Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5005/12. The contractual start date was in October 2013. The final report began editorial review in October 2015 and was accepted for publication in February 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
A Toby Prevost was a member of National Institute for Health and Care Excellence Public Health Advisory committees during the period of this research and has been a member of the National Institute for Health Research Public Health Research funding board since January 2014.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Gulliford et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Obesity is a growing global health concern1 and is second to smoking as a leading cause of preventable death globally. The prevalence of obesity is increasing in most countries, with especially rapid increases in high- and middle-income countries. 1 There is some evidence that the rate of increase in obesity may have decelerated in recent years. 2 This is not so for severe obesity, with body mass index (BMI) ≥ 35 kg/m2, and morbid obesity, with BMI ≥ 40 kg/m2, which continue to increase very rapidly. People with morbid obesity are at increased risk of several forms of morbidity, including type 2 diabetes mellitus (T2DM), cardiovascular diseases and depression, leading to the development of multiple morbidity at young ages3 and heightened risk of mortality. 4 These processes lead to reduced quality of life and reduced longevity, as well as increased health-care costs.
Surgical interventions for obesity, referred to as bariatric surgery, have emerged as offering important potential benefits. Randomised controlled trials (RCTs) with generally small samples, and up to 2 years’ follow-up, have shown important early reductions in body weight,5 with a mean weight reduction of 26 kg following the procedure. Remission of T2DM is also well documented. 6 Longer-term follow-up in cohort studies has suggested reduced incidence of T2DM7,8 and other long-term conditions,9 as well as reduced mortality. 5,10 The types of surgical procedure used have evolved over time, with declining use of adjustable gastric banding and increasing use of gastric bypass (GBP) and sleeve gastrectomy (SG) procedures. 11 New evidence has emerged concerning longer-term outcomes and costs12,13 of bariatric surgery, including effects on mortality,10 disease incidence7 and diabetes remission. 14,15 This has been accompanied by a growing recognition that the primary purpose of bariatric surgery is not a cosmetic one of reducing body weight; use of surgery should aim to improve the prognosis of patients with morbid obesity by reducing the incidence, and improving the control, of long-term conditions through incompletely understood mechanisms that are not entirely weight-dependent.
Overweight and obesity in the UK
In England, the proportion of adults who are overweight or obese has increased from 52.9% in 1993 to 62.8% in 2010, while obesity has increased from 14.9% to 26.1% of adults over the same period. 16 Individuals with obesity are classified as having severe obesity when their BMI is 35–39.9 kg/m2 and morbid obesity when their BMI is ≥ 40 kg/m2. The increase in severe and morbid obesity in England has been extremely rapid. From 1993 to 2010, morbid obesity increased eightfold from 0.2% to 1.6% of all men, and nearly tripled from 1.4% to 3.8% of all women. 16 Cardiovascular mortality is declining, and life expectancy is increasing, but these favourable trends are threatened by the increase in obesity and diabetes.
Obesity is associated with a wide range of negative health consequences, and these risks increase with increasing BMI. Severe and morbid obesity are independently associated with increased incidence of long-term conditions including T2DM, cardiovascular diseases and multiple cancer types. 17,18 In the UK, obesity may account for 4% of all cancers in men (including 27% of oesophageal cancer and 25% of kidney cancer) and 7% of cancers in women (including 9% of breast cancers and 34% of endometrial cancers). 19 Symptomatic conditions associated with obesity include asthma, joint problems, back pain and depressive symptoms. Obesity is associated with states of elevated risk and pre-disease including hypertension and hyperlipidaemia. Morbidity generally begins at younger ages in obese people and multiple morbidity becomes frequent as the condition progresses. 3 Increasing BMI is associated with shorter lifespan, with each additional 5 kg/m2 related to a 30% increase in mortality. 20 Excess weight has been estimated to account for 8% of premature deaths in men and 12% in women, with these figures rising to 18% when smokers are excluded. 21 The impacts of obesity are unequally distributed, being more frequent in women and lower socioeconomic groups, contributing to inequalities in health. 22
Severe and morbid obesity are associated with substantial increases in health-care utilisation and costs related to the management of obesity and associated comorbidities. The health-care costs of diabetes alone are estimated to be about £14B per year in the UK. In 2006–7 it was estimated that over £5B was spent on ill health due to overweight and obesity. 23 More recently, it has been predicted that an excess 545,000 cases of diabetes, 331,000 of coronary heart disease (CHD) and stroke, and 87,000 of cancer will occur in the UK as a result of the continuing increase in obesity. This would amount to an additional £613M in health-care spending by 2020. 24 These estimates do not consider the substantial indirect costs of obesity. 25 Given the negative impact of severe and morbid obesity on health and health-care costs, weight loss has been proposed to lead to important health and economic benefits. The Office for Health Economics estimated that wider use of obesity surgery may give economic benefits through reduced welfare payments and additional paid work. 26
Management of obesity
Reductions in the body weight of overweight and obese individuals are associated with improvements in cardiovascular risk factors such as cholesterol and blood pressure. A reduced risk of death from cardiovascular disease, cancer and T2DM together with lower risk of new-onset T2DM have also been linked to intentional weight loss. 27 The majority of obesity management interventions utilise lifestyle changes focused on diet and physical activity to reduce body weight, with weight-loss drugs also playing a more limited role. At present only a small proportion of obese patients access these interventions through UK primary health-care services, with > 80% of obese and > 50% of morbidly obese patients having no weight management intervention recorded over a 7-year period. 28 When these interventions are utilised, clinically meaningful weight loss is rarely achieved, with systematic reviews identifying only small reductions in body weight, with weight regain being frequent. 29,30
Bariatric surgery for obesity
An alternative and increasingly used strategy for the treatment of obesity is bariatric surgery.
The term bariatric surgery refers to surgical procedures that are designed to promote weight loss in obese individuals. A number of different bariatric surgical procedures are in current use; these have traditionally been classified as ‘restrictive’, ‘malabsorptive’ or ‘mixed’ procedures. Restrictive procedures reduce gastric volume, leading to reduced dietary intakes. Laparoscopic-adjustable gastric banding (LAGB) was the most common procedure before 2009 but now accounts for < 20% of procedures. 11 LAGB is associated with fewer complications than more invasive procedures, but also has a smaller effect on body weight. The Roux-en-Y GBP operation employs a mixed approach, with the creation of a reduced stomach pouch connected to the distal small intestine. The GBP procedure generally has a greater effect on body weight, but carries a greater risk of short- and long-term complications than a restrictive procedure. In the USA, GBP accounts for half to two-thirds of procedures, while in the UK it accounts for 46.6%. 11 However, the use of SG, where a large portion of the stomach is removed, has been increasing and now makes up 21% of operations at UK centres. 11 Publication of the UK National Bariatric Surgery Register (NBSR) data, which covered the years 2011–13, found that these three operations constitute 95% of bariatric surgeries in the UK. These operations are conducted laparoscopically in 95.4% of cases. 11
Weight loss after bariatric surgery can lead to a mean weight reduction of 26 kg [95% confidence interval (CI) –31 kg to –21 kg] when compared with non-surgical treatment6 and this effect can be maintained over 10 years. 5 GBP and SG are associated with greater weight loss than gastric banding, although operative complication rates are lower with the latter procedure. 31,32 Surgery is associated with lower incidence of comorbidity than non-surgical management,33 and mortality is reduced. 5 Resolution of comorbidity may be more relevant to health-care costs than weight loss, and is not always directly associated with weight loss. After GBP surgery there is a high rate of early resolution of T2DM which may precede maximal weight loss.
The literature suggests that bariatric surgery is generally more costly than non-surgical management of obesity. 31 Ackroyd et al. 34 reported incremental costs of more than £2000 per participant over the first 5 years. This highlights a concern for policy-makers: that the health-care costs of surgical intervention are generally immediate or short-term while gains, in terms of health benefits and costs, are delayed. A Health Technology Assessment conducted by Picot et al. 31 in 2009 investigated the clinical effectiveness and cost-effectiveness of bariatric surgery for obesity. Their findings showed a low to moderate probability that surgery is cost-effective within 2 years, but that over a 20-year time horizon there is a very high probability that bariatric surgery will prove cost-effective at thresholds of £20,000 or £30,000 per quality-adjusted life-year (QALY). 31,34 The Picot review investigated use of surgery for obesity from an individual patient perspective, which this analysis will complement by estimating the cost-effectiveness of different levels of surgery uptake from a population perspective. In addition, we will utilise more recent evidence from the growing literature on bariatric surgery and our own epidemiological analyses to update the findings of Picot et al. , in areas where there were previously few data reported. The value of estimates varies in different health-care systems and according to the type of surgical procedure and duration of follow-up. However, although a range of estimates have been produced by different studies,25 most suggest that bariatric surgery will generally be cost-effective in the treatment of individuals with severe or morbid obesity. An Office for Health Economics26 model reported that economic impacts were appreciable when indirect costs including estimated hours worked and welfare benefits were considered.
Adverse effects of bariatric surgery
The mortality rate associated with GBP surgery is approximately 0.5%. In addition, there are longer-term morbidity concerns associated with bariatric operations. Gastric banding is associated with a significant risk of erosion and band slippage rate; GBP patients can re-present with internal hernias. Other complications may include vomiting, leaks and gastrointestinal symptoms. Patients who have received bariatric surgery require long-term monitoring and this has significant cost implications.
Eligibility and access to bariatric surgery
The increasing evidence for the clinical effectiveness and cost-effectiveness of bariatric surgery raises questions concerning the selection of patients for surgery. The National Institute for Health and Care Excellence (NICE),35 in its guidelines on obesity, recommended that bariatric surgery should be considered (1) for individuals who have a BMI ≥ 40 kg/m2, or (2) for individuals with BMI 35–40 kg/m2 if comorbidities that could be improved through weight loss are present, and (3) if non-surgical management has not achieved sufficient weight loss over 6 months, the individual is committed to long-term follow-up, is fit for surgery and can be treated in a specialist surgical service.
Presently, access to bariatric surgery in the UK is restricted. Based on the age-specific prevalence for morbid obesity reported in the Health Survey for England,16 there were approximately 336,000 men and 806,000 women with morbid obesity alone in England in 2010, of whom 303,000 men and 676,000 women were aged 25–74 years. Approximately 8000 procedures for obesity are implemented annually in England,36 accounting for about 0.5% of morbidly obese individuals. This contrasts with about 28,000 coronary artery bypass grafts performed annually. Bariatric operation rates vary widely among English regions37 and there is also significant uptake of surgery in the private sector. 36 Based on a combination of epidemiological data, current clinical practice and expert opinion, NICE guidance suggested a population benchmark rate for bariatric surgical procedures, to be achieved in 5 years’ time, of 0.01% of the general population per year. 38 This implies that only a small minority of people with severe or morbid obesity would receive bariatric surgery. However, the long-term costs and outcomes of deploying bariatric surgery across the population at the rate suggested by NICE, or other rates, are not known.
The International Diabetes Federation (IDF) has recently challenged prevailing thinking by advancing a more liberal approach to the use of bariatric surgery in relation to diabetes. The IDF39 now recommends surgery for people with type 2 diabetes and a BMI of ≥ 35 kg/m2 and suggests surgery may be considered as a treatment option in patients with a BMI between 30 and 35 kg/m2 and poorly controlled T2DM on medical treatment. Its position statement proposes a significant expansion in the criteria for utilisation of bariatric surgery, specifically for people who have both T2DM and obesity.
At the population level, the role of surgery in the treatment of obesity will depend on the costs and health benefits achieved at different levels of uptake of bariatric surgery in the population at risk. Thus, a population strategy for bariatric surgery should consider the benefits, harms and costs that accrue both to those who do not receive surgery, and to those who do. This requires consideration of the impact of intervention on the prevalence of different categories of obesity, the occurrence of morbidity and mortality and the impact on the quality and duration of life in relation to the expenditure of health-care resources. The societal distribution of outcomes and costs in terms of inequalities in health must also be considered. Groups who live in conditions of social and material deprivation have a higher prevalence of morbid obesity, especially in women, as well as higher mortality and shorter healthy life expectancy. This suggests that obesity surgery is likely to be more cost-effective in lower socioeconomic groups or in areas of greater deprivation. We acknowledge that the private sector plays a significant role in the delivery of bariatric surgery. According to the NBSR, approximately one-third of the operations registered were done in the private sector, although this may underestimate the true level of activity. We do not explicitly consider private sector activity in this report.
The research asks to what extent a publicly funded health-care system, such as the NHS, should facilitate access to bariatric surgery? What are the impacts of different levels of bariatric surgery activity on health-care costs and health outcomes across the population at risk? This research will provide policy-makers and commissioners of services with evidence on the potential cost-effectiveness of facilitating access to bariatric surgery in a population, such as England, that has a high prevalence of severe and morbid obesity.
Chapter 2 Aims and objectives
This project aims to evaluate the extent to which the NHS, as a publicly funded health-care system, would be justified in facilitating increased access to bariatric surgery, and for which groups of patients.
Specific objectives
The research aimed to develop a Markov model to evaluate the costs and outcomes of bariatric surgery in order to estimate the cost-effectiveness of the procedure.
The research specifically aimed to evaluate three intervention strategies:
-
Expanding access within existing recognised indications for bariatric surgery as defined by NICE.
-
Expanding access to bariatric surgery for people with T2DM as proposed by the IDF.
-
Expanding access with a focus on the distributional consequences of different intervention strategies. The research aimed to evaluate to what extent health outcomes and costs of bariatric surgery vary by gender, age group and among socioeconomic groups, thus evaluating the potential impacts on inequalities in health related to obesity.
The research drew on analysis of electronic health records (EHRs) data for a large population. These analyses, required for the health economic model, enabled us to address several other substantive research questions, including:
-
What is the probability of an obese person transitioning to normal body weight, or maintaining clinically important weight loss, in the absence of bariatric surgery?
-
What are the health-care costs associated with obesity? What are the drivers of health-care costs in obese people?
-
What are the current rates of utilisation of bariatric surgery? What complications may follow bariatric surgery procedures?
-
What effect does bariatric surgery have on the development of new T2DM in obese people?
-
What are the long-term effects of bariatric surgery on diabetes control and antidiabetes drug utilisation in obese people with T2DM?
-
What effect does bariatric surgery have on measures of clinical depression?
The outputs from this research aimed to provide those responsible for commissioning and organising surgical services, as well as patients and the public, with evidence to inform policies on the utilisation of bariatric surgery for populations in which severe and morbid obesity are frequent.
Chapter 3 Methods
Design and conceptual framework
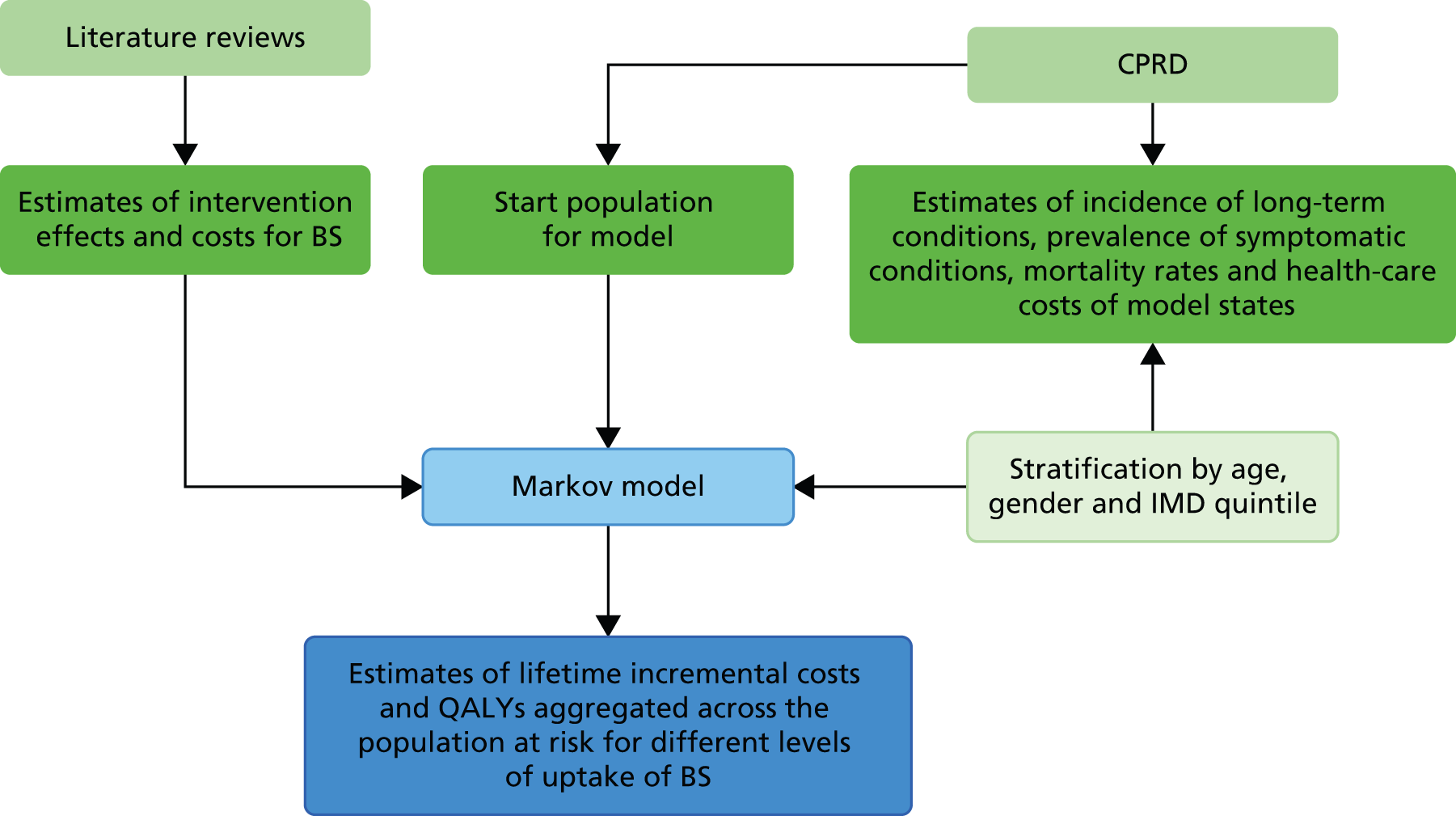
The overall design of the research is outlined in Figure 1. A Markov model was designed. Empirical data inputs to the model were provided through analysis of data for a large population registered in primary care, derived from the Clinical Practice Research Datalink (CPRD) (formerly known as General Practice Research Database). Estimates for the clinical effectiveness of bariatric surgery were derived from CPRD data analysis and updated systematic reviews. Probabilistic simulations, run using the model, provided estimates of lifetime incremental costs and QALYs aggregated across the population at risk.
FIGURE 1.
Schematic diagram outlining research. BS, bariatric surgery; IMD, Index of Multiple Deprivation.
Setting
The setting for the study was the general population with obesity, aged ≥ 20 years, in the UK.
Perspective
The research took the perspective of the NHS. Health service costs were included but social care costs were excluded. Wider societal costs, including changes in productivity, which are hard to estimate precisely, were not included. The research adopted a lifetime time horizon. Utilisation of bariatric surgical procedures in the private sector was not explicitly considered, though evidence emerged that a substantial proportion of procedures recorded in primary care electronic records may be performed privately.
Outcomes
The primary outcome was QALYs, after taking into account the incremental costs associated with intervention. Some simulations might be associated with negative incremental costs (where the intervention is cost saving) or negative incremental QALYs (as when standard care dominates), net health benefits40 were, therefore, estimated as:
where the threshold is the maximal acceptable value of cost per QALY; in the UK this is often taken as £30,000 per QALY. 41 Cost-effectiveness acceptability curves were also constructed using a range of values for the threshold.
Data source
The UK CPRD provided the source of EHRs for this study. The CPRD is the world’s largest primary care database, comprising anonymised longitudinal patient records from UK family practices. The CPRD presently holds more than 80 million person-years of research-quality data from 1990 onwards from more than 600 family practices. Data held within CPRD are considered to be broadly representative of the UK population based on the demographic characteristics of patients and the size and distribution of practices. 42,43 Scientific and ethics approval of the protocol for the study were given by the CPRD Independent Scientific Advisory Committee (ISAC 13_089).
Patient and public involvement
We organised a meeting for patients who had undergone bariatric surgery at St George’s Hospital to give an overview of the research, elicit any thoughts or concerns they had over the project and receive any potential research ideas. Interim findings were disseminated through new media as well as through scientific publications and meetings.
Empirical analysis of electronic health records data
The registered population of the CPRD was used to represent the target population for the study. CPRD general practices are located throughout the UK. CPRD data have been shown to be representative of the UK population in terms of age and sex distribution and deprivation category. The annual count of the CPRD registered population aged ≥ 20 years peaked at 3.7 million during this period, with a total of 7.1 million individual participants aged ≥ 20 years registered at any time between 2004 and 2014. Clinical diagnoses recorded into CPRD have high validity. 42 We have recently reported on clinical BMI recording in CPRD. 44 These analyses showed that there is under-recording of BMI in primary care, but individuals without BMI records have a low incidence of morbidity which suggests that under-recording may be more frequent in healthy individuals with normal body weight. A sample of CPRD general practices in England participate in the data-linkage scheme and offer linked data including Index of Multiple Deprivation (IMD) 2010 deprivation scores45 at individual participant postcode level, and Hospital Episode Statistics (HES) data. 46
Three cohorts of participants were selected from CPRD.
Bariatric surgery cohort
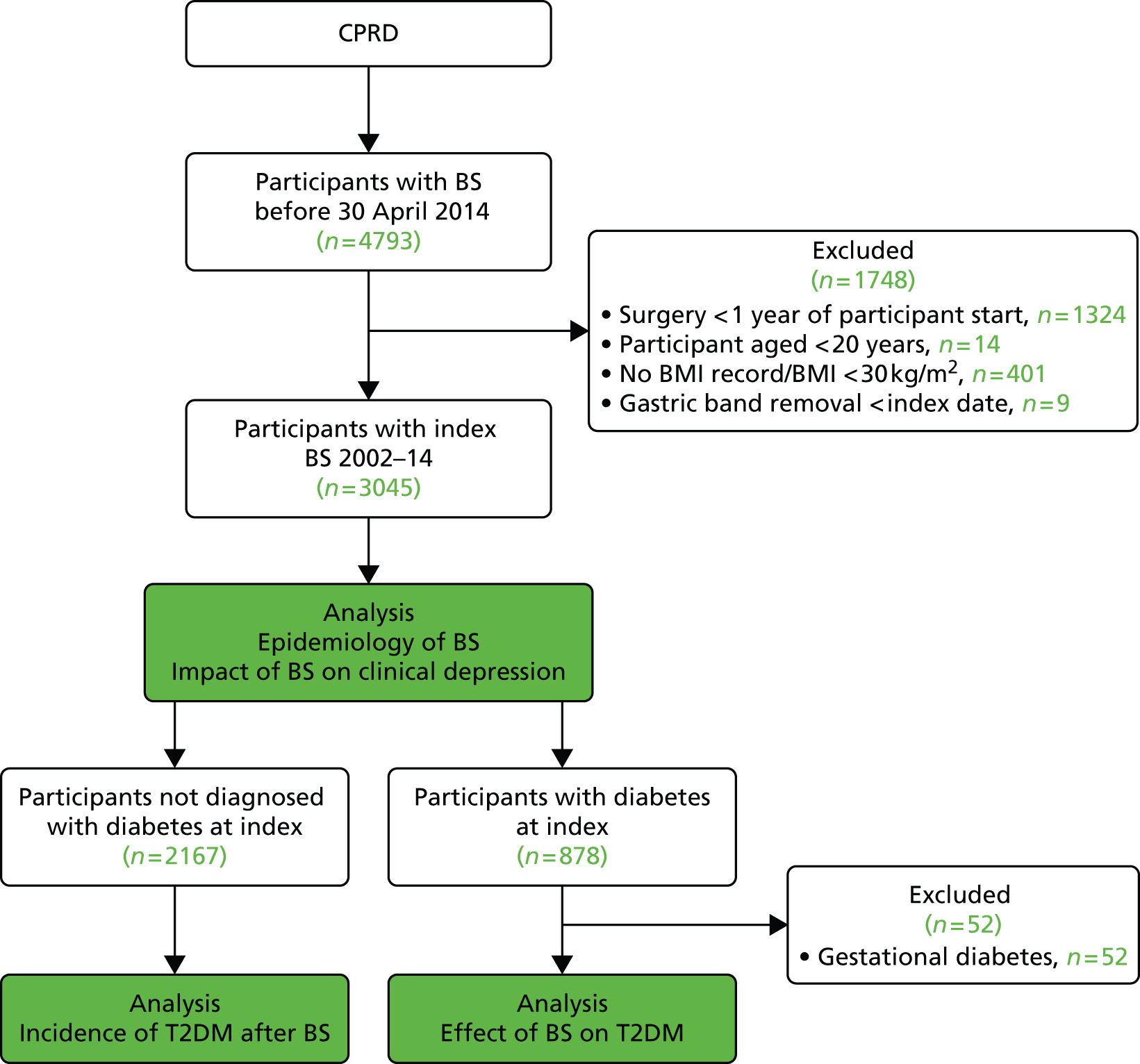
The sample comprised a cohort of adult obese patients with first bariatric surgery procedures performed, including all participants with LAGB, GBP or SG recorded before 30 April 2014. The date of the first procedure was taken as the index date. The earliest index event was in 2002. Participants were excluded if they were < 20 years of age. A lower age limit of 20 years was specified in the study protocol because we aimed to evaluate the use of bariatric surgery in adults. Use of bariatric surgery for individuals in their teens may be viewed as an outcome of childhood obesity and requires separate consideration. The mean age of 14 patients excluded was 18.5 years, range 17–19 years. Procedures recorded within 1 year of the participant start date in CPRD were also excluded because such records might refer to procedures performed before the patient’s registration at a CPRD practice. (When a patient joins a general practice, their previous notes are summarised and any significant diagnoses noted. A record of bariatric surgery within 12 months of joining a practice may refer to a procedure performed before the patient joined the practice.) Participants were excluded if they did not have a BMI record for obesity (BMI ≥ 30 kg/m2) prior to surgery. A minimum BMI value of 30 kg/m2 was employed to ensure that all participants were obese, but some BMI records dated from several years before operation and might not reflect preoperative BMI. Baseline BMI values were recorded a median of 1.6 years [interquartile range (IQR) 0.6 to 5.4 years] before surgery. Participants with gastric band removal recorded before the index date were also excluded. Additional exclusion criteria were employed in selected analyses, which are described in Figure 2. In the estimation of rates of bariatric surgical procedures, six participants with more than one procedure type coded on the index date were also excluded. In the analysis of T2DM incidence following bariatric surgery, participants with diabetes diagnosed on or before the index date were also excluded. In the analysis of bariatric surgery and diabetes remission, we included only participants diagnosed with T2DM prior to the index date. A diagnosis of T2DM was taken as the earlier of a medical diagnosis of diabetes, a prescription for antidiabetes medicines or a glycated haemoglobin (HbA1c) value of 48 mmol/mol (> 6.5%). Participants who were diagnosed with polycystic ovary syndrome and prescribed diabetes medicines, but not diagnosed with diabetes, were excluded from a diabetes diagnosis. Participants who were ever recorded as having a medical code for gestational diabetes were also excluded from these analyses. Blood glucose values were not used for diagnosis, nor assessment of remission, because distinctions between fasting or post-meal values are rarely clear in clinical records.
FIGURE 2.
Selection of bariatric surgical cohort. BS, bariatric surgery.
Reliability study
A sample of 102 participants who had bariatric surgery recorded in their CPRD records was selected for a reliability study in which EHR data were compared with general practitioner (GP)-reported information. The sample selected for study included approximately equal numbers of participants with EHR records for LAGB, SG or GBP. LAGB patients who had records of gastric band removal and GBP and SG patients who had repeat procedures were oversampled. Participants specifically selected included all 16 patients with GBP or SG who had repeat procedures recorded; all 43 LAGB patients who had band removal recorded and the initial procedure was in 2008 or later; and a random sample of 40 each with GBP and SG who had index date in 2008 or later. Participants with procedures from 2008 or later were selected because these were more likely to remain registered with the same general practice than patients treated longer ago. This gave 139 participants, of whom one was duplicated between the first and third criterion, and 138 participants remained eligible for the validation study. However, 36 were excluded because their general practice no longer contributed to CPRD, leaving 102 for further evaluation. The GP for each patient was sent a questionnaire (see Appendix 1) which included items concerning whether or not the patient had bariatric surgery, the date of surgery, the type of procedure, complications experienced, gastric band removal, operation reversal and repeat procedures. Multiple reminders were sent in order to optimise the response rate.
General population cohort stratified by body mass index category
We sampled a second cohort of participants from CPRD to act as a general population comparison sample. The sample was drawn from the list of all acceptable patients who were registered with CPRD practices that contributed to the data linkage scheme at any time between 1 January 2008 and 31 December 2014. For each participant, the mean of all of his or her BMI records was estimated. Then a stratified random sample was taken of up to 50,000 participants in each category of BMI: normal weight (18.5–24.9 kg/m2); overweight (25.0–29.9 kg/m2); obese (30.0–34.9 kg/m2); severe obesity (35.0–39.9 kg/m2); morbid obesity (40.0–44.9 kg/m2); and super-obese (≥ 45.0 kg/m2). Full CPRD data records were then extracted for this sample. There were 1819 participants who ever had bariatric surgery recorded who were excluded from the sample, together with one participant with undefined gender, leaving 257,187 patients for further analysis. The distribution of the sample by gender and BMI category is shown in Table 1. For selected analyses, we further restricted this sample to those participants who were eligible for linkage of IMD quintile and linked HES data to CPRD records. There were 250,046 participants eligible for HES data linkage and 247,537 eligible for linkage of IMD 2010 deprivation category.
| BMI category (kg/m2)a | Gender | Total | |
|---|---|---|---|
| Male | Female | ||
| 18.5–24.9 | 19,939 (18.6) | 30,058 (20.1) | 49,997 |
| 25.0–29.9 | 26,850 (25.0) | 23,139 (15.5) | 49,989 |
| 30.0–34.9 | 24,080 (22.4) | 25,855 (17.3) | 49,935 |
| 35.0–39.9 | 19,114 (17.8) | 30,604 (20.4) | 49,718 |
| 40.0–44.9 | 12,552 (11.7) | 27,317 (18.2) | 39,869 |
| ≥ 45.0 | 4934 (4.6) | 12,745 (8.5) | 17,679 |
| All | 107,469 | 149,718 | 257,187 |
The start was the later of 1 January 2008, the participant registration date, or the general practice CPRD start date. The end date was the earliest of 31 October 2014, the date death or end of registration, or the last data collection date for the general practice.
Sample for analysis of the probability of attaining normal body weight
Analysis of the general population sample yielded transition probabilities required for the health economic model. We recognised that further in-depth analysis of BMI transitions in this sample would be of interest. We therefore drew a sample that enabled analysis over a longer period of time, with the inclusion of participants with a larger number of BMI records.
There were 2,006,296 patients registered in CPRD between 1 November 2004 and 31 October 2014, who were aged ≥ 20 years and had three or more BMI records. A minimum of three BMI records per patient was required to estimate weight changes, including weight regain following weight loss, but most obese patients had more than three BMI records. Participants were classified according to the BMI value of their first record into the categories: 18.5–24.9 kg/m2 (normal weight); 25.0–29.9 kg/m2 (overweight); 30.0–34.9 kg/m2 (simple obesity); 35.0–35.9 kg/m2 (severe obesity); 40.0–44.9 kg/m2 (morbid obesity); and ≥ 45.0 kg/m2 (super obesity). A random sample of up to 30,000 participants was selected, using the ‘sample’ command in Stata version 13 (StataCorp LP, College Station, TX, USA), from each category of BMI and gender, resulting in a total of 314,477 participants. There were fewer than 30,000 women with BMI ≥ 45 kg/m2 and fewer than 30,000 men with either BMI 40–45 kg/m2 or ≥ 45 kg/m2. Full CPRD data records were then extracted for this sample. Data were analysed for research quality records for each participant. The start was the later of 1 November 2004, the participant registration date, or the general practice CPRD start date. The end date was the earliest of 31 October 2014, the date death or end of registration, or the last data collection date for the general practice. There were 2738 (1%) participants with bariatric surgery who were excluded, as were 32,757 (10%) who had fewer than three BMI values recorded between 1 November 2004 and 31 October 2014, leaving 278,982 participants for further analysis.
Sample size considerations
The large size of the general population cohort was sufficient to provide precise estimates of the parameters required for the Markov model. Estimates were expected to have acceptable precision, even after stratification by gender and six age groups. Table 2 provides our initial estimates, but the anticipated sample size was generally exceeded in this study.
| Measure | Assumptions | Precision (2 × standard error) | |
|---|---|---|---|
| Sample in obesity category | Stratified by age group and gender | ||
| Number | 80,000 | 6667 | |
| Prevalence | 50% | ±0.35% | ±1.2% |
| Prevalence | 2% | ±0.1% | ±0.34% |
| Incidence, stroke | 1 per 100047 | ||
| Person-years analysis over 5 years’ data | Upper limit: 1.10 | Upper limit: 1.4 | |
| Lower limit: 0.91 | Lower limit: 0.7 | ||
Statistical analysis methods
The aim of statistical analyses was to provide empirical inputs requires for the Markov model.
Body mass index probability analysis
In order to estimate the probability of a person transitioning to normal body weight in the absence of bariatric surgery, a longitudinal analysis of BMI records was conducted using a general population sample as outlined above. The start date for each participant was the later of 1 November 2004 or the beginning of the patient’s CPRD record. The end date was the earlier of 31 October 2014 or the end of the patient’s CPRD record. The first BMI record after the participant start date was used as the index BMI and the date of this record was used as the index date. The number of BMI records was evaluated for each BMI category and the number of records showing either an increase or a decrease in BMI category was calculated. For patients who showed a decrease in BMI category, we evaluated whether subsequent changes in BMI category represented increases or further decreases. Data were analysed in a time-to-event framework to evaluate, first, the proportion of patients from each starting BMI category who attained either normal body weight, or, second, a 5% reduction in body weight during 9 years’ follow-up. A 5% reduction in body weight was not envisaged in the original study protocol but was added as a minor amendment because this is a widely recommended target for body weight reduction. 35 In the first analysis, the annual probability of attaining normal body weight was estimated. The number of events (BMI category recorded as < 25 kg/m2) and the person-years of follow-up were used to estimate the annual rates, and their CIs, which were converted to annual probabilities using the formula, 1-exp-rate. Among participants who reduced BMI category, the direction of the next change in BMI category was evaluated. In the second analysis, to examine the participants who had lost 5% of body weight, the development of a body weight that was more than 95% of the initial body weight was also evaluated in a time-to-event framework. Analyses were conducted in Stata version 13 using the stset, sts list and stcox commands.
Health-care costs in obesity
To assess costs related to obesity in the general population sample, person-time was allocated to BMI category according to the most recent BMI value, combining records ≥ 40 kg/m2 into a single group to represent morbid obesity. Comorbidity status was evaluated using medical diagnoses coded into EHRs during general practice consultations. Person-time was classified according to morbidity status using the first diagnosis of T2DM, CHD, stroke or cancer. Depression was re-evaluated in each year of follow-up and patients were considered to have depression if they were diagnosed with depression in year or if they were prescribed antidepressants in year and were ever diagnosed with depression. Morbidities, including T2DM,48 CHD,49 stroke50 and depression,51 were evaluated using medical codes reported previously, while cancer diagnoses were evaluated using codes for malignant neoplasms.
Health-care utilisation was estimated from participants’ EHRs, with linked HES data. Primary and secondary care utilisation was evaluated including primary care consultations at the practice, by telephone, at home, emergency and out-of-hours. Secondary care utilisation included admissions to hospital, outpatient, day-case and emergency visits. All drug prescriptions issued by the practice were evaluated. Utilisation rates were calculated using person-time at risk. Age-standardised rates were estimated using direct standardisation and the European Standard Population for reference.
The costs of health-care utilisation were evaluated for participants by morbidity and depression status within BMI categories for the period 2008 to 2013. The annual costs were estimated by multiplying the health-care utilisation associated with each state by the costs of each unit of health care. Unit costs were derived from reference sources based on 2013 price estimates. The Personal Social Service Research Unit (PSSRU) publication Unit Costs of Health and Social Care 2013 was used as the main reference source (Table 3). 52 The same unit costs were applied across different ages, genders, BMI categories and morbidity status. Primary care GP consultation, emergency or out-of-hours, home visit and telephone consultations were priced at £45, £45, £114 and £27, respectively. Unit costs of secondary care inpatient, outpatient, day-case and emergency visits were priced at £1400, £135, £697 and £135, respectively. To assess prescription costs, drug codes for prescriptions in the EHRs were linked with costs from a dictionary compiled by RESIP UK (RESIP UK, Chertsey, Surrey, UK).
| Type of care | Unit cost 2013 (£) | Comment |
|---|---|---|
| GP consultations | 45 | Includes emergency consultations |
| Telephone consultations | 27 | |
| Home visits | 114 | |
| Day case | 697 | |
| Emergency referral | 135 | From outpatient |
| Inpatient | 1400 | Weighted average of all stays |
| Outpatient | 135 | Weighted average of all outpatients |
A two-part model53,54 was used to analyse health-care utilisation costs. In the first stage, a probit model was employed to estimate the probability of health-care utilisation being non-zero. In the second stage, a generalised linear model (GLM) with a log link and gamma errors was used to evaluate the distribution of costs in participants who utilised health care. This approach provided estimates of the predicted mean costs for men and women in different BMI and morbidity categories for each year of age. In the final stage of analysis, a linear regression model was employed to estimate the effects of BMI category, comorbidity and depression on predicted health-care costs controlling for patient gender and age. Interaction terms for comorbidity and depression and for comorbidity and BMI category were included. In order to make the data sufficiently concise for presentation, diabetes, CHD, stroke and cancer were combined into a single category of ‘comorbidity’ present or absent for the linear regression analysis. We combined ‘comorbidity’ into a single category in order to facilitate interaction terms with BMI status.
Epidemiology of bariatric surgery
The bariatric surgical cohort was analysed in order to understand the epidemiology of bariatric surgery over the period of study. The rate of utilisation of bariatric surgical procedures was estimated for men and women and for three age groups: 20–34 years, 35–54 years and 55–84 years. These represented young, middle-aged and older adults. The denominator was person-years at risk for the general population registered in CPRD. Participants were classified, according to the procedure recorded on the index date, into LAGB, SG or GBP. Utilisation of the three procedures, as a proportion of all bariatric surgical procedures, was evaluated from 2002 to 2014. Bariatric surgical codes recorded after the index date were evaluated and participants were classified as having a second operation if a procedure of a different type was recorded more than 30 days after the index date. In participants whose initial procedure was LAGB, we evaluated whether or not a code for removal of gastric band was recorded. The occurrence of repeat operations and band removal were evaluated in a time-to-event framework and annual incidence rates were estimated. Records of body weight, height and BMI were identified in order to estimate changes in body weight following the index date.
