Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/1017/07. The contractual start date was in September 2012. The final report began editorial review in June 2015 and was accepted for publication in October 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Goodman reports grants from the National Institute for Health Research (NIHR) during the course of the study. Dr Norton reports grants from the NIHR Health Services and Delivery Research programme during the course of the study and grants from the NIHR Research for Patient Benefit programme and the Multiple Sclerosis Society, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Bunn et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Introduction
Dementia is primarily a condition of old age and as such many people with dementia will have other long-term health conditions. 1,2 As the population ages and the proportion of people with both dementia and multimorbidity increases, the delivery of health care becomes increasingly complex and challenging. 3 Despite this, health-care systems and research often treat dementia as an isolated condition with little understanding of how other complex health needs might impact on patient and carer experiences or service use and provision. 4 There is a need to consider what kind of system-based support can enable different health-care professionals (HCPs), patients and their carers to navigate the multiple systems of care that they might encounter; to explore decision-making processes; and to understand barriers to and facilitators of the development of an integrated approach to care across services for people living with dementia (PLWD).
Dementia and comorbidity
Dementia affects one in 20 people aged > 65 years and one in five people aged > 80 years. 5 Over 800,000 people in the UK have dementia,6 with the most common form being Alzheimer’s disease. 7 This number is forecast to increase by 40% over the next 12 years. 8 Although there are significant differences in the physical and cognitive effects of the different types of dementia, all are progressive, involve increasing physical and mental deterioration and lead to PLWD becoming increasingly dependent. The prevalence of dementia in patients aged > 65 years in general hospitals is high and it is estimated that at any one time one-quarter of acute hospital beds are occupied by PLWD. 9
There are different conceptualisations of comorbidity but they are based on the core concept of more than one distinct condition in an individual. 10 The existence of several long-term conditions in one individual is common in PLWD1,2,11 and a study in the UK found that, on average, people with dementia had 4.6 chronic illnesses in addition to their dementia. 4 In addition, delirium, infections, falls, urinary and faecal incontinence and constipation occur more frequently in PLWD. 1,12
Common health conditions in PLWD include diabetes, vascular or heart disease, hypertension, chronic obstructive pulmonary disease, musculoskeletal disorders and chronic cardiac failure. 1,13,14 There is increasing evidence to support an association between Alzheimer’s disease and cardiovascular risk factors such as hypertension and hypercholesterolaemia. 15–17 It has been argued that in older age groups Alzheimer’s disease should be considered as a diffuse clinical syndrome representing the gradual accumulation of multiple pathologies rather than as a discrete neuropathological entity. 15
Health-care delivery for people with dementia and comorbidity
Comorbidity among people with dementia presents particular challenges for primary and secondary care. Certain comorbid medical conditions may exacerbate the progression of dementia. For example, there is evidence that cognitive decline may be accelerated in older people with type 2 diabetes. 18,19 Moreover, the presence of dementia may adversely affect the clinical care of other conditions and undermine a patient’s ability to self-manage chronic conditions and engage in health maintenance activities. 20,21 The presence of dementia can be a key factor in how different specialist and emergency services are used and in decision-making about transfer to long-term care (nursing homes).
Despite this, most research has been concerned with the effect of multimorbidity on physical functioning and its measurement, with little research investigating the effect on processes of care or what constitutes ‘best care’ for PLWD. 22 In addition, little is known about patients’ perspectives. A review of 126 qualitative research papers on patients’ experiences of the diagnosis and treatment of dementia23 found very little evidence relating to the experiences of people diagnosed with dementia who have an accompanying comorbid condition. In fact, this group are often actively excluded from research even though it is likely that a diagnosis of dementia may affect their ability to self-manage other conditions. There is a lack of research on patients’ views on the ways in which multiple conditions affect their health, well-being and clinical care. 10
Rationale for the research
It has been consistently highlighted that NHS professionals who do not work in mental health have very little understanding of the needs and experiences of PLWD and that the care needs of PLWD are frequently not being met. 24–26 Suboptimal systems and insufficient guidance for generalist and specialist services that encounter patients with dementia and other comorbid conditions result in the duplication of services, delays in the identification of problems and ultimately patients being admitted to hospital or transferred to long-term care earlier than is necessary. 9,27
Improving the organisation and delivery of services for PLWD remains a key government target. 25,28 A report from the Ministerial Advisory Group on Dementia Research Subgroup 129 identified several priority topics for dementia research, one of which was a need for more research addressing comorbidities, especially in relation to vascular disease, and a need to improve the physical health of patients with dementia. In addition, there is a growing recognition that current health-care services may not be designed to meet the needs of older people with complex health needs such as multimorbidity. 3,4
To date, research has tended to focus on the experience of living with dementia as a single disease. Moreover, although there is a growing awareness of the needs of patients with multimorbidity and frailty,30 there is little research on the specific needs of PLWD. The aim of this research was to add to our understanding of how having dementia impacts on the management of other health conditions. It summarises current evidence in this field, provides valuable information about how dementia impacts on the management of comorbid health conditions and provides suggestions for how services should be organised and delivered to improve quality of care for people with complex health conditions who are diagnosed with dementia.
Aims and objectives
The overall aims of the study were to:
-
explore the impact of comorbidities for a PLWD on access to non-dementia services
-
identify ways to improve the integration of services for this population and reduce fragmentation and the inappropriate use of care.
The research objectives were to map what is already known about comorbidity and dementia, identify how the presence of dementia impacts on access to health care and service delivery for comorbid conditions, identify barriers to and facilitators of service delivery for people with dementia and comorbidities and identify models of service delivery that are best suited to meet the needs of people with dementia who have other complex health-care needs. The research questions were:
-
What is best practice/effective care for service delivery for people with dementia and a comorbid condition [i.e. diabetes, stroke and vision impairment (VI)]?
-
How does the presence of one or more comorbidity impact on access to health care and service delivery for people with dementia, their carers and health and social care professionals?
-
What are the barriers to and facilitators of service delivery for people with dementia and comorbidities?
-
How can current services adapt to meet the needs of people with dementia who have other complex health-care needs?
Structure of the report
Chapter 2 describes the conceptual approach adopted and the framework used to structure the qualitative analysis and Chapter 3 details the methods of each phase of the study. The findings of the scoping review are presented in Chapter 4, the analysis of the Cognitive Function and Ageing Studies (CFAS) data is presented in Chapter 5, the interviews and focus groups are presented in Chapter 6 and the consensus conference is described in Chapter 7. Finally, Chapter 8 summarises the study findings and looks at their implications, in particular how current services can adapt to meet the needs of PLWD who have other complex health needs.
Chapter 2 Conceptual framework and approach
We used a mixed-method approach that was informed by theories about continuity of care31–33 and access to care. 34
Continuity of care
In its simplest terms continuity may be considered as the degree to which a series of discrete health-care events is experienced as coherent and connected and consistent with the patient’s needs and personal context. 31 There is evidence that increased continuity is associated with improved outcomes and satisfaction. 35,36 Although continuity may be important to all health service users, it is thought to be particularly important for those with complex health needs such as dementia, long-term conditions, multimorbidity and frailty. 37,38
Dementia is a long-term condition characterised by progressive deterioration and dependency. Navigating the different systems of care is particularly difficult for this population, not least because they receive advice and support from health and social care and increasingly third-sector providers. 39 Previous research on continuity has identified a need to prioritise the needs of vulnerable people who are unable to negotiate their own continuity as they wish. 40 Processes of care may be further complicated for PLWD who have other comorbid health conditions. Increasing specialisation of care for long-term conditions means that they are likely to see a variety of different HCPs in a number of different places. 3 This leads to further fragmentation of care and reduced continuity. 41
Continuity of care is a complex multidimensional concept that can refer to relationships between patients and practitioners, co-ordination across services, information transfer and co-ordination of care over time, and the coherent delivery of services for people with long-term conditions. 31 Theories about continuity of care provide an appropriate framework for exploring the health-care experiences of PLWD and ensure that activities that support coproduction of care are identified and considered. Older people with complex health needs value continuity and consistency of services, timely communication and follow-up between services, respectful delivery of services and HCPs who are familiar with their needs and can help them navigate multiple services. 42–44 Recent discourse around continuity has moved towards a partnership paradigm in which continuity is recognised to be coconstructed by patients, families and professionals, all of whom have an active part to play in its accomplishment. 33,45 In this model ‘professionals do not deliver continuity to service users but work with them and their carers/families to assess needs and preferences and facilitate contact and continuity’ (p. 597). 46
Freeman and colleagues40 have identified three main aspects of continuity: relationship continuity, management continuity and informational continuity. These categories are clearly not mutually exclusive and key concepts are common to all of them. The main aspects of each type of continuity are outlined in the following sections.
Relationship continuity
Relationship continuity refers to the continuous therapeutic relationship with one or more health professionals over time. 40 The relationship might be established with a single provider or with a team. 31 Relationship continuity with a trusted provider is seen to be a key attribute of high-performing primary care and is known to be important to patients and carers. 41,47,48 Moreover, good relationship continuity facilitates management and informational continuity. 41 Despite this, it is an aspect of care that has been relatively neglected in the literature. 47 Implied in the concept of relationship continuity is the idea that the health-care provider involved knows the patient as a person first. 47 This has particular importance for people with dementia where a focus on person-centred care that emphasises a good knowledge of the person’s priorities, preferences and previous history is the bedrock of good dementia care. 49 Individuals’ families are acknowledged to be a vital source of support for people with dementia, and care for people with dementia has evolved to recognise that relationships often involve triads including service users, their family carers and health and social care professionals. 50,51
Management continuity
Management continuity refers to processes involved in co-ordinating, integrating and personalising care, involving communicating both facts and judgements across team, institutional and professional boundaries and between professionals and patients. In the USA the National Quality Forum52 defines co-ordination as ‘a function that ensures that the patient’s needs and preferences for health services and information sharing across people, function and sites are met over time’. Management continuity includes the personalisation of care. The idea that personalising care is linked to improving the delivery of health care is a core tenet of many policy documents in the UK. 53 This links with concepts about the importance of providing person-centred care for people with dementia, core components of which include valuing the person with dementia and those who care for him or her and treating people as individuals. 54,55
Informational continuity
Informational continuity refers to record-keeping and the transfer of information, the timely availability of relevant information and patients’ and their carers’ understanding of their condition and treatment. Documented information tends to focus on the medical condition but this information is complemented by clinicians’ knowledge of the patients’ preferences, values and context, knowledge that accumulates over the course of a number of interactions with the patient. 31,41 However, the increasing complexity of care delivered by a variety of providers means that continuity can no longer rely on relationships with individual providers. 56 It has been argued, therefore, that as information continuity is conducive to automation and systematisation it could be the principal tool underpinning the co-ordination of care. 57
Individuals’ capacity to receive and understand information is a factor that influences continuity and concepts of informational continuity have been widened to include the recognition that people have different capacities for receiving information. 46 For PLWD, family carers may often be responsible for the transfer of information between different health providers, particularly as the dementia progresses. Despite this there is currently a lack of knowledge about the roles that family caregivers play in maintaining informational continuity. 57 People may live with dementia and other chronic conditions for a considerable time58 and it may be necessary for informational continuity to be maintained over sustained periods of time.
Access to care
In addition to continuity, issues around access to health and social care for PLWD emerged as particularly important in our scoping review of the literature (see Chapter 4). 59 This included issues around access to services, quality of care and appropriateness or comprehensiveness of care for PLWD. These have all been recognised as core dimensions of good care. 60 Commentators have argued that continuity should not be promoted over access but that both should be seen as equally important. 37,61
Access is concerned with the processes of gaining entry to the health-care system. This may be extended to include the processes of gaining entry to higher levels of care for those already admitted to lower levels of care, sometimes referred to as ‘in-system’ access. 62 The availability of services (having access) and utilisation of services (gaining access) are generally seen as key dimensions. Pechansky and Thomas63 suggested that the concept of access described the ‘degree of fit’ between clients and the health system. They identified five relevant dimensions to the client–service interaction: acceptability, affordability, availability, physical accessibility and accommodation. In high-income countries such as the UK, access to care can concern the degree of comprehensiveness that can be offered by heath-care systems, the extent to which equity is achieved and the timeliness and outcomes of care. 64 The appropriateness of services and their effectiveness at achieving the desired health outcomes are also considered to fall within the scope of conventional access models. 34
There are a number of organisational, geographical and financial barriers to access to care. For example, there may be costs associated with travelling to appointments and payment for some services, such as social care or eye checks, may be a barrier to access. Organisational barriers might include long waiting times for services or appointments and a failure to design services around the needs of patients. 34 In addition, personal, social and cultural influences may facilitate or impede the uptake of services. 34 Access is dependent on a person recognising a need for services and seeking help. 34,65 In the same way that dementia impacts on an individual’s ability to negotiate his or her own continuity of care, it may also impact on access to care.
Dixon-Woods and colleagues66 argue that equity of access is often measured by the use of health services but they suggest that access should be understood using the concept of candidacy. Candidacy describes how eligibility for health care is jointly negotiated between individuals and health services. Individuals need to view themselves as legitimate candidates for particular services, navigate gatekeeping within a service and have their candidacy validated by HCPs. 66,67 However, PLWD may lack the agency required for candidacy and may be dependent on family members or HCPs to identify a need for services and negotiate access to care on their behalf.
Framework
We used the different dimensions of continuity of care and access to care as a framework to structure how we asked questions and organised the analysis about the impact of living with dementia and other conditions for patients, carers and health-care providers at different points of the disease trajectory; to identify how continuity and access may be enhanced for this vulnerable group; and to inform how we considered service delivery from health-care providers. 32,40
The key aspects of the framework for this study were:
-
continuity:
-
relationship continuity
-
management continuity
-
information continuity
-
-
access to care:
-
appropriateness of care
-
comprehensiveness of care
-
candidacy
-
equity.
-
Summary
This chapter describes the conceptual framework that informed the original design of the study and provides a critique of the different components of continuity and access to care. It has highlighted what supports and inhibits access to health care and continuity of care with particular reference to the needs of PLWD and their family carers.
Chapter 3 Research plan and methods
Overview of the research plan
The research was undertaken in three phases (Figure 1).
FIGURE 1.
Diagrammatic summary of methods.
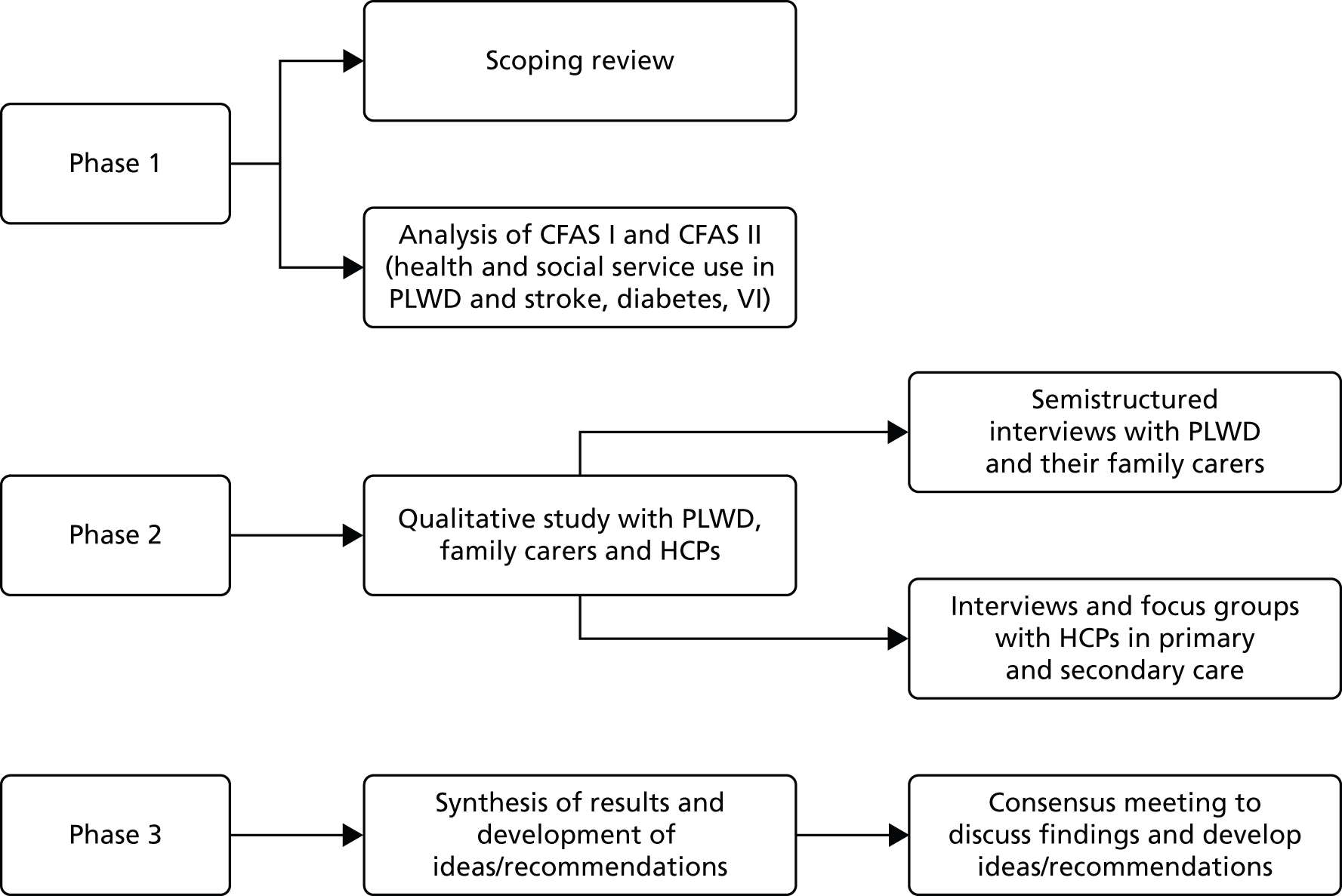
Phase 1
To understand current knowledge on the range and type of comorbid disease among PLWD and the impact of comorbidity on experiences and service use we:
-
scoped current evidence on the prevalence of comorbidities in PLWD, the experiences and attitudes of patients and carers and the systems and structures that exist for the care of PLWD and comorbid medical conditions
-
undertook a cross-sectional analysis of a population cohort database to explore health and social service use in people with a diagnosis of dementia and a comorbid medication condition.
Phase 2
To understand current models of service delivery, the points in the disease trajectory that pose the greatest challenges for service providers and barriers to and facilitators of access and continuity of care we undertook:
-
interviews with PLWD and a comorbid medical condition and their family carers
-
focus groups and interviews with clinicians involved in the care of PLWD and a comorbid medical condition.
Phase 3
In the final phase of the study we brought together the findings from phases 1 and 2 to:
-
map current models of care and how the presence of dementia with one or more comorbid condition is addressed by service providers
-
highlight interventions that support continuity and equity of access to care that can be incorporated into current models of service delivery
-
develop and refine ideas/recommendations about how services should engage with PLWD and their (family) carers.
We focused on three exemplar comorbid medical conditions, stroke, diabetes and VI, all of which generally involve some form of external clinical monitoring and require collaboration between primary and secondary care. Stroke and diabetes were chosen because they are common in older people68,69 and are thought to exacerbate or influence the progression of dementia. 70 Moreover, management of these conditions, in particular self-management, is likely to be complicated by the presence of dementia. 21 VI is also prevalent in older people71 and may exacerbate confusion. 72 In addition, the ability to cope with VI is reduced if a person also has dementia. Furthermore, although it is vital that eyesight is optimal in older people with dementia to maintain orientation and independence, there is a lack of uptake of services for this group, with few undergoing regular eye examinations. 73,74
Phase 1: scoping review
The scoping review was guided by Arksey and O’Malley’s75 methodological framework,76 which includes identifying the research question, searching for relevant studies, selecting studies, charting the data, and collating, and summarising and reporting the results. This approach allowed us to incorporate a range of study designs and address questions beyond those related to treatment efficacy. Although the scoping review has a number of similarities to a systematic review it does not typically involve quality assessment and findings are reported in a narrative format. 77,78 The inclusion criteria and methods for the review were prespecified in a protocol. 79
Identifying the research question
Inclusion criteria
We included studies involving PLWD who had an additional comorbid health condition. Although we included all types of comorbidities there was a particular focus on our three exemplar comorbid medical conditions: diabetes, stroke and VI. We focused on community-dwelling participants and excluded studies in long-term care settings. We looked for studies relating to the prevalence of comorbidities in people with dementia or cognitive impairment; current systems, structures and other issues relating to service organisation and delivery; patient and carer experiences; and the experiences and attitudes of service providers. We included all study types including systematic reviews, randomised controlled trials (RCTs), controlled studies, observation studies and qualitative studies using any recognisable qualitative methodology. In addition, we included non-research items such as clinical guidelines. We excluded studies disseminated in languages other than English.
Searching for relevant studies
We searched for a representative range of material that provided an overview of current knowledge and that identified some key examples of developments in the organisation and delivery of care for people with dementia and comorbid conditions. We included published and unpublished literature with no date restrictions. Studies were identified by computerised searches of the Allied and Complementary Medicine Database (AMED), The Cochrane Library [including the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects and the Health Technology Assessment database], the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (1980–2012), PubMed (1950–2012), NHS Evidence and Scopus (1966–2012). The electronic search strategy was developed by an experienced information scientist with input from the project team. An example of the search query for PubMed is given in Appendix 1. In addition, we employed extensive lateral search techniques such as checking reference lists, performing keyword searches in Google Scholar (Google Inc., Mountain View, CA, USA) and using the ‘cited by’ option in PubMed. We also contacted experts and those with an interest in dementia such as the Alzheimer’s Society, the Thomas Pocklington Trust, the Royal National Institute for the Blind, Diabetes UK, the Stroke Association and the Dementia and Sight Loss Interest Group. Such lateral strategies have been shown to be particularly important for identifying non-randomised studies. 80 The original electronic database searches were conducted in September 2012, with the PubMed search updated in November 2013 and lateral searches updated in February 2015.
Selecting studies and charting the data
Electronic search results were downloaded into EndNote bibliographic software (X7; Thomson Reuters, CA, USA) and, when possible, duplicates deleted. Two authors (FB and AB) independently screened titles and abstracts identified by the electronic search and applied the selection criteria to identify potentially relevant papers. Data were extracted by one author using a standardised checklist and checked by a second. Any disagreements were resolved by consensus or by discussion with a third author (CG). When the results of a study were reported in more than one publication, we grouped the reports together and marked the publication with the most complete data as the primary reference; the other papers describing the same study were classified as associated papers. Data extracted included type of item (e.g. empirical study, review, guideline), aims/research questions, methods, study focus, participants, setting and relevant outcome data (e.g. rates of comorbidities, access to treatment, patient and carer views).
Reporting the results
Studies were grouped into the following categories: (1) prevalence, (2) quality of care, (3) views and experiences (patients, carers and HCPs) and (4) health service organisation and delivery. Data were primarily reported in a narrative format. For the data on the prevalence of diabetes and stroke, forest plots have been used to graphically display (1) the prevalence of dementia comorbid with each of the target conditions in studies in which the target condition was the index condition and (2) each target condition comorbid with dementia in studies in which dementia was the index condition. Because of heterogeneity in the study designs and the lack of availability of age- and sex-standardised prevalence rates in the manuscripts, pooled estimates were not calculated.
The results of the scoping review were presented to and discussed with stakeholders at the University of Hertfordshire AgeNet research group. This group attracts an audience of older people, voluntary sector representatives, staff from health and social care and academics.
Phase 1: secondary data analysis
Sample and procedure
Data from two longitudinal multicentre population-based studies in the UK – CFAS I and CFAS II – were analysed. A detailed description of the samples and methodology is provided on the CFAS website [see www.cfas.ac.uk (accessed 7 December 2015)].
The CFAS I cohort consists of a sample of 13,004 individuals aged ≥ 65 years recruited between 1991 and 1993. Participants were randomly sampled from Family Health Service Authority lists in Cambridgeshire, Gwynedd, Newcastle, Nottingham, Liverpool and Oxford. These areas were selected to provide good geographical spread across urban and rural locations. Baseline interviews included questions about sociodemographic characteristics, lifestyle, health, activities of daily living and cognition. For CFAS I, repeated follow-up interviews took place on several occasions, with attempts to interview the entire sample at 2 and 10 years. The presence of health conditions was ascertained at each interview, although questions on service use were introduced for the whole sample during the 10-year follow-up interview. Data at 10 years were naturally available only for survivors who were aged ≥ 75 years (n = 3145).
Recruitment to CFAS II took place between 2008 and 2011 using the same strategy as for CFAS I. In total, 7796 participants were recruited from three of the original CFAS I locations in England. There was no repeated sampling from Liverpool. Furthermore, a separate but linked study in the Gwynedd area between 2011 and 2013 (CFAS II Wales) was not included in this analysis.
For the comparative analysis, to examine change between CFAS I and CFAS II, the analysis sample was restricted to the same age group (i.e. those aged ≥ 75 years) and centres (Cambridgeshire, Newcastle, Nottingham). Individuals living in residential or care homes were excluded from each sample as some services are provided by the residential and care homes themselves (CFAS, n = 109, CFAS I, n = 197). In total, 1619 and 3984 individuals from CFAS I and CFAS II, respectively, were included in the comparative analysis.
Ascertainment of health conditions and service use
Dementia was diagnosed using the Geriatric Mental State – Automated Geriatric Examination for Computer Assisted Taxonomy (GMS-AGECAT). 81 This standardised interview provides high agreement with the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition82 (DSM-IV) criteria (kappa = 0.86) [see http://allpsych.com/disorders/dsm/ (accessed December 2015)]. When it was not possible to determine a diagnosis of dementia using the GMS-AGECAT, a diagnostician would provide a study diagnosis using DSM-IV criteria. Cognitive function was assessed using the Mini Mental State Examination (MMSE). 83
Health conditions were self-reported by the individual or by an informant if a proxy interview was conducted. The conditions assessed included stroke, diabetes, VI, hearing difficulties, angina, heart attack, transient ischaemic attack, peripheral vascular disease, hypertension, Parkinson’s disease, anaemia and breathing difficulties with a focus on stroke, diabetes and VI. In line with other parts of the study, the impact of three exemplar ‘target’ conditions was considered to help determine the influence of comorbid dementia on service use. These were diabetes, stroke and VI.
All services, including health, social and informal care, were self-reported (or informant reported in CFAS II) with questions focusing on use of day-to-day services (e.g. home help, meals on wheels), service use in the 4 weeks prior to interview [e.g. day centre, social worker, general practitioner (GP)] and hospital service use (e.g. day hospital, inpatient). Use of unpaid care was considered separately for the analysis.
Statistical analysis
The analysis was undertaken in two stages. First, a comparative analysis compared service use one decade apart using data from both CFAS cohorts, restricting the sample to those aged ≥ 75 years in the CFAS II cohort (sample size: CFAS I, n = 1619; CFAS I, n = 3984). Second, a detailed analysis was conducted in CFAS II (sample size, n = 7796). Both analyses were conducted on those living in the community, with those living in residential or nursing homes excluded, as some services are provided by the residential and nursing homes themselves. Although service user data were collected from both the respondent and the informant interviews in CFAS II, data were collected from only the respondent interviews in CFAS I. Therefore, to ensure comparability, only respondent information was used in the comparison analysis.
Prevalence estimates with 95% confidence intervals (CIs) were generated for service use and the comorbidities considered. Logistic regression was used to estimate service use by individuals with dementia and a comorbidity in comparison to service use by (1) those with dementia alone and (2) those with the comorbidity alone. All models were adjusted for age and sex. Estimates were inverse probability weighted in both CFAS I and CFAS II to account for the oversampling of those aged ≥ 75 years and the differences in age, sex and deprivation in those who participated. As the CFAS I analysis was conducted with the 10-year follow-up wave, the analysis also had to be weighted for attrition based on age, sex, stroke, diabetes, VI and latest cognitive status (using the MMSE).
Phase 2: focus groups and interviews
In phase 2 we addressed research questions 2 and 3 (see Chapter 1) through in-depth semistructured interviews with service users with a long-term condition and their family carers and focus groups or interviews with staff who organise and deliver care in a range of different specialities. This included:
-
people diagnosed with dementia who had at least one of our target non-dementia-specific health-related problems (i.e. stroke, diabetes and VI)
-
family/unpaid carers of people with dementia and one of our target non-dementia-specific health-related problems
-
clinicians in both primary and secondary care who organised and delivered care for people with stroke, diabetes and VI.
Sampling
People with dementia and their family carers were recruited from two geographical regions: (1) the area covered by North Thames Clinical Research Network Dementias and neurodegeneration (DeNDRoN) (including the London boroughs north of the Thames, South Bedfordshire, Hertfordshire and Essex) and (2) the north-east of England (Newcastle). HCPs were also primarily recruited from these areas although focus groups were also held in Cambridgeshire and Leicestershire. We aimed to carry out 15–25 interviews per site with people with dementia and their family carers and a total of six focus groups across the sites to capture the different experiences of primary and specialist service professionals.
Recruitment of patients and family carers
Older people with a diagnosis of dementia with at least one of the target comorbid conditions (i.e. stroke, diabetes and VI) were recruited for the study. We also recruited family carers of people with dementia with at least one of the target comorbidities. Participants were purposively sampled to capture our specified comorbid conditions and a range of experience along the dementia pathway.
As stated above, recruitment took place in two geographical regions: the south-east of England (University of Hertfordshire) and the north-east of England (Newcastle University). In the south-east, participants were recruited through the North Thames Dementia Registry, general practices, local memory clinics in Hertfordshire and Bedfordshire and voluntary organisations (i.e. the Alzheimer’s Society, Thomas Pocklington Trust and Stroke Association). In Newcastle, participants were recruited through general practices and the North East DeNDRoN patient list. Participating general practices identified potentially eligible participants for the relevant long-term illnesses (i.e. dementia, diabetes) from the practice Quality and Outcomes Framework (QOF) registers. This list was then screened by one of the practice GPs to identify those who met the inclusion criteria.
Potentially eligible participants were approached via an invitation letter from DeNDRoN or from the appropriate gatekeeper (such as a GP or memory clinic clinician). Invitation letters were accompanied by an information sheet and a reply slip for those who were interested in taking part in the study. Participating patients were asked whether or not they received any significant help from an informal carer and, if so, permission was sought from patients to invite the carer for interview. Participants who expressed an interest in participating were contacted by one of the research team to discuss the study further and arrange a time and place for the interview.
Inclusion and exclusion criteria for people with dementia and family carers
Inclusion criteria
-
Having a range of experiences along the dementia pathway.
-
Similar numbers with each of the target conditions of stroke, diabetes and VI.
-
More than one comorbidity (e.g. stroke and heart disease) as long as one of the comorbidities was a target condition.
-
Any type of dementia but excluding mild cognitive impairment (MCI).
-
A confirmed diagnosis of dementia or taking dementia medication.
-
Stroke – confirmed diagnosis from secondary care (regardless of aetiology).
-
Diabetes – confirmed diagnosis of type 1 or type 2 diabetes.
-
VI – defined as being registered blind or partially sighted or having a secondary care diagnosis of a condition that leads to VI, for example cataracts or macular degeneration.
-
Interviews could be paired (person with dementia and his or her carer) or with the person with dementia or the carer alone.
Exclusion criteria
-
Unable to speak English.
-
Terminally ill or on the palliative care register.
Recruitment of health-care professionals
Clinicians in both primary and secondary care who organised and delivered care for people with stroke, diabetes and VI were recruited for interviews or focus groups. Individuals were purposively sampled to capture a range of experiences and interests. This included GPs with a specialist interest in long-term conditions, secondary care doctors at consultant or senior registrar level who specialised in the care of people with stroke, diabetes or VI and clinical nurse specialists/therapists and practice nurses responsible for the management of people with long-term conditions such as diabetes. Potential participants were identified and recruited through the clinical networks of the Research Management Group and the Advisory Group and through local clinical research networks.
Procedures
The focus of data collection was to identify characteristics of services that respond appropriately to patient and carer needs, positive and negative examples of patient care, areas where patient needs are not met and barriers to and facilitators of effective service provision for people with dementia and a comorbid condition. It enabled us to explore how the presence of dementia impacts on clinical decision-making processes. Different interview schedules/focus group prompts were designed for use with patients, carers and clinicians and according to the comorbidity involved (see Appendix 2). Interview and focus group schedules were guided by literature from the scoping study and consultation with members of the User Reference Group and were further refined based on content and findings from early interviews. Ethical approval was obtained from the National Research Ethics Service Committee East of England (Research Ethics Committee reference 13/EE/0091).
Interviews with service users
Most interviews with service users took place in the participants’ own home, with one interview taking place in a participating memory clinic. Initially, the researcher introduced herself to the participant and explained that the study was looking at how memory problems affect how people manage other health conditions, for example diabetes, stroke or sight loss. Participants were given a copy of the study information sheet, which provided contact details of the research team, and a consent form, which they were asked to read and sign. Permission to record the interview was requested and it was explained to participants that the interview would remain anonymous and confidential. Participants were informed that they could have a break from the interview or withdraw at any time.
Once consent had been obtained, the researcher gathered demographic information (age, education level, previous employment, family support, social services support) for both the PLWD and the family carer. In addition, consent was obtained from participants for the researchers to contact their HCP (e.g. GP or doctor at a memory clinic) to obtain information about the type of dementia that they had been diagnosed with. The semistructured interview schedule was designed to gather information about the patients’ health conditions; how they managed their conditions; their health-care experiences relating to their target comorbidities; the range of services used; and their views about what was working well or what they thought could be improved. The interview schedule was adapted according to the comorbidities involved. At the end of the interview the participants were asked if they had any questions and were given a £10 voucher in appreciation of their time.
Interviews and focus groups with service providers
Five focus groups with HCPs were conducted in the clinical setting, with each lasting for about an hour and facilitated by two researchers. For the interviews, one was conducted face to face and the rest were conducted by telephone, with interviews lasting for up to 30 minutes. The focus groups and telephone interviews followed the same procedures. HCPs were given the study information sheet together with a consent form to sign. The researchers requested permission to audio tape the sessions, explaining that all data would remain anonymous and confidential. After a brief description of the study, participants were asked to introduce themselves and describe their current role. The discussion focused on how dementia impacted on their service and clinical decisions; ways to improve services for people with dementia and comorbidities; care pathways and clinical guidance; and knowledge gaps for HCPs in non-dementia services.
Analysis
Qualitative data analysis drawing on thematic content analysis84 was used to enable key features of patients’, carers’ and clinicians’ experiences to be elicited from the data. Two (out of three) researchers (AB, FB, MP) independently scrutinised and coded a selection of transcripts. They compared codes and discrepancies were resolved by discussion. 85 Members of the User Reference Group also read several transcripts and met with the researchers involved in the qualitative data analysis to discuss the emerging themes. Emerging themes were also discussed with the Research Management Group and the Advisory Group. A coding framework, guided by the different characteristics of continuity of care and access to care, was developed and transcripts were entered into NVivo software (version 10; QSR International, Warrington, UK) for further qualitative data analysis. We used a constant comparison method to look for similarities and differences between patients, carers and clinicians and between different conditions.
Phase 3: development of ideas/recommendations
Consensus conference
The findings from the first two phases of the study were discussed with key stakeholders at a consensus conference. The purpose of consulting with stakeholders in this way was to validate the findings and assist in the development of guidance. Participants invited to the meeting included clinicians and service user representatives who took part in focus groups or interviews; members of the Research Management Group, Advisory Group and User Reference Group; practitioners specialising in the care of people with our target comorbidities (diabetes, stroke and VI); service managers and commissioners; and representatives from the third sector involved in supporting people with dementia (e.g. Alzheimer’s Society, Carers in Herts) or people with our target comorbid conditions (e.g. Stroke Association, Diabetes UK, Thomas Pocklington Trust). The meeting began with a presentation from the research team that drew on the findings from the study. The presentation covered what is known about the impact of dementia on the receipt of non-dementia health services; barriers to and facilitators of service delivery for people with dementia and comorbidities; and evidence around best practice/effective care for service delivery for people with dementia and a comorbid medical condition. This was followed by a group discussion on the implications of the findings.
Nominal group technique
To structure the discussion and rank the importance of the findings and their relevance for service improvement and delivery we used a nominal group technique. A nominal group technique is a process that promotes the generation of ideas to develop a set of prioritised ideas/recommendations, enabling the participation of all group members and preventing the domination of one or two participants in the discussion. The process involves four stages: (1) the generation of ideas, (2) the recording of ideas, (3) discussing ideas and (4) prioritising ideas. 86,87 The nominal group technique was used to enable stakeholders, including members of the public, to meaningfully participate in the development of ideas/recommendations.
Participants were split into four groups (seven to nine participants in each) based on their area of specialty (i.e. stroke, diabetes, VI, general practice). Two service user representatives were placed in each group, one from the Public Involvement in Research Group at the University of Hertfordshire and the other from a dementia voluntary organisation (e.g. Alzheimer’s Society, Dementia UK). Each group included two members of the research team, one to facilitate the group and one to take notes. The facilitator directed the discussion and focused attention on achieving a common understanding of the questions and their answers.
Based on the evidence presented and their own experiences, participants were asked to consider what changes they would recommend to improve care for people with dementia and comorbidity. They were told that this could be general or specific to one of the three target comorbidities. To stimulate free thinking of ideas, participants were told that no ideas were bad ideas and that they should not be restricted by cost, current systems or time frames. They were told that ideas/recommendations could be easily achievable or could be challenging to implement. To encourage a quick generation of ideas, participants had just 10 minutes to write their ideas/recommendations onto the ideas templates (see Appendix 3). The template required participants to name their idea, give a brief explanation and identify the barriers to and facilitators of the idea/recommendation.
After the generation of ideas, the group moderator asked the participants one by one to share one of their ideas/recommendations and these were recorded by the note taker. Each idea/recommendation was discussed, specifically focusing around how these concepts could be developed and implemented. The participants discussed and prioritised the ideas and decided which two they would present to the wider group. The note taker recorded the two ideas (agreed by the group) on large A3 templates in the centre of the table. These were discussed further to develop the ideas more fully (name the idea, explain the idea, benefits, barriers). Finally, the conference facilitator consolidated the ideas/recommendations by asking each of the tables (diabetes, stroke, VI and general practice) to put forward their ‘best idea’ to the larger group. Ideas/recommendations were discussed and recorded on a flip chart at the front of the room.
After the meeting ideas were summarised and grouped around key ideas or themes, such as integration, information sharing, carer support and education and training. They were also categorised into whether they related to primary or secondary care and if they were specific to one of our comorbidities. A report of the findings from the consensus meeting was circulated to all participants for feedback.
The development of ideas/recommendations
The findings of the scoping review, the results of the interviews and focus groups, and the outputs from the consensus meeting were used as a basis to map current models of care and to identify interventions that had the potential to support continuity and equity of access to care for people with dementia and comorbidity. After this mapping we ran searches in PubMed, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Google Scholar to identify systematic reviews evaluating the models of care identified (e.g. case management, carer support, integration) (see Appendix 1 for search terms). From this we developed ideas/recommendations about how services should engage with PLWD and their carers, indicators of quality that could inform the commissioning of services for older people and specialty-specific guidance on assessment and decision-making.
Patient and public involvement
A well-established Public Involvement in Research Group at the University of Hertfordshire has a broad membership of service users and carers. A member of this group chaired the project Advisory Group. In addition, three members of the Public Involvement in Research Group were part of a study User Reference Group. This group was involved in guiding the development of the interview schedule, guiding and challenging our interpretation of the qualitative findings and facilitating understanding of the implications for continuity from a service user perspective. Four members of the Public Involvement in Research Group attended the consensus conference in phase 3.
Chapter 4 Results of the scoping review
An earlier version of this review has been published elsewhere. 59 Since then the review has been updated to include an additional 12 papers.
Description of studies
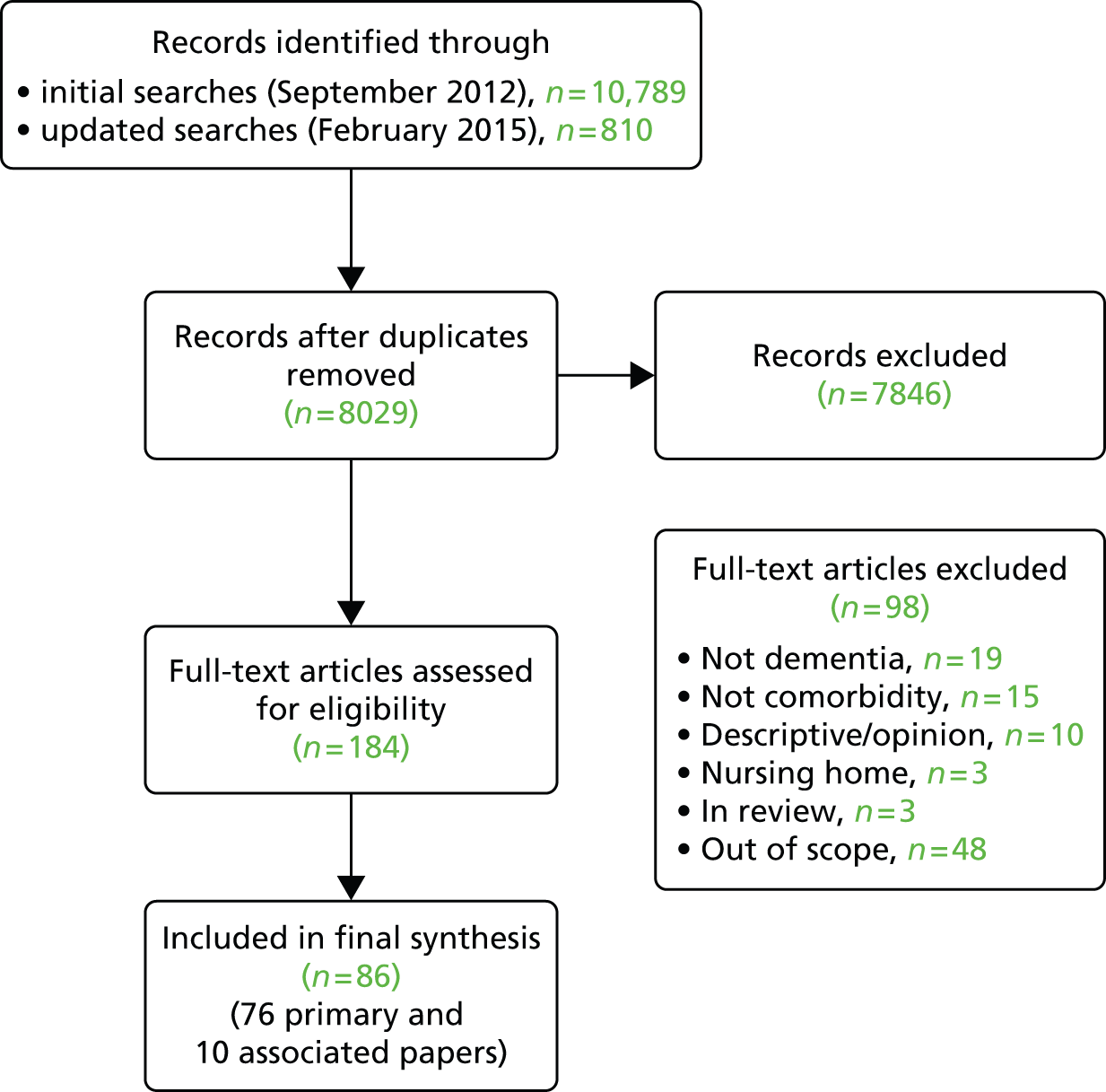
We included 86 papers, 76 of which were classified as primary references1,2,12,14,20,21,69,72,88–155 and 10 of which were classified as associated papers. 4,73,74,156–162 An overview of the selection process can be seen in Figure 2. Studies had been conducted in 12 different countries but 61% were from the UK and USA. The majority of the studies were observational or qualitative studies or reviews. Participants were predominantly community dwelling although some studies included mixed samples and those living in long-term care. The majority of studies included people with an average age of > 70 years. Ethnicity was not reported in 55% (n = 42) of studies. In 63% of studies (n = 47) all of the participants had dementia,1,2,12,14,69,72,89–94,96,98,99,102,105,108,110–112,116–118,122,123,125,127,129,130,133,136–139,142,144–149,151,153–155 in 24% (n = 18) the studies included mixed populations including people with dementia, cognitive impairment and delirium88,95,100,104,106,107,109,113,115,119,121,126,128,131,132,134,135,143 and in 13% (n = 10) participants had cognitive impairment or MCI. 20,21,101,103,114,120,124,140,141,150 In the remaining study152 the focus was on clinicians’ attitudes rather than people with dementia. In total, 44 studies20,21,69,72,89,90,92–96,98,100,102–104,108,112,114–117,122,126,129,130,133,136–141,144–147,149–152,154,155 (58%) focused on a single comorbidity and the rest of the studies focused on more than one comorbidity or on general comorbidity/multimorbidity (e.g. papers relating to the experiences of people with dementia in acute hospitals). Most of the evidence related to prevalence and quality of care, with less evidence on service organisation and delivery or views and experiences of patients, carers or HCPs. The study characteristics are summarised in Table 1 and details of the individual studies, including the links between primary and associated papers, are provided in Appendix 4 (see Table 12).
FIGURE 2.
