Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 10/2002/29. The contractual start date was in October 2012. The final report began editorial review in April 2015 and was accepted for publication in September 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Katherine Brown, Rodney Franklin, David Barron and David Cunningham are on the Steering Committee of the National Congenital Heart Diseases Audit (NCHDA). Sonya Crowe is a Health Improvement Science Fellow funded by the Health Foundation.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Major technological advances in paediatric surgical and intensive care in recent decades, particularly for neonates, have resulted in the survival of children with previously life-threatening congenital heart defects,1 yet congenital heart disease (CHD) remains the most common cause of infant death because of congenital anomalies in the UK. 2,3 Although many of the deaths that do occur early are in association with surgery, the outcomes for this immediate phase of in hospital care have improved overall in recent years4 with current 30-day mortality rates at historically low levels. The scope of audit beyond 30 days after operation is limited; however, UK National Congenital Heart Diseases Audit (NCHDA) data indicate that significant numbers of deaths do occur more than 30 days after neonatal cardiac surgery but within the first postoperative year. 5 Moreover, analysis of 1018 neonates undergoing all types of cardiac surgery in two London hospitals from 2000 to 2009 found that of 176 deaths during the first year of life, 116 (66%) occurred during the initial postsurgical hospital stay and 37 (21%) were unexpected late deaths in infants who had been discharged home after apparently successful cardiac surgery. 6 Thus, cardiac infants remain vulnerable even after surgery; one reason previously proposed for this is that certain CHD types necessitate a series of staged procedures within the first few months of life. 7 It is also important to note that babies with CHDs who survive past their first birthday are subsequently at a lower mortality risk during childhood. 3,8
Although considerable emphasis has been placed on quantifying and exploring what risk factors impact on early postoperative and in-hospital mortality9–12 and on improving treatment strategies for within hospital care, including surgical techniques13 and postoperative management approaches,14 resulting in net benefit to patients, less attention has been paid to the risks and care pathways post discharge. An exception to this is the case of hypoplastic left heart syndrome (HLHS), for which teams from the USA have reported the benefits of enhanced surveillance known as home monitoring programmes (HMPs). 15 However, despite the obvious importance to patients and families of this phase in the patient journey, uncertainty has previously existed around which diagnostic groups across the spectrum of infant CHD are at risk of adverse postdischarge outcomes and what can be done to improve upon them, while considering various elements and stages of relevant care pathways. Guidance on hospital discharge for high-risk neonates from the American Academy of Pediatrics is informed by a robust evidence base describing the specific needs of vulnerable neonates at the time of hospital discharge and hence what should be put into place. 16 Although neonates with complex congenital anomalies are highlighted as a vulnerable population, specific guidance for the postdischarge care of neonates with CHD is not provided. In the UK, the management of children with CHD, including infants after being discharged home, falls within the responsibility of ‘congenital heart networks of care’, including designated ‘outreach’ services provided or supported by the specialist centres. 17 These services have been subject to several reviews, including the ‘Safe and Sustainable Review’18 and most recently the ‘New Review of Congenital Heart Services’, which delivered its final report on 2 March 2015. 19 We hope that our study findings may be taken forwards within the context of this evolving service provision for children with CHD.
The original aims and objectives for the Infant Heart Study (IHS) were as follows.
Aims
To use a mixed-methods approach including quantitative analyses of national audit data and qualitative approaches to gather information from key individuals, in order to establish an evidence-based and realistic guideline for community-based surveillance of fragile infants with CHD.
Objectives
-
To perform a literature review exploring risk factors for death in infancy following cardiac surgery (rather than early postoperative death in hospital), to identify examples of successful surveillance or intervention programmes for infants with CHD and to explore evidence for social, ethnic and economic factors that may reduce access to health care for children with complex medical disorders.
-
To perform a quantitative analysis of risk factors, including both medical and social variables available from routine data sources, that may be related to the outcome measures: late death or unplanned readmission to intensive care, in infants that have undergone surgery for CHD. This analysis used national audit data from NCHDA and the Paediatric Intensive Care Audit Network (PICANet).
-
To perform a qualitative study drawing on a series of sources for information, including an online discussion forum (OF) through a patient and user group’s Facebook web page (www.facebook.com; Facebook, Inc., Menlo Park, CA, USA), the user group’s helpline staff, professionals caring for infants with CHD, and parents from high-risk groups or of children that experienced one of the outcome measures. Qualitative data from the last three sources were gathered via semistructured interviews. The objective here was to identify actual barriers to health care for infants with CHD, with particular focus on socioeconomic challenges and to inform subsequent intervention development. Two focus groups reviewed and discussed the proposed intervention designs.
-
To combine the data and information acquired in the first three objectives to generate the evidence-based protocol or guideline for surveillance of infants with CHD, including the ‘who?’ ‘when?’ and ‘how?’ this should best be delivered. The ultimate objective was to produce a workable and effective follow-up surveillance protocol for infants discharged into the community after cardiac surgery, with appropriate targeting of higher-risk patients and consideration of measures that will be acceptable and useful to parents and community-based health-care professionals. Intervention development included consideration of measures of success.
Approvals for the study
Research Ethics Committee
The study was approved by the London Central Research Ethics Committee on 4 October 2012 (reference 12/LO/1398).
National Information Governance Board
The study was approved by the National Information Governance Board now known as the Health Research Authority (HRA) and permission was granted for the use of identifiable data for the purposes of record linkage within specified limits (reference 12/LO/1398, November 2012).
Healthcare Quality Improvement Partnership
The use of national audit data from NCHDA and PICANet, including the use of identifiable data for the purposes of record linkage within specified limits, was approved by Healthcare Quality Improvement Partnership.
National Congenital Heart Diseases Audit
The use of national audit data for the specified purposes stated in the study protocol was approved by the NCHDA and is subject to a data sharing agreement (reference 12/CONG/03).
Chapter 2 Unexpected deaths and unplanned readmissions in infants discharged home after major surgery for congenital anomalies: a systematic review of potential risk factors
Some of the text of this chapter has been published previously as Tregay et al. , 2015. 20 Reproduced with permission. © Cambridge University Press 2014.
Introduction
In order to achieve optimal outcomes post discharge in infants going home after intervention, and to offer targeted support to vulnerable infants and their families at home in the community, it is also important to understand the risk factors for these late outcomes. We therefore undertook a systematic review of the published literature with the intent of capturing all studies of infants with congenital anomalies, to identify the key risk factors, identifiable at the time of discharge home after surgery, that are associated with unexpected death in the community or unplanned readmission to hospital. In setting up the systematic review, we aimed to capture studies from other specialist areas of practice, including but not exclusive to CHD, as we sought transferable knowledge as well as information regarding our specific population of interest.
Methods
Protocol and registration
The protocol and search strategy is registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42013003483. 21
Search strategy
To ensure a comprehensive review of the evidence, and to capture any pertinent risk factors that may not yet have been identified in studies with CHD patients, we used a broad search strategy that included other life-threatening congenital malformations requiring major surgery in the first year of life, for example gastroschisis or diaphragmatic hernia. We used key terms relating to children, congenital abnormalities, surgical procedures, hospital discharge and adverse outcomes to electronically search MEDLINE (1980 to 1 February 2013), EMBASE (1980 to 1 February 2013), Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1981 to 1 February 2013), The Cochrane Library (1999 to 1 February 2013), Web of Knowledge (1980 to 1 February 2013) and PsycINFO (1980 to 1 February 2013). Conference abstracts from the Association for European Paediatric Cardiology, the American Heart Association and the European Surveillance of Congenital Anomalies symposia were searched for the period 2008 to 2012. A forward citation search was carried out on the reference lists of all selected studies to identify additional published studies for review.
Selection of studies
Studies were eligible for inclusion only if they separately reported outcomes for children discharged from hospital and in-hospital surgical mortality. To ensure relevance to infant survival, only studies involving children up to the age of 5 years were included in the review and major surgery was defined as requiring intensive or high-dependency care in the postoperative period. Inclusion criteria for the studies included in the review are summarised in Box 1.
The titles and abstracts for all studies were independently reviewed by two reviewers (J Tregay and J Wray) then full-text papers of selected studies assessed by three reviewers (J Tregay, K Brown and R Knowles) to determine whether or not they met the inclusion criteria. Any discrepancies between reviewers were resolved through discussion with a fourth reviewer (J Wray) (see Figure 1).
Studies that include children:
-
aged from birth up to and including 5 years of age
-
with a life-threatening congenital abnormality
-
who have undergone major surgery (involving intensive care) for potentially life-threatening congenital disease
-
who were discharged home from hospital following their successful surgery.
Studies that:
-
refer exclusively to adults, children over the age of 5 years old or where the age group of interest is not clearly defined
-
include previously healthy children who had major surgery as a consequence of traumatic injury
-
do not refer to specified adverse outcomes (e.g. death, unplanned hospital readmission)
-
do not present postdischarge events and risk factors separately from in-hospital events
-
included children discharged home for palliative medical care
-
were case series of fewer than 20 cases, personal communications, letters and commentaries
-
have no available English-language abstract.
Data extraction
Data extraction was independently completed by two reviewers (J Tregay and R Knowles) using a standard pro forma that included information on study design, population, diagnosis, comparison groups, outcomes and risk factors.
Quality assessment
Studies were assessed for methodological quality of study design using levels of evidence rated from one (most rigorous, e.g. randomised controlled trial) to four (least rigorous, e.g. retrospective uncontrolled case series). 22 Within each evidence level, studies were assessed as A (high quality) to C (lowest quality), using predetermined criteria such as confounding, completeness of follow-up and objective measurement of outcomes (see Appendix 2).
Data analysis
The outcomes of interest were unexpected death or unplanned readmission to hospital in the first year of life after discharge following cardiac surgery. Factors associated with increased mortality or readmission risk are presented in a narrative synthesis.
Results
Study selection
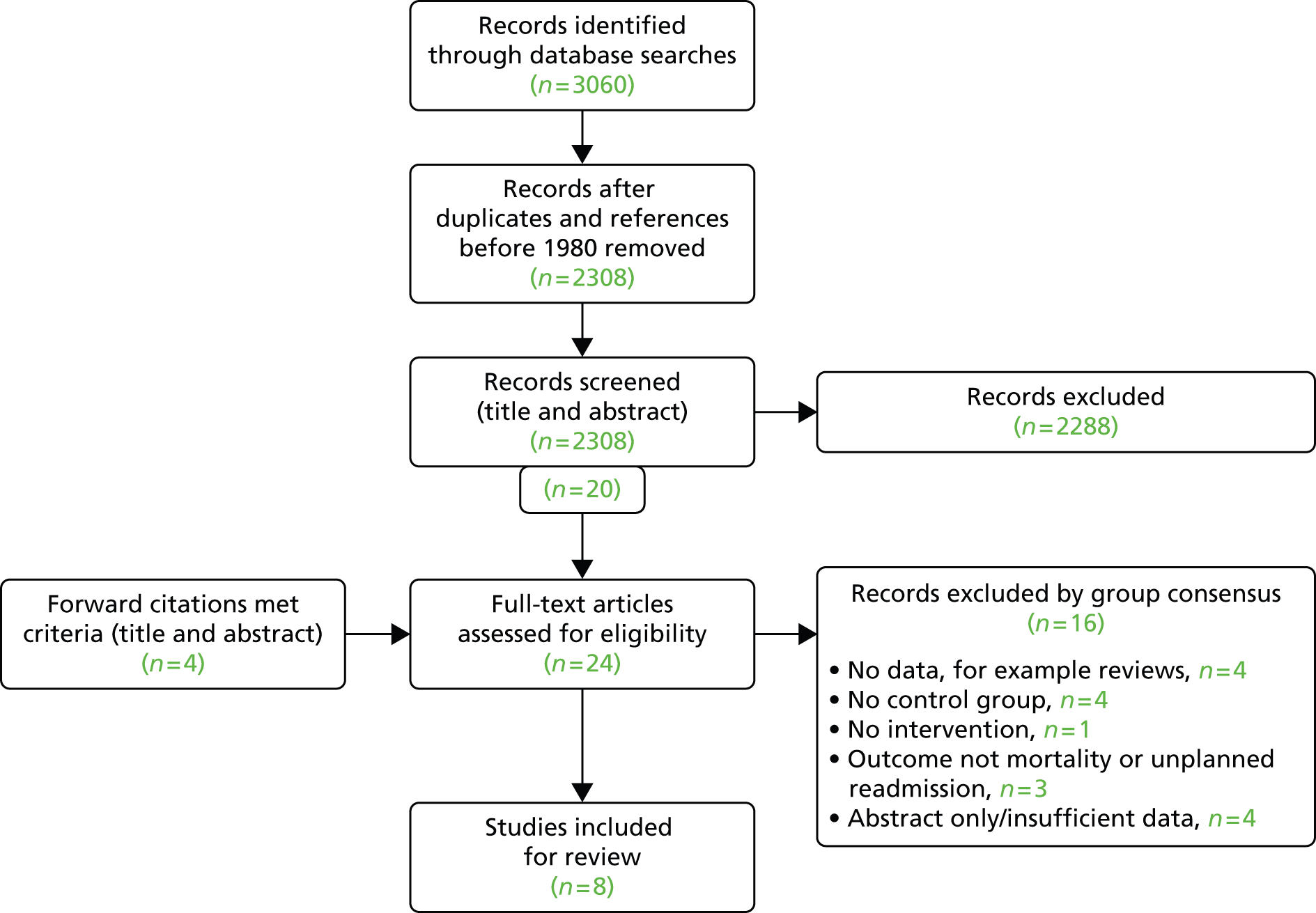
There were 17 studies identified through systematic searches and a further six studies through forward citations. Of the 23 full-text papers reviewed, eight studies failed to meet inclusion criteria resulting in 15 studies eligible for review (Figure 1). Despite our inclusive search strategy, no studies of post-surgical outcomes for children with non-cardiac congenital anomalies met the inclusion criteria.
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) flow chart showing study selection process. Reproduced with permission from Tregay et al. , 2015. 20 © Cambridge University Press 2014.

The review included eight retrospective reviews of surgical cases,23–30 four retrospective cohort studies,31–34 two case–control studies35,36 and one randomised controlled trial that was reported in two papers. 26,37 Only three studies29,36,37 included a prospective element. Although study designs differed, all studies were rated as good quality (Table 1); studies that were assigned a lower rating failed to address some potential confounding factors. Ten reports were of patients with a functional single-ventricle (SV) diagnosis,23,24,26–28,30,33,35–37 which was most often HLHS. Fourteen papers involved patients who underwent cardiac surgery during the first year of life and the remaining study31 included cardiac patients operated up to the age of 18 years with results provided separately for each age group. Only two studies were not conducted in the USA. 23,27 In total, 29,019 patients were followed up for mortality outcomes, of whom 1113 (4%) died. Of the 3672 children who underwent SV surgery, 452 (12%) died, compared with 661 (3%) of 25,347 children who underwent other types of cardiac surgery. Of the 1639 children who were observed in three studies of unplanned readmission, 173 (11%) were readmitted to hospital during the study follow-up period. Table 2 summarises the included papers.
| First author, year | LOE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOE 1: prospective cohort study, RCT or meta-analysis | LOE 2: prospective case–control study (including records based) | LOE 3: retrospective cohort or case–control study (including records based) | LOE 4: retrospective review of cases without control patients | |||||||||
| Study quality | Study quality | Study quality | Study quality | |||||||||
| A | B | C | A | B | C | A | B | C | A | B | C | |
| Ashburn, 200323 | ✓ | |||||||||||
| Carlo, 201124 | ✓ | |||||||||||
| Chang, 200631 | ✓ | |||||||||||
| Edwards, 201025 | ✓ | |||||||||||
| Fixler, 201232 | ✓ | |||||||||||
| Ghanayem, 201226 | ✓ | |||||||||||
| Hansen, 201127 | ✓ | |||||||||||
| Hebson, 201233 | ✓ | |||||||||||
| Hehir, 200835 | ✓ | |||||||||||
| Kogon, 201234 | ✓ | |||||||||||
| Mackie, 200436 | ✓ | |||||||||||
| Mahle, 200128 | ✓ | |||||||||||
| Ohye, 201237 | ✓ | |||||||||||
| Pinto, 201229 | ✓ | |||||||||||
| Simsic, 200530 | ✓ | |||||||||||
| First author, year | Participants | Setting and study design | Quality rating | Follow-up period | Primary outcome measure | Mortality rate (post-hospital discharge) | Factors associated with mortality/hospital readmission |
|---|---|---|---|---|---|---|---|
| Ashburn, 200323 | 710 HLHS with critical aortic stenosis or aortic valve atresia who underwent Norwood stage 1 procedure (512 infants discharged alive after stage 1 surgery) | Canadian multicentre study; retrospective records review of surgical cases; risk analysis for outcomes including death, further surgery | 4A | Follow-up at 1 month, 6 months, 1 year and 5 years after stage 1 procedure | Time to transition to death, further planned surgery, transplantation or other outcome | 12% of 512 infants died between stage 1 and stage 2 surgery. Survival after stage 1 surgery for whole cohort: 72% at 1 month; 62% at 6 months; 60% at 1 year; and 54% at 5 years | Patient-specific factors linked to mortality: low birthweight, smaller ascending aorta, older age at Norwood Institutional factors linked to mortality: institutions enrolling ≤ 10 neonates higher risk than institutions enrolling ≥ 40 neonates Procedural factors linked to mortality: shunt originating from the aorta, longer circulatory arrest time, management of ascending aorta |
| Carlo, 201124 | 85 HLHS (65% male) who underwent Norwood stage 1 followed by BDG stage 2 procedure | Single US centre; retrospective records review of surgical cases; comparison between survivors and deceased | 4A | From BDG (stage 2) until Fontan (stage 3) procedure, cardiac transplant or death. Follow-up for mean 3.4 years (range 1.6–5.8 years) after stage 2 procedure | Interstage attrition (death or cardiac transplantation) after hospital discharge from BDG (stage 2 procedure) and before Fontan (stage 3 procedure) | Eight died unexpectedly at home (9.4% mortality rate) and three underwent cardiac transplantation (13% overall attrition) | Factors associated with inter-stage mortality: longer intubation times (median 2 days vs. 1 day, p < 0.01), hospital length of stay (median 19 days vs. 6 days; p < 0.01) Higher mortality risk on multivariable analysis: lower weight z-score at BDG and moderate to severe tricuspid regurgitation Interstage mortality not associated with: sex, anatomic subtype in HLHS, use of antegrade cerebral perfusion, restrictive atrial septum at birth, age or weight at stage 1 palliation, stage 1 operative characteristics, age at BDG, haemodynamic data obtained at cardiac catheterisation, aortic arch obstruction, right ventricular dysfunction |
| Chang, 200631 | 23,897 children < 18 years (55% male) with ICD-9-CM procedure codes indicating any cardiac surgery; includes cardiac surgery for non-congenital diagnoses | All US Californian state hospitals; retrospective cohort follow-up using state-wide database; multivariate analysis of risk factors | 3A | Up to 365 days post discharge | Postoperative deaths within 1 year of surgery (included in-hospital death). Late deaths (occurring 31–365 days after hospital discharge) were reported separately | 23,987 ‘alive’ hospital discharges, 148 deaths (0.62%) occurred within 365 days after discharge (37 deaths within 30 days; 44 deaths at 31–90 days; 67 deaths at 91–365 days) | Factors associated with postdischarge death: younger age and procedure type in terms of neonates and infants undergoing Norwood procedure, aortopulmonary shunt with atrial septostomy, total anomalous pulmonary veins or truncus arteriosus repair, thoracic vessel procedures and open valvotomy Factors not associated with postdischarge death: ethnicity, sex, income, hospital care volume |
| Edwards, 201025 | 35 CHD (various) (46% male) who underwent any CHD surgery and were in a home mechanical ventilation programme | Single US centre; retrospective review using hospital records; survival analysis | 4A | 2–168 months after starting home mechanical ventilation | Mortality | 12 (34%) died | Higher mortality risk on univariable analysis: bronchopulmonary dysplasia, neurological disorder Higher mortality risk on multivariable analysis: adjusted RACHS score of ≥ 4 |
| Fixler, 201232 | 1213 CHD diagnosis associated with > 25% mortality | US state (Texas) registry; observational cohort follow-up using linked records; multivariate analysis of risk factors | 3A | Up to 1 year after birth | First-year mortality | Overall first-year survival was 59.9% | Overall ethnicity was not associated with survival, but Hispanic infants with HLHS had decreased survival Factors associated with worse survival after adjustment for defect type: living on Mexican border (proxy for deprivation), low birth weight (< 2500 kg) and gestational age, extracardiac defects Factors not associated with first year mortality: distance to cardiac centre, parental birthplace, sex, maternal education and marital status |
| Ghanayem, 201226 | 426 SV diagnosis; survived to hospital discharge after Norwood stage 1 surgery | US multicentre; retrospective case records analysis within a controlled trial; multivariate risk factor analysis | 3A | Until stage 2 surgery or death (up to 14 months after stage 1 surgery) | Interstage mortality post hospital discharge | 50 (12%) died | Factors associated with worse survival: preterm delivery, Hispanic ethnicity, aortic/mitral atresia, higher number of postoperative complications, percentage below poverty line (US census data), shunt type (MBTS linked to worse survival than RVPA) |
| Hansen, 201127 | 115 HLHS (67% male); underwent superior cavopulmonary anastomosis (stage 2) surgery | Single centre, Germany; retrospective surgical case series; multivariate analysis of risk factors | 4A | Follow-up over a 14-year period – minimum follow-up of 2 years | Death/cardiac transplant; postoperative complication and adverse events | Late adverse outcome in 10 (8.7%) patients (death n = 8; cardiac transplant n = 2) | Factors associated with death/cardiac transplant: longer cardiopulmonary bypass time, moderate or greater tricuspid regurgitation on post-operative echocardiogram (OR 16.5, 95% CI 4.4 to 62.6; p < 0.001) Factors not associated with death/cardiac transplant: aged < 4 months at surgery (OR 1.2, 95% CI 0.4 to 3.6; p = 0.78) |
| Hebson, 201233 | 334 neonates aged < 30 days at surgery; SV diagnosis (varied); underwent Norwood stage 1 (n = 165), pulmonary artery band (n = 17), or MBTS (n = 152) procedures | Single US centre; retrospective cohort study; analysis of different feeding modalities post discharge | 3A | Until stage 2 surgery (follow-up ranged from 1 to 8 years overall, but was not specified for individual patients) | Interstage mortality | 26 (7.8%) interstage deaths. Nine deaths with NF or GT; 17 without NF/GT. Seven died in hospital and 19 died at home/local facilities | Higher risk of interstage mortality on multivariate analysis (adjusted for age, weight, genetic syndromes, prematurity, heterotaxy, postoperative arrhythmia; and ventricular function at discharge): feeding with GT with/without NF (relative risk 2.38, 95% CI 1.05 to 5.40; p = 0.04) |
| Hehir, 200835 | 313 ‘hospital survivors’ with HLHS (and variants); underwent Norwood stage 1 procedure | Single US centre; retrospective, nested, case–control study; multivariate analysis of risk factors | 3A | Follow-up was for 1 year after stage 1 surgery or until stage 2 surgery or death if earlier | Interstage mortality (post discharge and before stage 2 procedure) | 33 interstage deaths (10.5%) | Higher risk of death on univariate analyses: restrictive atrial septum, older age at operation, postoperative arrhythmias and respiratory complications Higher risk of death on multivariate analyses: highly restrictive atrial septum (OR 7.6, 95% CI 1.9 to 29.6), age at operation > 7 days (OR 3.8, 95% CI 1.3 to 11.2) Factors not associated with interstage death: intra-operative factors, cardiac status at discharge, sex, birth weight, gestation, prenatal diagnosis, distance from centre, feeding at discharge, non-cardiac anomalies, oxygen at discharge, reoperation, discharge on > three medications, seizures, postoperative ECMO or cardiac arrest Higher risk of readmission on univariate analysis: younger age; lower weight at surgery; Hispanic; genetic syndrome; failure to thrive; pre-operative ventilation; higher RACHS-1 score; nasogastric feeding at discharge; palliative surgery; longer length of stay in ICU/hospital Higher risk of readmission on multivariate analysis: Hispanic ethnicity; failure to thrive; hospital length of stay >10 days Factors not associated with unplanned readmission: arrhythmia, gastro-oesophageal reflux and developmental delay |
| Kogon, 201234 | 685 any CHD diagnosis requiring surgery (57% male) | Single US centre; retrospective observational cohort study; multivariate analysis of risk factors | 3B | Until 30 days after hospital discharge | Unplanned readmissions within 30 days of hospital discharge | 70 patients (10.2%) were readmitted (total 74 readmissions in 70 patients) | Risk factors for unplanned readmission on univariable analysis: younger age; lower weight at surgery; Hispanic; genetic syndrome; failure to thrive; pre-operative ventilation; higher RACHS-1 score; nasogastric feeding at discharge; palliative surgery; longer length of stay in ICU/hospital Risk factors for unplanned readmission on multivariable analysis: Hispanic ethnicity; failure to thrive; hospital length of stay > 10 days Factors not associated with unplanned readmission: arrhythmia, gastro-oesophageal reflux and developmental delay |
| Mackie, 200436 | 162 (54 cases; 108 control patients) HLHS, other SV diagnosis or transposition of the great arteries; underwent Norwood stage 1 or arterial switch procedure | Single US centre; case–control study; multivariate analysis of risk factors | 2B | Until 30 days after hospital discharge | Unplanned readmission or death (combined outcome used to explore risk factors) | 54 cases (from 752 operated children) included: 48 readmissions (29.6% of 752); six deaths (3.7% of 752) | Factors associated with death or readmission: residual haemodynamic problems (OR 4.10), ICU stay > 7 days (OR 5.17), establishment of full oral intake < 2 days before. Combining with the control group, living in a low income areas was associated with a lower likelihood of readmission (OR 0.25, 95% CI 0.07 to 0.85; p = 0.027) |
| Mahle, 200128 | 536 HLHS and variants; underwent stage 1 surgery for SV reconstruction | Single US centre; retrospective records-based identification of cohort with prospective confirmation of outcomes; multivariate analysis of risk | 3B | Deaths within the first year after stage 1 (range 25–227 days post surgery) | ‘Unexpected’ death (defined as cardiovascular collapse without regaining consciousness) | 22 unexpected deaths (4.1%) and 63 non-surgery-related deaths (11.8%) from 536 infants discharged home after stage 1 surgery. Median age at unexpected death was 79 (25–227) days | Factors associated with late mortality: perioperative arrhythmia and earlier year of surgery Factors not associated with late mortality: prenatal diagnosis, aortic atresia, age at admission, age at each surgical stage, perioperative seizure, ventricular function measures and feeding difficulties |
| Ohye, 201237 | 549 SV; underwent Norwood stage 1 (randomised to two shunt types) | USA; multicentre; prospective RCT comparing two types of SV surgery; multivariate analysis of risk factors | 1B | Until 12 months after stage 1 surgery | Deaths (including ‘unexpected’ death) in the 12 months following Norwood stage 1 surgery | 164 died: 88 in-hospital at stage 1; 54 between stages 1 and 2; 16 in-hospital at stage 2; and 6 within 12 months of stage 2 discharge. A total of 29 deaths were ‘unexpected’ | Factor associated with inter-stage mortality: shunt type, with MBTS higher risk than RVPA. Twelve (41%) of the 29 ‘unexpected’ (postdischarge) deaths had prodromal illness including poor feeding/vomiting, fussiness, diarrhoea, cyanosis, fever and increased work of breathing |
| Pinto, 201229 | 202 any CHD requiring surgery (51.5% male); underwent neonatal congenital heart surgery (not minor surgery) | Single US centre; retrospective records review and follow-up survey; multivariate analysis of risk factors | 4A | 23.9 (± 3.4) months post discharge after neonatal congenital heart surgery | Mortality + ‘adverse events’ (unplanned readmissions and cardiac reinterventions) | 16 deaths (8%). Postdischarge adverse events were reported for surviving patients by telephone survey (contact rate 59%). Of those, 49 (45%) had an unplanned readmission | Patients resident 90–300 minutes from the surgical centre were less likely to experience an adverse event than those living < 90 minutes away but there was a non-significant trend toward higher mortality in this same group when compared with those living < 90 minutes and > 30 minutes away. Residence > 300 minutes from the hospital not associated with higher risk of postdischarge death |
| Simsic, 200530 | 50 HLHS/SV diagnosis; underwent Norwood stage 1 procedure | Single US centre; retrospective review of surgical cases and outcomes; multivariable analysis of risk factors | 4B | For 1 year after stage 1 surgery (until stage 2 surgery or death) | Interstage mortality between Norwood stage 1 discharge and stage 2 surgery | Eight deaths (16%) within 1 year after Norwood procedure | Factors associated with interstage mortality: postoperative arrhythmias; decreased ventricular function. Factors not associated with interstage mortality: duration of cardiopulmonary bypass, cross clamp or circulatory arrest; moderate valve regurgitation; postoperative epinephrine, length of mechanical ventilation, length of hospital stay and discharge medication |
Adverse outcomes
Reported mortality rates varied markedly and were influenced by the study population at risk as well as the duration of follow-up. Five studies23,26,30,33,35 involved children with SV diagnoses undergoing staged palliative surgery and reported ‘interstage’ mortality between first- and second-stage surgery that ranged from 8% to 16%, with follow-up ending at around 1 year after surgery in most studies. Two further studies focused on ‘unexpected’ interstage deaths, defined as acute events or sudden cardiovascular collapse, and reported 4–5% unexpected deaths in neonates discharged home between stages 1 and 2. 28,37 Carlo et al. 24 and Hansen et al. 27 reported interstage mortality of 9% for children discharged home between second (superior cavopulmonary anastomosis or bidirectional Glenn) and third stage (Fontan) surgery. Two studies investigated postdischarge mortality after all types of cardiac surgery: Chang et al. 31 reported a low mortality rate of 0.62% at 1 year after any cardiac surgery undertaken in children up to age 18 years, whereas Pinto et al. 29 found a higher mortality rate of 8% in the 2 years following discharge after neonatal congenital heart surgery. Mortality rates over 10% were reported in studies focusing on specific higher-risk cardiac defect subgroups32 or patients discharged home on mechanical ventilation. 25
Two studies evaluated unplanned hospital readmissions as distinct from mortality29,34 and one further study36 reported unplanned readmissions as part of a combined outcome measure of mortality and readmission. Readmission rates within 30 days of hospital discharge ranged from 10%34 to 30%36 and at 2 years post discharge were 45%;29 variations were influenced by duration of follow-up and differences in data collection methods, which included hospital records review34,36 and telephone survey. 29
Risk factors associated with adverse outcomes
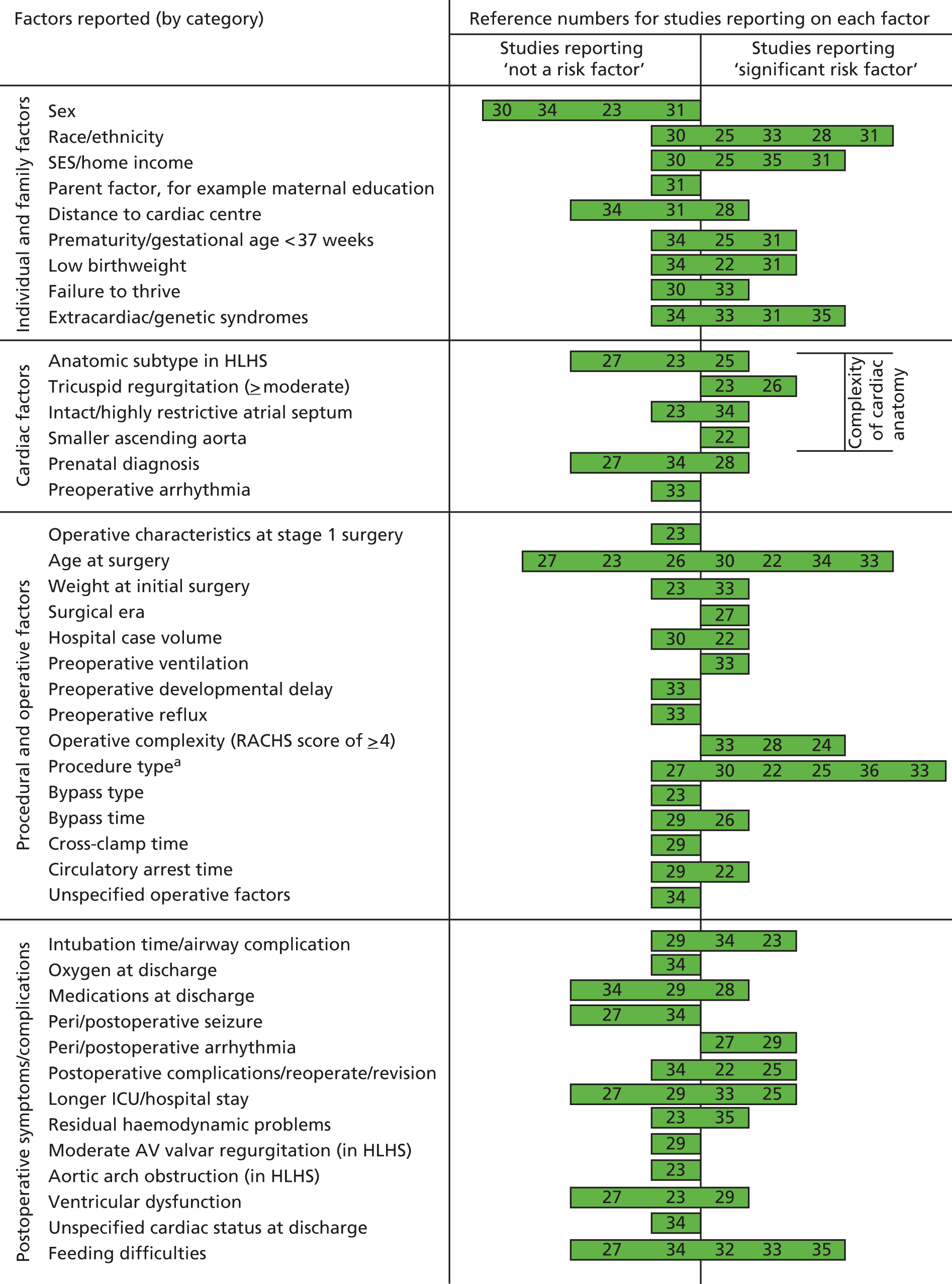
Although many different factors were investigated, the findings relating to individual factors were inconsistent; this may reflect the heterogeneity of participant characteristics and study designs. Figure 2 summarises the factors investigated by different studies and indicates whether or not these were found to increase mortality risk.
FIGURE 2.
Summary of factors examined in the 15 studies. AV, atrioventricular; ICU, intensive care unit; SES, socioeconomic status; RACHS category, Risk Adjustment in Congenital Heart Surgery category. a, The cardiac surgical procedures defined in these studies were palliative procedures, Norwood stage 1, bidirectional Glenn, shunt construction, thoracic vessel procedures, truncus arteriosus repair, total anomalous pulmonary venous return repair and open valvotomy. Reproduced with permission from Tregay et al. , 2015. 20 © Cambridge University Press 2014.
Individual and family factors
Ethnicity,26,29,32,34 socioeconomic status26,32,36 and non-cardiac malformations or genetic syndromes32,34,36 were the most frequently evaluated individual factors. Hispanic ethnicity14,19,23 and socioeconomic deprivation, as assessed through measures such as household income,36 family income below the national poverty threshold26 and deprivation index of the residential area32 were highlighted as risk factors for mortality and unplanned hospital readmission in the US studies. Preterm birth26,32 and low birthweight23,32 were risk factors for mortality, but patient sex was not significantly associated with adverse outcomes. 24,31,32,35 Children living 90–300 minutes from the cardiac centre were at significantly lower risk of unplanned readmission29 compared with families living under 90 minutes away, but there was no association with mortality. 29,32 Family factors, including maternal education, marital status and country of birth, were not associated with adverse outcomes. 32
Cardiac diagnosis and procedural factors
Infants with more complex hypoplastic left heart variants23,24,26,27,35 were at higher risk of mortality or readmission. Children undergoing more complex operations, based on the Risk Adjustment in Congenital Heart Surgery (RACHS) – 1 system,9,38 were at greater risk. 25,29,34 Several studies reported that Norwood procedures,31 specific shunt operations,23,26,31,37 total anomalous pulmonary venous connection (TAPVC) repair31 and truncus arteriosus repair31 were associated with significantly higher mortality, whereas palliative operations34 increased the risk of unplanned readmission. There was insufficient evidence to suggest that intraoperative characteristics, such as cardiopulmonary bypass or circulatory arrest time, had a negative impact on outcome. 23,30,35
In children undergoing staged palliative operations, older age at first procedure was associated with higher mortality risk,23,35 whereas younger age (under 4 months) at the second-stage Glenn procedure increased the risk of postoperative complications. 27 In two papers26,37 reporting findings from the Single Ventricle Reconstruction (SVR) trial, in which patients with HLHS were randomised to receive different surgical interventions, higher mortality rates after hospital discharge were observed in the group receiving a modified Blalock–Taussig shunt (MBTS) than in the group undergoing a right ventricle-to-pulmonary artery conduit (RVPA); this difference was no longer significant after adjustment for severity of postoperative atrioventricular valvar regurgitation.
Postoperative symptoms/complications
Five studies explored postoperative feeding difficulties;28,33–36 three of these identified feeding difficulties,33,34,36 including the need for gastrostomy tube placement,33 as a risk factor for mortality or unplanned readmission. Peri- and postoperative arrhythmias were also a significant risk factor for mortality in two studies;28,30 although airway complications, prolonged postoperative length of stay (LOS), postoperative complications and medications at discharge were not found to influence outcomes post discharge.
Discussion
We identified 15 studies that evaluated the potential risk factors associated with mortality or unplanned hospital readmission in children successfully discharged from hospital after cardiac surgery for serious CHDs. Factors identified most frequently by these studies as predicting significantly increased risk of adverse events were non-white ethnicity,26,29,32,34 lower socioeconomic status,26,32,36 comorbid conditions including non-cardiac malformations and syndromes,32,34,36 age at surgery,23,31,34,35 operative complexity or procedure type,23,25,26,29,31,34,37 and postoperative feeding difficulties. 33,34,36 Patient sex, parent factors, intraoperative factors and postoperative complications were also investigated but not found to be independent predictors of postdischarge outcomes.
Context and limitations
Our review confirms the significant lack of research into adverse outcomes after hospital discharge following surgery and highlights the fact that the evidence base to inform postdischarge clinical care and identify infants at high risk for focused support is extremely limited. As many reports derive from North American studies, and the research population is often limited to infants who have severe and complex cardiac diagnoses requiring staged surgery, care must be taken in generalising the findings from these existing studies to the wider UK population of infants with CHDs.
Despite our broad search strategy, which was intended to capture research into other life-threatening anomalies that require surgery during infancy, such as gastroschisis and diaphragmatic hernia,39 the only studies of postdischarge outcomes that met the inclusion criteria concerned CHDs. Of all congenital anomaly subgroups, the highest rate of infant deaths is associated with CHDs, and this may account for the greater interest in monitoring outcomes after hospital discharge. It is also notable that postdischarge outcomes of infants with CHDs came to prominence with the introduction of staged palliative surgery for HLHS, which led to improved early in-hospital outcomes40,41 and highlighted later interstage mortality as an important concern. 15,26
A limitation of our review was the rigour of our eligibility criteria, which excluded any studies that did not clearly differentiate between deaths that occurred before and after hospital discharge, and thus may have excluded from the review some studies that evaluated additional risk factors to those reported here. The relative lack of studies reporting postdischarge surgical outcomes may also simply reflect the limited monitoring of late adverse events and, specifically, of events occurring in the community or primary care setting. 31
Ethnicity and deprivation
In three US studies26,32,34 included in our review, patients of Hispanic ethnicity were found to be at a greater risk of adverse outcomes relative to white patients post hospital discharge. This confirms previous research which has shown that US Hispanic communities are more likely to experience multiple barriers to health care including language and immigration status, and financial barriers, such as lack of health insurance or low family incomes. 42,43 However, the impact of ethnicity and socioeconomic deprivation demonstrated in US-based studies may be influenced by an individual family’s ability to pay for care42–49 and the relevance of these findings for the UK health-care system is uncertain. Nevertheless, there is evidence that lower-income families in the UK also experience a considerable financial burden when caring for their child with CHD and that this may affect care-seeking behaviours. 49 The results of our review therefore add to the growing body of evidence suggesting that patients from minority ethnic and lower socioeconomic groups are more likely to experience barriers to timely and appropriate access to care and underlines the relevance of these factors to the population of infants with CHDs following hospital discharge.
Medical factors
Postoperative feeding and growth were also significantly associated with adverse outcome in several studies33,34,36 identified within the review, consistent with previous research. 50 However, the relationship between feeding difficulties and adverse outcomes post discharge is likely to be complex because of potential confounding with poorer cardiac status and other comorbidities; therefore, it requires further investigation.
Readmission to hospital will depend on both the child’s clinical state and the response to this by parents and medical staff. It is possible that readmission signified a timely response to a child’s deteriorating clinical state in some cases, whereas in others it was a response to a child who became seriously unwell. In our review, we considered an unplanned readmission to be an adverse event indicating that a child deteriorated unexpectedly at home and so did not experience a stable clinical course after discharge. Nevertheless, it is possible that the risk factors for readmission may differ from those for deaths and this may have contributed to the breadth of different risk factors identified.
Conclusion
We identified several key medical and social factors associated with a higher risk of mortality or unplanned hospital readmission for children discharged from hospital after paediatric cardiac surgery. Some of these risk factors, such as feeding difficulties, would be amenable to modification through specific interventions, whereas others enable health professionals (HPs) to identify children who are at greatest risk of adverse outcomes and to offer additional support, such as HMPs, targeted more effectively at vulnerable children and their families within the community setting. Although there were no studies of social and financial factors within the UK health-care context, unequal access to care may disproportionately affect minority ethnic communities and low-income families and should be a focus for future research in the UK. Crucially this review highlights an evidence gap and important need for longer-term studies to investigate the risk factors for out-of-hospital outcomes after surgery separately from in-hospital outcomes. Such evidence would better inform postdischarge care and community-based interventions to improve long-term survival and quality of life of infants with CHDs.
Chapter 3 A systematic review of non-invasive interventions for infants discharged from hospital after major surgery for congenital anomalies
Introduction
The purpose of this systematic review was to identify and evaluate the effectiveness of non-invasive interventions, both in and out of hospital, aimed at reducing adverse outcomes when children are discharged home following major surgery for congenital anomalies. Although the options for intervention during hospital admissions for surgery and associated early outcomes are reasonably well understood in this patient population, less is known about non-invasive interventions such as education, training and home monitoring, which are used in preparation for discharge from hospital and post discharge outside the hospital setting. We elected to broaden our systematic review beyond the specific patient group of interest, to incorporate all studies related to major surgery for congenital anomalies, as we considered the possibility of translatable knowledge between this wider area of practice and the postdischarge management of babies undergoing surgery for CHD. Information about the range and effectiveness of treatment packages available is critical to the development of interventions that could reduce out-of-hospital mortality rates and preventable unplanned readmission in these vulnerable children.
Methods
Protocol and registration
The protocol for this review is registered with the International Prospective Register of Systematic Reviews (PROSPERO) CRD42013003484. 51
Search strategy
Electronic databases
Electronic database searches were carried out on 8 March 2013. MEDLINE (1980 to March 2013), EMBASE (1980 to March 2013), CINAHL (1981 to March 2013), The Cochrane Library (1999 to 1 February 2013), Web of Knowledge (1980 to March 2013) and PsycINFO (1980 to March 2013) databases were searched using the search strategy detailed in Appendix 3.
Reference checking
A forward citation search was carried out on the reference lists of all selected studies to identify additional published studies for review.
Hand searching of specialist conference abstracts from the Association for European Paediatric Cardiology and the American Heart Association was also undertaken for the period of 2008–12.
Selection of studies
Eligibility criteria
Studies published between January 1980 and 8 March 2013 were included. Major surgery was defined as surgery requiring intensive or high-dependency care in the postoperative period and studies were eligible for inclusion only if they reported outcomes for children who were discharged from hospital separately from in-hospital surgical outcomes. To ensure relevance to neonatal and infant survival, only studies involving children from birth up to the age of 5 years were included in the review. Box 2 includes the list of criteria.
Studies that include children:
-
aged from birth up to 5 years of age
-
with a life-threatening congenital abnormality
-
who have undergone major surgery (involving intensive care) for potentially life-threatening congenital disease
-
who were discharged home from hospital following their successful surgery.
Studies that:
-
refer exclusively to adults, children over the age of 5 years or for which the group of interest is not clearly defined
-
have no abstract or for which the abstract was not available in English
-
include previously healthy children who had major surgery as a consequence of traumatic injury
-
do not refer to adverse outcomes (e.g. death or unplanned hospital readmission)
-
included children discharged home after surgery on a palliative care pathway
-
do not refer to an intervention or for which the intervention was invasive
-
do not have a control group.
The following were also excluded from the review:
-
single case studies and case series of fewer than 20 cases
-
personal communications, letters and commentaries.
Titles and abstracts for all studies were scanned by two reviewers (J Tregay and J Wray) against the eligibility criteria. Full-text articles of studies meeting eligibility criteria were independently assessed by three authors (J Tregay, K Brown and R Knowles) to determine whether or not they met criteria for inclusion. Discrepancies between reviewers were resolved through discussion with a fourth reviewer (J Wray).
Data extraction
Data extraction was independently completed by two reviewers (J Tregay and K Brown) and included information on study design, population, diagnosis, comparison groups, outcomes and the intervention characteristics.
Quality assessment
Two reviewers (J Tregay and K Brown) assessed the methodological quality of studies included for review. Studies were assessed on the basis of study design using levels of evidence (LOEs) rated from 1 to 4; with more rigorous study designs, for example randomised control trials, given a rating of 1 and the least rigorous, such as case series, given a rating of 4. Studies within each LOE were assessed as A (high quality) to C (lowest quality), dependent upon predetermined criteria such as confounding, completeness or follow-up and objective measurement of outcomes (see Appendix 2).
Data analysis
Data were not combined in a meta-analysis and are presented below in a qualitative synthesis. This approach to analysis was selected given the small number of studies found and their methodological quality. Studies were qualitatively reviewed by two researchers (K Brown and J Tregay) attempting to answer the following questions:
-
What were the constituents of the interventions described in terms of inclusion criteria and protocols deployed?