Type 2 diabetes mellitus incidence
The bariatric surgical cohort was analysed to evaluate the incidence of diabetes following the procedure. Matched controls for comparison were drawn from the general population cohort, which included 103,502 obese non-diabetic individuals sampled from CPRD who did not receive bariatric surgery and were not older than the maximum age of the bariatric surgery participants. Controls were matched for age, BMI, sex, index year and HbA1c category. The maximum HbA1c value before the index date was included using the categories < 42 mmol/mol (6%), 42–47 mmol/mol (6.0–6.49%) and not known. Nearest neighbour matching was performed without replacement. 14 The index date for controls was the date of the earliest BMI record on which the patient attained their highest BMI category.
For bariatric surgery participants and controls, new diagnoses of clinical diabetes were identified using medical diagnoses, drug prescriptions and HbA1c values. Participants were identified as being diagnosed with clinical diabetes if a medical code for diabetes was recorded or if insulin or oral hypoglycaemic drugs were prescribed or, using World Health Organization criteria,55 if a HbA1c value was 48 mmol/mol (> 6.5%). Oral hypoglycaemic drugs included sulphonylurea drugs, metformin, acarbose, dipeptidyl-peptidase-4 inhibitors, glitazones and glinide drugs. Participants with recorded diagnoses of polycystic ovary syndrome, who were prescribed antidiabetic drugs but never diagnosed with diabetes, were coded as non-diabetic. The date of the earliest medical, therapy or test event was taken as the diabetes diagnosis date. All new diagnoses of diabetes were included because different diabetes phenotypes cannot always be clearly distinguished in clinical practice. We evaluated new cases of diabetes for the recording of codes for type 1 diabetes, for prescription of insulin within 6 months of the diagnosis date and for diagnoses of gestational diabetes. The latter were excluded as a sensitivity analysis. The index BMI was the most recent recorded value prior to the index date. Records for smoking status, blood pressure and cholesterol were identified and the most recently recorded prior to the index date were used to describe baseline values. Depression, CHD and stroke were identified using previously described medical codes. 49–51
Baseline characteristics of the bariatric surgery participants and controls were described. A time-to-event framework was used to assess T2DM onset, using the Cox proportional hazards model. Failure was a new diagnosis of T2DM. Records were censored at the end of participants’ registration, the last date of CPRD data collection or death. Follow-up was censored after 7 years because few participants remained. Models were adjusted for matching variables, prevalent CHD, stroke and depression ever diagnosed, smoking status, whether or not total cholesterol was > 5 mmol/l, blood pressure > 140/90 mmHg, use of statins and antihypertensive drugs before surgery. Quadratic terms for age and BMI did not improve goodness of fit. Indicator variables were used for missing data on blood pressure and cholesterol. However, 591 (27%) of controls and 18 (1%) of bariatric surgery participants had missing values for blood pressure and 1466 (68%) of controls and 557 (26%) of bariatric surgery participants had missing values for cholesterol. The proportional hazards assumption was evaluated with no evidence that this was violated. Robust variance estimates were employed to allow for clustering of responses by family practice. Several sensitivity analyses were performed: using the unmatched cohort of 103,502 obese non-diabetic individuals for comparison; excluding participants diagnosed with diabetes within 12 months of the index date; and excluding participants diagnosed with gestational diabetes. An analysis was performed to evaluate the impact of competing risk from mortality using the method of Fine and Gray. 56
Diabetes treatment
The bariatric surgical cohort was used to evaluate changes in T2DM control and remission after surgery by selecting participants who had diabetes diagnosed prior to the index date. Control participants were selected from the general population cohort who never had bariatric surgery recorded, but were obese and had T2DM diagnosed before the index date. As the distribution of BMI differed greatly between bariatric surgery cases and the CPRD obese population, control participants were individually matched with cases using nearest neighbour matching on BMI, age, sex and index year. The index date for controls was the date of the first BMI record on which they entered their highest recorded BMI category. Participant records ended if they terminated their registration with a CPRD general practice; if their general practice ended participation in CPRD; if the latest data collection date was reached; or if the patient died.
Glycated haemoglobin records and prescriptions for oral hypoglycaemic drugs and insulin were evaluated for bariatric surgery cases and controls. The person-time for each participant was divided into study years from 3 years before the procedure to 6 years after the procedure. This allowed us to conduct an interrupted time-series analysis. Follow-up was censored at 6 years because few cases remained under follow-up for SG and GBP. The highest HbA1c value and the total number of diabetes prescriptions were evaluated in each study year. For each year of follow-up, participants were classified as being in remission if the maximum HbA1c value recorded in year was 48 mmol/mol (< 6.5%) and there were no diabetes prescriptions issued in the year. This is consistent with the definition of ‘complete and partial remission’ suggested by Buse et al. 57 Complete remission [HbA1c 42 mmol/mol (< 6.0%) and not on medication] was also evaluated. Relative rates were estimated for each year following the bariatric surgery procedure by using a Poisson model with person-time as the exposure. Interpretation of these results requires consideration of the relationship between year of procedure and duration of follow-up, with longer duration follow-up being available only for patients operated on longer ago. A model was fitted to evaluate the effect of group (bariatric surgery or control) and time after surgery, included as indicator variables for each postoperative year. 58 Confounders included age, gender, BMI, quartile of diabetes duration before surgery, whether CHD, stroke or depression were diagnosed before the index date, whether blood pressure was ≥ 140/90 mmHg or serum total cholesterol was ≥ 5 mmol/l, and whether or not antihypertensive drugs and lipid-lowering drugs were prescribed before the index date, and smoking status recorded before the index date. Missing values for covariates were accounted for using indicator variables.
Changes in depression
A controlled interrupted time-series study was conducted, drawing on data from the bariatric surgery cohort and matched control participants from the general population cohort, by evaluating multiple time points both before and after the bariatric surgical procedure. EHRs from primary care were evaluated for clinical diagnoses of depression and prescription of antidepressant drugs in the 3 years before, and 7 years after, bariatric surgery.
Clinical depression was identified through medical diagnoses for depression recorded in clinical or referral records as well as through prescriptions for antidepressant drugs. Participant records were divided into person-years before and after surgery. Individuals were classified as having clinical depression in a given person-year if they had a medical diagnosis of depression recorded in that year, or if they were prescribed antidepressant drugs in the year and were ever diagnosed with depression. 51 BMI was categorised using the World Health Organization criteria55 using the most recent record prior to the index date. Comorbidities were evaluated including stroke, CHD and T2DM. Records for hypertension (blood pressure ≥ 140/90 mmHg), high cholesterol (total cholesterol ≥ 5 mmol/l) and current smoking status were evaluated. Participants were also classified according to whether they were treated with antihypertensive or lipid-lowering medications.
Baseline characteristics in the surgery and control groups were compared. Participant records were divided into 1-year periods from up to 3 years before, to a maximum of 7 years after, the index date. The presence or absence of clinical depression was evaluated for each 1-year period. A multiple logistic regression analysis was conducted using person-years as observations and the presence or absence of clinical depression in each year as the outcome. A model was fitted to evaluate the effect of group (bariatric surgery or control), study year from 3 years before to 7 years after surgery, and time after surgery, included, for improved goodness of fit, as indicator variables for each postoperative year. 58 The reference category was all person-time without surgery from the control group and the bariatric surgery group before operation. The model was adjusted for gender, age, baseline BMI, index year, type of bariatric surgery procedure, prevalent diabetes mellitus, CHD and stroke, smoking status, high blood pressure and cholesterol, and treatment with antihypertensive or lipid-lowering drugs. Robust variance estimates were employed to adjust standard errors for clustering of person-years by participant.
Data analysis to inform health economic modelling
Data to populate the health economic model were derived using epidemiological analysis of the general population cohort, among whom obese patients may have received weight management interventions available in routine primary care. The incidence, prevalence, mortality and costs of health-care utilisation in this group were estimated for each state in the model. Incidence and mortality rates were estimated in a time-to-event framework using a Weibull model. Covariates were age, age squared, gender and BMI category. The prevalence of depression was estimated for each state in the model. 51 Health-care utilisation and associated costs were estimated for each state in the model as described above.
Evidence search and synthesis
The research drew on previous systematic reviews and primary research publications to provide evidence of the health outcomes and cost-effectiveness of bariatric surgery.
Purpose
The purpose of the review was to systematically retrieve, appraise and synthesise available research evidence. We aimed to provide information for the model by determining the projected short- and long-term effects of bariatric surgical procedures on health states included in the model.
Eligibility criteria
We conducted a rapid review of previous systematic reviews that evaluated bariatric surgery as an intervention for obesity. Subsequently, we identified literature not included in the reviews because of later publication dates or restrictive inclusion criteria of reviews. A single literature search was conducted to identify both systematic reviews and primary reports. We included systematic reviews, controlled clinical trials and controlled observational studies published in English. Modelling studies were included for cost-effectiveness evaluations. Participants were obese adults. The intervention was a bariatric surgical procedure; and a comparator of standard care or comparisons between different surgical procedures were accepted. The study outcomes were incidence of T2DM, CHD, stroke and cancer; remission of T2DM and changes in depression; long-term mortality (more than 30 days post surgery); and cost-effectiveness.
Search strategy
We used the following search terms: (obes* or overweight) and (bariatric surgery or weight*loss surgery or gastric band or gastric bypass or sleeve gastrectomy) and (diabetes or coronary heart disease or CHD or stroke or depression or cancer) or (cost-effectiveness or cost-utility). We searched for papers published after 2000 as there were sufficient recent systematic reviews. The searches were implemented in EMBASE (1980–present), MEDLINE (1946–present), MEDLINE In Process & Other Non-Indexed Citations, PubMed and the Cochrane Database of Systematic Reviews. Reference lists of included studies were checked for further publications.
Study selection and data extraction
The search results were checked for eligibility by one reviewer. The full text was consulted when eligibility was not evident from the abstract alone. Data were abstracted by one reviewer and checked by a second reviewer. For disease incidence, T2DM remission and mortality, the summary measure was ideally presented as a risk ratio, although we also considered odds ratios (ORs) and hazard ratios (HRs). We also included publications which presented rates in the intervention and control groups. Changes in continuous measures, such as changes in fasting blood glucose in T2DM or depression scales, were not included. For the cost-effectiveness data the summary measures were incremental cost-effectiveness ratios (ICERs) or costs per QALY. The data were abstracted by one reviewer into a spreadsheet.
Quality assessment
The AMSTAR (Assessing the Methodological Quality of Systematic Reviews) checklist was used to assess the quality of systematic reviews. 59 The assessment of study quality is presented in Table 4.
| Study (year) | A priori design provided? | Duplicate study selection and data extraction? | Comprehensive literature search performed? | Publication status as inclusion criteria? | Included and excluded studies listed? | Study characteristics outlined? | Study quality assessed? | Study quality considered in drawing conclusions? | Methods of combining findings appropriate? | Publication bias assessed? | Conflict of interest declared? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Picot et al. (2009)31 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ |
| Merlotti et al. (2014)60 | ✗ | ? | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ricci et al. (2015)61 | ✗ | ? | ✓ | ? | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Kwok et al. (2014)62 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Tee et al. (2013)63 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Casagrande et al. (2014)64 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Maestro et al. (2015)65 | ✗ | ? | ? | ? | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Yang et al. (2015)66 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✗ | ✓ | N/A | ✓ |
| Afshar et al. (2014)67 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ |
| Clegg et al. (2003)68 | ✗ | ✓ | ✓ | ? | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Ferchak and Meneghini (2004)69 | ✗ | ? | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ |
| Buchwald et al. (2009)70 | ✗ | ? | ✓ | ? | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Gloy et al. (2013)6 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Guo et al. (2013)71 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Ribaric et al. (2014)72 | ✗ | ? | ✓ | ? | ✗ | ✓ | ? | ✗ | ✓ | ✓ | ✓ |
| Colquitt et al. (2014)73 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ |
| Maggard-Gibbons et al. (2013)74 | ✓ | ? | ✓ | ? | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ |
| Picot et al. (2012)75 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | N/A | ✓ |
| Müller-Stich et al. (2015)76 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Yip et al. (2013)77 | ✗ | ? | ✓ | ? | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ |
| Zhang et al. (2014)78 | ✗ | ? | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cho et al. (2015)79 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | |
| Zhang et al. (2015)80 | ✗ | ? | ✓ | ? | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ |
| Kubik et al. (2013)81 | ✗ | ? | ✓ | ? | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | |
| Herpertz et al. (2003)82 | ✗ | ? | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | |
| Pontiroli and Morabito (2011)83 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ |
| Wang and Furnback (2013)84 | ✗ | ? | ✗ | ? | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Padwal et al. (2011)85 | ✗ | ✓ | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
Data synthesis
A narrative synthesis was conducted summarising results included in systematic reviews and summarising primary research studies of significance.
Markov modelling for health economic evaluation
Model structure
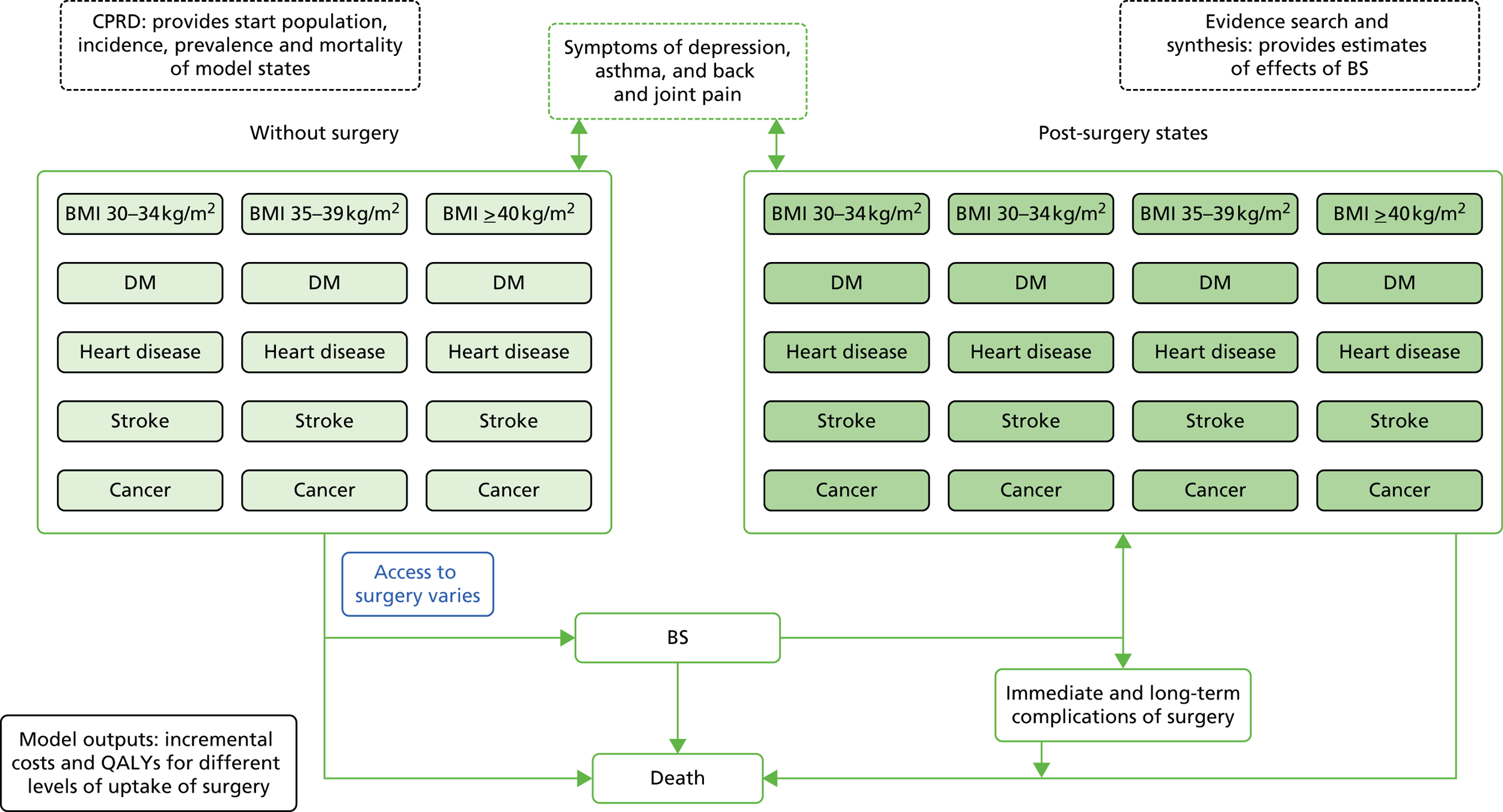
A Markov model was employed to conduct a cost–utility analysis to compare a strategy in which all eligible participants received bariatric surgery, with standard non-surgical weight management. A diagrammatic representation of the Markov model is shown in Figure 3. The model structure was informed by previously reported research86 and enabled us to include the main influences on costs and outcomes of bariatric surgery identified in the literature search. Healthy subjects, referred to as ‘at risk’, may develop one of the disease states of interest, including T2DM, CHD, stroke and cancer. Onset of disease was irreversible except for diabetes which was allowed to remit with individuals returning to the ‘at risk’ state. Individuals who entered remission then had the same risk of diabetes as all others in the ‘at risk’ state. Participants in each state were allowed to progress to depression, with each main state divided into substates representing ‘not depressed’ and ‘depressed’. Depression was included because it occurs frequently in chronic illness and is associated with higher health-care costs. 51 The model was stratified by BMI category, comprising morbid obesity, severe obesity, simple obesity, overweight and normal weight, and allowed participants to transition between BMI categories. There were, therefore, 50 states in the model that represented all potential combinations of the included BMI categories, morbidities and depression. Each state was further stratified by single year of age and gender. All states might lead to death. The perspective of the model is that of health-care services and only health-care costs were included. Inputs to the model for the standard care group were derived from the sources presented in Table 5.
FIGURE 3.
Simplified schematic diagram of Markov model. BS, bariatric surgery; DM, diabetes mellitus.
| Measure | Data source | Comment |
|---|---|---|
| Base population | CPRD | Data for approximately 250,000 adults aged ≥ 20 years, stratified by age, sex, IMD quintile and BMI category |
| Incidence of long-term conditions | CPRD | Diabetes, heart disease, stroke, obesity-related cancer |
| Prevalence of symptomatic conditions | CPRD | Depression |
| Mortality | CPRD | Mortality for each state estimated from CPRD |
| Health-care utilisation | CPRD | Estimated from CPRD records for each state |
| Unit costs of health-care utilisation | PSSRU52 | Reference source. Additional costs of surgery will also be estimated from NHS sources |
| Unit prescription costs for medicines | RESIP UK | Dictionary of costs provided by RESIP UK |
| Utility values | Sullivan et al.87 | Compendium of values provides utility of each state |
| Intervention effects | Literature reviews and CPRD analysis |
Model estimation
The probabilistic Markov model was estimated by cohort simulation, implemented through a program written in R software (The R Foundation for Statistical Computing, Vienna, Austria). The initial population had ages ranging from 20 to 74 years, as we observed that there were few bariatric surgical procedures over the age of 74 years in CPRD. The proportion of the start population with morbidity was also informed by analysis of a CPRD cohort.
All simulations were stratified by single year of age with the initial population ageing by 1 year per cycle. Participants exited the model when they died or reached 100 years of age. The model was run for each sex separately. Outcomes and costs were compared for scenarios in which all participants received bariatric surgery in the first annual cycle of the model or no participants received bariatric surgery. Annual transition probabilities for the model were obtained by sampling from the beta-binomial distribution, using CPRD data as inputs. The costs of each state were sampled from the gamma distribution, with the predicted mean value estimated from a two-part model as outlined above. Utilities for each state were obtained from data published in a compendium of values. 87 Utility values for each state were stratified by single year of age but were the same for men and women. Utility values were sampled from the beta distribution. The utility values used in the model are presented in Table 6. Total costs and QALYs were obtained by summing across the 81 cycles of the model included in each simulation. There were 1000 simulations run for each scenario. Results are expressed as rates per 1000 participants entering the model. Mean costs, and the 95% range, were obtained from the data for 1000 simulations. Costs and QALYs were discounted using a rate of 3.5%, but undiscounted values and values discounted at 1.5% are also shown as sensitivity analyses. 88 Net monetary benefits and net health benefits were estimated at threshold values of £20,000 and £30,000 per QALY. 88
| Condition | Utility |
|---|---|
| Mean adult utility at age 43 years | 0.828 |
| Age (per year increase) | –0.00029 |
| T2DM | –0.0714 |
| CHD | –0.0671 |
| Stroke | –0.1171 |
| Cancer | –0.04347 |
| Depression | –0.1123 |
| BMI category (kg/m2) | |
| 30–34 | –0.085 |
| 35–39 | –0.17 |
| ≥ 40 | –0.255 |
Intervention effects and costs of bariatric surgery
The effect of bariatric surgery was modelled as a reduction in disease incidence and mortality. The effect of bariatric surgery on the incidence of T2DM was drawn from CPRD data analyses7 that gave very similar results to data from the Swedish Obese Subjects (SOS) study. 9 Effects on incidence of CHD, stroke and cancer were also drawn from the SOS study,9,89 which showed a reduction in cancer incidence in women but not in men. 89 The effect on depression prevalence was drawn from CPRD data analyses90 and is also consistent with other reports. Based on CPRD data analyses (see Chapter 8), 40% of T2DM patients were estimated to enter remission following the procedure. Bariatric surgery was modelled as being associated with a positive impact on patient utility equivalent to a two-unit change in BMI category. 6,91 However, this effect was modelled to decline over time, according to year–0.25, consistent with the known reduction in the initial quality of life improvement following bariatric surgery. 92 The costs of bariatric surgery were drawn from NHS tariffs and included preoperative weight management, the cost of the procedure, and postoperative reviews (Table 7). Bariatric surgery was assumed to comprise one-third each of gastric banding, GBP and SG. The cost of leaks was included as an average cost across all patients. 93 Two per cent of patients were assumed to require repeat procedures each year, slightly higher than the 1.2% observed in CPRD records. Mortality from surgery was estimated at 0.07% from the NBSR report. 11 Costs of health-care utilisation were estimated from CPRD. Costs of health-care utilisation following bariatric surgery were determined by age, sex and morbidity category, and were not modelled as associated with body weight reduction, consistent with the results of empirical studies. 12,13
| Item | Adjustable gastric banding | GBP | SG | All |
|---|---|---|---|---|
| Proportion | 0.3333 | 0.3333 | 0.3333 | 1 |
| Preoperative Tier 3 weight management programme (£) | 1024 | 1024 | 1024 | |
| Surgical procedure | ||||
| Tariff: code | FZ05A | FZ04A | FZ04A | |
| Tariff: cost (£) | 3620 | 8713 | 8713 | |
| Postoperative reviews (£) | 875 | 875 | 875 | |
| Total (£) | 5519 | 10,612 | 10,612 | |
| Average total (£) | 8914.50 | |||
| Cost of leaks (£)a | 250 | |||
| Total (£) | 9164.50 | |||
| Cost of reoperations (£)b | FZ05A | 3620 | 2% of patients per year | |
Subgroup and sensitivity analyses
In the base case we investigated the effect of bariatric surgery in participants with BMI ≥ 40 kg/m2, compared with no participants undergoing surgery. This was because the majority of bariatric surgery procedures are performed for morbid obesity at present. The initial population included 19% with T2DM and 4% with CHD, similar to the distribution observed in CPRD at the start of the study period (see Chapter 6). Additional simulations were performed to estimate costs and outcomes separately for men and women; for separate age groups, including 20–34 years, 35–54 years and 55–64 years; and categories of deprivation, comparing the most- and least-deprived quintiles of deprivation according to the IMD 2010 score. IMD scores were linked at patient lower super-output area level. 94 A summary of the simulations performed is presented in Table 8.
| BMI category (kg/m2) | Condition | Category | Start population |
|---|---|---|---|
| ≥ 40 | All | Synthetic population of 100,000 each of men and women, aged 20–74 years, with morbid obesity, including 19% with T2DM and 4% with CHD | |
| ≥ 40 | Gender | Men | 100,000 men with morbid obesity as above |
| Women | 100,000 women with morbid obesity as above | ||
| ≥ 40 | Age group (years) | 20–34 | Synthetic population of 100,000 each of men and women, in the specified age group, with morbid obesity including 19% with T2DM and 4% with CHD |
| 35–54 | |||
| 55–74 | |||
| ≥ 40 | Deprivation category | Least deprived | As in ‘All’ above |
| Most deprived | As in ‘All’ above | ||
| ≥ 40 | Diabetes BMI ≥ 40 kg/m2 | BMI ≥ 40 kg/m2 | Synthetic population of 75,000 each of men and women, in the specified age group, with morbid obesity and T2DM |
| 35–39 | BMI 35–39 kg/m2 | BMI 35–39 kg/m2 | Synthetic population of 75,000 each of men and women, aged 20–74 years, with morbid obesity and no comorbidity |
| ≥ 40 | Costs of procedure | 50% higher | As in ‘All’ above |
| 100% higher | As in ‘All’ above | ||
| Zero procedure cost | As in ‘All’ above | ||
| ≥ 40 | Decline of intervention effect over time | Year–0.25 | As in ‘All’ above |
| Year–0.50 | As in ‘All’ above |
Sensitivity analyses were implemented to explore the effects of varying the unit costs of bariatric surgery, including values 50% and 100% higher than the base case; varying the discount rate including values of 0%, 1.5% and 3.5% following NICE recommendations; and to estimate outcomes assuming that intervention effects following bariatric surgery might diminish with time following the operation. This was implemented by allowing intervention effects from bariatric surgery to diminish by year–0.25 or year–0.5. The former implies that the effect of bariatric surgery will decline by 44% over 10 years, while the latter indicates that the intervention effect will decline by 68% over 10 years. Sensitivity analysis was also used to test intervention cost-effectiveness for patients with severe obesity (BMI 35–39 kg/m2) or with morbid obesity and T2DM.
Chapter 4 What is the probability of an obese person attaining normal body weight?
Introduction
This chapter describes changes in body weight of obese participants who did not undergo bariatric surgery. It assesses possible body weight trajectories among obese patients who are managed in primary care and provides a context against which bariatric surgery may be compared. A target of 5% body weight loss is often recommended for obese subjects who intend to lose weight. 95 In practice, access to weight-management interventions may be limited28 and systematic reviews show that weight-management interventions have only small and poorly maintained effects on body weight. 29,96 In order to understand the frequency with which reductions in BMI may occur in a large population, this part of the study aimed to estimate the probability of an obese individual attaining normal body weight, or a reduction of 5% in body weight in the absence of bariatric surgery. The results of this study have been published open access in the American Journal of Public Health (© American Public Health Association). 97
Results
The analysis comprised 278,982 participants, including 129,194 men and 149,788 women, who were registered between 1 November 2004 and 31 October 2014, and had three or more BMI records recorded during this period. The initial distribution of the sample by gender and BMI is shown in Table 9. Mean age was 55 years for men and 49 years for women. At the index date (date of the first BMI record in the study period) there were a minimum of 25,000 male and 23,000 female participants each for the BMI categories 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight), 30.0–34.9 kg/m2 (obese) and 35.0–39.9 kg/m2 (severely obese). There were similarly high numbers of female participants with an index BMI of 40.0–44.9 kg/m2 (morbidly obese) but fewer male participants in this category at baseline (14,767). There were 6481 men and 18,451 women with a baseline BMI ≥ 45.0 kg/m2 (super obese).
| Initial BMI category (kg/m2) | n | Mean (SD) age (years) | Number of BMI records, median (IQR) | All records show no change in BMI category, n (%) | One or more decreases in BMI category and no increases, n (%) | One or more increases in BMI category and no decreases, n (%) | Records show both increases and decreases in BMI category, n (%) |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| 18.5–24.9 | 25,082 | 58 (18) | 5 (3–7) | 14,217 (57) | 799 (3) | 5032 (20) | 5034 (20) |
| 25.0–29.9 | 27,408 | 58 (15) | 5 (3–8) | 13,281 (48) | 3243 (12) | 3428 (13) | 7456 (27) |
| 30.0–34.9 | 27,966 | 56 (14) | 6 (4–10) | 10,320 (37) | 4620 (17) | 2901 (10) | 10,125 (36) |
| 35.0–39.9 | 27,490 | 53 (13) | 7 (4–12) | 7200 (26) | 5070 (18) | 2525 (9) | 12,695 (46) |
| 40.0–44.9 | 14,767 | 50 (13) | 8 (4–14) | 2761 (19) | 2810 (19) | 1596 (11) | 7600 (51) |
| ≥ 45.0 | 6481 | 47 (13) | 8 (4–14) | 2828 (44) | 1353 (21) | N/A | 2300 (35) |
| All | 129,194 | 55 (15) | 6 (4–10) | 50,607 (39) | 17,895 (14) | 15,482 (12) | 45,210 (35) |
| Women | |||||||
| 18.5–24.9 | 23,640 | 46 (20) | 4 (3–7) | 14,047 (59) | 844 (4) | 4346 (18) | 4403 (19) |
| 25.0–29.9 | 26,357 | 52 (19) | 5 (3–8) | 10,140 (38) | 3696 (14) | 4197 (16) | 8324 (32) |
| 30.0–34.9 | 27,251 | 52 (17) | 6 (4–10) | 8275 (30) | 4621 (17) | 3626 (13) | 10,729 (39) |
| 35.0–39.9 | 27,373 | 49 (16) | 7 (4–11) | 6322 (23) | 4910 (18) | 3304 (12) | 12,837 (47) |
| 40.0–44.9 | 26,716 | 48 (15) | 7 (4–13) | 4680 (18) | 5009 (19) | 3108 (12) | 13,919 (52) |
| ≥ 45.0 | 18,451 | 46 (14) | 8 (5–14) | 8945 (48) | 3472 (19) | N/A | 6034 (33) |
| All | 149,788 | 49 (17) | 6 (4–10) | 52,409 (35) | 22,552 (15) | 18,581 (12) | 56,246 (38) |
Table 9 also shows the frequency and proportion of participants recorded as having no change in BMI category, increases in BMI category, decreases in BMI category or weight cycling (both increases and decreases) over 9 years following first BMI record. The number of BMI records per participant increased with baseline BMI category. The proportion of participants showing no change was greatest among participants in the normal weight category (men 57%; women 59%) and decreased with higher baseline BMI, with the exception of those initially categorised as super obese. Only 14% of all men and 15% of women showed decreases in BMI category without increases over the same period. The proportion of participants with records indicating only decreases in BMI increased with baseline BMI category, with the highest proportions observed for those initially categorised as morbidly obese (men 19%; women 19%) and super obese (men 21%; women 19%). A small proportion of participants (12% each of men and women) had only BMI category increases recorded, with the highest proportion found among those initially categorised as normal weight (men 20%; women 18%). Weight cycling was observed in over one-third of participants (35% of men; 38% of women) and was most common among severely obese (men 46%; women 47%) and morbidly obese (men 51%; women 52%) participants.
Table 10 shows the frequency of transitioning to normal body weight during up to 9.9 years’ follow-up after the first BMI record. During a maximum of 9 years’ follow-up, 1283 men and 2245 women attained normal body weight records. The annual probability of achieving normal body weight was 1 in 210 for men and 1 in 124 for women with simple obesity. The probability declined with increasing BMI category. In patients with morbid obesity, the annual probability of achieving normal weight was 1 in 1290 for men and 1 in 677 for women. In women, the probability of achieving normal weight among super-obese participants was 1 in 608, similar to that observed in morbid obesity. In the smaller number of super-obese men, the probability was higher, at 1 in 362.
| Initial BMI category (kg/m2) | Number of participants | Number of person-years during follow-up | Number attaining normal BMI | Annual probability of attaining normal BMI | ||
|---|---|---|---|---|---|---|
| Estimate | Lower 95% confidence limit | Upper 95% confidence limit | ||||
| Men | ||||||
| 30.0–34.9 | 27,966 | 179,746 | 857 | 1 in 210 | 1 in 197 | 1 in 225 |
| 35.0–39.9 | 27,490 | 174,386 | 249 | 1 in 701 | 1 in 619 | 1 in 797 |
| 40.0–44.9 | 14,767 | 91,528 | 71 | 1 in 1290 | 1 in 1023 | 1 in 1651 |
| ≥ 45.0 | 6481 | 38,367 | 106 | 1 in 362 | 1 in 300 | 1 in 442 |
| Women | ||||||
| 30.0–34.9 | 27,251 | 173,066 | 1398 | 1 in 124 | 1 in 118 | 1 in 131 |
| 35.0–39.9 | 27,373 | 175,356 | 408 | 1 in 430 | 1 in 390 | 1 in 475 |
| 40.0–44.9 | 26,716 | 170,483 | 252 | 1 in 677 | 1 in 599 | 1 in 769 |
| ≥ 45.0 | 18,451 | 113,540 | 187 | 1 in 608 | 1 in 527 | 1 in 704 |
Annual probabilities of achieving a clinically relevant 5% reduction in body weight are shown in Table 11. The annual probability of experiencing a 5% weight reduction was 1 in 12 for men and 1 in 10 for women with simple obesity. Probability increased with increasing BMI category. For patients with morbid obesity, the annual probability of achieving 5% reduction in body weight was 1 in 8 for men and 1 in 7 for women. The highest annual probability was observed among patients with super obesity (1 in 5 for men and 1 in 6 for women). However, among participants who lost 5% body weight, 52.7% (95% CI 52.4% to 53.0%) at 2 years, and 78.0% (95% CI 77.7% to 78.3%) at 5 years, had BMI records that indicated weight gain to values above the 5% weight loss threshold.
| Initial BMI category (kg/m2) | Number of participants | Number of person years during follow-up | Number attaining 5% reduction in body weight | Annual probability of attaining 5% reduction in body weight |
|---|---|---|---|---|
| Men | ||||
| 30.0–34.9 | 27,966 | 135,394 | 11,869 | 1 in 12 |
| 35.0–39.9 | 27,490 | 118,266 | 13,805 | 1 in 9 |
| 40.0–44.9 | 14,767 | 57,099 | 8100 | 1 in 8 |
| ≥ 45.0 | 6481 | 20,900 | 4177 | 1 in 5 |
| Women | ||||
| 30.0–34.9 | 27,251 | 123,567 | 12,792 | 1 in 10 |
| 35.0–39.9 | 27,373 | 116,042 | 13,972 | 1 in 9 |
| 40.0–44.9 | 26,716 | 103,849 | 15,208 | 1 in 7 |
| ≥ 45.0 | 18,451 | 63,397 | 11,340 | 1 in 6 |
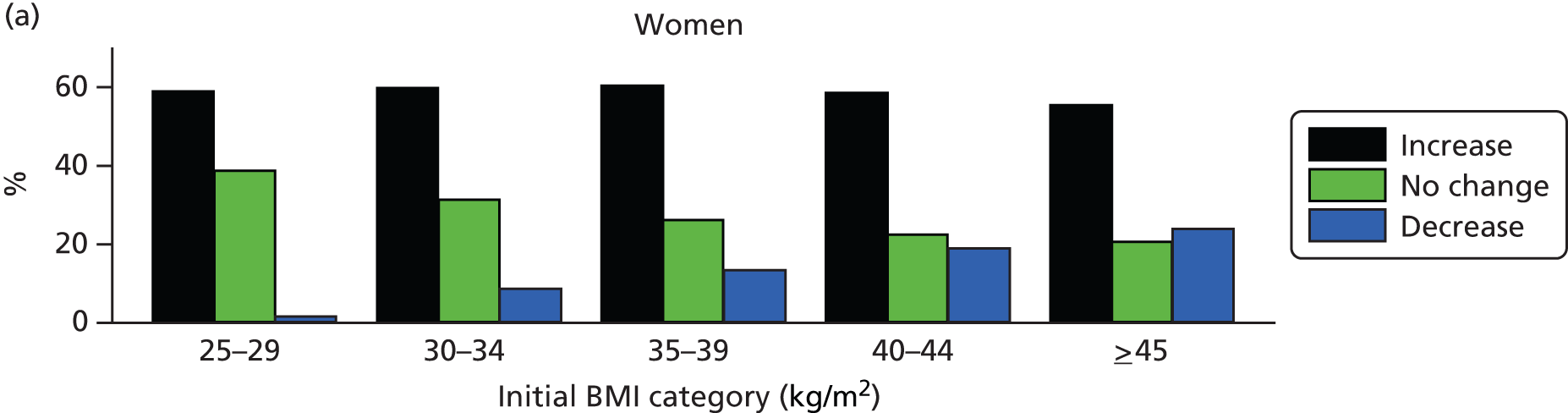
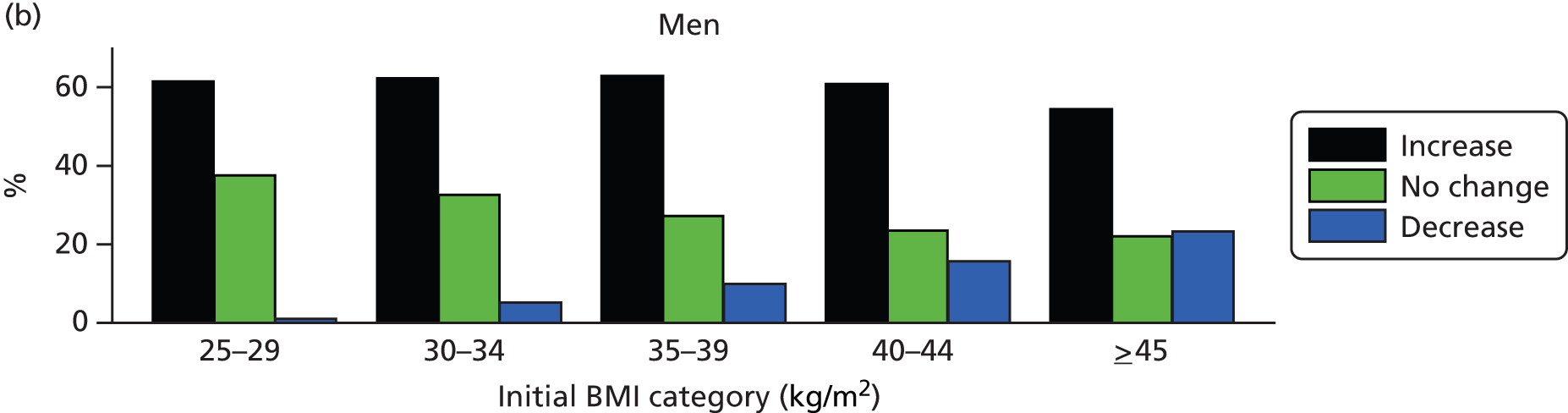
Among patients with a recorded decrease in BMI category over the study period, Figure 4 shows the percentage of men and women whose later BMI records revealed an increase, a further decrease or no change in BMI category. The majority of patients (men 61%; women 59%) whose records showed a decrease in BMI category went on to record a subsequent increase in BMI category. These proportions were similar for men and women and across BMI categories. The proportion of patients who showed a second decrease in BMI category was highest among patients with morbid (men 16%; women 19%) and super obesity (men 23%; women 24%) and was considerably less frequent in lower BMI categories. Overweight patients and those with simple obesity were the most likely to display no further BMI category change following a recorded decrease.
FIGURE 4.
Changes in BMI category following an initial decrease in BMI category. Data are presented by gender and initial BMI category.