Flow chart detailing the study selection process.
| Characteristic | Detailsa |
|---|---|
| Study information | |
| Year of publication, range | 1989–2015 |
| Country, n | |
| UK | 26 |
| USA | 20 |
| Europe (not UK) | 10 |
| Australia | 3 |
| Canada | 5 |
| Japan | 2 |
| International reviews/guidance | 10 |
| Type of study, n | |
| Case–control | 8 |
| Cohort study | 13 |
| Cross-sectional | 19 |
| Guideline/policy document | 4 |
| Qualitative | 8 |
| RCT | 3 |
| Survey | 4 |
| Review/scoping | 11 |
| Other | 6 |
| Areas covered in study, nb | |
| Prevalence | 30 |
| Service organisation and delivery | 17 |
| Views and experiences | 13 |
| Access to care | 25 |
| Setting, n | |
| Hospital/outpatient clinic | 34 |
| Community | 15 |
| Primary care | 7 |
| Mixed community/residential care/hospital | 7 |
| Population-based sample | 8 |
| Not specified (e.g. review) | 5 |
| Type of participants | |
| Type of cognitive impairment, n | |
| Dementia | 47 |
| Mixed populations | 18 |
| MCI or cognitive impairment | 10 |
| Age (years) | Range 43–102 but majority > 70; of 38 studies that gave a mean age, majority was in 70s |
| Ethnicity, n | |
| Not specified | 41 |
| White (or majority white) | 15 |
| Mixed | 4 |
| Mixed but majority black | 2 |
| NA (e.g. review) | 14 |
| Comorbidities included in studiesb | |
| Diabetes | 16 |
| Stroke | 9 |
| VI | 14 |
| Other comorbidities (e.g. cancer, MI, hypertension) | 8 |
| General comorbidity | 15 |
| More than one comorbidity (but includes one of our target conditions) | 14 |
| Type of dementia | |
| Alzheimer’s disease | 8 |
| Mixture of different types | 5 |
| Not reported | 63 |
Prevalence
Our main aim was to look at the prevalence of comorbidity (in particular stroke, diabetes and VI) in people with dementia. Fourteen studies1,2,12,14,91,118,119,121,127,128,137,142–144 provided data on the prevalence of comorbidities in people with dementia and two studies101,134 provided data on the prevalence of comorbidities in people with MCI (Table 2).
| Study and country | Study type | Dementia/cognitive impairment | Control/comparison | Eligibility criteria | Recruited from | n whole sample | n dementia/cognitive impairment | Diabetes (%) | Stroke (%) | VI (%) | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barnett et al., 2012,91 UK (Scotland) | Cross-sectional | Dementia | NA | Alive, permanently registered with a participating practice | Primary care – national data set | 1,751,841 | 11,139 | 13.3 | 18.8 | 3.8 | |
| Doraiswamy et al., 2002,1 USA | Cross-sectional | Dementia | NA | Diagnosis of Alzheimer’s disease, age ≥ 50 years | Community health-care sites | 679 | 679 | – | – | – | 61% had three or more comorbidities and 30% had vascular or heart disease. Sample included mixture of community-dwelling and long-term care participants |
| Feil et al., 2003,101 USA | Longitudinal cross-sectional | Cognitive impairment | No cognitive impairment | Geographically defined, age ≥ 65 years | Population-derived sample | 7482 | 1774 | 26 | 34 | – | |
| Heun et al., 201312 UK | Retrospective case–control | Dementia | No dementia | Diagnosis of Alzheimer’s disease, age ≥ 70 years, inpatient care for at least 24 hours | Hospital inpatients | 72,878 | 634 | 6 | 3 | 1 | VI was glaucoma. Diagnosis of diabetes was less common in those with Alzheimer’s disease than in control patients (RR 0.7, 95% CI 0.5 to 0.9). No significant difference in prevalence of ischaemic stroke (RR 1.3, 95% CI 0.9 to 2.0) or glaucoma (RR 2.0, 95% CI 1.0 to 4.3) |
| Jara et al., 2011144 UK | Retrospective cohort | Dementia | No dementia | Age ≥ 64 years, at least 24 months’ enrolment, no cataract diagnosis at baseline | Primary care – national data set | 650,325 | 8124 | – | – | – | Lower rate of any cataract in Alzheimer’s disease group than in control group (HR 0.52, 95% CI 0.47 to 0.58) |
| Löppönen et al., 2004,118 Finland | Cross-sectional | Dementia | NA | Geographically defined, age ≥ 65 years | Population based | 1260 | 112 | 16 | 24 | 29 | PLWD less likely to be diagnosed with glaucoma (OR 0.36, 95% CI 0.15 to 0.86); no difference in rates of cataract (p = 0.287) |
| Lyketsos et al., 2005,119 USA | Case–control | Dementia/cognitive impairment | No dementia/cognitive impairment | Geographically defined, age ≥ 65 years | Population based | 695 | 374 | 20 | 16 | – | Stroke more common in people with dementia (p < 0.001) |
| McCormick et al., 1994,121 USA | Case–control | Dementia/cognitive impairment | No dementia/cognitive impairment | Age ≥ 60 years, member of HMO, geographically defined | HMO database | 154 | 154 | 6 | 3a | 10 | Vision problems less common in PLWD (10% vs. 24%) |
| Poblador-Plou et al., 2014,14 Spain | Cross-sectional | Dementia | No dementia | Age ≥ 65 years, consulted physician at least once during the 12-month period of the study | Database of 19 primary health-care centres | 72,815 | 3971 | 20 M, 16 F | 7 M, 5 F | 7 (M and F) | Cataracts 7%, glaucoma 4% in M and F |
| Rait et al., 2010,127 UK | Cohort | Dementia | No dementia | Age ≥ 60 years with first code for dementia during the study period, at least 6 months of data | Primary care – national data set | 135,174 | 22,529 | 14 | 29a | – | No difference in prevalence of diabetes (13.9% vs. 14.5%) but cardiovascular disease more common in people with dementia (29.3% vs. 13.3%) |
| Sakurai et al., 2010,128 Japan | Cross-sectional | Dementia/cognitive impairment | NA | Dementia or MCI | Memory clinic | 160 | 160 | 19 | – | – | Dementia and CI |
| Schubert et al., 2006,2 USA | Cross-sectional | Dementia | No dementia | Age ≥ 65, seen primary care physician within 2 year. Excluded nursing home residents and non-English-speaking patients | Primary care | 3013 | 107 | 39 | 10 | – | No significant difference in prevalence of diabetes (p = 0.19) or stroke (p = 0.89) between those with and those without dementia |
| Stephan et al., 2011,134 UK | Cross-sectional | MCI | No MCI | Age ≥ 65 years | Population based | 13,004 | 1486 | 7 | 19 | – | |
| Uhlmann et al., 1991,137 USA | Case–control | Dementia | No dementia | Age ≥ 65 years, English speaking, eighth grade of higher level of education, ability to complete audiometric evaluation | Adult medicine clinic | 174 | 87 | – | – | – | Prevalence of VI significantly higher in cases than in control subjects (OR 2, 95% CI 1.2 to 3.4) |
| Zamrini et al., 2004,142 USA | Case–control | Dementia | NA | Probable Alzheimer’s disease, black or white (white participants matched non-randomly to black participants) | Memory clinic database | 334 | 334 | 18 | 9 | 10 | Includes all eye diseases |
| Zekry et al., 2008,143 Switzerland | Cohort | Dementia/MCI | No dementia | Age ≥ 75 years. Excluded those with a terminal illness or disorders interfering with psychometric assessment | Hospital inpatients | 349 | 188 | 19 | 22 | – | MCI and dementia |
The representativeness of the samples varied. Four studies101,118,119,134 included population-based samples seven1,2,14,91,121,127,144 recruited populations from primary care databases and five12,128,137,142,143 used samples from hospitals or outpatient clinics (see Appendix 4, Table 13). Data were collected from medical records in seven studies,12,14,91,127,137,142,144 from clinical examination or interviews in six studies1,101,118,119,128,134 and from a mixture of medical records and clinical examination in three studies. 2,121,143 The presence of dementia and comorbid medical conditions was assessed using a variety of measures (see Appendix 4, Table 14).
We also included a further nine primary studies20,95,103,104,129,140,141,145,146 and one systematic review69 that reported the prevalence of dementia in people with one of our three target comorbidities (Table 3); five studies20,95,103,104,145 included people with diabetes, three69,129,146 included people with stroke and two140,141 included people with VI. Three studies103,140,141 included samples from outpatient clinics, one20 included data from a RCT in primary care, two included data from research administration or health claims databases in the USA104 and Australia,145 one129 included data from a stroke register in Canada, one95 included a population-derived community-based sample in Australia and one146 included data from a population-based register of vascular events in the UK. Three studies104,129,145 collected data from medical or database records and the rest collected data from face-to-face interviews or clinical examinations.
| Study and country | Type of study | Type of population | Control/comparison | Eligibility criteria | Recruited from | n whole sample | n dementia/cognitive impairment |
|---|---|---|---|---|---|---|---|
| Bruce et al., 2003,95 Australia | Longitudinal cross-sectional | Diabetes | NA | Defined by postcode, age ≥ 70 years, diabetes | Patients living in catchment area of hospital (63% of eligible patients recruited) | 223 | 34 (15.2%) |
| Feil et al., 2009,103 USA | Longitudinal cross-sectional | Diabetes | NA | Diagnosis of type 2 diabetes, age ≥ 60 years | Geriatric clinic | 51 | 23 (45%) cognitive impairment |
| Feil et al., 2011,104 USA | Cross-sectional | Diabetes | NA | Veterans aged ≥ 65 with diabetes | Research administration database (Veterans Health Administration) | 497,000 | 65,107 (13%) dementia/cognitive impairment |
| Hewitt et al., 2010,20 UK | Questionnaire | Diabetes | NA | Type 2 diabetes, age ≥ 75 years, not resident in a nursing home | Data from RCT in primary care | 1047 | 235 (22.4%) dementia/cognitive impairment |
| Pendlebury and Rothwell, 2009,70 UK | Systematic review (73 studies) | Stroke | NA | Dementia and stroke measured by standard criteria | 22 hospital-based and eight population-based studies (7511 patients) | NA | Pooled prevalence of pre-stroke dementia 14% in hospital-based studies and 9% in population-based studies. Post-stroke rates ranged from 7% to 41% |
| Pendlebury et al. 2015,146 UK | Prospective cohort study | Stroke | NA | TIA or stroke; dementia defined as pre and post event | Register collected for study – data from primary care (nine GP practices) | 1236 | 93 (8%) pre-event dementia, 173/1143 (15%) post-stroke dementia |
| Saposnik et al., 2011,129 Canada | Retrospective cohort study | Stroke and dementia | Stroke no dementia | Age ≥ 18 years, first ischaemic stroke | Stroke register (included patients admitted to 12 regional stroke centres in Ontario, Canada) | 10,658 | 966 (9.1%) |
| Whitson et al., 2010,140 USA | Cross-sectional | VI (macular disease) | NA | Age ≥ 65 years, macular disease diagnoses | Low-vision rehabilitation clinic | 101 | 19 (19%) |
| Yochim et al., 2012,141 USA | Case series | VI (glaucoma) | NA | Age ≥ 50 years, diagnosis of glaucoma | Glaucoma clinic | 41 | 44% MCI |
| Zhang et al., 2010,145 Australia | Retrospective cohort study | Diabetes | NA | Veterans, age ≥ 65, received prescription for diabetes in previous 6 months | Health claims database | 17,095 | 4.4% |
Prevalence of target comorbidities in people with dementia
Diabetes
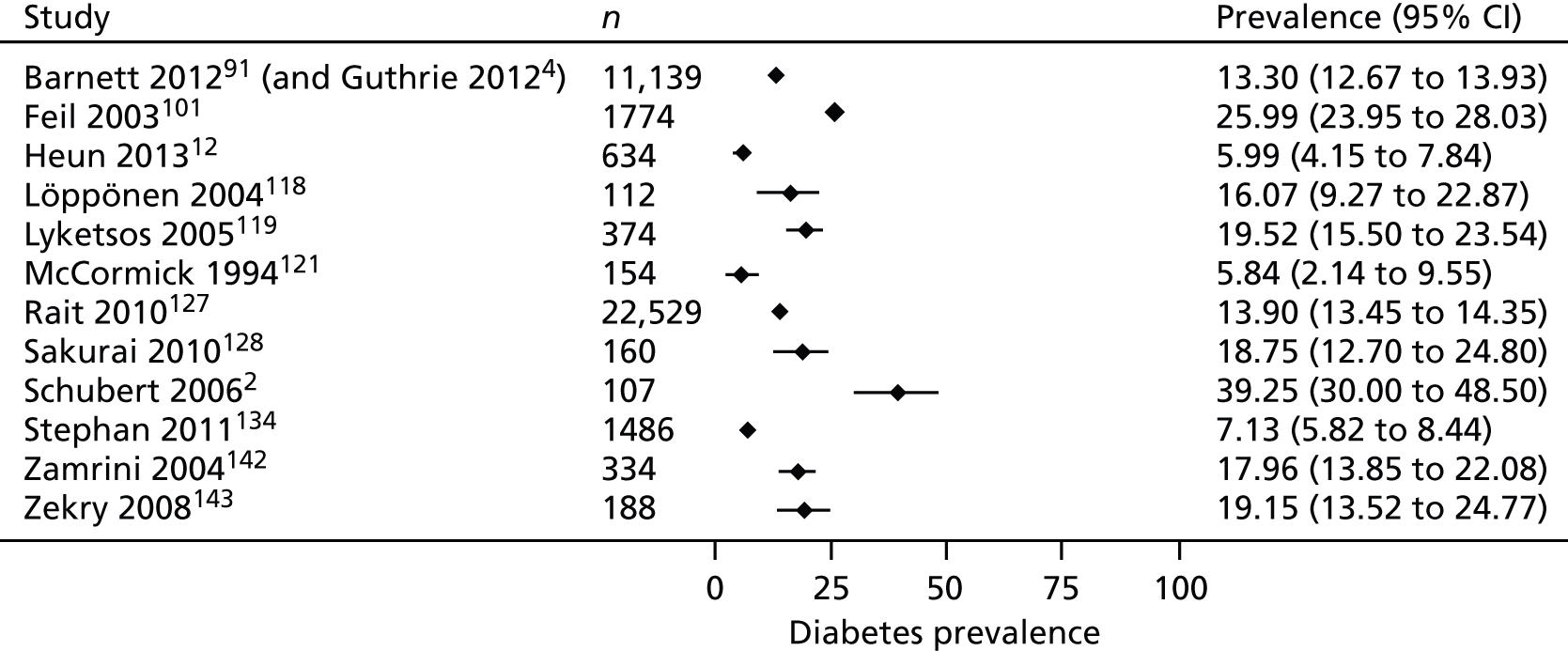
Thirteen studies reported the prevalence of diabetes in populations of people with dementia2,12,14,91,118,119,121,127,128,142,143 or MCI. 101,134 Prevalence rates varied from 6% to 39%. A visual representation of the prevalence of diabetes in PLWD is provided in Figure 3. The two largest studies, both of which involved participants from national primary care data sets in the UK, one from Scotland91 and one from the UK (primarily England),127 reported similar prevalence rates of 13%91 and 14%. 127 A Spanish study14 reported a prevalence rate of 20% in men (95% CI 18% to 22.5%) and 16% in women (95% CI 14% to 17%). Although diabetes was one of the most frequent comorbidities in the study, the authors did not demonstrate a significant association between dementia and diabetes. Five studies compared rates of diabetes in those with and those without dementia. Three studies with samples recruited from primary care databases, two from the USA2,121 and one from the UK,127 found similar rates of diabetes between groups and a study in hospital inpatients in Switzerland143 found no significant difference in the rate of diabetes between those with and those without dementia. In contrast, a study of hospital inpatients in the UK12 found significantly fewer people with dementia diagnosed with type 2 diabetes than control subjects without dementia.
FIGURE 3.
Prevalence of diabetes in PLWD.
Stroke
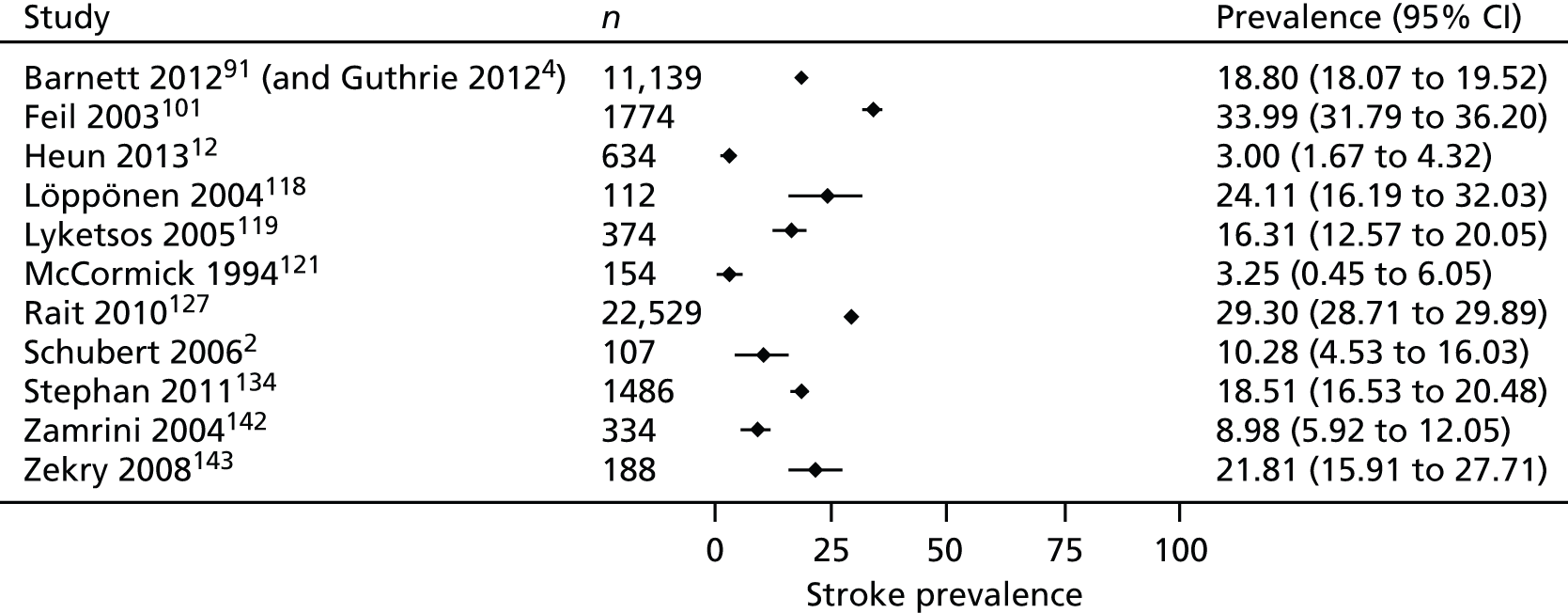
Ten studies2,12,14,91,118,119,121,127,142,143 reported the prevalence of stroke in people with dementia and two studies101,134 reported the prevalence of stroke in people with cognitive impairment. Prevalence rates varied from 3% in hospitalised older people in the UK12 to 34% in a sample of urban and rural community-dwelling people with cognitive impairment in the USA (Figure 4). 101 Two studies used records from large primary care databases in the UK, with one reporting that the rate of stroke in PLWD was 19%91 and the other reporting that the rate of cerebrovascular disease (including stroke) was 29%. 127 A study using a primary care data set in Spain found a prevalence of cardiovascular disease of 7% in men and 5% in women. 14 Five studies compared the rate of stroke in people with and without dementia. Three2,12,143 found no significant difference in the prevalence of stroke but one study of hospital inpatients119 found that stroke was more common in people with dementia and a primary care-based study in the UK127 found a greater prevalence of cerebrovascular disease in people with dementia.
FIGURE 4.
Prevalence of stroke in PLWD.
Vision impairment
Eight studies reported the prevalence of some form of VI in people with dementia, including all eye diseases,91,121,137,142 glaucoma12,14,118 and cataracts. 14,118,144 Differences in the populations studied and the way that cases were identified make comparisons across studies difficult. Two studies compared rates of glaucoma in people with dementia and those without dementia; prevalence in people with dementia was lower in a population sample in Finland118 but no different in hospital inpatients in a retrospective case–control study in the UK. 12 Two studies compared rates of cataracts. A large primary care cohort study in the UK found a lower incidence rate for cataracts in people with Alzheimer’s disease than in control subjects144 but a smaller population-based study in Finland reported no difference in the rates of cataracts. 118 A cross-sectional study in Spain found that the prevalence of cataracts was 7% and that of glaucoma was 4%. 14
Prevalence of dementia in people with stroke, diabetes and vision impairment
Ten studies20,69,95,103,104,129,140,141,145,146 provided some data on the prevalence of dementia in people with one of our target comorbidities, but only eight provided data that could be included in a forest plot (Figure 5).
FIGURE 5.
Prevalence of dementia/cognitive impairment in people with the target comorbidities.
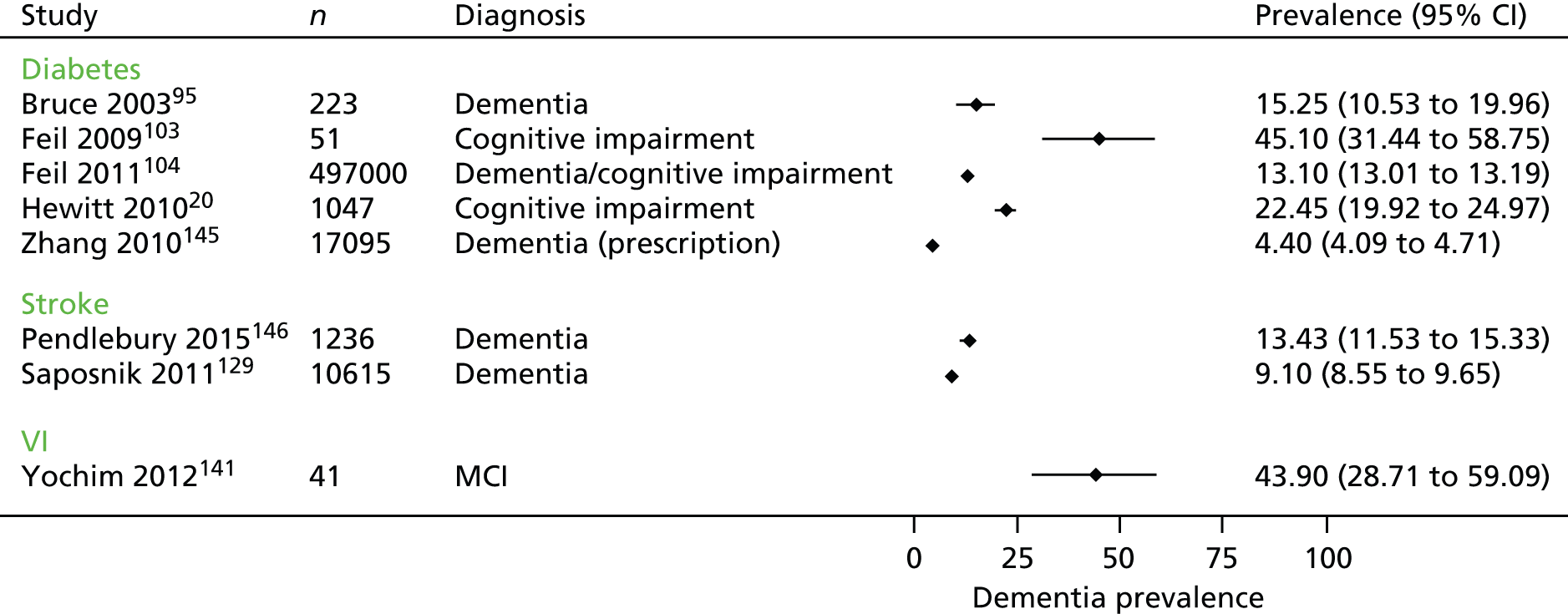
Five studies20,95,103,104,145 looked at the prevalence of dementia or cognitive impairment in populations of people with diabetes. In a large population-based study in the USA,104 13% of people with diabetes had dementia or cognitive impairment whereas, in a UK sample recruited through primary care,20 23% of people with diabetes had dementia or cognitive impairment. Two studies reported the prevalence of dementia in people with VI recruited through eye clinics. 140,141 In one,140 19% of people with macular disease had dementia and in the other,141 20% of people with glaucoma had memory impairment and 22% had impaired executive functioning.
Two studies129,146 and one systematic review69 looked at the prevalence of dementia among people who had had a stroke. Of over 10,000 people on a stroke register, 9% were reported to have dementia. 129 A study using hospital and primary care data in the UK146 found a prevalence of pre-stroke dementia of 8% and of post-stroke dementia of 15% and a systematic review69 reported a pooled prevalence of pre-stroke dementia of 14% in hospital-based studies and 9% in population-based studies. Post-stroke rates of dementia ranged from 7% to 41%. 69
Access to care
We categorised 25 studies21,90,98,100,103,104,110,111,114–118,123,129,130,136,139,145,150–152,154,155,161 as relating to access of care, for example comprehensiveness, equity and outcomes of care.
Comprehensiveness and equity
Eleven studies98,110,117,118,123,129,136,139,145,155,161 compared access to treatment or receipt of services in groups with and without dementia. Ten98,110,117,118,129,136,139,145,155,161 of the 11 studies found some evidence that people with dementia were less likely to receive the same quality of care or access to services as those without dementia. For instance, studies found that people with dementia were less likely to receive monitoring for diabetes-related problems97,136 and had reduced access to treatment such as intravenous thrombolysis for stroke,129 surgery for cataracts,110 treatment for age-related macular degeneration (AMD)98,117 or services for diabetes. 139,145 More details are provided in Table 4. In addition, a German study reported that older people with a greater number of comorbidities were less likely to receive cholinesterase inhibitors for dementia111 and a Canadian study found evidence that pain is undertreated in people with dementia and arthritis. 90 In addition, a study from the USA151 found that only 0.58% of a cohort of patients with acute ischaemic stroke (AIS) and dementia had received thrombolysis compared with a previous study which reported that 1.28% of all ischaemic stroke patients in the elderly population received thrombolysis. 163
| Study | Country | Comorbidity | Study type | n participants | Aspect of quality of care | Evidence that care is different | Reported differences in care/treatment |
|---|---|---|---|---|---|---|---|
| Connolly et al., 2013161 | UK | Diabetes, stroke | Cross-sectional | 700 PLWD (compared with people without dementia on QOF register) | Monitoring and treatment | Yes | PLWD scored significantly lower on 73% of the QOF indicators, including peripheral pulses checks, neuropathy testing and cholesterol measures for stroke |
| Curtis et al., 201298 | USA | VI (AMD) | Retrospective cohort | 284,380 | Treatment | Yes | PLWD were significantly less likely to receive anti-VEGF (RR 0.88, 95% CI 0.88 to 0.89) |
| Guijarro et al., 2010110 | Spain | VI, general | Cohort | 40,482 | Treatment | Yes | PLWD had some procedures less frequently than those without dementia, e.g. cataract surgery (p < 0.001), hernia repair, orthopaedic surgery |
| Keenan et al., 2014117 | UK | VI (AMD) | Cohort | 65,894 AMD cohort, 168,092 dementia cohort | Treatment | Yes | PLWD showed a significant decrease in the likelihood of hospital admission for AMD (p < 0.001) |
| Löppönen et al., 2004118 | Finland | VI, general | Cross-sectional (survey) | 1260 older people (112 PLWD) | Diagnosis and treatment | Yes | PLWD had more undiagnosed diseases than those without dementia (p = 0.041) and were less likely to be diagnosed with glaucoma (p = 0.022) |
| Müther et al., 2010123 | Germany | Diabetes, hypertension | Retrospective matched control | 216 PLWD, 216 matched control subjects | Treatment | No | No significant differences in treatment for those with and without dementia. PLWD were more likely not to receive medication for hypertension (not significant) |
| Saposnik et al., 2011129 | Canada | Stroke | Cohort | 877 pre-existing dementia, 877 control subjects (no pre-existing dementia) | Treatment | Yes | Patients with pre-existing dementia were less likely to receive intravenous thrombolysis |
| Sloan et al., 2004155 | USA | AMI | Cross-sectional | 5851 AMI with dementia, 123,241 AMI no dementia | Treatment | Yes | PLWD were less likely to have a range of invasive procedures than those without a history of dementia |
| Thorpe et al., 2012136 | USA | Diabetes, VI | Cohort | 288,805 (44,717 PLWD) | Monitoring | Yes | PLWD were less likely to receive HbA1c tests (73% vs. 81%), LSC-C tests (61% vs. 79%) and eye examinations (52% vs. 63%) |
| Vitry et al., 2010139 | Australia | Diabetes | Cohort | 20,134 veterans with diabetes (includes people with dementia/cognitive impairment but numbers not clear) | Treatment | Yes | Presence of dementia was associated with a decreased likelihood of treatment intensification (e.g. addition of antidiabetic medicine or switch to insulin/different medication) |
| Zhang et al., 2010145 | Australia | Diabetes, VI | Cohort | 17,095 veterans with and without diabetes (4.4% on dementia medication) | Treatment, access to services | Yes | Patients receiving medications prescribed for dementia were less likely to use diabetic and optometry/ophthalmology services |
Outcomes
A number of studies looked at the impact of health-care treatments on health-related outcomes for older people with dementia or cognitive impairment. One systematic review149 evaluated the evidence on the treatment of hypertension in older people with dementia. This review included six RCTs and concluded that, although there was evidence to suggest that antihypertensives are effective in lowering blood pressure in people with mild to moderate dementia, there was no consistent evidence of a benefit in terms of cognitive outcomes. Moreover, most trials excluded participants with substantial physical or mental health problems, which makes it difficult to generalise findings to those with more advanced dementia or multimorbidity.
Thrombolytic therapy for stroke
Although age is a known risk factor for stroke, most trials of intravenous tissue plasminogen activator (IV tPA) have excluded or under-represented patients aged > 80 years154 and the impacts of thrombolysis in people with dementia are unclear. We found three observational studies150,151,154 that had looked at outcomes in patients with dementia or cognitive impairment following thrombolysis for AIS. In one154 the odds of death were increased in patients with pre-stroke dementia, which suggests that pre-stroke dementia is an independent predictor of in-hospital mortality after acute reperfusion therapy for stroke. However, this was a small retrospective analysis of 153 patients of whom only 21 had pre-stroke dementia. A prospective observational study in French and Japanese patients treated with IV tPA for cerebral ischaemia150 found that patients with pre-stroke cognitive impairment had more symptomatic intracerebral haemorrhage and were less frequently independent 3 months after stroke than those without pre-stroke cognitive impairment, but they did not differ for any of the outcome measures after adjustment for age, baseline National Institutes of Health Stroke Scale score and onset to needle time. The authors concluded that, in patients with pre-stroke cognitive impairment presenting with AIS, IV tPA improves outcomes. A cohort study in the USA151 identified admissions for AIS from a national database. Of 35,557 patients with AIS and a diagnosis of dementia, only 207 (0.58%) had received thrombolysis. The authors found that thrombolysis was associated with increased mortality and intracerebral haemorrhage in both those with and those without dementia and that the risks in both groups were similar. They concluded that the administration of thrombolysis for AIS in patients with dementia was not associated with an increased risk of intracerebral haemorrhage or death compared with their counterparts without dementia.
One survey of Canadian neurologists attempted to better understand the decision-making process surrounding the administration of IV tPA. 152 They found that 79% of respondents were less likely to administer IV tPA to patients with dementia and many were less likely to treat patients from nursing homes, those with more severe stroke or those aged > 80 years. Post-hoc subgroup analyses suggested that more experienced physicians – those in practice for > 10 years – were more likely to administer IV tPA to patients with dementia. However, this study did not explore why neurologists might be less likely to treat people with dementia.
Views and experiences
We included 13 studies looking at views and experiences of PIWD, their family carers and HCPs. Seven72,92,102,125,132,148,162 were qualitative studies, one107 was a mixed study including a review and a qualitative study, three93,99,153 were reviews and two20,94 were questionnaire studies. Three studies20,94,102 focused on people with dementia and diabetes, two72,93 focused on those with dementia and VI, one89 looked at those with dementia and deafness, one92 focused on those with dementia and cancer, two148,153 looked at family carers’ views on medication management and four99,107,125,132 looked at the needs of people with dementia in general hospitals.
Literature on the experiences of older people with dementia in acute general hospitals has highlighted shortcomings in the care provided, the attitudes and training of staff, and the physical environment and problems with care cultures. 99,107,125 Poor communication is a major barrier to the provision of good care for people with dementia and a comorbid health condition. 72,99,107 Practitioners reported that they found it difficult to communicate with PLWD and communication difficulties were compounded when PLWD had additional comorbidities that made communication difficult, such as hearing loss or sight loss. 72,89,92 There were also problems with communication between different professionals and specialist teams, with a lack of co-ordinated working between practitioners in different specialities. 72 A lack of appropriate knowledge and training was also a major barrier, with those working in acute care hospitals,107 palliative care92 and diabetes104 lacking knowledge about dementia. Conversely, those in dementia services may lack awareness about how to support people with dementia and VI. 72,158
A qualitative study involving the views of 21 caregivers of people with dementia and type 2 diabetes found that behavioural and psychological symptoms of dementia disrupted diabetes care. 102 In addition, carers felt that they received inadequate support in planning their relatives’ care, that their contribution to managing their relatives’ care was not always recognised and that they were often excluded from decision-making. A systematic review153 investigating the role of informal caregivers of PLWD found that family carers are often expected to manage medication regimens, the complexity of which are increasing. However, health-care systems and structures were often not helpful, for example family caregivers might not be included in communications about medication regimen changes and were not given the information and support that they needed to carry out their medication management roles. A focus group with carers of PLWD148 found that carers felt unsupported by health and social care teams in terms of dealing with the practicalities of medication administration. There were also issues around the difficulties of recognising when a PLWD was no longer able to safely administer medication and ambiguity about where responsibility should lie for monitoring their use of medication.
Service organisation and delivery
Guidelines and care pathways
Current pathways for diabetes and other long-term conditions tend to focus on models of self-management. However, there is evidence that having dementia or cognitive impairment impacts on a person’s ability to undertake self-care management. For example, people with dementia and diabetes had problems understanding their condition, managing medication and monitoring their blood glucose. 20,21,94,102,103 A review published in 201388 identified 16 guidelines/position statements or standards (from the UK, USA and Australia) for the care of PLWD but these generally focused on standards for providing optimal care for older people with cognitive impairment in acute hospitals or on specific issues such as hydration, nutrition or wandering. They did not cover issues relating to the care of people with dementia and specific medical conditions (such as stroke). Moreover, most models of practice for work with PLWD do not mention VI. 158
Guidance on the care of older people with diabetes112 highlights the need to balance the benefits of diabetes treatment while minimising the risks in people with dementia, as this group may be at increased risk of hypoglycaemia. 112,131 A National Expert Working Group147 has recently published a best clinical practice statement relating to the management of diabetes in people with dementia. It has outlined a number of key principles of care for people with both diabetes and dementia. This includes regular reassessment to identify additional care needs, consideration of problems of adherence to therapy, use of ‘safer’ glucose-lowering medications and the recognition of when ‘severe’ dementia supervenes and priorities of management may need revision. Reduction in risk of hypoglycaemia should revolve around assessment of all risk factors for hypoglycaemia, identification of whether ‘hypo’ awareness exists, provision of tailored education, use of lower-risk glucose-lowering medications and adequate carer support. The statement suggests that diabetes specialists require training to ensure that they have the necessary competencies to identify and manage people with dementia and diabetes in a safe and supportive fashion.
Currently, there is little guidance for VI specialists when caring for PLWD, but the College of Optometrists96 states that when examining patients with dementia optometrists have a duty to carry out whatever tests are necessary to determine their need for vision care. To date, most guidelines are condition specific and generally fail to take into account multimorbidity or the needs of people with dementia. 4,20,103,134,139,158
Models of care for older people with cognitive impairment
A number of initiatives have been developed to improve the care of older people with dementia in acute hospitals, including liaison psychiatric services113,135 or specialist units that combine medical and mental health care for older people. 106,109 Much of the work in this area is descriptive,106 although a specialist medical and mental health unit for PLWD has recently been evaluated in a RCT in the UK. 109 This study randomised 600 patients aged > 65 years who were identified as confused on admission to either the specialist unit or standard care on a general medical ward. The authors found no difference in the primary outcome (days spent at home) but there were significant differences in process items in favour of the intervention and family carers were more satisfied. However, the study excluded patients with a clinical need for specialist services such as surgery or a stroke unit.
A scoping review of interventions for cognitively impaired older people in emergency departments found no evaluations of organisational or system-level interventions and little evidence of appropriate interventions. 124 We found no evaluations of interventions aimed specifically at our three target comorbidities, although a retrospective analysis of stroke patients suggested that cognitively impaired patients benefit from admission to an acute rehabilitation stroke unit. 126
Conclusions
The prevalence of comorbid conditions in PLWD is high. Although current evidence suggests that PLWD may have poorer access to services for conditions such as stroke, diabetes and VI, the reasons for this are not clear. There is a need for more research looking at the ways in which having dementia impacts on clinical care for other conditions, how processes of care and different services can adapt to the needs of people with dementia and comorbidity and what interventions might improve access to services and the physical health of PLWD. Clinical guidance should consider the particular needs of those with dementia and comorbid health conditions, particularly when a concomitant diagnosis of dementia is common. In addition, there is evidence that, although family carers are frequently expected to take on responsibility for managing medication and other health-related tasks, they often feel poorly supported by HCPs.
Chapter 5 Results from the Cognitive Functioning and Ageing Studies analysis
Chapter overview
The first half of this chapter concerns analysis using data from CFAS II to examine the prevalence of each of the target health conditions (stroke, diabetes and VI) with and without dementia and service use across groups with dementia and any of the health conditions. The sample was restricted to those living in the community as some services would be provided by care homes for those living in care settings.
The results shown in the second half of the chapter are for the comparison analysis between CFAS I and CFAS II. This allows inferences concerning changes in patterns of service use between the turn of the century and a decade later. The analysis was restricted to the three CFAS II centres and, for the same reason as above, those living in the community. Questions on service use were introduced in the 10-year follow-up wave in CFAS I, which meant that all participants were aged ≥ 75 years and therefore CFAS II was also restricted to individuals aged ≥ 75 years.
Cognitive Functioning and Ageing Studies II analysis
Box 1 shows the key messages of this analysis.
-
Comorbid stroke, diabetes and VI are common in people with dementia.
-
Inpatient hospital services were used more by those with dementia and a target health condition.
-
A chiropodist was used more by those with dementia and a target health condition.
-
A home care assistant, day centre and care worker were used more by those with dementia and stroke.
-
Any nursing service was used more by those with dementia and either diabetes or VI.
-
Unpaid care was used more by those with dementia and a target health condition.
-
A home care assistant, day centre and care worker were all used more by those with dementia and a target health condition.
Prevalence
The prevalence of each of the target conditions in people with and without dementia in the CFAS II sample (excluding those living in residential care) is shown in Table 5. For people with dementia, approximately one in six had all of the target health conditions and over one-third had at least one of the target health conditions. This compares to just under one-third for those without dementia. As expected, because of its strong causal link, the prevalence of stroke in patients with dementia was approximately 2.5 times greater than the prevalence in those without dementia. The prevalence rates for diabetes and VI were broadly similar between the groups.
| Health condition | Dementiaa | No dementia | ||||
|---|---|---|---|---|---|---|
| n | Weighted prevalence (%) | 95% CI (%) | n | Weighted prevalence (%) | 95% CI (%) | |
| Stroke | 58 | 17.8 | 13.6 to 23.0 | 536 | 7.7 | 7.1 to 8.4 |
| Diabetes | 53 | 16.5 | 12.5 to 21.6 | 999 | 14.0 | 13.2 to 14.8 |
| VI | 51 | 16.6 | 12.5 to 21.7 | 970 | 14.2 | 13.4 to 15.1 |
| Target comorbidities | 126 | 36.2 | 30.8 to 42.0 | 2173 | 30.8 | 29.8 to 31.9 |
Service use
Overall service use by presence of a target health condition is shown in Figure 6 and across individual categories is shown in Figure 7. Additional details of relative differences in service use between the dementia alone group and the dementia with a target condition group are provided in Appendix 5.
FIGURE 6.
Percentage of grouped service use with 95% CIs by those with dementia only and those with dementia and a target comorbidity. (a) Dementia and no target comorbidity; (b) dementia and stroke; (c) dementia and diabetes; and (d) dementia and VI.
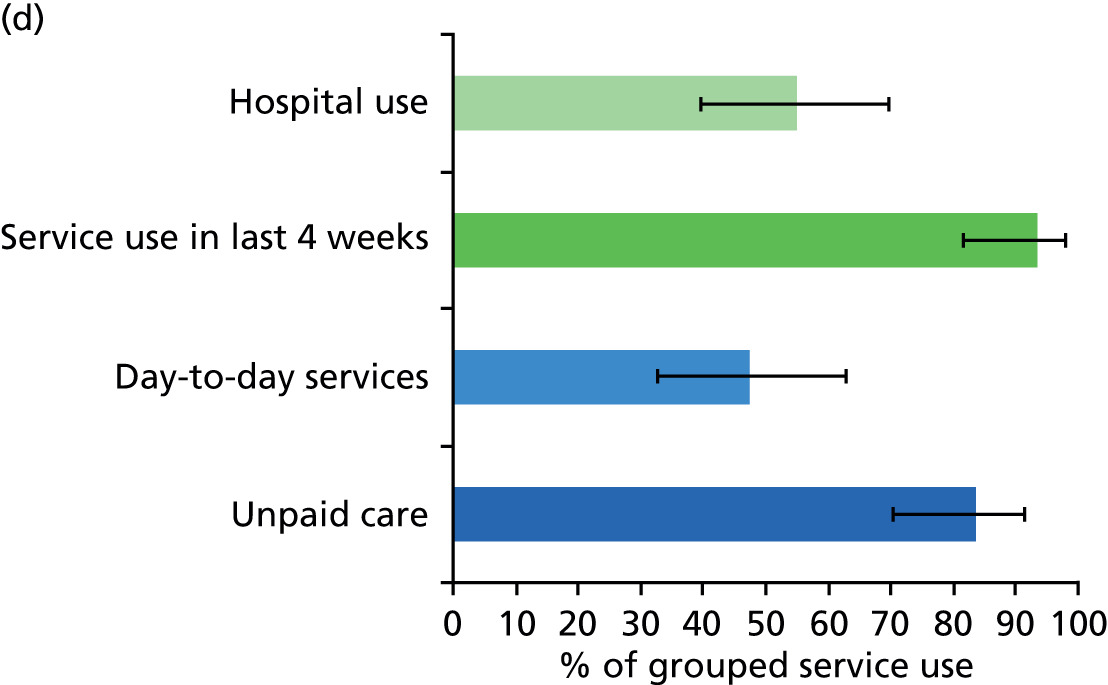
FIGURE 7.
Percentage of individual service use and 95% CIs by those with dementia only and those with dementia and a target comorbidity. (a) Dementia and no target comorbidity; (b) dementia and stroke; (c) dementia and diabetes; and (d) dementia and VI.