-
Was there evidence in respect of treatment effect: what outcome measures have been used and were there any important biases?
-
Do any important limitations, concerns or constraints exist when considering wider application of the intervention described?
Results
Presentation of results
Studies included in the review are summarised in Tables 3–6. Table 3 contains information about study quality; Table 4 provides a comparison between monitored patients and historical control patients in each of the studies; and Tables 5 and 6 summarise the protocols for the interventions concerned and their outcomes. Studies conducted by the same research group using the same patients are combined together for presentation in the tables.
| First author, year | LOE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LOE 1 | LOE 2 | LOE 3 | |||||||
| Study quality | A | B | C | A | B | C | A | B | C |
| Dobrolet, 201152 | ✓ | ||||||||
| Ghanayem, 200315 | ✓ | ||||||||
| Ghanayem, 200453 | ✓ | ||||||||
| Ghanayem, 200654 | ✓ | ||||||||
| Hansen, 201255 | ✓ | ||||||||
| Husain, 201256 | ✓ | ||||||||
| Petit, 201157 | ✓ | ||||||||
| Srinivasan, 200958 | ✓ | ||||||||
| First author, year | Patient group | Data collection period | Patient diagnoses | Surgical era | Age at first surgery in days (rangea) | Median weight at first surgery in kilograms (rangea) | Norwood type surgery: % RVPA versus % MBTS | Median age at discharge: entry into HMP in days (rangea) | Median age at second-stage surgery (rangea) |
|---|---|---|---|---|---|---|---|---|---|
| Dobrolet, 201152 | Intervention group | January 2002–January 2010 | 19 HLHS + 40 SV | January 2006–January 2010 | 5 (± 1)b | Birthweight 3.2 (± 0.1) | Not stated | 39b | – |
| Control group | 36 HLHS + 68 SV | January 2002–December 2005 | 12 (± 2)b | – | Not stated | 42b | – | ||
| Hansen, 201255 | Intervention group | January 1996–September 2009 | 45 HLHS | October 2005–September 2009 | 5 (3–29)b | 3.3 (± 0.5) | 3% RVPA, 97% MBTS | 42 (26–76) | 102 days (67–299 days)b |
| Control group | 97 HLHS | January 1996–October 2005 | 7 (1–52)b | 3.26 (± 0.58) | 1% RVPA, 99% MBTS | 41 (9–185) | 152 days (77–1372 days)b | ||
| Not discharged | 20 HLHS | January 1996–September 2009 | 7.0 (4–47)b | 3.19 ± 0.55 | 100% MBTS | N/A | 77 days (34–207 days) | ||
| Husain, 201256 | Intervention group | 2006–11 | 24 HLHS | 2008–11 | – | – | Hybrid | – | 176 days |
| Control group | 27 HLHS | 2006–8 | – | – | Hybrid | – | 168 days | ||
| Ghanayem, 200315/200453/200654 | Intervention group | July 1996–October 2004 | 70 HLHS | September 2000–October 2004 | Matched in 2003 paper | Matched in 2003 paper | MBTS only is mentioned | 37.4 approximately (2003) | 4.2 months (± 1.4 months)b |
| Control group | 54 HLHS | July 1996–September 2000 | Matched in 2003 paper | Matched in 2003 paper | MBTS only is mentioned | 43.1 approximately (2003) | 5.6 months (± 2.1 months)b | ||
| Petit, 201157 | Intervention group | January 2002–January 2010 | 88 of which 49 HLHS | September 2007–January 2010 | 8.5 ± 6.6b | 3.09 ± 0.51 | 24% RVPA, 64% MBTS | 37.5 ± 19 | 157 days (± 49.4 days)b |
| Control group | 116, of which 82 HLHS | January 2002–August 2007 | 13.8 ± 12.4b | 3.17 ± 0.66 | 1% RVPA, 99% MBTS | 43.8 ± 30 | 221 days (± 112 days)b | ||
| Srinivasan, 200958 | Intervention group | 2001–7 | 38 HLHS | 2005–7 | 6 (2–64) | 3.3 (23–4.3) | 70% RVPA, 30% MBTS | – | 153 days (71–325 days)b |
| Control group | 28 HLHS | 2001–4 | 10 (3–218) | 3.2 (2.0–5.7) | 58% RVPA, 42% MBTS | – | 206 days (102–441 days)b |
| First author, year | Data collection period | Parent education | Daily O2 saturation | Daily weight | Daily fluid intake | Feeding intervention? | Breach criteria/protocol | How monitored |
|---|---|---|---|---|---|---|---|---|
| Dobrolet, 201152 | January 2006–January 2010 | ✓ | ✓ | ✓ | – | Feeding protocol | Yes, but not specified | Weekly telephone call from a nurse practitioner |
| Hansen, 201255 | January 1996–September 2009 | ✓ | ✓ | ✓ | ✓ | No tube feeding at discharge | Saturation < 75% | Weekly telephone call from a cardiologist |
| Weight gain < 20 g in 3 days | ||||||||
| Weight loss of > 30 g in 1 day | ||||||||
| Husain, 201256 | 2006–11 | – | ✓ | ✓ | – | – | Yes, but not specified | Weekly telephone call from a nurse practitioner |
| Ghanayem, 200315/200453/200654 | July 1996–October 2004 | ✓ | ✓ | ✓ | – | 25% had gastrostomy | Saturation < 75% or acutely reduced | Parents call specialist centre if criteria are breached |
| Weight loss of 30 g | ||||||||
| Failure to gain weight of 20 g over 3 days | ||||||||
| Petit, 201157 | January 2002–January 2010 | ✓ | ✓ | ✓ | – | Feeding intervention including gastrostomy and nutritionist | Saturation < 75% | Weekly telephone call from nurse practitioner; multidisciplinary team meeting × 1 per week |
| Failure to gain weight for 3 days | ||||||||
| Srinivasan, 200958 | 2001–7 | ✓ | ✓ | ✓ | – | 80% had gastrostomy | Saturation < 75% or acutely reduced | Parents call specialist centre if criteria are breached |
| Weight loss of 30 g | ||||||||
| Failure to gain weight of 20 g over 3 days |
| First author, year | Data collection period | Patients | Breach % | Outcomes, noted where significant difference between groups p < 0.05 | ||||
|---|---|---|---|---|---|---|---|---|
| Earlier stage 2 surgery | Detection of residual lesions | Improvements in weight gain | Improved interstage mortality (% HMP vs. % control) | Improved longer-term survival with HMP | ||||
| Dobrolet, 201152 | January 2006–January 2010 | 54 HLHS + other SV; 104 historic control patients | – | – | ✓ | – | Yes (2.5% vs. 7%) | – |
| Hansen, 201255 | January 1996–September 2009 | 45 HLHS; 97 historic control patients | 31 | ✓ | – | ✓ | Yes (2.5% vs.12.4%) | – |
| Husain, 201256 | 2006–11 | 24 HLHS; 27 historic control patients | 62.5 | – | – | ✓ | No (21% vs. 26%) | – |
| Ghanayem, 200315/200453/200654 | July 1996–October 2004 | 70 HLHS; 54 historic control patients | 57 | ✓ | – | – | Yes (1% vs.15.5%) | Actuarial survival |
| Petit, 201157 | January 2002–January 2010 | 88 HLHS + other SV; 116 historic control patients | – | ✓ | – | ✓ | No (8% vs.12%) | 1-year survival |
| Srinivasan, 200958 | 2001–7 | 38 HLHS; 28 historic control patients | – | – | – | – | No (10% vs.12%) | Survival to stage 2 |
Studies selected
Title and abstract screening identified 20 studies for potential inclusion in the review. A further four studies were identified through forward citations. Following full-text screening of these 24 studies, 16 studies did not meet eligibility criteria and were excluded, leaving a total of eight studies for inclusion in the review (Figure 3).
FIGURE 3.
Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) flow chart showing study selection process.
Methodological quality
Eligible studies were all prospective cohort studies using retrospective historic controls and were of fair to good quality (rated A or B; see Table 3). Typically, controls were drawn from the time period immediately preceding introduction of the intervention.
Study populations
Despite a broad search strategy designed to encompass a range of congenital conditions requiring surgery, all studies (eight studies from six research groups) that were eligible for inclusion related to patients with CHD, specifically SV diagnoses, such as HLHS. HLHS is complex congenital heart defect necessitating a series of palliative surgical procedures to sustain life, including two in the first year. Infants undergoing these palliative procedures remain fragile, with mortality between their first and second operations a recognised complication for a range of reasons, many of which relate to the underlying heart disease30,35 becoming more stable after the second-stage operation.
The terminology used to describe diagnoses and operative procedures differed slightly between studies: this information is summarised in Table 4, which indicates that four of the research groups studied infants with diagnoses of predominantly HLHS and other related lesions that went down the pathway of a Norwood type stage 1 operation with either MBTS or RVPA15,53–55,58 or a hybrid procedure56 (the hybrid approach is a more recently developed treatment pathway applicable to HLHS and related conditions, which incorporates a series of palliative procedures involving both surgery and interventional cardiology or catheterisation), and two of the research groups studied both infants with HLHS type diagnoses who underwent a Norwood type stage 1 operation and infants with other SV diagnoses who underwent an appropriate operation based on diagnosis including isolated MBTS, Damus–Kaye–Stansel repair and pulmonary artery banding. 52,57 Five of six research groups were from the USA and one was from Germany.
Comparison between monitored patients and control patients
All patients in HMP groups were compared with historical control patients, typically taken from the period immediately prior to the commencement of the HMP under evaluation (see Table 4). This means that the studies under review are covering the surgical period between 1996 and 2009, with the shortest period of time between the first historical control patient and the last patient to be included in the HMP being 5 years in the study of infants undergoing the hybrid procedure56 and the longest being 13 years in the study from Germany. 55 There appeared to be a trend in practice towards slightly younger age at initial palliative operation by era across the studies, leaving the patients in the historic control groups slightly older at surgery and at discharge than the patients in the more recently recruited HMP group.
Intervention types
All studies meeting criteria for inclusion involved the evaluation of HMPs, which represent detailed protocols for management from hospital discharge (details in Home monitoring programmes) aiming to enhance patient supervision and respond early to any deterioration. No studies involving any other intervention types focusing on the post-hospital discharge period were identified as meeting inclusion criteria; however, in one study58 home monitoring was only one component of a broader protocol-based intervention that began in hospital prior to surgery. In this study, Srinivasan et al. 58 detail a standardised management protocol for the treatment and follow-up of infants undergoing the Norwood procedure, a complex surgical intervention for patients with SV congenital heart diagnoses incorporating all aspects of in-hospital and early post-hospital management including a HMP.
Home monitoring programmes
Although information about the content of the monitoring was inconsistently reported across studies, it was clear that all HMP included daily oxygen saturation and weight monitoring with the use of ‘breach criteria’ as detailed first by Ghanayem in 2003:15 see Table 5 for details.
-
All studies excepting one, which was a briefer conference abstract,56 explicitly mentioned parent education as a component of the HMP. This education typically involved giving information about how to operate any monitoring equipment, feeding, medications and signs and symptoms including ‘breach criteria’. The way in which this information was given to families was not always specified but may have involved different approaches. One study52 described a comprehensive written guideline outlining when to contact their cardiologist or high-risk team in addition to a ‘medical passport’ which included information about their child’s anatomy, surgical procedures and expected oxygen saturations. In the same study parental empowerment to initiate contact with HPs is highlighted as a key factor. In another study,55 parent education involved one night of ‘rooming in’ with their baby for at least 24 hours in order to learn how to operate the vital sign monitor they were to use to monitor their child at home.
Despite similarities in the content of monitoring and ‘breach criteria’, the studies differed in respect of:
-
Feeding, which in particular was approached very differently across the studies. Gastrostomy was explicitly mentioned as being a primary strategy for feeding at home for infants with feeding difficulties by three research groups across four studies,53,54,57,58 with uptake of gastrostomy as high as 80% in one study. 58 One study52 incorporated a postdischarge feeding protocol concerning the type of formula and target intake, and another involved a feeding team to oversee this aspect. 57 In contrast to these US-based studies, the German study55 specified that infants should meet home surveillance criteria, which included the ability to feed without tube supplementation for at least 1 week prior to discharge: 12% of patients remained in hospital interstage because they did not achieve this milestone.
-
The approach to contact post discharge: infants were typically monitored by the specialist paediatric cardiac centre by way of both routine follow-up appointments and weekly telephone calls from either a nurse practitioner52,56,57 or a cardiologist. 55 In two studies, details of the specialist centre follow-up were not reported, although parents were instructed to telephone the centre if criteria were breached. 53,58 In one study, a multidisciplinary team met weekly to review all patients on the HMP. 57 This team consisted of six paediatric cardiologists, six nurse practitioners, a social worker, a nutritionist and a developmental paediatrician. The same study noted increased resource use in terms of clinic visits and hospital admissions in the HMP group.
Outcomes
All studies reported a degree of positive effect linked to HMP:
-
Three research groups reported reduced interstage mortality rates in monitored patients versus control patients. 53,55,56 Two studies reported improved initial survival after stage 1 surgery, a non-significant difference (trend to improvement with HMP) in interstage outcome and improved survival at 1 year. 57,58 The latest study by Ghanayem et al. 54 reported improved actuarial survival with HMP in addition to reduced interstage mortality. Interestingly, the only study including patients that underwent a hybrid procedure reports the highest interstage mortality and the highest rate of breaches in both HMP and control groups (non-significant trend towards improvement with HMP). 56
-
The three research groups which included data on age at stage 2 operations reported that stage 2 surgery was performed at a younger age in monitored patients than in control patients;15,53–55,57 two of the relevant research groups noted that the earlier, and in their view more optimal, patient-specific timing of stage 2 operations was linked to a reduction in interstage mortality. 53,55
-
Three studies reported that monitored patients showed improvements in weight gain relative to control patients. 55–57
-
Three studies reported the statistics for breaches of the HMP: these occurred in 31%,55 57%15,53,54 and 62.5%56 of patients (most commonly desaturation) with interventions being enabled for the patients concerned potentially in a more timely manner. One study noted that HMP enabled the timely detection of residual lesions, which could then be treated. 52 The study reporting interstage emergency admissions in patients from the control groups (notably the study on hybrid patients) stated that the number of readmissions was similar in the HMP and control groups;56 however, comparisons between the HMP and the control groups are difficult in this regard because control participants did not have breach criteria.
Discussion
The systematic review aimed to identify and evaluate postdischarge interventions for infants following major congenital surgery. Despite using a search strategy designed to encompass all congenital abnormalities requiring surgery, only eight studies from six research groups relating to HMP for infants with CHD diagnoses (HLHS and SV) met criteria for inclusion in the review. There was a paucity of such studies based in Europe and, notably, none was from the UK. The included studies shared similarities in terms of the HMP deployed in that all of them involved standardisation of discharge and follow-up processes including the designation of professionals responsible for this, parental education, and enhanced patient monitoring including regular measurement of weights and saturations. It was noted in a recent review of HMP that, since the causes of interstage death in these infants are multifactorial, a single intervention on one aspect of care is unlikely to help hence favouring a package of care. 59
Outcomes with home monitoring programme
All studies reported benefit in terms of outcome; however, comparisons between patient groups were based on historic control patients in an era during which outcomes for children with CHD have improved. 3,60 Although it seems likely that interventions such as HMP have contributed to improved long-term outcomes over the era in which these have been introduced, the lack of randomised studies means that this cannot be confirmed.
The mortality rate at 1 year is an outcome measure that offsets the effect of earlier stage 2 surgeries in the context of HMP, and hence a shortened period of interstage follow-up, which is a bias to consider when the reduced interstage mortality rate is presented as a primary outcome measure. Three of the research groups presented evidence for improved longer-term survival (to stage 2 in the form of competing hazards over time,58 to 1 year57 and actuarial survival54) in the context of HMP; however, two of the research groups attributed these improvements to the whole patient pathway, in particular immediate post-stage 1 outcomes. These two studies57,58 found non-significant reductions in interstage mortality with HMP; given that these are single-centre studies involving a maximum of 204 patients, and that mortality in the control group was 12% in each case, studies may be underpowered unless there is very large drop in interstage mortality in the HMP group.
Specific patient types
Hypoplastic left heart syndrome
Three of the study populations in the review (in five of the papers15,53–55,58) were infants with HLHS-type conditions being managed using the Norwood pathway, among whom the interstage mortality rate, in the absence of a HMP, has been reported as between 10% and 14%. 35 Additional informative data on interstage mortality in HLHS come from the SVR trial, which is a large North American multicentre randomised trial of 426 patients comparing surgical techniques (MBTS vs. RVPA) for the stage 1 operation, in which the interstage mortality was 12% in 2012. 26 Although HMP is reportedly widely accepted as the standard practice for patients post Norwood operation in the USA, the protocols for HMP may vary. 61 The SVR trial data indicate that in the North American population concerned, interstage death rates also vary depending on the surgical approach for stage 1 operation (see Table 2; MBTS is higher risk than RVPA), specific cardiac risk factors, prematurity, ethnicity and deprivation (see Chapter 2). Among the eight studies reviewed, the surgical approach to stage 1 was reportedly broadly similar in HMP and control groups; however, from the presented data it is not possible to be sure that other important patient factors did not differ between groups. That said, the data do support the benefit of HMP for HLHS-type conditions.
Other types of functionally univentricular heart (not hypoplastic left heart syndrome)
Two of the included study populations52,57 were a mixture of infants with HLHS and other SV diagnoses who underwent, for example, MBTS procedure or pulmonary artery banding only, and hence the interstage mortality may have been influenced by a slightly lower average patient complexity. One of these studies reported the lowest interstage mortality for the control group at 7%, although with a HMP this was significantly reduced, to 2%. 52 SV infants undoubtedly suffer interstage mortality without HMP, as evidenced by the control groups in the included two studies52,57 and other reports of interstage mortality in SV infants without HMP which may be as high as 10%. 62 Although suggestive of benefit, there are fewer data available on HMP for SV infants who are not on a Norwood pathway (two studies in the review that also contained HLHS patients compared with five studies devoted to HLHS patients). SV patients have a diverse range of diagnoses and undergo several different surgical pathways depending on what is appropriate for the individual patient; this is a further challenge in terms of evaluating the issue.
Hybrid patients
To date the information on infants with a hybrid circulation in terms of benefit from HMP is very limited, comprising only one conference abstract56 (included in this review), which, as discussed in Outcomes, showed the worst patient outcomes in both HMP and control groups (see Table 6). Intuitively, infants with hybrid circulations are as vulnerable as, if not more vulnerable than, infants with a Norwood circulation, and hence it would be difficult to exclude them from HMP if advocating this for Norwood infants.
Practical and resource implications
The included studies indicated that HMPs involve the commitment of considerable dedicated resources; for example, one study reported that a multidisciplinary team comprising six paediatric cardiologists as well as several other professionals met weekly to discuss patients. 57 In addition, studies noted that the burden in terms of clinical visits and other patient contacts was increased with HMP. However, conversely, information about the health-care costs of infants who deteriorate in the absence of surveillance, and for example who require intensive interstage care, is lacking. Interestingly, the use of gastrostomy to provide feeding assistance in SV infants and infants with HLHS appears to be higher in the USA than in Europe (26% of infants in the SVR trial were fed by gastrostomy tube between stages);26 in Europe infants may be kept in hospital as inpatients because of feeding problems55 or, alternatively, given nasogastric feeding at home but are very rarely fitted with a gastrostomy tube (personal communication from Dr R Franklin, Royal Brompton Hospital, and L Smith, Great Ormond Street Hospital, both in London, UK, 2013). These alternative approaches may have differing resource implications, and a more detailed evaluation of this aspect is required in order to understand it better.
Studies of interest that did not meet inclusion criteria
Studies that were detected by the search strategy and reviewed as part of the study, but did not meet inclusion criteria included the following.
Discharge of the high-risk neonate (protocol from USA)
Discharge and follow-up protocols for high-risk infants have been issued by the American Academy of Pediatrics. 16,63,64 These guidelines address the needs of (1) the preterm infant; (2) the infant with special health-care needs or dependence on technology; (3) the infant at risk because of family issues; and (4) the infant in whom early death is expected; however, no specific studies were found detailing the implementation or effectiveness of these guidelines.
Telecardiology
One study65 described the use of novel methods, including ‘telecardiology’, for surveillance of high-risk infants in a low-resource rural setting. ‘Telecardiology’ was found to be a successful method of obtaining a diagnosis in infants with CHD. In another study, ‘telecardiology’ was used to aid follow-up in developed-world setting, including in a rural population. 66 However, these studies did not report data on the effectiveness of use of these methods in a post-procedural context and hence did not meet inclusion criteria for the review.
Conclusion
This review set out to describe and evaluate postdischarge interventions for infants who have undergone major congenital surgery. Surprisingly, only studies involving HMP for infants with CHD (HLHS or SV) met the criteria for inclusion in the review. The prominence of CHD in this review may be a result of the relatively high risk of postdischarge morbidity and mortality in these patients, particularly those undergoing staged palliative procedures. The evidence base for postdischarge interventions in this population would be strengthened by further prospective studies, such as randomised controlled trials, in patients with congenital heart disease and those with other complex congenital conditions; studies from research groups outside the USA; reporting of longer-term outcomes, to at least a year; and more detailed reporting of the content of intervention programmes and of demographic information such as ethnicity and socioeconomic status, which are known to be associated with adverse outcomes in paediatric patients with cardiac disease.
Chapter 4 Development of a risk model for death or emergency readmission within 1 year following hospital discharge from infant cardiac intervention for congenital heart disease and identification of patient risk groups for the purposes of service improvement
Some of the text of this chapter has been published previously as Crowe et al. , 2016. 67 © 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell. This is an open access article under the terms of the Creative Commons Attribution-Non Commercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Introduction
The main focus for the audit of paediatric cardiac surgery outcomes in registries or multi-institutional databases to date has been operative mortality, expressed as either 30-day11 or discharge outcome. 68 These early mortality outcomes have improved over time to the current, historically low, levels. 4 However, although important, these outcome measures are relatively limited in scope, and longer-term measures of outcome and metrics such as morbidity or complications are also essential to consider in quality assurance and improvement. A challenge for the audit of longer-term events for patients with CHD at a population level, in national or international registries outside the UK, such as the European Association of Cardiac Surgery database and the Society of Thoracic and Cardiovascular Surgery database in North America, is the reliable capture of appropriate data. The UK has a unique resource in mandatory national audit data sets for both paediatric cardiac procedures, represented by NCHDA,69 and paediatric intensive care unit (PICU) admissions, represented by PICANet,70 as well as life status tracking that enables late deaths to be reliably and independently ascertained. In respect of life status ascertainment, the NCHDA submits regular requests to the Central Register of NHS patients, as approved by the National Health Research Authority, in order to ascertain the survival or life status of patients. This information is reliably forthcoming for all patients possessing an NHS number who are based in England and Wales. Unfortunately, life status tracking is not currently available in Scotland or in Northern Ireland, where the two relevant specialist centres are responsible for ascertaining the life status of their own patients.
These UK national audit data sources enabled a national study addressing the following aims.
-
To explore patient-level risk factors for postdischarge death outside a planned PICU admission (outcome 1) and for postdischarge death or emergency readmission to PICU (outcome 2) in infants with CHD undergoing interventions in the UK.
-
To develop a clinically meaningful classification of patients in terms of the level and nature of their risk of outcome 2, with a view to informing improvements to the services provided for infants discharged alive following an initial major intervention for CHD.
Methods
Constructing the data set for analyses
Data sources and patient population
Data submissions to NCHDA have been mandatory since 2000 for all hospitals performing cardiac surgery in the UK, with data validated and subject to a quality assurance processes (all hospitals are inspected annually with local records examined to ensure every case has been submitted and a random sample of case notes examined to assess data quality). 71 All UK PICUs submit data to PICANet, which is also validated and subject to a quality assurance processes (PICUs receive annual visits from a PICANet team, during which submitted data are verified against hospital notes and admissions numbers are checked). 72
For this study, all children who received their first recorded interventional catheterisation or cardiac surgery procedure for a congenital heart defect at < 1 year of age and in the period 1 January 2005 to 31 December 2010 were identified by NCHDA. (Data were requested for the period 1 January 2005 to 31 March 2011 but were supplied for the time period specified above because of reporting restrictions within NCHDA.) In accordance with governance approvals, records within PICANet for these patients were then identified by a trusted third party by matching of unique patient NHS number. Data extracts fulfilling these criteria from each audit were provided to the study team as two separate data sets with the same patient-level identifier (pseudonymised NHS number). A single patient potentially had multiple procedure-based records in the NCHDA extract and multiple admission-based records in the PICANet extract. A flow diagram indicating the number of records present for each stage of this process is provided in Appendix 4.
The study team then constructed a single patient-based analysis data set by linking events pertaining to the same patient within each of the extracts and between the two using the patient-level identifier. This enabled information from both sources to be used in the analysis, with cardiac-related details and life status available in NCHDA and rich comorbidity and emergency PICU admission information available in PICANet.
Defining the index admission period and index procedure
The index admission period for each child was defined as the continuous period as an in-patient within a specialist paediatric cardiac hospital or PICU that started with or included their first surgical or interventional catheter procedure (see Appendix 5 for the list of procedures and their subgroupings; we included interventional cardiology procedures applicable to neonates or small infants that are undertaken with the aim of either primary correction or primary palliation such that a child can be discharged home). This period defined the index LOS within the specialist hospital (and associated PICU care).
Within the NCHDA data set, each procedure is described by a combination of up to eight individual procedural International Pediatric and Congenital Cardiac Codes (IPCCCs). 73 An algorithm developed by the NCHDA Steering Committee (which includes experienced paediatric cardiac surgeons and cardiologists) links the individual IPCCCs for a given record to one of 57 specific procedures, that is recognisable surgical operations or catheter procedures (see Appendix 5). The algorithm imposes a hierarchy, with the record assigned the most complex specific procedure consistent with the collection of codes recorded. Approximately 85% of procedures fall into one of these 57 specific procedures.
As some children undergo more than one procedure within a single index admission period, an individual’s index procedure was defined by applying the NCHDA-specific procedure hierarchy across all of the interventions carried out during the index admission. Therefore, the index procedure for each patient was defined as the most complex (specific) procedure (either surgery or interventional cardiology procedure) undergone during the index admission period. In a small subset of patients, an interventional catheter procedure was logged in NCHDA before the index admission. Based on clinical knowledge of the patient histories the research team considered that these early interventional catheters, which in most case were balloon atrial septostomy for a neonatal transposition of the great arteries (TGA), should not represent the index procedure. Instead the index procedure was considered to be an arterial switch operation carried out during a subsequent admission.
Exclusions
Children undergoing an excluded catheter procedure only (see Appendix 5 for the list of catheters included and excluded; rationale stated above), and therefore without an index admission, were excluded from the analysis. Premature babies who underwent only ligation of patent ductus arteriosus were also excluded because, unlike other infants with disease, the majority of these premature infants would have been subject to discharge and follow-up processes run mainly from neonatal intensive care units. Similarly, transplant patients have their own specific pathway of care and so were removed from the analysis. Finally, patients with unknown life status were removed (this included all patients within two UK cardiac units outside England whose life status was not linked to the NHS Central Register; see comment in Introduction).
Patient variables
Potential risk factors available in the patient-based analysis data set that would be known at the point of discharge were prespecified and are listed in Table 7, grouped according to whether they were non-medical, preoperative or postoperative factors. As detailed in the following sections, some variables were simplified prior to the statistical analyses in order to reduce the number of values (degrees of freedom) in the model and hence the risk of overfitting (see Harrell et al. 76). An appropriate power calculation was performed. The origin of each risk factor (either the PICANet or the NCHDA data set) is noted in Table 2, along with whether it relates to the index procedure or index admission period or is a characteristic of the child. Where both audits contained information on a particular risk factor, the risk factor was taken from the source that was most descriptive and complete or, in the case of ethnicity, a combination of the two (see Table 7). Some post-procedural factors originating from different audits overlap but were considered to provide sufficiently different information to include within multivariate analyses. In what follows, we briefly describe each of the patient variables considered in the analysis.
| Prespecified potential risk factor | Description | Variable level | Values | Source data set |
|---|---|---|---|---|
| Non-medical | ||||
| Deprivation | Quintiles of the English IMD 201074 as recorded for the index procedure | Child | 1–5 (1 most deprived, 5 least deprived) | NCHDA |
| Ethnicity | Census 2001 classification used by the Office for National Statistics. If the child had multiple records, the most frequently recorded ethnic group was used | Child | White, mixed, Asian, black, Chinese, other, not stated | PICANet |
| Preoperative | ||||
| Cardiac diagnosis group | Aggregated groupings of the cardiac diagnosis categories assigned through application of a modified version of the hierarchical mapping scheme developed by Brown et al.75 across all cardiac records for a child (see Appendix 6) | Child | VSD, HLHS, UVH/PA, other | NCHDA |
| Acquired cardiac diagnosis | Assigned if any cardiac record in the index admission period had an IPCCC diagnostic code corresponding to the acquired cardiac diagnosis category (see Appendix 6) | Index admission | Yes, no | NCHDA |
| Specific procedure group | Aggregated groupings of the index specific procedures assigned through application of the NCHDA-specific procedure hierarchy across all interventions within the index admission period (see Appendix 5) | Index procedure | Corrective, palliative, ungrouped | NCHDA |
| Congenital anomaly | Assigned if any PICU record during the index admission period contained a Read Code corresponding to the congenital anomaly category (see Appendix 6) | Index admission | Yes, no | PICANet |
| Neurodevelopment condition | Assigned if any PICU record during the index admission period contained a Read Code corresponding to the neurodevelopmental category (see Appendix 6) | Index admission | Yes, no | PICANet |
| Prematurity | Assigned if any PICU record for the child contained a Read Code corresponding to prematurity or preterm birth (see Appendix 6) | Child | Yes, no, missing | PICANet |
| Sex | Most frequently occurring sex across all cardiac records for a patient (or sex at index procedure if tied) | Child | Male, female | NCHDA |
| Age at index procedure | Age recorded in cardiac record for the index procedure | Index procedure | Continuous variable | NCHDA |
| Weight-for-age z-score | Standardised weight-for-age at index procedure, calculated from index procedure weight and age using World Health Organization reference standards | Index procedure | Continuous variable | NCHDA |
| Antenatal diagnosis | Assigned if coded in any cardiac record for the child | Child | Yes, no, missing | NCHDA |
| Clinical deterioration | Assigned if any PICU admissions prior to discharge from the index admission were urgent and unplanned | Index admission | Yes, no | PICANet |
| Postoperative | ||||
| LOS | The length in days of the continuous period as an in-patient within a specialist paediatric cardiac hospital or PICU that surrounds a child’s first interventional cardiac procedure in infancy | Index admission | Continuous variable | NCHDA and PICANet |
| Additional surgical procedures | Assigned if any surgical procedures were performed during the index admission in addition to the index procedure | Index admission | Yes, no | NCHDA |
| Additional catheter procedures | Assigned if any catheter procedures were performed during the index admission in addition to the index procedure | Index admission | Yes, no | NCHDA |
| Need for renal support | Assigned if any PICU record within the index admission period indicated renal support was required (including dialysis and haemofiltration) | Index admission | Yes, no, missing | PICANet |
| Need for ECMO | Assigned if any PICU record within the index admission period indicated ECMO support was required | Index admission | Yes, no, missing | PICANet |
| Acquired comorbidity | Assigned if any PICU record during the index admission period contained a Read Code identified as an acquired condition (categories 1–8 in Appendix 7) | Index admission | Yes, no, missing | PICANet |
| Events in PICU | Assigned if any PICU record during the index admission period contained a Read Code corresponding to collapse or cardiac arrest, acquired injury or complications, or a non-cardiac operation in PICU (categories 9–11 in Appendix 7) | Index admission | Yes, no, missing | PICANet |
| Post-operative morbidity | Assigned if any cardiac record during the index admission period contained an IPCCC code identified as a post-procedural morbidity | Index admission | Yes, no | NCHDA |
Non-medical variables
Deprivation was defined using quintiles of the English Index of Multiple Deprivation (IMD) 2010,74 which is calculated at the level of small (≈ 1500 people) geographic areas covering England and combines deprivation indicators across income, employment, health and disability, education skills and training, barriers to housing and other services, crime and living environment. 77
Ethnicity information is recorded in both audits, with NCHDA using a bespoke classification scheme and PICANet using the Census 2001 classification used by the Office for National Statistics. 78 PICANet was therefore used as the primary source for our ethnicity variable for the purposes of potential comparability with the Office for National Statistics population statistics, with further collapsing of codes into seven groups for considerations of model stability: white, mixed, Asian, black, Chinese, other and not stated. The most frequently recorded ethnic group was assigned to the child if they had multiple admission records.
Preprocedural variables
Cardiac diagnoses and procedural information
Each NCHDA record contains up to six IPCCC diagnostic codes, the combination of which can be mapped to 1 of 24 primary cardiac diagnoses using a hierarchical scheme developed by Brown et al. 75 For the purposes of this study, this mapping scheme was implemented with two minor adjustments (see Appendix 6 for details). In addition to their overall primary cardiac diagnosis, a child was identified as having an acquired cardiac diagnosis if any of his or her records included an IPCCC diagnostic code mapping to this category, for example ventricular dysfunction and ventricular hypoplasia. Some non-cardiac IPCCC diagnostic codes were identified as post-procedural morbidities (see Non-cardiac diagnosis and comorbidity information and Appendix 7 for details).
For reasons of model reliability and validity of predictive discrimination (i.e. to reduce the risk of overfitting), we grouped the diagnostic categories into four cardiac diagnosis groups considered clinically meaningful to the study focus (see Appendix 6 for mappings, including the relevant references underpinning our selection of the groupings). The choice of groupings was informed by the literature reviews in that the majority of the included studies referred to HLHS as being at higher risk and of special interest for late mortality. Other studies, although a smaller number, noted higher risk with other types of SV conditions and palliated circulations (see procedural groupings that follow):
-
HLHS
-
functionally univentricular heart (UVH) or pulmonary atresia (PA) (including PA with an intact ventricular septum)
-
ventricular septal defect (VSD)
-
‘other’ (the remaining 22 diagnosis categories).
Alongside this, the index (specific) procedures were aggregated into three procedural groups (see Appendix 5 for mappings including the relevant references underpinning our selection of the groupings); again this grouping was based on the findings of the literature review in Chapter 2, which noted a higher risk in babies with palliated circulations:
-
palliative (e.g. Norwood, bidirectional cavopulmonary shunt, arterial shunt)
-
corrective [e.g. truncus arteriosus repair, atrioventricular septal defect (AVSD) complete repair, tetralogy repair]
-
‘ungrouped’ if not a specific procedure.
Non-cardiac diagnosis and comorbidity information
Non-cardiac diagnosis and comorbidity information was primarily sourced from PICANet, in which any given PICU admission can record up to 24 clinical Read Codes (a clinical coding system used as standard in general practice in the UK and maintained by the Health and Social Care Information Centre). In total 3325 discrete Read Codes were present in the data set and so to explore the potential for this information to add discriminatory power to the risk model, we developed a new clinically intuitive scheme linking each code to at most 1 of 17 system-based categories (see Appendix 7).
If any PICU admission within the index admission period contained a Read Code in the congenital anomaly category, then the child was assigned this attribute. This included syndromes such as Down and DiGeorge and non-syndromic congenital anomalies such as urogenital/renal malformations, tracheal/tracheo-oesophageal malformations, vision/hearing deficits and exomphalos/gastrointestinal malformations. Similarly, if any Read Code within the index admission period was linked to the neurodevelopmental category, then the child was assigned this attribute. These comprised a range of acquired and congenital conditions including epilepsy/seizures, developmental delay, sleep apnoea, hydrocephalus, retinopathy of prematurity, stroke, hemiparesis/hemiplegia, anoxic encephalopathy, cerebral venous sinus thrombosis and cerebral palsy. Finally, a child was assigned the attribute of prematurity (< 37 completed weeks’ gestation) if a Read Code linked to this category appeared within any of his or her recorded PICU admissions.
Additional preprocedural patient variables
Additional preprocedural factors considered on the basis of potential clinical relevance and availability within the NCHDA data set were patient sex, age at procedure, weight-for-age at procedure (calculated using World Health Organization reference standards) and whether or not there was an antenatal diagnosis. From the PICANet data set it was determined if the patient had clinically deteriorated prior to the index intervention (assigned as such if the index admission or any prior PICU admissions were urgent and unplanned).
Post-procedural variables
The following post-procedural patient variables known at the point of discharge were considered: LOS for the index admission period; whether or not any surgical or catheter procedures were performed during the index admission in addition to the index procedure; whether or not renal support was required during the index admission (including dialysis and haemofiltration); whether or not extracorporeal membrane oxygenation (ECMO) support was required during the index admission; whether or not the index admission was associated with any intensive care unit events (assigned as such if any admission to PICU during the index admission period contained a Read Code category for collapse or cardiac arrest, acquired injury or complications or a non-cardiac operation in PICU); whether or not the index admission was associated with any acquired comorbidities [assigned if any admission to PICU during the index admission period contained a Read Code acquired category (1–8 in Appendix 7)]; and whether or not the index admission was associated with post-procedural morbidity (assigned if any NCHDA record during the index admission period contained an IPCCC code corresponding to a post-procedural morbidity).
Missing data
When ethnic group was not available from PICANet, the NCHDA ethnic code was used to assign white, Asian or black ethnicity (which showed strong concordance across the two audits) but not to assign Chinese, other or mixed ethnicity (which showed poorer concordance). Sensitivity analyses were performed excluding records without PICANet-derived ethnicity. Index procedure weights recorded as zero (i.e. missing) were replaced by weight recorded within the same index admission (if available). Weight-for-age z-scores outside the range ± 5 z-scores were assumed erroneous and treated as missing.
Outcomes
Two nested outcomes of interest were defined.
-
Outcome 1: death within 1 year following discharge from the index admission and not during a planned admission.
-
Outcome 2: either death (outside a planned admission) or an emergency unplanned readmission to PICU within 1 year following discharge from the index admission. Outcome 2 was viewed as including ‘near misses’ for death, given that all patients experiencing emergency intensive care unit admission are gravely ill. A decision was made to combine deaths and near misses for this section of the analyses in order to increase the number of events, and to form a useful basis for classification and regression tree (CART) analysis was used to identify patient groups with different ‘profiles of risk’ that could usefully inform the development and prioritisation of interventions aimed at improving outcomes within this patient population.
In order to ascertain these outcomes the following information was used: age at death (if applicable) and life status, which were available in NCHDA; emergency unplanned admissions to PICU were extracted from PICANet (defined as admission type ‘unplanned’ or ‘unplanned after surgery’ AND identified as a retrieval from another unit as an emergency transfer AND not defined as an elective admission). Note that death within 1 year of discharge from index admission that occurred during a planned readmission to intensive care was not considered an adverse outcome in this analysis designed to inform improvements in postdischarge services.
Statistical methods
Descriptive and univariate analyses
Descriptive analyses were performed to characterise the data set and univariate logistic regression analysis was used to assess the relationship of each candidate predictor with each outcome using fractional polynomials to investigate departure from linearity. This informed which variables were considered in two further, complementary, strands of analysis: the development of risk models for outcomes 1 and 2; and the identification of patient groups differentiated by risk of outcome 2.
Developing risk models for outcomes 1 and 2
The significant variables from the univariate analysis were investigated in a multivariable model for each outcome in turn. Initially, models for outcomes 1 and 2 were developed in which the continuous predictors were used in their original form. For the final model development, however, the continuous predictors were categorised through a process of discussion between clinicians and analysts influenced by considerations of model interpretability as well as statistical performance.
Multiple imputation assuming data were missing at random was used to investigate missing data when fitting the models. We generated 20 data sets and ran a backward stepwise logistic regression (factors where p < 0.05 remained in the model), implementing a bootstrap (200 samples) for each imputed data set. This was done for preoperative and postoperative factors only. Factors were selected based on the inclusion frequency of each predictor, over the imputed data sets, that is the proportion of times that the factor appeared in the model. 79 A threshold of 50% was set and estimates were combined using the Rubin rules. 80 The final models were estimated by taking up the factors whose inclusion frequency exceeded the threshold, and estimating the regression weights of this predictor set on the imputed data.
Sensitivity analysis for the multiple models derived on the complete case data was also performed to explore the effect of including and excluding prematurity from the set of potential predictors (using Akaike criteria to assess model fit). Sensitivity of the results to adjustment for clustering at both the hospital level and regional level (English Primary Care Trusts81) was assessed for each model. The Hosmer–Lemeshow statistic was used to test calibration (goodness of fit)82 and discrimination was described using the c-statistic (area under the receiver-operator curve), corrected for overfitting using bootstrapping (an internal validation method that is an alternative to data-splitting and cross-validation83).
All analyses were performed in Stata statistical software (Version 12.1 StataCorp LP, College Station, TX, USA) and a p-value of < 0.05 was considered statistically significant.
Identifying patient groups differentiated by risk of outcome 2
Classification and regression tree analysis was used to identify patient groups with different ‘profiles of risk’ that could usefully inform the development and prioritisation of interventions aimed at improving outcomes within this patient population. The analysis was designed to fulfil this purpose rather than, for example, the development of a prediction tool (which was not our intention). For other examples of CART applications in health care, see Ridley et al. ,84 Garbe et al. 85 and Fonarow et al. 86 Given our aim here was to inform service improvement, we focused on outcome 2 so as to include the ‘near misses’ as well as deaths (with the combined higher incidence rate also providing greater model stability). The technique recursively partitioned the data into subsets that were as homogeneous as possible with respect to outcome 2, that is into subsets of increasing ‘purity’. 87 This was implemented using a Gini impurity measure with minimum change in improvement of 0.0001. All variables significantly associated with outcome 2 in univariate analysis (and weight-for-age z-score) were included in the CART analysis. The continuous variables were entered in their categorised form used in the risk model development (see Table 9). To prevent overfitting, the CART groups were developed in a random 60% of the data. In order to limit the number of groups created (for reasons of statistical robustness and potential usability), we restricted the tree depth to 4 and required a minimum of 100 cases for branching to continue, with at least 50 cases in either branch. The resulting classification tree was applied to the patients in the remaining 40% of the data set and the occurrence of outcome 2 among patients at each node was compared with the corresponding group in the development set to assess model stability. All analysis was performed in SPSS 22 (IBM SPSS statistical software 2013, Armonk, NY, USA).
Results
Data set and headline outcomes
Approximately 20% of records in NCHDA did not have a valid NHS number (the unique patient identifier used in this study to match across the two audits); many of these records pertain to overseas patients as only patients resident in England or Wales are allocated an NHS number. A total of 12,390 infants with a valid patient identifier and meeting the inclusion criteria for the study were identified in NCHDA, of whom 9385 (76%) were linked to at least one record in PICANet. Failure to link a record from NCHDA with one in PICANet could be accounted for by a procedure (in general a catheter) which occurred without an intensive care admission or the lack of an NHS number for a given patient in PICANet. A total of 115 children who underwent only an excluded catheter procedure were excluded from the analysis, as were 765 premature babies who underwent ligation of patent ductus arteriosus (PDA) only and 24 transplant patients. A further 505 patients with unknown life status were also removed.
Of the remaining 7976 patients, 333 [4.2%, 95% confidence interval (CI) 3.7 to 4.6] died during their index admission period and were excluded from our analyses, leaving a final data set comprising 7643 infants discharged alive from their index admission. Of these, 246 (3.2%, 95% CI 2.8 to 3.6) died within 1 year following discharge from the index admission and not during a planned admission (outcome 1). A total of 514 children (6.7%, 95% CI 6.2 to 7.3) either died or were admitted unplanned, as an emergency, to a PICU within 1 year following discharge from the index admission (outcome 2). Finally, 115 children (1.5%, 95% CI 1.2% to 1.8%) died during a planned admission to PICU within 1 year following discharge from the index admission (which was not considered an outcome in this analysis), giving an overall mortality within the year following discharge from index admission of 4.7% (95% CI 4.2% to 5.2%).
Ethnic group was not available from PICANet for 1703 children in the final data set. Of these, the NCHDA ethnic code was used to assign white (n = 1001), Asian (n = 243), black (n = 113) or missing (n = 346) ethnicity. Excluding records without PICANet-derived ethnicity from the analyses did not significantly affect the results. In a total of 528 children weight-for-age was missing or anomalous (assumed erroneous and treated as missing). The variable for which the level of missing data was highest, markedly so, was prematurity [n = 2101 (27.5%)]; sensitivity analysis comparing models with and without prematurity showed a marginally better fit if it was included (based on Akaike criteria) and very little difference in the odds ratio coefficients for all other factors.