Discussion
Summary of findings
Analysis of primary care EHRs for a large population based sample of men and women over a 9-year period revealed that the probability of obese patients attaining normal weight was very low. The annual probability of patients with simple obesity attaining a normal body weight was only 1 in 131 for women and 1 in 225 for men. The likelihood of attaining normal body weight declined with increasing BMI category, with the lowest probability observed for patients with morbid obesity. The smaller group of patients with super obesity represented a departure from this trend, but nevertheless showed a low probability of attaining normal body weight. Although the probability of patients achieving a 5% reduction in body weight was considerably higher, the majority of these patients went on to regain lost weight, as evidenced by BMI records of > 95% of the initial value, within 2–5 years of the first record that was lower than 95% of the initial value.
These findings raise questions concerning whether or not current obesity treatment frameworks, grounded in weight management programmes accessed through primary care, may be expected to achieve clinically relevant and sustained reductions in BMI for the vast majority of obese patients and whether or not they could be expected to do so in the future. The lack of sustained BMI reductions could be driven by low intervention uptake rates or their lack of effectiveness. In a previous study, we reported that weight-loss interventions are currently offered only to a minority of patients in primary care. 28 Efforts are under way to improve this situation, with the proportion of patients with obesity offered multicomponent weight-loss interventions included among potential new indicators in the 2016/17 consultation for the Clinical Commissioning Group Indicator Set (CCG OIS). 98 However, even when treatment is accessed, evidence suggests that behavioural weight-loss interventions focusing on caloric restriction and increased physical activity are unlikely to yield clinically significant reductions in body weight. 29,99 A recent series of reviews documented the limited progress in reversing the global obesity epidemic and called for regulatory actions from governments as well as co-ordinated efforts across industry and society to reduce obesity. 100–103 Dietz et al. 104 warn that preventative strategies are unlikely to reduce weight in people living with severe obesity and stress the need for changes in the delivery of care for these patients. In combination with previous research, this study highlights the current failures in combating existing obesity cases at a population level.
Comparison with other results
Reductions in BMI category were observed more frequently among patients with a higher baseline BMI but these decreases were more likely to be followed by subsequent increases rather than further decreases or stability in BMI category. Weight cycling, evidenced by both increases and decreases in BMI category, was most common among men and women with baseline BMIs in the morbidly obese category. Greater instability in weight trajectories among patients with higher BMIs has been reported previously. 105 Weight cycling has been linked to a higher risk of morbidity and mortality compared with stable obesity,106–108 although evidence of causality remains inconclusive. 109
The higher likelihood of decreases in BMI category and of 5% weight loss among the more severely obese participants in this study is consistent with results from clinical trials110 and previous cohort studies111 in which higher BMI predicted greater weight loss. The increased probability of weight reduction among patients with more severe obesity may reflect more accurate perceptions of personal weight status112,113 and higher treatment rates among these patients. It is also possible that BMI decreases in severely obese patients reflect unintentional weight loss resulting from greater comorbidity. The finding that a high proportion of patients in this analysis experienced a period of weight regain following weight loss is also consistent with previous research. At least 50% of patients who achieved 5% weight loss were shown to have regained this weight within 2 years. It has previously been reported that approximately 80% of people who intentionally achieve weight loss ≥ 10% of their body weight will regain that weight within 1 year. 114
Strengths and limitations
This analysis had the strengths of a large population-based cohort with prolonged follow-up.
Slightly lower numbers at the highest levels of BMI might have made these estimates slightly less precise. Data are presented for adults aged > 20 years. Inspection of age-specific values revealed, as expected, greater weight gain at younger ages and a somewhat greater tendency to weight loss at older ages. It was not possible to evaluate intentionality of weight loss. Previous studies have reported that the majority of obese individuals would like to lose weight and a large proportion are actively attempting to reduce their weight,115,116 so a relatively high level of intentionality among obese participants may be assumed. Additionally, monitoring of BMI among obese patients in primary care has been shown to positively predict treatment. 117 Patients in the present study were required to have a minimum of three BMI measurements recorded, suggesting that an inflated proportion of patients in this sample might have been involved in and interested in weight-management interventions. Nevertheless, we acknowledge that unintentional weight loss was also included and might result from physical disorders such as cancer, or psychological concerns such as bereavement. 118–120 Additional in-depth analyses might evaluate patterns of weight change in relation to comorbidity.
Recording of body weight in primary care is generally opportunistic and dependent on patients attending the practice. We acknowledge that weight measurements in EHRs may be associated with error and bias including measurement error; confounding by indication, if weight changes prompt weight measurements; variation between professionals and family practices in measurement recording;44 and weight-management strategies. 28 Higher patient baseline BMI was associated with a higher frequency of BMI measurements recorded over the study period. UK general practices have contractual financial incentives to provide a register of adult patients who have a BMI ≥ 30 kg/m2 measured in the last 15 months121 which may lead to more frequent recording of BMI for obese patients. We reported on the recording of BMI in primary care in a previous study. 44 For this study, we selected participants with a minimum of three BMI records. We acknowledge that participants with fewer than three BMI records may show different patterns of weight change and the present results might be biased through their omission. However, we believe that this is one of the largest studies yet reported on body weight changes in the general population. The relatively high levels of comorbidity seen in obese compared with normal weight patients would also likely result in more regular consultations and more frequent recording of BMI. However, it is possible that patients from all BMI categories with three or more BMI measurements recorded over the 9-year study period represent a biased, less healthy sample than the general population. If this is the case then unintentional weight loss, along with comorbidities contributing to weight gain such as mobility impairment, may have influenced BMI changes disproportionately in the current sample.
Conclusions
Findings from this analysis indicate that current non-surgical obesity treatment strategies are failing to achieve sustained weight loss for the majority of obese patients. For patients with a BMI of ≥ 30 kg/m2, maintaining weight loss was rare and the probability of achieving normal weight was extremely low. Research to develop new and more effective approaches to obesity management and prevention are urgently required. Obesity treatment programmes should prioritise prevention of further weight gain, along with the maintenance of weight loss in those who achieve it. However, in the absence of effective interventions targeted at the level of the individual, the greatest opportunity for tackling the current obesity epidemic may be found outside primary care. Research to develop wider-reaching public health policies is needed to prevent obesity at the population level.
Chapter 5 Costs associated with obesity in primary care
Introduction
This chapter presents data for health-care costs in relation to obesity in order to assess the key drivers of costs relating to obesity. This will help to establish the impact that increasing the level of bariatric surgery offered to obese patients might have on associated costs. This analysis investigated the association between BMI category and health-care costs, focusing on the question of whether it is BMI, obesity-related comorbidity and/or depression that determines costs related to obesity. The results in this chapter were published in the journal Clinical Obesity in April 2016122 under the terms of the Creative Commons Attribution Licence CC-BY-NC.
Results
A breakdown of the cohort characteristics by person-years is presented in Table 12. The sample was majority female (62%) and person-time was distributed evenly among age groups and BMI categories. Person-time was mostly spent with no comorbidities (66%). Depression was prevalent in 16% of person-years. The results from the two-part model used to estimate health-care costs based on the CPRD cohort are presented in Table 13. More strongly positive coefficients indicate greater probability of utilising health care (probit model) or greater health-care costs (GLM) associated with that covariate. Significant interactions between BMI category and comorbidity and between comorbidity and depression on predicted health-care costs were identified.
| Variable | Category | Person-years | Per cent of total |
|---|---|---|---|
| Total | 873,809 | 100 | |
| Gender | Male | 335,610 | 38 |
| Female | 538,199 | 62 | |
| Age group (years) | 20–34 | 118,364 | 14 |
| 35–44 | 151,810 | 17 | |
| 45–54 | 170,562 | 20 | |
| 55–64 | 170,362 | 19 | |
| 65–74 | 143,723 | 16 | |
| 75–84 | 90,972 | 10 | |
| ≥ 85 | 28,017 | 3 | |
| BMI category (kg/m2) | 18.5–24.9 | 130,806 | 15 |
| 25.0–29.9 | 171,622 | 20 | |
| 30.0–34.9 | 181,283 | 21 | |
| 35.0–39.9 | 173,470 | 20 | |
| ≥ 40.0 | 216,629 | 25 | |
| Comorbidity | None | 573,998 | 66 |
| Diabetes | 144,089 | 16 | |
| CHD | 76,144 | 9 | |
| Stroke | 19,978 | 2 | |
| Cancer | 59,600 | 7 | |
| Depression | Not depressed | 731,526 | 84 |
| Depressed | 142,282 | 16 |
| Predictor | Probit model coefficient (95% CI) | p-value | GLM coefficient (95% CI) | p-value |
|---|---|---|---|---|
| Age per year | –0.019 (–0.022 to –0.17) | < 0.001 | –0.017 (–0.020 –0.015) | |
| Age-squared | 0.00034 (0.00031 to 0.00037) | < 0.001 | 0.00027 (0.00025 to 0.00030) | < 0.001 |
| Gender | ||||
| Female | 0.033 (0.031 to 0.034) | 0.02 (–0.00 to 0.03) | 0.094 | |
| BMI category (kg/m2) | ||||
| 18.5–24.9 | Reference | Reference | ||
| 25.0–29.9 | 0.08 (0.06 to 0.10) | < 0.001 | 0.01 (–0.02 to 0.03) | 0.660 |
| 30.0–34.9 | 0.18 (0.16 to 0.20) | < 0.001 | 0.11 (0.08 to 0.14) | < 0.001 |
| 35.0–39.9 | 0.21 (0.19 to 0.23) | < 0.001 | 0.18 (0.15 to 0.21) | < 0.001 |
| ≥ 40.0 | 0.25 (0.23 to 0.27) | < 0.001 | 0.29 (0.26 to 0.32) | < 0.001 |
| Depression | ||||
| Present | 1.68 (1.56 to 1.80) | < 0.001 | 0.61 (0.54 to 0.67) | < 0.001 |
| Comorbidity | ||||
| None | Reference | Reference | ||
| Diabetes mellitus | 0.64 (0.53 to 0.75) | < 0.001 | 0.84 (0.70 to 0.98) | < 0.001 |
| CHD | 0.54 (0.44 to 0.65) | < 0.001 | 0.79 (0.69 to 0.89) | < 0.001 |
| Stroke | 0.35 (0.21 to 0.49) | < 0.001 | 1.01 (0.81 to 1.21) | < 0.001 |
| Cancer | 0.35 (0.28 to 0.43) | < 0.001 | 0.84 (0.78 to 0.89) | < 0.001 |
| BMI × depression | χ2 = 11.9, df = 4 | 0.018 | χ2 = 1.6, df = 4 | 0.81 |
| BMI × comorbidity | χ2 = 33.6, df = 16 | 0.006 | χ2 = 54.5, df = 16 | < 0.001 |
| Comorbidity × depression | χ2 = 112.4, df = 4 | < 0.001 | χ2 = 130.1, df = 4 | < 0.001 |
The predicted costs by BMI category, gender, comorbidity and depression status are presented in Table 14. Depression was consistently associated with greater health-care costs in all states. Overall, there is a largely positive linear relationship between health-care costs and BMI category. However, there are some notable exceptions to this trend which are more suggestive of a ‘J’-shaped relationship. Normal-weight participants frequently had higher predicted health-care costs than their overweight counterparts. In participants with diabetes this result is further pronounced, with normal-weight men and women, with or without depression, showing higher predicted health-care costs than all but the most obese category of participants. In men with depression and diabetes, the normal-weight participants had the highest estimated cost of all BMI categories, at £3940 compared with £3796 in the morbidly obese category. Similarly, in men with stroke and depression the annual estimated cost was highest in the normal-weight category, at £4442 compared with £4385 in the morbidly obese category. Stroke appears to be related to the highest overall costs, with the exception of not-depressed men with a BMI ≥ 25 kg/m2 who have been diagnosed with cancer and not-depressed women who are obese and have been diagnosed with cancer.
| Gender | Depression | Condition | BMI category (kg/m2) | ||||
|---|---|---|---|---|---|---|---|
| 18.5–24 | 25–29 | 30–34 | 35–39 | ≥ 40 | |||
| Men | Not depressed | At risk | 997 | 1016 | 1134 | 1206 | 1376 |
| DM | 2471 | 2242 | 2267 | 2356 | 2559 | ||
| CHD | 2341 | 2121 | 2469 | 2602 | 2871 | ||
| Stroke | 2864 | 2551 | 2882 | 2962 | 3074 | ||
| Cancer | 2406 | 2566 | 2936 | 3059 | 3280 | ||
| Depressed | At risk | 1985 | 1981 | 2128 | 2282 | 2506 | |
| DM | 3940 | 3527 | 3530 | 3621 | 3796 | ||
| CHD | 3645 | 3204 | 3719 | 3876 | 4227 | ||
| Stroke | 4422 | 3876 | 4339 | 4343 | 4385 | ||
| Cancer | 3458 | 3690 | 4097 | 4227 | 4274 | ||
| Women | Not depressed | At risk | 1057 | 1072 | 1194 | 1289 | 1439 |
| DM | 2548 | 2306 | 2332 | 2421 | 2659 | ||
| CHD | 2421 | 2177 | 2537 | 2667 | 3061 | ||
| Stroke | 2983 | 2676 | 3020 | 3054 | 3229 | ||
| Cancer | 2505 | 2662 | 3040 | 3162 | 3419 | ||
| Depressed | At risk | 2040 | 2029 | 2225 | 2441 | 2602 | |
| DM | 4007 | 3588 | 3617 | 3786 | 4055 | ||
| CHD | 3741 | 3276 | 3810 | 4001 | 4513 | ||
| Stroke | 4492 | 3969 | 4407 | 4528 | 4750 | ||
| Cancer | 3577 | 3759 | 4207 | 4435 | 4527 | ||
Linear regression identified having a comorbidity as the single largest predictor of health-care costs with a £1366 (95% CI £1296 to £1463) mean increase in annual patient costs if a morbidity is present (Table 15). The second greatest predictor was depression, at £1044 (95% CI £973 to £1115) per patient per year. The remaining factors included in the analysis, including BMI group, have a demonstrably lower influence on patient costs, but are nonetheless measurable and important influences. BMI group was positively associated with health-care costs but at a much smaller magnitude, with a £456 increase in morbidly obese participants compared with normal weight. Being overweight did not increase average health-care costs. An illustration of the increase in costs associated with comorbidity and depression by BMI group in women aged 46 years is presented in Figure 5. Age and female gender were related to higher costs but of a lower magnitude (£51 per year and £113 in females). Finally, there was evidence of a multiplicative effect on costs with the presence of depression or high BMI alongside a comorbidity. Having depression alongside a comorbidity was associated with an additional cost of £243 (95% CI £164 to £322) per year, while being obese, severely obese or morbidly obese increased costs by a further £168, £152 or £199, respectively. Depression had a greater additive effect on cost in conjunction with a comorbidity than BMI. The additive cost impact of a comorbidity was also greatest for those at normal weight.
| Variable | Category | Adjusted mean difference (£) (95% CI) | p-value |
|---|---|---|---|
| Gender | Male | Reference | |
| Female | 113 (81 to 144) | < 0.001 | |
| Age | Per year | 51 (50 to 52) | < 0.001 |
| Depression | Not depressed | Reference | |
| Depressed | 1044 (973 to 1115) | < 0.001 | |
| BMI category (kg/m2) | Reference | ||
| 25–29 | 5 (–117 to 107) | 0.934 | |
| 30–34 | 146 (34 to 258) | 0.011 | |
| 35–39 | 280 (168 to 392) | < 0.001 | |
| ≥ 40 | 456 (344 to 568) | < 0.001 | |
| Comorbiditya | Absent | Reference | |
| Present | 1366 (1269 to 1463) | < 0.001 | |
| Comorbidity × depressionb | 243 (164 to 322) | < 0.001 | |
| Comorbidity × BMI categoryc | 18.5–24 | 232 (106 to 35) | < 0.001 |
| 25–29 | Reference | ||
| 30–34 | 168 (43 to 293) | 0.009 | |
| 35–39 | 152 (17 to 277) | 0.017 | |
| ≥ 40 | 199 (74 to 325) | 0.002 | |
| BMI category × depression | 18.5–24 | 121 (20 to 221) | 0.018 |
| 25–29 | Reference | ||
| 30–34 | 76 (–24 to 176) | 0.137 | |
| 35–39 | 125 (25 to 225) | 0.014 | |
| ≥ 40 | 116 (16 to 216) | 0.024 |
FIGURE 5.
Costs of health-care utilisation by BMI category in women aged 46 years.
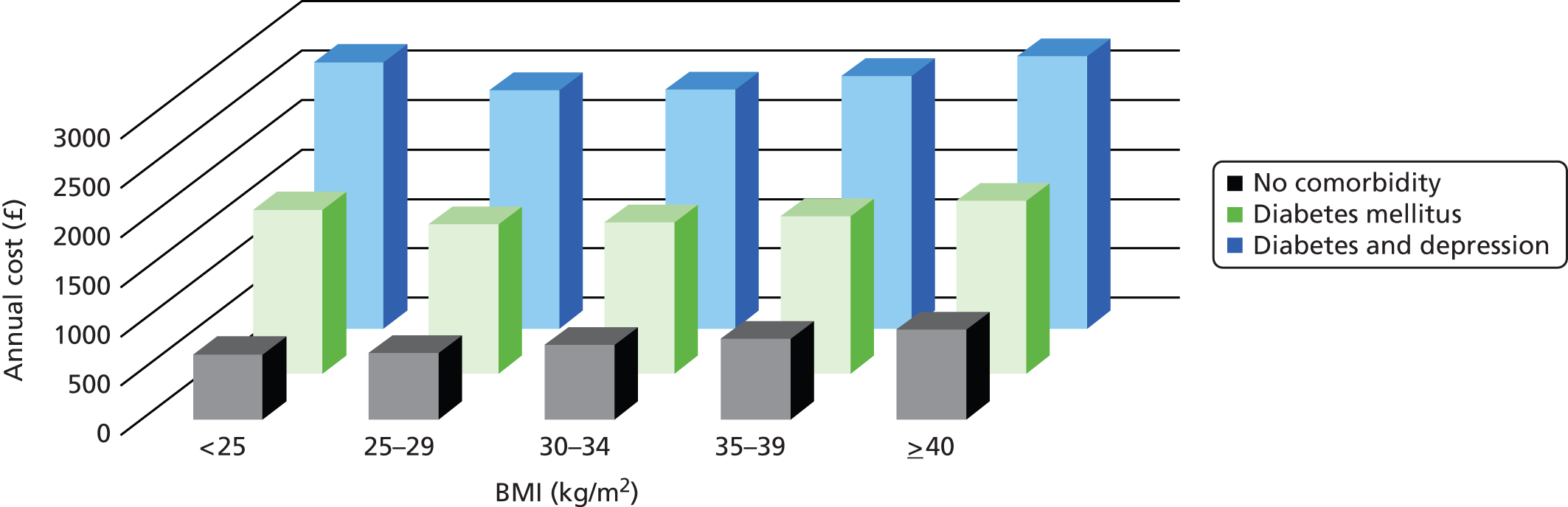
Discussion
Summary of findings
This analysis investigated health-care costs in relation to BMI and is the first we are aware of to isolate depression as a cost separate from other common obesity-related morbidities. Previous studies investigating health-care costs relating to obesity using patient cohorts have attempted to estimate the marginal effect of obesity on costs. 123,124 Understanding the causal association between BMI and health-care utilisation is important; however, in the context of treating patients in a clinical environment there is little benefit in divorcing obesity from related morbidities and socioeconomic factors.
We found that having a comorbidity was the greatest predictor of increased health-care costs, at £1366 per year, followed by clinical depression at £1044 per year. Of the four obesity-related morbidities we assessed, stroke was found to be associated with the highest absolute costs, which is likely to be a result of acute secondary care required after a stroke. This finding is supported by a previous paper that identified cardiovascular diseases and cardiovascular agents as the biggest drivers of health-care claims costs per unit of BMI. 125
We found that depression was the second greatest driver of costs and its presence increased costs in all states, in line with a previous investigation conducted into depression and health-care utilisation. 51 Overall, having high BMI in addition to a comorbidity appeared to increase costs exponentially, although having depression was an even greater contributor to high health-care costs alongside a comorbidity. In spite of this, being overweight was not associated with higher health-care costs than normal weight, and in fact, participants who were normal weight and diabetic had similar costs as diabetic participants who were morbidly obese. A majority of adults are now either overweight or obese. ‘Normal’ weight may sometimes result from weight loss associated with comorbidity and this might account for elevated health-care utilisation in this group. Therefore, diabetes and not just weight alone appears to be behind cost figures.
Comparison with other results
There is large variation in cost estimates for obesity based on population sample studies, ranging from an additional €160 per year for severe obesity compared with normal weight,124 to US$2741 for any obesity compared with normal weight. 123 Our findings estimated an increase of £146 per year for mild obesity in comparison with normal weight, and £456 for morbid obesity. These estimates were for the influence of BMI separate from the considerations of obesity-related morbidities and depression.
One unexpected finding was the high cost of health care in normal-weight diabetic patients compared with obese diabetics. In fact, this phenomenon has been reported elsewhere, and the possibility of greater morbidity in diabetics who are normal weight has been suggested as an explanatory factor. 126,127 The lack of statistically significant difference in costs between normal and overweight BMI categories has also been found in other studies. 128,129
Strengths and limitations
This analysis has a number of strengths that make it a valuable addition to the literature on health-care-related costs of obesity. First, it is based on a large, longitudinal, nationally representative data set and uses costs associated directly with provision of care rather than based on insurance claims, which are an indirect measure of morbidity and may be less accurate. It is also one of few analyses to use clinically measured height and weight values in the calculation of BMI rather than self-reported values which are prone to reporting bias. 130 This is also the only analysis we are aware of to use UK sample data to estimate the health-care costs associated with obesity. One possible limitation of the study is selection bias from inclusion of participants with a BMI recorded during a clinical consultation, who may be more frequent users of health-care services and, therefore, less healthy. Participants’ morbidity status was classified using the first diagnosis they received and we have not accounted for additional diagnoses. The costs associated with the conditions we have highlighted may represent costs from multiple morbidities, which are more frequent in obese patients and we know were present in 18% of the observations. 3
The economics literature has moved towards using an instrumental variables approach to examine the causal effect of obesity on medical costs by using the weight of a biological relative as an instrument. 123,124 The argument for this method is that the instrument predicts the participants’ weight, but not their morbidity status, meaning that the effect of weight on costs can be isolated. Such papers have found higher health-care costs for obesity than non-instrumented methods, so it is possible that our models underestimate the magnitude of the relationship.
Conclusions
The findings of this analysis emphasise that health-care costs for obesity are largely driven by a few key obesity-related morbidities and not by high BMI alone. Persistently high obesity rates and evidence from the literature7 suggest that preventative and reactive treatment strategies are currently of limited use. Until greater success is observed in lowering obesity levels, perhaps clinical focus should be on prevention of secondary conditions as a way of improving the health status of obese patients and lowering resulting costs. In particular, increasing uptake of successful diabetes prevention programmes131 may prove beneficial given the high costs observed across BMI categories in this analysis. Similarly, successful interventions to focus on the mental health of obese might also stem resultant health-care and non-health-care costs related to depression.
Chapter 6 Epidemiology of bariatric surgery in the UK
Introduction
This chapter contributes to the epidemiological context for the research by evaluating the epidemiology of bariatric surgery from 2002 to 2015. The analysis provides a population-based investigation of the changing epidemiology of bariatric surgery in the UK, drawing on primary care EHRs. We estimate utilisation rates for different procedures, changes in case mix over time, and the rate of reoperation. A reliability study was also performed to compare EHR data with GP questionnaire responses for the same patients. The results in this chapter were published in the journal Obesity Surgery in January 2016132 under the terms of the Creative Commons Attribution 4.0 International Licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Results
Reliability study results
Completed questionnaires were received for 78 patients (Table 16). All 78 responses confirmed that bariatric surgery had been performed on the date indicated in EHR data. The type of bariatric surgical procedure was confirmed for all 30 (100%) patients recorded with LAGB, for 24 out of 25 (96%) patients recorded with SG, and for 19 out of 23 (83%) patients recorded with GBP. Gastric band removal was confirmed for 27 out of 30 (90%) of cases. Among nine patients with second procedures recorded in EHRs following GBP or SG, six were confirmed in GP questionnaire responses. The most common complications after surgery reported by GPs were infection and wound site problems (13%), digestive issues including diarrhoea and vomiting (10%), pain (8%) and difficulties with the throat and swallowing (6%). High rates of complication in this group may be expected as patients requiring further procedures were oversampled. GPs reported that the surgery was privately funded in 32 (41%) of cases.
| Measure | EHR | GP questionnaire | Per cent agreement (95% CI) |
|---|---|---|---|
| Bariatric surgery performed | 78 | 78 | 100 (–) |
| Surgery type | |||
| Adjustable gastric banding | 30 | 30 | 100 (–) |
| GBP | 23 | 19 | 83 (61 to 95) |
| SG | 25 | 24 | 96 (80 to 100) |
| Gastric band removal | 30 | 27 | 90 (73 to 98) |
| Procedure secondary to GBP | 3 | 1 | 33 (1 to 91) |
| Procedure secondary to SG | 6 | 5 | 83 (36 to 100) |
| Difference in date, days (median, IQR) | |||
| Date of primary bariatric surgical procedure | 0 (0–0) | ||
Utilisation of bariatric surgical procedures
The number of procedures recorded increased over time, with only 104 procedures recorded between 2002 and 2005; 607 between 2006 and 2008; 1406 between 2009 and 2011; and 922 between 2012 and April 2014 (Table 17). The rate of surgery was highest in men and women aged 35–54 years. Rates of bariatric surgical procedures by age group and gender are presented in Figure 6. Rates of bariatric surgery were greatest for women in 2010 at 37 per 100,000 population per year, and in 2012 for men at 10 per 100,000 population per year. Disparity between genders was greatest in the youngest patients, aged 20–34 years, with peak rates of 15 per 100,000 per year in women and 3 per 100,000 per year in men. LAGB was the most frequent procedure, accounting for 1297 (43%) of cases, followed by GBP in 1265 (42%) participants and SG in 477 (16%). LAGB accounted for 97% of 104 procedures performed from 2002 to 2005. The use of GBP and SG increased over time while LAGB declined (Figure 7). During 2012–14, GBP accounted for 55% of procedures, while SG accounted for 25% and LAGB accounted for 20% (see Table 17).
| Measure | 2002–5 | 2006–8 | 2009–11 | 2012–4 | p-value |
|---|---|---|---|---|---|
| Number of procedures | 104 | 607 | 1406 | 922 | |
| Type of procedure | < 0.001 | ||||
| Gastric banding | 101 (97) | 518 (85) | 497 (35) | 181 (20) | |
| GBP | 2 (2) | 51 (8) | 701 (50) | 511 (55) | |
| SG | 1 (1) | 38 (6) | 208 (15) | 230 (25) | |
| Age at procedure (years), mean (SD) | 43.4 (8.6) | 44.4 (10.0) | 46.1 (10.4) | 46.8 (10.0) | < 0.001 |
| Female | 89 (86) | 504 (83) | 1118 (80) | 691 (75) | < 0.001 |
| BMI (kg/m2), mean (SD) | 40.6 (7.1) | 42.7 (8.3) | 44.2 (8.2) | 44.8 (8.3) | < 0.001 |
| BMI category (kg/m2) | |||||
| 30–34.9 | 29 (28) | 108 (18) | 162 (12) | 95 (10) | < 0.001 |
| 35.0–39.9 | 24 (23) | 161 (27) | 301 (21) | 189 (21) | |
| ≥ 40 | 51 (49) | 338 (56) | 943 (67) | 638 (69) | |
| Diabetes | 20 (19) | 124 (20) | 428 (30) | 302 (33) | < 0.001 |
| Depression | 61 (59) | 320 (53) | 762 (54) | 540 (59) | 0.148 |
| Current smoking | 20 (19) | 104 (17) | 231 (16) | 146 (16) | 0.323 |
| Antihypertensive | 42 (40) | 278 (46) | 728 (52) | 509 (55) | < 0.001 |
| Statins | 20 (19) | 123 (20) | 418 (30) | 301 (33) | < 0.001 |
FIGURE 6.
Rates of first bariatric surgery procedures in a large primary care population for (a) men; and (b) women. The denominator is the population registered in CPRD. Blue line, 20–34 years; green line, 35–54 years; black line 55–84 years.
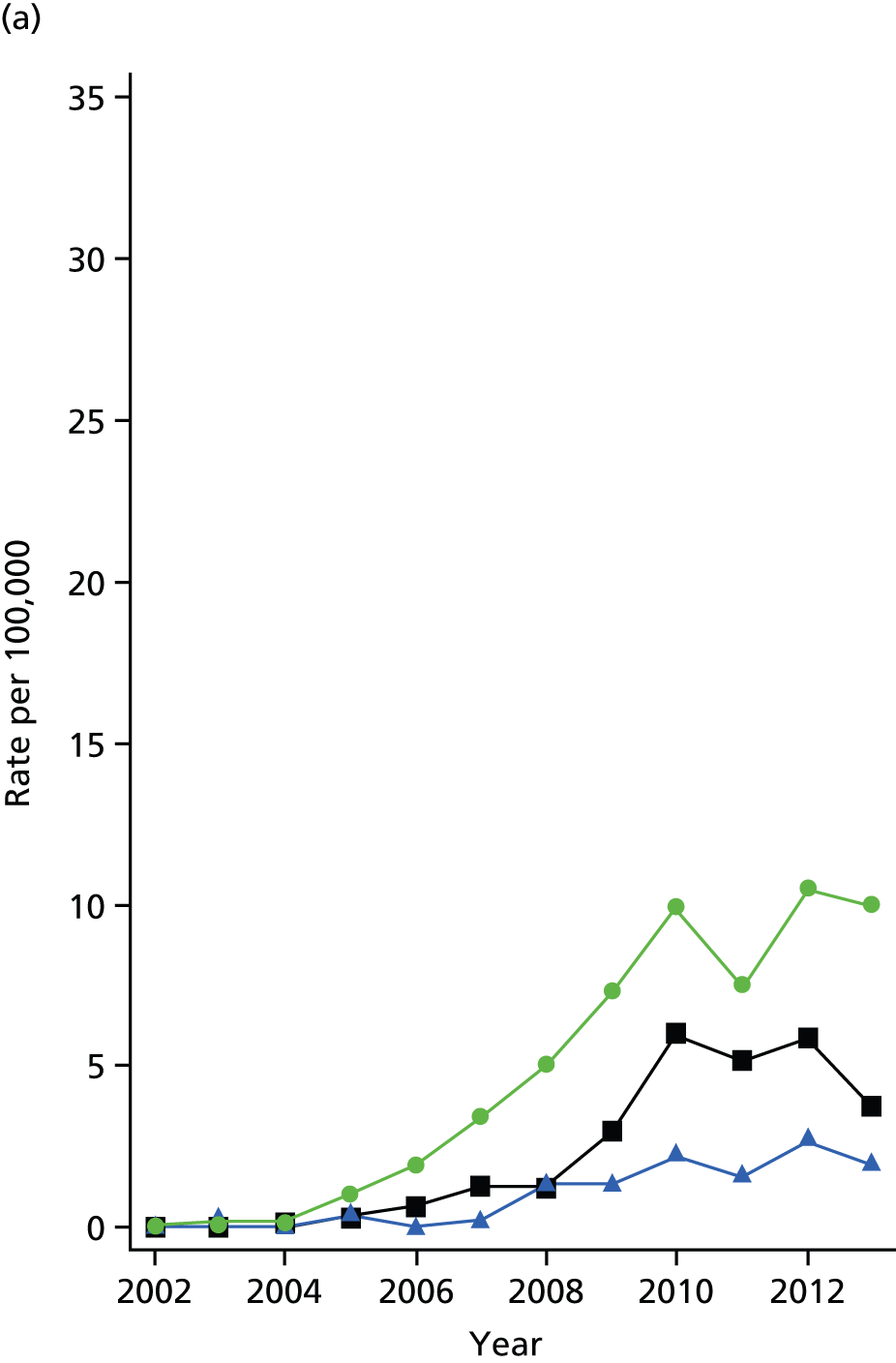
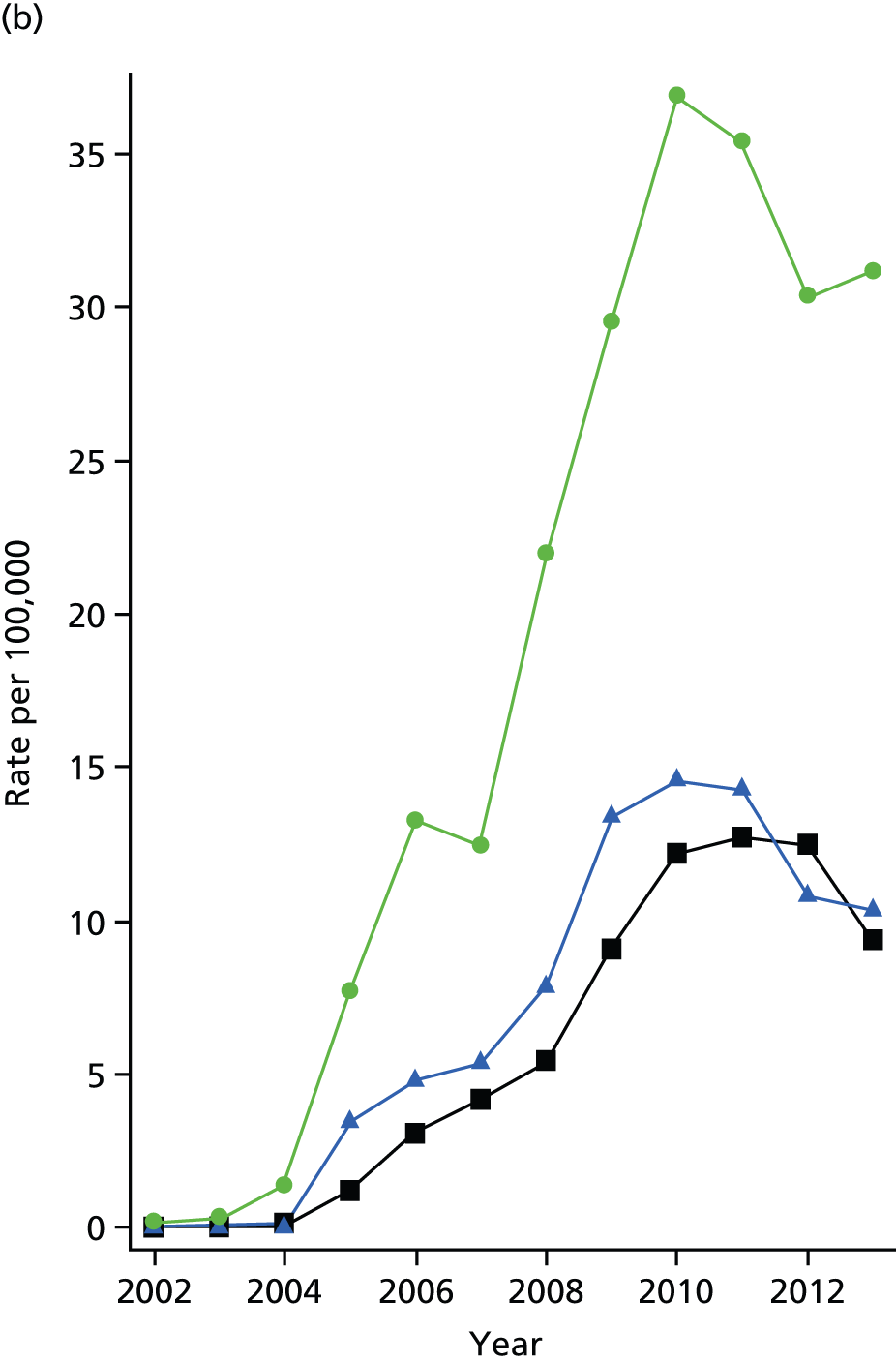
FIGURE 7.
(a) Trends in the utilisation of different bariatric surgical procedures from 2002 to April 2014; and (b) total number of procedures per year. Green line, LAGB; blue line, GBP; black line, SG.