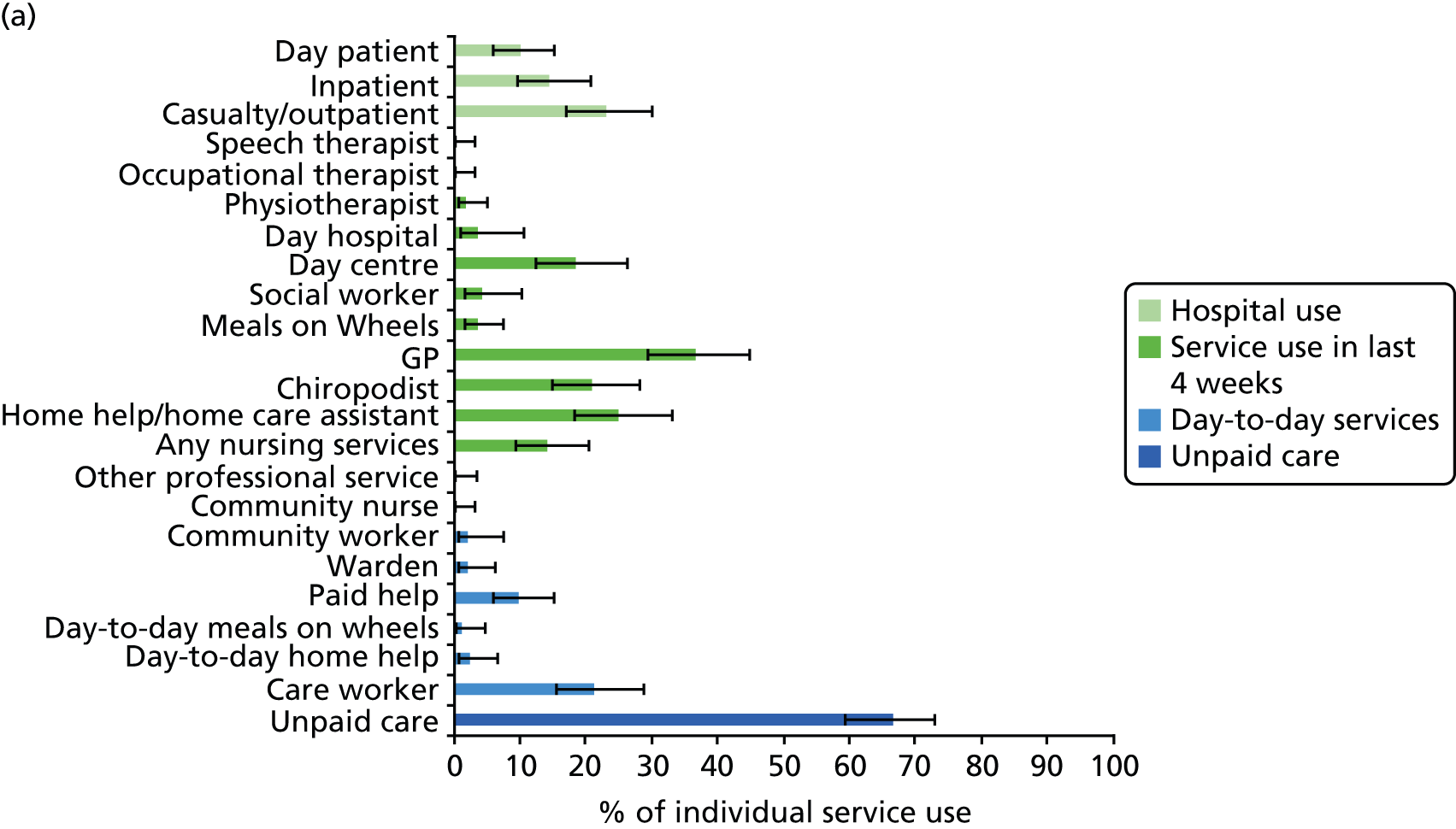
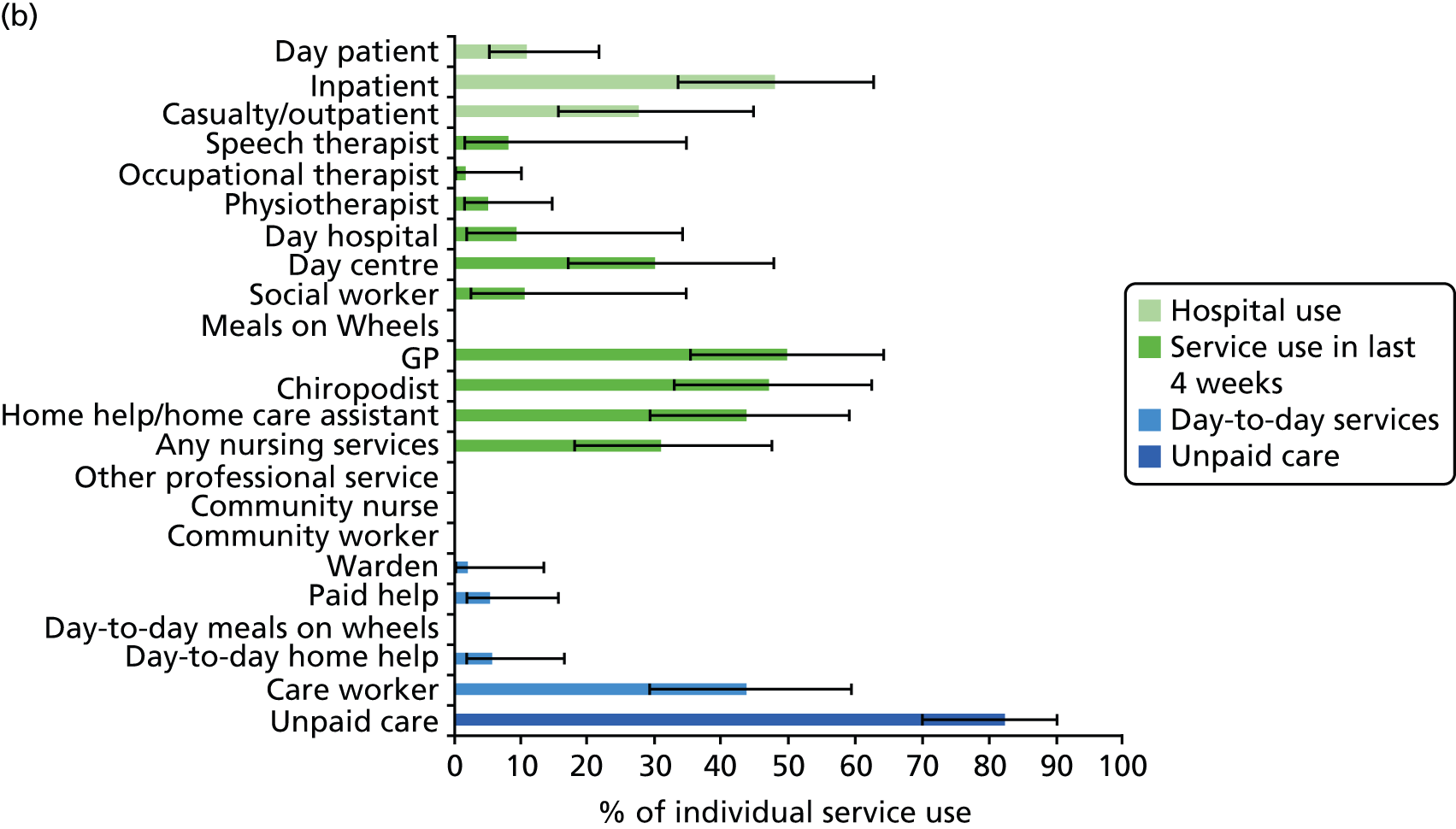
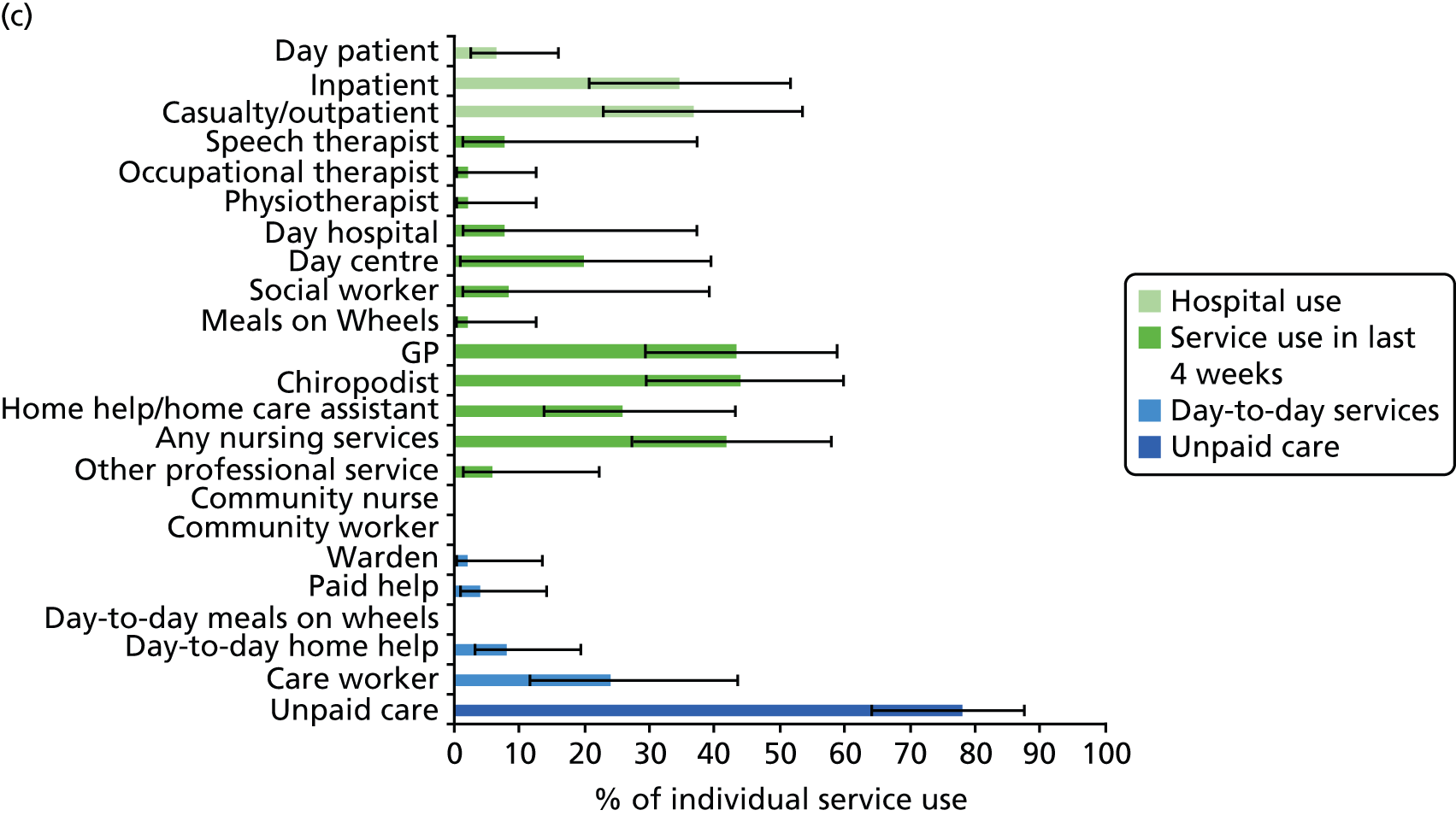
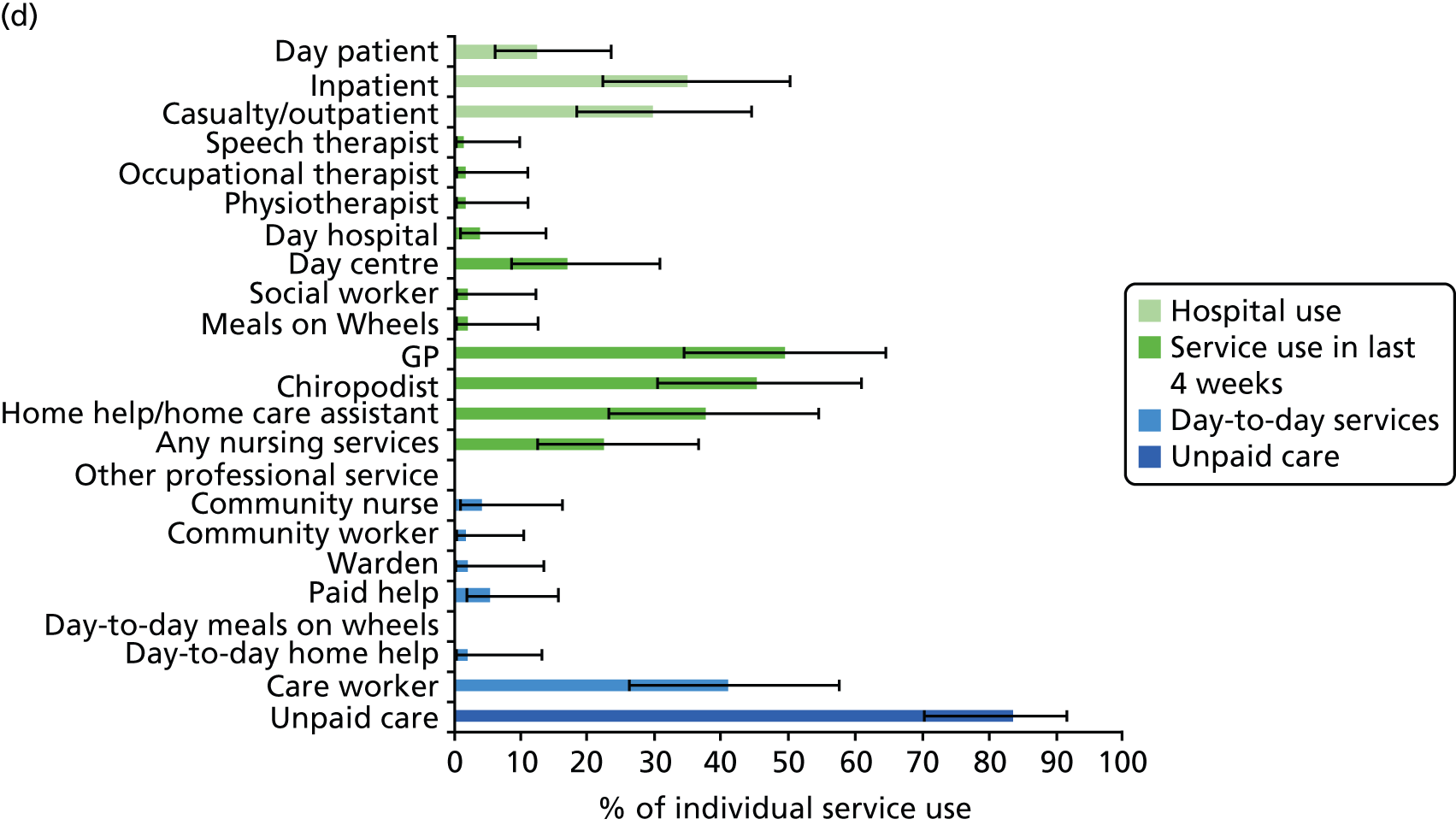
Dementia and comorbidity compared with dementia
Approximately one-third of individuals with dementia and no target condition used hospital services in the last 12 months. In comparison, between 45% and 58% of individuals with dementia and one of the target health conditions used hospital services, with the highest use seen in those with dementia and stroke (see Figure 6). This was mainly driven by the use of inpatient services, as day patient and outpatient service use was largely the same in both groups (see Figure 7). Those with dementia and stroke used inpatient services more than those with only dementia; this was also the case for those with dementia and either diabetes or VI, but the level of service use was not quite as high as it was for those with dementia and stroke (see Appendix 5).
As with hospital use, the use of services in the previous 4 weeks was higher for those with dementia and one of the target health conditions than for those with dementia alone (see Figure 6). For those with dementia but none of the target health conditions, six out of 10 people used some form of service in the last 4 weeks, whereas, for those with dementia and one of the target health conditions, around eight out of 10 people used some form of service in the last 4 weeks. Use of services was higher for those with dementia and stroke because of home care assistant, chiropodist and day centre use (see Appendix 5). The use of any nursing service and chiropodists was the reason for higher service use in the previous 4 weeks for those with dementia and diabetes and this was also the case for those with dementia and VI (see Appendix 5).
Around one-quarter of people with dementia and none of the target conditions used day-to-day services. This compared with more than one-third of those with dementia and diabetes and almost half of those with either dementia and stroke or dementia and VI (see Figure 6). Those with dementia and stroke had day-to-day help from a care worker a considerable amount more than those with dementia alone. Although this was also true for those with dementia and either diabetes or VI, it was not of the same magnitude (see Appendix 5).
Unpaid care was common, with over two-thirds of people with dementia alone reporting use of informal help from family and friends. Use of unpaid care was even higher for individuals with dementia and one of the target health conditions, with around eight out of 10 people reporting informal help (see Figure 7).
Dementia and comorbidity compared with comorbidity alone
When comparing those with dementia and a target health condition with those with just the health condition, hospital use mostly does not change (see Appendix 5). The only case in which there is a difference is for those with dementia and stroke, with use of inpatient services being higher than for those with stroke alone. The use of a home care assistant and a day centre in the previous 4 weeks was higher for those with dementia and the target health condition than for those with the target health condition alone. Of all of the day-to-day services, the use of a care worker was higher for those with dementia and a target health condition than for those with the target health condition alone. Unpaid care was also used more by those with dementia and any of the target health conditions than by those with the health condition alone. However, caution should be exercised when interpreting these results as the estimates have broad CIs because of instability introduced by having a small reference group (the target health condition).
Cognitive Functioning and Ageing Studies I and II comparison analysis
Prevalence
Box 2 shows the key messages relating to prevalence.
Between CFAS I baseline and CFAS II baseline:
-
the prevalence of diabetes more than doubled
-
the prevalence of dementia reduced
-
the prevalence of stroke and VI remained the same.
The CFAS I analysis was conducted using the 10-year follow-up wave only as the questions on service use were introduced only at this point. Even with the adjustments of the analysis using weights there was still some uncertainty in the estimates because of the low number of people who had the target comorbidities. This also meant that it was not possible to formally test the difference in service use between CFAS I and CFAS II.
The overall prevalence of the health conditions considered is shown in Table 6 for those aged ≥ 75 years. Two time points are given for CFAS I: the baseline assessment, which is more directly comparable to the CFAS II baseline assessment, and the 10-year follow-up wave in which service use was measured. Consistent with the main findings from CFAS II,164 the prevalence of dementia reduced slightly between the CFAS I baseline and the CFAS II baseline assessments. Over the same period the prevalence of stroke and VI remained similar. In contrast, the prevalence of diabetes more than doubled between CFAS I baseline and CFAS II baseline, with CIs that did not overlap. Comparing the CFAS I 10-year follow-up estimates with the CFAS I baseline estimates, the prevalence of dementia and VI decreased. This is likely to be the result of a survivor effect: those with these conditions may have been less likely to survive to the 10-year assessment.
| Health condition | CFAS I baseline (1991–3) | CFAS I 10-year follow-up wave (2001–3) | CFAS II baseline (2008–11) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Weighted prevalence (%) | 95% CI (%) | n | Weighted prevalence (%) | 95% CI (%) | n | Weighted prevalence (%) | 95% CI (%) | |
| Dementia | 165 | 9.9 | 7.7 to 12.7 | 83 | 6.4 | 3.9 to 10.3 | 277 | 8.5 | 7.5 to 9.6 |
| Stroke | 333 | 9.4 | 8.5 to 10.4 | 160 | 9.7 | 7.6 to 12.4 | 377 | 10.4 | 9.4 to 11.4 |
| Diabetes | 240 | 6.8 | 6.0 to 7.6 | 152 | 8.1 | 6.3 to 10.4 | 540 | 14.5 | 13.3 to 15.7 |
| VI | 662 | 18.9 | 17.7 to 20.3 | 178 | 12.5 | 9.3 to 16.5 | 602 | 17.3 | 16.0 to 18.6 |
| Target comorbidities | 1059 | 30.1 | 28.6 to 31.7 | 416 | 26.3 | 22.3 to 30.6 | 1294 | 34.5 | 33.0 to 36.1 |
Table 7 gives the prevalence of each of the target health conditions for individuals with and without dementia separately. The likelihood of having one of the health conditions was generally higher for people with dementia across all three time points. The difference in prevalence between those with and those without dementia was smaller in CFAS II and, for VI, there was no difference in prevalence between those with and those without dementia. The prevalence of having at least one of the target conditions was higher in those with dementia than in those without dementia at CFAS I baseline; however, at baseline in CFAS II the prevalence of having at least one target condition was the same in those with and those without dementia.
| Health condition | Dementia | No dementia | ||||
|---|---|---|---|---|---|---|
| n | Weighted prevalence (%) | 95% CI (%) | n | Weighted prevalence (%) | 95% CI (%) | |
| CFAS I baseline | ||||||
| Stroke | 38 | 22.8 | 14.2 to 34.6 | 61 | 9.4 | 6.6 to 13.3 |
| Diabetes | 12 | 8.1 | 4.3 to 14.6 | 33 | 6.2 | 3.8 to 10.1 |
| VI | 46 | 24.3 | 17.2 to 33.3 | 126 | 20.9 | 16.3 to 26.4 |
| Target comorbidities | 77 | 44.5 | 33.2 to 56.4 | 186 | 31.1 | 25.7 to 37.2 |
| CFAS I 10-year follow-up wave | ||||||
| Stroke | 17 | 22.6 | 14.0 to 34.4 | 143 | 9.7 | 8.3 to 11.4 |
| Diabetes | 13 | 15.6 | 6.6 to 34.8 | 139 | 8.6 | 7.3 to 10.1 |
| VI | 19 | 21.7 | 13.5 to 32.9 | 159 | 11.4 | 9.7 to 13.2 |
| Target comorbidities | 41 | 47.6 | 36.5 to 58.8 | 375 | 25.4 | 23.2 to 27.7 |
| CFAS II baseline | ||||||
| Stroke | 47 | 17.9 | 13.3 to 23.8 | 330 | 9.7 | 8.8 to 10.8 |
| Diabetes | 42 | 16.2 | 11.8 to 21.9 | 498 | 14.3 | 13.2 to 15.6 |
| VI | 39 | 16.7 | 12.1 to 22.6 | 563 | 17.3 | 16.0 to 18.7 |
| Target comorbidities | 100 | 35.7 | 29.7 to 42.1 | 1194 | 35.1 | 33.5 to 36.8 |
Service use
Overall service use
Box 3 shows the key messages relating to overall service use.
-
Hospital service use has increased in the last decade for those with dementia alone and those with dementia and stroke.
-
Use of unpaid care has increased from CFAS I to CFAS II for those with dementia and either diabetes or VI.
Figure 8 gives the overall grouped service use for individuals with dementia and one of the target health conditions. Additional details of differences in service use between CFAS I and CFAS II comparing dementia alone with dementia plus a target condition are provided in Appendix 5. One of the limitations was that hospital use data were collected for a different time period from that for day patient or outpatient service use data. For example, for day patient and outpatient services participants were asked if they had used them in the previous few months but for inpatient services participants were asked if they had been admitted in the last year. However, it is clear that hospital use increased between CFAS I and CFAS II for those with dementia alone and for those with dementia and stroke.
FIGURE 8.
Percentage of grouped service use in CFAS I and CFAS II with 95% CIs by those with dementia alone and dementia with each of the target comorbidities. (a) Dementia and no target comorbidity; (b) dementia and stroke; (c) dementia and diabetes; and (d) dementia and VI.
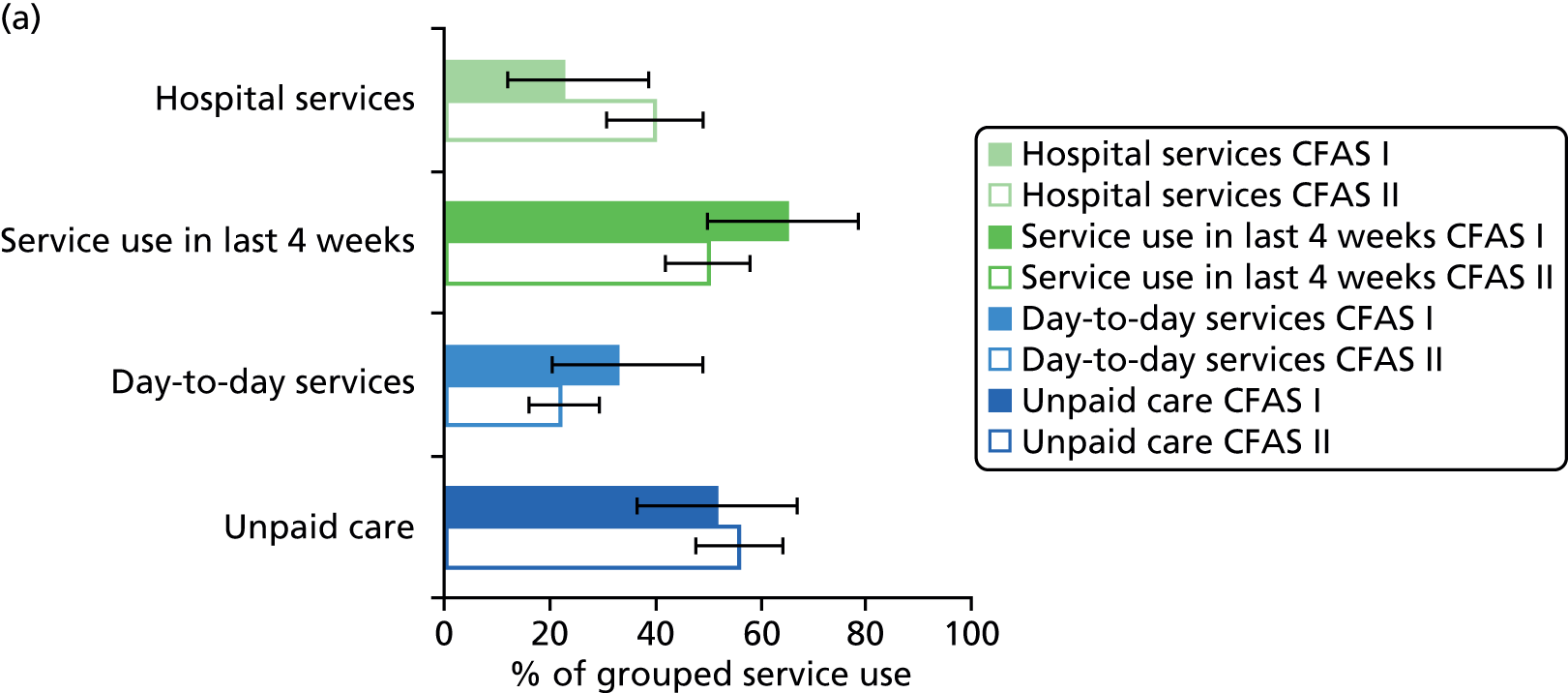


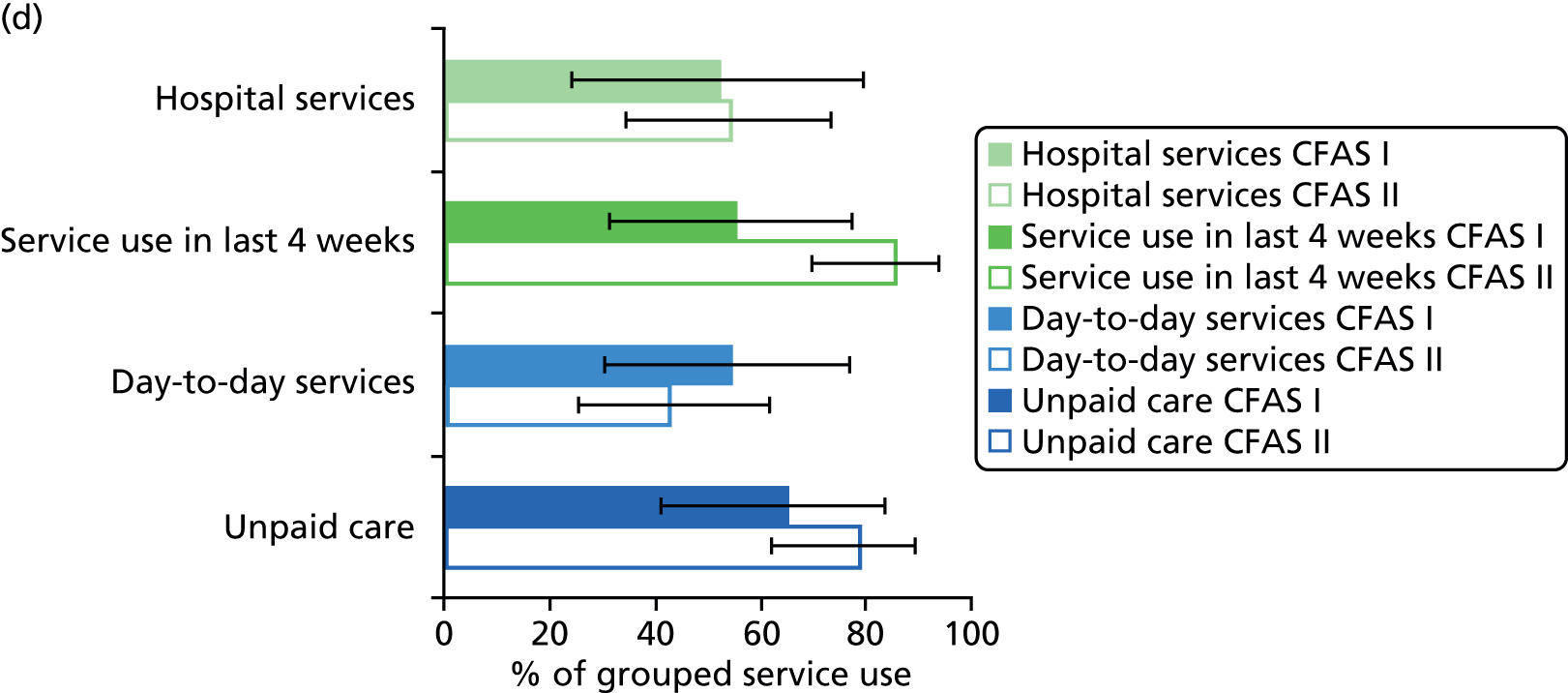
Although hospital service use increased for those with only dementia, the use of day-to-day services and service use in the 4 weeks before interview decreased slightly between CFAS I and CFAS II. Day-to-day service use decreased between CFAS I and CFAS II for all those with dementia and a target condition. Apart from day-to-day service use, those with dementia and stroke had similar service use in CFAS I and CFAS II. This was also the case for those with dementia and diabetes, although there was a slight increase in the use of unpaid care in CFAS II. More change was seen for those with dementia and VI: there was an increase in the use of unpaid care and services in the 4 weeks before interview between CFAS I and CFAS II as well as the decrease in day-to-day service use. Unfortunately, because of non-response this could not be tested formally.
Across all target conditions in CFAS I and CFAS II there was an increased use of unpaid care compared with those having dementia alone.
Separate service use
Box 4 shows the key messages relating to separate service use.
-
There was an increase in the use of unpaid care for those with dementia and either diabetes or VI.
-
Decreases in the use of day-to-day services mainly stem from decreases in the use of paid help and meals on wheels.
More detailed results on separate services are provided in Figure 9. For those with dementia but none of the target health conditions the overall increase in hospital use resulted from an increase in the use of all hospital services but mainly from an increase in day patient and outpatient admissions. The large increase in day patient service use was also present for those with dementia and stroke. Although hospital service use overall increased for people with dementia and stroke, reductions in inpatient and outpatient admissions were observed between CFAS I and II. These decreases were also observed in those with dementia and VI but not in those with dementia and diabetes.
FIGURE 9.
Percentage of individual service use in CFAS I and CFAS II with 95% CIs by those with dementia alone and dementia with each of the target comorbidities. (a) Dementia and no target comorbidity; (b) dementia and stroke; (c) dementia and diabetes; and (d) dementia and VI.
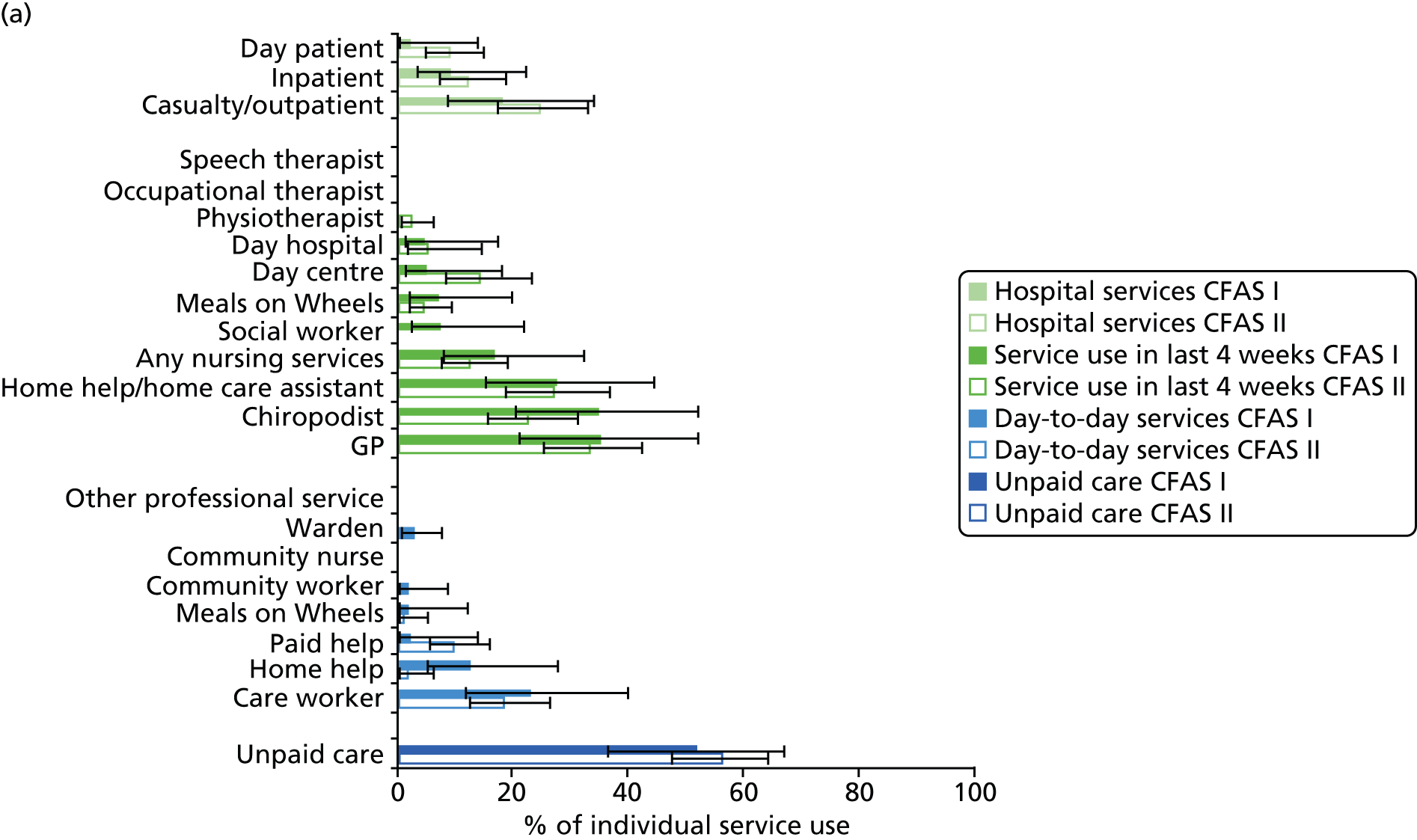

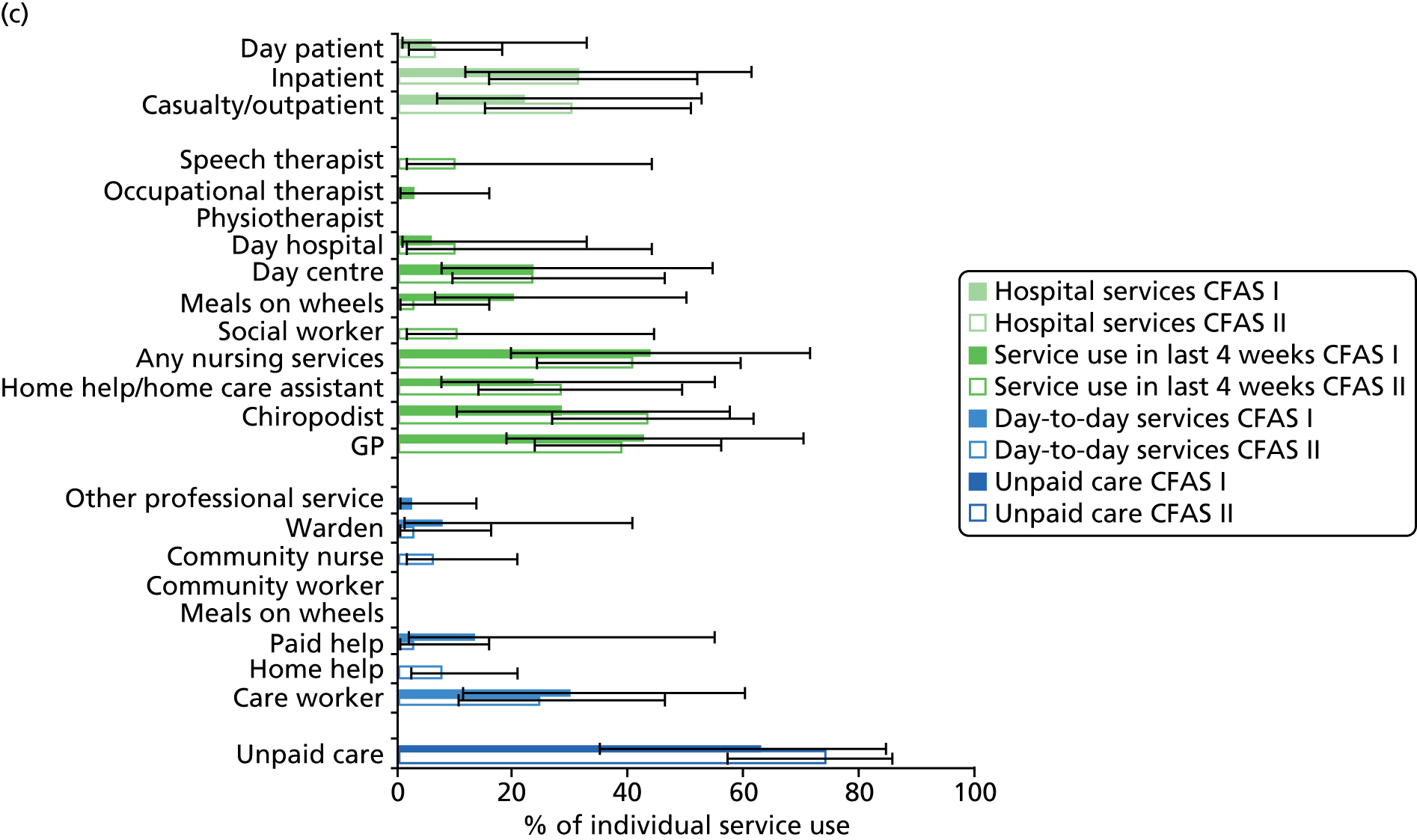
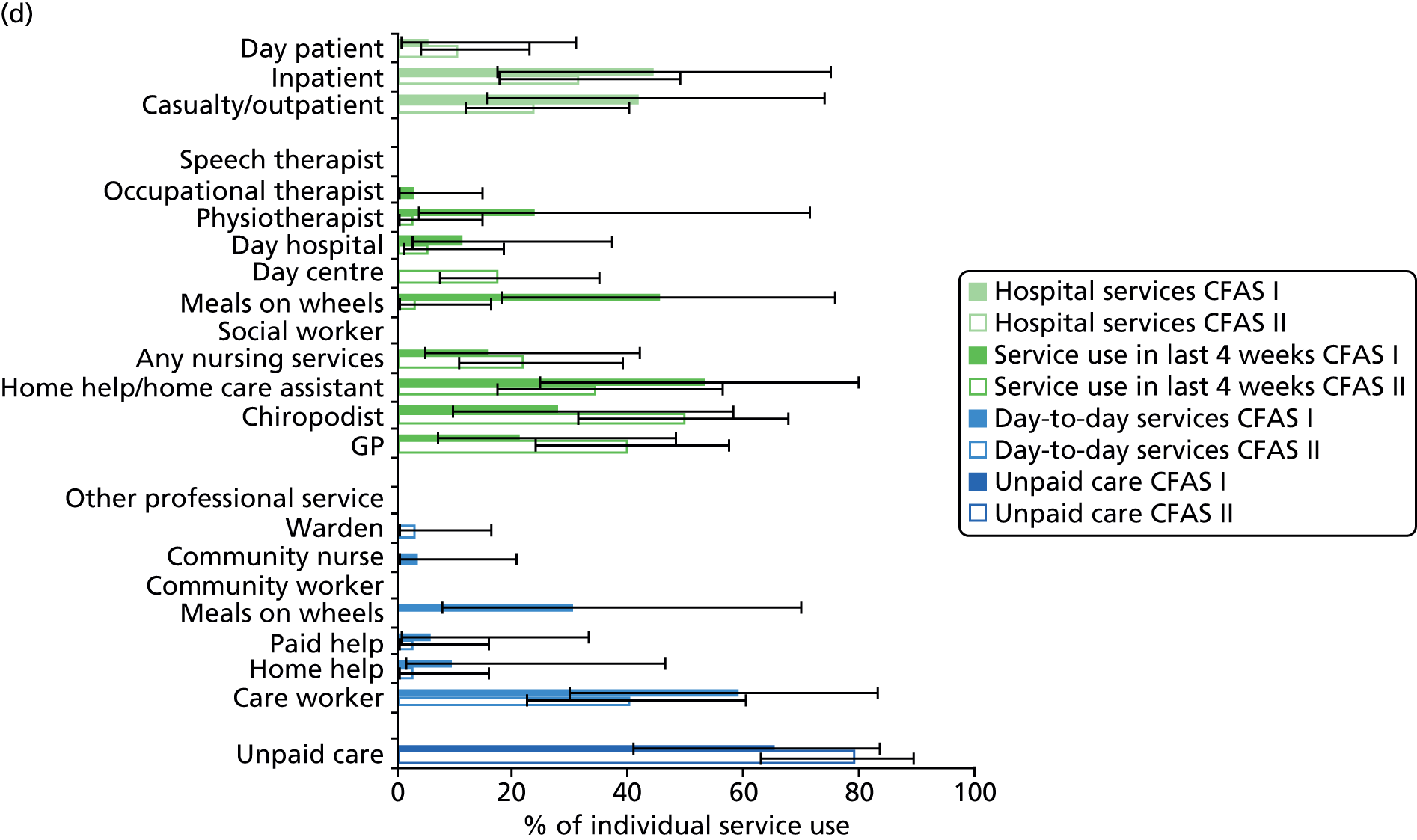
For people with dementia and without any of the target conditions, decreases in service use in the 4 weeks before interview were mainly driven by decreases in chiropodist and social worker use. Increases in day centre use were mirrored in people with dementia and either stroke or VI and there was also an increase in the use of nursing services by those with dementia and stroke. For people with dementia and VI, the overall increase in service use in the 4 weeks before interview was mainly driven by an increase in chiropodist use and GP visits, as well as greater use of day centres. A reduction in the use of meals on wheels was observed across all groups.
Differences in the proportion of people using care workers, paid help or home help on a day-to-day basis accounted for the majority of the change in overall use of day-to-day services across groups. For people with dementia and no target condition, reductions in the use of daily care workers and home help accounted for the majority of the reduction in day-to-day services. Decreases in the use of meals on wheels and care workers accounted for the overall decrease in use of day-to-day services seen for people with dementia and either stroke or VI.
Separate services within each condition
Although overall hospital service use remained similar in CFAS I and CFAS II for those with dementia and stroke, there was a decrease in inpatient and outpatient admissions, which was balanced out by an increase in day patient admissions. Between CFAS I and CFAS II those with dementia and stroke used more speech therapists, occupational therapists and social workers, whereas use was low in CFAS I. For this group, there was also an increase in the use of day hospitals and day centres, GPs, chiropodists and nursing services. There was a decrease in the use of physiotherapists, meals on wheels and home help by those with dementia and stroke during this time. The use of day-to-day services decreased for those with dementia and stroke. This was mainly because of a reduction in the use of care workers, home help, paid help and meals on wheels. The use of unpaid care remained similar between CFAS I and CFAS II.
For those with dementia and diabetes there was only a slight increase in the use of outpatient services between CFAS I and CFAS II. Overall, the use of services in the 4 weeks before interview was similar between CFAS I and CFAS II but there were increases in the use of speech therapists, social workers and chiropodists, which were mainly offset by the decrease in the use of meals on wheels. The decrease in day-to-day service use seen in those with dementia and diabetes was the result of a decrease in the use of care workers, wardens and paid help, although increases were seen in the use of community nurses and home help. The use of unpaid care increased substantially between CFAS I and CFAS II for those with dementia and diabetes.
All service use apart from day-to-day service use increased between CFAS I and CFAS II for individuals with dementia and VI. For hospital service use this resulted from an increase in day patient admissions although decreases were also seen in inpatient and outpatient admissions. There was a decrease in the use of physiotherapists, meals on wheels and home help in the 4 weeks before the interview between CFAS I and CFAS II but large increases in visits to chiropodists, GPs and day centres. For day-to-day service use, all service use decreased between CFAS I and CFAS II apart from use of wardens and community nurses. The use of unpaid care increased between CFAS I and CFAS II.
Conclusions
Cognitive Functioning and Ageing Studies II analysis
The CFAS II-only analysis aimed to determine differences in service use between those with dementia and a target health condition and those with either dementia alone or the target health condition alone. The use of unpaid care from family or friends was considerably greater for those with dementia and any one of the target health conditions than for those having the health condition alone. Home care assistants, day centres and care workers were also all used more by those with dementia and a target health condition than by those with the health condition alone. Compared with those with dementia alone, those with dementia and a target health condition visited inpatient hospital services more.
Cognitive Functioning and Ageing Studies I and II comparison analysis
A comparison of service use between CFAS I and CFAS II indicates that use of some services has changed substantially. Over the past decade there have been large increases in the use of unpaid care by some of those with dementia and a target comorbidity whereas a decrease has been seen in the use of day-to-day services, mainly stemming from decreases in the use of paid help and Meals on Wheels.
Chapter 6 Findings from the interviews and focus groups
Characteristics of participants
We interviewed 28 PLWD and comorbidity and 33 family carers. Six PLWD and six carers were interviewed individually (n = 12), two interviews were carried out with pairs of family carers (sons/daughters of patient) (n = 4) and one interview was carried out with a PLWD and two carers (n = 3), but the majority of interviews (n = 21) were with patient–carer dyads (n = 42). We conducted five focus groups (n = 29) and carried out telephone and face-to-face interviews with a further 27 HCPs from a range of different specialities working in primary and secondary care. Further details of the participants are provided in the following sections.
People living with dementia and comorbidity and family carers
People with dementia and their family carers were recruited to the study through GP practices, dementia registers, memory clinics and voluntary organisations (Alzheimer’s Society, Thomas Pocklington Trust, Stroke Association) in the north-east and south-east of England. The numbers of completed interviews by region and comorbidity are provided in Table 8. The numbers of PLWD and carers by comorbidity and region are presented in Table 9.
| Region | Diabetes | Diabetes/VI | Stroke | VI | Diabetes/stroke/VI | Total |
|---|---|---|---|---|---|---|
| South-east | 7 | 5 | 7 | 7 | 1 | 27 |
| North-east | 5 | 1 | 2 | 1 | 0 | 9 |
| Total | 12 | 6 | 9 | 8 | 1 | 36 |
| Region | Diabetes | Diabetes/VI | Stroke | VI | Diabetes/stroke/VI | Total number of participants | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLWD | Carer | PLWD | Carer | PLWD | Carer | PLWD | Carer | PLWD | Carer | PLWD | Carer | |
| South-east | 4 | 8 | 4 | 5 | 5 | 5 | 5 | 6 | 1 | 1 | 19 | 25 |
| North-east | 5 | 3 | 1 | 1 | 2 | 3 | 1 | 1 | 0 | 0 | 9 | 8 |
| Total number of interviews | 12 | 6 | 9 | 8 | 1 | 61 | ||||||
In total, 64% of the PLWD were male and 72% were white. The age range of the PLWD was 59–94 years (median 82.5 years). The most common form of dementia was Alzheimer’s disease (56%), followed by mixed dementia (19%) and vascular dementia (17%). A total of 8% of participants had Parkinson’s disease with dementia. The majority of PLWD lived with a carer (78%), either a spouse (64%) or an adult child (14%). Carers were aged between 46 and 90 years (median 65 years). The types of comorbidity present are provided in Figure 10, which shows that > 50% of patients had diabetes, 45% had VI and 28% had had a stroke.
FIGURE 10.
Types of comorbidity.
Health-care professionals
We recruited 56 HCPs, comprising 10 GPs, 18 nurses (specialist and general), 12 consultants/senior clinicians specialising in stroke, diabetes and VI, eight therapists, two managers, one old-age psychiatrist, one health-care assistant and four vision specialists (two orthoptists, one optometrist, one intravitreal co-ordinator). Table 10 provides details of the numbers of interviews by region and specialty and Table 11 provides details of the focus groups by region and specialty.
| Region | Primary care | Diabetes | Stroke | VI | Dementia | Total number of interviews |
|---|---|---|---|---|---|---|
| South-east/London | 5 GPsa | 2 nurses, 1 GP – diabetes lead for CCG | 1 physiotherapist, 1 manager CCG, 1 manager social services | 1 consultant, 1 clinician/researcher | 1 consultant | 14 |
| North-east | 4 GPs,b 1 practice nurse | 2 nurses, 1 consultant | 2 nurses, 2 consultants | 0 | 1 CPN (specialising in dementia) | 12 (13 HCPs) |
| Total | 10 | 6 | 7 | 2 | 2 | 26 (27 HCPs) |
| Region | Specialism | Setting | Roles of HCPs | Number of HCPs |
|---|---|---|---|---|
| Midlands | Stroke | Secondary care | 3 stroke consultants, 1 rehabilitation lead | 4 |
| South-east | Stroke | Community | 5 specialist neurological physiotherapists, 1 occupational therapist | 6 |
| London | Diabetes | Community | 4 specialist diabetes nurse consultants | 4 |
| South-east | Diabetes | Secondary care | 3 consultants, 5 diabetes specialist nurses | 8 |
| East of England | VI | Secondary care | 1 consultant ophthalmologist, 2 orthoptists, 1 specialist optometrist, 1 staff nurse ophthalmology, 1 senior health-care assistant specialising in VI, 1 intravitreal co-ordinator | 7 |
| Total | 29 |
Results of the thematic analysis
Qualitative data were analysed using a framework informed by theories of continuity of care and access to care (see Chapter 2 for further details). Our two overarching themes were negotiating continuity of care and negotiating access to care. The themes and subthemes are summarised in Figure 11 and are detailed in the following sections; quotations are provided to illustrate the themes.
FIGURE 11.
Themes and subthemes. IC, informational continuity; MC, management continuity; RC, relationship continuity, SM, self management.
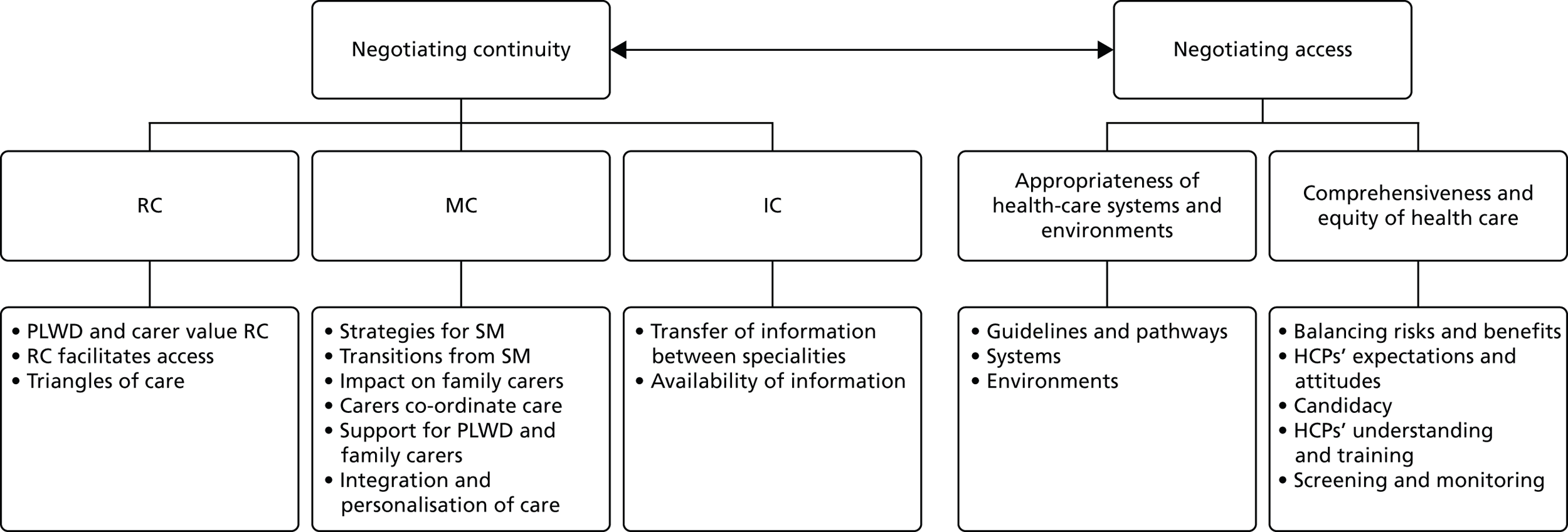
Theme 1: negotiating continuity of care
Relationship continuity
People living with dementia and their family carers value relationship continuity
A common thread in the interviews with PLWD and their carers was that they valued a continuous relationship with HCPs and health-care teams who knew their medical history and were aware of their health-care needs. Successful interactions with HCPs were usually associated with positive personality characteristics such as friendliness, warmth and being treated with consideration and respect. Good communication skills, taking time to explain things and including PLWD and their carers in decisions were valued. There were examples in which a rapport was developed with a HCP because he or she had taken the time to get to know the patient:
and he struck up a really warm relationship with this doctor and now he really likes going there and I expect they have the same conversation every time he goes.