Descriptive and univariate analyses
The following variables showed no univariate association with either outcome and so were not considered in further analyses: sex, and whether or not any catheter procedures were performed during the index admission in addition to the index procedure. Both outcome 1 and outcome 2 were significantly associated with all of the other candidate variables in univariate analysis (p-value < 0.05). Table 8 shows the observed numbers of patients and rate of outcomes 1 and 2 in the data set for those parameters significant in univariate analysis (and weight-for-age), along with the results of the univariate analysis.
| Patient variable | n (%) overall | Outcome 1 | Outcome 2 | ||
|---|---|---|---|---|---|
| n (%) | Univariate odds ratio (p-value) | n (%) | Univariate odds ratio (p-value) | ||
| Non-medical | |||||
| Deprivation | |||||
| 1 – most | 2205 (28.9) | 79 (3.6) | 1 | 157 (7.1) | 1 |
| 2 | 1563 (20.4) | 51 (3.3) | 0.91 (0.60) | 104 (6.7) | 0.93 (0.58) |
| 3 | 1242 (16.3) | 41 (3.3) | 0.92 (0.66) | 77 (6.2) | 0.86 (0.30) |
| 4 | 1078 (14.1) | 38 (3.5) | 0.98 (0.93) | 79 (7.3) | 1.03 (0.83) |
| 5 – least | 1085 (14.2) | 23 (2.1) | 0.58 (0.02) | 64 (5.9) | 0.82 (0.19) |
| Missing | 470 (6.1) | 14 (3.0) | – | 33 (7.0) | – |
| Ethnicity | |||||
| White | 5728 (75.0) | 166 (2.9) | 1 | 348 (6.1) | 1 |
| Mixed | 196 (2.6) | 4 (2.0) | 0.70 (0.48) | 9 (4.6) | 0.74 (0.39) |
| Asian | 867 (11.3) | 38 (4.4) | 1.54 (0.02) | 73 (8.4) | 1.42 (< 0.01) |
| Black | 345 (4.5) | 12 (3.5) | 1.21 (0.54) | 34 (9.9) | 1.69 (< 0.01) |
| Chinese | 28 (0.4) | 1 (3.6) | 1.24 (0.83) | 1 (3.6) | 0.57 (0.59) |
| Other | 133 (1.7) | 12 (9.0) | 3.32 (< 0.001) | 19 (14.3) | 2.58 (< 0.001) |
| Not stated | 346 (4.5) | 13 (3.8) | 1.31 (0.36) | 30 (8.7) | 1.47 (0.05) |
| Preoperative | |||||
| Cardiac diagnosis group | |||||
| VSD | 1348 (17.6) | 25 (1.9) | 1 | 60 (4.5) | 1 |
| HLHS | 390 (5.1) | 48 (12.3) | 7.43 (< 0.001) | 70 (18.0) | 4.70 (< 0.001) |
| UVH/PA | 531 (7.0) | 41 (7.7) | 4.43 (< 0.001) | 73 (13.8) | 3.42 (< 0.001) |
| Other | 5374 (70.3) | 132 (2.5) | 1.33 (0.19) | 311 (5.8) | 1.32 (0.06) |
| Acquired diagnosis | |||||
| Yes | 479 (6.3) | 25 (5.2) | 1.73 (0.01) | 57 (11.9) | 1.98 (< 0.001) |
| No | 7164 (93.7) | 221 (3.1) | 1 | 457 (6.4) | 1 |
| Specific procedure group | |||||
| Corrective | 4973 (65.1) | 86 (1.7) | 1 | 219 (4.4) | 1 |
| Palliative | 1629 (21.3) | 119 (7.3) | 4.48 (< 0.001) | 205 (12.6) | 3.13 (< 0.001) |
| Ungrouped | 1041 (13.6) | 41 (3.9) | 2.33 (< 0.001) | 90 (8.7) | 2.05 (< 0.001) |
| Congenital anomaly | |||||
| Yes | 1608 (21.0) | 90 (5.6) | 2.23 (< 0.001) | 209 (13.0) | 2.81 (< 0.001) |
| No | 6035 (79.0) | 156 (2.6) | 1 | 305 (5.1) | 1 |
| Neurodevelopment condition | |||||
| Yes | 307 (4.0) | 27 (8.8) | 3.13 (< 0.001) | 75 (24.4) | 5.08 (< 0.001) |
| No | 7336 (96.0) | 219 (3.0) | 1 | 439 (6.0) | 1 |
| Prematurity | |||||
| Yes | 828 (10.8) | 44 (5.3) | 1.59 (< 0.01) | 93 (11.2) | 1.63 (< 0.001) |
| No | 4714 (61.7) | 161 (3.4) | 1 | 340 (7.2) | 1 |
| Missing | 2101 (27.5) | 41 (2.0) | – | 81 (3.9) | – |
| Age at index procedure (continuous) | Median 64 days; IQR 11 to 153; missing = 0 | Not shown | 0.99 (< 0.001) | Not shown | 0.99 (< 0.001) |
| Weight-for-age z-score (continuous) | Median –1.7; IQR –2.8 to –0.6; missing = 528 | Not shown | 1.02 (0.69)b | Not shown | 0.95 (0.13)b |
| Antenatal diagnosis | |||||
| Yes | 2146 (8.1) | 105 (4.9) | 2.04 (< 0.001) | 219 (10.2) | 2.03 (< 0.001) |
| No | 5046 (66.0) | 124 (2.5) | 1 | 268 (5.3) | 1 |
| Missing | 451 (5.9) | 17 (3.8) | – | 27 (6.0) | – |
| Clinical deterioration | |||||
| Yes | 1469 (19.2) | 85 (5.8) | 2.29 (< 0.001) | 154 (10.5) | 1.89 (< 0.001) |
| No | 6174 (80.8) | 161 (2.6) | 1 | 360 (5.8) | 1 |
| Postoperative | |||||
| LOS (continuous) | Median 10 days; IQR 7 to 17; missing = 0 | Not shown | (< 0.001) | Not shown | (< 0.001) |
| Additional surgical procedures | |||||
| Yes | 414 (5.4) | 23 (5.6) | 1.85 (< 0.01) | 54 (13.0) | 2.21 (< 0.001) |
| No | 7229 (94.6) | 223 (3.1) | 1 | 460 (6.4) | 1 |
| Need for renal support | |||||
| Yes | 522 (6.8) | 31 (5.9) | 1.99 (< 0.01) | 57 (10.9) | 1.82 (< 0.001) |
| No | 6721 (88.0) | 207 (3.1) | 1 | 425 (6.3) | 1 |
| Missing | 400 (5.2) | 8 (2.0) | – | 32 (8.0) | – |
| Need for ECMO | |||||
| Yes | 64 (0.8) | 5 (7.8) | 2.53 (0.05) | 9 (14.1) | 2.32 (0.02) |
| No | 7290 (95.4) | 236 (3.2) | 1 | 480 (6.6) | 1 |
| Missing | 289 (3.8) | 5 (1.7) | – | 25 (8.7) | – |
| Acquired comorbidity | |||||
| Yes | 1481 (19.4) | 84 (5.7) | 2.19 (< 0.001) | 165 (11.1) | 2.15 (< 0.001) |
| No | 5918 (77.4) | 158 (2.7) | 1 | 326 (5.5) | 1 |
| Missing | 244 (3.2) | 4 (1.6) | – | 23 (9.4) | – |
| Events in PICU | |||||
| Yes | 634 (8.3) | 47 (7.4) | 2.70 (< 0.001) | 89 (14.0) | 2.58 (< 0.001) |
| No | 6765 (88.5) | 195 (2.9) | 1 | 402 (5.9) | 1 |
| Missing | 244 (3.2) | 4 (1.6) | – | 23 (9.4) | – |
| Post-operative morbidity | |||||
| Yes | 113 (1.5) | 9 (8.0) | 2.66 (< 0.01) | 17 (15.0) | 2.51 (< 0.01) |
| No | 7530 (98.5) | 237 (3.2) | 1 | 497 (6.6) | 1 |
The relationship between LOS and both outcomes was non-linear and the fractional polynomial transformation was used in the subsequent risk model development analyses; for age we used a log transformation. The relationship between weight-for-age and each outcome did not depart significantly from linearity.
Developing risk models for outcomes 1 and 2
In the multivariate analysis in which continuous variables were treated as such, the significant risk factors for both outcomes were age at procedure (continuous), weight-for-age z-score (continuous), index procedure group, cardiac diagnosis group, non-cardiac congenital anomaly, prematurity, ethnicity and LOS in a specialist centre (continuous). Preprocedure clinical deterioration was additionally significant to outcome 1, whereas neurodevelopmental condition and acquired cardiac diagnoses were additionally significant to outcome 2.
In the final model development, in which ease of interpretation was considered as well as statistical performance, the continuous predictors (age at procedure, weight-for-age z-score and LOS) were categorised as shown in Table 9 (along with observed numbers of patients and rate of outcomes 1 and 2 in the data set). The final risk model for outcome 1 comprises age at procedure (categorical), weight-for-age z-score (categorical), index procedure group, cardiac diagnosis group, non-cardiac congenital anomaly, prematurity, ethnicity, LOS in specialist centre (categorical) and clinical deterioration. The final risk model for outcome 2 comprises age at procedure (categorical), weight-for-age z-score (categorical), index procedure group, cardiac diagnosis group, non-cardiac congenital anomaly, prematurity, ethnicity, LOS in specialist centre (categorical), neurodevelopmental condition and acquired cardiac diagnoses. Details of the regression models (odds ratio, standard errors and 95% CIs) are shown in Tables 10 and 11.
| Patient variable | n (%) overall | n (%) outcome 1 | n (%) outcome 2 |
|---|---|---|---|
| Age at index procedure | |||
| > 3 months | 3202 (41.9) | 55 (1.7) | 129 (4.0) |
| 1–2 months | 1427 (18.7) | 45 (3.2) | 110 (7.7) |
| 10–30 days | 1114 (14.6) | 43 (3.9) | 90 (8.1) |
| 0–10 days | 1900 (24.8) | 103 (5.4) | 185 (9.7) |
| Weight-for-age z-score | |||
| > –2 SDs | 4064 (53.2) | 128 (3.1) | 243 (6.0) |
| –2 to –4 SDs | 2467 (32.3) | 71 (2.9) | 168 (6.8) |
| < –4 SDs | 584 (7.6) | 19 (3.3) | 50 (8.6) |
| Missing | 528 (6.9) | 28 (5.3) | 53 (10.0) |
| LOS | |||
| 0–7 days | 2564 (33.6) | 35 (1.4) | 84 (3.3) |
| 7–30 days | 4327 (56.6) | 146 (3.4) | 302 (7.0) |
| > 1 month | 752 (9.8) | 65 (8.6) | 128 (17.0) |
| Patient variable | Odds ratio | Standard error | 95% CI |
|---|---|---|---|
| Ethnicity | |||
| White | Reference category | ||
| Mixed | 0.68 | 0.35 | 0.25 to 1.88 |
| Asian | 1.38 | 0.26 | 0.95 to 2.01 |
| Black | 1.00 | 0.31 | 0.54 to 1.85 |
| Chinese | 1.46 | 1.53 | 0.19 to 11.43 |
| Other | 2.82 | 0.94 | 1.46 to 5.44 |
| Not stated | 1.53 | 0.47 | 0.85 to 2.78 |
| Cardiac diagnosis group | |||
| VSD | Reference category | ||
| HLHS | 3.07 | 0.97 | 1.65 to 5.71 |
| Functionally UVH/PA | 2.31 | 0.69 | 1.29 to 4.15 |
| Other | 1.12 | 0.26 | 0.70 to 1.77 |
| Specific procedure group | |||
| Corrective | Reference category | ||
| Palliative | 2.14 | 0.38 | 1.50 to 3.04 |
| Ungrouped | 1.77 | 0.36 | 1.20 to 2.63 |
| Congenital anomaly | |||
| No | Reference category | ||
| Yes | 2.43 | 0.37 | 1.81 to 3.27 |
| Prematurity | |||
| No | Reference category | ||
| Yes | 1.64 | 0.30 | 1.16 to 2.34 |
| Clinical deterioration | |||
| No | Reference category | ||
| Yes | 1.66 | 0.24 | 1.25 to 2.22 |
| Age at index procedure | |||
| > 3 months | Reference category | ||
| 1–2 months | 1.32 | 0.28 | 0.87 to 2.01 |
| 10–30 days | 1.89 | 0.45 | 1.19 to 3.02 |
| 0–10 days | 2.54 | 0.60 | 1.61 to 4.03 |
| Weight-for-age z-score | |||
| > –2 SDs | Reference category | ||
| –2 to –4 SDs | 1.59 | 0.28 | 1.12 to 2.26 |
| < –4 SDs | 2.28 | 0.61 | 1.34 to 3.87 |
| LOS | |||
| 0–7 days | Reference category | ||
| 7–30 days | 1.56 | 0.31 | 1.06 to 2.31 |
| > 1 month | 2.70 | 0.63 | 1.71 to 4.26 |
| Patient variable | Odds ratio | Standard error | 95% CI |
|---|---|---|---|
| Ethnicity | |||
| White | Reference category | ||
| Mixed | 0.63 | 0.23 | 0.31 to 1.29 |
| Asian | 1.21 | 0.17 | 0.92 to 1.61 |
| Black | 1.43 | 0.29 | 0.96 to 2.12 |
| Chinese | 0.65 | 0.68 | 0.09 to 5.02 |
| Other | 2.39 | 0.65 | 1.40 to 4.08 |
| Not stated | 1.76 | 0.37 | 1.16 to 2.65 |
| Cardiac diagnosis group | |||
| VSD | Reference category | ||
| HLHS | 2.46 | 0.58 | 1.55 to 3.90 |
| UVH/PA | 2.15 | 0.46 | 1.41 to 3.28 |
| Other | 1.20 | 0.19 | 0.88 to 1.64 |
| Specific procedure group | |||
| Corrective | Reference category | ||
| Palliative | 1.65 | 0.21 | 1.28 to 2.13 |
| Ungrouped | 1.61 | 0.22 | 1.23 to 2.11 |
| Congenital anomaly | |||
| No | Reference category | ||
| Yes | 2.71 | 0.29 | 2.19 to 3.35 |
| Neurodevelopment condition | |||
| No | Reference category | ||
| Yes | 2.81 | 0.44 | 2.06 to 3.82 |
| Prematurity | |||
| No | Reference category | ||
| Yes | 1.59 | 0.21 | 1.22 to 2.06 |
| Acquired diagnosis | |||
| No | Reference category | ||
| Yes | 1.85 | 0.30 | 1.35 to 2.53 |
| Age at index procedure | |||
| > 3 months old | Reference category | ||
| 1–2 months old | 1.59 | 0.23 | 1.20 to 2.10 |
| 10–30 days old | 2.21 | 0.37 | 1.59 to 3.06 |
| 0–10 days old | 2.93 | 0.48 | 2.12 to 4.04 |
| Weight-for-age z-score | |||
| > –2 SDs | Reference category | ||
| –2 to –4 SDs | 1.72 | 0.22 | 1.34 to 2.21 |
| < –4 SDs | 2.60 | 0.48 | 1.81 to 3.75 |
| LOS | |||
| 0–7 days | Reference category | ||
| 7–30 days | 1.54 | 0.21 | 1.19 to 2.00 |
| > 1 month | 2.73 | 0.44 | 1.99 to 3.75 |
For each patient variable the multivariate odds ratios, standard errors and 95% CIs are presented and the reference category indicated.
We note that, although variable selection was based on > 50% inclusion frequency over the imputed data sets, in practice there was clear discrimination between those factors that were included in the model (≥ 90%) and those that were not included (< 40%). For both outcomes, sensitivity analysis of the models derived on complete case data showed a marginally better fit to the data when prematurity was included, based on Akaike criteria. However, for models with and without prematurity the odds ratios for all other factors remained very similar, indicating robustness of model variable selection. Furthermore, for all models, adjusting for clustering at either the hospital level or regional (primary care trust) level had no significant impact on results so we have presented the unadjusted results.
Risk model performance
The final (categorical) model for outcome 1 gave a combined c-index of 0.78 (95% CI 0.75 to 0.82), indicating good discrimination. This was only marginally less discriminative than the continuous model (c-index 0.80). The final (categorical) model for outcome 2 also showed good discrimination with a combined c-index of 0.78 (95% CI 0.75 to 0.80), compared with a c-index of 0.78 for the continuous model. Calibration of the final categorical and continuous models for both outcomes was also good, with Hosmer–Lemeshow p-values ranging from 0.10 to 0.75 across the models fitted on the 20 imputed data sets, indicating no statistically significant differences between observed and expected number of deaths when calculated in deciles of predicted risk for each of the imputed data sets.
Identifying patient groups differentiated by risk of outcome 2
Figure 4 depicts the final tree generated by the CART analysis along with, for each node, the test set figures for the number of patients and rate of outcome 2. The rate of outcome 2 evaluated across the entire data set is shown below the box for each final patient group. The rate of outcome 2 across the entire analysis data set (total number of patients 7643) was 6.7%. The rate of outcome 2 within a 60% development subset was 7.0%, compared with 6.3% in the 40% test set.
FIGURE 4.
Patient groups derived from classification and regression tree analysis. The final tree generated by the CART analysis along with, for each node, the test set figures for the number of patients and rate of outcome 2. The rate of outcome 2 evaluated across the entire data set is shown below the box for each final patient group. Reproduced from Crowe et al. , 2016. 67 © 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell. This is an open access article under the terms of the Creative Commons Attribution-Non Commercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes
Of the 18 variables entered in the analysis, CART identified presence/absence of a neurodevelopmental condition as the best single discriminator between patients experiencing outcome 2 or not. For those without a neurodevelopmental condition, the next best discriminator is whether the cardiac diagnosis is high risk (HLHS, UVH or PA) or low risk (VSD or ‘other’). Of the latter, the next best discriminator is presence/absence of a congenital anomaly followed by the LOS in the specialist hospital (threshold 1 month). This branching creates six discrete patient groups, details of which are set out in Table 12.
| Patient group | Shared patient characteristics | Possible additional risk factors (% of patient group) | % of patient population in test (development) set | % of patient population in entire data set | % with outcome 2 in test (development) set | % with outcome 2 in entire data set |
|---|---|---|---|---|---|---|
| 1 | Neurodevelopmental condition(s) | May also have: congenital anomalies (52%); HLHS, functionally UVH or PA + IVS (17%); LOS > 1 month (26%) | 4 (4) | 4 | 19 (28) | 24 |
| 2 | No neurodevelopmental conditions; VSD/other; congenital anomalies; LOS > 1 month | – | 2 (2) | 2 | 28 (21) | 24 |
| 3 | No neurodevelopmental conditions; HLHS, functionally UVH or PA + IVS | May also have: congenital anomalies (10%); LOS > 1 month (20%) | 11 (11) | 11 | 15 (16) | 15 |
| 4 | No neurodevelopmental conditions; VSD/other; no congenital anomalies; LOS > 1 month | – | 4 (4) | 4 | 7 (11) | 9 |
| 5 | No neurodevelopmental conditions VSD/other; congenital anomalies; LOS < 1 month | – | 16 (15) | 16 | 9 (9) | 9 |
| 6 | No neurodevelopmental conditions; VSD/other; no congenital anomalies; LOS < 1 month | – | 62 (63) | 63 | 2 (3) | 3 |
Discussion
As far as we are aware, based on the systematic review reported in Chapter 2, this is the first study of its kind to explore adverse postdischarge outcomes for infants with CHD based on national audit data with equivalent universal coverage. Of 7643 infants discharged alive following an initial major intervention for CHD, representing the entire case load for England and Wales over a 6-year period, 246 (3.2%) died within 1 year and not during a planned readmission (outcome 1) and 514 (6.7%) either died or had an emergency unplanned readmission to PICU within 1 year (outcome 2). The following risk factors were associated with increased multivariate risk of both adverse outcomes: younger age at procedure, lower weight-for-age z-score, index procedure group (palliative procedures higher risk), cardiac diagnosis group (HLHS, UVH, PA higher risk), non-cardiac congenital anomaly, prematurity, ethnicity (‘other’ ethnicity higher risk), LOS in specialist centre (> 1 month higher risk). In addition, for outcome 1 preprocedure clinical deterioration was associated with increased multivariate risk whereas for outcome 2 neurodevelopmental conditions and acquired cardiac diagnoses were additionally associated with increased multivariate risk.
When considering the results of the two multivariable models in the context of previous evidence identified within the systematic review outlined in Chapter 2, one barrier is that very few of the previous studies presented risk factors for a large diverse group of CHD patients. There was concordance for the following risk factors being linked to adverse outcome: index procedure group (palliative),31,34 which relates to cardiac diagnosis group (HLHS was the predominant condition represented in the systematic review), non-cardiac congenital anomaly,34,36 prematurity32 and ethnicity26 (albeit in relation to different ethnic populations based in the USA).
The risk models in our study further identified the following factors: lower weight at operation, which is a factor in risk models for adverse early outcome11 and may be correlated with feeding difficulties that featured in the systematic review as a higher risk;33 acquired cardiac diagnoses and preoperative clinical deterioration, which would suggest that individual patients with more severe forms of a given CHD type are at higher late risk (as was the case for HLHS within systematic review studies26,88); and neurodevelopmental conditions, which did not feature in the systematic review, although these may have overlapped with congenital anomalies in previous studies (see previous paragraph).
One patient characteristic that featured as an adverse risk factor in the systematic review (in relation to populations from the USA)26,32 but was not linked to risk in our study was lower socioeconomic status [we included a measure of deprivation (IMD) as a potential risk factor in our analyses]. Further descriptive information on deprivation and a wider discussion of ethnicity is presented in Chapter 5, but we note that our data originate from a different health-care system to the USA (i.e. the NHS) and speculate that this may have a role in these observed differences.
Prolonged LOS in hospital was an important adverse risk factor in our study, but within the systematic review evidence in respect of this was conflicting: two studies indicated LOS to be a risk factor34,36 and one smaller single-centre study presenting the opposite viewpoint. 30 Prolonged LOS may reflect numerous different aspects of a patient’s condition and journey, but generally indicates greater complexity including potentially being a surrogate measure of postprocedural complications,89 which may lead to greater fragility post discharge. Younger age at surgery was associated with higher risk in our study, and in two studies in the systematic review that included a range of different CHD types,31,34 yet the systematic review indicated older age to be associated with higher risk of HLHS and aortic stenosis. 23,35 It is likely that the finding of higher risk at younger age represents a broad effect, such as neonates being generally more vulnerable than older infants post discharge.
Our results reflect outcomes within the context of recent historical provision of services for major forms of CHD requiring treatment in infancy, and are potentially insightful for ongoing quality improvement initiatives. To this end, we identified distinct patient groups using CART analysis with a view to informing the design (and potential targeting) of appropriate interventions and service improvements. The six groups that were identified span a wide range of risk of late death or emergency readmission to PICU (3–24% outcome 2). The groups are defined in terms of the following patient characteristics: neurodevelopmental conditions; cardiac diagnosis of HLHS, UVH or PA + intact ventricular septum (IVS); congenital anomalies; and LOS > 1 month. The two highest-risk groups are made up of patients with a neurodevelopmental condition (group 1: 24% outcome 2) and patients with a low-risk cardiac diagnosis who have a congenital anomaly and long LOS (but no neurodevelopmental condition) (group 2: 24% outcome 2). Group 3 comprises those patients most widely recognised as vulnerable to late death (see Chapters 2 and 3), namely patients with cardiac diagnoses of HLHS, UVH or PA + IVS (and no neurodevelopmental condition) (15% outcome 2), although we note that some patients in group 1 may also have these cardiac diagnoses.
Therefore, the groups identified by these population-based data as high risk include patients with diagnoses other than those conventionally recognised as benefiting from enhanced surveillance, that is HLHS52–54 and in small number of studies, other UVH conditions. 57 Our findings suggest that as well as HLHS, infants with UVH and PA + IVS are also at significant risk of poor interstage outcome and that these patient groups are candidates for home monitoring. Our study also indicates that it is important to mitigate risks arising from patient factors beyond simply cardiac diagnosis, in particular we note the very significant risks of poor postdischarge outcome in the presence of neurodevelopmental conditions and congenital anomalies with prolonged LOS. The implications of this for service provision are discussed further in Chapter 10.
Strengths and weaknesses
The national audit data underpinning this study offer a unique opportunity for a population-based analysis, and the study was designed, conducted and reported based on the Strobe Statement for observational studies. 90 First, the data are of high quality (as demonstrated by the results of a regular systematic independent validation process). Second, life-status tracking based on NHS number enables late deaths outside treatment centres to be reliably ascertained. Third, the mandatory and universal nature of the data submission means that all relevant cases are captured. Our findings may therefore be considered more generalisable than those based on single-centre studies or those from a more limited geographical area. That said, our study outcomes inevitably reflect recent historic services provision by the NHS in addition to intrinsic medical risk; hence, findings may differ to some extent between geographical regions. The study also reflects the demographics of the UK in terms of ethnicity, which may in turn have implications for the distribution of CHD types and other congenital anomalies. 91
A further positive feature of the study is that the combined data set used is more valuable for the purposes of this study than either of the individual audit data sets in isolation, since each contains different types of information concerning potential risk and, when variables in the two data extracts overlapped, the item with the highest data completeness and discrimination was selected. The strength of the NCHDA data set includes the detailed cardiac-specific information as determined by IPCCC codes related to CHD and the cardiac interventions undertaken. The strengths of the PICANet data set include the rich medical coding and information pertaining to the type of admission (such as where an emergency occurred), as well as intensive-care supports and interventions.
We note, however, that our results reflect only those elements that are captured within the national audit data and so certain known risk factors, for example postprocedural weight gain33 and selected abnormalities based on echocardiography,36 could not be incorporated. Furthermore, there may be other as yet unknown risk factors that are not captured in the registry data.
It was necessary to group cardiac diagnoses and procedures in order to render them tractable for statistical analysis (to reduce the degrees of freedom in the data set). Although this was performed with a strong clinical sense of what was pertinent, based on literature and clinical experience, we note that such groupings are to an extent subjective and, as in other registry-based studies, there remained a subset of ungrouped procedures. The choice of diagnosis group ‘HLHS’ reflects the body of literature related to ‘interstage’ deaths in this population. A separate group of ‘UVH or PA + IVS’ was chosen because of the high-risk nature of these conditions, although with a view to distinguishing these from HLHS patients. Of the remaining cardiac diagnosis groups, we elected to review the VSD group separately in order for us to evaluate the face validity of comparisons between HLHS or UVH/PA + IVS and the much larger, less homogeneous group of mainly biventricular diagnoses. We note that results for the VSD group and the diverse ‘other’ group were comparable.
We note that the ethnicity group classified as ‘other’ was associated with higher risk: this is discussed in more detail in Chapter 5. The audit data will not have captured the scenario in which babies were discharged to home on a palliative care pathway, and hence our outcomes may have incorporated a subset of babies that were expected to die. However, local audit of cases at two English tertiary centres suggests this occurs in only a minority of cases. 6 Finally, given its focus on the population of infants who have undergone an intervention, the analysis does not include infants awaiting intervention who are also known to be potentially vulnerable.
Chapter 5 Ethnic influences on the prevalence and outcomes of infants undergoing paediatric cardiac surgery for congenital heart defects
Introduction
In the IHS, we have used national audit data to explore the medical and sociodemographic variables that may be related to adverse outcomes for infants with CHD discharged home following cardiac surgery. The risk model presented in Chapter 4 highlighted the increased mortality risk for children in the ‘other’ ethnic group and, in order to better understand this phenomenon, we undertook additional analyses to investigate the influence of ethnic group on incidence, associated non-cardiac comorbidities and timing of death in the linked audit data set. Our objective was to identify ethnic variations in the distribution of CHD and associated health outcomes, and to determine whether ethnicity-related factors might present additional challenges to health-care provision for affected infants. Although racial and ethnic variations in CHD prevalence and outcomes have been reported globally,92 there are significant differences in the ethnic composition of different national populations, which often reflect historical patterns of migration; therefore, it was important to investigate the impact of ethnicity within a UK population.
The prevalence of all CHDs, as well as specific CHD subtypes, varies by ethnic, or racial, group. 93–96 It is reported that hospital stays after cardiac surgery are longer, and in-hospital mortality rates are higher, among black and Hispanic children than among white children,97,98 and ethnic variations in mortality rates appear to persist into later childhood. 99–101 Although genetic, biological or socioeconomic factors may partly explain racial and ethnic variations, some US authors have suggested that unequal access to diagnosis and care also contributes to differences in mortality. 102,103 However, these findings may not apply within the context of the UK health-care model. It is also important to note that some large minority ethnic populations in the USA do not exist in the UK, for example the Hispanic, Native American or Pacific Islander populations, and the Asian population of the UK has very different migratory origins to Asian populations in North America. Moreover, Flores46 has emphasised the importance of understanding these as diverse individual populations rather than grouping them under the term non-white.
Few population-based studies have been undertaken in the UK to explore ethnic variations in CHD incidence and outcomes. In a single-centre UK study, Sadiq et al. 104 demonstrated a higher prevalence of complex CHDs in children of Asian origin, which may imply a requirement for complex interventions and specific considerations when designing health-care provision appropriate to the cultural and language needs of this patient group. An audit of data from two London hospitals for the years 2000 to 2009 has also provided evidence that babies with CHDs who are of non-white British ethnicity experience a higher mortality risk after hospital discharge. 19
The linked national audit data from NCHDA and PICANet provided us with a unique patient-based data set to investigate ethnic variations in the incidence and distribution of CHD, as well as the mortality risk associated with cardiac surgery for affected infants. UK national paediatric audit data have not previously been analysed on an individual patient basis with a view to informing quality improvement and care pathways after hospital discharge.
Methods
Our investigation is based on a linked data set included individual records for 7529 infants with CHDs who underwent a bypass operation or interventional catheterisation for structural CHDs from 1 January 2005 to 31 December 2010, as described for the risk model and CART analyses in Chapter 4.
As the PICANet and NCHDA ethnicity classifications were not directly comparable, we investigated concordance using Cohen’s kappa statistic. Furthermore, taking the PICANet ethnic category as the reference standard, we calculated the proportion of patients whose NCHDA ethnic group was concordant with the PICANet-recorded ethnicity (sensitivity) and the probability that a patient with a given NCHDA ethnic group had the corresponding PICANet ethnic group (positive predictive value). For analyses of the concordance of ethnic classifications, we excluded 1954 children who had missing ethnicity either from NCHDA or from PICANet. However, for our analyses of the distribution of patient-based characteristics and outcomes associated with ethnicity, we excluded children for whom ethnicity was not available in PICANet and could not be inferred from the NCHDA ethnicity record.
Descriptive statistics are presented as numbers and percentages, or median and interquartile ranges (IQR), and 95% CIs were estimated for the difference between two proportions. Weight-at-index procedure was converted to age- and sex-standardised z-scores (British 1990 growth reference105) to facilitate comparison across age groups. 105 z-score values that were outside 5 standard deviations (SD) were considered to be clinically anomalous and treated as missing.
Estimates of the incidence and relative rates of children operated for CHD within the first year of life were calculated by sex and ethnic group for all CHD and each specific CHD subgroup, using mid-year population estimates for the years 2006 to 2009 as the denominator. Binomial exact 95% CIs were estimated for rates. To ensure that analyses of incidence included only audit years in which there was complete ascertainment of infant procedures as well as the period from birth to age 1 year, we based incidence analyses only on children whose index procedure was between 1 January 2006 and 31 December 2009 (excluding 2179 children whose index procedure was before or after this date). The incidence data set included 266 children who lacked any record of ethnicity. We investigated the relative risk of infant mortality by ethnic group using the white ethnic group as the reference.
We explored the distribution by ethnic group of specific cardiac diagnosis and individual patient characteristics defined for our analyses in Chapter 4, including sex, cardiac diagnosis, gestation at birth, ethnicity, area deprivation, associated comorbidities at the time of index procedure, weight at admission, antenatal diagnosis and clinical codes describing the child’s status during the index hospital admission. Given the lack of knowledge on this topic and our interest in building up a better descriptive picture of the links between ethnicity and various key factors within the population, in this section of the analyses we included the more detailed cardiac diagnosis (all primary cardiac diagnosis groups listed in Appendix 6) as a variable in our analysis of ethnic variations. Given the larger number of diagnosis groups analysed in this section, and the close links between cardiac intervention performed and detailed cardiac diagnosis, as well as the knowledge that some children undergo more than one procedure even over the first year, we excluded procedure type and procedure-related variables from these analyses. The risk model and CART analyses used a simplified diagnostic categorisation including only four broad diagnostic categories, and furthermore we explored risk after the first index operation only, again using very broad operative groups such as ‘palliative, corrrective’; therefore, additional procedure variables (in respect of that index operation) were included in the risk models detailed in Chapter 4.
We investigated the risk ratio (RR) for mortality (both within-hospital and for outcome 1) by ethnic group using white ethnicity as the reference. The factors associated with each mortality outcome were investigated by fitting a univariate generalised linear model (binomial log-linear)106 to determine the RR coefficients for ethnicity, with white as a reference category.
Results
Our results are presented in three parts: concordance of ethnic classifications between the two audit databases; incidence of CHD by ethnic group; and infant characteristics and outcomes by ethnic group. Data reported in tables omits cells in which the number of individuals is < 5 to reduce disclosure risk. All analyses were undertaken using Stata SE version 12.1.
Concordance of the ethnic classifications used in the National Congenital Heart Diseases Audit and Paediatric Intensive Care Audit Network data sets
During the development of the risk model data set, we explored the concordance of ethnic classification within the NCHDA and PICANet audit databases using a similar methodology to Saunders et al. 107 This determined the ethnic classification subsequently used in the risk model and CART analysis.
The NCHDA database uses a bespoke ethnic classification comprising five ethnic groups: Caucasian, Asian, black, Oriental and ‘other’ (‘other’ in NCHDA also includes children of mixed ethnicity; therefore, we refer to it below as ‘other – mixed’). Ethnicity is recorded in the PICANet database using the detailed (16 category) UK census classification (Table 13), which is often aggregated into six large groups. As ethnicity was recorded for each admission, the recorded ethnic group varied between admissions for some children; in these cases, the most frequently reported ethnic group (mode) for each child was selected as the child’s ethnic group.
As the PICANet and NCHDA ethnicity classifications were not directly comparable, we estimated Cohen’s kappa statistic as a measure of agreement. Furthermore, taking the PICANet ethnic category as the reference standard because of its more widespread use and comparability with national population statistics, we calculated the proportion of patients whose NCHDA ethnic group was concordant with the PICANet-recorded ethnicity (sensitivity) and the probability that a patient with a given NCHDA ethnic group had the corresponding PICANet ethnic group (positive predictive value).
| Ethnic group | n |
|---|---|
| White (total) | 4624 |
| White British | 4410 |
| White Irish | 19 |
| Any other white | 195 |
| Mixed (total) | 197 |
| Mixed white/black Caribbean | 51 |
| Mixed white/black African | 22 |
| Mixed white/Asian | 66 |
| Any other mixed | 58 |
| Asian (total) | 624 |
| Asian/British-Indian | 173 |
| Asian/British-Pakistani | 271 |
| Asian/British-Bangladeshi | 68 |
| Any other Asian | 112 |
| Black (total) | 218 |
| Black/British Caribbean | 31 |
| Black/British African | 143 |
| Any other black | 44 |
| Chinese (total) | 26 |
| Chinese | 26 |
| Other (total) | 127 |
| Any other ethnicity | 127 |
| Missing (total) | 1713 |
| Not stated | 1713 |
| Total | 7529 |
Ethnicity was not recorded for 1713 (22.8%) children in the PICANet data set (see Table 13); however, ethnic group was available from NCHDA for 1438 of these, of whom the majority were Caucasian (n = 1002; Table 14). Using a data set including only children who had a record in both NCHDA and PICANet (n = 5575), we explored the concordance between the two classifications to determine whether or not the NCHDA ethnic group could inform the missing PICANet record. The PICANet categories white, black, Asian, Chinese, other and mixed were matched against the NCHDA categories of Caucasian, black, Asian, Oriental and ‘other – mixed’, respectively. Cohen’s kappa statistic for agreement of PICANet and NCHDA recorded ethnicity was 0.83 overall.
| Ethnic group (PICANet) | Ethnic group (NCHDA) | ||||||
|---|---|---|---|---|---|---|---|
| Caucasian | Black | Asian | Oriental | Other – mixed | Ethnicity missing | Total | |
| White | 4368 | 6 | 21 | a | 37 | 189 | |
| Black | 10 | 189 | 5 | a | a | 10 | |
| Asian | 18 | a | 562 | 12 | 9 | 20 | |
| Chinese | a | a | a | 19 | a | a | |
| Other and mixedb | 98 | 39 | 58 | a | 104 | 21 | |
| Ethnicity missing | 1002 | 113 | 250 | 20 | 53 | 275 | |
| Total | 7529 | ||||||
The sensitivity and positive predictive value of each NCHDA ethnic category with respect to the corresponding PICANet category, was evaluated using the pairings white/Caucasian, black/black, Asian/Asian, Chinese/Oriental, other/‘other – mixed’ (Figure 5).
FIGURE 5.
Concordance between NCHDA ethnic group and corresponding PICANet ethnic group. NICOR, National Institute for Cardiovascular Outcomes Research; PPV, positive predictive value.
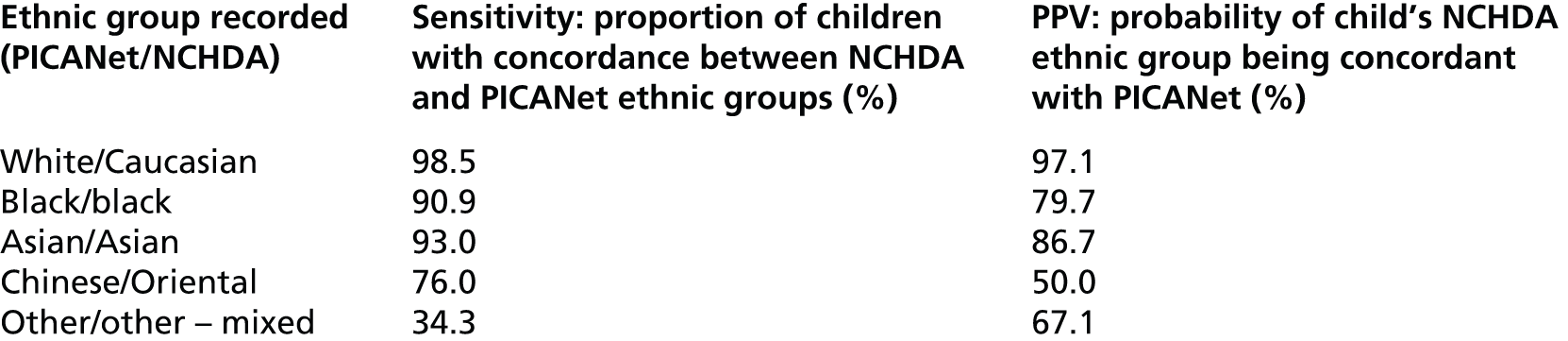
Around half of the children in the NCHDA Oriental category were classified as Asian (Pakistani, Bangladeshi, Indian or other Asian) in PICANet (see Table 14). The PICANet ‘mixed’ category does not exist in NCHDA; 58 (31.0%) of these children were classified as white in NCHDA and the remainder were distributed equally between the black, Asian and other categories.
The Caucasian, black and Asian categories in NCHDA demonstrated good concordance with the PICANet ethnic groups of white, black and Asian, respectively (see Figure 5), and risk of misclassification was low (positive predictive value ≥ 79.7%). The Chinese/Oriental and other/‘other – mixed’ categories were poorly concordant and the NCHDA category predicted the corresponding PICANet group in < 68% of cases. To facilitate comparison with population denominator data and reduce missing data, children with ethnicity missing in PICANet but recorded in NCHDA as Caucasian, black or Asian were assigned to the ethnic groups of white, black or Asian, respectively, whereas children in the Oriental and ‘other – mixed’ NCHDA categories who had no PICANet ethnicity record remained unchanged in PICANet. Using this method to map children to a PICANet ethnic group, we created a data set in which only 4.6% (348 out of 7529) of children had missing data for ethnic group; further analyses described here, including the risk model, CART analysis and analyses of ethnic variation, use this data set. Of 348 children with missing data, 20 were classified as Oriental and 53 as ‘other – mixed’ in NCHDA, while the remainder had no ethnicity record in either data set.
Incidence of congenital heart disease types by ethnicity
The incidence of CHD surgery in the first year of life in England and Wales from 2006 to 2009 was 2.0 (95% CI 1.9 to 2.0) per 1000 children (n = 5350; Table 15). Although this incidence rate approximates birth prevalence, actual birth prevalence would be slightly higher, as some children will survive the first year of life without an intervention, will remain undiagnosed, or will die without an intervention.
| Year | Mid-year population aged under 1 year | Number of children operated on | Rate per 1000 infants (95% CIa) |
|---|---|---|---|
| 2006 | 653,400 | 1250 | 1.9 (1.8 to 2.0) |
| 2007 | 674,700 | 1292 | 1.9 (1.8 to 2.0) |
| 2008 | 702,800 | 1344 | 1.9 (1.8 to 2.0) |
| 2009 | 698,800 | 1464 | 2.1 (2.0 to 2.2) |
| 2006–9 | 2,729,700 | 5350 | 2.0 (1.9 to 2.0) |
Compared with the white ethnic group, the relative rate of CHD was higher in children of Asian, black and ‘other’ ethnicity and lower in children of Chinese or mixed ethnicity (Table 16). In sensitivity analyses including only children with ethnicity recorded in PICANet (n = 4127), these relationships did not change; however, the CIs around the relative rate for the black and Chinese groups included 1.
| Ethnic group (n = 5084)a | CHD cases (%) | Total mid-year populationb (aged 0–1 year) | Annual incidence per 1000 infants (95% CI) | Relative rate (95% CI) |
|---|---|---|---|---|
| White | 3968 (78.0) | 2,230,400 | 1.8 (1.7 to 1.8) | Reference |
| Asian | 604 (11.9) | 220,100 | 2.7 (2.5 to 3 0) | 1.54 (1.41 to 1.68) |
| Black | 240 (4.7) | 93,700 | 2.6 (2.2 to 2.9) | 1.44 (1.26 to 1.64) |
| Chinese | 22 (0.4) | 19,700 | 1.1 (0.70 to 1.7) | 0.63 (0.39 to 0.95) |
| Mixed | 146 (2.9) | 151,500 | 1.0 (0.8 to 1.1) | 0.54 (0.46 to 0.64) |
| Other | 104 (2.0) | 14,300 | 7.3 (5.9 to 8.8) | 4.09 (3.33 to 4.97) |
The incidence of specific CHD subtypes varies by ethnic group (Figure 6). Compared with the white reference group, the following subtypes were more common: in the Asian ethnic group the relative rate of UVH, TGA + IVS, PA, tetralogy of Fallot, TAPVC, VSD, atrial septal defect (ASD) and PDA; in the black ethnic group HLHS, UVH, AVSD and VSD; and, in the ‘other’ ethnic group, HLHS, truncus arteriosus, TGA, PA + VSD, AVSD, tetralogy of Fallot and VSD (Table 17). Although specific defects appeared to be under-represented in non-white ethnic groups compared with the white ethnic group, as a result of small numbers there was insufficient precision to identify any significant differences.
FIGURE 6.
Distribution of CHD subgroups by ethnicity for children operated between 2006 and 2009. (a) white; (b) Asian; (c) black; (d) Chinese; (e) mixed; and (f) other. AAO, aortic arch obstruction; AS, aortic valve stenosis; ASD, atrial septal defect; AR, aortic regurgitation; IAA, interrupted aortic arch; misc. C, miscellaneous congenital CHD; misc. PC, miscellaneous primary CHDs; MV, mitral valve abnormalities; PS, pulmonary stenosis; TOF, tetralogy of Fallot; Tr, truncus arteriosus; TR, tricuspid valve insufficiency; SaS, subaortic stenosis.