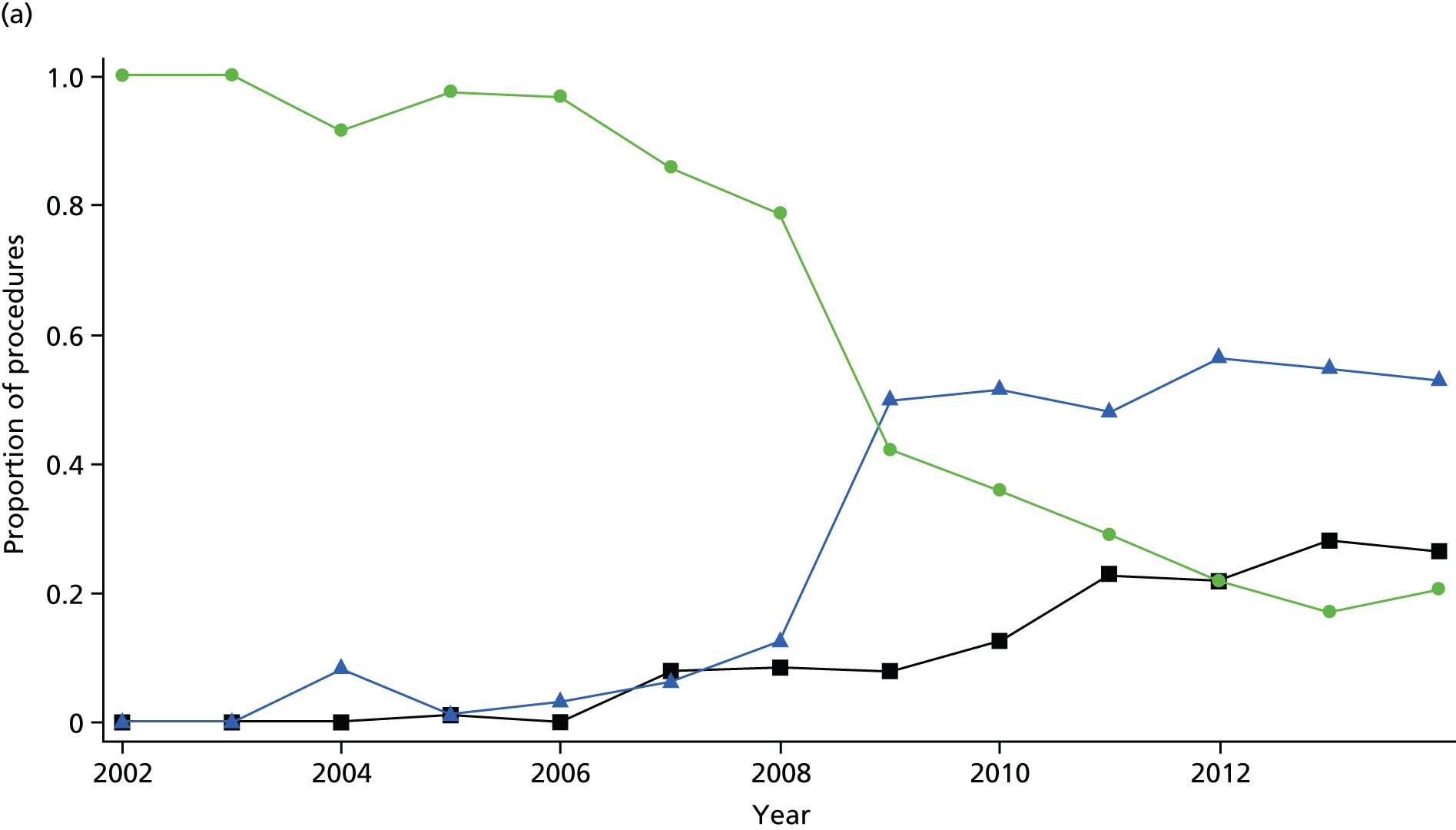
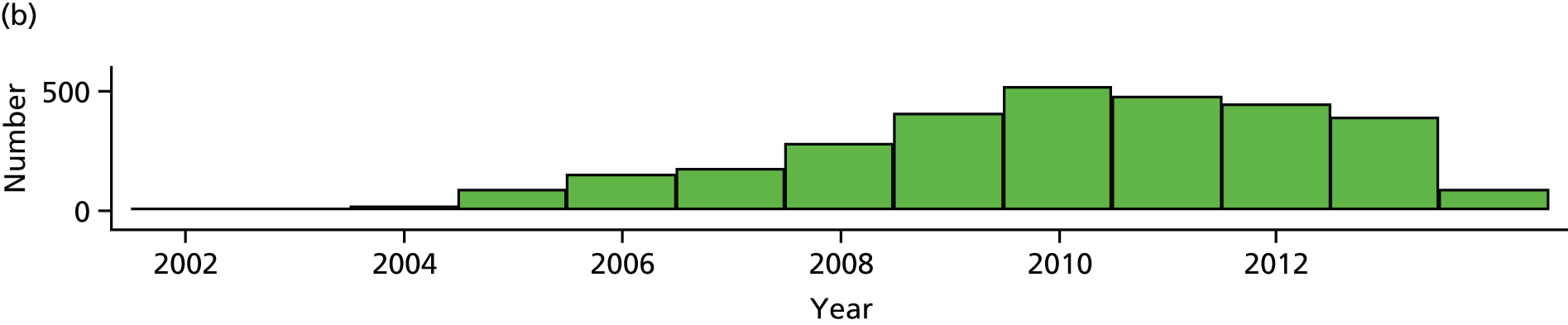
Changes in case mix
Patient characteristics at the index date are presented in Table 17. The mean age at operation increased from 43.4 to 46.8 years during the study (p < 0.001), and the proportion of women declined from 86% to 75% (p < 0.001). The mean recorded BMI increased from 40.6 kg/m2 to 44.8 kg/m2 (p < 0.001). The proportion of participants with diabetes increased from 19% to 33%, while the proportion of patients prescribed antihypertensive drugs and statins also increased (all p < 0.001). More than half of all participants had depression recorded at some time before the procedure. As a consequence of these trends, there were important differences in case mix for patients undergoing LAGB as compared with GBP and SG (Table 18). LAGB patients were generally operated on in an earlier period, were younger, more often female, less obese and less likely to have diabetes, hypertension or hypercholesterolaemia.
| Variables | LAGB (N = 1297) | GBP/SG (N = 1742) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Period of procedure, n (%) | ||||
| 2002–5 | 101 (8) | 3 (0) | 0.21 (0.06 to 0.68) | 0.009 |
| 2006–8 | 518 (40) | 89 (5) | Reference | |
| 2009–11 | 497 (38) | 909 (52) | 11.7 (8.61 to 15.9) | < 0.001 |
| 2012–14 | 181 (14) | 741 (43) | 26.0 (18.7 to 36.3) | < 0.001 |
| Age (years), mean (SD) | 44.3 (10.0) | 47.1 (10.2) | 1.017 (1.007 to 1.027) | 0.001 |
| Female, n (%) | 1103 (85) | 1299 (75) | 0.66 (0.53 to 0.82) | < 0.001 |
| BMI (kg/m2), mean (SD) | 41.3 (7.3) | 46.0 (8.4) | 1.08 (1.07 to 1.09) | < 0.001 |
| Diabetes, n (%) | 244 (19) | 630 (36) | 1.49 (1.18 to 1.89) | 0.001 |
| Depression, n (%) | 715 (55) | 968 (56) | 1.03 (0.86 to 1.24) | 0.754 |
| Current smoking, n (%) | 229 (18) | 272 (16) | 0.95 (0.75 to 1.21) | 0.663 |
| Antihypertensive, n (%) | 572 (44) | 985 (57) | 1.00 (0.83 to 1.22) | 0.960 |
| Statins, n (%) | 255 (20) | 607 (35) | 1.20 (0.91 to 1.59) | 0.198 |
Secondary procedures
There were three deaths within 30 days of the date of the initial procedure. Rates of band removal and reoperation following LAGB are presented in Table 19. The most common procedure was removal of a gastric band, found in 82 (6.3%) cases. This was equivalent to a rate of 1.6 (95% CI 1.3 to 2.0) per 100 person-years and the median time between gastric band insertion and removal was 144 weeks (IQR) 69–203 weeks). There were 60 (4.6%) of LAGB patients who had a subsequent medical code recorded indicating a GBP or SG procedure, with a rate of 1.2 (95% CI 0.9 to 1.5) per 100 patient-years. There were 10 patients who received SG, who later had codes for GBP recorded; and six patients with GBP, who later had codes for LAGB (n = 4) or SG recorded (n = 2). The validation study confirmed 86% and 33% of these reoperations, respectively, with two procedures secondary to SG attributed to hiatal hernia repair.
| First procedure | Subsequent procedure | Frequency (%) | Median interval (IQR, weeks) | Rate per 100 patient-years (95% CI) |
|---|---|---|---|---|
| LAGB (1297) | Band removed | 82 (6.3) | 144 (69–203) | 1.6 (1.3 to 2.0) |
| Subsequent bypass or sleeve | 60 (4.6) | 108 (58–200) | 1.2 (0.9 to 1.5) |
Long-term follow-up
Recording of BMI values into primary care EHRs after bariatric surgical procedures was generally poor. From 3039 participants, 486 had BMI values recorded in the first year following the procedure and 332 and 241 in the second and third years, representing 18%, 15% and 13% of those remaining under observation, respectively. Evaluation of body weight values indicated a mean reduction in BMI of 6.98 kg/m2 (95% CI 6.3 to 7.6 kg/m2) in the first postoperative year and 9.99 kg/m2 (95% CI 9.1 to 10.9 kg/m2) in the second postoperative year.
Discussion
Summary of findings
This analysis provides a large-scale population-based evaluation of the utilisation of bariatric surgical procedures in the UK. The study complements a recently published bariatric surgical registry report,133 which presents data reported by bariatric surgeons. The present results demonstrate that EHRs, including those from primary care, represent a valuable resource for evaluating the utilisation and outcomes of bariatric surgery. The reliability study demonstrated high levels of agreement between primary care EHRs and questionnaire responses obtained directly from GPs. We caution that errors may be present in either data source, and we do not have evidence to show which is the more likely to be correct, but high levels of agreement between the two sources of information lend support to the validity of EHR data.
The rate of bariatric surgery recorded in primary care medical records increased rapidly between 2002 and 2014. Initially, LAGB accounted for most procedures, but the use of GBP and, to a lesser extent, SG has increased since 2008. There have been changes in case mix, with procedures now being performed in older patients, with greater BMI and a higher prevalence of diabetes.
Comparison with other results
The large increase in number of bariatric surgery procedures identified in CPRD over the last 10 years is consistent with findings reported from analysis of hospital utilisation statistics. 36 The gender disparity, age profile of surgery patients and changing patterns of surgery were also comparable with the trends seen in data for hospital utilisation and the bariatric surgical registry. 36,133,134 Depression was recorded in > 50% of participants at some time prior to surgery, a higher rate than reported elsewhere. 135 This difference may relate to using primary care rather than hospital setting as a data source, with a diagnosis of depression more likely to be recorded in the former.
Following gastric banding, gastric band removal was observed in 1.6% of patients per year and 1.2% per year were recorded as having a further additional procedure of GBP or SG, with a high level of validation from GP responses. These findings confirm in population-based data that there is significant incidence of band slippage or band intolerance requiring removal. The bariatric surgical registry recorded a much lower proportion of patients undergoing revisional bariatric surgery (0.3%) after gastric banding. 133 This discrepency may reflect the short period (3 years) covered by registry data, problems with data linkage occuring when reoperations are performed at different hospitals or under-reporting of reoperations and revisions.
There were 10 patients who underwent SG who later had codes for GBP recorded, and six patients who received GBP with subsequent codes for LAGB (n = 4) or SG recorded (n = 2). Validation confirmed the majority of these secondary procedures, but coding errors may account for some of these, as misclassification can arise through errors of recording in primary care records, especially when primary care physicians may be unfamiliar with different bariatric surgical procedures. Nonetheless, it is clear that surgical reintervention rate following either SG or GBP is lower than that following gastric banding.
Recording of weight data for bariatric surgery patients in primary care was poor. Clinical guidelines suggest that all bariatric surgery patients are expected to undergo lifelong follow-up to monitor their weight, as well as micronutrient monitoring in GBP patients. 35 Recording of weight and BMI in primary care records is known to be generally limited,44 but it is especially concerning that the majority of patients who have undergone bariatric surgery are not being weighed regularly by their GP. The observed maximum weight loss at 2 years is comparable with data from clinical trials,5,6,136 and gastric banding was associated with lower weight loss that other surgery types in accordance with the literature. 31,133 However, the high rate of attrition in this study means that caution is required when interpreting these results. Additionally, long-term follow-up in primary care may be biased by better attendance of patients whose surgery has proved less successful or who have remained overweight. 44
Strengths and limitations
This study had the strengths of a large nationally representative data source with extended periods of longitudinal follow-up. We acknowledge that clinical information has several limitations when used for research purposes, including missing data values due to opportunistic data collection and recording, but a reliability study suggested a high level of agreement between EHR records and GP reports for primary surgeries. Recording of body weight in primary care was poor subsequent to bariatric surgery.
Conclusions
This is the first large-scale analysis to use EHRs for the evaluation of bariatric surgical utilisation for obesity; and demonstrates rapid increases in the use of such procedures and a move away from gastric banding towards GBP and SG, with a shift in case mix towards more severely affected patients.
Chapter 7 Reduced incidence of clinical diabetes mellitus after bariatric surgery
Introduction
This chapter uses primary care EHRs to analyse the incidence of new diabetes following bariatric surgery. The possible impact of bariatric surgery on T2DM is of particular importance because some 3% of morbidly obese individuals develop diabetes each year8 and we have demonstrated that comorbidity is a key driver of health-care costs in obesity (see Chapter 5). In the SOS study,8 a total of 1658 non-diabetic patients were followed over a 15-year period, showing a 76% reduction in the incidence of diabetes following bariatric surgery. However, the majority of participants in the Swedish study underwent vertical band gastroplasty, an operation which is no longer widely performed because of the high incidence of weight regain. Participants in research studies may undergo intensive follow-up that may not be typical of care provided in routine clinical practice. The aim of this study, therefore, was to employ a population-based cohort, with matched controls, drawn from a database of primary care EHRs to provide a pragmatic evaluation of the effect of bariatric surgery on the development of clinical diabetes in obese individuals. This analysis has been published open access in The Lancet Diabetes and Endocrinology as ‘Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study’7 under the terms of the Creative Commons Licence CC BY 3.0.
Results
There were 4793 participants with bariatric surgery recorded; 1324 participants with bariatric surgery first recorded < 1 year after the start of the patient record were excluded, as were 14 participants aged < 20 years at the index date, and 401 participants with either no BMI record before surgery or BMI values < 30 kg/m2 prior to surgery. There remained 3054 obese participants aged ≥ 20 years who received bariatric surgery while their EHR was active in CPRD. There were 878 participants who were already diagnosed with diabetes before the date of surgery, who were excluded. Nine participants with a record of gastric band removal before the index date were also excluded. There were then 2167 obese, non-diabetic participants who received first bariatric surgery procedures who were compared with 2167 matched control participants. The median duration of follow-up was 2.8 years (IQR 1.3–4.5 years), with a maximum of 7 years’ follow-up.
The type of bariatric surgery was classified according to the procedure recorded on the index date. There were 1053 (49%) participants who received LAGB, 795 (37%) received GBP procedures and 317 (15%) received SG as the index procedure. Two participants had codes for more than one type of procedure recorded on the index date.
Baseline characteristics for the bariatric surgery participants and controls are presented in Table 20. Values are those most recently recorded before the index date. The two groups were generally well matched for age, gender and BMI. The mean [standard deviation (SD)] age of participants undergoing surgery was 44.4 years (SD 10.1 years), and 84% were women. The index BMI was > 40 kg/m2 in 60% of participants. A diagnosis of depression was ever recorded before the index date for 55% of bariatric surgery participants and 32% of controls. Bariatric surgery participants were more likely to have recorded elevated blood pressure or raised total cholesterol values and to be treated with antihypertensive or lipid-lowering drugs.
| Characteristics | BS cases | Controls | p-value |
|---|---|---|---|
| Number | 2167 | 2167 | |
| Female | 1812 (84) | 1878 (87) | Matched |
| Age (years), mean (SD) | 44.4 (10.1) | 44.6 (14.1) | Matched |
| BMI (kg/m2), mean (SD) | 43.0 (8.1) | 43.2 (8.6) | Matched |
| BMI category | Matched | ||
| Obese (BMI 30–34.9 kg/m2) | 339 (16) | 332 (15) | |
| Severe obesity (BMI 35–39.9 kg/m2) | 535 (25) | 551 (24) | |
| Morbid obesity (BMI ≥ 40 kg/m2) | 1293 (60) | 1284 (59) | |
| Index year | |||
| 2002–5 | 84 (4) | 113 (5) | Matched |
| 2006–8 | 483 (22) | 442 (20) | |
| 2009–11 | 979 (45) | 1014 (47) | |
| 2012–14 | 621 (29) | 598 (28) | |
| HbA1c category | |||
| 42 mmol/mol | 208 (10) | 195 (9) | |
| 42 to < 48 mmol/mol | 54 (2) | 50 (2) | |
| Not recorded | 1905 (88) | 1922 (89) | |
| CHD | 60 (3) | 58 (3) | 0.847 |
| Stroke | 20 (1) | 26 (1) | 0.381 |
| Previous depression diagnosis | 1198 (55) | 701 (32) | < 0.001 |
| Current smoker | 376 (17) | 386 (18) | 0.583 |
| BP recorded > 140/90 mmHg | 581 (27) | 464 (21) | < 0.001 |
| Total cholesterol recorded > 5 mmol/l | 845 (39) | 387 (18) | < 0.001 |
| Treatment for hypertension | 906 (42) | 519 (24) | < 0.001 |
| Lipid-lowering treatment | 270 (12) | 183 (8) | < 0.001 |
The onset of diabetes in bariatric surgery and control participants is presented in Table 21/Figure 8. In the first 7 years, there were 38 new diagnoses of diabetes in bariatric surgery participants and 177 in control participants. None of the diabetes participants was recorded as being type 1 diabetes. Three controls and one bariatric surgery participant with diabetes were treated with insulin within 6 months of the diabetes diagnosis date. There were 10 controls and three cases whose diabetes was associated with codes for gestational diabetes, which were excluded as a sensitivity analysis. A Kaplan–Meier graph showing the development of diabetes in bariatric surgery and control participants is presented in Figure 8. By the end of the seventh year of follow-up, 4.3% (95% CI 2.9% to 6.5%) of bariatric surgery participants and 16.2% (95% CI 13.3% to 19.6%) of non-surgery controls had developed diabetes. The incidence of diabetes was 28.2 (95% CI 24.4 to 32.7) per 1000 person-years in control participants and 5.7 (95% CI 4.2 to 7.8) per 1000 in BS participants.
| Year | BS cases | Controls | ||||
|---|---|---|---|---|---|---|
| At risk, N | Diabetes diagnoses, n | Per cent with diabetes (95% CI)a | At risk, N | Diabetes diagnoses, n | Per cent with diabetes (95% CI)a | |
| 1 | 1759 | 14 | 0.7 (0.4 to 1.2) | 1847 | 73 | 3.5 (2.8 to 4.4) |
| 2 | 1369 | 4 | 1.0 (0.6 to 1.5) | 1317 | 41 | 5.9 (5.0 to 7.1) |
| 3 | 1017 | 5 | 1.4 (0.9 to 2.1) | 857 | 23 | 7.9 (6.7 to 9.3) |
| 4 | 692 | 6 | 2.1 (1.4 to 3.0) | 558 | 20 | 10.4 (8.8 to 12.2) |
| 5 | 440 | 4 | 2.7 (1.9 to 4.0) | 358 | 10 | 12.4 (10.5 to 14.7) |
| 6 | 264 | 3 | 3.6 (2.4 to 5.2) | 238 | 5 | 13.8 (11.6 to 16.3) |
| 7 | 154 | 2 | 4.3 (2.9 to 6.5) | 132 | 5 | 16.2 (13.3 to 19.6) |
FIGURE 8.
Incidence of T2DM in bariatric surgery participants and matched controls within 7 years after procedure. Green line, bariatric surgery; black line, non-surgery controls.
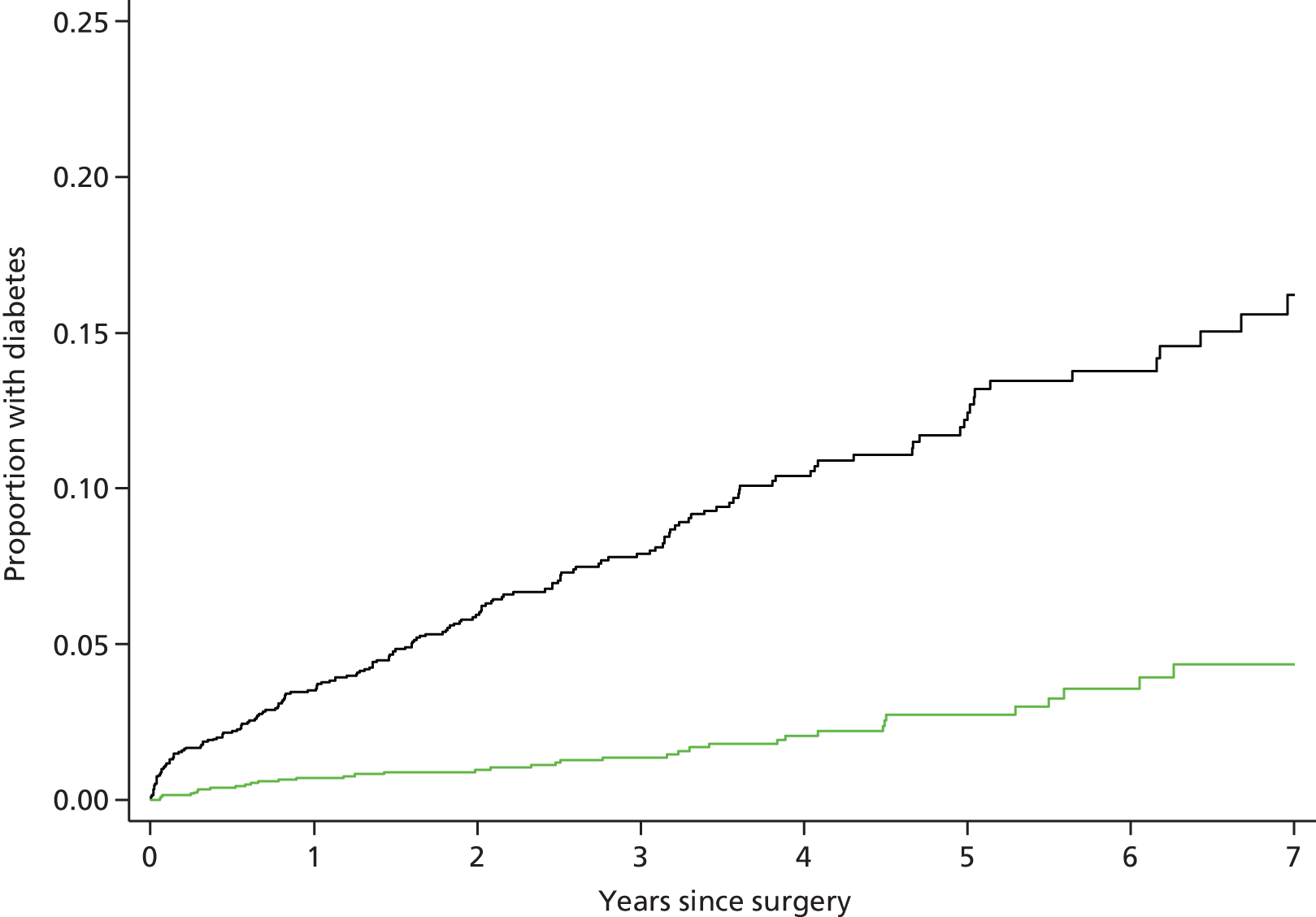
The unadjusted HR for diabetes in bariatric surgery participants compared with controls was 0.20 (95% CI 0.14 to 0.30; p < 0.001). Multivariable adjustment for baseline characteristics including matching variables, comorbid cardiovascular disease and depression, smoking, hypertension and hypercholesterolemia and associated treatment had negligible effect on the magnitude of the estimated HR. The fully adjusted HR for bariatric surgery was 0.20 (95% CI 0.13 to 0.30; p < 0.001). After allowing for bariatric surgery, baseline characteristics were generally not associated with the risk of developing diabetes except for an increased hazard associated with elevated baseline HbA1c (Table 22).
| Variable | HRa (95% CI) | p-value |
|---|---|---|
| Bariatric surgery | 0.20 (0.13 to 0.30) | < 0.001 |
| Female | 0.73 (0.50 to 1.07) | Matched |
| Age (years) | 1.02 (1.01 to 1.04) | Matched |
| BMI (kg/m2) | 1.03 (1.02 to 1.04) | Matched |
| Prevalent CHD | 1.25 (0.65 to 2.40) | 0.513 |
| Prevalent stroke | 1.23 (0.46 to 3.30) | 0.675 |
| Previous depression diagnosis | 1.26 (0.96 to 1.66) | 0.092 |
| Current smoker | 1.17 (0.81 to 1.70) | 0.399 |
| BP recorded > 140/90 mmHg before index date | 1.27 (0.91 to 1.79) | 0.163 |
| Total cholesterol recorded > 5 mmol/l before index date | 0.92 (0.61 to 1.41) | 0.716 |
| HbA1c 42 to < 48 mmol/mol | 3.73 (1.69 to 8.25) | Matched |
| Treatment for hypertension before index date | 1.23 (0.86 to 1.76) | 0.249 |
| Lipid-lowering treatment before index date | 1.15 (0.69 to 1.93) | 0.595 |
Table 23 shows HRs for diabetes divided by subgroups of age, gender and BMI category. The effect of bariatric surgery was generally similar in men and women and across age groups. There was no interaction of bariatric surgery and age. Estimates were also similar for time periods between 2002 and 2014 even though gastric banding accounted for 96% of procedures in the earliest period and only 24% in the latest. However, there was evidence of a trend towards greater effect of bariatric surgery with increasing BMI category (interaction term, p = 0.0819). Table 3 presents the incidence of diabetes by type of procedure. In the fully-adjusted model, which included adjustment for index year as well as other covariates, HRs were slightly lower for both GBP and SG than for LAGB (p = 0.0714), although each type of procedure was associated with lower incidence of diabetes than in the control group. There were small numbers for some subgroups; LAGB, GBP and SG were associated with 30, six and two new diagnoses of diabetes, respectively.
| Variable | Diabetes incidence rate per 1000 person-years (95% CI) | Adjusteda HR (95% CI) | p-value | |
|---|---|---|---|---|
| BS | Controls | |||
| All participants | 5.7 (4.2 to 7.8) | 28.2 (24.4 to 32.7) | 0.20 (0.13 to 0.30) | < 0.001 |
| Sex | ||||
| Men | 6.8 (3.2 to 14.3) | 38.5 (26.7 to 55.4) | 0.17 (0.06 to 0.46) | < 0.001 |
| Women | 5.5 (3.9 to 7.8) | 26.8 (22.8 to 31.5) | 0.21 (0.13 to 0.33) | < 0.001 |
| Baseline BMI category (kg/m2) | ||||
| 30–34.9 | 6.1 (2.9 to 12.9) | 15.7 (9.7 to 25.2) | 0.39 (0.11 to 1.42) | 0.153 |
| 35–39.9 | 5.9 (3.2 to 10.9) | 22.1 (16.0 to 30.5) | 0.24 (0.12 to 0.49) | < 0.001 |
| ≥ 40 | 5.5 (3.6 to 8.4) | 35.0 (29.3 to 41.7) | 0.15 (0.09 to 0.25) | < 0.001 |
| Age group (years) | ||||
| 20 to 34 | 1.7 (0.4 to 7.0) | 17.3 (11.8 to 25.2) | 0.14 (0.03 to 0.63) | 0.010 |
| 35 to 54 | 6.3 (4.4 to 9.3) | 26.3 (21.1 to 32.7) | 0.21 (0.13 to 0.34) | < 0.001 |
| ≥ 55 | 7.0 (3.5 to 14.1) | 42.1 (33.3 to 53.2) | 0.18 (0.08 to 0.38) | < 0.001 |
| Type of procedureb | ||||
| Laparoscopic gastric banding | 7.3 (5.1 to 10.5) | 24.9 (20.2 to 30.6) | 0.29 (0.18 to 0.48) | < 0.001 |
| GBP | 3.2 (1.4 to 7.1) | 33.3 (26.0 to 42.7) | 0.10 (0.04 to 0.25) | < 0.001 |
| SG | 2.9 (0.7 to 11.7) | 31.7 (21.4 to 47.0) | 0.07 (0.01 to 0.30) | < 0.001 |
| Index period | ||||
| 2002–5 | 3.8 (1.0 to 15.3) | 29.7 (18.9 to 46.5) | 0.12 (0.03 to 0.52) | 0.004 |
| 2006–8 | 8.3 (5.4 to 12.9) | 25.8 (19.7 to 33.9) | 0.32 (0.18 to 0.57) | 0.002 |
| 2009–11 | 4.2 (2.5 to 7.3) | 28.2 (22.6 to 35.4) | 0.14 (0.06 to 0.31) | < 0.001 |
| 2012–14 | 4.4 (1.4 to 13.7) | 32.4 (22.5 to 46.6) | 0.10 (0.03 to 0.36) | < 0.001 |
Sensitivity analyses
The association of bariatric surgery with lower incidence of diabetes was found to be robust in a number of sensitivity analyses. When the entire comparison cohort of 103,502 obese non-diabetic individuals, rather than selected matched controls, was used for reference, the adjusted HR associating bariatric surgery with diabetes incidence was 0.16 (95% CI 0.11 to 0.22; p < 0·001). Diagnosis of diabetes might be more likely soon after the index date because of increased medical surveillance. However, when cases of diabetes diagnosed within the first year following the index date were excluded, the adjusted HR was 0.20 (95% CI 0.12 to 0.32; p < 0.001). Exclusion of participants associated with gestational diabetes diagnoses gave an adjusted HR of 0.19 (95% CI 0.13 to 0.30; p < 0.001). In a competing risks analysis, allowing for the competing risk of death, the adjusted HR was 0.20 (95% CI 0.13 to 0.31; p < 0.001). Analyses that used only clinical criteria, and not HbA1c, for diagnosis of diabetes gave similar results.
Discussion
Summary of findings
This analysis demonstrates that in a large population-based cohort of obese participants undergoing bariatric surgery using contemporary procedures, the risk of developing T2DM is substantially reduced over a maximum of 7 years of follow-up. The risk of diabetes was reduced by 80%, compared with control participants who did not undergo surgery. This is perhaps the first large-scale pragmatic study to evaluate the impact of current bariatric surgical procedures on diabetes incidence in the context of usual care settings. Even in patients observed in routine clinical practice, our results demonstrate that newer bariatric surgical procedures have particular efficacy for diabetes prevention in obese individuals.
An effect of bariatric surgery on diabetes incidence was observed in both men and women and across all ages. GBP and SG procedures were associated with slightly lower relative hazards for diabetes than LAGB but we caution against drawing firm conclusions concerning the comparative effectiveness of different procedures from a non-randomised study because selection for different procedures may be associated with the underlying risk of developing diabetes and some of these subgroup analyses were based on small numbers of outcome events.
Comparison with other results
Previous studies of bariatric surgery and the prevention of T2DM include the SOS study8 as well as several case series. 137–139 In the SOS study,8 69% of patients underwent vertical banded gastroplasty, 19% underwent banding and 12% received GBP surgery. Both the incidence of diabetes in bariatric surgical patients and controls, and the relative risk reduction associated with bariatric surgery, are very similar to those observed in the present study. The present results, therefore, provide important confirmation from a population-based sample, with intervention using current surgical procedures.
Strengths and limitations
Our analysis had the strengths of a large population-based sample with prolonged follow-up and prospective documentation of clinical diabetes in primary care. However, we acknowledge several limitations. We identified the three major procedures used in bariatric surgical practice in the UK. However, there may be less frequently performed procedures, such as the duodenal switch, which were not included. The control group did not receive standardised non-surgical intervention for obesity, and during the study period intensive multimodal weight-loss programmes in primary care were rare. Ascertainment of diabetes outcomes was comprehensive; diagnoses recorded into CPRD records are generally valid. 42 However, we were not able to document subclinical diabetes that might have been confirmed through testing all participants for evidence of hyperglycaemia. We excluded cases in which antidiabetes drugs were prescribed for polycystic ovary syndrome but it is possible that some prescriptions might be for diabetes prevention rather than treatment. Body weight, and other relevant measures, during the period of study were not recorded consistently. Preoperative BMI category might have been misclassified and it was not possible to relate differences in diabetes incidence to changes in body weight, though it should be noted that the effects of bariatric surgery are not entirely mediated by changes in body weight. 136 Observed weight reductions following surgery were consistent with those of previous reports.
Access to bariatric surgery is presently very limited in the UK and, consequently, those receiving surgery represent a highly selected group. The higher prevalence of previous depression diagnoses in the surgical group suggests that patients with depression were more likely to be referred with the belief that weight loss may improve their depressive symptoms. This belief is likely to be misplaced in the long term. 90 Patients who received surgery might have been more adherent than controls to other diabetes prevention advice including diet or exercise. However, we observed that participants who received surgery were more likely to be prescribed antihypertensive drugs or statins, which may sometimes be associated with diabetes. Control participants more often had missing values for blood pressure and plasma cholesterol, suggesting that medical surveillance was lower in this group. Differential medical surveillance might have made detection of diabetes or pre-diabetes more likely in patients considered for surgery. However, the higher proportion of surgical cases on antihypertensive or lipid-lowering therapy might suggest a selection bias in the opposite direction. We included relevant confounders in the analysis but misclassification and missing values might lead to residual bias. In a non-randomised study, residual confounding from unmeasured genetic, social or environmental variables is a concern. However, we fitted several different models and performed sensitivity analyses that provided evidence that the main findings of the study were robust. Nearest neighbour matching gave a comparison group that was not exactly matched for key variables. However, covariate adjustment had minimal effect and very similar results were obtained when the entire source cohort was used for reference. A method of analysis that did not allow for matching might give slightly wider CIs and larger p-values than a matched analysis,24 but the present results did not raise concerns of statistical error.
Conclusions
The present results, together with those of previous studies,60 suggest that bariatric surgery may be a highly effective method for preventing diabetes in patients with severe and morbid obesity. This raises questions concerning how surgery for obesity should be integrated into strategies for the control of obesity and prevention of diabetes in the population at risk. Further research is needed to understand the outcomes of different levels of uptake of obesity surgery, as well as the long-term outcomes for patients who receive current surgical procedures for obesity.
Chapter 8 Bariatric surgery in people with diabetes: diabetes control and utilisation of antidiabetes drugs
Introduction
This chapter presents a pragmatic analysis of changes in diabetes control and antidiabetic drug utilisation following bariatric surgery. Remission of diabetes will be included as an input to the model as comorbidity is the greatest contributor to health-care costs in obesity. The potential role of bariatric surgery in the treatment of diabetes in individuals with severe obesity is increasingly recognised. 35,74,140 A systematic review found that use of bariatric surgery in obese patients with T2DM was associated with remission of diabetes in approximately 70% of patients over the first 2 years following surgery. 74 However, most randomised trials have evaluated outcomes for small samples of patients, with outcomes reported more than 2 years following surgery only for a few patients. 6 There are similarly few non-randomised studies that have reported on outcomes of diabetic patients more than 2 years after bariatric surgery. 140 The largest and longest-running study, the SOS study, reported rates of remission of 38% at 10 years’ and 30% at 15 years’ follow-up, with fewer microvascular and macrovascular complications of diabetes in patients receiving surgery. 141 The majority of participants in the SOS cohort received the vertical band gastroplasty procedure, which is no longer widely utilised. More recently, Brethauer et al. 142 reported a long-term complete remission rate of 24% in 217 patients undergoing bariatric surgery; Adams et al. 14 reported remission rates of 62% in 418 participants following GBP, while Sultan et al. 15 reported remission in 40% of 102 participants up to 5 years following gastric banding. Data remain especially limited for SG, the most recently introduced procedure. Few studies have provided direct comparisons of the outcomes of different bariatric surgical procedures. 140 Most reported studies have been carried out in selected patients at specialist centres and there are few pragmatic studies of the outcomes of patients treated in usual clinical practice.
The present analysis utilised primary care EHRs from a large database of UK family practices. This data resource enabled us to conduct a population-based study of adult patients receiving currently used bariatric surgical procedures, including adjustable gastric banding, GBP and SG, with comparison with matched obese subjects who did not undergo surgery. In a previous report,7 we evaluated the effect of bariatric surgery in non-diabetic subjects. In this study, we aimed to compare the effect of the three different bariatric surgical procedures, gastric banding, GBP and SG, on diabetes remission and utilisation of antidiabetes medications over a maximum of 6 years of follow-up.
Results
There were 4793 obese participants with bariatric surgery recorded; 1324 were excluded as prevalent cases because the index code was within 12 months of the start of the record, 14 were excluded with age < 20 years, 401 were excluded because their BMI was < 30 kg/m2 or no values were recorded before surgery, and 2176 were excluded as non-diabetic, leaving 878 obese participants with T2DM of whom 52 were excluded with gestational diabetes ever recorded. There were then 826 obese participants with T2DM diagnosed before surgery who were matched with 826 obese diabetic controls who did not receive surgery.
Bariatric surgery participants had procedures performed between 2002 and 2014. In the years up to and including 2007, procedures numbered 77. Procedures increased over time reaching a maximum of 160 in 2012. The mean duration of follow-up was more than 6 years for participants receiving procedures in 2007 or before. Participants were registered at 360 general practices, of which 92% continued their participation in CPRD until 2013 or later; there were 69 participants that ended their registration with a CPRD practice before 2013. There were 20 bariatric surgery participants and 33 controls who died during the period of follow-up, including nine bariatric surgery participants and six controls who died within 12 months of the index date. Registered causes of death were not available for analysis.
Baseline characteristics of the bariatric surgery participants and controls are shown in Table 24. Bariatric surgery participants and controls were generally similar with respect to age, gender and index year but baseline BMI was higher in the bariatric surgery participants. Bariatric surgery participants also had longer duration of diabetes, with a median duration of 5.5 years since diagnosis, compared with 3.1 years for controls. Bariatric surgery participants were more likely to be treated with statins and antihypertensive drugs, with lower blood pressure and cholesterol values, and were less likely to be current smokers. There were 672 (81%) bariatric surgery participants, and 240 (29%) controls, who received antiobesity drugs, including sibutramine or orlistat, before the index date and 154 (19%) BS participants and 76 (9%) controls who received antiobesity drugs after the index date, indicating that not all antiobesity drugs were stopped immediately after surgery.
| Characteristics | Control participants | BS participants | p-valuea | LAGB | GBP | SG | p-valueb |
|---|---|---|---|---|---|---|---|
| Number | 826 | 826 | 220 | 449 | 153 | ||
| Female | 524 (63) | 542 (66) | 0.390 | 165 (75) | 288 (64) | 87 (57) | 0.001 |
| Age (years), mean (SD) | 49.1 (13.8) | 50.0 (9.6) | 0.118 | 49.0 (9.8) | 50.1 (9.4) | 51.3 (10.1) | 0137 |
| BMI (kg/m2) | 44.2 (6.5) | 46.7 (8.3) | < 0.001 | 44.8 (8.0) | 46.8 (7.8) | 49.3 (9.7) | < 0.001 |
| Index year, median (IQR) | 2011 (2010 to 2012) | 2011 (2009 to 2012) | 0.495 | 2009 (2007 to 2010) | 2011 (2010 to 2012) | 2011 (2010 to 2012) | < 0.001 |
| Diabetes duration (years), median (IQR) | 3.1 (0.3–7.1) | 5.5 (2.4–9.3) | < 0.001 | 5.2 (2.6–8.4) | 6.4 (2.5–10.2) | 4.0 (2.1–7.6) | 0.003 |
| Mean HbA1c (mmol/mol) | 8.3 (2.1) | 8.0 (2.0) | 8.1 (1.9) | 8.1 (2.0) | 7.7 (2.2) | 0.204 | |
| Antidiabetes treatment | |||||||
| Insulin | 147 (18) | 201 (24) | 0.074 | 48 (22) | 127 (28) | 26 (17) | 0.012 |
| Sulphonylureas | 164 (20) | 182 (22) | 0.634 | 49 (22) | 101 (22) | 32 (21) | 0.126 |
| Metformin | 476 (58) | 588 (71) | 0.008 | 145 (66) | 332 (74) | 110 (72) | 0.038 |
| Other antidiabetes drug | 157 (19) | 250 (30) | < 0.001 | 41 (19) | 156 (35) | 52 (34) | < 0.001 |
| None | 261 (32) | 161 (19) | < 0.001 | 53 (24) | 70 (15) | 35 (23) | 0.014 |
| Antihypertensive therapy | 517 (63) | 632 (77) | < 0.001 | 169 (77) | 347 (77) | 114 (75) | 0.781 |
| Blood pressure > 140/90 mmHg | 287 (35) | 247 (30) | 0.027 | 72 (33) | 127 (28) | 48 (31) | 0.445 |
| Statin therapy | 451 (55) | 579 (70) | < 0.001 | 136 (62) | 336 (75) | 105 (69) | 0.002 |
| Total cholesterol > 5 mmol/l | 257 (31) | 217 (26) | < 0.001 | 69 (31) | 107 (24) | 39 (25) | 0.031 |
| Current smoking | 189 (23) | 117 (14) | < 0.001 | 30 (14) | 69 (15) | 17 (11) | 0.487 |
There were 220 (27%) bariatric surgery patients who received LAGB, 449 (54%) who received GBP procedures and 153 (19%) who received SG. Type of procedure was undefined for four participants with more than one operation type coded on the index date. LAGB procedures were used in clinical practice at an earlier time having a median index year of 2009, compared with 2011 for GBP or SG.