Carer VI 3, south-east
Likewise, less successful interactions were often attributed to HCPs’ negative personality characteristics and poor communication skills. Examples given were that the HCP was stern, abrupt and rude and spoke with no eye contact or in a patronising manner:
he was quite rude to us and we weren’t happy, he sort of didn’t look up, he was looking down.
Carer diabetes/VI 4, south-east
For many of our participants their comorbid health condition predated the diagnosis of dementia. Despite this, participants gave very few examples of when a diagnosis of dementia was discussed in relation to its implications for and impact on their overall health and the management of any existing conditions. Moreover, patients sometimes felt that the diagnosis of dementia had been delivered in an insensitive way. This carer talks of being ‘catapulted into despair’:
. . . in fact a nurse, I think looking back she was very out of order to say this, I think she said ‘I think it’s small vessel disease’, and I said ‘how do you treat that?’, and she said ‘there’s no treatment’ . . . I mean this nurse really catapulted me into despair.
Carer stroke 7, south-east
Continuity with social carers was also very important for PLWD and their family carers. Previously well-documented problems associated with paid carers’ erratic schedules and multiple people visiting the same person posed particular challenges for PLWD. It threatened their ability to manage their medication (especially a problem for people with diabetes) and stay in their own home. One carer gave an example of how she had negotiated with an agency to limit the number of carers visiting to a maximum of three, a number that accommodated times of staff leave and sickness:
I said to them, ‘We cannot have different carers coming in, we cannot have different,’ so they’ve said they’ll keep it to three which is fair enough because you have sickness.
Carer diabetes 7, south-east
Relationship continuity facilitates access to care
Relationship continuity with both the clinician and the practice management or support staff meant that additional administrative practices could be applied to address the potential for missed appointments because of memory loss. When HCPs knew patients and were aware that they had dementia, they were more likely to remind them of upcoming appointments, give longer appointments and make allowances when they missed appointments. However, we found no examples of when a dementia diagnosis would automatically trigger the kind of practice described by this GP:
In fact when I know that I’ve got one of my patients with dementia booked in I will ask, I will send an email in advance to the administrator, to the receptionist to sort of call them on the day to remind them.
GP 2, London
In addition, relationship continuity could facilitate assessment and treatment, for example making it easier for VI specialists to conduct and interpret tests, although, as the following quote demonstrates, such approaches were reliant on individual clinician’s knowledge of the patient. How this knowledge of the person could be shared more widely was less clear.
I get some dementia patients come to the AMD clinic and usually with a carer of some description, and then we have the difficulty of assessing their vision because of them not being able to verbalise what they’re seeing but usually those patients I’ve got to know and I can tell if they’re making a reasonable assessment or whether they’ve just switched off and on the whole I can tell, you know, that they’re understanding and I can understand what they’re seeing.
Staff nurse, VI focus group, East of England
Many PLWD and their carers reported positive continuous relationships with their GP and recognised the role that GPs played in overseeing and co-ordinating their care and facilitating access to care. There were multiple examples of GPs helping to organise social care for PLWD, following up with memory clinics, contacting pharmacies for prescriptions, chasing referrals, contacting transport providers and providing PLWD with signposting and information. This would suggest that clinicians in regular contact with PLWD were aware of the compensatory and anticipatory care that these patients needed when visiting other services. However, this was discretionary, required individuals to be engaged and proactive and did not necessarily translate into systems of care.
Triangles of care
In many cases interactions involved a triad of the PLWD, the family carer and the HCP. HCPs in our study were aware of the role of family carers in co-ordinating and managing their relative’s health care. In the focus group with VI specialists participants said that they actively encouraged family carers to accompany the PLWD to the eye clinic as they could provide important information and help the PLWD feel at ease during eye tests:
Well really important, really important, and I think they need to be involved in sort of every step of the way.
Ophthalmologist, VI focus group, East of England (speaking about involving family carers)
Stroke consultants reported that they involved family carers in decisions about whether to thrombolyse a PLWD. HCPs’ awareness of the importance of involving family carers was reflected in some of the accounts given by family carers:
when I rang to make the appointment I said to them, ‘My dad’s got Alzheimer’s disease but he won’t acknowledge that, but he does need extra time and I’d like to come with him because he may need my support, emotional support but he may also need me to help interpret some of the questions that he’s being asked’, and they were great, they gave him a double appointment, so he’d got twice as long as he would normally have, and they were quite happy for me to go.
Carer VI 3, south-east
However, the interviews with PLWD and their carers provided a number of examples in which carers felt undervalued or excluded from decision-making about their relative’s care. For example, there were several accounts in which carers felt ignored by HCPs when they struggled to get treatment for their relative:
And in fact I can remember it took at least two, if not three phone calls to the practice nurse, because at first you came off the phone . . . That’s right, because we’d been telling them there’s a problem, put it in writing, we thought we’d got the message across didn’t we?
Carer diabetes 4, south-east
I did get a bit annoyed two years ago because all they do is we bring him in, they check the pressures at the back of his eye and then they say to him, ‘Can you read that card?’, they put a few drops in, we see someone and we come home, and that’s been happening for five years. Now this year I asked for a second opinion and he’s never seen the glaucoma people.
Carer diabetes/VI 4, south-east
In one account, a district nurse gave a patient with dementia and diabetes a new blood testing kit without giving the family carers information about how to use it:
Do you remember that mum, you know your method for testing your blood that you’d used for years, last Easter the nurse came on Maundy Thursday, the day before Easter and she gave you a new machine to do it . . . And you could not fathom it at all . . . No, no, none of us could, could we? It was chaos.
Carer diabetes 4, south-east
Sometimes PLWD would also feel excluded and ignored when HCPs communicated only with their family carer:
Yeah, but I mean really when I sit in on that with Dr [consultant], really I could almost be invisible at some times because he doesn’t concentrate on me at all, he’s always concentrating on you.
PLWD diabetes/VI 6, north-east
An example of good relationship continuity with a GP and how this can facilitate access to services can be seen in the vignette in Box 5. This describes a particular case from the data and illustrates the complex health needs of some people with dementia.
Mr A is a 62-year-old man with vascular dementia and multiple conditions, including all three of our target comorbidities. He believes that one condition has led to another, that is, an operation on his pancreas caused his diabetes, which in turn caused his eye condition and his stroke. He has had numerous surgical procedures and has a complex medication regime that he manages by having a strict routine. His dependency on his wife for managing his health care and his conditions has increased as his dementia has become worse.
The patient has a positive relationship with his GP, who sees him every month for blood tests and to review his conditions. The GP will find time to see him, even at 7 a.m. before surgery starts. His stroke was diagnosed after his wife noticed something was wrong and encouraged him to see his GP. His GP sent him for a computed tomography scan and he was then seen at the stroke clinic. The patient was pleased with the quick response and said the stroke consultants were very good at explaining things to him. As a result of his stroke he has difficulties speaking. He devises strategies to cope with this but says that the effects of the stroke have caused him to be depressed. The stroke has affected his sense of taste and his appetite and he is losing weight. This has implications for his diabetes. He was referred to a physiotherapist for rehabilitation but finds the exercises too difficult and demanding for him.
Management continuity
Strategies for self-management of comorbidities
Some PLWD were still able to self-manage their care, including booking their own appointments, attending appointments alone and remembering to take medication. PLWD developed a variety of strategies to facilitate self-management of conditions such as diabetes and glaucoma. This included taking tablets or administering eye drops at the same time each day or putting medication in a particular place once it had been taken. One man with dementia and glaucoma described how he kept his eye drops in one place on a chest of drawers and, once he had administered them, he would move the bottle to the other side of the drawers. This would indicate to him that he had used the eye drops even though he might not remember doing so:
His plan is that he has them on this side in the morning, and he puts them over there, and then in the evening he puts them back on that side.
Carer VI 1, south-east
Transitions from self-management to dependency
As their dementia got worse, the ability of PLWD to self-manage their condition declined and management moved from the PLWD to the carer. This transition was often a gradual process, with carers initially noticing small changes in their relative’s ability to manage that could be dealt with by adopting strategies to facilitate self-management, for example using memory aids, diaries and dosette boxes. However, over time, these strategies ceased to be effective:
gradually I took over the medication, each step was really painful, you know ‘cos he always used, he was on by the time when he started sort of losing grip on things he was on a lot of medication, six or eight different pills a day and he would line them up and take them one at a time and so on, and then I started putting them in dosette boxes and then he started not remembering to take them and then he would take them at random so gradually I took over the whole thing and I mean there were a lot of tears and agony.
Carer stroke 7, south-east
we had a timer at the beginning and it bleeped when he should take a tablet, well he would go and turn the bleeper off and forget to take the tablet . . .
Carer VI 6, south-east
In some instances people with diabetes had successfully managed their condition for many years but had began to forget to take their medication or to lose the ability to control their diet. Carers of people with dementia and diabetes reported how it became difficult to regulate blood sugar levels because their relative often forgot to eat and drink, ate too much or ate the wrong kind of foods. There were safety concerns for people in this period of transition, with accounts of people forgetting to take medication or taking too many tablets or too much insulin:
Another risk that was highlighted to me recently was a patient in this circumstance who was previously self-managing, district nurses had to take over, but the insulin has to stay in-house and the nurses don’t carry it around, so this patient was, it transpired this patient was given her own insulin and the district nurses were coming in after and administering again, it took a while to establish that.
Diabetes consultant, diabetes focus group, south-east
An example of the transition from self-management to dependency for a woman with diabetes and dementia is provided in Figure 12. As a result of her dementia, this patient gradually became unable to manage her diabetes. This resulted in a hypoglycaemic attack and hospitalisation, after which she deteriorated and could no longer live alone.
FIGURE 12.
Transition from self-management to dependency.
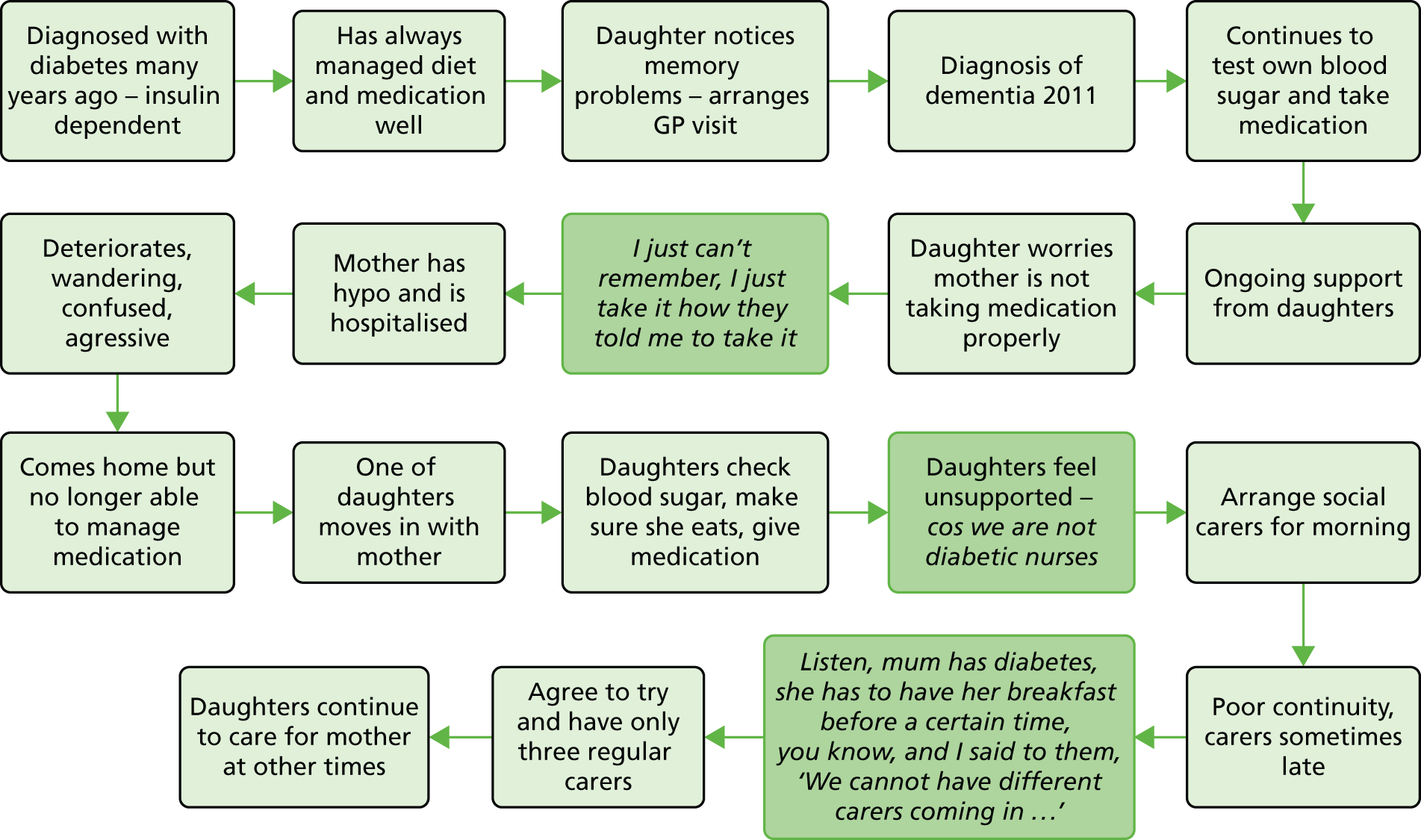
Impact of managing dementia and comorbidity on family carers
Many family carers, in particular spouses, had their own health problems, which made it difficult for them to help manage their relative’s conditions. Other carers, such as adult children, sometimes lived a distance away or had work and family commitments which meant that they found it difficult to take on responsibility for day-to-day care. Although many family carers have to care at a distance this is particularly problematic for carers of PLWD as it is difficult for them to offer support or to monitor adherence to medication over the telephone:
I know yesterday you had a bit of a problem because you thought, when I phoned you up in mid-morning you thought that the lady hadn’t been to give you your medications and your Cornflakes but in fact she had, hadn’t she?
She had, yeah.
So mum ended up having two breakfasts yesterday.
Carer and PLWD diabetes 4, south-east
For carers of people with dementia and diabetes, coping with the administration of insulin or testing blood sugar could be particularly challenging, because they felt that they lacked the necessary skills, because their relative became distressed or because they did not feel comfortable with the task:
And I’m waiting for someone to realise [laughs] ‘cos we’re not diabetic nurses.
Carer diabetes 7, south-east
‘now mam, you’ve got to do this’, I said ‘look I’m not a nurse’.
Carer diabetes 8, north-east
He doesn’t want it, he tries to refuse it and it is all horrible because it’s needles all the time and it hurts, you know, he doesn’t like it and I have to take bloods, and I say, ‘I’m sorry dad, you have to have it, your health will be worse, you’ll deteriorate even quicker’, he doesn’t care, he doesn’t want to be on anything.
Carer diabetes/VI 4, south-east
the minute you start asking them [referring to family carers] to do something technical or in their eyes complicated, such as pricking their finger, doing a blood sugar test or giving insulin, they kind of go, ‘No, I can’t do that’, and they’d be capable of doing it because we can teach them and support them and so on but there’s a barrier that they can’t get beyond where, no that’s beyond what I’m prepared to do, happy to do, comfortable to do.
Diabetes consultant, diabetes focus group, south-east
It was clear that there were emotional as well as practical and physical challenges for family carers as their role changed from spouse or child to carer:
Oh it was, she was like [laughs], . . ., you know, tantrums, you know, ‘I’m not doing that’, you know, flat refused [laughs] . . . Oh yeah, honestly, it’s quite funny, I mean sometimes you sort of think it definitely has been a role reversal, you know, you become the parent.
Carer stroke 4, south-east
Family carers co-ordinate care and navigate health-care systems
Family carers clearly play a significant role in co-ordinating their relative’s care and navigating health-care systems, for example managing multiple appointments, organising transport and keeping records of appointments, test results and medication:
And now I go with him for all his appointments . . . I have got a notebook there which I use to note everything, you know, when it started [sound of paper rustling] for myself, for my own, you know . . . I used to record everything, ‘seen by so and so, what prescribe and when to be seen again’ and all these things.
Carer diabetes/VI 2, south-east
Carers reported that they often had to chase up results or referrals and ‘battle’ to get appointments or access to treatments and tests. The following quote illustrates the frustration of a carer whose father’s electrocardiogram results had not been sent to the memory clinic:
I have to chase everything up in the surgery . . . the week before last for them to actually get the ECG [electrocardiogram] results because I had to go and pick them up and physically take them there myself.
Carer stroke 2, south-east
Although such experiences are not unique to the carers of PLWD, they may be more acute for them as evidence suggests that PLWD are a particularly vulnerable group who may have poorer access to services than people without dementia. They are also a group who generally lack the ability to co-ordinate their own care and are reliant on family carers to negotiate systems on their behalf. Moreover, HCPs’ concerns about confidentiality meant that carers sometimes had trouble accessing the information that they needed to manage their relative’s care, for example being unable to collect prescriptions or being refused copies of letters or details of hospital appointments. Some carers had proactively managed to add their contact details to health files so that they would receive copies of any letters sent to the PLWD, but this was not carried out routinely. Although a number of carers and PLWD mentioned lasting power of attorney, this was seen as facilitating management of financial affairs rather than health care.
Support for people living with dementia and family carers
Many HCPs recognised the importance of supporting family carers. One local initiative cited by a HCP was a carer-friendly hospital programme piloted on the stroke unit. This included a carer’s agreement that was completed on admission by the family carer in conjunction with a member of ward staff:
one of the things that ran very successfully in [name] Hospital this year was a carers, the Carer Friendly Hospital, and they piloted it on the stroke unit and I think that’s also about making sure that the carers of people who have dementia as well as stroke or who have multiple other comorbidities have the support.
HCP stroke CCG 1, south-east
However, often support for carers was limited and there appeared to be little in the way of formal support for carers managing their relative’s diabetes. For example, the daughters of a PLWD with diabetes felt that a telephone call with a diabetic nurse every 2 weeks was not adequate support:
I think ‘cos remember we’re just carers, and I think we have someone professional that comes in to do, especially the insulin at night to actually check to say, ‘Well yeah this is okay’, you know, I don’t think we get anything like that.
Carer diabetes 7, south-east
Informal social networks, such as extended family, friends and church groups, were clearly important to PLWD and their carers. In addition, voluntary organisations such as the Stroke Association, the Alzheimer’s Society and Parkinson’s UK provided social and practical support to people with dementia and comorbidity. Voluntary groups were able to provide condition-specific information, peer support and guidance about the support available.
One physiotherapist noticed that the Stroke Association was having more impact in supporting patients and carers.:
and certainly some of the stroke patients I’ve been seeing more recently, whatever age, young or elderly, with issues, have had contact from a Stroke Association visitor or phone call, and, oh and some of them are using them more and I think that’s very helpful to the spouses and carers ‘cos it, as a form of support.
Physiotherapist 1, south-east
the Alzheimer’s Society have been fantastic . . . Oh the Alzheimer’s Society, . . . that’s a godsend that is, absolutely godsend, yeah.
Carer stroke 4, south-east
Local groups could be a good source of peer support. For example, one PLWD who had been suffering with depression since having a stroke said that he appreciated the peer support he received from attending his local Stroke Association group.
Integration of services
The participants with dementia generally had complex health needs and were often seen by a number of different services and specialties. Carers and PLWD felt that specialists tended to focus on their own area and did not share information with other specialities. As the following quote shows, they did not necessarily see this as a problem but recognised the value of having one professional (in this case the GP) who could look at the ‘whole picture’:
memory loss, no, they’re not interested in that, they’re interested in treating the symptoms of diabetes not somebody else’s, it’s almost like somebody else’s problem but I don’t mean that hard-heartedly, I mean that we are dealing with this bit, there’s nobody, other than my GP looking at the whole picture.
PLWD diabetes/stroke/VI 1, south-east
Health-care professionals from the specialties studied admitted that they had a limited understanding of the needs of PLWD. For example, diabetes specialist nurses said that they were unsure about when to refer someone with cognitive problems:
when you identify somebody who’s having a problem with their memory and you’re following them up, . . . at what point should they be referred to this sort of agency, you know, what parameters do you use to measure it, you know, all of those things that I can’t say really what that is and I don’t know if I could say if any of my colleagues do either.
Diabetes specialist nurse, diabetes focus group, London
Several HCPs recognised that the organisation of services around single diseases was a problem for PLWD with complex health needs. The current infrastructure did not support the exchange of information and in particular participants said that poor integration of mental and physical health services, with different information technology systems, made it impossible to share medical records:
And I think that’s a key point I was going to make is one of the big stumbling blocks we have is the fact that services or parts of different trusts so the Mental Health Services sit within the [name] Partnership Trust so they don’t use the same system as us so we can’t share notes, the GPs use a different system again so it makes it very difficult to communicate to even find out what services people are under, you know, if that could be improved, if we could all be on the same system that would be good [laughs].
Physiotherapist, stroke focus group, south-east
There were some examples of successful integrated working, such as community matrons, or a community multidisciplinary team involving a geriatrician, an old-age psychiatrist, elderly care nurse specialists, GPs and a social worker who had regular face-to-face meetings. The presence of a key person or lead clinician to co-ordinate care was consistently identified by different participants as compensating for the known problems and challenges of services defined by single diseases and PLWD’s multiple encounters with different professionals:
I think new services like in L1 [London Borough] we have the community matrons have actually been of great help because they are more of care co-ordinators which I think do help these people with comorbidities in the community.
GP 2, London
Personalisation of care
Health-care professionals spoke about the importance of personalising care for PLWD. For example, diabetes specialists said that they would personalise targets for blood sugar control for PLWD:
And some of the nursing staff have been working with their community colleagues quite intensively to develop individualised regimes to try and, you know, if they eat half their meal to give this amount of insulin, if they eat all of the meal to give a different amount of insulin, so it’s almost like a sliding scale, it’s individualised, it’s not for every patient but for those who sometimes refuse food it’s sometimes really quite a helpful way to prevent them having to keep coming back in with hypos or highs.
Diabetes consultant, diabetes focus group, south-east
Vision impairment HCPs also said that they would simplify medication regimes for PLWD by reducing the number of times that they would need to administer eye drops. Stroke specialists said that stroke rehabilitation regimes needed to be personalised for PLWD and take into account the kinds of activities that they like to do:
And you might need to take more of a sort of automaticity approach so just, you know, find out what the patient likes to do, whether they like dancing or just, you know, try and go on the automatic things that aren’t too cognitively challenging to try and engage them as much as possible.
Physiotherapist, stroke focus group, south-east
Although HCPs acknowledged the importance of personalised care, it was not clear to what extent this was happening in practice. As this GP observed, there was a common understanding of the importance of having a person-centred approach but they were unsure what the practical application of these ideas looked like:
you get the vocabulary established but then people latch onto the vocabulary and not the meaning, ‘the holistic care’, you know brilliant one, but then what do people mean by it?
GP clinical commissioning group 1, Midlands
There were clear examples in which there was a failure to know the person’s story and tailor care accordingly, for example not adjusting stroke rehabilitation for a PLWD who had been a keen walker but who struggled with the rehabilitation exercises he was given:
we went three or four times and they said you know, ‘these exercises will help you’, well exercises are not part of what D does and they didn’t really help him, you know, . . . and I mean he just found it ridiculous to be doing these things.
Carer stroke 7, south-east
Information continuity
Transfer of information between specialities
People living with dementia, family carers and HCPs all gave examples of poor information transfer between systems and specialities. Of particular concern was that HCPs specialising in diabetes, stroke and VI were often unaware that someone had a diagnosis of dementia:
But obviously anywhere new that we go, like for this colonoscopy and all that sort of thing, I always mention, you know, ‘he has dementia quite, quite severe dementia’, I think when we went for a blood test for this colonoscopy it wasn’t on his notes there, although it was on the original colonoscopy referral sort of thing. So it seems that within the hospital set-up they don’t always transfer all relevant information between departments.
Carer diabetes 1, south-east
Diabetes specialists sometimes became aware that someone had dementia only when they realised that they were having problems self-managing their condition or taking on new information. VI specialists reported that they were frequently not made aware that a patient had dementia before they saw him or her in clinic, which made assessments and treatment more difficult.
Family carers were facilitating informational continuity by collating records and actively transferring information between HCPs and different services. Carers and PLWD were frustrated that they often had to repeat the same information to different HCPs:
You see one person one time and then you’d have, tell them what they need to know and then you see the next person and they don’t know, do they. You have to go all through it.
Yeah, you have to start again. But I mean, that actually is a problem with the NHS all the way through, I mean, because it’s a kind of, you know, you’re not always treated as a whole person, you’re treated as individual bits, aren’t you.
PLWD and Carer VI 7, south-east
Carers also reported problems with record-keeping, which may be particularly problematic for PLWD who are likely to be unable to provide an accurate medical history to clinicians:
but then what happened was it was, we came back here and he didn’t have no notes or recollection of my dad seeing that doctor in October.
Carer diabetes/VI 4, south-east
Health-care professionals from all specialities suggested that PLWD could have a hand-held record to take to appointments, similar to the ones carried by people with learning difficulties. It was clear, however, that the interviews were the triggers for these suggestions and that patient-held records had not been developed for PLWD or their carers, as this quote demonstrates:
I guess maybe the purple folder I’ve just mentioned, I’ve not thought of it before but it’s potentially something that can be passed, you know, from one professional to another even if you just write a few words, entry, at least then people know who else is involved with the care and especially if their usual carer isn’t about for whatever reason, you know easily who to contact.
GP 5, south-east
However, although hand-held personalised documents such as the ‘This is me’ passports exist as a way of ensuring that PLWD can share key information about themselves with HCPs [see http://alzheimers.org.uk/thisisme (accessed 7 January 2016)], we found that very few PLWD, carers or HCPs were aware of them. One carer thought that she had been given a ‘This is me’ passport but said that it wasn’t filled in and she didn’t use it. One patient mentioned having had a small card that detailed that he had Alzheimer’s disease and his next of kin, medication and doctor’s details, but he had misplaced it.
Availability of resources/information
There were clearly issues about the timely delivery and availability of information for people with dementia and another health condition. HCPs acknowledged that there is often a lack of support, information and training for people with dementia and diabetes and their carers. One HCP said that diabetes educational programmes vary across different regions and not all programmes are open to carers or PLWD who cannot self-manage:
But structured education varies in its availability anyway and the programmes vary and access to the programmes in different areas will vary, so whether they’re happy to accept carers or whether it’s only open to a person with diabetes and it’s therefore not open to somebody who has diabetes and dementia or who can’t actually self-care.
Diabetes nurse 1, south-east
There was a suggestion from some HCPs that PLWD would not be considered suitable for some of the educational programmes that were offered for stroke:
We also run a huge sub-management programme which does have a module for people who have had strokes as well as two general modules and somebody with dementia we wouldn’t invite necessarily to those modules so they themselves would miss out on a huge bit of secondary intervention and advice that they might get otherwise and because we don’t invite the carers automatically the carer also doesn’t get the secondary intervention advice.
Physiotherapist, stroke focus group, south-east
Some of the problems with relationship, management and informational continuity that have been detailed in the previous sections can be seen in the vignette in Box 6, which describes a specific case from an interview with a PLWD and her daughter. In particular, it highlights the transition from self-management to dependency and the problems that this cause for the family carer, who lives at some distance and feels excluded from decision-making about her mother’s condition.
Mrs B is a 92-year-old woman with type 1 diabetes and Alzheimer’s disease. She lives alone but has the support of her daughter, who lives a long distance away. Her daughter finds it difficult to manage and co-ordinate her mother’s health care from a distance.
Mrs B managed her diabetes well for 20 years, testing her bloods and injecting insulin twice a day. The daughter noticed that something was wrong when her mother was no longer able to manage her diabetes, that is, forgetting to eat, eating too much and forgetting medication. The daughter had to ‘battle’ (two or three telephone calls and a letter) with the GP practice to obtain a diagnosis of dementia for her mother. Mrs B does not always see the same GP and there appears to be poor relationship continuity.
Mrs B is now visited by a district nurse, who checks her bloods but the daughter is concerned that these visits are not regular enough and is worried that her mother’s diabetes is not being monitored properly. Recently, her mother has lost a lot of weight and has been taken off insulin. They were not sure if she had received any foot checks for diabetic neuropathy. However, the daughter said that her mother was seen by a private chiropodist and they assumed that she would notify them if there was a problem with her mother’s feet.
On a number of occasions changes have been made to Mrs B’s medication or care without informing her daughter. For example, one GP made changes to her medication that she was unable to cope with. On another occasion the district nurse issued a new blood testing kit to Mrs B but did not leave written instructions or inform the family. Mrs B was unable to use the kit and the family could not get in touch with the surgery for help as it was closed for 4 days over the Easter break. The daughter feels that she is not included in the decisions about her mother’s medical care and would like to be more informed.
The daughter now pays for private carers to visit her mother every morning and evening to give medication.
Theme 2: negotiating access to care
Appropriateness of health-care systems and environments
Appropriateness of guidelines and pathways
The data from the interviews and focus groups suggest that current guidelines and care pathways for diabetes, stroke and VI are often not appropriate for people with dementia and comorbidity:
You know, we have clinical pathways for the various specialties, and conditions, but not particularly aimed at patients with memory loss. I guess one could have sort of a subset of, yeah, within various guidelines, but I don’t think we have any in that at the moment in ophthalmology.
Ophthalmic surgeon, VI focus group, East of England
I think in terms of thinking about stroke pathways, in terms of dementia I think that one of the things is, one of my criticisms if you like of the stroke pathway is it’s very much related to the stroke and in relation to looking at comorbidities, not just dementia but a lot of the comorbidities, sometimes perhaps it’s not as robust as it could be.
Stroke clinical commissioning group 1, south-east
Health-care professionals also said that dementia pathways do not take into account those patients with comorbidities such as diabetes or VI:
I think that’s my next step is to try to raise a bit more of an awareness of vision within the dementia pathway.
Ophthalmologist 1, London
Some of the problems associated with current stroke pathways are illustrated in Figure 13. Many stroke pathways are geared to early discharge. Although reducing the amount of time that a PLWD spends in hospital may be a good thing, they may need more support at home than is generally available. They also need access to appropriate specialist rehabilitation.
FIGURE 13.
Potential problems with current stroke pathways for PLWD. ED, early discharge.
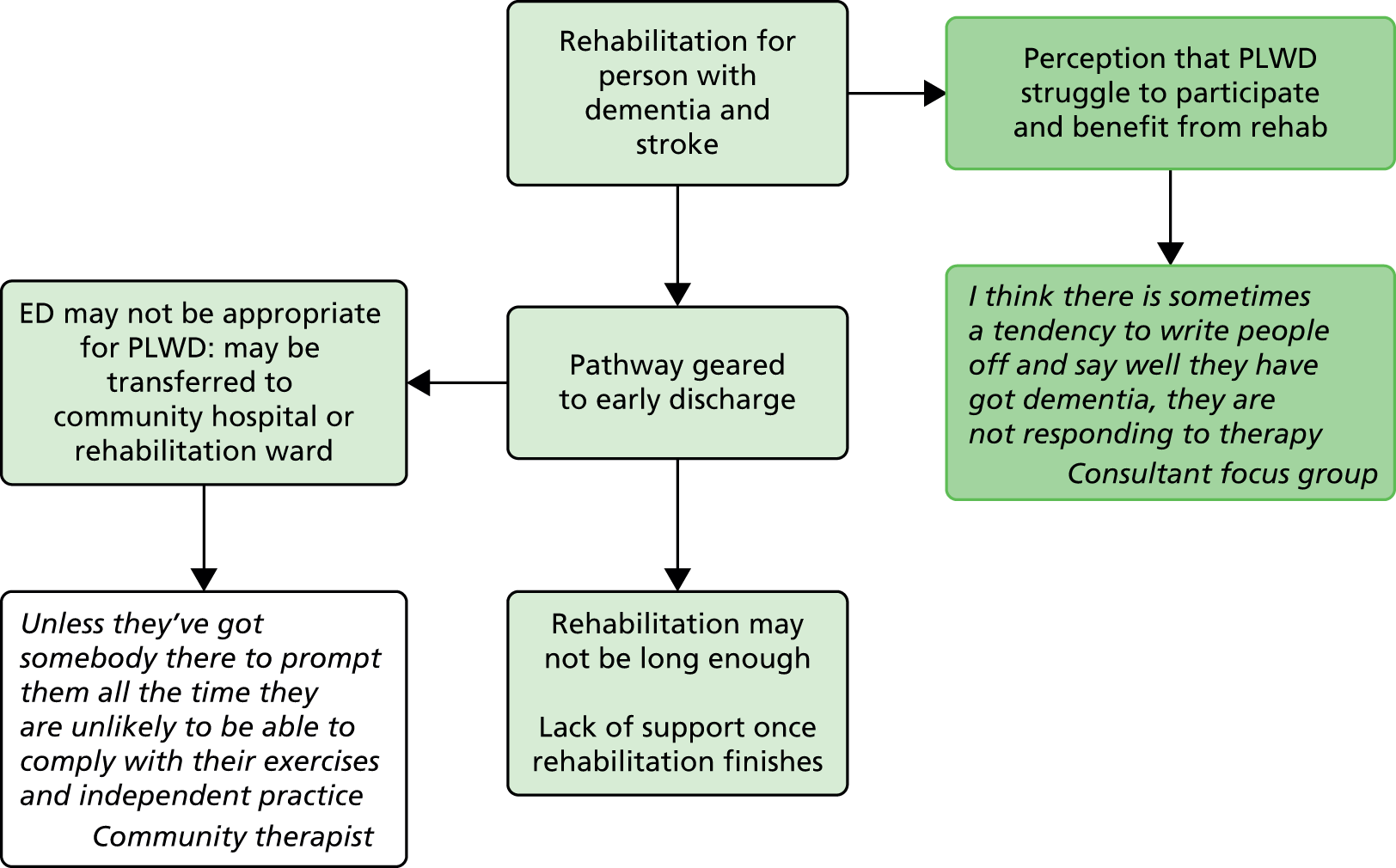
Systems not appropriate (not dementia friendly)
Health-care systems are often not dementia friendly. One frequently occurring example was around appointments, particularly in secondary care. PLWD and their family carers reported problems with remembering appointments (especially if they were booked over the telephone), difficulties with co-ordinating multiple appointments and problems with rescheduling and cancellations:
there was one clinic that he went to who wanted to make all the appointments by phone, so they would ring my parents . . . and say, ‘Right, we’d like you to come in next Tuesday at ten o’clock’, and dad would say, ‘Yeah, right okay’, and then he’d put the phone down and my mother would say, ‘Who was that?’ and he’d say, ‘I don’t know’, ‘What did they want?’, ‘Don’t know’.
Carer VI 3, south-east
Missed appointments could result in PLWD being taken off the clinic list and having to be re-referred by their GP. HCPs reported that pressures to meet targets could lead to a zero tolerance approach to patients who failed to turn up for appointments.
Health-care professionals were aware that people with dementia often needed longer appointments but time pressures meant that this generally did not happen:
one of the things about eye clinics is you have to be aware that the normal process which is fairly intensive of getting people through the system and it takes an hour or so to get through, if someone’s got dementia or learning disabilities you have to take that into account and how you handle them in the environment of the clinic, you know, they need more time.
Ophthalmologist 1, London
but actually it really requires time of talking to the patient I think to uncover those sort of issues to do with memory problems and we’re not always very good at that I think.
Ophthalmologist 2, East of England
There was a lack of flexibility in health and social care that made it difficult for people with dementia and comorbidities. For example, HCPs reported that in some instances insulin regimens had to be altered to fit with the schedules of district nurses who could only visit at certain times of the day:
but if you’re reliant on district nurses for example who got their own, you know, they‘ve got their timetable of what they need to do in their work to get through, and they have to administer it at a set time and that can be incredibly disruptive to the individual.
Diabetes consultant, diabetes focus group, south-east
The split between social care and health care was also identified as a particular problem. Supporting PLWD to live independently at home is invariably seen as social care. This broke down when social services carers were not able to test blood sugar or oversee medication for people with diabetes, making it difficult to co-ordinate meals and medication and putting PLWD at risk of hypoglycaemia:
Yeah. Silly things like a simple blood glucose test or being taught how to recognise signs and symptoms of a hypo could prevent a whole admission or, yeah.
Diabetes consultant, diabetes focus group, south-east
A more cohesive provision of social and health services to patients in the community was seen to be particularly important by HCPs for PLWD and diabetes:
then just to simply tie up the medication and monitoring of diabetes with the provision of meals is basically all that needs to be done. And I think that’s where it falls apart a lot of the time because people who can’t self-manage will often be reliant on a district nurse or a community nurse to perhaps come in and oversee the medication or give them their insulin, but they won’t be responsible for ensuring that that person has their breakfast or, so you get big gaps between one and the other and that really is not helpful. And that’s how people do end up having falls and being admitted to hospital, yeah.
Diabetes consultant, diabetes focus group, south-east
Some of the problems with systems that are detailed above can be seen in the vignette in Box 7, which describes a specific case from an interview with a PLWD and his daughter. It also highlights how some HCPs do not have the skills and knowledge to deal sensitively with PLWD.
Mr C is an 89-year-old retired airline pilot with AMD and Alzheimer’s disease. Mr C was already living with AMD when his daughter noticed that he was starting to forget things. She reports that it took her three or four telephones calls to the GP before she got her father referred for assessment. Her father was then diagnosed with Alzheimer’s disease but they felt that the diagnosis was delivered in a blunt and insensitive way. Mr C was upset at this appointment and refused to attend the follow-up appointment. He currently receives no care from the Community Mental Health Team.
His daughter, who lives a long distance away, said that they struggled without any support for over a year. They then had help from social services but were unsatisfied with the support provided: multiple carers who came at various times and sometimes did not turn up at all. They now no longer use social services but pay for a private carer who comes in on a daily basis.
Mr C continues to get support for his AMD and reports a positive relationship with the consultant treating him for his vision problems. He feels that the consultant has taken the time to get to know him as a person and, as a consequence, he likes going there. However, the daughter recounts problems with hospital appointments being booked over the telephone. Mr C would forget to write down appointments and as the system allowed for only one telephone number to be recorded she was not aware when appointments had been made. She also said that hospital transport staff did not understand the needs of her father.
They reported a visit to an optician that had clearly distressed both Mr C and his daughter. When booking the appointment Mr C’s daughter had explained that her father had Alzheimer’s disease and may need more time. He was given a double appointment but during the eye assessment Mr C found it difficult to understand what he was supposed to do and this was upsetting for him. Mr C’s daughter said that the optician did not understand her father’s dementia and he kept communicating with her instead of her father. The optician then suggested that her father should go into a care home because of the risks of living at home with dementia and VI. The daughter was unhappy that he had made this comment in the presence of her father.
Environments not appropriate (not dementia friendly)
People living with dementia and their family carers reported a number of negative inpatient experiences including multiple bed moves, long waiting times for procedures, staff not being aware of the patient’s dementia and staff not understanding the needs of PLWD. This often led to negative outcomes for the PLWD such as wandering and increased agitation, confusion and distress. There were also examples of a lack of understanding of the specific needs of people with dementia and particular comorbidities such as diabetes:
She had to leave here five in the morning, nil by mouth, she was told to get to H for eight and she didn’t go down for her surgery until after three.
She was distressed because she . . .
By the time . . .
. . . was so hungry and she, you know, with people with diabetes and they’re hungry they get pain don’t they in their head and things.
Carers diabetes 7, south-east
Health-care professionals also had concerns that hospital environments may not be safe or suitable for PLWD and that hospitalisation could lead to a deterioration in their condition. There appeared to be an expectation among some HCPs that PLWD will exhibit challenging behaviour while in hospital and that this will impact on the care of other patients. Some HCPs provided examples of dementia specialist wards or units where the focus was on person-centred care and where the environment was geared towards PLWD:
Actually on [name] ward it’s fine, there’s space and no one complains about them walking around the ward, it’s actually quite fine simply walking around the ward. So I think that’s hugely important that actually people aren’t being penalised for trying to be mobile or hey, people are actively encouraged almost.
Psychiatrist 1, south-east
However, environments that specialised in care for our three comorbid conditions tended to be less dementia friendly. For example, in eye clinics patients often have to complete multiple tests over long periods and may need to attend appointments on a number of different days. There is also an emphasis on moving many patients through the clinic as quickly as possible. VI HCPs felt that PLWD would benefit from longer appointments but the current structure of their service made this difficult to implement.
Some of the potential barriers and facilitators for people with dementia and VI can be seen in Figure 14. The barriers are common themes that arose in the literature and have been summarised to illustrate a typical patient journey. The facilitators are things that were suggested as good practice by HCPs but which were often aspirational rather than current practice.
FIGURE 14.
Barriers to and facilitators of care for people with dementia and VI.
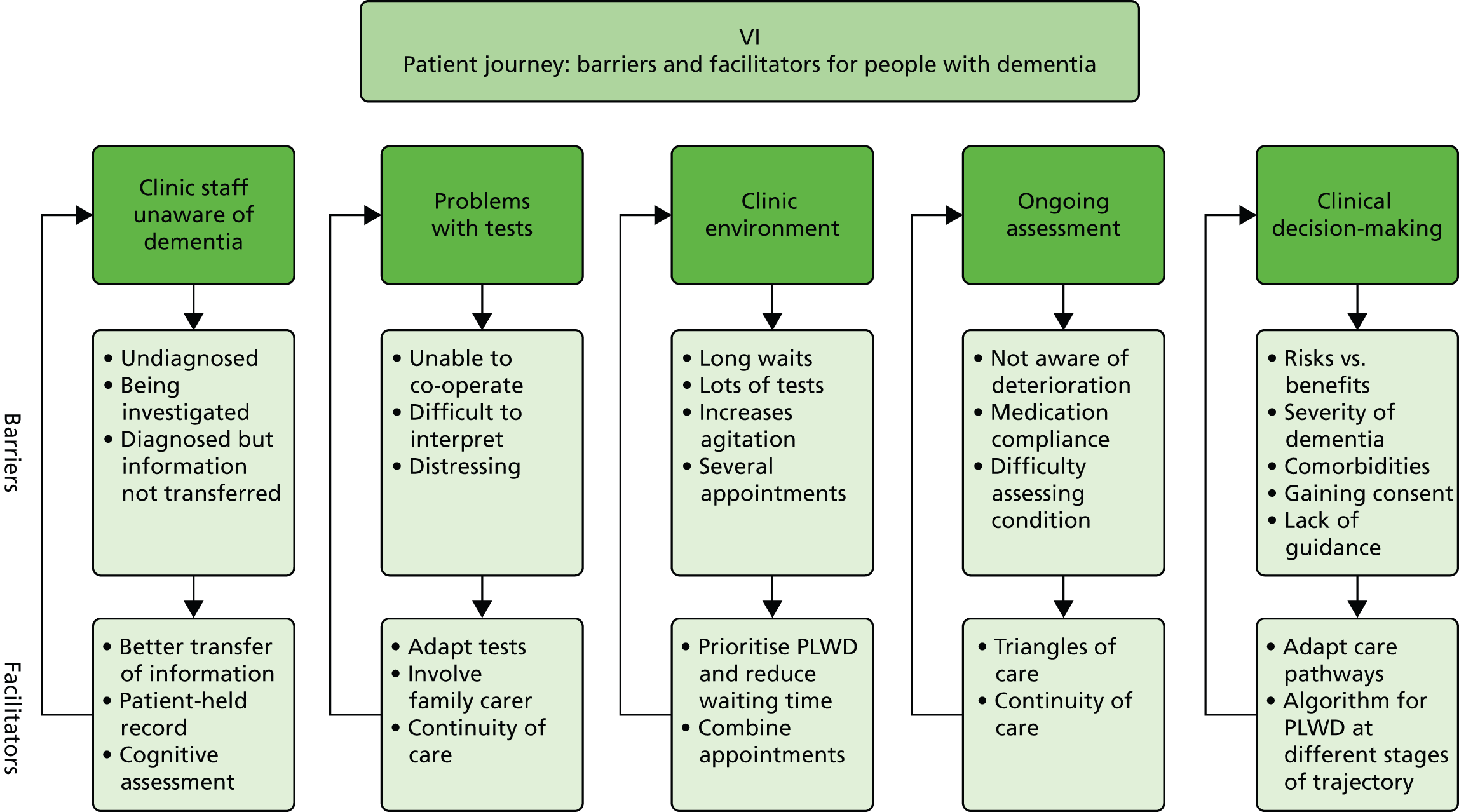
Comprehensiveness and equity of health care
Balancing risks and benefits
Access to care was affected by the ways in which HCPs and family carers weighed up the risks and benefits of treatments for PLWD, such as surgery for cataracts or thrombolysis for stroke. Some of the factors involved in making decisions about care for people with dementia who have had a stroke are illustrated in Figure 15.
FIGURE 15.
Factors involved in decision-making for people with dementia who have had a stroke.