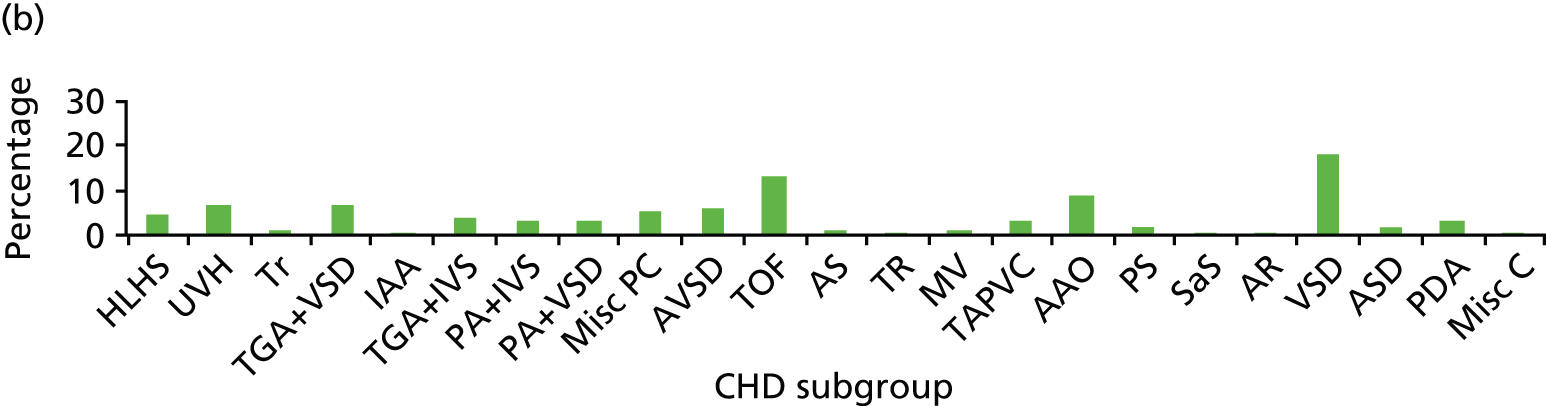
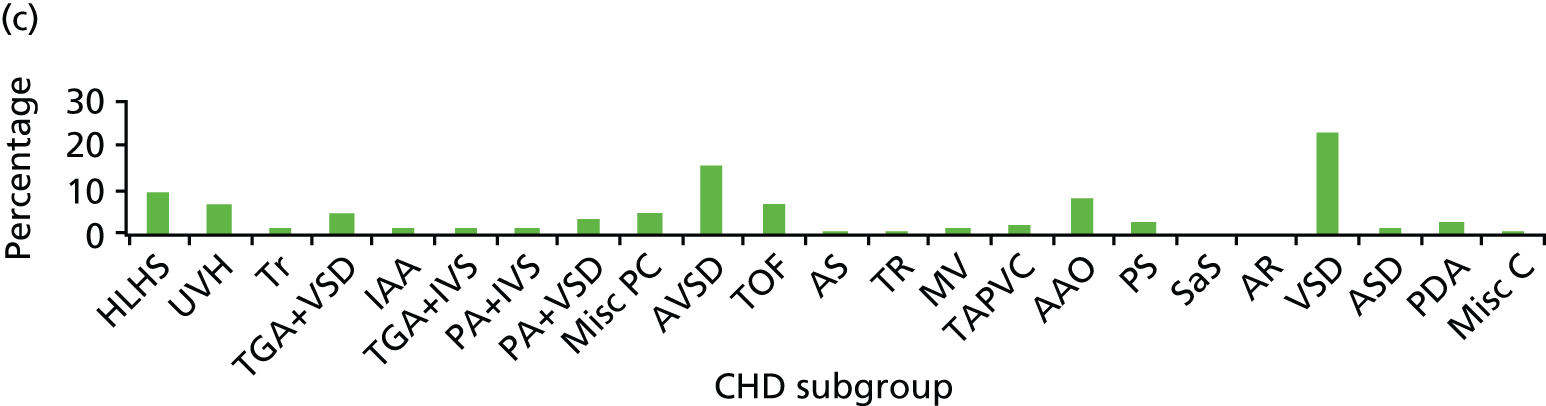
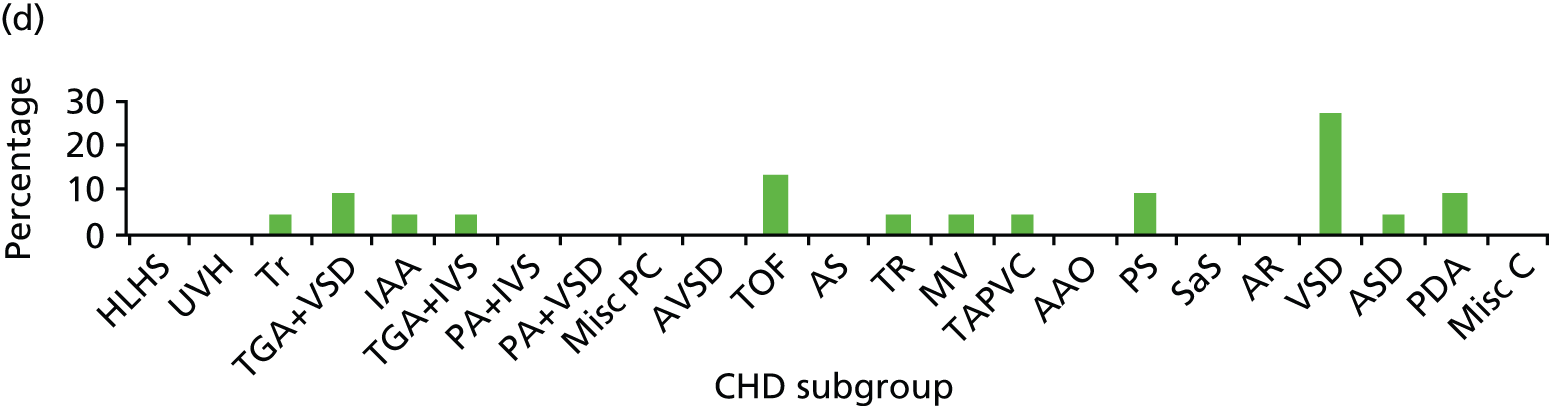
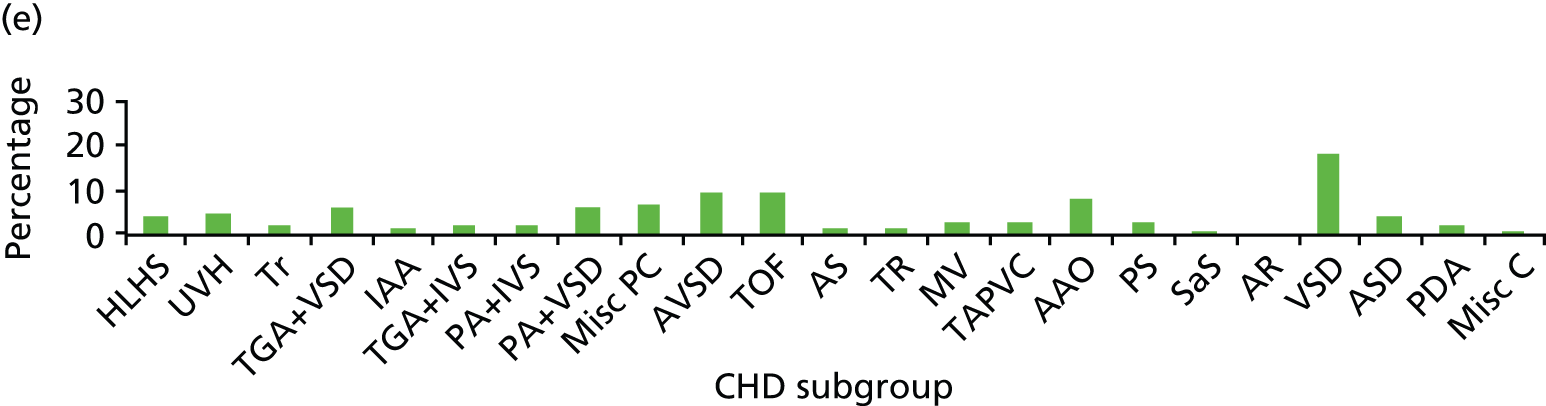
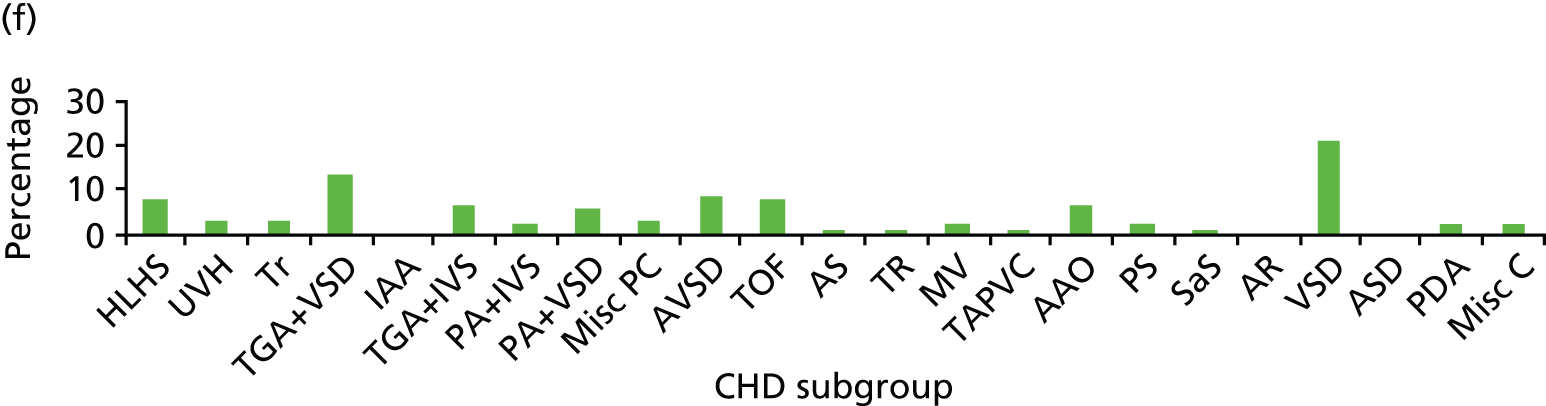
| Cardiac diagnosis | Ethnic group, sample size (population) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White, n = 3968 (N = 223,0400) | Asian, n = 604 (N = 220,100) | Black, n = 240 (N = 93,700) | Chinese, n = 22 (N = 19,700) | Mixed, n = 146 (N = 151,500) | Other, n = 104 (N = 14,300) | |||||||||||||||||
| n | IR | n | IR | RR | 95% CI | n | IR | RR | 95% CI | n | IR | RR | 95% CI | n | IR | RR | 95% CI | n | IR | RR | 95% CI | |
| HLHS | 231 | 1.0 | 30 | 1.4 | 1.3 | 0.9 to 1.9 | 22 | 2.3 | 2.3 | 1.4 to 3.5 | 0 | – | – | – | 6 | 0.4 | 0.4 | 0.1 to 0.8 | 8 | 5.6 | 5.4 | 2.3 to 10.8 |
| Functionally UVH | 193 | 0.9 | 41 | 1.9 | 2.2 | 1.5 to 3.0 | 16 | 1.7 | 2.0 | 1.1 to 3.3 | 0 | – | – | – | 7 | 0.5 | 0.5 | 0.2 to 1.1 | < 5 | – | – | – |
| Truncus arteriosus | 72 | 0.3 | 8 | 0.4 | 1.1 | 0.5 to 2.3 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| TGA with VSD (DORV–TGA) | 354 | 1.6 | 44 | 2.0 | 1.3 | 0.9 to 1.7 | 11 | 1.2 | 0.7 | 0.4 to 1.3 | < 5 | – | – | – | 9 | 0.6 | 0.4 | 0.2 to 0.7 | 14 | 9.8 | 6.2 | 3.3 to 10.5 |
| Interrupted aortic arch | 51 | 0.2 | 5 | 0.2 | 1.0 | 0.3 to 2.5 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – | 0 | – | – | – |
| TGA and IVS | 128 | 0.6 | 27 | 1.2 | 2.1 | 1.4 to 3.3 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – | 7 | 4.9 | 8.5 | 3.4 to 18.1 |
| PA and IVS | 104 | 0.5 | 22 | 1.0 | 2.1 | 1.3 to 3.4 | < 5 | – | – | – | 0 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| PA and VSD | 129 | 0.6 | 23 | 1.0 | 1.8 | 1.1 to 2.8 | 9 | 1.0 | 1.7 | 0.7 to 3.3 | 0 | – | – | – | 9 | 0.6 | 1.0 | 0.5 to 2.0 | 6 | 4.2 | 7.3 | 2.6 to 16.2 |
| Miscellaneous primary CHDs | 222 | 1.0 | 33 | 1.5 | 1.5 | 1.0 to 2.2 | 11 | 1.2 | 1.2 | 0.6 to 2.2 | 0 | – | – | – | 10 | 0.7 | 0.7 | 0.3 to 1.2 | < 5 | – | – | – |
| AVSD | 360 | 1.7 | 36 | 1.6 | 1.0 | 0.7 to 1.4 | 37 | 3.9 | 2.4 | 1.7 to 3.4 | 0 | – | – | – | 14 | 0.9 | 0.6 | 0.3 to 1.0 | 9 | 6.3 | 3.9 | 1.8 to 7.5 |
| Tetralogy of Fallot | 416 | 1.9 | 80 | 3.6 | 1.9 | 1.5 to 2.5 | 16 | 1.7 | 0.9 | 0.5 to 1.5 | < 5 | – | – | – | 14 | 0.9 | 0.5 | 0.3 to 0.8 | 8 | 5.6 | 3.0 | 1.3 to 6.0 |
| AS | 106 | 0.5 | 8 | 0.4 | 0.8 | 0.3 to 1.6 | < 5 | – | – | – | – | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| Tricuspid valve insufficiency | 35 | 0.2 | 5 | 0.2 | 1.4 | 0.4 to 3.7 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| Mitral valve abnormalities | 38 | 0.2 | 7 | 0.3 | 1.9 | 0.7 to 4.2 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| TAPVC | 90 | 0.4 | 20 | 0.9 | 2.3 | 1.3 to 3.7 | 5 | 0.5 | 1.3 | 0.4 to 3.2 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| Aortic arch obstruction | 467 | 2.1 | 54 | 2.5 | 1.2 | 0.9 to 1.6 | 19 | 2.0 | 1.0 | 0.6 to 1.5 | 0 | – | – | – | 12 | 0.8 | 0.4 | 0.2 to 0.7 | 7 | 4.9 | 2.3 | 0.9 to 4.9 |
| Pulmonary stenosis | 143 | 0.6 | 12 | 0.5 | 0.9 | 0.4 to 1.5 | 7 | 0.7 | 1.2 | 0.5 to 2.5 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| Subaortic stenosis | 6 | < 0.1 | < 5 | – | – | – | 0 | – | – | – | 0 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| Aortic regurgitation | 7 | < 0.1 | < 5 | – | – | – | 0 | – | – | – | 0 | – | – | – | 0 | – | – | – | 0 | – | – | – |
| VSD | 661 | 3.0 | 111 | 5.0 | 1.7 | 1.4 to 2.1 | 55 | 5.9 | 2.0 | 1.5 to 2.6 | 6 | 3.0 | 1.0 | 0.4 to 2.2 | 27 | 1.8 | 0.6 | 0.4 to 0.9 | 22 | 15.4 | 5.2 | 3.2 to 7.9 |
| ASD | 40 | 0.2 | 12 | 0.5 | 3.0 | 1.5 to 5.9 | < 5 | – | – | – | < 5 | – | – | – | 6 | 0.4 | 2.2 | 0.8 to 5.2 | 0 | – | – | – |
| PDA | 74 | 0.3 | 19 | 0.9 | 2.6 | 1.5 to 4.4 | 6 | 0.6 | 1.9 | 0.7 to 4.4 | < 5 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
| Miscellaneous congenital CHD | 41 | 0.2 | < 5 | – | – | – | < 5 | – | – | – | 0 | – | – | – | < 5 | – | – | – | < 5 | – | – | – |
Association between ethnicity and individual characteristics of children operated on (2005 to 2010)
Descriptive statistics are presented in Table 18. Although characteristics of children with no record of ethnicity in either data set (n = 348; ‘ethnicity not stated’) were explored, it is difficult to draw inferences about this group because of its potential heterogeneity and a lack of information about why ethnicity was not recorded. However, it is relevant to note that a higher percentage of children without a record of ethnicity appear to have died during the index hospital admission; therefore, information may be lacking because of an early death or this may represent a particularly vulnerable group.
| Characteristics | Ethnic group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White, N = 5626 | Asian, N = 874 | Black, N = 331 | Chinese, N = 26 | Mixed, N = 197 | Other, N = 127 | Ethnicity not stated, N = 348 | |||||||||||||||
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| Demographics | |||||||||||||||||||||
| Male | 3158 | 56.1 | 54.8 to 57.4 | 500 | 57.2 | 53.9 to 60.5 | 160 | 48.3 | 42.8 to 53.9 | 14 | 53.8 | 33.4 to 73.4 | 96 | 48.7 | 41.6 to 55.9 | 68 | 53.5 | 44.5 to 62.4 | 193 | 55.5 | 50.1 to 60.8 |
| Preterm | 564 | 10.0 | 9.3 to 10.8 | 88 | 10.1 | 8.2 to 12.3 | 39 | 11.8 | 8.5 to 15.8 | 2 | 7.7 | 0.9 to 25.1 | 32 | 16.2 | 11.4 to 22.2 | 9 | 7.1 | 3.3 to 13.0 | 32 | 9.2 | 6.4 to 12.7 |
| Clinical diagnoses and care | |||||||||||||||||||||
| Antenatally diagnosed | 1629 | 29.0 | 27.8 to 30.5 | 280 | 32.0 | 29.0 to 35.2 | 116 | 35.0 | 29.9 to 40.5 | 3 | 11.5 | 2.4 to 30.2 | 54 | 27.4 | 21.3 to 34.2 | 39 | 30.7 | 22.8 to 39.5 | 132 | 37.9 | 32.8 to 43.3 |
| Neurodevelopmental problems | 197 | 3.5 | 3.0 to 4.0 | 56 | 6.4 | 4.9 to 8.2 | 18 | 5.4 | 3.3 to 8.5 | 0 | – | – | 12 | 6.1 | 3.2 to 10.4 | 3 | 2.4 | 0.5 to 6.7 | 9 | 2.6 | 1.2 to 4.9 |
| Non-cardiac congenital anomalies | 1157 | 20.6 | 19.5 to 21.6 | 182 | 20.8 | 18.2 to 23.7 | 92 | 27.8 | 23.0 to 33.0 | 10 | 38.5 | 20.2 to 59.4 | 54 | 27.4 | 21.3 to 34.2 | 30 | 23.6 | 16.5 to 32.0 | 44 | 12.6 | 9.3 to 16.6 |
| Clinical deterioration | 1103 | 19.6 | 18.6 to 20.7 | 182 | 20.8 | 18.2 to 23.7 | 71 | 21.5 | 17.1 to 26.3 | 9 | 34.6 | 17.2 to 55.7 | 35 | 17.8 | 12.7 to 23.8 | 29 | 22.8 | 15.9 to 31.1 | 75 | 21.6 | 17.3 to 26.2 |
| Acquired comorbidities | 344 | 6.1 | 5.5 to 6.8 | 61 | 7.0 | 5.4 to 8.9 | 29 | 8.8 | 5.9 to 12.3 | 1 | 3.8 | 0.1 to 19.6 | 13 | 6.6 | 3.6 to 11.0 | 7 | 5.5 | 2.2 to 11.0 | 18 | 5.2 | 3.1 to 8.1 |
| IMD quintilea | n = 5219 | n = 859 | n = 325 | n = 25 | n = 188 | n = 125 | n = 324 | ||||||||||||||
| 1: most deprived | 1314 | 25.1 | 24.0 to 26.4 | 437 | 50.9 | 47.5 to 54.3 | 179 | 55.2 | 49.5 to 60.6 | 12 | 48.0 | 27.8 to 68.7 | 77 | 41.0 | 33.9 to 48.3 | 56 | 44.8 | 35.9 to 54.0 | 91 | 28.1 | 23.3 to 33.3 |
| 2 | 1107 | 21.2 | 20.1 to 22.3 | 203 | 23.6 | 20.8 to 26.6 | 88 | 27.1 | 22.3 to 32.3 | 7 | 28.0 | 12.1 to 49.4 | 34 | 18.1 | 12.9 to 24.3 | 24 | 19.2 | 12.7 to 27.2 | 78 | 24.1 | 19.5 to 29.1 |
| 3 | 946 | 18.1 | 17.1 to 19.2 | 112 | 13.0 | 10.9 to 15.5 | 35 | 10.8 | 7.6 to 14.7 | 4 | 16.0 | 4.5 to 36.1 | 34 | 18.1 | 12.9 to 24.3 | 22 | 17.6 | 11.4 to 25.4 | 63 | 19.4 | 15.3 to 24.2 |
| 4 | 910 | 17.4 | 16.4 to 18.5 | 67 | 7.8 | 6.1 to 9.8 | 13 | 4.0 | 2.0 to 6.7 | 0 | – | – | 27 | 14.4 | 9.7 to 20.2 | 13 | 10.4 | 5.7 to 17.1 | 42 | 13.0 | 9.5 to 17.1 |
| 5: least deprived | 942 | 18.0 | 17.0 to 19.1 | 40 | 4.7 | 3.3 to 6.3 | 10 | 3.1 | 1.5 to 5.6 | 2 | 8.0 | 0.1 to 26.0 | 16 | 8.5 | 4.9 to 13.5 | 10 | 8.0 | 3.9 to 14.2 | 50 | 15.4 | 11.7 to 19.8 |
| Weight z-score at index procedureb | n = 5088 | n = 744 | n = 294 | n = 23 | n = 176 | n = 112 | n = 305 | ||||||||||||||
| > –2 SDs (normal) | 3003 | 59.0 | 57.7 to 60.4 | 399 | 53.6 | 50.0 to 57.3 | 146 | 49.7 | 43.8 to 55.5 | 12 | 52.2 | 30.6 to 73.2 | 92 | 52.3 | 44.6 to 59.8 | 65 | 58.0 | 48.3 to 67.3 | 182 | 59.7 | 53.9 to 65.2 |
| –2 to –4 SDs (low) | 1703 | 33.5 | 32.2 to 34.8 | 276 | 37.1 | 33.6 to 40.7 | 122 | 41.5 | 35.8 to 47.4 | 9 | 39.1 | 19.7 to 61.5 | 70 | 39.8 | 32.5 to 47.4 | 33 | 29.5 | 21.2 to 38.8 | 95 | 31.1 | 26.0 to 36.7 |
| < –4 SDs (very low) | 382 | 7.5 | 6.8 to 8.3 | 69 | 9.3 | 7.3 to 11.6 | 26 | 7.9 | 5.2 to 11.3 | 2 | 8.7 | 1.1 to 28.0 | 14 | 8.0 | 4.4 to 13.0 | 14 | 7.9 | 7.0 to 20.1 | 28 | 9.2 | 6.2 to 13.0 |
| Age categories at index procedure | |||||||||||||||||||||
| > 3 months | 2190 | 38.9 | 37.6 to 40.2 | 357 | 40.8 | 37.6 to 44.2 | 165 | 49.8 | 44.3 to 55.4 | 11 | 42.3 | 23.4 to 63.1 | 76 | 38.6 | 31.7 to 45.8 | 41 | 32.2 | 24.3 to 41.2 | 125 | 35.9 | 30.9 to 41.2 |
| 1–2 months | 1051 | 18.7 | 17.7 to 19.7 | 138 | 15.8 | 13.4 to 18.4 | 58 | 17.5 | 13.6 to 22.1 | 6 | 23.1 | 9.0 to 43.6 | 50 | 25.4 | 19.5 to 32.1 | 34 | 26.8 | 19.3 to 35.4 | 62 | 17.8 | 13.9 to 22.2 |
| 10–30 days | 857 | 15.2 | 14.3 to 16.2 | 153 | 17.5 | 15.0 to 20.2 | 38 | 11.5 | 8.3 to 15.4 | 6 | 23.1 | 9.0 to 43.6 | 27 | 13.7 | 9.2 to 19.3 | 13 | 10.2 | 5.6 to 16.9 | 54 | 15.5 | 11.9 to 19.8 |
| < 10 days | 1528 | 27.2 | 26.0 to 28.3 | 226 | 25.9 | 23.0 to 28.9 | 70 | 21.2 | 16.9 to 25.9 | 3 | 11.5 | 2.4 to 30.2 | 44 | 22.3 | 16.7 to 28.8 | 39 | 30.7 | 22.8 to 39.5 | 107 | 30.7 | 25.9 to 35.9 |
| Outcomes of index procedure | |||||||||||||||||||||
| In-hospital death | 207 | 3.7 | 3.2 to 4.2 | 52 | 5.9 | 4.5 to 7.7 | 18 | 5.4 | 3.3 to 8.5 | 1 | 3.8 | 1.0 to 19.6 | 12 | 6.1 | 3.2 to 10.4 | 6 | 4.7 | 1.8 to 10.0 | 22 | 6.3 | 4.0 to 9.4 |
| Death within 1 year after discharge (outcome 1) | 158 | 2.8 | 2.4 to 3.3 | 34 | 3.9 | 2.7 to 5.4 | 10 | 3.0 | 1.5 to 5.5 | 1 | 3.8 | 1.0 to 19.6 | 4 | 2.0 | 0.6 to 5.1 | 12 | 9.4 | 5.0 to 15.9 | 13 | 3.7 | 2.0 to 6.3 |
| Death or unplanned readmission within 1 year after discharge (outcome 2) | 330 | 5.8 | 5.3 to 6.5 | 68 | 7.8 | 6.1 to 9.8 | 31 | 9.4 | 6.5 to 13.0 | 1 | 3.8 | 0.1 to 20.0 | 9 | 4.6 | 2.1 to 8.5 | 16 | 12.6 | 7.4 to 19.7 | 29 | 8.3 | 5.7 to 11.7 |
There were more boys than girls in the data set overall [n = 4189; 55.6% (95% CI 54.5% to 56.8%)] and within each ethnic group except the black and mixed ethnic groups, which each had a preponderance of girls [black ethnic group: boys 48.3% (95% CI 42.8% to 53.9%); mixed ethnic group: boys 48.7% (95% CI 41.6% to 55.9%)].
Children with CHD admitted to the PICU were more likely to be resident in the most deprived areas if they were from non-white ethnic groups than from the white ethnic group (Figure 7). Analysis of distribution across the quintiles by detailed 16-category ethnic groups demonstrated that there was variation within these broad ethnic groups, such that, within the Asian group the proportion of children of Asian-Pakistani and Asian-Bangladeshi ethnicity living in the most deprived areas was higher than the proportion of Asian-Indian ethnicity, more white-British children than white-Irish were found in the most deprived quintile, and children of black-African ethnicity were more likely than those of black-Caribbean ethnicity to be found living in deprived areas.
FIGURE 7.
Distribution of cases by ethnic group and deprivation quintile.
Compared with the white ethnic group, the proportion of children recorded as having neurodevelopmental abnormalities during the index admission was significantly higher within the Asian, black and mixed ethnic groups (white 3.5%, Asian 6.4%, black 5.4%, mixed 6.1%; see Table 18).
Variation by ethnic group in the proportion of children with associated non-cardiac congenital anomalies and acquired comorbid conditions was not significant (see Table 18). The proportion with congenital anomalies ranged from 20.6% to 38.3% between ethnic groups, and we do not currently have a breakdown by type of congenital anomaly between ethnic groups (the range of possible anomalies was very wide).
The proportion of infants born preterm (before 37 completed weeks’ gestation) was 10.2% (n = 766), which is higher than in the general population, but no significant variation by ethnic group was observed (see Table 18). Overall, 2253 children (29.9%) were diagnosed antenatally with CHD and 1504 children (20.0%) showed evidence of clinical deterioration prior to their index admission; no significant differences were found by ethnic group.
Age at index procedure was categorised into four groups: under 10 days, 10–30 days, 31 days to 3 months, and over 3 months. Most children were operated aged either > 3 months or < 10 days and this distribution was similar for all ethnic groups (see Table 18).
Infants were also categorised by their weight z-score at the time of their index procedure: more than –2 SDs of the mean weight for their age (normal), between –2 SDs and –4 SDs of the mean (low) and more than –4 SDs below the mean (very low). Within all ethnic groups, most children (49.7% to 59.7%) were within the normal weight-for-age category (see Table 18).
Ethnic variations in mortality and unplanned readmission outcomes
There were 318 deaths during the index hospital admission; 232 deaths occurred within the first year after discharge home from hospital following the index procedure and were associated with an unexpected collapse in the community or an unplanned readmission to hospital (outcome 1 in the risk model; see Table 18). A further 252 children who went home after the index admission had an unplanned emergency readmission but did not die within 1 year of discharge; therefore, a total of 484 children experienced outcome 2 (see Table 18).
In univariate analyses (Table 19), children of Asian ethnicity had a higher mortality risk [RR 1.6 (95% CI 1.2 to 2.2)] than the white reference group during the period of the index hospital admission. Following discharge, the ethnic group that experienced a higher rate of unexpected deaths (outcome 1) than the white ethnic group were children of ‘other’ ethnicity [RR 3.4, (95% CI 1.9 to 5.9)]. In the first year following discharge from hospital, children of Asian, black and other ethnicity had a significantly higher univariate risk of outcome 2 [Asian RR 1.3 (95% CI 1.0 to 1.7), black RR 1.6 (95% CI 1.1 to 2.3), other RR 2.1 (95% CI 1.3 to 3.4)] than the white reference group.
| RRs for different outcomes | RR | 95% CI | p-value |
|---|---|---|---|
| RRs for mortality during the index hospital admission | |||
| Asian | 1.6 | 1.2 to 2.2 | < 0.001 |
| Black | 1.5 | 0.9 to 2.4 | 0.10 |
| Chinese | 1.0 | 0.2 to 7.2 | 0.96 |
| Mixed | 1.7 | 0.9 to 2.9 | 0.08 |
| Other | 1.3 | 0.6 to 2.8 | 0.54 |
| RRs for outcome 1 (unexpected death following index hospital discharge) | |||
| Asian | 1.3 | 1.0 to 2.0 | 0.08 |
| Black | 1.1 | 0.6 to 2.0 | 0.82 |
| Chinese | 1.4 | 0.2 to 9.4 | 0.75 |
| Mixed | 0.7 | 0.3 to 1.9 | 0.52 |
| Other | 3.4 | 1.9 to 5.9 | < 0.001 |
| RRs for outcome 2 (unexpected death or readmission following index hospital discharge) | |||
| Asian | 1.3 | 1.0 to 1.7 | < 0.03 |
| Black | 1.6 | 1.1 to 2.3 | < 0.01 |
| Chinese | 0.7 | 0.1 to 4.5 | 0.67 |
| Mixed | 0.8 | 0.4 to 1.5 | 0.45 |
| Other | 2.1 | 1.3 to 3.4 | < 0.001 |
Discussion
Linkage of the national paediatric cardiac interventional and intensive care audit data provided us with a unique patient-based data set with which to investigate ethnic variations in the incidence of CHD, associated comorbidities and outcomes for infants. This extended analysis was pertinent to the findings of the risk model, which suggested a higher likelihood of postdischarge death for infants of ‘other’ ethnicity. Moreover, our initial analysis of the concordance of ethnicity records within the two data sets contributed to the risk model and CART analysis by informing methods to address missing ethnic group data.
In the analyses presented in this chapter, we identified a higher incidence of CHD overall within the Asian, black and other ethnic groups than in the white ethnic group, as well as a higher relative rate of specific complex and severe defects that carry a high early mortality risk, for example HLHS in the black and other ethnic groups, and UVH in the Asian and black ethnic groups. Furthermore, the Asian, black and other ethnic groups were more likely to live in more deprived areas and Asian and black ethnic groups had higher rates of neurodevelopmental problems (which based on Chapter 4 place them at higher risk). These distributions of additional features within the infant CHD population by ethnic group need to be borne in mind when designing postdischarge care pathways for higher-risk infants; for example, the potential for language barriers or cultural differences to play a role in the level of success or acceptability of a HMP needs to be considered.
The comparison of deaths among children of non-white ethnicity and those of white ethnicity demonstrated that children of Asian ethnicity experienced a higher mortality risk during the hospital stay. We present here univariable analyses only, as in-hospital mortality was outwith the scope of this project and is being further explored as an extension to the original proposed analyses. Children of ‘other’ ethnicity experienced higher mortality in the community or during an unexpected hospital readmission in the first year after discharge home, and this finding remained after multivariable analyses as laid out in Chapter 4. The univariate risk for outcome 2 in Asian and black patients presented above dropped out during the multivariable analyses presented in Chapter 4, leaving only the ‘other’ ethnic group at higher risk.
Data in context
In the UK, infant mortality (death in the first year after birth) is highest in the black Caribbean, Bangladeshi and Pakistani ethnic groups (at over 6 deaths per 1000 live births); this is twice as high as in the white ethnic groups. In 2012 UK data from the Office for National Statistics, the most common cause of death reported for most ethnic groups (black 54%, mixed/Chinese/any other ethnic group 44% and white 43%) was ‘immaturity-related conditions’, whereas for Asian infants the most common cause of death was congenital anomalies (41%). 108
Analysis of UK infant mortality statistics2 has also shown ethnic variation in mortality risk for babies born with congenital anomalies. Compared with affected babies born to mothers of white British ethnicity, there is evidence for a fourfold increased risk of death in the first year for infants born to mothers of Pakistani ethnic origin and a 45% higher infant death risk for babies born to mothers of ‘all other’ ethnicity (a group that includes other, Chinese and mixed ethnicity in our analysis). Some authors have suggested that excess infant mortality in the Pakistani population may be because of consanguinity91 and, based on a meta-analysis across different populations, Bittles109 suggests that children of closely related parents have a 3.3% median excess risk of congenital anomalies compared with children of unrelated parents. Nevertheless, Bittles also emphasises noticeable variations in the baseline prevalence of congenital anomalies between different populations.
It is possible that our risk model did not demonstrate a higher infant mortality or emergency admission rates associated with Asian ethnicity, as has been demonstrated in some previous studies,19,110 because the higher death rate among Asian babies during the index hospital admission meant that many infants did not survive to hospital discharge. These early in-hospital deaths are likely to have disproportionately affected babies with more severe cardiac defects or associated comorbid conditions, such as more severe types of non-cardiac congenital anomalies or prematurity. This aspect has not to date been explored in detail and goes beyond the scope of our research project.
Different mortality rates after birth will also be influenced by the likelihood of prenatal detection for different defects, and views about the acceptability of pregnancy termination will determine whether or not the diagnosis of a severely affected infant leads to a live birth. In 1999, Bull reported that a serious CHD was diagnosed in 23% of all pregnancies, but only 12% of live births were affected by a CHD; pregnancies affected by CHDs were terminated in around 50% of cases, and this varied by gestation at diagnosis but not by region of birth. 111 Although analyses of termination rates for pregnancies affected by CHDs by ethnic group have not been reported in the UK, a recent analysis of pregnancy terminations for neural tube defect reported to two English regional data sets suggested that UK mothers of Pakistani, black African and black Caribbean ethnicity were less likely to terminate an affected pregnancy than mothers of white, Indian or Bangladeshi ethnicity. 112
It is more difficult to determine the underlying reasons for the higher postdischarge mortality of babies within the ‘other’ ethnic group, because this group is heterogeneous and has not often been described in detail. An analysis of UK Census data,113 which reviewed the country of birth of individuals identified within this group, found that over half were born in the Far East (including the Philippines, Japan, Thailand and Vietnam), 10% were born in the Middle East (mainly Iran and Iraq) and 7% were born in North Africa (including Egypt, Morocco, Algeria and Libya). The ethnic group classed as ‘other’ warrants further evaluation and exploration in order to better understand what factors are at play, given that we do not know how to explain the finding with any certainty. An unproven hypothesis is that a proportion of families of patients in the ‘other’ ethnic group within this study may have recently travelled or migrated to the UK, in some cases for treatment, although all those included in this data set had an NHS number and hence were residents in the UK at the time of surgery. The particularly high incidence of CHD (highest incidence of all ethnicities) in the ‘other’ ethnic group supports this hypothesis. Recent migrants to the UK may have less awareness of and access to services important post discharge, and this may have an adverse effect upon outcome. Further qualitative assessment of families in the ‘other’ ethnic group is required to address these questions.
Conclusion
Although this detailed analysis of routine audit data has highlighted a lack of consistency and completeness in ethnic coding, it has nevertheless provided very valuable insights into the influence of ethnicity on the distribution of CHD in the UK infant population and on outcomes for affected infants. Further investigation of the impact of ethnicity on pregnancy termination rates and infant mortality for babies diagnosed with CHDs is merited. Further investigation of the links between ethnicity, other aspects of case mix and outcome incorporating different stages of the patient journey over the first year of life (incorporating multivariable modelling) is warranted.
Chapter 6 Qualitative study of family viewpoints expressed via an online discussion forum hosted by the Children’s Heart Federation
Introduction
Recent years have seen a significant increase in the use of online social networks, with evidence suggesting that 72% of adults worldwide now use social media. 114,115 Social networks provide a quick and easy means of sharing ideas, information and opinions, have a broad population reach and are widely used in every sphere of life. 114,116 Developments in technology have also resulted in an increasing use of electronic methods to collect data for research and there is evidence to support their feasibility, the prompt response of participants, the richness of data collected by these means and the reduction of human errors. 117–119 One method of electronic data collection is the online forum, which allows asynchronous interactions, whereby participants are able to join discussions at their own convenience, in contrast to methods that require synchronous interactions, such as chat groups. They have been reported to be relatively easy to use, safe, accessible and observable, and it has also been suggested that they offer a more comfortable mechanism for the discussion of sensitive or personal health issues and are a feasible alternative to more traditional research tools, such as face-to-face focus groups. 120–122 Furthermore, they offer flexibility to researchers and participants alike, thus reducing participant burden and pressures of time.
Parents of children with significant health conditions can find it challenging to participate in face-to-face research for a variety of practical reasons; however, those who are harder to reach via traditional data collection methods often have important and salient contributions to make. As we were interested in exploring parents’ experiences of taking a baby home after congenital heart surgery, we identified that an online forum would enable a greater number of parents to participate, particularly those who would find it more difficult to attend a focus group or take part in an interview. We decided that the most appropriate way to facilitate the online forum was via the Children’s Heart Federation (CHF) and that this should happen via its Facebook page (www.facebook.com). Facebook is the dominant social network worldwide and many health-related groups have arisen on Facebook that are predominantly used for raising awareness, social support and fundraising. The CHF has an active presence and following on Facebook and Twitter (www.twitter.com) and at the time of setting up the forum the CHF Facebook page had around 3120 members (75% female, most aged 25–40 years). Furthermore, the CHF has experience of running OFs and, being a national charity, its forums are accessed by parents of children treated at all of the specialist paediatric cardiac centres in the UK.
The aims were:
-
to set up an OF to ask parents about their experiences of caring for a baby at home after congenital heart surgery
-
to collect information that could inform the development of questions for the indepth family interviews (FIs).
Methods
A closed online discussion group was set up via the main Facebook page of the CHF. The discussion group was advertised through the charity’s web page and interested participants were then directed to the charity’s Facebook page. Participants were required to provide some basic demographic information (their age, sex, ethnicity and geographical region). Once this information was received by the CHF, the participants were given access to a private or ‘closed’ Facebook group and were able to begin responding to questions posted there. The CHF was responsible for all day-to-day running and moderation of the forum in line with a standard operating procedure developed in collaboration with the research team. The standard operating procedure included processes for managing inappropriate and/or offensive messaging and distressed users, as well as procedures for the day-to-day running of the forum. The research team provided questions to be posted on the forum (Box 3). The CHF was asked to probe further if it noticed any of the following issues in participant responses:
-
social and practical issues, for example socioeconomic status, financial, educational issues, transport
-
issues to do with language or cultural differences
-
difficulties accessing support in the community
-
understanding information from health-care providers.
In-hospital information and support:
What information were you given about looking after your baby at home after his/her surgery?
Community support and hospital follow-up:
What was your experience of the support available in the community when you took your baby home from hospital?
When there was a problem:
Could you tell us a bit about your experience of getting support when you were worried about your baby?
Final question:
If there was one thing that would have made a difference (or did make a difference) to you and your family when you took your baby home from hospital, what would that be?
Forum responses were anonymised by the CHF before being sent to the research team in a weekly update.
Data analysis
Responses were collated into a single transcript and thematic analysis was used to analyse forum responses. Thematic analysis involves:
-
familiarisation with the data
-
generating initial codes
-
searching for themes among these codes (but not by counting them)
-
reviewing themes
-
defining and naming themes.
Analysis was conducted by members of the research team working as a group.
Results
Participants and participation
The forum ran from February to May 2013. During this period, a total of 91 participants (mean age 35 years; range 23–58 years; 89 female; 89 parents, two grandparents) submitted demographic information and were given access to the closed forum group. Participants came from all over the UK and were predominantly of white British ethnicity (85 out of 91). Of these, 73 parents participated in the forum discussion and most responded to between one and five questions. Neither of the two grandparents contributed to the forum discussion. The questions with the most responses were those about information provided about caring for their baby at home (symptoms and how prepared they felt); community support [particularly experiences with general practitioners (GPs) and health visitors (HVs)]; and support from cardiac liaison nurses (CLNs). Parents also responded to others’ posts, offering support and sharing experiences, and towards the end of the forum parents became more open in identifying with other parents’ experiences: ‘After reading this it was like reading my own story’; ‘It is so good to read about someone who has done so well’. Selected quotations are included in Boxes 4–9.
I felt it too risky to go to clinic, with infections.
I didn’t see a soul . . . from leaving hospital to returning for review.
It was all such a huge shock, on discharge we were told she was like any other baby, but obviously there were differences. I was very scared about taking her out and catching a cold or something, or visitors bringing in germs.
. . . home visits were lovely but made me feel more isolated.
[My health visitor] was nervous about me going to baby clinics, etc. for weekly weights but I needed to get out.
Loads of mum formed their friendships at that time. I was left behind and still am because of his additional needs.
Didn’t have any support wasn’t even allowed to take her to baby group so was very isolated.
Absolutely no support groups . . . or help . . . feel quite isolated sometimes.
Other mums looking at me as he was tube fed, etc. felt very isolated.
No one where I lived had a CHD child.
For a long time I struggled to feel like his mummy and not a nurse because that was all I seemed to be doing . . . medications, meds and more meds.
Very disappointed with my health visitors. I had three visits in total. Seemed more interested in me filling in post natal depression form than in my son.
Health visitor only decided to visit 4 months after he was discharged home. Prescriptions again were a problem. No contact from GP. Local consultant at hospital didn’t even know he’d had open heart surgery or a stroke post-op! V v frustrating!
I’ve had good support from local GP’s practice and the local hospital when needed. No one has ever made me feel like I’m wasting time and have always reassured me that if I am worried they are more than happy to see her.
She [the CLN] was brilliant, with information, care and support. In hospital and afterwards. If there was any questions she would be there and if needs be go and find information and get back to us quickly.
My health visitor is and continues to be a positive influence in relation to our situation. Only ever a phone call away and visits on a regular basis.
There has been no one in the community or local hospital that had the answers.
GP has been very supportive but tends to send my daughter to hospital rather than make decisions himself.
HV lack of experience with CHD made her slightly apprehensive with us which came across as dismissive.
I felt a bit overwhelmed and like I didn’t know what I was doing at all.
I found that we know lots more than they [community HPs] did about her condition, which was both understandable . . . and terrifying in equal measures.
. . . just sick of explaining to everyone. I wish someone could tell me about him not the other way around.
My [health visitor] was good too & did the best she could for us despite not having all the answers about concerns regarding baby’s heart defect. She contacted other professionals to seek advice or support for us.
She helped as much as she could . . . she admitted she didn’t know much about chds . . . but was there when she saw I was starting to have anxiety . . .
We were [nasogastric] feeding, everything we needed to be told we were told and if we needed support/help they were there for us.
Complete lack of information. One general leaflet about chd’s and that was it.
We was given a folder with phone numbers of ward and heart charities in it also how to care for your child’s scar and what to look out for with the scar. I would of found it helpful if id been told what sats to look out for as i didn’t have a clue.
Felt out of my depth and very scared.
It was a lonely scary time and was just left to it.
It has been the most traumatic year of my life and yet I feel I can’t really talk to anyone else about it.
The child is discharged and the parents are left walking around in an often traumatised state with no suitable support.
Your whole life changes and no one tells you that . . . I think I had a bit of post-traumatic stress but I was so grateful my baby was home I didn’t want to say how terrified I was constantly and how much I relived every moment.
The last few months have been like walking in a fog, feeling completely lost at times.
I was having panic attacks and quite ‘fog-like’ for months, felt quite isolated but there seemed to be nobody really asking about the parents.
We got bombarded with info in hospital but once home there’s no information as to what to look out for.
A rollercoaster of information and procedures.
Always chasing receptionists for prescriptions. Even when I have explained long lead time for meds they don’t make any accommodations. Computer says no!
At one point we were left without heart meds for one week. Doctor refused to prescribe because the strength of medicine needed wasn’t exactly as it was written on his notes (even tho the dosage was the same). They told me they would phone the cardiologist to check. They didn’t. Needless to say my baby was getting sicker & sicker. I ended up crying down the phone pleading for a prescription.
GP was supportive but nowhere local could do instant INR checks on a baby so had to travel to [paediatric specialist centre] every couple of days which was 100 mile round trip
I had . . . this baby that struggled to feed, cried if I touched her and lost weight constantly. All I got was ‘just persevere and top her up with her [nasogastric]’
The first few days were a nightmare . . . I ended up syringing milk into her mouth as she wouldn’t take a bottle at all.
. . . very time-consuming and lots of pressure to keep it up.
I remember feeling so lost and helpless as I tried to breast feed without hurting her.
We had a horrendous 6 months and it was me that sought out support [for feeding issues] it wasn’t just there for us.
We weren’t offered anything. I researched myself and paid to go on a babies/children first aid course. Think it would’ve been massively useful (if only to instill some confidence in us as parents that we could cope if a situation had arisen) to have had some basic training or advice.
. . . we feared an emergency could happen at any time.
We were not prepared for complications other than cardiac ones so again felt lost, scared to death that something horrendous was going to happen every time she became slightly unwell.
I was very scared about taking her out and catching a cold or something or visitors bring in germs.
Went to mother and baby group once to get him weighed and never went again as was so upsetting by HV and other mums looking at you child’s scar and not talking.
I found my doctor very ‘stand-offish’ as if scared of him [baby].
I feel they [GP] think you are an over-reactive mum.
We were asked in the hospital to give her meds under the nurse’s supervision so she could check we were administering them correctly. We were given all doses and medicines written down and plenty of syringes to take home. We were given a lot of info on care of her wound, what to do if she went blue, etc. and numbers for the CLN, the ward and were told any queries just to call the ward direct which we did on a couple of occasions and got great and prompt advice.
The cardiac unit made sure we knew what was normal for him and what we should act on if we saw it.
. . . told to look for blue lips. fingers. if she got breathless tired sweaty while feeding also if oxygen levels go low.
We have open access at local hospitals and have [been] made to feel very welcome and nothing ever too big or small to come and see them.
Our local hospital told us their doors were always open to us . . . we were always taken seriously.
We could phone the ward or community nurses at any time & discuss any concerns.
She [the CLN] was always on hand via phone or in person to answer questions and help explain stuff to us in layman’s terms. I honestly believe she made a world of difference to our ability to cope.
Local hospital would have [baby] in for 1–2 nights so THEY knew what to expect and how to treat.
No problem was too small for the GP, she would phone the hospital if she needed to while I was waiting and send me straight away if she was worried about her.
My health visitor is and continues to be a positive influence in relation to our situation. Only ever a phone call away and visits on a regular basis.
Our community nurse was brilliant, she even gave me her home number just in case I needed her. The health visitor was great, ENT [Ear, Nose and Throat] feeding specialist was really helpful and still helps if I need her.
. . . nothing is ever too small a question.
She [the CLN] was a familiar face in a whirlwind of unknowns . . . a friend in the know.
Our cardiac liaison nurses are worth their weight in gold. They are always there if I have any concerns . . . I could not do without them now.
I will ask the other heart mums and dads first as they usually know what’s what.
My lifeline throughout the whole experience was the ‘Heartline’ charity forum [online] I got a tremendous amount of support and got in contact with 2 mums (both with heart children) who have supported me through the whole process.
. . . most of my support and advice has come from heart mums/dads on Facebook and the support groups there.
. . . it is the emotional support of friends and family that pulls you through.
We used to go to our local to see friends just to try and get some normal life and conversation.
Findings
The core theme emerging from the data was the family experience of isolation, epitomised by the following quote: ‘It’s a pretty lonely place’. This was described in terms of the way in which that isolation was experienced (physical, social and knowledge) and the resulting psychological impact, together with the factors that made that worse or better. There was also a theme of time, as parents moved from feeling overwhelmed and lacking in knowledge and skills to becoming ‘expert parents’ with a corresponding increase in their knowledge and skills. Figure 8 is a schematic representation of this.
FIGURE 8.
Themes from the online forum: ‘It’s a pretty lonely place’.
Physical isolation
A number of parents described being physically isolated as a result of having a baby with CHD, which was sometimes because of their own anxieties and concerns, particularly about the risk of infection. In other situations, parents identified that professionals, particularly HVs, were anxious about mothers taking their babies to a busy clinic, preferring instead to make home visits, but this in turn could compound the challenges that the families were facing.
Social isolation
Social isolation was described in terms of interactions with other parents and about the support parents of a child with CHD received from professionals. Being unable to participate in ‘normal’ mother and baby activities resulted in mothers feeling isolated from other new mothers, with the impact sometimes having consequences beyond the first weeks and months. Some parents described a lack of support and how the particular needs of their baby singled them out from other parents but also had an impact on their parenting role.
A number of parents were very specific in their descriptions of what they considered to be failings in the support they were given, identifying specific professionals and what they thought was lacking, together with the lack of communication between professionals about their child. In contrast, other parents reported feeling well supported by their local community team and by the CLNs.
Knowledge
Some parents described their own and others’ knowledge, or lack of knowledge, as both challenging and isolating. There was a lot of agreement that professionals in both secondary and primary care did not have sufficient information about CHD and/or their child’s specific heart condition. Parents also described their own stress associated with knowledge, in terms of either feeling that they did not know enough about their child’s condition or, conversely, the responsibility of needing to communicate knowledge to local HPs, which in itself could be burdensome. Others talked about the efforts that HPs in the community went to in order to become more knowledgeable about CHD or to get advice from other professionals. Finally, parents talked about the information they were given by the specialist centre, highlighting the degree to which tertiary centres varied in the information they gave families about local services and support networks, both in terms of what they provided and how they provided it.