Table 25 shows data for the number of participants contributing person time to the analysis, from 3 years before to 6 years after the bariatric surgery procedure. The proportion of participants contributing person-time in each postoperative year declined rapidly because > 50% of procedures were within the last 4 years and only 13% of participants contributed person-time in the sixth year of follow-up. Participants receiving LAGB contributed a higher proportion of person-time at long-term follow-up. The proportion of participants with HbA1c values recorded generally ranged between 60% and 80% (see Table 25). At the time of surgery, slightly more bariatric surgery patients had HbA1c values recorded, but at longer durations of follow-up HbA1c recording was more complete in controls than in bariatric surgery patients.
| Measure | Years from procedure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| –3 to –2 | –2 to –1 | –1 to 0 | 0 to 1 | 1 to 2 | 2 to 3 | 3 to 4 | 4 to 5 | 5 to 6 | |
| Controls | |||||||||
| Participants contributing PT | 399 | 567 | 826 | 826 | 733 | 464 | 272 | 161 | 101 |
| HbA1c recorded | 278 (70) | 388 (68) | 626 (76) | 612 (74) | 536 (73) | 309 (67) | 185 (68) | 113 (70) | 81 (80) |
| In diabetes remission | 20 (5) | 29 (5) | 32 (4) | 34 (4) | 27 (4) | 27 (4) | 9 (3) | 8 (5) | 6 (6) |
| All BS cases | |||||||||
| Participants contributing | 692 | 752 | 826 | 826 | 674 | 499 | 336 | 212 | 109 |
| HbA1c recorded | 506 (80) | 607 (81) | 698 (85) | 663 (80) | 499 (74) | 321 (64) | 321 (64) | 125 (59) | 69 (63) |
| In diabetes remission | 26 (4) | 32 (4) | 39 (5) | 177 (21) | 204 (30) | 124 (25) | 71 (21) | 44 (21) | 18 (17) |
| RR (95% CI)a | Reference | Reference | Reference | 4.66 (3.80 to 5.73) | 7.16 (5.79 to 8.86) | 6.68 (5.21 to 8.56) | 6.17 (4.60 to 8.28) | 7.05 (5.03 to 9.88) | 5.90 (3.72 to 9.34) |
| LAGB | |||||||||
| Participants contributing PT | 175 | 197 | 220 | 220 | 201 | 177 | 143 | 115 | 81 |
| In diabetes remission | 7 (4) | 9 (5) | 7 (3) | 15 (7) | 40 (20) | 32 (18) | 28 (20) | 21 (18) | 11 (14) |
| RR (95% CI)a | Reference | Reference | Reference | 1.40 (0.80 to 2.44) | 4.16 (2.84 to 6.11) | 4.01 (2.56 to 6.29) | 4.34 (2.73 to 6.89) | 4.06 (2.44 to 6.75) | 3.19 (1.70 to 5.97) |
| GBP | |||||||||
| Participants contributing | 344 | 413 | 449 | 449 | 361 | 249 | 155 | 81 | 17 |
| In diabetes remission | 13 (4) | 14 (3) | 19 (4) | 112 (25) | 122 (34) | 70 (28) | 33 (21) | 18 (22) | 3 (18) |
| RR (95% CI)a | Reference | Reference | Reference | 5.83 (4.55 to 7.48) | 8.55 (6.67 to 11.0) | 7.77 (5.82 to 10.4) | 6.27 (4.36 to 9.02) | 8.78 (5.68 to 13.6) | 11.7 (5.15 to 26.6) |
| SG | |||||||||
| Participants contributing | 107 | 138 | 153 | 153 | 109 | 70 | 36 | 14 | 10 |
| In diabetes remission | 6 (6) | 8 (6) | 13 (9) | 48 (31) | 41 (38) | 22 (31) | 9 (25) | 5 (36) | 4 (40) |
| RR (95% CI)a | Reference | Reference | Reference | 6.21 (4.59 to 8.41) | 6.21 (4.59 to 8.41) | 6.96 (4.65 to 10.4) | 6.15 (3.27 to 11.6) | 8.63 (4.07 to 18.3) | 8.63 (4.07 to 18.3) |
Trends in mean HbA1c and proportion with HbA1c < 48 mmol/mol (< 6.5%) are shown by year following surgery in Figure 9. The mean HbA1c value in the year before the index date was 64 mmol/mol (8.0%) in bariatric surgery cases and 67 mmol/mol (8.3%) in controls. The mean HbA1c value in bariatric surgery cases declined to 51 mmol/mol (6.8%), 48 mmol/mol (6.5%) and 51 mmol/mol (6.8%) in the first 3 years following surgery, but remained unchanged at 65 mmol/mol (8.1%), 66 mmol/mol (8.2%) and 66 mmol/mol (8.2%), respectively, in controls. The proportion of bariatric surgery cases with HbA1c values < 48 mmol/mol (< 6.5%) was 17% before operation, increasing to 44%, 47% and 39% in the first 3 postoperative years. No consistent trend in the proportion of controls with HbA1c < 48 mmol/mol (< 6.5%) was observed. Trends in the proportion of participants without antidiabetes drug prescriptions and the mean number of drug prescriptions per participant year are also shown in Figure 9. The proportion of bariatric surgery cases without antidiabetic drugs or insulin prescriptions increased from 15% before operation to 41%, 53% and 55% in the first 3 years after surgery, while the opposite trend was observed in controls.
FIGURE 9.
Changes by year before and after bariatric surgery. (a) Mean (standard error) HbA1c; (b) mean (standard error) diabetes prescriptions per year; (c) per cent with HbA1c < 6.5%; and (d) percentage not prescribed insulin or oral hypoglycaemic drugs. BS, bariatric surgery. Green symbols, bariatric surgery participants; black symbols, control participants.
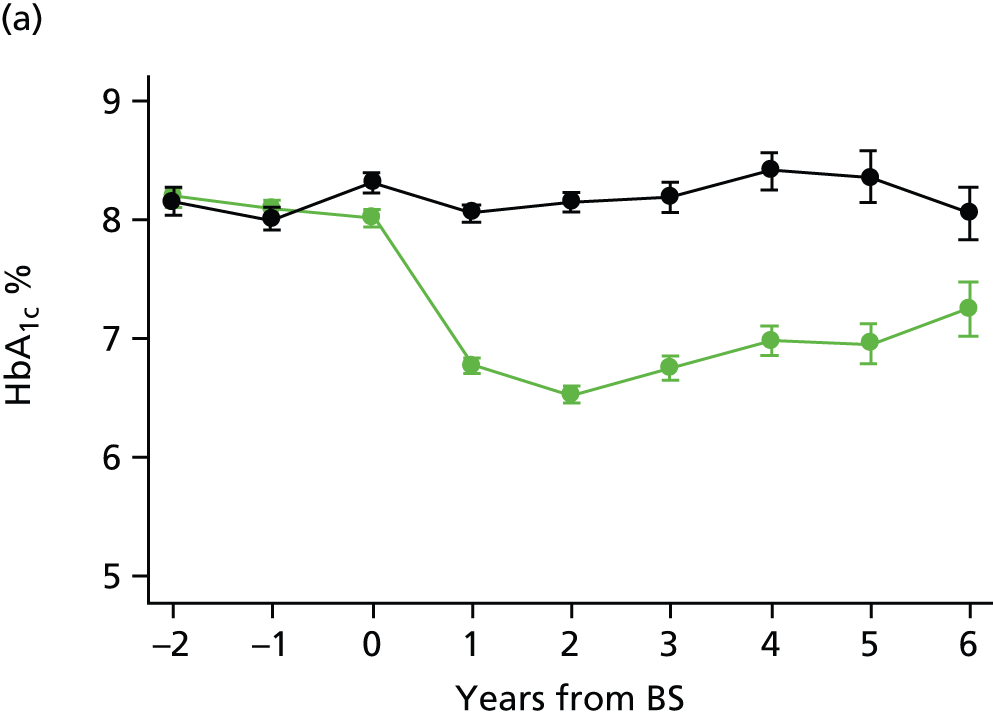

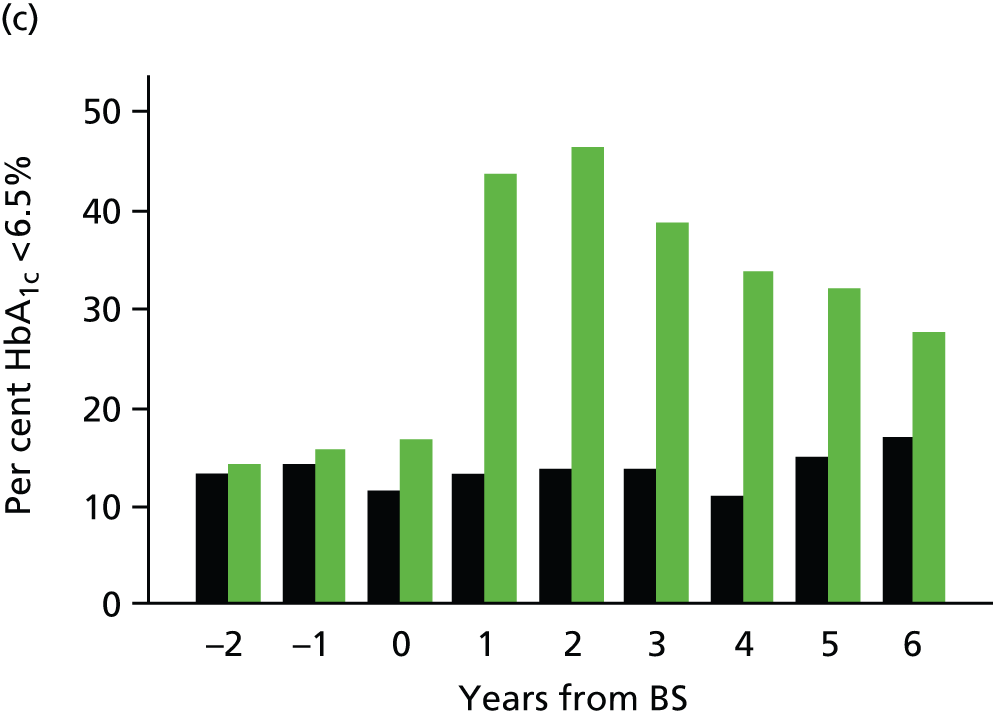
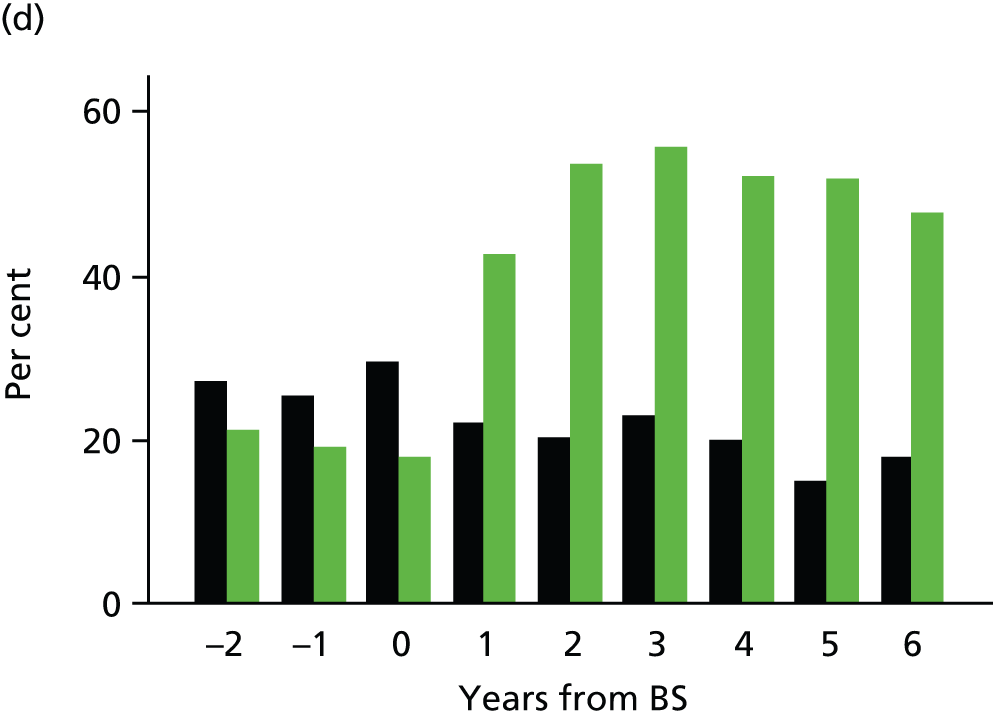
The proportion of bariatric surgery cases in remission was 5% before operation, increasing to 21%, 30% and 25% in the first 3 years after surgery (see Table 25), while the proportion of controls in remission tended to remain constant over time. In the first year after surgery, the proportion of participants in remission was lower for LAGB patients (7%, 95% CI 4% to 11%) than for patients receiving either GBP (25%, 95% CI 21% to 29%) or SG (31%, 95% CI 24% to 39%). HbA1c values were not recorded for every participant in every year. When remission was evaluated only including participants with HbA1c values recorded in a given year, then the proportion of participants in remission for the first and subsequent years following the procedure was 27%, 41%, 39%, 33%, 35% and 26%; the equivalent figures for controls were 6%, 5%, 7%, 5%, 7% and 7%. The proportion of participants either with HbA1c < 6.5% or not taking medications was higher than the proportion of participants in remission, which required both criteria to be met. Of the 744 person-years in remission among bariatric surgery cases, 175 (24%) were not in complete remission (HbA1c < 6.0%). The proportion of bariatric surgery participants in complete remission in the second year after the procedure was 26%.
Multivariable adjusted analyses were conducted using participant years as observations (see Table 25). The adjusted relative rate of diabetes remission across all types of bariatric surgery, relative to person time without surgery, was 4.66 (95% CI 3.80 to 5.73) in the first year after surgery increasing to 7.16 (95% CI 5.79 to 8.86) in the second year. By the sixth year of follow-up, the adjusted relative rate was 5.90 (95% CI 3.72 to 9.34). In participants receiving LAGB, compared with all controls, the adjusted relative rate of diabetes remission was not elevated in the first postoperative year but increased to 4.16 (95% CI 2.84 to 6.11) in the second postoperative year. The rate of remission remained elevated for the sixth year of follow-up (3.19, 95% CI 1.70 to 5.97). For either GBP or SG, the rate of diabetes remission was increased in the first year following the procedure (GBP 5.83, 95% CI 4.55 to 7.48; SG 6.21, 95% CI 4.59 to 8.41) and remained elevated until the end of the sixth year of follow-up (GBP 11.7, 95% CI 5.15 to 26.6; SG 8.53, 95% CI 3.75 to 19.4). For these procedures, adjusted rate ratios were higher than for LAGB, though CIs overlapped. There were small numbers of observations for the later years of follow-up for GBP and SG. Associations of confounders with remission were generally of small magnitude except for a graded association of duration of diabetes, with the highest quartile of diabetes duration (diabetes diagnosed more than 8.6 years before surgery) being associated with reduced relative risk of remission of 0.23 (95% CI 0.16 to 0.33; p < 0.001).
Table 26 presents adjusted rate ratios for diabetes remission for combining the 6 years of follow-up. The adjusted relative rate of diabetes remission after bariatric surgery compared with controls was 5.97 (95% CI 4.86 to 7.33; p < 0.001). The three types of bariatric surgery each resulted in a significantly higher rates of diabetes remission compared with controls (all p < 0.001). However, the rate of remission was lower after LAGB 3.32 (95% CI 2.27 to 4.86) than after GBP (7.16, 95% CI 5.64 to 9.08) or SG (6.82, 95% CI 5.05 to 9.19). Among participants who received bariatric surgical procedures, compared with LAGB as reference, either GBP (1.94, 95% CI 1.42 to 2.65; p < 0.001) or SG (2.00, 95% CI 1.40 to 2.86; p < 0.001) was associated with higher rates of remission than LAGB. SG was associated with similar rates of remission as GBP as reference (1.03, 95% CI 0.82 to 1.30; p = 0.781). The relative rate of diabetes remission was slightly higher in men than in women. The relative rate of diabetes remission increased with increasing BMI category, being 6.74 (95% CI 5.29 to 8.58) at BMI ≥ 40 kg/m2 but 4.23 (95% CI 1.98 to 9.03) at BMI ≥ 30 kg/m2. The relative rate of diabetes remission was highest in participants aged 35–54 years.
| Variable | Adjusteda rate ratio (95% CI) | p-value |
|---|---|---|
| All BS procedures | 5.97 (4.86 to 7.33) | < 0.001 |
| Type of procedureb | ||
| Laparoscopic gastric banding | 3.32 (2.27 to 4.86) | < 0.001 |
| GBP | 7.16 (5.64 to 9.08) | < 0.001 |
| SG | 6.82 (5.05 to 9.19) | < 0.001 |
| Sex | ||
| Men | 7.70 (5.26 to 11.3) | < 0.001 |
| Women | 5.32 (4.16 to 6.81) | < 0.001 |
| Baseline BMI category (kg/m2) | ||
| 30–34.9 | 4.23 (1.98 to 9.03) | < 0.001 |
| 35–39.9 | 3.59 (2.16 to 5.97) | < 0.001 |
| ≥ 40 | 6.74 (5.29 to 8.58) | < 0.001 |
| Age group (years) | ||
| 20–34 | 5.38 (2.81 to 10.3) | < 0.001 |
| 35–54 | 6.69 (5.05 to 8.86) | < 0.001 |
| ≥ 55 | 5.52 (3.91 to 7.80) | < 0.001 |
There were 204 bariatric surgery participants in remission in the second postoperative year. Of those remaining under follow-up, 41%, 48%, 46% and 52% had a HbA1c value ≥ 48 mmol/mol (≥ 6.5%) recorded in the third to sixth postoperative years, respectively. However, additional patients were recorded in remission in these later years.
Discussion
Summary of findings
In a population of obese patients with T2DM undergoing bariatric surgery there is evidence of improved blood glucose control, reduced requirement for antidiabetic medicines and an increased proportion of participants in diabetes remission for up to 6 years following the procedure. These benefits appear to be greatest among those with class III (morbid) obesity but procedure type was associated with BMI and this might have introduced confounding by indication. Participants receiving either GBP or SG procedures, rather than gastric banding, showed earlier onset and higher rates of diabetes remission. The SG and GBP groups differed at baseline, with low numbers beyond 3 years for SG, making it difficult to draw conclusions with respect to the comparative effectiveness of these procedures.
Bariatric surgery was associated with a fourfold increase in diabetes remission compared with no bariatric surgery. The proportion of patients in remission declined over time following surgery and there was biochemical evidence that individual patients showed relapse of diabetes after a period of remission. About one-third of all participants who received bariatric surgery were diabetic, indicating positive selection of diabetes patients for this intervention, consistent with current policy recommendations that bariatric surgery should be utilised in the management of obesity-associated comorbidity.
Comparison with other results
Changes in mean HbA1c in our study were similar to those reported in previous studies,72 but rates of diabetes remission after surgery reported in the present study were generally lower than those noted in published reviews, which ranged from 59% to 92%. 6,32,70 However, case definitions have varied. We used a stringent definition of remission that required a normal HbA1c value to be recorded in each year. Patient-years with missing values for HbA1c in the present sample could have led to underestimation of remission rates. When remission rates were re-estimated, including only participants with HbA1c values recorded in year, the proportion of participants in remission was higher. However, the pattern of missing HbA1c values may be related to diabetes control, possibly with less frequent recording in participants who are either in remission or in good control. In clinical practice, some patients may be continued on metformin therapy even in the setting of apparent biochemical remission but such participants were not classified as being in remission for this study. Underestimation of remission may also occur if patients receive repeat prescriptions for medicines they no longer require. Conversely, relapse after previous remission may not be detected if blood glucose control is not evaluated. Even allowing for these limitations, this pragmatic study raises questions concerning whether or not the results achieved in routine practice may be as favourable as those reported from research studies. Control participants generally had poorly controlled diabetes, as did bariatric surgery patients before the procedure, raising questions concerning the quality of diabetes care received. We do not have information on whether the primary purpose of surgery was weight reduction or improved diabetic control. We caution against concluding that bariatric surgery is the only method for improving blood glucose control in obese patients.
Previous studies suggest that GBP is more effective for the treatment of diabetes than LAGB. 42,74,142 Fewer data have been reported for SG and data with long follow-up are not available. A randomised trial of 60 participants suggested that GBP might be more effective than SG. 143 A recent randomised trial found biliopancreatic diversion may have greater success than GBP, but was also associated with more nutritional side effects and lower quality of life. 144 Our results suggest that both SG and GBP may be more effective than LAGB and do not support the hypothesis that duodenal exclusion is important. We caution that in a non-randomised study procedures may be used selectively for patients with different baseline risks, and are more susceptible to bias than results from randomised trials. Higher remission rates with greater weight loss have also been reported. 74 Although we did not investigate weight loss as a predictor of diabetes remission due to limited follow-up, a higher baseline BMI category was positively associated with remission and greater baseline BMI is known to be associated with greater weight loss. 6 Our results offer some suggestion that the effect of bariatric surgery may decline over time. However, LAGB comprised a higher proportion of procedures at long follow-up. There is a clear need for longer-term follow-up studies to evaluate the long-term diabetes outcomes of bariatric surgery.
The safety of bariatric surgery also requires careful consideration. The report of the UK National Bariatric Surgery Register11 estimated that postoperative mortality in hospital following bariatric surgery was approximately 0.07%, with rates of complications, including anastomotic leakages, of approximately 2.9% following first procedures. In the longer term, band removal may be required following adjustable gastric banding, and there are risks of intestinal herniation and strangulation, which may have serious consequences. These risks require better quantification.
Strengths and limitations
This analysis was based on primary care EHRs of a large population-based sample of patients, enabling comparison of the benefits of bariatric surgery over standard medical care for the treatment of diabetes. However, we acknowledge several limitations. As the analysis was based on primary care data, there was limited information concerning surgical details including whether operations were open or laparoscopic or whether or not there were surgical complications, and assessment of the safety of bariatric surgery was beyond the scope of this research. The problem of missing and inconsistently recorded data has been referred to above. Weight records in primary care were insufficiently recorded to relate study outcomes to weight loss. Preoperative BMI might have been misclassified through infrequent recording.
Control participants were matched with bariatric surgery patients on BMI, age, sex and index year, but there remained differences between groups with respect to important confounders including length of diagnosis of diabetes, smoking history and hypertension. Analyses were adjusted for these confounders in a multiple regression model. An alternative approach might have employed propensity score matching, but results obtained using this technique are generally similar to those obtained with the regression adjustment approach adopted here. 145 Multiple controls per case could have been sampled but there was no evidence of lack of statistical power. We acknowledge that diabetes care was not standardised and quality of diabetes care might have differed between bariatric surgery patients and controls. The control patients may have had a different length of diagnosis or may have suffered different levels of complications from the surgery group. Given the highly selective access to bariatric surgery in the UK we would anticipate that surgery patients would have diabetes for longer and possibly experience more complications than controls. It is possible that surgery patients had better access to specialist diabetes care in the run-up to their surgery. In this pragmatic study, we used HbA1c values to evaluate diabetes remission, but we acknowledge that greater sensitivity for diabetes might have been achieved through systematic use of glucose tolerance tests. 146
Cases and controls were matched but there were residual differences for some variables, including BMI. This may be expected as patients who receive surgery differ from those who do not receive surgery in many ways. Analyses were additionally adjusted for BMI, as well as other confounders. Nevertheless, residual confounding might bias the results. The median year of procedure was 2011 and only a minority of participants had operations more than 5 years before the study close in April 2014. We also acknowledge that the available duration of follow-up was generally shorter for more recently adopted procedures, including GBP and SG, than for LAGB. The analysis was on an ‘intention-to-treat’ basis and although small numbers of participants might have had conversion, revisional or reversal procedures, these were not excluded. A randomised trial remains the preferred design for evaluating the outcome of these procedures and results from a non-randomised comparison must be treated with caution, as noted above.
Conclusions
The findings of this analysis suggest that bariatric surgery may facilitate diabetes control in obese patients treated in routine primary care settings. The three most commonly used surgical techniques were associated with increased rates of remission, improved blood glucose control and reduced use of antidiabetes medications for T2DM over a maximum of 6 years’ follow-up period. SG and GBP were generally associated with more favourable diabetes outcomes than LAGB. Rates of remission tended to decline over time and there was biochemical evidence of relapse in some participants. These results, from a pragmatic non-randomised evaluation, add to current evidence, which is composed largely of data from specialist centres. Further investigation is required to evaluate the effect of bariatric surgery on longer-term clinical and patient outcomes, including the incidence of complications and mortality.
Chapter 9 Impact of bariatric surgery on clinical depression
Introduction
In Chapter 5, we identified depression as a major driver of health-care costs in obese patients. This analysis uses EHRs to investigate the possible impact of bariatric surgery on clinical depression. Obese people are at higher risk of depression but depression is also a predictor of weight gain and future obesity. 147 People who are obese experience the onset of morbidity at younger ages than those with lower body weight3 and the multiple morbidities associated with obesity may contribute to a higher prevalence of depression. 51 Patients selected for bariatric surgery often have a high prevalence of clinical depression. 18,148 However, evidence for an effect of bariatric surgery on depression is limited. Several longitudinal studies have explored the relationship between bariatric surgery and depression, identifying significant reductions in depression149 and depressive symptoms150,151 following surgery. One study found a decrease in depression from 32.7% at baseline, to 16.5% at 6–12 months, and 14.3% at 2–3 years following surgery. 152 However, other studies suggest that improvements following surgery may not be maintained after the first postoperative year151 and depressive symptoms may deteriorate in some patients. 153 Previous reports have often drawn on data from hospital-based series that did not include control groups, often with short durations of follow-up.
This analysis aimed to evaluate whether or not bariatric surgery is associated with a reduction in clinical depression up to a maximum of 7 years following the procedure. A population-based cohort provided the data source for an interrupted time-series design with matched controls, facilitating a pragmatic evaluation of the impact of bariatric surgery on clinical depression recorded in primary care EHRs. The results in this chapter were published open access in the Journal of Affective Disorders in 201590 under the terms of the Creative Commons Licence CC BY-NC-ND 4.0.
Results
There were 4793 participants with bariatric surgery recorded; 1324 participants with bariatric surgery first recorded < 1 year after the start of the patient record were excluded, as were 14 participants aged < 20 years at the index date, and 401 participants with either no BMI record before surgery or BMI values < 30 kg/m2 prior to surgery. Nine participants with a record of gastric band removal before the index date were also excluded. There were then 3045 participants identified as having bariatric surgery for obesity and 3045 matched controls. Bariatric surgery procedures included LAGB in 1297 (43%), GBP in 1265 (42%), SG in 477 (16%) and six of undefined type. Utilisation of bariatric surgery increased over the period and LAGB accounted for 97% of 104 procedures before 2006, but only 20% of 924 procedures from 2012 onwards, with increasing use of GBP and SG. The median year of procedure was 2010 and, consequently, only a minority of participants contributed more than 3 years of follow-up data.
Characteristics of the surgery and control participants at the index date are presented in Table 27. The majority of surgical procedures were conducted in women (79%) and in participants with morbid obesity (65%). The mean age at surgery was 45.9 years. Participants undergoing bariatric surgery more frequently had T2DM (29% vs. 14%; p < 0.001), hypercholesterolemia (35% vs. 25%; p = 0.022) and were more likely than controls to be prescribed antihypertensive drugs and statins.
| Variable | BS cases | Controls | p-value |
|---|---|---|---|
| Frequency | 3045 | 3045 | |
| Female (%) | 2406 (79) | 2521 (83) | Matched |
| Age (years), mean (SD) | 45.9 (10.2) | 44.3 (14.8) | Matched |
| BMI (kg/m2), mean (SD) | 44.0 (8.3) | 43.5 (7.6) | Matched |
| BMI category (kg/m2) | |||
| 30.0 to 34.9 | 394 (13) | 351 (12) | Matched |
| 35.0 to 39.9 | 677 (22) | 681 (22) | |
| ≥ 40.0 | 1974 (65) | 2013 (66) | |
| Comorbidity | |||
| Diabetes | 878 (29) | 424 (14) | < 0.001 |
| CHD | 134 (4) | 102 (3) | 0.034 |
| Stroke | 36 (1) | 38 (1) | 0.815 |
| Current smoking | 502 (16) | 675 (22) | < 0.001 |
| Blood pressure ≥ 140/90 mmHg | 834 (27) | 900 (30) | 0.001 |
| Cholesterol ≥ 5 mmol/l | 1080 (35) | 760 (25) | 0.022 |
| Antihypertensive drugs prescribed | 1561 (51) | 1074 (35) | < 0.001 |
| Statins prescribed | 864 (28) | 486 (16) | < 0.001 |
| Index year (median IQR) | 2010 (2009 to 2012) | 2010 (2009 to 2012) | Matched |
Table 28 shows the number of participants analysed by year before and after surgery. There were 63% contributing to follow-up after the end of 2 years and 31% in the fifth year of follow-up. In the year prior to surgery, 36% of surgery participants met the criteria for prevalent clinical depression in comparison with 21% of control participants (Figure 10 and see Table 28). In the 2 years following surgery, this reduced to 32% in the participants who underwent surgery before rising to pre-surgery levels (37%) in the seventh year of follow-up. Rates of depression in control participants remained stable. In the surgery group, 41% of participants were prescribed antidepressants in the year leading up to surgery, falling to 36% in the subsequent year. The proportion of participants prescribed antidepressants began to rise again after the first year and surpassed pre-surgery levels in the fifth year following bariatric surgery.
| Year | Participants contributing person-time in year | Antidepressants prescribed in year (row %) | Depression recorded in year (row %) | Meeting criteria of clinical depression in year (row %) | ||||
|---|---|---|---|---|---|---|---|---|
| BS cases | Controls | BS cases | Controls | BS cases | Controls | BS cases | Controls | |
| –3 to –2 | 2856 | 2560 | 38 | 19 | 12 | 6 | 34 | 16 |
| –2 to –1 | 3045 | 3045 | 40 | 22 | 12 | 7 | 34 | 19 |
| –1 to 0 | 3045 | 3045 | 41 | 25 | 9 | 7 | 36 | 21 |
| 0 to 1 | 3045 | 3045 | 36 | 25 | 6 | 6 | 32 | 21 |
| 1 to 2 | 2488 | 2786 | 37 | 24 | 7 | 4 | 32 | 20 |
| 2 to 3 | 1916 | 2014 | 38 | 24 | 7 | 5 | 33 | 20 |
| 3 to 4 | 1392 | 1369 | 40 | 23 | 6 | 4 | 34 | 19 |
| 4 to 5 | 935 | 914 | 43 | 23 | 6 | 4 | 34 | 18 |
| 5 to 6 | 574 | 570 | 44 | 24 | 7 | 4 | 37 | 19 |
| 6 to 7 | 335 | 369 | 43 | 26 | 6 | 2 | 37 | 20 |
FIGURE 10.
Prevalence of clinical depression for bariatric surgery cases (black) and controls (green) for 3 years before and 7 years after index date.
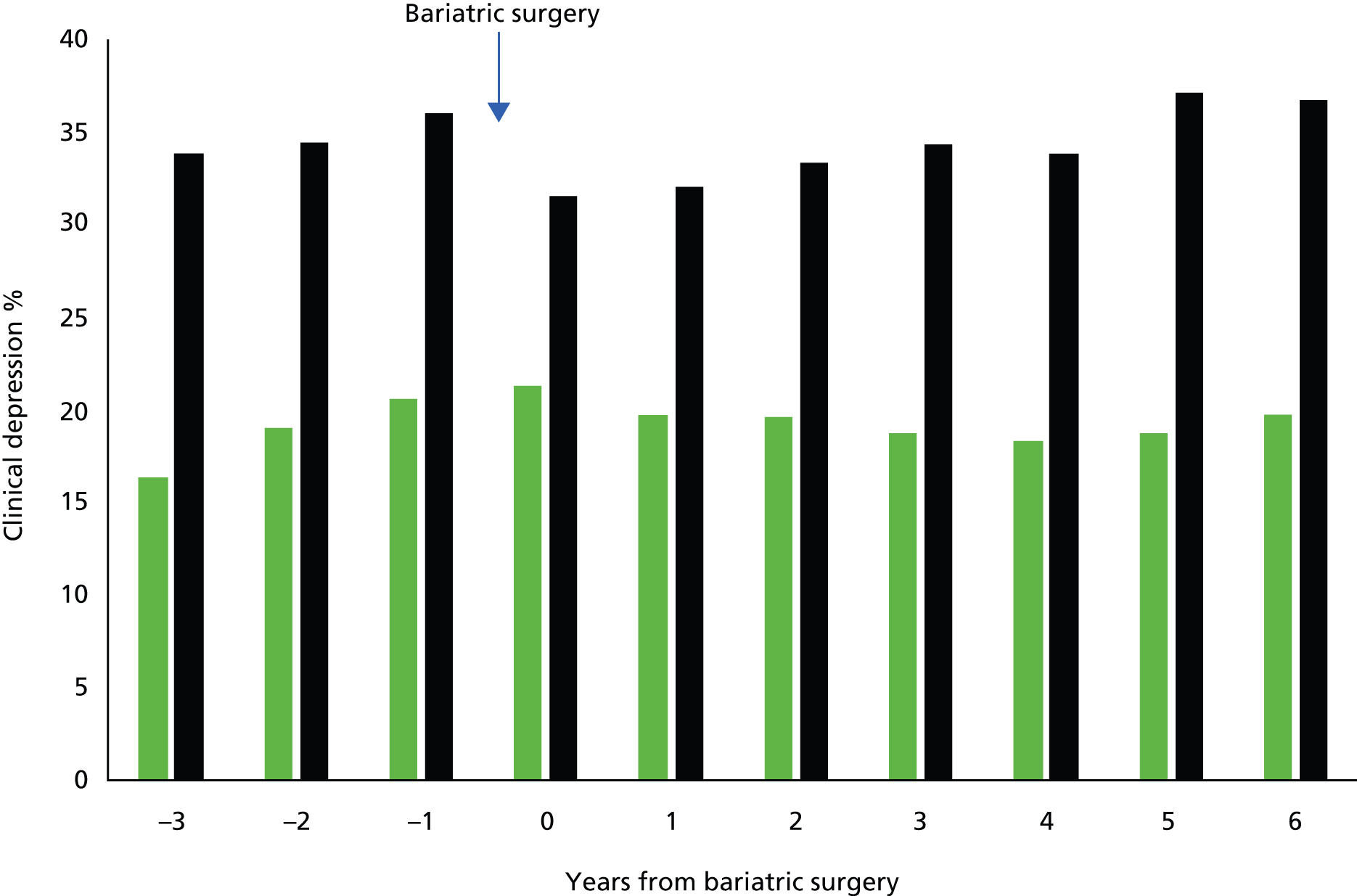
Table 29 presents the results of the multiple logistic regression model for the outcomes of clinical depression and antidepressant prescribing. Compared with control participants, the between-group effect shows that bariatric surgery participants were more likely to be diagnosed with clinical depression (OR 2.02, 95% CI 1.75 to 2.33; p < 0.001) or to be prescribed antidepressant drugs (OR 1.97, 95% CI 1.72 to 2.25; p < 0.001). There was evidence of increasing trends in diagnosis of depression and prescription of antidepressant drugs over the study period. Estimation of the effect of time since surgery, in comparison with all person-time without surgery from both groups, revealed a reduction in clinical depression and antidepressant prescribing in the first 3 years following the procedure. The adjusted relative odds of clinical depression were 0.82 (95% CI 0.78 to 0.87; p < 0.001) and 0.83 (95% CI 0.76 to 0.90; p < 0.001) in the first 2 years following the procedure. Similar changes were observed for the related outcome of antidepressant prescribing. However, from the fourth postoperative year onwards there was no longer any evidence for a reduction in clinical depression or antidepressant prescribing.
| Measure | Clinical depression, OR (95% CI) | p-value | Antidepressant prescribing, OR (95% CI) | p-value |
|---|---|---|---|---|
| Effect of groupa | ||||
| Control group | Reference | Reference | ||
| Bariatric surgery group | 2.02 (1.75 to 2.33) | < 0.001 | 1.97 (1.72 to 2.25) | < 0.001 |
| Effect of study yearb | ||||
| Year | 1.02 (1.01 to 1.04) | 0.007 | 1.04 (1.03 to 1.06) | < 0.001 |
| Effect of time since surgeryc | ||||
| Time without surgery | Reference | Reference | ||
| First postoperative year | 0.82 (0.78 to 0.87) | < 0.001 | 0.79 (0.74 to 0.84) | < 0.001 |
| Second postoperative year | 0.83 (0.76 to 0.90) | < 0.001 | 0.79 (0.72 to 0.86) | < 0.001 |
| Third postoperative year | 0.87 (0.78 to 0.97) | 0.014 | 0.82 (0.73 to 0.91) | < 0.001 |
| Fourth postoperative year | 0.89 (0.78 to 1.02) | 0.100 | 0.87 (0.76 to 0.99) | 0.039 |
| Fifth postoperative year | 0.87 (0.74 to 1.04) | 0.123 | 0.87 (0.74 to 1.03) | 0.107 |
| Sixth postoperative year | 1.01 (0.82 to 1.25) | 0.910 | 0.99 (0.80 to 1.22) | 0.912 |
| Seventh postoperative year | 0.99 (0.76 to 1.29) | 0.959 | 1.04 (0.81 to 1.35) | 0.747 |
There was no evidence that the effect of bariatric surgery varied by type of procedure (test for interaction, p = 0.2885). Table 30 presents the prevalence of depression for each of the three procedures included in the study, after omitting six with undefined procedure type. There were more participants with LAGB at long durations of follow-up while fewer than 25% of participants receiving SG, and 35% receiving GBP, contributed data after the end of 3 years’ follow-up because these procedures were utilised more recently. The effect in each subgroup was generally similar to the one observed overall, and in the absence of an interaction effect, possible subgroup differences were not explored further.
| Year | LAGB | GBP | SG | |||
|---|---|---|---|---|---|---|
| n | Depression (%) | n | Depression (%) | n | Depression (%) | |
| –3 to –2 | 1207 | 32 | 1195 | 36 | 448 | 34 |
| –2 to –1 | 1297 | 33 | 1265 | 35 | 477 | 35 |
| –1 to 0 | 1297 | 37 | 1265 | 36 | 477 | 33 |
| 0 to 1 | 1297 | 31 | 1265 | 33 | 477 | 31 |
| 1 to 2 | 1160 | 32 | 980 | 32 | 344 | 29 |
| 2 to 3 | 1003 | 35 | 682 | 33 | 227 | 30 |
| 3 to 4 | 826 | 34 | 444 | 36 | 119 | 27 |
| 4 to 5 | 645 | 35 | 229 | 32 | 59 | 27 |
| 5 to 6 | 478 | 38 | 65 | 37 | 30 | 27 |
| 6 to 7 | 300 | 37 | 18 | 33 | 17 | 29 |
There was no evidence that the effect of bariatric surgery on clinical depression varied by type of procedure (test for interaction, p = 0.2885).