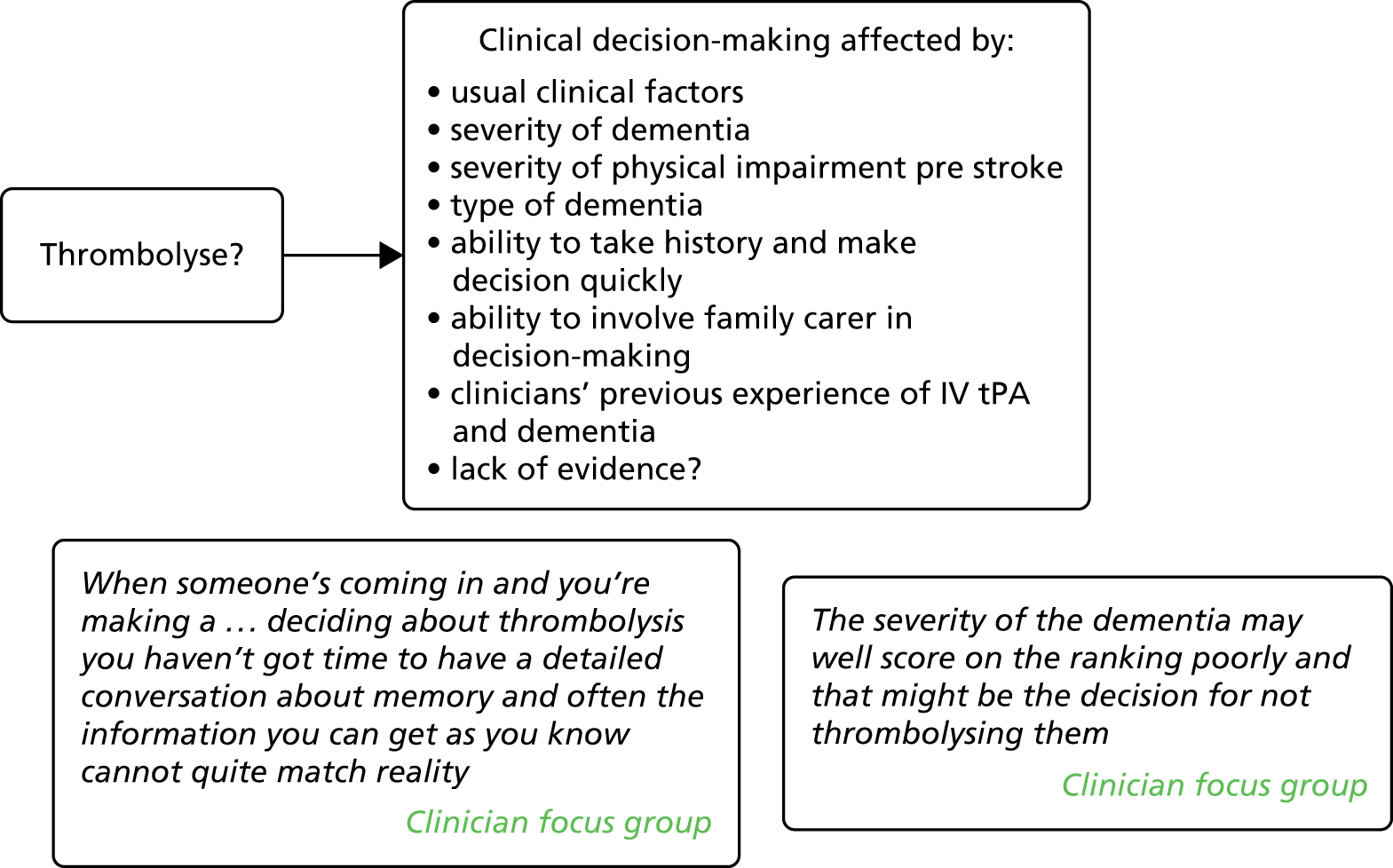
Factors that impacted on clinical decision-making across the three comorbidities included concerns about polypharmacy, a PLWD’s ability to co-operate with treatment, the ability to gain meaningful consent, severity of the dementia, levels of multimorbidity, the availability of support from a family carer and the presence of a family carer to act as a proxy and provide information.
It was clear that attitudes towards risk differed among clinicians. For example, in one focus group there were different opinions about the safety of taking someone off insulin:
it’s also going to be very risky to take them off insulin.
Diabetes consultant, diabetes focus group, south-east
you may decide that a blood sugar range of 5–15 is quite adequate for that person and you may be able to achieve that without insulin, so it is a question I do ask myself when I look after people’s diabetes, you know, is that something that we could safely offer.
Diabetes consultant, diabetes focus group, south-east
There were some data to suggest that attitudes towards risk were affected by previous experiences:
I wouldn’t refer someone who was uncooperative . . . I have had a patient who got up in the middle of a cataract operation and refused to have anything further done and lost the vision in his eye.
GP 4, south-east
It is also possible that attitudes towards risk change over time and as a HCP gains experience:
I think as you get more experienced, it’s quite a difficult decision but as you get more experienced your decision changes. I’m certainly quite . . . personally, I don’t know how others are but I certainly am quite aggressive about cataract surgery in people with dementia, I think that it’s got a very low downside, the chances of something going wrong are very remote and if you make it work and you make them function better then fine.
Ophthalmologist 1, London
Health-care professionals’ expectations and attitudes
Clinical decisions were influenced by HCPs’ attitudes and previous experiences of treating PLWD. There were contrasting attitudes towards the benefits of performing cataract surgery for PLWD. For example, one consultant was concerned about a lack of co-operation during surgery and thought that the benefits of improving vision for PLWD were sometimes ‘overplayed a bit’ (ophthalmic surgeon, VI focus group, East of England), whereas another was more positive about the benefits of surgery for PLWD:
I’ll do cataract surgery on most people because I believe it’s a very powerful intervention to make and the downsides are very low, different people have different attitudes, say in terms of the surgeon.
Ophthalmologist 1, London
There appeared to be an expectation among stroke clinicians and therapists that PLWD would not be able to co-operate with or benefit from rehabilitation, particularly if they had challenging behaviour.
Candidacy
Candidacy refers to the concept that access to health care is jointly negotiated between individuals and health services. For PLWD, family carers were key to this process, often acting as an advocate for their family member with dementia:
As a family member you’re the person who knows that person better than anyone else so you can see when it’s not, when it’s not right, when it’s going wrong.
Carer diabetes 4, south-east
It was like when she had her cataract done, I actually went into the room with her . . . you know, because one nurse at the cataract kind of looked at me and she said ‘no, if you wait in the waiting room’, I went ‘well, no – my sister has a memory problem so I’ll have to stay’.
Carer diabetes/VI 6, south-east
They were often also responsible for noticing when something was wrong and seeking help from the appropriate services. For example, one daughter noticed that her mother’s feet were going black:
Her feet were black and I was concerned, because we’ve got, in the paternal side of my family, she’s got aunts and her mother was blind, aunt had amputation of the toes.
Carer diabetes/VI 3, south-east
However, it had often taken carers some time to understand how systems worked, who to contact and what questions to ask. HCPs also recognised that those PLWD who lived alone or who did not have support from a carer were particularly vulnerable and may have poorer access to care:
the greatest difficulty is when that individual lives alone and doesn’t have an able partner, because then their care can become very disjointed or they’re not, they’re not able, often they, an appointment’s made or they, and they won’t answer the door or they forget and so it’s when somebody’s on their own that you have the biggest issues and lack of joined-up care.
Physiotherapist 1, south-east
Health-care professionals’ understanding/appropriate training
Many of the HCPs in our study, and across the three target conditions, appeared to feel unprepared to care for PLWD. Some of the HCPs referenced learning or training that was specific to the treatment and care of PLWD but this had been brief and did not appear to have impacted on practice:
I think a lot of people are aware that there hasn’t been apart from that one-off training that I’ve had recently, I haven’t seen a huge change in practice for dementia patients.
Physiotherapist, stroke focus group, south-east
Diabetes nurses in one focus group admitted that they had very little knowledge about dementia, and stroke physiotherapists in another group said that they had not received any dementia training. HCPs in the VI focus group said that they would like some dementia training to raise awareness in their field:
Just training really, just I think we just need that extra training just to, in this particular aspect, clinical and awareness of what to do.
Senior orthoptist, VI focus group, East of England
People living with dementia and their family carers provided many examples in which they felt that HCPs had not understood their needs, for example transport staff with no knowledge of dementia, clinic staff asking PLWD for details such as their medical history or date of birth that they had difficulty remembering or HCPs giving patients complicated medication regimes that they were unable to manage.
Screening and monitoring for long-term conditions
Access to appropriate screening and monitoring for people with conditions such as diabetes appeared to vary considerably, for example ranging from weekly home visits for some patients to irregular telephone monitoring for others. Some people reported that they were satisfied with the monitoring that they received for their condition whereas some carers of PLWD with diabetes were concerned that their relative was not receiving adequate monitoring. Diabetes specialist nurses and GPs expressed concerns that patients were not getting appropriate screening for their condition, such as foot checks. This was particularly a concern for housebound patients and those who lived alone:
And I think it’s only when [name of diabetes nurse in focus group] goes in that she can actually see, you know, that the devastation or whatever that’s been caused by possibly years and lack of input.
Diabetes specialist nurse, diabetes focus group, London
People living with dementia and their carers were often unclear about whether or not they were getting adequate foot checks and there was confusion over the different roles of chiropodists and podiatrists.
There were also problems with tests that were not geared towards PLWD:
she said you know, ‘can you read the third row of the chart’ or whatever he couldn’t, and she twigged that there might be a problem and called me in, I said ‘well he’s got dementia’, it’s on his diabetic notes but it obviously hadn’t gone through to the eye screening bit, so I think she tried him with numbers, he’s probably a bit better with numbers than he is with letters.
Carer diabetes 1, south-east
Health-care professionals also reported difficulties carrying out eye tests with PLWD and in some cases tests could not be carried out:
I get some dementia patients come the AMD clinic and usually with a carer of some description, and then we have the difficulty of assessing their vision because of them not being able to verbalise what they’re seeing, difficulty reporting symptoms.
Staff nurse ophthalmology, VI focus group, East of England
Patients with diabetes, stroke and dementia should receive routine monitoring as part of the QOF. However, it was clear that there was variation in the way that these assessments were delivered and GPs expressed concerns about whether or not all patients received these assessments, particularly those who were housebound. The dementia QOF appeared to be considered less of a priority than those for diabetes and stroke:
I think the diabetes, the heart disease, the stroke, you know, all those you know are more mainstream as it were, the ones that were given a lot of time and I think yes the dementia one would probably have been left, I certainly think we do achieve the QOF targets for it, but it is perhaps not at the forefront of people’s mind in the way that the diabetes one is, you know I suppose in terms that we don’t have a protocol for it, you know that wasn’t something I even and it hasn’t even kind of crossed my mind to be honest.
GP 8, north-east
Conclusions
Continuity and appropriate access to care were key for people with dementia and comorbid conditions such as stroke, diabetes and VI. Clearly, continuity and access are linked, with continuity of care being a facilitator of appropriate and comprehensive health care for people with dementia. However, although continuity is important to people with dementia and their family carers, it is often lacking. The transfer of information between specialties, such as a diagnosis of dementia, seems to be a particular problem. Family carers in our study played a significant role in facilitating both continuity of care and access to care.
Many of the issues highlighted in this chapter are applicable more generally to people with long-term conditions or complex health needs, not just those living with dementia. However, many of these problems are compounded by dementia. For example, although many carers may be playing a role in co-ordinating and managing care for a relative, this is perhaps more acute for PLWD who lack candidacy and are often completely reliant on family carers for facilitating continuity and access. Care for PLWD needs to be reframed as a three-way process between the HCP, the PLWD and the family carer.
There are a number of barriers to continuity and access to care for PLWD. Some of these are condition specific but many are applicable across the three conditions. For example, although there are specific concerns about preventing hypoglycaemia for people with dementia and diabetes, many of the issues are applicable to all three comorbidities, such as managing medication, providing co-ordinated services and supporting carers. The data provide some examples of effective care for PLWD but many of these examples seemed to be about the individual health providers that patients and carers encountered rather than about systems-based approaches.
Chapter 7 Consensus conference
The consensus conference
Findings from phases 1 and 2 of the study were discussed with key stakeholders at a consensus conference at the Royal College of General Practitioners in December 2014. This was a half-day event and was attended by 40 participants, including practitioners specialising in the care of people with our target comorbidities (diabetes, stroke and VI), GPs, service managers and commissioners, members of the public and representatives from the third sector involved in supporting people with dementia (e.g. Alzheimer’s Society, Carers in Herts) or people with our target comorbid conditions (e.g. Stroke Association, Diabetes UK, Thomas Pocklington Trust). The event was facilitated by Professor Steve Iliffe.
The more specific aims of the consensus meeting were to:
-
consider how current services can adapt to meet the needs of PLWD who have other complex health-care needs
-
develop guidance about how services might be developed to engage better with PLWD and their unpaid carers, indicators of quality that can inform commissioning of services for older people and specialty-specific guidance on assessment and decision-making.
To structure the discussion and rank the importance of the findings and their relevance for service improvement and delivery we used a nominal group technique. 86,87 Participants were split into four small groups around their area of specialty (i.e. stroke, diabetes, VI and general practice). Based on the evidence presented by the research team and their own experiences, participants were asked to consider what changes they would recommend to improve care for people with dementia and comorbidity. Group facilitators asked each person to silently and independently generate ideas/recommendations. Ideas/recommendations could be general or specific to one of the three target comorbidities. After the generation of ideas, group facilitators invited participants to share their ideas with the rest of the table and once all ideas had been presented the facilitators encouraged discussion around how these concepts could be developed and implemented. To filter and prioritise the ideas/recommendations, the facilitators asked the groups to choose the top two ideas and these were refined through further group discussion. Finally, the event facilitator consolidated the ideas/recommendations by asking each table to put forward their ‘best ideas’ to the larger group.
After the meeting, ideas from each group were documented and summarised.
Cross-cutting themes
There were a number of cross-cutting messages that arose from the discussion (Box 8).
-
The presence of dementia complicates the delivery of health care in what is often already a complex situation involving patients with multimorbidity and/or frailty.
-
Many HCPs reported that they and their colleagues feel underprepared to care for PLWD and better education and training for staff is required.
-
Better integration and co-ordination of care are needed, between primary and secondary care and between different specialities in secondary care.
-
There is a need for better record sharing and transfer of information; current systems such as ‘This is me’ could be expanded to include health-care information.
-
PLWD and their family carers need support (from either a professional or a trained lay person) to help navigate health-care systems in primary and secondary care.
-
The QOF dementia review needs standardisation and could include physical health checks (e.g. vision), assessment and care planning.
-
Disease-specific pathways (e.g. diabetes, stroke, VI) need to be adapted to meet the needs of PLWD.
-
There may be transferable learning from other areas, in particular the area of learning disability.
-
When appropriate unpaid carers need to be included in care planning and decision-making and provided with adequate support.
Ideas/recommendations and supporting evidence
The outputs of the discussion were grouped into five overarching categories: (1) integration (including information transfer), (2) co-ordination and navigation, (3) optimising current systems and care pathways, (4) workforce development and (5) including and supporting family carers. In the following sections we outline the main implications for practice in each of the categories and discuss them in light of existing evidence (see Chapter 3).
Integration
Evidence from the consensus meeting and the interviews and focus groups highlighted the problems caused by barriers between primary and secondary care and between different specialities in secondary care (e.g. between mental health services for dementia and physical health/older people’s services). Participants at the consensus meeting suggested a number of strategies for improving the integration of care.
Integrated practice
One suggestion for integrating primary and secondary care was the extension of the concept of integrated practice units (IPUs), with co-located care and access to shared health and social care budgets. 165 Porter and Lee165 suggest that the IPU should provide the full care cycle and treat not only the main condition but also related conditions and complications (such as eye disorders in people with diabetes). Within the UK there is a strong policy push towards better integration of care,166–169 which is seen as particularly important for addressing the needs of older people with complex health needs. 170 The Five-Year Forward View166 sets out the new care models programme, which includes integrated primary and acute care systems and multispecialty community providers. The aim of primary and acute care systems is to join up GP, hospital, community and mental health services and the aim of multispecialty community providers is to shift specialist care out of hospitals into the community. A number of vanguard sites will trial these models of care. 171
Although there are currently few formal evaluations of IPUs, as described by Porter and Lee,165 there are a number of systematic reviews that have looked at the effectiveness of initiatives for integrating care systems for older people. 172–174 One review of integrated funds for health and social care found mixed evidence of impacts on health outcomes, costs or resource use. 173 Barriers to integrated budgets included difficulties in implementing financial integration and differences in performance frameworks, priorities, governance and information systems. However, none of these reviews focused specifically on integrated care for people with dementia and comorbid health conditions and there is limited evidence on the care of patients with multimorbidity. 174
Integration on a smaller level was also recommended, for example linking community opticians and optometrists with memory clinics. A recent retrospective audit found evidence to suggest that a combined clinic with neurological, ophthalmic and psychiatric input is a potentially effective way to diagnose and manage complex vision problems in older people, including those with cognitive impairment. 175
Multidisciplinary assessment
Several groups recommended the use of multidisciplinary assessment and care. For example, diabetes specialists suggested a programme of joint assessment and review (with old-age psychiatrists) for people with diabetes who are diagnosed with dementia. There is evidence to support the use of comprehensive geriatric assessment (CGA) for older people. 176,177 A Cochrane review of CGA concluded that older people were more likely to be alive and in their own home after CGA. 178 The authors suggested that wards designed for CGA seem to be more effective than mobile teams. A UK study of CGA for older people considered to be at high risk of future health problems failed to show an effect on patient outcomes or subsequent use of secondary care, but the authors concluded that this was because their intervention did not deliver truly comprehensive CGA. 179
Information transfer and personalised information
Our qualitative data suggest that the transfer of patient information between professionals in different specialities and settings is a problem for patients, family carers and HCPs. This was reiterated by participants at the consensus conference. VI specialists recommended a system for flagging up a dementia diagnosis on records to ensure that specialists such as optometrists know when a person has received a dementia diagnosis. It was also suggested that the next of kin details for people given a dementia diagnosis should be clearly marked on GP records and that, with the consent of patients, family carers should be routinely copied into letters. This would help keep family carers informed and help to facilitate access to services for people with dementia.
Ideas/recommendations for improving information transfer also included the use of patient-held records, either electronic or paper, that could facilitate the sharing of information among patients and their carers, primary and secondary health care and social care. User-held records are part of an innovative memory service provided within a GP practice in Staffordshire. 180 Older people or their carers are supplied with hand-held applications to help co-ordinate their care, control their own records and trigger appropriate urgent support when required. The scheme has been well received by older people and their carers and there is some evidence to suggest that delivering this model has released savings in acute hospital activity. 181
It was also suggested that information transfer for people with dementia could be facilitated by expanding the ‘This is me’ document to include more health-related information. Participants cited schemes for people with a learning disability, such as the purple folders used in the West Hertfordshire Hospitals NHS Trust. 182 The ‘This is me’ document was designed for people with dementia who are receiving professional care in any setting. Although its use is supported by the Alzheimer’s Society and the Royal College of Nursing, it has not been formally evaluated.
Co-ordination and navigation
Case management and care co-ordination
Case management for people with dementia and comorbidity was suggested by several groups as a way of assisting people with dementia to navigate health systems and improve their access to care. A number of systematic reviews have been reported of studies assessing the impact of case management on older people with dementia. 183–189 In general, studies have provided conflicting information about the potential benefits and duration of effect of case management for people with dementia. A recent Cochrane review of case management for PLWD found that the case management group was significantly less likely to be institutionalised at 6 and 12 months than those not receiving case management, although the longer-term effects were uncertain. 187 The authors found no significant differences in hospital admissions, mortality or patients’ or carers’ quality of life.
Assessing the impact of case management is complicated by the variety of models and differences in the way that the role is interpreted. 188,190 Differences include the type of care provided, the degree of collaboration and integration with other HCPs and the professional background of case managers. 191 Furthermore, it is not clear which population of people with dementia is most likely to benefit from case management and at what stage of the dementia trajectory. 185 Studies have, however, identified a number of characteristics that appear to be important for the success of case management and care co-ordination. These include strong provider and care networks, good personal connections with other HCPs, expert knowledge, embedding in the multidisciplinary team, the involvement of GPs, intensive and proactive approaches and clearly defined skills and responsibilities. 184,188,192–194
An example in which care for people with dementia is reported to have been successfully co-ordinated in the UK is the Oxleas Advanced Dementia Service, which provides care co-ordination, palliative care and support to people with advanced dementia living at home. 195 The authors of this report provide key lessons that they have learned: the need to provide care and advice to family carers; strong links between physical and mental health services; acceptance of referrals from a wide range of HCPs; a single comprehensive assessment of the patient and carer that addresses physical, mental health and social care needs; for a personalised care plan; and dedicated care co-ordination by a specialist nurse with suitable case management skills.
Dementia co-ordinators/support workers
Another recurring idea/recommendation involved the use of dementia co-ordinators or support workers to help people with dementia navigate health services. For example, one group suggested the use of dementia nurses in each hospital who would liaise between staff, relatives and the community and follow patients through their hospital stay. However, although it has been suggested that dementia specialist nurses with clearly defined roles could benefit people with dementia, there is little direct evidence for the effectiveness of dementia specialist nurses in hospital settings. 48,196 Moreover, there is anecdotal evidence to suggest that some specialist teams, such as those for palliative care, can have negative impacts, such as disempowering or deskilling other HCPs.
Parallels were drawn with learning disability liaison nursing, in which specialist nurses provide support for people with learning disability admitted to hospital. However, although there is evidence to suggest that learning disability liaison nursing is valued by stakeholders,197 and that promoting access and equity is an important part of the role,198 there appears to have been little formal evaluation of the role199 and its impact is unclear. Others suggested that the role of a dementia navigator could be non-clinical. For example, it was suggested that the role of the Stroke Association Life After Stroke Coordinators could be extended to include support for people with dementia who have had a stroke. There is some evidence to suggest that non-clinical dementia advisors can have a positive impact on PLWD and their family carers200,201 but there is a need for further testing of such schemes.
Optimising current systems and care pathways
There were ideas/recommendations for all groups that focused on ways of improving and optimising current systems. One such example was around the QOF in primary care. Currently, the structure or content of a dementia review is not clearly specified and there is variability in practice. It was suggested that the dementia QOF could be standardised to include physical and mental health checks, including checks for vision and hearing. Again, parallels were drawn with services for people with a learning disability, in which health checks have led to the detection of unmet need and allow for targeted actions to address health needs. 202
There was recognition from participants that current pathways may not be appropriate or suitable for PLWD. Diabetes specialists suggested that there is a need to reframe what ‘good’ diabetes care is for PLWD and a need for better guidance about what is important and essential in diabetes management, for example whether or not it is possible to simplify diabetes management for PLWD. A recent best clinical practice statement by a multidisciplinary National Expert Working Group provides a pathway that integrates diabetes and dementia care147 and which is less reliant on self-management than standard diabetes pathways. 203 However, there is little in the way of formal evaluations of strategies for managing diabetes in people with dementia. 59
Suggestions for stroke care included improving the accident and emergency pathway for transient ischaemic attack patients with pre-existing dementia to ensure that scans were carried out on the same day rather than assessing patients and then sending them home with a referral form. Participants also proposed that current assessments such as the 6-month post-stroke review and the annual review in primary care could be improved, for example being used to pick up changes in cognition. VI specialists suggested that the eye care pathway for PLWD should include information about eye examinations (including the availability of domiciliary eye tests), longer appointments, fast tracking for treatment such as cataract operations and subsidies for spectacles. However, implementing longer appointments would require additional funding as opticians are currently paid based on appointment times of 20 minutes.
Workforce development
Participants in both primary and secondary care suggested that there was a need for improved education and training for staff to raise awareness around dementia and improve services for PLWD. In some instances, training needed to be specific rather than generic, for example training staff working in stroke care how to modify rehabilitation for PLWD. VI specialists recognised a need to raise awareness of dementia among staff with training that included information on recognising and managing dementia, tailoring assessments to PLWD, signposting to appropriate services and guidance on how to optimise the environment for PLWD. Although there has been considerable investment in dementia awareness and training this has tended to focus on support workers and nurses204 and has been generic rather than geared to the specific needs of people with dementia and comorbidities such as diabetes or stroke.
Including and supporting family carers
Participants at the consensus conference recognised the central role that family carers often play in the care of PLWD. Stroke specialists suggested that family support could be used to improve access by PLWD to services such as rehabilitation, for example by developing partnership rehabilitation involving PLWD, family carers and professionals. It was also recognised that family carers of people with dementia and diabetes often take on responsibility for giving medication, managing diet, etc., and that they may need additional education and support to equip them for this role. Although there has been research around support for family carers, this has tended to involve more generic interventions such as case management and educational and psychosocial support. 48 There is little research that looks at the specific needs of carers of people with dementia and medical comorbidities such as diabetes or VI.
Conclusions
It is clear that the presence of dementia complicates the delivery of health care in what is often already a complex situation involving patients with multimorbidity and/or frailty. Many of the ideas that arose were not just applicable to people with dementia and it is challenging to disentangle what are essential components of dementia care and what are components that are applicable to all older people with complex health and social care needs. The situation is further complicated as some people will develop comorbidity in the presence of already diagnosed dementia and others will develop dementia subsequent to a comorbidity such as diabetes, stroke or VI. The profiles and needs of these groups may be different. Furthermore, dementia is a syndrome and not one condition and, within that, there will be a range of people, from those who are able to self-care to those who are very frail and need palliative care.
Although some of the ideas/recommendations were specific to our three tracer conditions, many of the ideas/recommendations drew on similar concepts, in particular the need for better integration and co-ordination of care, improved transfer of patient information, support for PLWD and their unpaid carers to navigate health systems, training and education for HCPs, recognition and support for unpaid carers and the improvement of current systems to better meet the needs of PLWD who have other health-care needs.
In the next chapter we bring together the findings from each phase of the study to map current models of care, look at how the presence of dementia with one or more comorbid conditions is currently being addressed by service providers, highlight interventions that might support continuity and equity of access to care and outline the implications for clinical practice and research.
Chapter 8 Discussion
The overall aims of this study were to explore the impact of comorbidities for a PLWD on access to non-dementia services and identify ways of improving the integration of services and reducing fragmentation of care. We focused specifically on three conditions: diabetes, stroke and VI.
We used a mixed-methods approach including:
-
a scoping review of relevant literature to map what is currently known about comorbidity and dementia
-
a cross-sectional analysis of a population cohort database to explore health and social service use in people with a diagnosis of dementia and a comorbid medical condition
-
a qualitative study exploring the views and experiences of people with dementia, their family carers and HCPs
-
consensus methods to help develop ideas/recommendations for practice.
In this chapter we start by giving an overview of the findings and then go on to discuss the limitations of the study and the potential implications of the findings. We finish by outlining the implications of our findings for practice and future research.
Summary of the findings
Scoping review
We included 76 studies or reports that addressed issues around dementia and comorbidities (diabetes, stroke and VI). There was evidence of a lack of continuity in health-care systems and structures for people with dementia and comorbidity, with little integration or communication between different teams and specialities. 72 Moreover, many models of care are focused on single diseases and do not take into account the needs of those with multimorbidity. 4,91 Twenty-six studies reported prevalence data,1,2,12,14,20,69,91,101,103,104,118,119,121,127–129,134,137,140–146, either of one of our three target comorbidities in people with dementia or of dementia in people with stroke, diabetes or VI. Although heterogeneity in the populations and differences in the way that conditions were ascertained make comparisons across studies difficult, the data do suggest that the rate of diabetes in people with dementia may be between 13% and 20% and the rate of stroke may be between 16% and 29%. Of the 11 studies98,110,117,118,123,129,136,139,145,155,161 that compared access to treatment or receipt of services in groups with and without dementia, 1098,110,117,118,129,136,139,145,155,161 found some evidence that people with dementia were less likely to receive the same quality of care or access to services than those without dementia.
Analysis of the Cognitive Function and Ageing Study data
In CFAS II the prevalence of dementia in those living in the community was 5.3%. Of these people with dementia 17% had diabetes, 18% had had a stroke and 17% had VI. The aim of the CFAS II-only analysis was to see whether or not there was any difference in service use between those with dementia and a target health condition and those with dementia alone or the health condition alone. Of all of the services, unpaid care was the most commonly used service in CFAS II. When comparing unpaid care use between those with dementia and a target health condition and those with only the health condition, in every case unpaid care was used considerably more by those with dementia and a target health condition (see Appendix 5). As well as unpaid care, those with dementia and a target health condition also used a home care assistant, a care worker and a day centre more than those with the health condition alone. When comparing hospital service use between those with dementia and a target health condition and those with dementia alone, inpatient services were used more by those with dementia and a target health condition.
The comparison analysis between CFAS I and CFAS II looked at whether or not there were any differences in service use over the last decade. The main difference seen was in the use of day-to-day services, which decreased substantially over this time period because of a decrease in the use of meals on wheels, paid help and care workers. At the same time there was a marked increase in the use of unpaid care by those with dementia and either diabetes or VI. Unfortunately, the numbers were not large enough to test this formally.
Interviews and focus groups
We conducted interviews with 28 people with dementia and 33 family carers, and focus groups or interviews with 56 HCPs. The analysis was informed by the different dimensions of continuity46 and access to care. 34 Our two overarching themes were (1) negotiating continuity with respect to relationship, management and informational issues and (2) negotiating access to care, including appropriateness, comprehensiveness and equity for PLWD and comorbidity. For a summary of the themes and subthemes see Figure 11.
Negotiating continuity
People living with dementia and their family carers valued relationship continuity. In particular, the personal characteristics of HCPs, and the communication of information in a timely and sensitive manner, appeared to be key to developing a trusting relationship with a HCP. Relationship continuity did not always have to be with an individual but could be with a team, although this was dependent on good record-keeping and transfer of information between members of the team. Interviews with HCPs highlighted the way that relationship continuity facilitates access to health care for vulnerable patients.
Although some PLWD were still able to manage their condition and navigate health services, this was either because they were in the early stages of dementia or because of support provided by family carers. Family carers were often playing a significant role in managing and co-ordinating the care of their relative; this sometimes involved having to learn new skills such as checking blood sugar or giving insulin injections. The transition from self-management to dependency could be gradual or sudden (e.g. after a hospitalisation) and was often unpredictable or understood only in hindsight. HCPs whom we spoke to acknowledged the vital role that family carers played but from carers’ accounts it was clear that this recognition did not translate into their routine involvement in patient appointments or decision-making about the care of their family member. As in other studies we found that staff concerns about confidentiality may impede carers receiving information. 205
The absence of a standardised approach to sharing information about a person’s dementia and how it might affect the management of other conditions was a recurrent issue. This had implications for how appointments were planned and organised and how carers were involved. There appeared to be no cross-discipline approaches that supported the meaningful transfer of information about a person’s dementia and related disabilities; HCPs involved in delivering care for people with our target comorbidities commented that they were often unaware that someone had dementia. Instead, informational continuity was often provided by family carers attending appointments and transferring information between specialities. Despite evidence of awareness among staff that PLWD could need more time for consultations, clinic structures and pressures of patient numbers meant that there was generally little capacity to do this for PLWD. We found little evidence of services developing processes to support informational continuity or using tools such as ‘This is me’ to support continuity of information.
Negotiating access
Our interviews and focus groups provided many examples in which systems or environments had unintentionally blocked access to care for PLWD, for example appointments made over the telephone, long waits in busy clinic environments, tests that were not appropriate for PLWD, new technology introduced without proper explanation, lack of involvement of family carers and a failure to engage with social care. Pathways and guidelines for our three target conditions did not address the possibility of a dementia diagnosis or provide decision-making support for practitioners trying to weigh up the risks and benefits of treatment for PLWD. Moreover, many HCPs in our study reported that they felt underprepared to care for PLWD. There were examples of good practice but this tended to be about the behaviour of individual practitioners rather than about system-based approaches.
The care and treatment that PLWD received were influenced by the expectations and attitudes of clinicians. Decisions about treatment were made in the context of concerns about polypharmacy, consent, concordance (including for medication, tests, rehabilitation and surgery), the severity of dementia, multimorbidity and frailty. Clinicians also had varying perceptions of the potential benefits of treatment for PLWD, with some expressing positive views about treating PLWD and others being more pessimistic and risk adverse. There was a suggestion from the interviews and focus groups that HCPs may sometimes have a negative attitude towards the ability of PLWD to participate in, or benefit from, certain treatments, such as post-stroke rehabilitation.
The consensus conference
The consensus conference was a half-day event attended by 40 participants including practitioners, service user representatives and academics. Discussions from the meeting were grouped into five overarching categories. These categories and some of the associated models of care are shown in Figure 16.
FIGURE 16.
Summary of outputs from the consensus meeting. CM, case management; MDA, multidisciplinary assessment.
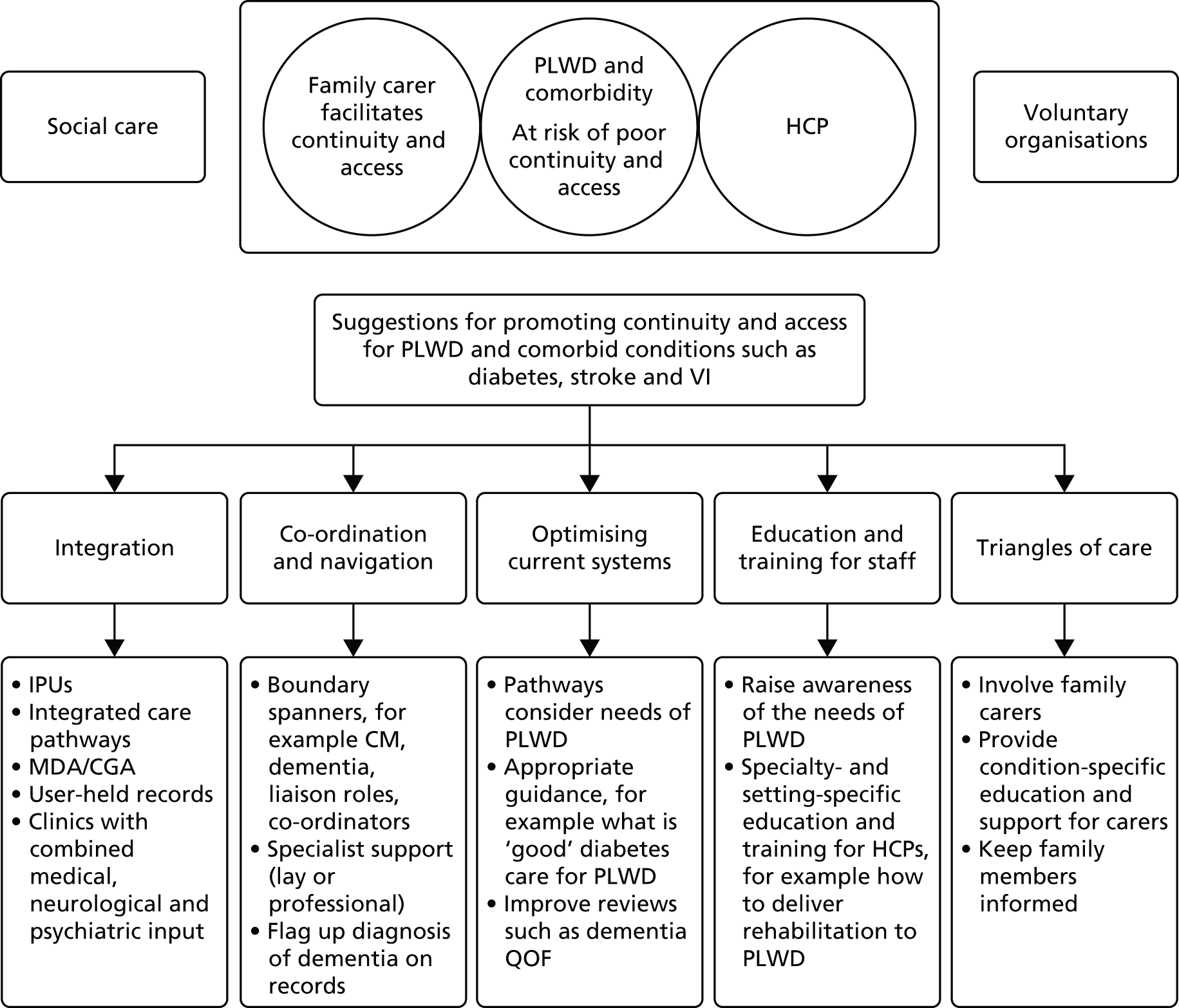
Although some of the models of care referred to by participants were specific to our three tracer conditions, many drew on similar concepts around integration, co-ordination, support for family carers and workforce development. However, evidence for many of the interventions identified, such as integrated or collaborative models of care, is currently limited, local and based on case study approaches. 206
Limitations
We focused on three tracer conditions and it is possible that our findings may not be applicable to people with dementia who have other long-term conditions such as arthritis or chronic obstructive pulmonary disease. However, there were many cross-cutting themes and issues across our three conditions that are likely to be generalisable to other comorbidities. The focus of our study was on single comorbidities rather than multimorbidity and we excluded those living in care homes where there are likely to be considerable levels of dementia and comorbidity. 207 The support that people with dementia and multiple health conditions need may be different from the support required by those with less complex needs. Moreover, our study did not distinguish between those people who developed a comorbidity in the presence of already diagnosed dementia and those who had a health condition such as diabetes and who then developed dementia. The profiles and needs of these groups may be different.
The scoping review
We undertook a scoping review rather than a systematic review. A limitation of such an approach is that the review did not include an assessment of the quality of the included studies or evaluate the effectiveness of interventions. However, evaluative research in this area is limited and our aim was to examine the extent, range and nature of research activity around patient and carer need, health-care provision and service organisation for people with dementia and comorbidity. Although we did not undertake formal quality assessment we did extract methodological information on study populations and data collection methods that aided in the interpretation of the evidence. We set out to include a representative rather than an exhaustive range of literature and it is possible that we have missed relevant studies or guidelines. However, our search strategy was guided by systematic review methodology and we employed extensive database and lateral searches. We are confident, therefore, that the scoping review provides a comprehensive summary of current evidence relating to dementia and comorbidity for people living in the community, particularly in relation to diabetes, stroke and VI.
The Cognitive Function and Ageing Study analysis
Both CFAS I and CFAS II were large population-based studies that included random samples of primary care lists representing institutions and community-living people aged ≥ 65 years. The advantage of this is that they provide an accurate representation of the population at both time points. Over the last two decades clinical consensus on the diagnosis of dementia has been prone to change but the use of the GMS-AGECAT algorithm to give a study diagnosis of dementia in both CFAS I and CFAS II means that the results from the two studies are directly comparable. Unfortunately, low numbers in the target health condition groups meant that formal testing of any changes between CFAS I and CFAS II was not possible. The main limitation of this phase of the study is that, in CFAS I, to conduct analysis on service use only the 10-year follow-up wave could be used as the questions on service use were only introduced at this stage. This automatically meant that only survivors at the 10-year follow-up were included in the analysis. As well as restricting the CFAS II comparison analysis to those aged ≥ 75 years, inverse probability weights for attrition were used for the CFAS I analysis. However, even with the use of weights there was still some uncertainty around the estimates that could not be accounted for because of the low numbers of people who had stroke, diabetes and VI.
The cross-sectional design of this study means that no direction of causality can be inferred. Although CFAS I does include longitudinal data, the rate of attrition at the 10-year follow-up, when the service use questions were introduced, resulted in numbers that were too low for longitudinal analysis to be carried out. Between CFAS I and CFAS II, non-response at baseline increased from 23% to 44%. Inverse probability weighting was introduced to account for this population representation at each time point. Because of the low numbers services were grouped together for a more general analysis, but a limitation of this is that hospital use data were collected for a different time period from data on the use of day patient or outpatient services. Item non-response meant that in certain cases the analysis for some of the services could not be carried out. If information was not available from the participant interviews the informant interviews were used to fill in gaps when possible. Although informants may not always give the same responses that participants with dementia would have given,208 this did still provide valuable extra information.
Interviews and focus groups
Our original intention was to recruit equal numbers of participants with stroke, diabetes and VI. However, we found it easier to identify and recruit people with dementia who had diabetes and more difficult to recruit people with dementia who had had a stroke. Furthermore, we recruited most participants through memory clinics or primary care and in most cases the stroke was not a recent event. Participants’ recall of the care and treatment that they had received may not have been completely accurate and/or may not reflect current practice in stroke care.
Although qualitative research does not generally set out to be representative, it is appropriate to consider the transferability of findings. HCPs in our study were a self-selecting sample who were willing to have their practice examined. As such, it is possible that they may have had more awareness of the needs of PLWD and more interest in their care needs than their colleagues. However, the accounts of HCPs were validated against the accounts of PLWD and their family carers.
Like much qualitative research with people with dementia,23 the majority (78%) of our participants with dementia lived with a family carer and we are able to say less about the experiences of those who live alone. Moreover, although we included two geographical areas, most participants lived in urban areas. PLWD in rural locations may face additional barriers to accessing services. The majority (72%) of PLWD were white and older people from other ethnic groups may have different experiences and needs. However, a previous systematic review of qualitative studies found that there were many similarities in the experiences of people with dementia and their family carers regardless of culture and context. 23 Our findings, therefore, should have resonance for the wider community of older people with dementia and comorbidity.
Implications of the findings
To our knowledge this is the first study to focus on research and practice in relation to comorbidity in dementia. Most research on multimorbidity has been concerned with its effect on prevalence and physical functioning and its measurement, with little research investigating patient and carer preferences and experiences, the effect on processes of care or what constitutes ‘best care’ for these patients. 22,59 Moreover, qualitative research on dementia to date has tended to focus on the experience of living with dementia as a single disease. 23 This study sets out current research and knowledge about the impact of comorbidities on people diagnosed with dementia and what is known about their experiences of health care. It highlights what experiences are common to PLWD and those with other complex health and social care needs. It also demonstrates how a diagnosis of dementia creates extra and different needs from those experienced by people with a long-term condition but without dementia. Based on these findings it is reasonable to argue that a service that is accessible and provides continuity of information and management for someone living with dementia and their carer is a service that could address the needs of all patients with a long-term condition and one that should be a benchmark of good practice.
Continuity of care
Our study supports previous research that relationship continuity and the personal characteristics of HCPs are important to older people with complex health needs209,210 and their family carers. 48 We found that, for PLWD, relationship continuity can facilitate access to some services, for example a patient may not automatically be taken off a clinic list if they miss an appointment or clinicians may more easily assess a patient’s progress. It has been argued that in some settings such as primary care there may be a trade-off between continuity and access,211 although people with chronic and serious conditions may be willing to trade off delays in access to routine care against increased continuity. 46
The estimated median survival time for people with a diagnosis of dementia in the UK is 4.5 years,58 but this may be greater, particularly in those who are diagnosed younger. 127 Many PLWD will have comorbid health conditions and, as such, are likely to require management continuity that spans a number of years. Despite this, PLWD and their family carers often experienced poor management and informational continuity. Of particular concern was that HCPs specialising in diabetes, stroke and VI were often unaware that someone had a diagnosis of dementia. In many cases our participants had a long-standing health condition before the development and diagnosis of dementia and this might explain why the diagnosis of dementia had not been transferred from one service to another. There may be a need for automated systems that trigger condition updates in clinics.
Access to care
It is well established that people with dementia are less likely than people without dementia to receive equivalent care for similar health conditions and experiences. Previous research has shown that PLWD may have poorer end-of-life care than those with conditions such as cancer. 162,212 There was evidence from the scoping review that people with dementia do not have the same access as those without dementia to treatment and monitoring for conditions such as VI and diabetes. This may be because people with dementia may be less likely to attend regular appointments or to notice or report relevant symptoms and they may be more reliant on carers to manage and facilitate appointments. 117 Our study supports the idea that people with dementia often lack candidacy and may be reliant on family carers to facilitate continuity and access. This clearly has implications for people with dementia who live alone or who do not have family support. Our study found that housebound patients and those who lived alone were a particular concern for GPs, who worried that they may be missing out on care. Although research suggests that people with dementia who live alone have an increased risk for unmet social, environmental, psychological and medical needs,213 we were unable to identify examples of research or practice that indicated how this problem was being addressed by different services.
Access to health care has been defined as the ‘degree of fit between the clients and the system’ (p. 128). 63 For people with dementia the degree of fit may be poor because systems and the clinical environment do not meet their needs, leading to the exacerbation of symptoms and distress. There may be transferable learning from services for people with learning disability, who are another group who have problems with access and continuity. 214 The key difference, however, is that for PLWD there is a history of having been able to manage independently and for people with a learning disability there is a formal recognition of their need for advocacy and support in the navigation of health care.
Conditions such as diabetes or VI may not be recognised in people with dementia because their symptoms are misinterpreted, especially if the person with dementia is experiencing the behavioural and psychological symptoms associated with dementia. 215,216 For example, people with dementia who develop diabetes may appear to have a worsening of their dementia because the symptoms of diabetes, such as confusion because of elevated blood glucose or incontinence, are wrongly attributed to dementia. 112 Problems such as falling or not recognising objects may be misinterpreted as signs of dementia, meaning that HCPs fail to investigate the possibility that they are the result of some form of VI. This is a major issue as some interventions, for example cataract operations, have the potential to improve quality of life and physical functioning for someone with dementia. 217
Clinicians may be more reluctant to investigate and treat VI in patients with dementia either because of the difficulties in securing patient co-operation or because treatments are considered inappropriate, or difficult to perform, in patients with dementia. 117 Clinicians in our study across the three conditions expressed concerns about compliance, consent and the appropriateness of treatment in older people with dementia, especially for those with multimorbidity or more advanced dementia. For conditions such as VI it would be better to treat people with dementia earlier rather than later and there may be an argument for fast-tracking people with dementia for treatments such as surgery for cataracts. Our study also suggests that there is a need for further research that addresses how rehabilitation might be delivered to people with dementia who have had a stroke, for example including family carers218 or adjusting rehabilitation strategies for people with dementia.
Initiatives to improve continuity and access for people with dementia and comorbidities
Hospital-based initiatives
In the scoping review (see Chapter 4) we considered some of the initiatives that have been developed with the aim of improving care for older people with dementia in acute hospitals. This includes liaison psychiatric services113 and specialist units that combine medical and mental health care for older people. 109 As yet, it is not clear if specialist units lead to better outcomes for PLWD or if these are the most appropriate care environments for PLWD who have acute care needs. For example, a person with dementia being admitted after a stroke may need to be in an acute stroke unit, or a person with dementia undergoing surgery for cataracts may need to be on an ophthalmology ward. The challenge from this study’s findings is how to make all staff practice and health-care environments appropriate for PLWD as well as investing in specialist dementia services to ensure that the behavioural and psychological symptoms of dementia are addressed within mainstream health-care provision.
Dementia-friendly environments
Recent years have seen a growing interest in initiatives designed to make health-care environments more dementia friendly. The UK government recently spent £50M on 116 projects aimed at improving environments for people with dementia in NHS and local authority settings. 219 There are a variety of tools and resources designed to improve the identification of people with dementia in busy acute environments and make health-care settings dementia friendly (e.g. the Butterfly Scheme,220 the Shining the Spotlight programme,221 the Pabulum Blue Book,222 ‘This is me’, Carer’s Passport,223,224 trust dementia portals). However, there have been few evaluations of their effectiveness and we found no evidence to suggest that these initiatives were being used in specialist services for diabetes, stroke or VI.
Ideas on person-centred care49,54,225 have had a considerable impact on approaches to the care of people with dementia in health-care settings. However, despite policy support for person-centred approaches to care for people with cognitive impairment,55,226 health services have struggled to deliver it. 227 The aim of initiatives such as ‘Getting to know me’ [see www.scottishcare.org/news/getting-to-know-me-dementia-resource-launched/ (accessed 5 January 2016)] or ‘This is me’ is to encourage health and social care professionals to see the person with dementia as an individual and deliver person-centred care that is tailored specifically to the person’s needs. 228 Although a number of HCPs in our study were aware of the ‘This is me’ tool, very few PLWD and their carers were actively using it. Moreover, dementia specialists reported that they were often not properly filled in. It was not clear from this study why such tools were not being used and more work is needed on their evaluation and promotion.