Psychological and emotional impact
Caring for a child after cardiac surgery can have a significant psychological and emotional impact, and parents described their anxiety, which for some developed into symptoms of post-traumatic stress or served to heighten their feelings of isolation. One parent talked about feeling unprepared for how life had changed and the tension between feeling grateful that her child had come through the surgery and was back home and not feeling able to tell the team how anxious she felt. A number of parents also described feeling in a ‘fog’ when they got home after their baby’s surgery.
Challenges
Parents provided insight into a number of challenges that they faced once home after their child’s surgery, which contributed to and compounded the overall feeling of isolation. Some of these challenges were related to practical issues, such as information, although other parents described a number of practical difficulties associated with aspects of the treatment regimen, such as getting prescriptions or having to have blood levels checked. Such perceived difficulties can undermine local health services and reduce parents’ trust and confidence in them.
Feeding was identified as a significant challenge by a number of the parents, which was related to the difficulties the baby had in feeding, the time it took and some of the practicalities for those children who were tube fed. Some mothers also talked about breastfeeding; for example, one mother described her worry about whether or not feeding would hurt her baby, whereas others discussed the lack of support they experienced around feeding issues.
Taking home a vulnerable baby after surgery was a further source of stress for a number of parents, related to the risk of complications, infections or the fear of something going wrong, and several parents identified that a lack of training had contributed to their anxiety.
Finally, parents described the challenge of dealing with the reactions of others, both professionals and other parents, to them and their baby.
Mitigating factors
Although some parents described the challenges of caring for a baby with a heart condition after cardiac surgery, many also talked about what helped and the factors that lessened feelings of isolation. A number of the mitigating factors were related to the same topics that were challenges for other parents. For example, in a number of cases parents were provided with, or had access to, training and information before they left the specialist centre. Several parents were also given specific information about signs and symptoms to look for in their baby. A number of parents had open-access arrangements with their local hospital, which were clearly highly valued and helped to reassure parents. Others described the accessibility of advice and the importance of that to the parents.
Although some parents had experienced negative reactions from HPs, others had a very positive experience of care and support after hospital discharge from professionals in both primary and secondary care. Parents also valued ongoing support from the CLNs in the specialist centres. Support from people other than HPs was also identified as an important factor for reducing isolation and facilitating coping. Parents described three main sources of non-medical support: the support of other heart families, online and charity support, and the support of family and friends. One mother talked about wishing that she had had ‘. . . contact with someone who had “been there done that” ’. Another mother saw parents in similar situations to hers as her first port of call for support. Online and charity contact were recognised as important and helpful sources of support, primarily as a means of having contact with other parents. The support from family and friends was mentioned less frequently than other sources of support, but those parents who did describe it saw it as an important facilitator of coping with the experience and it was also attributed to helping parents find some normality outside of their child’s heart condition and care.
Discussion
As far as we are aware, using a charity online forum as a systematic means of eliciting views from parents about their experiences of having a child with a health condition has not been undertaken previously within a research project. Collection of data using this method enabled us to reach a large number of potential participants, including those harder to reach families who may find it more difficult or not wish to participate in projects that use more common methods of data collection such as focus groups, interviews or questionnaires. Furthermore, the ability to see other participants’ posts may have had a positive impact on reluctant responders and encouraged and empowered them to engage.
The core theme emerging from the forum was one of isolation, which parents described in terms of social and physical isolation, and isolation related to knowledge. Physical and social isolation are common themes expressed by parents of children with health needs including parents of children with other chronic illnesses,123 autism124 and mothers of extremely preterm babies. 125 Parents also talked about the stress associated with knowledge about their child’s condition, in terms of feeling that they did not have enough knowledge, particularly in the early stages after hospital discharge, but also the burden of responsibility as the ‘keeper’ of the knowledge about their child’s condition and the need to inform less knowledgeable professionals in primary care in particular. Over time parents became ‘expert parents’ in relation to the specifics of their child’s condition and treatment, a phenomenon seen in parents of children with other chronic conditions,126,127 but there was also some ambivalence about this role associated with the need to assume responsibility for informing HPs outside the tertiary centre.
The psychological and emotional impact of CHD on parents is well documented128–131 and parents’ descriptions of their anxiety and symptoms of post-traumatic stress on the online forum corroborate findings in the literature. 132,133 What we were able to additionally elicit, however, were parents’ views about the specific challenges they faced following discharge after their child’s surgery and their perceptions about what helped mitigate their feelings of isolation and psychological distress. Key themes that emerged were related to information, training, practical issues and support, and examples were provided in all of these areas of when things went well and when things went badly. Interventions to ameliorate some of the negative factors and enhance some of the positive ones could focus on aspects such as improving communication between HPs in tertiary, secondary and primary care; providing appropriate training and information to parents and also to HPs, who are not specialists in CHD and its treatment, about what to look for and what to do if a problem arises; ensuring practical aspects are in place prior to discharge, such as open access at the local hospital, having necessary equipment locally such as INR machines, ensuring that prescriptions are organised appropriately; and managing parent expectations of community health services so that they are prepared for their local HPs having less specialist knowledge without assuming that this means that their child will not receive good support.
Limitations
There were a number of limitations with this element of the study, related to the method of collecting the data. The sample was predominantly white British, most of whom were mothers, corroborating research which has identified that the majority of internet users are women and participants in online interactions tend to be predominantly white, younger and highly educated. 134 In order to participate they required access to the CHF website and familiarity with Facebook, which meant that parents who did not speak English and were not able or willing to use social media could not participate. Furthermore, participants required a certain level of computer skills because of the requirement to register and login to the site. It is probable that parents from a more ethnically diverse population face additional challenges and have different experiences, which this method of data collection prevented us from capturing. Furthermore, families who are in contact with charities offering support may be more likely to engage with this type of research, thus limiting the representativeness of the sample. Finally, although questions posted on the forum related to discharge after infant cardiac surgery, some of the parents provided information and views about other stages of their journey (e.g. after surgery in later childhood). Although we did not include these posts in our analysis when this was made explicit, it is possible that some posts were included when the time that parents were referring to was not identified as being other than following cardiac surgery in infancy.
Online forums also have some inherent limitations compared with more traditional face-to-face methods of data collection. For example, non-verbal and contextual cues cannot be picked up on and specific participant comments cannot be probed, resulting in the potential loss of some richness of the data. Moreover, responses were generally much shorter than would be elicited in an interview. The automatically generated transcript, while being a benefit of this research method, was also not perfect, as has been identified previously in online forum research. 135 We did not collect information about the time spent on the site or number of visits participants made while the forum was running and we also did not know if any participants had technical problems accessing the site at any time or whether or not potential participants failed to join the forum at all. However, it is also important to consider the aims of this part of the study and the fact that we were not conducting a qualitative study requiring data saturation but wanted to elicit information about parental experiences to inform the development of a topic guide for the in-depth interview in the next phase of the study.
Conclusion
Use of an online forum provided a means of eliciting a large number of parents’ experiences of caring for their child after discharge from hospital following cardiac surgery. Parents engaged with the forum and were able to articulate what went well and what went less well, in addition to sharing their stories and supporting each other through doing so. Information gained from the forum was used to shape the questions for the parent interviews in a subsequent phase of the study and has been used to inform the development of an intervention, in conjunction with other findings from the study, for parents of high-risk babies following discharge after cardiac surgery.
Chapter 7 Signs of deterioration in infants discharged home following congenital heart surgery in the first year of life: a qualitative study
Some of the text of this chapter has been published previously as Tregay et al. , 2016. 136 © Article author (or their employer) 2016. Produced by BMJ Publishing Group Ltd (& RCPCH) under licence. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Background
To understand more about parent perspectives of caring for a child with complex needs after congenital heart surgery, we undertook a qualitative study involving semistructured interviews with parents. Our study aimed to describe the ways in which parents recognise and make decisions about their child’s symptoms, their experience of seeking help when there was a concern, and any barriers they encountered in engaging with services to support their child.
Methods
Parents were invited to take part in the study if their child underwent major congenital heart surgery in their first year of life at one of three UK children’s hospitals who subsequently died or was readmitted unexpectedly to intensive care following their initial discharge. Parents were invited to interview if their child had their index surgery in the last 5 years and were initially approached by local specialist nurses who obtained consent to pass on their details to the research team.
All interviews were conducted face to face by a single researcher (JT) and all but one took place in parents’ own homes. Parents were asked about caring for their child at home following surgery, the support they received and the events leading up to any emergency readmissions. Interviews were tape-recorded and transcribed verbatim before being analysed using framework analysis.
Framework analysis
Framework analysis137 is a structured approach to managing qualitative data that allows researchers to organise and extract themes from the data more easily. It is a systematic approach that aims to reduce bias and make analysis of large data sets more manageable and involves entering qualitative data (e.g. quotes and summaries) into charts to aid interpretation, ensuring key themes are systematically searched for across each transcript.
Analysis using the framework approach consists of five stages.
-
Familiarisation: listening to audiotapes, reading transcripts, becoming aware of key ideas/recurrent themes and noting them.
-
Identifying a thematic framework: recognising emerging themes in the data set; may be guided by a priori issues but must also be open-minded to new themes; making judgments about meaning, relevance and importance of issues; may be tentative at this stage.
-
Indexing: identifying data (e.g. quotes) that correspond to a particular theme.
-
Charting: data identified during indexing are now arranged in charts of themes.
-
Mapping and interpretation: analysis of key characteristics laid out in the charts; should be able to provide a schematic diagram.
Members of the core research team worked collaboratively and iteratively on the development of the frameworks, mapping and interpretation of the data for each of the participant groups.
Results
Descriptive information
Specialist nurses contacted 25 families, 21 of whom agreed to be interviewed. One family was excluded, as they did not meet inclusion criteria, leaving a total of 20 families who were interviewed for the study. Of these families, 12 were bereaved. Fourteen interviews were conducted with one parent alone (n = 14 mothers), and six with both parents together. A range of ethnic, educational and socioeconomic backgrounds was represented in the sample (Table 20). One parent did not speak English as a first language and two were bilingual.
| Demographic information | Number |
|---|---|
| Ethnicity (of child) | |
| White | |
| British | 14 |
| European | 1 |
| Asian | |
| Bangladeshi | 1 |
| Pakistani | 1 |
| Other | 1 |
| Mixed | |
| White/black African | 1 |
| White/black other | 1 |
| Educational history (primary caregiver) | |
| Learning disability | 1 |
| Primary/secondary | 11 |
| Graduate | 5 |
| Postgraduate | 3 |
All children had their first surgery between September 2009 and October 2013. Following their initial surgery, 12 children were discharged home directly from the specialist surgical centre; the remainder were discharged to their local hospital in a ‘step-down’ arrangement. A case-by-case summary of complications and symptoms for each patient is provided in Table 21.
| ID | Diagnosis | Complication | Symptoms |
|---|---|---|---|
| FR01 | SV disease (not HLHS) | Right diaphragm palsy with plication | Respiratory distress |
| FR02 | HLHS | Wound infection (emergency sternal wound debridement) | Reduced feeding |
| Severe vomiting | |||
| Abdomen ‘not quite right’ (respiratory distress) | |||
| FB03a | TGA (plus or minus other features) | Out-of-hospital cardiac arrest (cause unknown) | Collapsed at home after a feed |
| No other symptoms | |||
| FB04a | HLHS | Sudden collapse at home. Died in A&E | Excessive crying and screaming |
| Collapsed at home | |||
| FB05a | HLHS | Aspirated at home | Reduced feeding |
| Severe vomiting | |||
| Dry nappy | |||
| FB06a | VSD plus significant medical comorbidity | Sudden collapse at home | Slight cough |
| FR07 | SV disease (not HLHS) | Blocked left shunt (urgent redo) | Respiratory distress |
| Recessing chest | |||
| Purple fingertips and lips | |||
| Did not notice first time but symptoms pointed out at routine scan at local hospital | |||
| FR08 | Tetralogy of Fallot | Blocked shunt (urgent central shunt) | No obvious symptoms |
| Low saturation detected during home visit | |||
| FR09 | TGA (plus or minus other features) | Resection of aortic aneurysm | No obvious symptoms |
| Mother felt something was wrong but did not know what it was, ‘mother’s instinct’ | |||
| FB10a | SV disease (not HLHS) and significant medical co morbidity | Aspirated at home | Breathing |
| Pale colouring | |||
| Severe vomiting | |||
| Crying | |||
| Found in the night in respiratory distress | |||
| FB11a | HLHS | Sudden collapse at home. Died in A&E | Excessive crying |
| Unable to settle | |||
| FB12a | SV disease (not HLHS) | Blocked shunt | Breathlessness noted by paediatrician 2 days before, appeared ‘normal’ to parents |
| Sleepier after feeds | |||
| Screaming and unable to comfort | |||
| Collapsed at home | |||
| FB13a | HLHS | Blocked shunt | No obvious symptoms |
| Routine vaccinations day before and following this: | |||
| More lethargic than usual | |||
| Diarrhoea in night | |||
| Deteriorated over the course of the day | |||
| Respiratory distress leading to collapse at home | |||
| FB14a | Anomalous coronary artery from pulmonary artery | Sudden collapse at home. Died in A&E | Severe sweating and vomiting after feeds |
| ‘Spelling’ intermittently (pale/blue/grey lips) | |||
| Quiet and weak in the morning | |||
| Grunting/straining sounds | |||
| Started screaming during feed and collapsed | |||
| FR15 | SV disease (not HLHS) | Severe mitral regurgitation and left ventricular failure | Reduced appetite |
| Lethargic | |||
| Vomiting | |||
| Cough | |||
| ‘Grunting’ breath sounds | |||
| FR16a | SV disease (not HLHS) | Pacemaker pocket infection and dehydration | Severe crying and restless at night |
| Rapid breathing | |||
| Blue face/hands/lips | |||
| Recessing under ribs | |||
| FB17a | SV disease (not HLHS) | Sudden collapse at home. Died in A&E | Appeared ‘agitated’ and generally less settled |
| Taking less feed in one go | |||
| Slight cough | |||
| Routine vaccinations week prior to death | |||
| Collapsed during feed | |||
| FB18a | SV disease (not HLHS) | Sudden collapse at home. Died in A&E | More ‘emotional’ and ‘moody’ |
| More difficult to comfort | |||
| Easily tired | |||
| Breathless on activity | |||
| Napping more during daytime | |||
| More unsettled at night | |||
| Blue hands and feet at times | |||
| Vomiting in hot weather | |||
| Sweating | |||
| Woke in night crying, vomiting and short of breath, then screamed and collapsed | |||
| FR19 | TGA (plus or minus other features) | Readmitted for coarctation repair | High BP picked up at local review resulting in readmission to tertiary centre |
| No clinical signs observed | |||
| FR20 | TAPVD | Increasing LUPV stenosis, ongoing tachypnoea and hepatomegaly with increased right heart pressures secondary to increased pulmonary venous pressures | General ‘grumpiness’ |
| Mother feeling that something was not right | |||
| High rate of breathing | |||
| Vomiting |
Data are presented in three main sections. The first describes symptoms of deterioration noted by families and is divided into five subsections: no symptoms, feeding, respiratory distress, appearance and behaviour. The second section focuses on decision-making about symptoms and the final section describes families’ experiences of seeking help. Additional quotes are presented in Boxes 10–16.
The whole thing of, ‘You will know when your child is so unwell.’ Well, clearly he deals with it pretty well.
FR09
Everything was normal, literally, until the morning that we lost her.
FB12
She was absolutely fine, she was feeding, she was doing everything that we needed her to do, and she was kind of growing nicely. So I think it came as a bit of a shock.
FR19
The last week of her life, she started being sick and she’s never been sick. And I said to the community nurse ‘she’s been a bit sick and she’s not bringing up her wind . . . There’s something wrong.
FB05
He was sick the whole time. He couldn’t drink a bottle without being sick.
FR09
I think it was around that time he started to take off his feeds as well.
FB17
One thing we did notice was that if it was quite a hot day, he would be a bit sick.
FB18
It was his tummy and he was just, it wasn’t he was breathing funny, but it was just something-, he wasn’t quite comfortable. Something wasn’t quite right.
FB02
That little bit of the whistle in her chest again and basically just the breathing, you know, with the ribs. I don’t know how to describe it. The tummy sort of goes right in and then you can see, like, the outline of the ribs when someone’s breathing a bit funny.
FR07
In the morning, when he’d wake up, he’d cough. I now learned that that’s because he had lots of fluid resting on his lungs . . . At the end of August he started wheezing, but really randomly, to the point that I knew something was wrong.
FR09
We woke up in the night time, and we noticed that [his] breathing-, he looked different and his breathing was very hard.
FB10
‘Is she more breathless?’ I don’t actually know whether she was or not . . . I think it’s quite hard if it’s a gradual thing. To this day I still don’t really know whether she was more breathless or not, but she was her normal usual self.
FB12
Sometimes she would do a noise, as if she was straining.
FB14
. . . his nose will flare.
FR16
I noticed around seven months he would be a bit more breathless, he would have to nap more often because he would get tired more often . . . His stomach was sinking in.
FB18
He was breathing quite fast and I was like, ‘Slow down, slow down’, I didn’t know breaths could get that fast.
FR20
We, kind of noticed her fingernails at the end were going a little bit purple. Just around here, of her lips, they were a little bit purple.
FB02
He was smiling and happy but when he was lying down he just looked a bit-, in hindsight, he was incredibly puffy.
FR09
. . . sometimes he was a bit pale.
FB10
I noticed that she was sweating in her hair . . . When I stripped her off, then I noticed her chest was blue, and that-, I would have said she was sweating, but actually in retrospect she was clammy.
FB12
Her colour is a dark grey, blue and yellow. You see? There is no colour in her lips . . . My husband couldn’t tell . . . nobody could tell, except me.
FB14
I found that he would, even if it was very cool . . . in his sleep, he would sweat an awful lot. Around his back, his shoulders, his neck. I started to notice the blueing of the hands and his mouth . . . I think from six months on I mentioned at every appointment that I would notice more often that his hands would get blue, his feet would get blue, his lips.
FB18
It was quite sudden for us. He cried quite a lot and we could not know that he is not well. There was not any sign that he was not well.
FB10
. . . apart from the day he passed away, that was the only time [we noticed anything]. Then I wasn’t overly concerned, to me he was just crying, he didn’t look any different. [We] just couldn’t stop him crying …Probably for a normal child, baby, that’s normal. I don’t know, being a new mother, whether that’s normal or not normal. He was crying, and after everything I’d tried, he still wouldn’t stop crying.
FB11
I did wonder if she was head bobbing a little bit, but thought ‘She’s really tired,’ . . . so I took her back up with me and literally within five minutes of putting her down she just started screaming.
FB12
. . . she wasn’t herself, she was a lot quieter than she usually was, a lot drowsier.
FB13
He’ll start crying a lot more, he’ll wake himself up . . . He was really unsettled, he wouldn’t sleep . . . [later:] He kept hurling himself over, screaming like he was in pain.
FR16
He would have to nap more often . . . Also he started to get more unsettled at night. He was just more unsettled generally.
FB18
I felt something was wrong, but I couldn’t put my finger on it.
We just said [baby] just needs to be known, that’s all it is, just get known because if you present him in an iller condition, he’s deteriorating, they need a baseline to compare it against.
FR01
It felt like total care to none at all.
FB11
I think that was the problem in the end because I was so focused on the paperwork than actually my baby. I couldn’t see what else was going on with her because I was so worried about every drop of milk.
FB05
That’s the first time I’d seen anything like that, so I wasn’t really aware of what was going on until they said, ‘well okay, this is the kind of stuff you need to look for’ . . . I think once you’ve seen it once you can tell after that.
FR07
When I really feel strongly about something I just have to act on it and I need to take him to see someone and then I can go, ‘Look, I just think that there’s something wrong here. Help me out, because I can’t tell you what it is’.
FR09
They told you what to look out for, his blue lips and his eyes, but I think from a parent point of view, you do not really see it as much as a medic would.
FB11
I think it’s quite hard if it’s a gradual thing. To this day I still don’t really know whether she was more breathless or not, but she was her normal usual self.
FB12
[Our cardiologist] gave him some antibiotics and it cleared up, but he said ‘At what point were you going to go to your GP?’ We said, ‘We see you.’ . . . there’s a threshold – we went the wrong side of it and [the cardiologist] reigned us back in.
FR01
I feel like my community, my borough itself was the biggest let down for me, the hospital being the first one.
FB04
They need to listen, ’cause that nurse that came here, she didn’t listen to me that day . . . and she was saying ‘Wait until the meeting next week’ [review appointment at the specialist centre] . . . They’re drumming it into me ‘make sure this, make sure that’ . . . and then they let her down.
FB05
I have always questioned, ‘Should I contact her?’ . . . ’Maybe I’m just being a little paranoid,’ but who cares? She’s never ever gone, ‘I really wouldn’t worry about it’.
FR09
. . . what gets me is that, I have all the information, as in I know all the symptoms, I know the nitty gritty, and I feel, sometimes, that I’m not listened to very well.
FR15
They had originally told me that being his mum . . . I would know the signs . . . then when I started to notice things . . . and I told them, I just felt like I wasn’t being listened to.
FB18
It didn’t seem like there was any plan in place at all. They didn’t know how to deal with it or what to do . . . They were actually trying to look through [his] red health record book for answers . . .
FR19
If he is sick, I have to decide by 5 o’clock how sick I think he is. Even in [accident and emergency] then, there is nobody around except some overworked registrar who might not know him so well and not be so experienced, so it has got to be done in the day or not at all . . . You have to be pushy, you have to be proactive, otherwise I don’t know what would happen.
FR20
Symptoms
No symptoms
A small number of families reported very mild or no obvious symptoms at all followed by the very sudden collapse and deterioration of their child. Two apparently non-symptomatic children were readmitted as a result of routine saturation and blood pressure monitoring, which identified a blocked shunt in one case and the imminent need for further palliative surgery in another.
Feeding and gastrointestinal symptoms
Many families noted changes in their child’s feeding behaviour, which included a reduction in feeding, becoming more tired during feeds and the presence or an increase in vomiting. For many families these were symptoms that came on gradually and occurred within the context of challenging feeding behaviour characteristic of cardiac babies. In one case a prolonged bout of diarrhoea following routine vaccinations the previous day resulted in the very rapid decline of a previously well child.
Respiratory distress
A variety of terms were used by parents to describe respiratory symptoms in their baby. These included descriptions of breath sounds such as ‘wheezing’, ‘grunting’, ‘straining’ and ‘whistle’; changes in the rate, pattern or work of breathing including ‘breathlessness’; or their child’s appearance such as flaring nostrils and recessing under the ribs, which was sometimes described by parents as an abdominal, rather than a respiratory, symptom.
Appearance
It was common for parents to describe changes in their child’s appearance. This included changes in colour around the lips and extremities, which in the earlier stages may have been transitory, appearing in ‘spells’ or during exertion. Interestingly, there was variation in the ways that parents described the colour of their child: ‘blue’, ‘purple’, ‘pale’, ‘grey’ and ‘yellow’. Some parents reported that the colour changes were so subtle that they either missed them completely or the changes were not apparent to anyone but them. The colour change was sometimes noted in conjunction with cold hands and feet. Some parents also reported that their child had started to become more ‘sweaty’ or ‘clammy’, particularly at night or during a feed.
Behaviour
One of the more difficult areas to quantify was parent reports of behaviour changes in their child, many of which were subtle and not dissimilar to behaviours exhibited by healthy babies. This made these symptoms particularly difficult for parents to evaluate. Sometimes these subtle behavioural changes were the sole indicator that the child was unwell. In the early stages of their child’s deterioration, several parents noticed their child becoming increasingly weak and lethargic and tiring more quickly during exertion or feeds. Some parents noted changes to their child’s sleep pattern, sleeping more during the day and waking more frequently in the night.
Another early sign was their child being generally more ‘moody’, ‘grouchy’, ‘emotional’, ‘agitated’ or ‘unsettled’, and generally ‘not themselves’. Parents described babies that cried more frequently and were more difficult to comfort than usual. The changes they described were not out of keeping with what would be expected for a healthy baby but rather they were unusual for their own child. Several parents found this very difficult to interpret, and described a feeling of knowing something was wrong but being unable to identify what it was. In the late stages, only hours before their child’s collapse, a number of parents reported these behavioural symptoms increasing dramatically into persistent crying followed by high-pitched ‘screaming’ that preceded their child’s rapid deterioration.
Decision-making about symptoms
Although many, but not all, parents recalled being given information about signs and symptoms during their child’s hospital admission this was not always sufficient to enable them to recognise these symptoms out in the community. Even when symptoms were recognised, parents sometimes struggled to describe these and to make decisions about a course of action. This was particularly true if symptoms appeared very subtle or had a gradual onset. One parent also commented that as this was her first child she found it difficult to evaluate symptoms, as she did not know what was ‘normal’ for a healthy baby. Several parents spoke of the burden of completing monitoring forms at home, particularly in relation to feeding, with one parent explicitly stating that she felt she may have missed early warning signs in her child as a result. There was also some difficulty identifying change when the child’s baseline was atypical. A small number of parents said that they did not recognise the symptoms on the first occasion but that once these had been pointed out to them on their own child they found it much easier to identify them on subsequent occasions.
Often decision-making about symptoms took place in the context of local services that were relatively unfamiliar with CHD in comparison with the specialist centres where their child had their surgery and, in some cases this had a detrimental effect on parent’s trust in their local hospital. Despite this, some families still recognised the need for their child to be known to local services.
Experience of seeking help
For symptoms that parents judged to be non-urgent, their first point of contact would typically be the HP with whom they felt they had the best relationship, often the CLN at their specialist centre or the community nurse. However, in some cases parents waited until their next follow-up appointment to discuss their concerns with their local paediatrician or their cardiologist, resulting in a delay in their child receiving treatment. Several parents mentioned fear of appearing ‘silly’ or ‘paranoid’, particularly to more senior HPs, although this could be countered by positive experiences of seeking help and reassurances at an early stage, typically from the liaison nurse, that they should telephone with any concern no matter how small.
Parents reported an overwhelmingly positive experience of the support they received from their CLN with this link being described by one family as a ‘lifeline’. This was particularly true if this was someone they had met during their hospital admission. In some cases the liaison nurse was able to intervene with a family’s local hospital to facilitate more rapid access and treatment in an emergency, discuss treatment plans with local HPs and arrange transport back to the specialist centre when required.
Not all families had a good experience of seeking help when they were concerned about their child, and several families said they felt that their concerns were not taken seriously by their local services. In some cases, parents were falsely reassured about symptoms or told to wait until their next follow-up appointment to discuss it with their cardiologist. Several parents described occasions when they had to be particularly assertive with HPs in order to get the right care for their baby.
In a number of cases parents felt that their local accident and emergency (A&E) staff were unprepared to manage a child with CHD in an emergency, and in some cases there were also problems with A&E staff gaining access to the information they needed to treat the child. Out-of-hours service at A&E was also raised by some parents, with many detailing long wait times and one parent describing having to make decisions about the severity of her child’s symptoms within working hours in order to get the best care.
Discussion
Our study has described the ways in which parents recognise and make decisions about their child’s symptoms at home after congenital heart interventions in the first year of life and details parental accounts of seeking help. Our findings suggest that, although the potential for postdischarge deterioration in these children is well known (see Chapter 2), information given to parents may not always sufficient for them to make decisions about their child’s symptoms in the community.
One difficulty is in the language used to describe symptoms to parents. For example, some classic descriptions of heart failure suggest that parents look out for ‘blue’ skin colour in their child. A number of parents in our study found this vocabulary ambiguous and difficult to interpret with several different descriptions of colour being used by parents to describe cyanosis in their child. Decision-making may be particularly challenging if symptoms appear gradually or if parents have no previous experience of seeing their child unwell. Our study also highlighted behavioural symptoms as being a potentially under-recognised sign of deterioration in these children. Within our sample many parents described subtle changes in their child’s behaviour such as changes in sleep pattern, lethargy, crying and general irritability. These were some of the more difficult symptoms to interpret as they often presented subtly at first and could be difficult to distinguish from behaviour typical of a healthy infant. It is important that parents are encouraged to seek advice at the earliest opportunity and that those HPs at the front line have access to the information they need in order to respond in an appropriate and timely way. We suggest that such subtle signs as a behaviour change should be considered within the wider context of an infant’s medial history, normal or usual clinical state and physical examination at a given time point, since these may represent an early warning of true deterioration.
Although it is important for parents to be trained to recognise symptoms of deterioration in their child it is also important that they are able to summon prompt and appropriate medical care when they have a concern. Several barriers to accessing prompt medical assistance were identified and these included parents’ fear of appearing ‘silly’ or ‘paranoid’, parents feeling that their concerns were not taken seriously, and long wait times and lack of protocols at A&E. Several parents described feeling let down by their local services after flagging symptoms of concern and either being falsely reassured or advised to wait until their next follow-up appointment to discuss their concerns with their cardiologist. Factors that facilitated access to appropriate support included parents having a trusted point of contact with whom they could safely discuss their concerns and having the confidence to assert themselves with HPs when they were not satisfied that their concerns had been addressed. A role for home monitoring (Ghanayem et al. 15) was also noted as potentially useful in identifying high-risk children who appear clinically well. In some cases, apparently asymptomatic children were identified with the aid of home monitoring or measurements taken at routine follow-up appointments suggesting that these more objective forms of surveillance may be effective for identifying children who require intervention but appear clinically well.
Limitations
Our study has a number of limitations. First, parents approached to take part in the study were those known to the specialist nurses who assisted with recruitment. As this meant that parents were approached by someone familiar to them, resulting in a high opt-in rate, it is possible that those families who opted into the study are those who had a better relationship with the specialist nurses at their hospital. An important consideration of qualitative research methods is to describe, rather than quantify, the views held by a population of interest; therefore, it is important to ensure that the study sample represents the diversity present in the population being described. Our study included parents of children with a range of diagnoses, outcomes and discharge pathways. We also attempted to achieve diversity in our sampling of ethnicity and parent educational level, although, as is often typical in UK research, our sample included parents of predominantly white British children. We also struggled to recruit parents’ whose first language was not English, despite offering access to interpretation, and it is likely that these parents face additional challenges not captured by this study.
Conclusion
Many of the complications that can arise following congenital heart surgery may lead to relatively rapid deterioration in a small infant, thus leaving a small window of opportunity within which to intervene. This makes it particularly important for parents to be supported to recognise symptoms in their child and for them to be able to summon help quickly when there is a concern. The family burden of caring for a child with complex health needs is well known,138–144 and as families responding to their infant’s symptoms are likely to be acting under stress, it is important that the information they are provided with is straightforward and that help is readily accessible. Therefore, our study has implications for HPs involved in the discharge and follow-up of babies after congenital heart surgery, in relation to both interpretation of reported symptoms and the processes they follow in response.
Chapter 8 Going home after intervention for congenital heart disease in infancy: qualitative analyses of family and health professional viewpoints
Some of the text of this chapter has been published previously as Tregay et al. , 2016. 145 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Introduction
UK paediatric cardiac services work within ‘congenital heart networks’, partly to improve postdischarge care and outreach services. 17 Some evidence indicates that increased surveillance at home (known as HMPs) may improve outcomes in complex cardiac babies52,53,55,57,58 (see Chapter 3); however, the extent of implementation of HMPs in the UK is unclear and other elements of the patient pathway after discharge may also warrant improvement. We aimed to qualitatively assess the discharge process and subsequent care of infants undergoing major interventions for CHD based on the interview data collected within our wider study from a range of HPs and families.
Methods
Parents whose child had undergone cardiac interventions in infancy in one of three hospitals and who subsequently had an emergency readmission to a PICU or died outside the tertiary centre were interviewed (see Chapter 7 for details of families that were interviewed).
Cardiologists from all paediatric cardiac centres received a mail-shot through the British Congenital Cardiac Association (BCCA), followed by a direct approach. Tertiary nurses were recruited through cardiologists or one another, and community nurses and HVs through their links with a cardiac centre: this ensured that they had experience of providing support for at least one infant following paediatric cardiac surgery. Paediatricians, including paediatricians with expertise in cardiology (PECs), and GPs were approached via a study collaborator. We attempted to gain representation from both rural and urban communities. Interviews with HPs were conducted face to face or by telephone and lasted between 30 and 90 minutes. Topic guides for these interviews included identification of ‘high-risk’ patients; discharge planning; handover between tertiary and non-tertiary services; specialist centre follow-up; maintenance in the community, including details of any HMP; and systems for readmission if a child becomes unwell in the community.
Interviews were tape-recorded, transcribed verbatim and organised using framework analysis,131 a structured approach to managing and reducing bias in qualitative data. Our frameworks used a priori research questions and review of the transcripts to ensure all key themes were captured; each transcript was read by at least three members of the research team. Data from each transcript were then entered into the framework and the completed frameworks used to summarise key themes.
Results
Descriptive information
Information in respect of participating families is reported in Chapter 7.
The health professionals
A total of 36 HPs were interviewed: 25 were from the tertiary centres and 11 were involved in the care of children after discharge from the tertiary centres (Table 22). One cardiologist was interviewed from each tertiary centre (12 surgical and two non-surgical centres).
| HPs interviewed | Number |
|---|---|
| Tertiary centres | |
| Cardiologists | 14 |
| Specialist cardiac nursesa | 10 |
| Allied HPs | 1 |
| Total | 25 |
| Non-tertiary | |
| PECs | 3 |
| General paediatricians | 2 |
| GPs | 2 |
| HVs | 2 |
| Community nurses | 2 |
| Total | 11 |
Qualitative data from interviews
Data are presented below in sections corresponding to the patient journey, with illustrative quotations. Each section first comments on the areas of concern that were raised by respondents, and then highlights aspects of service provision they perceived to be effective. Illustrative quotes for each section are shown in Boxes 17–19.
They’re sometimes given so much stuff that it’s just chucked in a bag and never got out.
Q031; GP
Everything was such a blur really . . . it was all very, very raw and difficult to understand.
FR01; parent
I often have concerns particularly if it’s the woman who doesn’t speak English, about what they are translating because you think ‘I don't think you are getting everything I am saying to you’.
HP017; specialist nurse
I don’t get any communication from [tertiary centre] at all . . . Families get paperwork themselves. I’ll read their paperwork when I go and see them. I don’t get any directed to me.
Q025; HV
It’s not necessarily a ‘one size fits all’ amount of information. That’s possibly where the discharge procedures can fall down . . . I also think information like that is quite difficult to give in one hit – it needs to be given more than once for people to really understand.
Q033; GP
Until you’re actually doing it, it doesn’t really mean anything to you.
FR09; parent
I’m not a medical expert, so I’d like to be told in layman’s terms . . . it’s nice to know the long word, but it’s nice to know what it actually means.
FR07; parent
Some units don’t communicate very well. It may be that they’re not in our network and they function differently.
Q030; general paediatrician
It would be nice if there was consistency, but the bottom line is we’re dealing with 6 or 7 local health boards that will have different priorities and community services may not be a priority for that particular area . . . we have to adapt what we do according to where they are.
F005; cardiologist
They were hoping my local hospital would give me a paediatrician by the time we left, which didn’t happen . . . they tried to get me an A&E passport card . . . but unless you have a paediatrician it’s hard for you to have that.
F04; parent
The patient has gone home and I have no idea who this is. If this patient crashes they will come to my local hospital and we would have no information at all about this patient and we would be managing them.
Q027; PEC
If they give the information to parents, well give it to us as well. Don’t let us go in blind.
Q026; community nurse
There was an element of me having to liaise between the different organisations in order for everybody to have the information, which didn’t strike me as something I should be doing.
FR08; parent
I think life would be easier [if there were] national standards for the monitoring of babies . . . I do think it would be much easier if everybody did the same . . . it would make community nurses lives much easier because some of the community nurses I’ve spoken to they have patients from several different centres . . . it is really confusing for people.
CLNO017; cardiac liaison nurse
. . . I was more focused on the paperwork than my baby. I couldn't see what else was going on because I was so worried about every drop of milk.
FB05: parent
If local services aren’t familiar with CHD then they can miss the opportunity to intervene before that child crashes a day later.
E008; cardiologist
That’s one of the more difficult things to get right I think – who to call and when.
D007; cardiologist
[The CLN] was the main point of contact really . . . she was like a lifeline. I don’t know how we would have been [if they didn’t have a CLN].
FB03; parent
Preparing for discharge
At all specialist centres, parents receive information and training using a range of verbal and written materials, delivered by several types of HP. There was significant variability between centres, but even within centres, content and emphasis could vary from day to day or from patient to patient, and even between children with the same condition. Some specialist centres had developed bespoke leaflets in-house, whereas others used those generated by charities, including the British Heart Foundation or Little Hearts Matter. HPs varied in their views about the quality of information and training given to families, noting difficulties in prioritising the information most crucial to an individual patient.
Families remarked that it could be ‘a lot to take on board all at once’, or ‘overwhelming’. Both parents and professionals felt that information about the signs and symptoms that might warn of a baby’s deterioration might be missing, vague or not documented in writing. Although several HPs had used interpreters, both professionals and families noted the adverse impact of language barriers; interpreters were not always available and information transfer often seemed incomplete. Interestingly, non-tertiary HPs interviewed rarely knew what information or training families were given before discharge.
Service provision perceived to be effective
Health professionals felt that information and training was best given in chunks when families had the time and energy to concentrate, that it should be patient specific and that parents should have a chance to ask questions later. HPs noted that checklists used when a patient was entering a HMP offered advantages of consistency in terms of practice between professionals (see Box 19). Both HPs and families valued hands-on training for parents taking responsibility for care involving medical technologies (sometimes called ‘competencies’). Clear ‘lay terms’ were seen as beneficial by families, as was practical information about ‘what to look out for’.
Discharge process from the specialist centre
It can be difficult for HPs in tertiary centres to identify the correct secondary and primary HPs to contact before a patient goes home; this leads to confusion, missed opportunities for communication and wasted time. Communication works better when clinical networks are well established. Late delivery and variable quality and content of discharge documents from the tertiary centres compounded difficulties. Specialist technical terminology and the absence of key basic information rendered discharge documents less useful to community professionals and indeed families.
Families were aware that crucial opportunities for information handover were sometimes missed and that co-ordination of care after discharge could be unclear and could delay their accessing help when they needed it. Community teams noted their scarcity of resources and how rarely individual HPs see babies with CHD in their practice. This meant HPs valued clear, regular and consistent guidance, which was not always provided.
Service provision perceived to be effective
Some respondents felt that transition was more effective when the network for a specialist centre was clearly defined, and a paediatrician, particularly a PEC, co-ordinated postdischarge care. ‘Network days’ held by the tertiary centre for HPs in their linked network and a policy of early notice for HPs before a complex baby is discharged were advocated by some. Various HPs expressed the view that ‘step-down care’, achieved by discharging potentially precarious babies from the tertiary centre to their local hospital before going home, helped make this transition safer and improved communication. Early contact with their particular GP was valued, even if the child seemed stable. Non-specialist HPs and parents appreciated clear discharge documents written in non-technical language.
Maintenance at home
The default arrangements for follow-up and routine surveillance varied; who next sees the patient and when and where they are reviewed differ enormously between diagnoses and between services. Problems about information sharing undermined the confidence that some families had in local services and presented challenges to HPs responsible for patient care at home.
Professionals varied in their identification of patients as ‘high risk’. This could impact their subsequent management, for example whether or not they were flagged as having ‘open access’ to paediatric wards rather than having to attend A&E departments. Many HPs view babies with palliated or uncorrected heart defects and those with comorbidities or feeding difficulties as ‘high risk’. Family characteristics including poor English, recent migration, cultural pressures and chaotic or difficult economic circumstances could also impair communication or challenge a family’s ability to cope, adding to a child’s risk. Table 23 summarises the responses of interviewees asked about the current provision of HMPs at their centre, which suggest there is considerable variation across the UK. Importantly, HPs and some families expressed concern over the pressure of complying with the requirements of HMPs for multiple measurements and documentation.
| Tertiary centre | Formal HMP? | Eligibility for formal HMP or closer monitoring at home | Routine measurements | Measurements taken by | Breach criteria | Specialist centre monitoring | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Saturations | Weight | Parent | HV | Community nurse | None | Standard | Individualised | Regular calls from CNS | Parents call CNS | High-risk clinic | |||
| Centre A | ✓ | Functionally SV | Daily | ✓ | ✓ | ✓ | ✓ | – | – | ✓ | – | ✓ | – | ✓ |
| Centre B | ✗ | Functionally SV | Weekly | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | – | – | ✓ | – |
| Centre C | ✓ | Functionally SV + systemic-to-pulmonary arterial shunt | Weekly | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | – | – |
| Centre D | ✓ | Functionally SV, but flexible | Weekly | ✓ | – | ✓ | – | – | – | ✓ | – | ✓ | – | ✓ |
| Centre E | ✗ | Functionally SV + systemic-to-pulmonary arterial shunt | Daily | ✓ | – | ✓ | – | – | ✓ | – | – | ✓ | – | – |
| Centre F | ✓ | Functionally SV + systemic-to-pulmonary arterial shunt | Daily | ✓ | ✓ | ✓ | – | – | – | ✓ | – | – | ✓ | – |
| Centre G | ✓ | HLHS only | Weekly | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | ✓ | – |
| Centre H | ✗ | Functionally SV + systemic-to-pulmonary arterial shunt | Weekly | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | – | ✓ | – | – |
| Centre I | ✓ | HLHS only | Daily | ✓ | ✓ | ✓ | – | – | – | ✓ | – | ✓ | – | – |
| Centre J | ✗ | All shunt dependent | Weekly | ✓ | – | – | – | ✓ | ✓ | – | – | ✓ | – | ✓ |
| Centre K | ✓ | Functionally SV + systemic-to-pulmonary arterial shunt | Twice weekly | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | – | – | ✓ | – |
| Centre L | ✓ | Functionally SV | Twice weekly | ✓ | ✓ | – | ✓ | ✓ | – | – | ✓ | ✓ | ✓ | – |
| Centre M | ✓ | HLHS only | 1–2 per week | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | – | – |
| Centre N | ✗ | HLHS only | Weekly | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | – | ✓ | – | – |
| Centre O | ✗ | Follow protocol of surgical centres | Not stated | – | – | – | – | – | – | – | – | ✓ | – | – |
| Centre P | ✗ | Clinical judgement | Weekly | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | – | ✓ | – | – |
When a baby becomes unwell, it may be challenging for the families to adequately articulate their concerns (discussed in more detail elsewhere136). Both families and HPs noted difficulties encountered by non-specialists as they triaged babies who presented to primary or secondary care. Lack of confidence about the need for specialist input meant that action could be either over-zealous (‘calling for everything’) or initiated ‘too late’.
Service provision perceived to be effective
Families valued the role of the CLNs, who act to mitigate some of the difficulties previously described with telephone calls to/from parents and relevant HPs. PECs were reported by some as able to bridge some of the information gaps between tertiary and secondary care; however, this role is not universally available. ‘Open access’ to general paediatric wards for complex babies was viewed as beneficial by both parents and HPs. Standardised follow-up with explicit triggers for action (known as ‘breach criteria’) were viewed by some HPs as helpful.
Non-medical support
Families may face practical difficulties after their baby’s discharge (child care, transport or financial) but, unless there are safeguarding or significant psychological issues, psychosocial support is unlikely to be provided. Parents struggle with the demands of caring for their baby, including maintaining complex regimens of medication or measurements, with some reporting difficulties obtaining repeat prescriptions from their GP. The words ‘fear’ and ‘scary’ recurred when parents and HPs described their emotions dealing with a sick baby in the home.
In practice, opting out of the role of being an ‘expert parent’ may not be an option for parents.
One thing [tertiary centre] said is you will become the expert as a parent. I didn’t believe that at the start but then . . . the moment you start going into the community you realise you’re telling people what to do, rather than them telling you.
FB04; parent
Service provision perceived to be effective
Although not universally available, non-medical support was obtained from individual HPs in primary and secondary care with particularly strong approval expressed in respect of support provided by tertiary CLNs. The potential of the internet as a means of keeping in touch with HPs or accessing specialist charity helplines was noted.
Discussion
This study is a qualitative appraisal of discharge and postdischarge care for babies undergoing intervention for CHD within the UK. The data are informed by professionals from secondary, primary and community care and every UK specialist centre, and by families who had first-hand experience of ‘testing the systems’: all had either lost a baby or their baby had needed emergency readmission to PICU.
Limitations
The study has several limitations. The parents approached were already known to the specialist nurses assisting with recruitment so potentially represent those with a good relationship to the local team. Despite our attempts to achieve ethnic diversity, three-quarters of our sample were ‘white British’ families. Professionals approached were purposively sampled so may not be typical of all clinicians involved in the care of cardiac infants; furthermore, we were limited to one or two professionals per centre. Although we interviewed professionals from secondary and primary care and from rural and urban settings, given the small number of subjects and the large extent of the services they represent, we may not have captured a complete range of views.
Implications of our findings
Our study demonstrates certain ‘system problems’ within the discharge and follow-up pathways for infants going home following cardiac interventions.
Paediatric cardiac network services incorporate multiple team interfaces with corresponding steep knowledge gradients and opportunities for information loss. Infants with CHD may be medically fragile and subject to dangerous deterioration; many non-specialist HPs and parents find this responsibility challenging and extremely stressful. The pressures resulting from the system problems that our study identified fall particularly on parents, CLNs and PECs.
Our study suggests that implementation of HMP (see Chapter 3) in the UK is variable and the complex regimens of feeding, medications, weights and saturation monitoring with ‘breach criteria’ place considerable burdens on those responsible for them.
In practice, infants with heart disease who become acutely unwell at home are likely to present to a GP, to a local hospital via A&E or through ‘open access’ to the paediatric department. Our study identified problems with correct identification of the deteriorating child, and difficulties determining what appropriate steps to take from the perspective of both primary and secondary health-care professionals. Within this context, interview participants reported the potential benefit to both parents and local medical personnel of local ‘step-down’ care before an infant’s final discharge home after cardiac surgery, such that the child is known when in a stable condition.
Our findings also highlighted the effective role that named and informed PECs with responsibility for local children with heart disease could play in strengthening networks. PECs are well placed to recognise postpericardiotomy syndrome or discriminate a respiratory infection from decompensated ventricular function, perhaps with support from a regional cardiologist. PECs are also well placed to address the less acute but nevertheless important aspects of managing the postoperative infant, particularly other comorbidities and dealing with feeding difficulties, reflux or lung disease in context.
