as a member of the team? | 32 |
| Yes | 25 (78.1) |
| To some extent | 6 (18.8) |
| No | 1 (3.1) |
| 24. Did you experience any problems during your involvement as a PPI representative? (Tick all that apply) | 32 |
| Lack of clarity about the role specification | 2 (6.3) |
| Lack of training (for example training to develop research skills or knowledge) | 5 (15.6) |
| Lack of support (for example not receiving any feedback on contribution to the trial) | 1 (3.1) |
| Lack of information (for example information about the trial) | 1 (3.1) |
| Problems with payments | 2 (6.3) |
| Issues with confidentiality | 0 (0.0) |
| Inability to attend meetings | 4 (12.5) |
| Problems or clashes with other PPI representatives | 0 (0.0) |
| Problems or clashes with members of the research team | 0 (0.0) |
| None | 20 (62.5) |
| Other (please explain) | 5 (15.6) |
| It was not easy being the only PPI representative among a group of researchers. I was able to hold my own, but I imagine this could have been daunting. There was a sense it which my role felt like a tick box exercise for the research team, though they did allow me to contribute ideas at meetingsMy health went through a blip and I was therefore unable to attend many meetings, however we had several teleconference meetings which meant that I was able to continue my involvementReally none, except the permanent feeling that PPIs are tolerated rather than regarded as potential sources of assistance and balanceSometimes meetings/telephone conferences clashed with my normal work commitments. Use of terminology as advised earlierThe ticked item here simply reflects my earlier comment about having a list of acronyms and what they stood for and it wasn’t a problem, merely a slight inconvenience | |
| 25. Did you receive any form of payment in your PPI role? (Tick all that apply) | 32 |
| Travel expenses | 17 (53.1) |
| Payment for my time | 5 (15.6) |
| Carer costs | 0 (0.0) |
| None | 11 (34.4) |
| Other (please explain) | 4 (12.5) |
| I sit on the CSG and travel to London is paid otherwise, I bear the cost willinglyI think the research study was before the INVOLVE payments were common practiceI was offered travel expenses, and during the last year of the trial I have been able to claim a fixed fee for attendance at meetingsPPI representatives should be well funded a lot of them don’t get paid but they are unworking carers. It takes a lot of experience to know about doing PPI in researchers. There should be a clear budget in the funding for PPI, they should be adequately compensated | |
| 26. How satisfied were you with your personal commitment and involvement to contributing to the trial? | 32 |
| Very dissatisfied | 0 (0.0) |
| Dissatisfied | 2 (6.3) |
| Satisfied | 14 (43.8) |
| Very satisfied | 16 (50.0) |
| 27. How satisfied were you with the researchers’ commitment to work with and maintain PPI throughout the trial? | 32 |
| Very dissatisfied | 2 (6.3) |
| Dissatisfied | 3 (9.4) |
| Satisfied | 5 (15.6) |
| Very satisfied | 22 (68.8) |
| 29. As a result of your involvement in this trial, would you recommend becoming a PPI representative to others? | 32 |
| Yes | 32 (100.0) |
| No | 0 (0.0) |
| 30. Are you willing to be contacted to take part in an interview to further explore your experiences of PPI in this trial? (Answering ‘yes’ does not commit you to taking part in the interview) | 32 |
| Yes | 30 (93.8) |
| No | 2 (6.3) |
List of abbreviations
- CI
- chief investigator
- CTU
- clinical trials unit
- DMC
- Data Monitoring Committee
- EPIC
- Evidence base for Patient and public Involvement in Clinical trials
- HTA
- Health Technology Assessment
- NIHR
- National Institute for Health Research
- portable document format
- PiiAF
- Public Involvement Impact Assessment Framework
- PPI
- patient and public involvement
- RCTU
- UK Clinical Research Collaboration Registered Clinical Trials Unit
- TM
- trial manager
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UKCRC
- UK Clinical Research Collaboration