Holistic care
One approach that has been suggested for delivering holistic, integrated care to PLWD is collaborative care or case management. 229 In this approach a care/case manager co-ordinates care between professionals, liaises between primary and secondary care and utilises evidence-based care pathways to address both physical and psychosocial needs. Despite positive benefits for both PLWD and their carers in some individual trials,230 recent systematic reviews have found little clinical effectiveness or cost-effectiveness evidence to support widespread case management implementation beyond some quality-of-life benefits. 186,188,189 A recent pilot trial to evaluate the feasibility of case management in the UK struggled to successfully implement a nurse/social worker case manager intervention despite considerable support for this care approach from both patients and primary care teams. 190 However, this was largely because of the study coinciding with considerable organisational change within primary care. 231 In view of our findings of increasing physical comorbidity in people with dementia, such a model may require further testing as similar European health-care systems have managed successful integration into existing care. 192
Workforce development
In the UK, most chronic illness care of PLWD will be provided by GPs and their primary care teams. However, the 2007 National Audit Office report on improving services for PLWD232 found that, in its national GP survey, less than one-third of GPs felt that they had sufficient training to provide care for PLWD. Despite our rapidly ageing society, even current generations of doctors are not being adequately prepared to meet patient population needs, as medical student surveys have shown. 233 A recent recommendation from the Royal College of General Practitioners234 that GP training be extended to 4 years, to specifically include care of older people and management of multimorbidity, has not yet been implemented.
Despite a national policy push on dementia care through a national strategy25 and the Prime Minister’s dementia challenge,235 it would seem that we are still not adequately training our key community-based HCPs to meet societal need. 206 HCPs in our study consistently reported feeling underprepared to care for people with dementia. They raised concerns about generic issues such as behavioural and psychological symptoms but also condition-specific concerns, for example the best strategies for performing eye tests for PLWD, the best medication regimens for people with dementia and diabetes and approaches to rehabilitation for people with dementia who had had a stroke. This is consistent with a recent survey of dementia champions which suggests that dementia training should be specific to the clinical setting. 204
An assessment of dementia care between 2009 and 2014206 suggests that, although training initiatives such as that delivered via Health Education England236 and the Royal Colleges have led to improvements in care, more work needs to be carried out to improve training and education curricula. An audit of training in Hertfordshire and Bedfordshire found that uptake of dementia training may be greater among more junior staff, who have the most patient contact but who may lack authority to change practices. 204 Senior clinicians in our study showed an awareness of the needs of PLWD but many of the suggestions that they made for improving care for PLWD were aspirational rather than routinely implemented in current practice. It would appear that initiatives to increase staff awareness of dementia need to be further developed so that the needs of someone with a comorbid condition and dementia can be routinely accommodated into assessment, planning and review of care across all services.
The management of risk
There is a great deal of literature on the management of risk in dementia, particularly for those who are living independently at home. 237 For people with a comorbid condition such as diabetes, VI or stroke, the risk is even greater. Older people with diabetes and dementia are at greater risk of hypoglycaemia than older people without dementia. 238 Family carers of people with dementia and diabetes reported a number of ways in which dementia impacted on blood sugar control, resulting in hypoglycaemia and, in some instances, hospitalisation. This included family members with dementia forgetting that they had already eaten and eating twice or forgetting to eat at all. Carers also reported instances in which family members had forgotten to take medication or had taken medication (including insulin) more than once. The management of risk was a common theme in HCPs’ narratives, generally expressed in the context of uncertainty about what would be best for the patient, the family carer and the service. A more structured approach to the assessment of risk is needed that can help professionals focus on the PLWD and consider systematically the risk–benefit balance of different treatment options. The study findings suggested that at the moment this is very much at the discretion of the individual practitioner.
Carer inclusion and support
The CFAS analysis showed an increase in use of hospital services and unpaid care by people with dementia and a comorbid condition over the past decade. Analysis within CFAS II only showed that unpaid care was used substantially more by those with dementia and a target health condition than by those with the health condition alone. Previous analysis in CFAS has shown that fewer people are moving into care settings,164 which could, in turn, influence the use of unpaid care. It is well documented that those with dementia and their carers have difficulties with accessing the support that they need day to day. 239,240 This lack of appropriate community-based support could mean that individuals access health services at only their greatest times of need or crisis and may explain the increased hospital use. Some of the greatest barriers reported in terms of access to day-to-day services were the lack of communication between health and social care and a lack of formal training on dementia care available to those in community-based day-to-day services. Suggestions on how to improve access to day-to-day services included education and training on the impact of dementia on the management of other health conditions. 241
The challenges that carers of PLWD face are well documented. 23,242 Family caregivers of people who have dementia and other health conditions face additional challenges in managing both conditions and dealing with the impact of the accompanying behavioural and psychological symptoms of dementia on care routines. 102 Our study suggests that care pathways need to recognise the contribution of family members and, when available, incorporate their contribution in the care of PLWD. Pathways should include appropriate support for carers that addresses the possibility that carers do not want or cannot absorb the extra responsibilities of caring for someone with a condition such as diabetes. This support might need to focus on times of transition, for example from hospital to home after a stroke, or on the transition from self-management to dependency for a person with a long-term condition such as diabetes. Although services such as those provided by Admiral Nurses focus on the support of carers, there is relatively little understanding of which models of support are likely to be most effective or how they should be evaluated. 48 Moreover, it is not clear whether or not such services would be able to provide the appropriate support for carers of PLWD and a physical comorbidity such as diabetes or VI.
There is an increasing recognition of the importance of strengthening relationships between patients, carers and professionals. 243,244 In the USA the Institute for Patient- and Family-Centered Care245 set out four core concepts of patient- and family-centred care: dignity, respect, information sharing, and participation and collaboration. Its approach is based on the premise that families are crucial allies for quality and safety and that the individuals most dependent on health care, such as the very old and those with chronic conditions, are also those who are most dependent on families.
In the UK the triangle of care model50 focuses on carer inclusion and support. Originally developed for mental health service users the model has been adapted to meet the needs of carers of people with dementia in acute hospital. The model (Box 9) requires that staff are willing to collaborate and engage with PLWD and their family carers and involve the carers in decisions about the care and treatment of their relative. Although HCPs in our study acknowledged the importance of family carers, they did not generally articulate this in a way that addressed the clear expectations outlined in the triangle of care model. The model requires further testing and development, specifically on how it is implemented and how it can address issues such as confidentiality, the involvement of multiple carers or divergence of opinion between the PLWD and the carer. It also cannot address the needs of someone whose carer network involves loose ties or shifting patterns of unpaid support.
-
Carers and the essential role that they play are identified at first contact or as soon as possible thereafter.
-
Staff are ‘carer aware’ and trained in carer engagement strategies.
-
Policy and practice protocols regarding confidentiality and sharing information are in place.
-
Defined post(s) responsible for carers are in place.
-
A carer introduction to the service and staff is available, with a relevant range of information across the care pathway.
-
A range of carer support services is available.
Conclusions
Significant numbers of PLWD have comorbid conditions such as stroke, diabetes and VI and many of them have multimorbidities. The presence of dementia complicates the delivery of health care and magnifies the known difficulties that people with long-term conditions experience when navigating health and social care. The situation is further complicated as some people will develop comorbidity in the presence of already diagnosed dementia and others will develop dementia subsequent to a comorbidity such as diabetes, stroke or VI.
The delivery of good-quality care to PLWD demands a particularly high standard of care across multiple domains, including communication, multidisciplinary care, clinical decision-making and engagement with families and carers. Effective care for older patients with dementia will help set a standard of care of universal relevance to vulnerable adults. 246 Good care for PLWD and comorbidity may vary according to the type of condition(s) that they have. However, key elements include the PLWD and the family carer at the centre, flexibility around processes, good communication between services, ensuring that all services are aware when someone has a diagnosis of dementia and taking into account the impact of a diagnosis of dementia on pre-existing conditions, and incorporating this into guidelines and care planning.
The role of family carers in managing health-care conditions of PLWD and their contribution in facilitating continuity and access to care are indisputable. It is important, therefore, that HCPs conceptualise the provision of care for people with dementia and a comorbidity as a complex phenomenon that affects not just individuals but also dyads and families. The challenges of being a carer have been exhaustively documented, and legislation and staff training to ensure that carers are recognised and supported has been in place for some time. What is not so well understood is how to involve and support family carers at different stages of the dementia trajectory.
People with dementia may have poorer access to services than people with the same comorbidities but without dementia. There appears to be a number of reasons for this. One reason is a poor fit with current pathways and services. Despite an increasing range of initiatives to make health-care environments more ‘dementia friendly’, it is clear that many health-care environments do not meet the needs of PLWD and their family carers and the impact of these initiatives on patient and carer outcomes is unclear. Furthermore, we found no evidence to suggest that they are being widely used in clinical environments for conditions such as diabetes, stroke and VI. Access to care is also affected by the way that HCPs balance the risks and benefits of treatments for PLWD. Clearly, HCPs will sometimes have legitimate concerns about the risks of interventions or treatments for PLWD but it has been suggested that the construction and definition of risk by health and social care professionals may be different from the perceptions of PLWD and their family carers. 247 Moreover, there is a need to address negative perceptions about PLWD that may impact on how they are perceived and treated. 248
There is already a great deal of descriptive work on the experiences of PLWD and their family carers. 23 This study adds to that by providing information about the experiences of people with dementia and comorbid health conditions, the views of HCPs caring for them and how dementia impacts on the management of comorbid health conditions in PLWD. It also provides data on the prevalence of comorbidities and service use among people with dementia and comorbidities. We provide a number of ideas/recommendations for practice and research, some of which are generic to people with dementia and comorbidity and some of which are condition specific. We suggest that future work needs to focus on the development and evaluation of interventions rather than on further descriptive studies. PLWD should be included in the debate about the management of comorbidities in older populations and there needs to be greater consideration given to including them in studies that focus on age-related health-care issues.
Implications for practice
-
The evidence suggests that the use of models such as the triangle of care model may be helpful in ensuring that the input of family carers is properly recognised. This should include the identification of family carers, appropriate training in carer engagement for staff, and policy and practice protocols regarding confidentiality and information sharing.
-
Our study suggests that systems for booking appointments need to be made more ‘dementia friendly’, for example not booking appointments by telephone, sending reminders and including nominated family carers in all correspondence (this may not be the primary carer if the primary carer is a spouse with memory problems).
-
The evidence suggests that staff at all levels, including more senior staff, need appropriate training on dementia. Some training may need to be tailored to specific conditions, for example identifying the best strategies for the rehabilitation of PLWD who have had a stroke.
-
Professional bodies for HCPs may need to consider how the current provision of dementia training on undergraduate programmes can be improved.
-
HCPs in specialist areas are often unaware that someone has dementia. Our evidence suggests that a diagnosis of dementia should be flagged up on medical/electronic records. This should include systems for automatic updates of a dementia diagnosis to be transferred to health-care services that the PLWD is already attending.
-
PLWD who live alone or who do not have family support may be particularly disadvantaged and may need additional help to navigate systems and access care.
-
PLWD are likely to benefit from longer appointments, both in primary and in secondary care.
-
PLWD may need a suitably trained staff member to help them navigate clinic environments; they may also benefit from assessments being carried out by specialists in their own home or at their local GP surgery.
-
HCPs caring for people with cognitive impairment and long-term conditions such as diabetes need to regularly assess patients’ ability to self-manage and identify when they may need additional support.
-
Evidence suggests that there is a need for better integration of physical and mental health-care systems, that is, old-age psychiatry teams and geriatric teams working together and community-based geriatric teams having specialist mental health as an integral part of the team.
-
Our study suggests that, for people with dementia and diabetes, who need support from health and social care, there is a need to link medication and monitoring of diabetes with the provision of meals.
-
The evidence suggests that people with dementia and diabetes may not be undergoing regular eye and foot checks.
Suggestions for future research
Potential areas for future research identified by the study include the following:
-
What makes a ‘good’ dementia QOF, for example what components should be routinely included and should they include physical health checks?
-
What is the impact of providing PLWD and their family carers with support, either from a professional or from a trained lay person, to help navigate health-care systems?
-
What are the impacts on PLWD, their family carers and other HCPs of specialist dementia nurses, such as Admiral Nurses, working in hospital and community settings?
-
Is a collaborative care approach, with a case manager to provide integrated physical and psychological care, an effective approach to the provision of dementia care for people with dementia and comorbidities and which population of PLWD are most likely to benefit from case management and at what stage?
-
What is the impact of dementia case finding for older people with stroke, diabetes and VI, for example the use of case finding on admission to hospital or at the first clinic appointment?
-
What is the impact of expanding the ‘This is me’ document to include health-related information?
-
How can patients, carers and HCPs be encouraged to use the ‘This is me’ document?
-
What interventions can be used to improve medication management in PLWD, for example what is the impact of pharmacists carrying out short cognitive screening on older patients with multiple medications?
-
Diabetes – how can HCPs caring for people with long-term conditions and dementia be helped to recognise when a person is no longer able to self-manage?
-
Diabetes – what is the impact of self-management interventions for diabetes that involve family carers of adults with diabetes and cognitive impairment?
-
Diabetes – what is the impact of personalised glycaemic targets for PLWD on outcomes such as hypoglycaemic attacks, hospital admissions and falls?
-
Stroke – what are the most effective and cost-effective approaches to stroke rehabilitation for people with dementia?
-
VI – how can tests for VI be made appropriate or adapted for PLWD?
-
VI – is it possible to fast track PLWD for treatment such as surgery for cataracts and if so what are the impacts of this?
-
VI – how can ophthalmology clinics and other health-care environments be made to be more dementia friendly so that they are suitable for people with VI or sight loss as well as dementias?
Acknowledgements
The authors would like to thank the following:
-
Dr Simon Conroy, Dr Catherine Dennison, Professor Murna Downs, Heather Maggs, Dr James Pickett and Dr Jill Rasmussen – members of the Project Advisory Group
-
Dr Vina Mayor, Marion Cowe, Dr Paul Millac and Diane Munday – members of the Public Involvement in Research Group at the University of Hertfordshire who acted as the User Reference Group for the study and attended the consensus conference
-
HCPs who took part in our interviews and focus groups and those who attended the consensus conference
-
Reinhard Wentz (Information Scientist) for advice on the search strategy for the scoping review.
This report presents independent research commissioned by the National Institute for Health Research (NIHR). The views and opinions expressed by the authors in this publication are those of the authors and do not necessarily reflect those of the NHS, NIHR, NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC), Health Services and Delivery Research programme or Department of Health. The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, the NHS, the NIHR, the Medical Research Council (MRC), the Central Commissioning Facility, NETSCC, the Health Services and Delivery Research programme or the Department of Health.
Contributions of authors
Frances Bunn, Claire Goodman, Louise Robinson, Greta Rait, Sam Norton, Johan Schoeman and Carol Brayne wrote the protocol. Frances Bunn, Anne-Marie Burn and Marie Poole undertook the analysis of the qualitative data. Sam Norton and Holly Bennett undertook the analysis and writing up of the CFAS data. Carol Brayne is the principal investigator on the CFAS study that generated the data for analysis. Frances Bunn and Anne-Marie Burn wrote the first draft of the report. Claire Goodman, Louise Robinson, Greta Rait, Sam Norton, Holly Bennett and Carol Brayne contributed to the writing of the report.
All authors critically reviewed the manuscript and agreed the final version.
Publications
Bunn F, Burn A-M, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med 2014;12:192.
Bunn F, Goodman C, Burn A-M. Multimorbidity and frailty in people with dementia. Nurs Standard 2015;30:45–50.
Data sharing statement
Data from the MRC CFAS studies are available on request. Information on the data available and the process for requesting MRC CFAS data are provided at www.cfas.ac.uk (accessed 9 December 2015). Other data can be obtained from the corresponding author subject to safeguarding the confidentiality and anonymity of participants.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Doraiswamy PM, Leon J, Cummings JL, Marin D, Neumann PJ. Prevalence and impact of medical comorbidity in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 2002;57:M173-7. http://dx.doi.org/10.1093/gerona/57.3.M173.
- Schubert CC, Boustani M, Callahan CM, Perkins AJ, Carney CP, Fox C, et al. Comorbidity profile of dementia patients in primary care: are they sicker?. J Am Geriatr Soc 2006;54:104-9. http://dx.doi.org/10.1111/j.1532-5415.2005.00543.x.
- Banerjee S. Multimorbidity – older adults need health care that can count past one. Lancet 2014;385:587-9. http://dx.doi.org/10.1016/S0140-6736(14)61596-8.
- Guthrie B, Payne K, Alderson P, McMurdo ME, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6341.
- World Alzheimer’s Report 2010. The Global Economic Impact of Dementia. London: Alzheimer’s Disease International; 2010.
- Luengo-Fernandez R, Leal J, Gray A. Alzheimer’s Research Trust Dementia 2010: The Economic Burden of Dementia and Associated Research Funding in the United Kingdom 2010. www.alzheimersresearchuk.org/wp-content/uploads/2015/01/Dementia2010Full.pdf (accessed 28 May 2015).
- Summary of Key Findings: a Report to the Alzheimer’s Society on the Prevalence and Economic Cost of Dementia in the UK Produced by King’s College London and London School of Economics. London: Alzheimer’s Society; 2007.
- Dementia UK Update. London: Alzheimer’s Society; 2014.
- National Audit of Dementia Care in General Hospitals 2012–13: Second Round Audit Report and Update. London: Healthcare Quality Improvement Partnership; 2013.
- Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 2009;7:357-63. http://dx.doi.org/10.1370/afm.983.
- Kuo T-C, Zhao Y, Weir S, Kramer MS, Ash AS. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care 2008;46:839-46. http://dx.doi.org/10.1097/MLR.0b013e318178940b.
- Heun R, Schoepf D, Potluri R, Natalwala A. Alzheimer’s disease and co-morbidity: increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry 2013;28:40-8. http://dx.doi.org/10.1016/j.eurpsy.2011.06.001.
- Leon J, Cheng CK, Neumann PJ. Alzheimer’s disease care: costs and potential savings. Health Aff (Millwood) 1998;17:206-16. http://dx.doi.org/10.1377/hlthaff.17.6.206.
- Poblador-Plou B, Calderon-Larranaga A, Marta-Moreno J, Hancco-Saavedra J, Sicras-Mainar A, Soljak M, et al. Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry 2014;14. http://dx.doi.org/10.1186/1471-244X-14-84.
- Richards M, Brayne C. What do we mean by Alzheimer’s disease?. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4670.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788-94. http://dx.doi.org/10.1016/S1474-4422(14)70136-X.
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819-28. http://dx.doi.org/10.1016/S1474-4422(11)70072-2.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64-7. http://dx.doi.org/10.1016/S1474-4422(05)70284-2.
- Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565-72. http://dx.doi.org/10.1001/jama.2009.460.
- Hewitt J, Smeeth L, Chaturvedi N, Bulpitt CJ, Fletcher AE. Self management and patient understanding of diabetes in the older person. Diabet Med 2010;28:117-22. http://dx.doi.org/10.1111/j.1464-5491.2010.03142.x.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. Diabetes Res Clin Pract 2000;50:203-12. http://dx.doi.org/10.1016/S0168-8227(00)00195-9.
- Fortin M, Soubhi H, Hudon C, Bayliss EA, Van Den Akker M. Multimorbidity’s many challenges. BMJ 2007;334:1016-17. http://dx.doi.org/10.1136/bmj.39201.463819.2C.
- Bunn F, Goodman C, Sworn K, Rait G, Brayne C, Robinson L, et al. Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: a systematic review of qualitative studies. PLOS Med 2012;9. http://dx.doi.org/10.1371/journal.pmed.1001331.
- Iliffe S, Manthorpe J, Eden A. Sooner or later? Issues in the early diagnosis of dementia in general practice: a qualitative study. Fam Pract 2003;20:376-81. http://dx.doi.org/10.1093/fampra/cmg407.
- National Dementia Strategy. London: HMSO; 2009.
- Report of the National Audit of Dementia Care in General Hospitals. London: Healthcare Quality Improvement Partnership; 2011.
- Who Cares Wins, Improving the Outcome for Older People Admitted to the General Hospital. Guidelines for the Development of Liaison Mental Health Services for Older People. London: Royal College of Psychiatrists; 2005.
- Prime Minister’s Challenge on Dementia: Delivering Major Improvements in Dementia Care and Research by 2015. London: Department of Health; 2012.
- Final Report from the MAGDR Subgroup 1 on Priority Topics in Dementia Research, February 2011. London: Medical Research Council; 2011.
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h176.
- Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ 2003;327:1219-21. http://dx.doi.org/10.1136/bmj.327.7425.1219.
- Fulop N, Allen P. National Listening Exercise: Report of the Findings. London: NHS Service Delivery and Organisation; 2000.
- Parker G, Corden A, Heaton J. Synthesis and Conceptual Analysis of the SDO Programme’s Research on Continuity of Care. Report for the National Institute for Health Research Service Delivery and Organisation Programme. Southampton: National Institute for Health Research Service Delivery and Organisation programme; 2009.
- Gulliford M, Figueroa-Munoz J, Morgan M, Hughes D, Gibson B, Beech R, et al. What does ‘access to health care’ mean?. J Health Serv Res Policy 2002;7:186-8. http://dx.doi.org/10.1258/135581902760082517.
- Van Walraven C, Oake N, Jennings A, Forster AJ. The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract 2010;16:947-56. http://dx.doi.org/10.1111/j.1365-2753.2009.01235.x.
- Björkelund C, Maun A, Murante AM, Hoffmann K, De Maeseneer J, Farkas-Pall Z. Impact of continuity on quality of primary care: from the perspective of citizens’ preferences and multimorbidity – position paper of the European Forum for Primary Care. Qual Prim Care 2013;21:193-204.
- Salisbury C, Goodall S, Montgomery AA, Pickin DM, Edwards S, Sampson F, et al. Does advanced access improve access to primary health care? Questionnaire survey of patients. Br J Gen Pract 2007;57:615-21.
- Nutting PA. Continuity of primary care: to whom does it matter and when?. Ann Fam Med 2003;1:149-55. http://dx.doi.org/10.1370/afm.63.
- Pratt R, Clare L, Kirchner V. ‘It’s like a revolving door syndrome’: professional perspectives on models of access to services for people with early-stage dementia. Aging Ment Health 2006;10:55-62. http://dx.doi.org/10.1080/13607860500307530.
- Freeman GK, Woloshynowych M, Baker R, Boulton M, Guthrie B, Car J, et al. Continuity of Care 2006: What Have We Learned since 2000 and What Are Policy Imperatives Now. London: National Coordinating Center for NHS Service Delivery and Organisation R&D; 2007.
- Guthrie B, Saultz JW, Freeman GK, Haggerty JL. Continuity of care matters. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a867.
- Goodman C. The organisational culture of nursing staff providing long-term dementia care is related to quality of care. Evid Based Nurs 2011;14:88-9. http://dx.doi.org/10.1136/ebn1158.
- Ellins J, Glasby J, Tanner D, McIver S, Davidson D, Littlechild R, et al. Understanding and Improving Transitions of Older People: a User and Carer Centred Approach. Final Report. Southampton: National Institute for Health Research Service Delivery and Organisation programme; 2012.
- Goodman C, Drennan V, Manthorpe J, Gage H, D. T, Shah D, et al. A Study of the Effectiveness of Inter Professional Working for Community Dwelling Older People. Southampton: National Institute for Health Research Service Delivery and Organisation programme; 2012.
- Heaton J, Corden A, Parker G. ‘Continuity of care’: a critical interpretive synthesis of how the concept was elaborated by a national research programme. Int J Integr Care 2012;12.
- Parker G, Corden A, Heaton J. Experiences of and influences on continuity of care for service users and carers: synthesis of evidence from a research programme. Health Soc Care Community 2011;19:576-601. http://dx.doi.org/10.1111/j.1365-2524.2011.01001.x.
- Spenceley SM, Sedgwick N, Keenan J. Dementia care in the context of primary care reform: an integrative review. Aging Ment Health 2015;19:107-20. http://dx.doi.org/10.1080/13607863.2014.920301.
- Bunn F, Goodman C, Pinkney E, Drennan V. Specialist nursing and community support for the carers of people with dementia living at home: an evidence synthesis [published online ahead of print 16 February 2015]. Health Soc Care Community 2015. http://dx.doi.org/10.1111/hsc.12189.
- Brooker D. Person-Centred Dementia Care: Making Services Better. London: Jessica Kingsley; 2007.
- Hannan R, Thompson R, Worthington A, Rooney P. The Triangle of Care. Carers Included: A Guide to Best Practice for Dementia Care. London: Carers Trust; 2013.
- Adams T, Gardiner P. Communication and interaction within dementia care triads: developing a theory for relationship-centred care. Dementia 2005;4:185-20. http://dx.doi.org/10.1177/1471301205051092.
- National Quality Forum . National Voluntary Consensus Standards for Coordination of Care Across Episodes of Care and Care Transitions 2012. www.qualityforum.org/Projects/c-d/Care_Coordination_Endorsement_Maintenance/Care_Coordination_Endorsement_Maintenance.aspx (accessed 22 December 2015).
- Our NHS Our Future: NHS Next Stage Review – Interim Report. London: Department of Health; 2007.
- Brooker D. What is person-centred care in dementia?. Rev Clin Gerontol 2004;13:215-22. http://dx.doi.org/10.1017/S095925980400108X.
- Living Well with Dementia: A National Dementia Strategy. London: Department of Health; 2009.
- Bodenheimer T. Coordinating care – a perilous journey through the health care system. N Engl J Med 2008;358:1064-71. http://dx.doi.org/10.1056/NEJMhpr0706165.
- Crooks VA, Agarwal G. What are the roles involved in establishing and maintaining informational continuity of care within family practice? A systematic review. BMC Fam Pract 2008;9. http://dx.doi.org/10.1186/1471-2296-9-65.
- Xie J, Brayne C, Matthews FE. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 2008;336:258-62. http://dx.doi.org/10.1136/bmj.39433.616678.25.
- Bunn F, Burn A-M, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med 2014;12. http://dx.doi.org/10.1186/s12916-014-0192-4.
- Kringos DS, Boerma WG, Hutchinson A, van der Zee J, Groenewegen PP. The breadth of primary care: a systematic literature review of its core dimensions. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-65.
- Benett IJ. Access, continuity, or both. Br J Gen Pract 2014;64:388-9. http://dx.doi.org/10.3399/bjgp14X680845.
- Gulliford M. Modernizing concepts of access and equity. Health Econ Policy Law 2009;4:223-30. http://dx.doi.org/10.1017/S1744133109004940.
- Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care 1981;19:127-40. http://dx.doi.org/10.1097/00005650-198102000-00001.
- Gulliford M, Morgan M. Access to Health Care. London: Routledge; 2013.
- McIntyre D, Thiede M, Birch S. Access as a policy-relevant concept in low- and middle-income countries. Health Econ Policy Law 2009;4:179-93. http://dx.doi.org/10.1017/S1744133109004836.
- Dixon-Woods M, Cavers D, Agarwal S, Annandale E, Arthur A, Harvey J, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-35.
- Mackenzie M, Conway E, Hastings A, Munro M, O’Donnell C. Is ‘candidacy’ a useful concept for understanding journeys through public services? A critical interpretive literature synthesis. Soc Policy Adm 2013;47:806-25. http://dx.doi.org/10.1111/j.1467-9515.2012.00864.x.
- Fagot-Campagna A, Bourdel-Marchasson I, Simon D. Burden of diabetes in an aging population: prevalence, incidence, mortality, characteristics and quality of care. Diabetes Metab 2005;31:5S35-52.
- Pendlebury S, Rothwell P. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8:1006-18. http://dx.doi.org/10.1016/S1474-4422(09)70236-4.
- Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484-91. http://dx.doi.org/10.1111/j.1445-5994.2012.02758.x.
- Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4904.
- Lawrence V, Murray J, Ffytche D, Banerjee S. Out of sight, out of mind: a qualitative study of visual impairment and dementia from three perspectives. Int Psychogeriatr 2009;21:511-18. http://dx.doi.org/10.1017/S1041610209008424.
- Trigg R, Jones R. Dementia and Blindness. Research findings no. 6. London: Thomas Pocklington Trust; 2005.
- McKeefry D, Bartlett R. Improving Vision and Eye Health Care to People With Dementia. Research discussion paper no. 8. London: Thomas Pocklington Trust; 2010.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19-32. http://dx.doi.org/10.1080/1364557032000119616.
- Levac D, Colquhoun H, O’Brien K. Scoping studies: advancing the methodology. Implement Sci 2010;5:1-9. http://dx.doi.org/10.1186/1748-5908-5-69.
- Rumrill PD, Fitzgerald SM, Merchant WR. Using scoping literature reviews as a means of understanding and interpreting existing literature. Work 2010;35:399-404. http://dx.doi.org/10.3233/WOR-2010-0998.
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91-108. http://dx.doi.org/10.1111/j.1471-1842.2009.00848.x.
- Bunn F, Goodman C, Brayne C, Norton S, Rait G, Robinson L, et al. Comorbidity and Dementia: Improving Healthcare for People with Dementia (CoDem). Southampton: National Institute for Health Research Health Services and Delivery Research programme; 2012.
- Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 2005;331:1064-5. http://dx.doi.org/10.1136/bmj.38636.593461.68.
- Copeland JRM, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med 1986;16:89-9. http://dx.doi.org/10.1017/S0033291700057779.
- Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks, CA: Sage Publications; 1996.
- Barbour RS. Checklists for improving rigour in qualitative research: a case of the tail wagging the dog?. BMJ 2001;322:1115-17. http://dx.doi.org/10.1136/bmj.322.7294.1115.
- Delbecq A, Van de Ven A. A group process model for problem identification and program planning. J Appl Behav Sci 1971;7:466-92. http://dx.doi.org/10.1177/002188637100700404.
- Bartunek JM, Murninghan JK. The nominal group technique: expanding the basic procedure and underlying assumptions. Group Organ Manag 1984;9:417-32. http://dx.doi.org/10.1177/105960118400900307.
- Evidence for the Safety and Quality Issues Associated with the Care of Patients with Cognitive Impairment in Acute Care Settings: a Rapid Review. Sydney, NSW: Australian Commission on Safety and Quality in Health Care; 2013.
- Allan K, Stapleton K, McLean F. Dementia and Deafness: An Exploratory Study. Stirling: Queensbury House Trust and the Scottish Executive; 2005.
- Balfour J, O’Rourke N. Older adults with Alzheimer disease, comorbid arthritis and prescription of psychotropic medications. Pain 2003;8:198-204.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. http://dx.doi.org/10.1016/S0140-6736(12)60240-2.
- Bartlett A, Clarke B. An exploration of healthcare professionals’ beliefs about caring for older people dying from cancer with a coincidental dementia. Dementia 2012;11:559-65. http://dx.doi.org/10.1177/1471301212437824.
- Bartlett R, McKeefry D. Rethinking the experiences and entitlements of people with dementia: taking vision into account. J Care Serv Manag 2011;5:105-14. http://dx.doi.org/10.1179/175016811X12966389037637.
- Bayer AJ, Johnston J, Sinclair AJ. Impact of dementia on diabetic care in the aged. J R Soc Med 1994;87:619-21.
- Bruce DG, Casey GP, Grange V, Clarnette RC, Almeida OP, Foster JK, et al. Cognitive impairment, physical disability and depressive symptoms in older diabetic patients: the Fremantle Cognition in Diabetes Study. Diabetes Res Clin Pract 2003;61:59-67. http://dx.doi.org/10.1016/S0168-8227(03)00084-6.
- Examining the Patient with Dementia or other Acquired Cognitive Impairment. London: College of Optometrists; 2012.
- Connolly A, Iliffe S, Gaehl E, Campbell S, Drake R, Morris J, et al. Quality of care provided to people with dementia: utilisation and quality of the annual dementia review in general practice. Br J Gen Pract 2012;62:e91-8. http://dx.doi.org/10.3399/bjgp12X625148.
- Curtis LH, Hammill BG, Qualls LG, Dimartino LD, Wang F, Schulman KA, et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 Medicare beneficiaries. Am J Opthalmol 2012;153:1116-24. http://dx.doi.org/10.1016/j.ajo.2011.11.032.
- Dewing J, Dijk S. What is the current state of care for older people with dementia in general hospitals? A literature review [published online ahead of print 23 January 2014]. Dementia (London) 2014. http://dx.doi.org/10.1177/1471301213520172.
- Doucet J, Druesne L, Capet C, Gréboval E, Landrin I, Moirot P, et al. Risk factors and management of diabetes in elderly French patients. Diabetes Metab 2008;34:574-80. http://dx.doi.org/10.1016/j.diabet.2008.05.007.
- Feil D, Marmon T, Unutzer J. Cognitive impairment, chronic medical illness, and risk of mortality in an elderly cohort. Am J Geriatr Psychiatry 2003;11:551-60. http://dx.doi.org/10.1097/00019442-200309000-00010.
- Feil DG, Lukman R, Simon B, Walston A, Vickrey B. Impact of dementia on caring for patients’ diabetes. Aging Ment Health 2011;15:894-903. http://dx.doi.org/10.1080/13607863.2011.569485.
- Feil DG, Pearman A, Victor T, Harwood D, Weinreb J, Kahle K, et al. The role of cognitive impairment and caregiver support in diabetes management of older outpatients. Int J Psychiatry Med 2009;39:199-214. http://dx.doi.org/10.2190/PM.39.2.h.
- Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with type 2 diabetes. J Behav Med 2011;35:190-9. http://dx.doi.org/10.1007/s10865-011-9344-6.
- Formiga F, Fort I, Robles MJ, Riu S, Sabartes O, Barranco E, et al. Comorbidity and clinical features in elderly patients with dementia: differences according to dementia severity. J Nutr Health Aging 2009;13:423-7. http://dx.doi.org/10.1007/s12603-009-0078-x.
- George J, Adamson J, Woodford H. Joint geriatric and psychiatric wards: a review of the literature. Age Ageing 2011;40:543-8. http://dx.doi.org/10.1093/ageing/afr080.
- Gladman J, Porock D, Griffiths A, Clissett P, Harwood RH, Knight A, et al. Care of Older People with Cognitive Impairment in General Hospitals. Southampton: National Institute for Health Research Service Delivery and Organisation programme; 2012.
- Gold M, Lightfoot LA, Hnath-Chisolm T. Hearing loss in a memory disorders clinic: a specially vulnerable population. Arch Neurol 1996;53:922-8. http://dx.doi.org/10.1001/archneur.1996.00550090134019.
- Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PE, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4132.
- Guijarro R, San Roman CM, Gomez-Huelgas R, Villalobos A, Martin M, Guil M, et al. Impact of dementia on hospitalization. Neuroepidemiology 2010;35:101-8. http://dx.doi.org/10.1159/000311032.
- Hoffmann F, van den Bussche H, Wiese B, Schon G, Koller D, Eisele M, et al. Impact of geriatric comorbidity and polypharmacy on cholinesterase inhibitors prescribing in dementia. BMC Psychiatry 2011;11. http://dx.doi.org/10.1186/1471-244X-11-190.
- Hill J, Hicks D, James J, Vanterpool G, Gillespie C, Fox C, et al. Diabetes and Dementia Guidance on Practical Management: Training, Research and Education For Nurses in Diabetes and Institute of Diabetes in Older People. London: Institute of Diabetes for Older People and Trend UK; 2013.
- Holmes J, Montana C, Powell G, Hewison J, House A, Mason J, et al. Liaison Mental Health Services for Older People: a Literature Review, Service Mapping and In-Depth Evaluation of Service Models. Southampton: National Institute for Health Research Service Delivery and Organisation programme; 2010.
- Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Opthalmol 2008;146:404-9. http://dx.doi.org/10.1016/j.ajo.2008.05.014.
- Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol 2011;95:17-23. http://dx.doi.org/10.1136/bjo.2009.165902.
- Jones R, Trigg R. Dementia and Serious Sight Loss. Occasional paper. London: Thomas Pocklington Trust; 2007.
- Keenan TD, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, Alzheimer disease, and dementia: record linkage study of hospital admissions. JAMA Ophthalmol 2014;132:63-8. http://dx.doi.org/10.1001/jamaophthalmol.2013.5696.
- Löppönen MK, Isoaho RE, Räihä IJ, Vahlberg TJ, Loikas SM, Takala TI, et al. Undiagnosed diseases in patients with dementia – a potential target group for intervention. Dement Geriatr Cogn Disord 2004;18:321-9. http://dx.doi.org/10.1159/000080126.
- Lyketsos CG, Toone L, Tschanz J, Rabins PV, Steinberg M, Onyike CU, et al. Population-based study of medical comorbidity in early dementia and ‘cognitive impairment, no dementia (CIND)’: association with functional and cognitive impairment: the Cache County study. Am J Geriatr Soc 2005;13:656-64.
- MacKenzie G, Ireland S, Moore S, Heinz I, Johnson R, Sahlas D. Tailored interventions to improve hypertension management after stroke or TIA – Phase II (Tims II). Stroke 2011;42.
- McCormick WC, Kukull WA, van Belle G, Bowen JD. Symptom patterns and comorbidity in the early stages of Alzheimer’s disease. J Am Geriatr Soc 1994;42:517-21. http://dx.doi.org/10.1111/j.1532-5415.1994.tb04974.x.
- McKeefry D, Bartlett R. The Development of Professional Guidelines for the Eye Examination of People with Dementia. London: University of Bradford and Thomas Pocklington Trust; 2010.
- Müther J, Abholz H-H, Wiese B, Fuchs A, Wollny A, Pentzek M. Are patients with dementia treated as well as patients without dementia for hypertension, diabetes, and hyperlipidaemia?. Br J Gen Pract 2010;60:671-4. http://dx.doi.org/10.3399/bjgp10X515395.
- Parke B, Beaith A, Slater L, Clarke AM. Contextual factors influencing success or failure of emergency department interventions for cognitively impaired older people: a scoping and integrative review. J Adv Nurs 2011;67:1426-48. http://dx.doi.org/10.1111/j.1365-2648.2011.05611.x.
- Parke B, Hunter KF, Strain LA, Marck PB, Waugh EH, McClelland AJ. Facilitators and barriers to safe emergency department transitions for community dwelling older people with dementia and their caregivers: a social ecological study. Int J Nurs Stud 2013;50:1206-18. http://dx.doi.org/10.1016/j.ijnurstu.2012.11.005.
- Rabadi MH, Rabadi FM, Edelstein L, Peterson M. Cognitively impaired stroke patients do benefit from admission to an acute rehabilitation unit. Arch Phys Med Rehabil 2008;89:441-8. http://dx.doi.org/10.1016/j.apmr.2007.11.014.
- Rait G, Walters K, Bottomley C, Petersen I, Iliffe S, Nazareth I. Survival of people with clinical diagnosis of dementia in primary care: cohort study. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3584.
- Sakurai H, Hanyu H, Kanetaka H, Sato T, Shimizu S, Hirao K, et al. Prevalence of coexisting diseases in patients with Alzheimer’s disease. Geriatr Gerontol Intl 2010;10:216-17. http://dx.doi.org/10.1111/j.1447-0594.2010.00609.x.
- Saposnik G, Cote R, Rochon PA, Mamdani M, Liu Y, Raptis S, et al. Care and outcomes in patients with ischemic stroke with and without preexisting dementia. Neurology 2011;77:1664-73. http://dx.doi.org/10.1212/WNL.0b013e31823648f1.
- Shah R, Evans BJW, Edgar D. A survey of the availability of state-funded primary eye care in the UK for the very young and very old. Ophthalmic Physiol Opt 2007;27:473-81. http://dx.doi.org/10.1111/j.1475-1313.2007.00506.x.
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Manas L. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011;37:S27-38. http://dx.doi.org/10.1016/S1262-3636(11)70962-4.
- Spencer K, Foster P, Whittamore KH, Goldberg SE, Harwood RH. Delivering dementia care differently – evaluating the differences and similarities between a specialist medical and mental health unit and standard acute care wards: a qualitative study of family carers’ perceptions of quality of care. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-004198.
- Stenvall M, Berggren M, Lundström M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia – subgroup analyses of a randomized controlled trial. Arch Gerontol Geriatr 2012;54:e284-9. http://dx.doi.org/10.1016/j.archger.2011.08.013.
- Stephan B, Brayne C, Savva GM, Matthews FE. Occurrence of medical co-morbidity in mild cognitive impairment: implications for generalisation of MCI research. Age Ageing 2011;40:501-7. http://dx.doi.org/10.1093/ageing/afr057.
- Tadros G, Salama RA, Kingston P, Mustafa N, Johnson E, Pannell R, et al. Impact of an integrated rapid response psychiatric liaison team on quality improvement and cost savings: the Birmingham RAID model. Psychiatrist 2013;37:4-10. http://dx.doi.org/10.1192/pb.bp.111.037366.
- Thorpe CT, Thorpe JM, Kind AJH, Bartels CM, Everett CM, Smith MA. Receipt of monitoring of diabetes mellitus in older adults with comorbid dementia. J Am Geriatr Soc 2012;60:644-51. http://dx.doi.org/10.1111/j.1532-5415.2012.03907.x.
- Uhlmann R, Larson E, Koepsell T, Rees T, Duckert L. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J Gen Intern Med 1991;6:126-32. http://dx.doi.org/10.1007/BF02598307.
- Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA 1989;261:1916-19. http://dx.doi.org/10.1001/jama.1989.03420130084028.
- Vitry AI, Roughead EE, Preiss AK, Ryan P, Ramsay EN, Gilbert AL, et al. Influence of comorbidities on therapeutic progression of diabetes treatment in Australian veterans: a cohort study. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0014024.
- Whitson HE, Ansah D, Whitaker D, Potter G, Cousins SW, MacDonald H, et al. Prevalence and patterns of comorbid cognitive impairment in low vision rehabilitation for macular disease. Arch Gerontol Geriatr 2010;50:209-12. http://dx.doi.org/10.1016/j.archger.2009.03.010.
- Yochim BP, Mueller AE, Kane KD, Kahook MY. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J Glaucoma 2012;21:250-4. http://dx.doi.org/10.1097/IJG.0b013e3182071b7e.
- Zamrini E, Parrish JA, Parsons D, Harrell LE. Medical comorbidity in black and white patients with Alzheimer’s disease. South Med J 2004;97:2-6. http://dx.doi.org/10.1097/01.SMJ.0000077061.01235.42.
- Zekry D, Herrmann FR, Grandjean R, Meynet M-P, Michel J-P, Gold G, et al. Demented versus non-demented very old inpatients: the same comorbidities but poorer functional and nutritional status. Age Ageing 2008;37:83-9. http://dx.doi.org/10.1093/ageing/afm132.
- Jara M, Zhang I, Tcherny-Lessenot S, Wanju S. Less or more cataracts in Alzheimer’s disease. Pharmacoepidemiol Drug Saf 2011;20:S302-3.
- Zhang Y, Vitry A, Roughead E, Ryan P, Gilbert A. Co-morbidity and the utilization of health care for Australian veterans with diabetes. Diabet Med 2010;27:65-71. http://dx.doi.org/10.1111/j.1464-5491.2009.02872.x.
- Pendlebury ST, Chen PJ, Bull L, Silver L, Mehta Z, Rothwell PM. Methodological factors in determining rates of dementia in transient ischemic attack and stroke: (I) impact of baseline selection bias. Stroke 2015;46:641-6. http://dx.doi.org/10.1161/STROKEAHA.114.008043.
- Sinclair AJ, Hillson R, Bayer AJ. National Expert Working Group . Diabetes and dementia in older people: a best clinical practice statement by a multidisciplinary National Expert Working Group. Diabet Med 2014;31:1024-31. http://dx.doi.org/10.1111/dme.12467.
- Poland F, Mapes S, Pinnock H, Katona C, Sorensen S, Fox C, et al. Perspectives of carers on medication management in dementia: lessons from collaboratively developing a research proposal. BMC Res Notes 2014;7. http://dx.doi.org/10.1186/1756-0500-7-463.
- Beishon LC, Harrison JK, Harwood RH, Robinson TG, Gladman JRF, Conroy SP. The evidence for treating hypertension in older people with dementia: a systematic review. J Hum Hypertens 2014;28:283-7. http://dx.doi.org/10.1038/jhh.2013.107.
- Murao K, Leys D, Jacquin A, Kitazono T, Bordet R, Bejot Y, et al. Thrombolytic therapy for stroke in patients with preexisting cognitive impairment. Neurology 2014;82:2048-54. http://dx.doi.org/10.1212/WNL.0000000000000493.
- Alshekhlee A, Li CC, Chuang SY, Vora N, Edgell RC, Kitchener JM, et al. Does dementia increase risk of thrombolysis?: a case–control study. Neurology 2011;76:1575-80. http://dx.doi.org/10.1212/WNL.0b013e3182190d37.
- Shamy MC, Jaigobin CS. The complexities of acute stroke decision-making: a survey of neurologists. Neurology 2013;81:1130-3. http://dx.doi.org/10.1212/WNL.0b013e3182a55ec7.
- Gillespie R, Mullan J, Harrison L. Managing medications: the role of informal caregivers of older adults and people living with dementia. A review of the literature. J Clin Nurs 2014;23:3296-308. http://dx.doi.org/10.1111/jocn.12519.
- Busl KM, Nogueira RG, Yoo AJ, Hirsch JA, Schwamm LH, Rost NS. Prestroke dementia is associated with poor outcomes after reperfusion therapy among elderly stroke patients. J Stroke Cerebrovasc Dis 2013;22:718-24. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2011.11.005.
- Sloan FA, Trogdon JG, Curtis LH, Schulman KA. The effect of dementia on outcomes and process of care for Medicare beneficiaries admitted with acute myocardial infarction. J Am Geriatr Soc 2004;52:173-81. http://dx.doi.org/10.1111/j.1532-5415.2004.52052.x.
- Lawrence V, Murray J, Samsi K, Banerjee S. Attitudes and support needs of black Caribbean, south Asian and white British carers of people with dementia in the UK. Br J Psychiatry 2008;193:240-6. http://dx.doi.org/10.1192/bjp.bp.107.045187.
- Lawrence V, Samsi K, Banerjee S, Morgan C, Murray J. Threat to valued elements of life: the experience of dementia across three ethnic groups. Gerontologist 2011;51:39-50. http://dx.doi.org/10.1093/geront/gnq073.
- Bartlett D, McKeefry R. People with Dementia and Sight Loss: A Scoping Study of Models of Care. London: Thomas Pocklington Trust; 2009.
- Lawrence F, Murray J, Banerjee S. The Experiences and Needs of People with Dementia and Serious Visual Impairment: A Qualitative Study. London: Thomas Pocklington Trust; 2008.
- Lawrence V, Murray J. Balancing independence and safety: the challenge of supporting older people with dementia and sight loss. Age Ageing 2010;39:476-80. http://dx.doi.org/10.1093/ageing/afq054.
- Connolly A, Campbell S, Gaehl E, Iliffe S, Drake R, Morris J, et al. Under-provision of medical care for vascular diseases for people with dementia in primary care: a cross-sectional review. Br J Gen Pract 2013;63:e88-96. http://dx.doi.org/10.3399/bjgp13X663046.
- Allan K. Deafness and dementia: consulting on the issues. J Dement Care 2006;14:35-7.
- Alshekhlee A, Mohammadi A, Mehta S, Edgell RC, Vora N, Feen E, et al. Is thrombolysis safe in the elderly? Analysis of a national database. Stroke 2010;41:2259-64. http://dx.doi.org/10.1161/STROKEAHA.110.588632.
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405-12. http://dx.doi.org/10.1016/S0140-6736(13)61570-6.
- Porter M, Lee T. The Strategy That Will Fix Health Care. Harvard Business Review 2013. https://hbr.org/2013/10/the-strategy-that-will-fix-health-care/ (accessed 9 December 2015).
- NHS England . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 28 May 2015).