Paediatricians with expertise in cardiology, however, may not be the first point of contact for the deteriorating infant with CHD, and there may be potential for learning from related secondary care examples, such as scoring tools that have been deployed in A&E and utilised by non-specialist HPs to detect signs of deterioration in presenting children. 146
Finally, at present the focus of national and international audit remains on 30-day mortality rates for paediatric cardiac surgery68,69,147 and we note that additional audit metrics focusing on the postdischarge stage of the patient journey may be a useful lever for quality improvement in the future.
Chapter 9 Congenital heart charity helpline staff viewpoints: a qualitative study
Introduction
Charities have a key role to play in providing support, information and practical assistance to patients with health conditions. Many charities run a telephone helpline, usually staffed by volunteers, and there is evidence (predominantly in the field of cancer) that patients and their carers/families rate these helplines positively in terms of information provision and reducing anxiety. 148 Furthermore, it has been suggested that helplines fulfil a different function to websites, with website users less likely to request information on sensitive topics but more likely to request factual information than helpline users. 149 Helplines offer callers anonymity, convenience, time to discuss their concerns and are also an outlet for other people indirectly affected by a condition, such as family members and friends. 150 However, previous research into the role of helplines in health care, including those staffed by HPs, has focused on the user perspective, involving interviews or surveys with callers rather than with the helpline staff. 151,152
The CHF is a parent-led national charity that acts as the umbrella organisation for more than 20 other organisations and charities providing support to children with heart conditions and their families in the UK. The CHF has links to more than 12,000 families of children with CHD and provides support via its website, e-mail and a telephone helpline. Three of the charities affiliated to the CHF are Little Hearts Matter, which specifically supports children with SV conditions and their families; the Down’s Heart Group, which offers support and information relating to heart conditions associated with Down syndrome; and Max Appeal!, which supports families affected by DiGeorge syndrome/VCFS/22q11.2 deletion (which may be associated with a congenital heart lesion).
In view of the unique perspective that helpline staff have of the issues faced by families of children with CHD, we wanted to interview helpline staff from a number of charities to understand their experiences of talking to families whose baby had recently been discharged after infant cardiac surgery. We also wanted to elicit their perceptions of the key reasons/issues/concerns that families called the helpline to discuss in relation to their baby’s cardiac surgery.
Our aims were to:
-
access information about parents’ experiences with services/trying to access support (as reported to helpline staff)
-
elicit helpline staff’s perceptions about the key issues that parents call the helpline to discuss.
Methods
The CHF and three other UK charities that support families of children with CHD were approached about participation. Each charity was provided with written information about the project and asked to provide contact details for any helpline staff willing to participate in a short interview. All participants were assured of anonymity in the reporting of anything they told us and it was made explicit that we were not asking them about their responses to any issues raised by families but rather were interested in what the families told them.
All interviews were semistructured and followed a topic guide. Interviews focused on the following areas:
-
families’ concerns when the child is initially discharged
-
the information families are given about their child’s condition, what to look for and who to contact if they are worried
-
support that families access in the community
-
barriers to families getting the support they need.
Participants were asked to provide either written or verbal consent concerning their participation in the study. All interviews were tape-recorded (with participants’ agreement) and transcribed verbatim (with all identifying information removed). Framework analysis was used to process the transcripts.
Results
Key findings from the helpline interviews
The core theme identified from the helpline interviews was communication and knowledge (Figure 9), which was described in terms of patient and family factors, sources of support and systems. Quotes for each theme are presented in Boxes 20–29.
FIGURE 9.
Themes from the helpline interviews.
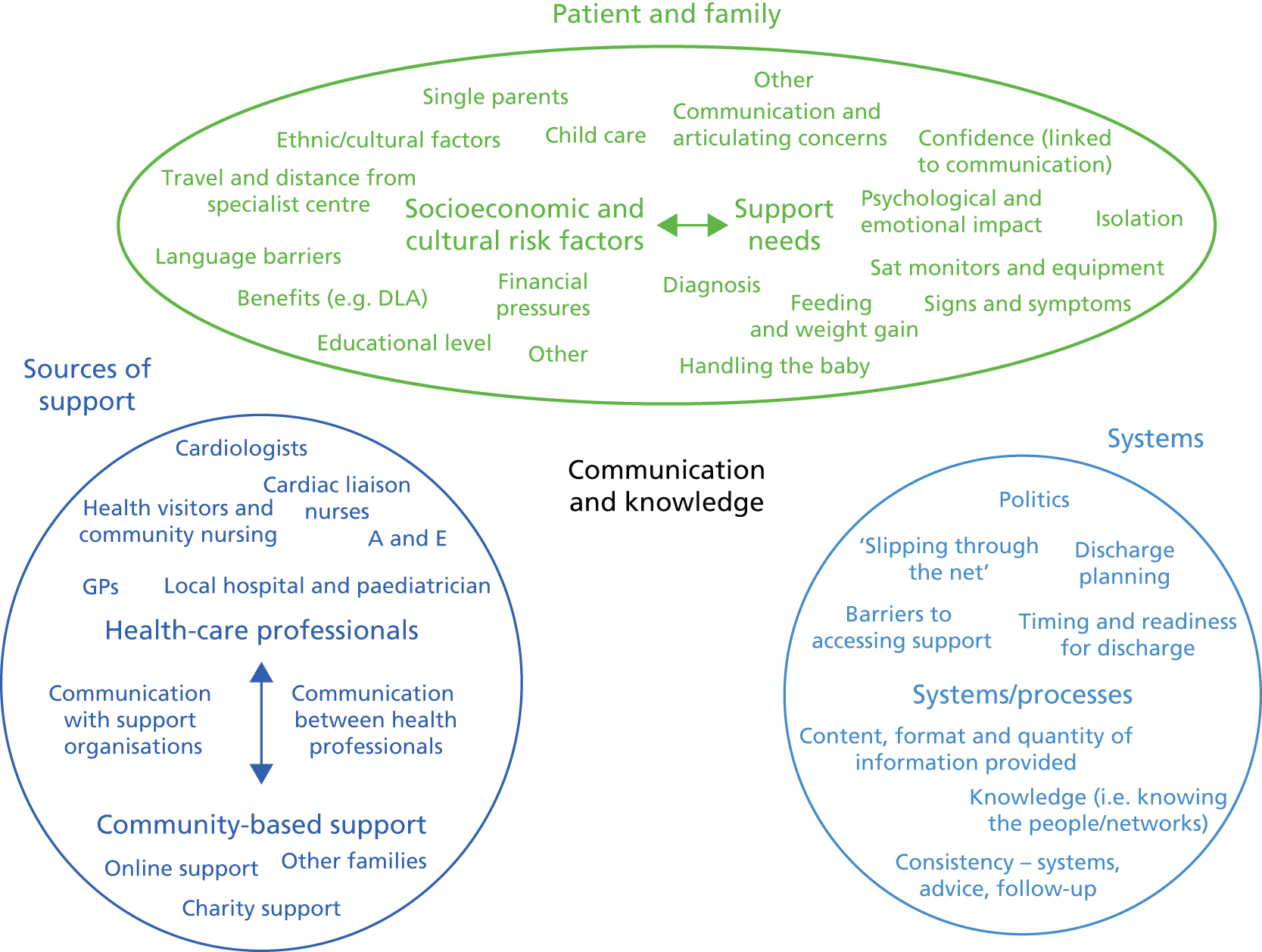
Discharge information needs to be given in the language that they understand otherwise they’re never going to get it are they?
06
They get there where they’re 100 miles from home. What do they do? . . . But I think it’s assumed that once you’re on your way home, then you’re on your way home and that’s your problem.
07
They couldn’t afford to get from A to B and going back for their follow-up appointments. Subsequently they weren’t able to make the appointments . . . It does tend to be the less educated end that manages it worse because they don’t think ahead, generally, to write things down or write questions down in advance or write what they have been told. Because people don’t absorb information straight away.
02
And the ethnic communities like that can be quite difficult – they are really hard to reach. There are a lot of pressures . . . we have a mum who had a child with a very complex heart defect who for a number of years . . . her family . . . really almost banned her from taking any outside help. And it’s the cultural barriers that’s probably the greater issue (rather than language barriers).
07
You get people from minority backgrounds who will I guess in their communities will hold back from accessing support so perhaps wouldn’t necessarily phone a charity, wouldn’t go to find a support group. They wouldn’t feel that that is acceptable in their own community. We have people we are in contact with who have a child with an illness and they are disowned from their community and find it very difficult to know where to go. It all comes down to when they are leaving the hospital, if they aren’t given the information then they may never find it . . . so yes, there is definitely a big gap depending on their background.
01
We do get calls, especially single families, who have other siblings, who are very worried about how to cope with child care arrangements and financially when they are taking time off work to care for their child, especially if they are in more isolated communities and don’t necessarily have any kind of support.
03
We have them phoning up about feeding – feeding is a VERY big issue. It is incredibly stressful and families feel very responsible.
06
We have in the past supplied pulse oximeters because bizarrely the hospitals like the families to look at the child and see how the child is looking. So ‘look at your baby and learn to know your baby, are they blue around the lips are they sweaty on the head, are they breathless, is their pulse racing?’ Whereas if you’re a qualified nurse or a consultant cardiologist then you use a pulse oximeter, but parents are encouraged not to do so – ironic isn’t it? But the parents are supposed to look at their baby and know them from their colour and behaviour.
07
We also get a lot of calls from people who have had recent discharges and they are very worried about even the slightest thing so they are not sure– I don’t want to say they are paranoid because that sounds negative . . . Totally understandably but they are like ‘Like oh my god I don’t know if my child’s lips are blue. How blue is blue. I don’t know what you are talking about’.
03
A lot of them are concerned about symptoms to look out for. So we get lots of calls from specifically mums or dads who’ve got home from the hospital who have been given lots of information but feel very lost so their questions is usually ‘what should I do if’, ‘What should I look out for’.
04
Things that parents sometimes say ‘I don’t know if this is normal or not’ something I hear a lot ‘Is this normal?’ and of course you can’t answer that. You don’t want to say ‘oh that’s fine’ because something might happen to them and you don’t want to panic them and say ‘Go to A&E’. When I try and say take them to A&E you have to say it so calmly. There are certain times that you think why are the parents phoning a charity when they should be phoning a medical professional. That’s what worries me. Why are they phoning us they should be phoning . . . or taking their child to a doctor?
01
They’ll go home with all the information about the medication for their children and they know exactly when they should be giving them and how much but they don’t know why. They don’t understand that if a child vomits what their next action should be regarding the medication they’ve given. They don’t understand the signs and symptoms they need to be looking for so that they can trigger a concern with someone else – they don’t understand about the respirations, they don’t understand about the colour, the way the baby feeds, because we all know that feeding for cardiac babies gives you a whole history.
06
It’s when people don’t have the confidence to communicate what they need and they end up getting intimidated or think ‘oh I must be wrong’. That’s the single most vulnerable thing and I think that can happen to anybody.
03
I think it depends a lot on how they are coping themselves, what family support network they have because obviously if you have got a good supportive family that are all rallying around . . . you know if you are a single family with no support then you have complete isolation.
08
I think [they need] the acceptance that it’s ok for them to get support as well, so breaking that barrier . . . For me the hardest helpline calls to answer are the ones where the Dad is in absolute bits saying ‘I need to fix this – my son’s got this condition and we are out of hospital but my wife’s had to leave work and she is in a real emotional state and I’ve got to fix this because I’m the dad’ . . . But I think something around making it ok or making it the normal for people to ask for help. This is the helpline number whenever you have problems, contact the [organisation] . . .
04
They [parents] are very overly aware, highly vigilant and very anxious . . . I think what people really want when they phone us up is support, they want help, they want a face, they want somebody who can tell them it’s going to be ok or can tell them this is what is going to happen, this is what you can expect, or even just somebody to say ‘I understand, I will listen to you’ or ‘I don’t understand, but tell me.’ They just want someone to talk to.
03
The ones that had clear access to a CLN or something similar seemed to do best because at least they could go back and ask.
02
What a lot of parents find very useful is where . . . the CLN has been able to talk and support the parent in the handover to the community team – and some are better at that than others.
05
What I’m always shocked at is that some of them aren’t even aware they have the CLN as that point of contact . . . When I contact the nurses on behalf of the family they’re amazing – they give a lot of their time, they will speak for a long, long time and give me a very detailed response, but I just think that families aren’t aware of them being there.
01
You might get luck and have a GP that is tuned in, switched on, proactive, or you might get one that isn’t really bothered. Sometimes you’re at the mercy of a belligerent receptionist . . . It would be really nice if you could have your GP out without having to go through 2 hours of NHS phone triage and take them out to somebody else in a community centre 5 miles up the road. So lack of personalised GP support is so poor.
07
They [HV] ask the parent to check this and the parent comes to us and that really diminishes the trust and faith that the parent has in the people that are supposed to be caring for their child.
03
There is the occasional fantastic HV and the occasional fantastic GP and it would be terrible to tar them all with the same brush. But in most cases they are out of their league with knowledge base. They don’t understand.
06
If they’ve [the family] been granted open access to the ward then that makes them feel so secure because they’re not required to go through A&E. They can just pop up onto the ward.
07
We work as hard as we can to make links really firm with centres because that really helps us. A lot of times we might ring cardiac liaison nurses or doctors ourselves and ask them about helpline calls if we had a particularly difficult one come in and we feel the parents won’t make that contact themselves. It will depend on the unit, how easy that is. Some of the communication of some units is absolutely amazing and we [are] very confident referring families to those units and other times the after care of some units are very questionable.
04
We’re often giving people advice on how to speak to their cardiologists because more often than not, and I completely understand, you go in to speak to someone and just sit there and get completely overwhelmed by what you’re listening to and not understanding what they’re saying and then leave with more questions . . . So what we try and do is prepare people before they see the cardiologists – try and get them to write down all the questions they’re asking us, try and get them to write down all of those questions because again we can’t answer them.
01
Quite a bit of my time on the phone is spent helping people to see that to get what they want they are going to have to stamp their feet and make a nuisance of themselves because it won’t be offered up on a plate.
02
They need to feel that confidence that the person that they are ringing won’t go oh for goodness sake how stupid are you. Sometimes . . . the staff . . . can be a little bit condescending and expect that the family really ought to know this now and the attitude is a little bit like what on earth are you asking me this for again.
08
When I take those types of calls I view that as a way of giving parents . . . the permission or confidence if you like to go back and ask questions . . . They’re not stupid questions and how about raising it like this, this and this.
05
And also somebody communicating with their community team because if you don’t build that then the community team don’t know what they’re doing and either avoid the family like the plague or they give them all the wrong information.
06
What I would really like is if there was a much closer link between the clinical team . . . with a parent support group and there is almost an automatic referral.
05
It’s that hand-over. It may well be that the community team themselves, they may not see many children with . . . any of these conditions. So it’s outside of their experience and then you’ve got a worried mother that’s when it can become quite difficult.
04
So to meet another Mum, to be given those opportunities, to be one on one or in a group. I think it’s just invaluable. Just talking it through with someone.
01
They wanted to chat to families that would get the worries that they were going through and maybe see people that had already been through that and were already on to the next stage and how you can come through that and any tips they had to help each other.
10
I think a lot of the parents are quite lonely . . . So they feel quite alone and that’s where social media has come in because you often see things posted on Facebook and so on . . . you see questions and that sort of thing so they get support that way. They can build up friendships. Sometimes you are a bit worried about the information that is given and we try to step in. So we try to monitor that.
05
I don’t know how that information is given in all centres. So that might vary. I think it’s been varied . . . some people come out feeling quite informed and others don’t . . .
09
Inconsistent provisions . . . some areas are better than others and have support while other don’t.
02
I think the quality of the CLN team and the quality of their relationships with external care providers . . . that really fuels whether somebody will receive that support.
03
We get some patients who go into their GPs a couple of weeks later and the GP hasn’t even got a letter so they don’t even know what surgery the child has had. I think that can just send parents into a real panic mode because they feel like there should be joined-up information.
04
If they are seen at a local centre not at a specialist unit then you might have someone who doesn’t specialise in cardiology. So again, they might not understand the full extent. So that’s the kind of barriers I see.
01
[We need to] get a point of contact. Someone who can guide them at least – whether they stick with the CLN or somebody that can guide them through the right paths to get to the point where they need to be. Possibly on discharge some mechanism for connecting people to more local support and indeed local after care services and local hospital and whether their care is provided because that seems to be a gap.
. . . the last call I had from a family couldn’t be worse. Because they were suddenly discharged. They didn’t think they were going for another 3 days, and they suddenly went and they didn’t have any mechanisms in place at all at that point in time. There’s a newborn baby . . . and they had to travel so they weren’t where their family were. So I would suggest that that one failed miserably . . . and they were really upset, I mean REALLY upset. Nobody knew that they were going home, they didn’t know they were going home and it was all rushed and they were frightened.
06
We have the horror stories of children who have just had open heart surgery being sent home on the tube and getting home and the parents feeling completely let down because the treatment and the care have been amazing and then it’s almost like ‘ok you’re done, goodbye.
04
. . . when people make the effort to put in phone calls to patients periodically, keep that communication going which happens in some centres. I think that’s really important, that the whole of the community involved with that child are on board with the support and the advice and the care . . . in vulnerable situations that relinquishes some of the pressure on the parent and I think that opens up the opportunities for the vulnerable person to access support for themselves as well as for their child . . . I think when people have got it right is when people actively, really actively, follow-up ‘do not attends’ so when people haven’t gone to their appointments for various reasons . . . we do often get calls from people saying ‘my appointment is in three days and I haven’t got any money to get the train’ . . . If somebody phoned the family before (follow-up appointment) saying ‘How are you going to get there? What’s your plan? . . . Have you thoughts about childcare?’ I think there should be really consistent questions which are being asked everywhere . . . We have heard of people being discharged from hospital . . . but then they are bunged on the tube or they are doing a two and a half hour journey home on the train . . . Consideration for that family, if you want the family to remain engaged with the services in accessing the right support. It has to start from day dot [because] if they feel abandoned, kicked out of hospital . . .
03
Non-medical support
As well as discussing support provided by HPs and the patterns of communication between professionals and charities in different health-care sectors, the importance of other heart families, patient support groups and social media was identified by helpline staff.
Patient and family factors
Socioeconomic and cultural risk factors
Helpline staff discussed knowledge and communication in relation to characteristics of the patient and family, in terms of socioeconomic and cultural risk factors such as language barriers, ethnicity, financial difficulties and the problems associated with being so far from the tertiary centre.
Practical support
Staff described calls they received requesting direct support or advice about accessing support in relation to practical topics such as feeding and the requirement for specific equipment. The CHF provides some funding for small grants to purchase particular pieces of equipment such as INR machines, and some of the participants talked about receiving a lot of requests for financial help, both from families and from nurses on behalf of families. In relation to support with equipment, one helpline staff member specifically talked about the advice that some parents were given about looking out for signs and symptoms in their baby but not necessarily knowing how to do so.
Signs and symptoms
Parents frequently used the helpline as a means of accessing support about specific signs and symptoms they were concerned about, particularly soon after hospital discharge. Furthermore, some of the participants mentioned calls from parents unsure about what to look for which were not in response to a particular situation but were reflecting their more general anxiety about caring for their baby after being discharged from hospital. Helpline staff also talked about the difficulty they have when parents call seeking medical advice when they are not in a position to provide that advice and their own anxieties around that. They described the tension they experience between wanting to reassure parents but at the same time not giving them false assurances. Some helpline staff also expressed their concerns about parents’ lack of knowledge about signs and symptoms and what to look for.
Emotional support
As well as receiving calls about particular queries and concerns, helpline staff discussed calls that they received that were about the support needs of the family related to more general issues such as confidence and isolation. A number of participants also talked about the psychological and emotional impact of CHD and subsequent treatment on parents of affected children and the need for parents to feel able to access support for themselves.
Sources of support
Participants discussed the individual sources of support that families access or call about, such as HPs in primary, secondary and tertiary care as well as non-professional support such as social media, other families and charities. Most of the participants talked specifically about the role of the CLN, with both good and poor examples of how the role worked in practice.
Staff also commented on the varying levels of knowledge of, and support provided by, HPs in the community and at the local hospital.
Communication
As well as describing sources of support, helpline staff also talked about how links were made between professionals and families. For example, one participant described their own role, as one of the charity helpline staff, in developing links with centres, sometimes with a view to giving more specific information to families (see Box 26, participant 4a). Others talked about the advice they provide to parents to enable them to talk to HPs, recognising that as helpline staff they are not able to provide the answers to parents’ medical questions but that they can have a role in facilitating communication. The level of confidence of parents was also mentioned by some of the participants in relation to how parents interacted with HPs and the role of the helpline staff in empowering parents. Finally, helpline staff described the importance of the communication between the different sources of support, such as between HPs and charities or between community and hospital professionals, and how they thought this could work more effectively.
Systems and processes
The third component of knowledge and communication focused on the systems and processes, in terms of the content, format and quantity of information provided, knowing the network and people who should be providing support to parents, together with barriers to obtaining support and the consequences of that. The overarching theme was consistency and continuity – or, conversely, the individual approaches in each centre, each community and of each professional, with the result that helpline staff perceived families to have very different experiences of ‘the system’ and how it works. This lack of consistency was perceived to extend across the network, from the tertiary centres and into primary and secondary care, and staff identified the need for a more joined-up and co-ordinated approach to care for individual families.
Participants also talked about their perceptions of what happens to families at discharge, in terms of the planning that happens prior to discharge, the practicalities at the time of discharge and communication with local HPs. In particular, staff described the lack of consistency and the impact on families of a poorly planned discharge. A number of staff identified processes that they thought worked and did not work for families once they were back home, which were primarily focused on the processes of communication and keeping the family engaged with services.
Discussion
Helpline staff provided a unique perspective on the issues faced by parents taking children home after cardiac surgery and identified a number of examples of when things work and do not work for families. Knowledge and communication are clearly key elements for the families and those working with them, and the insights of the helpline staff provide evidence to inform the development of more structured interventions to optimise outcomes for children and their parents.
Although the core theme from the data was knowledge and communication, the overarching factor described throughout all of the interviews was a lack of consistency in the way that families are supported after discharge from hospital, both in terms of their information and psychosocial needs. Families themselves clearly have different needs but the ways in which support was provided and their needs addressed varied enormously, despite many similarities in the patient journey for infants undergoing cardiac surgery. This variability in response was perceived to exist in all areas of care, in terms of both the place of care (tertiary, secondary or primary) and the professionals providing it (with respect to both individuals and generic professional groups). Helpline staff articulated many examples of good communication and provision of knowledge by HPs but also identified many examples where families had been failed or support had been less than optimal, however unintentional that might have been.
Limitations and strengths of the study
There are some limitations to this part of the overall study. First, although CHF and the three affiliated charities providing support to families were involved, the overall number of participants was small. The three organisations provide support in different ways and their access to specialised medical knowledge varies. Participants from three of the groups provide support to specific patient groups, whereas the remainder are there for a generic heart population covering the whole CHD spectrum. Participants could also comment only on the support and information needs of families who contacted the helpline, which is likely to result in the needs of some parents not being represented, such as those of parents from non-English-speaking populations or parents who were less able, for financial, social or other reasons, to contact the helpline. A further limitation was that not every participant had experience in each of the areas covered by the interview; for example, one helpline staff member had not taken any calls in relation to discharge after surgery. Finally, parents invariably call a helpline because they have a problem so the views expressed by the helpline staff are inevitably skewed towards the things that do not work or have gone wrong for families, rather than reflecting a more balanced view.
Despite these limitations, there are a number of unique elements with the approach adopted in this part of the study. The helpline staff are part of national charities and are therefore in contact with families from all over the UK, rather than only being exposed to families from a limited number of specialist centres. They do not have affiliations with any individual centre and, although individual helpline staff will inevitably hold opinions about aspects of care in specific centres, they were providing us with a national overview. Furthermore, charities do not provide clinical care and in most cases helpline staff are not medically trained, thus reducing the likelihood of medical or clinical bias influencing their responses.
Previous research into the role of helplines in health care has focused on the user perspective, involving interviews or surveys with callers rather than with the helpline staff. 151,152 We focused instead on accessing information about families’ needs and their experiences of using services by tapping into the staff’s body of experience. In common with studies of cancer patients’ use of helplines,150 interviews with helpline staff in our study indicated that parents’ reasons for calling charity helplines were multifaceted, with their psychosocial needs being intertwined with their needs for information and advice. Thus helplines clearly have a unique and important role in the support of families of children with CHD. Furthermore, the views of the helpline staff we interviewed provided important and previously uncaptured evidence to help inform and develop interventions to better support children and families after hospital discharge following infant cardiac surgery.
Chapter 10 Intervention development: suggestions for health care, proposed metrics for future monitoring and recommendations for future research directions
Introduction
The overarching aim for this study was:
To use a mixed methods approach including quantitative analyses of national audit data and qualitative approaches to gather information from key individuals, in order to establish an evidence based and realistic guideline for community based surveillance of fragile infants with congenital heart disease. 153
With the specific study objective:
To combine the data and information acquired to generate the evidence based protocol or guideline for surveillance of infants with CHD, including the ‘who’, ‘when’ and ‘how’ this should best be delivered. The ultimate objective is to produce a workable and effective follow-up surveillance protocol for infants discharged into the community after cardiac surgery, with appropriate targeting of higher risk patients and consideration of measures that will be acceptable and useful to parents and community based health care professionals. Intervention development will include consideration of measures of success. 153
This chapter discusses the development of our suggestions for service improvement in respect of the postdischarge management of fragile infants with CHD, which represent the main deliverable of the project, fulfilling the overarching study aim and objective. The evidence that was collected and analysed in each phase of the study over the course of 2 years, including the two systematic reviews of the literature (objective 1), the national audit data that generated a risk model for our outcomes of interest and CART risk groups (objective 2), and our interviews and other qualitative explorations (objective 3), were presented and discussed in an iterative fashion to a multidisciplinary group, over the course of the entire study. Each of these elements of research evidence informed and fed into subsequent stages of work, ultimately generating our final suggestions for service improvement (objective 4) and informing the implications for future practice and research that are discussed in this chapter.
Methods
To the end of fulfilling the above stated aim and objective, an expert advisory group to IHS was established to review the emerging evidence generated from the systematic reviews, quantitative analyses and qualitative analyses relating to the outcomes and discharge/postdischarge management of neonates and infants undergoing intervention for CHD and propose candidate suggestions for improving services based on that evidence. This multidisciplinary group comprised professionals (with a diverse skill set) from the three tertiary cardiac centres involved in the study (K Brown, K Bull, D Barron, R Franklin and P Daubeney), representatives from primary (S Hull) and secondary care (N Barnes), patient group representatives (R Simbodyal) and academics from the disciplines of psychology (J Tregay and J Wray), statistics (D Ridout), epidemiology (R Knowles) and operational research (S Crowe).
The group met on five occasions (each lasting 2–3 hours) between March 2013 and June 2014 to consider:
-
the results of mixed-methods research regarding UK service provision and outcomes specifically in this patient population as discussed in Chapters 2 and 3 (systematic reviews), Chapters 4 and 5 (quantitative analyses of national audit data) and Chapters 6–9 (qualitative analyses of OF data, as well as family, professional and charity helpline views)
-
quality improvement initiatives in related areas such as the discharge of premature and high-risk infants.
In July 2014, these candidate suggestions and a summary of the evidence were shared at a facilitated workshop with parents of infants who had undergone surgery for CHD and subsequently either died after discharge or required emergency readmission to intensive care (10 parents representing five babies). Their views on the acceptability of the candidate suggestions and additional ones were captured (notes from the meeting are included in full in Appendix 9).
This phase of the project drew upon the skills and input of an operational researcher trained in quality improvement methods (S Crowe) who was funded by the Health Foundation and joined the study team with the express purpose of bringing operational research expertise to bear upon this phase of the study.
Establishing the final set of suggestions for health care
A working group comprising selected members of the expert advisory group and invited additional representatives from the community and charitable sector was convened to assess the draft suggestions for health care and propose a final set for endorsement by the IHS expert advisory group (see Appendix 10 for the working group’s terms of reference and list of members). A facilitated all-day workshop was held in September 2014 (tape-recorded and with live minutes), in which the group was tasked with:
-
reviewing the draft suggestions for health care: assessing the feasibility and acceptability of each for service improvement
-
assessing the set of draft suggestions for health care as a whole within the context of patient risk groups and targeting these (setting priorities)
-
agreeing a final set of suggestions for health-care improvement to circulate among the IHS expert advisory group for comments and endorsement.
On the second point, the working group explicitly considered both the size and nature of the risk associated with each of the patient groups identified in our analysis of national audit data reported in Chapter 4 (summarised in Table 24). The groups are characterised by patient risk factors and stratified by occurrence of outcome 2 (death or emergency readmission to paediatric intensive care within the first year post discharge from infant cardiac surgery). Any decision about the types of interventions supported for different patient groups will have implications for resource use. Given the limited resources available for providing these services, the working group therefore agreed that it was important to consider the relative size of the patient groups alongside their relative risks and the nature of those risks when thinking about how limited resources might be targeted most effectively.
| Patient group | Group characteristics | Occurrence of adverse events (%)a | % overall patient population | % overall adverse eventsa |
|---|---|---|---|---|
| 1 | Neurodevelopmental condition(s). May also have: congenital anomalies (52%); HLHS, functionally UVH or PA + IVS (17%); LOS > 1 month (26%) | 24 | 4 | 15 |
| 2 | No neurodevelopmental conditions; VSD/other; congenital anomalies; LOS > 1 month | 24 | 2 | 8 |
| 3 | No neurodevelopmental conditions; HLHS, functionally UVH or PA. May also have: congenital anomalies (10%); LOS > 1 month (20%) | 15 | 11 | 26 |
| 4 | No neurodevelopmental conditions; VSD/other; no congenital anomalies; LOS > 1 month | 9 | 4 | 6 |
| 5 | No neurodevelopmental conditions; VSD/other; congenital anomalies; LOS < 1 month | 8 | 16 | 20 |
| 6 | No neurodevelopmental conditions; VSD/other; no congenital anomalies; LOS < 1 month | 3 | 63 | 26 |
Results
The proposals for service improvement that represent the main output of the project are set out in Table 25. The table is structured to show the aspects of the service that might benefit from improvement alongside the source of the evidence on which each individual statement is based, as collected over the various phases of the study in the left-hand column. The proposals put forward by the intervention development group, which are linked to each individual area for improvement, are listed in the right-hand column. The elements of the patient journey that are covered within Table 25 are training and information for families predischarge, discharge and transferring to non-specialist services, medical follow-up services, non-medical support, provision of patient information, accessing support when a baby is sick and knowledge gaps, weak links and poor communication between HPs.
| Identified service challenges and barriers to support | Suggestions and comments from the IHS |
|---|---|
| 1. Training and information for families predischarge | |
| Information overload: lots of information, some of which families find difficult to understand and absorb [FI, OF] Poor timing: information is often rushed before discharge [FI, HPI] Insufficient training on ‘signs, symptoms, responses’: often missed, vague or unstructured, and no written material to take away [FI, HPI] Barriers for non-English speakers: limited funding and/or access to interpreters and most resources only available in English [HPI] Some families miss out: ‘hit and miss’ which families are offered what information and training, depending on the HP at bedside and resource shortages [HPI] |
1.1 For all patients, training and information should start as early as possible, (antenatal if this is applicable) repeating as necessary and checking that families have taken it on board 1.2 For all patients, information should be targeted towards the individual child, for example through hands-on demonstrations with their baby and involvement in the baby’s care while in hospital. Nationally standardised generic information should also be provided, for example in written form and as a web-based resource 1.3 For all patients, training and information should be provided as far as possible in the format most helpful for the family and should therefore be available in a range of formats (e.g. verbal, written, visual and digital). Video and other visual information was considered easier to understand, particularly for non-English speakers. For example, mobile phone videos recorded at discharge of the ‘normal’ status of the child could be used by families as a comparison when child is unwell 1.4 For all patients, HPs should use a nationally standardised checklist in order to plan, deliver and audit the provision of training and information for families prior to discharge. The IHS will propose a checklist (based on the evidence gathered in the IHS), which they recommend for piloting and evaluation 1.5 The IHS notes that the following patient/family groups would benefit from more frequent/intensive provision of suggestions 1.1–1.4:
1.7 For non-English speakers, the IHS recommends interpreters and translations of written material where this is feasible and appropriate for the family but notes that where this is not possible visual information (e.g. videos of their own child; see suggestion 3) should be used |
| 2. Discharge and transferring to non-specialist services | |
| Poor access to local support services: it is difficult for specialist centres to know which local and community services are available and how to contact them (e.g. named individuals rather than teams), particularly when links are not well established. Community teams are often short of resources and it’s harder to get support for social (rather than medical) issues [HPI] Inadequate planning: can be ad hoc and strongly influenced by the availability and accessibility of local resources, leading to variation across the country in terms of who is offered what follow-up care. Contact with local services is often made on the day of discharge, at which point it is difficult to organise appropriate, timely support [HPI] Poor-quality discharge letters/summaries: letters are often very delayed, do not go to all HPs, contain too much specialist information and terminology and often do not include basic information (e.g. wound care, immunisations), what training families have received, details on what needs to be monitored and any associated breach criteria, what to look out for and how to respond [HPI] Ad-hoc planning for high-risk patients: there is often no protocol in place for identifying high-risk babies and the (extra) care that is offered to them – large variability across the country [HPI] |
2.1 At discharge from the specialist centre, all patients should have a named cardiologist, named paediatrician (with expertise in cardiology when possible) and named specialist nurse (e.g. cardiac liaison role or equivalent). When it is not possible to allocate a named specialist nurse, there should be a named specialist nursing team. Responsibility for ensuring this lies with the specialist centre 2.2 At discharge home, either the specialist centre or the local hospital if step-down, all patients should also have a named GP and a named pharmacy (if discharged with a long-term prescription) 2.3 For all patients, responsibility for care co-ordination should be transferred to the named paediatrician at discharge from the specialist centre. The named paediatrician and GP are responsible for referring to local services and maintaining effective communication between HPs 2.4 A multidisciplinary team should be established as early as possible (2–3 days prior to discharge or earlier) for the following groups:
2.5 All patients should have a nationally standardised structured discharge document that is distributed electronically to all relevant HPs. The IHS will propose the minimum content for this discharge document (based on the evidence gathered in the IHS), which they recommend should be piloted and evaluated 2.6 For all patients, the structured discharge document should be used for:
2.8 Patient groups 1–4 should receive ‘step-down’ care, that is discharge via their local hospital. Ideally this should be as an in-patient (even if just for 24 hours). If this is infeasible because of the lack of bed space they should be admitted as a day case. At a minimum (given resource constraints) they should be seen as an outpatient as soon as possible (e.g. within 48 hours) |
| 3. Medical follow-up services | |
| Problems with clinics: clinics are often full and running late. Specialist centre outpatient clinics can be difficult for families to get to and are not always attended by specialist nurses. Not all outreach clinics are jointly run with paediatricians or attended by specialist nurses and, in general, there is no multiprofessional follow-up [FI, HPI] Inconsistent specialist support between clinics: many families (particularly ‘high risk’) get regular calls from CLNs/CNSs, but some do not and can find it hard to get in touch with them. Families often do not speak with the same nurse each time [FI, HPI] Variability and resource challenges: the use of local services is not standardised, the support available varies across the country and there are often insufficient resources. In particular, there are not enough PEC (or out-of hours/annual leave cover) and often newly trained or less-experienced community nurses/HVs attend visits (sometimes from a pooled resource so there is a lack consistency of care). Babies must have a medical need to get a community nurse but it can be difficult to maintain regular home visits from HV, as the baby may not be considered high priority (i.e. child protection) [HPI] No protocol for home monitoring: large variation between centres in the provision of HMPs (which babies and what it consists of) and generally no clear protocol. Often a lack of clarity among community nurses/HVs/families as to what to do with the information monitored. Some families find it helpful, others a distraction or too complicated [FI, HPI] Feeding/weight gain: very stressful aspect of care for many families, who often feel unsupported and receive conflicting advice from the specialist centre, local hospital and HV. Replacing nasogastric tubes out of hours is particularly stressful [FI] |
3.1 All patients should be seen by their named paediatrician and named cardiologist at joint outreach clinics. A specialist nurse should attend all outpatient clinics and outreach clinics 3.2 All families should receive ‘check-in’ telephone calls from their named specialist nurse (team), the frequency of which should be determined by their needs 3.3 All families should have access to a telephone support service led by specialist nurses The IHS notes that recommendations 3.1–3.3 are broadly in line with the proposed standards from the NHS England Congenital Heart Disease review. The IHS further note that in order to meet these recommendations, additional resources may be required in some areas 3.4 All patients with a medical need (such as weight gain/feeding difficulties) should have access to community nursing and all patients should be referred to a HV team (via the GP or through community child health services). It is important that the community nurses/HVs are supported by the specialist centre (see 7.1–7.3) 3.5 Home monitoring should be provided for all patients with a primary diagnosis of HLHS, functionally univentricular heart or PA (including PA + IVS). This will include all patients in CART group 3 and some in group 1 (see above and Chapter 3) 3.6 There should be a nationally agreed protocol for home monitoring of these patients, based on the best available evidence. The IHS recommends that further research is conducted on the effectiveness of constituent components of home monitoring. The IHS notes that in order to meet the needs of a larger number of home monitoring patients, community nurses may need to run clinics or video and voice call clinics rather than provide home visits 3.7 The structured discharge document that is shared electronically with all HPs should contain:
|
| 4. Non-medical support | |
| Practical difficulties: families sometimes experience practical difficulties in the community that may not have been identified prior to discharge. These include child care for siblings, access to transport to get to follow-up appointments, financial difficulties due to long hospital stays, debts, loss of earnings and inability to return to work. Some families struggle to adhere to medication regimes and can experience difficultly getting prescriptions because GPs are not always clear what has been prescribed or what to do about off-licence medications [FI, HLI, OF] Fear and isolation: parents often live in fear of an emergency and the worry of infection isolates them from other parents and support groups in their community [FI, OF] Families lack confidence: some families lack the confidence to approach or challenge HPs, fail to ask questions during appointments for fear of appearing ignorant or incapable, or lack the ability to articulate their concerns (particularly non-English speakers) [HLI, HPI, OF] The strain of ‘expert parenting’/lack of confidence in local services: many families have to explain/pass on information about their child’s condition to HPs that do not have specialist knowledge and sometimes (as the holders of knowledge) feel they are battling with local services. Many families take on an ‘expert parent’ role, which can be alienating and frightening [FI, HPI, OF] Insufficient psychosocial support: the support offered to families is often purely related to the medical needs of their child with no specific protocol for assessing their psychosocial needs and resources harder to get for social (rather than medical) support unless they meet criteria for safeguarding [FI, HPI, OF] |
4.1 All families should have a named GP and named pharmacy prior to discharge. Changes to medication in hospital or as an outpatient should be sent electronically (e-mail/fax) to the named GP and pharmacy within an agreed timeframe (e.g. 72 hours) 4.2 Families with non-medical needs should be guided by their local HPs (e.g. GP, HV or community nurse) towards local support services appropriate to their needs (e.g. charity support or a family support worker). The IHS notes that statutory services for non-medical support are limited and declining and that this role is increasingly met by non-statutory services. The IHS recommends that further information is established regarding:
4.4 All patients should be referred to a HV (via the GP or through community child health services), who can act as an advocate for them, for example in helping them to articulate concerns/questions at appointments 4.5 All patients should be provided with information regarding patient support groups, both by the specialist centre (in particular cardiac support groups) and local HPs (local support services that may be more generic such as child development clinics) 4.6 For all patients, the named GP and named paediatrician should act as consistent points of contact in their locality 4.7 As part of the discharge planning process, families’ expectations of local/community health services should be managed, with relationships established as early as possible. Specifically, all patients should see their named GP within 2 weeks of discharge 4.8 Psychosocial meetings should be held after ward rounds in the specialist centre (led by the lead specialist nurse and psychologist) in order to determine needs and liaise with local or referral services as appropriate 4.9 Families with psychosocial needs should receive more frequent telephone calls (‘checking in’) from their named specialist nurse (team) and additional visits from a HV who is able to provide support and refer on to a psychologist if necessary |
| 5. Patient information | |
| Poor sharing of patient information: there are very few shared electronic patient record systems across services and notes from clinics/visits are often not sent to the other HPs involved (often assuming that they will be forwarded on by someone else, e.g. GP). Information is often relayed through the families, although there is inconsistency in the extent to which HPs use red books, hand-held records, health booklets, etc. [FI, HPI] Not flagged or fast-tracked: there is often no formal system for flagging (high-risk) babies to local HPs or for enabling them to have quick access to services [FI, HPI] |
5.1 For all patients, every HP involved in their care should receive electronic versions of:
5.3 For all families, their clinic letters should be sent electronically to the entire multidisciplinary team within a nationally agree standard timeframe (e.g. 72 hours) 5.4 The postdischarge death of any patient outside a specialist centre should be reported to the specialist centre and reviewed at a Network Mortality and Morbidity meeting for quality improvement purposes 5.5 All patients should have open access to their local hospital children’s ward 5.6 All patients should be flagged on their local hospital A&E system (e.g. using flags on electronic patient records or patient management systems), with fast-track referral to a secondary care paediatrician 5.7 All patients should be flagged on their GP practice system with clear instructions for receptionists/other GPs regarding appropriate fast-tracking |
| 6. Accessing support when a baby is sick | |
| Not knowing ‘Signs, symptoms, response’: parents and all local HPs are often unclear on what signs and symptoms to look for (or threshold criteria) and how to respond, with insufficient guidance from specialist centres [FI, HPI] Families not taken seriously: families sometimes find it difficult to verbalise their concerns, lack the confidence to seek help or do not feel listened to by HPs when they do [FI, OF] Failing to seek specialist advice: sometimes local HPs fail to notify the PEC or specialist centre of an incident (e.g. A&E visit) or contact them when there is a concern even when they lack the specialist knowledge they need (particularly out of hours) [FI, HPI] |
6.1 All families and all of the HPs involved in their support should receive the same clear guidance on ‘what is normal’ for that child, signs and symptoms to look for, how to respond and important contact numbers, for example in the form of a traffic-light tool. Ideally the format and content of this guidance should be standardised nationally, with scope for tailoring to local areas/networks as appropriate 6.2 The IHS agreed that there is an urgent need for such guidance (e.g. traffic-light tool) to be developed, that it should be evidence based as far as possible and that its implementation should be formally evaluated (i.e. its effectiveness and impact on families and HPs should be monitored) 6.3 The IHS recommends that guidance addresses out-of-hours procedures and specifies that the named specialist nurse or cardiologist must be informed if any patient attends hospital (for any reason). The IHS also recommends that guidance is short and self-contained (e.g. a sheet in the red book, fridge magnet, credit card for wallet or telephone application) and notes that it is likely to require different content for the following four sets of patients:
6.4 All families should receive hands-on training in the guidance prior to discharge. The IHS suggests that the guidance (e.g. traffic-light tool) is also made available to families in a diary format, as this may empower them to seek help and articulate their concerns with HPs in a timely fashion Note that some of the recommendations in sections 4 and 7 are also relevant here |
| 7. Knowledge gaps, weak links and poor communication between HPs | |
Qualitative evidence of knowledge gaps: all strands of the qualitative evidence highlighted the existence (across the entire patient journey) of large knowledge gaps between specialist and non-specialist HPs, weak links across different sectors and poor communication between HPs, identifying these as major potential or actual causes of failures in care. Examples reported in the data include:
Effective communication and co-ordination: examples of effective communication and co-ordination were also reported, typically when there was a PEC running joint clinics with the cardiologist who was familiar with the specialist centre processes and had direct contact with individuals there. Often CLNs/CNSs provided the link and point of contact between local and specialist centres and this was reported to work particularly well when they attended local clinics and/or trained key link nurses in the local team [FI, HPI] |
7.1 The IHS highlights the importance of strengthening networks and building local capacity in order to address the large knowledge gaps between specialist and non-specialist HPs, weak links across different sectors and poor communication between HPs, which the IHS identified as major potential or actual causes of failures in care 7.2 In particular, the IHS recommends that all HPs involved in caring for any patient should have the direct contact number and e-mail for the lead clinician co-ordinating care, that is the named paediatrician, in order to ask questions or raise concerns. They should additionally have the contact number/e-mail for the named specialist nurse and cardiologist at the specialist centre. This contact information will be contained on the discharge document (see 2.4) 7.3 Further suggestions include:
|
It is worth noting that the original overarching study objective was focused on the community-based surveillance of fragile infants with CHD, and as listed in Table 25, this area has been covered in detail in our suggestions for service improvement (see the sections on medical follow-up services, non-medical support, provision of patient information and accessing support when a baby is sick). In undertaking the iterative series of reviews of the emerging evidence from the study it became apparent that optimal community-based surveillance of these infants necessitates further inter-related improvements to additional elements of the patient journey (see sections on training and information for families predischarge and discharge and transferring to non-specialist services). Hence our focus widened somewhat to incorporate aspects of the patient journey that start in the tertiary centre (including training of families and communication between professionals in different sectors) and these additional aspects are discussed in detail in Table 25.
Many of the service improvements proposed and refined by the working group were suggested for all infants as they were considered to have lower resource implications and/or to be important for everyone, irrespective of their risk (e.g. a structured discharge document or standardised training). However, interventions considered particularly beneficial for certain patient groups were suggested selectively (e.g. multidisciplinary care teams for children with long term complex needs in addition to their primary cardiac diagnosis). In addition, given that over half of all adverse events occur in the 21% of patients in risk groups 1–4 (see Table 24), suggestions regarding some of the potentially higher resource aspects of service provision were prioritised for these high-risk patient groups (e.g. discharge home via the local hospital). Finally, on the basis of qualitative evidence (Chapters 7 and 8), suggesting that problems in accessing support can be exacerbated for families who face cultural or language barriers or have learning difficulties, and on the basis that National Audit data presented in Chapter 4 indicates that more complex heart defects, deprivation and higher-risk medical conditions such as neurodevelopmental problems are over-represented in particular ethnic minority groups, some suggestions were prioritised for these groups (e.g. offering and supporting family buddying).
The service challenges and barriers identified during the course of the IHS are listed below, accompanied by the suggestions for service improvements endorsed by the IHS expert advisory group.