Table 31 shows the results divided by depression status in the preoperative year. Among participants who were not depressed in the preoperative year, the prevalence of depression increased to 18% in the sixth postoperative year, while up to 9% were depressed 2 years before the procedure. Among participants who were depressed in the preoperative year, the prevalence of depression was generally close to 75% postoperatively. However, in the second preoperative year, 77% were depressed. These results are consistent with depression being episodic and frequent in this population.
| Year | Depressed in preoperative year | Not depressed in preoperative year | ||
|---|---|---|---|---|
| n | Depression (%) | n | Depression (%) | |
| –3 to –2 | 1036 | 77 | 1820 | 9 |
| –2 to –1 | 1097 | 84 | 1948 | 6 |
| –1 to 0 | 1097 | 100 | 1948 | 0 |
| 0 to 1 | 1097 | 79 | 1948 | 5 |
| 1 to 2 | 889 | 74 | 1599 | 8 |
| 2 to 3 | 664 | 74 | 1252 | 12 |
| 3 to 4 | 479 | 75 | 913 | 13 |
| 4 to 5 | 320 | 73 | 615 | 14 |
| 5 to 6 | 203 | 73 | 371 | 18 |
| 6 to 7 | 113 | 77 | 222 | 16 |
Discussion
Summary of findings
Patients undergoing bariatric surgery have higher levels of depression than other obese patients with similar BMI and of the same age and sex. Frequent comorbidities, including diabetes mellitus, might be associated with this increased frequency of depression. The results of this analysis indicate that bariatric surgery in obese patients may be associated with a modest reduction in the prevalence of depression, and the use of antidepressant medications in primary care, but these effects do not appear to persist more than 3 years following the procedure. The reasons why patients with depressive illness are disproportionately represented among patients undergoing bariatric surgery in this population are unclear. In the UK, only a very small minority of patients with severe obesity undergo bariatric surgery and it is possible that psychological symptoms may be one of the considerations that influence whether or not an obese patient receives surgery. Obese patients seeking treatment may generally have higher levels of psychological distress. In a study in Germany, Herpertz et al. 154 found that patients undergoing bariatric surgery had similar levels of depression to those receiving non-surgical weight-loss therapy, with both being higher than obese controls. Comparison of patients who were either depressed or not depressed in the preoperative year showed a decline in depression in the former and an increase in the latter after surgery. This is compatible with regression to the mean and is consistent with the episodic nature of depression symptoms. Our results do not provide strong evidence that patients should be prioritised in the hope that bariatric surgery will provide long-term relief of clinical features of depression, even though short-term effects might be judged clinically relevant.
Comparison with other results
Previous studies have generally shown larger effects than the present study but these generally used samples drawn from specialist centres that might be susceptible to bias. One of the largest studies was conducted by the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) group. The included 2148 participants from 10 US hospitals with Beck Depression Inventory recorded at baseline and at least 1 follow-up within 3 years of the procedure. There were 40% who reported treatment for depression before surgery, while 28% had mild depressive symptoms and 5% moderate or severe depressive symptoms. The proportion with any depressive symptoms was < 10% in the first year after surgery but increased to 16% at 3 years. A similar pattern of change in depressive symptoms over time was reported by Burgmer et al. ,155 in a 4-year study of 148 participants. However, changes in use of antidepressant medication in the LABS-2 study were modest, with 35% using antidepressant medication before operation and 28% in the second and third years following the procedure. These results emphasise the importance of long follow-up in future studies as well as the evaluation of different measures of depression. In the SOS study, a battery of health-related quality of life measures, including the Hospital Anxiety and Depression Scale, showed improvement followed by deterioration. 92 An initial improvement is associated with the period of greatest weight loss in the first 2 years following surgery, sometimes viewed as a ‘honeymoon period’,156 followed by a subsequent deterioration associated with weight regain. 92 However, in the SOS study there was evidence of some improvement in depression symptoms up to 10 years of follow-up. Premorbid depression was less frequent in the SOS study than in the present sample.
Strengths and limitations
This analysis had the strengths of a large, population-based sample of patients undergoing bariatric surgery with prospectively recorded data for depression diagnoses and antidepressant prescribing. The interrupted time-series design is generally considered to be more resistant to bias than other non-randomised designs. 157 However, there were clear differences between the intervention and matched comparison group with respect to the outcome of interest, indicating that individuals undergoing surgery represent a highly selected group that is not typical of all patients with the same BMI, age and sex. Patients undergoing surgery may receive a package of supportive care and improved clinical management to prepare them for surgery, which may confound the effect of the surgical procedure. Patients in the control group received the usual care offered at their general practice, which was unlikely to include standardised management of obesity or depression.
The types of surgical procedure and patient case mix changed over time and, though access remained restricted, the numbers of procedures increased. There were small numbers of patients with long follow-up with reduced statistical power for evaluation of later time points. Furthermore, there is a risk of bias because patients operated on longer ago may have different characteristics from those operated on more recently, with shorter periods of follow-up. Use of clinical data may also introduce bias because patients must consult with their physician, and have their symptoms recognised, before a depression diagnosis may be recorded. Some evidence shows rates of diagnosed clinical depression are lower than those found in epidemiological studies. 158 An important limitation is that there was very limited recording of body weight during the period of follow-up and we cannot determine whether changes in depression were associated with weight loss or regain.
Our assessment of depression was based on clinical diagnoses and antidepressant prescribing; the limited changes observed over time might result from difficulty in stopping antidepressant therapy once initiated. A previous CPRD study found that depression is often treated with short-term courses of antidepressant medications, with only a small proportion of patients being prescribed drugs over a long period for chronic depression. 157 The high rates of antidepressant use observed in this study may represent met need and not merely the result of repeat prescribing to patients who might no longer require clinical treatment for depression. Several previous studies used self-report measures to evaluate depressive symptoms and these measures might be associated with greater sensitivity for depression and responsiveness to change. 159,160 For all of the reasons, it would be desirable to test hypotheses using well-designed randomised trials with prospective documentation of depression.
Conclusions
The results of this analysis suggest that bariatric surgery may have only a limited and short-lived effect on clinical depression. However, we caution that patients are presently highly selected for bariatric surgery. If bariatric surgery were to be more widely accessible, it is possible that different effects might be observed. Randomised studies of the effect of bariatric surgery on depression are required.
Chapter 10 Rapid review of the clinical effectiveness and cost-effectiveness of bariatric surgery
Research objectives
The objectives of the rapid review were to:
-
quantify the effect of bariatric surgery on the incidence of obesity-related morbidities in comparison to standard weight management. The morbidities investigated were diabetes, CHD, stroke and cancer
-
establish the effect of bariatric surgery on diabetes remission in participants who have diabetes at the time of surgery
-
quantify the effect of bariatric surgery on the prevalence of clinical depression and use of antidepressant medications
-
assess changes in mortality after bariatric surgery compared with standard weight management
-
investigate the cost-effectiveness of bariatric surgery for the treatment of obesity.
Results
The literature search identified 11,996 articles (with duplicates), of which 247 were selected on the title for further investigation. After checking the abstracts 87 full-text papers were checked for inclusion. The final analysis included 31 systematic reviews and 7 single studies. A flow chart outlining study selection is presented in Figure 11. A summary of the key results from the systematic reviews is presented in Table 32.
FIGURE 11.
Flow chart showing selection of studies for the rapid review.
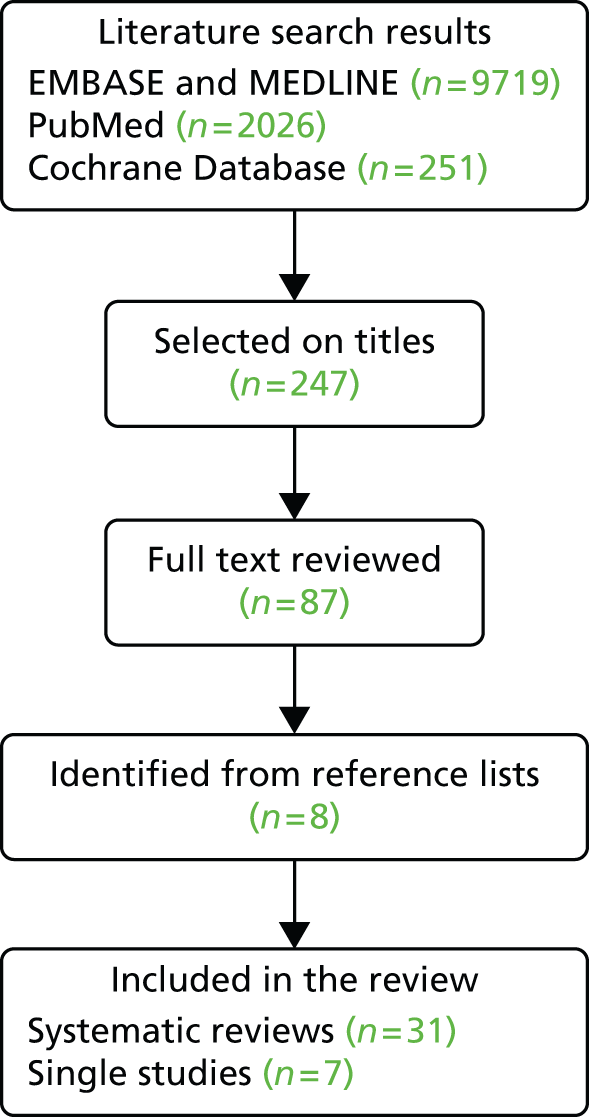
| Study details | Intervention and study designs included | Main findings |
|---|---|---|
| Picot (2009),31 narrative synthesis | GBP; BPD; SG; AGB; VBG Systematic reviews, controlled clinical trials and prospective (controlled) cohort studies |
T2DM incidence: one cohort study (SOS) included at 2- and 10-year follow-up. Two-year rate in surgery group 1% vs. control 8% (p < 0.001); 10-year 7% vs. 24% (p < 0.001) Cancer incidence: one cohort study (SOS), 11-year follow-up, HR 0.74 adjusted (p = 0.011) T2DM remission: one RCT, 2-year follow-up, RR 5.5 (95% CI 2.2 to 14.0); one cohort (SOS) at 2- and 10-year follow-up. Two-year rate in surgery group 72% vs. control 21% (p < 0.001); 10-year 36% vs. 13% (p < 0.001) Mortality: one cohort study (SOS), < 16-year follow-up, HR 0.76 (95% CI 0.59 to 0.99) |
| Padwal (2011),85 narrative synthesis | BPD; SG; GBP; AGB; VBG Economic analyses |
Cost-effectiveness: 13 studies; ICERs ranged from US$1000 to US$40,000 per QALY. More cost-effective in diabetes |
| Pontiroli (2011),83 meta-analysis | GBP; SG; AGB Controlled clinical trials (RCT and cohort) |
Mortality: eight cohorts < 12-year follow-up, OR 0.55 (95% CI 0.49 to 0.63) |
| Picot (2012),75 narrative synthesis | LAGB Controlled clinical trials (RCT and cohort) BMI 30–40 kg/m2 |
T2DM remission: one cohort study (SOS), 2-year follow-up remission in > 70% of surgery, 13% in control (p < 0.001) |
| Chang (2013),32 meta-analysis | GBP; AGB; VBG; SG RCT and observational studies |
T2DM remission: eight RCTs, rate in surgery group 92% vs. control 18%; 43 cohorts rate in surgery group 86% vs. control Mortality: 15 RCTs, rate in surgery group 0.31% vs. control?; 32 RCTs, rate in surgery group 0.35% vs. control |
| Gloy (2013),6 meta-analysis | GBP; BPD; SG; AGB RCTs |
T2DM remission: four RCTs > 2-year follow-up, RR 5.3 (95% CI 1.8 to 15.8); p = 0.003 |
| Guo (2013),71 meta-analysis | GBP; SG; BPD; gastric band RCT |
T2DM remission: two RCTs, RR 9.74 (95% CI 1.36 to 69.66) |
| Maggard-Gibbons (2013),74 narrative analysis | GBP; AGB; SG Systematic reviews, RCTs and controlled cohorts BMI 30–35 kg/m2 |
T2DM remission: three CCT vs. non-surgery, two head-to-head, one matched trial, 26 observational. BS in BMI 30–35 kg/m2 with diabetes have better intermediate glucose outcomes. Insufficient evidence to say more |
| Tee (2013),63 meta-analysis | Gastric band; VBG; GBP RCT, controlled cohort, case–control |
Cancer incidence: six cohorts, RR 0.55 (95% CI 0.41 to 0.73); women 0.68 (95% CI 0.60 to 0.77), men 0.99 (95% CI 0.74 to 1.32) |
| Wang (2013),84 narrative synthesis | GBP; SG; AGB Economic analyses |
Cost-effectiveness: six studies ICERs ranged from around US$1500 to ≈US$17,000 per QALY |
| Yip (2013),77 meta-analysis | SG; GBP Clinical studies (excluding case reports) |
T2DM remission: comparison of SG and GBP two RCTs, OR 5.0 (95% CI 0.7 to 38.1) |
| Afshar (2014),67 meta-analysis | GBP; VBG RCTs, controlled cohort, case–control |
Cancer incidence (colorectal cancer): four cohorts, RR 0.73 (95% CI 0.58 to 0.90); p = 0.004 |
| Casagrande (2014),64 meta-analysis | GBP; AGB; VGB Controlled studies |
Cancer incidence: four cohorts, OR 0.40 (95% CI 0.23 to 0.72), two studies removed to lower heterogeneity, OR 0.74 (95% CI 0.65 to 0.85) |
| Colquitt (2014),73 narrative synthesis | GBP; AGB; SG RCT |
T2DM remission: five RCTs < 12 months’ follow-up reported greater remission in surgery |
| Kwok (2014),62 meta-analysis | Gastric band; VBG; GBP RCTs, controlled cohorts |
Stroke incidence: four cohorts, OR 0.63 (95% CI 0.49 to 0.80) Mortality: 10 cohorts, OR 0.60 (95% CI 0.49 to 0.74) based on adjusted estimates |
| Merlotti (2014),60 meta-analysis | AGB; GBP; VBG RCT, controlled non-randomised studies BMI ≥ 30 kg/m2 |
T2DM incidence: three controlled clinical trials, OR 0.1 (95% CI 0.02 to 0.49) |
| Merlotti (2014),60 meta-analysis | AGB; GBP; VBG RCT, controlled non-randomised studies Included non-obese |
T2DM incidence: four controlled clinical trials, OR 0.16 (95% CI 0.11 to 0.24) |
| Ribaric (2014),72 meta-analysis | AGB; GBP; VBG RCTs and controlled cohort studies |
T2DM remission: 11 cohorts and five RCTs, 1- to 2-year follow-up, OR 9.81 (95% CI 6.06 to 15.87) |
| Zhang (2014),80 meta-analysis | GBP; SG RCT, controlled trials and cohort studies |
T2DM remission: comparison of SG and GBP at 2 years’ follow-up, seven cohorts, RR 1.05 (95% CI 0.90 to 1.23) |
| Cho (2015),79 meta-analysis | SG; GBP RCT and cohort studies |
T2DM remission: comparison of SG and GBP, two RCTs, nine cohorts < 1-year follow-up, RR 0.90 (95% CI 0.81 to 1.01) |
| Maestro (2015),65 narrative synthesis | GBP; VBG; gastric band Systematic reviews and observational studies |
Cancer incidence: one meta-analysis, three narrative reviews and nine cohorts. Surgery associated with lower morbidity and mortality from cancer |
| Müller-Stich (2015),76 meta-analysis | BPD; SG; GBP; AGB RCTs and cohort studies BMI < 40 kg/m2 |
T2DM remission: five RCTs, three cohorts < 3-year follow-up, OR 14.11 (95% CI 6.67 to 29.86) |
| Ricci (2015),61 meta-analysis | BPD; AGB; GBP; SG; VBG RCT and cohort studies |
T2DM incidence: 22 cohorts, RR 0.33 (95% CI 0.26 to 0.41) |
| Upala (2015),161 meta-analysis | GBP; VGB RCT and non-randomised trials and observational studies |
Cancer incidence (endometrial cancer): three female cohorts, RR 0.43 (95% CI 0.26 to 0.72) |
| Yang (2015),66 meta-analysis | GBP; VBG RCTs and observational studies |
Cancer incidence (obesity-related): five cohorts, OR 0.22 (95% CI 0.14 to 0.35) |
| Zhang (2015),78 meta-analysis | GBP; SG RCT and controlled studies |
T2DM remission: comparison of SG and GBP four cohorts, five RCTs, OR 3.29 (95% CI 1.98 to 5.49) |
Disease incidence
Type 2 diabetes mellitus
Four systematic reviews reported on the incidence of T2DM after bariatric surgery. Picot et al. 31 reported on the incidence of T2DM based on the SOS study at 2 and 10 years for this outcome. 162 There were two systematic reviews produced on T2DM prevention by various methods by a single team, with one focusing on obese participants (BMI ≥ 30 kg/m2) and the other without this criterion. In the bariatric surgery component they included three and four controlled clinical trials, respectively, and reported ORs of 0.10 (95% CI 0.02 to 0.49) and 0.16 (95% CI 0.11 to 0.24). 60,163 The most recent review was published in 2014 and summarised 22 randomised and non-randomised trials, giving a relative risk of 0.33 (95% CI 0.26 to 0.41). In a subgroup analysis of four malabsorptive procedures, the relative risk was 0.14 (95% CI 0.03 to 0.73). 61 The result from the CPRD analysis conducted in Chapter 7 gave a HR of 0.20 (95% CI 0.13 to 0.30) for follow-up of up to 7 years after surgery. This finding is comparable with results from the SOS study and was employed in the health economic model.
Coronary heart disease and stroke
We did not identify any systematic reviews or individual studies that reported the incidence of CHD after bariatric surgery. One systematic review reported on stroke incidence after bariatric surgery. 62 A meta-analysis of adjusted HRs from three cohort studies gave a result of 0.63 (95% CI 0.49 to 0.80), with the inclusion of an additional non-adjusted estimate altering the result to 0.49 (95% CI 0.32 to 0.75). For the health economic model we utilised the relative risk estimate of 0.67 (95% CI 0.54 to 0.83) from the SOS study.
Cancer
There were four systematic reviews that investigated incidence of cancers after bariatric surgery. Two used meta-analysis to combine the results of controlled cohort studies. The first, based on six papers, reported a relative risk of 0.55 (95% CI 0.41 to 0.73), and performed subgroup analysis by gender finding that bariatric surgery was protective in women but not in men. 63 However, four of the papers included in this meta-analysis used data from the same two cohorts. The second review using meta-analysis was based on the four non-duplicate cohorts and gave a pooled OR of 0.42 (95% CI 0.24 to 0.73). 64 Two of the studies were conducted in morbidly obese patients only. The association between surgery and reduced risk of cancer was maintained when two studies, one conducted only in women and one with controls taken from a hospital population, were removed to reduce heterogeneity. One older review identified just one relevant study,31 and another summarised the existing reviews. 65
Three additional recently published reviews were identified that focused on the relationship between bariatric surgery and specific cancers. A meta-analysis describing incidence of obesity specific cancers produced a pooled OR of 0.43 (95% CI 0.27 to 0.69) based on five studies. 66 Four studies on incidence of colorectal cancer gave a pooled relative rate of 0.73 (95% CI 0.58 to 0.90),67 while three studies of endometrial cancer gave a pooled relative rate of 0.43 (95% CI 0.26 to 0.72). 161 For the health economic model, we utilised the cancer incidence result reported by the SOS, as this is a large, well-established study with long-follow-up. 89
Type 2 diabetes remission
Remission of T2DM was the most commonly reported comorbidity measure in papers investigating the clinical impact of bariatric surgery, reflecting the potential importance of surgery as a T2DM treatment. In total we identified 18 systematic reviews published since 2000 that reported on remission of T2DM after bariatric surgery. We focused on the more recently published reviews, as the older literature covered a smaller evidence base. 31,68–70,164
A number of reviews reported on T2DM remission results from RCTs and included largely the same studies in their meta-analyses. Gloy et al. 6 used meta-analysis to combine the results of four RCTs to give a relative risk of 5.3 (95% CI 1.8 to 15.8) for T2DM remission at 1–2 years of follow-up when comparing surgery with medical management, while a review by Guo et al. 71 published in the same year included two RCTs to give an OR of 9.74 (95% CI 1.36 to 69.66). One further review, again published in 2013, employed meta-analysis to combine the results of eight RCTs to show a pooled estimate of 92% remission of T2DM after surgical intervention compared with 18% in control groups. 32 Subsequently, two further systematic reviews of T2DM remission have been published. One incorporated RCTs and controlled cohorts both separately and together into random-effect meta-analyses. 72 Combining the 11 observational studies and five RCTs together gave a pooled OR of 9.8 (95% CI 6.1 to 15.9). The other used narrative analysis to discuss the results of five RCTs, four of which matched the former review. 73
Three reviews focused on the potential of bariatric surgery to treat T2DM in patients who were not morbidly obese (BMI < 40 kg/m2). 74–76 The two older reviews identified one RCT of 60 patients with a BMI of 30–40 kg/m2 who underwent adjustable gastric banding that reported on remission. 165 After 2 years, 73% of the surgery group and 13% of the controls were in remission from T2DM (p < 0.001). A statistically significant difference in remission was also reported in patients with a BMI < 35 kg/m2, although this was based on just 13 patients. However, the most recent review identified two RCTs and six observational studies that were conducted in patients with a BMI < 35 kg/m2, with five of these reporting on T2DM remission. 76 Meta-analyses of the five studies, combined with an additional three publications that included patients with mild obesity non-exclusively, gave a pooled OR of 14.1 (95% CI 6.7 to 29.9).
Four reviews compared SG with GBP. Three of these concluded that there was no difference in T2DM remission after the two procedures, and were based on RCTs alone77 and by combining randomised and non-randomised studies. 78,79 The fourth review included four RCTs and five controlled studies to give a pooled OR of 3.29 (95% CI 1.98 to 5.49) favouring GBP over SG. 80
We identified four additional recently published studies through the literature search that were not included in existing literature reviews. Two prospective cohorts compared GBP and SG for T2DM remission. The first focused on patients with a BMI of 28–35 kg/m2 and was conducted on 64 Chinese diabetic patients. They found complete T2DM remission at 3 years in 85% of GBP and 78% of SG patients (p = 0.525). A third arm who received lifestyle treatment had no patients in remission at the end of the study. 166 The second larger study did find a significant difference in remission rates at 1 year after surgery in patients with a BMI ≥ 50 kg/m2, with remission in 48% of SG and 71% of GBP patients. 167 The third study, a RCT of patients with BMI 30–40 kg/m2, compared GBP, gastric banding and an intensive lifestyle intervention. At 3-year follow-up complete remission was found in 15% of bypass, 5% of banding and 0% of lifestyle participants. 168 A second RCT was conducted in 60 patients with BMI ≥ 35 kg/m2. After 5 years, 50% of the bariatric surgery group were in remission from T2DM: 37% of the GBP patients and 63% of those who underwent biliopancreatic diversion. 144
In our own analyses of CPRD data we identified a relative rate of remission from T2DM of 5.90 (95% CI 3.72 to 9.34) after 6 years. The heterogeneity of studies relating to the length of follow-up, choice of measure and different procedures makes it difficult to compare our results against the literature, although the majority of systematic reviews included papers that generally had follow-up periods of just a few years.
Depression
We identified two systematic reviews that focused on psychological health in relation to bariatric surgery. 81,82 Both included uncontrolled studies which did not fit with our inclusion criteria. Both also highlighted the SOS study as presenting the highest-quality evidence available on bariatric surgery and depressive symptoms, which has found a dose–response relationship between weight loss and depressive symptoms up to 4 years. 169
More recently, we identified one cohort study that compared patients who underwent surgery (gastric banding or vertical banded gastroplasty) with medical management of obesity and a no treatment group. 170 At 9 years’ follow-up they found that the surgical group had higher depression scores, using the Hospital Anxiety and Depression Scale, than the other groups after adjustment for baseline scores. Additionally, they found that scores in the surgery group were not significantly different from baseline, although they had been lower at a 4-year follow-up.
The CPRD analysis identified a reduction in clinical depression in surgery patients for the first 3 years after the procedure. Although we relied on clinical data rather than research questionnaires, our findings are similar to the literature in showing only a temporary effect as a result of surgery.
Mortality
Four systematic reviews presented data on long-term mortality after bariatric surgery. One of these reported data from just one study,31 and another included uncontrolled studies and so was able to report mortality rates in the treatment arm only. 32 Pontiroli and Morabito83 pooled eight controlled clinical trials to give an OR of 0.55 (95% CI 0.49 to 0.63) for mortality after the perioperative period. These results were also divided into deaths from cardiovascular disease (OR 0.58, 95% CI 0.46 to 0.73) and not from cardiovascular disease (OR 0.70, 95% CI 0.59 to 0.84), and by surgery type with similar results from gastric banding and bypass. More recently, Kwok et al. 62 reviewed the literature and combined the adjusted results from 10 cohorts to give a pooled OR of 0.60 (95% CI 0.49 to 0.74). There was a high level of overlap with studies identified by Pontiroli and Morabito, plus the addition of more recently published papers.
Two further studies of interest have been published since the reviews. Both were retrospective cohorts with the first comparing patients who had GBP to non-surgical controls. They found an OR of 0.43 (95% CI 0.25 to 0.73) for long-term mortality in favour of surgery at 10 years, with similar results when just diabetic participants were included. A significant difference in mortality was apparent at 5 years of follow-up but not at 2 years. 171 The second study had a mean follow-up of 7 years, and found a HR of 0.45 (95% CI 0.36 to 0.56) in years 1–4 after surgery, and 0.47 (95% CI 0.39 to 0.58) at 5–10 years. 10
Cost-effectiveness
Two systematic reviews evaluated the cost-effectiveness of bariatric surgery for obesity. The most recent was a narrative review of six economic models. 84 The models varied in structure, time horizon, comparators and setting, and the ICERs ranged from US$1500 to US$17,000. The highest ICER was attributed to open Roux-en-Y GBP. The second review was a narrative analysis of 13 studies, including RCTs, cohorts and literature reviews. 85 Again, the heterogeneity of the sources led to a large variation in estimated ICERs, from US$1000 to US$40,000 per QALY. Surgery was found to be more cost-effectiveness in diabetic study populations.
The literature search identified five modelling studies that evaluated the cost-effectiveness of bariatric surgery that were not included in the above reviews. Two of these were UK-based; the first estimated the ICER to be £20,159 at 2 years, £4969 at 5 years and £1635 at 20 years. 75 The second focused only on diabetic patients undergoing LAGB and calculated the ICER as £3602 using a 40-year time horizon. 172 An Australian study considering LAGB for BMI ≥ 35 kg/m2 and using disability adjusted life-years rather than QALYs estimated the mean ICER using a lifetime horizon to be AU$2154. 173 A US study predicted ICERs of US$6600 for GBP and US$6300 for gastric banding over a lifetime perspective and based on normal BMI trajectories post surgery. For open GBP the estimate was far higher at US$17,300. The authors also produced separate estimates for the post-surgery BMI being maintained and maximum weight regain. 174 Finally, a Markov model using Swedish data estimated the ICER at 2 years to be €26,985. 175
Chapter 11 Costs and outcomes of offering greater access to bariatric surgery for morbid obesity
Introduction
In spite of the important potential benefits, the role of bariatric surgery in the management of obesity remains controversial. Access to bariatric surgery is often limited in publicly funded health-care systems. In the UK, approximately 10,000 bariatric surgery procedures are performed annually11 but there are more than 1 million individuals with morbid obesity who could potentially benefit from the procedure. 176 This limited access to bariatric surgery may be related to perceptions of the cost of surgery and the resources required to offer it more readily. A recent study concluded that ‘bariatric surgery does not reduce overall health-care costs in the long-term’ and suggested that ‘future studies should focus on the potential benefit of improved health and well-being of persons undergoing the procedure rather than cost-savings.’177
The purpose of the present research was to explore the potential impacts of bariatric surgery deployed at scale for severe and morbid obesity. We aimed to evaluate the cost-effectiveness of bariatric surgery, including laparoscopic gastric banding, GBP and SG procedures, in comparison with standard non-surgical health-care management of obesity. We aimed to incorporate new evidence concerning the long-term effects of bariatric surgery on morbidity and mortality outcomes. We also aimed to determine whether there were population subgroups defined by age, gender, socioeconomic position, obesity category and comorbidity for whom bariatric surgery might be more, or less, cost-effective.
Methods
The methods for the health economic modelling study are given in Chapter 3 (see Markov modelling for health economic evaluation). A Markov model was designed. Empirical data inputs to the model were provided through analysis of data for a large population registered in primary care, derived from the CPRD. Estimates for the clinical effectiveness of bariatric surgery were derived from CPRD data analysis and updated systematic reviews. Probabilistic simulations, run using the model, provided estimates of lifetime incremental costs and QALYs aggregated across the population at risk. A summary of the simulation scenarios is provided in Table 33.
| BMI category | Condition | Category | Start population |
|---|---|---|---|
| ≥ 40 kg/m2 | All | Synthetic population of 100,000 each of men and women, aged 20–74 years, with morbid obesity including 19% with T2DM and 4% with CHD | |
| ≥ 40 kg/m2 | Gender | Men | 100,000 men with morbid obesity as above |
| Women | 100,000 women with morbid obesity as above | ||
| ≥ 40 kg/m2 | Age group (years) | 20–34 | Synthetic population of 100,000 each of men and women, in the specified age group, with morbid obesity including 19% with T2DM and 4% with CHD |
| 35–54 | |||
| 55–74 | |||
| ≥ 40 kg/m2 | Deprivation category | Least deprived | As in ‘All’ above |
| Most deprived | As in ‘All’ above | ||
| ≥ 40 kg/m2 | Diabetes BMI ≥ 40 kg/m2 | BMI ≥ 40 kg/m2 | Synthetic population of 75,000 each of men and women, in the specified age group, with morbid obesity and T2DM |
| 35–39 kg/m2 | BMI 35–39 kg/m2 | BMI 35–39 kg/m2 | Synthetic population of 75,000 each of men and women, aged 20–74 years, with morbid obesity and no comorbidity |
| ≥ 40 kg/m2 | Costs of procedure | 50% higher | As in ‘All’ above |
| 100% higher | As in ‘All’ above | ||
| Zero procedure cost | As in ‘All’ above | ||
| ≥ 40 kg/m2 | Decline of intervention effect over time | Year–0.25 | As in ‘All’ above |
| Year–0.50 | As in ‘All’ above |
In the base case, the population entering the Markov model comprised 200,000 participants with BMI ≥ 40 kg/m2. These included equal numbers of men and women, with mean age of 46 years and ages ranging from 20 to 74 years. There were 19% with T2DM and 4% with CHD, the remainder having no chronic comorbidity (Table 34). The structure of the model is presented in Figure 12.
| Measure | Bariatric surgery | No bariatric surgery |
|---|---|---|
| Number | 200,000 | 200,000 |
| Age (years), mean (range) | 46 (20–74) | 46 (20–74) |
| Men | 100,000 | 100,000 |
| Women | 100,000 | 100,000 |
| No morbidity | 153,846 (77%) | 153,846 (77%) |
| Diabetes mellitus | 38,462 (19%) | 38,462 (19%) |
| CHD | 7692 (4%) | 7692 (4%) |
FIGURE 12.
Showing the structure of the model. Each state is stratified by BI category. Model states are further divided into ‘depressed’ and ‘not depressed’. Participants occupying each state are stratified by gender and single year of age. ‘At risk’ denotes participants without comorbidities included in the model; BS, bariatric surgery; DM, diabetes mellitus.
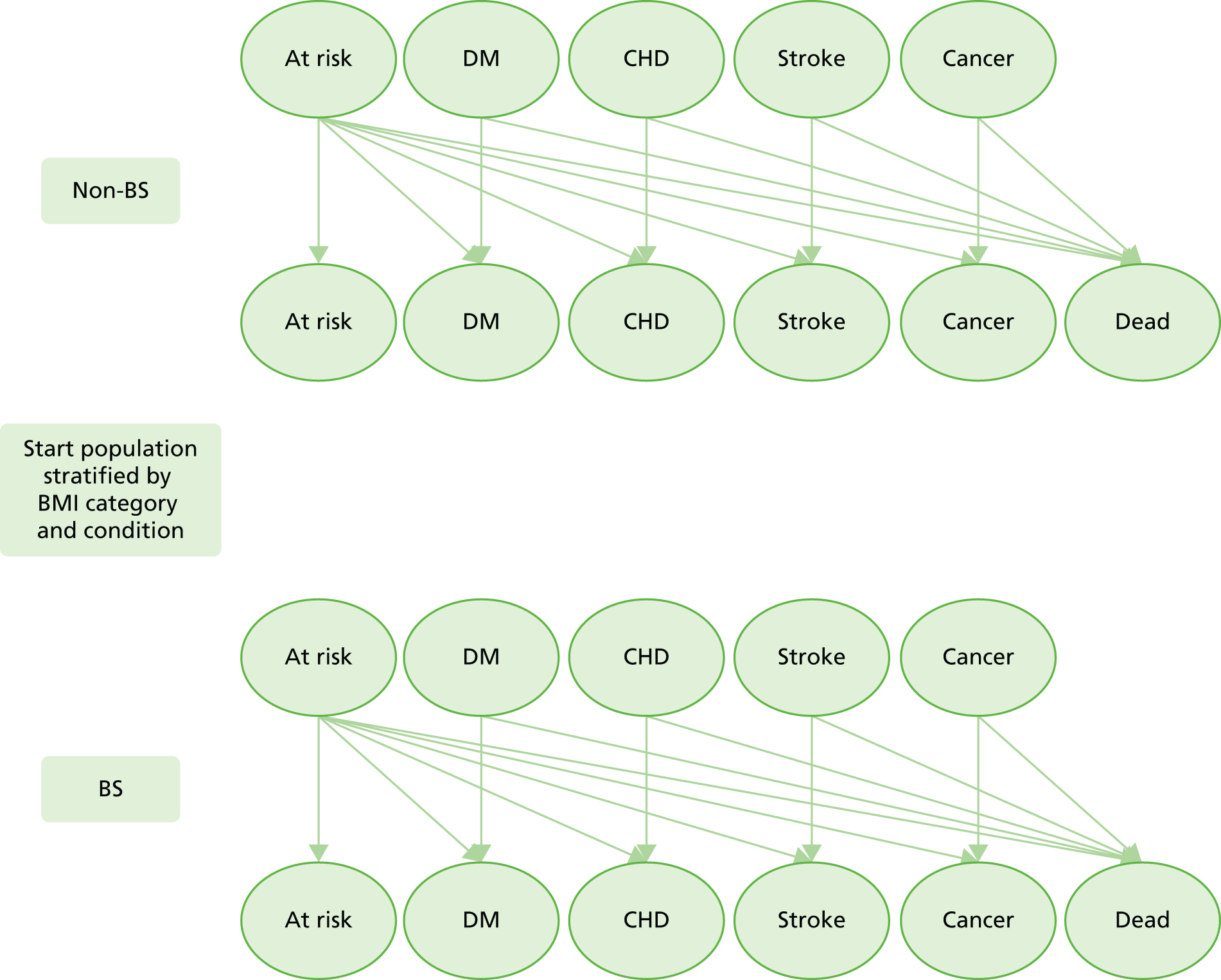
Estimates of cost-effectiveness were sensitive to the cost of the surgical procedure. However, even when the cost of the procedure was 100% higher than in the base case (£18,328 instead of £9164), bariatric surgery was cost-effective at £11,365 per QALY. Simulations in which the procedure cost was set at zero confirmed that incremental health-care costs remained positive. When the intervention effect from bariatric surgery was allowed to diminish markedly over time following the procedure, there was only a modest impact on estimated cost-effectiveness (see Table 38), reflecting the smaller contribution made by discounted costs and QALYs from later periods of follow-up.
Estimates of intervention effects of bariatric surgery on disease incidence and mortality that were incorporated into the model are outlined in Table 35. CPRD derived estimates were employed for T2DM incidence and remission, and for the effect of bariatric surgery on depression. Bariatric surgery was modelled as associated with 40% of patients entering remission; this was based on analysis of patients with complete data. The choice of estimate was also influenced by recognition that RCTs have generally reported higher remission rates than were observed in CPRD. Estimates from the SOS study were used for cardiovascular disease and cancer. Estimates from the SOS study were used as the largest study with long follow-up. The effect of bariatric surgery on mortality was derived from the study by Arterburn et al. 10 as the most recent large, authoritative study on bariatric surgery and mortality. It was considered to be beyond the scope of the present project to pursue in-depth analysis of each of CHD, stroke, cancer and mortality within CPRD. This was because problems of confounding may be more difficult to address with these outcomes. There have been large changes in uptake of interventions that contribute to cardiovascular prevention including antihypertensive therapy, statins and smoking cessation. There are also important issues of confounding by indication and contraindication that might require to be addressed. For these reasons, we preferred to draw on previously published reports for these measures.
| Item | Value | Source |
|---|---|---|
| Operative mortality (%) | 0.07 | NBSR11 |
| T2DM remission (%) | 40 | CPRD (see Chapter 8) |
| Incidence, relative risk (95% CI) | ||
| DM | 0.20 (0.13 to 0.30) | CPRD7 (see Chapter 7) |
| CHD | 0.67 (0.54 to 0.83) | SOS9 |
| Stroke | 0.67 (0.54 to 0.83) | SOS9 |
| Cancer | 0.58 (0.44 to 0.77) | SOS89 (women only) |
| Mortality, relative risk (95% CI) | 0.45 (0.36 to 0.56) | Arterburn et al.10 |
| Depression, relative risk (95% CI) | ||
| Year 1 | 0.82 (0.78 to 087) | CPRD90 |
| Year 2 | 0.83 (0.76 to 0.90) | |
| Year 3 | 0.87 (0.78 to 0.97) | |
| Decrement in utility associated with BMI category (kg/m2) | ||
| 25–29 | 0 | Hakim et al.91 |
| 30–34 | –0.085 | |
| 35–39 | –0.17 | |
| ≥ 40 | –0.255 | |
Results
Results are presented in Table 36 for a comparison of bariatric surgery with no bariatric surgery in morbid obesity. Results are presented as the mean and 95% range for 1000 simulations, with values expressed as rates per 1000 persons entering the model. Bariatric surgery was associated with an increase in total life-years, accumulated over the lifetime of participants entering the model, of 6097 per 1000. There was a substantial increase in the number of life-years lived free from chronic comorbidities of 10,297 per 1000 persons entering the model. There was a decrease in life-years lived with diabetes mellitus of 8320 per 1000 participants entering the model. There were modest increases in life-years lived with CHD, stroke and cancer following bariatric surgery. This resulted from the increase in the population at risk for these conditions over the lifetime of the model.
| Measure | Bariatric surgery | No bariatric surgery | Incremental value (mean, 95% range) |
|---|---|---|---|
| Total person-years lived | 41,869.28 | 35,772.21 | 6097 (6022 to 6171) |
| No morbidity, person-years | 22,296.44 | 11,998.61 | 10,297 (10,152 to 10,452) |
| Diabetes mellitus, person-years | 9434.01 | 17,754.62 | –8320 (–8502 to –8123) |
| CHD, person-years | 5321.58 | 3771.50 | 1550 (1473 to 1626) |
| Stroke, person-years | 1309.54 | 633.92 | 676 (647 to 705) |
| Cancer, person-years | 3507.70 | 1613.56 | 1894 (1830 to 1957) |
| Depression, person-years | 4393.11 | 4385.58 | 8 (–8 to 23) |
Table 37 presents the results of the cost–utility analysis. Figures are presented as values per 1000 persons entering the model. The total undiscounted health-care costs over a lifetime for 1000 persons with morbid obesity were estimated to be £97.82M in the absence of bariatric surgery and £126.84M with bariatric surgery. The undiscounted incremental cost associated with bariatric surgery was £29.01M, or £15.26M when discounted at 3.5%. As the cost of the bariatric surgical procedure is estimated to be £9.16M per 1000 participants, it can be concluded that bariatric surgery is associated with increased lifetime health-care costs associated with greater longevity. This is reflected in the greater estimated undiscounted QALYs after bariatric surgery of 28,345 per 1000 persons, compared with 22,772 in the absence of bariatric surgery. The net gain in discounted QALYs from bariatric surgery was 2142 per 1000 persons. The estimated value for discounted cost per QALY gained was £7129 per QALY, with a 95% range from 1000 simulations of £6775 to £7506 per QALY. Graphs of the cost-effectiveness plane and the cost-effectiveness acceptability curve are presented in Figure 13. If each QALY gained is valued at £30,000, then the net benefit associated with bariatric surgery performed in 1000 persons with morbid obesity is approximately £49M, or £28M if a value of £20,000 per QALY is used.
| Measure | Bariatric surgery | No bariatric surgery | Incremental value (mean, 95% range) |
|---|---|---|---|
| Health-care costs per 1000 (UK£M) | |||
| Not discounted | 126.84 | 97.82 | 29.01 (28.78 to 29.23) |
| Discounted 1.5% | 93.06 | 72.38 | 20.68 (20.53 to 20.81) |
| Discounted 3.5% | 67.25 | 51.99 | 15.26 (15.18 to 15.33) |
| QALYs per 1000 (QALYs) | |||
| Not discounted | 28,345 | 22,772 | 5572 (5422 to 5728) |
| Discounted 1.5% | 20,547 | 17,022 | 3524 (3397 to 3655) |
| Discounted 3.5% | 14,509 | 12,367 | 2142 (2032 to 2256) |
| Cost (£) per QALY | |||
| Not discounted | 5208 (5075 to 5338) | ||
| Discounted 1.5% | 5868 (5662 to 6073) | ||
| Discounted 3.5% | 7129 (6775 to 7506) | ||
| Net monetary benefit per 1000 (UK£M) | |||
| £30,000 per QALY | 49.02 (45.72 to 52.41) | ||
| £20,000 per QALY | 27.59 (25.40 to 29.85) | ||
| Net health benefit per 1000 (QALYs) | |||
| £30,000 per QALY | 1634 (1524 to 1747) | ||
| £20,000 per QALY | 1380 (1270 to 1493) | ||
FIGURE 13.