- Goodwin N, Smith J, Davies A, Perry C, Rosen R, Dixon A, et al. Integrated Care For Patients and Populations: Improving Outcomes by Working Together. London: The King’s Fund; 2011.
- Dowrick A, Southern A. Dementia 2014: Opportunity for Change. London: Alzheimer’s Society; 2014.
- Field S. NHS Future Forum: Summary Report on Proposed Changes to the NH 2011. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_127540.pdf (accessed 22 April 2015).
- Shaw S, Rosen R, Rumbold B. An Overview of Integrated Care in the NHS. What is Integrated Care?. London: Nuffield Trust; 2011.
- Dementia Partnerships . Vanguard Sites Selected to Deliver New Models of Care 2015. http://dementiapartnerships.com/vanguard-sites-selected-to-deliver-new-models-of-care/ (accessed 9 December 2015).
- Béland F, Hollander MJ. Integrated models of care delivery for the frail elderly: international perspectives. Gac Sanit 2011;25:138-46. http://dx.doi.org/10.1016/j.gaceta.2011.09.003.
- Mason A, Goddard M, Weatherly H, Chalkley M. Integrating funds for health and social care: an evidence review. J Health Serv Res Policy 2015;20:177-88. http://dx.doi.org/10.1177/1355819614566832.
- Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5205.
- Han RC, Jefferis JM, Taylor JP, Archibald NK, Clarke MP. A novel, multidisciplinary clinic for complex visual problems in older people. Eye 2012;26:1536-41. http://dx.doi.org/10.1038/eye.2012.205.
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008;371:725-35. http://dx.doi.org/10.1016/S0140-6736(08)60342-6.
- Stuck AE, Siu AL, Wieland GD, Rubenstein LZ, Adams J. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032-6. http://dx.doi.org/10.1016/0140-6736(93)92884-V.
- Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;7. http://dx.doi.org/10.1002/14651858.cd006211.pub2.
- Edmans J, Bradshaw L, Franklin M, Gladman J, Conroy S. Specialist geriatric medical assessment for patients discharged from hospital acute assessment units: randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5874.
- Clark M, Moreland N, Greaves I, Greaves N, Jolley D. Putting personalisation and integration into practice in primary care. J Integr Care 2013;21:105-20. http://dx.doi.org/10.1108/14769011311316033.
- Oliver D, Foot C, Humphries R. Making our Health and Care Systems Fit for an Ageing Population. London: The King’s Fund; 2014.
- My Purple Folder. Improving Health Care for People with a Learning Disability Living in Hertfordshire. Welwyn Garden City: NHS Hertfordshire; 2015.
- Khanassov V, Vedel I, Pluye P. Case management for dementia in primary health care: a systematic mixed studies review based on the diffusion of innovation model. Clin Interv Aging 2014;9:915-28. http://dx.doi.org/10.2147/CIA.S64723.
- Khanassov V, Vedel I, Pluye P. Barriers to implementation of case management for patients with dementia: a systematic mixed studies review. Ann Fam Med 2014;12:456-65. http://dx.doi.org/10.1370/afm.1677.
- Koch T, Iliffe S, Manthorpe J, Stephens B, Fox C, Robinson L, et al. The potential of case management for people with dementia: a commentary. Int J Geriatr Psychiatry 2012;27:1305-14. http://dx.doi.org/10.1002/gps.3783.
- Pimouguet C, Lavaud T, Dartigues JF, Helmer C. Dementia case management effectiveness on health care costs and resource utilization: a systematic review of randomized controlled trials. J Nutr Health Aging 2010;14:669-76. http://dx.doi.org/10.1007/s12603-010-0314-4.
- Reilly S, Miranda-Castillo C, Malouf R, Hoe J, Toot S, Challis D, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev 2015;1. http://dx.doi.org/10.1002/14651858.cd008345.pub2.
- Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimer’s Dement 2012;8:426-36. http://dx.doi.org/10.1016/j.jalz.2011.06.004.
- Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2013;28:889-902. http://dx.doi.org/10.1002/gps.3906.
- Iliffe S, Waugh A, Poole M, Bamford C, Brittain K, Chew-Graham C, et al. The effectiveness of collaborative care for people with memory problems in primary care: results of the CAREDEM case management modelling and feasibility study. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18520.
- Van Mierlo L, Meiland F, Van Hout H, Droes R-M. Towards personalized integrated dementia care: a qualitative study into the implementation of different models of case management. BMC Geriatr 2014;14. http://dx.doi.org/10.1186/1471-2318-14-84.
- Minkman MMN, Ligthart SA, Huijsman R. Integrated dementia care in the Netherlands: a multiple case study of case management programmes. Health Soc Care Community 2009;17:485-94. http://dx.doi.org/10.1111/j.1365-2524.2009.00850.x.
- Goodwin N, Sonola L, Thiel V, Kodner DL. Co-ordinated Care for People with Complex Chronic Conditions. London: Aetna Foundation; 2013.
- Goodman C, Drennan V, Davies S, Masey H, Gage H, Scott C, et al. Nurses as Case Managers in Primary Care: the Contribution to Chronic Disease Management. Southampton: National Institute for Health Research Service Delivery and Organisation programme; 2012.
- Sonola L, Thiel V, Goodwin N, Kodner DL. Oxleas Advanced Dementia Service Supporting Carers and Building Resilience. London: The King’s Fund; 2013.
- Griffiths P, Bridges J, Sheldon H, Thompson R. The role of the dementia specialist nurse in acute care: a scoping review. J Clin Nurs 2015;24:1394-405. http://dx.doi.org/10.1111/jocn.12717.
- Brown M, MacArthur J, McKechanie A, Mack S, Hayes M, Fletcher J. Learning disability liaison nursing services in south-east Scotland: a mixed-methods impact and outcome study. J Intellect Disabil Res 2012;56:1161-74. http://dx.doi.org/10.1111/j.1365-2788.2011.01511.x.
- MacArthur J, Brown M, McKechanie A, Mack S, Hayes M, Fletcher J. Making reasonable and achievable adjustments: the contributions of learning disability liaison nurses in ‘getting it right’ for people with learning disabilities receiving general hospitals care. J Adv Nurs 2015;71:1552-63. http://dx.doi.org/10.1111/jan.12629.
- Phillips L. Improving care for people with learning disabilities in hospital. Nurs Stand 2012;26:42-8. http://dx.doi.org/10.7748/ns2012.02.26.23.42.c8933.
- La Fontaine J, Brooker D, Bray J, Milosevic S. A Local Evaluation of Dementia Advisers (National Demonstrator Site). Worcestershire Report for Dementia Adviser Service Implementation Team. Worcester: Association for Dementia Studies, University of Worcester; 2011.
- Clarke CL, Keyes SE, Wilinson H, Alexjuk J, Wilcockson J, Robinson L, et al. HEALTHBRIDGE. The National Evaluation of Peer Support Networks and Dementia Advisers in Implementation of the National Dementia Strategy for England. London: Department of Health; 2013.
- Robertson J, Roberts H, Emerson E. Health Checks for People With Learning Disability: A Systematic Review of Evidence 2010. www.improvinghealthandlives.org.uk/uploads/doc/vid_7646_IHAL2010–04HealthChecksSystemticReview.pdf (accessed 14 April 2015).
- National Institute for Health and Care Excellence . NICE Pathways: Diabetes Overview 2014. http://pathways.nice.org.uk/pathways/diabetes (accessed 28 May 2015).
- Mayrhofer A, Goodman C, Sharpe R, Dye H. Dementia Education in Hertfordshire and Bedfordshire: an Organisational Audit Commissioned by Health Education East of England. Hatfield: Centre for Research in Primary and Community Care (CRIPACC), University of Hertfordshire; 2014.
- Livingston G, Leavey G, Manela M, Livingston D, Rait G, Sampson E, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ 2011;341. http://dx.doi.org/10.1136/bmj.c4184.
- Knapp M, Black N, Dixon J, Damant J, Rehill A, Tan S. Independent Assessment of Improvements in Dementia Care and Support Since 2009. London: PIRU; 2014.
- Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JR. Health status of UK care home residents: a cohort study. Age Ageing 2014;43:97-103. http://dx.doi.org/10.1093/ageing/aft077.
- Boyer F, Novella JL, Morrone I, Jolly D, Blanchard F. Agreement between dementia patient report and proxy reports using the Nottingham Health Profile. Int J Geriatr Psychiatry 2004;19:1026-34. http://dx.doi.org/10.1002/gps.1191.
- Freeman G, Hughes J. Continuity of Care and the Patient Experience. London: The King’s Fund; 2010.
- Aboulghate A, Abel G, Elliott MN, Parker RA, Campbell J, Lyratzopoulos G, et al. Do English patients want continuity of care, and do they receive it?. Br J Gen Pract 2012;62:e567-75. http://dx.doi.org/10.3399/bjgp12X653624.
- Freeman GK. Progress with relationship continuity 2012, a British perspective. Int J Integr Care 2012;12.
- McCarthy M, Addington-Hall J, Altmann D. The experience of dying with dementia: a retrospective study. Int J Geriatr Psychiatry 1997;12:404-9. http://dx.doi.org/10.1002/(SICI)1099-1166(199703)12:3<404::AID-GPS529>3.0.CO;2-2.
- Miranda-Castillo C, Woods B, Orrell M. People with dementia living alone: what are their needs and what kind of support are they receiving?. Int Psychogeriatr 2010;22:607-17. http://dx.doi.org/10.1017/S104161021000013X.
- Redley M, Banks C, Foody K, Holland A. Healthcare for men and women with learning disabilities: understanding inequalities in access. Disabil Soc 2012;27:747-59. http://dx.doi.org/10.1080/09687599.2012.673080.
- Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006;29:725-31. http://dx.doi.org/10.2337/diacare.29.03.06.dc05-2078.
- Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management?. J Gen Intern Med 2007;22:1635-40. http://dx.doi.org/10.1007/s11606-007-0313-2.
- Jefferis JM, Taylor JP, Clarke MP. Does cognitive impairment influence outcomes from cataract surgery? Results from a 1-year follow-up cohort study. Br J Opthalmol 2015;99:412-17. http://dx.doi.org/10.1136/bjophthalmol-2014-305657.
- Creasy KR, Lutz BJ, Young ME, Stacciarini J-MR. Clinical implications of family-centered care in stroke rehabilitation [published online ahead of print 3 February 2015]. Rehabil Nurs 2015. http://dx.doi.org/10.1002/rnj.188.
- Dementia Friendly Environments Funding: Successful Bids. London: Department of Health; 2013.
- The Butterfly Scheme n.d. http://butterflyscheme.org.uk/ (accessed 5 January 2016).
- Spotlight on Dementia Care. A Health Foundation Improvement Report. London: The Health Foundation; 2011.
- Norfolk Age UK. Pabulum Blue Book n.d. www.ageuk.org.uk/norfolk/dementia-support--services/pabulum-blue-book/ (accessed 5 January 2016).
- The Charity for Civil Servants . Carer’s Passport n.d. www.foryoubyyou.org.uk/helping-you/caring/carers-passport (accessed 5 January 2016).
- Bray J, Evans S, Thompson R, Bruce M, Carter C, Brooker D, et al. Understanding the needs of people with dementia and family carers. Nurs Older People 2015;27:18-23. http://dx.doi.org/10.7748/nop.27.7.18.e699.
- Kitwood T. Towards a theory of dementia care: the interpersonal process. Ageing Soc 2008;13:51-67. http://dx.doi.org/10.1017/S0144686X00000647.
- Guidelines on Supporting People with Dementia and their Carers. London: British Psychological Society and the Royal College of Psychiatrists; 2007.
- Clissett P, Porock D, Harwood RH, Gladman JR. The challenges of achieving person-centred care in acute hospitals: a qualitative study of people with dementia and their families. Int J Nurs Stud 2013;50:1495-503. http://dx.doi.org/10.1016/j.ijnurstu.2013.03.001.
- This is Me – Tool for People with Dementia Receiving Professional Care. London: Alzheimer’s Society; 2015.
- World Alzheimer Report 2011. The Benefits of Early Diagnosis and Intervention. London: Alzheimer’s Disease International; 2011.
- Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA 2006;295:2148-57. http://dx.doi.org/10.1001/jama.295.18.2148.
- Bamford C, Poole M, Brittain K, Chew-Graham C, Fox C, Iliffe S, et al. Understanding the challenges to implementing case management for people with dementia in primary care in England: a qualitative study using normalization process theory. BMC Health Serv Res 2014;14. http://dx.doi.org/10.1186/s12913-014-0549-6.
- Improving Services and Support for People with Dementia. London: HMSO; 2007.
- Tullo E, Allan L. What should we be teaching medical students about dementia?. Int Psychogeriatr 2011;23:1044-50. http://dx.doi.org/10.1017/S1041610211000536.
- The 2022 GP. A Vision for General Practice in the Future NHS. London: Royal College of General Practitioners; 2013.
- Prime Minister’s Challenge on Dementia: Delivering Major Improvements in Dementia Care and Research by 2015. London: Department of Health; 2012.
- Department of Health . Delivering High Quality, Effective, Compassionate Care: Developing the Right People With the Right Skills and the Right Values. A Mandate from the Government to Health Education England: April 2014 to March 2015 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/310170/DH_HEE_Mandate.pdf (accessed 4 January 2016).
- Nothing Ventured, Nothing Gained: Risk Guidance for Dementia. London: Department of Health; 2010.
- Bloomfield HE, Greer N, Newman D, MacDonald R, Carlyle M, Fitzgerald P, et al. Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – a Systematic Review of the Evidence. Washington, DC: Institute for Family-Centered Care; 2012.
- Weber SR, Pirraglia PA, Kunik ME. Use of services by community-dwelling patients with dementia: a systematic review. Am J Alzheimers Dis Other Demen 2011;26:195-204. http://dx.doi.org/10.1177/1533317510392564.
- Peel E, Harding R. ‘It’s a huge maze, the system, it’s a terrible maze’: dementia carers’ constructions of navigating health and social care services. Dementia (London) 2014;13:642-61. http://dx.doi.org/10.1177/1471301213480514.
- Ibrahim JE, Davis M-C. Availability of education and training for medical specialists about the impact of dementia on comorbid disease management. Educ Gerontol 2013;39:925-41. http://dx.doi.org/10.1080/03601277.2013.767657.
- Ory MG, Hoffman RR, Yee JL, Tennstedt S, Schulz R. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist 1999;39:177-85. http://dx.doi.org/10.1093/geront/39.2.177.
- Frampton S, Guastello S, Brady C, Hale M, Horowitz S, Bennett Smith S, et al. Patient-Centered Care: Improvement Guide. Derby, CT: Planetree/Picker Institute; 2008.
- Jurgens FJ, Clissett P, Gladman JR, Harwood RH. Why are family carers of people with dementia dissatisfied with general hospital care? A qualitative study. BMC Geriatr 2012;12. http://dx.doi.org/10.1186/1471-2318-12-57.
- Johnson B, Abraham M, Conway J, Simmons L, Edgman-Levitan S, Sodomka P, et al. Partnering with Patients and Families to Design a Patient- and Family-Centered Health Care System. Bethesda, MD: Institute for Patient- and Family-Centered Care and Institute for Healthcare Improvement; 2008.
- Future Hospital Commission . Future Hospital: Caring for Medical Patients 2013. www.rcplondon.ac.uk/projects/future-hospital-commission (accessed 8 April 2015).
- Clarke CL. Risk: constructing care and care environments in dementia. Health Risk Soc 2000;2:83-9. http://dx.doi.org/10.1080/136985700111477.
- Manthorpe J. Risk Taking. London: Jessica Kingsley; 2004.
- Rubenstein RS, Lohr ICN, Brook RH, Goldberg GA. Conceptualisation and Measurement of Physiological Health for Adults: Volume 12 Vision Impairments. Bethesda, MD: US Department of Health and Human Sciences/Rand Corporation; 1982.
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York, NY: Oxford University Press; 1998.
Appendix 1 Details of search terms
Search terms for the scoping review: PubMed
| Search component | Search terms |
|---|---|
| #1 Dementia and diabetes | ((Dementia OR Alzheimer OR cognitive impairment OR delirium) AND (Diabetes OR blood glucose self-monitoring) (Self management OR Self Care OR Self monitoring OR Service delivery OR Service organization OR Activities of daily living OR Caregivers OR Quality Assessment OR Quality OR Quality Indicators OR Quality of life OR Disease Progression OR Behaviour OR Impact OR Geriatric Assessment OR Severity of Illness OR Nursing Assessment OR Interprofessional OR Standard of Care OR Risk Factors OR Treatment outcome OR patient Experience) AND (Humans[Mesh])) |
| #2 Dementia and stroke | (Dementia[ti] OR Alzheimer[ti]) AND (stroke OR cerebrovascular OR CVA OR cerebrovascular disorders) AND (Self management OR Self Care OR Self monitoring OR Service delivery OR Service organization OR Activities of daily living OR Caregivers OR Quality Assessment OR Quality OR Quality Indicators OR Quality of life OR Disease Progression OR Behaviour OR Impact OR Geriatric Assessment OR Severity of Illness OR Nursing Assessment OR Interprofessional OR Standard of Care OR Risk Factors OR Treatment outcome OR patient Experience) AND (Humans[Mesh])) |
| #3 Dementia and visual impairment | (Dementia OR Alzheimer OR cognitive impairment OR delirium) AND (Eye diseases OR vision disorders OR Blindness OR visually impaired OR Nystagmus OR retinopathy OR macular degeneration OR glaucoma or cataract) AND (Self management OR Self Care OR Self monitoring OR Service delivery OR Service organization OR Activities of daily living OR Caregivers OR Quality Assessment OR Quality OR Quality Indicators OR Quality of life OR Disease Progression OR Behaviour OR Impact OR Geriatric Assessment OR Severity of Illness OR Nursing Assessment OR Interprofessional OR Standard of Care OR Risk Factors OR Treatment outcome OR patient Experience) |
| #4 Dementia and comorbidity | ((Dement*[ti] OR Alzheimer*[ti]) AND ((comorbidity OR co-morbidity OR comorbid OR "other medical conditions" OR "other chronic disease*" OR multimorbidiy[ti] OR multi-morbidity[ti] OR multiple disease*[ti] OR multiple morbid*[ti] OR polypathology[ti] OR associated disease*[ti] OR associated disorder*[ti]) OR co-existence[ti] OR co-existing[ti] OR concomitant[ti] OR co-occurring[ti])) |
| #5 | #1 OR #2 OR #3 OR #4 OR #5 |
Search strategy to support ideas/recommendations from the consensus conference
The following search terms were used in PubMed (searched March 2015):
((continuity[Title] OR access[Title] OR integration[Title] OR integrated[Title] OR management[Title] OR navigate*[Title]) AND ((dementia[Title] OR alzheimer*[Title] OR MCI[Title] OR cognitive[Title] OR multimorbid*[Title] OR comorbid*[Title] OR frail*[Title] OR complex[Title]) AND (Review[ptyp]))
The following terms were used in The Cochrane Library (searched March 2015):
(continuity OR access OR integration OR integrated OR management OR navigate*) AND (dementia OR alzheimer* OR MCI OR cognitive OR multimorbid* OR comorbid* OR frail* OR complex)
The following terms were used in Google Scholar (searched March 2015):
Case management AND dementia
Integration OR integrated AND dementia
Continuity AND dementia
Care pathway AND dementia
Patient held records AND dementia
Shared records AND dementia
Electronic records AND dementia
Comprehensive geriatric assessment AND dementia
Learning disability AND health care
Learning disability AND continuity
Appendix 2 Interview and focus group schedules
Interview schedule for people living with dementia: process summary and core questions
-
Introduction/warm-up and gaining consent
-
Researcher will introduce themselves and explain that they are from the University of Hertfordshire/Newcastle University.
-
Thank participant for taking part in the research and explain that this is a study looking at how memory problems affect how people manage other health conditions, for example diabetes, stroke or sight loss.
-
Provide details on how the research team can be contacted after the interview.
-
Give details about the format of the interview (semistructured) and time.
-
Travel expenses forms are available from us should you require one (if applicable).
-
We will give you your £10 voucher at the end of the interview.
-
Are there any questions?
Consent – Assess person with dementia’s capacity to participate in the interview and record this.
-
Please look at this information and then tell me how you feel. Ask that participant (person with dementia and/or carer) reads and signs the consent form (consent can range from signing the consent form to recording verbal consent).
-
Explanation of anonymity and confidentiality and that the interview will be recorded.
-
You may stop the interview at any point, you don’t have to share personal experiences if you don’t want to.
-
You don’t have to answer any questions you don’t want to.
-
Note ways in which decreasing level of well-being can be recognised.
-
Participant background/bio (conversational to find out about the person)
Probe for:
-
Have they always lived in current area or are they from another area.
-
Previous employment.
-
Education level.
-
Family – married/widowed, children/grandchildren.
-
What carers there are? Who else lives with them?
-
Ask about family support – how often do they see/speak to family and friends?
-
Any help from social services/day centres/carers coming to house/community nurses?
-
Health problems and comorbidities
-
I’d like to know about your health problems.
-
Ask about the medication they are taking.
-
Do you take any tablets specifically for your memory problems?
Prompt:
Find out when difficulty with memory began – before or after comorbidity? Could affect how they self-manage. Diabetes, sight loss and stroke – how long have they had these conditions (avoid spending too much time on other health conditions).
| Diabetes | Stroke | VI |
|---|---|---|
|
|
|
-
Management of condition
-
Do your memory problems affect how you manage your diabetes/sight problems/stroke recovery?
-
(Note any problems with self-management or any effective strategies to self-manage condition.)
-
Does having sight a problem make it more difficult to remember things? (VI only) Probe for whether comorbidity causes memory to be worse, e.g. a person with a sight problem may forget to take medication because of a lack of visual cues/not having information in large print.
-
Health-care experiences
I’d like to know a bit more about the health care/treatment that you have received for your diabetes/sight problems/stroke:
-
Do you see a regular team about your diabetes/sight problems/stroke? (Prompt: do they see the same professional, is there continuity of care?)
-
Who is the health professional you see most often about your diabetes/sight problems/stroke? (Prompt: GP/practice nurse/nurse specialist/consultant in hospital practice and community. Explore whether the HCP has dementia expertise as well as comorbidity expertise)
-
Does he/she spend enough time with you? Explain things to you? (About condition, how to take medication, health-care services available.) Do they seem to know about you and your problems?
-
What sort of information have you been given about your diabetes/sight problems/stroke? (Prompt: leaflets/information sheets/verbal explanation) (pursue the information they receive, any training courses, e.g. diabetes management). Prompt for ‘This is me’.
-
Do you have regular check-ups for your diabetes/sight problems/stroke? [Prompt: interested in whether they are neglected for regular check-ups, e.g. regular visits to diabetes clinic, eye examinations, annual blood tests for diabetes with follow-up appointments and annual eye screening (as required by guidelines)]. Do they get reminders?
-
Have you missed any appointments and what happened? (Have they been taken off waiting lists?)
-
Do you see a regular team about your memory problems? Do you think that the different health teams share information with each other? (Prompt: about how their care is managed/co-ordinated across different specialist teams, e.g. diabetes teams sharing information with dementia specialists; anything like ‘This is me’ being used?)
-
Have you joined any voluntary organisations like Diabetes UK, RNIB, Stroke Association, etc.? Do they get specialist advice from these organisations?
| Diabetes | Stroke | VI |
|---|---|---|
|
|
|
I’d like to know what it is like when you visit the hospital or a clinic about your diabetes/sight problems/stroke:
-
What was your experience like? (Prompts: find out which health-care services they have access to)
-
Did the doctors and nurses understand about your memory problems? (Prompt: did they know about the person’s dementia/provide extra help)
-
Does he/she/they spend enough time with you? Explain things to you?
-
What is working well and what could be improved? (re-orient back to examples given by participant)
-
What has been good about the care you have received?
-
What do you think has not been good about the care you have received?
-
Is there any treatment that you would like to have that isn’t available to you?
-
Are there any health-care services that you think would be helpful to you?
-
Would you like any support to help with your diabetes/stroke/sight problems?
Help the person with dementia to make the transition from the research context back into their day-to-day context.
I can explain anything if you need me to. Many thanks for your time and support of this study.
Focus group schedule
Introduction
-
Welcome and thanks for attending.
-
Who we are – the researchers in attendance introduce themselves (AB can be contacted following the focus group).
-
Summary of what we are trying to achieve – want to begin by looking generally at the extent to which you feel that dementia is an issue, move onto some issues relating more specifically to stroke care and pathways and what you think a good service would look like and then finish with looking at general initiatives relating to the improvement of hospital care for people with dementia.
Consent
-
Ask that everyone reads and signs the consent form.
-
Consent form for each individual.
-
Explanation of anonymity and confidentiality.
-
The focus groups will be recorded.
-
You may leave at any point, you don’t have to share personal experiences if you don’t want to.
Other instructions
-
Once you are sitting down with your group you may like to begin by introducing yourselves to the other people in your group.
-
When we begin the focus groups, everyone should have a say, please don’t interrupt each other.
Questions for stroke health-care professionals (schedule was adapted for each specialism)
-
In your experience to what extent is dementia an issue when providing care for stroke patients?
-
How does dementia impact on the service you provide?
-
Is the problem increasing?
-
-
How does it impact on the stroke care pathway if someone has dementia?
-
Does it affect decisions about brain scanning?
-
Does it affect decisions about thrombolysis or other treatment?
-
Where would people go – e.g. hyperacute unit, rehabilitation unit, geriatric ward or specialist dementia unit?
-
Who would be responsible for the care of people with dementia who have had a stroke?
-
How would it impact on decisions about rehabilitation?
-
-
In an ideal world what do you think a good stroke service for people with dementia would look like?
-
Would it be the same as the current NICE [National Institute for Health and Care Excellence] pathway or would it differ in any way?
-
How would you see family carers being involved?
-
-
How does the presence of dementia impact on secondary stroke prevention?
-
Compliance with medication, diet, etc.
-
-
Are there any initiatives that you are aware of in the hospital to improve the care of people with dementia?
-
Dementia champions?
-
Wrist bands?
-
‘This is me’?
-
Special facilities for patients with dementia?
-
-
What are the knowledge gaps among providers of stroke services about dementia?
-
Are there areas where you feel that you need more information/education?
-
-
Is there anything else you would like to add? Anything we haven’t covered?
Thank participants for their time.
Appendix 3 Template for nominal group technique at the consensus conference
Appendix 4 Additional tables for the scoping review
| Study ID | Country | Study design | Number of participants | Aims/research questions | Study focusa | Comorbidity | Age (years)b | Sex (% female) |
|---|---|---|---|---|---|---|---|---|
| Allan et al., 200589 (Allan 2006162) | UK | Qualitative | Total: 43 (n = 12 service users with hearing impairment, n = 16 practitioners, n = 8 relatives, n = 7 British Sign Language interpreters) | Consultation exercise looking at issues for people with dementia with hearing loss | V | Hearing impairment | NR | NR |
| Australian Commission on Safety and Quality in Health Care 201388 | Australia | Rapid review | NA | To identify best practice in caring for patients with cognitive impairment in acute hospital settings | S | General | NA | NA |
| Alshekhlee et al., 2011151 | USA | Cohort study | 35,557 patients with a diagnosis of dementia; 207 (0.58%) had received thrombolysis | To establish the impact of dementia on hospital mortality and intracerebral haemorrhage rates associated with thrombolysis therapy for AIS | A | Stroke | Most aged > 80 | 63 |
| Balfour and O’Rourke 200390 | Canada | Cross-sectional | Total: 460 PLWD (n = 245 with arthritis, n = 215 no arthritis) | Are patients with Alzheimer’s disease inappropriately prescribed neuroleptics and benzodiazepines | Q | Arthritis | 84.33 | 73 |
| Barnett et al., 201291 (Guthrie et al., 20124) | UK | Cross-sectional | 1,751,841 patients from database of primary care practices | Examined distribution of multimorbidity and comorbidity in relation to age and socioeconomic deprivation | P | General multimorbidity but includes information on diabetes and stroke | 16.6% aged ≥ 65 | 50.5 |
| Bartlett and McKeefry 201193 (Bartlett and McKeefry 2009158) | UK | Qualitative and scoping | 7 practitioners, 4 students | To increase knowledge of visual issues and eye health for people with dementia | V | VI | NR | NR |
| Bartlett and Clarke 201292 | UK | Qualitative | 5 HCPs | How HCPs assess the needs of and communicate with a person dying from cancer with a coincidental dementia | V | Cancer | NR | 100 |
| Bayer et al., 199494 | UK | Prevalence study | 26 older people with diabetes (in all but five, diabetes predated dementia) | Impact of dementia on diabetic care | V | Diabetes | Median 78.5 (range 70–91) | 73 |
| Beishon et al., 2014149 | NA | Systematic review | Includes six RCTs | Assess the evidence for the treatment of hypertension in older people with dementia | A | Hypertension | ≥ 65 | NR |
| Bruce et al., 200395 | Australia | Cross-sectional | 223 older people with diabetes | Determine whether or not the prevalence of dementia in older diabetics warrants an active screening approach | P | Dementia and depression in people with diabetes | 76.5 | 48.8 |
| Busl et al., 2013154 | USA | Retrospective cross-sectional | 153 (13.6% pre-stroke dementia) | To determine whether or not pre-stroke dementia contributed to poor outcomes in stroke patients aged > 80 years who underwent intravenous and/or IAT reperfusion | A | Stroke | 86 | NR |
| College of Optometrists 201296 | UK | Guidelines | NA | Guidelines for optometrists examining a patient with dementia or cognitive impairment | S | VI | NA | NA |
| Connolly et al., 2013161 (Connolly et al., 201297) | UK | Observational, cross-sectional review of primary care records | 700 PLWD (compared with people without dementia on QOF register) | Evaluate the quality of medical care for vascular diseases provided to people with dementia | Q | Stroke and diabetes | 82.1 (43–102) | 66 |
| Curtis et al., 201298 | USA | Retrospective cohort study | 284,380 (with and without dementia) | Examine use of treatments for AMD | Q | VI (AMD) | 81 | 64 |
| Dewing and Dijk 201499 | UK | Literature review | NA | Review of the literature on the acute care of PLWD in general hospitals | V, S | General | NA | NA |
| Doraiswamy et al., 20021 | USA | Cross-sectional | 679 older people with Alzheimer’s disease | Examine the prevalence of comorbid medical illness in Alzheimer’s disease patients | P | General | 87 | Community 55.6, assisted living 75.8, nursing home 83.5 |
| Doucet et al., 2008100 | France | Cross-sectional | 238 older people with diabetes | Identify characteristics of elderly diabetic patients and evaluate relationship between glycaemic control and complications of diabetes | Q | Diabetes | 82.2 | 58 |
| Feil et al., 2003101 | USA | Longitudinal cross-sectional | 7482 older adults | Examine relationship between cognitive impairment, common chronic medical illnesses and risk of mortality in older people | P | Several but includes diabetes and stroke | 51% aged ≥ 85 | 61 |
| Feil et al., 2009103 | USA | Longitudinal cross-sectional | 51 people with diabetes (n = 27 with caregiver) | Examine role of cognitive impairment and caregiver support in diabetes care adherence and glycaemic control | P, Q | Diabetes | 78 (62–90) | 0 |
| Feil et al., 2011102 | USA | Qualitative | 21 caregivers of PLWD and diabetes | Explore caregivers’ challenges and quality-of-life issues managing diabetes in PLWD | V | Diabetes | 65–90 | Majority female (numbers not given) |
| Feil et al., 2011104 | USA | Cross-sectional database analysis | 497,000 veterans with diabetes (with and without cognitive impairment/dementia) | Examine the relationship between management of diabetes and hypoglycaemia in older adults with dementia | P, Q | Diabetes | 100% aged > 65, 44% aged > 75 | 2 |
| Formiga et al., 2009105 | Spain | Prospective population-based survey | 515 PLWD | Evaluate comorbidity in elderly with dementia to determine differences according to dementia severity | P | General | 81 | 70 |
| George et al., 2011106 | UK | Systematic review | Examine literature to determine the evidence for the effectiveness of joint geriatric/psychiatric wards | S | General | NA | NA | |
| Gillespie et al., 2014153 | NA | Systematic review/scoping | 10 studies (n = 5 USA, n = 2 UK, n = 2 Australia, n = 1 Canada) | To explore published literature that describes what is known about the role of informal caregivers as they manage medications for older adults and/or PLWD resident in the community | V | General | NR | NR |
| Gladman et al., 2012107 | UK | Qualitative study (and review) | 60 HCPs, 36 patient and carer interviews | Elicit staff and organisational attitudes to dealing with older patients with cognitive impairment, understand the impact on patients, carers and staff and identify potential improvements | V | General | Patients 86.8 (70–99), carers 63 (46–79) | HCPs 80, patients 56 |
| Gold et al., 1996108 | USA | Case–control | 52 patients attending a memory clinic (n = 30 dementia, n = 22 cognitive impairment) | Determine prevalence and characteristics of hearing loss and whether screening tools are adequate and patients with Alzheimer’s disease can adequately report hearing problems | P | Hearing loss | NR | 83 |
| Goldberg et al., 2013109 | UK | RCT | 310 intervention group, 290 control group | Develop and evaluate a best practice model of general hospital acute medical care for older people with cognitive impairment | S | General | Median 85 | Intervention group 55, control group 40 |
| Guijarro et al., 2010110 | Spain | Cohort | 40,482 PLWD | Determine prevalence and clinical characteristics of hospitalised dementia patients | P, Q | Several including cataracts and diabetes | 78 | NR |
| Heun et al., 201312 | UK | Retrospective case–control | 634 with Alzheimer’s disease, 72,244 control group | Differences between Alzheimer’s disease and non-Alzheimer’s disease patients in terms of comorbid diseases at hospital admission and which comorbidities contribute to mortality | P | General | Alzheimer’s disease 85.1 (SE 8.2), control 80.8 (SE 7.4) | Alzheimer’s disease 65, control 51 |
| Hewitt et al., 201020 | UK | Questionnaire survey | 1047 older people with type 2 diabetes | Examine knowledge and management of diabetes in older people | P, V | Diabetes | 80.9 (75–100) | 53.3 |
| Hill et al., 2013112 | UK | Guidance | NA | Highlight importance of recognising relationship between diabetes and dementia, the impact one condition has on the other and maximising the benefits and safety of diabetes treatments while minimising risks | Q | Diabetes | NA | NA |
| Hoffman et al., 2011111 | Germany | Cohort | 1848 PLWD | Determine whether or not comorbidity and polypharmacy influence prescription of cholinesterase inhibitors in PLWD | Q | General comorbidity but some mention of visual disturbances | 78.7 | 47.6 |
| Holmes et al., 2010113 | UK | Literature review, mapping and case study | 10 case study sites: recruited 757 participants in the referred cohort and 975 participants in the comparison cohort | Establish what service models are being used to improve the care of older people with mental health problems in general hospitals and what impact these models might have on outcomes | S | General | 80 | Comparison cohort 55, referred cohort 64% |
| Ishii et al., 2008114 | Japan | Case series | 88 people with VI | Evaluate the influence of cataract surgery on cognitive function and depressive mental status of elderly patients | Q | VI | 75.3 (55–93) | 64 |
| Jara et al., 2011144 | USA (but UK data) | Retrospective cohort | 8124 Alzheimer’s disease cohort, 642,325 non-Alzheimer’s disease cohort | Evaluate the occurrence of cataracts in people with Alzheimer’s disease compared with the general population | P | VI | 64+ | Alzheimer’s disease cohort 68, non-Alzheimer’s disease cohort 54 |
| Jefferis et al., 2011115 | UK | Literature review | NA | What are the implications for practice relating to the benefits of cataract surgery for PLWD? | Q | VI (cataracts) | NA | NA |
| Jones and Trigg 2007116 (Trigg and Jones 200573) | UK | Scoping review | NA | Review of research on PLWD and serious sight loss | P, Q | VI | NA | NA |
| Keenan 2014117 | UK | Cohort | 65,894 AMD cohort, 168,092 dementia cohort | Are PLWD more or less likely to be admitted to hospital for AMD treatment | Q | VI (AMD) | Majority > 65 | 61 |
| Lawrence et al., 200972 (Lawrence et al., 2008,156 2010,160 2011157) | UK | Qualitative | 17 PLWD and VI, 17 family caregivers, 18 HCPs | The experiences and needs of older adults with VI and dementia | V | VI | 65–99 (18/19 aged ≥ 75) | 63 |
| Löppönen et al., 2004118 | Finland | Cross-sectional population-based study | 1260 older people, 112 PLWD | Study of undiagnosed diseases in older people with and without dementia | P, Q | Several including diabetes, stroke and VI | 64+ | 58 |
| Lyketsos et al., 2005119 | USA | Case–control | 695 older people; 149 PLWD, 225 without cognitive impairment, 321 cognitive impairment no dementia | Investigated medical comorbidity in persons with dementia and cognitive impairment | P | General | Dementia 83.89, CIND 82.38, no dementia 79.93 | Dementia 64.4, CIND 53.8, no dementia 54.8 |
| MacKenzie et al., 2011120 | Canada | RCT | 56 stroke and MCI patients | Investigate whether nurse case management interventions result in lowered blood pressure | S | Mainly stroke but includes diabetes | 59% > 65 | 30 |
| McCormick et al., 1994121 | USA | Case–control | Total: 375 (n = 154 with dementia, n = 92 with cognitive impairment, n = 129 control group) | Compare comorbidity in Alzheimer’s disease patients and non-Alzheimer’s disease patients | P | General | 76 | Dementia 68, cognitive impairment 57, control 63 |
| McKeefry and Bartlett 2010122 (McKeefry and Bartlett 201074) | UK | Scoping review | NA | To develop guidelines for optometrists for best practice with patients with dementia and sight loss | S | VI | NA | NA |
| Müther et al., 2010123 | Germany | Retrospective matched control study | 216 PLWD, 216 matched control subjects without dementia | Are patients with dementia treated differently from patients without dementia? | Q | Several, includes diabetes | dementia group 82.7, non-dementia group 82.2 | 77.3 |
| Parke et al., 2011124 | Canada | Scoping review | Included 15 evaluation studies | Scope research on cognitive impairment in older adults who visited the emergency department of an acute care hospital and evaluation of effectiveness of programmes | S | General | 65+ | NA |
| Parke et al., 2013125 | Canada | Qualitative | 10 adult–family caregiver dyads, 10 emergency department nurses, 4 nurse practitioners | Identify factors that facilitate or impede safe transitional care in the emergency department for community-dwelling older adults | S, V | General | PLWD 83.17 (77–90), carer 57.81 (51–84) | Not specified |
| Pendlebury and Rothwell 200969 | NA | Systematic review | 22 hospital-based and 8 population-based cohorts | Identify the risk factors for dementia and whether or not there are any differences in the risk factors associated with pre-stroke and post-stroke dementia | P | Stroke | NA | NA |
| Pendlebury et al., 2015146 | UK | Cross-sectional | 1236 patients with acute transient ischemic attack or stroke | To determine the impact of study entry criteria on measured rates of pre- and post-event dementia | P | Stroke | 75 | 53 |
| Poblador-Plou et al., 201414 | Spain | Cross-sectional | 72,815 [3971 (5.45%) diagnosed with dementia] | To look at the prevalence of chronic conditions in PLWD | P | General | 70 whole sample, 80 PLWD | 70 |
| Poland et al., 2014148 | UK | Qualitative | 9 carers of PLWD | To identify carers’ views gained from experiences of medication management in dementia | V | General | NR | 89 |
| Rabadi et al., 2008126 | USA | Retrospective analysis | 668 (n = 435 with cognitive impairment) | Can stroke patients with cognitive impairment benefit from admission to an acute rehabilitation unit? | S | Stroke | 70.3 (22–96) | 53 |
| Rait et al., 2010127 | UK | Cohort | 22,529 PLWD, 112,645 matched non-dementia participants. Ratio of 1 : 5 dementia : non-dementia | Estimate survival after diagnosis of dementia in primary care compared with people without dementia and determine incidence of dementia | P, Q | Several including stroke and diabetes | 82.2 | Dementia 67.9, no dementia 55.8 |
| Sakurai et al., 2010128 | Japan | Prevalence study | 113 PLWD | Investigated prevalence of coexisting diseases in PLWD | P | Several including diabetes | 78.6 | 73 |
| Saposnik et al., 2011129 | Canada | Retrospective cohort | 877 with pre-existing dementia, 877 control subjects (no pre-existing dementia) | Determine if pre-existing dementia is an independent predictor of all-cause mortality and disability after ischaemic stroke | P, Q | Stroke | 82 | 60 |
| Schubert et al., 20062 | USA | Cross-sectional | Total: 3013 (107 PLWD) | Compare the medical comorbidity of older patients with and without dementia in primary care | P | Several including stroke and diabetes | 73.4 | 66.6 |
| Shah et al., 2007130 | UK | Survey | 100 optometry practices | Investigate the accessibility of a sight test for an older person with dementia | Q | VI | NA | NA |
| Shamy and Jaigobin 2013152 | Canada | Survey | NR but say they had a response rate of 69% | To better understand the decision-making process surrounding the administration of IV tPA | A | Stroke | Not known | Not known |
| Sinclair et al., 200021 | UK | Case–control | 396 with diabetes, 393 matched control subjects | Whether cognitive impairment is associated with changes in self-care behaviour and health service use in older diabetics | Q | Diabetes | Diabetics 74.9, control 74.8 | 51 |
| Sinclair et al., 2011131 | Europe | Guidance | NA | Support clinical decisions in older people with diabetes and enhance high-quality diabetes care by the use of best available evidence | S | Diabetes | NA | NA |
| Sinclair et al., 2014147 | UK | Guidance | NA | Outline key steps in integrated care pathway for dementia and diabetes, produce guidance on identifying each condition, deal with potentially hazardous issue of hypoglycaemia and outline important competencies for HCPs in both settings | S | Diabetes | NA | NA |
| Sloan et al., 2004155 | USA | Cross-sectional (retrospective chart review) | 5851 admitted for acute myocardial infarction with dementia, 123,241 admitted for AMI without dementia | Differences in mortality after admission for AMI and in treatments for AMI between patients with and without dementia | S | AMI | Dementia 81.6, no dementia 75.5 | Dementia 57.2, no dementia 46.3 |
| Spencer et al., 2013132 | UK | Qualitative | Total: 40 (n = 20 from specialist unit, n = 20 standard care) | Examine carers’ views and experiences of delivery of patient care for PLWD in acute general hospital in order to evaluate specialist medical and mental health unit compared with standard hospital wards | V | General | NR | Not clear |
| Stenvall et al., 2012133 | Sweden | RCT | 64 patients with fractured neck of femur (n = 28 intervention group, n = 36 control group) | Whether or not a multidisciplinary postoperative intervention programme reduced postoperative complications and improved functional recovery among people with dementia | S | Hip fracture | > 70 | Intervention group 79, control group 69 |
| Stephan et al., 2011134 | UK | Cross-sectional | Total: 13,004 (n = 587 PLWD, n = 319 MCI, n = 608 no cognitive impairment) | Compared the pattern of disease comorbidity across different cognitive groups and whether or not comorbidity is a risk factor for dementia progression | P | Several but includes diabetes and stroke | Dementia group – with health conditions 79.8, no health conditions 81.9 | Dementia group – with health conditions 60.1, no health conditions 73.2 |
| Tadros et al., 2013135 | UK | Retrospective analysis | Not given | Evaluate whether or not implementation of the RAID integrated model improves access to psychiatric assessment and reduces costs of health service provision in an acute hospital | S | General | Mean age of referrals from all wards 65.7; 23% of RAID group and 20% of control group were aged > 75 | RAID group 54, comparison group 40 |
| Thorpe et al., 2012136 | USA | Retrospective cohort | Total: 288,805 (n = 44,717 PLWD) | Examined how recommended monitoring of diabetes differed for people with and without comorbid dementia | P, Q | Diabetes | 26% aged 65–69, 48% aged 70–79, 25.9% aged 80+ | 60 |
| Uhlmann et al., 1989138 | USA | Case–control | 200 with hearing impairment (n = 100 with dementia, n = 100 control subjects) | Whether or not hearing impairment contributes to cognitive dysfunction in older adults | P | Hearing impairment | 77 | 58 |
| Uhlmann et al., 1991137 | USA | Case–control | 87 PLWD, 87 matched control subjects | How impaired visual acuity is associated with dementia and cognitive dysfunction in older adults | P | VI | 77 | 58 |
| Vitry et al., 2010139 | Australia | Retrospective cohort | 20,134 veterans with diabetes (includes people with dementia/cognitive impairment but numbers not clear) | Whether or not the number of comorbid conditions unrelated to diabetes delays therapeutic progression of diabetes treatment | Q | Diabetes | 77.3 | 36 |
| Whitson et al., 2010140 | USA | Cross-sectional | 101 people with macular disease | Prevalence of comorbid cognitive impairment among older adults referred to low-vision rehabilitation for macular disease | P | VI (macular disease) | 80.1 | 65 |
| Yochim et al., 2012141 | USA | Case series | 41 glaucoma patients | Prevalence of cognitive impairment, depression and anxiety in older adults with glaucoma | P | VI | 70 | 70 |
| Zamrini et al., 2004142 | USA | Case–control | 999 people with Alzheimer’s disease | Prevalence of comorbid illness in black and white patients with probable Alzheimer’s disease | P | Several but includes diabetes and eye disease | Female patients 75.1, male patients 73.1 | 49 |
| Zekry et al., 2008143 | Switzerland | Cohort | 349 inpatients (43.3% dementia, 10.6% MCI) | Comorbid conditions and functional and nutritional status in hospitalised patients with dementia and MCI | P | Several but includes data on stroke and diabetes | 85 | 76 |
| Zhang et al., 2010145 | Australia | Retrospective cohort | 17,095 veterans with and without diabetes (4.4% on dementia medication) | Impact of comorbidity on health service utilisation by Australian veterans with diabetes | Q | Diabetes | 81 | 44 |
| Study and country | Type of study | Type of population | Eligibility criteria defined? | Method of selection | Nature of population | Number of participants | Participation rate |
|---|---|---|---|---|---|---|---|
| Barnett et al., 2012,91 UK (Scotland) | Cross-sectional | General | Alive, permanently registered with a participating practice | National data set | Primary care (about one-third of all Scottish population) | 11,139 | All patients registered with primary care practice |
| Bruce et al., 2003,95 Australia | Longitudinal cross-sectional | Diabetes | Defined by postcode, age ≥ 70 years, diabetes | Initial 223 members of a cohort of 529 participants | Community-based volunteers who consented to take part | 223 | Initially recruited 63% of those who were eligible |
| Doraiswamy et al., 2002,1 USA | Cross-sectional | Dementia | Diagnosis of Alzheimer’s disease, age ≥ 50 years | From a variety of community health-care sites | Volunteers who consented to take part | 679 | Not clear |
| Feil et al., 2003,101 USA | Longitudinal cross-sectional | Cognitive impairment | Geographically defined, age ≥ 65 years | Secondary data analysis of data set from community-based study | Community-based volunteers who consented to take part | 7482 (1774 with cognitive impairment) | 80% (baseline interview) |
| Feil et al., 2009,103 USA | Longitudinal cross-sectional | Diabetes | Diagnosis of type 2 diabetes, age ≥ 60 years | Electronic medical diagnosis of type 2 diabetes | Geriatric medical clinic | 51 | Not clear |
| Feil et al., 2011,104 USA | Cross-sectional | Diabetes | Veterans aged ≥ 65 with diabetes | Secondary data analysis of research administration database (Veterans Health Administration, Medicare and Medicaid) | Population-based sample – large national health-care system database | 497,000 | All patients on database |
| Heun et al., 2013,12 UK | Retrospective case–control | Dementia | Diagnosis of Alzheimer’s disease, age 70+ years, inpatient care for at least 24 hours | Consecutively admitted inpatients | Hospital | 634 | All patients who met criteria were included |
| Hewitt et al., 2010,20 UK | Questionnaire | Diabetes (cognitive impairment) | Type 2 diabetes, age ≥ 75 years, not resident in a nursing home | Secondary data analysis of intervention arm of RCT | Primary care | 1047 | Not clear |
| Jara et al., 2011,144 UK | Retrospective cohort | Dementia | Age ≥ 64 years, at least 24 months’ enrolment, no cataract diagnosis at baseline | National data set | Primary care | 650,325 (8124 with dementia) | All patients registered with primary care practice |
| Löppönen et al., 2004,118 Finland | Cross-sectional | Dementia | Geographically defined, age ≥ 65 years | All those who met criteria invited in random order | Population based | 112 | 82% |
| Lyketsos et al., 2005,119 USA | Case–control | Dementia/cognitive impairment | Geographically defined, age ≥ 65 years | All those who met criteria invited to participate | Population based | 695 (374 with dementia) | 90% |
| McCormick et al., 1994,121 USA | Case–control | Dementia/cognitive impairment | Age ≥ 60 years, member of HMO, geographically defined | Subsample recruited from database (not clear how chosen) | From HMO database | 154 | Not clear |
| Pendlebury et al., 2015146 | Cross-sectional | Stroke | All patients with stroke or TIA | All those with stroke or TIA within defined population area. Recruited through general practice or secondary care | Population based | 1236 (from a population of 92,728) | 92% |
| Poblador-Plou et al., 2014,14 Spain | Cross-sectional | Dementia | Age ≥ 65 years, consulted physician at least once during the 12-month period of the study | Primary care data set (19 primary health-care centres) | Primary care | 72,815 (3971 with dementia) | All patients who visit GP at least once during 2008 |
| Rait et al., 2010,127 UK | Cohort | Dementia | Age 60+ years with first code for dementia during the study period, at least 6 months of data | National data set (practices that met standards for acceptable levels of data recording) | Primary care | 135,174 (22,529 with dementia) | All patients registered with primary care practice |
| Sakurai et al., 2010,128 Japan | Cross-sectional | Dementia | Dementia or MCI | Consecutive outpatients attending memory clinic | Memory clinic | 160 | Not clear |
| Saposnik et al., 2011,129 Canada | Retrospective cohort | Stroke and dementia | Age ≥ 18 years, first ischaemic stroke | Stroke register | Clinical database | 10,658 | All stroke patients attending 12 regional stroke centres |
| Schubert et al., 2006,2 USA | Cross-sectional | Dementia | Age ≥ 65, seen primary care physician within 2 year. Excluded nursing home residents and non-English-speaking patients | Primary care practice centres | Primary care | 3013 (107 dementia) | Not clear |
| Stephan et al., 2011,134 UK | Cross-sectional | MCI | Age ≥ 65 years | Randomly selected from health authority lists in five areas of the UK | Population based | 13,004 (1486 dementia) | Not clear |
| Uhlmann et al., 1991,137 USA | Case–control | Dementia | Age ≥ 65 years, English speaking, eighth grade of higher level of education, ability to complete audiometric evaluation | Computer searches of clinic records | Outpatient clinic | 174 (87 dementia) | 70% |
| Whitson et al., 2010,140 USA | Cross-sectional | VI (macular disease) | Age ≥ 65 years, macular disease diagnoses | All eligible patients attending clinic invited to participate | Outpatient clinic | 101 | 74% |
| Yochim et al., 2012,141 USA | Case series | VI (glaucoma) | Age ≥ 50 years, diagnosis of glaucoma | All eligible patients attending clinic invited to participate | Outpatient clinic | 41 | Not reported |
| Zamrini et al., 2004,142 USA | Case–control | Dementia | Probable Alzheimer’s disease, black or white (white participants matched non-randomly to black participants) | Computer searches of clinic records | Memory clinic | 334 | All those eligible during study period |
| Zekry et al., 2008,143 Switzerland | Cohort | Dementia | Age ≥ 75 years. Excluded those with a terminal illness or disorders interfering with psychometric assessment | Random sample of all patients admitted selected each day | Hospital inpatients | 349 (188 dementia) | 85% |
| Zhang et al., 2010,145 Australia | Retrospective cohort | Diabetes | Veterans, age ≥ 65, received prescription for diabetes in previous 6 months | Research administration database (Department of Veterans Affairs) | From database of veterans | All eligible patients on database |
| Study and country | Type of study | Type of population | Method of data collection | How dementia/cognitive impairment defined/assessed? | How diabetes defined/recorded? | How stroke defined/recorded? | How VI defined/recorded? | Other |
|---|---|---|---|---|---|---|---|---|
| Barnett et al., 2012,91 UK (Scotland) | Cross-sectional | General | Electronic medical records | Read code ever recorded | Read code ever recorded | Read code ever recorded | NA | |
| Bruce et al., 2003,95 Australia | Longitudinal cross-sectional | Diabetes | Face-to-face interview and clinical examination | MMSE (< 24/30), IQCODE (> 3.61), Cambridge Examination for Mental Disorders of the Elderly and diagnostic examination | Clinical and biochemical assessment | |||
| Doraiswamy et al., 2002,1 USA | Cross-sectional | Dementia | Face-to-face interview and medical records | Diagnostic criteria for probable Alzheimer’s disease (NINADS-ADRDA) | NA | NA | NA | Cumulative Illness Rating Scale–Geriatric |
| Feil et al., 2003,101 USA | Longitudinal cross-sectional | Cognitive impairment | Face-to-face interview | Modified version of Pfeiffer’s Short Portable Mental Status Questionnaire (SPMSQ) | Self-reported | Self-reported | NA | |
| Feil et al., 2009,103 USA | Longitudinal cross-sectional | Diabetes | Face-to-face interview | Cognitive Abilities Screening Instrument (CASI) | Diagnosis confirmed by physician and patient | NA | NA | |
| Feil et al., 2011,104 USA | Cross-sectional | Diabetes | Medical records | ICD codes | Two or more diabetes-specific ICD codes (ICD-9-CM) | NA | NA | |
| Heun et al., 2013,12 UK | Retrospective case–control | Dementia | Retrospective data from clinical records | ICD-10 diagnostic category | Registered diagnosis in medical records | Registered diagnosis in medical notes | Registered diagnosis in medical notes | |
| Jara et al., 2011,144 UK | Retrospective cohort | Dementia | Electronic medical records | Not specified | Cataract-related surgical/diagnostic codes | |||
| Löppönen et al., 2004,118 Finland | Cross-sectional | Dementia | Face-to-face interview and clinical examination | Diagnostic criteria for probable Alzheimer’s disease (NINADS-ADRDA), DSM-IV – clinical examination | Diagnosis in medical records and/or history of stroke verified by clinical examination | Diagnosis in medical records and/or treatment with antidiabetic agents and/or fasting plasma glucose level ≥ 7.0 mmol/l | NA | |
| Lyketsos et al., 2005,119 USA | Case–control | Dementia/cognitive impairment | Face-to-face interview and clinical examination | Modified MMSE (3MS) or IQCODE plus clinical examination | Self- or informant response | Self- or informant response | ||
| McCormick et al., 1994,121 USA | Case–control | Dementia/cognitive impairment | Clinical examination and medical records | Diagnostic criteria for probable Alzheimer’s disease (NINADS-ADRDA), DSM-III-R (clinical examination) | Diagnosis in medical records | Diagnosis in medical records | Diagnosis in medical records | Charleston Comorbidity Index |
| Poblador-Plou et al., 2014,14 Spain | Cross Sectional | Dementia | Electronic medical records | Expanded Diagnostic Clusters NUR11 category ‘dementia and delirium’ | Diagnosis in medical records (ICD codes) | Diagnosis in medical records (ICD codes) | medical records (ICD codes) | |
| Rait et al., 2010,127 UK | Cohort | Dementia | Electronic medical records | Read codes | Read codes | Read codes | ||
| Sakurai et al., 2010,128 Japan | Cross-sectional | Dementia | Face-to-face interview and clinical examination | Diagnostic criteria for probable Alzheimer’s disease (NINADS-ADRDA) – clinical examination | Receiving diabetes therapy or HbA1c > 5.9% or diagnosis according to ADA guidelines | NA | NA | |
| Saposnik et al., 2011,129 Canada | Retrospective cohort | Stroke and dementia | Medical records and clinical examination | Any type of dementia recorded in notes | NA | Canadian Neurological Scale – clinical examination | NA | |
| Schubert et al., 2006,2 USA | Cross-sectional | Dementia | Medical records and clinical examination | ICD-10 – clinical examination | Coded ICD diagnosis | Coded ICD diagnosis | NA | Chronic Disease Score |
| Stephan et al., 2011,134 UK | Cross-sectional | MCI | Face-to-face interview | Mayo Clinic criteria for MCI | Self- or information response | Self- or information response | ||
| Uhlmann et al., 1991,137 USA | Case–control | Dementia | Medical records | Diagnostic criteria for probable Alzheimer’s disease (NINADS-ADRDA) | NA | NA | Snellen and Rosenbaum methods249 | |
| Whitson et al., 2010,140 USA | Cross-sectional | VI (macular disease) | Clinical examination | Telephone Interview for Cognitive Status–Modified, Wechsler Memory Scale–Revised, letter fluency (FASa) | NA | NA | Diagnosis in medical records | |
| Yochim et al., 2012,141 USA | Case series | VI (glaucoma) | Data collection face to face or by telephone | California Verbal Learning Test Short Form, verbal fluency test (Delis–Kaplan Executive Function System) | Diagnosis in medical records | |||
| Zamrini et al., 2004,142 USA | Case–control | Dementia | Medical records | Diagnostic criteria for probable Alzheimer’s disease (NINADS-ADRDA) | Diagnosis in medical records | Diagnosis in medical records | Diagnosis in medical records | |
| Zekry et al., 2008,143 Switzerland | Cohort | Dementia | Clinical examination and medical records | MMSE and short cognitive evaluation; diagnosis based on clinical criteria (clinical examination) | Diagnosis in medical records | Diagnosis in medical records | Diagnosis in medical records | Charleston Comorbidity Index |
| Zhang et al., 2010,145 Australia | Retrospective cohort | Diabetes | Medical claims database | Database information on whether or not medication for dementia was dispensed | ICD codes |
Appendix 5 Additional tables for the Cognitive Function and Ageing Studies analysis