Discussion
Over the course of the IHS a set of evidence-informed guidelines were developed for managing the discharge and postdischarge care of infants undergoing major cardiac intervention, which were directly aimed at addressing challenges in this previously neglected section of the care pathway. The development of this guideline was, indeed, the overarching aim of the study, and as stated above in the Introduction section of this chapter, formed the basis of study objective 4. In the completion of study objectives 1–3 and the gathering of multimethods research evidence that generated information regarding patient risk characteristics and the challenges encountered in accessing and providing services for this patient population presented in Chapters 2–9, it became clear that the guideline would need to incorporate a series of comments and suggestions related to discharge and postdischarge management, including community-based surveillance of fragile infants with heart disease. These guidelines, which are presented in full in Table 25, represent the final output of an evidence-informed, multistakeholder process, and go beyond the original aims of our study in that they are somewhat broader in scope. This was necessary in order to address comprehensively all of the evidence that came to light over the course of the programme of research.
Strengths and limitations
A limitation of our research project was that the development of optimal guidelines for services across sectors is challenging, not least because there is often limited or disparate evidence that is difficult to synthesise (for example with no established methods equivalent to the meta-analysis of randomised controlled trials), and inevitably involves an element of subjectivity. In this context, our study had two key strengths. First, developing and applying a systematic and transparent process for synthesising and incorporating a broad range of available evidence covering multiple aspects of the problem enhanced the richness and breadth of the guidelines. For example, the qualitative evidence was very useful in specifying what the problems in services were and how they might be improved, and the quantitative evidence enabled prioritisation of patient groups according to their risk. Second, representatives from across the entire patient pathway critiqued the feasibility and acceptability of the recommendations, and the needs of service users remained of central importance through incorporating findings from an OF and interviews, views captured in a family workshop and involving a parent representative on the expert group.
Implications for practice
Our proposed guidelines for services are of direct relevance to all health-care professionals caring for infants with CHD including GPs, community nurses, HVs, secondary care paediatricians and clinicians in specialist surgical centres, as well as patients, their families and support groups. The proposals will resonate with clinicians, patient families and user groups from other geographical areas when later postdischarge CHD deaths have been reported as a concern in certain diagnostic groups, for example Germany55 and the USA. 26
As discussed in more detail in Table 25, there is a need for reduced variability and improved overall standards in respect of the training and information for families with infants affected by CHD predischarge, the process for discharge and transfer of infants with CHD to non-specialist services and their medical follow-up services, the non-medical support for patient families, the provision of patient information, the access to support when a baby is sick, and in reducing knowledge gaps and strengthening communication between HPs. It is likely that such interventions could have a material impact in terms of reducing postdischarge deaths and readmissions to intensive care, as well as improving the perceptions and experiences of services by patients’ families.
There is a role for the relevant professional groups (e.g. the British Congenital Cardiac Association and the PEC Special Interest Group), research funders, the national audit body and service users in taking forward certain aspects that require further research and development (see Recommendations for research). CHD services in the UK are currently under national review by NHS England and the findings and conclusions reported in this article fed into the review’s public consultation on the care standards and service specification to be used in commissioning specialist CHD services (see Appendix 11).
Suggestions for audit metrics
The evidence and suggestions from the IHS would support monitoring of the process and outcome measures, in order to increase the focus on this neglected phase of the patient journey and hence, it is hoped, to drive up standards of service provision. One potential option for such monitoring is the Quality Dashboard, which is guided by the Clinical Reference Group. Please note, however, that while the IHS team suggests the monitoring of out-of-hospital outcomes was highlighted as important within the IHS data, the analytical steps and processes required to do this are beyond the scope of the IHS team and would need to be considered further by, for example, the NCHDA steering committee.
The IHS findings would first need to be disseminated more widely within the community and agreement reached about the steps required for implementing the recommendations; for example, a number of metrics relate to standardised documents/protocols that would need to be developed and appropriately piloted beforehand. Others require further consideration as to how the information could be collected and/or aggregated. Furthermore, some findings may be considered more appropriate for local monitoring for improvement purposes, rather than for inclusion on the national Quality Dashboard.
The IHS therefore suggests that the feasibility and appropriateness of including any or all of the following metrics from Box 30 on the Quality Dashboard should be revisited in the next 1 to 2 years and, in the meantime, steps taken to develop these areas.
Emergency unplanned readmissions to PICU within 1 year following infant heart surgery (for all infants).
Process measuresPercentage of infants with HLHS/UVH/PA/neurodevelopmental conditions/LOS in the specialist centre > 1 month that receive ‘step-down’ care (i.e. discharge via their local hospital).
Percentage of infant deaths outside the specialist hospital that are discussed at a Network Mortality and Morbidity conference, with details recorded on a nationally standardised pro forma.
Percentage of infants for whom a nationally standardised structured discharge document is completed prior to discharge and distributed electronically to all of the HPs involved in their care.
Percentage of infants with HLHS/UVH/PA who are following a nationally agreed protocol for home monitoring.
Percentage of families that receive nationally agreed guidance on ‘what is normal’ for that child, signs and symptoms to look for, how to respond and important contact numbers (e.g. in the form of a traffic-light tool).
Percentage of infants for whom all of the HPs involved in their care receive nationally agreed guidance on ‘what is normal’ for that child, signs and symptoms to look for, how to respond and important contact numbers (e.g. in the form of a traffic-light tool).
Percentage of families that receive all required training and information prior to discharge (facilitated using a nationally standardised checklist).
Percentage of infants who, at discharge home (either from specialist or local hospital), have a named GP.
Percentage of infants who, at discharge home (either from specialist or local hospital), have a named pharmacist (if discharged with a long-term prescription).
Percentage of families offered an opportunity to connect with other families (e.g. through social media or charity support groups).
Percentage of families more likely to experience language/cultural barriers to accessing support that are offered buddying.
Patient-reported experience measures
The IHS supports the monitoring of patient-reported experience measures alongside the process and outcome measures in Box 30, for example adopting a similar approach to the specialist Quality Dashboards (e.g. heart transplant and ECMO) that include three questions relating to patient-reported experience measures. This area would require further development work.
The importance of joined-up service specifications/commissioning across specialist, local and community services
We emphasise that the IHS findings demonstrate the need and potential for improvements across the entire patient journey spanning community, primary, secondary and specialist services. This would require service specifications and commissioning to be addressed not only for the specialist services commissioned by the Congenital Heart Services clinical reference group, but for all of these sectors. The IHS’s evidence of weak links across sectors and poor communication between different HPs further suggests the need for joined-up service specifications and models of commissioning across the whole patient journey, including local and community settings.
Recommendations for research
The study highlighted areas where further specific research is needed, these being as follows.
Home monitoring programmes
The systematic review (see Chapter 3) revealed that data on the effectiveness of HMPs are limited: most of the data originate from the USA (where health systems differ), with only one study available from Germany; in addition, the data’s effectiveness is not completely certain as control patients were based on historic data, which means that resultant biases should be considered, such as the role of other changes in the patient journey apart from HMPs contributing to improvements. The qualitative data in our study from the family and professional interviews reported in Chapters 7 and 8 indicated that although many informants thought that HMPs were beneficial because they helped everyone to follow consistent standards and increased awareness across various HP groups of high-risk patients, some parents found HMPs burdensome. The studies in the systematic review did not contain user viewpoints, so we cannot ascertain what these were. Furthermore, current practice across the UK in respect of HMP protocols varies widely (see Chapter 8). Overall, the studies in the systematic review support the hypothesis that HMP are effective (with some remaining doubts and caveats), and our study findings support the use of HMP for HLHS, UVH and PA patients with shunt dependence. However, further national consensus building and research is required to establish the optimal protocol and components of HMP incorporating specific consideration of user views and cultural or language barriers before taking these forward. Additional work is also needed to review the inclusion criteria for HMP, particularly when these necessitate additional patients being monitored over current practice, including an assessment of resource implications. As HMP are implemented more uniformly within UK centres, it will be vital to collect prospective information about effectiveness.
Development and evaluation of proposed tools for improvement
Additional health-care evaluation is required of the best format, applications and effectiveness of the proposed traffic-light tool to detect early warning signs of deterioration, as well as health-care evaluation of the proposed structured discharge document, discharge checklist and step-down care. Cultural and language barriers should form part of this evaluation.
Metrics for national audit
The study working group has clearly endorsed the need to include metrics for national audit and benchmarking, which go beyond 30-day mortality rates and address the need to monitor postdischarge outcomes for infant CHD. Given the availability of life status tracking to the national audit (NCHDA) and the possibility being open for record linkage to occur between NCHDA and PICANet as a means to underpin future audit (this would enable the audit of postdischarge emergency readmissions to PICU), this is technically feasible. That said, a series of analytical steps would need to be undertaken for this to occur, which are not insignificant.
Another important issue to consider, which is likely to require further research, is adjustment for case mix when monitoring postdischarge outcomes. The first step that the working group proposed, of auditing postdischarge interstage deaths in infants with HLHS, UVH and PA, incorporates case mix adjustment, as these diagnoses represent a high-risk group as determined by our analyses. However, we note that the risks of adverse outcome differ even between HLHS (odds ratio of outcome 1 : 7.4) and UVH–PA (odds ratio of outcome 1 : 4.4). In this example, the national distribution of children with HLHS across specialist centres is uneven in the UK with certain centres taking care of the majority of patients with HLHS. If outcome is audited for HLHS together with UVH and PA, then there is the potential for an unfairly negative assessment of outcome in a centre with a large number of patients that have HLHS. Hence, finally, we note that further analytical and statistical steps are required in order to adequately address case mix complexity, building upon the work we have set out in Chapter 4, such that these outcomes and further postdischarge outcome measures proposed above for future audit, may effectively form part of the portfolio of metrics subjected to national audit.
Acknowledgements
We would like to thank Nurse Specialists Helen Silk, Christie Fox and Kay Dyer for their assistance with recruitment and to extend our deepest gratitude to all of the parents who kindly agreed to be interviewed and made our study possible.
We would like to thank the professionals from tertiary, secondary and primary care who agreed to be interviewed for the study.
We would like to thank the CHF and Little Hearts Matter for their invaluable assistance with the study, and to thank the family members who posted on the online forum.
Author contributions
Katherine L Brown: study design, participated in all stages of conducting and writing up the research.
Jo Wray: study design, participated in all stages of conducting and writing up the research.
Rachel L Knowles: study design, participated in all stages of conducting and writing up the research.
Sonya Crowe: contributed to study design, primary analytical role for national audit data and writing up quantitative research, participated in conducting and writing up the qualitative research and facilitated the intervention development.
Jenifer Tregay: contributed to study design, primary role in qualitative research and systematic reviews, participated in all stages of conducting and writing up the research.
Deborah Ridout: contributed to study design, performed statistical analyses, contributed to writing up the quantitative research.
David J Barron: contributed to study design and writing up clinical aspects of the research.
David Cunningham: performed the data search in NCHDA and the data linkage, and contributed to the quantitative research.
Roger Parslow: contributed to the data search within PICANet and to the quantitative research.
Rodney Franklin: contributed to study design, intervention development and writing up clinical aspects of the research.
Nick Barnes: contributed to intervention development and writing up clinical aspects of the research.
Sally Hull: contributed to intervention development and writing up clinical aspects of the research.
Catherine Bull: study design, participated in all stages of conducting and writing up the the research.
Contributor roles
Liz Smith (Great Ormond Street Hospital NHS Foundation Trust): attended intervention development workshops, co-led family workshop and commented on intervention materials.
Martin Utley (Clinical Operational Research Unit, University College London): operational research contributor.
Faith Gibson (Great Ormond Street Hospital NHS Foundation Trust): qualitative research contributor.
Piers Daubeney (Royal Brompton and Harefield NHS Foundation Trust): tertiary professional consultant.
Rohini Simbodyal (CHF): user group consultant.
Suzie Hutchinson (Little Hearts Matter): attended intervention development workshop and commented on intervention materials.
Hannah Charrot (Cambridgeshire Community Services NHS Trust): attended intervention development workshop and commented on intervention materials.
Jan Pennington (Barts Health NHS Trust): attended intervention development workshop and commented on intervention materials.
Publications
Tregay J, Wray J, Bull C, Franklin RC, Daubeney P, Barron DJ, et al. Unexpected deaths and unplanned re-admissions in infants discharged home after cardiac surgery: a systematic review of potential risk factors. Cardiol Young 2015;25:839–52.
Tregay J, Brown KL, Crowe S, Bull C, Knowles RL, Smith L, et al. Signs of deterioration in infants discharged home following congenital heart surgery in the first year of life: a qualitative study [published online ahead of print 28 January 2016]. Arch Dis Child 2016
Tregay J, Wray J, Crowe S, Knowles R, Daubeney P, Franklin R, et al. Going home after infant cardiac surgery: a UK qualitative study. Arch Dis Child 2016;101:320–5.
Tregay J, Brown K, Crowe S, Bull C, Knowles R, Wray J. “I was so worried about every drop of milk” - feeding problems at home are a significant concern for parents after major heart surgery in infancy [published online ahead of print 19 February 2016]. Matern Child Nutr 2016.
Crowe S, Ridout DA, Knowles R, Tregay J, Wray J, Barron DJ, et al. Death and emergency readmission of infants discharged after interventions for congenital heart disease: a national study of 7643 infants to inform service improvement. J Am Heart Assoc 2016; in press.
Crowe S, Knowles R, Wray J, Tregay J, Ridout DA, Utley M, et al. Identifying ways to improvements to complex multi-sector pathways: systematic evidence synthesis and multi-stakeholder engagement in infant congenital heart disease. BMJ Open 2016; in press.
Data sharing statement
The quantitative data and analyses presented as part of this study were undertaken with specific approvals from the Health Research Authority, the Health Quality Improvement Partnership and the National Audits, NCHDA and PICANet. The research dataset that underpins this work is available for future research as long as equivalent approvals from these organizations are sought and granted. Given the complexity of this dataset, members of the infant heart study team would be required to act in an advisory capacity should further work be undertaken involving this dataset. Further information can be obtained from the corresponding author.
The qualitative data presented as part of this study are not suitable for sharing beyond that contained within the report. Further information can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Knowles RL, Bull C, Wren C, Dezateux C. Mortality with congenital heart defects in England and Wales, 1959–2009: exploring technological change through period and birth cohort analysis. Arch Dis Child 2012;97:861-5. http://dx.doi.org/10.1136/archdischild-2012-301662.
- Kurinczuk JJ, Hollowell J, Boyd PA, Oakley L, Brocklehurst P, Gray R. Inequalities in Infant Mortality Project Briefing Paper 4: the Contribution of Congenital Anomalies to Infant Mortality. University of Oxford: National Perinatal Epidemiology Unit; 2010.
- Billett J, Majeed A, Gatzoulis M, Cowie M. Trends in hospital admissions, in-hospital case fatality and population mortality from congenital heart disease in England, 1994 to 2004. Heart 2008;94:342-8. http://dx.doi.org/10.1136/hrt.2006.113787.
- Brown KL, Crowe S, Franklin R, Mclean A, Cunningham D, Barron D, et al. Trends in 30-day mortality rate and case mix for paediatric cardiac surgery in the UK between 2000 and 2010. Open Heart 2015;2:1-11. http://dx.doi.org/10.1136/openhrt-2014-000157.
- National Institute for Cardiovascular Outcomes Research (NICOR), University College London . NICOR: National Institute for Cardiovascular Outcomes Research Congenital Heart Disease Website 2014. https://nicor4.nicor.org.uk/CHD/an_paeds.nsf/vwContent/home?Opendocument (accessed 29 August 2014).
- Hindocha NBA. When, Where and Why Do Babies Die After Neonatal Surgery for Congenital Heart Disease? n.d.
- Azakie T, Merklinger SL, McCrindle BW, Van Arsdell GS, Lee KJ, Benson LN, et al. Evolving strategies and improving outcomes of the modified Norwood procedure: a 10-year single-institution experience. Ann Thorac Surg 2001;72:1349-53. http://dx.doi.org/10.1016/S0003-4975(01)02795-3.
- Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation 2010;122:2254-63. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.947002.
- Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002;123:110-18. http://dx.doi.org/10.1067/mtc.2002.119064.
- Lacour-Gayet F, Clarke D, Jacobs J, Gaynor W, Hamilton L, Jacobs M, et al. The Aristotle score for congenital heart surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann 2004;7:185-91. http://dx.doi.org/10.1053/j.pcsu.2004.02.011.
- Crowe S, Brown KL, Pagel C, Muthialu N, Cunningham D, Gibbs J, et al. Development of a diagnosis- and procedure-based risk model for 30-day outcome after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2013;145:1270-8. http://dx.doi.org/10.1016/j.jtcvs.2012.06.023.
- O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009;138:1139-53. http://dx.doi.org/10.1016/j.jtcvs.2009.03.071.
- Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 2010;362:1980-92. http://dx.doi.org/10.1056/NEJMoa0912461.
- Burkhardt BE, Rucker G, Stiller B. Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease. Cochrane Database Syst Rev 2015;3. http://dx.doi.org/10.1002/14651858.cd009515.pub2.
- Ghanayem NS, Hoffman GM, Mussatto KA, Cava JR, Frommelt PC, Rudd NA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg 2003;126:1367-77. http://dx.doi.org/10.1016/S0022-5223(03)00071-0.
- Committee on Fetus and Newborn . Hospital discharge of the high-risk neonate. Pediatrics 2008;122:1119-26. http://dx.doi.org/10.1542/peds.2008-2174.
- Qureshi SA. Requirements for Provision of Outreach Paediatric Cardiology Service. London: BCCA; 2009.
- NHS . Safe and Sustainable: Childrens Congenital Cardiac Services 2011. www.specialisedservices.nhs.uk/safe_sustainable/childrens-congenital-cardiac-services (accessed 26 February 2011).
- England N. New Review of Congenital Heart Services. Board Paper – NHS England: NHSE180713 n.d. www.england.nhs.uk/wp-content/uploads/2013/07/180713-item13.pdf (accessed 2 March 2015).
- Tregay J, Wray J, Bull C, Franklin RC, Daubeney P, Barron DJ, et al. Unexpected deaths and unplanned readmissions in infants discharged home after cardiac surgery: a systematic review of potential risk factors. Cardiol Young 2015;25:839-52. http://dx.doi.org/10.1017/S1047951114002492.
- Tregay J, Wray J, Brown KL, Knowles RL. A Systematic Review of Risk Factors Associated With Adverse Outcomes for Infants Discharged from Hospital After Major Surgery for Congenital Anomalies n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013003483 (accessed 2 March 2015).
- Morley PT. Quality Assessment for Individual Studies to be Used for the Review of Resuscitation Science for 2010. American Heart Association International Liaison Committee on Resuscitation (ILCOR); 2008.
- Ashburn DA, McCrindle BW, Tchervenkov CI, Jacobs ML, Lofland GK, Bove EL, et al. Outcomes after the Norwood operation in neonates with critical aortic stenosis or aortic valve atresia. J Thorac Cardiovasc Surg 2003;125:1070-82. http://dx.doi.org/10.1067/mtc.2003.183.
- Carlo WF, Carberry KE, Heinle JS, Morales DL, McKenzie ED, Fraser CD, et al. Interstage attrition between bidirectional Glenn and Fontan palliation in children with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2011;142:511-16. http://dx.doi.org/10.1016/j.jtcvs.2011.01.030.
- Edwards JD, Kun SS, Keens TG, Khemani RG, Moromisato DY. Children with corrected or palliated congenital heart disease on home mechanical ventilation. Pediatr Pulmonol 2010;45:645-9. http://dx.doi.org/10.1002/ppul.21214.
- Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg 2012;144:896-90. http://dx.doi.org/10.1016/j.jtcvs.2012.05.020.
- Hansen JH, Uebing A, Furck AK, Scheewe J, Jung O, Fischer G, et al. Risk factors for adverse outcome after superior cavopulmonary anastomosis for hypoplastic left heart syndrome. Eur J Cardiothoracic Surg 2011;40:e43-9. http://dx.doi.org/10.1016/j.ejcts.2011.02.044.
- Mahle WT, Spray TL, Gaynor JW, Clark BJ. Unexpected death after reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg 2001;71:61-5. http://dx.doi.org/10.1016/S0003-4975(00)02324-9.
- Pinto NM, Lasa J, Dominguez TE, Wernovsky G, Tabbutt S, Cohen MS. Regionalization in neonatal congenital heart surgery: the impact of distance on outcome after discharge. Pediatric Cardiol 2012;33:229-38. http://dx.doi.org/10.1007/s00246-011-0116-4.
- Simsic JM, Bradley SM, Stroud MR, Atz AM. Risk factors for interstage death after the Norwood procedure. Pediatr Cardiol 2005;26:400-3. http://dx.doi.org/10.1007/s00246-004-0776-4.
- Chang RK, Rodriguez S, Lee M, Klitzner TS. Risk factors for deaths occurring within 30 days and 1 year after hospital discharge for cardiac surgery among pediatric patients. Am Heart J 2006;152:386-93. http://dx.doi.org/10.1016/j.ahj.2005.12.016.
- Fixler DE, Nembhard WN, Xu P, Ethen MK, Canfield MA. Effect of acculturation and distance from cardiac center on congenital heart disease mortality. Pediatrics 2012;129:1118-24. http://dx.doi.org/10.1542/peds.2011-3114.
- Hebson CL, Oster ME, Kirshbom PM, Clabby ML, Wulkan ML, Simsic JM. Association of feeding modality with interstage mortality after single-ventricle palliation. J Thorac Cardiovasc Surg 2012;144:173-7. http://dx.doi.org/10.1016/j.jtcvs.2011.12.027.
- Kogon B, Jain A, Oster M, Woodall K, Kanter K, Kirshbom P. Risk factors associated with readmission after pediatric cardiothoracic surgery. Ann Thorac Surg 2012;94:865-73. http://dx.doi.org/10.1016/j.athoracsur.2012.04.025.
- Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, et al. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg 2008;136:94-9. http://dx.doi.org/10.1016/j.jtcvs.2007.12.012.
- Mackie AS, Gauvreau K, Newburger JW, Mayer JE, Erickson LC. Risk factors for readmission after neonatal cardiac surgery. Ann Thorac Surg 2004;78:1972-8.
- Ohye RG, Schonbeck JV, Eghtesady P, Laussen PC, Pizarro C, Shrader P, et al. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg 2012;144:907-14. http://dx.doi.org/10.1016/j.jtcvs.2012.04.028.
- Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:180-4. http://dx.doi.org/10.1053/j.pcsu.2004.02.009.
- Davis PJ, Firmin RK, Manktelow B, Goldman AP, Davis CF, Smith JH, et al. Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr 2004;144:309-15. http://dx.doi.org/10.1016/j.jpeds.2003.11.031.
- Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation 2002;106:I82-9.
- McGuirk SP, Griselli M, Stumper OF, Rumball EM, Miller P, Dhillon R, et al. Staged surgical management of hypoplastic left heart syndrome: a single institution 12 year experience. Heart 2006;92:364-70. http://dx.doi.org/10.1136/hrt.2005.068684.
- Cristancho S, Garces DM, Peters KE, Mueller BC. Listening to rural Hispanic immigrants in the Midwest: a community-based participatory assessment of major barriers to health care access and use. Qual Health Res 2008;18:633-46. http://dx.doi.org/10.1177/1049732308316669.
- Valdez RB, Giachello A, Rodriguez-Trias H, Gomez P, de la Rocha C. Improving access to health care in Latino communities. Public Health Rep 1993;108:534-9.
- DeMone JA, Gonzalez PC, Gauvreau K, Piercey GE, Jenkins KJ. Risk of death for Medicaid recipients undergoing congenital heart surgery. Pediatr Cardiol 2003;24:97-102. http://dx.doi.org/10.1007/s00246-002-0243-z.
- Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation 2010;121:644-50. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.881904.
- Flores G. Technical Report – Racial and Ethnic Disparities in the Health and Health Care of Children. Pediatrics 2010;125:e979-1020. http://dx.doi.org/10.1542/peds.2010-0188.
- Flores G, Lin H. Trends in racial/ethnic disparities in medical and oral health, access to care, and use of services in US children: has anything changed over the years?. Int J Equity Health 2013;12. http://dx.doi.org/10.1186/1475-9276-12-10.
- Nembhard WN, Salemi JL, Ethen MK, Fixler DE, Dimaggio A, Canfield MA. Racial/Ethnic disparities in risk of early childhood mortality among children with congenital heart defects. Pediatrics 2011;127:e1128-38. http://dx.doi.org/10.1542/peds.2010-2702.
- Connor JA, Kline NE, Mott S, Harris SK, Jenkins KJ. The meaning of cost for families of children with congenital heart disease. J Pediatr Health Care 2010;24:318-25. http://dx.doi.org/10.1016/j.pedhc.2009.09.002.
- Golbus JR, Wojcik BM, Charpie JR, Hirsch JC. Feeding complications in hypoplastic left heart syndrome after the Norwood procedure: a systematic review of the literature. Pediatr Cardiol 2011;32:539-52. http://dx.doi.org/10.1007/s00246-011-9907-x.
- Tregay J, Wray J, Knowles R, Brown K. A Systematic Review of Non-Invasive Interventions for Infants Discharged from Hospital After Major Surgery for Congenital Anomalies n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013003484 (accessed 2 March 2015).
- Dobrolet NC, Nieves JA, Welch EM, Khan D, Rossi AF, Burke RP, et al. New approach to interstage care for palliated high-risk patients with congenital heart disease. J Thorac Cardiovasc Surg 2011;142:855-60. http://dx.doi.org/10.1016/j.jtcvs.2011.01.054.
- Ghanayem NS, Cava JR, Jaquiss RD, Tweddell JS. Home monitoring of infants after stage one palliation for hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:32-8. http://dx.doi.org/10.1053/j.pcsu.2004.02.017.
- Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RD. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young 2006;16:61-6. http://dx.doi.org/10.1017/S1047951105002349.
- Hansen JH, Furck AK, Petko C, Buchholz-Berdau R, Voges I, Scheewe J, et al. Use of surveillance criteria reduces interstage mortality after the Norwood operation for hypoplastic left heart syndrome. Eur J Cardiothorac Surg 2012;41:1013-18. http://dx.doi.org/10.1093/ejcts/ezr190.
- Husain N, Texter K, Hershenson J, Allen R, Miller-Tate H, Stewart J, et al. Impact of Inter Stage Home Monitoring After Hybrid Palliation of Hypoplastic Left Heart Syndrome n.d.
- Petit CJ, Fraser CD, Mattamal R, Slesnick TC, Cephus CE, Ocampo EC. The impact of a dedicated single-ventricle home-monitoring program on interstage somatic growth, interstage attrition, and 1-year survival. J Thorac Cardiovasc Surg 2011;142:1358-66. http://dx.doi.org/10.1016/j.jtcvs.2011.04.043.
- Srinivasan C, Sachdeva R, Morrow WR, Gossett J, Chipman CW, Imamura M, et al. Standardized management improves outcomes after the Norwood procedure. Congenit Heart Dis 2009;4:329-37. http://dx.doi.org/10.1111/j.1747-0803.2009.00323.x.
- Hehir DA, Ghanayem NS. Single-ventricle infant home monitoring programs: outcomes and impact. Curr Opin Cardiol 2013;28:97-102. http://dx.doi.org/10.1097/HCO.0b013e32835dceaf.
- Welke KF, Diggs BS, Karamlou T, Ungerleider RM. Comparison of pediatric cardiac surgical mortality rates from national administrative data to contemporary clinical standards. Ann Thorac Surg 2009;87:216-22. http://dx.doi.org/10.1016/j.athoracsur.2008.10.032.
- Schidlow DN, Anderson JB, Klitzner TS, Beekman RH, Jenkins KJ, Kugler JD, et al. Variation in interstage outpatient care after the Norwood procedure: a report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenit Heart Dis 2011;6:98-107. http://dx.doi.org/10.1111/j.1747-0803.2011.00509.x.
- Fenton KN, Siewers RD, Rebovich B, Pigula FA. Interim mortality in infants with systemic-to-pulmonary artery shunts. Ann Thorac Surg 2003;76:152-6. http://dx.doi.org/10.1016/S0003-4975(03)00168-1.
- Zarbock SF. Hospital discharge of the high-risk neonate. Home Care Provid 1998;3:302-3. http://dx.doi.org/10.1016/S1084-628X(98)90006-5.
- Committee on Fetus and Newborn; American Academy of Pediatrics . Hospital discharge of the high-risk neonate – proposed guidelines. Pediatrics 1998;102:411-17. http://dx.doi.org/10.1542/peds.102.2.411.
- Sekar P, Vilvanathan V. Telecardiology: effective means of delivering cardiac care to rural children. Asian Cardiovasc Thorac Ann 2007;15:320-3. http://dx.doi.org/10.1177/021849230701500411.
- McCrossan B, Morgan G, Grant B, Sands AJ, Craig BG, Doherty NN, et al. A randomised trial of a remote home support programme for infants with major congenital heart disease. Heart 2012;98:1523-8. http://dx.doi.org/10.1136/heartjnl-2012-302350.
- Crowe S, Ridout DA, Knowles R, Tregay J, Wray J, Barron DJ, et al. Death and Emergency Readmission of Infants Discharged After Interventions for Congenital Heart Disease: National Study of 7643 Infants to Inform Service Improvement. J Am Heart Assoc 2016;5. http://dx.doi.org/10.1161/JAHA.116.003369.
- Jacobs JP, O’Brien SM, Pasquali SK, Jacobs ML, Lacour-Gayet FG, Tchervenkov CI, et al. Variation in outcomes for risk-stratified pediatric cardiac surgical operations: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg 2012;94:564-71. http://dx.doi.org/10.1016/j.athoracsur.2012.01.105.
- NICOR: National Institute for Cardiovascular Outcomes Research: Congenital Heart Diseases Website. University College London; 2015.
- (PICANet) PICAN . Paediatric Intensive Care Audit Network (PICANet) 2015. www.picanet.org.uk (accessed 8 February 2015).
- Clarke DR, Breen LS, Jacobs ML, Franklin RC, Tobota Z, Maruszewski B, et al. Verification of data in congenital cardiac surgery. Cardiol Young 2008;18:177-87. http://dx.doi.org/10.1017/S1047951108002862.
- Annual Report of the Paediatric Intensive Care Audit Network 2014 – Tables and Figures. Leeds: PICANet; 2014.
- IPCCC . International Paediatric and Congenital Cardiac Code (IPCCC) Home Page 2012. www.ipccc.net (accessed 12 April 2013).
- English indices of deprivation 2010. London: Department for Communities and Local Government; 2011.
- Brown KL, Crowe S, Pagel C, Bull C, Muthialu N, Gibbs J, et al. Use of diagnostic information submitted to the United Kingdom Central Cardiac Audit Database: development of categorisation and allocation algorithms. Cardiol Young 2013;23:491-8. http://dx.doi.org/10.1017/S1047951112001369.
- Harrell FE, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med 1984;3:143-52. http://dx.doi.org/10.1002/sim.4780030207.
- English Indices of Deprivation 2010 – Deprivation Category Lookups and Average Scores for Higher Geographies. London: London Health Observatory; n.d.
- Office for National Statistics . Guidance and Methodology: Ethnic Group n.d. http://ons.gov.uk/ons/guide-method/measuring-equality/equality/ethnic-nat-identity-religion/ethnic-group/index.html#1 (accessed 13 February 2015).
- Heymans MW, van Buuren S, Knol DL, van Mechelen W, de Vet HC. Variable selection under multiple imputation using the bootstrap in a prognostic study. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-33.
- Rubin D. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley and Sons, Inc; 1987.
- The NHS Plan: A Plan for Investment, A Plan for Reform. London: Department of Health; 2000.
- Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer–Lemeshow test revisited. Crit Care Med 2007;35:2052-6. http://dx.doi.org/10.1097/01.CCM.0000275267.64078.B0.
- Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. http://dx.doi.org/10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4.
- Ridley S, Jones S, Shahani A, Brampton W, Nielsen M, Rowan K. Classification trees. A possible method for iso-resource grouping in intensive care. Anaesthesia 1998;53:833-40. http://dx.doi.org/10.1046/j.1365-2044.1998.t01-1-00564.x.
- Garbe C, Buttner P, Bertz J, Burg G, d’Hoedt B, Drepper H, et al. Primary cutaneous melanoma. Identification of prognostic groups and estimation of individual prognosis for 5093 patients. Cancer 1995;75:2484-91. http://dx.doi.org/10.1002/1097-0142(19950515)75:10<2484::AID-CNCR2820751014>3.0.CO;2-U.
- Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572-80. http://dx.doi.org/10.1001/jama.293.5.572.
- Breiman LF, Friedman J, Stone CJ, Olshen RA. CART: Classification and Regression Trees. Monterey, CA: Wadsworth and Brooks/Cole Advanced Books and Software; 1983.
- Hehir DA, Rudd N, Slicker J, Mussatto KA, Simpson P, Li SH, et al. Normal interstage growth after the Norwood operation associated with interstage home monitoring. Pediatr Cardiol 2012;33:1315-22. http://dx.doi.org/10.1007/s00246-012-0320-x.
- Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ. Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med 2003;31:28-33. http://dx.doi.org/10.1097/00003246-200301000-00004.
- STROBE . STROBE Checklists, Version 4 n.d. www.strobe-statement.org/index.php?id=available-checklists (accessed 21 June 2012).
- Sheridan E, Wright J, Small N, Corry PC, Oddie S, Whibley C, et al. Risk factors for congenital anomaly in a multiethnic birth cohort: an analysis of the Born in Bradford study. Lancet 2013;382:1350-9. http://dx.doi.org/10.1016/S0140-6736(13)61132-0.
- Hoffman JIE. The global burden of congenital heart disease. Cardiovasc J Africa 2013;24:141-5. http://dx.doi.org/10.5830/CVJA-2013-028.
- Fixler DE, Pastor P, Sigman E, Eifler CW. Ethnicity and socioeconomic status: impact on the diagnosis of congenital heart disease. J Am Coll Cardiol 1993;21:1722-6. http://dx.doi.org/10.1016/0735-1097(93)90393-F.
- Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res Part A Clin Mol Teratol 2006;76:747-56. http://dx.doi.org/10.1002/bdra.20294.
- Nembhard WN, Wang T, Loscalzo ML, Salemi JL. Variation in the prevalence of congenital heart defects by maternal race/ethnicity and infant sex. J Pediatr 2010;156:259-64. http://dx.doi.org/10.1016/j.jpeds.2009.07.058.
- Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics 2001;107. http://dx.doi.org/10.1542/peds.107.3.e32.
- Benavidez OJ, Gauvreau K, Jenkins KJ. Racial and ethnic disparities in mortality following congenital heart surgery. Pediatr Cardiol 2006;27:321-8. http://dx.doi.org/10.1007/s00246-005-7121-4.
- Oster ME, Strickland MJ, Mahle WT. Racial and ethnic disparities in post-operative mortality following congenital heart surgery. J Pediatr 2011;159:222-6. http://dx.doi.org/10.1016/j.jpeds.2011.01.060.
- Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation 2001;103:2376-81. http://dx.doi.org/10.1161/01.CIR.103.19.2376.
- Nembhard WN, Xu P, Ethen MK, Fixler DE, Salemi JL, Canfield MA. Racial/ethnic disparities in timing of death during childhood among children with congenital heart defects. Birth Defects Res Part A Clin Mol Teratol 2013;97:628-40. http://dx.doi.org/10.1002/bdra.23169.
- Wang Y, Liu G, Druschel CM, Kirby RS. Maternal race/ethnicity and survival experience of children with congenital heart disease. J Pediatr 2013;163:1437-42.
- Gonzalez PC, Gauvreau K, Demone JA, Piercey GE, Jenkins KJ. Regional racial and ethnic differences in mortality for congenital heart surgery in children may reflect unequal access to care. Pediatr Cardiol 2003;24:103-8. http://dx.doi.org/10.1007/s00246-002-0244-y.
- Flores G, Tomany-Korman SC. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics 2008;121:e286-98. http://dx.doi.org/10.1542/peds.2007-1243.
- Sadiq M, Stumper O, Wright JG, De Giovanni JV, Billingham C, Silove ED. Influence of ethnic origin on the pattern of congenital heart defects in the first year of life. Br Heart J 1995;73:173-6. http://dx.doi.org/10.1136/hrt.73.2.173.
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407-29. http://dx.doi.org/10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L.
- Cummings P. Methods for estimating adjusted risk ratios. Stata J 2009;9:175-96.
- Saunders CL, Abel GA, El Turabi A, Ahmed F, Lyratzopoulos G. Accuracy of routinely recorded ethnic group information compared with self-reported ethnicity: evidence from the English Cancer Patient Experience survey. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002882.
- Statistical Bulletin: Gestation-Specific Infant Mortality, 2012. London: Office for National Statistics; 2015.
- Bittles AH. Consanguineous marriages and congenital anomalies. Lancet 2013;382:1316-17. http://dx.doi.org/10.1016/S0140-6736(13)61503-2.
- Parslow RC, Tasker RC, Draper ES, Parry GJ, Jones S, Chater T, et al. Epidemiology of critically ill children in England and Wales: incidence, mortality, deprivation and ethnicity. Arch Dis Child 2009;94:210-15. http://dx.doi.org/10.1136/adc.2007.134403.
- Bull C. Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. British Paediatric Cardiac Association. Lancet 1999;354:1242-7. http://dx.doi.org/10.1016/S0140-6736(99)01167-8.
- Peake JN, Knowles RL, Shawe J, Copp AJ. Pregnancy Outcomes for Neural Tube Defect Affected Pregnancies in Different Ethnic Groups in the UK n.d.
- Gardener D, Connolly H. 2001 Census: Ethnicity and Identity in the UK, Who are the ‘Other’ ethnic groups?. London: Office for National Statistics; 2005.
- Chou WY, Hunt YM, Beckjord EB, Moser RP, Hesse BW. Social media use in the United States: implications for health communication. J Med Internet Res 2009;11. http://dx.doi.org/10.2196/jmir.1249.
- The Social Skinny . 103 Crazy Social Media Statistics to Kick Off 2014 2014. http://thesocialskinny.com/103-crazy-media-statistics-to-kick-off-2014/ (accessed 23 March 2015).
- Gibbons MC, Casale CR. Reducing disparities in health care quality: the role of health IT in underresourced settings. MCRR 2010;67:155S-62S. http://dx.doi.org/10.1177/1077558710376202.
- Fawcett J, Buhle EL. Using the Internet for data collection. An innovative electronic strategy. Comput Nurs 1995;13:273-9.
- Curl M, Robinson D. Hand-held computers in clinical audit: a comparison with established paper and pencil methods. Int J Health Care Qual Assur 1994;7:16-20. http://dx.doi.org/10.1108/09526869410059709.
- Lakeman R. Using the Internet for data collection in nursing research. Comput Nurs 1997;15:269-75.
- Kramish Campbell M, Meier A, Carr C, Enga Z, James AS, Reedy J, et al. Health behavior changes after colon cancer: a comparison of findings from face-to-face and on-line focus groups. Fam Community Health 2001;24:88-103. http://dx.doi.org/10.1097/00003727-200110000-00010.
- Hsiung RC. The best of both worlds: an on-line self help group hosted by a mental health professional. Cyberpsychol Behavior 2000;3:935-50. http://dx.doi.org/10.1089/109493100452200.
- Anderson T, Kanuka H. On-line forums: new platforms for professional development and group collaboration. J Comput-Mediat Commun 1997;3. http://dx.doi.org/10.1111/j.1083-6101.1997.tb00078.x.
- Kratz L, Uding N, Trahms CM, Villareale N, Kieckhefer GM. Managing childhood chronic illness: parent perspectives and implications for parent–provider relationships. Fam Syst Health 2009;27:303-13. http://dx.doi.org/10.1037/a0018114.
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008;18:1075-83. http://dx.doi.org/10.1177/1049732308320112.
- Howe TH, Sheu CF, Wang TN, Hsu YW. Parenting stress in families with very low birth weight preterm infants in early infancy. Res Dev Disabil 2014;35:1748-56. http://dx.doi.org/10.1016/j.ridd.2014.02.015.
- Nightingale R, Sinha MD, Swallow V. Using focused ethnography in paediatric settings to explore professionals’ and parents’ attitudes towards expertise in managing chronic kidney disease stage 3–5. BMC Health Serv Res 2014;14. http://dx.doi.org/10.1186/1472-6963-14-403.
- Smith J, Cheater F, Bekker H. Parents’ experiences of living with a child with hydrocephalus: a cross-sectional interview-based study. Health Expect 2013;18:1709-20. http://dx.doi.org/10.1111/hex.12164.
- Vrijmoet-Wiersma CM, Ottenkamp J, van Roozendaal M, Grootenhuis MA, Koopman HM. A multicentric study of disease-related stress, and perceived vulnerability, in parents of children with congenital cardiac disease. Cardiol Young 2009;19:608-14. http://dx.doi.org/10.1017/S1047951109991831.
- Lawoko S, Soares JJ. Psychosocial morbidity among parents of children with congenital heart disease: a prospective longitudinal study. Heart Lung 2006;35:301-14. http://dx.doi.org/10.1016/j.hrtlng.2006.01.004.
- Hearps SJ, McCarthy MC, Muscara F, Burke K, Jones B, Anderson VA. Psychosocial risk in families of infants undergoing surgery for a serious congenital heart disease. Cardiol Young 2014;24:632-9. http://dx.doi.org/10.1017/S1047951113000760.
- Torowicz D, Irving SY, Hanlon AL, Sumpter DF, Medoff-Cooper B. Infant temperament and parental stress in 3-month-old infants after surgery for complex congenital heart disease. J Dev Behav Pediatr 2010;31:202-8. http://dx.doi.org/10.1097/DBP.0b013e3181d3deaa.
- Fischer AL, Butz C, Nicholson L, Blankenship A, Dyke P, Cua CL. Caregiver anxiety upon discharge for neonates with congenital heart disease. Congenit Heart Dis 2012;7:41-5. http://dx.doi.org/10.1111/j.1747-0803.2011.00600.x.
- Helfricht S, Latal B, Fischer JE, Tomaske M, Landolt MA. Surgery-related posttraumatic stress disorder in parents of children undergoing cardiopulmonary bypass surgery: a prospective cohort study. Pediatr Crit Care Med 2008;9:217-23. http://dx.doi.org/10.1097/PCC.0b013e318166eec3.
- Fallows D. How Women and Men Use the Internet n.d. www.pewtrusts.org/uploadedFiles/wwwpewtrustsorg/Reports/Society_and_the_Internet/PIP_Women_Men_122805.pdf (accessed 30 April 2015).
- Im EO, Chee W. An online forum as a qualitative research method: practical issues. Nurs Res 2006;55:267-73. http://dx.doi.org/10.1097/00006199-200607000-00007.
- Tregay J, Brown KL, Crowe S, Bull C, Knowles RL, Smith L, et al. Signs of deterioration in infants discharged home following congenital heart surgery in the first year of life: a qualitative study. Arch Dis Childhood 2016. http://dx.doi.org/10.1136/archdischild-2014-308092.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. JOAAG 2009;4:72-9.
- Counting the Costs 2012: The Financial Reality for Families with Disabled Children across the UK. London: Contact a Family; 2012.
- Moola FJ. ‘This is the best fatal illness that you can have’: contrasting and comparing the experiences of parenting youth with cystic fibrosis and congenital heart disease. Qual Health Research 2012;22:212-25. http://dx.doi.org/10.1177/1049732311421486.
- Goldberg S, Morris P, Simmons RJ, Fowler RS, Levison H. Chronic illness in infancy and parenting stress: a comparison of three groups of parents. J Pediatr Psychol 1990;15:347-58. http://dx.doi.org/10.1093/jpepsy/15.3.347.
- Wray J, Maynard L. Living with congenital or acquired cardiac disease in childhood: maternal perceptions of the impact on the child and family. Cardiol Young 2005;15:133-40. http://dx.doi.org/10.1017/S1047951105000302.
- Lawoko S, Soares JJ. Distress and hopelessness among parents of children with congenital heart disease, parents of children with other diseases, and parents of healthy children. J Psychosom Res 2002;52:193-208. http://dx.doi.org/10.1016/S0022-3999(02)00301-X.
- Mussatto K. Adaptation of the child and family to life with a chronic illness. Cardiol Young 2006;16:110-16. http://dx.doi.org/10.1017/S104795110600103X.
- Hartman DM, Medoff-Cooper B. Transition to home after neonatal surgery for congenital heart disease. MCN Am J Matern Child Nurs 2012;37:95-100. http://dx.doi.org/10.1097/NMC.0b013e318241dac1.
- Tregay J, Wray J, Crowe S, Knowles R, Daubeney P, Franklin R, et al. Going home after infant cardiac surgery: a UK qualitative study. Arch Dis Child 2016;101:320-5. http://dx.doi.org/10.1136/archdischild-2015-30882.
- Bradman K, Borland M, Pascoe E. Predicting patient disposition in a paediatric emergency department. J Paediatr Child Health 2014;50:E39-44. http://dx.doi.org/10.1111/jpc.12011.
- EACTS Congenital Database Gold Standards. Warsaw, Poland: Children’s Memorial Health Institute; 2013.
- Reubsaet A, Lechner L, De Vries H. The Dutch cancer information helpline: more critical patients after 10 years. Patient Educ Couns 2006;63:215-22. http://dx.doi.org/10.1016/j.pec.2005.10.011.
- Hardyman R, Hardy P, Brodie J, Stephens R. It’s good to talk: comparison of a telephone helpline and website for cancer information. Patient Educ Couns 2005;57:315-20. http://dx.doi.org/10.1016/j.pec.2004.08.009.
- Ekberg K, McDermott J, Moynihan C, Brindle L, Little P, Leydon GM. The role of helplines in cancer care: intertwining emotional support with information or advice-seeking needs. J Psychosoc Oncol 2014;32:359-81. http://dx.doi.org/10.1080/07347332.2014.897294.
- Venn MJ, Darling E, Dickens C, Quine L, Rutter DR, Slevin ML. The experience and impact of contacting a cancer information service. Eur J Cancer Care (Engl) 1996;5:38-42. http://dx.doi.org/10.1111/j.1365-2354.1996.tb00204.x.
- Collett A, Kent W, Swain S. The role of a telephone helpline in provision of patient information. Nurs Stand 2006;20:41-4. http://dx.doi.org/10.7748/ns2006.04.20.32.41.c4125.
- Brown K, Knowles R, Bull C, Daubeney P, Wray J, Barron D, et al. Infant Deaths in the UK Community Following Successful Cardiac Surgery – Building Evidence Base for Optimal Surveillance [Protocol] n.d. www.nets.nihr.ac.uk/__data/assets/pdf_file/0009/81729/PRO-10-2002-29.pdf.