Graphs showing (a) cost-effectiveness plane of incremental cost per QALY; and (b) cost-effectiveness acceptability curve.

Table 38 presents the results of analyses in subgroups of the population, as well as sensitivity analyses that varied underlying assumptions. Additional data are presented in Appendix 2. All estimates were discounted at 3.5%. Incremental costs and QALYs were slightly lower in men than in women, reflecting their general lower life expectancy, but estimates of costs per QALY were similar in men and women. Older participants generally incurred lower total costs and fewer total QALYs, consistent with their shorter life expectancy, but incremental costs and QALYs were higher as a result of the higher absolute risk reductions obtained in a population at higher baseline risk. Nevertheless, cost-effectiveness estimates were generally consistent across age groups. Comparing the use of bariatric surgery in most and least deprived quintiles of deprivation, total costs were greater and total QALYs were lower in the most deprived quintile but incremental costs and QALYs were similar in each deprivation category as were cost-effectiveness estimates. The model was run using an initial population with severe obesity and the procedure was found to be only slightly less cost-effective in this group, with estimated cost of £7669 per QALY. When the initial population was confined to morbidly obese persons with T2DM the estimated cost per QALY was £6176 (95% CI £5894 to £6457).
| Variable | Condition | Bariatric surgery arm | Incremental | Cost per QALY (mean, 95% range) (UK£) | ||
|---|---|---|---|---|---|---|
| Total costs (UK£M) | Total QALYs | Incremental costs (UK£M) | Incremental QALYs | |||
| All | BMI ≥ 40 kg/m2 | 67.25 | 14,509 | 15.26 | 2142 | 7129 (6775 to 7506) |
| Gender | Men | 63.99 | 14,332 | 14.97 | 2087 | 7188 (6662 to 7796) |
| Women | 70.51 | 14,680 | 15.55 | 2201 | 7076 (6581 to 7638) | |
| Age group (years) | 20–34 | 68.18 | 17,153 | 13.62 | 1866 | 7344 (6478 to 8421) |
| 35–54 | 70.79 | 15,030 | 15.00 | 2139 | 7027 (6511 to 7569) | |
| 55–74 | 59.49 | 11,545 | 17.01 | 2355 | 7230 (6862 to 7613) | |
| Deprivation category | Least deprived | 61.49 | 14,791 | 14.46 | 2052 | 7056 (6688 to 7448) |
| Most deprived | 70.00 | 14,187 | 16.32 | 2242 | 7287 (6930 to 7665) | |
| Diabetes BMI ≥ 40 kg/m2 | BMI ≥ 40 kg/m2 | 68.47 | 14,468 | 15.04 | 2437 | 6176 (5894 to 6457) |
| BMI 35–39 kg/m2 | BMI 35–39 kg/m2 | 68.08 | 14,708 | 15.00 | 1995 | 7675 (7339 to 8037) |
| Costs of procedure | 50% higher | 71.83 | 14,511 | 19.84 | 2144 | 9261 (8800 to 9795) |
| 100% higher | 76.41 | 14,512 | 24.42 | 2148 | 11,376 (10,763 to 11,950) | |
| Zero procedure cost | 58.09 | 14,512 | 6.10 | 2148 | 2842 (2701 to 2998) | |
| Decline of intervention effect over time | Year–0.25 | 64.25 | 13,786 | 12.25 | 1422 | 8637 (8009 to 9400) |
| Year–0.50 | 63.15 | 13,516 | 11.16 | 1152 | 9720 (8860 to 10,706) | |
Discussion
Summary of findings
This study drew on recently published evidence for the effects of bariatric surgery on incidence of disease,7,8 mortality10 and health-care costs12,13 following the procedure. This research modelled the lifetime health benefits from bariatric surgery. The results project substantial increases in life-years and reductions in years lived with diabetes. Bariatric surgery is expected to be associated with increased health-care costs arising from the costs of the procedure, as well as increased lifetime health-care costs associated with increased life expectancy. When health benefits and costs are combined into a single metric, using accepted values of cost per QALY, use of bariatric surgery is expected to yield substantial net monetary benefits amounting, over a lifetime, to £49M per 1000 persons. Net health benefits may amount to 1634 QALYs per 1000 persons. Bariatric surgery has similar cost-effectiveness in men and women, at different ages and in different deprivation categories. Bariatric surgery is also expected to be cost-effective in individuals with severe obesity (BMI 35–39 kg/m2). Bariatric surgery will be cost-effective even if the cost of the procedure is twice as high as we have estimated, or if the effect of the procedure declines over time so that only 32% of the initial effect remains after 10 years.
Comparison with other results
There have been several previous cost-effectiveness analyses of bariatric surgery for morbid obesity. One of the most authoritative was the 2009 study by Picot et al. 31 Their study reported a model in which weight loss following surgery was viewed as the main mediator of longer-term changes in health outcomes. The study found that bariatric surgery was a cost-effective intervention for morbid obesity, with ICERs ranging between £2000 and £4000 per QALY gained over a 20-year time horizon. 31 A more recent study suggested that bariatric surgery may be cost saving for health-care systems through reduced morbidity,175 though this conclusion has been disputed. 178 Although bariatric surgery does not appear to generate cost savings, its use is associated with substantial health gains at costs that are well below accepted thresholds for cost-effectiveness.
This analysis updates previous studies by including those surgical procedures that are currently utilised including adjustable gastric banding, GBP and SG. The analysis also updated the costs of surgery to present-day values. The present model incorporated direct evidence concerning the long-term outcomes of bariatric surgery in contrast to previous studies, which have used changes in intermediate measures of surgical effect including body weight, blood pressure and lipid profiles to model the long-term outcomes of bariatric surgery. Our analysis recognised that the effects of surgery, particularly those on diabetes, are not entirely weight dependent and our model therefore did not include weight change as an intermediate outcome. The model was informed by recent measures of the effects of surgery on substantive long-term health outcomes and health-care costs, additionally informed by analysis of CPRD EHRs. In spite of this difference of approach, our analyses are consistent with previous reports in showing that bariatric surgery is likely to be very cost-effective. We do not find that bariatric surgery is cost saving. This would not be expected, however, as it is unlikely for a procedure that reduces mortality in a population that experiences a heavy burden of morbidity to reduce lifetime health expenditures. 177
Strengths and limitations
This research was based on empirical data for disease incidence, mortality and costs of health-care utilisation estimated from the EHRs of a large sample of participants managed in primary care in the UK between 2008 and 2013. We caution that the small proportion of very obese patients who had bariatric surgery means there is a real possibility that they differ from other patients whose characteristics on health records are similar. The study also drew on recently published and authoritative estimates of the effects and costs of bariatric surgery with an emphasis on those bariatric surgical procedures that are currently utilised. Both the empirical and abstracted inputs were consistent with estimates from systematic reviews of the effects of surgery on related morbidities. We have used conservative assumptions including that costs of health-care utilisation following surgery are not associated with weight loss; that any gain in utility associated with BMI reduction declines rapidly over time; and that remission from diabetes following surgery occurs in 40% of patients, as estimated from CPRD, which is lower than levels observed in other reported studies. We acknowledge that any model represents a simplification of reality. There are other forms of morbidity that were not represented in the model. However, the costs of health-care utilisation from such conditions will have been included in cost estimates from CPRD, which encompassed all health-care utilisation. We included the major complications of surgery including operative mortality, costs of leaks following surgery, and reoperations in 2% of patients per year. There may be additional costs associated with surgery but a sensitivity analysis showed that even if total costs of surgery were to be twice as high as estimated in the base case, bariatric surgery would still be cost-effective. We modelled bariatric surgery as having a constant effect in the postoperative period, but we showed that even if the effects declined over time surgery would still be cost-effective.
Conclusions
Bariatric surgery is not cost saving but increased immediate and long-term health-care costs are outweighed by health benefits to obese individuals. This study shows that bariatric surgery is highly cost-effective and that substantial net health or monetary benefits may be anticipated from wider used of bariatric surgical procedures in patients with severe and morbid obesity. Similar cost-effectiveness may be anticipated in diverse groups of obese individuals including men and women, wide ranges of ages and different levels of deprivation. Bariatric surgery may have the potential to reduce obesity-related inequalities in health. This is in contrast to presently available non-surgical interventions for obesity, which generally have only small and generally short-lived effects. 29,96,97 Based on these results, the wider use of bariatric surgery may be justified in the management of people with severe and morbid obesity. A major concern remains that the social and environmental drivers of the increase in morbid obesity should not remain unchecked. 100 As a ‘downstream’ procedure, bariatric surgery will not stem the global increase in obesity. The role of surgery in treating disorders apparently rooted in individual lifestyle is also questioned. 179 There are also concerns relating to the capacity of health services to deliver safe, high-quality services for patients with severe and morbid obesity,180 which were recognised in national guidance on bariatric surgery. 181 Nevertheless, people with severe and morbid obesity share the right of all individuals to the highest attainable standard of health, and bariatric surgery may often offer important health gains at a reasonable level of investment.
Chapter 12 Conclusions
Statement of principal findings
This study used EHRs to analyse changes in disease status and health-care utilisation in obese patients who underwent bariatric surgery. These results were incorporated into a health economic model to estimate the cost-effectiveness of increasing access to bariatric surgical procedures for the treatment of obesity.
For patients with morbid obesity, the chance of attaining normal weight or maintaining clinically relevant weight loss is very low, with the implication that present obesity treatment frameworks grounded in community-based weight management programmes are ineffective. The evidence on transitions between obesity categories, and weight cycling, from the large number of electronic records analysed in the study provide robust estimates which add to the evidence base for radical action on obesity and associated morbidity. Health-care costs are considerably increased in obesity, primarily because of the greater burden of comorbidity.
The numbers of bariatric surgical procedures performed have increased since 2002, with increasing use of GBP and SG and declining use of LAGB. Rates of bariatric surgery per 100,000 population remain low and provide evidence of limited access to bariatric surgical procedures in relation to need. Bariatric surgery is associated with reduced incidence of clinical diabetes, in obese participants without diabetes at baseline, and remission of diabetes in obese patients with T2DM. Diabetes outcomes may be generally more favourable after GBP or SG than LAGB. Psychological comorbidity is frequent among individuals selected to undergo bariatric surgery, but any modest improvement over the initial postoperative years is not maintained.
Bariatric surgery is cost-effective relative to standard weight management across a wider range of BMI levels than currently recommended, and is more cost-effective in diabetes mellitus, with results robust to gender, age and deprivation differences.
Increasing access to bariatric surgery for patients with obesity is associated with increased health-care costs, but these are outweighed by expected health benefits to obese individuals. Bariatric surgery is a very cost-effective intervention. Bariatric surgery appears unlikely to reduce the population prevalence of severe and morbid obesity unless factors driving the increase in obesity are addressed. Development of more effective obesity prevention strategies is urgently required.
Box 1 presents a summary of findings.
-
The probability of an obese person attaining normal body weight is very small.
-
Maintaining a 5% loss of body weight over 2–5 years is also unlikely.
-
Obese people incur higher health costs, which are largely associated with physical and psychological comorbidity.
-
Utilisation of BS is presently at very low levels.
-
Over time, the use of BS has shifted to older, heavier patients with greater comorbidity.
-
Sleeve and partial gastrectomy procedures, which are more costly but more effective, are increasingly utilised.
-
Men are much less likely to receive BS than women.
-
In a sample of 78 procedures sampled from CPRD, 32 (41%) were performed privately.
-
In obese patients without diabetes, BS is associated with lower incidence of diabetes for up to 7 years following the procedure, when compared with controls of the same age, sex and BMI. These results confirm findings from the SOS study.
-
In obese patients with diabetes, remission is substantially higher than in controls for at least up to 6 years following the procedure. There is improved blood glucose control and lower utilisation of antidiabetes medications. These results confirm findings from small RCTs.
-
BS may be associated with a transient reduction in clinical depression following the procedure but many patients have significant psychological comorbidity.
-
The immediate cost of a bariatric surgical procedure is approximately £9164.
-
In people with morbid obesity, BS is a highly cost-effective intervention.
-
If QALY gains are valued at £30,000 per QALY, use of bariatric surgery is expected to yield substantial net monetary benefits amounting, over a lifetime, to £49M per 1000 persons.
-
BS would be cost effective even if it were to be more costly than is presently the case.
-
BS would be cost-effective even if the benefits of surgery declined over time.
-
BS is equally cost-effective in men and women.
-
BS has similar cost-effectiveness at different ages between 20 and 74 years.
-
BS has similar cost-effectiveness at different levels of deprivation including the most and least deprived.
-
BS is not cost saving owing to the increased life-expectancy and associated increase in life time health-care costs.
BS, bariatric surgery.
Implications of these research findings
The prevalence of morbid obesity in England is 1.8% in men and 3.9% in women aged 20–75 years, based on data for the Health Survey for England from 2011 to 2013. 182 In a primary care organisation with a population of 250,000 in this age group, there will be about 7163 people with morbid obesity, comprising 4875 women and 2288 men (Table 39). In this group of people, there will be approximately 200 new cases of diabetes each year, based on incidence rates reported in Chapter 7. There are important socioeconomic differences in the prevalence of morbid obesity (Figure 14). Among the least deprived, 1.3% of men and 2.3% of women have morbid obesity, but among the most deprived the prevalence of morbid obesity is 2.5% in men and 6.1% in women. A primary care organisation with an affluent population may have only 4413 people with morbid obesity, compared with 10,813 in a deprived area.
| Measure | Estimate | Source |
|---|---|---|
| Total population aged 20–74 years | 250,000 | |
| Number with morbid obesity | 7163 (4875 women; 2288 men) | Prevalence of obesity from Health Survey for England 2011–13 combined182 |
| Number with morbid obesity and diabetes | 1406 (763 women; 643 men) | Prevalence of diabetes by BMI category from Health Survey for England 2011–13 combined182 |
| Cost of 1000 bariatric surgical procedures, with 50% in people with diabetes | £9.164M | Table 7 in this report |
| Total increase in NHS costs over patients’ lifetime | £15.260M | See Chapter 11 |
| Number of new cases of diabetes prevented over 10 years | 112 | See Chapter 7 |
| Number of diabetes cases in remission over next 5 years | 200 | See Chapter 8 |
| Health gain in QALYs | 2142 | See Chapter 11 |
| Net monetary benefits over patients’ lifetime (£30,000 per QALY) | £49M | See Chapter 11 |
| Number with morbid obesity if the CCG is in the most deprived quintile (IMD2010) | 10,813 (7663 women; 3150 men) | Prevalence of obesity by deprivation quintile from Health Survey for England 2011–13 combined182 |
| Number with morbid obesity if the CCG is in the least deprived quintile (IMD2010) | 4413 (2838 women; 1575 men) |
FIGURE 14.
Prevalence of morbid obesity by gender and deprivation category. Data are for adults aged 20–75 years from Health Survey for England 2011–13 (n = 18,454). 182
Among adults, T2DM is largely a condition affecting those who are overweight or obese (Figure 15). In England, the prevalence of diabetes is 3.3% in men of normal weight but 28.1% among those with morbid obesity. In women, the equivalent figures are 1.4% and 15.6%. Among ethnic minority groups, diabetes may be more frequent among women. In a typical primary care organisation, there may be 1406 people with morbid obesity and diabetes mellitus.
FIGURE 15.
Prevalence of diabetes mellitus by obesity category. Data are for adults aged 20–75 years from Health Survey for England 2011–13 (n = 18,454).
At present rates of bariatric surgery, reported in Chapter 6, there may be 25 bariatric surgery procedures per year, of which between 8 and 12 may be performed privately (see Chapter 6). The empirical data and modelling results presented in this report identify the likely costs and outcomes of increasing access to bariatric surgery within a primary care organisation (see Table 39). If a decision is made to invest in 1000 bariatric surgical procedures over a defined period of time, then the immediate NHS costs will amount to £9.2M at 2014 prices. The total additional costs to the NHS, over the patients’ lifetime, are estimated to be £15.3M. If bariatric surgery procedures are divided equally between those with diabetes and those without, then there will be 112 fewer new diagnoses of diabetes over the next 10 years among the 500 non-diabetic patients who receive bariatric surgery (see Chapter 7). Among the 500 diabetic patients who receive bariatric surgery, at least 200 are expected to enter remission, with improved diabetes control and reduced antidiabetic drug utilisation among the remainder (see Chapter 8). Other modelled benefits from bariatric surgery include reduced incidence of cardiovascular diseases and cancer, lower mortality and increased well-being (see Chapter 11). Together, these are predicted to contribute 2142 additional QALYs over these patients’ lifetime. There may be additional benefits from bariatric surgery, for example on respiratory disorders or arthritis, that have not been included in the model. If the modelled health gains are valued at £30,000 per QALY, then these may be equated with a gain of £64.3M. There will be a net monetary benefit of £49M.
Efforts to expand access to bariatric surgery will need to address the question of which patients should receive surgery:
-
Morbid obesity is strongly associated with deprivation, as noted above. If patients are referred and selected for surgery on an equitable basis, then health benefits from bariatric surgery will generally flow to more deprived groups. The cost-effectiveness of bariatric surgery is not expected to differ greatly between socioeconomic groups (see Chapter 11).
-
Bariatric surgery has mainly been utilised by women, with 80% of the CPRD cohort being female (see Chapter 6). Estimates of cost-effectiveness do not suggest that there are important gender differences that could justify continuing gender differences in access to bariatric surgery (see Chapter 11).
-
Bariatric surgery is presently utilised most frequently between the ages of 35 and 54 years (see Chapter 6). Estimates of cost-effectiveness do not favour a clear strategy with respect to age. Younger patients have a greater life expectancy, but lower incidence of disease, while older adults have a higher incidence of disease that may be associated with greater absolute risk reductions from intervention. However, there is presently insufficient evidence to stratify intervention effects of bariatric surgery by age.
-
Use of bariatric surgery by people with diabetes appears to be slightly more cost-effective, with higher incremental QALYs and slightly lower incremental costs, than for non-diabetic individuals. As noted above, bariatric surgery in 500 individuals without diabetes may reduce new diabetes diagnoses by 112 over 10 years, while bariatric surgery in 500 diabetic individuals might lead to 200 cases of diabetes remission. This calculation might suggest that a secondary prevention strategy is to be preferred, but the simplifying assumption that the prognosis of individuals who previously had diabetes is similar to that of individuals who never had diabetes may not be justified. Research has not been conducted to compare policies of targeting bariatric surgery at diabetic as compared with non-diabetic individuals. Both diabetic and non-diabetic individuals have clear capacity to benefit from bariatric surgery and the present results do not determine whether a primary or a secondary prevention strategy is to be preferred with respect to diabetes. Within the diabetic population, evidence may be insufficient to distinguish policies of intervening with bariatric surgery for morbid, severe or simple obesity, as proposed by the IDF, with bariatric surgery presently being utilised only at low levels even among people with morbid obesity.
Strengths and limitations
Empirical data analyses for this study were based on representative patient cohorts, with long-term follow-up of participants. Health-care costs were derived prospectively and represented actual use rather than relying on an indirect measure such as subsequent insurance claims data. However, as a consequence of using clinical data there are also limitations to consider. First, bariatric surgery is offered to a very highly selected group of eligible patients in the UK, and these patients are likely to have characteristics that distinguish them from other very obese patients who have not been selected clinically, or through self-referral, for bariatric surgery. For instance, they may have a different morbidity profile and receive greater levels of care and surveillance than other patients. These factors may have introduced bias into our estimates of morbidity improvement when compared with matched controls who did not undergo surgery. We estimated a lower rate of diabetes remission than much of the published literature. Despite the potential differences in medical care provided to surgery patients, recording of body weight subsequent to surgery was poor. Although it would have been of great interest to be able to investigate changes in body weight after surgery using primary care records, we were less concerned about their importance for estimating changes in morbidity, as these effects are at least partly independent of weight loss. Nonetheless, we relied on matched cohort studies to assess the relationship between surgery and disease outcomes. Although the majority of these estimates were supported by the published literature, this does not reverse the need for more long-term studies on the outcomes of bariatric surgery, including RCTs. We acknowledge that we did not conduct a full systematic review of the outcomes of bariatric surgery because this was beyond the scope of this research. Instead, we conducted a rapid review that focused on recently reported systematic reviews and meta-analyses. In addition to this qualification, bariatric surgical procedures continue to evolve with new procedures developed and changes in the epidemiology of those popularly used and the patients upon which they are performed. Although our results are based on the three most commonly used procedures currently, these changes need to be considered and up-to-date evidence provided over time.
Patient and public involvement
Below we outline the ways in which we have sought to both include patient perspectives into the study and its findings as well as engage with the public.
Media interaction
The findings of this research have been reported widely within the media. The research team gave radio interviews to BBC London Live and the Today programme on BBC Radio 4. A summary of newspaper and online articles is presented in Box 2.
Eunjung Cha A. Could radical weight-loss surgery help prevent type 2 diabetes? The Washington Post, 3 November 2014. URL: www.washingtonpost.com/news/to-your-health/wp/2014/11/03/could-radical-weight-loss-surgery-help-prevent-type-2-diabetes/.
Lehman S. Weight loss surgery may help prevent diabetes. Reuters, 18 November 2014. URL: www.reuters.com/article/us-bariatric-surgery-diabetes-idUSKCN0J22MU20141118.
Willey J. New research says surgery could be key to beating diabetes. Express, 3 November 2014. URL: www.express.co.uk/life-style/health/530689/Surgery-key-to-beating-diabetes.
Weight loss surgery reduces diabetes risk. The Times of India, 3 November 2014. URL: http://timesofindia.indiatimes.com/life-style/health-fitness/health-news/Weight-loss-surgery-reduces-diabetes-risk/articleshow/45020847.cms.
Sifferlin A. Weight loss surgery lowers risk for Type 2 Diabetes, study suggests. TIME, 3 November 2014. URL: http://time.com/3554426/weight-loss-type-2-diabetes/.
Weight loss surgery cuts diabetes risk in very obese. NHS Choices, 3 November 2014. URL: www.nhs.uk/news/2014/11November/Pages/Weight-loss-surgery-cuts-diabetes-risk-in-very-obese.aspx.
Gallagher J, Weight loss surgery reduces diabetes risk. BBC News, 3 November 2014. URL: www.bbc.co.uk/news/health-29847059.
Monbiot G. Obesity is an incurable disease. So why is the government intent on punishing its sufferers? The Guardian, 11 August 2015. URL: www.theguardian.com/commentisfree/2015/aug/11/obesity-incurable-disease-cameron-punishing-sufferers.
Norton A. Most obese people will never reach normal weight. WebMD, 16 July 2015. URL: www.webmd.com/diet/obesity/20150716/most-obese-people-will-never-reach-normal-weight-study.
Lay K. Obese men have a one in 210 chance of slimming down. The Times, 17 July 2015. URL: www.thetimes.co.uk/tto/health/news/article4500533.ece.
Spencer B. Losing weight really IS impossible: the vast majority of people who pile on the pounds never lose them in the long run. Daily Mail, 16 July 2015. URL: www.dailymail.co.uk/health/article-3164042/Majority-people-pile-pounds-never-lose-them.html.
Obesity: ‘slim chance’ of return to normal weight. BBC News, 17 July 2015. URL: www.bbc.co.uk/news/health-33551498.
Odds are against reaching normal body weight for adults with obesity. Healio, Endocrine Today, 17 July 2015. URL: www.healio.com/endocrinology/obesity/news/online/%7B71c46386-9b44-4eb8-8506-0c9f05b96e57%7D/odds-are-against-reaching-normal-body-weight-for-adults-with-obesity.
McCall B. Only one in 210 obese men reach healthy weight. Medscape, 23 July 2015. URL: www.medscape.com/viewarticle/848544.
Last accessed date for URLs: 4 March 2016.
Patient engagement
Members of the research team attended a meeting of post-bariatric surgery patients at St George’s Hospital in London. The team presented information relating to the study and invited the 16 patients present to feedback comments and ideas relating to the project and future research. The study was well received by the group and elicited a good deal of feedback. Areas that the patients commented on included the need for better support both prior and subsequent to the procedure, particularly in relation to dealing with the psychosocial issues that encouraged their overeating. A key point was the need to improve support for patients in addition to increasing access to surgery. The demotivating effect of trying to reach a ‘normal’ BMI rather than focusing on weight lost was also discussed. Some patients reported their anxiety about weight regain and unpleasant side effects from surgery, such as loose skin and hair loss. Favourable comments were also made about the weight loss and diabetes remission. We plan to disseminate findings of the study to patients by producing a short summary leaflet and attending a further patient group meeting.
Presentation of results to professionals
Members of the research team have presented aspects of the study findings at the following conferences and meetings:
-
International Federation for the Surgery of Obesity and Metabolic Disorders, Montreal, Canada, 26–30 August 2014
-
Social Society for Medicine, Oxford, UK, 10–12 September 2014
-
Obesity Week, California, USA, 2–6 November 2015.
Overview of patient and public involvement
It was very informative for the team to attend the patient meeting and hear their thoughts on the research. We took on board their suggestions for future research, and although we were not able to include them in the current analyses we have included them in the research recommendations and have referred to them in the interpretation of our results where possible. We were delighted that the publications produced during the study received attention in the mainstream media. As a result of this coverage we were contacted by health professionals who work with bariatric surgery patients, who said that they would be communicating the findings to their patients.
Were the initial research objectives of the project met?
The research has provided new knowledge concerning the expected costs and outcomes of increasing access to bariatric surgery. The initial objectives of the research specifically aimed to evaluate three intervention strategies:
-
Expanding access within existing recognised indications for bariatric surgery as defined by NICE: this objective has been fully met. We have evaluated the costs and outcomes of increasing access to bariatric surgery for patients with morbid obesity, as well as severe obesity and diabetes. Diabetes is the chronic disease that is most strongly associated with obesity.
-
Expanding access to bariatric surgery for people with T2DM as proposed by the IDF: the recommendations of the IDF are for increased use of bariatric surgery among people with severe and morbid obesity. This has been evaluated as part of the research for objective 1. The IDF also made a recommendation that bariatric surgery should be considered for patients with BMI 30–34.9 kg/m2 who had poorly controlled diabetes with medical therapy, or who were at increased risk of cardiovascular disease. This second part of the IDF recommendation has not been fully addressed. We judged that the group of patients with BMI ≥ 35 kg/m2 would generally take priority in the selection of patients for bariatric surgery. We also concluded that issues of clinical judgement in patient selection would be of critical importance in implementing the second part of the IDF recommendation. We also concluded that trial evidence would require reviewing separately for patients with simple obesity and diabetes. There were therefore several reasons why this part of the IDF recommendation was not considered in this project, which aimed to have a broad population focus.
-
Expanding access with a focus on the distributional consequences of different intervention strategies: the research provided evidence of the extent to which health outcomes and costs of bariatric surgery vary by gender, age group and among socioeconomic groups, thus evaluating the potential impacts on inequalities in health related to obesity.
Research recommendations
Methodological
This research demonstrates the value of EHRs for long-term follow-up of participants who have received defined health care interventions. Future research might aim to recruit patients into randomised studies with long-term follow-up of substantive clinical outcomes.
Basic research
Improved understanding of the mechanisms of effect of bariatric surgical procedures may yield insights that can be used to inform more effective interventions for people with morbid obesity.
Health technology assessment
-
Research is needed, using efficient study designs, to evaluate the long-term effects of currently used bariatric surgical procedures, across a comprehensive range of outcome measures.
-
Estimates of intervention effects from bariatric surgery should be stratified by age group and morbidity category (especially diabetes status). This might enable evaluation of optimal age and morbidity distributions to be prioritised to receive bariatric surgery and inform patient selection.
-
The value of CPRD for deriving current clinical data on bariatric surgery use and outcomes has been established during this project. Continued observation of this cohort over time may provide further insights into the evolving use of procedures and related outcomes.
-
Research to define interventions to improve mental health outcomes of people with morbid obesity.
Service organisation and delivery research
-
Research to map and quantify disparities in access to bariatric surgery in relation to need.
-
Multi-method research on organisational and service barriers to accessing bariatric surgery, incorporating views and experiences of policy-makers, commissioners, managers, clinicians and patients.
-
Research on patient experience including pre- and post-operative patient support, weight regain after surgery, weight loss targets and how this information is presented to patients.
-
Evaluation of strategies for scaling up bariatric surgery for the treatment of obesity, while maintaining a safe service.
-
Research to define strategies for organising and delivering care to optimise mental and physical health outcomes of people with morbid obesity. This includes primary disease prevention, in the context of NHS Health Checks, and secondary prevention, in the context of primary care management of multi-morbidity.
Acknowledgements
This study is based in part on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The views expressed are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Contributions of authors
Martin C Gulliford (Professor of Public Health) designed the study, conducted the analyses, interpreted the results and drafted the report. He is guarantor of the study.
Judith Charlton (Research Associate) helped to design the study, conducted the analyses, programmed the health economic model and commented and approved the report.
Helen P Booth (Research Associate) contributed to CPRD data analysis and reporting and assisted in drafting the report.
Alison Fildes (Research Associate) assisted in drafting the report.
Omar Khan (Consultant Surgeon) helped with the design of the study, advised on clinical aspects and costing of surgery, contributed to interpretation of the results and commented on and approved the report.
Marcus Reddy (Consultant Surgeon) helped with the design of the study, advised on clinical aspects and costing of surgery, contributed to interpretation of the results and commented on and approved the report.
Mark Ashworth (Clinical Senior Lecturer) helped to design the study, interpret the results and commented on and approved the report.
Peter Littlejohns (Professor of Public Health) helped with the study design, advised on ethical issues relating to use of bariatric surgery, assisted with interpretation of the results and commented on and approved the report.
A Toby Prevost (Professor of Medical Statistics) helped to design the study, advised on the design and conduct of the statistical analyses, helped to interpret the results and commented on and approved the report.
Caroline Rudisill (Associate Professor of Health Economics) helped to design the study, advised on health economics and commented on and approved the report.
Publications
Booth HP, Khan O, Prevost AT, Reddy M, Charlton J, Gulliford MC. Impact of bariatric surgery on clinical depression. Interrupted time series study with matched controls. J Affect Dis 2014;174:644–9.
Booth HP, Khan O, Prevost AT, Reddy M, Dregan A, Charlton J, et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol 2014;2:963–8.
Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health 2015;105:e54–9.
Booth HP, Khan O, Fildes A, Prevost AT, Reddy M, Charlton J, et al. Changing epidemiology of bariatric surgery in the UK: cohort study using primary care electronic health records [published online ahead of print 12 January 2016]. Obesity Surgery 2016.
Rudisill C, Charlton J, Booth HP, Gulliford MC. Are healthcare costs from obesity associated with body mass index, comorbidity or depression? Cohort study using electronic health records [published online ahead of print 21 April 2016]. Clin Obes 2016.
Data sharing statement
Clinical Practice Research Datalink data were analysed under licence and are not available for sharing.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet n.d.;377:557-67. http://dx.doi.org/10.1016/S0140-6736(10)62037-5.
- van Jaarsveld CHM, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population-based cohort study. Arch Dis Child 2015;100:214-19. http://dx.doi.org/10.1136/archdischild-2014-307151.
- Booth HP, Prevost AT, Gulliford MC. Impact of body mass index on prevalence of multimorbidity in primary care: cohort study. Fam Pract 2014;31:38-43. http://dx.doi.org/10.1093/fampra/cmt061.
- Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet n.d.;368:666-78. http://dx.doi.org/10.1016/S0140-6736(06)69251-9.
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Eng J Med 2007;357:741-52. http://dx.doi.org/10.1056/NEJMoa066254.
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5934.
- Booth H, Khan O, Prevost T, Reddy M, Dregan A, Charlton J, et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diab Endocrin 2014;2:963-8. http://dx.doi.org/10.1016/S2213-8587(14)70214-1.
- Carlsson LMS, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, . Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Eng J Med 2012;367:695-704. http://dx.doi.org/10.1056/NEJMoa1112082.
- Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219-34. http://dx.doi.org/10.1111/joim.12012.
- Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62-70. http://dx.doi.org/10.1001/jama.2014.16968.
- Welbourn R, Small P, Hewin D, Reddy M, Somers S, Sedman P, et al. National Bariatric Surgery Register Second Registry Report. Henley-on-Thames: Dendrite Clinical Systems Ltd; 2014.
- Neovius M, Narbro K, Keating C, Peltonen M, Sjoholm K, Agren G, et al. Health care use during 20 years following bariatric surgery. JAMA 2012;308:1132-41. http://dx.doi.org/10.1001/2012.jama.11792.
- Weiner JP, Goodwin SM, Chang H, Bolen SD, Richards TM, Johns RA, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surgery 2013;148:555-61. http://dx.doi.org/10.1001/jamasurg.2013.1504.
- Adams TD, Davidson LE, Litwin SE, Kolotkin RA, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012;308:1122-31. http://dx.doi.org/10.1001/2012.jama.11164.
- Sultan S, Gupta D, Parikh M, Youn H, Kurian M, Fielding G, et al. Five-year outcomes of patients with type 2 diabetes who underwent laparoscopic adjustable gastric banding. Surg Obes Rel Dis 2010;6:373-6. http://dx.doi.org/10.1016/j.soard.2010.02.043.
- Health Survey for England 2008 Trend Tables. NHS Information Centre; 2009.
- James P, Detels R, Beaglehole R, Lansang MA, Gulliford M. Oxford Textbook of Public Health. Oxford: Oxford University Press; 2009.
- Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014;384:755-65. http://dx.doi.org/10.1016/S0140-6736(14)60892-8.
- Parkin DB, Boyd L. Cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer 2011;105:S34-7. http://dx.doi.org/10.1038/bjc.2011.481.
- Prospective Studies Collaboration . Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. http://dx.doi.org/10.1016/S0140-6736(09)60318-4.
- Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Eng J Med 2006;355:763-78. http://dx.doi.org/10.1056/NEJMoa055643.
- McLaren L. Socioeconomic status and obesity. Epidemiol Rev 2007;29:29-48. http://dx.doi.org/10.1093/epirev/mxm001.
- Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006–07 NHS costs. J Pub Health 2011;33:527-35. http://dx.doi.org/10.1093/pubmed/fdr033.
- Wang YC, McPherson K, Marsh, T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815-25. http://dx.doi.org/10.1016/S0140-6736(11)60814-3.
- Terranova L, Busetto L, Vestri A, Zappa M. Bariatric surgery: cost-effectiveness and budget impact. Obes Surg 2012;22:646-53. http://dx.doi.org/10.1007/s11695-012-0608-1.
- O’Neill P. Shedding the Pounds: Obesity Management and Bariatric Surgery in England. London: Office for Health Economics; 2010.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8210.
- Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2014-006642.
- Booth HP, Prevost AT, Wright AJ, Gulliford MC. Effectiveness of behavioral weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam Pract 2014;31:643-53. http://dx.doi.org/10.1093/fampra/cmu064.
- Loveman E, Frampton G, Shepherd J, Picot J, Cooper K, Bryant J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15020.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13410.
- Chang S, Stoll CT, Song J, Varela J, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg 2014;149:275-87. http://dx.doi.org/10.1001/jamasurg.2013.3654.
- Kaul A, Sharma J. Impact of bariatric surgery on comorbidities. Surg Clin North Am 2011;91:1295-312. http://dx.doi.org/10.1016/j.suc.2011.08.003.
- Ackroyd R, Mouiel J, Chevallier JM, Daoud F. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg 2006;16:1488-503. http://dx.doi.org/10.1381/096089206778870067.
- Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults. London: NICE; 2014.
- Dent M, Chrisopolous S, Mulhall C, Ridler C. Bariatric Surgery for Obesity. National Obesity Observatory; 2010.
- Statistics on Obesity, Physical Activity and Diet 2015. London: Health and Social Care Information Centre; 2015.
- Bariatric Surgical Service for the Treatment of People with Severe Obesity. Commissioning Guide. NICE; 2012.
- Bariatric Surgical and Procedural Intervention in the Treatment of Obese Patients with type 2 Diabetes. Brussels: IDF; 2012.
- Briggs ACK, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Judging Whether Public Health Interventions Offer Value for Money. London: NICE; 2013.
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4-14. http://dx.doi.org/10.1111/j.1365-2125.2009.03537.x.
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012;3:89-9. http://dx.doi.org/10.1177/2042098611435911.
- Booth HP, Prevost AT, Gulliford MC. Epidemiology of clinical body mass index recording in an obese population in primary care: a cohort study. J Public Health (Oxf) 2013;35:67-74. http://dx.doi.org/10.1093/pubmed/fds063.
- English Indices of Deprivation 2010. Department for Communities and Local Government; 2011.
- Health and Social Care Information Centre . Hospital Episode Statistics n.d. www.hscic.gov.uk/hes.
- Stewart JA, Dundas R, Howard RS, Rudd AG, Wolfe CDA. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ 1999;318:967-71. http://dx.doi.org/10.1136/bmj.318.7189.967.
- Charlton J, Latinovic R, Gulliford MC. Explaining the decline in early mortality in men and women with type 2 diabetes. Population-based cohort study. Diabetes Care 2008;31:1761-6. http://dx.doi.org/10.2337/dc08-0137.
- Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Coding, Recording and Incidence of different forms of coronary heart disease in primary care. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0029776.
- Gulliford MC, Charlton J, Ashworth M, Rudd AG, Toschke AM. eCRT Research Team . Selection of medical diagnostic codes for analysis of electronic patient records. Application to stroke in a primary care database. PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0007168.
- Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Prevalence of depression and utilization of health care in single and multiple morbidity: a population-based cohort study. Psychol Med 2013;43:1423-31. http://dx.doi.org/10.1017/S0033291712002498.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Duan N, Manning WG, Morris CN, Newhouse JP. A comparison of alternative models for the demand of medical care. J Bus Econ Stat 1983;1:115-26.
- Dunn G, Mirandola M, Amaddeo F, Tansella M. Describing, explaining or predicting mental health care costs: a guide to regression models. Br J Psychiatry 2003;183:398-404. http://dx.doi.org/10.1192/bjp.183.5.398.
- World Health Organization . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation 2011. www.who.int/diabetes/publications/report-hba1c_2011.pdf (accessed 4 September 2014).
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999;94:496-509. http://dx.doi.org/10.1080/01621459.1999.10474144.
- Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, et al. how do we define cure of diabetes?. Diabetes Care 2009;32:2133-5. http://dx.doi.org/10.2337/dc09-9036.
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Academic Pediatrics 2013;13:S38-44. http://dx.doi.org/10.1016/j.acap.2013.08.002.
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20. http://dx.doi.org/10.1016/j.jclinepi.2008.10.009.
- Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diab Obes Metabol 2014;16:719-27. http://dx.doi.org/10.1111/dom.12270.
- Ricci C, Gaeta M, Rausa E, Asti E, Bandera F, Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg 2015;25:397-405. http://dx.doi.org/10.1007/s11695-014-1442-4.
- Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 2014;173:20-8. http://dx.doi.org/10.1016/j.ijcard.2014.02.026.
- Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surgical Endoscopy 2013;27:4449-56. http://dx.doi.org/10.1007/s00464-013-3127-9.
- Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg 2014;24:1499-509. http://dx.doi.org/10.1007/s11695-014-1276-0.
- Maestro A, Rigla M, Caixas A. Does bariatric surgery reduce cancer risk? A review of the literature. Endocrinologia Y Nutricion 2015;62:138-43. http://dx.doi.org/10.1016/j.endonu.2014.12.005.
- Yang XW, Li PZ, Zhu LY, Zhu S. Effects of bariatric surgery on incidence of obesity-related cancers: a meta-analysis. Med Sci Monit 2015;21:1350-7. http://dx.doi.org/10.12659/MSM.893553.
- Afshar S, Kelly SB, Seymour K, Lara J, Woodcock S, Mathers JC. The effects of bariatric surgery on colorectal cancer risk: systematic review and meta-analysis. Obes Surg 2014;24:1793-9. http://dx.doi.org/10.1007/s11695-014-1359-y.
- Clegg A, Colquitt J, Sidhu M, Royle P, Walker A. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. Int J Obes Relat Metab Disord 2003;27:1167-77. http://dx.doi.org/10.1038/sj.ijo.0802394.
- Ferchak CV, Meneghini LF. Obesity, bariatric surgery and type 2 diabetes – a systematic review. Diabetes Metab Res Rev 2004;20:438-45. http://dx.doi.org/10.1002/dmrr.507.
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-56. http://dx.doi.org/10.1016/j.amjmed.2008.09.041.
- Guo X, Liu X, Wang M, Wei F, Zhang Y, Zhang Y. The effects of bariatric procedures versus medical therapy for obese patients with type 2 diabetes: meta-analysis of randomized controlled trials. BioMed Res Int 2013;2013. http://dx.doi.org/10.1155/2013/410609.
- Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg 2014;24:437-55. http://dx.doi.org/10.1007/s11695-013-1160-3.
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;8. http://dx.doi.org/10.1002/14651858.cd003641.pub4.
- Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AH, Hu J, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA 2013;309:2250-61. http://dx.doi.org/10.1001/jama.2013.4851.
- Picot J, Jones J, Colquitt J, Loveman E, Clegg A. Weight loss surgery for mild to moderate obesity: a systematic review and economic evaluation. Obes Surg 2012;22:1496-506. http://dx.doi.org/10.1007/s11695-012-0679-z.
- Müller-Stich BP, Senft JD, Warschkow R, Kenngott HG, Billeter AT, Vit G, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg 2015;261:421-9. http://dx.doi.org/10.1097/SLA.0000000000001014.
- Yip S, Plank LD, Murphy R. Gastric bypass and sleeve gastrectomy for type 2 diabetes: a systematic review and meta-analysis of outcomes. Obes Surg 2013;23. http://dx.doi.org/10.1007/s11695-013-1030-z.
- Zhang C, Yuan Y, Qiu C, Zhang W. A meta-analysis of 2-year effect after surgery: laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus. Obes Surg 2014;24:1528-35. http://dx.doi.org/10.1007/s11695-014-1303-1.
- Cho JM, Kim HJ, Menzo EL, Park S, Szomstein S, Rosenthal RJ. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Rel Dis 2015;11:1273-80. http://dx.doi.org/10.1016/j.soard.2015.03.001.
- Zhang Y, Ju W, Sun X, Cao Z, Xinsheng X, Daquan L, et al. Laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass for morbid obesity and related comorbidities: a meta-analysis of 21 studies. Obes Surg 2015;25:19-26. http://dx.doi.org/10.1007/s11695-014-1385-9.
- Kubik JF, Gill RS, Laffin M, Karmali S. The impact of bariatric surgery on psychological health. J Obes 2013;2013. http://dx.doi.org/10.1155/2013/837989.
- Herpertz S, Kielmann R, Wolf AM, Langkafel M, Senf W, Hebebrand J. Does obesity surgery improve psychosocial functioning? A systematic review. Int J Obes Relat Metab Disord 2003;27:1300-14. http://dx.doi.org/10.1038/sj.ijo.0802410.
- Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg 2011;253:484-7. http://dx.doi.org/10.1097/SLA.0b013e31820d98cb.
- Wang BCM, Furnback W. Modelling the long-term outcomes of bariatric surgery: a review of cost-effectiveness studies. Best Pract Res Clin Gastroenterol 2013;27:987-95. http://dx.doi.org/10.1016/j.bpg.2013.08.022.
- Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, et al. Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med 2011;26:1183-94. http://dx.doi.org/10.1007/s11606-011-1721-x.
- Gulliford MC, Charlton J, Bhattarai N, Charlton C, Rudisill C. Impact and cost-effectiveness of a universal strategy to promote physical activity in primary care: population-based cohort study and Markov model. Eur J Health Econ 2014;15:341-51. http://dx.doi.org/10.1007/s10198-013-0477-0.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. http://dx.doi.org/10.1177/0272989X11401031.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. Section 6: The Appraisal of the Evidence and Structured Decision-Making n.d. www.nice.org.uk/article/pmg9/chapter/6-The-appraisal-of-the-evidence-and-structured-decision-making (accessed August 2015).
- Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653-62. http://dx.doi.org/10.1016/S1470-2045(09)70159-7.
- Booth H, Khan O, Prevost AT, Reddy M, Charlton J, Gulliford MC. King’s Bariatric Surgery Group . Impact of bariatric surgery on clinical depression. Interrupted time series study with matched controls. J Affect Disord 2015;174:644-9. http://dx.doi.org/10.1016/j.jad.2014.12.050.
- Hakim Z, Wolf A, Garrison LP. Estimating the effect of changes in body mass index on health state preferences. Pharmacoeconomics 2002;20:393-404. http://dx.doi.org/10.2165/00019053-200220060-00004.
- Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes 2007;31:1248-61. http://dx.doi.org/10.1038/sj.ijo.0803573.
- Bransen J, Gilissen LL, van Rutte PJ, Nienhuijs S. Costs of leaks and bleeding after sleeve gastrectomies. Obes Surg 2015;10:1767-71. http://dx.doi.org/10.1007/s11695-015-1584-z.
- Charlton J, Rudisill AC, Bhattarai N, Gulliford MC. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J Health Serv Res Policy 2013;18:215-23. http://dx.doi.org/10.1177/1355819613493772.
- Centers for Disease Control and Prevention . Losing Weight n.d. www.cdc.gov/healthyweight/losing_weight/index.html (accessed 24 February 2015).
- Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA 2014;312:1779-91. http://dx.doi.org/10.1001/jama.2014.14173.
- Fildes A CJ, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Pub Health 2015;105:e54-9. http://dx.doi.org/10.2105/AJPH.2015.302773.
- Consultation on Potential New Indicators for Inclusion in the NICE Clinical Commissioning Group Outcomes Indicator Set (CCG OIS) Indicator Menu. London: NICE; 2015.
- Dombrowski SU, Avenell A, Sniehotta FF. Behavioural interventions for obese adults with additional risk factors for morbidity: systematic review of effects on behaviour, weight and disease risk factors. Obesity Facts 2010;3:377-96. http://dx.doi.org/10.1159/000323076.
- Kleinert S, Horton R. Rethinking and reframing obesity. Lancet 2015;385:2326-8. http://dx.doi.org/10.1016/S0140-6736(15)60163-5.
- Huang TTK, Cawley JH, Ashe M, Costa SA, Frerichs LM, Zwicker L, et al. Mobilisation of public support for policy actions to prevent obesity. Lancet 2015;385:2422-31. http://dx.doi.org/10.1016/S0140-6736(14)61743-8.
- Hawkes C, Smith TG, Jewell J, Wardle J, Hammond RA, Friel S, et al. Smart food policies for obesity prevention. Lancet 2015;385:2410-21. http://dx.doi.org/10.1016/S0140-6736(14)61745-1.
- Roberto CA, Swinburn B, Hawkes C, Huang TTK, Costa SA, Ashe M, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet 2015;385:2400-9. http://dx.doi.org/10.1016/S0140-6736(14)61744-X.
- Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, et al. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet 2015;385:2521-33. http://dx.doi.org/10.1016/S0140-6736(14)61748-7.
- Zajacova A, Ailshire J. Body mass trajectories and mortality among older adults: a joint growth mixture-discrete-time survival analysis. Gerontologist 2014;54:221-31. http://dx.doi.org/10.1093/geront/gns164.
- Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Newman AB, Koster A, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc 2014;62:1476-83. http://dx.doi.org/10.1111/jgs.12954.
- Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol 2007;22:665-73. http://dx.doi.org/10.1007/s10654-007-9167-5.
- Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med 2002;162:2575-80. http://dx.doi.org/10.1001/archinte.162.22.2575.
- Mehta T, Smith DL, Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes Rev 2014;15:870-81. http://dx.doi.org/10.1111/obr.12222.
- Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obes Rev 2005;6:43-65. http://dx.doi.org/10.1111/j.1467-789X.2005.00166.x.
- Jackson SE, Beeken RJ, Wardle J. Predictors of weight loss in obese older adults: findings from the USA and the UK. Obes Facts 2014;7:102-10. http://dx.doi.org/10.1159/000362196.
- Gregory CO, Blanck HM, Gillespie C, Maynard LM, Serdula MK. Health perceptions and demographic characteristics associated with underassessment of body weight. Obesity 2008;16:979-86. http://dx.doi.org/10.1038/oby.2008.22.
- Duncan D, Wolin K, Scharoun-Lee M, Ding E, Warner E, Bennett G. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. Int J Behav Nut Phys Act 2011;8. http://dx.doi.org/10.1186/1479-5868-8-20.
- Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nut 2005;82:222S-5S.
- Yaemsiri S, Slining MM, Agarwal SK. Perceived weight status, overweight diagnosis, and weight control among US adults: the NHANES 2003–2008 Study. Int J Obes 2011;35:1063-70. http://dx.doi.org/10.1038/ijo.2010.229.
- Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese U.S. adults. Am J Prevent Med 2012;42:481-5. http://dx.doi.org/10.1016/j.amepre.2012.01.005.
- Ma J, Xiao L, Stafford RS. Adult obesity and office-based quality of care in the United States. Obesity 2009;17:1077-85. http://dx.doi.org/10.1038/oby.2008.653.
- Reife CM. Involuntary weight loss. Med Clin North Am 1995;79:299-313.
- Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet 2007;370:1960-73. http://dx.doi.org/10.1016/S0140-6736(07)61816-9.
- Lankisch PG, Gerzmann M, Gerzmann JF, Lehnick D. Unintentional weight loss: diagnosis and prognosis. The first prospective follow-up study from a secondary referral centre. J Intern Med 2001;249:41-6. http://dx.doi.org/10.1046/j.1365-2796.2001.00771.x.
- Quality and Outcomes Framework. Leeds: Health and Social Care Information Centre; 2015.
- Rudisill C, Charlton J, Booth HP, Gulliford MC. Are healthcare costs from obesity associated with body mass index, comorbidity or depression? Cohort study using electronic health records [published online ahead of print 21 April 2016]. Clin Obes 2016.
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econom 2012;31:219-30. http://dx.doi.org/10.1016/j.jhealeco.2011.10.003.
- Mora T, Gil J, Sicras-Mainar A. The influence of obesity and overweight on medical costs: a panel data perspective. Eur J Health Econom 2015;16:161-73. http://dx.doi.org/10.1007/s10198-014-0562-z.
- Østbye T, Stroo M, Eisenstein EL, Peterson B, Dement J. Is overweight and class I obesity associated with increased health claims costs?. Obesity 2014;22:1179-86. http://dx.doi.org/10.1002/oby.20669.
- Shen Y, Sambamoorthi U, Rajan M, Miller D, Banerjea R, Pogach L. Obesity and expenditures among elderly veterans health administration users with diabetes. Popul Health Manag 2009;12:255-64. http://dx.doi.org/10.1089/pop.2008.0022.
- Jerant A, Bertakis KD, Franks P. Body mass index and health care utilization in diabetic and nondiabetic individuals. Med Care 2015;53:409-16. http://dx.doi.org/10.1097/MLR.0000000000000343.
- Wee CC, Phillips RS, Legedza ATR, Davis RB, Soukup JR, Colditz GA, et al. Health care expenditures associated with overweight and obesity among us adults: importance of age and race. Am J Public Health 2005;95:159-65. http://dx.doi.org/10.2105/AJPH.2003.027946.
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs 2009;28:w822-31. http://dx.doi.org/10.1377/hlthaff.28.5.w822.
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8:307-26. http://dx.doi.org/10.1111/j.1467-789X.2007.00347.x.
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37:922-33. http://dx.doi.org/10.2337/dc13-2195.
- Booth HP, Khan O, Fildes A, Prevost AT, Reddy M, Charlton J, et al. Changing epidemiology of bariatric surgery in the UK: cohort study using primary care electronic health records [published online ahead of print 12 January 2016]. Obes Surg 2016. http://dx.doi.org/10.1007/s11695-015-2032-9.
- The United Kingdom National Bariatric Surgery Registry: Second Registry Report. Henley-on-Thames: The British Obesity and Metabolic Surgery Society; 2014.
- Welbourn R, Fiennes A, Walton P, Kinsman R. National Bariatric Surgery Registry: First Registry Report. Henley-on-Thames: Dendrite Clinical Systems Ltd; 2010.
- Davis MM, Slish K, Chao C, Cabana MD. National trends in bariatric surgery, 1996–2002. Arch Surg 2006;141:71-4. http://dx.doi.org/10.1001/archsurg.141.1.71.
- Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416-25. http://dx.doi.org/10.1001/jama.2013.280928.
- Long SD, O’Brien K, MacDonald KG, Leggett-Frazier N, Swanson MS, Pories WJ, et al. Weight loss in severely obese subjects prevents the progression of impaired glucose tolerance to type II diabetes. A longitudinal interventional study. Diabetes Care 1994;17:372-5. http://dx.doi.org/10.2337/diacare.17.5.372.
- Pontiroli AE, Folli F, Paganelli M, Micheletto G, Pizzocri P, Vedani P, et al. Laparoscopic gastric banding prevents type 2 diabetes and arterial hypertension and induces their remission in morbid obesity: a 4-year case-controlled study. Diabetes Care 2005;28:2703-9. http://dx.doi.org/10.2337/diacare.28.11.2703.
- Wentworth JM, Playfair J, Laurie C, Ritchie ME, Brown WA, Burton P, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diab Endocrinol 2014;2:545-52. http://dx.doi.org/10.1016/S2213-8587(14)70066-X.
- Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet 2012;379:2300-11. http://dx.doi.org/10.1016/S0140-6736(12)60401-2.
- Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297-304. http://dx.doi.org/10.1001/jama.2014.5988.
- Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, et al. Can diabetes be surgically cured?: long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:628-37. http://dx.doi.org/10.1097/sla.0b013e3182a5034b.
- Lee W, Chong K, Ser K, Lee YC, Chen SC, Chen JC, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg 2011;146:143-8. http://dx.doi.org/10.1001/archsurg.2010.326.
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964-73. http://dx.doi.org/10.1016/S0140-6736(15)00075-6.
- Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol 2005;58:550-9. http://dx.doi.org/10.1016/j.jclinepi.2004.10.016.
- Guo F, Moellering DR, Garvey WT. Use of HbA1c for diagnoses of diabetes and prediabetes: comparison with diagnoses based on fasting and 2-hr glucose values and effects of gender, race, and age. Metab Syndr Relat Disord 2014;12:258-68. http://dx.doi.org/10.1089/met.2013.0128.
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psych 2010;67:220-9. http://dx.doi.org/10.1001/archgenpsychiatry.2010.2.
- Sarwer D, Cohn N, Gibbons L, Magee L, Crerand C, Raper S, et al. Psychiatric diagnoses and psychiatric treatment among bariatric surgery candidates. Obes Surg 2004;14:1148-56. http://dx.doi.org/10.1381/0960892042386922.
- Burgmer R, Petersen I, Burgmer M, de Zwaan M, Wolf A, Herpertz S. Psychological outcome two years after restrictive bariatric surgery. Obes Surg 2007;17:785-91. http://dx.doi.org/10.1007/s11695-007-9144-9.
- Dixon JB, Dixon ME, O’Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Int Med 2003;163:2058-65. http://dx.doi.org/10.1001/archinte.163.17.2058.
- Mitchell JE, King WC, Chen J-Y, Devlin MJ, Flum D, Garcia L, et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity 2014;22:1799-806. http://dx.doi.org/10.1002/oby.20738.
- De Zwaan M, Enderle J, Wagner S, Muhlhans B, Ditzen B, Gefeller O, et al. Anxiety and depression in bariatric surgery patients: a prospective, follow-up study using structured clinical interviews. J Affect Disord 2011;133:61-8. http://dx.doi.org/10.1016/j.jad.2011.03.025.
- Ivezaj V, Grilo C. When mood worsens after gastric bypass surgery: characterization of bariatric patients with increases in depressive symptoms following surgery. Obes Surg 2015;25:423-9. http://dx.doi.org/10.1007/s11695-014-1402-z.
- Herpertz S, Burgmer R, Stang A, de Zwaan M, Wolf AM, Chen-Stute A, et al. Prevalence of mental disorders in normal-weight and obese individuals with and without weight loss treatment in a German urban population. J Psychosom Res 2006;61:95-103. http://dx.doi.org/10.1016/j.jpsychores.2005.10.003.
- Burgmer R, Legenbauer T, Müller A, de Zwaan M, Fischer C, Herpertz S. Psychological outcome 4 years after restrictive bariatric surgery. Obes Surg 2014;24:1670-8. http://dx.doi.org/10.1007/s11695-014-1226-x.
- Andersen JR. Quality of life following laparoscopic sleeve gastrectomy. Surg Obes Rel Dis 2014;11:76-8. http://dx.doi.org/10.1016/j.soard.2014.06.003.
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 2003;19:613-23. http://dx.doi.org/10.1017/S0266462303000576.
- Rait G, Walters K, Griffin M, Buszewicz M, Petersen I, Nazareth I. Recent trends in the incidence of recorded depression in primary care. Br J Psych 2009;195:520-4. http://dx.doi.org/10.1192/bjp.bp.108.058636.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002;52:69-77. http://dx.doi.org/10.1016/S0022-3999(01)00296-3.
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77-100. http://dx.doi.org/10.1016/0272-7358(88)90050-5.
- Upala S, Anawin S. Bariatric surgery and risk of postoperative endometrial cancer: a systematic review and meta-analysis. Surg Obes Rel Dis 2015;11:949-55. http://dx.doi.org/10.1016/j.soard.2014.09.024.
- Sjöström CD, Peltonen M, Wedel H, Sjöström L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-5. http://dx.doi.org/10.1161/01.HYP.36.1.20.
- Merlotti C, Morabito A, Ceriani V, Pontiroli A. Prevention of type 2 diabetes in obese at-risk subjects: a systematic review and meta-analysis. Acta Diabetol 2014;51:853-63. http://dx.doi.org/10.1007/s00592-014-0624-9.
- Levy P, Fried M, Santini F, Finer N. The comparative effects of bariatric surgery on weight and type 2 diabetes. Obes Surg 2007;17:1248-56. http://dx.doi.org/10.1007/s11695-007-9214-z.
- Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-23. http://dx.doi.org/10.1001/jama.299.3.316.
- Yang J, Wang C, Cao G, Yang W, Yu S, Zhai H, et al. Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28–35 kg/m2. BMC Surg 2015;15. http://dx.doi.org/10.1186/s12893-015-0074-5.
- Thereaux J, Corigliano N, Poitou C, Oppert J-M, Czernichow S, Bouillot J-L. Comparison of results after one year between sleeve gastrectomy and gastric bypass in patients with BMI ≥ 50 kg/m2. Surg Obes Rel Dis 2015;11:785-90. http://dx.doi.org/10.1016/j.soard.2014.11.022.
- Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931-40. http://dx.doi.org/10.1001/jamasurg.2015.1534.
- Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS) – an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes 1998;22:113-26. http://dx.doi.org/10.1038/sj.ijo.0800553.
- Herpertz S, Müller A, Burgmer R, Crosby RD, de Zwaan M, Legenbauer T. Health-related quality of life and psychological functioning 9 years after restrictive surgical treatment for obesity. Surg Obes Rel Dis 2015;11:1361-70. http://dx.doi.org/10.1016/j.soard.2015.04.008.
- Guidry CA, Davies SW, Sawyer RG, Schirmer BD, Hallowell PT. Gastric bypass improves survival compared with propensity-matched controls: a cohort study with over 10-year follow-up. Am J Surg 2015;209:463-7. http://dx.doi.org/10.1016/j.amjsurg.2014.10.009.
- Pollock RF, Muduma G, Valentine WJ. Evaluating the cost-effectiveness of laparoscopic adjustable gastric banding versus standard medical management in obese patients with type 2 diabetes in the UK. Diabetes Obes Metab 2013;15:121-9. http://dx.doi.org/10.1111/j.1463-1326.2012.01692.x.
- Lee YY, Veerman JL, Barendregt JJ. The cost-effectiveness of laparoscopic adjustable gastric banding in the morbidly obese adult population of Australia. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0064965.
- Wang BM, Wong E, Alfonso-Cristancho R, He H, Flum D, Arterburn D, et al. Cost-effectiveness of bariatric surgical procedures for the treatment of severe obesity. Eur Journal Health Econ 2014;15:253-63. http://dx.doi.org/10.1007/s10198-013-0472-5.
- Borisenko O, Adam D, Funch-Jensen P, Ahmed A, Zhang R, Colpan Z, et al. Bariatric surgery can lead to net cost savings to health care systems: results from a comprehensive European decision analytic model. Obes Surg 2015;25:1559-68. http://dx.doi.org/10.1007/s11695-014-1567-5.
- Health Survey for England – 2012 Trend Tables. Leeds: Health and Social Care Information Centre; 2013.
- Maciejewski ML, Arterburn DE. Cost-effectiveness of bariatric surgery. JAMA 2013;310:742-3. http://dx.doi.org/10.1001/jama.2013.276131.
- Fildes A, Rudisill C, Charlton J, Gulliford M. Comment on ‘bariatric surgery can lead to net cost savings to health care systems: results from a comprehensive European decision analytic model’. Obes Surg 2015;25:1254-5. http://dx.doi.org/10.1007/s11695-015-1694-7.
- Report: Impact of Physical Activity and Diet on Health. London: The Stationery Office; 2015.
- Bariatric Surgery: Too Lean a Service?. London: NCEPOD; 2012.
- Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: NICE; 2006.
- Health Survey for England – 2013 Trend Tables. Leeds: Health and Social Care Information Centre; 2014.
Appendix 1 General practitioner questionnaire on bariatric surgery patients
Appendix 2 Additional results tables
| Measure | All | Male | Female | |||
|---|---|---|---|---|---|---|
| BS | No BS | BS | No BS | BS | No BS | |
| Costs | 126,836,121.81 | 97,822,340.46 | 118,400,495.64 | 91,241,063.75 | 135,276,143.77 | 104,409,362.78 |
| Costs DC35 | 67,251,683.64 | 51,993,824.43 | 63,992,984.42 | 49,024,080.05 | 70,511,968.19 | 54,965,655.38 |
| CostDC15 | 93,060,030.17 | 72,384,665.60 | 87,708,897.53 | 67,884,550.81 | 98,413,843.50 | 76,888,406.05 |
| QALYs | 28,345.05 | 22,772.64 | 27,526.08 | 22,284.14 | 29,158.05 | 23,253.09 |
| QALYs DC35 | 14,509.41 | 12,366.93 | 14,332.44 | 12,245.47 | 14,680.41 | 12,479.21 |
| QALYs DC15 | 20,547.16 | 17,022.33 | 20,128.72 | 16,757.24 | 20,959.56 | 17,278.53 |
| All life-years | 41,869.28 | 35,772.21 | 40,407.82 | 34,770.96 | 43,332.07 | 36,775.17 |
| Depression life-years | 4393.11 | 4385.58 | 2758.75 | 2788.49 | 6026.91 | 5982.95 |
| Life-years at risk | 22,296.44 | 11,998.61 | 19,548.83 | 10,699.63 | 25,046.19 | 13,296.59 |
| Life-years DM | 9434.01 | 17,754.62 | 9556.05 | 17,985.37 | 9310.55 | 17,527.22 |
| Life-years CHD | 5321.58 | 3771.50 | 6031.45 | 4167.85 | 4607.14 | 3374.40 |
| Life-years stroke | 1309.54 | 633.92 | 1264.04 | 624.09 | 1354.88 | 643.91 |
| Life-years cancer | 3507.70 | 1613.56 | 4007.44 | 1294.02 | 3013.32 | 1933.06 |
| Measure | Age 20–34 years | Age 35–54 years | Age 55–74 years | |||
|---|---|---|---|---|---|---|
| BS | No BS | BS | No BS | BS | No BS | |
| Costs | 162,802,898.40 | 130,904,334.92 | 132,488,841.51 | 103,034,399.09 | 88,562,965.62 | 62,590,058.83 |
| Costs DC35 | 68,175,090.26 | 54,554,520.65 | 70,788,350.76 | 55,790,378.00 | 59,487,707.66 | 42,481,582.10 |
| Costs DC15 | 106,243,818.53 | 86,314,167.65 | 97,908,327.02 | 77,166,636.27 | 73,479,839.77 | 52,375,922.36 |
| QALYs | 39,870.46 | 33,278.70 | 29,223.34 | 23,491.96 | 18,055.45 | 13,586.00 |
| QALYs DC35 | 17,153.23 | 15,286.99 | 15,029.65 | 12,890.59 | 11,545.05 | 9190.42 |
| QALYs DC15 | 26,444.01 | 22,878.23 | 21,308.87 | 17,707.97 | 14,684.95 | 11,352.60 |
| All life-years | 58,730.38 | 51,778.08 | 43,178.24 | 36,892.51 | 26,609.23 | 21,528.20 |
| Depression life-years | 6670.78 | 6728.21 | 4633.74 | 4629.18 | 2202.50 | 2141.60 |
| Life-years at risk | 33,304.16 | 18,911.61 | 22,506.81 | 11,947.94 | 13,618.81 | 6914.29 |
| Life-years DM | 13,262.11 | 25,823.41 | 9817.97 | 18,480.57 | 5794.04 | 10,254.75 |
| Life-years CHD | 6274.47 | 4340.60 | 5725.48 | 4060.51 | 3795.53 | 2763.76 |
| Life-years stroke | 1461.65 | 691.69 | 1347.01 | 650.45 | 1117.75 | 557.43 |
| Life-years cancer | 4427.99 | 2010.77 | 3780.97 | 1753.04 | 2283.10 | 1037.97 |
| Measure | BS | No BS |
|---|---|---|
| Costs | 128,590,497.64 | 100,136,128.52 |
| Costs DC35 | 68,079,271.04 | 53,082,707.75 |
| Costs DC15 | 94,294,469.84 | 74,019,668.93 |
| QALYs | 28,750.58 | 23,440.04 |
| QALYs DC35 | 14,708.08 | 12,752.62 |
| QALYs DC15 | 20,836.07 | 17,534.38 |
| All life-years | 42,373.22 | 36,526.57 |
| Depression life-years | 4195.70 | 4176.40 |
| Life-years at risk | 23,389.70 | 13,875.47 |
| Life-years DM | 8521.00 | 15,902.53 |
| Life-years CHD | 5356.61 | 4077.79 |
| Life-years stroke | 1342.90 | 736.52 |
| Measure | BS | No BS |
|---|---|---|
| Costs | 129,642,865.44 | 100,534,065.50 |
| Costs DC35 | 68,469,090 | 53,428,942 |
| Costs DC15 | 94,912,795 | 74,358,651 |
| QALYs | 28,518.57 | 22,383.33 |
| QALYs DC35 | 14,467.6 | 12,030.81 |
| QALYs DC15 | 20,579.67 | 16,650.46 |
| 36,055.78 | ||
| All life-years | 42,278.46 | 4949.024 |
| Depression life-years | 4890.988 | 0 |
| Life-years At Risk | 10,542.28 | 36,055.78 |
| Life-years DM | 27,745.59 | 0 |
| Life-years CHD | 1711.461 | 0 |
| Life-years stroke | 618.7119 | 0 |
| Life-years cancer | 1660.417 | 0 |
| Measure | Procedure cost £9164.50 | Procedure cost £13,746.75 | Procedure cost £18,329.01 | Procedure cost £0 | ||||
|---|---|---|---|---|---|---|---|---|
| BS | No BS | BS | No BS | BS | No BS | BS | No BS | |
| Costs | 126,836,121.81 | 97,822,340.46 | 131,420,946.21 | 97,823,996.34 | 135,997,173.35 | 97,823,162.75 | 117,679,548.60 | 97,823,233.99 |
| Costs DC35 | 67,251,683.64 | 51,993,824.43 | 71,831,895.04 | 51,994,297.06 | 76,410,753.84 | 51,994,000.59 | 58,093,442.76 | 51,994,289.69 |
| Costs DC15 | 93,060,030.17 | 72,384,665.60 | 97,642,112.75 | 72,385,641.51 | 102,220,005.82 | 72,385,082.26 | 83,902,400.34 | 72,385,319.78 |
| QALYs | 28,345.05 | 22,772.64 | 28,348.00 | 22,772.36 | 28,349.04 | 22,770.13 | 28,348.70 | 22,768.81 |
| QALYs DC35 | 14,509.41 | 12,366.93 | 14,510.76 | 12,366.42 | 14,512.37 | 12,363.89 | 14,511.63 | 12,363.63 |
| QALYs DC15 | 20,547.16 | 17,022.33 | 20,549.25 | 17,021.87 | 20,550.68 | 17,019.40 | 20,550.05 | 17,018.71 |
| All life-years | 41,869.28 | 35,772.21 | 41,871.88 | 35,772.76 | 41,870.76 | 35,772.24 | 41,870.13 | 35,772.48 |
| Depression life-years | 4393.11 | 4385.58 | 4392.66 | 4385.73 | 4392.49 | 4385.80 | 4,392.59 | 4385.67 |
| Life-years at risk | 22,296.44 | 11,998.61 | 22,298.90 | 11,998.21 | 22,302.07 | 11,997.72 | 22,296.63 | 11,998.25 |
| Life-years DM | 9434.01 | 17,754.62 | 9431.49 | 17,755.85 | 9422.88 | 17,755.88 | 9432.08 | 17,756.37 |
| Life-years CHD | 5321.58 | 3771.50 | 5321.65 | 3771.41 | 5324.31 | 3771.18 | 5319.60 | 3770.68 |
| Life-years stroke | 1309.54 | 633.92 | 1309.22 | 633.85 | 1309.11 | 634.04 | 1308.76 | 633.65 |
| Life-years cancer | 3507.70 | 1613.56 | 3510.63 | 1613.45 | 3512.40 | 1613.43 | 3513.06 | 1613.54 |
| Measure | No decay | Decay parameter 0.25 | Decay parameter 0.5 | |||
|---|---|---|---|---|---|---|
| BS | No BS | BS | No BS | BS | No BS | |
| Costs | 126,836,121.81 | 97,822,340.46 | 115,432,021.05 | 97,824,395.51 | 111,739,915.22 | 97,822,175.85 |
| Costs DC35 | 67,251,683.64 | 51,993,824.43 | 64,248,388.82 | 51,994,746.65 | 63,152,883.89 | 51,994,120.43 |
| Costs DC15 | 93,060,030.17 | 72,384,665.60 | 86,840,368.81 | 72,386,082.30 | 84,717,546.61 | 72,384,838.15 |
| QALYs | 28,345.05 | 22,772.64 | 25,712.42 | 22,770.51 | 24,838.51 | 22,770.49 |
| QALYs DC35 | 14,509.41 | 12,366.93 | 13,786.42 | 12,364.36 | 13,515.67 | 12,363.83 |
| QALYs DC15 | 20,547.16 | 17,022.33 | 19,086.64 | 17,019.87 | 18,575.23 | 17,019.61 |
| All life-years | 41,869.28 | 35,772.21 | 37,996.29 | 35,772.62 | 36,716.33 | 35,771.92 |
| Depression life-years | 4393.11 | 4385.58 | 4281.87 | 4385.85 | 4242.26 | 4385.69 |
| Life-years at risk | 22,296.44 | 11,998.61 | 17,432.68 | 11,997.65 | 15,720.39 | 11,998.08 |
| Life-years DM | 9434.01 | 17,754.62 | 12,649.89 | 17,756.23 | 13,753.63 | 17,755.13 |
| Life-years CHD | 5321.58 | 3771.50 | 4561.94 | 3771.01 | 4303.96 | 3771.05 |
| Life-years stroke | 1309.54 | 633.92 | 933.13 | 633.77 | 823.67 | 633.81 |
| Life-years cancer | 3507.70 | 1613.56 | 2418.66 | 1613.96 | 2114.68 | 1613.84 |
| Measure | Least deprived | Most deprived | ||
|---|---|---|---|---|
| BS | No BS | BS | No BS | |
| Costs | 117,745,667.26 | 91,107,021.29 | 131,069,648.77 | 99,175,584.91 |
| Costs DC35 | 61,493,790.82 | 47,034,946.43 | 69,995,133.48 | 53,670,281.45 |
| Costs DC15 | 85,705,625.18 | 66,476,663.19 | 96,595,623.78 | 74,078,377.12 |
| QALYs | 29,252.01 | 23,877.93 | 27,258.26 | 21,506.09 |
| QALYs DC35 | 14,790.50 | 12,738.97 | 14,186.56 | 11,944.26 |
| QALYs DC15 | 21,073.32 | 17,689.68 | 19,927.40 | 16,260.29 |
| All life-years | 43,082.07 | 37,342.02 | 40,398.39 | 33,977.36 |
| Depression life-years | 3703.37 | 3732.77 | 5161.74 | 5057.53 |
| Life-years at risk | 23,052.80 | 12,791.42 | 20,894.34 | 10,834.98 |
| Life-years DM | 9444.79 | 18,077.73 | 9540.41 | 17,464.53 |
| Life-years CHD | 5069.20 | 3761.28 | 5874.08 | 3895.64 |
| Life-years stroke | 1195.94 | 608.61 | 1363.94 | 612.80 |
| Life-years cancer | 4319.33 | 2102.97 | 2725.63 | 1169.41 |
Appendix 3 Protocol amendments
Amendment 1: dated 10 February 2014
On page 12 of the proposal, it reads ‘An additional sample that only includes individuals who have received bariatric surgery procedures will also be sampled to provide empirical data inputs to the model’. The protocol already specifies substantive outcome measures of disease incidence and mortality. The purpose of this amendment is to add as measures of interest, intermediate measures of outcome of bariatric surgery (BS). We wish to add that ‘the analysis will include comparison of changes over time in intermediate measures, and their management, between obese participants who received BS and those who did not receive BS. These measures include body weight, body mass index, blood pressure, serum cholesterol, HbA1c and smoking. Other medical therapies that impact on these measures will be evaluated including: diet advice and obesity drugs, use of antihypertensive drugs (including beta-clockers, calcium channel antagonists, diuretics and drugs acting on the renin-angiotensin system), cholesterol lowering drugs (including primarily statins), diabetes drugs (including insulin and oral hypoglycaemic drugs) and smoking advice and smoking prescriptions. Smoking is included because of its importance as a cause of morbidity in obese individuals. This will enable us to present observational data from CPRD on these intermediate measures in obese individuals and those who had bariatric surgery’. We note that several of these measures were covered in an earlier protocol (07_054).
Amendment 2: dated 14 July 2014
One page 13 of the proposal it reads ‘Incidence and mortality rates will be estimated in a time-to-event framework which will provide estimates from which probabilities can be estimated for the model.(54)’.
On evaluating the data it is clear that the distribution of BMI values is considerably different for bariatric surgery cases as compared to controls. Results may seem to lack credibility if mean BMI values differ greatly between surgery cases and controls. Therefore, we will draw a sample of matched controls for comparison including obese individuals sampled from CPRD who did not receive bariatric surgery and were not older than the maximum age of the bariatric surgery cases. Controls will be matched for age, body mass index, sex and index year. Nearest neighbour matching will be performed without replacement. The index date for controls will be the date of the earliest BMI record on which the patient attained their highest BMI category. Since prevalent cases of disease must be excluded from analyses to estimate the incidence of each outcome, a separate set of controls will be sampled for the analysis of each outcome measure.
With respect to outcome measures, in order to simplify the model, asthma and back and joint pain will be omitted. All malignant neoplasms (Read code B0 to B6) will be included in place of obesity associated cancers.
Amendment 3: dated 26 November 2014
This proposed amendment is for a validation study aiming to confirm the date, type of procedure, occurrence of complications, revision/reversal and repeat procedures following bariatric surgery.
The proposed questionnaire for the study is appended.
The questionnaire will be sent to GPs relating to patients with CPRD records of bariatric surgery. The sample will be stratified according to procedure type recorded in CPRD, participants with undefined type of initial procedure or records of second procedures will be specifically sampled. The sample size will be up to 150.
Amendment 4: dated 28 January 2015
This proposed amendment is to utilise linked Hospital Episode Statistics data in the estimation of health care utilisation.
On page 13 the text should be altered to read ‘We will estimate rates of health care utilisation from CPRD records with linked Hospital Episode Statistics data. Utilisation rates will be based on person-time at risk. Estimates for the unit cost of health service use will be obtained from reference sources (55). Unit costs will be applied to each category of health care utilisation in order to estimate health care costs’.
List of abbreviations
- AMSTAR
- Assessing the Methodological Quality of Systematic Reviews
- BMI
- body mass index
- CHD
- coronary heart disease
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- EHR
- electronic health record
- GBP
- gastric bypass
- GLM
- generalised linear model
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- IDF
- International Diabetes Federation
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- LABS-2
- Longitudinal Assessment of Bariatric Surgery-2
- LAGB
- laparoscopic-adjustable gastric banding
- NBSR
- National Bariatric Surgery Register
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SG
- sleeve gastrectomy
- SOS
- Swedish Obese Subjects
- T2DM
- type 2 diabetes mellitus