Service questions used
Q554. Who usually helps (with day-to-day tasks)? CODE MAIN HELPER
A. No one.
B. Spouse.
C. Daughter.
D. Daughter-in-law.
E. Son.
F. Son-in-law.
G. Brother.
H. Sister.
I. Other relative.
J. Friend or neighbour.
K. Home help.
L. Care worker.
M. Meals on wheels.
N. Community worker.
O. Community nurse.
P. Warden.
Q. Paid help.
R. Other.
S. Not applicable.
IF A OR S SKIP TO Q559
Q556. Does anyone else help? CODE UP TO THREE OTHER HELPERS. First helper
A. No one.
B. Spouse.
C. Daughter.
D. Daughter-in-law.
E. Son.
F. Son-in-law.
G. Brother.
H. Sister.
I. Other relative.
J. Friend or neighbour.
K. Home help.
L. Care worker.
M. Meals on wheels.
N. Community worker.
O. Community nurse.
P. Warden.
Q. Paid help.
R. Other.
S. Not applicable.
Q557. Does anyone else help? Second helper
A. No one.
B. Spouse.
C. Daughter.
D. Daughter-in-law.
E. Son.
F. Son-in-law.
G. Brother.
H. Sister.
I. Other relative.
J. Friend or neighbour.
K. Home help.
L. Care worker.
M. Meals on wheels.
N. Community worker.
O. Community nurse.
P. Warden.
Q. Paid help.
R. Other.
S. Not applicable.
Q558. Does anyone else help? Third helper
A. No one.
B. Spouse.
C. Daughter.
D. Daughter-in-law.
E. Son.
F. Son-in-law.
G. Brother.
H. Sister.
I. Other relative.
J. Friend or neighbour.
K. Home help.
L. Care worker.
M. Meals on wheels.
N. Community worker.
O. Community nurse.
P. Warden.
Q. Paid help.
R. Other.
S. Not applicable.
Lastly, I’d like to ask you whether you have received various health or local authority services or any private help in recent weeks. So, in the last 4 weeks, have you seen or had a visit from or to any of the following services?
Q562. Local authority home help or home care assistant
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q563. Any nursing services
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q564. Chiropodist
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q565. Meals on wheels
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q566. Physiotherapist
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q567. Occupational therapist
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q568. Speech therapist
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q569. Social worker
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q570. Day centre
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q571. Day hospital
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q572. GP (the doctor)
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q573. During the last 3 complete calendar months, did you attend the casualty or outpatient department of a hospital (as a patient)?
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q574. Which month was this?
Month:
Q575. How many times did you attend the casualty or outpatient department during that month?
Q579. During the last year, have you been in hospital for treatment as a day patient (i.e. admitted to a hospital bed or day ward, but not required to stay overnight)?
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q580. How many separate days in hospital have you had as a day patient (in the last year)?
____________ Rate no of days
Q581. During the last year, have you been in hospital as an inpatient, overnight or longer?
0. No.
1. Yes.
8. No answer.
9. Not asked.
Q582. How many separate stays in hospital have you had as an inpatient (in the last year)?
____________ Rate no of days
Cognitive Function and Ageing Studies II-only analysis results (restricted to those living in the community)
Dementia and comorbidity compared with dementia
Stroke
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.3 | 0.9 to 1.8 |
| Any service in previous 4 weeks (count) | 1.8 | 1.3 to 2.4 |
| Outpatient in last 3 months (count) | 1.2 | 0.6 to 2.2 |
| Day patient in last year (count) | 1.6 | 0.6 to 4.3 |
| Inpatient in last year (count) | 2.4 | 1.5 to 3.8 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 1.9 | 0.4 to 9.1 |
| Care worker | 3.1 | 1.4 to 6.6 |
| Warden | 1.2 | 0.1 to 12.0 |
| Paid help | 0.6 | 0.1 to 2.4 |
| Any professional service | 2.6 | 1.3 to 5.2 |
| Unpaid care | 1.7 | 0.8 to 3.6 |
| Services in previous 4 weeks | ||
| Home help/home care assistant | 2.7 | 1.3 to 5.7 |
| Any nursing services | 1.9 | 0.9 to 4.1 |
| Chiropodist | 2.6 | 1.3 to 5.2 |
| Physiotherapist | 2.9 | 0.7 to 12.8 |
| Occupational therapist | 1.5 | 0.1 to 14.6 |
| Social worker | 3.2 | 0.7 to 15.3 |
| Day centre | 2.2 | 1.0 to 5.1 |
| Day hospital | 2.9 | 0.5 to 17.9 |
| GP | 1.5 | 0.8 to 2.9 |
| Any service | 2.0 | 0.9 to 4.1 |
| Hospital services | ||
| Casualty/outpatient | 1.2 | 0.5 to 2.6 |
| Day patient | 1.2 | 0.5 to 3.0 |
| Inpatient | 4.4 | 2.2 to 8.9 |
Diabetes
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.2 | 0.8 to 2.0 |
| Any service in previous 4 weeks (count) | 1.5 | 1.0 to 2.2 |
| Outpatient in last 3 months (count) | 2.1 | 1.1 to 3.9 |
| Day patient in last year (count) | 1.7 | 0.4 to 7.2 |
| Inpatient in last year (count) | 1.2 | 0.7 to 2.2 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 3.4 | 0.8 to 14.6 |
| Care worker | 0.8 | 0.3 to 2.2 |
| Community nurse | 8.4 | 1.1 to 65.4 |
| Warden | 1.2 | 0.1 to 12.6 |
| Paid help | 0.4 | 0.1 to 2.0 |
| Any service | 1.2 | 0.5 to 2.8 |
| Unpaid care | 1.4 | 0.6 to 3.0 |
| Services in previous 4 weeks | ||
| Home help | 0.9 | 0.4 to 2.1 |
| Any nursing service | 3.5 | 1.7 to 7.5 |
| Chiropodist | 2.3 | 1.0 to 5.1 |
| Meals on wheels | 0.8 | 0.1 to 7.0 |
| Physiotherapist | 0.8 | 0.1 to 6.6 |
| Occupation therapist | 2.0 | 0.2 to 19.7 |
| Social worker | 2.0 | 0.3 to 13.6 |
| Day centre | 1.1 | 0.4 to 3.0 |
| Day hospital | 2.1 | 0.3 to 16.6 |
| GP | 1.1 | 0.6 to 2.2 |
| Any service | 1.7 | 0.7 to 3.8 |
| Hospital services | ||
| Casualty/outpatient | 1.9 | 0.9 to 4.2 |
| Day patient | 0.6 | 0.2 to 1.8 |
| Inpatient | 2.0 | 0.9 to 4.3 |
Vision impairment
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.1 | 0.7 to 1.5 |
| Any service in previous 4 weeks (count) | 1.2 | 0.9 to 1.5 |
| Outpatient in last 3 months (count) | 1.2 | 0.7 to 2.1 |
| Day patient in last year (count) | 1.4 | 0.6 to 3.6 |
| Inpatient in last year (count) | 1.7 | 0.9 to 3.1 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 0.4 | 0.0 to 3.2 |
| Care worker | 1.6 | 0.7 to 3.5 |
| Community worker | 1.0 | 0.1 to 11.7 |
| Community nurse | 4.1 | 0.6 to 30.0 |
| Warden | 0.9 | 0.1 to 8.6 |
| Paid help | 0.4 | 0.1 to 1.7 |
| Any professional day-to-day service | 1.7 | 0.8 to 3.6 |
| Unpaid care | 2.0 | 0.9 to 4.7 |
| Services in previous 4 weeks | ||
| Home help/home care assistant | 1.4 | 0.6 to 3.1 |
| Any nursing service | 1.0 | 0.4 to 2.1 |
| Chiropodist | 2.1 | 1.0 to 4.3 |
| Meals on wheels | 0.6 | 0.1 to 5.1 |
| Physiotherapist | 0.7 | 0.1 to 5.5 |
| Occupational therapist | 1.6 | 0.2 to 15.9 |
| Speech therapist | 0.5 | 0.0 to 8.9 |
| Social worker | 0.3 | 0.0 to 2.9 |
| Day centre | 0.8 | 0.3 to 1.9 |
| Day hospital | 0.7 | 0.1 to 4.3 |
| GP | 1.5 | 0.8 to 3.0 |
| Any service | 6.1 | 1.7 to 21.4 |
| Hospital services | ||
| Casualty/outpatient | 1.3 | 0.7 to 2.7 |
| Day patient | 1.4 | 0.6 to 3.6 |
| Inpatient | 1.9 | 0.9 to 4.0 |
Target comorbidities
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.4 | 1.0 to 2.0 |
| Any service in previous 4 weeks (count) | 1.8 | 1.4 to 2.3 |
| Outpatient in last 3 months (count) | 1.7 | 1.0 to 2.8 |
| Day patient in last year (count) | 1.4 | 0.6 to 3.4 |
| Inpatient in last year (count) | 2.1 | 1.3 to 3.5 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 2.4 | 0.6 to 9.2 |
| Care worker | 2.1 | 1.1 to 4.2 |
| Community worker | 0.3 | 0.0 to 3.4 |
| Warden | 0.4 | 0.0 to 4.3 |
| Paid help | 0.6 | 0.2 to 1.7 |
| Any professional day-to-day service | 2.3 | 1.2 to 4.2 |
| Unpaid care | 2.3 | 1.3 to 4.1 |
| Services in previous 4 weeks | ||
| Home help/home care assistant | 1.5 | 0.8 to 2.8 |
| Any nursing service | 2.8 | 1.5 to 5.2 |
| Chiropodist | 3.4 | 1.8 to 6.2 |
| Meals on wheels | 0.2 | 0.0 to 1.8 |
| Physiotherapist | 1.5 | 0.4 to 6.4 |
| Social worker | 1.3 | 0.3 to 6.0 |
| Day centre | 1.1 | 0.5 to 2.4 |
| Day hospital | 1.5 | 0.3 to 7.9 |
| GP | 1.5 | 0.9 to 2.5 |
| Any service | 3.4 | 1.9 to 6.2 |
| Hospital services | ||
| Casualty/outpatient | 1.3 | 0.7 to 2.4 |
| Day patient | 0.8 | 0.4 to 1.9 |
| Inpatient | 3.2 | 1.7 to 5.9 |
Dementia and comorbidity compared with comorbidity alone
Dementia as well as stroke
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.3 | 0.9 to 1.7 |
| Any service in previous 4 weeks (count) | 1.7 | 1.2 to 2.2 |
| Outpatient in last 3 months (count) | 0.6 | 0.4 to 1.1 |
| Day patient in last year (count) | 1.2 | 0.5 to 2.9 |
| Inpatient in last year (count) | 1.8 | 1.2 to 2.6 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 1.4 | 0.3 to 6.1 |
| Care worker | 9.2 | 4.3 to 19.5 |
| Warden | 0.8 | 0.1 to 7.1 |
| Paid help | 0.3 | 0.1 to 1.0 |
| Any professional day-to-day service | 2.8 | 1.5 to 5.3 |
| Unpaid care | 2.7 | 1.3 to 5.5 |
| Services in the previous 4 weeks | ||
| Home help/home care assistant | 6.1 | 3.0 to 12.4 |
| Any nursing service | 2.0 | 1.0 to 4.2 |
| Chiropodist | 1.4 | 0.8 to 2.7 |
| Physiotherapist | 0.8 | 0.2 to 3.0 |
| Occupational therapist | 0.7 | 0.1 to 6.0 |
| Day centre | 6.8 | 3.1 to 14.9 |
| Day hospital | 4.9 | 1.0 to 24.6 |
| GP | 1.2 | 0.7 to 2.2 |
| Any service | 1.6 | 0.8 to 3.2 |
| Hospital services | ||
| Casualty/outpatient | 0.8 | 0.3 to 1.6 |
| Day patient | 0.7 | 0.3 to 1.7 |
| Inpatient | 2.3 | 1.2 to 4.3 |
Dementia as well as diabetes
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.3 | 0.8 to 2.1 |
| Any service in previous 4 weeks (count) | 1.5 | 1.1 to 2.2 |
| Outpatient in last 3 months (count) | 1.1 | 0.6 to 2.1 |
| Day patient in last year (count) | 1.3 | 0.3 to 5.3 |
| Inpatient in last year (count) | 1.4 | 0.8 to 2.3 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 2.4 | 0.6 to 9.1 |
| Care worker | 6.5 | 2.3 to 18.2 |
| Warden | 0.8 | 0.1 to 6.3 |
| Paid help | 0.2 | 0.0 to 1.0 |
| Other | 3.3 | 0.6 to 18.3 |
| Any professional day-to-day service | 1.9 | 0.9 to 4.0 |
| Unpaid care | 3.6 | 1.8 to 7.5 |
| Services in previous 4 weeks | ||
| Home help/home care assistant | 4.1 | 1.8 to 9.6 |
| Any nursing service | 2.9 | 1.5 to 5.8 |
| Chiropodist | 1.0 | 0.5 to 2.1 |
| Meals on wheels | 0.6 | 0.1 to 5.3 |
| Physiotherapist | 0.6 | 0.1 to 4.6 |
| Occupational therapist | 1.9 | 0.2 to 15.4 |
| Day centre | 7.8 | 2.8 to 21.8 |
| Day hospital | 4.4 | 0.7 to 28.0 |
| GP | 1.0 | 0.5 to 1.9 |
| Any service | 1.5 | 0.7 to 3.2 |
| Hospital services | ||
| Casualty/outpatient | 1.3 | 0.6 to 2.7 |
| Day patient | 0.4 | 0.1 to 1.2 |
| Inpatient | 1.6 | 0.8 to 3.3 |
Dementia as well as vision impairment
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.3 | 0.9 to 1.8 |
| Any service in previous 4 weeks (count) | 1.5 | 1.2 to 1.8 |
| Outpatient in last 3 months (count) | 0.7 | 0.5 to 1.1 |
| Day patient in last year (count) | 1.2 | 0.5 to 2.7 |
| Inpatient in last year (count) | 2.2 | 1.3 to 3.9 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 0.4 | 0.1 to 3.7 |
| Care worker | 9.1 | 4.3 to 19.5 |
| Warden | 0.9 | 0.1 to 6.8 |
| Paid help | 0.2 | 0.1 to 0.9 |
| Any professional day-to-day service | 2.6 | 1.2 to 5.3 |
| Unpaid care | 4.8 | 2.1 to 10.8 |
| Services in previous 4 weeks | ||
| Home help/home care assistant | 5.3 | 2.5 to 11.4 |
| Any nursing service | 1.2 | 0.6 to 2.6 |
| Chiropodist | 1.8 | 1.0 to 3.4 |
| Meals on wheels | 0.6 | 0.1 to 5.2 |
| Physiotherapist | 0.3 | 0.0 to 2.3 |
| Occupational therapist | 1.0 | 0.1 to 8.1 |
| Day centre | 7.4 | 2.8 to 19.7 |
| Day hospital | 2.0 | 0.4 to 9.2 |
| GP | 1.6 | 0.8 to 3.0 |
| Any service | 7.7 | 2.2 to 26.5 |
| Hospital services | ||
| Casualty/outpatient | 0.9 | 0.5 to 1.8 |
| Day patient | 1.0 | 0.4 to 2.3 |
| Inpatient | 2.2 | 1.1 to 4.2 |
Dementia as well as any one of the target comorbidities
| Service use | IRR | 95% CI |
|---|---|---|
| Any day-to-day service (count) | 1.4 | 1.0 to 1.8 |
| Any service in previous 4 weeks (count) | 1.6 | 1.3 to 2.0 |
| Outpatient in last 3 months (count) | 0.8 | 0.5 to 1.2 |
| Day patient in last year (count) | 1.1 | 0.5 to 2.2 |
| Inpatient in last year (count) | 1.8 | 1.3 to 2.6 |
| OR | 95% CI | |
| Day-to-day services | ||
| Home help | 1.5 | 0.5 to 4.0 |
| Care worker | 8.0 | 4.6 to 14.0 |
| Warden | 0.4 | 0.1 to 3.2 |
| Paid help | 0.3 | 0.1 to 0.8 |
| Other day-to-day services | 1.9 | 0.4 to 9.9 |
| Any service | 2.3 | 1.5 to 3.8 |
| Unpaid services | 4.1 | 2.5 to 6.6 |
| Services in previous 4 weeks | ||
| Home help/home care assistant | 5.2 | 3.1 to 8.7 |
| Any nursing services | 2.2 | 1.4 to 3.5 |
| Chiropodist | 1.5 | 1.0 to 2.4 |
| Meals on wheels | 0.3 | 0.0 to 2.6 |
| Physiotherapist | 0.6 | 0.2 to 1.8 |
| Occupational therapist | 1.8 | 0.5 to 6.2 |
| Day centre | 7.2 | 3.8 to 13.6 |
| Day hospital | 3.4 | 1.0 to 10.9 |
| GP | 1.3 | 0.9 to 2.0 |
| Any service | 2.3 | 1.4 to 3.8 |
| Hospital services | ||
| Casualty/outpatient | 0.9 | 0.6 to 1.4 |
| Day patient | 0.6 | 0.3 to 1.2 |
| Inpatient | 1.9 | 1.2 to 3.0 |
Comparison analysis results (restricted to those aged ≥ 75 years and living in the community)
Dementia and comorbidity compared with dementia
Stroke
| Service use | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 3.2 | 1.9 to 5.3 | 1.5 | 1.0 to 2.4 |
| Any service in previous 4 weeks (count) | 1.5 | 1.0 to 2.4 | 2.0 | 1.3 to 3.0 |
| Outpatient in last 3 months (count) | 1.0 | 0.2 to 4.2 | 0.9 | 0.3 to 2.2 |
| Day patient in last year (count) | – | – | 2.2 | 0.7 to 6.6 |
| Inpatient in last year (count) | 3.9 | 1.2 to 12.2 | 3.3 | 1.8 to 5.9 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | 1.8 | 0.3 to 11.4 | 1.8 | 0.3 to 11.3 |
| Care worker | 4.7 | 1.4 to 15.2 | 3.8 | 1.5 to 9.6 |
| Warden | – | – | 1.2 | 0.1 to 12.7 |
| Paid help | – | – | 0.8 | 0.2 to 3.6 |
| Any professional service | 7.6 | 2.1 to 27.7 | 2.7 | 1.2 to 6.0 |
| Unpaid care | 3.3 | 1.0 to 10.9 | 1.9 | 0.8 to 4.3 |
| Services in previous 4 weeks | ||||
| Home help/home care assistant | 3.7 | 1.1 to 12.5 | 2.4 | 1.0 to 5.8 |
| Any nursing services | 0.9 | 0.2 to 3.6 | 2.4 | 1.0 to 6.0 |
| Chiropodist | 0.6 | 0.1 to 3.1 | 3.3 | 1.5 to 7.1 |
| Meals on wheels | 6.6 | 1.6 to 26.7 | – | – |
| Day centre | – | – | 4.0 | 1.5 to 10.5 |
| GP | 0.7 | 0.2 to 2.9 | 1.6 | 0.7 to 3.5 |
| Any service | 1.3 | 0.4 to 4.3 | 0.9 | 0.7 to 3.3 |
| Hospital services | ||||
| Casualty/outpatient | 1.4 | 0.3 to 7.3 | 0.9 | 0.3 to 2.8 |
| Day patient | – | – | 1.7 | 0.6 to 5.0 |
| Inpatient | 5.6 | 1.4 to 22.8 | 5.9 | 2.6 to 13.9 |
Diabetes
| Service use | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 1.1 | 0.5 to 2.1 | 1.3 | 0.8 to 2.2 |
| Any service in previous 4 weeks (count) | 1.5 | 0.8 to 2.5 | 1.8 | 1.1 to 2.9 |
| Outpatient in last 3 months (count) | 1.1 | 0.3 to 3.9 | 1.3 | 0.6 to 2.8 |
| Day patient in last year (count) | – | – | 1.7 | 0.4 to 7.9 |
| Inpatient in last year (count) | 1.2 | 0.3 to 4.0 | 1.1 | 0.6 to 2.2 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | – | – | 3.7 | 0.7 to 19.7 |
| Care worker | 0.9 | 0.2 to 3.7 | 1.0 | 0.3 to 2.7 |
| Warden | – | – | 1.3 | 0.1 to 13.1 |
| Paid help | – | – | 0.3 | 0.0 to 2.1 |
| Any service | 1.6 | 0.4 to 5.8 | 1.5 | 0.7 to 3.5 |
| Unpaid care | 1.7 | 0.5 to 6.0 | 1.7 | 0.8 to 3.8 |
| Services in previous 4 weeks | ||||
| Home help | 0.7 | 0.1 to 2.9 | 1.0 | 0.4 to 2.5 |
| Any nursing service | 3.7 | 1.0 to 14.2 | 3.6 | 1.5 to 8.6 |
| Chiropodist | 0.9 | 0.2 to 4.0 | 1.8 | 0.8 to 4.1 |
| Meals on wheels | 1.1 | 0.2 to 5.2 | 0.8 | 0.1 to 7.2 |
| Day centre | 3.8 | 0.7 to 20.9 | 1.6 | 0.5 to 5.0 |
| Day hospital | – | – | – | – |
| GP | 1.6 | 0.4 to 5.6 | 1.1 | 0.5 to 2.3 |
| Any service | 1.9 | 0.4 to 8.2 | 1.9 | 0.8 to 4.4 |
| Hospital services | ||||
| Casualty/outpatient | 0.8 | 0.2 to 3.8 | 1.5 | 0.6 to 4.0 |
| Day patient | – | – | 0.6 | 0.2 to 2.3 |
| Inpatient | 1.4 | 0.3 to 5.6 | 2.0 | 0.8 to 5.1 |
Vision impairment
| Service use | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 1.4 | 0.7 to 2.5 | 1.1 | 0.7 to 1.7 |
| Any service in previous 4 weeks (count) | 1.0 | 0.6 to 1.7 | 1.2 | 0.8 to 1.6 |
| Outpatient in last 3 months (count) | 1.5 | 0.4 to 5.4 | 1.2 | 0.6 to 2.4 |
| Day patient in last year (count) | – | – | 1.2 | 0.4 to 3.8 |
| Inpatient in last year (count) | 3.1 | 0.9 to 11.3 | 1.9 | 0.9 to 3.9 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | 0.8 | 0.1 to 7.7 | 0.5 | 0.1 to 4.9 |
| Care worker | 3.4 | 1.0 to 12.3 | 1.5 | 0.6 to 3.9 |
| Warden | – | – | 0.9 | 0.1 to 8.7 |
| Paid help | 0.4 | 0.0 to 4.7 | 0.2 | 0.0 to 1.5 |
| Any professional day-to-day service | 1.6 | 0.5 to 5.0 | 1.5 | 0.6 to 3.6 |
| Unpaid care | 1.6 | 0.5 to 5.0 | 2.1 | 0.9 to 4.9 |
| Services in previous 4 weeks | ||||
| Home help/home care assistant | 2.8 | 0.8 to 10.5 | 1.0 | 0.4 to 2.6 |
| Any nursing service | 0.6 | 0.1 to 2.7 | 1.0 | 0.4 to 2.5 |
| Chiropodist | 0.6 | 0.1 to 3.1 | 2.1 | 1.0 to 4.7 |
| Meals on wheels | 4.1 | 1.0 to 17.4 | 0.6 | 0.1 to 5.9 |
| Day centre | – | – | 0.8 | 0.3 to 2.6 |
| Day hospital | – | – | – | – |
| GP | 0.4 | 0.1 to 1.7 | 1.1 | 0.5 to 2.5 |
| Any service | 0.6 | 0.2 to 1.9 | 3.3 | 1.2 to 8.7 |
| Hospital services | ||||
| Casualty/outpatient | 2.9 | 0.6 to 14.0 | 1.0 | 0.4 to 2.5 |
| Day patient | – | – | 1.3 | 0.4 to 3.9 |
| Inpatient | 3.4 | 0.7 to 16.1 | 1.9 | 0.8 to 4.7 |
Target comorbidities
| Service counts | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 2.0 | 1.1 to 3.6 | 1.5 | 1.0 to 2.4 |
| Any service in previous 4 weeks (count) | 1.4 | 0.9 to 2.1 | 2.1 | 1.5 to 2.9 |
| Outpatient in last 3 months (count) | 0.8 | 0.3 to 2.5 | 1.1 | 0.6 to 2.0 |
| Day patient in last year (count) | 0.9 | 0.1 to 12.9 | 1.6 | 0.5 to 4.9 |
| Inpatient in last year (count) | 4.6 | 1.2 to 18.3 | 2.3 | 1.2 to 4.4 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | 0.6 | 0.1 to 3.3 | 3.6 | 0.6 to 20.2 |
| Care worker | 2.9 | 0.9 to 9.1 | 2.2 | 1.0 to 4.8 |
| Warden | – | – | 0.5 | 0.0 to 4.6 |
| Paid help | – | – | 0.6 | 0.2 to 1.8 |
| Any professional day-to-day service | 2.4 | 0.9 to 6.7 | 2.3 | 1.2 to 4.6 |
| Unpaid care | 1.8 | 0.7 to 4.8 | 2.5 | 1.3 to 4.7 |
| Services in previous 4 weeks | ||||
| Home help/home care assistant | 1.6 | 0.5 to 4.9 | 1.2 | 0.6 to 2.6 |
| Any nursing service | 2.6 | 0.8 to 8.9 | 3.1 | 1.5 to 6.6 |
| Chiropodist | 0.8 | 0.3 to 2.5 | 3.3 | 1.7 to 6.6 |
| Meals on wheels | – | – | 0.2 | 0.0 to 2.1 |
| Day centre | 3.5 | 0.6 to 21.8 | 1.7 | 0.7 to 4.3 |
| Day hospital | – | – | – | – |
| GP | 1.1 | 0.4 to 3.1 | 1.4 | 0.8 to 2.6 |
| Any service | 0.9 | 0.3 to 2.3 | 2.8 | 1.6 to 5.1 |
| Hospital services | ||||
| Casualty/outpatient | 1.5 | 0.4 to 5.4 | 0.8 | 0.4 to 1.8 |
| Day patient | 0.8 | 0.0 to 13.9 | 0.9 | 0.3 to 2.3 |
| Inpatient | 7.1 | 1.8 to 27.4 | 3.1 | 1.5 to 6.6 |
Dementia and comorbidity compared with comorbidity alone
Dementia as well as stroke
| Service counts | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 2.0 | 1.3 to 3.1 | 1.2 | 0.8 to 1.9 |
| Any service in previous 4 weeks (count) | 1.1 | 0.7 to 1.6 | 1.6 | 1.1 to 2.3 |
| Outpatient in last 3 months (count) | 0.6 | 0.1 to 2.4 | 0.4 | 0.2 to 1.0 |
| Day patient in last year (count) | – | – | 1.4 | 0.6 to 3.4 |
| Inpatient in last year (count) | 3.5 | 1.4 to 9.0 | 1.9 | 1.2 to 3.1 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | 2.8 | 0.4 to 18.0 | 1.1 | 0.2 to 6.0 |
| Care worker | 5.5 | 1.9 to 16.1 | 9.3 | 3.8 to 22.5 |
| Warden | – | – | 1.0 | 0.1 to 9.0 |
| Paid help | 1.4 | 0.3 to 7.5 | 0.3 | 0.1 to 1.2 |
| Any professional day-to-day service | 4.1 | 1.2 to 13.7 | 2.1 | 1.0 to 4.3 |
| Unpaid care | 1.2 | 0.4 to 3.6 | 1.9 | 0.9 to 4.3 |
| Services in the previous 4 weeks | ||||
| Home help/home care assistant | 4.1 | 1.3 to 12.7 | 5.8 | 2.5 to 13.6 |
| Any nursing service | 0.7 | 0.2 to 2.6 | 2.2 | 0.9 to 5.2 |
| Chiropodist | 0.4 | 0.1 to 1.8 | 2.1 | 1.0 to 4.2 |
| Day centre | 2.4 | 0.5 to 11.2 | 10.0 | 4.0 to 25.1 |
| GP | 0.6 | 0.2 to 2.2 | 1.1 | 0.5 to 2.4 |
| Any service | 0.6 | 0.2 to 1.8 | 0.8 | 0.4 to 1.7 |
| Hospital services | ||||
| Casualty/outpatient | 0.9 | 0.2 to 4.5 | 0.6 | 0.2 to 1.8 |
| Day patient | – | – | 0.9 | 0.4 to 2.4 |
| Inpatient | 4.9 | 1.4 to 17.7 | 2.5 | 1.2 to 5.4 |
Dementia as well as diabetes
| Service counts | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 1.2 | 0.6 to 2.2 | 1.1 | 0.7 to 1.8 |
| Any service in previous 4 weeks (count) | 1.0 | 0.6 to 1.7 | 1.4 | 0.9 to 2.2 |
| Outpatient in last 3 months (count) | 0.5 | 0.2 to 1.4 | 0.6 | 0.3 to 1.2 |
| Day patient in last year (count) | 0.4 | 0.1 to 2.7 | 1.0 | 0.2 to 4.3 |
| Inpatient in last year (count) | 1.3 | 0.5 to 3.6 | 0.9 | 0.5 to 1.6 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | – | – | 2.1 | 0.5 to 9.7 |
| Care worker | 1.8 | 0.5 to 6.8 | 5.0 | 1.8 to 14.1 |
| Warden | – | – | 0.8 | 0.1 to 7.1 |
| Paid help | 0.8 | 0.1 to 7.0 | 0.1 | 0.0 to 0.8 |
| Other | – | – | 1.1 | 0.1 to 9.6 |
| Any professional day-to-day service | 1.9 | 0.5 to 6.3 | 1.4 | 0.7 to 3.1 |
| Unpaid care | 0.9 | 0.3 to 2.8 | 2.8 | 1.3 to 5.9 |
| Services in previous 4 weeks | ||||
| Home help/home care assistant | 1.3 | 0.3 to 5.6 | 3.7 | 1.4 to 9.4 |
| Any nursing service | 1.3 | 0.4 to 4.4 | 2.4 | 1.1 to 5.4 |
| Chiropodist | 0.4 | 0.1 to 1.5 | 0.9 | 0.4 to 2.0 |
| Meals on wheels | 1.6 | 0.4 to 6.8 | 0.6 | 0.1 to 5.7 |
| Day centre | 2.8 | 0.6 to 12.6 | 6.8 | 2.2 to 21.0 |
| GP | 1.0 | 0.3 to 3.4 | 0.9 | 0.4 to 1.8 |
| Any service | 0.6 | 0.1 to 2.4 | 1.0 | 0.5 to 2.2 |
| Hospital services | ||||
| Casualty/outpatient | 0.3 | 0.1 to 1.4 | 0.9 | 0.4 to 2.4 |
| Day patient | 0.5 | 0.1 to 4.1 | 0.4 | 0.1 to 1.4 |
| Inpatient | 1.4 | 0.4 to 4.8 | 1.3 | 0.5 to 3.0 |
Dementia as well as vision impairment
| Service counts | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 1.5 | 0.9to 2.6 | 1.1 | 0.7 to 1.7 |
| Any service in previous 4 weeks (count) | 0.9 | 0.6 to 1.6 | 1.3 | 1.0 to 1.6 |
| Outpatient in last 3 months (count) | 0.9 | 0.3 to 3.0 | 0.6 | 0.3 to 1.1 |
| Day patient in last year (count) | 0.4 | 0.1 to 2.7 | 0.8 | 0.3 to 2.1 |
| Inpatient in last year (count) | 3.5 | 1.1 to 11.5 | 1.7 | 0.9 to 3.2 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | 2.1 | 0.2 to 19.3 | 0.5 | 0.1 to 4.4 |
| Care worker | 5.8 | 1.8 to 19.2 | 6.4 | 2.6 to 15.5 |
| Warden | – | – | 0.9 | 0.1 to 7.8 |
| Paid help | 0.2 | 0.0 to 1.9 | 0.1 | 0.0 to 0.7 |
| Any professional day-to-day service | 2.0 | 0.7 to 5.9 | 1.6 | 0.7 to 3.7 |
| Unpaid care | 0.9 | 0.3 to 2.5 | 2.9 | 1.3 to 6.7 |
| Services in previous 4 weeks | ||||
| Home help/home care assistant | 4.4 | 1.3 to 15.0 | 3.4 | 1.3 to 8.5 |
| Any nursing service | 0.5 | 0.1 to 1.9 | 1.0 | 0.4 to 2.4 |
| Chiropodist | 0.5 | 0.1 to 2.5 | 1.8 | 0.9 to 3.8 |
| Meals on wheels | – | – | 0.8 | 0.1 to 6.6 |
| Day centre | – | – | 6.5 | 2.0 to 20.7 |
| GP | 0.4 | 0.1 to 1.6 | 1.1 | 0.5 to 2.2 |
| Any service | 0.4 | 0.1 to 1.1 | 2.9 | 1.1 to 7.5 |
| Hospital services | ||||
| Casualty/outpatient | 1.9 | 0.4 to 8.5 | 0.7 | 0.3 to 1.5 |
| Day patient | 0.5 | 0.1 to 4.5 | 0.8 | 0.3 to 2.3 |
| Inpatient | 3.4 | 0.8 to 14.7 | 1.5 | 0.7 to 3.4 |
Dementia as well as any one of the target comorbidities
| Service counts | CFAS I | CFAS II | ||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Any day-to-day service (count) | 1.6 | 1.1 to 2.4 | 1.1 | 0.8 to 1.6 |
| Any service in previous 4 weeks (count) | 1.1 | 0.8 to 1.5 | 1.4 | 1.1 to 1.8 |
| Outpatient in last 3 months (count) | 0.5 | 0.2 to 1.2 | 0.5 | 0.3 to 0.8 |
| Day patient in last year (count) | 0.1 | 0.0 to 1.0 | 0.9 | 0.4 to 2.1 |
| Inpatient in last year (count) | 2.6 | 1.3 to 5.5 | 1.4 | 0.9 to 2.2 |
| OR | 95% CI | OR | 95% CI | |
| Day-to-day services | ||||
| Home help | 1.6 | 0.3 to 8.1 | 1.5 | 0.5 to 4.4 |
| Care worker | 4.2 | 1.9 to 9.0 | 5.6 | 3.0 to 10.4 |
| Warden | – | – | 0.5 | 0.1 to 3.8 |
| Paid help | 0.8 | 0.2 to 2.6 | 0.2 | 0.1 to 0.6 |
| Other day-to-day services | – | – | 0.7 | 0.1 to 5.7 |
| Any service | 2.0 | 1.0 to 4.3 | 1.5 | 0.9 to 2.6 |
| Unpaid services | 0.8 | 0.4 to 1.6 | 2.7 | 1.6 to 4.5 |
| Services in previous 4 weeks | ||||
| Home help/home care assistant | 2.9 | 1.2 to 6.7 | 4.1 | 2.3 to 7.6 |
| Any nursing services | 1.2 | 0.5 to 2.6 | 2.0 | 1.1 to 3.5 |
| Chiropodist | 0.5 | 0.2 to 1.4 | 1.6 | 1.0 to 2.7 |
| Meals on wheels | 5.3 | 2.2 to 12.8 | 0.4 | 0.0 to 3.2 |
| Physiotherapist | 4.5 | 0.6 to 36.0 | 0.6 | 0.2 to 2.1 |
| Occupational therapist | – | – | 2.3 | 0.6 to 8.6 |
| Day centre | 1.8 | 0.6 to 5.5 | 7.2 | 3.4 to 15.1 |
| GP | 0.9 | 0.4 to 1.9 | 1.1 | 0.7 to 1.7 |
| Any service | 0.5 | 0.2 to 1.0 | 1.2 | 0.8 to 2.0 |
| Hospital services | ||||
| Casualty/outpatient | 0.8 | 0.3 to 2.1 | 0.6 | 0.3 to 1.1 |
| Day patient | 0.2 | 0.0 to 1.3 | 0.6 | 0.3 to 1.2 |
| Inpatient | 3.1 | 1.3 to 7.1 | 1.5 | 0.9 to 2.6 |
List of abbreviations
- AIS
- acute ischaemic stroke
- AMD
- age-related macular degeneration
- CFAS
- Cognitive Function and Ageing Studies
- CGA
- comprehensive geriatric assessment
- CI
- confidence interval
- DeNDRoN
- Clinical Research Network Dementias and neurodegeneration
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- GMS-AGECAT
- Geriatric Mental State – Automated Geriatric Examination for Computer Assisted Taxonomy
- GP
- general practitioner
- HCP
- health-care professional
- IPU
- integrated practice unit
- IV tPA
- intravenous tissue plasminogen activator
- MCI
- mild cognitive impairment
- MMSE
- Mini Mental State Examination
- MRC
- Medical Research Council
- NETSCC
- NIHR Evaluation, Trials and Studies Coordinating Centre
- NIHR
- National Institute for Health Research
- PLWD
- person/people living with dementia
- QOF
- Quality Outcomes Framework
- RCT
- randomised controlled trial
- VI
- vision impairment