Appendix 1 Electronic search strategy for risk factors systematic review
The Cochrane Library (1999 to present), MEDLINE (1980 to present), EMBASE (1980 to present), CINAHL (1981 to present), Web of Knowledge (1980 to present) and PsycINFO (1980 to present) databases will be searched using the following search strategy (MEDLINE search strategy):
-
neonate OR newborn OR child* OR infant* OR baby OR babies OR pediatric* OR paediatric*.ti,ab,sh
-
exp. CONGENITAL ABNORMALITIES
-
‘congenital abnormalit*’ OR ‘birth defect*’ OR ‘congenital malformation*’ OR ‘congenital anomal*’
-
2 OR 3
-
1 AND 4
-
exp SURGICAL PROCEDURES, OPERATIVE
-
surgery OR ‘surgical procedure*’ OR ‘surgical intervention’ OR operation* OR ‘shunt’ OR ‘arterial shunt’ OR Norwood OR ‘arterial switch’ OR ‘truncus repair’ OR ‘interrupted adj3 arch repair’ OR ‘complete adj3 atrioventricular septal defect repair’ OR ‘complete AVSD repair’ OR ‘pulmonary artery band’ OR ‘total anomalous pulmonary venous adj2 repair’ OR ‘tetralogy adj3 fallot repair’ OR ‘coarctation repair’ OR ‘ventricular septal defect repair’ OR ‘VSD repair’ OR ‘diaphragmatic hernia repair’ OR ‘pulmonary adj5 lobectomy’ OR ‘tracheal resection’ OR ‘tracheal stenosis repair’ OR ‘oesophageal atresia repair’ OR ‘esophageal atresia repair’ OR ‘duodenal atresia repair’ OR ‘anal atresia repair’ OR ‘Ladd* procedure’ OR ‘malrotation adj5 repair’ OR ‘omphalocoele repair’ OR ‘omphalocele repair’ OR ‘gastroschisis repair’ OR ‘spina bifida adj2 repair’ OR ‘Myelomeningocele adj2 repair’ OR ‘Kasai procedure’ OR ‘Kasai portoenterostomy’.ti,ab,sh
-
6 OR 7
-
5 AND 8
-
community OR home OR discharge*.ti,ab,sh
-
9 AND 10
-
morbidity OR mortality OR death OR ‘adverse outcome’ OR readmi*.ti,ab,sh
-
11 AND 12
-
exp. AMBULATORY SURGICAL PROCEDURES
-
‘minor surgery’ OR ‘day surgery’ OR ‘day case’.ti,ab,sh
-
14 OR 15
-
13 NOT 16
-
‘injury’.ti,ab
-
17 NOT 18
Appendix 2 Evidence quality assessment framework
This appendix has been reproduced from Morley PT. Quality Assessment For Individual Studies To Be Used For The Review Of Resuscitation Science For 2010. American Heart Association International Liaison Committee on Resuscitation (ILCOR), 2008. URL: www.heart.org/idc/groups/heart-public/@wcm/@private/@ecc/documents/downloadable/ucm_308201.pdf (accessed 29 August 2014). Reproduced with permission.
| LOEs in order of strength: |
|---|
| LOE P1: inception (prospective) cohort studies (or meta-analyses of inception cohort studies), or validation of a Clinical Decision Rule (CDR) At study inception, the cohort is either non-diseased or all at the same stage of the disease or where groups of people (cohorts) are observed at a point in time to be exposed or not exposed to an intervention (or the factor under study) and then are followed prospectively with further outcomes recorded as they happen |
| LOE P2: follow-up of untreated control groups in RCTs (or meta-analyses of follow-up studies), or derivation of CDR, or validated on split-sample only |
| LOE P3: retrospective cohort studies Where the cohorts (groups of people exposed and not exposed-including surgical case series) are defined at a point of time in the past and information collected on subsequent outcomes |
| LOE P4: studies without a control group (e.g. case series) A single group of people exposed to the intervention (factor under study). Only outcomes after the intervention (factor under study) are recorded in the series of people, so no comparisons can be made |
After being categorised according to the levels of evidence above, studies were assessed for methodological quality according to the presence of particular quality items relevant to that particular level of evidence:
-
the study has most/all of the relevant quality items
-
the study has some of the relevant quality items
-
the study has few of the relevant quality items (but is of sufficient value to include in the review).
Level of evidence P1
Level of evidence P1: quality assessment for inception (prospective) cohort studies (or meta-analyses of inception cohort studies), or validation of a Clinical Decision Rule (CDR)
| Were comparison groups clearly defined? | Yes/no | |||
| Were outcomes measured in the same (preferably blinded), objective way in both groups? | Yes/no | |||
| Were known confounders identified and appropriately controlled for? | Yes/no | |||
| Was follow-up of patients sufficiently long and complete (e.g. > 80%) | Yes/no | |||
| Quality of evidence (please circle) | A (4 factors) | B (3 factors) | C (≤ 2 factors) | |
Level of evidence P1: quality assessment for meta-analyses of inception/prospective cohort studies
A meta-analyses of these types of studies is also allocated a LOE P1.
| Were specific objectives of the review stated (based on a specific clinical question in which patient, intervention, comparator, outcome (PICO) were specified) | Yes/no | |||
| Was the study design defined? | Yes/no | |||
| Were selection criteria stated for studies to be included (based on trial design and methodological quality)? | Yes/no | |||
| Were inclusive searches undertaken (using appropriately crafted search strategies)? | Yes/no | |||
| Were characteristics and methodological quality of each trial identified? | Yes/no | |||
| Were selection criteria applied and a log of excluded studies with reasons for exclusion reported? | Yes/no | |||
| Quality of evidence (please circle) | A (most of/all relevant quality items) | B (some relevant quality items) | C (few/no relevant quality items) | |
Level of evidence P2
Level of evidence P2: quality assessment for studies involving follow-up of untreated control groups in randomised controlled trials (or meta-analyses of follow-up studies), or derivation of Clinical Decision Rule, or validated on split-sample only
| Were comparison groups clearly defined? | Yes/no | |||
| Were outcomes measured in the same (preferably blinded), objective way in both groups? | Yes/no | |||
| Were known confounders identified and appropriately controlled for? | Yes/no | |||
| Was follow-up of patients sufficiently long and complete (e.g. > 80%) | Yes/no | |||
| Quality of evidence (please circle) | A (4 factors) | B (3 factors) | C (≤ 2 factors) | |
Level of evidence P2: quality assessment for meta-analyses of follow-up studies
A meta-analyses of these types of studies is also allocated a level of evidence P2
| Were specific objectives of the review stated (based on a specific clinical question in which patient, intervention, comparator, outcome (PICO) were specified) | Yes/no | |||
| Was the study design defined? | Yes/No | |||
| Were selection criteria stated for studies to be included (based on trial design and methodological quality)? | Yes/No | |||
| Were inclusive searches undertaken (using appropriately crafted search strategies)? | Yes/NO | |||
| Were characteristics and methodological quality of each trial identified? | Yes/NO | |||
| Were selection criteria applied and a log of excluded studies with reasons for exclusion reported? | YES/NO | |||
| Quality of evidence (please circle) | A (most/all relevant quality items) | B (some relevant quality items) | C (few/no relevant quality items) | |
Level of evidence P3
Level of evidence P3: quality assessment for retrospective cohort studies
| Were comparison groups clearly defined? | Yes/no | |||
| Were outcomes measured in the same (preferably blinded), objective way in both groups? | Yes/no | |||
| Were known confounders identified and appropriately controlled for? | Yes/no | |||
| Was follow-up of patients sufficiently long and complete? | Yes/no | |||
| Quality of evidence (please circle) | A (4 factors) | B (3 factors) | C (≤ 2 factors) | |
Level of evidence P4
Level of evidence P4: quality assessment for case series
| Were outcomes measured in an objective way? | Yes/no | |||
| Were known confounders identified and appropriately controlled for? | Yes/no | |||
| Was follow-up of patients sufficiently long and complete (e.g. > 80%)? | Yes/no | |||
| Quality of evidence (please circle) | A (3 factors) | B (2 factors) | C (≤ 1 factors) | |
Appendix 3 Electronic search strategy for interventions systematic review
The Cochrane Library (1999 to present), MEDLINE (1980 to present), EMBASE (1980 to present), CINAHL (1981 to present), Web of Knowledge (dates 1980 to present) and PsycINFO (1980 to present) databases will be searched using the following search strategy (in MEDLINE format):
-
neonate OR newborn OR child* OR infant* OR baby OR babies OR pediatric* OR paediatric*.ti,ab,sh
-
exp. CONGENITAL ABNORMALITIES
-
‘congenital abnormalit*’ OR ‘birth defect*’ OR ‘congenital malformation*’ OR ‘congenital anomal*’.ti,ab,sh
-
2 OR 3
-
1 AND 4
-
exp HOME CARE
-
exp TELEMEDICINE
-
‘community intervention’ OR ‘monitoring’ OR ‘outreach’ OR ‘surveillance’ OR ‘telemedicine’ OR ‘tele-medicine’ OR ‘telecare’ OR ‘tele-care’ OR ‘telehealth’.ti,ab,sh
-
6 OR 7 OR 8
-
morbidity OR mortality OR death OR ‘adverse outcome’ OR readmi*.ti,ab,sh
-
9 AND 10
Appendix 4 Record of data search carried out within NCHDA and the trusted third-party site
FIGURE 10.
Record of data search carried out within NCHDA and the trusted third-party site (final step) in order to generate the data set sent to the research team. CCAD, Central Cardiac Audit Database. n denotes the number of procedures.
Appendix 5 Specific procedure hierarchy and groupings used for risk model and CART analyses in Chapter 4
Reproduced from Crowe et al. , 2016. 67 © 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell. This is an open access article under the terms of the Creative Commons Attribution-Non Commercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
| Specific procedurea | NCHDA hierarchyb | Procedure groupc |
|---|---|---|
| Norwood | 1 | Palliative |
| Heart transplant | 2 | Palliative |
| TAPVC repair + arterial shunt | 3 | Palliative |
| Fontan procedure | 4 | Palliative |
| Bidirectional cavopulmonary shunt | 5 | Palliative |
| Senning or Mustard procedure | 6 | Palliative |
| Truncus and interruption repair | 7 | Corrective |
| Truncus arteriosus repair | 8 | Corrective |
| Tricuspid valve replacement | 9 | Ungrouped |
| Interrupted aortic arch repair | 10 | Corrective |
| Multiple VSD closure | 11 | Corrective |
| Mitral valve replacement | 12 | Corrective |
| Repair of TAPVC | 13 | Corrective |
| AVSD and tetralogy repair | 14 | Corrective |
| AVSD (complete) repair | 15 | Corrective |
| AVSD (partial) repair | 16 | Corrective |
| Aortic valvotomy (surgical) | 17 | Corrective |
| Aortic valvoplasty | 18 | Corrective |
| Anomalous coronary artery repair | 19 | Corrective |
| Cor triatriatum repair | 20 | Corrective |
| Arterial switch + VSD closure | 21 | Corrective |
| Arterial switch (for isolated transposition) | 22 | Corrective |
| PA VSD repair | 23 | Corrective |
| Pulmonary valve replacement | 24 | Corrective |
| Tetralogy with absent pulmonary valve repair | 25 | Corrective |
| Tetralogy repair | 26 | Corrective |
| Isolated coarctation repair | 27 | Corrective |
| Aortic valve replacement – non-Ross | 28 | Corrective |
| Supravalvar aortic stenosis repair | 29 | Corrective |
| Rastelli procedure | 30 | Corrective |
| Aortic valve replacement – Ross | 31 | Corrective |
| Aortic root replacement (not Ross) | 32 | Corrective |
| Subvalvar aortic stenosis repair | 33 | Corrective |
| Aortopulmonary window repair | 34 | Corrective |
| ASD repair | 35 | Corrective |
| VSD repair | 36 | Corrective |
| Arterial shunt | 37 | Palliative |
| Isolated pulmonary artery band | 38 | Palliative |
| PDA ligation (surgical) | 39 | Corrective |
| Transcatheter pulmonary valve replacementd | 40 | Excluded |
| VSD closure (catheter)d | 41 | Excluded |
| Aortic balloon valvotomy | 42 | Corrective |
| Coarctation angioplasty | 43 | Corrective |
| Pulmonary artery stentingd | 44 | Excluded |
| ASD closure (catheter)d | 45 | Excluded |
| PDA closure (catheter) | 46 | Corrective |
| Recoarctation angioplastyd | 47 | Excluded |
| Pulmonary balloon valvoplasty | 48 | Corrective |
| Blade atrial septostomyd | 49 | Excluded |
| Coarctation stenting | 50 | Corrective |
| PFO closure (catheter)d | 51 | Excluded |
| Pulmonary valvotomy (radiofrequency) | 52 | Corrective |
| Duct stenting | 53 | Palliative |
| RVOT stenting | 54 | Palliative |
| Radiofrequency ablation for supraventricular tachycardiad | 55 | Excluded |
| Implantable cardioverter defibrillatord | 56 | Excluded |
| Minor and excluded proceduresd | 57 | Excluded |
| Not a specific procedure: surgicale | 58 | Ungrouped |
| Not a specific procedure: catheterd | 59 | Excluded |
Appendix 6 Cardiac diagnosis hierarchy and groupings used for risk model and CART analyses in Chapter 4
Reproduced from Crowe et al. , 2016. 67 © 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell. This is an open access article under the terms of the Creative Commons Attribution-Non Commercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Within the NCHDA data set, each interventional procedure can be described by up to six individual diagnostic International Paediatric and Congenital Cardiac Codes (IPCCCs). The combination of these can be mapped to 1 of 24 primary cardiac diagnoses using a hierarchical scheme developed by Brown et al. 75 For the purposes of this study, this mapping scheme was implemented with two minor adjustments: a new category of ‘arrhythmia’ was created (ranked 24th in the modified hierarchy) and the ‘miscellaneous congenital’ diagnostic category was split into ‘major miscellaneous diagnoses’ (ranked ninth in the modified hierarchy) and ‘minor miscellaneous diagnoses’ (ranked 25th in the modified hierarchy).
| Primary cardiac diagnosis category | Hierarchy ranka | Cardiac diagnosis groupb |
|---|---|---|
| HLHS | 1 | HLHS |
| Functionally univentricular heart | 2 | UVH or PA + IVS |
| Common arterial trunk (truncus arteriosus) | 3 | Other |
| TGA + VSD/DORV-TGA type | 4 | Other |
| Interrupted aortic arch | 5 | Other |
| TGA (concordant AV and discordant VA connections) and intact ventricular septum | 6 | Other |
| PA with an intact ventricular septum | 7 | UVH or PA + IVS |
| PA + VSD (including Fallot type) | 8 | Other |
| Miscellaneous congenital primary diagnoses | 9 | Other |
| AVSD | 10 | Other |
| Fallot/DORV-Fallot type | 11 | Other |
| Aortic valve stenosis (isolated) | 12 | Other |
| Tricuspid valve abnormality (including Ebstein’s) | 13 | Other |
| Mitral valve abnormality (including supravalvar, subvalvar) | 14 | Other |
| TAPVC | 15 | Other |
| Aortic arch obstruction ± VSD/ASD | 16 | Other |
| Pulmonary stenosis | 17 | Other |
| Subaortic stenosis (isolated) | 18 | Other |
| Aortic regurgitation | 19 | Other |
| VSD | 20 | VSD |
| Interatrial communication (ASD) | 21 | Other |
| Patent ductus arteriosus | 22 | Other |
| Acquired | 23 | Other |
| Arrhythmia | 24 | Other |
| Miscellaneous congenital terms | 25 | Other |
| Non-cardiac or uncoded diagnosis | 26 | Other |
Appendix 7 Non-cardiac diagnosis and comorbidity information used for risk model and CART analyses in Chapter 4
Reproduced from Crowe et al. , 2016. 67 © 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell. This is an open access article under the terms of the Creative Commons Attribution-Non Commercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
| Category number | Category | Number of codes | Examples of included clinical conditions |
|---|---|---|---|
| 1 | Acquired endocrine, nutritional and metabolic conditions | 82 | Diabetes mellitus, alpha-1-antitrypsin disorder, rickets, failure to thrive |
| 2 | Acquired gastrointestinal (digestive system) conditions | 166 | Gastritis, constipation, liver failure, hernia, jaundice, perianal fistula |
| 3 | Acquired infections (in any system except respiratory infections which are included within the category for acquired respiratory system conditions) | 144 | Cytomegalovirus, Escherichia coli infection, MRSA, meningitis, otitis media, wound abscess |
| 4 | Conditions related to haematology, oncology or immunology, which may be acquired or congenital | 97 | Acute myeloid leukaemia, factor VIII deficiency, teratoma, sickle cell anaemia |
| 5 | Acquired musculoskeletal, connective tissue or skin conditions | 29 | Atopic dermatitis, scoliosis, systemic onset juvenile chronic arthritis |
| 6 | Acquired genitourinary system conditions | 42 | Acute renal failure, hydronephrosis, rectovaginal fistula |
| 7 | Acquired respiratory system conditions | 229 | Stridor, asthma, bronchiolitis, pulmonary oedema, pneumonia, haemothorax |
| 8 | Conditions originating in, or specific to, the perinatal period | 109 | Birth asphyxia, gestational diabetes, meconium ileus, shoulder dystocia |
| 9 | Non-cardiac intervention or operation, excluding procedures that are part of routine intensive care | 478 | Adenoidectomy, bone marrow transplant, splenectomy, plication of diaphragm |
| 10 | Collapse or cardiac arrest | 14 | Cardiac arrest, hypovolaemic shock, fainting, respiratory arrest |
| 11 | Acquired injury or complication of surgery/other condition | 145 | Brain injury, anaesthetic shock, closed rib fracture, vocal cord palsy, limb ischaemia |
| 12 | Congenital heart disease or cardiac procedures | 875 | Heart transplant, cardiac pacemaker, catheter procedure, Ebstein’s anomaly |
| 13 | Congenital anomalies (all severity) | 342 | Trisomy 18, Pierre Robin syndrome, cleft palate, club foot, oesophageal atresia |
| 14 | Neurological or neurodevelopmental conditions – may be congenital or acquired | 126 | Cataract, cerebral palsy, autistic spectrum disorder, epilepsy, optic atrophy |
| 15 | Additional codes which are non-specific or do not have standardised coding | 429 | Family history of hypothyroidism, child in foster care, central line feeding |
| 16 | Premature birth (< 37 completed weeks’ gestation) | 11 | Baby born premature/very premature |
| 17 | Supportive procedures | 7 | ECMO, ventricular assist device |
Non-cardiac diagnosis and comorbidity information was primarily sourced from PICANet, in which any given PICU admission can record up to 24 Read Codes (a clinical coding system used as standard in general practice in the UK and maintained by the Health and Social care Information Centre). In total, 3325 discrete Read Codes were present in the data set and so to explore the potential for this information to add discriminatory power to the risk model, we developed a new scheme linking each code to at most 1 of 17 system-based categories (see Appendix 6).
Appendix 8 Incidence of individual CHD subgroups by ethnic group (infants operated between 2006 and 2009)
| Primary cardiac diagnosis group | Ethnic group, sample size (population) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White, n = 3968 (N = 2,230,400) | Asian, n = 604 (N = 220,100) | Black, n = 240 (N = 93,700) | Chinese, n = 22 (N = 19,700) | Mixed, n = 146 (N = 151,500) | Other, n = 104 (N = 14,300) | |||||||||||||
| n | IR | 95% CI | n | IR | 95% CI | n | IR | 95% CI | n | IR | 95% CI | n | IR | 95% CI | n | IR | 95% CI | |
| HLHS | 231 | 1.0 | 0.9 to 1.2 | 30 | 1.4 | 1.0 to 1.9 | 22 | 2.3 | 1.5 to 3.6 | 0 | – | – | 6 | 0.4 | 0.2 to 0.9 | 8 | 5.6 | 2.4 to 11.0 |
| Functionally UVH | 193 | 0.9 | 0.7 to 1.0 | 41 | 1.9 | 1.3 to 2.5 | 16 | 1.7 | 1.0 to 2.8 | 0 | – | – | 7 | 0.5 | 0.2 to 1.0 | < 5 | – | – |
| Truncus arteriosus | 72 | 0.3 | 0.3 to 0.4 | 8 | 0.4 | 0.2 to 0.7 | < 5 | – | – | < 5 | – | – | < 5 | – | – | < 5 | – | – |
| TGA with VSD (DORV-TGA) | 354 | 1.6 | 1.4 to 1.8 | 44 | 2.0 | 1.5 to 2.7 | 11 | 1.2 | 0.6 to 2.1 | < 5 | – | – | 9 | 0.6 | 0.3 to 1.1 | 14 | 9.8 | 5.4 to 16.4 |
| Interrupted aortic arch | 51 | 0.2 | 0.2 to 0.3 | 5 | 0.2 | 0.1 to 0.5 | < 5 | – | – | < 5 | – | – | < 5 | – | – | 0 | – | – |
| TGA and IVS | 128 | 0.6 | 0.5 to 0.7 | 27 | 1.2 | 0.8 to 1.8 | < 5 | – | – | < 5 | – | – | < 5 | – | – | 7 | 4.9 | 2.0 to 10.1 |
| PA and IVS | 104 | 0.5 | 0.4 to 0.6 | 22 | 1.0 | 0.6 to 1.5 | < 5 | – | – | 0 | – | – | < 5 | – | – | < 5 | – | – |
| PA and VSD | 129 | 0.6 | 0.4 to 0.7 | 23 | 1.0 | 0.7 to 1.6 | 9 | 1.0 | 0.4 to 1.8 | 0 | – | – | 9 | 0.6 | 0.3 to 1.1 | 6 | 4.2 | 1.5 to 9.1 |
| Miscellaneous primary CHDs | 222 | 1.0 | 0.9 to 1.1 | 33 | 1.5 | 1.0 to 2.1 | 11 | 1.2 | 0.6 to 2.1 | 0 | – | – | 10 | 0.7 | 0.3 to 1.2 | < 5 | – | – |
| AVSD | 360 | 1.7 | 1.7 to 1.8 | 36 | 1.6 | 1.1 to 2.3 | 37 | 3.9 | 2.8 to 5.4 | 0 | – | – | 14 | 0.9 | 0.5 to 1.6 | 9 | 6.3 | 2.9 to 11.9 |
| Tetralogy of Fallot | 416 | 1.9 | 1.7 to 2.1 | 80 | 3.6 | 2.9 to 4.5 | 16 | 1.7 | 1.0 to 2.8 | < 5 | – | – | 14 | 0.9 | 0.5 to 1.6 | 8 | 5.6 | 2.4 to 11.0 |
| AS | 106 | 0.5 | 0.4 to 0.6 | 8 | 0.4 | 0.2 to 0.7 | < 5 | – | – | 0 | – | – | < 5 | – | – | < 5 | – | – |
| Tricuspid valve insufficiency | 35 | 0.2 | 0.1 to 0.2 | 5 | 0.2 | 0.1 to 0.5 | < 5 | – | – | < 5 | – | – | < 5 | – | – | < 5 | – | – |
| Mitral valve abnormalities | 38 | 0.2 | 0.1 to 0.2 | 7 | 0.3 | 0.1 to 0.7 | < 5 | – | – | < 5 | – | – | < 5 | – | – | < 5 | – | – |
| TAPVC | 90 | 0.4 | 0.3 to 0.5 | 20 | 0.9 | 0.6 to 1.4 | 5 | 0.5 | 0.2 to 1.2 | < 5 | – | – | < 5 | – | – | < 5 | – | – |
| Aortic arch obstruction | 467 | 2.1 | 1.9 to 2.3 | 54 | 2.5 | 1.8 to 3.2 | 19 | 2.0 | 1.2 to 3.2 | 0 | – | 12 | 0.8 | 0.4 to 1.4 | 7 | 4.9 | 2.0 to 10.1 | |
| Pulmonary stenosis | 143 | 0.6 | 0.5 to 0.8 | 12 | 0.5 | 0.3 to 0.9 | 7 | 0.7 | 0.3 to 1.5 | < 5 | – | – | < 5 | – | – | < 5 | – | – |
| Subaortic stenosis | 6 | < 0.1 | < 5 | – | – | – | 0 | – | – | 0 | – | – | < 5 | – | – | < 5 | – | – |
| Aortic regurgitation | 7 | < 0.1 | < 5 | – | – | – | 0 | – | – | 0 | – | – | 0 | – | – | 0 | – | – |
| VSD | 661 | 3.0 | 2.7 to 3.2 | 111 | 5.0 | 4.1 to 6.1 | 55 | 5.9 | 4.4 to 7.6 | 6 | 3.0 | 1.1 to 6.6 | 27 | 1.8 | 1.2 to 2.6 | 22 | 15.4 | 9.6 to 23.3 |
| ASD | 40 | 0.2 | 0.1 to 0.2 | 12 | 0.5 | 0.3 to 0.9 | < 5 | – | – | < 5 | – | – | 6 | 0.4 | 0.2 to 0.9 | 0 | – | – |
| PDA | 74 | 0.3 | 0.3 to 0.4 | 19 | 0.9 | 0.5 to 1.3 | 6 | 0.6 | 0.2 to 1.4 | < 5 | – | – | < 5 | – | – | < 5 | – | – |
| Miscellaneous congenital | 41 | 0.2 | 0.1 to 0.2 | < 5 | – | – | < 5 | – | – | 0 | – | – | < 5 | – | – | < 5 | – | – |
Appendix 9 Notes from the ‘Infant Heart Study Parent Workshop’ (19 July 2014)
Flip chart 1: what do parents want information about? (partial list)
-
Diagnosis.
-
Surgery.
-
Feeding.
-
How is the condition going to affect normal life?
-
Access to knowledge/troubleshooting [e.g. who to ask (named person)]; someone who can act as ‘back up’ both in and out of hospital and help parents to feel empowered.
-
Education for the local team.
-
Having criteria milestones to aim for.
-
Specialists focusing only on the heart – need to share information with the appropriate teams if they pick up something else.
Flip chart 2: how do parents decide who to contact when they have a problem?
-
Depends on the problem (e.g. if think it’s cardiac – CLN or if not then the community nurse).
-
Knowledge – someone they have confidence in and believe has the knowledge needed to deal with the problem and advise them appropriately.
-
Someone who is going to act in the best interests of their child and who can connect them to the right person.
-
Someone they believe in.
-
A&E – not valuable/helpful, but sometimes is the only choice.
-
Did not know who to contact (lost link with CLN team due to problems with handover).
-
Open access at local hospital – very helpful.
Flip chart 3: seeking help and feedback about the traffic-light system
-
What is normal for your child? – liked this.
-
Need someone refreshing your memory about symptoms on a regular basis – can be easy to forget.
-
Checklist as a ‘passport’ to getting access to the right support.
-
Scary time and worry that you are not going to notice the signs – good to have a list/guide.
-
Good to have something more structured to give credibility to what parents say about symptoms (e.g. ‘I know something is wrong but I’m not quite sure what it is – the chart says I needed to bring him to see you . . .’)
-
Need to make the top line re: ‘what to do’ look different from the symptoms – break this up from the rest.
-
Have a sheet in the red book with info about the condition and signs/symptoms/traffic light, etc. (like there is for Down Syndrome).
-
Give traffic lights to GP.
-
Good to have something separate from the rest of the information – self-contained info just about what to look for and what to do – just the information that is REALLY important.
-
Would need someone to go over it a week/few days before discharge and repeated several times – not just before when parents just want to get home.
-
Could give them a chance to practice with the traffic-light system while still in hospital.
-
Could bring it to appointments to help review how baby has been and document any deterioration.
-
Portable sats machine at home –? Different opinions among parents re: helpful/not helpful.
-
Could be bound together as a diary.
-
Traffic lights as a magnet for the fridge?
-
One parent had something very similar from the Lullaby Trust – bound diary and sheet for red book – very easy to use and reassuring.
Appendix 10 Terms of reference and composition of the final intervention development meeting as referred to in Chapter 10
Composition of the subgroup
The composition of the subgroup was designed to reflect the range of professions and sectors that provide support to babies and their families following discharge from heart surgery in the first year of life, including specialist centres, primary and secondary care providers, community health services and charitable organisations. The subgroup consists of selected members of the Intervention Development Group, co-applicants on the IHS and invited representatives from other professions who have had previous involvement in IHS.
-
Dr Nick Barnes, Consultant PEC (Northampton General Hospital).
-
Hannah Charrot, Community Children’s Nurse (Cambridgeshire Community Services NHS Trust).
-
Jan Pennington, HV (Barts Health NHS Trust).
-
Sally Hull, GP and Reader in Primary Care (Jubilee Street Practice, NHS East London and the City).
-
Rodney Franklin, Consultant Paediatric Cardiologist (The Royal Brompton Hospital).
-
Liz Smith, Lead Advanced Nurse Practitioner (Great Ormond Street Hospital for Children NHS Trust).
-
Suzie Hutchinson, Parent/Patient Representative (Chief Executive, Little Heart Matter).
-
Kate Bull, Acting Principal Investigator on the Infant Heart Project (Great Ormond Street Hospital for Children NHS Trust).
Facilitator
Sonya Crowe, Operational Researcher (University College London).
Minutes
Rachel Knowles, Paediatric Epidemiologist (UCL Institute of Child Health).
Observers
Jo Wray, Health Psychologist (Great Ormond Street Hospital); Simon Turner, Health Services Researcher (University College London).
Background to the subgroup
Over the last 2 years, the IHS [funded by the National Institute for Health Research (NIHR), NIHR project number 10/2002/29] has been gathering evidence about the barriers to accessing, and challenges to providing, support for infants and their families following discharge from cardiac surgery in the first year of life. It has also generated evidence to inform the identification of high risk infants based on factors available to clinicians at the time of discharge. This evidence base was intended to inform recommendations for service improvements aimed at reducing adverse events (death in the community or emergency readmission within the first year post discharge) in this patient population.
The Intervention Development Group has met on five occasions to review and critique the evidence gathered from the study and to propose service improvements to address the challenges and barriers identified in the data. Subsequently, a workshop was held for families of babies that had experienced an adverse event in order to elicit their suggestions for service improvements to address these challenges too.
The study research team has drafted a set of data-driven ‘service challenges’ linked to ‘recommended service improvements’ using the gathered evidence and proposals from the Family Workshop and Intervention Development Group.
The subgroup’s responsibilities
Building on the previous work of the Intervention Development Group and the IHS research team, the subgroup has been convened in order to assess the draft recommendations for service improvements and agree upon a final set that they will propose to the wider Intervention Development Group for endorsement.
More specifically, the subgroup is tasked with the following:
-
to review the draft recommendations – assess the feasibility and acceptability of each draft recommendation for service improvement
-
to assess the set of draft recommendations as a whole within the context of patient risk groups and targeting recommendations (setting priorities)
-
to agree a final set of recommendations to circulate amongst the wider Intervention Development Group for comments and endorsement
-
to develop a strategy for wider engagement and dissemination of the agreed recommendations (within the context of the NHS England CHD Review).
These terms of reference were reviewed and agreed by the subgroup in their meeting on Monday 29 September 2014.
Appendix 11 Infant Heart Study: recommendations and suggested metrics
Document containing main recommendations of the intervention development group and suggested metrics from the Infant Heart Study as circulated to the Clinical Reference Group prior to their meeting on 26 March 2015
This document is based on the headline findings of IHS, a 2-year multicentre multidisciplinary research study funded by the NIHR with additional support from the Health Foundation. The primary funding for the IHS was from the NIHR Health Services and Delivery Research programme (project number 10/2002/29). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR Health Services and Delivery Research programme or the Department of Health. Please note that these are emerging findings, since the underpinning research data will be peer reviewed in 2015 within the grant report for this project.
Main suggestions
Structured discharge and transfer of care
-
All infants should eventually have a nationally standardised structured discharge document that is distributed electronically to all of the health professionals involved in their care. The IHS has proposed the minimum content for this discharge document based on the evidence gathered. A national template would need to be appropriately reviewed, piloted and evaluated. Meanwhile, the following recommendations are all amenable to auditing within the current disparate discharge documents:
-
At discharge from the specialist centre, all infants should have a named cardiologist, named paediatrician (with expertise in cardiology where possible) and named specialist nurse (e.g. cardiac liaison role or equivalent). Where it is not possible to allocate a named specialist nurse, there should be a named specialist nursing team. Responsibility for ensuring this lies with the specialist centre.
-
This should be documented in the discharge summary. The use of a template would facilitate local provision of audit data but in principle a template is not absolutely necessary. However, responsibility for providing audit figures for the proportions of discharge summaries containing this information should lie with the specialist centre.
-
-
Infants in the following groups should receive ‘step-down’ care, that is discharge via their local hospital:
-
all infants with HLHS, UVH or PA
-
all infants with neurodevelopmental conditions
-
all infants with length of stay in the specialist centre > 1 month.
-
Ideally this ‘step-down’ should be as an in-patient transfer (even if just for 24 hours). If this is infeasible owing to bed shortages then they should be admitted as a day case. At a minimum (given severe resource constraints) they should be seen as an outpatient as soon as possible (e.g. within 48 hours). In principle this can be audited using returns from the specialist centres.
-
-
At discharge home, either from the specialist centre or local hospital if step-down, all patients should also have a named GP and a named pharmacist (if discharged with a long-term prescription). It is more difficult to aggregate audit data for this recommendation for patients who have ‘stepped down’, although it is identified as an important aim. Pending better information exchange, it should still be possible for specialist centres to report the proportion of patients discharged without a named GP.
Home monitoring
-
Home monitoring should be provided for all infants with a primary diagnosis of HLHS, functionally univentricular heart or pulmonary atresia (including PA + intact ventricular septum). There should be a nationally agreed protocol for home monitoring of these patients that is based on the best available evidence; this may take some time to achieve. The IHS recommends that further research is conducted on the effectiveness of constituent components of home monitoring. However, in the meantime, it should be possible for specialist centres to provide audit data showing the proportion of patients in these categories who are on home monitoring pathways, however defined locally.
Guidance on signs, symptoms and response (e.g. a traffic-light tool)
-
All families and all of the health professionals involved in their support should receive the same clear guidance on ‘what is normal’ for that child, signs and symptoms to look for, how to respond and important contact numbers, for example in the form of a traffic-light tool. Ideally the format and content of this guidance should be standardised nationally, with scope for tailoring to local areas/networks as appropriate. This may take some time and cannot be implemented immediately, although it will be worth considering how best to take this recommendation forward nationally.
-
The IHS demonstrated that there is an urgent need for such guidance (e.g. traffic-light tool) to be developed and recommends that it should be evidence-based as far as possible and that its implementation should be evaluated (i.e. its impact on families and health professionals monitored).
Information and training for families prior to discharge
-
Health professionals should use a nationally standardised checklist in order to plan, deliver and audit the provision of training and information for all families prior to discharge. The IHS will propose the content of a checklist based on the evidence gathered. A national checklist would need to be appropriately reviewed, piloted and evaluated. Again, this may take some time to agree nationally and it will be worth considering how best to take this recommendation forward.
Network review of deaths outside specialist centre
-
The post discharge death of any infant outside a specialist centre should be reported to the specialist centre and reviewed at a Network Mortality and Morbidity meeting attended in person, by teleconference or at least a written summary be distributed for quality improvement purposes. In principle, the paediatric team with a relationship to the deceased patient should perform the fact-checking and lead the discussion. Looking ahead, every death outside the specialist hospital should be recorded on a standard pro forma documenting the antecedents of every death. This could potentially be aggregated nationally, although further discussion about how this could be done is required.
Family buddying
-
All families should be offered an opportunity to connect with other families (e.g. through social media or charity support groups) and those families more likely to experience language/cultural barriers to accessing support should be offered buddying. The IHS notes that there would need to be appropriate infrastructure to support this (e.g. training for buddies) and that it may be best facilitated through the charity sector.
This is not easily audited in the first instance, but if the charity sector is prepared to evolve such a facility, it may be possible eventually to provide information about the beneficiaries.
Suggested metrics: for immediate consideration for the Quality Dashboard
The evidence and suggestions from IHS would support inclusion of the following process and outcome measures on the Quality Dashboard.
Please note, however, that while IHS suggests the monitoring of out-of-hospital outcomes, the analytical steps and processes required to do this are beyond the scope of IHS and would need to be considered by the National Congenital Heart Diseases Audit [at National Institute for Cardiovascular Outcomes Research (NICOR)].
Deaths outside a specialist centre within 1 year following infant heart surgery.
Emergency unplanned readmissions to PICU within 1 year following infant heart surgery for children with a primary diagnosis of HLHS, functionally univentricular heart or pulmonary atresia (including PA + IVS). b
Process measuresPercentage of infants who, at discharge from the specialist centre, have a named cardiologist.
Percentage of infants who, at discharge from the specialist centre, have a named paediatrician (with expertise in cardiology where possible).
Percentage of infants who, at discharge from the specialist centre, have a named specialist nurse (e.g. cardiac liaison role or equivalent) or named specialist nursing team.
Percentage of infants who, at discharge from the specialist centre, have a named cardiologist.
Percentage of infants who do not have a named GP at discharge from specialist centre.
Percentage of infants with a primary diagnosis of HLHS, functionally univentricular heart or pulmonary atresia (including PA + IVS) who are on home monitoring pathways, however defined locally.
Although IHS strongly recommends the monitoring of out-of-hospital outcomes, the analytical steps and processes required to do this are beyond the scope of the Study and would need to be considered by the National Congenital Heart Diseases Audit (at NICOR).
This is the patient subgroup for which home monitoring is recommended.
Suggested metrics: to revisit next year for the Quality Dashboard
The IHS surfaced a number of important and potentially problematic service areas for which there is considerable variability across the country and for which there are currently no guidelines or protocols. Evidence from the IHS supports the monitoring of metrics relating to these, but acknowledges that it would not be feasible to include them on the Quality Dashboard without some further work, and that indeed it may not be feasible to include all of them. The IHS findings would first need to be disseminated more widely within the community and agreement reached about the steps required for implementing the recommendations. For example, a number of metrics relate to standardised documents/protocols that would need to be developed and appropriately piloted beforehand. Others require further consideration as to how the information could be collected and/or aggregated. Furthermore, some may be considered more appropriate for local monitoring for improvement purposes, or linked into a CQUIN, rather than for inclusion on the national Quality Dashboard.
The IHS therefore suggests that the feasibility and appropriateness of including any or all of the following metrics on the Quality Dashboard should be revisited in the next 1 to 2 years and, in the meantime, steps taken to develop these areas.
Emergency unplanned readmissions to PICU within 1 year following infant heart surgery (for all infants).
Process measuresPercentage of infants with HLHS/UVH/PA/neurodevelopmental conditions/length of stay in the specialist centre > 1 month that receive ‘step-down’ care (i.e. discharge via their local hospital).
Percentage of infant deaths outside the specialist hospital that are discussed at a Network Mortality and Morbidity meeting, with details recorded on a nationally standardised pro forma.
Percentage of infants for whom a nationally standardised structured discharge document is completed prior to discharge and distributed electronically to all of the health professionals involved in their care.
Percentage of infants with HLHS/UVH/PA who are following a nationally agreed protocol for home monitoring.
Percentage of families that receive nationally agreed guidance on ‘what is normal’ for that child, signs and symptoms to look for, how to respond and important contact numbers (e.g. in the form of a traffic-light tool).
Percentage of infants for whom all of the health professionals involved in their care receive nationally agreed guidance on ‘what is normal’ for that child, signs and symptoms to look for, how to respond and important contact numbers (e.g. in the form of a traffic-light tool).
Percentage of families that receive all required training and information prior to discharge (facilitated using a nationally standardised checklist).
Percentage of infants who, at discharge home (either from specialist or local hospital), have a named GP.
Percentage of infants who, at discharge home (either from specialist or local hospital), have a named pharmacist (if discharged with a long-term prescription).
Percentage of families offered an opportunity to connect with other families (e.g. through social media or charity support groups).
Percentage of families more likely to experience language/cultural barriers to accessing support that are offered buddying.
Although IHS strongly recommends the monitoring of out-of-hospital outcomes, the analytical steps and processes required to do this are beyond the scope of the IHS and would need to be considered by the National Congenital Heart Diseases Audit (at NICOR).
Patient-Reported Experience Measures
The IHS supports the monitoring of patient reported experience measures alongside the process and outcome measures above, for example adopting a similar approach to the specialist Quality Dashboards (heart transplant, ECMO), which include three questions relating to patient-reported experience measures. This area would require further development work.
The importance of joined up service specifications/commissioning across specialist, local and community services
We emphasise that IHS findings demonstrate the need and potential for improvements across the entire patient journey spanning community, primary, secondary and specialist services. This would require service specifications and commissioning to be addressed not only for the specialist services commissioned by the Congenital Heart Services clinical reference group, but for all of these sectors. The IHS’s evidence of weak links across sectors and poor communication between different health professionals further suggests the need for joined-up service specifications and models of commissioning across the whole patient journey, including local and community settings.
Further information about the IHS is available from:
Sonya Crowe: xxxx
Kate Brown: xxxx
Kate Bull: xxxxx
Appendix 12 NHS England congenital heart disease review consultation report: references to the Infant Heart Study
Quotations in this appendix have been reproduced from: NHS England. Consultation on Draft Standards and Service Specifications for Congenital Heart Disease Services: Final Report. Dialogue by Design. 2015.
Page 59: suggestions on the proposed standards for nursing
One organisation makes a list of specific suggestions based on the findings of a recent two-year multicentre multidisciplinary research study. The study found that specialist nurses provide essential support and are often the link between local and specialist centres. This link was best demonstrated in cases where specialist nurses attended local clinics or trained key link nurses in local teams. The study makes a list of recommendations, some of which are already included in the draft standards:
at discharge from the specialist centre, all infants should have a named specialist nurse (e.g. cardiac liaison role or equivalent). Where it is not possible to allocate a named specialist nurse, there should be a named specialist nursing team;
all families should receive ‘check-in’ telephone calls from their named specialist nurse (team), the frequency of which should be determined by their needs;
a specialist nurse should attend all outpatient clinics and outreach clinics;
all families should have access to a telephone support service led by specialist nurses;
having cardiac trained nurses in the community (or formal training once a year for community paediatric nurses); and
training key link nurses in local hospitals to establish direct links and familiarity with specialist centre protocols.
The same organisation makes recommendations on how to improve the community nursing service, which the study found to be inconsistent:
all patients with a medical need should have access to community nursing which should be supported by the specialist centre; and
home monitoring should be provided for all patients with a primary diagnosis of HLHS5, functionally univentricular heart or pulmonary atresia; community nurses may need to run Skype clinics (or just clinics) rather than provide home visits.
Page 60: suggestions on the proposed standards for psychologists
The organisation drawing on the study mentioned above makes a number of recommendations for psychological support. The study found that psychological support was insufficient and focused mainly on patients’ medical rather than psychological needs. The recommendations are:
psychosocial meetings should be held after ward rounds in the specialist centre (led by the lead specialist nurse and psychologist) in order to determine needs and liaise with local or referral services as appropriate;
for families identified to have psychosocial needs, a multidisciplinary team including psychosocial involvement should be established as early as possible (2–3 days prior to discharge or earlier) with all team members invited to a discharge planning meeting (either in person or via teleconference/Skype);
families with psychosocial needs should receive more frequent phone calls (‘checking in’) from their named specialist nurse (team) and additional visits from a health visitor who is able to provide support and refer on to a psychologist if necessary; and
for families with psychosocial concerns, learning difficulties or difficulty communicating in English, the Study recommended referral to a health visitor/social work team to assist in ongoing training support (in their own home).
Page 61: suggestions on the proposed standards for cardiologists and paediatricians
The study mentioned above identified knowledge gaps between specialists and non-specialists as well as poor communication between health professionals, which could result in specialist centres not knowing what local and community services are available. The findings also showed that the level of local support across the country varied and that the number of available Paediatricians with Expertise in Cardiology was often insufficient. The positive counterexamples were cases where a Paediatrician with Expertise in Cardiology had links with the specialist centres and often ran joint outreach clinics with cardiologists. Drawing on this study, the organisation makes the following recommendations:
all patients should have a named paediatrician (with expertise in cardiology where possible);
for all patients, responsibility for care co-ordination should be transferred to the named paediatrician at discharge from the specialist centre. The named paediatrician and GP are responsible for referring to local services and maintaining effective communication between health professionals and should act as a consistent point of contact in their locality; and all patients should be seen by their named paediatrician and named cardiologist at joint outreach clinics.
Page 116: information sharing
Another refers to a research suggesting that referral processes and sharing of patient information between different professionals involved in CHD care are inconsistent and often poorly coordinated.
Page 118: organisation, governance and audit
One respondent makes a specific suggestion relating to audit. They suggest a number of data fields should be added to routine cardiac audit including birth gestation and birth weight.
Page 119: home monitoring
One research team makes specific reference to their study which recommends that further research is conducted on the effectiveness of the constituent components of home monitoring.
Page 122: information for patients
Respondents believe patients/parents/carers should be given clear guidance about what to expect with a diagnosis of CHD. Information provided should include comprehensive material about the signs and symptoms that are considered ‘normal’ and how to cope in unfamiliar situations, with the inclusion of important contact numbers and helpful advice.
List of abbreviations
- A&E
- accident and emergency
- ASD
- atrial septal defect
- AVSD
- atrioventricular septal defect
- CART
- categorisation and regression tree
- CHD
- congenital heart disease
- CHF
- Children’s Heart Federation
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLN
- cardiac liaison nurse
- ECMO
- extracorporeal membrane oxygenation
- FI
- family interview
- GP
- general practitioner
- HLHS
- hypoplastic left heart syndrome
- HLI
- helpline staff interview
- HMP
- home monitoring programme
- HP
- health professional
- HPI
- health professional interview
- HV
- health visitor
- IHS
- Infant Heart Study
- IMD
- Index of Multiple Deprivation
- IPCCC
- International Pediatric and Congenital Cardiac Code
- IVS
- intact ventricular septum
- LOE
- level of evidence
- LOS
- length of stay
- MBTS
- modified Blalock–Taussig shunt
- NCHDA
- National Congenital Heart Diseases Audit
- NICOR
- National Institute for Cardiovascular Outcomes Research
- NIHR
- National Institute for Health Research
- OF
- online discussion forum
- PA
- pulmonary atresia
- PDA
- patent ductus arteriosus
- PEC
- paediatrician with expertise in cardiology
- PICANet
- Paediatric Intensive Care Audit Network
- PICU
- paediatric intensive care unit
- RR
- risk ratio
- RVPA
- right ventricle-to-pulmonary artery conduit
- SD
- standard deviation
- SV
- single ventricle
- SVR
- single ventricle reconstruction
- TAPVC
- total anomalous pulmonary venous connection
- TGA
- transposition of the great arteries
- UVH
- univentricular heart
- VSD
- ventricular septal defect